
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 26
STYRENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR STYRENE
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Uses and sources of exposure
1.1.2. Chemobiokinetics, biotransformation and
biological monitoring
1.1.3. Adverse health effects
1.1.3.1 Acute effects
1.1.3.2 Nervous system effects
1.1.3.3 Genotoxic effects
1.1.3.4 Carcinogenic effects
1.1.3.5 Effects on reproduction
1.2. Recommendations for further studies
2. PROPERTIES AND ANALYTICAL METHODS
2.1. Analytical procedures
2.1.1. Measurement of styrene in air
2.1.2. Measurement of styrene and styrene metabolites
in biological samples
2.1.2.1 Exhaled air
2.1.2.2 Blood
2.1.2.3 Subcutaneous adipose tissue
2.1.2.4 Urine
3. SOURCES OF STYRENE IN THE ENVIRONMENT
3.1. Production of styrene
3.2. Uses
3.3. Main sources of environmental pollution
4. ENVIRONMENTAL EXPOSURE LEVELS
4.1. General environment
4.1.1. Ambient air
4.1.2. Water
4.1.3. Food
4.2. Working environment
4.2.1. Styrene and polystyrene manufacture
4.2.2. Reinforced plastics applications
4.2.3. Styrene-butadiene applications
4.2.4. Summary of occupational exposure
5. CHEMOBIOKINETICS AND METABOLISM
5.1. Uptake
5.1.1. Human studies
5.1.1.1 Uptake by inhalation
5.1.1.2 Uptake from the gastrointestinal tract
5.1.1.3 Uptake through the skin
5.1.2. Experimental animal studies
5.1.2.1 Uptake by inhalation
5.1.2.2 Uptake from the gastrointestinal tract
5.1.2.3 Uptake through the skin
5.2. Distribution and storage
5.2.1. Human studies
5.2.1.1 Controlled human studies
5.2.1.2 Occupational exposure studies
5.2.1.3 General population studies
5.2.2. Experimental animal studies
5.3. Biotransformation
5.4. Elimination
5.4.1. Human studies
5.4.1.1 Controlled human studies
5.4.1.2 Occupational exposure studies
5.4.1.3 General population studies
5.4.2. Experimental animal studies
5.5. Biomonitoring of styrene uptake
6 EFFECTS ON EXPERIMENTAL SYSTEMS
6.1. Haematopoietic and immune systems
6.2. Nervous system and behaviour
6.3. Kidneys and the urinary tract
6.4. Gastrointestinal tract
6.5. Liver
6.6. Cardiovascular system
6.7. Respiratory system
6.8. Endocrine organs
6.9. Carcinogenic effects
6.9.1. Styrene
6.9.1.1 Oral administration
6.9.1.2 Inhalation exposure
6.9.1.3 Pre- and post-natal exposure
6.9.2. Styrene 7,8-oxide
6.9.2.1 Dermal exposure
6.9.2.2 Oral administration
6.9.3. Summary and conclusions
6.10. Genetic effects
6.10.1. Chemical reactivity of styrene and
styrene oxides
6.10.2. Mutagenic effects of styrene and styrene
oxides in bacterial assay systems
6.10.3. Genetic effects of styrene and
styrene 7,8-oxide in eukaryotic non-mammalian systems
6.10.4. Genetic effects of styrene and
styrene 7,8-oxide in mammalian cells in vitro
6.10.5. Genetic effects of styrene and styrene
7,8-oxide in mammalian systems in vivo
6.10.6. Conclusions on the genetic effects of styrene
6.11. Effects on reproductive function and teratogenic effects
7. EFFECTS OF STYRENE IN MAN
7.1. Controlled human studies
7.2. Epidemiological studies
7.2.1. Haematopoietic and immune system
7.2.2. Nervous system
7.2.3. Kidneys and the urinary tract
7.2.4. Gastrointestinal tract
7.2.5. Liver
7.2.6. Cardiovascular system
7.2.7. Respiratory system
7.2.8. Endocrine organs
7.2.9. Carcinogenic effects
7.2.9.1 Summary and conclusions
7.2.10. Genetic effects in somatic cells
7.2.10.1 Structural chromosome aberrations
7.2.10.2 Other indicators of genetic damage
7.2.10.3 Conclusions
7.3. Effects on reproductive function and teratogenic effects
8. EXPOSURE-EFFECT/EXPOSURE-RESPONSE RELATIONSHIPS, AND
EVALUATION OF HEALTH EFFECTS
8.1. Data from experimental animal studies
8.1.1. Metabolic pathways and kinetics
8.1.2. General toxicity
8.1.2.1 Acute toxicity
8.1.2.2 Subacute and chronic toxicity
8.1.3. Genetic effects
8.1.4. Carcinogenic effects
8.2. Human studies
8.2.1. Effects on organs and systems
8.2.2. Genetic effects in somatic cells
8.2.3. Carcinogenic effects
REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in
the criteria documents as accurately as possible without
unduly delaying their publication, mistakes might have
occurred and are likely to occur in the future. In the
interest of all users of the environmental health criteria
documents, readers are kindly requested to communicate any
errors found to the Manager of the International Programme on
Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda
which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in
the criteria documents are kindly requested to make available
to the WHO Secretariat any important published information
that may have inadvertently been omitted and which may change
the evaluation of health risks from exposure to the
environmental agent under examination, so that the information
may be considered in the event of updating and re-evaluation
of the conclusions contained in the criteria documents.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA ON STYRENE
Members
Professor Z. Bardodej, Department of Medical Chemistry, Medical
Faculty of Hygiene, Charles University, Prague, Czechoslovakia
Dr I. Chouroulinkov, Laboratory for Applied Research of
Chemical Carcinogens, Villejuif, Cedex, France
Professor H.A. Greim, Institute of Toxicology and Biochemistry,
Society for Radiation and Environment Research, Neuherberg,
Federal Republic of Germany
Professor M. Ikeda, Tohoku University, School of Medicine,
Sendai, Japan (Chairman)
Professor R.J. Jaeger, Institute of Environmental Medicine, New
York University Medical Centre, New York, USA (Rapporteur)
Dr L.M. Jambaya, Hazardous Substances and Articles Department,
Ministry of Health, Harare, Zimbabwe
Professor N. Loprieno, Institute of Biochemistry, Biophysics
and Genetics, University of Pisa, Pisa, Italy
Professor M. Spasovski, Department of Industrial Toxicology and
Chemistry, Institute of Occupational Health, Sofia, Bulgaria
Dr J.F. Stara, Office of Environmental Criteria and
Assessment, US Environmental Protection Agency, Cincinnati,
Ohio, USA
Dr D. Wasserman, Department of Occupational Health, Hedasha
Medical School, Hebrew University, Jerusalem, Israel
Representatives of Other Organizations
Dr A. Berlin, Commission of the European Communities,
Luxembourg
Dr C.M. Bishop, Commission of the European Communities,
Luxembourg
Professor P. Grasso, European Chemical Industry Ecology and
Toxicology Centre
Dr H. Härkonen, Permanent Commission and International
Association on Occupational Health
Secretariat
Dr H. Vainio, Institute of Occupational Health, Helsinki,
Finland (Temporary Adviser)
Dr F. Valic, World Health Organization, Geneva, Switzerland,
(Secretary)
Dr J.D. Wilbourn, International Agency for Research on Cancer,
Lyons, France
ENVIRONMENTAL HEALTH CRITERIA FOR STYRENE
Further to the recommendations of the Stockholm United
Nations Conference on the Human Environment in 1972, and in
response to a number of World Health Assembly resolutions
(WHA23.60, WHA24.47, WHA25.58, WHA26.68) and the
recommendation of the Governing Council of the United Nations
Environment Programme, (UNEP/GC/10, July 3 1973), a programme
on the integrated assessment of the health effects of
environmental pollution was initiated in 1973. The programme,
known as the WHO Environmental Health Criteria Programme, has
been implemented with the support of the Environment Fund of
the United Nations Environment Programme. In 1980, the
Environmental Health Criteria Programme was incorporated into
the International Programme on Chemical Safety (IPCS). The
result of the Environmental Health Criteria Programme is a
series of criteria documents.
The Institute of Occupational Health (Director,
Dr J. Rantanen), Helsinki, was responsible, as a Lead Institution
of the IPCS, for the preparation of the first and second drafts of
the Environmental Health Criteria document on styrene. Dr H.
Vainio co-ordinated the work.
The Task Group for the Environmental Health Criteria for
Styrene met in Helsinki from 8 to 15 November, 1982. The
meeting was opened by Dr J. Rantanen and Dr F. Valic welcomed
the participants and representatives of other organizations on
behalf of the three organizations co-sponsoring the IPCS
(UNEP/ILO/WHO). The Task Group reviewed and revised the
second draft criteria document and made an evaluation of the
health risks of exposure to styrene.
The efforts of all who helped in the preparation and the
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract
from the National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA - a WHO
Collaborating Centre for Environmental Health Effects.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Uses and sources of exposure
Styrene (ethenylbenzene) is a commercially important
chemical used in the production of polymers, copolymers, and
reinforced plastics. Exposure mainly occurs in industries and
operations using styrene, and industrial sources are the most
likely cause of general population exposure. Other potential
sources of general population exposure include motor vehicle
exhaust, tobacco smoke, and other combustion/pyrolysis
processes. Low-level exposure of the general population can
occur through the ingestion of food products packaged in
polystyrene containers.
General population exposure levels are usually orders of
magnitude lower than occupational exposure levels - though the
latter vary considerably depending on the operations
concerned. While some exposure occurs in styrene/polystyrene
manufacturing plants, the highest levels of exposure are found
in the industries and operations concerned with the
fabrication and application of plastics. Thus, industrial
processes, such as those in the reinforced plastics industry,
require the greatest attention. In addition, clean-up and
maintenance procedures in many related industries may result
in significant exposures.
1.1.2. Chemobiokinetics, biotransformation, and biological
monitoring
The results of controlled laboratory studies on animals
and human beings have shown that uptake of styrene is rapid
and that it is widely distributed throughout the body. Uptake
is mainly via the pulmonary and, to a lesser extent, the
dermal and oral routes.
Styrene is distributed through the whole body and stored
in lipid depots. Its subsequent slow elimination from the
tissue indicates a potential for bioaccumulation following
repeated daily exposure.
Styrene is biotransformed largely via the 7,8-epoxide by
the mixed function oxidase system. The principal urinary
metabolites are alpha-hydroxybenzeneacetic (mandelic) and
phenylglyoxylic acids. Recent evidence suggests that the
pattern of urinary metabolite excretion varies with mammalian
species. Other minor metabolic pathways may also be important
in the toxicological assessment of this compound.
The elimination of styrene and its metabolites appears to
involve a two-compartment kinetic model that becomes
monophasic in experimental animals at high exposure levels.
This suggests the existence of saturable metabolic pathways.
Exposure levels can be assessed by the quantitative analysis of
alveolar air or determination of the urinary metabolites, mandelic
and phenylglyoxylic acid. At present, urinary mandelic acid
appears to be the most reliable biological indicator of exposure in
human beings.
1.1.3. Adverse health effects
1.1.3.1. Acute effects
Exposure levels of 420 mg/m3 (100 ppm) and above cause
irritation of the mucous membranes of the eyes and the upper
respiratory tract in man. Similar effects have been observed in
experimental animals. Exposure of human volunteers to levels
exceeding 840 mg/m3 (200 ppm) resulted in drowsiness, nausea, and
disturbed balance, within a few minutes. Prolonged reaction times
have been reported in connection with short-term exposure of human
volunteers to 840 mg/m3 (200 ppm).
1.1.3.2. Nervous system effects
Epidemiological studies on workers with long-term occupational
exposure to styrene have shown an increased prevalence of abnormal
EEGs associated with urinary mandelic acid concentrations of 700
mg/litre or more; at 1600 mg/litre, a reduction in psychomotor
performance and visuomotor accuracy in psychological tests has been
observed. However, definitive evidence of adverse effects on the
peripheral nervous system is still not available.
1.1.3.3. Genetic effects
When metabolically activated, styrene may be mutagenic and
clastogenic in many experimental systems. Conflicting results
obtained in some in vitro mutagenicity assays, were presumably due
to differences in metabolic activation and inactivation of styrene.
Styrene 7,8-oxide is the main reactive intermediate of styrene
biotransformation. It is an alkylating agent that is mutagenic and
clastogenic in many in vitro test systems.
Several studies have indicated an increased frequency of
structural chromosome aberrations in the peripheral blood
lymphocytes of workers exposed to styrene in the reinforced
plastics industry. Negative results have been reported in workers
employed in the production of styrene monomer and polystyrene,
where exposure to styrene is lower.
Because of the complexity of the evaluation of the total
exposure of workers in the reinforced plastics industry, it has not
been possible to show, unequivocally, that styrene is the cause of
the observed somatic chromosome aberrations. Even though the
biological and health significance of somatic chromosome damage is
not understood at present, the increase in such effects is
considered to be an indicator of possible adverse health effects.
1.1.3.4. Carcinogenic effects
Several case reports and epidemiological studies have
implied an increased risk of cancers of the lymphatic and
haematopoietic systems in workers involved in the manufacture
of styrene and polystyrene and styrene-butadiene rubber.
However, at present, there is insufficient evidence to
establish a direct cause and effect relationship between
styrene exposure and cancer in human beings.
A single experimental study in mice indicated limited
evidence for the carcinogenicity of styrene in this species.
Orally administered styrene 7,8-oxide, the primary metabolite
of styrene, was carcinogenic in rats.
1.1.3.5. Effects on reproduction
Results of a few studies on mammals (rats, mice, and
Chinese hamsters) suggest that inhaled styrene has embryotoxic
effects. Only a limited number of studies on women are
available, with inconclusive results.
1.2. Recommendations for Further Studies
Technological studies are needed to develop methods of
resin production with reduced exposure of workers.
Further elucidation of urinary metabolite excretion
kinetics and their relationship with styrene exposure is
recommended.
Morphological studies of the effects of styrene on the
central and peripheral nervous systems in experimental animals
are needed. Well-conducted human epidemiological investigations,
with monitored individual exposure levels, are necessary to
evaluate nervous system effects and should include
neurophysiological and psychological tests.
Studies on the genetic effects of styrene should be aimed
at defining the relationship between dose and the induction of
chromosome damage in workers with various types of
occupational styrene exposure. More experimental studies on
styrene are needed to verify the suspected role of styrene as
a genetic hazard in the occupational environment.
Research on the health effects of styrene should primarily
be devoted to resolving the issue of carcinogenesis both in
experimental animal studies and in epidemiological studies on
styrene-exposed workers, particularly in industries with high
styrene exposure.
The effects of styrene on the endocrine system and
reproduction should continue to be evaluated by means of
experimental animal studies and epidemiological investigations.
Further validation of experimental methods and analytical
techniques should be undertaken as well as the establishment
of quality control programmes.
2. PROPERTIES AND ANALYTICAL METHODS
Chemical and physical properties of styrenea
Chem. Abstr. Services Reg. No.: 100-42-5
Chem. Abstr. Name: Ethenylbenzene
Synonyms: phenethylene; phenylethene; phenylethylene;
styrol; styrole; styrolene; vinylbenzene;
vinylbenzol; cinnamene; cinnamol
Molecular formula: C8H8
Relative molecular mass: 104.14
Description: Colourless, viscous liquid with a pungent odour
Boiling point: 145.2°C; 33.6°C at 1.33 kPa (10 mmHg)
Freezing point: - 30.63°C
Density: (d420) 0.9060
Refractive index: (nD20) 1.5468
1
Spectroscopy data: lambdamax 245.3 (E = 1514)
1
Solubility: slightly soluble in water (30 mg/100 ml at 20°C);
soluble in ethanol, diethylether and acetone; very
soluble in benzene and petroleum ether
Volatility: Vapour pressure: 0.8666 kPa (6.45 mmHg) at
25°C; 1.33 kPa (10.0 mmHg) at 35°C
Stability: Flash-point, 31°C; polymerizes easily at room
temperature in the presence of oxygen and oxidizes
on exposure to light and air: must be stored
in an inert atmosphere or with added inhibitors
(e.g., 0.001% tertiary butylcatechol)
Autoignition temperature: 490°C
Inflammability range (20°C): 1.1-6.1%
Vapour density: (air = 1) 3.6
Conversion factors: 1 ppm in air = 4.2 mg/m3;
1 mg/m3 in air = 0.24 ppm
-------------------------------------------------------------------
a Chemical and physical properties of the pure substance; from
Foerst (1965), IARC (1979), Iti (1975), and Weast &
Astle (1981).
2.1. Analytical Procedures
Various methods are available for measuring styrene and
styrene metabolites in environmental media and biological
fluids and tissues.
A number of laboratories have shown the reproducibility of
analytical results. However, there have not been any
systematic interlaboratory analysis comparison programmes.
2.1.1. Measurement of styrene in air
Several methods have been developed for the measurement of
styrene vapour in the working environment.
Sampling can be carried out by means of absorption in a
solvent such as ethanol (Yamamoto & Cook, 1968; Leithe, 1971);
adsorption on a solid material such as activated carbon
(NIOSH, 1974; Kalliokoski & Pfäffli, 1975; Burnett, 1976;) or
porous polymer beads (Dietrich et al., 1978) and by grab
sampling either into empty tubes (White et al., 1970) or with
a motor-driven syringe (Bergman, 1977). Passive monitoring
badges can also be used for sampling (Ikeda et al., 1982).
Styrene is recovered from adsorption tubes by solvent
desorption (NIOSH, 1974; Kalliokoski & Pfäffli, 1975) or
heating (Dietrich et al., 1978). Samples taken by a syringe
require prompt analysis. Continuous monitoring of the
airborne styrene concentration is possible using an infrared
spectrometer equipped with a gas cell and pump.
Adsorption tubes have replaced absorption bottles to a
great extent in sampling. They can be incorporated into
small, portable sampling devices that do not contain liquids
and are well adapted for either personnel or area monitoring.
Activated carbon is the most widely used solid adsorbent
(Dietrich et al., 1978). Though styrene polymerizes easily,
samples of styrene on activated carbon are stable, even when
exposed to moderate heat and sunlight (Saalwaechter et al.,
1977). In the standard method of NIOSH (1974), the styrene is
desorbed from the activated carbon tubes with carbon
disulfide, which offers the advantage that the solvent peak is
insignificant when a flame ionization detector is used (McNair
& Bonelli, 1968). The desorption procedure does not remove
all the styrene and the results must be corrected for
desorption efficiency. According to Burnett (1976), the
average desorption efficiency of carbon disulfide for styrene
is 85%. The desorption efficiency varies somewhat from one
batch of activated carbon to another. It is therefore
advisable to determine the desorption efficiency for each
batch of activated carbon or commercial tubes (NIOSH, 1974).
If activated carbon is also added to the standards, the
desorption efficiency correction is not needed (Kalliokoski &
Pfäffli, 1975). Choi & Fung (1979) recommended the use of an
internal standard (ethylbenzene) to avoid the effects caused
by changes in the instrumental conditions. In addition to
carbon disulfide, other solvents, such as dimethyl formamide
(Kalliokoski & Pfäffli, 1975) or cyclohexanone (Saalwaechter
et al., 1977) can be used for desorption.
The use of porous polymer tubes, such as Tenax GC
(Dietrich et al., 1978), with subsequent thermal desorption
and gas chromatographic analysis offers the advantage that the
entire sample can be analysed at one time and in the absence
of the solvent peak. Commercial instrumentation for thermal
desorption is available. Choi & Fung (1979) warn of the
partial polymerization of styrene during thermal desorption.
When styrene is determined by UV-spectrometry, wavelengths of
245 and 290 nm can be used. The former wavelength gives
stronger absorption but is more sensitive to interferences
(Manson et al., 1965; Yamamoto & Cook, 1968; Babina, 1969;
Leithe, 1971).
Analysis of samples can be made by visible, ultraviolet,
and infrared spectrophotometry, or gas chromatography
(Fishbein, 1979). The direct determination of styrene in air
without a preceeding concentration step is possible using
IR-spectrometry or gas chromatography. Commercial
variable-path, IR analysers are available. The recommended
wavelengths are 11.0 and 13.0 µm (Thompson, 1974). Styrene
also has absorption bands at 3.3, 6.7, 10.2, and 14.8 µm. The
major absorption bands of the most usual interfering
substance, acetone, are at 3.4, 5.8, 7.4, and 8.2 µm (Wilti,
1970); also acetone has minor bands at 11.0 and 13.0 µm (the
latter is weaker). The band with the least interference is
14.8 µm. However, the interference does not prevent the use
of the other bands; in fact, it may be advantageous to perform
a multicomponent analysis by repeating the measurement using
different wavelengths. Commercial microprocessor control
devices are available with which it is possible to carry out
automatic, simultaneous multi-point monitoring of several air
pollutants (Jacobs & Syrjala, 1978). The lowest detectable
concentration of styrene is about 1 mg/m3 (0.2 ppm), in the
absence of interference, using 11.0 µm as the analytical
wavelength with a 20 m cell.
Gas chromatographic analysis with a flame ionization
detector is the most sensitive analytical method for styrene.
Several column stationary phases are suitable including (10%)
FFAP (NIOSH, 1974), (15%) Carbowax 1540 (Götell et al., 1972),
(10%) XF 1150 (Kalliokoski & Pfäffli, 1975), and (5%) SE-30
(Bergman, 1977). The limit of detection, which varies
according to the gas chromatographic conditions, is about 0.1
mg/m3 (0.02 ppm) for direct air analysis (Stewart et al.,
1968) and 0.05-0.1 mg for desorbed sample injections (NIOSH
1974; Kalliokoski & Pfäffli, 1975). The coefficient of
variation for gas chromatographic analysis of solutions
containing 0.1-1 mg styrene in 1 ml of solvent is about 2%.
2.1.2. Measurement of styrene and styrene metabolites in
biological samples
Styrene has been measured in blood, exhaled air, and
subcutaneous fat. The concentration of styrene in blood and
exhaled air falls rapidly during the first hour following
termination of exposure. Thus, a single sample of exhaled air
or blood is of limited use. A number of samples are required
for the establishment of a kinetic "decay curve" from which
the assessment of a whole day's exposure may be possible
(Stewart et al., 1968; Fernandez & Caperos, 1977; Fields &
Horstman, 1979).
2.1.2.1. Exhaled air
Gas chromatography is the method of choice for the rapid
determination of styrene concentrations in exhaled air (Götell
et al., 1972; Fernandéz & Caperos, 1977). The detection limit
of the method is 0.04 mg/m3 (0.01 ppm) (Fernandez & Caperos,
1977). The limitation of sample collection in polyethylene
bags is the instability of the sample, due to polymerization
of the styrene within the bag, after sampling (Fields &
Horstman, 1979). Brooks et al. (1980) have developed an
improved method for the measurement of styrene in exhaled air,
with increased stability of samples as well as reliability of
sample collection. For sampling, they used a bubbler system
instead of polyethylene bags and were able to reduce the
detection limit to 0.02 mg/m3 (0.005 ppm).
2.1.2.2. Blood
Styrene concentrations in venous blood have been
determined by gas chromatography using the "head space"
technique (Astrand et al., 1974; Schaller et al., 1976; Withey
& Collins, 1977; Bencev & Rizov, 1981) or after previous
extraction (Stewart et al., 1968; Brooks et al., 1980). Wolff
et al. (1978) have developed a direct spectrofluorometric
method for the determination of styrene in blood with a very
low reported detection limit.
2.1.2.3. Subcutaneous adipose tissue
Estimation of styrene concentrations in adipose tissue is
possible in selected cases using a non-surgical, needle biopsy
technique to obtain the tissue sample. After extraction, the
styrene absorbed in the adipose tissue can be determined
easily using gas chromatographic methods (Savolainen &
Pfäffli, 1977; Wolff et al., 1977). The detection limit for a
10 mg sample is 40 µg styrene/kg adipose tissue (Wolff et al.,
1977) with a recovery of 98 ± 1.9% (Savolainen & Pfäffli,
1977). Determination of styrene in adipose tissue has also
been performed using a modified "headspace" gas
chromatographic technique (Engström et al., 1978a).
2.1.2.4. Urine
Evaluation of styrene exposure may be based on the urine
concentration of phenylglyoxylic acid (Ohtsuji & Ikeda, 1970;
Götell et al., 1972; Härkönen et al., 1974), on the combined
concentrations of mandelic and phenylglyoxylic acids
(Guillemin & Bauer, 1978; Elia et al., 1980), or on the ratio
of mandelic to phenylglyoxylic acid (Philippe et al., 1974).
Most of the methods for the determination of mandelic and
phenylglyoxylic acid in urine are based on gas chromatographic
techniques (Slob, 1973; Buchet et al., 1974; Engström &
Rantanen, 1974; Guillemin & Bauer, 1976; Flek & Sedivec,
1980). Derivatization of the acids is necessary before the
gas chromatographic analysis and both methylation (Buchet et
al., 1974; Flek & Sedivec, 1980) and silylation (Slob, 1973;
Engström & Rantanen, 1974) have been used for this purpose.
Gas chromatographic determination of mandelic acid is reliable
and simple, whereas the determination of phenylglyoxylic acid
involves problems of derivatization, that can only be overcome
by special techniques (Bauer & Guillemin, 1976; Flek &
Sedivec, 1980). Detection limits ranging from 0.6 to 6
mg/litre and variation coefficients ranging from 1 to 3% have
been reported for the different gas chromatographic procedures
(Slob, 1973; Engström & Rantanen, 1974; Flek & Sedivec, 1980).
Determination of mandelic and phenylglyoxylic acid without
derivatization can be performed using methods based on
isotachoforesis (Sollenberg & Baldesten, 1977) and high
performance liquid chromatography (Ogata & Sugihara, 1978;
Poggi et al., 1982).
Instability of samples may be a problem in urine analysis
for mandelic and phenylglyoxylic acid, because both acids are
prone to change during storage at room temperature (Flek &
Sedivec, 1980). This is especially the case with phenyl-
glyoxylic acid, which should not be stored for periods longer
than 24 h at room temperature; freezer storage is recommended.
3. SOURCES OF STYRENE IN THE ENVIRONMENT
3.1. Production of Styrene
The first step in styrene production is the catalytic
alkylation of benzene with ethylene, both raw materials being
supplied primarily from the petroleum industries. The
following two processes are in use for the production of
styrene (US EPA, 1980).
(a) Dehydrogenation of ethylbenzene
Ethylbenzene is dehydrogenated to styrene by the following
reaction:
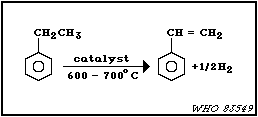 This is the desired selective reaction; however, various
non-selective reactions will occur. For example, a reactor
effluent contains an average of 0.7% benzene and 1.0% toluene
(Faith et al., 1975).
The crude styrene, with an average composition of 37%
styrene, 61% ethylbenzene, 1.0% toluene, 0.7% benzene, and
0.3% tars, is passed through a pot containing sulfur or some
other polymerization inhibitor and is then fed into a vacuum
column system. The overhead from a primary fractionating
column is fractionated to separate the ethylbenzene, which is
recycled, from the benzene and toluene, which are separated by
distillation. The bottoms from a primary fractionating column
are distilled to obtain the pure styrene product (US EPA,
1980).
(b) Co-product with propylene oxide
In late 1977, production began in a plant that makes
styrene by the following method (Soder, 1977):
C6H5CH2CH3 + O2 ------> C6H5CHOOHCH3
(ethylbenzene) (ethylbenzene hydroperoxide)
O
C6H5CHOOHCH3 + CH3CH=CH2 -------> C6H5CHOHCH3 + CH3CHCH2
(propylene) (methyl phenyl (propylene
carbinol) oxide)
C6H5CHOHCH3 -------> C6H5CH=CH2 + H2O
(styrene)
Ethylbenzene is oxidized to the hydroperoxide, which is
then reacted with propylene to yield the propylene oxide and a
co-product, methyl phenyl carbinol. The carbinol is then
dehydrated to styrene (US EPA, 1980). Expected impurities may
include propylbenzene, isopropylbenzene, and alpha-methylstyrene.
Table 1 shows the annual production of styrene monomer in
the world. The Federal Republic of Germany, Japan, and the
USA are the major producers (Tossavainen, 1978). It is
estimated that the world styrene consumption will grow at an
average of 5.1% per year in the period 1982-90 reaching 13.6
million tonnes by 1990 (Chemical Market Review, 1981).
Table 1. Production of styrenea
------------------------------------------
Country Year Production
(tonnes)
------------------------------------------
Canada 1974 146 000
France 1976 270 000
Germany, Federal 1976 860 000
Republic of
Italy 1976 325 000
Japan 1976 1 090 000
Mexico 1974 30 000
Spain 1976 60 000
USA 1976 2 864 000
Others 1976 approx. 1 000 000
------------------------------------------
World 1977 7 000 000
------------------------------------------
a From: Tossavainen (1978).
3.2. Uses
The uses of styrene (monomer) in the USA in 1980 are
listed in Table 2. The pattern of consumption is essentially
the same elsewhere in the world including Europe and Japan
(Tossavainen, 1978) and no significant change is foreseen as
far as 1990 (European Chemical News, 1982).
3.3. Main Sources of Environmental Pollution
According to Pervier et al. (1974), the major sources of
styrene contamination of the environment are the petrochemical
industries. Emissions from styrene production may result from
vents on distillation columns and other processing equipment,
storage tank losses, miscellaneous leaks and spills, waste-waters,
and solid process wastes. However, losses from production are low
in comparison with other petrochemical losses. Some styrene can
also be emitted from polymerization processes.
Table 2. Use pattern of styrene in the
USA in 1980
-----------------------------------------
Material %
-----------------------------------------
polystyrene 45
acrylonitrile-butadiene-styrene plus 10
styrene-acrylonitrile resin
styrene-butadiene rubber 8
styrene-butadiene latex 6
unsaturated polyester 5
miscellaneous 4
exports 17
-----------------------------------------
Styrene can be released into the environment during
various disposal procedures, e.g., during the incineration of
many types of styrene polymers. Styrene has been detected in
hydrocarbon exhausts from spark-ignition engines (Flemming,
1970), in oxyacetylene and oxyethylene flames (Crittenden &
Long, 1976), and in cigarette smoke (section 4.1.1). The
domestic use of polyester resins has increased potential
exposure patterns. Exposure may occur when styrene is used as
a solvent during the preparation of resin flooring (Gadalina
et al., 1969; Kaznina, 1969); or through the use of styrene in
various hobbies, crafts, and toys (Smirnova & Yatakova, 1966).
4. ENVIRONMENTAL EXPOSURE LEVELS
4.1. General Environment
Some data are available on styrene concentrations in air,
cigarette smoke, water, and food. However, they are not
sufficiently systematic to provide an overall picture and an
estimate of the main routes of human exposure.
Some of the following data for styrene levels in air and
water are drawn from a survey of literature, released in 1980
by the US Environmental Protection Agency (US EPA, 1980),
concerning exposure to several potential environmental
contaminants.
4.1.1. Ambient air
Styrene has been detected in ambient air in a wide variety
of locations.
In a study to develop procedures for determining specific
contaminants, Neligan et al. (1965) measured the amounts of a
number of hydrocarbons in the urban atmosphere in the USA.
The results of analysis of air from southern California in
1965 showed styrene to be present at levels of 0.008 - 0.063
mg/m3 (0.002 - 0.015 ppm) with an average level of 0.021 mg/m3
(0.005 ppm).
Styrene has also been found in the urban atmosphere in
Japan, (Hoshika, 1977) at levels of about 0.0008 µg/m3 (0.0002
ppm). Valenta (1966) measured the air and water contamination
near a butadiene-styrene rubber plant in Czechoslovakia. One
of the main sources of contamination was the plant warehouse
for liquid materials. A styrene concentration of 0.07 mg/m3
(0.017 ppm) was found in the immediate downwind vicinity of
the warehouse, compared with a concentration of 0.03 mg/m3
(0.007 ppm) at a distance of 800 m.
Schofield (1974) analysed hydrocarbon emissions from
vehicles and found that 0.76% of total hydrocarbons was in the
form of styrene in the exhaust of conventional engines, and
2.67% in the exhaust of rotary engines. Styrene has also been
identified in cigarette smoke, reported levels ranging from 18
to 48 µg per cigarette (Johnstone et al., 1962; Baggett et
al., 1974; Jermini et al., 1976).
4.1.2. Water
Styrene, detected in drinking water (US EPA, 1980) and in
river water (Rosén et al., 1963; Gordon & Goodly, 197l;
Bertsch et al., 1975), was usually traceable to an industrial
source or to improper disposal. An example of serious
contamination of a water supply through improper disposal of
styrene was reported by Grossman (1970) in which well water
close to the site where two drums of styrene had been buried
was found to have a disagreeable odour and contained a styrene
concentration of 0.1 - 0.2 mg/litre. Water from another well
close to the site of a waste dump from a styrene-butadiene
plant contained a styrene concentration of 0.01 - 0.02 mg/litre
(Valenta, 1966).
It seems that while styrene can be detected in water, it
is not one of the frequent contaminants, nor is it present in
large amounts.
4.1.3. Food
Polystyrene and its copolymers such as acrylonitrile-
butadiene-styrene (ABS), have been widely used as food
packaging materials. Currently available analytical surveys of
food and food packaging have shown that the styrene monomer
migrates into food from both rigid and expanded polystyrene foam
containers. According to Withey & Collins (1978), the lowest
concentration of monomer in rigid containers was 700 ppm and, the
highest was 3300 ppm. Other studies give figures of a similar
order of magnitude (Hamidullin et al., 1968). The lowest
concentration of monomer in expanded polystyrene foam was 87 ppm
(Withey, 1976). The highest migration figure (245.5 ppb) was found
in samples of sour cream contained in rigid polystyrene containers
(Withey & Collins, 1978). Styrene leached from containers at
0.2 - 0.5 ppm conveyed a disagreeable odour and taste to dairy
products (Jensen, 1972). However, styrene was not detected in milk
stored in polystyrene containers for up to 8 days (detection limit
50 ppb) (Finley & White, 1967). In another study, styrene rates of
leaching ranged from 0.0077, 0.0078, and 0.0078 µg/cm2
respectively, for foam cups containing water, tea, and coffee, to
0.036, 0.064, and 0.210 µg/cm2, respectively for foam, impact, and
crystal polystyrene cups containing 8% ethanol (Varner & Breder,
1981a,b).
In general, styrene concentrations in food are between 3
and 4 orders of magnitude lower than that in the package. It
appears that the contribution to the total body burden of
styrene via food is minimal.
4.2. Work Environment
Occupational exposure to styrene can be classified
according to the types of operations in which it is present.
In polystyrene manufacture, occupational chemical exposure is
mainly to styrene. In reinforced plastics applications, where
styrene is a solvent-reactant for copolymerization, styrene is
also the major air contaminant; however, there may be
concomitant exposure to glass fibres, catalysts, accelerators,
cleaning agents, and other chemicals. During
styrene-butadiene rubber production, workers may also be
exposed to such chemical substances as benzene, butadiene,
carbon disulfide, and trichloroethylene and, in factories that
produce styrene, there may be additional exposure to benzene
and ethyl benzene. In many of the applications, the
operations involve potential skin contact with liquid styrene.
4.2.1. Styrene and polystyrene manufacture
Concentrations of styrene recorded in a number of
factories producing styrene or polystyrene are summarized in
Table 3.
According to available reports, the styrene concentrations
found in polystyrene production are generally less than
21 mg/m3 (5 ppm); though occasional values of 210 mg/m3
(50 ppm) or more have been reported.
Table 3. Concentration of styrene in air of polystyrene manufacturing
plants
-----------------------------------------------------------------------
Year Authors Exposure levels Work area/process
mg/m3 (ppm)
-----------------------------------------------------------------------
1952 Barsotti et al. 800 (192) loading into
polymerization tower
1963 Zlobina et al. 2-9 (0.5-2) block production
4-9 (1-2) emulsion
< 50 (< 12) cleaning
1972 Ponomareva & 10 (2) polymerization
Zlobina <5 (< 1) polystyrene filament
production
< 5 (< 1) short & intermittent
operation
0.47-0.68 (0.11-0.16) drying & packaging
1974 Maier et al.a < 21 (< 5) at breathing zone of
1978 Wolff et al.a (11 samples were workers (involved in
between 21-378 mg/m3 routine operations)
(5-90 ppm)
1979 Thiess & Friedheim 244 (58) technical services
operation
4-50 (1-12) laboratory operation
-----------------------------------------------------------------------
a These investigators also identified benzene, ethylbenzene, toluene,
acetone. The highest measured concentrations of these solvents,
including styrene, resulted from spills and leaks.
4.2.2. Reinforced plastics applications
Data concerning air levels of styrene in reinforced
plastics plants, presented in Table 4, demonstrate that higher
air levels of styrene are monitored in this industry than in
styrene polymerization processes.
Tables 5, 6, 7, and 8 are designed to provide additional
information, useful in the assessment of occupational exposure
and to complement data presented in Table 4.
Table 4. Concentration of styrene in air of reinforced plastics plants
------------------------------------------------------------------------------
Year Author(s) No. of Exposure levels Work area/process
plants mg/m3 (ppm)
------------------------------------------------------------------------------
1967 Huzl et al. 5 210-420 (50-100) styrene containing polyester
resin applied by hand in 4
plants and sprayed in 1
1960 Bardodej et al. 2 63-840 (15-200) polyester resin applied by
hand
1964 Zielhuis et al. 3 101-378 (24-90) manufacturing of boats,
automobile bodies
ca. 29 (ca. 7) production of small objects
and upholstery; laboratory
1966 Simko et al. 3 21-819 (5-195) manufacture of reinforced
plastics
1968 Matsushita et 1 > 2520 (> 600) lamination of plywood
al. with styrene-containing resin
1972 Gotell et al. 4 84-1218 (20-290) production of reinforced
plastics
1974 Bodnei et al. 1 189-2130(45-550) reinforced plastics and
(1973-1974)a bathtub manufacturing
1976 Kallioski 22 84-1260 (20-300) boat laminating and other
reinforced plastics
manufacturing
1977 Rosensteel et 1 147-462 (35-110) reinforced plastic boat
(1975-1976)a plant (peak conc. of
al. 1260-1680 mg/m3 (300-400 ppm)
for approx. 5 min)
1977 Bergman 4 42-714 (10-170) Hand layup & spraying in the
reinforced plastic boat
industry (peak conc. of
256-2146 mg/m3 (61-511 ppm)
for approx. 60 min)
1979 Brooks et al. 3 168-966 (40-230)
(1977)a
1979 Kjellberg et 1 13-59 (3-14) boat fabrication
al.
1981 Crandall 7 8-756 (2-180) hull, deck & small part
(1978-1979)a lamination and gelcoating
process
------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------
Year Author(s) No. of Exposure levels Work area/process
plants mg/m3 (ppm)
------------------------------------------------------------------------------
1981 Schumacher et 12 < 1260 (< 300) Table 7
al.
1982 Ikeda et al. 5 < 4-1075 reinforced plastic boat
(< 1-256) plants
------------------------------------------------------------------------------
a Years when the study was conducted.
The resin manufacturers have tried to decrease styrene
emissions by reducing the styrene content of resins and by
using thixotropic resins and film-forming additives. They
(Nylander, 1979; Synthetic Resins Limited, 1980; Voskamp &
Studenberg, 1981) claim that significant decreases in styrene
emission can be achieved using these new resins, often called
low styrene emission resins (LSE resins). In the study of
Schumacher et al. (1981), the results of laboratory tests on
such resins suggested a 30% reduction in styrene vapour, but
when tested in the workplace there was essentially no
difference in the exposure levels of the workers and the
authors suggested the need for further studies to record any
effects from the use of the new resins.
Concentrations of styrene found during the production of
reinforced plastics (Zielhuis et al., 1964; Götell et al.,
1972; Bodnei et al., 1974; Rosensteel & Meyer, 1977) were
generally much higher than those found in the polystyrene
production plants, with peak concentrations as high as 6300
mg/m3 (1500 ppm). Most of the environmental concentrations of
styrene in reinforced plastic plants, summarized in Table 4,
are 8-h time-weighted-average (TWA) concentrations, but some
values represent shorter periods. It can be seen from the
table that exposure occurs not only in hand layup and spraying
operations using open moulds, but also in mechanical and
closed mould work. Furthermore, workers who do not handle the
polyester resin themselves but work in the laminating room
undergo passive exposure. It should be noted that the
sampling strategy used influences the results; for example,
breathing zone concentrations may differ considerably from the
general air concentrations.
4.2.3. Styrene-butadiene applications
Hazards encountered in the synthetic rubber industry
during the Second World War were discussed by Wilson (1944).
The principal chemicals used in the manufacture of rubber, at
that time, were styrene, butadiene, and acrylonitrile.
More recently, other studies of synthetic rubber
manufacture have shown that levels of styrene are low.
Basirov (1968) reported concentrations of 60-130 mg/m3 (14-31
ppm) in a 1968 study of a synthetic rubber plant. In a health
risk evaluation in a similar type of plant, Gunter & Lucas
(1973) were unable to find any measurable concentrations of
styrene. However, styrene levels varying between 42 and 840
mg/m3 (10 and 200 ppm) were measured in a synthetic rubber
plant by Spinazzola et al. (1980).
4.2.4. Summary of occupational exposure
In summary, occupational exposure to styrene varies
considerably depending on the operations concerned. While styrene
is present in detectable amounts in styrene/polystyrene
manufacturing plants, the greatest exposures occur in the
operations and industries that use unsaturated polyester resins
dissolved in styrene. However, in all industrial operations using
styrene, high levels of exposures occur during the clean-up and
maintenance procedures.
Table 5. Airborne styrene concentrations at 36 Finnish
factories using hand layup methoda
(i) Mean 8-h TWA breathing zone styrene concentrations at 22
factories
---------------------------------------------
Factory mg/m3 ppm Factory mg/m3 ppm
---------------------------------------------
1 1109 264 12 223 53
2 995 237 13 214 51
3 836 199 14 197 47
4 613 146 15 193 46
5 433 103 16 189 45
6 382 91 17 185 44
7 382 91 18 169 38
8 340 81 19 134 32
9 336 80 20 134 32
10 294 70 21 126 30
11 273 65 22 97 23
---------------------------------------------
(ii) Mean breathing zone styrene concentrations during shorter
(1-5 h) industrial hygiene surveys at 14 factories
---------------------------------------------
Factory mg/m3 ppm Factory mg/m3 ppm
---------------------------------------------
23 1029 244 30 416 99
24 819 195 31 302 72
25 50 12 32 235 56
26 769 183 33 193 46
27 622 148 34 105 25
28 521 124 35 92 22
29 491 117 36 67 16
---------------------------------------------
(iii) Distribution of styrene concentrations by product type
----------------------------------------------------------
8-h TWA styrene TWA styrene conc.
conc. for periods of 1-5h
Product type (factories 1-22) (factories 23-36)
mg/m3 ppm mg/m3 ppm
----------------------------------------------------------
Boats 483 115 865 206
Other products
- small objects 210 50 424 101
- large objects 357 85 248 59
----------------------------------------------------------
a Adapted from: Kallioski (1976).
Table 6. Airborne styrene concentrations at a factory in the USAa
---------------------------------------------------
8-h TWA styrene exposure
Job category (mean ± SD)
mg/m3 (ppm)
---------------------------------------------------
Prefabrication 11.8 ± 16.8 (2.8 ± 4)
Gel coating 288 ± 251 (68.5 ± 59.7)
Hand layup 350 ± 179 (83.4 ± 42.7)
Hand layup, other areas 108 ± 110 (25.7 ± 26.2)
Woodwork/upholstery 14.3 ± 8.8 (3.4 ± 2.1)
Final assembly 14.3 ± 8.8 (3.4 ± 2.1)
Custom moulding 27.3 ± 16.4 (6.5 ± 3.9)
Small boat 17.2 ± 4.2 (4.1 ± 1.0)
Miscellaneous 16.4 ± 13.0 (3.9 ± 3.1)
---------------------------------------------------
a Adapted from: Brooks et al. (1979).
Table 7. Airborne styrene concentrations at 12 factories in the USA. The figures in the columns
represent the number of workers in each exposure groupa
-------------------------------------------------------------------------------------------------------
Styrene concentration mg/m3 (ppm)
Classification
0-210 210-420 420-630 630-840 840-1050 1050-1260 > 1260 Totals
(0-50) (50-100) (100-150) (150-200) (200-250) (250-300) (> 300)
-------------------------------------------------------------------------------------------------------
Plant Type
Boat plants 127 144 80 32 25 23 48 479
Non-boat plants 26 47 29 5 3 1 2 113
Totals 153 191 109 37 28 24 50 592
Job Category
Laminators 61 70 61 27 21 11 22 273
Chopper gun operators 37 62 40 25 13 17 30 224
Gel coaters 12 6 4 3 2 27
Totals 110 138 105 55 34 28 54 524
Plant Type (Exposure of Laminators)
Boat plants 52 48 42 26 18 13 20 219
Non-boat plants 6 24 17 2 3 1 1 54
Totals 58 72 59 28 21 14 21 273
-------------------------------------------------------------------------------------------------------
a Modified from: Schumacher et al. (1981).
Table 8. Styrene concentration during various processes in boat production
-----------------------------------------------------------------------------------------------------------------------------
Process Case Personal sampling Stationary sampling
---------------------------------------------- ------------------------------------------------
Na GMb GSDc Ranged Nc GMb GSDc Ranged Nc
-----------------------------------------------------------------------------------------------------------------------------
Lamination A 25 500 (119) 6.8 (1.6) 143-1075 (34-256) 17 550 (131) 5.5 (1.3) 307-731 (73-174)
over boat B 9 273 (65) 5.8 (1.2) 193-378 (46-90) 15 281 (67) 5.9 (1.4) 160-546 (38-130)
shell mould
Installation laminators 3 71.4 (17) 7.1 (1.7) 42-118 (10-28) 20 33.6 (8) 10 (2.4) 8-214 (2-51)
of ribs helpers 5 < 4.2 (< 1) 23.5 (5.6) 8 (D1-2)
Installation laminators 5 92.4 (22) 8.8 (2.1) 25-185 (6-44) 6 29.4 (7) 12.2 (2.9) 8-92 (2-22)
of division helpers 5 D - (D-D)
plates
Auxilary 5 54.6 (13) 16.8 (4) 8-181 (2-43) 5 96.6 (23) 7.1 (1.7) 59-164 (14-39)
lamination
on deck
Lamination on A 4 128 1.4 (104-211) 3 269 (64) 19.3 (4.6) 55-1172 (13-279)
hold walls B 21 127 1.3 (87-215) 20 269 (64) 7.6 (1.8) 101-1088 (24-259)
Equipment 8 2 2.8 (1-17) 15 < 4.2 64.7 (15.4) Ng-38 (Ng-9)
(< 1)
Plant floor A 39 6 24.8 (5.9) 8-76 (2-18)
in general B 14 < 1 69.7 (16.6) Na-38 (Na-9)
-----------------------------------------------------------------------------------------------------------------------------
a The number of workers equipped with personal samplers for 4 h work (1300-1700).
b The geometric mean.
c The geometric standard deviation.
d The minimum and maximum concentration observed.
e The number of sites monitored by stationary samplers for 4 h (1300-1700).
f D = detected but not measurable (less than 4.2 mg/m3 (1 ppm).
g Not detected.
5. CHEMOBIOKINETICS AND BIOTRANSFORMATION
5.1. Uptake
5.1.1. Human studies
5.1.1.1. Uptake by inhalation
Pulmonary uptake of styrene concentrations of 67-164 mg/m3
(16-39 ppm), after a single breath or during exposures of up
to 8 h, has been studied in human volunteers. Depending on
exposure conditions, uptake varied from 45 to 66% (Bardodej et
al., 1961; Fiserova-Bergerova & Teisinger, 1965; Bardodej &
Bardodejova, 1970).
Astrand et al. (1974) examined the pulmonary uptake of
inhaled styrene (210 and 630 mg/m3; 50 and l50 ppm) in 14
healthy subjects at rest and during exercise over 30-min
periods. Blood concentrations of styrene were measurable
shortly after the onset of exposure indicating a rapid
uptake. With light work (50 W exercise), the alveolar
ventilation increased 3-fold, while the alveolar concentration
of styrene barely changed. The concentration of styrene in
arterial blood tripled.
Fernandez & Caperos (1977) measured the uptake of styrene
in 6 volunteers at exposure levels ranging from 294 to
840 mg/m3 (70 to 200 ppm) for periods ranging from 4 to 8 h.
The retention of styrene, measured from alveolar ventilation,
varied from 82 to 93%.
In studies by Ramsey & Young (1978) and Ramsey et al.
(1980) on 4 human volunteers, a single exposure to a styrene
concentration of 336 mg/m3 (80 ppm) for 6 h resulted in a
blood concentration at the end of exposure of about
800 µg/litre. A steady state concentration of about
900 µg/litre was predicted for repeated daily exposure to the
same atmospheric concentrations. The authors assumed a
2-compartment model that allowed a zeroth order uptake rate of
99.6 mg/h to be calculated.
5.1.1.2. Uptake from the gastrointestinal tract
No studies have been found in the literature related to
the uptake of styrene from the human gastrointestinal tract.
5.1.1.3. Uptake through the skin
Styrene is absorbed through human skin when applied in the
form of a liquid, an aqueous solution, or a vapour. Rates of
uptake of between 9 and 15 mg/cm2 per h were reported when
liquid styrene was applied to the skin of the hands and
forearm (Dutkiewicz & Tyras, 1967; 1968). Rates of uptake of
0.004-0.180 mg/cm2 per h were reported when aqueous solutions
containing mean styrene concentrations of between 66.5 and 269
mg/litre were applied. Skin absorption of styrene was some 30
times greater than that of aniline, benzene, or nitrobenzene.
Riihimäki & Pfäffli (1978) measured the percutaneous
uptake of styrene vapour in 2 human volunteers, wearing thin
cloth pyjamas and socks, who were exposed to a styrene vapour
concentration of 2520 mg/m3 (600 ppm) for 3.5 h, including 3
work intervals of 10 min each. Full-face respirators were
used to prevent inhalation uptake and the temperature was
maintained at 25°C with 50% relative humidity. Blood levels
reached a peak 3 h after the start of exposure and then
declined over the next 4 h. These results were equivalent to
a pulmonary exposure of about 84 mg/m3 (20 ppm) for an equal
period of time.
5.1.2. Experimental animal studies
5.1.2.1. Uptake by inhalation
A recent review of studies of the uptake of styrene in
several animal species indicated that styrene is distributed
rapidly throughout the body, is stored in lipid-rich tissues,
and is extensively metabolized (Santodonato et al., 1980). In
a study on rats exposed to styrene concentrations in air of
336, 840, 2520, and 5040 mg/m3 (80, 200, 600, and 1200 ppm)
for up to 24 h, kinetic data obtained by Ramsey & Young (1978)
obeyed a 2-compartment model. From the kinetic coefficients
derived from the data, the authors predicted that there was
little or no potential for bioaccumulation with repeated 8 h
per day exposure to 336 mg/m3 (80 ppm). They claimed that 95%
of the predicted maximum blood concentration of styrene would
be reached during the first day of exposure.
In another study in which rats with an indwelling jugular
cannula were exposed to styrene atmospheres (Withey, 1978;
Withey & Collins, 1979), a 2-compartment model was observed at
exposure concentrations ranging from 210 to 8400 mg/m3 (50 to
2000 ppm) for a period of 5 h. From the assumed zeroth-order
kinetics for uptake together with the rate coefficients for
distribution and elimination, it was possible to predict that
the time to equilibrium or steady-state would be longer at
higher exposure levels.
An autoradiographic study on the uptake of 14C-styrene-8
and 14C-styrene-ring by mice was carried out by Bergman
(1979). Each animal inhaled the vapours from 10 µl of styrene
in a small inhalation apparatus for 10 min. Extensive
uptake was observed in all organs and the potential for
enterohepatic recycling was demonstrated by the appearance of
the radiolabel in the bile. Styrene uptake in the mice was
reported to be low compared with that in human subjects (less
than one half) and this was attributed to the reduced
respiratory frequency in the mouse, induced by the irritant
action of the styrene vapour on the respiratory tract.
5.1.2.2. Uptake from the gastrointestinal tract
There have been few reports on the kinetics and the extent
of uptake of styrene from the gastrointestinal tract in
animals.
A study by Sauerhoff et al. (1976) on male and female
rats, administered a single oral dose of 14C-labelled styrene
at 500 or 50 mg/kg body weight, showed that a greater fraction
of unchanged styrene was excreted via the lung after
administration of the higher dose, a result suggesting that
saturable metabolic pathways were involved. The authors found
that the uptake of styrene monomer from oral doses was greater
for the female than for the male rats.
In a report of a preliminary investigation on the uptake
kinetics of styrene from the gastrointestinal tract of rats
(Withey, 1976), it was noted that the uptake and kinetic
profile after a single dose, administered as an aqueous
solution, was so rapid (peak values being obtained less than 4
min after the administration of the dose) that it was almost
indistinguishable from the kinetics of an intravenous bolus
dose. In contrast, when a similar dose was administered as a
solution in vegetable oil, peak values were not obtained until
4 h after dosing.
5.1.2.3. Uptake through the skin
Wolf et al. (1956) applied unspecified amounts of liquid
styrene to the ear or to the shaved abdomen of rabbits during
periods of 2-4 weeks (10-20 applications). Moderate irritation
and slight necrosis were observed. The authors claimed that, at
the doses applied, there was no evidence that styrene was absorbed
through the skin in amounts sufficient to cause acute, systemic
toxicity.
The percutaneous uptake of styrene in rats has been
measured by immersing the tail of the animal in liquid styrene
for 1 h (Shugaev, 1969). Inhalation exposure was carefully
avoided. Significant uptake of styrene by the liver and brain
was estimated to be between 50 and 70 % of the concentrations
found in the same organs after a 4-h inhalation exposure to a
vapour concentration of 11.8 gm/m3.
5.2. Distribution and Storage
5.2.1. Human studies
5.2.1.1. Controlled human studies
Studies on experimental animals have demonstrated that
styrene is widely distributed throughout the tissues and
organs and that it may accumulate in adipose tissue. Studies
on the distribution of styrene in human volunteers have,
necessarily, been limited to its quantitative analysis in
blood, expired air, and adipose tissue.
In a study by Engström (1978), 7 male subjects were
exposed to a styrene concentration of 210 mg/m3 (50 ppm)
during 30 min at rest and three, 30-min periods on a bicycle
ergometer set at a work intensity of 50, 100, or 150 W. The
mean uptake of styrene was 490 mg. Specimens of subcutaneous
adipose tissue were taken before and after exposure and at 0.5, 2,
4, and 21 h after exposure. Mean concentrations in the adipose
tissue, up to 21 h after exposure, were of the same magnitude (3.6
mg/kg). From the concentration of styrene still detectable l3 days
after exposure, it was possible to estimate an elimination half-
time of between 2.2 and 4.0 days.
Ramsey & Young (1978) exposed volunteers to a styrene
concentration of 336 mg/m3 (80 ppm) for 6 h and observed that
the decay of styrene concentration in the blood following the
exposure fitted a 2-compartment model. They speculated that
accumulation of styrene would not occur following repeated
exposure to concentrations up to 840 mg/m3 (200 ppm).
5.2.1.2. Occupational exposure studies
There have been a number of studies involving the
determination of styrene concentrations in the blood and
adipose tissue of workers occupationally exposed to styrene
monomer. These studies were not only intended to investigate the
correlation between levels in tissues and previous exposure to
styrene but also to see if there was a potential for its
accumulation and storage in adipose tissue. Wolff et al. (1977)
studied 25 workers who allowed from 3 to 66 mg of subcutaneous
gluteal adipose tissue to be taken. It was demonstrated that
styrene persisted in this tissue for as long as 3 days following
exposure, whereas urine metabolites and styrene in expired air
persisted for up to 16 h after exposure. In an extension of this
study (Wolff et al., 1978a), data on levels of styrene in the
gluteal adipose tissue of an additional 25 workers suggested a
correlation with blood styrene and urinary metabolite levels.
Engström et al. (1978a,b) measured air concentrations of
styrene in a polymerization plant and obtained subcutaneous
adipose samples from 3 workers. The TWA air concentrations
ranged from 32 to 84 mg/m3 (7.6 to 20.2 ppm) and the mean
daily styrene uptake of the 3 volunteers was 193, 343, and 558
mg, respectively. Adipose tissue concentrations were 2.8,
4.0, and 8.1 mg/kg, respectively, at the beginning of the
working week, and 4.7, 7.7, and 11.6 mg/kg, at the end.
5.2.1.3. General population studies
Reports on the uptake of styrene in the general population
are lacking.
5.2.2. Experimental animal studies
There have been a number of reports on the distribution of
controlled doses of styrene monomer administered by different
routes in several animal species.
In studies on mice and rats, Shugaev (1969) showed that,
after inhalation exposure to the median lethal concentration
(LC50), for 2 and 4 h, respectively, there was a positive
correlation between the brain concentration of styrene and
lethality. Styrene levels in the perirenal adipose tissue
were some 5 times higher than those in the brain, liver,
kidney, and spleen.
Savolainen & Vainio (1977) studied the organ distribution
of radiolabelled styrene and styrene epoxide following an
intraperitoneal injection of 577 µmol, 3, 6, and 24 h after
dosing. Three hours after dosing, the highest concentration of
styrene was found in the kidneys followed by the brain, duodenum,
liver, spinal cord, lungs, and blood. The kidney concentration was
19 times higher than that in the blood. After 6 h, levels in the
duodenum and brain had decreased whereas levels in the blood had
increased.
The distribution of 14C-styrene-8 and 14C-styrene-ring was
studied in male mice after a 10-min inhalation exposure
(Bergman, 1979). Animals were killed at 0.5, 1, 2, 4, 8, 24,
and 48 h after exposure and subjected to whole-body auto-
radiography. The autoradiograms obtained shortly after
exposure showed that the radioactivity was localized in the
bronchi, lungs, and liver, for both styrene labelled in the
side chain and the ring-labelled compound. Styrene was
apparently cleared from the nervous tissues in less than 1 h,
since no radiolabelled material was detected at 0.5 h.
However, it was retained in adipose tissues for up to 48 h
after dosing. Again, a large proportion of the radioactivity
was found in the bile and kidney suggesting that these routes
are the primary routes of excretion. Radiolabelled material
appeared in the intestinal contents within 1 h of exposure and
persisted for at least 24 h.
A recent inhalation study on rats (Carlsson, 1981) showed
that the largest amounts of 14C-styrene and its metabolites
were found in the kidneys, after an 8-h exposure to 184 mg/m3
(45 ppm). The styrene concentrations were also high in
subcutaneous adipose tissue; the concentrations in the
cerebrum, cerebellum, and muscles amounted to about 70% of the
styrene concentration in arterial blood.
The distribution of styrene was examined in male Wistar
rats after intravenous administration at 4.01 or 13.37 mg/kg
body weight or after a 5 h inhalation exposure at 6
concentrations between 227 and 9408 mg/m3 (54 and 2240 ppm)
(Withey, 1976; Withey & Collins, 1977, 1979). Animals were
killed immediately after the termination of the vapour
exposure, and the blood, heart, kidney, liver, spleen, brain,
and perirenal fat were analysed for their styrene content. At all
exposure levels except the lowest, styrene concentrations in all
organs were higher than that in the blood. At the lowest level of
exposure, the concentration in the kidney was at least 10 times
greater than that in any other organ except the perirenal fat. At
higher levels of exposure, the highest concentration was found in
the liver. Levels of styrene in organs, 8 min after intravenous
administration of 13.37 mg/kg were found in the following
descending order: liver > kidney > brain > blood > heart and
spleen. For the lower dose (4.01 mg/kg), the levels in these
organs were of the same order of distribution, but lower than that
in the blood, suggesting that, as in the case of the inhalation
study, the distribution to the major organs varied with dose.
5.3. Biotransformation
The first step in the metabolic transformation of styrene
is its oxidation, catalysed by cytochrome P-450 dependent
monooxygenases, to oxirane derivatives in the aliphatic chain
or in the aromatic ring (Fig. 1).
This is the desired selective reaction; however, various
non-selective reactions will occur. For example, a reactor
effluent contains an average of 0.7% benzene and 1.0% toluene
(Faith et al., 1975).
The crude styrene, with an average composition of 37%
styrene, 61% ethylbenzene, 1.0% toluene, 0.7% benzene, and
0.3% tars, is passed through a pot containing sulfur or some
other polymerization inhibitor and is then fed into a vacuum
column system. The overhead from a primary fractionating
column is fractionated to separate the ethylbenzene, which is
recycled, from the benzene and toluene, which are separated by
distillation. The bottoms from a primary fractionating column
are distilled to obtain the pure styrene product (US EPA,
1980).
(b) Co-product with propylene oxide
In late 1977, production began in a plant that makes
styrene by the following method (Soder, 1977):
C6H5CH2CH3 + O2 ------> C6H5CHOOHCH3
(ethylbenzene) (ethylbenzene hydroperoxide)
O
C6H5CHOOHCH3 + CH3CH=CH2 -------> C6H5CHOHCH3 + CH3CHCH2
(propylene) (methyl phenyl (propylene
carbinol) oxide)
C6H5CHOHCH3 -------> C6H5CH=CH2 + H2O
(styrene)
Ethylbenzene is oxidized to the hydroperoxide, which is
then reacted with propylene to yield the propylene oxide and a
co-product, methyl phenyl carbinol. The carbinol is then
dehydrated to styrene (US EPA, 1980). Expected impurities may
include propylbenzene, isopropylbenzene, and alpha-methylstyrene.
Table 1 shows the annual production of styrene monomer in
the world. The Federal Republic of Germany, Japan, and the
USA are the major producers (Tossavainen, 1978). It is
estimated that the world styrene consumption will grow at an
average of 5.1% per year in the period 1982-90 reaching 13.6
million tonnes by 1990 (Chemical Market Review, 1981).
Table 1. Production of styrenea
------------------------------------------
Country Year Production
(tonnes)
------------------------------------------
Canada 1974 146 000
France 1976 270 000
Germany, Federal 1976 860 000
Republic of
Italy 1976 325 000
Japan 1976 1 090 000
Mexico 1974 30 000
Spain 1976 60 000
USA 1976 2 864 000
Others 1976 approx. 1 000 000
------------------------------------------
World 1977 7 000 000
------------------------------------------
a From: Tossavainen (1978).
3.2. Uses
The uses of styrene (monomer) in the USA in 1980 are
listed in Table 2. The pattern of consumption is essentially
the same elsewhere in the world including Europe and Japan
(Tossavainen, 1978) and no significant change is foreseen as
far as 1990 (European Chemical News, 1982).
3.3. Main Sources of Environmental Pollution
According to Pervier et al. (1974), the major sources of
styrene contamination of the environment are the petrochemical
industries. Emissions from styrene production may result from
vents on distillation columns and other processing equipment,
storage tank losses, miscellaneous leaks and spills, waste-waters,
and solid process wastes. However, losses from production are low
in comparison with other petrochemical losses. Some styrene can
also be emitted from polymerization processes.
Table 2. Use pattern of styrene in the
USA in 1980
-----------------------------------------
Material %
-----------------------------------------
polystyrene 45
acrylonitrile-butadiene-styrene plus 10
styrene-acrylonitrile resin
styrene-butadiene rubber 8
styrene-butadiene latex 6
unsaturated polyester 5
miscellaneous 4
exports 17
-----------------------------------------
Styrene can be released into the environment during
various disposal procedures, e.g., during the incineration of
many types of styrene polymers. Styrene has been detected in
hydrocarbon exhausts from spark-ignition engines (Flemming,
1970), in oxyacetylene and oxyethylene flames (Crittenden &
Long, 1976), and in cigarette smoke (section 4.1.1). The
domestic use of polyester resins has increased potential
exposure patterns. Exposure may occur when styrene is used as
a solvent during the preparation of resin flooring (Gadalina
et al., 1969; Kaznina, 1969); or through the use of styrene in
various hobbies, crafts, and toys (Smirnova & Yatakova, 1966).
4. ENVIRONMENTAL EXPOSURE LEVELS
4.1. General Environment
Some data are available on styrene concentrations in air,
cigarette smoke, water, and food. However, they are not
sufficiently systematic to provide an overall picture and an
estimate of the main routes of human exposure.
Some of the following data for styrene levels in air and
water are drawn from a survey of literature, released in 1980
by the US Environmental Protection Agency (US EPA, 1980),
concerning exposure to several potential environmental
contaminants.
4.1.1. Ambient air
Styrene has been detected in ambient air in a wide variety
of locations.
In a study to develop procedures for determining specific
contaminants, Neligan et al. (1965) measured the amounts of a
number of hydrocarbons in the urban atmosphere in the USA.
The results of analysis of air from southern California in
1965 showed styrene to be present at levels of 0.008 - 0.063
mg/m3 (0.002 - 0.015 ppm) with an average level of 0.021 mg/m3
(0.005 ppm).
Styrene has also been found in the urban atmosphere in
Japan, (Hoshika, 1977) at levels of about 0.0008 µg/m3 (0.0002
ppm). Valenta (1966) measured the air and water contamination
near a butadiene-styrene rubber plant in Czechoslovakia. One
of the main sources of contamination was the plant warehouse
for liquid materials. A styrene concentration of 0.07 mg/m3
(0.017 ppm) was found in the immediate downwind vicinity of
the warehouse, compared with a concentration of 0.03 mg/m3
(0.007 ppm) at a distance of 800 m.
Schofield (1974) analysed hydrocarbon emissions from
vehicles and found that 0.76% of total hydrocarbons was in the
form of styrene in the exhaust of conventional engines, and
2.67% in the exhaust of rotary engines. Styrene has also been
identified in cigarette smoke, reported levels ranging from 18
to 48 µg per cigarette (Johnstone et al., 1962; Baggett et
al., 1974; Jermini et al., 1976).
4.1.2. Water
Styrene, detected in drinking water (US EPA, 1980) and in
river water (Rosén et al., 1963; Gordon & Goodly, 197l;
Bertsch et al., 1975), was usually traceable to an industrial
source or to improper disposal. An example of serious
contamination of a water supply through improper disposal of
styrene was reported by Grossman (1970) in which well water
close to the site where two drums of styrene had been buried
was found to have a disagreeable odour and contained a styrene
concentration of 0.1 - 0.2 mg/litre. Water from another well
close to the site of a waste dump from a styrene-butadiene
plant contained a styrene concentration of 0.01 - 0.02 mg/litre
(Valenta, 1966).
It seems that while styrene can be detected in water, it
is not one of the frequent contaminants, nor is it present in
large amounts.
4.1.3. Food
Polystyrene and its copolymers such as acrylonitrile-
butadiene-styrene (ABS), have been widely used as food
packaging materials. Currently available analytical surveys of
food and food packaging have shown that the styrene monomer
migrates into food from both rigid and expanded polystyrene foam
containers. According to Withey & Collins (1978), the lowest
concentration of monomer in rigid containers was 700 ppm and, the
highest was 3300 ppm. Other studies give figures of a similar
order of magnitude (Hamidullin et al., 1968). The lowest
concentration of monomer in expanded polystyrene foam was 87 ppm
(Withey, 1976). The highest migration figure (245.5 ppb) was found
in samples of sour cream contained in rigid polystyrene containers
(Withey & Collins, 1978). Styrene leached from containers at
0.2 - 0.5 ppm conveyed a disagreeable odour and taste to dairy
products (Jensen, 1972). However, styrene was not detected in milk
stored in polystyrene containers for up to 8 days (detection limit
50 ppb) (Finley & White, 1967). In another study, styrene rates of
leaching ranged from 0.0077, 0.0078, and 0.0078 µg/cm2
respectively, for foam cups containing water, tea, and coffee, to
0.036, 0.064, and 0.210 µg/cm2, respectively for foam, impact, and
crystal polystyrene cups containing 8% ethanol (Varner & Breder,
1981a,b).
In general, styrene concentrations in food are between 3
and 4 orders of magnitude lower than that in the package. It
appears that the contribution to the total body burden of
styrene via food is minimal.
4.2. Work Environment
Occupational exposure to styrene can be classified
according to the types of operations in which it is present.
In polystyrene manufacture, occupational chemical exposure is
mainly to styrene. In reinforced plastics applications, where
styrene is a solvent-reactant for copolymerization, styrene is
also the major air contaminant; however, there may be
concomitant exposure to glass fibres, catalysts, accelerators,
cleaning agents, and other chemicals. During
styrene-butadiene rubber production, workers may also be
exposed to such chemical substances as benzene, butadiene,
carbon disulfide, and trichloroethylene and, in factories that
produce styrene, there may be additional exposure to benzene
and ethyl benzene. In many of the applications, the
operations involve potential skin contact with liquid styrene.
4.2.1. Styrene and polystyrene manufacture
Concentrations of styrene recorded in a number of
factories producing styrene or polystyrene are summarized in
Table 3.
According to available reports, the styrene concentrations
found in polystyrene production are generally less than
21 mg/m3 (5 ppm); though occasional values of 210 mg/m3
(50 ppm) or more have been reported.
Table 3. Concentration of styrene in air of polystyrene manufacturing
plants
-----------------------------------------------------------------------
Year Authors Exposure levels Work area/process
mg/m3 (ppm)
-----------------------------------------------------------------------
1952 Barsotti et al. 800 (192) loading into
polymerization tower
1963 Zlobina et al. 2-9 (0.5-2) block production
4-9 (1-2) emulsion
< 50 (< 12) cleaning
1972 Ponomareva & 10 (2) polymerization
Zlobina <5 (< 1) polystyrene filament
production
< 5 (< 1) short & intermittent
operation
0.47-0.68 (0.11-0.16) drying & packaging
1974 Maier et al.a < 21 (< 5) at breathing zone of
1978 Wolff et al.a (11 samples were workers (involved in
between 21-378 mg/m3 routine operations)
(5-90 ppm)
1979 Thiess & Friedheim 244 (58) technical services
operation
4-50 (1-12) laboratory operation
-----------------------------------------------------------------------
a These investigators also identified benzene, ethylbenzene, toluene,
acetone. The highest measured concentrations of these solvents,
including styrene, resulted from spills and leaks.
4.2.2. Reinforced plastics applications
Data concerning air levels of styrene in reinforced
plastics plants, presented in Table 4, demonstrate that higher
air levels of styrene are monitored in this industry than in
styrene polymerization processes.
Tables 5, 6, 7, and 8 are designed to provide additional
information, useful in the assessment of occupational exposure
and to complement data presented in Table 4.
Table 4. Concentration of styrene in air of reinforced plastics plants
------------------------------------------------------------------------------
Year Author(s) No. of Exposure levels Work area/process
plants mg/m3 (ppm)
------------------------------------------------------------------------------
1967 Huzl et al. 5 210-420 (50-100) styrene containing polyester
resin applied by hand in 4
plants and sprayed in 1
1960 Bardodej et al. 2 63-840 (15-200) polyester resin applied by
hand
1964 Zielhuis et al. 3 101-378 (24-90) manufacturing of boats,
automobile bodies
ca. 29 (ca. 7) production of small objects
and upholstery; laboratory
1966 Simko et al. 3 21-819 (5-195) manufacture of reinforced
plastics
1968 Matsushita et 1 > 2520 (> 600) lamination of plywood
al. with styrene-containing resin
1972 Gotell et al. 4 84-1218 (20-290) production of reinforced
plastics
1974 Bodnei et al. 1 189-2130(45-550) reinforced plastics and
(1973-1974)a bathtub manufacturing
1976 Kallioski 22 84-1260 (20-300) boat laminating and other
reinforced plastics
manufacturing
1977 Rosensteel et 1 147-462 (35-110) reinforced plastic boat
(1975-1976)a plant (peak conc. of
al. 1260-1680 mg/m3 (300-400 ppm)
for approx. 5 min)
1977 Bergman 4 42-714 (10-170) Hand layup & spraying in the
reinforced plastic boat
industry (peak conc. of
256-2146 mg/m3 (61-511 ppm)
for approx. 60 min)
1979 Brooks et al. 3 168-966 (40-230)
(1977)a
1979 Kjellberg et 1 13-59 (3-14) boat fabrication
al.
1981 Crandall 7 8-756 (2-180) hull, deck & small part
(1978-1979)a lamination and gelcoating
process
------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------
Year Author(s) No. of Exposure levels Work area/process
plants mg/m3 (ppm)
------------------------------------------------------------------------------
1981 Schumacher et 12 < 1260 (< 300) Table 7
al.
1982 Ikeda et al. 5 < 4-1075 reinforced plastic boat
(< 1-256) plants
------------------------------------------------------------------------------
a Years when the study was conducted.
The resin manufacturers have tried to decrease styrene
emissions by reducing the styrene content of resins and by
using thixotropic resins and film-forming additives. They
(Nylander, 1979; Synthetic Resins Limited, 1980; Voskamp &
Studenberg, 1981) claim that significant decreases in styrene
emission can be achieved using these new resins, often called
low styrene emission resins (LSE resins). In the study of
Schumacher et al. (1981), the results of laboratory tests on
such resins suggested a 30% reduction in styrene vapour, but
when tested in the workplace there was essentially no
difference in the exposure levels of the workers and the
authors suggested the need for further studies to record any
effects from the use of the new resins.
Concentrations of styrene found during the production of
reinforced plastics (Zielhuis et al., 1964; Götell et al.,
1972; Bodnei et al., 1974; Rosensteel & Meyer, 1977) were
generally much higher than those found in the polystyrene
production plants, with peak concentrations as high as 6300
mg/m3 (1500 ppm). Most of the environmental concentrations of
styrene in reinforced plastic plants, summarized in Table 4,
are 8-h time-weighted-average (TWA) concentrations, but some
values represent shorter periods. It can be seen from the
table that exposure occurs not only in hand layup and spraying
operations using open moulds, but also in mechanical and
closed mould work. Furthermore, workers who do not handle the
polyester resin themselves but work in the laminating room
undergo passive exposure. It should be noted that the
sampling strategy used influences the results; for example,
breathing zone concentrations may differ considerably from the
general air concentrations.
4.2.3. Styrene-butadiene applications
Hazards encountered in the synthetic rubber industry
during the Second World War were discussed by Wilson (1944).
The principal chemicals used in the manufacture of rubber, at
that time, were styrene, butadiene, and acrylonitrile.
More recently, other studies of synthetic rubber
manufacture have shown that levels of styrene are low.
Basirov (1968) reported concentrations of 60-130 mg/m3 (14-31
ppm) in a 1968 study of a synthetic rubber plant. In a health
risk evaluation in a similar type of plant, Gunter & Lucas
(1973) were unable to find any measurable concentrations of
styrene. However, styrene levels varying between 42 and 840
mg/m3 (10 and 200 ppm) were measured in a synthetic rubber
plant by Spinazzola et al. (1980).
4.2.4. Summary of occupational exposure
In summary, occupational exposure to styrene varies
considerably depending on the operations concerned. While styrene
is present in detectable amounts in styrene/polystyrene
manufacturing plants, the greatest exposures occur in the
operations and industries that use unsaturated polyester resins
dissolved in styrene. However, in all industrial operations using
styrene, high levels of exposures occur during the clean-up and
maintenance procedures.
Table 5. Airborne styrene concentrations at 36 Finnish
factories using hand layup methoda
(i) Mean 8-h TWA breathing zone styrene concentrations at 22
factories
---------------------------------------------
Factory mg/m3 ppm Factory mg/m3 ppm
---------------------------------------------
1 1109 264 12 223 53
2 995 237 13 214 51
3 836 199 14 197 47
4 613 146 15 193 46
5 433 103 16 189 45
6 382 91 17 185 44
7 382 91 18 169 38
8 340 81 19 134 32
9 336 80 20 134 32
10 294 70 21 126 30
11 273 65 22 97 23
---------------------------------------------
(ii) Mean breathing zone styrene concentrations during shorter
(1-5 h) industrial hygiene surveys at 14 factories
---------------------------------------------
Factory mg/m3 ppm Factory mg/m3 ppm
---------------------------------------------
23 1029 244 30 416 99
24 819 195 31 302 72
25 50 12 32 235 56
26 769 183 33 193 46
27 622 148 34 105 25
28 521 124 35 92 22
29 491 117 36 67 16
---------------------------------------------
(iii) Distribution of styrene concentrations by product type
----------------------------------------------------------
8-h TWA styrene TWA styrene conc.
conc. for periods of 1-5h
Product type (factories 1-22) (factories 23-36)
mg/m3 ppm mg/m3 ppm
----------------------------------------------------------
Boats 483 115 865 206
Other products
- small objects 210 50 424 101
- large objects 357 85 248 59
----------------------------------------------------------
a Adapted from: Kallioski (1976).
Table 6. Airborne styrene concentrations at a factory in the USAa
---------------------------------------------------
8-h TWA styrene exposure
Job category (mean ± SD)
mg/m3 (ppm)
---------------------------------------------------
Prefabrication 11.8 ± 16.8 (2.8 ± 4)
Gel coating 288 ± 251 (68.5 ± 59.7)
Hand layup 350 ± 179 (83.4 ± 42.7)
Hand layup, other areas 108 ± 110 (25.7 ± 26.2)
Woodwork/upholstery 14.3 ± 8.8 (3.4 ± 2.1)
Final assembly 14.3 ± 8.8 (3.4 ± 2.1)
Custom moulding 27.3 ± 16.4 (6.5 ± 3.9)
Small boat 17.2 ± 4.2 (4.1 ± 1.0)
Miscellaneous 16.4 ± 13.0 (3.9 ± 3.1)
---------------------------------------------------
a Adapted from: Brooks et al. (1979).
Table 7. Airborne styrene concentrations at 12 factories in the USA. The figures in the columns
represent the number of workers in each exposure groupa
-------------------------------------------------------------------------------------------------------
Styrene concentration mg/m3 (ppm)
Classification
0-210 210-420 420-630 630-840 840-1050 1050-1260 > 1260 Totals
(0-50) (50-100) (100-150) (150-200) (200-250) (250-300) (> 300)
-------------------------------------------------------------------------------------------------------
Plant Type
Boat plants 127 144 80 32 25 23 48 479
Non-boat plants 26 47 29 5 3 1 2 113
Totals 153 191 109 37 28 24 50 592
Job Category
Laminators 61 70 61 27 21 11 22 273
Chopper gun operators 37 62 40 25 13 17 30 224
Gel coaters 12 6 4 3 2 27
Totals 110 138 105 55 34 28 54 524
Plant Type (Exposure of Laminators)
Boat plants 52 48 42 26 18 13 20 219
Non-boat plants 6 24 17 2 3 1 1 54
Totals 58 72 59 28 21 14 21 273
-------------------------------------------------------------------------------------------------------
a Modified from: Schumacher et al. (1981).
Table 8. Styrene concentration during various processes in boat production
-----------------------------------------------------------------------------------------------------------------------------
Process Case Personal sampling Stationary sampling
---------------------------------------------- ------------------------------------------------
Na GMb GSDc Ranged Nc GMb GSDc Ranged Nc
-----------------------------------------------------------------------------------------------------------------------------
Lamination A 25 500 (119) 6.8 (1.6) 143-1075 (34-256) 17 550 (131) 5.5 (1.3) 307-731 (73-174)
over boat B 9 273 (65) 5.8 (1.2) 193-378 (46-90) 15 281 (67) 5.9 (1.4) 160-546 (38-130)
shell mould
Installation laminators 3 71.4 (17) 7.1 (1.7) 42-118 (10-28) 20 33.6 (8) 10 (2.4) 8-214 (2-51)
of ribs helpers 5 < 4.2 (< 1) 23.5 (5.6) 8 (D1-2)
Installation laminators 5 92.4 (22) 8.8 (2.1) 25-185 (6-44) 6 29.4 (7) 12.2 (2.9) 8-92 (2-22)
of division helpers 5 D - (D-D)
plates
Auxilary 5 54.6 (13) 16.8 (4) 8-181 (2-43) 5 96.6 (23) 7.1 (1.7) 59-164 (14-39)
lamination
on deck
Lamination on A 4 128 1.4 (104-211) 3 269 (64) 19.3 (4.6) 55-1172 (13-279)
hold walls B 21 127 1.3 (87-215) 20 269 (64) 7.6 (1.8) 101-1088 (24-259)
Equipment 8 2 2.8 (1-17) 15 < 4.2 64.7 (15.4) Ng-38 (Ng-9)
(< 1)
Plant floor A 39 6 24.8 (5.9) 8-76 (2-18)
in general B 14 < 1 69.7 (16.6) Na-38 (Na-9)
-----------------------------------------------------------------------------------------------------------------------------
a The number of workers equipped with personal samplers for 4 h work (1300-1700).
b The geometric mean.
c The geometric standard deviation.
d The minimum and maximum concentration observed.
e The number of sites monitored by stationary samplers for 4 h (1300-1700).
f D = detected but not measurable (less than 4.2 mg/m3 (1 ppm).
g Not detected.
5. CHEMOBIOKINETICS AND BIOTRANSFORMATION
5.1. Uptake
5.1.1. Human studies
5.1.1.1. Uptake by inhalation
Pulmonary uptake of styrene concentrations of 67-164 mg/m3
(16-39 ppm), after a single breath or during exposures of up
to 8 h, has been studied in human volunteers. Depending on
exposure conditions, uptake varied from 45 to 66% (Bardodej et
al., 1961; Fiserova-Bergerova & Teisinger, 1965; Bardodej &
Bardodejova, 1970).
Astrand et al. (1974) examined the pulmonary uptake of
inhaled styrene (210 and 630 mg/m3; 50 and l50 ppm) in 14
healthy subjects at rest and during exercise over 30-min
periods. Blood concentrations of styrene were measurable
shortly after the onset of exposure indicating a rapid
uptake. With light work (50 W exercise), the alveolar
ventilation increased 3-fold, while the alveolar concentration
of styrene barely changed. The concentration of styrene in
arterial blood tripled.
Fernandez & Caperos (1977) measured the uptake of styrene
in 6 volunteers at exposure levels ranging from 294 to
840 mg/m3 (70 to 200 ppm) for periods ranging from 4 to 8 h.
The retention of styrene, measured from alveolar ventilation,
varied from 82 to 93%.
In studies by Ramsey & Young (1978) and Ramsey et al.
(1980) on 4 human volunteers, a single exposure to a styrene
concentration of 336 mg/m3 (80 ppm) for 6 h resulted in a
blood concentration at the end of exposure of about
800 µg/litre. A steady state concentration of about
900 µg/litre was predicted for repeated daily exposure to the
same atmospheric concentrations. The authors assumed a
2-compartment model that allowed a zeroth order uptake rate of
99.6 mg/h to be calculated.
5.1.1.2. Uptake from the gastrointestinal tract
No studies have been found in the literature related to
the uptake of styrene from the human gastrointestinal tract.
5.1.1.3. Uptake through the skin
Styrene is absorbed through human skin when applied in the
form of a liquid, an aqueous solution, or a vapour. Rates of
uptake of between 9 and 15 mg/cm2 per h were reported when
liquid styrene was applied to the skin of the hands and
forearm (Dutkiewicz & Tyras, 1967; 1968). Rates of uptake of
0.004-0.180 mg/cm2 per h were reported when aqueous solutions
containing mean styrene concentrations of between 66.5 and 269
mg/litre were applied. Skin absorption of styrene was some 30
times greater than that of aniline, benzene, or nitrobenzene.
Riihimäki & Pfäffli (1978) measured the percutaneous
uptake of styrene vapour in 2 human volunteers, wearing thin
cloth pyjamas and socks, who were exposed to a styrene vapour
concentration of 2520 mg/m3 (600 ppm) for 3.5 h, including 3
work intervals of 10 min each. Full-face respirators were
used to prevent inhalation uptake and the temperature was
maintained at 25°C with 50% relative humidity. Blood levels
reached a peak 3 h after the start of exposure and then
declined over the next 4 h. These results were equivalent to
a pulmonary exposure of about 84 mg/m3 (20 ppm) for an equal
period of time.
5.1.2. Experimental animal studies
5.1.2.1. Uptake by inhalation
A recent review of studies of the uptake of styrene in
several animal species indicated that styrene is distributed
rapidly throughout the body, is stored in lipid-rich tissues,
and is extensively metabolized (Santodonato et al., 1980). In
a study on rats exposed to styrene concentrations in air of
336, 840, 2520, and 5040 mg/m3 (80, 200, 600, and 1200 ppm)
for up to 24 h, kinetic data obtained by Ramsey & Young (1978)
obeyed a 2-compartment model. From the kinetic coefficients
derived from the data, the authors predicted that there was
little or no potential for bioaccumulation with repeated 8 h
per day exposure to 336 mg/m3 (80 ppm). They claimed that 95%
of the predicted maximum blood concentration of styrene would
be reached during the first day of exposure.
In another study in which rats with an indwelling jugular
cannula were exposed to styrene atmospheres (Withey, 1978;
Withey & Collins, 1979), a 2-compartment model was observed at
exposure concentrations ranging from 210 to 8400 mg/m3 (50 to
2000 ppm) for a period of 5 h. From the assumed zeroth-order
kinetics for uptake together with the rate coefficients for
distribution and elimination, it was possible to predict that
the time to equilibrium or steady-state would be longer at
higher exposure levels.
An autoradiographic study on the uptake of 14C-styrene-8
and 14C-styrene-ring by mice was carried out by Bergman
(1979). Each animal inhaled the vapours from 10 µl of styrene
in a small inhalation apparatus for 10 min. Extensive
uptake was observed in all organs and the potential for
enterohepatic recycling was demonstrated by the appearance of
the radiolabel in the bile. Styrene uptake in the mice was
reported to be low compared with that in human subjects (less
than one half) and this was attributed to the reduced
respiratory frequency in the mouse, induced by the irritant
action of the styrene vapour on the respiratory tract.
5.1.2.2. Uptake from the gastrointestinal tract
There have been few reports on the kinetics and the extent
of uptake of styrene from the gastrointestinal tract in
animals.
A study by Sauerhoff et al. (1976) on male and female
rats, administered a single oral dose of 14C-labelled styrene
at 500 or 50 mg/kg body weight, showed that a greater fraction
of unchanged styrene was excreted via the lung after
administration of the higher dose, a result suggesting that
saturable metabolic pathways were involved. The authors found
that the uptake of styrene monomer from oral doses was greater
for the female than for the male rats.
In a report of a preliminary investigation on the uptake
kinetics of styrene from the gastrointestinal tract of rats
(Withey, 1976), it was noted that the uptake and kinetic
profile after a single dose, administered as an aqueous
solution, was so rapid (peak values being obtained less than 4
min after the administration of the dose) that it was almost
indistinguishable from the kinetics of an intravenous bolus
dose. In contrast, when a similar dose was administered as a
solution in vegetable oil, peak values were not obtained until
4 h after dosing.
5.1.2.3. Uptake through the skin
Wolf et al. (1956) applied unspecified amounts of liquid
styrene to the ear or to the shaved abdomen of rabbits during
periods of 2-4 weeks (10-20 applications). Moderate irritation
and slight necrosis were observed. The authors claimed that, at
the doses applied, there was no evidence that styrene was absorbed
through the skin in amounts sufficient to cause acute, systemic
toxicity.
The percutaneous uptake of styrene in rats has been
measured by immersing the tail of the animal in liquid styrene
for 1 h (Shugaev, 1969). Inhalation exposure was carefully
avoided. Significant uptake of styrene by the liver and brain
was estimated to be between 50 and 70 % of the concentrations
found in the same organs after a 4-h inhalation exposure to a
vapour concentration of 11.8 gm/m3.
5.2. Distribution and Storage
5.2.1. Human studies
5.2.1.1. Controlled human studies
Studies on experimental animals have demonstrated that
styrene is widely distributed throughout the tissues and
organs and that it may accumulate in adipose tissue. Studies
on the distribution of styrene in human volunteers have,
necessarily, been limited to its quantitative analysis in
blood, expired air, and adipose tissue.
In a study by Engström (1978), 7 male subjects were
exposed to a styrene concentration of 210 mg/m3 (50 ppm)
during 30 min at rest and three, 30-min periods on a bicycle
ergometer set at a work intensity of 50, 100, or 150 W. The
mean uptake of styrene was 490 mg. Specimens of subcutaneous
adipose tissue were taken before and after exposure and at 0.5, 2,
4, and 21 h after exposure. Mean concentrations in the adipose
tissue, up to 21 h after exposure, were of the same magnitude (3.6
mg/kg). From the concentration of styrene still detectable l3 days
after exposure, it was possible to estimate an elimination half-
time of between 2.2 and 4.0 days.
Ramsey & Young (1978) exposed volunteers to a styrene
concentration of 336 mg/m3 (80 ppm) for 6 h and observed that
the decay of styrene concentration in the blood following the
exposure fitted a 2-compartment model. They speculated that
accumulation of styrene would not occur following repeated
exposure to concentrations up to 840 mg/m3 (200 ppm).
5.2.1.2. Occupational exposure studies
There have been a number of studies involving the
determination of styrene concentrations in the blood and
adipose tissue of workers occupationally exposed to styrene
monomer. These studies were not only intended to investigate the
correlation between levels in tissues and previous exposure to
styrene but also to see if there was a potential for its
accumulation and storage in adipose tissue. Wolff et al. (1977)
studied 25 workers who allowed from 3 to 66 mg of subcutaneous
gluteal adipose tissue to be taken. It was demonstrated that
styrene persisted in this tissue for as long as 3 days following
exposure, whereas urine metabolites and styrene in expired air
persisted for up to 16 h after exposure. In an extension of this
study (Wolff et al., 1978a), data on levels of styrene in the
gluteal adipose tissue of an additional 25 workers suggested a
correlation with blood styrene and urinary metabolite levels.
Engström et al. (1978a,b) measured air concentrations of
styrene in a polymerization plant and obtained subcutaneous
adipose samples from 3 workers. The TWA air concentrations
ranged from 32 to 84 mg/m3 (7.6 to 20.2 ppm) and the mean
daily styrene uptake of the 3 volunteers was 193, 343, and 558
mg, respectively. Adipose tissue concentrations were 2.8,
4.0, and 8.1 mg/kg, respectively, at the beginning of the
working week, and 4.7, 7.7, and 11.6 mg/kg, at the end.
5.2.1.3. General population studies
Reports on the uptake of styrene in the general population
are lacking.
5.2.2. Experimental animal studies
There have been a number of reports on the distribution of
controlled doses of styrene monomer administered by different
routes in several animal species.
In studies on mice and rats, Shugaev (1969) showed that,
after inhalation exposure to the median lethal concentration
(LC50), for 2 and 4 h, respectively, there was a positive
correlation between the brain concentration of styrene and
lethality. Styrene levels in the perirenal adipose tissue
were some 5 times higher than those in the brain, liver,
kidney, and spleen.
Savolainen & Vainio (1977) studied the organ distribution
of radiolabelled styrene and styrene epoxide following an
intraperitoneal injection of 577 µmol, 3, 6, and 24 h after
dosing. Three hours after dosing, the highest concentration of
styrene was found in the kidneys followed by the brain, duodenum,
liver, spinal cord, lungs, and blood. The kidney concentration was
19 times higher than that in the blood. After 6 h, levels in the
duodenum and brain had decreased whereas levels in the blood had
increased.
The distribution of 14C-styrene-8 and 14C-styrene-ring was
studied in male mice after a 10-min inhalation exposure
(Bergman, 1979). Animals were killed at 0.5, 1, 2, 4, 8, 24,
and 48 h after exposure and subjected to whole-body auto-
radiography. The autoradiograms obtained shortly after
exposure showed that the radioactivity was localized in the
bronchi, lungs, and liver, for both styrene labelled in the
side chain and the ring-labelled compound. Styrene was
apparently cleared from the nervous tissues in less than 1 h,
since no radiolabelled material was detected at 0.5 h.
However, it was retained in adipose tissues for up to 48 h
after dosing. Again, a large proportion of the radioactivity
was found in the bile and kidney suggesting that these routes
are the primary routes of excretion. Radiolabelled material
appeared in the intestinal contents within 1 h of exposure and
persisted for at least 24 h.
A recent inhalation study on rats (Carlsson, 1981) showed
that the largest amounts of 14C-styrene and its metabolites
were found in the kidneys, after an 8-h exposure to 184 mg/m3
(45 ppm). The styrene concentrations were also high in
subcutaneous adipose tissue; the concentrations in the
cerebrum, cerebellum, and muscles amounted to about 70% of the
styrene concentration in arterial blood.
The distribution of styrene was examined in male Wistar
rats after intravenous administration at 4.01 or 13.37 mg/kg
body weight or after a 5 h inhalation exposure at 6
concentrations between 227 and 9408 mg/m3 (54 and 2240 ppm)
(Withey, 1976; Withey & Collins, 1977, 1979). Animals were
killed immediately after the termination of the vapour
exposure, and the blood, heart, kidney, liver, spleen, brain,
and perirenal fat were analysed for their styrene content. At all
exposure levels except the lowest, styrene concentrations in all
organs were higher than that in the blood. At the lowest level of
exposure, the concentration in the kidney was at least 10 times
greater than that in any other organ except the perirenal fat. At
higher levels of exposure, the highest concentration was found in
the liver. Levels of styrene in organs, 8 min after intravenous
administration of 13.37 mg/kg were found in the following
descending order: liver > kidney > brain > blood > heart and
spleen. For the lower dose (4.01 mg/kg), the levels in these
organs were of the same order of distribution, but lower than that
in the blood, suggesting that, as in the case of the inhalation
study, the distribution to the major organs varied with dose.
5.3. Biotransformation
The first step in the metabolic transformation of styrene
is its oxidation, catalysed by cytochrome P-450 dependent
monooxygenases, to oxirane derivatives in the aliphatic chain
or in the aromatic ring (Fig. 1).
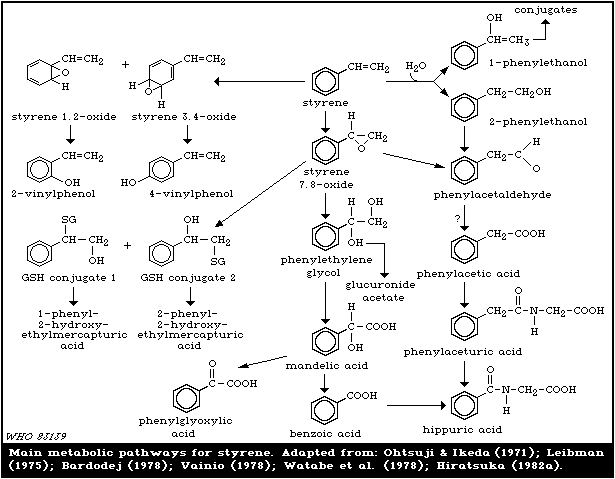 The oxidation of styrene to styrene 7,8-oxide has been
demonstrated in vitro using rat or rabbit liver microsomes
(Leibman & Ortiz, 1969; Leibman, 1975; Belvedere et al., 1977;
Duverger-van Bogaert et al., 1978; Watabe et al., 1978), rat
liver nuclei (Gazzotti et al., 1980) or rat, rabbit, or
guinea-pig extrahepatic microsomes (Cantoni et al., 1978). The
major route of styrene metabolism is believed to proceed via
this epoxide intermediate.
Styrene has been shown to be epoxidized to ( R)- and
( S)-styrene 7,8-oxides in the ratio 1:1.3 by rat liver microsomal
cytochrome P-450 (Watabe et al., 1981a). The ( R)-and ( S)-oxides
are hydrolysed in a regiospecific manner by rat liver microsomal
epoxide hydrolase to ( R)- and ( S)-phenyl-ethylene glycols in the
ratio of 1:4 (Watabe et al., 1981a). Styrene oxides are also
conjugated with glutathione (Oesch, 1973; James et al., 1975;
Pacheka et al., 1979). Rat liver cytosolic glutathione
transferases convert the ( R)- and ( S)-oxides stereoselectively
to S-(1-phenyl-2-hydroxyethyl)-glutathione (GSH conjugate 1) and
S-(2-phenyl-2-hydroxyethyl)-glutathione (GSH conjugate 2),
respectively (Watabe et al., 1981b). Conjugates 1 and 2 would be
directly correlated with the urinary N-acetyl- S-cysteine
conjugates, namely 1-phenyl-2-hydroxyethyl and 2-phenyl-2-
hydroxyethylmercapturic acids, respectively (James & White, 1967;
Seutter-Berlage et al. 1978; Watabe et al., 1982c).
Rats administered styrene intraperitoneally (i.p.) also
excreted 2-vinylphenol and 4-vinylphenol in conjugated forms
in the urine (Hiratsuka et al., 1982a). Styrene 1,2- and
3,4-oxides administered i.p. to rats have been demonstrated in
the urine as 2-vinylphenol and 4-vinylphenol, respectively
(Hiratsuka et al., 1982a). However, 3-vinylphenol has not
been detected in the urine (Bakke & Scheline, 1970; Pantarotto
et al., 1978; Watabe et al., 1978). Other minor metabolites
are 1- and 2-phenylethanol (Delbressine et al., 1980).
Comparing the kinetic parameters of styrene monooxygenase
and styrene 7,8-oxide hydrolase in rat liver microsomes
(Cantoni et al., 1978; Duverger-van Bogaert et al., 1978), it
appears that the conversion of styrene 7,8-oxide into styrene
glycol is preferred. The ratio between styrene epoxide
hydrolase and monooxygenase in rat liver was 2-3 times higher
than those found in mouse and rabbit liver (Cantoni et al.,
1978).
Styrene glycol is partly transformed into a glucuronide
that has been detected in the urine of styrene-treated rabbits
(El Masri et al., 1958). The major metabolic pathway involves
the sequential oxidation to mandelic, phenylglyoxylic, and
benzoic acids, which have been identified in urine (Bardodej,
1964; James & White, 1967; Ohtsuji & Ikeda, 1971).
Quantitative differences have been observed between various
species, hippuric acid being quantitatively the most relevant
urinary metabolite in the rabbit (El Masri et al., 1958),
phenylglyoxylic acid in the rat (Bardodej et al., 1971;
Watanabe et al., 1982c) and mandelic acid in man (Bardodej,
1964; Bardodej & Bardodejova, 1970). Minor metabolites
identified include parahydroxymandelic, parahydroxyphenyl-
glyoxylic, and parahydroxybenzoic acids (Bakke & Sheline,
1970; Pantarotto et al., 1978).
The metabolism of styrene is enhanced by phenobarbital
pretreatment and is inhibited by SKF 525-A, an inhibitor of
monooxygenases (Ohtsuji & Ikeda, 1971).
Styrene 7,8-oxide either binds covalently to macro-
molecules such as microsomal proteins (Marniemi et al., 1977;
Watabe et al., 1978) or conjugates with glutathione
non-enzymatically and in the presence of a transferase (Oesch,
1973; James et al., 1975; Pachecka et al., 1979). Pheno-
barbital pretreatment of the rats enhances, whereas carbon
tetrachloride administration reduces, the NADPH dependent-
binding to microsomal protein in vitro (Watabe et al., 1978).
Prolonged exposure to a styrene vapour concentration of
1260 mg/m3 (300 ppm) leads to an enhancement of the metabolic
elimination of the compound (Vainio et al., 1979) resulting in
decreasing body burden during continued exposure.
Though numerous metabolic pathways have been found in
animal studies (mainly in the rat and rabbit), only a few have
been identified in human beings. Analysis of the urine of
human subjects exposed to styrene has revealed mandelic and
phenylglyoxylic acids as the major metabolites indicating
that, at least qualitatively, styrene metabolism in human
beings follows pathways similar to those previously described
in animal studies. The results of a study by Pfäffli et al.
(1981) suggest that 4-vinylphenol is also excreted in the
urine of workers exposed to styrene. Though this metabolite
represented only 0.3 % of the urinary concentration of
mandelic acid, there was a good correlation between the
excretion of the two metabolites.
Recent data indicate that human whole blood lymphocyte
cultures apparently catalyse the oxidation of styrene to
styrene 7,8-oxide (Norppa et al., 1980a). Belvedere & Tursi
(1981) have shown that both human lymphocytes and erythrocytes
are capable of converting styrene to styrene 7,8-oxide. In
whole blood, red blood cells may be more important in this
conversion, because they are present in greater numbers than
lymphocytes.
5.4. Elimination
5.4.1. Human studies
5.4.1.1. Controlled human studies
In an inhalation study by Fiserova-Bergerova & Teisinger
(1965), 7 human volunteers were exposed to styrene levels of
63-160 mg/m3 (15-38 ppm) for 5 h. From analysis of the
alveolar air, the authors found that pulmonary elimination
followed a bi-exponential curve indicating a 2-compartment
model.
Stewart et al. (1968) found that styrene elimination from
the lungs, after inhalation exposure, was rapid and bi-
exponential but that the rates of elimination varied with the
level and duration of exposure. It was apparent that subjects
who were exposed to a styrene concentration of 1579 mg/m3 (376
ppm) for 1 h had lower elimination rate coefficients for the
terminal phase than those exposed to 907 mg/m3 (216 ppm) and
214 mg/m3 (51 ppm) for 1 h. No values for the rate
coefficients were given in this report but it was calculated
that one subject, who had been exposed to 491 mg/m3 (117 ppm)
for 2 h, exhaled 1.2% of the total amount of styrene absorbed
during the first 4 h after exposure. Exhaled styrene was
detected up to 24 h after the termination of a 30-min exposure
to concentrations of 210 or 630 mg/m3 (50 or 150 ppm) (Astrand
et al., 1974).
The exhalation of styrene after pulmonary exposure was
also examined in human volunteers by Fernandéz & Caperos
(1977). Six subjects were exposed to vapour concentrations of
294-840 mg/m3 (70-200 ppm) for periods of 4 or 8 h. The
concentration of styrene in the expired alveolar air was
reported to decline rapidly during the first hour following
termination of exposure, at all exposure concentrations, and
2.6% of the total absorbed dose was considered to be
eliminated via the lung.
Exhalation of styrene after inhalation exposure to a
styrene concentration of 210 mg/m3 (50 ppm) was measured in 7
volunteers for 4 consecutive 30-min periods either resting or
in a work cycle (Engström et al., 1978b). The exhalation was
followed for up to 19 h after exposure by monitoring styrene
concentrations in the expired air; some 31% of the retained
styrene was estimated to be eliminated via the lungs. In the
same study, styrene eliminated from adipose tissue was
reported to have a half-life of from 2 to 4 days, which led
the authors to conclude that repeated daily exposure to
styrene could lead to bioaccumulation.
In a study by Ramsey & Young (1978), 4 male human
volunteers were exposed to a styrene concentration of 336
mg/m3 (80 ppm) for 6 h and the styrene in the blood and
expired air monitored for 41 h after exposure. The styrene
concentration in blood was found to decay in a bi-exponential
fashion, typical of a linear 2-compartment model. The rapid
initial elimination phase yielded a half-time of 0.58 h and,
for the slower terminal phase, a value of 13.0 h was
reported. The concentration of styrene in the expired air for
the same volunteers showed a biphasic log-linear decline
similar to that observed for the blood concentrations.
In a study designed to investigate the skin absorption of
styrene (Riihimäki & Pfäffli, 1978), two subjects wearing air-
supplied respirators were exposed to a styrene concentration
of 2520 mg/m3 (600 ppm) for 3.5 h while performing light
work. Bi-exponential elimination data were obtained both from
analysis of venous blood and of alveolar air and the authors
reported a value of about l h for the half-time of the rapid
phase and about 10 h for the slower phase.
Studies conducted on 6 male volunteers at styrene
exposures varying from 865 mg/m3 (206 ppm) for 8 h to 294
mg/m3 (70 ppm) for 4 h gave alveolar concentration decay
curves that the authors claimed were best described by a
3-compartment model (Fernandez & Caperos, 1977). The rate
coefficients for the three phases had half-lives that ranged
from 0.06 to 0.61 h for the rapid phase, 1.01 to 3.75 h for
the intermediate phase, and 10.05 to 24.75 h for the slowest
phase.
A study on the rate of excretion of the urinary
metabolites of styrene, phenylglyoxylic and mandelic acids
(Guillemin & Bauer, 1978) was carried out on 9 human subjects
after pulmonary exposure to styrene at 168 or 840 mg/m3 (40 or
200 ppm) for 4-8 h. The authors found that the rate
coefficients for the urinary excretion of metabolites were
unaffected by the duration or level of exposure and claimed
that if the amounts of both metabolites were totalled over 4
days following exposure, reliable measurements of exposure
could be obtained. For mandelic acid, the first and second
phase half-times were evaluated to be respectively 3.9 h and
24.7 h, while for phenylglyoxylic acid the half-time was
calculated to be 10.5 h (Guillemin & Bauer, 1979).
5.4.1.2. Occupational exposure studies
Of the numerous studies that have been conducted on
styrene-exposed workers (Härkönen, 1978), only a few have been
devoted to the assessment of metabolite production.
In a study by Götell et al. (1972), a group of 17 workers
was divided into 3 exposure subgroups, the highest at 987-1226
mg/m3 (235-292 ppm), an intermediate group at 374-584 mg/m3
(89-139 ppm), and the lowest group at 71-134 mg/m3 (17-32
ppm). Decay curves of styrene in expired air for the 3 groups
had a multi-exponential form but no kinetic parameters were
cited. It was evident, however, that the terminal elimination
phase was markedly faster for the group receiving the highest
exposure.
The rate of appearance of mandelic acid in the urine has been
shown to be biphasic, half times being 9 h and 16 h, respectively
(Engström, K., et al., 1976). In a preliminary report on workers,
with limited time points, monophasic elimination with half-times of
8.5 and 7.8 h, respectively for phenylglyoxylic and mandelic acid,
was found (Ikeda & Imamura, 1974). Considerable inter-subject
variability has been reported by several authors (Philippe et al.,
1971; Götell et al., 1972; Härkönen et al., 1974; Gompertz, 1978).
On the basis of findings in animal studies, the excretion
of hippuric acid was investigated in styrene-exposed workers.
An increase in hippuric acid excretion was noted in styrene-
exposed workers (Ikeda et al., 1974); similar levels and
important fluctuations have been found in unexposed controls
(Engström, K., et al., 1978).
5.4.1.3. General population studies
There have not been any reported studies on the
elimination of styrene in the general population.
5.4.2. Experimental animal studies
Results of studies on rats subcutaneously injected with
500 mg/kg 14C-labelled styrene showed that there was rapid
distribution to tissues and organs within 1 h and that 85% of
the radioactivity was eliminated within 24 h; 71% of the
radioactivity appeared in the urine, 12% in expired air as
carbon dioxide, 3% in the faeces, and about 3% as unchanged
styrene in expired air (Danishefsky & Willhite, 1954).
A later study (Sauerhoff et al., 1976) demonstrated a
similar rapid clearance of ring-labelled 14C-styrene in male
and female rats after oral administration of 50 or 500 mg/kg
body weight. More than 90% of the radioactivity appeared in
the urine within 72 h and no residual styrene was found in the
tissues examined at that time. The urinary excretion of
radioactivity followed a 2-compartment model at the low dose
level with half-times of 1.29 and 8.13 h for the initial
(alpha) and terminal (beta) phases, respectively. At the high
dose, a mono-exponential urinary excretion was observed with a
half-time of 6.74 h. About twice as much styrene was
eliminated by the pulmonary route in male compared with female
rats.
Biphasic excretion of radioactivity in the urine of rats
was observed after inhalation of 14C-styrene (252 or
2520 mg/m3; 60 or 600 ppm) for 6 h with half-time values of
14.9 h for the lower and 19.2 h for the higher exposure
concentration (Sauerhoff & Braun, 1976).
Inhalation exposure of rats to styrene concentrations
varying from 336 to 5040 mg/m3 (80 to 1200 ppm) for 6 h
(Ramsey & Young, 1978) also showed evidence of dose-dependent
kinetics. For the 15-fold increase in exposure level, the
area generated under the blood level-time curve increased by a
factor of 112. The elimination kinetics in the post-exposure
period appeared to change from a bi-exponential curve to a
log-linear form with increasing exposure concentrations. At
the 336 mg/m3 (80 ppm) dose level, the hybrid elimination rate
coefficients for the initial and terminal phases were 0.26 h
and 3.60 h, respectively.
Withey (1978) and Withey & Collins (1979) described the
elimination kinetics of styrene in rats after 5 h inhalation
exposure at levels that varied from 188 to 10 151 mg/m3 (44.8
to 2417 ppm). In contrast to the study by Ramsey & Young
(1978), these authors reported a bi-exponential curve of
elimination for all levels of exposure. The reported
half-times for the initial and terminal phases ranged from 3.4
to 6.3 min and about 50 to 350 min, respectively.
Teramoto et al. (1978) examined the elimination rates of
styrene from samples of the liver, brain, kidney, blood,
spleen, muscle, and adipose tissue in rats after an inhalation
exposure to 2940 mg/m3 (700 ppm) for 4 h. They found that all
tissues had a similar elimination half-time of about 2 h with
the exception of adipose tissue for which the styrene
half-time was about 6 h.
To examine the accumulation of styrene in tissues,
Savolainen & Pfäffli (1978) exposed rats to 1260 mg/m3 (300
ppm), for 6 h per day, and 5 days per week, for up to 11 weeks
and then monitored the styrene concentrations in adipose
tissue. Concentrations increased almost linearly, during the
first 4 weeks of exposure and then decreased exponentially.
In the study reported by Bergman (1979) (section 5.2.2),
mice were exposed through inhalation to 10 µl of volatilized
radiolabelled styrene over a 10-min period. The amount of
unchanged styrene exhaled over an 8-h period following
exposure amounted to 2.9% of the calculated dose. The
kinetics of elimination of radioactivity in exhaled air gave a
biphasic curve with half-times of 15 and 275 min for the
initial and terminal phases, respectively. Eight hours after
dosing, high levels of radioactivity were found in the liver,
kidney, lung, and adipose tissue.
Results of a study by Withey & Collins (1977) showed that
the elimination of styrene after intravenous administration in
rats also obeyed a 2-compartment model. Approximate rates of
elimination from some individual organs were obtained for dose
levels of approximately 1.3 and 4 mg/kg body weight. At the
higher dose, the kinetics of styrene disappearance from blood,
heart, brain, liver, spleen, and kidney appeared to follow a
1-compartment model with an elimination half-time of about
14.4 h in the same organs. At the lower dose, a biphasic
elimination was observed with half-times of about 4.78 and 26
h for the rapid and slow phases, respectively.
5.5. Biological Monitoring of Styrene Uptake
The measurement of styrene in blood, subcutaneous fat, as
well as in exhaled air has been suggested for the biological
monitoring of styrene uptake. Because of the efficient metabolism
of styrene and rapid excretion of the metabolites, mandelic and
phenylglyoxylic acid are present in the urine in easily measurable
amounts. The evaluation of the absorption level has usually been
based either on the concentration of one of these metabolites
(Ohtsuji & Ikeda, 1970; Burkiewicz et al., 1974; Härkönen et al.,
1974; Vivoli & Vechhi, 1974; Engström, K., et al., 1976, 1978;
Fernandez & Caperos, 1977; Caperos et al., 1979; Elia et al., 1980)
or on their sum (Bardodej & Bardodejova, 1970; Philippe et al.,
1971; Guillemin & Bauer, 1978, 1979; Wolff et al., 1978a,b; Elia et
al., 1980). "End of exposure" spot specimens have usually been
sampled and results corrected for the dilution of urine. Only this
type of sample is dealt with in the following discussions.
A number of authors have attempted to correlate levels of
styrene in air with levels of mandelic acid in urine, either
from experimental data or field studies, and calculations of
mandelic acid levels corresponding to styrene levels in air of
210, 420, and 630 mg/m3 (50, 100, and 150 ppm) are presented
in Table 9. The differences between the calculated values
could be explained by such factors as: type of exposure,
sampling strategy, individual variability, and differences in
analytical methods.
The use of unspecific analytical methods influences the
results, especially at low levels of styrene exposure, giving
too high values for metabolite concentrations in the urine
(Engström & Rantanen, 1974).
In the case of occupational exposure, a number of
additional elements may have to be taken into account. For
example, in work situations, exposure is usually repeated and
it has been shown that about 30% of the maximal mandelic acid
concentration of the previous day can still be found in urine
samples taken before work on the following day (Engström, K.,
et al., 1976). In another study, the amount of mandelic acid
excreted at the end of the working shift was shown to increase
in the course of the working week (Fernandez & Caperos, 1977).
Increased pulmonary ventilation with increased physical
work load results in a larger total absorption of styrene and
thus increased excretion of metabolites in the urine (Åstrand
et al., 1974; Droz & Fernandez, 1977).
Fluctuations in styrene concentrations in the workroom air
are very common in occupational exposure and may also
contribute to the differences observed in Table 9. A
short-term high peak exposure occurring shortly before the
sampling time may result in a high mandelic acid concentration
in the urine, even though the time-weighted average
concentration of styrene is low (Engström, K., 1983).
Table 9. Mandelic Acid levels* corresponding to various concentrations of
styrene in air
------------------------------------------------------------------------------
Styrene in air
mg/m3 (ppm)
-----------------
210 420 630 Remarks Reference
(50) (100) (150)
------------------------------------------------------------------------------
mandelic (a) 6 12 18 mandelic acid expressed Spasovski
acid as mg/g creatinine; (1982)
spectrophotometry
(a) - 7 - mandelic acid expressed Guillemin &
as mg/g creatinine; Bonet (1979)
gas chromatography
experimental study
(b) 8 14 20 Density 1.024; high Ikeda et al
performance liquid (1982)
chromatography field
study
(b) 10 20 - Density 1.024; Bardodej (1978)
polarography;
experimental study
(b) 9 19 27 Density 1.024; Engström, K.
gas chromatography; et al. (1978)
field study
(b) 14 26 34 Density 1.024 Härkönen et al.
spectrophotometry (1974)
field study
(b) 7-9 12-13 15-19 Density 1.024; Götell et al.
spectrophotometry; (1972)
field study
(b) 6 14 22 Density 1.024; Elia et al.
gas chromatography (1980)
field study
(b) 3 11 19 Density 1.024; Fields et al.
gas chromatography (1979)
field study
------------------------------------------------------------------------------
*(a) = mg mandelic acid/g creatinine.
(b) = mmol mandelic acid/litre urine.
The ratio of mandelic to phenylglyoxylic acid excreted at
the end of exposure varied from about 1 to 4 in different
studies. The ratio appears to increase at higher exposure
levels (Ohtsuji & Ikeda, 1970; Riihimäki & Pfäffli, 1978;
Caperos et al., 1979; Elia et al., 1980). The mandelic
acid/phenylglyoxylic acid ratios were higher in urine samples
taken at the end of exposure than in samples taken later
(Philippe et al., 1971; Guillemin & Bauer, 1979). In terms of
biological monitoring, it is claimed that the mandelic
acid/phenylglyoxylic acid ratio in the urine may be of help in
interpreting the exposure pattern (Philippe et al., 1971;
Guillemin & Bauer, 1979).
The various factors influencing the excretion of these
metabolites are not well known. It may be that such a
difficulty could be overcome by determining mandelic and
phenylglyoxylic acids and combining the results. This may
explain the good agreement obtained in the two studies in
Table l0 based on the sum of the levels of the two acids
corresponding to styrene concentrations in air of 210, 420,
and 630 mg/m3 (50, 100 and 150 ppm).
Table 10. Sum of mandelic acid and phenylglyoxylic acid levels
(mmol/litre) corresponding to various concentrations of styrene in air
---------------------------------------------------------------------------
Styrene in air
mg/m3 (ppm)
-------------------
210 420 630 Remarks Reference
(50) (100) (150)
---------------------------------------------------------------------------
Mandelic acid 8 16 24 density 1.024; Elia et al.
+ gas chromatography; (1980)
phenylglyoxylic field study
acid 11 19 27 density 1.024 Ikeda et al.
aigu performance (1982)
liquid chromatography;
field study
---------------------------------------------------------------------------
The determination of minor metabolites (4-vinylphenol and
hippuric acid) for the biological monitoring of styrene
exposure has also been reported (Ikeda et al., 1974; Pfäffli
et al., 1981).
The difficulties of the biological monitoring of styrene
uptake using urine samples cannot be overcome by selecting
another medium, as even less experience is available with
other media. Styrene concentrations in blood decline rapidly
during the first hour following exposure, which makes the
results strongly dependent on sampling time.
Styrene concentrations in exhaled air are in equilibrium
with blood concentrations, which means that styrene levels in
exhaled air are also sensitive to the changes in concentration
in ambient air. A single sample of exhaled air at the end of
exhalation can be used only for the evaluation of immediate
past exposure. It has been suggested that extrapolation of a
whole day's exposure is possible using several samples taken
after exposure, from which a "decay curve" can be established
(Fernandez & Caperos, 1977; Fields & Horstman, 1979). This
method is inconvenient for the routine monitoring of workers.
The estimation of styrene concentrations in subcutaneous
fat has been proposed as a method of indicating styrene body
burden, even a long time after exposure, when styrene and its
metabolites can no longer be detected in other specimens
(Wolff et al., 1978). However, much more information about
the kinetics of styrene in fat is needed before the relevance
of this method can be evaluated. For obvious practical
reasons, this technique cannot be used routinely.
6. EFFECTS ON EXPERIMENTAL SYSTEMS
6.1. Haematopoietic and Immune Systems
Spencer et al. (1942) did not observe any differences in
erythrocyte count, haemoglobin concentration, total leukocyte
count, and differential count between 18 rats exposed
repeatedly for 7 h a day for 6 months to a styrene
concentration of about 5460 mg/m3 (1300 ppm) and 18 control
animals. The test animals received a total of 137-139
exposures. The same authors did not see any significant
haematological changes in 8 rabbits and 4 monkeys similarly
exposed.
Wolf (1956) gave styrene orally at 66.7-667 mg/kg body
weight to female rats for 6 months, 5 days a week, but did not
observe any effects on the haematopoietic system.
In a study by Quast et al. (1978), styrene was
administered orally to beagle dogs at 200, 400, and 600 mg/kg
body weight for up to 19 months. At the highest dose, Heinz
bodies were regularly detected in the blood erythrocytes of
the dogs. At 400 mg/kg body weight, Heinz bodies were still
frequently seen, but at 200 mg/kg body weight they were
observed only sporadically in female dogs. Heinz bodies
disappeared after withdrawal of styrene. Decreases in packed
cell volume, red blood cell count, haemoglobin levels, and the
erythrocyte sedimentation rate were also observed.
When styrene was fed to rabbits at doses of up to 250
mg/kg body weight for up to 216 days, severe impairment of the
immunological defence system was indicated with the blood
complement titre reduced and leukocyte phagocytic activity
depressed (Sinitskij, 1969).
6.2. Nervous System and Behaviour
The acute neurotoxic effect of styrene is depression of
the central nervous system (Roth, 1979). However, the
narcotic effect of styrene glycol is at least twice as potent
as that of styrene (Parkki et al., 1976) and styrene 7,8-oxide
is more toxic than both styrene and styrene glycol with a
lethal dose 4-5 times lower than that of the parent compound
(Ohtsuji & Ikeda, 1971). Central nervous system depression
follows treatment with styrene 7,8-oxide. After a single
intraperitoneal injection both styrene and styrene oxide have
been shown to bind covalently to macromolecules in the central
nervous system (Savolainen & Vainio, 1977). In a study on
rats by Shugaev (1969), in which styrene was injected intra-
peritoneally, the maximum concentration of styrene in brain
tissue was 250 mg/kg (range 180-324 mg/kg).
Toxic action in the central nervous system in prolonged
(11 weeks) low-level inhalation exposure to a styrene
concentration of 1260 mg/m3 (300 ppm) led to minor changes in
axonal proteins (Savolainen & Pfäffli, 1977). Concomitant
neurophysiological studies, however, failed to demonstrate any
reduction in the peripheral motor conduction velocities
(Seppäläinen, 1978), a suggested clinical feature of the
toxicity of styrene in human subjects.
In studies by Zaprianov & Bainova (1979) on male rats,
repeated oral doses of styrene (1250 mg/kg body weight for 7
days) decreased monoamine oxidase (EC 1.4.3.4) activity. The
authors concluded that the inhibition of monoamine oxidase
activity in total brain may be regarded as a possible link in
the pathogenesis of reported occupational neurophysiological
effects of styrene.
Polystyrene containers were toxic to nerve cells in a
closed tissue culture system, possibly because of the release
of unreacted styrene in the medium (Mithen et al., 1980).
In an open-field test, styrene vapour at 1260 mg/m3
(300 ppm) had marginal effects on animal behaviour, even when
pronounced neurochemical effects were taken into account
(Savolainen et al., 1980).
In a 6-month study, female rats exposed to 200 and 2000
mg/m3 of styrene exhibited enhanced spontaneous activity.
Long-term memory was impaired in male rats after a 4-month
exposure (Vergieva & Zajkov, 1981).
6.3. Kidneys and the Urinary Tract
Oral treatment of rats with styrene at 400 mg/kg body
weight (administered in olive oil, once a day for 132 days
during 6 months) caused an increase in kidney weight. A lower
daily dose of 133 mg/kg body weight did not have any apparent
effect (Wolff et al., 1956).
Vainio et al. (1979) reported that intermittent 11-week
inhalation exposure of rats to low styrene vapour
concentrations of 1260 mg/m3 (300 ppm), 6 h/day, 5 days a
week, enhanced the activities of both drug conjugating
(epoxide hydratase (EC 4.2.1.63), UDP glucuronosyl transferase
(EC 2.4.1.17), and hydroxylating (ethoxycoumarin 0-deethylase,
cytochrome P-450) enzymes in the kidneys.
6.4. Gastrointestinal Tract
No information was available concerning the effect of
styrene on the gastrointestinal tract.
6.5. Liver
Mild liver damage, particularly in the parenchymal cells,
was reported after a single exposure of rats to styrene vapour
at 10 500 mg/m3 (2500 ppm) for up to 21 h (Spencer et al.,
1942). The same authors also reported increases in liver
weight in rats repeatedly exposed to styrene vapour at
5460 mg/m3 (1300 ppm) for 130-139 days. However, they did not
find any liver damage in guinea-pigs exposed for similar
periods to a styrene concentration of 2730 mg/m3 (650 ppm) or
in rabbits and monkeys exposed to 5460 mg/m3 (1300 ppm).
Intermittent 11-week inhalation exposure of rats to a
styrene concentration of 1260 mg/m3 (300 ppm) (6 h/day, 5
days/week) caused histological liver alterations consisting of
hydropic degeneration, and steatosis of parenchymal cells and
congestion. Enhanced activity of both the drug hydroxylating
(ethoxycoumarin O-deethylase, cytochrome P-450) and
conjugating (epoxide hydrolase (EC 3.3.2.3), UDP
glucuronosyltransferase (EC 2.4.1.42)) enzymes in the liver
was detected (Vainio et al., 1979). In a skin-painting study
on rats, styrene at 4 and 8 ml/kg body weight induced
dose-related fatty infiltration of liver cells that was
reversible after 2 weeks without exposure (Burkova et al.,
1982).
Liver cell necrosis was noted in a long-term
carcinogenicity bioassay in several rats in a group exposed to
a high dose of styrene (2000 mg/kg body weight by gavage, 5
days a week, for 78 weeks) (US National Cancer Institute,
1979).
The intermittent inhalation of styrene vapour at a
concentration of 1260 mg/m3 (300 ppm) in air decreased
significantly the reduced glutathione (GSH) concentration in
rat liver (Elovaara et al., 1979). Vainio & Mäkinen (1977)
demonstrated a clear species difference in sensitivity to
styrene-induced depletion of GSH content. The mouse was the
most sensitive and the rat the most resistant species. The
decrease in GSH in liver was dose-dependent. A slight decrease
was observed in rats exposed to a styrene concentration in air
of 420 mg/m3 (100 ppm) (Vainio et al., 1979). When isolated
hepatocytes were incubated in the presence of styrene, there
was also a dose- and time-dependent decrease in GSH
concentrations (Zitting et al., 1980).
When the hepatic GSH in Syrian hamsters decreased to less
than 1 mmol/litre as a result of a high intragastric dose of
styrene (4.5 g/kg body weight), serum aminotransferase
activity increased (Parkki, 1978).
Quast et al. (1979) observed an increased amount of
hemosiderin pigment in liver reticuloendothelial cells of male
and female beagle dogs administered styrene orally at 400 or
600 mg/kg body weight, 7 days a week, for up to 19 months.
The higher dose also increased the number of hepatocellular
intranuclear acidophilic crystalline inclusions.
6.6. Cardiovascular System
No information was available concerning possible effects
of styrene on the cardiovascular system.
6.7. Respiratory System
A single exposure of rats to styrene at 5460-42 000 mg/m3
(1300-10 000 ppm) for 1-4 h caused mucous membrane irritation
as evidenced by lacrymation, nasal discharge, and salivation,
accompanied by aggressive scratching and rubbing of the eyes
and nose. The irritation was particularly severe at higher
concentrations (Spencer et al., 1942). The degree of acute
pulmonary damage varied, in general, with the vapour
concentration of styrene and the length of exposure. When the
exposure was long enough to cause death in some of the exposed
animals, marked pulmonary lesions were induced at all
concentrations studied. Pulmonary changes varied from slight
congestion to congestion, multiple haemorrhage, exudation, and
various degrees of leukocytic infiltration. When another
group of animals (60 rats, 94 guinea-pigs, 12 rabbits, 4
monkeys) were exposed to a styrene concentration of 5460 mg/m3
(1300 ppm) for 7-8 h/day, 5 days a week, over a period of at
least 6 months, microscopic examination of various tissues,
including the lung, did not reveal specific changes in any of
the animals except the guinea-pigs. About 10% of all
guinea-pigs that were exposed repeatedly to styrene at 5460
mg/m3 (1300 ppm) died after several exposures; microscopic
examination of the dead animals revealed pronounced lung
irritation, which was characterized by general acute
inflammatory response. Respiratory insufficiency was the
cause of death (Spencer et al., 1942).
Wolf et al. (1956) exposed animals by inhalation to a
styrene concentration of 5460 mg/m3 (1300 ppm) for 7 h/day,
for 214-360 days. They found slight eye and nasal irritation
in rats and guinea-pigs, but no effects in rabbits and rhesus
monkeys. However, when rats and guinea-pigs were exposed to a
styrene concentration of 2730 mg/m3 (650 ppm), no effects were
observed.
According to Alarie (1973), the concentration of styrene
in air that reduced the respiratory frequency in mice to 50%
was 666 mg/m3 (95% confidence limits 574-758 mg/m3).
When rats were exposed to styrene at 1260 mg/m3 (300 ppm)
for 6 h daily, 5 days a week for 2-11 weeks, no significant
increase in UDP glucuronosyltransferase activity was observed
in the lungs but, after 2 and 4 weeks exposure,
the free glutathione content in the lung was lower (P < 0.05)
than that in the controls (Vainio et al., 1979).
6.8. Endocrine Organs
The adverse effects of styrene on the endocrine organs
have been insufficiently studied. While Izjumova (1972)
reported that repeated exposure to styrene concentrations of
5-50 mg/m3 (1-12 ppm) for up to 4 months prolonged the estrus
cycle in rats, the data were inconsistent from month to month,
and appeared not to be dose-dependent.
6.9. Carcinogenic Effects
6.9.1. Styrene
6.9.1.1. Oral administration
(a) Mouse
In a carcinogenesis bioassay (US National Cancer
Institute, 1979; Chu et al., 1981), 2 groups each of 50 male
and 50 female B6C3F1 mice, 6 weeks of age, were given styrene
at 150 and 300 mg/kg body weight (at least 0.3% impurities) in
corn oil by gavage, 5 days per week, for 78 weeks, and then
observed for an additional 13 weeks. A group of 20 males and
20 females receiving corn oil only (10 ml/kg body weight)
served as vehicle controls. At the end of the study, 78%,
92%, and 100% of the male mice and 76%, 80%, and 80% of the
females were alive in the high-dose, low-dose, and control
groups, respectively. An increased incidence of adenomas and
carcinomas of the lung was seen in treated male mice, i.e., 3
adenomas and 3 carcinomas in 44 animals in the low-dose group,
and 4 adenomas and 5 carcinomas in 43 animals in the high-dose
group, compared with none in the 20 controls. The increased
incidence of both types of lung tumours combined was
statistically significant (P = 0.02) in the high-dose group
compared with the vehicle-treated controls and also in the
Cochrane-Armitage test for linear trend (P = 0.02). It should
be noted that the number of control animals was small (40)
compared with the treated group (100), that the historical
vehicle-treated controls were inadequate, and that the
incidence of neoplasms in historical untreated controls was
12% (32/271). For these reasons, it was not possible for the
Task Group to conclude that styrene was carcinogenic in this
study, even though the results of the study were positive.
(b) Rat
In carcinogenic bioassays (US National Cancer Institute,
1979; Chu et al., 1981), groups of 50 male and 50 female
Fischer 344 rats, 6 weeks of age, were given styrene (at least
0.3% impurities) in corn oil, by gavage, 5 days per week,
either at 1000 or 2000 mg/kg body weight for 78 weeks with
further observation for 27 weeks, or at 500 mg/kg body weight
for 103 weeks with further observation for 1 week. Two
groups, each of 20 males and 20 females served as vehicle
controls. A high mortality was seen in the high-dose group;
only 12% of the males survived 53 weeks of treatment and 14%
of the females survived 70 weeks of treatment. Hepatic
necrosis was observed in several rats. At the 90th week of
the study, 94%, 88%, 85%, and 90% of the males and 92%, 92%,
75%, and 90% of the females were alive in the medium-dose,
low-dose, and the 2 control groups, respectively. There was
no statistically significant difference between the groups in
tumour incidence at any site (National Cancer Institute,
1979). The Task Group noted the high mortality in the
high-dose group and the small numbers of animals in the
control groups.
6.9.1.2. Inhalation exposure
Rat
In a study conducted by Jersey et al. (1978), 2 groups of
84 and 86 male, and 2 groups each of 85 female Sprague-Dawley
rats of the Spartan substrain, 7-8 weeks of age, were exposed
by inhalation to "production grade" styrene (minimum purity
99.5%) containing approximately 5 ppm t-butylcatechol. They
were exposed 6 h/day, 5 days/week, for 18.3 months, if male,
and for 20.7 months, if female. Exposure duration was based
on the time when mortality reached 50% in one of the test
groups of that sex. Initial concentrations of styrene in air
were either 5040 mg/m3 (1200 ppm) or 2520 mg/m3 (600 ppm).
The high level was reduced to 4200 mg/m3 (1000 ppm) after 2
months because of excessive toxicity. Another group of 85
males and 85 females served as untreated controls. At the end
of the 2-year study, 5, 18, and 6 males and 30, 30, and 22
females had survived in the control, low-, and high-dose
groups, respectively. An excessive mortality from chronic
pneumonia occurred in the control and high-dose male groups.
An increased (but not statistically significant) combined
incidence of leukaemias and lymphosarcomas was seen: 1/84
males and 6/85 females in the high-dose groups, 5/86 males and
6/85 females in the low-dose groups, 1/85 males and 1/85
females in the control groups. If the total incidence of
leukaemias/lymphosarcomas in females is compared with
historical controls, it becomes statistically significant
(P < 0.05). A statistically significant increase in the
incidence of mammary adenocarcinomas was seen in the low-dose
female group (7/85 versus 1/85 in controls), but the incidence
was not statistically significant in comparison with
historical controls. Though these data are inconclusive, they
are interpreted by Jersey et al. (1978) as suggesting an
association between the exposure of the female rats to styrene
and an increased incidence of tumours of the leukaemia/
lymphosarcoma types. The Task Group was unable to evaluate
this study because of the high mortality.
6.9.1.3. Pre- and postnatal exposure
(a) Mouse
In the studies of Ponomarkov & Tomatis (1978), 29 pregnant
O20 mice were each given a single treatment of styrene at 1350
mg/kg body weight (99% pure dissolved in 0.1 ml olive oil) by
stomach tube on the 17th day of gestation. A control group of
9 pregnant animals received olive oil alone. The neonatal
mortality rate in the offspring of styrene-treated mice was
43%, compared with 22% in the controls. The same dose of
styrene was then administered weekly, by stomach tube, to 45
male and 39 female progeny from weaning to 16 weeks of age, at
which time the treatment was stopped because of the high rate
of mortality (64% alive at 20 weeks). The study was
terminated at 100 weeks, when all the animals had died. No
differences in tumour incidence were found between mothers
treated with styrene and those given olive oil. In the
progeny that had received weekly treatments, lung tumours
(adenomas and adenocarcinomas) were found in 20/23 males and
32/32 females, compared with 8/19 and 14/21 in olive
oil-treated controls (P < 0.01, P < 0.01) and compared with
34/53 and 25/47 in untreated controls (P < 0.05, P < 0.001).
No differences in tumour incidence at sites other than the
lung were seen in the progeny, compared with olive oil-treated
or untreated controls.
In the same studies (Ponomarkov & Tomatis, 1978), 15
pregnant C57 black mice were each given a single treatment of
styrene at 300 mg/kg body weight dissolved in 0.1 ml olive
oil, by stomach tube, on the 17th day of gestation. A control
group of 5 pregnant animals received olive oil alone. The
same amount of styrene was then given weekly by stomach tube
to 27 male and 27 female progeny from weaning up to 120 weeks
of age, at which time the survivors, 15 males and 12 females,
were killed. Control progeny (12 males and 13 females)
received olive oil for 120 weeks at which time 7 males and 4
females survived. In mothers treated with a single dose of
styrene, lymphomas were observed in 10/12 animals, compared
with 3/5 in the olive oil-treated controls. In treated male
progeny, liver tumours (hepatocellular carcinomas) were found
in 3/24 animals, compared with 1/12 olive oil-treated and 1/47
untreated controls (both hepatocellular adenomas). The
increased incidence of these tumours in styrene-treated
animals compared with controls was not statistically
significant. The small number of animals was noted by the
Task Group.
(b) Rat
Twenty-one female BDIV rats were each given a single
treatment of styrene at 1350 mg/kg body weight dissolved in
olive oil, by stomach tube, on the 17th day of gestation
(Ponomarkov & Tomatis, 1978). A control group of 10 pregnant
rats received 0.3 ml olive oil alone. The neonatal mortality
rate in the offspring was 10% in treated rats and 2.5% in
olive oil-treated controls. Subsequently, doses of 500 mg/kg
body weight were administered weekly by stomach tube to 73
male and 71 female progeny from weaning up to 120 weeks of
age, at which time the survivors, 8 males and 20 females, were
killed. Controls (36 males and 39 females) received olive oil
alone for 120 weeks. Two stomach tumours and 1 hepatocellular
adenoma were seen in styrene-treated females and one stomach
tumour in styrene-treated males. One stomach tumour was also
found in olive oil-treated female controls. The difference in
the incidence of these and other tumours in styrene-treated
rats compared with controls was not statistically significant.
6.9.2. Styrene 7,8-oxide
6.9.2.1. Dermal exposure
Mouse
Forty 13-week-old C3H mice were painted on the clipped
dorsal skin with a 5% solution of styrene 7,8-oxide in
acetone, 3 times weekly for life (Weil et al., 1963). No skin
tumours were observed in 33 animals that survived for 17-24
months (37 mice were alive at 12 months). Another group of
C3H mice were similarly painted with a 10% solution of styrene
oxide in acetone; 18 mice survived 12 months, only 2 mice
survived 17 months, and no skin tumours were observed.
In another study by Van Duuren et al. (1963), the clipped
dorsal skin of 30, 8-week-old male Swiss ICR/Ha mice was
treated 3 times weekly, throughout the life span, with 0.1 ml
of a 10% solution of styrene oxide in benzene. Median
survival time was 431 days in the styrene oxide-treated group
and 262 and 412 days, respectively, in the groups of 30 and 60
benzene-treated controls. Three styrene oxide-treated animals
and 11 (out of 150) benzene controls developed skin tumours.
This difference was not statistically significant. One of the
tumours in each group was a squamous-cell carcinoma.
6.9.2.2. Oral administration
Rat
Groups of 40 male and 40 female Sprague-Dawley rats, 13
weeks of age, were given either 50 or 250 mg/kg body weight,
per day, of styrene oxide (purity unspecified) dissolved in
olive oil, by stomach tube, 4 or 5 days per week, for 52 weeks
(Maltoni et al., 1979). Forty male and 40 female controls
received olive oil alone. The animals were allowed to live
until natural death or were killed 156 weeks after the start
of the study. Preliminary results included only stomach
cancer incidence in animals dying within the first 135 weeks.
The numbers of animals alive at the appearance of the first
tumour (51 weeks) were: controls, 37 males and 28 females;
low-dose, 31 males and 31 females; high-dose, 28 males and 30
females. The numbers of papillomas of the stomach were: 0/37
and 0/28 in controls; 0/31 and 2/31 in low-dose animals; and
3/28 and 6/30 in high-dose animals. The respective numbers
for squamous-cell carcinomas in situ were: 0/37 and 0/28;
5/31 and 6/31; and 11/28 and 12/30. Invasive squamous-cell
carcinomas occurred in 0/37 and 0/28; 2/31 and 1/31; and 4/2;
and 6/30, respectively.
Fourteen female rats were given, by gavage, a single dose
of styrene oxide at 200 mg/kg body weight in olive oil (0.3
ml) on day 17 of pregnancy (estimated total dose, 0.04 g).
Their progeny (62 females and 43 males) received 96 weekly
doses of 100-150 mg/kg body weight in olive oil (0.2 ml), from
weaning (4 weeks of age) until termination of the study (120
weeks) (estimated total doses, 2.5 g for females and 5.0 g for
males). Similar groups of controls (14 pregnant females, 55
female and 49 male progeny) were given only olive oil. All
survivors were killed at the end of the study. There were
fewer tumour-bearing animals among mothers treated with
styrene oxide (31%) than among rats given only olive oil
(57%); mammary tumours were seen only in the olive-oil-treated
animals. The incidences of other tumours were similar.
Forestomach tumours were seen only in treated progeny;
Carcinomas or early carcinomas of the forestomach occurred in
10/42 males and 16/60 females compared with 1/49 and 1/55
controls respectively. Four tumours of the nervous system
occurred in treated groups, whereas only 1 was observed in a
male control. Eight lung tumours were seen in treated
animals, and 2 in controls; 7 of these tumours (6 malignant +
1 benign) occurred among treated female progeny, giving an
incidence of 7/60 (12%) compared with 1/55 (2%) in control
females. The incidences of other types of tumour did not show
any marked differences between control and treated groups
(Ponomarkov et al., 1983; Ponomarkov et al., personal
communication).
6.9.3. Summary and conclusions
Of the 6 studies cited, 3 in mice and 3 in rats, only one
(O20 mice) gave results that indicated a significant increase
in lung tumour incidence. The others either did not show any
significant increase in tumour incidence or were performed in
such a way that conclusions could not be drawn from the
results.
The results of studies concerning styrene 7,8-oxide
indicated: that there was no development of, or increase in,
skin tumours, when the compound was applied to mouse skin;
that tumours (benign and malignant) developed in the
forestomach, when styrene 7,8-oxide was given by gavage in
rats; and that there was an increase in lung tumour incidence
in female progeny, when styrene 7,8-oxide was administered to
rats pre- and post-natally.
6.10. Genetic effects
Several reviews have been published on the mutagenic and
related effects of styrene (Vainio, 1978; IARC, 1979; Norppa,
1981a; Vainio et al., 1982; Zetterberg, 1982; Norppa & Vainio,
1983a).
6.10.1. Chemical reactivity of styrene and styrene oxides
Styrene needs metabolic activation to bind covalently with
nucleophilic biological macromolecules. Styrene 7,8-oxide,
which has been considered to be a primary mammalian metabolite
of styrene (section 5.3), is spontaneously reactive because of
the lability of the oxirane ring. Styrene 7,8-oxide can bind
covalently with proteins and nucleic acids (Marniemi et al.,
1977; van Anda et al., 1979) and is able to alkylate in vitro
4-( p-nitrobenzyl)-pyridine, a synthetic nucleophile, and
deoxyguanosine, a biological nucleophile (Hemminki, 1979;
Hemminki & Falck, 1979; Hemminki et al., 1981).
Hemminki (1979) measured the electrophilic reactivity of
styrene 7,8-oxide by detecting alkylation of 2-amino-1,7-di-
hydro-6 H-purin-6-one (guanine) fluorometrically. The rate of
reaction was roughly equal for guanosine, and deoxyguanosine.
Styrene 7,8-oxide formed a covalent 7-alkyl derivative with
guanine (Hemminki et al., 1980b). With an identical
concentration of guanine, RNA and single-stranded DNA were
substantially less reactive than guanosine, possibly as a
consequence of the tertiary structures of the large relative
molecular mass polymers. Double-stranded DNA was even less
reactive.
Two highly reactive arene oxide derivatives of styrene,
styrene 1,2- and 3,4-oxides were recently synthesized
(Hiratsuka & Watabe, 1982; Watabe et al., 1982a,b). These
arene oxides have a very brief half-life and they are very
hard to detect, even in an in vitro system consisting of
styrene, microsomes, and NADPH.
6.10.2. Mutagenic effects of styrene and styrene oxides in
bacterial assay systems
Styrene has not been shown to induce reverse mutations in
any of the Salmonella typhimurium tester strains used in the
Ames' test, in the absence of a metabolic activation system
(Table 11). However, in the presence of a metabolic
activation system, some investigators have found styrene to be
mutagenic in the Salmonella strains (TA 100, TA 1530, TA 1535)
used to detect base-substitution-inducing mutagens; others
have obtained negative results (Table 11). No positive
results have been reported for styrene in the Salmonella
strains (TA 98, TA 1537, TA 1538) detecting frame-
shift-inducing mutagens. The divergent results on the
mutagenicity of styrene in Salmonella could be explained, in
part, by metabolic differences between the microsomal
preparations (S-9 mix, S-10 mix) used. The high volatility,
poor solubility, and bacterial toxicity of styrene may also
affect the results (de Meester et al., 1981).
Styrene was reported to be positive in the rec assay with
Bacillus subtilis without metabolic activation, in a review by
Kawachi et al. (1979), but no specific data were given.
The primary metabolite of styrene, styrene 7,8-oxide, has
been shown to be mutagenic in a number of bacterial studies
with base-pair strains, without a metabolic activation system
(Table 11). Moreover, styrene 7,8-oxide was mutagenic in the
fluctuation test with Escherichia coli WP2 uvrA (for detecting
base-substitution mutagens) and with Klebsiella pneumoniae
(Hemminki & Falck, 1979; Voogd et al., 1981). The frame-shift
mutagen sensitive strains of Salmonella gave only negative
results with styrene 7,8-oxide, either with or without
metabolic activation systems (de Meester et al., 1977; Stolz &
Withey, 1977; Drinkwater et al., 1978; Wade et al., 1978;
Watabe et al., 1978; Busk, 1979; Hemminki & Falck, 1979;
El-Tantawy & Hammock, 1980). Watabe et al. (1981) did not
find any appreciable difference in the bacterial (Salmonella)
mutagenicity of R-and S-enantiomers of styrene 7,8-oxide and
their racemic mixture. However, Pagano et al. (1982) reported
that the R-enantiomer was more mutagenic to Salmonella than
the S-form, while the racemic styrene 7,8-oxide had an
intermediate mutagenic activity.
Table 11. Mutagenicity of styrene and styrene-7,8-oxide in the base-
pair substitution strains of Salmonella typhimurium and E. colia
------------------------------------------------------------------------
Results of mutagenicity studies
Styrene styrene 7,8-oxide
Metabolic activation Metabolic activation
system: system:
None Present None Present
------------------------------------------------------------------------
Drinkwater et al. (1978) .. .. - -
Glatt et al. (1979) .. .. + ..
Hemminki & Falck (1979) .. .. + ..
Pagano et al. (1982) .. .. + ..
Planche et al. (1979) .. .. + ..
Sugiura & Goto (1981) .. .. + ..
Sugiura et al. (1978a,b) .. .. + ..
Turchi et al. (1981) .. .. + ..
Voogd et al. (1981) .. .. + ..
Wade et al. (1978) .. .. + ..
Watabe et al. (1981a) .. .. + ..
El-Tantawy & Hammock (1980) .. .. + +b
Yoshikawa et al. (1980) .. .. + +b
Kawachi et al. (1979) - - .. ..
Milvy & Garro (1976) - .. + ..
Greim et al. (1977) - - + ..
Busk (1979) - - + +b
Loprieno et al. (1978) - - + +
Stolz & Withey (1977)
Watabe et al. (1981, 1982b) - +c + -
de Meester et al. (1977, - + + +b
1981)
Vainio et al. (1976) -d + + +b
Poncelet et al. (1980) - + .. ..
Cerna & Kypenova (1977) .. +e .. ..
------------------------------------------------------------------------
.. No data.
a Modified from: Norppa (1981a).
b A decrease reported in mutagenic activity, as compared to treatment
without metabolic activation.
c Only with trichloropropane oxide present.
d A weak activity reported also without S-9 mix in TA 1535.
e Host mediated assay, Salmonella strain TA 1950, mice as hosts.
Two arene oxide derivatives of styrene, styrene 1,2- and
3,4-oxide, both of which have been considered to be the
precursors of isolated urinary metabolites of styrene in the
rat, have been demonstrated to be mutagenic towards a
base-pair strain (TA 100) of Salmonella, but not towards a
frame-shift strain (TA 98) (Watabe et al., 1982b). The 1,2-
and 3,4-oxides were more mutagenic than the 7,8-oxide, but
only after sequential addition to the medium during pre-
incubation.
Thus, the results obtained with various bacterial assay
systems show that styrene 7,8-oxide is a direct base-pair
substitution type mutagen. The extremely labile minor
metabolites of styrene, 1,2- and 3,4-oxide, seem to be highly
reactive and are mutagenic for Salmonella. However, with
styrene, even in the presence of a metabolic activation
system, contradictory results have been reported.
6.10.3. Genetic effects of styrene and styrene 7,8-oxide
in eukaryotic non-mammalian systems
Various non-mammalian assay systems, including point
mutations and gene conversion in yeast, point mutations in
silk worm, recessive lethal mutations, sex chromosome loss and
non-disjunction in Drosophila melanogaster, and chromosome
damage in root tip cells of Allium cepa, have been applied to
study the mutagenic and genetic effects of styrene and styrene
7,8-oxide.
In the yeast assays, styrene did not induce mitotic gene
conversions at the adenine and tryptophane loci of
Saccharomyces cerevisiae or forward mutations at the adenine
loci of Schizosaccharomyces pombe, even in the presence of a
mouse liver microsome mix (Loprieno et al., 1976; Loprieno,
1981; Loprieno & Abbondandolo, 1980). According to Bauer et
al. (1980, 1981), this negative result could be because the
epoxide hydrotase (EC 4.2.1.63) (inactivation enzyme) is more
stable than the monooxygenase, because the concentration of
styrene 7,8-oxide in the incubation mixture could not reach a
mutagenic level. When a yeast strain ( Saccharomyces
cerevisiae D7) capable of metabolic activation was used to
test the induction of gene conversions by styrene, a positive
result was reported (Del Carratore et al., 1982). Styrene
7,8-oxide has been found positive in all the yeast systems
(Loprieno et al., 1978; Loprieno, 1981).
When tested in a host-mediated assay, with mice as the
host animal and yeast as the target cells, styrene and styrene
7,8-oxide were weakly mutagenic for some genetic end points
(Loprieno et al., 1978), but these results were later
considered negative for styrene (Loprieno & Abbondandolo,
1980) when a higher number of historical control values became
available.
Table 12. Point mutations, chromosome aberrations and genetic damage induced in cultured mammalian
cells by styrene and styrene 7,8-oxide
-------------------------------------------------------------------------------------------------------
Styrene Styrene 7,8-oxide
Test-system Type of damage References
assayed Metabolic Metabolic
activation system: activation system:
None Present None Present
-------------------------------------------------------------------------------------------------------
Rodent cell lines
V79 HGPRT mutations .. .. + .. Bonatti et al. (1978);
Sugiura et al. (1979)
- - + .. Loprieno et al. (1976,
1978)
- + + -c Beije & Jenssen (1982)
aberrations .. .. + .. Turchi et al. (1981)
micronuclei .. .. + ..
anaphase bridges .. .. + ..
CHL aberrations - +a + .. Ishidate & Yoshikawa
(1980);
Ishidate et al. (1981);
Kawachi et al. (1979);
Matsuoka et al. (1979)
CHO SCEs - +b + +d de Raat (1978)
.. .. + .. Kubiak et al. (1981)
L5178Y TK mutations .. .. + - Amacher & Turner (1982)
Human cells
Lymphocytes aberrations +e .. + .. Linnainmaa et al.
(1978a,b);
micronuclei +e .. + .. Meretoja & Vainio (1979)
aberrations .. .. + .. Fabry et al. (1978)
-------------------------------------------------------------------------------------------------------
Table 12. (contd.)
-------------------------------------------------------------------------------------------------------
Styrene Styrene 7,8-oxide
Test-system Type of damage References
assayed Metabolic Metabolic
activation system: activation system:
None Present None Present
-------------------------------------------------------------------------------------------------------
aberrations +e .. + .. Norppa et al.(1980a,
SCEs +e .. + .. 1981, 1982);
Norppa (1981a);
Norppa & Vainio (l983b)
unscheduled DNA -f .. .. .. Pero et al. (1982)
synthesis
EUE unscheduled DNA .. - + .. Loprieno et al. (1978)
synthesis
Wistar rat
hepatocytes unscheduled .. .. .. - Brouns et al. (1979)
DNA synthesis
-------------------------------------------------------------------------------------------------------
.. No data.
a With methylcholantrene preinduced rat liver S-9.
b Only with cyclohexene oxide present.
c In the liver perfusion system.
d A decrease in activity.
e In vitro activation by erythrocytes suggested.
f Purified lymphocytes.
In Drosophila, a significant increase in the frequency of
X-linked recessive lethals was observed in the offspring of
males fed styrene or styrene 7,8-oxide (Donner et al., 1979).
The test for sex chromosome loss and non-disjunction in
Drosophila gave negative results for styrene (Penttilä et al.,
1980). In a review (Kawachi et al., 1979), styrene was
reported not to induce point mutations in silk worm.
Results of cytogenetic studies in meristematic root cells
of Allium cepa demonstrated that styrene induces metaphase
chromosome breaks and micronuclei (Linnainmaa et al., 1978a,b).
Styrene 7,8-oxide induced micronuclei and anaphase fragments and
bridges in these growing root-tip cells (Linnainmaa et al.,
1978a,b).
6.10.4. Genetic effects of styrene and styrene 7,8-oxide in
mammalian cells in vitro
Point mutations, chromosome damage, and unscheduled DNA
synthesis (UDS) have been studied after treatment of mammalian
cell cultures with styrene or styrene 7,8-oxide (Table 12).
In the Chinese hamster V79 cell line, styrene did not
induce point mutations at the HPRT locus, with or without a
metabolic activation system (S-10 mix) from mouse liver
(Loprieno et al., 1976, 1978; Loprieno, 1981). On the other
hand, Beije & Jenssen (1982) found styrene weakly mutagenic in
the same system after metabolic activation by rat liver S-9
mix. When a liver perfusion system was used for the metabolic
activation of styrene, there was a clear increase in point
mutations at the HRPT locus of V79 cells (Beije & Jenssen,
1982).
Loprieno et al. (1978) examined the effects of styrene (with
mouse liver S-10 mix) and styrene 7,8-oxide on UDS in human
heteroploid EUE cell line. There was an increase in UDS with
styrene 7,8-oxide but not with styrene. Brouns et al. (1979) used
freshly isolated hepatocytes of Wistar rats for UDS detection after
styrene 7,8-oxide treatment. The results were negative, which
according to the authors was due to the rapid inactivation of
styrene 7,8-oxide in the hepatocytes. Pero et al. (1982) did not
find any effects of styrene (without metabolic activation) on UDS
in purified resting human lymphocytes.
Styrene induced chromosome aberrations, micronuclei, and
SCEs in human whole blood lymphocyte cultures, and chromosome
aberrations in Chinese hamster CHL cells in the presence of
metabolic activation (Table 12). Styrene 7,8-oxide induced
chromosome aberrations, micronuclei, and SCEs, in rodent cell
lines and in human lymphocyte cultures without metabolizing
systems (Table 12). The positive effects of styrene in human
whole blood lymphocyte cultures are explained by the ability
of erythrocytes to convert this compound into styrene
7,8-oxide (Norppa et al., 1980a; Belvedere & Tursi, l981;
Norppa et al., l982; Vainio et al., l982).
Without metabolizing systems, styrene 7,8-oxide has been
clearly shown to induce HGPRT mutations in V79 cells (Loprieno
et al., 1976; 1978; Bonatti et al., 1978; Sugiura et al.,
1979; Loprieno & Abbondandolo, 1980; Loprieno, 1981; Beije &
Jenssen, 1982) and point mutations in the TK locus of the
mouse lymphoma cell line L5178 Y (Amacher & Turner, 1982).
With the liver perfusion technique, styrene 7,8-oxide did not
induce point mutations at the HPRT locus of V-79 cells (Beije
& Jenssen, l982).
6.10.5. Genetic effects of styrene and styrene 7,8-oxide in
mammalian systems in vivo
Styrene and styrene 7,8-oxide have been tested in vivo for
their ability to induce genetic damage in somatic and germ
cells in different species (mouse, rat, Chinese hamster) using
different genetic endpoints (chromosome aberrations,
micronuclei, dominant lethals, SCEs and translocations in
spermatocytes; Table 13). Most of the studies concerning
chromosome aberrations in the bone marrow cells of animals
were negative for styrene. No increases in chromosome
aberrations in bone marrow were observed in mice after high
single or repeated doses of styrene administered by gavage or
intraperitoneally (i.p.), or in rats after an unspecified
dose. However, a positive effect for chromosome aberrations
in rat bone marrow was found after inhalation exposure, and
for SCEs in mouse bone marrow, alveolar macrophages, and
regenerating liver cells. In the micronucleus test, styrene
gave negative results in Chinese hamsters but positive results
in mice (Penttilä et al., 1980; Norppa, 1981b).
Studies on the cytogenetic effects of styrene 7,8-oxide in
whole mammals also gave contradictory results (Table 13).
Loprieno et al. (1978) observed a dose-dependent clastogenic
effect in mouse bone marrow cells after oral administration of
styrene 7,8-oxide. However, Fabry et al. (1978) did not find
any chromosome aberrations or micronuclei in the bone marrow
of mice after an i.p. administration. Penttilä et al. (1980)
also failed to detect any effects on micronuclei in Chinese
hamster bone marrow erythrocytes after an i.p. administration.
Negative findings were reported by Norppa et al. (1979) for
chromosome aberrations and SCEs in the bone marrow cells of
Chinese hamsters after inhalation exposure. A slight increase
in SCEs was observed after i.p. injection of a lethal dose;
chromosomal aberrations were also elevated. Conner et al.
(1982) noticed a slight increase in SCEs in regenerating liver
cells and alveolar macrophages (but not in bone marrow) in
hepatectomized mice exposed through inhalation to styrene
7,8-oxide.
Table 13. Cytogenetic damage induced by styrene and styrene 7,8-oxide in various rodent studies in vivo
------------------------------------------------------------------------------------------------------------
Exposure
Species Duration of Type of
(strain) Routea Dose time after damage Result Reference
Cell type treatment studied
------------------------------------------------------------------------------------------------------------
STYRENE
Rat
(Wistar)
bone marrow inhalation 1260 mg/m3 (300 ppm) 9-11 weeks Chromosomal + Meretoja et al. (1978)
(Wistar) aberrations
bone marrow inhalation .. .. Chromosomal - Kawachi et al. (1979)
aberrations
Mouse
(BDF1)
bone marrow inhalation 1625-3872 mg/m3 4 days SCEs + Conner et al. (1979;
alveolar (387-922 ppm) 1980; 1982)
macrophages,
regenerating
liver cells 3872 mg/m3 (922 ppm) 2 days SCEs +
(C57BL/6)
polychromatic i.p. 250 or 1000 mg/kg 30 h Micronuclei + Norppa (1981b)
erythrocytes 500 or 1500 mg/kg 30 h Micronuclei -
(CD-1)
bone marrow p.o. 500 or 1000 mg/kg 24 h Chromosomal - Loprieno et al. (1978)
aberrations
p.o. 4 x 500 mg/kg 4 days Chromosomal - Sbrana et al. (1982)
aberrations
70 x 200 mg/kg 70 days Chromosomal -
aberrations
(ICR)
bone Marrow i.p. LD50 or 5xLD50 6, 24, or Chromosomal - Cerna & Kypenova
2 6 48 h aberrations (1977)
Chinese hamster
bone marrow inhalation 1260 mg/m3 (300 ppm) 4 or 21 Chromosomal - Norppa et al. (1980)
days
polychromatic i.p. 1000 mg/kg 30 h Micronuclei - Penttilä et al. (1980)
erythrocytes
-----------------------------------------------------------------------------------------------------------
Table 13. (contd.)
------------------------------------------------------------------------------------------------------------
Exposure
Species Duration of Type of
(strain) Routea Dose time after damage Result Reference
Cell type treatment studied
------------------------------------------------------------------------------------------------------------
STYRENE 7,8-OXIDE
Mouse
(BDF1)
bone marrow, inhalation 210 mg/m3 (50 ppm) 5 h SCEs +b Conner et al. (1982)
alveolar
macrophages,
regenerating
liver cells
(CD-1)
bone marrow p.o. 500-1000 mg/kg 24 h Chromosomal + Loprieno et al.
aberrations (1978)
(BALB/c)
bone marrow i.p. 250 mg/kg 1-13 days Chromosomal - Fabry et al. (1978)
aberrations
polychromatid 30 h Micronuclei -
erythrocytes
primary 2.5-3 Trans- -
spermatocytes monthsc locations
post-meiotic 1-3 weeks Dominant -
sperm cells lethals
Chinese hamster
bone marrow inhalation 105-420 mg/m3 2,4, or 20 Chromosomal - Norppa et al. (1979)
(25-100 ppm) days aberrations
SCEs -
i.p. 500 mg/kg 24 h Chromosomal -d
aberrations
7 h SCEs +e
------------------------------------------------------------------------------------------------------------
.. No data.
a i.p. = intraperitoneal, p.o. = peroral.
b Negative in bone marrow.
c Considered to be too long time for a positive result.
d Positive in animals who died of the treatment.
e A slight effect.
Only one study has appeared concerning the possible
effects of styrene 7,8-oxide on germinal cells. Fabry et al.
(1978) reported negative results for translocations in primary
spermatocytes after a single i.p. injection and for dominant
lethals in male postmeiotic germ cells of mice.
In summary, the results concerning the genetic effects of
styrene and styrene 7,8-oxide in vivo are conflicting. Positive
results for both compounds have been obtained mainly at toxic or
nearly toxic doses. The studies have dealt with different
indicators of chromosome damage (aberrations, micronuclei, SCEs) in
actively dividing somatic cells. The effects of styrene 7,8-oxide
on germ cells have been examined only in one study, with negative
results. On the basis of the activity of monooxygenase and epoxide
hydrolase in different species, mice would be expected to be more
sensitive to styrene than rats or Chinese hamsters (Cantoni et al.,
1978; Norppa et al., 1979; Norppa, 1981a). Even in mice, styrene
and styrene 7,8-oxide disappeared rather rapidly after
intraperitoneal or oral administration (Bidoli et al., 1980;
Pantorotto et al., 1980; Sbrana et al., 1982). However, the
results of the mutagenicity studies available cannot at present be
interpreted solely according to such differences in metabolism.
6.10.6. Conclusions on the genetic effects of styrene
Styrene is a potential mutagen only after metabolic
activation. Its most important active metabolite seems to be
styrene 7,8-oxide. Arene oxides (styrene 1,2-oxide and
styrene 3,4-oxide) may also be of importance, but at present
their role cannot be evaluated.
Genetic effect tests in bacteria, yeasts, and mammalian cells
in vitro have yielded contradictory results for styrene, in the
presence of microsomal fraction of rodent liver (usually S-9 mix).
This appears to be mostly due to differences in the efficiency of
activation of styrene in different studies.
Results of in vivo studies on the genetic effects of styrene in
mammals are also contradictory. Some of the discrepancies can be
explained by variations in metabolic capacity among the rodent
species used, divergence in dose levels, and by the different
routes of administration.
Styrene 7,8-oxide is a direct mutagen that is clearly positive
in vitro without metabolic activation, but gives contradictory
results in mammals in vivo.
6.11. Effects on Reproductive Function and Teratogenic Effects
(a) Non-mammalian systems
Styrene and styrene 7,8-oxide have been reported to be
embryotoxic and teratogenic in chick embryos (Vainio et al., 1977).
Kankaanpää et al. (1979) found that trichloropropylene oxide
increased the embryo toxicity and teratogenicity of styrene and
styrene oxide. The LD50s were 40 µmol/egg for styrene and 1.5
µmol/egg for styrene oxide. Depending on the dose, malformations
were found in up to 20% of the test embryos but not in the
controls. The types of malformation included: absence of one or
both eyes, eyelid deformation, deformation of the skull,
exencephaly, exteriorization of viscera, etc. Styrene and styrene
oxide have also been found to interfere with the development of sea
urchin embryos (Pagano et al., 1978).
(b) Mammalian systems
When male rats were exposed to a styrene concentration of
200 mg/m3 (48 ppm) for 5 h/day, 5 days per week for 4 months,
Ivanova-Tchemichanska et al. (1982) found decreased osmotic
resistance and mobility of spermatozoa, decreased sulfhydryl
content, and desquamated epithelium in the seminiferous
tubules.
Ragyl'ye (1974) studied the effects of styrene on pregnant
rats exposed to 1.47, 5, and 50 mg/m3 (0.35, 1.2, and 12 ppm)
for 4 h/day throughout gestation. Resorptions were noted at
all exposure levels but the number of malformations was not
significantly increased. Murray et al. (1978) exposed rats
and rabbits to styrene through inhalation (1260 and 2520
mg/m3; 300 and 600 ppm) for 7 h/day from day 6 to 15 (rats) or
from day 6 to 18 (rabbits), and by gavage (in peanut oil, 180
and 300 mg/kg body weight/day). No increase in the incidence
of resorptions or major malformations, compared with controls,
was reported. Delayed ossification was noted in rabbits
exposed to 2520 mg/m3 (600 ppm). In an inhalation study,
Vergieva et al. (l979) exposed rats to styrene at 200 and 400
mg/m3 (48 and 96 ppm) for 4 h daily, 5 days a week, throughout
pregnancy. There was not any evidence of embryotoxicity or
teratogenicity. Mice were exposed to a styrene level of 1050
mg/m3 (250 ppm) and Chinese hamsters to 1260, 2100, 3150, and
4200 mg/m3 (300, 500, 750, and 1000 ppm) for 6 h daily on days
6-16 (18 for hamsters) of gestation. An increased number of
resorptions was noted in both species at the highest
concentration tested. A slight increase (not statistically
significant) was observed in the umber of minor skeletal
malformations (rib fusion, extra ribs) in mice but not in
hamsters (Kankaanpää et al., 1980).
Quast et al. (1978) exposed pregnant Sprague-Dawley rats
and New Zealand white rabbits to styrene concentrations of
1260 or 2520 mg/m3 (300 or 600 ppm) for 7 h/day on days 6-15
(rats) and 6-10 (rabbits) of gestation. Additional groups of
rats were given styrene (90 or 150 mg/kg body weight daily) on
days 6-15 of gestation. No teratogenic effects were detected.
In a 3-generation reproduction study in rats, styrene was
administered in the drinking water at l25 and 250 mg/litre
(estimated daily intake 14 and 21 mg/kg body weight per day
for males and females, respectively, at the high dose). No
adverse effects on reproductive capacity were observed. Some
significant effects on early survival in the second generation
offspring at the high dose were found in 2 specific litters.
No further evaluation could be made, since data for
individuals were not available (Litton Bionetics, 1980).
7. EFFECTS OF STYRENE IN MAN
7.1. Controlled Human Studies
Few controlled exposure studies have been reported in
which the effects of styrene on physiological functions in man
have been investigated. Attention has been focused on the
irritant effects of styrene vapour on the mucous membranes and
on the central nervous system effects caused by styrene
inhalation.
The odour perception threshold for styrene in air was
determined by Smith & Hochstettler (1969) to be as low as
0.2-0.34 mg/m3 (0.05-0.08 ppm). At higher concentrations, the
odour of styrene was clearly perceptible and it was reported
as "strong but not objectionable" at about 420 mg/m3 (100 ppm)
(Stewart et al., 1968). The ability to detect the odour
typically fades as the exposed individuals become adapted
(Stewart et al., 1968). Styrene vapour was irritating to the
eye and the nose at concentrations exceeding 840 mg/m3 (200
ppm) and, when the styrene concentration approached 1596 mg/m3
(380 ppm), it was poorly tolerated (Stewart et al., 1968).
Strong immediate irritation of the eyes and the respiratory
tract has been reported at styrene concentrations in air of
2520-3360 mg/m3 (600-800 ppm) (Carpenter et al., 1944; Wolf et
al., 1956).
Oltramare et al. (1974) exposed 6 volunteers to styrene at
210, 420, and 840 mg/m3 (50, 100, and 200 ppm) over 1-3 h and
noted that for a combination of symptoms of the mucous
membrane (irritation of eyes, nose, and lips) and central
nervous system (dizziness, headache, drowsiness, difficulty in
concentrating, lightheadedness, fatigue), the number of
symptoms increased with dose. This increase was still evident
at the 210 mg/m3 (50 ppm level). At the 2 higher levels,
digestive disturbances also occurred. In studies by Gamberale
& Hultengren (1974), 12 volunteer subjects were exposed to
styrene vapour through a mouth tube at concentrations
increasing stepwise 210, 630, 1050, and 1470 mg/m3 (50, 150,
250, and 350 ppm), each successive step lasting about 30 min.
When the volunteers were asked afterwards to rate their
subjective sensations (during the exposure) it appeared that
exposure to styrene had made them feel more tense and more
"affected" compared with control conditions. The authors also
studied the psycho- physiological performance of the
subjects. Simple reaction times tended to be prolonged with
increasing exposure. The changes became statistically
significant during the final 30 min, when the environmental
concentration reached 1470 mg/m3 (350 ppm). Tests on
perceptual speed and manual dexterity did not show any
impairment in relation to styrene exposure.
Stewart et al. (1968), however, noted an impairment in
performance of tasks involving manual dexterity and eye-hand
coordination in some subjects exposed to a styrene
concentration of about 1596 mg/m3 (380 ppm) over a 1-h
period. On the basis of a study on 3 volunteers, Oltramare et
al. (1974) reported impaired reaction time to visual stimuli,
to combined visual-acoustic stimuli, and in a test of diffuse
attention during and immediately after exposure to styrene at
210, 420, and 840 mg/m3 (50, 100, and 200 ppm) for 1.5 h.
There was no clear dose dependance. Inhalation exposure to
high concentrations of styrene (3360 mg/m3; 800 ppm) caused
symptoms of impairment of balance in 2 subjects and a marked
unsteadiness in posture was observed (Carpenter et al.,
1944). At a styrene level of about 1596 mg/m3 (380 ppm) for 1
h, 2 out of 5 subjects showed abnormal results in the modified
Romberg test indicating difficulty in maintaining balance.
Furthermore, after a 7-h exposure to about 420 mg/m3 (100
ppm), 3 out of six subjects reported that they had transitory
difficulty in performing the modified Romberg test, though no
objective signs of impairment of balance were found (Stewart
et al., 1968). Oltramare et al. (1974), in a study on 3
subjects, showed impairment of balance on a body sway platform
at a styrene concentration of 840 mg/m3 (200 ppm) but not at
420 mg/m3 (100 ppm).
Five volunteers were exposed by Odkvist et al. (1980) to
styrene at about 1260 mg/m3 (300 ppm) for 1 h, using a mouth
tube. Treatment was combined with exercise on a bicycle
ergometer with a 50 W workload, and observations were made on
both equilibrium ability and functioning of the vestibular
system immediately after exposure. None of the subjects
exhibited spontaneous, fixation or positional nystagmus,
whereas an impairment of eye-tracking ability was found in all
individuals in an optokinetic test. The difference in
eye-movement changes between styrene-exposed and control
groups was not statistically significant. No styrene-induced
changes were found in conventional clinical tests of balance
(standing on one leg with eyes closed, walking a line with
eyes closed). The results were thought to suggest that
exposure to styrene (inducing blood levels of approximately 80
µmol/litre) decreased the inhibitory effect of the cerebellum
on the motor function of the eyes.
In summary, the odour threshold for styrene was found to be
0.2 - 0.34 mg/m3 (0.05-0.08 ppm) and the odour was uncomfortable at
elevated concentrations. Styrene induced subjective symptoms of
irritation of the mucous membranes at concentrations exceeding
420 - 840 mg/m3 (100-200 ppm). In the same concentration range,
subjective symptoms of the central nervous system, such as
dizziness, lightheadedness, headache, and drowsiness may occur.
Reaction time, performance, and body balance tend to be impaired by
short-term inhalation exposure to styrene at concentrations of
630 - 840 mg/m3 (150-200 ppm) and definite impairment occurs at
concentrations exceeding 1470 mg/m3 (350 ppm).
7.2. Epidemiological Studies
7.2.1. Haematopoietic and immune system
Chmielewski & Renke (1975), who studied a group of 101
workers exposed for at least one year to a styrene
concentration of 100-300 mg/m3 (24-72 ppm), did not
demonstrate any appreciable effects on the haemoglobin
concentration, erythrocyte count, leukocyte count, or
differential count. Workers exposed for more than 10 years,
however, had a slightly decreased thrombocyte count compared
with workers exposed for shorter periods.
In a study on 494 workers at different levels and
durations of exposure, Lorimer et al. (1976) observed a random
distribution of abnormal haemoglobin concentrations, and
leukocyte or platelet counts between the groups. Thiess &
Friedheim (1978), who investigated 84 workers exposed to
210-2100 mg/m3 (50-500 ppm) for 1-36 years, did not notice any
appreciable differences in haemoglobin concentrations, or
leukocyte, erythrocyte, or platelet counts compared with a
reference group.
In the studies of Chmielewski & Renke (1975), and Renke &
Chmielewski (1976), 20 styrene workers did not show any
differences in bleeding time or fibrinogen level compared with
a reference group. However, the coagulation time and the
adhesivity of the platelets were somewhat increased in the
styrene-exposed group and the prothrombin index was slightly
reduced.
There is very little information on the immunological
effects of styrene. In the immunophoresis study of
Chmielewski et al. (1973), no dose-related differences were
observed in concentrations of serum gamma globulin among
workers exposed to different concentrations of styrene.
7.2.2. Nervous system
Four studies have dealt with reaction times among workers
occupationally exposed to styrene. One study (Götell et al.,
1972) in which 17 men were exposed to a styrene concentration
of 630 mg/m3 (150 ppm) showed prolonged simple reaction times
in the styrene-exposed workers, both in the morning and in the
afternoon, compared with an age-matched control group. A
second study of 106 workers in 4 work places indicated
longer and more irregular reaction times in workers exposed to
styrene than in controls (Gamberale et al., 1975). The
differences were still present after a night's rest. The mean
styrene concentration determined by continuous measurement in
the workers' breathing zone was 57-426 mg/m3 (13.6-101.4
ppm). The mean duration of exposure was 2.7 years (range
0.1-11.0 years).
Another study with a similar study design, also revealed
prolonged reaction times during the working day among styrene-
and acetone-exposed boat manufacturers (Kjellberg et al.,
1979). The exposed group (7 workers, average styrene
concentration 37 mg/m3 (9 ppm), acetone concentration
82 mg/m3, mean employment time 10.5 years) did not show any
deterioration in sensory motor functions in relation to
styrene exposure and the addition task. There was a
correlation between the reaction-time impairment and the total
uptake of styrene divided by the estimated amount of adipose
tissue.
In the study of Kjellberg et al. (1979), the reaction
times of 3 subjects were followed after removal from exposure.
Reaction times improved after 4 days and there was a further
improvement after 35 days.
The study by Cherry et al. (1980) dealt with 27 workers
(mean age, 23 years), who were exposed to a time-weighted
average level of styrene of 386 mg/m3 (92 ppm). Among the
psychological and behavioural tests applied, differences
between the exposed and unexposed groups were seen only in
reaction times.
Several case reports have described neurasthenic symptoms
among patients who had been occupationally exposed to styrene
(Klimkova-Deutschova, 1962; Axelson et al., 1974; Spasovski et
al., 1976).
A considerable percentage of 101 workers employed in the
polyester laminate industry had disturbances of the nervous
system, particularly of the vegetative nervous system. The
exposure level of the workers ranged from approximately 100 to
300 mg/m3 (24 to 72 ppm). The urinary mandelic acid excretion
was more than 400 mg/litre in 36 of the workers (Chmielewski
et al., 1973).
Schneider & Seeber (1979) used psychological tests on 2
groups occupationally exposed to styrene, one group (35
persons) having a mean exposure of 700 mg/m3 (168 ppm), and
the other (46 persons), a mean exposure of 300 mg/m3
(72 ppm). Exposure was associated with poor visual attention,
interferences with attention span, perceptual inaccuracy, and
decreased manual dexterity.
In studies concerning 98 male laminating workers having a
mean urinary mandelic acid concentration that varied from 7 to
4715 mg/litre (median concentration 808 mg/litre), symptoms of
tiredness, and difficulty in concentrating were mentioned more
often than among a reference population (Härkönen, 1977). The
duration of styrene exposure varied from 0.5 to 14 years
(median 5.1 years). In psychological tests, the same
styrene-exposed workers showed visuomotor inaccuracy and poor
psychomotor performance (Lindström et al., 1976).
Exposure-effect relationships were drawn between the urinary
mandelic acid concentration and a combined score for 3 tests
(symmetry drawing, the Bourdon-Wiersma test, and the Mira
test). Analysis of the exposure-response relationship for
these tests showed a significant increase in response for
workers with levels of urinary mandelic acid exceeding 1600
mg/litre (75% having mandelic acid levels of more than 2000
mg/litre) (Härkönen et al., 1978). The proportion of abnormal
EEGs also increased with increasing exposure levels
(Seppäläinen & Härkönen, 1976). Workers with urinary mandelic
acid concentrations above 700 mg/litre had significantly
(P < 0.05) more frequent abnormal EEGs than those with lower
levels (Seppäläinen & Härkönen, 1976; Härkönen et al., 1978).
EEG abnormalities have been reported to be more prevalent
among young styrene workers, especially during the first years
of exposure (Dolmierski et al., 1974; Chmielewski et al.,
1977). EEG abnormalities, indicated mainly by increased slow
activity in the theta range, did not increase with longer
exposure (Roth & Klimkova-Deutschova, 1963; Seppäläinen &
Härkönen, 1976), though paroxysmal episodes have also been
evident (Dolmierski et al., 1974; Seppäläinen & Härkönen,
1976). Results of a study by Rosén et al. (1978) in which 33
men were exposed to styrene concentrations ranging from less
than 21 mg/m3 (5 ppm) to 735 mg/m3 (175 ppm), showed an
increased beta activity in 9 subjects and slow activity (theta
waves) in 6. The EEG abnormalities were non-specific and
resembled those caused by various other solvents (Rosén et
al., 1978; Seppäläinen et al., 1980).
Styrene exposure may also affect peripheral nerves
(Matsushita et al., 1968, Lilis et al., 1978; Rosén et al.,
1978). Lilis et al. (1978) found distal hypoaesthesia in the
lower extremities of 412 styrene-exposed workers (persons with
diabetes mellitus, a history of back injury, and/or excess
alcohol intake were excluded). The frequency of this symptom
varied from 4.1% among those exposed for less than 7 years to
8.5% among those exposed for more than 20 years. Abnormally
low motor conduction velocity (MCV) of the radial nerve was
found in 15 out of 80 persons tested and a slowed MCV of the
peroneal nerve in 12 out of 73 tested. The MCV of the
peroneal nerve decreased with increasing length of exposure,
but the effect of age was not considered. However, the level
of exposure did not have any effect on the peroneal nerve
conduction. The MCV of the radial nerve was not correlated
with the length or level of exposure. Rosén et al. (1978) did
not find differences in the MCVs of the median, ulnar,
peroneal, or posterior tibial nerves of 33 workers exposed to
styrene, compared with normal controls. However, the
amplitude of the sensory action potential of the median nerve
was reduced and the duration of this potential increased among
styrene-exposed workers, even in 10 individuals who had been
exposed to styrene levels of less than 21 mg/m3 (5 ppm). The
dose-effect relationships were conflicting. Disorders in
attention span and movement coordination in psychological
tests were reported in 12 out of 102 workers, occupationally
exposed to styrene concentrations of 10-100 mg/m3, who had
urinary mandelic acid concentrations of 30.9 - 268 g/kg
creatinine (Spasovski et al., 1980). The same authors also
reported diminished alcohol tolerance among styrene workers.
In summary, the results of 3 studies showed that simple
reaction times were prolonged in workers occupationally exposed to
styrene at levels below 630 mg/m3 (150 ppm), while one study gave
equivocal results at a time-weighted average level of styrene in
air of 386 mg/m3 (92 ppm). Another study suggested that the effect
on reaction time was reversible.
Surveys of styrene-exposed workers have shown that an increased
incidence of abnormalities in electroencephalographic recordings
at mean styrene levels of less than 420 mg/m3 (100 ppm) was related
to styrene exposure levels. Some slight disturbances in visuomotor
accuracy and psychomotor performance were noted in workers exposed
to levels of the order of 210 mg/m3 (50 ppm) or more. Various
neurasthenic and autonomic symptoms were also reported among these
workers.
7.2.3. Kidneys and the urinary tract
There are only a few studies of the effects of styrene
exposure on the human kidney. Härkönen (1977), who studied
laminating workers exposed to styrene for periods ranging from
0.5 to 14 years, could not find any cytological changes
differing from Papanicolau I in 35 urine specimens. Results
of studies concerning mortality and morbidity rates due to
kidney and urinary tract disorders have not revealed any
differences between subjects exposed to styrene and unexposed
subjects (Lorimer et al., 1978; Thiess & Friedheim, 1978).
In studies on organic solvent exposure in relation to
kidney function, Askergren et al. (1981) reported that workers
exposed to organic solvents, especially to styrene, excreted
significantly larger quantities of albumin in the urine than
unexposed workers. No differences were observed in beta-2-
microglobulin excretion. The same author compared the
excretion of erythrocytes and leukocytes in the urine of 101
men, occupationally exposed to styrene or toluene or to a
combination of xylene and toluene, with the cell excretion in
the urine from 39 unexposed controls. While no changes in
glomerular filtration rates were observed, the urine of
exposed men contained significantly more cells than that of
the controls.
7.2.4. Gastrointestinal tract
Basirov (1975) reported studies on the digestive system of 130
workers (89 men, 41 women) engaged in styrene-butadiene synthetic
rubber manufacture. The workers were mostly 20-40 years old and
the length of exposure varied from less than 5 years (16 workers)
to more than 10 years (28 workers). Average styrene concentrations
were 60-130 mg/m3 (14-31 ppm). Tests of secretory, excretory,
motor, and pepsinogen-generating functions of the stomach were
conducted on 20 unexposed people and on 80 workers who first
developed symptoms related to the digestive system after working in
the plant. Thirty-six had decreased digestive function, 25 had
decreased peristalsis, and 51 had decreased acidity. In further
studies, chronic gastritis was diagnosed in 35 of these workers.
7.2.5. Liver
Lorimer et al. (1976) assessed the liver function of 493
styrene-exposed workers. Exposure concentrations were not
measured. The activities of alkaline phosphatase (AP) (EC
3.1.3.1), aspartate aminotransferase (ASAT or GOT) (EC 2.6.1.1),
alanine aminotransferase (ALAT or GPT) (EC 2.6.1.2), and gamma
glutamyltranspeptidase (gamma-GTP) (EC 2.3.2.1) and the amount of
serum bilirubin were determined. Only the gamma-GTP activity
showed a significant elevation in the high-exposure group compared
with the low-exposure category, even when alcohol intake was taken
into account (1.7% versus 7.2%; 0.01 < P < 0.02).
Axelson & Gustavson (1978) gathered data on ASAT, ALAT in
serum from 35 styrene-exposed male workers and 12 unexposed
controls. The time-weighted average exposure levels were less
than or about 420 mg/m3 (100 ppm). The average transferase
levels were higher among men who had been exposed, but only
ASAT was significantly elevated (P < 0.001). No differences
were found in the average levels of AP.
The serum levels of ALATP, ASAT, gamma-GTP, LDH, and SDH
(sorbitol dehydrogenase (EC 1.1.1.14)) enzymes were studied in 72
workers exposed to styrene in the manufacture of plastic boats.
The exposure levels varied between very low concentrations < 21
mg/m3 (< 5 ppm) and about 170 mg/m3 (40.8 ppm). The styrene-
exposed groups had a higher mean gamma-GTP value than the control
group (P < 0.05). However, there was no dose dependence
(Lundberg, 1981).
Vihko et al. (1983) studied 25 persons exposed to styrene at
about 126 - 168 mg/m3 (30 - 40 ppm) (mean exposure time 3 years,
all exposures exceeding 1 year). The serum activities of ALAT,
ASAT, gamma-GTP, and LD and the concentrations of serum total
bilirubin and conjugated bilirubin were determined, as well as
serum bile acids, cholic acid, and chenodeoxycholic acid. An
elevated concentration of chenodeoxycholic acid in serum was the
most frequently found parameter among styrene-exposed workers.
Spasovski (1976) showed that the serum protein profile changed and
that serum transaminase activity was elevated at a styrene
concentration of 2000 mg/m3 (500 ppm).
In conclusion, a clear-cut trend towards altered liver
function was not demonstrated. At low exposure levels, the
commonly used parameters such as serum activity of enzymes of
hepatic origin have given equivocal results.
7.2.6. Cardiovascular system
The thorax radiographs of 84 workers were evaluated before
the beginning of employment and after exposure to styrene at
210 - 1260 mg/m3 (50 - 300 ppm) for 1-36 years (Thiess &
Friedheim, 1978). The authors could not attribute any grossly
observable changes to styrene exposure, nor did they observe
any "gross pathological indications in the electrocardiograms"
compared with those of a reference group of 62 subjects.
7.2.7. Respiratory system
In clinical studies, Wilson (1944) found that styrene-
exposed workers reported irritation of the eyes, throat, and
respiratory tract. It has frequently been reported that
people in areas of high styrene exposure complain of
irritation of the eyes and nasopharynx. Götell et al. (1972)
examined 15 workers occupationally exposed to a time-weighted
average styrene concentration of 71 - 1218 mg/m3 (17 - 290 ppm)
and found that lung function tests (forced vital capacity and
forced expiratory volume in 1 s) were normal and did not
change during the working day. However, the concentrations of
styrene that gave rise to complaints varied from person to
person, apparently depending on individual tolerance. In a
study on styrene-exposed workers who had respiratory tract
symptoms, it was suggested that long-term exposure to styrene
could cause obstructive pulmonary changes (Chmielewski &
Renke, 1975).
In a cross-sectional study of clinical signs and symptoms
of irritation (Lorimer et al., 1978) in 488 styrene-exposed
workers, the workers were divided into 2 groups, based on
estimated exposures of 4.2 mg/m3 (1 ppm) and > 21 mg/m3 (> 5
ppm), mostly above 84 mg/m3 (20 ppm). When asked about lower
respiratory symptoms, significantly more workers in the
high-dose group responded in the affirmative. There were no
differences in length of employment and the prevalence of
upper respiratory symptoms between the 2 groups.
In a symptom survey (Härkönen, 1977) of 98 male laminating
workers occupationally exposed to styrene, symptoms that
fulfilled the British Medical Research Council's criteria for
simple chronic bronchitis were more common in the exposed than
in an unexposed group. In the exposed group, 28% had simple
chronic bronchitis, compared with 12% in the control group
(P < 0.05). The smoking habits of the groups did not differ.
7.2.8. Endocrine organs
In a study that involved 25 rubber workers, exposed to
styrene, and 26 age-matched controls, Wink (1972) did not
observe any significant differences in the urinary excretion
of 17-ketosteroids or 17-ketogenic steroids. Exposure data
were not given. However, Chmielewski et al. (1973) reported
reduced excretion of 17-ketosteroids in the urine in 27 out of
67 workers exposed to styrene concentrations of 100-300 mg/m3
(24 - 72 ppm), but these findings were not convincingly
documented. The evaluation of possible effects of styrene on
the steroid metabolism should be studied using more refined
techniques.
In the study of Chmielewski et al. (1973), glucose
tolerance was observed to be somewhat higher in the exposed
group than in the control group, and even more in the group
with higher mandelic acid concentrations in the urine (limit
400 mg/litre). Evidence of effects of styrene on the
endocrine glands was lacking.
7.2.9. Carcinogenic effects
(a) Styrene production and polymerization processes
A survey of 560 individuals from a styrene production and
polymerization plant was reported by Lilis & Nicholson (l976)
and Nicholson et al. (1978). In 1960, the workers had been
employed in the plant for at least 5 years. It is known that
benzene, coke-oven products, butadiene, and ethyl-benzene had
been used in this plant prior to 1965. Among 83 deaths, 17
were due to cancer, including 2 cases of leukaemia and one of
a lymphoma. When the total cancer mortality in this cohort
was compared with that in the general population, no excess of
cancer incidence was found, but the follow-up period may have
been too short to detect an increased risk of neoplasia. One
case of leukaemia and one of lymphoma were found in 21
additional death certificates of individuals who had been
employed for less than 5 years in 1960. In an additional
analysis of 444 death certificates on all individuals who had
been employed for at least 6 months in the plant, 7 cases of
leukaemia and 5 cancers of the lymphatic system were found.
However, the populations at risk are not known.
A cohort of 1960 workers employed for at least 1 month in
a styrene-polystyrene manufacturing plant was followed from
1931 to 1976 (Frentzel-Beyme et al., 1978). Analysis of
deaths occurring (after a minimum period of 5 years) in groups
exposed for 5, 10, 15, and over 15 years did not indicate an
increase in cancer mortality in any of the groups compared
with the reference population. The Task Group noted, however,
that the mortality rates for the reference population were
only available for the period 1970-75, and that follow-up was
incomplete.
Ott et al. (1980) carried out a retrospective cohort study
in workers involved in the production of styrene and
polystyrene. Mortality experience was followed from 1935 to
1975. Among 282 deaths, 6 cancers of the lymphatic and
haematopoietic tissues (except leukaemia) and 6 cases of
lymphocytic leukaemia were observed, compared with 4.5 and
2.9, respectively, expected on the basis of US white male
mortality rates and 2.6 and 1.6 deaths expected from a
comparison industrial cohort. Four deaths from leukaemia were
observed in a sub-group of workers exposed to styrene, ethyl
benzene, oligomers of styrene, mineral oil, polymer dusts, and
extrusion fumes; 0.5 deaths would have been expected on the
basis of US white male mortality experience. Only one of the
4 individuals had spent at least 5 years in the working
environments under study. Inclusion of cases of leukaemia
still alive at the end of the study period or identified
through other conditions listed on the death certificate
increased the total number of lymphoreticular malignancies to
21 cases. These included 7 lymphocytic leukaemias, 4 other
leukaemias, 4 multiple myelomas, 4 Hodgkin's diseases, and 2
other lymphomas. Five of the subjects with lymphocytic
leukaemia had worked in the area of polymer extrusion and for
4 of them the disease developed 20 or more years after the
first exposure. According to the authors, there was no
increase in the general cancer incidence. The incidence of
lymphatic leukaemia was, however, more than that expected.
(b) Lamination and related processes in boat building
An epidemiological study was carried out on 1500 workers
from 36 companies in the reinforced plastics and plastic boat
industry. The majority of the workers were between 30 and 40
years old. The average period of employment was from 6 to 7
years but half of the workers had been in this type of work
for less than 3 years, while 54 had been employed for over 20
years. Exposure was estimated to be to an average styrene
concentration of 1050-1470 mg/m3 (250-350 ppm) at the end of
the sixties and the beginning of the seventies. Seventeen
cases of cancer were found in this group. In 3 of these, the
cancer was present before their employment. In the remaining
l4 cases, the sites and distribution of the various types of
tumours were in accordance with data from the cancer
registry. Among the deaths, one case of neoplasm of the
lymphatic and haematopoietic system (plasmocytoma) was
identified. However, because of the short follow-up period
and the small number of deaths it was not possible for the
authors to calculate any observed/expected figures (Ahlmark,
l978).
A study is in progress (Tola et al., 1981) in which the
tumour incidence in a cohort of 2209 workers, employed in the
manufacture of reinforced plastics in 160 workplaces, is being
investigated. Exposure to styrene vapour commenced in 1960
and the level of exposure has been estimated to range between
126 and 1260 mg/m3 (30 and 300 ppm). The majority of workers
have been exposed since 1967. Among 27 deaths, 6 deaths from
cancer were observed in this cohort (versus 8.l expected) and
the cancers were found in tissues other than lymphatic or
bone-marrow tissue. Five of the cancers appeared in workers
with 5 or fewer years of exposure. The Task Group noted the
low mortality rate.
(c) Styrene-butadiene rubber manufacture
McMichael et al. (1976) analysed the mortality experience
during the period 1940-60 in a sample of workers (exact number
not specified) taken from a population of 1482 workers in the
rubber industry. The subgroup of workers was involved in the
manufacture of styrene-butadiene rubber. For malignancies of
the lymphatic and haematopoietic systems, a relative risk
ratio of 6.2 was observed compared with other workers.
Subsequently, Smith & Ellis (1977) reported that this excess
was based on 4 cases. A case control analysis of the same
data was performed by Spirtas et al. (1976) who calculated a
relative risk ratio of 2.4 for lymphatic and haematopoietic
neoplasms among these styrene-butadiene workers. Past
exposure to solvents and other monomers was suspected.
The mortality experience of 2 cohorts of rubber workers
was analysed by Taulbee et al. (1976) using both cohort and
case-control analysis. No significant excess of lymphatic and
haematopoietic neoplasms was observed.
Case reports of leukaemias and lymphomas among styrene-
butadiene rubber workers were reported by Block (1976), Lemen
& Young (l976), and Meinhardt et al. (1978).
7.2.9.1. Summary and conclusions
There have been anecdotal reports of a small number of
cases of leukaemia and lymphoma in workers employed in the
manufacture of styrene-butadiene rubber, but the study
populations have been ill-defined and exposure to solvents and
other monomers is known to have occurred.
An association between leukaemia and exposure to styrene
in the production and polymerization process industries has
been suggested in another study. However, the cases of
leukaemia occurred in a group with a concomitant past history
of exposure to colourants, polymer fumes and, possibly,
benzene.
The effects of long-term exposure to styrene are under
investigation in at least 2 epidemiological studies on workers
employed in the industries involving styrene lamination of
glass fibre materials and related activities.
It was the opinion of the Task Group that the data
necessary to form an evaluation were inadequate and that a
causal relationship between exposure to styrene and human
cancer could not be demonstrated at present.
7.2.10. Genetic effects in somatic cells
7.2.10.1. Structural chromosome aberrations
Several studies have been published on structural
chromosome aberrations in the peripheral lymphocytes of
workers employed in the reinforced plastics industry or in the
production of styrene and polystyrene (Table 14). In these
studies, exposures have been estimated by measuring styrene
air concentrations in the workplace or by determining
concentrations of styrene metabolites, in most cases mandelic
acid, in the urine of the workers.
In Table 14, the studies are listed according to the
industrial process. Positive results have been obtained among
workers employed in polyester processing. Nine studies
included detailed data on chromosome aberrations in
individuals. One report by Watanabe et al. (1981) was
considered inconclusive because of the low number (50 or less)
of metaphases analysed per person. The other 8 studies with
detailed information were used for the final evaluation.
Three studies were reported only in abstract or review
articles (Sorsa et al., 1979; Sram, 1981; Vainio et al., 1982;
Brogger, 1982).
The study of Meretoja et al. (1978a) was an extension of
an earlier study (Meretoja et al., 1977) and included
additional analyses of samples taken from the same workers a
year later, after continuous workplace exposure.
One of the abstracts was concerned with a study on
children exposed during the fetal period, while their mothers
were working in hand lamination of polyester resin.
The only available study on chromosome aberrations among
workers employed in the production of styrene or polystyrene
was negative (Fleig & Thiess, 1978).
7.2.10.2. Other indicators of genetic damage
Seven studies were available on SCE induction in the
lymphocytes of styrene-exposed workers employed in polyester
processing (Table 14). Two of the studies were reported only
in abstracts or reviews (Sorsa et al., 1979; Brogger, 1982;
Vainio et al., 1982). A slight increase in SCEs was reported
in 2 studies (Andersson et al., 1980; Camurri et al., 1982).
In 2 of the studies, peripheral lymphocytes of polyester
processing workers were analysed for micronuclei. Meretoja et
al. (1978a) and Meretoja & Vainio (1979) reported an increase
in micronuclei and "cells connected with a nuclear bridge" in
the cultured lymphocytes of 10 workers in polyester plastic
product plants; the study included 5 controls (Table 14).
Högstedt & Mitelman (1983) found that the frequency of
micronuclei increased in 38 workers exposed for 1-23 years to
styrene (mean of time-weighted average concentrations in
breathing zone 55 mg/m3 or l3 ppm, range 4-168 mg/m3 or 1-40
ppm) compared with 20 controls.
Pero et al. (1982) did not find any increase in
unscheduled DNA synthesis (UDS) in isolated lymphocytes of 38
workers in a fibreglass-reinforced polyester plastic factory
compared with 20 unexposed controls. Styrene air
concentrations in the workroom varied between 4 and 168 mg/m3
(1 and 40 ppm).
In an evaluation of the incidence of malformed children
and miscarriages among spouses of men exposed to styrene in a
reinforced-plastic boat factory, Andersson et al. (1980)
concluded that the number of pregnancies (39 exposed and 41
controls) was too small to reveal mutagenic effects.
7.2.10.3. Conclusions
The available evidence suggests that styrene exposure in
the reinforced plastics industry with its more intensive
exposures is associated with an increased frequency of
structural chromosomal abnormalities.
7.3. Effects on Reproductive Function and Teratogenic Effects
In a case-reference study on 63 pairs of mothers from a
register of congenital malformations (Holmberg, 1977), 2
mothers with children having central nervous system defects
had been employed in the reinforced plastics industry during
pregnancy. A third case-mother was also found, who had been
exposed to styrene at home.
In a study on spontaneous abortions registered in
hospitals (Hemminki et al., 1980a) during the period 1973-76,
the following numbers and rates of abortion were found:
- General population 15 482 (5.52%);
- Union of Chemical Workers 52 (8.54%);
- Plastics industry 21 (8.94%);
- Styrene production and use 6 (15%).
The abortion rates for the occupational groups were
statistically different from the controls. The abortion rates
increased with age in the general population, while they were
found to decrease with age among the Union of Chemical
Workers. No data were given concerning the relationship with
age of spontaneous abortions among women employed in the
plastics industry or in styrene production and use; thus the
significance of the increased abortion rates among these
groups cannot be assessed.
Table 14. Summary on studies of structural chromosome aberrations and sister chromatid exchanges (SCEs) in the lymphocytes of
workers exposed to styrene.
------------------------------------------------------------------------------------------------------------------------------------
No. persons Measured Years of Urinary man- Cells with SCEs/cell
studied concentration exposure delic acid chromosome
Industry styrene in (mg/g of aberrations,
branch air (mg/m3) creatinine) gaps included (%)a
---------------------------------------------------------------------------------------------------------------------
Reference Exposed Controls Averagea Range Meana Range Meana Range Exposed Controls Resultb Exposed Controls Resultb
------------------------------------------------------------------------------------------------------------------------------------
Styrene
manufacture
Flieg & 5 20 ~2c 0-29c 21.6 14-25 ~30d 19- 3.8 5.5 - .. .. ..
Thiess (1978) 40d
Polystyrene
manufacture
Flieg & 12 20 ~8c 0- 20.3 3-39 ~32d <5- 5.1 5.5 - .. .. ..
Thiess (1978) (12)e 197c 100d (5.5)e .. .. ..
Polyester
processing
Meretoja et 10 5 .. .. 3.2 0.6- 721 23- 16.6 1.8 + .. .. ..
al. (1977); 8.5 3257
Meretoja &
Vainio (1979)
Meretoja et 16 6 .. up to 6.3 1-15 570 23- 15.1 2.0 + .. .. ..
al. (1978b) 1260 3257
(a year 11 3f .. up to 8.1 2-16 329 52- 16.2 .. + 5.3 4.4 -
later) 1260 1646
Flieg & 14 20 ~477c <210- 7.9 2-24 ~593 42- 9.2 5.5 + .. .. ..
Thiess (1978) 1260c >1500
Andersson et 36 37 ~197 0- 5.0 0.3- .. .. 12.3 6.7 + 9.7 8.7 +
al. (1980) 20f 20f 1008 12
Watanabe et 16 13 151- 0-886 ~4.5 0.6- ~594 90- 2.9 3.2 (-)g 13.5 13.9 -
al. (1981) <294 9.3 2640
Watanabe et 18 6 168- <5- 9.7 <1-30 332 1- 6.5 4.7 ± 8.9 8.5 -
al. (1983) 210 1075 ~1040
Thiess et al. 24 24 25- 3-748 ~14.4 4-27 .. 0- 5.1 3.8 +h .. .. ..
(1980, 1982) 244 320c
------------------------------------------------------------------------------------------------------------------------------------
Table 14. (contd.)
------------------------------------------------------------------------------------------------------------------------------------
No. persons Measured Years of Urinary man- Cells with SCEs/cell
studied concentration exposure delic acid chromosome
Industry styrene in (mg/g of aberrations,
branch air (mg/m3) creatinine) gaps included (%)a
---------------------------------------------------------------------------------------------------------------------
Reference Exposed Controls Averagea Range Meana Range Meana Range Exposed Controls Resultb Exposed Controls Resultb
------------------------------------------------------------------------------------------------------------------------------------
Camurri et al. 24 21 .. 30- 9.4 1-22 472c 45- ~35.1i ~8.4i + 13.9 10.8 +
(1982)k (22)f (20)f >400 1108c
Abstracts
Sram (1981) 36 19 .. .. .. .. .. .. 1.4i 1.3 - .. .. ..
(cited data 34j (1.4)ij
of Pchlova & 22 22 .. .. .. .. .. .. 1.7i 1.4i - .. .. ..
Sram)
Brogger 13 0 129 15- 9.8 1-24 527 292- 6.4 .. (-)g 6.6 .. (-)g
(1982)k (8)f 364 688
Sorsa et al. 6 10 .. .. .. 2-7 .. .. 8.8 3.0 + 7.5 8.5 -
(1979);
Vainio et al. 7 10 .. .. .. (5-8 .. .. 4.9 1.6 + 4.9 5.2 -
(1982) ( in months)
utero exposed
children)
Högstedt et 6 6 ~162 60- 4.0 0.5- 490 225- 10.2 4.9 + .. .. ..
al. (1979) 800 10 2100
------------------------------------------------------------------------------------------------------------------------------------
a Estimated from the data given if not indicated in the report.
b Compared to response in control persons: + increase; - no effect.
c From Thiess & Friedheim (1978).
d mg/litre urine.
e Controls in Thiess & Fleig (1978).
f For the analysis of sister chromatid exchanges only.
g Inconclusive because of the low number of cells analysed (Watanabe et al., 1981) or lack of controls (Brogger, 1982).
h Negative if gaps are excluded.
i Gaps excluded.
j 6 months later.
k Details, not mentioned in the report, were obtained from the author(s).
In a small-scale study, Härkönen & Holmberg (1982)
recently interviewed 67 female lamination workers of child-
bearing age and a similar number of textile and food
production workers who were thought to be proper controls.
The groups were reported not to differ either in their
menstrual behaviour or in the number of spontaneous
abortions. The number of deliveries was found to be lower
among the styrene workers, which may be partially explained by
the higher number of induced abortions in the styrene-exposed
group.
8. EXPOSURE-EFFECT/EXPOSURE-RESPONSE RELATIONSHIPS, AND
EVALUATION OF HEALTH EFFECTS
8.1. Data from Experimental Animal Studies
8.1.1. Metabolic pathways and kinetics
Pulmonary uptake in man is of greatest importance though uptake
through the skin occurs. Styrene is biotransformed largely via the
7,8-epoxide by the mixed function oxidase system.
The kinetic data show that exposure of experimental animals to
increasing levels of styrene results in progressive saturation of
the metabolic pathways. The half-time of styrene disappearance
from the body varies with the dose. The range of exposures tested
was from 189 mg/m3 (45 ppm) to 10 500 mg/m3 (2500 ppm). The
consequence of such metabolic saturation, which has been reported
to occur at about 2520 mg/m3 (600 ppm) in air, is an increase in
the proportional deposition of styrene in fat. In human beings the
half-time for the elimination of styrene from adipose tissue is 2-3
days.
The principal urinary metabolites are mandelic and
phenylglyoxylic acids. Recent evidence suggests that the
pattern of urinary metabolite excretion varies with mammalian
species.
8.1.2. General toxicity
8.1.2.1. Acute toxicity
A mean odour detection threshold for styrene of 3.06 mg/m3
(0.73 ppm) was found for unadapted subjects. The odour was
reported to be strong but not objectionable at about 420 mg/m3
(l00 ppm). With short-term exposures at concentrations
exceeding 840 mg/m3 (200 ppm), styrene vapour was irritating
to the eyes and nose.
The central nervous system effects begin to appear in the
range of 210-840 mg/m3 (50-200 ppm) and distinct impairment of
reaction time and body balance were found at concentrations
exceeding 840 mg/m3 (200 ppm).
The acute toxicity of styrene in animals is rather low:
the LD50 in rats has been reported to be 5 g/kg body weight
after oral administration and 2-3 g/kg body weight after
intraperitoneal injection. Exposure of rats to styrene vapour
at 1300 to 10 000 ppm for up to 4 h caused nasal mucous
membrane and eye irritation as well as acute depression of the
central nervous system. Marked pulmonary lesions were also
noted.
There were no kidney changes in animals exposed to 5460
mg/m3 (l300 ppm) after a single exposure. Severe irritation
of the eyes and nose was observed in rats and guinea-pigs
after exposure to concentrations of 2730-5460 mg/m3 (650 - 1300
ppm) and general weakness and unsteadiness gradually developed
after 12 h.
Acute toxic effects were observed in mice exposed to a
styrene level of 21 000 mg/m3 (5000 ppm) for 2 h and
guinea-pigs exposed to 10 920 mg/m3 (2600 ppm) for 8 h. A
short-term exposure to 1260 mg/m3 (300 ppm) in the air caused
only very slight behavioural effects in rats, even though
pronounced neurochemical changes were observed.
The high exposure levels used in studies on experimental
animals have not been observed in the human environment.
8.1.2.2. Subacute and chronic toxicity
Inhalation of styrene vapour by rats at a concentration of
5460 mg/m3 (1300 ppm) for 7 h/day over several months did not
have any deleterious effects on the kidney and did not cause
any changes in the erythrocyte count, haemoglobin
concentration, and leukocyte count. There were not any
essential morphological changes in the rat lungs. Similar
results were obtained with rabbits, monkeys, and guinea-pigs
except that 10% of the guinea-pigs died after the first
exposure with signs of lung irritation. An exposure level of
2730 mg/m3 (650 ppm), for 7 h/day for 214-360 days, appeared
not to have any effects on the lungs of guinea-pigs.
Rats exposed by inhalation to a styrene concentration of
1260 mg/m3 (300 ppm) (6 h/day, 5 days a week) for up to 11
weeks developed axonal protein changes in the brain, an
induction of drug metabolizing enzymes in the kidney and the
liver, histological alterations in the liver, and a depletion
of the glutathione (GSH) contents of the kidney and the liver.
No significant depletion of GSH occurred at 420 mg/m3 (100
ppm).
Oral subacute studies were carried out on rats and beagle
dogs. The rat study was carried out on females and exposure
induced only weight increases in the liver and kidney. No
effect was observed at a dose of 133 mg/kg body weight. The
study on dogs lasted more than 19 months and showed a mild
haemolytic anaemia characterized by Heinz body production.
Effects were observed down to the lowest dose tested (200
mg/kg body weight).
8.1.3. Genetic effects
Styrene is a potential mutagen only after metabolic activation.
Mutagenicity tests in bacteria, yeasts and mammalian cells in vitro
have yielded contradictory results for styrene that may be due to
differences in the efficiency of activation and inactivation of
styrene in different studies.
Conclusive dose-effect relationships could only be established
in several in vitro studies.
The results of tests for chromosomal damage induced by
styrene in experimental mammals have been contradictory.
Three positive studies have been reported; 2 in mice, and 1 in
rats. A dose-effect relationship was only observed for the
induction of SCE in mice exposed to styrene in air levels of
more than 1260 mg/m3 (300 ppm). Five other animal studies
have given negative results. The different end-points, routes
of exposure, durations of treatment, species and strains of
animals used, and the small number of mammalian studies
available, make it impossible to draw definitive conclusions
concerning dose-effect relationships.
8.1.4. Carcinogenic effects
Oral administration of styrene to mice induced a
significant increase in pulmonary tumours in the O20 strain at
a dose of 1350 mg/kg body weight and a doubtful increase in
B6C3F1 mice at 300 mg/kg body weight. No significant increase
in tumour incidence was observed in the C57B1 strain, when
styrene was administered pre- and post-natally at 300 mg/kg
body weight.
In 2 studies on rats, styrene given orally at doses
ranging from 500 to 1350 mg/kg body weight did not induce a
significant increase in tumour incidence. A greater incidence
of lymphomas, observed in an inhalation study on rats exposed
to a styrene concentration of 2520 or 4200 mg/m3(600 or 1000 ppm),
could not be causally related to styrene.
Thus, the evidence available indicates that styrene at the
doses administered (1350 mg/kg body weight for 020 strain)
caused an increase in pulmonary tumours in mice.
Styrene 7,8-oxide induced squamous cell carcinomas in the
forestomach of rats given doses of 50 and 250 mg/kg body weight.
In a second study on rats, both squamous cell carcinomas and
pulmonary carcinomas were induced, when styrene oxide was given at
a dose of 100 - 150 mg/kg body weight per day, 4-5 days a week.
When styrene oxide was painted on the skin of mice at
concentrations of 50 or 100 g/kg, no tumours of the skin were
induced.
8.2. Human Studies
8.2.1. Effects on organs and systems
Effects on the following organ systems were investigated:
nervous, haematopoietic and immune systems, the kidney and
urinary tract, the gastrointestinal tract, liver,
cardiovascular and respiratory systems, and the endocrine
organs.
Most studies of the haematopoietic system did not produce
any positive data; in one study, a slight decrease in
thrombocyte count in workers exposed to styrene for more than
ten years was detected.
A clear-cut trend towards altered liver function has not been
demonstrated. At low exposure concentrations, the commonly used
parameters, such as activity of serum enzymes of hepatic origin,
except for gamma-GTP, have given equivocal results.
Slight effects on the lower respiratory system were noted
in some studies.
No adequate data were available to the Task Group to
establish dose-effect or dose-response relationships for the
aforementioned systems.
Slight disturbances of visuomotor accuracy and psychomotor
performance were noted at styrene levels exceeding 210 mg/m3
(50 ppm), and an increased incidence of abnormalities in
electroencephalograph recordings was detected at styrene
concentrations below 420 mg/m3 (100 ppm); relationships
between the exposure levels and the severity of these effects
and the response rates were observed.
While the reaction times were shown to be prolonged for
exposure levels below 630 mg/m3 (150 ppm), dose-response
relationships could not be established.
8.2.2. Genetic effects in somatic cells
Knowledge concerning the possible genetic effects of
styrene exposure on man is still inadequate for any
dose-response extrapolations. Available information is
limited to structural somatic chromosome damage in the
peripheral blood lymphocytes of workers occupationally exposed
to high concentrations of styrene in the reinforced plastics
industry.
Data on individual exposure profiles are necessary for
meaningful interpretation of the results of somatic cell
analysis. Furthermore, the role of high occasional peak
exposures and low continuous workplace exposures in the
induction of chromosome damage in lymphocytes in styrene-
exposed workers is not clear. Considering the survival time
of the target lymphocytes, exposure data should be available
for a period of at least 2-3 years preceding chromosome
analysis.
Based on available published data, the following
conclusions can be made:
1. No clear dose-response pattern can be recognized.
Positive results of chromosome aberrations in
styrene-exposed workers were restricted to the
reinforced plastics industry, where styrene
concentrations in air were high. Negative results
have been obtained in the manufacture of styrene or
polystyrene where exposures to styrene are lower.
2. Variability in the chromosome aberration frequencies
reported in different studies is great. This
suggests that several factors such as exposure
conditions, the method, and the analytical criteria
selected, influence the results.
3. Individual differences are great and may reflect
variations in exposure or differences in
susceptibility.
The Task Group concluded that the health significance of
structural chromosomal aberrations in the somatic cells of
persons exposed to styrene could not be assessed at present,
but that such effects were undesirable.
8.2.3. Carcinogenic effects
Several case reports and epidemiological investigations
have implied an increased risk of lymphatic and haematopoietic
system cancer in workers involved in the application of
styrene, polystyrene, and styrene-butadiene rubber. However,
at present, there is not sufficient evidence to establish a
direct cause and effect relationship between styrene exposure
and cancer in human beings. Assessment of data has frequently
been complicated by concomitant exposure to other volatile
substances.
The Task Group concluded that, to date, a causal
relationship between styrene exposure and cancer could not be
established in man.
REFERENCES
AHLMARK, A. (1978) [Styrene study. Epidemiological report.]
Stockholm, Sveriges Plastförbund, 21 pp (in Swedish).
ALARIE, Y. (1973) Sensory irritation of the upper airways by
airborne chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
AMACHER, D.E. & TURNER, G.N. (1982) Mutagenic evaluation of
carcinogens and non-carcinogens in the L5178Y/TK assay
utilizing postmitochondrial fractions (S9) from normal rat
liver. Mutat. Res., 97: 49-65.
ANDERSSON, H.C., TRANBERG, E.C., UGGLA, A.H., & ZETTERBERG, G.
(1980) Chromosomal aberrations and sister-chromatid exchanges
in lymphocytes of men occupationally exposed to styrene in a
plastic-boat factory. Mutat. Res., 73: 387-401.
ASKERGREN, A., ALLEN, L.-G., KARLSSON, D., LUNDBERG, I., &
NYBERG, E. (1981) Studies on kidney function in subjects
exposed to organic solvents. I. Excretion of albumin and -2
microglobulin in the urine. Acta Med. Scand., 209: 479-483
ASTRAND, I., KILBOM, C., OVRUM, P., & WAHLBERG, I. (1974)
Exposure to styrene. I. Concentration in alveolar air and
blood at rest and during exercise and metabolism. Work
environ. Health, 11: 69-85.
AXELSON, O. & GUSTAVSON, J. (1978) Some hygienic and clinical
observations on styrene exposure. Scand. J. Work Environ.
Health, 4 (Suppl. 2): 215-219.
AXELSON, O., FROBARJ, G., & WEDEFELT, U. (1974) [Can styrene
exposure cause states with cerebral lesion.] Läkartidningen,
71: 137-138 (in Swedish).
BABINA, M.D. (1969) [Spectrophotometric determination of
styrene and benzaldehyde in the work environment.] Gig. i
Sanit., 34: 105-106 (in Russian).
BAGGET, M.S., MORIA, G.P., SIMMONS, M.W., & LEWIS, J.S. (1974)
Quantitative determination of semivolatile compounds in
cigarette smoke. J. Chromatogr., 97: 79-82.
BAKKE, O.M. & SCHELINE, R.R. (1970) Hydroxylation of aromatic
hydrocarbons in the rat. Toxicol. appl. Pharmacol., 16:
691-700.
BARDODEJ, Z. (1964) [Styrene metabolism.] Cesk. Hyg., 9:
223-239 (in Czech).
BARDODEJ, Z. (1978) Styrene, its metabolism and the
evaluation of hazards in industry. Scand. J. Work Environ.
Health, 4 (Suppl. 2): 95-103.
BARDODEJ, Z. & BARDODEJOVA, E. (1970) Biotransformation of
ethyl benzene, styrene and alphamethyl styrene in man.
Am. Ind. Hyg. Assoc. J., 31: 206-209.
BARDODEJ, A., MALEK, B., VOLFOVA, B., & ZELENA, E. (1960)
[The hazard of styrene in the production of glass laminates.
Cesk. Hyg., 5: 541-546 (in Czech).
BARDODEJ, Z., BARDODEJOVA, E., & MALEK, B. (1961) [Value and
application of exposure tests. XI. Exposure test for
styrene.] Cesk. Hyg., 6: 546-552 (in Czech).
BARDODEJ, Z., BARDODEJOVA, E., & GUT, I. (1971) [Metabolosm
of styrene in rats.] Cesk. Hyg., 16: 243-245 (in Czech).
BARSOTTI, M., PARMEGGIANI, L., & SASSI, C. (1952)
[Observations on occupational pathology in a polystyrene resin
factory.] Med. Lav., 43: 418-424 (in Italian).
BASIROV, A.A. (1968) [Gastric function in workers of the
synthetic rubber industry.] Vrac. Delo, 4: 200-203 (in
Russian).
BASIROV, A.A. (1975) [Biochemical indexes of the gastric
juice in the early diagnosis of stomach illnesses under the
effect of toxic substances (1,3-butadiene and styrene).
Azerb. Med. Zh., 52: 60-66 (in Russian).
BAUER, C., LEPORINI, C., BRONZETTI, G., CORSI., C., NIERI, R.,
DEL CARRATONE, R., & TONARELLI, S. (1980) The problem of
negative results for styrene in the in vitro mutagenesis test
with metabolic activation (microsomal assay): explanation by
gas chromatographic analysis. Boll. Soc. It. Biol. Sper., 56:
203-207.
BAUER, C., LEPORINI, C., BRONZETTI, G., CORSI, C., NIERI, R.,
DEL CARRATONE, R., & TONARELLI, S. (1981) Studies on the
incubation mixtures for the in vitro mutagenesis test with
metabolic activation (microsomal assay) - behaviour of some
activating and detoxifying enzyme systems during incubations.
The case of styrene. Mutat. Res., 85: 268.
BAUER, D. & GUILLEMIN, M. (1976) Human exposure to styrene:
I. The gas-chromatographic determination of urinary
phenylglyoxylic acid using diazomethane derivatization.
Int. Arch. occup. environ. Health, 37: 47-55.
BEIJE, B. & JENSSEN, D. (1982) Investigation of styrene in
the liver perfusion/cell culture system. No indication of
styrene-7,8-oxide as the principal mutagenic metabolite
produced by the intact rat liver. Chem. biol. Interact., 39:
57-76.
BELVEDERE, G. & TURSI, F. (1981) Styrene oxidation to styrene
oxide in human blood erythrocytes and lymphocytes. Res. Comm.
Chem. Pathol. Pharmacol., 33: 273-282.
BELVEDERE, G., CANTONI, L., TACCHINETTI, T., & SALMONA, M.
(1977) Kinetic behaviour of microsomal styrene monooxygenase
and styrene epoxide hydratase in different animal species.
Experientia (Basle), 33: 708-709.
BENCEV, I.V. & RIZOV, N. (1981) [Gas-chromatographic method
for styrene determination blood (headspace method).]
Prob. Hyg., VI: 88-91 (in Russian).
BERGMAN, K. (1977) [Exposure to styrene in plastic boat
industry, I. Technical-hygienic study.] Arbete Hälsa, 3: 1-9
(in Swedish).
BERGMAN, K. (1979) Whole body autoradiography and allied
techniques. Scand. J. Work Environ. Health, 5 (Suppl. 1):
93-120.
BERTSCH, W., ANDERSON, E., & HOLZER, G. (1975) Trace analysis
of organic volatiles in water by gas chromatography-mass
spectrometry with glass capillary columns. J. Chromatogr.,
112: 701-718.
BIDOLI, F., AIROLDI, L., & PANTAROTTO, C. (1980) Quantitative
determination of styrene-7,8-oxide in blood by combined gas
chromatography-multiple ion detection mass fragmatography.
J. Chromatogr., 196: 314-318.
BLOCK, J.B. (1976) A Kentucky study: 1950-1975. In: Ede, L.,
ed. Proceedings of NIOSH Styrene-Butadiene Briefing,
Covington, Kentucky, USA, 1976, Cincinnati, Ohio, US
Department of Health, Education and Welfare pp. 28-32 (HEW
Publ. No. (NIOSH) 77-129).
BODNEI, A.H., BUTLER, G.J., & OKAWA, M.T. (1974) Health
hazard evaluation/toxicity determination. Cincinnati, US
Department of Health, Education and Welfare, Public Health
Service, Center for Disease Control, National Institute for
Occupational Safety and Health, 10 pp (Report no. 73-103-128 -
American Standard Fiberglass Inc., Stockton, California).
BONATTI, S., ABBONDANDOLO, A., CORTI, G., FIORIO, R., &
MAZZACCARO, A. (1978) The expression curve of mutants induced
by styrene oxide at the HGPRT locus in V79 cells.
Mutat. Res., 52: 295-300.
BROGGER, A. (1982) Application of SCE to public health. In:
Sandberg, A., ed. Sister chromatid exchange, New York, A.R.
Liss, pp. 655-673.
BROOKS, S.M., ANDERSON, L.A., TSAY, J.-Y., CARSON, A.,
BUNCHER, C.R., ELIA, V., & EMMETT, E.A. (1979) Investigation
of workers exposed to styrene in the reinforced plastic
industry - health and psychomotor status, toxicological and
industrial hygiene data and effect of protective equipment as
it relates to exposures through lung and skin routes.
Cincinnati, University of Cincinnati, College of Medicine,
pp. 330. (Institute of Environmental Health and Kettering
Laboratory Report Prepared for the Society of Plastics
Industries).
BROOKS, S., ANDERSON, L., EMMETT, E., CARSON, A., TSAY, J.-Y.,
ELIA, V., BUNCHER, R., & KARBOWSKY, R. (1980) The effects of
protective equipment on styrene exposure in workers in the
reinforced plastics industry. Arch. environ. Health, 35:
287-294.
BROUNS, R.E., POOT, M., DE VRIND, R., V. HOEK-KON, T.,
HENDERSON, P.T., & KUYPER, C.M.A. (1979) Measurement of
DNA-excision repair in suspensions of freshly isolated rat
hepatocytes after exposure to some carcinogenic compounds.
Its possible use in carcinogenicity screening. Mutat. Res.,
64: 425-432.
BUCHET, J.-P., LAUWERYS, R., ROELS, H., & DEFELD, J.-M.
(1974) [Evaluation of the exposure of workers to styrene by
means of the determination of its urinary metabolites:
mandelic and phenylglyoxylic acids: I. Technique of
determination of the metabolites by gas chromarography.
Arch. Mal. prof. Med. Tra. Secur. soc., 35: 511-516.
BURKIEWICZ, C., RYBKOWSKA, J., & ZIELINSKA, H. (1974)
[Assessment of exposure to styrene in human beings under
industrial conditions.] Med. Prac., 3: 305-310 (in Polish).
BURKOVA, T., BAJNOVA, A., & KAPURDOV, V. (1982) Occupational
risk with repeated styrene dermal contact. Prob. Hyg., VII:
51-59.
BURNETT, R.D. (1976) Evaluation of charcoal sampling tubes.
Am Ind. Hyg. Assoc. J., 37: 37-45.
BUSK, L. (1979) Mutagenic effects of styrene and styrene
oxide. Mutat. Res., 67: 201-208.
CAMURRI, L., CODELUPPI, S., & PEDRONI, C. (1982) Chromosomal
aberrations and sister chromatid exchanges in styrene exposed
workers. In: 12th Annual Meeting of European Environmental
Mutagen Society, Espoo, Finland, 20-24 June, 1982 Abstracts,
Helsinki, Institute of Occupational Health, p. 159.
CANTONI, L., SALMONA, M., FACCHINETTI, T., PANTAROTTO, C., &
BELVEDERE, G. (1978) Hepatic and extrahepatic formation and
hydration of styrene oxide in vitro in animals of different
species and sex. Toxicol. Lett., 2: 179-186.
CAPEROS, J.R., HUMBERT, B., & DROZ, P.O. (1979) Exposition on
styrene. II. Bilan de l'absorption, de l'excrétion et du
métabolisme sur des sujets humains. Int. Arch. occup.
environ. Health, 42: 223-230.
CARLSSON, A. (1981) Distribution and elimination of
14C-styrene in rat. Scand. J. Work Environ. Health, 7: 45-50.
CARPENTER, C.P., SHAFFER, C.B., WEIL, C.S., & SMYTH, H.F.
(1944) Studies on the inhalation of 1:3-butadiene; with a
comparison on its narcotic effect with benzol, tuluol, and
styrene, and a note on the elimination of styrene by the
human. J. ind. Hyg., 26: 69-78.
CERNA, M. & KYPENOVA, H. (1977) Mutagenic activity of
chloroethylenes analysed by screening system tests.
Mutat. Res., 46: 35-36.
CHEMICAL MARKET REVIEW (1981) Oct. 26, p. 7.
CHERRY, N., WALDRON, H.A., WELLS, G.G., WILKINSON, R.T.,
WILSON, H.K., & JONES, S. (1980) An investigation of the
acute behavioral effects of styrene on factory workers.
Br. J. ind. Med., 37: 234-240.
CHMIELEWSKI, J. & RENKE, W. (1975) Clinical and experimental
research into the pathogenesis of toxic effects of styrene
III. Morphology, coagulation and fibrinolisis systems of the
blood in persons exposed to the action of styrene during their
work. Biul. Inst. Med. Morskej, 26: 299-302.
CHMIELEWSKI, J., MIKULSKI, P., USELIS, J., & WIGLUSZ, R.
(1973) Rating of the exposure to styrene of persons working
at the production of polyester laminates. Biul. Inst. Med.
Morskej Gdansku, 24: 203-209.
CHMIELEWSKI, J., DOLMIERSKI, R., RENKE, W., & KWIATKOWSKI,
S.R. (1977) [Long-term occupational effects of styrene on
working people.] Z. gesamte Hyg. Grenzgeb., 23: 639-643 (in
German).
CHOI, K.K. & FUNG, K.W. (1979) Determination of styrene in
the atmosphere near industrial sites by gas chromatography.
Analyst, 104: 455-457.
CHU, K.C., CUETO, C., Jr, & WARD, J.M. (1981) Factors in the
evaluation of 200 National Cancer Institute Bioassays.
J. Toxicol. Environ. Health, 8: 251-280.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1979) Sister
chromatid exchange in regenerating liver an bone marrow cells
of mice exposed to styrene. Toxicol. appl. Pharmacol., 50:
365-367.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1980) Sister
chromatid exchange in murine alveolar macrophages, bone
marrow, and regenerating liver cells induced by styrene
inhalation. Toxicol. appl. Pharmacol., 55: 37-42.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1982) Multiple
tissue comparisons of sister chromatid exchanges induced by
inhaled styrene. In: Tice. R., Costa, D.L., & Schaich, K.M.,
ed. Genotoxic effects of airborne agents, New York, Plenum
Press, pp. 433-441.
CRANDALL, M.S. (1981) Worker exposure to styrene monomer in
the reinforced plastic boat-making industry. Am. Ind. Hyg.
Assoc. J., 42: 499-502.
CRITTENDEN, B.D. & LONG, R. (1976) The mechanisms of
formation of polynuclear aromatic compounds in combustion
systems. In: Carcinogenesis - A comprehensive survey - Volume
I, New York, Raven Press, pp. 209-223.
DANISHEFSKY, I. & WILLHITE, M. (1954) The metabolism of
styrene in the rat. J. Biol. Chem., 211: 549-553.
DELBRESSINE, L.P.C., KETELAARS, H.C.J., SEUTER-BERLAGE, F., &
SMEETS, F.L.M. (1980) Phenaceturic acid a new urinary
metabolite of styrene in the rat. Xenobiotica, 10: 337-342.
DEL CARRATORE, R., BRONZETTI, G., BAUER, C., CORSI, C., NIERI,
R., PAOLINI, M., & GIAGONI, P. (1982) Study of cytochrome
P450 in yeast D7 strain. An alternative model to microsomal
assay. Mutagenicity of styrene. In: 12th Annual Meeting of
the European Environmental Mutagen Society, Espoo, Finland,
20-24 June, 1982, Abstracts, Helsinki, Institute of
Occupational Health, p. 160.
DE MEESTER, C., PONCELET, F., ROBERFROID, M., RONDELET, J., &
MERCIER, M. (1977) Mutagenicity of styrene and styrene oxide.
Mutat. Res., 56: 147-152.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER,
M., MERCIER, M., & PONCELET, F. (1981) Mutagenicity of
styrene in the Salmonella typhimurium test system.
Mutat. Res., 90: 443-450.
DIETRICH, M.W., CHAPMAN, L.M., & MIEURE, J.P. (1978) Sampling
for organic chemicals in workplace athmosphere with porous
polymer beads. Am. Ind. Hyg. Assoc. J., 39: 385-392.
DOLMIERSKI, R., KWIATKOWSKI, S.R., & NITKA, J. (1974) A
preliminary evaluation of the studies on central nervous
system in workers exposed to styrene. Biul. Inst. Med.
Morskiej Gdansku, Gdansk, 25: 399-406.
DONNER, M., SORSA, M., & VAINIO, H. (1979) Recessive lethals
induced by styrene and styrene oxide in Drosophila
melanogaster. Mutat. Res., 67: 373-376.
DRINKWATER, N.R., MILLER, J.A., MILLER, E.C., & YANG, N.-C.
(1978) Covalent intercalative binding to DNA in relation to
the mutagenicity of hydrocarbon epoxides and N-acetoxy-2-
acetylaminofluorene. Cancer Res., 38: 3247-3255.
DROZ, P.O. & FERNANDEZ, J.G. (1977) Effect of physical work
load on retention and metabolism of inhaled organic solvents.
A comparative theoretical approach and its application with
regards to exposure monitoring. Int. Arch. occup. environ.
Health, 38: 231-246.
DUTKIEWICZ, T. & TYRAS, H. (1967) A study of the skin
absorption of ethylbenzene in man. Br. J. ind. Med., 24:
330-332.
DUTKIEWICZ, T. & TYRAS, H. (1968) Skin absorption of toluene,
styrene and xylene by man. Br. J. ind. Med., 25: 243.
DUVERGER-VAN BOGAERT, M., NOEL, G., ROLLMAN, B., CUMPS, J.,
ROBERFROID, M., & MERCIER, M. (1978) Determination of oxide
synthetase and hydratase activities by a new highly sensitive
gas chromatographic method using styrene and styrene oxide as
substrates. Biochim. Biophys. Acta, 526: 77-84.
ELIA, V.J., ANDERSON, L.A., MacDONALD, T.J., CARSON, A.,
BUNCHER, C.R., & BROOKS, S.M. (1980) Determination of urinary
mandelic and phenylglyoxylic acids in styrene exposed workers
and a control population. Am. Ind. Hyg. Assoc. J., 41:
922-926.
EL-MASRI, A.M., SMITH, J.N., & WILLIAMS, R.T. (1958) Studies
on detoxication. The metabolism of alkylbenzenes:
phenylacetylene and phenylethylene (styrene). Biochem. J.,
68: 199-204.
ELOVAARA, E., VAINIO, H., PFAFFLI, P., & COLLAN, Y. (1979)
Effects of intermittent styrene inhalation, ethanol intake and
their combination on drug biotransformation in rat liver and
kidneys. Med. Biol., 57: 321-327.
EL-TANTAWY, M.A. & HAMMOCK, B.D. (1980) The effect of hepatic
microsomal and cytosolic subcellular fractions on the
mutagenic activity of epoxide-containing compounds in the
Salmonella assay. Mutat. Res., 79: 59-71.
ENGSTROM, J. (1978) Styrene in subcutaneous adipose tissue
after experimental and industrial exposure. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 119-120.
ENGSTROM, J., USTRAND, I., & WIGAEUS, E. (1978a) Exposure to
styrene in a polymerization plant. Uptake in the organism and
concentration in subcutaneous adipose tissue. Scand. J. Work
Environ. Health, 4: 324-329.
ENGSTROM, J., BJURSTROM, R., USTRAND, I., & OVRUM, P. (1978b)
Uptake, distribution and elimination of styrene in man.
Concentration in subacutaneous adipose tissue. Scand. J. Work
Environ. Health, 4: 315-323.
ENGSTROM, K. (1983) In: Aitio, A., Riihimäki, V., & Vainio,
H., ed. Biological monitoring of exposure to industrial
chemicals, New York, Hemisphere (in press).
ENGSTROM, K. & RANTANEN, J. (1974) A new gas chromatographic
method for determination of mandelic acid in urine. Int.
Arch. Arbeitsmed., 33: 163-167.
ENGSTROM, K., HARKONEN, H., PEKARI, K., & RANTANEN, J. (1978)
Evaluation of occupational styrene exposure by ambient air and
urine analysis. Scand. J. Work Environ. Health, 4 (Suppl. 2):
121-123.
ENGSTROM, K., HARKONEN, H., KALLIOKOSKI, P., & RANTANEN, J.
(1976) Urinary mandelic acid concentration after occupational
exposure to styrene and its use as a biological exposure test.
Scand. J. Work Environ. Health, 2: 21-26.
FABRY, L., LEONARD, A., & ROBERFROID, M. (1978) Mutagenicity
tests with styrene oxide in mammals. Mutat. Res., 51: 377-381.
FAITH, W.L., KLYES, D.B., & CLARK, R.L. (1975) Industrial
chemicals, 4th ed., New York, John Wiley and Sons, Inc., pp.
365-370, 779-784.
FERNANDEZ, J.G. & CAPEROS, J.R. (1977) Exposition au styrène.
I. Etude expérimentale de l'absorption et l'excrétion
pulmonaires sur des sujets humains. Int. Arch. occup.
environ. Health, 40: 1-12.
FIELDS, R.L. & HORSTMAN, S.W. (1979) Biomonitoring of
industrial styrene exposures. Am. Ind. Hyg. Assoc. J., 40:
451-459.
FINLEY, J.W. & WHITE, J.C. (1967) Two methods to determine if
styrene monomer is present in milk, Bull. environ. Contam.
Toxicol., 2(1): 41-46.
FISEROVA-BERGEROVA, V. & TEISINGER, J. (1965) Pulmonary
styrene vapor retention. Ind. Med. Surg., 34: 620-622.
FISHBEIN, L. (1979) Chromatography of environmental hazards,
Volume II, Amsterdam, Elsevier, pp. 455-469.
FLEIG, I. & THIESS, A.M. (1978) Mutagenicity study of workers
employed in the styrene and polystyrene processing and
manufacturing industry. Scand. J. Work Environ. Health, 4
(Suppl. 2): 254-258.
FLEK, J. & SEDIVEC, V. (1980) Simultaneous gas
chromatographic determination of urinary mandelic and
phenylglyoxylic acids using diazomethane derivatization. Int.
Arch. occup. environ. Health, 45: 181-188.
FLEMING, R.D. (1970) Effect of fuel composition on exhaust
emissions from a spark-ignition engine, Washington DC, US
Department of the Interior, Bureau of Mines (Report of
Investigations 7423).
FOERST, W. (1965) [Styrene.] In: Ullmans Encyklopädie der
technischen Chemie (Vol. 16), Munich, Urban et Schwarzenberg,
pp. 460-476 (in German).
FRENTZEL-BEYME, R., THEISS, A.M., & WIELAND, R. (1978) Survey
of mortality among employees engaged in the manufacture of
styrene and polystyrene at the BASF Ludwigshafen works.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 231-239.
GADALINA, I.D., KAZNINA, N.I., KUZNETSOVA, G.M., &
SMIRNISTSKII, N.S. (1969) Hygienic assessment of polyester
plastics for flooring. Hyg. Sanit., 34: 25-29.
GAMBERALE, F. & HULTENGREN, M. (1974) Exposure to styrene
II. Psychological functions. Work Environ. Health, 11: 86-93.
GAMBERALE, F., LISPER, H.O., & ANSHELM-OLSON, B. (1975)
[Effect of styrene gases on reaction time among workers in
plastic boat industry.] Arbete och Hälsa, 8: 23 pp (in
Swedish).
GAZZOTTI, G., GARATTINI, E., & SALMONA, M. (1980) Nuclear
metabolism I. Determination of styrene monooxygenase activity
in rat liver nuclei. Chem. Biol. Interact., 29: 189-195.
GOMPERTZ, D. (1978) Comments made at the end of the session.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 227.
GORDON, M. & GOODLEY, P.C. (1971) Isolation and
characterization of industrial organic pollutants in Water.
Am. Chem. Soc., Div. Water, Air, Waste Chem., Gen. Pap.,
11(1): 91-94.
GOTELL, P., AXELSSON, O., & LINDELOF, B. (1972) Field studies
on human styrene exposure. Work Environ. Health, 9: 76-83.
GREIM, H., BIMBOES, D., EGERT, G., GOGGELMAN, W., & KRAMER, M.
(1977) Mutagenicity and chromosomal aberrations as an
analytical tool for in vitro detection of mammalian enzyme-
mediated formation of reactive metabolites. Arch. Toxicol.,
39: 159-169.
GROSSMAN, I.G. (1970) Waterborne styrene in a crystalline
bedrock aquifer in the Gales Ferry Area, Ledyard, Southeastern
Connecticut. US Geol. Surv. Prof. Pap., 700-B : 203-209.
GUILLEMIN, M. & BAUER, D. (1976) Human exposure to styrene.
II. Quantitative and specific gas chromatographic analysis of
urinary mandelic and phenylglyoxylic acids as an index of
styrene exposure. Int. Arch. occup. environ. Health, 37:
57-64.
GUILLEMIN, M.P. & BAUER, D. (1978) Biological monitoring of
exposure to styrene by analysis of combined urinary mandelic
and phenylglyoxylic acids. Am. Ind. Hyg. Assoc. J., 39:
873-879.
GUILLEMIN, M.P. & BAUER, D. (1979) Human exposure to styrene.
III. Elimination kinetics of urinary mandelic and
phenylglyoxylic acids after single experimental exposure.
Int. Arch. occup. environ. Health, 44: 249-263.
GUNTER, B.J. & LUCAS, J.B. (1973) Health hazard
evaluation/toxicity determination. Cincinnati, US Department
of Health, Education and Welfare, Public Health Service,
Center for Disease Control, National Institute for
Occupational Safety and Health, 17 pp (Report No. 72-86-38 -
Gates Rubber Co. Denver, CO.).
HAMIDULLIN, R.S., FELDMAN, N.G., & VENDILO, M.V. (1968)
Hygienic assessment of articles made of PS-S suspension
polystyrene, Hyg. Sanit., 33: 331-334.
HARKONEN, H. (1977) Relationship of symptoms to occupational
styrene exposure and to the findings of electroencephalo-
graphic and psychological examinations. Int. Arch. occup.
environ. Health, 40: 231-239.
HARKONEN, H. (1978) Styrene, its experimental and clinical
toxicology. Scand. J. Work Environ. Health, 4 (Suppl. 2):
104-113.
HARKONEN, H. & HOLMBERG, P. (1982) Obstetric histories of
workers occupationally exposed to styrene. Scand. J. Work
Environ. Health, 8: 74-77.
HARKONEN, H., KALLIOKOSKI, P., HIETALA, S., & HERNBERG, S.
(1974) Concentration of mandelic and phenylglyoxylic acid in
urine as indicators of styrene exposure. Work Environ.
Health, 11: 162-165.
HARKONEN, H., LINDSTROM, K., SEPPALAINEN, A.M., ASP, S., &
HERNBERG, S. (1978) Exposure-response relationships between
styrene exposure and central nervous functions. Scand. J.
Work Environ. Health, 4: 53-59.
HEMMINKI, K. (1979) Fluorescence study of DNA alkylation by
epoxides. Chem.-biol. Interact., 28: 269-278.
HEMMINKI, K. & FALCK, K. (1979) Correlation of mutagenicity
and 4-( p-nitrobenzyl)-pyridine alkylation by epoxides.
Toxicol. Lett., 4: 103-106.
HEMMINKI, K., FRANSSILA, E., & VAINIO, H. (1980a) Spontaneous
abortion among female chemical workers in Finland. Int. Arch.
occup. environ. Health, 45: 123-126.
HEMMINKI, K., PAASIVIRTA, J., KURKIRINNE, T., & VIRKKI, L.
(1980b) Alkylation products of DNA bases by simple epoxides.
Chem. biol. Interact., 30: 259-270.
HEMMINKI, K., HEINONEN, T., & VAINIO, H. (1981) Alkylation of
guanosine and 4-( p-nitrobenzyl)-pyridine by styrene oxide
analogues in vitro . Arch. Toxicol., 49: 35-41.
HIRATSUKA, A. & WATABE, T. (1982) [Reaction of 1-vinylbenzene
3,4-oxide with ethyl mercaptan.] In: [Proceedings of the
102nd Annual Meeting for the Pharmaceutical Society of Japan,
Osaka, Japan, 3-5 April, 1982]. Japan, p. 480 (in Japanese).
HIRATSUKA, A., AIZAWA, T., OZAWA, N., ISOBE, M., WATABE, T., &
TAKABATAKE, E. (1982a) The role of epoxides in the metabolic
activation of styrene to mutagens. Eisei Kagaku, 28: 34.
HOGSTEDT, B. & MITELMAN, F. (1983) Micronuclei in cultured
lymphocytes as an indicator of genotoxic exposure. In: De
Serres, F. & Pero, R.W., ed. Individual susceptibility to
genotoxic agents in the human population, New York, Plenum
Publishing Corporation, (in press).
HOLMBERG, P.C. (1977) Central nervous defects in two children
of mothers exposed to chemicals in the reinforced plastics
industry: chance or causal relation? Scand. J. Work Environ.
Health, 3: 212-214.
HOSHIKA, Y. (1977) Gas chromatographic determination of
styrene as its dibromide. J. Chromatogr., 136: 95-103.
HUZL, F., SYKOVA, J., MAINEVOVA, J., JANKOVA, J., SRUTEK, J.,
JUNGER, V., & LAHN, V. (1967) [The question of health hazards
in working with styrene.] Prac. Lek., 19: 121-125 (in Czech).
IARC (1979) Some monomers, plastics and synthetic elastomers,
and acrolein, Lyons, International Agency for Research on
Cancer, pp. 231-274 (IARC Monographs on the evaluation of the
carcinogenic risk of chemicals to humans. Vol. 19).
IKEDA, M. & IMAMURA, T. (1974) Evaluation of hippuric,
phenylglyoxylic and mandelic acids in urine as indices of
styrene exposure. Int. Arch. Arbeitsmed., 32: 93-101.
IKEDA, M., IMAMURA, T., HAYASHI, M., TABUCHI, T., & HARA, I.
(1974) Evaluation of hippuric, phenylglyoxylic and mandelic
acids in urine as indices of styrene exposure. Int. Arch.
Arbeitsmed., 32: 93-101.
IKEDA, M., KOIZUMI, A., MIYASAKA, M., & WATABE, T. (1982)
Styrene exposure and biologic monitoring in FRP production
plants. Int. Arch. occup. environ. Health, 49: 325-339.
ISHIDATE, M., Jr & YOSHIKAWA, K. (1980) Chromosome aberration
tests with Chinese hamster cells in vitro with and without
metabolic activation - a comparative study on mutagens and
carcinogens. Arch. Toxicol., Suppl. 4: 41-44.
ISHIDATE, M., Jr, SOFUNI, T., & YOSHIKAWA, K. (1981)
Chromosomal aberration tests in vitro as a primary screening
tool for environmental mutagens and/or carcinogens. GANN
Monogr. Cancer Res., 27: 95-108.
ITI (1975) Toxic and hazardous industrial chemicals safety
manual, Tokyo, The International Technical Information
Institute, pp. 494-495.
IZJUMOVA, A.S. (1972) [The action of small concentrations of
styrol on the sexual function of albino rats.] Gig. i Sanit.,
37: 29-30 (in Russian).
IVANOVA-TCHEMICHANSKA, L., ANTOV, G., & ILIEVA, P. (1982)
[Gonadotropic action of some organic solvents.] In: Resumes
de la XVIIe Semaine, August 29-September 3, 1982, Sofia,
Bulgaria, Sofia, L. Union Médicale Balkanique, pp. 50-51 (in
French).
JACOBS, H.W. & SYRJALA, R.H. (1978) The use of infrared
analyzers for monitoring acrylonitrile. Am. Ind. Hyg. Assoc.
J., 39: 161-165.
JAMES, M.O., FOUTS, J.R., & BEND, J.R. (1975) Hepatic and
extrahepatic metabolism in vitro of an epoxide (8-14C-
styrene oxide) in the rabbit. Biochem. Pharmacol., 25:
187-193.
JAMES, S.D. & WHITE, D.A. (1967) The metabolism of phenethyl
bromide, styrene and styrene oxide in the rabbit and rat.
Biochem. J., 104: 914-921.
JENSEN, F. (1972) Determination of monomers from polystyrene
in milk products, Ann. 1st Super. Sanita, 8: 443-448.
JERMINI, C., WEBER, A., & GRANDJEAN, E. (1976) [Quantitative
determination of various gas-phase components of the side-
stream smoke of cigarettes in the room air as a contribution
to the problem of passive-smoking.] Int. Arch. occup.
environ. Health, 36: l69-181 (in German).
JERSEY, G.C., BALMER, M.F., QUAST, J.F., PARK, C.N., SCHUETZ,
D.J., BEYER, J.E., OLSEN, K.J., MCCOLLISTER, S.B., & RAMPY,
L.W. (1978) Two-Year chronic inhalation toxicity and
carcinogenicity study of monomeric styrene in rats - Final
report. (2yr), Midland, MI, Dow Chemical Co., 150 pp
(Manufacturing Chemists Association MCA No: Sty 1.1-Tox-INH).
JOHNSTONE, R.A.W., QUAN, P.M., & CARRUTHERS, W. (1962)
Composition of cigarette smoke: Some low-boiling components.
Nature (Lond.), 195: 1267-1269.
KALLIOSKI, P. (1976) [The reinforced plastic industry - a
problem work environment.] (Työterveyslaitoksen tutkimuksia
122) (in Finnish).
KALLIOSKI, P. & PFAFFLI, P. (1975) Charcoal sampling method
for determining the concentration of styrene in air. Scand.
J. Work Environ. Health, 1: 193-198.
KANKAANPAA, J.T.J., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity and teratogenicity of styrene and styrene oxide
on chick embryos enhanced by trichloropropylene oxide. Acta
Pharmacol. Toxicol., 45: 399-402.
KANKAANPAA, J.T.J., ELOVAARA, E., HEMMINKI, K., & VAINIO, H.
(1980) The effect of maternally inhaled styrene on embryonal
and foetal development in mice and Chinese hamsters. Acta
Pharmacol. Toxicol., 47: 127-129.
KAWACHI, T., YAHAGI, T., KADA, T., TAZIMA, Y., ISHIDATE, M.,
SASAKI, M., & SUGIYAMA, T. (1979) Cooperative programme on
short-term assays for carcinogenicity in Japan. In:
Montesano, R., Bartsch, H., & Tomatis, L., ed. Molecular and
cellular aspects of carcinogen screening tests, IARC
Scientific Publication No. 27, Lyons, IARC, pp. 323-330.
KAZNINA, N.I. (1969) Air pollution by volatiles liberated by
some plastics. Hyg. Sanit., 33: 267-269.
KJELLBERG, A., WIGAEUS, E., ENGSTROM, J., CSTRAND, I., &
LJUNGQUIST, E. (1979) [Long-term effects of styrene exposure
in plastic industry.] Arbete och Hälsa, 18: 1-25 (in Swedish).
KLIMKOVA-DEUTSCHOVA, E. (1962) [Neurological findings in the
plastics industry among styrene workers.] Int. Arch.
Gewerbepath. Gewerbehyg., 19: 35-50 (in German).
LEIBMAN. K.C. (1975) Metabolism and toxicity of styrene.
Environ. health Perspect., 11: 115-119.
LEIBMAN, K.C. & ORTIZ, E. (1969) Oxidation of styrene in
liver microsomes. Biochem. Pharmacol., 18: 552-554.
LEITHE, W. (1971) Analysis of air pollutants, Ann Arbor, Ann
Arbor Science Publishers, p. 246.
LEMEN, R.A. & YOUNG, R. (1976) Investigations of health
hazards in styrene butadiene rubber facilities. In: Ede, L.,
ed. Proceedings of the NIOSH Styrene-Butadiene Briefing,
Covington, Kentucky, USA, 1976, Cincinnati, Ohio, US
Department of Health, Education and Welfare pp. 3-8 (HEW
Publ. No. (NIOSH) 77-129).
LILIS, R. & NICHOLSON, W.J. (1976) Cancer experience among
workers in a chemical plant producing styrene monomers. In:
Ede, L., ed., Proceedings of the NIOSH Styrene-Butadiene
Briefing, Covington, Kentucky, USA, 1976, Cincinnati, Ohio,
Department of Health, Education and Welfare pp. 22-27 (HEW
Publ. No. (NIOSH) 77-129).
LILIS, R., LORIMER, W.V., DIAMOND, S., & SELIKOFF, I.J. (1978)
Neurotoxicity of styrene in production and polymerization
workers. Environ. Res., 15: 133-138.
LINDSTROM, K., HARKONEN, H., & HERNBERG, S. (1976)
Disturbances in psychological functions of workers
occupationally exposed to styrene. Scand. J. Work Environ.
Health, 2: 129-139.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978a)
Cytogenetic effects of styrene and styrene oxide. Mutat.
Res., 58: 277-286.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978b)
Cytogenetic effects of styrene and styrene oxide on human
lymphocytes and Allium cepa. Scand. J. Work Environ. Health,
4 (Suppl. 2): 156-162.
LITTON BIONETICS (1980) Toxicological study on styrene
incorporated in drinking water of rats for two years in
conjuction with a three-generation reproduction study.
(Submitted to Chemical Manufacturers Association.)
LOPRIENO, N. (1981) Environmental mutagens and comparative
short-term mutagenicity studies. In: Molecular bases of
genetic processes. Proceedings of the XIV International
Congress of Genetics, Moscow, MIR Publishers, Vol. III, Book
1, pp. 164-180.
LOPRIENO, N. & ABBONDANDOLO, A. (1980) Comparative mutagenic
evaluation of some industrial compounds. In: Norpoth, K.H. &
Garner, R.C., ed. Short-term test systems for detecting
carcinogens, Berlin, Springer-Verlag, pp. 333-356.
LOPRIENO, N., ABBONDANDOLO, A., BARALE, R., BARONCELLI, S.,
BONATTI, S., BRONZETTI, G., CAMMELLINI, A., CORSI, C., CORTI,
G., FREZZA, D., LEPORINI, C., MAZZACCARO, A., NIERI, R.,
ROSELLINI, R., & ROSSI, A.M. (1976) Mutagenicity of
industrial compounds: styrene and its possible metabolite
styrene oxide. Mutat. Res., 40: 317-324.
LOPRIENO, N., PRESCIUTTINI, S., SBRANA, I., STRETTI, G.,
ZACCARO, L., ABBONDANDOLO, A., BONATTI, S., FIORIO, R., &
MAZZACCARO, A. (1978) Mutagenicity of industrial compounds
VII. Styrene and styrene oxide: II. Point mutations,
chromosome aberrations and DNA repair induction analyses.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 169-178.
LORIMER, W.V., LILIS, R., NICHOLSON, W.J., ANDERSON, H.,
FISCHBEIN, A. DAUM, S., ROM, W., RICE, C., & SELIKOFF, I.J.
(1976) Clinical studies of styrene workers: Initial
findings. Environ. health Perspect., 17: 171-181.
LORIMER, W., LILIS, R., FISCHBEIN, A., DAUM, S., ANDERSON, H.,
WOLFF, M., & SELIKOFF, I. (1978) Health status of
styrene-polystyrene polymerization workers. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 220-226.
LUNDBERG, I. (1981) [Serumenzyme levels in workers exposed to
styrene.] Arbete och Hälsa, 1: 19 pp (in Swedish).
MAIER, A. RUHE, R., TOSENSTEEL, R., & LUCAS, J.B. (1974)
Health hazard evaluation/toxicity determintion. Arco Polymer
Incorporated (Sinclair-Koppers Company, Inc.), Monaco, PA.,
Cincinnati, US Department of Health, Education, and Welfare,
Center for Disease Control, National Institute for
Occupational Safety and Health, 28 pp (Report No. 72-90--107).
MALTONI, C., FAILLA, G., & KASSAPIDIS, G. (1979) First
experimental demonstration of the carcinogenic effects of
styrene oxide. Long-term bioassays on Spraque-Dawley rats by
oral administration. Med. Lav., 70(5): 358-362.
MANSON, N.W., REILLY, D.A., & STAGG, H.E. (1965) The
determination of toxic substances in air, manual of ICI
practice, Cambridge, W. Heffer & Sons, p. 181.
MARNIEMI, J., SUOLINNA, E.M., KAARTINEN, N., & VAINIO, H.
(1977) Covalent binding of styrene oxide to rat liver
macromolecules in vivo and in vitro. In: Ullrich, V., Roots,
I., Hildebrandt, A., Estabrook, R.W., & Conney, A.H., ed.
Microsomes and drug oxidations, London, Oxford, Pergamon
press, pp. 698-702.
MATSUOKA, A., HAYASHI, M., & ISHIDATE, M., Jr (1979)
Chromosomal aberration tests on 29 chemicals combined with S9
mix in vitro. Mutat. Res., 66: 277-290.
MATSUSHITA, T., MATSUMOTO. T., MIYAGAKI, J., MAEDA, K.,
TAKEUCHI, Y., & KATAJIMA, J. (1968) [Nervous disorders
considered to be symptoms of chronic styrol poisoning.
Saigai Igaku, 11: 173-179 (in Japanese).
McMICHAEL, A.J., SPIRTAS, R., GAMBLE, J.F., & TOUSEY, P.M.
(1976) Mortality among rubber workers: relationship to
specific jobs. J. occup. Med., 18: 178-185.
McNAIR, H.M. & BONELLI, E.J. (1968) Basic Gas Chromatography,
5th Ed., Walnut Creek, Varian Aerograph., p. 101.
MEINHARDT, T.J., YOUNG, R.J., & HARTLE, R.W. (1978)
Epidemiologic investigations of styrene-butadiene rubber
production and reinforced plastics production. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 240-246.
MERETOJA, T. & VAINIO, H. (1979) The use of human lymphocyte
tests in the evaluation of potential mutagens: clastogenic
activity of styrene in occupational exposure. In: Berg, K.,
ed. Genetic damage in man caused by environmental agents, New
York, Academic Press, pp. 213-225.
MERETOJA, T., VAINIO, H., SORSA. M., & HARKONEN, H. (1977)
Occupational styrene exposure and chromosomal aberrations.
Mutation Res., 56: 193-197.
MERETOJA, T., JARVENTAUS, H., SORSA, M., & VAINIO, H. (1978a)
Chromosome aberrations in lymphocytes of workers exposed to
styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
259-264.
MERETOJA, T., VAINIO, H., & JARVENTAUS, H. (1978b)
Clastogenic effects of styrene exposure on bone marrow cells
of rat. Toxicol. Lett., 1: 315-318.
MILVY, P. & GARRO, A.J. (1976) Mutagenic activity of styrene
oxide (1,2-epoxyethylbenzene), a presumed styrene metabolite.
Mutat. Res., 40: 15-18.
MITHEN, F.A., COCHRAN, M., JOHNSON, M.I., & BUNGE, R.P. (1980)
Neurotoxicity of polystyrene containers detected in a closed
tissue culture system. Neurosci. Lett., 17: 107-111.
MURRAY, F. J., JOHN, J. A., BALMER, M. F., & SCHWELTZ, B. A.
(1978) Teratologic evaluation of styrene given to rats and
rabbits by inhalation or by gavage, Toxicology, 11: 335-343.
NELIGAN, R.E., LEONARD, M.J., & BRYAN, R.J. (1965) The gas
chromatographic determination of aromatic hydrocarbons in the
atmosphere. Am. Chem. Soc. Div. Water, Air. Waste Chem.
Preprints, 5: 118-121.
NICHOLSON, W.J., SELIKOFF, I.J., & SEIDMAN, H. (1978)
Mortality experience of styrene-polystyrene polymerization
workers. Initial findings. Scand. J. Work Environ. Health, 4
(Suppl. 2): 247-252.
NIOSH (1974) NIOSH manual of analytical methods, Cincinnati,
National Institute for Occupational Safety and Health, (Method
No. 127).
NORPPA, H. (1981a) Chromosome damage induced by styrene,
styrene oxide and some analogues, Academic dissertation,
Helsinki, Institute of Occupational Health and University of
Helsinki, 56 pp.
NORPPA, H. (1981b) Styrene and vinyltoluene induce
micronuclei in mouse bone marrow. Toxicol. Lett., 8: 247-251.
NORPPA, H. & VAINIO, H. (1983a) Genetic toxicity of styrene
and some of its derivatives. Scand. J. Work Environ. Health,
9: 108-114.
NORPPA, H. & VAINIO, H. (1983b) Induction of sister-chromatid
exchanges by styrene analogues in cultured human lymphocytes.
Mutat. Res., 116: 379-387.
NORPPA, H., ELOVAARA, E., HUSGAFVEL-PURSIAINEN, K., SORSA, M.,
& VAINIO, H. (1979) Effects of styrene oxide on chromosome
aberrations, sister chromatid exchange and hepatic drug
biotransformation in Chinese hamsters in vivo. Chem.-biol.
Interact., 26: 305-315.
NORPPA, H., SORSA, M., PFAFFLI, P., & VAINIO, H. (1980a)
Styrene and styrene oxide induce SCEs and are metabolised in
human lymphocyte cultures. Carcinogenesis, 1: 357-361.
NORPPA, H., SORSA, M., & VAINIO, H. (1980b) Chromosomal
aberrations in bone marrow of Chinese hamsters exposed to
styrene and ethanol. Toxicol. Lett., 5: 241-244.
NORPPA, H., VAINIO, H., SORSA, M., & BELVEDERE, G. (1982)
Metabolic activation of styrene by erythrocytes in human
lymphocyte cultures. In: 12th Annual Meeting of the European
Environmental Mutagen Society, Espoo, Finland, 20-24 June,
1982, Abstracts, Helsinki, Institute of Occupational Health,
p. 148.
NYLANDER, P. (1979) [LSE polyesters - environmental
effects.] Plastforum Scandinavia, 9: 130-132 (in Swedish).
ODKVIST, L.M., CSTRAND, I., LARSBY, B., & KALL, C. (1980)
[Does styrene incude disturbancies in the human balance
mechanisms?] Arbete och Hälsa, 2: 5-19 (in Swedish).
OESCH, F. (1973) Mammalian epoxide hydrolase. Inducable
enzyme catalyzing the inactivation of carcinogenic and
cytotoxic metabolites derived from aromatic and olefinic
compounds. Xenobiotica, 3: 305-360.
OGATA, M. & SUGIHARA, R. (1978) High performance liquid
chromatographic procedure for quantitative determination or
urinary phenylglyoxylic, mandelic and hippuric acids as
indices of styrene exposure. Int. Arch. occup. environ.
Health, 42: 11-19.
OHTSUJI, H. & IKEDA, M. (1970) A rapid colorimetric method
for the determination of phenylglyoxylic and mandelic acids.
Its application to the urinanalysis of workers exposed to
styrene vapour. Br. J. ind. Med., 27: 150-154.
OHTSUJI, H. & IKEDA, M. (1971) The metabolism of styrene in
rat and the stimulatory effect of phenobarbital. Toxicol.
appl. Pharmacol., 18: 321-328.
OLTRAMARE, M., DESBAUMES, E., IMHOFF, C., & MICHIELS, W.
(1974) Toxicologie du styrene monomère. Geneva, Editions
Mèdicine et Hygiène, 100 pp.
OTT, M.G., KOLESAR, R.C., SCHARNWEBER, H.C., SCHNEIDER, E.J.,
& VENABLE, J.R. (1980) A mortality survey of employees
engaged in the development or manufacture of styrene-based
products. J. occup. Med., 22: 445-460.
PACHECKA, J., GARIBOLDI, P., CANTONI, L., BELVEDERE, G.,
MUSSINI, E., & SALMONA, M. (1979) Isolation and structure
determination of enzymatically formed styrene oxide
glutathione conjugates. Chem. Biol. Interact., 27: 313-321.
PAGANO, D.A., YAGEN, B., HERNANDEZ, O. BEND, J.R., & ZEIGER,
E. (1982) Mutagenicity of ( R) and ( S) styrene 7,8-oxide and
the intermediary mercapturic acid metabolites formed from
styrene 7,8-oxide. Environ. Mutag., 4(5): 575-584.
PAGANO, G., ESPOSITO, A., GIORDANO, G.G., & HAGSTROM, B. E.
(1978) Embryotoxic and teratogenic effects of styrene
derivatives on sea urchin development. Scand. J. Work,
Environ. Health, 4 (Suppl. 2): 136-141.
PANTAROTTO, E., FANELLI, R., BIDOLI, F., MANZONI, P., SALMONA,
M., & SZCZAWINSKI, K. (1978) Areneoxides in styrene
metabolism. New perspective in styrene toxicity? Scand. J.
Work Environ. Health, 4 (Suppl. 2): 67-77.
PANTAROTTO, C., SALMONA, M., SZCZAWINSKA, K., & BIDOLI, F.
(1980) Gas chromatography-mass spectrometric studies on
styrene toxicity. In: Albatges, J., ed. Analytical techniques
in environmental chemistry, New York, Pergamon Press, pp.
245-279.
PARKKI, M.G. (1978) The role of glutathione in the toxicity
of styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
53-59.
PARKKI, M.G., MARNIEMI, J., & VAINIO, H. (1976) Action of
styrene and its metabolites styrene oxide and styrene glycol
on activities of xenobiotic biotransformation enzymes in rat
liver in vivo. Toxicol. appl. Pharmacol., 38: 59-70.
PENTTILA, M., SORSA, M., & VAINIO, H. (1980) Inability of
styrene to induce nondisjunction in Drosophila or a positive
micronucleus test in the Chinese hamster. Toxicol. Lett., 6:
119-123.
PERO, R.W., BRYNGELSSON, T., HOGSTEDT, B., & CKESON, B. (1982)
Occupational and in vitro exposure to styrene assessed by
unscheduled DNA synthesis in resting human lymphocytes.
Carcinogenesis, 3: 681-685.
PERVIER, J.W., BARLEY, R.C., FIELD, D.E., FRIEDMAN, B.M., &
MORRIS, R.B. (1974) Survey reports on atmospheric emissions
from the petrochemical industry, Vol. IV. 287 pp (US NTIS, PB
Rep. PB-245630).
PFAFFLI, P., HESSO, A., VAINIO, H., & HYVONEN, M. (1981)
4-Vinylphenol excretion suggestive of arene oxide formation in
workers occupationally exposed to styrene. Toxicol. appl.
Pharmacol., 60: 85-90.
PHILIPPE, R., LAUWERYS, R., BUCHET, J.P., ROELS, H., & DEFELD,
J.M. (1974) [Evaluation of the exposure of workers to styrene
by means of the determination of its urinary metabolites:
mandelic and phenylglyoxylic acids: II. Application to
polyester fiber workers.] Arch. Mal. Prof. Méd. Tra. Secur.
Soc., 35: 631-640.
PLANCHE, G., CROISY, A., MALAVEILLE, C., TOMATIS, L., &
BARTSCH, H. (1979) Metabolic and mutagenicity studies on DDT
and 15 derivatives. Detection of 1,1-bis(p-chlorophenyl)-2,2-
dichloroethane and 1,1-bis(p-chlorophenyl)-2,2,2-trichloro-
ethyl acetate (kelthane acetate) as mutagens in Salmonella
typhimurium and of 1,1-bis(p-chlorophenyl)ethylene oxide, a
likely metabolite, as an alkylating agent. Chem. biol.
Interact., 25: 157-175.
POGGI, G., GUISIANI, M., PALAGI, U., PAGGIARO, P.L., LOI,
A.M., DAZZI, F., SICLARI, C., & BASCHIERI, L. (1982) High-
performance liquid chromatography for the quantitative
determination of the urinary metabolites of toluene, xylene,
and styrene. Int. Arch. occup. environ. Health, 50: 25-31.
PONCELET, F., DE MEESTER, C., DUVERGER-VAN BOGAERT, M.,
LAMBOTTE-VANDEPAER, M., ROBERTFROID, M., & MERCIER, M. (1980)
Influence of experimental factors on the mutagenicity of
vinylic monomers. Arch. Toxicol., Suppl. 4: 63-66.
PONOMAREVA, N.I. & ZLOBINA, N.S. (1972) [Working conditions
and the state of the upper respiratory tract in workers
engaged in the production of block and emulsion polystyrene
and its copolymers.] Gig. Tr. Prof. Zabol., 15: 22-26 (in
Russian).
PONOMARKOV, V. & TOMATIS, L. (1978) Effects of long-term oral
administration of styrene to mice and rats. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 127-135.
PONOMARKOV, V., CABRAL, J.R.P., WAHRENDORF, J., & GALENDO, D.
(1983) A carcinogenicity study of styrene oxide in rats.
Toxicolgist, 3: 46 (Abstract No. 184).
QUAST, J.F., KALNINS, R.P., OLSON, K.J., HUMISTON, C.G.,
MURRAY, F.J., JOHN, J.A., & SCHWETZ, B.A. (1978) Results of a
toxicity study in dogs and teratogenicity studies in rabbits
and rats administered monomeric styrene. In: Meeting of the
Society of Toxicology, San Francisco, USA, March 1978.
QUAST, J.F., HUMISTON, C.G., KALNINS, R.V., OLSON, K.J.,
McCOLLISTER, S.B., WADE, C.E., BEYER, J.E., & SCHWETZ, B.A.
(1979) Results of a toxicity study of monomeric styrene
administered to beagle dogs by oral intubation for 19 months -
Midland, MI, Dow Chemical Co., 199 pp (MCA (Manufacturing
Chemists Association) No: Sty 1.2-Tox-Gav-Dow.)
RAGYL'YE, N. (1974) [Problems concerning the embryotropic
effects of styrene.] Gig. i Sanit., 11: 65-66 (in Russian).
RAMSEY, J.C. & YOUNG, J.D. (1978) Pharmacokinetics of inhaled
styrene in rats and humans. Scand. J. Work Environ. Health, 4
(Suppl. 2): 84-91.
RAMSEY, J.C., YOUNG, J.D., KARBOWSKI, R.J., CHENOWETH, M.B.,
MCCARTY, L.P., & BRAUN, W.H. (1980) Pharmacokinetics of
inhaled styrene in human volunteers. Toxicol. appl.
Pharmacol., 53: 54-63.
RENKE, W. & CHEMIELEWSKI, J. (1976) Blood coagulation and
fibronolysis in occupational exposure to some chemical
factors. Biul. Inst. Med. Morskiej, 27: 289-298.
RIIHIMAKI, V. & PFAFFLI, P. (1978) Percutaneous absorption of
solvent vapors in man. Scand. J. Work Environ. Health, 4:
73-85.
ROSEN, A.A., SKEEL, R.T., & ETTINGER, M.B. (1963)
Relationship of river water odor to specific organic
contaminants. J. Water Pollut. Control Fed., 35: 777-82.
ROSEN, I., HAEGER-ARONSEN, B., REHNSTROM, S., & WELINDER, H.
(1978) Neurophysiological observations after chronic styrene
exposure. Scand. J. Work Environ. & Health, 4 (Suppl. 2):
184-194.
ROSENSTEEL, R.E. & MEYER, C.R. (1977) Health hazard
evaluation determination. Cincinnati, US Department of
Health, Education and Welfare, Public Health Service, Center
for Disease Control, National Institute for Occupational
Safety and Health. 52 pp (Report no. 75-150-378 - Reinell
Boats, Inc., Poplar Bluff, Missouri).
ROTH, B. & KLIMKOVA-DEUTSCHOVA, E. (1963) The effect of the
chronic action of industrial poisons on the electro-
encephalogram of man. Rev. Czech. Med., 9: 217-227.
ROTH, S.H. (1979) Physical mechanisms of anesthesia. Ann.
Rev. Pharmacol. Toxicol., 19: 159-178.
SAALWAECHTER, A.T., McCAMMON, C.S., ROPER, C.P. Jr, &
CARLBERG, K.S. (1977) Performance testing of the NIOSH
charcoal tube technique for the determination of air
concentrations of organic vapors. Am. Ind. Hyg. Assoc. J.,
38(9): 476-486.
SANTODONATO, J., MEYLAN, W.M., DAVIS, L.N., HOWARD, P.H.,
ORZEL, D.M., & BOGYO, D.A. (1980) Investigation of selected
potential environmental contaminants: Styrene, ethyl benzene
and related compounds. 261 pp (A report prepared for the
Office of Toxic Substances, US Environmental Protection
Agency, Washington, DC by Syracuse Research Corporation;
Contract No. 68-01-3250.)
SAUERHOFF, M.W. & BRAUN, W.H. (1976) The fate of styrene in
rats following an inhalation exposure to 14 C-styrene.
Midland, Michigan, Toxicology Research Laboratory, Health and
Environmental Research, Dow Chemical USA, 26 pp.
SAUERHOFF, M.W., MADRID, E.O., & BRAUN, W.H. (1976) The fate
of orally administered styrene in rats. Midland, Michigan,
Toxicology Research Laboratory, Health and Environmental
Research, Dow Chemical USA, 44 pp.
SAVOLAINEN, H. & PFAFFLI, P. (1977) Effects of chronic
styrene inhalation on rat brain protein metabolism. Acta
Neuropath., 40: 237-241.
SAVOLAINEN, H. & PFAFFLI, P. (1978) Accumulation of styrene
monomer and neurochemical effects of long-term inhalation
exposure in rats. Scand. J. Work Environ. Health, 4 (Suppl.
2): 78-83.
SAVOLAINEN, H. & VAINIO, H. (1977) Organ distribution and
nervous system binding of styrene and styrene oxide.
Toxicology, 8: 135-141.
SAVOLAINEN, H., HELOJOKI, M., & TENGEN-JUNNILA, M. (1980)
Behavioural and glial cell effects of inhalation exposure to
styrene vapour with special reference to interactions of
simultaneous ethanol intake. Acta Pharmacol. Toxicol., 46:
51-56.
SBRANA, I., LASCIALFARI, D., ROSSI, A.M., & LOPRIENO, N.
(1982) Bone marrow cell chromosomal aberrations and styrene
biotransformation in mice given styrene on a repeated oral
schedule. Submitted for publication.
SCHALLER, K.-H., GOSSLER, K., BOST, H.-P., & VALENTIN, H.
(1976) [Gas chromatographic methods for the determination of
styrene in the blood and of mandelic acid and phenylglyoxylic
acid in the urine.] Arbeits-, Sozial-, Präventivmedizin, 11:
64-64 (in German).
SCHNEIDER, H. & SEEBER, A. (1979) [Psychodiagnostics in the
understanding of neurotoxic effects of harmful chemical
substances.] Z. Psychol. Bd., 187: 178-205 (in German).
SCHOFIELD, K. (1974) Problems with flame ionization detectors
in automotive exhaust hydrocarbon measurements. Environ. Sci.
Technol., 8: 826-834.
SCHUMACHER, R.L., BREYSSE, P.A., CARLYON, W.R., HIBBARD, R.P.,
& KLEINMAN, G.D. (1981) Styrene exposure in the fiberglass
fabrication industry in Washington State. Am. Ind. Hyg.
Assoc. J., 42: 143-149.
SEPPALAINEN, A.M. (1978) Neurotoxicity of styrene in
occupational and experimental exposure. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 181-183.
SEPPALAINEN, A.M. & HARKONEN, H. (1976) Neurophysiological
findings among workers occupationally exposed to styrene.
Scand. J. Work Environ. Health, 2: 140-146.
SEPPALAINEN, A.M., LINDSTROM, K., & MARTELIN, T. (1980)
Neurophysiological and psychological picture of solvent
poisoning. Am. J. ind. Med., 1: 31-42.
SEUTTER-BERLAGE, F., DELBRESSINE, L.P.C., SMEETS, F.L.N., &
KETELAARS, H.C.J. (1978) Identification of three sulphur-
containing urinary metabolites of styrene in the rat.
Xenobiotica, 8: 413-418.
SHUGAEV, B.B. (1969) Concentration of hydrocarbons in tissues
as a measure of toxicity. Arch. environ. Health, 18: 878-882.
SIMKO, A., JINDRICHOVA, J., & PULTAROVA, H. (1966) [The
effect of styrene on the health state of workers employed in
laminate-production.] Prac. Lek., 18: 348-352 (in Czech).
SINITSKIJ, V.G. (1969) [Indexes for immunological reactivity
in rabbits during the long-term exposure to small doses of
styrene.] Gig. Primen. Polim. Mater, Izdelii Nikh, 1: 394-398
(in Russian).
SLOB, A. (1973) A new method for determination of mandelic
acid excretion at low level styrene exposure. Br. J. ind.
Med., 30: 390-393.
SMIRNOVA, E.T. & YATAKOVA, Z.M. (1966) Health hazard of
polystyrene toys. Hyg. Sanit., 31: 135-137.
SMITH, A.H. & ELLIS, L. (1977) Styrene butadiene rubber
synthetic plants and leukaemia. J. occup. Med., 19: 441.
SMITH, H.O. & HOCHSTETTLER, A.D. (1969) Determination of odor
thresholds in air using C14-labeled compounds to monitor
concentrations. Environ. Sci. Technol., 3: 169-170.
SODER, S.L. (1977) "Styrene" Chemical economics handbook,
California, Stanford Research Institute.
SOLLENBERG, J. & BALDESTEN, A. (1977) Isotachophoretic
analysis of mandelic acid, phenylglyoxylic acid, hippuric acid
and methylhippuric acid in urine after occupational exposure
to styrene, toluene and/or xylene. J. Chromatogr., 132:
469-476.
SORSA, M., HYVONEN, M., JARVENTAUS, H., & VAINIO, H. (1979)
Chromosomal aberrations and sister chromatid exchange in
children of women in reinforced plastic industry. In:
Symposium on Toxicology, Abstracts, Turku, Finland 29-20 May,
1979, Turku, University of Turku, p. 16.
SPASOVSKI, M. (1976) Health hazards in the production and
processing of some fibers, resins, and plastics in Bulgaria.
Environ. Health Perspect., 17: 199-202.
SPASOVSKI, M., HINKOVA, L., & BENCHEV, I. (1980) Exposure to
styrene - field studies. Toxicol. Lett., (suppl. 1): 218.
SPENCER, H.C., IRISH, D.D., ADAMS, E.M., & ROWE, V.K. (1942)
The response of laboratory animals to monomeric styrene. J.
ind. Hyg. Toxicol., 24: 295-301.
SPINAZZOLA et al. (1980) Petrolchimica. Tecnologia, ambiente
di lavoro, prevenzione e patologia. Atti del 43° Congresso
Nazionale della Società Italiana di Medicina del Lavoro ed
Igiene Industriale. Parma, Edizioni Tecnografica, Vol. 2.
SPIRTAS, R., VAN ERT, M., GAMBLE, J.F., WOLF, P., & McMICHAEL,
A.J. (1976) Toxicologic, industrial hygiene and
epidemiological consideration in the possible association
between SBR manufacturing and neoplasms of the lymphatic and
hematopoietic tissues. In: Ede, L., ed. Proceedings of NIOSH
Styrene-Butadiene Briefing, Covington, Kentucky, USA, 1976,
Cincinnati, Ohio, Department of Health, Education and Welfare,
pp. 67-112 (HEW Publ. No. (NIOSH) 77-129).
SRAM, R.J. (1981) Cytogenetic analysis of peripheral
lymphocytes as a method for monitoring environmental levels of
mutagens. In: Gut, I., Cikrt, M., & Plaa, G.L., ed.
Industrial and environmental xenobiotics. Metabolism and
pharmacokinetics of organic chemicals and metals., Berlin,
Springer-Verlag, pp. 187-194.
STEWART, R.D., DODD, H.C., BARETTA, E.D., & SCHAFFER, A.W.
(1968) Human exposure to styrene vapor. Arch. environ.
Health, 16: 656-662.
STOLZ, D.R. & WITHEY, R.J. (1977) Mutagenicity testing of
styrene and styrene epoxide in Salmonella typhimurium. Bull.
environ. contamin. Toxicol., 17: 739-742.
SUGIURA, K. & GOTO, M. (1981) Mutagenicities of styrene oxide
derivatives on bacterial test systems: relationship between
mutagenic potencies and chemical reactivity. Chem. biol.
Interact., 35: 71-91.
SUGIURA, K., KIMURA, T., & GOTO, M. (1978a) Mutagenicities of
styrene oxide derivatives on Salmonella typhimurium (TA100).
Relationship between mutagenic potencies and chemical
reactivity. Mutat. Res., 58: 159-165.
SUGIURA, K., YAMANAKA, S., FUKUSAWA, S., & GOTO, M. (1978b)
The mutagenicity of substituted and unsubstituted styrene
oxides in E. coli: Relationship between mutagenic potencies
and physico-chemical properties. Chemosphere, 7: 737-742.
SUGIURA, K., MAEDA, A., & GOTO, M. (1979) Substitutional
effects of styrene oxides on survival and mutation induction
in cultured Chinese hamster cells (V-79). Chemosphere, 8:
369-372.
SYNTHETIC RESINS LIMITED (1980) LSE capability with good
interlaminal adhesion. Reinf. Plast., 3: 72-73.
TAULBEE, J., ANDJELKOVIC, D., WILLIAMS, T., GAMBLE, J.F., &
WOLF, P. (1976) A study of possible associations between
exposure to SBR processes and industrial hygiene and
epidemiologic considerations (for workers in the 1951 and 1964
cohorts and deaths 1964-1973). In: Ede, L., ed. Proceedings
of NIOSH Styrene-Butadiene Briefing, Covington, Kentucky, USA,
1976, Cincinnati, Ohio, Department of Health, Education and
Welfare, pp. 113-162 (HEW Publ. No. (NIOSH) 77-129).
TERAMOTO, K., HORIGUCHI, S., & KITIBATAKE, S. (1978) [Studies
on industrial styrene poisoning. (Part VIII). Distribution and
elimination of styrene in rats exposed to styrene.] Jpn. J.
ind. Health, 20(2): 118-119 (in Japanese).
THEISS, A.M. & FLEIG, I. (1978) Chromosome investigations on
workers exposed to styrene/polystyrene. J. occup. Med., 20:
747-749.
THIESS, A.M. & FRIEDHEIM, M. (1978) Morbidity among persons
employed in styrene production, polymerization and processing
plants. Scand. J. Work Environ. Health, 4 (Suppl. 2): 203-214.
THIESS, A.M. & FRIEDHEIM, M. (1979) [Morbidity study in
coworkers of the polyester laboratory and of the technical
service exposed to styrene.] Zentralbl. Arbeitsmed., 9:
238-241 (in German).
THIESS, A.M., SCHWEGLER, H., & FLEIG, I. (1980) Chromosome
investigations in lymphocytes of workers employed in areas in
which styrene-containing unsaturated polyester resins are
manufactured. Am. J. ind. Med., 1: 205-210.
THOMPSON, B. (1974) Hazardous gases and vapors: Infrared
spectra and physical constants, Fullerton, Beckman
Instruments, Inc., pp. 154, 255 (Technical report 595).
TOLA, S., HARKONEN, H., KORKALA, M.L., & JARVINEN, E. (in
press) Mortality of workers exposed to styrene. Egypt. J.
occup. Med., (in press).
TOSSAVAINEN, A. (1978) Styrene use and occupational exposure
in the plastics industry. Scand. J. Work Environ. Health, 4
(Suppl. 2): 7-13.
TURCHI, G., BONATTI, S., CITTI, L., GERVASI, P.G.,
ABBONDANDOLO, A., & PRESCIUTTINI, S. (1981) Alkylating
properties and genetic activity of 4-vinylcyclohexane
metabolites and structurally related epoxides. Mutat. Res.,
83: 419-430.
US EPA (1980) Investigation of Selected Potential
Environmental Contaminants: Styrene Ethylbenzene, and Related
Compounds. Washington DC, US Environmental Protection Agency,
261 pp (Publication No. EPA 5601 11-80---018).
US NATIONAL CANCER INSTITUTE (1979) Bioassay of styrene for
possible carcinogenicity. Washington, DC, Department of
Health, Education and Welfare, 46 pp (Publ. No. (NIH)
79-1741, Washington DC, (Techn. Rep. ser. No. 185)).
VAINIO, H. (1978) Vinyl chloride and vinyl benzene (styrene)-
metabolism, mutagenicity and carcinogenicity. Chem.-biol.
Interact., 22: 117-124.
VAINIO, H. & MAKINEN, A. (1977) Styrene and acrylonitrile
induced depression of hepatic nonprotein sulfhydryl content in
various rodent species. Res. Commun. Chem. Pathol.
Pharmacol., 17: 115-124
VAINIO, H., PAAKKONEN, R., RONNHOLM, K., RAUNIO, V., &
PELKONEN, O. (1976) A study on the mutagenic activity of
styrene and styrene oxide. Scand. J. Work Environ. Health, 3:
147-151.
VAINIO, H., HEMMINKI, K., & ELOVAARA, E. (1977) Toxicity of
styrene and styrene oxide on chick embryos. Toxicology, 8:
319-325.
VAINIO, H., JARVISALO, J., & TASKINEN, E. (1979) Adaptive
changes caused by intermittent styrene inhalation on
xenobiotic biotransformation. Toxicol. appl. Pharmacol., 49:
7-14.
VAINIO, H., NORPPA, H., & BELVEDERE, G. (1982) The role of
cytochrome P-450 mediated metabolism in SCEs induced by
styrene in human lymphocytes. In: Proceedings of the l3th
International Cancer Congress, Abstracts, Seattle, USA, 8-l5
September, l982, Seattle, International Union Against Cancer,
p. 118.
VALENTA, J. (1966) [Air and water pollution by styrene with
the butadiene-styrene rubber production.] Cesk. Hyg., 11:
349-352 (in Czech).
VAN ANDA, J., SMITH, B.R., FOUTS, J.R., & BEND, J.R. (1979)
Concentration-dependent metabolism and toxicity of [14C
styrene oxide in the isolated perfused rat liver. J.
Pharmacol. exp. Ther., 211: 207-211.
VAN DUUREN, B.L., NELSON, N., ORRIS, L., PALMES, E.D., &
SCHMITT, F.L. (1963) Carcinogenicity of epoxides, lactones
and peroxy compounds. J. Natl Cancer Inst., 31: 41-55.
VARNER, S.L. & BREDER, C.V. (1981a) Liquid chromatographic
determination of residual styrene in polystyrene food
packaging. J. Assoc. Off. Anal. Chem., 64: 647-652.
VARNER, S.L. & BREDER, C.V. (1981b) Head space sampling and
gas chromatographic determination of styrene migration from
food contact polystyrene cups into beverages and food
simulants. J. Assoc. Off. Anal. Chem., 64: 1122-1130.
VERGIEVA, T. & ZAJKOV, H. (1981) [Behavioural changes in rats
with inhalation styrene effect.] Hyg. Zdrav., 24: 242-247 (in
Bulgarian).
VERGIEVA, T., ZAIKOV, Hr., & PALATOV, S. (1979) [A study on
the embryotoxic action of styrol.] Hyg. Zdrav., XXII: 39-43
(in Russian).
VIHKO , R., VIHKO, P., MAENTAUSTA, O., PAKARINEN, A., JANNE,
O., & YRJANHEIKKI, E. (1983) Assessment of early
hepatotoxicity. In: Aitio, A., Riihimäki, V., & Vainio, H.,
ed. Biological monitoring and surveillance of workers exposed
to chemicals, Washington, Hemisphere Publ. Co. (in press).
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J.J.A.A. (1981)
The mutagenic action of aliphatic epoxides. Mutat. Res., 89:
269-282.
VOSKAMP, A.J. & STUDENBERG, J.E. (1981) A new low styrene
emission laminating resin. In: 36th Annual Conference,
Reinforced Plastics/Composites Institute, the Society of the
Plastics Industry, Inc., February 16-20, 1981, Session 19-D.
WADE, D.R., AIRY, S.C., & SINSHEIMER, J.E. (1978)
Mutagenicity of aliphatic epoxides. Mutat. Res., 58: 217-223.
WATABE, T., ISOBE, M., SAWAHATA, T., YOSHIKAWA, K., YAMADA,
S., & TAKABATAKE, E. (1978) Metabolism and mutagenicity of
styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
142-155.
WATABE, T., OZAWA, N., & YOSHIKAWA, K. (1981a)
Stereochemistry in the oxidative metabolism of styrene by
hepatic microsomes. Biochem. Pharmacol., 30: 1695-1698.
WATABE, T., HIRATSUKA, A., OZAWA, N., & ISOBE, M. (1981b)
Glutathione S-conjugates of phenyloxirane. Biochem.
Pharmacol., 30: 390-392.
WATABE, T., HIRATSUKA, A., AIZAWA, T., & SAWAHATA, T. (1982a)
1-vinylbenzene 1,2- and 3,4-oxides. Tetrahedron Lett., 23:
1185-1188.
WATABE, T., HIRATSUKA, A., AIZAWA, T., SAWAHATA, T., OZAWA,
N., ISOBE, M., & TAKABATAKE, E. (1982b) Studies on the
metabolism and toxicity of styrene IV. 1-vinylbenzene
3,4-oxide, a potent mutagen formed as a possible intermediate
in the metabolism in vivo of styrene to 4-vinylphenol. Mutat.
Res., 93: 45-55.
WATABE, T., OZAWA, N., & YOSHIKAWA, K. (1982c) Studies on
metabolism and toxicity of styrene. V. The metabolism of
styrene, racemic, ( R)-(+), and ( S)-(-) -phenyloxiranes in the
rat. J. Pharm. Dyn., 5: 129-133
WATANABE, T. ENDO, A., SATO, K., OHTSUKI, T., MIYASAKA, M.,
KOIZUMI, A., & IKEDA, M. (1981) Mutagenic potential of
styrene in man. Ind. Health, 19: 37-45.
WATANABE, T., ENDO; A., KUMAI, M., & IKEDA, M. (1983)
Chromosome aberrations and sister chromatid exchanges in
styrene-exposed workers with reference to their smoking
habits. Environmental Mutagenesis, 5: 299-309
WEAST, R.C. & ASTLE, M.J. ed. (1981) Handbook of chemistry
and physics, Boca Raton, CRS Press, p. c-500.
WEIL, C.S., CONDRA, N., HAUN, C., & STRIEGEL, J.A. (1963)
Experimental carcinogenicity and acute toxicity of
representative epoxides. Am. Ind. Hyg. Assoc. J., 24: 305-325.
WHITE, L.D., TAYLOR, D.G., MAUER, P.A., & KUPEL, R.E. (1970)
A convenient optimized method for the analysis of selected
solvent vapors in the industrial atmosphere. Am. Ind. Hyg.
Assoc. J., 31: 225-232.
WILSON, R.H. (1944) Health hazards encountered in the
manufacture of synthetic rubber. J. Am. Med. Assoc., 124:
701-703.
WILTI, D. (1970) Infrared vapour spectra, London, Heyden &
Son Ltd. (Index No. 180).
WINK, A. (1972) Effect of long-term exposure to toxic
substances on urinary excretion of 17-oxogenic steroids and
17-oxosteroids. Ann. occup. Hyg., 15: 211-215.
WITHEY, J.R. (1976) Quantitative analysis of styrene monomer
in polystyrene in foods including some preliminary studies of
the uptake and pharmacodynamics of the monomer in rats.
Environ. Health Perspect., 17: 125-133.
WITHEY, J.R. (1978) The toxicology of styrene monomer and its
pharmacokinetics and distribution in the rat. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 31-40.
WITHEY, J. & COLLINS, P.G. (1977) Pharmakokinetics and
distribution of styrene monomer in rats after intravenous
administration. J. Toxicol. environ. Health, 3: l0ll-l020.
WITHEY, J.R. & COLLINS, P.G. (1978) Styrene monomer in foods:
A limited Canadian survey, Bull. environ. Contam. Toxicol.,
19: 86-94.
WITHEY, J.R. & COLLINS, P.G. (1979) The distribution and
pharmacokinetics of styrene monomer in rats by the pulmonary
route. J. environ. Pathol. Toxicol., 2: 1329-1342.
WOLF, M.A., ROWE, V.K., MCCOLLISTER, D.D., HOLLINGSWORTH,
R.L., & OYEN, F. (1956) Toxicological studies of certain
alkylated benzenes and benzene. Am. Med. Assoc. Arch. ind.
Health, 14: 387-398.
WOLFF, M.S., DAUM, S.M., LORIMER, W.V., & SELIKOFF, I.J.
(1977) Styrene and related hydrocarbons in subacutaneous fat
from polymerization workers. J. Toxicol. environ. Health, 2:
997-1005.
WOLFF, M.S., LILIS, R., LORIMER, W.V., & SELIKOFF, I.J.
(1978a) Biological indicators of exposure in styrene
polymerization workers. Styrene in blood and adipose tissue
and mandelic and phenylglyoxylic acids in urine. Scand. J.
Work Environ. Health, 4 (Suppl. 2): 114-118.
WOLFF, M.S., LORIMER, W.V., LILIS, R., & SELIKOFF, I.J.
(1978b) Blood styrene and urinary metabolites in styrene
polymerization. Br. J. ind. Med., 35: 318-329.
YAMAMOTO, R.K. & COOK, W.A. (1968) Determination of
ethylbenzene and styrene in air by ultraviolet
spectrophotometry. Am. Ind. Hyg. Assoc. J., 29: 283-241.
YOSHIKAWA, K., ISOBE, M., WATANABE, T., & TAKABATAKE, E.
(1980) Studies on metabolism and toxicity of styrene III.
The effect of metabolic inactivation by rat-liver S9 on the
mutagenicity of phenyloxirane toward Salmonella typhimurium.
Mutat. Res., 78: 219-226.
ZAPRIANOV, Z & BAINOVA, A. (1979) Changes in monoamine
oxidase activity (MAO) after styrene and ethanol combined
treatment of rats. Activ. nerv. Sup. (Praha), 21(4): 262-264.
ZETTERBERG, G. (1982) Styrene - a widespread mutagen:
conclusions from the results of testing. In: Sugimura, T.,
Kondo, S. & Takabe, H., ed. Environmental Mutag. Carcinog.,
Tokyo, University of Tokyo Press, pp. 316-322.
ZIELHUIS, R.L., HARTOGENSIS, F., JONGH, J., KALSBEEK, J.W.H.,
& VAN REES, H. (1964) The health of workers processing
reinforced polyesters. In: XIVth International Congress of
Occupational Health, Madrid, Spain, 16-21 September, 1963,
Volume III, pp. 1092-1097.
ZITTING, A., HEINONEN, T., & VAINIO, H. (1980) Glutathione
depletion in isolated rat hepatocytes caused by styrene and
the thermal degradation products of polystyrene. Chem. biol.
Interact., 31: 313-318.
ZLOBINA, N.S. (1963) [On the toxicity of small concentrations
of styrol vapors.] Gig. i Sanit., 28: 29-35 (in Russian).
The oxidation of styrene to styrene 7,8-oxide has been
demonstrated in vitro using rat or rabbit liver microsomes
(Leibman & Ortiz, 1969; Leibman, 1975; Belvedere et al., 1977;
Duverger-van Bogaert et al., 1978; Watabe et al., 1978), rat
liver nuclei (Gazzotti et al., 1980) or rat, rabbit, or
guinea-pig extrahepatic microsomes (Cantoni et al., 1978). The
major route of styrene metabolism is believed to proceed via
this epoxide intermediate.
Styrene has been shown to be epoxidized to ( R)- and
( S)-styrene 7,8-oxides in the ratio 1:1.3 by rat liver microsomal
cytochrome P-450 (Watabe et al., 1981a). The ( R)-and ( S)-oxides
are hydrolysed in a regiospecific manner by rat liver microsomal
epoxide hydrolase to ( R)- and ( S)-phenyl-ethylene glycols in the
ratio of 1:4 (Watabe et al., 1981a). Styrene oxides are also
conjugated with glutathione (Oesch, 1973; James et al., 1975;
Pacheka et al., 1979). Rat liver cytosolic glutathione
transferases convert the ( R)- and ( S)-oxides stereoselectively
to S-(1-phenyl-2-hydroxyethyl)-glutathione (GSH conjugate 1) and
S-(2-phenyl-2-hydroxyethyl)-glutathione (GSH conjugate 2),
respectively (Watabe et al., 1981b). Conjugates 1 and 2 would be
directly correlated with the urinary N-acetyl- S-cysteine
conjugates, namely 1-phenyl-2-hydroxyethyl and 2-phenyl-2-
hydroxyethylmercapturic acids, respectively (James & White, 1967;
Seutter-Berlage et al. 1978; Watabe et al., 1982c).
Rats administered styrene intraperitoneally (i.p.) also
excreted 2-vinylphenol and 4-vinylphenol in conjugated forms
in the urine (Hiratsuka et al., 1982a). Styrene 1,2- and
3,4-oxides administered i.p. to rats have been demonstrated in
the urine as 2-vinylphenol and 4-vinylphenol, respectively
(Hiratsuka et al., 1982a). However, 3-vinylphenol has not
been detected in the urine (Bakke & Scheline, 1970; Pantarotto
et al., 1978; Watabe et al., 1978). Other minor metabolites
are 1- and 2-phenylethanol (Delbressine et al., 1980).
Comparing the kinetic parameters of styrene monooxygenase
and styrene 7,8-oxide hydrolase in rat liver microsomes
(Cantoni et al., 1978; Duverger-van Bogaert et al., 1978), it
appears that the conversion of styrene 7,8-oxide into styrene
glycol is preferred. The ratio between styrene epoxide
hydrolase and monooxygenase in rat liver was 2-3 times higher
than those found in mouse and rabbit liver (Cantoni et al.,
1978).
Styrene glycol is partly transformed into a glucuronide
that has been detected in the urine of styrene-treated rabbits
(El Masri et al., 1958). The major metabolic pathway involves
the sequential oxidation to mandelic, phenylglyoxylic, and
benzoic acids, which have been identified in urine (Bardodej,
1964; James & White, 1967; Ohtsuji & Ikeda, 1971).
Quantitative differences have been observed between various
species, hippuric acid being quantitatively the most relevant
urinary metabolite in the rabbit (El Masri et al., 1958),
phenylglyoxylic acid in the rat (Bardodej et al., 1971;
Watanabe et al., 1982c) and mandelic acid in man (Bardodej,
1964; Bardodej & Bardodejova, 1970). Minor metabolites
identified include parahydroxymandelic, parahydroxyphenyl-
glyoxylic, and parahydroxybenzoic acids (Bakke & Sheline,
1970; Pantarotto et al., 1978).
The metabolism of styrene is enhanced by phenobarbital
pretreatment and is inhibited by SKF 525-A, an inhibitor of
monooxygenases (Ohtsuji & Ikeda, 1971).
Styrene 7,8-oxide either binds covalently to macro-
molecules such as microsomal proteins (Marniemi et al., 1977;
Watabe et al., 1978) or conjugates with glutathione
non-enzymatically and in the presence of a transferase (Oesch,
1973; James et al., 1975; Pachecka et al., 1979). Pheno-
barbital pretreatment of the rats enhances, whereas carbon
tetrachloride administration reduces, the NADPH dependent-
binding to microsomal protein in vitro (Watabe et al., 1978).
Prolonged exposure to a styrene vapour concentration of
1260 mg/m3 (300 ppm) leads to an enhancement of the metabolic
elimination of the compound (Vainio et al., 1979) resulting in
decreasing body burden during continued exposure.
Though numerous metabolic pathways have been found in
animal studies (mainly in the rat and rabbit), only a few have
been identified in human beings. Analysis of the urine of
human subjects exposed to styrene has revealed mandelic and
phenylglyoxylic acids as the major metabolites indicating
that, at least qualitatively, styrene metabolism in human
beings follows pathways similar to those previously described
in animal studies. The results of a study by Pfäffli et al.
(1981) suggest that 4-vinylphenol is also excreted in the
urine of workers exposed to styrene. Though this metabolite
represented only 0.3 % of the urinary concentration of
mandelic acid, there was a good correlation between the
excretion of the two metabolites.
Recent data indicate that human whole blood lymphocyte
cultures apparently catalyse the oxidation of styrene to
styrene 7,8-oxide (Norppa et al., 1980a). Belvedere & Tursi
(1981) have shown that both human lymphocytes and erythrocytes
are capable of converting styrene to styrene 7,8-oxide. In
whole blood, red blood cells may be more important in this
conversion, because they are present in greater numbers than
lymphocytes.
5.4. Elimination
5.4.1. Human studies
5.4.1.1. Controlled human studies
In an inhalation study by Fiserova-Bergerova & Teisinger
(1965), 7 human volunteers were exposed to styrene levels of
63-160 mg/m3 (15-38 ppm) for 5 h. From analysis of the
alveolar air, the authors found that pulmonary elimination
followed a bi-exponential curve indicating a 2-compartment
model.
Stewart et al. (1968) found that styrene elimination from
the lungs, after inhalation exposure, was rapid and bi-
exponential but that the rates of elimination varied with the
level and duration of exposure. It was apparent that subjects
who were exposed to a styrene concentration of 1579 mg/m3 (376
ppm) for 1 h had lower elimination rate coefficients for the
terminal phase than those exposed to 907 mg/m3 (216 ppm) and
214 mg/m3 (51 ppm) for 1 h. No values for the rate
coefficients were given in this report but it was calculated
that one subject, who had been exposed to 491 mg/m3 (117 ppm)
for 2 h, exhaled 1.2% of the total amount of styrene absorbed
during the first 4 h after exposure. Exhaled styrene was
detected up to 24 h after the termination of a 30-min exposure
to concentrations of 210 or 630 mg/m3 (50 or 150 ppm) (Astrand
et al., 1974).
The exhalation of styrene after pulmonary exposure was
also examined in human volunteers by Fernandéz & Caperos
(1977). Six subjects were exposed to vapour concentrations of
294-840 mg/m3 (70-200 ppm) for periods of 4 or 8 h. The
concentration of styrene in the expired alveolar air was
reported to decline rapidly during the first hour following
termination of exposure, at all exposure concentrations, and
2.6% of the total absorbed dose was considered to be
eliminated via the lung.
Exhalation of styrene after inhalation exposure to a
styrene concentration of 210 mg/m3 (50 ppm) was measured in 7
volunteers for 4 consecutive 30-min periods either resting or
in a work cycle (Engström et al., 1978b). The exhalation was
followed for up to 19 h after exposure by monitoring styrene
concentrations in the expired air; some 31% of the retained
styrene was estimated to be eliminated via the lungs. In the
same study, styrene eliminated from adipose tissue was
reported to have a half-life of from 2 to 4 days, which led
the authors to conclude that repeated daily exposure to
styrene could lead to bioaccumulation.
In a study by Ramsey & Young (1978), 4 male human
volunteers were exposed to a styrene concentration of 336
mg/m3 (80 ppm) for 6 h and the styrene in the blood and
expired air monitored for 41 h after exposure. The styrene
concentration in blood was found to decay in a bi-exponential
fashion, typical of a linear 2-compartment model. The rapid
initial elimination phase yielded a half-time of 0.58 h and,
for the slower terminal phase, a value of 13.0 h was
reported. The concentration of styrene in the expired air for
the same volunteers showed a biphasic log-linear decline
similar to that observed for the blood concentrations.
In a study designed to investigate the skin absorption of
styrene (Riihimäki & Pfäffli, 1978), two subjects wearing air-
supplied respirators were exposed to a styrene concentration
of 2520 mg/m3 (600 ppm) for 3.5 h while performing light
work. Bi-exponential elimination data were obtained both from
analysis of venous blood and of alveolar air and the authors
reported a value of about l h for the half-time of the rapid
phase and about 10 h for the slower phase.
Studies conducted on 6 male volunteers at styrene
exposures varying from 865 mg/m3 (206 ppm) for 8 h to 294
mg/m3 (70 ppm) for 4 h gave alveolar concentration decay
curves that the authors claimed were best described by a
3-compartment model (Fernandez & Caperos, 1977). The rate
coefficients for the three phases had half-lives that ranged
from 0.06 to 0.61 h for the rapid phase, 1.01 to 3.75 h for
the intermediate phase, and 10.05 to 24.75 h for the slowest
phase.
A study on the rate of excretion of the urinary
metabolites of styrene, phenylglyoxylic and mandelic acids
(Guillemin & Bauer, 1978) was carried out on 9 human subjects
after pulmonary exposure to styrene at 168 or 840 mg/m3 (40 or
200 ppm) for 4-8 h. The authors found that the rate
coefficients for the urinary excretion of metabolites were
unaffected by the duration or level of exposure and claimed
that if the amounts of both metabolites were totalled over 4
days following exposure, reliable measurements of exposure
could be obtained. For mandelic acid, the first and second
phase half-times were evaluated to be respectively 3.9 h and
24.7 h, while for phenylglyoxylic acid the half-time was
calculated to be 10.5 h (Guillemin & Bauer, 1979).
5.4.1.2. Occupational exposure studies
Of the numerous studies that have been conducted on
styrene-exposed workers (Härkönen, 1978), only a few have been
devoted to the assessment of metabolite production.
In a study by Götell et al. (1972), a group of 17 workers
was divided into 3 exposure subgroups, the highest at 987-1226
mg/m3 (235-292 ppm), an intermediate group at 374-584 mg/m3
(89-139 ppm), and the lowest group at 71-134 mg/m3 (17-32
ppm). Decay curves of styrene in expired air for the 3 groups
had a multi-exponential form but no kinetic parameters were
cited. It was evident, however, that the terminal elimination
phase was markedly faster for the group receiving the highest
exposure.
The rate of appearance of mandelic acid in the urine has been
shown to be biphasic, half times being 9 h and 16 h, respectively
(Engström, K., et al., 1976). In a preliminary report on workers,
with limited time points, monophasic elimination with half-times of
8.5 and 7.8 h, respectively for phenylglyoxylic and mandelic acid,
was found (Ikeda & Imamura, 1974). Considerable inter-subject
variability has been reported by several authors (Philippe et al.,
1971; Götell et al., 1972; Härkönen et al., 1974; Gompertz, 1978).
On the basis of findings in animal studies, the excretion
of hippuric acid was investigated in styrene-exposed workers.
An increase in hippuric acid excretion was noted in styrene-
exposed workers (Ikeda et al., 1974); similar levels and
important fluctuations have been found in unexposed controls
(Engström, K., et al., 1978).
5.4.1.3. General population studies
There have not been any reported studies on the
elimination of styrene in the general population.
5.4.2. Experimental animal studies
Results of studies on rats subcutaneously injected with
500 mg/kg 14C-labelled styrene showed that there was rapid
distribution to tissues and organs within 1 h and that 85% of
the radioactivity was eliminated within 24 h; 71% of the
radioactivity appeared in the urine, 12% in expired air as
carbon dioxide, 3% in the faeces, and about 3% as unchanged
styrene in expired air (Danishefsky & Willhite, 1954).
A later study (Sauerhoff et al., 1976) demonstrated a
similar rapid clearance of ring-labelled 14C-styrene in male
and female rats after oral administration of 50 or 500 mg/kg
body weight. More than 90% of the radioactivity appeared in
the urine within 72 h and no residual styrene was found in the
tissues examined at that time. The urinary excretion of
radioactivity followed a 2-compartment model at the low dose
level with half-times of 1.29 and 8.13 h for the initial
(alpha) and terminal (beta) phases, respectively. At the high
dose, a mono-exponential urinary excretion was observed with a
half-time of 6.74 h. About twice as much styrene was
eliminated by the pulmonary route in male compared with female
rats.
Biphasic excretion of radioactivity in the urine of rats
was observed after inhalation of 14C-styrene (252 or
2520 mg/m3; 60 or 600 ppm) for 6 h with half-time values of
14.9 h for the lower and 19.2 h for the higher exposure
concentration (Sauerhoff & Braun, 1976).
Inhalation exposure of rats to styrene concentrations
varying from 336 to 5040 mg/m3 (80 to 1200 ppm) for 6 h
(Ramsey & Young, 1978) also showed evidence of dose-dependent
kinetics. For the 15-fold increase in exposure level, the
area generated under the blood level-time curve increased by a
factor of 112. The elimination kinetics in the post-exposure
period appeared to change from a bi-exponential curve to a
log-linear form with increasing exposure concentrations. At
the 336 mg/m3 (80 ppm) dose level, the hybrid elimination rate
coefficients for the initial and terminal phases were 0.26 h
and 3.60 h, respectively.
Withey (1978) and Withey & Collins (1979) described the
elimination kinetics of styrene in rats after 5 h inhalation
exposure at levels that varied from 188 to 10 151 mg/m3 (44.8
to 2417 ppm). In contrast to the study by Ramsey & Young
(1978), these authors reported a bi-exponential curve of
elimination for all levels of exposure. The reported
half-times for the initial and terminal phases ranged from 3.4
to 6.3 min and about 50 to 350 min, respectively.
Teramoto et al. (1978) examined the elimination rates of
styrene from samples of the liver, brain, kidney, blood,
spleen, muscle, and adipose tissue in rats after an inhalation
exposure to 2940 mg/m3 (700 ppm) for 4 h. They found that all
tissues had a similar elimination half-time of about 2 h with
the exception of adipose tissue for which the styrene
half-time was about 6 h.
To examine the accumulation of styrene in tissues,
Savolainen & Pfäffli (1978) exposed rats to 1260 mg/m3 (300
ppm), for 6 h per day, and 5 days per week, for up to 11 weeks
and then monitored the styrene concentrations in adipose
tissue. Concentrations increased almost linearly, during the
first 4 weeks of exposure and then decreased exponentially.
In the study reported by Bergman (1979) (section 5.2.2),
mice were exposed through inhalation to 10 µl of volatilized
radiolabelled styrene over a 10-min period. The amount of
unchanged styrene exhaled over an 8-h period following
exposure amounted to 2.9% of the calculated dose. The
kinetics of elimination of radioactivity in exhaled air gave a
biphasic curve with half-times of 15 and 275 min for the
initial and terminal phases, respectively. Eight hours after
dosing, high levels of radioactivity were found in the liver,
kidney, lung, and adipose tissue.
Results of a study by Withey & Collins (1977) showed that
the elimination of styrene after intravenous administration in
rats also obeyed a 2-compartment model. Approximate rates of
elimination from some individual organs were obtained for dose
levels of approximately 1.3 and 4 mg/kg body weight. At the
higher dose, the kinetics of styrene disappearance from blood,
heart, brain, liver, spleen, and kidney appeared to follow a
1-compartment model with an elimination half-time of about
14.4 h in the same organs. At the lower dose, a biphasic
elimination was observed with half-times of about 4.78 and 26
h for the rapid and slow phases, respectively.
5.5. Biological Monitoring of Styrene Uptake
The measurement of styrene in blood, subcutaneous fat, as
well as in exhaled air has been suggested for the biological
monitoring of styrene uptake. Because of the efficient metabolism
of styrene and rapid excretion of the metabolites, mandelic and
phenylglyoxylic acid are present in the urine in easily measurable
amounts. The evaluation of the absorption level has usually been
based either on the concentration of one of these metabolites
(Ohtsuji & Ikeda, 1970; Burkiewicz et al., 1974; Härkönen et al.,
1974; Vivoli & Vechhi, 1974; Engström, K., et al., 1976, 1978;
Fernandez & Caperos, 1977; Caperos et al., 1979; Elia et al., 1980)
or on their sum (Bardodej & Bardodejova, 1970; Philippe et al.,
1971; Guillemin & Bauer, 1978, 1979; Wolff et al., 1978a,b; Elia et
al., 1980). "End of exposure" spot specimens have usually been
sampled and results corrected for the dilution of urine. Only this
type of sample is dealt with in the following discussions.
A number of authors have attempted to correlate levels of
styrene in air with levels of mandelic acid in urine, either
from experimental data or field studies, and calculations of
mandelic acid levels corresponding to styrene levels in air of
210, 420, and 630 mg/m3 (50, 100, and 150 ppm) are presented
in Table 9. The differences between the calculated values
could be explained by such factors as: type of exposure,
sampling strategy, individual variability, and differences in
analytical methods.
The use of unspecific analytical methods influences the
results, especially at low levels of styrene exposure, giving
too high values for metabolite concentrations in the urine
(Engström & Rantanen, 1974).
In the case of occupational exposure, a number of
additional elements may have to be taken into account. For
example, in work situations, exposure is usually repeated and
it has been shown that about 30% of the maximal mandelic acid
concentration of the previous day can still be found in urine
samples taken before work on the following day (Engström, K.,
et al., 1976). In another study, the amount of mandelic acid
excreted at the end of the working shift was shown to increase
in the course of the working week (Fernandez & Caperos, 1977).
Increased pulmonary ventilation with increased physical
work load results in a larger total absorption of styrene and
thus increased excretion of metabolites in the urine (Åstrand
et al., 1974; Droz & Fernandez, 1977).
Fluctuations in styrene concentrations in the workroom air
are very common in occupational exposure and may also
contribute to the differences observed in Table 9. A
short-term high peak exposure occurring shortly before the
sampling time may result in a high mandelic acid concentration
in the urine, even though the time-weighted average
concentration of styrene is low (Engström, K., 1983).
Table 9. Mandelic Acid levels* corresponding to various concentrations of
styrene in air
------------------------------------------------------------------------------
Styrene in air
mg/m3 (ppm)
-----------------
210 420 630 Remarks Reference
(50) (100) (150)
------------------------------------------------------------------------------
mandelic (a) 6 12 18 mandelic acid expressed Spasovski
acid as mg/g creatinine; (1982)
spectrophotometry
(a) - 7 - mandelic acid expressed Guillemin &
as mg/g creatinine; Bonet (1979)
gas chromatography
experimental study
(b) 8 14 20 Density 1.024; high Ikeda et al
performance liquid (1982)
chromatography field
study
(b) 10 20 - Density 1.024; Bardodej (1978)
polarography;
experimental study
(b) 9 19 27 Density 1.024; Engström, K.
gas chromatography; et al. (1978)
field study
(b) 14 26 34 Density 1.024 Härkönen et al.
spectrophotometry (1974)
field study
(b) 7-9 12-13 15-19 Density 1.024; Götell et al.
spectrophotometry; (1972)
field study
(b) 6 14 22 Density 1.024; Elia et al.
gas chromatography (1980)
field study
(b) 3 11 19 Density 1.024; Fields et al.
gas chromatography (1979)
field study
------------------------------------------------------------------------------
*(a) = mg mandelic acid/g creatinine.
(b) = mmol mandelic acid/litre urine.
The ratio of mandelic to phenylglyoxylic acid excreted at
the end of exposure varied from about 1 to 4 in different
studies. The ratio appears to increase at higher exposure
levels (Ohtsuji & Ikeda, 1970; Riihimäki & Pfäffli, 1978;
Caperos et al., 1979; Elia et al., 1980). The mandelic
acid/phenylglyoxylic acid ratios were higher in urine samples
taken at the end of exposure than in samples taken later
(Philippe et al., 1971; Guillemin & Bauer, 1979). In terms of
biological monitoring, it is claimed that the mandelic
acid/phenylglyoxylic acid ratio in the urine may be of help in
interpreting the exposure pattern (Philippe et al., 1971;
Guillemin & Bauer, 1979).
The various factors influencing the excretion of these
metabolites are not well known. It may be that such a
difficulty could be overcome by determining mandelic and
phenylglyoxylic acids and combining the results. This may
explain the good agreement obtained in the two studies in
Table l0 based on the sum of the levels of the two acids
corresponding to styrene concentrations in air of 210, 420,
and 630 mg/m3 (50, 100 and 150 ppm).
Table 10. Sum of mandelic acid and phenylglyoxylic acid levels
(mmol/litre) corresponding to various concentrations of styrene in air
---------------------------------------------------------------------------
Styrene in air
mg/m3 (ppm)
-------------------
210 420 630 Remarks Reference
(50) (100) (150)
---------------------------------------------------------------------------
Mandelic acid 8 16 24 density 1.024; Elia et al.
+ gas chromatography; (1980)
phenylglyoxylic field study
acid 11 19 27 density 1.024 Ikeda et al.
aigu performance (1982)
liquid chromatography;
field study
---------------------------------------------------------------------------
The determination of minor metabolites (4-vinylphenol and
hippuric acid) for the biological monitoring of styrene
exposure has also been reported (Ikeda et al., 1974; Pfäffli
et al., 1981).
The difficulties of the biological monitoring of styrene
uptake using urine samples cannot be overcome by selecting
another medium, as even less experience is available with
other media. Styrene concentrations in blood decline rapidly
during the first hour following exposure, which makes the
results strongly dependent on sampling time.
Styrene concentrations in exhaled air are in equilibrium
with blood concentrations, which means that styrene levels in
exhaled air are also sensitive to the changes in concentration
in ambient air. A single sample of exhaled air at the end of
exhalation can be used only for the evaluation of immediate
past exposure. It has been suggested that extrapolation of a
whole day's exposure is possible using several samples taken
after exposure, from which a "decay curve" can be established
(Fernandez & Caperos, 1977; Fields & Horstman, 1979). This
method is inconvenient for the routine monitoring of workers.
The estimation of styrene concentrations in subcutaneous
fat has been proposed as a method of indicating styrene body
burden, even a long time after exposure, when styrene and its
metabolites can no longer be detected in other specimens
(Wolff et al., 1978). However, much more information about
the kinetics of styrene in fat is needed before the relevance
of this method can be evaluated. For obvious practical
reasons, this technique cannot be used routinely.
6. EFFECTS ON EXPERIMENTAL SYSTEMS
6.1. Haematopoietic and Immune Systems
Spencer et al. (1942) did not observe any differences in
erythrocyte count, haemoglobin concentration, total leukocyte
count, and differential count between 18 rats exposed
repeatedly for 7 h a day for 6 months to a styrene
concentration of about 5460 mg/m3 (1300 ppm) and 18 control
animals. The test animals received a total of 137-139
exposures. The same authors did not see any significant
haematological changes in 8 rabbits and 4 monkeys similarly
exposed.
Wolf (1956) gave styrene orally at 66.7-667 mg/kg body
weight to female rats for 6 months, 5 days a week, but did not
observe any effects on the haematopoietic system.
In a study by Quast et al. (1978), styrene was
administered orally to beagle dogs at 200, 400, and 600 mg/kg
body weight for up to 19 months. At the highest dose, Heinz
bodies were regularly detected in the blood erythrocytes of
the dogs. At 400 mg/kg body weight, Heinz bodies were still
frequently seen, but at 200 mg/kg body weight they were
observed only sporadically in female dogs. Heinz bodies
disappeared after withdrawal of styrene. Decreases in packed
cell volume, red blood cell count, haemoglobin levels, and the
erythrocyte sedimentation rate were also observed.
When styrene was fed to rabbits at doses of up to 250
mg/kg body weight for up to 216 days, severe impairment of the
immunological defence system was indicated with the blood
complement titre reduced and leukocyte phagocytic activity
depressed (Sinitskij, 1969).
6.2. Nervous System and Behaviour
The acute neurotoxic effect of styrene is depression of
the central nervous system (Roth, 1979). However, the
narcotic effect of styrene glycol is at least twice as potent
as that of styrene (Parkki et al., 1976) and styrene 7,8-oxide
is more toxic than both styrene and styrene glycol with a
lethal dose 4-5 times lower than that of the parent compound
(Ohtsuji & Ikeda, 1971). Central nervous system depression
follows treatment with styrene 7,8-oxide. After a single
intraperitoneal injection both styrene and styrene oxide have
been shown to bind covalently to macromolecules in the central
nervous system (Savolainen & Vainio, 1977). In a study on
rats by Shugaev (1969), in which styrene was injected intra-
peritoneally, the maximum concentration of styrene in brain
tissue was 250 mg/kg (range 180-324 mg/kg).
Toxic action in the central nervous system in prolonged
(11 weeks) low-level inhalation exposure to a styrene
concentration of 1260 mg/m3 (300 ppm) led to minor changes in
axonal proteins (Savolainen & Pfäffli, 1977). Concomitant
neurophysiological studies, however, failed to demonstrate any
reduction in the peripheral motor conduction velocities
(Seppäläinen, 1978), a suggested clinical feature of the
toxicity of styrene in human subjects.
In studies by Zaprianov & Bainova (1979) on male rats,
repeated oral doses of styrene (1250 mg/kg body weight for 7
days) decreased monoamine oxidase (EC 1.4.3.4) activity. The
authors concluded that the inhibition of monoamine oxidase
activity in total brain may be regarded as a possible link in
the pathogenesis of reported occupational neurophysiological
effects of styrene.
Polystyrene containers were toxic to nerve cells in a
closed tissue culture system, possibly because of the release
of unreacted styrene in the medium (Mithen et al., 1980).
In an open-field test, styrene vapour at 1260 mg/m3
(300 ppm) had marginal effects on animal behaviour, even when
pronounced neurochemical effects were taken into account
(Savolainen et al., 1980).
In a 6-month study, female rats exposed to 200 and 2000
mg/m3 of styrene exhibited enhanced spontaneous activity.
Long-term memory was impaired in male rats after a 4-month
exposure (Vergieva & Zajkov, 1981).
6.3. Kidneys and the Urinary Tract
Oral treatment of rats with styrene at 400 mg/kg body
weight (administered in olive oil, once a day for 132 days
during 6 months) caused an increase in kidney weight. A lower
daily dose of 133 mg/kg body weight did not have any apparent
effect (Wolff et al., 1956).
Vainio et al. (1979) reported that intermittent 11-week
inhalation exposure of rats to low styrene vapour
concentrations of 1260 mg/m3 (300 ppm), 6 h/day, 5 days a
week, enhanced the activities of both drug conjugating
(epoxide hydratase (EC 4.2.1.63), UDP glucuronosyl transferase
(EC 2.4.1.17), and hydroxylating (ethoxycoumarin 0-deethylase,
cytochrome P-450) enzymes in the kidneys.
6.4. Gastrointestinal Tract
No information was available concerning the effect of
styrene on the gastrointestinal tract.
6.5. Liver
Mild liver damage, particularly in the parenchymal cells,
was reported after a single exposure of rats to styrene vapour
at 10 500 mg/m3 (2500 ppm) for up to 21 h (Spencer et al.,
1942). The same authors also reported increases in liver
weight in rats repeatedly exposed to styrene vapour at
5460 mg/m3 (1300 ppm) for 130-139 days. However, they did not
find any liver damage in guinea-pigs exposed for similar
periods to a styrene concentration of 2730 mg/m3 (650 ppm) or
in rabbits and monkeys exposed to 5460 mg/m3 (1300 ppm).
Intermittent 11-week inhalation exposure of rats to a
styrene concentration of 1260 mg/m3 (300 ppm) (6 h/day, 5
days/week) caused histological liver alterations consisting of
hydropic degeneration, and steatosis of parenchymal cells and
congestion. Enhanced activity of both the drug hydroxylating
(ethoxycoumarin O-deethylase, cytochrome P-450) and
conjugating (epoxide hydrolase (EC 3.3.2.3), UDP
glucuronosyltransferase (EC 2.4.1.42)) enzymes in the liver
was detected (Vainio et al., 1979). In a skin-painting study
on rats, styrene at 4 and 8 ml/kg body weight induced
dose-related fatty infiltration of liver cells that was
reversible after 2 weeks without exposure (Burkova et al.,
1982).
Liver cell necrosis was noted in a long-term
carcinogenicity bioassay in several rats in a group exposed to
a high dose of styrene (2000 mg/kg body weight by gavage, 5
days a week, for 78 weeks) (US National Cancer Institute,
1979).
The intermittent inhalation of styrene vapour at a
concentration of 1260 mg/m3 (300 ppm) in air decreased
significantly the reduced glutathione (GSH) concentration in
rat liver (Elovaara et al., 1979). Vainio & Mäkinen (1977)
demonstrated a clear species difference in sensitivity to
styrene-induced depletion of GSH content. The mouse was the
most sensitive and the rat the most resistant species. The
decrease in GSH in liver was dose-dependent. A slight decrease
was observed in rats exposed to a styrene concentration in air
of 420 mg/m3 (100 ppm) (Vainio et al., 1979). When isolated
hepatocytes were incubated in the presence of styrene, there
was also a dose- and time-dependent decrease in GSH
concentrations (Zitting et al., 1980).
When the hepatic GSH in Syrian hamsters decreased to less
than 1 mmol/litre as a result of a high intragastric dose of
styrene (4.5 g/kg body weight), serum aminotransferase
activity increased (Parkki, 1978).
Quast et al. (1979) observed an increased amount of
hemosiderin pigment in liver reticuloendothelial cells of male
and female beagle dogs administered styrene orally at 400 or
600 mg/kg body weight, 7 days a week, for up to 19 months.
The higher dose also increased the number of hepatocellular
intranuclear acidophilic crystalline inclusions.
6.6. Cardiovascular System
No information was available concerning possible effects
of styrene on the cardiovascular system.
6.7. Respiratory System
A single exposure of rats to styrene at 5460-42 000 mg/m3
(1300-10 000 ppm) for 1-4 h caused mucous membrane irritation
as evidenced by lacrymation, nasal discharge, and salivation,
accompanied by aggressive scratching and rubbing of the eyes
and nose. The irritation was particularly severe at higher
concentrations (Spencer et al., 1942). The degree of acute
pulmonary damage varied, in general, with the vapour
concentration of styrene and the length of exposure. When the
exposure was long enough to cause death in some of the exposed
animals, marked pulmonary lesions were induced at all
concentrations studied. Pulmonary changes varied from slight
congestion to congestion, multiple haemorrhage, exudation, and
various degrees of leukocytic infiltration. When another
group of animals (60 rats, 94 guinea-pigs, 12 rabbits, 4
monkeys) were exposed to a styrene concentration of 5460 mg/m3
(1300 ppm) for 7-8 h/day, 5 days a week, over a period of at
least 6 months, microscopic examination of various tissues,
including the lung, did not reveal specific changes in any of
the animals except the guinea-pigs. About 10% of all
guinea-pigs that were exposed repeatedly to styrene at 5460
mg/m3 (1300 ppm) died after several exposures; microscopic
examination of the dead animals revealed pronounced lung
irritation, which was characterized by general acute
inflammatory response. Respiratory insufficiency was the
cause of death (Spencer et al., 1942).
Wolf et al. (1956) exposed animals by inhalation to a
styrene concentration of 5460 mg/m3 (1300 ppm) for 7 h/day,
for 214-360 days. They found slight eye and nasal irritation
in rats and guinea-pigs, but no effects in rabbits and rhesus
monkeys. However, when rats and guinea-pigs were exposed to a
styrene concentration of 2730 mg/m3 (650 ppm), no effects were
observed.
According to Alarie (1973), the concentration of styrene
in air that reduced the respiratory frequency in mice to 50%
was 666 mg/m3 (95% confidence limits 574-758 mg/m3).
When rats were exposed to styrene at 1260 mg/m3 (300 ppm)
for 6 h daily, 5 days a week for 2-11 weeks, no significant
increase in UDP glucuronosyltransferase activity was observed
in the lungs but, after 2 and 4 weeks exposure,
the free glutathione content in the lung was lower (P < 0.05)
than that in the controls (Vainio et al., 1979).
6.8. Endocrine Organs
The adverse effects of styrene on the endocrine organs
have been insufficiently studied. While Izjumova (1972)
reported that repeated exposure to styrene concentrations of
5-50 mg/m3 (1-12 ppm) for up to 4 months prolonged the estrus
cycle in rats, the data were inconsistent from month to month,
and appeared not to be dose-dependent.
6.9. Carcinogenic Effects
6.9.1. Styrene
6.9.1.1. Oral administration
(a) Mouse
In a carcinogenesis bioassay (US National Cancer
Institute, 1979; Chu et al., 1981), 2 groups each of 50 male
and 50 female B6C3F1 mice, 6 weeks of age, were given styrene
at 150 and 300 mg/kg body weight (at least 0.3% impurities) in
corn oil by gavage, 5 days per week, for 78 weeks, and then
observed for an additional 13 weeks. A group of 20 males and
20 females receiving corn oil only (10 ml/kg body weight)
served as vehicle controls. At the end of the study, 78%,
92%, and 100% of the male mice and 76%, 80%, and 80% of the
females were alive in the high-dose, low-dose, and control
groups, respectively. An increased incidence of adenomas and
carcinomas of the lung was seen in treated male mice, i.e., 3
adenomas and 3 carcinomas in 44 animals in the low-dose group,
and 4 adenomas and 5 carcinomas in 43 animals in the high-dose
group, compared with none in the 20 controls. The increased
incidence of both types of lung tumours combined was
statistically significant (P = 0.02) in the high-dose group
compared with the vehicle-treated controls and also in the
Cochrane-Armitage test for linear trend (P = 0.02). It should
be noted that the number of control animals was small (40)
compared with the treated group (100), that the historical
vehicle-treated controls were inadequate, and that the
incidence of neoplasms in historical untreated controls was
12% (32/271). For these reasons, it was not possible for the
Task Group to conclude that styrene was carcinogenic in this
study, even though the results of the study were positive.
(b) Rat
In carcinogenic bioassays (US National Cancer Institute,
1979; Chu et al., 1981), groups of 50 male and 50 female
Fischer 344 rats, 6 weeks of age, were given styrene (at least
0.3% impurities) in corn oil, by gavage, 5 days per week,
either at 1000 or 2000 mg/kg body weight for 78 weeks with
further observation for 27 weeks, or at 500 mg/kg body weight
for 103 weeks with further observation for 1 week. Two
groups, each of 20 males and 20 females served as vehicle
controls. A high mortality was seen in the high-dose group;
only 12% of the males survived 53 weeks of treatment and 14%
of the females survived 70 weeks of treatment. Hepatic
necrosis was observed in several rats. At the 90th week of
the study, 94%, 88%, 85%, and 90% of the males and 92%, 92%,
75%, and 90% of the females were alive in the medium-dose,
low-dose, and the 2 control groups, respectively. There was
no statistically significant difference between the groups in
tumour incidence at any site (National Cancer Institute,
1979). The Task Group noted the high mortality in the
high-dose group and the small numbers of animals in the
control groups.
6.9.1.2. Inhalation exposure
Rat
In a study conducted by Jersey et al. (1978), 2 groups of
84 and 86 male, and 2 groups each of 85 female Sprague-Dawley
rats of the Spartan substrain, 7-8 weeks of age, were exposed
by inhalation to "production grade" styrene (minimum purity
99.5%) containing approximately 5 ppm t-butylcatechol. They
were exposed 6 h/day, 5 days/week, for 18.3 months, if male,
and for 20.7 months, if female. Exposure duration was based
on the time when mortality reached 50% in one of the test
groups of that sex. Initial concentrations of styrene in air
were either 5040 mg/m3 (1200 ppm) or 2520 mg/m3 (600 ppm).
The high level was reduced to 4200 mg/m3 (1000 ppm) after 2
months because of excessive toxicity. Another group of 85
males and 85 females served as untreated controls. At the end
of the 2-year study, 5, 18, and 6 males and 30, 30, and 22
females had survived in the control, low-, and high-dose
groups, respectively. An excessive mortality from chronic
pneumonia occurred in the control and high-dose male groups.
An increased (but not statistically significant) combined
incidence of leukaemias and lymphosarcomas was seen: 1/84
males and 6/85 females in the high-dose groups, 5/86 males and
6/85 females in the low-dose groups, 1/85 males and 1/85
females in the control groups. If the total incidence of
leukaemias/lymphosarcomas in females is compared with
historical controls, it becomes statistically significant
(P < 0.05). A statistically significant increase in the
incidence of mammary adenocarcinomas was seen in the low-dose
female group (7/85 versus 1/85 in controls), but the incidence
was not statistically significant in comparison with
historical controls. Though these data are inconclusive, they
are interpreted by Jersey et al. (1978) as suggesting an
association between the exposure of the female rats to styrene
and an increased incidence of tumours of the leukaemia/
lymphosarcoma types. The Task Group was unable to evaluate
this study because of the high mortality.
6.9.1.3. Pre- and postnatal exposure
(a) Mouse
In the studies of Ponomarkov & Tomatis (1978), 29 pregnant
O20 mice were each given a single treatment of styrene at 1350
mg/kg body weight (99% pure dissolved in 0.1 ml olive oil) by
stomach tube on the 17th day of gestation. A control group of
9 pregnant animals received olive oil alone. The neonatal
mortality rate in the offspring of styrene-treated mice was
43%, compared with 22% in the controls. The same dose of
styrene was then administered weekly, by stomach tube, to 45
male and 39 female progeny from weaning to 16 weeks of age, at
which time the treatment was stopped because of the high rate
of mortality (64% alive at 20 weeks). The study was
terminated at 100 weeks, when all the animals had died. No
differences in tumour incidence were found between mothers
treated with styrene and those given olive oil. In the
progeny that had received weekly treatments, lung tumours
(adenomas and adenocarcinomas) were found in 20/23 males and
32/32 females, compared with 8/19 and 14/21 in olive
oil-treated controls (P < 0.01, P < 0.01) and compared with
34/53 and 25/47 in untreated controls (P < 0.05, P < 0.001).
No differences in tumour incidence at sites other than the
lung were seen in the progeny, compared with olive oil-treated
or untreated controls.
In the same studies (Ponomarkov & Tomatis, 1978), 15
pregnant C57 black mice were each given a single treatment of
styrene at 300 mg/kg body weight dissolved in 0.1 ml olive
oil, by stomach tube, on the 17th day of gestation. A control
group of 5 pregnant animals received olive oil alone. The
same amount of styrene was then given weekly by stomach tube
to 27 male and 27 female progeny from weaning up to 120 weeks
of age, at which time the survivors, 15 males and 12 females,
were killed. Control progeny (12 males and 13 females)
received olive oil for 120 weeks at which time 7 males and 4
females survived. In mothers treated with a single dose of
styrene, lymphomas were observed in 10/12 animals, compared
with 3/5 in the olive oil-treated controls. In treated male
progeny, liver tumours (hepatocellular carcinomas) were found
in 3/24 animals, compared with 1/12 olive oil-treated and 1/47
untreated controls (both hepatocellular adenomas). The
increased incidence of these tumours in styrene-treated
animals compared with controls was not statistically
significant. The small number of animals was noted by the
Task Group.
(b) Rat
Twenty-one female BDIV rats were each given a single
treatment of styrene at 1350 mg/kg body weight dissolved in
olive oil, by stomach tube, on the 17th day of gestation
(Ponomarkov & Tomatis, 1978). A control group of 10 pregnant
rats received 0.3 ml olive oil alone. The neonatal mortality
rate in the offspring was 10% in treated rats and 2.5% in
olive oil-treated controls. Subsequently, doses of 500 mg/kg
body weight were administered weekly by stomach tube to 73
male and 71 female progeny from weaning up to 120 weeks of
age, at which time the survivors, 8 males and 20 females, were
killed. Controls (36 males and 39 females) received olive oil
alone for 120 weeks. Two stomach tumours and 1 hepatocellular
adenoma were seen in styrene-treated females and one stomach
tumour in styrene-treated males. One stomach tumour was also
found in olive oil-treated female controls. The difference in
the incidence of these and other tumours in styrene-treated
rats compared with controls was not statistically significant.
6.9.2. Styrene 7,8-oxide
6.9.2.1. Dermal exposure
Mouse
Forty 13-week-old C3H mice were painted on the clipped
dorsal skin with a 5% solution of styrene 7,8-oxide in
acetone, 3 times weekly for life (Weil et al., 1963). No skin
tumours were observed in 33 animals that survived for 17-24
months (37 mice were alive at 12 months). Another group of
C3H mice were similarly painted with a 10% solution of styrene
oxide in acetone; 18 mice survived 12 months, only 2 mice
survived 17 months, and no skin tumours were observed.
In another study by Van Duuren et al. (1963), the clipped
dorsal skin of 30, 8-week-old male Swiss ICR/Ha mice was
treated 3 times weekly, throughout the life span, with 0.1 ml
of a 10% solution of styrene oxide in benzene. Median
survival time was 431 days in the styrene oxide-treated group
and 262 and 412 days, respectively, in the groups of 30 and 60
benzene-treated controls. Three styrene oxide-treated animals
and 11 (out of 150) benzene controls developed skin tumours.
This difference was not statistically significant. One of the
tumours in each group was a squamous-cell carcinoma.
6.9.2.2. Oral administration
Rat
Groups of 40 male and 40 female Sprague-Dawley rats, 13
weeks of age, were given either 50 or 250 mg/kg body weight,
per day, of styrene oxide (purity unspecified) dissolved in
olive oil, by stomach tube, 4 or 5 days per week, for 52 weeks
(Maltoni et al., 1979). Forty male and 40 female controls
received olive oil alone. The animals were allowed to live
until natural death or were killed 156 weeks after the start
of the study. Preliminary results included only stomach
cancer incidence in animals dying within the first 135 weeks.
The numbers of animals alive at the appearance of the first
tumour (51 weeks) were: controls, 37 males and 28 females;
low-dose, 31 males and 31 females; high-dose, 28 males and 30
females. The numbers of papillomas of the stomach were: 0/37
and 0/28 in controls; 0/31 and 2/31 in low-dose animals; and
3/28 and 6/30 in high-dose animals. The respective numbers
for squamous-cell carcinomas in situ were: 0/37 and 0/28;
5/31 and 6/31; and 11/28 and 12/30. Invasive squamous-cell
carcinomas occurred in 0/37 and 0/28; 2/31 and 1/31; and 4/2;
and 6/30, respectively.
Fourteen female rats were given, by gavage, a single dose
of styrene oxide at 200 mg/kg body weight in olive oil (0.3
ml) on day 17 of pregnancy (estimated total dose, 0.04 g).
Their progeny (62 females and 43 males) received 96 weekly
doses of 100-150 mg/kg body weight in olive oil (0.2 ml), from
weaning (4 weeks of age) until termination of the study (120
weeks) (estimated total doses, 2.5 g for females and 5.0 g for
males). Similar groups of controls (14 pregnant females, 55
female and 49 male progeny) were given only olive oil. All
survivors were killed at the end of the study. There were
fewer tumour-bearing animals among mothers treated with
styrene oxide (31%) than among rats given only olive oil
(57%); mammary tumours were seen only in the olive-oil-treated
animals. The incidences of other tumours were similar.
Forestomach tumours were seen only in treated progeny;
Carcinomas or early carcinomas of the forestomach occurred in
10/42 males and 16/60 females compared with 1/49 and 1/55
controls respectively. Four tumours of the nervous system
occurred in treated groups, whereas only 1 was observed in a
male control. Eight lung tumours were seen in treated
animals, and 2 in controls; 7 of these tumours (6 malignant +
1 benign) occurred among treated female progeny, giving an
incidence of 7/60 (12%) compared with 1/55 (2%) in control
females. The incidences of other types of tumour did not show
any marked differences between control and treated groups
(Ponomarkov et al., 1983; Ponomarkov et al., personal
communication).
6.9.3. Summary and conclusions
Of the 6 studies cited, 3 in mice and 3 in rats, only one
(O20 mice) gave results that indicated a significant increase
in lung tumour incidence. The others either did not show any
significant increase in tumour incidence or were performed in
such a way that conclusions could not be drawn from the
results.
The results of studies concerning styrene 7,8-oxide
indicated: that there was no development of, or increase in,
skin tumours, when the compound was applied to mouse skin;
that tumours (benign and malignant) developed in the
forestomach, when styrene 7,8-oxide was given by gavage in
rats; and that there was an increase in lung tumour incidence
in female progeny, when styrene 7,8-oxide was administered to
rats pre- and post-natally.
6.10. Genetic effects
Several reviews have been published on the mutagenic and
related effects of styrene (Vainio, 1978; IARC, 1979; Norppa,
1981a; Vainio et al., 1982; Zetterberg, 1982; Norppa & Vainio,
1983a).
6.10.1. Chemical reactivity of styrene and styrene oxides
Styrene needs metabolic activation to bind covalently with
nucleophilic biological macromolecules. Styrene 7,8-oxide,
which has been considered to be a primary mammalian metabolite
of styrene (section 5.3), is spontaneously reactive because of
the lability of the oxirane ring. Styrene 7,8-oxide can bind
covalently with proteins and nucleic acids (Marniemi et al.,
1977; van Anda et al., 1979) and is able to alkylate in vitro
4-( p-nitrobenzyl)-pyridine, a synthetic nucleophile, and
deoxyguanosine, a biological nucleophile (Hemminki, 1979;
Hemminki & Falck, 1979; Hemminki et al., 1981).
Hemminki (1979) measured the electrophilic reactivity of
styrene 7,8-oxide by detecting alkylation of 2-amino-1,7-di-
hydro-6 H-purin-6-one (guanine) fluorometrically. The rate of
reaction was roughly equal for guanosine, and deoxyguanosine.
Styrene 7,8-oxide formed a covalent 7-alkyl derivative with
guanine (Hemminki et al., 1980b). With an identical
concentration of guanine, RNA and single-stranded DNA were
substantially less reactive than guanosine, possibly as a
consequence of the tertiary structures of the large relative
molecular mass polymers. Double-stranded DNA was even less
reactive.
Two highly reactive arene oxide derivatives of styrene,
styrene 1,2- and 3,4-oxides were recently synthesized
(Hiratsuka & Watabe, 1982; Watabe et al., 1982a,b). These
arene oxides have a very brief half-life and they are very
hard to detect, even in an in vitro system consisting of
styrene, microsomes, and NADPH.
6.10.2. Mutagenic effects of styrene and styrene oxides in
bacterial assay systems
Styrene has not been shown to induce reverse mutations in
any of the Salmonella typhimurium tester strains used in the
Ames' test, in the absence of a metabolic activation system
(Table 11). However, in the presence of a metabolic
activation system, some investigators have found styrene to be
mutagenic in the Salmonella strains (TA 100, TA 1530, TA 1535)
used to detect base-substitution-inducing mutagens; others
have obtained negative results (Table 11). No positive
results have been reported for styrene in the Salmonella
strains (TA 98, TA 1537, TA 1538) detecting frame-
shift-inducing mutagens. The divergent results on the
mutagenicity of styrene in Salmonella could be explained, in
part, by metabolic differences between the microsomal
preparations (S-9 mix, S-10 mix) used. The high volatility,
poor solubility, and bacterial toxicity of styrene may also
affect the results (de Meester et al., 1981).
Styrene was reported to be positive in the rec assay with
Bacillus subtilis without metabolic activation, in a review by
Kawachi et al. (1979), but no specific data were given.
The primary metabolite of styrene, styrene 7,8-oxide, has
been shown to be mutagenic in a number of bacterial studies
with base-pair strains, without a metabolic activation system
(Table 11). Moreover, styrene 7,8-oxide was mutagenic in the
fluctuation test with Escherichia coli WP2 uvrA (for detecting
base-substitution mutagens) and with Klebsiella pneumoniae
(Hemminki & Falck, 1979; Voogd et al., 1981). The frame-shift
mutagen sensitive strains of Salmonella gave only negative
results with styrene 7,8-oxide, either with or without
metabolic activation systems (de Meester et al., 1977; Stolz &
Withey, 1977; Drinkwater et al., 1978; Wade et al., 1978;
Watabe et al., 1978; Busk, 1979; Hemminki & Falck, 1979;
El-Tantawy & Hammock, 1980). Watabe et al. (1981) did not
find any appreciable difference in the bacterial (Salmonella)
mutagenicity of R-and S-enantiomers of styrene 7,8-oxide and
their racemic mixture. However, Pagano et al. (1982) reported
that the R-enantiomer was more mutagenic to Salmonella than
the S-form, while the racemic styrene 7,8-oxide had an
intermediate mutagenic activity.
Table 11. Mutagenicity of styrene and styrene-7,8-oxide in the base-
pair substitution strains of Salmonella typhimurium and E. colia
------------------------------------------------------------------------
Results of mutagenicity studies
Styrene styrene 7,8-oxide
Metabolic activation Metabolic activation
system: system:
None Present None Present
------------------------------------------------------------------------
Drinkwater et al. (1978) .. .. - -
Glatt et al. (1979) .. .. + ..
Hemminki & Falck (1979) .. .. + ..
Pagano et al. (1982) .. .. + ..
Planche et al. (1979) .. .. + ..
Sugiura & Goto (1981) .. .. + ..
Sugiura et al. (1978a,b) .. .. + ..
Turchi et al. (1981) .. .. + ..
Voogd et al. (1981) .. .. + ..
Wade et al. (1978) .. .. + ..
Watabe et al. (1981a) .. .. + ..
El-Tantawy & Hammock (1980) .. .. + +b
Yoshikawa et al. (1980) .. .. + +b
Kawachi et al. (1979) - - .. ..
Milvy & Garro (1976) - .. + ..
Greim et al. (1977) - - + ..
Busk (1979) - - + +b
Loprieno et al. (1978) - - + +
Stolz & Withey (1977)
Watabe et al. (1981, 1982b) - +c + -
de Meester et al. (1977, - + + +b
1981)
Vainio et al. (1976) -d + + +b
Poncelet et al. (1980) - + .. ..
Cerna & Kypenova (1977) .. +e .. ..
------------------------------------------------------------------------
.. No data.
a Modified from: Norppa (1981a).
b A decrease reported in mutagenic activity, as compared to treatment
without metabolic activation.
c Only with trichloropropane oxide present.
d A weak activity reported also without S-9 mix in TA 1535.
e Host mediated assay, Salmonella strain TA 1950, mice as hosts.
Two arene oxide derivatives of styrene, styrene 1,2- and
3,4-oxide, both of which have been considered to be the
precursors of isolated urinary metabolites of styrene in the
rat, have been demonstrated to be mutagenic towards a
base-pair strain (TA 100) of Salmonella, but not towards a
frame-shift strain (TA 98) (Watabe et al., 1982b). The 1,2-
and 3,4-oxides were more mutagenic than the 7,8-oxide, but
only after sequential addition to the medium during pre-
incubation.
Thus, the results obtained with various bacterial assay
systems show that styrene 7,8-oxide is a direct base-pair
substitution type mutagen. The extremely labile minor
metabolites of styrene, 1,2- and 3,4-oxide, seem to be highly
reactive and are mutagenic for Salmonella. However, with
styrene, even in the presence of a metabolic activation
system, contradictory results have been reported.
6.10.3. Genetic effects of styrene and styrene 7,8-oxide
in eukaryotic non-mammalian systems
Various non-mammalian assay systems, including point
mutations and gene conversion in yeast, point mutations in
silk worm, recessive lethal mutations, sex chromosome loss and
non-disjunction in Drosophila melanogaster, and chromosome
damage in root tip cells of Allium cepa, have been applied to
study the mutagenic and genetic effects of styrene and styrene
7,8-oxide.
In the yeast assays, styrene did not induce mitotic gene
conversions at the adenine and tryptophane loci of
Saccharomyces cerevisiae or forward mutations at the adenine
loci of Schizosaccharomyces pombe, even in the presence of a
mouse liver microsome mix (Loprieno et al., 1976; Loprieno,
1981; Loprieno & Abbondandolo, 1980). According to Bauer et
al. (1980, 1981), this negative result could be because the
epoxide hydrotase (EC 4.2.1.63) (inactivation enzyme) is more
stable than the monooxygenase, because the concentration of
styrene 7,8-oxide in the incubation mixture could not reach a
mutagenic level. When a yeast strain ( Saccharomyces
cerevisiae D7) capable of metabolic activation was used to
test the induction of gene conversions by styrene, a positive
result was reported (Del Carratore et al., 1982). Styrene
7,8-oxide has been found positive in all the yeast systems
(Loprieno et al., 1978; Loprieno, 1981).
When tested in a host-mediated assay, with mice as the
host animal and yeast as the target cells, styrene and styrene
7,8-oxide were weakly mutagenic for some genetic end points
(Loprieno et al., 1978), but these results were later
considered negative for styrene (Loprieno & Abbondandolo,
1980) when a higher number of historical control values became
available.
Table 12. Point mutations, chromosome aberrations and genetic damage induced in cultured mammalian
cells by styrene and styrene 7,8-oxide
-------------------------------------------------------------------------------------------------------
Styrene Styrene 7,8-oxide
Test-system Type of damage References
assayed Metabolic Metabolic
activation system: activation system:
None Present None Present
-------------------------------------------------------------------------------------------------------
Rodent cell lines
V79 HGPRT mutations .. .. + .. Bonatti et al. (1978);
Sugiura et al. (1979)
- - + .. Loprieno et al. (1976,
1978)
- + + -c Beije & Jenssen (1982)
aberrations .. .. + .. Turchi et al. (1981)
micronuclei .. .. + ..
anaphase bridges .. .. + ..
CHL aberrations - +a + .. Ishidate & Yoshikawa
(1980);
Ishidate et al. (1981);
Kawachi et al. (1979);
Matsuoka et al. (1979)
CHO SCEs - +b + +d de Raat (1978)
.. .. + .. Kubiak et al. (1981)
L5178Y TK mutations .. .. + - Amacher & Turner (1982)
Human cells
Lymphocytes aberrations +e .. + .. Linnainmaa et al.
(1978a,b);
micronuclei +e .. + .. Meretoja & Vainio (1979)
aberrations .. .. + .. Fabry et al. (1978)
-------------------------------------------------------------------------------------------------------
Table 12. (contd.)
-------------------------------------------------------------------------------------------------------
Styrene Styrene 7,8-oxide
Test-system Type of damage References
assayed Metabolic Metabolic
activation system: activation system:
None Present None Present
-------------------------------------------------------------------------------------------------------
aberrations +e .. + .. Norppa et al.(1980a,
SCEs +e .. + .. 1981, 1982);
Norppa (1981a);
Norppa & Vainio (l983b)
unscheduled DNA -f .. .. .. Pero et al. (1982)
synthesis
EUE unscheduled DNA .. - + .. Loprieno et al. (1978)
synthesis
Wistar rat
hepatocytes unscheduled .. .. .. - Brouns et al. (1979)
DNA synthesis
-------------------------------------------------------------------------------------------------------
.. No data.
a With methylcholantrene preinduced rat liver S-9.
b Only with cyclohexene oxide present.
c In the liver perfusion system.
d A decrease in activity.
e In vitro activation by erythrocytes suggested.
f Purified lymphocytes.
In Drosophila, a significant increase in the frequency of
X-linked recessive lethals was observed in the offspring of
males fed styrene or styrene 7,8-oxide (Donner et al., 1979).
The test for sex chromosome loss and non-disjunction in
Drosophila gave negative results for styrene (Penttilä et al.,
1980). In a review (Kawachi et al., 1979), styrene was
reported not to induce point mutations in silk worm.
Results of cytogenetic studies in meristematic root cells
of Allium cepa demonstrated that styrene induces metaphase
chromosome breaks and micronuclei (Linnainmaa et al., 1978a,b).
Styrene 7,8-oxide induced micronuclei and anaphase fragments and
bridges in these growing root-tip cells (Linnainmaa et al.,
1978a,b).
6.10.4. Genetic effects of styrene and styrene 7,8-oxide in
mammalian cells in vitro
Point mutations, chromosome damage, and unscheduled DNA
synthesis (UDS) have been studied after treatment of mammalian
cell cultures with styrene or styrene 7,8-oxide (Table 12).
In the Chinese hamster V79 cell line, styrene did not
induce point mutations at the HPRT locus, with or without a
metabolic activation system (S-10 mix) from mouse liver
(Loprieno et al., 1976, 1978; Loprieno, 1981). On the other
hand, Beije & Jenssen (1982) found styrene weakly mutagenic in
the same system after metabolic activation by rat liver S-9
mix. When a liver perfusion system was used for the metabolic
activation of styrene, there was a clear increase in point
mutations at the HRPT locus of V79 cells (Beije & Jenssen,
1982).
Loprieno et al. (1978) examined the effects of styrene (with
mouse liver S-10 mix) and styrene 7,8-oxide on UDS in human
heteroploid EUE cell line. There was an increase in UDS with
styrene 7,8-oxide but not with styrene. Brouns et al. (1979) used
freshly isolated hepatocytes of Wistar rats for UDS detection after
styrene 7,8-oxide treatment. The results were negative, which
according to the authors was due to the rapid inactivation of
styrene 7,8-oxide in the hepatocytes. Pero et al. (1982) did not
find any effects of styrene (without metabolic activation) on UDS
in purified resting human lymphocytes.
Styrene induced chromosome aberrations, micronuclei, and
SCEs in human whole blood lymphocyte cultures, and chromosome
aberrations in Chinese hamster CHL cells in the presence of
metabolic activation (Table 12). Styrene 7,8-oxide induced
chromosome aberrations, micronuclei, and SCEs, in rodent cell
lines and in human lymphocyte cultures without metabolizing
systems (Table 12). The positive effects of styrene in human
whole blood lymphocyte cultures are explained by the ability
of erythrocytes to convert this compound into styrene
7,8-oxide (Norppa et al., 1980a; Belvedere & Tursi, l981;
Norppa et al., l982; Vainio et al., l982).
Without metabolizing systems, styrene 7,8-oxide has been
clearly shown to induce HGPRT mutations in V79 cells (Loprieno
et al., 1976; 1978; Bonatti et al., 1978; Sugiura et al.,
1979; Loprieno & Abbondandolo, 1980; Loprieno, 1981; Beije &
Jenssen, 1982) and point mutations in the TK locus of the
mouse lymphoma cell line L5178 Y (Amacher & Turner, 1982).
With the liver perfusion technique, styrene 7,8-oxide did not
induce point mutations at the HPRT locus of V-79 cells (Beije
& Jenssen, l982).
6.10.5. Genetic effects of styrene and styrene 7,8-oxide in
mammalian systems in vivo
Styrene and styrene 7,8-oxide have been tested in vivo for
their ability to induce genetic damage in somatic and germ
cells in different species (mouse, rat, Chinese hamster) using
different genetic endpoints (chromosome aberrations,
micronuclei, dominant lethals, SCEs and translocations in
spermatocytes; Table 13). Most of the studies concerning
chromosome aberrations in the bone marrow cells of animals
were negative for styrene. No increases in chromosome
aberrations in bone marrow were observed in mice after high
single or repeated doses of styrene administered by gavage or
intraperitoneally (i.p.), or in rats after an unspecified
dose. However, a positive effect for chromosome aberrations
in rat bone marrow was found after inhalation exposure, and
for SCEs in mouse bone marrow, alveolar macrophages, and
regenerating liver cells. In the micronucleus test, styrene
gave negative results in Chinese hamsters but positive results
in mice (Penttilä et al., 1980; Norppa, 1981b).
Studies on the cytogenetic effects of styrene 7,8-oxide in
whole mammals also gave contradictory results (Table 13).
Loprieno et al. (1978) observed a dose-dependent clastogenic
effect in mouse bone marrow cells after oral administration of
styrene 7,8-oxide. However, Fabry et al. (1978) did not find
any chromosome aberrations or micronuclei in the bone marrow
of mice after an i.p. administration. Penttilä et al. (1980)
also failed to detect any effects on micronuclei in Chinese
hamster bone marrow erythrocytes after an i.p. administration.
Negative findings were reported by Norppa et al. (1979) for
chromosome aberrations and SCEs in the bone marrow cells of
Chinese hamsters after inhalation exposure. A slight increase
in SCEs was observed after i.p. injection of a lethal dose;
chromosomal aberrations were also elevated. Conner et al.
(1982) noticed a slight increase in SCEs in regenerating liver
cells and alveolar macrophages (but not in bone marrow) in
hepatectomized mice exposed through inhalation to styrene
7,8-oxide.
Table 13. Cytogenetic damage induced by styrene and styrene 7,8-oxide in various rodent studies in vivo
------------------------------------------------------------------------------------------------------------
Exposure
Species Duration of Type of
(strain) Routea Dose time after damage Result Reference
Cell type treatment studied
------------------------------------------------------------------------------------------------------------
STYRENE
Rat
(Wistar)
bone marrow inhalation 1260 mg/m3 (300 ppm) 9-11 weeks Chromosomal + Meretoja et al. (1978)
(Wistar) aberrations
bone marrow inhalation .. .. Chromosomal - Kawachi et al. (1979)
aberrations
Mouse
(BDF1)
bone marrow inhalation 1625-3872 mg/m3 4 days SCEs + Conner et al. (1979;
alveolar (387-922 ppm) 1980; 1982)
macrophages,
regenerating
liver cells 3872 mg/m3 (922 ppm) 2 days SCEs +
(C57BL/6)
polychromatic i.p. 250 or 1000 mg/kg 30 h Micronuclei + Norppa (1981b)
erythrocytes 500 or 1500 mg/kg 30 h Micronuclei -
(CD-1)
bone marrow p.o. 500 or 1000 mg/kg 24 h Chromosomal - Loprieno et al. (1978)
aberrations
p.o. 4 x 500 mg/kg 4 days Chromosomal - Sbrana et al. (1982)
aberrations
70 x 200 mg/kg 70 days Chromosomal -
aberrations
(ICR)
bone Marrow i.p. LD50 or 5xLD50 6, 24, or Chromosomal - Cerna & Kypenova
2 6 48 h aberrations (1977)
Chinese hamster
bone marrow inhalation 1260 mg/m3 (300 ppm) 4 or 21 Chromosomal - Norppa et al. (1980)
days
polychromatic i.p. 1000 mg/kg 30 h Micronuclei - Penttilä et al. (1980)
erythrocytes
-----------------------------------------------------------------------------------------------------------
Table 13. (contd.)
------------------------------------------------------------------------------------------------------------
Exposure
Species Duration of Type of
(strain) Routea Dose time after damage Result Reference
Cell type treatment studied
------------------------------------------------------------------------------------------------------------
STYRENE 7,8-OXIDE
Mouse
(BDF1)
bone marrow, inhalation 210 mg/m3 (50 ppm) 5 h SCEs +b Conner et al. (1982)
alveolar
macrophages,
regenerating
liver cells
(CD-1)
bone marrow p.o. 500-1000 mg/kg 24 h Chromosomal + Loprieno et al.
aberrations (1978)
(BALB/c)
bone marrow i.p. 250 mg/kg 1-13 days Chromosomal - Fabry et al. (1978)
aberrations
polychromatid 30 h Micronuclei -
erythrocytes
primary 2.5-3 Trans- -
spermatocytes monthsc locations
post-meiotic 1-3 weeks Dominant -
sperm cells lethals
Chinese hamster
bone marrow inhalation 105-420 mg/m3 2,4, or 20 Chromosomal - Norppa et al. (1979)
(25-100 ppm) days aberrations
SCEs -
i.p. 500 mg/kg 24 h Chromosomal -d
aberrations
7 h SCEs +e
------------------------------------------------------------------------------------------------------------
.. No data.
a i.p. = intraperitoneal, p.o. = peroral.
b Negative in bone marrow.
c Considered to be too long time for a positive result.
d Positive in animals who died of the treatment.
e A slight effect.
Only one study has appeared concerning the possible
effects of styrene 7,8-oxide on germinal cells. Fabry et al.
(1978) reported negative results for translocations in primary
spermatocytes after a single i.p. injection and for dominant
lethals in male postmeiotic germ cells of mice.
In summary, the results concerning the genetic effects of
styrene and styrene 7,8-oxide in vivo are conflicting. Positive
results for both compounds have been obtained mainly at toxic or
nearly toxic doses. The studies have dealt with different
indicators of chromosome damage (aberrations, micronuclei, SCEs) in
actively dividing somatic cells. The effects of styrene 7,8-oxide
on germ cells have been examined only in one study, with negative
results. On the basis of the activity of monooxygenase and epoxide
hydrolase in different species, mice would be expected to be more
sensitive to styrene than rats or Chinese hamsters (Cantoni et al.,
1978; Norppa et al., 1979; Norppa, 1981a). Even in mice, styrene
and styrene 7,8-oxide disappeared rather rapidly after
intraperitoneal or oral administration (Bidoli et al., 1980;
Pantorotto et al., 1980; Sbrana et al., 1982). However, the
results of the mutagenicity studies available cannot at present be
interpreted solely according to such differences in metabolism.
6.10.6. Conclusions on the genetic effects of styrene
Styrene is a potential mutagen only after metabolic
activation. Its most important active metabolite seems to be
styrene 7,8-oxide. Arene oxides (styrene 1,2-oxide and
styrene 3,4-oxide) may also be of importance, but at present
their role cannot be evaluated.
Genetic effect tests in bacteria, yeasts, and mammalian cells
in vitro have yielded contradictory results for styrene, in the
presence of microsomal fraction of rodent liver (usually S-9 mix).
This appears to be mostly due to differences in the efficiency of
activation of styrene in different studies.
Results of in vivo studies on the genetic effects of styrene in
mammals are also contradictory. Some of the discrepancies can be
explained by variations in metabolic capacity among the rodent
species used, divergence in dose levels, and by the different
routes of administration.
Styrene 7,8-oxide is a direct mutagen that is clearly positive
in vitro without metabolic activation, but gives contradictory
results in mammals in vivo.
6.11. Effects on Reproductive Function and Teratogenic Effects
(a) Non-mammalian systems
Styrene and styrene 7,8-oxide have been reported to be
embryotoxic and teratogenic in chick embryos (Vainio et al., 1977).
Kankaanpää et al. (1979) found that trichloropropylene oxide
increased the embryo toxicity and teratogenicity of styrene and
styrene oxide. The LD50s were 40 µmol/egg for styrene and 1.5
µmol/egg for styrene oxide. Depending on the dose, malformations
were found in up to 20% of the test embryos but not in the
controls. The types of malformation included: absence of one or
both eyes, eyelid deformation, deformation of the skull,
exencephaly, exteriorization of viscera, etc. Styrene and styrene
oxide have also been found to interfere with the development of sea
urchin embryos (Pagano et al., 1978).
(b) Mammalian systems
When male rats were exposed to a styrene concentration of
200 mg/m3 (48 ppm) for 5 h/day, 5 days per week for 4 months,
Ivanova-Tchemichanska et al. (1982) found decreased osmotic
resistance and mobility of spermatozoa, decreased sulfhydryl
content, and desquamated epithelium in the seminiferous
tubules.
Ragyl'ye (1974) studied the effects of styrene on pregnant
rats exposed to 1.47, 5, and 50 mg/m3 (0.35, 1.2, and 12 ppm)
for 4 h/day throughout gestation. Resorptions were noted at
all exposure levels but the number of malformations was not
significantly increased. Murray et al. (1978) exposed rats
and rabbits to styrene through inhalation (1260 and 2520
mg/m3; 300 and 600 ppm) for 7 h/day from day 6 to 15 (rats) or
from day 6 to 18 (rabbits), and by gavage (in peanut oil, 180
and 300 mg/kg body weight/day). No increase in the incidence
of resorptions or major malformations, compared with controls,
was reported. Delayed ossification was noted in rabbits
exposed to 2520 mg/m3 (600 ppm). In an inhalation study,
Vergieva et al. (l979) exposed rats to styrene at 200 and 400
mg/m3 (48 and 96 ppm) for 4 h daily, 5 days a week, throughout
pregnancy. There was not any evidence of embryotoxicity or
teratogenicity. Mice were exposed to a styrene level of 1050
mg/m3 (250 ppm) and Chinese hamsters to 1260, 2100, 3150, and
4200 mg/m3 (300, 500, 750, and 1000 ppm) for 6 h daily on days
6-16 (18 for hamsters) of gestation. An increased number of
resorptions was noted in both species at the highest
concentration tested. A slight increase (not statistically
significant) was observed in the umber of minor skeletal
malformations (rib fusion, extra ribs) in mice but not in
hamsters (Kankaanpää et al., 1980).
Quast et al. (1978) exposed pregnant Sprague-Dawley rats
and New Zealand white rabbits to styrene concentrations of
1260 or 2520 mg/m3 (300 or 600 ppm) for 7 h/day on days 6-15
(rats) and 6-10 (rabbits) of gestation. Additional groups of
rats were given styrene (90 or 150 mg/kg body weight daily) on
days 6-15 of gestation. No teratogenic effects were detected.
In a 3-generation reproduction study in rats, styrene was
administered in the drinking water at l25 and 250 mg/litre
(estimated daily intake 14 and 21 mg/kg body weight per day
for males and females, respectively, at the high dose). No
adverse effects on reproductive capacity were observed. Some
significant effects on early survival in the second generation
offspring at the high dose were found in 2 specific litters.
No further evaluation could be made, since data for
individuals were not available (Litton Bionetics, 1980).
7. EFFECTS OF STYRENE IN MAN
7.1. Controlled Human Studies
Few controlled exposure studies have been reported in
which the effects of styrene on physiological functions in man
have been investigated. Attention has been focused on the
irritant effects of styrene vapour on the mucous membranes and
on the central nervous system effects caused by styrene
inhalation.
The odour perception threshold for styrene in air was
determined by Smith & Hochstettler (1969) to be as low as
0.2-0.34 mg/m3 (0.05-0.08 ppm). At higher concentrations, the
odour of styrene was clearly perceptible and it was reported
as "strong but not objectionable" at about 420 mg/m3 (100 ppm)
(Stewart et al., 1968). The ability to detect the odour
typically fades as the exposed individuals become adapted
(Stewart et al., 1968). Styrene vapour was irritating to the
eye and the nose at concentrations exceeding 840 mg/m3 (200
ppm) and, when the styrene concentration approached 1596 mg/m3
(380 ppm), it was poorly tolerated (Stewart et al., 1968).
Strong immediate irritation of the eyes and the respiratory
tract has been reported at styrene concentrations in air of
2520-3360 mg/m3 (600-800 ppm) (Carpenter et al., 1944; Wolf et
al., 1956).
Oltramare et al. (1974) exposed 6 volunteers to styrene at
210, 420, and 840 mg/m3 (50, 100, and 200 ppm) over 1-3 h and
noted that for a combination of symptoms of the mucous
membrane (irritation of eyes, nose, and lips) and central
nervous system (dizziness, headache, drowsiness, difficulty in
concentrating, lightheadedness, fatigue), the number of
symptoms increased with dose. This increase was still evident
at the 210 mg/m3 (50 ppm level). At the 2 higher levels,
digestive disturbances also occurred. In studies by Gamberale
& Hultengren (1974), 12 volunteer subjects were exposed to
styrene vapour through a mouth tube at concentrations
increasing stepwise 210, 630, 1050, and 1470 mg/m3 (50, 150,
250, and 350 ppm), each successive step lasting about 30 min.
When the volunteers were asked afterwards to rate their
subjective sensations (during the exposure) it appeared that
exposure to styrene had made them feel more tense and more
"affected" compared with control conditions. The authors also
studied the psycho- physiological performance of the
subjects. Simple reaction times tended to be prolonged with
increasing exposure. The changes became statistically
significant during the final 30 min, when the environmental
concentration reached 1470 mg/m3 (350 ppm). Tests on
perceptual speed and manual dexterity did not show any
impairment in relation to styrene exposure.
Stewart et al. (1968), however, noted an impairment in
performance of tasks involving manual dexterity and eye-hand
coordination in some subjects exposed to a styrene
concentration of about 1596 mg/m3 (380 ppm) over a 1-h
period. On the basis of a study on 3 volunteers, Oltramare et
al. (1974) reported impaired reaction time to visual stimuli,
to combined visual-acoustic stimuli, and in a test of diffuse
attention during and immediately after exposure to styrene at
210, 420, and 840 mg/m3 (50, 100, and 200 ppm) for 1.5 h.
There was no clear dose dependance. Inhalation exposure to
high concentrations of styrene (3360 mg/m3; 800 ppm) caused
symptoms of impairment of balance in 2 subjects and a marked
unsteadiness in posture was observed (Carpenter et al.,
1944). At a styrene level of about 1596 mg/m3 (380 ppm) for 1
h, 2 out of 5 subjects showed abnormal results in the modified
Romberg test indicating difficulty in maintaining balance.
Furthermore, after a 7-h exposure to about 420 mg/m3 (100
ppm), 3 out of six subjects reported that they had transitory
difficulty in performing the modified Romberg test, though no
objective signs of impairment of balance were found (Stewart
et al., 1968). Oltramare et al. (1974), in a study on 3
subjects, showed impairment of balance on a body sway platform
at a styrene concentration of 840 mg/m3 (200 ppm) but not at
420 mg/m3 (100 ppm).
Five volunteers were exposed by Odkvist et al. (1980) to
styrene at about 1260 mg/m3 (300 ppm) for 1 h, using a mouth
tube. Treatment was combined with exercise on a bicycle
ergometer with a 50 W workload, and observations were made on
both equilibrium ability and functioning of the vestibular
system immediately after exposure. None of the subjects
exhibited spontaneous, fixation or positional nystagmus,
whereas an impairment of eye-tracking ability was found in all
individuals in an optokinetic test. The difference in
eye-movement changes between styrene-exposed and control
groups was not statistically significant. No styrene-induced
changes were found in conventional clinical tests of balance
(standing on one leg with eyes closed, walking a line with
eyes closed). The results were thought to suggest that
exposure to styrene (inducing blood levels of approximately 80
µmol/litre) decreased the inhibitory effect of the cerebellum
on the motor function of the eyes.
In summary, the odour threshold for styrene was found to be
0.2 - 0.34 mg/m3 (0.05-0.08 ppm) and the odour was uncomfortable at
elevated concentrations. Styrene induced subjective symptoms of
irritation of the mucous membranes at concentrations exceeding
420 - 840 mg/m3 (100-200 ppm). In the same concentration range,
subjective symptoms of the central nervous system, such as
dizziness, lightheadedness, headache, and drowsiness may occur.
Reaction time, performance, and body balance tend to be impaired by
short-term inhalation exposure to styrene at concentrations of
630 - 840 mg/m3 (150-200 ppm) and definite impairment occurs at
concentrations exceeding 1470 mg/m3 (350 ppm).
7.2. Epidemiological Studies
7.2.1. Haematopoietic and immune system
Chmielewski & Renke (1975), who studied a group of 101
workers exposed for at least one year to a styrene
concentration of 100-300 mg/m3 (24-72 ppm), did not
demonstrate any appreciable effects on the haemoglobin
concentration, erythrocyte count, leukocyte count, or
differential count. Workers exposed for more than 10 years,
however, had a slightly decreased thrombocyte count compared
with workers exposed for shorter periods.
In a study on 494 workers at different levels and
durations of exposure, Lorimer et al. (1976) observed a random
distribution of abnormal haemoglobin concentrations, and
leukocyte or platelet counts between the groups. Thiess &
Friedheim (1978), who investigated 84 workers exposed to
210-2100 mg/m3 (50-500 ppm) for 1-36 years, did not notice any
appreciable differences in haemoglobin concentrations, or
leukocyte, erythrocyte, or platelet counts compared with a
reference group.
In the studies of Chmielewski & Renke (1975), and Renke &
Chmielewski (1976), 20 styrene workers did not show any
differences in bleeding time or fibrinogen level compared with
a reference group. However, the coagulation time and the
adhesivity of the platelets were somewhat increased in the
styrene-exposed group and the prothrombin index was slightly
reduced.
There is very little information on the immunological
effects of styrene. In the immunophoresis study of
Chmielewski et al. (1973), no dose-related differences were
observed in concentrations of serum gamma globulin among
workers exposed to different concentrations of styrene.
7.2.2. Nervous system
Four studies have dealt with reaction times among workers
occupationally exposed to styrene. One study (Götell et al.,
1972) in which 17 men were exposed to a styrene concentration
of 630 mg/m3 (150 ppm) showed prolonged simple reaction times
in the styrene-exposed workers, both in the morning and in the
afternoon, compared with an age-matched control group. A
second study of 106 workers in 4 work places indicated
longer and more irregular reaction times in workers exposed to
styrene than in controls (Gamberale et al., 1975). The
differences were still present after a night's rest. The mean
styrene concentration determined by continuous measurement in
the workers' breathing zone was 57-426 mg/m3 (13.6-101.4
ppm). The mean duration of exposure was 2.7 years (range
0.1-11.0 years).
Another study with a similar study design, also revealed
prolonged reaction times during the working day among styrene-
and acetone-exposed boat manufacturers (Kjellberg et al.,
1979). The exposed group (7 workers, average styrene
concentration 37 mg/m3 (9 ppm), acetone concentration
82 mg/m3, mean employment time 10.5 years) did not show any
deterioration in sensory motor functions in relation to
styrene exposure and the addition task. There was a
correlation between the reaction-time impairment and the total
uptake of styrene divided by the estimated amount of adipose
tissue.
In the study of Kjellberg et al. (1979), the reaction
times of 3 subjects were followed after removal from exposure.
Reaction times improved after 4 days and there was a further
improvement after 35 days.
The study by Cherry et al. (1980) dealt with 27 workers
(mean age, 23 years), who were exposed to a time-weighted
average level of styrene of 386 mg/m3 (92 ppm). Among the
psychological and behavioural tests applied, differences
between the exposed and unexposed groups were seen only in
reaction times.
Several case reports have described neurasthenic symptoms
among patients who had been occupationally exposed to styrene
(Klimkova-Deutschova, 1962; Axelson et al., 1974; Spasovski et
al., 1976).
A considerable percentage of 101 workers employed in the
polyester laminate industry had disturbances of the nervous
system, particularly of the vegetative nervous system. The
exposure level of the workers ranged from approximately 100 to
300 mg/m3 (24 to 72 ppm). The urinary mandelic acid excretion
was more than 400 mg/litre in 36 of the workers (Chmielewski
et al., 1973).
Schneider & Seeber (1979) used psychological tests on 2
groups occupationally exposed to styrene, one group (35
persons) having a mean exposure of 700 mg/m3 (168 ppm), and
the other (46 persons), a mean exposure of 300 mg/m3
(72 ppm). Exposure was associated with poor visual attention,
interferences with attention span, perceptual inaccuracy, and
decreased manual dexterity.
In studies concerning 98 male laminating workers having a
mean urinary mandelic acid concentration that varied from 7 to
4715 mg/litre (median concentration 808 mg/litre), symptoms of
tiredness, and difficulty in concentrating were mentioned more
often than among a reference population (Härkönen, 1977). The
duration of styrene exposure varied from 0.5 to 14 years
(median 5.1 years). In psychological tests, the same
styrene-exposed workers showed visuomotor inaccuracy and poor
psychomotor performance (Lindström et al., 1976).
Exposure-effect relationships were drawn between the urinary
mandelic acid concentration and a combined score for 3 tests
(symmetry drawing, the Bourdon-Wiersma test, and the Mira
test). Analysis of the exposure-response relationship for
these tests showed a significant increase in response for
workers with levels of urinary mandelic acid exceeding 1600
mg/litre (75% having mandelic acid levels of more than 2000
mg/litre) (Härkönen et al., 1978). The proportion of abnormal
EEGs also increased with increasing exposure levels
(Seppäläinen & Härkönen, 1976). Workers with urinary mandelic
acid concentrations above 700 mg/litre had significantly
(P < 0.05) more frequent abnormal EEGs than those with lower
levels (Seppäläinen & Härkönen, 1976; Härkönen et al., 1978).
EEG abnormalities have been reported to be more prevalent
among young styrene workers, especially during the first years
of exposure (Dolmierski et al., 1974; Chmielewski et al.,
1977). EEG abnormalities, indicated mainly by increased slow
activity in the theta range, did not increase with longer
exposure (Roth & Klimkova-Deutschova, 1963; Seppäläinen &
Härkönen, 1976), though paroxysmal episodes have also been
evident (Dolmierski et al., 1974; Seppäläinen & Härkönen,
1976). Results of a study by Rosén et al. (1978) in which 33
men were exposed to styrene concentrations ranging from less
than 21 mg/m3 (5 ppm) to 735 mg/m3 (175 ppm), showed an
increased beta activity in 9 subjects and slow activity (theta
waves) in 6. The EEG abnormalities were non-specific and
resembled those caused by various other solvents (Rosén et
al., 1978; Seppäläinen et al., 1980).
Styrene exposure may also affect peripheral nerves
(Matsushita et al., 1968, Lilis et al., 1978; Rosén et al.,
1978). Lilis et al. (1978) found distal hypoaesthesia in the
lower extremities of 412 styrene-exposed workers (persons with
diabetes mellitus, a history of back injury, and/or excess
alcohol intake were excluded). The frequency of this symptom
varied from 4.1% among those exposed for less than 7 years to
8.5% among those exposed for more than 20 years. Abnormally
low motor conduction velocity (MCV) of the radial nerve was
found in 15 out of 80 persons tested and a slowed MCV of the
peroneal nerve in 12 out of 73 tested. The MCV of the
peroneal nerve decreased with increasing length of exposure,
but the effect of age was not considered. However, the level
of exposure did not have any effect on the peroneal nerve
conduction. The MCV of the radial nerve was not correlated
with the length or level of exposure. Rosén et al. (1978) did
not find differences in the MCVs of the median, ulnar,
peroneal, or posterior tibial nerves of 33 workers exposed to
styrene, compared with normal controls. However, the
amplitude of the sensory action potential of the median nerve
was reduced and the duration of this potential increased among
styrene-exposed workers, even in 10 individuals who had been
exposed to styrene levels of less than 21 mg/m3 (5 ppm). The
dose-effect relationships were conflicting. Disorders in
attention span and movement coordination in psychological
tests were reported in 12 out of 102 workers, occupationally
exposed to styrene concentrations of 10-100 mg/m3, who had
urinary mandelic acid concentrations of 30.9 - 268 g/kg
creatinine (Spasovski et al., 1980). The same authors also
reported diminished alcohol tolerance among styrene workers.
In summary, the results of 3 studies showed that simple
reaction times were prolonged in workers occupationally exposed to
styrene at levels below 630 mg/m3 (150 ppm), while one study gave
equivocal results at a time-weighted average level of styrene in
air of 386 mg/m3 (92 ppm). Another study suggested that the effect
on reaction time was reversible.
Surveys of styrene-exposed workers have shown that an increased
incidence of abnormalities in electroencephalographic recordings
at mean styrene levels of less than 420 mg/m3 (100 ppm) was related
to styrene exposure levels. Some slight disturbances in visuomotor
accuracy and psychomotor performance were noted in workers exposed
to levels of the order of 210 mg/m3 (50 ppm) or more. Various
neurasthenic and autonomic symptoms were also reported among these
workers.
7.2.3. Kidneys and the urinary tract
There are only a few studies of the effects of styrene
exposure on the human kidney. Härkönen (1977), who studied
laminating workers exposed to styrene for periods ranging from
0.5 to 14 years, could not find any cytological changes
differing from Papanicolau I in 35 urine specimens. Results
of studies concerning mortality and morbidity rates due to
kidney and urinary tract disorders have not revealed any
differences between subjects exposed to styrene and unexposed
subjects (Lorimer et al., 1978; Thiess & Friedheim, 1978).
In studies on organic solvent exposure in relation to
kidney function, Askergren et al. (1981) reported that workers
exposed to organic solvents, especially to styrene, excreted
significantly larger quantities of albumin in the urine than
unexposed workers. No differences were observed in beta-2-
microglobulin excretion. The same author compared the
excretion of erythrocytes and leukocytes in the urine of 101
men, occupationally exposed to styrene or toluene or to a
combination of xylene and toluene, with the cell excretion in
the urine from 39 unexposed controls. While no changes in
glomerular filtration rates were observed, the urine of
exposed men contained significantly more cells than that of
the controls.
7.2.4. Gastrointestinal tract
Basirov (1975) reported studies on the digestive system of 130
workers (89 men, 41 women) engaged in styrene-butadiene synthetic
rubber manufacture. The workers were mostly 20-40 years old and
the length of exposure varied from less than 5 years (16 workers)
to more than 10 years (28 workers). Average styrene concentrations
were 60-130 mg/m3 (14-31 ppm). Tests of secretory, excretory,
motor, and pepsinogen-generating functions of the stomach were
conducted on 20 unexposed people and on 80 workers who first
developed symptoms related to the digestive system after working in
the plant. Thirty-six had decreased digestive function, 25 had
decreased peristalsis, and 51 had decreased acidity. In further
studies, chronic gastritis was diagnosed in 35 of these workers.
7.2.5. Liver
Lorimer et al. (1976) assessed the liver function of 493
styrene-exposed workers. Exposure concentrations were not
measured. The activities of alkaline phosphatase (AP) (EC
3.1.3.1), aspartate aminotransferase (ASAT or GOT) (EC 2.6.1.1),
alanine aminotransferase (ALAT or GPT) (EC 2.6.1.2), and gamma
glutamyltranspeptidase (gamma-GTP) (EC 2.3.2.1) and the amount of
serum bilirubin were determined. Only the gamma-GTP activity
showed a significant elevation in the high-exposure group compared
with the low-exposure category, even when alcohol intake was taken
into account (1.7% versus 7.2%; 0.01 < P < 0.02).
Axelson & Gustavson (1978) gathered data on ASAT, ALAT in
serum from 35 styrene-exposed male workers and 12 unexposed
controls. The time-weighted average exposure levels were less
than or about 420 mg/m3 (100 ppm). The average transferase
levels were higher among men who had been exposed, but only
ASAT was significantly elevated (P < 0.001). No differences
were found in the average levels of AP.
The serum levels of ALATP, ASAT, gamma-GTP, LDH, and SDH
(sorbitol dehydrogenase (EC 1.1.1.14)) enzymes were studied in 72
workers exposed to styrene in the manufacture of plastic boats.
The exposure levels varied between very low concentrations < 21
mg/m3 (< 5 ppm) and about 170 mg/m3 (40.8 ppm). The styrene-
exposed groups had a higher mean gamma-GTP value than the control
group (P < 0.05). However, there was no dose dependence
(Lundberg, 1981).
Vihko et al. (1983) studied 25 persons exposed to styrene at
about 126 - 168 mg/m3 (30 - 40 ppm) (mean exposure time 3 years,
all exposures exceeding 1 year). The serum activities of ALAT,
ASAT, gamma-GTP, and LD and the concentrations of serum total
bilirubin and conjugated bilirubin were determined, as well as
serum bile acids, cholic acid, and chenodeoxycholic acid. An
elevated concentration of chenodeoxycholic acid in serum was the
most frequently found parameter among styrene-exposed workers.
Spasovski (1976) showed that the serum protein profile changed and
that serum transaminase activity was elevated at a styrene
concentration of 2000 mg/m3 (500 ppm).
In conclusion, a clear-cut trend towards altered liver
function was not demonstrated. At low exposure levels, the
commonly used parameters such as serum activity of enzymes of
hepatic origin have given equivocal results.
7.2.6. Cardiovascular system
The thorax radiographs of 84 workers were evaluated before
the beginning of employment and after exposure to styrene at
210 - 1260 mg/m3 (50 - 300 ppm) for 1-36 years (Thiess &
Friedheim, 1978). The authors could not attribute any grossly
observable changes to styrene exposure, nor did they observe
any "gross pathological indications in the electrocardiograms"
compared with those of a reference group of 62 subjects.
7.2.7. Respiratory system
In clinical studies, Wilson (1944) found that styrene-
exposed workers reported irritation of the eyes, throat, and
respiratory tract. It has frequently been reported that
people in areas of high styrene exposure complain of
irritation of the eyes and nasopharynx. Götell et al. (1972)
examined 15 workers occupationally exposed to a time-weighted
average styrene concentration of 71 - 1218 mg/m3 (17 - 290 ppm)
and found that lung function tests (forced vital capacity and
forced expiratory volume in 1 s) were normal and did not
change during the working day. However, the concentrations of
styrene that gave rise to complaints varied from person to
person, apparently depending on individual tolerance. In a
study on styrene-exposed workers who had respiratory tract
symptoms, it was suggested that long-term exposure to styrene
could cause obstructive pulmonary changes (Chmielewski &
Renke, 1975).
In a cross-sectional study of clinical signs and symptoms
of irritation (Lorimer et al., 1978) in 488 styrene-exposed
workers, the workers were divided into 2 groups, based on
estimated exposures of 4.2 mg/m3 (1 ppm) and > 21 mg/m3 (> 5
ppm), mostly above 84 mg/m3 (20 ppm). When asked about lower
respiratory symptoms, significantly more workers in the
high-dose group responded in the affirmative. There were no
differences in length of employment and the prevalence of
upper respiratory symptoms between the 2 groups.
In a symptom survey (Härkönen, 1977) of 98 male laminating
workers occupationally exposed to styrene, symptoms that
fulfilled the British Medical Research Council's criteria for
simple chronic bronchitis were more common in the exposed than
in an unexposed group. In the exposed group, 28% had simple
chronic bronchitis, compared with 12% in the control group
(P < 0.05). The smoking habits of the groups did not differ.
7.2.8. Endocrine organs
In a study that involved 25 rubber workers, exposed to
styrene, and 26 age-matched controls, Wink (1972) did not
observe any significant differences in the urinary excretion
of 17-ketosteroids or 17-ketogenic steroids. Exposure data
were not given. However, Chmielewski et al. (1973) reported
reduced excretion of 17-ketosteroids in the urine in 27 out of
67 workers exposed to styrene concentrations of 100-300 mg/m3
(24 - 72 ppm), but these findings were not convincingly
documented. The evaluation of possible effects of styrene on
the steroid metabolism should be studied using more refined
techniques.
In the study of Chmielewski et al. (1973), glucose
tolerance was observed to be somewhat higher in the exposed
group than in the control group, and even more in the group
with higher mandelic acid concentrations in the urine (limit
400 mg/litre). Evidence of effects of styrene on the
endocrine glands was lacking.
7.2.9. Carcinogenic effects
(a) Styrene production and polymerization processes
A survey of 560 individuals from a styrene production and
polymerization plant was reported by Lilis & Nicholson (l976)
and Nicholson et al. (1978). In 1960, the workers had been
employed in the plant for at least 5 years. It is known that
benzene, coke-oven products, butadiene, and ethyl-benzene had
been used in this plant prior to 1965. Among 83 deaths, 17
were due to cancer, including 2 cases of leukaemia and one of
a lymphoma. When the total cancer mortality in this cohort
was compared with that in the general population, no excess of
cancer incidence was found, but the follow-up period may have
been too short to detect an increased risk of neoplasia. One
case of leukaemia and one of lymphoma were found in 21
additional death certificates of individuals who had been
employed for less than 5 years in 1960. In an additional
analysis of 444 death certificates on all individuals who had
been employed for at least 6 months in the plant, 7 cases of
leukaemia and 5 cancers of the lymphatic system were found.
However, the populations at risk are not known.
A cohort of 1960 workers employed for at least 1 month in
a styrene-polystyrene manufacturing plant was followed from
1931 to 1976 (Frentzel-Beyme et al., 1978). Analysis of
deaths occurring (after a minimum period of 5 years) in groups
exposed for 5, 10, 15, and over 15 years did not indicate an
increase in cancer mortality in any of the groups compared
with the reference population. The Task Group noted, however,
that the mortality rates for the reference population were
only available for the period 1970-75, and that follow-up was
incomplete.
Ott et al. (1980) carried out a retrospective cohort study
in workers involved in the production of styrene and
polystyrene. Mortality experience was followed from 1935 to
1975. Among 282 deaths, 6 cancers of the lymphatic and
haematopoietic tissues (except leukaemia) and 6 cases of
lymphocytic leukaemia were observed, compared with 4.5 and
2.9, respectively, expected on the basis of US white male
mortality rates and 2.6 and 1.6 deaths expected from a
comparison industrial cohort. Four deaths from leukaemia were
observed in a sub-group of workers exposed to styrene, ethyl
benzene, oligomers of styrene, mineral oil, polymer dusts, and
extrusion fumes; 0.5 deaths would have been expected on the
basis of US white male mortality experience. Only one of the
4 individuals had spent at least 5 years in the working
environments under study. Inclusion of cases of leukaemia
still alive at the end of the study period or identified
through other conditions listed on the death certificate
increased the total number of lymphoreticular malignancies to
21 cases. These included 7 lymphocytic leukaemias, 4 other
leukaemias, 4 multiple myelomas, 4 Hodgkin's diseases, and 2
other lymphomas. Five of the subjects with lymphocytic
leukaemia had worked in the area of polymer extrusion and for
4 of them the disease developed 20 or more years after the
first exposure. According to the authors, there was no
increase in the general cancer incidence. The incidence of
lymphatic leukaemia was, however, more than that expected.
(b) Lamination and related processes in boat building
An epidemiological study was carried out on 1500 workers
from 36 companies in the reinforced plastics and plastic boat
industry. The majority of the workers were between 30 and 40
years old. The average period of employment was from 6 to 7
years but half of the workers had been in this type of work
for less than 3 years, while 54 had been employed for over 20
years. Exposure was estimated to be to an average styrene
concentration of 1050-1470 mg/m3 (250-350 ppm) at the end of
the sixties and the beginning of the seventies. Seventeen
cases of cancer were found in this group. In 3 of these, the
cancer was present before their employment. In the remaining
l4 cases, the sites and distribution of the various types of
tumours were in accordance with data from the cancer
registry. Among the deaths, one case of neoplasm of the
lymphatic and haematopoietic system (plasmocytoma) was
identified. However, because of the short follow-up period
and the small number of deaths it was not possible for the
authors to calculate any observed/expected figures (Ahlmark,
l978).
A study is in progress (Tola et al., 1981) in which the
tumour incidence in a cohort of 2209 workers, employed in the
manufacture of reinforced plastics in 160 workplaces, is being
investigated. Exposure to styrene vapour commenced in 1960
and the level of exposure has been estimated to range between
126 and 1260 mg/m3 (30 and 300 ppm). The majority of workers
have been exposed since 1967. Among 27 deaths, 6 deaths from
cancer were observed in this cohort (versus 8.l expected) and
the cancers were found in tissues other than lymphatic or
bone-marrow tissue. Five of the cancers appeared in workers
with 5 or fewer years of exposure. The Task Group noted the
low mortality rate.
(c) Styrene-butadiene rubber manufacture
McMichael et al. (1976) analysed the mortality experience
during the period 1940-60 in a sample of workers (exact number
not specified) taken from a population of 1482 workers in the
rubber industry. The subgroup of workers was involved in the
manufacture of styrene-butadiene rubber. For malignancies of
the lymphatic and haematopoietic systems, a relative risk
ratio of 6.2 was observed compared with other workers.
Subsequently, Smith & Ellis (1977) reported that this excess
was based on 4 cases. A case control analysis of the same
data was performed by Spirtas et al. (1976) who calculated a
relative risk ratio of 2.4 for lymphatic and haematopoietic
neoplasms among these styrene-butadiene workers. Past
exposure to solvents and other monomers was suspected.
The mortality experience of 2 cohorts of rubber workers
was analysed by Taulbee et al. (1976) using both cohort and
case-control analysis. No significant excess of lymphatic and
haematopoietic neoplasms was observed.
Case reports of leukaemias and lymphomas among styrene-
butadiene rubber workers were reported by Block (1976), Lemen
& Young (l976), and Meinhardt et al. (1978).
7.2.9.1. Summary and conclusions
There have been anecdotal reports of a small number of
cases of leukaemia and lymphoma in workers employed in the
manufacture of styrene-butadiene rubber, but the study
populations have been ill-defined and exposure to solvents and
other monomers is known to have occurred.
An association between leukaemia and exposure to styrene
in the production and polymerization process industries has
been suggested in another study. However, the cases of
leukaemia occurred in a group with a concomitant past history
of exposure to colourants, polymer fumes and, possibly,
benzene.
The effects of long-term exposure to styrene are under
investigation in at least 2 epidemiological studies on workers
employed in the industries involving styrene lamination of
glass fibre materials and related activities.
It was the opinion of the Task Group that the data
necessary to form an evaluation were inadequate and that a
causal relationship between exposure to styrene and human
cancer could not be demonstrated at present.
7.2.10. Genetic effects in somatic cells
7.2.10.1. Structural chromosome aberrations
Several studies have been published on structural
chromosome aberrations in the peripheral lymphocytes of
workers employed in the reinforced plastics industry or in the
production of styrene and polystyrene (Table 14). In these
studies, exposures have been estimated by measuring styrene
air concentrations in the workplace or by determining
concentrations of styrene metabolites, in most cases mandelic
acid, in the urine of the workers.
In Table 14, the studies are listed according to the
industrial process. Positive results have been obtained among
workers employed in polyester processing. Nine studies
included detailed data on chromosome aberrations in
individuals. One report by Watanabe et al. (1981) was
considered inconclusive because of the low number (50 or less)
of metaphases analysed per person. The other 8 studies with
detailed information were used for the final evaluation.
Three studies were reported only in abstract or review
articles (Sorsa et al., 1979; Sram, 1981; Vainio et al., 1982;
Brogger, 1982).
The study of Meretoja et al. (1978a) was an extension of
an earlier study (Meretoja et al., 1977) and included
additional analyses of samples taken from the same workers a
year later, after continuous workplace exposure.
One of the abstracts was concerned with a study on
children exposed during the fetal period, while their mothers
were working in hand lamination of polyester resin.
The only available study on chromosome aberrations among
workers employed in the production of styrene or polystyrene
was negative (Fleig & Thiess, 1978).
7.2.10.2. Other indicators of genetic damage
Seven studies were available on SCE induction in the
lymphocytes of styrene-exposed workers employed in polyester
processing (Table 14). Two of the studies were reported only
in abstracts or reviews (Sorsa et al., 1979; Brogger, 1982;
Vainio et al., 1982). A slight increase in SCEs was reported
in 2 studies (Andersson et al., 1980; Camurri et al., 1982).
In 2 of the studies, peripheral lymphocytes of polyester
processing workers were analysed for micronuclei. Meretoja et
al. (1978a) and Meretoja & Vainio (1979) reported an increase
in micronuclei and "cells connected with a nuclear bridge" in
the cultured lymphocytes of 10 workers in polyester plastic
product plants; the study included 5 controls (Table 14).
Högstedt & Mitelman (1983) found that the frequency of
micronuclei increased in 38 workers exposed for 1-23 years to
styrene (mean of time-weighted average concentrations in
breathing zone 55 mg/m3 or l3 ppm, range 4-168 mg/m3 or 1-40
ppm) compared with 20 controls.
Pero et al. (1982) did not find any increase in
unscheduled DNA synthesis (UDS) in isolated lymphocytes of 38
workers in a fibreglass-reinforced polyester plastic factory
compared with 20 unexposed controls. Styrene air
concentrations in the workroom varied between 4 and 168 mg/m3
(1 and 40 ppm).
In an evaluation of the incidence of malformed children
and miscarriages among spouses of men exposed to styrene in a
reinforced-plastic boat factory, Andersson et al. (1980)
concluded that the number of pregnancies (39 exposed and 41
controls) was too small to reveal mutagenic effects.
7.2.10.3. Conclusions
The available evidence suggests that styrene exposure in
the reinforced plastics industry with its more intensive
exposures is associated with an increased frequency of
structural chromosomal abnormalities.
7.3. Effects on Reproductive Function and Teratogenic Effects
In a case-reference study on 63 pairs of mothers from a
register of congenital malformations (Holmberg, 1977), 2
mothers with children having central nervous system defects
had been employed in the reinforced plastics industry during
pregnancy. A third case-mother was also found, who had been
exposed to styrene at home.
In a study on spontaneous abortions registered in
hospitals (Hemminki et al., 1980a) during the period 1973-76,
the following numbers and rates of abortion were found:
- General population 15 482 (5.52%);
- Union of Chemical Workers 52 (8.54%);
- Plastics industry 21 (8.94%);
- Styrene production and use 6 (15%).
The abortion rates for the occupational groups were
statistically different from the controls. The abortion rates
increased with age in the general population, while they were
found to decrease with age among the Union of Chemical
Workers. No data were given concerning the relationship with
age of spontaneous abortions among women employed in the
plastics industry or in styrene production and use; thus the
significance of the increased abortion rates among these
groups cannot be assessed.
Table 14. Summary on studies of structural chromosome aberrations and sister chromatid exchanges (SCEs) in the lymphocytes of
workers exposed to styrene.
------------------------------------------------------------------------------------------------------------------------------------
No. persons Measured Years of Urinary man- Cells with SCEs/cell
studied concentration exposure delic acid chromosome
Industry styrene in (mg/g of aberrations,
branch air (mg/m3) creatinine) gaps included (%)a
---------------------------------------------------------------------------------------------------------------------
Reference Exposed Controls Averagea Range Meana Range Meana Range Exposed Controls Resultb Exposed Controls Resultb
------------------------------------------------------------------------------------------------------------------------------------
Styrene
manufacture
Flieg & 5 20 ~2c 0-29c 21.6 14-25 ~30d 19- 3.8 5.5 - .. .. ..
Thiess (1978) 40d
Polystyrene
manufacture
Flieg & 12 20 ~8c 0- 20.3 3-39 ~32d <5- 5.1 5.5 - .. .. ..
Thiess (1978) (12)e 197c 100d (5.5)e .. .. ..
Polyester
processing
Meretoja et 10 5 .. .. 3.2 0.6- 721 23- 16.6 1.8 + .. .. ..
al. (1977); 8.5 3257
Meretoja &
Vainio (1979)
Meretoja et 16 6 .. up to 6.3 1-15 570 23- 15.1 2.0 + .. .. ..
al. (1978b) 1260 3257
(a year 11 3f .. up to 8.1 2-16 329 52- 16.2 .. + 5.3 4.4 -
later) 1260 1646
Flieg & 14 20 ~477c <210- 7.9 2-24 ~593 42- 9.2 5.5 + .. .. ..
Thiess (1978) 1260c >1500
Andersson et 36 37 ~197 0- 5.0 0.3- .. .. 12.3 6.7 + 9.7 8.7 +
al. (1980) 20f 20f 1008 12
Watanabe et 16 13 151- 0-886 ~4.5 0.6- ~594 90- 2.9 3.2 (-)g 13.5 13.9 -
al. (1981) <294 9.3 2640
Watanabe et 18 6 168- <5- 9.7 <1-30 332 1- 6.5 4.7 ± 8.9 8.5 -
al. (1983) 210 1075 ~1040
Thiess et al. 24 24 25- 3-748 ~14.4 4-27 .. 0- 5.1 3.8 +h .. .. ..
(1980, 1982) 244 320c
------------------------------------------------------------------------------------------------------------------------------------
Table 14. (contd.)
------------------------------------------------------------------------------------------------------------------------------------
No. persons Measured Years of Urinary man- Cells with SCEs/cell
studied concentration exposure delic acid chromosome
Industry styrene in (mg/g of aberrations,
branch air (mg/m3) creatinine) gaps included (%)a
---------------------------------------------------------------------------------------------------------------------
Reference Exposed Controls Averagea Range Meana Range Meana Range Exposed Controls Resultb Exposed Controls Resultb
------------------------------------------------------------------------------------------------------------------------------------
Camurri et al. 24 21 .. 30- 9.4 1-22 472c 45- ~35.1i ~8.4i + 13.9 10.8 +
(1982)k (22)f (20)f >400 1108c
Abstracts
Sram (1981) 36 19 .. .. .. .. .. .. 1.4i 1.3 - .. .. ..
(cited data 34j (1.4)ij
of Pchlova & 22 22 .. .. .. .. .. .. 1.7i 1.4i - .. .. ..
Sram)
Brogger 13 0 129 15- 9.8 1-24 527 292- 6.4 .. (-)g 6.6 .. (-)g
(1982)k (8)f 364 688
Sorsa et al. 6 10 .. .. .. 2-7 .. .. 8.8 3.0 + 7.5 8.5 -
(1979);
Vainio et al. 7 10 .. .. .. (5-8 .. .. 4.9 1.6 + 4.9 5.2 -
(1982) ( in months)
utero exposed
children)
Högstedt et 6 6 ~162 60- 4.0 0.5- 490 225- 10.2 4.9 + .. .. ..
al. (1979) 800 10 2100
------------------------------------------------------------------------------------------------------------------------------------
a Estimated from the data given if not indicated in the report.
b Compared to response in control persons: + increase; - no effect.
c From Thiess & Friedheim (1978).
d mg/litre urine.
e Controls in Thiess & Fleig (1978).
f For the analysis of sister chromatid exchanges only.
g Inconclusive because of the low number of cells analysed (Watanabe et al., 1981) or lack of controls (Brogger, 1982).
h Negative if gaps are excluded.
i Gaps excluded.
j 6 months later.
k Details, not mentioned in the report, were obtained from the author(s).
In a small-scale study, Härkönen & Holmberg (1982)
recently interviewed 67 female lamination workers of child-
bearing age and a similar number of textile and food
production workers who were thought to be proper controls.
The groups were reported not to differ either in their
menstrual behaviour or in the number of spontaneous
abortions. The number of deliveries was found to be lower
among the styrene workers, which may be partially explained by
the higher number of induced abortions in the styrene-exposed
group.
8. EXPOSURE-EFFECT/EXPOSURE-RESPONSE RELATIONSHIPS, AND
EVALUATION OF HEALTH EFFECTS
8.1. Data from Experimental Animal Studies
8.1.1. Metabolic pathways and kinetics
Pulmonary uptake in man is of greatest importance though uptake
through the skin occurs. Styrene is biotransformed largely via the
7,8-epoxide by the mixed function oxidase system.
The kinetic data show that exposure of experimental animals to
increasing levels of styrene results in progressive saturation of
the metabolic pathways. The half-time of styrene disappearance
from the body varies with the dose. The range of exposures tested
was from 189 mg/m3 (45 ppm) to 10 500 mg/m3 (2500 ppm). The
consequence of such metabolic saturation, which has been reported
to occur at about 2520 mg/m3 (600 ppm) in air, is an increase in
the proportional deposition of styrene in fat. In human beings the
half-time for the elimination of styrene from adipose tissue is 2-3
days.
The principal urinary metabolites are mandelic and
phenylglyoxylic acids. Recent evidence suggests that the
pattern of urinary metabolite excretion varies with mammalian
species.
8.1.2. General toxicity
8.1.2.1. Acute toxicity
A mean odour detection threshold for styrene of 3.06 mg/m3
(0.73 ppm) was found for unadapted subjects. The odour was
reported to be strong but not objectionable at about 420 mg/m3
(l00 ppm). With short-term exposures at concentrations
exceeding 840 mg/m3 (200 ppm), styrene vapour was irritating
to the eyes and nose.
The central nervous system effects begin to appear in the
range of 210-840 mg/m3 (50-200 ppm) and distinct impairment of
reaction time and body balance were found at concentrations
exceeding 840 mg/m3 (200 ppm).
The acute toxicity of styrene in animals is rather low:
the LD50 in rats has been reported to be 5 g/kg body weight
after oral administration and 2-3 g/kg body weight after
intraperitoneal injection. Exposure of rats to styrene vapour
at 1300 to 10 000 ppm for up to 4 h caused nasal mucous
membrane and eye irritation as well as acute depression of the
central nervous system. Marked pulmonary lesions were also
noted.
There were no kidney changes in animals exposed to 5460
mg/m3 (l300 ppm) after a single exposure. Severe irritation
of the eyes and nose was observed in rats and guinea-pigs
after exposure to concentrations of 2730-5460 mg/m3 (650 - 1300
ppm) and general weakness and unsteadiness gradually developed
after 12 h.
Acute toxic effects were observed in mice exposed to a
styrene level of 21 000 mg/m3 (5000 ppm) for 2 h and
guinea-pigs exposed to 10 920 mg/m3 (2600 ppm) for 8 h. A
short-term exposure to 1260 mg/m3 (300 ppm) in the air caused
only very slight behavioural effects in rats, even though
pronounced neurochemical changes were observed.
The high exposure levels used in studies on experimental
animals have not been observed in the human environment.
8.1.2.2. Subacute and chronic toxicity
Inhalation of styrene vapour by rats at a concentration of
5460 mg/m3 (1300 ppm) for 7 h/day over several months did not
have any deleterious effects on the kidney and did not cause
any changes in the erythrocyte count, haemoglobin
concentration, and leukocyte count. There were not any
essential morphological changes in the rat lungs. Similar
results were obtained with rabbits, monkeys, and guinea-pigs
except that 10% of the guinea-pigs died after the first
exposure with signs of lung irritation. An exposure level of
2730 mg/m3 (650 ppm), for 7 h/day for 214-360 days, appeared
not to have any effects on the lungs of guinea-pigs.
Rats exposed by inhalation to a styrene concentration of
1260 mg/m3 (300 ppm) (6 h/day, 5 days a week) for up to 11
weeks developed axonal protein changes in the brain, an
induction of drug metabolizing enzymes in the kidney and the
liver, histological alterations in the liver, and a depletion
of the glutathione (GSH) contents of the kidney and the liver.
No significant depletion of GSH occurred at 420 mg/m3 (100
ppm).
Oral subacute studies were carried out on rats and beagle
dogs. The rat study was carried out on females and exposure
induced only weight increases in the liver and kidney. No
effect was observed at a dose of 133 mg/kg body weight. The
study on dogs lasted more than 19 months and showed a mild
haemolytic anaemia characterized by Heinz body production.
Effects were observed down to the lowest dose tested (200
mg/kg body weight).
8.1.3. Genetic effects
Styrene is a potential mutagen only after metabolic activation.
Mutagenicity tests in bacteria, yeasts and mammalian cells in vitro
have yielded contradictory results for styrene that may be due to
differences in the efficiency of activation and inactivation of
styrene in different studies.
Conclusive dose-effect relationships could only be established
in several in vitro studies.
The results of tests for chromosomal damage induced by
styrene in experimental mammals have been contradictory.
Three positive studies have been reported; 2 in mice, and 1 in
rats. A dose-effect relationship was only observed for the
induction of SCE in mice exposed to styrene in air levels of
more than 1260 mg/m3 (300 ppm). Five other animal studies
have given negative results. The different end-points, routes
of exposure, durations of treatment, species and strains of
animals used, and the small number of mammalian studies
available, make it impossible to draw definitive conclusions
concerning dose-effect relationships.
8.1.4. Carcinogenic effects
Oral administration of styrene to mice induced a
significant increase in pulmonary tumours in the O20 strain at
a dose of 1350 mg/kg body weight and a doubtful increase in
B6C3F1 mice at 300 mg/kg body weight. No significant increase
in tumour incidence was observed in the C57B1 strain, when
styrene was administered pre- and post-natally at 300 mg/kg
body weight.
In 2 studies on rats, styrene given orally at doses
ranging from 500 to 1350 mg/kg body weight did not induce a
significant increase in tumour incidence. A greater incidence
of lymphomas, observed in an inhalation study on rats exposed
to a styrene concentration of 2520 or 4200 mg/m3(600 or 1000 ppm),
could not be causally related to styrene.
Thus, the evidence available indicates that styrene at the
doses administered (1350 mg/kg body weight for 020 strain)
caused an increase in pulmonary tumours in mice.
Styrene 7,8-oxide induced squamous cell carcinomas in the
forestomach of rats given doses of 50 and 250 mg/kg body weight.
In a second study on rats, both squamous cell carcinomas and
pulmonary carcinomas were induced, when styrene oxide was given at
a dose of 100 - 150 mg/kg body weight per day, 4-5 days a week.
When styrene oxide was painted on the skin of mice at
concentrations of 50 or 100 g/kg, no tumours of the skin were
induced.
8.2. Human Studies
8.2.1. Effects on organs and systems
Effects on the following organ systems were investigated:
nervous, haematopoietic and immune systems, the kidney and
urinary tract, the gastrointestinal tract, liver,
cardiovascular and respiratory systems, and the endocrine
organs.
Most studies of the haematopoietic system did not produce
any positive data; in one study, a slight decrease in
thrombocyte count in workers exposed to styrene for more than
ten years was detected.
A clear-cut trend towards altered liver function has not been
demonstrated. At low exposure concentrations, the commonly used
parameters, such as activity of serum enzymes of hepatic origin,
except for gamma-GTP, have given equivocal results.
Slight effects on the lower respiratory system were noted
in some studies.
No adequate data were available to the Task Group to
establish dose-effect or dose-response relationships for the
aforementioned systems.
Slight disturbances of visuomotor accuracy and psychomotor
performance were noted at styrene levels exceeding 210 mg/m3
(50 ppm), and an increased incidence of abnormalities in
electroencephalograph recordings was detected at styrene
concentrations below 420 mg/m3 (100 ppm); relationships
between the exposure levels and the severity of these effects
and the response rates were observed.
While the reaction times were shown to be prolonged for
exposure levels below 630 mg/m3 (150 ppm), dose-response
relationships could not be established.
8.2.2. Genetic effects in somatic cells
Knowledge concerning the possible genetic effects of
styrene exposure on man is still inadequate for any
dose-response extrapolations. Available information is
limited to structural somatic chromosome damage in the
peripheral blood lymphocytes of workers occupationally exposed
to high concentrations of styrene in the reinforced plastics
industry.
Data on individual exposure profiles are necessary for
meaningful interpretation of the results of somatic cell
analysis. Furthermore, the role of high occasional peak
exposures and low continuous workplace exposures in the
induction of chromosome damage in lymphocytes in styrene-
exposed workers is not clear. Considering the survival time
of the target lymphocytes, exposure data should be available
for a period of at least 2-3 years preceding chromosome
analysis.
Based on available published data, the following
conclusions can be made:
1. No clear dose-response pattern can be recognized.
Positive results of chromosome aberrations in
styrene-exposed workers were restricted to the
reinforced plastics industry, where styrene
concentrations in air were high. Negative results
have been obtained in the manufacture of styrene or
polystyrene where exposures to styrene are lower.
2. Variability in the chromosome aberration frequencies
reported in different studies is great. This
suggests that several factors such as exposure
conditions, the method, and the analytical criteria
selected, influence the results.
3. Individual differences are great and may reflect
variations in exposure or differences in
susceptibility.
The Task Group concluded that the health significance of
structural chromosomal aberrations in the somatic cells of
persons exposed to styrene could not be assessed at present,
but that such effects were undesirable.
8.2.3. Carcinogenic effects
Several case reports and epidemiological investigations
have implied an increased risk of lymphatic and haematopoietic
system cancer in workers involved in the application of
styrene, polystyrene, and styrene-butadiene rubber. However,
at present, there is not sufficient evidence to establish a
direct cause and effect relationship between styrene exposure
and cancer in human beings. Assessment of data has frequently
been complicated by concomitant exposure to other volatile
substances.
The Task Group concluded that, to date, a causal
relationship between styrene exposure and cancer could not be
established in man.
REFERENCES
AHLMARK, A. (1978) [Styrene study. Epidemiological report.]
Stockholm, Sveriges Plastförbund, 21 pp (in Swedish).
ALARIE, Y. (1973) Sensory irritation of the upper airways by
airborne chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
AMACHER, D.E. & TURNER, G.N. (1982) Mutagenic evaluation of
carcinogens and non-carcinogens in the L5178Y/TK assay
utilizing postmitochondrial fractions (S9) from normal rat
liver. Mutat. Res., 97: 49-65.
ANDERSSON, H.C., TRANBERG, E.C., UGGLA, A.H., & ZETTERBERG, G.
(1980) Chromosomal aberrations and sister-chromatid exchanges
in lymphocytes of men occupationally exposed to styrene in a
plastic-boat factory. Mutat. Res., 73: 387-401.
ASKERGREN, A., ALLEN, L.-G., KARLSSON, D., LUNDBERG, I., &
NYBERG, E. (1981) Studies on kidney function in subjects
exposed to organic solvents. I. Excretion of albumin and -2
microglobulin in the urine. Acta Med. Scand., 209: 479-483
ASTRAND, I., KILBOM, C., OVRUM, P., & WAHLBERG, I. (1974)
Exposure to styrene. I. Concentration in alveolar air and
blood at rest and during exercise and metabolism. Work
environ. Health, 11: 69-85.
AXELSON, O. & GUSTAVSON, J. (1978) Some hygienic and clinical
observations on styrene exposure. Scand. J. Work Environ.
Health, 4 (Suppl. 2): 215-219.
AXELSON, O., FROBARJ, G., & WEDEFELT, U. (1974) [Can styrene
exposure cause states with cerebral lesion.] Läkartidningen,
71: 137-138 (in Swedish).
BABINA, M.D. (1969) [Spectrophotometric determination of
styrene and benzaldehyde in the work environment.] Gig. i
Sanit., 34: 105-106 (in Russian).
BAGGET, M.S., MORIA, G.P., SIMMONS, M.W., & LEWIS, J.S. (1974)
Quantitative determination of semivolatile compounds in
cigarette smoke. J. Chromatogr., 97: 79-82.
BAKKE, O.M. & SCHELINE, R.R. (1970) Hydroxylation of aromatic
hydrocarbons in the rat. Toxicol. appl. Pharmacol., 16:
691-700.
BARDODEJ, Z. (1964) [Styrene metabolism.] Cesk. Hyg., 9:
223-239 (in Czech).
BARDODEJ, Z. (1978) Styrene, its metabolism and the
evaluation of hazards in industry. Scand. J. Work Environ.
Health, 4 (Suppl. 2): 95-103.
BARDODEJ, Z. & BARDODEJOVA, E. (1970) Biotransformation of
ethyl benzene, styrene and alphamethyl styrene in man.
Am. Ind. Hyg. Assoc. J., 31: 206-209.
BARDODEJ, A., MALEK, B., VOLFOVA, B., & ZELENA, E. (1960)
[The hazard of styrene in the production of glass laminates.
Cesk. Hyg., 5: 541-546 (in Czech).
BARDODEJ, Z., BARDODEJOVA, E., & MALEK, B. (1961) [Value and
application of exposure tests. XI. Exposure test for
styrene.] Cesk. Hyg., 6: 546-552 (in Czech).
BARDODEJ, Z., BARDODEJOVA, E., & GUT, I. (1971) [Metabolosm
of styrene in rats.] Cesk. Hyg., 16: 243-245 (in Czech).
BARSOTTI, M., PARMEGGIANI, L., & SASSI, C. (1952)
[Observations on occupational pathology in a polystyrene resin
factory.] Med. Lav., 43: 418-424 (in Italian).
BASIROV, A.A. (1968) [Gastric function in workers of the
synthetic rubber industry.] Vrac. Delo, 4: 200-203 (in
Russian).
BASIROV, A.A. (1975) [Biochemical indexes of the gastric
juice in the early diagnosis of stomach illnesses under the
effect of toxic substances (1,3-butadiene and styrene).
Azerb. Med. Zh., 52: 60-66 (in Russian).
BAUER, C., LEPORINI, C., BRONZETTI, G., CORSI., C., NIERI, R.,
DEL CARRATONE, R., & TONARELLI, S. (1980) The problem of
negative results for styrene in the in vitro mutagenesis test
with metabolic activation (microsomal assay): explanation by
gas chromatographic analysis. Boll. Soc. It. Biol. Sper., 56:
203-207.
BAUER, C., LEPORINI, C., BRONZETTI, G., CORSI, C., NIERI, R.,
DEL CARRATONE, R., & TONARELLI, S. (1981) Studies on the
incubation mixtures for the in vitro mutagenesis test with
metabolic activation (microsomal assay) - behaviour of some
activating and detoxifying enzyme systems during incubations.
The case of styrene. Mutat. Res., 85: 268.
BAUER, D. & GUILLEMIN, M. (1976) Human exposure to styrene:
I. The gas-chromatographic determination of urinary
phenylglyoxylic acid using diazomethane derivatization.
Int. Arch. occup. environ. Health, 37: 47-55.
BEIJE, B. & JENSSEN, D. (1982) Investigation of styrene in
the liver perfusion/cell culture system. No indication of
styrene-7,8-oxide as the principal mutagenic metabolite
produced by the intact rat liver. Chem. biol. Interact., 39:
57-76.
BELVEDERE, G. & TURSI, F. (1981) Styrene oxidation to styrene
oxide in human blood erythrocytes and lymphocytes. Res. Comm.
Chem. Pathol. Pharmacol., 33: 273-282.
BELVEDERE, G., CANTONI, L., TACCHINETTI, T., & SALMONA, M.
(1977) Kinetic behaviour of microsomal styrene monooxygenase
and styrene epoxide hydratase in different animal species.
Experientia (Basle), 33: 708-709.
BENCEV, I.V. & RIZOV, N. (1981) [Gas-chromatographic method
for styrene determination blood (headspace method).]
Prob. Hyg., VI: 88-91 (in Russian).
BERGMAN, K. (1977) [Exposure to styrene in plastic boat
industry, I. Technical-hygienic study.] Arbete Hälsa, 3: 1-9
(in Swedish).
BERGMAN, K. (1979) Whole body autoradiography and allied
techniques. Scand. J. Work Environ. Health, 5 (Suppl. 1):
93-120.
BERTSCH, W., ANDERSON, E., & HOLZER, G. (1975) Trace analysis
of organic volatiles in water by gas chromatography-mass
spectrometry with glass capillary columns. J. Chromatogr.,
112: 701-718.
BIDOLI, F., AIROLDI, L., & PANTAROTTO, C. (1980) Quantitative
determination of styrene-7,8-oxide in blood by combined gas
chromatography-multiple ion detection mass fragmatography.
J. Chromatogr., 196: 314-318.
BLOCK, J.B. (1976) A Kentucky study: 1950-1975. In: Ede, L.,
ed. Proceedings of NIOSH Styrene-Butadiene Briefing,
Covington, Kentucky, USA, 1976, Cincinnati, Ohio, US
Department of Health, Education and Welfare pp. 28-32 (HEW
Publ. No. (NIOSH) 77-129).
BODNEI, A.H., BUTLER, G.J., & OKAWA, M.T. (1974) Health
hazard evaluation/toxicity determination. Cincinnati, US
Department of Health, Education and Welfare, Public Health
Service, Center for Disease Control, National Institute for
Occupational Safety and Health, 10 pp (Report no. 73-103-128 -
American Standard Fiberglass Inc., Stockton, California).
BONATTI, S., ABBONDANDOLO, A., CORTI, G., FIORIO, R., &
MAZZACCARO, A. (1978) The expression curve of mutants induced
by styrene oxide at the HGPRT locus in V79 cells.
Mutat. Res., 52: 295-300.
BROGGER, A. (1982) Application of SCE to public health. In:
Sandberg, A., ed. Sister chromatid exchange, New York, A.R.
Liss, pp. 655-673.
BROOKS, S.M., ANDERSON, L.A., TSAY, J.-Y., CARSON, A.,
BUNCHER, C.R., ELIA, V., & EMMETT, E.A. (1979) Investigation
of workers exposed to styrene in the reinforced plastic
industry - health and psychomotor status, toxicological and
industrial hygiene data and effect of protective equipment as
it relates to exposures through lung and skin routes.
Cincinnati, University of Cincinnati, College of Medicine,
pp. 330. (Institute of Environmental Health and Kettering
Laboratory Report Prepared for the Society of Plastics
Industries).
BROOKS, S., ANDERSON, L., EMMETT, E., CARSON, A., TSAY, J.-Y.,
ELIA, V., BUNCHER, R., & KARBOWSKY, R. (1980) The effects of
protective equipment on styrene exposure in workers in the
reinforced plastics industry. Arch. environ. Health, 35:
287-294.
BROUNS, R.E., POOT, M., DE VRIND, R., V. HOEK-KON, T.,
HENDERSON, P.T., & KUYPER, C.M.A. (1979) Measurement of
DNA-excision repair in suspensions of freshly isolated rat
hepatocytes after exposure to some carcinogenic compounds.
Its possible use in carcinogenicity screening. Mutat. Res.,
64: 425-432.
BUCHET, J.-P., LAUWERYS, R., ROELS, H., & DEFELD, J.-M.
(1974) [Evaluation of the exposure of workers to styrene by
means of the determination of its urinary metabolites:
mandelic and phenylglyoxylic acids: I. Technique of
determination of the metabolites by gas chromarography.
Arch. Mal. prof. Med. Tra. Secur. soc., 35: 511-516.
BURKIEWICZ, C., RYBKOWSKA, J., & ZIELINSKA, H. (1974)
[Assessment of exposure to styrene in human beings under
industrial conditions.] Med. Prac., 3: 305-310 (in Polish).
BURKOVA, T., BAJNOVA, A., & KAPURDOV, V. (1982) Occupational
risk with repeated styrene dermal contact. Prob. Hyg., VII:
51-59.
BURNETT, R.D. (1976) Evaluation of charcoal sampling tubes.
Am Ind. Hyg. Assoc. J., 37: 37-45.
BUSK, L. (1979) Mutagenic effects of styrene and styrene
oxide. Mutat. Res., 67: 201-208.
CAMURRI, L., CODELUPPI, S., & PEDRONI, C. (1982) Chromosomal
aberrations and sister chromatid exchanges in styrene exposed
workers. In: 12th Annual Meeting of European Environmental
Mutagen Society, Espoo, Finland, 20-24 June, 1982 Abstracts,
Helsinki, Institute of Occupational Health, p. 159.
CANTONI, L., SALMONA, M., FACCHINETTI, T., PANTAROTTO, C., &
BELVEDERE, G. (1978) Hepatic and extrahepatic formation and
hydration of styrene oxide in vitro in animals of different
species and sex. Toxicol. Lett., 2: 179-186.
CAPEROS, J.R., HUMBERT, B., & DROZ, P.O. (1979) Exposition on
styrene. II. Bilan de l'absorption, de l'excrétion et du
métabolisme sur des sujets humains. Int. Arch. occup.
environ. Health, 42: 223-230.
CARLSSON, A. (1981) Distribution and elimination of
14C-styrene in rat. Scand. J. Work Environ. Health, 7: 45-50.
CARPENTER, C.P., SHAFFER, C.B., WEIL, C.S., & SMYTH, H.F.
(1944) Studies on the inhalation of 1:3-butadiene; with a
comparison on its narcotic effect with benzol, tuluol, and
styrene, and a note on the elimination of styrene by the
human. J. ind. Hyg., 26: 69-78.
CERNA, M. & KYPENOVA, H. (1977) Mutagenic activity of
chloroethylenes analysed by screening system tests.
Mutat. Res., 46: 35-36.
CHEMICAL MARKET REVIEW (1981) Oct. 26, p. 7.
CHERRY, N., WALDRON, H.A., WELLS, G.G., WILKINSON, R.T.,
WILSON, H.K., & JONES, S. (1980) An investigation of the
acute behavioral effects of styrene on factory workers.
Br. J. ind. Med., 37: 234-240.
CHMIELEWSKI, J. & RENKE, W. (1975) Clinical and experimental
research into the pathogenesis of toxic effects of styrene
III. Morphology, coagulation and fibrinolisis systems of the
blood in persons exposed to the action of styrene during their
work. Biul. Inst. Med. Morskej, 26: 299-302.
CHMIELEWSKI, J., MIKULSKI, P., USELIS, J., & WIGLUSZ, R.
(1973) Rating of the exposure to styrene of persons working
at the production of polyester laminates. Biul. Inst. Med.
Morskej Gdansku, 24: 203-209.
CHMIELEWSKI, J., DOLMIERSKI, R., RENKE, W., & KWIATKOWSKI,
S.R. (1977) [Long-term occupational effects of styrene on
working people.] Z. gesamte Hyg. Grenzgeb., 23: 639-643 (in
German).
CHOI, K.K. & FUNG, K.W. (1979) Determination of styrene in
the atmosphere near industrial sites by gas chromatography.
Analyst, 104: 455-457.
CHU, K.C., CUETO, C., Jr, & WARD, J.M. (1981) Factors in the
evaluation of 200 National Cancer Institute Bioassays.
J. Toxicol. Environ. Health, 8: 251-280.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1979) Sister
chromatid exchange in regenerating liver an bone marrow cells
of mice exposed to styrene. Toxicol. appl. Pharmacol., 50:
365-367.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1980) Sister
chromatid exchange in murine alveolar macrophages, bone
marrow, and regenerating liver cells induced by styrene
inhalation. Toxicol. appl. Pharmacol., 55: 37-42.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1982) Multiple
tissue comparisons of sister chromatid exchanges induced by
inhaled styrene. In: Tice. R., Costa, D.L., & Schaich, K.M.,
ed. Genotoxic effects of airborne agents, New York, Plenum
Press, pp. 433-441.
CRANDALL, M.S. (1981) Worker exposure to styrene monomer in
the reinforced plastic boat-making industry. Am. Ind. Hyg.
Assoc. J., 42: 499-502.
CRITTENDEN, B.D. & LONG, R. (1976) The mechanisms of
formation of polynuclear aromatic compounds in combustion
systems. In: Carcinogenesis - A comprehensive survey - Volume
I, New York, Raven Press, pp. 209-223.
DANISHEFSKY, I. & WILLHITE, M. (1954) The metabolism of
styrene in the rat. J. Biol. Chem., 211: 549-553.
DELBRESSINE, L.P.C., KETELAARS, H.C.J., SEUTER-BERLAGE, F., &
SMEETS, F.L.M. (1980) Phenaceturic acid a new urinary
metabolite of styrene in the rat. Xenobiotica, 10: 337-342.
DEL CARRATORE, R., BRONZETTI, G., BAUER, C., CORSI, C., NIERI,
R., PAOLINI, M., & GIAGONI, P. (1982) Study of cytochrome
P450 in yeast D7 strain. An alternative model to microsomal
assay. Mutagenicity of styrene. In: 12th Annual Meeting of
the European Environmental Mutagen Society, Espoo, Finland,
20-24 June, 1982, Abstracts, Helsinki, Institute of
Occupational Health, p. 160.
DE MEESTER, C., PONCELET, F., ROBERFROID, M., RONDELET, J., &
MERCIER, M. (1977) Mutagenicity of styrene and styrene oxide.
Mutat. Res., 56: 147-152.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER,
M., MERCIER, M., & PONCELET, F. (1981) Mutagenicity of
styrene in the Salmonella typhimurium test system.
Mutat. Res., 90: 443-450.
DIETRICH, M.W., CHAPMAN, L.M., & MIEURE, J.P. (1978) Sampling
for organic chemicals in workplace athmosphere with porous
polymer beads. Am. Ind. Hyg. Assoc. J., 39: 385-392.
DOLMIERSKI, R., KWIATKOWSKI, S.R., & NITKA, J. (1974) A
preliminary evaluation of the studies on central nervous
system in workers exposed to styrene. Biul. Inst. Med.
Morskiej Gdansku, Gdansk, 25: 399-406.
DONNER, M., SORSA, M., & VAINIO, H. (1979) Recessive lethals
induced by styrene and styrene oxide in Drosophila
melanogaster. Mutat. Res., 67: 373-376.
DRINKWATER, N.R., MILLER, J.A., MILLER, E.C., & YANG, N.-C.
(1978) Covalent intercalative binding to DNA in relation to
the mutagenicity of hydrocarbon epoxides and N-acetoxy-2-
acetylaminofluorene. Cancer Res., 38: 3247-3255.
DROZ, P.O. & FERNANDEZ, J.G. (1977) Effect of physical work
load on retention and metabolism of inhaled organic solvents.
A comparative theoretical approach and its application with
regards to exposure monitoring. Int. Arch. occup. environ.
Health, 38: 231-246.
DUTKIEWICZ, T. & TYRAS, H. (1967) A study of the skin
absorption of ethylbenzene in man. Br. J. ind. Med., 24:
330-332.
DUTKIEWICZ, T. & TYRAS, H. (1968) Skin absorption of toluene,
styrene and xylene by man. Br. J. ind. Med., 25: 243.
DUVERGER-VAN BOGAERT, M., NOEL, G., ROLLMAN, B., CUMPS, J.,
ROBERFROID, M., & MERCIER, M. (1978) Determination of oxide
synthetase and hydratase activities by a new highly sensitive
gas chromatographic method using styrene and styrene oxide as
substrates. Biochim. Biophys. Acta, 526: 77-84.
ELIA, V.J., ANDERSON, L.A., MacDONALD, T.J., CARSON, A.,
BUNCHER, C.R., & BROOKS, S.M. (1980) Determination of urinary
mandelic and phenylglyoxylic acids in styrene exposed workers
and a control population. Am. Ind. Hyg. Assoc. J., 41:
922-926.
EL-MASRI, A.M., SMITH, J.N., & WILLIAMS, R.T. (1958) Studies
on detoxication. The metabolism of alkylbenzenes:
phenylacetylene and phenylethylene (styrene). Biochem. J.,
68: 199-204.
ELOVAARA, E., VAINIO, H., PFAFFLI, P., & COLLAN, Y. (1979)
Effects of intermittent styrene inhalation, ethanol intake and
their combination on drug biotransformation in rat liver and
kidneys. Med. Biol., 57: 321-327.
EL-TANTAWY, M.A. & HAMMOCK, B.D. (1980) The effect of hepatic
microsomal and cytosolic subcellular fractions on the
mutagenic activity of epoxide-containing compounds in the
Salmonella assay. Mutat. Res., 79: 59-71.
ENGSTROM, J. (1978) Styrene in subcutaneous adipose tissue
after experimental and industrial exposure. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 119-120.
ENGSTROM, J., USTRAND, I., & WIGAEUS, E. (1978a) Exposure to
styrene in a polymerization plant. Uptake in the organism and
concentration in subcutaneous adipose tissue. Scand. J. Work
Environ. Health, 4: 324-329.
ENGSTROM, J., BJURSTROM, R., USTRAND, I., & OVRUM, P. (1978b)
Uptake, distribution and elimination of styrene in man.
Concentration in subacutaneous adipose tissue. Scand. J. Work
Environ. Health, 4: 315-323.
ENGSTROM, K. (1983) In: Aitio, A., Riihimäki, V., & Vainio,
H., ed. Biological monitoring of exposure to industrial
chemicals, New York, Hemisphere (in press).
ENGSTROM, K. & RANTANEN, J. (1974) A new gas chromatographic
method for determination of mandelic acid in urine. Int.
Arch. Arbeitsmed., 33: 163-167.
ENGSTROM, K., HARKONEN, H., PEKARI, K., & RANTANEN, J. (1978)
Evaluation of occupational styrene exposure by ambient air and
urine analysis. Scand. J. Work Environ. Health, 4 (Suppl. 2):
121-123.
ENGSTROM, K., HARKONEN, H., KALLIOKOSKI, P., & RANTANEN, J.
(1976) Urinary mandelic acid concentration after occupational
exposure to styrene and its use as a biological exposure test.
Scand. J. Work Environ. Health, 2: 21-26.
FABRY, L., LEONARD, A., & ROBERFROID, M. (1978) Mutagenicity
tests with styrene oxide in mammals. Mutat. Res., 51: 377-381.
FAITH, W.L., KLYES, D.B., & CLARK, R.L. (1975) Industrial
chemicals, 4th ed., New York, John Wiley and Sons, Inc., pp.
365-370, 779-784.
FERNANDEZ, J.G. & CAPEROS, J.R. (1977) Exposition au styrène.
I. Etude expérimentale de l'absorption et l'excrétion
pulmonaires sur des sujets humains. Int. Arch. occup.
environ. Health, 40: 1-12.
FIELDS, R.L. & HORSTMAN, S.W. (1979) Biomonitoring of
industrial styrene exposures. Am. Ind. Hyg. Assoc. J., 40:
451-459.
FINLEY, J.W. & WHITE, J.C. (1967) Two methods to determine if
styrene monomer is present in milk, Bull. environ. Contam.
Toxicol., 2(1): 41-46.
FISEROVA-BERGEROVA, V. & TEISINGER, J. (1965) Pulmonary
styrene vapor retention. Ind. Med. Surg., 34: 620-622.
FISHBEIN, L. (1979) Chromatography of environmental hazards,
Volume II, Amsterdam, Elsevier, pp. 455-469.
FLEIG, I. & THIESS, A.M. (1978) Mutagenicity study of workers
employed in the styrene and polystyrene processing and
manufacturing industry. Scand. J. Work Environ. Health, 4
(Suppl. 2): 254-258.
FLEK, J. & SEDIVEC, V. (1980) Simultaneous gas
chromatographic determination of urinary mandelic and
phenylglyoxylic acids using diazomethane derivatization. Int.
Arch. occup. environ. Health, 45: 181-188.
FLEMING, R.D. (1970) Effect of fuel composition on exhaust
emissions from a spark-ignition engine, Washington DC, US
Department of the Interior, Bureau of Mines (Report of
Investigations 7423).
FOERST, W. (1965) [Styrene.] In: Ullmans Encyklopädie der
technischen Chemie (Vol. 16), Munich, Urban et Schwarzenberg,
pp. 460-476 (in German).
FRENTZEL-BEYME, R., THEISS, A.M., & WIELAND, R. (1978) Survey
of mortality among employees engaged in the manufacture of
styrene and polystyrene at the BASF Ludwigshafen works.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 231-239.
GADALINA, I.D., KAZNINA, N.I., KUZNETSOVA, G.M., &
SMIRNISTSKII, N.S. (1969) Hygienic assessment of polyester
plastics for flooring. Hyg. Sanit., 34: 25-29.
GAMBERALE, F. & HULTENGREN, M. (1974) Exposure to styrene
II. Psychological functions. Work Environ. Health, 11: 86-93.
GAMBERALE, F., LISPER, H.O., & ANSHELM-OLSON, B. (1975)
[Effect of styrene gases on reaction time among workers in
plastic boat industry.] Arbete och Hälsa, 8: 23 pp (in
Swedish).
GAZZOTTI, G., GARATTINI, E., & SALMONA, M. (1980) Nuclear
metabolism I. Determination of styrene monooxygenase activity
in rat liver nuclei. Chem. Biol. Interact., 29: 189-195.
GOMPERTZ, D. (1978) Comments made at the end of the session.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 227.
GORDON, M. & GOODLEY, P.C. (1971) Isolation and
characterization of industrial organic pollutants in Water.
Am. Chem. Soc., Div. Water, Air, Waste Chem., Gen. Pap.,
11(1): 91-94.
GOTELL, P., AXELSSON, O., & LINDELOF, B. (1972) Field studies
on human styrene exposure. Work Environ. Health, 9: 76-83.
GREIM, H., BIMBOES, D., EGERT, G., GOGGELMAN, W., & KRAMER, M.
(1977) Mutagenicity and chromosomal aberrations as an
analytical tool for in vitro detection of mammalian enzyme-
mediated formation of reactive metabolites. Arch. Toxicol.,
39: 159-169.
GROSSMAN, I.G. (1970) Waterborne styrene in a crystalline
bedrock aquifer in the Gales Ferry Area, Ledyard, Southeastern
Connecticut. US Geol. Surv. Prof. Pap., 700-B : 203-209.
GUILLEMIN, M. & BAUER, D. (1976) Human exposure to styrene.
II. Quantitative and specific gas chromatographic analysis of
urinary mandelic and phenylglyoxylic acids as an index of
styrene exposure. Int. Arch. occup. environ. Health, 37:
57-64.
GUILLEMIN, M.P. & BAUER, D. (1978) Biological monitoring of
exposure to styrene by analysis of combined urinary mandelic
and phenylglyoxylic acids. Am. Ind. Hyg. Assoc. J., 39:
873-879.
GUILLEMIN, M.P. & BAUER, D. (1979) Human exposure to styrene.
III. Elimination kinetics of urinary mandelic and
phenylglyoxylic acids after single experimental exposure.
Int. Arch. occup. environ. Health, 44: 249-263.
GUNTER, B.J. & LUCAS, J.B. (1973) Health hazard
evaluation/toxicity determination. Cincinnati, US Department
of Health, Education and Welfare, Public Health Service,
Center for Disease Control, National Institute for
Occupational Safety and Health, 17 pp (Report No. 72-86-38 -
Gates Rubber Co. Denver, CO.).
HAMIDULLIN, R.S., FELDMAN, N.G., & VENDILO, M.V. (1968)
Hygienic assessment of articles made of PS-S suspension
polystyrene, Hyg. Sanit., 33: 331-334.
HARKONEN, H. (1977) Relationship of symptoms to occupational
styrene exposure and to the findings of electroencephalo-
graphic and psychological examinations. Int. Arch. occup.
environ. Health, 40: 231-239.
HARKONEN, H. (1978) Styrene, its experimental and clinical
toxicology. Scand. J. Work Environ. Health, 4 (Suppl. 2):
104-113.
HARKONEN, H. & HOLMBERG, P. (1982) Obstetric histories of
workers occupationally exposed to styrene. Scand. J. Work
Environ. Health, 8: 74-77.
HARKONEN, H., KALLIOKOSKI, P., HIETALA, S., & HERNBERG, S.
(1974) Concentration of mandelic and phenylglyoxylic acid in
urine as indicators of styrene exposure. Work Environ.
Health, 11: 162-165.
HARKONEN, H., LINDSTROM, K., SEPPALAINEN, A.M., ASP, S., &
HERNBERG, S. (1978) Exposure-response relationships between
styrene exposure and central nervous functions. Scand. J.
Work Environ. Health, 4: 53-59.
HEMMINKI, K. (1979) Fluorescence study of DNA alkylation by
epoxides. Chem.-biol. Interact., 28: 269-278.
HEMMINKI, K. & FALCK, K. (1979) Correlation of mutagenicity
and 4-( p-nitrobenzyl)-pyridine alkylation by epoxides.
Toxicol. Lett., 4: 103-106.
HEMMINKI, K., FRANSSILA, E., & VAINIO, H. (1980a) Spontaneous
abortion among female chemical workers in Finland. Int. Arch.
occup. environ. Health, 45: 123-126.
HEMMINKI, K., PAASIVIRTA, J., KURKIRINNE, T., & VIRKKI, L.
(1980b) Alkylation products of DNA bases by simple epoxides.
Chem. biol. Interact., 30: 259-270.
HEMMINKI, K., HEINONEN, T., & VAINIO, H. (1981) Alkylation of
guanosine and 4-( p-nitrobenzyl)-pyridine by styrene oxide
analogues in vitro . Arch. Toxicol., 49: 35-41.
HIRATSUKA, A. & WATABE, T. (1982) [Reaction of 1-vinylbenzene
3,4-oxide with ethyl mercaptan.] In: [Proceedings of the
102nd Annual Meeting for the Pharmaceutical Society of Japan,
Osaka, Japan, 3-5 April, 1982]. Japan, p. 480 (in Japanese).
HIRATSUKA, A., AIZAWA, T., OZAWA, N., ISOBE, M., WATABE, T., &
TAKABATAKE, E. (1982a) The role of epoxides in the metabolic
activation of styrene to mutagens. Eisei Kagaku, 28: 34.
HOGSTEDT, B. & MITELMAN, F. (1983) Micronuclei in cultured
lymphocytes as an indicator of genotoxic exposure. In: De
Serres, F. & Pero, R.W., ed. Individual susceptibility to
genotoxic agents in the human population, New York, Plenum
Publishing Corporation, (in press).
HOLMBERG, P.C. (1977) Central nervous defects in two children
of mothers exposed to chemicals in the reinforced plastics
industry: chance or causal relation? Scand. J. Work Environ.
Health, 3: 212-214.
HOSHIKA, Y. (1977) Gas chromatographic determination of
styrene as its dibromide. J. Chromatogr., 136: 95-103.
HUZL, F., SYKOVA, J., MAINEVOVA, J., JANKOVA, J., SRUTEK, J.,
JUNGER, V., & LAHN, V. (1967) [The question of health hazards
in working with styrene.] Prac. Lek., 19: 121-125 (in Czech).
IARC (1979) Some monomers, plastics and synthetic elastomers,
and acrolein, Lyons, International Agency for Research on
Cancer, pp. 231-274 (IARC Monographs on the evaluation of the
carcinogenic risk of chemicals to humans. Vol. 19).
IKEDA, M. & IMAMURA, T. (1974) Evaluation of hippuric,
phenylglyoxylic and mandelic acids in urine as indices of
styrene exposure. Int. Arch. Arbeitsmed., 32: 93-101.
IKEDA, M., IMAMURA, T., HAYASHI, M., TABUCHI, T., & HARA, I.
(1974) Evaluation of hippuric, phenylglyoxylic and mandelic
acids in urine as indices of styrene exposure. Int. Arch.
Arbeitsmed., 32: 93-101.
IKEDA, M., KOIZUMI, A., MIYASAKA, M., & WATABE, T. (1982)
Styrene exposure and biologic monitoring in FRP production
plants. Int. Arch. occup. environ. Health, 49: 325-339.
ISHIDATE, M., Jr & YOSHIKAWA, K. (1980) Chromosome aberration
tests with Chinese hamster cells in vitro with and without
metabolic activation - a comparative study on mutagens and
carcinogens. Arch. Toxicol., Suppl. 4: 41-44.
ISHIDATE, M., Jr, SOFUNI, T., & YOSHIKAWA, K. (1981)
Chromosomal aberration tests in vitro as a primary screening
tool for environmental mutagens and/or carcinogens. GANN
Monogr. Cancer Res., 27: 95-108.
ITI (1975) Toxic and hazardous industrial chemicals safety
manual, Tokyo, The International Technical Information
Institute, pp. 494-495.
IZJUMOVA, A.S. (1972) [The action of small concentrations of
styrol on the sexual function of albino rats.] Gig. i Sanit.,
37: 29-30 (in Russian).
IVANOVA-TCHEMICHANSKA, L., ANTOV, G., & ILIEVA, P. (1982)
[Gonadotropic action of some organic solvents.] In: Resumes
de la XVIIe Semaine, August 29-September 3, 1982, Sofia,
Bulgaria, Sofia, L. Union Médicale Balkanique, pp. 50-51 (in
French).
JACOBS, H.W. & SYRJALA, R.H. (1978) The use of infrared
analyzers for monitoring acrylonitrile. Am. Ind. Hyg. Assoc.
J., 39: 161-165.
JAMES, M.O., FOUTS, J.R., & BEND, J.R. (1975) Hepatic and
extrahepatic metabolism in vitro of an epoxide (8-14C-
styrene oxide) in the rabbit. Biochem. Pharmacol., 25:
187-193.
JAMES, S.D. & WHITE, D.A. (1967) The metabolism of phenethyl
bromide, styrene and styrene oxide in the rabbit and rat.
Biochem. J., 104: 914-921.
JENSEN, F. (1972) Determination of monomers from polystyrene
in milk products, Ann. 1st Super. Sanita, 8: 443-448.
JERMINI, C., WEBER, A., & GRANDJEAN, E. (1976) [Quantitative
determination of various gas-phase components of the side-
stream smoke of cigarettes in the room air as a contribution
to the problem of passive-smoking.] Int. Arch. occup.
environ. Health, 36: l69-181 (in German).
JERSEY, G.C., BALMER, M.F., QUAST, J.F., PARK, C.N., SCHUETZ,
D.J., BEYER, J.E., OLSEN, K.J., MCCOLLISTER, S.B., & RAMPY,
L.W. (1978) Two-Year chronic inhalation toxicity and
carcinogenicity study of monomeric styrene in rats - Final
report. (2yr), Midland, MI, Dow Chemical Co., 150 pp
(Manufacturing Chemists Association MCA No: Sty 1.1-Tox-INH).
JOHNSTONE, R.A.W., QUAN, P.M., & CARRUTHERS, W. (1962)
Composition of cigarette smoke: Some low-boiling components.
Nature (Lond.), 195: 1267-1269.
KALLIOSKI, P. (1976) [The reinforced plastic industry - a
problem work environment.] (Työterveyslaitoksen tutkimuksia
122) (in Finnish).
KALLIOSKI, P. & PFAFFLI, P. (1975) Charcoal sampling method
for determining the concentration of styrene in air. Scand.
J. Work Environ. Health, 1: 193-198.
KANKAANPAA, J.T.J., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity and teratogenicity of styrene and styrene oxide
on chick embryos enhanced by trichloropropylene oxide. Acta
Pharmacol. Toxicol., 45: 399-402.
KANKAANPAA, J.T.J., ELOVAARA, E., HEMMINKI, K., & VAINIO, H.
(1980) The effect of maternally inhaled styrene on embryonal
and foetal development in mice and Chinese hamsters. Acta
Pharmacol. Toxicol., 47: 127-129.
KAWACHI, T., YAHAGI, T., KADA, T., TAZIMA, Y., ISHIDATE, M.,
SASAKI, M., & SUGIYAMA, T. (1979) Cooperative programme on
short-term assays for carcinogenicity in Japan. In:
Montesano, R., Bartsch, H., & Tomatis, L., ed. Molecular and
cellular aspects of carcinogen screening tests, IARC
Scientific Publication No. 27, Lyons, IARC, pp. 323-330.
KAZNINA, N.I. (1969) Air pollution by volatiles liberated by
some plastics. Hyg. Sanit., 33: 267-269.
KJELLBERG, A., WIGAEUS, E., ENGSTROM, J., CSTRAND, I., &
LJUNGQUIST, E. (1979) [Long-term effects of styrene exposure
in plastic industry.] Arbete och Hälsa, 18: 1-25 (in Swedish).
KLIMKOVA-DEUTSCHOVA, E. (1962) [Neurological findings in the
plastics industry among styrene workers.] Int. Arch.
Gewerbepath. Gewerbehyg., 19: 35-50 (in German).
LEIBMAN. K.C. (1975) Metabolism and toxicity of styrene.
Environ. health Perspect., 11: 115-119.
LEIBMAN, K.C. & ORTIZ, E. (1969) Oxidation of styrene in
liver microsomes. Biochem. Pharmacol., 18: 552-554.
LEITHE, W. (1971) Analysis of air pollutants, Ann Arbor, Ann
Arbor Science Publishers, p. 246.
LEMEN, R.A. & YOUNG, R. (1976) Investigations of health
hazards in styrene butadiene rubber facilities. In: Ede, L.,
ed. Proceedings of the NIOSH Styrene-Butadiene Briefing,
Covington, Kentucky, USA, 1976, Cincinnati, Ohio, US
Department of Health, Education and Welfare pp. 3-8 (HEW
Publ. No. (NIOSH) 77-129).
LILIS, R. & NICHOLSON, W.J. (1976) Cancer experience among
workers in a chemical plant producing styrene monomers. In:
Ede, L., ed., Proceedings of the NIOSH Styrene-Butadiene
Briefing, Covington, Kentucky, USA, 1976, Cincinnati, Ohio,
Department of Health, Education and Welfare pp. 22-27 (HEW
Publ. No. (NIOSH) 77-129).
LILIS, R., LORIMER, W.V., DIAMOND, S., & SELIKOFF, I.J. (1978)
Neurotoxicity of styrene in production and polymerization
workers. Environ. Res., 15: 133-138.
LINDSTROM, K., HARKONEN, H., & HERNBERG, S. (1976)
Disturbances in psychological functions of workers
occupationally exposed to styrene. Scand. J. Work Environ.
Health, 2: 129-139.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978a)
Cytogenetic effects of styrene and styrene oxide. Mutat.
Res., 58: 277-286.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978b)
Cytogenetic effects of styrene and styrene oxide on human
lymphocytes and Allium cepa. Scand. J. Work Environ. Health,
4 (Suppl. 2): 156-162.
LITTON BIONETICS (1980) Toxicological study on styrene
incorporated in drinking water of rats for two years in
conjuction with a three-generation reproduction study.
(Submitted to Chemical Manufacturers Association.)
LOPRIENO, N. (1981) Environmental mutagens and comparative
short-term mutagenicity studies. In: Molecular bases of
genetic processes. Proceedings of the XIV International
Congress of Genetics, Moscow, MIR Publishers, Vol. III, Book
1, pp. 164-180.
LOPRIENO, N. & ABBONDANDOLO, A. (1980) Comparative mutagenic
evaluation of some industrial compounds. In: Norpoth, K.H. &
Garner, R.C., ed. Short-term test systems for detecting
carcinogens, Berlin, Springer-Verlag, pp. 333-356.
LOPRIENO, N., ABBONDANDOLO, A., BARALE, R., BARONCELLI, S.,
BONATTI, S., BRONZETTI, G., CAMMELLINI, A., CORSI, C., CORTI,
G., FREZZA, D., LEPORINI, C., MAZZACCARO, A., NIERI, R.,
ROSELLINI, R., & ROSSI, A.M. (1976) Mutagenicity of
industrial compounds: styrene and its possible metabolite
styrene oxide. Mutat. Res., 40: 317-324.
LOPRIENO, N., PRESCIUTTINI, S., SBRANA, I., STRETTI, G.,
ZACCARO, L., ABBONDANDOLO, A., BONATTI, S., FIORIO, R., &
MAZZACCARO, A. (1978) Mutagenicity of industrial compounds
VII. Styrene and styrene oxide: II. Point mutations,
chromosome aberrations and DNA repair induction analyses.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 169-178.
LORIMER, W.V., LILIS, R., NICHOLSON, W.J., ANDERSON, H.,
FISCHBEIN, A. DAUM, S., ROM, W., RICE, C., & SELIKOFF, I.J.
(1976) Clinical studies of styrene workers: Initial
findings. Environ. health Perspect., 17: 171-181.
LORIMER, W., LILIS, R., FISCHBEIN, A., DAUM, S., ANDERSON, H.,
WOLFF, M., & SELIKOFF, I. (1978) Health status of
styrene-polystyrene polymerization workers. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 220-226.
LUNDBERG, I. (1981) [Serumenzyme levels in workers exposed to
styrene.] Arbete och Hälsa, 1: 19 pp (in Swedish).
MAIER, A. RUHE, R., TOSENSTEEL, R., & LUCAS, J.B. (1974)
Health hazard evaluation/toxicity determintion. Arco Polymer
Incorporated (Sinclair-Koppers Company, Inc.), Monaco, PA.,
Cincinnati, US Department of Health, Education, and Welfare,
Center for Disease Control, National Institute for
Occupational Safety and Health, 28 pp (Report No. 72-90--107).
MALTONI, C., FAILLA, G., & KASSAPIDIS, G. (1979) First
experimental demonstration of the carcinogenic effects of
styrene oxide. Long-term bioassays on Spraque-Dawley rats by
oral administration. Med. Lav., 70(5): 358-362.
MANSON, N.W., REILLY, D.A., & STAGG, H.E. (1965) The
determination of toxic substances in air, manual of ICI
practice, Cambridge, W. Heffer & Sons, p. 181.
MARNIEMI, J., SUOLINNA, E.M., KAARTINEN, N., & VAINIO, H.
(1977) Covalent binding of styrene oxide to rat liver
macromolecules in vivo and in vitro. In: Ullrich, V., Roots,
I., Hildebrandt, A., Estabrook, R.W., & Conney, A.H., ed.
Microsomes and drug oxidations, London, Oxford, Pergamon
press, pp. 698-702.
MATSUOKA, A., HAYASHI, M., & ISHIDATE, M., Jr (1979)
Chromosomal aberration tests on 29 chemicals combined with S9
mix in vitro. Mutat. Res., 66: 277-290.
MATSUSHITA, T., MATSUMOTO. T., MIYAGAKI, J., MAEDA, K.,
TAKEUCHI, Y., & KATAJIMA, J. (1968) [Nervous disorders
considered to be symptoms of chronic styrol poisoning.
Saigai Igaku, 11: 173-179 (in Japanese).
McMICHAEL, A.J., SPIRTAS, R., GAMBLE, J.F., & TOUSEY, P.M.
(1976) Mortality among rubber workers: relationship to
specific jobs. J. occup. Med., 18: 178-185.
McNAIR, H.M. & BONELLI, E.J. (1968) Basic Gas Chromatography,
5th Ed., Walnut Creek, Varian Aerograph., p. 101.
MEINHARDT, T.J., YOUNG, R.J., & HARTLE, R.W. (1978)
Epidemiologic investigations of styrene-butadiene rubber
production and reinforced plastics production. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 240-246.
MERETOJA, T. & VAINIO, H. (1979) The use of human lymphocyte
tests in the evaluation of potential mutagens: clastogenic
activity of styrene in occupational exposure. In: Berg, K.,
ed. Genetic damage in man caused by environmental agents, New
York, Academic Press, pp. 213-225.
MERETOJA, T., VAINIO, H., SORSA. M., & HARKONEN, H. (1977)
Occupational styrene exposure and chromosomal aberrations.
Mutation Res., 56: 193-197.
MERETOJA, T., JARVENTAUS, H., SORSA, M., & VAINIO, H. (1978a)
Chromosome aberrations in lymphocytes of workers exposed to
styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
259-264.
MERETOJA, T., VAINIO, H., & JARVENTAUS, H. (1978b)
Clastogenic effects of styrene exposure on bone marrow cells
of rat. Toxicol. Lett., 1: 315-318.
MILVY, P. & GARRO, A.J. (1976) Mutagenic activity of styrene
oxide (1,2-epoxyethylbenzene), a presumed styrene metabolite.
Mutat. Res., 40: 15-18.
MITHEN, F.A., COCHRAN, M., JOHNSON, M.I., & BUNGE, R.P. (1980)
Neurotoxicity of polystyrene containers detected in a closed
tissue culture system. Neurosci. Lett., 17: 107-111.
MURRAY, F. J., JOHN, J. A., BALMER, M. F., & SCHWELTZ, B. A.
(1978) Teratologic evaluation of styrene given to rats and
rabbits by inhalation or by gavage, Toxicology, 11: 335-343.
NELIGAN, R.E., LEONARD, M.J., & BRYAN, R.J. (1965) The gas
chromatographic determination of aromatic hydrocarbons in the
atmosphere. Am. Chem. Soc. Div. Water, Air. Waste Chem.
Preprints, 5: 118-121.
NICHOLSON, W.J., SELIKOFF, I.J., & SEIDMAN, H. (1978)
Mortality experience of styrene-polystyrene polymerization
workers. Initial findings. Scand. J. Work Environ. Health, 4
(Suppl. 2): 247-252.
NIOSH (1974) NIOSH manual of analytical methods, Cincinnati,
National Institute for Occupational Safety and Health, (Method
No. 127).
NORPPA, H. (1981a) Chromosome damage induced by styrene,
styrene oxide and some analogues, Academic dissertation,
Helsinki, Institute of Occupational Health and University of
Helsinki, 56 pp.
NORPPA, H. (1981b) Styrene and vinyltoluene induce
micronuclei in mouse bone marrow. Toxicol. Lett., 8: 247-251.
NORPPA, H. & VAINIO, H. (1983a) Genetic toxicity of styrene
and some of its derivatives. Scand. J. Work Environ. Health,
9: 108-114.
NORPPA, H. & VAINIO, H. (1983b) Induction of sister-chromatid
exchanges by styrene analogues in cultured human lymphocytes.
Mutat. Res., 116: 379-387.
NORPPA, H., ELOVAARA, E., HUSGAFVEL-PURSIAINEN, K., SORSA, M.,
& VAINIO, H. (1979) Effects of styrene oxide on chromosome
aberrations, sister chromatid exchange and hepatic drug
biotransformation in Chinese hamsters in vivo. Chem.-biol.
Interact., 26: 305-315.
NORPPA, H., SORSA, M., PFAFFLI, P., & VAINIO, H. (1980a)
Styrene and styrene oxide induce SCEs and are metabolised in
human lymphocyte cultures. Carcinogenesis, 1: 357-361.
NORPPA, H., SORSA, M., & VAINIO, H. (1980b) Chromosomal
aberrations in bone marrow of Chinese hamsters exposed to
styrene and ethanol. Toxicol. Lett., 5: 241-244.
NORPPA, H., VAINIO, H., SORSA, M., & BELVEDERE, G. (1982)
Metabolic activation of styrene by erythrocytes in human
lymphocyte cultures. In: 12th Annual Meeting of the European
Environmental Mutagen Society, Espoo, Finland, 20-24 June,
1982, Abstracts, Helsinki, Institute of Occupational Health,
p. 148.
NYLANDER, P. (1979) [LSE polyesters - environmental
effects.] Plastforum Scandinavia, 9: 130-132 (in Swedish).
ODKVIST, L.M., CSTRAND, I., LARSBY, B., & KALL, C. (1980)
[Does styrene incude disturbancies in the human balance
mechanisms?] Arbete och Hälsa, 2: 5-19 (in Swedish).
OESCH, F. (1973) Mammalian epoxide hydrolase. Inducable
enzyme catalyzing the inactivation of carcinogenic and
cytotoxic metabolites derived from aromatic and olefinic
compounds. Xenobiotica, 3: 305-360.
OGATA, M. & SUGIHARA, R. (1978) High performance liquid
chromatographic procedure for quantitative determination or
urinary phenylglyoxylic, mandelic and hippuric acids as
indices of styrene exposure. Int. Arch. occup. environ.
Health, 42: 11-19.
OHTSUJI, H. & IKEDA, M. (1970) A rapid colorimetric method
for the determination of phenylglyoxylic and mandelic acids.
Its application to the urinanalysis of workers exposed to
styrene vapour. Br. J. ind. Med., 27: 150-154.
OHTSUJI, H. & IKEDA, M. (1971) The metabolism of styrene in
rat and the stimulatory effect of phenobarbital. Toxicol.
appl. Pharmacol., 18: 321-328.
OLTRAMARE, M., DESBAUMES, E., IMHOFF, C., & MICHIELS, W.
(1974) Toxicologie du styrene monomère. Geneva, Editions
Mèdicine et Hygiène, 100 pp.
OTT, M.G., KOLESAR, R.C., SCHARNWEBER, H.C., SCHNEIDER, E.J.,
& VENABLE, J.R. (1980) A mortality survey of employees
engaged in the development or manufacture of styrene-based
products. J. occup. Med., 22: 445-460.
PACHECKA, J., GARIBOLDI, P., CANTONI, L., BELVEDERE, G.,
MUSSINI, E., & SALMONA, M. (1979) Isolation and structure
determination of enzymatically formed styrene oxide
glutathione conjugates. Chem. Biol. Interact., 27: 313-321.
PAGANO, D.A., YAGEN, B., HERNANDEZ, O. BEND, J.R., & ZEIGER,
E. (1982) Mutagenicity of ( R) and ( S) styrene 7,8-oxide and
the intermediary mercapturic acid metabolites formed from
styrene 7,8-oxide. Environ. Mutag., 4(5): 575-584.
PAGANO, G., ESPOSITO, A., GIORDANO, G.G., & HAGSTROM, B. E.
(1978) Embryotoxic and teratogenic effects of styrene
derivatives on sea urchin development. Scand. J. Work,
Environ. Health, 4 (Suppl. 2): 136-141.
PANTAROTTO, E., FANELLI, R., BIDOLI, F., MANZONI, P., SALMONA,
M., & SZCZAWINSKI, K. (1978) Areneoxides in styrene
metabolism. New perspective in styrene toxicity? Scand. J.
Work Environ. Health, 4 (Suppl. 2): 67-77.
PANTAROTTO, C., SALMONA, M., SZCZAWINSKA, K., & BIDOLI, F.
(1980) Gas chromatography-mass spectrometric studies on
styrene toxicity. In: Albatges, J., ed. Analytical techniques
in environmental chemistry, New York, Pergamon Press, pp.
245-279.
PARKKI, M.G. (1978) The role of glutathione in the toxicity
of styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
53-59.
PARKKI, M.G., MARNIEMI, J., & VAINIO, H. (1976) Action of
styrene and its metabolites styrene oxide and styrene glycol
on activities of xenobiotic biotransformation enzymes in rat
liver in vivo. Toxicol. appl. Pharmacol., 38: 59-70.
PENTTILA, M., SORSA, M., & VAINIO, H. (1980) Inability of
styrene to induce nondisjunction in Drosophila or a positive
micronucleus test in the Chinese hamster. Toxicol. Lett., 6:
119-123.
PERO, R.W., BRYNGELSSON, T., HOGSTEDT, B., & CKESON, B. (1982)
Occupational and in vitro exposure to styrene assessed by
unscheduled DNA synthesis in resting human lymphocytes.
Carcinogenesis, 3: 681-685.
PERVIER, J.W., BARLEY, R.C., FIELD, D.E., FRIEDMAN, B.M., &
MORRIS, R.B. (1974) Survey reports on atmospheric emissions
from the petrochemical industry, Vol. IV. 287 pp (US NTIS, PB
Rep. PB-245630).
PFAFFLI, P., HESSO, A., VAINIO, H., & HYVONEN, M. (1981)
4-Vinylphenol excretion suggestive of arene oxide formation in
workers occupationally exposed to styrene. Toxicol. appl.
Pharmacol., 60: 85-90.
PHILIPPE, R., LAUWERYS, R., BUCHET, J.P., ROELS, H., & DEFELD,
J.M. (1974) [Evaluation of the exposure of workers to styrene
by means of the determination of its urinary metabolites:
mandelic and phenylglyoxylic acids: II. Application to
polyester fiber workers.] Arch. Mal. Prof. Méd. Tra. Secur.
Soc., 35: 631-640.
PLANCHE, G., CROISY, A., MALAVEILLE, C., TOMATIS, L., &
BARTSCH, H. (1979) Metabolic and mutagenicity studies on DDT
and 15 derivatives. Detection of 1,1-bis(p-chlorophenyl)-2,2-
dichloroethane and 1,1-bis(p-chlorophenyl)-2,2,2-trichloro-
ethyl acetate (kelthane acetate) as mutagens in Salmonella
typhimurium and of 1,1-bis(p-chlorophenyl)ethylene oxide, a
likely metabolite, as an alkylating agent. Chem. biol.
Interact., 25: 157-175.
POGGI, G., GUISIANI, M., PALAGI, U., PAGGIARO, P.L., LOI,
A.M., DAZZI, F., SICLARI, C., & BASCHIERI, L. (1982) High-
performance liquid chromatography for the quantitative
determination of the urinary metabolites of toluene, xylene,
and styrene. Int. Arch. occup. environ. Health, 50: 25-31.
PONCELET, F., DE MEESTER, C., DUVERGER-VAN BOGAERT, M.,
LAMBOTTE-VANDEPAER, M., ROBERTFROID, M., & MERCIER, M. (1980)
Influence of experimental factors on the mutagenicity of
vinylic monomers. Arch. Toxicol., Suppl. 4: 63-66.
PONOMAREVA, N.I. & ZLOBINA, N.S. (1972) [Working conditions
and the state of the upper respiratory tract in workers
engaged in the production of block and emulsion polystyrene
and its copolymers.] Gig. Tr. Prof. Zabol., 15: 22-26 (in
Russian).
PONOMARKOV, V. & TOMATIS, L. (1978) Effects of long-term oral
administration of styrene to mice and rats. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 127-135.
PONOMARKOV, V., CABRAL, J.R.P., WAHRENDORF, J., & GALENDO, D.
(1983) A carcinogenicity study of styrene oxide in rats.
Toxicolgist, 3: 46 (Abstract No. 184).
QUAST, J.F., KALNINS, R.P., OLSON, K.J., HUMISTON, C.G.,
MURRAY, F.J., JOHN, J.A., & SCHWETZ, B.A. (1978) Results of a
toxicity study in dogs and teratogenicity studies in rabbits
and rats administered monomeric styrene. In: Meeting of the
Society of Toxicology, San Francisco, USA, March 1978.
QUAST, J.F., HUMISTON, C.G., KALNINS, R.V., OLSON, K.J.,
McCOLLISTER, S.B., WADE, C.E., BEYER, J.E., & SCHWETZ, B.A.
(1979) Results of a toxicity study of monomeric styrene
administered to beagle dogs by oral intubation for 19 months -
Midland, MI, Dow Chemical Co., 199 pp (MCA (Manufacturing
Chemists Association) No: Sty 1.2-Tox-Gav-Dow.)
RAGYL'YE, N. (1974) [Problems concerning the embryotropic
effects of styrene.] Gig. i Sanit., 11: 65-66 (in Russian).
RAMSEY, J.C. & YOUNG, J.D. (1978) Pharmacokinetics of inhaled
styrene in rats and humans. Scand. J. Work Environ. Health, 4
(Suppl. 2): 84-91.
RAMSEY, J.C., YOUNG, J.D., KARBOWSKI, R.J., CHENOWETH, M.B.,
MCCARTY, L.P., & BRAUN, W.H. (1980) Pharmacokinetics of
inhaled styrene in human volunteers. Toxicol. appl.
Pharmacol., 53: 54-63.
RENKE, W. & CHEMIELEWSKI, J. (1976) Blood coagulation and
fibronolysis in occupational exposure to some chemical
factors. Biul. Inst. Med. Morskiej, 27: 289-298.
RIIHIMAKI, V. & PFAFFLI, P. (1978) Percutaneous absorption of
solvent vapors in man. Scand. J. Work Environ. Health, 4:
73-85.
ROSEN, A.A., SKEEL, R.T., & ETTINGER, M.B. (1963)
Relationship of river water odor to specific organic
contaminants. J. Water Pollut. Control Fed., 35: 777-82.
ROSEN, I., HAEGER-ARONSEN, B., REHNSTROM, S., & WELINDER, H.
(1978) Neurophysiological observations after chronic styrene
exposure. Scand. J. Work Environ. & Health, 4 (Suppl. 2):
184-194.
ROSENSTEEL, R.E. & MEYER, C.R. (1977) Health hazard
evaluation determination. Cincinnati, US Department of
Health, Education and Welfare, Public Health Service, Center
for Disease Control, National Institute for Occupational
Safety and Health. 52 pp (Report no. 75-150-378 - Reinell
Boats, Inc., Poplar Bluff, Missouri).
ROTH, B. & KLIMKOVA-DEUTSCHOVA, E. (1963) The effect of the
chronic action of industrial poisons on the electro-
encephalogram of man. Rev. Czech. Med., 9: 217-227.
ROTH, S.H. (1979) Physical mechanisms of anesthesia. Ann.
Rev. Pharmacol. Toxicol., 19: 159-178.
SAALWAECHTER, A.T., McCAMMON, C.S., ROPER, C.P. Jr, &
CARLBERG, K.S. (1977) Performance testing of the NIOSH
charcoal tube technique for the determination of air
concentrations of organic vapors. Am. Ind. Hyg. Assoc. J.,
38(9): 476-486.
SANTODONATO, J., MEYLAN, W.M., DAVIS, L.N., HOWARD, P.H.,
ORZEL, D.M., & BOGYO, D.A. (1980) Investigation of selected
potential environmental contaminants: Styrene, ethyl benzene
and related compounds. 261 pp (A report prepared for the
Office of Toxic Substances, US Environmental Protection
Agency, Washington, DC by Syracuse Research Corporation;
Contract No. 68-01-3250.)
SAUERHOFF, M.W. & BRAUN, W.H. (1976) The fate of styrene in
rats following an inhalation exposure to 14 C-styrene.
Midland, Michigan, Toxicology Research Laboratory, Health and
Environmental Research, Dow Chemical USA, 26 pp.
SAUERHOFF, M.W., MADRID, E.O., & BRAUN, W.H. (1976) The fate
of orally administered styrene in rats. Midland, Michigan,
Toxicology Research Laboratory, Health and Environmental
Research, Dow Chemical USA, 44 pp.
SAVOLAINEN, H. & PFAFFLI, P. (1977) Effects of chronic
styrene inhalation on rat brain protein metabolism. Acta
Neuropath., 40: 237-241.
SAVOLAINEN, H. & PFAFFLI, P. (1978) Accumulation of styrene
monomer and neurochemical effects of long-term inhalation
exposure in rats. Scand. J. Work Environ. Health, 4 (Suppl.
2): 78-83.
SAVOLAINEN, H. & VAINIO, H. (1977) Organ distribution and
nervous system binding of styrene and styrene oxide.
Toxicology, 8: 135-141.
SAVOLAINEN, H., HELOJOKI, M., & TENGEN-JUNNILA, M. (1980)
Behavioural and glial cell effects of inhalation exposure to
styrene vapour with special reference to interactions of
simultaneous ethanol intake. Acta Pharmacol. Toxicol., 46:
51-56.
SBRANA, I., LASCIALFARI, D., ROSSI, A.M., & LOPRIENO, N.
(1982) Bone marrow cell chromosomal aberrations and styrene
biotransformation in mice given styrene on a repeated oral
schedule. Submitted for publication.
SCHALLER, K.-H., GOSSLER, K., BOST, H.-P., & VALENTIN, H.
(1976) [Gas chromatographic methods for the determination of
styrene in the blood and of mandelic acid and phenylglyoxylic
acid in the urine.] Arbeits-, Sozial-, Präventivmedizin, 11:
64-64 (in German).
SCHNEIDER, H. & SEEBER, A. (1979) [Psychodiagnostics in the
understanding of neurotoxic effects of harmful chemical
substances.] Z. Psychol. Bd., 187: 178-205 (in German).
SCHOFIELD, K. (1974) Problems with flame ionization detectors
in automotive exhaust hydrocarbon measurements. Environ. Sci.
Technol., 8: 826-834.
SCHUMACHER, R.L., BREYSSE, P.A., CARLYON, W.R., HIBBARD, R.P.,
& KLEINMAN, G.D. (1981) Styrene exposure in the fiberglass
fabrication industry in Washington State. Am. Ind. Hyg.
Assoc. J., 42: 143-149.
SEPPALAINEN, A.M. (1978) Neurotoxicity of styrene in
occupational and experimental exposure. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 181-183.
SEPPALAINEN, A.M. & HARKONEN, H. (1976) Neurophysiological
findings among workers occupationally exposed to styrene.
Scand. J. Work Environ. Health, 2: 140-146.
SEPPALAINEN, A.M., LINDSTROM, K., & MARTELIN, T. (1980)
Neurophysiological and psychological picture of solvent
poisoning. Am. J. ind. Med., 1: 31-42.
SEUTTER-BERLAGE, F., DELBRESSINE, L.P.C., SMEETS, F.L.N., &
KETELAARS, H.C.J. (1978) Identification of three sulphur-
containing urinary metabolites of styrene in the rat.
Xenobiotica, 8: 413-418.
SHUGAEV, B.B. (1969) Concentration of hydrocarbons in tissues
as a measure of toxicity. Arch. environ. Health, 18: 878-882.
SIMKO, A., JINDRICHOVA, J., & PULTAROVA, H. (1966) [The
effect of styrene on the health state of workers employed in
laminate-production.] Prac. Lek., 18: 348-352 (in Czech).
SINITSKIJ, V.G. (1969) [Indexes for immunological reactivity
in rabbits during the long-term exposure to small doses of
styrene.] Gig. Primen. Polim. Mater, Izdelii Nikh, 1: 394-398
(in Russian).
SLOB, A. (1973) A new method for determination of mandelic
acid excretion at low level styrene exposure. Br. J. ind.
Med., 30: 390-393.
SMIRNOVA, E.T. & YATAKOVA, Z.M. (1966) Health hazard of
polystyrene toys. Hyg. Sanit., 31: 135-137.
SMITH, A.H. & ELLIS, L. (1977) Styrene butadiene rubber
synthetic plants and leukaemia. J. occup. Med., 19: 441.
SMITH, H.O. & HOCHSTETTLER, A.D. (1969) Determination of odor
thresholds in air using C14-labeled compounds to monitor
concentrations. Environ. Sci. Technol., 3: 169-170.
SODER, S.L. (1977) "Styrene" Chemical economics handbook,
California, Stanford Research Institute.
SOLLENBERG, J. & BALDESTEN, A. (1977) Isotachophoretic
analysis of mandelic acid, phenylglyoxylic acid, hippuric acid
and methylhippuric acid in urine after occupational exposure
to styrene, toluene and/or xylene. J. Chromatogr., 132:
469-476.
SORSA, M., HYVONEN, M., JARVENTAUS, H., & VAINIO, H. (1979)
Chromosomal aberrations and sister chromatid exchange in
children of women in reinforced plastic industry. In:
Symposium on Toxicology, Abstracts, Turku, Finland 29-20 May,
1979, Turku, University of Turku, p. 16.
SPASOVSKI, M. (1976) Health hazards in the production and
processing of some fibers, resins, and plastics in Bulgaria.
Environ. Health Perspect., 17: 199-202.
SPASOVSKI, M., HINKOVA, L., & BENCHEV, I. (1980) Exposure to
styrene - field studies. Toxicol. Lett., (suppl. 1): 218.
SPENCER, H.C., IRISH, D.D., ADAMS, E.M., & ROWE, V.K. (1942)
The response of laboratory animals to monomeric styrene. J.
ind. Hyg. Toxicol., 24: 295-301.
SPINAZZOLA et al. (1980) Petrolchimica. Tecnologia, ambiente
di lavoro, prevenzione e patologia. Atti del 43° Congresso
Nazionale della Società Italiana di Medicina del Lavoro ed
Igiene Industriale. Parma, Edizioni Tecnografica, Vol. 2.
SPIRTAS, R., VAN ERT, M., GAMBLE, J.F., WOLF, P., & McMICHAEL,
A.J. (1976) Toxicologic, industrial hygiene and
epidemiological consideration in the possible association
between SBR manufacturing and neoplasms of the lymphatic and
hematopoietic tissues. In: Ede, L., ed. Proceedings of NIOSH
Styrene-Butadiene Briefing, Covington, Kentucky, USA, 1976,
Cincinnati, Ohio, Department of Health, Education and Welfare,
pp. 67-112 (HEW Publ. No. (NIOSH) 77-129).
SRAM, R.J. (1981) Cytogenetic analysis of peripheral
lymphocytes as a method for monitoring environmental levels of
mutagens. In: Gut, I., Cikrt, M., & Plaa, G.L., ed.
Industrial and environmental xenobiotics. Metabolism and
pharmacokinetics of organic chemicals and metals., Berlin,
Springer-Verlag, pp. 187-194.
STEWART, R.D., DODD, H.C., BARETTA, E.D., & SCHAFFER, A.W.
(1968) Human exposure to styrene vapor. Arch. environ.
Health, 16: 656-662.
STOLZ, D.R. & WITHEY, R.J. (1977) Mutagenicity testing of
styrene and styrene epoxide in Salmonella typhimurium. Bull.
environ. contamin. Toxicol., 17: 739-742.
SUGIURA, K. & GOTO, M. (1981) Mutagenicities of styrene oxide
derivatives on bacterial test systems: relationship between
mutagenic potencies and chemical reactivity. Chem. biol.
Interact., 35: 71-91.
SUGIURA, K., KIMURA, T., & GOTO, M. (1978a) Mutagenicities of
styrene oxide derivatives on Salmonella typhimurium (TA100).
Relationship between mutagenic potencies and chemical
reactivity. Mutat. Res., 58: 159-165.
SUGIURA, K., YAMANAKA, S., FUKUSAWA, S., & GOTO, M. (1978b)
The mutagenicity of substituted and unsubstituted styrene
oxides in E. coli: Relationship between mutagenic potencies
and physico-chemical properties. Chemosphere, 7: 737-742.
SUGIURA, K., MAEDA, A., & GOTO, M. (1979) Substitutional
effects of styrene oxides on survival and mutation induction
in cultured Chinese hamster cells (V-79). Chemosphere, 8:
369-372.
SYNTHETIC RESINS LIMITED (1980) LSE capability with good
interlaminal adhesion. Reinf. Plast., 3: 72-73.
TAULBEE, J., ANDJELKOVIC, D., WILLIAMS, T., GAMBLE, J.F., &
WOLF, P. (1976) A study of possible associations between
exposure to SBR processes and industrial hygiene and
epidemiologic considerations (for workers in the 1951 and 1964
cohorts and deaths 1964-1973). In: Ede, L., ed. Proceedings
of NIOSH Styrene-Butadiene Briefing, Covington, Kentucky, USA,
1976, Cincinnati, Ohio, Department of Health, Education and
Welfare, pp. 113-162 (HEW Publ. No. (NIOSH) 77-129).
TERAMOTO, K., HORIGUCHI, S., & KITIBATAKE, S. (1978) [Studies
on industrial styrene poisoning. (Part VIII). Distribution and
elimination of styrene in rats exposed to styrene.] Jpn. J.
ind. Health, 20(2): 118-119 (in Japanese).
THEISS, A.M. & FLEIG, I. (1978) Chromosome investigations on
workers exposed to styrene/polystyrene. J. occup. Med., 20:
747-749.
THIESS, A.M. & FRIEDHEIM, M. (1978) Morbidity among persons
employed in styrene production, polymerization and processing
plants. Scand. J. Work Environ. Health, 4 (Suppl. 2): 203-214.
THIESS, A.M. & FRIEDHEIM, M. (1979) [Morbidity study in
coworkers of the polyester laboratory and of the technical
service exposed to styrene.] Zentralbl. Arbeitsmed., 9:
238-241 (in German).
THIESS, A.M., SCHWEGLER, H., & FLEIG, I. (1980) Chromosome
investigations in lymphocytes of workers employed in areas in
which styrene-containing unsaturated polyester resins are
manufactured. Am. J. ind. Med., 1: 205-210.
THOMPSON, B. (1974) Hazardous gases and vapors: Infrared
spectra and physical constants, Fullerton, Beckman
Instruments, Inc., pp. 154, 255 (Technical report 595).
TOLA, S., HARKONEN, H., KORKALA, M.L., & JARVINEN, E. (in
press) Mortality of workers exposed to styrene. Egypt. J.
occup. Med., (in press).
TOSSAVAINEN, A. (1978) Styrene use and occupational exposure
in the plastics industry. Scand. J. Work Environ. Health, 4
(Suppl. 2): 7-13.
TURCHI, G., BONATTI, S., CITTI, L., GERVASI, P.G.,
ABBONDANDOLO, A., & PRESCIUTTINI, S. (1981) Alkylating
properties and genetic activity of 4-vinylcyclohexane
metabolites and structurally related epoxides. Mutat. Res.,
83: 419-430.
US EPA (1980) Investigation of Selected Potential
Environmental Contaminants: Styrene Ethylbenzene, and Related
Compounds. Washington DC, US Environmental Protection Agency,
261 pp (Publication No. EPA 5601 11-80---018).
US NATIONAL CANCER INSTITUTE (1979) Bioassay of styrene for
possible carcinogenicity. Washington, DC, Department of
Health, Education and Welfare, 46 pp (Publ. No. (NIH)
79-1741, Washington DC, (Techn. Rep. ser. No. 185)).
VAINIO, H. (1978) Vinyl chloride and vinyl benzene (styrene)-
metabolism, mutagenicity and carcinogenicity. Chem.-biol.
Interact., 22: 117-124.
VAINIO, H. & MAKINEN, A. (1977) Styrene and acrylonitrile
induced depression of hepatic nonprotein sulfhydryl content in
various rodent species. Res. Commun. Chem. Pathol.
Pharmacol., 17: 115-124
VAINIO, H., PAAKKONEN, R., RONNHOLM, K., RAUNIO, V., &
PELKONEN, O. (1976) A study on the mutagenic activity of
styrene and styrene oxide. Scand. J. Work Environ. Health, 3:
147-151.
VAINIO, H., HEMMINKI, K., & ELOVAARA, E. (1977) Toxicity of
styrene and styrene oxide on chick embryos. Toxicology, 8:
319-325.
VAINIO, H., JARVISALO, J., & TASKINEN, E. (1979) Adaptive
changes caused by intermittent styrene inhalation on
xenobiotic biotransformation. Toxicol. appl. Pharmacol., 49:
7-14.
VAINIO, H., NORPPA, H., & BELVEDERE, G. (1982) The role of
cytochrome P-450 mediated metabolism in SCEs induced by
styrene in human lymphocytes. In: Proceedings of the l3th
International Cancer Congress, Abstracts, Seattle, USA, 8-l5
September, l982, Seattle, International Union Against Cancer,
p. 118.
VALENTA, J. (1966) [Air and water pollution by styrene with
the butadiene-styrene rubber production.] Cesk. Hyg., 11:
349-352 (in Czech).
VAN ANDA, J., SMITH, B.R., FOUTS, J.R., & BEND, J.R. (1979)
Concentration-dependent metabolism and toxicity of [14C
styrene oxide in the isolated perfused rat liver. J.
Pharmacol. exp. Ther., 211: 207-211.
VAN DUUREN, B.L., NELSON, N., ORRIS, L., PALMES, E.D., &
SCHMITT, F.L. (1963) Carcinogenicity of epoxides, lactones
and peroxy compounds. J. Natl Cancer Inst., 31: 41-55.
VARNER, S.L. & BREDER, C.V. (1981a) Liquid chromatographic
determination of residual styrene in polystyrene food
packaging. J. Assoc. Off. Anal. Chem., 64: 647-652.
VARNER, S.L. & BREDER, C.V. (1981b) Head space sampling and
gas chromatographic determination of styrene migration from
food contact polystyrene cups into beverages and food
simulants. J. Assoc. Off. Anal. Chem., 64: 1122-1130.
VERGIEVA, T. & ZAJKOV, H. (1981) [Behavioural changes in rats
with inhalation styrene effect.] Hyg. Zdrav., 24: 242-247 (in
Bulgarian).
VERGIEVA, T., ZAIKOV, Hr., & PALATOV, S. (1979) [A study on
the embryotoxic action of styrol.] Hyg. Zdrav., XXII: 39-43
(in Russian).
VIHKO , R., VIHKO, P., MAENTAUSTA, O., PAKARINEN, A., JANNE,
O., & YRJANHEIKKI, E. (1983) Assessment of early
hepatotoxicity. In: Aitio, A., Riihimäki, V., & Vainio, H.,
ed. Biological monitoring and surveillance of workers exposed
to chemicals, Washington, Hemisphere Publ. Co. (in press).
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J.J.A.A. (1981)
The mutagenic action of aliphatic epoxides. Mutat. Res., 89:
269-282.
VOSKAMP, A.J. & STUDENBERG, J.E. (1981) A new low styrene
emission laminating resin. In: 36th Annual Conference,
Reinforced Plastics/Composites Institute, the Society of the
Plastics Industry, Inc., February 16-20, 1981, Session 19-D.
WADE, D.R., AIRY, S.C., & SINSHEIMER, J.E. (1978)
Mutagenicity of aliphatic epoxides. Mutat. Res., 58: 217-223.
WATABE, T., ISOBE, M., SAWAHATA, T., YOSHIKAWA, K., YAMADA,
S., & TAKABATAKE, E. (1978) Metabolism and mutagenicity of
styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
142-155.
WATABE, T., OZAWA, N., & YOSHIKAWA, K. (1981a)
Stereochemistry in the oxidative metabolism of styrene by
hepatic microsomes. Biochem. Pharmacol., 30: 1695-1698.
WATABE, T., HIRATSUKA, A., OZAWA, N., & ISOBE, M. (1981b)
Glutathione S-conjugates of phenyloxirane. Biochem.
Pharmacol., 30: 390-392.
WATABE, T., HIRATSUKA, A., AIZAWA, T., & SAWAHATA, T. (1982a)
1-vinylbenzene 1,2- and 3,4-oxides. Tetrahedron Lett., 23:
1185-1188.
WATABE, T., HIRATSUKA, A., AIZAWA, T., SAWAHATA, T., OZAWA,
N., ISOBE, M., & TAKABATAKE, E. (1982b) Studies on the
metabolism and toxicity of styrene IV. 1-vinylbenzene
3,4-oxide, a potent mutagen formed as a possible intermediate
in the metabolism in vivo of styrene to 4-vinylphenol. Mutat.
Res., 93: 45-55.
WATABE, T., OZAWA, N., & YOSHIKAWA, K. (1982c) Studies on
metabolism and toxicity of styrene. V. The metabolism of
styrene, racemic, ( R)-(+), and ( S)-(-) -phenyloxiranes in the
rat. J. Pharm. Dyn., 5: 129-133
WATANABE, T. ENDO, A., SATO, K., OHTSUKI, T., MIYASAKA, M.,
KOIZUMI, A., & IKEDA, M. (1981) Mutagenic potential of
styrene in man. Ind. Health, 19: 37-45.
WATANABE, T., ENDO; A., KUMAI, M., & IKEDA, M. (1983)
Chromosome aberrations and sister chromatid exchanges in
styrene-exposed workers with reference to their smoking
habits. Environmental Mutagenesis, 5: 299-309
WEAST, R.C. & ASTLE, M.J. ed. (1981) Handbook of chemistry
and physics, Boca Raton, CRS Press, p. c-500.
WEIL, C.S., CONDRA, N., HAUN, C., & STRIEGEL, J.A. (1963)
Experimental carcinogenicity and acute toxicity of
representative epoxides. Am. Ind. Hyg. Assoc. J., 24: 305-325.
WHITE, L.D., TAYLOR, D.G., MAUER, P.A., & KUPEL, R.E. (1970)
A convenient optimized method for the analysis of selected
solvent vapors in the industrial atmosphere. Am. Ind. Hyg.
Assoc. J., 31: 225-232.
WILSON, R.H. (1944) Health hazards encountered in the
manufacture of synthetic rubber. J. Am. Med. Assoc., 124:
701-703.
WILTI, D. (1970) Infrared vapour spectra, London, Heyden &
Son Ltd. (Index No. 180).
WINK, A. (1972) Effect of long-term exposure to toxic
substances on urinary excretion of 17-oxogenic steroids and
17-oxosteroids. Ann. occup. Hyg., 15: 211-215.
WITHEY, J.R. (1976) Quantitative analysis of styrene monomer
in polystyrene in foods including some preliminary studies of
the uptake and pharmacodynamics of the monomer in rats.
Environ. Health Perspect., 17: 125-133.
WITHEY, J.R. (1978) The toxicology of styrene monomer and its
pharmacokinetics and distribution in the rat. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 31-40.
WITHEY, J. & COLLINS, P.G. (1977) Pharmakokinetics and
distribution of styrene monomer in rats after intravenous
administration. J. Toxicol. environ. Health, 3: l0ll-l020.
WITHEY, J.R. & COLLINS, P.G. (1978) Styrene monomer in foods:
A limited Canadian survey, Bull. environ. Contam. Toxicol.,
19: 86-94.
WITHEY, J.R. & COLLINS, P.G. (1979) The distribution and
pharmacokinetics of styrene monomer in rats by the pulmonary
route. J. environ. Pathol. Toxicol., 2: 1329-1342.
WOLF, M.A., ROWE, V.K., MCCOLLISTER, D.D., HOLLINGSWORTH,
R.L., & OYEN, F. (1956) Toxicological studies of certain
alkylated benzenes and benzene. Am. Med. Assoc. Arch. ind.
Health, 14: 387-398.
WOLFF, M.S., DAUM, S.M., LORIMER, W.V., & SELIKOFF, I.J.
(1977) Styrene and related hydrocarbons in subacutaneous fat
from polymerization workers. J. Toxicol. environ. Health, 2:
997-1005.
WOLFF, M.S., LILIS, R., LORIMER, W.V., & SELIKOFF, I.J.
(1978a) Biological indicators of exposure in styrene
polymerization workers. Styrene in blood and adipose tissue
and mandelic and phenylglyoxylic acids in urine. Scand. J.
Work Environ. Health, 4 (Suppl. 2): 114-118.
WOLFF, M.S., LORIMER, W.V., LILIS, R., & SELIKOFF, I.J.
(1978b) Blood styrene and urinary metabolites in styrene
polymerization. Br. J. ind. Med., 35: 318-329.
YAMAMOTO, R.K. & COOK, W.A. (1968) Determination of
ethylbenzene and styrene in air by ultraviolet
spectrophotometry. Am. Ind. Hyg. Assoc. J., 29: 283-241.
YOSHIKAWA, K., ISOBE, M., WATANABE, T., & TAKABATAKE, E.
(1980) Studies on metabolism and toxicity of styrene III.
The effect of metabolic inactivation by rat-liver S9 on the
mutagenicity of phenyloxirane toward Salmonella typhimurium.
Mutat. Res., 78: 219-226.
ZAPRIANOV, Z & BAINOVA, A. (1979) Changes in monoamine
oxidase activity (MAO) after styrene and ethanol combined
treatment of rats. Activ. nerv. Sup. (Praha), 21(4): 262-264.
ZETTERBERG, G. (1982) Styrene - a widespread mutagen:
conclusions from the results of testing. In: Sugimura, T.,
Kondo, S. & Takabe, H., ed. Environmental Mutag. Carcinog.,
Tokyo, University of Tokyo Press, pp. 316-322.
ZIELHUIS, R.L., HARTOGENSIS, F., JONGH, J., KALSBEEK, J.W.H.,
& VAN REES, H. (1964) The health of workers processing
reinforced polyesters. In: XIVth International Congress of
Occupational Health, Madrid, Spain, 16-21 September, 1963,
Volume III, pp. 1092-1097.
ZITTING, A., HEINONEN, T., & VAINIO, H. (1980) Glutathione
depletion in isolated rat hepatocytes caused by styrene and
the thermal degradation products of polystyrene. Chem. biol.
Interact., 31: 313-318.
ZLOBINA, N.S. (1963) [On the toxicity of small concentrations
of styrol vapors.] Gig. i Sanit., 28: 29-35 (in Russian).
 This is the desired selective reaction; however, various
non-selective reactions will occur. For example, a reactor
effluent contains an average of 0.7% benzene and 1.0% toluene
(Faith et al., 1975).
The crude styrene, with an average composition of 37%
styrene, 61% ethylbenzene, 1.0% toluene, 0.7% benzene, and
0.3% tars, is passed through a pot containing sulfur or some
other polymerization inhibitor and is then fed into a vacuum
column system. The overhead from a primary fractionating
column is fractionated to separate the ethylbenzene, which is
recycled, from the benzene and toluene, which are separated by
distillation. The bottoms from a primary fractionating column
are distilled to obtain the pure styrene product (US EPA,
1980).
(b) Co-product with propylene oxide
In late 1977, production began in a plant that makes
styrene by the following method (Soder, 1977):
C6H5CH2CH3 + O2 ------> C6H5CHOOHCH3
(ethylbenzene) (ethylbenzene hydroperoxide)
O
C6H5CHOOHCH3 + CH3CH=CH2 -------> C6H5CHOHCH3 + CH3CHCH2
(propylene) (methyl phenyl (propylene
carbinol) oxide)
C6H5CHOHCH3 -------> C6H5CH=CH2 + H2O
(styrene)
Ethylbenzene is oxidized to the hydroperoxide, which is
then reacted with propylene to yield the propylene oxide and a
co-product, methyl phenyl carbinol. The carbinol is then
dehydrated to styrene (US EPA, 1980). Expected impurities may
include propylbenzene, isopropylbenzene, and alpha-methylstyrene.
Table 1 shows the annual production of styrene monomer in
the world. The Federal Republic of Germany, Japan, and the
USA are the major producers (Tossavainen, 1978). It is
estimated that the world styrene consumption will grow at an
average of 5.1% per year in the period 1982-90 reaching 13.6
million tonnes by 1990 (Chemical Market Review, 1981).
Table 1. Production of styrenea
------------------------------------------
Country Year Production
(tonnes)
------------------------------------------
Canada 1974 146 000
France 1976 270 000
Germany, Federal 1976 860 000
Republic of
Italy 1976 325 000
Japan 1976 1 090 000
Mexico 1974 30 000
Spain 1976 60 000
USA 1976 2 864 000
Others 1976 approx. 1 000 000
------------------------------------------
World 1977 7 000 000
------------------------------------------
a From: Tossavainen (1978).
3.2. Uses
The uses of styrene (monomer) in the USA in 1980 are
listed in Table 2. The pattern of consumption is essentially
the same elsewhere in the world including Europe and Japan
(Tossavainen, 1978) and no significant change is foreseen as
far as 1990 (European Chemical News, 1982).
3.3. Main Sources of Environmental Pollution
According to Pervier et al. (1974), the major sources of
styrene contamination of the environment are the petrochemical
industries. Emissions from styrene production may result from
vents on distillation columns and other processing equipment,
storage tank losses, miscellaneous leaks and spills, waste-waters,
and solid process wastes. However, losses from production are low
in comparison with other petrochemical losses. Some styrene can
also be emitted from polymerization processes.
Table 2. Use pattern of styrene in the
USA in 1980
-----------------------------------------
Material %
-----------------------------------------
polystyrene 45
acrylonitrile-butadiene-styrene plus 10
styrene-acrylonitrile resin
styrene-butadiene rubber 8
styrene-butadiene latex 6
unsaturated polyester 5
miscellaneous 4
exports 17
-----------------------------------------
Styrene can be released into the environment during
various disposal procedures, e.g., during the incineration of
many types of styrene polymers. Styrene has been detected in
hydrocarbon exhausts from spark-ignition engines (Flemming,
1970), in oxyacetylene and oxyethylene flames (Crittenden &
Long, 1976), and in cigarette smoke (section 4.1.1). The
domestic use of polyester resins has increased potential
exposure patterns. Exposure may occur when styrene is used as
a solvent during the preparation of resin flooring (Gadalina
et al., 1969; Kaznina, 1969); or through the use of styrene in
various hobbies, crafts, and toys (Smirnova & Yatakova, 1966).
4. ENVIRONMENTAL EXPOSURE LEVELS
4.1. General Environment
Some data are available on styrene concentrations in air,
cigarette smoke, water, and food. However, they are not
sufficiently systematic to provide an overall picture and an
estimate of the main routes of human exposure.
Some of the following data for styrene levels in air and
water are drawn from a survey of literature, released in 1980
by the US Environmental Protection Agency (US EPA, 1980),
concerning exposure to several potential environmental
contaminants.
4.1.1. Ambient air
Styrene has been detected in ambient air in a wide variety
of locations.
In a study to develop procedures for determining specific
contaminants, Neligan et al. (1965) measured the amounts of a
number of hydrocarbons in the urban atmosphere in the USA.
The results of analysis of air from southern California in
1965 showed styrene to be present at levels of 0.008 - 0.063
mg/m3 (0.002 - 0.015 ppm) with an average level of 0.021 mg/m3
(0.005 ppm).
Styrene has also been found in the urban atmosphere in
Japan, (Hoshika, 1977) at levels of about 0.0008 µg/m3 (0.0002
ppm). Valenta (1966) measured the air and water contamination
near a butadiene-styrene rubber plant in Czechoslovakia. One
of the main sources of contamination was the plant warehouse
for liquid materials. A styrene concentration of 0.07 mg/m3
(0.017 ppm) was found in the immediate downwind vicinity of
the warehouse, compared with a concentration of 0.03 mg/m3
(0.007 ppm) at a distance of 800 m.
Schofield (1974) analysed hydrocarbon emissions from
vehicles and found that 0.76% of total hydrocarbons was in the
form of styrene in the exhaust of conventional engines, and
2.67% in the exhaust of rotary engines. Styrene has also been
identified in cigarette smoke, reported levels ranging from 18
to 48 µg per cigarette (Johnstone et al., 1962; Baggett et
al., 1974; Jermini et al., 1976).
4.1.2. Water
Styrene, detected in drinking water (US EPA, 1980) and in
river water (Rosén et al., 1963; Gordon & Goodly, 197l;
Bertsch et al., 1975), was usually traceable to an industrial
source or to improper disposal. An example of serious
contamination of a water supply through improper disposal of
styrene was reported by Grossman (1970) in which well water
close to the site where two drums of styrene had been buried
was found to have a disagreeable odour and contained a styrene
concentration of 0.1 - 0.2 mg/litre. Water from another well
close to the site of a waste dump from a styrene-butadiene
plant contained a styrene concentration of 0.01 - 0.02 mg/litre
(Valenta, 1966).
It seems that while styrene can be detected in water, it
is not one of the frequent contaminants, nor is it present in
large amounts.
4.1.3. Food
Polystyrene and its copolymers such as acrylonitrile-
butadiene-styrene (ABS), have been widely used as food
packaging materials. Currently available analytical surveys of
food and food packaging have shown that the styrene monomer
migrates into food from both rigid and expanded polystyrene foam
containers. According to Withey & Collins (1978), the lowest
concentration of monomer in rigid containers was 700 ppm and, the
highest was 3300 ppm. Other studies give figures of a similar
order of magnitude (Hamidullin et al., 1968). The lowest
concentration of monomer in expanded polystyrene foam was 87 ppm
(Withey, 1976). The highest migration figure (245.5 ppb) was found
in samples of sour cream contained in rigid polystyrene containers
(Withey & Collins, 1978). Styrene leached from containers at
0.2 - 0.5 ppm conveyed a disagreeable odour and taste to dairy
products (Jensen, 1972). However, styrene was not detected in milk
stored in polystyrene containers for up to 8 days (detection limit
50 ppb) (Finley & White, 1967). In another study, styrene rates of
leaching ranged from 0.0077, 0.0078, and 0.0078 µg/cm2
respectively, for foam cups containing water, tea, and coffee, to
0.036, 0.064, and 0.210 µg/cm2, respectively for foam, impact, and
crystal polystyrene cups containing 8% ethanol (Varner & Breder,
1981a,b).
In general, styrene concentrations in food are between 3
and 4 orders of magnitude lower than that in the package. It
appears that the contribution to the total body burden of
styrene via food is minimal.
4.2. Work Environment
Occupational exposure to styrene can be classified
according to the types of operations in which it is present.
In polystyrene manufacture, occupational chemical exposure is
mainly to styrene. In reinforced plastics applications, where
styrene is a solvent-reactant for copolymerization, styrene is
also the major air contaminant; however, there may be
concomitant exposure to glass fibres, catalysts, accelerators,
cleaning agents, and other chemicals. During
styrene-butadiene rubber production, workers may also be
exposed to such chemical substances as benzene, butadiene,
carbon disulfide, and trichloroethylene and, in factories that
produce styrene, there may be additional exposure to benzene
and ethyl benzene. In many of the applications, the
operations involve potential skin contact with liquid styrene.
4.2.1. Styrene and polystyrene manufacture
Concentrations of styrene recorded in a number of
factories producing styrene or polystyrene are summarized in
Table 3.
According to available reports, the styrene concentrations
found in polystyrene production are generally less than
21 mg/m3 (5 ppm); though occasional values of 210 mg/m3
(50 ppm) or more have been reported.
Table 3. Concentration of styrene in air of polystyrene manufacturing
plants
-----------------------------------------------------------------------
Year Authors Exposure levels Work area/process
mg/m3 (ppm)
-----------------------------------------------------------------------
1952 Barsotti et al. 800 (192) loading into
polymerization tower
1963 Zlobina et al. 2-9 (0.5-2) block production
4-9 (1-2) emulsion
< 50 (< 12) cleaning
1972 Ponomareva & 10 (2) polymerization
Zlobina <5 (< 1) polystyrene filament
production
< 5 (< 1) short & intermittent
operation
0.47-0.68 (0.11-0.16) drying & packaging
1974 Maier et al.a < 21 (< 5) at breathing zone of
1978 Wolff et al.a (11 samples were workers (involved in
between 21-378 mg/m3 routine operations)
(5-90 ppm)
1979 Thiess & Friedheim 244 (58) technical services
operation
4-50 (1-12) laboratory operation
-----------------------------------------------------------------------
a These investigators also identified benzene, ethylbenzene, toluene,
acetone. The highest measured concentrations of these solvents,
including styrene, resulted from spills and leaks.
4.2.2. Reinforced plastics applications
Data concerning air levels of styrene in reinforced
plastics plants, presented in Table 4, demonstrate that higher
air levels of styrene are monitored in this industry than in
styrene polymerization processes.
Tables 5, 6, 7, and 8 are designed to provide additional
information, useful in the assessment of occupational exposure
and to complement data presented in Table 4.
Table 4. Concentration of styrene in air of reinforced plastics plants
------------------------------------------------------------------------------
Year Author(s) No. of Exposure levels Work area/process
plants mg/m3 (ppm)
------------------------------------------------------------------------------
1967 Huzl et al. 5 210-420 (50-100) styrene containing polyester
resin applied by hand in 4
plants and sprayed in 1
1960 Bardodej et al. 2 63-840 (15-200) polyester resin applied by
hand
1964 Zielhuis et al. 3 101-378 (24-90) manufacturing of boats,
automobile bodies
ca. 29 (ca. 7) production of small objects
and upholstery; laboratory
1966 Simko et al. 3 21-819 (5-195) manufacture of reinforced
plastics
1968 Matsushita et 1 > 2520 (> 600) lamination of plywood
al. with styrene-containing resin
1972 Gotell et al. 4 84-1218 (20-290) production of reinforced
plastics
1974 Bodnei et al. 1 189-2130(45-550) reinforced plastics and
(1973-1974)a bathtub manufacturing
1976 Kallioski 22 84-1260 (20-300) boat laminating and other
reinforced plastics
manufacturing
1977 Rosensteel et 1 147-462 (35-110) reinforced plastic boat
(1975-1976)a plant (peak conc. of
al. 1260-1680 mg/m3 (300-400 ppm)
for approx. 5 min)
1977 Bergman 4 42-714 (10-170) Hand layup & spraying in the
reinforced plastic boat
industry (peak conc. of
256-2146 mg/m3 (61-511 ppm)
for approx. 60 min)
1979 Brooks et al. 3 168-966 (40-230)
(1977)a
1979 Kjellberg et 1 13-59 (3-14) boat fabrication
al.
1981 Crandall 7 8-756 (2-180) hull, deck & small part
(1978-1979)a lamination and gelcoating
process
------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------
Year Author(s) No. of Exposure levels Work area/process
plants mg/m3 (ppm)
------------------------------------------------------------------------------
1981 Schumacher et 12 < 1260 (< 300) Table 7
al.
1982 Ikeda et al. 5 < 4-1075 reinforced plastic boat
(< 1-256) plants
------------------------------------------------------------------------------
a Years when the study was conducted.
The resin manufacturers have tried to decrease styrene
emissions by reducing the styrene content of resins and by
using thixotropic resins and film-forming additives. They
(Nylander, 1979; Synthetic Resins Limited, 1980; Voskamp &
Studenberg, 1981) claim that significant decreases in styrene
emission can be achieved using these new resins, often called
low styrene emission resins (LSE resins). In the study of
Schumacher et al. (1981), the results of laboratory tests on
such resins suggested a 30% reduction in styrene vapour, but
when tested in the workplace there was essentially no
difference in the exposure levels of the workers and the
authors suggested the need for further studies to record any
effects from the use of the new resins.
Concentrations of styrene found during the production of
reinforced plastics (Zielhuis et al., 1964; Götell et al.,
1972; Bodnei et al., 1974; Rosensteel & Meyer, 1977) were
generally much higher than those found in the polystyrene
production plants, with peak concentrations as high as 6300
mg/m3 (1500 ppm). Most of the environmental concentrations of
styrene in reinforced plastic plants, summarized in Table 4,
are 8-h time-weighted-average (TWA) concentrations, but some
values represent shorter periods. It can be seen from the
table that exposure occurs not only in hand layup and spraying
operations using open moulds, but also in mechanical and
closed mould work. Furthermore, workers who do not handle the
polyester resin themselves but work in the laminating room
undergo passive exposure. It should be noted that the
sampling strategy used influences the results; for example,
breathing zone concentrations may differ considerably from the
general air concentrations.
4.2.3. Styrene-butadiene applications
Hazards encountered in the synthetic rubber industry
during the Second World War were discussed by Wilson (1944).
The principal chemicals used in the manufacture of rubber, at
that time, were styrene, butadiene, and acrylonitrile.
More recently, other studies of synthetic rubber
manufacture have shown that levels of styrene are low.
Basirov (1968) reported concentrations of 60-130 mg/m3 (14-31
ppm) in a 1968 study of a synthetic rubber plant. In a health
risk evaluation in a similar type of plant, Gunter & Lucas
(1973) were unable to find any measurable concentrations of
styrene. However, styrene levels varying between 42 and 840
mg/m3 (10 and 200 ppm) were measured in a synthetic rubber
plant by Spinazzola et al. (1980).
4.2.4. Summary of occupational exposure
In summary, occupational exposure to styrene varies
considerably depending on the operations concerned. While styrene
is present in detectable amounts in styrene/polystyrene
manufacturing plants, the greatest exposures occur in the
operations and industries that use unsaturated polyester resins
dissolved in styrene. However, in all industrial operations using
styrene, high levels of exposures occur during the clean-up and
maintenance procedures.
Table 5. Airborne styrene concentrations at 36 Finnish
factories using hand layup methoda
(i) Mean 8-h TWA breathing zone styrene concentrations at 22
factories
---------------------------------------------
Factory mg/m3 ppm Factory mg/m3 ppm
---------------------------------------------
1 1109 264 12 223 53
2 995 237 13 214 51
3 836 199 14 197 47
4 613 146 15 193 46
5 433 103 16 189 45
6 382 91 17 185 44
7 382 91 18 169 38
8 340 81 19 134 32
9 336 80 20 134 32
10 294 70 21 126 30
11 273 65 22 97 23
---------------------------------------------
(ii) Mean breathing zone styrene concentrations during shorter
(1-5 h) industrial hygiene surveys at 14 factories
---------------------------------------------
Factory mg/m3 ppm Factory mg/m3 ppm
---------------------------------------------
23 1029 244 30 416 99
24 819 195 31 302 72
25 50 12 32 235 56
26 769 183 33 193 46
27 622 148 34 105 25
28 521 124 35 92 22
29 491 117 36 67 16
---------------------------------------------
(iii) Distribution of styrene concentrations by product type
----------------------------------------------------------
8-h TWA styrene TWA styrene conc.
conc. for periods of 1-5h
Product type (factories 1-22) (factories 23-36)
mg/m3 ppm mg/m3 ppm
----------------------------------------------------------
Boats 483 115 865 206
Other products
- small objects 210 50 424 101
- large objects 357 85 248 59
----------------------------------------------------------
a Adapted from: Kallioski (1976).
Table 6. Airborne styrene concentrations at a factory in the USAa
---------------------------------------------------
8-h TWA styrene exposure
Job category (mean ± SD)
mg/m3 (ppm)
---------------------------------------------------
Prefabrication 11.8 ± 16.8 (2.8 ± 4)
Gel coating 288 ± 251 (68.5 ± 59.7)
Hand layup 350 ± 179 (83.4 ± 42.7)
Hand layup, other areas 108 ± 110 (25.7 ± 26.2)
Woodwork/upholstery 14.3 ± 8.8 (3.4 ± 2.1)
Final assembly 14.3 ± 8.8 (3.4 ± 2.1)
Custom moulding 27.3 ± 16.4 (6.5 ± 3.9)
Small boat 17.2 ± 4.2 (4.1 ± 1.0)
Miscellaneous 16.4 ± 13.0 (3.9 ± 3.1)
---------------------------------------------------
a Adapted from: Brooks et al. (1979).
Table 7. Airborne styrene concentrations at 12 factories in the USA. The figures in the columns
represent the number of workers in each exposure groupa
-------------------------------------------------------------------------------------------------------
Styrene concentration mg/m3 (ppm)
Classification
0-210 210-420 420-630 630-840 840-1050 1050-1260 > 1260 Totals
(0-50) (50-100) (100-150) (150-200) (200-250) (250-300) (> 300)
-------------------------------------------------------------------------------------------------------
Plant Type
Boat plants 127 144 80 32 25 23 48 479
Non-boat plants 26 47 29 5 3 1 2 113
Totals 153 191 109 37 28 24 50 592
Job Category
Laminators 61 70 61 27 21 11 22 273
Chopper gun operators 37 62 40 25 13 17 30 224
Gel coaters 12 6 4 3 2 27
Totals 110 138 105 55 34 28 54 524
Plant Type (Exposure of Laminators)
Boat plants 52 48 42 26 18 13 20 219
Non-boat plants 6 24 17 2 3 1 1 54
Totals 58 72 59 28 21 14 21 273
-------------------------------------------------------------------------------------------------------
a Modified from: Schumacher et al. (1981).
Table 8. Styrene concentration during various processes in boat production
-----------------------------------------------------------------------------------------------------------------------------
Process Case Personal sampling Stationary sampling
---------------------------------------------- ------------------------------------------------
Na GMb GSDc Ranged Nc GMb GSDc Ranged Nc
-----------------------------------------------------------------------------------------------------------------------------
Lamination A 25 500 (119) 6.8 (1.6) 143-1075 (34-256) 17 550 (131) 5.5 (1.3) 307-731 (73-174)
over boat B 9 273 (65) 5.8 (1.2) 193-378 (46-90) 15 281 (67) 5.9 (1.4) 160-546 (38-130)
shell mould
Installation laminators 3 71.4 (17) 7.1 (1.7) 42-118 (10-28) 20 33.6 (8) 10 (2.4) 8-214 (2-51)
of ribs helpers 5 < 4.2 (< 1) 23.5 (5.6) 8 (D1-2)
Installation laminators 5 92.4 (22) 8.8 (2.1) 25-185 (6-44) 6 29.4 (7) 12.2 (2.9) 8-92 (2-22)
of division helpers 5 D - (D-D)
plates
Auxilary 5 54.6 (13) 16.8 (4) 8-181 (2-43) 5 96.6 (23) 7.1 (1.7) 59-164 (14-39)
lamination
on deck
Lamination on A 4 128 1.4 (104-211) 3 269 (64) 19.3 (4.6) 55-1172 (13-279)
hold walls B 21 127 1.3 (87-215) 20 269 (64) 7.6 (1.8) 101-1088 (24-259)
Equipment 8 2 2.8 (1-17) 15 < 4.2 64.7 (15.4) Ng-38 (Ng-9)
(< 1)
Plant floor A 39 6 24.8 (5.9) 8-76 (2-18)
in general B 14 < 1 69.7 (16.6) Na-38 (Na-9)
-----------------------------------------------------------------------------------------------------------------------------
a The number of workers equipped with personal samplers for 4 h work (1300-1700).
b The geometric mean.
c The geometric standard deviation.
d The minimum and maximum concentration observed.
e The number of sites monitored by stationary samplers for 4 h (1300-1700).
f D = detected but not measurable (less than 4.2 mg/m3 (1 ppm).
g Not detected.
5. CHEMOBIOKINETICS AND BIOTRANSFORMATION
5.1. Uptake
5.1.1. Human studies
5.1.1.1. Uptake by inhalation
Pulmonary uptake of styrene concentrations of 67-164 mg/m3
(16-39 ppm), after a single breath or during exposures of up
to 8 h, has been studied in human volunteers. Depending on
exposure conditions, uptake varied from 45 to 66% (Bardodej et
al., 1961; Fiserova-Bergerova & Teisinger, 1965; Bardodej &
Bardodejova, 1970).
Astrand et al. (1974) examined the pulmonary uptake of
inhaled styrene (210 and 630 mg/m3; 50 and l50 ppm) in 14
healthy subjects at rest and during exercise over 30-min
periods. Blood concentrations of styrene were measurable
shortly after the onset of exposure indicating a rapid
uptake. With light work (50 W exercise), the alveolar
ventilation increased 3-fold, while the alveolar concentration
of styrene barely changed. The concentration of styrene in
arterial blood tripled.
Fernandez & Caperos (1977) measured the uptake of styrene
in 6 volunteers at exposure levels ranging from 294 to
840 mg/m3 (70 to 200 ppm) for periods ranging from 4 to 8 h.
The retention of styrene, measured from alveolar ventilation,
varied from 82 to 93%.
In studies by Ramsey & Young (1978) and Ramsey et al.
(1980) on 4 human volunteers, a single exposure to a styrene
concentration of 336 mg/m3 (80 ppm) for 6 h resulted in a
blood concentration at the end of exposure of about
800 µg/litre. A steady state concentration of about
900 µg/litre was predicted for repeated daily exposure to the
same atmospheric concentrations. The authors assumed a
2-compartment model that allowed a zeroth order uptake rate of
99.6 mg/h to be calculated.
5.1.1.2. Uptake from the gastrointestinal tract
No studies have been found in the literature related to
the uptake of styrene from the human gastrointestinal tract.
5.1.1.3. Uptake through the skin
Styrene is absorbed through human skin when applied in the
form of a liquid, an aqueous solution, or a vapour. Rates of
uptake of between 9 and 15 mg/cm2 per h were reported when
liquid styrene was applied to the skin of the hands and
forearm (Dutkiewicz & Tyras, 1967; 1968). Rates of uptake of
0.004-0.180 mg/cm2 per h were reported when aqueous solutions
containing mean styrene concentrations of between 66.5 and 269
mg/litre were applied. Skin absorption of styrene was some 30
times greater than that of aniline, benzene, or nitrobenzene.
Riihimäki & Pfäffli (1978) measured the percutaneous
uptake of styrene vapour in 2 human volunteers, wearing thin
cloth pyjamas and socks, who were exposed to a styrene vapour
concentration of 2520 mg/m3 (600 ppm) for 3.5 h, including 3
work intervals of 10 min each. Full-face respirators were
used to prevent inhalation uptake and the temperature was
maintained at 25°C with 50% relative humidity. Blood levels
reached a peak 3 h after the start of exposure and then
declined over the next 4 h. These results were equivalent to
a pulmonary exposure of about 84 mg/m3 (20 ppm) for an equal
period of time.
5.1.2. Experimental animal studies
5.1.2.1. Uptake by inhalation
A recent review of studies of the uptake of styrene in
several animal species indicated that styrene is distributed
rapidly throughout the body, is stored in lipid-rich tissues,
and is extensively metabolized (Santodonato et al., 1980). In
a study on rats exposed to styrene concentrations in air of
336, 840, 2520, and 5040 mg/m3 (80, 200, 600, and 1200 ppm)
for up to 24 h, kinetic data obtained by Ramsey & Young (1978)
obeyed a 2-compartment model. From the kinetic coefficients
derived from the data, the authors predicted that there was
little or no potential for bioaccumulation with repeated 8 h
per day exposure to 336 mg/m3 (80 ppm). They claimed that 95%
of the predicted maximum blood concentration of styrene would
be reached during the first day of exposure.
In another study in which rats with an indwelling jugular
cannula were exposed to styrene atmospheres (Withey, 1978;
Withey & Collins, 1979), a 2-compartment model was observed at
exposure concentrations ranging from 210 to 8400 mg/m3 (50 to
2000 ppm) for a period of 5 h. From the assumed zeroth-order
kinetics for uptake together with the rate coefficients for
distribution and elimination, it was possible to predict that
the time to equilibrium or steady-state would be longer at
higher exposure levels.
An autoradiographic study on the uptake of 14C-styrene-8
and 14C-styrene-ring by mice was carried out by Bergman
(1979). Each animal inhaled the vapours from 10 µl of styrene
in a small inhalation apparatus for 10 min. Extensive
uptake was observed in all organs and the potential for
enterohepatic recycling was demonstrated by the appearance of
the radiolabel in the bile. Styrene uptake in the mice was
reported to be low compared with that in human subjects (less
than one half) and this was attributed to the reduced
respiratory frequency in the mouse, induced by the irritant
action of the styrene vapour on the respiratory tract.
5.1.2.2. Uptake from the gastrointestinal tract
There have been few reports on the kinetics and the extent
of uptake of styrene from the gastrointestinal tract in
animals.
A study by Sauerhoff et al. (1976) on male and female
rats, administered a single oral dose of 14C-labelled styrene
at 500 or 50 mg/kg body weight, showed that a greater fraction
of unchanged styrene was excreted via the lung after
administration of the higher dose, a result suggesting that
saturable metabolic pathways were involved. The authors found
that the uptake of styrene monomer from oral doses was greater
for the female than for the male rats.
In a report of a preliminary investigation on the uptake
kinetics of styrene from the gastrointestinal tract of rats
(Withey, 1976), it was noted that the uptake and kinetic
profile after a single dose, administered as an aqueous
solution, was so rapid (peak values being obtained less than 4
min after the administration of the dose) that it was almost
indistinguishable from the kinetics of an intravenous bolus
dose. In contrast, when a similar dose was administered as a
solution in vegetable oil, peak values were not obtained until
4 h after dosing.
5.1.2.3. Uptake through the skin
Wolf et al. (1956) applied unspecified amounts of liquid
styrene to the ear or to the shaved abdomen of rabbits during
periods of 2-4 weeks (10-20 applications). Moderate irritation
and slight necrosis were observed. The authors claimed that, at
the doses applied, there was no evidence that styrene was absorbed
through the skin in amounts sufficient to cause acute, systemic
toxicity.
The percutaneous uptake of styrene in rats has been
measured by immersing the tail of the animal in liquid styrene
for 1 h (Shugaev, 1969). Inhalation exposure was carefully
avoided. Significant uptake of styrene by the liver and brain
was estimated to be between 50 and 70 % of the concentrations
found in the same organs after a 4-h inhalation exposure to a
vapour concentration of 11.8 gm/m3.
5.2. Distribution and Storage
5.2.1. Human studies
5.2.1.1. Controlled human studies
Studies on experimental animals have demonstrated that
styrene is widely distributed throughout the tissues and
organs and that it may accumulate in adipose tissue. Studies
on the distribution of styrene in human volunteers have,
necessarily, been limited to its quantitative analysis in
blood, expired air, and adipose tissue.
In a study by Engström (1978), 7 male subjects were
exposed to a styrene concentration of 210 mg/m3 (50 ppm)
during 30 min at rest and three, 30-min periods on a bicycle
ergometer set at a work intensity of 50, 100, or 150 W. The
mean uptake of styrene was 490 mg. Specimens of subcutaneous
adipose tissue were taken before and after exposure and at 0.5, 2,
4, and 21 h after exposure. Mean concentrations in the adipose
tissue, up to 21 h after exposure, were of the same magnitude (3.6
mg/kg). From the concentration of styrene still detectable l3 days
after exposure, it was possible to estimate an elimination half-
time of between 2.2 and 4.0 days.
Ramsey & Young (1978) exposed volunteers to a styrene
concentration of 336 mg/m3 (80 ppm) for 6 h and observed that
the decay of styrene concentration in the blood following the
exposure fitted a 2-compartment model. They speculated that
accumulation of styrene would not occur following repeated
exposure to concentrations up to 840 mg/m3 (200 ppm).
5.2.1.2. Occupational exposure studies
There have been a number of studies involving the
determination of styrene concentrations in the blood and
adipose tissue of workers occupationally exposed to styrene
monomer. These studies were not only intended to investigate the
correlation between levels in tissues and previous exposure to
styrene but also to see if there was a potential for its
accumulation and storage in adipose tissue. Wolff et al. (1977)
studied 25 workers who allowed from 3 to 66 mg of subcutaneous
gluteal adipose tissue to be taken. It was demonstrated that
styrene persisted in this tissue for as long as 3 days following
exposure, whereas urine metabolites and styrene in expired air
persisted for up to 16 h after exposure. In an extension of this
study (Wolff et al., 1978a), data on levels of styrene in the
gluteal adipose tissue of an additional 25 workers suggested a
correlation with blood styrene and urinary metabolite levels.
Engström et al. (1978a,b) measured air concentrations of
styrene in a polymerization plant and obtained subcutaneous
adipose samples from 3 workers. The TWA air concentrations
ranged from 32 to 84 mg/m3 (7.6 to 20.2 ppm) and the mean
daily styrene uptake of the 3 volunteers was 193, 343, and 558
mg, respectively. Adipose tissue concentrations were 2.8,
4.0, and 8.1 mg/kg, respectively, at the beginning of the
working week, and 4.7, 7.7, and 11.6 mg/kg, at the end.
5.2.1.3. General population studies
Reports on the uptake of styrene in the general population
are lacking.
5.2.2. Experimental animal studies
There have been a number of reports on the distribution of
controlled doses of styrene monomer administered by different
routes in several animal species.
In studies on mice and rats, Shugaev (1969) showed that,
after inhalation exposure to the median lethal concentration
(LC50), for 2 and 4 h, respectively, there was a positive
correlation between the brain concentration of styrene and
lethality. Styrene levels in the perirenal adipose tissue
were some 5 times higher than those in the brain, liver,
kidney, and spleen.
Savolainen & Vainio (1977) studied the organ distribution
of radiolabelled styrene and styrene epoxide following an
intraperitoneal injection of 577 µmol, 3, 6, and 24 h after
dosing. Three hours after dosing, the highest concentration of
styrene was found in the kidneys followed by the brain, duodenum,
liver, spinal cord, lungs, and blood. The kidney concentration was
19 times higher than that in the blood. After 6 h, levels in the
duodenum and brain had decreased whereas levels in the blood had
increased.
The distribution of 14C-styrene-8 and 14C-styrene-ring was
studied in male mice after a 10-min inhalation exposure
(Bergman, 1979). Animals were killed at 0.5, 1, 2, 4, 8, 24,
and 48 h after exposure and subjected to whole-body auto-
radiography. The autoradiograms obtained shortly after
exposure showed that the radioactivity was localized in the
bronchi, lungs, and liver, for both styrene labelled in the
side chain and the ring-labelled compound. Styrene was
apparently cleared from the nervous tissues in less than 1 h,
since no radiolabelled material was detected at 0.5 h.
However, it was retained in adipose tissues for up to 48 h
after dosing. Again, a large proportion of the radioactivity
was found in the bile and kidney suggesting that these routes
are the primary routes of excretion. Radiolabelled material
appeared in the intestinal contents within 1 h of exposure and
persisted for at least 24 h.
A recent inhalation study on rats (Carlsson, 1981) showed
that the largest amounts of 14C-styrene and its metabolites
were found in the kidneys, after an 8-h exposure to 184 mg/m3
(45 ppm). The styrene concentrations were also high in
subcutaneous adipose tissue; the concentrations in the
cerebrum, cerebellum, and muscles amounted to about 70% of the
styrene concentration in arterial blood.
The distribution of styrene was examined in male Wistar
rats after intravenous administration at 4.01 or 13.37 mg/kg
body weight or after a 5 h inhalation exposure at 6
concentrations between 227 and 9408 mg/m3 (54 and 2240 ppm)
(Withey, 1976; Withey & Collins, 1977, 1979). Animals were
killed immediately after the termination of the vapour
exposure, and the blood, heart, kidney, liver, spleen, brain,
and perirenal fat were analysed for their styrene content. At all
exposure levels except the lowest, styrene concentrations in all
organs were higher than that in the blood. At the lowest level of
exposure, the concentration in the kidney was at least 10 times
greater than that in any other organ except the perirenal fat. At
higher levels of exposure, the highest concentration was found in
the liver. Levels of styrene in organs, 8 min after intravenous
administration of 13.37 mg/kg were found in the following
descending order: liver > kidney > brain > blood > heart and
spleen. For the lower dose (4.01 mg/kg), the levels in these
organs were of the same order of distribution, but lower than that
in the blood, suggesting that, as in the case of the inhalation
study, the distribution to the major organs varied with dose.
5.3. Biotransformation
The first step in the metabolic transformation of styrene
is its oxidation, catalysed by cytochrome P-450 dependent
monooxygenases, to oxirane derivatives in the aliphatic chain
or in the aromatic ring (Fig. 1).
This is the desired selective reaction; however, various
non-selective reactions will occur. For example, a reactor
effluent contains an average of 0.7% benzene and 1.0% toluene
(Faith et al., 1975).
The crude styrene, with an average composition of 37%
styrene, 61% ethylbenzene, 1.0% toluene, 0.7% benzene, and
0.3% tars, is passed through a pot containing sulfur or some
other polymerization inhibitor and is then fed into a vacuum
column system. The overhead from a primary fractionating
column is fractionated to separate the ethylbenzene, which is
recycled, from the benzene and toluene, which are separated by
distillation. The bottoms from a primary fractionating column
are distilled to obtain the pure styrene product (US EPA,
1980).
(b) Co-product with propylene oxide
In late 1977, production began in a plant that makes
styrene by the following method (Soder, 1977):
C6H5CH2CH3 + O2 ------> C6H5CHOOHCH3
(ethylbenzene) (ethylbenzene hydroperoxide)
O
C6H5CHOOHCH3 + CH3CH=CH2 -------> C6H5CHOHCH3 + CH3CHCH2
(propylene) (methyl phenyl (propylene
carbinol) oxide)
C6H5CHOHCH3 -------> C6H5CH=CH2 + H2O
(styrene)
Ethylbenzene is oxidized to the hydroperoxide, which is
then reacted with propylene to yield the propylene oxide and a
co-product, methyl phenyl carbinol. The carbinol is then
dehydrated to styrene (US EPA, 1980). Expected impurities may
include propylbenzene, isopropylbenzene, and alpha-methylstyrene.
Table 1 shows the annual production of styrene monomer in
the world. The Federal Republic of Germany, Japan, and the
USA are the major producers (Tossavainen, 1978). It is
estimated that the world styrene consumption will grow at an
average of 5.1% per year in the period 1982-90 reaching 13.6
million tonnes by 1990 (Chemical Market Review, 1981).
Table 1. Production of styrenea
------------------------------------------
Country Year Production
(tonnes)
------------------------------------------
Canada 1974 146 000
France 1976 270 000
Germany, Federal 1976 860 000
Republic of
Italy 1976 325 000
Japan 1976 1 090 000
Mexico 1974 30 000
Spain 1976 60 000
USA 1976 2 864 000
Others 1976 approx. 1 000 000
------------------------------------------
World 1977 7 000 000
------------------------------------------
a From: Tossavainen (1978).
3.2. Uses
The uses of styrene (monomer) in the USA in 1980 are
listed in Table 2. The pattern of consumption is essentially
the same elsewhere in the world including Europe and Japan
(Tossavainen, 1978) and no significant change is foreseen as
far as 1990 (European Chemical News, 1982).
3.3. Main Sources of Environmental Pollution
According to Pervier et al. (1974), the major sources of
styrene contamination of the environment are the petrochemical
industries. Emissions from styrene production may result from
vents on distillation columns and other processing equipment,
storage tank losses, miscellaneous leaks and spills, waste-waters,
and solid process wastes. However, losses from production are low
in comparison with other petrochemical losses. Some styrene can
also be emitted from polymerization processes.
Table 2. Use pattern of styrene in the
USA in 1980
-----------------------------------------
Material %
-----------------------------------------
polystyrene 45
acrylonitrile-butadiene-styrene plus 10
styrene-acrylonitrile resin
styrene-butadiene rubber 8
styrene-butadiene latex 6
unsaturated polyester 5
miscellaneous 4
exports 17
-----------------------------------------
Styrene can be released into the environment during
various disposal procedures, e.g., during the incineration of
many types of styrene polymers. Styrene has been detected in
hydrocarbon exhausts from spark-ignition engines (Flemming,
1970), in oxyacetylene and oxyethylene flames (Crittenden &
Long, 1976), and in cigarette smoke (section 4.1.1). The
domestic use of polyester resins has increased potential
exposure patterns. Exposure may occur when styrene is used as
a solvent during the preparation of resin flooring (Gadalina
et al., 1969; Kaznina, 1969); or through the use of styrene in
various hobbies, crafts, and toys (Smirnova & Yatakova, 1966).
4. ENVIRONMENTAL EXPOSURE LEVELS
4.1. General Environment
Some data are available on styrene concentrations in air,
cigarette smoke, water, and food. However, they are not
sufficiently systematic to provide an overall picture and an
estimate of the main routes of human exposure.
Some of the following data for styrene levels in air and
water are drawn from a survey of literature, released in 1980
by the US Environmental Protection Agency (US EPA, 1980),
concerning exposure to several potential environmental
contaminants.
4.1.1. Ambient air
Styrene has been detected in ambient air in a wide variety
of locations.
In a study to develop procedures for determining specific
contaminants, Neligan et al. (1965) measured the amounts of a
number of hydrocarbons in the urban atmosphere in the USA.
The results of analysis of air from southern California in
1965 showed styrene to be present at levels of 0.008 - 0.063
mg/m3 (0.002 - 0.015 ppm) with an average level of 0.021 mg/m3
(0.005 ppm).
Styrene has also been found in the urban atmosphere in
Japan, (Hoshika, 1977) at levels of about 0.0008 µg/m3 (0.0002
ppm). Valenta (1966) measured the air and water contamination
near a butadiene-styrene rubber plant in Czechoslovakia. One
of the main sources of contamination was the plant warehouse
for liquid materials. A styrene concentration of 0.07 mg/m3
(0.017 ppm) was found in the immediate downwind vicinity of
the warehouse, compared with a concentration of 0.03 mg/m3
(0.007 ppm) at a distance of 800 m.
Schofield (1974) analysed hydrocarbon emissions from
vehicles and found that 0.76% of total hydrocarbons was in the
form of styrene in the exhaust of conventional engines, and
2.67% in the exhaust of rotary engines. Styrene has also been
identified in cigarette smoke, reported levels ranging from 18
to 48 µg per cigarette (Johnstone et al., 1962; Baggett et
al., 1974; Jermini et al., 1976).
4.1.2. Water
Styrene, detected in drinking water (US EPA, 1980) and in
river water (Rosén et al., 1963; Gordon & Goodly, 197l;
Bertsch et al., 1975), was usually traceable to an industrial
source or to improper disposal. An example of serious
contamination of a water supply through improper disposal of
styrene was reported by Grossman (1970) in which well water
close to the site where two drums of styrene had been buried
was found to have a disagreeable odour and contained a styrene
concentration of 0.1 - 0.2 mg/litre. Water from another well
close to the site of a waste dump from a styrene-butadiene
plant contained a styrene concentration of 0.01 - 0.02 mg/litre
(Valenta, 1966).
It seems that while styrene can be detected in water, it
is not one of the frequent contaminants, nor is it present in
large amounts.
4.1.3. Food
Polystyrene and its copolymers such as acrylonitrile-
butadiene-styrene (ABS), have been widely used as food
packaging materials. Currently available analytical surveys of
food and food packaging have shown that the styrene monomer
migrates into food from both rigid and expanded polystyrene foam
containers. According to Withey & Collins (1978), the lowest
concentration of monomer in rigid containers was 700 ppm and, the
highest was 3300 ppm. Other studies give figures of a similar
order of magnitude (Hamidullin et al., 1968). The lowest
concentration of monomer in expanded polystyrene foam was 87 ppm
(Withey, 1976). The highest migration figure (245.5 ppb) was found
in samples of sour cream contained in rigid polystyrene containers
(Withey & Collins, 1978). Styrene leached from containers at
0.2 - 0.5 ppm conveyed a disagreeable odour and taste to dairy
products (Jensen, 1972). However, styrene was not detected in milk
stored in polystyrene containers for up to 8 days (detection limit
50 ppb) (Finley & White, 1967). In another study, styrene rates of
leaching ranged from 0.0077, 0.0078, and 0.0078 µg/cm2
respectively, for foam cups containing water, tea, and coffee, to
0.036, 0.064, and 0.210 µg/cm2, respectively for foam, impact, and
crystal polystyrene cups containing 8% ethanol (Varner & Breder,
1981a,b).
In general, styrene concentrations in food are between 3
and 4 orders of magnitude lower than that in the package. It
appears that the contribution to the total body burden of
styrene via food is minimal.
4.2. Work Environment
Occupational exposure to styrene can be classified
according to the types of operations in which it is present.
In polystyrene manufacture, occupational chemical exposure is
mainly to styrene. In reinforced plastics applications, where
styrene is a solvent-reactant for copolymerization, styrene is
also the major air contaminant; however, there may be
concomitant exposure to glass fibres, catalysts, accelerators,
cleaning agents, and other chemicals. During
styrene-butadiene rubber production, workers may also be
exposed to such chemical substances as benzene, butadiene,
carbon disulfide, and trichloroethylene and, in factories that
produce styrene, there may be additional exposure to benzene
and ethyl benzene. In many of the applications, the
operations involve potential skin contact with liquid styrene.
4.2.1. Styrene and polystyrene manufacture
Concentrations of styrene recorded in a number of
factories producing styrene or polystyrene are summarized in
Table 3.
According to available reports, the styrene concentrations
found in polystyrene production are generally less than
21 mg/m3 (5 ppm); though occasional values of 210 mg/m3
(50 ppm) or more have been reported.
Table 3. Concentration of styrene in air of polystyrene manufacturing
plants
-----------------------------------------------------------------------
Year Authors Exposure levels Work area/process
mg/m3 (ppm)
-----------------------------------------------------------------------
1952 Barsotti et al. 800 (192) loading into
polymerization tower
1963 Zlobina et al. 2-9 (0.5-2) block production
4-9 (1-2) emulsion
< 50 (< 12) cleaning
1972 Ponomareva & 10 (2) polymerization
Zlobina <5 (< 1) polystyrene filament
production
< 5 (< 1) short & intermittent
operation
0.47-0.68 (0.11-0.16) drying & packaging
1974 Maier et al.a < 21 (< 5) at breathing zone of
1978 Wolff et al.a (11 samples were workers (involved in
between 21-378 mg/m3 routine operations)
(5-90 ppm)
1979 Thiess & Friedheim 244 (58) technical services
operation
4-50 (1-12) laboratory operation
-----------------------------------------------------------------------
a These investigators also identified benzene, ethylbenzene, toluene,
acetone. The highest measured concentrations of these solvents,
including styrene, resulted from spills and leaks.
4.2.2. Reinforced plastics applications
Data concerning air levels of styrene in reinforced
plastics plants, presented in Table 4, demonstrate that higher
air levels of styrene are monitored in this industry than in
styrene polymerization processes.
Tables 5, 6, 7, and 8 are designed to provide additional
information, useful in the assessment of occupational exposure
and to complement data presented in Table 4.
Table 4. Concentration of styrene in air of reinforced plastics plants
------------------------------------------------------------------------------
Year Author(s) No. of Exposure levels Work area/process
plants mg/m3 (ppm)
------------------------------------------------------------------------------
1967 Huzl et al. 5 210-420 (50-100) styrene containing polyester
resin applied by hand in 4
plants and sprayed in 1
1960 Bardodej et al. 2 63-840 (15-200) polyester resin applied by
hand
1964 Zielhuis et al. 3 101-378 (24-90) manufacturing of boats,
automobile bodies
ca. 29 (ca. 7) production of small objects
and upholstery; laboratory
1966 Simko et al. 3 21-819 (5-195) manufacture of reinforced
plastics
1968 Matsushita et 1 > 2520 (> 600) lamination of plywood
al. with styrene-containing resin
1972 Gotell et al. 4 84-1218 (20-290) production of reinforced
plastics
1974 Bodnei et al. 1 189-2130(45-550) reinforced plastics and
(1973-1974)a bathtub manufacturing
1976 Kallioski 22 84-1260 (20-300) boat laminating and other
reinforced plastics
manufacturing
1977 Rosensteel et 1 147-462 (35-110) reinforced plastic boat
(1975-1976)a plant (peak conc. of
al. 1260-1680 mg/m3 (300-400 ppm)
for approx. 5 min)
1977 Bergman 4 42-714 (10-170) Hand layup & spraying in the
reinforced plastic boat
industry (peak conc. of
256-2146 mg/m3 (61-511 ppm)
for approx. 60 min)
1979 Brooks et al. 3 168-966 (40-230)
(1977)a
1979 Kjellberg et 1 13-59 (3-14) boat fabrication
al.
1981 Crandall 7 8-756 (2-180) hull, deck & small part
(1978-1979)a lamination and gelcoating
process
------------------------------------------------------------------------------
Table 4. (contd.)
------------------------------------------------------------------------------
Year Author(s) No. of Exposure levels Work area/process
plants mg/m3 (ppm)
------------------------------------------------------------------------------
1981 Schumacher et 12 < 1260 (< 300) Table 7
al.
1982 Ikeda et al. 5 < 4-1075 reinforced plastic boat
(< 1-256) plants
------------------------------------------------------------------------------
a Years when the study was conducted.
The resin manufacturers have tried to decrease styrene
emissions by reducing the styrene content of resins and by
using thixotropic resins and film-forming additives. They
(Nylander, 1979; Synthetic Resins Limited, 1980; Voskamp &
Studenberg, 1981) claim that significant decreases in styrene
emission can be achieved using these new resins, often called
low styrene emission resins (LSE resins). In the study of
Schumacher et al. (1981), the results of laboratory tests on
such resins suggested a 30% reduction in styrene vapour, but
when tested in the workplace there was essentially no
difference in the exposure levels of the workers and the
authors suggested the need for further studies to record any
effects from the use of the new resins.
Concentrations of styrene found during the production of
reinforced plastics (Zielhuis et al., 1964; Götell et al.,
1972; Bodnei et al., 1974; Rosensteel & Meyer, 1977) were
generally much higher than those found in the polystyrene
production plants, with peak concentrations as high as 6300
mg/m3 (1500 ppm). Most of the environmental concentrations of
styrene in reinforced plastic plants, summarized in Table 4,
are 8-h time-weighted-average (TWA) concentrations, but some
values represent shorter periods. It can be seen from the
table that exposure occurs not only in hand layup and spraying
operations using open moulds, but also in mechanical and
closed mould work. Furthermore, workers who do not handle the
polyester resin themselves but work in the laminating room
undergo passive exposure. It should be noted that the
sampling strategy used influences the results; for example,
breathing zone concentrations may differ considerably from the
general air concentrations.
4.2.3. Styrene-butadiene applications
Hazards encountered in the synthetic rubber industry
during the Second World War were discussed by Wilson (1944).
The principal chemicals used in the manufacture of rubber, at
that time, were styrene, butadiene, and acrylonitrile.
More recently, other studies of synthetic rubber
manufacture have shown that levels of styrene are low.
Basirov (1968) reported concentrations of 60-130 mg/m3 (14-31
ppm) in a 1968 study of a synthetic rubber plant. In a health
risk evaluation in a similar type of plant, Gunter & Lucas
(1973) were unable to find any measurable concentrations of
styrene. However, styrene levels varying between 42 and 840
mg/m3 (10 and 200 ppm) were measured in a synthetic rubber
plant by Spinazzola et al. (1980).
4.2.4. Summary of occupational exposure
In summary, occupational exposure to styrene varies
considerably depending on the operations concerned. While styrene
is present in detectable amounts in styrene/polystyrene
manufacturing plants, the greatest exposures occur in the
operations and industries that use unsaturated polyester resins
dissolved in styrene. However, in all industrial operations using
styrene, high levels of exposures occur during the clean-up and
maintenance procedures.
Table 5. Airborne styrene concentrations at 36 Finnish
factories using hand layup methoda
(i) Mean 8-h TWA breathing zone styrene concentrations at 22
factories
---------------------------------------------
Factory mg/m3 ppm Factory mg/m3 ppm
---------------------------------------------
1 1109 264 12 223 53
2 995 237 13 214 51
3 836 199 14 197 47
4 613 146 15 193 46
5 433 103 16 189 45
6 382 91 17 185 44
7 382 91 18 169 38
8 340 81 19 134 32
9 336 80 20 134 32
10 294 70 21 126 30
11 273 65 22 97 23
---------------------------------------------
(ii) Mean breathing zone styrene concentrations during shorter
(1-5 h) industrial hygiene surveys at 14 factories
---------------------------------------------
Factory mg/m3 ppm Factory mg/m3 ppm
---------------------------------------------
23 1029 244 30 416 99
24 819 195 31 302 72
25 50 12 32 235 56
26 769 183 33 193 46
27 622 148 34 105 25
28 521 124 35 92 22
29 491 117 36 67 16
---------------------------------------------
(iii) Distribution of styrene concentrations by product type
----------------------------------------------------------
8-h TWA styrene TWA styrene conc.
conc. for periods of 1-5h
Product type (factories 1-22) (factories 23-36)
mg/m3 ppm mg/m3 ppm
----------------------------------------------------------
Boats 483 115 865 206
Other products
- small objects 210 50 424 101
- large objects 357 85 248 59
----------------------------------------------------------
a Adapted from: Kallioski (1976).
Table 6. Airborne styrene concentrations at a factory in the USAa
---------------------------------------------------
8-h TWA styrene exposure
Job category (mean ± SD)
mg/m3 (ppm)
---------------------------------------------------
Prefabrication 11.8 ± 16.8 (2.8 ± 4)
Gel coating 288 ± 251 (68.5 ± 59.7)
Hand layup 350 ± 179 (83.4 ± 42.7)
Hand layup, other areas 108 ± 110 (25.7 ± 26.2)
Woodwork/upholstery 14.3 ± 8.8 (3.4 ± 2.1)
Final assembly 14.3 ± 8.8 (3.4 ± 2.1)
Custom moulding 27.3 ± 16.4 (6.5 ± 3.9)
Small boat 17.2 ± 4.2 (4.1 ± 1.0)
Miscellaneous 16.4 ± 13.0 (3.9 ± 3.1)
---------------------------------------------------
a Adapted from: Brooks et al. (1979).
Table 7. Airborne styrene concentrations at 12 factories in the USA. The figures in the columns
represent the number of workers in each exposure groupa
-------------------------------------------------------------------------------------------------------
Styrene concentration mg/m3 (ppm)
Classification
0-210 210-420 420-630 630-840 840-1050 1050-1260 > 1260 Totals
(0-50) (50-100) (100-150) (150-200) (200-250) (250-300) (> 300)
-------------------------------------------------------------------------------------------------------
Plant Type
Boat plants 127 144 80 32 25 23 48 479
Non-boat plants 26 47 29 5 3 1 2 113
Totals 153 191 109 37 28 24 50 592
Job Category
Laminators 61 70 61 27 21 11 22 273
Chopper gun operators 37 62 40 25 13 17 30 224
Gel coaters 12 6 4 3 2 27
Totals 110 138 105 55 34 28 54 524
Plant Type (Exposure of Laminators)
Boat plants 52 48 42 26 18 13 20 219
Non-boat plants 6 24 17 2 3 1 1 54
Totals 58 72 59 28 21 14 21 273
-------------------------------------------------------------------------------------------------------
a Modified from: Schumacher et al. (1981).
Table 8. Styrene concentration during various processes in boat production
-----------------------------------------------------------------------------------------------------------------------------
Process Case Personal sampling Stationary sampling
---------------------------------------------- ------------------------------------------------
Na GMb GSDc Ranged Nc GMb GSDc Ranged Nc
-----------------------------------------------------------------------------------------------------------------------------
Lamination A 25 500 (119) 6.8 (1.6) 143-1075 (34-256) 17 550 (131) 5.5 (1.3) 307-731 (73-174)
over boat B 9 273 (65) 5.8 (1.2) 193-378 (46-90) 15 281 (67) 5.9 (1.4) 160-546 (38-130)
shell mould
Installation laminators 3 71.4 (17) 7.1 (1.7) 42-118 (10-28) 20 33.6 (8) 10 (2.4) 8-214 (2-51)
of ribs helpers 5 < 4.2 (< 1) 23.5 (5.6) 8 (D1-2)
Installation laminators 5 92.4 (22) 8.8 (2.1) 25-185 (6-44) 6 29.4 (7) 12.2 (2.9) 8-92 (2-22)
of division helpers 5 D - (D-D)
plates
Auxilary 5 54.6 (13) 16.8 (4) 8-181 (2-43) 5 96.6 (23) 7.1 (1.7) 59-164 (14-39)
lamination
on deck
Lamination on A 4 128 1.4 (104-211) 3 269 (64) 19.3 (4.6) 55-1172 (13-279)
hold walls B 21 127 1.3 (87-215) 20 269 (64) 7.6 (1.8) 101-1088 (24-259)
Equipment 8 2 2.8 (1-17) 15 < 4.2 64.7 (15.4) Ng-38 (Ng-9)
(< 1)
Plant floor A 39 6 24.8 (5.9) 8-76 (2-18)
in general B 14 < 1 69.7 (16.6) Na-38 (Na-9)
-----------------------------------------------------------------------------------------------------------------------------
a The number of workers equipped with personal samplers for 4 h work (1300-1700).
b The geometric mean.
c The geometric standard deviation.
d The minimum and maximum concentration observed.
e The number of sites monitored by stationary samplers for 4 h (1300-1700).
f D = detected but not measurable (less than 4.2 mg/m3 (1 ppm).
g Not detected.
5. CHEMOBIOKINETICS AND BIOTRANSFORMATION
5.1. Uptake
5.1.1. Human studies
5.1.1.1. Uptake by inhalation
Pulmonary uptake of styrene concentrations of 67-164 mg/m3
(16-39 ppm), after a single breath or during exposures of up
to 8 h, has been studied in human volunteers. Depending on
exposure conditions, uptake varied from 45 to 66% (Bardodej et
al., 1961; Fiserova-Bergerova & Teisinger, 1965; Bardodej &
Bardodejova, 1970).
Astrand et al. (1974) examined the pulmonary uptake of
inhaled styrene (210 and 630 mg/m3; 50 and l50 ppm) in 14
healthy subjects at rest and during exercise over 30-min
periods. Blood concentrations of styrene were measurable
shortly after the onset of exposure indicating a rapid
uptake. With light work (50 W exercise), the alveolar
ventilation increased 3-fold, while the alveolar concentration
of styrene barely changed. The concentration of styrene in
arterial blood tripled.
Fernandez & Caperos (1977) measured the uptake of styrene
in 6 volunteers at exposure levels ranging from 294 to
840 mg/m3 (70 to 200 ppm) for periods ranging from 4 to 8 h.
The retention of styrene, measured from alveolar ventilation,
varied from 82 to 93%.
In studies by Ramsey & Young (1978) and Ramsey et al.
(1980) on 4 human volunteers, a single exposure to a styrene
concentration of 336 mg/m3 (80 ppm) for 6 h resulted in a
blood concentration at the end of exposure of about
800 µg/litre. A steady state concentration of about
900 µg/litre was predicted for repeated daily exposure to the
same atmospheric concentrations. The authors assumed a
2-compartment model that allowed a zeroth order uptake rate of
99.6 mg/h to be calculated.
5.1.1.2. Uptake from the gastrointestinal tract
No studies have been found in the literature related to
the uptake of styrene from the human gastrointestinal tract.
5.1.1.3. Uptake through the skin
Styrene is absorbed through human skin when applied in the
form of a liquid, an aqueous solution, or a vapour. Rates of
uptake of between 9 and 15 mg/cm2 per h were reported when
liquid styrene was applied to the skin of the hands and
forearm (Dutkiewicz & Tyras, 1967; 1968). Rates of uptake of
0.004-0.180 mg/cm2 per h were reported when aqueous solutions
containing mean styrene concentrations of between 66.5 and 269
mg/litre were applied. Skin absorption of styrene was some 30
times greater than that of aniline, benzene, or nitrobenzene.
Riihimäki & Pfäffli (1978) measured the percutaneous
uptake of styrene vapour in 2 human volunteers, wearing thin
cloth pyjamas and socks, who were exposed to a styrene vapour
concentration of 2520 mg/m3 (600 ppm) for 3.5 h, including 3
work intervals of 10 min each. Full-face respirators were
used to prevent inhalation uptake and the temperature was
maintained at 25°C with 50% relative humidity. Blood levels
reached a peak 3 h after the start of exposure and then
declined over the next 4 h. These results were equivalent to
a pulmonary exposure of about 84 mg/m3 (20 ppm) for an equal
period of time.
5.1.2. Experimental animal studies
5.1.2.1. Uptake by inhalation
A recent review of studies of the uptake of styrene in
several animal species indicated that styrene is distributed
rapidly throughout the body, is stored in lipid-rich tissues,
and is extensively metabolized (Santodonato et al., 1980). In
a study on rats exposed to styrene concentrations in air of
336, 840, 2520, and 5040 mg/m3 (80, 200, 600, and 1200 ppm)
for up to 24 h, kinetic data obtained by Ramsey & Young (1978)
obeyed a 2-compartment model. From the kinetic coefficients
derived from the data, the authors predicted that there was
little or no potential for bioaccumulation with repeated 8 h
per day exposure to 336 mg/m3 (80 ppm). They claimed that 95%
of the predicted maximum blood concentration of styrene would
be reached during the first day of exposure.
In another study in which rats with an indwelling jugular
cannula were exposed to styrene atmospheres (Withey, 1978;
Withey & Collins, 1979), a 2-compartment model was observed at
exposure concentrations ranging from 210 to 8400 mg/m3 (50 to
2000 ppm) for a period of 5 h. From the assumed zeroth-order
kinetics for uptake together with the rate coefficients for
distribution and elimination, it was possible to predict that
the time to equilibrium or steady-state would be longer at
higher exposure levels.
An autoradiographic study on the uptake of 14C-styrene-8
and 14C-styrene-ring by mice was carried out by Bergman
(1979). Each animal inhaled the vapours from 10 µl of styrene
in a small inhalation apparatus for 10 min. Extensive
uptake was observed in all organs and the potential for
enterohepatic recycling was demonstrated by the appearance of
the radiolabel in the bile. Styrene uptake in the mice was
reported to be low compared with that in human subjects (less
than one half) and this was attributed to the reduced
respiratory frequency in the mouse, induced by the irritant
action of the styrene vapour on the respiratory tract.
5.1.2.2. Uptake from the gastrointestinal tract
There have been few reports on the kinetics and the extent
of uptake of styrene from the gastrointestinal tract in
animals.
A study by Sauerhoff et al. (1976) on male and female
rats, administered a single oral dose of 14C-labelled styrene
at 500 or 50 mg/kg body weight, showed that a greater fraction
of unchanged styrene was excreted via the lung after
administration of the higher dose, a result suggesting that
saturable metabolic pathways were involved. The authors found
that the uptake of styrene monomer from oral doses was greater
for the female than for the male rats.
In a report of a preliminary investigation on the uptake
kinetics of styrene from the gastrointestinal tract of rats
(Withey, 1976), it was noted that the uptake and kinetic
profile after a single dose, administered as an aqueous
solution, was so rapid (peak values being obtained less than 4
min after the administration of the dose) that it was almost
indistinguishable from the kinetics of an intravenous bolus
dose. In contrast, when a similar dose was administered as a
solution in vegetable oil, peak values were not obtained until
4 h after dosing.
5.1.2.3. Uptake through the skin
Wolf et al. (1956) applied unspecified amounts of liquid
styrene to the ear or to the shaved abdomen of rabbits during
periods of 2-4 weeks (10-20 applications). Moderate irritation
and slight necrosis were observed. The authors claimed that, at
the doses applied, there was no evidence that styrene was absorbed
through the skin in amounts sufficient to cause acute, systemic
toxicity.
The percutaneous uptake of styrene in rats has been
measured by immersing the tail of the animal in liquid styrene
for 1 h (Shugaev, 1969). Inhalation exposure was carefully
avoided. Significant uptake of styrene by the liver and brain
was estimated to be between 50 and 70 % of the concentrations
found in the same organs after a 4-h inhalation exposure to a
vapour concentration of 11.8 gm/m3.
5.2. Distribution and Storage
5.2.1. Human studies
5.2.1.1. Controlled human studies
Studies on experimental animals have demonstrated that
styrene is widely distributed throughout the tissues and
organs and that it may accumulate in adipose tissue. Studies
on the distribution of styrene in human volunteers have,
necessarily, been limited to its quantitative analysis in
blood, expired air, and adipose tissue.
In a study by Engström (1978), 7 male subjects were
exposed to a styrene concentration of 210 mg/m3 (50 ppm)
during 30 min at rest and three, 30-min periods on a bicycle
ergometer set at a work intensity of 50, 100, or 150 W. The
mean uptake of styrene was 490 mg. Specimens of subcutaneous
adipose tissue were taken before and after exposure and at 0.5, 2,
4, and 21 h after exposure. Mean concentrations in the adipose
tissue, up to 21 h after exposure, were of the same magnitude (3.6
mg/kg). From the concentration of styrene still detectable l3 days
after exposure, it was possible to estimate an elimination half-
time of between 2.2 and 4.0 days.
Ramsey & Young (1978) exposed volunteers to a styrene
concentration of 336 mg/m3 (80 ppm) for 6 h and observed that
the decay of styrene concentration in the blood following the
exposure fitted a 2-compartment model. They speculated that
accumulation of styrene would not occur following repeated
exposure to concentrations up to 840 mg/m3 (200 ppm).
5.2.1.2. Occupational exposure studies
There have been a number of studies involving the
determination of styrene concentrations in the blood and
adipose tissue of workers occupationally exposed to styrene
monomer. These studies were not only intended to investigate the
correlation between levels in tissues and previous exposure to
styrene but also to see if there was a potential for its
accumulation and storage in adipose tissue. Wolff et al. (1977)
studied 25 workers who allowed from 3 to 66 mg of subcutaneous
gluteal adipose tissue to be taken. It was demonstrated that
styrene persisted in this tissue for as long as 3 days following
exposure, whereas urine metabolites and styrene in expired air
persisted for up to 16 h after exposure. In an extension of this
study (Wolff et al., 1978a), data on levels of styrene in the
gluteal adipose tissue of an additional 25 workers suggested a
correlation with blood styrene and urinary metabolite levels.
Engström et al. (1978a,b) measured air concentrations of
styrene in a polymerization plant and obtained subcutaneous
adipose samples from 3 workers. The TWA air concentrations
ranged from 32 to 84 mg/m3 (7.6 to 20.2 ppm) and the mean
daily styrene uptake of the 3 volunteers was 193, 343, and 558
mg, respectively. Adipose tissue concentrations were 2.8,
4.0, and 8.1 mg/kg, respectively, at the beginning of the
working week, and 4.7, 7.7, and 11.6 mg/kg, at the end.
5.2.1.3. General population studies
Reports on the uptake of styrene in the general population
are lacking.
5.2.2. Experimental animal studies
There have been a number of reports on the distribution of
controlled doses of styrene monomer administered by different
routes in several animal species.
In studies on mice and rats, Shugaev (1969) showed that,
after inhalation exposure to the median lethal concentration
(LC50), for 2 and 4 h, respectively, there was a positive
correlation between the brain concentration of styrene and
lethality. Styrene levels in the perirenal adipose tissue
were some 5 times higher than those in the brain, liver,
kidney, and spleen.
Savolainen & Vainio (1977) studied the organ distribution
of radiolabelled styrene and styrene epoxide following an
intraperitoneal injection of 577 µmol, 3, 6, and 24 h after
dosing. Three hours after dosing, the highest concentration of
styrene was found in the kidneys followed by the brain, duodenum,
liver, spinal cord, lungs, and blood. The kidney concentration was
19 times higher than that in the blood. After 6 h, levels in the
duodenum and brain had decreased whereas levels in the blood had
increased.
The distribution of 14C-styrene-8 and 14C-styrene-ring was
studied in male mice after a 10-min inhalation exposure
(Bergman, 1979). Animals were killed at 0.5, 1, 2, 4, 8, 24,
and 48 h after exposure and subjected to whole-body auto-
radiography. The autoradiograms obtained shortly after
exposure showed that the radioactivity was localized in the
bronchi, lungs, and liver, for both styrene labelled in the
side chain and the ring-labelled compound. Styrene was
apparently cleared from the nervous tissues in less than 1 h,
since no radiolabelled material was detected at 0.5 h.
However, it was retained in adipose tissues for up to 48 h
after dosing. Again, a large proportion of the radioactivity
was found in the bile and kidney suggesting that these routes
are the primary routes of excretion. Radiolabelled material
appeared in the intestinal contents within 1 h of exposure and
persisted for at least 24 h.
A recent inhalation study on rats (Carlsson, 1981) showed
that the largest amounts of 14C-styrene and its metabolites
were found in the kidneys, after an 8-h exposure to 184 mg/m3
(45 ppm). The styrene concentrations were also high in
subcutaneous adipose tissue; the concentrations in the
cerebrum, cerebellum, and muscles amounted to about 70% of the
styrene concentration in arterial blood.
The distribution of styrene was examined in male Wistar
rats after intravenous administration at 4.01 or 13.37 mg/kg
body weight or after a 5 h inhalation exposure at 6
concentrations between 227 and 9408 mg/m3 (54 and 2240 ppm)
(Withey, 1976; Withey & Collins, 1977, 1979). Animals were
killed immediately after the termination of the vapour
exposure, and the blood, heart, kidney, liver, spleen, brain,
and perirenal fat were analysed for their styrene content. At all
exposure levels except the lowest, styrene concentrations in all
organs were higher than that in the blood. At the lowest level of
exposure, the concentration in the kidney was at least 10 times
greater than that in any other organ except the perirenal fat. At
higher levels of exposure, the highest concentration was found in
the liver. Levels of styrene in organs, 8 min after intravenous
administration of 13.37 mg/kg were found in the following
descending order: liver > kidney > brain > blood > heart and
spleen. For the lower dose (4.01 mg/kg), the levels in these
organs were of the same order of distribution, but lower than that
in the blood, suggesting that, as in the case of the inhalation
study, the distribution to the major organs varied with dose.
5.3. Biotransformation
The first step in the metabolic transformation of styrene
is its oxidation, catalysed by cytochrome P-450 dependent
monooxygenases, to oxirane derivatives in the aliphatic chain
or in the aromatic ring (Fig. 1).
 The oxidation of styrene to styrene 7,8-oxide has been
demonstrated in vitro using rat or rabbit liver microsomes
(Leibman & Ortiz, 1969; Leibman, 1975; Belvedere et al., 1977;
Duverger-van Bogaert et al., 1978; Watabe et al., 1978), rat
liver nuclei (Gazzotti et al., 1980) or rat, rabbit, or
guinea-pig extrahepatic microsomes (Cantoni et al., 1978). The
major route of styrene metabolism is believed to proceed via
this epoxide intermediate.
Styrene has been shown to be epoxidized to ( R)- and
( S)-styrene 7,8-oxides in the ratio 1:1.3 by rat liver microsomal
cytochrome P-450 (Watabe et al., 1981a). The ( R)-and ( S)-oxides
are hydrolysed in a regiospecific manner by rat liver microsomal
epoxide hydrolase to ( R)- and ( S)-phenyl-ethylene glycols in the
ratio of 1:4 (Watabe et al., 1981a). Styrene oxides are also
conjugated with glutathione (Oesch, 1973; James et al., 1975;
Pacheka et al., 1979). Rat liver cytosolic glutathione
transferases convert the ( R)- and ( S)-oxides stereoselectively
to S-(1-phenyl-2-hydroxyethyl)-glutathione (GSH conjugate 1) and
S-(2-phenyl-2-hydroxyethyl)-glutathione (GSH conjugate 2),
respectively (Watabe et al., 1981b). Conjugates 1 and 2 would be
directly correlated with the urinary N-acetyl- S-cysteine
conjugates, namely 1-phenyl-2-hydroxyethyl and 2-phenyl-2-
hydroxyethylmercapturic acids, respectively (James & White, 1967;
Seutter-Berlage et al. 1978; Watabe et al., 1982c).
Rats administered styrene intraperitoneally (i.p.) also
excreted 2-vinylphenol and 4-vinylphenol in conjugated forms
in the urine (Hiratsuka et al., 1982a). Styrene 1,2- and
3,4-oxides administered i.p. to rats have been demonstrated in
the urine as 2-vinylphenol and 4-vinylphenol, respectively
(Hiratsuka et al., 1982a). However, 3-vinylphenol has not
been detected in the urine (Bakke & Scheline, 1970; Pantarotto
et al., 1978; Watabe et al., 1978). Other minor metabolites
are 1- and 2-phenylethanol (Delbressine et al., 1980).
Comparing the kinetic parameters of styrene monooxygenase
and styrene 7,8-oxide hydrolase in rat liver microsomes
(Cantoni et al., 1978; Duverger-van Bogaert et al., 1978), it
appears that the conversion of styrene 7,8-oxide into styrene
glycol is preferred. The ratio between styrene epoxide
hydrolase and monooxygenase in rat liver was 2-3 times higher
than those found in mouse and rabbit liver (Cantoni et al.,
1978).
Styrene glycol is partly transformed into a glucuronide
that has been detected in the urine of styrene-treated rabbits
(El Masri et al., 1958). The major metabolic pathway involves
the sequential oxidation to mandelic, phenylglyoxylic, and
benzoic acids, which have been identified in urine (Bardodej,
1964; James & White, 1967; Ohtsuji & Ikeda, 1971).
Quantitative differences have been observed between various
species, hippuric acid being quantitatively the most relevant
urinary metabolite in the rabbit (El Masri et al., 1958),
phenylglyoxylic acid in the rat (Bardodej et al., 1971;
Watanabe et al., 1982c) and mandelic acid in man (Bardodej,
1964; Bardodej & Bardodejova, 1970). Minor metabolites
identified include parahydroxymandelic, parahydroxyphenyl-
glyoxylic, and parahydroxybenzoic acids (Bakke & Sheline,
1970; Pantarotto et al., 1978).
The metabolism of styrene is enhanced by phenobarbital
pretreatment and is inhibited by SKF 525-A, an inhibitor of
monooxygenases (Ohtsuji & Ikeda, 1971).
Styrene 7,8-oxide either binds covalently to macro-
molecules such as microsomal proteins (Marniemi et al., 1977;
Watabe et al., 1978) or conjugates with glutathione
non-enzymatically and in the presence of a transferase (Oesch,
1973; James et al., 1975; Pachecka et al., 1979). Pheno-
barbital pretreatment of the rats enhances, whereas carbon
tetrachloride administration reduces, the NADPH dependent-
binding to microsomal protein in vitro (Watabe et al., 1978).
Prolonged exposure to a styrene vapour concentration of
1260 mg/m3 (300 ppm) leads to an enhancement of the metabolic
elimination of the compound (Vainio et al., 1979) resulting in
decreasing body burden during continued exposure.
Though numerous metabolic pathways have been found in
animal studies (mainly in the rat and rabbit), only a few have
been identified in human beings. Analysis of the urine of
human subjects exposed to styrene has revealed mandelic and
phenylglyoxylic acids as the major metabolites indicating
that, at least qualitatively, styrene metabolism in human
beings follows pathways similar to those previously described
in animal studies. The results of a study by Pfäffli et al.
(1981) suggest that 4-vinylphenol is also excreted in the
urine of workers exposed to styrene. Though this metabolite
represented only 0.3 % of the urinary concentration of
mandelic acid, there was a good correlation between the
excretion of the two metabolites.
Recent data indicate that human whole blood lymphocyte
cultures apparently catalyse the oxidation of styrene to
styrene 7,8-oxide (Norppa et al., 1980a). Belvedere & Tursi
(1981) have shown that both human lymphocytes and erythrocytes
are capable of converting styrene to styrene 7,8-oxide. In
whole blood, red blood cells may be more important in this
conversion, because they are present in greater numbers than
lymphocytes.
5.4. Elimination
5.4.1. Human studies
5.4.1.1. Controlled human studies
In an inhalation study by Fiserova-Bergerova & Teisinger
(1965), 7 human volunteers were exposed to styrene levels of
63-160 mg/m3 (15-38 ppm) for 5 h. From analysis of the
alveolar air, the authors found that pulmonary elimination
followed a bi-exponential curve indicating a 2-compartment
model.
Stewart et al. (1968) found that styrene elimination from
the lungs, after inhalation exposure, was rapid and bi-
exponential but that the rates of elimination varied with the
level and duration of exposure. It was apparent that subjects
who were exposed to a styrene concentration of 1579 mg/m3 (376
ppm) for 1 h had lower elimination rate coefficients for the
terminal phase than those exposed to 907 mg/m3 (216 ppm) and
214 mg/m3 (51 ppm) for 1 h. No values for the rate
coefficients were given in this report but it was calculated
that one subject, who had been exposed to 491 mg/m3 (117 ppm)
for 2 h, exhaled 1.2% of the total amount of styrene absorbed
during the first 4 h after exposure. Exhaled styrene was
detected up to 24 h after the termination of a 30-min exposure
to concentrations of 210 or 630 mg/m3 (50 or 150 ppm) (Astrand
et al., 1974).
The exhalation of styrene after pulmonary exposure was
also examined in human volunteers by Fernandéz & Caperos
(1977). Six subjects were exposed to vapour concentrations of
294-840 mg/m3 (70-200 ppm) for periods of 4 or 8 h. The
concentration of styrene in the expired alveolar air was
reported to decline rapidly during the first hour following
termination of exposure, at all exposure concentrations, and
2.6% of the total absorbed dose was considered to be
eliminated via the lung.
Exhalation of styrene after inhalation exposure to a
styrene concentration of 210 mg/m3 (50 ppm) was measured in 7
volunteers for 4 consecutive 30-min periods either resting or
in a work cycle (Engström et al., 1978b). The exhalation was
followed for up to 19 h after exposure by monitoring styrene
concentrations in the expired air; some 31% of the retained
styrene was estimated to be eliminated via the lungs. In the
same study, styrene eliminated from adipose tissue was
reported to have a half-life of from 2 to 4 days, which led
the authors to conclude that repeated daily exposure to
styrene could lead to bioaccumulation.
In a study by Ramsey & Young (1978), 4 male human
volunteers were exposed to a styrene concentration of 336
mg/m3 (80 ppm) for 6 h and the styrene in the blood and
expired air monitored for 41 h after exposure. The styrene
concentration in blood was found to decay in a bi-exponential
fashion, typical of a linear 2-compartment model. The rapid
initial elimination phase yielded a half-time of 0.58 h and,
for the slower terminal phase, a value of 13.0 h was
reported. The concentration of styrene in the expired air for
the same volunteers showed a biphasic log-linear decline
similar to that observed for the blood concentrations.
In a study designed to investigate the skin absorption of
styrene (Riihimäki & Pfäffli, 1978), two subjects wearing air-
supplied respirators were exposed to a styrene concentration
of 2520 mg/m3 (600 ppm) for 3.5 h while performing light
work. Bi-exponential elimination data were obtained both from
analysis of venous blood and of alveolar air and the authors
reported a value of about l h for the half-time of the rapid
phase and about 10 h for the slower phase.
Studies conducted on 6 male volunteers at styrene
exposures varying from 865 mg/m3 (206 ppm) for 8 h to 294
mg/m3 (70 ppm) for 4 h gave alveolar concentration decay
curves that the authors claimed were best described by a
3-compartment model (Fernandez & Caperos, 1977). The rate
coefficients for the three phases had half-lives that ranged
from 0.06 to 0.61 h for the rapid phase, 1.01 to 3.75 h for
the intermediate phase, and 10.05 to 24.75 h for the slowest
phase.
A study on the rate of excretion of the urinary
metabolites of styrene, phenylglyoxylic and mandelic acids
(Guillemin & Bauer, 1978) was carried out on 9 human subjects
after pulmonary exposure to styrene at 168 or 840 mg/m3 (40 or
200 ppm) for 4-8 h. The authors found that the rate
coefficients for the urinary excretion of metabolites were
unaffected by the duration or level of exposure and claimed
that if the amounts of both metabolites were totalled over 4
days following exposure, reliable measurements of exposure
could be obtained. For mandelic acid, the first and second
phase half-times were evaluated to be respectively 3.9 h and
24.7 h, while for phenylglyoxylic acid the half-time was
calculated to be 10.5 h (Guillemin & Bauer, 1979).
5.4.1.2. Occupational exposure studies
Of the numerous studies that have been conducted on
styrene-exposed workers (Härkönen, 1978), only a few have been
devoted to the assessment of metabolite production.
In a study by Götell et al. (1972), a group of 17 workers
was divided into 3 exposure subgroups, the highest at 987-1226
mg/m3 (235-292 ppm), an intermediate group at 374-584 mg/m3
(89-139 ppm), and the lowest group at 71-134 mg/m3 (17-32
ppm). Decay curves of styrene in expired air for the 3 groups
had a multi-exponential form but no kinetic parameters were
cited. It was evident, however, that the terminal elimination
phase was markedly faster for the group receiving the highest
exposure.
The rate of appearance of mandelic acid in the urine has been
shown to be biphasic, half times being 9 h and 16 h, respectively
(Engström, K., et al., 1976). In a preliminary report on workers,
with limited time points, monophasic elimination with half-times of
8.5 and 7.8 h, respectively for phenylglyoxylic and mandelic acid,
was found (Ikeda & Imamura, 1974). Considerable inter-subject
variability has been reported by several authors (Philippe et al.,
1971; Götell et al., 1972; Härkönen et al., 1974; Gompertz, 1978).
On the basis of findings in animal studies, the excretion
of hippuric acid was investigated in styrene-exposed workers.
An increase in hippuric acid excretion was noted in styrene-
exposed workers (Ikeda et al., 1974); similar levels and
important fluctuations have been found in unexposed controls
(Engström, K., et al., 1978).
5.4.1.3. General population studies
There have not been any reported studies on the
elimination of styrene in the general population.
5.4.2. Experimental animal studies
Results of studies on rats subcutaneously injected with
500 mg/kg 14C-labelled styrene showed that there was rapid
distribution to tissues and organs within 1 h and that 85% of
the radioactivity was eliminated within 24 h; 71% of the
radioactivity appeared in the urine, 12% in expired air as
carbon dioxide, 3% in the faeces, and about 3% as unchanged
styrene in expired air (Danishefsky & Willhite, 1954).
A later study (Sauerhoff et al., 1976) demonstrated a
similar rapid clearance of ring-labelled 14C-styrene in male
and female rats after oral administration of 50 or 500 mg/kg
body weight. More than 90% of the radioactivity appeared in
the urine within 72 h and no residual styrene was found in the
tissues examined at that time. The urinary excretion of
radioactivity followed a 2-compartment model at the low dose
level with half-times of 1.29 and 8.13 h for the initial
(alpha) and terminal (beta) phases, respectively. At the high
dose, a mono-exponential urinary excretion was observed with a
half-time of 6.74 h. About twice as much styrene was
eliminated by the pulmonary route in male compared with female
rats.
Biphasic excretion of radioactivity in the urine of rats
was observed after inhalation of 14C-styrene (252 or
2520 mg/m3; 60 or 600 ppm) for 6 h with half-time values of
14.9 h for the lower and 19.2 h for the higher exposure
concentration (Sauerhoff & Braun, 1976).
Inhalation exposure of rats to styrene concentrations
varying from 336 to 5040 mg/m3 (80 to 1200 ppm) for 6 h
(Ramsey & Young, 1978) also showed evidence of dose-dependent
kinetics. For the 15-fold increase in exposure level, the
area generated under the blood level-time curve increased by a
factor of 112. The elimination kinetics in the post-exposure
period appeared to change from a bi-exponential curve to a
log-linear form with increasing exposure concentrations. At
the 336 mg/m3 (80 ppm) dose level, the hybrid elimination rate
coefficients for the initial and terminal phases were 0.26 h
and 3.60 h, respectively.
Withey (1978) and Withey & Collins (1979) described the
elimination kinetics of styrene in rats after 5 h inhalation
exposure at levels that varied from 188 to 10 151 mg/m3 (44.8
to 2417 ppm). In contrast to the study by Ramsey & Young
(1978), these authors reported a bi-exponential curve of
elimination for all levels of exposure. The reported
half-times for the initial and terminal phases ranged from 3.4
to 6.3 min and about 50 to 350 min, respectively.
Teramoto et al. (1978) examined the elimination rates of
styrene from samples of the liver, brain, kidney, blood,
spleen, muscle, and adipose tissue in rats after an inhalation
exposure to 2940 mg/m3 (700 ppm) for 4 h. They found that all
tissues had a similar elimination half-time of about 2 h with
the exception of adipose tissue for which the styrene
half-time was about 6 h.
To examine the accumulation of styrene in tissues,
Savolainen & Pfäffli (1978) exposed rats to 1260 mg/m3 (300
ppm), for 6 h per day, and 5 days per week, for up to 11 weeks
and then monitored the styrene concentrations in adipose
tissue. Concentrations increased almost linearly, during the
first 4 weeks of exposure and then decreased exponentially.
In the study reported by Bergman (1979) (section 5.2.2),
mice were exposed through inhalation to 10 µl of volatilized
radiolabelled styrene over a 10-min period. The amount of
unchanged styrene exhaled over an 8-h period following
exposure amounted to 2.9% of the calculated dose. The
kinetics of elimination of radioactivity in exhaled air gave a
biphasic curve with half-times of 15 and 275 min for the
initial and terminal phases, respectively. Eight hours after
dosing, high levels of radioactivity were found in the liver,
kidney, lung, and adipose tissue.
Results of a study by Withey & Collins (1977) showed that
the elimination of styrene after intravenous administration in
rats also obeyed a 2-compartment model. Approximate rates of
elimination from some individual organs were obtained for dose
levels of approximately 1.3 and 4 mg/kg body weight. At the
higher dose, the kinetics of styrene disappearance from blood,
heart, brain, liver, spleen, and kidney appeared to follow a
1-compartment model with an elimination half-time of about
14.4 h in the same organs. At the lower dose, a biphasic
elimination was observed with half-times of about 4.78 and 26
h for the rapid and slow phases, respectively.
5.5. Biological Monitoring of Styrene Uptake
The measurement of styrene in blood, subcutaneous fat, as
well as in exhaled air has been suggested for the biological
monitoring of styrene uptake. Because of the efficient metabolism
of styrene and rapid excretion of the metabolites, mandelic and
phenylglyoxylic acid are present in the urine in easily measurable
amounts. The evaluation of the absorption level has usually been
based either on the concentration of one of these metabolites
(Ohtsuji & Ikeda, 1970; Burkiewicz et al., 1974; Härkönen et al.,
1974; Vivoli & Vechhi, 1974; Engström, K., et al., 1976, 1978;
Fernandez & Caperos, 1977; Caperos et al., 1979; Elia et al., 1980)
or on their sum (Bardodej & Bardodejova, 1970; Philippe et al.,
1971; Guillemin & Bauer, 1978, 1979; Wolff et al., 1978a,b; Elia et
al., 1980). "End of exposure" spot specimens have usually been
sampled and results corrected for the dilution of urine. Only this
type of sample is dealt with in the following discussions.
A number of authors have attempted to correlate levels of
styrene in air with levels of mandelic acid in urine, either
from experimental data or field studies, and calculations of
mandelic acid levels corresponding to styrene levels in air of
210, 420, and 630 mg/m3 (50, 100, and 150 ppm) are presented
in Table 9. The differences between the calculated values
could be explained by such factors as: type of exposure,
sampling strategy, individual variability, and differences in
analytical methods.
The use of unspecific analytical methods influences the
results, especially at low levels of styrene exposure, giving
too high values for metabolite concentrations in the urine
(Engström & Rantanen, 1974).
In the case of occupational exposure, a number of
additional elements may have to be taken into account. For
example, in work situations, exposure is usually repeated and
it has been shown that about 30% of the maximal mandelic acid
concentration of the previous day can still be found in urine
samples taken before work on the following day (Engström, K.,
et al., 1976). In another study, the amount of mandelic acid
excreted at the end of the working shift was shown to increase
in the course of the working week (Fernandez & Caperos, 1977).
Increased pulmonary ventilation with increased physical
work load results in a larger total absorption of styrene and
thus increased excretion of metabolites in the urine (Åstrand
et al., 1974; Droz & Fernandez, 1977).
Fluctuations in styrene concentrations in the workroom air
are very common in occupational exposure and may also
contribute to the differences observed in Table 9. A
short-term high peak exposure occurring shortly before the
sampling time may result in a high mandelic acid concentration
in the urine, even though the time-weighted average
concentration of styrene is low (Engström, K., 1983).
Table 9. Mandelic Acid levels* corresponding to various concentrations of
styrene in air
------------------------------------------------------------------------------
Styrene in air
mg/m3 (ppm)
-----------------
210 420 630 Remarks Reference
(50) (100) (150)
------------------------------------------------------------------------------
mandelic (a) 6 12 18 mandelic acid expressed Spasovski
acid as mg/g creatinine; (1982)
spectrophotometry
(a) - 7 - mandelic acid expressed Guillemin &
as mg/g creatinine; Bonet (1979)
gas chromatography
experimental study
(b) 8 14 20 Density 1.024; high Ikeda et al
performance liquid (1982)
chromatography field
study
(b) 10 20 - Density 1.024; Bardodej (1978)
polarography;
experimental study
(b) 9 19 27 Density 1.024; Engström, K.
gas chromatography; et al. (1978)
field study
(b) 14 26 34 Density 1.024 Härkönen et al.
spectrophotometry (1974)
field study
(b) 7-9 12-13 15-19 Density 1.024; Götell et al.
spectrophotometry; (1972)
field study
(b) 6 14 22 Density 1.024; Elia et al.
gas chromatography (1980)
field study
(b) 3 11 19 Density 1.024; Fields et al.
gas chromatography (1979)
field study
------------------------------------------------------------------------------
*(a) = mg mandelic acid/g creatinine.
(b) = mmol mandelic acid/litre urine.
The ratio of mandelic to phenylglyoxylic acid excreted at
the end of exposure varied from about 1 to 4 in different
studies. The ratio appears to increase at higher exposure
levels (Ohtsuji & Ikeda, 1970; Riihimäki & Pfäffli, 1978;
Caperos et al., 1979; Elia et al., 1980). The mandelic
acid/phenylglyoxylic acid ratios were higher in urine samples
taken at the end of exposure than in samples taken later
(Philippe et al., 1971; Guillemin & Bauer, 1979). In terms of
biological monitoring, it is claimed that the mandelic
acid/phenylglyoxylic acid ratio in the urine may be of help in
interpreting the exposure pattern (Philippe et al., 1971;
Guillemin & Bauer, 1979).
The various factors influencing the excretion of these
metabolites are not well known. It may be that such a
difficulty could be overcome by determining mandelic and
phenylglyoxylic acids and combining the results. This may
explain the good agreement obtained in the two studies in
Table l0 based on the sum of the levels of the two acids
corresponding to styrene concentrations in air of 210, 420,
and 630 mg/m3 (50, 100 and 150 ppm).
Table 10. Sum of mandelic acid and phenylglyoxylic acid levels
(mmol/litre) corresponding to various concentrations of styrene in air
---------------------------------------------------------------------------
Styrene in air
mg/m3 (ppm)
-------------------
210 420 630 Remarks Reference
(50) (100) (150)
---------------------------------------------------------------------------
Mandelic acid 8 16 24 density 1.024; Elia et al.
+ gas chromatography; (1980)
phenylglyoxylic field study
acid 11 19 27 density 1.024 Ikeda et al.
aigu performance (1982)
liquid chromatography;
field study
---------------------------------------------------------------------------
The determination of minor metabolites (4-vinylphenol and
hippuric acid) for the biological monitoring of styrene
exposure has also been reported (Ikeda et al., 1974; Pfäffli
et al., 1981).
The difficulties of the biological monitoring of styrene
uptake using urine samples cannot be overcome by selecting
another medium, as even less experience is available with
other media. Styrene concentrations in blood decline rapidly
during the first hour following exposure, which makes the
results strongly dependent on sampling time.
Styrene concentrations in exhaled air are in equilibrium
with blood concentrations, which means that styrene levels in
exhaled air are also sensitive to the changes in concentration
in ambient air. A single sample of exhaled air at the end of
exhalation can be used only for the evaluation of immediate
past exposure. It has been suggested that extrapolation of a
whole day's exposure is possible using several samples taken
after exposure, from which a "decay curve" can be established
(Fernandez & Caperos, 1977; Fields & Horstman, 1979). This
method is inconvenient for the routine monitoring of workers.
The estimation of styrene concentrations in subcutaneous
fat has been proposed as a method of indicating styrene body
burden, even a long time after exposure, when styrene and its
metabolites can no longer be detected in other specimens
(Wolff et al., 1978). However, much more information about
the kinetics of styrene in fat is needed before the relevance
of this method can be evaluated. For obvious practical
reasons, this technique cannot be used routinely.
6. EFFECTS ON EXPERIMENTAL SYSTEMS
6.1. Haematopoietic and Immune Systems
Spencer et al. (1942) did not observe any differences in
erythrocyte count, haemoglobin concentration, total leukocyte
count, and differential count between 18 rats exposed
repeatedly for 7 h a day for 6 months to a styrene
concentration of about 5460 mg/m3 (1300 ppm) and 18 control
animals. The test animals received a total of 137-139
exposures. The same authors did not see any significant
haematological changes in 8 rabbits and 4 monkeys similarly
exposed.
Wolf (1956) gave styrene orally at 66.7-667 mg/kg body
weight to female rats for 6 months, 5 days a week, but did not
observe any effects on the haematopoietic system.
In a study by Quast et al. (1978), styrene was
administered orally to beagle dogs at 200, 400, and 600 mg/kg
body weight for up to 19 months. At the highest dose, Heinz
bodies were regularly detected in the blood erythrocytes of
the dogs. At 400 mg/kg body weight, Heinz bodies were still
frequently seen, but at 200 mg/kg body weight they were
observed only sporadically in female dogs. Heinz bodies
disappeared after withdrawal of styrene. Decreases in packed
cell volume, red blood cell count, haemoglobin levels, and the
erythrocyte sedimentation rate were also observed.
When styrene was fed to rabbits at doses of up to 250
mg/kg body weight for up to 216 days, severe impairment of the
immunological defence system was indicated with the blood
complement titre reduced and leukocyte phagocytic activity
depressed (Sinitskij, 1969).
6.2. Nervous System and Behaviour
The acute neurotoxic effect of styrene is depression of
the central nervous system (Roth, 1979). However, the
narcotic effect of styrene glycol is at least twice as potent
as that of styrene (Parkki et al., 1976) and styrene 7,8-oxide
is more toxic than both styrene and styrene glycol with a
lethal dose 4-5 times lower than that of the parent compound
(Ohtsuji & Ikeda, 1971). Central nervous system depression
follows treatment with styrene 7,8-oxide. After a single
intraperitoneal injection both styrene and styrene oxide have
been shown to bind covalently to macromolecules in the central
nervous system (Savolainen & Vainio, 1977). In a study on
rats by Shugaev (1969), in which styrene was injected intra-
peritoneally, the maximum concentration of styrene in brain
tissue was 250 mg/kg (range 180-324 mg/kg).
Toxic action in the central nervous system in prolonged
(11 weeks) low-level inhalation exposure to a styrene
concentration of 1260 mg/m3 (300 ppm) led to minor changes in
axonal proteins (Savolainen & Pfäffli, 1977). Concomitant
neurophysiological studies, however, failed to demonstrate any
reduction in the peripheral motor conduction velocities
(Seppäläinen, 1978), a suggested clinical feature of the
toxicity of styrene in human subjects.
In studies by Zaprianov & Bainova (1979) on male rats,
repeated oral doses of styrene (1250 mg/kg body weight for 7
days) decreased monoamine oxidase (EC 1.4.3.4) activity. The
authors concluded that the inhibition of monoamine oxidase
activity in total brain may be regarded as a possible link in
the pathogenesis of reported occupational neurophysiological
effects of styrene.
Polystyrene containers were toxic to nerve cells in a
closed tissue culture system, possibly because of the release
of unreacted styrene in the medium (Mithen et al., 1980).
In an open-field test, styrene vapour at 1260 mg/m3
(300 ppm) had marginal effects on animal behaviour, even when
pronounced neurochemical effects were taken into account
(Savolainen et al., 1980).
In a 6-month study, female rats exposed to 200 and 2000
mg/m3 of styrene exhibited enhanced spontaneous activity.
Long-term memory was impaired in male rats after a 4-month
exposure (Vergieva & Zajkov, 1981).
6.3. Kidneys and the Urinary Tract
Oral treatment of rats with styrene at 400 mg/kg body
weight (administered in olive oil, once a day for 132 days
during 6 months) caused an increase in kidney weight. A lower
daily dose of 133 mg/kg body weight did not have any apparent
effect (Wolff et al., 1956).
Vainio et al. (1979) reported that intermittent 11-week
inhalation exposure of rats to low styrene vapour
concentrations of 1260 mg/m3 (300 ppm), 6 h/day, 5 days a
week, enhanced the activities of both drug conjugating
(epoxide hydratase (EC 4.2.1.63), UDP glucuronosyl transferase
(EC 2.4.1.17), and hydroxylating (ethoxycoumarin 0-deethylase,
cytochrome P-450) enzymes in the kidneys.
6.4. Gastrointestinal Tract
No information was available concerning the effect of
styrene on the gastrointestinal tract.
6.5. Liver
Mild liver damage, particularly in the parenchymal cells,
was reported after a single exposure of rats to styrene vapour
at 10 500 mg/m3 (2500 ppm) for up to 21 h (Spencer et al.,
1942). The same authors also reported increases in liver
weight in rats repeatedly exposed to styrene vapour at
5460 mg/m3 (1300 ppm) for 130-139 days. However, they did not
find any liver damage in guinea-pigs exposed for similar
periods to a styrene concentration of 2730 mg/m3 (650 ppm) or
in rabbits and monkeys exposed to 5460 mg/m3 (1300 ppm).
Intermittent 11-week inhalation exposure of rats to a
styrene concentration of 1260 mg/m3 (300 ppm) (6 h/day, 5
days/week) caused histological liver alterations consisting of
hydropic degeneration, and steatosis of parenchymal cells and
congestion. Enhanced activity of both the drug hydroxylating
(ethoxycoumarin O-deethylase, cytochrome P-450) and
conjugating (epoxide hydrolase (EC 3.3.2.3), UDP
glucuronosyltransferase (EC 2.4.1.42)) enzymes in the liver
was detected (Vainio et al., 1979). In a skin-painting study
on rats, styrene at 4 and 8 ml/kg body weight induced
dose-related fatty infiltration of liver cells that was
reversible after 2 weeks without exposure (Burkova et al.,
1982).
Liver cell necrosis was noted in a long-term
carcinogenicity bioassay in several rats in a group exposed to
a high dose of styrene (2000 mg/kg body weight by gavage, 5
days a week, for 78 weeks) (US National Cancer Institute,
1979).
The intermittent inhalation of styrene vapour at a
concentration of 1260 mg/m3 (300 ppm) in air decreased
significantly the reduced glutathione (GSH) concentration in
rat liver (Elovaara et al., 1979). Vainio & Mäkinen (1977)
demonstrated a clear species difference in sensitivity to
styrene-induced depletion of GSH content. The mouse was the
most sensitive and the rat the most resistant species. The
decrease in GSH in liver was dose-dependent. A slight decrease
was observed in rats exposed to a styrene concentration in air
of 420 mg/m3 (100 ppm) (Vainio et al., 1979). When isolated
hepatocytes were incubated in the presence of styrene, there
was also a dose- and time-dependent decrease in GSH
concentrations (Zitting et al., 1980).
When the hepatic GSH in Syrian hamsters decreased to less
than 1 mmol/litre as a result of a high intragastric dose of
styrene (4.5 g/kg body weight), serum aminotransferase
activity increased (Parkki, 1978).
Quast et al. (1979) observed an increased amount of
hemosiderin pigment in liver reticuloendothelial cells of male
and female beagle dogs administered styrene orally at 400 or
600 mg/kg body weight, 7 days a week, for up to 19 months.
The higher dose also increased the number of hepatocellular
intranuclear acidophilic crystalline inclusions.
6.6. Cardiovascular System
No information was available concerning possible effects
of styrene on the cardiovascular system.
6.7. Respiratory System
A single exposure of rats to styrene at 5460-42 000 mg/m3
(1300-10 000 ppm) for 1-4 h caused mucous membrane irritation
as evidenced by lacrymation, nasal discharge, and salivation,
accompanied by aggressive scratching and rubbing of the eyes
and nose. The irritation was particularly severe at higher
concentrations (Spencer et al., 1942). The degree of acute
pulmonary damage varied, in general, with the vapour
concentration of styrene and the length of exposure. When the
exposure was long enough to cause death in some of the exposed
animals, marked pulmonary lesions were induced at all
concentrations studied. Pulmonary changes varied from slight
congestion to congestion, multiple haemorrhage, exudation, and
various degrees of leukocytic infiltration. When another
group of animals (60 rats, 94 guinea-pigs, 12 rabbits, 4
monkeys) were exposed to a styrene concentration of 5460 mg/m3
(1300 ppm) for 7-8 h/day, 5 days a week, over a period of at
least 6 months, microscopic examination of various tissues,
including the lung, did not reveal specific changes in any of
the animals except the guinea-pigs. About 10% of all
guinea-pigs that were exposed repeatedly to styrene at 5460
mg/m3 (1300 ppm) died after several exposures; microscopic
examination of the dead animals revealed pronounced lung
irritation, which was characterized by general acute
inflammatory response. Respiratory insufficiency was the
cause of death (Spencer et al., 1942).
Wolf et al. (1956) exposed animals by inhalation to a
styrene concentration of 5460 mg/m3 (1300 ppm) for 7 h/day,
for 214-360 days. They found slight eye and nasal irritation
in rats and guinea-pigs, but no effects in rabbits and rhesus
monkeys. However, when rats and guinea-pigs were exposed to a
styrene concentration of 2730 mg/m3 (650 ppm), no effects were
observed.
According to Alarie (1973), the concentration of styrene
in air that reduced the respiratory frequency in mice to 50%
was 666 mg/m3 (95% confidence limits 574-758 mg/m3).
When rats were exposed to styrene at 1260 mg/m3 (300 ppm)
for 6 h daily, 5 days a week for 2-11 weeks, no significant
increase in UDP glucuronosyltransferase activity was observed
in the lungs but, after 2 and 4 weeks exposure,
the free glutathione content in the lung was lower (P < 0.05)
than that in the controls (Vainio et al., 1979).
6.8. Endocrine Organs
The adverse effects of styrene on the endocrine organs
have been insufficiently studied. While Izjumova (1972)
reported that repeated exposure to styrene concentrations of
5-50 mg/m3 (1-12 ppm) for up to 4 months prolonged the estrus
cycle in rats, the data were inconsistent from month to month,
and appeared not to be dose-dependent.
6.9. Carcinogenic Effects
6.9.1. Styrene
6.9.1.1. Oral administration
(a) Mouse
In a carcinogenesis bioassay (US National Cancer
Institute, 1979; Chu et al., 1981), 2 groups each of 50 male
and 50 female B6C3F1 mice, 6 weeks of age, were given styrene
at 150 and 300 mg/kg body weight (at least 0.3% impurities) in
corn oil by gavage, 5 days per week, for 78 weeks, and then
observed for an additional 13 weeks. A group of 20 males and
20 females receiving corn oil only (10 ml/kg body weight)
served as vehicle controls. At the end of the study, 78%,
92%, and 100% of the male mice and 76%, 80%, and 80% of the
females were alive in the high-dose, low-dose, and control
groups, respectively. An increased incidence of adenomas and
carcinomas of the lung was seen in treated male mice, i.e., 3
adenomas and 3 carcinomas in 44 animals in the low-dose group,
and 4 adenomas and 5 carcinomas in 43 animals in the high-dose
group, compared with none in the 20 controls. The increased
incidence of both types of lung tumours combined was
statistically significant (P = 0.02) in the high-dose group
compared with the vehicle-treated controls and also in the
Cochrane-Armitage test for linear trend (P = 0.02). It should
be noted that the number of control animals was small (40)
compared with the treated group (100), that the historical
vehicle-treated controls were inadequate, and that the
incidence of neoplasms in historical untreated controls was
12% (32/271). For these reasons, it was not possible for the
Task Group to conclude that styrene was carcinogenic in this
study, even though the results of the study were positive.
(b) Rat
In carcinogenic bioassays (US National Cancer Institute,
1979; Chu et al., 1981), groups of 50 male and 50 female
Fischer 344 rats, 6 weeks of age, were given styrene (at least
0.3% impurities) in corn oil, by gavage, 5 days per week,
either at 1000 or 2000 mg/kg body weight for 78 weeks with
further observation for 27 weeks, or at 500 mg/kg body weight
for 103 weeks with further observation for 1 week. Two
groups, each of 20 males and 20 females served as vehicle
controls. A high mortality was seen in the high-dose group;
only 12% of the males survived 53 weeks of treatment and 14%
of the females survived 70 weeks of treatment. Hepatic
necrosis was observed in several rats. At the 90th week of
the study, 94%, 88%, 85%, and 90% of the males and 92%, 92%,
75%, and 90% of the females were alive in the medium-dose,
low-dose, and the 2 control groups, respectively. There was
no statistically significant difference between the groups in
tumour incidence at any site (National Cancer Institute,
1979). The Task Group noted the high mortality in the
high-dose group and the small numbers of animals in the
control groups.
6.9.1.2. Inhalation exposure
Rat
In a study conducted by Jersey et al. (1978), 2 groups of
84 and 86 male, and 2 groups each of 85 female Sprague-Dawley
rats of the Spartan substrain, 7-8 weeks of age, were exposed
by inhalation to "production grade" styrene (minimum purity
99.5%) containing approximately 5 ppm t-butylcatechol. They
were exposed 6 h/day, 5 days/week, for 18.3 months, if male,
and for 20.7 months, if female. Exposure duration was based
on the time when mortality reached 50% in one of the test
groups of that sex. Initial concentrations of styrene in air
were either 5040 mg/m3 (1200 ppm) or 2520 mg/m3 (600 ppm).
The high level was reduced to 4200 mg/m3 (1000 ppm) after 2
months because of excessive toxicity. Another group of 85
males and 85 females served as untreated controls. At the end
of the 2-year study, 5, 18, and 6 males and 30, 30, and 22
females had survived in the control, low-, and high-dose
groups, respectively. An excessive mortality from chronic
pneumonia occurred in the control and high-dose male groups.
An increased (but not statistically significant) combined
incidence of leukaemias and lymphosarcomas was seen: 1/84
males and 6/85 females in the high-dose groups, 5/86 males and
6/85 females in the low-dose groups, 1/85 males and 1/85
females in the control groups. If the total incidence of
leukaemias/lymphosarcomas in females is compared with
historical controls, it becomes statistically significant
(P < 0.05). A statistically significant increase in the
incidence of mammary adenocarcinomas was seen in the low-dose
female group (7/85 versus 1/85 in controls), but the incidence
was not statistically significant in comparison with
historical controls. Though these data are inconclusive, they
are interpreted by Jersey et al. (1978) as suggesting an
association between the exposure of the female rats to styrene
and an increased incidence of tumours of the leukaemia/
lymphosarcoma types. The Task Group was unable to evaluate
this study because of the high mortality.
6.9.1.3. Pre- and postnatal exposure
(a) Mouse
In the studies of Ponomarkov & Tomatis (1978), 29 pregnant
O20 mice were each given a single treatment of styrene at 1350
mg/kg body weight (99% pure dissolved in 0.1 ml olive oil) by
stomach tube on the 17th day of gestation. A control group of
9 pregnant animals received olive oil alone. The neonatal
mortality rate in the offspring of styrene-treated mice was
43%, compared with 22% in the controls. The same dose of
styrene was then administered weekly, by stomach tube, to 45
male and 39 female progeny from weaning to 16 weeks of age, at
which time the treatment was stopped because of the high rate
of mortality (64% alive at 20 weeks). The study was
terminated at 100 weeks, when all the animals had died. No
differences in tumour incidence were found between mothers
treated with styrene and those given olive oil. In the
progeny that had received weekly treatments, lung tumours
(adenomas and adenocarcinomas) were found in 20/23 males and
32/32 females, compared with 8/19 and 14/21 in olive
oil-treated controls (P < 0.01, P < 0.01) and compared with
34/53 and 25/47 in untreated controls (P < 0.05, P < 0.001).
No differences in tumour incidence at sites other than the
lung were seen in the progeny, compared with olive oil-treated
or untreated controls.
In the same studies (Ponomarkov & Tomatis, 1978), 15
pregnant C57 black mice were each given a single treatment of
styrene at 300 mg/kg body weight dissolved in 0.1 ml olive
oil, by stomach tube, on the 17th day of gestation. A control
group of 5 pregnant animals received olive oil alone. The
same amount of styrene was then given weekly by stomach tube
to 27 male and 27 female progeny from weaning up to 120 weeks
of age, at which time the survivors, 15 males and 12 females,
were killed. Control progeny (12 males and 13 females)
received olive oil for 120 weeks at which time 7 males and 4
females survived. In mothers treated with a single dose of
styrene, lymphomas were observed in 10/12 animals, compared
with 3/5 in the olive oil-treated controls. In treated male
progeny, liver tumours (hepatocellular carcinomas) were found
in 3/24 animals, compared with 1/12 olive oil-treated and 1/47
untreated controls (both hepatocellular adenomas). The
increased incidence of these tumours in styrene-treated
animals compared with controls was not statistically
significant. The small number of animals was noted by the
Task Group.
(b) Rat
Twenty-one female BDIV rats were each given a single
treatment of styrene at 1350 mg/kg body weight dissolved in
olive oil, by stomach tube, on the 17th day of gestation
(Ponomarkov & Tomatis, 1978). A control group of 10 pregnant
rats received 0.3 ml olive oil alone. The neonatal mortality
rate in the offspring was 10% in treated rats and 2.5% in
olive oil-treated controls. Subsequently, doses of 500 mg/kg
body weight were administered weekly by stomach tube to 73
male and 71 female progeny from weaning up to 120 weeks of
age, at which time the survivors, 8 males and 20 females, were
killed. Controls (36 males and 39 females) received olive oil
alone for 120 weeks. Two stomach tumours and 1 hepatocellular
adenoma were seen in styrene-treated females and one stomach
tumour in styrene-treated males. One stomach tumour was also
found in olive oil-treated female controls. The difference in
the incidence of these and other tumours in styrene-treated
rats compared with controls was not statistically significant.
6.9.2. Styrene 7,8-oxide
6.9.2.1. Dermal exposure
Mouse
Forty 13-week-old C3H mice were painted on the clipped
dorsal skin with a 5% solution of styrene 7,8-oxide in
acetone, 3 times weekly for life (Weil et al., 1963). No skin
tumours were observed in 33 animals that survived for 17-24
months (37 mice were alive at 12 months). Another group of
C3H mice were similarly painted with a 10% solution of styrene
oxide in acetone; 18 mice survived 12 months, only 2 mice
survived 17 months, and no skin tumours were observed.
In another study by Van Duuren et al. (1963), the clipped
dorsal skin of 30, 8-week-old male Swiss ICR/Ha mice was
treated 3 times weekly, throughout the life span, with 0.1 ml
of a 10% solution of styrene oxide in benzene. Median
survival time was 431 days in the styrene oxide-treated group
and 262 and 412 days, respectively, in the groups of 30 and 60
benzene-treated controls. Three styrene oxide-treated animals
and 11 (out of 150) benzene controls developed skin tumours.
This difference was not statistically significant. One of the
tumours in each group was a squamous-cell carcinoma.
6.9.2.2. Oral administration
Rat
Groups of 40 male and 40 female Sprague-Dawley rats, 13
weeks of age, were given either 50 or 250 mg/kg body weight,
per day, of styrene oxide (purity unspecified) dissolved in
olive oil, by stomach tube, 4 or 5 days per week, for 52 weeks
(Maltoni et al., 1979). Forty male and 40 female controls
received olive oil alone. The animals were allowed to live
until natural death or were killed 156 weeks after the start
of the study. Preliminary results included only stomach
cancer incidence in animals dying within the first 135 weeks.
The numbers of animals alive at the appearance of the first
tumour (51 weeks) were: controls, 37 males and 28 females;
low-dose, 31 males and 31 females; high-dose, 28 males and 30
females. The numbers of papillomas of the stomach were: 0/37
and 0/28 in controls; 0/31 and 2/31 in low-dose animals; and
3/28 and 6/30 in high-dose animals. The respective numbers
for squamous-cell carcinomas in situ were: 0/37 and 0/28;
5/31 and 6/31; and 11/28 and 12/30. Invasive squamous-cell
carcinomas occurred in 0/37 and 0/28; 2/31 and 1/31; and 4/2;
and 6/30, respectively.
Fourteen female rats were given, by gavage, a single dose
of styrene oxide at 200 mg/kg body weight in olive oil (0.3
ml) on day 17 of pregnancy (estimated total dose, 0.04 g).
Their progeny (62 females and 43 males) received 96 weekly
doses of 100-150 mg/kg body weight in olive oil (0.2 ml), from
weaning (4 weeks of age) until termination of the study (120
weeks) (estimated total doses, 2.5 g for females and 5.0 g for
males). Similar groups of controls (14 pregnant females, 55
female and 49 male progeny) were given only olive oil. All
survivors were killed at the end of the study. There were
fewer tumour-bearing animals among mothers treated with
styrene oxide (31%) than among rats given only olive oil
(57%); mammary tumours were seen only in the olive-oil-treated
animals. The incidences of other tumours were similar.
Forestomach tumours were seen only in treated progeny;
Carcinomas or early carcinomas of the forestomach occurred in
10/42 males and 16/60 females compared with 1/49 and 1/55
controls respectively. Four tumours of the nervous system
occurred in treated groups, whereas only 1 was observed in a
male control. Eight lung tumours were seen in treated
animals, and 2 in controls; 7 of these tumours (6 malignant +
1 benign) occurred among treated female progeny, giving an
incidence of 7/60 (12%) compared with 1/55 (2%) in control
females. The incidences of other types of tumour did not show
any marked differences between control and treated groups
(Ponomarkov et al., 1983; Ponomarkov et al., personal
communication).
6.9.3. Summary and conclusions
Of the 6 studies cited, 3 in mice and 3 in rats, only one
(O20 mice) gave results that indicated a significant increase
in lung tumour incidence. The others either did not show any
significant increase in tumour incidence or were performed in
such a way that conclusions could not be drawn from the
results.
The results of studies concerning styrene 7,8-oxide
indicated: that there was no development of, or increase in,
skin tumours, when the compound was applied to mouse skin;
that tumours (benign and malignant) developed in the
forestomach, when styrene 7,8-oxide was given by gavage in
rats; and that there was an increase in lung tumour incidence
in female progeny, when styrene 7,8-oxide was administered to
rats pre- and post-natally.
6.10. Genetic effects
Several reviews have been published on the mutagenic and
related effects of styrene (Vainio, 1978; IARC, 1979; Norppa,
1981a; Vainio et al., 1982; Zetterberg, 1982; Norppa & Vainio,
1983a).
6.10.1. Chemical reactivity of styrene and styrene oxides
Styrene needs metabolic activation to bind covalently with
nucleophilic biological macromolecules. Styrene 7,8-oxide,
which has been considered to be a primary mammalian metabolite
of styrene (section 5.3), is spontaneously reactive because of
the lability of the oxirane ring. Styrene 7,8-oxide can bind
covalently with proteins and nucleic acids (Marniemi et al.,
1977; van Anda et al., 1979) and is able to alkylate in vitro
4-( p-nitrobenzyl)-pyridine, a synthetic nucleophile, and
deoxyguanosine, a biological nucleophile (Hemminki, 1979;
Hemminki & Falck, 1979; Hemminki et al., 1981).
Hemminki (1979) measured the electrophilic reactivity of
styrene 7,8-oxide by detecting alkylation of 2-amino-1,7-di-
hydro-6 H-purin-6-one (guanine) fluorometrically. The rate of
reaction was roughly equal for guanosine, and deoxyguanosine.
Styrene 7,8-oxide formed a covalent 7-alkyl derivative with
guanine (Hemminki et al., 1980b). With an identical
concentration of guanine, RNA and single-stranded DNA were
substantially less reactive than guanosine, possibly as a
consequence of the tertiary structures of the large relative
molecular mass polymers. Double-stranded DNA was even less
reactive.
Two highly reactive arene oxide derivatives of styrene,
styrene 1,2- and 3,4-oxides were recently synthesized
(Hiratsuka & Watabe, 1982; Watabe et al., 1982a,b). These
arene oxides have a very brief half-life and they are very
hard to detect, even in an in vitro system consisting of
styrene, microsomes, and NADPH.
6.10.2. Mutagenic effects of styrene and styrene oxides in
bacterial assay systems
Styrene has not been shown to induce reverse mutations in
any of the Salmonella typhimurium tester strains used in the
Ames' test, in the absence of a metabolic activation system
(Table 11). However, in the presence of a metabolic
activation system, some investigators have found styrene to be
mutagenic in the Salmonella strains (TA 100, TA 1530, TA 1535)
used to detect base-substitution-inducing mutagens; others
have obtained negative results (Table 11). No positive
results have been reported for styrene in the Salmonella
strains (TA 98, TA 1537, TA 1538) detecting frame-
shift-inducing mutagens. The divergent results on the
mutagenicity of styrene in Salmonella could be explained, in
part, by metabolic differences between the microsomal
preparations (S-9 mix, S-10 mix) used. The high volatility,
poor solubility, and bacterial toxicity of styrene may also
affect the results (de Meester et al., 1981).
Styrene was reported to be positive in the rec assay with
Bacillus subtilis without metabolic activation, in a review by
Kawachi et al. (1979), but no specific data were given.
The primary metabolite of styrene, styrene 7,8-oxide, has
been shown to be mutagenic in a number of bacterial studies
with base-pair strains, without a metabolic activation system
(Table 11). Moreover, styrene 7,8-oxide was mutagenic in the
fluctuation test with Escherichia coli WP2 uvrA (for detecting
base-substitution mutagens) and with Klebsiella pneumoniae
(Hemminki & Falck, 1979; Voogd et al., 1981). The frame-shift
mutagen sensitive strains of Salmonella gave only negative
results with styrene 7,8-oxide, either with or without
metabolic activation systems (de Meester et al., 1977; Stolz &
Withey, 1977; Drinkwater et al., 1978; Wade et al., 1978;
Watabe et al., 1978; Busk, 1979; Hemminki & Falck, 1979;
El-Tantawy & Hammock, 1980). Watabe et al. (1981) did not
find any appreciable difference in the bacterial (Salmonella)
mutagenicity of R-and S-enantiomers of styrene 7,8-oxide and
their racemic mixture. However, Pagano et al. (1982) reported
that the R-enantiomer was more mutagenic to Salmonella than
the S-form, while the racemic styrene 7,8-oxide had an
intermediate mutagenic activity.
Table 11. Mutagenicity of styrene and styrene-7,8-oxide in the base-
pair substitution strains of Salmonella typhimurium and E. colia
------------------------------------------------------------------------
Results of mutagenicity studies
Styrene styrene 7,8-oxide
Metabolic activation Metabolic activation
system: system:
None Present None Present
------------------------------------------------------------------------
Drinkwater et al. (1978) .. .. - -
Glatt et al. (1979) .. .. + ..
Hemminki & Falck (1979) .. .. + ..
Pagano et al. (1982) .. .. + ..
Planche et al. (1979) .. .. + ..
Sugiura & Goto (1981) .. .. + ..
Sugiura et al. (1978a,b) .. .. + ..
Turchi et al. (1981) .. .. + ..
Voogd et al. (1981) .. .. + ..
Wade et al. (1978) .. .. + ..
Watabe et al. (1981a) .. .. + ..
El-Tantawy & Hammock (1980) .. .. + +b
Yoshikawa et al. (1980) .. .. + +b
Kawachi et al. (1979) - - .. ..
Milvy & Garro (1976) - .. + ..
Greim et al. (1977) - - + ..
Busk (1979) - - + +b
Loprieno et al. (1978) - - + +
Stolz & Withey (1977)
Watabe et al. (1981, 1982b) - +c + -
de Meester et al. (1977, - + + +b
1981)
Vainio et al. (1976) -d + + +b
Poncelet et al. (1980) - + .. ..
Cerna & Kypenova (1977) .. +e .. ..
------------------------------------------------------------------------
.. No data.
a Modified from: Norppa (1981a).
b A decrease reported in mutagenic activity, as compared to treatment
without metabolic activation.
c Only with trichloropropane oxide present.
d A weak activity reported also without S-9 mix in TA 1535.
e Host mediated assay, Salmonella strain TA 1950, mice as hosts.
Two arene oxide derivatives of styrene, styrene 1,2- and
3,4-oxide, both of which have been considered to be the
precursors of isolated urinary metabolites of styrene in the
rat, have been demonstrated to be mutagenic towards a
base-pair strain (TA 100) of Salmonella, but not towards a
frame-shift strain (TA 98) (Watabe et al., 1982b). The 1,2-
and 3,4-oxides were more mutagenic than the 7,8-oxide, but
only after sequential addition to the medium during pre-
incubation.
Thus, the results obtained with various bacterial assay
systems show that styrene 7,8-oxide is a direct base-pair
substitution type mutagen. The extremely labile minor
metabolites of styrene, 1,2- and 3,4-oxide, seem to be highly
reactive and are mutagenic for Salmonella. However, with
styrene, even in the presence of a metabolic activation
system, contradictory results have been reported.
6.10.3. Genetic effects of styrene and styrene 7,8-oxide
in eukaryotic non-mammalian systems
Various non-mammalian assay systems, including point
mutations and gene conversion in yeast, point mutations in
silk worm, recessive lethal mutations, sex chromosome loss and
non-disjunction in Drosophila melanogaster, and chromosome
damage in root tip cells of Allium cepa, have been applied to
study the mutagenic and genetic effects of styrene and styrene
7,8-oxide.
In the yeast assays, styrene did not induce mitotic gene
conversions at the adenine and tryptophane loci of
Saccharomyces cerevisiae or forward mutations at the adenine
loci of Schizosaccharomyces pombe, even in the presence of a
mouse liver microsome mix (Loprieno et al., 1976; Loprieno,
1981; Loprieno & Abbondandolo, 1980). According to Bauer et
al. (1980, 1981), this negative result could be because the
epoxide hydrotase (EC 4.2.1.63) (inactivation enzyme) is more
stable than the monooxygenase, because the concentration of
styrene 7,8-oxide in the incubation mixture could not reach a
mutagenic level. When a yeast strain ( Saccharomyces
cerevisiae D7) capable of metabolic activation was used to
test the induction of gene conversions by styrene, a positive
result was reported (Del Carratore et al., 1982). Styrene
7,8-oxide has been found positive in all the yeast systems
(Loprieno et al., 1978; Loprieno, 1981).
When tested in a host-mediated assay, with mice as the
host animal and yeast as the target cells, styrene and styrene
7,8-oxide were weakly mutagenic for some genetic end points
(Loprieno et al., 1978), but these results were later
considered negative for styrene (Loprieno & Abbondandolo,
1980) when a higher number of historical control values became
available.
Table 12. Point mutations, chromosome aberrations and genetic damage induced in cultured mammalian
cells by styrene and styrene 7,8-oxide
-------------------------------------------------------------------------------------------------------
Styrene Styrene 7,8-oxide
Test-system Type of damage References
assayed Metabolic Metabolic
activation system: activation system:
None Present None Present
-------------------------------------------------------------------------------------------------------
Rodent cell lines
V79 HGPRT mutations .. .. + .. Bonatti et al. (1978);
Sugiura et al. (1979)
- - + .. Loprieno et al. (1976,
1978)
- + + -c Beije & Jenssen (1982)
aberrations .. .. + .. Turchi et al. (1981)
micronuclei .. .. + ..
anaphase bridges .. .. + ..
CHL aberrations - +a + .. Ishidate & Yoshikawa
(1980);
Ishidate et al. (1981);
Kawachi et al. (1979);
Matsuoka et al. (1979)
CHO SCEs - +b + +d de Raat (1978)
.. .. + .. Kubiak et al. (1981)
L5178Y TK mutations .. .. + - Amacher & Turner (1982)
Human cells
Lymphocytes aberrations +e .. + .. Linnainmaa et al.
(1978a,b);
micronuclei +e .. + .. Meretoja & Vainio (1979)
aberrations .. .. + .. Fabry et al. (1978)
-------------------------------------------------------------------------------------------------------
Table 12. (contd.)
-------------------------------------------------------------------------------------------------------
Styrene Styrene 7,8-oxide
Test-system Type of damage References
assayed Metabolic Metabolic
activation system: activation system:
None Present None Present
-------------------------------------------------------------------------------------------------------
aberrations +e .. + .. Norppa et al.(1980a,
SCEs +e .. + .. 1981, 1982);
Norppa (1981a);
Norppa & Vainio (l983b)
unscheduled DNA -f .. .. .. Pero et al. (1982)
synthesis
EUE unscheduled DNA .. - + .. Loprieno et al. (1978)
synthesis
Wistar rat
hepatocytes unscheduled .. .. .. - Brouns et al. (1979)
DNA synthesis
-------------------------------------------------------------------------------------------------------
.. No data.
a With methylcholantrene preinduced rat liver S-9.
b Only with cyclohexene oxide present.
c In the liver perfusion system.
d A decrease in activity.
e In vitro activation by erythrocytes suggested.
f Purified lymphocytes.
In Drosophila, a significant increase in the frequency of
X-linked recessive lethals was observed in the offspring of
males fed styrene or styrene 7,8-oxide (Donner et al., 1979).
The test for sex chromosome loss and non-disjunction in
Drosophila gave negative results for styrene (Penttilä et al.,
1980). In a review (Kawachi et al., 1979), styrene was
reported not to induce point mutations in silk worm.
Results of cytogenetic studies in meristematic root cells
of Allium cepa demonstrated that styrene induces metaphase
chromosome breaks and micronuclei (Linnainmaa et al., 1978a,b).
Styrene 7,8-oxide induced micronuclei and anaphase fragments and
bridges in these growing root-tip cells (Linnainmaa et al.,
1978a,b).
6.10.4. Genetic effects of styrene and styrene 7,8-oxide in
mammalian cells in vitro
Point mutations, chromosome damage, and unscheduled DNA
synthesis (UDS) have been studied after treatment of mammalian
cell cultures with styrene or styrene 7,8-oxide (Table 12).
In the Chinese hamster V79 cell line, styrene did not
induce point mutations at the HPRT locus, with or without a
metabolic activation system (S-10 mix) from mouse liver
(Loprieno et al., 1976, 1978; Loprieno, 1981). On the other
hand, Beije & Jenssen (1982) found styrene weakly mutagenic in
the same system after metabolic activation by rat liver S-9
mix. When a liver perfusion system was used for the metabolic
activation of styrene, there was a clear increase in point
mutations at the HRPT locus of V79 cells (Beije & Jenssen,
1982).
Loprieno et al. (1978) examined the effects of styrene (with
mouse liver S-10 mix) and styrene 7,8-oxide on UDS in human
heteroploid EUE cell line. There was an increase in UDS with
styrene 7,8-oxide but not with styrene. Brouns et al. (1979) used
freshly isolated hepatocytes of Wistar rats for UDS detection after
styrene 7,8-oxide treatment. The results were negative, which
according to the authors was due to the rapid inactivation of
styrene 7,8-oxide in the hepatocytes. Pero et al. (1982) did not
find any effects of styrene (without metabolic activation) on UDS
in purified resting human lymphocytes.
Styrene induced chromosome aberrations, micronuclei, and
SCEs in human whole blood lymphocyte cultures, and chromosome
aberrations in Chinese hamster CHL cells in the presence of
metabolic activation (Table 12). Styrene 7,8-oxide induced
chromosome aberrations, micronuclei, and SCEs, in rodent cell
lines and in human lymphocyte cultures without metabolizing
systems (Table 12). The positive effects of styrene in human
whole blood lymphocyte cultures are explained by the ability
of erythrocytes to convert this compound into styrene
7,8-oxide (Norppa et al., 1980a; Belvedere & Tursi, l981;
Norppa et al., l982; Vainio et al., l982).
Without metabolizing systems, styrene 7,8-oxide has been
clearly shown to induce HGPRT mutations in V79 cells (Loprieno
et al., 1976; 1978; Bonatti et al., 1978; Sugiura et al.,
1979; Loprieno & Abbondandolo, 1980; Loprieno, 1981; Beije &
Jenssen, 1982) and point mutations in the TK locus of the
mouse lymphoma cell line L5178 Y (Amacher & Turner, 1982).
With the liver perfusion technique, styrene 7,8-oxide did not
induce point mutations at the HPRT locus of V-79 cells (Beije
& Jenssen, l982).
6.10.5. Genetic effects of styrene and styrene 7,8-oxide in
mammalian systems in vivo
Styrene and styrene 7,8-oxide have been tested in vivo for
their ability to induce genetic damage in somatic and germ
cells in different species (mouse, rat, Chinese hamster) using
different genetic endpoints (chromosome aberrations,
micronuclei, dominant lethals, SCEs and translocations in
spermatocytes; Table 13). Most of the studies concerning
chromosome aberrations in the bone marrow cells of animals
were negative for styrene. No increases in chromosome
aberrations in bone marrow were observed in mice after high
single or repeated doses of styrene administered by gavage or
intraperitoneally (i.p.), or in rats after an unspecified
dose. However, a positive effect for chromosome aberrations
in rat bone marrow was found after inhalation exposure, and
for SCEs in mouse bone marrow, alveolar macrophages, and
regenerating liver cells. In the micronucleus test, styrene
gave negative results in Chinese hamsters but positive results
in mice (Penttilä et al., 1980; Norppa, 1981b).
Studies on the cytogenetic effects of styrene 7,8-oxide in
whole mammals also gave contradictory results (Table 13).
Loprieno et al. (1978) observed a dose-dependent clastogenic
effect in mouse bone marrow cells after oral administration of
styrene 7,8-oxide. However, Fabry et al. (1978) did not find
any chromosome aberrations or micronuclei in the bone marrow
of mice after an i.p. administration. Penttilä et al. (1980)
also failed to detect any effects on micronuclei in Chinese
hamster bone marrow erythrocytes after an i.p. administration.
Negative findings were reported by Norppa et al. (1979) for
chromosome aberrations and SCEs in the bone marrow cells of
Chinese hamsters after inhalation exposure. A slight increase
in SCEs was observed after i.p. injection of a lethal dose;
chromosomal aberrations were also elevated. Conner et al.
(1982) noticed a slight increase in SCEs in regenerating liver
cells and alveolar macrophages (but not in bone marrow) in
hepatectomized mice exposed through inhalation to styrene
7,8-oxide.
Table 13. Cytogenetic damage induced by styrene and styrene 7,8-oxide in various rodent studies in vivo
------------------------------------------------------------------------------------------------------------
Exposure
Species Duration of Type of
(strain) Routea Dose time after damage Result Reference
Cell type treatment studied
------------------------------------------------------------------------------------------------------------
STYRENE
Rat
(Wistar)
bone marrow inhalation 1260 mg/m3 (300 ppm) 9-11 weeks Chromosomal + Meretoja et al. (1978)
(Wistar) aberrations
bone marrow inhalation .. .. Chromosomal - Kawachi et al. (1979)
aberrations
Mouse
(BDF1)
bone marrow inhalation 1625-3872 mg/m3 4 days SCEs + Conner et al. (1979;
alveolar (387-922 ppm) 1980; 1982)
macrophages,
regenerating
liver cells 3872 mg/m3 (922 ppm) 2 days SCEs +
(C57BL/6)
polychromatic i.p. 250 or 1000 mg/kg 30 h Micronuclei + Norppa (1981b)
erythrocytes 500 or 1500 mg/kg 30 h Micronuclei -
(CD-1)
bone marrow p.o. 500 or 1000 mg/kg 24 h Chromosomal - Loprieno et al. (1978)
aberrations
p.o. 4 x 500 mg/kg 4 days Chromosomal - Sbrana et al. (1982)
aberrations
70 x 200 mg/kg 70 days Chromosomal -
aberrations
(ICR)
bone Marrow i.p. LD50 or 5xLD50 6, 24, or Chromosomal - Cerna & Kypenova
2 6 48 h aberrations (1977)
Chinese hamster
bone marrow inhalation 1260 mg/m3 (300 ppm) 4 or 21 Chromosomal - Norppa et al. (1980)
days
polychromatic i.p. 1000 mg/kg 30 h Micronuclei - Penttilä et al. (1980)
erythrocytes
-----------------------------------------------------------------------------------------------------------
Table 13. (contd.)
------------------------------------------------------------------------------------------------------------
Exposure
Species Duration of Type of
(strain) Routea Dose time after damage Result Reference
Cell type treatment studied
------------------------------------------------------------------------------------------------------------
STYRENE 7,8-OXIDE
Mouse
(BDF1)
bone marrow, inhalation 210 mg/m3 (50 ppm) 5 h SCEs +b Conner et al. (1982)
alveolar
macrophages,
regenerating
liver cells
(CD-1)
bone marrow p.o. 500-1000 mg/kg 24 h Chromosomal + Loprieno et al.
aberrations (1978)
(BALB/c)
bone marrow i.p. 250 mg/kg 1-13 days Chromosomal - Fabry et al. (1978)
aberrations
polychromatid 30 h Micronuclei -
erythrocytes
primary 2.5-3 Trans- -
spermatocytes monthsc locations
post-meiotic 1-3 weeks Dominant -
sperm cells lethals
Chinese hamster
bone marrow inhalation 105-420 mg/m3 2,4, or 20 Chromosomal - Norppa et al. (1979)
(25-100 ppm) days aberrations
SCEs -
i.p. 500 mg/kg 24 h Chromosomal -d
aberrations
7 h SCEs +e
------------------------------------------------------------------------------------------------------------
.. No data.
a i.p. = intraperitoneal, p.o. = peroral.
b Negative in bone marrow.
c Considered to be too long time for a positive result.
d Positive in animals who died of the treatment.
e A slight effect.
Only one study has appeared concerning the possible
effects of styrene 7,8-oxide on germinal cells. Fabry et al.
(1978) reported negative results for translocations in primary
spermatocytes after a single i.p. injection and for dominant
lethals in male postmeiotic germ cells of mice.
In summary, the results concerning the genetic effects of
styrene and styrene 7,8-oxide in vivo are conflicting. Positive
results for both compounds have been obtained mainly at toxic or
nearly toxic doses. The studies have dealt with different
indicators of chromosome damage (aberrations, micronuclei, SCEs) in
actively dividing somatic cells. The effects of styrene 7,8-oxide
on germ cells have been examined only in one study, with negative
results. On the basis of the activity of monooxygenase and epoxide
hydrolase in different species, mice would be expected to be more
sensitive to styrene than rats or Chinese hamsters (Cantoni et al.,
1978; Norppa et al., 1979; Norppa, 1981a). Even in mice, styrene
and styrene 7,8-oxide disappeared rather rapidly after
intraperitoneal or oral administration (Bidoli et al., 1980;
Pantorotto et al., 1980; Sbrana et al., 1982). However, the
results of the mutagenicity studies available cannot at present be
interpreted solely according to such differences in metabolism.
6.10.6. Conclusions on the genetic effects of styrene
Styrene is a potential mutagen only after metabolic
activation. Its most important active metabolite seems to be
styrene 7,8-oxide. Arene oxides (styrene 1,2-oxide and
styrene 3,4-oxide) may also be of importance, but at present
their role cannot be evaluated.
Genetic effect tests in bacteria, yeasts, and mammalian cells
in vitro have yielded contradictory results for styrene, in the
presence of microsomal fraction of rodent liver (usually S-9 mix).
This appears to be mostly due to differences in the efficiency of
activation of styrene in different studies.
Results of in vivo studies on the genetic effects of styrene in
mammals are also contradictory. Some of the discrepancies can be
explained by variations in metabolic capacity among the rodent
species used, divergence in dose levels, and by the different
routes of administration.
Styrene 7,8-oxide is a direct mutagen that is clearly positive
in vitro without metabolic activation, but gives contradictory
results in mammals in vivo.
6.11. Effects on Reproductive Function and Teratogenic Effects
(a) Non-mammalian systems
Styrene and styrene 7,8-oxide have been reported to be
embryotoxic and teratogenic in chick embryos (Vainio et al., 1977).
Kankaanpää et al. (1979) found that trichloropropylene oxide
increased the embryo toxicity and teratogenicity of styrene and
styrene oxide. The LD50s were 40 µmol/egg for styrene and 1.5
µmol/egg for styrene oxide. Depending on the dose, malformations
were found in up to 20% of the test embryos but not in the
controls. The types of malformation included: absence of one or
both eyes, eyelid deformation, deformation of the skull,
exencephaly, exteriorization of viscera, etc. Styrene and styrene
oxide have also been found to interfere with the development of sea
urchin embryos (Pagano et al., 1978).
(b) Mammalian systems
When male rats were exposed to a styrene concentration of
200 mg/m3 (48 ppm) for 5 h/day, 5 days per week for 4 months,
Ivanova-Tchemichanska et al. (1982) found decreased osmotic
resistance and mobility of spermatozoa, decreased sulfhydryl
content, and desquamated epithelium in the seminiferous
tubules.
Ragyl'ye (1974) studied the effects of styrene on pregnant
rats exposed to 1.47, 5, and 50 mg/m3 (0.35, 1.2, and 12 ppm)
for 4 h/day throughout gestation. Resorptions were noted at
all exposure levels but the number of malformations was not
significantly increased. Murray et al. (1978) exposed rats
and rabbits to styrene through inhalation (1260 and 2520
mg/m3; 300 and 600 ppm) for 7 h/day from day 6 to 15 (rats) or
from day 6 to 18 (rabbits), and by gavage (in peanut oil, 180
and 300 mg/kg body weight/day). No increase in the incidence
of resorptions or major malformations, compared with controls,
was reported. Delayed ossification was noted in rabbits
exposed to 2520 mg/m3 (600 ppm). In an inhalation study,
Vergieva et al. (l979) exposed rats to styrene at 200 and 400
mg/m3 (48 and 96 ppm) for 4 h daily, 5 days a week, throughout
pregnancy. There was not any evidence of embryotoxicity or
teratogenicity. Mice were exposed to a styrene level of 1050
mg/m3 (250 ppm) and Chinese hamsters to 1260, 2100, 3150, and
4200 mg/m3 (300, 500, 750, and 1000 ppm) for 6 h daily on days
6-16 (18 for hamsters) of gestation. An increased number of
resorptions was noted in both species at the highest
concentration tested. A slight increase (not statistically
significant) was observed in the umber of minor skeletal
malformations (rib fusion, extra ribs) in mice but not in
hamsters (Kankaanpää et al., 1980).
Quast et al. (1978) exposed pregnant Sprague-Dawley rats
and New Zealand white rabbits to styrene concentrations of
1260 or 2520 mg/m3 (300 or 600 ppm) for 7 h/day on days 6-15
(rats) and 6-10 (rabbits) of gestation. Additional groups of
rats were given styrene (90 or 150 mg/kg body weight daily) on
days 6-15 of gestation. No teratogenic effects were detected.
In a 3-generation reproduction study in rats, styrene was
administered in the drinking water at l25 and 250 mg/litre
(estimated daily intake 14 and 21 mg/kg body weight per day
for males and females, respectively, at the high dose). No
adverse effects on reproductive capacity were observed. Some
significant effects on early survival in the second generation
offspring at the high dose were found in 2 specific litters.
No further evaluation could be made, since data for
individuals were not available (Litton Bionetics, 1980).
7. EFFECTS OF STYRENE IN MAN
7.1. Controlled Human Studies
Few controlled exposure studies have been reported in
which the effects of styrene on physiological functions in man
have been investigated. Attention has been focused on the
irritant effects of styrene vapour on the mucous membranes and
on the central nervous system effects caused by styrene
inhalation.
The odour perception threshold for styrene in air was
determined by Smith & Hochstettler (1969) to be as low as
0.2-0.34 mg/m3 (0.05-0.08 ppm). At higher concentrations, the
odour of styrene was clearly perceptible and it was reported
as "strong but not objectionable" at about 420 mg/m3 (100 ppm)
(Stewart et al., 1968). The ability to detect the odour
typically fades as the exposed individuals become adapted
(Stewart et al., 1968). Styrene vapour was irritating to the
eye and the nose at concentrations exceeding 840 mg/m3 (200
ppm) and, when the styrene concentration approached 1596 mg/m3
(380 ppm), it was poorly tolerated (Stewart et al., 1968).
Strong immediate irritation of the eyes and the respiratory
tract has been reported at styrene concentrations in air of
2520-3360 mg/m3 (600-800 ppm) (Carpenter et al., 1944; Wolf et
al., 1956).
Oltramare et al. (1974) exposed 6 volunteers to styrene at
210, 420, and 840 mg/m3 (50, 100, and 200 ppm) over 1-3 h and
noted that for a combination of symptoms of the mucous
membrane (irritation of eyes, nose, and lips) and central
nervous system (dizziness, headache, drowsiness, difficulty in
concentrating, lightheadedness, fatigue), the number of
symptoms increased with dose. This increase was still evident
at the 210 mg/m3 (50 ppm level). At the 2 higher levels,
digestive disturbances also occurred. In studies by Gamberale
& Hultengren (1974), 12 volunteer subjects were exposed to
styrene vapour through a mouth tube at concentrations
increasing stepwise 210, 630, 1050, and 1470 mg/m3 (50, 150,
250, and 350 ppm), each successive step lasting about 30 min.
When the volunteers were asked afterwards to rate their
subjective sensations (during the exposure) it appeared that
exposure to styrene had made them feel more tense and more
"affected" compared with control conditions. The authors also
studied the psycho- physiological performance of the
subjects. Simple reaction times tended to be prolonged with
increasing exposure. The changes became statistically
significant during the final 30 min, when the environmental
concentration reached 1470 mg/m3 (350 ppm). Tests on
perceptual speed and manual dexterity did not show any
impairment in relation to styrene exposure.
Stewart et al. (1968), however, noted an impairment in
performance of tasks involving manual dexterity and eye-hand
coordination in some subjects exposed to a styrene
concentration of about 1596 mg/m3 (380 ppm) over a 1-h
period. On the basis of a study on 3 volunteers, Oltramare et
al. (1974) reported impaired reaction time to visual stimuli,
to combined visual-acoustic stimuli, and in a test of diffuse
attention during and immediately after exposure to styrene at
210, 420, and 840 mg/m3 (50, 100, and 200 ppm) for 1.5 h.
There was no clear dose dependance. Inhalation exposure to
high concentrations of styrene (3360 mg/m3; 800 ppm) caused
symptoms of impairment of balance in 2 subjects and a marked
unsteadiness in posture was observed (Carpenter et al.,
1944). At a styrene level of about 1596 mg/m3 (380 ppm) for 1
h, 2 out of 5 subjects showed abnormal results in the modified
Romberg test indicating difficulty in maintaining balance.
Furthermore, after a 7-h exposure to about 420 mg/m3 (100
ppm), 3 out of six subjects reported that they had transitory
difficulty in performing the modified Romberg test, though no
objective signs of impairment of balance were found (Stewart
et al., 1968). Oltramare et al. (1974), in a study on 3
subjects, showed impairment of balance on a body sway platform
at a styrene concentration of 840 mg/m3 (200 ppm) but not at
420 mg/m3 (100 ppm).
Five volunteers were exposed by Odkvist et al. (1980) to
styrene at about 1260 mg/m3 (300 ppm) for 1 h, using a mouth
tube. Treatment was combined with exercise on a bicycle
ergometer with a 50 W workload, and observations were made on
both equilibrium ability and functioning of the vestibular
system immediately after exposure. None of the subjects
exhibited spontaneous, fixation or positional nystagmus,
whereas an impairment of eye-tracking ability was found in all
individuals in an optokinetic test. The difference in
eye-movement changes between styrene-exposed and control
groups was not statistically significant. No styrene-induced
changes were found in conventional clinical tests of balance
(standing on one leg with eyes closed, walking a line with
eyes closed). The results were thought to suggest that
exposure to styrene (inducing blood levels of approximately 80
µmol/litre) decreased the inhibitory effect of the cerebellum
on the motor function of the eyes.
In summary, the odour threshold for styrene was found to be
0.2 - 0.34 mg/m3 (0.05-0.08 ppm) and the odour was uncomfortable at
elevated concentrations. Styrene induced subjective symptoms of
irritation of the mucous membranes at concentrations exceeding
420 - 840 mg/m3 (100-200 ppm). In the same concentration range,
subjective symptoms of the central nervous system, such as
dizziness, lightheadedness, headache, and drowsiness may occur.
Reaction time, performance, and body balance tend to be impaired by
short-term inhalation exposure to styrene at concentrations of
630 - 840 mg/m3 (150-200 ppm) and definite impairment occurs at
concentrations exceeding 1470 mg/m3 (350 ppm).
7.2. Epidemiological Studies
7.2.1. Haematopoietic and immune system
Chmielewski & Renke (1975), who studied a group of 101
workers exposed for at least one year to a styrene
concentration of 100-300 mg/m3 (24-72 ppm), did not
demonstrate any appreciable effects on the haemoglobin
concentration, erythrocyte count, leukocyte count, or
differential count. Workers exposed for more than 10 years,
however, had a slightly decreased thrombocyte count compared
with workers exposed for shorter periods.
In a study on 494 workers at different levels and
durations of exposure, Lorimer et al. (1976) observed a random
distribution of abnormal haemoglobin concentrations, and
leukocyte or platelet counts between the groups. Thiess &
Friedheim (1978), who investigated 84 workers exposed to
210-2100 mg/m3 (50-500 ppm) for 1-36 years, did not notice any
appreciable differences in haemoglobin concentrations, or
leukocyte, erythrocyte, or platelet counts compared with a
reference group.
In the studies of Chmielewski & Renke (1975), and Renke &
Chmielewski (1976), 20 styrene workers did not show any
differences in bleeding time or fibrinogen level compared with
a reference group. However, the coagulation time and the
adhesivity of the platelets were somewhat increased in the
styrene-exposed group and the prothrombin index was slightly
reduced.
There is very little information on the immunological
effects of styrene. In the immunophoresis study of
Chmielewski et al. (1973), no dose-related differences were
observed in concentrations of serum gamma globulin among
workers exposed to different concentrations of styrene.
7.2.2. Nervous system
Four studies have dealt with reaction times among workers
occupationally exposed to styrene. One study (Götell et al.,
1972) in which 17 men were exposed to a styrene concentration
of 630 mg/m3 (150 ppm) showed prolonged simple reaction times
in the styrene-exposed workers, both in the morning and in the
afternoon, compared with an age-matched control group. A
second study of 106 workers in 4 work places indicated
longer and more irregular reaction times in workers exposed to
styrene than in controls (Gamberale et al., 1975). The
differences were still present after a night's rest. The mean
styrene concentration determined by continuous measurement in
the workers' breathing zone was 57-426 mg/m3 (13.6-101.4
ppm). The mean duration of exposure was 2.7 years (range
0.1-11.0 years).
Another study with a similar study design, also revealed
prolonged reaction times during the working day among styrene-
and acetone-exposed boat manufacturers (Kjellberg et al.,
1979). The exposed group (7 workers, average styrene
concentration 37 mg/m3 (9 ppm), acetone concentration
82 mg/m3, mean employment time 10.5 years) did not show any
deterioration in sensory motor functions in relation to
styrene exposure and the addition task. There was a
correlation between the reaction-time impairment and the total
uptake of styrene divided by the estimated amount of adipose
tissue.
In the study of Kjellberg et al. (1979), the reaction
times of 3 subjects were followed after removal from exposure.
Reaction times improved after 4 days and there was a further
improvement after 35 days.
The study by Cherry et al. (1980) dealt with 27 workers
(mean age, 23 years), who were exposed to a time-weighted
average level of styrene of 386 mg/m3 (92 ppm). Among the
psychological and behavioural tests applied, differences
between the exposed and unexposed groups were seen only in
reaction times.
Several case reports have described neurasthenic symptoms
among patients who had been occupationally exposed to styrene
(Klimkova-Deutschova, 1962; Axelson et al., 1974; Spasovski et
al., 1976).
A considerable percentage of 101 workers employed in the
polyester laminate industry had disturbances of the nervous
system, particularly of the vegetative nervous system. The
exposure level of the workers ranged from approximately 100 to
300 mg/m3 (24 to 72 ppm). The urinary mandelic acid excretion
was more than 400 mg/litre in 36 of the workers (Chmielewski
et al., 1973).
Schneider & Seeber (1979) used psychological tests on 2
groups occupationally exposed to styrene, one group (35
persons) having a mean exposure of 700 mg/m3 (168 ppm), and
the other (46 persons), a mean exposure of 300 mg/m3
(72 ppm). Exposure was associated with poor visual attention,
interferences with attention span, perceptual inaccuracy, and
decreased manual dexterity.
In studies concerning 98 male laminating workers having a
mean urinary mandelic acid concentration that varied from 7 to
4715 mg/litre (median concentration 808 mg/litre), symptoms of
tiredness, and difficulty in concentrating were mentioned more
often than among a reference population (Härkönen, 1977). The
duration of styrene exposure varied from 0.5 to 14 years
(median 5.1 years). In psychological tests, the same
styrene-exposed workers showed visuomotor inaccuracy and poor
psychomotor performance (Lindström et al., 1976).
Exposure-effect relationships were drawn between the urinary
mandelic acid concentration and a combined score for 3 tests
(symmetry drawing, the Bourdon-Wiersma test, and the Mira
test). Analysis of the exposure-response relationship for
these tests showed a significant increase in response for
workers with levels of urinary mandelic acid exceeding 1600
mg/litre (75% having mandelic acid levels of more than 2000
mg/litre) (Härkönen et al., 1978). The proportion of abnormal
EEGs also increased with increasing exposure levels
(Seppäläinen & Härkönen, 1976). Workers with urinary mandelic
acid concentrations above 700 mg/litre had significantly
(P < 0.05) more frequent abnormal EEGs than those with lower
levels (Seppäläinen & Härkönen, 1976; Härkönen et al., 1978).
EEG abnormalities have been reported to be more prevalent
among young styrene workers, especially during the first years
of exposure (Dolmierski et al., 1974; Chmielewski et al.,
1977). EEG abnormalities, indicated mainly by increased slow
activity in the theta range, did not increase with longer
exposure (Roth & Klimkova-Deutschova, 1963; Seppäläinen &
Härkönen, 1976), though paroxysmal episodes have also been
evident (Dolmierski et al., 1974; Seppäläinen & Härkönen,
1976). Results of a study by Rosén et al. (1978) in which 33
men were exposed to styrene concentrations ranging from less
than 21 mg/m3 (5 ppm) to 735 mg/m3 (175 ppm), showed an
increased beta activity in 9 subjects and slow activity (theta
waves) in 6. The EEG abnormalities were non-specific and
resembled those caused by various other solvents (Rosén et
al., 1978; Seppäläinen et al., 1980).
Styrene exposure may also affect peripheral nerves
(Matsushita et al., 1968, Lilis et al., 1978; Rosén et al.,
1978). Lilis et al. (1978) found distal hypoaesthesia in the
lower extremities of 412 styrene-exposed workers (persons with
diabetes mellitus, a history of back injury, and/or excess
alcohol intake were excluded). The frequency of this symptom
varied from 4.1% among those exposed for less than 7 years to
8.5% among those exposed for more than 20 years. Abnormally
low motor conduction velocity (MCV) of the radial nerve was
found in 15 out of 80 persons tested and a slowed MCV of the
peroneal nerve in 12 out of 73 tested. The MCV of the
peroneal nerve decreased with increasing length of exposure,
but the effect of age was not considered. However, the level
of exposure did not have any effect on the peroneal nerve
conduction. The MCV of the radial nerve was not correlated
with the length or level of exposure. Rosén et al. (1978) did
not find differences in the MCVs of the median, ulnar,
peroneal, or posterior tibial nerves of 33 workers exposed to
styrene, compared with normal controls. However, the
amplitude of the sensory action potential of the median nerve
was reduced and the duration of this potential increased among
styrene-exposed workers, even in 10 individuals who had been
exposed to styrene levels of less than 21 mg/m3 (5 ppm). The
dose-effect relationships were conflicting. Disorders in
attention span and movement coordination in psychological
tests were reported in 12 out of 102 workers, occupationally
exposed to styrene concentrations of 10-100 mg/m3, who had
urinary mandelic acid concentrations of 30.9 - 268 g/kg
creatinine (Spasovski et al., 1980). The same authors also
reported diminished alcohol tolerance among styrene workers.
In summary, the results of 3 studies showed that simple
reaction times were prolonged in workers occupationally exposed to
styrene at levels below 630 mg/m3 (150 ppm), while one study gave
equivocal results at a time-weighted average level of styrene in
air of 386 mg/m3 (92 ppm). Another study suggested that the effect
on reaction time was reversible.
Surveys of styrene-exposed workers have shown that an increased
incidence of abnormalities in electroencephalographic recordings
at mean styrene levels of less than 420 mg/m3 (100 ppm) was related
to styrene exposure levels. Some slight disturbances in visuomotor
accuracy and psychomotor performance were noted in workers exposed
to levels of the order of 210 mg/m3 (50 ppm) or more. Various
neurasthenic and autonomic symptoms were also reported among these
workers.
7.2.3. Kidneys and the urinary tract
There are only a few studies of the effects of styrene
exposure on the human kidney. Härkönen (1977), who studied
laminating workers exposed to styrene for periods ranging from
0.5 to 14 years, could not find any cytological changes
differing from Papanicolau I in 35 urine specimens. Results
of studies concerning mortality and morbidity rates due to
kidney and urinary tract disorders have not revealed any
differences between subjects exposed to styrene and unexposed
subjects (Lorimer et al., 1978; Thiess & Friedheim, 1978).
In studies on organic solvent exposure in relation to
kidney function, Askergren et al. (1981) reported that workers
exposed to organic solvents, especially to styrene, excreted
significantly larger quantities of albumin in the urine than
unexposed workers. No differences were observed in beta-2-
microglobulin excretion. The same author compared the
excretion of erythrocytes and leukocytes in the urine of 101
men, occupationally exposed to styrene or toluene or to a
combination of xylene and toluene, with the cell excretion in
the urine from 39 unexposed controls. While no changes in
glomerular filtration rates were observed, the urine of
exposed men contained significantly more cells than that of
the controls.
7.2.4. Gastrointestinal tract
Basirov (1975) reported studies on the digestive system of 130
workers (89 men, 41 women) engaged in styrene-butadiene synthetic
rubber manufacture. The workers were mostly 20-40 years old and
the length of exposure varied from less than 5 years (16 workers)
to more than 10 years (28 workers). Average styrene concentrations
were 60-130 mg/m3 (14-31 ppm). Tests of secretory, excretory,
motor, and pepsinogen-generating functions of the stomach were
conducted on 20 unexposed people and on 80 workers who first
developed symptoms related to the digestive system after working in
the plant. Thirty-six had decreased digestive function, 25 had
decreased peristalsis, and 51 had decreased acidity. In further
studies, chronic gastritis was diagnosed in 35 of these workers.
7.2.5. Liver
Lorimer et al. (1976) assessed the liver function of 493
styrene-exposed workers. Exposure concentrations were not
measured. The activities of alkaline phosphatase (AP) (EC
3.1.3.1), aspartate aminotransferase (ASAT or GOT) (EC 2.6.1.1),
alanine aminotransferase (ALAT or GPT) (EC 2.6.1.2), and gamma
glutamyltranspeptidase (gamma-GTP) (EC 2.3.2.1) and the amount of
serum bilirubin were determined. Only the gamma-GTP activity
showed a significant elevation in the high-exposure group compared
with the low-exposure category, even when alcohol intake was taken
into account (1.7% versus 7.2%; 0.01 < P < 0.02).
Axelson & Gustavson (1978) gathered data on ASAT, ALAT in
serum from 35 styrene-exposed male workers and 12 unexposed
controls. The time-weighted average exposure levels were less
than or about 420 mg/m3 (100 ppm). The average transferase
levels were higher among men who had been exposed, but only
ASAT was significantly elevated (P < 0.001). No differences
were found in the average levels of AP.
The serum levels of ALATP, ASAT, gamma-GTP, LDH, and SDH
(sorbitol dehydrogenase (EC 1.1.1.14)) enzymes were studied in 72
workers exposed to styrene in the manufacture of plastic boats.
The exposure levels varied between very low concentrations < 21
mg/m3 (< 5 ppm) and about 170 mg/m3 (40.8 ppm). The styrene-
exposed groups had a higher mean gamma-GTP value than the control
group (P < 0.05). However, there was no dose dependence
(Lundberg, 1981).
Vihko et al. (1983) studied 25 persons exposed to styrene at
about 126 - 168 mg/m3 (30 - 40 ppm) (mean exposure time 3 years,
all exposures exceeding 1 year). The serum activities of ALAT,
ASAT, gamma-GTP, and LD and the concentrations of serum total
bilirubin and conjugated bilirubin were determined, as well as
serum bile acids, cholic acid, and chenodeoxycholic acid. An
elevated concentration of chenodeoxycholic acid in serum was the
most frequently found parameter among styrene-exposed workers.
Spasovski (1976) showed that the serum protein profile changed and
that serum transaminase activity was elevated at a styrene
concentration of 2000 mg/m3 (500 ppm).
In conclusion, a clear-cut trend towards altered liver
function was not demonstrated. At low exposure levels, the
commonly used parameters such as serum activity of enzymes of
hepatic origin have given equivocal results.
7.2.6. Cardiovascular system
The thorax radiographs of 84 workers were evaluated before
the beginning of employment and after exposure to styrene at
210 - 1260 mg/m3 (50 - 300 ppm) for 1-36 years (Thiess &
Friedheim, 1978). The authors could not attribute any grossly
observable changes to styrene exposure, nor did they observe
any "gross pathological indications in the electrocardiograms"
compared with those of a reference group of 62 subjects.
7.2.7. Respiratory system
In clinical studies, Wilson (1944) found that styrene-
exposed workers reported irritation of the eyes, throat, and
respiratory tract. It has frequently been reported that
people in areas of high styrene exposure complain of
irritation of the eyes and nasopharynx. Götell et al. (1972)
examined 15 workers occupationally exposed to a time-weighted
average styrene concentration of 71 - 1218 mg/m3 (17 - 290 ppm)
and found that lung function tests (forced vital capacity and
forced expiratory volume in 1 s) were normal and did not
change during the working day. However, the concentrations of
styrene that gave rise to complaints varied from person to
person, apparently depending on individual tolerance. In a
study on styrene-exposed workers who had respiratory tract
symptoms, it was suggested that long-term exposure to styrene
could cause obstructive pulmonary changes (Chmielewski &
Renke, 1975).
In a cross-sectional study of clinical signs and symptoms
of irritation (Lorimer et al., 1978) in 488 styrene-exposed
workers, the workers were divided into 2 groups, based on
estimated exposures of 4.2 mg/m3 (1 ppm) and > 21 mg/m3 (> 5
ppm), mostly above 84 mg/m3 (20 ppm). When asked about lower
respiratory symptoms, significantly more workers in the
high-dose group responded in the affirmative. There were no
differences in length of employment and the prevalence of
upper respiratory symptoms between the 2 groups.
In a symptom survey (Härkönen, 1977) of 98 male laminating
workers occupationally exposed to styrene, symptoms that
fulfilled the British Medical Research Council's criteria for
simple chronic bronchitis were more common in the exposed than
in an unexposed group. In the exposed group, 28% had simple
chronic bronchitis, compared with 12% in the control group
(P < 0.05). The smoking habits of the groups did not differ.
7.2.8. Endocrine organs
In a study that involved 25 rubber workers, exposed to
styrene, and 26 age-matched controls, Wink (1972) did not
observe any significant differences in the urinary excretion
of 17-ketosteroids or 17-ketogenic steroids. Exposure data
were not given. However, Chmielewski et al. (1973) reported
reduced excretion of 17-ketosteroids in the urine in 27 out of
67 workers exposed to styrene concentrations of 100-300 mg/m3
(24 - 72 ppm), but these findings were not convincingly
documented. The evaluation of possible effects of styrene on
the steroid metabolism should be studied using more refined
techniques.
In the study of Chmielewski et al. (1973), glucose
tolerance was observed to be somewhat higher in the exposed
group than in the control group, and even more in the group
with higher mandelic acid concentrations in the urine (limit
400 mg/litre). Evidence of effects of styrene on the
endocrine glands was lacking.
7.2.9. Carcinogenic effects
(a) Styrene production and polymerization processes
A survey of 560 individuals from a styrene production and
polymerization plant was reported by Lilis & Nicholson (l976)
and Nicholson et al. (1978). In 1960, the workers had been
employed in the plant for at least 5 years. It is known that
benzene, coke-oven products, butadiene, and ethyl-benzene had
been used in this plant prior to 1965. Among 83 deaths, 17
were due to cancer, including 2 cases of leukaemia and one of
a lymphoma. When the total cancer mortality in this cohort
was compared with that in the general population, no excess of
cancer incidence was found, but the follow-up period may have
been too short to detect an increased risk of neoplasia. One
case of leukaemia and one of lymphoma were found in 21
additional death certificates of individuals who had been
employed for less than 5 years in 1960. In an additional
analysis of 444 death certificates on all individuals who had
been employed for at least 6 months in the plant, 7 cases of
leukaemia and 5 cancers of the lymphatic system were found.
However, the populations at risk are not known.
A cohort of 1960 workers employed for at least 1 month in
a styrene-polystyrene manufacturing plant was followed from
1931 to 1976 (Frentzel-Beyme et al., 1978). Analysis of
deaths occurring (after a minimum period of 5 years) in groups
exposed for 5, 10, 15, and over 15 years did not indicate an
increase in cancer mortality in any of the groups compared
with the reference population. The Task Group noted, however,
that the mortality rates for the reference population were
only available for the period 1970-75, and that follow-up was
incomplete.
Ott et al. (1980) carried out a retrospective cohort study
in workers involved in the production of styrene and
polystyrene. Mortality experience was followed from 1935 to
1975. Among 282 deaths, 6 cancers of the lymphatic and
haematopoietic tissues (except leukaemia) and 6 cases of
lymphocytic leukaemia were observed, compared with 4.5 and
2.9, respectively, expected on the basis of US white male
mortality rates and 2.6 and 1.6 deaths expected from a
comparison industrial cohort. Four deaths from leukaemia were
observed in a sub-group of workers exposed to styrene, ethyl
benzene, oligomers of styrene, mineral oil, polymer dusts, and
extrusion fumes; 0.5 deaths would have been expected on the
basis of US white male mortality experience. Only one of the
4 individuals had spent at least 5 years in the working
environments under study. Inclusion of cases of leukaemia
still alive at the end of the study period or identified
through other conditions listed on the death certificate
increased the total number of lymphoreticular malignancies to
21 cases. These included 7 lymphocytic leukaemias, 4 other
leukaemias, 4 multiple myelomas, 4 Hodgkin's diseases, and 2
other lymphomas. Five of the subjects with lymphocytic
leukaemia had worked in the area of polymer extrusion and for
4 of them the disease developed 20 or more years after the
first exposure. According to the authors, there was no
increase in the general cancer incidence. The incidence of
lymphatic leukaemia was, however, more than that expected.
(b) Lamination and related processes in boat building
An epidemiological study was carried out on 1500 workers
from 36 companies in the reinforced plastics and plastic boat
industry. The majority of the workers were between 30 and 40
years old. The average period of employment was from 6 to 7
years but half of the workers had been in this type of work
for less than 3 years, while 54 had been employed for over 20
years. Exposure was estimated to be to an average styrene
concentration of 1050-1470 mg/m3 (250-350 ppm) at the end of
the sixties and the beginning of the seventies. Seventeen
cases of cancer were found in this group. In 3 of these, the
cancer was present before their employment. In the remaining
l4 cases, the sites and distribution of the various types of
tumours were in accordance with data from the cancer
registry. Among the deaths, one case of neoplasm of the
lymphatic and haematopoietic system (plasmocytoma) was
identified. However, because of the short follow-up period
and the small number of deaths it was not possible for the
authors to calculate any observed/expected figures (Ahlmark,
l978).
A study is in progress (Tola et al., 1981) in which the
tumour incidence in a cohort of 2209 workers, employed in the
manufacture of reinforced plastics in 160 workplaces, is being
investigated. Exposure to styrene vapour commenced in 1960
and the level of exposure has been estimated to range between
126 and 1260 mg/m3 (30 and 300 ppm). The majority of workers
have been exposed since 1967. Among 27 deaths, 6 deaths from
cancer were observed in this cohort (versus 8.l expected) and
the cancers were found in tissues other than lymphatic or
bone-marrow tissue. Five of the cancers appeared in workers
with 5 or fewer years of exposure. The Task Group noted the
low mortality rate.
(c) Styrene-butadiene rubber manufacture
McMichael et al. (1976) analysed the mortality experience
during the period 1940-60 in a sample of workers (exact number
not specified) taken from a population of 1482 workers in the
rubber industry. The subgroup of workers was involved in the
manufacture of styrene-butadiene rubber. For malignancies of
the lymphatic and haematopoietic systems, a relative risk
ratio of 6.2 was observed compared with other workers.
Subsequently, Smith & Ellis (1977) reported that this excess
was based on 4 cases. A case control analysis of the same
data was performed by Spirtas et al. (1976) who calculated a
relative risk ratio of 2.4 for lymphatic and haematopoietic
neoplasms among these styrene-butadiene workers. Past
exposure to solvents and other monomers was suspected.
The mortality experience of 2 cohorts of rubber workers
was analysed by Taulbee et al. (1976) using both cohort and
case-control analysis. No significant excess of lymphatic and
haematopoietic neoplasms was observed.
Case reports of leukaemias and lymphomas among styrene-
butadiene rubber workers were reported by Block (1976), Lemen
& Young (l976), and Meinhardt et al. (1978).
7.2.9.1. Summary and conclusions
There have been anecdotal reports of a small number of
cases of leukaemia and lymphoma in workers employed in the
manufacture of styrene-butadiene rubber, but the study
populations have been ill-defined and exposure to solvents and
other monomers is known to have occurred.
An association between leukaemia and exposure to styrene
in the production and polymerization process industries has
been suggested in another study. However, the cases of
leukaemia occurred in a group with a concomitant past history
of exposure to colourants, polymer fumes and, possibly,
benzene.
The effects of long-term exposure to styrene are under
investigation in at least 2 epidemiological studies on workers
employed in the industries involving styrene lamination of
glass fibre materials and related activities.
It was the opinion of the Task Group that the data
necessary to form an evaluation were inadequate and that a
causal relationship between exposure to styrene and human
cancer could not be demonstrated at present.
7.2.10. Genetic effects in somatic cells
7.2.10.1. Structural chromosome aberrations
Several studies have been published on structural
chromosome aberrations in the peripheral lymphocytes of
workers employed in the reinforced plastics industry or in the
production of styrene and polystyrene (Table 14). In these
studies, exposures have been estimated by measuring styrene
air concentrations in the workplace or by determining
concentrations of styrene metabolites, in most cases mandelic
acid, in the urine of the workers.
In Table 14, the studies are listed according to the
industrial process. Positive results have been obtained among
workers employed in polyester processing. Nine studies
included detailed data on chromosome aberrations in
individuals. One report by Watanabe et al. (1981) was
considered inconclusive because of the low number (50 or less)
of metaphases analysed per person. The other 8 studies with
detailed information were used for the final evaluation.
Three studies were reported only in abstract or review
articles (Sorsa et al., 1979; Sram, 1981; Vainio et al., 1982;
Brogger, 1982).
The study of Meretoja et al. (1978a) was an extension of
an earlier study (Meretoja et al., 1977) and included
additional analyses of samples taken from the same workers a
year later, after continuous workplace exposure.
One of the abstracts was concerned with a study on
children exposed during the fetal period, while their mothers
were working in hand lamination of polyester resin.
The only available study on chromosome aberrations among
workers employed in the production of styrene or polystyrene
was negative (Fleig & Thiess, 1978).
7.2.10.2. Other indicators of genetic damage
Seven studies were available on SCE induction in the
lymphocytes of styrene-exposed workers employed in polyester
processing (Table 14). Two of the studies were reported only
in abstracts or reviews (Sorsa et al., 1979; Brogger, 1982;
Vainio et al., 1982). A slight increase in SCEs was reported
in 2 studies (Andersson et al., 1980; Camurri et al., 1982).
In 2 of the studies, peripheral lymphocytes of polyester
processing workers were analysed for micronuclei. Meretoja et
al. (1978a) and Meretoja & Vainio (1979) reported an increase
in micronuclei and "cells connected with a nuclear bridge" in
the cultured lymphocytes of 10 workers in polyester plastic
product plants; the study included 5 controls (Table 14).
Högstedt & Mitelman (1983) found that the frequency of
micronuclei increased in 38 workers exposed for 1-23 years to
styrene (mean of time-weighted average concentrations in
breathing zone 55 mg/m3 or l3 ppm, range 4-168 mg/m3 or 1-40
ppm) compared with 20 controls.
Pero et al. (1982) did not find any increase in
unscheduled DNA synthesis (UDS) in isolated lymphocytes of 38
workers in a fibreglass-reinforced polyester plastic factory
compared with 20 unexposed controls. Styrene air
concentrations in the workroom varied between 4 and 168 mg/m3
(1 and 40 ppm).
In an evaluation of the incidence of malformed children
and miscarriages among spouses of men exposed to styrene in a
reinforced-plastic boat factory, Andersson et al. (1980)
concluded that the number of pregnancies (39 exposed and 41
controls) was too small to reveal mutagenic effects.
7.2.10.3. Conclusions
The available evidence suggests that styrene exposure in
the reinforced plastics industry with its more intensive
exposures is associated with an increased frequency of
structural chromosomal abnormalities.
7.3. Effects on Reproductive Function and Teratogenic Effects
In a case-reference study on 63 pairs of mothers from a
register of congenital malformations (Holmberg, 1977), 2
mothers with children having central nervous system defects
had been employed in the reinforced plastics industry during
pregnancy. A third case-mother was also found, who had been
exposed to styrene at home.
In a study on spontaneous abortions registered in
hospitals (Hemminki et al., 1980a) during the period 1973-76,
the following numbers and rates of abortion were found:
- General population 15 482 (5.52%);
- Union of Chemical Workers 52 (8.54%);
- Plastics industry 21 (8.94%);
- Styrene production and use 6 (15%).
The abortion rates for the occupational groups were
statistically different from the controls. The abortion rates
increased with age in the general population, while they were
found to decrease with age among the Union of Chemical
Workers. No data were given concerning the relationship with
age of spontaneous abortions among women employed in the
plastics industry or in styrene production and use; thus the
significance of the increased abortion rates among these
groups cannot be assessed.
Table 14. Summary on studies of structural chromosome aberrations and sister chromatid exchanges (SCEs) in the lymphocytes of
workers exposed to styrene.
------------------------------------------------------------------------------------------------------------------------------------
No. persons Measured Years of Urinary man- Cells with SCEs/cell
studied concentration exposure delic acid chromosome
Industry styrene in (mg/g of aberrations,
branch air (mg/m3) creatinine) gaps included (%)a
---------------------------------------------------------------------------------------------------------------------
Reference Exposed Controls Averagea Range Meana Range Meana Range Exposed Controls Resultb Exposed Controls Resultb
------------------------------------------------------------------------------------------------------------------------------------
Styrene
manufacture
Flieg & 5 20 ~2c 0-29c 21.6 14-25 ~30d 19- 3.8 5.5 - .. .. ..
Thiess (1978) 40d
Polystyrene
manufacture
Flieg & 12 20 ~8c 0- 20.3 3-39 ~32d <5- 5.1 5.5 - .. .. ..
Thiess (1978) (12)e 197c 100d (5.5)e .. .. ..
Polyester
processing
Meretoja et 10 5 .. .. 3.2 0.6- 721 23- 16.6 1.8 + .. .. ..
al. (1977); 8.5 3257
Meretoja &
Vainio (1979)
Meretoja et 16 6 .. up to 6.3 1-15 570 23- 15.1 2.0 + .. .. ..
al. (1978b) 1260 3257
(a year 11 3f .. up to 8.1 2-16 329 52- 16.2 .. + 5.3 4.4 -
later) 1260 1646
Flieg & 14 20 ~477c <210- 7.9 2-24 ~593 42- 9.2 5.5 + .. .. ..
Thiess (1978) 1260c >1500
Andersson et 36 37 ~197 0- 5.0 0.3- .. .. 12.3 6.7 + 9.7 8.7 +
al. (1980) 20f 20f 1008 12
Watanabe et 16 13 151- 0-886 ~4.5 0.6- ~594 90- 2.9 3.2 (-)g 13.5 13.9 -
al. (1981) <294 9.3 2640
Watanabe et 18 6 168- <5- 9.7 <1-30 332 1- 6.5 4.7 ± 8.9 8.5 -
al. (1983) 210 1075 ~1040
Thiess et al. 24 24 25- 3-748 ~14.4 4-27 .. 0- 5.1 3.8 +h .. .. ..
(1980, 1982) 244 320c
------------------------------------------------------------------------------------------------------------------------------------
Table 14. (contd.)
------------------------------------------------------------------------------------------------------------------------------------
No. persons Measured Years of Urinary man- Cells with SCEs/cell
studied concentration exposure delic acid chromosome
Industry styrene in (mg/g of aberrations,
branch air (mg/m3) creatinine) gaps included (%)a
---------------------------------------------------------------------------------------------------------------------
Reference Exposed Controls Averagea Range Meana Range Meana Range Exposed Controls Resultb Exposed Controls Resultb
------------------------------------------------------------------------------------------------------------------------------------
Camurri et al. 24 21 .. 30- 9.4 1-22 472c 45- ~35.1i ~8.4i + 13.9 10.8 +
(1982)k (22)f (20)f >400 1108c
Abstracts
Sram (1981) 36 19 .. .. .. .. .. .. 1.4i 1.3 - .. .. ..
(cited data 34j (1.4)ij
of Pchlova & 22 22 .. .. .. .. .. .. 1.7i 1.4i - .. .. ..
Sram)
Brogger 13 0 129 15- 9.8 1-24 527 292- 6.4 .. (-)g 6.6 .. (-)g
(1982)k (8)f 364 688
Sorsa et al. 6 10 .. .. .. 2-7 .. .. 8.8 3.0 + 7.5 8.5 -
(1979);
Vainio et al. 7 10 .. .. .. (5-8 .. .. 4.9 1.6 + 4.9 5.2 -
(1982) ( in months)
utero exposed
children)
Högstedt et 6 6 ~162 60- 4.0 0.5- 490 225- 10.2 4.9 + .. .. ..
al. (1979) 800 10 2100
------------------------------------------------------------------------------------------------------------------------------------
a Estimated from the data given if not indicated in the report.
b Compared to response in control persons: + increase; - no effect.
c From Thiess & Friedheim (1978).
d mg/litre urine.
e Controls in Thiess & Fleig (1978).
f For the analysis of sister chromatid exchanges only.
g Inconclusive because of the low number of cells analysed (Watanabe et al., 1981) or lack of controls (Brogger, 1982).
h Negative if gaps are excluded.
i Gaps excluded.
j 6 months later.
k Details, not mentioned in the report, were obtained from the author(s).
In a small-scale study, Härkönen & Holmberg (1982)
recently interviewed 67 female lamination workers of child-
bearing age and a similar number of textile and food
production workers who were thought to be proper controls.
The groups were reported not to differ either in their
menstrual behaviour or in the number of spontaneous
abortions. The number of deliveries was found to be lower
among the styrene workers, which may be partially explained by
the higher number of induced abortions in the styrene-exposed
group.
8. EXPOSURE-EFFECT/EXPOSURE-RESPONSE RELATIONSHIPS, AND
EVALUATION OF HEALTH EFFECTS
8.1. Data from Experimental Animal Studies
8.1.1. Metabolic pathways and kinetics
Pulmonary uptake in man is of greatest importance though uptake
through the skin occurs. Styrene is biotransformed largely via the
7,8-epoxide by the mixed function oxidase system.
The kinetic data show that exposure of experimental animals to
increasing levels of styrene results in progressive saturation of
the metabolic pathways. The half-time of styrene disappearance
from the body varies with the dose. The range of exposures tested
was from 189 mg/m3 (45 ppm) to 10 500 mg/m3 (2500 ppm). The
consequence of such metabolic saturation, which has been reported
to occur at about 2520 mg/m3 (600 ppm) in air, is an increase in
the proportional deposition of styrene in fat. In human beings the
half-time for the elimination of styrene from adipose tissue is 2-3
days.
The principal urinary metabolites are mandelic and
phenylglyoxylic acids. Recent evidence suggests that the
pattern of urinary metabolite excretion varies with mammalian
species.
8.1.2. General toxicity
8.1.2.1. Acute toxicity
A mean odour detection threshold for styrene of 3.06 mg/m3
(0.73 ppm) was found for unadapted subjects. The odour was
reported to be strong but not objectionable at about 420 mg/m3
(l00 ppm). With short-term exposures at concentrations
exceeding 840 mg/m3 (200 ppm), styrene vapour was irritating
to the eyes and nose.
The central nervous system effects begin to appear in the
range of 210-840 mg/m3 (50-200 ppm) and distinct impairment of
reaction time and body balance were found at concentrations
exceeding 840 mg/m3 (200 ppm).
The acute toxicity of styrene in animals is rather low:
the LD50 in rats has been reported to be 5 g/kg body weight
after oral administration and 2-3 g/kg body weight after
intraperitoneal injection. Exposure of rats to styrene vapour
at 1300 to 10 000 ppm for up to 4 h caused nasal mucous
membrane and eye irritation as well as acute depression of the
central nervous system. Marked pulmonary lesions were also
noted.
There were no kidney changes in animals exposed to 5460
mg/m3 (l300 ppm) after a single exposure. Severe irritation
of the eyes and nose was observed in rats and guinea-pigs
after exposure to concentrations of 2730-5460 mg/m3 (650 - 1300
ppm) and general weakness and unsteadiness gradually developed
after 12 h.
Acute toxic effects were observed in mice exposed to a
styrene level of 21 000 mg/m3 (5000 ppm) for 2 h and
guinea-pigs exposed to 10 920 mg/m3 (2600 ppm) for 8 h. A
short-term exposure to 1260 mg/m3 (300 ppm) in the air caused
only very slight behavioural effects in rats, even though
pronounced neurochemical changes were observed.
The high exposure levels used in studies on experimental
animals have not been observed in the human environment.
8.1.2.2. Subacute and chronic toxicity
Inhalation of styrene vapour by rats at a concentration of
5460 mg/m3 (1300 ppm) for 7 h/day over several months did not
have any deleterious effects on the kidney and did not cause
any changes in the erythrocyte count, haemoglobin
concentration, and leukocyte count. There were not any
essential morphological changes in the rat lungs. Similar
results were obtained with rabbits, monkeys, and guinea-pigs
except that 10% of the guinea-pigs died after the first
exposure with signs of lung irritation. An exposure level of
2730 mg/m3 (650 ppm), for 7 h/day for 214-360 days, appeared
not to have any effects on the lungs of guinea-pigs.
Rats exposed by inhalation to a styrene concentration of
1260 mg/m3 (300 ppm) (6 h/day, 5 days a week) for up to 11
weeks developed axonal protein changes in the brain, an
induction of drug metabolizing enzymes in the kidney and the
liver, histological alterations in the liver, and a depletion
of the glutathione (GSH) contents of the kidney and the liver.
No significant depletion of GSH occurred at 420 mg/m3 (100
ppm).
Oral subacute studies were carried out on rats and beagle
dogs. The rat study was carried out on females and exposure
induced only weight increases in the liver and kidney. No
effect was observed at a dose of 133 mg/kg body weight. The
study on dogs lasted more than 19 months and showed a mild
haemolytic anaemia characterized by Heinz body production.
Effects were observed down to the lowest dose tested (200
mg/kg body weight).
8.1.3. Genetic effects
Styrene is a potential mutagen only after metabolic activation.
Mutagenicity tests in bacteria, yeasts and mammalian cells in vitro
have yielded contradictory results for styrene that may be due to
differences in the efficiency of activation and inactivation of
styrene in different studies.
Conclusive dose-effect relationships could only be established
in several in vitro studies.
The results of tests for chromosomal damage induced by
styrene in experimental mammals have been contradictory.
Three positive studies have been reported; 2 in mice, and 1 in
rats. A dose-effect relationship was only observed for the
induction of SCE in mice exposed to styrene in air levels of
more than 1260 mg/m3 (300 ppm). Five other animal studies
have given negative results. The different end-points, routes
of exposure, durations of treatment, species and strains of
animals used, and the small number of mammalian studies
available, make it impossible to draw definitive conclusions
concerning dose-effect relationships.
8.1.4. Carcinogenic effects
Oral administration of styrene to mice induced a
significant increase in pulmonary tumours in the O20 strain at
a dose of 1350 mg/kg body weight and a doubtful increase in
B6C3F1 mice at 300 mg/kg body weight. No significant increase
in tumour incidence was observed in the C57B1 strain, when
styrene was administered pre- and post-natally at 300 mg/kg
body weight.
In 2 studies on rats, styrene given orally at doses
ranging from 500 to 1350 mg/kg body weight did not induce a
significant increase in tumour incidence. A greater incidence
of lymphomas, observed in an inhalation study on rats exposed
to a styrene concentration of 2520 or 4200 mg/m3(600 or 1000 ppm),
could not be causally related to styrene.
Thus, the evidence available indicates that styrene at the
doses administered (1350 mg/kg body weight for 020 strain)
caused an increase in pulmonary tumours in mice.
Styrene 7,8-oxide induced squamous cell carcinomas in the
forestomach of rats given doses of 50 and 250 mg/kg body weight.
In a second study on rats, both squamous cell carcinomas and
pulmonary carcinomas were induced, when styrene oxide was given at
a dose of 100 - 150 mg/kg body weight per day, 4-5 days a week.
When styrene oxide was painted on the skin of mice at
concentrations of 50 or 100 g/kg, no tumours of the skin were
induced.
8.2. Human Studies
8.2.1. Effects on organs and systems
Effects on the following organ systems were investigated:
nervous, haematopoietic and immune systems, the kidney and
urinary tract, the gastrointestinal tract, liver,
cardiovascular and respiratory systems, and the endocrine
organs.
Most studies of the haematopoietic system did not produce
any positive data; in one study, a slight decrease in
thrombocyte count in workers exposed to styrene for more than
ten years was detected.
A clear-cut trend towards altered liver function has not been
demonstrated. At low exposure concentrations, the commonly used
parameters, such as activity of serum enzymes of hepatic origin,
except for gamma-GTP, have given equivocal results.
Slight effects on the lower respiratory system were noted
in some studies.
No adequate data were available to the Task Group to
establish dose-effect or dose-response relationships for the
aforementioned systems.
Slight disturbances of visuomotor accuracy and psychomotor
performance were noted at styrene levels exceeding 210 mg/m3
(50 ppm), and an increased incidence of abnormalities in
electroencephalograph recordings was detected at styrene
concentrations below 420 mg/m3 (100 ppm); relationships
between the exposure levels and the severity of these effects
and the response rates were observed.
While the reaction times were shown to be prolonged for
exposure levels below 630 mg/m3 (150 ppm), dose-response
relationships could not be established.
8.2.2. Genetic effects in somatic cells
Knowledge concerning the possible genetic effects of
styrene exposure on man is still inadequate for any
dose-response extrapolations. Available information is
limited to structural somatic chromosome damage in the
peripheral blood lymphocytes of workers occupationally exposed
to high concentrations of styrene in the reinforced plastics
industry.
Data on individual exposure profiles are necessary for
meaningful interpretation of the results of somatic cell
analysis. Furthermore, the role of high occasional peak
exposures and low continuous workplace exposures in the
induction of chromosome damage in lymphocytes in styrene-
exposed workers is not clear. Considering the survival time
of the target lymphocytes, exposure data should be available
for a period of at least 2-3 years preceding chromosome
analysis.
Based on available published data, the following
conclusions can be made:
1. No clear dose-response pattern can be recognized.
Positive results of chromosome aberrations in
styrene-exposed workers were restricted to the
reinforced plastics industry, where styrene
concentrations in air were high. Negative results
have been obtained in the manufacture of styrene or
polystyrene where exposures to styrene are lower.
2. Variability in the chromosome aberration frequencies
reported in different studies is great. This
suggests that several factors such as exposure
conditions, the method, and the analytical criteria
selected, influence the results.
3. Individual differences are great and may reflect
variations in exposure or differences in
susceptibility.
The Task Group concluded that the health significance of
structural chromosomal aberrations in the somatic cells of
persons exposed to styrene could not be assessed at present,
but that such effects were undesirable.
8.2.3. Carcinogenic effects
Several case reports and epidemiological investigations
have implied an increased risk of lymphatic and haematopoietic
system cancer in workers involved in the application of
styrene, polystyrene, and styrene-butadiene rubber. However,
at present, there is not sufficient evidence to establish a
direct cause and effect relationship between styrene exposure
and cancer in human beings. Assessment of data has frequently
been complicated by concomitant exposure to other volatile
substances.
The Task Group concluded that, to date, a causal
relationship between styrene exposure and cancer could not be
established in man.
REFERENCES
AHLMARK, A. (1978) [Styrene study. Epidemiological report.]
Stockholm, Sveriges Plastförbund, 21 pp (in Swedish).
ALARIE, Y. (1973) Sensory irritation of the upper airways by
airborne chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
AMACHER, D.E. & TURNER, G.N. (1982) Mutagenic evaluation of
carcinogens and non-carcinogens in the L5178Y/TK assay
utilizing postmitochondrial fractions (S9) from normal rat
liver. Mutat. Res., 97: 49-65.
ANDERSSON, H.C., TRANBERG, E.C., UGGLA, A.H., & ZETTERBERG, G.
(1980) Chromosomal aberrations and sister-chromatid exchanges
in lymphocytes of men occupationally exposed to styrene in a
plastic-boat factory. Mutat. Res., 73: 387-401.
ASKERGREN, A., ALLEN, L.-G., KARLSSON, D., LUNDBERG, I., &
NYBERG, E. (1981) Studies on kidney function in subjects
exposed to organic solvents. I. Excretion of albumin and -2
microglobulin in the urine. Acta Med. Scand., 209: 479-483
ASTRAND, I., KILBOM, C., OVRUM, P., & WAHLBERG, I. (1974)
Exposure to styrene. I. Concentration in alveolar air and
blood at rest and during exercise and metabolism. Work
environ. Health, 11: 69-85.
AXELSON, O. & GUSTAVSON, J. (1978) Some hygienic and clinical
observations on styrene exposure. Scand. J. Work Environ.
Health, 4 (Suppl. 2): 215-219.
AXELSON, O., FROBARJ, G., & WEDEFELT, U. (1974) [Can styrene
exposure cause states with cerebral lesion.] Läkartidningen,
71: 137-138 (in Swedish).
BABINA, M.D. (1969) [Spectrophotometric determination of
styrene and benzaldehyde in the work environment.] Gig. i
Sanit., 34: 105-106 (in Russian).
BAGGET, M.S., MORIA, G.P., SIMMONS, M.W., & LEWIS, J.S. (1974)
Quantitative determination of semivolatile compounds in
cigarette smoke. J. Chromatogr., 97: 79-82.
BAKKE, O.M. & SCHELINE, R.R. (1970) Hydroxylation of aromatic
hydrocarbons in the rat. Toxicol. appl. Pharmacol., 16:
691-700.
BARDODEJ, Z. (1964) [Styrene metabolism.] Cesk. Hyg., 9:
223-239 (in Czech).
BARDODEJ, Z. (1978) Styrene, its metabolism and the
evaluation of hazards in industry. Scand. J. Work Environ.
Health, 4 (Suppl. 2): 95-103.
BARDODEJ, Z. & BARDODEJOVA, E. (1970) Biotransformation of
ethyl benzene, styrene and alphamethyl styrene in man.
Am. Ind. Hyg. Assoc. J., 31: 206-209.
BARDODEJ, A., MALEK, B., VOLFOVA, B., & ZELENA, E. (1960)
[The hazard of styrene in the production of glass laminates.
Cesk. Hyg., 5: 541-546 (in Czech).
BARDODEJ, Z., BARDODEJOVA, E., & MALEK, B. (1961) [Value and
application of exposure tests. XI. Exposure test for
styrene.] Cesk. Hyg., 6: 546-552 (in Czech).
BARDODEJ, Z., BARDODEJOVA, E., & GUT, I. (1971) [Metabolosm
of styrene in rats.] Cesk. Hyg., 16: 243-245 (in Czech).
BARSOTTI, M., PARMEGGIANI, L., & SASSI, C. (1952)
[Observations on occupational pathology in a polystyrene resin
factory.] Med. Lav., 43: 418-424 (in Italian).
BASIROV, A.A. (1968) [Gastric function in workers of the
synthetic rubber industry.] Vrac. Delo, 4: 200-203 (in
Russian).
BASIROV, A.A. (1975) [Biochemical indexes of the gastric
juice in the early diagnosis of stomach illnesses under the
effect of toxic substances (1,3-butadiene and styrene).
Azerb. Med. Zh., 52: 60-66 (in Russian).
BAUER, C., LEPORINI, C., BRONZETTI, G., CORSI., C., NIERI, R.,
DEL CARRATONE, R., & TONARELLI, S. (1980) The problem of
negative results for styrene in the in vitro mutagenesis test
with metabolic activation (microsomal assay): explanation by
gas chromatographic analysis. Boll. Soc. It. Biol. Sper., 56:
203-207.
BAUER, C., LEPORINI, C., BRONZETTI, G., CORSI, C., NIERI, R.,
DEL CARRATONE, R., & TONARELLI, S. (1981) Studies on the
incubation mixtures for the in vitro mutagenesis test with
metabolic activation (microsomal assay) - behaviour of some
activating and detoxifying enzyme systems during incubations.
The case of styrene. Mutat. Res., 85: 268.
BAUER, D. & GUILLEMIN, M. (1976) Human exposure to styrene:
I. The gas-chromatographic determination of urinary
phenylglyoxylic acid using diazomethane derivatization.
Int. Arch. occup. environ. Health, 37: 47-55.
BEIJE, B. & JENSSEN, D. (1982) Investigation of styrene in
the liver perfusion/cell culture system. No indication of
styrene-7,8-oxide as the principal mutagenic metabolite
produced by the intact rat liver. Chem. biol. Interact., 39:
57-76.
BELVEDERE, G. & TURSI, F. (1981) Styrene oxidation to styrene
oxide in human blood erythrocytes and lymphocytes. Res. Comm.
Chem. Pathol. Pharmacol., 33: 273-282.
BELVEDERE, G., CANTONI, L., TACCHINETTI, T., & SALMONA, M.
(1977) Kinetic behaviour of microsomal styrene monooxygenase
and styrene epoxide hydratase in different animal species.
Experientia (Basle), 33: 708-709.
BENCEV, I.V. & RIZOV, N. (1981) [Gas-chromatographic method
for styrene determination blood (headspace method).]
Prob. Hyg., VI: 88-91 (in Russian).
BERGMAN, K. (1977) [Exposure to styrene in plastic boat
industry, I. Technical-hygienic study.] Arbete Hälsa, 3: 1-9
(in Swedish).
BERGMAN, K. (1979) Whole body autoradiography and allied
techniques. Scand. J. Work Environ. Health, 5 (Suppl. 1):
93-120.
BERTSCH, W., ANDERSON, E., & HOLZER, G. (1975) Trace analysis
of organic volatiles in water by gas chromatography-mass
spectrometry with glass capillary columns. J. Chromatogr.,
112: 701-718.
BIDOLI, F., AIROLDI, L., & PANTAROTTO, C. (1980) Quantitative
determination of styrene-7,8-oxide in blood by combined gas
chromatography-multiple ion detection mass fragmatography.
J. Chromatogr., 196: 314-318.
BLOCK, J.B. (1976) A Kentucky study: 1950-1975. In: Ede, L.,
ed. Proceedings of NIOSH Styrene-Butadiene Briefing,
Covington, Kentucky, USA, 1976, Cincinnati, Ohio, US
Department of Health, Education and Welfare pp. 28-32 (HEW
Publ. No. (NIOSH) 77-129).
BODNEI, A.H., BUTLER, G.J., & OKAWA, M.T. (1974) Health
hazard evaluation/toxicity determination. Cincinnati, US
Department of Health, Education and Welfare, Public Health
Service, Center for Disease Control, National Institute for
Occupational Safety and Health, 10 pp (Report no. 73-103-128 -
American Standard Fiberglass Inc., Stockton, California).
BONATTI, S., ABBONDANDOLO, A., CORTI, G., FIORIO, R., &
MAZZACCARO, A. (1978) The expression curve of mutants induced
by styrene oxide at the HGPRT locus in V79 cells.
Mutat. Res., 52: 295-300.
BROGGER, A. (1982) Application of SCE to public health. In:
Sandberg, A., ed. Sister chromatid exchange, New York, A.R.
Liss, pp. 655-673.
BROOKS, S.M., ANDERSON, L.A., TSAY, J.-Y., CARSON, A.,
BUNCHER, C.R., ELIA, V., & EMMETT, E.A. (1979) Investigation
of workers exposed to styrene in the reinforced plastic
industry - health and psychomotor status, toxicological and
industrial hygiene data and effect of protective equipment as
it relates to exposures through lung and skin routes.
Cincinnati, University of Cincinnati, College of Medicine,
pp. 330. (Institute of Environmental Health and Kettering
Laboratory Report Prepared for the Society of Plastics
Industries).
BROOKS, S., ANDERSON, L., EMMETT, E., CARSON, A., TSAY, J.-Y.,
ELIA, V., BUNCHER, R., & KARBOWSKY, R. (1980) The effects of
protective equipment on styrene exposure in workers in the
reinforced plastics industry. Arch. environ. Health, 35:
287-294.
BROUNS, R.E., POOT, M., DE VRIND, R., V. HOEK-KON, T.,
HENDERSON, P.T., & KUYPER, C.M.A. (1979) Measurement of
DNA-excision repair in suspensions of freshly isolated rat
hepatocytes after exposure to some carcinogenic compounds.
Its possible use in carcinogenicity screening. Mutat. Res.,
64: 425-432.
BUCHET, J.-P., LAUWERYS, R., ROELS, H., & DEFELD, J.-M.
(1974) [Evaluation of the exposure of workers to styrene by
means of the determination of its urinary metabolites:
mandelic and phenylglyoxylic acids: I. Technique of
determination of the metabolites by gas chromarography.
Arch. Mal. prof. Med. Tra. Secur. soc., 35: 511-516.
BURKIEWICZ, C., RYBKOWSKA, J., & ZIELINSKA, H. (1974)
[Assessment of exposure to styrene in human beings under
industrial conditions.] Med. Prac., 3: 305-310 (in Polish).
BURKOVA, T., BAJNOVA, A., & KAPURDOV, V. (1982) Occupational
risk with repeated styrene dermal contact. Prob. Hyg., VII:
51-59.
BURNETT, R.D. (1976) Evaluation of charcoal sampling tubes.
Am Ind. Hyg. Assoc. J., 37: 37-45.
BUSK, L. (1979) Mutagenic effects of styrene and styrene
oxide. Mutat. Res., 67: 201-208.
CAMURRI, L., CODELUPPI, S., & PEDRONI, C. (1982) Chromosomal
aberrations and sister chromatid exchanges in styrene exposed
workers. In: 12th Annual Meeting of European Environmental
Mutagen Society, Espoo, Finland, 20-24 June, 1982 Abstracts,
Helsinki, Institute of Occupational Health, p. 159.
CANTONI, L., SALMONA, M., FACCHINETTI, T., PANTAROTTO, C., &
BELVEDERE, G. (1978) Hepatic and extrahepatic formation and
hydration of styrene oxide in vitro in animals of different
species and sex. Toxicol. Lett., 2: 179-186.
CAPEROS, J.R., HUMBERT, B., & DROZ, P.O. (1979) Exposition on
styrene. II. Bilan de l'absorption, de l'excrétion et du
métabolisme sur des sujets humains. Int. Arch. occup.
environ. Health, 42: 223-230.
CARLSSON, A. (1981) Distribution and elimination of
14C-styrene in rat. Scand. J. Work Environ. Health, 7: 45-50.
CARPENTER, C.P., SHAFFER, C.B., WEIL, C.S., & SMYTH, H.F.
(1944) Studies on the inhalation of 1:3-butadiene; with a
comparison on its narcotic effect with benzol, tuluol, and
styrene, and a note on the elimination of styrene by the
human. J. ind. Hyg., 26: 69-78.
CERNA, M. & KYPENOVA, H. (1977) Mutagenic activity of
chloroethylenes analysed by screening system tests.
Mutat. Res., 46: 35-36.
CHEMICAL MARKET REVIEW (1981) Oct. 26, p. 7.
CHERRY, N., WALDRON, H.A., WELLS, G.G., WILKINSON, R.T.,
WILSON, H.K., & JONES, S. (1980) An investigation of the
acute behavioral effects of styrene on factory workers.
Br. J. ind. Med., 37: 234-240.
CHMIELEWSKI, J. & RENKE, W. (1975) Clinical and experimental
research into the pathogenesis of toxic effects of styrene
III. Morphology, coagulation and fibrinolisis systems of the
blood in persons exposed to the action of styrene during their
work. Biul. Inst. Med. Morskej, 26: 299-302.
CHMIELEWSKI, J., MIKULSKI, P., USELIS, J., & WIGLUSZ, R.
(1973) Rating of the exposure to styrene of persons working
at the production of polyester laminates. Biul. Inst. Med.
Morskej Gdansku, 24: 203-209.
CHMIELEWSKI, J., DOLMIERSKI, R., RENKE, W., & KWIATKOWSKI,
S.R. (1977) [Long-term occupational effects of styrene on
working people.] Z. gesamte Hyg. Grenzgeb., 23: 639-643 (in
German).
CHOI, K.K. & FUNG, K.W. (1979) Determination of styrene in
the atmosphere near industrial sites by gas chromatography.
Analyst, 104: 455-457.
CHU, K.C., CUETO, C., Jr, & WARD, J.M. (1981) Factors in the
evaluation of 200 National Cancer Institute Bioassays.
J. Toxicol. Environ. Health, 8: 251-280.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1979) Sister
chromatid exchange in regenerating liver an bone marrow cells
of mice exposed to styrene. Toxicol. appl. Pharmacol., 50:
365-367.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1980) Sister
chromatid exchange in murine alveolar macrophages, bone
marrow, and regenerating liver cells induced by styrene
inhalation. Toxicol. appl. Pharmacol., 55: 37-42.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1982) Multiple
tissue comparisons of sister chromatid exchanges induced by
inhaled styrene. In: Tice. R., Costa, D.L., & Schaich, K.M.,
ed. Genotoxic effects of airborne agents, New York, Plenum
Press, pp. 433-441.
CRANDALL, M.S. (1981) Worker exposure to styrene monomer in
the reinforced plastic boat-making industry. Am. Ind. Hyg.
Assoc. J., 42: 499-502.
CRITTENDEN, B.D. & LONG, R. (1976) The mechanisms of
formation of polynuclear aromatic compounds in combustion
systems. In: Carcinogenesis - A comprehensive survey - Volume
I, New York, Raven Press, pp. 209-223.
DANISHEFSKY, I. & WILLHITE, M. (1954) The metabolism of
styrene in the rat. J. Biol. Chem., 211: 549-553.
DELBRESSINE, L.P.C., KETELAARS, H.C.J., SEUTER-BERLAGE, F., &
SMEETS, F.L.M. (1980) Phenaceturic acid a new urinary
metabolite of styrene in the rat. Xenobiotica, 10: 337-342.
DEL CARRATORE, R., BRONZETTI, G., BAUER, C., CORSI, C., NIERI,
R., PAOLINI, M., & GIAGONI, P. (1982) Study of cytochrome
P450 in yeast D7 strain. An alternative model to microsomal
assay. Mutagenicity of styrene. In: 12th Annual Meeting of
the European Environmental Mutagen Society, Espoo, Finland,
20-24 June, 1982, Abstracts, Helsinki, Institute of
Occupational Health, p. 160.
DE MEESTER, C., PONCELET, F., ROBERFROID, M., RONDELET, J., &
MERCIER, M. (1977) Mutagenicity of styrene and styrene oxide.
Mutat. Res., 56: 147-152.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER,
M., MERCIER, M., & PONCELET, F. (1981) Mutagenicity of
styrene in the Salmonella typhimurium test system.
Mutat. Res., 90: 443-450.
DIETRICH, M.W., CHAPMAN, L.M., & MIEURE, J.P. (1978) Sampling
for organic chemicals in workplace athmosphere with porous
polymer beads. Am. Ind. Hyg. Assoc. J., 39: 385-392.
DOLMIERSKI, R., KWIATKOWSKI, S.R., & NITKA, J. (1974) A
preliminary evaluation of the studies on central nervous
system in workers exposed to styrene. Biul. Inst. Med.
Morskiej Gdansku, Gdansk, 25: 399-406.
DONNER, M., SORSA, M., & VAINIO, H. (1979) Recessive lethals
induced by styrene and styrene oxide in Drosophila
melanogaster. Mutat. Res., 67: 373-376.
DRINKWATER, N.R., MILLER, J.A., MILLER, E.C., & YANG, N.-C.
(1978) Covalent intercalative binding to DNA in relation to
the mutagenicity of hydrocarbon epoxides and N-acetoxy-2-
acetylaminofluorene. Cancer Res., 38: 3247-3255.
DROZ, P.O. & FERNANDEZ, J.G. (1977) Effect of physical work
load on retention and metabolism of inhaled organic solvents.
A comparative theoretical approach and its application with
regards to exposure monitoring. Int. Arch. occup. environ.
Health, 38: 231-246.
DUTKIEWICZ, T. & TYRAS, H. (1967) A study of the skin
absorption of ethylbenzene in man. Br. J. ind. Med., 24:
330-332.
DUTKIEWICZ, T. & TYRAS, H. (1968) Skin absorption of toluene,
styrene and xylene by man. Br. J. ind. Med., 25: 243.
DUVERGER-VAN BOGAERT, M., NOEL, G., ROLLMAN, B., CUMPS, J.,
ROBERFROID, M., & MERCIER, M. (1978) Determination of oxide
synthetase and hydratase activities by a new highly sensitive
gas chromatographic method using styrene and styrene oxide as
substrates. Biochim. Biophys. Acta, 526: 77-84.
ELIA, V.J., ANDERSON, L.A., MacDONALD, T.J., CARSON, A.,
BUNCHER, C.R., & BROOKS, S.M. (1980) Determination of urinary
mandelic and phenylglyoxylic acids in styrene exposed workers
and a control population. Am. Ind. Hyg. Assoc. J., 41:
922-926.
EL-MASRI, A.M., SMITH, J.N., & WILLIAMS, R.T. (1958) Studies
on detoxication. The metabolism of alkylbenzenes:
phenylacetylene and phenylethylene (styrene). Biochem. J.,
68: 199-204.
ELOVAARA, E., VAINIO, H., PFAFFLI, P., & COLLAN, Y. (1979)
Effects of intermittent styrene inhalation, ethanol intake and
their combination on drug biotransformation in rat liver and
kidneys. Med. Biol., 57: 321-327.
EL-TANTAWY, M.A. & HAMMOCK, B.D. (1980) The effect of hepatic
microsomal and cytosolic subcellular fractions on the
mutagenic activity of epoxide-containing compounds in the
Salmonella assay. Mutat. Res., 79: 59-71.
ENGSTROM, J. (1978) Styrene in subcutaneous adipose tissue
after experimental and industrial exposure. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 119-120.
ENGSTROM, J., USTRAND, I., & WIGAEUS, E. (1978a) Exposure to
styrene in a polymerization plant. Uptake in the organism and
concentration in subcutaneous adipose tissue. Scand. J. Work
Environ. Health, 4: 324-329.
ENGSTROM, J., BJURSTROM, R., USTRAND, I., & OVRUM, P. (1978b)
Uptake, distribution and elimination of styrene in man.
Concentration in subacutaneous adipose tissue. Scand. J. Work
Environ. Health, 4: 315-323.
ENGSTROM, K. (1983) In: Aitio, A., Riihimäki, V., & Vainio,
H., ed. Biological monitoring of exposure to industrial
chemicals, New York, Hemisphere (in press).
ENGSTROM, K. & RANTANEN, J. (1974) A new gas chromatographic
method for determination of mandelic acid in urine. Int.
Arch. Arbeitsmed., 33: 163-167.
ENGSTROM, K., HARKONEN, H., PEKARI, K., & RANTANEN, J. (1978)
Evaluation of occupational styrene exposure by ambient air and
urine analysis. Scand. J. Work Environ. Health, 4 (Suppl. 2):
121-123.
ENGSTROM, K., HARKONEN, H., KALLIOKOSKI, P., & RANTANEN, J.
(1976) Urinary mandelic acid concentration after occupational
exposure to styrene and its use as a biological exposure test.
Scand. J. Work Environ. Health, 2: 21-26.
FABRY, L., LEONARD, A., & ROBERFROID, M. (1978) Mutagenicity
tests with styrene oxide in mammals. Mutat. Res., 51: 377-381.
FAITH, W.L., KLYES, D.B., & CLARK, R.L. (1975) Industrial
chemicals, 4th ed., New York, John Wiley and Sons, Inc., pp.
365-370, 779-784.
FERNANDEZ, J.G. & CAPEROS, J.R. (1977) Exposition au styrène.
I. Etude expérimentale de l'absorption et l'excrétion
pulmonaires sur des sujets humains. Int. Arch. occup.
environ. Health, 40: 1-12.
FIELDS, R.L. & HORSTMAN, S.W. (1979) Biomonitoring of
industrial styrene exposures. Am. Ind. Hyg. Assoc. J., 40:
451-459.
FINLEY, J.W. & WHITE, J.C. (1967) Two methods to determine if
styrene monomer is present in milk, Bull. environ. Contam.
Toxicol., 2(1): 41-46.
FISEROVA-BERGEROVA, V. & TEISINGER, J. (1965) Pulmonary
styrene vapor retention. Ind. Med. Surg., 34: 620-622.
FISHBEIN, L. (1979) Chromatography of environmental hazards,
Volume II, Amsterdam, Elsevier, pp. 455-469.
FLEIG, I. & THIESS, A.M. (1978) Mutagenicity study of workers
employed in the styrene and polystyrene processing and
manufacturing industry. Scand. J. Work Environ. Health, 4
(Suppl. 2): 254-258.
FLEK, J. & SEDIVEC, V. (1980) Simultaneous gas
chromatographic determination of urinary mandelic and
phenylglyoxylic acids using diazomethane derivatization. Int.
Arch. occup. environ. Health, 45: 181-188.
FLEMING, R.D. (1970) Effect of fuel composition on exhaust
emissions from a spark-ignition engine, Washington DC, US
Department of the Interior, Bureau of Mines (Report of
Investigations 7423).
FOERST, W. (1965) [Styrene.] In: Ullmans Encyklopädie der
technischen Chemie (Vol. 16), Munich, Urban et Schwarzenberg,
pp. 460-476 (in German).
FRENTZEL-BEYME, R., THEISS, A.M., & WIELAND, R. (1978) Survey
of mortality among employees engaged in the manufacture of
styrene and polystyrene at the BASF Ludwigshafen works.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 231-239.
GADALINA, I.D., KAZNINA, N.I., KUZNETSOVA, G.M., &
SMIRNISTSKII, N.S. (1969) Hygienic assessment of polyester
plastics for flooring. Hyg. Sanit., 34: 25-29.
GAMBERALE, F. & HULTENGREN, M. (1974) Exposure to styrene
II. Psychological functions. Work Environ. Health, 11: 86-93.
GAMBERALE, F., LISPER, H.O., & ANSHELM-OLSON, B. (1975)
[Effect of styrene gases on reaction time among workers in
plastic boat industry.] Arbete och Hälsa, 8: 23 pp (in
Swedish).
GAZZOTTI, G., GARATTINI, E., & SALMONA, M. (1980) Nuclear
metabolism I. Determination of styrene monooxygenase activity
in rat liver nuclei. Chem. Biol. Interact., 29: 189-195.
GOMPERTZ, D. (1978) Comments made at the end of the session.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 227.
GORDON, M. & GOODLEY, P.C. (1971) Isolation and
characterization of industrial organic pollutants in Water.
Am. Chem. Soc., Div. Water, Air, Waste Chem., Gen. Pap.,
11(1): 91-94.
GOTELL, P., AXELSSON, O., & LINDELOF, B. (1972) Field studies
on human styrene exposure. Work Environ. Health, 9: 76-83.
GREIM, H., BIMBOES, D., EGERT, G., GOGGELMAN, W., & KRAMER, M.
(1977) Mutagenicity and chromosomal aberrations as an
analytical tool for in vitro detection of mammalian enzyme-
mediated formation of reactive metabolites. Arch. Toxicol.,
39: 159-169.
GROSSMAN, I.G. (1970) Waterborne styrene in a crystalline
bedrock aquifer in the Gales Ferry Area, Ledyard, Southeastern
Connecticut. US Geol. Surv. Prof. Pap., 700-B : 203-209.
GUILLEMIN, M. & BAUER, D. (1976) Human exposure to styrene.
II. Quantitative and specific gas chromatographic analysis of
urinary mandelic and phenylglyoxylic acids as an index of
styrene exposure. Int. Arch. occup. environ. Health, 37:
57-64.
GUILLEMIN, M.P. & BAUER, D. (1978) Biological monitoring of
exposure to styrene by analysis of combined urinary mandelic
and phenylglyoxylic acids. Am. Ind. Hyg. Assoc. J., 39:
873-879.
GUILLEMIN, M.P. & BAUER, D. (1979) Human exposure to styrene.
III. Elimination kinetics of urinary mandelic and
phenylglyoxylic acids after single experimental exposure.
Int. Arch. occup. environ. Health, 44: 249-263.
GUNTER, B.J. & LUCAS, J.B. (1973) Health hazard
evaluation/toxicity determination. Cincinnati, US Department
of Health, Education and Welfare, Public Health Service,
Center for Disease Control, National Institute for
Occupational Safety and Health, 17 pp (Report No. 72-86-38 -
Gates Rubber Co. Denver, CO.).
HAMIDULLIN, R.S., FELDMAN, N.G., & VENDILO, M.V. (1968)
Hygienic assessment of articles made of PS-S suspension
polystyrene, Hyg. Sanit., 33: 331-334.
HARKONEN, H. (1977) Relationship of symptoms to occupational
styrene exposure and to the findings of electroencephalo-
graphic and psychological examinations. Int. Arch. occup.
environ. Health, 40: 231-239.
HARKONEN, H. (1978) Styrene, its experimental and clinical
toxicology. Scand. J. Work Environ. Health, 4 (Suppl. 2):
104-113.
HARKONEN, H. & HOLMBERG, P. (1982) Obstetric histories of
workers occupationally exposed to styrene. Scand. J. Work
Environ. Health, 8: 74-77.
HARKONEN, H., KALLIOKOSKI, P., HIETALA, S., & HERNBERG, S.
(1974) Concentration of mandelic and phenylglyoxylic acid in
urine as indicators of styrene exposure. Work Environ.
Health, 11: 162-165.
HARKONEN, H., LINDSTROM, K., SEPPALAINEN, A.M., ASP, S., &
HERNBERG, S. (1978) Exposure-response relationships between
styrene exposure and central nervous functions. Scand. J.
Work Environ. Health, 4: 53-59.
HEMMINKI, K. (1979) Fluorescence study of DNA alkylation by
epoxides. Chem.-biol. Interact., 28: 269-278.
HEMMINKI, K. & FALCK, K. (1979) Correlation of mutagenicity
and 4-( p-nitrobenzyl)-pyridine alkylation by epoxides.
Toxicol. Lett., 4: 103-106.
HEMMINKI, K., FRANSSILA, E., & VAINIO, H. (1980a) Spontaneous
abortion among female chemical workers in Finland. Int. Arch.
occup. environ. Health, 45: 123-126.
HEMMINKI, K., PAASIVIRTA, J., KURKIRINNE, T., & VIRKKI, L.
(1980b) Alkylation products of DNA bases by simple epoxides.
Chem. biol. Interact., 30: 259-270.
HEMMINKI, K., HEINONEN, T., & VAINIO, H. (1981) Alkylation of
guanosine and 4-( p-nitrobenzyl)-pyridine by styrene oxide
analogues in vitro . Arch. Toxicol., 49: 35-41.
HIRATSUKA, A. & WATABE, T. (1982) [Reaction of 1-vinylbenzene
3,4-oxide with ethyl mercaptan.] In: [Proceedings of the
102nd Annual Meeting for the Pharmaceutical Society of Japan,
Osaka, Japan, 3-5 April, 1982]. Japan, p. 480 (in Japanese).
HIRATSUKA, A., AIZAWA, T., OZAWA, N., ISOBE, M., WATABE, T., &
TAKABATAKE, E. (1982a) The role of epoxides in the metabolic
activation of styrene to mutagens. Eisei Kagaku, 28: 34.
HOGSTEDT, B. & MITELMAN, F. (1983) Micronuclei in cultured
lymphocytes as an indicator of genotoxic exposure. In: De
Serres, F. & Pero, R.W., ed. Individual susceptibility to
genotoxic agents in the human population, New York, Plenum
Publishing Corporation, (in press).
HOLMBERG, P.C. (1977) Central nervous defects in two children
of mothers exposed to chemicals in the reinforced plastics
industry: chance or causal relation? Scand. J. Work Environ.
Health, 3: 212-214.
HOSHIKA, Y. (1977) Gas chromatographic determination of
styrene as its dibromide. J. Chromatogr., 136: 95-103.
HUZL, F., SYKOVA, J., MAINEVOVA, J., JANKOVA, J., SRUTEK, J.,
JUNGER, V., & LAHN, V. (1967) [The question of health hazards
in working with styrene.] Prac. Lek., 19: 121-125 (in Czech).
IARC (1979) Some monomers, plastics and synthetic elastomers,
and acrolein, Lyons, International Agency for Research on
Cancer, pp. 231-274 (IARC Monographs on the evaluation of the
carcinogenic risk of chemicals to humans. Vol. 19).
IKEDA, M. & IMAMURA, T. (1974) Evaluation of hippuric,
phenylglyoxylic and mandelic acids in urine as indices of
styrene exposure. Int. Arch. Arbeitsmed., 32: 93-101.
IKEDA, M., IMAMURA, T., HAYASHI, M., TABUCHI, T., & HARA, I.
(1974) Evaluation of hippuric, phenylglyoxylic and mandelic
acids in urine as indices of styrene exposure. Int. Arch.
Arbeitsmed., 32: 93-101.
IKEDA, M., KOIZUMI, A., MIYASAKA, M., & WATABE, T. (1982)
Styrene exposure and biologic monitoring in FRP production
plants. Int. Arch. occup. environ. Health, 49: 325-339.
ISHIDATE, M., Jr & YOSHIKAWA, K. (1980) Chromosome aberration
tests with Chinese hamster cells in vitro with and without
metabolic activation - a comparative study on mutagens and
carcinogens. Arch. Toxicol., Suppl. 4: 41-44.
ISHIDATE, M., Jr, SOFUNI, T., & YOSHIKAWA, K. (1981)
Chromosomal aberration tests in vitro as a primary screening
tool for environmental mutagens and/or carcinogens. GANN
Monogr. Cancer Res., 27: 95-108.
ITI (1975) Toxic and hazardous industrial chemicals safety
manual, Tokyo, The International Technical Information
Institute, pp. 494-495.
IZJUMOVA, A.S. (1972) [The action of small concentrations of
styrol on the sexual function of albino rats.] Gig. i Sanit.,
37: 29-30 (in Russian).
IVANOVA-TCHEMICHANSKA, L., ANTOV, G., & ILIEVA, P. (1982)
[Gonadotropic action of some organic solvents.] In: Resumes
de la XVIIe Semaine, August 29-September 3, 1982, Sofia,
Bulgaria, Sofia, L. Union Médicale Balkanique, pp. 50-51 (in
French).
JACOBS, H.W. & SYRJALA, R.H. (1978) The use of infrared
analyzers for monitoring acrylonitrile. Am. Ind. Hyg. Assoc.
J., 39: 161-165.
JAMES, M.O., FOUTS, J.R., & BEND, J.R. (1975) Hepatic and
extrahepatic metabolism in vitro of an epoxide (8-14C-
styrene oxide) in the rabbit. Biochem. Pharmacol., 25:
187-193.
JAMES, S.D. & WHITE, D.A. (1967) The metabolism of phenethyl
bromide, styrene and styrene oxide in the rabbit and rat.
Biochem. J., 104: 914-921.
JENSEN, F. (1972) Determination of monomers from polystyrene
in milk products, Ann. 1st Super. Sanita, 8: 443-448.
JERMINI, C., WEBER, A., & GRANDJEAN, E. (1976) [Quantitative
determination of various gas-phase components of the side-
stream smoke of cigarettes in the room air as a contribution
to the problem of passive-smoking.] Int. Arch. occup.
environ. Health, 36: l69-181 (in German).
JERSEY, G.C., BALMER, M.F., QUAST, J.F., PARK, C.N., SCHUETZ,
D.J., BEYER, J.E., OLSEN, K.J., MCCOLLISTER, S.B., & RAMPY,
L.W. (1978) Two-Year chronic inhalation toxicity and
carcinogenicity study of monomeric styrene in rats - Final
report. (2yr), Midland, MI, Dow Chemical Co., 150 pp
(Manufacturing Chemists Association MCA No: Sty 1.1-Tox-INH).
JOHNSTONE, R.A.W., QUAN, P.M., & CARRUTHERS, W. (1962)
Composition of cigarette smoke: Some low-boiling components.
Nature (Lond.), 195: 1267-1269.
KALLIOSKI, P. (1976) [The reinforced plastic industry - a
problem work environment.] (Työterveyslaitoksen tutkimuksia
122) (in Finnish).
KALLIOSKI, P. & PFAFFLI, P. (1975) Charcoal sampling method
for determining the concentration of styrene in air. Scand.
J. Work Environ. Health, 1: 193-198.
KANKAANPAA, J.T.J., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity and teratogenicity of styrene and styrene oxide
on chick embryos enhanced by trichloropropylene oxide. Acta
Pharmacol. Toxicol., 45: 399-402.
KANKAANPAA, J.T.J., ELOVAARA, E., HEMMINKI, K., & VAINIO, H.
(1980) The effect of maternally inhaled styrene on embryonal
and foetal development in mice and Chinese hamsters. Acta
Pharmacol. Toxicol., 47: 127-129.
KAWACHI, T., YAHAGI, T., KADA, T., TAZIMA, Y., ISHIDATE, M.,
SASAKI, M., & SUGIYAMA, T. (1979) Cooperative programme on
short-term assays for carcinogenicity in Japan. In:
Montesano, R., Bartsch, H., & Tomatis, L., ed. Molecular and
cellular aspects of carcinogen screening tests, IARC
Scientific Publication No. 27, Lyons, IARC, pp. 323-330.
KAZNINA, N.I. (1969) Air pollution by volatiles liberated by
some plastics. Hyg. Sanit., 33: 267-269.
KJELLBERG, A., WIGAEUS, E., ENGSTROM, J., CSTRAND, I., &
LJUNGQUIST, E. (1979) [Long-term effects of styrene exposure
in plastic industry.] Arbete och Hälsa, 18: 1-25 (in Swedish).
KLIMKOVA-DEUTSCHOVA, E. (1962) [Neurological findings in the
plastics industry among styrene workers.] Int. Arch.
Gewerbepath. Gewerbehyg., 19: 35-50 (in German).
LEIBMAN. K.C. (1975) Metabolism and toxicity of styrene.
Environ. health Perspect., 11: 115-119.
LEIBMAN, K.C. & ORTIZ, E. (1969) Oxidation of styrene in
liver microsomes. Biochem. Pharmacol., 18: 552-554.
LEITHE, W. (1971) Analysis of air pollutants, Ann Arbor, Ann
Arbor Science Publishers, p. 246.
LEMEN, R.A. & YOUNG, R. (1976) Investigations of health
hazards in styrene butadiene rubber facilities. In: Ede, L.,
ed. Proceedings of the NIOSH Styrene-Butadiene Briefing,
Covington, Kentucky, USA, 1976, Cincinnati, Ohio, US
Department of Health, Education and Welfare pp. 3-8 (HEW
Publ. No. (NIOSH) 77-129).
LILIS, R. & NICHOLSON, W.J. (1976) Cancer experience among
workers in a chemical plant producing styrene monomers. In:
Ede, L., ed., Proceedings of the NIOSH Styrene-Butadiene
Briefing, Covington, Kentucky, USA, 1976, Cincinnati, Ohio,
Department of Health, Education and Welfare pp. 22-27 (HEW
Publ. No. (NIOSH) 77-129).
LILIS, R., LORIMER, W.V., DIAMOND, S., & SELIKOFF, I.J. (1978)
Neurotoxicity of styrene in production and polymerization
workers. Environ. Res., 15: 133-138.
LINDSTROM, K., HARKONEN, H., & HERNBERG, S. (1976)
Disturbances in psychological functions of workers
occupationally exposed to styrene. Scand. J. Work Environ.
Health, 2: 129-139.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978a)
Cytogenetic effects of styrene and styrene oxide. Mutat.
Res., 58: 277-286.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978b)
Cytogenetic effects of styrene and styrene oxide on human
lymphocytes and Allium cepa. Scand. J. Work Environ. Health,
4 (Suppl. 2): 156-162.
LITTON BIONETICS (1980) Toxicological study on styrene
incorporated in drinking water of rats for two years in
conjuction with a three-generation reproduction study.
(Submitted to Chemical Manufacturers Association.)
LOPRIENO, N. (1981) Environmental mutagens and comparative
short-term mutagenicity studies. In: Molecular bases of
genetic processes. Proceedings of the XIV International
Congress of Genetics, Moscow, MIR Publishers, Vol. III, Book
1, pp. 164-180.
LOPRIENO, N. & ABBONDANDOLO, A. (1980) Comparative mutagenic
evaluation of some industrial compounds. In: Norpoth, K.H. &
Garner, R.C., ed. Short-term test systems for detecting
carcinogens, Berlin, Springer-Verlag, pp. 333-356.
LOPRIENO, N., ABBONDANDOLO, A., BARALE, R., BARONCELLI, S.,
BONATTI, S., BRONZETTI, G., CAMMELLINI, A., CORSI, C., CORTI,
G., FREZZA, D., LEPORINI, C., MAZZACCARO, A., NIERI, R.,
ROSELLINI, R., & ROSSI, A.M. (1976) Mutagenicity of
industrial compounds: styrene and its possible metabolite
styrene oxide. Mutat. Res., 40: 317-324.
LOPRIENO, N., PRESCIUTTINI, S., SBRANA, I., STRETTI, G.,
ZACCARO, L., ABBONDANDOLO, A., BONATTI, S., FIORIO, R., &
MAZZACCARO, A. (1978) Mutagenicity of industrial compounds
VII. Styrene and styrene oxide: II. Point mutations,
chromosome aberrations and DNA repair induction analyses.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 169-178.
LORIMER, W.V., LILIS, R., NICHOLSON, W.J., ANDERSON, H.,
FISCHBEIN, A. DAUM, S., ROM, W., RICE, C., & SELIKOFF, I.J.
(1976) Clinical studies of styrene workers: Initial
findings. Environ. health Perspect., 17: 171-181.
LORIMER, W., LILIS, R., FISCHBEIN, A., DAUM, S., ANDERSON, H.,
WOLFF, M., & SELIKOFF, I. (1978) Health status of
styrene-polystyrene polymerization workers. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 220-226.
LUNDBERG, I. (1981) [Serumenzyme levels in workers exposed to
styrene.] Arbete och Hälsa, 1: 19 pp (in Swedish).
MAIER, A. RUHE, R., TOSENSTEEL, R., & LUCAS, J.B. (1974)
Health hazard evaluation/toxicity determintion. Arco Polymer
Incorporated (Sinclair-Koppers Company, Inc.), Monaco, PA.,
Cincinnati, US Department of Health, Education, and Welfare,
Center for Disease Control, National Institute for
Occupational Safety and Health, 28 pp (Report No. 72-90--107).
MALTONI, C., FAILLA, G., & KASSAPIDIS, G. (1979) First
experimental demonstration of the carcinogenic effects of
styrene oxide. Long-term bioassays on Spraque-Dawley rats by
oral administration. Med. Lav., 70(5): 358-362.
MANSON, N.W., REILLY, D.A., & STAGG, H.E. (1965) The
determination of toxic substances in air, manual of ICI
practice, Cambridge, W. Heffer & Sons, p. 181.
MARNIEMI, J., SUOLINNA, E.M., KAARTINEN, N., & VAINIO, H.
(1977) Covalent binding of styrene oxide to rat liver
macromolecules in vivo and in vitro. In: Ullrich, V., Roots,
I., Hildebrandt, A., Estabrook, R.W., & Conney, A.H., ed.
Microsomes and drug oxidations, London, Oxford, Pergamon
press, pp. 698-702.
MATSUOKA, A., HAYASHI, M., & ISHIDATE, M., Jr (1979)
Chromosomal aberration tests on 29 chemicals combined with S9
mix in vitro. Mutat. Res., 66: 277-290.
MATSUSHITA, T., MATSUMOTO. T., MIYAGAKI, J., MAEDA, K.,
TAKEUCHI, Y., & KATAJIMA, J. (1968) [Nervous disorders
considered to be symptoms of chronic styrol poisoning.
Saigai Igaku, 11: 173-179 (in Japanese).
McMICHAEL, A.J., SPIRTAS, R., GAMBLE, J.F., & TOUSEY, P.M.
(1976) Mortality among rubber workers: relationship to
specific jobs. J. occup. Med., 18: 178-185.
McNAIR, H.M. & BONELLI, E.J. (1968) Basic Gas Chromatography,
5th Ed., Walnut Creek, Varian Aerograph., p. 101.
MEINHARDT, T.J., YOUNG, R.J., & HARTLE, R.W. (1978)
Epidemiologic investigations of styrene-butadiene rubber
production and reinforced plastics production. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 240-246.
MERETOJA, T. & VAINIO, H. (1979) The use of human lymphocyte
tests in the evaluation of potential mutagens: clastogenic
activity of styrene in occupational exposure. In: Berg, K.,
ed. Genetic damage in man caused by environmental agents, New
York, Academic Press, pp. 213-225.
MERETOJA, T., VAINIO, H., SORSA. M., & HARKONEN, H. (1977)
Occupational styrene exposure and chromosomal aberrations.
Mutation Res., 56: 193-197.
MERETOJA, T., JARVENTAUS, H., SORSA, M., & VAINIO, H. (1978a)
Chromosome aberrations in lymphocytes of workers exposed to
styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
259-264.
MERETOJA, T., VAINIO, H., & JARVENTAUS, H. (1978b)
Clastogenic effects of styrene exposure on bone marrow cells
of rat. Toxicol. Lett., 1: 315-318.
MILVY, P. & GARRO, A.J. (1976) Mutagenic activity of styrene
oxide (1,2-epoxyethylbenzene), a presumed styrene metabolite.
Mutat. Res., 40: 15-18.
MITHEN, F.A., COCHRAN, M., JOHNSON, M.I., & BUNGE, R.P. (1980)
Neurotoxicity of polystyrene containers detected in a closed
tissue culture system. Neurosci. Lett., 17: 107-111.
MURRAY, F. J., JOHN, J. A., BALMER, M. F., & SCHWELTZ, B. A.
(1978) Teratologic evaluation of styrene given to rats and
rabbits by inhalation or by gavage, Toxicology, 11: 335-343.
NELIGAN, R.E., LEONARD, M.J., & BRYAN, R.J. (1965) The gas
chromatographic determination of aromatic hydrocarbons in the
atmosphere. Am. Chem. Soc. Div. Water, Air. Waste Chem.
Preprints, 5: 118-121.
NICHOLSON, W.J., SELIKOFF, I.J., & SEIDMAN, H. (1978)
Mortality experience of styrene-polystyrene polymerization
workers. Initial findings. Scand. J. Work Environ. Health, 4
(Suppl. 2): 247-252.
NIOSH (1974) NIOSH manual of analytical methods, Cincinnati,
National Institute for Occupational Safety and Health, (Method
No. 127).
NORPPA, H. (1981a) Chromosome damage induced by styrene,
styrene oxide and some analogues, Academic dissertation,
Helsinki, Institute of Occupational Health and University of
Helsinki, 56 pp.
NORPPA, H. (1981b) Styrene and vinyltoluene induce
micronuclei in mouse bone marrow. Toxicol. Lett., 8: 247-251.
NORPPA, H. & VAINIO, H. (1983a) Genetic toxicity of styrene
and some of its derivatives. Scand. J. Work Environ. Health,
9: 108-114.
NORPPA, H. & VAINIO, H. (1983b) Induction of sister-chromatid
exchanges by styrene analogues in cultured human lymphocytes.
Mutat. Res., 116: 379-387.
NORPPA, H., ELOVAARA, E., HUSGAFVEL-PURSIAINEN, K., SORSA, M.,
& VAINIO, H. (1979) Effects of styrene oxide on chromosome
aberrations, sister chromatid exchange and hepatic drug
biotransformation in Chinese hamsters in vivo. Chem.-biol.
Interact., 26: 305-315.
NORPPA, H., SORSA, M., PFAFFLI, P., & VAINIO, H. (1980a)
Styrene and styrene oxide induce SCEs and are metabolised in
human lymphocyte cultures. Carcinogenesis, 1: 357-361.
NORPPA, H., SORSA, M., & VAINIO, H. (1980b) Chromosomal
aberrations in bone marrow of Chinese hamsters exposed to
styrene and ethanol. Toxicol. Lett., 5: 241-244.
NORPPA, H., VAINIO, H., SORSA, M., & BELVEDERE, G. (1982)
Metabolic activation of styrene by erythrocytes in human
lymphocyte cultures. In: 12th Annual Meeting of the European
Environmental Mutagen Society, Espoo, Finland, 20-24 June,
1982, Abstracts, Helsinki, Institute of Occupational Health,
p. 148.
NYLANDER, P. (1979) [LSE polyesters - environmental
effects.] Plastforum Scandinavia, 9: 130-132 (in Swedish).
ODKVIST, L.M., CSTRAND, I., LARSBY, B., & KALL, C. (1980)
[Does styrene incude disturbancies in the human balance
mechanisms?] Arbete och Hälsa, 2: 5-19 (in Swedish).
OESCH, F. (1973) Mammalian epoxide hydrolase. Inducable
enzyme catalyzing the inactivation of carcinogenic and
cytotoxic metabolites derived from aromatic and olefinic
compounds. Xenobiotica, 3: 305-360.
OGATA, M. & SUGIHARA, R. (1978) High performance liquid
chromatographic procedure for quantitative determination or
urinary phenylglyoxylic, mandelic and hippuric acids as
indices of styrene exposure. Int. Arch. occup. environ.
Health, 42: 11-19.
OHTSUJI, H. & IKEDA, M. (1970) A rapid colorimetric method
for the determination of phenylglyoxylic and mandelic acids.
Its application to the urinanalysis of workers exposed to
styrene vapour. Br. J. ind. Med., 27: 150-154.
OHTSUJI, H. & IKEDA, M. (1971) The metabolism of styrene in
rat and the stimulatory effect of phenobarbital. Toxicol.
appl. Pharmacol., 18: 321-328.
OLTRAMARE, M., DESBAUMES, E., IMHOFF, C., & MICHIELS, W.
(1974) Toxicologie du styrene monomère. Geneva, Editions
Mèdicine et Hygiène, 100 pp.
OTT, M.G., KOLESAR, R.C., SCHARNWEBER, H.C., SCHNEIDER, E.J.,
& VENABLE, J.R. (1980) A mortality survey of employees
engaged in the development or manufacture of styrene-based
products. J. occup. Med., 22: 445-460.
PACHECKA, J., GARIBOLDI, P., CANTONI, L., BELVEDERE, G.,
MUSSINI, E., & SALMONA, M. (1979) Isolation and structure
determination of enzymatically formed styrene oxide
glutathione conjugates. Chem. Biol. Interact., 27: 313-321.
PAGANO, D.A., YAGEN, B., HERNANDEZ, O. BEND, J.R., & ZEIGER,
E. (1982) Mutagenicity of ( R) and ( S) styrene 7,8-oxide and
the intermediary mercapturic acid metabolites formed from
styrene 7,8-oxide. Environ. Mutag., 4(5): 575-584.
PAGANO, G., ESPOSITO, A., GIORDANO, G.G., & HAGSTROM, B. E.
(1978) Embryotoxic and teratogenic effects of styrene
derivatives on sea urchin development. Scand. J. Work,
Environ. Health, 4 (Suppl. 2): 136-141.
PANTAROTTO, E., FANELLI, R., BIDOLI, F., MANZONI, P., SALMONA,
M., & SZCZAWINSKI, K. (1978) Areneoxides in styrene
metabolism. New perspective in styrene toxicity? Scand. J.
Work Environ. Health, 4 (Suppl. 2): 67-77.
PANTAROTTO, C., SALMONA, M., SZCZAWINSKA, K., & BIDOLI, F.
(1980) Gas chromatography-mass spectrometric studies on
styrene toxicity. In: Albatges, J., ed. Analytical techniques
in environmental chemistry, New York, Pergamon Press, pp.
245-279.
PARKKI, M.G. (1978) The role of glutathione in the toxicity
of styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
53-59.
PARKKI, M.G., MARNIEMI, J., & VAINIO, H. (1976) Action of
styrene and its metabolites styrene oxide and styrene glycol
on activities of xenobiotic biotransformation enzymes in rat
liver in vivo. Toxicol. appl. Pharmacol., 38: 59-70.
PENTTILA, M., SORSA, M., & VAINIO, H. (1980) Inability of
styrene to induce nondisjunction in Drosophila or a positive
micronucleus test in the Chinese hamster. Toxicol. Lett., 6:
119-123.
PERO, R.W., BRYNGELSSON, T., HOGSTEDT, B., & CKESON, B. (1982)
Occupational and in vitro exposure to styrene assessed by
unscheduled DNA synthesis in resting human lymphocytes.
Carcinogenesis, 3: 681-685.
PERVIER, J.W., BARLEY, R.C., FIELD, D.E., FRIEDMAN, B.M., &
MORRIS, R.B. (1974) Survey reports on atmospheric emissions
from the petrochemical industry, Vol. IV. 287 pp (US NTIS, PB
Rep. PB-245630).
PFAFFLI, P., HESSO, A., VAINIO, H., & HYVONEN, M. (1981)
4-Vinylphenol excretion suggestive of arene oxide formation in
workers occupationally exposed to styrene. Toxicol. appl.
Pharmacol., 60: 85-90.
PHILIPPE, R., LAUWERYS, R., BUCHET, J.P., ROELS, H., & DEFELD,
J.M. (1974) [Evaluation of the exposure of workers to styrene
by means of the determination of its urinary metabolites:
mandelic and phenylglyoxylic acids: II. Application to
polyester fiber workers.] Arch. Mal. Prof. Méd. Tra. Secur.
Soc., 35: 631-640.
PLANCHE, G., CROISY, A., MALAVEILLE, C., TOMATIS, L., &
BARTSCH, H. (1979) Metabolic and mutagenicity studies on DDT
and 15 derivatives. Detection of 1,1-bis(p-chlorophenyl)-2,2-
dichloroethane and 1,1-bis(p-chlorophenyl)-2,2,2-trichloro-
ethyl acetate (kelthane acetate) as mutagens in Salmonella
typhimurium and of 1,1-bis(p-chlorophenyl)ethylene oxide, a
likely metabolite, as an alkylating agent. Chem. biol.
Interact., 25: 157-175.
POGGI, G., GUISIANI, M., PALAGI, U., PAGGIARO, P.L., LOI,
A.M., DAZZI, F., SICLARI, C., & BASCHIERI, L. (1982) High-
performance liquid chromatography for the quantitative
determination of the urinary metabolites of toluene, xylene,
and styrene. Int. Arch. occup. environ. Health, 50: 25-31.
PONCELET, F., DE MEESTER, C., DUVERGER-VAN BOGAERT, M.,
LAMBOTTE-VANDEPAER, M., ROBERTFROID, M., & MERCIER, M. (1980)
Influence of experimental factors on the mutagenicity of
vinylic monomers. Arch. Toxicol., Suppl. 4: 63-66.
PONOMAREVA, N.I. & ZLOBINA, N.S. (1972) [Working conditions
and the state of the upper respiratory tract in workers
engaged in the production of block and emulsion polystyrene
and its copolymers.] Gig. Tr. Prof. Zabol., 15: 22-26 (in
Russian).
PONOMARKOV, V. & TOMATIS, L. (1978) Effects of long-term oral
administration of styrene to mice and rats. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 127-135.
PONOMARKOV, V., CABRAL, J.R.P., WAHRENDORF, J., & GALENDO, D.
(1983) A carcinogenicity study of styrene oxide in rats.
Toxicolgist, 3: 46 (Abstract No. 184).
QUAST, J.F., KALNINS, R.P., OLSON, K.J., HUMISTON, C.G.,
MURRAY, F.J., JOHN, J.A., & SCHWETZ, B.A. (1978) Results of a
toxicity study in dogs and teratogenicity studies in rabbits
and rats administered monomeric styrene. In: Meeting of the
Society of Toxicology, San Francisco, USA, March 1978.
QUAST, J.F., HUMISTON, C.G., KALNINS, R.V., OLSON, K.J.,
McCOLLISTER, S.B., WADE, C.E., BEYER, J.E., & SCHWETZ, B.A.
(1979) Results of a toxicity study of monomeric styrene
administered to beagle dogs by oral intubation for 19 months -
Midland, MI, Dow Chemical Co., 199 pp (MCA (Manufacturing
Chemists Association) No: Sty 1.2-Tox-Gav-Dow.)
RAGYL'YE, N. (1974) [Problems concerning the embryotropic
effects of styrene.] Gig. i Sanit., 11: 65-66 (in Russian).
RAMSEY, J.C. & YOUNG, J.D. (1978) Pharmacokinetics of inhaled
styrene in rats and humans. Scand. J. Work Environ. Health, 4
(Suppl. 2): 84-91.
RAMSEY, J.C., YOUNG, J.D., KARBOWSKI, R.J., CHENOWETH, M.B.,
MCCARTY, L.P., & BRAUN, W.H. (1980) Pharmacokinetics of
inhaled styrene in human volunteers. Toxicol. appl.
Pharmacol., 53: 54-63.
RENKE, W. & CHEMIELEWSKI, J. (1976) Blood coagulation and
fibronolysis in occupational exposure to some chemical
factors. Biul. Inst. Med. Morskiej, 27: 289-298.
RIIHIMAKI, V. & PFAFFLI, P. (1978) Percutaneous absorption of
solvent vapors in man. Scand. J. Work Environ. Health, 4:
73-85.
ROSEN, A.A., SKEEL, R.T., & ETTINGER, M.B. (1963)
Relationship of river water odor to specific organic
contaminants. J. Water Pollut. Control Fed., 35: 777-82.
ROSEN, I., HAEGER-ARONSEN, B., REHNSTROM, S., & WELINDER, H.
(1978) Neurophysiological observations after chronic styrene
exposure. Scand. J. Work Environ. & Health, 4 (Suppl. 2):
184-194.
ROSENSTEEL, R.E. & MEYER, C.R. (1977) Health hazard
evaluation determination. Cincinnati, US Department of
Health, Education and Welfare, Public Health Service, Center
for Disease Control, National Institute for Occupational
Safety and Health. 52 pp (Report no. 75-150-378 - Reinell
Boats, Inc., Poplar Bluff, Missouri).
ROTH, B. & KLIMKOVA-DEUTSCHOVA, E. (1963) The effect of the
chronic action of industrial poisons on the electro-
encephalogram of man. Rev. Czech. Med., 9: 217-227.
ROTH, S.H. (1979) Physical mechanisms of anesthesia. Ann.
Rev. Pharmacol. Toxicol., 19: 159-178.
SAALWAECHTER, A.T., McCAMMON, C.S., ROPER, C.P. Jr, &
CARLBERG, K.S. (1977) Performance testing of the NIOSH
charcoal tube technique for the determination of air
concentrations of organic vapors. Am. Ind. Hyg. Assoc. J.,
38(9): 476-486.
SANTODONATO, J., MEYLAN, W.M., DAVIS, L.N., HOWARD, P.H.,
ORZEL, D.M., & BOGYO, D.A. (1980) Investigation of selected
potential environmental contaminants: Styrene, ethyl benzene
and related compounds. 261 pp (A report prepared for the
Office of Toxic Substances, US Environmental Protection
Agency, Washington, DC by Syracuse Research Corporation;
Contract No. 68-01-3250.)
SAUERHOFF, M.W. & BRAUN, W.H. (1976) The fate of styrene in
rats following an inhalation exposure to 14 C-styrene.
Midland, Michigan, Toxicology Research Laboratory, Health and
Environmental Research, Dow Chemical USA, 26 pp.
SAUERHOFF, M.W., MADRID, E.O., & BRAUN, W.H. (1976) The fate
of orally administered styrene in rats. Midland, Michigan,
Toxicology Research Laboratory, Health and Environmental
Research, Dow Chemical USA, 44 pp.
SAVOLAINEN, H. & PFAFFLI, P. (1977) Effects of chronic
styrene inhalation on rat brain protein metabolism. Acta
Neuropath., 40: 237-241.
SAVOLAINEN, H. & PFAFFLI, P. (1978) Accumulation of styrene
monomer and neurochemical effects of long-term inhalation
exposure in rats. Scand. J. Work Environ. Health, 4 (Suppl.
2): 78-83.
SAVOLAINEN, H. & VAINIO, H. (1977) Organ distribution and
nervous system binding of styrene and styrene oxide.
Toxicology, 8: 135-141.
SAVOLAINEN, H., HELOJOKI, M., & TENGEN-JUNNILA, M. (1980)
Behavioural and glial cell effects of inhalation exposure to
styrene vapour with special reference to interactions of
simultaneous ethanol intake. Acta Pharmacol. Toxicol., 46:
51-56.
SBRANA, I., LASCIALFARI, D., ROSSI, A.M., & LOPRIENO, N.
(1982) Bone marrow cell chromosomal aberrations and styrene
biotransformation in mice given styrene on a repeated oral
schedule. Submitted for publication.
SCHALLER, K.-H., GOSSLER, K., BOST, H.-P., & VALENTIN, H.
(1976) [Gas chromatographic methods for the determination of
styrene in the blood and of mandelic acid and phenylglyoxylic
acid in the urine.] Arbeits-, Sozial-, Präventivmedizin, 11:
64-64 (in German).
SCHNEIDER, H. & SEEBER, A. (1979) [Psychodiagnostics in the
understanding of neurotoxic effects of harmful chemical
substances.] Z. Psychol. Bd., 187: 178-205 (in German).
SCHOFIELD, K. (1974) Problems with flame ionization detectors
in automotive exhaust hydrocarbon measurements. Environ. Sci.
Technol., 8: 826-834.
SCHUMACHER, R.L., BREYSSE, P.A., CARLYON, W.R., HIBBARD, R.P.,
& KLEINMAN, G.D. (1981) Styrene exposure in the fiberglass
fabrication industry in Washington State. Am. Ind. Hyg.
Assoc. J., 42: 143-149.
SEPPALAINEN, A.M. (1978) Neurotoxicity of styrene in
occupational and experimental exposure. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 181-183.
SEPPALAINEN, A.M. & HARKONEN, H. (1976) Neurophysiological
findings among workers occupationally exposed to styrene.
Scand. J. Work Environ. Health, 2: 140-146.
SEPPALAINEN, A.M., LINDSTROM, K., & MARTELIN, T. (1980)
Neurophysiological and psychological picture of solvent
poisoning. Am. J. ind. Med., 1: 31-42.
SEUTTER-BERLAGE, F., DELBRESSINE, L.P.C., SMEETS, F.L.N., &
KETELAARS, H.C.J. (1978) Identification of three sulphur-
containing urinary metabolites of styrene in the rat.
Xenobiotica, 8: 413-418.
SHUGAEV, B.B. (1969) Concentration of hydrocarbons in tissues
as a measure of toxicity. Arch. environ. Health, 18: 878-882.
SIMKO, A., JINDRICHOVA, J., & PULTAROVA, H. (1966) [The
effect of styrene on the health state of workers employed in
laminate-production.] Prac. Lek., 18: 348-352 (in Czech).
SINITSKIJ, V.G. (1969) [Indexes for immunological reactivity
in rabbits during the long-term exposure to small doses of
styrene.] Gig. Primen. Polim. Mater, Izdelii Nikh, 1: 394-398
(in Russian).
SLOB, A. (1973) A new method for determination of mandelic
acid excretion at low level styrene exposure. Br. J. ind.
Med., 30: 390-393.
SMIRNOVA, E.T. & YATAKOVA, Z.M. (1966) Health hazard of
polystyrene toys. Hyg. Sanit., 31: 135-137.
SMITH, A.H. & ELLIS, L. (1977) Styrene butadiene rubber
synthetic plants and leukaemia. J. occup. Med., 19: 441.
SMITH, H.O. & HOCHSTETTLER, A.D. (1969) Determination of odor
thresholds in air using C14-labeled compounds to monitor
concentrations. Environ. Sci. Technol., 3: 169-170.
SODER, S.L. (1977) "Styrene" Chemical economics handbook,
California, Stanford Research Institute.
SOLLENBERG, J. & BALDESTEN, A. (1977) Isotachophoretic
analysis of mandelic acid, phenylglyoxylic acid, hippuric acid
and methylhippuric acid in urine after occupational exposure
to styrene, toluene and/or xylene. J. Chromatogr., 132:
469-476.
SORSA, M., HYVONEN, M., JARVENTAUS, H., & VAINIO, H. (1979)
Chromosomal aberrations and sister chromatid exchange in
children of women in reinforced plastic industry. In:
Symposium on Toxicology, Abstracts, Turku, Finland 29-20 May,
1979, Turku, University of Turku, p. 16.
SPASOVSKI, M. (1976) Health hazards in the production and
processing of some fibers, resins, and plastics in Bulgaria.
Environ. Health Perspect., 17: 199-202.
SPASOVSKI, M., HINKOVA, L., & BENCHEV, I. (1980) Exposure to
styrene - field studies. Toxicol. Lett., (suppl. 1): 218.
SPENCER, H.C., IRISH, D.D., ADAMS, E.M., & ROWE, V.K. (1942)
The response of laboratory animals to monomeric styrene. J.
ind. Hyg. Toxicol., 24: 295-301.
SPINAZZOLA et al. (1980) Petrolchimica. Tecnologia, ambiente
di lavoro, prevenzione e patologia. Atti del 43° Congresso
Nazionale della Società Italiana di Medicina del Lavoro ed
Igiene Industriale. Parma, Edizioni Tecnografica, Vol. 2.
SPIRTAS, R., VAN ERT, M., GAMBLE, J.F., WOLF, P., & McMICHAEL,
A.J. (1976) Toxicologic, industrial hygiene and
epidemiological consideration in the possible association
between SBR manufacturing and neoplasms of the lymphatic and
hematopoietic tissues. In: Ede, L., ed. Proceedings of NIOSH
Styrene-Butadiene Briefing, Covington, Kentucky, USA, 1976,
Cincinnati, Ohio, Department of Health, Education and Welfare,
pp. 67-112 (HEW Publ. No. (NIOSH) 77-129).
SRAM, R.J. (1981) Cytogenetic analysis of peripheral
lymphocytes as a method for monitoring environmental levels of
mutagens. In: Gut, I., Cikrt, M., & Plaa, G.L., ed.
Industrial and environmental xenobiotics. Metabolism and
pharmacokinetics of organic chemicals and metals., Berlin,
Springer-Verlag, pp. 187-194.
STEWART, R.D., DODD, H.C., BARETTA, E.D., & SCHAFFER, A.W.
(1968) Human exposure to styrene vapor. Arch. environ.
Health, 16: 656-662.
STOLZ, D.R. & WITHEY, R.J. (1977) Mutagenicity testing of
styrene and styrene epoxide in Salmonella typhimurium. Bull.
environ. contamin. Toxicol., 17: 739-742.
SUGIURA, K. & GOTO, M. (1981) Mutagenicities of styrene oxide
derivatives on bacterial test systems: relationship between
mutagenic potencies and chemical reactivity. Chem. biol.
Interact., 35: 71-91.
SUGIURA, K., KIMURA, T., & GOTO, M. (1978a) Mutagenicities of
styrene oxide derivatives on Salmonella typhimurium (TA100).
Relationship between mutagenic potencies and chemical
reactivity. Mutat. Res., 58: 159-165.
SUGIURA, K., YAMANAKA, S., FUKUSAWA, S., & GOTO, M. (1978b)
The mutagenicity of substituted and unsubstituted styrene
oxides in E. coli: Relationship between mutagenic potencies
and physico-chemical properties. Chemosphere, 7: 737-742.
SUGIURA, K., MAEDA, A., & GOTO, M. (1979) Substitutional
effects of styrene oxides on survival and mutation induction
in cultured Chinese hamster cells (V-79). Chemosphere, 8:
369-372.
SYNTHETIC RESINS LIMITED (1980) LSE capability with good
interlaminal adhesion. Reinf. Plast., 3: 72-73.
TAULBEE, J., ANDJELKOVIC, D., WILLIAMS, T., GAMBLE, J.F., &
WOLF, P. (1976) A study of possible associations between
exposure to SBR processes and industrial hygiene and
epidemiologic considerations (for workers in the 1951 and 1964
cohorts and deaths 1964-1973). In: Ede, L., ed. Proceedings
of NIOSH Styrene-Butadiene Briefing, Covington, Kentucky, USA,
1976, Cincinnati, Ohio, Department of Health, Education and
Welfare, pp. 113-162 (HEW Publ. No. (NIOSH) 77-129).
TERAMOTO, K., HORIGUCHI, S., & KITIBATAKE, S. (1978) [Studies
on industrial styrene poisoning. (Part VIII). Distribution and
elimination of styrene in rats exposed to styrene.] Jpn. J.
ind. Health, 20(2): 118-119 (in Japanese).
THEISS, A.M. & FLEIG, I. (1978) Chromosome investigations on
workers exposed to styrene/polystyrene. J. occup. Med., 20:
747-749.
THIESS, A.M. & FRIEDHEIM, M. (1978) Morbidity among persons
employed in styrene production, polymerization and processing
plants. Scand. J. Work Environ. Health, 4 (Suppl. 2): 203-214.
THIESS, A.M. & FRIEDHEIM, M. (1979) [Morbidity study in
coworkers of the polyester laboratory and of the technical
service exposed to styrene.] Zentralbl. Arbeitsmed., 9:
238-241 (in German).
THIESS, A.M., SCHWEGLER, H., & FLEIG, I. (1980) Chromosome
investigations in lymphocytes of workers employed in areas in
which styrene-containing unsaturated polyester resins are
manufactured. Am. J. ind. Med., 1: 205-210.
THOMPSON, B. (1974) Hazardous gases and vapors: Infrared
spectra and physical constants, Fullerton, Beckman
Instruments, Inc., pp. 154, 255 (Technical report 595).
TOLA, S., HARKONEN, H., KORKALA, M.L., & JARVINEN, E. (in
press) Mortality of workers exposed to styrene. Egypt. J.
occup. Med., (in press).
TOSSAVAINEN, A. (1978) Styrene use and occupational exposure
in the plastics industry. Scand. J. Work Environ. Health, 4
(Suppl. 2): 7-13.
TURCHI, G., BONATTI, S., CITTI, L., GERVASI, P.G.,
ABBONDANDOLO, A., & PRESCIUTTINI, S. (1981) Alkylating
properties and genetic activity of 4-vinylcyclohexane
metabolites and structurally related epoxides. Mutat. Res.,
83: 419-430.
US EPA (1980) Investigation of Selected Potential
Environmental Contaminants: Styrene Ethylbenzene, and Related
Compounds. Washington DC, US Environmental Protection Agency,
261 pp (Publication No. EPA 5601 11-80---018).
US NATIONAL CANCER INSTITUTE (1979) Bioassay of styrene for
possible carcinogenicity. Washington, DC, Department of
Health, Education and Welfare, 46 pp (Publ. No. (NIH)
79-1741, Washington DC, (Techn. Rep. ser. No. 185)).
VAINIO, H. (1978) Vinyl chloride and vinyl benzene (styrene)-
metabolism, mutagenicity and carcinogenicity. Chem.-biol.
Interact., 22: 117-124.
VAINIO, H. & MAKINEN, A. (1977) Styrene and acrylonitrile
induced depression of hepatic nonprotein sulfhydryl content in
various rodent species. Res. Commun. Chem. Pathol.
Pharmacol., 17: 115-124
VAINIO, H., PAAKKONEN, R., RONNHOLM, K., RAUNIO, V., &
PELKONEN, O. (1976) A study on the mutagenic activity of
styrene and styrene oxide. Scand. J. Work Environ. Health, 3:
147-151.
VAINIO, H., HEMMINKI, K., & ELOVAARA, E. (1977) Toxicity of
styrene and styrene oxide on chick embryos. Toxicology, 8:
319-325.
VAINIO, H., JARVISALO, J., & TASKINEN, E. (1979) Adaptive
changes caused by intermittent styrene inhalation on
xenobiotic biotransformation. Toxicol. appl. Pharmacol., 49:
7-14.
VAINIO, H., NORPPA, H., & BELVEDERE, G. (1982) The role of
cytochrome P-450 mediated metabolism in SCEs induced by
styrene in human lymphocytes. In: Proceedings of the l3th
International Cancer Congress, Abstracts, Seattle, USA, 8-l5
September, l982, Seattle, International Union Against Cancer,
p. 118.
VALENTA, J. (1966) [Air and water pollution by styrene with
the butadiene-styrene rubber production.] Cesk. Hyg., 11:
349-352 (in Czech).
VAN ANDA, J., SMITH, B.R., FOUTS, J.R., & BEND, J.R. (1979)
Concentration-dependent metabolism and toxicity of [14C
styrene oxide in the isolated perfused rat liver. J.
Pharmacol. exp. Ther., 211: 207-211.
VAN DUUREN, B.L., NELSON, N., ORRIS, L., PALMES, E.D., &
SCHMITT, F.L. (1963) Carcinogenicity of epoxides, lactones
and peroxy compounds. J. Natl Cancer Inst., 31: 41-55.
VARNER, S.L. & BREDER, C.V. (1981a) Liquid chromatographic
determination of residual styrene in polystyrene food
packaging. J. Assoc. Off. Anal. Chem., 64: 647-652.
VARNER, S.L. & BREDER, C.V. (1981b) Head space sampling and
gas chromatographic determination of styrene migration from
food contact polystyrene cups into beverages and food
simulants. J. Assoc. Off. Anal. Chem., 64: 1122-1130.
VERGIEVA, T. & ZAJKOV, H. (1981) [Behavioural changes in rats
with inhalation styrene effect.] Hyg. Zdrav., 24: 242-247 (in
Bulgarian).
VERGIEVA, T., ZAIKOV, Hr., & PALATOV, S. (1979) [A study on
the embryotoxic action of styrol.] Hyg. Zdrav., XXII: 39-43
(in Russian).
VIHKO , R., VIHKO, P., MAENTAUSTA, O., PAKARINEN, A., JANNE,
O., & YRJANHEIKKI, E. (1983) Assessment of early
hepatotoxicity. In: Aitio, A., Riihimäki, V., & Vainio, H.,
ed. Biological monitoring and surveillance of workers exposed
to chemicals, Washington, Hemisphere Publ. Co. (in press).
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J.J.A.A. (1981)
The mutagenic action of aliphatic epoxides. Mutat. Res., 89:
269-282.
VOSKAMP, A.J. & STUDENBERG, J.E. (1981) A new low styrene
emission laminating resin. In: 36th Annual Conference,
Reinforced Plastics/Composites Institute, the Society of the
Plastics Industry, Inc., February 16-20, 1981, Session 19-D.
WADE, D.R., AIRY, S.C., & SINSHEIMER, J.E. (1978)
Mutagenicity of aliphatic epoxides. Mutat. Res., 58: 217-223.
WATABE, T., ISOBE, M., SAWAHATA, T., YOSHIKAWA, K., YAMADA,
S., & TAKABATAKE, E. (1978) Metabolism and mutagenicity of
styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
142-155.
WATABE, T., OZAWA, N., & YOSHIKAWA, K. (1981a)
Stereochemistry in the oxidative metabolism of styrene by
hepatic microsomes. Biochem. Pharmacol., 30: 1695-1698.
WATABE, T., HIRATSUKA, A., OZAWA, N., & ISOBE, M. (1981b)
Glutathione S-conjugates of phenyloxirane. Biochem.
Pharmacol., 30: 390-392.
WATABE, T., HIRATSUKA, A., AIZAWA, T., & SAWAHATA, T. (1982a)
1-vinylbenzene 1,2- and 3,4-oxides. Tetrahedron Lett., 23:
1185-1188.
WATABE, T., HIRATSUKA, A., AIZAWA, T., SAWAHATA, T., OZAWA,
N., ISOBE, M., & TAKABATAKE, E. (1982b) Studies on the
metabolism and toxicity of styrene IV. 1-vinylbenzene
3,4-oxide, a potent mutagen formed as a possible intermediate
in the metabolism in vivo of styrene to 4-vinylphenol. Mutat.
Res., 93: 45-55.
WATABE, T., OZAWA, N., & YOSHIKAWA, K. (1982c) Studies on
metabolism and toxicity of styrene. V. The metabolism of
styrene, racemic, ( R)-(+), and ( S)-(-) -phenyloxiranes in the
rat. J. Pharm. Dyn., 5: 129-133
WATANABE, T. ENDO, A., SATO, K., OHTSUKI, T., MIYASAKA, M.,
KOIZUMI, A., & IKEDA, M. (1981) Mutagenic potential of
styrene in man. Ind. Health, 19: 37-45.
WATANABE, T., ENDO; A., KUMAI, M., & IKEDA, M. (1983)
Chromosome aberrations and sister chromatid exchanges in
styrene-exposed workers with reference to their smoking
habits. Environmental Mutagenesis, 5: 299-309
WEAST, R.C. & ASTLE, M.J. ed. (1981) Handbook of chemistry
and physics, Boca Raton, CRS Press, p. c-500.
WEIL, C.S., CONDRA, N., HAUN, C., & STRIEGEL, J.A. (1963)
Experimental carcinogenicity and acute toxicity of
representative epoxides. Am. Ind. Hyg. Assoc. J., 24: 305-325.
WHITE, L.D., TAYLOR, D.G., MAUER, P.A., & KUPEL, R.E. (1970)
A convenient optimized method for the analysis of selected
solvent vapors in the industrial atmosphere. Am. Ind. Hyg.
Assoc. J., 31: 225-232.
WILSON, R.H. (1944) Health hazards encountered in the
manufacture of synthetic rubber. J. Am. Med. Assoc., 124:
701-703.
WILTI, D. (1970) Infrared vapour spectra, London, Heyden &
Son Ltd. (Index No. 180).
WINK, A. (1972) Effect of long-term exposure to toxic
substances on urinary excretion of 17-oxogenic steroids and
17-oxosteroids. Ann. occup. Hyg., 15: 211-215.
WITHEY, J.R. (1976) Quantitative analysis of styrene monomer
in polystyrene in foods including some preliminary studies of
the uptake and pharmacodynamics of the monomer in rats.
Environ. Health Perspect., 17: 125-133.
WITHEY, J.R. (1978) The toxicology of styrene monomer and its
pharmacokinetics and distribution in the rat. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 31-40.
WITHEY, J. & COLLINS, P.G. (1977) Pharmakokinetics and
distribution of styrene monomer in rats after intravenous
administration. J. Toxicol. environ. Health, 3: l0ll-l020.
WITHEY, J.R. & COLLINS, P.G. (1978) Styrene monomer in foods:
A limited Canadian survey, Bull. environ. Contam. Toxicol.,
19: 86-94.
WITHEY, J.R. & COLLINS, P.G. (1979) The distribution and
pharmacokinetics of styrene monomer in rats by the pulmonary
route. J. environ. Pathol. Toxicol., 2: 1329-1342.
WOLF, M.A., ROWE, V.K., MCCOLLISTER, D.D., HOLLINGSWORTH,
R.L., & OYEN, F. (1956) Toxicological studies of certain
alkylated benzenes and benzene. Am. Med. Assoc. Arch. ind.
Health, 14: 387-398.
WOLFF, M.S., DAUM, S.M., LORIMER, W.V., & SELIKOFF, I.J.
(1977) Styrene and related hydrocarbons in subacutaneous fat
from polymerization workers. J. Toxicol. environ. Health, 2:
997-1005.
WOLFF, M.S., LILIS, R., LORIMER, W.V., & SELIKOFF, I.J.
(1978a) Biological indicators of exposure in styrene
polymerization workers. Styrene in blood and adipose tissue
and mandelic and phenylglyoxylic acids in urine. Scand. J.
Work Environ. Health, 4 (Suppl. 2): 114-118.
WOLFF, M.S., LORIMER, W.V., LILIS, R., & SELIKOFF, I.J.
(1978b) Blood styrene and urinary metabolites in styrene
polymerization. Br. J. ind. Med., 35: 318-329.
YAMAMOTO, R.K. & COOK, W.A. (1968) Determination of
ethylbenzene and styrene in air by ultraviolet
spectrophotometry. Am. Ind. Hyg. Assoc. J., 29: 283-241.
YOSHIKAWA, K., ISOBE, M., WATANABE, T., & TAKABATAKE, E.
(1980) Studies on metabolism and toxicity of styrene III.
The effect of metabolic inactivation by rat-liver S9 on the
mutagenicity of phenyloxirane toward Salmonella typhimurium.
Mutat. Res., 78: 219-226.
ZAPRIANOV, Z & BAINOVA, A. (1979) Changes in monoamine
oxidase activity (MAO) after styrene and ethanol combined
treatment of rats. Activ. nerv. Sup. (Praha), 21(4): 262-264.
ZETTERBERG, G. (1982) Styrene - a widespread mutagen:
conclusions from the results of testing. In: Sugimura, T.,
Kondo, S. & Takabe, H., ed. Environmental Mutag. Carcinog.,
Tokyo, University of Tokyo Press, pp. 316-322.
ZIELHUIS, R.L., HARTOGENSIS, F., JONGH, J., KALSBEEK, J.W.H.,
& VAN REES, H. (1964) The health of workers processing
reinforced polyesters. In: XIVth International Congress of
Occupational Health, Madrid, Spain, 16-21 September, 1963,
Volume III, pp. 1092-1097.
ZITTING, A., HEINONEN, T., & VAINIO, H. (1980) Glutathione
depletion in isolated rat hepatocytes caused by styrene and
the thermal degradation products of polystyrene. Chem. biol.
Interact., 31: 313-318.
ZLOBINA, N.S. (1963) [On the toxicity of small concentrations
of styrol vapors.] Gig. i Sanit., 28: 29-35 (in Russian).
The oxidation of styrene to styrene 7,8-oxide has been
demonstrated in vitro using rat or rabbit liver microsomes
(Leibman & Ortiz, 1969; Leibman, 1975; Belvedere et al., 1977;
Duverger-van Bogaert et al., 1978; Watabe et al., 1978), rat
liver nuclei (Gazzotti et al., 1980) or rat, rabbit, or
guinea-pig extrahepatic microsomes (Cantoni et al., 1978). The
major route of styrene metabolism is believed to proceed via
this epoxide intermediate.
Styrene has been shown to be epoxidized to ( R)- and
( S)-styrene 7,8-oxides in the ratio 1:1.3 by rat liver microsomal
cytochrome P-450 (Watabe et al., 1981a). The ( R)-and ( S)-oxides
are hydrolysed in a regiospecific manner by rat liver microsomal
epoxide hydrolase to ( R)- and ( S)-phenyl-ethylene glycols in the
ratio of 1:4 (Watabe et al., 1981a). Styrene oxides are also
conjugated with glutathione (Oesch, 1973; James et al., 1975;
Pacheka et al., 1979). Rat liver cytosolic glutathione
transferases convert the ( R)- and ( S)-oxides stereoselectively
to S-(1-phenyl-2-hydroxyethyl)-glutathione (GSH conjugate 1) and
S-(2-phenyl-2-hydroxyethyl)-glutathione (GSH conjugate 2),
respectively (Watabe et al., 1981b). Conjugates 1 and 2 would be
directly correlated with the urinary N-acetyl- S-cysteine
conjugates, namely 1-phenyl-2-hydroxyethyl and 2-phenyl-2-
hydroxyethylmercapturic acids, respectively (James & White, 1967;
Seutter-Berlage et al. 1978; Watabe et al., 1982c).
Rats administered styrene intraperitoneally (i.p.) also
excreted 2-vinylphenol and 4-vinylphenol in conjugated forms
in the urine (Hiratsuka et al., 1982a). Styrene 1,2- and
3,4-oxides administered i.p. to rats have been demonstrated in
the urine as 2-vinylphenol and 4-vinylphenol, respectively
(Hiratsuka et al., 1982a). However, 3-vinylphenol has not
been detected in the urine (Bakke & Scheline, 1970; Pantarotto
et al., 1978; Watabe et al., 1978). Other minor metabolites
are 1- and 2-phenylethanol (Delbressine et al., 1980).
Comparing the kinetic parameters of styrene monooxygenase
and styrene 7,8-oxide hydrolase in rat liver microsomes
(Cantoni et al., 1978; Duverger-van Bogaert et al., 1978), it
appears that the conversion of styrene 7,8-oxide into styrene
glycol is preferred. The ratio between styrene epoxide
hydrolase and monooxygenase in rat liver was 2-3 times higher
than those found in mouse and rabbit liver (Cantoni et al.,
1978).
Styrene glycol is partly transformed into a glucuronide
that has been detected in the urine of styrene-treated rabbits
(El Masri et al., 1958). The major metabolic pathway involves
the sequential oxidation to mandelic, phenylglyoxylic, and
benzoic acids, which have been identified in urine (Bardodej,
1964; James & White, 1967; Ohtsuji & Ikeda, 1971).
Quantitative differences have been observed between various
species, hippuric acid being quantitatively the most relevant
urinary metabolite in the rabbit (El Masri et al., 1958),
phenylglyoxylic acid in the rat (Bardodej et al., 1971;
Watanabe et al., 1982c) and mandelic acid in man (Bardodej,
1964; Bardodej & Bardodejova, 1970). Minor metabolites
identified include parahydroxymandelic, parahydroxyphenyl-
glyoxylic, and parahydroxybenzoic acids (Bakke & Sheline,
1970; Pantarotto et al., 1978).
The metabolism of styrene is enhanced by phenobarbital
pretreatment and is inhibited by SKF 525-A, an inhibitor of
monooxygenases (Ohtsuji & Ikeda, 1971).
Styrene 7,8-oxide either binds covalently to macro-
molecules such as microsomal proteins (Marniemi et al., 1977;
Watabe et al., 1978) or conjugates with glutathione
non-enzymatically and in the presence of a transferase (Oesch,
1973; James et al., 1975; Pachecka et al., 1979). Pheno-
barbital pretreatment of the rats enhances, whereas carbon
tetrachloride administration reduces, the NADPH dependent-
binding to microsomal protein in vitro (Watabe et al., 1978).
Prolonged exposure to a styrene vapour concentration of
1260 mg/m3 (300 ppm) leads to an enhancement of the metabolic
elimination of the compound (Vainio et al., 1979) resulting in
decreasing body burden during continued exposure.
Though numerous metabolic pathways have been found in
animal studies (mainly in the rat and rabbit), only a few have
been identified in human beings. Analysis of the urine of
human subjects exposed to styrene has revealed mandelic and
phenylglyoxylic acids as the major metabolites indicating
that, at least qualitatively, styrene metabolism in human
beings follows pathways similar to those previously described
in animal studies. The results of a study by Pfäffli et al.
(1981) suggest that 4-vinylphenol is also excreted in the
urine of workers exposed to styrene. Though this metabolite
represented only 0.3 % of the urinary concentration of
mandelic acid, there was a good correlation between the
excretion of the two metabolites.
Recent data indicate that human whole blood lymphocyte
cultures apparently catalyse the oxidation of styrene to
styrene 7,8-oxide (Norppa et al., 1980a). Belvedere & Tursi
(1981) have shown that both human lymphocytes and erythrocytes
are capable of converting styrene to styrene 7,8-oxide. In
whole blood, red blood cells may be more important in this
conversion, because they are present in greater numbers than
lymphocytes.
5.4. Elimination
5.4.1. Human studies
5.4.1.1. Controlled human studies
In an inhalation study by Fiserova-Bergerova & Teisinger
(1965), 7 human volunteers were exposed to styrene levels of
63-160 mg/m3 (15-38 ppm) for 5 h. From analysis of the
alveolar air, the authors found that pulmonary elimination
followed a bi-exponential curve indicating a 2-compartment
model.
Stewart et al. (1968) found that styrene elimination from
the lungs, after inhalation exposure, was rapid and bi-
exponential but that the rates of elimination varied with the
level and duration of exposure. It was apparent that subjects
who were exposed to a styrene concentration of 1579 mg/m3 (376
ppm) for 1 h had lower elimination rate coefficients for the
terminal phase than those exposed to 907 mg/m3 (216 ppm) and
214 mg/m3 (51 ppm) for 1 h. No values for the rate
coefficients were given in this report but it was calculated
that one subject, who had been exposed to 491 mg/m3 (117 ppm)
for 2 h, exhaled 1.2% of the total amount of styrene absorbed
during the first 4 h after exposure. Exhaled styrene was
detected up to 24 h after the termination of a 30-min exposure
to concentrations of 210 or 630 mg/m3 (50 or 150 ppm) (Astrand
et al., 1974).
The exhalation of styrene after pulmonary exposure was
also examined in human volunteers by Fernandéz & Caperos
(1977). Six subjects were exposed to vapour concentrations of
294-840 mg/m3 (70-200 ppm) for periods of 4 or 8 h. The
concentration of styrene in the expired alveolar air was
reported to decline rapidly during the first hour following
termination of exposure, at all exposure concentrations, and
2.6% of the total absorbed dose was considered to be
eliminated via the lung.
Exhalation of styrene after inhalation exposure to a
styrene concentration of 210 mg/m3 (50 ppm) was measured in 7
volunteers for 4 consecutive 30-min periods either resting or
in a work cycle (Engström et al., 1978b). The exhalation was
followed for up to 19 h after exposure by monitoring styrene
concentrations in the expired air; some 31% of the retained
styrene was estimated to be eliminated via the lungs. In the
same study, styrene eliminated from adipose tissue was
reported to have a half-life of from 2 to 4 days, which led
the authors to conclude that repeated daily exposure to
styrene could lead to bioaccumulation.
In a study by Ramsey & Young (1978), 4 male human
volunteers were exposed to a styrene concentration of 336
mg/m3 (80 ppm) for 6 h and the styrene in the blood and
expired air monitored for 41 h after exposure. The styrene
concentration in blood was found to decay in a bi-exponential
fashion, typical of a linear 2-compartment model. The rapid
initial elimination phase yielded a half-time of 0.58 h and,
for the slower terminal phase, a value of 13.0 h was
reported. The concentration of styrene in the expired air for
the same volunteers showed a biphasic log-linear decline
similar to that observed for the blood concentrations.
In a study designed to investigate the skin absorption of
styrene (Riihimäki & Pfäffli, 1978), two subjects wearing air-
supplied respirators were exposed to a styrene concentration
of 2520 mg/m3 (600 ppm) for 3.5 h while performing light
work. Bi-exponential elimination data were obtained both from
analysis of venous blood and of alveolar air and the authors
reported a value of about l h for the half-time of the rapid
phase and about 10 h for the slower phase.
Studies conducted on 6 male volunteers at styrene
exposures varying from 865 mg/m3 (206 ppm) for 8 h to 294
mg/m3 (70 ppm) for 4 h gave alveolar concentration decay
curves that the authors claimed were best described by a
3-compartment model (Fernandez & Caperos, 1977). The rate
coefficients for the three phases had half-lives that ranged
from 0.06 to 0.61 h for the rapid phase, 1.01 to 3.75 h for
the intermediate phase, and 10.05 to 24.75 h for the slowest
phase.
A study on the rate of excretion of the urinary
metabolites of styrene, phenylglyoxylic and mandelic acids
(Guillemin & Bauer, 1978) was carried out on 9 human subjects
after pulmonary exposure to styrene at 168 or 840 mg/m3 (40 or
200 ppm) for 4-8 h. The authors found that the rate
coefficients for the urinary excretion of metabolites were
unaffected by the duration or level of exposure and claimed
that if the amounts of both metabolites were totalled over 4
days following exposure, reliable measurements of exposure
could be obtained. For mandelic acid, the first and second
phase half-times were evaluated to be respectively 3.9 h and
24.7 h, while for phenylglyoxylic acid the half-time was
calculated to be 10.5 h (Guillemin & Bauer, 1979).
5.4.1.2. Occupational exposure studies
Of the numerous studies that have been conducted on
styrene-exposed workers (Härkönen, 1978), only a few have been
devoted to the assessment of metabolite production.
In a study by Götell et al. (1972), a group of 17 workers
was divided into 3 exposure subgroups, the highest at 987-1226
mg/m3 (235-292 ppm), an intermediate group at 374-584 mg/m3
(89-139 ppm), and the lowest group at 71-134 mg/m3 (17-32
ppm). Decay curves of styrene in expired air for the 3 groups
had a multi-exponential form but no kinetic parameters were
cited. It was evident, however, that the terminal elimination
phase was markedly faster for the group receiving the highest
exposure.
The rate of appearance of mandelic acid in the urine has been
shown to be biphasic, half times being 9 h and 16 h, respectively
(Engström, K., et al., 1976). In a preliminary report on workers,
with limited time points, monophasic elimination with half-times of
8.5 and 7.8 h, respectively for phenylglyoxylic and mandelic acid,
was found (Ikeda & Imamura, 1974). Considerable inter-subject
variability has been reported by several authors (Philippe et al.,
1971; Götell et al., 1972; Härkönen et al., 1974; Gompertz, 1978).
On the basis of findings in animal studies, the excretion
of hippuric acid was investigated in styrene-exposed workers.
An increase in hippuric acid excretion was noted in styrene-
exposed workers (Ikeda et al., 1974); similar levels and
important fluctuations have been found in unexposed controls
(Engström, K., et al., 1978).
5.4.1.3. General population studies
There have not been any reported studies on the
elimination of styrene in the general population.
5.4.2. Experimental animal studies
Results of studies on rats subcutaneously injected with
500 mg/kg 14C-labelled styrene showed that there was rapid
distribution to tissues and organs within 1 h and that 85% of
the radioactivity was eliminated within 24 h; 71% of the
radioactivity appeared in the urine, 12% in expired air as
carbon dioxide, 3% in the faeces, and about 3% as unchanged
styrene in expired air (Danishefsky & Willhite, 1954).
A later study (Sauerhoff et al., 1976) demonstrated a
similar rapid clearance of ring-labelled 14C-styrene in male
and female rats after oral administration of 50 or 500 mg/kg
body weight. More than 90% of the radioactivity appeared in
the urine within 72 h and no residual styrene was found in the
tissues examined at that time. The urinary excretion of
radioactivity followed a 2-compartment model at the low dose
level with half-times of 1.29 and 8.13 h for the initial
(alpha) and terminal (beta) phases, respectively. At the high
dose, a mono-exponential urinary excretion was observed with a
half-time of 6.74 h. About twice as much styrene was
eliminated by the pulmonary route in male compared with female
rats.
Biphasic excretion of radioactivity in the urine of rats
was observed after inhalation of 14C-styrene (252 or
2520 mg/m3; 60 or 600 ppm) for 6 h with half-time values of
14.9 h for the lower and 19.2 h for the higher exposure
concentration (Sauerhoff & Braun, 1976).
Inhalation exposure of rats to styrene concentrations
varying from 336 to 5040 mg/m3 (80 to 1200 ppm) for 6 h
(Ramsey & Young, 1978) also showed evidence of dose-dependent
kinetics. For the 15-fold increase in exposure level, the
area generated under the blood level-time curve increased by a
factor of 112. The elimination kinetics in the post-exposure
period appeared to change from a bi-exponential curve to a
log-linear form with increasing exposure concentrations. At
the 336 mg/m3 (80 ppm) dose level, the hybrid elimination rate
coefficients for the initial and terminal phases were 0.26 h
and 3.60 h, respectively.
Withey (1978) and Withey & Collins (1979) described the
elimination kinetics of styrene in rats after 5 h inhalation
exposure at levels that varied from 188 to 10 151 mg/m3 (44.8
to 2417 ppm). In contrast to the study by Ramsey & Young
(1978), these authors reported a bi-exponential curve of
elimination for all levels of exposure. The reported
half-times for the initial and terminal phases ranged from 3.4
to 6.3 min and about 50 to 350 min, respectively.
Teramoto et al. (1978) examined the elimination rates of
styrene from samples of the liver, brain, kidney, blood,
spleen, muscle, and adipose tissue in rats after an inhalation
exposure to 2940 mg/m3 (700 ppm) for 4 h. They found that all
tissues had a similar elimination half-time of about 2 h with
the exception of adipose tissue for which the styrene
half-time was about 6 h.
To examine the accumulation of styrene in tissues,
Savolainen & Pfäffli (1978) exposed rats to 1260 mg/m3 (300
ppm), for 6 h per day, and 5 days per week, for up to 11 weeks
and then monitored the styrene concentrations in adipose
tissue. Concentrations increased almost linearly, during the
first 4 weeks of exposure and then decreased exponentially.
In the study reported by Bergman (1979) (section 5.2.2),
mice were exposed through inhalation to 10 µl of volatilized
radiolabelled styrene over a 10-min period. The amount of
unchanged styrene exhaled over an 8-h period following
exposure amounted to 2.9% of the calculated dose. The
kinetics of elimination of radioactivity in exhaled air gave a
biphasic curve with half-times of 15 and 275 min for the
initial and terminal phases, respectively. Eight hours after
dosing, high levels of radioactivity were found in the liver,
kidney, lung, and adipose tissue.
Results of a study by Withey & Collins (1977) showed that
the elimination of styrene after intravenous administration in
rats also obeyed a 2-compartment model. Approximate rates of
elimination from some individual organs were obtained for dose
levels of approximately 1.3 and 4 mg/kg body weight. At the
higher dose, the kinetics of styrene disappearance from blood,
heart, brain, liver, spleen, and kidney appeared to follow a
1-compartment model with an elimination half-time of about
14.4 h in the same organs. At the lower dose, a biphasic
elimination was observed with half-times of about 4.78 and 26
h for the rapid and slow phases, respectively.
5.5. Biological Monitoring of Styrene Uptake
The measurement of styrene in blood, subcutaneous fat, as
well as in exhaled air has been suggested for the biological
monitoring of styrene uptake. Because of the efficient metabolism
of styrene and rapid excretion of the metabolites, mandelic and
phenylglyoxylic acid are present in the urine in easily measurable
amounts. The evaluation of the absorption level has usually been
based either on the concentration of one of these metabolites
(Ohtsuji & Ikeda, 1970; Burkiewicz et al., 1974; Härkönen et al.,
1974; Vivoli & Vechhi, 1974; Engström, K., et al., 1976, 1978;
Fernandez & Caperos, 1977; Caperos et al., 1979; Elia et al., 1980)
or on their sum (Bardodej & Bardodejova, 1970; Philippe et al.,
1971; Guillemin & Bauer, 1978, 1979; Wolff et al., 1978a,b; Elia et
al., 1980). "End of exposure" spot specimens have usually been
sampled and results corrected for the dilution of urine. Only this
type of sample is dealt with in the following discussions.
A number of authors have attempted to correlate levels of
styrene in air with levels of mandelic acid in urine, either
from experimental data or field studies, and calculations of
mandelic acid levels corresponding to styrene levels in air of
210, 420, and 630 mg/m3 (50, 100, and 150 ppm) are presented
in Table 9. The differences between the calculated values
could be explained by such factors as: type of exposure,
sampling strategy, individual variability, and differences in
analytical methods.
The use of unspecific analytical methods influences the
results, especially at low levels of styrene exposure, giving
too high values for metabolite concentrations in the urine
(Engström & Rantanen, 1974).
In the case of occupational exposure, a number of
additional elements may have to be taken into account. For
example, in work situations, exposure is usually repeated and
it has been shown that about 30% of the maximal mandelic acid
concentration of the previous day can still be found in urine
samples taken before work on the following day (Engström, K.,
et al., 1976). In another study, the amount of mandelic acid
excreted at the end of the working shift was shown to increase
in the course of the working week (Fernandez & Caperos, 1977).
Increased pulmonary ventilation with increased physical
work load results in a larger total absorption of styrene and
thus increased excretion of metabolites in the urine (Åstrand
et al., 1974; Droz & Fernandez, 1977).
Fluctuations in styrene concentrations in the workroom air
are very common in occupational exposure and may also
contribute to the differences observed in Table 9. A
short-term high peak exposure occurring shortly before the
sampling time may result in a high mandelic acid concentration
in the urine, even though the time-weighted average
concentration of styrene is low (Engström, K., 1983).
Table 9. Mandelic Acid levels* corresponding to various concentrations of
styrene in air
------------------------------------------------------------------------------
Styrene in air
mg/m3 (ppm)
-----------------
210 420 630 Remarks Reference
(50) (100) (150)
------------------------------------------------------------------------------
mandelic (a) 6 12 18 mandelic acid expressed Spasovski
acid as mg/g creatinine; (1982)
spectrophotometry
(a) - 7 - mandelic acid expressed Guillemin &
as mg/g creatinine; Bonet (1979)
gas chromatography
experimental study
(b) 8 14 20 Density 1.024; high Ikeda et al
performance liquid (1982)
chromatography field
study
(b) 10 20 - Density 1.024; Bardodej (1978)
polarography;
experimental study
(b) 9 19 27 Density 1.024; Engström, K.
gas chromatography; et al. (1978)
field study
(b) 14 26 34 Density 1.024 Härkönen et al.
spectrophotometry (1974)
field study
(b) 7-9 12-13 15-19 Density 1.024; Götell et al.
spectrophotometry; (1972)
field study
(b) 6 14 22 Density 1.024; Elia et al.
gas chromatography (1980)
field study
(b) 3 11 19 Density 1.024; Fields et al.
gas chromatography (1979)
field study
------------------------------------------------------------------------------
*(a) = mg mandelic acid/g creatinine.
(b) = mmol mandelic acid/litre urine.
The ratio of mandelic to phenylglyoxylic acid excreted at
the end of exposure varied from about 1 to 4 in different
studies. The ratio appears to increase at higher exposure
levels (Ohtsuji & Ikeda, 1970; Riihimäki & Pfäffli, 1978;
Caperos et al., 1979; Elia et al., 1980). The mandelic
acid/phenylglyoxylic acid ratios were higher in urine samples
taken at the end of exposure than in samples taken later
(Philippe et al., 1971; Guillemin & Bauer, 1979). In terms of
biological monitoring, it is claimed that the mandelic
acid/phenylglyoxylic acid ratio in the urine may be of help in
interpreting the exposure pattern (Philippe et al., 1971;
Guillemin & Bauer, 1979).
The various factors influencing the excretion of these
metabolites are not well known. It may be that such a
difficulty could be overcome by determining mandelic and
phenylglyoxylic acids and combining the results. This may
explain the good agreement obtained in the two studies in
Table l0 based on the sum of the levels of the two acids
corresponding to styrene concentrations in air of 210, 420,
and 630 mg/m3 (50, 100 and 150 ppm).
Table 10. Sum of mandelic acid and phenylglyoxylic acid levels
(mmol/litre) corresponding to various concentrations of styrene in air
---------------------------------------------------------------------------
Styrene in air
mg/m3 (ppm)
-------------------
210 420 630 Remarks Reference
(50) (100) (150)
---------------------------------------------------------------------------
Mandelic acid 8 16 24 density 1.024; Elia et al.
+ gas chromatography; (1980)
phenylglyoxylic field study
acid 11 19 27 density 1.024 Ikeda et al.
aigu performance (1982)
liquid chromatography;
field study
---------------------------------------------------------------------------
The determination of minor metabolites (4-vinylphenol and
hippuric acid) for the biological monitoring of styrene
exposure has also been reported (Ikeda et al., 1974; Pfäffli
et al., 1981).
The difficulties of the biological monitoring of styrene
uptake using urine samples cannot be overcome by selecting
another medium, as even less experience is available with
other media. Styrene concentrations in blood decline rapidly
during the first hour following exposure, which makes the
results strongly dependent on sampling time.
Styrene concentrations in exhaled air are in equilibrium
with blood concentrations, which means that styrene levels in
exhaled air are also sensitive to the changes in concentration
in ambient air. A single sample of exhaled air at the end of
exhalation can be used only for the evaluation of immediate
past exposure. It has been suggested that extrapolation of a
whole day's exposure is possible using several samples taken
after exposure, from which a "decay curve" can be established
(Fernandez & Caperos, 1977; Fields & Horstman, 1979). This
method is inconvenient for the routine monitoring of workers.
The estimation of styrene concentrations in subcutaneous
fat has been proposed as a method of indicating styrene body
burden, even a long time after exposure, when styrene and its
metabolites can no longer be detected in other specimens
(Wolff et al., 1978). However, much more information about
the kinetics of styrene in fat is needed before the relevance
of this method can be evaluated. For obvious practical
reasons, this technique cannot be used routinely.
6. EFFECTS ON EXPERIMENTAL SYSTEMS
6.1. Haematopoietic and Immune Systems
Spencer et al. (1942) did not observe any differences in
erythrocyte count, haemoglobin concentration, total leukocyte
count, and differential count between 18 rats exposed
repeatedly for 7 h a day for 6 months to a styrene
concentration of about 5460 mg/m3 (1300 ppm) and 18 control
animals. The test animals received a total of 137-139
exposures. The same authors did not see any significant
haematological changes in 8 rabbits and 4 monkeys similarly
exposed.
Wolf (1956) gave styrene orally at 66.7-667 mg/kg body
weight to female rats for 6 months, 5 days a week, but did not
observe any effects on the haematopoietic system.
In a study by Quast et al. (1978), styrene was
administered orally to beagle dogs at 200, 400, and 600 mg/kg
body weight for up to 19 months. At the highest dose, Heinz
bodies were regularly detected in the blood erythrocytes of
the dogs. At 400 mg/kg body weight, Heinz bodies were still
frequently seen, but at 200 mg/kg body weight they were
observed only sporadically in female dogs. Heinz bodies
disappeared after withdrawal of styrene. Decreases in packed
cell volume, red blood cell count, haemoglobin levels, and the
erythrocyte sedimentation rate were also observed.
When styrene was fed to rabbits at doses of up to 250
mg/kg body weight for up to 216 days, severe impairment of the
immunological defence system was indicated with the blood
complement titre reduced and leukocyte phagocytic activity
depressed (Sinitskij, 1969).
6.2. Nervous System and Behaviour
The acute neurotoxic effect of styrene is depression of
the central nervous system (Roth, 1979). However, the
narcotic effect of styrene glycol is at least twice as potent
as that of styrene (Parkki et al., 1976) and styrene 7,8-oxide
is more toxic than both styrene and styrene glycol with a
lethal dose 4-5 times lower than that of the parent compound
(Ohtsuji & Ikeda, 1971). Central nervous system depression
follows treatment with styrene 7,8-oxide. After a single
intraperitoneal injection both styrene and styrene oxide have
been shown to bind covalently to macromolecules in the central
nervous system (Savolainen & Vainio, 1977). In a study on
rats by Shugaev (1969), in which styrene was injected intra-
peritoneally, the maximum concentration of styrene in brain
tissue was 250 mg/kg (range 180-324 mg/kg).
Toxic action in the central nervous system in prolonged
(11 weeks) low-level inhalation exposure to a styrene
concentration of 1260 mg/m3 (300 ppm) led to minor changes in
axonal proteins (Savolainen & Pfäffli, 1977). Concomitant
neurophysiological studies, however, failed to demonstrate any
reduction in the peripheral motor conduction velocities
(Seppäläinen, 1978), a suggested clinical feature of the
toxicity of styrene in human subjects.
In studies by Zaprianov & Bainova (1979) on male rats,
repeated oral doses of styrene (1250 mg/kg body weight for 7
days) decreased monoamine oxidase (EC 1.4.3.4) activity. The
authors concluded that the inhibition of monoamine oxidase
activity in total brain may be regarded as a possible link in
the pathogenesis of reported occupational neurophysiological
effects of styrene.
Polystyrene containers were toxic to nerve cells in a
closed tissue culture system, possibly because of the release
of unreacted styrene in the medium (Mithen et al., 1980).
In an open-field test, styrene vapour at 1260 mg/m3
(300 ppm) had marginal effects on animal behaviour, even when
pronounced neurochemical effects were taken into account
(Savolainen et al., 1980).
In a 6-month study, female rats exposed to 200 and 2000
mg/m3 of styrene exhibited enhanced spontaneous activity.
Long-term memory was impaired in male rats after a 4-month
exposure (Vergieva & Zajkov, 1981).
6.3. Kidneys and the Urinary Tract
Oral treatment of rats with styrene at 400 mg/kg body
weight (administered in olive oil, once a day for 132 days
during 6 months) caused an increase in kidney weight. A lower
daily dose of 133 mg/kg body weight did not have any apparent
effect (Wolff et al., 1956).
Vainio et al. (1979) reported that intermittent 11-week
inhalation exposure of rats to low styrene vapour
concentrations of 1260 mg/m3 (300 ppm), 6 h/day, 5 days a
week, enhanced the activities of both drug conjugating
(epoxide hydratase (EC 4.2.1.63), UDP glucuronosyl transferase
(EC 2.4.1.17), and hydroxylating (ethoxycoumarin 0-deethylase,
cytochrome P-450) enzymes in the kidneys.
6.4. Gastrointestinal Tract
No information was available concerning the effect of
styrene on the gastrointestinal tract.
6.5. Liver
Mild liver damage, particularly in the parenchymal cells,
was reported after a single exposure of rats to styrene vapour
at 10 500 mg/m3 (2500 ppm) for up to 21 h (Spencer et al.,
1942). The same authors also reported increases in liver
weight in rats repeatedly exposed to styrene vapour at
5460 mg/m3 (1300 ppm) for 130-139 days. However, they did not
find any liver damage in guinea-pigs exposed for similar
periods to a styrene concentration of 2730 mg/m3 (650 ppm) or
in rabbits and monkeys exposed to 5460 mg/m3 (1300 ppm).
Intermittent 11-week inhalation exposure of rats to a
styrene concentration of 1260 mg/m3 (300 ppm) (6 h/day, 5
days/week) caused histological liver alterations consisting of
hydropic degeneration, and steatosis of parenchymal cells and
congestion. Enhanced activity of both the drug hydroxylating
(ethoxycoumarin O-deethylase, cytochrome P-450) and
conjugating (epoxide hydrolase (EC 3.3.2.3), UDP
glucuronosyltransferase (EC 2.4.1.42)) enzymes in the liver
was detected (Vainio et al., 1979). In a skin-painting study
on rats, styrene at 4 and 8 ml/kg body weight induced
dose-related fatty infiltration of liver cells that was
reversible after 2 weeks without exposure (Burkova et al.,
1982).
Liver cell necrosis was noted in a long-term
carcinogenicity bioassay in several rats in a group exposed to
a high dose of styrene (2000 mg/kg body weight by gavage, 5
days a week, for 78 weeks) (US National Cancer Institute,
1979).
The intermittent inhalation of styrene vapour at a
concentration of 1260 mg/m3 (300 ppm) in air decreased
significantly the reduced glutathione (GSH) concentration in
rat liver (Elovaara et al., 1979). Vainio & Mäkinen (1977)
demonstrated a clear species difference in sensitivity to
styrene-induced depletion of GSH content. The mouse was the
most sensitive and the rat the most resistant species. The
decrease in GSH in liver was dose-dependent. A slight decrease
was observed in rats exposed to a styrene concentration in air
of 420 mg/m3 (100 ppm) (Vainio et al., 1979). When isolated
hepatocytes were incubated in the presence of styrene, there
was also a dose- and time-dependent decrease in GSH
concentrations (Zitting et al., 1980).
When the hepatic GSH in Syrian hamsters decreased to less
than 1 mmol/litre as a result of a high intragastric dose of
styrene (4.5 g/kg body weight), serum aminotransferase
activity increased (Parkki, 1978).
Quast et al. (1979) observed an increased amount of
hemosiderin pigment in liver reticuloendothelial cells of male
and female beagle dogs administered styrene orally at 400 or
600 mg/kg body weight, 7 days a week, for up to 19 months.
The higher dose also increased the number of hepatocellular
intranuclear acidophilic crystalline inclusions.
6.6. Cardiovascular System
No information was available concerning possible effects
of styrene on the cardiovascular system.
6.7. Respiratory System
A single exposure of rats to styrene at 5460-42 000 mg/m3
(1300-10 000 ppm) for 1-4 h caused mucous membrane irritation
as evidenced by lacrymation, nasal discharge, and salivation,
accompanied by aggressive scratching and rubbing of the eyes
and nose. The irritation was particularly severe at higher
concentrations (Spencer et al., 1942). The degree of acute
pulmonary damage varied, in general, with the vapour
concentration of styrene and the length of exposure. When the
exposure was long enough to cause death in some of the exposed
animals, marked pulmonary lesions were induced at all
concentrations studied. Pulmonary changes varied from slight
congestion to congestion, multiple haemorrhage, exudation, and
various degrees of leukocytic infiltration. When another
group of animals (60 rats, 94 guinea-pigs, 12 rabbits, 4
monkeys) were exposed to a styrene concentration of 5460 mg/m3
(1300 ppm) for 7-8 h/day, 5 days a week, over a period of at
least 6 months, microscopic examination of various tissues,
including the lung, did not reveal specific changes in any of
the animals except the guinea-pigs. About 10% of all
guinea-pigs that were exposed repeatedly to styrene at 5460
mg/m3 (1300 ppm) died after several exposures; microscopic
examination of the dead animals revealed pronounced lung
irritation, which was characterized by general acute
inflammatory response. Respiratory insufficiency was the
cause of death (Spencer et al., 1942).
Wolf et al. (1956) exposed animals by inhalation to a
styrene concentration of 5460 mg/m3 (1300 ppm) for 7 h/day,
for 214-360 days. They found slight eye and nasal irritation
in rats and guinea-pigs, but no effects in rabbits and rhesus
monkeys. However, when rats and guinea-pigs were exposed to a
styrene concentration of 2730 mg/m3 (650 ppm), no effects were
observed.
According to Alarie (1973), the concentration of styrene
in air that reduced the respiratory frequency in mice to 50%
was 666 mg/m3 (95% confidence limits 574-758 mg/m3).
When rats were exposed to styrene at 1260 mg/m3 (300 ppm)
for 6 h daily, 5 days a week for 2-11 weeks, no significant
increase in UDP glucuronosyltransferase activity was observed
in the lungs but, after 2 and 4 weeks exposure,
the free glutathione content in the lung was lower (P < 0.05)
than that in the controls (Vainio et al., 1979).
6.8. Endocrine Organs
The adverse effects of styrene on the endocrine organs
have been insufficiently studied. While Izjumova (1972)
reported that repeated exposure to styrene concentrations of
5-50 mg/m3 (1-12 ppm) for up to 4 months prolonged the estrus
cycle in rats, the data were inconsistent from month to month,
and appeared not to be dose-dependent.
6.9. Carcinogenic Effects
6.9.1. Styrene
6.9.1.1. Oral administration
(a) Mouse
In a carcinogenesis bioassay (US National Cancer
Institute, 1979; Chu et al., 1981), 2 groups each of 50 male
and 50 female B6C3F1 mice, 6 weeks of age, were given styrene
at 150 and 300 mg/kg body weight (at least 0.3% impurities) in
corn oil by gavage, 5 days per week, for 78 weeks, and then
observed for an additional 13 weeks. A group of 20 males and
20 females receiving corn oil only (10 ml/kg body weight)
served as vehicle controls. At the end of the study, 78%,
92%, and 100% of the male mice and 76%, 80%, and 80% of the
females were alive in the high-dose, low-dose, and control
groups, respectively. An increased incidence of adenomas and
carcinomas of the lung was seen in treated male mice, i.e., 3
adenomas and 3 carcinomas in 44 animals in the low-dose group,
and 4 adenomas and 5 carcinomas in 43 animals in the high-dose
group, compared with none in the 20 controls. The increased
incidence of both types of lung tumours combined was
statistically significant (P = 0.02) in the high-dose group
compared with the vehicle-treated controls and also in the
Cochrane-Armitage test for linear trend (P = 0.02). It should
be noted that the number of control animals was small (40)
compared with the treated group (100), that the historical
vehicle-treated controls were inadequate, and that the
incidence of neoplasms in historical untreated controls was
12% (32/271). For these reasons, it was not possible for the
Task Group to conclude that styrene was carcinogenic in this
study, even though the results of the study were positive.
(b) Rat
In carcinogenic bioassays (US National Cancer Institute,
1979; Chu et al., 1981), groups of 50 male and 50 female
Fischer 344 rats, 6 weeks of age, were given styrene (at least
0.3% impurities) in corn oil, by gavage, 5 days per week,
either at 1000 or 2000 mg/kg body weight for 78 weeks with
further observation for 27 weeks, or at 500 mg/kg body weight
for 103 weeks with further observation for 1 week. Two
groups, each of 20 males and 20 females served as vehicle
controls. A high mortality was seen in the high-dose group;
only 12% of the males survived 53 weeks of treatment and 14%
of the females survived 70 weeks of treatment. Hepatic
necrosis was observed in several rats. At the 90th week of
the study, 94%, 88%, 85%, and 90% of the males and 92%, 92%,
75%, and 90% of the females were alive in the medium-dose,
low-dose, and the 2 control groups, respectively. There was
no statistically significant difference between the groups in
tumour incidence at any site (National Cancer Institute,
1979). The Task Group noted the high mortality in the
high-dose group and the small numbers of animals in the
control groups.
6.9.1.2. Inhalation exposure
Rat
In a study conducted by Jersey et al. (1978), 2 groups of
84 and 86 male, and 2 groups each of 85 female Sprague-Dawley
rats of the Spartan substrain, 7-8 weeks of age, were exposed
by inhalation to "production grade" styrene (minimum purity
99.5%) containing approximately 5 ppm t-butylcatechol. They
were exposed 6 h/day, 5 days/week, for 18.3 months, if male,
and for 20.7 months, if female. Exposure duration was based
on the time when mortality reached 50% in one of the test
groups of that sex. Initial concentrations of styrene in air
were either 5040 mg/m3 (1200 ppm) or 2520 mg/m3 (600 ppm).
The high level was reduced to 4200 mg/m3 (1000 ppm) after 2
months because of excessive toxicity. Another group of 85
males and 85 females served as untreated controls. At the end
of the 2-year study, 5, 18, and 6 males and 30, 30, and 22
females had survived in the control, low-, and high-dose
groups, respectively. An excessive mortality from chronic
pneumonia occurred in the control and high-dose male groups.
An increased (but not statistically significant) combined
incidence of leukaemias and lymphosarcomas was seen: 1/84
males and 6/85 females in the high-dose groups, 5/86 males and
6/85 females in the low-dose groups, 1/85 males and 1/85
females in the control groups. If the total incidence of
leukaemias/lymphosarcomas in females is compared with
historical controls, it becomes statistically significant
(P < 0.05). A statistically significant increase in the
incidence of mammary adenocarcinomas was seen in the low-dose
female group (7/85 versus 1/85 in controls), but the incidence
was not statistically significant in comparison with
historical controls. Though these data are inconclusive, they
are interpreted by Jersey et al. (1978) as suggesting an
association between the exposure of the female rats to styrene
and an increased incidence of tumours of the leukaemia/
lymphosarcoma types. The Task Group was unable to evaluate
this study because of the high mortality.
6.9.1.3. Pre- and postnatal exposure
(a) Mouse
In the studies of Ponomarkov & Tomatis (1978), 29 pregnant
O20 mice were each given a single treatment of styrene at 1350
mg/kg body weight (99% pure dissolved in 0.1 ml olive oil) by
stomach tube on the 17th day of gestation. A control group of
9 pregnant animals received olive oil alone. The neonatal
mortality rate in the offspring of styrene-treated mice was
43%, compared with 22% in the controls. The same dose of
styrene was then administered weekly, by stomach tube, to 45
male and 39 female progeny from weaning to 16 weeks of age, at
which time the treatment was stopped because of the high rate
of mortality (64% alive at 20 weeks). The study was
terminated at 100 weeks, when all the animals had died. No
differences in tumour incidence were found between mothers
treated with styrene and those given olive oil. In the
progeny that had received weekly treatments, lung tumours
(adenomas and adenocarcinomas) were found in 20/23 males and
32/32 females, compared with 8/19 and 14/21 in olive
oil-treated controls (P < 0.01, P < 0.01) and compared with
34/53 and 25/47 in untreated controls (P < 0.05, P < 0.001).
No differences in tumour incidence at sites other than the
lung were seen in the progeny, compared with olive oil-treated
or untreated controls.
In the same studies (Ponomarkov & Tomatis, 1978), 15
pregnant C57 black mice were each given a single treatment of
styrene at 300 mg/kg body weight dissolved in 0.1 ml olive
oil, by stomach tube, on the 17th day of gestation. A control
group of 5 pregnant animals received olive oil alone. The
same amount of styrene was then given weekly by stomach tube
to 27 male and 27 female progeny from weaning up to 120 weeks
of age, at which time the survivors, 15 males and 12 females,
were killed. Control progeny (12 males and 13 females)
received olive oil for 120 weeks at which time 7 males and 4
females survived. In mothers treated with a single dose of
styrene, lymphomas were observed in 10/12 animals, compared
with 3/5 in the olive oil-treated controls. In treated male
progeny, liver tumours (hepatocellular carcinomas) were found
in 3/24 animals, compared with 1/12 olive oil-treated and 1/47
untreated controls (both hepatocellular adenomas). The
increased incidence of these tumours in styrene-treated
animals compared with controls was not statistically
significant. The small number of animals was noted by the
Task Group.
(b) Rat
Twenty-one female BDIV rats were each given a single
treatment of styrene at 1350 mg/kg body weight dissolved in
olive oil, by stomach tube, on the 17th day of gestation
(Ponomarkov & Tomatis, 1978). A control group of 10 pregnant
rats received 0.3 ml olive oil alone. The neonatal mortality
rate in the offspring was 10% in treated rats and 2.5% in
olive oil-treated controls. Subsequently, doses of 500 mg/kg
body weight were administered weekly by stomach tube to 73
male and 71 female progeny from weaning up to 120 weeks of
age, at which time the survivors, 8 males and 20 females, were
killed. Controls (36 males and 39 females) received olive oil
alone for 120 weeks. Two stomach tumours and 1 hepatocellular
adenoma were seen in styrene-treated females and one stomach
tumour in styrene-treated males. One stomach tumour was also
found in olive oil-treated female controls. The difference in
the incidence of these and other tumours in styrene-treated
rats compared with controls was not statistically significant.
6.9.2. Styrene 7,8-oxide
6.9.2.1. Dermal exposure
Mouse
Forty 13-week-old C3H mice were painted on the clipped
dorsal skin with a 5% solution of styrene 7,8-oxide in
acetone, 3 times weekly for life (Weil et al., 1963). No skin
tumours were observed in 33 animals that survived for 17-24
months (37 mice were alive at 12 months). Another group of
C3H mice were similarly painted with a 10% solution of styrene
oxide in acetone; 18 mice survived 12 months, only 2 mice
survived 17 months, and no skin tumours were observed.
In another study by Van Duuren et al. (1963), the clipped
dorsal skin of 30, 8-week-old male Swiss ICR/Ha mice was
treated 3 times weekly, throughout the life span, with 0.1 ml
of a 10% solution of styrene oxide in benzene. Median
survival time was 431 days in the styrene oxide-treated group
and 262 and 412 days, respectively, in the groups of 30 and 60
benzene-treated controls. Three styrene oxide-treated animals
and 11 (out of 150) benzene controls developed skin tumours.
This difference was not statistically significant. One of the
tumours in each group was a squamous-cell carcinoma.
6.9.2.2. Oral administration
Rat
Groups of 40 male and 40 female Sprague-Dawley rats, 13
weeks of age, were given either 50 or 250 mg/kg body weight,
per day, of styrene oxide (purity unspecified) dissolved in
olive oil, by stomach tube, 4 or 5 days per week, for 52 weeks
(Maltoni et al., 1979). Forty male and 40 female controls
received olive oil alone. The animals were allowed to live
until natural death or were killed 156 weeks after the start
of the study. Preliminary results included only stomach
cancer incidence in animals dying within the first 135 weeks.
The numbers of animals alive at the appearance of the first
tumour (51 weeks) were: controls, 37 males and 28 females;
low-dose, 31 males and 31 females; high-dose, 28 males and 30
females. The numbers of papillomas of the stomach were: 0/37
and 0/28 in controls; 0/31 and 2/31 in low-dose animals; and
3/28 and 6/30 in high-dose animals. The respective numbers
for squamous-cell carcinomas in situ were: 0/37 and 0/28;
5/31 and 6/31; and 11/28 and 12/30. Invasive squamous-cell
carcinomas occurred in 0/37 and 0/28; 2/31 and 1/31; and 4/2;
and 6/30, respectively.
Fourteen female rats were given, by gavage, a single dose
of styrene oxide at 200 mg/kg body weight in olive oil (0.3
ml) on day 17 of pregnancy (estimated total dose, 0.04 g).
Their progeny (62 females and 43 males) received 96 weekly
doses of 100-150 mg/kg body weight in olive oil (0.2 ml), from
weaning (4 weeks of age) until termination of the study (120
weeks) (estimated total doses, 2.5 g for females and 5.0 g for
males). Similar groups of controls (14 pregnant females, 55
female and 49 male progeny) were given only olive oil. All
survivors were killed at the end of the study. There were
fewer tumour-bearing animals among mothers treated with
styrene oxide (31%) than among rats given only olive oil
(57%); mammary tumours were seen only in the olive-oil-treated
animals. The incidences of other tumours were similar.
Forestomach tumours were seen only in treated progeny;
Carcinomas or early carcinomas of the forestomach occurred in
10/42 males and 16/60 females compared with 1/49 and 1/55
controls respectively. Four tumours of the nervous system
occurred in treated groups, whereas only 1 was observed in a
male control. Eight lung tumours were seen in treated
animals, and 2 in controls; 7 of these tumours (6 malignant +
1 benign) occurred among treated female progeny, giving an
incidence of 7/60 (12%) compared with 1/55 (2%) in control
females. The incidences of other types of tumour did not show
any marked differences between control and treated groups
(Ponomarkov et al., 1983; Ponomarkov et al., personal
communication).
6.9.3. Summary and conclusions
Of the 6 studies cited, 3 in mice and 3 in rats, only one
(O20 mice) gave results that indicated a significant increase
in lung tumour incidence. The others either did not show any
significant increase in tumour incidence or were performed in
such a way that conclusions could not be drawn from the
results.
The results of studies concerning styrene 7,8-oxide
indicated: that there was no development of, or increase in,
skin tumours, when the compound was applied to mouse skin;
that tumours (benign and malignant) developed in the
forestomach, when styrene 7,8-oxide was given by gavage in
rats; and that there was an increase in lung tumour incidence
in female progeny, when styrene 7,8-oxide was administered to
rats pre- and post-natally.
6.10. Genetic effects
Several reviews have been published on the mutagenic and
related effects of styrene (Vainio, 1978; IARC, 1979; Norppa,
1981a; Vainio et al., 1982; Zetterberg, 1982; Norppa & Vainio,
1983a).
6.10.1. Chemical reactivity of styrene and styrene oxides
Styrene needs metabolic activation to bind covalently with
nucleophilic biological macromolecules. Styrene 7,8-oxide,
which has been considered to be a primary mammalian metabolite
of styrene (section 5.3), is spontaneously reactive because of
the lability of the oxirane ring. Styrene 7,8-oxide can bind
covalently with proteins and nucleic acids (Marniemi et al.,
1977; van Anda et al., 1979) and is able to alkylate in vitro
4-( p-nitrobenzyl)-pyridine, a synthetic nucleophile, and
deoxyguanosine, a biological nucleophile (Hemminki, 1979;
Hemminki & Falck, 1979; Hemminki et al., 1981).
Hemminki (1979) measured the electrophilic reactivity of
styrene 7,8-oxide by detecting alkylation of 2-amino-1,7-di-
hydro-6 H-purin-6-one (guanine) fluorometrically. The rate of
reaction was roughly equal for guanosine, and deoxyguanosine.
Styrene 7,8-oxide formed a covalent 7-alkyl derivative with
guanine (Hemminki et al., 1980b). With an identical
concentration of guanine, RNA and single-stranded DNA were
substantially less reactive than guanosine, possibly as a
consequence of the tertiary structures of the large relative
molecular mass polymers. Double-stranded DNA was even less
reactive.
Two highly reactive arene oxide derivatives of styrene,
styrene 1,2- and 3,4-oxides were recently synthesized
(Hiratsuka & Watabe, 1982; Watabe et al., 1982a,b). These
arene oxides have a very brief half-life and they are very
hard to detect, even in an in vitro system consisting of
styrene, microsomes, and NADPH.
6.10.2. Mutagenic effects of styrene and styrene oxides in
bacterial assay systems
Styrene has not been shown to induce reverse mutations in
any of the Salmonella typhimurium tester strains used in the
Ames' test, in the absence of a metabolic activation system
(Table 11). However, in the presence of a metabolic
activation system, some investigators have found styrene to be
mutagenic in the Salmonella strains (TA 100, TA 1530, TA 1535)
used to detect base-substitution-inducing mutagens; others
have obtained negative results (Table 11). No positive
results have been reported for styrene in the Salmonella
strains (TA 98, TA 1537, TA 1538) detecting frame-
shift-inducing mutagens. The divergent results on the
mutagenicity of styrene in Salmonella could be explained, in
part, by metabolic differences between the microsomal
preparations (S-9 mix, S-10 mix) used. The high volatility,
poor solubility, and bacterial toxicity of styrene may also
affect the results (de Meester et al., 1981).
Styrene was reported to be positive in the rec assay with
Bacillus subtilis without metabolic activation, in a review by
Kawachi et al. (1979), but no specific data were given.
The primary metabolite of styrene, styrene 7,8-oxide, has
been shown to be mutagenic in a number of bacterial studies
with base-pair strains, without a metabolic activation system
(Table 11). Moreover, styrene 7,8-oxide was mutagenic in the
fluctuation test with Escherichia coli WP2 uvrA (for detecting
base-substitution mutagens) and with Klebsiella pneumoniae
(Hemminki & Falck, 1979; Voogd et al., 1981). The frame-shift
mutagen sensitive strains of Salmonella gave only negative
results with styrene 7,8-oxide, either with or without
metabolic activation systems (de Meester et al., 1977; Stolz &
Withey, 1977; Drinkwater et al., 1978; Wade et al., 1978;
Watabe et al., 1978; Busk, 1979; Hemminki & Falck, 1979;
El-Tantawy & Hammock, 1980). Watabe et al. (1981) did not
find any appreciable difference in the bacterial (Salmonella)
mutagenicity of R-and S-enantiomers of styrene 7,8-oxide and
their racemic mixture. However, Pagano et al. (1982) reported
that the R-enantiomer was more mutagenic to Salmonella than
the S-form, while the racemic styrene 7,8-oxide had an
intermediate mutagenic activity.
Table 11. Mutagenicity of styrene and styrene-7,8-oxide in the base-
pair substitution strains of Salmonella typhimurium and E. colia
------------------------------------------------------------------------
Results of mutagenicity studies
Styrene styrene 7,8-oxide
Metabolic activation Metabolic activation
system: system:
None Present None Present
------------------------------------------------------------------------
Drinkwater et al. (1978) .. .. - -
Glatt et al. (1979) .. .. + ..
Hemminki & Falck (1979) .. .. + ..
Pagano et al. (1982) .. .. + ..
Planche et al. (1979) .. .. + ..
Sugiura & Goto (1981) .. .. + ..
Sugiura et al. (1978a,b) .. .. + ..
Turchi et al. (1981) .. .. + ..
Voogd et al. (1981) .. .. + ..
Wade et al. (1978) .. .. + ..
Watabe et al. (1981a) .. .. + ..
El-Tantawy & Hammock (1980) .. .. + +b
Yoshikawa et al. (1980) .. .. + +b
Kawachi et al. (1979) - - .. ..
Milvy & Garro (1976) - .. + ..
Greim et al. (1977) - - + ..
Busk (1979) - - + +b
Loprieno et al. (1978) - - + +
Stolz & Withey (1977)
Watabe et al. (1981, 1982b) - +c + -
de Meester et al. (1977, - + + +b
1981)
Vainio et al. (1976) -d + + +b
Poncelet et al. (1980) - + .. ..
Cerna & Kypenova (1977) .. +e .. ..
------------------------------------------------------------------------
.. No data.
a Modified from: Norppa (1981a).
b A decrease reported in mutagenic activity, as compared to treatment
without metabolic activation.
c Only with trichloropropane oxide present.
d A weak activity reported also without S-9 mix in TA 1535.
e Host mediated assay, Salmonella strain TA 1950, mice as hosts.
Two arene oxide derivatives of styrene, styrene 1,2- and
3,4-oxide, both of which have been considered to be the
precursors of isolated urinary metabolites of styrene in the
rat, have been demonstrated to be mutagenic towards a
base-pair strain (TA 100) of Salmonella, but not towards a
frame-shift strain (TA 98) (Watabe et al., 1982b). The 1,2-
and 3,4-oxides were more mutagenic than the 7,8-oxide, but
only after sequential addition to the medium during pre-
incubation.
Thus, the results obtained with various bacterial assay
systems show that styrene 7,8-oxide is a direct base-pair
substitution type mutagen. The extremely labile minor
metabolites of styrene, 1,2- and 3,4-oxide, seem to be highly
reactive and are mutagenic for Salmonella. However, with
styrene, even in the presence of a metabolic activation
system, contradictory results have been reported.
6.10.3. Genetic effects of styrene and styrene 7,8-oxide
in eukaryotic non-mammalian systems
Various non-mammalian assay systems, including point
mutations and gene conversion in yeast, point mutations in
silk worm, recessive lethal mutations, sex chromosome loss and
non-disjunction in Drosophila melanogaster, and chromosome
damage in root tip cells of Allium cepa, have been applied to
study the mutagenic and genetic effects of styrene and styrene
7,8-oxide.
In the yeast assays, styrene did not induce mitotic gene
conversions at the adenine and tryptophane loci of
Saccharomyces cerevisiae or forward mutations at the adenine
loci of Schizosaccharomyces pombe, even in the presence of a
mouse liver microsome mix (Loprieno et al., 1976; Loprieno,
1981; Loprieno & Abbondandolo, 1980). According to Bauer et
al. (1980, 1981), this negative result could be because the
epoxide hydrotase (EC 4.2.1.63) (inactivation enzyme) is more
stable than the monooxygenase, because the concentration of
styrene 7,8-oxide in the incubation mixture could not reach a
mutagenic level. When a yeast strain ( Saccharomyces
cerevisiae D7) capable of metabolic activation was used to
test the induction of gene conversions by styrene, a positive
result was reported (Del Carratore et al., 1982). Styrene
7,8-oxide has been found positive in all the yeast systems
(Loprieno et al., 1978; Loprieno, 1981).
When tested in a host-mediated assay, with mice as the
host animal and yeast as the target cells, styrene and styrene
7,8-oxide were weakly mutagenic for some genetic end points
(Loprieno et al., 1978), but these results were later
considered negative for styrene (Loprieno & Abbondandolo,
1980) when a higher number of historical control values became
available.
Table 12. Point mutations, chromosome aberrations and genetic damage induced in cultured mammalian
cells by styrene and styrene 7,8-oxide
-------------------------------------------------------------------------------------------------------
Styrene Styrene 7,8-oxide
Test-system Type of damage References
assayed Metabolic Metabolic
activation system: activation system:
None Present None Present
-------------------------------------------------------------------------------------------------------
Rodent cell lines
V79 HGPRT mutations .. .. + .. Bonatti et al. (1978);
Sugiura et al. (1979)
- - + .. Loprieno et al. (1976,
1978)
- + + -c Beije & Jenssen (1982)
aberrations .. .. + .. Turchi et al. (1981)
micronuclei .. .. + ..
anaphase bridges .. .. + ..
CHL aberrations - +a + .. Ishidate & Yoshikawa
(1980);
Ishidate et al. (1981);
Kawachi et al. (1979);
Matsuoka et al. (1979)
CHO SCEs - +b + +d de Raat (1978)
.. .. + .. Kubiak et al. (1981)
L5178Y TK mutations .. .. + - Amacher & Turner (1982)
Human cells
Lymphocytes aberrations +e .. + .. Linnainmaa et al.
(1978a,b);
micronuclei +e .. + .. Meretoja & Vainio (1979)
aberrations .. .. + .. Fabry et al. (1978)
-------------------------------------------------------------------------------------------------------
Table 12. (contd.)
-------------------------------------------------------------------------------------------------------
Styrene Styrene 7,8-oxide
Test-system Type of damage References
assayed Metabolic Metabolic
activation system: activation system:
None Present None Present
-------------------------------------------------------------------------------------------------------
aberrations +e .. + .. Norppa et al.(1980a,
SCEs +e .. + .. 1981, 1982);
Norppa (1981a);
Norppa & Vainio (l983b)
unscheduled DNA -f .. .. .. Pero et al. (1982)
synthesis
EUE unscheduled DNA .. - + .. Loprieno et al. (1978)
synthesis
Wistar rat
hepatocytes unscheduled .. .. .. - Brouns et al. (1979)
DNA synthesis
-------------------------------------------------------------------------------------------------------
.. No data.
a With methylcholantrene preinduced rat liver S-9.
b Only with cyclohexene oxide present.
c In the liver perfusion system.
d A decrease in activity.
e In vitro activation by erythrocytes suggested.
f Purified lymphocytes.
In Drosophila, a significant increase in the frequency of
X-linked recessive lethals was observed in the offspring of
males fed styrene or styrene 7,8-oxide (Donner et al., 1979).
The test for sex chromosome loss and non-disjunction in
Drosophila gave negative results for styrene (Penttilä et al.,
1980). In a review (Kawachi et al., 1979), styrene was
reported not to induce point mutations in silk worm.
Results of cytogenetic studies in meristematic root cells
of Allium cepa demonstrated that styrene induces metaphase
chromosome breaks and micronuclei (Linnainmaa et al., 1978a,b).
Styrene 7,8-oxide induced micronuclei and anaphase fragments and
bridges in these growing root-tip cells (Linnainmaa et al.,
1978a,b).
6.10.4. Genetic effects of styrene and styrene 7,8-oxide in
mammalian cells in vitro
Point mutations, chromosome damage, and unscheduled DNA
synthesis (UDS) have been studied after treatment of mammalian
cell cultures with styrene or styrene 7,8-oxide (Table 12).
In the Chinese hamster V79 cell line, styrene did not
induce point mutations at the HPRT locus, with or without a
metabolic activation system (S-10 mix) from mouse liver
(Loprieno et al., 1976, 1978; Loprieno, 1981). On the other
hand, Beije & Jenssen (1982) found styrene weakly mutagenic in
the same system after metabolic activation by rat liver S-9
mix. When a liver perfusion system was used for the metabolic
activation of styrene, there was a clear increase in point
mutations at the HRPT locus of V79 cells (Beije & Jenssen,
1982).
Loprieno et al. (1978) examined the effects of styrene (with
mouse liver S-10 mix) and styrene 7,8-oxide on UDS in human
heteroploid EUE cell line. There was an increase in UDS with
styrene 7,8-oxide but not with styrene. Brouns et al. (1979) used
freshly isolated hepatocytes of Wistar rats for UDS detection after
styrene 7,8-oxide treatment. The results were negative, which
according to the authors was due to the rapid inactivation of
styrene 7,8-oxide in the hepatocytes. Pero et al. (1982) did not
find any effects of styrene (without metabolic activation) on UDS
in purified resting human lymphocytes.
Styrene induced chromosome aberrations, micronuclei, and
SCEs in human whole blood lymphocyte cultures, and chromosome
aberrations in Chinese hamster CHL cells in the presence of
metabolic activation (Table 12). Styrene 7,8-oxide induced
chromosome aberrations, micronuclei, and SCEs, in rodent cell
lines and in human lymphocyte cultures without metabolizing
systems (Table 12). The positive effects of styrene in human
whole blood lymphocyte cultures are explained by the ability
of erythrocytes to convert this compound into styrene
7,8-oxide (Norppa et al., 1980a; Belvedere & Tursi, l981;
Norppa et al., l982; Vainio et al., l982).
Without metabolizing systems, styrene 7,8-oxide has been
clearly shown to induce HGPRT mutations in V79 cells (Loprieno
et al., 1976; 1978; Bonatti et al., 1978; Sugiura et al.,
1979; Loprieno & Abbondandolo, 1980; Loprieno, 1981; Beije &
Jenssen, 1982) and point mutations in the TK locus of the
mouse lymphoma cell line L5178 Y (Amacher & Turner, 1982).
With the liver perfusion technique, styrene 7,8-oxide did not
induce point mutations at the HPRT locus of V-79 cells (Beije
& Jenssen, l982).
6.10.5. Genetic effects of styrene and styrene 7,8-oxide in
mammalian systems in vivo
Styrene and styrene 7,8-oxide have been tested in vivo for
their ability to induce genetic damage in somatic and germ
cells in different species (mouse, rat, Chinese hamster) using
different genetic endpoints (chromosome aberrations,
micronuclei, dominant lethals, SCEs and translocations in
spermatocytes; Table 13). Most of the studies concerning
chromosome aberrations in the bone marrow cells of animals
were negative for styrene. No increases in chromosome
aberrations in bone marrow were observed in mice after high
single or repeated doses of styrene administered by gavage or
intraperitoneally (i.p.), or in rats after an unspecified
dose. However, a positive effect for chromosome aberrations
in rat bone marrow was found after inhalation exposure, and
for SCEs in mouse bone marrow, alveolar macrophages, and
regenerating liver cells. In the micronucleus test, styrene
gave negative results in Chinese hamsters but positive results
in mice (Penttilä et al., 1980; Norppa, 1981b).
Studies on the cytogenetic effects of styrene 7,8-oxide in
whole mammals also gave contradictory results (Table 13).
Loprieno et al. (1978) observed a dose-dependent clastogenic
effect in mouse bone marrow cells after oral administration of
styrene 7,8-oxide. However, Fabry et al. (1978) did not find
any chromosome aberrations or micronuclei in the bone marrow
of mice after an i.p. administration. Penttilä et al. (1980)
also failed to detect any effects on micronuclei in Chinese
hamster bone marrow erythrocytes after an i.p. administration.
Negative findings were reported by Norppa et al. (1979) for
chromosome aberrations and SCEs in the bone marrow cells of
Chinese hamsters after inhalation exposure. A slight increase
in SCEs was observed after i.p. injection of a lethal dose;
chromosomal aberrations were also elevated. Conner et al.
(1982) noticed a slight increase in SCEs in regenerating liver
cells and alveolar macrophages (but not in bone marrow) in
hepatectomized mice exposed through inhalation to styrene
7,8-oxide.
Table 13. Cytogenetic damage induced by styrene and styrene 7,8-oxide in various rodent studies in vivo
------------------------------------------------------------------------------------------------------------
Exposure
Species Duration of Type of
(strain) Routea Dose time after damage Result Reference
Cell type treatment studied
------------------------------------------------------------------------------------------------------------
STYRENE
Rat
(Wistar)
bone marrow inhalation 1260 mg/m3 (300 ppm) 9-11 weeks Chromosomal + Meretoja et al. (1978)
(Wistar) aberrations
bone marrow inhalation .. .. Chromosomal - Kawachi et al. (1979)
aberrations
Mouse
(BDF1)
bone marrow inhalation 1625-3872 mg/m3 4 days SCEs + Conner et al. (1979;
alveolar (387-922 ppm) 1980; 1982)
macrophages,
regenerating
liver cells 3872 mg/m3 (922 ppm) 2 days SCEs +
(C57BL/6)
polychromatic i.p. 250 or 1000 mg/kg 30 h Micronuclei + Norppa (1981b)
erythrocytes 500 or 1500 mg/kg 30 h Micronuclei -
(CD-1)
bone marrow p.o. 500 or 1000 mg/kg 24 h Chromosomal - Loprieno et al. (1978)
aberrations
p.o. 4 x 500 mg/kg 4 days Chromosomal - Sbrana et al. (1982)
aberrations
70 x 200 mg/kg 70 days Chromosomal -
aberrations
(ICR)
bone Marrow i.p. LD50 or 5xLD50 6, 24, or Chromosomal - Cerna & Kypenova
2 6 48 h aberrations (1977)
Chinese hamster
bone marrow inhalation 1260 mg/m3 (300 ppm) 4 or 21 Chromosomal - Norppa et al. (1980)
days
polychromatic i.p. 1000 mg/kg 30 h Micronuclei - Penttilä et al. (1980)
erythrocytes
-----------------------------------------------------------------------------------------------------------
Table 13. (contd.)
------------------------------------------------------------------------------------------------------------
Exposure
Species Duration of Type of
(strain) Routea Dose time after damage Result Reference
Cell type treatment studied
------------------------------------------------------------------------------------------------------------
STYRENE 7,8-OXIDE
Mouse
(BDF1)
bone marrow, inhalation 210 mg/m3 (50 ppm) 5 h SCEs +b Conner et al. (1982)
alveolar
macrophages,
regenerating
liver cells
(CD-1)
bone marrow p.o. 500-1000 mg/kg 24 h Chromosomal + Loprieno et al.
aberrations (1978)
(BALB/c)
bone marrow i.p. 250 mg/kg 1-13 days Chromosomal - Fabry et al. (1978)
aberrations
polychromatid 30 h Micronuclei -
erythrocytes
primary 2.5-3 Trans- -
spermatocytes monthsc locations
post-meiotic 1-3 weeks Dominant -
sperm cells lethals
Chinese hamster
bone marrow inhalation 105-420 mg/m3 2,4, or 20 Chromosomal - Norppa et al. (1979)
(25-100 ppm) days aberrations
SCEs -
i.p. 500 mg/kg 24 h Chromosomal -d
aberrations
7 h SCEs +e
------------------------------------------------------------------------------------------------------------
.. No data.
a i.p. = intraperitoneal, p.o. = peroral.
b Negative in bone marrow.
c Considered to be too long time for a positive result.
d Positive in animals who died of the treatment.
e A slight effect.
Only one study has appeared concerning the possible
effects of styrene 7,8-oxide on germinal cells. Fabry et al.
(1978) reported negative results for translocations in primary
spermatocytes after a single i.p. injection and for dominant
lethals in male postmeiotic germ cells of mice.
In summary, the results concerning the genetic effects of
styrene and styrene 7,8-oxide in vivo are conflicting. Positive
results for both compounds have been obtained mainly at toxic or
nearly toxic doses. The studies have dealt with different
indicators of chromosome damage (aberrations, micronuclei, SCEs) in
actively dividing somatic cells. The effects of styrene 7,8-oxide
on germ cells have been examined only in one study, with negative
results. On the basis of the activity of monooxygenase and epoxide
hydrolase in different species, mice would be expected to be more
sensitive to styrene than rats or Chinese hamsters (Cantoni et al.,
1978; Norppa et al., 1979; Norppa, 1981a). Even in mice, styrene
and styrene 7,8-oxide disappeared rather rapidly after
intraperitoneal or oral administration (Bidoli et al., 1980;
Pantorotto et al., 1980; Sbrana et al., 1982). However, the
results of the mutagenicity studies available cannot at present be
interpreted solely according to such differences in metabolism.
6.10.6. Conclusions on the genetic effects of styrene
Styrene is a potential mutagen only after metabolic
activation. Its most important active metabolite seems to be
styrene 7,8-oxide. Arene oxides (styrene 1,2-oxide and
styrene 3,4-oxide) may also be of importance, but at present
their role cannot be evaluated.
Genetic effect tests in bacteria, yeasts, and mammalian cells
in vitro have yielded contradictory results for styrene, in the
presence of microsomal fraction of rodent liver (usually S-9 mix).
This appears to be mostly due to differences in the efficiency of
activation of styrene in different studies.
Results of in vivo studies on the genetic effects of styrene in
mammals are also contradictory. Some of the discrepancies can be
explained by variations in metabolic capacity among the rodent
species used, divergence in dose levels, and by the different
routes of administration.
Styrene 7,8-oxide is a direct mutagen that is clearly positive
in vitro without metabolic activation, but gives contradictory
results in mammals in vivo.
6.11. Effects on Reproductive Function and Teratogenic Effects
(a) Non-mammalian systems
Styrene and styrene 7,8-oxide have been reported to be
embryotoxic and teratogenic in chick embryos (Vainio et al., 1977).
Kankaanpää et al. (1979) found that trichloropropylene oxide
increased the embryo toxicity and teratogenicity of styrene and
styrene oxide. The LD50s were 40 µmol/egg for styrene and 1.5
µmol/egg for styrene oxide. Depending on the dose, malformations
were found in up to 20% of the test embryos but not in the
controls. The types of malformation included: absence of one or
both eyes, eyelid deformation, deformation of the skull,
exencephaly, exteriorization of viscera, etc. Styrene and styrene
oxide have also been found to interfere with the development of sea
urchin embryos (Pagano et al., 1978).
(b) Mammalian systems
When male rats were exposed to a styrene concentration of
200 mg/m3 (48 ppm) for 5 h/day, 5 days per week for 4 months,
Ivanova-Tchemichanska et al. (1982) found decreased osmotic
resistance and mobility of spermatozoa, decreased sulfhydryl
content, and desquamated epithelium in the seminiferous
tubules.
Ragyl'ye (1974) studied the effects of styrene on pregnant
rats exposed to 1.47, 5, and 50 mg/m3 (0.35, 1.2, and 12 ppm)
for 4 h/day throughout gestation. Resorptions were noted at
all exposure levels but the number of malformations was not
significantly increased. Murray et al. (1978) exposed rats
and rabbits to styrene through inhalation (1260 and 2520
mg/m3; 300 and 600 ppm) for 7 h/day from day 6 to 15 (rats) or
from day 6 to 18 (rabbits), and by gavage (in peanut oil, 180
and 300 mg/kg body weight/day). No increase in the incidence
of resorptions or major malformations, compared with controls,
was reported. Delayed ossification was noted in rabbits
exposed to 2520 mg/m3 (600 ppm). In an inhalation study,
Vergieva et al. (l979) exposed rats to styrene at 200 and 400
mg/m3 (48 and 96 ppm) for 4 h daily, 5 days a week, throughout
pregnancy. There was not any evidence of embryotoxicity or
teratogenicity. Mice were exposed to a styrene level of 1050
mg/m3 (250 ppm) and Chinese hamsters to 1260, 2100, 3150, and
4200 mg/m3 (300, 500, 750, and 1000 ppm) for 6 h daily on days
6-16 (18 for hamsters) of gestation. An increased number of
resorptions was noted in both species at the highest
concentration tested. A slight increase (not statistically
significant) was observed in the umber of minor skeletal
malformations (rib fusion, extra ribs) in mice but not in
hamsters (Kankaanpää et al., 1980).
Quast et al. (1978) exposed pregnant Sprague-Dawley rats
and New Zealand white rabbits to styrene concentrations of
1260 or 2520 mg/m3 (300 or 600 ppm) for 7 h/day on days 6-15
(rats) and 6-10 (rabbits) of gestation. Additional groups of
rats were given styrene (90 or 150 mg/kg body weight daily) on
days 6-15 of gestation. No teratogenic effects were detected.
In a 3-generation reproduction study in rats, styrene was
administered in the drinking water at l25 and 250 mg/litre
(estimated daily intake 14 and 21 mg/kg body weight per day
for males and females, respectively, at the high dose). No
adverse effects on reproductive capacity were observed. Some
significant effects on early survival in the second generation
offspring at the high dose were found in 2 specific litters.
No further evaluation could be made, since data for
individuals were not available (Litton Bionetics, 1980).
7. EFFECTS OF STYRENE IN MAN
7.1. Controlled Human Studies
Few controlled exposure studies have been reported in
which the effects of styrene on physiological functions in man
have been investigated. Attention has been focused on the
irritant effects of styrene vapour on the mucous membranes and
on the central nervous system effects caused by styrene
inhalation.
The odour perception threshold for styrene in air was
determined by Smith & Hochstettler (1969) to be as low as
0.2-0.34 mg/m3 (0.05-0.08 ppm). At higher concentrations, the
odour of styrene was clearly perceptible and it was reported
as "strong but not objectionable" at about 420 mg/m3 (100 ppm)
(Stewart et al., 1968). The ability to detect the odour
typically fades as the exposed individuals become adapted
(Stewart et al., 1968). Styrene vapour was irritating to the
eye and the nose at concentrations exceeding 840 mg/m3 (200
ppm) and, when the styrene concentration approached 1596 mg/m3
(380 ppm), it was poorly tolerated (Stewart et al., 1968).
Strong immediate irritation of the eyes and the respiratory
tract has been reported at styrene concentrations in air of
2520-3360 mg/m3 (600-800 ppm) (Carpenter et al., 1944; Wolf et
al., 1956).
Oltramare et al. (1974) exposed 6 volunteers to styrene at
210, 420, and 840 mg/m3 (50, 100, and 200 ppm) over 1-3 h and
noted that for a combination of symptoms of the mucous
membrane (irritation of eyes, nose, and lips) and central
nervous system (dizziness, headache, drowsiness, difficulty in
concentrating, lightheadedness, fatigue), the number of
symptoms increased with dose. This increase was still evident
at the 210 mg/m3 (50 ppm level). At the 2 higher levels,
digestive disturbances also occurred. In studies by Gamberale
& Hultengren (1974), 12 volunteer subjects were exposed to
styrene vapour through a mouth tube at concentrations
increasing stepwise 210, 630, 1050, and 1470 mg/m3 (50, 150,
250, and 350 ppm), each successive step lasting about 30 min.
When the volunteers were asked afterwards to rate their
subjective sensations (during the exposure) it appeared that
exposure to styrene had made them feel more tense and more
"affected" compared with control conditions. The authors also
studied the psycho- physiological performance of the
subjects. Simple reaction times tended to be prolonged with
increasing exposure. The changes became statistically
significant during the final 30 min, when the environmental
concentration reached 1470 mg/m3 (350 ppm). Tests on
perceptual speed and manual dexterity did not show any
impairment in relation to styrene exposure.
Stewart et al. (1968), however, noted an impairment in
performance of tasks involving manual dexterity and eye-hand
coordination in some subjects exposed to a styrene
concentration of about 1596 mg/m3 (380 ppm) over a 1-h
period. On the basis of a study on 3 volunteers, Oltramare et
al. (1974) reported impaired reaction time to visual stimuli,
to combined visual-acoustic stimuli, and in a test of diffuse
attention during and immediately after exposure to styrene at
210, 420, and 840 mg/m3 (50, 100, and 200 ppm) for 1.5 h.
There was no clear dose dependance. Inhalation exposure to
high concentrations of styrene (3360 mg/m3; 800 ppm) caused
symptoms of impairment of balance in 2 subjects and a marked
unsteadiness in posture was observed (Carpenter et al.,
1944). At a styrene level of about 1596 mg/m3 (380 ppm) for 1
h, 2 out of 5 subjects showed abnormal results in the modified
Romberg test indicating difficulty in maintaining balance.
Furthermore, after a 7-h exposure to about 420 mg/m3 (100
ppm), 3 out of six subjects reported that they had transitory
difficulty in performing the modified Romberg test, though no
objective signs of impairment of balance were found (Stewart
et al., 1968). Oltramare et al. (1974), in a study on 3
subjects, showed impairment of balance on a body sway platform
at a styrene concentration of 840 mg/m3 (200 ppm) but not at
420 mg/m3 (100 ppm).
Five volunteers were exposed by Odkvist et al. (1980) to
styrene at about 1260 mg/m3 (300 ppm) for 1 h, using a mouth
tube. Treatment was combined with exercise on a bicycle
ergometer with a 50 W workload, and observations were made on
both equilibrium ability and functioning of the vestibular
system immediately after exposure. None of the subjects
exhibited spontaneous, fixation or positional nystagmus,
whereas an impairment of eye-tracking ability was found in all
individuals in an optokinetic test. The difference in
eye-movement changes between styrene-exposed and control
groups was not statistically significant. No styrene-induced
changes were found in conventional clinical tests of balance
(standing on one leg with eyes closed, walking a line with
eyes closed). The results were thought to suggest that
exposure to styrene (inducing blood levels of approximately 80
µmol/litre) decreased the inhibitory effect of the cerebellum
on the motor function of the eyes.
In summary, the odour threshold for styrene was found to be
0.2 - 0.34 mg/m3 (0.05-0.08 ppm) and the odour was uncomfortable at
elevated concentrations. Styrene induced subjective symptoms of
irritation of the mucous membranes at concentrations exceeding
420 - 840 mg/m3 (100-200 ppm). In the same concentration range,
subjective symptoms of the central nervous system, such as
dizziness, lightheadedness, headache, and drowsiness may occur.
Reaction time, performance, and body balance tend to be impaired by
short-term inhalation exposure to styrene at concentrations of
630 - 840 mg/m3 (150-200 ppm) and definite impairment occurs at
concentrations exceeding 1470 mg/m3 (350 ppm).
7.2. Epidemiological Studies
7.2.1. Haematopoietic and immune system
Chmielewski & Renke (1975), who studied a group of 101
workers exposed for at least one year to a styrene
concentration of 100-300 mg/m3 (24-72 ppm), did not
demonstrate any appreciable effects on the haemoglobin
concentration, erythrocyte count, leukocyte count, or
differential count. Workers exposed for more than 10 years,
however, had a slightly decreased thrombocyte count compared
with workers exposed for shorter periods.
In a study on 494 workers at different levels and
durations of exposure, Lorimer et al. (1976) observed a random
distribution of abnormal haemoglobin concentrations, and
leukocyte or platelet counts between the groups. Thiess &
Friedheim (1978), who investigated 84 workers exposed to
210-2100 mg/m3 (50-500 ppm) for 1-36 years, did not notice any
appreciable differences in haemoglobin concentrations, or
leukocyte, erythrocyte, or platelet counts compared with a
reference group.
In the studies of Chmielewski & Renke (1975), and Renke &
Chmielewski (1976), 20 styrene workers did not show any
differences in bleeding time or fibrinogen level compared with
a reference group. However, the coagulation time and the
adhesivity of the platelets were somewhat increased in the
styrene-exposed group and the prothrombin index was slightly
reduced.
There is very little information on the immunological
effects of styrene. In the immunophoresis study of
Chmielewski et al. (1973), no dose-related differences were
observed in concentrations of serum gamma globulin among
workers exposed to different concentrations of styrene.
7.2.2. Nervous system
Four studies have dealt with reaction times among workers
occupationally exposed to styrene. One study (Götell et al.,
1972) in which 17 men were exposed to a styrene concentration
of 630 mg/m3 (150 ppm) showed prolonged simple reaction times
in the styrene-exposed workers, both in the morning and in the
afternoon, compared with an age-matched control group. A
second study of 106 workers in 4 work places indicated
longer and more irregular reaction times in workers exposed to
styrene than in controls (Gamberale et al., 1975). The
differences were still present after a night's rest. The mean
styrene concentration determined by continuous measurement in
the workers' breathing zone was 57-426 mg/m3 (13.6-101.4
ppm). The mean duration of exposure was 2.7 years (range
0.1-11.0 years).
Another study with a similar study design, also revealed
prolonged reaction times during the working day among styrene-
and acetone-exposed boat manufacturers (Kjellberg et al.,
1979). The exposed group (7 workers, average styrene
concentration 37 mg/m3 (9 ppm), acetone concentration
82 mg/m3, mean employment time 10.5 years) did not show any
deterioration in sensory motor functions in relation to
styrene exposure and the addition task. There was a
correlation between the reaction-time impairment and the total
uptake of styrene divided by the estimated amount of adipose
tissue.
In the study of Kjellberg et al. (1979), the reaction
times of 3 subjects were followed after removal from exposure.
Reaction times improved after 4 days and there was a further
improvement after 35 days.
The study by Cherry et al. (1980) dealt with 27 workers
(mean age, 23 years), who were exposed to a time-weighted
average level of styrene of 386 mg/m3 (92 ppm). Among the
psychological and behavioural tests applied, differences
between the exposed and unexposed groups were seen only in
reaction times.
Several case reports have described neurasthenic symptoms
among patients who had been occupationally exposed to styrene
(Klimkova-Deutschova, 1962; Axelson et al., 1974; Spasovski et
al., 1976).
A considerable percentage of 101 workers employed in the
polyester laminate industry had disturbances of the nervous
system, particularly of the vegetative nervous system. The
exposure level of the workers ranged from approximately 100 to
300 mg/m3 (24 to 72 ppm). The urinary mandelic acid excretion
was more than 400 mg/litre in 36 of the workers (Chmielewski
et al., 1973).
Schneider & Seeber (1979) used psychological tests on 2
groups occupationally exposed to styrene, one group (35
persons) having a mean exposure of 700 mg/m3 (168 ppm), and
the other (46 persons), a mean exposure of 300 mg/m3
(72 ppm). Exposure was associated with poor visual attention,
interferences with attention span, perceptual inaccuracy, and
decreased manual dexterity.
In studies concerning 98 male laminating workers having a
mean urinary mandelic acid concentration that varied from 7 to
4715 mg/litre (median concentration 808 mg/litre), symptoms of
tiredness, and difficulty in concentrating were mentioned more
often than among a reference population (Härkönen, 1977). The
duration of styrene exposure varied from 0.5 to 14 years
(median 5.1 years). In psychological tests, the same
styrene-exposed workers showed visuomotor inaccuracy and poor
psychomotor performance (Lindström et al., 1976).
Exposure-effect relationships were drawn between the urinary
mandelic acid concentration and a combined score for 3 tests
(symmetry drawing, the Bourdon-Wiersma test, and the Mira
test). Analysis of the exposure-response relationship for
these tests showed a significant increase in response for
workers with levels of urinary mandelic acid exceeding 1600
mg/litre (75% having mandelic acid levels of more than 2000
mg/litre) (Härkönen et al., 1978). The proportion of abnormal
EEGs also increased with increasing exposure levels
(Seppäläinen & Härkönen, 1976). Workers with urinary mandelic
acid concentrations above 700 mg/litre had significantly
(P < 0.05) more frequent abnormal EEGs than those with lower
levels (Seppäläinen & Härkönen, 1976; Härkönen et al., 1978).
EEG abnormalities have been reported to be more prevalent
among young styrene workers, especially during the first years
of exposure (Dolmierski et al., 1974; Chmielewski et al.,
1977). EEG abnormalities, indicated mainly by increased slow
activity in the theta range, did not increase with longer
exposure (Roth & Klimkova-Deutschova, 1963; Seppäläinen &
Härkönen, 1976), though paroxysmal episodes have also been
evident (Dolmierski et al., 1974; Seppäläinen & Härkönen,
1976). Results of a study by Rosén et al. (1978) in which 33
men were exposed to styrene concentrations ranging from less
than 21 mg/m3 (5 ppm) to 735 mg/m3 (175 ppm), showed an
increased beta activity in 9 subjects and slow activity (theta
waves) in 6. The EEG abnormalities were non-specific and
resembled those caused by various other solvents (Rosén et
al., 1978; Seppäläinen et al., 1980).
Styrene exposure may also affect peripheral nerves
(Matsushita et al., 1968, Lilis et al., 1978; Rosén et al.,
1978). Lilis et al. (1978) found distal hypoaesthesia in the
lower extremities of 412 styrene-exposed workers (persons with
diabetes mellitus, a history of back injury, and/or excess
alcohol intake were excluded). The frequency of this symptom
varied from 4.1% among those exposed for less than 7 years to
8.5% among those exposed for more than 20 years. Abnormally
low motor conduction velocity (MCV) of the radial nerve was
found in 15 out of 80 persons tested and a slowed MCV of the
peroneal nerve in 12 out of 73 tested. The MCV of the
peroneal nerve decreased with increasing length of exposure,
but the effect of age was not considered. However, the level
of exposure did not have any effect on the peroneal nerve
conduction. The MCV of the radial nerve was not correlated
with the length or level of exposure. Rosén et al. (1978) did
not find differences in the MCVs of the median, ulnar,
peroneal, or posterior tibial nerves of 33 workers exposed to
styrene, compared with normal controls. However, the
amplitude of the sensory action potential of the median nerve
was reduced and the duration of this potential increased among
styrene-exposed workers, even in 10 individuals who had been
exposed to styrene levels of less than 21 mg/m3 (5 ppm). The
dose-effect relationships were conflicting. Disorders in
attention span and movement coordination in psychological
tests were reported in 12 out of 102 workers, occupationally
exposed to styrene concentrations of 10-100 mg/m3, who had
urinary mandelic acid concentrations of 30.9 - 268 g/kg
creatinine (Spasovski et al., 1980). The same authors also
reported diminished alcohol tolerance among styrene workers.
In summary, the results of 3 studies showed that simple
reaction times were prolonged in workers occupationally exposed to
styrene at levels below 630 mg/m3 (150 ppm), while one study gave
equivocal results at a time-weighted average level of styrene in
air of 386 mg/m3 (92 ppm). Another study suggested that the effect
on reaction time was reversible.
Surveys of styrene-exposed workers have shown that an increased
incidence of abnormalities in electroencephalographic recordings
at mean styrene levels of less than 420 mg/m3 (100 ppm) was related
to styrene exposure levels. Some slight disturbances in visuomotor
accuracy and psychomotor performance were noted in workers exposed
to levels of the order of 210 mg/m3 (50 ppm) or more. Various
neurasthenic and autonomic symptoms were also reported among these
workers.
7.2.3. Kidneys and the urinary tract
There are only a few studies of the effects of styrene
exposure on the human kidney. Härkönen (1977), who studied
laminating workers exposed to styrene for periods ranging from
0.5 to 14 years, could not find any cytological changes
differing from Papanicolau I in 35 urine specimens. Results
of studies concerning mortality and morbidity rates due to
kidney and urinary tract disorders have not revealed any
differences between subjects exposed to styrene and unexposed
subjects (Lorimer et al., 1978; Thiess & Friedheim, 1978).
In studies on organic solvent exposure in relation to
kidney function, Askergren et al. (1981) reported that workers
exposed to organic solvents, especially to styrene, excreted
significantly larger quantities of albumin in the urine than
unexposed workers. No differences were observed in beta-2-
microglobulin excretion. The same author compared the
excretion of erythrocytes and leukocytes in the urine of 101
men, occupationally exposed to styrene or toluene or to a
combination of xylene and toluene, with the cell excretion in
the urine from 39 unexposed controls. While no changes in
glomerular filtration rates were observed, the urine of
exposed men contained significantly more cells than that of
the controls.
7.2.4. Gastrointestinal tract
Basirov (1975) reported studies on the digestive system of 130
workers (89 men, 41 women) engaged in styrene-butadiene synthetic
rubber manufacture. The workers were mostly 20-40 years old and
the length of exposure varied from less than 5 years (16 workers)
to more than 10 years (28 workers). Average styrene concentrations
were 60-130 mg/m3 (14-31 ppm). Tests of secretory, excretory,
motor, and pepsinogen-generating functions of the stomach were
conducted on 20 unexposed people and on 80 workers who first
developed symptoms related to the digestive system after working in
the plant. Thirty-six had decreased digestive function, 25 had
decreased peristalsis, and 51 had decreased acidity. In further
studies, chronic gastritis was diagnosed in 35 of these workers.
7.2.5. Liver
Lorimer et al. (1976) assessed the liver function of 493
styrene-exposed workers. Exposure concentrations were not
measured. The activities of alkaline phosphatase (AP) (EC
3.1.3.1), aspartate aminotransferase (ASAT or GOT) (EC 2.6.1.1),
alanine aminotransferase (ALAT or GPT) (EC 2.6.1.2), and gamma
glutamyltranspeptidase (gamma-GTP) (EC 2.3.2.1) and the amount of
serum bilirubin were determined. Only the gamma-GTP activity
showed a significant elevation in the high-exposure group compared
with the low-exposure category, even when alcohol intake was taken
into account (1.7% versus 7.2%; 0.01 < P < 0.02).
Axelson & Gustavson (1978) gathered data on ASAT, ALAT in
serum from 35 styrene-exposed male workers and 12 unexposed
controls. The time-weighted average exposure levels were less
than or about 420 mg/m3 (100 ppm). The average transferase
levels were higher among men who had been exposed, but only
ASAT was significantly elevated (P < 0.001). No differences
were found in the average levels of AP.
The serum levels of ALATP, ASAT, gamma-GTP, LDH, and SDH
(sorbitol dehydrogenase (EC 1.1.1.14)) enzymes were studied in 72
workers exposed to styrene in the manufacture of plastic boats.
The exposure levels varied between very low concentrations < 21
mg/m3 (< 5 ppm) and about 170 mg/m3 (40.8 ppm). The styrene-
exposed groups had a higher mean gamma-GTP value than the control
group (P < 0.05). However, there was no dose dependence
(Lundberg, 1981).
Vihko et al. (1983) studied 25 persons exposed to styrene at
about 126 - 168 mg/m3 (30 - 40 ppm) (mean exposure time 3 years,
all exposures exceeding 1 year). The serum activities of ALAT,
ASAT, gamma-GTP, and LD and the concentrations of serum total
bilirubin and conjugated bilirubin were determined, as well as
serum bile acids, cholic acid, and chenodeoxycholic acid. An
elevated concentration of chenodeoxycholic acid in serum was the
most frequently found parameter among styrene-exposed workers.
Spasovski (1976) showed that the serum protein profile changed and
that serum transaminase activity was elevated at a styrene
concentration of 2000 mg/m3 (500 ppm).
In conclusion, a clear-cut trend towards altered liver
function was not demonstrated. At low exposure levels, the
commonly used parameters such as serum activity of enzymes of
hepatic origin have given equivocal results.
7.2.6. Cardiovascular system
The thorax radiographs of 84 workers were evaluated before
the beginning of employment and after exposure to styrene at
210 - 1260 mg/m3 (50 - 300 ppm) for 1-36 years (Thiess &
Friedheim, 1978). The authors could not attribute any grossly
observable changes to styrene exposure, nor did they observe
any "gross pathological indications in the electrocardiograms"
compared with those of a reference group of 62 subjects.
7.2.7. Respiratory system
In clinical studies, Wilson (1944) found that styrene-
exposed workers reported irritation of the eyes, throat, and
respiratory tract. It has frequently been reported that
people in areas of high styrene exposure complain of
irritation of the eyes and nasopharynx. Götell et al. (1972)
examined 15 workers occupationally exposed to a time-weighted
average styrene concentration of 71 - 1218 mg/m3 (17 - 290 ppm)
and found that lung function tests (forced vital capacity and
forced expiratory volume in 1 s) were normal and did not
change during the working day. However, the concentrations of
styrene that gave rise to complaints varied from person to
person, apparently depending on individual tolerance. In a
study on styrene-exposed workers who had respiratory tract
symptoms, it was suggested that long-term exposure to styrene
could cause obstructive pulmonary changes (Chmielewski &
Renke, 1975).
In a cross-sectional study of clinical signs and symptoms
of irritation (Lorimer et al., 1978) in 488 styrene-exposed
workers, the workers were divided into 2 groups, based on
estimated exposures of 4.2 mg/m3 (1 ppm) and > 21 mg/m3 (> 5
ppm), mostly above 84 mg/m3 (20 ppm). When asked about lower
respiratory symptoms, significantly more workers in the
high-dose group responded in the affirmative. There were no
differences in length of employment and the prevalence of
upper respiratory symptoms between the 2 groups.
In a symptom survey (Härkönen, 1977) of 98 male laminating
workers occupationally exposed to styrene, symptoms that
fulfilled the British Medical Research Council's criteria for
simple chronic bronchitis were more common in the exposed than
in an unexposed group. In the exposed group, 28% had simple
chronic bronchitis, compared with 12% in the control group
(P < 0.05). The smoking habits of the groups did not differ.
7.2.8. Endocrine organs
In a study that involved 25 rubber workers, exposed to
styrene, and 26 age-matched controls, Wink (1972) did not
observe any significant differences in the urinary excretion
of 17-ketosteroids or 17-ketogenic steroids. Exposure data
were not given. However, Chmielewski et al. (1973) reported
reduced excretion of 17-ketosteroids in the urine in 27 out of
67 workers exposed to styrene concentrations of 100-300 mg/m3
(24 - 72 ppm), but these findings were not convincingly
documented. The evaluation of possible effects of styrene on
the steroid metabolism should be studied using more refined
techniques.
In the study of Chmielewski et al. (1973), glucose
tolerance was observed to be somewhat higher in the exposed
group than in the control group, and even more in the group
with higher mandelic acid concentrations in the urine (limit
400 mg/litre). Evidence of effects of styrene on the
endocrine glands was lacking.
7.2.9. Carcinogenic effects
(a) Styrene production and polymerization processes
A survey of 560 individuals from a styrene production and
polymerization plant was reported by Lilis & Nicholson (l976)
and Nicholson et al. (1978). In 1960, the workers had been
employed in the plant for at least 5 years. It is known that
benzene, coke-oven products, butadiene, and ethyl-benzene had
been used in this plant prior to 1965. Among 83 deaths, 17
were due to cancer, including 2 cases of leukaemia and one of
a lymphoma. When the total cancer mortality in this cohort
was compared with that in the general population, no excess of
cancer incidence was found, but the follow-up period may have
been too short to detect an increased risk of neoplasia. One
case of leukaemia and one of lymphoma were found in 21
additional death certificates of individuals who had been
employed for less than 5 years in 1960. In an additional
analysis of 444 death certificates on all individuals who had
been employed for at least 6 months in the plant, 7 cases of
leukaemia and 5 cancers of the lymphatic system were found.
However, the populations at risk are not known.
A cohort of 1960 workers employed for at least 1 month in
a styrene-polystyrene manufacturing plant was followed from
1931 to 1976 (Frentzel-Beyme et al., 1978). Analysis of
deaths occurring (after a minimum period of 5 years) in groups
exposed for 5, 10, 15, and over 15 years did not indicate an
increase in cancer mortality in any of the groups compared
with the reference population. The Task Group noted, however,
that the mortality rates for the reference population were
only available for the period 1970-75, and that follow-up was
incomplete.
Ott et al. (1980) carried out a retrospective cohort study
in workers involved in the production of styrene and
polystyrene. Mortality experience was followed from 1935 to
1975. Among 282 deaths, 6 cancers of the lymphatic and
haematopoietic tissues (except leukaemia) and 6 cases of
lymphocytic leukaemia were observed, compared with 4.5 and
2.9, respectively, expected on the basis of US white male
mortality rates and 2.6 and 1.6 deaths expected from a
comparison industrial cohort. Four deaths from leukaemia were
observed in a sub-group of workers exposed to styrene, ethyl
benzene, oligomers of styrene, mineral oil, polymer dusts, and
extrusion fumes; 0.5 deaths would have been expected on the
basis of US white male mortality experience. Only one of the
4 individuals had spent at least 5 years in the working
environments under study. Inclusion of cases of leukaemia
still alive at the end of the study period or identified
through other conditions listed on the death certificate
increased the total number of lymphoreticular malignancies to
21 cases. These included 7 lymphocytic leukaemias, 4 other
leukaemias, 4 multiple myelomas, 4 Hodgkin's diseases, and 2
other lymphomas. Five of the subjects with lymphocytic
leukaemia had worked in the area of polymer extrusion and for
4 of them the disease developed 20 or more years after the
first exposure. According to the authors, there was no
increase in the general cancer incidence. The incidence of
lymphatic leukaemia was, however, more than that expected.
(b) Lamination and related processes in boat building
An epidemiological study was carried out on 1500 workers
from 36 companies in the reinforced plastics and plastic boat
industry. The majority of the workers were between 30 and 40
years old. The average period of employment was from 6 to 7
years but half of the workers had been in this type of work
for less than 3 years, while 54 had been employed for over 20
years. Exposure was estimated to be to an average styrene
concentration of 1050-1470 mg/m3 (250-350 ppm) at the end of
the sixties and the beginning of the seventies. Seventeen
cases of cancer were found in this group. In 3 of these, the
cancer was present before their employment. In the remaining
l4 cases, the sites and distribution of the various types of
tumours were in accordance with data from the cancer
registry. Among the deaths, one case of neoplasm of the
lymphatic and haematopoietic system (plasmocytoma) was
identified. However, because of the short follow-up period
and the small number of deaths it was not possible for the
authors to calculate any observed/expected figures (Ahlmark,
l978).
A study is in progress (Tola et al., 1981) in which the
tumour incidence in a cohort of 2209 workers, employed in the
manufacture of reinforced plastics in 160 workplaces, is being
investigated. Exposure to styrene vapour commenced in 1960
and the level of exposure has been estimated to range between
126 and 1260 mg/m3 (30 and 300 ppm). The majority of workers
have been exposed since 1967. Among 27 deaths, 6 deaths from
cancer were observed in this cohort (versus 8.l expected) and
the cancers were found in tissues other than lymphatic or
bone-marrow tissue. Five of the cancers appeared in workers
with 5 or fewer years of exposure. The Task Group noted the
low mortality rate.
(c) Styrene-butadiene rubber manufacture
McMichael et al. (1976) analysed the mortality experience
during the period 1940-60 in a sample of workers (exact number
not specified) taken from a population of 1482 workers in the
rubber industry. The subgroup of workers was involved in the
manufacture of styrene-butadiene rubber. For malignancies of
the lymphatic and haematopoietic systems, a relative risk
ratio of 6.2 was observed compared with other workers.
Subsequently, Smith & Ellis (1977) reported that this excess
was based on 4 cases. A case control analysis of the same
data was performed by Spirtas et al. (1976) who calculated a
relative risk ratio of 2.4 for lymphatic and haematopoietic
neoplasms among these styrene-butadiene workers. Past
exposure to solvents and other monomers was suspected.
The mortality experience of 2 cohorts of rubber workers
was analysed by Taulbee et al. (1976) using both cohort and
case-control analysis. No significant excess of lymphatic and
haematopoietic neoplasms was observed.
Case reports of leukaemias and lymphomas among styrene-
butadiene rubber workers were reported by Block (1976), Lemen
& Young (l976), and Meinhardt et al. (1978).
7.2.9.1. Summary and conclusions
There have been anecdotal reports of a small number of
cases of leukaemia and lymphoma in workers employed in the
manufacture of styrene-butadiene rubber, but the study
populations have been ill-defined and exposure to solvents and
other monomers is known to have occurred.
An association between leukaemia and exposure to styrene
in the production and polymerization process industries has
been suggested in another study. However, the cases of
leukaemia occurred in a group with a concomitant past history
of exposure to colourants, polymer fumes and, possibly,
benzene.
The effects of long-term exposure to styrene are under
investigation in at least 2 epidemiological studies on workers
employed in the industries involving styrene lamination of
glass fibre materials and related activities.
It was the opinion of the Task Group that the data
necessary to form an evaluation were inadequate and that a
causal relationship between exposure to styrene and human
cancer could not be demonstrated at present.
7.2.10. Genetic effects in somatic cells
7.2.10.1. Structural chromosome aberrations
Several studies have been published on structural
chromosome aberrations in the peripheral lymphocytes of
workers employed in the reinforced plastics industry or in the
production of styrene and polystyrene (Table 14). In these
studies, exposures have been estimated by measuring styrene
air concentrations in the workplace or by determining
concentrations of styrene metabolites, in most cases mandelic
acid, in the urine of the workers.
In Table 14, the studies are listed according to the
industrial process. Positive results have been obtained among
workers employed in polyester processing. Nine studies
included detailed data on chromosome aberrations in
individuals. One report by Watanabe et al. (1981) was
considered inconclusive because of the low number (50 or less)
of metaphases analysed per person. The other 8 studies with
detailed information were used for the final evaluation.
Three studies were reported only in abstract or review
articles (Sorsa et al., 1979; Sram, 1981; Vainio et al., 1982;
Brogger, 1982).
The study of Meretoja et al. (1978a) was an extension of
an earlier study (Meretoja et al., 1977) and included
additional analyses of samples taken from the same workers a
year later, after continuous workplace exposure.
One of the abstracts was concerned with a study on
children exposed during the fetal period, while their mothers
were working in hand lamination of polyester resin.
The only available study on chromosome aberrations among
workers employed in the production of styrene or polystyrene
was negative (Fleig & Thiess, 1978).
7.2.10.2. Other indicators of genetic damage
Seven studies were available on SCE induction in the
lymphocytes of styrene-exposed workers employed in polyester
processing (Table 14). Two of the studies were reported only
in abstracts or reviews (Sorsa et al., 1979; Brogger, 1982;
Vainio et al., 1982). A slight increase in SCEs was reported
in 2 studies (Andersson et al., 1980; Camurri et al., 1982).
In 2 of the studies, peripheral lymphocytes of polyester
processing workers were analysed for micronuclei. Meretoja et
al. (1978a) and Meretoja & Vainio (1979) reported an increase
in micronuclei and "cells connected with a nuclear bridge" in
the cultured lymphocytes of 10 workers in polyester plastic
product plants; the study included 5 controls (Table 14).
Högstedt & Mitelman (1983) found that the frequency of
micronuclei increased in 38 workers exposed for 1-23 years to
styrene (mean of time-weighted average concentrations in
breathing zone 55 mg/m3 or l3 ppm, range 4-168 mg/m3 or 1-40
ppm) compared with 20 controls.
Pero et al. (1982) did not find any increase in
unscheduled DNA synthesis (UDS) in isolated lymphocytes of 38
workers in a fibreglass-reinforced polyester plastic factory
compared with 20 unexposed controls. Styrene air
concentrations in the workroom varied between 4 and 168 mg/m3
(1 and 40 ppm).
In an evaluation of the incidence of malformed children
and miscarriages among spouses of men exposed to styrene in a
reinforced-plastic boat factory, Andersson et al. (1980)
concluded that the number of pregnancies (39 exposed and 41
controls) was too small to reveal mutagenic effects.
7.2.10.3. Conclusions
The available evidence suggests that styrene exposure in
the reinforced plastics industry with its more intensive
exposures is associated with an increased frequency of
structural chromosomal abnormalities.
7.3. Effects on Reproductive Function and Teratogenic Effects
In a case-reference study on 63 pairs of mothers from a
register of congenital malformations (Holmberg, 1977), 2
mothers with children having central nervous system defects
had been employed in the reinforced plastics industry during
pregnancy. A third case-mother was also found, who had been
exposed to styrene at home.
In a study on spontaneous abortions registered in
hospitals (Hemminki et al., 1980a) during the period 1973-76,
the following numbers and rates of abortion were found:
- General population 15 482 (5.52%);
- Union of Chemical Workers 52 (8.54%);
- Plastics industry 21 (8.94%);
- Styrene production and use 6 (15%).
The abortion rates for the occupational groups were
statistically different from the controls. The abortion rates
increased with age in the general population, while they were
found to decrease with age among the Union of Chemical
Workers. No data were given concerning the relationship with
age of spontaneous abortions among women employed in the
plastics industry or in styrene production and use; thus the
significance of the increased abortion rates among these
groups cannot be assessed.
Table 14. Summary on studies of structural chromosome aberrations and sister chromatid exchanges (SCEs) in the lymphocytes of
workers exposed to styrene.
------------------------------------------------------------------------------------------------------------------------------------
No. persons Measured Years of Urinary man- Cells with SCEs/cell
studied concentration exposure delic acid chromosome
Industry styrene in (mg/g of aberrations,
branch air (mg/m3) creatinine) gaps included (%)a
---------------------------------------------------------------------------------------------------------------------
Reference Exposed Controls Averagea Range Meana Range Meana Range Exposed Controls Resultb Exposed Controls Resultb
------------------------------------------------------------------------------------------------------------------------------------
Styrene
manufacture
Flieg & 5 20 ~2c 0-29c 21.6 14-25 ~30d 19- 3.8 5.5 - .. .. ..
Thiess (1978) 40d
Polystyrene
manufacture
Flieg & 12 20 ~8c 0- 20.3 3-39 ~32d <5- 5.1 5.5 - .. .. ..
Thiess (1978) (12)e 197c 100d (5.5)e .. .. ..
Polyester
processing
Meretoja et 10 5 .. .. 3.2 0.6- 721 23- 16.6 1.8 + .. .. ..
al. (1977); 8.5 3257
Meretoja &
Vainio (1979)
Meretoja et 16 6 .. up to 6.3 1-15 570 23- 15.1 2.0 + .. .. ..
al. (1978b) 1260 3257
(a year 11 3f .. up to 8.1 2-16 329 52- 16.2 .. + 5.3 4.4 -
later) 1260 1646
Flieg & 14 20 ~477c <210- 7.9 2-24 ~593 42- 9.2 5.5 + .. .. ..
Thiess (1978) 1260c >1500
Andersson et 36 37 ~197 0- 5.0 0.3- .. .. 12.3 6.7 + 9.7 8.7 +
al. (1980) 20f 20f 1008 12
Watanabe et 16 13 151- 0-886 ~4.5 0.6- ~594 90- 2.9 3.2 (-)g 13.5 13.9 -
al. (1981) <294 9.3 2640
Watanabe et 18 6 168- <5- 9.7 <1-30 332 1- 6.5 4.7 ± 8.9 8.5 -
al. (1983) 210 1075 ~1040
Thiess et al. 24 24 25- 3-748 ~14.4 4-27 .. 0- 5.1 3.8 +h .. .. ..
(1980, 1982) 244 320c
------------------------------------------------------------------------------------------------------------------------------------
Table 14. (contd.)
------------------------------------------------------------------------------------------------------------------------------------
No. persons Measured Years of Urinary man- Cells with SCEs/cell
studied concentration exposure delic acid chromosome
Industry styrene in (mg/g of aberrations,
branch air (mg/m3) creatinine) gaps included (%)a
---------------------------------------------------------------------------------------------------------------------
Reference Exposed Controls Averagea Range Meana Range Meana Range Exposed Controls Resultb Exposed Controls Resultb
------------------------------------------------------------------------------------------------------------------------------------
Camurri et al. 24 21 .. 30- 9.4 1-22 472c 45- ~35.1i ~8.4i + 13.9 10.8 +
(1982)k (22)f (20)f >400 1108c
Abstracts
Sram (1981) 36 19 .. .. .. .. .. .. 1.4i 1.3 - .. .. ..
(cited data 34j (1.4)ij
of Pchlova & 22 22 .. .. .. .. .. .. 1.7i 1.4i - .. .. ..
Sram)
Brogger 13 0 129 15- 9.8 1-24 527 292- 6.4 .. (-)g 6.6 .. (-)g
(1982)k (8)f 364 688
Sorsa et al. 6 10 .. .. .. 2-7 .. .. 8.8 3.0 + 7.5 8.5 -
(1979);
Vainio et al. 7 10 .. .. .. (5-8 .. .. 4.9 1.6 + 4.9 5.2 -
(1982) ( in months)
utero exposed
children)
Högstedt et 6 6 ~162 60- 4.0 0.5- 490 225- 10.2 4.9 + .. .. ..
al. (1979) 800 10 2100
------------------------------------------------------------------------------------------------------------------------------------
a Estimated from the data given if not indicated in the report.
b Compared to response in control persons: + increase; - no effect.
c From Thiess & Friedheim (1978).
d mg/litre urine.
e Controls in Thiess & Fleig (1978).
f For the analysis of sister chromatid exchanges only.
g Inconclusive because of the low number of cells analysed (Watanabe et al., 1981) or lack of controls (Brogger, 1982).
h Negative if gaps are excluded.
i Gaps excluded.
j 6 months later.
k Details, not mentioned in the report, were obtained from the author(s).
In a small-scale study, Härkönen & Holmberg (1982)
recently interviewed 67 female lamination workers of child-
bearing age and a similar number of textile and food
production workers who were thought to be proper controls.
The groups were reported not to differ either in their
menstrual behaviour or in the number of spontaneous
abortions. The number of deliveries was found to be lower
among the styrene workers, which may be partially explained by
the higher number of induced abortions in the styrene-exposed
group.
8. EXPOSURE-EFFECT/EXPOSURE-RESPONSE RELATIONSHIPS, AND
EVALUATION OF HEALTH EFFECTS
8.1. Data from Experimental Animal Studies
8.1.1. Metabolic pathways and kinetics
Pulmonary uptake in man is of greatest importance though uptake
through the skin occurs. Styrene is biotransformed largely via the
7,8-epoxide by the mixed function oxidase system.
The kinetic data show that exposure of experimental animals to
increasing levels of styrene results in progressive saturation of
the metabolic pathways. The half-time of styrene disappearance
from the body varies with the dose. The range of exposures tested
was from 189 mg/m3 (45 ppm) to 10 500 mg/m3 (2500 ppm). The
consequence of such metabolic saturation, which has been reported
to occur at about 2520 mg/m3 (600 ppm) in air, is an increase in
the proportional deposition of styrene in fat. In human beings the
half-time for the elimination of styrene from adipose tissue is 2-3
days.
The principal urinary metabolites are mandelic and
phenylglyoxylic acids. Recent evidence suggests that the
pattern of urinary metabolite excretion varies with mammalian
species.
8.1.2. General toxicity
8.1.2.1. Acute toxicity
A mean odour detection threshold for styrene of 3.06 mg/m3
(0.73 ppm) was found for unadapted subjects. The odour was
reported to be strong but not objectionable at about 420 mg/m3
(l00 ppm). With short-term exposures at concentrations
exceeding 840 mg/m3 (200 ppm), styrene vapour was irritating
to the eyes and nose.
The central nervous system effects begin to appear in the
range of 210-840 mg/m3 (50-200 ppm) and distinct impairment of
reaction time and body balance were found at concentrations
exceeding 840 mg/m3 (200 ppm).
The acute toxicity of styrene in animals is rather low:
the LD50 in rats has been reported to be 5 g/kg body weight
after oral administration and 2-3 g/kg body weight after
intraperitoneal injection. Exposure of rats to styrene vapour
at 1300 to 10 000 ppm for up to 4 h caused nasal mucous
membrane and eye irritation as well as acute depression of the
central nervous system. Marked pulmonary lesions were also
noted.
There were no kidney changes in animals exposed to 5460
mg/m3 (l300 ppm) after a single exposure. Severe irritation
of the eyes and nose was observed in rats and guinea-pigs
after exposure to concentrations of 2730-5460 mg/m3 (650 - 1300
ppm) and general weakness and unsteadiness gradually developed
after 12 h.
Acute toxic effects were observed in mice exposed to a
styrene level of 21 000 mg/m3 (5000 ppm) for 2 h and
guinea-pigs exposed to 10 920 mg/m3 (2600 ppm) for 8 h. A
short-term exposure to 1260 mg/m3 (300 ppm) in the air caused
only very slight behavioural effects in rats, even though
pronounced neurochemical changes were observed.
The high exposure levels used in studies on experimental
animals have not been observed in the human environment.
8.1.2.2. Subacute and chronic toxicity
Inhalation of styrene vapour by rats at a concentration of
5460 mg/m3 (1300 ppm) for 7 h/day over several months did not
have any deleterious effects on the kidney and did not cause
any changes in the erythrocyte count, haemoglobin
concentration, and leukocyte count. There were not any
essential morphological changes in the rat lungs. Similar
results were obtained with rabbits, monkeys, and guinea-pigs
except that 10% of the guinea-pigs died after the first
exposure with signs of lung irritation. An exposure level of
2730 mg/m3 (650 ppm), for 7 h/day for 214-360 days, appeared
not to have any effects on the lungs of guinea-pigs.
Rats exposed by inhalation to a styrene concentration of
1260 mg/m3 (300 ppm) (6 h/day, 5 days a week) for up to 11
weeks developed axonal protein changes in the brain, an
induction of drug metabolizing enzymes in the kidney and the
liver, histological alterations in the liver, and a depletion
of the glutathione (GSH) contents of the kidney and the liver.
No significant depletion of GSH occurred at 420 mg/m3 (100
ppm).
Oral subacute studies were carried out on rats and beagle
dogs. The rat study was carried out on females and exposure
induced only weight increases in the liver and kidney. No
effect was observed at a dose of 133 mg/kg body weight. The
study on dogs lasted more than 19 months and showed a mild
haemolytic anaemia characterized by Heinz body production.
Effects were observed down to the lowest dose tested (200
mg/kg body weight).
8.1.3. Genetic effects
Styrene is a potential mutagen only after metabolic activation.
Mutagenicity tests in bacteria, yeasts and mammalian cells in vitro
have yielded contradictory results for styrene that may be due to
differences in the efficiency of activation and inactivation of
styrene in different studies.
Conclusive dose-effect relationships could only be established
in several in vitro studies.
The results of tests for chromosomal damage induced by
styrene in experimental mammals have been contradictory.
Three positive studies have been reported; 2 in mice, and 1 in
rats. A dose-effect relationship was only observed for the
induction of SCE in mice exposed to styrene in air levels of
more than 1260 mg/m3 (300 ppm). Five other animal studies
have given negative results. The different end-points, routes
of exposure, durations of treatment, species and strains of
animals used, and the small number of mammalian studies
available, make it impossible to draw definitive conclusions
concerning dose-effect relationships.
8.1.4. Carcinogenic effects
Oral administration of styrene to mice induced a
significant increase in pulmonary tumours in the O20 strain at
a dose of 1350 mg/kg body weight and a doubtful increase in
B6C3F1 mice at 300 mg/kg body weight. No significant increase
in tumour incidence was observed in the C57B1 strain, when
styrene was administered pre- and post-natally at 300 mg/kg
body weight.
In 2 studies on rats, styrene given orally at doses
ranging from 500 to 1350 mg/kg body weight did not induce a
significant increase in tumour incidence. A greater incidence
of lymphomas, observed in an inhalation study on rats exposed
to a styrene concentration of 2520 or 4200 mg/m3(600 or 1000 ppm),
could not be causally related to styrene.
Thus, the evidence available indicates that styrene at the
doses administered (1350 mg/kg body weight for 020 strain)
caused an increase in pulmonary tumours in mice.
Styrene 7,8-oxide induced squamous cell carcinomas in the
forestomach of rats given doses of 50 and 250 mg/kg body weight.
In a second study on rats, both squamous cell carcinomas and
pulmonary carcinomas were induced, when styrene oxide was given at
a dose of 100 - 150 mg/kg body weight per day, 4-5 days a week.
When styrene oxide was painted on the skin of mice at
concentrations of 50 or 100 g/kg, no tumours of the skin were
induced.
8.2. Human Studies
8.2.1. Effects on organs and systems
Effects on the following organ systems were investigated:
nervous, haematopoietic and immune systems, the kidney and
urinary tract, the gastrointestinal tract, liver,
cardiovascular and respiratory systems, and the endocrine
organs.
Most studies of the haematopoietic system did not produce
any positive data; in one study, a slight decrease in
thrombocyte count in workers exposed to styrene for more than
ten years was detected.
A clear-cut trend towards altered liver function has not been
demonstrated. At low exposure concentrations, the commonly used
parameters, such as activity of serum enzymes of hepatic origin,
except for gamma-GTP, have given equivocal results.
Slight effects on the lower respiratory system were noted
in some studies.
No adequate data were available to the Task Group to
establish dose-effect or dose-response relationships for the
aforementioned systems.
Slight disturbances of visuomotor accuracy and psychomotor
performance were noted at styrene levels exceeding 210 mg/m3
(50 ppm), and an increased incidence of abnormalities in
electroencephalograph recordings was detected at styrene
concentrations below 420 mg/m3 (100 ppm); relationships
between the exposure levels and the severity of these effects
and the response rates were observed.
While the reaction times were shown to be prolonged for
exposure levels below 630 mg/m3 (150 ppm), dose-response
relationships could not be established.
8.2.2. Genetic effects in somatic cells
Knowledge concerning the possible genetic effects of
styrene exposure on man is still inadequate for any
dose-response extrapolations. Available information is
limited to structural somatic chromosome damage in the
peripheral blood lymphocytes of workers occupationally exposed
to high concentrations of styrene in the reinforced plastics
industry.
Data on individual exposure profiles are necessary for
meaningful interpretation of the results of somatic cell
analysis. Furthermore, the role of high occasional peak
exposures and low continuous workplace exposures in the
induction of chromosome damage in lymphocytes in styrene-
exposed workers is not clear. Considering the survival time
of the target lymphocytes, exposure data should be available
for a period of at least 2-3 years preceding chromosome
analysis.
Based on available published data, the following
conclusions can be made:
1. No clear dose-response pattern can be recognized.
Positive results of chromosome aberrations in
styrene-exposed workers were restricted to the
reinforced plastics industry, where styrene
concentrations in air were high. Negative results
have been obtained in the manufacture of styrene or
polystyrene where exposures to styrene are lower.
2. Variability in the chromosome aberration frequencies
reported in different studies is great. This
suggests that several factors such as exposure
conditions, the method, and the analytical criteria
selected, influence the results.
3. Individual differences are great and may reflect
variations in exposure or differences in
susceptibility.
The Task Group concluded that the health significance of
structural chromosomal aberrations in the somatic cells of
persons exposed to styrene could not be assessed at present,
but that such effects were undesirable.
8.2.3. Carcinogenic effects
Several case reports and epidemiological investigations
have implied an increased risk of lymphatic and haematopoietic
system cancer in workers involved in the application of
styrene, polystyrene, and styrene-butadiene rubber. However,
at present, there is not sufficient evidence to establish a
direct cause and effect relationship between styrene exposure
and cancer in human beings. Assessment of data has frequently
been complicated by concomitant exposure to other volatile
substances.
The Task Group concluded that, to date, a causal
relationship between styrene exposure and cancer could not be
established in man.
REFERENCES
AHLMARK, A. (1978) [Styrene study. Epidemiological report.]
Stockholm, Sveriges Plastförbund, 21 pp (in Swedish).
ALARIE, Y. (1973) Sensory irritation of the upper airways by
airborne chemicals. Toxicol. appl. Pharmacol., 24: 279-297.
AMACHER, D.E. & TURNER, G.N. (1982) Mutagenic evaluation of
carcinogens and non-carcinogens in the L5178Y/TK assay
utilizing postmitochondrial fractions (S9) from normal rat
liver. Mutat. Res., 97: 49-65.
ANDERSSON, H.C., TRANBERG, E.C., UGGLA, A.H., & ZETTERBERG, G.
(1980) Chromosomal aberrations and sister-chromatid exchanges
in lymphocytes of men occupationally exposed to styrene in a
plastic-boat factory. Mutat. Res., 73: 387-401.
ASKERGREN, A., ALLEN, L.-G., KARLSSON, D., LUNDBERG, I., &
NYBERG, E. (1981) Studies on kidney function in subjects
exposed to organic solvents. I. Excretion of albumin and -2
microglobulin in the urine. Acta Med. Scand., 209: 479-483
ASTRAND, I., KILBOM, C., OVRUM, P., & WAHLBERG, I. (1974)
Exposure to styrene. I. Concentration in alveolar air and
blood at rest and during exercise and metabolism. Work
environ. Health, 11: 69-85.
AXELSON, O. & GUSTAVSON, J. (1978) Some hygienic and clinical
observations on styrene exposure. Scand. J. Work Environ.
Health, 4 (Suppl. 2): 215-219.
AXELSON, O., FROBARJ, G., & WEDEFELT, U. (1974) [Can styrene
exposure cause states with cerebral lesion.] Läkartidningen,
71: 137-138 (in Swedish).
BABINA, M.D. (1969) [Spectrophotometric determination of
styrene and benzaldehyde in the work environment.] Gig. i
Sanit., 34: 105-106 (in Russian).
BAGGET, M.S., MORIA, G.P., SIMMONS, M.W., & LEWIS, J.S. (1974)
Quantitative determination of semivolatile compounds in
cigarette smoke. J. Chromatogr., 97: 79-82.
BAKKE, O.M. & SCHELINE, R.R. (1970) Hydroxylation of aromatic
hydrocarbons in the rat. Toxicol. appl. Pharmacol., 16:
691-700.
BARDODEJ, Z. (1964) [Styrene metabolism.] Cesk. Hyg., 9:
223-239 (in Czech).
BARDODEJ, Z. (1978) Styrene, its metabolism and the
evaluation of hazards in industry. Scand. J. Work Environ.
Health, 4 (Suppl. 2): 95-103.
BARDODEJ, Z. & BARDODEJOVA, E. (1970) Biotransformation of
ethyl benzene, styrene and alphamethyl styrene in man.
Am. Ind. Hyg. Assoc. J., 31: 206-209.
BARDODEJ, A., MALEK, B., VOLFOVA, B., & ZELENA, E. (1960)
[The hazard of styrene in the production of glass laminates.
Cesk. Hyg., 5: 541-546 (in Czech).
BARDODEJ, Z., BARDODEJOVA, E., & MALEK, B. (1961) [Value and
application of exposure tests. XI. Exposure test for
styrene.] Cesk. Hyg., 6: 546-552 (in Czech).
BARDODEJ, Z., BARDODEJOVA, E., & GUT, I. (1971) [Metabolosm
of styrene in rats.] Cesk. Hyg., 16: 243-245 (in Czech).
BARSOTTI, M., PARMEGGIANI, L., & SASSI, C. (1952)
[Observations on occupational pathology in a polystyrene resin
factory.] Med. Lav., 43: 418-424 (in Italian).
BASIROV, A.A. (1968) [Gastric function in workers of the
synthetic rubber industry.] Vrac. Delo, 4: 200-203 (in
Russian).
BASIROV, A.A. (1975) [Biochemical indexes of the gastric
juice in the early diagnosis of stomach illnesses under the
effect of toxic substances (1,3-butadiene and styrene).
Azerb. Med. Zh., 52: 60-66 (in Russian).
BAUER, C., LEPORINI, C., BRONZETTI, G., CORSI., C., NIERI, R.,
DEL CARRATONE, R., & TONARELLI, S. (1980) The problem of
negative results for styrene in the in vitro mutagenesis test
with metabolic activation (microsomal assay): explanation by
gas chromatographic analysis. Boll. Soc. It. Biol. Sper., 56:
203-207.
BAUER, C., LEPORINI, C., BRONZETTI, G., CORSI, C., NIERI, R.,
DEL CARRATONE, R., & TONARELLI, S. (1981) Studies on the
incubation mixtures for the in vitro mutagenesis test with
metabolic activation (microsomal assay) - behaviour of some
activating and detoxifying enzyme systems during incubations.
The case of styrene. Mutat. Res., 85: 268.
BAUER, D. & GUILLEMIN, M. (1976) Human exposure to styrene:
I. The gas-chromatographic determination of urinary
phenylglyoxylic acid using diazomethane derivatization.
Int. Arch. occup. environ. Health, 37: 47-55.
BEIJE, B. & JENSSEN, D. (1982) Investigation of styrene in
the liver perfusion/cell culture system. No indication of
styrene-7,8-oxide as the principal mutagenic metabolite
produced by the intact rat liver. Chem. biol. Interact., 39:
57-76.
BELVEDERE, G. & TURSI, F. (1981) Styrene oxidation to styrene
oxide in human blood erythrocytes and lymphocytes. Res. Comm.
Chem. Pathol. Pharmacol., 33: 273-282.
BELVEDERE, G., CANTONI, L., TACCHINETTI, T., & SALMONA, M.
(1977) Kinetic behaviour of microsomal styrene monooxygenase
and styrene epoxide hydratase in different animal species.
Experientia (Basle), 33: 708-709.
BENCEV, I.V. & RIZOV, N. (1981) [Gas-chromatographic method
for styrene determination blood (headspace method).]
Prob. Hyg., VI: 88-91 (in Russian).
BERGMAN, K. (1977) [Exposure to styrene in plastic boat
industry, I. Technical-hygienic study.] Arbete Hälsa, 3: 1-9
(in Swedish).
BERGMAN, K. (1979) Whole body autoradiography and allied
techniques. Scand. J. Work Environ. Health, 5 (Suppl. 1):
93-120.
BERTSCH, W., ANDERSON, E., & HOLZER, G. (1975) Trace analysis
of organic volatiles in water by gas chromatography-mass
spectrometry with glass capillary columns. J. Chromatogr.,
112: 701-718.
BIDOLI, F., AIROLDI, L., & PANTAROTTO, C. (1980) Quantitative
determination of styrene-7,8-oxide in blood by combined gas
chromatography-multiple ion detection mass fragmatography.
J. Chromatogr., 196: 314-318.
BLOCK, J.B. (1976) A Kentucky study: 1950-1975. In: Ede, L.,
ed. Proceedings of NIOSH Styrene-Butadiene Briefing,
Covington, Kentucky, USA, 1976, Cincinnati, Ohio, US
Department of Health, Education and Welfare pp. 28-32 (HEW
Publ. No. (NIOSH) 77-129).
BODNEI, A.H., BUTLER, G.J., & OKAWA, M.T. (1974) Health
hazard evaluation/toxicity determination. Cincinnati, US
Department of Health, Education and Welfare, Public Health
Service, Center for Disease Control, National Institute for
Occupational Safety and Health, 10 pp (Report no. 73-103-128 -
American Standard Fiberglass Inc., Stockton, California).
BONATTI, S., ABBONDANDOLO, A., CORTI, G., FIORIO, R., &
MAZZACCARO, A. (1978) The expression curve of mutants induced
by styrene oxide at the HGPRT locus in V79 cells.
Mutat. Res., 52: 295-300.
BROGGER, A. (1982) Application of SCE to public health. In:
Sandberg, A., ed. Sister chromatid exchange, New York, A.R.
Liss, pp. 655-673.
BROOKS, S.M., ANDERSON, L.A., TSAY, J.-Y., CARSON, A.,
BUNCHER, C.R., ELIA, V., & EMMETT, E.A. (1979) Investigation
of workers exposed to styrene in the reinforced plastic
industry - health and psychomotor status, toxicological and
industrial hygiene data and effect of protective equipment as
it relates to exposures through lung and skin routes.
Cincinnati, University of Cincinnati, College of Medicine,
pp. 330. (Institute of Environmental Health and Kettering
Laboratory Report Prepared for the Society of Plastics
Industries).
BROOKS, S., ANDERSON, L., EMMETT, E., CARSON, A., TSAY, J.-Y.,
ELIA, V., BUNCHER, R., & KARBOWSKY, R. (1980) The effects of
protective equipment on styrene exposure in workers in the
reinforced plastics industry. Arch. environ. Health, 35:
287-294.
BROUNS, R.E., POOT, M., DE VRIND, R., V. HOEK-KON, T.,
HENDERSON, P.T., & KUYPER, C.M.A. (1979) Measurement of
DNA-excision repair in suspensions of freshly isolated rat
hepatocytes after exposure to some carcinogenic compounds.
Its possible use in carcinogenicity screening. Mutat. Res.,
64: 425-432.
BUCHET, J.-P., LAUWERYS, R., ROELS, H., & DEFELD, J.-M.
(1974) [Evaluation of the exposure of workers to styrene by
means of the determination of its urinary metabolites:
mandelic and phenylglyoxylic acids: I. Technique of
determination of the metabolites by gas chromarography.
Arch. Mal. prof. Med. Tra. Secur. soc., 35: 511-516.
BURKIEWICZ, C., RYBKOWSKA, J., & ZIELINSKA, H. (1974)
[Assessment of exposure to styrene in human beings under
industrial conditions.] Med. Prac., 3: 305-310 (in Polish).
BURKOVA, T., BAJNOVA, A., & KAPURDOV, V. (1982) Occupational
risk with repeated styrene dermal contact. Prob. Hyg., VII:
51-59.
BURNETT, R.D. (1976) Evaluation of charcoal sampling tubes.
Am Ind. Hyg. Assoc. J., 37: 37-45.
BUSK, L. (1979) Mutagenic effects of styrene and styrene
oxide. Mutat. Res., 67: 201-208.
CAMURRI, L., CODELUPPI, S., & PEDRONI, C. (1982) Chromosomal
aberrations and sister chromatid exchanges in styrene exposed
workers. In: 12th Annual Meeting of European Environmental
Mutagen Society, Espoo, Finland, 20-24 June, 1982 Abstracts,
Helsinki, Institute of Occupational Health, p. 159.
CANTONI, L., SALMONA, M., FACCHINETTI, T., PANTAROTTO, C., &
BELVEDERE, G. (1978) Hepatic and extrahepatic formation and
hydration of styrene oxide in vitro in animals of different
species and sex. Toxicol. Lett., 2: 179-186.
CAPEROS, J.R., HUMBERT, B., & DROZ, P.O. (1979) Exposition on
styrene. II. Bilan de l'absorption, de l'excrétion et du
métabolisme sur des sujets humains. Int. Arch. occup.
environ. Health, 42: 223-230.
CARLSSON, A. (1981) Distribution and elimination of
14C-styrene in rat. Scand. J. Work Environ. Health, 7: 45-50.
CARPENTER, C.P., SHAFFER, C.B., WEIL, C.S., & SMYTH, H.F.
(1944) Studies on the inhalation of 1:3-butadiene; with a
comparison on its narcotic effect with benzol, tuluol, and
styrene, and a note on the elimination of styrene by the
human. J. ind. Hyg., 26: 69-78.
CERNA, M. & KYPENOVA, H. (1977) Mutagenic activity of
chloroethylenes analysed by screening system tests.
Mutat. Res., 46: 35-36.
CHEMICAL MARKET REVIEW (1981) Oct. 26, p. 7.
CHERRY, N., WALDRON, H.A., WELLS, G.G., WILKINSON, R.T.,
WILSON, H.K., & JONES, S. (1980) An investigation of the
acute behavioral effects of styrene on factory workers.
Br. J. ind. Med., 37: 234-240.
CHMIELEWSKI, J. & RENKE, W. (1975) Clinical and experimental
research into the pathogenesis of toxic effects of styrene
III. Morphology, coagulation and fibrinolisis systems of the
blood in persons exposed to the action of styrene during their
work. Biul. Inst. Med. Morskej, 26: 299-302.
CHMIELEWSKI, J., MIKULSKI, P., USELIS, J., & WIGLUSZ, R.
(1973) Rating of the exposure to styrene of persons working
at the production of polyester laminates. Biul. Inst. Med.
Morskej Gdansku, 24: 203-209.
CHMIELEWSKI, J., DOLMIERSKI, R., RENKE, W., & KWIATKOWSKI,
S.R. (1977) [Long-term occupational effects of styrene on
working people.] Z. gesamte Hyg. Grenzgeb., 23: 639-643 (in
German).
CHOI, K.K. & FUNG, K.W. (1979) Determination of styrene in
the atmosphere near industrial sites by gas chromatography.
Analyst, 104: 455-457.
CHU, K.C., CUETO, C., Jr, & WARD, J.M. (1981) Factors in the
evaluation of 200 National Cancer Institute Bioassays.
J. Toxicol. Environ. Health, 8: 251-280.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1979) Sister
chromatid exchange in regenerating liver an bone marrow cells
of mice exposed to styrene. Toxicol. appl. Pharmacol., 50:
365-367.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1980) Sister
chromatid exchange in murine alveolar macrophages, bone
marrow, and regenerating liver cells induced by styrene
inhalation. Toxicol. appl. Pharmacol., 55: 37-42.
CONNER, M.K., ALARIE, Y., & DOMBROSKE, R.L. (1982) Multiple
tissue comparisons of sister chromatid exchanges induced by
inhaled styrene. In: Tice. R., Costa, D.L., & Schaich, K.M.,
ed. Genotoxic effects of airborne agents, New York, Plenum
Press, pp. 433-441.
CRANDALL, M.S. (1981) Worker exposure to styrene monomer in
the reinforced plastic boat-making industry. Am. Ind. Hyg.
Assoc. J., 42: 499-502.
CRITTENDEN, B.D. & LONG, R. (1976) The mechanisms of
formation of polynuclear aromatic compounds in combustion
systems. In: Carcinogenesis - A comprehensive survey - Volume
I, New York, Raven Press, pp. 209-223.
DANISHEFSKY, I. & WILLHITE, M. (1954) The metabolism of
styrene in the rat. J. Biol. Chem., 211: 549-553.
DELBRESSINE, L.P.C., KETELAARS, H.C.J., SEUTER-BERLAGE, F., &
SMEETS, F.L.M. (1980) Phenaceturic acid a new urinary
metabolite of styrene in the rat. Xenobiotica, 10: 337-342.
DEL CARRATORE, R., BRONZETTI, G., BAUER, C., CORSI, C., NIERI,
R., PAOLINI, M., & GIAGONI, P. (1982) Study of cytochrome
P450 in yeast D7 strain. An alternative model to microsomal
assay. Mutagenicity of styrene. In: 12th Annual Meeting of
the European Environmental Mutagen Society, Espoo, Finland,
20-24 June, 1982, Abstracts, Helsinki, Institute of
Occupational Health, p. 160.
DE MEESTER, C., PONCELET, F., ROBERFROID, M., RONDELET, J., &
MERCIER, M. (1977) Mutagenicity of styrene and styrene oxide.
Mutat. Res., 56: 147-152.
DE MEESTER, C., DUVERGER-VAN BOGAERT, M., LAMBOTTE-VANDEPAER,
M., MERCIER, M., & PONCELET, F. (1981) Mutagenicity of
styrene in the Salmonella typhimurium test system.
Mutat. Res., 90: 443-450.
DIETRICH, M.W., CHAPMAN, L.M., & MIEURE, J.P. (1978) Sampling
for organic chemicals in workplace athmosphere with porous
polymer beads. Am. Ind. Hyg. Assoc. J., 39: 385-392.
DOLMIERSKI, R., KWIATKOWSKI, S.R., & NITKA, J. (1974) A
preliminary evaluation of the studies on central nervous
system in workers exposed to styrene. Biul. Inst. Med.
Morskiej Gdansku, Gdansk, 25: 399-406.
DONNER, M., SORSA, M., & VAINIO, H. (1979) Recessive lethals
induced by styrene and styrene oxide in Drosophila
melanogaster. Mutat. Res., 67: 373-376.
DRINKWATER, N.R., MILLER, J.A., MILLER, E.C., & YANG, N.-C.
(1978) Covalent intercalative binding to DNA in relation to
the mutagenicity of hydrocarbon epoxides and N-acetoxy-2-
acetylaminofluorene. Cancer Res., 38: 3247-3255.
DROZ, P.O. & FERNANDEZ, J.G. (1977) Effect of physical work
load on retention and metabolism of inhaled organic solvents.
A comparative theoretical approach and its application with
regards to exposure monitoring. Int. Arch. occup. environ.
Health, 38: 231-246.
DUTKIEWICZ, T. & TYRAS, H. (1967) A study of the skin
absorption of ethylbenzene in man. Br. J. ind. Med., 24:
330-332.
DUTKIEWICZ, T. & TYRAS, H. (1968) Skin absorption of toluene,
styrene and xylene by man. Br. J. ind. Med., 25: 243.
DUVERGER-VAN BOGAERT, M., NOEL, G., ROLLMAN, B., CUMPS, J.,
ROBERFROID, M., & MERCIER, M. (1978) Determination of oxide
synthetase and hydratase activities by a new highly sensitive
gas chromatographic method using styrene and styrene oxide as
substrates. Biochim. Biophys. Acta, 526: 77-84.
ELIA, V.J., ANDERSON, L.A., MacDONALD, T.J., CARSON, A.,
BUNCHER, C.R., & BROOKS, S.M. (1980) Determination of urinary
mandelic and phenylglyoxylic acids in styrene exposed workers
and a control population. Am. Ind. Hyg. Assoc. J., 41:
922-926.
EL-MASRI, A.M., SMITH, J.N., & WILLIAMS, R.T. (1958) Studies
on detoxication. The metabolism of alkylbenzenes:
phenylacetylene and phenylethylene (styrene). Biochem. J.,
68: 199-204.
ELOVAARA, E., VAINIO, H., PFAFFLI, P., & COLLAN, Y. (1979)
Effects of intermittent styrene inhalation, ethanol intake and
their combination on drug biotransformation in rat liver and
kidneys. Med. Biol., 57: 321-327.
EL-TANTAWY, M.A. & HAMMOCK, B.D. (1980) The effect of hepatic
microsomal and cytosolic subcellular fractions on the
mutagenic activity of epoxide-containing compounds in the
Salmonella assay. Mutat. Res., 79: 59-71.
ENGSTROM, J. (1978) Styrene in subcutaneous adipose tissue
after experimental and industrial exposure. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 119-120.
ENGSTROM, J., USTRAND, I., & WIGAEUS, E. (1978a) Exposure to
styrene in a polymerization plant. Uptake in the organism and
concentration in subcutaneous adipose tissue. Scand. J. Work
Environ. Health, 4: 324-329.
ENGSTROM, J., BJURSTROM, R., USTRAND, I., & OVRUM, P. (1978b)
Uptake, distribution and elimination of styrene in man.
Concentration in subacutaneous adipose tissue. Scand. J. Work
Environ. Health, 4: 315-323.
ENGSTROM, K. (1983) In: Aitio, A., Riihimäki, V., & Vainio,
H., ed. Biological monitoring of exposure to industrial
chemicals, New York, Hemisphere (in press).
ENGSTROM, K. & RANTANEN, J. (1974) A new gas chromatographic
method for determination of mandelic acid in urine. Int.
Arch. Arbeitsmed., 33: 163-167.
ENGSTROM, K., HARKONEN, H., PEKARI, K., & RANTANEN, J. (1978)
Evaluation of occupational styrene exposure by ambient air and
urine analysis. Scand. J. Work Environ. Health, 4 (Suppl. 2):
121-123.
ENGSTROM, K., HARKONEN, H., KALLIOKOSKI, P., & RANTANEN, J.
(1976) Urinary mandelic acid concentration after occupational
exposure to styrene and its use as a biological exposure test.
Scand. J. Work Environ. Health, 2: 21-26.
FABRY, L., LEONARD, A., & ROBERFROID, M. (1978) Mutagenicity
tests with styrene oxide in mammals. Mutat. Res., 51: 377-381.
FAITH, W.L., KLYES, D.B., & CLARK, R.L. (1975) Industrial
chemicals, 4th ed., New York, John Wiley and Sons, Inc., pp.
365-370, 779-784.
FERNANDEZ, J.G. & CAPEROS, J.R. (1977) Exposition au styrène.
I. Etude expérimentale de l'absorption et l'excrétion
pulmonaires sur des sujets humains. Int. Arch. occup.
environ. Health, 40: 1-12.
FIELDS, R.L. & HORSTMAN, S.W. (1979) Biomonitoring of
industrial styrene exposures. Am. Ind. Hyg. Assoc. J., 40:
451-459.
FINLEY, J.W. & WHITE, J.C. (1967) Two methods to determine if
styrene monomer is present in milk, Bull. environ. Contam.
Toxicol., 2(1): 41-46.
FISEROVA-BERGEROVA, V. & TEISINGER, J. (1965) Pulmonary
styrene vapor retention. Ind. Med. Surg., 34: 620-622.
FISHBEIN, L. (1979) Chromatography of environmental hazards,
Volume II, Amsterdam, Elsevier, pp. 455-469.
FLEIG, I. & THIESS, A.M. (1978) Mutagenicity study of workers
employed in the styrene and polystyrene processing and
manufacturing industry. Scand. J. Work Environ. Health, 4
(Suppl. 2): 254-258.
FLEK, J. & SEDIVEC, V. (1980) Simultaneous gas
chromatographic determination of urinary mandelic and
phenylglyoxylic acids using diazomethane derivatization. Int.
Arch. occup. environ. Health, 45: 181-188.
FLEMING, R.D. (1970) Effect of fuel composition on exhaust
emissions from a spark-ignition engine, Washington DC, US
Department of the Interior, Bureau of Mines (Report of
Investigations 7423).
FOERST, W. (1965) [Styrene.] In: Ullmans Encyklopädie der
technischen Chemie (Vol. 16), Munich, Urban et Schwarzenberg,
pp. 460-476 (in German).
FRENTZEL-BEYME, R., THEISS, A.M., & WIELAND, R. (1978) Survey
of mortality among employees engaged in the manufacture of
styrene and polystyrene at the BASF Ludwigshafen works.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 231-239.
GADALINA, I.D., KAZNINA, N.I., KUZNETSOVA, G.M., &
SMIRNISTSKII, N.S. (1969) Hygienic assessment of polyester
plastics for flooring. Hyg. Sanit., 34: 25-29.
GAMBERALE, F. & HULTENGREN, M. (1974) Exposure to styrene
II. Psychological functions. Work Environ. Health, 11: 86-93.
GAMBERALE, F., LISPER, H.O., & ANSHELM-OLSON, B. (1975)
[Effect of styrene gases on reaction time among workers in
plastic boat industry.] Arbete och Hälsa, 8: 23 pp (in
Swedish).
GAZZOTTI, G., GARATTINI, E., & SALMONA, M. (1980) Nuclear
metabolism I. Determination of styrene monooxygenase activity
in rat liver nuclei. Chem. Biol. Interact., 29: 189-195.
GOMPERTZ, D. (1978) Comments made at the end of the session.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 227.
GORDON, M. & GOODLEY, P.C. (1971) Isolation and
characterization of industrial organic pollutants in Water.
Am. Chem. Soc., Div. Water, Air, Waste Chem., Gen. Pap.,
11(1): 91-94.
GOTELL, P., AXELSSON, O., & LINDELOF, B. (1972) Field studies
on human styrene exposure. Work Environ. Health, 9: 76-83.
GREIM, H., BIMBOES, D., EGERT, G., GOGGELMAN, W., & KRAMER, M.
(1977) Mutagenicity and chromosomal aberrations as an
analytical tool for in vitro detection of mammalian enzyme-
mediated formation of reactive metabolites. Arch. Toxicol.,
39: 159-169.
GROSSMAN, I.G. (1970) Waterborne styrene in a crystalline
bedrock aquifer in the Gales Ferry Area, Ledyard, Southeastern
Connecticut. US Geol. Surv. Prof. Pap., 700-B : 203-209.
GUILLEMIN, M. & BAUER, D. (1976) Human exposure to styrene.
II. Quantitative and specific gas chromatographic analysis of
urinary mandelic and phenylglyoxylic acids as an index of
styrene exposure. Int. Arch. occup. environ. Health, 37:
57-64.
GUILLEMIN, M.P. & BAUER, D. (1978) Biological monitoring of
exposure to styrene by analysis of combined urinary mandelic
and phenylglyoxylic acids. Am. Ind. Hyg. Assoc. J., 39:
873-879.
GUILLEMIN, M.P. & BAUER, D. (1979) Human exposure to styrene.
III. Elimination kinetics of urinary mandelic and
phenylglyoxylic acids after single experimental exposure.
Int. Arch. occup. environ. Health, 44: 249-263.
GUNTER, B.J. & LUCAS, J.B. (1973) Health hazard
evaluation/toxicity determination. Cincinnati, US Department
of Health, Education and Welfare, Public Health Service,
Center for Disease Control, National Institute for
Occupational Safety and Health, 17 pp (Report No. 72-86-38 -
Gates Rubber Co. Denver, CO.).
HAMIDULLIN, R.S., FELDMAN, N.G., & VENDILO, M.V. (1968)
Hygienic assessment of articles made of PS-S suspension
polystyrene, Hyg. Sanit., 33: 331-334.
HARKONEN, H. (1977) Relationship of symptoms to occupational
styrene exposure and to the findings of electroencephalo-
graphic and psychological examinations. Int. Arch. occup.
environ. Health, 40: 231-239.
HARKONEN, H. (1978) Styrene, its experimental and clinical
toxicology. Scand. J. Work Environ. Health, 4 (Suppl. 2):
104-113.
HARKONEN, H. & HOLMBERG, P. (1982) Obstetric histories of
workers occupationally exposed to styrene. Scand. J. Work
Environ. Health, 8: 74-77.
HARKONEN, H., KALLIOKOSKI, P., HIETALA, S., & HERNBERG, S.
(1974) Concentration of mandelic and phenylglyoxylic acid in
urine as indicators of styrene exposure. Work Environ.
Health, 11: 162-165.
HARKONEN, H., LINDSTROM, K., SEPPALAINEN, A.M., ASP, S., &
HERNBERG, S. (1978) Exposure-response relationships between
styrene exposure and central nervous functions. Scand. J.
Work Environ. Health, 4: 53-59.
HEMMINKI, K. (1979) Fluorescence study of DNA alkylation by
epoxides. Chem.-biol. Interact., 28: 269-278.
HEMMINKI, K. & FALCK, K. (1979) Correlation of mutagenicity
and 4-( p-nitrobenzyl)-pyridine alkylation by epoxides.
Toxicol. Lett., 4: 103-106.
HEMMINKI, K., FRANSSILA, E., & VAINIO, H. (1980a) Spontaneous
abortion among female chemical workers in Finland. Int. Arch.
occup. environ. Health, 45: 123-126.
HEMMINKI, K., PAASIVIRTA, J., KURKIRINNE, T., & VIRKKI, L.
(1980b) Alkylation products of DNA bases by simple epoxides.
Chem. biol. Interact., 30: 259-270.
HEMMINKI, K., HEINONEN, T., & VAINIO, H. (1981) Alkylation of
guanosine and 4-( p-nitrobenzyl)-pyridine by styrene oxide
analogues in vitro . Arch. Toxicol., 49: 35-41.
HIRATSUKA, A. & WATABE, T. (1982) [Reaction of 1-vinylbenzene
3,4-oxide with ethyl mercaptan.] In: [Proceedings of the
102nd Annual Meeting for the Pharmaceutical Society of Japan,
Osaka, Japan, 3-5 April, 1982]. Japan, p. 480 (in Japanese).
HIRATSUKA, A., AIZAWA, T., OZAWA, N., ISOBE, M., WATABE, T., &
TAKABATAKE, E. (1982a) The role of epoxides in the metabolic
activation of styrene to mutagens. Eisei Kagaku, 28: 34.
HOGSTEDT, B. & MITELMAN, F. (1983) Micronuclei in cultured
lymphocytes as an indicator of genotoxic exposure. In: De
Serres, F. & Pero, R.W., ed. Individual susceptibility to
genotoxic agents in the human population, New York, Plenum
Publishing Corporation, (in press).
HOLMBERG, P.C. (1977) Central nervous defects in two children
of mothers exposed to chemicals in the reinforced plastics
industry: chance or causal relation? Scand. J. Work Environ.
Health, 3: 212-214.
HOSHIKA, Y. (1977) Gas chromatographic determination of
styrene as its dibromide. J. Chromatogr., 136: 95-103.
HUZL, F., SYKOVA, J., MAINEVOVA, J., JANKOVA, J., SRUTEK, J.,
JUNGER, V., & LAHN, V. (1967) [The question of health hazards
in working with styrene.] Prac. Lek., 19: 121-125 (in Czech).
IARC (1979) Some monomers, plastics and synthetic elastomers,
and acrolein, Lyons, International Agency for Research on
Cancer, pp. 231-274 (IARC Monographs on the evaluation of the
carcinogenic risk of chemicals to humans. Vol. 19).
IKEDA, M. & IMAMURA, T. (1974) Evaluation of hippuric,
phenylglyoxylic and mandelic acids in urine as indices of
styrene exposure. Int. Arch. Arbeitsmed., 32: 93-101.
IKEDA, M., IMAMURA, T., HAYASHI, M., TABUCHI, T., & HARA, I.
(1974) Evaluation of hippuric, phenylglyoxylic and mandelic
acids in urine as indices of styrene exposure. Int. Arch.
Arbeitsmed., 32: 93-101.
IKEDA, M., KOIZUMI, A., MIYASAKA, M., & WATABE, T. (1982)
Styrene exposure and biologic monitoring in FRP production
plants. Int. Arch. occup. environ. Health, 49: 325-339.
ISHIDATE, M., Jr & YOSHIKAWA, K. (1980) Chromosome aberration
tests with Chinese hamster cells in vitro with and without
metabolic activation - a comparative study on mutagens and
carcinogens. Arch. Toxicol., Suppl. 4: 41-44.
ISHIDATE, M., Jr, SOFUNI, T., & YOSHIKAWA, K. (1981)
Chromosomal aberration tests in vitro as a primary screening
tool for environmental mutagens and/or carcinogens. GANN
Monogr. Cancer Res., 27: 95-108.
ITI (1975) Toxic and hazardous industrial chemicals safety
manual, Tokyo, The International Technical Information
Institute, pp. 494-495.
IZJUMOVA, A.S. (1972) [The action of small concentrations of
styrol on the sexual function of albino rats.] Gig. i Sanit.,
37: 29-30 (in Russian).
IVANOVA-TCHEMICHANSKA, L., ANTOV, G., & ILIEVA, P. (1982)
[Gonadotropic action of some organic solvents.] In: Resumes
de la XVIIe Semaine, August 29-September 3, 1982, Sofia,
Bulgaria, Sofia, L. Union Médicale Balkanique, pp. 50-51 (in
French).
JACOBS, H.W. & SYRJALA, R.H. (1978) The use of infrared
analyzers for monitoring acrylonitrile. Am. Ind. Hyg. Assoc.
J., 39: 161-165.
JAMES, M.O., FOUTS, J.R., & BEND, J.R. (1975) Hepatic and
extrahepatic metabolism in vitro of an epoxide (8-14C-
styrene oxide) in the rabbit. Biochem. Pharmacol., 25:
187-193.
JAMES, S.D. & WHITE, D.A. (1967) The metabolism of phenethyl
bromide, styrene and styrene oxide in the rabbit and rat.
Biochem. J., 104: 914-921.
JENSEN, F. (1972) Determination of monomers from polystyrene
in milk products, Ann. 1st Super. Sanita, 8: 443-448.
JERMINI, C., WEBER, A., & GRANDJEAN, E. (1976) [Quantitative
determination of various gas-phase components of the side-
stream smoke of cigarettes in the room air as a contribution
to the problem of passive-smoking.] Int. Arch. occup.
environ. Health, 36: l69-181 (in German).
JERSEY, G.C., BALMER, M.F., QUAST, J.F., PARK, C.N., SCHUETZ,
D.J., BEYER, J.E., OLSEN, K.J., MCCOLLISTER, S.B., & RAMPY,
L.W. (1978) Two-Year chronic inhalation toxicity and
carcinogenicity study of monomeric styrene in rats - Final
report. (2yr), Midland, MI, Dow Chemical Co., 150 pp
(Manufacturing Chemists Association MCA No: Sty 1.1-Tox-INH).
JOHNSTONE, R.A.W., QUAN, P.M., & CARRUTHERS, W. (1962)
Composition of cigarette smoke: Some low-boiling components.
Nature (Lond.), 195: 1267-1269.
KALLIOSKI, P. (1976) [The reinforced plastic industry - a
problem work environment.] (Työterveyslaitoksen tutkimuksia
122) (in Finnish).
KALLIOSKI, P. & PFAFFLI, P. (1975) Charcoal sampling method
for determining the concentration of styrene in air. Scand.
J. Work Environ. Health, 1: 193-198.
KANKAANPAA, J.T.J., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity and teratogenicity of styrene and styrene oxide
on chick embryos enhanced by trichloropropylene oxide. Acta
Pharmacol. Toxicol., 45: 399-402.
KANKAANPAA, J.T.J., ELOVAARA, E., HEMMINKI, K., & VAINIO, H.
(1980) The effect of maternally inhaled styrene on embryonal
and foetal development in mice and Chinese hamsters. Acta
Pharmacol. Toxicol., 47: 127-129.
KAWACHI, T., YAHAGI, T., KADA, T., TAZIMA, Y., ISHIDATE, M.,
SASAKI, M., & SUGIYAMA, T. (1979) Cooperative programme on
short-term assays for carcinogenicity in Japan. In:
Montesano, R., Bartsch, H., & Tomatis, L., ed. Molecular and
cellular aspects of carcinogen screening tests, IARC
Scientific Publication No. 27, Lyons, IARC, pp. 323-330.
KAZNINA, N.I. (1969) Air pollution by volatiles liberated by
some plastics. Hyg. Sanit., 33: 267-269.
KJELLBERG, A., WIGAEUS, E., ENGSTROM, J., CSTRAND, I., &
LJUNGQUIST, E. (1979) [Long-term effects of styrene exposure
in plastic industry.] Arbete och Hälsa, 18: 1-25 (in Swedish).
KLIMKOVA-DEUTSCHOVA, E. (1962) [Neurological findings in the
plastics industry among styrene workers.] Int. Arch.
Gewerbepath. Gewerbehyg., 19: 35-50 (in German).
LEIBMAN. K.C. (1975) Metabolism and toxicity of styrene.
Environ. health Perspect., 11: 115-119.
LEIBMAN, K.C. & ORTIZ, E. (1969) Oxidation of styrene in
liver microsomes. Biochem. Pharmacol., 18: 552-554.
LEITHE, W. (1971) Analysis of air pollutants, Ann Arbor, Ann
Arbor Science Publishers, p. 246.
LEMEN, R.A. & YOUNG, R. (1976) Investigations of health
hazards in styrene butadiene rubber facilities. In: Ede, L.,
ed. Proceedings of the NIOSH Styrene-Butadiene Briefing,
Covington, Kentucky, USA, 1976, Cincinnati, Ohio, US
Department of Health, Education and Welfare pp. 3-8 (HEW
Publ. No. (NIOSH) 77-129).
LILIS, R. & NICHOLSON, W.J. (1976) Cancer experience among
workers in a chemical plant producing styrene monomers. In:
Ede, L., ed., Proceedings of the NIOSH Styrene-Butadiene
Briefing, Covington, Kentucky, USA, 1976, Cincinnati, Ohio,
Department of Health, Education and Welfare pp. 22-27 (HEW
Publ. No. (NIOSH) 77-129).
LILIS, R., LORIMER, W.V., DIAMOND, S., & SELIKOFF, I.J. (1978)
Neurotoxicity of styrene in production and polymerization
workers. Environ. Res., 15: 133-138.
LINDSTROM, K., HARKONEN, H., & HERNBERG, S. (1976)
Disturbances in psychological functions of workers
occupationally exposed to styrene. Scand. J. Work Environ.
Health, 2: 129-139.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978a)
Cytogenetic effects of styrene and styrene oxide. Mutat.
Res., 58: 277-286.
LINNAINMAA, K., MERETOJA, T., SORSA, M., & VAINIO, H. (1978b)
Cytogenetic effects of styrene and styrene oxide on human
lymphocytes and Allium cepa. Scand. J. Work Environ. Health,
4 (Suppl. 2): 156-162.
LITTON BIONETICS (1980) Toxicological study on styrene
incorporated in drinking water of rats for two years in
conjuction with a three-generation reproduction study.
(Submitted to Chemical Manufacturers Association.)
LOPRIENO, N. (1981) Environmental mutagens and comparative
short-term mutagenicity studies. In: Molecular bases of
genetic processes. Proceedings of the XIV International
Congress of Genetics, Moscow, MIR Publishers, Vol. III, Book
1, pp. 164-180.
LOPRIENO, N. & ABBONDANDOLO, A. (1980) Comparative mutagenic
evaluation of some industrial compounds. In: Norpoth, K.H. &
Garner, R.C., ed. Short-term test systems for detecting
carcinogens, Berlin, Springer-Verlag, pp. 333-356.
LOPRIENO, N., ABBONDANDOLO, A., BARALE, R., BARONCELLI, S.,
BONATTI, S., BRONZETTI, G., CAMMELLINI, A., CORSI, C., CORTI,
G., FREZZA, D., LEPORINI, C., MAZZACCARO, A., NIERI, R.,
ROSELLINI, R., & ROSSI, A.M. (1976) Mutagenicity of
industrial compounds: styrene and its possible metabolite
styrene oxide. Mutat. Res., 40: 317-324.
LOPRIENO, N., PRESCIUTTINI, S., SBRANA, I., STRETTI, G.,
ZACCARO, L., ABBONDANDOLO, A., BONATTI, S., FIORIO, R., &
MAZZACCARO, A. (1978) Mutagenicity of industrial compounds
VII. Styrene and styrene oxide: II. Point mutations,
chromosome aberrations and DNA repair induction analyses.
Scand. J. Work Environ. Health, 4 (Suppl. 2): 169-178.
LORIMER, W.V., LILIS, R., NICHOLSON, W.J., ANDERSON, H.,
FISCHBEIN, A. DAUM, S., ROM, W., RICE, C., & SELIKOFF, I.J.
(1976) Clinical studies of styrene workers: Initial
findings. Environ. health Perspect., 17: 171-181.
LORIMER, W., LILIS, R., FISCHBEIN, A., DAUM, S., ANDERSON, H.,
WOLFF, M., & SELIKOFF, I. (1978) Health status of
styrene-polystyrene polymerization workers. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 220-226.
LUNDBERG, I. (1981) [Serumenzyme levels in workers exposed to
styrene.] Arbete och Hälsa, 1: 19 pp (in Swedish).
MAIER, A. RUHE, R., TOSENSTEEL, R., & LUCAS, J.B. (1974)
Health hazard evaluation/toxicity determintion. Arco Polymer
Incorporated (Sinclair-Koppers Company, Inc.), Monaco, PA.,
Cincinnati, US Department of Health, Education, and Welfare,
Center for Disease Control, National Institute for
Occupational Safety and Health, 28 pp (Report No. 72-90--107).
MALTONI, C., FAILLA, G., & KASSAPIDIS, G. (1979) First
experimental demonstration of the carcinogenic effects of
styrene oxide. Long-term bioassays on Spraque-Dawley rats by
oral administration. Med. Lav., 70(5): 358-362.
MANSON, N.W., REILLY, D.A., & STAGG, H.E. (1965) The
determination of toxic substances in air, manual of ICI
practice, Cambridge, W. Heffer & Sons, p. 181.
MARNIEMI, J., SUOLINNA, E.M., KAARTINEN, N., & VAINIO, H.
(1977) Covalent binding of styrene oxide to rat liver
macromolecules in vivo and in vitro. In: Ullrich, V., Roots,
I., Hildebrandt, A., Estabrook, R.W., & Conney, A.H., ed.
Microsomes and drug oxidations, London, Oxford, Pergamon
press, pp. 698-702.
MATSUOKA, A., HAYASHI, M., & ISHIDATE, M., Jr (1979)
Chromosomal aberration tests on 29 chemicals combined with S9
mix in vitro. Mutat. Res., 66: 277-290.
MATSUSHITA, T., MATSUMOTO. T., MIYAGAKI, J., MAEDA, K.,
TAKEUCHI, Y., & KATAJIMA, J. (1968) [Nervous disorders
considered to be symptoms of chronic styrol poisoning.
Saigai Igaku, 11: 173-179 (in Japanese).
McMICHAEL, A.J., SPIRTAS, R., GAMBLE, J.F., & TOUSEY, P.M.
(1976) Mortality among rubber workers: relationship to
specific jobs. J. occup. Med., 18: 178-185.
McNAIR, H.M. & BONELLI, E.J. (1968) Basic Gas Chromatography,
5th Ed., Walnut Creek, Varian Aerograph., p. 101.
MEINHARDT, T.J., YOUNG, R.J., & HARTLE, R.W. (1978)
Epidemiologic investigations of styrene-butadiene rubber
production and reinforced plastics production. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 240-246.
MERETOJA, T. & VAINIO, H. (1979) The use of human lymphocyte
tests in the evaluation of potential mutagens: clastogenic
activity of styrene in occupational exposure. In: Berg, K.,
ed. Genetic damage in man caused by environmental agents, New
York, Academic Press, pp. 213-225.
MERETOJA, T., VAINIO, H., SORSA. M., & HARKONEN, H. (1977)
Occupational styrene exposure and chromosomal aberrations.
Mutation Res., 56: 193-197.
MERETOJA, T., JARVENTAUS, H., SORSA, M., & VAINIO, H. (1978a)
Chromosome aberrations in lymphocytes of workers exposed to
styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
259-264.
MERETOJA, T., VAINIO, H., & JARVENTAUS, H. (1978b)
Clastogenic effects of styrene exposure on bone marrow cells
of rat. Toxicol. Lett., 1: 315-318.
MILVY, P. & GARRO, A.J. (1976) Mutagenic activity of styrene
oxide (1,2-epoxyethylbenzene), a presumed styrene metabolite.
Mutat. Res., 40: 15-18.
MITHEN, F.A., COCHRAN, M., JOHNSON, M.I., & BUNGE, R.P. (1980)
Neurotoxicity of polystyrene containers detected in a closed
tissue culture system. Neurosci. Lett., 17: 107-111.
MURRAY, F. J., JOHN, J. A., BALMER, M. F., & SCHWELTZ, B. A.
(1978) Teratologic evaluation of styrene given to rats and
rabbits by inhalation or by gavage, Toxicology, 11: 335-343.
NELIGAN, R.E., LEONARD, M.J., & BRYAN, R.J. (1965) The gas
chromatographic determination of aromatic hydrocarbons in the
atmosphere. Am. Chem. Soc. Div. Water, Air. Waste Chem.
Preprints, 5: 118-121.
NICHOLSON, W.J., SELIKOFF, I.J., & SEIDMAN, H. (1978)
Mortality experience of styrene-polystyrene polymerization
workers. Initial findings. Scand. J. Work Environ. Health, 4
(Suppl. 2): 247-252.
NIOSH (1974) NIOSH manual of analytical methods, Cincinnati,
National Institute for Occupational Safety and Health, (Method
No. 127).
NORPPA, H. (1981a) Chromosome damage induced by styrene,
styrene oxide and some analogues, Academic dissertation,
Helsinki, Institute of Occupational Health and University of
Helsinki, 56 pp.
NORPPA, H. (1981b) Styrene and vinyltoluene induce
micronuclei in mouse bone marrow. Toxicol. Lett., 8: 247-251.
NORPPA, H. & VAINIO, H. (1983a) Genetic toxicity of styrene
and some of its derivatives. Scand. J. Work Environ. Health,
9: 108-114.
NORPPA, H. & VAINIO, H. (1983b) Induction of sister-chromatid
exchanges by styrene analogues in cultured human lymphocytes.
Mutat. Res., 116: 379-387.
NORPPA, H., ELOVAARA, E., HUSGAFVEL-PURSIAINEN, K., SORSA, M.,
& VAINIO, H. (1979) Effects of styrene oxide on chromosome
aberrations, sister chromatid exchange and hepatic drug
biotransformation in Chinese hamsters in vivo. Chem.-biol.
Interact., 26: 305-315.
NORPPA, H., SORSA, M., PFAFFLI, P., & VAINIO, H. (1980a)
Styrene and styrene oxide induce SCEs and are metabolised in
human lymphocyte cultures. Carcinogenesis, 1: 357-361.
NORPPA, H., SORSA, M., & VAINIO, H. (1980b) Chromosomal
aberrations in bone marrow of Chinese hamsters exposed to
styrene and ethanol. Toxicol. Lett., 5: 241-244.
NORPPA, H., VAINIO, H., SORSA, M., & BELVEDERE, G. (1982)
Metabolic activation of styrene by erythrocytes in human
lymphocyte cultures. In: 12th Annual Meeting of the European
Environmental Mutagen Society, Espoo, Finland, 20-24 June,
1982, Abstracts, Helsinki, Institute of Occupational Health,
p. 148.
NYLANDER, P. (1979) [LSE polyesters - environmental
effects.] Plastforum Scandinavia, 9: 130-132 (in Swedish).
ODKVIST, L.M., CSTRAND, I., LARSBY, B., & KALL, C. (1980)
[Does styrene incude disturbancies in the human balance
mechanisms?] Arbete och Hälsa, 2: 5-19 (in Swedish).
OESCH, F. (1973) Mammalian epoxide hydrolase. Inducable
enzyme catalyzing the inactivation of carcinogenic and
cytotoxic metabolites derived from aromatic and olefinic
compounds. Xenobiotica, 3: 305-360.
OGATA, M. & SUGIHARA, R. (1978) High performance liquid
chromatographic procedure for quantitative determination or
urinary phenylglyoxylic, mandelic and hippuric acids as
indices of styrene exposure. Int. Arch. occup. environ.
Health, 42: 11-19.
OHTSUJI, H. & IKEDA, M. (1970) A rapid colorimetric method
for the determination of phenylglyoxylic and mandelic acids.
Its application to the urinanalysis of workers exposed to
styrene vapour. Br. J. ind. Med., 27: 150-154.
OHTSUJI, H. & IKEDA, M. (1971) The metabolism of styrene in
rat and the stimulatory effect of phenobarbital. Toxicol.
appl. Pharmacol., 18: 321-328.
OLTRAMARE, M., DESBAUMES, E., IMHOFF, C., & MICHIELS, W.
(1974) Toxicologie du styrene monomère. Geneva, Editions
Mèdicine et Hygiène, 100 pp.
OTT, M.G., KOLESAR, R.C., SCHARNWEBER, H.C., SCHNEIDER, E.J.,
& VENABLE, J.R. (1980) A mortality survey of employees
engaged in the development or manufacture of styrene-based
products. J. occup. Med., 22: 445-460.
PACHECKA, J., GARIBOLDI, P., CANTONI, L., BELVEDERE, G.,
MUSSINI, E., & SALMONA, M. (1979) Isolation and structure
determination of enzymatically formed styrene oxide
glutathione conjugates. Chem. Biol. Interact., 27: 313-321.
PAGANO, D.A., YAGEN, B., HERNANDEZ, O. BEND, J.R., & ZEIGER,
E. (1982) Mutagenicity of ( R) and ( S) styrene 7,8-oxide and
the intermediary mercapturic acid metabolites formed from
styrene 7,8-oxide. Environ. Mutag., 4(5): 575-584.
PAGANO, G., ESPOSITO, A., GIORDANO, G.G., & HAGSTROM, B. E.
(1978) Embryotoxic and teratogenic effects of styrene
derivatives on sea urchin development. Scand. J. Work,
Environ. Health, 4 (Suppl. 2): 136-141.
PANTAROTTO, E., FANELLI, R., BIDOLI, F., MANZONI, P., SALMONA,
M., & SZCZAWINSKI, K. (1978) Areneoxides in styrene
metabolism. New perspective in styrene toxicity? Scand. J.
Work Environ. Health, 4 (Suppl. 2): 67-77.
PANTAROTTO, C., SALMONA, M., SZCZAWINSKA, K., & BIDOLI, F.
(1980) Gas chromatography-mass spectrometric studies on
styrene toxicity. In: Albatges, J., ed. Analytical techniques
in environmental chemistry, New York, Pergamon Press, pp.
245-279.
PARKKI, M.G. (1978) The role of glutathione in the toxicity
of styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
53-59.
PARKKI, M.G., MARNIEMI, J., & VAINIO, H. (1976) Action of
styrene and its metabolites styrene oxide and styrene glycol
on activities of xenobiotic biotransformation enzymes in rat
liver in vivo. Toxicol. appl. Pharmacol., 38: 59-70.
PENTTILA, M., SORSA, M., & VAINIO, H. (1980) Inability of
styrene to induce nondisjunction in Drosophila or a positive
micronucleus test in the Chinese hamster. Toxicol. Lett., 6:
119-123.
PERO, R.W., BRYNGELSSON, T., HOGSTEDT, B., & CKESON, B. (1982)
Occupational and in vitro exposure to styrene assessed by
unscheduled DNA synthesis in resting human lymphocytes.
Carcinogenesis, 3: 681-685.
PERVIER, J.W., BARLEY, R.C., FIELD, D.E., FRIEDMAN, B.M., &
MORRIS, R.B. (1974) Survey reports on atmospheric emissions
from the petrochemical industry, Vol. IV. 287 pp (US NTIS, PB
Rep. PB-245630).
PFAFFLI, P., HESSO, A., VAINIO, H., & HYVONEN, M. (1981)
4-Vinylphenol excretion suggestive of arene oxide formation in
workers occupationally exposed to styrene. Toxicol. appl.
Pharmacol., 60: 85-90.
PHILIPPE, R., LAUWERYS, R., BUCHET, J.P., ROELS, H., & DEFELD,
J.M. (1974) [Evaluation of the exposure of workers to styrene
by means of the determination of its urinary metabolites:
mandelic and phenylglyoxylic acids: II. Application to
polyester fiber workers.] Arch. Mal. Prof. Méd. Tra. Secur.
Soc., 35: 631-640.
PLANCHE, G., CROISY, A., MALAVEILLE, C., TOMATIS, L., &
BARTSCH, H. (1979) Metabolic and mutagenicity studies on DDT
and 15 derivatives. Detection of 1,1-bis(p-chlorophenyl)-2,2-
dichloroethane and 1,1-bis(p-chlorophenyl)-2,2,2-trichloro-
ethyl acetate (kelthane acetate) as mutagens in Salmonella
typhimurium and of 1,1-bis(p-chlorophenyl)ethylene oxide, a
likely metabolite, as an alkylating agent. Chem. biol.
Interact., 25: 157-175.
POGGI, G., GUISIANI, M., PALAGI, U., PAGGIARO, P.L., LOI,
A.M., DAZZI, F., SICLARI, C., & BASCHIERI, L. (1982) High-
performance liquid chromatography for the quantitative
determination of the urinary metabolites of toluene, xylene,
and styrene. Int. Arch. occup. environ. Health, 50: 25-31.
PONCELET, F., DE MEESTER, C., DUVERGER-VAN BOGAERT, M.,
LAMBOTTE-VANDEPAER, M., ROBERTFROID, M., & MERCIER, M. (1980)
Influence of experimental factors on the mutagenicity of
vinylic monomers. Arch. Toxicol., Suppl. 4: 63-66.
PONOMAREVA, N.I. & ZLOBINA, N.S. (1972) [Working conditions
and the state of the upper respiratory tract in workers
engaged in the production of block and emulsion polystyrene
and its copolymers.] Gig. Tr. Prof. Zabol., 15: 22-26 (in
Russian).
PONOMARKOV, V. & TOMATIS, L. (1978) Effects of long-term oral
administration of styrene to mice and rats. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 127-135.
PONOMARKOV, V., CABRAL, J.R.P., WAHRENDORF, J., & GALENDO, D.
(1983) A carcinogenicity study of styrene oxide in rats.
Toxicolgist, 3: 46 (Abstract No. 184).
QUAST, J.F., KALNINS, R.P., OLSON, K.J., HUMISTON, C.G.,
MURRAY, F.J., JOHN, J.A., & SCHWETZ, B.A. (1978) Results of a
toxicity study in dogs and teratogenicity studies in rabbits
and rats administered monomeric styrene. In: Meeting of the
Society of Toxicology, San Francisco, USA, March 1978.
QUAST, J.F., HUMISTON, C.G., KALNINS, R.V., OLSON, K.J.,
McCOLLISTER, S.B., WADE, C.E., BEYER, J.E., & SCHWETZ, B.A.
(1979) Results of a toxicity study of monomeric styrene
administered to beagle dogs by oral intubation for 19 months -
Midland, MI, Dow Chemical Co., 199 pp (MCA (Manufacturing
Chemists Association) No: Sty 1.2-Tox-Gav-Dow.)
RAGYL'YE, N. (1974) [Problems concerning the embryotropic
effects of styrene.] Gig. i Sanit., 11: 65-66 (in Russian).
RAMSEY, J.C. & YOUNG, J.D. (1978) Pharmacokinetics of inhaled
styrene in rats and humans. Scand. J. Work Environ. Health, 4
(Suppl. 2): 84-91.
RAMSEY, J.C., YOUNG, J.D., KARBOWSKI, R.J., CHENOWETH, M.B.,
MCCARTY, L.P., & BRAUN, W.H. (1980) Pharmacokinetics of
inhaled styrene in human volunteers. Toxicol. appl.
Pharmacol., 53: 54-63.
RENKE, W. & CHEMIELEWSKI, J. (1976) Blood coagulation and
fibronolysis in occupational exposure to some chemical
factors. Biul. Inst. Med. Morskiej, 27: 289-298.
RIIHIMAKI, V. & PFAFFLI, P. (1978) Percutaneous absorption of
solvent vapors in man. Scand. J. Work Environ. Health, 4:
73-85.
ROSEN, A.A., SKEEL, R.T., & ETTINGER, M.B. (1963)
Relationship of river water odor to specific organic
contaminants. J. Water Pollut. Control Fed., 35: 777-82.
ROSEN, I., HAEGER-ARONSEN, B., REHNSTROM, S., & WELINDER, H.
(1978) Neurophysiological observations after chronic styrene
exposure. Scand. J. Work Environ. & Health, 4 (Suppl. 2):
184-194.
ROSENSTEEL, R.E. & MEYER, C.R. (1977) Health hazard
evaluation determination. Cincinnati, US Department of
Health, Education and Welfare, Public Health Service, Center
for Disease Control, National Institute for Occupational
Safety and Health. 52 pp (Report no. 75-150-378 - Reinell
Boats, Inc., Poplar Bluff, Missouri).
ROTH, B. & KLIMKOVA-DEUTSCHOVA, E. (1963) The effect of the
chronic action of industrial poisons on the electro-
encephalogram of man. Rev. Czech. Med., 9: 217-227.
ROTH, S.H. (1979) Physical mechanisms of anesthesia. Ann.
Rev. Pharmacol. Toxicol., 19: 159-178.
SAALWAECHTER, A.T., McCAMMON, C.S., ROPER, C.P. Jr, &
CARLBERG, K.S. (1977) Performance testing of the NIOSH
charcoal tube technique for the determination of air
concentrations of organic vapors. Am. Ind. Hyg. Assoc. J.,
38(9): 476-486.
SANTODONATO, J., MEYLAN, W.M., DAVIS, L.N., HOWARD, P.H.,
ORZEL, D.M., & BOGYO, D.A. (1980) Investigation of selected
potential environmental contaminants: Styrene, ethyl benzene
and related compounds. 261 pp (A report prepared for the
Office of Toxic Substances, US Environmental Protection
Agency, Washington, DC by Syracuse Research Corporation;
Contract No. 68-01-3250.)
SAUERHOFF, M.W. & BRAUN, W.H. (1976) The fate of styrene in
rats following an inhalation exposure to 14 C-styrene.
Midland, Michigan, Toxicology Research Laboratory, Health and
Environmental Research, Dow Chemical USA, 26 pp.
SAUERHOFF, M.W., MADRID, E.O., & BRAUN, W.H. (1976) The fate
of orally administered styrene in rats. Midland, Michigan,
Toxicology Research Laboratory, Health and Environmental
Research, Dow Chemical USA, 44 pp.
SAVOLAINEN, H. & PFAFFLI, P. (1977) Effects of chronic
styrene inhalation on rat brain protein metabolism. Acta
Neuropath., 40: 237-241.
SAVOLAINEN, H. & PFAFFLI, P. (1978) Accumulation of styrene
monomer and neurochemical effects of long-term inhalation
exposure in rats. Scand. J. Work Environ. Health, 4 (Suppl.
2): 78-83.
SAVOLAINEN, H. & VAINIO, H. (1977) Organ distribution and
nervous system binding of styrene and styrene oxide.
Toxicology, 8: 135-141.
SAVOLAINEN, H., HELOJOKI, M., & TENGEN-JUNNILA, M. (1980)
Behavioural and glial cell effects of inhalation exposure to
styrene vapour with special reference to interactions of
simultaneous ethanol intake. Acta Pharmacol. Toxicol., 46:
51-56.
SBRANA, I., LASCIALFARI, D., ROSSI, A.M., & LOPRIENO, N.
(1982) Bone marrow cell chromosomal aberrations and styrene
biotransformation in mice given styrene on a repeated oral
schedule. Submitted for publication.
SCHALLER, K.-H., GOSSLER, K., BOST, H.-P., & VALENTIN, H.
(1976) [Gas chromatographic methods for the determination of
styrene in the blood and of mandelic acid and phenylglyoxylic
acid in the urine.] Arbeits-, Sozial-, Präventivmedizin, 11:
64-64 (in German).
SCHNEIDER, H. & SEEBER, A. (1979) [Psychodiagnostics in the
understanding of neurotoxic effects of harmful chemical
substances.] Z. Psychol. Bd., 187: 178-205 (in German).
SCHOFIELD, K. (1974) Problems with flame ionization detectors
in automotive exhaust hydrocarbon measurements. Environ. Sci.
Technol., 8: 826-834.
SCHUMACHER, R.L., BREYSSE, P.A., CARLYON, W.R., HIBBARD, R.P.,
& KLEINMAN, G.D. (1981) Styrene exposure in the fiberglass
fabrication industry in Washington State. Am. Ind. Hyg.
Assoc. J., 42: 143-149.
SEPPALAINEN, A.M. (1978) Neurotoxicity of styrene in
occupational and experimental exposure. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 181-183.
SEPPALAINEN, A.M. & HARKONEN, H. (1976) Neurophysiological
findings among workers occupationally exposed to styrene.
Scand. J. Work Environ. Health, 2: 140-146.
SEPPALAINEN, A.M., LINDSTROM, K., & MARTELIN, T. (1980)
Neurophysiological and psychological picture of solvent
poisoning. Am. J. ind. Med., 1: 31-42.
SEUTTER-BERLAGE, F., DELBRESSINE, L.P.C., SMEETS, F.L.N., &
KETELAARS, H.C.J. (1978) Identification of three sulphur-
containing urinary metabolites of styrene in the rat.
Xenobiotica, 8: 413-418.
SHUGAEV, B.B. (1969) Concentration of hydrocarbons in tissues
as a measure of toxicity. Arch. environ. Health, 18: 878-882.
SIMKO, A., JINDRICHOVA, J., & PULTAROVA, H. (1966) [The
effect of styrene on the health state of workers employed in
laminate-production.] Prac. Lek., 18: 348-352 (in Czech).
SINITSKIJ, V.G. (1969) [Indexes for immunological reactivity
in rabbits during the long-term exposure to small doses of
styrene.] Gig. Primen. Polim. Mater, Izdelii Nikh, 1: 394-398
(in Russian).
SLOB, A. (1973) A new method for determination of mandelic
acid excretion at low level styrene exposure. Br. J. ind.
Med., 30: 390-393.
SMIRNOVA, E.T. & YATAKOVA, Z.M. (1966) Health hazard of
polystyrene toys. Hyg. Sanit., 31: 135-137.
SMITH, A.H. & ELLIS, L. (1977) Styrene butadiene rubber
synthetic plants and leukaemia. J. occup. Med., 19: 441.
SMITH, H.O. & HOCHSTETTLER, A.D. (1969) Determination of odor
thresholds in air using C14-labeled compounds to monitor
concentrations. Environ. Sci. Technol., 3: 169-170.
SODER, S.L. (1977) "Styrene" Chemical economics handbook,
California, Stanford Research Institute.
SOLLENBERG, J. & BALDESTEN, A. (1977) Isotachophoretic
analysis of mandelic acid, phenylglyoxylic acid, hippuric acid
and methylhippuric acid in urine after occupational exposure
to styrene, toluene and/or xylene. J. Chromatogr., 132:
469-476.
SORSA, M., HYVONEN, M., JARVENTAUS, H., & VAINIO, H. (1979)
Chromosomal aberrations and sister chromatid exchange in
children of women in reinforced plastic industry. In:
Symposium on Toxicology, Abstracts, Turku, Finland 29-20 May,
1979, Turku, University of Turku, p. 16.
SPASOVSKI, M. (1976) Health hazards in the production and
processing of some fibers, resins, and plastics in Bulgaria.
Environ. Health Perspect., 17: 199-202.
SPASOVSKI, M., HINKOVA, L., & BENCHEV, I. (1980) Exposure to
styrene - field studies. Toxicol. Lett., (suppl. 1): 218.
SPENCER, H.C., IRISH, D.D., ADAMS, E.M., & ROWE, V.K. (1942)
The response of laboratory animals to monomeric styrene. J.
ind. Hyg. Toxicol., 24: 295-301.
SPINAZZOLA et al. (1980) Petrolchimica. Tecnologia, ambiente
di lavoro, prevenzione e patologia. Atti del 43° Congresso
Nazionale della Società Italiana di Medicina del Lavoro ed
Igiene Industriale. Parma, Edizioni Tecnografica, Vol. 2.
SPIRTAS, R., VAN ERT, M., GAMBLE, J.F., WOLF, P., & McMICHAEL,
A.J. (1976) Toxicologic, industrial hygiene and
epidemiological consideration in the possible association
between SBR manufacturing and neoplasms of the lymphatic and
hematopoietic tissues. In: Ede, L., ed. Proceedings of NIOSH
Styrene-Butadiene Briefing, Covington, Kentucky, USA, 1976,
Cincinnati, Ohio, Department of Health, Education and Welfare,
pp. 67-112 (HEW Publ. No. (NIOSH) 77-129).
SRAM, R.J. (1981) Cytogenetic analysis of peripheral
lymphocytes as a method for monitoring environmental levels of
mutagens. In: Gut, I., Cikrt, M., & Plaa, G.L., ed.
Industrial and environmental xenobiotics. Metabolism and
pharmacokinetics of organic chemicals and metals., Berlin,
Springer-Verlag, pp. 187-194.
STEWART, R.D., DODD, H.C., BARETTA, E.D., & SCHAFFER, A.W.
(1968) Human exposure to styrene vapor. Arch. environ.
Health, 16: 656-662.
STOLZ, D.R. & WITHEY, R.J. (1977) Mutagenicity testing of
styrene and styrene epoxide in Salmonella typhimurium. Bull.
environ. contamin. Toxicol., 17: 739-742.
SUGIURA, K. & GOTO, M. (1981) Mutagenicities of styrene oxide
derivatives on bacterial test systems: relationship between
mutagenic potencies and chemical reactivity. Chem. biol.
Interact., 35: 71-91.
SUGIURA, K., KIMURA, T., & GOTO, M. (1978a) Mutagenicities of
styrene oxide derivatives on Salmonella typhimurium (TA100).
Relationship between mutagenic potencies and chemical
reactivity. Mutat. Res., 58: 159-165.
SUGIURA, K., YAMANAKA, S., FUKUSAWA, S., & GOTO, M. (1978b)
The mutagenicity of substituted and unsubstituted styrene
oxides in E. coli: Relationship between mutagenic potencies
and physico-chemical properties. Chemosphere, 7: 737-742.
SUGIURA, K., MAEDA, A., & GOTO, M. (1979) Substitutional
effects of styrene oxides on survival and mutation induction
in cultured Chinese hamster cells (V-79). Chemosphere, 8:
369-372.
SYNTHETIC RESINS LIMITED (1980) LSE capability with good
interlaminal adhesion. Reinf. Plast., 3: 72-73.
TAULBEE, J., ANDJELKOVIC, D., WILLIAMS, T., GAMBLE, J.F., &
WOLF, P. (1976) A study of possible associations between
exposure to SBR processes and industrial hygiene and
epidemiologic considerations (for workers in the 1951 and 1964
cohorts and deaths 1964-1973). In: Ede, L., ed. Proceedings
of NIOSH Styrene-Butadiene Briefing, Covington, Kentucky, USA,
1976, Cincinnati, Ohio, Department of Health, Education and
Welfare, pp. 113-162 (HEW Publ. No. (NIOSH) 77-129).
TERAMOTO, K., HORIGUCHI, S., & KITIBATAKE, S. (1978) [Studies
on industrial styrene poisoning. (Part VIII). Distribution and
elimination of styrene in rats exposed to styrene.] Jpn. J.
ind. Health, 20(2): 118-119 (in Japanese).
THEISS, A.M. & FLEIG, I. (1978) Chromosome investigations on
workers exposed to styrene/polystyrene. J. occup. Med., 20:
747-749.
THIESS, A.M. & FRIEDHEIM, M. (1978) Morbidity among persons
employed in styrene production, polymerization and processing
plants. Scand. J. Work Environ. Health, 4 (Suppl. 2): 203-214.
THIESS, A.M. & FRIEDHEIM, M. (1979) [Morbidity study in
coworkers of the polyester laboratory and of the technical
service exposed to styrene.] Zentralbl. Arbeitsmed., 9:
238-241 (in German).
THIESS, A.M., SCHWEGLER, H., & FLEIG, I. (1980) Chromosome
investigations in lymphocytes of workers employed in areas in
which styrene-containing unsaturated polyester resins are
manufactured. Am. J. ind. Med., 1: 205-210.
THOMPSON, B. (1974) Hazardous gases and vapors: Infrared
spectra and physical constants, Fullerton, Beckman
Instruments, Inc., pp. 154, 255 (Technical report 595).
TOLA, S., HARKONEN, H., KORKALA, M.L., & JARVINEN, E. (in
press) Mortality of workers exposed to styrene. Egypt. J.
occup. Med., (in press).
TOSSAVAINEN, A. (1978) Styrene use and occupational exposure
in the plastics industry. Scand. J. Work Environ. Health, 4
(Suppl. 2): 7-13.
TURCHI, G., BONATTI, S., CITTI, L., GERVASI, P.G.,
ABBONDANDOLO, A., & PRESCIUTTINI, S. (1981) Alkylating
properties and genetic activity of 4-vinylcyclohexane
metabolites and structurally related epoxides. Mutat. Res.,
83: 419-430.
US EPA (1980) Investigation of Selected Potential
Environmental Contaminants: Styrene Ethylbenzene, and Related
Compounds. Washington DC, US Environmental Protection Agency,
261 pp (Publication No. EPA 5601 11-80---018).
US NATIONAL CANCER INSTITUTE (1979) Bioassay of styrene for
possible carcinogenicity. Washington, DC, Department of
Health, Education and Welfare, 46 pp (Publ. No. (NIH)
79-1741, Washington DC, (Techn. Rep. ser. No. 185)).
VAINIO, H. (1978) Vinyl chloride and vinyl benzene (styrene)-
metabolism, mutagenicity and carcinogenicity. Chem.-biol.
Interact., 22: 117-124.
VAINIO, H. & MAKINEN, A. (1977) Styrene and acrylonitrile
induced depression of hepatic nonprotein sulfhydryl content in
various rodent species. Res. Commun. Chem. Pathol.
Pharmacol., 17: 115-124
VAINIO, H., PAAKKONEN, R., RONNHOLM, K., RAUNIO, V., &
PELKONEN, O. (1976) A study on the mutagenic activity of
styrene and styrene oxide. Scand. J. Work Environ. Health, 3:
147-151.
VAINIO, H., HEMMINKI, K., & ELOVAARA, E. (1977) Toxicity of
styrene and styrene oxide on chick embryos. Toxicology, 8:
319-325.
VAINIO, H., JARVISALO, J., & TASKINEN, E. (1979) Adaptive
changes caused by intermittent styrene inhalation on
xenobiotic biotransformation. Toxicol. appl. Pharmacol., 49:
7-14.
VAINIO, H., NORPPA, H., & BELVEDERE, G. (1982) The role of
cytochrome P-450 mediated metabolism in SCEs induced by
styrene in human lymphocytes. In: Proceedings of the l3th
International Cancer Congress, Abstracts, Seattle, USA, 8-l5
September, l982, Seattle, International Union Against Cancer,
p. 118.
VALENTA, J. (1966) [Air and water pollution by styrene with
the butadiene-styrene rubber production.] Cesk. Hyg., 11:
349-352 (in Czech).
VAN ANDA, J., SMITH, B.R., FOUTS, J.R., & BEND, J.R. (1979)
Concentration-dependent metabolism and toxicity of [14C
styrene oxide in the isolated perfused rat liver. J.
Pharmacol. exp. Ther., 211: 207-211.
VAN DUUREN, B.L., NELSON, N., ORRIS, L., PALMES, E.D., &
SCHMITT, F.L. (1963) Carcinogenicity of epoxides, lactones
and peroxy compounds. J. Natl Cancer Inst., 31: 41-55.
VARNER, S.L. & BREDER, C.V. (1981a) Liquid chromatographic
determination of residual styrene in polystyrene food
packaging. J. Assoc. Off. Anal. Chem., 64: 647-652.
VARNER, S.L. & BREDER, C.V. (1981b) Head space sampling and
gas chromatographic determination of styrene migration from
food contact polystyrene cups into beverages and food
simulants. J. Assoc. Off. Anal. Chem., 64: 1122-1130.
VERGIEVA, T. & ZAJKOV, H. (1981) [Behavioural changes in rats
with inhalation styrene effect.] Hyg. Zdrav., 24: 242-247 (in
Bulgarian).
VERGIEVA, T., ZAIKOV, Hr., & PALATOV, S. (1979) [A study on
the embryotoxic action of styrol.] Hyg. Zdrav., XXII: 39-43
(in Russian).
VIHKO , R., VIHKO, P., MAENTAUSTA, O., PAKARINEN, A., JANNE,
O., & YRJANHEIKKI, E. (1983) Assessment of early
hepatotoxicity. In: Aitio, A., Riihimäki, V., & Vainio, H.,
ed. Biological monitoring and surveillance of workers exposed
to chemicals, Washington, Hemisphere Publ. Co. (in press).
VOOGD, C.E., VAN DER STEL, J.J., & JACOBS, J.J.J.A.A. (1981)
The mutagenic action of aliphatic epoxides. Mutat. Res., 89:
269-282.
VOSKAMP, A.J. & STUDENBERG, J.E. (1981) A new low styrene
emission laminating resin. In: 36th Annual Conference,
Reinforced Plastics/Composites Institute, the Society of the
Plastics Industry, Inc., February 16-20, 1981, Session 19-D.
WADE, D.R., AIRY, S.C., & SINSHEIMER, J.E. (1978)
Mutagenicity of aliphatic epoxides. Mutat. Res., 58: 217-223.
WATABE, T., ISOBE, M., SAWAHATA, T., YOSHIKAWA, K., YAMADA,
S., & TAKABATAKE, E. (1978) Metabolism and mutagenicity of
styrene. Scand. J. Work Environ. Health, 4 (Suppl. 2):
142-155.
WATABE, T., OZAWA, N., & YOSHIKAWA, K. (1981a)
Stereochemistry in the oxidative metabolism of styrene by
hepatic microsomes. Biochem. Pharmacol., 30: 1695-1698.
WATABE, T., HIRATSUKA, A., OZAWA, N., & ISOBE, M. (1981b)
Glutathione S-conjugates of phenyloxirane. Biochem.
Pharmacol., 30: 390-392.
WATABE, T., HIRATSUKA, A., AIZAWA, T., & SAWAHATA, T. (1982a)
1-vinylbenzene 1,2- and 3,4-oxides. Tetrahedron Lett., 23:
1185-1188.
WATABE, T., HIRATSUKA, A., AIZAWA, T., SAWAHATA, T., OZAWA,
N., ISOBE, M., & TAKABATAKE, E. (1982b) Studies on the
metabolism and toxicity of styrene IV. 1-vinylbenzene
3,4-oxide, a potent mutagen formed as a possible intermediate
in the metabolism in vivo of styrene to 4-vinylphenol. Mutat.
Res., 93: 45-55.
WATABE, T., OZAWA, N., & YOSHIKAWA, K. (1982c) Studies on
metabolism and toxicity of styrene. V. The metabolism of
styrene, racemic, ( R)-(+), and ( S)-(-) -phenyloxiranes in the
rat. J. Pharm. Dyn., 5: 129-133
WATANABE, T. ENDO, A., SATO, K., OHTSUKI, T., MIYASAKA, M.,
KOIZUMI, A., & IKEDA, M. (1981) Mutagenic potential of
styrene in man. Ind. Health, 19: 37-45.
WATANABE, T., ENDO; A., KUMAI, M., & IKEDA, M. (1983)
Chromosome aberrations and sister chromatid exchanges in
styrene-exposed workers with reference to their smoking
habits. Environmental Mutagenesis, 5: 299-309
WEAST, R.C. & ASTLE, M.J. ed. (1981) Handbook of chemistry
and physics, Boca Raton, CRS Press, p. c-500.
WEIL, C.S., CONDRA, N., HAUN, C., & STRIEGEL, J.A. (1963)
Experimental carcinogenicity and acute toxicity of
representative epoxides. Am. Ind. Hyg. Assoc. J., 24: 305-325.
WHITE, L.D., TAYLOR, D.G., MAUER, P.A., & KUPEL, R.E. (1970)
A convenient optimized method for the analysis of selected
solvent vapors in the industrial atmosphere. Am. Ind. Hyg.
Assoc. J., 31: 225-232.
WILSON, R.H. (1944) Health hazards encountered in the
manufacture of synthetic rubber. J. Am. Med. Assoc., 124:
701-703.
WILTI, D. (1970) Infrared vapour spectra, London, Heyden &
Son Ltd. (Index No. 180).
WINK, A. (1972) Effect of long-term exposure to toxic
substances on urinary excretion of 17-oxogenic steroids and
17-oxosteroids. Ann. occup. Hyg., 15: 211-215.
WITHEY, J.R. (1976) Quantitative analysis of styrene monomer
in polystyrene in foods including some preliminary studies of
the uptake and pharmacodynamics of the monomer in rats.
Environ. Health Perspect., 17: 125-133.
WITHEY, J.R. (1978) The toxicology of styrene monomer and its
pharmacokinetics and distribution in the rat. Scand. J. Work
Environ. Health, 4 (Suppl. 2): 31-40.
WITHEY, J. & COLLINS, P.G. (1977) Pharmakokinetics and
distribution of styrene monomer in rats after intravenous
administration. J. Toxicol. environ. Health, 3: l0ll-l020.
WITHEY, J.R. & COLLINS, P.G. (1978) Styrene monomer in foods:
A limited Canadian survey, Bull. environ. Contam. Toxicol.,
19: 86-94.
WITHEY, J.R. & COLLINS, P.G. (1979) The distribution and
pharmacokinetics of styrene monomer in rats by the pulmonary
route. J. environ. Pathol. Toxicol., 2: 1329-1342.
WOLF, M.A., ROWE, V.K., MCCOLLISTER, D.D., HOLLINGSWORTH,
R.L., & OYEN, F. (1956) Toxicological studies of certain
alkylated benzenes and benzene. Am. Med. Assoc. Arch. ind.
Health, 14: 387-398.
WOLFF, M.S., DAUM, S.M., LORIMER, W.V., & SELIKOFF, I.J.
(1977) Styrene and related hydrocarbons in subacutaneous fat
from polymerization workers. J. Toxicol. environ. Health, 2:
997-1005.
WOLFF, M.S., LILIS, R., LORIMER, W.V., & SELIKOFF, I.J.
(1978a) Biological indicators of exposure in styrene
polymerization workers. Styrene in blood and adipose tissue
and mandelic and phenylglyoxylic acids in urine. Scand. J.
Work Environ. Health, 4 (Suppl. 2): 114-118.
WOLFF, M.S., LORIMER, W.V., LILIS, R., & SELIKOFF, I.J.
(1978b) Blood styrene and urinary metabolites in styrene
polymerization. Br. J. ind. Med., 35: 318-329.
YAMAMOTO, R.K. & COOK, W.A. (1968) Determination of
ethylbenzene and styrene in air by ultraviolet
spectrophotometry. Am. Ind. Hyg. Assoc. J., 29: 283-241.
YOSHIKAWA, K., ISOBE, M., WATANABE, T., & TAKABATAKE, E.
(1980) Studies on metabolism and toxicity of styrene III.
The effect of metabolic inactivation by rat-liver S9 on the
mutagenicity of phenyloxirane toward Salmonella typhimurium.
Mutat. Res., 78: 219-226.
ZAPRIANOV, Z & BAINOVA, A. (1979) Changes in monoamine
oxidase activity (MAO) after styrene and ethanol combined
treatment of rats. Activ. nerv. Sup. (Praha), 21(4): 262-264.
ZETTERBERG, G. (1982) Styrene - a widespread mutagen:
conclusions from the results of testing. In: Sugimura, T.,
Kondo, S. & Takabe, H., ed. Environmental Mutag. Carcinog.,
Tokyo, University of Tokyo Press, pp. 316-322.
ZIELHUIS, R.L., HARTOGENSIS, F., JONGH, J., KALSBEEK, J.W.H.,
& VAN REES, H. (1964) The health of workers processing
reinforced polyesters. In: XIVth International Congress of
Occupational Health, Madrid, Spain, 16-21 September, 1963,
Volume III, pp. 1092-1097.
ZITTING, A., HEINONEN, T., & VAINIO, H. (1980) Glutathione
depletion in isolated rat hepatocytes caused by styrene and
the thermal degradation products of polystyrene. Chem. biol.
Interact., 31: 313-318.
ZLOBINA, N.S. (1963) [On the toxicity of small concentrations
of styrol vapors.] Gig. i Sanit., 28: 29-35 (in Russian).
