
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 43
CHLORDECONE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1984
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154183 0
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1984
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORDECONE
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Properties and analytical methods
1.1.2. Uses and sources of exposure
1.1.3. Environmental concentrations and exposures
1.1.4. Kinetics and metabolism
l.l.5 Studies on experimental animals
1.1.6. Effects in man
1.2. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1. Production and uses
3.2. Transport and distribution
3.2.1. Air
3.2.2. Water
3.2.3. Soil
3.2.4. Abiotic degradation
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. General population exposure
4.2. Occupational exposure
4.3. Wildlife
5. KINETICS AND METABOLISM
5.1. Animal studies
5.2. Human studies
6. STUDIES ON EXPERIMENTAL ANIMALS
6.1. Single exposures
6.2. Short-term exposures
6.2.1. Dermal toxicity
6.3. Long-term exposures and carcinogenicity studies
6.4. Reproduction and teratology studies
6.5. Mutagenicity
6.6. Behavioral studies
6.7. Neurotoxicity
6.8. Other studies
7. EFFECTS ON MAN
7.1. Poisoning incidents in the general population
7.2. Occupational exposure
7.3. Treatment of poisoning in man
8. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8.1. Aquatic organisms
8.2. Terrestrial organisms
8.3. Microorganisms
8.4. Bioaccumulation and biomagnification
8.5. Population and community effects
8.6. Effects on the abiotic environment
8.7. Appraisal
9. PREVIOUS EVALUATIONS OF CHLORDECONE BY INTERNATIONAL BODIES
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE ENVIRONMENT
10.1. Chlordecone toxicity
10.2. Exposure to chlordecone
10.3. Effects on the environment
10.4. Conclusions
REFERENCES
TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
ORGANOCHLORINE PESTICIDES OTHER THAN DDT (CHLORDANE, HEPTACHLOR,
MIREX, CHLORDECONE, KELEVAN, CAMPHECHLOR)
Members
Dr Z. Adamis, National Institute of Occupational Health,
Budapest, Hungary
Dr D.A. Akintonwa, Department of Biochemistry, Faculty of
Medicine, University of Calabar, Calabar, Nigeriaa
Dr R. Goulding, Chairman of the Scientific Sub-committee, UK
Pesticides Safety Precautions Scheme, Ministry of
Agriculture, Fisheries & Food, London, England (Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health
(Indian Council of Medical Research), Meghaninager,
Ahmedabad, India
Dr D.C. Villeneuve, Environmental Contaminants Section,
Environmental Health Centre, Tunney's Pasture, Ottawa,
Ontario, Canada (Rapporteur)
Dr D. Wassermann, Department of Occupational Health, The
Hebrew University, Haddassah Medical School, Jerusalem,
Israel (Vice-Chairman)
Representatives of Other Organizations
Dr C.J. Calo, European Chemical Industry Ecology and
Toxicology Centre (ECETOC), Brussels, Belgium
Mrs M.Th. van der Venne, Commission of the European
Communities, Health and Safety Directorate, Luxembourg
Dr D.M. Whitacre, International Group of National Associations
of Agrochemical Manufacturers (GIFAP), Brussels, Belgium
Secretariat
Dr M. Gilbert, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Mrs B. Goelzer, Division of Noncommunicable Diseases, Office
of Occupational Health, World Health Organization, Geneva,
Switzerland
Dr Y. Hasegawa, Division of Environmental Health,
Environmental Hazards and Food Protection, World Health
Organization, Geneva, Switzerland
--------------------------------------------------------------------
a Unable to attend.
Secretariat (contd.)
Dr K.W. Jager, Division of Environmental Health, International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland (Secretary)
Mr B. Labarthe, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr I.M. Lindquist, International Labour Organisation, Geneva,
Switzerland
Dr M. Vandekar, Division of Vector Biology and Control,
Pesticides Development and Safe Use Unit, World Health
Organization, Geneva, Switzerland
Mr J.D. Wilbourn, Unit of Carcinogen Identification and
Evaluation, International Agency for Research on Cancer,
Lyons, France
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in
the criteria documents as accurately as possible without unduly
delaying their publication, mistakes might have occurred and are
likely to occur in the future. In the interest of all users of
the environmental health criteria documents, readers are kindly
requested to communicate any errors found to the Manager of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in
the criteria documents are kindly requested to make available to
the WHO Secretariat any important published information that may
have inadvertently been omitted and which may change the
evaluation of health risks from exposure to the environmental
agent under examination, so that the information may be
considered in the event of updating and re-evaluation of the
conclusions contained in the criteria documents.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORDECONE
Following the recommendations of the United Nations
Conference on the Human Environment held in Stockholm in 1972,
and in response to a number of World Health Assembly Resolutions
(WHA23.60, WHA24.47, WHA25.58, WHA26.68), and the recommendation
of the Governing Council of the United Nations Environment
Programme, (UNEP/GC/10, 3 July 1973), a programme on the
integrated assessment of the health effects of environmental
pollution was initiated in 1973. The programme, known as the WHO
Environmental Health Criteria Programme, has been implemented
with the support of the Environment Fund of the United Nations
Environment Programme. In 1980, the Environmental Health
Criteria Programme was incorporated into the International
Programme on Chemical Safety (IPCS). The result of the
Environmental Health Criteria Programme is a series of criteria
documents.
A WHO Task Group on Environmental Health Criteria for
Organochlorine Pesticides other than DDT met in Geneva from 28
November to 2 December, 1983. Dr K.W. Jager opened the meeting
on behalf of the Director-General. The Task Group reviewed and
revised the draft criteria document and made an evaluation of the
health risks of exposure to chlordecone.
This document is a combination of drafts prepared by Dr D.C.
Villeneuve of Canada and Dr S. Dobson of the United Kingdom.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract from
the National Institute of Environmental Health Sciences, Research
Triangle Park, North Carolina, USA - a WHO Collaborating Centre
for Environmental Health Effects.
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Properties and analytical methods
Chlordecone (Kepone) is a tan- to white-coloured solid.
Gas chromatography with electron capture detection is the
method most widely used for the determination of chlordecone.
1.1.2. Uses and sources of exposure
Chlordecone was used as an insecticide and as a base material
in the manufacture of the insecticide kelevan. Its production in
the USA was discontinued in 1976; information about its production
elsewhere is lacking.
Exposure of the general population through its normal use can
be regarded as minimal and is mainly related to residues in food.
Poisoning amongst workers and severe contamination of the
surrounding area and rivers have occurred where manufacture and
formulation were carried out in a careless and unhygienic manner.
The exposure of people living near these plants must have been
considerable.
Small children may be exposed through playing with insect
traps containing chlordecone.
1.1.3. Environmental concentrations and exposures
Chlordecone presents a major hazard for aquatic ecosystems
because of its stability and persistence in sediments, its
bioaccumulation in food chains, and its acute and chronic
toxicity. Low concentrations cause reductions in both algal
growth and invertebrate populations, thereby affecting productivity
at other trophic levels. The few data available on terrestrial
ecosystems indicate low acute toxicity but some long-term effects
on vertebrate reproduction.
1.1.4. Kinetics and metabolism
Chlordecone is readily absorbed following ingestion by
animals and human beings. It is also absorbed following
inhalation and dermal exposure. It is widely distributed in the
body; accumulation occurs mainly in the liver. The half-life in
the body is of the order of several months and excretion is slow,
mainly via the faeces.
1.1.5. Studies on experimental animals
Chlordecone is moderately toxic with single exposures. Acute
toxic symptoms in all species tested included severe tremors. It
can cause skin irritation. In long-term studies, lower doses
caused tremors and other neurological symptoms, liver hypertrophy
with induction of mixed function oxidases, hepatobiliary
dysfunction, and centrilobular hepatocellular necrosis.
Chlordecone interferes with reproduction, and it is fetotoxic
in experimental animals.
It is not generally active in short-term tests for genetic
activity. Chlordecone is carcinogenic in both sexes of mice and
rats producing hepatocellular carcinomas.
1.1.6. Effects in man
No accidental poisonings have been reported.
A large number of cases of occupational poisoning were
reported in a manufacturing plant where work-hygiene and safety
precautions were insufficient. Neurological symptoms, especially
nervousness and tremors, together with oligospermia and joint
pains were reported.
1.2. Recommendations
1. Careful surveillance should be maintained over the future
production of chlordecone and the nature and extent of its
uses.
2. The levels in the environment should continue to be
monitored.
3. It is desirable that a long-term follow-up study should be
conducted on workers whose health has been affected by
chlordecone.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1. Identity
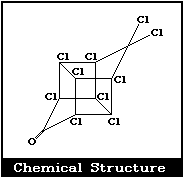 Molecular formula: C10Cl10O
CAS chemical name: 1,1a,3,3a,4,5,5,5a,5b,6-decachloro-
octahydro-1,3,4-metheno-2H-cyclo-
buta[cd]pentalen-2-one
Synonyms: decachloro-pentacyclo[5,2,1,02,6,03,
9,O5,8]decan-4-one, dec-
achloro-octahydro-1,3,4-metheno-
2H,5H-cyclobuta[cd]pentalen-2-one
Trade names: GC 1189, Kepone, Merex
CAS registry number: 143-50-0
Relative molecular mass: 490.6
2.2. Physical and Chemical Properties
Chlordecone is a tan- to white-coloured solid that sublimes
with some decomposition at 350 °C (IARC, 1979). Its vapour
pressure is less than 3 x 10-7 at 25 °C.
In the anhydrous form, chlordecone is soluble in organic
solvents such as benzene and hexane. The hydrated compound is
less soluble in apolar solvents. Oxygenated solvents such as
alcohols and ketones are recommended for the hydrated form
(Blanke et al., 1977). Chlordecone is also soluble in light
petroleum and may be recrystallized from 85 - 90% aqueous ethanol
(Information Canada, 1973). It is readily soluble in acetone
(IARC, 1979).
Early reports did not include any evidence of chlordecone
degradation in the natural environment (Dawson, 1978; Geer,
1978), but, in a more recent study, microbial action has been
shown to transform chlordecone into monohydro and possibly
dihydro-chlordecone (Orndorff & Colwell, 1980a).
Technical grade chlordecone contains from 88.6% to 99.4%
chlordecone (Blanke et al., 1977), 3.5 - 6.0% water (Dawson, 1978)
and 0.1% hexachlorocyclopentadiene. It has been formulated as a
wettable powder (50% chlordecone), emulsifiable concentrates,
granules, and dust (Information Canada, 1973).
2.3. Analytical Methods
Various methods for the determination of chlordecone are
summarized in Table 1.
Table 1. Methods for the determination of chlordecone
---------------------------------------------------------------------------------------------------
Sample type Sampling method, Analytical Limit of Reference
or medium extraction/clean-up method detection
---------------------------------------------------------------------------------------------------
general gas chromato- 0.005- Moseman et al.
graphy electron 0.01 µg (1978)
capture detection
(GC/ECD)
formulations, extract (acetone), infrared (IR) - Allied Chemicals
concentrations, decant, evaporate (c = o band) Corporation (1966)
wettable powders to dryness, dissolve
(decane), boil, cool
technical grade extract (acetone-decane), infrared (IR) - Allied Chemicals
heat to remove (c = o band) Corporation (1966)
acetone, boil, cool
air trap on filter and back up gas chromato- 0.1 µg/m3 NIOSH (1977)
up impinger containing graphy electron
sodium hydroxide solution, capture detection
extract filter (benzene- (GC/ECD)
methanol), acidify extract
(benzene), bulk extracts
food:
apples extract (benzene), decant, gas chromato- 80 µg/kg Brewerton & Slade
filter graphy electron (1964)
capture detection
(GC/ECD)
potatoes extract (methylene chloride), thin-layer 200 µg/kg Proszynska (1977)
column chromatography (CC) chromatography
(TLC) (revelation:
silver nitrate/
ultra-violet)
gas chromato-
graphy electron
capture detection
(GC/ECD)
---------------------------------------------------------------------------------------------------
Table 1. (contd.)
-------------------------------------------------------------------------------------------------------
Sample type Sampling method, Analytical Limit of Reference
or medium extraction/clean-up method detection
-------------------------------------------------------------------------------------------------------
bananas extract (isopropanol-benzene), gas chromato- 3 µg/kg Allied Chemicals
evaporate to dryness, dissolve graphy (GC)/ Corporation (1963)
(hexane), liquid/liquid micro coulo-
partition, extract (benzene) metric detection
water add XAD-2 resin to water, gas chromato- 0.015 Harris et al. (1980)
extract (toluene ethyl graphy electron µg/kg
acetate), column capture detection
chromatography (CC) (GC/ECD)
soil and extract (50% methanol gas chromato- 10-20 Blanke et al. (1977);
sediment in benzene), column graphy electron µg/kg Moseman et al. (1977);
chromatography (CC) capture detection Saleh & Lee (1978);
(GC/ECD) Orndorff & Colwell
(1980b)
biological extract (toluene in gas chromato- 10 µg/kg Mady et al. (1979)
tissues ethyl acetate), column graphy electron
chromatography (CC) capture detection
(GC/ECD)
-------------------------------------------------------------------------------------------------------
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1. Production and Uses
The synthesis of chlordecone was first reported in 1952 by
Gilbert & Giolito (1952). Commercial production in the USA
started in 1966 (IARC, 1979).
Chlordecone is manufactured by the condensation of 2
molecules of hexachlorocylopentadiene in the presence of sulfur
trioxide, followed by hydrolysis to the ketone. It is also
produced during the synthesis of mirex and is a contaminant of
technical grade mirex. From the 1950s until 1975, some 1 600 000
kg of chlordecone were produced in the USA, of which between 90%
(Sterrett & Boss, 1977) and 99.2% (US EPA, 1976b) was exported to
Africa, Europe, and Latin America. The bulk of the remainder,
12 000 - 70 000 kg (US EPA, 1976b) was used in ant and cockroach
traps in the USA or, after 1978, stored until it could be
disposed of safely. It has been reported that most of the
chlordecone exported was used in the manufacture of kelevan
(Cannon et al., 1978).
Chlordecone has been used extensively in the tropics for the
control of banana root borer (Anonymous, 1978a; Langford, 1978).
It is regarded as an effective insecticide against leaf-cutting
insects, but less effective against sucking insects (Information
Canada, 1973). It can be used as a fly larvicide, as a fungicide
against apple scab and powdery mildew (Information Canada, 1973),
and to control the Colorado potato beetle (Motl, 1977), rust mite
on non-bearing citrus, and potato and tobacco wireworm on gladioli
and other plants (Suta, 1978).
Life Science Products in Hopewell, Virginia, produced up to
2700 kg of chlordecone a day between April, 1974 and June, 1975,
when the plant was closed (Lewis & Lee, 1976). Chlordecone
production was discontinued in the USA in 1976. However, a year
later it was reported that a French company was considering the
establishment of production facilities in France (Anonymous,
1978b), but no further information on this proposal is available.
3.2. Transport and Distribution
3.2.1. Air
Laboratory and field observations indicate that chlordecone
does not volatilize to any significant extent (Dawson, 1978).
However, in the past, the release of copious quantities of
chlordecone dust from production facilities has represented a
major source of environmental and human contamination. It has
been suggested that chlordecone emissions from the Hopewell plant
"were of a fine particle size having a long residence time in the
atmosphere" (Lewis & Lee, 1976).
3.2.2. Water
The solubility of chlordecone in water is low (1 - 2 mg/litre)
and, as in the case of mirex, contamination is more likely to be
associated with the particulate matter in the water than with the
water itself (Orndorff & Colwell, 1980b). With the exception of
contamination in the James River system, very little information
is available on chlordecone residues in water. Sampling after
the closure of the Life Science Plant revealed chlordecone levels
of 1 -4 µg/litre in Bailey Creek, 0.1 µg/litre in the Appomattox
River, and 0.3 µg/litre in the James River and at the mouth of
Bailey Creek (Smith, 1976).a Chlordecone was not detected (limit
of determination 0.01 mg/kg) in samples taken from the James River
several months after the plant was shut down (Huggett et al.,
1977). However, it was detected periodically in the water table
of Hopewell at levels as high as 3.4 µg/litre but typically 0.1
µg/litre (Dawson, 1978) and was also detected in the New York
water supply of the Great Lakes Basin by Suta (1978).
Residues as high as 0.21 µg chlordecone/litre have been
reported in runoff from a banana plantation in Guadeloupe
(Snegaroff, 1977).
3.2.3. Soil
Chlordecone has a high affinity for soils and sediments such
that, at equilibrium in the environment, residue levels in
particulate matter will be 104 - 105 times that in any surrounding
water (Dawson, 1978). Consequently, sediments act as sink for
chlordecone-contaminated water and soils provide a sink for most
atmospheric contamination. Again, most of the residue data result
from work in and around Hopewell and the James River system.
Sediment levels were as high as 10 mg/kg in Bailey Bay, and it has
been estimated that as much as 47 000 kg of chlordecone lie on the
bottom of the James River (Chigges, unpublished data, 1977).
Soil residue levels in Hopewell ranged from as high as 10 000
to 20 000 mg/kg near the plant to 2 - 6 mg/kg at a distance of 1
km (US EPA, 1976a) and it was estimated (Anonymous, 1978) that
1000 kg of chlordecone lay within a 1 km radius of the plant.
Most of the soils tested in Hopewell contained detectable levels
of chlordecone with concentrations generally decreasing with
increasing distance from the plant (Dawson, 1978). Chlordecone
residues may be expected in sediments of waterways in the vicinity
of other production-formulation facilities, but no data are
available on this.
----------------------------------------------------------------------
a Smith, W.C. (1976) Kepone discharges from Allied Chemical
Corporation, Hopewell, Virginia, Denver, Colorado, US EPA,
National Field Center (Internal EPA Memorandum).
The US EPA (Anonymous, 1978a) estimated that a field that had
been treated with chlordecone (4.2 kg active ingredient/ha) should
have a residue level of 100 mg/kg in the top 3 cm of soil, after
application. Reports of actual determinations in soil are scarce,
but the United Fruit Company (Anonymous, 1978a) described a
residue level of 15 - 25 mg/kg, 6 months after an application of
6.73 kg active ingredient/ha. Snegaroff (1977) reported soil
residue levels of 9.5 mg/kg and a level of 0.135 mg/kg in
sediments in streams neighbouring on a banana plantation in
Guadeloupe.
3.2.4. Abiotic degradation
Chlordecone is an extremely stable compound and, as mentioned
in section 2, it is not expected to be degraded in the environment
to any significant extent. However, there have been reports of
trace amounts of monohydro chlordecone being found (Carver et al.,
1978, Orndorff & Colwell, 1980b), but the mechanism of its
formation is not clear. Solar irradiation of chlordecone in the
presence of ethylenediamine will result in 78% degradation after
10 days, but no study of the degradation products or their
toxicity has been undertaken (Dawson, 1978).
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. General Population Exposure
Precise information on general population exposure to
chlordecone is not available. However, a summary of the daily
exposure from several sources in different regions in the USA has
been compiled (Suta, 1978).
(a) Air
Airborne chlordecone has been known to spread 60 miles from a
point source (Feldmann, 1976), and the potential exists for
further dispersion of fine particles (Lewis & Lee, 1976).
(b) Water
At present, exposure via drinking-water does not present a
health hazard with the possible exception of that in the Hopewell
area. Values quoted for the lower James River ranged from 0.1 to
10 µg/litre (Suta, 1978).
(c) Food
The USA action levels for chlordecone residues in foods are
0.3 mg/kg for shellfish, 0.3 mg/kg for finfish, 0.4 mg/kg for
crabs, and 0.01 mg/kg for banana peels (Suta, 1978). While the
majority of shellfish taken from the polluted James river in 1976
contained less than the 0.3 mg/kg action level of chlordecone,
oyster and clam samples in certain areas contained 0.21 - 0.81
mg/kg, crab samples contained 0.45 - 3.44 mg/kg, and finfish
samples 0.02 - 14.4 mg/kg. These data prompted a fishing ban on
the James River (Shanholtz, 1976).
In 1978, samples of spot, flounder, mullet, trout, and croakers
from the James River contained chlordecone but in concentrations
below the 0.3 mg/kg action level (Suta, 1978). In bluefish, one
sample was above 0.1 mg/kg (0.2 mg/kg) (US FDA, 1977). The shell-
fish sampled in the same area contained chlordecone, but at levels
that could not be reliably determined (Reuber, 1977). All crabs
in the area contained chlordecone, but all levels were below the
action level.
In 1976, samples from the polluted Chesapeake Bay contained
levels of 0.037 mg/kg for 75 finfish samples and 0.61 mg/kg for
11 crab samples, and levels in 3 samples of oysters and one
sample of clams were non-detectable (US EPA, 1979).
Residues in Atlantic coast bluefish (66 samples) ranged from
0.01 to 0.06 mg/kg, with the higher concentrations found off the
Virginia coast (Peeler, 1976). South Atlantic coastal fish were
relatively free of chlordecone as only 1 out of 132 samples
contained detectable levels (Reuber, 1977).
Residues of chlordecone in edible plants have only been
reported in New Zealand (Brewerton & Slade, 1964). No data are
available in the literature for chlordecone residue levels in
bananas (Suta, 1978).
Chlordecone has been found in 9 out of 298 samples of human
milk, but the detection limit was relatively high (1 µg/kg)
(Suta, 1978). Samples were taken in the southern USA, and
chlordecone residues were only found in areas that had received
bait treatment for fire ants.
(d) Exposure in infants
Two major sources of chlordecone exposure for infants are
insect traps and human milk. The USDA (1977)a has reported that
of 56 cases of non-occupational exposure to chlordecone, 52 were
children under the age of 5, and all but 9 of these had come into
contact with insect traps. This is understandable as children of
this age group are fairly inquisitive and their activity areas
are likely to overlap target areas for ant and cockroach traps.
The same study also cited exposure of 2 adults and 2 persons of
unspecified age.
To date, chlordecone contamination of human milk has only
been reported in 9 samples (Suta, 1978) in the southeastern USA.
However, relatively few samples have been tested for chlordecone.
(e) Miscellaneous
Since tobacco plants were treated with chlordecone, this may
have also represented an exposure route, but again no residue
data are available.
4.2. Occupational Exposure
Chlordecone received its notoriety when severe and wide-
spread industrial poisoning was discovered at the Life Science
Plant (LSP) in Hopewell in 1975. From March 1974 to June 1975,
the LSP recorded output of chlordecone was 769 390 kg (Dawson,
1978). The total production was certainly above this figure, but
massive amounts of chlordecone found their way into the soil,
water, and air surrounding the plant. The workers in the plant
and the families in the area were exposed to extremely high
concentrations of chlordecone dust. High volume air samplers
(Pate & Tabor, 1962), 200 m from the plant, recorded chlordecone
levels as high as 54.8 mg/m3, which constituted 50% of the total
particulate load. Lower concentrations of chlordecone were
detected in the air 25 km away from the plant. Concentrations of
---------------------------------------------------------------------
a Comments of the Secretary of Agriculture in response to
the Notice of Intent to cancel pesticide products
containing chlordecone, trade name "Kepone". Washington DC
vs. USDA, January 11, 1977.
chlordecone dust within the plant were not monitored, but levels
reaching 11.8 mg/litre were found in blood samples of workers
from the LSP (Heath, 1978). Illness was found in 76 of the 133
current and former workers of the plant examined. Families of
the LSP workers were also examined, as well as Allied Chemical
Corporation people working in the area of the plant, workers from
the sewage treatment plant that received chlordecone sludge, and
residents of Hopewell. It was found that the blood levels of
workers who were ill, averaged 2.53 mg/litre, whereas the average
level in workers not reporting ill was 0.60 mg/litre (Heath,
1978).
4.3. Wildlife
Residue levels for phytoplankton in the James River were
found to average 1.3 mg/kg (Huggett et al., 1977).
Chlordecone residues were also found in several species of
birds that inhabit the southeastern USA coast, such as the blue
heron, mallard duck, coot, black duck, wood duck, herring gull,
Canada goose, hooded mersanger, and the bald eagle (Dawson,
1978). Residue levels were as high as 13.23 mg/kg (Dawson,
1978), but typically between 0.02 and 2 mg/kg. Eggs from the
bald eagles and the osprey in Virginia were also examined and
were found to contain residue levels ranging from 0.14 to 0.19
mg/kg, and 0.06 to 1.5 mg/kg, respectively (Dawson, 1978).
Studies on marsh plants in the James River Basin indicated
that there was no translocation of chlordecone from root to
aerial plant tissue (Lunz, 1978).
5. KINETICS AND METABOLISM
Only limited information is available on the absorption,
distribution, metabolism, and excretion of chlordecone in human
beings and animals. These aspects of the chemical are therefore
discussed together, rather than in separate sections.
5.1. Animal Studies
The results of earlier studies by Huber (1965) indicated that,
after dietary exposure, chlordecone was accumulated mainly in the
liver of mice. The brain, fat, and kidneys also contained some
residues. Chlordecone was well absorbed and distributed through-
out the body of rats after oral administration. Following a
single oral dose at 40 mg/kg body weight, the highest concentrations
were found in the adrenal glands and liver, followed by the fat
and lung (Egle et al., 1978). The compound had a long biological
half-life and disappeared more slowly from the liver that from
other tissues. Excretion occurred mainly in the faeces, a total
of 66% of the dose being removed in the faeces and 2% in the urine
urine in the 84 days following administration. Faecal excretion
of chlordecone in rats was increased by the adminstration of an
ionic exchange resin, cholestyramine (Boylan et al., 1977).
Excretion of chlordecone by the gastrointestinal tract, in addition
to the biliary route, occurs in rats as well as human beings
(Boylan et al., 1979). A small amount of chlordecone alcohol was
found in rat faeces suggesting that chlordecone under went
reductive biotransformation in the rat (Blanke et al., 1978).
5.2. Human Studies
A number of studies were conducted to investigate the
kinetics of chlordecone in workers who were exposed to this
chemical. Chlordecone was present in high concentrations in the
liver (mean and range) (75.9 mg/kg; 13.3 - 173 mg/kg), whole
blood (5.8 mg/litre, 0.6 - 32 mg/litre), and subcutaneous fat
(21.5 mg/kg, 2.2 - 62 mg/kg) of 32 male workers (Cohn et al.,
1976). Adir et al. (1978) reported that, in occupationally-
exposed workers, serum chlordecone concentrations ranged from 120
to 2109 µg/litre. Six to 7 months later, the concentration
dropped to 37 - 486 µg/litre. The half-life was estimated to be
63 - 148 days. Chlordecone was eliminated, primarily in the
faeces, at a mean daily rate of 0.075% of the estimated total
store in the body (Cohn et al., 1976). Cholestyramine was found
to increase the faecal excretion of chlordecone by a factor of 6
- 7, presumably by interfering with reabsorption from the
intestine. Chlordecone underwent extensive biliary excretion and
enterohepatic circulation. Elimination by the gastrointestinal
tract also played an important role (Boylan et al., 1979).
Chlordecone alcohol was identified in human faeces (Blanke et
al., 1978).
6. STUDIES ON EXPERIMENTAL ANIMALS
6.1. Single Exposures
Toxicity data resulting from single exposures to chlordecone
in several animal species are summarized in Table 2. Toxic
symptoms included severe tremors in all species tested. These
tremors usually reached a maximum within 2 - 3 days, then
gradually subsided. Tremors were exacerbated by excitement.
In dermal studies on rats and rabbits, no skin irritation was
observed when chlordecone was administered in oil, but in aqueous
solution it produced marked irritation, oedema, and scab formation
(Epstein, 1978).
6.2. Short-Term Exposures
The effects of chlordecone following short-term exposures are
summarized in Table 3. In general, they include nervous symptoms,
liver hypertrophy, induction of mixed-function oxidases (EC 1.14.14.1),
and structural and ultrastructural changes in the liver, thyroid,
adrenals, and testes. Death sometimes followed.
6.2.1. Dermal toxicity
A study has been reported (Epstein, 1978) in which
chlordecone concentrations equivalent to 5 and 10 mg/kg body
weight were tested on groups of 6 male albino rats for 3 weeks,
totalling 15 applications; the animals were killed 2 weeks after
termination of exposure. Two out of 6 animals in the low-dose
group and 1 out of 6 in the high-dose group showed testicular
atrophy. Otherwise, there were no consistent or significant
pathological changes.
6.3. Long-Term Exposures and Carcinogenicity Studies
The long-term and carcinogenic effects of chlordecone are
summarized in Table 4. Effects in these studies were similar to
those reported following short-term exposures. The data indicate
that chlordecone is carcinogenic in mice and rats. These studies
were reviewed by IARC (1979) and it was concluded that chlordecone
produced hepatocellular carcinomas in both sexes of mice and rats.
Table 2. Acute toxicity of chlordecone
-------------------------------------------------------------------------
Species Sex Route of LD50 (mg/kg Reference
administration body weight)
-------------------------------------------------------------------------
dog M & F oral 250 Larson et al. (1979b)
rabbit ? oral 65 NIOSH (1978)
chicken ? oral 480 NIOSH (1978)
rat ? oral 95 NIOSH (1978)
rabbit ? dermal 345 NIOSH (1978)
rat M oral (oil) 132 Larson et al. (1979b)
rat F oral (oil) 126 Larson et al. (1979b)
rat M oral (aqueous) 96 Epstein (1978)
rabbit M oral (oil) 71 Larson et al. (1979b)
rabbit M oral (aqueous) 65 Epstein (1978)
rabbit M dermal (oil) 410 Epstein (1978)
rabbit M dermal (aqueous) 435 Epstein (1978)
pig M oral (approx.) 250 Epstein (1978)
rat M oral (aqueous) 9.6a Epstein (1978)
rat M oral (peanut oil) 125 Gaines (1969)
rat F oral (peanut oil) 125 Gaines (1969)
rat M dermal (xylene) 2000 Gaines (1969)
rat F dermal (xylene) 2000 Gaines (1969)
-------------------------------------------------------------------------
a These animals were dosed for 20 consecutive days excluding Sundays.
Table 3. Summary of short-term studies with chlordecone
-----------------------------------------------------------------------------------------------------
Species Sex Duration Doses used Effects Reference
-----------------------------------------------------------------------------------------------------
mouse M 14 days 1, 10, or induction of hepatic mixed- Fabacher & Hodgson
50 mg/kg diet function oxidases (1976)
at 2 highest levels
rat M 8 days 200 mg/kg ultrastructural changes in Baggett et al.
diet the liver and adrenal (1977, 1980)
medulla, decreased adrenal
catecholamines, and
increased P-450 values
rat F 15 days 50, 100, or decreased body weight gain Mehendale et al.
150 mg/kg diet and induction of mixed- (1978)
function oxidases at all
3 levels of treatment
rat M 15 days 10, 50, or decreased biliary excretion Mehendale et al.
150 mg/kg diet at 10 mg/kg and higher; (1978)
body weight gain affected
at 50 mg/kg and higher;
liver enlargement at
all 3 levels of treatment
rat M & F 3 months 25 mg/kg diet tremors after 4 weeks; liver Cannon & Kimbrough
followed by hypertrophy; liver and adrenals (1979)
"clean" diets both showed histological
for 4.5 months changes; after recovery
period, liver still showed
histological abnormalities
rat 14 days 1 mg/kg diet induction of hepatic mixed- Baker et al.
function oxidases (1972)
-----------------------------------------------------------------------------------------------------
Table 4. Summary of long-term and carcinogenicity studies with chlordecone
---------------------------------------------------------------------------------------------------------
Species Duration Doses used Effects Reference
---------------------------------------------------------------------------------------------------------
rat 2 years 5, 10, 25, all rats on 2 highest doses died during first Larson et al.
50, or 80 6 months; depressed growth occurred at 10 mg/kg (1979b)
mg/kg diet and higher; liver hypertrophy occurred at levels
of 10 mg/kg and higher; histopathological find-
ings in liver, kidneys, and testes at 25 mg/kg;
haematological changes at 25 mg/kg
dog 127 weeks 1, 5, or 25 weight gain reduced at 25 mg/kg; no treatment- Larson et al.
mg/kg diet related histological abnormalities observed (1979b)
mouse 90 weeks 20-40 mg/kg survival reduced at high dose level in males; Anonymous
diet hepatocellular carcinomas induced in both males (1976)
and females
rat up to 24 months 1-80 mg/kg hepatocellular carcinomas observed in some Larson et al.
diet intermediate dose groups, but not all (1979b)
mouse 12 months 0-100 mg/kg tremors observed after 4 weeks in all mice Huber (1965)
diet fed 30 or more mg/kg; deaths observed
at 2 highest doses; liver enlargement
observed at 40 mg/kg and higher; micro-
scopic and electron microscopic changes
observed in dose-dependent manner
rat exposure for 80 8-26 mg/kg increased incidence of hepatocellular carcinomas Anonymous
weeks followed diet observed in high-dose females (1976)
by 16 weeks of
observation
rat up to 2 years 1 mg/kg increased incidence of malignant tumours in Reuber (1978,
diet male and female rats 1979)
---------------------------------------------------------------------------------------------------------
6.4. Reproduction and Teratology Studies
The reproductive performance of mice fed 0, 10, 30, or 37.5
mg chlordecone/kg diet was impaired in terms of offspring and
litter size (Huber, 1965). No litters were produced by females
fed 40 mg/kg, but litter production did resume within 7 weeks
following withdrawal of the chlordecone, although litters were
still smaller than those of untreated controls. Histological
examination of the testes showed they were normal, but corpora
lutea were virtually absent from the ovaries. The authors
concluded that reproductive failure was largely due to an effect
in females characterized by prolonged FSH and estrogen
stimulation, inducing constant estrus, large follicles and
absence of corpora lutea but with levels of LH subminimal for
ovulation.
In a study reported by Good et al. (1965), male and female
mice fed chlordecone in the diet at levels ranging from 10 to 375
mg/kg for 1 month, were randomly paired with animals at the same
feeding level and then maintained on the same diet for 4 months.
The results indicated that chlordecone caused a dose-dependent
effect on reproduction, even at 10 mg/kg.
Similar effects on reproduction were noted by Hammond et al.
(1978) in rats fed 30 mg/kg; the estrogenic properties of this
chemical were also noted (Couch et al., 1977; Bulger et al.,
1979; Hammond et al., 1979). In female rats fed 25 mg
chlordecone/kg diet for 3 months, followed by a control diet for
4.5 months, reproduction was completely inhibited during the
treatment period. Two months after exposure was discontinued,
reproduction was only partially restored (Cannon & Kimbrough,
1979). Chlordecone has also been shown to interfere with egg
production in both quails (McFarland & Lacy, 1969) and hens
(Naber & Ware, 1965).
Chlordecone was administered by gastric intubation in doses
of 2, 6, and 10 mg/kg body weight per day to rats and 2, 4, 8,
and 12 mg/kg body weight per day to mice on days 7 - 16 of
gestation (Chernoff & Rogers, 1976). In rats, the highest dose
caused 19% maternal mortality and fetuses exhibited reduced
weight, reduced degree of ossification, oedema, undescended
testes, enlarged renal pelvis, and enlarged cerebral ventricles.
Lower dose levels induced reductions in fetal weight and degree
of ossification. Male rats born to treated dams did not show any
reproductive impairment. In the mouse, fetotoxicity was observed
only at the highest dose level and consisted of increased fetal
mortality and clubfoot.
In a study by Rosenstein et al. (1977), rats were
administered chlordecone by gavage from day 2 of gestation at
levels of 1, 2, or 4 mg/kg body weight per day. At parturition,
all control pups and those from mothers receiving 1 mg/kg body
weight were normal. Two-thirds of the females receiving 2 mg/kg
and all females receiving 4 mg/kg aborted or had still births.
Chlordecone was administered to female rats at concentrations
of 2.5 mg/kg body weight per day and to mice at 6.0 - 24 mg/kg
body weight per day on days 7 - 16 of gestation and also
postpartum (Chernoff et al., 1979a). Although there were toxic
manifestations in the mother (death) and fetuses (litter
mortality, decreased litter weight), ophthalmological studies did
not reveal cataracts or outlined lenses.
6.5. Mutagenicity
Chlordecone was found to be negative at dose levels of 3.6 or
11.4 mg/kg body weight per day for 5 days in a dominant lethal
study on rats (Simon et al., 1978). Chlordane gave negative
results when tested for enhancement of unscheduled DNA synthesis
in primary cultures of adult rat hepatocytes (Williams, 1980;
Prohst et al., 1981) and was not mutagenic in Salmonella
typhimurium(Prohst et al., 1981).
6.6. Behavioural Studies
In studies on rats administered 40 - 80 mg chlordecone/kg
diet, behavioural changes including hyperactivity, decreased
ambulation in an open field, and delayed emergence from the home
cage were seen at both dose levels within one week (Reiter et
al., 1977; Reiter & Kidd, 1978; Tilson et al., 1979). Chlordecone
was given intragastrically, 5 - 6 days per week, at dosages of 1,
5, and 10 mg/kg body weight for 4 - 76 days to male and female
Zivic-Miller rats. A dose of 1 mg/kg body weight disrupted the
multiple-fixed-ratio test and the fixed-interval test after 3
injections and a dose of 5 mg/kg decreased the spaced-responding
test after 9 - 10 injections. Gradual recovery occurred after
discontinuation of treatment (Dietz & McMillan, 1978).
6.7. Neurotoxicity
Chickens (Naber & Ware, 1965), quail (McFarland & Lacy,
1969), fish (Couch et al., 1977), hamsters (Martinez et al.,
1976), mice (End et al., 1979), rats (Epstein, 1978), and man
(Martinez et al., 1978) have all displayed neurotoxic symptoms on
exposure to chlordecone. Biochemically, chlordecone has been
shown to inhibit Mg-ATPases in fish brain (IARC, 1979) and rat
liver (Desaiah et al., 1977) and also to cause disruption of rat
brain synaptosomal membranes (End et al., 1979).
6.8. Other Studies
Chlordecone has been shown to inhibit several enzymes (in
vitro) including maleate dehydrogenase (Anderson et al., 1977),
lactate dehydrogenase (EC 1.1.1.27) (Anderson & Noble, 1977;
Anderson et al., 1978), and succinic acid dehydrogenase (Kawatski
& Hecker, 1979).
Chlordecone has been demonstrated to enhance the hepatotoxic
effects of both chloroform and carbon tetrachloride (Cianflone et
al., 1980), but had no similar effect on the response of the rat
liver to polyhalogenated biphenyls (Chu et al., 1980). It was
able to increase the detoxification of lindane in weanling rats
(Chadwick et al., 1979). Pretreatment of rats with low non-toxic
levels of dietary chlordecone (10 mg/kg, 15 days) potentiated the
hepatotoxicity (Curtis et al., 1979) and lethality of carbon
tetrachloride (Klingensmith & Mehendale, 1982a) about 70-fold in
male rats and 25-fold in female rats (Agarwal & Mehendale,
1982a). Comparative doses of other inducers of microsomal
enzymes such as mirex, photomirex, and phenobarbital did not
potentiate carbon tetrachloride toxicity to such an extent
(Curtis & Mehendale, 1980; Klingensmith & Mehendale, 1982b).
Hepatobiliary dysfunction, elevation of hepatic enzymes in serum,
and centrilobular hepatocellular necrosis were the characteristic
features for the rat. The hepatotoxicity and lethality of
bromotrichloromethane were also potentiated about 5-fold by
chlordecone (Agarwal & Mehendale, 1982b).
Like mirex, chlordecone has been shown to modify
hepatobiliary function (Mehendale, 1979), possibly due to
interference with energy production and utilization.
In an inhalation study reported in a review (Epstein, 1978),
male rats were exposed to test and control dusts for 2 h per day
for 10 days and killed 2 weeks later. Air flow was maintained at
10 - 12 litre/min, and the effective chlordecone concentrations
were 3.7 and 15.4 µg/litre. The reviewer concluded, contrary to
the authors of the actual study, that chlordecone at both dose
levels induced toxic effects, including hepatomegaly and
histopathological changes in the liver and lungs.
7. EFFECTS ON MAN
7.1. Poisoning Incidents in the General Population
No information is available concerning such incidents.
7.2. Occupational Exposure
Life Sciences Products Co. (LSPC) was formed in November 1973
and went out of production in July 1975. In a study carried out
by the Center for Disease Control (Cannon et al., 1978), 133
employees, including 33 currently employed, were interviewed,
examined, had blood samples taken, and completed a standard
questionnaire. Of the 133 examined, 76 (57%) had developed
clinical illness described as nervousness, tremor, weight loss,
opsoclonus, pleuritic and joint pain, and oligospermia. Illness
rates were higher for production workers than non-production
workers, and the mean blood-chlordecone level for workers with
illness was 2.53 mg/litre compared with a level of 0.60 mg/litre
in workers without disease. Laboratory findings from the above
study showed an increase in serum alkaline phosphatase (EC
3.1.3.1) activity in several patients (Taylor et al., 1978) and
morphological changes in peripheral nervous tissue (Martinez et
al., 1978).
7.3. Treatment of Poisoning in Man
The treatment is symptomatic.
Administration of cholestyramine will increase the excretion
of chlordecone, and so reduce the body burden of the chemical
(Cohn et al., 1976, 1978; Anonymous, 1977).
8. EFFECTS ON ORGANISMS IN THE THE ENVIRONMENT
8.1. Aquatic Organisms
The results of studies on the toxicity of chlordecone for a
variety of algae are given in Table 5.
Acute and short-term toxicity values for invertebrate species
are also tabulated (Table 6). A more comprehensive table listing
different conditions and exposure times is available on request
from the IRPTC, Geneva. A life cycle study is available for
mysid shrimps, Mysidopsis bahia (Nimmo et al., 1977). This test
was long enough to cover the production of several broods. The
average number of young produced by each female was reduced from
the control level of 15.3 to 8.9 on exposure to 0.39 µg
chlordecone/litre. Juveniles produced grew more slowly than
controls. Young females exposed to as little as 0.072 µg
chlordecone/litre for 14 days were shorter than controls. The
authors pointed out that reproductive success was related to body
size, the number of eggs produced being greater in bigger
females. In a life cycle study of a copepod, Eurytemora affinis,
a dominant zooplankter, the intrinsic rate of natural increase
was reduced by all concentrations of chlordecone greater than
5 µg/litre (Allan & Daniels, 1982). This was due to a combination
of a reduced rate of survival, delayed onset of reproduction, and
reduced fecundity.
The toxicity of chlordecone for fish varies with species
(Table 6). Juvenile fish are generally less susceptible to
chlordecone than adults. Symptoms of chlordecone poisoning
(Hansen et al., 1976) progressed from scoliosis (darkening of the
posterior third of the body) through haemorrhaging near the brain
and anterior point of darkening, oedema, fin rot, incoordinated
swimming, and cessation of feeding. Symptoms increased in
severity before death, which occurred between 5 and 8 days after
initial exposure. Juveniles showed reduced growth at 0.08 µg
chlordecone/litre with some showing scoliosis during a 36-day
test. Embryo survival was reduced when adults were exposed to
chlordecone. When adults were exposed to 1.9 µg/litre, their
embryos developed abnormally or died, even when incubated in
chlordecone-free water. Fry from embryos exposed to 6.6 or 33 µg
chlordecone/litre were visibly affected within 24 h of hatching.
Symptoms of poisoning in fry less than 1 week old included
diminished activity, loss of equilibrium, cessation of feeding,
and emaciation. Fry more than 1 week old had symptoms identical
to those in adult fish, except for haemorrhaging and oedema.
Sixty percent of juvenile fish that had survived 36 days'
exposure to 0.08 µg chlordecone/ litre had scoliosis and
blackened tails. In clean water, symptoms persisted for more
than ten days (Hansen et al., 1976).
Table 5. Toxicity of chlordecone for algae
---------------------------------------------------------------------------------------------------
Alga Flow/ Temp Salinity End point Parameter Concentration Reference
stat (°C) o/oo (µg/litre)
---------------------------------------------------------------------------------------------------
Chlorococcum stat 20±0.5 30 growth 7-day EC50 0.35 Walsh et al. (1977)
sp. retardation
Dunaliella stat 20±0.5 30 growth 7-day EC50 0.58 Walsh et al. (1977)
tertiolecta retardation
Nitzschia stat 20±0.5 30 growth 7-day EC50 0.60 Walsh et al. (1977)
sp. retardation
Thalassiosira stat 20±0.5 30 growth 7-day EC50 0.60 Walsh et al. (1977)
pseudonana retardation
---------------------------------------------------------------------------------------------------
Table 6. Toxicity of chlordecone for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Salinity End point Parameter Concen- Reference
stat (°C) o/oo tration
(µg/litre)
---------------------------------------------------------------------------------------------------------
grass shrimp flow 96-h LC50 121 Schimmel & Wilson (1977)
(Palaemonetes flow 25-28 10-20 19-day LC50 1.4 Nimmo et al. (1977)
pugio)
blue crab flow 96-h LC50 >210 Schimmel & Wilson (1977)
(Callinectes flow 48-h LC50 1000a Butler (1963)
sapidus)
easter oyster flow 14 inhibition 96-h EC50 57a Butler (1963)
(Crassostrea shell depos-
virginica) ition
American eel flow 19 flesh 96-h LC50 35 Roberts & Bendl (1982)
(Anguilla
rostrata)
juvenile
stage VIA
sheepshead flow 96-h LC50 69.5 Schimmel & Wilson (1977)
minnow
(Cyprinodon
variegatus)
spot flow 96-h LC50 6.6 Schimmel & Wilson (1977)
(Leiostomus
xanthurus)
bluegill flow 19-21 96-h LC50 50 Roberts & Bendl (1982)
sunfish, juv
(Lepomis
macrochirus)
channel flow 20-23 96-h LC50 514 Roberts & Bendl (1982)
catfish, juv
(Ictalurus
punctatus)
---------------------------------------------------------------------------------------------------------
a Nominal concentration, not measured.
Estimation of the long-term effects of chlordecone on
juvenile fish, from the results of acute tests can result in
severe underestimation. When juvenile spot were fed sublethal
doses of chlordecone (0.3 and 0.7 mg/kg diet per day) for 56
days, they developed bone damage including fracturing and
thickening of vertebrae (Stehlik & Merriner, 1983).
Desaiah & Koch (1975) conducted in vitro studies on brain
ATPase activity in channel catfish (Ictalurus punctatus) and
demonstrated a significant inhibition of oligomysin-sensitive
(mitochondrial) Mg2+, oligomysin-insensitive Mg2+ and NA+-K+
ATPases with increasing concentrations of chlordecone. Inhibition
was 25.7% and 36.7% at chlordecone concentrations of 1.25 and 2.5
µM, respectively. The authors noted that the resulting reduction
in energy supply could have physiological consequences.
Winkelhake et al. (1983) showed the inducement of an acute phase
(C-reactive) protein in the serum of rainbow trout after
administration of chlordecone at 5 mg/kg. The formation of these
proteins is the initial reaction to bacteria or response to
foreign proteins.
8.2. Terrestrial Organisms
(a) Plants
Little work on the effects of chlordecone on plants has been
reported. In one experiment, chlordecone increased both the
quality and quantity of the cotton yield (Gawaad et al., 1976).
Residues in seeds were always, 1 mg/kg, despite different
application rates.
(b) Insects
In a study on bees, Atkins & Anderson (1962) reported an LT50
value for a 200 mg dose of chlordecone of 68 h. They tested
chlordecone in 1961 on bee colonies that had shown a progressive
resistance to DDT over a 5-year period. They obtained an LT50
value of 45 h for chlordecone, in 1952, when tested on a
different strain of DDT-susceptible bees. The authors implied
that DDT resistance carries over to other organochlorine
insecticides; but though lower susceptibility to chlordecone was
shown by DDT-resistant bees, the results do not directly
demonstrate this. The results of later studies by Atkins et al.
(1973) suggest that chlordecone would have to be used at 5 times
the recommended application rate to kill 50% of bee populations.
At the recommended usage rate of 2.25 kg/ha, chlordecone was not
harmful to 3 out of 4 predatory insect species and arthropods,
monitored in an apple orchard.
There is no information on the effects of chlordecone on
amphibians or reptiles.
(c) Birds
Chlordecone was shown not to be very toxic when fed to either
young or adult birds (Table 7). Species tested were not very
representative. Birds fed lethal doses of the insecticide
developed characteristic whole-body tremor, prior to death
(DeWitt et al., 1962; Naber & Ware, 1965; McFarland & Lacy,
1969). Japanese quail injected daily with 0.5 mg chlordecone/
bird showed liver damage (damage to hepatic parenchymal cells,
including disruption of mitochondria, with cellular debris in the
bile and bile ducts), with increased numbers of phagocytic Kupffer
cells lining the liver sinusoids (US EPA, 1979).
Sublethal effects of chlordecone on birds are pronounced
despite the compound's low acute toxicity. A sublethal dose of
200 mg chlordecone/kg diet administered to Japanese quail caused
structural changes in the liver, adrenals, and gonads (Eroschenko
& Wilson, 1975). Many sublethal effects of the compound are
attributable to its estrogenic effects. Dosing with chlordecone
caused oviduct maturation in sexually immature females held on
non-stimulatory daylengths, but mature females were not affected
(Eroschenko & Wilson, 1975). Ovaries from chlordecone-treated
females contained more primary oocytes and smaller follicles than
those from controls. A central effect on follicle-stimulating
hormone production was postulated by McFarland & Lacy (1969), but
direct hormone measurement does not seem to have been carried
out. Estrogen-like stimulation of secondary sexual characteristics
caused male pheasants to develop female plumage at dietary doses of
50, 100, and 150 mg chlordecone/kg (DeWitt et al., 1962). Males
also showed malformed sperm and reduced reproductive success.
Eroschenko & Wilson (1975) reported effects on the testicles in
both immature and adult quail; seminiferous tubules were distended
with watery fluid that caused a significant weight increase in the
testes, germinal epithelium and spermatozoa were reduced, and
abundant intraluminal cellular debris was common.
Both egg laying and chick survival were reduced in domestic
hens fed 75 or 150 mg chlordecone/kg diet for 12 weeks. Only 56%
of chicks hatched from hens treated with 75 mg/kg survived for 20
days, no chicks or hens treated with 100 mg/kg survived. Residues
were still detectable in eggs laid 3 weeks after treatment ceased
(Naber & Ware, 1965). Eggshell deposition was affected by
chlordecone. A peculiarly thick spongy layer developed leading to
blockage of shell pores and suffocation of the embryo (Erben,
1972). Changes in shell structure occurred in Japanese quail fed
225 mg chlordecone/kg diet (US EPA, 1979).
There is no information on the toxicity of chlordecone for
non-laboratory mammals.
Table 7. Toxicity of chlordecone for birds
---------------------------------------------------------------------------------------
Species Age Route Parameter Concentration Reference
(mg/kg)
---------------------------------------------------------------------------------------
mallard duck young diet LC50 400 Dewitt et al. (1962)
bobwhite quail young diet LC50 600 Dewitt et al. (1962)
bobwhite quail adult diet LC50 530 Dewitt et al. (1962)
ringnecked pheasant young diet LC50 600 Dewitt et al. (1962)
ringnecked pheasant adult diet LC50 115 Dewitt et al. (1962)
---------------------------------------------------------------------------------------
8.3. Microorganisms
Effects of chlordecone on soil microorganisms were investigated
by Gawaad et al. (1972a). Application of chlordecone to 3 soil
types in the Nile Delta altered fungal, actinomycete, and other
bacterial populations for as long as 45 days, compared with
controls (Gawaad et al., 1972a). Unfortunately, chlordecone was
applied at a very high rate (22.0 kg/ha) and therefore the results
are difficult to interpret in terms of likely effects on crops.
The magnitude and duration of effects on populations differed with
soil type, but the general pattern was a fall in numbers in the
first week followed by an increase in the second week with numbers
eventually returning to normal levels. In a second experiment in
which effects on nitrogen transformation in treated soils were
studied, chlordecone was shown to affect fungi and bacteria
responsible for ammonification, and Nitrobacter, which is
responsible for changing nitrite to nitrate, but not Nitrosomonas,
which is responsible for changing ammonia to nitrite (Gawaad et
al., 1972b).
Similar effects on microbial populations were found by Meyers
et al. (1982), when chlordecone at 0.5 mg/litre was applied to
static carbon metabolism microcosms; no significant total
treatment variation was seen in either bacterial or fungal
populations after 10 days incubation. Similar results were
obtained in response to continuous application of chlordecone.
Chlordecone is probably highly toxic for sludge microorganisms,
since massive amounts of beneficial bacteria in a sludge digester
were killed after chlordecone wastes were discharged into the
sewage system (Bray, 1975). Portier & Meyers (1982) stated that
microcosms (simulated aquatic microenvironmental systems) were
"sensitive" to chlordecone "under a variety of regimes". Among
response criteria used were microbial diversity, enzymatic
activity, ATP, and material turnover.
The toxicity of chlordecone for mixed populations of
microorganisms was determined by standard plate assays on Zobell
marine medium containing 0.02, 0.2, or 2 mg chlordecone/litre
(Mahaffey et al., 1982). All these concentrations of chlordecone
reduced the number of colony-forming aerobes but did not affect
anaerobes. Gram-positive organisms were more sensitive to
chlordecone than gram-negative organisms. Oxygen uptake by gram-
negative isolates was reduced by 25 - 100% by chlordecone at 20
mg/litre. A significant reduction in the specific activities of
NADH oxidase and succinooxidase by the addition of chlordecone at
0.49 mg/litre indicated that chlordecone can inhibit electron
transport.
8.4. Bioaccumulation and Biomagnification
Data on the bioconcentration of chlordecone are given in Table
8. It should be noted that none of the exposures were representative
of realistic environmental levels. Bioaccumulation in detritus,
such as decomposing Spartina cyanosuroide, was demonstrated by
Odum & Drifmeyer (1978). As detritus is a major energy source in
aquatic environments, this could represent an important entrance
for chlordecone into aquatic food webs. Both aquatic invertebrates
and fish bioaccumulate chlordecone to very high levels. Depuration
is slow in fish, thus residues tend to be high. Levels of chlordecone
accumulated in edible fillets were almost the same as the whole
body concentrations in sheepshead minnows and spot; therefore one
of the largest residue reserves in contaminated fish is in the
edible portion (Bahner et al., 1977).
Residues were higher in female sheepshead minnows than in males
(Bahner et al., 1977), and residues in juveniles tended to increase
with increasing concentrations of chlordecone in the water (Hansen
et al., 1976). When chlordecone was fed to juvenile spot for 28
days, the body burden of chlordecone increased additively and
equilibrium was not attained (Stehlik & Merriner, 1983). Chlordecone
accumulation in an estuarine food chain (composed of green algae,
oysters, mysids, grass shrimps, sheepshead minnows, and spot)
occurred at concentrations as low as 0.023 µg/litre (Bahner et al.,
1977). All species had equilibrated tissue concentrations of
chlordecone 8 - 17 days after the beginning of the exposure.
Clearance of chlordecone from oysters was rapid; levels were non-
detectable, 7 - 20 days after exposure ceased. Clearance was slow
in shrimp and fish, with tissue levels of chlordecone decreasing by
30 - 50% in 24 - 28 days. When oysters were fed chlordecone-
contaminated algae, the maximum overall accumulation and transfer
of chlordecone (or "food-chain potential") from water to algae and
then to oysters was 2.1 (Bahner et al., 1977). However, the
transfer potential (transfer from one trophic level to the next)
from algae to oysters was only 0.007; therefore, transfer of
chlordecone from algae to oyster and retention in oyster were
inefficient. When spot were fed mysids that had eaten chlordecone-
contaminated brine shrimp, the food-chain potential from water to
brine shrimp to mysids and finally to fish ranged from 3.9 to 10.5.
The transfer potential from shrimp to mysids was 0.53 and from
mysids to spot, 0.85. This indicated that much of the chlordecone
was being transferred through the trophic levels.
No data are available on the bioconcentration of chlordecone
by terrestrial organisms.
Table 8. Bioaccumulation of chlordecone
-------------------------------------------------------------------------------------------------------
Organism Temp Salinity Flow/ Bioconc. Exposure Time Reference
(°C) o/oo stat factor concentration
(BCF) (µg/litre)
-------------------------------------------------------------------------------------------------------
algae, 19.5- 30 stat 230-800 100 24 h Walsh et al. (1977)
unicellular 20.5
oyster 9354 0.03 19 d Bahner et al. (1977)
(Crassostrea 9278 0.39 21 day
virginica)
grass shrimp 698 12-121 96 h Schimmel & Wilson
(Palaemonetes (425-933) (1977)
pugio) 5127 0.023 28 day Bahner et al. (1977)
11425 0.4 28 day Bahner et al. (1977)
spot 3217 0.029 30 day Bahner et al. (1977)
(Leiostomus
xanthurus)
spot 1120 1.5 96 h Bahner et al. (1977)
(Leiostomus
xanthurus)
fathead minnow flow 16600 0.004 56 day Huckins et al. (1982)
(Pimephales
promelas)
sheepshead minnow, 28-32 11-31 flow 1800 0.041 life Goodman et al. (1982)
juv 21-day cycle test
(Cyprinodon
variegatus
sheepshead minnow, 28-32 11-31 flow 2400 0.041 life Goodman et al. (1982)
juv 42-day cycle test
(Cyprinodon
variegatus)
Table 8. (contd.)
-------------------------------------------------------------------------------------------------------
Organism Temp Salinity Flow/ Bioconc. Exposure Time Reference
(°C) o/oo stat factor concentration
(BCF) (µg/litre)
-------------------------------------------------------------------------------------------------------
sheepshead minnow 28-32 11-31 flow 3900 0.041 life Goodman et al. (1982)
adult male cycle test
(Cyprinodon
variegatus)
sheepshead minnow 28-32 11-31 flow 3700 0.041 life Goodman et al. (1982)
adult female cycle test
(Cyprinodon
variegatus)
sheepshead minnow, 28-32 11-31 flow 2900 0.041 life Goodman et al. (1982)
embryos cycle test
(Cyprinodon
variegatus)
sheepshead minnow, 28-32 11-31 flow 2400 0.041 life Goodman et al. (1982)
juvenile progeny cycle test
(Cyprinodon
variegatus)
-------------------------------------------------------------------------------------------------------
8.5. Population and Community Effects
Chlordecone is strongly adsorbed on sediment. Effects on
aquatic organisms are therefore partly from material in the water
and partly from material obtained from sediment. D'Asaro & Wilkes
(1982) examined the effects of sediments, previously exposed to
chlordecone at a known concentration, and of James River sediments
contaminated with chlordecone, on an estuarine community established
in aquaria supplied with non-filtered sea water. Mysid shrimps
showed a dose-related mortality rate, when exposed to sediments
previously equilibrated at 0.1, 1.0, or 10 µg chlordecone/litre.
Mysids were not affected by James River sediment. Oysters showed
dose-dependent reduced shell growth, when exposed to chlordecone-
equilibrated sediments, and also responded adversely to river
sediment. Lugworms Arenicola cristata disappeared from aquaria
after 28 days of treatment with sediment exposed to 10 µg
chlordecone/litre, though numbers were not affected by lower doses.
Both lugworms and oysters concentrated chlordecone from the
sediment.
8.6. Effects on the Abiotic Environment
No data are available on the effects of chlordecone on the
abiotic environment.
8.7. Appraisal
As actual levels of chlordecone in natural waters are extremely
low, because most of the chlordecone is transferred rapidly to
sediments, bioconcentration and toxicity test levels are often
unrealistically high. However, bearing in mind the potential for
bioaccumulation, data suggest that chlordecone is both acutely and
chronically toxic for aquatic organisms. A major omission in the
aquatic toxicity data is the toxicity of chlordecone for detritus
feeders that will be exposed to significant concentrations in
contaminated sediments. Exposure of the lowest level of the
aquatic food chain to concentrations of chlordecone above a
threshold of 0.35 - 1 mg/litre will cause disturbance or destruction,
sufficient to affect productivity at other levels of the food chain.
Few data are available on the sublethal effects of chlordecone on
aquatic organisms. In fish, such effects include: retardation of
growth, which will ultimately affect fecundity, scoliosis, inhibition
of ATPase; and stimulation of some immune response. Juvenile fish
appear to be less sensitive to chlordecone than adults.
Chlordecone appears to have little effect on soil
microorganisms at concentrations that would result from
agricultural use. However, discharges directly into sewage
systems are highly toxic for sludge microbes. Agricultural
application rates cause little acute toxicity to non-target
invertebrates or birds, but chlordecone at higher dosages can
have pronounced effects on many reproductive variables in birds.
No data are available on effects on amphibia, reptiles, or non-
laboratory mammals.
9. PREVIOUS EVALUATIONS OF CHLORDECONE BY INTERNATIONAL BODIES
IARC (1979) evaluated the carcinogenic hazard resulting from
exposure to chlordecone and concluded that "there is sufficient
evidence for its carcinogenicity in rats and mice. In the absence
of adequate data in humans, it is reasonable for practical
purposes to regard chlordecone as if it presented a carcinogenic
risk to humans".
No acceptable daily intake (ADI) for chlordecone has been
proposed by FAO/WHO.
In recent years, official registrations for a number of uses
of chlordecone have been withdrawn in certain countries for
various reasons (IRPTC, 1983).
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the
Federal Republic of Germany, India, Japan, Kenya, Mexico, Sweden,
the United Kingdom, the USA, and the USSR) and the EEC can be
found in the International Register of Potentially Toxic
Chemicals Legal File (IRPTC, 1983).
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Chlordecone Toxicity
Chlordecone is moderately toxic in acute studies on rats,
i.e. the oral LD50 values range from 95 to 132 mg/kg body weight.
It can enter the body via ingestion, inhalation, and via the
skin. It is not metabolized to any significant extent. It
bioaccumulates mainly in the liver, and it is excreted very
slowly via the faeces.
Toxic effects include neurological symptoms, especially
tremors, liver hypertrophy with enzyme induction, centrilobular
hepatocellular necrosis, and hepatobiliary dysfunction. It can
impair reproduction (mouse, 10 mg/kg diet or 0.5 mg/kg body
weight per day) and is fetotoxic (rat, 2 mg/kg body weight per
day).
Chlordecone was not generally active in short-term tests for
genetic activity. There is sufficient evidence of its
carcinogenicity for mice and rats.
Careless occupational handling in a manufacturing plant
caused a series of poisonings with neurological symptoms,
especially nervousness and tremors, oligospermia, and joint
pains.
10.2. Exposure to Chlordecone
Exposure of the general population through the normal use of
chlordecone can be regarded as minimal and is mainly related to
residues in food.
Small children may be exposed when playing with insect traps.
10.3. Effects on the Environment
The environmental hazard posed by chlordecone is associated
with its stability and persistence in sediments, which provide a
long-term source of contamination, in conjunction with its massive
bioaccumulation in aquatic food chains. One of the largest
reserves of chlordecone in food is in the edible portion of
contaminated fish. Although chlordecone has a low solubility in
water, between 0.35 and 1 mg/litre is sufficient to reduce algal
growth, thereby affecting productivity at other trophic levels.
Chlordecone is acutely and chronically toxic for aquatic
invertebrates and causes loss of equilibrium, reduction in
reproductive success, and decreased shell growth at sublethal
concentrations. Reduction in mysid populations due to low-level
chlordecone contamination has important consequences for fish
productivity. Symptoms of exposure range from diminished activity
and emaciation to abnormal development and death.
The few data available indicate that chlordecone is not
acutely toxic for terrestrial invertebrates. Subacute doses of
chlordecone induce significant toxic effects in birds including
tremors, liver damage, and reproductive failure.
Excretion of chlordecone is extremely slow.
10.4. Conclusions
1. Serious illness has been suffered by workers occupationally
over-exposed to chlordecone.
2. Based on the findings in mice and rats, this chemical should
be considered, for practical purposes, as being potentially
carcinogenic for human beings.
3. For the above reason, reservations must remain about the
occurrence of residues of chlordecone in food.
4. Adverse effects on the organisms studied, as well as
persistence, suggest that chlordecone presents a long-term
hazard for the environment.
5. Taking into account these considerations, it is felt that the
use of this chemical should be discouraged, except where there
is no adequate alternative.
REFERENCES
ADIR, J., CAPLAN, Y.H., & THOMPSON, B.C. (1978) Kepone serum
half-life in humans. Life Sci., 22: 699-702.
AGARWAL, A.K. & MEHENDALE, H.M. (1982a) Potentiation of CCl4
hepatotoxicity and lethality by chlordecone in female rats.
Toxicology, 26: 231-242.
AGARWAL, A.K. & MEHENDALE, H.M. (1982b) Potentiation of
bromotrichloromethane hepatotoxicity and lethality by
chlordecone pre-exposure in the rat. Fundam. appl. Toxicol.,
2: 161-167.
ALLAN, J.D. & DANIELS, R.E. (1982) Life table evaluation of
chronic exposure of Eurytemora affinis (Copepoda) to Kepone.
Mar. Biol. (Berlin), 66: 179-184.
ALLIED CHEMICAL CORPORATION (1963) Determination of kepone
(GC-1189). Residues in crops, 26 June, Morristown, New Jersey,
Allied Chemical Corporation.
ALLIED CHEMICAL CORPORATION (1966) Assay method for kepone,
8 March, Morristown, New Jersey, Allied Chemical Corporation.
ALLIED CHEMICAL CORPORATION (1977) Summary of basic
information - Kepone insecticide, Morristown, New Jersey,
Allied Chemical Corporation (Product Information Technical
Data Sheet 1189-01-1012. Source 120).
ANDERSON, B.M. & NOBLE, C., Jr (1977) In vitro inhibition of
lactate dehydrogenases by Kepone. J. agric. food Chem., 25:
28-31.
ANDERSON, B.M., NOBLE, C., Jr, & GREGORY, E.M. (1977) Kepone
inhibition of malate dehydrogenases. J. agric. food Chem., 25:
485-489.
ANDERSON, B.M., KOHLER, S.T., & YOUNG, R.W. (1978)
Interactions of Kepone with rabbit muscle lactate
dehydrogenase. J. agric. food Chem., 26: 130-133.
ANONYMOUS (1976) Report on (NCI) carcinogenesis bioassay of
technical grade chlordecone (Kepone). Am. Ind. Hyg. Assoc. J.,
37: 680-681.
ANONYMOUS (1977) Getting Kepone out of the body. Med. World
News, 18: 32.
ANONYMOUS (1978a) Kepone/mirex/hexachlorocyclopentadiene -
an environmental assessment, Washington DC, US NRC, US
Department of Commerce (US NTIS, PB 280-289).
ANONYMOUS (1978b) UK prohibits burning of Kepone. Chem.
Aust., 45: 142.
ATKINS, E.L. & ANDERSON, L.D. (1962) DDT resistance in honey
bees. J. econ. Entomol., 55: 791-792.
ATKINS, E.L. Jr, GRAYWOOD, E.A., & MACDONALD, R.L. (1973)
Toxicity of pesticides and other agricultural chemicals to
honey bees, California, 38 pp (Laboratory Studies, Calif.
Agric., Extension M-16).
BAGGETT, J.McC., KLEIN, R.L., MEHENDALE, H.M., & THURESON-
KLEIN, A.K. (1977) Acute Kepone treatment of rats: a
biochemical and ultrastructural study. Pharmacologist, 19:
199-205.
BAGGETT, J.McC., THURESON-KLEIN, A., & KLEIN, R.L. (1980)
Effects of chlordecone on the adrenal medulla of the rat.
Toxicol. appl. Pharmacol., 52: 313-322.
BAHNER, L.H., WILSON, A.J., Jr, SHEPPARD, J.M., PATRICK, J.M.,
Jr, GOODMAN, L.R., & WALSH, G.E. (1977) Kepone
bioconcentration, accumulation, loss, and transfer through
estuarine food chains. Chesapeake Sci., 18: 299-308.
BAKER, R.C., COONS, L.B., MAILMAN, R.B., & HODGSON, E.
(1972) Induction of hepatic mixed function oxidases by the
insecticide mirex. Environ. Res., 5: 418-424.
BENTLEY, R.E. (1975) Acute toxicity of kepone to Bluegill
Lepomis macrochirus and Rainbow Trout Salmo gairdneri, 7pp
(Toxicity Report submitted to Allied Chemical Corporation,
Morristown, New Jersey, EG & G Environmental Consultants,
Bionomics, Wareham, Massachusetts).
BLANKE, R.V., FARISS, M.W., GRIFFITH, F.D., Jr, & GUZELIAN,
P.S. (1977) Analysis of chlordecone (Kepone) in biological
specimens. J. anal. Toxicol., 1: 57-62.
BLANKE, R.V., FARISS, M.W., GUZELIAN, P.S., PATERSON, A.R., &
SMITH, D.E. (1978) Identification of a reduced form of
chlordecone (Kepone) in human stool. Bull. environ. Contam.
Toxicol., 20: 782-785.
BOYLAN, J.J., EGLE, J.L., & GUZELIAN, P.S. (1977)
Stimulation of chlordecone (Kepone) excretion by
cholestyramine in rats. Pharmacologist, 19: 210.
BOYLAN, J.J., COHN, W.J., EGLE, J.L., BLANKE, R.V., &
GUZELIAN, P.S. (1979) Excretion of chlordecone by the
gastrointestinal tract: evidence for a non-biliary mechanism.
Clin. Pharmacol. Ther., 25: 579-585.
BRAY, T.J. (1975) Health hazard. Chemical firm's story
underscores problems of cleaning up plants. Wall Street J.,
December 2.
BREWERTON, H.V. & SLADE, D.A. (1964) Kepone residues on
apples. N.Z. J. agric. Res., 7: 647-653.
BUCKLER, D.R., WITT, A., MAYE R, F.L., & HUCKINS, J.N. (1981)
Acute and chronic effects of Kepone and mirex on the fathead
minnow. Trans. Am. Fish. Soc., 110: 270-280.
BULGER, W.J., MUCCITELLI, R.M., & KUPFER, D. (1979) Studies
on the estrogenic activity of chlordecone (Kepone) in the rat:
effects on uterine estrogen receptor. Mol. Pharmacol., 15:
515-524.
BUTLER, P.A. (1963) A review of fish and wildlife service
investigations during 1961 and 1962. In: George, J.C., ed.
Commercial Fisheries Investigations, Pesticide-Wildlife
Series, US Department of the Interior, Fish and Wildlife
Services, Vol. 167, pp. 11-25.
CANNON, S.B. & KIMBROUGH, R.D. (1979) Short-term chlordecone
toxicity in rats including effects on reproduction,
pathological organ changes and their reversibility. Toxicol.
appl. Pharmacol., 47: 469-476.
CANNON, S.B., VEAZEY, J.M., Jr, JACKSON, R.S., BURSE, V.W.,
HAYES, C., STRAUB, W.E., LANDRIGAN, P.J., & LIDDLE, J.A.
(1978) Epidemic Kepone poisoning in chemical workers. Am. J.
Epidemiol., 107: 529-537.
CARVER, R.A., BORSETTI, A.P., & KAMPS, L.R. (1978)
Gas-liquid chromatographic determination of Kepone residues in
finfish, shellfish, and crustaceans. J. Assoc. Off. Anal.
Chem., 61: 877-883.
CHADWICK, R.W., COPELAND, M.F., & ROSENSTEIN, L. (1979)
Effect of Kepone exposure during gestation and lactation on
the metabolism of lindane by weanling rats. Toxicology Lett.,
4: 247-252.
CHERNOFF, N. & ROGERS, E.H. (1976) Fetal toxicity of Kepone
in rats and mice. Toxicol. appl. Pharmacol., 38: 189-194.
CHERNOFF, N., LINDER, R.E., SCOTTI, T.M., ROGERS, E.H.,
CARVER, B.D., & KAVLOCK, R.J. (1979a) Fetotoxicity and
cataractogenicity of mirex in rats and mice with notes on
Kepone. Environ. Res., 18: 257-269.
CHU, I., VILLENEUVE, D.C., VALLI, V.E., & REYNOLDS, L.M.
(1980) Short-term study of the combined effects of mirex,
photomirex, and Kepone to halogenated biphenyls in the rat. J.
Toxicol. environ. Health, 6: 421-432.
CIANFLONE, D.J., HEWITT, W.R., VILLENEUVE, D.C., & PLAA, G.L.
(1980) Role of biotransformation of the alterations of
chloroform hepatotoxicity produced by Kepone and mirex.
Toxicol. appl. Pharmacol., 53: 140-149.
COHN, W.J., BLANKE, R.V., GRIFFITH, F.D., Jr, & GUZELIAN,
P.S. (1976) Distribution and excretion of Kepone in humans.
Gastroenterology, 71: A8-901.
COHN, W.J., BOYLAN, J.J., BLANKE, R.V., FARISS, M.W., HOWELL,
J.R., & GUZELIAN, P.S. (1978) Treatment of chlordecone
(Kepone) toxicity with cholestyramine. Results of a controlled
clinical trial. N. Engl. J. Med., 298: 243-248.
COUCH, J.A., WINSTEAD, J.T., & GOODMAN, L.R. (1977)
Kepone-induced scoliosis and its histological consequences in
fish. Science, 197: 585-587.
CURTIS, L.R. & MEHENDALE, H.M. (1979) The effects of Kepone
pretreatment on biliary excretion of xenobiotics in the male
rat. Toxicol. appl. Pharmacol., 47: 295-303.
CURTIS, L.R. & MEHENDALE, H.M. (1980) Specificity of
chlordecone-induced potentiation of carbon tetrachloride
hepatotoxicity. Drug Metab. Dispos., 8: 23-27.
CURTIS, L.R., WILLIAMS, W.L., & MEHENDALE, H.M. (1979)
Potentiation of the hepatotoxicity of carbon tetrachloride
following pre-exposure to chlordecone (Kepone) in the male
rat. Toxicol. appl. Pharmacol., 51: 283-293.
D'ASARO, C.N. & WILKES, F.G. (1982) Cycling of xenobiotics
through marine and estuarine sediments, 51 pp (US EPA Report
No. 600/3-82-074; PB82-239252).
DAWSON, G.W. (1978) Kepone mitigation feasibility report:
Appendix A: The feasibility of mitigating kepone contamination
in the James River Basin (US NTIS PB Report, PB 286 085).
DESAIAH, D. & KOCH, R.B. (1975) Inhibition of ATPases
activity in channel catfish brain by Kepone and its reduction
product. Bull. environ. Contam. Toxicol., 13: 153-158.
DESAIAH, D., HO, I.K., & MEHENDALE, H.M. (1977) Effects of
Kepone and mirex on mitochondrial magnesium ion-dependent
ATPhase activity in rat liver. Toxicol. appl. Pharmacol., 39:
219-228.
DEWITT, J.B., CRABTREE, D.G., FINLEY, R.B. & GEORGE, J.L.
(1962) Effects on wildlife. Effects of pesticides on fish and
wildlife: a review of investigations during 1960, Washington
DC, US Department of the Interior, Bureau of Fish and Wildlife
Services, pp 4-15, 31-33, 49-52 (Circular No. 143).
DIETZ, D.D. & McMILLAN, D.E. (1978) Effects of mirex and
Kepone on schedule controlled responding. Pharmacologist, 20:
225.
EGLE, J.L., Jr, FERNANDEZ, S.B., GUZELIAN, P.S., & BORZELLECA,
J.F. (1978) Distribution and excretion of chlordecone
(Kepone) in the rat. Drug Metab. Dispos., 6: 91-95.
END, D.W., CARCHMAN, R.A., & DEWEY, W.L. (1979) Disruption
of rat brain synaptosomal membranes by the neurotoxic
insecticide Kepone. Fed. Proc., 38: 845.
EPSTEIN, S.S. (1978) Kepone-hazard evaluation. Sci. total
Environ., 9: 1-62.
ERBEN, H.K. (1972) [Ultrastructure and wall thickness of
pathological eggshells.] Abkhandl. Math. Naturwissensch.,
Lichen Klasse, 6: 192-216 (in German, Pestic. Abstr., 7:
74-0598-602).
EROSCHENKO, V.P. & WILSON, W.O. (1975) Cellular changes in
the gonads, livers and adrenal glands of Japanese quail as
affected by the insecticide Kepone. Toxicol. appl. Pharmacol.,
31: 491-504.
FABACHER, D.L. & HODGSON, E. (1976) Induction of hepatic
mixed-function oxidase enzymes in adult and neonatal mice by
Kepone and mirex. Toxicol. appl. Pharmacol., 38: 71-77.
FELDMANN, E.G. (1976) The lesson from Kepone. J. pharmacol.
Sci., 65: 1.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14: 5-534.
GAWAAD, A.A.A., HAMMAD, M.H., & EL-GAYAR, F.H. (1972a)
Studies on soil insecticides. X. Effect of some soil
insecticides on soil microorganisms. Zentr. Bakteriol.
Parasitenk. Infect and Hygien. Abt 2, 127: 290-295.
GAWAAD, A.A.A., HAMMAD, M.H., & EL-GAYAR, F.H. (1972b)
Studies on soil insecticides. XI. Effect of some soil
insecticides on the nitrogen transformation in treated soils.
Zentr. Bakteriol. Parasitenk. Infect and Hygien. Abt 2, 127:
296-300.
GAWAAD, A.A.A., EL-GAYAR, F.H., & KHADR, A.A. (1976) Effect
of certain soil insecticides on the germination of cotton
seeds, growth, dry weight, cotton yield and the quality of
yield. Biol. Abstr., 61: 5840.
GEER, R.D. (1978) Predicting the anaerobic degradation of
organic chemical pollutants in waste water treatment plants
from their electrochemical reduction behavior, Bozeman,
Montana, Montana University Joint Water Resources Research
Center (US NTIS Report No. ISS-MUJWRRC-95 MTIS PB-289224).
GILBERT, E.E. & GIOLITO, S.L. (1952) US Patent 2.616.825 and
US Patent 2.616.928, 4 November, to Allied Chemical and Dye
Corporation.
GOOD, E.E., WARE, G.W., & MILLER, D.F. (1965) Effects of
insecticides on reproduction in the laboratory mouse. I.
Kepone. J. Econom. Entomol., 58: 754-757.
GOODMAN, L.R., HANSEN, D.J., MANNING, C.S., & FAAS, L.F.
(1982) Effects of Kepone on the sheepshead minnow in an
entire lifecycle toxicity test. Arch. environ. Contam.
Toxicol., 11: 335-342.
HAMMOND, B., BAHR, J., DIAL, O., MCCONNEL, J., & METCALF, R.
(1978) Reproductive toxicology of mirex and Kepone. Fed.
Prod. Fed. Am. Soc. Exp. Biol., 37: 501.
HAMMOND, B., KATZENELLENBOGEN, B.S., KRAUTHAMMER, N., &
MCCONNELL, J. (1979) Estrogenic activity of the insecticide
chlordecone (Kepone) and interaction with uterine estrogen
receptors. Proc. Natl Acad. Sci., 76: 6641-6645.
HANSEN, D.J., GOODMAN, L.R., & WILSON, A.J., Jr (1976)
Kepone: Chronic effects on embryo, fry, juvenile and adult
sheepshead minnow Cyprinodon variegatus. In Hansen, D.J., ed.
Prepublications of kepone in the marine environment, Gulf
Breeze, Florida, US EPA Office of Research and Development,
Environmental Research Laboratory, 28 pp.
HARRIS, R.L., HUGGETT, R.J., & SLONE, H.D. (1980)
Determination of dissolved Kepone by direct addition of XAD-2
resin to water. Anal. Chem., 52: 779-780.
HEATH, C.W., Jr (1978) Industrial toxins and the community,
Washington DC, US Department of Health, Education and Welfare,
pp. 259-261 (US DHEW (NIOSH) Publication No. 78-169).
HEITMULLER, T. (1975) Acute toxicity of kepone to fiddler
crabs (Uca pugilator), 5 pp (Toxicity Test Report, submitted
to Allied Chemical Corporation, Morristown, New Jersey, EG &
G, Inc., Bionomics, Marine Research Laboratory, Pensacola,
Florida).
HUBER, J.J. (1965) Some physiological effects of the
insecticide Kepone in the laboratory mouse. Toxicol. appl.
Pharmacol., 1: 516-524.
HUCKINS, J.N., STALLING, D.L., PETTY, J.D., BUCKLER, D.R., &
JOHNSON, B.T. (1982) Fate of Kepone and mirex in the aquatic
environment. J. agric. food Chem., 30: 1020-1027.
HUGGETT, R.D., HAVEN, D., & NICHOLS, M. (1977) Kepone
sediment relationships in James River (Abstract) (Report to US
EPA Gulf Breeze Laboratory).
IARC (1979) Some halogenated hydrocarbons, Lyons,
International Agency for Research on Cancer (Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Vol. 20).
INFORMATION CANADA (1973) Guide to chemicals used in crop
protection, Vol. 6, p. 96.
IRPTC (1983) Legal file, Vols 1 & 2, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
KAWATSKI, J.A. & HECKER, R.J. (1979) Kepone inhibition of
succinic acid dehydrogenase in mouse liver mitochondrial
preparations. Proc. N. D. Acad. Sci., 33: 24.
KLINGENSMITH, J.S. & MEHENDALE, H.M. (1982a) Chlordecone-
induced mobilization of body fat and lipolysis in the male
rat. J. Toxicol. environ. Health, 10: 121-129.
KLINGENSMITH, J.S. & MEHENDALE, H.M. (1982b) Potentiation of
CCl4 lethality by chlordecone. Toxicol. Lett., 11: 149-154.
LANGFORD, H.D. (1978) Kepone, mirex pesticide residues
persist, full effects unknown. News Rep., 28: 1, 4-5.
LARSON, P.S., EGLE, J.L., Jr, HENNIGAR, G.R., LANE, R.W., &
BORZELLECA, J.F. (1979b) Acute, subchronic, and chronic
toxicity of chlordecone. Toxicol. appl. Pharmacol., 48: 29-41.
LEWIS, R.G. & LEE, R.E. (1976) Air pollution from
pesticides: sources, occurrence and dispersion. In: Air
pollution from pesticides and agricultural processes, Boca,
Florida, CRC Press Inc.
MADY, N., SMITH, D., SMITH, J., & WEZWICK, C. (1979)
Analysis of Kepone in biological samples. NBS (US) Spec.
Publ., 519: 341-343.
MAHAFFEY, W.R., PRITCHARD, P.H., & BOURQUIN, A.W. (1982)
Effects of Kepone on growth and respiration of several
estuarine bacteria. Appl. environ. Microbiol., 43: 1419-1424.
MARTINEZ, A.J., TAYLOR, J.R., ISAACS, E., DYCK, P.J., & HOUFF,
S.A. (1976) Kepone poisoning ultrastructure of nerves and
skeletal muscles. J. Neuropathol. exp. Neurol., 35: 323.
MARTINEZ, A.J., TAYLOR, J.R., DYCK, P.J., HOUFF, S.A., &
ISAACS, E. (1978) Chlordecone intoxication in man: II.
ultrastructure of peripheral nerves and skeletal muscle.
Neurology, 28: 631-635.
McFARLAND, L.Z. & LACY, P.B. (1969) Physiologic and
endocrinologic effects of the insecticide Kepone in the
Japanese quail. Toxicol. appl. Pharmacol., 15: 441-450.
MEHENDALE, H.M. (1979) Modification of hepatobiliary
function by toxic chemicals. Fed. Proc. Fed. Am. Soc. Exp.
Biol., 38: 2240-2245.
MEHENDALE, H.M, TAKANAKA, A., DESAIAH, D., & HO, I.K. (1978)
Effect of pre-exposure to Kepone on hepatic mixed-function
oxidases in the female rat. Toxicol. appl. Pharmacol., 44:
171-180.
MEYERS, S.P., GAMBRELL, & DAY, (1982) Determination of
environmental impact of several substitute chemicals in
agricultural affected wetlands, Washington DC, US
Environmental Protection Agency, 150 pp (US EPA Report No.
EPA-600/4-82-052; PB82-242017).
MOSEMAN, R.F., CRIST, H.L., EDGERTON, T.R., & WARD, M.K.
(1977) Electron capture gas chromatographic determination of
Kepone residues in environmental samples. Arch. environ.
Contam. Toxicol., 6: 221-231.
MOSEMAN, R.F., WARD, M.K., CRIST, H.L., & ZEHR, R.D. (1978)
A micro derivatization technique for the confirmation of trace
quantities of Kepone. J. agric. food Chem., 26: 965-968.
MOTL, M.L. (1977) EPA develops process to destroy Kepone.
Environ. Manage., 1: 491-493.
NABER, E.C. & WARE, G.W. (1965) Effect of Kepone and mirex
on reproductive performance in the laying hen. Poult. Sci.,
44: 875-880.
NIMMO, D.R., BAHNER, L.H., RIGBY, R.A., SHEPPARD, J.M., &
WILSON, A.J., Jr (1977) Mysidopsis bahia: An estuarine
species suitable for life cycle bioassays to determine
sublethal effects of a pollutant. In: Prepublications of
kepone in the marine environment, Gulf Breeze, Florida, US EPA
Office of Research and Development, Environmental Research
Laboratory.
NIOSH (1977) NIOSH manual of analytical methods, Part 1,
NIOSH monitoring methods, Vol. 1, 2nd ed., Washington DC,
National Institute for Occupational Safety and Health, pp
225-1-256-6 (Method No. P Cam 225, US DHEW (NIOSH) Publication
No. 77-157B).
NIOSH (1978) Registry of toxic effects of chemical
substances, Washington DC, US Department of Health, Education
and Welfare, p. 751.
ODUM, W.E. & DRIFMEYER, J.E. (1978) Sorption of pollutants
by plant detritus: a review. Environ. health Perspect., 27:
133-137.
ORNDORFF, S.A. & COLWELL, R.R. (1980a) Microbial
transformation of Kepone. Appl. environ. Microbiol., 39:
398-406.
ORNDORFF, S.A. & COLWELL, R.R. (1980b) Distribution and
characterization of Kepone-resistant bacteria in the aquatic
environment. Appl. environ. Microbiol., 39: 611-622.
PATE, J.B. & TABOR, E.C. (1962) Analytical aspects of the
use of glass fiber filters for the collection and analysis of
atmospheric particulate matter. Am. Ind. Hyg. Assoc. J., 23:
145-156.
PORTIER, R.J. & MEYERS, S.P. (1982) Microbial responses to
sequential and differential application of a xenobiotic in
aquatic microcosms. Abst. Annu. Meet. Am. Soc. Microbiol., 82:
Abstract Q63.
PROHST, G.S., McMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP,
J.K., & NEALS S.B. (1981) Chemically-induced unscheduled DNA
synthesis in primary rat hepatocyte cultures. A comparison
with bacterial mutagenicity using 218 compounds. Environ.
Mutat., 3: 11-32.
PROSZYNSKA, B. (1977) [Method for determining despirol
residues in potatoes.] Roozn. Panstw. Zakl. Hig., 28: 201-207
(in Polish).
REITER, L. & KIDD, K. (1978) The behavioral effects of
subacute exposure to Kepone or mirex on the weanling rat.
Toxicol. appl. Pharmacol., 45: 357.
REITER, L., KIDD, K., LEDBETTER, G., CHERNOFF, N., & GRAY,
L.E., Jr (1977) Comparative behavioural toxicology of mirex
and Kepone in the rat. Toxicol. appl. Pharmacol., 41: 143.
REUBER, M.D. (1977) Kepone carcinogenicity affirmed by
Reuber. Pest. Toxic Chem. News, 5: 6-7.
REUBER, M.D. (1978) Carcinogenicity of Kepone. J. Toxicol.
environ. Health, 4: 895-911.
REUBER, M.D. (1979) The carcinogenicity of Kepone. J.
environ. Path. Toxicol., 2: 671-686.
ROBERTS, M.H., Jr & BENDL., R.E. (1982) Acute toxicity of
Kepone to selected freshwater fish. Estuaries, 5: 158-164.
ROSENSTEIN, L., BRICE, A., ROGERS, N., & LAWRENCE, S. (1977)
Neurotoxicity of Kepone in perinatal rats following in utero
exposure. Toxicol. appl. Pharmacol., 41: 142-143 (Abstract 28).
SALEH, F.Y. & LEE, G.F. (1978) Analytical methodology for
Kepone in water and sediment. Environ. Sci. Technol., 12:
297-301.
SALEH, F.Y., LEE, G.F., & BUTLER, J.S. (1978) Kepone and
other selected chlorinated hydrocarbon pesticides and PCBs
behavior during hydraulic dredging of the James River near
Hopewell, Virginia. J. environ. Sci. Health, Part A, A13:
261-294.
SCHIMMEL, S.C. & WILSON, A.J. Jr (1977) Acute toxicity of
Kepone to four estuarine animals. Chesapeake Sci., 18: 224-227.
SHANHOLTZ, M.I. (1976) Emergency rule, Virginia State Board
of Health. Prohibiting the taking of crabs from the James
River and its tributaries and the taking of fish for human
consumption, Richmond, Virginia, Virginia Department of
Health, 3 pp.
SIMON, G.S., KIPPS, B.R., TARDIFF, R.G., & BORZELLECA, J.F.
(1978) Failure of Kepone and hexachlorobenzene to induce
dominant lethal mutations in the rat. Toxicol. appl.
Pharmacol., 45: 330-331.
SNEGAROFF, J. (1977) Organochlorine insecticidal residues in
the soil and rivers of banana-growing region of Guadeloupe.
Phytiatr. Phytopharm., 26: 251-267.
STEHLIK, L.L. & MERRINER, J.V. (1983) Effects of accumulated
dietary Kepone on spot (Leiostomus xanthurus). Aq.
Toxicol., 3: 345-358.
STERRETT, F.S. & BOSS, C.A. (1977) Careless Kepone.
Environment, 19: 30-37.
SUTA, B.E. (1978) Human population exposures to mirex and
kepone, Springfield, Virginia, US Department of Commerce (US
NTIS PB REP., PB-285, 430).
TAYLOR, J.R., SELHORST, J.B., HOUFF, S.A., & MARTINEZ, A.J.
(1978) Chlordecone intoxication in man. I. Clinical
observations. Neurology, 28: 626-630.
TILSON, H.A., BYRD, M., & RILEY, M. (1979) Neurobehavioral
effects of exposing rats to Kepone via the diet. Environ.
health Perspec., 33: 321.
US EPA (1976a) Preliminary report on Kepone levels found in
environmental samples from the Hopewell, Virginia area,
Research Triangle Park, North Carolina, US Environmental
Protection Agency, Health Effects Research Laboratory.
US EPA (1976b) Review of the Chesapeake Bay Program. Seminar
on kepone held at Virginia Institute of Marine Sciences, 12-13
October, 1976, Research Triangle Park, North Carolina, US
Environmental Protection Agency.
US EPA (1979) Reviews of the environmental effects of
pollutants. 1. Mirex and Kepone, Washington DC, US
Environmental Protection Agency (EPA Report No.
EPA-600/1-78-013; PB80-12595).
US FDA (1977) Compliance program evaluation-FY77 Kepone and
mirex contamination, Washington DC, US Department of Health,
Education and Welfare.
WALSH, G.E., AINSWORTH, K., & WILSON, A.J., Jr (1977)
Toxicity and uptake of Kepone in marine unicellular algae.
Chesapeake Sci., 18: 222-223.
WILLIAMS, G.M. (1980) Classification of genotoxic and
epigenetic hepatocarcinogens using liver culture assays. Ann.
N.Y. Acad. Sci., 349: 273-282.
WINKELHAKE, J.L., VODICNIK, M.J., & TAYLOR, J.L. (1983)
Induction in rainbow trout of an acute phase (C-reactive)
protein by chemicals of environmental concern. Comp. Biochem.
Physiol. C., 74: 55-58.
Molecular formula: C10Cl10O
CAS chemical name: 1,1a,3,3a,4,5,5,5a,5b,6-decachloro-
octahydro-1,3,4-metheno-2H-cyclo-
buta[cd]pentalen-2-one
Synonyms: decachloro-pentacyclo[5,2,1,02,6,03,
9,O5,8]decan-4-one, dec-
achloro-octahydro-1,3,4-metheno-
2H,5H-cyclobuta[cd]pentalen-2-one
Trade names: GC 1189, Kepone, Merex
CAS registry number: 143-50-0
Relative molecular mass: 490.6
2.2. Physical and Chemical Properties
Chlordecone is a tan- to white-coloured solid that sublimes
with some decomposition at 350 °C (IARC, 1979). Its vapour
pressure is less than 3 x 10-7 at 25 °C.
In the anhydrous form, chlordecone is soluble in organic
solvents such as benzene and hexane. The hydrated compound is
less soluble in apolar solvents. Oxygenated solvents such as
alcohols and ketones are recommended for the hydrated form
(Blanke et al., 1977). Chlordecone is also soluble in light
petroleum and may be recrystallized from 85 - 90% aqueous ethanol
(Information Canada, 1973). It is readily soluble in acetone
(IARC, 1979).
Early reports did not include any evidence of chlordecone
degradation in the natural environment (Dawson, 1978; Geer,
1978), but, in a more recent study, microbial action has been
shown to transform chlordecone into monohydro and possibly
dihydro-chlordecone (Orndorff & Colwell, 1980a).
Technical grade chlordecone contains from 88.6% to 99.4%
chlordecone (Blanke et al., 1977), 3.5 - 6.0% water (Dawson, 1978)
and 0.1% hexachlorocyclopentadiene. It has been formulated as a
wettable powder (50% chlordecone), emulsifiable concentrates,
granules, and dust (Information Canada, 1973).
2.3. Analytical Methods
Various methods for the determination of chlordecone are
summarized in Table 1.
Table 1. Methods for the determination of chlordecone
---------------------------------------------------------------------------------------------------
Sample type Sampling method, Analytical Limit of Reference
or medium extraction/clean-up method detection
---------------------------------------------------------------------------------------------------
general gas chromato- 0.005- Moseman et al.
graphy electron 0.01 µg (1978)
capture detection
(GC/ECD)
formulations, extract (acetone), infrared (IR) - Allied Chemicals
concentrations, decant, evaporate (c = o band) Corporation (1966)
wettable powders to dryness, dissolve
(decane), boil, cool
technical grade extract (acetone-decane), infrared (IR) - Allied Chemicals
heat to remove (c = o band) Corporation (1966)
acetone, boil, cool
air trap on filter and back up gas chromato- 0.1 µg/m3 NIOSH (1977)
up impinger containing graphy electron
sodium hydroxide solution, capture detection
extract filter (benzene- (GC/ECD)
methanol), acidify extract
(benzene), bulk extracts
food:
apples extract (benzene), decant, gas chromato- 80 µg/kg Brewerton & Slade
filter graphy electron (1964)
capture detection
(GC/ECD)
potatoes extract (methylene chloride), thin-layer 200 µg/kg Proszynska (1977)
column chromatography (CC) chromatography
(TLC) (revelation:
silver nitrate/
ultra-violet)
gas chromato-
graphy electron
capture detection
(GC/ECD)
---------------------------------------------------------------------------------------------------
Table 1. (contd.)
-------------------------------------------------------------------------------------------------------
Sample type Sampling method, Analytical Limit of Reference
or medium extraction/clean-up method detection
-------------------------------------------------------------------------------------------------------
bananas extract (isopropanol-benzene), gas chromato- 3 µg/kg Allied Chemicals
evaporate to dryness, dissolve graphy (GC)/ Corporation (1963)
(hexane), liquid/liquid micro coulo-
partition, extract (benzene) metric detection
water add XAD-2 resin to water, gas chromato- 0.015 Harris et al. (1980)
extract (toluene ethyl graphy electron µg/kg
acetate), column capture detection
chromatography (CC) (GC/ECD)
soil and extract (50% methanol gas chromato- 10-20 Blanke et al. (1977);
sediment in benzene), column graphy electron µg/kg Moseman et al. (1977);
chromatography (CC) capture detection Saleh & Lee (1978);
(GC/ECD) Orndorff & Colwell
(1980b)
biological extract (toluene in gas chromato- 10 µg/kg Mady et al. (1979)
tissues ethyl acetate), column graphy electron
chromatography (CC) capture detection
(GC/ECD)
-------------------------------------------------------------------------------------------------------
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1. Production and Uses
The synthesis of chlordecone was first reported in 1952 by
Gilbert & Giolito (1952). Commercial production in the USA
started in 1966 (IARC, 1979).
Chlordecone is manufactured by the condensation of 2
molecules of hexachlorocylopentadiene in the presence of sulfur
trioxide, followed by hydrolysis to the ketone. It is also
produced during the synthesis of mirex and is a contaminant of
technical grade mirex. From the 1950s until 1975, some 1 600 000
kg of chlordecone were produced in the USA, of which between 90%
(Sterrett & Boss, 1977) and 99.2% (US EPA, 1976b) was exported to
Africa, Europe, and Latin America. The bulk of the remainder,
12 000 - 70 000 kg (US EPA, 1976b) was used in ant and cockroach
traps in the USA or, after 1978, stored until it could be
disposed of safely. It has been reported that most of the
chlordecone exported was used in the manufacture of kelevan
(Cannon et al., 1978).
Chlordecone has been used extensively in the tropics for the
control of banana root borer (Anonymous, 1978a; Langford, 1978).
It is regarded as an effective insecticide against leaf-cutting
insects, but less effective against sucking insects (Information
Canada, 1973). It can be used as a fly larvicide, as a fungicide
against apple scab and powdery mildew (Information Canada, 1973),
and to control the Colorado potato beetle (Motl, 1977), rust mite
on non-bearing citrus, and potato and tobacco wireworm on gladioli
and other plants (Suta, 1978).
Life Science Products in Hopewell, Virginia, produced up to
2700 kg of chlordecone a day between April, 1974 and June, 1975,
when the plant was closed (Lewis & Lee, 1976). Chlordecone
production was discontinued in the USA in 1976. However, a year
later it was reported that a French company was considering the
establishment of production facilities in France (Anonymous,
1978b), but no further information on this proposal is available.
3.2. Transport and Distribution
3.2.1. Air
Laboratory and field observations indicate that chlordecone
does not volatilize to any significant extent (Dawson, 1978).
However, in the past, the release of copious quantities of
chlordecone dust from production facilities has represented a
major source of environmental and human contamination. It has
been suggested that chlordecone emissions from the Hopewell plant
"were of a fine particle size having a long residence time in the
atmosphere" (Lewis & Lee, 1976).
3.2.2. Water
The solubility of chlordecone in water is low (1 - 2 mg/litre)
and, as in the case of mirex, contamination is more likely to be
associated with the particulate matter in the water than with the
water itself (Orndorff & Colwell, 1980b). With the exception of
contamination in the James River system, very little information
is available on chlordecone residues in water. Sampling after
the closure of the Life Science Plant revealed chlordecone levels
of 1 -4 µg/litre in Bailey Creek, 0.1 µg/litre in the Appomattox
River, and 0.3 µg/litre in the James River and at the mouth of
Bailey Creek (Smith, 1976).a Chlordecone was not detected (limit
of determination 0.01 mg/kg) in samples taken from the James River
several months after the plant was shut down (Huggett et al.,
1977). However, it was detected periodically in the water table
of Hopewell at levels as high as 3.4 µg/litre but typically 0.1
µg/litre (Dawson, 1978) and was also detected in the New York
water supply of the Great Lakes Basin by Suta (1978).
Residues as high as 0.21 µg chlordecone/litre have been
reported in runoff from a banana plantation in Guadeloupe
(Snegaroff, 1977).
3.2.3. Soil
Chlordecone has a high affinity for soils and sediments such
that, at equilibrium in the environment, residue levels in
particulate matter will be 104 - 105 times that in any surrounding
water (Dawson, 1978). Consequently, sediments act as sink for
chlordecone-contaminated water and soils provide a sink for most
atmospheric contamination. Again, most of the residue data result
from work in and around Hopewell and the James River system.
Sediment levels were as high as 10 mg/kg in Bailey Bay, and it has
been estimated that as much as 47 000 kg of chlordecone lie on the
bottom of the James River (Chigges, unpublished data, 1977).
Soil residue levels in Hopewell ranged from as high as 10 000
to 20 000 mg/kg near the plant to 2 - 6 mg/kg at a distance of 1
km (US EPA, 1976a) and it was estimated (Anonymous, 1978) that
1000 kg of chlordecone lay within a 1 km radius of the plant.
Most of the soils tested in Hopewell contained detectable levels
of chlordecone with concentrations generally decreasing with
increasing distance from the plant (Dawson, 1978). Chlordecone
residues may be expected in sediments of waterways in the vicinity
of other production-formulation facilities, but no data are
available on this.
----------------------------------------------------------------------
a Smith, W.C. (1976) Kepone discharges from Allied Chemical
Corporation, Hopewell, Virginia, Denver, Colorado, US EPA,
National Field Center (Internal EPA Memorandum).
The US EPA (Anonymous, 1978a) estimated that a field that had
been treated with chlordecone (4.2 kg active ingredient/ha) should
have a residue level of 100 mg/kg in the top 3 cm of soil, after
application. Reports of actual determinations in soil are scarce,
but the United Fruit Company (Anonymous, 1978a) described a
residue level of 15 - 25 mg/kg, 6 months after an application of
6.73 kg active ingredient/ha. Snegaroff (1977) reported soil
residue levels of 9.5 mg/kg and a level of 0.135 mg/kg in
sediments in streams neighbouring on a banana plantation in
Guadeloupe.
3.2.4. Abiotic degradation
Chlordecone is an extremely stable compound and, as mentioned
in section 2, it is not expected to be degraded in the environment
to any significant extent. However, there have been reports of
trace amounts of monohydro chlordecone being found (Carver et al.,
1978, Orndorff & Colwell, 1980b), but the mechanism of its
formation is not clear. Solar irradiation of chlordecone in the
presence of ethylenediamine will result in 78% degradation after
10 days, but no study of the degradation products or their
toxicity has been undertaken (Dawson, 1978).
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. General Population Exposure
Precise information on general population exposure to
chlordecone is not available. However, a summary of the daily
exposure from several sources in different regions in the USA has
been compiled (Suta, 1978).
(a) Air
Airborne chlordecone has been known to spread 60 miles from a
point source (Feldmann, 1976), and the potential exists for
further dispersion of fine particles (Lewis & Lee, 1976).
(b) Water
At present, exposure via drinking-water does not present a
health hazard with the possible exception of that in the Hopewell
area. Values quoted for the lower James River ranged from 0.1 to
10 µg/litre (Suta, 1978).
(c) Food
The USA action levels for chlordecone residues in foods are
0.3 mg/kg for shellfish, 0.3 mg/kg for finfish, 0.4 mg/kg for
crabs, and 0.01 mg/kg for banana peels (Suta, 1978). While the
majority of shellfish taken from the polluted James river in 1976
contained less than the 0.3 mg/kg action level of chlordecone,
oyster and clam samples in certain areas contained 0.21 - 0.81
mg/kg, crab samples contained 0.45 - 3.44 mg/kg, and finfish
samples 0.02 - 14.4 mg/kg. These data prompted a fishing ban on
the James River (Shanholtz, 1976).
In 1978, samples of spot, flounder, mullet, trout, and croakers
from the James River contained chlordecone but in concentrations
below the 0.3 mg/kg action level (Suta, 1978). In bluefish, one
sample was above 0.1 mg/kg (0.2 mg/kg) (US FDA, 1977). The shell-
fish sampled in the same area contained chlordecone, but at levels
that could not be reliably determined (Reuber, 1977). All crabs
in the area contained chlordecone, but all levels were below the
action level.
In 1976, samples from the polluted Chesapeake Bay contained
levels of 0.037 mg/kg for 75 finfish samples and 0.61 mg/kg for
11 crab samples, and levels in 3 samples of oysters and one
sample of clams were non-detectable (US EPA, 1979).
Residues in Atlantic coast bluefish (66 samples) ranged from
0.01 to 0.06 mg/kg, with the higher concentrations found off the
Virginia coast (Peeler, 1976). South Atlantic coastal fish were
relatively free of chlordecone as only 1 out of 132 samples
contained detectable levels (Reuber, 1977).
Residues of chlordecone in edible plants have only been
reported in New Zealand (Brewerton & Slade, 1964). No data are
available in the literature for chlordecone residue levels in
bananas (Suta, 1978).
Chlordecone has been found in 9 out of 298 samples of human
milk, but the detection limit was relatively high (1 µg/kg)
(Suta, 1978). Samples were taken in the southern USA, and
chlordecone residues were only found in areas that had received
bait treatment for fire ants.
(d) Exposure in infants
Two major sources of chlordecone exposure for infants are
insect traps and human milk. The USDA (1977)a has reported that
of 56 cases of non-occupational exposure to chlordecone, 52 were
children under the age of 5, and all but 9 of these had come into
contact with insect traps. This is understandable as children of
this age group are fairly inquisitive and their activity areas
are likely to overlap target areas for ant and cockroach traps.
The same study also cited exposure of 2 adults and 2 persons of
unspecified age.
To date, chlordecone contamination of human milk has only
been reported in 9 samples (Suta, 1978) in the southeastern USA.
However, relatively few samples have been tested for chlordecone.
(e) Miscellaneous
Since tobacco plants were treated with chlordecone, this may
have also represented an exposure route, but again no residue
data are available.
4.2. Occupational Exposure
Chlordecone received its notoriety when severe and wide-
spread industrial poisoning was discovered at the Life Science
Plant (LSP) in Hopewell in 1975. From March 1974 to June 1975,
the LSP recorded output of chlordecone was 769 390 kg (Dawson,
1978). The total production was certainly above this figure, but
massive amounts of chlordecone found their way into the soil,
water, and air surrounding the plant. The workers in the plant
and the families in the area were exposed to extremely high
concentrations of chlordecone dust. High volume air samplers
(Pate & Tabor, 1962), 200 m from the plant, recorded chlordecone
levels as high as 54.8 mg/m3, which constituted 50% of the total
particulate load. Lower concentrations of chlordecone were
detected in the air 25 km away from the plant. Concentrations of
---------------------------------------------------------------------
a Comments of the Secretary of Agriculture in response to
the Notice of Intent to cancel pesticide products
containing chlordecone, trade name "Kepone". Washington DC
vs. USDA, January 11, 1977.
chlordecone dust within the plant were not monitored, but levels
reaching 11.8 mg/litre were found in blood samples of workers
from the LSP (Heath, 1978). Illness was found in 76 of the 133
current and former workers of the plant examined. Families of
the LSP workers were also examined, as well as Allied Chemical
Corporation people working in the area of the plant, workers from
the sewage treatment plant that received chlordecone sludge, and
residents of Hopewell. It was found that the blood levels of
workers who were ill, averaged 2.53 mg/litre, whereas the average
level in workers not reporting ill was 0.60 mg/litre (Heath,
1978).
4.3. Wildlife
Residue levels for phytoplankton in the James River were
found to average 1.3 mg/kg (Huggett et al., 1977).
Chlordecone residues were also found in several species of
birds that inhabit the southeastern USA coast, such as the blue
heron, mallard duck, coot, black duck, wood duck, herring gull,
Canada goose, hooded mersanger, and the bald eagle (Dawson,
1978). Residue levels were as high as 13.23 mg/kg (Dawson,
1978), but typically between 0.02 and 2 mg/kg. Eggs from the
bald eagles and the osprey in Virginia were also examined and
were found to contain residue levels ranging from 0.14 to 0.19
mg/kg, and 0.06 to 1.5 mg/kg, respectively (Dawson, 1978).
Studies on marsh plants in the James River Basin indicated
that there was no translocation of chlordecone from root to
aerial plant tissue (Lunz, 1978).
5. KINETICS AND METABOLISM
Only limited information is available on the absorption,
distribution, metabolism, and excretion of chlordecone in human
beings and animals. These aspects of the chemical are therefore
discussed together, rather than in separate sections.
5.1. Animal Studies
The results of earlier studies by Huber (1965) indicated that,
after dietary exposure, chlordecone was accumulated mainly in the
liver of mice. The brain, fat, and kidneys also contained some
residues. Chlordecone was well absorbed and distributed through-
out the body of rats after oral administration. Following a
single oral dose at 40 mg/kg body weight, the highest concentrations
were found in the adrenal glands and liver, followed by the fat
and lung (Egle et al., 1978). The compound had a long biological
half-life and disappeared more slowly from the liver that from
other tissues. Excretion occurred mainly in the faeces, a total
of 66% of the dose being removed in the faeces and 2% in the urine
urine in the 84 days following administration. Faecal excretion
of chlordecone in rats was increased by the adminstration of an
ionic exchange resin, cholestyramine (Boylan et al., 1977).
Excretion of chlordecone by the gastrointestinal tract, in addition
to the biliary route, occurs in rats as well as human beings
(Boylan et al., 1979). A small amount of chlordecone alcohol was
found in rat faeces suggesting that chlordecone under went
reductive biotransformation in the rat (Blanke et al., 1978).
5.2. Human Studies
A number of studies were conducted to investigate the
kinetics of chlordecone in workers who were exposed to this
chemical. Chlordecone was present in high concentrations in the
liver (mean and range) (75.9 mg/kg; 13.3 - 173 mg/kg), whole
blood (5.8 mg/litre, 0.6 - 32 mg/litre), and subcutaneous fat
(21.5 mg/kg, 2.2 - 62 mg/kg) of 32 male workers (Cohn et al.,
1976). Adir et al. (1978) reported that, in occupationally-
exposed workers, serum chlordecone concentrations ranged from 120
to 2109 µg/litre. Six to 7 months later, the concentration
dropped to 37 - 486 µg/litre. The half-life was estimated to be
63 - 148 days. Chlordecone was eliminated, primarily in the
faeces, at a mean daily rate of 0.075% of the estimated total
store in the body (Cohn et al., 1976). Cholestyramine was found
to increase the faecal excretion of chlordecone by a factor of 6
- 7, presumably by interfering with reabsorption from the
intestine. Chlordecone underwent extensive biliary excretion and
enterohepatic circulation. Elimination by the gastrointestinal
tract also played an important role (Boylan et al., 1979).
Chlordecone alcohol was identified in human faeces (Blanke et
al., 1978).
6. STUDIES ON EXPERIMENTAL ANIMALS
6.1. Single Exposures
Toxicity data resulting from single exposures to chlordecone
in several animal species are summarized in Table 2. Toxic
symptoms included severe tremors in all species tested. These
tremors usually reached a maximum within 2 - 3 days, then
gradually subsided. Tremors were exacerbated by excitement.
In dermal studies on rats and rabbits, no skin irritation was
observed when chlordecone was administered in oil, but in aqueous
solution it produced marked irritation, oedema, and scab formation
(Epstein, 1978).
6.2. Short-Term Exposures
The effects of chlordecone following short-term exposures are
summarized in Table 3. In general, they include nervous symptoms,
liver hypertrophy, induction of mixed-function oxidases (EC 1.14.14.1),
and structural and ultrastructural changes in the liver, thyroid,
adrenals, and testes. Death sometimes followed.
6.2.1. Dermal toxicity
A study has been reported (Epstein, 1978) in which
chlordecone concentrations equivalent to 5 and 10 mg/kg body
weight were tested on groups of 6 male albino rats for 3 weeks,
totalling 15 applications; the animals were killed 2 weeks after
termination of exposure. Two out of 6 animals in the low-dose
group and 1 out of 6 in the high-dose group showed testicular
atrophy. Otherwise, there were no consistent or significant
pathological changes.
6.3. Long-Term Exposures and Carcinogenicity Studies
The long-term and carcinogenic effects of chlordecone are
summarized in Table 4. Effects in these studies were similar to
those reported following short-term exposures. The data indicate
that chlordecone is carcinogenic in mice and rats. These studies
were reviewed by IARC (1979) and it was concluded that chlordecone
produced hepatocellular carcinomas in both sexes of mice and rats.
Table 2. Acute toxicity of chlordecone
-------------------------------------------------------------------------
Species Sex Route of LD50 (mg/kg Reference
administration body weight)
-------------------------------------------------------------------------
dog M & F oral 250 Larson et al. (1979b)
rabbit ? oral 65 NIOSH (1978)
chicken ? oral 480 NIOSH (1978)
rat ? oral 95 NIOSH (1978)
rabbit ? dermal 345 NIOSH (1978)
rat M oral (oil) 132 Larson et al. (1979b)
rat F oral (oil) 126 Larson et al. (1979b)
rat M oral (aqueous) 96 Epstein (1978)
rabbit M oral (oil) 71 Larson et al. (1979b)
rabbit M oral (aqueous) 65 Epstein (1978)
rabbit M dermal (oil) 410 Epstein (1978)
rabbit M dermal (aqueous) 435 Epstein (1978)
pig M oral (approx.) 250 Epstein (1978)
rat M oral (aqueous) 9.6a Epstein (1978)
rat M oral (peanut oil) 125 Gaines (1969)
rat F oral (peanut oil) 125 Gaines (1969)
rat M dermal (xylene) 2000 Gaines (1969)
rat F dermal (xylene) 2000 Gaines (1969)
-------------------------------------------------------------------------
a These animals were dosed for 20 consecutive days excluding Sundays.
Table 3. Summary of short-term studies with chlordecone
-----------------------------------------------------------------------------------------------------
Species Sex Duration Doses used Effects Reference
-----------------------------------------------------------------------------------------------------
mouse M 14 days 1, 10, or induction of hepatic mixed- Fabacher & Hodgson
50 mg/kg diet function oxidases (1976)
at 2 highest levels
rat M 8 days 200 mg/kg ultrastructural changes in Baggett et al.
diet the liver and adrenal (1977, 1980)
medulla, decreased adrenal
catecholamines, and
increased P-450 values
rat F 15 days 50, 100, or decreased body weight gain Mehendale et al.
150 mg/kg diet and induction of mixed- (1978)
function oxidases at all
3 levels of treatment
rat M 15 days 10, 50, or decreased biliary excretion Mehendale et al.
150 mg/kg diet at 10 mg/kg and higher; (1978)
body weight gain affected
at 50 mg/kg and higher;
liver enlargement at
all 3 levels of treatment
rat M & F 3 months 25 mg/kg diet tremors after 4 weeks; liver Cannon & Kimbrough
followed by hypertrophy; liver and adrenals (1979)
"clean" diets both showed histological
for 4.5 months changes; after recovery
period, liver still showed
histological abnormalities
rat 14 days 1 mg/kg diet induction of hepatic mixed- Baker et al.
function oxidases (1972)
-----------------------------------------------------------------------------------------------------
Table 4. Summary of long-term and carcinogenicity studies with chlordecone
---------------------------------------------------------------------------------------------------------
Species Duration Doses used Effects Reference
---------------------------------------------------------------------------------------------------------
rat 2 years 5, 10, 25, all rats on 2 highest doses died during first Larson et al.
50, or 80 6 months; depressed growth occurred at 10 mg/kg (1979b)
mg/kg diet and higher; liver hypertrophy occurred at levels
of 10 mg/kg and higher; histopathological find-
ings in liver, kidneys, and testes at 25 mg/kg;
haematological changes at 25 mg/kg
dog 127 weeks 1, 5, or 25 weight gain reduced at 25 mg/kg; no treatment- Larson et al.
mg/kg diet related histological abnormalities observed (1979b)
mouse 90 weeks 20-40 mg/kg survival reduced at high dose level in males; Anonymous
diet hepatocellular carcinomas induced in both males (1976)
and females
rat up to 24 months 1-80 mg/kg hepatocellular carcinomas observed in some Larson et al.
diet intermediate dose groups, but not all (1979b)
mouse 12 months 0-100 mg/kg tremors observed after 4 weeks in all mice Huber (1965)
diet fed 30 or more mg/kg; deaths observed
at 2 highest doses; liver enlargement
observed at 40 mg/kg and higher; micro-
scopic and electron microscopic changes
observed in dose-dependent manner
rat exposure for 80 8-26 mg/kg increased incidence of hepatocellular carcinomas Anonymous
weeks followed diet observed in high-dose females (1976)
by 16 weeks of
observation
rat up to 2 years 1 mg/kg increased incidence of malignant tumours in Reuber (1978,
diet male and female rats 1979)
---------------------------------------------------------------------------------------------------------
6.4. Reproduction and Teratology Studies
The reproductive performance of mice fed 0, 10, 30, or 37.5
mg chlordecone/kg diet was impaired in terms of offspring and
litter size (Huber, 1965). No litters were produced by females
fed 40 mg/kg, but litter production did resume within 7 weeks
following withdrawal of the chlordecone, although litters were
still smaller than those of untreated controls. Histological
examination of the testes showed they were normal, but corpora
lutea were virtually absent from the ovaries. The authors
concluded that reproductive failure was largely due to an effect
in females characterized by prolonged FSH and estrogen
stimulation, inducing constant estrus, large follicles and
absence of corpora lutea but with levels of LH subminimal for
ovulation.
In a study reported by Good et al. (1965), male and female
mice fed chlordecone in the diet at levels ranging from 10 to 375
mg/kg for 1 month, were randomly paired with animals at the same
feeding level and then maintained on the same diet for 4 months.
The results indicated that chlordecone caused a dose-dependent
effect on reproduction, even at 10 mg/kg.
Similar effects on reproduction were noted by Hammond et al.
(1978) in rats fed 30 mg/kg; the estrogenic properties of this
chemical were also noted (Couch et al., 1977; Bulger et al.,
1979; Hammond et al., 1979). In female rats fed 25 mg
chlordecone/kg diet for 3 months, followed by a control diet for
4.5 months, reproduction was completely inhibited during the
treatment period. Two months after exposure was discontinued,
reproduction was only partially restored (Cannon & Kimbrough,
1979). Chlordecone has also been shown to interfere with egg
production in both quails (McFarland & Lacy, 1969) and hens
(Naber & Ware, 1965).
Chlordecone was administered by gastric intubation in doses
of 2, 6, and 10 mg/kg body weight per day to rats and 2, 4, 8,
and 12 mg/kg body weight per day to mice on days 7 - 16 of
gestation (Chernoff & Rogers, 1976). In rats, the highest dose
caused 19% maternal mortality and fetuses exhibited reduced
weight, reduced degree of ossification, oedema, undescended
testes, enlarged renal pelvis, and enlarged cerebral ventricles.
Lower dose levels induced reductions in fetal weight and degree
of ossification. Male rats born to treated dams did not show any
reproductive impairment. In the mouse, fetotoxicity was observed
only at the highest dose level and consisted of increased fetal
mortality and clubfoot.
In a study by Rosenstein et al. (1977), rats were
administered chlordecone by gavage from day 2 of gestation at
levels of 1, 2, or 4 mg/kg body weight per day. At parturition,
all control pups and those from mothers receiving 1 mg/kg body
weight were normal. Two-thirds of the females receiving 2 mg/kg
and all females receiving 4 mg/kg aborted or had still births.
Chlordecone was administered to female rats at concentrations
of 2.5 mg/kg body weight per day and to mice at 6.0 - 24 mg/kg
body weight per day on days 7 - 16 of gestation and also
postpartum (Chernoff et al., 1979a). Although there were toxic
manifestations in the mother (death) and fetuses (litter
mortality, decreased litter weight), ophthalmological studies did
not reveal cataracts or outlined lenses.
6.5. Mutagenicity
Chlordecone was found to be negative at dose levels of 3.6 or
11.4 mg/kg body weight per day for 5 days in a dominant lethal
study on rats (Simon et al., 1978). Chlordane gave negative
results when tested for enhancement of unscheduled DNA synthesis
in primary cultures of adult rat hepatocytes (Williams, 1980;
Prohst et al., 1981) and was not mutagenic in Salmonella
typhimurium(Prohst et al., 1981).
6.6. Behavioural Studies
In studies on rats administered 40 - 80 mg chlordecone/kg
diet, behavioural changes including hyperactivity, decreased
ambulation in an open field, and delayed emergence from the home
cage were seen at both dose levels within one week (Reiter et
al., 1977; Reiter & Kidd, 1978; Tilson et al., 1979). Chlordecone
was given intragastrically, 5 - 6 days per week, at dosages of 1,
5, and 10 mg/kg body weight for 4 - 76 days to male and female
Zivic-Miller rats. A dose of 1 mg/kg body weight disrupted the
multiple-fixed-ratio test and the fixed-interval test after 3
injections and a dose of 5 mg/kg decreased the spaced-responding
test after 9 - 10 injections. Gradual recovery occurred after
discontinuation of treatment (Dietz & McMillan, 1978).
6.7. Neurotoxicity
Chickens (Naber & Ware, 1965), quail (McFarland & Lacy,
1969), fish (Couch et al., 1977), hamsters (Martinez et al.,
1976), mice (End et al., 1979), rats (Epstein, 1978), and man
(Martinez et al., 1978) have all displayed neurotoxic symptoms on
exposure to chlordecone. Biochemically, chlordecone has been
shown to inhibit Mg-ATPases in fish brain (IARC, 1979) and rat
liver (Desaiah et al., 1977) and also to cause disruption of rat
brain synaptosomal membranes (End et al., 1979).
6.8. Other Studies
Chlordecone has been shown to inhibit several enzymes (in
vitro) including maleate dehydrogenase (Anderson et al., 1977),
lactate dehydrogenase (EC 1.1.1.27) (Anderson & Noble, 1977;
Anderson et al., 1978), and succinic acid dehydrogenase (Kawatski
& Hecker, 1979).
Chlordecone has been demonstrated to enhance the hepatotoxic
effects of both chloroform and carbon tetrachloride (Cianflone et
al., 1980), but had no similar effect on the response of the rat
liver to polyhalogenated biphenyls (Chu et al., 1980). It was
able to increase the detoxification of lindane in weanling rats
(Chadwick et al., 1979). Pretreatment of rats with low non-toxic
levels of dietary chlordecone (10 mg/kg, 15 days) potentiated the
hepatotoxicity (Curtis et al., 1979) and lethality of carbon
tetrachloride (Klingensmith & Mehendale, 1982a) about 70-fold in
male rats and 25-fold in female rats (Agarwal & Mehendale,
1982a). Comparative doses of other inducers of microsomal
enzymes such as mirex, photomirex, and phenobarbital did not
potentiate carbon tetrachloride toxicity to such an extent
(Curtis & Mehendale, 1980; Klingensmith & Mehendale, 1982b).
Hepatobiliary dysfunction, elevation of hepatic enzymes in serum,
and centrilobular hepatocellular necrosis were the characteristic
features for the rat. The hepatotoxicity and lethality of
bromotrichloromethane were also potentiated about 5-fold by
chlordecone (Agarwal & Mehendale, 1982b).
Like mirex, chlordecone has been shown to modify
hepatobiliary function (Mehendale, 1979), possibly due to
interference with energy production and utilization.
In an inhalation study reported in a review (Epstein, 1978),
male rats were exposed to test and control dusts for 2 h per day
for 10 days and killed 2 weeks later. Air flow was maintained at
10 - 12 litre/min, and the effective chlordecone concentrations
were 3.7 and 15.4 µg/litre. The reviewer concluded, contrary to
the authors of the actual study, that chlordecone at both dose
levels induced toxic effects, including hepatomegaly and
histopathological changes in the liver and lungs.
7. EFFECTS ON MAN
7.1. Poisoning Incidents in the General Population
No information is available concerning such incidents.
7.2. Occupational Exposure
Life Sciences Products Co. (LSPC) was formed in November 1973
and went out of production in July 1975. In a study carried out
by the Center for Disease Control (Cannon et al., 1978), 133
employees, including 33 currently employed, were interviewed,
examined, had blood samples taken, and completed a standard
questionnaire. Of the 133 examined, 76 (57%) had developed
clinical illness described as nervousness, tremor, weight loss,
opsoclonus, pleuritic and joint pain, and oligospermia. Illness
rates were higher for production workers than non-production
workers, and the mean blood-chlordecone level for workers with
illness was 2.53 mg/litre compared with a level of 0.60 mg/litre
in workers without disease. Laboratory findings from the above
study showed an increase in serum alkaline phosphatase (EC
3.1.3.1) activity in several patients (Taylor et al., 1978) and
morphological changes in peripheral nervous tissue (Martinez et
al., 1978).
7.3. Treatment of Poisoning in Man
The treatment is symptomatic.
Administration of cholestyramine will increase the excretion
of chlordecone, and so reduce the body burden of the chemical
(Cohn et al., 1976, 1978; Anonymous, 1977).
8. EFFECTS ON ORGANISMS IN THE THE ENVIRONMENT
8.1. Aquatic Organisms
The results of studies on the toxicity of chlordecone for a
variety of algae are given in Table 5.
Acute and short-term toxicity values for invertebrate species
are also tabulated (Table 6). A more comprehensive table listing
different conditions and exposure times is available on request
from the IRPTC, Geneva. A life cycle study is available for
mysid shrimps, Mysidopsis bahia (Nimmo et al., 1977). This test
was long enough to cover the production of several broods. The
average number of young produced by each female was reduced from
the control level of 15.3 to 8.9 on exposure to 0.39 µg
chlordecone/litre. Juveniles produced grew more slowly than
controls. Young females exposed to as little as 0.072 µg
chlordecone/litre for 14 days were shorter than controls. The
authors pointed out that reproductive success was related to body
size, the number of eggs produced being greater in bigger
females. In a life cycle study of a copepod, Eurytemora affinis,
a dominant zooplankter, the intrinsic rate of natural increase
was reduced by all concentrations of chlordecone greater than
5 µg/litre (Allan & Daniels, 1982). This was due to a combination
of a reduced rate of survival, delayed onset of reproduction, and
reduced fecundity.
The toxicity of chlordecone for fish varies with species
(Table 6). Juvenile fish are generally less susceptible to
chlordecone than adults. Symptoms of chlordecone poisoning
(Hansen et al., 1976) progressed from scoliosis (darkening of the
posterior third of the body) through haemorrhaging near the brain
and anterior point of darkening, oedema, fin rot, incoordinated
swimming, and cessation of feeding. Symptoms increased in
severity before death, which occurred between 5 and 8 days after
initial exposure. Juveniles showed reduced growth at 0.08 µg
chlordecone/litre with some showing scoliosis during a 36-day
test. Embryo survival was reduced when adults were exposed to
chlordecone. When adults were exposed to 1.9 µg/litre, their
embryos developed abnormally or died, even when incubated in
chlordecone-free water. Fry from embryos exposed to 6.6 or 33 µg
chlordecone/litre were visibly affected within 24 h of hatching.
Symptoms of poisoning in fry less than 1 week old included
diminished activity, loss of equilibrium, cessation of feeding,
and emaciation. Fry more than 1 week old had symptoms identical
to those in adult fish, except for haemorrhaging and oedema.
Sixty percent of juvenile fish that had survived 36 days'
exposure to 0.08 µg chlordecone/ litre had scoliosis and
blackened tails. In clean water, symptoms persisted for more
than ten days (Hansen et al., 1976).
Table 5. Toxicity of chlordecone for algae
---------------------------------------------------------------------------------------------------
Alga Flow/ Temp Salinity End point Parameter Concentration Reference
stat (°C) o/oo (µg/litre)
---------------------------------------------------------------------------------------------------
Chlorococcum stat 20±0.5 30 growth 7-day EC50 0.35 Walsh et al. (1977)
sp. retardation
Dunaliella stat 20±0.5 30 growth 7-day EC50 0.58 Walsh et al. (1977)
tertiolecta retardation
Nitzschia stat 20±0.5 30 growth 7-day EC50 0.60 Walsh et al. (1977)
sp. retardation
Thalassiosira stat 20±0.5 30 growth 7-day EC50 0.60 Walsh et al. (1977)
pseudonana retardation
---------------------------------------------------------------------------------------------------
Table 6. Toxicity of chlordecone for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Flow/ Temp Salinity End point Parameter Concen- Reference
stat (°C) o/oo tration
(µg/litre)
---------------------------------------------------------------------------------------------------------
grass shrimp flow 96-h LC50 121 Schimmel & Wilson (1977)
(Palaemonetes flow 25-28 10-20 19-day LC50 1.4 Nimmo et al. (1977)
pugio)
blue crab flow 96-h LC50 >210 Schimmel & Wilson (1977)
(Callinectes flow 48-h LC50 1000a Butler (1963)
sapidus)
easter oyster flow 14 inhibition 96-h EC50 57a Butler (1963)
(Crassostrea shell depos-
virginica) ition
American eel flow 19 flesh 96-h LC50 35 Roberts & Bendl (1982)
(Anguilla
rostrata)
juvenile
stage VIA
sheepshead flow 96-h LC50 69.5 Schimmel & Wilson (1977)
minnow
(Cyprinodon
variegatus)
spot flow 96-h LC50 6.6 Schimmel & Wilson (1977)
(Leiostomus
xanthurus)
bluegill flow 19-21 96-h LC50 50 Roberts & Bendl (1982)
sunfish, juv
(Lepomis
macrochirus)
channel flow 20-23 96-h LC50 514 Roberts & Bendl (1982)
catfish, juv
(Ictalurus
punctatus)
---------------------------------------------------------------------------------------------------------
a Nominal concentration, not measured.
Estimation of the long-term effects of chlordecone on
juvenile fish, from the results of acute tests can result in
severe underestimation. When juvenile spot were fed sublethal
doses of chlordecone (0.3 and 0.7 mg/kg diet per day) for 56
days, they developed bone damage including fracturing and
thickening of vertebrae (Stehlik & Merriner, 1983).
Desaiah & Koch (1975) conducted in vitro studies on brain
ATPase activity in channel catfish (Ictalurus punctatus) and
demonstrated a significant inhibition of oligomysin-sensitive
(mitochondrial) Mg2+, oligomysin-insensitive Mg2+ and NA+-K+
ATPases with increasing concentrations of chlordecone. Inhibition
was 25.7% and 36.7% at chlordecone concentrations of 1.25 and 2.5
µM, respectively. The authors noted that the resulting reduction
in energy supply could have physiological consequences.
Winkelhake et al. (1983) showed the inducement of an acute phase
(C-reactive) protein in the serum of rainbow trout after
administration of chlordecone at 5 mg/kg. The formation of these
proteins is the initial reaction to bacteria or response to
foreign proteins.
8.2. Terrestrial Organisms
(a) Plants
Little work on the effects of chlordecone on plants has been
reported. In one experiment, chlordecone increased both the
quality and quantity of the cotton yield (Gawaad et al., 1976).
Residues in seeds were always, 1 mg/kg, despite different
application rates.
(b) Insects
In a study on bees, Atkins & Anderson (1962) reported an LT50
value for a 200 mg dose of chlordecone of 68 h. They tested
chlordecone in 1961 on bee colonies that had shown a progressive
resistance to DDT over a 5-year period. They obtained an LT50
value of 45 h for chlordecone, in 1952, when tested on a
different strain of DDT-susceptible bees. The authors implied
that DDT resistance carries over to other organochlorine
insecticides; but though lower susceptibility to chlordecone was
shown by DDT-resistant bees, the results do not directly
demonstrate this. The results of later studies by Atkins et al.
(1973) suggest that chlordecone would have to be used at 5 times
the recommended application rate to kill 50% of bee populations.
At the recommended usage rate of 2.25 kg/ha, chlordecone was not
harmful to 3 out of 4 predatory insect species and arthropods,
monitored in an apple orchard.
There is no information on the effects of chlordecone on
amphibians or reptiles.
(c) Birds
Chlordecone was shown not to be very toxic when fed to either
young or adult birds (Table 7). Species tested were not very
representative. Birds fed lethal doses of the insecticide
developed characteristic whole-body tremor, prior to death
(DeWitt et al., 1962; Naber & Ware, 1965; McFarland & Lacy,
1969). Japanese quail injected daily with 0.5 mg chlordecone/
bird showed liver damage (damage to hepatic parenchymal cells,
including disruption of mitochondria, with cellular debris in the
bile and bile ducts), with increased numbers of phagocytic Kupffer
cells lining the liver sinusoids (US EPA, 1979).
Sublethal effects of chlordecone on birds are pronounced
despite the compound's low acute toxicity. A sublethal dose of
200 mg chlordecone/kg diet administered to Japanese quail caused
structural changes in the liver, adrenals, and gonads (Eroschenko
& Wilson, 1975). Many sublethal effects of the compound are
attributable to its estrogenic effects. Dosing with chlordecone
caused oviduct maturation in sexually immature females held on
non-stimulatory daylengths, but mature females were not affected
(Eroschenko & Wilson, 1975). Ovaries from chlordecone-treated
females contained more primary oocytes and smaller follicles than
those from controls. A central effect on follicle-stimulating
hormone production was postulated by McFarland & Lacy (1969), but
direct hormone measurement does not seem to have been carried
out. Estrogen-like stimulation of secondary sexual characteristics
caused male pheasants to develop female plumage at dietary doses of
50, 100, and 150 mg chlordecone/kg (DeWitt et al., 1962). Males
also showed malformed sperm and reduced reproductive success.
Eroschenko & Wilson (1975) reported effects on the testicles in
both immature and adult quail; seminiferous tubules were distended
with watery fluid that caused a significant weight increase in the
testes, germinal epithelium and spermatozoa were reduced, and
abundant intraluminal cellular debris was common.
Both egg laying and chick survival were reduced in domestic
hens fed 75 or 150 mg chlordecone/kg diet for 12 weeks. Only 56%
of chicks hatched from hens treated with 75 mg/kg survived for 20
days, no chicks or hens treated with 100 mg/kg survived. Residues
were still detectable in eggs laid 3 weeks after treatment ceased
(Naber & Ware, 1965). Eggshell deposition was affected by
chlordecone. A peculiarly thick spongy layer developed leading to
blockage of shell pores and suffocation of the embryo (Erben,
1972). Changes in shell structure occurred in Japanese quail fed
225 mg chlordecone/kg diet (US EPA, 1979).
There is no information on the toxicity of chlordecone for
non-laboratory mammals.
Table 7. Toxicity of chlordecone for birds
---------------------------------------------------------------------------------------
Species Age Route Parameter Concentration Reference
(mg/kg)
---------------------------------------------------------------------------------------
mallard duck young diet LC50 400 Dewitt et al. (1962)
bobwhite quail young diet LC50 600 Dewitt et al. (1962)
bobwhite quail adult diet LC50 530 Dewitt et al. (1962)
ringnecked pheasant young diet LC50 600 Dewitt et al. (1962)
ringnecked pheasant adult diet LC50 115 Dewitt et al. (1962)
---------------------------------------------------------------------------------------
8.3. Microorganisms
Effects of chlordecone on soil microorganisms were investigated
by Gawaad et al. (1972a). Application of chlordecone to 3 soil
types in the Nile Delta altered fungal, actinomycete, and other
bacterial populations for as long as 45 days, compared with
controls (Gawaad et al., 1972a). Unfortunately, chlordecone was
applied at a very high rate (22.0 kg/ha) and therefore the results
are difficult to interpret in terms of likely effects on crops.
The magnitude and duration of effects on populations differed with
soil type, but the general pattern was a fall in numbers in the
first week followed by an increase in the second week with numbers
eventually returning to normal levels. In a second experiment in
which effects on nitrogen transformation in treated soils were
studied, chlordecone was shown to affect fungi and bacteria
responsible for ammonification, and Nitrobacter, which is
responsible for changing nitrite to nitrate, but not Nitrosomonas,
which is responsible for changing ammonia to nitrite (Gawaad et
al., 1972b).
Similar effects on microbial populations were found by Meyers
et al. (1982), when chlordecone at 0.5 mg/litre was applied to
static carbon metabolism microcosms; no significant total
treatment variation was seen in either bacterial or fungal
populations after 10 days incubation. Similar results were
obtained in response to continuous application of chlordecone.
Chlordecone is probably highly toxic for sludge microorganisms,
since massive amounts of beneficial bacteria in a sludge digester
were killed after chlordecone wastes were discharged into the
sewage system (Bray, 1975). Portier & Meyers (1982) stated that
microcosms (simulated aquatic microenvironmental systems) were
"sensitive" to chlordecone "under a variety of regimes". Among
response criteria used were microbial diversity, enzymatic
activity, ATP, and material turnover.
The toxicity of chlordecone for mixed populations of
microorganisms was determined by standard plate assays on Zobell
marine medium containing 0.02, 0.2, or 2 mg chlordecone/litre
(Mahaffey et al., 1982). All these concentrations of chlordecone
reduced the number of colony-forming aerobes but did not affect
anaerobes. Gram-positive organisms were more sensitive to
chlordecone than gram-negative organisms. Oxygen uptake by gram-
negative isolates was reduced by 25 - 100% by chlordecone at 20
mg/litre. A significant reduction in the specific activities of
NADH oxidase and succinooxidase by the addition of chlordecone at
0.49 mg/litre indicated that chlordecone can inhibit electron
transport.
8.4. Bioaccumulation and Biomagnification
Data on the bioconcentration of chlordecone are given in Table
8. It should be noted that none of the exposures were representative
of realistic environmental levels. Bioaccumulation in detritus,
such as decomposing Spartina cyanosuroide, was demonstrated by
Odum & Drifmeyer (1978). As detritus is a major energy source in
aquatic environments, this could represent an important entrance
for chlordecone into aquatic food webs. Both aquatic invertebrates
and fish bioaccumulate chlordecone to very high levels. Depuration
is slow in fish, thus residues tend to be high. Levels of chlordecone
accumulated in edible fillets were almost the same as the whole
body concentrations in sheepshead minnows and spot; therefore one
of the largest residue reserves in contaminated fish is in the
edible portion (Bahner et al., 1977).
Residues were higher in female sheepshead minnows than in males
(Bahner et al., 1977), and residues in juveniles tended to increase
with increasing concentrations of chlordecone in the water (Hansen
et al., 1976). When chlordecone was fed to juvenile spot for 28
days, the body burden of chlordecone increased additively and
equilibrium was not attained (Stehlik & Merriner, 1983). Chlordecone
accumulation in an estuarine food chain (composed of green algae,
oysters, mysids, grass shrimps, sheepshead minnows, and spot)
occurred at concentrations as low as 0.023 µg/litre (Bahner et al.,
1977). All species had equilibrated tissue concentrations of
chlordecone 8 - 17 days after the beginning of the exposure.
Clearance of chlordecone from oysters was rapid; levels were non-
detectable, 7 - 20 days after exposure ceased. Clearance was slow
in shrimp and fish, with tissue levels of chlordecone decreasing by
30 - 50% in 24 - 28 days. When oysters were fed chlordecone-
contaminated algae, the maximum overall accumulation and transfer
of chlordecone (or "food-chain potential") from water to algae and
then to oysters was 2.1 (Bahner et al., 1977). However, the
transfer potential (transfer from one trophic level to the next)
from algae to oysters was only 0.007; therefore, transfer of
chlordecone from algae to oyster and retention in oyster were
inefficient. When spot were fed mysids that had eaten chlordecone-
contaminated brine shrimp, the food-chain potential from water to
brine shrimp to mysids and finally to fish ranged from 3.9 to 10.5.
The transfer potential from shrimp to mysids was 0.53 and from
mysids to spot, 0.85. This indicated that much of the chlordecone
was being transferred through the trophic levels.
No data are available on the bioconcentration of chlordecone
by terrestrial organisms.
Table 8. Bioaccumulation of chlordecone
-------------------------------------------------------------------------------------------------------
Organism Temp Salinity Flow/ Bioconc. Exposure Time Reference
(°C) o/oo stat factor concentration
(BCF) (µg/litre)
-------------------------------------------------------------------------------------------------------
algae, 19.5- 30 stat 230-800 100 24 h Walsh et al. (1977)
unicellular 20.5
oyster 9354 0.03 19 d Bahner et al. (1977)
(Crassostrea 9278 0.39 21 day
virginica)
grass shrimp 698 12-121 96 h Schimmel & Wilson
(Palaemonetes (425-933) (1977)
pugio) 5127 0.023 28 day Bahner et al. (1977)
11425 0.4 28 day Bahner et al. (1977)
spot 3217 0.029 30 day Bahner et al. (1977)
(Leiostomus
xanthurus)
spot 1120 1.5 96 h Bahner et al. (1977)
(Leiostomus
xanthurus)
fathead minnow flow 16600 0.004 56 day Huckins et al. (1982)
(Pimephales
promelas)
sheepshead minnow, 28-32 11-31 flow 1800 0.041 life Goodman et al. (1982)
juv 21-day cycle test
(Cyprinodon
variegatus
sheepshead minnow, 28-32 11-31 flow 2400 0.041 life Goodman et al. (1982)
juv 42-day cycle test
(Cyprinodon
variegatus)
Table 8. (contd.)
-------------------------------------------------------------------------------------------------------
Organism Temp Salinity Flow/ Bioconc. Exposure Time Reference
(°C) o/oo stat factor concentration
(BCF) (µg/litre)
-------------------------------------------------------------------------------------------------------
sheepshead minnow 28-32 11-31 flow 3900 0.041 life Goodman et al. (1982)
adult male cycle test
(Cyprinodon
variegatus)
sheepshead minnow 28-32 11-31 flow 3700 0.041 life Goodman et al. (1982)
adult female cycle test
(Cyprinodon
variegatus)
sheepshead minnow, 28-32 11-31 flow 2900 0.041 life Goodman et al. (1982)
embryos cycle test
(Cyprinodon
variegatus)
sheepshead minnow, 28-32 11-31 flow 2400 0.041 life Goodman et al. (1982)
juvenile progeny cycle test
(Cyprinodon
variegatus)
-------------------------------------------------------------------------------------------------------
8.5. Population and Community Effects
Chlordecone is strongly adsorbed on sediment. Effects on
aquatic organisms are therefore partly from material in the water
and partly from material obtained from sediment. D'Asaro & Wilkes
(1982) examined the effects of sediments, previously exposed to
chlordecone at a known concentration, and of James River sediments
contaminated with chlordecone, on an estuarine community established
in aquaria supplied with non-filtered sea water. Mysid shrimps
showed a dose-related mortality rate, when exposed to sediments
previously equilibrated at 0.1, 1.0, or 10 µg chlordecone/litre.
Mysids were not affected by James River sediment. Oysters showed
dose-dependent reduced shell growth, when exposed to chlordecone-
equilibrated sediments, and also responded adversely to river
sediment. Lugworms Arenicola cristata disappeared from aquaria
after 28 days of treatment with sediment exposed to 10 µg
chlordecone/litre, though numbers were not affected by lower doses.
Both lugworms and oysters concentrated chlordecone from the
sediment.
8.6. Effects on the Abiotic Environment
No data are available on the effects of chlordecone on the
abiotic environment.
8.7. Appraisal
As actual levels of chlordecone in natural waters are extremely
low, because most of the chlordecone is transferred rapidly to
sediments, bioconcentration and toxicity test levels are often
unrealistically high. However, bearing in mind the potential for
bioaccumulation, data suggest that chlordecone is both acutely and
chronically toxic for aquatic organisms. A major omission in the
aquatic toxicity data is the toxicity of chlordecone for detritus
feeders that will be exposed to significant concentrations in
contaminated sediments. Exposure of the lowest level of the
aquatic food chain to concentrations of chlordecone above a
threshold of 0.35 - 1 mg/litre will cause disturbance or destruction,
sufficient to affect productivity at other levels of the food chain.
Few data are available on the sublethal effects of chlordecone on
aquatic organisms. In fish, such effects include: retardation of
growth, which will ultimately affect fecundity, scoliosis, inhibition
of ATPase; and stimulation of some immune response. Juvenile fish
appear to be less sensitive to chlordecone than adults.
Chlordecone appears to have little effect on soil
microorganisms at concentrations that would result from
agricultural use. However, discharges directly into sewage
systems are highly toxic for sludge microbes. Agricultural
application rates cause little acute toxicity to non-target
invertebrates or birds, but chlordecone at higher dosages can
have pronounced effects on many reproductive variables in birds.
No data are available on effects on amphibia, reptiles, or non-
laboratory mammals.
9. PREVIOUS EVALUATIONS OF CHLORDECONE BY INTERNATIONAL BODIES
IARC (1979) evaluated the carcinogenic hazard resulting from
exposure to chlordecone and concluded that "there is sufficient
evidence for its carcinogenicity in rats and mice. In the absence
of adequate data in humans, it is reasonable for practical
purposes to regard chlordecone as if it presented a carcinogenic
risk to humans".
No acceptable daily intake (ADI) for chlordecone has been
proposed by FAO/WHO.
In recent years, official registrations for a number of uses
of chlordecone have been withdrawn in certain countries for
various reasons (IRPTC, 1983).
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the
Federal Republic of Germany, India, Japan, Kenya, Mexico, Sweden,
the United Kingdom, the USA, and the USSR) and the EEC can be
found in the International Register of Potentially Toxic
Chemicals Legal File (IRPTC, 1983).
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Chlordecone Toxicity
Chlordecone is moderately toxic in acute studies on rats,
i.e. the oral LD50 values range from 95 to 132 mg/kg body weight.
It can enter the body via ingestion, inhalation, and via the
skin. It is not metabolized to any significant extent. It
bioaccumulates mainly in the liver, and it is excreted very
slowly via the faeces.
Toxic effects include neurological symptoms, especially
tremors, liver hypertrophy with enzyme induction, centrilobular
hepatocellular necrosis, and hepatobiliary dysfunction. It can
impair reproduction (mouse, 10 mg/kg diet or 0.5 mg/kg body
weight per day) and is fetotoxic (rat, 2 mg/kg body weight per
day).
Chlordecone was not generally active in short-term tests for
genetic activity. There is sufficient evidence of its
carcinogenicity for mice and rats.
Careless occupational handling in a manufacturing plant
caused a series of poisonings with neurological symptoms,
especially nervousness and tremors, oligospermia, and joint
pains.
10.2. Exposure to Chlordecone
Exposure of the general population through the normal use of
chlordecone can be regarded as minimal and is mainly related to
residues in food.
Small children may be exposed when playing with insect traps.
10.3. Effects on the Environment
The environmental hazard posed by chlordecone is associated
with its stability and persistence in sediments, which provide a
long-term source of contamination, in conjunction with its massive
bioaccumulation in aquatic food chains. One of the largest
reserves of chlordecone in food is in the edible portion of
contaminated fish. Although chlordecone has a low solubility in
water, between 0.35 and 1 mg/litre is sufficient to reduce algal
growth, thereby affecting productivity at other trophic levels.
Chlordecone is acutely and chronically toxic for aquatic
invertebrates and causes loss of equilibrium, reduction in
reproductive success, and decreased shell growth at sublethal
concentrations. Reduction in mysid populations due to low-level
chlordecone contamination has important consequences for fish
productivity. Symptoms of exposure range from diminished activity
and emaciation to abnormal development and death.
The few data available indicate that chlordecone is not
acutely toxic for terrestrial invertebrates. Subacute doses of
chlordecone induce significant toxic effects in birds including
tremors, liver damage, and reproductive failure.
Excretion of chlordecone is extremely slow.
10.4. Conclusions
1. Serious illness has been suffered by workers occupationally
over-exposed to chlordecone.
2. Based on the findings in mice and rats, this chemical should
be considered, for practical purposes, as being potentially
carcinogenic for human beings.
3. For the above reason, reservations must remain about the
occurrence of residues of chlordecone in food.
4. Adverse effects on the organisms studied, as well as
persistence, suggest that chlordecone presents a long-term
hazard for the environment.
5. Taking into account these considerations, it is felt that the
use of this chemical should be discouraged, except where there
is no adequate alternative.
REFERENCES
ADIR, J., CAPLAN, Y.H., & THOMPSON, B.C. (1978) Kepone serum
half-life in humans. Life Sci., 22: 699-702.
AGARWAL, A.K. & MEHENDALE, H.M. (1982a) Potentiation of CCl4
hepatotoxicity and lethality by chlordecone in female rats.
Toxicology, 26: 231-242.
AGARWAL, A.K. & MEHENDALE, H.M. (1982b) Potentiation of
bromotrichloromethane hepatotoxicity and lethality by
chlordecone pre-exposure in the rat. Fundam. appl. Toxicol.,
2: 161-167.
ALLAN, J.D. & DANIELS, R.E. (1982) Life table evaluation of
chronic exposure of Eurytemora affinis (Copepoda) to Kepone.
Mar. Biol. (Berlin), 66: 179-184.
ALLIED CHEMICAL CORPORATION (1963) Determination of kepone
(GC-1189). Residues in crops, 26 June, Morristown, New Jersey,
Allied Chemical Corporation.
ALLIED CHEMICAL CORPORATION (1966) Assay method for kepone,
8 March, Morristown, New Jersey, Allied Chemical Corporation.
ALLIED CHEMICAL CORPORATION (1977) Summary of basic
information - Kepone insecticide, Morristown, New Jersey,
Allied Chemical Corporation (Product Information Technical
Data Sheet 1189-01-1012. Source 120).
ANDERSON, B.M. & NOBLE, C., Jr (1977) In vitro inhibition of
lactate dehydrogenases by Kepone. J. agric. food Chem., 25:
28-31.
ANDERSON, B.M., NOBLE, C., Jr, & GREGORY, E.M. (1977) Kepone
inhibition of malate dehydrogenases. J. agric. food Chem., 25:
485-489.
ANDERSON, B.M., KOHLER, S.T., & YOUNG, R.W. (1978)
Interactions of Kepone with rabbit muscle lactate
dehydrogenase. J. agric. food Chem., 26: 130-133.
ANONYMOUS (1976) Report on (NCI) carcinogenesis bioassay of
technical grade chlordecone (Kepone). Am. Ind. Hyg. Assoc. J.,
37: 680-681.
ANONYMOUS (1977) Getting Kepone out of the body. Med. World
News, 18: 32.
ANONYMOUS (1978a) Kepone/mirex/hexachlorocyclopentadiene -
an environmental assessment, Washington DC, US NRC, US
Department of Commerce (US NTIS, PB 280-289).
ANONYMOUS (1978b) UK prohibits burning of Kepone. Chem.
Aust., 45: 142.
ATKINS, E.L. & ANDERSON, L.D. (1962) DDT resistance in honey
bees. J. econ. Entomol., 55: 791-792.
ATKINS, E.L. Jr, GRAYWOOD, E.A., & MACDONALD, R.L. (1973)
Toxicity of pesticides and other agricultural chemicals to
honey bees, California, 38 pp (Laboratory Studies, Calif.
Agric., Extension M-16).
BAGGETT, J.McC., KLEIN, R.L., MEHENDALE, H.M., & THURESON-
KLEIN, A.K. (1977) Acute Kepone treatment of rats: a
biochemical and ultrastructural study. Pharmacologist, 19:
199-205.
BAGGETT, J.McC., THURESON-KLEIN, A., & KLEIN, R.L. (1980)
Effects of chlordecone on the adrenal medulla of the rat.
Toxicol. appl. Pharmacol., 52: 313-322.
BAHNER, L.H., WILSON, A.J., Jr, SHEPPARD, J.M., PATRICK, J.M.,
Jr, GOODMAN, L.R., & WALSH, G.E. (1977) Kepone
bioconcentration, accumulation, loss, and transfer through
estuarine food chains. Chesapeake Sci., 18: 299-308.
BAKER, R.C., COONS, L.B., MAILMAN, R.B., & HODGSON, E.
(1972) Induction of hepatic mixed function oxidases by the
insecticide mirex. Environ. Res., 5: 418-424.
BENTLEY, R.E. (1975) Acute toxicity of kepone to Bluegill
Lepomis macrochirus and Rainbow Trout Salmo gairdneri, 7pp
(Toxicity Report submitted to Allied Chemical Corporation,
Morristown, New Jersey, EG & G Environmental Consultants,
Bionomics, Wareham, Massachusetts).
BLANKE, R.V., FARISS, M.W., GRIFFITH, F.D., Jr, & GUZELIAN,
P.S. (1977) Analysis of chlordecone (Kepone) in biological
specimens. J. anal. Toxicol., 1: 57-62.
BLANKE, R.V., FARISS, M.W., GUZELIAN, P.S., PATERSON, A.R., &
SMITH, D.E. (1978) Identification of a reduced form of
chlordecone (Kepone) in human stool. Bull. environ. Contam.
Toxicol., 20: 782-785.
BOYLAN, J.J., EGLE, J.L., & GUZELIAN, P.S. (1977)
Stimulation of chlordecone (Kepone) excretion by
cholestyramine in rats. Pharmacologist, 19: 210.
BOYLAN, J.J., COHN, W.J., EGLE, J.L., BLANKE, R.V., &
GUZELIAN, P.S. (1979) Excretion of chlordecone by the
gastrointestinal tract: evidence for a non-biliary mechanism.
Clin. Pharmacol. Ther., 25: 579-585.
BRAY, T.J. (1975) Health hazard. Chemical firm's story
underscores problems of cleaning up plants. Wall Street J.,
December 2.
BREWERTON, H.V. & SLADE, D.A. (1964) Kepone residues on
apples. N.Z. J. agric. Res., 7: 647-653.
BUCKLER, D.R., WITT, A., MAYE R, F.L., & HUCKINS, J.N. (1981)
Acute and chronic effects of Kepone and mirex on the fathead
minnow. Trans. Am. Fish. Soc., 110: 270-280.
BULGER, W.J., MUCCITELLI, R.M., & KUPFER, D. (1979) Studies
on the estrogenic activity of chlordecone (Kepone) in the rat:
effects on uterine estrogen receptor. Mol. Pharmacol., 15:
515-524.
BUTLER, P.A. (1963) A review of fish and wildlife service
investigations during 1961 and 1962. In: George, J.C., ed.
Commercial Fisheries Investigations, Pesticide-Wildlife
Series, US Department of the Interior, Fish and Wildlife
Services, Vol. 167, pp. 11-25.
CANNON, S.B. & KIMBROUGH, R.D. (1979) Short-term chlordecone
toxicity in rats including effects on reproduction,
pathological organ changes and their reversibility. Toxicol.
appl. Pharmacol., 47: 469-476.
CANNON, S.B., VEAZEY, J.M., Jr, JACKSON, R.S., BURSE, V.W.,
HAYES, C., STRAUB, W.E., LANDRIGAN, P.J., & LIDDLE, J.A.
(1978) Epidemic Kepone poisoning in chemical workers. Am. J.
Epidemiol., 107: 529-537.
CARVER, R.A., BORSETTI, A.P., & KAMPS, L.R. (1978)
Gas-liquid chromatographic determination of Kepone residues in
finfish, shellfish, and crustaceans. J. Assoc. Off. Anal.
Chem., 61: 877-883.
CHADWICK, R.W., COPELAND, M.F., & ROSENSTEIN, L. (1979)
Effect of Kepone exposure during gestation and lactation on
the metabolism of lindane by weanling rats. Toxicology Lett.,
4: 247-252.
CHERNOFF, N. & ROGERS, E.H. (1976) Fetal toxicity of Kepone
in rats and mice. Toxicol. appl. Pharmacol., 38: 189-194.
CHERNOFF, N., LINDER, R.E., SCOTTI, T.M., ROGERS, E.H.,
CARVER, B.D., & KAVLOCK, R.J. (1979a) Fetotoxicity and
cataractogenicity of mirex in rats and mice with notes on
Kepone. Environ. Res., 18: 257-269.
CHU, I., VILLENEUVE, D.C., VALLI, V.E., & REYNOLDS, L.M.
(1980) Short-term study of the combined effects of mirex,
photomirex, and Kepone to halogenated biphenyls in the rat. J.
Toxicol. environ. Health, 6: 421-432.
CIANFLONE, D.J., HEWITT, W.R., VILLENEUVE, D.C., & PLAA, G.L.
(1980) Role of biotransformation of the alterations of
chloroform hepatotoxicity produced by Kepone and mirex.
Toxicol. appl. Pharmacol., 53: 140-149.
COHN, W.J., BLANKE, R.V., GRIFFITH, F.D., Jr, & GUZELIAN,
P.S. (1976) Distribution and excretion of Kepone in humans.
Gastroenterology, 71: A8-901.
COHN, W.J., BOYLAN, J.J., BLANKE, R.V., FARISS, M.W., HOWELL,
J.R., & GUZELIAN, P.S. (1978) Treatment of chlordecone
(Kepone) toxicity with cholestyramine. Results of a controlled
clinical trial. N. Engl. J. Med., 298: 243-248.
COUCH, J.A., WINSTEAD, J.T., & GOODMAN, L.R. (1977)
Kepone-induced scoliosis and its histological consequences in
fish. Science, 197: 585-587.
CURTIS, L.R. & MEHENDALE, H.M. (1979) The effects of Kepone
pretreatment on biliary excretion of xenobiotics in the male
rat. Toxicol. appl. Pharmacol., 47: 295-303.
CURTIS, L.R. & MEHENDALE, H.M. (1980) Specificity of
chlordecone-induced potentiation of carbon tetrachloride
hepatotoxicity. Drug Metab. Dispos., 8: 23-27.
CURTIS, L.R., WILLIAMS, W.L., & MEHENDALE, H.M. (1979)
Potentiation of the hepatotoxicity of carbon tetrachloride
following pre-exposure to chlordecone (Kepone) in the male
rat. Toxicol. appl. Pharmacol., 51: 283-293.
D'ASARO, C.N. & WILKES, F.G. (1982) Cycling of xenobiotics
through marine and estuarine sediments, 51 pp (US EPA Report
No. 600/3-82-074; PB82-239252).
DAWSON, G.W. (1978) Kepone mitigation feasibility report:
Appendix A: The feasibility of mitigating kepone contamination
in the James River Basin (US NTIS PB Report, PB 286 085).
DESAIAH, D. & KOCH, R.B. (1975) Inhibition of ATPases
activity in channel catfish brain by Kepone and its reduction
product. Bull. environ. Contam. Toxicol., 13: 153-158.
DESAIAH, D., HO, I.K., & MEHENDALE, H.M. (1977) Effects of
Kepone and mirex on mitochondrial magnesium ion-dependent
ATPhase activity in rat liver. Toxicol. appl. Pharmacol., 39:
219-228.
DEWITT, J.B., CRABTREE, D.G., FINLEY, R.B. & GEORGE, J.L.
(1962) Effects on wildlife. Effects of pesticides on fish and
wildlife: a review of investigations during 1960, Washington
DC, US Department of the Interior, Bureau of Fish and Wildlife
Services, pp 4-15, 31-33, 49-52 (Circular No. 143).
DIETZ, D.D. & McMILLAN, D.E. (1978) Effects of mirex and
Kepone on schedule controlled responding. Pharmacologist, 20:
225.
EGLE, J.L., Jr, FERNANDEZ, S.B., GUZELIAN, P.S., & BORZELLECA,
J.F. (1978) Distribution and excretion of chlordecone
(Kepone) in the rat. Drug Metab. Dispos., 6: 91-95.
END, D.W., CARCHMAN, R.A., & DEWEY, W.L. (1979) Disruption
of rat brain synaptosomal membranes by the neurotoxic
insecticide Kepone. Fed. Proc., 38: 845.
EPSTEIN, S.S. (1978) Kepone-hazard evaluation. Sci. total
Environ., 9: 1-62.
ERBEN, H.K. (1972) [Ultrastructure and wall thickness of
pathological eggshells.] Abkhandl. Math. Naturwissensch.,
Lichen Klasse, 6: 192-216 (in German, Pestic. Abstr., 7:
74-0598-602).
EROSCHENKO, V.P. & WILSON, W.O. (1975) Cellular changes in
the gonads, livers and adrenal glands of Japanese quail as
affected by the insecticide Kepone. Toxicol. appl. Pharmacol.,
31: 491-504.
FABACHER, D.L. & HODGSON, E. (1976) Induction of hepatic
mixed-function oxidase enzymes in adult and neonatal mice by
Kepone and mirex. Toxicol. appl. Pharmacol., 38: 71-77.
FELDMANN, E.G. (1976) The lesson from Kepone. J. pharmacol.
Sci., 65: 1.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14: 5-534.
GAWAAD, A.A.A., HAMMAD, M.H., & EL-GAYAR, F.H. (1972a)
Studies on soil insecticides. X. Effect of some soil
insecticides on soil microorganisms. Zentr. Bakteriol.
Parasitenk. Infect and Hygien. Abt 2, 127: 290-295.
GAWAAD, A.A.A., HAMMAD, M.H., & EL-GAYAR, F.H. (1972b)
Studies on soil insecticides. XI. Effect of some soil
insecticides on the nitrogen transformation in treated soils.
Zentr. Bakteriol. Parasitenk. Infect and Hygien. Abt 2, 127:
296-300.
GAWAAD, A.A.A., EL-GAYAR, F.H., & KHADR, A.A. (1976) Effect
of certain soil insecticides on the germination of cotton
seeds, growth, dry weight, cotton yield and the quality of
yield. Biol. Abstr., 61: 5840.
GEER, R.D. (1978) Predicting the anaerobic degradation of
organic chemical pollutants in waste water treatment plants
from their electrochemical reduction behavior, Bozeman,
Montana, Montana University Joint Water Resources Research
Center (US NTIS Report No. ISS-MUJWRRC-95 MTIS PB-289224).
GILBERT, E.E. & GIOLITO, S.L. (1952) US Patent 2.616.825 and
US Patent 2.616.928, 4 November, to Allied Chemical and Dye
Corporation.
GOOD, E.E., WARE, G.W., & MILLER, D.F. (1965) Effects of
insecticides on reproduction in the laboratory mouse. I.
Kepone. J. Econom. Entomol., 58: 754-757.
GOODMAN, L.R., HANSEN, D.J., MANNING, C.S., & FAAS, L.F.
(1982) Effects of Kepone on the sheepshead minnow in an
entire lifecycle toxicity test. Arch. environ. Contam.
Toxicol., 11: 335-342.
HAMMOND, B., BAHR, J., DIAL, O., MCCONNEL, J., & METCALF, R.
(1978) Reproductive toxicology of mirex and Kepone. Fed.
Prod. Fed. Am. Soc. Exp. Biol., 37: 501.
HAMMOND, B., KATZENELLENBOGEN, B.S., KRAUTHAMMER, N., &
MCCONNELL, J. (1979) Estrogenic activity of the insecticide
chlordecone (Kepone) and interaction with uterine estrogen
receptors. Proc. Natl Acad. Sci., 76: 6641-6645.
HANSEN, D.J., GOODMAN, L.R., & WILSON, A.J., Jr (1976)
Kepone: Chronic effects on embryo, fry, juvenile and adult
sheepshead minnow Cyprinodon variegatus. In Hansen, D.J., ed.
Prepublications of kepone in the marine environment, Gulf
Breeze, Florida, US EPA Office of Research and Development,
Environmental Research Laboratory, 28 pp.
HARRIS, R.L., HUGGETT, R.J., & SLONE, H.D. (1980)
Determination of dissolved Kepone by direct addition of XAD-2
resin to water. Anal. Chem., 52: 779-780.
HEATH, C.W., Jr (1978) Industrial toxins and the community,
Washington DC, US Department of Health, Education and Welfare,
pp. 259-261 (US DHEW (NIOSH) Publication No. 78-169).
HEITMULLER, T. (1975) Acute toxicity of kepone to fiddler
crabs (Uca pugilator), 5 pp (Toxicity Test Report, submitted
to Allied Chemical Corporation, Morristown, New Jersey, EG &
G, Inc., Bionomics, Marine Research Laboratory, Pensacola,
Florida).
HUBER, J.J. (1965) Some physiological effects of the
insecticide Kepone in the laboratory mouse. Toxicol. appl.
Pharmacol., 1: 516-524.
HUCKINS, J.N., STALLING, D.L., PETTY, J.D., BUCKLER, D.R., &
JOHNSON, B.T. (1982) Fate of Kepone and mirex in the aquatic
environment. J. agric. food Chem., 30: 1020-1027.
HUGGETT, R.D., HAVEN, D., & NICHOLS, M. (1977) Kepone
sediment relationships in James River (Abstract) (Report to US
EPA Gulf Breeze Laboratory).
IARC (1979) Some halogenated hydrocarbons, Lyons,
International Agency for Research on Cancer (Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Vol. 20).
INFORMATION CANADA (1973) Guide to chemicals used in crop
protection, Vol. 6, p. 96.
IRPTC (1983) Legal file, Vols 1 & 2, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
KAWATSKI, J.A. & HECKER, R.J. (1979) Kepone inhibition of
succinic acid dehydrogenase in mouse liver mitochondrial
preparations. Proc. N. D. Acad. Sci., 33: 24.
KLINGENSMITH, J.S. & MEHENDALE, H.M. (1982a) Chlordecone-
induced mobilization of body fat and lipolysis in the male
rat. J. Toxicol. environ. Health, 10: 121-129.
KLINGENSMITH, J.S. & MEHENDALE, H.M. (1982b) Potentiation of
CCl4 lethality by chlordecone. Toxicol. Lett., 11: 149-154.
LANGFORD, H.D. (1978) Kepone, mirex pesticide residues
persist, full effects unknown. News Rep., 28: 1, 4-5.
LARSON, P.S., EGLE, J.L., Jr, HENNIGAR, G.R., LANE, R.W., &
BORZELLECA, J.F. (1979b) Acute, subchronic, and chronic
toxicity of chlordecone. Toxicol. appl. Pharmacol., 48: 29-41.
LEWIS, R.G. & LEE, R.E. (1976) Air pollution from
pesticides: sources, occurrence and dispersion. In: Air
pollution from pesticides and agricultural processes, Boca,
Florida, CRC Press Inc.
MADY, N., SMITH, D., SMITH, J., & WEZWICK, C. (1979)
Analysis of Kepone in biological samples. NBS (US) Spec.
Publ., 519: 341-343.
MAHAFFEY, W.R., PRITCHARD, P.H., & BOURQUIN, A.W. (1982)
Effects of Kepone on growth and respiration of several
estuarine bacteria. Appl. environ. Microbiol., 43: 1419-1424.
MARTINEZ, A.J., TAYLOR, J.R., ISAACS, E., DYCK, P.J., & HOUFF,
S.A. (1976) Kepone poisoning ultrastructure of nerves and
skeletal muscles. J. Neuropathol. exp. Neurol., 35: 323.
MARTINEZ, A.J., TAYLOR, J.R., DYCK, P.J., HOUFF, S.A., &
ISAACS, E. (1978) Chlordecone intoxication in man: II.
ultrastructure of peripheral nerves and skeletal muscle.
Neurology, 28: 631-635.
McFARLAND, L.Z. & LACY, P.B. (1969) Physiologic and
endocrinologic effects of the insecticide Kepone in the
Japanese quail. Toxicol. appl. Pharmacol., 15: 441-450.
MEHENDALE, H.M. (1979) Modification of hepatobiliary
function by toxic chemicals. Fed. Proc. Fed. Am. Soc. Exp.
Biol., 38: 2240-2245.
MEHENDALE, H.M, TAKANAKA, A., DESAIAH, D., & HO, I.K. (1978)
Effect of pre-exposure to Kepone on hepatic mixed-function
oxidases in the female rat. Toxicol. appl. Pharmacol., 44:
171-180.
MEYERS, S.P., GAMBRELL, & DAY, (1982) Determination of
environmental impact of several substitute chemicals in
agricultural affected wetlands, Washington DC, US
Environmental Protection Agency, 150 pp (US EPA Report No.
EPA-600/4-82-052; PB82-242017).
MOSEMAN, R.F., CRIST, H.L., EDGERTON, T.R., & WARD, M.K.
(1977) Electron capture gas chromatographic determination of
Kepone residues in environmental samples. Arch. environ.
Contam. Toxicol., 6: 221-231.
MOSEMAN, R.F., WARD, M.K., CRIST, H.L., & ZEHR, R.D. (1978)
A micro derivatization technique for the confirmation of trace
quantities of Kepone. J. agric. food Chem., 26: 965-968.
MOTL, M.L. (1977) EPA develops process to destroy Kepone.
Environ. Manage., 1: 491-493.
NABER, E.C. & WARE, G.W. (1965) Effect of Kepone and mirex
on reproductive performance in the laying hen. Poult. Sci.,
44: 875-880.
NIMMO, D.R., BAHNER, L.H., RIGBY, R.A., SHEPPARD, J.M., &
WILSON, A.J., Jr (1977) Mysidopsis bahia: An estuarine
species suitable for life cycle bioassays to determine
sublethal effects of a pollutant. In: Prepublications of
kepone in the marine environment, Gulf Breeze, Florida, US EPA
Office of Research and Development, Environmental Research
Laboratory.
NIOSH (1977) NIOSH manual of analytical methods, Part 1,
NIOSH monitoring methods, Vol. 1, 2nd ed., Washington DC,
National Institute for Occupational Safety and Health, pp
225-1-256-6 (Method No. P Cam 225, US DHEW (NIOSH) Publication
No. 77-157B).
NIOSH (1978) Registry of toxic effects of chemical
substances, Washington DC, US Department of Health, Education
and Welfare, p. 751.
ODUM, W.E. & DRIFMEYER, J.E. (1978) Sorption of pollutants
by plant detritus: a review. Environ. health Perspect., 27:
133-137.
ORNDORFF, S.A. & COLWELL, R.R. (1980a) Microbial
transformation of Kepone. Appl. environ. Microbiol., 39:
398-406.
ORNDORFF, S.A. & COLWELL, R.R. (1980b) Distribution and
characterization of Kepone-resistant bacteria in the aquatic
environment. Appl. environ. Microbiol., 39: 611-622.
PATE, J.B. & TABOR, E.C. (1962) Analytical aspects of the
use of glass fiber filters for the collection and analysis of
atmospheric particulate matter. Am. Ind. Hyg. Assoc. J., 23:
145-156.
PORTIER, R.J. & MEYERS, S.P. (1982) Microbial responses to
sequential and differential application of a xenobiotic in
aquatic microcosms. Abst. Annu. Meet. Am. Soc. Microbiol., 82:
Abstract Q63.
PROHST, G.S., McMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP,
J.K., & NEALS S.B. (1981) Chemically-induced unscheduled DNA
synthesis in primary rat hepatocyte cultures. A comparison
with bacterial mutagenicity using 218 compounds. Environ.
Mutat., 3: 11-32.
PROSZYNSKA, B. (1977) [Method for determining despirol
residues in potatoes.] Roozn. Panstw. Zakl. Hig., 28: 201-207
(in Polish).
REITER, L. & KIDD, K. (1978) The behavioral effects of
subacute exposure to Kepone or mirex on the weanling rat.
Toxicol. appl. Pharmacol., 45: 357.
REITER, L., KIDD, K., LEDBETTER, G., CHERNOFF, N., & GRAY,
L.E., Jr (1977) Comparative behavioural toxicology of mirex
and Kepone in the rat. Toxicol. appl. Pharmacol., 41: 143.
REUBER, M.D. (1977) Kepone carcinogenicity affirmed by
Reuber. Pest. Toxic Chem. News, 5: 6-7.
REUBER, M.D. (1978) Carcinogenicity of Kepone. J. Toxicol.
environ. Health, 4: 895-911.
REUBER, M.D. (1979) The carcinogenicity of Kepone. J.
environ. Path. Toxicol., 2: 671-686.
ROBERTS, M.H., Jr & BENDL., R.E. (1982) Acute toxicity of
Kepone to selected freshwater fish. Estuaries, 5: 158-164.
ROSENSTEIN, L., BRICE, A., ROGERS, N., & LAWRENCE, S. (1977)
Neurotoxicity of Kepone in perinatal rats following in utero
exposure. Toxicol. appl. Pharmacol., 41: 142-143 (Abstract 28).
SALEH, F.Y. & LEE, G.F. (1978) Analytical methodology for
Kepone in water and sediment. Environ. Sci. Technol., 12:
297-301.
SALEH, F.Y., LEE, G.F., & BUTLER, J.S. (1978) Kepone and
other selected chlorinated hydrocarbon pesticides and PCBs
behavior during hydraulic dredging of the James River near
Hopewell, Virginia. J. environ. Sci. Health, Part A, A13:
261-294.
SCHIMMEL, S.C. & WILSON, A.J. Jr (1977) Acute toxicity of
Kepone to four estuarine animals. Chesapeake Sci., 18: 224-227.
SHANHOLTZ, M.I. (1976) Emergency rule, Virginia State Board
of Health. Prohibiting the taking of crabs from the James
River and its tributaries and the taking of fish for human
consumption, Richmond, Virginia, Virginia Department of
Health, 3 pp.
SIMON, G.S., KIPPS, B.R., TARDIFF, R.G., & BORZELLECA, J.F.
(1978) Failure of Kepone and hexachlorobenzene to induce
dominant lethal mutations in the rat. Toxicol. appl.
Pharmacol., 45: 330-331.
SNEGAROFF, J. (1977) Organochlorine insecticidal residues in
the soil and rivers of banana-growing region of Guadeloupe.
Phytiatr. Phytopharm., 26: 251-267.
STEHLIK, L.L. & MERRINER, J.V. (1983) Effects of accumulated
dietary Kepone on spot (Leiostomus xanthurus). Aq.
Toxicol., 3: 345-358.
STERRETT, F.S. & BOSS, C.A. (1977) Careless Kepone.
Environment, 19: 30-37.
SUTA, B.E. (1978) Human population exposures to mirex and
kepone, Springfield, Virginia, US Department of Commerce (US
NTIS PB REP., PB-285, 430).
TAYLOR, J.R., SELHORST, J.B., HOUFF, S.A., & MARTINEZ, A.J.
(1978) Chlordecone intoxication in man. I. Clinical
observations. Neurology, 28: 626-630.
TILSON, H.A., BYRD, M., & RILEY, M. (1979) Neurobehavioral
effects of exposing rats to Kepone via the diet. Environ.
health Perspec., 33: 321.
US EPA (1976a) Preliminary report on Kepone levels found in
environmental samples from the Hopewell, Virginia area,
Research Triangle Park, North Carolina, US Environmental
Protection Agency, Health Effects Research Laboratory.
US EPA (1976b) Review of the Chesapeake Bay Program. Seminar
on kepone held at Virginia Institute of Marine Sciences, 12-13
October, 1976, Research Triangle Park, North Carolina, US
Environmental Protection Agency.
US EPA (1979) Reviews of the environmental effects of
pollutants. 1. Mirex and Kepone, Washington DC, US
Environmental Protection Agency (EPA Report No.
EPA-600/1-78-013; PB80-12595).
US FDA (1977) Compliance program evaluation-FY77 Kepone and
mirex contamination, Washington DC, US Department of Health,
Education and Welfare.
WALSH, G.E., AINSWORTH, K., & WILSON, A.J., Jr (1977)
Toxicity and uptake of Kepone in marine unicellular algae.
Chesapeake Sci., 18: 222-223.
WILLIAMS, G.M. (1980) Classification of genotoxic and
epigenetic hepatocarcinogens using liver culture assays. Ann.
N.Y. Acad. Sci., 349: 273-282.
WINKELHAKE, J.L., VODICNIK, M.J., & TAYLOR, J.L. (1983)
Induction in rainbow trout of an acute phase (C-reactive)
protein by chemicals of environmental concern. Comp. Biochem.
Physiol. C., 74: 55-58.
 Molecular formula: C10Cl10O
CAS chemical name: 1,1a,3,3a,4,5,5,5a,5b,6-decachloro-
octahydro-1,3,4-metheno-2H-cyclo-
buta[cd]pentalen-2-one
Synonyms: decachloro-pentacyclo[5,2,1,02,6,03,
9,O5,8]decan-4-one, dec-
achloro-octahydro-1,3,4-metheno-
2H,5H-cyclobuta[cd]pentalen-2-one
Trade names: GC 1189, Kepone, Merex
CAS registry number:
Molecular formula: C10Cl10O
CAS chemical name: 1,1a,3,3a,4,5,5,5a,5b,6-decachloro-
octahydro-1,3,4-metheno-2H-cyclo-
buta[cd]pentalen-2-one
Synonyms: decachloro-pentacyclo[5,2,1,02,6,03,
9,O5,8]decan-4-one, dec-
achloro-octahydro-1,3,4-metheno-
2H,5H-cyclobuta[cd]pentalen-2-one
Trade names: GC 1189, Kepone, Merex
CAS registry number: 