Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 2000
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer-review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
©World Health Organization 2000
Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria monographs as accurately as possible without unduly delaying their publication. In the interest of all users of the Environmental Health Criteria monographs, readers are requested to communicate any errors that may have occurred to the Director of the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland, in order that they may be included in corrigenda.
A detailed data profile and a legal file can be obtained from the International Register of Potentially Toxic Chemicals, Case postale 356, 1219 Chatelaine, Geneva, Switzerland (telephone no. + 41 22 9799111, fax no. + 41 22 7973460, E-mail irptcp@unep.ch).
This publication was made possible by grant number 5 U01 ES02617-15 from the National Institute of Environmental Health Sciences, National Institutes of Health, USA, and by financial support from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was initiated with the following objectives:
Since its inauguration the EHC Programme has widened its scope, and the importance of environmental effects, in addition to health effects, has been increasingly emphasized in the total evaluation of chemicals.
The original impetus for the Programme came from World Health Assembly resolutions and the recommendations of the 1972 UN Conference on the Human Environment. Subsequently the work became an integral part of the International Programme on Chemical Safety (IPCS), a cooperative programme of UNEP, ILO and WHO. In this manner, with the strong support of the new partners, the importance of occupational health and environmental effects was fully recognized. The EHC monographs have become widely established, used and recognized throughout the world.
The recommendations of the 1992 LTN Conference on Environment and Development and the subsequent establishment of the Intergovernmental Forum on Chemical Safety with the priorities for action in the six programme areas of Chapter 19, Agenda 2 1, all lend further weight to the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews on the effect on human health and the environment of chemicals and of combinations of chemicals and physical and biological agents. As such, they include and review studies that are of direct relevance for the evaluation. However, they do not describe every study carried out. Worldwide data are used and are quoted from original studies, not from abstracts or reviews. Both published and unpublished reports are considered and it is incumbent on the authors to assess all the articles cited in the references. Preference is always given to published data. Unpublished data are used only when relevant published data are absent or when they are pivotal to the risk assessment. A detailed policy statement is available that describes the procedures used for unpublished proprietary data so that this information can be used in the evaluation without compromising its confidential nature (WHO (1990) Revised Guidelines for the Preparation of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World Health Organization).
In the evaluation of human health risks, sound human data, whenever available, are preferred to animal data. Animal and in vitro studies provide support and are used mainly to supply evidence missing from human studies. It is mandatory that research on human subjects is conducted in full accord with ethical principles, including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and international authorities in making risk assessments and subsequent risk management decisions. They represent a thorough evaluation of risks and are not, in any sense, recommendations for regulation or standard setting. These latter are the exclusive purview of national and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
Since the inception of the EHC Programme, the IPCS has organized meetings of scientists to establish lists of priority chemicals for subsequent evaluation. Such meetings have been held in: Ispra, Italy, 1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North Carolina, USA, 1995. The selection of chemicals has been based on the following criteria: the existence of scientific evidence that the substance presents a hazard to human health and/or the environment; the possible use, persistence, accumulation or degradation of the substance shows that there may be significant human or environmental exposure; the size and nature of populations at risk (both human and other species) and risks for the environment; international concern, i.e. the substance is of major interest to several countries; adequate data on the hazards are available.
If an EHC monograph is proposed for a chemical not on the priority list, the IPCS Secretariat consults with the Cooperating Organizations and all the Participating Institutions before embarking on the preparation of the monograph.

Procedures
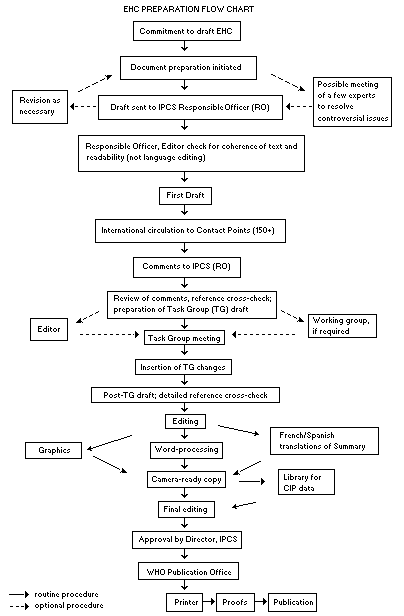
The order of procedures that result in the publication of an EHC monograph is shown in the flow chart. A designated staff member of IPCS, responsible for the scientific quality of the document, serves as Responsible Officer (RO). The IPCS Editor is responsible for layout and language. The first draft, prepared by consultants or, more usually, staff from an IPCS Participating Institution, is based initially on data provided from the International Register of Potentially Toxic Chemicals, and reference data bases such as Medline and Toxline.
The draft document, when received by the RO, may require an initial review by a small panel of experts to determine its scientific quality and objectivity. Once the RO finds the document acceptable as a first draft, it is distributed, in its unedited form, to well over 150 EHC contact points throughout the world who are asked to comment on its completeness and accuracy and, where necessary, provide additional material. The contact points, usually designated by governments, may be Participating Institutions, IPCS Focal Points, or individual scientists known for their particular expertise. Generally some four months are allowed before the comments are considered by the RO and author(s). A second draft incorporating comments received and approved by the Director, IPCS, is then distributed to Task Group members, who carry out the peer review, at least six weeks before their meeting.
The Task Group members serve as individual scientists, not as representatives of any organization, government or industry. Their function is to evaluate the accuracy, significance and relevance of the information in the document and to assess the health and environmental risks from exposure to the chemical. A summary and recommendations for further research and improved safety aspects are also required. The composition of the Task Group is dictated by the range of expertise required for the subject of the meeting and by the need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the important role played by non-govermental organizations. Representatives from relevant national and international associations may be invited to join the Task Group as observers. While observers may provide a valuable contribution to the process, they can speak only at the invitation of the Chairperson. Observers do not participate in the final evaluation of the chemical; this is the sole responsibility of the Task Group members. When the Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers participate in the preparation of the EHC monograph must, in addition to serving in their personal capacity as scientists, inform the RO if at any time a conflict of interest, whether actual or potential, could be perceived in their work. They are required to sign a conflict of interest statement. Such a procedure ensures the transparency and probity of the process.
When the Task Group has completed its review and the RO is satisfied as to the scientific correctness and completeness of the document, it then goes for language editing, reference checking, and preparation of camera-ready copy. After approval by the Director, IPCS, the monograph is submitted to the VMO Office of Publications for printing. At this time a copy of the final draft is sent to the Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the updating of an EHC monograph: new data are available that would substantially change the evaluation; there is public concern for health or environmental effects of the agent because of greater exposure; an appreciable time period has elapsed since the last evaluation.
All Participating Institutions are informed, through the EHC progress report, of the authors and institutions proposed for the drafting of the documents. A comprehensive file of all comments received on drafts of each EHC monograph is maintained and is available on request. The Chairpersons of Task Groups are briefed before each meeting on their role and responsibility in ensuring that these rules are followed.
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR dinitro-ortho-cresol
Members
Dr D. Anderson, British Industrial Biological Research Association (BIBRA) International, Carshalton, Surrey, United Kingdom
Dr B.H. Chen, Department of Environmental Health, School of Public Health, Shanghai Medical University, Shanghai, People’s Republic of China
Dr S. Dobson, The Institute of Terrestrial Ecology, Monks Wood Experimental Station, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Professor M.C.A. Lotti, Università degli Studi di Padova, Istituto di Medicina del Lavoro, Azienda Ospedaliera, Padova, Italy (Chairman)
Dr P. Lundberg, Risk Evaluation Group, Department of Occupational Medicine, National Institute for Working Life, Solna, Sweden
Dr L.R. Papa, National Center for Environmental Assessment – CIN, US Environmental Protection Agency, Cincinnati, Ohio, USA
Dr A.F. Pelfrène, The Agrochemicals Defense Network, La Marjolaine, Charbonnières-les-Bains, France (Rapporteur)
Professor S.A. Soliman, Department of Pesticide Chemistry, Faculty of Agriculture, Alexandria University, El-Shatby, Alexandria, Egypt
Secretariat
Mr Y. Hayashi, Scientist, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Dr Y. Uyama, Food Chemistry Division, Environmental Health Bureau, Ministry of Health and Welfare, Tokyo, Japan (On secondment to the International Programme on Chemical Safety)
Dr M. Younes, Acting Coordinator, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland (Secretary)
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DINITRO-ortho-CRESOL
A WHO Task Group on Environmental Health Criteria for Dinitro-ortho-cresol was held at the World Health Organization, Geneva, Switzerland from 20 to 23 April 1999. Dr R. Helmer, Director, Department for the Protection of the Human Environment, opened the meeting and welcomed the participants on behalf the IPCS and its three cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the draft criteria monograph and made an evaluation of the risks for human health and the environment from exposure to dinitro-ortho-cresol.
Dr A.F. Pelfrène prepared the first draft of this monograph. The second draft incorporated comments received following the circulation of the first draft to the IPCS Contact Points for Environmental Health Criteria monographs.
Dr B.H. Chen (IPCS) and Ms K. Lyle (Sheffield, England) were responsible for the overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation and finalization of the monograph are gratefully acknowledged.
* * *
Financial support for this Task Group was provided by the US Food and Drug Administration as part of its contributions to the IPCS.
ABBREVIATIONS
| 4-ANOC | 4-amino-6-nitro-o-cresol |
| 6-ANOC | 6-amino-4-nitro-o-cresol |
| 6-Ac ANOC | 6-acetamido-4-nitro-o-cresol |
| ADI | acceptable daily intake |
| ADP | adenosine disphosphate |
| AdSV | adsorptive stripping voltametric detector |
| a.i. | active ingredient |
| ALT | alanine aminotrasferase |
| 3-ANSA | 3-amino-5-nitrosalicyclic acid |
| AST | aspartate aminotransferase |
| ATP | adenosine triphosphate |
| b.w. | body weight |
| BMR | basal metabolic rate |
| BOEL | biological operation exposure limit |
| BSI | British Standards Institute |
| CA | Chemical Abstracts |
| CAS | Chemical Abstracts Services |
| DECOS | Dutch Expert Committee on Occupational Standards |
| DNC | synonym for DNOC |
| DNHMP | 4,6-dinitro-2-hydroxymethylphenol |
| DNOC | 4,6 dinitro-o-cresol |
| DPP | differential pulse polarographic detector |
| DT50 | median degradation time |
| EC | emulsifiable concentrate |
| EC50 | median effective concentration |
| ELCD | electrochemical detector |
| ENT 154 | synonym for DNOC |
| EPPO | European and Mediterranean Plant Protection Organization |
| FID | flame ionization detection |
| F0 | first filial generation |
| GC | gas chromatography |
| GLP | Good Laboratory Practice |
| GTZ | German Agency for Technical Cooperation |
| HPLC | high-performance liquid chromatography |
| HRGC | high-resolution gas chromatography |
| ISO | International Organization for Standardization |
| IUPAC | International Union of Pure and Applied Chemistry |
| JMAF | Japanese Ministry of Agriculture and Forestry |
| JMPR | FAO/WHO Joint Meeting on Pesticide Residues |
| LC50 | median lethal concentration |
| LC–MS | liquid chromatography–mass spectrometry |
| LD50 | median lethal dose |
| MACWZ | maximum allowable concentration in the working zone |
| MRL | maximum residue limit |
| MS | mass spectrometry |
| MS–MS | tandem mass spectrometry |
| MTD | maximum tolerated dose |
| NOAEL | no observed adverse effect level |
| NOEC | no observed effect concentration |
| NOEL | no effect level |
| NPD | nitrogen phosphorus detector |
| OECD | Organisation for Economic Co-operation and Development |
| OL | oil-miscible liquids |
| PA | Pastes |
| PDD | photodiode array detector |
| PND | phosphorus/nitrogen detector |
| PT50 | median photolysis time |
| RSD | relative standard deviation |
| SC | suspension concentrate |
| SGOT | see ALT |
| SGPT | see AST |
| SPE | solid phase extraction |
| SPME | solid phase microextraction |
| t½ | half-life |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| JMPR | FAO/WHO Joint Meeting on Pesticide Residues |
| TER | toxicity exposure ratio |
| TSELhm | tentatively safe exposure level in the atmosphere of residential areas |
| TWA | time weighted average |
| UV | Ultraviolet |
| v/v | volume per volume |
The solubility of DNOC in water is 6.94 g/litre at 20 °C and pH 7, and largely depends on pH.
DNOC is relatively stable in sterile water.
DNOC is analysed in environmental media by high-performance liqid chromatrography (HPLC) with ultraviolet (UV) detection or by gas chromatography (GC) with detection by nitrogen phosphorus dection (NPD), flame ionization detection (FID) or mass spectrometry (MS). In biological fluids, determination of DNOC is usually by spectrophotometry and more recently by either GC/NPD or HPLC/UV.
Occupational exposure is expected to occur in agriculture and in the chemical industry.
DNOC did not induce any teratogenic effects in pregnant rats receiving oral doses up to 25 mg/kg b.w. per day from gestation day 6 to day 15, inclusive. In rabbits, treated orally, the high dose of 25 mg/kg b.w. per day was maternally toxic, inducing mortality. At this dose level teratogenic effects, including microphthalmia or anophthalmia and hydrocephaly or microcephaly, were observed.
When administered to pregnant rabbits by cutaneous application during gestation, DNOC induced maternal toxicity at the high dose of 90 mg/kg b.w. per day, resulting in some embryotoxicity but not teratogenicity. No evidence of teratogenicity or embryotoxicity was recorded in mice treated orally or intraperitoneally during pregnancy.
DNOC is acutely toxic to honey bees but exposure is likely to be low; hazard quotients for honey bees indicate low risk. TER for earthworms (LC50 at 17 mg/kg soil) indicates moderate risk following use of DNOC as a desiccant.
The high acute toxicity of DNOC for birds and mammals is unlikely to be manifest in the environment because exposure is likely to be low. This conclusion is supported by limited reports of incidents in the field. Further characterization of risk is not possible because field information on residues and effects is not available.
Given the present use patterns of the plant protection product containing DNOC as the active ingredient, there are no detectable residues in treated crops, and thus no exposure of the general population.
DNOC is a skin sensitizer in guinea-pigs.
Agricultural use as a desiccant and on dormant fruit crops leads to calculated risk factors indicating possible adverse effects on aquatic organisms (from spray drift) and earthworms. Other organisms in the field are unlikely to be adversely affected because exposure will be low. No risk assessment was attempted for possible other uses of DNOC (such as locust control) because of lack of information on application rates and methods.
Chemical structure:
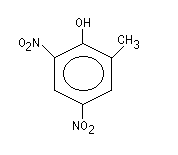
| Relative molecular mass: | 198.13 |
| Common name: | DNOC (ISO, WSSA, BSI, JMAF) |
| Chemical names: | 4,6-dinitro-ortho-cresol
(IUPAC)
2-methyl-4,6-dinitrophenol (CA) 2,4-dinitro-ortho-cresol 3,5-dinitro-2-hydroxytoluene 2,4-dinitro-6-methylphenol |
| Synonyms: | DNC; ENT 154 |
| Common trade names: | Antinonin; Bonitol; Dinitrol; Technolor; Trifocide; Trifina; Veraline |
| Trade names no longer in use: | Elgetol; Extar A; Nicyl; Nitrador; Sandoline; Selinon; Sinox |
| CAS registry number: | |
| CIPAC number: | 19 |
| EEC number: | 208 601 1 |
| UN number: | 1598 |
Table 1. Physical and chemical properties of DNOC
| Property | Characteristics | Reference |
| Physical state | yellow, crystalline, solid | Jongerius & Jongeneelen (1991) |
| Crystal structure | Triclinic | Jongerius & Jongeneelen (1991) |
| Purity of the technical product | 97.45%
95–98% |
Sainsbury et al. (1995)
Tomlin (1997) |
| Molecular weight | 198.13 | Tomlin (1997) |
| Melting point | 88.2–89.8 °C | Hope et al. (1995) |
| Boiling point | 312 C | Jongerius & Jongeneelen (1991) |
| Vapour pressure | 1.6 × 10–2 Pa at 25 ° C | Howarth et al. (1995) |
| Relative density | 1.58 at 20 ° C | Hope et al. (1995) |
| Solubility in water (20 ° C) | 0.214 g/litre at pH 4
6.94 g/litre at pH 7 33.3 g/litre at pH 10 |
Hope et al. (1995) |
| Solubility in organic solvents (at 20 ° C) | Hope et al. (1995), Tomlin (1997) | |
| toluene | 251 g/litre | |
| methanol | 58.4 g/litre | |
| dichloromethane | 503 g/l | |
| acetone | 514 g/litre | |
| hexane | 4.03 g/litre | |
| log Pow | 1.78 at pH 4
8.67 × 10–2 at pH 7 |
Hope et al. (1995) |
| Dissociation constant (pKa) | 4.48 at 20 ° C
4.9 and pH limits 3–8.5 |
Hope et al. (1995)
Heimlich & Nolte (1993) |
| Vapour density | 6.84 (air = 1) | Jongerius & Jongeneelen (1991) |
| Saturation vapour concentration (20–25 ° C) | 0.56–1.0 mg/m3 | Jongerius & Jongeneelen (1991) |
| Conversion factor
(at 760 mmHg and 20 ° C) |
1 mg/m3 = 0.12 ppm
1 ppm = 8.24 mg/m3 |
Jongerius & Jongeneelen (1991) |
| Flammability | no auto-ignition below 400 ° C | Tremain & Bartlett (1995) |
| Stability in water | DT50 >1 year | Tomlin (1997) |
| Photolysis | PT50 _ 253 h (20 ° C) | Tomlin (1997) |
Like all other dinitrophenols, DNOC is a pseudoacid and readily forms water-soluble salts with alkalis (Metcalf, 1978; HSDB, 1994). At pH 4.4, more than 50% of the DNOC in water exists as the free anion. The concentration of DNOC in ionized form increases as the pH increases, and at pH 7 or above 100% of DNOC will be in the ionized form. Therefore, at physiological pH DNOC is either ionized or bound to macromolecules (i.e., albumin) (King & Harvey, 1953b).
Levels of DNOC in environmental and biological samples can be measured following several extraction or clean-up steps. These steps might include liquid–liquid extraction, solid phase extraction or solid phase microextraction. Both HPLC and GC with several detection methods are used for final separation and quantification.
All analytical methods used for measuring DNOC in biological samples listed in Table 3 rely on spectrophotometry for final quantification, with the exception of those of Hopper et al. (1992) and Diepenhorst et al. (1995). False positive results may be obtained by these methods because of abnormally high bilirubin or carotene levels in the blood (Jongerius & Jongeleenen, 1991).
Table 2. Analytical methods for measuring DNOC in environmental samples
| Type of sample | Preparation | Analytical method | Detection limit | Recovery (%) | Reference |
| Technical and formulated products | Dissolve sample in methanol or acetone | HPLC/UV | 2 nga | No data | Farrington et al. (1982); Yao et al. (1991) |
| Technical products | Dissolve sample in methanol | HPLC/ELCD | 0.1 nga
(oxidative) 0.4 nga (reductive) |
No data | Yao et al. (1991) |
| Air | Draw air through filter and a midget bubbler in series. DNOC extracted into ethylene glycol and 2-propanol added before analysis | HPLC/UV (method S166) | 0.070 mg/m3 (8 ppb) for 180-litre sample | 104 for 0.07 mg loaded on to filter | NIOSH (1984) |
| Water | Sample adjusted to pH 6.1 by buffer | HPLC/AdSV
HPLC/DPP |
0.1 µg/litre (AdSV)
1.5 µg/litre (DPP) |
No data | Benadikova & Kalvoda, (1984) |
| Water | Extract reconstituted in methanol-acetonitrile acetic acid (20:78.5:1.5 v/v) | HPLC/UV | No data | 97 | Tripathi et al. (1989) |
| Drinking-water, atmospheric water | Acidify sample, add salt, and extract continuously with methylene chloride. Dry, reduce volume, and solvent exchange to hexane. Derivatize with acetic anhydride | GC/NPD | 0.20 µg/litre
(0.2 ppm) |
102 (5.5% RSD) | Herterich (1991) |
| Drinking-water, groundwater | Acidify water, add sodium sulfite, and pass through SPE cartridge of Carbopak. Elute with methanol/ methylene chloride; reduce volume | HPLC/UV | 0.009 µg/litre
(9 ppb) |
96 | Di Corcia & Marchetti (1992) |
| Groundwater | Acidify to pH 2, saturate with salt, and extract using SPME | GC/MS | 0.070 µg/litre (0.07 ppm) (5.6% RSD) | No data | Buchholz & Pawliszyn (1993) |
| Groundwater, sediment | Extract acidified water with methylene chloride, reduce volume and solvent exchange to 2-propanol | GC/FID
(Method 8040) |
160 µg/litre | 0.84C – 1.01 where C is the true value of concentration in µg/litre | US EPA (1986a) |
| Groundwater, soil, solid waste | Extract acidified water with methylene chloride, reduce volume and exchange into 2-propanol. For other matrices, mix with anhydrous sodium sulfate and extract (soxhlet or sonication) with methylene chloride. Reduce volume. Clean up with silica gel if needed | GC/MS
(Method 8270) |
50 µg/litre
(50 ppm water); 3.3 mg/kg (ppm soil/ sediment) |
1.04C – 28.04 where C is the true value of concentration in µg/litre | US EPA (1986b) |
| Waste water | Extract acidified sample with methylene chloride; concentrate and exchange solvent to 2-propanol | GC/FID
(Method 604) |
16 µg/litre
(16 ppm) |
83 at 100 µg/litre | US EPA (1984a) |
| Waste water | Extract acidified sample with methylene chloride; concentrate | GC/MS
(Method 625) |
24 µg/litre
(24 ppm) |
93 at 100 µg/litre | US EPA (1984b) |
| Waste water | Extract acidified sample with methylene chloride, dry and reduce volume. Add deuterated standards | GC/MS isotope dilution
(Method 1625) |
20 µg/litre
(20 ppm) |
77–133 at 100 µg/litre | US EPA (1984c) |
| Rain and snow | Extract acidified sample with methylene chloride; concentrate | HPLC/PDD | No data | No data | Alber et al. (1989) |
| Soil | Extract with methylene chloride; evaporate to dryness and dissolve residue in alkaline methanol/water | HPLC/UV | 0.005 mg/kg
(5 ppb) |
85–105 | Roseboom et al. (1981) |
| Soil | Soxhlet extraction of clay loam using hexane : acetone (1 : 1). Reduce volume | GC/MS | No data | 63.4 at 6 mg/kg | Lopez-Avila et al. (1993) |
| Various crops | Extract macerated or homogenized sample with methylene chloride; evaporate to dryness and dissolve in potassium carbonate/methanol mixture | HPLC/UV | 0.005 mg/kg
(5 ppb) |
82–105 at 0.05 mg/kg
%RSD range 4–13% |
Roseboom et al. (1981) |
| Various crops | Homogenize sample in blender, adding distilled water as needed. Add Florisil to form free flowing mixture and pack into a column with a sodium sulfate layer at bottom. Elute with methylene chloride : acetone (1 : 1) or ethyl acetate. Reduce volume | GC/ELCD | 0.001 mg/kg
(1 ppb) |
69–79 at 0.01–0.5 mg/kg | Kadenczki et al. (1992) |
| Fatty and non-fat foods | Mix fatty sample with methanol, sulfuric acid and potassium oxalate and, non-fat samples with sulfuric acid and methanol; extract both with petroleum ether or methylene chloride; clean-up by gel permeation chromatography, methylate and clean up with Florisil | GC/NPD | No data | 45–50 (fatty foods)
>80 (non-fat foods) |
Hopper et al. (1992) |
aThere are absolute detection limits.
AdSV, adsorptive stripping voltametric detector; DPP, differential pulse polarographic detector; ELCD, electrochemical detector; FID, flame ionization detection; GC, gas chromatography, HPLC, high-performance liquid chromatography; HRGC, high-resolution gas chromatography; MS, mass spectometry; NPD, nitrogen phosphorus detector; PDD, photodiode array detector; RSD, relative standard deviation; SPE, solid phase extraction; SPME, solid phase microextraction; UV, ultraviolet detector; v/v, volume per volume.
Table 3. Analytical methods for measuring DNOC in biological samples
|
Sample matrix |
Preparation method |
Analytical method |
Sample detection limit |
Recovery (%) |
Reference |
|
Animal tissue |
Extract sample mixed with methanol, sulfuric acid, and potassium oxalate with petroleum ether; clean up by gel permeation chromatography, methylate, and clean up with Florisil |
GC-NPD |
No data |
45–50 |
Hopper et al. (1992) |
|
Urine, kidney, liver, brain (DNOC and metabolite 4-amino-2-methyl-6-nitrophenol) |
Hydrolyse sample directly or after acetone extraction; extract with petroleum ether |
Spectrophotometric |
No data |
No data |
Truhaut & de Lavaur (1967) |
|
Serum |
Dilute with water; add sodium chloride and sodium carbonate and extract with methyl ethyl ketone |
Spectrophotometric |
<0.5 mg/litre |
No data |
Parker (1949) |
|
Serum |
Samples were acid coagulated then serum separated by centrifugation |
HPLC/UV |
0.05 m g/g |
91.0 |
Diepenhorst et al. (1995) |
|
Tissue |
Dilute homogenized tissue with water; add sodium chloride and sodium carbonate; extract with methyl ethyl ketone |
Spectrophotometric |
No data |
No data |
Parker (1949) |
|
Urine (DNOC and metabolite 4-amino-2-methyl-6-nitrophenol) |
Acidify and subject to continuous extraction with diethyl ether |
Spectrophotometric |
No data |
No data |
Smith et al. (1953) |
|
Urine |
Add sodium chloride and sodium carbonate; extract with methyl ethyl ketone |
Spectrophotometric |
No data |
No data |
Parker (1949) |
GC, gas chromatography; NPD, nitrogen phosphorus detection device.
DNOC is also used as a desiccant in potatoes. It is sprayed once or twice on seed potatoes between July and September to desiccate the haulms in order to prevent virus and disease contamination of the tubers, and incidentally to facilitate mechanical harvesting. The registered rates of application of DNOC range from 2.5 to 5.6 kg/ha.
DNOC is formulated as emulsifiable concentrate (EC) for use as a potato haulm desiccant and as a suspension concentrate (SC) for winter treatment on fruit trees. Other types of formulation include pastes (PA) and oil-miscible liquids (OL). It is understood that DNOC is still used as a desiccant for crop potatoes and in locust control in developing countries. However, details of sources, application rates and methods are not available.
Although the use of DNOC as a pesticide has currently declined, and also because it has been banned in some countries (see for instance EC, 1999), there are still significant volumes of obsolete stocks of this chemical around the world, especially in developing countries. The German Agency for Technical Cooperation (GTZ) has helped in disposing of 57.6 tonnes of DNOC in the United Republic of Tanzania by incineration in a cement kiln (GTZ, 1997). More than 14 tonnes of obsolete DNOC have been located in Zambia (Wodageneh, 1997).
The main current use of DNOC is in the plastics industry as an inhibitor of polymerization in styrene and vinyl aromatic compounds. It is also used as an intermediate for synthesis of other fungicides, dyes and pharmaceuticals (Hawley, 1981; US EPA, 1988).
On the basis of the Gustafson (1989) groundwater ubiquity score, DNOC is considered to have a limited potential to leach from soil to groundwater.
DNOC is metabolized in soil. One bacterium of the Arthrobacter species is capable of using the compound as its source of carbon and nitrogen (Gasiewicz, 1991). It was also demonstrated that DNOC is rapidly inactivated in soil by a form of Corynebacterium simplex with formation of nitrite (Jensen & Gundersen, 1955). The biological decomposition of DNOC in soils was reviewed by Jensen (1966).
Tewfik & Evans (1966) have isolated a Pseudomonas species able to degrade DNOC in soils. The degradation of DNOC by 31 strains of Rhizobium and 5 strains of Azotobacter has been described (Hamdi & Tewfik, 1970); this microflora is important in nitrogen fixation.
The degradation of DNOC in three types of standard soils was investigated over a period of 88 days, at 20 °C, in the dark, at an application rate of 4.9 mg 14C-labelled DNOC/kg (dry weight) of soil. This is equivalent to a field application rate of 5 kg DNOC/ha. The DT50 was determined to be 1.7, 5.9 or 12 days, depending on the soil type. The main final degradation product of the aromatic ring was carbon dioxide, representing 39% of the applied radioactive dose; the main non-volatile metabolite was 2-methyl-4-nitrophenol, representing 40% of the applied radiocarbon between day 10 and day 20, and declining thereafter. The amount of bound residues in soil after extraction with organic solvents increased over the course of the study to reach 37% (Bieber, 1995). The presence of 2-methyl-4-nitrophenol as a decomposition product of DNOC in soil was confirmed by Verheij & van der Graaf (1995) by combined liquid chromatography–mass spectrometry (LC–MS) and tandem mass spectrometry (MS–MS).
DNOC has been identified in extracts of rain (Leuenberger et al., 1988; Alber et al., 1989), and snow (Alber et al., 1989). One pathway through which DNOC can enter the atmosphere is from overspray during use on agricultural products. DNOC has been detected in rain throughout the year, and its concentrations in rain did not show a trend with seasonal applications to crops (Leuenberger et al., 1988). These observations, and its low volatility, indicate that DNOC most likely enters the atmosphere through another mechanism. The low air–water partition coefficient of DNOC allows it to be scavenged effectively by precipitation, and enriched in humid aerosols, fog, clouds and rain droplets.
Biodegradation is the most significant process for removal from water and soil.
In the plastics industry, workers may be exposed to dusts when the damping water is removed before use. DNOC is used to inhibit immediate polymerization of styrene during the distillation and purification stages of manufacture. During the process, DNOC remains in the distillation columns, thereby ensuring that the finished styrene monomer contains no residues. The distillation process allows recycling of some DNOC, and the remaining DNOC-rich by-products are incinerated, thereby greatly reducing the risk of occupational and environmental exposures.
Table 4 summarizes the time-weighted average (TWA) values for occupational exposures.
In the former USSR, a maximum allowable concentration in the working zone (MACWZ) of 0.05 mg/m3 as a mixture of vapour and aerosol; a value of 0.002 mg/m3 for the lightest short, single exposure, tentatively safe exposure level in the atmosphere of residential areas (TSELhm); and a value of 0.05 mg/litre for surface water were established (Izmerov et al., 1982).
WHO (1982) indicates: "there exists a fair agreement, although no adequately valid relationship has yet been established, that – on the basis of human data – a blood DNOC level below 20 mg/litre will probably not lead to manifest health impairment"; the Dutch Expert Committee on Occupational Standards recommended a biological operator exposure limit (BOEL) in whole blood of 10 µg/ml. In their report, prepared on behalf of the Industrial Medicine and Hygiene Unit of the Health and Safety Directorate of the Commission of the European Communities, Jongerius & Jongeneelen (1991) recommended, based on human exposure data, a BOEL of 10 µg/ml in serum or 5 µg/ml in whole blood for workers not exposed to heat stress.
Table 4. TWA values for DNOC occupational exposures
| Country | TWA
(mg/m3 per 8 h) |
Year established |
| Argentina | 0.2 | 1991 |
| Canada | 0.2 | 1994 |
| Finland | 0.2 | 1996 |
| Denmark | 0.2 | 1996 |
| Germany | 0.2 | 1996 |
| Mexico | 0.2 | 1991 |
| Netherlands | 0.2 | 1996 |
| Norway | 0.2 | 1996 |
| UK | 0.2 | 1996 |
| USA (OSHA) | 0.2 | 1996 |
| USA (NIOSH) | 0.2 (10 h) | 1996 |
Source: UNEP Chemicals (IRPTC) (1999).
DNOC may be absorbed through the skin as well as by ingestion or inhalation of aerosols. The skin is the principal route of exposure in agricultural workers. The metabolic pathway of DNOC is identical in several non-ruminant mammalian species, but the rate at which it is cleared from the organism varies between species. In ruminants, DNOC undergoes an initial phase of bacterial metabolism in the rumen before it is absorbed into the blood.When applied under the same conditions as an oily formulation, the peak plasma concentrations represent 5.0% of the applied dose in males and 5.8% in females (38–45 µg/ml of blood). The peak plasma level occurred after 8 h in males and 24 h in females. The average t½ absorption was 2.8 h and the average t½ elimination was 34 h.
DNOC is more readily absorbed through the skin in oily formulation than when in aqueous solution; the peak plasma concentration is higher and is reached earlier. However, elimination remains fairly rapid, and the residual plasma and skin levels are comparable for the two types of formulation (Fabreguettes, 1993).
Following a single dose by gavage, the maximum plasma concentration is reached in 2–4 h in rats, and in 4–6 h in rabbits (Gasiewicz, 1991).
A single oral dose of 14C-labelled DNOC (0.4 mg/kg b.w.) given to two rats resulted in the following tissue distribution:
A field study of 18 sprayers showed that a daily exposure to DNOC leads to continuous elevation of DNOC level in the blood. The plasma levels increased daily and, at the end of the season, plasma levels ranged from 11 to 88 µg/ml (van Noort, 1960).
Leegwater et al. (1982) and van der Greef & Leegwater (1983) have identified similar metabolites as well as two other new ones, not previously described: 4,6-diacetamido-o-cresol (DAcAOC) and 4,6-dinitro-2-hydroxymethylphenol (DNHMP) in the urine of rats treated with a single oral dose of 0.4 or 6.0 mg DNOC/kg b.w., and in the urine of a rabbit administered orally a single dose of 20 mg DNOC/kg b.w.
Based on these observations, it may be concluded that rats and rabbits metabolize DNOC along the same pathway (Fig. 1) as suggested by Leegwater et al. (1982) with slight modification.
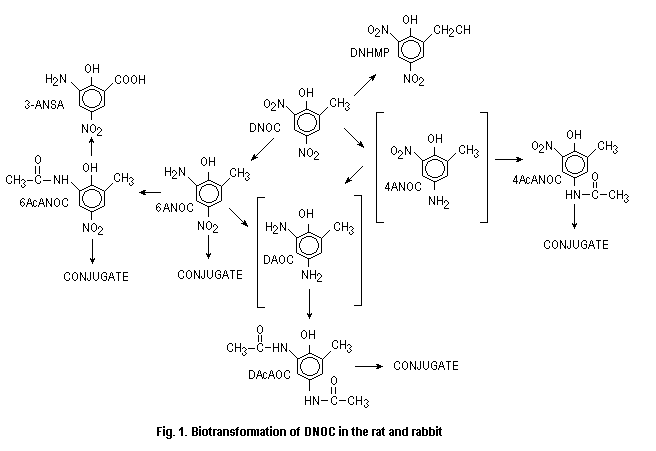
In an in vitro study in which DNOC was incubated with the contents of rat caecum, a rapid reduction to 6-ANOC occurred and 6-ANOC was then converted to DAOC. After 12 h of contact, 90% of the initial concentration of DNOC had been metabolized to DAOC (Ingebritsen & Froslie, 1980).
In ruminants (cattle), DNOC induces methaemoglobinaemia when administered intra-ruminally. This effect is related to the reduction process mediated by microflora that occurs in the rumen, leading to the formation of aminophenols and diaminophenols, which are known to be methaemoglobin-forming compounds (Harvey, 1958; Froslie & Karlog, 1970; Froslie 1973). The role of the microflora in the metabolism of DNOC by ruminants was confirmed experimentally in sheep by Jegatheeswaran & Harvey (1970).
In female rabbits, the half-life was determined to be approximately 6.5 h. After repeated dosing in humans, the DNOC level in blood increases more than that in laboratory animals (Harvey et al., 1951), because it is excreted at a slower rate in humans than in animals (Parker et al., 1951; Pollard & Filbee, 1951). In humans, the half-life of DNOC has been calculated from blood levels measured under circumstances related to heavy occupational exposure. The half-lives so determined varied from 96 h (van Noort, 1960) to 148 h (Jastroch et al., 1978) or 153.6 h (Pollard & Filbee, 1951) in severely poisoned sprayers. Lawford et al. (1954) have demonstrated that the elimination rate in descending order was:
Van den Berg et al. (1991) found that DNOC is an in vitro competitor for the thyroxine (T4) binding site on the plasma protein transthyretin. This plasma protein is a carrier for vitamin A and hormones, including T4. Speculations suggest that DNOC may alter thyroid hormone levels in plasma, thereby affecting thyroid functions.
Table 5. Acute toxicity of DNOC in laboratory animals
| Route | Species | LD50/LC50
(mg/kg b.w.)a |
Reference |
| Oral | rat | 20 (at 37–40 °C) | King & Harvey (1953a) |
| Oral | rat | 25 | Ben Dyke et al (1970) |
| Oral | rat | 30 (minimum lethal dose) | Ambrose (1942) |
| Oral | rat | 31 | Driscoll (1995 a) |
| Oral | rat | 50 | Spencer et al (1948) |
| Oral | rat | 85 | Burkatskaya (1965b) |
| Oral | cat | 50 | Jongerius & Jongeneelen (1991) |
| Oral | mouse | 16 | Jongerius & Jongeneelen (1991) |
| Oral | mouse | 47 | Jongerius & Jongeneelen (1991) |
| Oral | pig | 50–100 | McGirr & Papworth (1953) |
| Dermal | rat | 200–600 | Ben Dyke et al (1970) |
| Dermal | rat | >2000 | Driscoll (1995b) |
| Dermal | rabbit | 500 (no effect) | Burkatskaya (1965b) |
| Dermal | rabbit | 1000 | Burkatskaya (1965b) |
| Dermal | mouse | 187 | Arustamyn (1972) |
| Dermal | guinea-pig | 200 (no effect) | Jongerius & Jongeneelen (1991) |
| Dermal | guinea-pig | 500 (LD100) | Spencer et al (1948) |
| Inhalation | rat | 100 mg/m3 (4 h) (no effect) | King & Harvey (1953) |
| Inhalation | rat | 230 mg/m3 (4 h) | Dey-Hazra & Heisler (1981) |
| Inhalation | cat | 40 mg/m3 (4 h) | Burkatskaya (1965a) |
| Intraperitoneal | rat | 29. | Gasiewicz (1991) |
| Intraperitoneal | mouse | 24–26 | Gasiewicz (1991) |
| Intraperitoneal | rabbit | 24 | Jongerius & Jongeneelen (1991) |
| Intraperitoneal | guinea-pig | 23 | Jongerius & Jongeneelen (1991) |
No treatment-related mortality was recorded. No significant effects were observed on food consumption and haematology. The body-weight gain of the females of the two highest concentrations was significantly lower (p < 0.001) than that of the controls, whereas the food conversion factor in these two groups was slightly elevated. This effect is directly related to the particular mode of action of the compound. A slight, but statistically significant (p < 0.05), decrease in alanine aminotransferase (ALT) activity was observed in the animals treated with 80 and 200 mg/kg DNOC in the diet. The blood urea level was slightly, but significantly, elevated (p < 0.05) in females of the two high-dose groups. Body temperature was not affected. No abnormalities were seen at necropsy and consequently no histopathological examination was performed (Broadmeadow, 1988).
A 90-day feeding study was performed in Wistar rats. Groups of 10 males and 10 females were administered diets containing either 0 (controls), 10, 100, 200 or 400 mg DNOC of unspecified purity/kg in their diet (equivalent to 0, 2.5, 5.0, 10.0 or 20 mg/kg b.w. per day) for 90 consecutive days. Although not adequately reported, the following results were observed. At the highest feed concentration (400 mg/kg diet), 25% of the rats died during the course of the study. Mortality was also observed in the next 2 doses (2 rats at 200 mg/kg diet and 1 at 100 mg/kg diet). Body-weight gain was severely depressed at the high dose and also at the next lower dose, and to a lesser extent at the next lower dose of 100 mg/kg diet. Food consumption was depressed in the high-dose group and slightly in the next lower group of 200 mg/kg diet. There was a sharp increase in ALT activity in the high-dose group, but only one male and one female in this group were evaluated. Increased levels of glucose and urea were recorded in the two highest treatment concentrations in both sexes. Urea level was also increased in the 100 mg/kg diet group, but in male rats only. T3 and T4 levels were decreased at all dose levels. Pyruvate was decreased in a dose-related manner. No effects were seen on the lactate blood level or the organ weights. Some histopathological alterations were observed in the high-dose animals only: fewer acidophilic cells in the pituitary, atrophic islets of Langerhans, no corpora lutea in the ovaries, lower spermatogenesis and atrophic thymus (den Tonkelaar et al., 1983).
From these results it is obvious that the two highest concentrations of 400 and 200 mg/kg diet (equivalent to 20 and 10 mg/kg b.w. per day) were well above the maximum tolerated dose (MTD), and that the alterations observed in the animals treated at these levels were most likely related to the poor physiological and nutritional state of the sick animals, as indicated, in particular, by the high mortality rate and the severe body-weight depression in the high-dose group of animals. Some limited effects were seen in animals exposed to 100 mg/kg diet (equivalent to 5 mg/kg b.w. per day): decrease in body weight, increased blood urea level in males only, and some non-dose-related decrease in T3 and T4 levels. The lowest concentration of 10 mg/kg diet (equivalent to 2.5 mg/kg b.w. per day) may be considered as the no effect level (NOEL).
In a study by Spencer, concentrations up to and including 0.01% (100 mg/kg diet) did not affect the body-weight gain of the rats. At 0.02% (200 mg/kg diet) the body-weight gain was 7–9% below that of the controls. At 500 mg/kg diet the growth of the animals was well below that of the other groups. No effect was observed on the organ weights. Blood urea levels were increased when compared to controls. No histopathological alterations were recorded in the lung, heart, kidney, liver, adrenal, pancreas, testes and stomach. There was a depletion of the body fat (Spencer et al., 1948).
No mortality was recorded during the course of the study, nor was any sign of adverse effect noted. A poorly characterized hyperactivity was reported in an unspecified number of dogs of the mid- and high-dose groups during the last few weeks of the study (onset not specified). Food consumption was not changed at any dose level, and, although there is a reduction in body-weight gain in males and females, it is more pronounced in males. This effect is likely to be related to the mechanism of action of DNOC. The prothrombin time was slightly reduced in all groups at the 49-day dosing interval and, in the two highest concentrations, at day 85 of treatment. However, this observation has little importance in view of the wide variations observed in the reference values from the untreated controls, as well as within the groups. Some biochemical parameters showed some isolated, or not dose-related, variations during the course of the study. Kidney and liver functional tests did not show any alterations. No histopathological modifications attributable to the treatment were observed (Til, 1980).
DNOC has been demonstrated to be a skin sensitizer in the guinea-pig (Driscoll, 1995e).
No significant difference in the mortality rate was observed among the treated groups, or in comparison to the untreated control group. No clinical signs of adverse effects from the treatment were recorded. In male rats treated at the highest concentration, food consumption was found to be slightly higher than in the controls (+6%) from week 5 onwards. No effect was noted on the body-weight gain of the animals. No significant alterations were recorded in the haematological and biochemical parameters evaluated in the course of the experiment.
Histopathological examination did not reveal any alteration in any organ that could be attributable to an effect of the treatment with DNOC. No increase in the incidence of any type of tumour was recorded (Broadmeadow, 1991). A NOEL of 0.59 mg/kg b.w. per day was determined in males on the basis of increased food consumption, and 5.03 mg/kg b.w. per day in females (highest administered dose).
The concentrations administered were 15, 30 and 100 mg of DNOC/kg diet. At all dose levels there were no toxicologically meaningful effects on treated adults compared with untreated controls. From the results, the authors concluded that there were no toxicologically significant effects at any dose level for both generations and concluded that the NOEL for adults and reproductive parameters was 100 mg DNOC/kg diet (the highest concentration administered), corresponding to 7.20 mg/kg b.w. per day for F0 males, 9.24 mg/kg b.w. per day for F0 females, 10.1 mg/kg b.w. per day for F1 males and 10.55 mg/kg b.w. per day for F1 females. However, the reported data indicate that during the gestation phase of the F0 generations of the 100 mg DNOC/kg diet group there was a statistically significant reduction in group mean body weight (p < 0.05) compared to controls from days 7–21 of gestation. No such effect was observed in any other treated group.
During lactation of the F0 generations the same statistically significant effect, in addition to a statistically significant reduction in food consumption, was noted in the high-dose group only. In the same group (100 mg/kg diet) there was a statistically significant reduction in group mean litter size on days 14 and 21 of lactation (p < 0.01) compared to control values in the F1 generations. Litter size at birth was comparable to controls and the later reduction was mainly due to 3 litters which all had 6 or fewer pups left at days 14 and 21. This effect was also seen in the 30 mg/kg diet group on the same days of the lactation phase (p < 0.05).
In the F0 and F1 generation 100 mg/kg diet groups, there was a slight, but statistically significant, reduction in group mean litter weight (p < 0.01 on days 14 and 21 post-partum). However, the effects seen in the high-dose group of both F0 and F1 generations were limited in importance. Therefore, on this basis, the NOEL should be considered to be the intermediate concentration level of 30 mg/kg diet, equivalent to 1.73 mg/kg per day for the F0 males, 2.24 mg/kg per day for the F0 females, 2.40 mg/kg per day for the F1 males and 2.61 mg/kg per day for the F1 females.
Mortality was recorded in the high-dose group, in which four pregnant females died. These deaths occurred during the first 5 days and were not treatment-related. Two deaths occurred late in the study (days 26 and 27 of gestation); lung and intestinal infections were present at necropsy.
Laboured respiration in the high-dose group animals was observed. Food consumption was generally increased in the treated groups when compared to that of the controls, with no concomitant meaningful effects observed on the maternal body-weight gain of the treated animals indicating a metabolic stress in relation to the known mechanism of action of DNOC on the pregnant females.
No biologically significant effects were observed in fetal weights. At the high-dose level, 29 fetuses out of a total of 64 in 8 litters out of 10 showed either external or visceral malformations or skeletal variations. The most frequent malformations were microphthalmia or anophthalmia (24 fetuses in 8 litters) and hydrocephaly or microcephaly (21 fetuses in 6 litters). In only one of the affected litters, 6 fetuses out of 8 had multiple malformations (limbs, omphalocele or gastroschisis) in addition to microcephaly or hydrocephaly. The NOAEL was determined at 10 mg/kg b.w. per day for fetal effects, on the basis of the malformations observed in the high-dose group (Allen et al., 1990a).
No irritating effect was observed at the application sites throughout the course of the study. One mortality was recorded in the high-dose group on day 9 of gestation. No effect was seen on the food consumption. At the high-dose level, the body-weight gain decreased during the first week of treatment but was comparable to the controls afterwards.
Two females in the high-dose group had total resorption. No other adverse effects were observed in the maternal parameters. No effect was seen on the fetal body weights. No significant, or dose-related, teratogenic response was observed in this study. Two fetuses in two separate litters in the low-dose group had hydrocephaly or exencephaly. One isolated case of hydrocephaly was seen in the low-dose level and one in the mid-dose group. No teratogenic response was observed in the high-dose group.
The NOELs were determined as 10 mg/kg b.w. per day for embryotoxicity on the basis of the total resorptions seen in two females in the high-dose group, 30 mg/kg b.w. per day for maternal effects on the basis of early effects on the body weights and 90 mg/kg per day for teratogenicity (Allen et al., 1990b).
Table 6. Studies on mutagenicity of DNOC
| Test systems | Cells/species/ endpoint | Concentrations/ dosesa | Activation | Resultsb | Reference |
| Microbial systems and lower organisms | |||||
| Bacteria | Proteus mirabilis
repair deficient/repair proficient |
10 mg | without | + | Adler et al. (1976) |
| Salmonella typhimurium
TA1537, TA98, TA100 |
100 µg/plate | without | –TA1537
–TA98 –TA100 |
Somani et al. (1981) | |
| Salmonella typhimurium
TA98, TA100 |
1 µmol/plate | with
without |
+TA98
–TA100 –TA98 –TA100 |
Nishimura et al. (1982) | |
| Salmonella typhimurium
TA1535, TA1538, TA98, TA100 |
* | without | –TA1535
+TA1538 +TA98 +TA100 |
Sundvall et al. (1984) | |
| Salmonella typhimurium
TA98NR and TA100NR |
±TA98NR
–TA100NR |
||||
| Salmonella typhimurium
TA1535, TA1537, TA1538, TA98, TA100 |
5–500 µg/plate | With
without with and without |
+TA1535
–TA1535 +TA98 +TA1537 +TA1538 –TA100 |
Marzin (1991a) | |
| Salmonella typhimurium
TA97, TA98, TA100, TA102 |
3–1000 µg/plate | with and without | –TA97
–TA98 –TA100 –TA 102 |
Hrelia et al. (1994) | |
| Drosophila melanogaster | Sex-linked recessive lethal | 1/3–2 x LC50 | + | Müller and Haberzetti (1980) | |
| Mammalian cells in vitro | |||||
| HGPRT,c mouse lymphoma L5178Y cells | 31.3–500 µg/ml | with and without | +
– |
Martin (1981) | |
| HGPRT,c Chinese hamster V79 cells | 100–300–1000–3000 µg/ml | With
without |
+
– |
Marzin (1991b) | |
| chromosome damage, Chinese hamster ovary cells | 0.25–25 µg/ml | with and without | –
– |
Garner (1984) | |
| chromosome damage, human lymphocytes | 2 µg/mlc
0.02 µg/mlc |
+
+ |
Nehéz et al. (1977)
Nehéz et al. (1978) |
||
| chromosome damage, human lymphocytes | 100–300–1000–100 µg/ml
3–10–30 µg/ml |
With
without |
– | Marzin (1991d) | |
| sister chromatid exchange, human lymphocytes | 7.66 and 7.51
8.03 and 8.32 µg/ml |
With
without |
–
– |
Hrelia et al. (1994) | |
| unscheduled DNA synthesis human lymphocytes | 12–25–50–100 µg/ml | without | – | Hrelia et al. (1994) | |
| Mammalian cells in vivo | |||||
| Somatic cells | bone marrow micronucleus, mouse | 20 mg/kg b.w. c
10 mg/kg b.w. after 1 year,c i.p. |
+
+ |
Nehéz et al. (1984) | |
| bone marrow chromosomal aberrations, rat | 4–16 mg/kg b.w., oral | – | Kirland ( 1984) | ||
| bone marrow chromosomal aberrations, mice | 3–12 mg/kg b.w., i.p. | – | Kirland (1986) | ||
| bone marrow micronucleus, mouse | 20 mg/kg b.w., i.p. | – | Marzin (1991c) | ||
| bone marrow chromosomal aberrations, rat | 7.5, 15, 30 mg/kg b.w. i.p. | + | Hrelia et al. (1994) | ||
| DNA unwinding, rat hepatocytes | 1–9.3 mg/kg b.w., i.p. | + | Grilli et al. (1991) | ||
| unscheduled DNA synthesis, rat hepatocytes | 28, 70 mg/kg b.w. oral | – | Fellows 1998 | ||
| Germ cells | germ cells dominant lethal assay,
mouse
germ cells meiotic chromosomal, mouse |
8, 10, 15 mg/kg b.w. i.p.d | +
+ |
Nehéz et al. (1978) | |
| chromosomal aberration F1 embryo, mouse | 4 x 5 mg/kg b.w., p.o. to F0 femaled | + | Nehéz et al. (1981) | ||
| germ cells meiotic chromosomal, mouse | 0.6 mg/kg b.w. per dayd x 10 days, i.p. | ± | Nehéz et al. (1982) | ||
| chromosomal aberrations F1 embryo, mouse | 10 mg/kg b.w. per day to F0 male; i.p. | + | Nehéz et al. (1984) | ||
| testes wt., sperm counts, sperm abnormalities, mouse | 3–12 mg/kg b.w. i.p. or oral | – | Quinto et al. (1989) | ||
In conclusion, some positive responses have been detected in Salmonella, Drosophila and mammalian cells in vitro and in vivo. However, the weight of evidence in studies conducted to Good Laboratory Practice (GLP) standards in vivo have generally produced negative responses. On the basis of all the data available, the mutagenicity of DNOC remains equivocal.
It was shown that the amount of DNOC absorbed from the gastrointestinal tract of the rat, and the resulting blood level, depend on the type of fat administered shortly after dosing with DNOC (olive oil or castor oil). Gastrointestinal uptake of DNOC increases when 0.2 ml of olive oil is given, whereas 1.0 ml has no influence. Castor oil in a non- purgative dose (0.2 ml) inhibits resorption, and then slows down the decrease of DNOC blood level over a period of 48 h. Rapeseed oil, which is more slowly absorbed than olive oil, shows only a slight inhibitory effect on DNOC digestive absorption (Starek & Lepiarz, 1974).
By preventing phosphorylation of ADP without interfering with electron transfer, DNOC dissociates oxidation in the respiratory chain from phosphorylation. This results in respiration becoming uncontrolled since the concentrations of ADP and inorganic phosphate no longer limit the rate of respiration. Oxygen consumption and the release of energy in the form of heat therefore increase, because energy is no longer captured by ADP to form ATP. As a consequence the shortage of ATP in critical organs, such as heart and respiratory muscles, may lead to the blocking of their vital functions. Increased oxygen consumption and dissipation of energy in the form of heat represent the hallmark of the pharmacological and toxicological effects of DNOC.
The exaggeration of catabolic processes, involving glycolysis, glycogenolysis and fatty acid metabolism, has been shown to be linearly related in vivo in guinea-pigs with the dose of DNOC (Harvey, 1959). The increased oxygen consumption has been shown not only in mammals but also in birds, bees and urchin eggs (Parker et al., 1951). Dissipation of energy may result in elevation of the body temperature, leading eventually to severe hyperthermia. Shortage of ATP at muscular levels may lead to muscular paralysis and, in the case of death by DNOC poisoning, to early rigor mortis.
The pharmacological effect of DNOC, which has been used to induce weight loss associated with normal or increased food intake, results from increased degradation of fatty acids, inhibition of lipogenesis and increased glycolysis and glycogenolysis (Gasiewicz, 1991).
A case of poisoning in a 4-year old child resulting from a dosing error in the preparation of an ointment has been reported. Fifty grams of this ointment, containing 25% DNOC, was applied to the boy’s skin. This led to acute poisoning and death in 3.5 h. The applied dose was calculated to be 757 mg/kg b.w. (Gasiewicz, 1991).
Several old cases of subacute exposure during manufacturing can be found in the literature. Malter (1949) described the case of six workers exposed to DNOC dust for a month before the first symptoms occurred: general muscular lassitude; nausea; anorexia; profuse sweating; weight loss (an average of 3% of the initial body weight, with one subject losing 2 kg in 48 h) and insomnia. There was neither hyperthermia nor polypnoea. The workers were removed from exposure without any treatment, except for a high-calorie diet for 2 weeks. All signs subsided, apart from persistent neuritic-type pain in the legs in one subject. No indications of exposure doses, or levels of DNOC in the blood, were provided in the report.
Malter (1949) also mentioned several cases from the literature: four fatal cases in Germany, two in the Netherlands reported in 1946 and two cases in England in 1947. Bidstrup & Payne (1951) reported eight fatal cases of subacute poisoning that occurred in the United Kingdom (one manufacturing, and seven agricultural workers) between 1946 and 1950. The authors observed that: "both in Great Britain and in other countries, all the cases of fatal poisoning have occurred during unusually hot weather". All the clinical symptoms and necropsy findings described in the paper are consistent with those of DNOC poisoning. These are briefly described in the WHO (1982) review. An extensive analysis of the signs and manifestations of DNOC poisoning, as well as of their circumstances and conditions of occurrence, has been made by Hunter (1953) and by Stott (1953).
A recent field exposure study has been performed in the Netherlands on 11 male agricultural workers: 4 were private farmers, 6 contract sprayers and 1 truck driver carrying the chemical from the warehouse to the fields (Heuts, 1993). DNOC was sprayed as an oily formulation, containing 200 g a.i./litre, used in July and August for desiccating the haulm of seed potatoes before harvest. Blood samples were taken at regular intervals from the workers and DNOC levels were determined, together with several liver function parameters. The total exposure time during the spraying season varied from 4 to 20 h per person for the individual farmers, and from 2 to 22 h for the professional sprayers. The total quantity of formulated product sprayed during the 2-week period before the blood sampling ranged from 100 to 400 litres for the farmers, and from 145 to 2193 litres for the contractors.
DNOC concentrations in the blood ranged from < 0.5 mg DNOC/litre of blood to 0.6 and 0.8 mg/litre in two farmers, respectively. Less than 0.5 mg DNOC/litre of blood was detected in professional sprayers (the limit of detection of the analytical method used was 0.5 mg/litre). No biological alterations of the liver parameters were detected and no complaints of clinical signs of exposure were recorded during the study. This study shows that, under present agricultural use conditions, the exposure of sprayers is kept to low levels and is unlikely to induce acute toxic effects.
In 1956 Stott described two cases of polyneuritis observed among staff servicing aircraft that were used for spraying DNOC on locust swarms in eastern Africa (Stott, 1956). The product used was a 20% solution of DNOC in oil. Both men were heavily exposed and did not wear protective equipment. Skin was considered to be the main route of contamination, and both patients displayed marked yellow staining from DNOC both on the palms of their hands and on the sides of their feet. Serum DNOC levels in these patients were 7.6 lg/ml 1 week after the end of exposure and 11.5 lg/ml at the end of exposure, respectively.
The duration of exposures before they were seen by a physician were 2 weeks and 17 days, respectively. Both patients complained of sensations of pins and needles and tingling on the back of the hands and fingers, and numbness in the legs. One had evidence of excessive sweating in the arms and legs; the other also had a loss of sensation to pinprick and cotton wool on the back of his fingers and the dorsal aspects of his toes. There was no loss of motor function in either patient. Signs and symptoms disappeared 12 and 7 days, respectively, after the end of exposure to DNOC.
The characteristics of the clinical picture, the lack of signs of systemic poisoning, the rapid recovery after removal from exposure, the heavy contamination of hands and feet and the lack of correlation with serum DNOC levels suggest a local effect of DNOC.
A fatal case was reported by Steer (1951) where a 21-year-old man was brought to the hospital having felt sick after spraying DNOC for several days. His body had heavy yellow staining. The clinical description of the symptoms was consistent with DNOC poisoning. He had a sudden generalized convulsion with tonic spasms. After his heart fibrillated, the patient died. Shortly after death, postmortem lividity was noticed around the neck and rigor mortis was well marked within 45 min. The main finding at necropsy the next day was that the blood still had not clotted. A blood sample taken immediately after death contained 75 µg DNOC/g, whereas a sample taken at necropsy showed only 4.3 µg/g and DNOC levels in other tissues were comparable with the latter value.
A limited number of cases of poisoning, with hepatic alterations, have been reported following either agricultural or industrial exposure. In the case reported by Prost et al. (1973), a farmer, without any unusual history of health problems, felt sick after spraying his fruit trees with DNOC during the afternoon, without protective equipment. His face, hands, hair and work clothes were stained yellow and he complained of a headache. The next morning he was sweating profusely and had a fever of 39 °C. He sought medical attention, and the biological evaluation revealed a significant liver effect with increased bilirubin, increased activity of alanine aminotransferase (ALT; formerly known as SGOT) and aspartate aminotransferase (AST; formerly known as SGPT), and decreased cholesterol. During the following 2 months, the values progressively returned to normal.
A few comparable cases have been reported from Belgium (Herman & Heyndrickx, 1957; Heyndrickx et al., 1962; Heyndrickx et al., 1964). Necropsy findings and DNOC levels in the tissues were reported. Gaultier et al. (1974) have reported three cases of manufacturing workers exposed to DNOC for periods of 2–8 weeks before becoming ill. One case was fatal. The case with the longest exposure showed limited and transient kidney and liver effects, in addition to the usual symptomatology.
Similarly, Jastroch et al. (1978) described the case of a young farmer exposed for a total of 70 h. The description of the case indicates that the patient had a slight kidney and liver impairment. However, it is not known whether this condition existed before the poisoning, or was a consequence of it. At the time of admission to hospital, the blood level was 70 µg DNOC/ml, and the clinical symptomatology was typically that of subacute DNOC poisoning. A liver biopsy performed 14 days later showed some parenchymal disturbances which were not described.
In a review of pesticide poisoning cases in agricultural workers during the year 1991 by the National Dutch Poison Control Centre, van der Laar et al. (1993) identified one case of a local irritation and two others of systemic intoxication by DNOC. All three cases resulted from either technical imperfections or careless handling of the products (inadequate protective clothing).
In the two systemic cases, one was mild, causing only complaints of a feeling of heat, headache and malaise. The second case was more severe and occurred in a worker exposed for 3 weeks. This professional applicator complained of fever, deep sensation of malaise, shortness of breath and body-weight loss. However, the DNOC plasma level found was 0.8 µg/ml, which seems much too low to be responsible for such a typical picture of intoxication, since it appears from many data quoted in the literature that symptoms become apparent only when the concentration in the blood reaches 40 µg/ml.
Table 7 summarizes the measured blood levels and effects in humans after exposure to DNOC. No effects are seen when the blood level of DNOC is below 20 µg/g blood. (For details, see section 8.2.).
In their recommendation of health limits for pesticides, WHO (1982) stated that on the basis of human data a blood level below 20 µg/g will probably not lead to manifest health impairment.
The Dutch Expert Committee on Occupational Standards (DECOS), in their report prepared on behalf of the Industrial Medicine and Hygiene Unit of the Health and Safety Directorate of the Commission of the European Communities, recommended, based on human exposure data, a biological operation exposure limit (BOEL) of 10 mg/g in serum and 5 µg/g in whole blood for workers not exposed to heat stress (Jongerius & Jongeneelen, 1991).
The Task Group therefore concludes, on the basis of human data, that a blood DNOC level below 20 µg/g of whole blood will probably not lead to manifest health impairment.
Table 7. Measured blood levels in humans and effects after exposure
to DNOC
| Blood level
(µg/g) |
Comments | Reference |
| 75 | Fatal case. Blood sample taken immediately after death. Yellow staining all over the body. Blood level at necropsy 4.3 µg/g | Steer (1951) |
| 70 | Farmer exposed totally 70 h. Clinical symptomatology typical of subacute DNOC poisoning. Slight kidney and liver impairment | Jastroch et al. (1978) |
| 48 | One volunteer 75 mg/day, 7 days. No symptoms of poisoning | Harvey et al. (1951) |
| 20 | Volunteers 75 mg/day, 5 days. No symptoms of poisoning | Harvey et al. (1951) |
| 0.8 | One professional applicator exposed 3 weeks. Deep sensation of malaise, shortness of breath, body-weight loss | van der Laar et al. (1993) |
| <0.5–0.8 | Farmers spraying 4–20 h. No biological alterations of liver parameters. No complaints of clinical signs. | Heuts (1993) |
| <0.5 | Professional sprayers 2–22 h. No biological alterations of liver parameters. No complaints of clinical signs. 0.5 was limit of detection in method used | Heuts (1993) |
Early life stage exposure of embryos of the common carp (Cyprinus carpio) was carried out at 4 concentrations of DNOC (0.25, 0.5, 1.0 and 2.0 mg/litre) at 3 pH values (6.9, 7.8 and 9.0) over a 13 day period from fertilization. Mortality decreased markedly with increasing pH. The no observed effect concentrations (NOECs) of DNOC are at or below 0.25 mg/litre at pH 6.9, and 0.5–1 mg/litre at pH 7.8. At pH 9.0, no toxic effect was observed at the highest concentration (2.0 mg/l) (Ghillebaert et al., 1995).
Table 8 Acute toxicity of DNOC to aquatic organisms
| Organism | Endpoint | Concentration
(mg/litre) |
Reference |
| Micro-organisms | |||
| Bacterium
Pseudomonas putida |
Toxic threshold 16 h EC3 (growth) | 16 | Bringmann & Kühn (1980) |
| Cyanobacterium
Microcystis aeruginosa |
Toxic threshold 72 h EC3 (growth) | 0.15 | Bringmann & Kühn (1978) |
| Green algae
Scenedesmus quadricauda |
Toxic threshold 7 d EC3 (growth) | 13 | Bringmann & Kühn (1980) |
| Scenedesmus subspicatus | 96 h EC50(biomass)
48 h EC50 (growth rate) |
6
12 |
Sewell et al. (1995a)
Sewell et al. (1995a) |
| Protozoans
Entosiphon sulcatum |
Toxic threshold 72 h EC5 (growth) | 5.4 | Bringmann & Kühn (1980) |
| Chilomonas paramecium | Toxic threshold 72h EC5 (growth) | 5.4 | Bringmann & Kühn (1981) |
| Uronaemia parduczi | Toxic threshold 72 h EC5 (growth) | 0.012 | Bringmann & Kühn (1981) |
| Invertebrates | |||
| Water flea
Daphnia magna |
24 h LC50
14 d LC50 14 d NOEC (reproduction) 24 h LC50 24 h NOEC (mortality) 21 d NOEC (reproduction) 48 h LC50 |
5.7
1.6 0.6 2.3 1.5 1.3 |
van der Hoven (1984)
van der Hoven (1984) van der Hoven (1984) Kühn et al. (1989) Kühn et al. (1989) Kühn et al. (1989) |
| Daphnia pulex | 0.145 | Mayer & Ellersieck (1986) | |
| Scud
Gammarus fasciatus |
96 h LC50 | 0.11 | Mayer & Ellersieck (1986) |
| Insect | |||
| Pteronarcys californica | 96 h LC50 | 0.32 | Mayer & Ellersieck (1986) |
| Vertebrates (fish) | |||
| Bluegill sunfish
Lepomis macrochirus |
96 h LC50
96 h LC50 |
0.95
0.36 |
Sewell et al. (1995b)
Mayer & Ellersieck (1986) |
| Rainbow trout
Oncorhynchus mykiss |
96 h LC50
96 h NOEC 96 h LC50 |
0.45
0.32 0.066 |
Sewell et al. (1995c)
Sewell et al. (1995c) Mayer & Ellersieck (1986) |
Jones & Edwards (1952) reported the results of their experimental work on honey bees, both in the laboratory and in the field, under a range of varied conditions of temperature, humidity and formulations. Although DNOC was toxic to honey bees under laboratory conditions, in the field the repellent effect of the sprayed solutions, followed by the wilting and shrivelling effect, made sprayed flowers unattractive to bees. Below a certain temperature, the bees do not forage and DNOC is used in the autumn and winter; this combination of factors makes it unlikely that bees will forage on treated plants, thus reducing the risk of significant exposure and toxicity.
The subacute dietary toxicity was evaluated by feeding Japanese quail a diet containing concentrations of 555–960 mg DNOC/kg of feed for 5 consecutive days, followed by 3 days’ observation period. The LC50 (8 days) was determined to be 637 mg DNOC/kg of diet, equivalent to approximately 106 mg DNOC/kg b.w. (Til & Kengen, 1980).
Additional data on the acute toxicity of DNOC to wild birds have been reported by Janda (1970). Pheasants and partridges were treated with one gavage of a water dilution of a formulated product containing 35% DNOC. The LD50 values were 24 mg of formulation/kg b.w. (equivalent to 8.4 mg DNOC/kg b.w.) for pheasants, and 23.7 mg of formulation/kg b.w. (equivalent to 8.3 mg DNOC/kg b.w.) for partridges. In a subchronic study reported in the same paper, in which the product was administered by gavage during 3 consecutive days, the LD50 values were 7.1 DNOC/kg b.w. per day and 11.1 mg DNOC/kg b.w. per day for pheasants and partridges, respectively. These results indicate that DNOC does not accumulate in these birds. Some repulsive effect was observed when maize, barley and wheat, treated with a concentration equivalent to that sprayed in normal field use, were offered to the birds in a "no-choice" test (i.e., birds were offered only treated grain).
A relatively small number of human cases of acute poisoning have been reported in the literature during both manufacturing and agricultural occupational exposures. In all occasions these occurred from poor hygienic conditions.
The main route of exposure was through skin contact. These cases were reported more frequently several decades ago (see section 8.2). It was noticed that exposure during a period of hot weather increased the risk of acute poisoning. In today’s agricultural practices, exposure has been severely reduced through better education of farmers, making them aware of the toxicity of the product and of the necessity to wear personal protective clothing, and to spray with tractors equipped with closed cabins; spraying equipment, formulation and packaging have also been improved.
The onset of clinical symptoms occurred after a relatively short period of exposure. Symptoms included nausea, gastric distress, restlessness, sensation of heat, excessive sweating, thirst, deep respiration, tachycardia and hyperpyrexia. In severe cases, collapse, coma and death occurred within 24–48 h.
A small fraction of the general population was exposed for a limited period of time more than 50 years ago when DNOC was used as a therapeutic agent against obesity. Doses of 3 mg/kg b.w. per day induced an excessive metabolic rate with associated symptoms, including sweating, lethargy and sleep disturbances.
A recent, limited scale, field worker exposure study has shown that the plasma concentration did not exceed 0.8 mg/litre (in an isolated case) and even remained below 0.5 mg/litre (limit of detection) in the majority of workers.
The Group endorses the previous WHO conclusion for a biological threshold limit of 20 µg/ml of whole blood for DNOC (WHO, 1982). A TWA (mg/m3 per 8 h) of 0.2 has been established in several countries (see section 5.3).
In animal studies, the oral LD50 values, as determined in several species, range from 16 to 100 mg/kg b.w. Some positive responses have been detected in Salmonella, Drosophila and mammalian cells in vitro and in vivo. The weight of evidence in studies conducted to GLP standards in vivo has generally produced negative responses. On the basis of all the data available, the mutagenicity of DNOC remains equivocal. A rat carcinogenicity study did not show any carcinogenic effect. Teratogenicity studies performed in rats and rabbits by oral and percutaneous administration showed that DNOC induced embryotoxicity and teratogenic effects only at dose levels that were also maternally toxic.
Adsorption of undissociated DNOC to particulates in soil is high at low pH (4–5.5); adsorption of dissociated anions is much lower at higher environmentally significant pH (5.5–8) with commonly more than half of the DNOC in the aqueous phase. Adsorption to soil is concentration-dependent, with reduced sorption within the range expected from recommended application rates. Leaching appears to be limited in practice, with occasional low concentrations detected in groundwater.
A range of micro-organisms capable of degrading DNOC has been identified in soils. Half-life in surface waters is of the order of 3–5 weeks and DT50 values for soil have been measured at 4–15 days; DT100 for soil ranges from 2.5 to 62 days.
Use of the compound as a desiccant on potato and an insecticide on dormant fruit crops limits exposure of many organisms. DNOC used for locust control is likely to be sprayed over wide areas; however, details of this usage were not available and no risk assessment can be made.
DNOC is acutely toxic to honey bees. Exposure from winter use would not occur. Desiccant use would be on potato haulms past the flowering stage, and desiccant-treated foliage would not be attractive to bees. Even assuming overspray, the bee hazard quotient ([application rate in g a.i./ha]/[toxicity in µg/bee]) gives no cause for concern.
Little or no exposure of birds and mammals is expected from direct use of DNOC. Indirect exposure from contaminated earthworms is a possibility, but no risk assessment can be made because no residue information is available. Rapid biodegradation could be expected to reduce indirect exposure. Data from a wildlife incident scheme suggest very limited kills of wild mammals. The means of exposure of these animals is unknown.
Earthworms would be exposed to spray reaching the ground and desiccated haulms on the soil surface. Winter use is unlikely to expose worms. Calculated soil concentrations of 7.5 mg/kg are obtained following spraying at 5.6 kg a.i./ha assuming even distribution in the top 5 cm of soil (EPPO/COE Guidelines, EPPO, 1993). This gives a toxicity exposure ratio (TER) of 2.01, regarded as being of moderate concern.
Calculated water concentration following application as a desiccant at 5.6 kg a.i./ha is 0.093 mg/litre (EPPO/COE Guidelines, EPPO, 1993) assuming a level of spray drift of 5% at 1 m from the spray boom (Ganzelmeier et al., 1995). TER values for aquatic organisms are 0.7 for fish (based on rainbow trout), 1.6 for invertebrates (based on Daphnia magna) and 64.3 for algae. A spray buffer zone of 5 m from water courses increases the TER values to acceptable levels (59, 130, 5360 respectively). Application to dormant fruit crops is expected to be by drench with large droplet size; aquatic concentrations are assumed to be no higher than for boom application.
It can be concluded that DNOC could adversely affect organisms in the environment following acute exposure; chronic effects are not expected given the degradation of the compound. Field studies are not available for the organisms most likely to be exposed.
DNOC was evaluated by the FAO/WHO Joint Meeting on Pesticide Residues (JMPR) in 1963 and 1965. No ADI was established.DNOC is classified in class Ib, ‘highly hazardous’, in the WHO Recommended Classification of Pesticides by Hazards (WHO, 1999).
Adler B, Braun R, Schoneich J, & Bohme H (1976). Repair-defective mutants of Proteus mirabilis as a pre-screening system for the detection of potential carcinogens. Biol Zbl, 95:463–469.Alber M, Boehm HB, Brodesser J (1989) Determination of nitrophenols in rain and snow. Fresenius J Anal Chem, 334:540–545.
Allen PA, Biedermann K, & Terrier C (1990a) Embryotoxicity study (including teratogenicity) with DNOC Technical in the rabbit (dermal application). (RCC study no. 215638) Itingen, Switzerland (unpublished report prepared for Pennwalt Holland bv).
Allen PA, Biedermann K, & Terrier C (1990b) Embryotoxicity study (including teratogenicity) with DNOC Technical in the rabbit (oral administration). (RCC study no. 215651) Itingen, Switzerland (unpublished report prepared for Pennwalt Holland bv).
Ambrose AM (1942) Some toxicological and pharmacological studies on 3,5-dinitro-o-cresol. J Pharm Exp Ther, 76: 245–251.
Arustamyn AN (1972) [The toxicity of dinitro-ortho-cresols for warm-blooded animals and problems of industrial hygiene in its application.] Tr Inst Vet Sanit, 45:166–169 (in Russian).
Batchelor GS, Walker KC, & Elliott JW (1956) Dinitroorthocresol exposure from apple-thinning sprays. Arch Indust Health, 13:593–596.
Benadikova H, & Kalvoda R (1984) Adsorptive stripping voltammetry of some triazine- and nitro-group-containing pesticides. Anal Lett, 17:1519–1531.
Ben Dyke R, Sanderson DM, & Noakes DN (1970) Acute toxicity data for pesticides. World Rev Pest Contr, 9(3):119–127.
Beran F, & Neururer J (1955) [About understanding of the effects of pesticides on the honey bee (Apis mellifera L.) 1. Hazards of pesticides to honey bees.] Pflanzenschutzberichte, 15(8/12):97–147 (in German).
Bidstrup PL, & Payne DJH (1951) Poisoning by dinitro-ortho-cresol. Report of eight fatal cases occurring in Great Britain. BMJ, ii:16–19.
Bieber WD (1995) Degradation and metabolism of DNOC in soil (SGS Natec Institut, Hamburg Germany study no. 92 9633, unpublished report prepared for Elf Atochem Agri SA).
Bringmann G, & Kühn R (1978) Limiting values for the noxious effects of water pollutants material to blue-green algae and green algae. vom Wasser, 80:45–60.
Bringmann G, & Kühn R (1980) Comparison of the toxicity thresholds of water pollutants to bacteria, algae and protozoa in the cell multiplication inhibition test. Water Res, 14:231–241.
Bringmann G, & Kühn R (1981) [Comparison of the effects of harmful substances on flagellates and ciliates, as well as on holozoic bacteriophagic and saprozoic protozoa.] gwf-wasser/abwasser, 122:308–313 (in German).
Broadmeadow A (1988) Technical DNOC: Preliminary toxicity study by dietary administration to F-344 rats for six weeks (Life Science Research study no. 87/PTN 001/433). Eye, UK (unpublished report prepared for Pennwalt Corporation Agrichemicals Division).
Broadmeadow A (1991) Technical DNOC: Combined oncogenicity and toxicity study by dietary administration to F-344 rats for 104 weeks (Life Science Research study no. PTN/003/DNOC). Eye, UK (unpublished report prepared for Pennwalt Corporation).
Buchholz KD, & Pawliszyn J (1993) Determination of phenols by solid-phase microextraction and gas chromatographic analysis. Environ Sci Technol, 27: 2844–2848.
Burkatskaya EN (1965a) [Maximum permissible concentration of dinitro-o-cresol in air.] Gig Sanit, 30:34–37 (in Russian).
Burkatskaya EN (1965b) The toxicity of dinitro-ortho-cresols for warm-blooded animals and problems of industrial hygiene in its application. Gig Tr Prof Zobal, 9(4):56–57.
Coles RJ, & Brooks PN (1997) Technical DNOC: dietary two generations reproduction study in the rat (Safepharm Laboratory project no.764/010). Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
de Lavaur E (1967) Résidus de dinitro-4,6-orthocrésol sur blé après différents types de traitement. Phytiat Phytopharm, 16:15–21.
de Lavaur E, & Arnold A (1977) Pesticides et faune sauvage: Résultats des analyses toxicologiques effectuées sur le gibier de 1974 à 1976. Phytiat Phytopharm, 26:159–168.
de Lavaur E, & Arnold A (1981) Pollution des vertébrés terrestres sauvages par les pesticides et les PCB. Enquête 1977–1979. Phytiat Phytopharm, 30:89–95.
den Tonkelaar EM, van Leeuwen FXR, & Kuiper C (1983) Semichronic toxicity of DNOC in the rat. Med Fac Landbouww Rijksuniv, 48(4):1015–1022.
Dey-Hazra A, & Heisler E (1981) [Acute toxicity of trifocide 50% flowable, 50% DNOC ammonium salt in water by inhalation in the rat.] Pharmatox study no. 1–4-246–81 (unpublished report prepared for Elf Atochem Agri bv) (in German).
Dickhaus S, & Heisler E (1980) [Acute toxicity of technical active substance DNOC (99% ± 1%) after oral administration to quail.] Pharmatox study no. 1–8-239–80. Pharmatox, Hannover, Germany (unpublished report prepared for Ruhr-Stickstoff AG) (in German).
Dickhaus S, & Heisler E (1984) [Teratogenic/embryotoxic study with the product "Trifocide liquid 50%" following oral administration in the rat.] (Pharmatox study no. 2–4-240–83) Hamburg, Germany (unpublished report prepared for Pennwalt Holland bv) in German.
Di Corcia A, & Marchetti M (1992) Method development for monitoring pesticides in environmental waters. Liquid–solid extraction followed by liquid chromatography. Environ Sci Technol, 26: 66–74.
Diepenhorst PC, Kool P, & Luijdendijk HM (1995) DNOC. Determination by HPLC in human blood. Rotterdam Elf Atochem Agri bv Analytical Dept (unpublished report no. Anal. Meth. SOP DLA-011.7).
Dodds EC, & Robertson JD (1933a) The clinical applications of dinitro-o-cresol. Lancet, ii:1137–1139.
Dodds EC, & Robertson JD (1933b) The clinical applications of dinitro-o-cresol. II. A study of myxoedema. Lancet, ii:1197–1198.
Driscoll R (1995a) Technical DNOC: Acute oral toxicity test in the rat (Safepharm Laboratories study no. 765/4). Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
Driscoll R (1995b) Technical DNOC: Acute dermal toxicity (limit test) in the rat (Safepharm Laboratories study no. 764/5). Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
Driscoll R (1995c) Technical DNOC: Acute dermal irritation test in the rabbit (Safepharm Laboratories study no. 764/30). Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
Driscoll R (1995d) Technical DNOC: Acute eye irritation test in the rabbit (Safepharm Laboratories study no. 764/31). Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
Driscoll R (1995e) Technical DNOC: Magnusson and Kligmann maximisation study in the guinea pig (Safepharm Laboratories study no. 764/7). Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
EC (1999) O J EC, 2 March 1999, Commission decision 1999/164/EC.
EPPO (1993) European Plant Protection Organization. Decision-making scheme for the environmental risk assessment of plant protection products. Chapters 6–11. EPPO Bulletin, 23.
Fabreguettes C (1993) Pharmacokinetic study after single cutaneous application in rats (Centre International de Toxicologie, Evreux, France, study no. 10530/PAR) (unpublished report prepared for Elf Atochem Agri SA).
FAO/WHO (1964) Evaluation of the toxicity of pesticides residues in food; report of a Joint Meeting of the FAO Committee on Pesticides in Agriculture and the WHO Expert Committee on Pesticide Residues. Geneva, World Health Organization. FAO Meeting Report no. PL/1963/13; WHO/Food Add. 23.
FAO/WHO (1965) Evaluation of the toxicity of pesticide residues in food. FAO Meeting Report no. PL/1965/10/1; WHO/Food Add./27–65.
Farrington DS, Martindale RW, & Woolam CJ (1982) Determination of the active ingredient content of technical and formulated dinobuton, dinoseb, dinoterb and DNOC by high-performance liquid chromatography. Analyst, 107:71–75.
Fellows M (1998) DNOC technical measurement of unscheduled DNA synthesis in rat liver using an in vivo/in vitro procedure. Report No. 160711-DS140. unpublished report by Covance Laboratories Limited, North Yorkshire, UK Prepared for Elf Atochem Agri SA.
Froslie A (1973) Methaemoglobin formation by diamino metabolites of DNOC and DNBP. Acta Pharmacol Toxicol, 32:257–265.
Froslie A, & Karlog O (1970) Ruminal metabolism of DNOC and DNBP. Acta Vet Scand, 11:114–131.
Ganzelmeier H, Spangenberg R, Streloke M, Hermann M, Wenzelburger H-J, & Walter H-F (1995). Studies on the spray drift of plant protection products. Berlin, Blackwell Wissenschafts-Verlag.
Garner RC (1984) Study to evaluate the chromosome damaging potential of DNOC by its effects on cultured Chinese hamster ovary cells using an in vitro cytogenetics assay. (Microtest Research study no. PHARM 1/CYT/RCG 2) UK (unpublished report prepared for Pennwalt Holland bv).
Gasiewicz TA (1991) Nitro compounds and related phenolic pesticides. In: Hayes WJ Jr, & Laws ER Jr ed. Handbook of pesticide toxicology. San Diego, CA, Academic Press, vol. 3, pp. 1191–1269.
Gaultier M, Gervais P, & Conso F (1974) Intoxication aiguë par le dinitroorthocresol. J Eur Tox, 7(1):9–11.
Ghillebaert F, Chaillou C, Deschamps F, & Roubaud P (1995) Toxic effects at three pH levels of two reference molecules on common carp embryo. Ecotoxicol Env Safety, 32:19–28.
Grilli S, Ancora G, Valenti AM, Mazzullo M, & Colacci A (1991) In vivo unwinding fluorometric assay as evidence of the damage induced by fenarimol and DNOC in rat liver DNA. J Tox Environ Health, 34:485–494.
Grolleau G (1967) Intoxication experimentale de lièvres par le DNOC. Phytiat Phytopharm, 16:23–25.
GTZ, German Agency for Technical Cooperation (1997) The work of other agencies and organizations relating to prevention and disposal of obsolete and unwanted pesticide stocks. In: Prevention and disposal of obsolete and unwanted pesticide stocks in Africa and the Near East. Second Consultation Meeting. FAO Pesticide Series, 5:17–21.
Gustafson DI (1989) Ground water ubiquity score: a simple method for assessing pesticide leachability. Envir Tox Chem, 8:339–357.
Hamdi YA, & Tewfik MS (1970) Degradation of 3,5-dinitro-o-cresol by Rhizobium and Azobacter spp. Soil Biol Biochem, 2:163–166.
Hallberg GR (1989) Pesticide pollution of groundwater in the humid United States. Agricol Ecosyst Environ, 26:299–367.
Harvey DG (1952) The toxicity of the dinitro-cresols. J Pharm Pharmacol, 4:1062–1066.
Harvey DG (1958) Some aspects of the metabolism of 4:6-dinitro-o-cresol (DNC) by the ruminant. J Comp Pathol, 64:54–63.
Harvey DG (1959) The quantitative response of the oxygen consumption and weight of guinea pig to some metabolic stimulants. J Pharm Pharmacol, 11:681–688.
Harvey DG, Bidstrup PL, & Bonnel JAL (1951) Poisoning by dinitro-ortho-cresol. Some observations on the effects of dinitro-ortho-cresol administered by mouth to human volunteers. BMJ, 2:13–16.
Hawley GG (1981) The condensed chemical dictionary. 10th ed. New York, Van Nostrand Reinhold.
Hayes WJ Jr (1963) Clinical handbook on economic poisons. Washington, DC, US Department of Health, Education and Welfare (Public Health Service publication no. 476).
Heimlich F, & Nolte J (1993) Determination of the pK values of 2,4-dinitrophenol herbicides using UV spectroscopy. Science Total Envir, 132:125–131.
Herman M, & Heyndrickx A (1957) [Toxicological investigation of poisoning cases with dinitro-ortho-cresol (DNOC) with fatal outcome.]. Med Landb Hogeschool Gent, 22:647–653 (in Dutch).
Herterich R (1991) Gas chromatographic determination of nitrophenols in atmospheric liquid water and airborne particulates. J Chromatogr, 549: 313–324.
Heuts FJM (1993) Biological monitoring of DNOC in applicators after use as haulmkiller in seed potatoes. Rotterdam (unpublished report prepared for Elf Atochem Agri bv).
Heyndrickx A, Maes R, & Tyberghein F (1964) Fatal intoxication by man due to dinitro-ortho-cresol (DNOC) and dinitrobutylphenol (DNBP). Med Landb Hogeschool Gent, 29: 1189–1197.
Heyndrickx A, Avermaete M, Maes R, & Schauvliege F (1962) Determination of dinitro-ortho-cresol (DNOC) in post mortem material of a farmer. Med Landb Hogeschool Gent, 27:955–963.
Hope RL, Mullee DM, & Bartlett AJ (1995) Analytical DNOC and technical DNOC. Determination of general physico-chemical properties (Safepharm Laboratories study no. 764/001) Derby, UK, Safepharm Analytical Chemistry Laboratory (unpublished report prepared for Elf Atochem Agri SA).
Hopper ML, McMahon B, & Griffith KR (1992) Analysis of fatty and nonfat foods for chlorophenoxy alkyl acids and pentachlorophenol. J Am Org Anal Chem Int, 75: 707–713.
Howarth R, Tremain SP, & Bartlett AJ (1995) Technical DNOC (batch number 547–108). Determination of vapour pressure. Safepharm Laboratories study no. 764/002) Derby, UK, Safepharm Analytical Chemistry Laboratory (unpublished report prepared for Elf Atochem Agri SA).
Hrelia P, Vigagni F, Maffei F, Morotti M, Colacci A, Perocco P, Griili S, & Cantelli-Forti G (1994) Genetic safety evaluation of pesticides in different short-term tests. Mut Res, 321:219–228.
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of pesticides to wildlife. US Department of the Interior, Fish and Wildlife Service, Washington, DC Resource Publication No. 153, PE17–215.
Hunter D (1953) Industrial toxicology. J Pharm Pharmacol, 5:145–157.
HSDB (1994) Hazardous Substances Data Bank. National Library of Medicine, National Toxicology Information Program, Bethesda, MD, USA.
Ilivicky J, & Casida JE (1969) Uncoupling action of 2,4-dinitrophenols, 2-trifluoromethylbenzimidazols and certain other pesticide chemicals upon mitochondria from different sources and its relation to toxicity. Biochem Pharmacol, 18:1389–1401.
Ingebrigtsen K, & Froslie A (1980) Intestinal metabolism of DNOC and DNBP in the rat. Acta Pharmacol Toxicol, 46:326–328.
Izmerov NF, Sanotsky IV, & Sidorov KK (1982) Toxicometric parameters of industrial toxic chemicals under single exposure. Moscow, UNEP.
Jafvert CT (1990) Sorption of organic acid compounds to sediments: initial model development. Envir Toxicol Chem, 9:1259–1268.
Janda J (1970) On the toxicity of DNOC to pheasants, partridges and hares. Scien Agricult Bohem, 2(4):301–312.
Jastroch W, Knoll W, Lange B, Riemer F, & Thiele E (1978) [Results of a study on the exposure of agricultural workers to DNOC. Z Ges Hyg, 24:340–343 (in German).
Jegatheeswaran T, & Harvey DG (1970) The metabolism of DNOC in sheep. Vet Rec, 4 July, 19–20.
Jensen HL (1966) [Biological decomposition of herbicides in the soil. IV Dinitro-ortho-cresol.] Tidssk Planteal, 70:149–159 (in Danish).
Jensen HL, & Gundersen K (1955) Biological decomposition of aromatic nitro-compounds. Nature, 175:341.
Jonas W (1995) Adsorption/desorption of the test substance: [14C]-DNOC Study no. NA 93 9714, NATEC Institut, Hamburg, Germany (unpublished report prepared for Elf Atochem Agri SA).
Jones GDG, & Edwards RA (1952) Studies of toxicity of 3,5-dinitro-ortho-cresol and its sodium salt to the honey bee. Bull Entomol Res, 43:67–78.
Jongerius O & Jongeneelen FJ (1991) Criteria document for an occupational exposure limit value of 4,6-dinitro-o-cresol (CAS 534–52–1). Commission of the European Communities, Directorate General Employment, Industrial Relations and Social Affairs, Health and Safety Directorate, Industrial Medicine and Hygiene Unit. SEG/CDO/29.1992, Luxembourg.
Judah JD (1952) Mode of action of the nitrophenols. Proc R Soc Med, 45:574.
Kadenczki L, Arpad Z, & Gardi I (1992) Column extraction of residues of several pesticides from fruits and vegetables: a simple multiresidue analysis methods. J Am Org Anal Chem Int, 75:53–61.
Kelly J (1995) DNOC: 13-week oral (dietary administration) range-finding study in the mouse. (Corning Hazelton project no. CHE 1151/8). Harrogate, UK (unpublished report prepared for Elf Atochem Agri SA).
Keplinger ML, Lanier GE, & Deichmann WB (1959) Effects of environmental temperature on the acute toxicity of a number of compounds in rats. Tox Appl Pharmacol, 1:156–161.
King E, & Harvey DG (1953a) Some observations on the absorption and excretion of 4,6-dinitro-o-cresol (DNOC) I. Blood dinitro-o-cresol levels in the rat and the rabbit following different methods of absorption. Biochem J, 53:185–195.
King E, & Harvey DG (1953b) Some observations on the absorption and excretion of 4,6-dinitro-o-cresol. Biochem J, 53:196–200.
Kirkland DJ (1984) Study to evaluate the chromosome damaging potential of DNOC by its effects on the bone marrow cells of treated rats. (Pharmatox study no. PHM 6/RBM/AR/KF6) Hannover, Germany (unpublished report prepared for Pennwalt Holland bv).
Kirkland DJ (1986) Study to evaluate the chromosome damaging potential of 4,6-dinitro-o-cresol (DNOC) by its effects on the bone marrow cells of treated mice. (Microtest Research study no. PEN 1/MBM/KF27/MB1) York, UK (unpublished report prepared for Pennwalt Holland bv).
Kreczko S, Zwierz K, & Jaroszewicz K (1974) Glycoprotein biosynthesis by the guinea pig liver in chronic 4,6-dinitro-o-cresol poisoning. Acta Biol Acad Sci Hung, 25(3):167–171.
Kühn R, Monika P, Pernak K-D, & Winter A (1989) Results of the harmful effects of water pollutants to Daphnia magna in the 21-day reproduction test. Water Res, 23: 501–510.
Lawford DJ, King E, & Harvey DG (1954) On the metabolism of some aromatic nitro-compounds by different species of animals. Part II. The elimination of various nitro-compounds from the blood of different species of animals. J Pharm Pharmacol, 6:619–624.
Leegwater DC, van der Greef J, & Bos KD (1982) Integrated studies on the metabolic fate of DNOC. II. Biotransformation in mammals. Med Fac Landbouww Rijksuniv Gent, 47(1):401–408.
Leuenberger C, Czuczwa J, & Tremp J (1988) Nitrated phenols in rain: atmospheric occurrence of phototoxic pollutants. Chemosphere, 17:511–515.
Lopez-Avila V, Bauer K, & Milanes J (1993) Evaluation of Soxhlet extraction procedure for extracting organic compounds from soils and sediments. J Am Org Anal Chem Int, 76:864–880.
Malter A (1949) Intoxications survenues au cours de la fabrication d’un insecticide à base de dinitrocrésol. Arch Belg Med Soc Hyg, 7:475–481.
Martin CN (1981) Study to determine the ability of DNOC to induce mutations to ouabain and 6-thioguanine resistance in mouse lymphoma L5178Y cells. (Microtest Research study no. PHARM 2/ML/JC/JGL) York, UK (unpublished report prepared for Pennwalt Holland bv).
Marzin D (1991a) Recherche de mutagénicité sur Salmonella typhimurium his – selon la technique de B.N. Ames sur le produit dinitro-ortho-cresol. (Institut Pasteur de Lille study no. IPL-R 910801). Lille, France (unpublished report prepared for Elf Atochem Agri SA).
Marzin D (1991b) Etude de mutagenèse au locus HPRT sur cellules V79 de hamster chinois (résistance à la 6-thioguanine) sur le produit dinitro-ortho-cresol (2-méthyl-4,6-dinitrophénol). (Institut Pasteur de Lille study no. IPL-R 910904). Lille, France (unpublished report for Elf Atochem Agri SA).
Marzin D (1991c) Etude de l’activité génotoxique par la technique du micronucléus chez la souris sur le produit dinitro-ortho-crésol. (Institut Pasteur de Lille study no. IPL-R 910804) Lille, France (unpublished report prepared for Elf Atochem Agri SA).
Marzin D (1991d) Etude de l’activité génotoxique du produit dinitro-ortho-crésol par la recherche d’aberrations chromosomiques par analyse de métaphases sur lymphocytes humains en culture. (Institut Pasteur de Lille study no. IPL-R 911010). Lille, France (unpublished report prepared for Elf Atochem Agri SA).
Mayer FL, & Ellersieck MR (1986) Manual of acute toxicity: Interpretation and database of 410 chemicals and 66 species of freshwater animals. US Department of the Interior, Fish and Wildlife Services, Publication No. 160, PE17–2LS, Washington, DC
McGirr JL, & Papworth DS (1953) Toxic hazards of the newer insecticides and herbicides. Vet Rec 65(48):857–862.
Metcalf RL (1978) Insect control technology. In: Kirk-Othmer encyclopaedia of chemical technology. Vol. 13, 3rd ed. New York, John Wiley & Sons, p. 428.
Mogensen BB, & Spliid NH (1995) Pesticides in Danish watercourses: occurrence and effects. Chemosphere, 31(8):3977–3990.
Moreland DE (1980) Effects of toxicants on oxidative phosphorylation. In: Hogdson E, & Guthrie FE eds. Introduction to biochemical toxicology. New York, Elsevier, pp. 245–260.
Morgan DP (1982) Recognition and management of pesticide poisonings. 3rd ed. Washington, DC, US Environmental Protection Agency (EPA-540/9–80–005).
Müller J, & Haberzetti R (1980). Mutagenicity of DNOC in Drosophila melanogaster. Arch Toxicol Suppl, 4:59–61.
Nehéz M, Selypes A, & Paldy A (1977) [Examination of dinitro-o-cresol-containing fertilizer for mutagenic effects.] Egeszsegtudomany 21:237–243 (in Hungarian).
Nehéz M, Selypes A, Paldy A, & Berencsi G (1978) The mutagenic effect of a dinitro-o-cresol containing pesticide on mice germ cells. Ecotox Environ Safety, 2:401–405.
Nehéz M, Paldy A, & Selypes A (1981) The teratogenicity and mutagenicity effects of dinitro-o-cresol-containing herbicide on laboratory mouse. Ecotox Environ Safety, 5:38–44.
Nehéz M, Selypes A, Paldy A, Mezzag E, Berencsi G and Jarmay K (1982) The effects of five weeks treatment with dinitro-o-cresol or trifluralin containing pesticides on the germ cells of male mice. J Appl Toxicol, 2(4):179–180.
Nehéz M, Selypes A, Mazzag E and Berencsi G (1984) Additional data on the mutagenic effect of dinitro-o-cresol containing herbicides. Ecotox Environ Safety, 8:75–79.
NIOSH (1978) Criteria for a recommended standard: Occupational exposure to dinitro-ortho-cresol. Report No.78–131, PB80–17870–159. Washington, DC, National Institute for Occupational Safety and Health.
NIOSH (1984) Manual of analytical methods. 2nd ed. Vol 5. Method no. S166 Washington, DC, National Institute for Occupational Safety and Health.
Nishimura N, Nishimura H, & Oshima H (1982) Survey on mutagenicity of pesticides by the Salmonella microsome test. J Aichi Med Univ Assoc, 10(4):305–312.
Parker VH (1949) Method for routine estimation of 3:5-dinitro-o-cresol (DNOC) Analyst, 74: 646–647.
Parker VH (1952) Enzymic reduction of 2,4-dinitrophenol by rat-tissue homogenates. Biochem J, 51:363–370.
Parker VH, Barnes JM, & Denz FA (1951) Some observations on the toxic properties of 3,5-dinitro-ortho-cresol. Br J Industr Med, 8:226–235.
Pollard AB, & Filbee JF (1951) Recovery after poisoning with di-nitro-ortho-cresol. Lancet, II:618–619.
Prost G, Vial R, & Tolot F (1973) Intoxication par le dinitro-ortho-crésol avec atteinte hépatique. Arch Mal Prof, 34(9):556–557.
Quidet P (1975) Résultats des enquêtes en France en 1972, 1973, 1974 sur les causes de mortalités du gibier. Influence des pesticides et évaluation des risques selon la nature des produits. Phytoma, July:26–32.
Quinto I, DeMarinis E, Mallardo M, Arcucci A, Della Morte R, & Staiano N (1989) Effect of DNOC, ferbam and imidan exposure on mouse sperm morphology. Mutat Res, 224:405–408.
Roseboom H, Wammes JIJ, & Wegman RCC (1981) Determination of nitrophenol derivatives in various crops by reversed-phase ion-pair high-performance liquid chromatography. Anal Chim Acta, 132:195–199.
Sainsbury CR, Mullee DM, & Bartlett AJ (1995) Analytical DNOC and technical DNOC. Characterisation (Safepharm Laboratories study no. 764/029) Derby,UK Safepharm Analytical Chemistry Laboratory (unpublished report prepared for Elf Atochem Agri SA).
Sewell IG, Mead C, & Bartlett AJ (1995a) Technical DNOC. Algal inhibition test. Safepharm Laboratories study no. 764/15. Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
Sewell IG, Foulger J, & Bartlett AJ (1995b) Technical DNOC. Acute toxicity to bluegill sunfish (Lepomis macrochirus). Safepharm Laboratories study no. 764/12. Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
Sewell IG, Foulger J, & Bartlett AJ (1995c) Technical DNOC: Acute toxicity to Rainbow trout (Onchorhynchus mykiss). Safepharm Laboratories study no. 764/13. Derby, UK (unpublished report prepared for Elf Atochem Agri SA).
Smith JN, Smithies RH, & Williams RT (1953) Studies in detoxication. 48. Urinary metabolites of 4,6-dinitro-o-cresol in the rabbit. Biochemistry, 54:224–230.
Somani SM, Schaeffer DJ, & Mack JO (1981) Quantifying the toxic and mutagenic activity of complex mixtures with Salmonella typhimurium. J Tox Environ Health, 7:643–653.
Spencer HC, Rowe VK, Adams EM, & Irish DD (1948) Toxicological studies on laboratory animals of certain alkyldinitrophenols used in agriculture. J Industr Hyg Tox, 30(1):10–25.
Starek A, & Lepiarz W (1974) Influence of some fats on 4,6-dinitro-o-cresol (DNOC) resorption from alimentation tract of rats. Pol J Pharmacol Pharm, 26:485–491.
Steer C (1951) Death from di-nitro-ortho-cresol. Lancet, I:1419.
Stott H (1953) The use of certain toxic chemicals in agricultural practice. East African Med J, 30:59–67.
Stott H (1956) Polyneuritis after exposure to dinitro-ortho-cresol. BMJ, 1:900–901.
Sundvall A, Marklund H, & Rannung U (1984) The mutagenicity of Salmonella typhimium of nitrobenzoic acids and other wastewater components generated in the production of nitrobenzoic acids and nitrotoluenes. Mutat Res 137:71–78.
Tan GH, & Chong CL (1993) Trace monitoring of water-borne phenolics in the Klang river basin. Environ Monit Assess, 24:267–277.
Tesic D, Terzic LJ, Dimitrijevic B, Zivanov D, & Slavic M (1972) Experimental investigation on the effect of environmental temperature and some medicaments on the toxic effect of dinitroorthocresol. Acta Vet Beograd, 22(2):45–52.
Tewfik MS, & Evans WC (1966) The metabolism of 3,5-dinitro-o-cresol (DNOC) by soil micro-organisms. Biochem J, 99:31–32.
Til HP (1980) Sub-chronic (90-day) oral toxicity study with DNOC in dogs. CIVO TNO Institute study no. B 80/0359, Zeist, The Netherlands (unpublished report prepared for Pennwalt Holland bv).
Til HP, & Kengen MTF (1980) Subacute (8-day) dietary LC50 study with DNOC in Japanese quail. CIVO-TNO study no. R 6596. CIVO-TNO, Zeist, The Netherlands. (unpublished report prepared for Pennwalt Holland bv).
Tomlin C ed. (1997) The pesticide manual, a word compendium. 11th ed. Farnham, UK, British Crop Protection Council.
Tremain SP, & Bartlett AJ (1995) Technical DNOC (batch number 547–108). Determination of hazardous physico-chemical properties. (Safepharm Laboratories study no. 764/003) Derby, UK Safepharm Analytical Chemistry Laboratory (unpublished report prepared for Elf Atochem Agri SA).
Tremp J, Mattrel P, & Giger W (1993) Phenols and nitrophenols as tropospheric pollutants: Emissions from automobile exhausts and phase transfer in the atmosphere. Water, Air Soil Pollution, 68:113–123.
Tripathi AM, Mhalas JG, & Rama-Rao NV (1989) Determination of 2,6 and 4,6-dinitrocresols by high performance liquid chromatography on a betacyclodextrin bonded column. J Chromatogr, 466:442–445.
Truhaut R & de Lavaur E (1967) Etude du métabolisme du dinitro-4,6-orthocrésol chez le lapin. C R Acad Sci Paris Ser D, 264:1938–1940.
UNEP Chemicals (IRPTC) (1999) UNEP Chemicals data profile (legal) on 4,6 DNOC. Geneva, UNEP Chemicals.
US EPA (1984a) Method 604–phenols. Environmental Protection Agency. Code of Federal Regulations. 40 CFR 136.
US EPA (1984b) Method 625–Base/Neutrals and Acids. Environmental Protection Agency. Code of Federal Regulations. 40 CFR 136.
US EPA (1984c) Method 1625 Revision B–Semivolatile Organic Compounds by Isotope Dilution GC/MS. Environmental Protection Agency. Code of Federal Regulations. 40 CFR 136
US EPA (1986a) Phenols–method 8040. In: Test methods for evaluating solid wastes. SW-846. 3rd ed. Volume 1B: Laboratory Manual. Washington, DC: US Environmental Protection Agency, Office of Solid Waste and Emergency Response.
US EPA (1986b) Gas chromatography/mass spectrometry for semivolatile organics: capillary column technique – method 8270. In: Test methods for evaluating solid wastes. SW-846. 3rd ed. Volume 1B: Laboratory Manual. Washington, DC: US Environmental Protection Agency, Office of Solid Waste and Emergency Response.
US EPA (1988) Health and environmental effects profile for dinitrocresols. Cincinnati, OH: US Environmental Protection Agency. NTIS PB88–220769.
US EPA (1993) Status of pesticides in reregistration and special review. EPA 738/R/93/009. Washington, DC: Office of Prevention, Pesticides and Toxic Substances.
van de Berg KJ, van Raaij JAGM, Bragt PC, & Notten WRF (1991) Interactions of halogenated industrial chemicals with transthyretin and effects on thyroid hormone levels in vivo. Arch Toxicol, 65:15–19.
van der Greef J, & Leegwater DC (1983) Urine profile analysis by field desorption mass spectrophotometry, a technique for detecting metabolites of xenobiotics. Application to 3,5-dinitro-2-hydroxytoluene (DNOC). Biomed Mass Spectrom, 10:1–4.
van der Hoeven JCM (1984) Assessment of the effects of 4,6-dinitro-o-cresol (DNOC) on the reproduction of Daphnia magna (Notox Toxicological Research and Consultancy, s’Hertogenbosch, The Netherlands) (unpublished report prepared for Pennwalt Holland bv).
van der Hoeven JCM (1992) Acute toxicity of DNOC technical to the worm species Eisenia fetida. TNO study no. R91/324. TNO Institute of Delft, The Netherlands (unpublished report prepared for Elf Atochem Agri SA).
van der Laar RTH, de Vries I, & Meulenbelt J (1993) [Acute occupational intoxications through the use of pesticides in forestry, agriculture and horticulture.]. National Poison Control Center of the National Institute of Public Health and Environmental Protection, The Hague (in Dutch).
van Noort HR (1960) [Dinitro-ortho-cresol intoxication in sprayers.] Nederlands tijdschrift voor geneeskunde 104:676–684 (in Dutch).
Verheij ER, & van der Graaf J (1995) The identification of a DNOC metabolite in soil. Study no. 274514, TNO Nutrition and Food Research, Zeist, The Netherlands. (unpublished report prepared for Elf Atochem Agri bv).
Vonk JW, & van der Hoven A (1981) Degradation and conversion of DNOC in water. Adsorption of DNOC to bottom sludge. TNO Organisch Chemisch Intituut, The Netherlands (unpublished report prepared for Elf Atochem Agri bv).
Vos JG, Krajnc EI, Beekhof PK, & van Logten MJ (1983) Methods for testing immune effects of toxic chemicals: evaluation of the immunotoxicity of various pesticides in the rat. In: Miyamoto J ed. IUPAC Pesticide chemistry: human welfare and the environment. Kyoto, Japan, Pergamon Press, pp. 497–504.
Wodageneh A (1997) Obsolete and unwanted pesticide stocks. In: Prevention and disposal of obsolete and unwanted pesticide stocks in Africa and the Near East. Second Consultation Meeting. FAO Pesticide Series, 5:5–12.
WHO (1982) Recommended health limits in occupational exposure to pesticides. Technical Report Series 677. Geneva, World Health Organization.
WHO (1999) The WHO recommended classification of pesticide by hazards and guidelines to classification 1998–1999. WHO/PCS/98.21. Geneva, World Health Organization.
Yao S, Meyer A, & Henze G (1991) Comparison of amperometric and UV-spectrophotometric monitoring in HPLC analysis of pesticides. Fresenius J Anal Chem, 339:207–211.
See Also:
Toxicological Abbreviations