
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 62
1,2-DICHLOROETHANE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1987
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154262 4
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1987
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
1. SUMMARY AND CONCLUSIONS
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production, disposal of waste, and uses
3.2.1.1 Production levels
3.2.1.2 Production processes
3.2.1.3 Disposal of wastes
3.2.1.4 Uses
3.3. Transport and fate in the environment
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. Environmental levels
4.1.1. Water
4.1.2. Air
4.1.3. Food
4.1.4. Industrial wastes
4.2. General population exposure
4.3. Occupational exposure
5. KINETICS AND METABOLISM
5.1. Absorption
5.2. Distribution
5.3. Metabolism
5.4. Excretion and elimination
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.1.1. Acute toxicity
6.1.2. Short-term exposures
6.1.3. Long-term exposure
6.1.4. Bioconcentration
6.2. Microorganisms
6.3. Terrestrial toxicity
6.3.1. Birds
6.3.2. Plants
7. EFFECTS ON ANIMALS
7.1. Single exposures
7.1.1. Inhalation and oral exposure
7.1.2. Skin and eye irritation
7.2. Short-term exposures
7.2.1. Inhalation exposure
7.2.2. Oral exposure
7.3. Long-term exposure
7.3.1. Inhalation exposure
7.3.2. Oral exposure
7.4. Carcinogenicity
7.4.1. Inhalation exposure
7.4.2. Oral exposure
7.4.3. Dermal exposure
7.5. Mutagenicity and related end-points
7.5.1. Mutations
7.5.1.1 Bacteria
7.5.1.2 Fungi
7.5.1.3 Insects
7.5.1.4 Mammals/mammalian
7.5.2. Chromosome damage/DNA damage
7.5.3. Cell transformation
7.6. Reproduction and teratogenicity
7.6.1. Inhalation exposure
7.6.2. Oral exposure
7.7. Immunotoxicity
8. EFFECTS ON MAN
8.1. Accidental exposures
8.1.1. Inhalation exposure
8.1.2. Oral exposure
8.1.3. Acute effects on eyes and skin
8.2. Occupational exposure
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1. Evaluation of human health risks
9.2. Evaluation of effects on the environment
9.2.1. Air
9.2.2. Water
9.2.3. Soil
10. RECOMMENDATIONS FOR FURTHER STUDIES
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
WHO TASK GROUP ON 1,2-DICHLOROETHANE
Members
Dr B. Gilbert, CODETEC, University City, Campinas, Brazil
Professor P. Grasso, Robens Institute, University of Surrey,
Guildford, Surrey, United Kingdom
Mr M. Greenberg, Environmental and Criteria Assessment Office,
US Environmental Protection Agency MD-52, Research Triangle
Park, North Carolina, USA (Rapporteur)
Professor M. Ikeda, Department of Environmental Health, Tohoku
University School of Medicine, Sendai, Japan (Chairman)
Dr N.N. Litvinov, A.N. Sysin Institute of General and Community
Hygiene, USSR Academy of Medical Science, Moscow, USSR
(Vice-Chairman)
Dr G.B. Maru, Carcinogenesis Division, Cancer Research Insti-
tute, Tata Memorial Center, Parel, Bombay, India
Professor M. Noweir, Occupational Health Research Centre, High
Institute of Public Health, University of Alexandria,
Alexandria, Egypt
Dr E. Rauckman, Carcinogenesis and Toxicologica Evaluation
Branch, National Institute of Environmental Health Sciences,
National Toxicology Program, Research Triangle Park, North
Carolina, USA
Professor D.J. Reed, Environmental Health Sciences Center,
Oregon State University, Corvallis, Oregon, USA
Dr E. Rosskamp, Institute for Water, Soil and Air Hygiene of
the Federal Ministry of Health, Berlin (West)
Dr S. Susten, Document Development Branch, Division of Standards
Development and Technology Transfer, National Institute for
Occupational Safety and Health, Cincinnati, Ohio, USA
Secretariat
Professor F. Valic, Andrija Stampar School of Public Health,
University of Zagreb, Zagreb, Yugoslavia (Secretary)a
Dr T. Ng, Office of Occupational Health, World Health Organ-
ization, Geneva, Switzerland
Ms F. Ouane, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr T. Vermeire, National Institute of Public Health and
Environmental Hygiene, Bilthoven, Netherlands (Temporary
Adviser)
Mr J. Wilbourn, Unit of Carcinogen Identification and
Evaluation, International Agency for Research on Cancer,
Lyons, France
a IPCS Consultant.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to
the Manager of the International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland, in order that
they may be included in corrigenda, which will appear in
subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained
from the International Register of Potentially Toxic Chemicals,
Palais des Nations, 1211 Geneva 10, Switzerland (Telephone no.
988400 - 985850).
ENVIRONMENTAL HEALTH CRITERIA FOR 1,2-DICHLOROETHANE
A WHO Task Group on Environmental Health Criteria for
1,2-Dichloroethane met in Geneva from 25 to 30 August, 1986.
Professor F. Valic opened the meeting on behalf of the Director-
General. The Task Group reviewed and revised the draft criteria
document and made an evaluation of the health risks of exposure
to 1,2-dichloroethane.
The drafts of this document were prepared by DR T. VERMEIRE
of the National Institute of Public Health and Environmental
Hygiene, Bilthoven, the Netherlands.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract from
the National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA - a WHO
Collaborating Centre for Environmental Health Effects. The
United Kingdom Department of Health and Social Security
generously supported the costs of printing.
1. SUMMARY AND CONCLUSIONS
1,2-Dichloroethane (DCE) is a colourless, flammable, and
volatile liquid that decomposes slowly in the presence of air,
moisture, and light; its vapour decomposes in flame and on hot
surfaces yielding hydrogen chloride, phosgene, and other
chlorine-containing compounds.
Sensitive analytical methods have been developed for the
determination of 1,2-dichloroethane using gas chromatography.
Detection limits are in the range of 0.02 - 1.2 µg/m3 air,
0.001 - 10 µg/litre water, 25 µg/litre blood, and 44 - 100
µg/kg food or tissue. Methods used for air analysis
include direct ultraviolet or infrared spectroscopy and direct
reading colorimetry tubes.
World production of 1,2-dichloroethane in 1981 was estimated
to be about 23 000 kilotonnes. In 1983, the chemical was ranked
as the 15th highest volume chemical produced in the USA. It is
principally used in the synthesis of vinyl chloride. Human
exposure mainly occurs at, and in the vicinity of, production
facilities, through skin contact and inhalation. Almost 60% of
the total emission (about 0.2% of production) is estimated to be
lost to the air, water, and soil from these industries; nearly
one-third of this loss is estimated to occur via disposal of
heavy ends in vinyl chloride production (EDC tars). Human
exposure to the vapour as a result of dispersive uses of 1,2-
dichloroethane can occur when it is used in gasoline or as a
solvent or seed fumigant. Losses via dispersive uses account
for about 40% of the total emission. Emissions also occur from
contaminated water and from waste-disposal sites.
Average concentrations found in the vicinity of production
facilities have been below 40 µg/m3. In the air of cities,
average concentrations of between 0.3 and 6.5 µg/m3, with a
reported maximum of 30 µg/m3, have been measured. Only two old
reports on small groups of occupationally-exposed men are
available, indicating exposure levels of 40 - 800 mg/m3.
In air, 1,2-dichloroethane is degraded by sunlight fairly
rapidly yielding mainly carbon oxides and hydrogen chloride.
This prevents accumulation in the atmosphere.
Emissions of 1,2-dichloroethane entering water may amount to
about 0.1% of production volume; in addition, some of the
emissions from EDC tars will also contaminate water. However,
average levels in drinking-water are generally below 1
µg/litre. The main removal process from water is
evaporation, since chemical degradation, biodegradation, and
bioconcentration are unlikely to occur.
LC50 values for fish exposed for 1 - 4 days ranged between
85 and 550 mg/litre water, with bioaccumulation unlikely. A no-
observed-adverse-effect level of 11 mg/litre was found for
Daphnia magna, following long-term exposure. 1,2-Dichloro-
ethane does not pose a signficant hazard for the aquatic
environment, except in cases of accident or inappropriate
disposal.
1,2-Dichloroethane is readily absorbed via the dermal, oral,
or inhalation routes.
After oral administration, blood levels peak earlier with
low than with high doses, and are 5 times higher with oral
exposure to doses of the order of 150 mg/kg body weight than
with similar inhalation exposure. After inhalation, a
disproportionate increase in blood level occurs with increasing
dose. At an exposure level of 3200 mg/m3, a steady blood level
is achieved after 2 - 3 h. After oral dosing, 1,2-dichloro-
ethane showed a preference for adipose tissue and liver.
Following inhalation, accumulation was mainly observed in
adipose tissue, but not in the liver. At higher levels of
exposure, relatively more 1,2-dichloroethane accumulated.
1,2-Dichloroethane was found in fetal tissues and in the
placenta, when pregnant rats were exposed by inhalation to the
compound at 1000 mg/m3 for 3 days.
When administered orally, parenterally, or by inhalation to
rats and mice, it is extensively biotransformed to urinary
metabolites (55 - 90%). Relatively more is metabolized at lower
doses. Metabolism may occur via two known pathways: one via
cytochrome P-450-mediated oxidation and the other via
glutathione conjugation. The former pathway involves the
formation of 2-chloroacetaldehyde and 2-chloroethanol. Although
this pathway appears to be important in vitro in producing
intermediates capable of interacting with DNA, it does not
appear to be important in vivo. Reactive intermediates are
formed when 1,2-dichloroethane is metabolized via glutathione
conj ugation. The identity of these intermediates has not been
confirmed, though some evidence suggests the formation of S-(2-
chloroethyl) glutathione and its alkylating episulfonium ion,
which, by reaction with DNA, yield an indicated adduct, S-[2-
(N7-guanyl)-ethyl] glutathione.
Excretion of 1,2-dichloroethane or its metabolites from
rodents is rapid. At least 89% of the body burden was excreted
via the lungs or urine within 24 h in intraperitoneally-injected
mice and within 48 h in orally-dosed mice.
The oral LD50 was found to be 413 - 489 mg/kg body weight in
the mouse, 680 - 850 mg/kg body weight in the rat, and 2500
mg/kg body weight in the dog. The 6-h LC50 was estimated to be
1060 mg/m3 in the mouse and 5100 - 6660 mg/m3 in the rat.
Deaths occurred within a narrow range of concentrations.
In an exposure-response inhalation study on rats, no adverse
effects were observed in a 7-h exposure to 1200 mg/m3. At the
next higher exposure level (2400 mg/m3), depression of the
central nervous system (CNS) was observed, and some of the rats
died after 7 h. As the exposure levels increased, depression of
the CNS became more severe, and deaths occurred after
progressively shorter exposure periods. At the highest
concentration (81 000 mg/m3), rats became comatose and some died
within 0.3 h. Liver and kidney damage was found in most of the
animals that died.
After single oral doses of 615 - 770 mg/kg body weight,
liver damage was observed histologically in rats. Myocardial
oedema and damage to coronary vessels were observed.
In 3 short-term inhalation studies, various species were
exposed to concentrations of between 405 and 3900 mg 1,2-
dichloroethane/m3 air, 6 or 7 h per day, for 5 days/week. Mice
and rats appeared to be more sensitive than guinea-pigs,
rabbits, monkeys, dogs, and cats. The overall no-observed-
adverse-effect level for exposure periods ranging from 4 to 9
monhts in the rat was about 400 mg/m3. Signs of intoxication,
including central nervous system depression and death, were
observed in all species exposed to the higher concentrations of
between 1620 and 3900 mg/m3. For rats, liver damage, mainly
consisting of fatty changes, was observed after exposure to 1540
mg/m3 for up to 12 weeks, 1620 mg/m3 for up to 8 weeks, and 1900
mg/m3 for up to 1 week. In rats, guinea-pigs, and mice, an
increased mortality rate was observed at concentrations of 730
mg/m3 or more. In rabbits, there was an increase in the
mortality rate from 1540 mg/m3 and, in monkeys, from 1620 mg/m3.
Dogs and cats only showed increased mortality at 3900 mg/m3.
Repeated oral administration of 1,2-dichloroethane at a dose
of 300 mg/kg body weight was lethal for rats after 5 doses and
produced necrosis and fatty changes in the liver. No effects
were observed when the chemical was given orally to rats at 10
mg/kg body weight daily for 90 days or at 150 mg/kg, 5 times per
week, for 2 weeks.
1,2-Dichloroethane is weakly mutagenic in Salmonella
typhimurium TA 1535, both in the absence of, and in the presence
of, a microsomal activation system. However, in the presence of
cytosolic glutathione-S-transferase, a stronger positive
response was obtained. Negative results were obtained with
strains TA 1537, TA 1538, and TA 98. Mutagenicity occurs in
fungi, Drosophila, and mammalian cells in vitro. In two human
cell lines exposed to 1,2-dichloroethane, the incidence of gene
mutations was found to increase with increasing levels of
glutathione-S-transferase. Micronuclei or dominant lethals were
not induced, and a weak mutagenic effect was reported in a spot
test on mice. DNA damage has been observed in bacteria,
mammalian cells in vitro, and in mammals in vivo. 1,2-
Dichloroethane did not induce cell transformation in one of two
assays, and enhanced virus-induced cell transformation in the
other.
1,2-Dichloroethane is carcinogenic in B6C3F1 mice and
Osborne-Mendel rats following administration of doses of 50 -
300 mg/kg body weight, given by gavage, in oil. In male rats,
squamous cell carcinomas of the forestomach, subcutaneous
fibromas, and haemangiosarcomas in several organs (mainly the
spleen) were produced following gavage; in female rats, mammary
gland fibromas and mammary adenocarcinomas were increased. In
mice, increased incidences of hepatocellular carcinomas in males
and mammary gland adenocarcinomas in females, and lung adenomas
in both sexes were observed. No increase in tumour incidence
was reported in inhalation studies on Swiss mice and Sprague
Dawley rats exposed to concentrations of up to 607 mg/m3.
A prolongation of the estrus cycle, an increase in embryonal
mortality, pre-implantation losses, and haematomas were found
when female rats were exposed to 15 mg/m3, 4 h per day, 6
days/week, for 4 months prior to mating and during pregnancy.
While the fetal toxicity of 1,2-dichloroethane was not confirmed
at higher exposure levels, severe toxic effects on rats were
observed and all implantation sites resorbed. No fetal
abnormalities were observed in the rabbit. Oral adminstration
to male and female rats of up to 35 mg 1,2-dichloroethane/kg
body weight per day, via the food, for up to 2 years, did not
affect reproduction. No effects on fertility or gestation
index, and no teratological effects were observed in a 2-
generation study on mice treated with 5 - 50 mg 1,2-
dichloroethane/kg body weight per day, via drinking-water, for
up to 25 weeks.
1,2-Dichloroethane may cause severe corneal damage in
animals, but no gross skin reactions occurred in a 12-h occluded
patch test on guinea-pigs. Corneal opacity was observed in
dogs, following subcutaneous injection.
In man, immersion of the hands for 4 h at intermittent
intervals caused severe dermatitis. Conjunctivitis has been
reported from exposure to vapour, and corneal opacity from
accidental ingestion.
Two early reports describe human effects from occupational
exposure, and a number of fatal case histories through
accidental oral ingestion. Complaints referrable to the CNS,
liver, and gastrointestinal tract were reported in workers
occupationally exposed to concentrations of 1,2-dichloroethane
of 250 - 800 mg/m3. Similar complaints were reported less
frequently by workers exposed to concentrations of 40 - 150
mg/m3. Liver and bile-duct disorders, neurotic conditions,
autonomous dystonia, neuromyalgia, and hyperthyroidism have been
reported in workers exposed to 5 - 150 mg 1,2-dichlorethane/m3.
Accidental ingestion of 10 - 250 g 1,2-dichloroethane
resulted in death in all instances. Haemorrhage at various
sites, depression of the CNS, liver and kidney damage, and
pulmonary oedema occurred.
Making an overall evaluation, in the absence of human data,
and taking into account that: (a) 1,2-dichloroethane produces a
reactive intermediate that alkylates DNA, (b) it is positive in
a number of mutagenicity tests in vitro, though weakly so, and
(c) both rare and common tumours are produced in rats and mice,
it would be prudent to consider 1,2-dichloroethane as a possible
human carcinogen. Thus, it should be regarded, for practical
purposes, as if it presented a carcinogenic risk for man. In
evaluating reproduction hazards and teratogenicity, it is
necessary to rely on the limited data available from laboratory
investigations since there are no human data. The weight of
evidence does not suggest that exposure to prevailing
environmental levels poses a reproductive or teratogenic
hazard.
Degradation processes are rapid enough to prevent accumu-
lation of 1,2-dichloroethane in the atmosphere. Except in the
case of accidents or inappropriate disposal, 1,2-dichloroethane
does not present a significant hazard for the aquatic
environment. Available data are not sufficient to evaluate its
effects on soil.
Further studies are needed on: (a) DNA alkylation (adduct
identification); (b) sub-chronic toxicity by various routes of
exposure; (c) assessment of the extent to which EDC tars
contribute to contamination of groundwater by 1,2-dichloro-
ethane; and (d) dose-response on sensitive, commercially
important fish species (particularly studies relevant to EDC tar
spills).
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Structural formula: H H
| |
Cl--C---C--Cl
| |
H H
Molecular formula: C2H4Cl2
Abbreviation: EDC
Synonyms: alpha,beta-dichloroethane, 1,2-bi-
chloroethane, ethane dichloride,
ethylene chloride, ethylene dichlor-
ide, 1,2-ethylene dichloride, sym-
(metric)-dichlorethane
Common trade names: Borer-Sol, Brocide, Destruxol
Dichlor-emulsion, Dichlor-mulsion,
Dutch Liquid, Dutch Oil, ENT 1656,
Gaze Olefiant
CAS registry number: 107-06-2
Conversion factor: 1 ppm = 4.05 mg/m3 air at 25 °C and
101.3 kPa (760 mmHg)
2.2 Physical and Chemical Properties
1,2-Dichloroethane is a flammable compound that burns with a
smoky flame. When dry, 1,2-dichloroethane is stable at ordinary
temperatures. In the presence of air, moisture, and light, the
liquid decomposes slowly, yielding hydrogen chloride and other
corrosive products. Vapour-air mixtures are readily ignited.
In a flame, or at a hot surface, 1,2-dichloroethane decomposes,
yielding hydrogen chloride, phosgene, and other chlorine-
containing compounds. Some physical characteristics of 1,2-
dichloroethane are given in Table 1.
2.3 Analytical Methods
A summary of relevant methods of sampling and analysis is
presented in Table 2.
Table 1. Some physical characteristics of 1,2-dichloroethane
-------------------------------------------------------------
Physical state liquid
Colour colourless
Taste sweet
Odour chloroform-like
Odour threshold 25 - 450 mg/m3, for perception;
162 - 750 mg/m3 for recognitiona
Relative molecular mass 98.96
Melting point -35 °C
Boiling point 83 °C
Water solubility 8.69 g/litre, 20 °C
log n-Octanol/water 1.48
partition coefficient
Relative density 1.23, 20 °C
Relative vapour density 3.42
Vapour pressure 8.53 kPa (64 mmHg), 20 °C
Flash point 13 °C (closed cup)
Flammable limits 0.25 - 0.64 g/litre, 6 to 16 vol %
-------------------------------------------------------------
a From: May (1966), Hellmann & Small (1974),
and Kleinschmidt (1983).
Table 2. Sampling, preparation, analysis
---------------------------------------------------------------------------------------------------------
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
---------------------------------------------------------------------------------------------------------
air manual sampling colorimetry, not specific Saltzman
pump direct reading (1972)
indicating tube
air continuous monit- UV-photodetection approximately not specific, contin- Nelson &
oring with or 4 mg/m3 uous monitoring por- Shapiro
without built-in table halide meter (1971)
aspirator
air continuous monitoring infra-red spectro- subject to interfer- Baretta
and breath analysis scopy ence by similar com- et al.
pounds (1969)
air sampling on charcoal desorption by car- 0.013 ug 3 - 40 NIOSH
bon disulfide, gas per sample litre (1977)
chromatography with
flame ionization
detection
air sampling on charcoal thermal desorption; 1.2 ug/m3 10 litre Parkes
or Chromosorb gas chromatography et al.
with flame ioniza- (1976)
tion detection
air gas chromatography 0.02 ug/m3 20 ml direct injection Grimsrud &
with mass spectro- Rasmussen
metric detection (1975)
air sampling on Tenax thermal desorption; 0.032 ug/m3 Krost et
polymeric beads gas chromatography al. (1982)
with mass spectro-
metric detection
---------------------------------------------------------------------------------------------------------
Table 2 (contd).
---------------------------------------------------------------------------------------------------------
water stripping by helium thermal desorption; 0.001 ug/litre Sauer
adsorption on Tenax gas chromatography (1981)
with flame ioniza-
tion detection or
mass spectrometric
detection
water stripping by helium thermal desorption; 0.1 - 0.4 5 ml Symons
or nitrogen, sorption gas chromatography ug/litre et al.
on Tenax or Chromo- with microcoulo- (1975)
sorb metric detection
water gas chromatography 0.5 ug/litre 0.1 ml direct injection, di- Fujii
with mass spectro- glycerol precolumn (1977)
metric detection
water gas chromatography 10 ug/litre 1 litre headspace analysis Piet
with electron cap- et al.
ture detection (1978)
blood, gas chromatography 25 ug/litre 1 ml headspace analysis of Zuccato
tissue with flame ioniza- blood, blood, acidified blood and et al.
tion detection 50 ug/kg 0.5 g tissue homogenate (1980)
tissue (wet tissue
weight)
food extraction by gas chromatography 100 ug/kg 5 - analysis of fumigant Heuser &
acetone-water (5:1 with ß-ionization 10 g residues Scudamore
by volume) detection (1969)
food, stripping by nitrogen elution by pentane, 44 ug/kg 10 g also suitable for air Bauer
tissue, sorption on XAD-2 gas chromatography solid solid, analysis (elution by (1981)
water with electron cap- (wet weight), 2 litre pentane-ether, detec-
ture detection 0.2 ug/litre tion limit 2.9 ug/m3
water for a 0.3 m3 sample)
---------------------------------------------------------------------------------------------------------
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1 Natural Occurrence
There are no reports of 1,2-dichloroethane occurring as a
natural product.
3.2 Man-Made Sources
3.2.1 Production, disposal of waste, and uses
3.2.1.1 Production levels
In 1983, 1,2-dichloroethane was ranked as the 15th highest
volume chemical produced in the USA (Webber, 1984). World
production in 1981 was estimated to be 23 130 kilotonnes (Gold,
1980). In the countries of the European Community, the
production capacity was estimated to be 9446 kilotonnes in 1982
(DeQuinze et al., 1984), an increase over the estimated 5290
kilotonnes capacity reported for 1977 (Atri, 1984). In the USA,
production increased from 4750 kilotonnes in 1977 (Drury &
Hammons, 1979) to 5740 kilotonnes in 1983 (Webber, 1984). In
Japan, a total production of 1800 kilotonnes was reported in
1976 (IARC, 1979).
3.2.1.2 Production processes
Two processes are used, which are very often combined into
one so-called "balanced process". The first is the vapour or
liquid phase reaction of chlorine with ethene in the presence of
a catalyst, usually 1,2-dibromoethane or metal chlorides. The
second is the reaction of ethene with oxygen and hydrogen
chloride in the presence of the catalyst, copper (II) chloride
(Drury & Hammons, 1979).
Most commercial 1,2-dichloroethane is 97 - 99% pure and
contains approximately 0.1% by weight of alkylamines to inhibit
decomposition. Impure 1,2-dichloroethane may contain poly-
chlorinated ethanes, and the uninhibited product may also
contain chlorine and/or hydrogen chloride (Drury & Hammons,
1979; IARC, 1979).
The above production processes and the production of end-
products, mainly vinyl chloride, are important sources of
emission of 1,2-dichloroethane into the environment. In 1979,
in the USA, almost 60% of the total emission of 12 kilotonnes
was lost by these industries to the air, water, and soil and
about 40% via dispersive uses as a solvent (Seufert et al.,
1980). In the USA, in 1977, approximately 35% of the emissions
of 1,2-dichloroethane associated with the production of the
compound itself and end-products were estimated to occur via
disposal of heavy ends, the so-called EDC tars, a mixture of
low- and high-boiling chlorinated hydrocarbons (Gold, 1980).
3.2.1.3 Disposal of wastes
Large amounts of western European tars used to be dumped in
the North Sea, but incineration at sea seems to be the present
practice (Jensen et al., 1975). In the USA, disposal of EDC
tars is usually by burial in a landfill or incineration (Drury &
Hammons, 1979; Gold, 1980).
3.2.1.4 Uses
The major industrial use of the compound is in the synthesis
of vinyl chloride (approximately 90% of the total production in
Japan and approximately 85% of total production in the USA)
(IARC, 1979). Other chemicals produced from 1,2-dichloroethane
are 1,1,1-trichloroethane, ethyleneamines, vinylidene chloride,
trichloroethylene, and tetrachloroethylene. In 1977, 2 - 4% of
the total production of 1,2-dichloroethane in the USA was used
for the synthesis of each of these chemicals. Another 2% was
used in the USA as a lead scavenger in gasoline. This
application will decline in importance with the world-wide
conversion to unleaded fuel. A small fraction of the total
production, approximately 0.1% in the USA in 1977, was used for
solvent and fumigant applications (Gold, 1980). When used as a
fumigant, 1,2-dichloroethane is usually mixed with carbon
tetrachloride to reduce the fire hazard, and small portions of
other fumigants may be added (WHO, 1972).
3.3 Transport and Fate in the Environment
Out of the total production of 1,2-dichloroethane in the USA
in 1979, approximately 0.2% was estimated to be lost to the
atmosphere, 40% of this during dispersive use as a solvent or
fumigant (Seufert et al., 1980). Evaporation from disposal
sites also occurs. In 1977, losses to the atmosphere were
estimated to be higher (1% of total production). Minimal
estimates for emissions entering water and for emissions via
EDC tars in 1977 were 0.1 and 0.5% of total production,
respectively (Gold, 1980).
Evaporation appears to be the major pathway by which 1,2-
dichloroethane is lost from water. In a controlled outdoor
experiment, the half-life for the disappearance from running
river water was found to be 1.4 h (Scherb, 1978). This agrees
well with laboratory findings (Dilling et al., 1975). Loss by
chemical reaction with water is insignificant (Radding et al.,
1977).
In the troposphere, rain-out and adsorption on atmospheric
aerosols are unlikely because of the high vapour pressure and
the low solubility of the compound (Cupitt, 1980). The major
part of the 1,2-dichloroethane is removed from the atmosphere
via oxidation by hydroxyl radicals. On the basis of experi-
mentally-derived rate constants, and hydroxyl radical concen-
trations of 4.8 x 106 and 1.0 x 106 radicals/ml, respectively,
half-lives for this reaction have been calculated of 10 days
(Radding et al., 1977) and 36 days (Howard & Evenson, 1976). A
lifetime of 53 days was predicted, which would preclude
accumulation in the troposphere and transport to the strato-
sphere (Howard & Evenson, 1976). The reported degradation
products are formyl chloride, hydrogen chloride, carbon dioxide,
carbon monoxide, and monochloroacetyl chloride (Pearson &
McConnell, 1975; Spence & Hanst, 1978). Since 1,2-dichloro-
ethane absorbs light within the solar spectral region, photo-
lytic transformation is possible (Cupitt, 1980). However, the
extent of this reaction has not been verified experimentally.
Slow biodegradation of 1,2-dichloroethane was observed in
fresh water, seeded by settled domestic waste water. Non-
acclimated cultures caused a biological demand of 16% of the
theoretical oxygen demand for the compound in 10 days (Price et
al., 1974). 1,2-Dichloroethane was biodegraded in aqueous media
by acclimated aerobic mixed cultures from soil or sewage samples
(Stucki et al., 1981; Tabak et al., 1981). An aerobic bacterium
G10, a Pseudomonas strain, that was able to use 1,2-dichloro-
ethane as a sole source of carbon and energy for growth, was
isolated from samples containing a mixture of activated sludge
and soil samples (Janssen et al., 1984). In addition, slow
anaerobic biodegradation, mainly to carbon dioxide, was observed
in an aqueous medium with a mixed methanogenic culture, grown on
waste activated sludge with sodium acetate as a primary
substrate (Bouwer & McCarty, 1983).
In soil, 1,2-dichloroethane adsorbs aselectively to
bentonite clay and peat moss, but not to dolomitic limestone and
silica (Dilling et al., 1975).
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 Environmental Levels
4.1.1 Water
Concentrations of 1,2-dichloroethane measured at different
locations are summarized in Table 3. It will be noted that
Symons et al. (1975) observed more positive samples in finished
water than in untreated water. This suggests treatment-related
contamination during water chlorination.
4.1.2 Air
Concentrations of 1,2-dichloroethane measured in air at
various locations are summarized in Table 4.
4.1.3 Food
Reports on 1,2-dichloroethane in food are scarce. Bauer
(1981) found that levels were generally low in the Federal
Republic of Germany. Milk products with added fruits contained
an average of 0.8 µg/kg. In Canada, 15 out of 34 samples of
spice oleoresins contained between 2 and 34 mg of 1,2-
dichloroethane, used as an extractant, per kg (Page & Kennedy,
1975).
In residue studies, various amounts of 1,2-dichloroethane
were found to remain in fumigated grain, depending on the type
of grain and fumigation mixture, exposure conditions, and the
extent of subsequent ventilation (Berck, 1965, 1974). Wheat was
found to contain the highest residue levels, varying from 16 to
213 mg/kg, following common fumigation practices. Processing
reduces residue levels; for example, 1 - 10 mg/kg were recovered
in ground wheat flour; less than 2 mg/kg was present in bread
(Lynn & Vorhes, 1957; Wit et al., 1969). In the United Kingdom,
1 out of 281 samples of wheat contained 1,2-dichloroethane at a
level of 290 mg/kg; in the remaining samples, the concentration
was below the detection limit of 4.0 mg/kg (Bailey et al.,
1982). 1,2-Dichloroethane at a level of 51 mg/kg in fumigated
soybeans was completely extracted by hexane, during the
production of oil by solvent extraction (Storey et al., 1972).
4.1.4 Industrial wastes
EDC tar originating from vinyl chloride production in 1974
(400 000 tonnes on a global basis) contained up to 35% 1,2-
dichloroethane together with other components, seven of which
have been identified.
Table 3. Environmental levels in water
--------------------------------------------------------------------------------------
Type of water Location Detection Levels observeda Reference
limit (ug/litre)
(ug/litre)
--------------------------------------------------------------------------------------
Sea water Gulf of Mexico, open 0.001 nd Sauer (1981)
ocean;
near Mississippi mouth 0.05 - 0.21
Osaka Bay 0.69 (average) Okamoto &
Tatsukawa (1981)
River water Germany, Federal Rep- 1.0 1.0 (average) Bauer (1978)
ublic of; 3 rivers nd - 4.0
USA; 14 industrial 1.0 5.6 (average in Ewing et al.
river basins 25% of samples) (1977)
nd - 90
Untreated USA; 80 drinking-water 0.2 - 0.4 nd (86%) Symons et al.
water stations 3.0 (maximum) (1975)
Netherlands; 232 ground- 0.5 nd (229 stations) Zoeteman
water stations 0.8 - 1.7 et al. (1979)
(3 stations)
Drinking-water USA; 80 stations 0.2 - 0.4 nd (68%) Symons et
6.0 (maximum) al. (1975)
Japan; 5 locations 0.5 nd (4 locations) Fujii (1977)
0.9 (1 location)
Germany, Federal Rep- 1.0 nd Bauer (1981)
ublic of; 100 cities
--------------------------------------------------------------------------------------
a nd = not detected.
4.2 General Population Exposure
Daily intake from urban air in the USA has been estimated to
be between 8 and 140 µg per day (Singh et al., 1983). In the
Netherlands, the figure is 26.5 µg per day (Guicherit &
Schulting, 1985).
More specifically, people can be exposed via air at, or
near, sites of production and dispersive use, notably in anti-
knock agents in gasoline. At 12 locations near production
facilities in each of 3 areas in the USA, average air
concentrations gradually decreased from 61 µg/m3 to 2 µg/m3 at
distances of 1 km and 3 - 4 km, respectively. Thus, near
production facilities, approximately 12.5 million people in the
USA were estimated to be exposed to average annual concentra-
tions of up to 40 µg/m3 (Elfers, 1979; Kellam & Dusetzina,
1980).
The annual average population exposure to 1,2-dichloroethane
from gasoline, in the USA, has been estimated to remain below
0.12 µg/m3 (Kellam & Dusetzina, 1980).
4.3 Occupational Exposure
No data were available to the Task Group concerning exposure
levels in the 1,2-dichloroethane- and vinyl chloride-
synthesizing industries. Poisoning incidents following
inhalation or skin exposure have been reported frequently for
places of work where 1,2-dichloroethane is used as a solvent or
fumigant, but data concerning exposure levels are scarce
(Hadengue & Martin, 1953; Paparopoli & Cali, 1956; Suveev &
Babichenko, 1969).
1,2-Dichloroethane levels of up to 150 mg/m3 (Kozik, 1957)
and ranging from 40 to 800 mg/m3 (Cetnarowicz, 1959) were
detected in industrial plants using the chemical as a solvent.
Time-weighted averages of 0.1 and 1 mg/m3, respectively,
have been reported for 2 different jobs in an anti-knock agent
blending plant in the USA. The maximum exposure level measured
was 8.9 mg/m3 (Jacobs, 1980).
Table 4. Environmental levels in air
------------------------------------------------------------------------------
Type of site Location Detection Average Reference
limit levels
(ug/m3) observed
(ug/m3)
------------------------------------------------------------------------------
Marine Osaka Bay 8.4 Okamoto &
Tatsukawa (1981)
Pacific 0.168 Singh et al.
(1982)
Rural USA 0.02 nd Grimsrud &
Rasmussen (1975)
Japan 0.05 0.3 - 0.4a Environment Agency,
Japan (1983)
United Kingdom 0.08 Clark et al.
(1984a,b)
Netherlands 0.2 Guicherit &
Schulting (1985)
Urban United Kingdom 0.48 - 2.14a Tsani-Bazaca
et al. (1981)
USA; 10 cities 0.335 - 6.11 Singh et al.
30 (maximum) (1983)
United Kingdom 1.2 Clark et al.
(1984a,b)
Netherlands 1.2 Guicherit &
Schulting (1985)
Industrial area USA 0.02 nd Harkov et al.
(1984)
Heavily USA 5.3b Bozzelli &
industrialized 65 (maximum) Kebbekus (1982)
areas
Japan 0.05 0.09 - 3.5a Environment Agency,
Japan (1983)
Near gasoline USA 0.2b Tsani-Bazaca
station et al. (1982)
Gasoline station Sweden 0.01 4.0 Jonsson & Berg
(1980)
Table 4 (contd.)
------------------------------------------------------------------------------
Type of site Location Detection Average Reference
limit levels
(ug/m3) observed
(ug/m3)
------------------------------------------------------------------------------
Parking garage and Sweden 0.01 2.0 - 6.5 Jonsson & Berg
repair shop (1980)
Inside cars Sweden 0.01 0.4 - 1.2 Jonsson & Berg
(1980)
Exhaust gases United Kingdom 38 - 3250a Tsani-Bazaca
(cars) et al. (1981)
Airport vicinity USA nd Tsani-Bazaca
et al. (1982)
Motorway United Kingdom 0.08 Clark et al.
(1984a)
------------------------------------------------------------------------------
a Range of values (not average).
b Many sites with undetectable levels of 1,2-dichloroethane were not
considered in the average.
nd = not detected.
5. KINETICS AND METABOLISM
5.1 Absorption
1,2-Dichloroethane can be found in the blood of rodents,
almost immediately after dermal, oral, or inhalation exposure.
During a 12-h dermal exposure of guinea-pigs to undiluted
1,2-dichloroethane, blood concentrations of 1,2-dichloroethane
increased rapidly during the first half hour and then more
slowly, up to the end of exposure (Jakobson et al., 1982). In
mice, a dermal absorption rate of 47 µg/cm2 per min was
measured over the first 15 min following application of
undiluted 1,2-dichloroethane (Tsuruta, 1975).
Oral exposure of rats to 25 or 250 mg EDC/kg body weight in
corn oil produced peak blood levels within 9 and 90 min,
respectively. Blood levels appeared to increase linearly with
exposure from 13 mg/litre at 25 mg/kg up to levels of between 30
and 70 mg/litre at 150 and 250 mg/kg. Small quantities of
metabolites, but no detectable amounts of 1,2-dichloroethane,
were recovered in faeces (Sopikov & Gorshunova, 1979; Reitz et
al., 1982).
During inhalation at concentrations of up to 3200 mg/m3,
steady-state blood levels of the chemical in rats were reached
within 2 - 3 h. The blood levels increased disproportionally
with exposure from 1.4 mg/litre at 202 mg/m3 to 8.3 mg/litre at
607 mg/m3 and 56 mg/litre at 3200 mg/m3. These data suggest
saturation of the metabolic capacity at a blood level of
approximately 5 mg/litre. Peak blood levels of 1,2-dichloro-
ethane were almost 5 times higher following oral exposure to
150 mg/kg body weight than after inhalation exposure to
607 mg/m3, which appeared equivalent to 113 mg/kg body weight
(Sopikov & Gorshunova, 1979; Spreafico et al., 1980; Reitz et
al., 1982). These exposure concentrations were the high dose
levels for the NCI (1978) oral study and the Maltoni et al.
(1980) inhalation study.
5.2 Distribution
The distribution of 1,2-dichloroethane in tissue has mainly
been investigated during exposure of laboratory animals. How-
ever, one report, has been identified that gives an indication
of the relative distribution of 1,2-dichloroethane in human
tissues (Luznikov et al., 1985). As shown in Table 5, 1,2-
dichloroethane concentrations were measured in ten biological
compartments following acute oral poisoning. 2-Chloroacet-
aldehyde, a metabolite of 1,2-dichloroacetaldehyde, was not
detected. In addition to 1,2-dichloroethane, detectable
quantities of 2-chloroethanol and monochloroacetic acid were
reported. In this report, the omentum and stomach contained
similar high levels of 1,2-dichloroethane; liver and kidney
contents were comparable, but approximately 10 times less. The
detectable amounts of metabolites were too low to make
comparisons.
Table 5. Levels of 1,2-dichloroethane and its metabolites determined by
gas chromatography in cadaveric organs and tissues of 15 human beings
who died after acute oral poisoninga
-----------------------------------------------------------------------------
Tissues/ 1,2-dichloro- 2-chloro- 2-chloro- Monochloro-
organs ethane acetalde- ethanol acetic acid
hyde
(mg/kg) (mg/kg) (mg/kg) (mg/kg)
-----------------------------------------------------------------------------
Liver 5 - 100 nd nd nd
Kidney 5 - 80 nd 0.1 1.0
Myocardium 10 - 150 nd 0.12 - 1.1 2.3 - 3.8
Spleen 1 - 50 nd 0.1 1.0
Omentum 100 - 950 nd 0.1 1.0
Brain 10 - 100 nd 0.12 - 0.28 1.0 - 2.0
Stomach 100 - 1000 nd 0.14 - 0.56 1.0 - 2.0
Small 10 - 90 nd 0.13 - 0.21 0
intestine
Large 5 - 60 nd nd nd
intestine
Blood 10 - 150 nd nd nd
-----------------------------------------------------------------------------
a From: Luznikov et al. (1985).
nd = not detected.
Note: The analytical detection limit for 1,2-dichloroethane and all meta-
bolites except monochloroacetic acid was 100 g/litre or 100 g/kg
tissue; for monochloroacetic acid, the limit was 100 g/litre or 100
g/kg tissue.
After oral exposure of rats to 25, 50, or 150 mg 1,2-
dichloroethane/kg, in corn oil, peak levels of the parent
compound in adipose tissue at 45 - 60 min exceeded those in
blood by 3.9 - 8.3 times. Peak levels in the liver, 10 min
after exposure, exceeded those in blood by 1.3 - 2.2 times.
This accumulation was lower than expected at the 2 higher
exposure levels, indicating saturation of the tissues at higher
doses. During inhalation, steady-state levels in rat tissues
were reached within 2 - 3 h and increased 20- to 30-fold when
the exposure increased from 202 to 1012 mg/m3, suggesting a
saturable metabolic capacity (section 5.3). Levels in adipose
tissue, at steady-state, were 7 - 8 times higher than those in
blood, while levels in the liver were 20% below those in blood.
At comparable blood levels, the maximum concentration of 1,2-
dichloroethane after inhalation was lower in the liver and
higher in lung and adipose tissue than after oral exposure.
Levels in the spleen, brain, and kidney were similar to those in
the blood, irrespective of the route of administration
(Spreafico et al., 1980).
Forty-eight h after ingestion of 150 mg/kg body weight or
inhalation exposure to a concentration of 607 mg/m3, 3 - 4% of
the body burden of labelled 1,2-dichloroethane was recovered in
the carcass of rats. Most radioactivity was found in the liver
and kidneys. Residual radioactivity in selected tissues was 1 -
2 times higher after oral exposure than after inhalation.
Another difference between oral and inhalation exposure was the
higher residual activity in the forestomach, well after the oral
exposure. A similar distribution pattern emerged for macro-
molecular binding, as determined 4 h after oral ingestion or
directly after inhalation. At these times, oral exposure pro-
duced lower levels of total macromolecular binding, but higher
levels of DNA alkylation than inhalation exposure. The absolute
levels of DNA alkylation (2 - 14 µmol equivalents of 1,2-
dichloroethane per mol DNA at 1 mmol/kg body weight) were
considered low (Reitz et al., 1982).
In another study, rats and mice were compared with respect
to DNA binding in liver, kidney, stomach, and lung, 22 h after a
single intraperitoneal injection of 0.86 mg labelled 1,2-
dichloroethane/kg body weight in ethanol. Binding to lung DNA
was low compared with that in the other tissues. Binding to DNA
of mouse organs was always greater than that to DNA of rat
organs (Arfellini et al., 1984).
When pregnant rats inhaled 1,2-dichloroethane at a level of
1000 mg/m3, for 4 h per day, the compound was found to accumu-
late in the placental and fetal tissues over a period of 7 days
(Vosovaya, 1977). Withey & Karpinski (1985) also obtained
evidence that exposure of rats to 1,2-dichloroethane via
inhalation results in detectable levels in fetuses in a dose-
related manner.
Binding of 1,2-dichloroethane to protein, lipid, and DNA was
also observed in vitro (Guengerich et al., 1980).
5.3 Metabolism
Metabolism of 1,2-dichloroethane appears to have a signi-
ficant role in the manifestation of the toxic, carcinogenic, and
mutagenic effects of this chemical.
Biotransformation of 1,2-dichloroethane is extensive in the
mouse; ip doses of 50 and 170 mg/kg body weight were associated
with 88 and 55% conversion to metabolites, respectively (Yllner,
1971). The metabolites identified by Yllner (1971) are shown in
Table 6. Reitz et al. (1982) observed extensive metabolism of
1,2-dichloroethane in the rat, i.e., 70 and 91% transformation,
with oral (150 mg/kg) and inhalation (607 mg/m3; 6 h) exposures,
respectively, 85% of the metabolites appearing in the urine.
Biotransformation of 1,2-dichloroethane approaches saturation at
high blood levels.
Table 6. Non-volatile urinary metabolites of 1,2-dichloroethane in rodents
-----------------------------------------------------------------------------
Species Route Metabolite Fraction of Reference
total (%)
-----------------------------------------------------------------------------
mouse oral (conjugated) S-carboxy- 48 Yllner (1971)
(in methylcysteine
oil)
thiodiacetic acid 33
chloroacetic acid 16
S,S'-ethene-bis-cysteine 0.9
2-chloroethanol 0.3
rat inhal- thiodiacetic acid 67 - 68 Reitz et al.
ation (1982)
or
oral
(in thiodiacetic acid 26 - 29
oil) sulfoxide
oral S-(2-hydroxyethyl)mercap- Nachtomi et al.
(in turic acid (1966)
oil)
S-(2-hydroxyethyl)cysteine
2-chloroethanol Kokarovtseva &
Kiselyova (1978)
-----------------------------------------------------------------------------
1,2-Dichloroethane metabolism involves the formation of
sulfur-containing metabolites, which appear in the urine. Two
proposed pathways of metabolism of 1,2-dichloroethane are
depicted in Fig. 1; one pathway begins with cytochrome P-450-
mediated oxidation, and the other begins with glutathione
conjugation. There is a lack of evidence that doses of 1,2-
dichloroethane, either by gavage or inhalation, have any effects
on the distribution of its metabolites between these pathways.
Cytochrome P-450 enzymes catalyse an oxidative transformation of
1,2-dichloroethane to form reactive intermediates, which result
in the formation of 2-chloroacetaldehyde and 2-chloroethanol
(Guengerich et al., 1980) (Fig. 1). Johnson (1965, 1966, 1967)
has shown that 2-chloroacetaldehyde reacts both enzymatically
and non-enzymatically with glutathione (GSH).
 Rannug et al. (1978) first reported that mutagenic compounds
could be formed by the reaction of GSH with 1,2-dihaloalkanes in
the presence of cytosolic glutathione-S-transferases. This
observation led workers to investigate in greater detail the
role of glutathione-S-transferases in the metabolism and bio-
activation of both dibromoethane (DBE) and 1,2-dichloroethane
(Rannug, 1980; Sundheimer et al., 1982; Ozawa & Guengerich,
1983; Inskeep & Guengerich, 1984). This pathway (Fig. 1)
involves the direct reaction of GSH with 1,2-dichloroethane to
form S-(2-chloroethyl) glutathione, which is a half mustard with
a half-life of 69 min at 20 °C (Schasteen & Reed, 1983) and less
than 15 min at 37 °C (Foureman & Reed, 1985). Non-enzymic
conversion of the half mustard to the corresponding episulfonium
ion gives a putative alkylating agent (episulfonium ion) that
has several fates (Fig. 1). Reaction can occur with water to
form S-(2-hydroxyethyl) glutathione or reaction with thiols such
as GSH to form ethene bis-glutathione or with DNA to form
adducts. With the exception of DNA adducts, the reaction pro-
ducts are considered non-toxic and undergo further metabolism.
These reactions and subsequent metabolism of the products can
account for all of the known sulfur-containing metabolites found
in the urine of 1,2-dichloroethane-treated animals.
Although much evidence has been reported that supports the
P-450 mediated metabolism of 1,2-dihaloethanes, this branch of
the pathway (Fig. 1) does not appear relevant to DNA adduct
formation by 1,2-dichloroethane (Koga et al., 1986). Guengerich
et al. (1980) proposed the possibility of chloroso oxidation
products of 1,2-dichloroethane in DNA adduct formation (Fig. 1).
However, they observed that the apparent stimulation of P-450-
directed DNA adduct formation by GSH was a result of incomplete
removal of GSH conjugates during analysis (Koga et al., 1986).
In addition, they concluded that 2H and 18O studies on the
formation of 2-haloethanols and 2-haloacetaldehydes from 1,2-
dihaloethanes are inconsistent with a major role of such a
mechanism for DNA damage (Guengerich et al., 1986; Koga et al.,
1986).
It should be pointed out that the P-450 directed pathway can
presumably form considerable quantities of 2-haloacetaldehydes,
which readily bind to protein and non-protein thiols, as shown
for vinyl bromide and vinyl chloride (Guengerich et al., 1981)
and dibromoethane (DBE) (van Bladeren et al., 1981).
Although some DNA damage can be produced via the P-450 path-
way under in vitro conditions (Hill et al., 1978; Banerjee et
al., 1980; Guengerich et al., 1980; Lin et al., 1985), several
lines of evidence suggest that the GSH conjugation pathway is
probably of greater significance than the P-450 pathway as the
major in vivo route for DNA damage (Guengerich et al., 1980;
Rannug, 1980; Sundheimer et al., 1982; Inskeep et al., 1986).
It has been possible to correlate the 1,2-dichloroethane-
induced mutation frequency of two human cell lines with the
difference in levels of glutathione-S-transferase activities.
AHH-1 cell line mutation frequency was 25 times that in the TK6
cell line in the presence of 1,2-dichloroethane. The difference
was attributed to the fact that the AHH-1 cell line possesses 5
times more glutathione-S-transferase activity than the TK6 cell
line (Crespi et al., 1985).
Male B6C3F1 mice, pretreated with piperonyl butoxide (PIB),
were examined for the extent of hepatic DNA damage produced 4 h
after 1,2-dichloroethane administration (Storer & Conolly,
1985). PIB is a P-450 inhibitor. Hepatic DNA damage, as
measured by the alkaline DNA unwinding assay for single-strand
breaks and alkali-labile lesions, was potentiated by PIB.
Treatment of mice with high doses of 2-chloroethanol failed to
produce DNA damage, as measured by this assay. Diethylmaleate,
a GSH depletor, potentiated the hepatotoxicity of 2-chloro-
ethanol but not DNA damage. Although the significance of this
observation is uncertain, it is not inconsistent with the
hypothesis that reduction of GSH levels is associated with a
reduction in DNA damage.
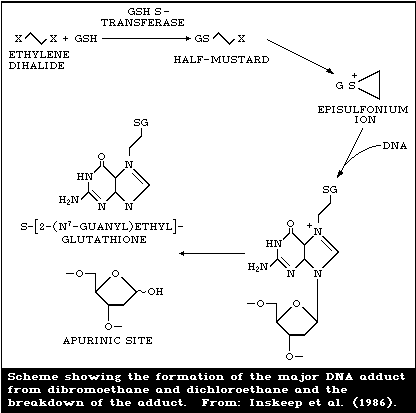
Recent evidence suggests that the putative episulfonium ion,
resulting from a non-enzymatic conversion of S-(2-chloroethyl)
glutathione, is a major intermediate in the formation of DNA
adducts in vivo from 1,2-dichloroethane exposures (Fig. 2)
(Inskeep et al., 1986). When rats were administered a single
dose of 14C-1,2-dichloroethane in vivo and the liver analysed 8
h later, 78% of the DNA adducts (0.25 nmol/mg DNA) could be
released by neutral thermal hydrolysis. A major adduct and
several minor adducts were present; the major adduct co-
chromatographed with S-[2-(N7-guanyl)ethyl] glutathione. DNA
adducts released from kidney preparations by neutral thermal
hydrolysis were represented by 5 different fractions containing
radioactivity after chromatography. The postulated adduct of
liver DNA after 14C-1,2-dichloroethane exposure, S-[2-(N7-
guanyl)ethyl] glutathione, appears to be chromatographically
identical to the major adduct in rats after exposure to DBE
(Koga et al., 1986). This DBE adduct, which has been isolated
and characterized by NMR and mass spectrometry, gives strong
support to an identical adduct being the principal DNA adduct
from exposure to 1,2-dihaloethanes. The formation of apurinic
sites, as this adduct cleaves from DNA, may be a key factor in
the mutagenic and carcinogenic effects of these compounds.
Rannug et al. (1978) first reported that mutagenic compounds
could be formed by the reaction of GSH with 1,2-dihaloalkanes in
the presence of cytosolic glutathione-S-transferases. This
observation led workers to investigate in greater detail the
role of glutathione-S-transferases in the metabolism and bio-
activation of both dibromoethane (DBE) and 1,2-dichloroethane
(Rannug, 1980; Sundheimer et al., 1982; Ozawa & Guengerich,
1983; Inskeep & Guengerich, 1984). This pathway (Fig. 1)
involves the direct reaction of GSH with 1,2-dichloroethane to
form S-(2-chloroethyl) glutathione, which is a half mustard with
a half-life of 69 min at 20 °C (Schasteen & Reed, 1983) and less
than 15 min at 37 °C (Foureman & Reed, 1985). Non-enzymic
conversion of the half mustard to the corresponding episulfonium
ion gives a putative alkylating agent (episulfonium ion) that
has several fates (Fig. 1). Reaction can occur with water to
form S-(2-hydroxyethyl) glutathione or reaction with thiols such
as GSH to form ethene bis-glutathione or with DNA to form
adducts. With the exception of DNA adducts, the reaction pro-
ducts are considered non-toxic and undergo further metabolism.
These reactions and subsequent metabolism of the products can
account for all of the known sulfur-containing metabolites found
in the urine of 1,2-dichloroethane-treated animals.
Although much evidence has been reported that supports the
P-450 mediated metabolism of 1,2-dihaloethanes, this branch of
the pathway (Fig. 1) does not appear relevant to DNA adduct
formation by 1,2-dichloroethane (Koga et al., 1986). Guengerich
et al. (1980) proposed the possibility of chloroso oxidation
products of 1,2-dichloroethane in DNA adduct formation (Fig. 1).
However, they observed that the apparent stimulation of P-450-
directed DNA adduct formation by GSH was a result of incomplete
removal of GSH conjugates during analysis (Koga et al., 1986).
In addition, they concluded that 2H and 18O studies on the
formation of 2-haloethanols and 2-haloacetaldehydes from 1,2-
dihaloethanes are inconsistent with a major role of such a
mechanism for DNA damage (Guengerich et al., 1986; Koga et al.,
1986).
It should be pointed out that the P-450 directed pathway can
presumably form considerable quantities of 2-haloacetaldehydes,
which readily bind to protein and non-protein thiols, as shown
for vinyl bromide and vinyl chloride (Guengerich et al., 1981)
and dibromoethane (DBE) (van Bladeren et al., 1981).
Although some DNA damage can be produced via the P-450 path-
way under in vitro conditions (Hill et al., 1978; Banerjee et
al., 1980; Guengerich et al., 1980; Lin et al., 1985), several
lines of evidence suggest that the GSH conjugation pathway is
probably of greater significance than the P-450 pathway as the
major in vivo route for DNA damage (Guengerich et al., 1980;
Rannug, 1980; Sundheimer et al., 1982; Inskeep et al., 1986).
It has been possible to correlate the 1,2-dichloroethane-
induced mutation frequency of two human cell lines with the
difference in levels of glutathione-S-transferase activities.
AHH-1 cell line mutation frequency was 25 times that in the TK6
cell line in the presence of 1,2-dichloroethane. The difference
was attributed to the fact that the AHH-1 cell line possesses 5
times more glutathione-S-transferase activity than the TK6 cell
line (Crespi et al., 1985).
Male B6C3F1 mice, pretreated with piperonyl butoxide (PIB),
were examined for the extent of hepatic DNA damage produced 4 h
after 1,2-dichloroethane administration (Storer & Conolly,
1985). PIB is a P-450 inhibitor. Hepatic DNA damage, as
measured by the alkaline DNA unwinding assay for single-strand
breaks and alkali-labile lesions, was potentiated by PIB.
Treatment of mice with high doses of 2-chloroethanol failed to
produce DNA damage, as measured by this assay. Diethylmaleate,
a GSH depletor, potentiated the hepatotoxicity of 2-chloro-
ethanol but not DNA damage. Although the significance of this
observation is uncertain, it is not inconsistent with the
hypothesis that reduction of GSH levels is associated with a
reduction in DNA damage.
Recent evidence suggests that the putative episulfonium ion,
resulting from a non-enzymatic conversion of S-(2-chloroethyl)
glutathione, is a major intermediate in the formation of DNA
adducts in vivo from 1,2-dichloroethane exposures (Fig. 2)
(Inskeep et al., 1986). When rats were administered a single
dose of 14C-1,2-dichloroethane in vivo and the liver analysed 8
h later, 78% of the DNA adducts (0.25 nmol/mg DNA) could be
released by neutral thermal hydrolysis. A major adduct and
several minor adducts were present; the major adduct co-
chromatographed with S-[2-(N7-guanyl)ethyl] glutathione. DNA
adducts released from kidney preparations by neutral thermal
hydrolysis were represented by 5 different fractions containing
radioactivity after chromatography. The postulated adduct of
liver DNA after 14C-1,2-dichloroethane exposure, S-[2-(N7-
guanyl)ethyl] glutathione, appears to be chromatographically
identical to the major adduct in rats after exposure to DBE
(Koga et al., 1986). This DBE adduct, which has been isolated
and characterized by NMR and mass spectrometry, gives strong
support to an identical adduct being the principal DNA adduct
from exposure to 1,2-dihaloethanes. The formation of apurinic
sites, as this adduct cleaves from DNA, may be a key factor in
the mutagenic and carcinogenic effects of these compounds.
 5.4 Excretion and Elimination
Excretion of 1,2-dichloroethane from rodents is rapid.
Approximately 89% or more of the body burden of the compound was
excreted within 24 h in ip-injected mice (Yllner, 1971), within
48 h in orally exposed mice (Mitoma et al., 1985), and within
48 h in rats exposed orally or via inhalation (Reitz et al.,
1982; Mitoma et al., 1985). In the 3 studies cited above,
excretion of 1,2-dichloroethane or its metabolites mainly
occurred in exhaled air via the lungs and in urine via the
kidneys. In both species, and at various exposure levels, 7 -
18% of the metabolized 1,2-dichloroethane was excreted as carbon
dioxide (CO2) and approximately 80 - 85% as non-volatile
metabolites (Table 6). The metabolism of 1,2-dichloroethane in
mice and rats is dose-dependent. For example, in mice, 11 and
45% of the body burdens of 1,2-dichloroethane, resulting from ip
exposure to 50 and 170 mg/kg body weight, respectively, were
excreted unchanged via the lungs within 72 h (Yllner, 1971). In
rats, 1.8, 11.5, and 29% of the body burdens were excreted
unchanged via the lungs within 48 h following: an inhalation
exposure at 607 mg/m3 for 6 h (equivalent to a dose of 113 mg/kg
body weight) (Reitz et al., 1982), an oral dose of 100 mg/kg
body weight (Mitoma et al., 1985), and an oral dose of 150 mg/kg
body weight (Reitz et al., 1982), respectively.
The rate of elimination from blood and tissues appears to
depend on the exposure level; the higher the exposure level, the
slower the elimination rate of 1,2-dichloroethane, after both
oral and inhalation exposure. Half-lives in the blood of rats,
exposed orally, increased from 25 min at 25 mg/kg body weight to
57 min at 150 mg/kg body weight. With inhalation exposure,
half-lives increased from 13 min at 202 mg/m3 to 22 min at 1012
mg/m3, after a 6-h inhalation exposure (Spreafico et al., 1980).
In addition, after oral exposure of rats to 150 mg/kg body
weight, an initial half-life of 90 min in blood decreased to
20 - 30 min, when blood levels fell below 5 - 10 mg/litre after
3 h (Reitz et al., 1982). Elimination of 1,2-dichloroethane
from blood, adipose tissue, lung, liver, brain, kidneys, and
spleen was comparable after oral exposures of up to 150 mg/kg
body weight. Elimination from the liver was reported to be
biphasic with a higher elimination rate just after reaching peak
levels of 1,2-dichloroethane. Elimination from other organs was
monophasic. Following inhalation, elimination was the slowest
in adipose tissue and the most rapid in the lung, up to an
exposure level of 1012 mg/m3 (Spreafico et al., 1980). Withey &
Collins (1980) also reported that the elimination of 1,2-
dichloroethane was dose-dependent. After iv administration of
from 3 to 15 mg/kg body weight to male Wistar rats, the authors
found that the elimination fitted a two-compartment model at a
low-dose level and a three-compartment model at high-dose
levels.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Aquatic Organisms
6.1.1 Acute toxicity
The acute toxicity of 1,2-dichloroethane for aquatic
organisms is summarized in Table 7. The concentration of 1,2-
dichloroethane was measured in four of the studies cited (see
footnotea in Table 7); the concentrations reported in the
other studies were nominal. It should be noted that, in open
systems, the toxic effects observed must have occurred at
concentrations lower than the nominal ones reported in latter
studies, due to the anticipated evaporation of 1,2-dichloro-
ethane in the aquatic media.
The species most sensitive to 1,2-dichloroethane were
members of the class Crustacea. A no-observed-adverse-effect
level below 68 mg/litre was found for Daphnia magna (Le Blanc,
1980). The shrimp Crangon crangon showed a 96-h LC50 of
85 mg/litre in sea water, measured by the flow-through method
(Adema, 1976). When the brine shrimp Artemia salina was exposed
to 1,2-dichloroethane at levels ranging from 0.25 to
25 mg/litre, growth inhibition was noted 24 h after cyst wetting
(Kerster & Schaeffer, 1983).
EDC tar is much more toxic for marine species than 1,2-
dichloroethane, the heavy fractions of the tar being responsible
for the high toxicity observed (Jernelöv et al., 1972; Rosen-
berg, 1972; Jensen et al., 1975; Rosenberg et al., 1975).
6.1.2 Short-term exposures
When blue algae Mycrocystis aerigunosa and green algae
Scenedesmus quadricauda were exposed in closed containers to
1,2-dichloroethane at 105 and 710 mg/litre, respectively, for 8
days, cell multiplication started to be inhibited (Bringmann &
Kühn, 1978). Guppies (Poecilia reticulata) were exposed for 7
days in a static test, and an LC50 of 106 mg/litre was found.
Solutions were renewed daily, but no water analysis data were
reported (Könemann, 1981). Finally, an early life stage flow-
through test was done. Fathead minnows (Pimephales promelas)
were exposed to concentrations of between 4 and 56 mg/litre
beginning from 2 to 5 days after spawning and continuing
throughout the subsequent embryonal, larval, and juvenile stages
up to 28 days after hatching. Water was analysed for 1,2-
dichloroethane. Body weight was reduced at 59 mg/litre. The
survival of juveniles, the percentage of normal larvae at hatch,
and the hatchability of embryos were not affected (Benoit et
al., 1982).
Table 7. Acute aquatic toxicity
---------------------------------------------------------------------------------------------------------
Organism Description t (°C) pH Dissolved Hardness Flow/b Parameter Concen- Reference
oxygen (mg CaCO3/ stat tration
(mg/litre) litre) open/ (mg/litre)
closed
---------------------------------------------------------------------------------------------------------
Fresh water
-----------
Bacteria Pseudomonas 25 7 stat 16-h MICc 135 Bringmann
putida closed & Kühn
(1980)
Protozoa Entosipon sulc- 25 7 stat 72-h MIC 943-1127 Bringmann
atum, Uronema closed & Kühn
parduczi, Chilo- (1981)
monas paramecium
Crustacea water flea 22 6.7-8.1 6.5-9.1 72 stat 24-h LC50 250 Le Blanc
(Daphnia magna) open no-observed- < 68 (1980)
adverse-
effect level
Crustacea water flea 20 8.0 > 2 - stat 24-h EC50 540a,d Bringmann
(Daphnia magna) open & Kühn
(1982)
Crustacea water flea 20 7.1-7.7 7.9-9.9 44.7 stat 48-h LC50 270a,d Richter
(Daphnia magna) closed 48-h EC50 160a,d et al.
20 7.0-7.5 4.1-8.4 44.7 stat 48-h LC50 320a,e (1983)
closed 48-h EC50 180a,e
Fish bluegill sunfish 21-23 6.5-7.9 32-48 stat 96-h LC50 430 Buccafusco
(Lepomis macro- closed et al.
chirus) (1981)
Fish bluegill sunfish 23 7.6-7.9 55 stat 96-h LC50 550 Dawson
(Lepomis macro- open et al.
chirus) (1977)
Fish fathead minnow 25 6.7-7.6 8.0 45.1 flow 96-h LC50 116a Walbridge
(Pimephales prom- open et al.
elas) (1983)
Table 7 (contd.)
---------------------------------------------------------------------------------------------------------
Sea water
---------
Alga Phaeodactylum stat EC50 340a Pearson &
tricornutum McConnell
(1975)f
Worm chaetopod 23 stat 96-h LC50 400 Rosenberg
(Ophryotrocha closed et al.
labronica) (1975)g
Crustacea shrimp 15 8.0 > 8.0 flow 96-h LC50 85a Adema
(Crangon crangon) open (1976)
Crustacea shrimp 16 stat 24-h LC50 170 Rosenberg
(Crangon crangon) open et al.
(1975)h
Mollusca barnacle nauplii stat 48-h LC50 186a Pearson &
(Elminius modestus) closed McConnell
(1975)
Fish dab flow 96-h LC50 115a Pearson &
(Limanda limanda) open McConnell
(1975)
Fish tidewater silver- 20 7.6-7.9 55 stat 96-h LC50 480 Dawson
sides (Menidia open et al.
beryllina) (1977)
Fish sheepshead minnow 25-31 stat 96-h LC50 130-230 Heitmüller
(Cyprinodon open no-observed- 130 et al.
variegatus) adverse- (1981)
effect level
Fish goby 15 8.0 > 8.0 flow 96-h LC50 185a Adema
(Gobius minutus) open (1976)
---------------------------------------------------------------------------------------------------------
a Water analysis for 1,2-dichloroethane was reported.
b Flow-through or static method; open or closed system.
c MIC = minimum inhibitory concentration for cell multipication. EC50 and LC50 = concentration,
causing an effect and death, respectively, in 50% of the population.
d Fleas unfed. Effect was complete immobilization.
e Fleas fed. Effect was complete immobilization.
f Effect was growth inhibition, measured by 4C uptake during photosynthesis.
g When the concentration was gradually built up during the first hour, the 96-h LC50 was
900 mg/litre, and the percentage of hatched eggs, laid within 15 days, was decreased by 90% at
400 mg/litre. The latter was not noted at 200 mg/litre.
h Concentration was gradually built up during the first hour.
6.1.3 Long-term exposure
No-observed-adverse-effect and effect concentrations were
determined on the basis of reproduction or length for Daphnia
magna in a 28-day test, in stoppered flasks. The test solution
was analysed by gas chromatography. The lowest observed adverse
effect concentrations were 21 mg/litre, on the basis of repro-
duction, and 72 mg/litre, on the basis of length. The no-
observed-adverse-effect concentrations were 11 mg/litre, on the
basis of reproduction and 42 mg/litre, on the basis of length
(Richter et al., 1983).
6.1.4 Bioconcentration
Bioconcentration of 1,2-dichloroethane in aquatic species is
unlikely in view of its physical and chemical properties. In a
tracer study, a bioconcentration factor of 2 was found for
bluegill sunfish (Lepomis macrochirus) in flowing water. The
half-life for the elimination of 1,2-dichloroethane from tissues
was 1 - 2 days (Barrows et al., 1980).
When tissues of several aquatic species, collected from near
the discharge zone of a wastewater treatment plant, were ana-
lysed for 1,2-dichloroethane, the concentration of the compound
was less than 0.5 µg/kg wet weight in all cases, while the
average effluent concentration was 41 µg/litre and the average
sediment concentration was less than 0.5 µg/kg dry weight
(Gossett et al., 1983).
6.2 Microorganisms
1,2-Dichloroethane at an influent concentration of
258 mg/litre did not affect the treatment efficiency of a bench-
scale activated sludge system. The compound itself was
virtually completely removed by stripping but not by biodegra-
dation (Stover & Kincannon, 1983). In a batch anaerobic
toxicity assay, 1,2-dichloroethane was slightly toxic to the
anaerobic digestion process from a concentration of
2.5 mg/litre, though acclimation was observed after several
days. A concentration of 20 mg/litre caused more severe
retardation, while acclimation was slow. In semi-continuous
assays, stress became evident at 1,2-dichloroethane concentra-
tions of between 5 and 7.5 mg/litre (Stuckey et al., 1980).
6.3 Terrestrial Organisms
6.3.1 Birds
The effects on reproduction were investigated in groups of
10 male and 20 female white leghorn chickens after 2 years of
oral exposure to 0, 250, or 500 mg 1,2-dichloroethane/kg feed
mash. From the fourth month of laying onwards, decreased egg
weight was observed at both dose levels, while at the higher
dose level, the number of eggs and the feed intake were also
reduced. 1,2-Dichloroethane did not affect serum composition
and growth, semen characteristics, or fertility of chickens
(Alumot et al., 1976b).
6.3.2 Plants
1,2-Dichloroethane is used as a seed fumigant, usually in
combination with compounds such as carbon tetrachloride, 1,2-
dibromomethane, or 2-chloroethanol. Such fumigants inhibited
the germination of seeds (Caswell & Clifford, 1958; Kamel,
1959), broke the dormancy period of potato tubers (Varga &
Ferenczy, 1956; Jolivet, 1968) and beech (Thorup, 1957), and
adversely affected the nodulation status and yield of groundnuts
treated with Rhizobium (Kulkarni et al., 1975). 1,2-Dichloro-
ethane vapour was both lethal and mutagenic for barley seeds at
3 mg/m3 during 24 h (Ehrenberg et al., 1974).
7. EFFECTS ON ANIMALS
7.1 Single Exposures
7.1.1 Inhalation and oral exposure
The available acute mortality data following inhalation and
oral exposure are summarized in Table 8.
Table 8. Acute mortality after inhalation or oral exposure to
1,2-dichloroethane
-----------------------------------------------------------------------------
Species/ Route Vehicle Parameter Result Reference
strain studied
-----------------------------------------------------------------------------
dog oral acacia LD50 2500 mg/kg Barsoum & Saad
gum (1934)
rat oral corn oil LD50 680 mg/kg McCollister et
al. (1956)
CD-1 oral water LD50 489 mg/kg Munson et al.
mouse (1982)
(male)
CD-1 oral water LD50 413 mg/kg Munson et al.
mouse (1982)
(female)
Wistar inhalation 6-h LC50 5100 mg/m3 Spencer et al.
rat (1951)
Sprague inhalation - 6-h LC50 6660 mg/m3 Bonnet et al.
Dawley (1980)
rat
OF1 inhalation 6-h LC50 1060 mg/m3 Gradiski et al.
mouse (1978)
(female)
rat oral LD50 850 mg/kg Larionov &
Kokarovtseva
(1976)
albino inhalation LC50(expo- 30 000 mg/m3 Nevrotsky et al.
rat sure period (1971)
unknown)
-----------------------------------------------------------------------------
Deaths occur within a narrow range of concentrations. In
rats, the difference between the 6-h LC10 and 6-h LC90 was
approximately 2800 mg/m3 (Bonnet et al., 1980). No deaths were
observed in rats after 6 h of exposure to 2000 mg/m3 (Spencer et
al., 1951). In mice, an extremely narrow range of about
500 mg/m3 was observed between the 6-h LC10 and the 6-h LC90
(Gradiski et al., 1978).
Exposure of rats to single high doses of 1,2-dichloroethane
resulted in adverse effects on the CNS, liver, kidneys,
adrenals, and lungs (Spencer et al., 1951). Groups of 10 - 52
Wistar rats were exposed to 81 000, 48 600, 12 100, 6100, 4000,
3200, 2400, or 1200 mg 1,2-dichloroethane/m3 for various lengths
of time. At the highest concentration (81 000 mg/m3), deaths
were observed in animals exposed for 0.3 h or longer. Deaths
were observed at all except the lowest of the other concentra-
tions with exposure periods of 0.4, 0.7, 3.0, 4.0, 7.0, and
7.0 h, respectively. No deaths were observed in rats exposed to
the lowest concentration for 7 h.
Severe depression of the central nervous system resulting in
coma was observed in rats exposed to the highest concentration.
At lower concentrations, this depressant action expressed itself
as various levels of "drunkeness". In this investigation,
Spencer et al. (1951) did not specify the sex of the rats.
In the same publication, the authors reported another study
in which groups of 4 - 6 female rats were exposed to 48 600,
12 100, 4000, 1200, or 800 mg/m3. "Adverse effects" (not
described by the authors) were observed at the 4 highest levels
in rats exposed for 0.2, 0.5, 3.0, and 5.5 h, respectively. No
adverse effects were observed in female rats exposed to the
lowest concentration, for 7 h.
Examination of internal organs in groups of rats killed,
either when moribund or 24 h after the last exposure, showed
that, after exposure to 1,2-dichloroethane levels of 2400 mg/m3
or more, the most severe damage occurred in the kidney and
consisted of haemorrhage and tubular necrosis. The liver showed
fatty changes and hepatocellular necrosis with haemorrhage, and
adrenal glands were haemorrhagic. Lung oedema was observed at
concentrations above 12 100 mg/m3. The injury to organs in this
study was accompanied by high blood-urea levels, a decrease in
serum-phosphatase activity, and increased lipid concentration in
the liver (Spencer et al., 1951).
Depression of the central nervous system was noted during
exposure of rats to 1,2-dichloroethane concentrations exceeding
1200 mg/m3 (Bonnet et al., 1980). From an exposure level of
4000 mg/m3, for 4 h, rats showed altered behaviour. A narcotic
effect was observed at 9100 mg/m3 (Wolff et al., 1979).
Albino rats, given a single oral dose of 615 mg/kg body
weight, showed congested livers with cloudy swelling and fatty
degeneration. The myocardium showed oedema and haemorrhaging in
the walls of the coronary vessels, stasis, and thrombi in the
vessels. These changes were associated with an increased
activity of alanine- and aspartate aminotransferase in the serum
and decreased tissue levels of nicotinamide coenzymes (Natsyuk &
Chekman, 1975). A single administration of 861 mg/kg, by
gavage, was reported to partially uncouple oxidative phosphory-
lation measured in vitro in albino rat livers, 1, 3, or 6 days
after exposure (Natsyuk et al., 1974). In rat liver microsomes,
cytochrome P-450 levels were slightly decreased after an oral
dose of 625 mg/kg (Moody et al., 1981). After a single oral
dose of 1,2-dichloroethane in corn oil at 770 mg/kg body weight
in rats, dystrophy in the cytoplasm and hyperchromatosis in the
nuclei of hepatocytes were observed. A decrease in protein
synthesis and lysosomal enzyme activity was also reported. The
same changes took place in renal nephrons (Boikova & Kravtsova,
1982). A single dose of 850 mg/kg in albino rats resulted in a
decrease in RBC count, haematocrit, and other haematological
changes (Larionov & Kokarovtseva, 1976). Oral administration of
1,2-dichloroethane (615 mg/kg) to rabbits induced pronounced
morphological changes in the liver in about 24 h (Nazikhi &
Skrizhinsky, 1973). Effects on fibrinolytic activity in the
blood of rabbits administered 1,2-dichloroethane at 1476 mg/kg
have been reported by Kagramanov & Kazieva (1972). Electro-
cardiographic changes in albino rats associated with doses of 1,
1.5, and 2 mg/kg have been reported (Saitanov & Arsenieva,
1969).
7.1.2 Skin and eye irritation
When undiluted 1,2-dichloroethane was applied directly on
the clipped skin of guinea-pigs for up to 12 h in occluded patch
tests, no gross skin reactions were visible (Jakobson et al.,
1982). Microscopic changes appeared 4 h after application,
comprising karyopyknosis, perinuclear oedema, spongiosis, and
junctional separation (Kronevi et al., 1981). In similar tests
on rabbits, moderate erythema and oedema were observed, 24 h
after application. Microscopy on the third day revealed
necrosis and other lesions such as ulcerations and acanthosis.
The severity of the changes was not indicated (Duprat et al.,
1976).
Instillation of 0.1 ml of undiluted 1,2-dichloroethane into
the conjunctival sac of the eye of rabbits generated reversible,
mild irritation characterized by conjunctivitis and epithelial
abrasion. Epithelial keratitis, described as being "in a state
of repair", was observed microscopically, 7 days after
application (Duprat et al., 1976). Reversible clouding of the
cornea was observed in dogs within 10 h of subcutaneous
administration of undiluted 1,2-dichloroethane at 0.9/kg body
weight. The clouding continued up to 48 h, but the corneas
appeared clear after 5 days. The histological changes,
including necrosis of the corneal endothelium, partially denuded
Descemet's membrane, formation of excess basement membrane, and
swelling of the corneal stroma, were also observed in dogs,
cats, and rabbits after ocular injection of 1.8 mg 1,2-
dichloroethane (0.15 ml of a 1% solution) into the anterior
chamber (Kuwabara et al., 1968).
7.2 Short-Term Exposures
7.2.1 Inhalation exposure
The effects of repeated exposure to 1,2-dichloroethane have
been studied in mice, rats, guinea-pigs, rabbits, cats, dogs,
and monkeys (Heppel et al., 1946; Spencer et al., 1951; Hofmann
et al., 1971).
Heppel et al. (1946) exposed rats of the Wistar and Osborne-
Mendel strains to 1,2-dichloroethane concentrations of 420, 730,
1540, or 3900 mg/m3 in air, 7 h daily, 5 days/week, for several
weeks. The duration varied with each exposure level. No loss
of weight and no deaths occurred in rats of either strain
exposed to 420 mg/m3 for up to 4 months (74 exposures). Seven
out of 12 Wistar rats exposed to 730 mg/m3 died within 15 weeks
(after 1 - 73 exposures) and 8/12 similarly treated rats of the
Osborne-Mendel strain died after 1 - 6 exposures. Nine out of
16 rats (strain unspecified) died within 12 weeks after 2 - 60
exposures to 1540 mg/m3; 20/26 (strain unspecified) died within
3 weeks after 3 - 15 exposures to 3900 mg/m3. Guinea-pigs were
exposed to the same concentrations of 1,2-dichloroethane as the
rats. Several deaths, which occurred in the lowest exposure
group (420 mg/m3) and in controls, were attributed to an
intercurrent disease. At higher dose levels, mortality was
related to 1,2-dichloroethane exposure. Five out of 14 guinea-
pigs died after 5 - 115 exposures to 730 mg/m3 within 23 weeks.
Fourteen out of 20 guinea-pigs died after 8 - 65 exposures to
1,2-dichloroethane at 1540 mg/m3 within 13 weeks. All guinea-
pigs exposed to 3900 mg/m3 had died by the 4th day of the
study.
Rabbits were exposed to the three higher concentrations of
1,2-dichloroethane only. No deaths were observed when rabbits
were exposed to 730 mg/m3 for 25 weeks. All 5 rabbits exposed
to 1540 mg/m3 died, one after one exposure and the rest after
89 - 97 exposures within 19 weeks. Five out of 6 rabbits
exposed to 3900 mg/m3 died after 2 - 43 exposures within 9
weeks.
A group of 19 mice survived for 4 weeks when exposed to 420
mg/m3, but 18/20 mice died within 10 days by the end of 7
exposures to 730 mg/m3. In other species, a group of 6 female
dogs survived up to 35 weeks of exposure to 1540 mg/m3. Two out
of 6 dogs exposed to 3900 mg/m3 died after 30 and 43 exposures,
respectively. Two out of six cats exposed to 3900 mg/m3 died
after 43 exposures. Cats were not exposed to lower
concentrations. Two monkeys died after 2 and 32 exposures,
respectively, to 3900 mg/m3, but 2 others exposed to 730 mg/m3
survived for 25 weeks.
Kidney and liver damage, consisting of fatty changes and
necrosis in both organs, was found in animals that died from
exposure to the highest dose level (3900 mg/m3). In addition,
the rats showed pulmonary congestion and haemorrhage; one monkey
that died after 32 exposures and one dog showed a focal
myocarditis. Approximately half of the animals that died after
exposure to 1540 mg/m3 showed similar histological changes in
the liver and kidneys, but no such changes were observed in rats
that died from exposure to lower levels. Hepatic fatty changes
were observed in guinea-pigs exposed to 730 mg/m3. Histological
examinations were not carried out on mice.
Spencer et al. (1951) exposed rats, guinea-pigs, monkeys,
and rabbits to 1,2-dichloroethane at concentrations of 1620,
810, or 405 mg/m3, 7 h per day, 5 days/week, for various lengths
of time. In a group of 15 male and 15 female rats, exposed to
the highest level, no animals survived for more than 8 weeks,
and 60% mortality occurred in a second group exposed to the same
regime after 2 or 3 exposures. Mortality was also high in a
group of 8 male and 8 female guinea-pigs exposed to 1620 mg/m3.
All males had died by the second week and all females by
approximately the 5th week. Two male monkeys experienced rapid
and severe intoxication. Both were killed when moribund after
10 - 12 exposures. On the other hand, 2 male and 1 female
rabbits tolerated 33 weeks exposure with no evidence of adverse
effects. No mortality was observed when groups of 15 male and
15 female rats and 8 male and 8 female guinea-pigs were exposed
to 810 mg/m3 for about 30 weeks (151 exposures) or 36 weeks
(180 exposures), respectively. Similarly, no clinical effects
were observed in groups of 15 male and female rats, 8 male or
female guinea-pigs, 2 male and 1 female rabbits, and 1 male and
1 female monkey exposed for approximately 30 - 36 weeks to 405
mg/m3.
Histological examination was carried out on animals exposed
to the three dose levels. In both guinea-pigs and rats exposed
to the highest dose level, liver changes consisting of cloudy
swelling and fatty changes were observed. None of the other
organs were affected. Similar but less marked changes were
observed in the monkeys, while no adverse changes were found in
the rabbits. In rats exposed to 810 mg/m3, there were no
adverse changes in the liver or other organs, but reduced growth
and some fatty changes were found in the liver of guinea-pigs.
No adverse changes were found in animals exposed to the lowest
dose level (405 mg/m3).
Hofmann et al. (1971) exposed cats, rabbits, guinea-pigs,
and rats to 1,2-dichloroethane at 1980 mg/m3 or 405 mg/m3 for
6 h/day, 5 days/week, for up to 17 weeks. At the higher
concentration, rats became dyspnoeic and guinea-pigs apathetic.
Three out of 4 rabbits died after 10 - 17 exposures, and 9/10
guinea-pigs died after 4 - 14 exposures. Rats were more
sensitive, dying after only 1 - 5 exposures. All cats survived
30 exposures. Histologically, rats showed pulmonary hyperaemia
and oedema, fatty liver, and adrenal and myocardial necrosis.
Cats and rabbits exhibited a heart lesion and guinea-pigs, fatty
changes in the myocardium, liver, and adrenals and necrosis in
the myocardium and liver. At the lower concentration (405
mg/m3), rats, guinea-pigs, rabbits, and cats exposed for 17
weeks did not show any clinical or histological changes.
Of the species studied, mice and rats appear to be more
sensitive than other species to the adverse effects of 1,2-
dichloroethane. The no-observed-adverse-effect level for short-
term exposures (4 - 9 months) in rats studied in the 3
investigations is about 400 mg/m3.
Signs of central nervous system depression observed in the
above studies were apathy in guinea-pigs at 1980 mg/m3 (Hofmann
et al., 1971) and 3900 mg/m3 (Heppel et al., 1946), and coma in
dogs and monkeys at 3900 mg/m3 (Heppel et al., 1946). When rats
were exposed continuously for 3.5 months to 5 mg/m3, changes in
EEG were observed (Dmitrieva & Kuleshova, 1971). However, the
significance of these findings is not known.
7.2.2 Oral exposure
The liver appeared to be the principal target organ
following oral exposure. Rats, treated by gavage with 1,2-
dichloroethane in corn oil for 2 weeks, 5 times per week, at
doses of 150 mg/kg body weight or less did not show any
treatment-related abnormalities in organ or body weights,
histology, clinical chemistry, or haematology (Van Esch et al.,
1977; Reitz et al., 1982).
Rats were also exposed for 90 days, 5 times per week, to 0,
10, 30, or 90 mg/kg body weight (Van Esch et al., 1977). At the
2 highest exposure levels, a tendency to decreased weight gain
was observed. At 90 mg/kg, rats of both sexes showed an
increase in the relative weight of kidneys, but only the females
on this dose showed increased relative weights of liver and
brain compared with controls. Histology and clinical chemistry
were normal. Some haematological parameters were altered, but
not in a dose-related manner. In another study, after 5 doses
of 300 mg 1,2-dichloroethane/kg body weight in 5 days, all 6
rats died, and their livers showed fatty degeneration with an
increase in the triglycerides level (Van Esch et al., 1977).
Total fat and triglycerides were elevated in the livers of
rats exposed to approximately 100 mg/kg body weight per day via
the feed, which was administered twice daily, for 7 weeks
(Alumot et al., 1976a).
No adverse effects related to liver and kidney function were
observed at the lowest dose (10 mg/kg).
7.3 Long-Term Exposure
7.3.1 Inhalation exposure
Clinical chemical investigations were performed on Sprague
Dawley rats exposed by inhalation to 1,2-dichloroethane at 0, 5,
40, 202, or 1012 mg/m3 for 7 h per day, 5 days/week (Spreafico
et al., 1980). The highest exposure level was reduced to 607
mg/m3 after a few weeks because of high mortality. Animals of
each sex were exposed, starting at 3 months of age, for 3, 6, or
18 months. In addition, animals starting at 14 months of age
were exposed for 12 months. Groups of 8 - 10 animals of each
sex were sacrificed at the specified time intervals and clinical
chemistry tests performed. Changes in SGOT, SGPT, and
c-glutamyl transpeptidase activities in the 12-month animals
were not observed in the 18-month animals. Likewise, increases
in serum-uric acid and blood urea-nitrogen levels in the 12-
month animals were not observed in the 18-month animals. In
addition, the 12-month animals, but not the 18-month animals,
displayed decreases in serum-cholesterol. On the basis of the
negative results obtained in the animals starting exposure at 3
months of age and sacrificed after 3, 6, or 18 months, the
authors suggested a lack of significant toxicity, in spite of
the biochemical changes found in the older 12-month animals.
Neurotoxic changes (conditioned reflexes) in albino rats
have been associated with a 1,2-dichloroethane exposure of 50
mg/m3, 4 h/day, for 6 months (Borissova, 1957, 1960).
7.3.2 Oral exposure
In a controlled feeding schedule for 2 years, three groups
of 18 locally-bred rats of each sex were provided with feed
fumigated with 1,2-dichloroethane. The doses of 1,2-
dichloroethane administered were estimated to be 0, 11 - 17, or
23 - 35 mg 1,2-dichloroethane/kg body weight per day. No
adverse effects were observed on growth, mortality rates, or
serum composition. The mean survival period was 18 months or
more (Alumot et al., 1976a).
7.4 Carcinogenicity
7.4.1 Inhalation exposure
Groups of 11-week-old Swiss mice and 12-week-old Sprague
Dawley rats, comprising 90 animals of each sex, were exposed to
20, 40, 202, and 1012 mg 1,2-dichloroethane/m3 air (5, 10, 50,
and 250 ppm) for 78 weeks, 7 h per day, 5 days per week, and
observed for a lifetime. Purity was reported to be greater than
99%. The highest exposure was reduced to 607 mg/m3 (150 ppm)
after a few weeks because of high mortality. Control groups
contained 115 male mice, 134 female mice, or 180 rats of each
sex. Percentage survival in male and female mice, 52 weeks
after the beginning of treatment, was, respectively, 63 and 84%
in the controls; 47 and 93% at 20 mg/m3; 66 and 80% at 40 mg/m3;
51 and 81% at 202 mg/m3; and 43 and 64% at 607 mg/m3. The last
mouse died about 100 weeks after initiation of treatment. In
male and female rats, survival at 52 weeks was, respectively, 67
and 73% in the controls; 75 and 85% at 20 mg/m3; 70 and 81% at
40 mg/m3; 70 and 84% at 202 mg/m3; and 67 and 79% at 607 mg/m3.
Most rats had died by about 140 weeks after the start of
treatment. No specific types of tumours or changes in the
incidence of tumours were found in either species, with the
exception of an increased incidence (not dose-related) of
fibromas and fibroadenomas of the mammary glands of female rats
at 20, 202, and 607 mg/m3. The average latency time for these
tumours was 83 weeks in control rats and in rats exposed at
20 mg/m3, and 79 weeks at the 2 highest exposures. The authors
ascribe the differences in the incidence of mammary tumours
between the groups to the different survival rates in the groups
(Maltoni et al., 1980).
7.4.2 Oral exposure
Groups of 50 Osborne-Mendel rats of each sex were exposed to
average doses of 1,2-dichloroethane (technical grade with
reported purity > 90%)a in corn oil of 47 or 95 mg/kg body
weight for 78 weeks (NCI, 1978). Treatment was usually given 5
times per week, and the animals were observed for another 15 -
32 weeks after the end of treatment. Control groups consisted
of 20 matched controls of each sex treated with corn oil and 60
pooled control-treated rats of each sex. Eleven minor
contaminants were detected in the test compound. The rats were
housed in the same room as rats intubated with other halogenated
hydrocarbons or carbon disulfide. Body weights were not
affected by the exposures. A dose-related increase was found in
mortality rate, which was 100%, 27 weeks after cessation of
exposure to 95 mg/kg body weight. As shown in Table 9, male
rats had a dose-related increased incidence of subcutaneous
fibromas, and forestomach squamous cell carcinomas were
observed. In treated females, the stomach showed hyperplastic
lesions. The incidence of haemangiosarcomas (in various organs,
mainly the spleen) was increased in a dose-related manner in
both sexes, but the increase was statistically significant in
males only. In females, an increase was found in the incidence
of adenocarcinomas of the mammary gland (NCI, 1978).
a Subsequent analysis indicated a purity of about 98 - 99%
(Hooper et al., 1980; Ward, 1980).
Table 9. Summary of main tumour types after oral administration of 1,2-dichoroethane to mice and ratsa
---------------------------------------------------------------------------------------------------------
Group Maximum Number of animals with:
number of Forestomach Subcutaneous Mammary Haemangiosarcomas
animals squamous cell fibromas adeno- (several organs)
examined carcinomas carcinomas
---------------------------------------------------------------------------------------------------------
Rat (male)
Pooled vehicle controls 59 0 0 0 0
Matched vehicle controls 19 0 0 1 0
Low dose 46 3 5 2 9
(P < 0.002)b (P = 0.003)b
High dose 47 9 6 0 7
(P < 0.04)c (P < 0.01)b (P < 0.02)b
Rat (female) Mammary gland
fibroma
Pooled vehicle controls 59 0 5 1 0
Matched vehicle controls 20 0 0 0 0
Low dose 50 0 14 1 4
(P < 0.01)b
High dose 50 0 8 18 4
(P < 0.001)b
---------------------------------------------------------------------------------------------------------
Group Maximum Number of animals with:
number of Hepatocellular Alveolar/ Forestomach Mammary gland
animals carcinomas bronchiolar squamous adenocarcinomas
examined adenomas cell carcinomas
---------------------------------------------------------------------------------------------------------
Mice (male)
Pooled vehicle controls 59 4 0 1 -
Matched vehicle controls 19 1 0 1 -
Low dose 46 6 1 1 -
High dose 47 12 15 2 -
(P < 0.01)b (P < 0.001)b (NS)
Mice (female)
Pooled vehicle controls 60 0 2 1 0
Matched vehicle controls 20 1 1 1 0
Low dose 50 0 7 2 9
(P = 0.001)b
High dose 48 1 15 5 7
(P < 0.001)b (NS) (P = 0.003)b
---------------------------------------------------------------------------------------------------------
a Adapted from: NCI (1978).
b Statistical analyses shown are in comparison with the pooled matched controls.
c Statistical analyses shown are in comparison with the matched vehicle controls.
In the same set of studies, B6C3F1 mice were exposed in a
similar fashion to average doses of 0, 97, or 195 mg technical
grade compound/kg body weight for males and 0, 149, or 299 mg/kg
body weight for females. They were housed in the same room
where several other hydrocarbons or other substances were
tested. After exposure, they were observed for another 12 - 13
weeks. In females, body weights were depressed at the highest
doses from week 15 onwards, while the survival rate decreased in
a dose-related manner. Mean survival exceeded 65 weeks in all
groups. Many treated mice suffered from bronchopneumonia. As
shown in Table 9, dose-related increased incidences of alveolar
or bronchiolar adenomas were found in both sexes. In males, a
dose-related increased incidence of hepatocellular carcinomas
was observed. Female mice showed slight, dose-related increases
in the incidence of squamous cell carcinomas of the forestomach,
but this was not statistically significant. In male mice,
hyperplastic changes were found at this site. The incidence of
adenocarcinomas of the mammary gland was significantly increased
in females at both doses (NCI, 1978).
7.4.3 Dermal exposure
Groups of 30 female Ha:ICR Swiss mice were treated with 42
or 126 mg 1,2-dichloroethane in acetone on the shaven dorsal
skin, 3 times per week for 440 - 594 days. In a third group,
each female received one application of 126 mg of the test
compound followed 2 weeks later by phorbol myristate acetate, a
promotor, in acetone 3 times per week for 428 - 576 days. There
were 3 control groups, a positive control, one for the promotor,
and one for no treatment. 1,2-Dichloroethane did not initiate
skin tumours. There was an elevated incidence of lung
papillomas at the highest dose compared with controls (Van
Duuren et al., 1979).
7.5 Mutagenicity and Related End-Points
7.5.1 Mutations
Information in this section is summarized in Table 10.
7.5.1.1 Bacteria
Several investigators have observed a weak or no effect of
1,2-dichloroethane in Salmonella typhimurium TA1535 or TA 100 in
spot tests or standard plate incorporation assays with or
without rat liver S9 fraction or pure microsomes (Brem et al.,
1974; McCann et al., 1975; King et al., 1979; Guengerich et al.,
1980; Principe et al., 1981). A weak mutagenic effect was
observed in 1,2-dichoroethane vapour-exposed S. typhimurium TA
1535 and TA 100, which did not increase further by the addition
of a metabolic activation system (Barber et al., 1981). However,
a stronger positive response was observed by others (Rannug et
al., 1978; Rannug & Beije, 1979; Principe et al., 1981) in TA
1535 in the presence of rat liver metabolic activation system.
It has been established that this effect has been caused by
cytosolic glutathione-S-transferases (Rannug et al., 1978; Guen-
gerich et al., 1980; Reitz et al., 1982), and similar results
have also been obtained in TA 100 (van Bladeren et al., 1981b).
In vitro Salmonella tests using the bile of mice or rats
exposed to 1,2-dichloroethane, probably containing active con-
jugates, have confirmed these results (Rannug & Beije, 1979).
No mutagenic effects were observed in forward mutation tests
with Escherichia coli (King et al., 1979).
Table 10. Tests for gene mutations/chromosome/DNA damage and cell transformation induced
by 1,2-dichloroethane
---------------------------------------------------------------------------------------------------------
Test description System description Activation Result Reference
Organism Strain/cell type system (S9)
---------------------------------------------------------------------------------------------------------
Gene mutations
reverse mutation bacteria S. typhimurium TA 1530 A + (weak) Brem et al. (1974)
TA 1535 A + (weak)
TA 1538 A + (weak)
reverse mutation bacteria S. typhimurium TA 100 A + (weak) McCann et al. (1975)
reverse mutation bacteria S. typhimurium TA 1535 P - King et al. (1979)
TA 100 P -
TA 1537 P -
TA 1538 P -
TA 98 P -
reverse mutation bacteria S. typhimurium TA 1535 P + Principe et al.
TA 1537 A or P - (1981)
TA 1538 A or P -
TA 98 A or P -
TA 100 A or P -
reverse mutation bacteria S. typhimurium TA 1535 Pa - Guengerich et al.
Pb + (1980)
reverse mutation bacteria S. typhimurium TA 1535 A or P + Barber et al.
TA 100 A or P ± (1981)
TA 1538 A or P -
TA 98 A or P -
---------------------------------------------------------------------------------------------------------
Table 10 (contd).
---------------------------------------------------------------------------------------------------------
Gene mutations (contd)
reverse mutation bacteria S. typhimurium TA 1535 A ± Rannug et al. (1978)
TA 1535 P +
TA 1535 Pb +
reverse mutation bacteria S. typhimurium TA 1535 P + Rannug & Beije (1979)
reverse mutation bacteria S. typhimurium TA 1535 P + Reitz et al. (1982)
reverse mutation bacteria S. typhimurium TA 100 Pb + van Bladeren et al.
(1981a)
forward mutation fungi S. coelicolor A - Principe et al.
A. nidulans A - (1981)
sex-linked lethals insect D. melanogaster + Rapoport (1960); Shak-
arnis (1969, 1970);
King et al. (1979)
somatic cell mutation insect D. melanogaster + Nylander et al.
(1978)
forward mutation Chinese ovary cells in vitro P ++ Tan & Hsie (1981)
hamster A +
P + Zamora et al. (1983)
forward mutation human lymphoblastoid cell line A + Crespi et al. (1985)
AHH-1 and TK6 in vitro
somatic cell mutation mouse C57BL/6J Han (female) NA +? Gocke et al. (1983)
(spot test) x T stock (male)/embryos
---------------------------------------------------------------------------------------------------------
Table 10 (contd).
---------------------------------------------------------------------------------------------------------
Test description System description Activation Result Reference
Organism Strain/cell type system (S9)
---------------------------------------------------------------------------------------------------------
Chromosome/DNA damage
micronucleus test mice NMRI/polychromatic NA - King et al. (1979)
(ip or gavage exposure) erythrocytes
micronucleus test mice CBA/polychromatic NA - Jenssen & Ramel
(ip exposure) erythrocytes (1980)
dominant lethal mice ICR Swiss/germ cells NA - Lane et al. (1982)
(ip exposure)
alkaline DNA mice B6C3F1/liver in vivo/ NA + Storer & Conolly
unwinding in vitro (1983)
alkaline DNA mice B6C3F1/liver in vivo/ NA + Storer et al. (1984)
unwinding in vitro
unscheduled DNA human lymphocytes in vitro NA + Perocco & Prodi
synthesis (exposure by (1981)
addition in the medium)
Cell transformation
cell transformation Syrian embryo cells in vitro A +c Hatch et al. (1983)
(gas/vapour exposure) golden hamster
cell transformation mice BALB/c-3T3 in vitro A - Tu et al. (1985)
(exposure by addition
in the medium)
---------------------------------------------------------------------------------------------------------
a Pure microsomes.
b Cytosol.
c Enhanced viral induced transformation.
NA = not applicable.
7.5.1.2 Fungi
Forward mutation tests conducted with Streptomyces coeli-
color and Aspergillus nidulans were negative in plate incor-
poration assays and spot tests (Principe et al., 1981). In
A. nidulans, 1,2-dichoroethane induced non-disjunction and
haploidization (Crebelli et al., 1984). 1,2-Dichloroethane was
found to be a weak inducer of mitotic crossing over
in Saccharomyces cerevisiae (Simmon, 1980).
7.5.1.3 Insects
Sex-linked recessive lethal mutations were induced
in Drosophila melanogaster by 1,2-dichloroethane (Rapoport,
1960; Shakarnis, 1970; King et al., 1979). Non-dysjunction was
observed inconsistently (Shakarnis, 1969, 1970), while the
frequency of somatic mutations for eye pigmentation increased
(Nylander et al., 1978).
7.5.1.4 Mammals/mammalian cells
A spot test using mice provided weak evidence that 1,2-
dichloroethane can induce somatic mutations (Gocke et al.,
1983).
In Chinese hamster ovary cells, 1,2-dichloroethane induced
mutations at the HGPRT-locus (Tan & Hsie, 1981; Zamora et al.,
1983). Metabolic activation increased the mutation frequency in
the presence of NADPH (Tan & Hsie, 1981). 1,2-Dichloroethane
also induced a dose-related increase in the frequency of
mutations at the HGPRT-locus in two human lymphoblastoid cell
lines, AHH-1 and TK6. The mutation frequency in the AHH-1 cell
line was 25 times that in the TK6 cell line. The difference in
sensitivity was attributed to the difference in the levels of
glutathione-S-transferase (EC 2.5.1.18) activity. The activity
of this enzyme in the AHH-1 cell line was 5 times that in the
TK6 cell line (Crespi et al., 1985).
7.5.2 Chromosome damage/DNA damage
In in vivo studies, no effects were observed in dominant
lethal assays in 2 generations of ICR Swiss mice (Lane et al.,
1982) and in the micronucleus test with CBA and NMRI mice (King
et al., 1979; Jenssen & Ramel, 1980).
1,2-Dichloroethane was a weak inducer of unscheduled DNA
synthesis in cultured human lymphocytes (Perocco & Prodi,
1981).
DNA alkylation in Salmonella by activated 1,2-dichloroethane
was directly related to the mutation frequency, but absolute
levels of DNA alkylation in Salmonella were considered low
(Reitz et al., 1982).
1,2-Dichloroethane weakly inhibited growth of DNA polymerase
deficient E. coli (Brem et al., 1974; Rosenkranz, 1977).
Administration of 1,2-dichloroethane to mice and rats
resulted in covalent binding to macromolecules (Reitz et al.,
1982; Arfellini et al., 1984; Inskeep et al., 1986). Absolute
levels of DNA alkylation in rats, either by gavage or
inhalation, were considered low (Reitz et al., 1982).
Hepatic DNA damage was demonstrated using the alkaline DNA
unwinding assay in male B6C3F1 mice after a single intraperi-
toneal or oral dose of 1,2-dichloroethane that failed to induce
toxic effects in the liver. It was also shown that, after one
inhalation exposure, hepatic DNA damage only occurred at
exposure levels that caused high mortality (Storer & Conolly,
1983; Storer et al., 1984).
7.5.3 Cell transformation
1,2-Dichloroethane did not transform BALB/c-3T3 mouse cells
in a test conducted without any exogenous metabolic activating
system (Tu et al., 1985). It enhanced transformation of Syrian
hamster embryo cells by simian adenovirus (Hatch et al., 1983).
7.6 Reproduction and Teratogenicity
7.6.1 Inhalation exposure
It has been reported that 1,2-dichloroethane was found in
the fetuses of rats after exposure to 600 mg/m3 for 5 h (Withey
& Karpinski, 1985).
Female rats (strain unspecified) were exposed to 15 mg 1,2-
dichloroethane/m3, for 4 h daily, 6 days/week, for 4 months
prior to mating. During this period, the estrus cycle became
longer than normal. The rats were then mated and exposure
continued. No information on the effects on fertility was
given, but the total embryonal mortality increased from
approximately 11% in controls to 27% in treated dams, while pre-
implantation losses were found to be 5 times greater in treated
animals than in the controls. No fetal abnormalities were
reported, with the exception of haematomas in the region of the
head, neck, and anterior extremities (Vozovaya, 1977).
In a second study, 1,2-dichloroethane was administered to
female albino rats at a concentration of 57 ± 10 mg/m3 in air,
for 4 h daily, 6 days/week, for 6 and 9 months. When the rats
were mated, a reduction in fertility was observed (6.5 fetuses
per treated female versus 9.7 in controls). The weight of
newborn rats was reduced (5.06 g versus 6.44 in unexposed
females). Perinatal mortality was increased (Vozovaya, 1974).
1,2-Dichloroethane was detected in placental and fetal
tissues after inhalation exposure to 1000 mg/m3 for 3 days
(daily duration not stated). It was also found in the stomach
of 12- to 14-day-old mice, when lactating females were exposed
to an unspecified concentration of 1,2-dichloroethane by
inhalation (Vozovaya, 1977).
While the above results indicate a possible adverse effect
of 1,2-dichloroethane on reproduction, the following repro-
ductive studies yielded negative results. Groups of 20 Sprague
Dawley rats of each sex were exposed for 60 days prior to
mating, for 6 h per day, and 5 days per week to 101, 304, or
607 mg 1,2-dichloroethane/m3 in air. After mating, they were
exposed similarly, for another 116 days, but for 7 days per
week. Females were not exposed between gestation day 21 and day
4 post partum. Control groups contained 30 rats each. Pups
were removed and examined at 21 days of age, and the females
were remated following the removal of the last litter. Each
female produced 2 litters. The parents did not show any toxic
effects and the fertility index and gestation period were
normal. In the pups, no effects were found on sex ratio,
survival indices, organ weights, or histology. A small decrease
was noted in the number of pups in the first litters at
304 mg/m3 (Rao et al., 1980).
A teratogenicity study was further performed with groups of
16 - 30 female rats and 19 - 21 female rabbits (Rao et al.,
1980). These groups were exposed through inhalation to 0, 405,
or 1215 mg 1,2-dichloroethane/m3 air, for 7 h per day, from the
6th day of pregnancy onwards (rats up to the 15th day and
rabbits up to the 18th day of gestation). Exposures at both
levels were toxic for rabbit dams. The higher exposure level
was severely toxic for the rat dams; only 1 of the few surviving
females was pregnant, and all the implantation sites were
resorbed. No adverse effects on reproduction were observed
among rats exposed at 405 mg/m3 and rabbits exposed at 405 or
1215 mg/m3. The only gross change observed in rat fetuses was a
decreased incidence of bilobed thoracic centra. There were no
significant alterations in rabbit fetuses.
7.6.2 Oral exposure
Groups of 18 female rats that had received 0, 11 - 17, or
23 - 35 mg 1,2-dichloroethane/kg body weight per day via the
feed, for up to 2 years, were mated with untreated males. The
purity of the substance was not reported. No effects on
reproduction were observed (Alumot et al., 1976a).
Lane et al. (1982) reported a 2-generation reproduction
study on groups of 10 male and 30 female ICR Swiss mice
receiving nominally 5, 15, or 50 mg 1,2-dichloroethane/kg body
weight per day via the drinking-water, for up to 25 weeks.
Control groups contained 20 male and 60 female mice. After 5
weeks of treatment, the mice were mated to produce F1A, F1B, and
F1C litters. After weaning and 11 weeks of treatment, the F1B
mice were mated to produce F2A and F2B litters. Remating always
occurred 2 weeks after weaning. In the F1C and F2B matings,
females were co-housed with untreated males for teratology
screening. The parents did not show any toxic effects and
fertility and gestation indexes were normal. There were no
effects on survival, litter size, postnatal body weight, and
gross pathology of pups. No congenital malformations were
detected. In the F1C and F2B litters, no exposure-related
reproductive effects were found. In the F2B litters, there was
no increase in the incidence of fetal visceral or skeletal
anomalies. F1C litters were not examined for skeletal
anomalies.
7.7 Immunotoxicity
When rabbits were exposed for 7.5 - 8 months to 1,2-
dichloroethane vapour of unspecified purity at a level of
100 mg/m3, for 3 h per day, 6 h per week, an 80% reduction in
the production of antibodies against typhoid vaccine was
observed. Concomitantly, there was a 2-fold increase in Forsman
sheep erythrocyte antibodies (Shmuter, 1977).
Immunosuppression was also noted in a later oral study, in
which groups of 32 male CD-1 mice were exposed to 3, 24, or
189 mg 1,2-dichloroethane/kg body weight (purity unknown) via
the drinking-water for 90 days. In addition, groups of 10 male
CD-1 mice were exposed, once a day, to 4.9 or 49 mg/kg body
weight by water gavage for 14 days. Control groups comprised 48
mice in the 90-day study and 12 mice in the 14-day study. Apart
from a 30% reduction in the leukocyte count after 14 days of
exposure to 49 mg/kg body weight, no effects were found on other
haematological parameters and relative organ weights. After the
90-day exposure, decreases in body weight and fluid consumption
were noted. There was a tendency towards a reduction in
immunoglobulin spleen antibody-forming cells and in the serum-
antibody level after sheep erythrocyte immunization, while no
effects were observed in the response to the B-cell mitogen
lipopolysaccharide S. After the 14-day exposure, 25% and 40%
suppression of antibody-forming cells were measured at 4.9 and
49 mg/kg body weight, respectively. After the 90-day exposure,
no effects were seen on the cell-mediated immunity, assessed by
measuring the delayed hypersensitivity response to sheep
erythrocytes and the spleen cell response to the T-cell mitogen
Concanavalin A. After the 14-day exposure, a slight suppression
of the delayed hypersensitivity response was found, which was
not dose-dependent (Munson et al., 1982).
8. EFFECTS ON MAN
Limited data are available on the effects on man of 1,2-
dichloroethane. There are no adequate controlled studies, no
recent occupational studies, and no mortality studies. However,
case studies of accidental exposures have been reported.
8.1 Accidental Exposures
8.1.1 Inhalation exposure
Many reports are concerned with mixed exposures. However,
in this publication, only case studies are considered in which
the exposure was reported to be to 1,2-dichloroethane alone.
According to a review by NIOSH (1976), no data on exposure
levels and duration of exposure were reported.
Inhalation of 1,2-dichloroethane vapour first afflicts the
central nervous system. Symptoms include headache, dizziness,
weakness, cyanosis, muscular spasms, hypotonia, vomiting, and
unconsciousness. Death often follows. The respiratory tract
can be irritated and inflamed with such symptoms as cough and
rales over the chest. Cyanosis may occur either as the result
of respiratory insufficiency due to depression of the central
nervous system or by bronchial obstruction due to inflammation.
Epigastric or visceral pains and diarrhoea have been observed.
Autopsy reports frequently mention damage to the lungs, liver,
and kidneys (Wirtschafter & Schwartz, 1939; Hadengue & Martin,
1953; Menschick, 1957; Troisi & Cavallazzi, 1961; Suveev &
Babichenko, 1969). Changes in heart rhythm have been reported,
which are probably secondary effects (section 8.1.2) (Suveev &
Babichenko, 1969). Clinical findings include elevated serum-
bilirubin levels and leukocytosis (Wirtschafter & Schwartz,
1939; Menschick, 1957) and elevations of blood-lactate, ammonia,
ornithine carbamyl transferase, serum aspartate transaminase (EC
2.6.1.1), lactate dehydrogenase, and creatine phosphokinase
(Nouchi et al., 1984).
8.1.2 Oral exposure
The effects of acute oral exposure are very similar to those
found after inhalation, but they are more pronounced. A summary
of acute oral intoxications was prepared by US NIOSH in 1976.
The lethal effects of 1,2-dichloroethane associated with oral
exposure are presented in Table 11. Oral doses of 20 - 50 ml
1,2-dichloroethane have been identified as being lethal (IRPTC,
1984). Several major syndromes can be identified including
central nervous system depression, gastroenteritis, and
disorders of the liver and kidneys. Frequently-observed
cardiovascular insufficiency and haemorrhagic diathesis may be
related to changes in oxygenation and effects on the liver
(Weiss, 1957; Morozov, 1958; Hinkel, 1965; Bogoyavlenski et al.,
1968; Martin et al., 1968; Schönborn et al., 1970; Yodaiken &
Babcock, 1973; Dorndorf et al., 1975; Andriukin, 1979).
Table 11. Effects associated with acute lethal oral doses of 1,2-dichloroethane in human beingsa
---------------------------------------------------------------------------------------------------------
Amount ingested (g) Findingsb Reference
---------------------------------------------------------------------------------------------------------
188 - 250 death of 4 males up to 35 h; internal Bryzhin (1945)c
haemorrhage at various sites; liver damage;
symptoms at 3 - 4 h
87 - 125 death of 3 males after 5 - 8 h; internal Kaira (1966)c
haemorrhage; symptoms immediate (uncon-
sciousness, vomiting, dizziness)
103 death after 6 h; haemorrhagic lesions Noetzel (1944)c
75 death after 22 h; symptoms at 2 h (cyanosis, Hueper & Smith (1935)
(cyanosis, vomiting, dilated pupils); brain
haemorrhage, liver damage, nephrosis
63 death at 91 h; lack of eye reflex to light Roubal (1947)c
on 4th day; vomiting; rapid pulse
63 death in 3 - 4 h; liver damage Secchi et al. (1968)c
63 death at 17 h; cyanosis, diarrhoea; impaired Schonborn et al. (1970)
blood coagulation
50 death at 24 h; haemorrhage at various sites; Martin et al. (1968)
signs of cardiac damage
---------------------------------------------------------------------------------------------------------
Table 11 (contd).
---------------------------------------------------------------------------------------------------------
Amount ingested (g) Findingsb Reference
---------------------------------------------------------------------------------------------------------
50 death at 28 h; internal haemorrhage Garrison & Leadingham (1954)c
37 death at 10 h; internal haemorrhage at Lochhead & Close (1951)
various sites; adverse lung effects
25 death at 24 h; epigastric pain; slow Roubal (1947)c
pulse
25 death at 13 h; symptoms at 1 h (cyanosis, Flowtow (1952)c
vomiting)
25 death within 12 h; symptoms not reported Flowtow (1952)c
19 death after 6 days; liver, kidney, renal Yodaiken & Babcock (1973)
damage; pulmonary oedema; hypoglycaemia;
clotting time decreased; some haemorrhaging
10 death after 56 h; delirium; pulse deterior- Bogoyavlenski et al. (1968)
ation
---------------------------------------------------------------------------------------------------------
a Studies in which doses could not be estimated have not been cited. The reader is referred to
NIOSH (1976) for a description of these case reports.
b Unless otherwise stated, death refers to single male individuals.
c The reader is referred to NIOSH (1976) for a more complete description of symptomology.
Symptoms of central nervous system depression commonly
appear within 1 h, frequently with cyanosis, nausea, vomiting,
diarrhoea, epigastric and abdominal pains, and irritation of the
mucous membranes. Irreversible brain damage has been reported
in one case, and brain damage has been found in several fatal
cases (Rohmann et al., 1969; Dorndorf et al., 1975). In some of
the cases, an interval relatively free of symptoms followed
ingestion (Hinkel, 1965; Martin et al., 1968; Komarov et al.,
1973; Dorndorf et al., 1975). In the next phase, decreasing
consciousness and circulatory and respiratory failure may occur,
often leading to death some hours to some days after exposure.
During the intoxication, heart rhythm disturbances can lead to
cardiac arrest (Morozov, 1958; Martin et al., 1968; Yodaiken &
Babcock, 1973; Andriukin, 1979). Autopsy reports have revealed
damage to the mucosae of the gastrointestinal tract, liver,
kidney, lung, heart, and brain. Livers can be enlarged. Liver
and kidney epithelium can show fatty degeneration and necrosis.
Renal insufficiency has been reported to follow development of
hepatic insufficiency and has been known to progress to uraemic
coma (Natsyuk & Mudritsky, 1974). Lung oedema is frequently
found. Hyperaemia and haemorrhagic lesions are found in some
organs. According to some authors (Martin et al., 1968;
Schönborn et al., 1970; Yodaiken & Babcock, 1973), it appeared
that the blood coagulation time was increased because of a
decrease in blood clotting factors and thrombocytes. These
effects appear secondary to liver cell necrosis complicated
further by intravascular coagulation. Biochemically, liver
damage is illustrated by increased serum levels of bilirubin,
transaminases, and lactate dehydrogenase (Martin et al., 1968;
Yodaiken & Babcock, 1973; Dorndorf et al., 1975; Andriukin,
1979). Kidney damage is expressed by anuria or oliguria
(Morozov, 1958; Bogoyavlenski et al., 1968; Yodaiken & Babcock,
1973) and albumin, leukocytes, and epithelium cells in the
urine. Together with the histopathology, this points to acute
necrosis of the kidney tubule, possibly as a result of the liver
cell necrosis and the changes in circulation (Morozov, 1958;
Hinkel, 1965; Yodaiken & Babcock, 1973). Haematological changes
include decreases in the erythrocyte count and haemoglobin
content (Morozov, 1958; Dorndorf et al., 1975).
8.1.3 Acute effects on eyes and skin
Dysfunction of the central nervous system, which could be
caused by brain oedema, can lead to effects on the eyes such as
dilation or constriction of the pupils and impairment of eye
reflexes (Weiss, 1957; Troisi & Cavallazzi, 1961). Weiss (1957)
reported a cloudy cornea in 2 cases of oral exposure; this has
also been reported in dogs (section 7.1.2). Conjunctivitis was
found in 2 out of 4 patients, who had been exposed to 1,2-
dichloroethane vapour (Menschick, 1957). After intermittent
immersion of the hands of 3 men in 1,2-dichloroethane for 4 h,
severe dermatitis developed (Wirtschafter & Schwartz, 1939).
8.2 Occupational Exposure
Only 2 reports are available, and these date from before
1960. In the first study (Cetnarowicz, 1959), 16 male workers
from an oil refinery, exposed for 2 - 8 months, were selected
for close examination. A group of 6 workers was exposed to
concentrations of between 40 and 150 mg 1,2-dichloroethane/m3
air, and a group of 10 workers was exposed to concentrations
between 250 and 800 mg/m3. The workers were also exposed to
benzene at levels between 10 and 25 mg/m3, which was considered
not significant by the authors. A general reduction in body
weight was observed. Complaints came mainly from the group with
the higher exposure and included a burning sensation of the
eyes, lachrymation, dizziness, lassitude, sleepiness, nausea,
vomiting, constipation, poor appetite, epigastric pain, and
weight loss. There was no control group, but symptoms repor-
tedly disappeared when workers were removed from exposure;
symptoms returned upon re-exposure. Most, but not all, abnorm-
alities were found in the group with higher exposure and
involved the liver (8 workers), central nervous system (3
workers), gastrointestinal tract (7 workers), and haematological
parameters (1 - 7 workers). No lesions were found in the eye,
respiratory tract, lung, or heart.
The second study (Kozik, 1957), was conducted on workers
employed in an aircraft factory using a gum dissolved in 1,2-
dichloroethane. No data were given on the composition of the
glue or on the employment status of the workers. Air concentra-
tions of 1,2-dichloroethane varied considerably, the level being
5 mg/m3 or less during 70 - 75% of the working time, and 80 -
150 mg/m3 during 25 - 30% of the working time. The morbidity of
the workers in this department during the whole period under
study (1951-55) was increased for all disease categories in
comparison with that in workers in the entire factory. A group
of 83 workers was examined further, in the absence of controls.
There were 19 workers with diseases of the liver and bile duct,
13 workers with neurotic conditions, 11 workers with autonomous
dystonia, and 10 workers with hyperthyroidism and goitre.
These reports are difficult to evaluate, because no
indication is given of the prevalence in unexposed workers of
the symptoms and signs described in the case studies.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Evaluation of Human Health Risks
In spite of the high volume of production of 1,2-dichloro-
ethane, there are no quantitative exposure-effect data on human
beings. Frequently, 1,2-dichloroethane is not the only chemical
involved in an exposure and thus, cause-effect relationships are
difficult to derive. No epidemiological or mortality studies
are available. There are only two old reports on small groups
of occupationally-exposed men (section 8.2). These data indi-
cate that repeated inhalation exposures in the range of approx-
imately 40 - 800 mg/m3 may lead to central nervous system
depression and gastrointestinal and liver abnormalities. As
might be expected, such symptoms were more prevalent in indivi-
duals exposed to high levels. The only available data regarding
oral exposure are those involving fatal intoxications (Table 11,
section 8.1).
Because of the limitations of the human data base, it is
necessary to rely on the available experimental animal data to
derive a no-observed-adverse-effect level for human beings.
This is possible because of the similarity in the spectrum of
adverse effects in man and laboratory animals, which include
central nervous system depression, liver and possibly kidney
abnormalties, lung oedema, and cardiovascular disorders. The
dose-response data from animal studies include a no-observed-
adverse-effect level for the rat of about 400 mg 1,2-dichloro-
ethane/m3 air (section 7.2.1), equivalent to an intake of about
35 mg/kg body weight per day (assuming inhalation of 42 litres
over 7 h for a 500-g rat and 100% absorption). The highest
registered air exposure level of the general population is
65 µg/m3 (Table 4, section 4.1.2). This level leads to a
calculated intake of 1.3 mg per person per day (20 m3/day x
65 µg/m3). A comparable intake from drinking-water containing
the highest level recorded (6 µg/litre) (Table 3, section
4.1.1) would amount to a daily intake of 12 µg (6 µg/litre
litre/day). Thus, a combined air and water exposure, in the
context of a worst-case scenario for the general population,
when compared with the no-observed-adverse-effect level for
animals, is lower by a factor of well over 1000. Consequently,
for the biological end-points considered so far, it can be
concluded that 1,2-dichloroethane is unlikely to present a toxic
hazard for the general population, under prevailing exposure
conditions.
In an oral administration study, 1,2-dichloroethane produced
a statistically-significant increase in squamous cell carcinoma
of the forestomach, haemangiosarcoma, and mammary adenocarcinoma
in rats, and mammary adenocarcinoma and hepatocellular carcinoma
in mice (section 7.4.2). In one carcinogenicity study, inhala-
tion exposure did not result in an increase in tumour incidence
(section 7.4.1). The natural occurrence of forestomach squamous
cell carcinomas in rats and haemangiosarcomas in laboratory
rodents is unusual. Taking into consideration that cancer has
been produced in two species of experimental animals and in
several target organs, it can be concluded that 1,2-dichloro-
ethane is carcinogenic for rats and mice, when administered by
gavage.
In the absence of human data, and taking into account the
fact that 1,2-dichloroethane produces a reactive intermediate
that alkylates DNA, that it is positive in a number of in vitro
mutagenicity tests, though weakly so (section 7.5), and that it
results in the production of both rare and common tumours in
rats and mice, it would be prudent to consider 1,2-dichloro-
ethane as a possible human carcinogen. Therefore, 1,2-dichloro-
ethane should be regarded, for practical purposes, as if it
presented a carcinogenic risk for man. Thus, levels in the
environment should be kept as low as feasible.
Since there are no human data, it is necessary to rely on
the limited data available from experimental animal studies in
evaluating reproduction hazards and teratogenicity. The weight
of evidence (section 7.6) does not suggest that exposure to
prevailing environmental levels would pose a human reproductive
or teratogenic hazard.
9.2 Evaluation of Effects on the Environment
9.2.1 Air
Emissions of 1,2-dichloroethane into the air mainly occur in
process industries. Other emissions occur during its use as a
fumigant, solvent, and lead scavenger, and via evaporation from
contaminated water and from waste disposal sites. Total emis-
sions are estimated to amount to 0.2% of the production volume.
Photochemical degradation via oxidation by hydroxyl radicals is
the most important route of elimination from air. Rainout and
adsorption on atmospheric particles are unlikely to be important
processes of elimination. Photolysis is theoretically a possib-
ility, but no evidence of this is available. The products of
the photochemical degradation are carbon monoxide, carbon
dioxide, hydrogen chloride, formyl chloride, and chloroacetyl-
chloride. The two last compounds will degrade further. The
process is rapid enough to prevent accumulation of the compound
in the atmosphere (section 3.3).
9.2.2 Water
Emissions of 1,2-dichloroethane into water may amount to
0.1% of the production volume. Some of the emissions from EDC
tars, which total about 0.5% of the production volume, will
contaminate water. The main process of removal of 1,2-dichloro-
ethane from water is evaporation. Chemical degradation is not
expected, nor is biodegradation fast enough to be of any signi-
ficance (section 3.1). Bioconcentration in aquatic species is
unlikely in view of the rather low octanol/water partition
coefficient. This conclusion is supported by a low bioconcen-
tration factor found experimentally (section 6.1.4).
The compound was only slightly toxic for aquatic species
tested. A no-observed-adverse-effect concentration for Daphnia
magna in a long-term test was 11 mg/litre (section 6.1.3). The
lowest LC50 value (85 mg/litre) was found for the shrimp Crangon
crangon (section 6.1.1). Average environmental levels of the
compound in surface water are generally below 1 µg/litre, but,
in heavily polluted surface water, average levels of 5.6
µg/litre have been measured with a maximum of 90 µ g/litre
(Table 3, section 4.1.1).
On the basis of the above data, it can be concluded that,
except in case of accidents and inappropriate disposal, 1,2-
dichloroethane does not pose a significant hazard for the
aquatic environment. However, it should be noted that EDC tars
are much more toxic than 1,2-dichloroethane (section 6.1.1).
9.2.3 Soil
Data are not sufficient to evaluate the effects of 1,2-
dichloroethane in soil.
10. RECOMMENDATIONS FOR FURTHER STUDIES
1. DNA alkylation (adduct identification).
2. Studies on sub-chronic toxicity using various routes of
exposure.
3. Assessment of the extent to which EDC tars contribute to
contamination of groundwater by 1,2-dichloroethane.
4. Dose-response studies on sensitive, commercially important
fish species (particularly studies relevant to EDC tar
spills).
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,2-Dichloroethane was evaluated by IARC in 1979 (Volume 20
of the IARC Monographs). It was concluded that:
"There is sufficient evidence that 1,2-dichloroethane is
carcinogenic in mice and rats. In the absence of adequate data
in humans, it is reasonable, for practical purposes, to regard
1,2-dichloroethane as if it presented a carcinogenic risk to
humans."
REFERENCES
ADEMA, D.M.M. (1976) [Acute toxicity tests with 1,2-
dichloroethane, phenol, acrylonitrile, and alkyl benzene-
sulfonate in sea water,] Delft, the Netherlands, Central
Laboratory TNO (TNO Report No. MD-N & E 76/1) (in Dutch).
ALUMOT, E., NACHTOMI, E., MANDEL, E., HOLSTEIN, P., BONDI, A., &
HERZBERG, M. (1976a) Tolerance and acceptable daily intake of
chlorinated fumigants in the rat diet. Food Cosmet. Toxicol.,
14: 105-110.
ALUMOT, E., MEIDLER, M., HOLSTEIN, P., & HERZBERG, M. (1976b)
Tolerance and acceptable daily intake of ethylene dichloride in
the chicken diet. Food Cosmet. Toxicol., 14: 111-114.
ANDRIUKIN, A.A. (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klin. Med. (Mosk.), 57: 43-47 (in
Russian).
ARFELLINI, G., BARTOLI, S., COLACCI, A., MAZZULO, M., GALLI,
M.C., PRODI, G., & GRILLI, S. (1984) In vivo and in vitro
binding of 1,2-dibromethane and 1,2-dichloroethane to
macromolecules in rat and mouse organs. J. Cancer Res. clin.
Oncol., 108: 204-213.
ATRI, F.R. (1984) [ Study to assess the toxicity of water
pollutants. III. 1,2-Dichloroethane,] Bonn, Federal Environment
Agency (Research Report No. 106/07/047) (in German).
BAILEY, S., COLLINS, G.B., FISHWICK, F.B., HART, H.V., HORLER,
D.F., & SCUDAMORE, K.A. (1982) Pesticide residues in
foodstuffs in Great Britain; organochlorine pesticides,
organophosphorus pesticides, and fumigant residues in home-
produced and imported wheat. Pestic. Sci., 13: 373-378.
BANERJEE, S., VAN DUUREN, B.L., & ORUAMBO, F.I. (1980)
Microsome-mediated covalent binding of 1,2-dichloroethane to
lung microsomal protein and salmon sperm DNA. Cancer Res., 40:
2170-2173.
BARBER, R.D., DONISH, W.H., & MUELLER, K.R. (1981) A procedure
for the quantitative measurement of the mutagenicity of volatile
liquids in the Ames Salmonella/microsome assay. Mutat. Res.,
90: 31-48.
BARETTA, E.D., STEWARD, R.D., & MUTCHLER, J.E. (1969)
Monitoring exposures to vinyl chloride vapour: breath analysis
and continuous air sampling. Am. Ind. Hyg. Assoc. J., 30: 537-
544.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CAROLL, J.J.
(1980) Bioconcentration and elimination of selected water
pollutants by bluegill sunfish Lepomis macrochirus. In:
Dynamics, Exposure and Hazard Assessment of Toxic Chemicals,
Paper Symposium 1978, Ann Arbor, Michigan, Ann Arbor Science,
pp. 379-392.
BARSOUM, G.S. & SAAD, K. (1934) Relative toxicity of certain
chlorine derivatives of the aliphatic series. Q. J. Pharm.
Pharmacol., 7: 205-214.
BAUER, U. (1978) [Halogenated hydrocarbons in drinking and
surface water. Analysis results 1976-77 in the Federal Republic
of Germany. Drinking water from 100 cities; surface water from
the Ruhr, Lippe, Main, and Rhine.] WaBoLu-Berichte, 3: 64-74 (in
German).
BAUER, U. (1981) [Human exposure to harmful components in
environmental studies on water, air, food, and human tissues.
II, IV.] Zentralbl. Bakteriol. Hyg. I. Abt. Orig. B, 174: 39-55,
556-583 (in German).
BENOIT, D.A., PUGLISI, F.A., & OLSON, D.L. (1982) A fathead
minnow Pimephales promelas early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ.
Pollut., 28(Series A): 189-197.
BERCK, B. (1965) Sorption of ethylene dibromide, ethylene
dichloride, and carbon tetrachloride by cereal products. J.
agric. food Chem., 13: 248-254.
BERCK, B. (1974) Fumigant residues of carbon tetrachloride,
ethylene dichloride, and ethylene dibromide in wheat, flour
bran, middlings, and bread. J. agric. food Chem., 22: 977-984.
BOGOYAVLENSKI, V.F., SALIKHOVA, S.K.H., & KARPOVA, E.V. (1968)
[Clinical aspects and therapy for ethylene dichloride
poisoning.] Sov. Med., 31: 107-109 (in Russian).
BOIKOVA, N.Y. & KRAVTSOVA, G.V. (1982) Histoenzymologic
characteristics of liver and kidney after acute and chronic
poisoning by 1,2-dichloroethane. In: Proceedings of the
Leningrad Scientific Society of Pathologoanatoms, Leningrad,
Medicine, Vol. 23, pp. 158-160.
BONNET, P., FRANCIN, J.-M., GRADISKI, D., RAOULT, G., & ZISSU,
D. (1980) Détermination de la concentration léthale 50 des
principaux hydrocarbures aliphatiques chlorés chez le rat.
Arch. Mal. prof. Méd. Trav. Sécur. soc., 41: 317-321.
BORISSOVA, M.K. (1957) [Experimental inputs to evaluation of
maximum allowable dichloroethane concentration in the air.] Gig.
i Sanit., 3: 13-19 (in Russian).
BORISSOVA, M.K. (1960) Some inputs to evaluation of maximum
allowable dichloroethane concentration in the air. In: Maximum
allowable concentrations of air pollutants, Vol. 4, pp. 21-24.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformations of 1- and
2-carbon halogenated aliphatic organic compounds under methano-
genic conditions. Appl. environ. Microbiol., 45: 1286-1294.
BOZZELLI, J.W. & KEBBEKUS, B.B. (1982) A study of some
aromatic and halocarbon vapors in the ambient atmosphere of New
Jersey. J. environ. Sci. Health, A17: 693-711.
BREM, H., STEIN, A.B., & ROSENKRANZ, H.S. (1974) The muta-
genicity and DNA-modifying effect of haloalkanes. Cancer Res.,
34: 2576-2579.
BRINGMANN, G. & KUHN, R. (1978) Testing of substances for
their toxicity threshold: model organisms Microcystis
(Diplocystis) aeruginosa and Scenedesmus quadricauda. Mitt.
Int. Verein Limnol., 21: 275-284.
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa
in the cell multiplication inhibition test. Water Res., 14:
231-241.
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effect of
hazardous substances on flagellated and ciliated, respectively,
holozoic bactivorous and saprozoic protozoa.] GWF Wasser-
Abwasser, 122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Data on the toxicity of water
pollutants to Daphnia magna in an improved standard test.] Z.
Wasser Abwasser Forsch., 15: 1-6 (in German).
BRYZHIN, F.F. (1945) Pathomorphological changes in internal
organs in connection with poisoning by ethylene dichloride
through the digestive tract. Pharmacol. Toxicol., 8: 43-49.
BUCCAFUSCO, R.J., ELLS, S.J., & LE BLANC, G.A. (1981) Acute
toxicity of priority pollutants of bluegill Lepomis macro-
chirus. Bull. environ. Contam. Toxicol., 26: 446-452.
CASWELL, G.H. & CLIFFORD, H.T. (1958) The effect of ethylene
dichloride and carbon tetrachloride on the germination and early
growth of maize. Emp. J. exp. Agric., 26: 365-372.
CETNAROWICZ, J. (1959) Experimental and clinical investiga-
tions on the action of dichloroethane. Folia Med. Cracov., 1:
169-192.
CLARK, A.I., MCINTYRE, A.E., LESTER, J.N., & PERRY, R. (1984a)
Ambient air measurement of aromatic and halogenated hydrocarbons
at urban, rural, and motorway locations. Sci. total Environ.,
39: 265-279.
CLARK, A.I., MCINTYRE, A.E., PERRY, R., & LESTER, J.N. (1984b)
Monitoring and assessment of ambient atmospheric concentrations
of aromatic and halogenated hydrocarbons at urban, rural, and
motorway locations. Environ. Pollut. Ser. B, 7: 141-158.
CREBELLI, R., CONTI, G., CONTI, L., & CARERE, A. (1984)
Induction of somatic segregation by halogenated aliphatic
hydrocarbons in Aspergillus nidulans. Mutat. Res., 138(1): 33-
38.
CRESPI, C.L., SEIXAS, G.M., TURNER, T.R., RYAN, C.G., & PENMAN,
B.W. (1985) Mutagenicity of 1,2-dichloroethane and 1,2-
dibromoethane in two human lymphoblastoid cell lines. Mutat.
Res., 142: 133-140.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA 600/3-80-
084, PB 221948).
DAWSON, G.W., JENNINGS, A.L., DROSDOWSKI, D., & RIDER, E.
(1977) The acute toxicity of 47 industrial chemicals to fresh
and saltwater fish. J. hazard. Mater., 1: 303-318.
DEQUINZE, J., SZIMAR, C., & EDELINE, F. (1984) Identification
of the substances and their derivative products, on the list of
129 substances (list 1 of the directive 76/464/EEC) present in
the refuse of chlorine derivatives by organic chemistry,
Brussels, European Economic Community.
DILLING, W.L., TEFERTILLER, N.B., & KALLOS, G.J. (1975)
Evaporation rates and reactivities of methylene chloride,
chloroform, 1,1,1-trichloroethane, trichloroethylene,
tetrachloroethylene, and other chlorinated compounds in dilute
aqueous solutions. Environ. Sci. Technol., 9: 833-838.
DMITRIEVA, N.V. & KULESHOV, E.V. (1971) [Changes in the
cerebral bioelectric activity and electric conductivity in rats
in chronic intoxications with certain chlorinated hydrocarbons.]
Gig. i Sanit., 36: 20-25 (in Russian).
DORNDORF, W., KRESSE, M., CHRISTIAN, W., & KATRITZKI, KG.
(1975) [Dichloroethane poisoning with myoclinic syndrome,
epileptic attacks, and irreversible cerebral effects.] Arch.
Psychiatr. Nervenkr., 220: 373-379 (in German).
DRURY, J.S. & HAMMONS, A.S. (1979) Investigations of selected
environmental pollutants: 1,2-dichloroethane, Washington DC, US
Environmental Protection Agency, Office of Toxic Substances (EPA
560/2-78-006, PB 295865).
DUPRAT, P., DELSAUT, L., & GRADISKI, D. (1976) Pouvoir
irritant des principaux solvants chlorés aliphatiques sur la
peau et les muqueuses oculaires du lapin. Eur. J. Toxicol., 9:
171-177.
EHRENBERG, Z., OSTERMAN-GOLKAR, S., SINGH, D., & LUNDQVIST, U.
(1974) On the reaction kinetics and mutagenic activity of
methylating and beta-halogenoethylating gasoline additives.
Radiat. Bot., 15: 184-194.
ELFERS, L.A. (1979) Monitoring of ambient levels of EDC in the
vicinity of EDC production and user facilities, Research
Triangle Park, North Carolina, US Environmental Protection
Agency, Environmental Monitoring and Support Laboratory (EPA
600/4-79-029, PB 298303).
ENVIRONMENT AGENCY, JAPAN (1983) Environmental monitoring of
chemicals: environmental survey report of fiscal years 1980 and
1981, Tokyo, Environment Agency.
EWING, B.B., CHIAN, E.S.K., COOK, J.C., DEWALLE, F.B., EVANS,
C.A., HOPKE, P.K., KIM, J.H., MEANS, J.C., MILBERG, R., PERKINS,
E.G., SHERWOOD, J.D., & WADLIN, W.H. (1977) Monitoring to
detect previously unrecognized pollutants in surface waters,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA 560/6-77-015, PB 273349).
FLOWTOW, E. (1952) Poisoning due to chlorinated hydrocarbon
compounds, particularly 1,2-dichloroethane. Chem. Tech.
(Leipzig), 6: 253-254.
FOUREMAN, G.L. & REED, D.J. (1985) Evidence for a non-
episulfonium ion intermediate during alkylation by S-(2-
chloroethyl) cysteine but not by S-(2-chloroethyl) glutathione.
Fed. Proc. Fed. Am. Exp. Biol., 44: 517.
FUJII, T. (1977) Direct aqueous injection gas chromato-
graphy-mass spectrometry for analysis of organohalides in water
at concentrations below the parts per billion level. J.
Chromatogr., 139: 297-302.
GARRISON, S.C. & LEADINGHAM, R.S. (1954) A fatal case of
ethylene dichloride poisoning in an occupational therapy
department of a neuro-psychiatric hospital. Am. J. phys. Med.,
33: 230-237.
GOCKE, E., WILD, D., ECKHARDT, K., & KING, M.-T. (1983)
Mutagenicity studies with the mouse spot test. Mutat. Res., 117:
201-212.
GOLD, L.S. (1980) Human exposures to ethylene dichloride. In:
Ames, B., Infante, P., & Reitz, R., ed. Ethylene dichloride: a
potential health risk? Cold Spring Harbor, Cold Spring Harbor
Laboratory, pp. 209-225 (Banbury Report No. 5).
GOSSETT, R.W., BROWN, D.A., & YOUNG, D.R. (1983) Predicting
the bioaccumulation of organic compounds in marine organisms
using octanol/water partition coefficients. Mar. Pollut. Bull.,
14: 387-392.
GRADISKI, D., BONNET, P., RAOULT, G., MAGADUR, J.L., & FRANCIN,
J.-M. (1978) Toxicité aiguë comparée par inhalation des
principaux solvants aliphatiques chlorés. Arch. Mal. prof. Méd.
Trav. Sécur. soc., 39: 249-257.
GRIMSRUD, E.P. & RASMUSSEN, R.A. (1975) Survey and analysis of
halocarbons in the atmosphere by gas chromatography-mass
spectrometry. Atmos. Environ., 9: 1014-1017.
GUENGERICH, F.P., CRAWFORD, W.M., DOMORADZKI, J.Y., MACDONALD,
T.L., & WATANABE, P.G. (1980) In vitro activation of 1,2-
dichloroethane by microsomal and cytosolic enzymes. Toxicol.
appl. Pharmacol., 55: 303-317.
GUENGERICH, F.P., MASON, P.S., STOTT, W.T., FOX, T.R., &
WATANABE, P.G. (1981) Role of 2-haloethylene oxides and vinyl
chloride in irreversible binding to protein and DNA. Cancer
Res., 41: 4391-4398.
GUENGERICH, F.P., KOGA, N., INSKEEP, P.B., & OZAWA, N. (1986)
Proceedings of the Second International Symposium on the
Synthesis and Application of Isotopically-Labelled Compounds,
New York, Elsevier.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of
organic chemicals in the atmosphere of the Netherlands. Sci.
total Environ., 43: 193-219.
HADENGUE, M.A. & MARTIN, R. (1953) Un cas d'intoxication
mortelle par le dichloréthane. Soc. Med. Leg., 33: 247-249.
HARKOV, R., KEBBEKUS, B., BOZZELLI, J.W., LLOY, P.J., & DAISEY,
J. (1984) Comparison of selected volatile organic compounds
during the summer and winter at urban sites in New Jersey. Sci.
total Environ., 38: 259-274.
HATCH, G.G., MAMAY, P.D., AYER, M.L., CASTO, B.C., & NESNOW, S.
(1983) Chemical enhancement of viral transformation in Syrian
hamster embryo cells by gaseous and volatile chlorinated
methanes and ethanes. Cancer Res., 43: 1945-1950.
HEITMULLER, P.T., HOLLISTER, T.A., & PARRISH, P.R. (1981)
Acute toxicity of 54 industrial chemicals to sheepshead minnows
Cyprinodon variegatus. Bull. environ. Contam. Toxicol., 27:
596-604.
HELLMANN, T.M. & SMALL, F.H. (1974) Characterization of the
odour properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
HEPPEL, L.A., NEAL, P.A., PERRIN, T.L., ENDICOTT, K.M., &
PORTERFIELD, V.T. (1946) The toxicology of 1,2-dichloro-
ethane (ethylene dichloride). V. The effect of daily
inhalations. J. ind. Hyg. Toxicol., 28: 113-120.
HILL, D.L., SHIH, T.-W., JOHNSTON, T.P., & STRUCK, R.F. (1978)
Macromolecular binding and metabolism of the carcinogen 1,2-
dibromoethane. Cancer Res., 38: 2438-2442.
HINKEL, G.K. (1965) [Oral dichloroethane intoxication of
children.] Dtsch Gesudheitswes., 20: 1327-1331 (in German).
HOFMANN, H.T., BIRNSTIEL, H., & JOBST, P. (1971) [On the
inhalation toxicity of 1,2-dichloroethane.] Arch. Toxikol., 27:
248-265 (in German).
HOOPER, K., GOLD, I., & AMES, B. (1980) The carcinogenic
potency of ethylene dichloride in two animal bioassays: a
comparison of inhalation and gavage studies. In: Ames, B.N.,
Infante, P., & Reitz, R., ed. Ethylene dichloride: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 65-81 (Banbury Report No. 5).
HOWARD, C.J. & EVENSON, K.M. (1976) Rate constants for the
reactions of OH with ethane and some halogen substituted ethanes
at 296 K. J. Chem. Phys., 64: 4303-4306.
HUEPER, W.C. & SMITH, C. (1935) Fatal ethylene dichloride
poisoning. Am. J. med. Sci., 189: 778-784.
IARC (1979) 1,2-Dichloroethane. In: Some halogenated hydro-
carbons, Lyons, International Agency for Research on Cancer, pp.
429-446 (IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Vol. 20).
INSKEEP, P.B. & GUENGERICH, F.P. (1984) Glutathione mediated
binding of dibromoalkanes to DNA: specificity of rat glutathione
S-transferases and dibromoalkane structure. Carcinogenesis, 5:
805-808.
INSKEEP, P.B., KOGA, N., CMARIK, J.L., & GUENGERICH, F.P.
(1986) Covalent binding of 1,2-dihaloalkanes to DNA and
stability of the major DNA adduct S-[2-(N7/guanyl)ethyl]
glutathione. Cancer Res., 46: 2839-2844.
IRPTC (1982) Izmerov, N.F., ed. Dichloroethane, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme (Scientific Review of Soviet
Literature on Toxicity and Hazards of Chemicals No. 25).
IRPTC (1984) Data profile of 1,2-dichloroethane, Geneva,
International Register of Potentially Toxic Chemicals.
JACOBS, E.S. (1980) Use and air quality impact of ethylene
dichloride and ethylene dibromide scavengers in leaded gasoline.
In: Ames, B., Infante, P., & Reitz, R., ed. Ethylene dichloride:
a potential health risk?, Cold Spring Harbor, New York, Cold
Spring Harbor Laboratory, pp. 239-255 (Banbury Report No. 5).
JAKOBSON, I., WAHLBERG, J.E., HOLMBERG, B., & JOHANSSON, G.
(1982) Uptake via the blood and elimination of 10 organic
solvents following epicutaneous exposure of anesthetized guinea-
pigs. Toxicol. appl. Pharmacol., 63: 181-187.
JANSSEN, D.B., SCHEPER, A., & WITHOLT, B. (1984) Biodegra-
dation of 2-chloroethanol and 1,2-dichloroethane by pure
bacterial cultures. Prog. Med. Microbiol., 20: 169-178.
JENSEN, S., LANGE, R., BERGE, G., PALMORK, K.H., & RENBERG, L.
(1975) On the chemistry of EDC-tar and its biological
significance in the sea. Proc. R. Soc. Lond. B, 189: 333-346.
JENSSEN, D. & RAMEL, C. (1980) The micronucleus test as part
of a short-term mutagenicity test program for the prediction of
carcinogenicity evaluated by 143 agents tested. Mutat. Res., 75:
191-202.
JERNELOV, A., ROSENBERG, R., & JENSEN, S. (1972) Biological
effects and physical properties in the marine environment of
aliphatic chlorinated by-products from vinyl chloride
production. Water Res., 6: 1181-1191.
JOHNSON, M.K. (1965) The influence of some aliphatic compounds
on rat liver glutathione levels. Biochem. Pharmacol., 14: 1383-
1385.
JOHNSON, M.K. (1966) Studies on glutathione-S-alkyl transferase
of the rat. Biochem. J., 98: 44-56.
JOHNSON, M.K. (1967) Metabolism of chloroethanol in the rats.
Biochem. Pharmacol., 16: 185-199.
JOLIVET, E. (1968) Métabolisme des acides organiques et des
acides aminés libres dans le tubercule de pomme de terre au
cours de la rupture provoquée de son repos végétatif par la
"Rindite". Physiol. vég., 6: 221-233.
JONSSON, A. & BERG, S. (1980) Determination of 1,2-dibromo-
ethane, 1,2-dichloroethane, and benzene in ambient air using
porous polymer traps and gas chromatographic-mass spectro-
metric analysis with selected ion monitoring. J. Chromatogr.,
190: 97-106.
KAGRAMANOV, V.G. & KAZIEVA, N.K. (1972) Condition of
fibrinolytic blood activity in acute dichloroethane poisoning.
Azerb. State Med. Inst. Baku, 36: 30-31.
KAIRA, F.M. (1966) [Alimentary oral dichloroethane poisoning.]
Klin. Med. (Mosk.), 44: 143-146 (in Russian).
KAMEL, A. (1959) The effect of carbon bisulphide and ethylene
dichloride-carbon tetrachloride mixture on the germination of
some seeds of certain crops. Agric. Res. Rev. (Cairo), 37: 330-
350.
KELLAM, R.G. & DUSETZINA, M.G. (1980) Human exposure to
ethylene dichloride: potential for regulation via EPA's proposed
airborne carcinogen policy. In: Ames, B., Infante, P., & Reitz,
R., ed. Ethylene dichloride: a potential health risk?, Cold
Spring Harbor, New York, Cold Spring Harbor Laboratory, pp.
265-276 (Banbury Report No. 5).
KERSTER, H.W. & SCHAEFFER, D.J. (1983) Brine shrimp (Artemia
salina nauplii) as a teratogen test system. Ecotoxicol.
environ. Saf., 7: 342-349.
KING, M.-T., BEIKIRCH, H., ECKHARDT, K., GOCKE, E., & WILD, D.
(1979) Mutagenicity studies with X-ray contrast media,
analgesics, antipyretics, antirheumatics, and some other
pharmaceutical drugs in bacterial, Drosophila, and mammalian
test systems. Mutat. Res., 66: 33-43.
KLEINSCHMIDT, E.-G., VON (1983) [Investigations into the
relation between odour thresholds of human beings for some
substances and their chemical structure.] Wiss. Z. Wilhelm-
Piech-Univ. Rostock, Naturwiss. Reihe, 32: 54-58 (in German).
KOGA, N., INSKEEP, P.B., HARRIS, T.M., & GUENGERICH, F.P.
(1986) S-[2-(N7-guanyl)ethyl] glutathione, the major DNA adduct
formed from 1,2-dichloroethane. Biochemistry, 25: 2192-2198.
KOKAROVTSEVA, M.G. & KISELYOVA, N. (1978) [Chloroethanol
(ethylenechlorohydrin) - a toxic metabolite of 1,2-dichloro-
ethane.] Farmakol. Toksikol., 41: 118-120 (in Russian).
KOMAROV, V.P., ROMANCHAK, M.N., SHCHERBAN, I.I., & GAPONTSEV,
S.N. (1973) [On the clinical features and therapy of acute
dichloroethane intoxications.] Zdravookhr. Belorussi, 4: 55-57
(in Belorussian).
KONEMANN, H. (1981) Quantitative structure-activity
relationships in fish toxicity studies. I. Relationship for 50
industrial pollutants. Toxicology, 19: 209-221.
KOZIK, I.V. (1957) [Some problems of occupational hygiene in
the use of dichloroethane in the aircraft industry.] Gig. Tr.
Prof. Zabol., 1: 31-38 (in Russian).
KRONEVI, T., WAHLBERG, J.E., & HOLMBERG, B. (1981) Skin
pathology following epicutaneous exposure to seven organic
solvents. Int. J. Tissue React., 3: 21-30.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A.
(1982) Collection and analysis of hazardous organic emissions.
Anal. Chem., 54: 810-817.
KULKARNI, J.H., SARDESHPANDE, J.S., & BAGYARAJ, D.J. (1975)
Effect of seed fumigation on the symbiosis of Rhizobium sp.
with Arachis hypogaea Linn. Zentralbl. Bakteriol. Parasitenkr.
Infektionskr. Hyg. Abt. II, 130: 41-44.
KUWABARA, T., QUEVEDO, A.R., & COGAN, D. (1968) An experi-
mental study of dichloroethane poisoning. Arch. Ophtalmol., 79:
321-330.
LANE, R.W., RIDDLE, B.L., & BORZELLECA, J.F. (1982) Effects of
1,2-dichloroethane and 1,1,1-trichlorethane in drinking-water on
reproduction and development in mice. Toxicol. appl. Pharmacol.,
63: 409-421.
LARIONOV, V.G. & KOKAROVTSEVA, M.G. (1976) Morphological
constitution of peripheral blood in intoxication with
dichloroethane and its metabolites. In: Actual problems of
pesticide application in different climatographic zones,
Yerevan, Aiastan Publishers, pp. 131-133.
LE BLANC, G.A. (1980) Acute toxicity of priority pollutants to
water flea Daphnia magna. Bull. environ. Contam. Toxicol., 24:
684-691.
LIN, E.L.C., MATTOX, J.K., & PEREIRA, M.A. (1985) Glutathione
plus cytosol- and microsome-mediated binding of 1,2-dichloro-
ethane to polynucleotides. Toxicol. appl. Pharmacol., 78: 428-
435.
LIVESEY, J.C. & ANDERS, M.W. (1979) In vitro metabolism of
1,2-dihaloethanes to ethylene. Drug Metab. Dispos., 7: 100-203.
LOCHHEAD, H.B. & CLOSE, H.P. (1951) Ethylene dichloride
plastic cement: a case of fata poisoning. J. Am. Med. Assoc.,
146: 1323.
LUZNIKOV, E.A., LISOVIK, G.A., & NOVIKOVSKAYA, T.V. (1985)
[Metabolism of 1,2-dichloroethane in human organisms after acute
poisonings.] [ Forensic Med. Expert.,] 2: 47-49 (in Russian
with English summary).
LYNN, G.E. & VORHES, F.A., ed. (1957) Symposium on residues in
foods and feeds resulting from fumigation of grains with the
commoner liquid formulations of carbon disulphide, carbon
tetrachloride, ethylene dichloride, and ethylene dibromide. J.
Assoc. Off. Agric. Chem., 40: 163-209.
MCCANN, J., SIMMON, V., STREITWIESER, D., & AMES, B. (1975)
Mutagenicity of chloroacetaldehyde, a possible metabolic product
of 1,2-dichloroethane (ethylene dichloride), chloroethanol
(ethylene chlorohydrin), vinyl chloride, and cyclophosphamide.
Proc. Natl Acad. Sci. (USA), 72: 3190-3193.
MCCOLLISTER, D.D., HOLLINGSWORTH, R.L., OYEN, F., & ROWE, V.K.
(1956) Comparative inhalation toxicity of fumigant mixtures.
Individual and joint effects of ethylene dichloride, carbon
tetrachloride, and ethylene dibromide. Arch. ind. Health, 13:
1-7.
MALTONI, C., VALGIMIGLI, L., & SCARNATO, C. (1980) Long-term
carcinogenic bioassays on ethylene dichloride administered by
inhalation to rats and mice. In: Ames, B., Infante, P., & Reitz,
R., ed. Ethylene dichloride: a potential health risk?, Cold
Spring Harbor, New York, Cold Spring Harbor Laboratory, pp. 5-30
(Banbury Report No. 5).
MARTIN, G., KNORPP, K., HUTH, K., HEINRICHT, F., & MITTERMAYER,
C. (1968) [Clinical features, pathogenesis and therapy of
dichloroethane poisoning.] Dtsch med. Wschr., 93: 2002-2010 (in
German).
MAY, J. (1966) [Odour thresholds of solvents for the judgement
of solvent odour in air.] Staub, 26: 385-389 (in German).
MENSCHICK, H. (1957) [Acute inhalation intoxications by
symmetric dichloroethane.] Arch. Gewerbepathol. Gewerbehyg., 15:
241-252 (in German).
MITOMA, C., STEEGER, T., JACKSON, S.E., WHEELER, K.P., ROGERS,
J.H., & MILMAN, H.A. (1985) Metabolic disposition study of
chlorinated hydrocarbons in rats and mice. Drug chem. Toxicol.,
8: 183-194.
MOODY, E.D., JAMES, J.L., CLAWSON, G.A., & SMUCKLER, E.A.
(1981) Correlations among the changes in hepatic microsomal
components after intoxication with alkyl halides and other
heptatotoxins. Mol. Pharmacol., 20: 685-693.
MOROZOV, G.N. (1958) [On acute dichloroethane poisoning.]
Farmakol. Toksicol., 21(1): 76-78 (in Russian).
MUNSON, A.E., SANDERS, V.M., DOUGLAS, K.A., SAIN, L.E.,
KAUFFMANN, B.M., & WHITE, K.L. (1982) In vivo assessment of
immunotoxicity. Environ. Health Perspect., 43: 41-52.
NACHTOMI, E., ALUMOT, E., & BONDI, A. (1966) The metabolism of
ethylene dibromide in the rat. I. Identification of detoxifi-
cation products in urine. Isr. J. Chem., 4: 329-246.
NATSYUK, M.V. & CHEKMAN, I.S. (1975) [Content of nicotinamide
coenzymes in the liver and myocardium of rats poisoned with
dichloroethane.] Byull. eksp. Biol. Med., 79: 58-60 (in
Russian).
NATSYUK, M.V. & MUDRITSKY, A.D. (1974) Clinical and
biochemical characteristics of hepatic and renal disorders in
acute dichloroethane intoxications. Mil. med. J., 10: 48-50.
NATSYUK, M.V., CHERNUKHA, F.A., BASENKO, G.A., & FEDUROV, V.V.
(1974) [Effect of acute dichloroethane intoxication on
oxidative phosphorylation in rat liver mitochondria.] Farmakol.
Toksikol., 37: 92-93 (in Russian).
NAZIKHI, F. & SKRIZHINSKY, S.F. (1973) Activity of succinate-
toxicodase system enzymes of myocardium in experimental
dichloroethane intoxication. In: Current issues of physiology,
pathology, and clinical diseases of the cardiovascular system,
Leningrad, pp. 62-63.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute,
US Department of Health, Education and Welfare, Public Health
Services, National Institute of Health (HEW (NIH) Publication
No. 78-1305).
NELSON, G.O. & SHAPIRO, E.G. (1971) A field instrument for
detecting airborne halogen compounds. Am. Ind. Hyg. Assoc. J.,
32: 757-765.
NEVROTSKY, V.K., KASHIN, L.M., KULINSKAYA, I.L., MIKHAILOVSKAYA,
L.F., SHMUTER, L.M., BURLAKA-VOVK, Z.I., & ZADOROZHNY, B.V.
(1971) Comparative toxicity evaluation of several industrial
poisons in protracted inhalation exposure to low concentrations.
In: Proceedings of the 8th Ukrainian Hygiene Congress, Kiev,
1971, pp. 224-226.
NIOSH (1976) Criteria for a recommended standard -
occupational exposure to ethylene dichloride (1,2-dichloro-
ethane), Cincinnati, Ohio, National Institute of Occupational
Safety and Health, US Department of Health, Education and
Welfare, Public Health Services, Center for Disease Control
(DHEW (NIOSH) Publication No. 76-139).
NIOSH (1977) Manual of analytical methods. II. Standards
completion program validated methods, Cincinnati, Ohio, National
Institute of Occupational Safety and Health, Vol. 2, pp. S122/1-
S122/9 (DHEW (NIOSH) Publication No. 77-157-B).
NOETZEL, O. (1944) Lethal poisoning with ethylene dichloride.
Chem. Ztg., 68: 146-147.
NOUCHI, T., MIURA, H., KANAYAMA, M., MIZUGUCHI, O., & TAKANO, T.
(1984) Fatal intoxication by 1,2-dichloroethane: a case report.
Int. Arch. occup. environ. Health, 54: 111-113.
NYLANDER, P.-O., OLOFSSON, H., RASMUSON, B., & SVAHLIN, H.
(1978) Mutagenic effects of petrol in Drosophila melanogaster:
effects of benzene and 1,2-dichloroethane. Mutat. Res., 57:
163-167.
OKAMOTO, T. & TATSUKAWA, R. (1981) [Distribution and fate of
low molecular weight chlorinated hydrocarbons in Osaka Bay.]
Chikyukagaku, 15: 17-24 (in Japanese).
OZAWA, N. & GUENGERICH, F.P. (1983) Evidence for formation of
an S-[2-(N7-guanyl)ethyl] glutathione adduct in glutathione-
mediated binding of the carcinogen 1,2-dibromoethane to DNA.
Proc. Natl Acad. Sci. (USA), 80: 5268-5270.
PAGE, B.D. & KENNEDY, B.P.C. (l975) Determination of methylene
chloride, ethylene dichloride and trichloroethylene as solvent
residues in spice oleoresins, using vacuum distillation and
electron capture. J. Assoc. Off. Anal. Chem., 58: 1062-1068.
PAPAROPOLI, G. & CALI, V. (l956) [Collective intoxications
with chlorinated hydrocarbons in dock workers.] Folia Med., 39:
819-831 (in Italian).
PARKES, D.G., GANZ, C.R., POLINSKY, A., & SCHULZE, J. (l976) A
simple gas chromatographic method for the analysis of trace
organics in ambient air. Am. Ind. Hyg. Assoc. J., 37: 165-173.
PEARSON, C.R. & MCCONNELL, G. (l975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proc. R. Soc. Lond. B,
189: 305-332.
PEROCCO, P. & PARODI, G. (1981) DNA damage by haloalkanes in
human lymphocytes cultures in vitro. Cancer Lett., 13: 213-218.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
PRINCIPE, P., DOGLIOTTI, E., BIGNAMI, M., CREBELLI, R., FALCONE,
E., FABRIZI, M., CONTI, G., & COMBA, P. (1981) Mutagenicity of
chemicals of industrial and agricultural relevance in
Salmonella, Streptomyces, and Aspergillus. J. Sci. food Agric.,
32: 826-832.
RADDING, S.B., LUI, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA report no. 560/5-77-003, PB 267121.
RANNUG, U. (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichoroethane. Mutat. Res., 76: 269-295.
RANNUG, U. & BEIJE, B. (1979) The mutagenic effect of 1,2-
dichloroethane on Salmonella typhimurium. II. Activation by the
isolated perfused rat liver. Chem.-biol. Interact., 24: 265-
285.
RANNUG, U., SUNDVALL, A., & RAMEL, C. (1978) The mutagenic
effect of 1,2-dichloroethane on Salmonella typhimurium. I.
Activation through conjunction with glutathione in vitro. Chem.-
biol. Interact., 20: 1-16.
RAO, K.S., MURRAY, J.S., DEACON, M.M., JOHN, J.A., CALHOUN,
L.L., & YOUNG, J.T. (1980) Teratogenicity and reproduction
studies in animals inhaling ethylene dichloride. In: Ames, B.,
Infante, P., & Reitz, R., ed. Ethylene dichloride: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 145-161 (Banbury Report No. 5).
RAPOPORT, I.A. (1960) [The reaction of genic proteins with
1,2-dichloroethane.] Dokl. Akad. Nauk. SSSR, 134: 1214-1217 (in
Russian).
REITZ, R.H., FOX, T.R., RAMSEY, J.C., QUAST, J.F., LANGVARDT,
P.W., & WATANABE, P.G. (1982) Pharmacokinetics and macro-
molecular interactions of ethylene dichloride in rats after
inhalation or gavage. Toxicol. appl. Pharmacol., 62: 190-204.
RICHTER, J.E., PETERSON, S.F., & KLEINER, C.F. (1983) Acute
and chronic toxicity of some chlorinated benzenes, chlorinated
ethanes and tetrachloroethylene to Daphnia magna. Arch. environ.
Contam. Toxicol., 12: 679-684.
ROHMANN, E., VON, ZINN, D., & KULZ, J. (1969) [Electro-
encephalographic observations in child blood poisonings and
their therapeutic consequences.] Kinderaerztl. Prax., 37: 209-
216 (in German).
ROSENBERG, R. (1972) Effects of chlorinated aliphatic hydro-
carbons on larval and juvenile Balanus balanoides L. Environ.
Pollut., 3: 313-318.
ROSENBERG, R., GRAHN, O., & JOHANSSON, L. (1975) Toxic effects
of aliphatic chlorinated by-products from vinyl chloride
production on marine animals. Water Res., 9: 607-612.
ROUBAL, J. (1947) Two fatal cases of intoxication with
symmetric dichloroethane ingestion. Cas. Lek. Cesk., 86: 203-
206.
SAITANOV, A.O. & ARSENIEVA, S.S. (1969) Electrocardiographic
changes due to acute dichloroethane poisoning: experimental
investigation. Gig. Tr. Prof. Zabol., 7: 49-50.
SALTZMAN, B.E. (1972) Direct reading colorimetric indicators.
In: Air sampling instruments for evaluation of atmospheric
contamination, 4th ed., Cincinnati, Ohio, American Conference of
Governmental Hygienists, pp. S22-23.
SAUER, T.C. (1981) Volatile organic compounds in open ocean
and coastal surface waters. Org. Geochem., 3: 91-101.
SCHASTEEN, C.S. & REED, D.J. (1983) The hydrolysis and
alkylation activities of S-(2-haloethyl)-L-cysteine analogues -
evidence for extended half-life. Toxicol. appl. Pharmacol., 70:
423-432.
SCHERB, K. (1978) [Investigations on the evaporation of low
molecular weight chlorinated hydrocarbons from flow-gutter.]
Munch. Beitr. Abwasser-Fisch. Flussbiol., 30: 235-248 (in
German).
SCHONBORN, H., PRELLWITZ, W., & BAUM, P. (1970) [Consumption
coagulation pathology of 1,2-dichloroethane poisoning.] Klin.
Wochenschr., 48: 822-824 (in German).
SECCHI, G.C., CHIAPPINO, G., LOTTO, A., & ZURLO, N. (1968)
[Actual chemical composition of the "commercial trieline" and
their hepatotoxic effect: clinical and enzymological studies.]
Med. Lav., 59: 486-497 (in Italian).
SEUFERT, F.B., BROWN, P., OATWAY, J.A., BORNSTEIN, M.,
OSTROWSKI, W., & HORNE, R. (1980) 1,2-Dichloroethane technical
control options analysis, Bedford, Massachusetts, CGA
Corporation (EPA Contract No. 68-01-5960).
SHAKARNIS, V.F. (1969) [1,2-Dichloroethane-induced chromosome
non-disjunction and recessive sex-linked lethal mutations in
Drosophila melanogaster.] Genetika, 5: 89-95 (in Russian).
SHAKARNIS, V.F. (1970) [Effect of 1,2-dichloroethane on
chromosome non-disjunction and recessive sex-linked lethals in a
radioresistant strain of Drosophila melanogaster.] [ Vestn.
Leningrad Univ. Biol.,] 25: 153-156 (in Russian).
SHMUTER, L.M. (1977) [Effect of chronic exposure to low
concentrations of chlorinated hydrocarbons of the ethane series
on specific and non-specific reactivity of animals in vivo.]
Gig. Tr. Prof. Zabol., 8: 38-42 (in Russian).
SIMMON, V.F. (1980) Review of non-bacterial tests of the
genotoxic activity of ethylene chloride. In: Ames, B., Infante,
P., & Reitz, R., ed. Ethylene chlorde: a potential health risk?,
Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp. 97-103 (Banbury Report No. 5).
SINGH, H.B., SALAS, L.J., & STILES, R.E. (1982) Distribution
of selected gaseous organic mutagens and suspect carcinogens in
ambient air. Environ. Sci. Technol., 16: 872-880.
SINGH, H.B., SALAS, L.J., & STILES, R.E. (1983) Selected man-
made halogenated chemicals in the air and oceanic environment.
J. geophys. Res., 88: 3675-3683.
SOPIKOV, N.G. & GORSHUNOVA, A.I. (1979) [Investigation of the
intake, distribution and excretion of ethylene dichloride in
rats.] Gig. Tr. Prof. Zabol., 4: 36-40 (in Russian).
SPENCE, J.W. & HANST, P.L. (1978) Oxidation of chlorinated
ethanes. J. Air Pollut. Control Assoc., 28: 250-253.
SPENCER, H.C., ROWE, V.K., ADAMS, E.M., MCCOLLISTER, D.D., &
IRISH, D.D. (1951) Vapor toxicity of ethylene dichloride
determined by experiments on laboratory animals. Arch. ind.
Hyg. occup. Med., 4: 482-493.
SPREAFICO, F., ZUCCATO, E., MARCUCCI, F., SIRONI, M.,
PAGLIALUNGA, S., MADONNA, M., & MUSSINI, E. (1980) Pharmaco-
kinetics of ethylene dichloride in rats treated by different
routes and its long-term inhalatory toxicity. In: Ames, B.,
Infante, P., & Reitz, R., ed. Ethylene chlorde: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 107-129 (Banbury Report No. 5).
STORER, R.D. & CONOLLY, R.B. (1983) Comparative in vivo
genotoxicity and hepatotoxicity of three 1,2-dihaloethanes.
Carcinogenesis, 4: 1491-1494.
STORER, R.D. & CONOLLY, R.B. (1985) An investigation of the
role of microsomal oxidative metabolism in the in vivo
genotoxicity of 1,2-dichloroethane. Toxicol. appl. Pharmacol.,
77: 36-46.
STORER, R.D., JACKSON, N.M., & CONOLLY, R.B. (1984) In vivo
genotoxicity and acute hepatotoxicity of 1,2-dichloroethane in
mice: comparison of oral, intraperitoneal, and inhalation routes
of exposure. Cancer Res., 44: 4267-4271.
STOREY, C.L., KIRK, L.D., & MUSTAKAS, G.C. (1972) Fate of EDC-
CC14 (75:25) residues during milling and oil extraction of
soybeans. J. econ. Entomol., 65: 1126-1129.
STOVER, E.L. & KINCANNON, D.F. (1983) Biological treatability
of specific organic compounds found in chemical industry
wastewaters. J. Water Pollut. Control Fed., 55: 97-105.
STUCKEY, D.C., OWEN, W.F., MCCARTY, P.L., & PARKIN, G.F. (1980)
Anaerobic toxicity evaluation by batch and semi-continuous
assays. J. Water Pollut. Control Fed., 52: 720-729.
STUCKI, G., BRUNNER, W., STAUB, D., & LEISINGER, T. (1981)
Microbial degradation of chlorinated C1 and C2 hydrocarbons.
In: Leisinger, T., Cook, A.M., Hütter, R., & Nüesch, J., ed.
FEMS Symposium No. 12, Microbial degradation of xenobiotics and
recalcitrant compounds, London, Academic Press, pp. 131-137.
SUNDHEIMER, D.W., WHITE, R.D., BRENDEL, K., & SIPES, I.C.
(1982) The bioactivation of 1,2-dibromoethane in rat
hepatocytes: covalent binding to nucleic acids. Carcinogenesis,
3: 1129-1133.
SUVEEV, I.M. & BABICHENKO, M.E. (1969) [On the clinic and cure
of acute intoxication with dichloroethane vapours.] Gig. Tr.
Prof. Zabol., 13: 50-51 (in Russian).
SYMONS, J.M., BELLAR, T.A., CARSWELL, J.K., DEMARCO, J., KROPP,
K.L., ROBECK, G.G., SEEGER, D.R., SLOCUM, C.J., SMITH, B.L., &
STEVENS, A.A. (1975) National organics reconnaissance survey
for halogenated organics. J. Am. Water Works Assoc., 67: 634-
648.
TABAK, H.H., QUAVE, S.A., MASHNI, C.E., & BARTH, E.F. (1981)
Biodegradability studies with organic priority pollutant
compounds. J. Water Pollut. Control Fed., 53: 1503-1517.
TAN, E.-L. & HSIE, A.W. (1981) Mutagenicity and cytotoxicity
of haloethanes as studied in the CHO-HGPRT system. Mutat. Res.,
90: 183-191.
THORUP, S. (1957) Breaking dormancy of beech. Physiol. Plant,
10: 728-731.
TROISI, F.M. & CAVALLAZZI, D. (1961) [A fatal case of
poisoning from inhalation of dichloroethane vapours.] Med.
Lav., 52: 612-618 (in Italian).
TSANI-BAZACA, E., MCINTYRE, A.E., LESTER, J.N., & PERRY, R.
(1981) Concentrations and correlations of 1,2-dibromoethane,
1,2-dichloroethane, benzene, and toluene in vehicle exhaust and
ambient air. Sci. Technol. Lett., 2: 303-316.
TSANI-BAZACA, E., MCINTYRE, A.E., LESTER, J.N., PERRY, R.
(1982) Ambient concentrations and correlations of hydrocarbons
and halocarbons in the vicinity of an airport. Chemosphere, 11:
11-23.
TSURUTA, H. (1975) Percutaneous absorption of organic solvents
I. Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind. Health, 13: 227-236.
TU, A.S., MURRAY, T.A., HATCH, K.M., SIVAK, A., & MILMAN, H.A.
(1985) In vitro transformation of BALB/c-3T3 cells by
chlorinated ethanes and ethylenes. Cancer Lett., 28: 85-92.
VAN BLADEREN, P.J., BREIMER, D.D., ROTTEVEEL-SMIJS, G.M.T.,
KNIJFF, P. DE, MOHN, G.R., MEETEREN-WALCHLI, B. VAN, BUIJS, W.,
& VAN DER GEN, A. (1981a) The relation between the structure
of vicinal dihalogen compounds and their mutagenic activation
via conjugation to glutathione. Carcinogenesis, 2: 499-505.
VAN BLADEREN, P.J., HODGETERP, J.J., BREIMER, D.O., & VAN DER
GEN, A. (1981b) The influence of disulfiram and other
inhibitors of oxidative metabolism on the formation of 2-
hydroxyethyl-mercapturic acid from 1,2-dibromoethane by the rat.
Biochem. Pharmacol., 30: 2983-2987.
VAN DUUREN, B.L., GOLDSCHMIDT, B.M., LOEWENGART, G., SMITH,
A.C., MELCHLONNE, S., LEIDMAN, I., & ROTH, D. (1979)
Carcinogenicity of halogenated olefinic and aliphatic
hydrocarbons in mice. J. Natl Cancer Inst., 63: 1433-1439.
VAN ESCH, G.J., KROES, R., LOGTEN, M.J., VAN, & TONKELAAR, E.M.,
DEN (1977) Ninety-day toxicity study with 1,2-dichloroethane
(DCE) in rats, Bilthoven, the Netherlands, National Institute of
Public Health and Environmental Hygiene (Report 195/77 Al.
Tox.).
VARGA, M.B. & FERENCZY, L. (1956) Effect of "Rindite" on the
development of the growth-substances in potato tubers. Nature
(Lond.), 175: 1075.
VOSOVAYA, M.A. (1974) [Development of posterity of two
generations obtained from females subjected to the action of
dichloroethane.] Gig. i Sanit., 7: 25-28 (in Russian).
VOSOVOYA, M.A. (1977) [Effect of dichloroethane on the
reproductive cycle and embryogenesis in experimental animals.]
Akush. Ginekol., 2: 57-59 (in Russian).
WALBRIDGE, C.T., FIANDT, J.T., PHIPPS, G.L., & HOLCOMBE, G.W.
(1983) Acute toxicity of ten chlorinated aliphatic hydrocarbons
to the fathead minnow (Pimephales promelas). Arch. environ.
Contam. Toxicol., 12: 661-666.
WARD, J.M. (1980) The carcinogenicity of ethylene dichloride
in Osborne-Mendel rats and B6C3F1 mice. In: Ames, B., Infante,
P., & Reitz, R., ed. Ethylene dichloride: a potential health
risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 35-53 (Banbury Report No. 5).
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. eng. News, May 7: 8-10.
WEISS, F. (1957) [Lethal oral intoxications by
dichloroethane.] Gewerbehygiene, 15: 253-264 (in German).
WHO (1972) Evaluations of some pesticide residues in food,
Geneva, World Health Organization, pp. 276-279, 325-329 (The
Monographs: WHO Pesticide Residues Series, No. 1).
WIRTSCHAFTER, Z.T. & SCHWARTZ, W.D. (1939) Acute ethylene
dichloride poisoning. J. ind. Hyg. Toxicol., 21: 126-131.
WIT, S.L., BESEMER, F.H., DAS, H.A., GOEDKOOP, W., LOOSJES,
F.E., & MEPPELIN, E.K. (1969) Results of an investigation on
the regression of three fumigants (carbon tetrachloride,
ethylene dibromide and ethylene dichloride) in wheat during
processing to bread, Bilthoven, The Netherlands, National
Institute of Public Health and Environmental Hygiene (Report No.
36/69 Tox).
WITHEY, J.R. & COLLINS, B.T. (1980) Chlorinated aliphatic
hydrocarbons used in the foods industry: the comparative
pharmacokinetics of methylene chloride, 1,2-dichloroethane,
chloroform, and trichloroethylene after iv administration in the
rat. J. environ. Pathol. Toxicol., 3: 313-332.
WITHEY, J.R. & KARPINSKI, K. (1985) The fetal distribution of
some aliphatic chlorinated hydrocarbons in the rat after vapor
phase exposure. Biol. Res. Preg., 6(2): 79-88.
WOLFF, D.L., IVANOV, N.G., KLJACKINA, A.M., & MEL'NIKOVA, L.V.
(1979) [Evidence of the effect of significant industrial
toxicological substances on the nervous system by animal
behavioural tests.] Zool. Jahrb. Physiol., 83: 82-91 (in
German).
YLLNER, S. (1971) Metabolism of 1,2-dichloroethane-4C in the
mouse. Acta pharmacol. toxicol., 30: 257-265.
YODAIKEN, R.E. & BABCOCK, J.R. (1973) 1,2-Dichloroethane
poisoning. Arch. environ. Health, 26: 281-284.
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly-volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
ZOETEMAN, B.C.J., PIET, G.J., SLINGERLAND, P., FONDS, A.W., &
WEGMAN, R.C.C. (1979) [ Investigation into the presence of
volatile organic halogenated compounds in groundwater used as a
drinking water supply. Final report: results on the period
November 1976 to August 1978,] Bilthoven, The Netherlands,
National Institute of Drinking Water Supply, National Institute
of Public Health and Environmental Hygiene (Report no. RID-CBH-
79/01 and no. RIV 10/79) (in Dutch).
ZUCCATO, E., MARCUCCI, F., & MUSSINI, E. (1980) GLC
determination of ethylene dichloride (EDC) in biological
samples. Anal. Lett., 13(B5): 371-379.
5.4 Excretion and Elimination
Excretion of 1,2-dichloroethane from rodents is rapid.
Approximately 89% or more of the body burden of the compound was
excreted within 24 h in ip-injected mice (Yllner, 1971), within
48 h in orally exposed mice (Mitoma et al., 1985), and within
48 h in rats exposed orally or via inhalation (Reitz et al.,
1982; Mitoma et al., 1985). In the 3 studies cited above,
excretion of 1,2-dichloroethane or its metabolites mainly
occurred in exhaled air via the lungs and in urine via the
kidneys. In both species, and at various exposure levels, 7 -
18% of the metabolized 1,2-dichloroethane was excreted as carbon
dioxide (CO2) and approximately 80 - 85% as non-volatile
metabolites (Table 6). The metabolism of 1,2-dichloroethane in
mice and rats is dose-dependent. For example, in mice, 11 and
45% of the body burdens of 1,2-dichloroethane, resulting from ip
exposure to 50 and 170 mg/kg body weight, respectively, were
excreted unchanged via the lungs within 72 h (Yllner, 1971). In
rats, 1.8, 11.5, and 29% of the body burdens were excreted
unchanged via the lungs within 48 h following: an inhalation
exposure at 607 mg/m3 for 6 h (equivalent to a dose of 113 mg/kg
body weight) (Reitz et al., 1982), an oral dose of 100 mg/kg
body weight (Mitoma et al., 1985), and an oral dose of 150 mg/kg
body weight (Reitz et al., 1982), respectively.
The rate of elimination from blood and tissues appears to
depend on the exposure level; the higher the exposure level, the
slower the elimination rate of 1,2-dichloroethane, after both
oral and inhalation exposure. Half-lives in the blood of rats,
exposed orally, increased from 25 min at 25 mg/kg body weight to
57 min at 150 mg/kg body weight. With inhalation exposure,
half-lives increased from 13 min at 202 mg/m3 to 22 min at 1012
mg/m3, after a 6-h inhalation exposure (Spreafico et al., 1980).
In addition, after oral exposure of rats to 150 mg/kg body
weight, an initial half-life of 90 min in blood decreased to
20 - 30 min, when blood levels fell below 5 - 10 mg/litre after
3 h (Reitz et al., 1982). Elimination of 1,2-dichloroethane
from blood, adipose tissue, lung, liver, brain, kidneys, and
spleen was comparable after oral exposures of up to 150 mg/kg
body weight. Elimination from the liver was reported to be
biphasic with a higher elimination rate just after reaching peak
levels of 1,2-dichloroethane. Elimination from other organs was
monophasic. Following inhalation, elimination was the slowest
in adipose tissue and the most rapid in the lung, up to an
exposure level of 1012 mg/m3 (Spreafico et al., 1980). Withey &
Collins (1980) also reported that the elimination of 1,2-
dichloroethane was dose-dependent. After iv administration of
from 3 to 15 mg/kg body weight to male Wistar rats, the authors
found that the elimination fitted a two-compartment model at a
low-dose level and a three-compartment model at high-dose
levels.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Aquatic Organisms
6.1.1 Acute toxicity
The acute toxicity of 1,2-dichloroethane for aquatic
organisms is summarized in Table 7. The concentration of 1,2-
dichloroethane was measured in four of the studies cited (see
footnotea in Table 7); the concentrations reported in the
other studies were nominal. It should be noted that, in open
systems, the toxic effects observed must have occurred at
concentrations lower than the nominal ones reported in latter
studies, due to the anticipated evaporation of 1,2-dichloro-
ethane in the aquatic media.
The species most sensitive to 1,2-dichloroethane were
members of the class Crustacea. A no-observed-adverse-effect
level below 68 mg/litre was found for Daphnia magna (Le Blanc,
1980). The shrimp Crangon crangon showed a 96-h LC50 of
85 mg/litre in sea water, measured by the flow-through method
(Adema, 1976). When the brine shrimp Artemia salina was exposed
to 1,2-dichloroethane at levels ranging from 0.25 to
25 mg/litre, growth inhibition was noted 24 h after cyst wetting
(Kerster & Schaeffer, 1983).
EDC tar is much more toxic for marine species than 1,2-
dichloroethane, the heavy fractions of the tar being responsible
for the high toxicity observed (Jernelöv et al., 1972; Rosen-
berg, 1972; Jensen et al., 1975; Rosenberg et al., 1975).
6.1.2 Short-term exposures
When blue algae Mycrocystis aerigunosa and green algae
Scenedesmus quadricauda were exposed in closed containers to
1,2-dichloroethane at 105 and 710 mg/litre, respectively, for 8
days, cell multiplication started to be inhibited (Bringmann &
Kühn, 1978). Guppies (Poecilia reticulata) were exposed for 7
days in a static test, and an LC50 of 106 mg/litre was found.
Solutions were renewed daily, but no water analysis data were
reported (Könemann, 1981). Finally, an early life stage flow-
through test was done. Fathead minnows (Pimephales promelas)
were exposed to concentrations of between 4 and 56 mg/litre
beginning from 2 to 5 days after spawning and continuing
throughout the subsequent embryonal, larval, and juvenile stages
up to 28 days after hatching. Water was analysed for 1,2-
dichloroethane. Body weight was reduced at 59 mg/litre. The
survival of juveniles, the percentage of normal larvae at hatch,
and the hatchability of embryos were not affected (Benoit et
al., 1982).
Table 7. Acute aquatic toxicity
---------------------------------------------------------------------------------------------------------
Organism Description t (°C) pH Dissolved Hardness Flow/b Parameter Concen- Reference
oxygen (mg CaCO3/ stat tration
(mg/litre) litre) open/ (mg/litre)
closed
---------------------------------------------------------------------------------------------------------
Fresh water
-----------
Bacteria Pseudomonas 25 7 stat 16-h MICc 135 Bringmann
putida closed & Kühn
(1980)
Protozoa Entosipon sulc- 25 7 stat 72-h MIC 943-1127 Bringmann
atum, Uronema closed & Kühn
parduczi, Chilo- (1981)
monas paramecium
Crustacea water flea 22 6.7-8.1 6.5-9.1 72 stat 24-h LC50 250 Le Blanc
(Daphnia magna) open no-observed- < 68 (1980)
adverse-
effect level
Crustacea water flea 20 8.0 > 2 - stat 24-h EC50 540a,d Bringmann
(Daphnia magna) open & Kühn
(1982)
Crustacea water flea 20 7.1-7.7 7.9-9.9 44.7 stat 48-h LC50 270a,d Richter
(Daphnia magna) closed 48-h EC50 160a,d et al.
20 7.0-7.5 4.1-8.4 44.7 stat 48-h LC50 320a,e (1983)
closed 48-h EC50 180a,e
Fish bluegill sunfish 21-23 6.5-7.9 32-48 stat 96-h LC50 430 Buccafusco
(Lepomis macro- closed et al.
chirus) (1981)
Fish bluegill sunfish 23 7.6-7.9 55 stat 96-h LC50 550 Dawson
(Lepomis macro- open et al.
chirus) (1977)
Fish fathead minnow 25 6.7-7.6 8.0 45.1 flow 96-h LC50 116a Walbridge
(Pimephales prom- open et al.
elas) (1983)
Table 7 (contd.)
---------------------------------------------------------------------------------------------------------
Sea water
---------
Alga Phaeodactylum stat EC50 340a Pearson &
tricornutum McConnell
(1975)f
Worm chaetopod 23 stat 96-h LC50 400 Rosenberg
(Ophryotrocha closed et al.
labronica) (1975)g
Crustacea shrimp 15 8.0 > 8.0 flow 96-h LC50 85a Adema
(Crangon crangon) open (1976)
Crustacea shrimp 16 stat 24-h LC50 170 Rosenberg
(Crangon crangon) open et al.
(1975)h
Mollusca barnacle nauplii stat 48-h LC50 186a Pearson &
(Elminius modestus) closed McConnell
(1975)
Fish dab flow 96-h LC50 115a Pearson &
(Limanda limanda) open McConnell
(1975)
Fish tidewater silver- 20 7.6-7.9 55 stat 96-h LC50 480 Dawson
sides (Menidia open et al.
beryllina) (1977)
Fish sheepshead minnow 25-31 stat 96-h LC50 130-230 Heitmüller
(Cyprinodon open no-observed- 130 et al.
variegatus) adverse- (1981)
effect level
Fish goby 15 8.0 > 8.0 flow 96-h LC50 185a Adema
(Gobius minutus) open (1976)
---------------------------------------------------------------------------------------------------------
a Water analysis for 1,2-dichloroethane was reported.
b Flow-through or static method; open or closed system.
c MIC = minimum inhibitory concentration for cell multipication. EC50 and LC50 = concentration,
causing an effect and death, respectively, in 50% of the population.
d Fleas unfed. Effect was complete immobilization.
e Fleas fed. Effect was complete immobilization.
f Effect was growth inhibition, measured by 4C uptake during photosynthesis.
g When the concentration was gradually built up during the first hour, the 96-h LC50 was
900 mg/litre, and the percentage of hatched eggs, laid within 15 days, was decreased by 90% at
400 mg/litre. The latter was not noted at 200 mg/litre.
h Concentration was gradually built up during the first hour.
6.1.3 Long-term exposure
No-observed-adverse-effect and effect concentrations were
determined on the basis of reproduction or length for Daphnia
magna in a 28-day test, in stoppered flasks. The test solution
was analysed by gas chromatography. The lowest observed adverse
effect concentrations were 21 mg/litre, on the basis of repro-
duction, and 72 mg/litre, on the basis of length. The no-
observed-adverse-effect concentrations were 11 mg/litre, on the
basis of reproduction and 42 mg/litre, on the basis of length
(Richter et al., 1983).
6.1.4 Bioconcentration
Bioconcentration of 1,2-dichloroethane in aquatic species is
unlikely in view of its physical and chemical properties. In a
tracer study, a bioconcentration factor of 2 was found for
bluegill sunfish (Lepomis macrochirus) in flowing water. The
half-life for the elimination of 1,2-dichloroethane from tissues
was 1 - 2 days (Barrows et al., 1980).
When tissues of several aquatic species, collected from near
the discharge zone of a wastewater treatment plant, were ana-
lysed for 1,2-dichloroethane, the concentration of the compound
was less than 0.5 µg/kg wet weight in all cases, while the
average effluent concentration was 41 µg/litre and the average
sediment concentration was less than 0.5 µg/kg dry weight
(Gossett et al., 1983).
6.2 Microorganisms
1,2-Dichloroethane at an influent concentration of
258 mg/litre did not affect the treatment efficiency of a bench-
scale activated sludge system. The compound itself was
virtually completely removed by stripping but not by biodegra-
dation (Stover & Kincannon, 1983). In a batch anaerobic
toxicity assay, 1,2-dichloroethane was slightly toxic to the
anaerobic digestion process from a concentration of
2.5 mg/litre, though acclimation was observed after several
days. A concentration of 20 mg/litre caused more severe
retardation, while acclimation was slow. In semi-continuous
assays, stress became evident at 1,2-dichloroethane concentra-
tions of between 5 and 7.5 mg/litre (Stuckey et al., 1980).
6.3 Terrestrial Organisms
6.3.1 Birds
The effects on reproduction were investigated in groups of
10 male and 20 female white leghorn chickens after 2 years of
oral exposure to 0, 250, or 500 mg 1,2-dichloroethane/kg feed
mash. From the fourth month of laying onwards, decreased egg
weight was observed at both dose levels, while at the higher
dose level, the number of eggs and the feed intake were also
reduced. 1,2-Dichloroethane did not affect serum composition
and growth, semen characteristics, or fertility of chickens
(Alumot et al., 1976b).
6.3.2 Plants
1,2-Dichloroethane is used as a seed fumigant, usually in
combination with compounds such as carbon tetrachloride, 1,2-
dibromomethane, or 2-chloroethanol. Such fumigants inhibited
the germination of seeds (Caswell & Clifford, 1958; Kamel,
1959), broke the dormancy period of potato tubers (Varga &
Ferenczy, 1956; Jolivet, 1968) and beech (Thorup, 1957), and
adversely affected the nodulation status and yield of groundnuts
treated with Rhizobium (Kulkarni et al., 1975). 1,2-Dichloro-
ethane vapour was both lethal and mutagenic for barley seeds at
3 mg/m3 during 24 h (Ehrenberg et al., 1974).
7. EFFECTS ON ANIMALS
7.1 Single Exposures
7.1.1 Inhalation and oral exposure
The available acute mortality data following inhalation and
oral exposure are summarized in Table 8.
Table 8. Acute mortality after inhalation or oral exposure to
1,2-dichloroethane
-----------------------------------------------------------------------------
Species/ Route Vehicle Parameter Result Reference
strain studied
-----------------------------------------------------------------------------
dog oral acacia LD50 2500 mg/kg Barsoum & Saad
gum (1934)
rat oral corn oil LD50 680 mg/kg McCollister et
al. (1956)
CD-1 oral water LD50 489 mg/kg Munson et al.
mouse (1982)
(male)
CD-1 oral water LD50 413 mg/kg Munson et al.
mouse (1982)
(female)
Wistar inhalation 6-h LC50 5100 mg/m3 Spencer et al.
rat (1951)
Sprague inhalation - 6-h LC50 6660 mg/m3 Bonnet et al.
Dawley (1980)
rat
OF1 inhalation 6-h LC50 1060 mg/m3 Gradiski et al.
mouse (1978)
(female)
rat oral LD50 850 mg/kg Larionov &
Kokarovtseva
(1976)
albino inhalation LC50(expo- 30 000 mg/m3 Nevrotsky et al.
rat sure period (1971)
unknown)
-----------------------------------------------------------------------------
Deaths occur within a narrow range of concentrations. In
rats, the difference between the 6-h LC10 and 6-h LC90 was
approximately 2800 mg/m3 (Bonnet et al., 1980). No deaths were
observed in rats after 6 h of exposure to 2000 mg/m3 (Spencer et
al., 1951). In mice, an extremely narrow range of about
500 mg/m3 was observed between the 6-h LC10 and the 6-h LC90
(Gradiski et al., 1978).
Exposure of rats to single high doses of 1,2-dichloroethane
resulted in adverse effects on the CNS, liver, kidneys,
adrenals, and lungs (Spencer et al., 1951). Groups of 10 - 52
Wistar rats were exposed to 81 000, 48 600, 12 100, 6100, 4000,
3200, 2400, or 1200 mg 1,2-dichloroethane/m3 for various lengths
of time. At the highest concentration (81 000 mg/m3), deaths
were observed in animals exposed for 0.3 h or longer. Deaths
were observed at all except the lowest of the other concentra-
tions with exposure periods of 0.4, 0.7, 3.0, 4.0, 7.0, and
7.0 h, respectively. No deaths were observed in rats exposed to
the lowest concentration for 7 h.
Severe depression of the central nervous system resulting in
coma was observed in rats exposed to the highest concentration.
At lower concentrations, this depressant action expressed itself
as various levels of "drunkeness". In this investigation,
Spencer et al. (1951) did not specify the sex of the rats.
In the same publication, the authors reported another study
in which groups of 4 - 6 female rats were exposed to 48 600,
12 100, 4000, 1200, or 800 mg/m3. "Adverse effects" (not
described by the authors) were observed at the 4 highest levels
in rats exposed for 0.2, 0.5, 3.0, and 5.5 h, respectively. No
adverse effects were observed in female rats exposed to the
lowest concentration, for 7 h.
Examination of internal organs in groups of rats killed,
either when moribund or 24 h after the last exposure, showed
that, after exposure to 1,2-dichloroethane levels of 2400 mg/m3
or more, the most severe damage occurred in the kidney and
consisted of haemorrhage and tubular necrosis. The liver showed
fatty changes and hepatocellular necrosis with haemorrhage, and
adrenal glands were haemorrhagic. Lung oedema was observed at
concentrations above 12 100 mg/m3. The injury to organs in this
study was accompanied by high blood-urea levels, a decrease in
serum-phosphatase activity, and increased lipid concentration in
the liver (Spencer et al., 1951).
Depression of the central nervous system was noted during
exposure of rats to 1,2-dichloroethane concentrations exceeding
1200 mg/m3 (Bonnet et al., 1980). From an exposure level of
4000 mg/m3, for 4 h, rats showed altered behaviour. A narcotic
effect was observed at 9100 mg/m3 (Wolff et al., 1979).
Albino rats, given a single oral dose of 615 mg/kg body
weight, showed congested livers with cloudy swelling and fatty
degeneration. The myocardium showed oedema and haemorrhaging in
the walls of the coronary vessels, stasis, and thrombi in the
vessels. These changes were associated with an increased
activity of alanine- and aspartate aminotransferase in the serum
and decreased tissue levels of nicotinamide coenzymes (Natsyuk &
Chekman, 1975). A single administration of 861 mg/kg, by
gavage, was reported to partially uncouple oxidative phosphory-
lation measured in vitro in albino rat livers, 1, 3, or 6 days
after exposure (Natsyuk et al., 1974). In rat liver microsomes,
cytochrome P-450 levels were slightly decreased after an oral
dose of 625 mg/kg (Moody et al., 1981). After a single oral
dose of 1,2-dichloroethane in corn oil at 770 mg/kg body weight
in rats, dystrophy in the cytoplasm and hyperchromatosis in the
nuclei of hepatocytes were observed. A decrease in protein
synthesis and lysosomal enzyme activity was also reported. The
same changes took place in renal nephrons (Boikova & Kravtsova,
1982). A single dose of 850 mg/kg in albino rats resulted in a
decrease in RBC count, haematocrit, and other haematological
changes (Larionov & Kokarovtseva, 1976). Oral administration of
1,2-dichloroethane (615 mg/kg) to rabbits induced pronounced
morphological changes in the liver in about 24 h (Nazikhi &
Skrizhinsky, 1973). Effects on fibrinolytic activity in the
blood of rabbits administered 1,2-dichloroethane at 1476 mg/kg
have been reported by Kagramanov & Kazieva (1972). Electro-
cardiographic changes in albino rats associated with doses of 1,
1.5, and 2 mg/kg have been reported (Saitanov & Arsenieva,
1969).
7.1.2 Skin and eye irritation
When undiluted 1,2-dichloroethane was applied directly on
the clipped skin of guinea-pigs for up to 12 h in occluded patch
tests, no gross skin reactions were visible (Jakobson et al.,
1982). Microscopic changes appeared 4 h after application,
comprising karyopyknosis, perinuclear oedema, spongiosis, and
junctional separation (Kronevi et al., 1981). In similar tests
on rabbits, moderate erythema and oedema were observed, 24 h
after application. Microscopy on the third day revealed
necrosis and other lesions such as ulcerations and acanthosis.
The severity of the changes was not indicated (Duprat et al.,
1976).
Instillation of 0.1 ml of undiluted 1,2-dichloroethane into
the conjunctival sac of the eye of rabbits generated reversible,
mild irritation characterized by conjunctivitis and epithelial
abrasion. Epithelial keratitis, described as being "in a state
of repair", was observed microscopically, 7 days after
application (Duprat et al., 1976). Reversible clouding of the
cornea was observed in dogs within 10 h of subcutaneous
administration of undiluted 1,2-dichloroethane at 0.9/kg body
weight. The clouding continued up to 48 h, but the corneas
appeared clear after 5 days. The histological changes,
including necrosis of the corneal endothelium, partially denuded
Descemet's membrane, formation of excess basement membrane, and
swelling of the corneal stroma, were also observed in dogs,
cats, and rabbits after ocular injection of 1.8 mg 1,2-
dichloroethane (0.15 ml of a 1% solution) into the anterior
chamber (Kuwabara et al., 1968).
7.2 Short-Term Exposures
7.2.1 Inhalation exposure
The effects of repeated exposure to 1,2-dichloroethane have
been studied in mice, rats, guinea-pigs, rabbits, cats, dogs,
and monkeys (Heppel et al., 1946; Spencer et al., 1951; Hofmann
et al., 1971).
Heppel et al. (1946) exposed rats of the Wistar and Osborne-
Mendel strains to 1,2-dichloroethane concentrations of 420, 730,
1540, or 3900 mg/m3 in air, 7 h daily, 5 days/week, for several
weeks. The duration varied with each exposure level. No loss
of weight and no deaths occurred in rats of either strain
exposed to 420 mg/m3 for up to 4 months (74 exposures). Seven
out of 12 Wistar rats exposed to 730 mg/m3 died within 15 weeks
(after 1 - 73 exposures) and 8/12 similarly treated rats of the
Osborne-Mendel strain died after 1 - 6 exposures. Nine out of
16 rats (strain unspecified) died within 12 weeks after 2 - 60
exposures to 1540 mg/m3; 20/26 (strain unspecified) died within
3 weeks after 3 - 15 exposures to 3900 mg/m3. Guinea-pigs were
exposed to the same concentrations of 1,2-dichloroethane as the
rats. Several deaths, which occurred in the lowest exposure
group (420 mg/m3) and in controls, were attributed to an
intercurrent disease. At higher dose levels, mortality was
related to 1,2-dichloroethane exposure. Five out of 14 guinea-
pigs died after 5 - 115 exposures to 730 mg/m3 within 23 weeks.
Fourteen out of 20 guinea-pigs died after 8 - 65 exposures to
1,2-dichloroethane at 1540 mg/m3 within 13 weeks. All guinea-
pigs exposed to 3900 mg/m3 had died by the 4th day of the
study.
Rabbits were exposed to the three higher concentrations of
1,2-dichloroethane only. No deaths were observed when rabbits
were exposed to 730 mg/m3 for 25 weeks. All 5 rabbits exposed
to 1540 mg/m3 died, one after one exposure and the rest after
89 - 97 exposures within 19 weeks. Five out of 6 rabbits
exposed to 3900 mg/m3 died after 2 - 43 exposures within 9
weeks.
A group of 19 mice survived for 4 weeks when exposed to 420
mg/m3, but 18/20 mice died within 10 days by the end of 7
exposures to 730 mg/m3. In other species, a group of 6 female
dogs survived up to 35 weeks of exposure to 1540 mg/m3. Two out
of 6 dogs exposed to 3900 mg/m3 died after 30 and 43 exposures,
respectively. Two out of six cats exposed to 3900 mg/m3 died
after 43 exposures. Cats were not exposed to lower
concentrations. Two monkeys died after 2 and 32 exposures,
respectively, to 3900 mg/m3, but 2 others exposed to 730 mg/m3
survived for 25 weeks.
Kidney and liver damage, consisting of fatty changes and
necrosis in both organs, was found in animals that died from
exposure to the highest dose level (3900 mg/m3). In addition,
the rats showed pulmonary congestion and haemorrhage; one monkey
that died after 32 exposures and one dog showed a focal
myocarditis. Approximately half of the animals that died after
exposure to 1540 mg/m3 showed similar histological changes in
the liver and kidneys, but no such changes were observed in rats
that died from exposure to lower levels. Hepatic fatty changes
were observed in guinea-pigs exposed to 730 mg/m3. Histological
examinations were not carried out on mice.
Spencer et al. (1951) exposed rats, guinea-pigs, monkeys,
and rabbits to 1,2-dichloroethane at concentrations of 1620,
810, or 405 mg/m3, 7 h per day, 5 days/week, for various lengths
of time. In a group of 15 male and 15 female rats, exposed to
the highest level, no animals survived for more than 8 weeks,
and 60% mortality occurred in a second group exposed to the same
regime after 2 or 3 exposures. Mortality was also high in a
group of 8 male and 8 female guinea-pigs exposed to 1620 mg/m3.
All males had died by the second week and all females by
approximately the 5th week. Two male monkeys experienced rapid
and severe intoxication. Both were killed when moribund after
10 - 12 exposures. On the other hand, 2 male and 1 female
rabbits tolerated 33 weeks exposure with no evidence of adverse
effects. No mortality was observed when groups of 15 male and
15 female rats and 8 male and 8 female guinea-pigs were exposed
to 810 mg/m3 for about 30 weeks (151 exposures) or 36 weeks
(180 exposures), respectively. Similarly, no clinical effects
were observed in groups of 15 male and female rats, 8 male or
female guinea-pigs, 2 male and 1 female rabbits, and 1 male and
1 female monkey exposed for approximately 30 - 36 weeks to 405
mg/m3.
Histological examination was carried out on animals exposed
to the three dose levels. In both guinea-pigs and rats exposed
to the highest dose level, liver changes consisting of cloudy
swelling and fatty changes were observed. None of the other
organs were affected. Similar but less marked changes were
observed in the monkeys, while no adverse changes were found in
the rabbits. In rats exposed to 810 mg/m3, there were no
adverse changes in the liver or other organs, but reduced growth
and some fatty changes were found in the liver of guinea-pigs.
No adverse changes were found in animals exposed to the lowest
dose level (405 mg/m3).
Hofmann et al. (1971) exposed cats, rabbits, guinea-pigs,
and rats to 1,2-dichloroethane at 1980 mg/m3 or 405 mg/m3 for
6 h/day, 5 days/week, for up to 17 weeks. At the higher
concentration, rats became dyspnoeic and guinea-pigs apathetic.
Three out of 4 rabbits died after 10 - 17 exposures, and 9/10
guinea-pigs died after 4 - 14 exposures. Rats were more
sensitive, dying after only 1 - 5 exposures. All cats survived
30 exposures. Histologically, rats showed pulmonary hyperaemia
and oedema, fatty liver, and adrenal and myocardial necrosis.
Cats and rabbits exhibited a heart lesion and guinea-pigs, fatty
changes in the myocardium, liver, and adrenals and necrosis in
the myocardium and liver. At the lower concentration (405
mg/m3), rats, guinea-pigs, rabbits, and cats exposed for 17
weeks did not show any clinical or histological changes.
Of the species studied, mice and rats appear to be more
sensitive than other species to the adverse effects of 1,2-
dichloroethane. The no-observed-adverse-effect level for short-
term exposures (4 - 9 months) in rats studied in the 3
investigations is about 400 mg/m3.
Signs of central nervous system depression observed in the
above studies were apathy in guinea-pigs at 1980 mg/m3 (Hofmann
et al., 1971) and 3900 mg/m3 (Heppel et al., 1946), and coma in
dogs and monkeys at 3900 mg/m3 (Heppel et al., 1946). When rats
were exposed continuously for 3.5 months to 5 mg/m3, changes in
EEG were observed (Dmitrieva & Kuleshova, 1971). However, the
significance of these findings is not known.
7.2.2 Oral exposure
The liver appeared to be the principal target organ
following oral exposure. Rats, treated by gavage with 1,2-
dichloroethane in corn oil for 2 weeks, 5 times per week, at
doses of 150 mg/kg body weight or less did not show any
treatment-related abnormalities in organ or body weights,
histology, clinical chemistry, or haematology (Van Esch et al.,
1977; Reitz et al., 1982).
Rats were also exposed for 90 days, 5 times per week, to 0,
10, 30, or 90 mg/kg body weight (Van Esch et al., 1977). At the
2 highest exposure levels, a tendency to decreased weight gain
was observed. At 90 mg/kg, rats of both sexes showed an
increase in the relative weight of kidneys, but only the females
on this dose showed increased relative weights of liver and
brain compared with controls. Histology and clinical chemistry
were normal. Some haematological parameters were altered, but
not in a dose-related manner. In another study, after 5 doses
of 300 mg 1,2-dichloroethane/kg body weight in 5 days, all 6
rats died, and their livers showed fatty degeneration with an
increase in the triglycerides level (Van Esch et al., 1977).
Total fat and triglycerides were elevated in the livers of
rats exposed to approximately 100 mg/kg body weight per day via
the feed, which was administered twice daily, for 7 weeks
(Alumot et al., 1976a).
No adverse effects related to liver and kidney function were
observed at the lowest dose (10 mg/kg).
7.3 Long-Term Exposure
7.3.1 Inhalation exposure
Clinical chemical investigations were performed on Sprague
Dawley rats exposed by inhalation to 1,2-dichloroethane at 0, 5,
40, 202, or 1012 mg/m3 for 7 h per day, 5 days/week (Spreafico
et al., 1980). The highest exposure level was reduced to 607
mg/m3 after a few weeks because of high mortality. Animals of
each sex were exposed, starting at 3 months of age, for 3, 6, or
18 months. In addition, animals starting at 14 months of age
were exposed for 12 months. Groups of 8 - 10 animals of each
sex were sacrificed at the specified time intervals and clinical
chemistry tests performed. Changes in SGOT, SGPT, and
c-glutamyl transpeptidase activities in the 12-month animals
were not observed in the 18-month animals. Likewise, increases
in serum-uric acid and blood urea-nitrogen levels in the 12-
month animals were not observed in the 18-month animals. In
addition, the 12-month animals, but not the 18-month animals,
displayed decreases in serum-cholesterol. On the basis of the
negative results obtained in the animals starting exposure at 3
months of age and sacrificed after 3, 6, or 18 months, the
authors suggested a lack of significant toxicity, in spite of
the biochemical changes found in the older 12-month animals.
Neurotoxic changes (conditioned reflexes) in albino rats
have been associated with a 1,2-dichloroethane exposure of 50
mg/m3, 4 h/day, for 6 months (Borissova, 1957, 1960).
7.3.2 Oral exposure
In a controlled feeding schedule for 2 years, three groups
of 18 locally-bred rats of each sex were provided with feed
fumigated with 1,2-dichloroethane. The doses of 1,2-
dichloroethane administered were estimated to be 0, 11 - 17, or
23 - 35 mg 1,2-dichloroethane/kg body weight per day. No
adverse effects were observed on growth, mortality rates, or
serum composition. The mean survival period was 18 months or
more (Alumot et al., 1976a).
7.4 Carcinogenicity
7.4.1 Inhalation exposure
Groups of 11-week-old Swiss mice and 12-week-old Sprague
Dawley rats, comprising 90 animals of each sex, were exposed to
20, 40, 202, and 1012 mg 1,2-dichloroethane/m3 air (5, 10, 50,
and 250 ppm) for 78 weeks, 7 h per day, 5 days per week, and
observed for a lifetime. Purity was reported to be greater than
99%. The highest exposure was reduced to 607 mg/m3 (150 ppm)
after a few weeks because of high mortality. Control groups
contained 115 male mice, 134 female mice, or 180 rats of each
sex. Percentage survival in male and female mice, 52 weeks
after the beginning of treatment, was, respectively, 63 and 84%
in the controls; 47 and 93% at 20 mg/m3; 66 and 80% at 40 mg/m3;
51 and 81% at 202 mg/m3; and 43 and 64% at 607 mg/m3. The last
mouse died about 100 weeks after initiation of treatment. In
male and female rats, survival at 52 weeks was, respectively, 67
and 73% in the controls; 75 and 85% at 20 mg/m3; 70 and 81% at
40 mg/m3; 70 and 84% at 202 mg/m3; and 67 and 79% at 607 mg/m3.
Most rats had died by about 140 weeks after the start of
treatment. No specific types of tumours or changes in the
incidence of tumours were found in either species, with the
exception of an increased incidence (not dose-related) of
fibromas and fibroadenomas of the mammary glands of female rats
at 20, 202, and 607 mg/m3. The average latency time for these
tumours was 83 weeks in control rats and in rats exposed at
20 mg/m3, and 79 weeks at the 2 highest exposures. The authors
ascribe the differences in the incidence of mammary tumours
between the groups to the different survival rates in the groups
(Maltoni et al., 1980).
7.4.2 Oral exposure
Groups of 50 Osborne-Mendel rats of each sex were exposed to
average doses of 1,2-dichloroethane (technical grade with
reported purity > 90%)a in corn oil of 47 or 95 mg/kg body
weight for 78 weeks (NCI, 1978). Treatment was usually given 5
times per week, and the animals were observed for another 15 -
32 weeks after the end of treatment. Control groups consisted
of 20 matched controls of each sex treated with corn oil and 60
pooled control-treated rats of each sex. Eleven minor
contaminants were detected in the test compound. The rats were
housed in the same room as rats intubated with other halogenated
hydrocarbons or carbon disulfide. Body weights were not
affected by the exposures. A dose-related increase was found in
mortality rate, which was 100%, 27 weeks after cessation of
exposure to 95 mg/kg body weight. As shown in Table 9, male
rats had a dose-related increased incidence of subcutaneous
fibromas, and forestomach squamous cell carcinomas were
observed. In treated females, the stomach showed hyperplastic
lesions. The incidence of haemangiosarcomas (in various organs,
mainly the spleen) was increased in a dose-related manner in
both sexes, but the increase was statistically significant in
males only. In females, an increase was found in the incidence
of adenocarcinomas of the mammary gland (NCI, 1978).
a Subsequent analysis indicated a purity of about 98 - 99%
(Hooper et al., 1980; Ward, 1980).
Table 9. Summary of main tumour types after oral administration of 1,2-dichoroethane to mice and ratsa
---------------------------------------------------------------------------------------------------------
Group Maximum Number of animals with:
number of Forestomach Subcutaneous Mammary Haemangiosarcomas
animals squamous cell fibromas adeno- (several organs)
examined carcinomas carcinomas
---------------------------------------------------------------------------------------------------------
Rat (male)
Pooled vehicle controls 59 0 0 0 0
Matched vehicle controls 19 0 0 1 0
Low dose 46 3 5 2 9
(P < 0.002)b (P = 0.003)b
High dose 47 9 6 0 7
(P < 0.04)c (P < 0.01)b (P < 0.02)b
Rat (female) Mammary gland
fibroma
Pooled vehicle controls 59 0 5 1 0
Matched vehicle controls 20 0 0 0 0
Low dose 50 0 14 1 4
(P < 0.01)b
High dose 50 0 8 18 4
(P < 0.001)b
---------------------------------------------------------------------------------------------------------
Group Maximum Number of animals with:
number of Hepatocellular Alveolar/ Forestomach Mammary gland
animals carcinomas bronchiolar squamous adenocarcinomas
examined adenomas cell carcinomas
---------------------------------------------------------------------------------------------------------
Mice (male)
Pooled vehicle controls 59 4 0 1 -
Matched vehicle controls 19 1 0 1 -
Low dose 46 6 1 1 -
High dose 47 12 15 2 -
(P < 0.01)b (P < 0.001)b (NS)
Mice (female)
Pooled vehicle controls 60 0 2 1 0
Matched vehicle controls 20 1 1 1 0
Low dose 50 0 7 2 9
(P = 0.001)b
High dose 48 1 15 5 7
(P < 0.001)b (NS) (P = 0.003)b
---------------------------------------------------------------------------------------------------------
a Adapted from: NCI (1978).
b Statistical analyses shown are in comparison with the pooled matched controls.
c Statistical analyses shown are in comparison with the matched vehicle controls.
In the same set of studies, B6C3F1 mice were exposed in a
similar fashion to average doses of 0, 97, or 195 mg technical
grade compound/kg body weight for males and 0, 149, or 299 mg/kg
body weight for females. They were housed in the same room
where several other hydrocarbons or other substances were
tested. After exposure, they were observed for another 12 - 13
weeks. In females, body weights were depressed at the highest
doses from week 15 onwards, while the survival rate decreased in
a dose-related manner. Mean survival exceeded 65 weeks in all
groups. Many treated mice suffered from bronchopneumonia. As
shown in Table 9, dose-related increased incidences of alveolar
or bronchiolar adenomas were found in both sexes. In males, a
dose-related increased incidence of hepatocellular carcinomas
was observed. Female mice showed slight, dose-related increases
in the incidence of squamous cell carcinomas of the forestomach,
but this was not statistically significant. In male mice,
hyperplastic changes were found at this site. The incidence of
adenocarcinomas of the mammary gland was significantly increased
in females at both doses (NCI, 1978).
7.4.3 Dermal exposure
Groups of 30 female Ha:ICR Swiss mice were treated with 42
or 126 mg 1,2-dichloroethane in acetone on the shaven dorsal
skin, 3 times per week for 440 - 594 days. In a third group,
each female received one application of 126 mg of the test
compound followed 2 weeks later by phorbol myristate acetate, a
promotor, in acetone 3 times per week for 428 - 576 days. There
were 3 control groups, a positive control, one for the promotor,
and one for no treatment. 1,2-Dichloroethane did not initiate
skin tumours. There was an elevated incidence of lung
papillomas at the highest dose compared with controls (Van
Duuren et al., 1979).
7.5 Mutagenicity and Related End-Points
7.5.1 Mutations
Information in this section is summarized in Table 10.
7.5.1.1 Bacteria
Several investigators have observed a weak or no effect of
1,2-dichloroethane in Salmonella typhimurium TA1535 or TA 100 in
spot tests or standard plate incorporation assays with or
without rat liver S9 fraction or pure microsomes (Brem et al.,
1974; McCann et al., 1975; King et al., 1979; Guengerich et al.,
1980; Principe et al., 1981). A weak mutagenic effect was
observed in 1,2-dichoroethane vapour-exposed S. typhimurium TA
1535 and TA 100, which did not increase further by the addition
of a metabolic activation system (Barber et al., 1981). However,
a stronger positive response was observed by others (Rannug et
al., 1978; Rannug & Beije, 1979; Principe et al., 1981) in TA
1535 in the presence of rat liver metabolic activation system.
It has been established that this effect has been caused by
cytosolic glutathione-S-transferases (Rannug et al., 1978; Guen-
gerich et al., 1980; Reitz et al., 1982), and similar results
have also been obtained in TA 100 (van Bladeren et al., 1981b).
In vitro Salmonella tests using the bile of mice or rats
exposed to 1,2-dichloroethane, probably containing active con-
jugates, have confirmed these results (Rannug & Beije, 1979).
No mutagenic effects were observed in forward mutation tests
with Escherichia coli (King et al., 1979).
Table 10. Tests for gene mutations/chromosome/DNA damage and cell transformation induced
by 1,2-dichloroethane
---------------------------------------------------------------------------------------------------------
Test description System description Activation Result Reference
Organism Strain/cell type system (S9)
---------------------------------------------------------------------------------------------------------
Gene mutations
reverse mutation bacteria S. typhimurium TA 1530 A + (weak) Brem et al. (1974)
TA 1535 A + (weak)
TA 1538 A + (weak)
reverse mutation bacteria S. typhimurium TA 100 A + (weak) McCann et al. (1975)
reverse mutation bacteria S. typhimurium TA 1535 P - King et al. (1979)
TA 100 P -
TA 1537 P -
TA 1538 P -
TA 98 P -
reverse mutation bacteria S. typhimurium TA 1535 P + Principe et al.
TA 1537 A or P - (1981)
TA 1538 A or P -
TA 98 A or P -
TA 100 A or P -
reverse mutation bacteria S. typhimurium TA 1535 Pa - Guengerich et al.
Pb + (1980)
reverse mutation bacteria S. typhimurium TA 1535 A or P + Barber et al.
TA 100 A or P ± (1981)
TA 1538 A or P -
TA 98 A or P -
---------------------------------------------------------------------------------------------------------
Table 10 (contd).
---------------------------------------------------------------------------------------------------------
Gene mutations (contd)
reverse mutation bacteria S. typhimurium TA 1535 A ± Rannug et al. (1978)
TA 1535 P +
TA 1535 Pb +
reverse mutation bacteria S. typhimurium TA 1535 P + Rannug & Beije (1979)
reverse mutation bacteria S. typhimurium TA 1535 P + Reitz et al. (1982)
reverse mutation bacteria S. typhimurium TA 100 Pb + van Bladeren et al.
(1981a)
forward mutation fungi S. coelicolor A - Principe et al.
A. nidulans A - (1981)
sex-linked lethals insect D. melanogaster + Rapoport (1960); Shak-
arnis (1969, 1970);
King et al. (1979)
somatic cell mutation insect D. melanogaster + Nylander et al.
(1978)
forward mutation Chinese ovary cells in vitro P ++ Tan & Hsie (1981)
hamster A +
P + Zamora et al. (1983)
forward mutation human lymphoblastoid cell line A + Crespi et al. (1985)
AHH-1 and TK6 in vitro
somatic cell mutation mouse C57BL/6J Han (female) NA +? Gocke et al. (1983)
(spot test) x T stock (male)/embryos
---------------------------------------------------------------------------------------------------------
Table 10 (contd).
---------------------------------------------------------------------------------------------------------
Test description System description Activation Result Reference
Organism Strain/cell type system (S9)
---------------------------------------------------------------------------------------------------------
Chromosome/DNA damage
micronucleus test mice NMRI/polychromatic NA - King et al. (1979)
(ip or gavage exposure) erythrocytes
micronucleus test mice CBA/polychromatic NA - Jenssen & Ramel
(ip exposure) erythrocytes (1980)
dominant lethal mice ICR Swiss/germ cells NA - Lane et al. (1982)
(ip exposure)
alkaline DNA mice B6C3F1/liver in vivo/ NA + Storer & Conolly
unwinding in vitro (1983)
alkaline DNA mice B6C3F1/liver in vivo/ NA + Storer et al. (1984)
unwinding in vitro
unscheduled DNA human lymphocytes in vitro NA + Perocco & Prodi
synthesis (exposure by (1981)
addition in the medium)
Cell transformation
cell transformation Syrian embryo cells in vitro A +c Hatch et al. (1983)
(gas/vapour exposure) golden hamster
cell transformation mice BALB/c-3T3 in vitro A - Tu et al. (1985)
(exposure by addition
in the medium)
---------------------------------------------------------------------------------------------------------
a Pure microsomes.
b Cytosol.
c Enhanced viral induced transformation.
NA = not applicable.
7.5.1.2 Fungi
Forward mutation tests conducted with Streptomyces coeli-
color and Aspergillus nidulans were negative in plate incor-
poration assays and spot tests (Principe et al., 1981). In
A. nidulans, 1,2-dichoroethane induced non-disjunction and
haploidization (Crebelli et al., 1984). 1,2-Dichloroethane was
found to be a weak inducer of mitotic crossing over
in Saccharomyces cerevisiae (Simmon, 1980).
7.5.1.3 Insects
Sex-linked recessive lethal mutations were induced
in Drosophila melanogaster by 1,2-dichloroethane (Rapoport,
1960; Shakarnis, 1970; King et al., 1979). Non-dysjunction was
observed inconsistently (Shakarnis, 1969, 1970), while the
frequency of somatic mutations for eye pigmentation increased
(Nylander et al., 1978).
7.5.1.4 Mammals/mammalian cells
A spot test using mice provided weak evidence that 1,2-
dichloroethane can induce somatic mutations (Gocke et al.,
1983).
In Chinese hamster ovary cells, 1,2-dichloroethane induced
mutations at the HGPRT-locus (Tan & Hsie, 1981; Zamora et al.,
1983). Metabolic activation increased the mutation frequency in
the presence of NADPH (Tan & Hsie, 1981). 1,2-Dichloroethane
also induced a dose-related increase in the frequency of
mutations at the HGPRT-locus in two human lymphoblastoid cell
lines, AHH-1 and TK6. The mutation frequency in the AHH-1 cell
line was 25 times that in the TK6 cell line. The difference in
sensitivity was attributed to the difference in the levels of
glutathione-S-transferase (EC 2.5.1.18) activity. The activity
of this enzyme in the AHH-1 cell line was 5 times that in the
TK6 cell line (Crespi et al., 1985).
7.5.2 Chromosome damage/DNA damage
In in vivo studies, no effects were observed in dominant
lethal assays in 2 generations of ICR Swiss mice (Lane et al.,
1982) and in the micronucleus test with CBA and NMRI mice (King
et al., 1979; Jenssen & Ramel, 1980).
1,2-Dichloroethane was a weak inducer of unscheduled DNA
synthesis in cultured human lymphocytes (Perocco & Prodi,
1981).
DNA alkylation in Salmonella by activated 1,2-dichloroethane
was directly related to the mutation frequency, but absolute
levels of DNA alkylation in Salmonella were considered low
(Reitz et al., 1982).
1,2-Dichloroethane weakly inhibited growth of DNA polymerase
deficient E. coli (Brem et al., 1974; Rosenkranz, 1977).
Administration of 1,2-dichloroethane to mice and rats
resulted in covalent binding to macromolecules (Reitz et al.,
1982; Arfellini et al., 1984; Inskeep et al., 1986). Absolute
levels of DNA alkylation in rats, either by gavage or
inhalation, were considered low (Reitz et al., 1982).
Hepatic DNA damage was demonstrated using the alkaline DNA
unwinding assay in male B6C3F1 mice after a single intraperi-
toneal or oral dose of 1,2-dichloroethane that failed to induce
toxic effects in the liver. It was also shown that, after one
inhalation exposure, hepatic DNA damage only occurred at
exposure levels that caused high mortality (Storer & Conolly,
1983; Storer et al., 1984).
7.5.3 Cell transformation
1,2-Dichloroethane did not transform BALB/c-3T3 mouse cells
in a test conducted without any exogenous metabolic activating
system (Tu et al., 1985). It enhanced transformation of Syrian
hamster embryo cells by simian adenovirus (Hatch et al., 1983).
7.6 Reproduction and Teratogenicity
7.6.1 Inhalation exposure
It has been reported that 1,2-dichloroethane was found in
the fetuses of rats after exposure to 600 mg/m3 for 5 h (Withey
& Karpinski, 1985).
Female rats (strain unspecified) were exposed to 15 mg 1,2-
dichloroethane/m3, for 4 h daily, 6 days/week, for 4 months
prior to mating. During this period, the estrus cycle became
longer than normal. The rats were then mated and exposure
continued. No information on the effects on fertility was
given, but the total embryonal mortality increased from
approximately 11% in controls to 27% in treated dams, while pre-
implantation losses were found to be 5 times greater in treated
animals than in the controls. No fetal abnormalities were
reported, with the exception of haematomas in the region of the
head, neck, and anterior extremities (Vozovaya, 1977).
In a second study, 1,2-dichloroethane was administered to
female albino rats at a concentration of 57 ± 10 mg/m3 in air,
for 4 h daily, 6 days/week, for 6 and 9 months. When the rats
were mated, a reduction in fertility was observed (6.5 fetuses
per treated female versus 9.7 in controls). The weight of
newborn rats was reduced (5.06 g versus 6.44 in unexposed
females). Perinatal mortality was increased (Vozovaya, 1974).
1,2-Dichloroethane was detected in placental and fetal
tissues after inhalation exposure to 1000 mg/m3 for 3 days
(daily duration not stated). It was also found in the stomach
of 12- to 14-day-old mice, when lactating females were exposed
to an unspecified concentration of 1,2-dichloroethane by
inhalation (Vozovaya, 1977).
While the above results indicate a possible adverse effect
of 1,2-dichloroethane on reproduction, the following repro-
ductive studies yielded negative results. Groups of 20 Sprague
Dawley rats of each sex were exposed for 60 days prior to
mating, for 6 h per day, and 5 days per week to 101, 304, or
607 mg 1,2-dichloroethane/m3 in air. After mating, they were
exposed similarly, for another 116 days, but for 7 days per
week. Females were not exposed between gestation day 21 and day
4 post partum. Control groups contained 30 rats each. Pups
were removed and examined at 21 days of age, and the females
were remated following the removal of the last litter. Each
female produced 2 litters. The parents did not show any toxic
effects and the fertility index and gestation period were
normal. In the pups, no effects were found on sex ratio,
survival indices, organ weights, or histology. A small decrease
was noted in the number of pups in the first litters at
304 mg/m3 (Rao et al., 1980).
A teratogenicity study was further performed with groups of
16 - 30 female rats and 19 - 21 female rabbits (Rao et al.,
1980). These groups were exposed through inhalation to 0, 405,
or 1215 mg 1,2-dichloroethane/m3 air, for 7 h per day, from the
6th day of pregnancy onwards (rats up to the 15th day and
rabbits up to the 18th day of gestation). Exposures at both
levels were toxic for rabbit dams. The higher exposure level
was severely toxic for the rat dams; only 1 of the few surviving
females was pregnant, and all the implantation sites were
resorbed. No adverse effects on reproduction were observed
among rats exposed at 405 mg/m3 and rabbits exposed at 405 or
1215 mg/m3. The only gross change observed in rat fetuses was a
decreased incidence of bilobed thoracic centra. There were no
significant alterations in rabbit fetuses.
7.6.2 Oral exposure
Groups of 18 female rats that had received 0, 11 - 17, or
23 - 35 mg 1,2-dichloroethane/kg body weight per day via the
feed, for up to 2 years, were mated with untreated males. The
purity of the substance was not reported. No effects on
reproduction were observed (Alumot et al., 1976a).
Lane et al. (1982) reported a 2-generation reproduction
study on groups of 10 male and 30 female ICR Swiss mice
receiving nominally 5, 15, or 50 mg 1,2-dichloroethane/kg body
weight per day via the drinking-water, for up to 25 weeks.
Control groups contained 20 male and 60 female mice. After 5
weeks of treatment, the mice were mated to produce F1A, F1B, and
F1C litters. After weaning and 11 weeks of treatment, the F1B
mice were mated to produce F2A and F2B litters. Remating always
occurred 2 weeks after weaning. In the F1C and F2B matings,
females were co-housed with untreated males for teratology
screening. The parents did not show any toxic effects and
fertility and gestation indexes were normal. There were no
effects on survival, litter size, postnatal body weight, and
gross pathology of pups. No congenital malformations were
detected. In the F1C and F2B litters, no exposure-related
reproductive effects were found. In the F2B litters, there was
no increase in the incidence of fetal visceral or skeletal
anomalies. F1C litters were not examined for skeletal
anomalies.
7.7 Immunotoxicity
When rabbits were exposed for 7.5 - 8 months to 1,2-
dichloroethane vapour of unspecified purity at a level of
100 mg/m3, for 3 h per day, 6 h per week, an 80% reduction in
the production of antibodies against typhoid vaccine was
observed. Concomitantly, there was a 2-fold increase in Forsman
sheep erythrocyte antibodies (Shmuter, 1977).
Immunosuppression was also noted in a later oral study, in
which groups of 32 male CD-1 mice were exposed to 3, 24, or
189 mg 1,2-dichloroethane/kg body weight (purity unknown) via
the drinking-water for 90 days. In addition, groups of 10 male
CD-1 mice were exposed, once a day, to 4.9 or 49 mg/kg body
weight by water gavage for 14 days. Control groups comprised 48
mice in the 90-day study and 12 mice in the 14-day study. Apart
from a 30% reduction in the leukocyte count after 14 days of
exposure to 49 mg/kg body weight, no effects were found on other
haematological parameters and relative organ weights. After the
90-day exposure, decreases in body weight and fluid consumption
were noted. There was a tendency towards a reduction in
immunoglobulin spleen antibody-forming cells and in the serum-
antibody level after sheep erythrocyte immunization, while no
effects were observed in the response to the B-cell mitogen
lipopolysaccharide S. After the 14-day exposure, 25% and 40%
suppression of antibody-forming cells were measured at 4.9 and
49 mg/kg body weight, respectively. After the 90-day exposure,
no effects were seen on the cell-mediated immunity, assessed by
measuring the delayed hypersensitivity response to sheep
erythrocytes and the spleen cell response to the T-cell mitogen
Concanavalin A. After the 14-day exposure, a slight suppression
of the delayed hypersensitivity response was found, which was
not dose-dependent (Munson et al., 1982).
8. EFFECTS ON MAN
Limited data are available on the effects on man of 1,2-
dichloroethane. There are no adequate controlled studies, no
recent occupational studies, and no mortality studies. However,
case studies of accidental exposures have been reported.
8.1 Accidental Exposures
8.1.1 Inhalation exposure
Many reports are concerned with mixed exposures. However,
in this publication, only case studies are considered in which
the exposure was reported to be to 1,2-dichloroethane alone.
According to a review by NIOSH (1976), no data on exposure
levels and duration of exposure were reported.
Inhalation of 1,2-dichloroethane vapour first afflicts the
central nervous system. Symptoms include headache, dizziness,
weakness, cyanosis, muscular spasms, hypotonia, vomiting, and
unconsciousness. Death often follows. The respiratory tract
can be irritated and inflamed with such symptoms as cough and
rales over the chest. Cyanosis may occur either as the result
of respiratory insufficiency due to depression of the central
nervous system or by bronchial obstruction due to inflammation.
Epigastric or visceral pains and diarrhoea have been observed.
Autopsy reports frequently mention damage to the lungs, liver,
and kidneys (Wirtschafter & Schwartz, 1939; Hadengue & Martin,
1953; Menschick, 1957; Troisi & Cavallazzi, 1961; Suveev &
Babichenko, 1969). Changes in heart rhythm have been reported,
which are probably secondary effects (section 8.1.2) (Suveev &
Babichenko, 1969). Clinical findings include elevated serum-
bilirubin levels and leukocytosis (Wirtschafter & Schwartz,
1939; Menschick, 1957) and elevations of blood-lactate, ammonia,
ornithine carbamyl transferase, serum aspartate transaminase (EC
2.6.1.1), lactate dehydrogenase, and creatine phosphokinase
(Nouchi et al., 1984).
8.1.2 Oral exposure
The effects of acute oral exposure are very similar to those
found after inhalation, but they are more pronounced. A summary
of acute oral intoxications was prepared by US NIOSH in 1976.
The lethal effects of 1,2-dichloroethane associated with oral
exposure are presented in Table 11. Oral doses of 20 - 50 ml
1,2-dichloroethane have been identified as being lethal (IRPTC,
1984). Several major syndromes can be identified including
central nervous system depression, gastroenteritis, and
disorders of the liver and kidneys. Frequently-observed
cardiovascular insufficiency and haemorrhagic diathesis may be
related to changes in oxygenation and effects on the liver
(Weiss, 1957; Morozov, 1958; Hinkel, 1965; Bogoyavlenski et al.,
1968; Martin et al., 1968; Schönborn et al., 1970; Yodaiken &
Babcock, 1973; Dorndorf et al., 1975; Andriukin, 1979).
Table 11. Effects associated with acute lethal oral doses of 1,2-dichloroethane in human beingsa
---------------------------------------------------------------------------------------------------------
Amount ingested (g) Findingsb Reference
---------------------------------------------------------------------------------------------------------
188 - 250 death of 4 males up to 35 h; internal Bryzhin (1945)c
haemorrhage at various sites; liver damage;
symptoms at 3 - 4 h
87 - 125 death of 3 males after 5 - 8 h; internal Kaira (1966)c
haemorrhage; symptoms immediate (uncon-
sciousness, vomiting, dizziness)
103 death after 6 h; haemorrhagic lesions Noetzel (1944)c
75 death after 22 h; symptoms at 2 h (cyanosis, Hueper & Smith (1935)
(cyanosis, vomiting, dilated pupils); brain
haemorrhage, liver damage, nephrosis
63 death at 91 h; lack of eye reflex to light Roubal (1947)c
on 4th day; vomiting; rapid pulse
63 death in 3 - 4 h; liver damage Secchi et al. (1968)c
63 death at 17 h; cyanosis, diarrhoea; impaired Schonborn et al. (1970)
blood coagulation
50 death at 24 h; haemorrhage at various sites; Martin et al. (1968)
signs of cardiac damage
---------------------------------------------------------------------------------------------------------
Table 11 (contd).
---------------------------------------------------------------------------------------------------------
Amount ingested (g) Findingsb Reference
---------------------------------------------------------------------------------------------------------
50 death at 28 h; internal haemorrhage Garrison & Leadingham (1954)c
37 death at 10 h; internal haemorrhage at Lochhead & Close (1951)
various sites; adverse lung effects
25 death at 24 h; epigastric pain; slow Roubal (1947)c
pulse
25 death at 13 h; symptoms at 1 h (cyanosis, Flowtow (1952)c
vomiting)
25 death within 12 h; symptoms not reported Flowtow (1952)c
19 death after 6 days; liver, kidney, renal Yodaiken & Babcock (1973)
damage; pulmonary oedema; hypoglycaemia;
clotting time decreased; some haemorrhaging
10 death after 56 h; delirium; pulse deterior- Bogoyavlenski et al. (1968)
ation
---------------------------------------------------------------------------------------------------------
a Studies in which doses could not be estimated have not been cited. The reader is referred to
NIOSH (1976) for a description of these case reports.
b Unless otherwise stated, death refers to single male individuals.
c The reader is referred to NIOSH (1976) for a more complete description of symptomology.
Symptoms of central nervous system depression commonly
appear within 1 h, frequently with cyanosis, nausea, vomiting,
diarrhoea, epigastric and abdominal pains, and irritation of the
mucous membranes. Irreversible brain damage has been reported
in one case, and brain damage has been found in several fatal
cases (Rohmann et al., 1969; Dorndorf et al., 1975). In some of
the cases, an interval relatively free of symptoms followed
ingestion (Hinkel, 1965; Martin et al., 1968; Komarov et al.,
1973; Dorndorf et al., 1975). In the next phase, decreasing
consciousness and circulatory and respiratory failure may occur,
often leading to death some hours to some days after exposure.
During the intoxication, heart rhythm disturbances can lead to
cardiac arrest (Morozov, 1958; Martin et al., 1968; Yodaiken &
Babcock, 1973; Andriukin, 1979). Autopsy reports have revealed
damage to the mucosae of the gastrointestinal tract, liver,
kidney, lung, heart, and brain. Livers can be enlarged. Liver
and kidney epithelium can show fatty degeneration and necrosis.
Renal insufficiency has been reported to follow development of
hepatic insufficiency and has been known to progress to uraemic
coma (Natsyuk & Mudritsky, 1974). Lung oedema is frequently
found. Hyperaemia and haemorrhagic lesions are found in some
organs. According to some authors (Martin et al., 1968;
Schönborn et al., 1970; Yodaiken & Babcock, 1973), it appeared
that the blood coagulation time was increased because of a
decrease in blood clotting factors and thrombocytes. These
effects appear secondary to liver cell necrosis complicated
further by intravascular coagulation. Biochemically, liver
damage is illustrated by increased serum levels of bilirubin,
transaminases, and lactate dehydrogenase (Martin et al., 1968;
Yodaiken & Babcock, 1973; Dorndorf et al., 1975; Andriukin,
1979). Kidney damage is expressed by anuria or oliguria
(Morozov, 1958; Bogoyavlenski et al., 1968; Yodaiken & Babcock,
1973) and albumin, leukocytes, and epithelium cells in the
urine. Together with the histopathology, this points to acute
necrosis of the kidney tubule, possibly as a result of the liver
cell necrosis and the changes in circulation (Morozov, 1958;
Hinkel, 1965; Yodaiken & Babcock, 1973). Haematological changes
include decreases in the erythrocyte count and haemoglobin
content (Morozov, 1958; Dorndorf et al., 1975).
8.1.3 Acute effects on eyes and skin
Dysfunction of the central nervous system, which could be
caused by brain oedema, can lead to effects on the eyes such as
dilation or constriction of the pupils and impairment of eye
reflexes (Weiss, 1957; Troisi & Cavallazzi, 1961). Weiss (1957)
reported a cloudy cornea in 2 cases of oral exposure; this has
also been reported in dogs (section 7.1.2). Conjunctivitis was
found in 2 out of 4 patients, who had been exposed to 1,2-
dichloroethane vapour (Menschick, 1957). After intermittent
immersion of the hands of 3 men in 1,2-dichloroethane for 4 h,
severe dermatitis developed (Wirtschafter & Schwartz, 1939).
8.2 Occupational Exposure
Only 2 reports are available, and these date from before
1960. In the first study (Cetnarowicz, 1959), 16 male workers
from an oil refinery, exposed for 2 - 8 months, were selected
for close examination. A group of 6 workers was exposed to
concentrations of between 40 and 150 mg 1,2-dichloroethane/m3
air, and a group of 10 workers was exposed to concentrations
between 250 and 800 mg/m3. The workers were also exposed to
benzene at levels between 10 and 25 mg/m3, which was considered
not significant by the authors. A general reduction in body
weight was observed. Complaints came mainly from the group with
the higher exposure and included a burning sensation of the
eyes, lachrymation, dizziness, lassitude, sleepiness, nausea,
vomiting, constipation, poor appetite, epigastric pain, and
weight loss. There was no control group, but symptoms repor-
tedly disappeared when workers were removed from exposure;
symptoms returned upon re-exposure. Most, but not all, abnorm-
alities were found in the group with higher exposure and
involved the liver (8 workers), central nervous system (3
workers), gastrointestinal tract (7 workers), and haematological
parameters (1 - 7 workers). No lesions were found in the eye,
respiratory tract, lung, or heart.
The second study (Kozik, 1957), was conducted on workers
employed in an aircraft factory using a gum dissolved in 1,2-
dichloroethane. No data were given on the composition of the
glue or on the employment status of the workers. Air concentra-
tions of 1,2-dichloroethane varied considerably, the level being
5 mg/m3 or less during 70 - 75% of the working time, and 80 -
150 mg/m3 during 25 - 30% of the working time. The morbidity of
the workers in this department during the whole period under
study (1951-55) was increased for all disease categories in
comparison with that in workers in the entire factory. A group
of 83 workers was examined further, in the absence of controls.
There were 19 workers with diseases of the liver and bile duct,
13 workers with neurotic conditions, 11 workers with autonomous
dystonia, and 10 workers with hyperthyroidism and goitre.
These reports are difficult to evaluate, because no
indication is given of the prevalence in unexposed workers of
the symptoms and signs described in the case studies.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Evaluation of Human Health Risks
In spite of the high volume of production of 1,2-dichloro-
ethane, there are no quantitative exposure-effect data on human
beings. Frequently, 1,2-dichloroethane is not the only chemical
involved in an exposure and thus, cause-effect relationships are
difficult to derive. No epidemiological or mortality studies
are available. There are only two old reports on small groups
of occupationally-exposed men (section 8.2). These data indi-
cate that repeated inhalation exposures in the range of approx-
imately 40 - 800 mg/m3 may lead to central nervous system
depression and gastrointestinal and liver abnormalities. As
might be expected, such symptoms were more prevalent in indivi-
duals exposed to high levels. The only available data regarding
oral exposure are those involving fatal intoxications (Table 11,
section 8.1).
Because of the limitations of the human data base, it is
necessary to rely on the available experimental animal data to
derive a no-observed-adverse-effect level for human beings.
This is possible because of the similarity in the spectrum of
adverse effects in man and laboratory animals, which include
central nervous system depression, liver and possibly kidney
abnormalties, lung oedema, and cardiovascular disorders. The
dose-response data from animal studies include a no-observed-
adverse-effect level for the rat of about 400 mg 1,2-dichloro-
ethane/m3 air (section 7.2.1), equivalent to an intake of about
35 mg/kg body weight per day (assuming inhalation of 42 litres
over 7 h for a 500-g rat and 100% absorption). The highest
registered air exposure level of the general population is
65 µg/m3 (Table 4, section 4.1.2). This level leads to a
calculated intake of 1.3 mg per person per day (20 m3/day x
65 µg/m3). A comparable intake from drinking-water containing
the highest level recorded (6 µg/litre) (Table 3, section
4.1.1) would amount to a daily intake of 12 µg (6 µg/litre
litre/day). Thus, a combined air and water exposure, in the
context of a worst-case scenario for the general population,
when compared with the no-observed-adverse-effect level for
animals, is lower by a factor of well over 1000. Consequently,
for the biological end-points considered so far, it can be
concluded that 1,2-dichloroethane is unlikely to present a toxic
hazard for the general population, under prevailing exposure
conditions.
In an oral administration study, 1,2-dichloroethane produced
a statistically-significant increase in squamous cell carcinoma
of the forestomach, haemangiosarcoma, and mammary adenocarcinoma
in rats, and mammary adenocarcinoma and hepatocellular carcinoma
in mice (section 7.4.2). In one carcinogenicity study, inhala-
tion exposure did not result in an increase in tumour incidence
(section 7.4.1). The natural occurrence of forestomach squamous
cell carcinomas in rats and haemangiosarcomas in laboratory
rodents is unusual. Taking into consideration that cancer has
been produced in two species of experimental animals and in
several target organs, it can be concluded that 1,2-dichloro-
ethane is carcinogenic for rats and mice, when administered by
gavage.
In the absence of human data, and taking into account the
fact that 1,2-dichloroethane produces a reactive intermediate
that alkylates DNA, that it is positive in a number of in vitro
mutagenicity tests, though weakly so (section 7.5), and that it
results in the production of both rare and common tumours in
rats and mice, it would be prudent to consider 1,2-dichloro-
ethane as a possible human carcinogen. Therefore, 1,2-dichloro-
ethane should be regarded, for practical purposes, as if it
presented a carcinogenic risk for man. Thus, levels in the
environment should be kept as low as feasible.
Since there are no human data, it is necessary to rely on
the limited data available from experimental animal studies in
evaluating reproduction hazards and teratogenicity. The weight
of evidence (section 7.6) does not suggest that exposure to
prevailing environmental levels would pose a human reproductive
or teratogenic hazard.
9.2 Evaluation of Effects on the Environment
9.2.1 Air
Emissions of 1,2-dichloroethane into the air mainly occur in
process industries. Other emissions occur during its use as a
fumigant, solvent, and lead scavenger, and via evaporation from
contaminated water and from waste disposal sites. Total emis-
sions are estimated to amount to 0.2% of the production volume.
Photochemical degradation via oxidation by hydroxyl radicals is
the most important route of elimination from air. Rainout and
adsorption on atmospheric particles are unlikely to be important
processes of elimination. Photolysis is theoretically a possib-
ility, but no evidence of this is available. The products of
the photochemical degradation are carbon monoxide, carbon
dioxide, hydrogen chloride, formyl chloride, and chloroacetyl-
chloride. The two last compounds will degrade further. The
process is rapid enough to prevent accumulation of the compound
in the atmosphere (section 3.3).
9.2.2 Water
Emissions of 1,2-dichloroethane into water may amount to
0.1% of the production volume. Some of the emissions from EDC
tars, which total about 0.5% of the production volume, will
contaminate water. The main process of removal of 1,2-dichloro-
ethane from water is evaporation. Chemical degradation is not
expected, nor is biodegradation fast enough to be of any signi-
ficance (section 3.1). Bioconcentration in aquatic species is
unlikely in view of the rather low octanol/water partition
coefficient. This conclusion is supported by a low bioconcen-
tration factor found experimentally (section 6.1.4).
The compound was only slightly toxic for aquatic species
tested. A no-observed-adverse-effect concentration for Daphnia
magna in a long-term test was 11 mg/litre (section 6.1.3). The
lowest LC50 value (85 mg/litre) was found for the shrimp Crangon
crangon (section 6.1.1). Average environmental levels of the
compound in surface water are generally below 1 µg/litre, but,
in heavily polluted surface water, average levels of 5.6
µg/litre have been measured with a maximum of 90 µ g/litre
(Table 3, section 4.1.1).
On the basis of the above data, it can be concluded that,
except in case of accidents and inappropriate disposal, 1,2-
dichloroethane does not pose a significant hazard for the
aquatic environment. However, it should be noted that EDC tars
are much more toxic than 1,2-dichloroethane (section 6.1.1).
9.2.3 Soil
Data are not sufficient to evaluate the effects of 1,2-
dichloroethane in soil.
10. RECOMMENDATIONS FOR FURTHER STUDIES
1. DNA alkylation (adduct identification).
2. Studies on sub-chronic toxicity using various routes of
exposure.
3. Assessment of the extent to which EDC tars contribute to
contamination of groundwater by 1,2-dichloroethane.
4. Dose-response studies on sensitive, commercially important
fish species (particularly studies relevant to EDC tar
spills).
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,2-Dichloroethane was evaluated by IARC in 1979 (Volume 20
of the IARC Monographs). It was concluded that:
"There is sufficient evidence that 1,2-dichloroethane is
carcinogenic in mice and rats. In the absence of adequate data
in humans, it is reasonable, for practical purposes, to regard
1,2-dichloroethane as if it presented a carcinogenic risk to
humans."
REFERENCES
ADEMA, D.M.M. (1976) [Acute toxicity tests with 1,2-
dichloroethane, phenol, acrylonitrile, and alkyl benzene-
sulfonate in sea water,] Delft, the Netherlands, Central
Laboratory TNO (TNO Report No. MD-N & E 76/1) (in Dutch).
ALUMOT, E., NACHTOMI, E., MANDEL, E., HOLSTEIN, P., BONDI, A., &
HERZBERG, M. (1976a) Tolerance and acceptable daily intake of
chlorinated fumigants in the rat diet. Food Cosmet. Toxicol.,
14: 105-110.
ALUMOT, E., MEIDLER, M., HOLSTEIN, P., & HERZBERG, M. (1976b)
Tolerance and acceptable daily intake of ethylene dichloride in
the chicken diet. Food Cosmet. Toxicol., 14: 111-114.
ANDRIUKIN, A.A. (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klin. Med. (Mosk.), 57: 43-47 (in
Russian).
ARFELLINI, G., BARTOLI, S., COLACCI, A., MAZZULO, M., GALLI,
M.C., PRODI, G., & GRILLI, S. (1984) In vivo and in vitro
binding of 1,2-dibromethane and 1,2-dichloroethane to
macromolecules in rat and mouse organs. J. Cancer Res. clin.
Oncol., 108: 204-213.
ATRI, F.R. (1984) [ Study to assess the toxicity of water
pollutants. III. 1,2-Dichloroethane,] Bonn, Federal Environment
Agency (Research Report No. 106/07/047) (in German).
BAILEY, S., COLLINS, G.B., FISHWICK, F.B., HART, H.V., HORLER,
D.F., & SCUDAMORE, K.A. (1982) Pesticide residues in
foodstuffs in Great Britain; organochlorine pesticides,
organophosphorus pesticides, and fumigant residues in home-
produced and imported wheat. Pestic. Sci., 13: 373-378.
BANERJEE, S., VAN DUUREN, B.L., & ORUAMBO, F.I. (1980)
Microsome-mediated covalent binding of 1,2-dichloroethane to
lung microsomal protein and salmon sperm DNA. Cancer Res., 40:
2170-2173.
BARBER, R.D., DONISH, W.H., & MUELLER, K.R. (1981) A procedure
for the quantitative measurement of the mutagenicity of volatile
liquids in the Ames Salmonella/microsome assay. Mutat. Res.,
90: 31-48.
BARETTA, E.D., STEWARD, R.D., & MUTCHLER, J.E. (1969)
Monitoring exposures to vinyl chloride vapour: breath analysis
and continuous air sampling. Am. Ind. Hyg. Assoc. J., 30: 537-
544.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CAROLL, J.J.
(1980) Bioconcentration and elimination of selected water
pollutants by bluegill sunfish Lepomis macrochirus. In:
Dynamics, Exposure and Hazard Assessment of Toxic Chemicals,
Paper Symposium 1978, Ann Arbor, Michigan, Ann Arbor Science,
pp. 379-392.
BARSOUM, G.S. & SAAD, K. (1934) Relative toxicity of certain
chlorine derivatives of the aliphatic series. Q. J. Pharm.
Pharmacol., 7: 205-214.
BAUER, U. (1978) [Halogenated hydrocarbons in drinking and
surface water. Analysis results 1976-77 in the Federal Republic
of Germany. Drinking water from 100 cities; surface water from
the Ruhr, Lippe, Main, and Rhine.] WaBoLu-Berichte, 3: 64-74 (in
German).
BAUER, U. (1981) [Human exposure to harmful components in
environmental studies on water, air, food, and human tissues.
II, IV.] Zentralbl. Bakteriol. Hyg. I. Abt. Orig. B, 174: 39-55,
556-583 (in German).
BENOIT, D.A., PUGLISI, F.A., & OLSON, D.L. (1982) A fathead
minnow Pimephales promelas early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ.
Pollut., 28(Series A): 189-197.
BERCK, B. (1965) Sorption of ethylene dibromide, ethylene
dichloride, and carbon tetrachloride by cereal products. J.
agric. food Chem., 13: 248-254.
BERCK, B. (1974) Fumigant residues of carbon tetrachloride,
ethylene dichloride, and ethylene dibromide in wheat, flour
bran, middlings, and bread. J. agric. food Chem., 22: 977-984.
BOGOYAVLENSKI, V.F., SALIKHOVA, S.K.H., & KARPOVA, E.V. (1968)
[Clinical aspects and therapy for ethylene dichloride
poisoning.] Sov. Med., 31: 107-109 (in Russian).
BOIKOVA, N.Y. & KRAVTSOVA, G.V. (1982) Histoenzymologic
characteristics of liver and kidney after acute and chronic
poisoning by 1,2-dichloroethane. In: Proceedings of the
Leningrad Scientific Society of Pathologoanatoms, Leningrad,
Medicine, Vol. 23, pp. 158-160.
BONNET, P., FRANCIN, J.-M., GRADISKI, D., RAOULT, G., & ZISSU,
D. (1980) Détermination de la concentration léthale 50 des
principaux hydrocarbures aliphatiques chlorés chez le rat.
Arch. Mal. prof. Méd. Trav. Sécur. soc., 41: 317-321.
BORISSOVA, M.K. (1957) [Experimental inputs to evaluation of
maximum allowable dichloroethane concentration in the air.] Gig.
i Sanit., 3: 13-19 (in Russian).
BORISSOVA, M.K. (1960) Some inputs to evaluation of maximum
allowable dichloroethane concentration in the air. In: Maximum
allowable concentrations of air pollutants, Vol. 4, pp. 21-24.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformations of 1- and
2-carbon halogenated aliphatic organic compounds under methano-
genic conditions. Appl. environ. Microbiol., 45: 1286-1294.
BOZZELLI, J.W. & KEBBEKUS, B.B. (1982) A study of some
aromatic and halocarbon vapors in the ambient atmosphere of New
Jersey. J. environ. Sci. Health, A17: 693-711.
BREM, H., STEIN, A.B., & ROSENKRANZ, H.S. (1974) The muta-
genicity and DNA-modifying effect of haloalkanes. Cancer Res.,
34: 2576-2579.
BRINGMANN, G. & KUHN, R. (1978) Testing of substances for
their toxicity threshold: model organisms Microcystis
(Diplocystis) aeruginosa and Scenedesmus quadricauda. Mitt.
Int. Verein Limnol., 21: 275-284.
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa
in the cell multiplication inhibition test. Water Res., 14:
231-241.
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effect of
hazardous substances on flagellated and ciliated, respectively,
holozoic bactivorous and saprozoic protozoa.] GWF Wasser-
Abwasser, 122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Data on the toxicity of water
pollutants to Daphnia magna in an improved standard test.] Z.
Wasser Abwasser Forsch., 15: 1-6 (in German).
BRYZHIN, F.F. (1945) Pathomorphological changes in internal
organs in connection with poisoning by ethylene dichloride
through the digestive tract. Pharmacol. Toxicol., 8: 43-49.
BUCCAFUSCO, R.J., ELLS, S.J., & LE BLANC, G.A. (1981) Acute
toxicity of priority pollutants of bluegill Lepomis macro-
chirus. Bull. environ. Contam. Toxicol., 26: 446-452.
CASWELL, G.H. & CLIFFORD, H.T. (1958) The effect of ethylene
dichloride and carbon tetrachloride on the germination and early
growth of maize. Emp. J. exp. Agric., 26: 365-372.
CETNAROWICZ, J. (1959) Experimental and clinical investiga-
tions on the action of dichloroethane. Folia Med. Cracov., 1:
169-192.
CLARK, A.I., MCINTYRE, A.E., LESTER, J.N., & PERRY, R. (1984a)
Ambient air measurement of aromatic and halogenated hydrocarbons
at urban, rural, and motorway locations. Sci. total Environ.,
39: 265-279.
CLARK, A.I., MCINTYRE, A.E., PERRY, R., & LESTER, J.N. (1984b)
Monitoring and assessment of ambient atmospheric concentrations
of aromatic and halogenated hydrocarbons at urban, rural, and
motorway locations. Environ. Pollut. Ser. B, 7: 141-158.
CREBELLI, R., CONTI, G., CONTI, L., & CARERE, A. (1984)
Induction of somatic segregation by halogenated aliphatic
hydrocarbons in Aspergillus nidulans. Mutat. Res., 138(1): 33-
38.
CRESPI, C.L., SEIXAS, G.M., TURNER, T.R., RYAN, C.G., & PENMAN,
B.W. (1985) Mutagenicity of 1,2-dichloroethane and 1,2-
dibromoethane in two human lymphoblastoid cell lines. Mutat.
Res., 142: 133-140.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA 600/3-80-
084, PB 221948).
DAWSON, G.W., JENNINGS, A.L., DROSDOWSKI, D., & RIDER, E.
(1977) The acute toxicity of 47 industrial chemicals to fresh
and saltwater fish. J. hazard. Mater., 1: 303-318.
DEQUINZE, J., SZIMAR, C., & EDELINE, F. (1984) Identification
of the substances and their derivative products, on the list of
129 substances (list 1 of the directive 76/464/EEC) present in
the refuse of chlorine derivatives by organic chemistry,
Brussels, European Economic Community.
DILLING, W.L., TEFERTILLER, N.B., & KALLOS, G.J. (1975)
Evaporation rates and reactivities of methylene chloride,
chloroform, 1,1,1-trichloroethane, trichloroethylene,
tetrachloroethylene, and other chlorinated compounds in dilute
aqueous solutions. Environ. Sci. Technol., 9: 833-838.
DMITRIEVA, N.V. & KULESHOV, E.V. (1971) [Changes in the
cerebral bioelectric activity and electric conductivity in rats
in chronic intoxications with certain chlorinated hydrocarbons.]
Gig. i Sanit., 36: 20-25 (in Russian).
DORNDORF, W., KRESSE, M., CHRISTIAN, W., & KATRITZKI, KG.
(1975) [Dichloroethane poisoning with myoclinic syndrome,
epileptic attacks, and irreversible cerebral effects.] Arch.
Psychiatr. Nervenkr., 220: 373-379 (in German).
DRURY, J.S. & HAMMONS, A.S. (1979) Investigations of selected
environmental pollutants: 1,2-dichloroethane, Washington DC, US
Environmental Protection Agency, Office of Toxic Substances (EPA
560/2-78-006, PB 295865).
DUPRAT, P., DELSAUT, L., & GRADISKI, D. (1976) Pouvoir
irritant des principaux solvants chlorés aliphatiques sur la
peau et les muqueuses oculaires du lapin. Eur. J. Toxicol., 9:
171-177.
EHRENBERG, Z., OSTERMAN-GOLKAR, S., SINGH, D., & LUNDQVIST, U.
(1974) On the reaction kinetics and mutagenic activity of
methylating and beta-halogenoethylating gasoline additives.
Radiat. Bot., 15: 184-194.
ELFERS, L.A. (1979) Monitoring of ambient levels of EDC in the
vicinity of EDC production and user facilities, Research
Triangle Park, North Carolina, US Environmental Protection
Agency, Environmental Monitoring and Support Laboratory (EPA
600/4-79-029, PB 298303).
ENVIRONMENT AGENCY, JAPAN (1983) Environmental monitoring of
chemicals: environmental survey report of fiscal years 1980 and
1981, Tokyo, Environment Agency.
EWING, B.B., CHIAN, E.S.K., COOK, J.C., DEWALLE, F.B., EVANS,
C.A., HOPKE, P.K., KIM, J.H., MEANS, J.C., MILBERG, R., PERKINS,
E.G., SHERWOOD, J.D., & WADLIN, W.H. (1977) Monitoring to
detect previously unrecognized pollutants in surface waters,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA 560/6-77-015, PB 273349).
FLOWTOW, E. (1952) Poisoning due to chlorinated hydrocarbon
compounds, particularly 1,2-dichloroethane. Chem. Tech.
(Leipzig), 6: 253-254.
FOUREMAN, G.L. & REED, D.J. (1985) Evidence for a non-
episulfonium ion intermediate during alkylation by S-(2-
chloroethyl) cysteine but not by S-(2-chloroethyl) glutathione.
Fed. Proc. Fed. Am. Exp. Biol., 44: 517.
FUJII, T. (1977) Direct aqueous injection gas chromato-
graphy-mass spectrometry for analysis of organohalides in water
at concentrations below the parts per billion level. J.
Chromatogr., 139: 297-302.
GARRISON, S.C. & LEADINGHAM, R.S. (1954) A fatal case of
ethylene dichloride poisoning in an occupational therapy
department of a neuro-psychiatric hospital. Am. J. phys. Med.,
33: 230-237.
GOCKE, E., WILD, D., ECKHARDT, K., & KING, M.-T. (1983)
Mutagenicity studies with the mouse spot test. Mutat. Res., 117:
201-212.
GOLD, L.S. (1980) Human exposures to ethylene dichloride. In:
Ames, B., Infante, P., & Reitz, R., ed. Ethylene dichloride: a
potential health risk? Cold Spring Harbor, Cold Spring Harbor
Laboratory, pp. 209-225 (Banbury Report No. 5).
GOSSETT, R.W., BROWN, D.A., & YOUNG, D.R. (1983) Predicting
the bioaccumulation of organic compounds in marine organisms
using octanol/water partition coefficients. Mar. Pollut. Bull.,
14: 387-392.
GRADISKI, D., BONNET, P., RAOULT, G., MAGADUR, J.L., & FRANCIN,
J.-M. (1978) Toxicité aiguë comparée par inhalation des
principaux solvants aliphatiques chlorés. Arch. Mal. prof. Méd.
Trav. Sécur. soc., 39: 249-257.
GRIMSRUD, E.P. & RASMUSSEN, R.A. (1975) Survey and analysis of
halocarbons in the atmosphere by gas chromatography-mass
spectrometry. Atmos. Environ., 9: 1014-1017.
GUENGERICH, F.P., CRAWFORD, W.M., DOMORADZKI, J.Y., MACDONALD,
T.L., & WATANABE, P.G. (1980) In vitro activation of 1,2-
dichloroethane by microsomal and cytosolic enzymes. Toxicol.
appl. Pharmacol., 55: 303-317.
GUENGERICH, F.P., MASON, P.S., STOTT, W.T., FOX, T.R., &
WATANABE, P.G. (1981) Role of 2-haloethylene oxides and vinyl
chloride in irreversible binding to protein and DNA. Cancer
Res., 41: 4391-4398.
GUENGERICH, F.P., KOGA, N., INSKEEP, P.B., & OZAWA, N. (1986)
Proceedings of the Second International Symposium on the
Synthesis and Application of Isotopically-Labelled Compounds,
New York, Elsevier.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of
organic chemicals in the atmosphere of the Netherlands. Sci.
total Environ., 43: 193-219.
HADENGUE, M.A. & MARTIN, R. (1953) Un cas d'intoxication
mortelle par le dichloréthane. Soc. Med. Leg., 33: 247-249.
HARKOV, R., KEBBEKUS, B., BOZZELLI, J.W., LLOY, P.J., & DAISEY,
J. (1984) Comparison of selected volatile organic compounds
during the summer and winter at urban sites in New Jersey. Sci.
total Environ., 38: 259-274.
HATCH, G.G., MAMAY, P.D., AYER, M.L., CASTO, B.C., & NESNOW, S.
(1983) Chemical enhancement of viral transformation in Syrian
hamster embryo cells by gaseous and volatile chlorinated
methanes and ethanes. Cancer Res., 43: 1945-1950.
HEITMULLER, P.T., HOLLISTER, T.A., & PARRISH, P.R. (1981)
Acute toxicity of 54 industrial chemicals to sheepshead minnows
Cyprinodon variegatus. Bull. environ. Contam. Toxicol., 27:
596-604.
HELLMANN, T.M. & SMALL, F.H. (1974) Characterization of the
odour properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
HEPPEL, L.A., NEAL, P.A., PERRIN, T.L., ENDICOTT, K.M., &
PORTERFIELD, V.T. (1946) The toxicology of 1,2-dichloro-
ethane (ethylene dichloride). V. The effect of daily
inhalations. J. ind. Hyg. Toxicol., 28: 113-120.
HILL, D.L., SHIH, T.-W., JOHNSTON, T.P., & STRUCK, R.F. (1978)
Macromolecular binding and metabolism of the carcinogen 1,2-
dibromoethane. Cancer Res., 38: 2438-2442.
HINKEL, G.K. (1965) [Oral dichloroethane intoxication of
children.] Dtsch Gesudheitswes., 20: 1327-1331 (in German).
HOFMANN, H.T., BIRNSTIEL, H., & JOBST, P. (1971) [On the
inhalation toxicity of 1,2-dichloroethane.] Arch. Toxikol., 27:
248-265 (in German).
HOOPER, K., GOLD, I., & AMES, B. (1980) The carcinogenic
potency of ethylene dichloride in two animal bioassays: a
comparison of inhalation and gavage studies. In: Ames, B.N.,
Infante, P., & Reitz, R., ed. Ethylene dichloride: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 65-81 (Banbury Report No. 5).
HOWARD, C.J. & EVENSON, K.M. (1976) Rate constants for the
reactions of OH with ethane and some halogen substituted ethanes
at 296 K. J. Chem. Phys., 64: 4303-4306.
HUEPER, W.C. & SMITH, C. (1935) Fatal ethylene dichloride
poisoning. Am. J. med. Sci., 189: 778-784.
IARC (1979) 1,2-Dichloroethane. In: Some halogenated hydro-
carbons, Lyons, International Agency for Research on Cancer, pp.
429-446 (IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Vol. 20).
INSKEEP, P.B. & GUENGERICH, F.P. (1984) Glutathione mediated
binding of dibromoalkanes to DNA: specificity of rat glutathione
S-transferases and dibromoalkane structure. Carcinogenesis, 5:
805-808.
INSKEEP, P.B., KOGA, N., CMARIK, J.L., & GUENGERICH, F.P.
(1986) Covalent binding of 1,2-dihaloalkanes to DNA and
stability of the major DNA adduct S-[2-(N7/guanyl)ethyl]
glutathione. Cancer Res., 46: 2839-2844.
IRPTC (1982) Izmerov, N.F., ed. Dichloroethane, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme (Scientific Review of Soviet
Literature on Toxicity and Hazards of Chemicals No. 25).
IRPTC (1984) Data profile of 1,2-dichloroethane, Geneva,
International Register of Potentially Toxic Chemicals.
JACOBS, E.S. (1980) Use and air quality impact of ethylene
dichloride and ethylene dibromide scavengers in leaded gasoline.
In: Ames, B., Infante, P., & Reitz, R., ed. Ethylene dichloride:
a potential health risk?, Cold Spring Harbor, New York, Cold
Spring Harbor Laboratory, pp. 239-255 (Banbury Report No. 5).
JAKOBSON, I., WAHLBERG, J.E., HOLMBERG, B., & JOHANSSON, G.
(1982) Uptake via the blood and elimination of 10 organic
solvents following epicutaneous exposure of anesthetized guinea-
pigs. Toxicol. appl. Pharmacol., 63: 181-187.
JANSSEN, D.B., SCHEPER, A., & WITHOLT, B. (1984) Biodegra-
dation of 2-chloroethanol and 1,2-dichloroethane by pure
bacterial cultures. Prog. Med. Microbiol., 20: 169-178.
JENSEN, S., LANGE, R., BERGE, G., PALMORK, K.H., & RENBERG, L.
(1975) On the chemistry of EDC-tar and its biological
significance in the sea. Proc. R. Soc. Lond. B, 189: 333-346.
JENSSEN, D. & RAMEL, C. (1980) The micronucleus test as part
of a short-term mutagenicity test program for the prediction of
carcinogenicity evaluated by 143 agents tested. Mutat. Res., 75:
191-202.
JERNELOV, A., ROSENBERG, R., & JENSEN, S. (1972) Biological
effects and physical properties in the marine environment of
aliphatic chlorinated by-products from vinyl chloride
production. Water Res., 6: 1181-1191.
JOHNSON, M.K. (1965) The influence of some aliphatic compounds
on rat liver glutathione levels. Biochem. Pharmacol., 14: 1383-
1385.
JOHNSON, M.K. (1966) Studies on glutathione-S-alkyl transferase
of the rat. Biochem. J., 98: 44-56.
JOHNSON, M.K. (1967) Metabolism of chloroethanol in the rats.
Biochem. Pharmacol., 16: 185-199.
JOLIVET, E. (1968) Métabolisme des acides organiques et des
acides aminés libres dans le tubercule de pomme de terre au
cours de la rupture provoquée de son repos végétatif par la
"Rindite". Physiol. vég., 6: 221-233.
JONSSON, A. & BERG, S. (1980) Determination of 1,2-dibromo-
ethane, 1,2-dichloroethane, and benzene in ambient air using
porous polymer traps and gas chromatographic-mass spectro-
metric analysis with selected ion monitoring. J. Chromatogr.,
190: 97-106.
KAGRAMANOV, V.G. & KAZIEVA, N.K. (1972) Condition of
fibrinolytic blood activity in acute dichloroethane poisoning.
Azerb. State Med. Inst. Baku, 36: 30-31.
KAIRA, F.M. (1966) [Alimentary oral dichloroethane poisoning.]
Klin. Med. (Mosk.), 44: 143-146 (in Russian).
KAMEL, A. (1959) The effect of carbon bisulphide and ethylene
dichloride-carbon tetrachloride mixture on the germination of
some seeds of certain crops. Agric. Res. Rev. (Cairo), 37: 330-
350.
KELLAM, R.G. & DUSETZINA, M.G. (1980) Human exposure to
ethylene dichloride: potential for regulation via EPA's proposed
airborne carcinogen policy. In: Ames, B., Infante, P., & Reitz,
R., ed. Ethylene dichloride: a potential health risk?, Cold
Spring Harbor, New York, Cold Spring Harbor Laboratory, pp.
265-276 (Banbury Report No. 5).
KERSTER, H.W. & SCHAEFFER, D.J. (1983) Brine shrimp (Artemia
salina nauplii) as a teratogen test system. Ecotoxicol.
environ. Saf., 7: 342-349.
KING, M.-T., BEIKIRCH, H., ECKHARDT, K., GOCKE, E., & WILD, D.
(1979) Mutagenicity studies with X-ray contrast media,
analgesics, antipyretics, antirheumatics, and some other
pharmaceutical drugs in bacterial, Drosophila, and mammalian
test systems. Mutat. Res., 66: 33-43.
KLEINSCHMIDT, E.-G., VON (1983) [Investigations into the
relation between odour thresholds of human beings for some
substances and their chemical structure.] Wiss. Z. Wilhelm-
Piech-Univ. Rostock, Naturwiss. Reihe, 32: 54-58 (in German).
KOGA, N., INSKEEP, P.B., HARRIS, T.M., & GUENGERICH, F.P.
(1986) S-[2-(N7-guanyl)ethyl] glutathione, the major DNA adduct
formed from 1,2-dichloroethane. Biochemistry, 25: 2192-2198.
KOKAROVTSEVA, M.G. & KISELYOVA, N. (1978) [Chloroethanol
(ethylenechlorohydrin) - a toxic metabolite of 1,2-dichloro-
ethane.] Farmakol. Toksikol., 41: 118-120 (in Russian).
KOMAROV, V.P., ROMANCHAK, M.N., SHCHERBAN, I.I., & GAPONTSEV,
S.N. (1973) [On the clinical features and therapy of acute
dichloroethane intoxications.] Zdravookhr. Belorussi, 4: 55-57
(in Belorussian).
KONEMANN, H. (1981) Quantitative structure-activity
relationships in fish toxicity studies. I. Relationship for 50
industrial pollutants. Toxicology, 19: 209-221.
KOZIK, I.V. (1957) [Some problems of occupational hygiene in
the use of dichloroethane in the aircraft industry.] Gig. Tr.
Prof. Zabol., 1: 31-38 (in Russian).
KRONEVI, T., WAHLBERG, J.E., & HOLMBERG, B. (1981) Skin
pathology following epicutaneous exposure to seven organic
solvents. Int. J. Tissue React., 3: 21-30.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A.
(1982) Collection and analysis of hazardous organic emissions.
Anal. Chem., 54: 810-817.
KULKARNI, J.H., SARDESHPANDE, J.S., & BAGYARAJ, D.J. (1975)
Effect of seed fumigation on the symbiosis of Rhizobium sp.
with Arachis hypogaea Linn. Zentralbl. Bakteriol. Parasitenkr.
Infektionskr. Hyg. Abt. II, 130: 41-44.
KUWABARA, T., QUEVEDO, A.R., & COGAN, D. (1968) An experi-
mental study of dichloroethane poisoning. Arch. Ophtalmol., 79:
321-330.
LANE, R.W., RIDDLE, B.L., & BORZELLECA, J.F. (1982) Effects of
1,2-dichloroethane and 1,1,1-trichlorethane in drinking-water on
reproduction and development in mice. Toxicol. appl. Pharmacol.,
63: 409-421.
LARIONOV, V.G. & KOKAROVTSEVA, M.G. (1976) Morphological
constitution of peripheral blood in intoxication with
dichloroethane and its metabolites. In: Actual problems of
pesticide application in different climatographic zones,
Yerevan, Aiastan Publishers, pp. 131-133.
LE BLANC, G.A. (1980) Acute toxicity of priority pollutants to
water flea Daphnia magna. Bull. environ. Contam. Toxicol., 24:
684-691.
LIN, E.L.C., MATTOX, J.K., & PEREIRA, M.A. (1985) Glutathione
plus cytosol- and microsome-mediated binding of 1,2-dichloro-
ethane to polynucleotides. Toxicol. appl. Pharmacol., 78: 428-
435.
LIVESEY, J.C. & ANDERS, M.W. (1979) In vitro metabolism of
1,2-dihaloethanes to ethylene. Drug Metab. Dispos., 7: 100-203.
LOCHHEAD, H.B. & CLOSE, H.P. (1951) Ethylene dichloride
plastic cement: a case of fata poisoning. J. Am. Med. Assoc.,
146: 1323.
LUZNIKOV, E.A., LISOVIK, G.A., & NOVIKOVSKAYA, T.V. (1985)
[Metabolism of 1,2-dichloroethane in human organisms after acute
poisonings.] [ Forensic Med. Expert.,] 2: 47-49 (in Russian
with English summary).
LYNN, G.E. & VORHES, F.A., ed. (1957) Symposium on residues in
foods and feeds resulting from fumigation of grains with the
commoner liquid formulations of carbon disulphide, carbon
tetrachloride, ethylene dichloride, and ethylene dibromide. J.
Assoc. Off. Agric. Chem., 40: 163-209.
MCCANN, J., SIMMON, V., STREITWIESER, D., & AMES, B. (1975)
Mutagenicity of chloroacetaldehyde, a possible metabolic product
of 1,2-dichloroethane (ethylene dichloride), chloroethanol
(ethylene chlorohydrin), vinyl chloride, and cyclophosphamide.
Proc. Natl Acad. Sci. (USA), 72: 3190-3193.
MCCOLLISTER, D.D., HOLLINGSWORTH, R.L., OYEN, F., & ROWE, V.K.
(1956) Comparative inhalation toxicity of fumigant mixtures.
Individual and joint effects of ethylene dichloride, carbon
tetrachloride, and ethylene dibromide. Arch. ind. Health, 13:
1-7.
MALTONI, C., VALGIMIGLI, L., & SCARNATO, C. (1980) Long-term
carcinogenic bioassays on ethylene dichloride administered by
inhalation to rats and mice. In: Ames, B., Infante, P., & Reitz,
R., ed. Ethylene dichloride: a potential health risk?, Cold
Spring Harbor, New York, Cold Spring Harbor Laboratory, pp. 5-30
(Banbury Report No. 5).
MARTIN, G., KNORPP, K., HUTH, K., HEINRICHT, F., & MITTERMAYER,
C. (1968) [Clinical features, pathogenesis and therapy of
dichloroethane poisoning.] Dtsch med. Wschr., 93: 2002-2010 (in
German).
MAY, J. (1966) [Odour thresholds of solvents for the judgement
of solvent odour in air.] Staub, 26: 385-389 (in German).
MENSCHICK, H. (1957) [Acute inhalation intoxications by
symmetric dichloroethane.] Arch. Gewerbepathol. Gewerbehyg., 15:
241-252 (in German).
MITOMA, C., STEEGER, T., JACKSON, S.E., WHEELER, K.P., ROGERS,
J.H., & MILMAN, H.A. (1985) Metabolic disposition study of
chlorinated hydrocarbons in rats and mice. Drug chem. Toxicol.,
8: 183-194.
MOODY, E.D., JAMES, J.L., CLAWSON, G.A., & SMUCKLER, E.A.
(1981) Correlations among the changes in hepatic microsomal
components after intoxication with alkyl halides and other
heptatotoxins. Mol. Pharmacol., 20: 685-693.
MOROZOV, G.N. (1958) [On acute dichloroethane poisoning.]
Farmakol. Toksicol., 21(1): 76-78 (in Russian).
MUNSON, A.E., SANDERS, V.M., DOUGLAS, K.A., SAIN, L.E.,
KAUFFMANN, B.M., & WHITE, K.L. (1982) In vivo assessment of
immunotoxicity. Environ. Health Perspect., 43: 41-52.
NACHTOMI, E., ALUMOT, E., & BONDI, A. (1966) The metabolism of
ethylene dibromide in the rat. I. Identification of detoxifi-
cation products in urine. Isr. J. Chem., 4: 329-246.
NATSYUK, M.V. & CHEKMAN, I.S. (1975) [Content of nicotinamide
coenzymes in the liver and myocardium of rats poisoned with
dichloroethane.] Byull. eksp. Biol. Med., 79: 58-60 (in
Russian).
NATSYUK, M.V. & MUDRITSKY, A.D. (1974) Clinical and
biochemical characteristics of hepatic and renal disorders in
acute dichloroethane intoxications. Mil. med. J., 10: 48-50.
NATSYUK, M.V., CHERNUKHA, F.A., BASENKO, G.A., & FEDUROV, V.V.
(1974) [Effect of acute dichloroethane intoxication on
oxidative phosphorylation in rat liver mitochondria.] Farmakol.
Toksikol., 37: 92-93 (in Russian).
NAZIKHI, F. & SKRIZHINSKY, S.F. (1973) Activity of succinate-
toxicodase system enzymes of myocardium in experimental
dichloroethane intoxication. In: Current issues of physiology,
pathology, and clinical diseases of the cardiovascular system,
Leningrad, pp. 62-63.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute,
US Department of Health, Education and Welfare, Public Health
Services, National Institute of Health (HEW (NIH) Publication
No. 78-1305).
NELSON, G.O. & SHAPIRO, E.G. (1971) A field instrument for
detecting airborne halogen compounds. Am. Ind. Hyg. Assoc. J.,
32: 757-765.
NEVROTSKY, V.K., KASHIN, L.M., KULINSKAYA, I.L., MIKHAILOVSKAYA,
L.F., SHMUTER, L.M., BURLAKA-VOVK, Z.I., & ZADOROZHNY, B.V.
(1971) Comparative toxicity evaluation of several industrial
poisons in protracted inhalation exposure to low concentrations.
In: Proceedings of the 8th Ukrainian Hygiene Congress, Kiev,
1971, pp. 224-226.
NIOSH (1976) Criteria for a recommended standard -
occupational exposure to ethylene dichloride (1,2-dichloro-
ethane), Cincinnati, Ohio, National Institute of Occupational
Safety and Health, US Department of Health, Education and
Welfare, Public Health Services, Center for Disease Control
(DHEW (NIOSH) Publication No. 76-139).
NIOSH (1977) Manual of analytical methods. II. Standards
completion program validated methods, Cincinnati, Ohio, National
Institute of Occupational Safety and Health, Vol. 2, pp. S122/1-
S122/9 (DHEW (NIOSH) Publication No. 77-157-B).
NOETZEL, O. (1944) Lethal poisoning with ethylene dichloride.
Chem. Ztg., 68: 146-147.
NOUCHI, T., MIURA, H., KANAYAMA, M., MIZUGUCHI, O., & TAKANO, T.
(1984) Fatal intoxication by 1,2-dichloroethane: a case report.
Int. Arch. occup. environ. Health, 54: 111-113.
NYLANDER, P.-O., OLOFSSON, H., RASMUSON, B., & SVAHLIN, H.
(1978) Mutagenic effects of petrol in Drosophila melanogaster:
effects of benzene and 1,2-dichloroethane. Mutat. Res., 57:
163-167.
OKAMOTO, T. & TATSUKAWA, R. (1981) [Distribution and fate of
low molecular weight chlorinated hydrocarbons in Osaka Bay.]
Chikyukagaku, 15: 17-24 (in Japanese).
OZAWA, N. & GUENGERICH, F.P. (1983) Evidence for formation of
an S-[2-(N7-guanyl)ethyl] glutathione adduct in glutathione-
mediated binding of the carcinogen 1,2-dibromoethane to DNA.
Proc. Natl Acad. Sci. (USA), 80: 5268-5270.
PAGE, B.D. & KENNEDY, B.P.C. (l975) Determination of methylene
chloride, ethylene dichloride and trichloroethylene as solvent
residues in spice oleoresins, using vacuum distillation and
electron capture. J. Assoc. Off. Anal. Chem., 58: 1062-1068.
PAPAROPOLI, G. & CALI, V. (l956) [Collective intoxications
with chlorinated hydrocarbons in dock workers.] Folia Med., 39:
819-831 (in Italian).
PARKES, D.G., GANZ, C.R., POLINSKY, A., & SCHULZE, J. (l976) A
simple gas chromatographic method for the analysis of trace
organics in ambient air. Am. Ind. Hyg. Assoc. J., 37: 165-173.
PEARSON, C.R. & MCCONNELL, G. (l975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proc. R. Soc. Lond. B,
189: 305-332.
PEROCCO, P. & PARODI, G. (1981) DNA damage by haloalkanes in
human lymphocytes cultures in vitro. Cancer Lett., 13: 213-218.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
PRINCIPE, P., DOGLIOTTI, E., BIGNAMI, M., CREBELLI, R., FALCONE,
E., FABRIZI, M., CONTI, G., & COMBA, P. (1981) Mutagenicity of
chemicals of industrial and agricultural relevance in
Salmonella, Streptomyces, and Aspergillus. J. Sci. food Agric.,
32: 826-832.
RADDING, S.B., LUI, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA report no. 560/5-77-003, PB 267121.
RANNUG, U. (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichoroethane. Mutat. Res., 76: 269-295.
RANNUG, U. & BEIJE, B. (1979) The mutagenic effect of 1,2-
dichloroethane on Salmonella typhimurium. II. Activation by the
isolated perfused rat liver. Chem.-biol. Interact., 24: 265-
285.
RANNUG, U., SUNDVALL, A., & RAMEL, C. (1978) The mutagenic
effect of 1,2-dichloroethane on Salmonella typhimurium. I.
Activation through conjunction with glutathione in vitro. Chem.-
biol. Interact., 20: 1-16.
RAO, K.S., MURRAY, J.S., DEACON, M.M., JOHN, J.A., CALHOUN,
L.L., & YOUNG, J.T. (1980) Teratogenicity and reproduction
studies in animals inhaling ethylene dichloride. In: Ames, B.,
Infante, P., & Reitz, R., ed. Ethylene dichloride: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 145-161 (Banbury Report No. 5).
RAPOPORT, I.A. (1960) [The reaction of genic proteins with
1,2-dichloroethane.] Dokl. Akad. Nauk. SSSR, 134: 1214-1217 (in
Russian).
REITZ, R.H., FOX, T.R., RAMSEY, J.C., QUAST, J.F., LANGVARDT,
P.W., & WATANABE, P.G. (1982) Pharmacokinetics and macro-
molecular interactions of ethylene dichloride in rats after
inhalation or gavage. Toxicol. appl. Pharmacol., 62: 190-204.
RICHTER, J.E., PETERSON, S.F., & KLEINER, C.F. (1983) Acute
and chronic toxicity of some chlorinated benzenes, chlorinated
ethanes and tetrachloroethylene to Daphnia magna. Arch. environ.
Contam. Toxicol., 12: 679-684.
ROHMANN, E., VON, ZINN, D., & KULZ, J. (1969) [Electro-
encephalographic observations in child blood poisonings and
their therapeutic consequences.] Kinderaerztl. Prax., 37: 209-
216 (in German).
ROSENBERG, R. (1972) Effects of chlorinated aliphatic hydro-
carbons on larval and juvenile Balanus balanoides L. Environ.
Pollut., 3: 313-318.
ROSENBERG, R., GRAHN, O., & JOHANSSON, L. (1975) Toxic effects
of aliphatic chlorinated by-products from vinyl chloride
production on marine animals. Water Res., 9: 607-612.
ROUBAL, J. (1947) Two fatal cases of intoxication with
symmetric dichloroethane ingestion. Cas. Lek. Cesk., 86: 203-
206.
SAITANOV, A.O. & ARSENIEVA, S.S. (1969) Electrocardiographic
changes due to acute dichloroethane poisoning: experimental
investigation. Gig. Tr. Prof. Zabol., 7: 49-50.
SALTZMAN, B.E. (1972) Direct reading colorimetric indicators.
In: Air sampling instruments for evaluation of atmospheric
contamination, 4th ed., Cincinnati, Ohio, American Conference of
Governmental Hygienists, pp. S22-23.
SAUER, T.C. (1981) Volatile organic compounds in open ocean
and coastal surface waters. Org. Geochem., 3: 91-101.
SCHASTEEN, C.S. & REED, D.J. (1983) The hydrolysis and
alkylation activities of S-(2-haloethyl)-L-cysteine analogues -
evidence for extended half-life. Toxicol. appl. Pharmacol., 70:
423-432.
SCHERB, K. (1978) [Investigations on the evaporation of low
molecular weight chlorinated hydrocarbons from flow-gutter.]
Munch. Beitr. Abwasser-Fisch. Flussbiol., 30: 235-248 (in
German).
SCHONBORN, H., PRELLWITZ, W., & BAUM, P. (1970) [Consumption
coagulation pathology of 1,2-dichloroethane poisoning.] Klin.
Wochenschr., 48: 822-824 (in German).
SECCHI, G.C., CHIAPPINO, G., LOTTO, A., & ZURLO, N. (1968)
[Actual chemical composition of the "commercial trieline" and
their hepatotoxic effect: clinical and enzymological studies.]
Med. Lav., 59: 486-497 (in Italian).
SEUFERT, F.B., BROWN, P., OATWAY, J.A., BORNSTEIN, M.,
OSTROWSKI, W., & HORNE, R. (1980) 1,2-Dichloroethane technical
control options analysis, Bedford, Massachusetts, CGA
Corporation (EPA Contract No. 68-01-5960).
SHAKARNIS, V.F. (1969) [1,2-Dichloroethane-induced chromosome
non-disjunction and recessive sex-linked lethal mutations in
Drosophila melanogaster.] Genetika, 5: 89-95 (in Russian).
SHAKARNIS, V.F. (1970) [Effect of 1,2-dichloroethane on
chromosome non-disjunction and recessive sex-linked lethals in a
radioresistant strain of Drosophila melanogaster.] [ Vestn.
Leningrad Univ. Biol.,] 25: 153-156 (in Russian).
SHMUTER, L.M. (1977) [Effect of chronic exposure to low
concentrations of chlorinated hydrocarbons of the ethane series
on specific and non-specific reactivity of animals in vivo.]
Gig. Tr. Prof. Zabol., 8: 38-42 (in Russian).
SIMMON, V.F. (1980) Review of non-bacterial tests of the
genotoxic activity of ethylene chloride. In: Ames, B., Infante,
P., & Reitz, R., ed. Ethylene chlorde: a potential health risk?,
Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp. 97-103 (Banbury Report No. 5).
SINGH, H.B., SALAS, L.J., & STILES, R.E. (1982) Distribution
of selected gaseous organic mutagens and suspect carcinogens in
ambient air. Environ. Sci. Technol., 16: 872-880.
SINGH, H.B., SALAS, L.J., & STILES, R.E. (1983) Selected man-
made halogenated chemicals in the air and oceanic environment.
J. geophys. Res., 88: 3675-3683.
SOPIKOV, N.G. & GORSHUNOVA, A.I. (1979) [Investigation of the
intake, distribution and excretion of ethylene dichloride in
rats.] Gig. Tr. Prof. Zabol., 4: 36-40 (in Russian).
SPENCE, J.W. & HANST, P.L. (1978) Oxidation of chlorinated
ethanes. J. Air Pollut. Control Assoc., 28: 250-253.
SPENCER, H.C., ROWE, V.K., ADAMS, E.M., MCCOLLISTER, D.D., &
IRISH, D.D. (1951) Vapor toxicity of ethylene dichloride
determined by experiments on laboratory animals. Arch. ind.
Hyg. occup. Med., 4: 482-493.
SPREAFICO, F., ZUCCATO, E., MARCUCCI, F., SIRONI, M.,
PAGLIALUNGA, S., MADONNA, M., & MUSSINI, E. (1980) Pharmaco-
kinetics of ethylene dichloride in rats treated by different
routes and its long-term inhalatory toxicity. In: Ames, B.,
Infante, P., & Reitz, R., ed. Ethylene chlorde: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 107-129 (Banbury Report No. 5).
STORER, R.D. & CONOLLY, R.B. (1983) Comparative in vivo
genotoxicity and hepatotoxicity of three 1,2-dihaloethanes.
Carcinogenesis, 4: 1491-1494.
STORER, R.D. & CONOLLY, R.B. (1985) An investigation of the
role of microsomal oxidative metabolism in the in vivo
genotoxicity of 1,2-dichloroethane. Toxicol. appl. Pharmacol.,
77: 36-46.
STORER, R.D., JACKSON, N.M., & CONOLLY, R.B. (1984) In vivo
genotoxicity and acute hepatotoxicity of 1,2-dichloroethane in
mice: comparison of oral, intraperitoneal, and inhalation routes
of exposure. Cancer Res., 44: 4267-4271.
STOREY, C.L., KIRK, L.D., & MUSTAKAS, G.C. (1972) Fate of EDC-
CC14 (75:25) residues during milling and oil extraction of
soybeans. J. econ. Entomol., 65: 1126-1129.
STOVER, E.L. & KINCANNON, D.F. (1983) Biological treatability
of specific organic compounds found in chemical industry
wastewaters. J. Water Pollut. Control Fed., 55: 97-105.
STUCKEY, D.C., OWEN, W.F., MCCARTY, P.L., & PARKIN, G.F. (1980)
Anaerobic toxicity evaluation by batch and semi-continuous
assays. J. Water Pollut. Control Fed., 52: 720-729.
STUCKI, G., BRUNNER, W., STAUB, D., & LEISINGER, T. (1981)
Microbial degradation of chlorinated C1 and C2 hydrocarbons.
In: Leisinger, T., Cook, A.M., Hütter, R., & Nüesch, J., ed.
FEMS Symposium No. 12, Microbial degradation of xenobiotics and
recalcitrant compounds, London, Academic Press, pp. 131-137.
SUNDHEIMER, D.W., WHITE, R.D., BRENDEL, K., & SIPES, I.C.
(1982) The bioactivation of 1,2-dibromoethane in rat
hepatocytes: covalent binding to nucleic acids. Carcinogenesis,
3: 1129-1133.
SUVEEV, I.M. & BABICHENKO, M.E. (1969) [On the clinic and cure
of acute intoxication with dichloroethane vapours.] Gig. Tr.
Prof. Zabol., 13: 50-51 (in Russian).
SYMONS, J.M., BELLAR, T.A., CARSWELL, J.K., DEMARCO, J., KROPP,
K.L., ROBECK, G.G., SEEGER, D.R., SLOCUM, C.J., SMITH, B.L., &
STEVENS, A.A. (1975) National organics reconnaissance survey
for halogenated organics. J. Am. Water Works Assoc., 67: 634-
648.
TABAK, H.H., QUAVE, S.A., MASHNI, C.E., & BARTH, E.F. (1981)
Biodegradability studies with organic priority pollutant
compounds. J. Water Pollut. Control Fed., 53: 1503-1517.
TAN, E.-L. & HSIE, A.W. (1981) Mutagenicity and cytotoxicity
of haloethanes as studied in the CHO-HGPRT system. Mutat. Res.,
90: 183-191.
THORUP, S. (1957) Breaking dormancy of beech. Physiol. Plant,
10: 728-731.
TROISI, F.M. & CAVALLAZZI, D. (1961) [A fatal case of
poisoning from inhalation of dichloroethane vapours.] Med.
Lav., 52: 612-618 (in Italian).
TSANI-BAZACA, E., MCINTYRE, A.E., LESTER, J.N., & PERRY, R.
(1981) Concentrations and correlations of 1,2-dibromoethane,
1,2-dichloroethane, benzene, and toluene in vehicle exhaust and
ambient air. Sci. Technol. Lett., 2: 303-316.
TSANI-BAZACA, E., MCINTYRE, A.E., LESTER, J.N., PERRY, R.
(1982) Ambient concentrations and correlations of hydrocarbons
and halocarbons in the vicinity of an airport. Chemosphere, 11:
11-23.
TSURUTA, H. (1975) Percutaneous absorption of organic solvents
I. Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind. Health, 13: 227-236.
TU, A.S., MURRAY, T.A., HATCH, K.M., SIVAK, A., & MILMAN, H.A.
(1985) In vitro transformation of BALB/c-3T3 cells by
chlorinated ethanes and ethylenes. Cancer Lett., 28: 85-92.
VAN BLADEREN, P.J., BREIMER, D.D., ROTTEVEEL-SMIJS, G.M.T.,
KNIJFF, P. DE, MOHN, G.R., MEETEREN-WALCHLI, B. VAN, BUIJS, W.,
& VAN DER GEN, A. (1981a) The relation between the structure
of vicinal dihalogen compounds and their mutagenic activation
via conjugation to glutathione. Carcinogenesis, 2: 499-505.
VAN BLADEREN, P.J., HODGETERP, J.J., BREIMER, D.O., & VAN DER
GEN, A. (1981b) The influence of disulfiram and other
inhibitors of oxidative metabolism on the formation of 2-
hydroxyethyl-mercapturic acid from 1,2-dibromoethane by the rat.
Biochem. Pharmacol., 30: 2983-2987.
VAN DUUREN, B.L., GOLDSCHMIDT, B.M., LOEWENGART, G., SMITH,
A.C., MELCHLONNE, S., LEIDMAN, I., & ROTH, D. (1979)
Carcinogenicity of halogenated olefinic and aliphatic
hydrocarbons in mice. J. Natl Cancer Inst., 63: 1433-1439.
VAN ESCH, G.J., KROES, R., LOGTEN, M.J., VAN, & TONKELAAR, E.M.,
DEN (1977) Ninety-day toxicity study with 1,2-dichloroethane
(DCE) in rats, Bilthoven, the Netherlands, National Institute of
Public Health and Environmental Hygiene (Report 195/77 Al.
Tox.).
VARGA, M.B. & FERENCZY, L. (1956) Effect of "Rindite" on the
development of the growth-substances in potato tubers. Nature
(Lond.), 175: 1075.
VOSOVAYA, M.A. (1974) [Development of posterity of two
generations obtained from females subjected to the action of
dichloroethane.] Gig. i Sanit., 7: 25-28 (in Russian).
VOSOVOYA, M.A. (1977) [Effect of dichloroethane on the
reproductive cycle and embryogenesis in experimental animals.]
Akush. Ginekol., 2: 57-59 (in Russian).
WALBRIDGE, C.T., FIANDT, J.T., PHIPPS, G.L., & HOLCOMBE, G.W.
(1983) Acute toxicity of ten chlorinated aliphatic hydrocarbons
to the fathead minnow (Pimephales promelas). Arch. environ.
Contam. Toxicol., 12: 661-666.
WARD, J.M. (1980) The carcinogenicity of ethylene dichloride
in Osborne-Mendel rats and B6C3F1 mice. In: Ames, B., Infante,
P., & Reitz, R., ed. Ethylene dichloride: a potential health
risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 35-53 (Banbury Report No. 5).
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. eng. News, May 7: 8-10.
WEISS, F. (1957) [Lethal oral intoxications by
dichloroethane.] Gewerbehygiene, 15: 253-264 (in German).
WHO (1972) Evaluations of some pesticide residues in food,
Geneva, World Health Organization, pp. 276-279, 325-329 (The
Monographs: WHO Pesticide Residues Series, No. 1).
WIRTSCHAFTER, Z.T. & SCHWARTZ, W.D. (1939) Acute ethylene
dichloride poisoning. J. ind. Hyg. Toxicol., 21: 126-131.
WIT, S.L., BESEMER, F.H., DAS, H.A., GOEDKOOP, W., LOOSJES,
F.E., & MEPPELIN, E.K. (1969) Results of an investigation on
the regression of three fumigants (carbon tetrachloride,
ethylene dibromide and ethylene dichloride) in wheat during
processing to bread, Bilthoven, The Netherlands, National
Institute of Public Health and Environmental Hygiene (Report No.
36/69 Tox).
WITHEY, J.R. & COLLINS, B.T. (1980) Chlorinated aliphatic
hydrocarbons used in the foods industry: the comparative
pharmacokinetics of methylene chloride, 1,2-dichloroethane,
chloroform, and trichloroethylene after iv administration in the
rat. J. environ. Pathol. Toxicol., 3: 313-332.
WITHEY, J.R. & KARPINSKI, K. (1985) The fetal distribution of
some aliphatic chlorinated hydrocarbons in the rat after vapor
phase exposure. Biol. Res. Preg., 6(2): 79-88.
WOLFF, D.L., IVANOV, N.G., KLJACKINA, A.M., & MEL'NIKOVA, L.V.
(1979) [Evidence of the effect of significant industrial
toxicological substances on the nervous system by animal
behavioural tests.] Zool. Jahrb. Physiol., 83: 82-91 (in
German).
YLLNER, S. (1971) Metabolism of 1,2-dichloroethane-4C in the
mouse. Acta pharmacol. toxicol., 30: 257-265.
YODAIKEN, R.E. & BABCOCK, J.R. (1973) 1,2-Dichloroethane
poisoning. Arch. environ. Health, 26: 281-284.
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly-volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
ZOETEMAN, B.C.J., PIET, G.J., SLINGERLAND, P., FONDS, A.W., &
WEGMAN, R.C.C. (1979) [ Investigation into the presence of
volatile organic halogenated compounds in groundwater used as a
drinking water supply. Final report: results on the period
November 1976 to August 1978,] Bilthoven, The Netherlands,
National Institute of Drinking Water Supply, National Institute
of Public Health and Environmental Hygiene (Report no. RID-CBH-
79/01 and no. RIV 10/79) (in Dutch).
ZUCCATO, E., MARCUCCI, F., & MUSSINI, E. (1980) GLC
determination of ethylene dichloride (EDC) in biological
samples. Anal. Lett., 13(B5): 371-379.
 Rannug et al. (1978) first reported that mutagenic compounds
could be formed by the reaction of GSH with 1,2-dihaloalkanes in
the presence of cytosolic glutathione-S-transferases. This
observation led workers to investigate in greater detail the
role of glutathione-S-transferases in the metabolism and bio-
activation of both dibromoethane (DBE) and 1,2-dichloroethane
(Rannug, 1980; Sundheimer et al., 1982; Ozawa & Guengerich,
1983; Inskeep & Guengerich, 1984). This pathway (Fig. 1)
involves the direct reaction of GSH with 1,2-dichloroethane to
form S-(2-chloroethyl) glutathione, which is a half mustard with
a half-life of 69 min at 20 °C (Schasteen & Reed, 1983) and less
than 15 min at 37 °C (Foureman & Reed, 1985). Non-enzymic
conversion of the half mustard to the corresponding episulfonium
ion gives a putative alkylating agent (episulfonium ion) that
has several fates (Fig. 1). Reaction can occur with water to
form S-(2-hydroxyethyl) glutathione or reaction with thiols such
as GSH to form ethene bis-glutathione or with DNA to form
adducts. With the exception of DNA adducts, the reaction pro-
ducts are considered non-toxic and undergo further metabolism.
These reactions and subsequent metabolism of the products can
account for all of the known sulfur-containing metabolites found
in the urine of 1,2-dichloroethane-treated animals.
Although much evidence has been reported that supports the
P-450 mediated metabolism of 1,2-dihaloethanes, this branch of
the pathway (Fig. 1) does not appear relevant to DNA adduct
formation by 1,2-dichloroethane (Koga et al., 1986). Guengerich
et al. (1980) proposed the possibility of chloroso oxidation
products of 1,2-dichloroethane in DNA adduct formation (Fig. 1).
However, they observed that the apparent stimulation of P-450-
directed DNA adduct formation by GSH was a result of incomplete
removal of GSH conjugates during analysis (Koga et al., 1986).
In addition, they concluded that 2H and 18O studies on the
formation of 2-haloethanols and 2-haloacetaldehydes from 1,2-
dihaloethanes are inconsistent with a major role of such a
mechanism for DNA damage (Guengerich et al., 1986; Koga et al.,
1986).
It should be pointed out that the P-450 directed pathway can
presumably form considerable quantities of 2-haloacetaldehydes,
which readily bind to protein and non-protein thiols, as shown
for vinyl bromide and vinyl chloride (Guengerich et al., 1981)
and dibromoethane (DBE) (van Bladeren et al., 1981).
Although some DNA damage can be produced via the P-450 path-
way under in vitro conditions (Hill et al., 1978; Banerjee et
al., 1980; Guengerich et al., 1980; Lin et al., 1985), several
lines of evidence suggest that the GSH conjugation pathway is
probably of greater significance than the P-450 pathway as the
major in vivo route for DNA damage (Guengerich et al., 1980;
Rannug, 1980; Sundheimer et al., 1982; Inskeep et al., 1986).
It has been possible to correlate the 1,2-dichloroethane-
induced mutation frequency of two human cell lines with the
difference in levels of glutathione-S-transferase activities.
AHH-1 cell line mutation frequency was 25 times that in the TK6
cell line in the presence of 1,2-dichloroethane. The difference
was attributed to the fact that the AHH-1 cell line possesses 5
times more glutathione-S-transferase activity than the TK6 cell
line (Crespi et al., 1985).
Male B6C3F1 mice, pretreated with piperonyl butoxide (PIB),
were examined for the extent of hepatic DNA damage produced 4 h
after 1,2-dichloroethane administration (Storer & Conolly,
1985). PIB is a P-450 inhibitor. Hepatic DNA damage, as
measured by the alkaline DNA unwinding assay for single-strand
breaks and alkali-labile lesions, was potentiated by PIB.
Treatment of mice with high doses of 2-chloroethanol failed to
produce DNA damage, as measured by this assay. Diethylmaleate,
a GSH depletor, potentiated the hepatotoxicity of 2-chloro-
ethanol but not DNA damage. Although the significance of this
observation is uncertain, it is not inconsistent with the
hypothesis that reduction of GSH levels is associated with a
reduction in DNA damage.
Recent evidence suggests that the putative episulfonium ion,
resulting from a non-enzymatic conversion of S-(2-chloroethyl)
glutathione, is a major intermediate in the formation of DNA
adducts in vivo from 1,2-dichloroethane exposures (Fig. 2)
(Inskeep et al., 1986). When rats were administered a single
dose of 14C-1,2-dichloroethane in vivo and the liver analysed 8
h later, 78% of the DNA adducts (0.25 nmol/mg DNA) could be
released by neutral thermal hydrolysis. A major adduct and
several minor adducts were present; the major adduct co-
chromatographed with S-[2-(N7-guanyl)ethyl] glutathione. DNA
adducts released from kidney preparations by neutral thermal
hydrolysis were represented by 5 different fractions containing
radioactivity after chromatography. The postulated adduct of
liver DNA after 14C-1,2-dichloroethane exposure, S-[2-(N7-
guanyl)ethyl] glutathione, appears to be chromatographically
identical to the major adduct in rats after exposure to DBE
(Koga et al., 1986). This DBE adduct, which has been isolated
and characterized by NMR and mass spectrometry, gives strong
support to an identical adduct being the principal DNA adduct
from exposure to 1,2-dihaloethanes. The formation of apurinic
sites, as this adduct cleaves from DNA, may be a key factor in
the mutagenic and carcinogenic effects of these compounds.
Rannug et al. (1978) first reported that mutagenic compounds
could be formed by the reaction of GSH with 1,2-dihaloalkanes in
the presence of cytosolic glutathione-S-transferases. This
observation led workers to investigate in greater detail the
role of glutathione-S-transferases in the metabolism and bio-
activation of both dibromoethane (DBE) and 1,2-dichloroethane
(Rannug, 1980; Sundheimer et al., 1982; Ozawa & Guengerich,
1983; Inskeep & Guengerich, 1984). This pathway (Fig. 1)
involves the direct reaction of GSH with 1,2-dichloroethane to
form S-(2-chloroethyl) glutathione, which is a half mustard with
a half-life of 69 min at 20 °C (Schasteen & Reed, 1983) and less
than 15 min at 37 °C (Foureman & Reed, 1985). Non-enzymic
conversion of the half mustard to the corresponding episulfonium
ion gives a putative alkylating agent (episulfonium ion) that
has several fates (Fig. 1). Reaction can occur with water to
form S-(2-hydroxyethyl) glutathione or reaction with thiols such
as GSH to form ethene bis-glutathione or with DNA to form
adducts. With the exception of DNA adducts, the reaction pro-
ducts are considered non-toxic and undergo further metabolism.
These reactions and subsequent metabolism of the products can
account for all of the known sulfur-containing metabolites found
in the urine of 1,2-dichloroethane-treated animals.
Although much evidence has been reported that supports the
P-450 mediated metabolism of 1,2-dihaloethanes, this branch of
the pathway (Fig. 1) does not appear relevant to DNA adduct
formation by 1,2-dichloroethane (Koga et al., 1986). Guengerich
et al. (1980) proposed the possibility of chloroso oxidation
products of 1,2-dichloroethane in DNA adduct formation (Fig. 1).
However, they observed that the apparent stimulation of P-450-
directed DNA adduct formation by GSH was a result of incomplete
removal of GSH conjugates during analysis (Koga et al., 1986).
In addition, they concluded that 2H and 18O studies on the
formation of 2-haloethanols and 2-haloacetaldehydes from 1,2-
dihaloethanes are inconsistent with a major role of such a
mechanism for DNA damage (Guengerich et al., 1986; Koga et al.,
1986).
It should be pointed out that the P-450 directed pathway can
presumably form considerable quantities of 2-haloacetaldehydes,
which readily bind to protein and non-protein thiols, as shown
for vinyl bromide and vinyl chloride (Guengerich et al., 1981)
and dibromoethane (DBE) (van Bladeren et al., 1981).
Although some DNA damage can be produced via the P-450 path-
way under in vitro conditions (Hill et al., 1978; Banerjee et
al., 1980; Guengerich et al., 1980; Lin et al., 1985), several
lines of evidence suggest that the GSH conjugation pathway is
probably of greater significance than the P-450 pathway as the
major in vivo route for DNA damage (Guengerich et al., 1980;
Rannug, 1980; Sundheimer et al., 1982; Inskeep et al., 1986).
It has been possible to correlate the 1,2-dichloroethane-
induced mutation frequency of two human cell lines with the
difference in levels of glutathione-S-transferase activities.
AHH-1 cell line mutation frequency was 25 times that in the TK6
cell line in the presence of 1,2-dichloroethane. The difference
was attributed to the fact that the AHH-1 cell line possesses 5
times more glutathione-S-transferase activity than the TK6 cell
line (Crespi et al., 1985).
Male B6C3F1 mice, pretreated with piperonyl butoxide (PIB),
were examined for the extent of hepatic DNA damage produced 4 h
after 1,2-dichloroethane administration (Storer & Conolly,
1985). PIB is a P-450 inhibitor. Hepatic DNA damage, as
measured by the alkaline DNA unwinding assay for single-strand
breaks and alkali-labile lesions, was potentiated by PIB.
Treatment of mice with high doses of 2-chloroethanol failed to
produce DNA damage, as measured by this assay. Diethylmaleate,
a GSH depletor, potentiated the hepatotoxicity of 2-chloro-
ethanol but not DNA damage. Although the significance of this
observation is uncertain, it is not inconsistent with the
hypothesis that reduction of GSH levels is associated with a
reduction in DNA damage.
Recent evidence suggests that the putative episulfonium ion,
resulting from a non-enzymatic conversion of S-(2-chloroethyl)
glutathione, is a major intermediate in the formation of DNA
adducts in vivo from 1,2-dichloroethane exposures (Fig. 2)
(Inskeep et al., 1986). When rats were administered a single
dose of 14C-1,2-dichloroethane in vivo and the liver analysed 8
h later, 78% of the DNA adducts (0.25 nmol/mg DNA) could be
released by neutral thermal hydrolysis. A major adduct and
several minor adducts were present; the major adduct co-
chromatographed with S-[2-(N7-guanyl)ethyl] glutathione. DNA
adducts released from kidney preparations by neutral thermal
hydrolysis were represented by 5 different fractions containing
radioactivity after chromatography. The postulated adduct of
liver DNA after 14C-1,2-dichloroethane exposure, S-[2-(N7-
guanyl)ethyl] glutathione, appears to be chromatographically
identical to the major adduct in rats after exposure to DBE
(Koga et al., 1986). This DBE adduct, which has been isolated
and characterized by NMR and mass spectrometry, gives strong
support to an identical adduct being the principal DNA adduct
from exposure to 1,2-dihaloethanes. The formation of apurinic
sites, as this adduct cleaves from DNA, may be a key factor in
the mutagenic and carcinogenic effects of these compounds.
 5.4 Excretion and Elimination
Excretion of 1,2-dichloroethane from rodents is rapid.
Approximately 89% or more of the body burden of the compound was
excreted within 24 h in ip-injected mice (Yllner, 1971), within
48 h in orally exposed mice (Mitoma et al., 1985), and within
48 h in rats exposed orally or via inhalation (Reitz et al.,
1982; Mitoma et al., 1985). In the 3 studies cited above,
excretion of 1,2-dichloroethane or its metabolites mainly
occurred in exhaled air via the lungs and in urine via the
kidneys. In both species, and at various exposure levels, 7 -
18% of the metabolized 1,2-dichloroethane was excreted as carbon
dioxide (CO2) and approximately 80 - 85% as non-volatile
metabolites (Table 6). The metabolism of 1,2-dichloroethane in
mice and rats is dose-dependent. For example, in mice, 11 and
45% of the body burdens of 1,2-dichloroethane, resulting from ip
exposure to 50 and 170 mg/kg body weight, respectively, were
excreted unchanged via the lungs within 72 h (Yllner, 1971). In
rats, 1.8, 11.5, and 29% of the body burdens were excreted
unchanged via the lungs within 48 h following: an inhalation
exposure at 607 mg/m3 for 6 h (equivalent to a dose of 113 mg/kg
body weight) (Reitz et al., 1982), an oral dose of 100 mg/kg
body weight (Mitoma et al., 1985), and an oral dose of 150 mg/kg
body weight (Reitz et al., 1982), respectively.
The rate of elimination from blood and tissues appears to
depend on the exposure level; the higher the exposure level, the
slower the elimination rate of 1,2-dichloroethane, after both
oral and inhalation exposure. Half-lives in the blood of rats,
exposed orally, increased from 25 min at 25 mg/kg body weight to
57 min at 150 mg/kg body weight. With inhalation exposure,
half-lives increased from 13 min at 202 mg/m3 to 22 min at 1012
mg/m3, after a 6-h inhalation exposure (Spreafico et al., 1980).
In addition, after oral exposure of rats to 150 mg/kg body
weight, an initial half-life of 90 min in blood decreased to
20 - 30 min, when blood levels fell below 5 - 10 mg/litre after
3 h (Reitz et al., 1982). Elimination of 1,2-dichloroethane
from blood, adipose tissue, lung, liver, brain, kidneys, and
spleen was comparable after oral exposures of up to 150 mg/kg
body weight. Elimination from the liver was reported to be
biphasic with a higher elimination rate just after reaching peak
levels of 1,2-dichloroethane. Elimination from other organs was
monophasic. Following inhalation, elimination was the slowest
in adipose tissue and the most rapid in the lung, up to an
exposure level of 1012 mg/m3 (Spreafico et al., 1980). Withey &
Collins (1980) also reported that the elimination of 1,2-
dichloroethane was dose-dependent. After iv administration of
from 3 to 15 mg/kg body weight to male Wistar rats, the authors
found that the elimination fitted a two-compartment model at a
low-dose level and a three-compartment model at high-dose
levels.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Aquatic Organisms
6.1.1 Acute toxicity
The acute toxicity of 1,2-dichloroethane for aquatic
organisms is summarized in Table 7. The concentration of 1,2-
dichloroethane was measured in four of the studies cited (see
footnotea in Table 7); the concentrations reported in the
other studies were nominal. It should be noted that, in open
systems, the toxic effects observed must have occurred at
concentrations lower than the nominal ones reported in latter
studies, due to the anticipated evaporation of 1,2-dichloro-
ethane in the aquatic media.
The species most sensitive to 1,2-dichloroethane were
members of the class Crustacea. A no-observed-adverse-effect
level below 68 mg/litre was found for Daphnia magna (Le Blanc,
1980). The shrimp Crangon crangon showed a 96-h LC50 of
85 mg/litre in sea water, measured by the flow-through method
(Adema, 1976). When the brine shrimp Artemia salina was exposed
to 1,2-dichloroethane at levels ranging from 0.25 to
25 mg/litre, growth inhibition was noted 24 h after cyst wetting
(Kerster & Schaeffer, 1983).
EDC tar is much more toxic for marine species than 1,2-
dichloroethane, the heavy fractions of the tar being responsible
for the high toxicity observed (Jernelöv et al., 1972; Rosen-
berg, 1972; Jensen et al., 1975; Rosenberg et al., 1975).
6.1.2 Short-term exposures
When blue algae Mycrocystis aerigunosa and green algae
Scenedesmus quadricauda were exposed in closed containers to
1,2-dichloroethane at 105 and 710 mg/litre, respectively, for 8
days, cell multiplication started to be inhibited (Bringmann &
Kühn, 1978). Guppies (Poecilia reticulata) were exposed for 7
days in a static test, and an LC50 of 106 mg/litre was found.
Solutions were renewed daily, but no water analysis data were
reported (Könemann, 1981). Finally, an early life stage flow-
through test was done. Fathead minnows (Pimephales promelas)
were exposed to concentrations of between 4 and 56 mg/litre
beginning from 2 to 5 days after spawning and continuing
throughout the subsequent embryonal, larval, and juvenile stages
up to 28 days after hatching. Water was analysed for 1,2-
dichloroethane. Body weight was reduced at 59 mg/litre. The
survival of juveniles, the percentage of normal larvae at hatch,
and the hatchability of embryos were not affected (Benoit et
al., 1982).
Table 7. Acute aquatic toxicity
---------------------------------------------------------------------------------------------------------
Organism Description t (°C) pH Dissolved Hardness Flow/b Parameter Concen- Reference
oxygen (mg CaCO3/ stat tration
(mg/litre) litre) open/ (mg/litre)
closed
---------------------------------------------------------------------------------------------------------
Fresh water
-----------
Bacteria Pseudomonas 25 7 stat 16-h MICc 135 Bringmann
putida closed & Kühn
(1980)
Protozoa Entosipon sulc- 25 7 stat 72-h MIC 943-1127 Bringmann
atum, Uronema closed & Kühn
parduczi, Chilo- (1981)
monas paramecium
Crustacea water flea 22 6.7-8.1 6.5-9.1 72 stat 24-h LC50 250 Le Blanc
(Daphnia magna) open no-observed- < 68 (1980)
adverse-
effect level
Crustacea water flea 20 8.0 > 2 - stat 24-h EC50 540a,d Bringmann
(Daphnia magna) open & Kühn
(1982)
Crustacea water flea 20 7.1-7.7 7.9-9.9 44.7 stat 48-h LC50 270a,d Richter
(Daphnia magna) closed 48-h EC50 160a,d et al.
20 7.0-7.5 4.1-8.4 44.7 stat 48-h LC50 320a,e (1983)
closed 48-h EC50 180a,e
Fish bluegill sunfish 21-23 6.5-7.9 32-48 stat 96-h LC50 430 Buccafusco
(Lepomis macro- closed et al.
chirus) (1981)
Fish bluegill sunfish 23 7.6-7.9 55 stat 96-h LC50 550 Dawson
(Lepomis macro- open et al.
chirus) (1977)
Fish fathead minnow 25 6.7-7.6 8.0 45.1 flow 96-h LC50 116a Walbridge
(Pimephales prom- open et al.
elas) (1983)
Table 7 (contd.)
---------------------------------------------------------------------------------------------------------
Sea water
---------
Alga Phaeodactylum stat EC50 340a Pearson &
tricornutum McConnell
(1975)f
Worm chaetopod 23 stat 96-h LC50 400 Rosenberg
(Ophryotrocha closed et al.
labronica) (1975)g
Crustacea shrimp 15 8.0 > 8.0 flow 96-h LC50 85a Adema
(Crangon crangon) open (1976)
Crustacea shrimp 16 stat 24-h LC50 170 Rosenberg
(Crangon crangon) open et al.
(1975)h
Mollusca barnacle nauplii stat 48-h LC50 186a Pearson &
(Elminius modestus) closed McConnell
(1975)
Fish dab flow 96-h LC50 115a Pearson &
(Limanda limanda) open McConnell
(1975)
Fish tidewater silver- 20 7.6-7.9 55 stat 96-h LC50 480 Dawson
sides (Menidia open et al.
beryllina) (1977)
Fish sheepshead minnow 25-31 stat 96-h LC50 130-230 Heitmüller
(Cyprinodon open no-observed- 130 et al.
variegatus) adverse- (1981)
effect level
Fish goby 15 8.0 > 8.0 flow 96-h LC50 185a Adema
(Gobius minutus) open (1976)
---------------------------------------------------------------------------------------------------------
a Water analysis for 1,2-dichloroethane was reported.
b Flow-through or static method; open or closed system.
c MIC = minimum inhibitory concentration for cell multipication. EC50 and LC50 = concentration,
causing an effect and death, respectively, in 50% of the population.
d Fleas unfed. Effect was complete immobilization.
e Fleas fed. Effect was complete immobilization.
f Effect was growth inhibition, measured by 4C uptake during photosynthesis.
g When the concentration was gradually built up during the first hour, the 96-h LC50 was
900 mg/litre, and the percentage of hatched eggs, laid within 15 days, was decreased by 90% at
400 mg/litre. The latter was not noted at 200 mg/litre.
h Concentration was gradually built up during the first hour.
6.1.3 Long-term exposure
No-observed-adverse-effect and effect concentrations were
determined on the basis of reproduction or length for Daphnia
magna in a 28-day test, in stoppered flasks. The test solution
was analysed by gas chromatography. The lowest observed adverse
effect concentrations were 21 mg/litre, on the basis of repro-
duction, and 72 mg/litre, on the basis of length. The no-
observed-adverse-effect concentrations were 11 mg/litre, on the
basis of reproduction and 42 mg/litre, on the basis of length
(Richter et al., 1983).
6.1.4 Bioconcentration
Bioconcentration of 1,2-dichloroethane in aquatic species is
unlikely in view of its physical and chemical properties. In a
tracer study, a bioconcentration factor of 2 was found for
bluegill sunfish (Lepomis macrochirus) in flowing water. The
half-life for the elimination of 1,2-dichloroethane from tissues
was 1 - 2 days (Barrows et al., 1980).
When tissues of several aquatic species, collected from near
the discharge zone of a wastewater treatment plant, were ana-
lysed for 1,2-dichloroethane, the concentration of the compound
was less than 0.5 µg/kg wet weight in all cases, while the
average effluent concentration was 41 µg/litre and the average
sediment concentration was less than 0.5 µg/kg dry weight
(Gossett et al., 1983).
6.2 Microorganisms
1,2-Dichloroethane at an influent concentration of
258 mg/litre did not affect the treatment efficiency of a bench-
scale activated sludge system. The compound itself was
virtually completely removed by stripping but not by biodegra-
dation (Stover & Kincannon, 1983). In a batch anaerobic
toxicity assay, 1,2-dichloroethane was slightly toxic to the
anaerobic digestion process from a concentration of
2.5 mg/litre, though acclimation was observed after several
days. A concentration of 20 mg/litre caused more severe
retardation, while acclimation was slow. In semi-continuous
assays, stress became evident at 1,2-dichloroethane concentra-
tions of between 5 and 7.5 mg/litre (Stuckey et al., 1980).
6.3 Terrestrial Organisms
6.3.1 Birds
The effects on reproduction were investigated in groups of
10 male and 20 female white leghorn chickens after 2 years of
oral exposure to 0, 250, or 500 mg 1,2-dichloroethane/kg feed
mash. From the fourth month of laying onwards, decreased egg
weight was observed at both dose levels, while at the higher
dose level, the number of eggs and the feed intake were also
reduced. 1,2-Dichloroethane did not affect serum composition
and growth, semen characteristics, or fertility of chickens
(Alumot et al., 1976b).
6.3.2 Plants
1,2-Dichloroethane is used as a seed fumigant, usually in
combination with compounds such as carbon tetrachloride, 1,2-
dibromomethane, or 2-chloroethanol. Such fumigants inhibited
the germination of seeds (Caswell & Clifford, 1958; Kamel,
1959), broke the dormancy period of potato tubers (Varga &
Ferenczy, 1956; Jolivet, 1968) and beech (Thorup, 1957), and
adversely affected the nodulation status and yield of groundnuts
treated with Rhizobium (Kulkarni et al., 1975). 1,2-Dichloro-
ethane vapour was both lethal and mutagenic for barley seeds at
3 mg/m3 during 24 h (Ehrenberg et al., 1974).
7. EFFECTS ON ANIMALS
7.1 Single Exposures
7.1.1 Inhalation and oral exposure
The available acute mortality data following inhalation and
oral exposure are summarized in Table 8.
Table 8. Acute mortality after inhalation or oral exposure to
1,2-dichloroethane
-----------------------------------------------------------------------------
Species/ Route Vehicle Parameter Result Reference
strain studied
-----------------------------------------------------------------------------
dog oral acacia LD50 2500 mg/kg Barsoum & Saad
gum (1934)
rat oral corn oil LD50 680 mg/kg McCollister et
al. (1956)
CD-1 oral water LD50 489 mg/kg Munson et al.
mouse (1982)
(male)
CD-1 oral water LD50 413 mg/kg Munson et al.
mouse (1982)
(female)
Wistar inhalation 6-h LC50 5100 mg/m3 Spencer et al.
rat (1951)
Sprague inhalation - 6-h LC50 6660 mg/m3 Bonnet et al.
Dawley (1980)
rat
OF1 inhalation 6-h LC50 1060 mg/m3 Gradiski et al.
mouse (1978)
(female)
rat oral LD50 850 mg/kg Larionov &
Kokarovtseva
(1976)
albino inhalation LC50(expo- 30 000 mg/m3 Nevrotsky et al.
rat sure period (1971)
unknown)
-----------------------------------------------------------------------------
Deaths occur within a narrow range of concentrations. In
rats, the difference between the 6-h LC10 and 6-h LC90 was
approximately 2800 mg/m3 (Bonnet et al., 1980). No deaths were
observed in rats after 6 h of exposure to 2000 mg/m3 (Spencer et
al., 1951). In mice, an extremely narrow range of about
500 mg/m3 was observed between the 6-h LC10 and the 6-h LC90
(Gradiski et al., 1978).
Exposure of rats to single high doses of 1,2-dichloroethane
resulted in adverse effects on the CNS, liver, kidneys,
adrenals, and lungs (Spencer et al., 1951). Groups of 10 - 52
Wistar rats were exposed to 81 000, 48 600, 12 100, 6100, 4000,
3200, 2400, or 1200 mg 1,2-dichloroethane/m3 for various lengths
of time. At the highest concentration (81 000 mg/m3), deaths
were observed in animals exposed for 0.3 h or longer. Deaths
were observed at all except the lowest of the other concentra-
tions with exposure periods of 0.4, 0.7, 3.0, 4.0, 7.0, and
7.0 h, respectively. No deaths were observed in rats exposed to
the lowest concentration for 7 h.
Severe depression of the central nervous system resulting in
coma was observed in rats exposed to the highest concentration.
At lower concentrations, this depressant action expressed itself
as various levels of "drunkeness". In this investigation,
Spencer et al. (1951) did not specify the sex of the rats.
In the same publication, the authors reported another study
in which groups of 4 - 6 female rats were exposed to 48 600,
12 100, 4000, 1200, or 800 mg/m3. "Adverse effects" (not
described by the authors) were observed at the 4 highest levels
in rats exposed for 0.2, 0.5, 3.0, and 5.5 h, respectively. No
adverse effects were observed in female rats exposed to the
lowest concentration, for 7 h.
Examination of internal organs in groups of rats killed,
either when moribund or 24 h after the last exposure, showed
that, after exposure to 1,2-dichloroethane levels of 2400 mg/m3
or more, the most severe damage occurred in the kidney and
consisted of haemorrhage and tubular necrosis. The liver showed
fatty changes and hepatocellular necrosis with haemorrhage, and
adrenal glands were haemorrhagic. Lung oedema was observed at
concentrations above 12 100 mg/m3. The injury to organs in this
study was accompanied by high blood-urea levels, a decrease in
serum-phosphatase activity, and increased lipid concentration in
the liver (Spencer et al., 1951).
Depression of the central nervous system was noted during
exposure of rats to 1,2-dichloroethane concentrations exceeding
1200 mg/m3 (Bonnet et al., 1980). From an exposure level of
4000 mg/m3, for 4 h, rats showed altered behaviour. A narcotic
effect was observed at 9100 mg/m3 (Wolff et al., 1979).
Albino rats, given a single oral dose of 615 mg/kg body
weight, showed congested livers with cloudy swelling and fatty
degeneration. The myocardium showed oedema and haemorrhaging in
the walls of the coronary vessels, stasis, and thrombi in the
vessels. These changes were associated with an increased
activity of alanine- and aspartate aminotransferase in the serum
and decreased tissue levels of nicotinamide coenzymes (Natsyuk &
Chekman, 1975). A single administration of 861 mg/kg, by
gavage, was reported to partially uncouple oxidative phosphory-
lation measured in vitro in albino rat livers, 1, 3, or 6 days
after exposure (Natsyuk et al., 1974). In rat liver microsomes,
cytochrome P-450 levels were slightly decreased after an oral
dose of 625 mg/kg (Moody et al., 1981). After a single oral
dose of 1,2-dichloroethane in corn oil at 770 mg/kg body weight
in rats, dystrophy in the cytoplasm and hyperchromatosis in the
nuclei of hepatocytes were observed. A decrease in protein
synthesis and lysosomal enzyme activity was also reported. The
same changes took place in renal nephrons (Boikova & Kravtsova,
1982). A single dose of 850 mg/kg in albino rats resulted in a
decrease in RBC count, haematocrit, and other haematological
changes (Larionov & Kokarovtseva, 1976). Oral administration of
1,2-dichloroethane (615 mg/kg) to rabbits induced pronounced
morphological changes in the liver in about 24 h (Nazikhi &
Skrizhinsky, 1973). Effects on fibrinolytic activity in the
blood of rabbits administered 1,2-dichloroethane at 1476 mg/kg
have been reported by Kagramanov & Kazieva (1972). Electro-
cardiographic changes in albino rats associated with doses of 1,
1.5, and 2 mg/kg have been reported (Saitanov & Arsenieva,
1969).
7.1.2 Skin and eye irritation
When undiluted 1,2-dichloroethane was applied directly on
the clipped skin of guinea-pigs for up to 12 h in occluded patch
tests, no gross skin reactions were visible (Jakobson et al.,
1982). Microscopic changes appeared 4 h after application,
comprising karyopyknosis, perinuclear oedema, spongiosis, and
junctional separation (Kronevi et al., 1981). In similar tests
on rabbits, moderate erythema and oedema were observed, 24 h
after application. Microscopy on the third day revealed
necrosis and other lesions such as ulcerations and acanthosis.
The severity of the changes was not indicated (Duprat et al.,
1976).
Instillation of 0.1 ml of undiluted 1,2-dichloroethane into
the conjunctival sac of the eye of rabbits generated reversible,
mild irritation characterized by conjunctivitis and epithelial
abrasion. Epithelial keratitis, described as being "in a state
of repair", was observed microscopically, 7 days after
application (Duprat et al., 1976). Reversible clouding of the
cornea was observed in dogs within 10 h of subcutaneous
administration of undiluted 1,2-dichloroethane at 0.9/kg body
weight. The clouding continued up to 48 h, but the corneas
appeared clear after 5 days. The histological changes,
including necrosis of the corneal endothelium, partially denuded
Descemet's membrane, formation of excess basement membrane, and
swelling of the corneal stroma, were also observed in dogs,
cats, and rabbits after ocular injection of 1.8 mg 1,2-
dichloroethane (0.15 ml of a 1% solution) into the anterior
chamber (Kuwabara et al., 1968).
7.2 Short-Term Exposures
7.2.1 Inhalation exposure
The effects of repeated exposure to 1,2-dichloroethane have
been studied in mice, rats, guinea-pigs, rabbits, cats, dogs,
and monkeys (Heppel et al., 1946; Spencer et al., 1951; Hofmann
et al., 1971).
Heppel et al. (1946) exposed rats of the Wistar and Osborne-
Mendel strains to 1,2-dichloroethane concentrations of 420, 730,
1540, or 3900 mg/m3 in air, 7 h daily, 5 days/week, for several
weeks. The duration varied with each exposure level. No loss
of weight and no deaths occurred in rats of either strain
exposed to 420 mg/m3 for up to 4 months (74 exposures). Seven
out of 12 Wistar rats exposed to 730 mg/m3 died within 15 weeks
(after 1 - 73 exposures) and 8/12 similarly treated rats of the
Osborne-Mendel strain died after 1 - 6 exposures. Nine out of
16 rats (strain unspecified) died within 12 weeks after 2 - 60
exposures to 1540 mg/m3; 20/26 (strain unspecified) died within
3 weeks after 3 - 15 exposures to 3900 mg/m3. Guinea-pigs were
exposed to the same concentrations of 1,2-dichloroethane as the
rats. Several deaths, which occurred in the lowest exposure
group (420 mg/m3) and in controls, were attributed to an
intercurrent disease. At higher dose levels, mortality was
related to 1,2-dichloroethane exposure. Five out of 14 guinea-
pigs died after 5 - 115 exposures to 730 mg/m3 within 23 weeks.
Fourteen out of 20 guinea-pigs died after 8 - 65 exposures to
1,2-dichloroethane at 1540 mg/m3 within 13 weeks. All guinea-
pigs exposed to 3900 mg/m3 had died by the 4th day of the
study.
Rabbits were exposed to the three higher concentrations of
1,2-dichloroethane only. No deaths were observed when rabbits
were exposed to 730 mg/m3 for 25 weeks. All 5 rabbits exposed
to 1540 mg/m3 died, one after one exposure and the rest after
89 - 97 exposures within 19 weeks. Five out of 6 rabbits
exposed to 3900 mg/m3 died after 2 - 43 exposures within 9
weeks.
A group of 19 mice survived for 4 weeks when exposed to 420
mg/m3, but 18/20 mice died within 10 days by the end of 7
exposures to 730 mg/m3. In other species, a group of 6 female
dogs survived up to 35 weeks of exposure to 1540 mg/m3. Two out
of 6 dogs exposed to 3900 mg/m3 died after 30 and 43 exposures,
respectively. Two out of six cats exposed to 3900 mg/m3 died
after 43 exposures. Cats were not exposed to lower
concentrations. Two monkeys died after 2 and 32 exposures,
respectively, to 3900 mg/m3, but 2 others exposed to 730 mg/m3
survived for 25 weeks.
Kidney and liver damage, consisting of fatty changes and
necrosis in both organs, was found in animals that died from
exposure to the highest dose level (3900 mg/m3). In addition,
the rats showed pulmonary congestion and haemorrhage; one monkey
that died after 32 exposures and one dog showed a focal
myocarditis. Approximately half of the animals that died after
exposure to 1540 mg/m3 showed similar histological changes in
the liver and kidneys, but no such changes were observed in rats
that died from exposure to lower levels. Hepatic fatty changes
were observed in guinea-pigs exposed to 730 mg/m3. Histological
examinations were not carried out on mice.
Spencer et al. (1951) exposed rats, guinea-pigs, monkeys,
and rabbits to 1,2-dichloroethane at concentrations of 1620,
810, or 405 mg/m3, 7 h per day, 5 days/week, for various lengths
of time. In a group of 15 male and 15 female rats, exposed to
the highest level, no animals survived for more than 8 weeks,
and 60% mortality occurred in a second group exposed to the same
regime after 2 or 3 exposures. Mortality was also high in a
group of 8 male and 8 female guinea-pigs exposed to 1620 mg/m3.
All males had died by the second week and all females by
approximately the 5th week. Two male monkeys experienced rapid
and severe intoxication. Both were killed when moribund after
10 - 12 exposures. On the other hand, 2 male and 1 female
rabbits tolerated 33 weeks exposure with no evidence of adverse
effects. No mortality was observed when groups of 15 male and
15 female rats and 8 male and 8 female guinea-pigs were exposed
to 810 mg/m3 for about 30 weeks (151 exposures) or 36 weeks
(180 exposures), respectively. Similarly, no clinical effects
were observed in groups of 15 male and female rats, 8 male or
female guinea-pigs, 2 male and 1 female rabbits, and 1 male and
1 female monkey exposed for approximately 30 - 36 weeks to 405
mg/m3.
Histological examination was carried out on animals exposed
to the three dose levels. In both guinea-pigs and rats exposed
to the highest dose level, liver changes consisting of cloudy
swelling and fatty changes were observed. None of the other
organs were affected. Similar but less marked changes were
observed in the monkeys, while no adverse changes were found in
the rabbits. In rats exposed to 810 mg/m3, there were no
adverse changes in the liver or other organs, but reduced growth
and some fatty changes were found in the liver of guinea-pigs.
No adverse changes were found in animals exposed to the lowest
dose level (405 mg/m3).
Hofmann et al. (1971) exposed cats, rabbits, guinea-pigs,
and rats to 1,2-dichloroethane at 1980 mg/m3 or 405 mg/m3 for
6 h/day, 5 days/week, for up to 17 weeks. At the higher
concentration, rats became dyspnoeic and guinea-pigs apathetic.
Three out of 4 rabbits died after 10 - 17 exposures, and 9/10
guinea-pigs died after 4 - 14 exposures. Rats were more
sensitive, dying after only 1 - 5 exposures. All cats survived
30 exposures. Histologically, rats showed pulmonary hyperaemia
and oedema, fatty liver, and adrenal and myocardial necrosis.
Cats and rabbits exhibited a heart lesion and guinea-pigs, fatty
changes in the myocardium, liver, and adrenals and necrosis in
the myocardium and liver. At the lower concentration (405
mg/m3), rats, guinea-pigs, rabbits, and cats exposed for 17
weeks did not show any clinical or histological changes.
Of the species studied, mice and rats appear to be more
sensitive than other species to the adverse effects of 1,2-
dichloroethane. The no-observed-adverse-effect level for short-
term exposures (4 - 9 months) in rats studied in the 3
investigations is about 400 mg/m3.
Signs of central nervous system depression observed in the
above studies were apathy in guinea-pigs at 1980 mg/m3 (Hofmann
et al., 1971) and 3900 mg/m3 (Heppel et al., 1946), and coma in
dogs and monkeys at 3900 mg/m3 (Heppel et al., 1946). When rats
were exposed continuously for 3.5 months to 5 mg/m3, changes in
EEG were observed (Dmitrieva & Kuleshova, 1971). However, the
significance of these findings is not known.
7.2.2 Oral exposure
The liver appeared to be the principal target organ
following oral exposure. Rats, treated by gavage with 1,2-
dichloroethane in corn oil for 2 weeks, 5 times per week, at
doses of 150 mg/kg body weight or less did not show any
treatment-related abnormalities in organ or body weights,
histology, clinical chemistry, or haematology (Van Esch et al.,
1977; Reitz et al., 1982).
Rats were also exposed for 90 days, 5 times per week, to 0,
10, 30, or 90 mg/kg body weight (Van Esch et al., 1977). At the
2 highest exposure levels, a tendency to decreased weight gain
was observed. At 90 mg/kg, rats of both sexes showed an
increase in the relative weight of kidneys, but only the females
on this dose showed increased relative weights of liver and
brain compared with controls. Histology and clinical chemistry
were normal. Some haematological parameters were altered, but
not in a dose-related manner. In another study, after 5 doses
of 300 mg 1,2-dichloroethane/kg body weight in 5 days, all 6
rats died, and their livers showed fatty degeneration with an
increase in the triglycerides level (Van Esch et al., 1977).
Total fat and triglycerides were elevated in the livers of
rats exposed to approximately 100 mg/kg body weight per day via
the feed, which was administered twice daily, for 7 weeks
(Alumot et al., 1976a).
No adverse effects related to liver and kidney function were
observed at the lowest dose (10 mg/kg).
7.3 Long-Term Exposure
7.3.1 Inhalation exposure
Clinical chemical investigations were performed on Sprague
Dawley rats exposed by inhalation to 1,2-dichloroethane at 0, 5,
40, 202, or 1012 mg/m3 for 7 h per day, 5 days/week (Spreafico
et al., 1980). The highest exposure level was reduced to 607
mg/m3 after a few weeks because of high mortality. Animals of
each sex were exposed, starting at 3 months of age, for 3, 6, or
18 months. In addition, animals starting at 14 months of age
were exposed for 12 months. Groups of 8 - 10 animals of each
sex were sacrificed at the specified time intervals and clinical
chemistry tests performed. Changes in SGOT, SGPT, and
c-glutamyl transpeptidase activities in the 12-month animals
were not observed in the 18-month animals. Likewise, increases
in serum-uric acid and blood urea-nitrogen levels in the 12-
month animals were not observed in the 18-month animals. In
addition, the 12-month animals, but not the 18-month animals,
displayed decreases in serum-cholesterol. On the basis of the
negative results obtained in the animals starting exposure at 3
months of age and sacrificed after 3, 6, or 18 months, the
authors suggested a lack of significant toxicity, in spite of
the biochemical changes found in the older 12-month animals.
Neurotoxic changes (conditioned reflexes) in albino rats
have been associated with a 1,2-dichloroethane exposure of 50
mg/m3, 4 h/day, for 6 months (Borissova, 1957, 1960).
7.3.2 Oral exposure
In a controlled feeding schedule for 2 years, three groups
of 18 locally-bred rats of each sex were provided with feed
fumigated with 1,2-dichloroethane. The doses of 1,2-
dichloroethane administered were estimated to be 0, 11 - 17, or
23 - 35 mg 1,2-dichloroethane/kg body weight per day. No
adverse effects were observed on growth, mortality rates, or
serum composition. The mean survival period was 18 months or
more (Alumot et al., 1976a).
7.4 Carcinogenicity
7.4.1 Inhalation exposure
Groups of 11-week-old Swiss mice and 12-week-old Sprague
Dawley rats, comprising 90 animals of each sex, were exposed to
20, 40, 202, and 1012 mg 1,2-dichloroethane/m3 air (5, 10, 50,
and 250 ppm) for 78 weeks, 7 h per day, 5 days per week, and
observed for a lifetime. Purity was reported to be greater than
99%. The highest exposure was reduced to 607 mg/m3 (150 ppm)
after a few weeks because of high mortality. Control groups
contained 115 male mice, 134 female mice, or 180 rats of each
sex. Percentage survival in male and female mice, 52 weeks
after the beginning of treatment, was, respectively, 63 and 84%
in the controls; 47 and 93% at 20 mg/m3; 66 and 80% at 40 mg/m3;
51 and 81% at 202 mg/m3; and 43 and 64% at 607 mg/m3. The last
mouse died about 100 weeks after initiation of treatment. In
male and female rats, survival at 52 weeks was, respectively, 67
and 73% in the controls; 75 and 85% at 20 mg/m3; 70 and 81% at
40 mg/m3; 70 and 84% at 202 mg/m3; and 67 and 79% at 607 mg/m3.
Most rats had died by about 140 weeks after the start of
treatment. No specific types of tumours or changes in the
incidence of tumours were found in either species, with the
exception of an increased incidence (not dose-related) of
fibromas and fibroadenomas of the mammary glands of female rats
at 20, 202, and 607 mg/m3. The average latency time for these
tumours was 83 weeks in control rats and in rats exposed at
20 mg/m3, and 79 weeks at the 2 highest exposures. The authors
ascribe the differences in the incidence of mammary tumours
between the groups to the different survival rates in the groups
(Maltoni et al., 1980).
7.4.2 Oral exposure
Groups of 50 Osborne-Mendel rats of each sex were exposed to
average doses of 1,2-dichloroethane (technical grade with
reported purity > 90%)a in corn oil of 47 or 95 mg/kg body
weight for 78 weeks (NCI, 1978). Treatment was usually given 5
times per week, and the animals were observed for another 15 -
32 weeks after the end of treatment. Control groups consisted
of 20 matched controls of each sex treated with corn oil and 60
pooled control-treated rats of each sex. Eleven minor
contaminants were detected in the test compound. The rats were
housed in the same room as rats intubated with other halogenated
hydrocarbons or carbon disulfide. Body weights were not
affected by the exposures. A dose-related increase was found in
mortality rate, which was 100%, 27 weeks after cessation of
exposure to 95 mg/kg body weight. As shown in Table 9, male
rats had a dose-related increased incidence of subcutaneous
fibromas, and forestomach squamous cell carcinomas were
observed. In treated females, the stomach showed hyperplastic
lesions. The incidence of haemangiosarcomas (in various organs,
mainly the spleen) was increased in a dose-related manner in
both sexes, but the increase was statistically significant in
males only. In females, an increase was found in the incidence
of adenocarcinomas of the mammary gland (NCI, 1978).
a Subsequent analysis indicated a purity of about 98 - 99%
(Hooper et al., 1980; Ward, 1980).
Table 9. Summary of main tumour types after oral administration of 1,2-dichoroethane to mice and ratsa
---------------------------------------------------------------------------------------------------------
Group Maximum Number of animals with:
number of Forestomach Subcutaneous Mammary Haemangiosarcomas
animals squamous cell fibromas adeno- (several organs)
examined carcinomas carcinomas
---------------------------------------------------------------------------------------------------------
Rat (male)
Pooled vehicle controls 59 0 0 0 0
Matched vehicle controls 19 0 0 1 0
Low dose 46 3 5 2 9
(P < 0.002)b (P = 0.003)b
High dose 47 9 6 0 7
(P < 0.04)c (P < 0.01)b (P < 0.02)b
Rat (female) Mammary gland
fibroma
Pooled vehicle controls 59 0 5 1 0
Matched vehicle controls 20 0 0 0 0
Low dose 50 0 14 1 4
(P < 0.01)b
High dose 50 0 8 18 4
(P < 0.001)b
---------------------------------------------------------------------------------------------------------
Group Maximum Number of animals with:
number of Hepatocellular Alveolar/ Forestomach Mammary gland
animals carcinomas bronchiolar squamous adenocarcinomas
examined adenomas cell carcinomas
---------------------------------------------------------------------------------------------------------
Mice (male)
Pooled vehicle controls 59 4 0 1 -
Matched vehicle controls 19 1 0 1 -
Low dose 46 6 1 1 -
High dose 47 12 15 2 -
(P < 0.01)b (P < 0.001)b (NS)
Mice (female)
Pooled vehicle controls 60 0 2 1 0
Matched vehicle controls 20 1 1 1 0
Low dose 50 0 7 2 9
(P = 0.001)b
High dose 48 1 15 5 7
(P < 0.001)b (NS) (P = 0.003)b
---------------------------------------------------------------------------------------------------------
a Adapted from: NCI (1978).
b Statistical analyses shown are in comparison with the pooled matched controls.
c Statistical analyses shown are in comparison with the matched vehicle controls.
In the same set of studies, B6C3F1 mice were exposed in a
similar fashion to average doses of 0, 97, or 195 mg technical
grade compound/kg body weight for males and 0, 149, or 299 mg/kg
body weight for females. They were housed in the same room
where several other hydrocarbons or other substances were
tested. After exposure, they were observed for another 12 - 13
weeks. In females, body weights were depressed at the highest
doses from week 15 onwards, while the survival rate decreased in
a dose-related manner. Mean survival exceeded 65 weeks in all
groups. Many treated mice suffered from bronchopneumonia. As
shown in Table 9, dose-related increased incidences of alveolar
or bronchiolar adenomas were found in both sexes. In males, a
dose-related increased incidence of hepatocellular carcinomas
was observed. Female mice showed slight, dose-related increases
in the incidence of squamous cell carcinomas of the forestomach,
but this was not statistically significant. In male mice,
hyperplastic changes were found at this site. The incidence of
adenocarcinomas of the mammary gland was significantly increased
in females at both doses (NCI, 1978).
7.4.3 Dermal exposure
Groups of 30 female Ha:ICR Swiss mice were treated with 42
or 126 mg 1,2-dichloroethane in acetone on the shaven dorsal
skin, 3 times per week for 440 - 594 days. In a third group,
each female received one application of 126 mg of the test
compound followed 2 weeks later by phorbol myristate acetate, a
promotor, in acetone 3 times per week for 428 - 576 days. There
were 3 control groups, a positive control, one for the promotor,
and one for no treatment. 1,2-Dichloroethane did not initiate
skin tumours. There was an elevated incidence of lung
papillomas at the highest dose compared with controls (Van
Duuren et al., 1979).
7.5 Mutagenicity and Related End-Points
7.5.1 Mutations
Information in this section is summarized in Table 10.
7.5.1.1 Bacteria
Several investigators have observed a weak or no effect of
1,2-dichloroethane in Salmonella typhimurium TA1535 or TA 100 in
spot tests or standard plate incorporation assays with or
without rat liver S9 fraction or pure microsomes (Brem et al.,
1974; McCann et al., 1975; King et al., 1979; Guengerich et al.,
1980; Principe et al., 1981). A weak mutagenic effect was
observed in 1,2-dichoroethane vapour-exposed S. typhimurium TA
1535 and TA 100, which did not increase further by the addition
of a metabolic activation system (Barber et al., 1981). However,
a stronger positive response was observed by others (Rannug et
al., 1978; Rannug & Beije, 1979; Principe et al., 1981) in TA
1535 in the presence of rat liver metabolic activation system.
It has been established that this effect has been caused by
cytosolic glutathione-S-transferases (Rannug et al., 1978; Guen-
gerich et al., 1980; Reitz et al., 1982), and similar results
have also been obtained in TA 100 (van Bladeren et al., 1981b).
In vitro Salmonella tests using the bile of mice or rats
exposed to 1,2-dichloroethane, probably containing active con-
jugates, have confirmed these results (Rannug & Beije, 1979).
No mutagenic effects were observed in forward mutation tests
with Escherichia coli (King et al., 1979).
Table 10. Tests for gene mutations/chromosome/DNA damage and cell transformation induced
by 1,2-dichloroethane
---------------------------------------------------------------------------------------------------------
Test description System description Activation Result Reference
Organism Strain/cell type system (S9)
---------------------------------------------------------------------------------------------------------
Gene mutations
reverse mutation bacteria S. typhimurium TA 1530 A + (weak) Brem et al. (1974)
TA 1535 A + (weak)
TA 1538 A + (weak)
reverse mutation bacteria S. typhimurium TA 100 A + (weak) McCann et al. (1975)
reverse mutation bacteria S. typhimurium TA 1535 P - King et al. (1979)
TA 100 P -
TA 1537 P -
TA 1538 P -
TA 98 P -
reverse mutation bacteria S. typhimurium TA 1535 P + Principe et al.
TA 1537 A or P - (1981)
TA 1538 A or P -
TA 98 A or P -
TA 100 A or P -
reverse mutation bacteria S. typhimurium TA 1535 Pa - Guengerich et al.
Pb + (1980)
reverse mutation bacteria S. typhimurium TA 1535 A or P + Barber et al.
TA 100 A or P ± (1981)
TA 1538 A or P -
TA 98 A or P -
---------------------------------------------------------------------------------------------------------
Table 10 (contd).
---------------------------------------------------------------------------------------------------------
Gene mutations (contd)
reverse mutation bacteria S. typhimurium TA 1535 A ± Rannug et al. (1978)
TA 1535 P +
TA 1535 Pb +
reverse mutation bacteria S. typhimurium TA 1535 P + Rannug & Beije (1979)
reverse mutation bacteria S. typhimurium TA 1535 P + Reitz et al. (1982)
reverse mutation bacteria S. typhimurium TA 100 Pb + van Bladeren et al.
(1981a)
forward mutation fungi S. coelicolor A - Principe et al.
A. nidulans A - (1981)
sex-linked lethals insect D. melanogaster + Rapoport (1960); Shak-
arnis (1969, 1970);
King et al. (1979)
somatic cell mutation insect D. melanogaster + Nylander et al.
(1978)
forward mutation Chinese ovary cells in vitro P ++ Tan & Hsie (1981)
hamster A +
P + Zamora et al. (1983)
forward mutation human lymphoblastoid cell line A + Crespi et al. (1985)
AHH-1 and TK6 in vitro
somatic cell mutation mouse C57BL/6J Han (female) NA +? Gocke et al. (1983)
(spot test) x T stock (male)/embryos
---------------------------------------------------------------------------------------------------------
Table 10 (contd).
---------------------------------------------------------------------------------------------------------
Test description System description Activation Result Reference
Organism Strain/cell type system (S9)
---------------------------------------------------------------------------------------------------------
Chromosome/DNA damage
micronucleus test mice NMRI/polychromatic NA - King et al. (1979)
(ip or gavage exposure) erythrocytes
micronucleus test mice CBA/polychromatic NA - Jenssen & Ramel
(ip exposure) erythrocytes (1980)
dominant lethal mice ICR Swiss/germ cells NA - Lane et al. (1982)
(ip exposure)
alkaline DNA mice B6C3F1/liver in vivo/ NA + Storer & Conolly
unwinding in vitro (1983)
alkaline DNA mice B6C3F1/liver in vivo/ NA + Storer et al. (1984)
unwinding in vitro
unscheduled DNA human lymphocytes in vitro NA + Perocco & Prodi
synthesis (exposure by (1981)
addition in the medium)
Cell transformation
cell transformation Syrian embryo cells in vitro A +c Hatch et al. (1983)
(gas/vapour exposure) golden hamster
cell transformation mice BALB/c-3T3 in vitro A - Tu et al. (1985)
(exposure by addition
in the medium)
---------------------------------------------------------------------------------------------------------
a Pure microsomes.
b Cytosol.
c Enhanced viral induced transformation.
NA = not applicable.
7.5.1.2 Fungi
Forward mutation tests conducted with Streptomyces coeli-
color and Aspergillus nidulans were negative in plate incor-
poration assays and spot tests (Principe et al., 1981). In
A. nidulans, 1,2-dichoroethane induced non-disjunction and
haploidization (Crebelli et al., 1984). 1,2-Dichloroethane was
found to be a weak inducer of mitotic crossing over
in Saccharomyces cerevisiae (Simmon, 1980).
7.5.1.3 Insects
Sex-linked recessive lethal mutations were induced
in Drosophila melanogaster by 1,2-dichloroethane (Rapoport,
1960; Shakarnis, 1970; King et al., 1979). Non-dysjunction was
observed inconsistently (Shakarnis, 1969, 1970), while the
frequency of somatic mutations for eye pigmentation increased
(Nylander et al., 1978).
7.5.1.4 Mammals/mammalian cells
A spot test using mice provided weak evidence that 1,2-
dichloroethane can induce somatic mutations (Gocke et al.,
1983).
In Chinese hamster ovary cells, 1,2-dichloroethane induced
mutations at the HGPRT-locus (Tan & Hsie, 1981; Zamora et al.,
1983). Metabolic activation increased the mutation frequency in
the presence of NADPH (Tan & Hsie, 1981). 1,2-Dichloroethane
also induced a dose-related increase in the frequency of
mutations at the HGPRT-locus in two human lymphoblastoid cell
lines, AHH-1 and TK6. The mutation frequency in the AHH-1 cell
line was 25 times that in the TK6 cell line. The difference in
sensitivity was attributed to the difference in the levels of
glutathione-S-transferase (EC 2.5.1.18) activity. The activity
of this enzyme in the AHH-1 cell line was 5 times that in the
TK6 cell line (Crespi et al., 1985).
7.5.2 Chromosome damage/DNA damage
In in vivo studies, no effects were observed in dominant
lethal assays in 2 generations of ICR Swiss mice (Lane et al.,
1982) and in the micronucleus test with CBA and NMRI mice (King
et al., 1979; Jenssen & Ramel, 1980).
1,2-Dichloroethane was a weak inducer of unscheduled DNA
synthesis in cultured human lymphocytes (Perocco & Prodi,
1981).
DNA alkylation in Salmonella by activated 1,2-dichloroethane
was directly related to the mutation frequency, but absolute
levels of DNA alkylation in Salmonella were considered low
(Reitz et al., 1982).
1,2-Dichloroethane weakly inhibited growth of DNA polymerase
deficient E. coli (Brem et al., 1974; Rosenkranz, 1977).
Administration of 1,2-dichloroethane to mice and rats
resulted in covalent binding to macromolecules (Reitz et al.,
1982; Arfellini et al., 1984; Inskeep et al., 1986). Absolute
levels of DNA alkylation in rats, either by gavage or
inhalation, were considered low (Reitz et al., 1982).
Hepatic DNA damage was demonstrated using the alkaline DNA
unwinding assay in male B6C3F1 mice after a single intraperi-
toneal or oral dose of 1,2-dichloroethane that failed to induce
toxic effects in the liver. It was also shown that, after one
inhalation exposure, hepatic DNA damage only occurred at
exposure levels that caused high mortality (Storer & Conolly,
1983; Storer et al., 1984).
7.5.3 Cell transformation
1,2-Dichloroethane did not transform BALB/c-3T3 mouse cells
in a test conducted without any exogenous metabolic activating
system (Tu et al., 1985). It enhanced transformation of Syrian
hamster embryo cells by simian adenovirus (Hatch et al., 1983).
7.6 Reproduction and Teratogenicity
7.6.1 Inhalation exposure
It has been reported that 1,2-dichloroethane was found in
the fetuses of rats after exposure to 600 mg/m3 for 5 h (Withey
& Karpinski, 1985).
Female rats (strain unspecified) were exposed to 15 mg 1,2-
dichloroethane/m3, for 4 h daily, 6 days/week, for 4 months
prior to mating. During this period, the estrus cycle became
longer than normal. The rats were then mated and exposure
continued. No information on the effects on fertility was
given, but the total embryonal mortality increased from
approximately 11% in controls to 27% in treated dams, while pre-
implantation losses were found to be 5 times greater in treated
animals than in the controls. No fetal abnormalities were
reported, with the exception of haematomas in the region of the
head, neck, and anterior extremities (Vozovaya, 1977).
In a second study, 1,2-dichloroethane was administered to
female albino rats at a concentration of 57 ± 10 mg/m3 in air,
for 4 h daily, 6 days/week, for 6 and 9 months. When the rats
were mated, a reduction in fertility was observed (6.5 fetuses
per treated female versus 9.7 in controls). The weight of
newborn rats was reduced (5.06 g versus 6.44 in unexposed
females). Perinatal mortality was increased (Vozovaya, 1974).
1,2-Dichloroethane was detected in placental and fetal
tissues after inhalation exposure to 1000 mg/m3 for 3 days
(daily duration not stated). It was also found in the stomach
of 12- to 14-day-old mice, when lactating females were exposed
to an unspecified concentration of 1,2-dichloroethane by
inhalation (Vozovaya, 1977).
While the above results indicate a possible adverse effect
of 1,2-dichloroethane on reproduction, the following repro-
ductive studies yielded negative results. Groups of 20 Sprague
Dawley rats of each sex were exposed for 60 days prior to
mating, for 6 h per day, and 5 days per week to 101, 304, or
607 mg 1,2-dichloroethane/m3 in air. After mating, they were
exposed similarly, for another 116 days, but for 7 days per
week. Females were not exposed between gestation day 21 and day
4 post partum. Control groups contained 30 rats each. Pups
were removed and examined at 21 days of age, and the females
were remated following the removal of the last litter. Each
female produced 2 litters. The parents did not show any toxic
effects and the fertility index and gestation period were
normal. In the pups, no effects were found on sex ratio,
survival indices, organ weights, or histology. A small decrease
was noted in the number of pups in the first litters at
304 mg/m3 (Rao et al., 1980).
A teratogenicity study was further performed with groups of
16 - 30 female rats and 19 - 21 female rabbits (Rao et al.,
1980). These groups were exposed through inhalation to 0, 405,
or 1215 mg 1,2-dichloroethane/m3 air, for 7 h per day, from the
6th day of pregnancy onwards (rats up to the 15th day and
rabbits up to the 18th day of gestation). Exposures at both
levels were toxic for rabbit dams. The higher exposure level
was severely toxic for the rat dams; only 1 of the few surviving
females was pregnant, and all the implantation sites were
resorbed. No adverse effects on reproduction were observed
among rats exposed at 405 mg/m3 and rabbits exposed at 405 or
1215 mg/m3. The only gross change observed in rat fetuses was a
decreased incidence of bilobed thoracic centra. There were no
significant alterations in rabbit fetuses.
7.6.2 Oral exposure
Groups of 18 female rats that had received 0, 11 - 17, or
23 - 35 mg 1,2-dichloroethane/kg body weight per day via the
feed, for up to 2 years, were mated with untreated males. The
purity of the substance was not reported. No effects on
reproduction were observed (Alumot et al., 1976a).
Lane et al. (1982) reported a 2-generation reproduction
study on groups of 10 male and 30 female ICR Swiss mice
receiving nominally 5, 15, or 50 mg 1,2-dichloroethane/kg body
weight per day via the drinking-water, for up to 25 weeks.
Control groups contained 20 male and 60 female mice. After 5
weeks of treatment, the mice were mated to produce F1A, F1B, and
F1C litters. After weaning and 11 weeks of treatment, the F1B
mice were mated to produce F2A and F2B litters. Remating always
occurred 2 weeks after weaning. In the F1C and F2B matings,
females were co-housed with untreated males for teratology
screening. The parents did not show any toxic effects and
fertility and gestation indexes were normal. There were no
effects on survival, litter size, postnatal body weight, and
gross pathology of pups. No congenital malformations were
detected. In the F1C and F2B litters, no exposure-related
reproductive effects were found. In the F2B litters, there was
no increase in the incidence of fetal visceral or skeletal
anomalies. F1C litters were not examined for skeletal
anomalies.
7.7 Immunotoxicity
When rabbits were exposed for 7.5 - 8 months to 1,2-
dichloroethane vapour of unspecified purity at a level of
100 mg/m3, for 3 h per day, 6 h per week, an 80% reduction in
the production of antibodies against typhoid vaccine was
observed. Concomitantly, there was a 2-fold increase in Forsman
sheep erythrocyte antibodies (Shmuter, 1977).
Immunosuppression was also noted in a later oral study, in
which groups of 32 male CD-1 mice were exposed to 3, 24, or
189 mg 1,2-dichloroethane/kg body weight (purity unknown) via
the drinking-water for 90 days. In addition, groups of 10 male
CD-1 mice were exposed, once a day, to 4.9 or 49 mg/kg body
weight by water gavage for 14 days. Control groups comprised 48
mice in the 90-day study and 12 mice in the 14-day study. Apart
from a 30% reduction in the leukocyte count after 14 days of
exposure to 49 mg/kg body weight, no effects were found on other
haematological parameters and relative organ weights. After the
90-day exposure, decreases in body weight and fluid consumption
were noted. There was a tendency towards a reduction in
immunoglobulin spleen antibody-forming cells and in the serum-
antibody level after sheep erythrocyte immunization, while no
effects were observed in the response to the B-cell mitogen
lipopolysaccharide S. After the 14-day exposure, 25% and 40%
suppression of antibody-forming cells were measured at 4.9 and
49 mg/kg body weight, respectively. After the 90-day exposure,
no effects were seen on the cell-mediated immunity, assessed by
measuring the delayed hypersensitivity response to sheep
erythrocytes and the spleen cell response to the T-cell mitogen
Concanavalin A. After the 14-day exposure, a slight suppression
of the delayed hypersensitivity response was found, which was
not dose-dependent (Munson et al., 1982).
8. EFFECTS ON MAN
Limited data are available on the effects on man of 1,2-
dichloroethane. There are no adequate controlled studies, no
recent occupational studies, and no mortality studies. However,
case studies of accidental exposures have been reported.
8.1 Accidental Exposures
8.1.1 Inhalation exposure
Many reports are concerned with mixed exposures. However,
in this publication, only case studies are considered in which
the exposure was reported to be to 1,2-dichloroethane alone.
According to a review by NIOSH (1976), no data on exposure
levels and duration of exposure were reported.
Inhalation of 1,2-dichloroethane vapour first afflicts the
central nervous system. Symptoms include headache, dizziness,
weakness, cyanosis, muscular spasms, hypotonia, vomiting, and
unconsciousness. Death often follows. The respiratory tract
can be irritated and inflamed with such symptoms as cough and
rales over the chest. Cyanosis may occur either as the result
of respiratory insufficiency due to depression of the central
nervous system or by bronchial obstruction due to inflammation.
Epigastric or visceral pains and diarrhoea have been observed.
Autopsy reports frequently mention damage to the lungs, liver,
and kidneys (Wirtschafter & Schwartz, 1939; Hadengue & Martin,
1953; Menschick, 1957; Troisi & Cavallazzi, 1961; Suveev &
Babichenko, 1969). Changes in heart rhythm have been reported,
which are probably secondary effects (section 8.1.2) (Suveev &
Babichenko, 1969). Clinical findings include elevated serum-
bilirubin levels and leukocytosis (Wirtschafter & Schwartz,
1939; Menschick, 1957) and elevations of blood-lactate, ammonia,
ornithine carbamyl transferase, serum aspartate transaminase (EC
2.6.1.1), lactate dehydrogenase, and creatine phosphokinase
(Nouchi et al., 1984).
8.1.2 Oral exposure
The effects of acute oral exposure are very similar to those
found after inhalation, but they are more pronounced. A summary
of acute oral intoxications was prepared by US NIOSH in 1976.
The lethal effects of 1,2-dichloroethane associated with oral
exposure are presented in Table 11. Oral doses of 20 - 50 ml
1,2-dichloroethane have been identified as being lethal (IRPTC,
1984). Several major syndromes can be identified including
central nervous system depression, gastroenteritis, and
disorders of the liver and kidneys. Frequently-observed
cardiovascular insufficiency and haemorrhagic diathesis may be
related to changes in oxygenation and effects on the liver
(Weiss, 1957; Morozov, 1958; Hinkel, 1965; Bogoyavlenski et al.,
1968; Martin et al., 1968; Schönborn et al., 1970; Yodaiken &
Babcock, 1973; Dorndorf et al., 1975; Andriukin, 1979).
Table 11. Effects associated with acute lethal oral doses of 1,2-dichloroethane in human beingsa
---------------------------------------------------------------------------------------------------------
Amount ingested (g) Findingsb Reference
---------------------------------------------------------------------------------------------------------
188 - 250 death of 4 males up to 35 h; internal Bryzhin (1945)c
haemorrhage at various sites; liver damage;
symptoms at 3 - 4 h
87 - 125 death of 3 males after 5 - 8 h; internal Kaira (1966)c
haemorrhage; symptoms immediate (uncon-
sciousness, vomiting, dizziness)
103 death after 6 h; haemorrhagic lesions Noetzel (1944)c
75 death after 22 h; symptoms at 2 h (cyanosis, Hueper & Smith (1935)
(cyanosis, vomiting, dilated pupils); brain
haemorrhage, liver damage, nephrosis
63 death at 91 h; lack of eye reflex to light Roubal (1947)c
on 4th day; vomiting; rapid pulse
63 death in 3 - 4 h; liver damage Secchi et al. (1968)c
63 death at 17 h; cyanosis, diarrhoea; impaired Schonborn et al. (1970)
blood coagulation
50 death at 24 h; haemorrhage at various sites; Martin et al. (1968)
signs of cardiac damage
---------------------------------------------------------------------------------------------------------
Table 11 (contd).
---------------------------------------------------------------------------------------------------------
Amount ingested (g) Findingsb Reference
---------------------------------------------------------------------------------------------------------
50 death at 28 h; internal haemorrhage Garrison & Leadingham (1954)c
37 death at 10 h; internal haemorrhage at Lochhead & Close (1951)
various sites; adverse lung effects
25 death at 24 h; epigastric pain; slow Roubal (1947)c
pulse
25 death at 13 h; symptoms at 1 h (cyanosis, Flowtow (1952)c
vomiting)
25 death within 12 h; symptoms not reported Flowtow (1952)c
19 death after 6 days; liver, kidney, renal Yodaiken & Babcock (1973)
damage; pulmonary oedema; hypoglycaemia;
clotting time decreased; some haemorrhaging
10 death after 56 h; delirium; pulse deterior- Bogoyavlenski et al. (1968)
ation
---------------------------------------------------------------------------------------------------------
a Studies in which doses could not be estimated have not been cited. The reader is referred to
NIOSH (1976) for a description of these case reports.
b Unless otherwise stated, death refers to single male individuals.
c The reader is referred to NIOSH (1976) for a more complete description of symptomology.
Symptoms of central nervous system depression commonly
appear within 1 h, frequently with cyanosis, nausea, vomiting,
diarrhoea, epigastric and abdominal pains, and irritation of the
mucous membranes. Irreversible brain damage has been reported
in one case, and brain damage has been found in several fatal
cases (Rohmann et al., 1969; Dorndorf et al., 1975). In some of
the cases, an interval relatively free of symptoms followed
ingestion (Hinkel, 1965; Martin et al., 1968; Komarov et al.,
1973; Dorndorf et al., 1975). In the next phase, decreasing
consciousness and circulatory and respiratory failure may occur,
often leading to death some hours to some days after exposure.
During the intoxication, heart rhythm disturbances can lead to
cardiac arrest (Morozov, 1958; Martin et al., 1968; Yodaiken &
Babcock, 1973; Andriukin, 1979). Autopsy reports have revealed
damage to the mucosae of the gastrointestinal tract, liver,
kidney, lung, heart, and brain. Livers can be enlarged. Liver
and kidney epithelium can show fatty degeneration and necrosis.
Renal insufficiency has been reported to follow development of
hepatic insufficiency and has been known to progress to uraemic
coma (Natsyuk & Mudritsky, 1974). Lung oedema is frequently
found. Hyperaemia and haemorrhagic lesions are found in some
organs. According to some authors (Martin et al., 1968;
Schönborn et al., 1970; Yodaiken & Babcock, 1973), it appeared
that the blood coagulation time was increased because of a
decrease in blood clotting factors and thrombocytes. These
effects appear secondary to liver cell necrosis complicated
further by intravascular coagulation. Biochemically, liver
damage is illustrated by increased serum levels of bilirubin,
transaminases, and lactate dehydrogenase (Martin et al., 1968;
Yodaiken & Babcock, 1973; Dorndorf et al., 1975; Andriukin,
1979). Kidney damage is expressed by anuria or oliguria
(Morozov, 1958; Bogoyavlenski et al., 1968; Yodaiken & Babcock,
1973) and albumin, leukocytes, and epithelium cells in the
urine. Together with the histopathology, this points to acute
necrosis of the kidney tubule, possibly as a result of the liver
cell necrosis and the changes in circulation (Morozov, 1958;
Hinkel, 1965; Yodaiken & Babcock, 1973). Haematological changes
include decreases in the erythrocyte count and haemoglobin
content (Morozov, 1958; Dorndorf et al., 1975).
8.1.3 Acute effects on eyes and skin
Dysfunction of the central nervous system, which could be
caused by brain oedema, can lead to effects on the eyes such as
dilation or constriction of the pupils and impairment of eye
reflexes (Weiss, 1957; Troisi & Cavallazzi, 1961). Weiss (1957)
reported a cloudy cornea in 2 cases of oral exposure; this has
also been reported in dogs (section 7.1.2). Conjunctivitis was
found in 2 out of 4 patients, who had been exposed to 1,2-
dichloroethane vapour (Menschick, 1957). After intermittent
immersion of the hands of 3 men in 1,2-dichloroethane for 4 h,
severe dermatitis developed (Wirtschafter & Schwartz, 1939).
8.2 Occupational Exposure
Only 2 reports are available, and these date from before
1960. In the first study (Cetnarowicz, 1959), 16 male workers
from an oil refinery, exposed for 2 - 8 months, were selected
for close examination. A group of 6 workers was exposed to
concentrations of between 40 and 150 mg 1,2-dichloroethane/m3
air, and a group of 10 workers was exposed to concentrations
between 250 and 800 mg/m3. The workers were also exposed to
benzene at levels between 10 and 25 mg/m3, which was considered
not significant by the authors. A general reduction in body
weight was observed. Complaints came mainly from the group with
the higher exposure and included a burning sensation of the
eyes, lachrymation, dizziness, lassitude, sleepiness, nausea,
vomiting, constipation, poor appetite, epigastric pain, and
weight loss. There was no control group, but symptoms repor-
tedly disappeared when workers were removed from exposure;
symptoms returned upon re-exposure. Most, but not all, abnorm-
alities were found in the group with higher exposure and
involved the liver (8 workers), central nervous system (3
workers), gastrointestinal tract (7 workers), and haematological
parameters (1 - 7 workers). No lesions were found in the eye,
respiratory tract, lung, or heart.
The second study (Kozik, 1957), was conducted on workers
employed in an aircraft factory using a gum dissolved in 1,2-
dichloroethane. No data were given on the composition of the
glue or on the employment status of the workers. Air concentra-
tions of 1,2-dichloroethane varied considerably, the level being
5 mg/m3 or less during 70 - 75% of the working time, and 80 -
150 mg/m3 during 25 - 30% of the working time. The morbidity of
the workers in this department during the whole period under
study (1951-55) was increased for all disease categories in
comparison with that in workers in the entire factory. A group
of 83 workers was examined further, in the absence of controls.
There were 19 workers with diseases of the liver and bile duct,
13 workers with neurotic conditions, 11 workers with autonomous
dystonia, and 10 workers with hyperthyroidism and goitre.
These reports are difficult to evaluate, because no
indication is given of the prevalence in unexposed workers of
the symptoms and signs described in the case studies.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Evaluation of Human Health Risks
In spite of the high volume of production of 1,2-dichloro-
ethane, there are no quantitative exposure-effect data on human
beings. Frequently, 1,2-dichloroethane is not the only chemical
involved in an exposure and thus, cause-effect relationships are
difficult to derive. No epidemiological or mortality studies
are available. There are only two old reports on small groups
of occupationally-exposed men (section 8.2). These data indi-
cate that repeated inhalation exposures in the range of approx-
imately 40 - 800 mg/m3 may lead to central nervous system
depression and gastrointestinal and liver abnormalities. As
might be expected, such symptoms were more prevalent in indivi-
duals exposed to high levels. The only available data regarding
oral exposure are those involving fatal intoxications (Table 11,
section 8.1).
Because of the limitations of the human data base, it is
necessary to rely on the available experimental animal data to
derive a no-observed-adverse-effect level for human beings.
This is possible because of the similarity in the spectrum of
adverse effects in man and laboratory animals, which include
central nervous system depression, liver and possibly kidney
abnormalties, lung oedema, and cardiovascular disorders. The
dose-response data from animal studies include a no-observed-
adverse-effect level for the rat of about 400 mg 1,2-dichloro-
ethane/m3 air (section 7.2.1), equivalent to an intake of about
35 mg/kg body weight per day (assuming inhalation of 42 litres
over 7 h for a 500-g rat and 100% absorption). The highest
registered air exposure level of the general population is
65 µg/m3 (Table 4, section 4.1.2). This level leads to a
calculated intake of 1.3 mg per person per day (20 m3/day x
65 µg/m3). A comparable intake from drinking-water containing
the highest level recorded (6 µg/litre) (Table 3, section
4.1.1) would amount to a daily intake of 12 µg (6 µg/litre
litre/day). Thus, a combined air and water exposure, in the
context of a worst-case scenario for the general population,
when compared with the no-observed-adverse-effect level for
animals, is lower by a factor of well over 1000. Consequently,
for the biological end-points considered so far, it can be
concluded that 1,2-dichloroethane is unlikely to present a toxic
hazard for the general population, under prevailing exposure
conditions.
In an oral administration study, 1,2-dichloroethane produced
a statistically-significant increase in squamous cell carcinoma
of the forestomach, haemangiosarcoma, and mammary adenocarcinoma
in rats, and mammary adenocarcinoma and hepatocellular carcinoma
in mice (section 7.4.2). In one carcinogenicity study, inhala-
tion exposure did not result in an increase in tumour incidence
(section 7.4.1). The natural occurrence of forestomach squamous
cell carcinomas in rats and haemangiosarcomas in laboratory
rodents is unusual. Taking into consideration that cancer has
been produced in two species of experimental animals and in
several target organs, it can be concluded that 1,2-dichloro-
ethane is carcinogenic for rats and mice, when administered by
gavage.
In the absence of human data, and taking into account the
fact that 1,2-dichloroethane produces a reactive intermediate
that alkylates DNA, that it is positive in a number of in vitro
mutagenicity tests, though weakly so (section 7.5), and that it
results in the production of both rare and common tumours in
rats and mice, it would be prudent to consider 1,2-dichloro-
ethane as a possible human carcinogen. Therefore, 1,2-dichloro-
ethane should be regarded, for practical purposes, as if it
presented a carcinogenic risk for man. Thus, levels in the
environment should be kept as low as feasible.
Since there are no human data, it is necessary to rely on
the limited data available from experimental animal studies in
evaluating reproduction hazards and teratogenicity. The weight
of evidence (section 7.6) does not suggest that exposure to
prevailing environmental levels would pose a human reproductive
or teratogenic hazard.
9.2 Evaluation of Effects on the Environment
9.2.1 Air
Emissions of 1,2-dichloroethane into the air mainly occur in
process industries. Other emissions occur during its use as a
fumigant, solvent, and lead scavenger, and via evaporation from
contaminated water and from waste disposal sites. Total emis-
sions are estimated to amount to 0.2% of the production volume.
Photochemical degradation via oxidation by hydroxyl radicals is
the most important route of elimination from air. Rainout and
adsorption on atmospheric particles are unlikely to be important
processes of elimination. Photolysis is theoretically a possib-
ility, but no evidence of this is available. The products of
the photochemical degradation are carbon monoxide, carbon
dioxide, hydrogen chloride, formyl chloride, and chloroacetyl-
chloride. The two last compounds will degrade further. The
process is rapid enough to prevent accumulation of the compound
in the atmosphere (section 3.3).
9.2.2 Water
Emissions of 1,2-dichloroethane into water may amount to
0.1% of the production volume. Some of the emissions from EDC
tars, which total about 0.5% of the production volume, will
contaminate water. The main process of removal of 1,2-dichloro-
ethane from water is evaporation. Chemical degradation is not
expected, nor is biodegradation fast enough to be of any signi-
ficance (section 3.1). Bioconcentration in aquatic species is
unlikely in view of the rather low octanol/water partition
coefficient. This conclusion is supported by a low bioconcen-
tration factor found experimentally (section 6.1.4).
The compound was only slightly toxic for aquatic species
tested. A no-observed-adverse-effect concentration for Daphnia
magna in a long-term test was 11 mg/litre (section 6.1.3). The
lowest LC50 value (85 mg/litre) was found for the shrimp Crangon
crangon (section 6.1.1). Average environmental levels of the
compound in surface water are generally below 1 µg/litre, but,
in heavily polluted surface water, average levels of 5.6
µg/litre have been measured with a maximum of 90 µ g/litre
(Table 3, section 4.1.1).
On the basis of the above data, it can be concluded that,
except in case of accidents and inappropriate disposal, 1,2-
dichloroethane does not pose a significant hazard for the
aquatic environment. However, it should be noted that EDC tars
are much more toxic than 1,2-dichloroethane (section 6.1.1).
9.2.3 Soil
Data are not sufficient to evaluate the effects of 1,2-
dichloroethane in soil.
10. RECOMMENDATIONS FOR FURTHER STUDIES
1. DNA alkylation (adduct identification).
2. Studies on sub-chronic toxicity using various routes of
exposure.
3. Assessment of the extent to which EDC tars contribute to
contamination of groundwater by 1,2-dichloroethane.
4. Dose-response studies on sensitive, commercially important
fish species (particularly studies relevant to EDC tar
spills).
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,2-Dichloroethane was evaluated by IARC in 1979 (Volume 20
of the IARC Monographs). It was concluded that:
"There is sufficient evidence that 1,2-dichloroethane is
carcinogenic in mice and rats. In the absence of adequate data
in humans, it is reasonable, for practical purposes, to regard
1,2-dichloroethane as if it presented a carcinogenic risk to
humans."
REFERENCES
ADEMA, D.M.M. (1976) [Acute toxicity tests with 1,2-
dichloroethane, phenol, acrylonitrile, and alkyl benzene-
sulfonate in sea water,] Delft, the Netherlands, Central
Laboratory TNO (TNO Report No. MD-N & E 76/1) (in Dutch).
ALUMOT, E., NACHTOMI, E., MANDEL, E., HOLSTEIN, P., BONDI, A., &
HERZBERG, M. (1976a) Tolerance and acceptable daily intake of
chlorinated fumigants in the rat diet. Food Cosmet. Toxicol.,
14: 105-110.
ALUMOT, E., MEIDLER, M., HOLSTEIN, P., & HERZBERG, M. (1976b)
Tolerance and acceptable daily intake of ethylene dichloride in
the chicken diet. Food Cosmet. Toxicol., 14: 111-114.
ANDRIUKIN, A.A. (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klin. Med. (Mosk.), 57: 43-47 (in
Russian).
ARFELLINI, G., BARTOLI, S., COLACCI, A., MAZZULO, M., GALLI,
M.C., PRODI, G., & GRILLI, S. (1984) In vivo and in vitro
binding of 1,2-dibromethane and 1,2-dichloroethane to
macromolecules in rat and mouse organs. J. Cancer Res. clin.
Oncol., 108: 204-213.
ATRI, F.R. (1984) [ Study to assess the toxicity of water
pollutants. III. 1,2-Dichloroethane,] Bonn, Federal Environment
Agency (Research Report No. 106/07/047) (in German).
BAILEY, S., COLLINS, G.B., FISHWICK, F.B., HART, H.V., HORLER,
D.F., & SCUDAMORE, K.A. (1982) Pesticide residues in
foodstuffs in Great Britain; organochlorine pesticides,
organophosphorus pesticides, and fumigant residues in home-
produced and imported wheat. Pestic. Sci., 13: 373-378.
BANERJEE, S., VAN DUUREN, B.L., & ORUAMBO, F.I. (1980)
Microsome-mediated covalent binding of 1,2-dichloroethane to
lung microsomal protein and salmon sperm DNA. Cancer Res., 40:
2170-2173.
BARBER, R.D., DONISH, W.H., & MUELLER, K.R. (1981) A procedure
for the quantitative measurement of the mutagenicity of volatile
liquids in the Ames Salmonella/microsome assay. Mutat. Res.,
90: 31-48.
BARETTA, E.D., STEWARD, R.D., & MUTCHLER, J.E. (1969)
Monitoring exposures to vinyl chloride vapour: breath analysis
and continuous air sampling. Am. Ind. Hyg. Assoc. J., 30: 537-
544.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CAROLL, J.J.
(1980) Bioconcentration and elimination of selected water
pollutants by bluegill sunfish Lepomis macrochirus. In:
Dynamics, Exposure and Hazard Assessment of Toxic Chemicals,
Paper Symposium 1978, Ann Arbor, Michigan, Ann Arbor Science,
pp. 379-392.
BARSOUM, G.S. & SAAD, K. (1934) Relative toxicity of certain
chlorine derivatives of the aliphatic series. Q. J. Pharm.
Pharmacol., 7: 205-214.
BAUER, U. (1978) [Halogenated hydrocarbons in drinking and
surface water. Analysis results 1976-77 in the Federal Republic
of Germany. Drinking water from 100 cities; surface water from
the Ruhr, Lippe, Main, and Rhine.] WaBoLu-Berichte, 3: 64-74 (in
German).
BAUER, U. (1981) [Human exposure to harmful components in
environmental studies on water, air, food, and human tissues.
II, IV.] Zentralbl. Bakteriol. Hyg. I. Abt. Orig. B, 174: 39-55,
556-583 (in German).
BENOIT, D.A., PUGLISI, F.A., & OLSON, D.L. (1982) A fathead
minnow Pimephales promelas early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ.
Pollut., 28(Series A): 189-197.
BERCK, B. (1965) Sorption of ethylene dibromide, ethylene
dichloride, and carbon tetrachloride by cereal products. J.
agric. food Chem., 13: 248-254.
BERCK, B. (1974) Fumigant residues of carbon tetrachloride,
ethylene dichloride, and ethylene dibromide in wheat, flour
bran, middlings, and bread. J. agric. food Chem., 22: 977-984.
BOGOYAVLENSKI, V.F., SALIKHOVA, S.K.H., & KARPOVA, E.V. (1968)
[Clinical aspects and therapy for ethylene dichloride
poisoning.] Sov. Med., 31: 107-109 (in Russian).
BOIKOVA, N.Y. & KRAVTSOVA, G.V. (1982) Histoenzymologic
characteristics of liver and kidney after acute and chronic
poisoning by 1,2-dichloroethane. In: Proceedings of the
Leningrad Scientific Society of Pathologoanatoms, Leningrad,
Medicine, Vol. 23, pp. 158-160.
BONNET, P., FRANCIN, J.-M., GRADISKI, D., RAOULT, G., & ZISSU,
D. (1980) Détermination de la concentration léthale 50 des
principaux hydrocarbures aliphatiques chlorés chez le rat.
Arch. Mal. prof. Méd. Trav. Sécur. soc., 41: 317-321.
BORISSOVA, M.K. (1957) [Experimental inputs to evaluation of
maximum allowable dichloroethane concentration in the air.] Gig.
i Sanit., 3: 13-19 (in Russian).
BORISSOVA, M.K. (1960) Some inputs to evaluation of maximum
allowable dichloroethane concentration in the air. In: Maximum
allowable concentrations of air pollutants, Vol. 4, pp. 21-24.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformations of 1- and
2-carbon halogenated aliphatic organic compounds under methano-
genic conditions. Appl. environ. Microbiol., 45: 1286-1294.
BOZZELLI, J.W. & KEBBEKUS, B.B. (1982) A study of some
aromatic and halocarbon vapors in the ambient atmosphere of New
Jersey. J. environ. Sci. Health, A17: 693-711.
BREM, H., STEIN, A.B., & ROSENKRANZ, H.S. (1974) The muta-
genicity and DNA-modifying effect of haloalkanes. Cancer Res.,
34: 2576-2579.
BRINGMANN, G. & KUHN, R. (1978) Testing of substances for
their toxicity threshold: model organisms Microcystis
(Diplocystis) aeruginosa and Scenedesmus quadricauda. Mitt.
Int. Verein Limnol., 21: 275-284.
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa
in the cell multiplication inhibition test. Water Res., 14:
231-241.
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effect of
hazardous substances on flagellated and ciliated, respectively,
holozoic bactivorous and saprozoic protozoa.] GWF Wasser-
Abwasser, 122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Data on the toxicity of water
pollutants to Daphnia magna in an improved standard test.] Z.
Wasser Abwasser Forsch., 15: 1-6 (in German).
BRYZHIN, F.F. (1945) Pathomorphological changes in internal
organs in connection with poisoning by ethylene dichloride
through the digestive tract. Pharmacol. Toxicol., 8: 43-49.
BUCCAFUSCO, R.J., ELLS, S.J., & LE BLANC, G.A. (1981) Acute
toxicity of priority pollutants of bluegill Lepomis macro-
chirus. Bull. environ. Contam. Toxicol., 26: 446-452.
CASWELL, G.H. & CLIFFORD, H.T. (1958) The effect of ethylene
dichloride and carbon tetrachloride on the germination and early
growth of maize. Emp. J. exp. Agric., 26: 365-372.
CETNAROWICZ, J. (1959) Experimental and clinical investiga-
tions on the action of dichloroethane. Folia Med. Cracov., 1:
169-192.
CLARK, A.I., MCINTYRE, A.E., LESTER, J.N., & PERRY, R. (1984a)
Ambient air measurement of aromatic and halogenated hydrocarbons
at urban, rural, and motorway locations. Sci. total Environ.,
39: 265-279.
CLARK, A.I., MCINTYRE, A.E., PERRY, R., & LESTER, J.N. (1984b)
Monitoring and assessment of ambient atmospheric concentrations
of aromatic and halogenated hydrocarbons at urban, rural, and
motorway locations. Environ. Pollut. Ser. B, 7: 141-158.
CREBELLI, R., CONTI, G., CONTI, L., & CARERE, A. (1984)
Induction of somatic segregation by halogenated aliphatic
hydrocarbons in Aspergillus nidulans. Mutat. Res., 138(1): 33-
38.
CRESPI, C.L., SEIXAS, G.M., TURNER, T.R., RYAN, C.G., & PENMAN,
B.W. (1985) Mutagenicity of 1,2-dichloroethane and 1,2-
dibromoethane in two human lymphoblastoid cell lines. Mutat.
Res., 142: 133-140.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA 600/3-80-
084, PB 221948).
DAWSON, G.W., JENNINGS, A.L., DROSDOWSKI, D., & RIDER, E.
(1977) The acute toxicity of 47 industrial chemicals to fresh
and saltwater fish. J. hazard. Mater., 1: 303-318.
DEQUINZE, J., SZIMAR, C., & EDELINE, F. (1984) Identification
of the substances and their derivative products, on the list of
129 substances (list 1 of the directive 76/464/EEC) present in
the refuse of chlorine derivatives by organic chemistry,
Brussels, European Economic Community.
DILLING, W.L., TEFERTILLER, N.B., & KALLOS, G.J. (1975)
Evaporation rates and reactivities of methylene chloride,
chloroform, 1,1,1-trichloroethane, trichloroethylene,
tetrachloroethylene, and other chlorinated compounds in dilute
aqueous solutions. Environ. Sci. Technol., 9: 833-838.
DMITRIEVA, N.V. & KULESHOV, E.V. (1971) [Changes in the
cerebral bioelectric activity and electric conductivity in rats
in chronic intoxications with certain chlorinated hydrocarbons.]
Gig. i Sanit., 36: 20-25 (in Russian).
DORNDORF, W., KRESSE, M., CHRISTIAN, W., & KATRITZKI, KG.
(1975) [Dichloroethane poisoning with myoclinic syndrome,
epileptic attacks, and irreversible cerebral effects.] Arch.
Psychiatr. Nervenkr., 220: 373-379 (in German).
DRURY, J.S. & HAMMONS, A.S. (1979) Investigations of selected
environmental pollutants: 1,2-dichloroethane, Washington DC, US
Environmental Protection Agency, Office of Toxic Substances (EPA
560/2-78-006, PB 295865).
DUPRAT, P., DELSAUT, L., & GRADISKI, D. (1976) Pouvoir
irritant des principaux solvants chlorés aliphatiques sur la
peau et les muqueuses oculaires du lapin. Eur. J. Toxicol., 9:
171-177.
EHRENBERG, Z., OSTERMAN-GOLKAR, S., SINGH, D., & LUNDQVIST, U.
(1974) On the reaction kinetics and mutagenic activity of
methylating and beta-halogenoethylating gasoline additives.
Radiat. Bot., 15: 184-194.
ELFERS, L.A. (1979) Monitoring of ambient levels of EDC in the
vicinity of EDC production and user facilities, Research
Triangle Park, North Carolina, US Environmental Protection
Agency, Environmental Monitoring and Support Laboratory (EPA
600/4-79-029, PB 298303).
ENVIRONMENT AGENCY, JAPAN (1983) Environmental monitoring of
chemicals: environmental survey report of fiscal years 1980 and
1981, Tokyo, Environment Agency.
EWING, B.B., CHIAN, E.S.K., COOK, J.C., DEWALLE, F.B., EVANS,
C.A., HOPKE, P.K., KIM, J.H., MEANS, J.C., MILBERG, R., PERKINS,
E.G., SHERWOOD, J.D., & WADLIN, W.H. (1977) Monitoring to
detect previously unrecognized pollutants in surface waters,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA 560/6-77-015, PB 273349).
FLOWTOW, E. (1952) Poisoning due to chlorinated hydrocarbon
compounds, particularly 1,2-dichloroethane. Chem. Tech.
(Leipzig), 6: 253-254.
FOUREMAN, G.L. & REED, D.J. (1985) Evidence for a non-
episulfonium ion intermediate during alkylation by S-(2-
chloroethyl) cysteine but not by S-(2-chloroethyl) glutathione.
Fed. Proc. Fed. Am. Exp. Biol., 44: 517.
FUJII, T. (1977) Direct aqueous injection gas chromato-
graphy-mass spectrometry for analysis of organohalides in water
at concentrations below the parts per billion level. J.
Chromatogr., 139: 297-302.
GARRISON, S.C. & LEADINGHAM, R.S. (1954) A fatal case of
ethylene dichloride poisoning in an occupational therapy
department of a neuro-psychiatric hospital. Am. J. phys. Med.,
33: 230-237.
GOCKE, E., WILD, D., ECKHARDT, K., & KING, M.-T. (1983)
Mutagenicity studies with the mouse spot test. Mutat. Res., 117:
201-212.
GOLD, L.S. (1980) Human exposures to ethylene dichloride. In:
Ames, B., Infante, P., & Reitz, R., ed. Ethylene dichloride: a
potential health risk? Cold Spring Harbor, Cold Spring Harbor
Laboratory, pp. 209-225 (Banbury Report No. 5).
GOSSETT, R.W., BROWN, D.A., & YOUNG, D.R. (1983) Predicting
the bioaccumulation of organic compounds in marine organisms
using octanol/water partition coefficients. Mar. Pollut. Bull.,
14: 387-392.
GRADISKI, D., BONNET, P., RAOULT, G., MAGADUR, J.L., & FRANCIN,
J.-M. (1978) Toxicité aiguë comparée par inhalation des
principaux solvants aliphatiques chlorés. Arch. Mal. prof. Méd.
Trav. Sécur. soc., 39: 249-257.
GRIMSRUD, E.P. & RASMUSSEN, R.A. (1975) Survey and analysis of
halocarbons in the atmosphere by gas chromatography-mass
spectrometry. Atmos. Environ., 9: 1014-1017.
GUENGERICH, F.P., CRAWFORD, W.M., DOMORADZKI, J.Y., MACDONALD,
T.L., & WATANABE, P.G. (1980) In vitro activation of 1,2-
dichloroethane by microsomal and cytosolic enzymes. Toxicol.
appl. Pharmacol., 55: 303-317.
GUENGERICH, F.P., MASON, P.S., STOTT, W.T., FOX, T.R., &
WATANABE, P.G. (1981) Role of 2-haloethylene oxides and vinyl
chloride in irreversible binding to protein and DNA. Cancer
Res., 41: 4391-4398.
GUENGERICH, F.P., KOGA, N., INSKEEP, P.B., & OZAWA, N. (1986)
Proceedings of the Second International Symposium on the
Synthesis and Application of Isotopically-Labelled Compounds,
New York, Elsevier.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of
organic chemicals in the atmosphere of the Netherlands. Sci.
total Environ., 43: 193-219.
HADENGUE, M.A. & MARTIN, R. (1953) Un cas d'intoxication
mortelle par le dichloréthane. Soc. Med. Leg., 33: 247-249.
HARKOV, R., KEBBEKUS, B., BOZZELLI, J.W., LLOY, P.J., & DAISEY,
J. (1984) Comparison of selected volatile organic compounds
during the summer and winter at urban sites in New Jersey. Sci.
total Environ., 38: 259-274.
HATCH, G.G., MAMAY, P.D., AYER, M.L., CASTO, B.C., & NESNOW, S.
(1983) Chemical enhancement of viral transformation in Syrian
hamster embryo cells by gaseous and volatile chlorinated
methanes and ethanes. Cancer Res., 43: 1945-1950.
HEITMULLER, P.T., HOLLISTER, T.A., & PARRISH, P.R. (1981)
Acute toxicity of 54 industrial chemicals to sheepshead minnows
Cyprinodon variegatus. Bull. environ. Contam. Toxicol., 27:
596-604.
HELLMANN, T.M. & SMALL, F.H. (1974) Characterization of the
odour properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
HEPPEL, L.A., NEAL, P.A., PERRIN, T.L., ENDICOTT, K.M., &
PORTERFIELD, V.T. (1946) The toxicology of 1,2-dichloro-
ethane (ethylene dichloride). V. The effect of daily
inhalations. J. ind. Hyg. Toxicol., 28: 113-120.
HILL, D.L., SHIH, T.-W., JOHNSTON, T.P., & STRUCK, R.F. (1978)
Macromolecular binding and metabolism of the carcinogen 1,2-
dibromoethane. Cancer Res., 38: 2438-2442.
HINKEL, G.K. (1965) [Oral dichloroethane intoxication of
children.] Dtsch Gesudheitswes., 20: 1327-1331 (in German).
HOFMANN, H.T., BIRNSTIEL, H., & JOBST, P. (1971) [On the
inhalation toxicity of 1,2-dichloroethane.] Arch. Toxikol., 27:
248-265 (in German).
HOOPER, K., GOLD, I., & AMES, B. (1980) The carcinogenic
potency of ethylene dichloride in two animal bioassays: a
comparison of inhalation and gavage studies. In: Ames, B.N.,
Infante, P., & Reitz, R., ed. Ethylene dichloride: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 65-81 (Banbury Report No. 5).
HOWARD, C.J. & EVENSON, K.M. (1976) Rate constants for the
reactions of OH with ethane and some halogen substituted ethanes
at 296 K. J. Chem. Phys., 64: 4303-4306.
HUEPER, W.C. & SMITH, C. (1935) Fatal ethylene dichloride
poisoning. Am. J. med. Sci., 189: 778-784.
IARC (1979) 1,2-Dichloroethane. In: Some halogenated hydro-
carbons, Lyons, International Agency for Research on Cancer, pp.
429-446 (IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Vol. 20).
INSKEEP, P.B. & GUENGERICH, F.P. (1984) Glutathione mediated
binding of dibromoalkanes to DNA: specificity of rat glutathione
S-transferases and dibromoalkane structure. Carcinogenesis, 5:
805-808.
INSKEEP, P.B., KOGA, N., CMARIK, J.L., & GUENGERICH, F.P.
(1986) Covalent binding of 1,2-dihaloalkanes to DNA and
stability of the major DNA adduct S-[2-(N7/guanyl)ethyl]
glutathione. Cancer Res., 46: 2839-2844.
IRPTC (1982) Izmerov, N.F., ed. Dichloroethane, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme (Scientific Review of Soviet
Literature on Toxicity and Hazards of Chemicals No. 25).
IRPTC (1984) Data profile of 1,2-dichloroethane, Geneva,
International Register of Potentially Toxic Chemicals.
JACOBS, E.S. (1980) Use and air quality impact of ethylene
dichloride and ethylene dibromide scavengers in leaded gasoline.
In: Ames, B., Infante, P., & Reitz, R., ed. Ethylene dichloride:
a potential health risk?, Cold Spring Harbor, New York, Cold
Spring Harbor Laboratory, pp. 239-255 (Banbury Report No. 5).
JAKOBSON, I., WAHLBERG, J.E., HOLMBERG, B., & JOHANSSON, G.
(1982) Uptake via the blood and elimination of 10 organic
solvents following epicutaneous exposure of anesthetized guinea-
pigs. Toxicol. appl. Pharmacol., 63: 181-187.
JANSSEN, D.B., SCHEPER, A., & WITHOLT, B. (1984) Biodegra-
dation of 2-chloroethanol and 1,2-dichloroethane by pure
bacterial cultures. Prog. Med. Microbiol., 20: 169-178.
JENSEN, S., LANGE, R., BERGE, G., PALMORK, K.H., & RENBERG, L.
(1975) On the chemistry of EDC-tar and its biological
significance in the sea. Proc. R. Soc. Lond. B, 189: 333-346.
JENSSEN, D. & RAMEL, C. (1980) The micronucleus test as part
of a short-term mutagenicity test program for the prediction of
carcinogenicity evaluated by 143 agents tested. Mutat. Res., 75:
191-202.
JERNELOV, A., ROSENBERG, R., & JENSEN, S. (1972) Biological
effects and physical properties in the marine environment of
aliphatic chlorinated by-products from vinyl chloride
production. Water Res., 6: 1181-1191.
JOHNSON, M.K. (1965) The influence of some aliphatic compounds
on rat liver glutathione levels. Biochem. Pharmacol., 14: 1383-
1385.
JOHNSON, M.K. (1966) Studies on glutathione-S-alkyl transferase
of the rat. Biochem. J., 98: 44-56.
JOHNSON, M.K. (1967) Metabolism of chloroethanol in the rats.
Biochem. Pharmacol., 16: 185-199.
JOLIVET, E. (1968) Métabolisme des acides organiques et des
acides aminés libres dans le tubercule de pomme de terre au
cours de la rupture provoquée de son repos végétatif par la
"Rindite". Physiol. vég., 6: 221-233.
JONSSON, A. & BERG, S. (1980) Determination of 1,2-dibromo-
ethane, 1,2-dichloroethane, and benzene in ambient air using
porous polymer traps and gas chromatographic-mass spectro-
metric analysis with selected ion monitoring. J. Chromatogr.,
190: 97-106.
KAGRAMANOV, V.G. & KAZIEVA, N.K. (1972) Condition of
fibrinolytic blood activity in acute dichloroethane poisoning.
Azerb. State Med. Inst. Baku, 36: 30-31.
KAIRA, F.M. (1966) [Alimentary oral dichloroethane poisoning.]
Klin. Med. (Mosk.), 44: 143-146 (in Russian).
KAMEL, A. (1959) The effect of carbon bisulphide and ethylene
dichloride-carbon tetrachloride mixture on the germination of
some seeds of certain crops. Agric. Res. Rev. (Cairo), 37: 330-
350.
KELLAM, R.G. & DUSETZINA, M.G. (1980) Human exposure to
ethylene dichloride: potential for regulation via EPA's proposed
airborne carcinogen policy. In: Ames, B., Infante, P., & Reitz,
R., ed. Ethylene dichloride: a potential health risk?, Cold
Spring Harbor, New York, Cold Spring Harbor Laboratory, pp.
265-276 (Banbury Report No. 5).
KERSTER, H.W. & SCHAEFFER, D.J. (1983) Brine shrimp (Artemia
salina nauplii) as a teratogen test system. Ecotoxicol.
environ. Saf., 7: 342-349.
KING, M.-T., BEIKIRCH, H., ECKHARDT, K., GOCKE, E., & WILD, D.
(1979) Mutagenicity studies with X-ray contrast media,
analgesics, antipyretics, antirheumatics, and some other
pharmaceutical drugs in bacterial, Drosophila, and mammalian
test systems. Mutat. Res., 66: 33-43.
KLEINSCHMIDT, E.-G., VON (1983) [Investigations into the
relation between odour thresholds of human beings for some
substances and their chemical structure.] Wiss. Z. Wilhelm-
Piech-Univ. Rostock, Naturwiss. Reihe, 32: 54-58 (in German).
KOGA, N., INSKEEP, P.B., HARRIS, T.M., & GUENGERICH, F.P.
(1986) S-[2-(N7-guanyl)ethyl] glutathione, the major DNA adduct
formed from 1,2-dichloroethane. Biochemistry, 25: 2192-2198.
KOKAROVTSEVA, M.G. & KISELYOVA, N. (1978) [Chloroethanol
(ethylenechlorohydrin) - a toxic metabolite of 1,2-dichloro-
ethane.] Farmakol. Toksikol., 41: 118-120 (in Russian).
KOMAROV, V.P., ROMANCHAK, M.N., SHCHERBAN, I.I., & GAPONTSEV,
S.N. (1973) [On the clinical features and therapy of acute
dichloroethane intoxications.] Zdravookhr. Belorussi, 4: 55-57
(in Belorussian).
KONEMANN, H. (1981) Quantitative structure-activity
relationships in fish toxicity studies. I. Relationship for 50
industrial pollutants. Toxicology, 19: 209-221.
KOZIK, I.V. (1957) [Some problems of occupational hygiene in
the use of dichloroethane in the aircraft industry.] Gig. Tr.
Prof. Zabol., 1: 31-38 (in Russian).
KRONEVI, T., WAHLBERG, J.E., & HOLMBERG, B. (1981) Skin
pathology following epicutaneous exposure to seven organic
solvents. Int. J. Tissue React., 3: 21-30.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A.
(1982) Collection and analysis of hazardous organic emissions.
Anal. Chem., 54: 810-817.
KULKARNI, J.H., SARDESHPANDE, J.S., & BAGYARAJ, D.J. (1975)
Effect of seed fumigation on the symbiosis of Rhizobium sp.
with Arachis hypogaea Linn. Zentralbl. Bakteriol. Parasitenkr.
Infektionskr. Hyg. Abt. II, 130: 41-44.
KUWABARA, T., QUEVEDO, A.R., & COGAN, D. (1968) An experi-
mental study of dichloroethane poisoning. Arch. Ophtalmol., 79:
321-330.
LANE, R.W., RIDDLE, B.L., & BORZELLECA, J.F. (1982) Effects of
1,2-dichloroethane and 1,1,1-trichlorethane in drinking-water on
reproduction and development in mice. Toxicol. appl. Pharmacol.,
63: 409-421.
LARIONOV, V.G. & KOKAROVTSEVA, M.G. (1976) Morphological
constitution of peripheral blood in intoxication with
dichloroethane and its metabolites. In: Actual problems of
pesticide application in different climatographic zones,
Yerevan, Aiastan Publishers, pp. 131-133.
LE BLANC, G.A. (1980) Acute toxicity of priority pollutants to
water flea Daphnia magna. Bull. environ. Contam. Toxicol., 24:
684-691.
LIN, E.L.C., MATTOX, J.K., & PEREIRA, M.A. (1985) Glutathione
plus cytosol- and microsome-mediated binding of 1,2-dichloro-
ethane to polynucleotides. Toxicol. appl. Pharmacol., 78: 428-
435.
LIVESEY, J.C. & ANDERS, M.W. (1979) In vitro metabolism of
1,2-dihaloethanes to ethylene. Drug Metab. Dispos., 7: 100-203.
LOCHHEAD, H.B. & CLOSE, H.P. (1951) Ethylene dichloride
plastic cement: a case of fata poisoning. J. Am. Med. Assoc.,
146: 1323.
LUZNIKOV, E.A., LISOVIK, G.A., & NOVIKOVSKAYA, T.V. (1985)
[Metabolism of 1,2-dichloroethane in human organisms after acute
poisonings.] [ Forensic Med. Expert.,] 2: 47-49 (in Russian
with English summary).
LYNN, G.E. & VORHES, F.A., ed. (1957) Symposium on residues in
foods and feeds resulting from fumigation of grains with the
commoner liquid formulations of carbon disulphide, carbon
tetrachloride, ethylene dichloride, and ethylene dibromide. J.
Assoc. Off. Agric. Chem., 40: 163-209.
MCCANN, J., SIMMON, V., STREITWIESER, D., & AMES, B. (1975)
Mutagenicity of chloroacetaldehyde, a possible metabolic product
of 1,2-dichloroethane (ethylene dichloride), chloroethanol
(ethylene chlorohydrin), vinyl chloride, and cyclophosphamide.
Proc. Natl Acad. Sci. (USA), 72: 3190-3193.
MCCOLLISTER, D.D., HOLLINGSWORTH, R.L., OYEN, F., & ROWE, V.K.
(1956) Comparative inhalation toxicity of fumigant mixtures.
Individual and joint effects of ethylene dichloride, carbon
tetrachloride, and ethylene dibromide. Arch. ind. Health, 13:
1-7.
MALTONI, C., VALGIMIGLI, L., & SCARNATO, C. (1980) Long-term
carcinogenic bioassays on ethylene dichloride administered by
inhalation to rats and mice. In: Ames, B., Infante, P., & Reitz,
R., ed. Ethylene dichloride: a potential health risk?, Cold
Spring Harbor, New York, Cold Spring Harbor Laboratory, pp. 5-30
(Banbury Report No. 5).
MARTIN, G., KNORPP, K., HUTH, K., HEINRICHT, F., & MITTERMAYER,
C. (1968) [Clinical features, pathogenesis and therapy of
dichloroethane poisoning.] Dtsch med. Wschr., 93: 2002-2010 (in
German).
MAY, J. (1966) [Odour thresholds of solvents for the judgement
of solvent odour in air.] Staub, 26: 385-389 (in German).
MENSCHICK, H. (1957) [Acute inhalation intoxications by
symmetric dichloroethane.] Arch. Gewerbepathol. Gewerbehyg., 15:
241-252 (in German).
MITOMA, C., STEEGER, T., JACKSON, S.E., WHEELER, K.P., ROGERS,
J.H., & MILMAN, H.A. (1985) Metabolic disposition study of
chlorinated hydrocarbons in rats and mice. Drug chem. Toxicol.,
8: 183-194.
MOODY, E.D., JAMES, J.L., CLAWSON, G.A., & SMUCKLER, E.A.
(1981) Correlations among the changes in hepatic microsomal
components after intoxication with alkyl halides and other
heptatotoxins. Mol. Pharmacol., 20: 685-693.
MOROZOV, G.N. (1958) [On acute dichloroethane poisoning.]
Farmakol. Toksicol., 21(1): 76-78 (in Russian).
MUNSON, A.E., SANDERS, V.M., DOUGLAS, K.A., SAIN, L.E.,
KAUFFMANN, B.M., & WHITE, K.L. (1982) In vivo assessment of
immunotoxicity. Environ. Health Perspect., 43: 41-52.
NACHTOMI, E., ALUMOT, E., & BONDI, A. (1966) The metabolism of
ethylene dibromide in the rat. I. Identification of detoxifi-
cation products in urine. Isr. J. Chem., 4: 329-246.
NATSYUK, M.V. & CHEKMAN, I.S. (1975) [Content of nicotinamide
coenzymes in the liver and myocardium of rats poisoned with
dichloroethane.] Byull. eksp. Biol. Med., 79: 58-60 (in
Russian).
NATSYUK, M.V. & MUDRITSKY, A.D. (1974) Clinical and
biochemical characteristics of hepatic and renal disorders in
acute dichloroethane intoxications. Mil. med. J., 10: 48-50.
NATSYUK, M.V., CHERNUKHA, F.A., BASENKO, G.A., & FEDUROV, V.V.
(1974) [Effect of acute dichloroethane intoxication on
oxidative phosphorylation in rat liver mitochondria.] Farmakol.
Toksikol., 37: 92-93 (in Russian).
NAZIKHI, F. & SKRIZHINSKY, S.F. (1973) Activity of succinate-
toxicodase system enzymes of myocardium in experimental
dichloroethane intoxication. In: Current issues of physiology,
pathology, and clinical diseases of the cardiovascular system,
Leningrad, pp. 62-63.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute,
US Department of Health, Education and Welfare, Public Health
Services, National Institute of Health (HEW (NIH) Publication
No. 78-1305).
NELSON, G.O. & SHAPIRO, E.G. (1971) A field instrument for
detecting airborne halogen compounds. Am. Ind. Hyg. Assoc. J.,
32: 757-765.
NEVROTSKY, V.K., KASHIN, L.M., KULINSKAYA, I.L., MIKHAILOVSKAYA,
L.F., SHMUTER, L.M., BURLAKA-VOVK, Z.I., & ZADOROZHNY, B.V.
(1971) Comparative toxicity evaluation of several industrial
poisons in protracted inhalation exposure to low concentrations.
In: Proceedings of the 8th Ukrainian Hygiene Congress, Kiev,
1971, pp. 224-226.
NIOSH (1976) Criteria for a recommended standard -
occupational exposure to ethylene dichloride (1,2-dichloro-
ethane), Cincinnati, Ohio, National Institute of Occupational
Safety and Health, US Department of Health, Education and
Welfare, Public Health Services, Center for Disease Control
(DHEW (NIOSH) Publication No. 76-139).
NIOSH (1977) Manual of analytical methods. II. Standards
completion program validated methods, Cincinnati, Ohio, National
Institute of Occupational Safety and Health, Vol. 2, pp. S122/1-
S122/9 (DHEW (NIOSH) Publication No. 77-157-B).
NOETZEL, O. (1944) Lethal poisoning with ethylene dichloride.
Chem. Ztg., 68: 146-147.
NOUCHI, T., MIURA, H., KANAYAMA, M., MIZUGUCHI, O., & TAKANO, T.
(1984) Fatal intoxication by 1,2-dichloroethane: a case report.
Int. Arch. occup. environ. Health, 54: 111-113.
NYLANDER, P.-O., OLOFSSON, H., RASMUSON, B., & SVAHLIN, H.
(1978) Mutagenic effects of petrol in Drosophila melanogaster:
effects of benzene and 1,2-dichloroethane. Mutat. Res., 57:
163-167.
OKAMOTO, T. & TATSUKAWA, R. (1981) [Distribution and fate of
low molecular weight chlorinated hydrocarbons in Osaka Bay.]
Chikyukagaku, 15: 17-24 (in Japanese).
OZAWA, N. & GUENGERICH, F.P. (1983) Evidence for formation of
an S-[2-(N7-guanyl)ethyl] glutathione adduct in glutathione-
mediated binding of the carcinogen 1,2-dibromoethane to DNA.
Proc. Natl Acad. Sci. (USA), 80: 5268-5270.
PAGE, B.D. & KENNEDY, B.P.C. (l975) Determination of methylene
chloride, ethylene dichloride and trichloroethylene as solvent
residues in spice oleoresins, using vacuum distillation and
electron capture. J. Assoc. Off. Anal. Chem., 58: 1062-1068.
PAPAROPOLI, G. & CALI, V. (l956) [Collective intoxications
with chlorinated hydrocarbons in dock workers.] Folia Med., 39:
819-831 (in Italian).
PARKES, D.G., GANZ, C.R., POLINSKY, A., & SCHULZE, J. (l976) A
simple gas chromatographic method for the analysis of trace
organics in ambient air. Am. Ind. Hyg. Assoc. J., 37: 165-173.
PEARSON, C.R. & MCCONNELL, G. (l975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proc. R. Soc. Lond. B,
189: 305-332.
PEROCCO, P. & PARODI, G. (1981) DNA damage by haloalkanes in
human lymphocytes cultures in vitro. Cancer Lett., 13: 213-218.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
PRINCIPE, P., DOGLIOTTI, E., BIGNAMI, M., CREBELLI, R., FALCONE,
E., FABRIZI, M., CONTI, G., & COMBA, P. (1981) Mutagenicity of
chemicals of industrial and agricultural relevance in
Salmonella, Streptomyces, and Aspergillus. J. Sci. food Agric.,
32: 826-832.
RADDING, S.B., LUI, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA report no. 560/5-77-003, PB 267121.
RANNUG, U. (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichoroethane. Mutat. Res., 76: 269-295.
RANNUG, U. & BEIJE, B. (1979) The mutagenic effect of 1,2-
dichloroethane on Salmonella typhimurium. II. Activation by the
isolated perfused rat liver. Chem.-biol. Interact., 24: 265-
285.
RANNUG, U., SUNDVALL, A., & RAMEL, C. (1978) The mutagenic
effect of 1,2-dichloroethane on Salmonella typhimurium. I.
Activation through conjunction with glutathione in vitro. Chem.-
biol. Interact., 20: 1-16.
RAO, K.S., MURRAY, J.S., DEACON, M.M., JOHN, J.A., CALHOUN,
L.L., & YOUNG, J.T. (1980) Teratogenicity and reproduction
studies in animals inhaling ethylene dichloride. In: Ames, B.,
Infante, P., & Reitz, R., ed. Ethylene dichloride: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 145-161 (Banbury Report No. 5).
RAPOPORT, I.A. (1960) [The reaction of genic proteins with
1,2-dichloroethane.] Dokl. Akad. Nauk. SSSR, 134: 1214-1217 (in
Russian).
REITZ, R.H., FOX, T.R., RAMSEY, J.C., QUAST, J.F., LANGVARDT,
P.W., & WATANABE, P.G. (1982) Pharmacokinetics and macro-
molecular interactions of ethylene dichloride in rats after
inhalation or gavage. Toxicol. appl. Pharmacol., 62: 190-204.
RICHTER, J.E., PETERSON, S.F., & KLEINER, C.F. (1983) Acute
and chronic toxicity of some chlorinated benzenes, chlorinated
ethanes and tetrachloroethylene to Daphnia magna. Arch. environ.
Contam. Toxicol., 12: 679-684.
ROHMANN, E., VON, ZINN, D., & KULZ, J. (1969) [Electro-
encephalographic observations in child blood poisonings and
their therapeutic consequences.] Kinderaerztl. Prax., 37: 209-
216 (in German).
ROSENBERG, R. (1972) Effects of chlorinated aliphatic hydro-
carbons on larval and juvenile Balanus balanoides L. Environ.
Pollut., 3: 313-318.
ROSENBERG, R., GRAHN, O., & JOHANSSON, L. (1975) Toxic effects
of aliphatic chlorinated by-products from vinyl chloride
production on marine animals. Water Res., 9: 607-612.
ROUBAL, J. (1947) Two fatal cases of intoxication with
symmetric dichloroethane ingestion. Cas. Lek. Cesk., 86: 203-
206.
SAITANOV, A.O. & ARSENIEVA, S.S. (1969) Electrocardiographic
changes due to acute dichloroethane poisoning: experimental
investigation. Gig. Tr. Prof. Zabol., 7: 49-50.
SALTZMAN, B.E. (1972) Direct reading colorimetric indicators.
In: Air sampling instruments for evaluation of atmospheric
contamination, 4th ed., Cincinnati, Ohio, American Conference of
Governmental Hygienists, pp. S22-23.
SAUER, T.C. (1981) Volatile organic compounds in open ocean
and coastal surface waters. Org. Geochem., 3: 91-101.
SCHASTEEN, C.S. & REED, D.J. (1983) The hydrolysis and
alkylation activities of S-(2-haloethyl)-L-cysteine analogues -
evidence for extended half-life. Toxicol. appl. Pharmacol., 70:
423-432.
SCHERB, K. (1978) [Investigations on the evaporation of low
molecular weight chlorinated hydrocarbons from flow-gutter.]
Munch. Beitr. Abwasser-Fisch. Flussbiol., 30: 235-248 (in
German).
SCHONBORN, H., PRELLWITZ, W., & BAUM, P. (1970) [Consumption
coagulation pathology of 1,2-dichloroethane poisoning.] Klin.
Wochenschr., 48: 822-824 (in German).
SECCHI, G.C., CHIAPPINO, G., LOTTO, A., & ZURLO, N. (1968)
[Actual chemical composition of the "commercial trieline" and
their hepatotoxic effect: clinical and enzymological studies.]
Med. Lav., 59: 486-497 (in Italian).
SEUFERT, F.B., BROWN, P., OATWAY, J.A., BORNSTEIN, M.,
OSTROWSKI, W., & HORNE, R. (1980) 1,2-Dichloroethane technical
control options analysis, Bedford, Massachusetts, CGA
Corporation (EPA Contract No. 68-01-5960).
SHAKARNIS, V.F. (1969) [1,2-Dichloroethane-induced chromosome
non-disjunction and recessive sex-linked lethal mutations in
Drosophila melanogaster.] Genetika, 5: 89-95 (in Russian).
SHAKARNIS, V.F. (1970) [Effect of 1,2-dichloroethane on
chromosome non-disjunction and recessive sex-linked lethals in a
radioresistant strain of Drosophila melanogaster.] [ Vestn.
Leningrad Univ. Biol.,] 25: 153-156 (in Russian).
SHMUTER, L.M. (1977) [Effect of chronic exposure to low
concentrations of chlorinated hydrocarbons of the ethane series
on specific and non-specific reactivity of animals in vivo.]
Gig. Tr. Prof. Zabol., 8: 38-42 (in Russian).
SIMMON, V.F. (1980) Review of non-bacterial tests of the
genotoxic activity of ethylene chloride. In: Ames, B., Infante,
P., & Reitz, R., ed. Ethylene chlorde: a potential health risk?,
Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp. 97-103 (Banbury Report No. 5).
SINGH, H.B., SALAS, L.J., & STILES, R.E. (1982) Distribution
of selected gaseous organic mutagens and suspect carcinogens in
ambient air. Environ. Sci. Technol., 16: 872-880.
SINGH, H.B., SALAS, L.J., & STILES, R.E. (1983) Selected man-
made halogenated chemicals in the air and oceanic environment.
J. geophys. Res., 88: 3675-3683.
SOPIKOV, N.G. & GORSHUNOVA, A.I. (1979) [Investigation of the
intake, distribution and excretion of ethylene dichloride in
rats.] Gig. Tr. Prof. Zabol., 4: 36-40 (in Russian).
SPENCE, J.W. & HANST, P.L. (1978) Oxidation of chlorinated
ethanes. J. Air Pollut. Control Assoc., 28: 250-253.
SPENCER, H.C., ROWE, V.K., ADAMS, E.M., MCCOLLISTER, D.D., &
IRISH, D.D. (1951) Vapor toxicity of ethylene dichloride
determined by experiments on laboratory animals. Arch. ind.
Hyg. occup. Med., 4: 482-493.
SPREAFICO, F., ZUCCATO, E., MARCUCCI, F., SIRONI, M.,
PAGLIALUNGA, S., MADONNA, M., & MUSSINI, E. (1980) Pharmaco-
kinetics of ethylene dichloride in rats treated by different
routes and its long-term inhalatory toxicity. In: Ames, B.,
Infante, P., & Reitz, R., ed. Ethylene chlorde: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 107-129 (Banbury Report No. 5).
STORER, R.D. & CONOLLY, R.B. (1983) Comparative in vivo
genotoxicity and hepatotoxicity of three 1,2-dihaloethanes.
Carcinogenesis, 4: 1491-1494.
STORER, R.D. & CONOLLY, R.B. (1985) An investigation of the
role of microsomal oxidative metabolism in the in vivo
genotoxicity of 1,2-dichloroethane. Toxicol. appl. Pharmacol.,
77: 36-46.
STORER, R.D., JACKSON, N.M., & CONOLLY, R.B. (1984) In vivo
genotoxicity and acute hepatotoxicity of 1,2-dichloroethane in
mice: comparison of oral, intraperitoneal, and inhalation routes
of exposure. Cancer Res., 44: 4267-4271.
STOREY, C.L., KIRK, L.D., & MUSTAKAS, G.C. (1972) Fate of EDC-
CC14 (75:25) residues during milling and oil extraction of
soybeans. J. econ. Entomol., 65: 1126-1129.
STOVER, E.L. & KINCANNON, D.F. (1983) Biological treatability
of specific organic compounds found in chemical industry
wastewaters. J. Water Pollut. Control Fed., 55: 97-105.
STUCKEY, D.C., OWEN, W.F., MCCARTY, P.L., & PARKIN, G.F. (1980)
Anaerobic toxicity evaluation by batch and semi-continuous
assays. J. Water Pollut. Control Fed., 52: 720-729.
STUCKI, G., BRUNNER, W., STAUB, D., & LEISINGER, T. (1981)
Microbial degradation of chlorinated C1 and C2 hydrocarbons.
In: Leisinger, T., Cook, A.M., Hütter, R., & Nüesch, J., ed.
FEMS Symposium No. 12, Microbial degradation of xenobiotics and
recalcitrant compounds, London, Academic Press, pp. 131-137.
SUNDHEIMER, D.W., WHITE, R.D., BRENDEL, K., & SIPES, I.C.
(1982) The bioactivation of 1,2-dibromoethane in rat
hepatocytes: covalent binding to nucleic acids. Carcinogenesis,
3: 1129-1133.
SUVEEV, I.M. & BABICHENKO, M.E. (1969) [On the clinic and cure
of acute intoxication with dichloroethane vapours.] Gig. Tr.
Prof. Zabol., 13: 50-51 (in Russian).
SYMONS, J.M., BELLAR, T.A., CARSWELL, J.K., DEMARCO, J., KROPP,
K.L., ROBECK, G.G., SEEGER, D.R., SLOCUM, C.J., SMITH, B.L., &
STEVENS, A.A. (1975) National organics reconnaissance survey
for halogenated organics. J. Am. Water Works Assoc., 67: 634-
648.
TABAK, H.H., QUAVE, S.A., MASHNI, C.E., & BARTH, E.F. (1981)
Biodegradability studies with organic priority pollutant
compounds. J. Water Pollut. Control Fed., 53: 1503-1517.
TAN, E.-L. & HSIE, A.W. (1981) Mutagenicity and cytotoxicity
of haloethanes as studied in the CHO-HGPRT system. Mutat. Res.,
90: 183-191.
THORUP, S. (1957) Breaking dormancy of beech. Physiol. Plant,
10: 728-731.
TROISI, F.M. & CAVALLAZZI, D. (1961) [A fatal case of
poisoning from inhalation of dichloroethane vapours.] Med.
Lav., 52: 612-618 (in Italian).
TSANI-BAZACA, E., MCINTYRE, A.E., LESTER, J.N., & PERRY, R.
(1981) Concentrations and correlations of 1,2-dibromoethane,
1,2-dichloroethane, benzene, and toluene in vehicle exhaust and
ambient air. Sci. Technol. Lett., 2: 303-316.
TSANI-BAZACA, E., MCINTYRE, A.E., LESTER, J.N., PERRY, R.
(1982) Ambient concentrations and correlations of hydrocarbons
and halocarbons in the vicinity of an airport. Chemosphere, 11:
11-23.
TSURUTA, H. (1975) Percutaneous absorption of organic solvents
I. Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind. Health, 13: 227-236.
TU, A.S., MURRAY, T.A., HATCH, K.M., SIVAK, A., & MILMAN, H.A.
(1985) In vitro transformation of BALB/c-3T3 cells by
chlorinated ethanes and ethylenes. Cancer Lett., 28: 85-92.
VAN BLADEREN, P.J., BREIMER, D.D., ROTTEVEEL-SMIJS, G.M.T.,
KNIJFF, P. DE, MOHN, G.R., MEETEREN-WALCHLI, B. VAN, BUIJS, W.,
& VAN DER GEN, A. (1981a) The relation between the structure
of vicinal dihalogen compounds and their mutagenic activation
via conjugation to glutathione. Carcinogenesis, 2: 499-505.
VAN BLADEREN, P.J., HODGETERP, J.J., BREIMER, D.O., & VAN DER
GEN, A. (1981b) The influence of disulfiram and other
inhibitors of oxidative metabolism on the formation of 2-
hydroxyethyl-mercapturic acid from 1,2-dibromoethane by the rat.
Biochem. Pharmacol., 30: 2983-2987.
VAN DUUREN, B.L., GOLDSCHMIDT, B.M., LOEWENGART, G., SMITH,
A.C., MELCHLONNE, S., LEIDMAN, I., & ROTH, D. (1979)
Carcinogenicity of halogenated olefinic and aliphatic
hydrocarbons in mice. J. Natl Cancer Inst., 63: 1433-1439.
VAN ESCH, G.J., KROES, R., LOGTEN, M.J., VAN, & TONKELAAR, E.M.,
DEN (1977) Ninety-day toxicity study with 1,2-dichloroethane
(DCE) in rats, Bilthoven, the Netherlands, National Institute of
Public Health and Environmental Hygiene (Report 195/77 Al.
Tox.).
VARGA, M.B. & FERENCZY, L. (1956) Effect of "Rindite" on the
development of the growth-substances in potato tubers. Nature
(Lond.), 175: 1075.
VOSOVAYA, M.A. (1974) [Development of posterity of two
generations obtained from females subjected to the action of
dichloroethane.] Gig. i Sanit., 7: 25-28 (in Russian).
VOSOVOYA, M.A. (1977) [Effect of dichloroethane on the
reproductive cycle and embryogenesis in experimental animals.]
Akush. Ginekol., 2: 57-59 (in Russian).
WALBRIDGE, C.T., FIANDT, J.T., PHIPPS, G.L., & HOLCOMBE, G.W.
(1983) Acute toxicity of ten chlorinated aliphatic hydrocarbons
to the fathead minnow (Pimephales promelas). Arch. environ.
Contam. Toxicol., 12: 661-666.
WARD, J.M. (1980) The carcinogenicity of ethylene dichloride
in Osborne-Mendel rats and B6C3F1 mice. In: Ames, B., Infante,
P., & Reitz, R., ed. Ethylene dichloride: a potential health
risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 35-53 (Banbury Report No. 5).
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. eng. News, May 7: 8-10.
WEISS, F. (1957) [Lethal oral intoxications by
dichloroethane.] Gewerbehygiene, 15: 253-264 (in German).
WHO (1972) Evaluations of some pesticide residues in food,
Geneva, World Health Organization, pp. 276-279, 325-329 (The
Monographs: WHO Pesticide Residues Series, No. 1).
WIRTSCHAFTER, Z.T. & SCHWARTZ, W.D. (1939) Acute ethylene
dichloride poisoning. J. ind. Hyg. Toxicol., 21: 126-131.
WIT, S.L., BESEMER, F.H., DAS, H.A., GOEDKOOP, W., LOOSJES,
F.E., & MEPPELIN, E.K. (1969) Results of an investigation on
the regression of three fumigants (carbon tetrachloride,
ethylene dibromide and ethylene dichloride) in wheat during
processing to bread, Bilthoven, The Netherlands, National
Institute of Public Health and Environmental Hygiene (Report No.
36/69 Tox).
WITHEY, J.R. & COLLINS, B.T. (1980) Chlorinated aliphatic
hydrocarbons used in the foods industry: the comparative
pharmacokinetics of methylene chloride, 1,2-dichloroethane,
chloroform, and trichloroethylene after iv administration in the
rat. J. environ. Pathol. Toxicol., 3: 313-332.
WITHEY, J.R. & KARPINSKI, K. (1985) The fetal distribution of
some aliphatic chlorinated hydrocarbons in the rat after vapor
phase exposure. Biol. Res. Preg., 6(2): 79-88.
WOLFF, D.L., IVANOV, N.G., KLJACKINA, A.M., & MEL'NIKOVA, L.V.
(1979) [Evidence of the effect of significant industrial
toxicological substances on the nervous system by animal
behavioural tests.] Zool. Jahrb. Physiol., 83: 82-91 (in
German).
YLLNER, S. (1971) Metabolism of 1,2-dichloroethane-4C in the
mouse. Acta pharmacol. toxicol., 30: 257-265.
YODAIKEN, R.E. & BABCOCK, J.R. (1973) 1,2-Dichloroethane
poisoning. Arch. environ. Health, 26: 281-284.
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly-volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
ZOETEMAN, B.C.J., PIET, G.J., SLINGERLAND, P., FONDS, A.W., &
WEGMAN, R.C.C. (1979) [ Investigation into the presence of
volatile organic halogenated compounds in groundwater used as a
drinking water supply. Final report: results on the period
November 1976 to August 1978,] Bilthoven, The Netherlands,
National Institute of Drinking Water Supply, National Institute
of Public Health and Environmental Hygiene (Report no. RID-CBH-
79/01 and no. RIV 10/79) (in Dutch).
ZUCCATO, E., MARCUCCI, F., & MUSSINI, E. (1980) GLC
determination of ethylene dichloride (EDC) in biological
samples. Anal. Lett., 13(B5): 371-379.
5.4 Excretion and Elimination
Excretion of 1,2-dichloroethane from rodents is rapid.
Approximately 89% or more of the body burden of the compound was
excreted within 24 h in ip-injected mice (Yllner, 1971), within
48 h in orally exposed mice (Mitoma et al., 1985), and within
48 h in rats exposed orally or via inhalation (Reitz et al.,
1982; Mitoma et al., 1985). In the 3 studies cited above,
excretion of 1,2-dichloroethane or its metabolites mainly
occurred in exhaled air via the lungs and in urine via the
kidneys. In both species, and at various exposure levels, 7 -
18% of the metabolized 1,2-dichloroethane was excreted as carbon
dioxide (CO2) and approximately 80 - 85% as non-volatile
metabolites (Table 6). The metabolism of 1,2-dichloroethane in
mice and rats is dose-dependent. For example, in mice, 11 and
45% of the body burdens of 1,2-dichloroethane, resulting from ip
exposure to 50 and 170 mg/kg body weight, respectively, were
excreted unchanged via the lungs within 72 h (Yllner, 1971). In
rats, 1.8, 11.5, and 29% of the body burdens were excreted
unchanged via the lungs within 48 h following: an inhalation
exposure at 607 mg/m3 for 6 h (equivalent to a dose of 113 mg/kg
body weight) (Reitz et al., 1982), an oral dose of 100 mg/kg
body weight (Mitoma et al., 1985), and an oral dose of 150 mg/kg
body weight (Reitz et al., 1982), respectively.
The rate of elimination from blood and tissues appears to
depend on the exposure level; the higher the exposure level, the
slower the elimination rate of 1,2-dichloroethane, after both
oral and inhalation exposure. Half-lives in the blood of rats,
exposed orally, increased from 25 min at 25 mg/kg body weight to
57 min at 150 mg/kg body weight. With inhalation exposure,
half-lives increased from 13 min at 202 mg/m3 to 22 min at 1012
mg/m3, after a 6-h inhalation exposure (Spreafico et al., 1980).
In addition, after oral exposure of rats to 150 mg/kg body
weight, an initial half-life of 90 min in blood decreased to
20 - 30 min, when blood levels fell below 5 - 10 mg/litre after
3 h (Reitz et al., 1982). Elimination of 1,2-dichloroethane
from blood, adipose tissue, lung, liver, brain, kidneys, and
spleen was comparable after oral exposures of up to 150 mg/kg
body weight. Elimination from the liver was reported to be
biphasic with a higher elimination rate just after reaching peak
levels of 1,2-dichloroethane. Elimination from other organs was
monophasic. Following inhalation, elimination was the slowest
in adipose tissue and the most rapid in the lung, up to an
exposure level of 1012 mg/m3 (Spreafico et al., 1980). Withey &
Collins (1980) also reported that the elimination of 1,2-
dichloroethane was dose-dependent. After iv administration of
from 3 to 15 mg/kg body weight to male Wistar rats, the authors
found that the elimination fitted a two-compartment model at a
low-dose level and a three-compartment model at high-dose
levels.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Aquatic Organisms
6.1.1 Acute toxicity
The acute toxicity of 1,2-dichloroethane for aquatic
organisms is summarized in Table 7. The concentration of 1,2-
dichloroethane was measured in four of the studies cited (see
footnotea in Table 7); the concentrations reported in the
other studies were nominal. It should be noted that, in open
systems, the toxic effects observed must have occurred at
concentrations lower than the nominal ones reported in latter
studies, due to the anticipated evaporation of 1,2-dichloro-
ethane in the aquatic media.
The species most sensitive to 1,2-dichloroethane were
members of the class Crustacea. A no-observed-adverse-effect
level below 68 mg/litre was found for Daphnia magna (Le Blanc,
1980). The shrimp Crangon crangon showed a 96-h LC50 of
85 mg/litre in sea water, measured by the flow-through method
(Adema, 1976). When the brine shrimp Artemia salina was exposed
to 1,2-dichloroethane at levels ranging from 0.25 to
25 mg/litre, growth inhibition was noted 24 h after cyst wetting
(Kerster & Schaeffer, 1983).
EDC tar is much more toxic for marine species than 1,2-
dichloroethane, the heavy fractions of the tar being responsible
for the high toxicity observed (Jernelöv et al., 1972; Rosen-
berg, 1972; Jensen et al., 1975; Rosenberg et al., 1975).
6.1.2 Short-term exposures
When blue algae Mycrocystis aerigunosa and green algae
Scenedesmus quadricauda were exposed in closed containers to
1,2-dichloroethane at 105 and 710 mg/litre, respectively, for 8
days, cell multiplication started to be inhibited (Bringmann &
Kühn, 1978). Guppies (Poecilia reticulata) were exposed for 7
days in a static test, and an LC50 of 106 mg/litre was found.
Solutions were renewed daily, but no water analysis data were
reported (Könemann, 1981). Finally, an early life stage flow-
through test was done. Fathead minnows (Pimephales promelas)
were exposed to concentrations of between 4 and 56 mg/litre
beginning from 2 to 5 days after spawning and continuing
throughout the subsequent embryonal, larval, and juvenile stages
up to 28 days after hatching. Water was analysed for 1,2-
dichloroethane. Body weight was reduced at 59 mg/litre. The
survival of juveniles, the percentage of normal larvae at hatch,
and the hatchability of embryos were not affected (Benoit et
al., 1982).
Table 7. Acute aquatic toxicity
---------------------------------------------------------------------------------------------------------
Organism Description t (°C) pH Dissolved Hardness Flow/b Parameter Concen- Reference
oxygen (mg CaCO3/ stat tration
(mg/litre) litre) open/ (mg/litre)
closed
---------------------------------------------------------------------------------------------------------
Fresh water
-----------
Bacteria Pseudomonas 25 7 stat 16-h MICc 135 Bringmann
putida closed & Kühn
(1980)
Protozoa Entosipon sulc- 25 7 stat 72-h MIC 943-1127 Bringmann
atum, Uronema closed & Kühn
parduczi, Chilo- (1981)
monas paramecium
Crustacea water flea 22 6.7-8.1 6.5-9.1 72 stat 24-h LC50 250 Le Blanc
(Daphnia magna) open no-observed- < 68 (1980)
adverse-
effect level
Crustacea water flea 20 8.0 > 2 - stat 24-h EC50 540a,d Bringmann
(Daphnia magna) open & Kühn
(1982)
Crustacea water flea 20 7.1-7.7 7.9-9.9 44.7 stat 48-h LC50 270a,d Richter
(Daphnia magna) closed 48-h EC50 160a,d et al.
20 7.0-7.5 4.1-8.4 44.7 stat 48-h LC50 320a,e (1983)
closed 48-h EC50 180a,e
Fish bluegill sunfish 21-23 6.5-7.9 32-48 stat 96-h LC50 430 Buccafusco
(Lepomis macro- closed et al.
chirus) (1981)
Fish bluegill sunfish 23 7.6-7.9 55 stat 96-h LC50 550 Dawson
(Lepomis macro- open et al.
chirus) (1977)
Fish fathead minnow 25 6.7-7.6 8.0 45.1 flow 96-h LC50 116a Walbridge
(Pimephales prom- open et al.
elas) (1983)
Table 7 (contd.)
---------------------------------------------------------------------------------------------------------
Sea water
---------
Alga Phaeodactylum stat EC50 340a Pearson &
tricornutum McConnell
(1975)f
Worm chaetopod 23 stat 96-h LC50 400 Rosenberg
(Ophryotrocha closed et al.
labronica) (1975)g
Crustacea shrimp 15 8.0 > 8.0 flow 96-h LC50 85a Adema
(Crangon crangon) open (1976)
Crustacea shrimp 16 stat 24-h LC50 170 Rosenberg
(Crangon crangon) open et al.
(1975)h
Mollusca barnacle nauplii stat 48-h LC50 186a Pearson &
(Elminius modestus) closed McConnell
(1975)
Fish dab flow 96-h LC50 115a Pearson &
(Limanda limanda) open McConnell
(1975)
Fish tidewater silver- 20 7.6-7.9 55 stat 96-h LC50 480 Dawson
sides (Menidia open et al.
beryllina) (1977)
Fish sheepshead minnow 25-31 stat 96-h LC50 130-230 Heitmüller
(Cyprinodon open no-observed- 130 et al.
variegatus) adverse- (1981)
effect level
Fish goby 15 8.0 > 8.0 flow 96-h LC50 185a Adema
(Gobius minutus) open (1976)
---------------------------------------------------------------------------------------------------------
a Water analysis for 1,2-dichloroethane was reported.
b Flow-through or static method; open or closed system.
c MIC = minimum inhibitory concentration for cell multipication. EC50 and LC50 = concentration,
causing an effect and death, respectively, in 50% of the population.
d Fleas unfed. Effect was complete immobilization.
e Fleas fed. Effect was complete immobilization.
f Effect was growth inhibition, measured by 4C uptake during photosynthesis.
g When the concentration was gradually built up during the first hour, the 96-h LC50 was
900 mg/litre, and the percentage of hatched eggs, laid within 15 days, was decreased by 90% at
400 mg/litre. The latter was not noted at 200 mg/litre.
h Concentration was gradually built up during the first hour.
6.1.3 Long-term exposure
No-observed-adverse-effect and effect concentrations were
determined on the basis of reproduction or length for Daphnia
magna in a 28-day test, in stoppered flasks. The test solution
was analysed by gas chromatography. The lowest observed adverse
effect concentrations were 21 mg/litre, on the basis of repro-
duction, and 72 mg/litre, on the basis of length. The no-
observed-adverse-effect concentrations were 11 mg/litre, on the
basis of reproduction and 42 mg/litre, on the basis of length
(Richter et al., 1983).
6.1.4 Bioconcentration
Bioconcentration of 1,2-dichloroethane in aquatic species is
unlikely in view of its physical and chemical properties. In a
tracer study, a bioconcentration factor of 2 was found for
bluegill sunfish (Lepomis macrochirus) in flowing water. The
half-life for the elimination of 1,2-dichloroethane from tissues
was 1 - 2 days (Barrows et al., 1980).
When tissues of several aquatic species, collected from near
the discharge zone of a wastewater treatment plant, were ana-
lysed for 1,2-dichloroethane, the concentration of the compound
was less than 0.5 µg/kg wet weight in all cases, while the
average effluent concentration was 41 µg/litre and the average
sediment concentration was less than 0.5 µg/kg dry weight
(Gossett et al., 1983).
6.2 Microorganisms
1,2-Dichloroethane at an influent concentration of
258 mg/litre did not affect the treatment efficiency of a bench-
scale activated sludge system. The compound itself was
virtually completely removed by stripping but not by biodegra-
dation (Stover & Kincannon, 1983). In a batch anaerobic
toxicity assay, 1,2-dichloroethane was slightly toxic to the
anaerobic digestion process from a concentration of
2.5 mg/litre, though acclimation was observed after several
days. A concentration of 20 mg/litre caused more severe
retardation, while acclimation was slow. In semi-continuous
assays, stress became evident at 1,2-dichloroethane concentra-
tions of between 5 and 7.5 mg/litre (Stuckey et al., 1980).
6.3 Terrestrial Organisms
6.3.1 Birds
The effects on reproduction were investigated in groups of
10 male and 20 female white leghorn chickens after 2 years of
oral exposure to 0, 250, or 500 mg 1,2-dichloroethane/kg feed
mash. From the fourth month of laying onwards, decreased egg
weight was observed at both dose levels, while at the higher
dose level, the number of eggs and the feed intake were also
reduced. 1,2-Dichloroethane did not affect serum composition
and growth, semen characteristics, or fertility of chickens
(Alumot et al., 1976b).
6.3.2 Plants
1,2-Dichloroethane is used as a seed fumigant, usually in
combination with compounds such as carbon tetrachloride, 1,2-
dibromomethane, or 2-chloroethanol. Such fumigants inhibited
the germination of seeds (Caswell & Clifford, 1958; Kamel,
1959), broke the dormancy period of potato tubers (Varga &
Ferenczy, 1956; Jolivet, 1968) and beech (Thorup, 1957), and
adversely affected the nodulation status and yield of groundnuts
treated with Rhizobium (Kulkarni et al., 1975). 1,2-Dichloro-
ethane vapour was both lethal and mutagenic for barley seeds at
3 mg/m3 during 24 h (Ehrenberg et al., 1974).
7. EFFECTS ON ANIMALS
7.1 Single Exposures
7.1.1 Inhalation and oral exposure
The available acute mortality data following inhalation and
oral exposure are summarized in Table 8.
Table 8. Acute mortality after inhalation or oral exposure to
1,2-dichloroethane
-----------------------------------------------------------------------------
Species/ Route Vehicle Parameter Result Reference
strain studied
-----------------------------------------------------------------------------
dog oral acacia LD50 2500 mg/kg Barsoum & Saad
gum (1934)
rat oral corn oil LD50 680 mg/kg McCollister et
al. (1956)
CD-1 oral water LD50 489 mg/kg Munson et al.
mouse (1982)
(male)
CD-1 oral water LD50 413 mg/kg Munson et al.
mouse (1982)
(female)
Wistar inhalation 6-h LC50 5100 mg/m3 Spencer et al.
rat (1951)
Sprague inhalation - 6-h LC50 6660 mg/m3 Bonnet et al.
Dawley (1980)
rat
OF1 inhalation 6-h LC50 1060 mg/m3 Gradiski et al.
mouse (1978)
(female)
rat oral LD50 850 mg/kg Larionov &
Kokarovtseva
(1976)
albino inhalation LC50(expo- 30 000 mg/m3 Nevrotsky et al.
rat sure period (1971)
unknown)
-----------------------------------------------------------------------------
Deaths occur within a narrow range of concentrations. In
rats, the difference between the 6-h LC10 and 6-h LC90 was
approximately 2800 mg/m3 (Bonnet et al., 1980). No deaths were
observed in rats after 6 h of exposure to 2000 mg/m3 (Spencer et
al., 1951). In mice, an extremely narrow range of about
500 mg/m3 was observed between the 6-h LC10 and the 6-h LC90
(Gradiski et al., 1978).
Exposure of rats to single high doses of 1,2-dichloroethane
resulted in adverse effects on the CNS, liver, kidneys,
adrenals, and lungs (Spencer et al., 1951). Groups of 10 - 52
Wistar rats were exposed to 81 000, 48 600, 12 100, 6100, 4000,
3200, 2400, or 1200 mg 1,2-dichloroethane/m3 for various lengths
of time. At the highest concentration (81 000 mg/m3), deaths
were observed in animals exposed for 0.3 h or longer. Deaths
were observed at all except the lowest of the other concentra-
tions with exposure periods of 0.4, 0.7, 3.0, 4.0, 7.0, and
7.0 h, respectively. No deaths were observed in rats exposed to
the lowest concentration for 7 h.
Severe depression of the central nervous system resulting in
coma was observed in rats exposed to the highest concentration.
At lower concentrations, this depressant action expressed itself
as various levels of "drunkeness". In this investigation,
Spencer et al. (1951) did not specify the sex of the rats.
In the same publication, the authors reported another study
in which groups of 4 - 6 female rats were exposed to 48 600,
12 100, 4000, 1200, or 800 mg/m3. "Adverse effects" (not
described by the authors) were observed at the 4 highest levels
in rats exposed for 0.2, 0.5, 3.0, and 5.5 h, respectively. No
adverse effects were observed in female rats exposed to the
lowest concentration, for 7 h.
Examination of internal organs in groups of rats killed,
either when moribund or 24 h after the last exposure, showed
that, after exposure to 1,2-dichloroethane levels of 2400 mg/m3
or more, the most severe damage occurred in the kidney and
consisted of haemorrhage and tubular necrosis. The liver showed
fatty changes and hepatocellular necrosis with haemorrhage, and
adrenal glands were haemorrhagic. Lung oedema was observed at
concentrations above 12 100 mg/m3. The injury to organs in this
study was accompanied by high blood-urea levels, a decrease in
serum-phosphatase activity, and increased lipid concentration in
the liver (Spencer et al., 1951).
Depression of the central nervous system was noted during
exposure of rats to 1,2-dichloroethane concentrations exceeding
1200 mg/m3 (Bonnet et al., 1980). From an exposure level of
4000 mg/m3, for 4 h, rats showed altered behaviour. A narcotic
effect was observed at 9100 mg/m3 (Wolff et al., 1979).
Albino rats, given a single oral dose of 615 mg/kg body
weight, showed congested livers with cloudy swelling and fatty
degeneration. The myocardium showed oedema and haemorrhaging in
the walls of the coronary vessels, stasis, and thrombi in the
vessels. These changes were associated with an increased
activity of alanine- and aspartate aminotransferase in the serum
and decreased tissue levels of nicotinamide coenzymes (Natsyuk &
Chekman, 1975). A single administration of 861 mg/kg, by
gavage, was reported to partially uncouple oxidative phosphory-
lation measured in vitro in albino rat livers, 1, 3, or 6 days
after exposure (Natsyuk et al., 1974). In rat liver microsomes,
cytochrome P-450 levels were slightly decreased after an oral
dose of 625 mg/kg (Moody et al., 1981). After a single oral
dose of 1,2-dichloroethane in corn oil at 770 mg/kg body weight
in rats, dystrophy in the cytoplasm and hyperchromatosis in the
nuclei of hepatocytes were observed. A decrease in protein
synthesis and lysosomal enzyme activity was also reported. The
same changes took place in renal nephrons (Boikova & Kravtsova,
1982). A single dose of 850 mg/kg in albino rats resulted in a
decrease in RBC count, haematocrit, and other haematological
changes (Larionov & Kokarovtseva, 1976). Oral administration of
1,2-dichloroethane (615 mg/kg) to rabbits induced pronounced
morphological changes in the liver in about 24 h (Nazikhi &
Skrizhinsky, 1973). Effects on fibrinolytic activity in the
blood of rabbits administered 1,2-dichloroethane at 1476 mg/kg
have been reported by Kagramanov & Kazieva (1972). Electro-
cardiographic changes in albino rats associated with doses of 1,
1.5, and 2 mg/kg have been reported (Saitanov & Arsenieva,
1969).
7.1.2 Skin and eye irritation
When undiluted 1,2-dichloroethane was applied directly on
the clipped skin of guinea-pigs for up to 12 h in occluded patch
tests, no gross skin reactions were visible (Jakobson et al.,
1982). Microscopic changes appeared 4 h after application,
comprising karyopyknosis, perinuclear oedema, spongiosis, and
junctional separation (Kronevi et al., 1981). In similar tests
on rabbits, moderate erythema and oedema were observed, 24 h
after application. Microscopy on the third day revealed
necrosis and other lesions such as ulcerations and acanthosis.
The severity of the changes was not indicated (Duprat et al.,
1976).
Instillation of 0.1 ml of undiluted 1,2-dichloroethane into
the conjunctival sac of the eye of rabbits generated reversible,
mild irritation characterized by conjunctivitis and epithelial
abrasion. Epithelial keratitis, described as being "in a state
of repair", was observed microscopically, 7 days after
application (Duprat et al., 1976). Reversible clouding of the
cornea was observed in dogs within 10 h of subcutaneous
administration of undiluted 1,2-dichloroethane at 0.9/kg body
weight. The clouding continued up to 48 h, but the corneas
appeared clear after 5 days. The histological changes,
including necrosis of the corneal endothelium, partially denuded
Descemet's membrane, formation of excess basement membrane, and
swelling of the corneal stroma, were also observed in dogs,
cats, and rabbits after ocular injection of 1.8 mg 1,2-
dichloroethane (0.15 ml of a 1% solution) into the anterior
chamber (Kuwabara et al., 1968).
7.2 Short-Term Exposures
7.2.1 Inhalation exposure
The effects of repeated exposure to 1,2-dichloroethane have
been studied in mice, rats, guinea-pigs, rabbits, cats, dogs,
and monkeys (Heppel et al., 1946; Spencer et al., 1951; Hofmann
et al., 1971).
Heppel et al. (1946) exposed rats of the Wistar and Osborne-
Mendel strains to 1,2-dichloroethane concentrations of 420, 730,
1540, or 3900 mg/m3 in air, 7 h daily, 5 days/week, for several
weeks. The duration varied with each exposure level. No loss
of weight and no deaths occurred in rats of either strain
exposed to 420 mg/m3 for up to 4 months (74 exposures). Seven
out of 12 Wistar rats exposed to 730 mg/m3 died within 15 weeks
(after 1 - 73 exposures) and 8/12 similarly treated rats of the
Osborne-Mendel strain died after 1 - 6 exposures. Nine out of
16 rats (strain unspecified) died within 12 weeks after 2 - 60
exposures to 1540 mg/m3; 20/26 (strain unspecified) died within
3 weeks after 3 - 15 exposures to 3900 mg/m3. Guinea-pigs were
exposed to the same concentrations of 1,2-dichloroethane as the
rats. Several deaths, which occurred in the lowest exposure
group (420 mg/m3) and in controls, were attributed to an
intercurrent disease. At higher dose levels, mortality was
related to 1,2-dichloroethane exposure. Five out of 14 guinea-
pigs died after 5 - 115 exposures to 730 mg/m3 within 23 weeks.
Fourteen out of 20 guinea-pigs died after 8 - 65 exposures to
1,2-dichloroethane at 1540 mg/m3 within 13 weeks. All guinea-
pigs exposed to 3900 mg/m3 had died by the 4th day of the
study.
Rabbits were exposed to the three higher concentrations of
1,2-dichloroethane only. No deaths were observed when rabbits
were exposed to 730 mg/m3 for 25 weeks. All 5 rabbits exposed
to 1540 mg/m3 died, one after one exposure and the rest after
89 - 97 exposures within 19 weeks. Five out of 6 rabbits
exposed to 3900 mg/m3 died after 2 - 43 exposures within 9
weeks.
A group of 19 mice survived for 4 weeks when exposed to 420
mg/m3, but 18/20 mice died within 10 days by the end of 7
exposures to 730 mg/m3. In other species, a group of 6 female
dogs survived up to 35 weeks of exposure to 1540 mg/m3. Two out
of 6 dogs exposed to 3900 mg/m3 died after 30 and 43 exposures,
respectively. Two out of six cats exposed to 3900 mg/m3 died
after 43 exposures. Cats were not exposed to lower
concentrations. Two monkeys died after 2 and 32 exposures,
respectively, to 3900 mg/m3, but 2 others exposed to 730 mg/m3
survived for 25 weeks.
Kidney and liver damage, consisting of fatty changes and
necrosis in both organs, was found in animals that died from
exposure to the highest dose level (3900 mg/m3). In addition,
the rats showed pulmonary congestion and haemorrhage; one monkey
that died after 32 exposures and one dog showed a focal
myocarditis. Approximately half of the animals that died after
exposure to 1540 mg/m3 showed similar histological changes in
the liver and kidneys, but no such changes were observed in rats
that died from exposure to lower levels. Hepatic fatty changes
were observed in guinea-pigs exposed to 730 mg/m3. Histological
examinations were not carried out on mice.
Spencer et al. (1951) exposed rats, guinea-pigs, monkeys,
and rabbits to 1,2-dichloroethane at concentrations of 1620,
810, or 405 mg/m3, 7 h per day, 5 days/week, for various lengths
of time. In a group of 15 male and 15 female rats, exposed to
the highest level, no animals survived for more than 8 weeks,
and 60% mortality occurred in a second group exposed to the same
regime after 2 or 3 exposures. Mortality was also high in a
group of 8 male and 8 female guinea-pigs exposed to 1620 mg/m3.
All males had died by the second week and all females by
approximately the 5th week. Two male monkeys experienced rapid
and severe intoxication. Both were killed when moribund after
10 - 12 exposures. On the other hand, 2 male and 1 female
rabbits tolerated 33 weeks exposure with no evidence of adverse
effects. No mortality was observed when groups of 15 male and
15 female rats and 8 male and 8 female guinea-pigs were exposed
to 810 mg/m3 for about 30 weeks (151 exposures) or 36 weeks
(180 exposures), respectively. Similarly, no clinical effects
were observed in groups of 15 male and female rats, 8 male or
female guinea-pigs, 2 male and 1 female rabbits, and 1 male and
1 female monkey exposed for approximately 30 - 36 weeks to 405
mg/m3.
Histological examination was carried out on animals exposed
to the three dose levels. In both guinea-pigs and rats exposed
to the highest dose level, liver changes consisting of cloudy
swelling and fatty changes were observed. None of the other
organs were affected. Similar but less marked changes were
observed in the monkeys, while no adverse changes were found in
the rabbits. In rats exposed to 810 mg/m3, there were no
adverse changes in the liver or other organs, but reduced growth
and some fatty changes were found in the liver of guinea-pigs.
No adverse changes were found in animals exposed to the lowest
dose level (405 mg/m3).
Hofmann et al. (1971) exposed cats, rabbits, guinea-pigs,
and rats to 1,2-dichloroethane at 1980 mg/m3 or 405 mg/m3 for
6 h/day, 5 days/week, for up to 17 weeks. At the higher
concentration, rats became dyspnoeic and guinea-pigs apathetic.
Three out of 4 rabbits died after 10 - 17 exposures, and 9/10
guinea-pigs died after 4 - 14 exposures. Rats were more
sensitive, dying after only 1 - 5 exposures. All cats survived
30 exposures. Histologically, rats showed pulmonary hyperaemia
and oedema, fatty liver, and adrenal and myocardial necrosis.
Cats and rabbits exhibited a heart lesion and guinea-pigs, fatty
changes in the myocardium, liver, and adrenals and necrosis in
the myocardium and liver. At the lower concentration (405
mg/m3), rats, guinea-pigs, rabbits, and cats exposed for 17
weeks did not show any clinical or histological changes.
Of the species studied, mice and rats appear to be more
sensitive than other species to the adverse effects of 1,2-
dichloroethane. The no-observed-adverse-effect level for short-
term exposures (4 - 9 months) in rats studied in the 3
investigations is about 400 mg/m3.
Signs of central nervous system depression observed in the
above studies were apathy in guinea-pigs at 1980 mg/m3 (Hofmann
et al., 1971) and 3900 mg/m3 (Heppel et al., 1946), and coma in
dogs and monkeys at 3900 mg/m3 (Heppel et al., 1946). When rats
were exposed continuously for 3.5 months to 5 mg/m3, changes in
EEG were observed (Dmitrieva & Kuleshova, 1971). However, the
significance of these findings is not known.
7.2.2 Oral exposure
The liver appeared to be the principal target organ
following oral exposure. Rats, treated by gavage with 1,2-
dichloroethane in corn oil for 2 weeks, 5 times per week, at
doses of 150 mg/kg body weight or less did not show any
treatment-related abnormalities in organ or body weights,
histology, clinical chemistry, or haematology (Van Esch et al.,
1977; Reitz et al., 1982).
Rats were also exposed for 90 days, 5 times per week, to 0,
10, 30, or 90 mg/kg body weight (Van Esch et al., 1977). At the
2 highest exposure levels, a tendency to decreased weight gain
was observed. At 90 mg/kg, rats of both sexes showed an
increase in the relative weight of kidneys, but only the females
on this dose showed increased relative weights of liver and
brain compared with controls. Histology and clinical chemistry
were normal. Some haematological parameters were altered, but
not in a dose-related manner. In another study, after 5 doses
of 300 mg 1,2-dichloroethane/kg body weight in 5 days, all 6
rats died, and their livers showed fatty degeneration with an
increase in the triglycerides level (Van Esch et al., 1977).
Total fat and triglycerides were elevated in the livers of
rats exposed to approximately 100 mg/kg body weight per day via
the feed, which was administered twice daily, for 7 weeks
(Alumot et al., 1976a).
No adverse effects related to liver and kidney function were
observed at the lowest dose (10 mg/kg).
7.3 Long-Term Exposure
7.3.1 Inhalation exposure
Clinical chemical investigations were performed on Sprague
Dawley rats exposed by inhalation to 1,2-dichloroethane at 0, 5,
40, 202, or 1012 mg/m3 for 7 h per day, 5 days/week (Spreafico
et al., 1980). The highest exposure level was reduced to 607
mg/m3 after a few weeks because of high mortality. Animals of
each sex were exposed, starting at 3 months of age, for 3, 6, or
18 months. In addition, animals starting at 14 months of age
were exposed for 12 months. Groups of 8 - 10 animals of each
sex were sacrificed at the specified time intervals and clinical
chemistry tests performed. Changes in SGOT, SGPT, and
c-glutamyl transpeptidase activities in the 12-month animals
were not observed in the 18-month animals. Likewise, increases
in serum-uric acid and blood urea-nitrogen levels in the 12-
month animals were not observed in the 18-month animals. In
addition, the 12-month animals, but not the 18-month animals,
displayed decreases in serum-cholesterol. On the basis of the
negative results obtained in the animals starting exposure at 3
months of age and sacrificed after 3, 6, or 18 months, the
authors suggested a lack of significant toxicity, in spite of
the biochemical changes found in the older 12-month animals.
Neurotoxic changes (conditioned reflexes) in albino rats
have been associated with a 1,2-dichloroethane exposure of 50
mg/m3, 4 h/day, for 6 months (Borissova, 1957, 1960).
7.3.2 Oral exposure
In a controlled feeding schedule for 2 years, three groups
of 18 locally-bred rats of each sex were provided with feed
fumigated with 1,2-dichloroethane. The doses of 1,2-
dichloroethane administered were estimated to be 0, 11 - 17, or
23 - 35 mg 1,2-dichloroethane/kg body weight per day. No
adverse effects were observed on growth, mortality rates, or
serum composition. The mean survival period was 18 months or
more (Alumot et al., 1976a).
7.4 Carcinogenicity
7.4.1 Inhalation exposure
Groups of 11-week-old Swiss mice and 12-week-old Sprague
Dawley rats, comprising 90 animals of each sex, were exposed to
20, 40, 202, and 1012 mg 1,2-dichloroethane/m3 air (5, 10, 50,
and 250 ppm) for 78 weeks, 7 h per day, 5 days per week, and
observed for a lifetime. Purity was reported to be greater than
99%. The highest exposure was reduced to 607 mg/m3 (150 ppm)
after a few weeks because of high mortality. Control groups
contained 115 male mice, 134 female mice, or 180 rats of each
sex. Percentage survival in male and female mice, 52 weeks
after the beginning of treatment, was, respectively, 63 and 84%
in the controls; 47 and 93% at 20 mg/m3; 66 and 80% at 40 mg/m3;
51 and 81% at 202 mg/m3; and 43 and 64% at 607 mg/m3. The last
mouse died about 100 weeks after initiation of treatment. In
male and female rats, survival at 52 weeks was, respectively, 67
and 73% in the controls; 75 and 85% at 20 mg/m3; 70 and 81% at
40 mg/m3; 70 and 84% at 202 mg/m3; and 67 and 79% at 607 mg/m3.
Most rats had died by about 140 weeks after the start of
treatment. No specific types of tumours or changes in the
incidence of tumours were found in either species, with the
exception of an increased incidence (not dose-related) of
fibromas and fibroadenomas of the mammary glands of female rats
at 20, 202, and 607 mg/m3. The average latency time for these
tumours was 83 weeks in control rats and in rats exposed at
20 mg/m3, and 79 weeks at the 2 highest exposures. The authors
ascribe the differences in the incidence of mammary tumours
between the groups to the different survival rates in the groups
(Maltoni et al., 1980).
7.4.2 Oral exposure
Groups of 50 Osborne-Mendel rats of each sex were exposed to
average doses of 1,2-dichloroethane (technical grade with
reported purity > 90%)a in corn oil of 47 or 95 mg/kg body
weight for 78 weeks (NCI, 1978). Treatment was usually given 5
times per week, and the animals were observed for another 15 -
32 weeks after the end of treatment. Control groups consisted
of 20 matched controls of each sex treated with corn oil and 60
pooled control-treated rats of each sex. Eleven minor
contaminants were detected in the test compound. The rats were
housed in the same room as rats intubated with other halogenated
hydrocarbons or carbon disulfide. Body weights were not
affected by the exposures. A dose-related increase was found in
mortality rate, which was 100%, 27 weeks after cessation of
exposure to 95 mg/kg body weight. As shown in Table 9, male
rats had a dose-related increased incidence of subcutaneous
fibromas, and forestomach squamous cell carcinomas were
observed. In treated females, the stomach showed hyperplastic
lesions. The incidence of haemangiosarcomas (in various organs,
mainly the spleen) was increased in a dose-related manner in
both sexes, but the increase was statistically significant in
males only. In females, an increase was found in the incidence
of adenocarcinomas of the mammary gland (NCI, 1978).
a Subsequent analysis indicated a purity of about 98 - 99%
(Hooper et al., 1980; Ward, 1980).
Table 9. Summary of main tumour types after oral administration of 1,2-dichoroethane to mice and ratsa
---------------------------------------------------------------------------------------------------------
Group Maximum Number of animals with:
number of Forestomach Subcutaneous Mammary Haemangiosarcomas
animals squamous cell fibromas adeno- (several organs)
examined carcinomas carcinomas
---------------------------------------------------------------------------------------------------------
Rat (male)
Pooled vehicle controls 59 0 0 0 0
Matched vehicle controls 19 0 0 1 0
Low dose 46 3 5 2 9
(P < 0.002)b (P = 0.003)b
High dose 47 9 6 0 7
(P < 0.04)c (P < 0.01)b (P < 0.02)b
Rat (female) Mammary gland
fibroma
Pooled vehicle controls 59 0 5 1 0
Matched vehicle controls 20 0 0 0 0
Low dose 50 0 14 1 4
(P < 0.01)b
High dose 50 0 8 18 4
(P < 0.001)b
---------------------------------------------------------------------------------------------------------
Group Maximum Number of animals with:
number of Hepatocellular Alveolar/ Forestomach Mammary gland
animals carcinomas bronchiolar squamous adenocarcinomas
examined adenomas cell carcinomas
---------------------------------------------------------------------------------------------------------
Mice (male)
Pooled vehicle controls 59 4 0 1 -
Matched vehicle controls 19 1 0 1 -
Low dose 46 6 1 1 -
High dose 47 12 15 2 -
(P < 0.01)b (P < 0.001)b (NS)
Mice (female)
Pooled vehicle controls 60 0 2 1 0
Matched vehicle controls 20 1 1 1 0
Low dose 50 0 7 2 9
(P = 0.001)b
High dose 48 1 15 5 7
(P < 0.001)b (NS) (P = 0.003)b
---------------------------------------------------------------------------------------------------------
a Adapted from: NCI (1978).
b Statistical analyses shown are in comparison with the pooled matched controls.
c Statistical analyses shown are in comparison with the matched vehicle controls.
In the same set of studies, B6C3F1 mice were exposed in a
similar fashion to average doses of 0, 97, or 195 mg technical
grade compound/kg body weight for males and 0, 149, or 299 mg/kg
body weight for females. They were housed in the same room
where several other hydrocarbons or other substances were
tested. After exposure, they were observed for another 12 - 13
weeks. In females, body weights were depressed at the highest
doses from week 15 onwards, while the survival rate decreased in
a dose-related manner. Mean survival exceeded 65 weeks in all
groups. Many treated mice suffered from bronchopneumonia. As
shown in Table 9, dose-related increased incidences of alveolar
or bronchiolar adenomas were found in both sexes. In males, a
dose-related increased incidence of hepatocellular carcinomas
was observed. Female mice showed slight, dose-related increases
in the incidence of squamous cell carcinomas of the forestomach,
but this was not statistically significant. In male mice,
hyperplastic changes were found at this site. The incidence of
adenocarcinomas of the mammary gland was significantly increased
in females at both doses (NCI, 1978).
7.4.3 Dermal exposure
Groups of 30 female Ha:ICR Swiss mice were treated with 42
or 126 mg 1,2-dichloroethane in acetone on the shaven dorsal
skin, 3 times per week for 440 - 594 days. In a third group,
each female received one application of 126 mg of the test
compound followed 2 weeks later by phorbol myristate acetate, a
promotor, in acetone 3 times per week for 428 - 576 days. There
were 3 control groups, a positive control, one for the promotor,
and one for no treatment. 1,2-Dichloroethane did not initiate
skin tumours. There was an elevated incidence of lung
papillomas at the highest dose compared with controls (Van
Duuren et al., 1979).
7.5 Mutagenicity and Related End-Points
7.5.1 Mutations
Information in this section is summarized in Table 10.
7.5.1.1 Bacteria
Several investigators have observed a weak or no effect of
1,2-dichloroethane in Salmonella typhimurium TA1535 or TA 100 in
spot tests or standard plate incorporation assays with or
without rat liver S9 fraction or pure microsomes (Brem et al.,
1974; McCann et al., 1975; King et al., 1979; Guengerich et al.,
1980; Principe et al., 1981). A weak mutagenic effect was
observed in 1,2-dichoroethane vapour-exposed S. typhimurium TA
1535 and TA 100, which did not increase further by the addition
of a metabolic activation system (Barber et al., 1981). However,
a stronger positive response was observed by others (Rannug et
al., 1978; Rannug & Beije, 1979; Principe et al., 1981) in TA
1535 in the presence of rat liver metabolic activation system.
It has been established that this effect has been caused by
cytosolic glutathione-S-transferases (Rannug et al., 1978; Guen-
gerich et al., 1980; Reitz et al., 1982), and similar results
have also been obtained in TA 100 (van Bladeren et al., 1981b).
In vitro Salmonella tests using the bile of mice or rats
exposed to 1,2-dichloroethane, probably containing active con-
jugates, have confirmed these results (Rannug & Beije, 1979).
No mutagenic effects were observed in forward mutation tests
with Escherichia coli (King et al., 1979).
Table 10. Tests for gene mutations/chromosome/DNA damage and cell transformation induced
by 1,2-dichloroethane
---------------------------------------------------------------------------------------------------------
Test description System description Activation Result Reference
Organism Strain/cell type system (S9)
---------------------------------------------------------------------------------------------------------
Gene mutations
reverse mutation bacteria S. typhimurium TA 1530 A + (weak) Brem et al. (1974)
TA 1535 A + (weak)
TA 1538 A + (weak)
reverse mutation bacteria S. typhimurium TA 100 A + (weak) McCann et al. (1975)
reverse mutation bacteria S. typhimurium TA 1535 P - King et al. (1979)
TA 100 P -
TA 1537 P -
TA 1538 P -
TA 98 P -
reverse mutation bacteria S. typhimurium TA 1535 P + Principe et al.
TA 1537 A or P - (1981)
TA 1538 A or P -
TA 98 A or P -
TA 100 A or P -
reverse mutation bacteria S. typhimurium TA 1535 Pa - Guengerich et al.
Pb + (1980)
reverse mutation bacteria S. typhimurium TA 1535 A or P + Barber et al.
TA 100 A or P ± (1981)
TA 1538 A or P -
TA 98 A or P -
---------------------------------------------------------------------------------------------------------
Table 10 (contd).
---------------------------------------------------------------------------------------------------------
Gene mutations (contd)
reverse mutation bacteria S. typhimurium TA 1535 A ± Rannug et al. (1978)
TA 1535 P +
TA 1535 Pb +
reverse mutation bacteria S. typhimurium TA 1535 P + Rannug & Beije (1979)
reverse mutation bacteria S. typhimurium TA 1535 P + Reitz et al. (1982)
reverse mutation bacteria S. typhimurium TA 100 Pb + van Bladeren et al.
(1981a)
forward mutation fungi S. coelicolor A - Principe et al.
A. nidulans A - (1981)
sex-linked lethals insect D. melanogaster + Rapoport (1960); Shak-
arnis (1969, 1970);
King et al. (1979)
somatic cell mutation insect D. melanogaster + Nylander et al.
(1978)
forward mutation Chinese ovary cells in vitro P ++ Tan & Hsie (1981)
hamster A +
P + Zamora et al. (1983)
forward mutation human lymphoblastoid cell line A + Crespi et al. (1985)
AHH-1 and TK6 in vitro
somatic cell mutation mouse C57BL/6J Han (female) NA +? Gocke et al. (1983)
(spot test) x T stock (male)/embryos
---------------------------------------------------------------------------------------------------------
Table 10 (contd).
---------------------------------------------------------------------------------------------------------
Test description System description Activation Result Reference
Organism Strain/cell type system (S9)
---------------------------------------------------------------------------------------------------------
Chromosome/DNA damage
micronucleus test mice NMRI/polychromatic NA - King et al. (1979)
(ip or gavage exposure) erythrocytes
micronucleus test mice CBA/polychromatic NA - Jenssen & Ramel
(ip exposure) erythrocytes (1980)
dominant lethal mice ICR Swiss/germ cells NA - Lane et al. (1982)
(ip exposure)
alkaline DNA mice B6C3F1/liver in vivo/ NA + Storer & Conolly
unwinding in vitro (1983)
alkaline DNA mice B6C3F1/liver in vivo/ NA + Storer et al. (1984)
unwinding in vitro
unscheduled DNA human lymphocytes in vitro NA + Perocco & Prodi
synthesis (exposure by (1981)
addition in the medium)
Cell transformation
cell transformation Syrian embryo cells in vitro A +c Hatch et al. (1983)
(gas/vapour exposure) golden hamster
cell transformation mice BALB/c-3T3 in vitro A - Tu et al. (1985)
(exposure by addition
in the medium)
---------------------------------------------------------------------------------------------------------
a Pure microsomes.
b Cytosol.
c Enhanced viral induced transformation.
NA = not applicable.
7.5.1.2 Fungi
Forward mutation tests conducted with Streptomyces coeli-
color and Aspergillus nidulans were negative in plate incor-
poration assays and spot tests (Principe et al., 1981). In
A. nidulans, 1,2-dichoroethane induced non-disjunction and
haploidization (Crebelli et al., 1984). 1,2-Dichloroethane was
found to be a weak inducer of mitotic crossing over
in Saccharomyces cerevisiae (Simmon, 1980).
7.5.1.3 Insects
Sex-linked recessive lethal mutations were induced
in Drosophila melanogaster by 1,2-dichloroethane (Rapoport,
1960; Shakarnis, 1970; King et al., 1979). Non-dysjunction was
observed inconsistently (Shakarnis, 1969, 1970), while the
frequency of somatic mutations for eye pigmentation increased
(Nylander et al., 1978).
7.5.1.4 Mammals/mammalian cells
A spot test using mice provided weak evidence that 1,2-
dichloroethane can induce somatic mutations (Gocke et al.,
1983).
In Chinese hamster ovary cells, 1,2-dichloroethane induced
mutations at the HGPRT-locus (Tan & Hsie, 1981; Zamora et al.,
1983). Metabolic activation increased the mutation frequency in
the presence of NADPH (Tan & Hsie, 1981). 1,2-Dichloroethane
also induced a dose-related increase in the frequency of
mutations at the HGPRT-locus in two human lymphoblastoid cell
lines, AHH-1 and TK6. The mutation frequency in the AHH-1 cell
line was 25 times that in the TK6 cell line. The difference in
sensitivity was attributed to the difference in the levels of
glutathione-S-transferase (EC 2.5.1.18) activity. The activity
of this enzyme in the AHH-1 cell line was 5 times that in the
TK6 cell line (Crespi et al., 1985).
7.5.2 Chromosome damage/DNA damage
In in vivo studies, no effects were observed in dominant
lethal assays in 2 generations of ICR Swiss mice (Lane et al.,
1982) and in the micronucleus test with CBA and NMRI mice (King
et al., 1979; Jenssen & Ramel, 1980).
1,2-Dichloroethane was a weak inducer of unscheduled DNA
synthesis in cultured human lymphocytes (Perocco & Prodi,
1981).
DNA alkylation in Salmonella by activated 1,2-dichloroethane
was directly related to the mutation frequency, but absolute
levels of DNA alkylation in Salmonella were considered low
(Reitz et al., 1982).
1,2-Dichloroethane weakly inhibited growth of DNA polymerase
deficient E. coli (Brem et al., 1974; Rosenkranz, 1977).
Administration of 1,2-dichloroethane to mice and rats
resulted in covalent binding to macromolecules (Reitz et al.,
1982; Arfellini et al., 1984; Inskeep et al., 1986). Absolute
levels of DNA alkylation in rats, either by gavage or
inhalation, were considered low (Reitz et al., 1982).
Hepatic DNA damage was demonstrated using the alkaline DNA
unwinding assay in male B6C3F1 mice after a single intraperi-
toneal or oral dose of 1,2-dichloroethane that failed to induce
toxic effects in the liver. It was also shown that, after one
inhalation exposure, hepatic DNA damage only occurred at
exposure levels that caused high mortality (Storer & Conolly,
1983; Storer et al., 1984).
7.5.3 Cell transformation
1,2-Dichloroethane did not transform BALB/c-3T3 mouse cells
in a test conducted without any exogenous metabolic activating
system (Tu et al., 1985). It enhanced transformation of Syrian
hamster embryo cells by simian adenovirus (Hatch et al., 1983).
7.6 Reproduction and Teratogenicity
7.6.1 Inhalation exposure
It has been reported that 1,2-dichloroethane was found in
the fetuses of rats after exposure to 600 mg/m3 for 5 h (Withey
& Karpinski, 1985).
Female rats (strain unspecified) were exposed to 15 mg 1,2-
dichloroethane/m3, for 4 h daily, 6 days/week, for 4 months
prior to mating. During this period, the estrus cycle became
longer than normal. The rats were then mated and exposure
continued. No information on the effects on fertility was
given, but the total embryonal mortality increased from
approximately 11% in controls to 27% in treated dams, while pre-
implantation losses were found to be 5 times greater in treated
animals than in the controls. No fetal abnormalities were
reported, with the exception of haematomas in the region of the
head, neck, and anterior extremities (Vozovaya, 1977).
In a second study, 1,2-dichloroethane was administered to
female albino rats at a concentration of 57 ± 10 mg/m3 in air,
for 4 h daily, 6 days/week, for 6 and 9 months. When the rats
were mated, a reduction in fertility was observed (6.5 fetuses
per treated female versus 9.7 in controls). The weight of
newborn rats was reduced (5.06 g versus 6.44 in unexposed
females). Perinatal mortality was increased (Vozovaya, 1974).
1,2-Dichloroethane was detected in placental and fetal
tissues after inhalation exposure to 1000 mg/m3 for 3 days
(daily duration not stated). It was also found in the stomach
of 12- to 14-day-old mice, when lactating females were exposed
to an unspecified concentration of 1,2-dichloroethane by
inhalation (Vozovaya, 1977).
While the above results indicate a possible adverse effect
of 1,2-dichloroethane on reproduction, the following repro-
ductive studies yielded negative results. Groups of 20 Sprague
Dawley rats of each sex were exposed for 60 days prior to
mating, for 6 h per day, and 5 days per week to 101, 304, or
607 mg 1,2-dichloroethane/m3 in air. After mating, they were
exposed similarly, for another 116 days, but for 7 days per
week. Females were not exposed between gestation day 21 and day
4 post partum. Control groups contained 30 rats each. Pups
were removed and examined at 21 days of age, and the females
were remated following the removal of the last litter. Each
female produced 2 litters. The parents did not show any toxic
effects and the fertility index and gestation period were
normal. In the pups, no effects were found on sex ratio,
survival indices, organ weights, or histology. A small decrease
was noted in the number of pups in the first litters at
304 mg/m3 (Rao et al., 1980).
A teratogenicity study was further performed with groups of
16 - 30 female rats and 19 - 21 female rabbits (Rao et al.,
1980). These groups were exposed through inhalation to 0, 405,
or 1215 mg 1,2-dichloroethane/m3 air, for 7 h per day, from the
6th day of pregnancy onwards (rats up to the 15th day and
rabbits up to the 18th day of gestation). Exposures at both
levels were toxic for rabbit dams. The higher exposure level
was severely toxic for the rat dams; only 1 of the few surviving
females was pregnant, and all the implantation sites were
resorbed. No adverse effects on reproduction were observed
among rats exposed at 405 mg/m3 and rabbits exposed at 405 or
1215 mg/m3. The only gross change observed in rat fetuses was a
decreased incidence of bilobed thoracic centra. There were no
significant alterations in rabbit fetuses.
7.6.2 Oral exposure
Groups of 18 female rats that had received 0, 11 - 17, or
23 - 35 mg 1,2-dichloroethane/kg body weight per day via the
feed, for up to 2 years, were mated with untreated males. The
purity of the substance was not reported. No effects on
reproduction were observed (Alumot et al., 1976a).
Lane et al. (1982) reported a 2-generation reproduction
study on groups of 10 male and 30 female ICR Swiss mice
receiving nominally 5, 15, or 50 mg 1,2-dichloroethane/kg body
weight per day via the drinking-water, for up to 25 weeks.
Control groups contained 20 male and 60 female mice. After 5
weeks of treatment, the mice were mated to produce F1A, F1B, and
F1C litters. After weaning and 11 weeks of treatment, the F1B
mice were mated to produce F2A and F2B litters. Remating always
occurred 2 weeks after weaning. In the F1C and F2B matings,
females were co-housed with untreated males for teratology
screening. The parents did not show any toxic effects and
fertility and gestation indexes were normal. There were no
effects on survival, litter size, postnatal body weight, and
gross pathology of pups. No congenital malformations were
detected. In the F1C and F2B litters, no exposure-related
reproductive effects were found. In the F2B litters, there was
no increase in the incidence of fetal visceral or skeletal
anomalies. F1C litters were not examined for skeletal
anomalies.
7.7 Immunotoxicity
When rabbits were exposed for 7.5 - 8 months to 1,2-
dichloroethane vapour of unspecified purity at a level of
100 mg/m3, for 3 h per day, 6 h per week, an 80% reduction in
the production of antibodies against typhoid vaccine was
observed. Concomitantly, there was a 2-fold increase in Forsman
sheep erythrocyte antibodies (Shmuter, 1977).
Immunosuppression was also noted in a later oral study, in
which groups of 32 male CD-1 mice were exposed to 3, 24, or
189 mg 1,2-dichloroethane/kg body weight (purity unknown) via
the drinking-water for 90 days. In addition, groups of 10 male
CD-1 mice were exposed, once a day, to 4.9 or 49 mg/kg body
weight by water gavage for 14 days. Control groups comprised 48
mice in the 90-day study and 12 mice in the 14-day study. Apart
from a 30% reduction in the leukocyte count after 14 days of
exposure to 49 mg/kg body weight, no effects were found on other
haematological parameters and relative organ weights. After the
90-day exposure, decreases in body weight and fluid consumption
were noted. There was a tendency towards a reduction in
immunoglobulin spleen antibody-forming cells and in the serum-
antibody level after sheep erythrocyte immunization, while no
effects were observed in the response to the B-cell mitogen
lipopolysaccharide S. After the 14-day exposure, 25% and 40%
suppression of antibody-forming cells were measured at 4.9 and
49 mg/kg body weight, respectively. After the 90-day exposure,
no effects were seen on the cell-mediated immunity, assessed by
measuring the delayed hypersensitivity response to sheep
erythrocytes and the spleen cell response to the T-cell mitogen
Concanavalin A. After the 14-day exposure, a slight suppression
of the delayed hypersensitivity response was found, which was
not dose-dependent (Munson et al., 1982).
8. EFFECTS ON MAN
Limited data are available on the effects on man of 1,2-
dichloroethane. There are no adequate controlled studies, no
recent occupational studies, and no mortality studies. However,
case studies of accidental exposures have been reported.
8.1 Accidental Exposures
8.1.1 Inhalation exposure
Many reports are concerned with mixed exposures. However,
in this publication, only case studies are considered in which
the exposure was reported to be to 1,2-dichloroethane alone.
According to a review by NIOSH (1976), no data on exposure
levels and duration of exposure were reported.
Inhalation of 1,2-dichloroethane vapour first afflicts the
central nervous system. Symptoms include headache, dizziness,
weakness, cyanosis, muscular spasms, hypotonia, vomiting, and
unconsciousness. Death often follows. The respiratory tract
can be irritated and inflamed with such symptoms as cough and
rales over the chest. Cyanosis may occur either as the result
of respiratory insufficiency due to depression of the central
nervous system or by bronchial obstruction due to inflammation.
Epigastric or visceral pains and diarrhoea have been observed.
Autopsy reports frequently mention damage to the lungs, liver,
and kidneys (Wirtschafter & Schwartz, 1939; Hadengue & Martin,
1953; Menschick, 1957; Troisi & Cavallazzi, 1961; Suveev &
Babichenko, 1969). Changes in heart rhythm have been reported,
which are probably secondary effects (section 8.1.2) (Suveev &
Babichenko, 1969). Clinical findings include elevated serum-
bilirubin levels and leukocytosis (Wirtschafter & Schwartz,
1939; Menschick, 1957) and elevations of blood-lactate, ammonia,
ornithine carbamyl transferase, serum aspartate transaminase (EC
2.6.1.1), lactate dehydrogenase, and creatine phosphokinase
(Nouchi et al., 1984).
8.1.2 Oral exposure
The effects of acute oral exposure are very similar to those
found after inhalation, but they are more pronounced. A summary
of acute oral intoxications was prepared by US NIOSH in 1976.
The lethal effects of 1,2-dichloroethane associated with oral
exposure are presented in Table 11. Oral doses of 20 - 50 ml
1,2-dichloroethane have been identified as being lethal (IRPTC,
1984). Several major syndromes can be identified including
central nervous system depression, gastroenteritis, and
disorders of the liver and kidneys. Frequently-observed
cardiovascular insufficiency and haemorrhagic diathesis may be
related to changes in oxygenation and effects on the liver
(Weiss, 1957; Morozov, 1958; Hinkel, 1965; Bogoyavlenski et al.,
1968; Martin et al., 1968; Schönborn et al., 1970; Yodaiken &
Babcock, 1973; Dorndorf et al., 1975; Andriukin, 1979).
Table 11. Effects associated with acute lethal oral doses of 1,2-dichloroethane in human beingsa
---------------------------------------------------------------------------------------------------------
Amount ingested (g) Findingsb Reference
---------------------------------------------------------------------------------------------------------
188 - 250 death of 4 males up to 35 h; internal Bryzhin (1945)c
haemorrhage at various sites; liver damage;
symptoms at 3 - 4 h
87 - 125 death of 3 males after 5 - 8 h; internal Kaira (1966)c
haemorrhage; symptoms immediate (uncon-
sciousness, vomiting, dizziness)
103 death after 6 h; haemorrhagic lesions Noetzel (1944)c
75 death after 22 h; symptoms at 2 h (cyanosis, Hueper & Smith (1935)
(cyanosis, vomiting, dilated pupils); brain
haemorrhage, liver damage, nephrosis
63 death at 91 h; lack of eye reflex to light Roubal (1947)c
on 4th day; vomiting; rapid pulse
63 death in 3 - 4 h; liver damage Secchi et al. (1968)c
63 death at 17 h; cyanosis, diarrhoea; impaired Schonborn et al. (1970)
blood coagulation
50 death at 24 h; haemorrhage at various sites; Martin et al. (1968)
signs of cardiac damage
---------------------------------------------------------------------------------------------------------
Table 11 (contd).
---------------------------------------------------------------------------------------------------------
Amount ingested (g) Findingsb Reference
---------------------------------------------------------------------------------------------------------
50 death at 28 h; internal haemorrhage Garrison & Leadingham (1954)c
37 death at 10 h; internal haemorrhage at Lochhead & Close (1951)
various sites; adverse lung effects
25 death at 24 h; epigastric pain; slow Roubal (1947)c
pulse
25 death at 13 h; symptoms at 1 h (cyanosis, Flowtow (1952)c
vomiting)
25 death within 12 h; symptoms not reported Flowtow (1952)c
19 death after 6 days; liver, kidney, renal Yodaiken & Babcock (1973)
damage; pulmonary oedema; hypoglycaemia;
clotting time decreased; some haemorrhaging
10 death after 56 h; delirium; pulse deterior- Bogoyavlenski et al. (1968)
ation
---------------------------------------------------------------------------------------------------------
a Studies in which doses could not be estimated have not been cited. The reader is referred to
NIOSH (1976) for a description of these case reports.
b Unless otherwise stated, death refers to single male individuals.
c The reader is referred to NIOSH (1976) for a more complete description of symptomology.
Symptoms of central nervous system depression commonly
appear within 1 h, frequently with cyanosis, nausea, vomiting,
diarrhoea, epigastric and abdominal pains, and irritation of the
mucous membranes. Irreversible brain damage has been reported
in one case, and brain damage has been found in several fatal
cases (Rohmann et al., 1969; Dorndorf et al., 1975). In some of
the cases, an interval relatively free of symptoms followed
ingestion (Hinkel, 1965; Martin et al., 1968; Komarov et al.,
1973; Dorndorf et al., 1975). In the next phase, decreasing
consciousness and circulatory and respiratory failure may occur,
often leading to death some hours to some days after exposure.
During the intoxication, heart rhythm disturbances can lead to
cardiac arrest (Morozov, 1958; Martin et al., 1968; Yodaiken &
Babcock, 1973; Andriukin, 1979). Autopsy reports have revealed
damage to the mucosae of the gastrointestinal tract, liver,
kidney, lung, heart, and brain. Livers can be enlarged. Liver
and kidney epithelium can show fatty degeneration and necrosis.
Renal insufficiency has been reported to follow development of
hepatic insufficiency and has been known to progress to uraemic
coma (Natsyuk & Mudritsky, 1974). Lung oedema is frequently
found. Hyperaemia and haemorrhagic lesions are found in some
organs. According to some authors (Martin et al., 1968;
Schönborn et al., 1970; Yodaiken & Babcock, 1973), it appeared
that the blood coagulation time was increased because of a
decrease in blood clotting factors and thrombocytes. These
effects appear secondary to liver cell necrosis complicated
further by intravascular coagulation. Biochemically, liver
damage is illustrated by increased serum levels of bilirubin,
transaminases, and lactate dehydrogenase (Martin et al., 1968;
Yodaiken & Babcock, 1973; Dorndorf et al., 1975; Andriukin,
1979). Kidney damage is expressed by anuria or oliguria
(Morozov, 1958; Bogoyavlenski et al., 1968; Yodaiken & Babcock,
1973) and albumin, leukocytes, and epithelium cells in the
urine. Together with the histopathology, this points to acute
necrosis of the kidney tubule, possibly as a result of the liver
cell necrosis and the changes in circulation (Morozov, 1958;
Hinkel, 1965; Yodaiken & Babcock, 1973). Haematological changes
include decreases in the erythrocyte count and haemoglobin
content (Morozov, 1958; Dorndorf et al., 1975).
8.1.3 Acute effects on eyes and skin
Dysfunction of the central nervous system, which could be
caused by brain oedema, can lead to effects on the eyes such as
dilation or constriction of the pupils and impairment of eye
reflexes (Weiss, 1957; Troisi & Cavallazzi, 1961). Weiss (1957)
reported a cloudy cornea in 2 cases of oral exposure; this has
also been reported in dogs (section 7.1.2). Conjunctivitis was
found in 2 out of 4 patients, who had been exposed to 1,2-
dichloroethane vapour (Menschick, 1957). After intermittent
immersion of the hands of 3 men in 1,2-dichloroethane for 4 h,
severe dermatitis developed (Wirtschafter & Schwartz, 1939).
8.2 Occupational Exposure
Only 2 reports are available, and these date from before
1960. In the first study (Cetnarowicz, 1959), 16 male workers
from an oil refinery, exposed for 2 - 8 months, were selected
for close examination. A group of 6 workers was exposed to
concentrations of between 40 and 150 mg 1,2-dichloroethane/m3
air, and a group of 10 workers was exposed to concentrations
between 250 and 800 mg/m3. The workers were also exposed to
benzene at levels between 10 and 25 mg/m3, which was considered
not significant by the authors. A general reduction in body
weight was observed. Complaints came mainly from the group with
the higher exposure and included a burning sensation of the
eyes, lachrymation, dizziness, lassitude, sleepiness, nausea,
vomiting, constipation, poor appetite, epigastric pain, and
weight loss. There was no control group, but symptoms repor-
tedly disappeared when workers were removed from exposure;
symptoms returned upon re-exposure. Most, but not all, abnorm-
alities were found in the group with higher exposure and
involved the liver (8 workers), central nervous system (3
workers), gastrointestinal tract (7 workers), and haematological
parameters (1 - 7 workers). No lesions were found in the eye,
respiratory tract, lung, or heart.
The second study (Kozik, 1957), was conducted on workers
employed in an aircraft factory using a gum dissolved in 1,2-
dichloroethane. No data were given on the composition of the
glue or on the employment status of the workers. Air concentra-
tions of 1,2-dichloroethane varied considerably, the level being
5 mg/m3 or less during 70 - 75% of the working time, and 80 -
150 mg/m3 during 25 - 30% of the working time. The morbidity of
the workers in this department during the whole period under
study (1951-55) was increased for all disease categories in
comparison with that in workers in the entire factory. A group
of 83 workers was examined further, in the absence of controls.
There were 19 workers with diseases of the liver and bile duct,
13 workers with neurotic conditions, 11 workers with autonomous
dystonia, and 10 workers with hyperthyroidism and goitre.
These reports are difficult to evaluate, because no
indication is given of the prevalence in unexposed workers of
the symptoms and signs described in the case studies.
9. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
9.1 Evaluation of Human Health Risks
In spite of the high volume of production of 1,2-dichloro-
ethane, there are no quantitative exposure-effect data on human
beings. Frequently, 1,2-dichloroethane is not the only chemical
involved in an exposure and thus, cause-effect relationships are
difficult to derive. No epidemiological or mortality studies
are available. There are only two old reports on small groups
of occupationally-exposed men (section 8.2). These data indi-
cate that repeated inhalation exposures in the range of approx-
imately 40 - 800 mg/m3 may lead to central nervous system
depression and gastrointestinal and liver abnormalities. As
might be expected, such symptoms were more prevalent in indivi-
duals exposed to high levels. The only available data regarding
oral exposure are those involving fatal intoxications (Table 11,
section 8.1).
Because of the limitations of the human data base, it is
necessary to rely on the available experimental animal data to
derive a no-observed-adverse-effect level for human beings.
This is possible because of the similarity in the spectrum of
adverse effects in man and laboratory animals, which include
central nervous system depression, liver and possibly kidney
abnormalties, lung oedema, and cardiovascular disorders. The
dose-response data from animal studies include a no-observed-
adverse-effect level for the rat of about 400 mg 1,2-dichloro-
ethane/m3 air (section 7.2.1), equivalent to an intake of about
35 mg/kg body weight per day (assuming inhalation of 42 litres
over 7 h for a 500-g rat and 100% absorption). The highest
registered air exposure level of the general population is
65 µg/m3 (Table 4, section 4.1.2). This level leads to a
calculated intake of 1.3 mg per person per day (20 m3/day x
65 µg/m3). A comparable intake from drinking-water containing
the highest level recorded (6 µg/litre) (Table 3, section
4.1.1) would amount to a daily intake of 12 µg (6 µg/litre
litre/day). Thus, a combined air and water exposure, in the
context of a worst-case scenario for the general population,
when compared with the no-observed-adverse-effect level for
animals, is lower by a factor of well over 1000. Consequently,
for the biological end-points considered so far, it can be
concluded that 1,2-dichloroethane is unlikely to present a toxic
hazard for the general population, under prevailing exposure
conditions.
In an oral administration study, 1,2-dichloroethane produced
a statistically-significant increase in squamous cell carcinoma
of the forestomach, haemangiosarcoma, and mammary adenocarcinoma
in rats, and mammary adenocarcinoma and hepatocellular carcinoma
in mice (section 7.4.2). In one carcinogenicity study, inhala-
tion exposure did not result in an increase in tumour incidence
(section 7.4.1). The natural occurrence of forestomach squamous
cell carcinomas in rats and haemangiosarcomas in laboratory
rodents is unusual. Taking into consideration that cancer has
been produced in two species of experimental animals and in
several target organs, it can be concluded that 1,2-dichloro-
ethane is carcinogenic for rats and mice, when administered by
gavage.
In the absence of human data, and taking into account the
fact that 1,2-dichloroethane produces a reactive intermediate
that alkylates DNA, that it is positive in a number of in vitro
mutagenicity tests, though weakly so (section 7.5), and that it
results in the production of both rare and common tumours in
rats and mice, it would be prudent to consider 1,2-dichloro-
ethane as a possible human carcinogen. Therefore, 1,2-dichloro-
ethane should be regarded, for practical purposes, as if it
presented a carcinogenic risk for man. Thus, levels in the
environment should be kept as low as feasible.
Since there are no human data, it is necessary to rely on
the limited data available from experimental animal studies in
evaluating reproduction hazards and teratogenicity. The weight
of evidence (section 7.6) does not suggest that exposure to
prevailing environmental levels would pose a human reproductive
or teratogenic hazard.
9.2 Evaluation of Effects on the Environment
9.2.1 Air
Emissions of 1,2-dichloroethane into the air mainly occur in
process industries. Other emissions occur during its use as a
fumigant, solvent, and lead scavenger, and via evaporation from
contaminated water and from waste disposal sites. Total emis-
sions are estimated to amount to 0.2% of the production volume.
Photochemical degradation via oxidation by hydroxyl radicals is
the most important route of elimination from air. Rainout and
adsorption on atmospheric particles are unlikely to be important
processes of elimination. Photolysis is theoretically a possib-
ility, but no evidence of this is available. The products of
the photochemical degradation are carbon monoxide, carbon
dioxide, hydrogen chloride, formyl chloride, and chloroacetyl-
chloride. The two last compounds will degrade further. The
process is rapid enough to prevent accumulation of the compound
in the atmosphere (section 3.3).
9.2.2 Water
Emissions of 1,2-dichloroethane into water may amount to
0.1% of the production volume. Some of the emissions from EDC
tars, which total about 0.5% of the production volume, will
contaminate water. The main process of removal of 1,2-dichloro-
ethane from water is evaporation. Chemical degradation is not
expected, nor is biodegradation fast enough to be of any signi-
ficance (section 3.1). Bioconcentration in aquatic species is
unlikely in view of the rather low octanol/water partition
coefficient. This conclusion is supported by a low bioconcen-
tration factor found experimentally (section 6.1.4).
The compound was only slightly toxic for aquatic species
tested. A no-observed-adverse-effect concentration for Daphnia
magna in a long-term test was 11 mg/litre (section 6.1.3). The
lowest LC50 value (85 mg/litre) was found for the shrimp Crangon
crangon (section 6.1.1). Average environmental levels of the
compound in surface water are generally below 1 µg/litre, but,
in heavily polluted surface water, average levels of 5.6
µg/litre have been measured with a maximum of 90 µ g/litre
(Table 3, section 4.1.1).
On the basis of the above data, it can be concluded that,
except in case of accidents and inappropriate disposal, 1,2-
dichloroethane does not pose a significant hazard for the
aquatic environment. However, it should be noted that EDC tars
are much more toxic than 1,2-dichloroethane (section 6.1.1).
9.2.3 Soil
Data are not sufficient to evaluate the effects of 1,2-
dichloroethane in soil.
10. RECOMMENDATIONS FOR FURTHER STUDIES
1. DNA alkylation (adduct identification).
2. Studies on sub-chronic toxicity using various routes of
exposure.
3. Assessment of the extent to which EDC tars contribute to
contamination of groundwater by 1,2-dichloroethane.
4. Dose-response studies on sensitive, commercially important
fish species (particularly studies relevant to EDC tar
spills).
11. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
1,2-Dichloroethane was evaluated by IARC in 1979 (Volume 20
of the IARC Monographs). It was concluded that:
"There is sufficient evidence that 1,2-dichloroethane is
carcinogenic in mice and rats. In the absence of adequate data
in humans, it is reasonable, for practical purposes, to regard
1,2-dichloroethane as if it presented a carcinogenic risk to
humans."
REFERENCES
ADEMA, D.M.M. (1976) [Acute toxicity tests with 1,2-
dichloroethane, phenol, acrylonitrile, and alkyl benzene-
sulfonate in sea water,] Delft, the Netherlands, Central
Laboratory TNO (TNO Report No. MD-N & E 76/1) (in Dutch).
ALUMOT, E., NACHTOMI, E., MANDEL, E., HOLSTEIN, P., BONDI, A., &
HERZBERG, M. (1976a) Tolerance and acceptable daily intake of
chlorinated fumigants in the rat diet. Food Cosmet. Toxicol.,
14: 105-110.
ALUMOT, E., MEIDLER, M., HOLSTEIN, P., & HERZBERG, M. (1976b)
Tolerance and acceptable daily intake of ethylene dichloride in
the chicken diet. Food Cosmet. Toxicol., 14: 111-114.
ANDRIUKIN, A.A. (1979) [Toxic effect of dichloroethane on the
cardiovascular system.] Klin. Med. (Mosk.), 57: 43-47 (in
Russian).
ARFELLINI, G., BARTOLI, S., COLACCI, A., MAZZULO, M., GALLI,
M.C., PRODI, G., & GRILLI, S. (1984) In vivo and in vitro
binding of 1,2-dibromethane and 1,2-dichloroethane to
macromolecules in rat and mouse organs. J. Cancer Res. clin.
Oncol., 108: 204-213.
ATRI, F.R. (1984) [ Study to assess the toxicity of water
pollutants. III. 1,2-Dichloroethane,] Bonn, Federal Environment
Agency (Research Report No. 106/07/047) (in German).
BAILEY, S., COLLINS, G.B., FISHWICK, F.B., HART, H.V., HORLER,
D.F., & SCUDAMORE, K.A. (1982) Pesticide residues in
foodstuffs in Great Britain; organochlorine pesticides,
organophosphorus pesticides, and fumigant residues in home-
produced and imported wheat. Pestic. Sci., 13: 373-378.
BANERJEE, S., VAN DUUREN, B.L., & ORUAMBO, F.I. (1980)
Microsome-mediated covalent binding of 1,2-dichloroethane to
lung microsomal protein and salmon sperm DNA. Cancer Res., 40:
2170-2173.
BARBER, R.D., DONISH, W.H., & MUELLER, K.R. (1981) A procedure
for the quantitative measurement of the mutagenicity of volatile
liquids in the Ames Salmonella/microsome assay. Mutat. Res.,
90: 31-48.
BARETTA, E.D., STEWARD, R.D., & MUTCHLER, J.E. (1969)
Monitoring exposures to vinyl chloride vapour: breath analysis
and continuous air sampling. Am. Ind. Hyg. Assoc. J., 30: 537-
544.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CAROLL, J.J.
(1980) Bioconcentration and elimination of selected water
pollutants by bluegill sunfish Lepomis macrochirus. In:
Dynamics, Exposure and Hazard Assessment of Toxic Chemicals,
Paper Symposium 1978, Ann Arbor, Michigan, Ann Arbor Science,
pp. 379-392.
BARSOUM, G.S. & SAAD, K. (1934) Relative toxicity of certain
chlorine derivatives of the aliphatic series. Q. J. Pharm.
Pharmacol., 7: 205-214.
BAUER, U. (1978) [Halogenated hydrocarbons in drinking and
surface water. Analysis results 1976-77 in the Federal Republic
of Germany. Drinking water from 100 cities; surface water from
the Ruhr, Lippe, Main, and Rhine.] WaBoLu-Berichte, 3: 64-74 (in
German).
BAUER, U. (1981) [Human exposure to harmful components in
environmental studies on water, air, food, and human tissues.
II, IV.] Zentralbl. Bakteriol. Hyg. I. Abt. Orig. B, 174: 39-55,
556-583 (in German).
BENOIT, D.A., PUGLISI, F.A., & OLSON, D.L. (1982) A fathead
minnow Pimephales promelas early life stage toxicity test method
evaluation and exposure to four organic chemicals. Environ.
Pollut., 28(Series A): 189-197.
BERCK, B. (1965) Sorption of ethylene dibromide, ethylene
dichloride, and carbon tetrachloride by cereal products. J.
agric. food Chem., 13: 248-254.
BERCK, B. (1974) Fumigant residues of carbon tetrachloride,
ethylene dichloride, and ethylene dibromide in wheat, flour
bran, middlings, and bread. J. agric. food Chem., 22: 977-984.
BOGOYAVLENSKI, V.F., SALIKHOVA, S.K.H., & KARPOVA, E.V. (1968)
[Clinical aspects and therapy for ethylene dichloride
poisoning.] Sov. Med., 31: 107-109 (in Russian).
BOIKOVA, N.Y. & KRAVTSOVA, G.V. (1982) Histoenzymologic
characteristics of liver and kidney after acute and chronic
poisoning by 1,2-dichloroethane. In: Proceedings of the
Leningrad Scientific Society of Pathologoanatoms, Leningrad,
Medicine, Vol. 23, pp. 158-160.
BONNET, P., FRANCIN, J.-M., GRADISKI, D., RAOULT, G., & ZISSU,
D. (1980) Détermination de la concentration léthale 50 des
principaux hydrocarbures aliphatiques chlorés chez le rat.
Arch. Mal. prof. Méd. Trav. Sécur. soc., 41: 317-321.
BORISSOVA, M.K. (1957) [Experimental inputs to evaluation of
maximum allowable dichloroethane concentration in the air.] Gig.
i Sanit., 3: 13-19 (in Russian).
BORISSOVA, M.K. (1960) Some inputs to evaluation of maximum
allowable dichloroethane concentration in the air. In: Maximum
allowable concentrations of air pollutants, Vol. 4, pp. 21-24.
BOUWER, E.J. & MCCARTY, P.L. (1983) Transformations of 1- and
2-carbon halogenated aliphatic organic compounds under methano-
genic conditions. Appl. environ. Microbiol., 45: 1286-1294.
BOZZELLI, J.W. & KEBBEKUS, B.B. (1982) A study of some
aromatic and halocarbon vapors in the ambient atmosphere of New
Jersey. J. environ. Sci. Health, A17: 693-711.
BREM, H., STEIN, A.B., & ROSENKRANZ, H.S. (1974) The muta-
genicity and DNA-modifying effect of haloalkanes. Cancer Res.,
34: 2576-2579.
BRINGMANN, G. & KUHN, R. (1978) Testing of substances for
their toxicity threshold: model organisms Microcystis
(Diplocystis) aeruginosa and Scenedesmus quadricauda. Mitt.
Int. Verein Limnol., 21: 275-284.
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa
in the cell multiplication inhibition test. Water Res., 14:
231-241.
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effect of
hazardous substances on flagellated and ciliated, respectively,
holozoic bactivorous and saprozoic protozoa.] GWF Wasser-
Abwasser, 122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Data on the toxicity of water
pollutants to Daphnia magna in an improved standard test.] Z.
Wasser Abwasser Forsch., 15: 1-6 (in German).
BRYZHIN, F.F. (1945) Pathomorphological changes in internal
organs in connection with poisoning by ethylene dichloride
through the digestive tract. Pharmacol. Toxicol., 8: 43-49.
BUCCAFUSCO, R.J., ELLS, S.J., & LE BLANC, G.A. (1981) Acute
toxicity of priority pollutants of bluegill Lepomis macro-
chirus. Bull. environ. Contam. Toxicol., 26: 446-452.
CASWELL, G.H. & CLIFFORD, H.T. (1958) The effect of ethylene
dichloride and carbon tetrachloride on the germination and early
growth of maize. Emp. J. exp. Agric., 26: 365-372.
CETNAROWICZ, J. (1959) Experimental and clinical investiga-
tions on the action of dichloroethane. Folia Med. Cracov., 1:
169-192.
CLARK, A.I., MCINTYRE, A.E., LESTER, J.N., & PERRY, R. (1984a)
Ambient air measurement of aromatic and halogenated hydrocarbons
at urban, rural, and motorway locations. Sci. total Environ.,
39: 265-279.
CLARK, A.I., MCINTYRE, A.E., PERRY, R., & LESTER, J.N. (1984b)
Monitoring and assessment of ambient atmospheric concentrations
of aromatic and halogenated hydrocarbons at urban, rural, and
motorway locations. Environ. Pollut. Ser. B, 7: 141-158.
CREBELLI, R., CONTI, G., CONTI, L., & CARERE, A. (1984)
Induction of somatic segregation by halogenated aliphatic
hydrocarbons in Aspergillus nidulans. Mutat. Res., 138(1): 33-
38.
CRESPI, C.L., SEIXAS, G.M., TURNER, T.R., RYAN, C.G., & PENMAN,
B.W. (1985) Mutagenicity of 1,2-dichloroethane and 1,2-
dibromoethane in two human lymphoblastoid cell lines. Mutat.
Res., 142: 133-140.
CUPITT, L.T. (1980) Fate of toxic and hazardous materials in
the air environment, Research Triangle Park, North Carolina, US
Environmental Protection Agency, Environmental Sciences
Laboratory, Office of Research and Development (EPA 600/3-80-
084, PB 221948).
DAWSON, G.W., JENNINGS, A.L., DROSDOWSKI, D., & RIDER, E.
(1977) The acute toxicity of 47 industrial chemicals to fresh
and saltwater fish. J. hazard. Mater., 1: 303-318.
DEQUINZE, J., SZIMAR, C., & EDELINE, F. (1984) Identification
of the substances and their derivative products, on the list of
129 substances (list 1 of the directive 76/464/EEC) present in
the refuse of chlorine derivatives by organic chemistry,
Brussels, European Economic Community.
DILLING, W.L., TEFERTILLER, N.B., & KALLOS, G.J. (1975)
Evaporation rates and reactivities of methylene chloride,
chloroform, 1,1,1-trichloroethane, trichloroethylene,
tetrachloroethylene, and other chlorinated compounds in dilute
aqueous solutions. Environ. Sci. Technol., 9: 833-838.
DMITRIEVA, N.V. & KULESHOV, E.V. (1971) [Changes in the
cerebral bioelectric activity and electric conductivity in rats
in chronic intoxications with certain chlorinated hydrocarbons.]
Gig. i Sanit., 36: 20-25 (in Russian).
DORNDORF, W., KRESSE, M., CHRISTIAN, W., & KATRITZKI, KG.
(1975) [Dichloroethane poisoning with myoclinic syndrome,
epileptic attacks, and irreversible cerebral effects.] Arch.
Psychiatr. Nervenkr., 220: 373-379 (in German).
DRURY, J.S. & HAMMONS, A.S. (1979) Investigations of selected
environmental pollutants: 1,2-dichloroethane, Washington DC, US
Environmental Protection Agency, Office of Toxic Substances (EPA
560/2-78-006, PB 295865).
DUPRAT, P., DELSAUT, L., & GRADISKI, D. (1976) Pouvoir
irritant des principaux solvants chlorés aliphatiques sur la
peau et les muqueuses oculaires du lapin. Eur. J. Toxicol., 9:
171-177.
EHRENBERG, Z., OSTERMAN-GOLKAR, S., SINGH, D., & LUNDQVIST, U.
(1974) On the reaction kinetics and mutagenic activity of
methylating and beta-halogenoethylating gasoline additives.
Radiat. Bot., 15: 184-194.
ELFERS, L.A. (1979) Monitoring of ambient levels of EDC in the
vicinity of EDC production and user facilities, Research
Triangle Park, North Carolina, US Environmental Protection
Agency, Environmental Monitoring and Support Laboratory (EPA
600/4-79-029, PB 298303).
ENVIRONMENT AGENCY, JAPAN (1983) Environmental monitoring of
chemicals: environmental survey report of fiscal years 1980 and
1981, Tokyo, Environment Agency.
EWING, B.B., CHIAN, E.S.K., COOK, J.C., DEWALLE, F.B., EVANS,
C.A., HOPKE, P.K., KIM, J.H., MEANS, J.C., MILBERG, R., PERKINS,
E.G., SHERWOOD, J.D., & WADLIN, W.H. (1977) Monitoring to
detect previously unrecognized pollutants in surface waters,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA 560/6-77-015, PB 273349).
FLOWTOW, E. (1952) Poisoning due to chlorinated hydrocarbon
compounds, particularly 1,2-dichloroethane. Chem. Tech.
(Leipzig), 6: 253-254.
FOUREMAN, G.L. & REED, D.J. (1985) Evidence for a non-
episulfonium ion intermediate during alkylation by S-(2-
chloroethyl) cysteine but not by S-(2-chloroethyl) glutathione.
Fed. Proc. Fed. Am. Exp. Biol., 44: 517.
FUJII, T. (1977) Direct aqueous injection gas chromato-
graphy-mass spectrometry for analysis of organohalides in water
at concentrations below the parts per billion level. J.
Chromatogr., 139: 297-302.
GARRISON, S.C. & LEADINGHAM, R.S. (1954) A fatal case of
ethylene dichloride poisoning in an occupational therapy
department of a neuro-psychiatric hospital. Am. J. phys. Med.,
33: 230-237.
GOCKE, E., WILD, D., ECKHARDT, K., & KING, M.-T. (1983)
Mutagenicity studies with the mouse spot test. Mutat. Res., 117:
201-212.
GOLD, L.S. (1980) Human exposures to ethylene dichloride. In:
Ames, B., Infante, P., & Reitz, R., ed. Ethylene dichloride: a
potential health risk? Cold Spring Harbor, Cold Spring Harbor
Laboratory, pp. 209-225 (Banbury Report No. 5).
GOSSETT, R.W., BROWN, D.A., & YOUNG, D.R. (1983) Predicting
the bioaccumulation of organic compounds in marine organisms
using octanol/water partition coefficients. Mar. Pollut. Bull.,
14: 387-392.
GRADISKI, D., BONNET, P., RAOULT, G., MAGADUR, J.L., & FRANCIN,
J.-M. (1978) Toxicité aiguë comparée par inhalation des
principaux solvants aliphatiques chlorés. Arch. Mal. prof. Méd.
Trav. Sécur. soc., 39: 249-257.
GRIMSRUD, E.P. & RASMUSSEN, R.A. (1975) Survey and analysis of
halocarbons in the atmosphere by gas chromatography-mass
spectrometry. Atmos. Environ., 9: 1014-1017.
GUENGERICH, F.P., CRAWFORD, W.M., DOMORADZKI, J.Y., MACDONALD,
T.L., & WATANABE, P.G. (1980) In vitro activation of 1,2-
dichloroethane by microsomal and cytosolic enzymes. Toxicol.
appl. Pharmacol., 55: 303-317.
GUENGERICH, F.P., MASON, P.S., STOTT, W.T., FOX, T.R., &
WATANABE, P.G. (1981) Role of 2-haloethylene oxides and vinyl
chloride in irreversible binding to protein and DNA. Cancer
Res., 41: 4391-4398.
GUENGERICH, F.P., KOGA, N., INSKEEP, P.B., & OZAWA, N. (1986)
Proceedings of the Second International Symposium on the
Synthesis and Application of Isotopically-Labelled Compounds,
New York, Elsevier.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of
organic chemicals in the atmosphere of the Netherlands. Sci.
total Environ., 43: 193-219.
HADENGUE, M.A. & MARTIN, R. (1953) Un cas d'intoxication
mortelle par le dichloréthane. Soc. Med. Leg., 33: 247-249.
HARKOV, R., KEBBEKUS, B., BOZZELLI, J.W., LLOY, P.J., & DAISEY,
J. (1984) Comparison of selected volatile organic compounds
during the summer and winter at urban sites in New Jersey. Sci.
total Environ., 38: 259-274.
HATCH, G.G., MAMAY, P.D., AYER, M.L., CASTO, B.C., & NESNOW, S.
(1983) Chemical enhancement of viral transformation in Syrian
hamster embryo cells by gaseous and volatile chlorinated
methanes and ethanes. Cancer Res., 43: 1945-1950.
HEITMULLER, P.T., HOLLISTER, T.A., & PARRISH, P.R. (1981)
Acute toxicity of 54 industrial chemicals to sheepshead minnows
Cyprinodon variegatus. Bull. environ. Contam. Toxicol., 27:
596-604.
HELLMANN, T.M. & SMALL, F.H. (1974) Characterization of the
odour properties of 101 petrochemicals using sensory methods.
J. Air Pollut. Control Assoc., 24: 979-982.
HEPPEL, L.A., NEAL, P.A., PERRIN, T.L., ENDICOTT, K.M., &
PORTERFIELD, V.T. (1946) The toxicology of 1,2-dichloro-
ethane (ethylene dichloride). V. The effect of daily
inhalations. J. ind. Hyg. Toxicol., 28: 113-120.
HILL, D.L., SHIH, T.-W., JOHNSTON, T.P., & STRUCK, R.F. (1978)
Macromolecular binding and metabolism of the carcinogen 1,2-
dibromoethane. Cancer Res., 38: 2438-2442.
HINKEL, G.K. (1965) [Oral dichloroethane intoxication of
children.] Dtsch Gesudheitswes., 20: 1327-1331 (in German).
HOFMANN, H.T., BIRNSTIEL, H., & JOBST, P. (1971) [On the
inhalation toxicity of 1,2-dichloroethane.] Arch. Toxikol., 27:
248-265 (in German).
HOOPER, K., GOLD, I., & AMES, B. (1980) The carcinogenic
potency of ethylene dichloride in two animal bioassays: a
comparison of inhalation and gavage studies. In: Ames, B.N.,
Infante, P., & Reitz, R., ed. Ethylene dichloride: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 65-81 (Banbury Report No. 5).
HOWARD, C.J. & EVENSON, K.M. (1976) Rate constants for the
reactions of OH with ethane and some halogen substituted ethanes
at 296 K. J. Chem. Phys., 64: 4303-4306.
HUEPER, W.C. & SMITH, C. (1935) Fatal ethylene dichloride
poisoning. Am. J. med. Sci., 189: 778-784.
IARC (1979) 1,2-Dichloroethane. In: Some halogenated hydro-
carbons, Lyons, International Agency for Research on Cancer, pp.
429-446 (IARC Monographs on the Evaluation of the Carcinogenic
Risk of Chemicals to Humans, Vol. 20).
INSKEEP, P.B. & GUENGERICH, F.P. (1984) Glutathione mediated
binding of dibromoalkanes to DNA: specificity of rat glutathione
S-transferases and dibromoalkane structure. Carcinogenesis, 5:
805-808.
INSKEEP, P.B., KOGA, N., CMARIK, J.L., & GUENGERICH, F.P.
(1986) Covalent binding of 1,2-dihaloalkanes to DNA and
stability of the major DNA adduct S-[2-(N7/guanyl)ethyl]
glutathione. Cancer Res., 46: 2839-2844.
IRPTC (1982) Izmerov, N.F., ed. Dichloroethane, Geneva,
International Register of Potentially Toxic Chemicals, United
Nations Environment Programme (Scientific Review of Soviet
Literature on Toxicity and Hazards of Chemicals No. 25).
IRPTC (1984) Data profile of 1,2-dichloroethane, Geneva,
International Register of Potentially Toxic Chemicals.
JACOBS, E.S. (1980) Use and air quality impact of ethylene
dichloride and ethylene dibromide scavengers in leaded gasoline.
In: Ames, B., Infante, P., & Reitz, R., ed. Ethylene dichloride:
a potential health risk?, Cold Spring Harbor, New York, Cold
Spring Harbor Laboratory, pp. 239-255 (Banbury Report No. 5).
JAKOBSON, I., WAHLBERG, J.E., HOLMBERG, B., & JOHANSSON, G.
(1982) Uptake via the blood and elimination of 10 organic
solvents following epicutaneous exposure of anesthetized guinea-
pigs. Toxicol. appl. Pharmacol., 63: 181-187.
JANSSEN, D.B., SCHEPER, A., & WITHOLT, B. (1984) Biodegra-
dation of 2-chloroethanol and 1,2-dichloroethane by pure
bacterial cultures. Prog. Med. Microbiol., 20: 169-178.
JENSEN, S., LANGE, R., BERGE, G., PALMORK, K.H., & RENBERG, L.
(1975) On the chemistry of EDC-tar and its biological
significance in the sea. Proc. R. Soc. Lond. B, 189: 333-346.
JENSSEN, D. & RAMEL, C. (1980) The micronucleus test as part
of a short-term mutagenicity test program for the prediction of
carcinogenicity evaluated by 143 agents tested. Mutat. Res., 75:
191-202.
JERNELOV, A., ROSENBERG, R., & JENSEN, S. (1972) Biological
effects and physical properties in the marine environment of
aliphatic chlorinated by-products from vinyl chloride
production. Water Res., 6: 1181-1191.
JOHNSON, M.K. (1965) The influence of some aliphatic compounds
on rat liver glutathione levels. Biochem. Pharmacol., 14: 1383-
1385.
JOHNSON, M.K. (1966) Studies on glutathione-S-alkyl transferase
of the rat. Biochem. J., 98: 44-56.
JOHNSON, M.K. (1967) Metabolism of chloroethanol in the rats.
Biochem. Pharmacol., 16: 185-199.
JOLIVET, E. (1968) Métabolisme des acides organiques et des
acides aminés libres dans le tubercule de pomme de terre au
cours de la rupture provoquée de son repos végétatif par la
"Rindite". Physiol. vég., 6: 221-233.
JONSSON, A. & BERG, S. (1980) Determination of 1,2-dibromo-
ethane, 1,2-dichloroethane, and benzene in ambient air using
porous polymer traps and gas chromatographic-mass spectro-
metric analysis with selected ion monitoring. J. Chromatogr.,
190: 97-106.
KAGRAMANOV, V.G. & KAZIEVA, N.K. (1972) Condition of
fibrinolytic blood activity in acute dichloroethane poisoning.
Azerb. State Med. Inst. Baku, 36: 30-31.
KAIRA, F.M. (1966) [Alimentary oral dichloroethane poisoning.]
Klin. Med. (Mosk.), 44: 143-146 (in Russian).
KAMEL, A. (1959) The effect of carbon bisulphide and ethylene
dichloride-carbon tetrachloride mixture on the germination of
some seeds of certain crops. Agric. Res. Rev. (Cairo), 37: 330-
350.
KELLAM, R.G. & DUSETZINA, M.G. (1980) Human exposure to
ethylene dichloride: potential for regulation via EPA's proposed
airborne carcinogen policy. In: Ames, B., Infante, P., & Reitz,
R., ed. Ethylene dichloride: a potential health risk?, Cold
Spring Harbor, New York, Cold Spring Harbor Laboratory, pp.
265-276 (Banbury Report No. 5).
KERSTER, H.W. & SCHAEFFER, D.J. (1983) Brine shrimp (Artemia
salina nauplii) as a teratogen test system. Ecotoxicol.
environ. Saf., 7: 342-349.
KING, M.-T., BEIKIRCH, H., ECKHARDT, K., GOCKE, E., & WILD, D.
(1979) Mutagenicity studies with X-ray contrast media,
analgesics, antipyretics, antirheumatics, and some other
pharmaceutical drugs in bacterial, Drosophila, and mammalian
test systems. Mutat. Res., 66: 33-43.
KLEINSCHMIDT, E.-G., VON (1983) [Investigations into the
relation between odour thresholds of human beings for some
substances and their chemical structure.] Wiss. Z. Wilhelm-
Piech-Univ. Rostock, Naturwiss. Reihe, 32: 54-58 (in German).
KOGA, N., INSKEEP, P.B., HARRIS, T.M., & GUENGERICH, F.P.
(1986) S-[2-(N7-guanyl)ethyl] glutathione, the major DNA adduct
formed from 1,2-dichloroethane. Biochemistry, 25: 2192-2198.
KOKAROVTSEVA, M.G. & KISELYOVA, N. (1978) [Chloroethanol
(ethylenechlorohydrin) - a toxic metabolite of 1,2-dichloro-
ethane.] Farmakol. Toksikol., 41: 118-120 (in Russian).
KOMAROV, V.P., ROMANCHAK, M.N., SHCHERBAN, I.I., & GAPONTSEV,
S.N. (1973) [On the clinical features and therapy of acute
dichloroethane intoxications.] Zdravookhr. Belorussi, 4: 55-57
(in Belorussian).
KONEMANN, H. (1981) Quantitative structure-activity
relationships in fish toxicity studies. I. Relationship for 50
industrial pollutants. Toxicology, 19: 209-221.
KOZIK, I.V. (1957) [Some problems of occupational hygiene in
the use of dichloroethane in the aircraft industry.] Gig. Tr.
Prof. Zabol., 1: 31-38 (in Russian).
KRONEVI, T., WAHLBERG, J.E., & HOLMBERG, B. (1981) Skin
pathology following epicutaneous exposure to seven organic
solvents. Int. J. Tissue React., 3: 21-30.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A.
(1982) Collection and analysis of hazardous organic emissions.
Anal. Chem., 54: 810-817.
KULKARNI, J.H., SARDESHPANDE, J.S., & BAGYARAJ, D.J. (1975)
Effect of seed fumigation on the symbiosis of Rhizobium sp.
with Arachis hypogaea Linn. Zentralbl. Bakteriol. Parasitenkr.
Infektionskr. Hyg. Abt. II, 130: 41-44.
KUWABARA, T., QUEVEDO, A.R., & COGAN, D. (1968) An experi-
mental study of dichloroethane poisoning. Arch. Ophtalmol., 79:
321-330.
LANE, R.W., RIDDLE, B.L., & BORZELLECA, J.F. (1982) Effects of
1,2-dichloroethane and 1,1,1-trichlorethane in drinking-water on
reproduction and development in mice. Toxicol. appl. Pharmacol.,
63: 409-421.
LARIONOV, V.G. & KOKAROVTSEVA, M.G. (1976) Morphological
constitution of peripheral blood in intoxication with
dichloroethane and its metabolites. In: Actual problems of
pesticide application in different climatographic zones,
Yerevan, Aiastan Publishers, pp. 131-133.
LE BLANC, G.A. (1980) Acute toxicity of priority pollutants to
water flea Daphnia magna. Bull. environ. Contam. Toxicol., 24:
684-691.
LIN, E.L.C., MATTOX, J.K., & PEREIRA, M.A. (1985) Glutathione
plus cytosol- and microsome-mediated binding of 1,2-dichloro-
ethane to polynucleotides. Toxicol. appl. Pharmacol., 78: 428-
435.
LIVESEY, J.C. & ANDERS, M.W. (1979) In vitro metabolism of
1,2-dihaloethanes to ethylene. Drug Metab. Dispos., 7: 100-203.
LOCHHEAD, H.B. & CLOSE, H.P. (1951) Ethylene dichloride
plastic cement: a case of fata poisoning. J. Am. Med. Assoc.,
146: 1323.
LUZNIKOV, E.A., LISOVIK, G.A., & NOVIKOVSKAYA, T.V. (1985)
[Metabolism of 1,2-dichloroethane in human organisms after acute
poisonings.] [ Forensic Med. Expert.,] 2: 47-49 (in Russian
with English summary).
LYNN, G.E. & VORHES, F.A., ed. (1957) Symposium on residues in
foods and feeds resulting from fumigation of grains with the
commoner liquid formulations of carbon disulphide, carbon
tetrachloride, ethylene dichloride, and ethylene dibromide. J.
Assoc. Off. Agric. Chem., 40: 163-209.
MCCANN, J., SIMMON, V., STREITWIESER, D., & AMES, B. (1975)
Mutagenicity of chloroacetaldehyde, a possible metabolic product
of 1,2-dichloroethane (ethylene dichloride), chloroethanol
(ethylene chlorohydrin), vinyl chloride, and cyclophosphamide.
Proc. Natl Acad. Sci. (USA), 72: 3190-3193.
MCCOLLISTER, D.D., HOLLINGSWORTH, R.L., OYEN, F., & ROWE, V.K.
(1956) Comparative inhalation toxicity of fumigant mixtures.
Individual and joint effects of ethylene dichloride, carbon
tetrachloride, and ethylene dibromide. Arch. ind. Health, 13:
1-7.
MALTONI, C., VALGIMIGLI, L., & SCARNATO, C. (1980) Long-term
carcinogenic bioassays on ethylene dichloride administered by
inhalation to rats and mice. In: Ames, B., Infante, P., & Reitz,
R., ed. Ethylene dichloride: a potential health risk?, Cold
Spring Harbor, New York, Cold Spring Harbor Laboratory, pp. 5-30
(Banbury Report No. 5).
MARTIN, G., KNORPP, K., HUTH, K., HEINRICHT, F., & MITTERMAYER,
C. (1968) [Clinical features, pathogenesis and therapy of
dichloroethane poisoning.] Dtsch med. Wschr., 93: 2002-2010 (in
German).
MAY, J. (1966) [Odour thresholds of solvents for the judgement
of solvent odour in air.] Staub, 26: 385-389 (in German).
MENSCHICK, H. (1957) [Acute inhalation intoxications by
symmetric dichloroethane.] Arch. Gewerbepathol. Gewerbehyg., 15:
241-252 (in German).
MITOMA, C., STEEGER, T., JACKSON, S.E., WHEELER, K.P., ROGERS,
J.H., & MILMAN, H.A. (1985) Metabolic disposition study of
chlorinated hydrocarbons in rats and mice. Drug chem. Toxicol.,
8: 183-194.
MOODY, E.D., JAMES, J.L., CLAWSON, G.A., & SMUCKLER, E.A.
(1981) Correlations among the changes in hepatic microsomal
components after intoxication with alkyl halides and other
heptatotoxins. Mol. Pharmacol., 20: 685-693.
MOROZOV, G.N. (1958) [On acute dichloroethane poisoning.]
Farmakol. Toksicol., 21(1): 76-78 (in Russian).
MUNSON, A.E., SANDERS, V.M., DOUGLAS, K.A., SAIN, L.E.,
KAUFFMANN, B.M., & WHITE, K.L. (1982) In vivo assessment of
immunotoxicity. Environ. Health Perspect., 43: 41-52.
NACHTOMI, E., ALUMOT, E., & BONDI, A. (1966) The metabolism of
ethylene dibromide in the rat. I. Identification of detoxifi-
cation products in urine. Isr. J. Chem., 4: 329-246.
NATSYUK, M.V. & CHEKMAN, I.S. (1975) [Content of nicotinamide
coenzymes in the liver and myocardium of rats poisoned with
dichloroethane.] Byull. eksp. Biol. Med., 79: 58-60 (in
Russian).
NATSYUK, M.V. & MUDRITSKY, A.D. (1974) Clinical and
biochemical characteristics of hepatic and renal disorders in
acute dichloroethane intoxications. Mil. med. J., 10: 48-50.
NATSYUK, M.V., CHERNUKHA, F.A., BASENKO, G.A., & FEDUROV, V.V.
(1974) [Effect of acute dichloroethane intoxication on
oxidative phosphorylation in rat liver mitochondria.] Farmakol.
Toksikol., 37: 92-93 (in Russian).
NAZIKHI, F. & SKRIZHINSKY, S.F. (1973) Activity of succinate-
toxicodase system enzymes of myocardium in experimental
dichloroethane intoxication. In: Current issues of physiology,
pathology, and clinical diseases of the cardiovascular system,
Leningrad, pp. 62-63.
NCI (1978) Bioassay of 1,2-dichloroethane for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute,
US Department of Health, Education and Welfare, Public Health
Services, National Institute of Health (HEW (NIH) Publication
No. 78-1305).
NELSON, G.O. & SHAPIRO, E.G. (1971) A field instrument for
detecting airborne halogen compounds. Am. Ind. Hyg. Assoc. J.,
32: 757-765.
NEVROTSKY, V.K., KASHIN, L.M., KULINSKAYA, I.L., MIKHAILOVSKAYA,
L.F., SHMUTER, L.M., BURLAKA-VOVK, Z.I., & ZADOROZHNY, B.V.
(1971) Comparative toxicity evaluation of several industrial
poisons in protracted inhalation exposure to low concentrations.
In: Proceedings of the 8th Ukrainian Hygiene Congress, Kiev,
1971, pp. 224-226.
NIOSH (1976) Criteria for a recommended standard -
occupational exposure to ethylene dichloride (1,2-dichloro-
ethane), Cincinnati, Ohio, National Institute of Occupational
Safety and Health, US Department of Health, Education and
Welfare, Public Health Services, Center for Disease Control
(DHEW (NIOSH) Publication No. 76-139).
NIOSH (1977) Manual of analytical methods. II. Standards
completion program validated methods, Cincinnati, Ohio, National
Institute of Occupational Safety and Health, Vol. 2, pp. S122/1-
S122/9 (DHEW (NIOSH) Publication No. 77-157-B).
NOETZEL, O. (1944) Lethal poisoning with ethylene dichloride.
Chem. Ztg., 68: 146-147.
NOUCHI, T., MIURA, H., KANAYAMA, M., MIZUGUCHI, O., & TAKANO, T.
(1984) Fatal intoxication by 1,2-dichloroethane: a case report.
Int. Arch. occup. environ. Health, 54: 111-113.
NYLANDER, P.-O., OLOFSSON, H., RASMUSON, B., & SVAHLIN, H.
(1978) Mutagenic effects of petrol in Drosophila melanogaster:
effects of benzene and 1,2-dichloroethane. Mutat. Res., 57:
163-167.
OKAMOTO, T. & TATSUKAWA, R. (1981) [Distribution and fate of
low molecular weight chlorinated hydrocarbons in Osaka Bay.]
Chikyukagaku, 15: 17-24 (in Japanese).
OZAWA, N. & GUENGERICH, F.P. (1983) Evidence for formation of
an S-[2-(N7-guanyl)ethyl] glutathione adduct in glutathione-
mediated binding of the carcinogen 1,2-dibromoethane to DNA.
Proc. Natl Acad. Sci. (USA), 80: 5268-5270.
PAGE, B.D. & KENNEDY, B.P.C. (l975) Determination of methylene
chloride, ethylene dichloride and trichloroethylene as solvent
residues in spice oleoresins, using vacuum distillation and
electron capture. J. Assoc. Off. Anal. Chem., 58: 1062-1068.
PAPAROPOLI, G. & CALI, V. (l956) [Collective intoxications
with chlorinated hydrocarbons in dock workers.] Folia Med., 39:
819-831 (in Italian).
PARKES, D.G., GANZ, C.R., POLINSKY, A., & SCHULZE, J. (l976) A
simple gas chromatographic method for the analysis of trace
organics in ambient air. Am. Ind. Hyg. Assoc. J., 37: 165-173.
PEARSON, C.R. & MCCONNELL, G. (l975) Chlorinated C1 and C2
hydrocarbons in the marine environment. Proc. R. Soc. Lond. B,
189: 305-332.
PEROCCO, P. & PARODI, G. (1981) DNA damage by haloalkanes in
human lymphocytes cultures in vitro. Cancer Lett., 13: 213-218.
PRICE, K.S., WAGGY, G.T., & CONWAY, R.A. (1974) Brine shrimp
bioassay and seawater BOD of petrochemicals. J. Water Pollut.
Control Fed., 46: 63-77.
PRINCIPE, P., DOGLIOTTI, E., BIGNAMI, M., CREBELLI, R., FALCONE,
E., FABRIZI, M., CONTI, G., & COMBA, P. (1981) Mutagenicity of
chemicals of industrial and agricultural relevance in
Salmonella, Streptomyces, and Aspergillus. J. Sci. food Agric.,
32: 826-832.
RADDING, S.B., LUI, D.H., JOHNSON, H.L., & MILL, T. (1977)
Review of the environmental fate of selected chemicals,
Washington DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA report no. 560/5-77-003, PB 267121.
RANNUG, U. (1980) Genotoxic effects of 1,2-dibromoethane and
1,2-dichoroethane. Mutat. Res., 76: 269-295.
RANNUG, U. & BEIJE, B. (1979) The mutagenic effect of 1,2-
dichloroethane on Salmonella typhimurium. II. Activation by the
isolated perfused rat liver. Chem.-biol. Interact., 24: 265-
285.
RANNUG, U., SUNDVALL, A., & RAMEL, C. (1978) The mutagenic
effect of 1,2-dichloroethane on Salmonella typhimurium. I.
Activation through conjunction with glutathione in vitro. Chem.-
biol. Interact., 20: 1-16.
RAO, K.S., MURRAY, J.S., DEACON, M.M., JOHN, J.A., CALHOUN,
L.L., & YOUNG, J.T. (1980) Teratogenicity and reproduction
studies in animals inhaling ethylene dichloride. In: Ames, B.,
Infante, P., & Reitz, R., ed. Ethylene dichloride: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 145-161 (Banbury Report No. 5).
RAPOPORT, I.A. (1960) [The reaction of genic proteins with
1,2-dichloroethane.] Dokl. Akad. Nauk. SSSR, 134: 1214-1217 (in
Russian).
REITZ, R.H., FOX, T.R., RAMSEY, J.C., QUAST, J.F., LANGVARDT,
P.W., & WATANABE, P.G. (1982) Pharmacokinetics and macro-
molecular interactions of ethylene dichloride in rats after
inhalation or gavage. Toxicol. appl. Pharmacol., 62: 190-204.
RICHTER, J.E., PETERSON, S.F., & KLEINER, C.F. (1983) Acute
and chronic toxicity of some chlorinated benzenes, chlorinated
ethanes and tetrachloroethylene to Daphnia magna. Arch. environ.
Contam. Toxicol., 12: 679-684.
ROHMANN, E., VON, ZINN, D., & KULZ, J. (1969) [Electro-
encephalographic observations in child blood poisonings and
their therapeutic consequences.] Kinderaerztl. Prax., 37: 209-
216 (in German).
ROSENBERG, R. (1972) Effects of chlorinated aliphatic hydro-
carbons on larval and juvenile Balanus balanoides L. Environ.
Pollut., 3: 313-318.
ROSENBERG, R., GRAHN, O., & JOHANSSON, L. (1975) Toxic effects
of aliphatic chlorinated by-products from vinyl chloride
production on marine animals. Water Res., 9: 607-612.
ROUBAL, J. (1947) Two fatal cases of intoxication with
symmetric dichloroethane ingestion. Cas. Lek. Cesk., 86: 203-
206.
SAITANOV, A.O. & ARSENIEVA, S.S. (1969) Electrocardiographic
changes due to acute dichloroethane poisoning: experimental
investigation. Gig. Tr. Prof. Zabol., 7: 49-50.
SALTZMAN, B.E. (1972) Direct reading colorimetric indicators.
In: Air sampling instruments for evaluation of atmospheric
contamination, 4th ed., Cincinnati, Ohio, American Conference of
Governmental Hygienists, pp. S22-23.
SAUER, T.C. (1981) Volatile organic compounds in open ocean
and coastal surface waters. Org. Geochem., 3: 91-101.
SCHASTEEN, C.S. & REED, D.J. (1983) The hydrolysis and
alkylation activities of S-(2-haloethyl)-L-cysteine analogues -
evidence for extended half-life. Toxicol. appl. Pharmacol., 70:
423-432.
SCHERB, K. (1978) [Investigations on the evaporation of low
molecular weight chlorinated hydrocarbons from flow-gutter.]
Munch. Beitr. Abwasser-Fisch. Flussbiol., 30: 235-248 (in
German).
SCHONBORN, H., PRELLWITZ, W., & BAUM, P. (1970) [Consumption
coagulation pathology of 1,2-dichloroethane poisoning.] Klin.
Wochenschr., 48: 822-824 (in German).
SECCHI, G.C., CHIAPPINO, G., LOTTO, A., & ZURLO, N. (1968)
[Actual chemical composition of the "commercial trieline" and
their hepatotoxic effect: clinical and enzymological studies.]
Med. Lav., 59: 486-497 (in Italian).
SEUFERT, F.B., BROWN, P., OATWAY, J.A., BORNSTEIN, M.,
OSTROWSKI, W., & HORNE, R. (1980) 1,2-Dichloroethane technical
control options analysis, Bedford, Massachusetts, CGA
Corporation (EPA Contract No. 68-01-5960).
SHAKARNIS, V.F. (1969) [1,2-Dichloroethane-induced chromosome
non-disjunction and recessive sex-linked lethal mutations in
Drosophila melanogaster.] Genetika, 5: 89-95 (in Russian).
SHAKARNIS, V.F. (1970) [Effect of 1,2-dichloroethane on
chromosome non-disjunction and recessive sex-linked lethals in a
radioresistant strain of Drosophila melanogaster.] [ Vestn.
Leningrad Univ. Biol.,] 25: 153-156 (in Russian).
SHMUTER, L.M. (1977) [Effect of chronic exposure to low
concentrations of chlorinated hydrocarbons of the ethane series
on specific and non-specific reactivity of animals in vivo.]
Gig. Tr. Prof. Zabol., 8: 38-42 (in Russian).
SIMMON, V.F. (1980) Review of non-bacterial tests of the
genotoxic activity of ethylene chloride. In: Ames, B., Infante,
P., & Reitz, R., ed. Ethylene chlorde: a potential health risk?,
Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp. 97-103 (Banbury Report No. 5).
SINGH, H.B., SALAS, L.J., & STILES, R.E. (1982) Distribution
of selected gaseous organic mutagens and suspect carcinogens in
ambient air. Environ. Sci. Technol., 16: 872-880.
SINGH, H.B., SALAS, L.J., & STILES, R.E. (1983) Selected man-
made halogenated chemicals in the air and oceanic environment.
J. geophys. Res., 88: 3675-3683.
SOPIKOV, N.G. & GORSHUNOVA, A.I. (1979) [Investigation of the
intake, distribution and excretion of ethylene dichloride in
rats.] Gig. Tr. Prof. Zabol., 4: 36-40 (in Russian).
SPENCE, J.W. & HANST, P.L. (1978) Oxidation of chlorinated
ethanes. J. Air Pollut. Control Assoc., 28: 250-253.
SPENCER, H.C., ROWE, V.K., ADAMS, E.M., MCCOLLISTER, D.D., &
IRISH, D.D. (1951) Vapor toxicity of ethylene dichloride
determined by experiments on laboratory animals. Arch. ind.
Hyg. occup. Med., 4: 482-493.
SPREAFICO, F., ZUCCATO, E., MARCUCCI, F., SIRONI, M.,
PAGLIALUNGA, S., MADONNA, M., & MUSSINI, E. (1980) Pharmaco-
kinetics of ethylene dichloride in rats treated by different
routes and its long-term inhalatory toxicity. In: Ames, B.,
Infante, P., & Reitz, R., ed. Ethylene chlorde: a potential
health risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 107-129 (Banbury Report No. 5).
STORER, R.D. & CONOLLY, R.B. (1983) Comparative in vivo
genotoxicity and hepatotoxicity of three 1,2-dihaloethanes.
Carcinogenesis, 4: 1491-1494.
STORER, R.D. & CONOLLY, R.B. (1985) An investigation of the
role of microsomal oxidative metabolism in the in vivo
genotoxicity of 1,2-dichloroethane. Toxicol. appl. Pharmacol.,
77: 36-46.
STORER, R.D., JACKSON, N.M., & CONOLLY, R.B. (1984) In vivo
genotoxicity and acute hepatotoxicity of 1,2-dichloroethane in
mice: comparison of oral, intraperitoneal, and inhalation routes
of exposure. Cancer Res., 44: 4267-4271.
STOREY, C.L., KIRK, L.D., & MUSTAKAS, G.C. (1972) Fate of EDC-
CC14 (75:25) residues during milling and oil extraction of
soybeans. J. econ. Entomol., 65: 1126-1129.
STOVER, E.L. & KINCANNON, D.F. (1983) Biological treatability
of specific organic compounds found in chemical industry
wastewaters. J. Water Pollut. Control Fed., 55: 97-105.
STUCKEY, D.C., OWEN, W.F., MCCARTY, P.L., & PARKIN, G.F. (1980)
Anaerobic toxicity evaluation by batch and semi-continuous
assays. J. Water Pollut. Control Fed., 52: 720-729.
STUCKI, G., BRUNNER, W., STAUB, D., & LEISINGER, T. (1981)
Microbial degradation of chlorinated C1 and C2 hydrocarbons.
In: Leisinger, T., Cook, A.M., Hütter, R., & Nüesch, J., ed.
FEMS Symposium No. 12, Microbial degradation of xenobiotics and
recalcitrant compounds, London, Academic Press, pp. 131-137.
SUNDHEIMER, D.W., WHITE, R.D., BRENDEL, K., & SIPES, I.C.
(1982) The bioactivation of 1,2-dibromoethane in rat
hepatocytes: covalent binding to nucleic acids. Carcinogenesis,
3: 1129-1133.
SUVEEV, I.M. & BABICHENKO, M.E. (1969) [On the clinic and cure
of acute intoxication with dichloroethane vapours.] Gig. Tr.
Prof. Zabol., 13: 50-51 (in Russian).
SYMONS, J.M., BELLAR, T.A., CARSWELL, J.K., DEMARCO, J., KROPP,
K.L., ROBECK, G.G., SEEGER, D.R., SLOCUM, C.J., SMITH, B.L., &
STEVENS, A.A. (1975) National organics reconnaissance survey
for halogenated organics. J. Am. Water Works Assoc., 67: 634-
648.
TABAK, H.H., QUAVE, S.A., MASHNI, C.E., & BARTH, E.F. (1981)
Biodegradability studies with organic priority pollutant
compounds. J. Water Pollut. Control Fed., 53: 1503-1517.
TAN, E.-L. & HSIE, A.W. (1981) Mutagenicity and cytotoxicity
of haloethanes as studied in the CHO-HGPRT system. Mutat. Res.,
90: 183-191.
THORUP, S. (1957) Breaking dormancy of beech. Physiol. Plant,
10: 728-731.
TROISI, F.M. & CAVALLAZZI, D. (1961) [A fatal case of
poisoning from inhalation of dichloroethane vapours.] Med.
Lav., 52: 612-618 (in Italian).
TSANI-BAZACA, E., MCINTYRE, A.E., LESTER, J.N., & PERRY, R.
(1981) Concentrations and correlations of 1,2-dibromoethane,
1,2-dichloroethane, benzene, and toluene in vehicle exhaust and
ambient air. Sci. Technol. Lett., 2: 303-316.
TSANI-BAZACA, E., MCINTYRE, A.E., LESTER, J.N., PERRY, R.
(1982) Ambient concentrations and correlations of hydrocarbons
and halocarbons in the vicinity of an airport. Chemosphere, 11:
11-23.
TSURUTA, H. (1975) Percutaneous absorption of organic solvents
I. Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind. Health, 13: 227-236.
TU, A.S., MURRAY, T.A., HATCH, K.M., SIVAK, A., & MILMAN, H.A.
(1985) In vitro transformation of BALB/c-3T3 cells by
chlorinated ethanes and ethylenes. Cancer Lett., 28: 85-92.
VAN BLADEREN, P.J., BREIMER, D.D., ROTTEVEEL-SMIJS, G.M.T.,
KNIJFF, P. DE, MOHN, G.R., MEETEREN-WALCHLI, B. VAN, BUIJS, W.,
& VAN DER GEN, A. (1981a) The relation between the structure
of vicinal dihalogen compounds and their mutagenic activation
via conjugation to glutathione. Carcinogenesis, 2: 499-505.
VAN BLADEREN, P.J., HODGETERP, J.J., BREIMER, D.O., & VAN DER
GEN, A. (1981b) The influence of disulfiram and other
inhibitors of oxidative metabolism on the formation of 2-
hydroxyethyl-mercapturic acid from 1,2-dibromoethane by the rat.
Biochem. Pharmacol., 30: 2983-2987.
VAN DUUREN, B.L., GOLDSCHMIDT, B.M., LOEWENGART, G., SMITH,
A.C., MELCHLONNE, S., LEIDMAN, I., & ROTH, D. (1979)
Carcinogenicity of halogenated olefinic and aliphatic
hydrocarbons in mice. J. Natl Cancer Inst., 63: 1433-1439.
VAN ESCH, G.J., KROES, R., LOGTEN, M.J., VAN, & TONKELAAR, E.M.,
DEN (1977) Ninety-day toxicity study with 1,2-dichloroethane
(DCE) in rats, Bilthoven, the Netherlands, National Institute of
Public Health and Environmental Hygiene (Report 195/77 Al.
Tox.).
VARGA, M.B. & FERENCZY, L. (1956) Effect of "Rindite" on the
development of the growth-substances in potato tubers. Nature
(Lond.), 175: 1075.
VOSOVAYA, M.A. (1974) [Development of posterity of two
generations obtained from females subjected to the action of
dichloroethane.] Gig. i Sanit., 7: 25-28 (in Russian).
VOSOVOYA, M.A. (1977) [Effect of dichloroethane on the
reproductive cycle and embryogenesis in experimental animals.]
Akush. Ginekol., 2: 57-59 (in Russian).
WALBRIDGE, C.T., FIANDT, J.T., PHIPPS, G.L., & HOLCOMBE, G.W.
(1983) Acute toxicity of ten chlorinated aliphatic hydrocarbons
to the fathead minnow (Pimephales promelas). Arch. environ.
Contam. Toxicol., 12: 661-666.
WARD, J.M. (1980) The carcinogenicity of ethylene dichloride
in Osborne-Mendel rats and B6C3F1 mice. In: Ames, B., Infante,
P., & Reitz, R., ed. Ethylene dichloride: a potential health
risk?, Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp. 35-53 (Banbury Report No. 5).
WEBBER, D. (1984) Basic chemical output returns to growth.
Top 50 chemical products. Chem. eng. News, May 7: 8-10.
WEISS, F. (1957) [Lethal oral intoxications by
dichloroethane.] Gewerbehygiene, 15: 253-264 (in German).
WHO (1972) Evaluations of some pesticide residues in food,
Geneva, World Health Organization, pp. 276-279, 325-329 (The
Monographs: WHO Pesticide Residues Series, No. 1).
WIRTSCHAFTER, Z.T. & SCHWARTZ, W.D. (1939) Acute ethylene
dichloride poisoning. J. ind. Hyg. Toxicol., 21: 126-131.
WIT, S.L., BESEMER, F.H., DAS, H.A., GOEDKOOP, W., LOOSJES,
F.E., & MEPPELIN, E.K. (1969) Results of an investigation on
the regression of three fumigants (carbon tetrachloride,
ethylene dibromide and ethylene dichloride) in wheat during
processing to bread, Bilthoven, The Netherlands, National
Institute of Public Health and Environmental Hygiene (Report No.
36/69 Tox).
WITHEY, J.R. & COLLINS, B.T. (1980) Chlorinated aliphatic
hydrocarbons used in the foods industry: the comparative
pharmacokinetics of methylene chloride, 1,2-dichloroethane,
chloroform, and trichloroethylene after iv administration in the
rat. J. environ. Pathol. Toxicol., 3: 313-332.
WITHEY, J.R. & KARPINSKI, K. (1985) The fetal distribution of
some aliphatic chlorinated hydrocarbons in the rat after vapor
phase exposure. Biol. Res. Preg., 6(2): 79-88.
WOLFF, D.L., IVANOV, N.G., KLJACKINA, A.M., & MEL'NIKOVA, L.V.
(1979) [Evidence of the effect of significant industrial
toxicological substances on the nervous system by animal
behavioural tests.] Zool. Jahrb. Physiol., 83: 82-91 (in
German).
YLLNER, S. (1971) Metabolism of 1,2-dichloroethane-4C in the
mouse. Acta pharmacol. toxicol., 30: 257-265.
YODAIKEN, R.E. & BABCOCK, J.R. (1973) 1,2-Dichloroethane
poisoning. Arch. environ. Health, 26: 281-284.
ZAMORA, P.O., BENSON, J.M., LI, A.P., & BROOKS, A.L. (1983)
Evaluation of an exposure system using cells grown on collagen
gels for detecting highly-volatile mutagens in the CHO/HGPRT
mutation assay. Environ. Mutagen., 5: 795-801.
ZOETEMAN, B.C.J., PIET, G.J., SLINGERLAND, P., FONDS, A.W., &
WEGMAN, R.C.C. (1979) [ Investigation into the presence of
volatile organic halogenated compounds in groundwater used as a
drinking water supply. Final report: results on the period
November 1976 to August 1978,] Bilthoven, The Netherlands,
National Institute of Drinking Water Supply, National Institute
of Public Health and Environmental Hygiene (Report no. RID-CBH-
79/01 and no. RIV 10/79) (in Dutch).
ZUCCATO, E., MARCUCCI, F., & MUSSINI, E. (1980) GLC
determination of ethylene dichloride (EDC) in biological
samples. Anal. Lett., 13(B5): 371-379.
