
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 211
HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO
USE AND EXPOSURE TO OTHER AGENTS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 211
First draft prepared by Dr K. Rothwell, Knaresborough, Yorkshire,
United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS), established in
1980, is a joint venture of the United Nations Environment Programme
(UNEP), the International Labour Organisation (ILO), and the World
Health Organization (WHO). The overall objectives of the IPCS are to
establish the scientific basis for assessment of the risk to human
health and the environment from exposure to chemicals, through
international peer review processes, as a prerequisite for the
promotion of chemical safety, and to provide technical assistance in
strengthening national capacities for the sound management of
chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals
(IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture
Organization of the United Nations, WHO, the United Nations Industrial
Development Organization, the United Nations Institute for Training
and Research, and the Organisation for Economic Co-operation and
Development (Participating Organizations), following recommendations
made by the 1992 UN Conference on Environment and Development to
strengthen cooperation and increase coordination in the field of
chemical safety. The purpose of the IOMC is to promote coordination
of the policies and activities pursued by the Participating
Organizations, jointly or separately, to achieve the sound management
of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Health effects of interactions between tobacco use and exposure to
other agents.
(Environmental health criteria ; 211)
1.Smoking - adverse effects 2.Tobacco smoke pollution
3.Drug interactions 4.Environmental exposure
5.Occupational exposure 6.Risk factors
I.International Programme on Chemical Safety II.Series
ISBN 92 4 157211 6 (NLM Classification: QV 137)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE AND EXPOSURE TO
OTHER AGENTS
PREAMBLE
ABBREVIATIONS
1. OVERVIEW
1.1. Introduction
1.2. Examples of combined effects of tobacco smoking and other
exposures
1.3. Composition of tobacco leaf and tobacco smoke
1.4. Mainstream tobacco smoke
1.5. Sidestream tobacco smoke
1.6. Effects of ways of cigarette smoking on smoke
toxicity
1.7. Summary of conclusions and recommendations
2. EXPOSURE TO TOBACCO PRODUCTS AND HEALTH RISKS FROM TOBACCO USE
2.1. Tobacco and its uses
2.1.1. Introduction
2.1.2. Tobacco smoking
2.1.3. Tobacco chewing and snuff
2.2. Responses to mainstream smoke
2.2.1. Acute responses
2.2.1.1 Acute bronchitis
2.2.1.2 Asthma
2.2.2. Chronic responses
2.2.2.1 Chronic obstructive lung
diseases (COLD)
2.2.2.2 Chronic bronchitis
2.2.2.3 Small airways disease
2.2.2.4 Emphysema
2.2.2.5 Pulmonary fibrosis
2.2.2.6 Effects on the immune system
2.2.3. Cancer
2.2.4. Cardiovascular effects
2.2.5. Smoking and occupational accidents, injuries and
absenteeism
2.3. Health risks from smokeless tobacco use
2.3.1. Introduction
2.3.2. Cancer
2.3.3. Cardiovascular disease
3. EFFECTS ON HEALTH OF TOBACCO USE AND EXPOSURE TO OTHER CHEMICALS
3.1. Introduction
3.1.1. Interaction
3.1.2. Measuring interaction
3.1.3. Effects of tobacco smoking on lung deposition
and clearance of particles
3.2. Interactions between tobacco smoke and other agents
3.2.1. Asbestos
3.2.1.1 Asbestos and lung cancer
3.2.1.2 Asbestos and pleural
mesothelioma
3.2.1.3 Asbestos and other forms of cancer
3.2.1.4 Asbestosis
3.3. Non-asbestos fibres
3.3.1. Glass fibre
3.3.2. Rockwool, slagwool and ceramic fibres
3.4. Inorganic chemicals
3.4.1. Arsenic
3.4.2. Beryllium
3.4.3. Chromium
3.4.4. Nickel
3.4.5. Manganese
3.4.6. Platinum
3.4.7. Silica
3.5. Organic chemical agents
3.5.1. Chloromethyl ethers
3.5.2. Tetrachlorophthalic anhydride
3.5.3. Dyestuffs
3.5.4. Polycyclic aromatic hydrocarbons
3.5.5. Ethanol
3.5.6. Other organic compounds
3.6. Physical agents
3.6.1. Radiation
3.6.1.1 Radon in mines (high linear energy
transfer (LET) alpha-radiation)
3.6.1.2 Environmental radon (high linear
energy transfer (LET) alpha-radiation)
3.6.1.3 Atomic bomb site radiation
(low linear energy transfer (LET)
radiation)
3.6.1.4 Therapeutic X-rays (low linear
energy transfer (LET) radiation)
3.6.1.5 Nuclear plant
3.6.1.6 Summary
3.6.2. Vibration
3.6.3. Noise
3.6.4. Dupuytren's contracture
3.7. Biological agents
3.7.1. Biological (vegetable) dusts
3.7.1.1 Cotton dust
3.7.1.2 Wood dust
3.7.1.3 Allergic responses
3.7.2. Other biological agents
3.7.3. Agents found in factory farming
(animal confinement effects)
3.7.4. Laboratory animals
3.7.5. Schistosomiasis
3.7.6. Other urinary tract infections
3.7.7. Sarcoidosis
3.8. Vector effects
3.8.1. Polytetrafluoroethylene
3.8.2. Mercury
3.9. Effects of tobacco smoking and metabolism
of drugs and other chemicals
3.9.1. Oral contraceptive use
3.9.2. Drug and chemical metabolism
3.10. Animal studies of the interactions between
cigarette smoke exposure and other agents
3.10.1. Non-cancer end-points
3.10.2. Cancer studies: tobacco (cigarette)
smoke plus other chemicals
3.10.3. Cancer studies: cigarette smoke plus
radiation
4. EFFECTS OF EXPOSURE TO TOBACCO SMOKE AND OTHER AGENTS: SEPARATE
EFFECTS OR POSSIBLE INTERACTIONS
4.1. Coal mining
4.1.1. Coal dust
4.1.2. Bronchitis in coal miners
4.1.3. Emphysema and pneumoconiosis in coal miners
4.1.4. Lung cancer in coal miners
4.2. Other mineral dusts
4.2.1. Talc
4.2.2. Kaolin
4.2.3. Alumina
4.3. Fibrous minerals
4.4. Metals
4.4.1. Antimony
4.4.2. Cadmium
4.4.3. Cobalt
4.4.4. Lead
4.5. Rubber industry
4.6. Petroleum industry
4.7. Pesticides
5. CONCLUSIONS AND RECOMMENDATIONS
5.1. Conclusions
5.2. Recommendations for protection of human health
6. FURTHER RESEARCH
REFERENCES
SYNOPSIS
PANORAMA GENERAL
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22
- 9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number 5 U01 ES02617-15
from the National Institute of Environmental Health Sciences, National
Institutes of Health, USA, and by financial support from the European
Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was initiated
with the following objectives:
(i) to assess information on the relationship between exposure
to environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects
of pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on mercury,
was published in 1976 and since that time an ever-increasing number of
assessments of chemicals and of physical effects have been produced.
In addition, many EHC monographs have been devoted to evaluating
toxicological methodology, e.g., for genetic, neurotoxic, teratogenic
and nephrotoxic effects. Other publications have been concerned with
epidemiological guidelines, evaluation of short-term tests for
carcinogens, biomarkers, effects on the elderly and so forth.
Since its inauguration the EHC Programme has widened its scope, and
the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health Assembly
resolutions and the recommendations of the 1972 UN Conference on the
Human Environment. Subsequently the work became an integral part of
the International Programme on Chemical Safety (IPCS), a cooperative
programme of UNEP, ILO and WHO. In this manner, with the strong
support of the new partners, the importance of occupational health and
environmental effects was fully recognized. The EHC monographs have
become widely established, used and recognized throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews on
the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data, whenever
available, are preferred to animal data. Animal and in vitro
studies provide support and are used mainly to supply evidence missing
from human studies. It is mandatory that research on human subjects
is conducted in full accord with ethical principles, including the
provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and international
authorities in making risk assessments and subsequent risk management
decisions. They represent a thorough evaluation of risks and are not,
in any sense, recommendations for regulation or standard setting.
These latter are the exclusive purview of national and regional
governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary -- a review of the salient facts and the risk evaluation
of the chemical
* Identity -- physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the priority
list, the IPCS Secretariat consults with the Cooperating Organizations
and all the Participating Institutions before embarking on the
preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an initial
review by a small panel of experts to determine its scientific quality
and objectivity. Once the RO finds the document acceptable as a first
draft, it is distributed, in its unedited form, to well over 150 EHC
contact points throughout the world who are asked to comment on its
completeness and accuracy and, where necessary, provide additional
material. The contact points, usually designated by governments, may
be Participating Institutions, IPCS Focal Points, or individual
scientists known for their particular expertise. Generally some four
months are allowed before the comments are considered by the RO and
author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers participate in
the preparation of the EHC monograph must, in addition to serving in
their personal capacity as scientists, inform the RO if at any time a
conflict of interest, whether actual or potential, could be perceived
in their work. They are required to sign a conflict of interest
statement. Such a procedure ensures the transparency and probity of
the process.
When the Task Group has completed its review and the RO is satisfied
as to the scientific correctness and completeness of the document, it
then goes for language editing, reference checking, and preparation of
camera-ready copy. After approval by the Director, IPCS, the
monograph is submitted to the WHO Office of Publications for printing.
At this time a copy of the final draft is sent to the Chairperson and
Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC progress
report, of the authors and institutions proposed for the drafting of
the documents. A comprehensive file of all comments received on
drafts of each EHC monograph is maintained and is available on
request. The Chairpersons of Task Groups are briefed before each
meeting on their role and responsibility in ensuring that these rules
are followed.
 WHO WORKING GROUP ON HEALTH RISKS FROM THE COMBINED EFFECTS OF TOBACCO
SMOKING AND EXPOSURE TO OTHER CHEMICALS
(Geneva, 25-26 April 1966)
Members
Dr G. Finch, Inhalation Toxicology Research Institute, Lovelace
Biomedical and Environmental Research Institute, Albuquerque, New
Mexico, USA
Professor G. Pershagen, Division of Epidemiology, Institute of
Environmental Medicine, Karolinska Institute, Stockholm, Sweden
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
Professor H.-P. Witschi, Institute of Toxicology and Environmental
Health, University of California, Davis, California, USA
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr N.F. Collishaw, Tobacco or Health, Programme on Substance
Abuse, World Health Organization, Geneva, Switzerland
WHO TASK GROUP ON HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE
AND EXPOSURE TO OTHER AGENTS
(Geneva, 18-21 February 1997)
Members
Dr G. Finch, Lovelace Respiratory Research Institute, Inhalation
Toxicology Research Institute, Albuquerque, New Mexico, USA
( Chairman)
Dr L. Fishbein, Fairfax, VA, USA ( Joint Rapporteur)
Dr Dorota Jarosinska, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Professor G. Kazantzis, Royal School of Mines, London, United
Kingdom ( Joint Rapporteur)
Professor U. Keil, Institute for Epidemiology and Social Medicine,
Münster, Germany
Dr D. Krewski, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
( Vice-Chairman)
Dr L. van Bree, Laboratory of Health Effects Research, National
Institute of Public Health and the Environment, Bilthoven, The
Netherlands
Observers
Dr G. Minotti, Catholic University of the Sacred Heart, Rome, Italy
( representing the International Union of Pharmaceutical
Societies)
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
Mrs B.F. Goelzer, Occupational Health, World Health Organization,
Geneva, Switzerland
Mr N.E. Collishaw, Programme on Substance Abuse, World Health
Organization, Geneva, Switzerland
HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE AND EXPOSURE TO
OTHER AGENTS
A WHO Task Group on Health Effects of Interactions Between Tobacco Use
and Exposure to Other Agents met at the World Health Organization,
Geneva, from 18 to 21 February 1997. Dr E. Smith, IPCS, welcomed the
participants on behalf of Dr M. Mercier, Director IPCS, and the
cooperating organizations. The Task Group reviewed and revised the
draft monograph and developed a new text.
The first draft of the monograph was prepared by Dr K. Rothwell,
Knaresborough, Yorkshire, United Kingdom. This draft was further
developed by a Working Group held in WHO, Geneva, 25-26 April 1996,
and then circulated for international comment to IPCS contact points
for Environmental Health Criteria monographs. Comments were
incorporated in a second draft prepared by Dr K. Rothwell. This draft
was reviewed at the Task Group meeting and a text given further
limited circulation to Task Group members and a number of other
experts, including the US EPA National Center for Environmental
Assessment under the coordination of Dr D. Mukerjee, for final
comment. In the development of the monograph, contributions were made
by a number of authors listed below.
Dr E. Smith (IPCS Unit for the Assessment of Risk and Methods) was
responsible for the scientific content of the monograph and Dr P.G.
Jenkins (IPCS Central Unit) for the technical editing.
The efforts of all who helped in the preparation and finalization of
the monograph are gratefully acknowledged.
The authors were:
Main authors
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
( Coordinating author)
Dr G. Finch, Lovelace Respiratory Research Institute, Albuquerque,
New Mexico, USA
Dr L. Fishbein, Fairfax, Virginia, USA
Dr D. Krewski, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Professor G. Pershagen, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden
Professor H-P. Witschi, Institute of Toxicology and Environmental
Health, University of California, Davis, California, USA
Contributing authors
Dr D. Jarosinska, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Professor G. Kazantzis, Royal School of Mines, London, United
Kingdom
Professor U. Keil, Institute for Epidemiology and Social Medicine,
Munster, Germany
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr L. van Bree, National Institute of Public Health and the
Environment, Bilthoven, the Netherlands
* * *
The IPCS expresses its gratitude to the external reviewers who
provided comments and other relevant material, in particular the
United Kingdom Department of Health, London, and the US Environmental
Protection Agency's Office of Research and Development, National
Center for Environmental Assessment, Cincinnati, Ohio, USA.
The funds for the preparation, review and publication of this
monograph were generously provided by Health Canada.
ABBREVIATIONS
BCME bis(chloromethyl) ether
CMME chloromethyl methyl ether
COLD chronic obstructive lung disease
CHD coronary heart disease
CVD cardiovascular disease
ERR excess relative risk
FIV1 forced expiratory volume (1 second)
IARC International Agency for Research on Cancer
LET linear energy transfer
MMAD mass median aerodynamic diameter
NOx nitrogen oxides
SAD small airways disease
TSNA tobacco-specific nitroasamines
URT upper respiratory tract
1. OVERVIEW
1.1 Introduction
Tobacco use, particularly smoking, causes a range of adverse health
effects, is directly implicated in a number of serious diseases, and
can increase adverse effects of other chemical, physical and
biological agents. Chemicals and other agents in workplaces can cause,
if not controlled, disease, incapacity and early death. In the
workplace it is clear that adverse effects can be produced by the
synergistic interaction of tobacco smoking and other hazards. The
majority of interactions of harmful tobacco smoke constituents with
toxic chemicals occur when the latter are airborne, although
interactions of smoking with ingested and/or absorbed harmful agents
have also been reported.
Tobacco use is widespread throughout the world, from countries with
low income economies to the most affluent industrialized nations.
Tobacco is used by men and women, by children and adults, and millions
of people are involuntarily subjected to environmental tobacco smoke.
There are numerous explanations for the tobacco habit but the main
reason for its ubiquity is the addictive drug nicotine present in all
forms of tobacco leaf and delivered in varying amounts to the user by
the various methods of tobacco use (chapter 2). The advent of the
cigarette, mass produced, easily obtainable, relatively cheap and
light in weight, so it can be held in the mouth leaving the hands
free, has had a major impact on smoking habits, in general and in
workplaces.
In many countries tobacco smoking is recognized as a serious health
hazard and a major contributing factor to deaths from a number of
common diseases. In these countries health warning legislation and
measures to control consumption by taxation have been implemented, as
well as public education programmes on the dangers of smoking and the
benefits to be gained from not starting or from stopping. However,
there are still countries where decisive action has yet to be taken to
deal with the problem of tobacco use.
Many work situations involve an element of risk. The nature of the
work may generate harmful effects on health, and working activities
may cause environmental contamination. Tobacco growing itself involves
the use of pesticides, harvesting of tobacco leaf can cause sickness
due to skin absorption of nicotine, and processing exposes workers to
health hazards from airborne dust and fungal spores. A high male
cancer incidence has been reported in areas with tobacco industries.
In mining there are airborne mineral dusts and, in farming and
industries using biologically produced raw materials, biological dusts
are found. Fumes are produced during welding, and gases, smokes, mists
and vapours containing inorganic and/or organic toxic substances
present hazards in many industries. Excessive heat or exposure to
ultraviolet light can be detrimental to the well-being of workers.
Ionizing radiation in mining and modern technology is recognized as a
workplace hazard. In many occupations workers are subjected to
excessive noise or harmful mechanical vibration. Working conditions
can impact adversely on health to a greater extent in smokers than
non-smokers. In many countries, smoking at the workplace is
prohibited, primarily for reasons of fire/explosion safety. However,
in some countries, regulations are not always enforced. In some newly
industrializing countries health problems associated with work have
not yet been fully addressed and many employers and workers are
ignorant of the dangers to health of their occupations. In addition,
there is the large "informal sector" of industry, particularly in
developing countries, where the home is the workplace, chemicals are
used (including solvents, resins, and synthetic dyestuffs), the whole
family is exposed, and there are no restrictions on exposure to work
hazards or smoking.
The situation for adverse health effects resulting from combined
exposure to tobacco smoke, mainstream or environmental, and agents in
the domestic environment is much less defined. However, the incidence
of lung cancer and the concentration of radon in homes has a similar
dose-response to lung cancer and radon in mines, and the risk is
higher in smokers.
1.2 Examples of combined effects of tobacco smoking and other
exposures
There is evidence for synergism in the production of adverse effects
(cancer) between tobacco smoking and exposure to arsenic, asbestos,
ethanol, silica and radiation (radon, atomic bomb, X-ray). On the
other hand there is evidence for antagonism in the case of tobacco
smoking and the carcinogenic chloromethyl ethers, i.e. chloromethyl
methyl ether (CMME) and bis(chloromethyl) ether (BCME) (Hoffmann &
Wynder, 1976; IARC, 1986), tobacco smoking and allergic alveolitis,
and tobacco smoking and chronic beryllium disease. Tobacco smoking
affects the health risks of exposures in coal mining, pesticide
handling, and in the rubber and petroleum industries. Coal miners who
smoke are at greater risk of developing chronic bronchitis and
obstructive airway disease but not emphysema. Lung cancer in coal
miners has been attributed entirely to tobacco smoking. Tobacco
smoking can increase the health risks of exposure to vegetable dusts
that produce chronic respiratory conditions, such as byssinosis
produced by cotton dust, and nasal cancer caused by wood dusts.
1.3 Composition of tobacco leaf and tobacco smoke
More than 3040 chemical compounds have been isolated from processed
tobacco leaf (Roberts, 1988). Most are leaf constituents, but some
arise from growing conditions such as the soil and atmosphere in an
area, while others originate from the use of agricultural chemicals,
from casings, humectants and flavourings added to the leaves, and from
curing methods. Different tobacco varieties grown in different
countries, and cured and processed in various ways show differences.
The proportions of individual constituents may differ but not the
overall composition. Among important toxic compounds identified, other
than nicotine, are carcinogenic nitrosamines, derived from nitrites,
amines, proteins and alkaloids present in the leaf, polycyclic
aromatic hydrocarbons resulting from the curing process, radioactive
elements absorbed from the soil and the air, and cadmium in tobacco
grown on cadmium-rich soils. When tobacco is burned in the course of
smoking, many pyrolysis and other reaction products are formed.
1.4 Mainstream tobacco smoke
Tobacco smoke is an aerosol consisting of a particulate phase of
liquid droplets dispersed in a gas/vapour phase. When a cigarette is
smoked, many compounds are formed by pyrolysis of the tobacco. These
either pass through the cigarette as mainstream smoke, some being
condensed a short distance behind the burning cone, or they are
emitted into the air from the burning end as sidestream smoke. With
each puff the smoke becomes progressively stronger because previously
condensed material is added to the smoke and the length of cigarette
available for further condensation is decreasing. The physicochemical
nature of the smoke depends on the processing and burning of the
tobacco, the porosity and treatment of the paper wrapper, and on the
type of filter tip (Hoffmann & Hoffmann, 1997). In the case of a
cigarette or Asian "bidi" (tobacco wrapped in vegetable leaf), the
smoke chemistry is affected by such factors as dimensions, wrapper
porosity and the smoking parameters of puff volume, frequency and
duration (NIH, 1998). Variations in smoke chemistry are mainly in the
balance of smoke constituents rather than the presence or absence of
particular compounds.
Mainstream smoke is generated in a comparatively low-oxygen atmosphere
at a burning temperature of 850-950°C in the fire cone. Initially,
mainstream smoke particles have a mass median aerodynamic diameter
(MMAD) of 0.2 to 0.3 µm; however, as soon as they encounter the 100%
humidity of the respiratory tract, they coalesce into larger particles
and behave as if their MMAD was in the micrometre range. Between 50
and 90% of all inhaled particulate matter may be retained in the
respiratory tract (Wynder & Hoffmann, 1967; Hinds et al., 1983). From
size considerations, the aerosol particulate matter, the vapour phase
constituents and the permanent gases are capable of reaching the
alveoli when smoke is inhaled. Deposition in the tracheobronchial tree
is complicated by the behaviour of hydrophilic constituents in the
high humidity conditions, but smoke reaches every part of the airways.
Mainstream smoke contains nearly 4000 identified chemicals and an
unknown number of unidentified chemicals (Roberts, 1988). Mainstream
smoke can be divided into particulate and gas phases. Mainstream smoke
particulate phase contains nicotine, nitrosamines such as
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and
N-nitrosonornicotine (NNN), metals such as cadmium, nickel, zinc and
polonium-210, polycyclic hydrocarbons, and carcinogenic amines, such
as 4-aminobiphenyl. The vapour phase contains carbon monoxide, carbon
dioxide, benzene, ammonia, formaldehyde, hydrogen cyanide,
N-nitrosodimethylamine, N-nitrosodiethylamine and other compounds.
Compounds in tobacco smoke can be classified by their biological
activity as asphyxiants, irritants, ciliatoxins, mutagens,
carcinogens, enzyme inhibitors, neurotoxins or pharmacologically
active compounds. The main point of entry of cigarette smoke into the
body is via the airways, but many constituents, particularly from pipe
and cigar smoke, dissolve in saliva and are absorbed in the buccal
cavity or are swallowed. Cigar and pipe smokers generally do not
inhale the smoke and it remains in the oral cavity, is dissolved in
the saliva and absorbed through the mucous membranes or swallowed
(NIH, 1998). Alcoholic drinks have a solvent effect for the smoke
constituents facilitating their absorption.
1.5 Sidestream tobacco smoke
Sidestream smoke is generated at lower burning temperature (500-600°C)
in a reducing atmosphere. Fresh sidestream smoke particles are about
the same size as mainstream smoke particles with a mass median
aerodynamic diameter (MMAD) of approximately 0.2 µm. Qualitatively,
sidestream smoke composition is similar to the composition of
mainstream smoke. Some chemicals in sidestream smoke are emitted at
higher concentrations per gram of tobacco burned than in mainstream
smoke. This is particularly so for carcinogens such as
N-nitrosodimethylamine and N-nitrosodiethyl-amine, and for metals
such as nickel or cadmium. Many carcinogenic compounds are more
concentrated in sidestream than in mainstream smoke. Mouse
skin-painting bioassays have shown that condensate of sidestream smoke
is more carcinogenic than that of mainstream smoke (Wynder & Hoffmann,
1967; US Surgeon General, 1986; NIH, 1998).
1.6 Effects of ways of cigarette smoking on smoke toxicity
The nicotine content of different cigarettes varies, and the smoker
adjusts the smoking intensity and depth of inhalation to satisfy the
acquired nicotine need. Consequently, the smoker of a filter cigarette
with a low nicotine yield (<1.2 mg) smokes more intensively and this
influences toxicity (NIH, 1998).
1.7 Summary of conclusions and recommendations
Tobacco use, particularly smoking, is a most important public health
hazard and a major preventable cause of morbidity and mortality. In
addition to the adverse health effects of active tobacco use, adverse
health effects have been demonstrated to result from exposure to
environmental tobacco smoke. The risks from tobacco smoking are also
increased through interactions with certain chemical, physical and
biological hazards found in the workplace and general environment.
There are a few instances of antagonistic interactions, but the health
risks of tobacco smoke far outweigh any apparent protective effects.
All possible measures should be taken to eliminate tobacco use,
particularly smoking, and smoking in public places should be strongly
discouraged. To avoid interaction with occupational exposures and to
eliminate the risk of exposure to environmental tobacco smoke, smoking
in the workplace should be prohibited.
To protect health, in particular that of children, smoking in domestic
environments should be strongly discouraged. This will prevent
possible harmful interactions between tobacco smoke and residential
exposures to other hazards. There is a pressing need for educational
programmes on the health hazards of smoking. Health professionals
should provide assistance to help smokers quit. Since smoking may
result in altered response or adverse reactions to drugs and other
treatments, appropriate dose adjustments and patient surveillance
should be taken into consideration by clinicians.
2. EXPOSURE TO TOBACCO PRODUCTS AND HEALTH RISKS FROM TOBACCO USE
2.1 Tobacco and its uses
2.1.1 Introduction
The genus Nicotiana, a member of the plant family Solanaceae, is
represented by about 100 species and sub-species (Cromwell, 1955; Tso,
1990) widely distributed throughout the world. The species N.
tabacum and N. rustica are the principal sources of tobacco. The
primary intention in using tobacco is to obtain the alkaloid nicotine
and, once the habit has been established, nicotine appears to fulfil
both a pharmacological and psychological need (US Surgeon General,
1988; NIH, 1998).
Tobacco and its uses were unknown outside America before its discovery
by Columbus. In a description of tobacco use in South America at that
period, Wilbert (1987) used information from European explorers.
There were six types of tobacco use: chewing, drinking, licking,
rectal insertion, nasal and oral snuffing, and smoking. Smoking was by
far the most common, while rectal application was only used
occasionally. Tobacco smoke was inhaled via the mouth through tubes or
rolls of tobacco leaf, or through the nostrils using a Y-shaped tube.
Alternatively, it was swallowed and belched back, blown from one
person to another, or blown into the eyes. Leaves were chewed, alone
or mixed with ash, powdered shells or honey, or they were held in the
mouth and sucked. Infusions were drunk or a concentrated infusion was
licked or even used as an enema. Many ways of preparing and taking
snuff among different tribes were reported. Tobacco use was linked to
religious rituals.
The rest of the world adopted tobacco use in the forms of smoking,
chewing and snuffing. Smoking has remained the most popular. Tobacco
smoking consists of burning the cured leaf, perhaps mixed with
fragrant additives, in a pipe or tube. Cigarette smoking has largely
replaced other methods in many countries. Reasons for the popularity
of cigarettes include their ready availability resulting from
large-scale manufacture and their greater convenience over other forms
of tobacco use (IARC, 1986). In developed countries, cigarettes
account for at least 80% of overall tobacco consumption, but in most
developing countries other ways of using tobacco predominate, although
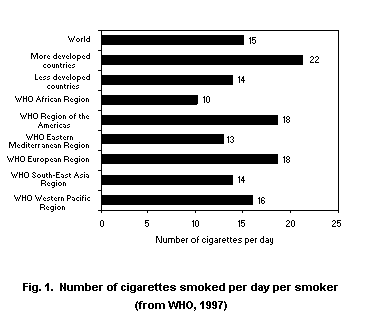
cigarette smoking is increasing. Fig. 1 shows estimated daily
cigarette consumption on a regional basis, Tables 1 and 2 show daily
smoking prevalences on a regional and national basis and Table 3 shows
trends in cigarette consumption.
WHO WORKING GROUP ON HEALTH RISKS FROM THE COMBINED EFFECTS OF TOBACCO
SMOKING AND EXPOSURE TO OTHER CHEMICALS
(Geneva, 25-26 April 1966)
Members
Dr G. Finch, Inhalation Toxicology Research Institute, Lovelace
Biomedical and Environmental Research Institute, Albuquerque, New
Mexico, USA
Professor G. Pershagen, Division of Epidemiology, Institute of
Environmental Medicine, Karolinska Institute, Stockholm, Sweden
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
Professor H.-P. Witschi, Institute of Toxicology and Environmental
Health, University of California, Davis, California, USA
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr N.F. Collishaw, Tobacco or Health, Programme on Substance
Abuse, World Health Organization, Geneva, Switzerland
WHO TASK GROUP ON HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE
AND EXPOSURE TO OTHER AGENTS
(Geneva, 18-21 February 1997)
Members
Dr G. Finch, Lovelace Respiratory Research Institute, Inhalation
Toxicology Research Institute, Albuquerque, New Mexico, USA
( Chairman)
Dr L. Fishbein, Fairfax, VA, USA ( Joint Rapporteur)
Dr Dorota Jarosinska, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Professor G. Kazantzis, Royal School of Mines, London, United
Kingdom ( Joint Rapporteur)
Professor U. Keil, Institute for Epidemiology and Social Medicine,
Münster, Germany
Dr D. Krewski, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
( Vice-Chairman)
Dr L. van Bree, Laboratory of Health Effects Research, National
Institute of Public Health and the Environment, Bilthoven, The
Netherlands
Observers
Dr G. Minotti, Catholic University of the Sacred Heart, Rome, Italy
( representing the International Union of Pharmaceutical
Societies)
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
Mrs B.F. Goelzer, Occupational Health, World Health Organization,
Geneva, Switzerland
Mr N.E. Collishaw, Programme on Substance Abuse, World Health
Organization, Geneva, Switzerland
HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE AND EXPOSURE TO
OTHER AGENTS
A WHO Task Group on Health Effects of Interactions Between Tobacco Use
and Exposure to Other Agents met at the World Health Organization,
Geneva, from 18 to 21 February 1997. Dr E. Smith, IPCS, welcomed the
participants on behalf of Dr M. Mercier, Director IPCS, and the
cooperating organizations. The Task Group reviewed and revised the
draft monograph and developed a new text.
The first draft of the monograph was prepared by Dr K. Rothwell,
Knaresborough, Yorkshire, United Kingdom. This draft was further
developed by a Working Group held in WHO, Geneva, 25-26 April 1996,
and then circulated for international comment to IPCS contact points
for Environmental Health Criteria monographs. Comments were
incorporated in a second draft prepared by Dr K. Rothwell. This draft
was reviewed at the Task Group meeting and a text given further
limited circulation to Task Group members and a number of other
experts, including the US EPA National Center for Environmental
Assessment under the coordination of Dr D. Mukerjee, for final
comment. In the development of the monograph, contributions were made
by a number of authors listed below.
Dr E. Smith (IPCS Unit for the Assessment of Risk and Methods) was
responsible for the scientific content of the monograph and Dr P.G.
Jenkins (IPCS Central Unit) for the technical editing.
The efforts of all who helped in the preparation and finalization of
the monograph are gratefully acknowledged.
The authors were:
Main authors
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
( Coordinating author)
Dr G. Finch, Lovelace Respiratory Research Institute, Albuquerque,
New Mexico, USA
Dr L. Fishbein, Fairfax, Virginia, USA
Dr D. Krewski, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Professor G. Pershagen, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden
Professor H-P. Witschi, Institute of Toxicology and Environmental
Health, University of California, Davis, California, USA
Contributing authors
Dr D. Jarosinska, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Professor G. Kazantzis, Royal School of Mines, London, United
Kingdom
Professor U. Keil, Institute for Epidemiology and Social Medicine,
Munster, Germany
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr L. van Bree, National Institute of Public Health and the
Environment, Bilthoven, the Netherlands
* * *
The IPCS expresses its gratitude to the external reviewers who
provided comments and other relevant material, in particular the
United Kingdom Department of Health, London, and the US Environmental
Protection Agency's Office of Research and Development, National
Center for Environmental Assessment, Cincinnati, Ohio, USA.
The funds for the preparation, review and publication of this
monograph were generously provided by Health Canada.
ABBREVIATIONS
BCME bis(chloromethyl) ether
CMME chloromethyl methyl ether
COLD chronic obstructive lung disease
CHD coronary heart disease
CVD cardiovascular disease
ERR excess relative risk
FIV1 forced expiratory volume (1 second)
IARC International Agency for Research on Cancer
LET linear energy transfer
MMAD mass median aerodynamic diameter
NOx nitrogen oxides
SAD small airways disease
TSNA tobacco-specific nitroasamines
URT upper respiratory tract
1. OVERVIEW
1.1 Introduction
Tobacco use, particularly smoking, causes a range of adverse health
effects, is directly implicated in a number of serious diseases, and
can increase adverse effects of other chemical, physical and
biological agents. Chemicals and other agents in workplaces can cause,
if not controlled, disease, incapacity and early death. In the
workplace it is clear that adverse effects can be produced by the
synergistic interaction of tobacco smoking and other hazards. The
majority of interactions of harmful tobacco smoke constituents with
toxic chemicals occur when the latter are airborne, although
interactions of smoking with ingested and/or absorbed harmful agents
have also been reported.
Tobacco use is widespread throughout the world, from countries with
low income economies to the most affluent industrialized nations.
Tobacco is used by men and women, by children and adults, and millions
of people are involuntarily subjected to environmental tobacco smoke.
There are numerous explanations for the tobacco habit but the main
reason for its ubiquity is the addictive drug nicotine present in all
forms of tobacco leaf and delivered in varying amounts to the user by
the various methods of tobacco use (chapter 2). The advent of the
cigarette, mass produced, easily obtainable, relatively cheap and
light in weight, so it can be held in the mouth leaving the hands
free, has had a major impact on smoking habits, in general and in
workplaces.
In many countries tobacco smoking is recognized as a serious health
hazard and a major contributing factor to deaths from a number of
common diseases. In these countries health warning legislation and
measures to control consumption by taxation have been implemented, as
well as public education programmes on the dangers of smoking and the
benefits to be gained from not starting or from stopping. However,
there are still countries where decisive action has yet to be taken to
deal with the problem of tobacco use.
Many work situations involve an element of risk. The nature of the
work may generate harmful effects on health, and working activities
may cause environmental contamination. Tobacco growing itself involves
the use of pesticides, harvesting of tobacco leaf can cause sickness
due to skin absorption of nicotine, and processing exposes workers to
health hazards from airborne dust and fungal spores. A high male
cancer incidence has been reported in areas with tobacco industries.
In mining there are airborne mineral dusts and, in farming and
industries using biologically produced raw materials, biological dusts
are found. Fumes are produced during welding, and gases, smokes, mists
and vapours containing inorganic and/or organic toxic substances
present hazards in many industries. Excessive heat or exposure to
ultraviolet light can be detrimental to the well-being of workers.
Ionizing radiation in mining and modern technology is recognized as a
workplace hazard. In many occupations workers are subjected to
excessive noise or harmful mechanical vibration. Working conditions
can impact adversely on health to a greater extent in smokers than
non-smokers. In many countries, smoking at the workplace is
prohibited, primarily for reasons of fire/explosion safety. However,
in some countries, regulations are not always enforced. In some newly
industrializing countries health problems associated with work have
not yet been fully addressed and many employers and workers are
ignorant of the dangers to health of their occupations. In addition,
there is the large "informal sector" of industry, particularly in
developing countries, where the home is the workplace, chemicals are
used (including solvents, resins, and synthetic dyestuffs), the whole
family is exposed, and there are no restrictions on exposure to work
hazards or smoking.
The situation for adverse health effects resulting from combined
exposure to tobacco smoke, mainstream or environmental, and agents in
the domestic environment is much less defined. However, the incidence
of lung cancer and the concentration of radon in homes has a similar
dose-response to lung cancer and radon in mines, and the risk is
higher in smokers.
1.2 Examples of combined effects of tobacco smoking and other
exposures
There is evidence for synergism in the production of adverse effects
(cancer) between tobacco smoking and exposure to arsenic, asbestos,
ethanol, silica and radiation (radon, atomic bomb, X-ray). On the
other hand there is evidence for antagonism in the case of tobacco
smoking and the carcinogenic chloromethyl ethers, i.e. chloromethyl
methyl ether (CMME) and bis(chloromethyl) ether (BCME) (Hoffmann &
Wynder, 1976; IARC, 1986), tobacco smoking and allergic alveolitis,
and tobacco smoking and chronic beryllium disease. Tobacco smoking
affects the health risks of exposures in coal mining, pesticide
handling, and in the rubber and petroleum industries. Coal miners who
smoke are at greater risk of developing chronic bronchitis and
obstructive airway disease but not emphysema. Lung cancer in coal
miners has been attributed entirely to tobacco smoking. Tobacco
smoking can increase the health risks of exposure to vegetable dusts
that produce chronic respiratory conditions, such as byssinosis
produced by cotton dust, and nasal cancer caused by wood dusts.
1.3 Composition of tobacco leaf and tobacco smoke
More than 3040 chemical compounds have been isolated from processed
tobacco leaf (Roberts, 1988). Most are leaf constituents, but some
arise from growing conditions such as the soil and atmosphere in an
area, while others originate from the use of agricultural chemicals,
from casings, humectants and flavourings added to the leaves, and from
curing methods. Different tobacco varieties grown in different
countries, and cured and processed in various ways show differences.
The proportions of individual constituents may differ but not the
overall composition. Among important toxic compounds identified, other
than nicotine, are carcinogenic nitrosamines, derived from nitrites,
amines, proteins and alkaloids present in the leaf, polycyclic
aromatic hydrocarbons resulting from the curing process, radioactive
elements absorbed from the soil and the air, and cadmium in tobacco
grown on cadmium-rich soils. When tobacco is burned in the course of
smoking, many pyrolysis and other reaction products are formed.
1.4 Mainstream tobacco smoke
Tobacco smoke is an aerosol consisting of a particulate phase of
liquid droplets dispersed in a gas/vapour phase. When a cigarette is
smoked, many compounds are formed by pyrolysis of the tobacco. These
either pass through the cigarette as mainstream smoke, some being
condensed a short distance behind the burning cone, or they are
emitted into the air from the burning end as sidestream smoke. With
each puff the smoke becomes progressively stronger because previously
condensed material is added to the smoke and the length of cigarette
available for further condensation is decreasing. The physicochemical
nature of the smoke depends on the processing and burning of the
tobacco, the porosity and treatment of the paper wrapper, and on the
type of filter tip (Hoffmann & Hoffmann, 1997). In the case of a
cigarette or Asian "bidi" (tobacco wrapped in vegetable leaf), the
smoke chemistry is affected by such factors as dimensions, wrapper
porosity and the smoking parameters of puff volume, frequency and
duration (NIH, 1998). Variations in smoke chemistry are mainly in the
balance of smoke constituents rather than the presence or absence of
particular compounds.
Mainstream smoke is generated in a comparatively low-oxygen atmosphere
at a burning temperature of 850-950°C in the fire cone. Initially,
mainstream smoke particles have a mass median aerodynamic diameter
(MMAD) of 0.2 to 0.3 µm; however, as soon as they encounter the 100%
humidity of the respiratory tract, they coalesce into larger particles
and behave as if their MMAD was in the micrometre range. Between 50
and 90% of all inhaled particulate matter may be retained in the
respiratory tract (Wynder & Hoffmann, 1967; Hinds et al., 1983). From
size considerations, the aerosol particulate matter, the vapour phase
constituents and the permanent gases are capable of reaching the
alveoli when smoke is inhaled. Deposition in the tracheobronchial tree
is complicated by the behaviour of hydrophilic constituents in the
high humidity conditions, but smoke reaches every part of the airways.
Mainstream smoke contains nearly 4000 identified chemicals and an
unknown number of unidentified chemicals (Roberts, 1988). Mainstream
smoke can be divided into particulate and gas phases. Mainstream smoke
particulate phase contains nicotine, nitrosamines such as
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and
N-nitrosonornicotine (NNN), metals such as cadmium, nickel, zinc and
polonium-210, polycyclic hydrocarbons, and carcinogenic amines, such
as 4-aminobiphenyl. The vapour phase contains carbon monoxide, carbon
dioxide, benzene, ammonia, formaldehyde, hydrogen cyanide,
N-nitrosodimethylamine, N-nitrosodiethylamine and other compounds.
Compounds in tobacco smoke can be classified by their biological
activity as asphyxiants, irritants, ciliatoxins, mutagens,
carcinogens, enzyme inhibitors, neurotoxins or pharmacologically
active compounds. The main point of entry of cigarette smoke into the
body is via the airways, but many constituents, particularly from pipe
and cigar smoke, dissolve in saliva and are absorbed in the buccal
cavity or are swallowed. Cigar and pipe smokers generally do not
inhale the smoke and it remains in the oral cavity, is dissolved in
the saliva and absorbed through the mucous membranes or swallowed
(NIH, 1998). Alcoholic drinks have a solvent effect for the smoke
constituents facilitating their absorption.
1.5 Sidestream tobacco smoke
Sidestream smoke is generated at lower burning temperature (500-600°C)
in a reducing atmosphere. Fresh sidestream smoke particles are about
the same size as mainstream smoke particles with a mass median
aerodynamic diameter (MMAD) of approximately 0.2 µm. Qualitatively,
sidestream smoke composition is similar to the composition of
mainstream smoke. Some chemicals in sidestream smoke are emitted at
higher concentrations per gram of tobacco burned than in mainstream
smoke. This is particularly so for carcinogens such as
N-nitrosodimethylamine and N-nitrosodiethyl-amine, and for metals
such as nickel or cadmium. Many carcinogenic compounds are more
concentrated in sidestream than in mainstream smoke. Mouse
skin-painting bioassays have shown that condensate of sidestream smoke
is more carcinogenic than that of mainstream smoke (Wynder & Hoffmann,
1967; US Surgeon General, 1986; NIH, 1998).
1.6 Effects of ways of cigarette smoking on smoke toxicity
The nicotine content of different cigarettes varies, and the smoker
adjusts the smoking intensity and depth of inhalation to satisfy the
acquired nicotine need. Consequently, the smoker of a filter cigarette
with a low nicotine yield (<1.2 mg) smokes more intensively and this
influences toxicity (NIH, 1998).
1.7 Summary of conclusions and recommendations
Tobacco use, particularly smoking, is a most important public health
hazard and a major preventable cause of morbidity and mortality. In
addition to the adverse health effects of active tobacco use, adverse
health effects have been demonstrated to result from exposure to
environmental tobacco smoke. The risks from tobacco smoking are also
increased through interactions with certain chemical, physical and
biological hazards found in the workplace and general environment.
There are a few instances of antagonistic interactions, but the health
risks of tobacco smoke far outweigh any apparent protective effects.
All possible measures should be taken to eliminate tobacco use,
particularly smoking, and smoking in public places should be strongly
discouraged. To avoid interaction with occupational exposures and to
eliminate the risk of exposure to environmental tobacco smoke, smoking
in the workplace should be prohibited.
To protect health, in particular that of children, smoking in domestic
environments should be strongly discouraged. This will prevent
possible harmful interactions between tobacco smoke and residential
exposures to other hazards. There is a pressing need for educational
programmes on the health hazards of smoking. Health professionals
should provide assistance to help smokers quit. Since smoking may
result in altered response or adverse reactions to drugs and other
treatments, appropriate dose adjustments and patient surveillance
should be taken into consideration by clinicians.
2. EXPOSURE TO TOBACCO PRODUCTS AND HEALTH RISKS FROM TOBACCO USE
2.1 Tobacco and its uses
2.1.1 Introduction
The genus Nicotiana, a member of the plant family Solanaceae, is
represented by about 100 species and sub-species (Cromwell, 1955; Tso,
1990) widely distributed throughout the world. The species N.
tabacum and N. rustica are the principal sources of tobacco. The
primary intention in using tobacco is to obtain the alkaloid nicotine
and, once the habit has been established, nicotine appears to fulfil
both a pharmacological and psychological need (US Surgeon General,
1988; NIH, 1998).
Tobacco and its uses were unknown outside America before its discovery
by Columbus. In a description of tobacco use in South America at that
period, Wilbert (1987) used information from European explorers.
There were six types of tobacco use: chewing, drinking, licking,
rectal insertion, nasal and oral snuffing, and smoking. Smoking was by
far the most common, while rectal application was only used
occasionally. Tobacco smoke was inhaled via the mouth through tubes or
rolls of tobacco leaf, or through the nostrils using a Y-shaped tube.
Alternatively, it was swallowed and belched back, blown from one
person to another, or blown into the eyes. Leaves were chewed, alone
or mixed with ash, powdered shells or honey, or they were held in the
mouth and sucked. Infusions were drunk or a concentrated infusion was
licked or even used as an enema. Many ways of preparing and taking
snuff among different tribes were reported. Tobacco use was linked to
religious rituals.
The rest of the world adopted tobacco use in the forms of smoking,
chewing and snuffing. Smoking has remained the most popular. Tobacco
smoking consists of burning the cured leaf, perhaps mixed with
fragrant additives, in a pipe or tube. Cigarette smoking has largely
replaced other methods in many countries. Reasons for the popularity
of cigarettes include their ready availability resulting from
large-scale manufacture and their greater convenience over other forms
of tobacco use (IARC, 1986). In developed countries, cigarettes
account for at least 80% of overall tobacco consumption, but in most
developing countries other ways of using tobacco predominate, although
cigarette smoking is increasing. Fig. 1 shows estimated daily
cigarette consumption on a regional basis, Tables 1 and 2 show daily
smoking prevalences on a regional and national basis and Table 3 shows
trends in cigarette consumption.
 2.1.2 Tobacco smoking
Smoking prevalence data and most studies into the effects of smoking
have concentrated on cigarette smoking, yet worldwide only about 55%
of tobacco is used for cigarettes. The rest, along with significant
amounts traded in "farm gate" and "local market" sales or "home
grown", is smoked in "bidis" or other hand-rolled devices, or in some
form of pipe (IARC, 1986). Tobacco consumption data give an insight
into tobacco-related disease distribution, as can information on ways
of smoking.
Table 1. Estimated smoking prevalence by WHO Region, early 1990s
(from WHO, 1997)
Men Women
(%) (%)
WHO Regions:
African Regiona 29 4
Region of the Americas 35 22
Eastern Mediterranean Region 35 4
European Region 46 26
South-East Asia Region 44 4
Western Pacific Region 60 8
More developed countries 42 24
Less developed countries 48 7
World 47 12
a Smoking prevalence estimates for African Region are based on
very limited information
Differences in smoke chemistry occur with different tobacco cultivars
and curing methods. Curing removes moisture so that the leaf can be
stored without fermenting or rotting, and the time and temperature of
curing influences enzyme reactions such as deamination and oxidation,
and the content of oils and resins. Air-, flue-, sun-, and fire-curing
methods are employed and each produces distinctive tobaccos which are
intended for smoking in a specific way or to suit a particular
preference.
Between 1933 and the late 1940s, the yields from an average cigarette
varied from 33 to 49 mg tar and from < 1 to 3 mg nicotine (Creek et
al., 1994). However, in the 1960s and 1970s, the average yield from
cigarettes in Western Europe and the USA was around 16 mg tar and 1.5
mg nicotine per cigarette. Current average levels are lower. Changes
in the levels of tar and nicotine have resulted in the characteristic
more intense puffing and smoke inhalation pattern of smokers of
low-yield cigarettes (Djordjevic et al., 1995; NIH, 1996). The
high tar yields measured in the smoke of cigarettes in developed
countries before 1950 are comparable with current tar yields of
"bidis", which range from 23 to 48 mg per cigarette (Hoffmann et al.,
1974; Jayant & Pakhale, 1985; Ball & Simpson, 1987.) The "kretek" (a
cigarette of strong tobacco and cloves) of Indonesia yields up to
71 mg tar (WHO, 1985). Smoking materials in northern Thailand
(cigarette or cigar of strong tobacco plus various vegetable
materials) produce high levels of tar and nicotine (Simarak et al.,
1977).
For smoking, tobacco leaf can be flue-cured, light air-cured,
fire-cured or sun-cured, and can be of dark, oriental or N. rustica
type. Flue-cured tobacco is used for cigarettes and pipe tobacco.
Air-cured tobacco is used in blended cigarettes. Dark tobaccos are
widely grown, usually air-cured, and mostly used in their countries of
origin for dark cigarettes, "bidis", cigars, in pipes and "sheeshas",
and for chewing tobaccos and snuff (Voges, 1984). Some tobaccos have
low nitrate levels ranging up to 0.9% (Neurath & Ehmke, 1964; Wynder &
Hoffmann, 1967) and their smoke contains low nitrogen oxide levels in
the range of 50-200 mg NOx/cigarette (Norman et al., 1983). In the
reducing atmosphere of the burning cone, NOx gives rise to amino
radicals, which react with benzene, biphenyl, naphthalene and other
ring hydrocarbons to form aromatic amines. Aniline, alkylated
anilines, aminobiphenyls, and naphthylamines are therefore found in
higher quantities in the smoke of tobaccos with a high nitrate content
(Patrianakos & Hoffmann, 1979; Pieraccini et al., 1992; Grimmer et
al., 1995). Smokers of these tobaccos can inhale greater quantities of
aromatic amines, such as the human bladder carcinogens
2-naphthyl-amine and 4-aminobiphenyl, and have higher concentrations
of haemoglobin adducts in their blood (Bartsch et al., 1993). This is
the basis for the higher risk of bladder cancer among the smokers of
cigarettes made from dark tobaccos (Vineis et al., 1984; D'Avanzo et
al., 1990; Vineis, 1992).
In the Indian subcontinent, the "bidi" is the common smoking device.
It consists of tobacco flakes, loosely packed and hand-rolled in a
tendu or temburni leaf ( Diospyros melanoxylon). It contains less
tobacco (0.223 g) than a cigarette (0.782 g) (Ramakrishnan et al.,
1995) but up to 8.2% nicotine compared with up to 3.7% in cigarette
tobacco. "Bidi" smoke contains 23 to 48 mg tar and 1.7 to 2.9 mg
nicotine per cigarette (Hoffmann et al., 1974; Jayant & Pakhale,
1985). In India, where around 7% of world tobacco is consumed (US DA,
1990), around 30% of tobacco is smoked as cigarettes, 50% as "bidis",
10% in other ways, and 10% is used for chewing. "Bidis" need to be
puffed frequently to ensure even burning and can generate up to 70 mg
of carbon monoxide, while a US non-filter cigarette smoked under
identical conditions generated 25 mg carbon monoxide (Hoffmann et al.,
1974; Jayant & Pakhale, 1985).
Table 2. Estimated smoking prevalence, ranked in order of male smoking prevalencea
Rank Countryb Men Women Rank Country Men Women
(%) (%) (%) (%)
1 Republic of Korea (1989) 68.2 6.7 21 Seychelles (1989) 50.9 10.3
2 Latvia (1993) 67 12 22 Bolivia (1992) 50 21.4
2 Russian Federation (1993) 67 30 23 Albania (1990) 49.8 7.9
4 Dominican Republic (1990) 66.3 13.6 24 Cuba (1990) 49.3 24.5
5 Tonga (1991) 65 14 25 Bulgaria (1989) 49 17
6 Turkey (1988) 63 24 25 Thailand (1995) 49 4
7 China (1984)c 61 7 27 Spain (1993) 48 25
8 Bangladesh (1990) 60 15 28 Mauritius (1992) 47.2 3.7
9 Fiji (1988) 59.3 30.6 29 Greece (1994) 46 28
10 Japan (1994) 59 14.8 29 Papua New Guinea (1990) 46 28
11 Sri Lanka (1988) 54.8 0.8 31 Israel (1989) 45 30
12 Algeria (1980) 53 10 32 Cook Islands (1988) 44 26
12 Indonesia (1986) 53 4 33 Czech Republic (1994) 43 31
12 Samoa (1994) 53 18.6 33 Jamaica (1990) 43 13
15 Saudi Arabia (1990) 52.7 N/Ad 33 Philippines (1987) 43 8
16 Estonia (1994) 52 24 33 Slovakia (1992) 43 26
16 Kuwait (1991) 52 12 37 Cyprus (1990) 42.5 7.2
16 Lithuania (1992) 52 10 38 Austria (1992) 42 27
16 South Africa (1995) 52 17 39 Malaysia (1986) 41 4
20 Poland (1993) 51 29 39 Peru (1989) 41 13
41 Uruguay (1990) 40.9 26.6 66 Colombia (1992) 35.1 19.1
42 Argentina (1992) 40 23 67 Costa Rica (1988) 35 20
42 France (1993) 40 27 67 Slovenia (1994) 35 23
42 Hungary 40 27 69 Swaziland (1989) 33 8
42 India (1980s) 40 3 70 Luxembourg (1993) 32 26
42 Iraq (1990) 40 5 71 Singapore (1995) 31.9 2.7
42 Malta (1992) 40 18 72 Belgium (1993) 31 19
50 Brazil (1989) 39.9 25.4 72 Canada (1991) 31 29
51 Egypt (1986) 39.8 1 72 Iceland (1994) 31 28
52 Morocco (1990) 39.6 9.1 75 Australia (1993) 29 21
53 Lesotho (1989) 38.3 1 75 Ireland (1993) 29 28
53 Mexico (1990) 38.3 14.4 77 UK (1994) 28 26
55 El Salvador (1988) 38 12 78 USA (1993) 27.7 22.5
Table 2. (cont'd)
Rank Countryb Men Women Rank Country Men Women
(%) (%) (%) (%)
55 Italy (1994) 38 26 79 Pakistan (1980) 27.4 4.4
55 Portugal (1994) 38 15 80 Finland (1994) 27 19
58 Chile (1990) 37.9 25.1 81 Turkmenistan (1992) 26.6 0.5
59 Guatemala (1989) 37.8 17.7 82 Nigeria (1990) 24.4 6.7
60 Denmark (1993) 37 37 83 Paraguay (1990) 24.1 5.5
61 Germany (1992) 36.8 21.5 84 Bahrain (1991) 24 6
62 Norway (1994) 36.4 35.5 84 New Zealand (1992) 24 22
63 Honduras (1988) 36 11 86 Sweden (1994) 22 24
63 Netherlands (1994) 36 29 87 Bahamas (1989) 19.3 3.8
63 Switzerland (1992) 36 26
a Adapted from: WHO (1997).
b The year given in parentheses is the latest available year for data.
c Some 1991 data suggest that there has been little change in smoking prevalence since 1984.
d Data not available.
Table 3. Trends in adult consumption of cigarettes from 1970-1972 to 1990-1992 (Adapted from WHO, 1997)
Annual % change
1970-1972 to 1990-1992
WHO Regions:
African Region +1.2
Region of the Americas -1.5
Eastern Mediterranean Region +1.4
European Region 0
South-East Asia Region +1.8
Western Pacific Region +3
More developed countries -0.5
Less developed countries +2.5
World -0.8
Tar yields for Russian cigarettes were found to be high (21.6-29.2
mg) and cigarettes containing N. rustica produced high smoke
concentrations of tobacco-specific nitrosamines (TSNA) (up to 620 ng
total TSNA/cigarette) (Djordjevic et al., 1991). Indigenous cigarettes
and cigars in Thailand, containing tobacco and other vegetable
materials, have up to 41 mg and 200 mg tar, up to 5.5 mg and 11.4 mg
nicotine, and 41 mg and 820 mg carbon monoxide in cigarette and cigar
smoke, respectively. Indonesian cigarette smoke contains up to 100 ng
of carcinogenic volatile N-nitrosamines and up to 1580 ng of
carcinogenic tobacco-specific N-nitrosamines (Mitacek et al., 1990,
1991), and high tar cigarettes contain up to 28.1 mg tobacco-specific
N-nitrosamines per cigarette (Brunnemann et al., 1996).
Many cigar-like devices are smoked throughout Asia. The tobacco can be
rolled in a tobacco leaf or in the leaves of the jackfruit tree
( Artocarpus integrifolia), banana ( Musa paradisiaca) or hansali
( Grewia microcos) (Bhonsle et al., 1976). They may be smoked
conventionally or in the reverse manner with the burning end inside
the mouth (Reddy, 1974). In Thailand they can contain strong tobacco
and a mixture of koi bark ( Streblus asper), dry tamarind pod
( Tamarindus indica), khai bark ( Homonoia riparia, Euphorbiaceae),
Areca palm bark ( Areca catechu) or other tree bark; they can be
rolled in a banana leaf or have fragrant additives such as sandalwood
Mougne et al. (1982). They contain high levels of tar and nicotine
(Simarak et al., 1977; Mitacek et al., 1999).
Additives are used to enhance the fragrance or taste of smoke
(Hoffmann & Hoffmann, 1997). "Casing sauces", consisting of sugars,
aromatic substances and compounds such as glycerol, propylene glycol,
ethylene glycol and diethylene glycol, which resist changes in
moisture content, are sprayed on the leaf before it is cut to
condition it for processing. Flavouring and dressing compounds are
added to cut tobacco after drying and include licorice, menthol,
cocoa, chocolate, ginger, cinnamon, vanilla, molasses, angelica,
honey, essential oils from anise, clove and juniper, resins and plant
extracts and organic compounds such as coumarins. Additives have been
widely used in pipe and chewing tobaccos. They have also become
important in cigarette tobacco with the development of low-tar and
low-nicotine tobaccos and the use of stem, midrib or reconstituted
leaf (which lacks the aromas and flavours of natural tobacco leaf
lamina) and of tobacco dust requiring additives to ensure its
adherence to cut tobacco.
Added glycerol is transferred to mainstream smoke: 3-6% in cigarette
smoke and 35-43% in pipe smoke (IARC, 1986) and one of its pyrolysis
products is acrolein. Levels of acrolein ranging from 69 mg to 230 mg
per cigarette have been reported and air concentrations as high as
0.46 mg/m3 have been found in smoke-filled rooms (Izard & Liberman,
1978). Acrolein is extremely irritating to the eyes and nasal mucosa,
it affects mitotic and ciliary activity, at the cellular level it has
cytotoxic and cilia-depressant effects, and it can act as a mutagen
(Izard & Liberman, 1978).
In the USA, additives used in cigarette manufacture are food additive
compounds that are "generally recognized as safe (GRAS)" and,
therefore, also considered "safe" as additives to tobacco. However,
the non-volatile additives are to some extent pyrolized during smoking
and can give rise to toxic and/or carcinogenic agents in the smoke. A
major group of compounds formed during pyrolysis is that of the
polynuclear aromatic hydrocarbons. Ethylene glycol is pyrolytically
converted to the human carcinogen ethylene oxide (IARC, 1994a).
Another example of carcinogen formation during tobacco curing and
smoking is the case of MH-30, a sucker growth control agent formulated
from maleic hydrazide in diethanolamine. Residual MH-30 on tobacco
leads to the formation of N-nitrosodiethanolamine (NDELA) in the
smoke (Brunnemann & Hoffmann, 1981). The use of MH-30 on tobacco has
been forbidden in the USA since 1981, and NDELA levels in tobacco and
its smoke have declined (Brunnemann & Hoffmann, 1991).
The use of cloves as a tobacco additive can have health effects. In
Indonesia, the smoke of "kreteks", a blend of ground cloves with
60-65% of tobacco, contains between 41 and 113 mg of tar, and between
1.2 and 4.5 mg nicotine (WHO, 1985; Wise & Guerin, 1986). Mainstream
smoke of kreteks without filter tips contains 19-23 mg of eugenol
released from the cloves, while filter-tipped kretek smoke contains up
to 15 mg (LGC, 1982; Wise & Guerin, 1986). Inhalation of eugenol in
the smoke of kreteks can lead to interstitial haemorrhaging and
congestion of the lung, acute emphysema and acute pulmonary oedema.
These effects were also seen in Syrian golden hamsters exposed to the
smoke of kreteks (LaVoie et al., 1986).
Menthol and other additives that produce a sensation of coolness but
without a mint flavour have also been used in cigarettes. There is no
evidence that these additives result in a higher risk (Cummings et
al., 1987; Sidney et al., 1989).
Many shapes and sizes of smoking pipes are found worldwide (Voges,
1984). Various types of tobacco are used and the smoke can range from
mild to very strong. In the sheesha water pipe the tobacco is kept
alight by pieces of glowing charcoal and the smoke is drawn through
water before being inhaled. Sheesha smoke is mild and low in
particulate matter, benzo( a) pyrene and volatile phenols (Hoffmann
et al., 1961), but it has a high level of carbon monoxide, in part
from the charcoal that keeps the tobacco burning, and smokers have
high carboxyhaemoglobin levels and a reduced FEV1 (8.5% in women, 45%
in men) (Zahran et al., 1985; Al-Fayez et al., 1988).
2.1.3 Tobacco chewing and snuff
The popularity of tobacco chewing and snuff (finely powdered tobacco
leaf) has varied. Nasal inhalation of snuff has given way to the oral
application of snuff and other tobacco-containing mixtures between the
gum and lip, or gum and cheeks, or under the tongue. Chewing tobacco
comes in several forms and may have flavour added from syrups,
liquorice and brandy. Tobacco chewing has retained its popularity in
heavy industries, such as steel and coal mining, woodworking and the
petroleum industry, where the flammability hazard precludes smoking.
In Sweden, 17% of the population uses oral snuff. In the USA, sales of
chewing tobacco have declined but sales of oral snuff have increased
by 61% (US DA, 1997) owing to the popularity of the latter with
teenagers and young adult men. Oral tobacco use in India is very
common but, generally, in developing countries it is declining because
urban populations and younger age groups are smoking cigarettes.
"Betel-quid" chewing is common in Asia and Africa. The basic contents
of a betel-quid are slices of areca nut ( Areca catechu), lime and
tobacco, wrapped in a betel pepper leaf ( Piper betle), but it may
also contain dried dates, menthol and spices such as cardamom, cloves,
coriander, mace and cinnamon.
Other tobacco preparations for oral use often contain lime, calcium
carbonate, sodium carbonate, some form of ash or flavouring materials.
The lime and other agents assist the release of nicotine (Voges, 1984;
Idris et al., 1991).
2.2 Responses to mainstream smoke
Tobacco use has direct effects on health and is responsible for a
variety of diseases. These form a basis for studying interactions
between tobacco use and chemical, physical and biological agents, and
associated effects on health.
2.2.1 Acute responses
Inhaled irritant chemicals cause inflammation of the upper respiratory
tract (URT) and paranasal sinuses, sore throat and bronchial oedema.
Pulmonary oedema may follow if irritants penetrate the lower
respiratory tract. Acute irritation of the URT usually follows
inhalation of highly soluble gases. Slightly less soluble gases cause
URT and bronchial irritation, while relatively insoluble gases
penetrate deeply into the lung and can have delayed effects including
pulmonary oedema (Miller & Kimbell, 1995). Cigarette smoke is a
complex mixture of organic and inorganic constituents with particulate
and vapour phases and highly reactive free radicals (Hocking & Golde,
1979). It contains many compounds with irritant properties, which can
affect all parts of the lung. Kremer et al. (1994) examined the
association between occupational exposures to a variety of airway
irritants and respiratory system effects, and whether the association
was modified by smoking, airway hyperresponsiveness and allergy. The
irritants were SO2, HCl, SO42-, polyester vapour, polyamide vapour,
and oil mist and vapour. Current smoking, airway hyperresponsiveness
and allergy were significantly associated with a higher prevalence of
chronic respiratory symptoms, independent of each other and of
irritant exposure. The association between exposure and the prevalence
of chronic respiratory symptoms was greater in smokers than in non- or
ex-smokers.
2.2.1.1 Acute bronchitis
Acute bronchitis is an inflammation of the bronchial mucous membrane,
initially accompanied by a dry painful cough and followed by
mucopurulent sputum. The cause may be infectious agents or chemical
agents such as tobacco smoke, dusts, fumes, vapours or gases.
2.2.1.2 Asthma
Asthma is a chronic pulmonary inflammatory disease associated with
bronchial hyperreactivity causing paroxysmal dyspnoea due to spasm of
the bronchial musculature, swelling of the mucous membranes and the
production of viscid mucus. In the majority of asthma cases a clear
association exists with atopic IgE-mediated hypersensitivity. Allergic
asthma is a response to a specific agent. Many chemical agents,
including mixtures such as tobacco smoke, can induce asthma. Smoking
enhances the effect of other agents and can reduce the latent period
from first exposure to onset of sensitization.
There has been an increase in the prevalence of asthma in many
countries, and roles for environmental factors, increased
susceptibility and tobacco smoking have been suggested (ISAAC, 1998).
Cigarette smoking together, with atopic status, age, URT infection and
genetic factors, has been considered to increase susceptibility
(Venables et al., 1985; Seaton et al., 1993). Studies have shown a
relationship between cigarette smoking and serum IgE levels. Smokers
have higher levels of IgE with increasing age, compared to non-smoking
controls. A relationship has also been observed between IgE level and
the number of cigarettes smoked (Sherrill et al., 1994).
2.2.2 Chronic responses
2.2.2.1 Chronic obstructive lung diseases
The chronic obstructive lung diseases (COLD) are chronic bronchitis,
small airways disease, toxic bronchiolitis obliterans, emphysema and
fibrosis (Niewoehner et al., 1974; Niewoehner, 1991).
These diseases form an important group of pulmonary diseases caused by
smoking (and by chemical atmospheric pollution) (US Surgeon General,
1984). In a study by Simecek et al. (1986) of 215 229 adults in a
region of former-Czechoslovakia, smoking was the most important risk
factor in COLD. Risks for male non-smokers and light smokers under 30
years of age were 1.18% and 2.28%, respectively; for men aged 50
years, smoking more than 20 cigarettes a day, the risk was 20.36%
compared with 3.31% for non-smokers of the same age. In the USA,
80-90% of the mortality from COLD has been attributed to cigarette
smoking. Cigar and pipe smokers who inhale the smoke also have an
increased death rate from COLD (US Surgeon General, 1984, 1989; NIH,
1998). Between 1979 and 1993, the age-adjusted annual death rate from
COLD in women increased by 122% to 17.1 per 100 000, while in men it
increased by 14% to 27.8 per 100 000. The greater increase of COLD
among women during this period reflected the increase in smoking by
women.
2.2.2.2 Chronic bronchitis
Chronic bronchitis has been variously defined. The United Kingdom
Medical Research Council (MRC, 1965) considered it to be a condition
with persistent production of sputum, which might be associated with
cough, occurring on most days for at least three months in the year
for at least two successive years. The Council recommended a
classification of simple chronic bronchitis, chronic or recurrent
mucopurulent bronchitis or chronic obstructive bronchitis. In a
workplace context, Morgan (1982) defined it as "a condition
characterized by cough and sputum for at least three months of the
year, which may or may not be accompanied by airways obstruction, and
which is a consequence of prolonged inhalation of dust or irritant
gases at the workplace". Fletcher & Pride (1984) suggested an improved
terminology with the term chronic bronchitis meaning chronic or
recurrent bronchial hypersecretion only, abandoning the term chronic
obstructive bronchitis because this implies a causal connection
between mucus hypersecretion and airflow obstruction. In smokers'
lungs the irritant constituents of tobacco smoke cause hypersecretion
of mucus, alter its physical properties and chemical structure, and
impair the mucociliary clearance mechanism. Removal of major
ciliatoxins (hydrogen cyanide and volatile aldehydes) from the smoke
stream by charcoal filter tips reduces the effects on the lung
epithelium (Friedman et al., 1972). Mineral dusts, particularly those
encountered in mining, many biological dusts, irritant vapours and
gases, inorganic and organic chemical dusts and sprays can all cause
chronic bronchitis.
2.2.2.3 Small airways disease
Small airways disease (SAD) is a widespread narrowing of membranous
bronchioli. It is inflammatory in origin and is often associated with
excess mucus and an accumulation of macrophages in the respiratory
bronchioli. SAD is mainly caused by smoking, but can be associated
with environmental and industrial pollutants (Cosio et al., 1980).
2.2.2.4 Emphysema
Emphysema has been defined as a condition of the lung characterized by
an abnormal enlargement of the airspaces distal to the terminal
non-respiratory bronchioli, accompanied by destructive changes in the
alveolar walls, and without obvious fibrosis. It tends to be prevalent
in older age groups and follows SAD. For purposes of postmortem
examination of lung slices of miners, Ruckley et al. (1984) defined
emphysema as the presence of air spaces of 1 mm or more in size.
Macrophages that have engulfed foreign particles, including smoke
particulate matter in the lungs of smokers, and which have been found
accumulated in the bronchioli (Niewoehner et al., 1974) and in the
lung parenchyma (McLaughlin & Tueller, 1971) have been implicated in
the pathogenesis of emphysema. There are different forms of emphysema,
which vary with the nature of the insult to the tissues. One
hypothesis is that tobacco smoke causes increased production and
release of proteolytic enzymes, such as elastase, and interferes with
normal antiproteolytic mechanisms. Emphysema has been induced
experimentally in animals by endotracheal installation of elastase or
homogenates of alveolar macrophages or polymorphonuclear leukocytes.
Chronic exposure to tobacco smoke has a number of effects on alveolar
macrophages, including changes in metabolism, alteration of the enzyme
content and impairment of RNA and protein synthesis (Hocking & Golde,
1979). The function of alveolar macrophages is to remove inhaled
foreign material from the alveoli and respiratory bronchioles, and
their numbers increase when the lungs are exposed to particles and
gases. It has been demonstrated that the macrophage count is higher in
people exposed to cigarette smoke than in non-exposed people (Harris
et al., 1974; Rylander et al., 1979). Alveolar macrophages from
smokers are more active than those from non-smokers, numbers are
increased, there are morphological changes with an increased cell
diameter, crystalline inclusions and surface membrane alteration
(Sopori et al., 1994).
2.2.2.5 Pulmonary fibrosis
Pulmonary fibrosis is the abnormal formation of fibrous or scar tissue
and is the response of bronchiolar tissue to the deposition of an
inhaled inciting agent. Mineral and other dusts are causes of
pulmonary fibrosis. The radiological changes seen in early stages of
dust fibroses are associated with relatively minor lung function
impairment, but continuous exposure leads to a greater degree of
fibrosis and to progressive massive fibrosis in some subjects. On
histological, animal experimental and radiographic evidence, Weiss
(1984) concluded that cigarette smoking could cause diffuse fibrosis.
2.2.2.6 Effects on the immune system
Tobacco smoking and exposure to environmental tobacco smoke increase
susceptibility to pulmonary infections, and changes in immune
processes may be involved. Tobacco smoking affects humoral and
cellular immunity in humans and experimental animals, but the
magnitude of the changes vary widely among studies. In humans,
cigarette smoke has marked effects on alveolar macrophage morphology
and physiology, it decreases serum immunoglobulin (IgA, IgG, IgM) but
increases IgE, and has a range of effects on B- and T-lymphocytes.
Similar effects are found in experimental animals (Sopori et al.,
1994). Studies in rats and mice show that cigarette smoke or nicotine
induces impaired responses of systemically distributed B- and
T-lymphocytes to antigen-induced signalling (Geng et al., 1995, 1996).
T-lymphocyte unresponsiveness, with decreased antibody response to
T-dependent antigens, is important in response to infection (Sopori et
al., 1998).
2.2.3 Cancer
Many forms of cancer have been associated with inhaled particulate
matter, vapours, fumes and gases. Examples are lung cancer associated
with tobacco smoking and inhalation of asbestos, fumes from metal
moulding and coking plants, particles and gases inhaled by motor
vehicle drivers, dusts in several types of mines where there is an
accumulation of alpha-emitting radioisotopes, and contact with
materials such as arsenic, chromates, nickel, chloromethyl ethers,
mustard gas and polycyclic aromatic hydrocarbons. Pleural mesothelioma
has been associated with asbestos, nasal and sinus cancer with nickel
refining and wood dust exposure, leukaemia with ionizing radiations
and benzene, bladder cancer with the manufacture and use of dyes and
in the rubber industry, and liver cancer with the use of vinyl
chloride (IARC, 1987).
Cigarette smoke contains many toxic chemicals, including chemicals
that are DNA reactive and cytotoxic or become DNA reactive upon
metabolic activation. Consequently, cigarette smoke has the potential
to initiate genetic lesions. Moolgavkar et al. (1989) postulated
mechanisms by which cigarette smoke induces lung cancer by fitting the
two-stage clonal expansion model of carcinogenesis to lung cancer
mortality data derived from a large cohort of British doctors who
smoked. This analysis suggested that tobacco smoke affected both the
rates of mutation and cell proliferation involved in the model,
supporting the hypothesis that tobacco smoke acts as a complete
carcinogen.
Cigarette smoking is causally associated with cancer of the lung,
larynx, pharynx, oesophagus, pancreas, kidney and urinary bladder. It
is also associated with cancer of the nasal cavity, liver, uterine
cervix and myeloid leukaemia (RCP, 1962; US Surgeon General, 1982,
1989; IARC, 1986; Winkelstein, 1990; Brownson et al., 1993; Roush,
1996).
In 1991, in the USA, it was estimated that 90.3% of the lung cancer
deaths in men and 78.5% of lung cancer deaths in women were
attributable to cigarette smoking. Deaths from oesophageal cancer were
linked to smoking in 78.2% of the cases in men and 74.3% of the cases
in women. Smoking was held responsible for 81.2% of deaths from
laryngeal cancer in men and 86.7% in women, for 91.5% and 61.2%,
respectively, of deaths from oral cancer, and 46.5% and 36.7%,
respectively, of deaths from bladder cancer. Smoking-attributable
deaths from cancer of the kidney in men and women were 47.6 and 12.3%,
respectively, and for pancreatic cancer the figures were 28.6% and
33.3% respectively. In women, 32.4% of uterine cervical cancer deaths
were attributed to cigarette smoking (Shopland et al., 1991).
There has been a change in the rates of different types of lung cancer
among smokers. In 1950, squamous cell carcinoma (SCC) occurred 17
times more often than adenocarcinoma (AC) (Wynder & Graham, 1950) in
cigarette smokers. In 1991, Devesa et al. (1991) reported that in male
cigarette smokers the ratio of SCC to AC was 2.4:1 between 1969 and
1971, and changed to 1.4:1 between 1984 and 1986; in cigarette-smoking
women, the SCC to AC ratio changed from 3.6:1 in 1950 to 0.57:1 in
1984-1986. Between 1970 and 1980 some studies showed a 20-50%
reduction in risk of lung cancer for long-term smokers of filter
cigarettes as compared to smokers of non-filter cigarettes (IARC,
1986) but later studies indicated a similar risk for lung cancer in
smokers of filter and non-filter cigarettes (Stellman et al., 1997;
Thun et al., 1997). The changes in the ratio of SCC to AC, and the
disappearance of an advantage of filter cigarette smoking in terms of
lung cancer risk, have been related to changes in smoke yields, which
have caused smokers to modify patterns of puff drawing and smoke
inhalation. Smokers regulate the speed and the quantity of their
nicotine uptake to achieve the desired pharmacological effects
(Benowitz et al., 1988; Djordjevic et al., 1995). Smokers of lower
nicotine cigarettes draw puffs of greater volume, at a higher
frequency, and inhale more deeply; this is governed by the amount of
nicotine in the smoke (Wynder & Hoffmann, 1994).
Smoking is a major risk factor for the early stage development of
oesophageal and gastric adenocarcinomas, accounting for 40% of cases,
and may have contributed to the increase in the incidence of these
cancers, especially in older people (Gammon et al., 1997).
Cigar smoking is causally associated with cancer of the oral cavity,
the pharynx and the lung, even though for cigar smokers, who do not or
only minimally inhale the smoke, the lung cancer risk is considerably
lower than that for cigarette smokers. Cigar smoking is also
associated with cancer of the pancreas and of the urinary bladder
(NIH, 1998). Oral snuff users have an increased risk of cancer of the
oral cavity and, possibly, cancer of the oesophagus, pancreas and
urinary bladder (US Surgeon General, 1986).
It has been suggested that around 4-5% of all lung cancer is related
to occupational exposure (Wynder & Gori, 1977; Doll & Peto, 1981;
Morgan, 1982 ). Occupational and any other forms of exposure to
chemical compounds are of limited importance in the etiology of lung
cancer whereas tobacco smoking is the cause of approximately 85-90% of
cases (Shopland et al., 1991).
"Reverse smoking", in which rolled tobacco leaves are smoked with the
burning end inside the mouth, has been linked to carcinoma of the hard
palate (Reddy & Rao, 1957; Mehta et al., 1971; Pindborg et al., 1971;
Reddy, 1974; Bhonsle et al., 1976). Reddy et al. (1960) simulated the
effect of reverse smoking experimentally by painting the skin of male
and female mice on alternate days with the tar from Indian cigars and
exposing the painted skin to a temperature of 58°C for 3 min; the heat
treatment enhanced the dermal tumour response.
2.2.4 Cardiovascular effects
Cigarette smoking is a major independent risk factor for
cardiovascular disease (CVD). Cigarette smoking acts synergistically
with other risk factors, such as elevated cholesterol levels and
hypertension (Wilhelmsen, 1977; Gibinski, 1977; US Surgeon General,
1983). Prospective studies indicated that elevated cholesterol and
hypertension appear to be prerequisites for CVD in cigarette smokers
(Kimura, 1977). It has been estimated that in countries with a long
history of cigarette smoking the tobacco habit is responsible for
26-30% of early deaths from CVD (US Surgeon General, 1983; Wald et
al., 1985). Those who smoke several cigars a day and inhale the smoke
also face an increased risk of CVD (NIH, 1998).
The major contributors to the cardiovascular effects of tobacco smoke
are carbon monoxide and nicotine (Lakier, 1992), as well as nitrogen
oxides (NOx), hydrogen cyanide and tar; minor contributors are
cadmium, zinc and carbon disulfide (US Surgeon General, 1983). The
smoke of cigarettes can be slightly acidic (pH 5.6-5.9) or weakly
alkaline (pH 6.7-7.9) depending on the type of tobacco and the blend
(Brunnemann & Hoffmann, 1974). In the slightly acidic smoke, nicotine
is protonated, i.e., bound to a salt or acid moiety and is part of the
particulate phase. Weakly alkaline smoke contains a small percentage
of protonated nicotine (often up to 50% of the nicotine is
unprotonated) in the vapour phase. In contrast to the protonated
variety, unprotonated nicotine is rapidly absorbed through the oral
mucosa and this is why smokers of cigarettes with weakly acidic smoke
and smokers of cigars do not need to inhale into the lung to
experience the pharmacological effects of nicotine. Protonated
nicotine in the particulate matter of slightly acidic cigarette smoke
is not or is only minimally absorbed through the oral mucosa, so that
smokers inhale the smoke and absorption into the bloodstream takes
place in the lung (Armitage & Turner, 1970; Russell, 1976; Benowitz et
al., 1988).
Smoking has been associated with a two-to-fourfold increased risk of
coronary heart disease (CHD), a greater than 70% excess rate of death
from CHD, and an elevated risk of sudden death (Lakier, 1992).
Nicotine causes increases in heart rate and blood pressure, stimulates
nerve endings that are activated by acetylcholine, causes increased
mobilization of free fatty acids in the serum and enhances platelet
adhesiveness. These effects increase cardiac load (which for
individuals with some forms of heart disease will not be met by
increased coronary blood flow) and interfere with metabolic exchange
across capillary walls, leading to ischaemic episodes and thrombosis.
Carbon monoxide increases carboxyhaemoglobin concentrations in the
blood and lowers its oxygen-carrying capacity: increased oxygen debt
after exercise and impairment of endurance performance are evident in
smokers, compared to non-smokers. Carbon monoxide also has an affinity
for myoglobin and interferes with oxygen uptake by the myocardium.
"Bidi" smoke contains a higher concentration of carbon monoxide than
cigarette smoke (Hoffmann et al., 1974; Jayant & Pakhale, 1985; Ball &
Simpson, 1987). Most of the Asian smoking devices with their
non-porous wrappers and dark tobacco contain higher levels of nicotine
(Simarak et al., 1977; WHO, 1985). High nicotine and carbon monoxide
are also obtained from many dark tobacco cigarettes and from cigars.
"Sheesha" water pipe smoke contains small amounts of nicotine but is
rich in carbon monoxide; the blood carboxyhaemoglobin concentration in
sheesha smokers is higher than in cigarette smokers (Zahran et al.,
1985).
Cigarette smoking has been considered to be the primary cause of
Buerger's disease (thromboangiitis obliterans), an inflammatory
obliterative, non-atherosclerotic, vascular disease. The disease
usually becomes quiescent if the patient stops smoking cigarettes
(Olin, 1994). It was rare in women but an increasing number of cases
has been observed and ascribed to the increased use of tobacco by
young women (Yorukoglu et al., 1993).
Cardiovascular disease has been associated with exposure to other
factors, which can be classified as physical, chemical and biological,
and with occupation or life-style. The combination of smoking with any
of these factors will predispose to an increased detrimental
cardiovascular effect.
In women who smoke, peripheral vasoconstriction (PV) leading to acute
intervillous placental blood flow was measured during smoking and
attributed to nicotine, which simultaneously caused increases in heart
rate and blood pressure. It was suggested that PV explained fetal
growth retardation and other complications of pregnancy (Lehtovirta &
Forss, 1978).
2.2.5 Smoking and occupational accidents, injuries and
absenteeism
A survey of public employees found that cigarette smokers took 23%
more sick leave than non-smokers (Van Tuinen & Land, 1986). In another
study among 2537 postal workers in the USA, cigarette smokers had a
1.29 times higher accident rate (CI 1.07-1.55), and a 1.55 times
higher injury rate (CI 1.11-1.77) than non-smokers (Ryan et al.,
1992). Other studies confirm the higher rates of injury, occupational
accidents and absenteeism in smokers (Naus et al., 1966; Parka, 1983;
US Surgeon General, 1985; Hawker & Holtby, 1988). There are higher
costs of illness for cigarette smoking employees compared to
non-smokers (Van Peenen et al., 1986; Penner & Penner, 1990.
2.3 Health risks from smokeless tobacco use
2.3.1 Introduction
Oral cancers have been linked with oral tobacco use. The consequences
of chewing tobacco can be reactional keratosis, irreversible gingival
recession, periodontitis, oral dysplasia and leukoplakia, cancer and
cardiovascular effects (Chakrabarti et al., 1991; Guggenheimer, 1991).
In India, chewing material containing tobacco has been shown to be a
primary cause of oral cancer (Jussawalla & Deshpande, 1971).
2.3.2 Cancer
Leukoplakia and oral cancer are common results of oral tobacco use,
particularly in the countries of Asia (IARC, 1985a). Simarak et al.
(1977) reported a strong association between betel chewing and oral
cancer in northern Thailand. Sankaranarayanan et al. (1989a,b, 1990)
associated oral cancers in southern India with tobacco chewing.
Chakrabarti et al. (1991) reported much higher levels of pre-malignant
and malignant lesions of the oral cavity in tobacco chewers;
Nandakumar et al. (1990) found the relative risk to be elevated in
both sexes, but appreciably higher in females. From chemical analysis
of Indian tobacco products, it was concluded that their use may lead
to high exposures to carcinogenic tobacco nitrosamines (Nair et al.,
1989).
Case-control studies reported a higher incidence of oral cancer in
oral snuff users than in those not using any form of tobacco. There
was up to a 50-fold excess risk of cancer of the cheek and gum in
long-term oral snuff users (Axéll et al., 1978; US Surgeon General,
1986a; Winn, 1997). Idris et al. (1991, 1994), reported a high
incidence rate of oral cancer in men in northern Sudan who used an
oral snuff with relatively high concentrations of nicotine (0.8-3.2%)
and nornicotine. In a study of baseball players who chewed or (the
majority) used snuff orally, there was higher prevalence of
leukoplakia, plaque formation, gingivitis and dental disorders
compared to non-users (Robertson et al., 1997).
A case-control study on nasal cavity and paranasal sinus cancer and
snuff use showed that snuff users have a significantly increased risk
for adenocarcinoma and squamous cell carcinoma in the nasal cavity
(Brinton et al., 1984). N'-nitrosonornicotine is present and is an
organ-specific carcinogen that induces benign and malignant tumours of
the nasal cavity in rats, hamsters and mink (Rivenson et al., 1991;
Koppang et al., 1992, 1997; Hoffmann et al., 1994). Studies conducted
in South Africa showed that people using local snuff made from tobacco
and aloe plant ash as nasal applications have an increased risk for
tumours of the maxillary antrum (Keen et al., 1955). It was thought
that this snuff mixture contained high levels of nickel and chromium,
which may be associated with the induction of these tumours (Baumslag
et al., 1971).
Instillation of snuff into the lips of rats induced benign and
malignant tumours in the oral cavity (Hirsch & Thilander, 1981; Hecht
et al., 1986; Johansson et al., 1989; Larsson et al., 1989).
Instillation of snuff into the buccal pouch of hamsters, which were
repeatedly infected with Herpes simplex virus type I or II, led to
oral tumours, although no tumours occurred when either the virus or
tobacco was applied alone (Park et al., 1991b).
More than 3050 chemical compounds have been identified as tobacco
constituents (Roberts, 1988) and 50 are known carcinogens (Brunnemann
& Hoffmann, 1992). Nitrosamines, especially the tobacco-specific
nitrosamines (TSNA), must be regarded as major oral carcinogens. Oral
tumours were elicited when an aqueous solution of N-nitrosonornicotine
(NNN) and 4(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was
applied to the oral surfaces of rats (Hecht et al., 1993).
2.3.3 Cardiovascular disease
The effect of tobacco chewing on cardiovascular disease has received
less attention than the effect of cancer. Benowitz et al. (1988)
compared the cardiovascular effects of smokeless tobacco, cigarettes
and nicotine gum and reported that all tobacco use increased heart
rate and blood pressure. Nanda & Sharma (1988) recorded incremental
increases in heart rate and blood pressure following tobacco chewing.
However, Eliasson et al. (1991) found no significant elevation of
diastolic blood pressure in young snuff users. Bolinder et al. (1994)
found an excess risk of death from cardiovascular and cerebrovascular
disease among smokeless tobacco users. Tobacco chewing has detrimental
effects on pregnancy (Krishna, 1978) which, in the absence of any
anoxia due to carbon monoxide found in smokers, could result from
nicotine-induced vasoconstriction.
O'Dell et al. (1987) reported a case of Buerger's disease in a
38-year-old man that was associated with the use of smokeless tobacco.
A regimen which included complete abstinence from tobacco resulted in
a resolution of the symptoms. Buerger's disease is the commonest
vascular disease in the Indian subcontinent where tobacco consumption
is high (Jindal & Patel, 1992). In a study of the disease in
Bangladesh, 39 patients (38 males, 1 female) were investigated. All
but two were current long-term smokers, one male had given up smoking
6 months previously and the one woman with the disease (it is uncommon
in women) was a tobacco chewer (Grove & Stansby, 1992).
3. EFFECTS ON HEALTH OF TOBACCO USE AND EXPOSURE TO OTHER CHEMICALS
3.1 Introduction
3.1.1 Interaction
To discuss the interaction between tobacco and other agents as risk
factors for cancer and other adverse health effects, it is necessary
to define what is meant by the term "interaction". In general terms,
interaction represents a departure from additivity, in which the
combined effect of exposure to two agents is in some sense the sum of
the effects of the individual agents (US EPA, 1988). Synergism occurs
when the combined effect is greater than the sum of the component
effects; antagonism occurs when the effect of the combination is less
than would be suggested by summing the effects of the components.
3.1.2 Measuring interaction
To make these concepts precise, the scale in which risk is measured
and the manner in which risks are summed must be specified (Kaldor &
L'Abbe, 1990). In epidemiological investigations, the age-adjusted
relative risk is often used to characterize risk. In risk assessment
applications involving chronic health effects such as cancer, the
cumulative risk over a period of time may be of more direct interest.
In laboratory studies of carcinogenicity, for example, the lifetime
probability of tumour induction is often used to describe risk. In
order to take into account the age at which an adverse effect is
induced, the expected loss in life expectancy has also been used to
evaluate risk (UNSCEAR, 1988).
Kodell & Pounds (1991) discuss interaction in terms of departures from
response additivity and dose additivity. Dose additivity occurs
when the combined effect of two agents can be expressed in terms of a
total dose of the two agents, taking into account their relative
potencies. Dose additivity presumes that the two agents act by the
same biological mechanism, and that the effective dose of one agent is
simply a dilution of the dose of the other agent. Response additivity
occurs when the two agents act independently of each other. In this
case, the probabilities of an adverse effect due to each of the agents
can be treated as statistically independent and can be combined
accordingly.
The quantification of interaction may be illustrated using the
age-specific relative risk (A,B) associated with exposure to two
agents A and B. The relative risk is formally defined as:
relative risk (A,B) = I(A,B)/I(0,0)
where I(A,B) denotes the incidence rate of the disease of
interest at a specified age in the presence of exposure and
I(0,0) is the incidence rate in the absence of exposure. Lack of
interaction then corresponds to additivity of the excess relative
risk (ERR = relative risk - 1).
Specifically,
ERR(A,B) = ERR(A,0) + ERR(0,B)
where ERR(A,0) and ERR(0,B) denote the excess relative risks for
A and B alone, respectively. In terms of relative risk,
additivity is equivalent to:
relative risk (A,B) = relative risk (A,0) + relative risk
(0,B) - 1
For a supra-additive relative risk relationship, such as the
multiplicative relative risk model:
relative risk (A,B) = relative risk (A,0)RR(0,B)
and reflects a synergistic effect.
It is of interest to note that as the relative risk (A,0) and relative
risk (0,B) become small, the multiplicative relative risk model
approximates the additive relative risk model. Under this
multiplicative model, a strong synergistic relationship may be
apparent at high exposures, yet become negligible at low exposures.
This, along with the realization that relative risks of less than
about two are difficult to detect epidemiologically, suggests that
relatively high exposures are likely to be required to evaluate
interactive effects reliably.
Brown & Chu (1989) and Kodell et al. (1991) investigated the type of
interactions that might be expected under both the Armitage-Doll
multi-stage model and the Moolgavkar-Venzon-Knudson two-stage model of
carcinogenesis following exposure to two carcinogens that may affect
different stages in the model. These theoretical results indicate that
a variety of synergistic interactions are possible, including
supra-additive, multiplicative, and supra-multiplicative.
Although the additive excess relative risk model described above
provides a useful baseline for evaluating interaction, it is not the
only model that has been proposed for this purpose. Kaldor & L'Abbe
(1990) pointed out that the multiplicative relative risk model will
become the baseline model for evaluating interactions following a
logarithmic transformation of the relative risk. Thomas & Whittemore
(1988) review arguments in favour of the additive and multiplicative
models as the basis for evaluating interaction. Steenland & Thun
(1986) illustrate how these two models can be applied in evaluating
tobacco/occupation interactions in the causation of lung cancer.
This brief overview illustrates that interaction can be measured in
different ways, with the most appropriate depending on the nature of
the problem at hand. In general, however, all of the commonly
encountered measures of synergism indicate that the risk associated
with combined exposure to two agents is in some sense greater than
would be expected based on the risks of individual exposures. Although
no attempt is made throughout this monograph to systematically specify
the precise nature of the interactive effects reported in the
literature, most interactive effects documented in this monograph have
been identified through epidemiological investigations, in which the
additive age-specific relative risk model is the predominant approach
to describing interaction (Saracci, 1987; Greenland, 1993).
There are four principal ways in which tobacco smoke can interact with
other chemicals to impair the health of the smoker. They are not
mutually exclusive and in fact there are many situations in which they
may occur together, particularly in the workplace or the environs of
industry.
a) Modification of effects
Cigarette smoke can modify the harmful effects associated with other
toxic agents, in some cases causing a highly elevated risk, e.g., the
effects of smoking on diseases related to asbestos, alpha-radiation,
arsenic and some organic compounds.
b) Increased concentration effects
Chemical compounds hazardous to health are often found in both tobacco
smoke and the working environment and each source can augment the dose
obtained from the other, e.g., carbon monoxide, acrolein, benzene and
heavy metal elements.
c) Vector effects
Materials used in the workplace that produce harmful chemical agents
when they are burnt or vaporized can contaminate smoking materials and
cause the smoke to be far more injurious when the tobacco is smoked,
e.g., polytetrafluoroethylene and methylparathion.
d) Other interactions
Tobacco smoke can affect a physiological process and increase the
impairment of physical or physiological functions caused by another
activity. For instance, impaired lung clearance will affect the
residence time of inhaled toxic materials, the effect of smoking on
the peripheral vascular system can enhance the detrimental effects of
vibration and noise, and smoking may alter the effect of drugs taken
for other purposes.
3.1.3 Effects of tobacco smoking on lung deposition and clearance of
particles
Pulmonary deposition of inspired particles depends on their
physicochemical properties and on airway structure and geometry.
Mathematical models describing the deposition of particles in the
various airway sections show that in compromised airways, as is the
case in patients suffering from asthma and chronic obstructive lung
diseases, particle deposition is enhanced several-fold (ICRP, 1994).
Tobacco smoking can be an indirect cause of enhanced deposition of
inspirable particles. In addition, during tobacco smoking the
breathing pattern is changed to more frequent and deeper inhalation,
especially in the case of low-nicotine cigarettes, which can result in
an increased inhaled dose and dose rate of inspirable compounds (IARC,
1990).
Clearance of particles deposited in the lung is a complex
physiological process involving relatively rapid tracheobronchial
clearance, in which mucus is moved upward by ciliary action to the
pharynx and swallowed, and slower deep-lung clearance, in which
phagocytic cells remove inhaled particles. These processes are
balanced by the solubility of the inhaled particles, with relatively
insoluble particles having a longer residence time in the alveolar
portions of the lungs. A longer residence time in the lung would be
accompanied by a greater possibility that harmful effects could occur.
Both rapid and slow clearance phases are reduced by smoking, although
probably by different mechanisms. Cigarette smoke contains significant
concentrations of ciliatoxic agents, such as hydrogen cyanide,
formaldehyde, acetaldehyde, acrolein and nitrogen oxides, which
greatly contribute to retarded clearance of inhaled particles by
inhibiting lung clearance mechanisms (Battista, 1976). Retardation of
clearance has been seen for airway-deposited particles, in which
decreased mucus transport velocities slow this normally relatively
rapid phase of clearance (Lourenco et al., 1971; Chopra et al., 1979).
The deep-lung clearance of relatively insoluble particles is retarded
in smokers. Cohen et al. (1979) found that 1 year after a tracer
particle exposure, some 50% and 10% of the original lung burden
remained in the lungs of smokers and non-smokers, respectively.
Bohning et al. (1982) and Philipson et al. (1996) found that smoking
retarded long-term particle clearance from the lungs. The mechanism(s)
for interference with this longer-term phase of clearance has not been
shown definitively, but may be related to impairment of phagocyte
function and/or smoke-induced lung damage.
3.2 Interactions between tobacco smoke and other agents
3.2.1 Asbestos
Asbestos is a generic name for a group of fibrous silicates, differing
in colour, fibre arrangement and length. Recognition of the health
risks of asbestos has led to major reductions in production and uses.
Asbestos types are classified according to their physical
characteristics as serpentine or amphibole and differ in their
relative carcinogenic potential. Amosite and crocidolite are
amphiboles and have short and straight needle-like fibres. Chrysotile
is a serpentine and consists of long, pliable white fibres. The longer
fibre varieties of asbestos can be spun into yarn which can be woven
into fabric; short fibre varieties can be incorporated into cement,
asbestos board and tiles. Asbestos products have been used in a
variety of applications including electrical and thermal insulation in
buildings, fire and safety equipment, brake linings of motor vehicles,
and shipbuilding. Workers in asbestos mining and processing and a wide
range of manufacturing industries are exposed to various forms of
asbestos, while others are exposed in maintenance work, demolition and
recycling operations.
Occupational exposure to asbestos is associated with asbestosis and
cancers at various sites, notably pleural mesothelioma and lung
cancer. Differences between the effect of asbestos on the health of
smokers and non-smokers have been reported, and studies have been
conducted aimed specifically at elucidating the combined effects of
smoking and asbestos exposure. Perioccupational exposure to asbestos
is a hazard to household contacts of asbestos workers, who bring home
dust on their clothes, and to people living in areas where there is
environmental contamination by asbestos dust from industry (Anderson
et al., 1979).
The amphibole varieties of asbestos (crocidolite and amosite) have the
highest carcinogenic risk. Crocidolite presents a greater risk than
amosite, which in turn is more dangerous than chrysotile, a serpentine
variety. Erionite and tremolite are non-asbestos fibrous minerals used
in building in some parts of the world and there is a high prevalence
of mesothelioma in these regions (Baris et al., 1979; Yazicioglu et
al., 1980).
Because there are many different occupations and environmental
situations in which asbestos exposure might occur, along with a wide
range of possible levels of exposure and variety of types of asbestos
in use, it is difficult to define clearly asbestos exposure or the
smoking habits of those exposed. The smoking history of the population
sampled is important, because there have been changes in smoking
materials and prevalences of smoking in many countries (Cheng & Kong,
1992). In many studies, only the number of smokers within sub-groups
of workers with asbestos-related disease have been reported, rather
than the detailed smoking habits of the exposed population. A widely
used assumption is that the smoking habits of asbestos-exposed workers
reflect those of blue collar workers and are thus higher than national
average figures. Table 5 gives examples of smoking prevalence in
different groups of asbestos-exposed workers.
3.2.1.1 Asbestos and lung cancer
Exposure to asbestos dust carries a risk of parenchymal and pleural
fibrosis, mesothelioma and lung cancer. Selikoff et al. (1968) and
Berry et al. (1972) showed that cigarette smoking was an added hazard
among asbestos workers. In combination, the two hazards are associated
with very high lung cancer rates. Studies were carried out (e.g.,
Hammond & Selikoff, 1973; Martischnig et al., 1977; Hammond et al.,
1979; Selikoff et al., 1980; Acheson et al., 1984; Berry et al., 1985)
to determine whether cigarette smoke and asbestos act independently,
Table 5. Smoking prevalence in asbestos-exposed workers
Exposure Smoking habits References
Asbestos textile workers 75% smokers Weiss (1971)
46% cigarette smokers
36% ex-cigarette smokers
5.5% pipe/cigar smokers
Electrochemical plant 84% to 87% were smokers
(two areas) or ex-smokers Kobusch et al. (1984)
Population in Telemark, Asbestos exposed: Hilt (1986)
Norway 44.6% smokers
36.0% ex-smokers
Not exposed:
40.95% smokers
28.6% ex-smokers
Survey of 800 000 American Asbestos exposed: Stellman et al. (1988)
men and women in 1982 33.6% smokers
47.3% ex-smokers
Lung cancer case-referent Men: 95% smokers Järvholm (1993)
study; Swedish industrial city Women: 78% smokers
Shipyard workers in 46% smokers Sanden et al. (1992)
Gothenburg, Sweden 31% ex-smokers
December 1987 21% non-smokers
2% not known
Asbestos factory workers Men: 74% smokers Newhouse & Berry (1979)
(male population average 66%)
Women: 49% smokers
(female population average 40%)
their combined effect being the sum of the individual effects, or
there is an interaction with the ultimate effect being a product of
the two risk factors. In some studies, the effects of smoke and
asbestos appeared to be additive, in others multiplicative and in
others somewhere between the two. Reasons for the lack of consistency
among the studies may relate to the size of the population sampled,
its average age, social class and residential area, the type of
asbestos involved, the time scale covered and the intensity of
exposure to asbestos. The weight of evidence favours a synergistic or
multiplicative model for the interaction of asbestos and smoking.
While the differences may be partly linked to the carcinogenic
potential of different types of asbestos and to different smoking
materials and ways of smoking, including passive smoking (Cheng &
Kong, 1992), they also reflect the complex nature of tobacco smoke,
which contains complete carcinogens, tumour promoters and
co-carcinogens and other compounds that can influence the multistage
carcinogenic process. However, whatever the type of smoking/asbestos
interaction influencing the incidence of lung cancer, there is a
greatly increased risk for the asbestos-exposed worker who smokes
(Table 6).
Hammond et al. (1979) found a very strong synergistic effect and this
was supported by studies of shipyard workers in Italy (Bovenzi et al.,
1993), asbestos factory workers in London (Newhouse & Berry, 1979),
Finnish anthophyllite miners and millers (Meurman et al., 1979),
chrysotile workers in China (Cheng & Kong, 1992; Zhu & Wang, 1993) and
workers exposed to crocidolite in Western Australia (de Klerk et al.,
1991). Cheng & Kong (1992) reported a lower ratio of non-smoking to
smoking lung cancer death rates and suggested that this reflected
passive smoking among non-smokers and the use by most smokers of
hand-rolled cigarettes. Liddell et al. (1984) found that their data
fitted both an additive model and a multiplicative model and concluded
that the combined relative risk lay somewhere between the two.
Selikoff et al. (1980), from a study of amosite factory workers, and
Berry et al. (1985), from a study of asbestos factory workers,
favoured an additive model. However, caution is required because of
the definitions of additive and multiplicative used by different
authors and the overlap between these terms and such words as
synergism and promoter.
Molecular biology studies of autopsy specimens of lung tumour tissue
from of cigarette smokers have revealed that cigarette smoking induces
K-ras mutation (Rodenhuis & Slebos, 1992). It has been suggested that
such cigarette-smoke-induced K-ras oncogene mutations are promoted by
the presence of asbestos, which creates selective growth conditions
for the mutated cells (Vainio et al., 1993). Vainio & Boffetta (1994)
concluded that both tobacco smoke and asbestos fibres can be genotoxic
and cytotoxic, and cause proliferative lesions in the lungs. Tobacco
smoke contains carcinogens that bind to critical genes and cause
mutations. Asbestos fibres cause chronic inflammation of the lungs,
which releases various cytokines and growth factors, and may provide a
selective growth advantage for mutated cells.
Table 6. Age-standardized lung cancer death ratesa for cigarette smoking and/or
occupational exposure to asbestos dust compared with no smoking and no occupational
exposure to asbestos dust (from: Hammond et al., 1979)
Group Exposure to History cigarette Death Mortality Mortality
asbestos? smoking? rate difference ratio
Control No No 1.3 0.0 1.00
Asbestos workers Yes No 58.4 +47.1 5.17
Control No Yes 122.6 +111.3 10.85
Asbestos workers Yes Yes 601.6 +590.3 53.24
a Rate per 100 000 man-years standardized for age on the distribution of the
man-years of all the asbestos workers; number of lung cancer deaths based on
death certificate information
3.2.1.2 Asbestos and pleural mesothelioma
There is an established relationship between exposure to asbestos
- crocidolite, amosite, chrysotile - and pleural mesothelioma
(Stellman, 1988). In shipyard workers mainly exposed to chrysotile,
Sanden et al. (1992) found an increase in pleural mesotheliomas up to
15 years after cessation of exposure. Asbestos is also linked with
peritoneal mesothelioma (Newhouse & Berry, 1976). The risk of lung
cancer was found to fall after exposure ceased, suggesting that
asbestos acted as a lung cancer promoter, but the risk of mesothelioma
long after cessation of exposure indicated that asbestos acted as a
complete carcinogen. Mesothelioma can have an extremely long latent
period, with cases presenting even 30 years or more after first
exposure (Newhouse & Berry, 1976). Up to 90% of cases of pleural
mesothelioma have been attributed to asbestos but there is no evidence
directly associating smoking with the disease, or showing that smoking
has any influence on the incidence of asbestos-related pleural
mesothelioma (Berry et al., 1985; Hughes & Weill, 1991; Sanden &
Jarvholm, 1991; Muscat & Wynder, 1991).
3.2.1.3 Asbestos and other forms of cancer
Asbestos fibres have been found in many tissues, other than the lungs,
of asbestos workers. There is evidence that an asbestos/smoking
interaction increases the incidence of cancer of the oesophagus,
pharynx, buccal cavity and larynx but not of pleural or peritoneal
mesothelioma, or of cancer of the stomach, colon-rectum or kidney, for
which smoking and non-smoking asbestos workers are at equal risk
(Hammond et al., 1979; Selikoff & Frank, 1983; US ATSDR, 1995).
3.2.1.4 Asbestosis
Asbestosis is a fibrotic reaction to asbestos in the lungs. In a
review of histological, animal experimental and radiological evidence,
Weiss (1984) concluded that cigarette smoking could result in diffuse
fibrosis similar to that caused by asbestos, and the fibrosis showed a
dose-response to the duration and degree of smoking. Prevalence
studies are consistent in showing a higher frequency of diffuse small
irregular opacities in asbestos workers who are smokers than in those
who are non-smokers. It has been suggested that the effects may be
additive. Tobacco smoke affects lung clearance and hence the retention
of asbestos fibres in the lungs. In asbestosis the intensity of
fibrosis correlates with the number of asbestos bodies in the lungs,
and Murai et al. (1994) concluded that reduction of lung clearance by
tobacco smoke could increase the intensity of fibrosis. Crocidolite
fibres are the most fibrogenic of the various types of asbestos but
De Klerk et al. (1991) concluded that smoking had no measurable effect
on crocidolite asbestosis.
An interaction between asbestos and smoking causing a greater
frequency of obstructive airways disease in asbestos workers who smoke
was found in a study of pulmonary function changes caused by
asbestosis (Selikoff & Frank, 1983). Miller (1993) presented similar
results suggesting an interaction between asbestos and smoking. In a
prospective mortality study, Hughes & Weill (1991) concluded that
asbestosis is a precursor of asbestos-related lung cancer, but they
were unable to assess an interaction between tobacco smoking and
asbestosis because all the cases were in smokers and there were no
non-smokers.
In rats, asbestos fibres stimulate alveolar macrophages to generate
the inflammatory and fibrogenic mediators, tumour necrosis
factor-alpha (TNF-alpha), and this may be the cause of inflammation
and lung fibrosis due to asbestos (Ljungman et al., 1994). In in vitro
studies Morimoto et al. (1993) found synergism between chrysotile
fibres and cigarette smoke in the stimulation of the formation of
TNF-alpha by rat alveolar macrophages.
3.3 Non-asbestos fibres
3.3.1 Glass fibre
IARC (1988) classified glasswool as possibly carcinogenic to humans
(Group 2B) and glass filaments as not classifiable as to their
carcinogenicity to humans (Group 3), based on sufficient evidence for
the carcinogenicity of glasswool and inadequate evidence for the
carcinogenicity of glass filaments in experimental animals and
inadequate evidence for the carcinogenicity of glasswool and glass
filaments in humans. There are data on exposure to glass fibre and
tobacco smoke. Enterline et al. (1987a) carried out a case control
study of 7586 glasswool workers in four plants producing small
diameter fibres, less than 3 µm in diameter. Smoking histories were
obtained for 75% of the workers. Analysis of data by logistic
regression showed that smoking was a powerful variable and multiplied
the effect of fibre exposure. In a case-control study of the influence
of non-workplace factors on respiratory disease in employees of a
glass fibre manufacturing facility, Chiazze et al. (1992, 1995)
concluded that smoking, and not exposure to glass fibre, was the most
important risk factor for the increased lung cancer risk but was not
as important for non-malignant respiratory disease. In a further
analysis, using data not previously available, Chiazze et al. (1995)
estimated the extent of confounding by cigarette smoking, and
suggested that adjusting for the confounding effect could reduce the
lung cancer standardized mortality ratio to a non-statistically
significant level.
3.3.2 Rockwool, slagwool and ceramic fibres
IARC (1988) concluded that there was limited evidence for the
carcinogenicity of rockwool and inadequate evidence for the
carcinogenicity of slagwool in experimental animals, with limited
evidence for the carcinogenicity of rock-/slagwool in humans: the
overall evaluation for both was Group 2B, possibly carcinogenic to
humans. For ceramic fibres there was sufficient evidence for their
carcinogenicity in experimental animals, with no data on their
carcinogenicity in humans: the overall evaluation for ceramic fibres
was also Group 2B, possibly carcinogenic to humans.
In a study of insulation workers using rock and glass wool, (Clausen
et al., 1993) concluded that exposure was associated with an increased
risk of developing obstructive lung disease. In a study of respiratory
health in 628 workers in seven European plants manufacturing ceramic
fibres, skin, eye and nasal irritation, breathlessness and wheezing
were common findings (Trethowan et al., 1995). Respiratory symptoms
were more frequent in smokers and increased with the amount smoked.
The authors concluded that exposure caused irritation, similar to that
caused by other man-made fibres, and that cumulative exposure could
cause airways obstruction by promoting the effects of cigarette smoke.
Ljungman et al. (1994) demonstrated in rats that rock wool, slag wool,
kaolin ceramic fibre and silicon carbide fibre stimulated alveolar
macrophages to generate tumour necrosis factor-alpha (TNF-alpha), a
potent inflammatory and fibrogenic mediator. In in vitro studies
Morimoto et al. (1993) found synergism between mineral fibres
(chrysotile and alumina silicate ceramic fibres) and cigarette smoke
in the stimulation of the formation of TNF-alpha by rat alveolar
macrophages. Leanderson & Tagesson (1989) found that cigarette smoke
potentiated the DNA-damaging effect of man-made mineral fibres
(rockwool, glasswool and ceramic fibres).
3.4 Inorganic chemicals
3.4.1 Arsenic
Compounds of arsenic have been used as pesticides and as preservatives
of wood and leather. Arsenic is present in many metal ores and is
released during smelting Radon progeny are frequently encountered as a
contaminant of arsenic. In some parts of the world arsenic is found in
drinking-water in relatively high concentrations.
Arsenic and its compounds are carcinogenic (WHO, 1980; IARC, 1987;
Tsuda et al., 1990, 1995). Skin cancer can occur after ingestion of
arsenic (Tseng et al., 1968; Smith et al., 1992), and lung cancer
after inhalation of arsenic by smelter workers or by people living
nearby (Welch et al., 1982; Pershagen, 1985; Pershagen et al., 1987)
or by agricultural workers exposed to the pesticide lead arsenate
(Wicklund et al., 1988). IARC (1987) classed arsenic and arsenic
compounds as Group 1, carcinogenic to humans. It has been suggested
that arsenic in drinking-water may also cause liver, lung, kidney and
bladder cancer (Smith et al., 1992).
A study of the lung cancer risk among cadmium-exposed workers
suggested that exposure to arsenic and tobacco smoke may have been the
cause of an increased rate of lung cancer, rather than exposure to
cadmium particulates (Lamm et al., 1992). Tsuda et al. (1990)
suggested an interaction between arsenic and smoking in exposed
workers in a small Japanese village where arsenic was mined and
refined. However, the village water and air were highly polluted by
emissions from the smelter and from slag disposal, making interaction
between arsenic and smoking difficult to assess. A study of copper
smelter workers in the USA indicated that the effect of arsenic was
probably more important in lung cancer than that of tobacco smoke
(Welch et al., 1982). Studies in Sweden showed increased lung cancer
risks from arsenic exposure at a copper smelter; a multiplicative
effect for smoking and arsenic was found and age-standardized rate
ratios for lung cancer mortality were 3.0 for arsenic-exposed workers,
4.9 for smokers with no arsenic exposure and 14.6 for arsenic-exposed
smokers (Pershagen et al., 1981). In a later study, Pershagen (1985)
reported an additive effect for smoking and arsenic exposure on lung
cancer incidence in situations where the arsenic exposure was lower.
In a cohort of 3916 Swedish copper smelter workers, the risk of
developing lung cancer from the interaction between arsenic and
smoking was intermediate between additive and multiplicative and
appeared to be less pronounced among heavy smokers (Jarup & Pershagen,
1991).
There was no evidence of synergism between arsenic and tobacco smoke
in tin miners in Yunan Province, China. The lung cancer risk was
greater for arsenic than for smoking, and simultaneous assessment of
arsenic and radon exposure revealed radon to be the greater risk
(Taylor et al., 1989). In Ontario it was concluded that the excess
lung cancer mortality of gold miners and uranium miners was probably
due to exposure to arsenic and short-lived radon decay products
(Kusiak et al., 1991). This was consistent with the hypothesis that
the risk of lung cancer from exposure to arsenic is enhanced by
exposure to other carcinogens (Kusiak et al., 1993).
Hertz-Picciotto et al. (1992) assembled data from several studies to
examine possible synergism between smoking and exposure to arsenic and
an increased risk of lung cancer. The joint effect from both exposures
consistently exceeded the sum of the separate effects: a minimum of
30% to 54% of lung cancer cases among those with both exposures could
not be attributed to either one or the other exposure alone. The
conclusion was that arsenic and smoking acted synergistically to cause
lung cancer. Arsenic-induced lung cancer was not limited to exposure
to inhaled arsenic because there was evidence of synergism between
ingested arsenic and smoking (Tsuda et al., 1995). An association of
arsenic exposures with bladder cancer was confined to subjects who had
been smokers Bates et al. (1995).
In a Swedish study of lung cancer in arsenic workers, it was found
that cases among smelter workers who had never smoked showed a
histological distribution resembling that of smokers, probably
reflecting an exposure to carcinogenic agents at the smelter which
influence the risk of different histological types in the same way as
smoking (Pershagen et al., 1987). Tobacco smoking primarily induces
epidermoid and small cell carcinomas but there are also increased
risks for other cell types. The proportion of small cell carcinomas
was greater in uranium miners than in the general population (Kusiak
et al., 1993). In smokers, there were no pronounced differences in the
histological type of lung carcinomas between arsenic exposed smelter
workers and controls (Pershagen et al., 1987).
It has been suggested that the potentiation of the carcinogenic
properties of arsenic by smoking could be due to inorganic arsenic
requiring a strong co-carcinogen to manifest a carcinogenic effect, or
that arsenic itself might be acting as co-carcinogen rather than as a
direct carcinogen (Stohrer, 1991; Tsuda et al., 1995).
3.4.2 Beryllium
Beryllium is a metal with a number of uses including alloys, nuclear
energy applications, and in the rocket and aerospace industry (IPCS,
1990; IARC, 1993). Fine dusts and fumes of the metal and some of its
salts are hazardous and when inhaled are deposited in the lungs from
where beryllium may be widely distributed throughout the body.
Beryllium metal, oxide and some salts give rise to acute inflammation
on skin contact, particularly when accompanied by friction or
perspiration. Short exposure to dusts and fumes can cause acute
inflammation of mucous membranes: conjunctivitis, bronchitis,
pneumonitis. Granulomatous reaction can follow chronic inflammation of
the skin, and lesions may appear in the liver and elsewhere after long
periods of absorption from the lungs. Beryllium and its compounds are
a cause of delayed pneumonitis and pulmonary granulomas. IARC (1993)
classified beryllium and beryllium compounds as Group 1, carcinogenic
to humans, on the basis of sufficient evidence in humans and in
experimental animals. However, in epidemiological studies the
information on smoking was incomplete and the data did not rule out
the possibility that the few excess deaths observed could have been
due to smoking rather than to any other cause (Steenland & Ward, 1991,
1993; Eisenbud, 1993; MacMahon, 1994; Kotin, 1994).
The prevalence of chronic beryllium disease (CBD) is reduced in
smokers compared with non-smokers. The disease is preceded by the
development of beryllium-specific sensitization. In two studies
examining different worker populations, the prevalence of smoking in
those with clinically diagnosed CBD was lower than in those that were
sensitized but did not have the disease (Kreiss et al., 1993; Kreiss
et al., 1996).
3.4.3 Chromium
Chromium and its compounds are used in metallurgical, chemical,
electroplating and leather tanning industries (IARC, 1990). The
principal route of entry to the body is through the lungs. Chromium
ulcers of the skin and dermatitis can result from handling chromium
products and deposition of chromates on mucous membranes can also
cause ulceration which, in the nasal septum, can lead to perforation
(Lindberg & Hedenstierna, 1983; IARC, 1990). Chromium and some
chromium compounds are respiratory tract sensitizers and a cause
asthma. Hexavalent chromium salts have been associated with lung
cancer both in experimental animals and in epidemiological studies.
IARC (1990) concluded that there is sufficient evidence in humans for
the carcinogenicity of hexavalent chromium compounds as encountered in
chromate production, chromate pigment production and chromium plating
industries.
Langård & Norseth (1975) suggested that cigarette smoking increases
the risk for lung cancer in workers exposed to chromate dust. Other
studies (Abe et al., 1982; Langård & Vigander, 1983; Yoshizawa, 1984;
Nishiyama et al., 1985) have suggested that workers with exposure to
chromium compounds who are also smokers may be at greater risk than
non-smokers. However, the numbers were too small for conclusions on
interactions to be drawn.
3.4.4 Nickel
Exposure to nickel or its compounds occurs in mining, refining,
smelting and alloying the metal, in nickel plating and in welding. It
is used in battery manufacture, electroplating, enamelling, ceramics,
the chemical and petroleum industries and in dyestuffs and ink making.
Exposure may be by skin contact or inhalation of dusts, fumes, mists
or gaseous nickel carbonyl (IPCS, 1991a). In occupational exposure the
daily intake and absorption/retention vary widely between industries
(IARC, 1990).
Nickel is absorbed from the soil by the tobacco plant. During smoking,
up to 20% of the nickel in the tobacco is transferred to mainstream
smoke. This high transfer rate, compared to the much lower transfer
rates of other metals, has been explained by the formation of the
volatile nickel carbonyl (Sunderman & Sunderman, 1961). Nickel
carbonyl is a strong lung carcinogen in rats (IARC, 1990).
IARC (1990) evaluated the carcinogenicity of nickel and nickel
compounds and classified nickel compounds as carcinogenic to humans
(Group 1) and nickel as possibly carcinogenic to humans (Group 2B). In
an epidemiological study on a cohort of 916 workers in a Norwegian
nickel refinery, four work categories were defined: i) roasting and
smelting; ii) electrolysis; iii) other processes; and iv) other work
groups. All groups showed an excess risk of respiratory cancer. In the
roasting and smelting department there were excess risks for lung
cancer (O/E = 12/2.5) and nasal cavity cancer (O/E = 5/0.1). In the
electrolysis department there were also excess risks of lung cancer
(O/E = 26/3.6) and nasal cavity cancer (0/E = 6/0.2) (Pedersen et al.,
1973). Magnus et al. (1982) updated this study and found evidence of
an additive effect of smoking and nickel exposure in the induction of
respiratory cancer. Histological examination of nasal biopsy specimens
from 59 retired nickel workers, 21 of whom were smokers and snuff
users, showed a higher score of nasal epithelial dysplasia in smokers
than in non-smokers, and 4 workers with nasal carcinoma were smokers
(Boysen et al., 1984). In monitoring nickel exposure by imaging
cytometry of nasal smears (Reith et al., 1994), it was possible to
distinguish between workers who were exposed to different nickel
compounds and to distinguish between smoking and non-smoking nickel
workers.
3.4.5 Manganese
There is occupational exposure to manganese in its mining, the
ferromanganese and iron and steel industries, the production of dry
cell batteries, and the manufacture and use of welding rods. Manganese
is released by the combustion of the gasoline additive,
methylcyclopentadienyl manganese tricarbonyl (MMT), used in some
countries. However, the amount in gasoline (approximately 10 mg/litre)
and emitted in vehicle exhausts is small and does not to lead to human
exposure (Health Canada, 1994). The principal route of entry of
manganese is through the lungs but, because most of the compounds are
insoluble, only the smallest particles, as contained in furnace and
welding fume, are capable of reaching the alveoli and being
phagocytosed and absorbed (IARC, 1986).
Manganese is present in tobacco leaves and it has been reported (IARC,
1986) that 0.003 µg of manganese appears in the mainstream smoke from
one cigarette and thus contributes to a smoker's manganese intake in
the form of small and dangerous particles.
Manganese is neurotoxic and long-term occupational exposure can cause
a condition resembling Parkinson's disease. It causes lung damage
leading to an increased incidence of pneumonia and a higher rate of
acute and chronic bronchitis. In studies of manganese alloy production
workers and chronic lung disease, smokers were more affected than
non-smokers and the relationship between the number of cigarettes
smoked and the prevalence of respiratory tract symptoms suggested that
smoking acted synergistically with manganese (Saris & Lucic-Palaic,
1977). In a study of workers producing manganese salts and oxide
(Roels et al., 1985) smoking and manganese exposure were additive in
producing preclinical toxic effects. In studies on workers producing
iron-manganese alloys, one concerned with chronic bronchitis
(Misiewicz et al., 1994) and the other with pulmonary ventilatory
disturbance (Misiewicz et al., 1992), there was no relationship
between the occupational exposure or its duration and health effects.
The chronic bronchitis and ventilatory disturbance were attributed to
cigarette smoking.
3.4.6 Platinum
Platinum is used as a catalyst in many chemical processes and in motor
vehicle exhausts. Chloroplatinic acid is an intermediate in the
preparation of a large number of complex salts, which are used in
platinum refining, the chemical industry and, therapeutically, in
cancer chemotherapy.
Platinum salt sensitivity is an IgE-mediated immune response (IPCS,
1991b). Ammonium hexachloroplatinate is used as an intermediate in
platinum refining, and its inhalation provokes asthmatic responses and
elicits immediate skin test responses in sensitized individuals (Pepys
et al., 1972). In a cohort study of 91 workers in a platinum refinery
(Venables et al., 1989), 22 developed respiratory symptoms and an
immediate skin test response to ammonium hexachloroplatinate. The risk
was greatest in the first year of employment and smokers had an
increased risk of becoming sensitized. In another study, out of 78
workers at a platinum refinery, 32 (41%) had developed platinum salts
sensitivity after 24 months of exposure and the risk of sensitization
was about 8 times greater for smokers (Calverley et al., 1995).
Baker et al. (1990) conducted a cross-sectional study of respiratory
and dermatological effects of platinum salt sensitization in 136
workers (107 current employees and 29 former employees) at a precious
metal refinery. Twenty three workers (22%) had become sensitized.
Platinum salts sensitivity was not associated with atopicity but was
strongly associated with cigarette smoking status.
3.4.7 Silica
Silicosis (lung fibrosis caused by silica) is not only a hazard of
mining. It is also found in bricklayers, cement makers, workers in
pottery, porcelain and ceramics, rock drillers, workers chipping,
grinding or polishing stone, in sandblasting, using grinding stones to
smooth or polish precious stones, metals or optical glass, and in the
manufacture of polishing materials such as metal polishes and
toothpaste. The number of industries generating silica dust is large.
The amount of respirable dust varies from one to another and, because
silica is an active adsorbent, it can become contaminated and have its
toxic potential changed. Furthermore, freshly fractured silica dust
may exhibit a different surface reactivity and cytotoxicity from that
of aged silica (Vallyathan et al., 1988).
Hnizdo & Sluis-Cremer (1991), in a study of gold miners, linked high
exposure to silica dust with lung cancer and found a combined effect
of dust and smoking that fitted a multiplicative model for lung
cancer. Amandus et al. (1992) studied lung cancer in men with
diagnosed silicosis and suggested that there was an association
between the two diseases. In a study of iron foundry workers
(Andjelkovich et al., 1994), cigarette smoking was a strong predictor
of lung cancer whereas silica exposure showed no association with the
disease.
Chronic silicosis develops after 20 to 40 years of exposure to silica
dust. There are also other types of pneumoconiosis related to the
nature of the dust, and chronic bronchitis and airways obstruction
have been associated with silica dust exposure. A hypothesis for the
pathogenesis of chronic silicosis is that silica particles are
phagocytosed by the alveolar macrophages for which they have a marked
selective toxicity. Permanent macrophage activation initiates
inflammatory reactions leading to the formation of collagenous fibres.
Acute silicosis arises from the inhalation of more highly reactive
silica (Vallyathan et al., 1988). The link between silicosis and
smoking was examined in a study of smoking and silica exposure on
pulmonary epithelial permeability. Faster clearance of a radioaerosol
from the upper lung regions was found for smokers (Nery et al., 1988,
1993). The question of silica clearance was considered in an analysis
of an association between silicosis and smoking: differences in
collagenization for smokers and non-smokers were attributed to
differences in the interception of silica particulate matter by mucus
(Hessel et al., 1991). In studies by Ng et al. (1987, 1992), smoking
was not considered to affect the progression of silicosis in granite
quarry workers. However, examining the association of silicosis with
lung cancer, Ng et al. (1990) concluded that the excess lung cancer
risk in silicosis is attributable to smoking, and there appeared to be
a synergistic effect between smoking and silica/silicosis regarding
the risk of developing lung cancer.
In a study of 562 South African gold miners exposed to low levels of
dust with a high (50-70%) silica content, the incidence of chronic
bronchitis was higher than in non-dust-exposed controls. Although the
percentage of smokers was higher in those with chronic bronchitis in
both groups, there was a significant excess in the dust-exposed
smokers (Sluis-Cremer et al., 1967). The authors concluded that there
was a factor in dust-exposed smokers that increased the incidence of
chronic bronchitis above that expected from smoking alone. In a study
of 2209 South African gold miners and 483 non-miners on the effect of
silica dust and tobacco smoking on mortality from chronic obstructive
lung disease, it was found that miners who smoked and were exposed to
silica dust were at higher risk of dying from chronic obstructive lung
disease than smokers not exposed to silica dust. In South African gold
mines about 30% of the respirable dust is free silica. It was
concluded that tobacco smoking and silica dust acted synergistically
in causing chronic obstructive lung disease (Hnizdo, 1990). Hnizdo et
al. (1990) applied additive and multiplicative relative risk models to
the same sample and found that departure from additivity increased
progressively with the severity of obstructive impairment. They
concluded that tobacco smoking potentiated the effect of dust in
causing respiratory impairment and that severe respiratory impairment
could have been prevented through elimination of tobacco smoking
(Hnizdo et al., 1990). Oxman et al., (1993) analysed the relationship
between occupational dust exposure and chronic obstructive lung
disease in both gold and coal miners and found a significant
association between loss of lung function and cumulative respirable
dust exposure, which was greater in gold miners. In this study there
was no evidence of interaction with tobacco smoking for gold miners,
and the authors suggested that the increased risk of lung function
loss was due to exposure to dust having a higher silica content than
coal dust. Among iron ore miners in Sweden, Jörgensen et al. (1988)
found a strong relationship between chronic bronchitis and smoking,
but not with working underground. The two risk factors, silica dust
and smoking, appeared to be additive but the smoking effect was far
greater than that of silica dust. In a study of small airway disease
in patients with silica dust exposure, with and without radiographic
evidence of silicosis, and smoking, Avolio et al. (1986) found no
differences in lung function and prevalence of small airways disease
with silicosis. However, in both groups small airway disease was
significantly related to tobacco smoking, indicating that this had a
more powerful effect than silicosis.
3.5 Organic chemical agents
Many organic compounds with properties covering a wide spectrum of
molecular structure and biological activity are encountered in a
variety of industries. The effects of a few compounds, some of which
are encountered in specific industries, and smoking have been studied.
Where organic compounds occur in both tobacco smoke and the workplace,
the effect of smoking becomes one of dose augmentation, although
modification of effect can also occur. Some organic compounds would
normally not be found in tobacco smoke but are present because
workplace materials have contaminated smoking materials and they are
then pyrolysed or volatilized during smoking.
3.5.1 Chloromethyl ethers
Chloromethyl methyl ether (CMME) and its contaminant bis(chloromethyl)
ether (BCME) are used in the synthetic chemical industry, in the
manufacture of ion exchange resins and in polymer production. They are
carcinogenic when inhaled, BCME more so than CMME. In a long-term
study of chemical workers, 93 had exposure to chloromethyl ethers and
22 died from lung cancer. Of 32 workers who had no exposure, 3 died
from lung cancer (Weiss & Boucot, 1975; Weiss, 1976, 1980, 1982). For
the 22 cases in of lung cancer in the exposed workers, a dose-response
relationship was established. In the groups with heavy occupational
exposure, there were fewer heavy smokers (>20 cigarettes per day)
than in the groups with lower occupational exposure. This
statistically significant shift might be explained by self-selection
of heavy smokers out of the high occupational exposure groups because
of the bronchial irritation that is caused by the exposure to
chloromethyl ethers, or cigarette smoking might have an antagonistic
activity (Steenland & Thun, 1986; Thomas & Whittemore, 1988; Weiss &
Nash, 1997).
3.5.2 Tetrachlorophthalic anhydride
Tetrachlorophthalic anhydride (TCPA) is used as an epoxy resin curing
agent. It is respiratory tract sensitizer and causes asthma (Schlueter
et al., 1978). In a study using a radio allergosorbent test with a
TCPA human serum albumin conjugate, specific IgE antibody was detected
in serum from 24 out of 300 factory floor workers exposed to TCPA. Of
these 24, 20 (83%) were current smokers, compared with 133 (48%) of
276 without antibody (p<0.01), and there was a weaker association
with atopy, defined by skin tests with common allergens. Smoking and
atopy interacted, the prevalence of antibody being 16% in atopic
smokers, 12% in non-atopic smokers, 8% in atopic non-smokers and none
in the non-atopic non-smokers. It was concluded that smoking may
predispose to, and interact with atopy in the production of specific
IgE antibody to this hapten protein conjugate.
3.5.3 Dyestuffs
There is an established relationship between bladder cancer and
exposure to certain aromatic amines encountered in the dyestuffs
industry, e.g., benzidine, 4-aminobiphenyl and 2-naphthylamine (IARC,
1987), and smoking is causally associated with bladder cancer (IARC,
1986; US Surgeon General, 1989). From an analysis of 991 cases by
Cartwright (1982), a significant risk of bladder cancer was associated
with cigarette smoking, and a dose-response relationship, based on
years of employment, was found in workers in dyestuffs manufacturing.
The risks were considered to be additive. Overall, there was a
significant risk of bladder cancer associated with cigarette smoking,
a risk ratio of 1.8 for males, and there were significant overall
risks associated with occupations such as those of process workers in
the dye manufacturing industry who had a risk of 2.9 for males. When
dye manufacturing process workers who were smokers were compared with
non-smoking workers, the risk for smokers was 4.6, while for
non-smokers the risk was 1.9.
Boyko et al. (1985) concluded that arylamines in the dyestuffs
industry posed a major threat of bladder cancer. However, there was
little evidence to support an effect due to smoking or an interaction
between smoking and occupational exposure.
In an area of Spain where 44% of the adult population worked in dyeing
and printing textile fabrics, there was an increased risk of bladder
cancer for smokers (OR 2.3) (Gonzalez et al., 1985). The estimated
risks for occupation and for smoking and occupational exposure were OR
5.5 and 11.7, respectively. The observed effect was multiplicative.
Tobacco smoke contains many amines, including the bladder carcinogens
4-aminobiphenyl (>9 ng/cigarette) and 2-naphthylamine (54
ng/cigarette) (Patrianakos & Hoffmann, 1979; Pieraccini et al., 1992;
Grimmer et al., 1995).
In a study of risk factors for bladder cancer in Spain (Bravo et al.,
1987), the results were considered to corroborate previous data that
bladder cancer does not have a single cause. Cigarette smoking was
considered an important cause but one which was additional to
urological disease or occupational exposure, among other factors. In a
study of men in Spain (Gonzalez et al., 1989), increased risks of
bladder cancer were found for textile workers (OR 1.97), mechanics and
maintenance workers (OR 1.86), and workers in the printing industry
(OR 2.06). The highest risk was in those who were employed in the
textile industry before the age of 25 and prior to 1960. Among
mechanics the highest risk was for those who started after the age of
25 and after 1960. The OR for smokers who had also been employed in
one of the high-risk occupations was 7.82, which is compatible with a
multiplicative effect of joint exposure to tobacco smoke and
occupational hazards. In an Italian study (D'Avanzo et al., 1990),
risk additivity was found for the interaction between tobacco smoke
and several occupations associated with bladder cancer but the
occupations were not specified. The bladder cancer risk for smokers of
black tobacco was higher (OR = 3.7) compared with smokers of blond
tobacco cigarettes (OR = 2.6). A higher risk for black tobacco than
for blond varieties and a protective effect for smokers of tipped
cigarettes was also reported in a study in Northern Italy where a
multiplicative effect for smoking and high risk occupations was also
found (Vineis et al., 1984).
Bartsch et al. (1993) correlated the higher incidence of bladder
cancer among smokers of black tobacco with high yield aromatic amines,
particularly 4-aminobiphenyl from black tobacco (5 times greater than
from blond tobacco). The concentrations of urinary mutagens and of
4-aminobiphenyl adducts in the blood were also higher in smokers of
black tobacco.
A Chinese study of bladder cancer incidence and mortality in workers
with benzidine exposure found a marked dose response and an elevated
risk for both producers and users of benzidine. Workers exposed to
benzidine who were smokers had a 31-fold risk, while the risk for
exposed workers who were non-smokers was 11-fold, and a multiplicative
interaction was suggested (Bi et al., 1992).
3.5.4 Polycyclic aromatic hydrocarbons
Tobacco smoke contains many polycyclic aromatic hydrocarbons (PAHs)
(IARC, 1986), a number of which, such as benz( a)pyrene and
dibenz( a,h)anthracene, are known to be carcinogenic (IARC, 1987;
Hoffmann & Hoffmann, 1997). PAHs are generated by incomplete
combustion of organic matter in many industrial processes and
constitute a hazard not only in occupations but also as environmental
pollutants, representing primary risk factors as lung and bladder
carcinogens. A tobacco smoker can obtain one dose of PAHs from tobacco
smoke and another from the industrial or environmental source.
Furthermore, an interaction of tobacco smoke and an occupational
hazard is a possibility. PAHs in the workplace are often accompanied
by many other toxic compounds, particularly irritants, and, in
addition to carcinogenic PAHs, tobacco smoke contains co-carcinogens
and tumour promoters as well as ciliatoxic agents, irritants and other
biologically active species.
PAHs occur in coal gas manufacture, coking oven fumes, aluminium
smelting, in the use of tar and asphalt, in oil refining and the
exhaust from internal combustion engines. They are frequently
accompanied by irritant fumes or aerosols and potentially harmful
particulate matter. There is a lack of smoking data for workers in
many of these industries, but it has been assumed that the smoking
prevalence is at least as high as the average for blue collar workers.
At a Norwegian smelter 69% of workers were smokers when the expected
prevalence of smoking was 52% (Abramson et al., 1989). The percentage
of smokers and ex-smokers among workers exposed to chemicals and coal
tar pitch in a 1982 survey of 800 000 men and women in the USA was
49.9% against 46.1% for the average worker (Stellman et al., 1988).
A Canadian study found a high prevalence of bladder cancer in
aluminium smelter workers, particularly among those employed in
Söderberg potrooms where carbon electrodes made from a mixture of
petroleum pitch and coal pitch are used and PAH levels are high
(Thériault et al., 1984). Changing electrodes, breaking the crust that
forms on top of the molten metal and cleaning out the "pots" are
activities that create air pollution by tar volatiles including
carcinogenic PAHs which, measured as benzo( a)pyrene, could reach a
concentration of 800 µg/m3/8 h (Bjorseth et al., 1978). High levels
of PAHs were found in urine samples from aluminium plant workers
(Haugen et al., 1986). Lung cancer rates among aluminium reduction
plant workers are also high (Gibbs & Horowitz, 1979). Tobacco smoke
appears to increase the risk. In a study by Thériault et al. (1984),
the numbers were too small to determine whether the interaction was
additive or multiplicative, but in another study (Bjorseth et al.,
1978) there was suggestive, but not conclusive, evidence that the
relative risks from combined exposure to tar volatiles and cigarette
smoke were multiplicative. In a study in which the preceding data were
augmented (Armstrong et al., 1986), the tar volatiles were confirmed
as the cause of bladder cancer and the results suggested that a
multiplicative risk arose from a combined exposure to tar volatiles
and cigarette smoke.
Gullvåg et al. (1985) found that the alveolar macrophage count for
workers in the potrooms of an aluminium reduction plant was elevated
and for workers who were also smokers the count was further elevated.
The conclusion was that smoking and workplace pollution act
synergistically in increasing the number of alveolar macrophages.
Workers in coke oven plants have a higher incidence of lung cancer
than the general population and a measurable concentration of PAHs in
urine, which is higher in smokers than non-smokers (Haugen et al.,
1986). Van Schooten et al. (1990) analysed blood samples from coke
oven workers for PAH-DNA adducts and urine for 1-hydroxypyrene and
compared the results with those of non-exposed workers. Levels were
elevated in coke oven workers and in both exposed and control groups
the PAH-DNA adduct levels were higher among smokers than among
non-smokers.
Professional drivers are exposed to benzene and carcinogenic PAHs and
nitroarenes through the exhaust of petrol (gasoline) and,
particularly, diesel engines. An excess of lung cancer has been found
in this occupational group, with a suggestion of a synergistic
interaction between smoking and occupational exposure (Damber &
Larsson, 1985a).
Diesel exhaust contains large quantities of carbonaceous particulates
with adsorbed PAHs. The association between lung cancer and diesel
exhaust and the contributing role of cigarette smoking has been
considered to be problematic (Garshick et al., 1987, 1988; Boffetta et
al., 1988; Stöber & Abel, 1996; IPCS, 1996). In its evaluation, IARC
(IARC, 1989c) considered diesel engine exhaust to be probably
carcinogenic to humans (group 2A) and gasoline engine exhaust as being
possibly carcinogenic to humans (group 2B).
In most of the workplaces where PAHs contaminate the atmosphere, there
are also gases, fumes and aerosols that contain other hazardous
materials that act as irritants; they may play a role in the etiology
of chronic obstructive lung disease. It is important to include
smoking in epidemiological studies. In a study of lung cancer
mortality rates and smoking patterns in workers in the motor vehicle
industry, proportionate mortality rates were considerably reduced when
smoking rates were taken into account. An increased lung cancer risk
has been described among foundry workers; PAHs and silica were
considered to be possible etiological factors (Sherson et al., 1992).
IARC (IARC, 1984, 1985b) considers the following technical products as
carcinogenic to humans: coal tar and coal tar pitches, shale oils,
soots, effluent aerosols from coal gasification, and emissions from
coke ovens. Exposure occurring in the production of aluminium is
classified as probably carcinogenic to humans, whereas the exposures
to aerosol emissions from iron and steel foundries are classified as
possibly carcinogenic to humans.
3.5.5 Ethanol
Clinical and epidemiological studies have established a strong
relationship between smoking and drinking (Istvan & Matarazzo, 1984;
Bien & Burge, 1990; Zacny, 1990).
Elevated tobacco and alcohol consumption are regarded as the major
risk factors for oropharyngeal and oesophageal cancer in many
developed countries (Herity et al., 1982; Tuyns, 1983, 1991; Boyle et
al., 1990; Muir & McKinney, 1992; Negri et al., 1992).
It has been difficult to distinguish the separate effects of these
agents since many smokers tend to consume alcoholic beverages and
vice versa. In addition, the consumption of one substance may have
an effect on the use of the other substance. The possible interactions
(e.g., multiplicative effect) of tobacco smoking and alcohol
consumption for cancers of the oral cavity, pharynx and larynx have
been evaluated by IARC (1986). IARC (1986) concluded that tobacco
smoking was an important cause of oral, oropharyngeal, hypopharyngeal,
laryngeal and oesophageal cancers and combined ethanol consumption
increased the risk substantially. In a case control study of 1114
patients with oropharyngeal cancer, Blot et al. (1988) showed that the
risk to consumers of tobacco and alcohol was multiplicative rather
than additive and increased 35-fold in those who consumed two or more
packs of cigarettes and more than four alcoholic drinks per day. The
risk was higher in those consuming spirits or beer than in those
consuming wine and was lower in lifetime smokers of filter cigarettes.
Alcohol consumption and smoking affect fetal outcome, leading to
infants with low birth weight (Wright et al., 1983, 1984; Smith et
al., 1986).
In contrast to the many studies in laboratory animals of the
interactions of ethanol with tobacco-smoke condensate (TSC) and
specific tobacco constituents, e.g., nicotine (receptor studies)
(Collins, 1990), (gastric-mucosal damage) (Wong et al., 1986; Cho et
al., 1990), tobacco-smoke-specific nitrosamines, e.g.,
N-nitrosonornicotine (metabolism and carcinogenicity) (McCoy et al.,
1981; Castonguay et al., 1983, 1984) and
4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK) (Jorquera et al.,
1992; Schüller et al., 1993), there has been a relative paucity of
studies involving ethanol and tobacco smoke per se. These latter
studies have included fetotoxicity in mice (Peterson et al., 1981),
gastric mucosal damage (Iwata et al., 1995; Chow et al., 1996), and
mechanisms underlying behavioural association between alcohol and
tobacco consumption (Zacny, 1990).
In in vitro studies on the effect of tobacco smoke condensate on rat
buccal mucosa cells following exposure to ethanol, the level of
adducts was higher than in controls, suggesting an increased uptake of
carcinogens in the condensate (Autrup et al., 1992). Hsu et al. (1991)
studied in vitro genotoxicity of tobacco smoke condensate in
conjunction with 2% and 4% ethanol in human lymphoid cell lines.
Ethanol potentiated clastogenicity, measured by frequency of
chromosome breaks per cell, in a dose-dependent manner, and the
results indicated that ethanol at relatively high doses inhibited DNA
and chromosome repair systems.
Swiss Albino mice fetuses prenatally exposed to both tobacco smoke and
ethanol had a high resorption frequency, a significant reduction in
fetal weight and length, and neonatal growth retardation, indicating
that ethanol and tobacco smoke may interact to produce fetotoxicity
(Peterson et al., 1981).
Both cigarette smoking and ethanol consumption individually have been
associated with gastric and duodenal ulcers in humans and animals.
Exposure to cigarette smoke significantly potentiated ethanol-induced
gastric mucosal damage in Sprague-Dawley rats (Iwata et al., 1995;
Chow et al., 1996).
The effect of smoking on the incidence of cancers of the oral cavity,
oropharynx, hypopharynx and larynx is often combined with other
factors, principally alcohol, in the Western world. The possibility of
interaction between cigarette smoking and alcohol consumption is
complex (Burch et al., 1981).
Rothman & Keller (1972) reviewed the effect of joint exposure to
tobacco and alcohol with regard to oral cancers alone (based on data
published earlier by Keller & Terris, 1965) and concluded that a
single multiplicative function of the relative risks associated with
alcohol and tobacco separately provided an adequate summary of their
joint effect.
Wynder & Bross (1961) studied etiological factors in cancer of the
oesophagus, considered the consumption and effects of tobacco and
alcohol separately and together, and considered that the combined
effect was multiplicative. Tuyns et al. (1977) reported a similar
pattern of joint effect of tobacco and alcohol in a retrospective
study of oesophageal cancer in Brittany, France. The relative risk of
developing oesophageal cancer increased linearly with daily
consumption of alcohol and tobacco independently. The combined effect
fitted a multiplicative model.
Wynder et al. (1976) analysed environmental factors in cancer of the
larynx and showed a combined effect of tobacco and alcohol. In the
presence of smoking, heavy drinking increased the risk of cancer of
the larynx, especially for cancer of the supraglottic portion of the
larynx. Similar findings were reported by Burch et al. (1981).
In a prospective epidemiological study, the relative risk of incurring
a single primary carcinoma of the oral cavity, pharynx, larynx and
oesophagus in any one of these sites was increased independently by
the duration and intensity of exposure to tobacco or alcohol and
sustained exposure enhanced the risk in a multiplicative or
synergistic fashion (Schottenfeld et al., 1974) The relative risk of
multiple primary cancers in the sub-group with combined exposures to
high levels of tobacco and alcohol was 3.9 times that of patients
exposed previously to low levels of alcohol and tobacco.
3.5.6 Other organic compounds
Exposure situations involving compounds and mixtures of organic
compounds for which no definite smoking interactions have been
established but which are known to present serious health hazards are
summarized in chapter 4.
3.6 Physical agents
3.6.1 Radiation
The harmful forms of ionizing radiation that are of concern are
alpha- and beta-particles and gamma- and X-rays. All cause cellular
damage and have been implicated in carcinogenesis. IARC (1988)
classified radon and its decay products as Group 1 (carcinogenic to
humans) on the basis of sufficient evidence in humans and in
laboratory animals. The interaction of the effects of these radiations
with the effects of tobacco smoke has been studied. Radium is present
in uranium and other minerals and in all rocks and soils. It emits
alpha- and beta-particles and gamma-rays and decays to form the
chemically inert radioactive gas radon, which is released in tiny
amounts into the atmosphere where its concentration is extremely small
because of dilution. It can, however, become more concentrated in some
locations, particularly in uranium and other mines and in residential
buildings. Radon is an inspirable gas and its radioactive decay
products are ionized metal atoms, which adhere to inspirable dust
particles. These atoms are themselves undergoing radioactive decay and
emitting damaging alpha- and beta-particles and gamma-rays. In
addition to interactions of tobacco smoke with radon in mines and
residential situations, other effects of tobacco smoke and radiation
interactions have been studied in atom-bomb survivors and in the low
energy transfer radiation involved in the use of therapeutic radiation
(X-rays).
3.6.1.1 Radon in mines (high linear energy transfer (LET)
alpha-radiation)
Unless mines are well ventilated, the atmospheric concentration of
radon becomes significant. The gas and its radioactive decay products,
the radon daughters, can be inhaled. The daughters have short
half-lives and their decay is proceeding while the particles to which
they adhere are resident in the lungs and before they can be removed
by normal lung clearance. Thus radiation is delivered directly to the
delicate lung tissues where it causes an excess of lung cancer among
some miners.
Observations in several mining communities, e.g., among uranium miners
in the USA, Czechoslovakia, Canada and France, workers in a niobium
mine in Norway, iron ore miners in Sweden, tin miners in China and the
United Kingdom, and fluorspar miners in Newfoundland, showed a
significant dose-related increase in lung cancer risk with exposure to
radon and radon daughter elements (Archer, 1988). In miners who were
cigarette smokers, there was an interaction between the radiation
exposure and the smoke exposure leading to more than the expected
number of cases of cancer. The latent period for induction of lung
cancer was longer when the exposure to radioactivity started at a
younger age, it was shortened by high exposure rates and by cigarette
smoking, and lung cancers developed at lower levels of exposure to
radioactivity in miners who smoked than in those who were non-smokers.
In Bulgaria, Michaylov et al. (1995) used sputum cytology to study
bronchial cell dysplasia in 334 miners (uranium and metal mines)
exposed to 222Rn progeny, and 100 control miners from a metal mine
where radon was virtually absent. The dust and silica concentrations
and exposure to diesel exhaust and explosion gases were similar. The
frequency of bronchial cell dysplasia was significantly higher in
radon-exposed miners than in controls and the frequency of dysplasia
in smokers was significantly greater than in non-smokers.
The lower prevalence of lung cancer among coal miners than among other
underground workers is probably because coal mines are well ventilated
to reduce fire and explosion risk, and no build up of radioactivity
occurs. Attempts to reduce silicosis by ventilation have achieved a
similar effect. In some Swedish mines, because freezing occurred when
outside air was used for ventilation, filtration was achieved in the
1920s by circulating the air through old underground mine workings,
with the result that the potential for silicosis was reduced. However,
an increase in lung cancer was found because radioactive materials
built up, a fact that only became evident many years later (Archer,
1988).
The nature of the interaction between radon and cigarette smoke is not
clear. In a study by Edling (1982), the effects of smoking and radon
were considered to be additive, whereas in another by Damber & Larsson
(1985b) the effect was multiplicative. From a long-term study on
Swedish iron ore miners (Jörgensen, 1984), it was concluded that
tobacco smoke acts as a tumour promoter, an effect that has been
demonstrated in almost all animal studies. The concept that radon
serves as a tumour initiator and tobacco smoke as the tumour promoter
for the induction and development of lung cancer is supported by a
sequence of studies. Tobacco contains small amounts of polonium-210
(210Po), which primarily originates from phosphate fertilizers (Tso et
al., 1966) and, to a minor extent, from airborne 210Po trapped by the
glandular hairs (trichomes) found on the soil-facing surfaces of
tobacco leaves (Martell, 1974). 210Po is a decay product of radon -222
and an emitter of alpha-particles. It is present in cigarette smoke,
and the bronchial epithelium of smokers contains 2-10 times more 210Po
than is found in these tissues in non-smokers (Harley et al., 1980).
The alpha-radiation of 210Po damages DNA in the bronchial airways and
serves as a tumour inhibitor, and the tar in the tobacco smoke acts as
a tumour promoter.
The frequencies of different histological types of lung cancer among
miners have varied with working conditions and follow-up time. It has
also been shown (Archer, 1988) that the age range of the population
under observation can influence the conclusion. Thus, the
smoking-radon relationship appears to be multiplicative only for the
group aged 35-65 years. Steenland (1994) found the death rates from
lung cancer in smoking uranium miners to be intermediate between
additive and multiplicative for the two exposures, but, when
stratified for age, the multiplicative model fitted well for the
youngest and oldest categories but poorly for the middle range. In a
comprehensive analysis of data from 11 studies of radon-induced health
risks (Lubin et al., 1995), it was concluded that the joint effect of
radon progeny exposure and smoking is greater than the sum of the
individual effects and for smokers is higher by a factor of at least
three. The tobacco of cigarettes contains 0.1-1.0 pCi of 210Po
(Cohen et al., 1985; Hoffmann et al., 1986).
The conclusion reached by the US Surgeon General (1985) was that the
smoking-radon interaction consists of two parts: an additive effect of
the contribution of the two agents on the number of tumours produced
and an accelerating effect due to tumour promoters in cigarette smoke.
Thus for a miner who smokes, not only is the chance of lung cancer
greater but the latent period is shorter and therefore the cancer
appears sooner in smokers.
3.6.1.2 Environmental radon (high linear energy transfer (LET)
alpha-radiation)
Alpha-radiation from radon daughters in the home or in other
situations where there are enclosed spaces with poor ventilation,
e.g., where strict energy conservation measures have been adopted,
presents an elevated health hazard to occupants, particularly smokers,
and is a matter of public health concern. The ease with which ionized
radon daughters could be attracted to environmental tobacco smoke
particles and the possibility of a higher than additive combined
effect of radon progeny and smoke clearly indicate the importance of
residential contamination by radon.
The relative risk in the range of exposure experienced by miners has
been found to be linear, and it has been suggested from extrapolation
that exposures at the lower levels found in homes would carry some
risk (Lubin et al., 1995). Steindorf et al. (1995) calculated that 7%
of all lung cancer deaths in the western part of Germany may be due to
residential radon.
Axelson (1995) reviewed cancer risks from exposure to radon in the
home and suggested that cancers other than lung cancer may also be
related to indoor radon, especially leukaemia, kidney cancer and
malignant melanoma. However, it was acknowledged that studies of radon
and miners gave no clear support for this. Alavanja et al. (1995)
listed other risk factors as being responsible for lung cancer in
lifetime non-smokers and found a small non-significant risk for
subjects exposed to domestic radon at median concentrations. In a
case-control study of lung cancer in relation to exposure to radon in
homes (Letourneau et al., 1994), no increase in the relative risk for
any of the histological types of lung cancer was detected in relation
to cumulative exposure to radon. On the other hand, Biberman et al.
(1993) found an increased risk for small cell lung cancer following
residential long-term exposure to radon at a low-dose level. In a
large case-control study in Sweden, Pershagen et al. (1994) reported
an increased relative risk of lung cancer within the highest exposure
group. In an attempt to resolve the conflicting epidemiological data,
Lubin & Boice (1997) conducted a meta-analysis of eight large-scale
case-control studies of residential radon and lung cancer. This
analysis was consistent with an excess lung cancer risk. Furthermore,
the slope of the exposure- response curve derived from this
meta-analysis was comparable with that obtained from a combined
analysis of eleven miner cohorts exposed to radon (Lubin et al.,
1997).
The combined analysis of the miner data also confirmed the strong
synergistic relationship between radon and tobacco at high levels of
exposure to these two agents (Lubin et al., 1997), although it is
difficult to determine whether the interaction is closer to additive
or multiplicative (Chaffey & Bowie, 1994). When extrapolated to lower
levels of exposure, however, the magnitude of this interaction is
substantially diminished (Moolgavkar et al., 1993). However, Pershagen
et al. (1994) reported some evidence of a synergistic effect between
tobacco and residential radon exposure, with the relative risk of
radon-induced lung cancer being highest among heavy smokers. In a
case-control study of 982 subjects with lung cancer and 3185 hospital
or population control subjects, lung cancer risk was examined in
relation to residential radon concentration and length of time that
subjects were resident, and adjusted for age, sex and smoking (Darby
et al., 1998). The relative risk increased in an exposure-related
manner with time-weighted residential radon concentration and fitted
the data from studies in miners and the effect of smoking. Regardless
of the magnitude of any interaction between tobacco smoking and
residential radon, the lung cancer risks due to smoking exceed the
risk associated with radon in homes.
3.6.1.3 Atomic bomb site radiation (low linear energy transfer (LET)
radiation)
In tobacco-smoking survivors of atomic bombing in Hiroshima and
Nagasaki, Japan, elevated levels of cancer of several sites have been
reported. In the case of lung cancer both additive and multiplicative
models fit the data (Prentice et al., 1983; US NRC, 1988).
3.6.1.4 Therapeutic X-rays (low linear energy transfer (LET)
radiation)
Lung cancer as a possible side effect of the radiation therapy used to
treat breast cancer has been studied by Neugut et al. (1993, 1994) and
discussed by Inskip & Boice (1994). Neugut et al. (1993) reported that
the risk was greater in the ipsilateral than in the contralateral
lung. In a second study (Neugut et al., 1994), a three-fold relative
risk was found for the effect of radiation therapy among 10-year
survivors, a 14-fold risk was associated with smoking alone, and a
33-fold risk was found among irradiated smokers; in each case the
effect was most pronounced for ipsilateral lung cancer. A
multiplicative interaction was proposed and the implications of the
results for the design of treatment of breast cancer in smokers was
considered. The increased risk of lung cancer among survivors of
Hodgkin's disease(HD) was studied by van Leeuwen et al. (1995). Their
overall conclusions were that the risk of lung cancer increased more
with increasing radiation dose in HD patients who smoked than among
those who did not smoke. Thus, smokers were at greater risk from the
radiotherapy than non-smokers. The interaction between the
carcinogenic effects of smoking and radiation was significantly
stronger than multiplicative, and the low lung cancer rate found among
women with HD was attributable to the delayed popularity of smoking
among Dutch women, a fact shown by the male/female lung cancer ratio
(13:5) in the Netherlands in 1980.
3.6.1.5 Nuclear plant
Ongoing epidemiological studies are being conducted on the workforce
exposed to radiation at the Mayak plant in Russia. Of 500 workers
examined in a case-control study (Tokarskaya et al., 1995), 162
workers had contracted lung cancer, and the remaining 338 served as
radiation-exposed, non-tumour-bearing controls. Both the incidence and
duration of smoking was significantly higher in workers contracting
tumours compared to combined male and female controls. The strongest
smoking-related effect was for squamous cell carcinomas, followed by
adenocarcinomas, then small cell carcinomas. However, the findings are
complicated by the fact that the great majority of the workforce was
male, and there was only one of the 148 "never smokers" among the male
lung cancer cases.
3.6.1.6 Summary
In summary, miners subjected to chronic exposure throughout a working
lifetime to high-LET radiation show a radiation/tobacco smoke
interaction greater than additive and sometimes multiplicative. Atomic
bomb survivors exposed instantaneously to low-LET radiation show in
some cases an additive and in others a multiplicative interaction. The
results for residentially exposed smokers, subjected to a lifetime of
very low dose exposure, tend to show similar interactions to those for
miners. Tobacco-smoking patients subjected to therapeutic radiation
(low-LET) show a multiplicative interaction.
3.6.2 Vibration
Raynaud's phenomenon is an episodic disorder that produces
intermittent attacks of blanching in the extremities and there may be
numbness or tingling in the hands and fingers. There are several
causes (the term "Raynaud's disease" is applied when the cause is not
known). It was first associated with the use of vibrating tools among
Italian miners in 1911, and the association has since been reported
for a wide range of hand-held vibrating tools such as impact hammers,
chipping hammers, grinders, riveters and the motor-driven chain-saws
used in forestry. The terms vibration white finger (VWF), vibration
syndrome, traumatic vasospastic disease and dead finger have been used
for this condition that begins with numbness and tingling, followed by
blanching and can include intermittent episodes of hand and finger
pain and flushing. With continuing exposure to vibration the symptoms
may become more severe and continue after the cessation of exposure.
Damage to digital arteries and narrowing of the lumen has been
associated with vibration syndrome (JOM, 1984) and, because nicotine
acts as a vasoconstrictor, it has been suggested (JOM, 1984) that
limiting smoking could aid blood flow to the extremities and thus
reduce the condition. In a survey of forestry workers in Quebec in
1977-1978 (Thériault et al., 1982), a prevalence of Raynaud's
phenomenon among 1540 woodcutters was found in 30.5% of chain-saw
users and there was a strong association between this and cigarette
smoking; the relative risks were 3.60 for non-smokers, 6.55 for
smokers and 1.72 for smokers who had not used a chain-saw:
corresponding to an additive effect for the two risk factors. From
another study of the effect of tobacco use on a cohort of men with
VWF, in which the extent of tobacco use was confirmed by blood
nicotine and cotinine measurements (Ekenvall & Lindblad, 1989), it was
shown that tobacco aggravates the symptoms of VWF. Patients with
advanced symptoms were found to use tobacco more frequently and to
have higher blood cotinine levels than patients with less advanced
disease. In a study of the prognosis of VWF (Petersen et al., 1995),
an improvement in the condition occurred when there was no exposure to
either vibration or smoking, whereas an aggravation of the condition
was most notable in smokers. Whole-body vibration has been associated
with persistent severe neck trouble, and smoking was an added
predictor for this condition (Viikari-Juntura et al., 1994). Finger
temperature changes have been measured after smoking a cigarette; in
all cases a reduction in temperature was recorded (Saumet et al.,1986;
Bornmyr & Svensson, 1991).
3.6.3 Noise
In a study of aviators in 1963 at the US Naval Aerospace Medical
Research Laboratory (Thomas et al., 1981), two hearing level groups
were identified, one with normal and the other with impaired hearing.
The impaired hearing group had smoked more cigarettes for a longer
period of time than had those in the normal hearing group. In another
study the relationship between cigarette smoking and hearing loss was
studied in 2348 noise-exposed workers at an aerospace company and it
was found that smoking was a clear risk factor in noise-induced
hearing loss: the OR was 1.27 for "ever smokers" and 1.39 for present
smokers, compared with non-smokers (Barone et al., 1987). Vascular
insufficiency of the cochlear organ has been cited as the predominant
cause of progressive hearing loss that occurs with age. It was
suggested that smoking reduces the cochlear blood supply by:
a) vasospasm induced by nicotine; b) atherosclerotic narrowing of
vessels; and c) thrombotic occlusions (Zelman, 1973). In a study of
1000 subjects at a Veterans Hospital, Zelman (1973) found that whilst
age and sex were the most important variables, at all measured
frequencies the percentage of loss was greater for smokers, the
differences being greater at higher frequencies. From a retrospective
analysis of audiograms taken between 1984 and 1990 of a cohort of 119
workers, 78.8% of smokers compared with 25.7% of non-smokers had
noise-induced hearing loss (ILO, 1991). Although these studies
demonstrated a positive correlation between smoking and hearing loss,
Friedman et al. (1969) and Pyykko et al. (1987) were not able to show
that smoking was a significant risk factor to hearing loss.
3.6.4 Dupuytren's contracture
Dupuytren's contracture is a contracture of some muscle membranes of
the palm of the hand that causes the little finger and ring finger to
be drawn into the palm from where they cannot be extended. It is
characterized by a retractile sclerosis of the palmar aponeurosis,
which may progress to an irreducible flexion of the fingers. Opinions
differ on the influence of occupation, handedness and hand injury on
Dupuytrens contracture which Dupuytren himself attributed to chronic
occupational injury. Some people assert that heavy work always causes
the disease, while others claim that it is not responsible. Mikkelsen
(1978) studied 901 cases in an epidemiological study of 15 950 and
concluded, after isolating hand trauma, that Dupuytren's contracture
is caused by heavy work. The contribution made by tobacco has been as
contentious as has the cause of Dupuytren's contracture. Hand
thermography of affected fingers in Dupuytren's contracture shows a
drop in temperature of up to 3 degrees; and hand temperature falls by
a similar amount during smoking. In a study of 84 men and 16 women
with Dupuytren's contracture, Fraser-Moodie (1976) found no evidence
that smoking was connected with the condition, a conclusion also
reached by Mackenney (1983). However, cigarette smoking was listed
among the responsible factors for Dupuytren's contracture by Attali et
al. (1987), as well as by An et al. (1988), who found that cigarette
smoking was linked statistically to Dupuytren's contracture and
suggested that it may be involved in its pathogenesis by producing
microvascular occlusion and subsequent fibrosis and contracture. An et
al. (1988) concluded that cigarette smoking was one of the most
significant factors in the development of peripheral vasculopathy
Abelin et al. (1990) showed a significant association between
Dupuytren's contracture and smoking habits.
3.7 Biological agents
3.7.1 Biological (vegetable) dusts
Uncontrolled exposure to airborne vegetable dusts can affect health
and occurs worldwide in many workplaces, e.g., in agricultural
operations, textile industries, construction, carpentry and the
furniture industry. The population exposed is large, particularly in
developing countries where whole families, from young children to the
elderly, may engage in agricultural activities and small scale
manufacturing operations using vegetable products. These agents can
take the form of vegetable dusts, airborne fungal spores and
microorganisms, animal danders and feathers, herbicides and pesticides
and their residues. Processing of agricultural products, such as
cotton, flax, hemp, grain, tobacco, paprika and tea, and the milling
of certain varieties of wood are occupations where vegetable dust
exposures have been associated with detrimental health effects.
In addition to the irritation and bronchitis that is associated with
exposure to almost any dust, biological dusts can cause byssinosis,
allergic and immunological responses and, in some cases, nasal and
paranasal cancer. All these conditions can be affected in different
measure by tobacco smoking.
3.7.1.1 Cotton dust
Byssinosis is a respiratory disease of textile workers. The disease is
found in many cotton processing countries. It is more prevalent in the
dusty stages of cotton processing, such as carding, than in weaving.
Byssinosis, or similar symptoms, and bronchitis have also been found
in flax, hemp, jute and sisal workers. The characteristic symptoms are
tightness of the chest and shortness of breath on returning to work
after a period of absence. There is the possibility of progression to
permanent respiratory disability.
In a study of textile workers in South Carolina, USA, in 1973, the
smoking prevalence was almost the same for workers as for controls
(Beck et al., 1984). In another study of cotton workers in 1963-1966
(Molyneux & Tombleson, 1970), the percentage of male current smokers
was 62.5% and of ex-smokers 16.4%; among females the figures were
33.9% and 6.1%, respectively. Among flax scutchers in Normandy in
1986-1987, 56% were smokers and 18% were ex-smokers; compared with 45%
and 15%, respectively, for the controls (Cinkotai et al., 1988a). In
31 Lancashire textile factories (1988) 47.5% of the 4656 workers
interviewed were smokers (Cinkotai et al., 1988b). Of 800 000 American
workers surveyed (Stellman et al., 1988) for smoking habits in 1982,
5.9% were exposed to textile fibres or dust: 28.5% of these were
regular cigarette smokers and 44.9% were former cigarette smokers;
compared with 23.5% and 43.5%, respectively, in other occupations not
exposed to textiles.
Increases in both byssinosis and bronchitis were attributed to cotton
dust exposure and smoking in the cotton industry (Molyneux &
Tombleson, 1970; Merchant et al., 1973). From an industrial study of
the effects of cotton dust and cigarette smoke, Merchant et al. (1972)
concluded that smokers showed an increase in both the prevalence and
severity of cotton dust-induced byssinosis and that cigarette smoke
also increased the detrimental effect of cotton dust on ventilatory
capacity. It was suggested that the impairment of lung clearance
mechanisms by cigarette smoke could be responsible for the deleterious
effect of cotton dust and that smoking might lower the threshold of
susceptibility to the effects of inhaled cotton dust. Additivity and
the equal importance of the effects of smoke and cotton dust have been
suggested (Beck et al., 1984) but since different lung function
parameters are affected it would seem that the two factors affect
different sites. The fact that workers who stopped smoking, whilst
remaining in the same job, lost their byssinotic symptoms was
significant. A survey by Cinkotai et al. (1988a) of workers in 31
textile factories in Lancashire, United Kingdom, showed that
byssinotic symptoms (in decreasing order) were related to years in the
industry, degree of dust exposure, quality of cotton in use, ethnic
origin of workers and smoking habits. Symptoms of chronic bronchitis
were related primarily to smoking habits and then to factors connected
with the occupation. In a study of hemp workers (Bouhuys & Zuskin,
1976), decline in ventilatory function was more pronounced in smokers.
It was suggested (Cinkotai et al., 1988b) that a surprisingly low
prevalence of byssinotic symptoms in 12 flax scutching mills in
Normandy may have been due to either self selection of the workforce
or to an absence of the causative agent in the dust. Persistent cough
and phlegm production were associated with tobacco use.
In textile-related pulmonary disease, smoking as a primary causative
factor was reported by Pratt et al. (1980), and a similar conclusion
was arrived at from lung function tests carried out over a 3-year
period on 153 women (103 smokers, 50 non-smokers) with grades 2 and 3
byssinosis by Honeybourne & Pickering (1986). Cancer deaths in general
and lung cancer in particular were lower in workers exposed to cotton
dust than in others (Enterline et al., 1985). Kilburn (1989) suggested
that the effect of byssinosis on mortality of textile workers from
pulmonary disease needed more comprehensive study.
3.7.1.2 Wood dust
IARC (1995) evaluated the carcinogenic risk of wood dust and
classified it as carcinogenic to humans (Group 1), based on sufficient
evidence in humans and inadequate evidence in animals. The risk of
developing cancer of the nasal cavity among workers manufacturing
wooden furniture has been shown to be up to 100 times greater than for
the general population (Rang & Acheson, 1981). The effect is worst in
the most dusty areas (Rang & Acheson, 1981; Hayes et al., 1986). The
association of risk with certain hardwoods and the finishing of fine
furniture, rather than with woodworking in general, suggests that it
may be allied to both the chemical and physical nature of the dust. In
the study where the nasal cancer incidence was 100 times greater than
for the general population, it did not appear to be affected by
smoking habits. A similar conclusion was reached in a study in an area
of Italy with a large number of cases of nasal cancer among wood and
leather workers (Cecchi et al., 1980). An association with smoking was
established in other studies, and current and past smoking habits were
shown to be a risk factor for developing squamous cell cancer of the
sinus in men (Fukuda et al., 1987). A case-control study of 121 male
woodworkers who were examined for cancer of the nasal cavity or
paranasal sinus, in British Columbia in Canada between 1939 and 1977
(Elwood, 1981), showed increased relative risks associated with
occupations involving exposure to wood (relative risk 2.5) and with
smoking (relative risk 4.9). In a study in North Carolina and Virginia
in the USA between 1970 and 1980 (Brinton et al., 1984), a major
finding was the elevated risk of nasal cavity and sinus cancer among
cigarette smokers. However, the nature of any interaction of wood dust
and tobacco smoking needs further study because adenocarcinomata
appear to be the tumour type associated with wood dust, whereas the
relative risks for squamous-cell and small-cell cancers tend to be
higher for smokers. The available data do not permit an assessment of
the degree of interaction between smoking and wood dust exposure.
3.7.1.3 Allergic responses
This type of response can occur in the upper airways, where it is
manifest as hay fever, in response to certain types of pollen, or in
the bronchi as asthma, or it may appear in both. Some of the dusts
that cause allergic airways responses (occupational asthma) are grain
dusts from various cereals and their products, wood dusts particularly
from red cedar and iroko, and dusts from teas and tobacco. Among
asthmatics, environmental cigarette smoke makes the effect of the
asthma worse (Shim & Williams, 1986), and smoking effects appear to be
additive to that of asthma from other causes (Conolly et al., 1988).
Grain dust exposure and smoking have been found to cause increases in
the prevalence of respiratory symptoms and reductions in pulmonary
function of grain elevator workers. The effect of smoking was slightly
more pronounced; the combined effect of grain dust and smoking
appeared to be additive, except in the least exposed workers (5 years
or less) where a synergistic effect was observed in tests for
peripheral airways dysfunction (Cotton et al., 1983). Chan-Yeung et
al. (1985) stated that the effect of grain dust and smoking was
additive and not synergistic in causing a decrease in lung function.
In 303 workers in the animal feed industry exposed to dusts of grains
and cassava, 61 (20%) showed respiratory symptoms; in office staff in
the same plant the prevalence was similar. The plant workers had a
higher prevalence of smoking than office staff. Current smoking was
strongly associated with respiratory complaints in both groups (Post
et al., 1994).
Occupational asthma also occurs in flour, tea, coffee and rice
handlers. In Italian bakers and pastry makers De Zotti et al. (1994)
found that 54 (23%) of the 226 subjects were atopic. Forty (18%) were
skin-test-positive to storage mites, 27 (12%) to wheat flour and 17
(8%) to alpha-amylase. Skin sensitization to these occupational
allergens was significantly associated with atopy, smoking and
duration of exposure. In a study of 401 workers in bakeries or flour
mills, Cullinan et al. (1994a) found that work-related symptoms of
allergy were more common in smokers, with little difference between
atopic and non-atopic workers. However, smoking was not independently
related to either symptoms or positive skin test.
Zetterstrom et al. (1981) skin-prick-tested 129 workers in a coffee
roastery with green coffee bean and castor bean extracts. There was a
significantly increased prevalence of positive skin prick tests in
smokers. In enzyme detergent workers with occupational asthma, it was
found that twice as many smokers as non-smokers exhibited asthmatic
symptoms (Greenberg et al., 1970; Mitchell & Gandevia, 1971).
Smokers are more likely to show higher specific antibody production
and correspondingly be more susceptible to asthma.
3.7.2 Other biological agents
Although many biological dusts are known to have detrimental health
effects, there have been few studies of any interaction of smoking
with these agents. Extrinsic allergic alveolitis may be caused by
spores from a number of fungi which are small enough to reach the
pulmonary compartment. There are several forms of allergic alveolitis,
of which farmer's lung, bagasse pneumonitis, and bird fancier's lung
are examples. These are caused by fungal spores in mouldy hay or
mouldy sugar cane or an agent in bird feathers, respectively, and are
immunologically mediated (Lancet, 1985). Extrinsic allergic alveolitis
is a rare example of a respiratory disease which is more prevalent in
non-smokers than in smokers. In one study, 14 of 18 patients (11 with
farmer's lung and 7 with bird fancier's lung) were non-smokers, twice
the proportion of non-smokers in patients with cryptogenic fibrosing
alveolitis or in the local population (Warren, 1977). Carrillo et al.
(1991) investigated IgG response to pigeon serum and its relation to
tobacco smoking in 160 pigeon fanciers. The sensitization rate was
31.9%. Pigeon fanciers who were current smokers had significantly
lower levels of IgG antibodies to pigeon serum (P<0.001).
Precipitating antibodies to Micropolyspora faeni, a common cause of
farmer's lung, were found to be twice as common in the area of
non-smokers in farming communities compared with smokers (Morgan et
al., 1975). Alveolar macrophage phagocytosis has been shown to be
depressed by cigarette smoke and it has been suggested this may
explain its apparent protective effect (Hocking & Golde, 1979).
3.7.3 Agents found in factory farming (animal
confinement effects)
Factory farming of pigs, where the animals are kept in confined
conditions, is common practice in livestock production in many
developed countries and it has been found to be accompanied by adverse
respiratory symptoms in workers. Donham et al. (1984) summarized the
effects as: acute toxicosis and inflammation of the respiratory tract
from inhaling hydrogen sulfide; acute asthma-like symptoms;
bronchitis; and delayed or hypersensitivity pneumonitis-like symptoms.
They found that smoking interacted additively with the bronchitis and
obstructive symptoms of the condition. In a study of workers in swine
confinement areas, Zuskin et al. (1992) reported similar effects. They
also found that smoking aggravated acute and chronic respiratory
symptoms and impairment of lung function.
3.7.4 Laboratory animals
Venables et al. (1988b) examined data from three cross-sectional
surveys of 296 laboratory workers around 30 years of age exposed to
small mammals. Two populations were of pharmaceutical research workers
(N = 133 and 140) and one of research workers in a tobacco company
(N = 23). One of the pharmaceutical research worker populations had a
laboratory animal allergy (LAA) prevalence rate of over 40% (Venables
et al., 1988a). The tobacco company research workers were exposed only
to rats while the other two populations were exposed to rats, mice,
guinea-pigs and rabbits. Atopy was determined by skin prick test to
non-animal aeroallergens. Sensitization to laboratory animals was
determined by response to skin prick tests using urinary extracts from
the species used in each laboratory. Radioallergoabsorbent tests
(RASTs) were used to measure serum IgE antibody concentration to the
urinary extracts in two of the populations.
Atopy in the three populations ranged from 30% to 44%, and positive
skin response to urinary extract ranged from 13% to 48%. Pooled data
from the three surveys showed an association between smoking and
positive skin response to urinary extract. Associations with smoking
persisted after stratifying by atopic status, suggesting that smoking
was a risk factor for developing laboratory animal allergy.
In other studies of 238 laboratory workers without previous
occupational exposure to rats in three institutions specializing in
small animal research, atopy was again determined by skin prick test
response to non-animal aeroallergens and sensitization to laboratory
rats by response to urinary extract. Exposure to total dust and rat
urinary aeroallergen was also measured. Allergy to rats was positively
related to exposure intensity and this was stronger in atopic
subjects. Positive responses to skin prick testing with rat urinary
extract were strongly related to atopy and to smoking at all levels of
exposure (Cullinan et al., 1994b).
Several studies from Europe have shown an association between
ownership of pet birds or pigeons and lung cancer (Holst et al., 1988;
Kohlmeier et al., 1992; Gardiner et al., 1992). The relative risk
adjusted for smoking was 6.7 (2.2-20.0). However, two community-based
case-control studies, one from the USA and one from Sweden, could not
confirm an association between pet birds and lung cancer (Alavanja et
al., 1996; Modigh et al., 1996). In 1998, a hospital-based
case-control study conducted in New York City and Washington, DC, with
887 cases and 1350 controls, did not show an association of keeping
pet birds with lung cancer in non-smokers. There was a ten-fold
increase of lung cancer among smokers who were not bird keepers over
non-smokers, but there was no indication of synergism between smoking
and keeping a pet bird (Morabia et al., 1998).
3.7.5 Schistosomiasis
In a study carried out in Spain, risk factors for urinary bladder
cancer were identified (Bravo et al., 1987). The factors were listed
in order of importance and the first three were total number of
cigarettes smoked, history of urological disease and exposure to an
occupational risk. Vineis (1992) summarized epidemiological,
biochemical and molecular evidence that clearly linked smoking with an
increased risk of bladder cancer. Cohen & Johansson (1992) considered
smoking to be the most important etiological factor for bladder
cancer. They also implicated a variety of occupational exposures and,
in some parts of the world, an association with various endemic
diseases including schistosomiasis. Schistosomiasis is a waterborne
parasitic disease found in many developing countries in Africa, Asia
and South America. It is a widespread occupational disease for
agricultural workers, and also affects members of the general
population.
A phase in the life-cycle of the trematodes responsible for the
disease lives in the blood vessels of visceral organs and their eggs
are discharged through the bladder or intestine in urine and faeces.
Some species live in the mesenteric veins and the eggs are discharged
in the faeces but the eggs of Schistosoma haematobium mature in the
veins of the bladder and are discharged in the urine. The eggs mature
in water and the resultant larvae infect freshwater snails. Within the
snail the parasites multiply to produce free swimming cercaria larvae
which can infect humans via skin penetration and repeat the cycle. S.
haematobium is found in nearly all countries in the African continent
and it has been found that the incidence of bladder cancer is higher
in areas with a high prevalence of infection than in areas with a low
prevalence. IARC classifies infection with S. haematobium as
carcinogenic to humans (Group 1) (IARC, 1994b). In Egypt, the most
common form of cancer is bladder cancer, accounting for 27.6% of all
malignancies encountered (38.5% of cancers in males and 11.3% in
females), and these high levels have been attributed to underlying
schistosomiasis (Tawfik, 1987). Makhyoun (1974) carried out a
case-control study of smoking among Egyptian males with and without a
previous history of S. haematobium infection. A smoking index was
calculated (average number of cigarettes per day × duration of smoking
in years) to categorize subjects. The smoking index (intensity and
duration of smoking) was higher in all the patients with bladder
cancer. In the patients with a previous schistosomiasis infection,
22.7% were moderate or heavy smokers compared with 79.3% of the
non-schistosomiasis patients. In the latter there was a good
correlation with the smoking index but in the bladder cancer patients
with previous schistosomiasis there was no significant difference in
smoking index between patients and matched controls. It was not
possible to identify an interaction between smoking and
schistosomiasis in the production of bladder cancer.
However, in a review of the role of S. haematobium in human bladder
cancer, Badawi et al. (1995) referred to several major studies that
implicated this infection with the subsequent development of bladder
cancer. Badawi et al. (1995) listed examples of a co-carcinogenic
effect of parasitic infection in the presence of chemical carcinogens
and it has been suggested (Hicks et al., 1980; Hicks, 1982) that
schistosomiasis could supply the necessary proliferative stimulus to
accelerate cancer growth from latent tumour foci on exposure to
carcinogenic nitrosamines. Nitrosamines have been implicated as
carcinogens among tobacco chewers and oral snuff users (Hecht &
Hoffmann, 1988, 1991; Hoffmann et al., 1991a), nitrosamines have been
demonstrated in smoke (Tricker & Preussmann, 1992; Hoffmann et al.,
1991b) and nicotine-derived N-nitrosamines cause cancer (Hoffmann &
Hoffmann, 1991; (IARC, 1991). Some N-nitrosamines are excreted as
esters via the urinary tract, e.g., N-nitroso-di- n-butanol or NNK
as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. They may hydrolyse
in the presence of infectious agents in the bladder, as is the case in
schistosomiasis, and thus appear in their precarcinogenic form,
thereby increasing the risk for cancer in the urinary tract.
Endogenous nitrosation, which is increased by tobacco smoking, chewing
and oral snuff use has been demonstrated by monitoring urinary
N-nitrosamino acids (Tsuda & Kurashima, 1991). Badawi & Mostafa
(1993) and Badawi et al. (1995) suggested that the evidence for an
association between urinary schistosomiasis and bladder cancer
development is sufficient to justify the conclusion that S.
haematobium infection is a factor in inducing preneoplastic changes
in the bladder. Infection can reduce detoxification mechanisms (and
thus prolong retention of carcinogens in the bladder), stimulate
nitrosamine synthesis, and alter the activity of
carcinogen-metabolizing enzymes.
The conclusion is that an interaction is likely between tobacco use
and schistosomiasis infection.
3.7.6 Other urinary tract infections
Kantor et al. (1984) found that the joint effect of urinary tract
infection and cigarette smoking on bladder cancer was slightly beyond
that expected under an additive model and suggested that patients with
cystitis may be especially prone to tobacco-derived carcinogens in the
urine. La Vecchia et al. (1991) studied cystitis, gonorrhoea and
condylomata acuminata and found an interaction between urinary tract
infection and tobacco that appeared to be multiplicative, with
relative risk 2.4 for "ever smoking", 3.2 for cystitis alone, and 10.3
for combined exposure. It was concluded that a relationship between
urinary tract infection (and possibly some genital infections) and
bladder cancer indicated a cancer promotion role of infection and a
multiplicative smoking interaction. There have been reports of
synergism between herpes simplex virus and tobacco-specific
N-nitrosamines in cell transformation (Park et al., 1991a); an
additive interaction between smoking and herpes simplex virus type 2
in the promotion of cervical abnormalities (Mayberry, 1985), and the
possibility of an interaction between cigarette smoking and herpes
virus infection leading to cancer of the uterine cervix (Winkelstein
et al., 1984).
3.7.7 Sarcoidosis
Bresnitz & Strom (1983) reviewed sarcoidosis and concluded that
tobacco smoking might have a protective effect for the pathogenesis of
sarcoidosis. Sarcoidosis is a generalized granulomatous disease
involving the reticulo-endothelial system with lesions predominantly
in the lymphatic system. Harf et al. (1986) investigated the smoking
habits of 113 cases of histologically confirmed sarcoidosis in a
case-control study in Lyon, France. Smoking habits were defined in 101
cases and these were compared with a control group of healthy
volunteers. There was a highly statistically significant negative
association between sarcoidosis and smoking (OR = 3.8; 95% confidence
interval: 2.4, 6.5) in both cases.
3.8 Vector effects
Toxic chemicals, as well as harmless materials that produce harmful
chemical agents when they are burnt or vaporized, can be inadvertently
transferred to cigarettes or other smoking materials and cause the
smoke to be more injurious when it is inhaled.
3.8.1 Polytetrafluoroethylene
Polytetrafluoroethylene (Teflon(R)) is used in coatings for cooking
utensils, for making chemical vessels, gaskets and bearings and in
sprays as a mould release agent. Polytetrafluoroethylene and polyvinyl
fluoride are inert materials but their thermal decomposition products
can be very biologically reactive. Cigarettes can be easily
contaminated in the workplace and, when smoked, the polymer burns to
form fumes which cause "polymer-fume fever": severe gripping chest
pain giving rise to difficulty in breathing; trembling and shaking;
elevated temperature; and severe diaphoresis. The symptoms pass after
a day or two, but recur on again smoking a contaminated cigarette.
Before the cause was recognized a case was recorded of a person, who
used the polymer in a mould release spray, having some 40 attacks
(Kuntz & McCord, 1974). Another case was a person who referred to the
disease as "mould machine pneumonia" (Kuntz & McCord, 1974). Other
cases have been reported (Albrecht & Bryant, 1987) and better
occupational hygiene and a ban on smoking in the workplace resulted in
the disappearance of symptoms in those previously affected.
3.8.2 Mercury
Inorganic mercury occurs in many industries, as elemental mercury in
scientific and electrical instruments, as amalgams with many other
metals, in paints and pigments and in the chemical industry, as well
as in mining and extraction of the metal. Organic mercury compounds
are used as antiseptics, disinfectants, fungicides, bactericides and
herbicides. Contamination of smoking materials can lead to the
inhalation of mercury vapour. Mercury has been detected by neutron
activation analysis as a naturally occurring trace element in tobacco
(<1.0 ng/g) and in the smoke of cigarettes (4 ng/cigarette)
(Schneider & Krivan, 1993; Krivan et al., 1994).
3.9 Effects of tobacco smoking and metabolism of drugs and other
chemicals
3.9.1 Oral contraceptive use
In the 1970s it was postulated that an interaction between tobacco use
and oral contraceptives increased the risk of myocardial infarction in
women. In a study of women less than 45 years of age, a greater
proportion of moderate to heavy smokers were found in oral
contraceptive users experiencing myocardial infarction, compared
against a control population (Mann et al., 1975, 1976). Sturtevant
(1982), however, found no "convincing evidence" for an interaction or
synergism between the factors smoking and oral contraceptive use for
the major classes of cardiovascular disease. Lidegaard (1993) reported
no difference in the proportion of smokers between users and non-users
of oral contraceptives in Danish women experiencing a cerebral
thromboembolic attack and age-matched controls. Regarding thrombosis,
the data of Vessey & Doll (1969) suggest an increased thromboembolism
risk from oral contraceptive use for heavy smokers compared to
non-smokers. However, other studies reviewed by Sturtevant (1982) and
Nevius et al. (1982) are said to be ambiguous regarding a possible
interaction between smoking and oral contraceptive use. On balance,
there is evidence for certain interactions between smoking and oral
contraceptive use: the US Surgeon General (1983a), concluded that
"women who use oral contraceptives and who smoke increase their risk
of a myocardial infarction by an approximately tenfold factor,
compared with women who neither use oral contraceptives nor smoke",
and "the use of both cigarettes and oral contraceptives greatly
increases the risk for subarachnoid haemorrhage among women."
3.9.2 Drug and chemical metabolism
A number of studies have demonstrated that the metabolism of various
drugs and other chemicals is influenced by the smoking status of the
individual. This effect is sufficiently noteworthy that the US Surgeon
General (1979) report concluded that it is "apparent that cigarette
smoking is one of the primary causes of drug interactions in humans".
The extensive review of the literature at that time led to the
conclusions that, with respect to the influence of smoking on the
disposition/metabolism of other compounds: (a) the dominant effect of
smoking is enhanced drug disposition caused by the induction of
hepatic enzymes; (b) tobacco smoke contains many enzyme inducers,
notably polynuclear aromatic hydrocarbons; and (c) smoking can induce
the metabolism of various therapeutic agents and their pharmacological
and/or clinical effects. The metabolism of chemical carcinogens
involves various isozymes of cytochromes P450 and differences in their
genotypes or phenotypes may be a main factor responsible for
differences among individuals in susceptibility to carcinogens.
Metabolic activation of the procarcinogens such as benzo( a)pyrene to
the ultimate form is accomplished by cytochrome P450IA1 (Kawajiri et
al., 1990; Kawajiri & Fujii-Kuriyama, 1991; Nakachi et al., 1991).
Although the most noteworthy effects of smoking cited were related to
enzyme induction, it should also be noted that other components of the
smoke, such as carbon monoxide, nicotine, cadmium, some pesticides,
cyanide and acrolein, may serve to inhibit the function of some
enzymes (Jusko, 1978). An example for this phenomenon is the
inhibition by nicotine of the P450 isozymes that are involved in the
metabolic activation of NNN and NNK (Murphy & Heiblum, 1990). Nicotine
levels exceed those of NNN and NNK by more than 500 times. Therefore,
it was not surprising that even increasing NNN and NNK levels in snuff
ten-fold by adding the synthetic compounds did not alter the
carcinogenic potency of snuff in the oral cavity of rats (Hecht et
al., 1986).
Miller (1990) reviewed how cigarette smoking affects the
pharmacokinetic and pharmacodynamic properties of various drugs. The
drugs pentazocine, phenylbutazone and heparin show increased
metabolism in smokers. Also in smokers, the metabolism of oestrogen
and theophylline is increased. In addition, although smoking does not
pharmacokinetically affect the drugs propranolol and pindolol, the
nicotine in smoke is associated with elevations in blood pressure, and
thus smoking might serve to inhibit the antihypertensive effects of
these beta-adrenergic receptor blockers. On the other hand, smoking
had no effect on the drug disposition and/or pharmacological effects
of various other drugs examined (Jusko, 1978; Miller, 1990).
3.10 Animal studies of the interactions between cigarette smoke
exposure and other agents
Fourteen chronic inhalation studies (whole-body or nose-only exposure)
with mainstream cigarette smoke in rats and mice were reviewed by
Coggins (1998) and the results and histopathological changes
contrasted with epidemiological studies in humans. In most of the
studies there were epithelial changes in conducting airways and
increased numbers of alveolar macrophages, occasionally associated
with alveolar metaplasia. Lung adenomas and adenocarcinomas were seen
in some of the studies but no statistically significant increase in
the incidence of malignant lung tumours was found in either rats or
mice. This contrasts with human epidemiology where there is an
increased lung cancer risk. In an invited commentary, Morgan (1998)
stated that, based on these differences, rodents may be ineffective
models for predicting human health risk, at least for certain inhaled
materials, but stressed the interspecies differences in respiratory
anatomy and physiology and the need for consideration of the many
factors that may lead to differences in the effects of tobacco smoke
exposure.
The existing animal toxicology studies regarding interactions between
tobacco smoke and other materials do not form a comprehensive body of
work on the topic. A number of combinations of exposures between smoke
and other agents have been studied. Results are at times inconsistent
or contradictory, and the mechanisms by which interactions occur are
often not understood. This section provides a brief review of the
literature. In general, the existing work: (a) was generally performed
using rodents; (b) usually (but not always) examined the effects of
cigarette smoking (or components of the smoke) combined with either
with specific chemical components of the smoke or with radiation; and
(c) usually examined cancer as the biological response end-point of
interest. Other studies have focused on the use of cigarette smoke
components or condensates, and have used models such as in vitro
cell systems or mouse skin; a discussion of these studies is beyond
the scope of this section.
3.10.1 Non-cancer end-points
Several studies in experimental animals have examined the effects of
cigarette smoke administered over short periods of time (from hours to
daily exposures over several weeks). The cigarette smoke-induced
increase in the number of pulmonary macrophages and leukocytes noted
in humans has also been seen in animals such as the guinea-pig, even
after short exposures (Rylander et al., 1979). In addition, Morimoto
et al. (1993) observed a synergistic increase between mineral fibres
and exposure to cigarette smoke in the production of tumour necrosis
factor by rat alveolar macrophages. On the other hand, a 10-week
tobacco smoke exposure in rats suppressed radiation- induced pulmonary
inflammation (Nilsson et al., 1992), and a 12-week exposure of rats to
smoke did not influence the lung damage caused by an intratracheal
instillation of cadmium (Lai & Diamond, 1992).
Nishikawa et al. (1992) studied in guinea-pigs the effects of combined
exposure and single exposure to ozone and cigarette smoke on airway
responsiveness and tracheal vascular permeability and found that the
combined exposure increased airway responsiveness and vascular
permeability to a greater extent in terms of magnitude, but not in
duration, than a single exposure. This indicated that combined
exposure was more harmful than exposure to either agent alone.
3.10.2 Cancer studies: tobacco (cigarette) smoke plus other chemicals
Mori (1964) studied rats receiving multiple subcutaneous injections of
the carcinogen 4-nitroquinoline-1-oxide (NQO) with or without 6-7
month inhalation of cigarette smoke. Six of eight rats had lung
carcinomas in the combined exposure group compared to 3 of 9 rats in
the NQO-only group, and tumours occurred earlier. Davis et al. (1975)
studied Wistar rats receiving single intratracheal instillations of
benzo( a)pyrene with or without cigarette smoke inhalation for most
of the lifespan. Slight elevations of pulmonary squamous neoplasia
were noted in the combined exposure group compared with the individual
agents alone. However, the effects were not statistically significant.
In mice, inhaled cigarette smoke did not influence the occurrence of
lung tumours in (a) B6C3F1 mice pretreated with 3-methylcholanthrene
or benzo( a)pyrene (Henry & Kouri, 1984), (b) C57BL mice receiving
benzo( a)pyrene or influenza virus(Harris & Negroni, 1967), (c) A/J
mice receiving intraperitoneal injections of the tobacco specific
nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and a
6-month inhalation of smoke using a whole-body mode of exposure (Finch
et al., 1996), or (d) A/J mice exposed to sidestream smoke plus the
carcinogen 3-methylcholanthrene or urethane (Witschi et al., 1997). On
the other hand, a carcinogenic synergism in lung adenoma formation was
reported in Strain A mice some 6 months after initiation of treatment
with intraperitoneally injected urethane and skin-painted cigarette
smoke tar (DiPaolo & Sheehe, 1962). However, the non- pulmonary route
of exposure makes it difficult to interpret the relevance of this
result.
Several studies in hamsters have combined cigarette smoke exposure
with other agents. Dontenwill et al. (1973) combined smoke exposure
with a single intratracheal instillation of asbestos, but found no
significant differences in laryngeal lesions or tumours in the group
exposed to both materials, compared with the group receiving smoke
alone; the asbestos alone was non-tumorigenic. The combination of a
single intratracheal administration of dimethylbenz( a)anthrene
(DMBA) and smoke was found to increase the tumour incidence in the
oral cavity, pharynx, trachea and non-pulmonary tissues, compared to
the individual agents alone (Dontenwill et al., 1973). A similar
synergism between DMBA and smoke in producing laryngeal papillomas and
other oral and laryngeal lesions was reported by Hoffmann et al.
(1979) and by Kobayashi et al. (1974), and a synergism was also noted
between the nitrosamine DENA and smoke (Dontenwill et al., 1973).
Hamsters pretreated with a single dose of either 1.0, 3.3 or 10.0 mg
of NNK, and subsequently exposed twice daily for up to 72 weeks to
diluted cigarette smoke developed significantly more tumours in the
respiratory tract than hamsters receiving the identical dosage of NNK
and subjected to sham-smoking (Hecht et al., 1983). This supports the
concept that tobacco smoke has tumour-promoting activity. A
combination of smoke plus benzo( a)-pyrene adsorbed onto haematite
produced tracheal and laryngeal hyperplasia, whereas either agent
alone did not (Hoffmann et al., 1979), but no tumours were produced.
3.10.3 Cancer studies: cigarette smoke plus radiation
Dogs were used to study the potential interactions between radon and
cigarette smoke inhalation in producing lung cancer (Cross et al.,
1982). The lung tumour incidence in dogs exposed to radon plus
cigarette smoke for 4-5 years was decreased compared to that of dogs
receiving a mixture of radon, radon daughters, and uranium ore dust.
Studies have examined the potential interactions between tobacco smoke
and the alpha-emitters radon or plutonium-239 in rats or mice. In a
series of studies, Chameaud et al. (1982) examined the effects of
combined exposures to radon and cigarette smoke in Sprague-Dawley
rats, as well as the effects of pre-treatment with either radon or
smoke exposure. Smoke exposures begun before radon exposure did not
influence the radon-induced lung tumour incidence, but this increased
by 2- to 3-fold in the combined exposure group receiving radon before
cigarette smoke compared to the group receiving radon alone.
Finch et al. (1995a) examined F-344 rats receiving a single inhalation
exposure to 239PuO2, combined with chronic (up to 30 months)
cigarette smoke exposure, and found a synergistic crude tumour
incidence in rats receiving both agents, compared with animals
receiving the radiation alone. Most of these effects could be
explained by a cigarette-smoke-induced retardation of lung clearance
of the 239PuO2 particles (Finch et al., 1995b). This group is
continuing to study the potential combined effects of smoke and X-ray
exposure in rats, and the potential effects of smoke combined with
either X- or alpha-irradiation in mice (Finch et al., 1995a). Talbot
et al. (1987) reported results of a study in which mice inhaled
cigarette smoke, 239PuO2 or both materials. As in the case of rats,
cigarette smoke exposure caused retarded lung clearance and thus led
to greater radiation doses in animals receiving both agents.
4. EFFECTS OF EXPOSURE TO TOBACCO SMOKE AND OTHER AGENTS: SEPARATE
EFFECTS OR POSSIBLE INTERACTIONS
4.1 Coal mining
4.1.1 Coal dust
Coal miners can suffer from chronic bronchitis, coal workers
pneumoconiosis, progressive massive fibrosis, and emphysema. The
respiratory impairment appears as radiologically visible and
functional changes in the lungs, but although some of these are
associated solely with coal dust, some are also closely associated
with smoking. In the past it has been difficult to apportion
attributable risk to the two causes due to the fact that coalface
workers, the group subjected to the highest dust exposure, have a
different smoking pattern and perhaps a different daily consumption of
smoking materials than non-coalface workers because smoking in the
mine is forbidden. With the limitation of dust in modern mining, the
effect of the two factors, dust and smoking, is becoming clearer.
However, difficulties arise in interpreting data because non-smokers
may accumulate more dust, as they have less absenteeism, a different
pattern of lung clearance and a longer life (NIOSH, 1995).
In most of the countries surveyed, the prevalence of smoking by miners
tends to have been somewhat higher than in either the male population
as a whole, or among most other occupational groups, although a
distinction is seldom drawn between miners and coal miners except in
studies centred on coal mining populations. Between 1963 and 1975 in
the United Kingdom, the smoking prevalence fell for the male
population (and for miners) from 54% (miners 77%) to 47% (miners 49%)
(Lee, 1976). The figures for 1988 and 1990 were 33% and 31% (Bennett
et al., 1996) and for face-trained coalminers 35.7% in 1989 (Elliott,
1995). In a study of 8555 American miners from 29 bituminous coal
mines (Kibelstis et al., 1973), over 50% were smokers and 25% were
ex-smokers. In a United Kingdom study (Love & Miller, 1982), only 13%
of 1677 coal miners from 5 British collieries, who were examined in a
lung function study, were non-smokers; 66% were regular smokers and
the remainder were intermittent or ex-smokers. In a 20 year follow-up
study of a population of coal British miners and others (Cochrane &
Moore, 1980), 69% of the coal miners were smokers. These examples
typify the smoking prevalence of coal miners prior to the early
1980's. A 1982 survey of 800 000 American men and women in relation to
their occupations (Stellman et al., 1988) found that among miners
(type unspecified) 29.4% had never smoked regularly, 31.5% were
current smokers and 39.1% were former cigarette smokers. This may
reflect a general trend in the countries with higher income economies
where smoking has been decreasing in many sections of the population.
4.1.2 Bronchitis in coal miners
Kibelstis et al. (1973) found that the prevalence of bronchitis in
coal miners who smoked was higher than in non-smoking coal miners.
Coalface workers had more bronchitis and more airway obstruction than
surface workers and the difference between smokers working at the
coalface and non-smoking surface workers showed that the effect of
smoking was five times greater than that of coal dust. A German
epidemiological evaluation of chronic obstructive bronchitis in 5605
miners, 1276 ex-miners and 3898 individuals who had never worked in a
mine showed that smoking has a more serious effect on miners than on
other groups (Roth et al., 1985). It is possible that, within a mining
community, the other two groups may have a predisposition to the
disease and have achieved their status by job selection or job escape.
In a study of new entrants into coal mining McLintock (1971) found
that those who drop out of mining tend to be less physically fit and
more prone to chest problems than those who remain.
Morgan (1982) analysed the effects of cigarette smoking, dust exposure
and environmental factors on respiratory disease, and concluded that
bronchitis and airways obstruction were two separate responses to
cigarette smoking. The airflow obstruction found in smokers is due to
small airways disease and an involvement of respiratory bronchioles
leading to the development of emphysema. In coal miners, the
prevalence of bronchitis among non-smokers is related to the degree of
dust exposure. Marine et al. (1988) analysed data from studies on 53
382 coal miners in the United Kingdom and found that smoking miners
were at greater risk of developing chronic bronchitis. In a study by
Selig & Nestler (1985) of the relationship between chronic bronchitis,
smoking and dust (unspecified source), heavy smoking was equated with
20 years of dust exposure. From postmortem examinations of coal mine
workers, a correlation was reported between clinical chronic
bronchitis and smoking (Selig & Nestler, 1985).
The chronic bronchitis of coal miners is probably a combination of (a)
mucus hypersecretion caused by dust; (b) mucus hypersecretion, mucus
modification and clearance impairment caused by tobacco smoke; (c)
small airways disease caused by tobacco smoke; and (d) the effect of
dust on small airways tissue already inflamed by smoking. Bronchitis
due to smoking causes mucus hypersecretion which is much greater than
that due to coal dust (Morgan, 1982). Cigarette smoke impairs lung
clearance by changing the physical and chemical properties of mucus
and causing ciliastasis. Rheological measurement show changes in the
viscoelastic properties of mucus; chemically the mucus glycoprotein
structure is changed (King et al., 1989) and the irritant gases in
smoke cause abnormal mucus secretion and ciliastasis (Holbrook, 1977).
Small airways disease and bronchiolitis, leading to emphysema, are due
to smoking rather than to dust (Morgan, 1982).
4.1.3 Emphysema and pneumoconiosis in coal miners
Dust in coal mining is considered to be the primary cause of coal
workers pneumoconiosis. In a study of coalworkers and non-coalworkers,
Cockroft et al. (1982) concluded, after taking any effect of smoking
into account, that there was a 7-fold excess of emphysema in
coalworkers. Results of postmortem examinations of 866 Australian
miners (Leigh et al., 1983) showed a positive correlation between dust
exposure and emphysema and pneumoconiosis, with the severity highest
in non-smokers. However, smoking and non-smoking coalface workers were
not compared. From a postmortem comparison of lungs from 450 coal
miners, Rockley et al. (1984) found that emphysema occurred more
frequently in smokers (72%) than in ex-smokers (65%) or in non-smokers
(42%) and the relative frequency increased with age at death. The
study considered the possibility that coal dust might cause emphysema
which inhibits clearance and, in turn, promotes fibrosis, or
alternatively that fibrosis caused by dust increases the chance of
emphysema. However, the findings of a study of South Wales coal miners
(Fletcher., 1972) militated against dust-induced emphysema. It has
been suggested that differences in emphysema between coalworkers and
non-coalworkers can be accounted for by taking into account current
smokers in the two groups. Morgan (1982) concluded that the evidence
militates against obstructive emphysema occurring more commonly in
coal miners than in the general population, or that more dust
inhalation leads to a greater likelihood of emphysema developing.
Reviews of small airways disease (SAD) suggested that emphysema
proceeds from smoking-induced SAD. Cosio et al. (1980) considered that
their observations supported the hypothesis that SAD is causally
related to centrilobular emphysema, but not necessarily to panlobular
emphysema.
4.1.4 Lung cancer in coal miners
Perhaps as a result of failure to control confounding factors, there
has been a lack of consistency among reports on the relationship
between coal mining and lung cancer incidence in miners. In a direct
evaluation of the relationship between lung cancer mortality and coal
mine dust exposure, controlling for smoking status, Ames et al. (1983)
found no evidence of a link between coal mine dust exposure and lung
cancer risk, nor of an interaction effect, although the expected lung
cancer risk in cigarette smokers was observed. From a study of dust
exposure, pneumoconiosis and mortality of coal miners (Miller &
Jacobsen, 1985) it was found that lung cancer mortality among miners
who smoked was 5.5 times higher than in "never smokers" but that the
effect was entirely due to smoking.
Radon and radon daughter contamination of the dust in coal mines might
be expected to be as prevalent as in all other mines, and thus the
apparent very low lung cancer risk in coal mining may seem unexpected.
However, because of the explosion risk in coal mines, the ventilation
is usually efficient and a build-up of radioactivity is probably less
likely than in other types of mine.
4.2 Other mineral dusts
4.2.1 Talc
Talc is a hydrated magnesium silicate, often contaminated with free
silica or fibrous asbestos-like minerals such as tremolite and
anthophyllite. The only significant difference in the effects on
exposed and non-exposed was in the number and severity of cases of
dyspnoea in the talc workers, and smoking was considered to be an
aggravating factor (Kleinfeld et al., 1973).
4.2.2 Kaolin
Kaolin (pure China clay) is a hydrated aluminium silicate used for
ceramics and as a filler in the paper, rubber and paint industries.
The dry powder can give rise to fibrotic nodules in the lungs (Seaton
et al., 1981; Wagner et al., 1986). Characteristic smoker's inclusions
have been seen in transmission electron micrographs of pulmonary
alveolar macrophages obtained from cigarette smokers. The contents of
these inclusions are heterogeneous and include electron-dense areas,
lipid material and needle-like structures. These have properties
consistent with the composition of kaolinite. Kaolinite is present in
cigarette smoke from different brands, and pulmonary alveolar
macrophages are able to ingest this material in vivo (Hocking &
Golde, 1979).
4.2.3 Alumina
Alumina (aluminium oxide) is extremely hard and is used as an abrasive
(corundum). A cross-sectional study of 788 employees of an aluminium
production company examined the relationship of radiographic
abnormalities to smoking and dust exposure during bauxite and alumina
mining and refining (Townsend et al., 1988). Chest radiographs showed
a moderate time trend of increasing prevalence of small opacities in
non-smokers with high cumulative dust exposures. In most exposure
categories, smokers had a higher prevalence of opacities than
non-smokers. For cumulative exposures of less than 100 mg/m3-years,
increasing trends with duration of exposure were accentuated in
smokers as compared to non-smokers. The stronger effects observed in
smokers were attributed to the joint effects of duration of smoking
and duration of occupational exposure (Townsend et al., 1988).
4.3 Fibrous minerals
Fibrous minerals have been implicated in pleural thickening, pulmonary
fibrosis, mesothelioma and lung cancer in some villages in the
Anatolian region of Turkey (Artvinli & Baris, 1979), where fibrous
zeolite minerals (chabazite and erionite) are present in volcanic
deposits and used in buildings. Erionite has been shown to induce
mesothelioma. The symptoms and pathology of the respiratory disorders
and malignant disease were similar to those of asbestos. Asbestos-type
diseases have also been described in communities exposed to zeolite
minerals and tremolite dust in other similar regions by Baris et al.
(1979) and Yazicioglu et al. (1980). Non-asbestos fibrous materials
have been associated with pulmonary fibrosis (Stanton et al., 1977).
There are no data on any interaction of these minerals with smoking
but there appears to be a potential for interaction.
Wollastonite is a fibrous monocalcium silicate, which has been used as
a substitute for asbestos, as a filler and flux in ceramics, in
grinding wheels, refractory products, building blocks and acoustic
tiles. It is weakly fibrogenic. Hanke et al. (1984) studied a small
population of workers exposed to wollastonite and attributed
significant levels of chronic cough, phlegm and bronchitis to smoking
and not to exposure to wollastonite.
4.4 Metals
4.4.1 Antimony
Antimony is chemically similar to arsenic. Arsenic is metalloid,
antimony is a metal, both have volatile hydrides and form halogen,
oxygen and sulfur derivatives. Compounds of both frequently occur
together, particularly in smelter fume. Biological effects are similar
to those of arsenic (De Wolff & Edelbroek, 1994; De Wolff, 1995).
The concentrations of antimony, arsenic, cadmium, chromium, cobalt,
lanthanum, lead, selenium and zinc measured in lung tissues of
deceased smelter workers suggested that lung cancer risk was
multifactorial, involving carcinogenic and anti-carcinogenic factors
(Gerhardsson & Nordberg, 1993). A 30-year study at an antimony smelter
did not specifically implicate antimony as the cause of excess lung
cancer because of concurrent exposure to other carcinogens (Jones,
1994). In another study of smelters, the data suggested an increase in
lung cancer and non-malignant respiratory and heart disease (Schnorr
et al., 1995).
Antimony can have harmful effects on lung tissues, including
pneumoconiosis. IARC (1989a) evaluated antimony trioxide and antimony
trisulfide and concluded that the trioxide was possibly carcinogenic
to humans (Group 2B) and the trisulfide was not classifiable (Group
3). It is likely that the effects of inhalation of antimony fume/dust
and tobacco smoke would be worse than inhaling either separately. The
similarities of antimony to arsenic, both chemically and in some
biological effects, leads to the conclusion that tobacco smoke and
antimony could interact like tobacco smoke and arsenic in producing
toxicity.
4.4.2 Cadmium
Most zinc and lead-zinc ores contain small amounts of cadmium. It is
used in electroplating, in metal alloys (with copper for overhead
wires, and aluminium for casting), in nickel-cadmium dry cells, for
pigment manufacture and use, added to silver to prevent staining, and
it is a hazard of welding. The main route of exposure for the
non-smoking general population is via food, while for exposed workers
it enters the body mainly by inhalation. Tobacco is an important
source of cadmium in smokers (IPCS, 1992), and the tobacco source
affects the level of cadmium exposure (Yue, 1992). Any intake is
important because cadmium has an extremely long biological half-life.
It was suggested (Hassler et al., 1983) that higher levels of cadmium
in the blood and urine of exposed workers could arise both from
workplace contamination of cigarettes and transfer as fume during
smoking.
Cadmium has various toxic effects, the earliest being impairment of
renal tubular function leading to failure of resorption and excretion
of low molecular weight protein, glycosuria, aminoaciduria and
hypercalciuria (IPCS, 1992; IARC, 1993). It has been associated with
some types of lung disorder (emphysema, obstructive pulmonary disease
and diffuse fibrosis) (IPCS, 1992) Exposure to cadmium compounds has
been associated with cancer of the lung. There is some evidence for an
association with prostatic cancer (IARC, 1993). Other epidemiological
studies did not confirm an increased risk of prostatic cancer
(Kazantzis et al., 1992). The evaluation by IARC (1993) concluded that
cadmium and cadmium compounds are carcinogenic to humans (Group 1).
The supposition that cadmium in cigarette tobacco or in the workplace
may cause lung cancer has been questioned (Lamm et al., 1992;
Hertz-Picciotto & Hu, 1994). In a cohort mortality study of cadmium
workers in England, an observed increase lung cancer risk could not be
attributed strictly to cadmium due to the presence of multiple
confounding factors, particularly arsenic (Kazantzis et al., 1992).
Lamm et al. (1992) also suggested that arsenic may be responsible for
the observed lung cancer increased. The many elements in the lung
tissues of deceased smelter workers (Gerhardsson & Nordberg, 1993)
illustrates the difficulty in apportioning a role to one material in a
multifactorial environment.
Cadmium affects the myocardium and produces hypertension in animal
studies but the induction of cardiovascular disease and hypertension
in humans has not been demonstrated in epidemiological studies
(Kristensen, 1989; IPCS, 1992)). Blood and urine cadmium levels are
higher in smokers than in non-smokers and were found to be
considerably elevated in smokers working in an alkaline battery
factory (Hassler et al., 1983) and in smelter workers who were also
smokers (Kazantzis & Armstrong, 1984; Lilis et al., 1984a,b).
Davison et al. (1988) reported a cadmium dose/effect relationship in
functional and radiological evidence of emphysema in 101 subjects.
Leduc et al. (1993) described the very rapid development of emphysema
in a smoker after exposure to very high levels of cadmium. Smoking by
cumulatively increasing the body burden and hindering the lung
clearance may have provided an additional cause for the emphysema.
However, an additive or synergistic mechanism for the cadmium plus
smoking effect could not be inferred in this case because of the high
cadmium dose.
4.4.3 Cobalt
Cobalt is used in the production of alloys, tungsten carbide tools,
permanent magnets, and in the electrical industry.
Cobalt has toxic effects (Beliles, 1994). Cobalt exposure has been
linked to various allergic reactions (Shirakawa et al., 1992); hard
metal exposure and smoking together arithmetically increased total IgE
levels. Interstitial lung disease has been associated with cobalt in
susceptible individuals (Sprince et al., 1988), although in a study
involving the manufacture of permanent magnets (Deng et al., 1991)
abnormalities in pulmonary function and respiratory symptoms were no
higher than those of a reference population, except for 4 subjects out
of 362, who showed diffuse patches consistent with pneumoconiosis.
4.4.4 Lead
Lead is used in batteries, paint, glass, ceramics, fuel additives and
other industrial applications. In lead-using industries the main route
of exposure is by inhalation, mainly as dust and fume. Lead has a
range of toxic effects on blood, and the renal and nervous systems
(IPCS, 1995).
Levels of lead in blood vary from one area to another, between urban,
rural and occupationally exposed populations, and between men and
women (IPCS, 1995). The tobacco plant absorbs lead from the soil and
around 5-6% of that in cigarettes is inhaled in the smoke. Lead
concentrations in the smoke from one cigarette were found to range
from 0.017 to 0.98 µg (IARC, 1986). Higher blood lead and erythrocyte
protoporphyrin levels have been demonstrated in heavy smokers exposed
to lead (Williams et al., 1983; Landrigan & Straub, 1985); these could
have been partly due to contaminated cigarettes acting as vectors.
Other studies on occupationally exposed workers showed a progressive
increase in blood lead with an increase in the number of cigarettes
smoked (Maheswaren et al., 1993).
The association between lead exposure, tobacco smoke exposure and
blood pressure was examined in a cross-sectional study on 809 men
occupationally exposed to lead in a battery factory but only a small
increase in systolic blood pressure was found (Maheswaren et al.,
1993). There was no evidence of interactive effects between smoking
and lead exposure, but the absorbed lead from cigarettes added to the
body burden.
4.5 Rubber industry
Some of the principal hazards in the industry are fumes, talc, carbon
black, chemical additives and organic solvents but the components of
the hazard mixture differ between different areas of work. A high risk
of pulmonary disease has been reported in the rubber industry. It was
elevated for smokers, particularly those employed in areas where there
were respirable particulates and/or solvents (Lednar et al., 1977).
The data suggested an interaction between smoking and hazards
encountered in mixing (particulates), extrusion (solvent sprays and
mould release agents), and curing (solvents and rubber reaction
products). A problem in epidemiological studies in the industry arises
because of movement of workers between jobs. Some high-risk workers
who were also smokers were involved in finishing and inspection but
they tended to be older employees who had worked in other areas before
moving to this particular job. Emphysema was the principal pulmonary
condition leading to premature termination of employment (Lednar et
al., 1977).
IARC (1987) classified the rubber industry as Group 1, based on
sufficient evidence for carcinogenicity to humans. Excess mortality
from cancers of various sites, the site usually being associated with
the nature of the work and types of exposure, has been reported with
bladder cancer being associated with exposure to aromatic amines (Fox
et al., 1974; Monson & Nakano, 1976a,b; Monson & Fine, 1978;
Kilpikari, 1982; IARC, 1987; Zhang et al., 1989; Weiland et al.,
1996). Lung cancer was associated with curing and inner tube
manufacture. The use of talc in the rubber industry has been
associated with pulmonary disease (Kleinfeld et al., 1973). In the
rubber industry the relative risk of lung cancer for talc-exposed
workers was 3.2 for men and 4.4 for women (Zhang et al., 1989),
compared with 2.5 times excess lung cancer risk in talc-using
industries not associated with rubber manufacture (Thomas & Stewart,
1986). In jobs where very high lung cancer rates were found, smoking
levels were also very high, but any possible interaction effect from
the two exposures could not be assessed.
Gastrointestinal cancer, bladder cancer and leukaemia were found to be
associated with jobs in the rubber industry (Monson & Fine, 1978;
IARC, 1987). Possible causes, such as exposure to carbon black,
plasticisers, antioxidants, arylamino compounds and benzene have been
suggested. Urine samples from rubber workers showed a higher mutagenic
activity in the middle of a working week than at the beginning of a
week in both smokers and non-smokers, indicating the presence of
mutagens in the work environment. In a study involving the analysis of
urine from rubber workers for mutagenic factors, a possible
synergistic effect of smoking and occupational exposure was found in
smokers (Wicklund et al., 1988). There was a relationship between skin
contamination and urinary mutagenicity (Bos et al., 1989). Other
studies involving the analysis of urine from rubber workers for
mutagenic factors also suggested a possible synergistic effect of
smoking and occupational exposure among smokers (Falk et al., 1980;
Crebelli et al., 1985).
Andjelkovich et al. (1988) carried out a case-control study of lung
cancer in workers at a rubber manufacturing plant. There was an
association between lung cancer mortality risk and work in certain
areas for smokers and non-smokers, and the risk was greater in
smokers. Zhang et al. (1989) studied a cohort of 957 men and 667 women
employed in a rubber factory. The relative risk of lung cancer for
smokers was 8.5 for men and 11.4 for women, and for those exposed to
curing agents or talc the relative risk was 3.2 for men and 4.6 for
women. Additive and multiplicative models were used to evaluate the
interaction between smoking and occupational exposure on lung cancer.
The additive interaction term was not statistically significant and
the multiplicative interaction was negative. Weiland et al. (1996)
were not able to find interactions between smoking and exposure in the
rubber industry.
4.6 Petroleum industry
Petroleum refining involves exposure of workers to a large number of
chemical compounds occurring in crude oil or encountered in production
processes as intermediates, catalysts, additives or in the final
products. Because of fire risk, there are sections of the industry
where smoking is not permitted. However, in a study of 10 923 male and
624 female employees of the Australian petroleum industry between 1981
and 1984, it was found that the smoking habits did not differ
substantially from those of the general population (Christie et al.,
1986), and continued surveillance of these workers showed that
mortality rates were lower than for the general population (Christie
et al., 1987).
IARC (1989b) concluded that there is limited evidence that working in
petroleum refineries entails a carcinogenic risk. This limited
evidence applies to skin cancer and leukaemia; for all other cancer
sites on which information was available, the evidence was inadequate.
The overall evaluation, taking account of sufficient or limited
evidence in experimental animals for the carcinogenicity of various
distillates produced during petroleum refining, was that occupational
exposures in petroleum refining are probably carcinogenic to humans
(Group 2A).
In a study of 92 men with histologically confirmed renal cell
carcinoma (Domiano et al., 1985), it was concluded that there could be
an interaction between long-term gasoline exposure and heavy smoking.
In a case-control study of bladder cancer in New Jersey, USA, Najem et
al. (1982) found a significantly elevated risk of bladder cancer in
patients who had worked in the petroleum industry (OR, 2.5, CI,
1.2-5.5). While the risk was increased in current smokers (OR, 2.6),
it was higher in "never smokers" (OR, 5.6) and only slightly elevated
in ex-smokers (OR, 1.4). In another hospital-based study in Argentina
an association was found between bladder cancer and oil refinery work,
along with an elevated risk of lung cancer in smokers, but the numbers
were too few for any evaluation of the interaction (Iscovich et al.,
1987).
There was no increased mortality from either kidney cancer or
leukaemia in employees exposed to gasoline (Wong et al., 1993). A low
frequency of bladder cancer among refinery workers was attributed to
an assumed lower level of smoking among the group of workers studied
(Higginson et al., 1984).
In the study of workers occupationally exposed to gasoline vapour, the
sister chromatid exchange (SCE) frequency in peripheral blood
lymphocytes was used as an indicator of genotoxic response. Workers
employed in gasoline retail outlets were classified according to
cigarette smoking habits. The SCE frequency in lymphocytes was
unaffected by cigarette smoke or gasoline exposure alone, but
increased with combined exposure. The increased SCE frequency observed
in smokers occupationally exposed to gasoline vapour could be due to
the activation of hepatic enzymes by cigarette smoke, leading to a
greater formation of reactive metabolites of gasoline vapour (Edwards
& Priestly, 1993).
4.7 Pesticides
A variety of chemical compounds, natural and synthetic, are used as
pesticides. The number of individuals exposed during manufacture is
relatively small, and processes are usually contained, but the
worldwide population of pesticide users is very large. Most of the
world's population is exposed to pesticide residues in food and
drinking-water (Ecobichon, 1995). There is some information concerning
effects on health from interaction of pesticide exposure and smoking.
"Vineyard sprayer's lung", is an occupational disease associated with
the spraying of copper-sulfate-based Bordeaux Mixture. In a study of
the cytological changes in the respiratory tract of vineyard workers
spraying Bordeaux Mixture (Plamenac et al., 1985), the macrophages of
control subjects contained no copper whereas copper was detected in
64% of the sprayers. Abnormal cytological changes in the sputum were
found in smokers, in sprayers and controls, and atypical squamous
metaplasia of the respiratory epithelium was observed in 29% of the
sprayers who were smokers.
In a study to evaluate the hypothesis that exposure to lead arsenate
resulted in an excess mortality from lung cancer, Wicklund et al.
(1988) compared 155 male orchard workers who had died of respiratory
cancer with 155 orchard workers who had died of other causes. Two
groups of non-orchard workers (620) were used as matched controls.
There was no difference in lead arsenate exposure between the orchard
worker group. Although cigarette smoking was common among the orchard
workers, their smoking habits were similar to the non-orchard worker
control groups. In both groups of orchard workers mortality from
respiratory cancer was higher in smokers than in non-smokers.
McDuffie et al. (1990) examined the possibility that pesticide use was
related to the risk of primary lung cancer. In a case-control study
using data from a population-based cancer registry, they interviewed
273 men and 103 women with diagnosed primary lung cancer and compared
their occupational exposures, medical history and working
characteristics with 187 male community control subjects. There was no
correlation of lung cancer risk with exposure to pesticides and
adjusting for smoking did not alter this.
5. CONCLUSIONS AND RECOMMENDATIONS
5.1 Conclusions
1. Tobacco use, particularly smoking, is the single most important
public health hazard in the world today, and a major preventable
cause of morbidity and mortality.
2. In addition to the adverse health effects of active tobacco use,
adverse health effects have also been demonstrated as a result of
exposure to environmental tobacco smoke.
3. The risks from tobacco smoke are demonstrably increased through
interactions with certain chemical, physical and biological
hazards found in the workplace and general environment.
4. Based on this review of the scientific literature, additional
interactive effects, not yet identified, may exist.
5. In addition to synergistic interactions between tobacco smoke and
other agents, a few instances of antagonistic interactions were
also noted. However, even in these cases, the health risks of
tobacco smoke far outweigh any apparent protective effects.
5.2 Recommendations for protection of human health
1. All possible measures should be taken to eliminate tobacco use,
particularly smoking.
2. In order to avoid interaction with occupational exposures, and
eliminate the risks of exposure to environmental tobacco smoke,
smoking in the workplace should be prohibited.
3. Smoking in public places should be strongly discouraged.
4. In order to reduce the risks of exposure to environmental tobacco
smoke, particularly for children, smoking in domestic
environments should be strongly discouraged. Such action will also
avoid possible deleterious interactions between tobacco smoke and
residential exposures to other hazards.
5. Awareness-building through active educational programmes on the
health hazards of smoking should be undertaken in both developed
and developing countries. This should include enhanced
communication about deleterious interactions between tobacco and
other agents. Governments, industry, health and educational
professionals, and the general public should share in this
responsibility.
6. Since smoking may result in altered response or adverse reactions
to drugs and other therapeutic treatments, appropriate dose
adjustments and patient surveillance should be taken into
consideration by clinicians.
7. Health professionals should provide assistance to smokers to stop
smoking. This may necessitate the allocation of additional
resources for this purpose.
6. FURTHER RESEARCH
1. A number of different methodological approaches to investigating
interactions currently exist. The feasibility of harmonizing
methodologies for the assessment of potential interactions
between two or more health hazards should be explored. In the
interim, investigators should make every effort to state clearly
which methodologies have been used when reporting their results.
2. In many of the observational studies included in this review,
exposure data were limited. In order to improve future
investigations of interactions among human health hazards, more
complete and accurate exposure assessments should be performed.
3. Further epidemiological investigations of potential interactions
between tobacco smoke and other hazards in both the occupational
and general environments are needed to identify additional
populations at risk. Such interactive effects can lead to risks
higher than would be predicted by separate analyses of the risks
associated with individual hazards.
Epidemiological investigations in countries where the health
effects of tobacco have not been extensively studied previously
are of particular importance. This information would be of value
in characterizing the morbidity and mortality due to tobacco use
in those countries.
4. Additional research is needed to clarify the toxicological
mechanisms by which tobacco smoke leads to adverse health
effects, and by which tobacco smoke and other agents interact.
This information will be of use in the design and interpretation
of epidemiological studies on the health effects of tobacco,
including interactive effects.
5. Additional epidemiological research on the health effects of
passive smoking, particularly carcinogenic, cardiorespiratory and
allergic effects, would be of value in characterizing the effects
of low levels of exposure to tobacco smoke. Interactions between
environmental tobacco smoke and other health hazards also warrant
further investigation.
6. Established biomarker methods should be applied to the monitoring
of workers for the early detection of harmful exposures to toxic
or carcinogenic agents, and, in many areas, new biomarker methods
should be developed to improve hazard identification.
Consideration should be given to evaluating the ongoing exposure
of workers to active or passive tobacco smoke by monitoring
salivary or urinary cotinine, one of the major nicotine
metabolites.
7. A comprehensive review of the adverse health effects of
environmental tobacco smoke should be undertaken. This review
would provide an authoritative summary of the health impacts of
environmental tobacco smoke, as well as interactions between
environmental tobacco smoke and other health hazards.
REFERENCES
Abe S, Ohsaki Y, Kimura K, Tsuneta Y, Mikami H, & Murao M (1982)
Chromate lung cancer with special reference to its cell type and
relation to the manufacturing process. Cancer, 49(4): 783-787.
Abelin T, Segmüller H, & Segmüller G (1990) Tobacco and alcohol
consumption as risk factors of Dupuytren's contracture. In: Tobacco
and health 1990 -- The global war: Proceedings of the 7th World
Conference on Tobacco and Health, Perth, Western Australia, 1-5 April
1990. Perth, Department of Health of Western Australia, pp 449-450.
Abramson MJ, Wlodarczyk JH, Saunders NA, & Hensley MJ (1989) Does
aluminium smelting cause lung disease. Am Rev Respir Dis, 139:
1042-1057.
Acheson ED, Gardner MJ, Winter PD, & Bennett C (1984) Cancer in a
factory using amosite asbestos. Int J Epidemiol, 13(1): 3-10.
Alavanja MC, Brownson RC, Benichou J, Swanson C, & Boice JD (1995)
Attributable risk of lung cancer in lifetime nonsmokers and long-term
ex-smokers (Missouri, United States). Cancer Causes Control, 6(3):
209-216.
Alavanja MC, Brownson RC, Berger E, Lubin J, & Modigh C (1996) Avian
exposure and risk of lung cancer in women in Missouri: population
based case-control study. Br Med J, 313(7067): 1233-1235.
Albrecht WN & Bryant CJ (1987) Polymer-fume fever associated with
smoking and use of a mold-release spray containing
polytetrafluoroethylene. J Occup Med, 29(10): 817-819.
Al-Fayez SF, Salleh M, Ardawi M, & Zahran FM (1988) Effects of sheesha
and cigarette smoking on pulmonary function of Saudi males and
females. Trop Geogr Med, 40(2): 115-123.
Amandus HE, Castellan RM, Shy C, Heineman EF, & Blair A (1992)
Reevaluation of silicosis and lung cancer in North Carolina dusty
trades workers. Am J Ind Med, 22(2): 147-153.
Ames RG, Amandus H, Attfield M, Green FY, & Vallyathan V (1983) Does
coal mine dust present a risk for lung cancer? A case-control study of
US coal miners. Arch Environ Health, 38(6): 331-333.
An HS, Southworth SR, Jackson WT, & Russ B (1988) Cigarette smoking
and Dupuytren's contracture of the hand. J Hand Surg, 13A: 872-874
Anderson HA, Lilis R, Daum SM, & Selikoff IJ (1979) Asbestosis among
household contacts of asbestos factory workers. Ann NY Acad Sci, 330:
387-399.
Andjelkovich DA, Abdelghany N, Mathew RM, & Blum S (1988) Lung cancer
case-control study in a rubber manufacturing plant. Am J Ind Med,
14(5): 559-574.
Andjelkovich DA, Shy CM, Brown MH, Janszen DB, Levine RJ, & Richardson
RB (1994) Mortality of iron foundry workers: III. Lung cancer
case-control study. J Occup Med, 36(12): 1301-1309.
Archer VE (1988) Lung cancer risk in underground miners: cohort
case-control studies. Yale J Biol Med, 61: 183-193.
Armitage AK & Turner DM (1970) Absorption of nicotine in cigarette and
cigar smoke through the oral mucosa. Nature (Lond), 226: 1231-1232.
Armstrong BG, Tremblay CG, Cyr D, & Theriault GP (1986) Estimating the
relationship between exposure to tar volatiles and the incidence of
bladder cancer in aluminium smelter workers. Scand J Work Environ
Health, 12: 486-493.
Artvinli M & Baris YI (1979) Malignant mesotheliomas in a small
village in the Anatolian Region of Turkey: An epidemiologic study. J
Natl Cancer Inst, 63(1): 17-20.
Attali P, Ink O, Pelletier G, Vernier C, Jean F, Moulton L, & Etienne
JP (1987) Dupuytren's contracture, alcohol consumption, and chronic
liver disease. Arch Int Med, 147: 1065-1067.
Autrup JI, Hansen C, & Autrup H (1992) Detection of tobacco smoke
carcinogen-DNA adducts in cultured rat buccal mucosa cells following
exposure to ethanol and total cigarette smoke condensate or chewing
tobacco. Chem-Biol Interact, 85: 141-150
Avolio G, Cacciabue M, Rolla G, Caria E, Oliaro A, Carlino F, Pepino
E, Polizzi S, & Bucca C (1986) [Functional compromise of the small
airways in subjects exposed to SiO2.] Minerva Med, 77(45-46):
2183-2185 (in Italian).
Axéll T, Mörnstad H, & Sundström B (1978) [Snuff and cancer of the
oral cavity.] Lakartidningen, 75: 2224-2226 (in Swedish).
Axelson O (1995) Cancer risks from exposure to radon in homes. Environ
Health Perspect, 103(suppl 2): 37-43.
Badawi AF & Mostafa MH (1993) Possible mechanisms of alteration in the
capacities of carcinogen metabolizing enzymes during schistosomiasis
and their role in bladder cancer induction. J Int Med Res, 21(6):
281-305.
Badawi AF, Mostafa MH, Probert A, & O'Connor PJ (1995) Role of
schistosomiasis in human bladder cancer: evidence of association,
aetiological factors, and basic mechanisms of carcinogenesis. Eur J
Cancer Prev, 4: 45-59.
Baker DB, Gann PH, Brooks SM, Gallagher J, & Bernstein IL (1990)
Cross-sectional study of platinum salts sensitization among precious
metals refinery workers. Am J Ind Med, 18: 653-664.
Ball J & Simpson D (1987) Round the world: India -- Tobacco and ill
health. Lancet, 18: 149.
Baris Y, Artvilini M, & Sahin AA (1979) Environmental mesothelioma in
Turkey. Ann NY Acad Sci, 330: 423-432.
Barone JA, Peters JM, Garabrant DH, Bernstein L, & Krebsbach R (1987)
Smoking as a risk factor in noise-induced hearing loss. J Occup Med,
29(9): 741-745
Bartsch H, Malaveille C, Friesen M, Kadlubar FF, & Vineis P (1993)
Black (air-cured) and blond (flue-cured) tobacco cancer risk. IV:
Molecular dosimetry studies implicate aromatic amines as bladder
carcinogens. Eur J Cancer, 29A(8): 1199-1207
Bates MN, Smith AH, & Cantor P (1995) Case-control study of bladder
cancer and arsenic in drinking water. Am J Epidemiol, 141(6): 523-530.
Battista SP (1976) Ciliatoxic components in cigarette smoke. In:
Proceedings of 3rd World Conference on Smoking and Health, New York
City, 2-5 June 1975 -- Volume 1: Modifying the risk for the smoker.
Washington, DC, US Department of Health, Education and Welfare, pp
517-534 (DHEW Publication No. (NIH) 76-1221).
Baumslag N, Keen P, & Petering HG (1971) Carcinoma of the maxillary
antrum and its relationship to trace metal content of snuff. Arch
Environ Health, 23: 1-5.
Beck GJ, Maunder LR, & Schachter EN (1984) Cotton dust and smoking
effects on lung function in cotton textile workers. Am J Epidemiol,
119(1): 33-43.
Beliles RP (1994) Cobalt. In: Clayton GD & Clayton FE ed. The metals
-- Patty's industrial hygiene and toxicology. New York,
Wiley-Interscience, chapter 27, pp 1986-1999.
Bennett N, Jarvis L, Rowlands O, Singleton N, & Haselden L (1996)
Office of Population Censuses and Surveys, Social Survey Division:
Results from the 1994 general household survey. London, Her Majesty's
Stationery Office (HMSO), pp 92-102.
Benowitz NL, Porchet H, Sheiner L, & Jacob P (1988) Nicotine
absorption and cardiovascular effects with smokeless tobacco use:
comparison with cigarettes and nicotine gum. Clin Pharmacol Ther,
44(1): 23-28.
Berry G, Newhouse ML, & Turok M (1972) Combined effects of asbestos
exposure and smoking on mortality from lung cancer in factory workers.
Lancet, 2: 476-479.
Berry G, Newhouse ML, & Antonis P (1985) Combined effect of asbestos
and smoking on mortality from lung cancer and mesothelioma in factory
workers. Br J Ind Med, 42: 12-18.
Bhonsle RB, Murti PR, Gupta PC, & Mehta FS (1976) Reverse dhumti
smoking in Goa: an epidemiologic study of 5,499 villagers for oral
precancerous lesions. Indian J Cancer, 13(4): 301-305.
Bi W, Hayes RB, Feng P, Qi Y, You X, Zhen J, Zhang M, Qu B, Fu Z, Chen
M, Chien HTC, & Blot WJ (1992) Mortality and incidence of bladder
cancer in benzidine-exposed workers in China. Am J Ind Med, 21(4):
481-489.
Biberman R, Lusky A, Schlesinger T, Margaloit M, Neeman E, & Modan B
(1993) Increased risk for small cell lung cancer following residential
exposure to low dose radon: a pilot study. Arch Environ Health, 48(4):
209-212.
Bien TH & Burge R (1990) Smoking and drinking: A review of the
literature. Int J Addict, 25: 1429-1454.
Bjorseth A, Bjorseth O, & Feldstad PE (1978) Polycyclic aromatic
hydrocarbons in the work atmosphere; I. Determination in an aluminium
reduction plant. Scand J Work Environ Health, 4: 212-223.
Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS,
Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, & Fraumeni
JF Jr (1988) Smoking and drinking in relation to oral and pharyngeal
cancer. Cancer Res, 48: 3282-3287.
Boffetta P, Stellman SD, & Garfinkel L (1988) Diesel exhaust exposure
and mortality among males in the American Cancer Society prospective
study. Am J Ind Med, 14: 403-415.
Bohning DE, Atkins HL, & Cohn SN (1982) Long-term particle clearance
in man: normal and impaired. Ann Occup Hyg, 26: 259-271.
Bolinder G, Alfredsson L, Englund A, & de Faire U (1994) Smokeless
tobacco use and increased cardiovascular mortality among Swedish
construction workers. Am J Public Health, 84(3): 399-404.
Bornmyr S & Svensson H (1991) Thermography and laser-Doppler flowmetry
for monitoring changes in finger skin blood flow upon cigarette
smoking. Clin Physiol, 11(2): 135-141.
Bos RP, Kromhout H, Ikink H, de Haan W, Koppejan J, & Theuws JLG
(1989) Mutagens in urine of non-smoking and smoking workers in an
aircraft tyre retreading plant: Skin exposure as a causal factor.
Mutat Res, 223(1): 41-48.
Bouhuys A & Zuskin E (1976) Chronic respiratory disease in hemp
workers. Ann Intern Med, 84: 398-405.
Bovenzi M, Stanta G, Antiga G, Perruzzo P, & Cavallieri F (1993)
Occupational exposure and lung cancer risk in a coastal area of
northeastern Italy. Int Arch Occup Environ Health, 65(1): 35-41.
Boyko RW, Cartwright RA, & Glashan RW (1985) Bladder cancer in dye
manufacturing workers. J Occup Med, 27(11): 799-803.
Boyle P, Macfarlane GJ, Maisonneuve P, Zheng T, Scully C, & Tedesco B
(1990) Epidemiology of mouth cancer in 1989: a review. J R Soc Med,
83: 724-730.
Boysen M, Solberg LA, Torjussen W, Poppe S, & Hogetveit AC (1984)
Histological changes, rhinoscopical findings and nickel concentration
in plasma and urine in retired nickel workers. Acta Oto-Laryngol,
97(´): 105-115.
Bravo MP, Del Rey Calero J, & Conde M (1987) Risk factors of bladder
cancer in Spain. Neoplasma, 34(5): 633-637.
Bresnitz EA & Strom BL (1983) Epidemiology of sarcoidosis. Epidemiol
Rev, 5: 124-156.
Brinton LA, Blot WJ, Becker JA, Winn DM, Browder JP, & Farmer JC Jr
(1984) A case-control study of cancers of the nasal cavity and
paranasal sinuses. Am J Epidemiol, 119(6): 896-906.
Brown CC & Chu KC (1989) Additive and multiplicative models and
multistage carcinogenesis theory. Risk Anal, 9: 9-105.
Brownson RC, Novotny TE, & Perry MC (1993) Cigarette smoking and adult
leukemia: A meta analysis. Arch Intern Med, 153: 469-475.
Brunnemann KD & Hoffmann D (1974) The pH of tobacco smoke. Food Cosmet
Toxicol, 12: 115-124.
Brunnemann KD & Hoffmann D (1981) Assessment of the carcinogenic
N-nitrosodiethanolamine in tobacco products and tobacco smoke.
Carcinogenesis, 2: 1123-1127.
Brunnemann KD & Hoffmann D (1991) Chemical studies on tobacco smoke
XCC: Decreased concentrations of N-nitrosodiethanilamine and
N-nitrosomorpholine in commercial tobacco products. J Agric Food
Chem, 39: 207-208.
Brunnemann KD & Hoffmann D (1992) Chemical composition of smokeless
tobacco products. Bethesda, Maryland, National Cancer Institute, pp
96-108 (Smoking and Tobacco Control Monographs, No. 2).
Brunnemann KD, Mitacek EJ, Liu Y, Limsila T, & Suttajit M (1996)
Assessment of major carcinogenic tobacco-specific N-nitrosoamines in
Thai cigarettes. Cancer Detect Prev, 20(2): 114-121.
Burch JD, Howe GR, Miller AB, & Semenciw R (1981) Tobacco, alcohol,
asbestos, and nickel in the etiology of cancer of the larynx: A
case-control study. J Natl Cancer Inst, 67: 1219-1224.
Calverley AE, Rees D, Dowdeswell RJ, Linnett PJ, & Kielkowski D (1995)
Platinum salt sensitivity in refinery workers: incidence and effects
of smoking and exposure. Occup Environ Med, 52: 661-666.
Carrillo T, de Castro R, Cuevas M, Diaz F, & Cabrera P (1991) Effect
of cigarette smoking on the humoral immune response in pigeon
fanciers. Allergy, 46: 241-244.
Cartwright R (1982) Occupational bladder cancer and cigarette smoking
in West Yorkshire. Scand J Work Environ Health, 8(suppl 1): 79-82.
Castonguay A, Tjalve H, & Hecht SS (1983) Tissue distribution of the
tobacco-specific carcinogen
4-(methyl-nitrosamino)-1-(pyridyl)-1-butanone and its metabolites in
F344 rats. Cancer Res, 43: 631-635.
Castonguay A, Rivenson A, Trushin N, Reinhardt J, Spathopoulos S,
Weiss CJ, Reiss B, & Hecht SS (1984) Effects of chronic ethanol
consumption on the metabolism and carcinogenicity of
N-nitrosonornicotine in F344 rats. Cancer Res, 44: 2285-2290.
Cecchi F, Buiatti E, Kriebel D, Nastasi L, & Santucci M (1980)
Adenocarcinoma of the nose and paranasal sinuses in shoemakers and
woodworkers in the province of Florence, Italy (1963-77). Br J Ind
Med, 37: 222-225.
Chaffey CM & Bowie C (1994) Radon and health -- an update [Review]. J
Public Health Med, 16(4): 465-470.
Chakrabarti RN, Dutta K, Sikdar S, & Ghosh K (1991) Smokeless tobacco
and premalignant and malignant lesions of the oral cavity. Indian J
Med Sci, 45(10): 273-275.
Chameaud J, Perraud R, Chretien J, Masse R, & Lafuma J (1982) Combined
effects of inhalation of radon daughter products and tobacco smoke.
In: Sanders CL ed. Pulmonary toxicology of respirable particles.
Washington, DC, US Department of Energy, Technical Information Center,
pp 551-557 (CONF-791002).
Chan-Yeung M, Enarson D, & Grzybowski S (1985) Grain dust and
respiratory health. Can Med Assoc J, 133(10): 969-973.
Cheng WN & Kong J (1992) Retrospective mortality cohort study of
chrysotile asbestos products workers in Tianjin 1972-1987. Environ
Res, 59(1): 271-278.
Chiazze L Jr, Watkins DK, & Fryar C (1992) A case-control study of
malignant and non-malignant respiratory disease among employees of a
fibreglass manufacturing facility. Br J Ind Med, 49(5): 326-331.
Chiazze L Jr, Watkins DK, & Fryar C (1995) Adjustment for the
confounding effect of cigarette smoking in an historical cohort
mortality study of workers in a fiberglass manufacturing facility. J
Occup Environ Med, 37(6): 744-748.
Chopra SK, Taplin GV, Elam D, Carson SA, & Golde D (1979) Measurement
of tracheal mucociliary transport velocity in humans -- smokers versus
non-smokers. Am Rev Resp Dis, 119(4): 205 (Abstract).
Cho CH, Chen BW, Hui WM, & Lam SL (1990) The influence of acute or
chronic nicotine treatment on ethanol-induced gastric mucosal damage
in rats. Dig Dis Sci, 35: 106-112.
Chow JYC, Ma L, & Cho CH (1996) An experimental model for studying
passive cigarette smoking effects on gastric ulceration. Life Sci, 58:
2415-2422.
Christie D, Gordon I, & Robinson K (1986) Smoking in an industrial
population: An analysis by birth cohort. Med J Aust, 145: 11-14.
Christie D, Robinson K, Gordon I, Webley C, & Bisby J (1987) Current
mortality in the Australian petroleum industry: the healthy worker
effect and the influence of life-style factors. Med J Aust, 147:
222-225.
Cinkotai FF, Rigby A, Pickering CAC, Seaborn D, & Faragher E (1988a)
Recent trends in the prevalence of byssinotic symptoms in the
Lancashire textile industry. Br J Ind Med, 45: 782-789.
Cinkotai FF, Emo P, Gibbs ACC, & Jouany JM (1988b) Low prevalence of
byssinotic symptoms in 12 flax scutching mills in Normandy, France. Br
J Ind Med, 45: 325-328.
Clausen J, Netterstrom B, & Wolff C (1993) Lung function in insulation
workers. Br J Ind Med, 50(3): 252-256.
Cochrane AL & Moore F (1980) A 20-year follow-up of a population
sample (aged 25-34) including coal-miners and foundry workers in
Stavely, Derbyshire. Br J Ind Med, 37: 230-233.
Cockroft A, Seal RME, Wagner JC, Lyons JP, Ryder R, & Andersson N
(1982) Post-mortem study of emphysema in coalworkers and
non-coalworkers. Lancet, 2(8298): 600-603.
Coggins CRE (1998) A review of chronic inhalation studies with
mainstream cigarette smoke in rats and mice. Toxicol Pathol, 26(3):
307-314.
Cohen SM & Johansson SL (1992) Epidemiology and etiology of bladder
cancer. Urol Clin North Am, 19(3): 421-428.
Cohen D, Arai SF, & Brain JD (1979) Smoking impairs long-term
clearance from the lung. Science, 204: 514-517.
Cohen BS, Harley NH, & Tso TC (1985) Clearance of polonium-210
enriched cigarette smoke from rat trachea and lung. Toxicol Appl
Pharmacol, 79: 314-322.
Collins AC (1990) Interactions of ethanol and nicotine at the receptor
level. Recent Dev Alcohol, 8: 221-231.
Conolly CK, Chan NS, & Prescott RJ (1988) The relationship between age
and duration of asthma and the presence of persistent obstruction in
asthma. Postgrad Med J, 64(752): 422-425.
Cosio MG, Hale KA, & Niewoehner DE (1980) Morphologic and morphometric
effects of prolonged cigarette smoking on the small airways. Am Rev
Respir Dis, 122: 265-271.
Cotton DJ, Graham BL, Li KYR, Froh F, Barnett GD, & Dosman JA (1983)
Effects of grain dust exposure and smoking on respiratory symptoms and
lung function. J Occup Med, 25(2): 131-141.
Crebelli R, Paoletti A, Falcone E, Aquilana G, Fabri G, & Carere A
(1985) Mutagenicity studies in a tyre plant: in vitro activity of
workers' urinary concentrates and raw materials. Br J Ind Med, 42(7):
481-487.
Creek L, Capehart T, & Griese V (1994) US tobacco statistics,
1935-1992. US Dep Agric Stat Bull, 869: 14.
Cromwell BT (1955) Modern methods of plant analysis -- Volume IV: The
alkaloids. Berlin, Gottingen, Heidelberg, Springer-Verlag, pp 367-516.
Cross FT, Palmer RF, Filipy RE, Dagle GE, & Stuart BO (1982)
Carcinogenic effects of radon daughters, uranium ore dust and
cigarette smoke in beagle dogs. Health Phys, 42(1): 33-52.
Cullinan P, Lowson D, Nieuwenhuijsen MJ, Sandiford C, Tee RD, Venables
KM, McDonald JC, & Newman-Taylor AJ (1994a) Work related symptoms,
sensitisation, and estimated exposure in workers not previously
exposed to flour. Occup Environ Med, 51: 579-583.
Cullinan P, Lowson D, Nieuwenhuijsen MJ, Gordon S, Tee RD, Venables
KM, McDonald JC, & Newman-Taylor AJ (1994b) Work related symptoms,
sensitisation, and estimated exposure in workers not previously
exposed to laboratory rats. Occup Environ Med, 51: 589-592.
Cummings KM, Giovino G, & Mendicino AJ (1987) Cigarette advertizing
and black white differences in brand preference. Public Health Rep,
102(6): 698-701.
Damber L & Larsson LG (1985a) Professional driving, smoking and lung
cancer: a case referent study. Br J Ind Med, 42: 246-252.
Damber L & Larsson LG (1985b) Underground mining, smoking and lung
cancer: a case-control study in the iron ore municipalities in
Northern Sweden. J Natl Cancer Inst, 74(6): 1207-1213.
Darby S, Whitley E, Silcocks P, Thakrar B, Green M, Lomas P, Miles J,
Reeves G, Fearn T, & Doll R (1998) Risk of lung cancer associated with
residential radon exposure in south-west England: a case control
study. Br J Cancer, 78(3): 394-408.
D'Avanzo B, Negri E, La Vecchia C, Gramenzi A, Bianchi C, Franceschi
S, & Boyle P (1990) Cigarette smoking and bladder cancer. Eur J
Cancer, 26(6): 714-718.
Davis BR, Whitehead JK, Gill ME, Lee PN, Butterworth AD, & Roe FJC
(1975) Response of rat lung to inhaled tobacco smoke with or without
prior exposure to 3,4-benzpyrene (BP) given by intratracheal
instillation. Br J Cancer, 31: 469-484.
Davison AG, Fayers PM, Newman Taylor AJ, Venables KM, Darbyshire J,
Pickering CA, Chettle DR, Franklin D, Guthrie CJ, Scott MC, O'Malley
D, Holden H, Mason HJ, Wright AL, & Gompertz D (1988) Cadmium fume
inhalation and emphysema. Lancet, 8587: 663-667.
De Klerk NH, Musk AW, Armstrong BK, & Hobbs MS (1991) Smoking,
exposure to crocidolite, and the incidence of lung cancer and
asbestosis. Br J Ind Med, 48(6): 412-417.
Deng JF, Sinks T, Elliot L, Smith D, Singal M, & Fine L (1991)
Characterisation of respiratory health and exposures at a sintered
permanent magnet manufacturer. Br J Ind Med, 48: 609-615.
Devesa SS, Shaw GL, & Blot WJ (1991) Changing patterns of lung cancer
incidence by histological type. Can Epi Bio Prev, 1: 29-43.
De Wolff FA & Edelbroek PM (1994) Neurotoxicity of arsenic and its
compounds. In: de Wolff FA ed. Vinken and Bruyn's handbook of clinical
neurology. Part I. Intoxications of the nervous system. Amsterdam,
Elsevier, pp 283-291.
De Wolff FA (1995) Antimony and health. Br Med J, 310: 1216-1217.
De Zotti R, Larese F, Bovenzi M, Negro C, & Molinari S (1994) Allergic
airway disease in Italian bakers and pastry makers. Occup Environ Med,
51: 548-552.
DiPaolo JA & Sheehe PR (1962) The combined carcinogenic effect of
cigarette smoke condensate and urethan. Cancer Res, 22: 1058-1060.
Djordjevic MV, Sigountos CW, Hoffmann D, Brunnemann KD, Kagan MR, Bush
LP, Safaev RD, Belitsky GA, & Zaridze D (1991) Assessment of major
carcinogens and alkaloids in the tobacco and mainstream smoke of USSR
cigarettes. Int J Cancer, 47: 348-351.
Djordjevic MV, Fan J, Ferguson S, & Hoffmann D (1995) Self-regulation
of smoking intensity: Smoke yields of the low-nicotine, "low-tar"
cigarettes. Carcinogenesis, 16: 2015-2021.
Doll R & Peto R (1981) The causes of cancer: Quantitative estimates of
avoidable risk of cancer in the United States today. Natl Cancer Inst,
66: 1191-1308.
Domiano SF, Vena JE, & Swanson MK (1985) Gasoline exposure, smoking
and kidney cancer. J Occup Med, 27(6): 398-399.
Donham KJ, Zavala DC, & Merchant JA (1984) Respiratory symptoms and
lung function among workers in swine confinement buildings: a cross
sectional epidemiological study. Arch Environ Health, 39(2): 96-101.
Dontenwill W, Chavalier H-J, Harke H-P, Lafrenz U, Rechzeh G, &
Schneider B (1973) Investigations on the effects of chronic
cigarette-smoke inhalation in Syrian Golden Hamsters. J Natl Cancer
Inst, 51(6): 1781-1832.
Ecobichon DJ (1995) Toxic effects of pesticides. In: Klaassen CD ed.
Casarett and Doull's toxicology, 5th ed. New York, McGraw-Hill,
chapter 22, pp 643-689.
Edling C (1982) Lung cancer and smoking in a group of iron ore miners.
Am J Ind Med, 3(2): 191-199.
Edwards JW & Priestly BG (1993) Sister chromatid exchanges in
lymphocytes of petroleum retailers. Br J Ind Med, 50: 149-154.
Eisenbud M (1993) Lung cancer incidence among patients with beryllium
disease. J Natl Cancer Inst, 85(2): 1697-1698.
Ekenvall L & Lindblad LE (1989) Effect of tobacco use on vibration
white finger disease. J Occup Med, 31(1): 13-16.
Eliasson M, Lundblad D, & Hägg E (1991) Cardiovascular risk factors in
young snuff-users and cigarette smokers. J Intern Med, 230(1): 17-22.
Elliott R (1995) Smoking drinking and occupation: Occupational Health.
In: Drever F ed. Office of Population Censuses and Surveys, Health and
Safety Executive: The registrar general's decennial supplement for
England and Wales. London, Her Majesty's Stationery Office (HMSO), pp
195-201.
Elwood MJ (1981) Wood exposure and smoking: Association with cancer of
the nasal cavity and paranasal sinuses in British Columbia. Can Med
Assoc J, 124: 1573-1577.
Enterline PE, Sykora JL, Keleti G, & Lange JH (1985) Endotoxins,
cotton dust, and cancer. Lancet, 2(8461): 934-935.
Enterline PE, Marsh GM, Henderson V, & Callahan C (1987a) Mortality
update of a cohort of US man-made mineral fibre workers. Ann Occup
Hyg, 31: 625-656.
Enterline PE, Marsh GM, Esmen NA, Henderson VL, Callahan CM, & Paik M
(1987b) Some effects of cigarette smoking, arsenic, and SO2 on
mortality among US copper smelter workers. J Occup Med, 29(10):
831-838.
Falk K, Sorsa M, Vainio H, & Kilpikari I (1980) Mutagenicity in urine
of workers in rubber industry. Mutat Res, 79(1): 45-52.
Finch GL, Nikula KJ, Barr EB, Bechtold WE, Chen BT, Griffith WC, Hahn
FF, Hobbs CH, Hoover MD, Lundgren DL, & Mauderly JL (1995a) Effects of
combined exposure of F344 rats to radiation and chronically inhaled
cigarette smoke: Annual report of the Inhalation Toxicology Research
Institute. Washington, DC, Department of Energy (ITRI-146).
Finch GL, Nikula KJ, Chen BT, Barr EB, Chang I-Y, & Hobbs CH (1995b)
Effect of chronic cigarette smoke exposure on lung clearance of tracer
particles inhaled by rats. Fundam Appl Toxicol, 24: 76-85.
Finch GL, Nikula K, Belinsky S, Barr EB, Stoner G, & Lechner J (1996)
Failure of cigarette smoke to induce or promote lung cancer in the A/J
mouse. Cancer Lett, 99: 161-167.
Fletcher CM (1972) Coal workers pneumoconiosis [letter]. Br Med J, 3:
116.
Fletcher CM & Pride NB (1984) Definitions of emphysema, chronic
bronchitis, asthma, and airflow obstruction. Thorax, 39: 81-85.
Fox AJ, Lindars DC, & Owen R (1974) A survey of occupational cancer in
the rubber and cable making industries: Results of five-year analysis,
1967-71. Br J Ind Med, 31: 140-151.
Fraser-Moodie A (1976) Dupuytren's contracture and cigarette smoking.
Br J Plastic Surg, 29: 214-215.
Friedman GD, Siegeland AB, & Seltzer CC (1969) Cigarette smoking and
exposure to occupational hazards. Am J Epidemiol, 98: 175-183.
Friedman S, Fletcher CM, & Field GB (1972) Effects of smoking modified
cigarettes on respiratory symptoms and ventilatory capacity. J Natl
Cancer Inst, 48: 1805-1810.
Fukuda K, Shibata A, & Harada K (1987) Squamous cell cancer of the
maxillary sinus in Hokkaido, Japan: A case-control study. Br J Ind
Med, 44: 263-266.
Gammon MD, Schoenberg JB, Ahian H, Risch HA, Vaughan TL, Chow WH,
Rotterdam H, West AB, Dubrow R, & Stanford JL (1997) Tobacco, alcohol,
and socioeconomic status and adenocarcinoma of the esophagus and
gastric cardia. J Natl Cancer Inst, 89(17): 1277-1284.
Gardiner AJS, Forey BA, & Lee PN (1992) Avian exposure and
bronchogenic carcinoma. Br Med J, 305: 989-992.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1987) A case-control study of lung cancer
and diesel exhaust exposure in railroad workers. Am Rev Respir Dis,
135: 1242-1248.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1988) A retrospective cohort study of lung
cancer and diesel exhaust exposure in railroad workers. Am Rev Respir
Dis, 137: 820-825.
Geng Y, Savage SM, Johnson LJ, Seagrave J, & Sopori ML (1995) Effects
of nicotine on the immune system: I. Chronic exposure to nicotine
impairs antigen receptor-mediated signal transduction in lymphocytes.
Toxicol Appl Pharmacol, 135: 268-278.
Geng Y, Savage SM, Razanai-Boroujerdi S, & Sopori ML (1996) Effects of
nicotine on the immune system: II. Chronic nicotine treatment induces
T cell anergy. J Immunol, 156(7): 2384-2390.
Gerhardsson L & Nordberg GF (1993) Lung cancer in smelter workers
- interactions of metals as indicated by tissue levels. Scand J Work
Environ Health, 19(suppl 1): 90-94.
Gibbs GW & Horowitz I (1979) Lung cancer mortality in aluminium
reduction plant workers. J Occup Med, 21(5): 347-353.
Gibinski K (1977) Studies on cardiovascular effects of smoking in
Poland and other Eastern European countries. In: Proceedings of 3rd
World Conference on Smoking and Health, New York City, 2-5 June 1975
- Volume II: Health consequence, education, cessation and social
actions. Washington, DC, US Department of Health, Education and
Welfare, pp 179-184 (DHEW Publication No. (NIH) 77:1413).
Gonzalez CA, Lopez-Abente G, Errezola M, Castejon J, Estrada A,
Garcia-Mila M, Gili P, Huguet M, Serrat M, Soler F, & Rodriguez C
(1985) Occupation, tobacco use, coffee and bladder cancer in the
county of Mataro (Spain). Cancer, 55: 2031-2034.
Gonzalez CA, Lopez-Abente G, Errezola M, Escolar A, Riboli E,
Izarzugaza I, & Nebot M (1989) Occupation and bladder cancer in Spain:
a multi-centre case control study. Int J Epidemiol, 18(3): 569-577.
Greenberg M, Milne JF, & Watt A (1970) A survey of workers exposed to
dusts containing derivatives of Bacillus subtilis. Br Med J, ii:
629-633.
Greenland S (1993) Basic problems in interaction assessment. Environ
Health Perspect, 101(suppl 4): 59-65.
Grimmer G, Schneider D, Nanjack KW, Deltbarn G, & Jacob J (1995)
Intercept-reactant method for the determination of aromatic amines in
mainstream tobacco smoke. Beitr Tabilk-Forsch, 16: 141-156.
Grove WJ & Stansby GP (1992) Buerger's disease and cigarette smoking
in Bangladesh. Ann R Coll Surg Engl, 74: 115-118.
Guggenheimer J (1991) Implications of smokeless tobacco use in
athletes [Review]. Dent Clin North Am, 35(4): 797-808.
Gullvåg B, Mylius E, & Nilsen A (1985) Counting alveolar macrophages
from expectorate samples of exposed workers as a test for lung
irritation from occupational exposure. Bull Environ Contam Toxicol,
34: 702-708.
Hammond EC & Selikoff IJ (1973) Relation of cigarette smoking to risk
of death of asbestos- associated disease among insulation workers in
the United States. In: Bogovski P, Gilson JC, Timbrell V, & Wagner JC
Biological effects of asbestos. Lyon, International Agency for
Research on Cancer, pp 312-317 (IARC Scientific Publication No. 8).
Hammond EC, Selikoff IJ, & Seidman H (1979) Asbestos exposure,
cigarette smoking and death rates. Ann NY Acad Sci, 333: 473-489.
Hanke W, Sepulveda MJ, Watson A, & Jankovic J (1984) Respiratory
morbidity in wollastonite workers. Br J Ind Med, 41: 474-479.
Harf RA, Ethevenaux C, Gleize J, Perrin-Fayolle M, Guerin JG, &
Ollagnier C (1986) Reduced prevalence of smokers in sarcoidosis:
Results of a case-control study. Ann NY Acad Sci, 465: 625-631.
Harley NH, Cohen BS, & Tso TC (1980) Polonium-210: A questionable risk
factor in smoking-related carcinogenesis. In: Gori GB & Bock FG ed. A
safe cigarette? Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp 93-104 (Banbury Report No. 3).
Harris RJC & Negroni G (1967) Production of lung carcinomas in C57BL
mice exposed to a cigarette smoke and air mixture. Br Med J, 4:
637-641.
Harris RJC, Negroni G, Ludgate S, Pick CR, Chesterman FC, & Maidment
BJ (1974) The incidence of lung tumours in C57BL mice exposed to
cigarette smoke: air mixtures for prolonged periods. Int J Cancer, 14:
130-136.
Hassler E, Lind B, & Piscator M (1983) Cadmium in blood and urine
related to present and past exposure: A study of workers in an
alkaline battery factory. Br J Ind Med, 40: 420-425.
Haugen A, Becher G, Benestad C, Vahakangas K, Trivers GE, Newman MJ, &
Harris CC (1986) Determination of polycyclic aromatic hydrocarbons in
the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA,
and antibodies to the adducts in sera from coke oven workers exposed
to measured amounts of polycyclic aromatic hydrocarbons in the work
atmosphere. Cancer Res, 46(8): 4178-4183.
Hawker R & Holtby I (1988) Smoking and absence from work in a
population of student nurses. Public Health (Lond), 102: 161-167.
Hayes RB, Gerin M, Raatgever JW, & de Bruyn A (1986) Wood-related
occupations, wood dust exposure, and sinonasal cancer. Am J Epidemiol,
124(4): 569-577.
Health Canada (1994) Risk assessment for the combustion products of
methylcyclo-pentadienyl manganese tricarbonyl (MMT) in gasoline.
Ottawa, Ontario, Health Canada, Environmental Health Directorate, 113
pp.
Hecht SS & Hoffmann D (1988) Tobacco specific nitrosamines, an
important group of carcinogens in tobacco and tobacco smoke.
Carcinogenesis, 9(6): 875-884.
Hecht SS & Hoffmann D (1991) N-nitroso compounds and tobacco-induced
cancers in man. In: O'Neill IK, Chen J, & Bartsch H ed. Relevance to
human cancer of N-nitroso compounds, tobacco smoke and mycotoxins.
Lyon, International Agency for Research on Cancer, pp 54-61 (IARC
Scientific Publications No. 105).
Hecht SS, Adams JD, Numoto S, & Hoffmann D (1983) Induction of
respiratory tract tumors in Syrian golden hamsters by a single dose of
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 4:
1287-1290.
Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, & Hoffmann D
(1986) Induction of oral cavity tumors in F344 rats by
tobacco-specific N-nitrosamines and snuff. Cancer Res, 46:
4162-4166.
Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, & Hoffmann
D (1993) A tobacco-specific lung carcinogen in the urine of men
exposed to cigarette smoke. N Engl J Med, 329: 1543-1546.
Henry CJ & Kouri RE (1984) Chronic exposure of mice to cigarette smoke
-- Final Report to the Council for Tobacco Research -- USA, Inc.
(Contract CTR-0030). New York, Field, Rich and Associates, Inc.
Herity B, Moriarity M, Daly L, Dunn J, & Bourke GJ (1982) The role of
tobacco and alcohol in the aetiology of lung and larynx cancer. Br J
Cancer, 46: 961-964.
Hertz-Picciotto I, & Hu SW (1994) Contribution of cadmium in
cigarettes to lung cancer: an evaluation of risk assessment
methodologies. Arch Environ Health, 49(4): 297-302.
Hertz-Picciotto I, Smith AH, Holtzman D, Lipsett M, & Alexeef G (1992)
Synergism between occupational arsenic exposure and smoking in the
induction of lung cancer. Epidemiology, 3(1): 23-31.
Hessel PA, Sluis-Cremer GK, & Lee SL (1991) Distribution of silicotic
collagenization in relation to smoking habits. Am Rev Respir Dis,
144(2): 297-301.
Hicks RM (1982) Nitrosamines as possible etiological agents in
bilharzial bladder cancer. In: Magee PM ed. Nitrosamines and human
cancer. Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp 455-471 (Banbury Report No. 12).
Hicks RM, James C, & Webbe G (1980) Effect of Schistosoma
haematobium and N-butyl- N-(4-hydroxybutyl)nitrosamine on the
development of urothelial neoplasia in baboon. Br J Cancer, 42:
730-755.
Higginson J, Muir C, & Buffler PA (1984) The epidemiology of renal
carcinoma in humans with a note on the effect of exposure to gasoline.
In: Mehlman MA, Hemstreet GP III, Thorp JJ, & Weaver NK ed. Advances
in modern environmental toxicology -- Volume VII: Renal effects of
petroleum hydrocarbons. Princeton, New Jersey, Princeton Scientific
Publishers, pp 203-226.
Hilt B, Langard S, Lund-Larsen PG, & Lien JT (1986) Previous asbestos
exposure and smoking habits in the county of Telemark, Norway -- A
cross-sectional population study. Scand J Work Environ Health, 12:
561-566.
Hinds WC, First MW, Huber GI, & Shea JW (1983) A method for measuring
respiratory deposition of cigarette smoke during smoking. Am Ind Hyg
J, 44: 113-118.
Hirsch JM & Thilander H (1981) Snuff-induced lesions of the oral
mucosa: an experimental model in the rat. J Oral Pathol, 10: 342-353.
Hnizdo E (1990) Combined effect of silica dust and tobacco smoking on
mortality from chronic obstructive lung disease in gold miners. Br J
Ind Med, 47: 656-664.
Hnizdo E & Sluis-Cremer GK (1991) Silica exposure, silicosis, and lung
cancer: a mortality study of South African gold miners. Br J Ind Med,
48(1): 53-60.
Hnizdo E, Baskind E, & Sluis-Cremer GK (1990) Combined effects of
silica dust exposure and tobacco smoking on the prevalence of
respiratory impairments among gold miners. Scand J Work Environ
Health, 16: 411-422.
Hocking WG & Golde DW (1979) The pulmonary-alveolar macrophage. N Engl
J Med, 301: 580-587, 639-645.
Hoffmann D & Hoffmann I (1991) Recent developments in smoking related
cancer research. Smok Relat Dis, 5(2): 77-93.
Hoffmann D & Hoffmann I (1997) The changing cigarette: 1950-1995. J
Toxicol Environ Health, 50: 307-364.
Hoffmann D & Wynder EL (1976) Smoking and occupational cancer. Prev
Med, 5: 245-261.
Hoffmann D, Rathkamp G, & Wynder EL (1961) Comparison of the yields of
several selected compounds in the smoke of different tobacco products.
J Natl Cancer Inst, 31: 627-637.
Hoffmann D, Sanghvi LD, & Wynder EL (1974) Chemical studies on tobacco
smoke: XXVIII. Comparative chemical analysis of Indian bidi and
American cigarette smoke. Int J Cancer, 14: 49-53.
Hoffmann D, Rivenson A, Hecht SS, Hilfrich J, Kobayashi N, & Wynder EL
(1979) Model studies in tobacco carcinogenesis with the Syrian Golden
Hamster. Prog Exp Tumor Res, 24: 370-390.
Hoffmann D, Harley NH, Fisenne I, Adams JD, & Brunnemann KD (1986)
Carcinogenic agents in snuff. J Natl Cancer Inst, 76: 435-437.
Hoffmann D, Rivenson A, Chung FL, & Hecht SS (1991a) Nicotine-derived
nitrosamines and their relevance in tobacco carcinogenesis. Crit Rev
Toxicol, 21(4): 305-311.
Hoffmann D, Melikian AA, & Brunnemann KD (1991b) Studies in tobacco
carcinogenesis. Lyon, International Agency for Research on Cancer, pp
482-484 (IARC Scientific Publications No. 105).
Hoffmann D, Brunnemann KD, Prokopczyk B, & Djordjevic MV (1994)
Tobacco-specific N-nitrosamines and areca-derived N-nitrosamines:
chemistry, biochemistry, carcinogenicity, and relevance to humans. J
Toxicol Environ Health, 41: 1-52.
Holbrook JH (1977) Tobacco and health. CA-Cancer J Clin, 27: 344-353.
Holst PA, Kromhout D, & Brand R (1988) For debate: pet birds as an
independent risk factor for lung cancer. Br Med J, 297: 1319-1324.
Honeybourne D & Pickering CAC (1986) Physiological evidence that
emphysema is not a feature of byssinosis. Thorax, 41(1): 6-11.
Hsu TC, Furlong C, & Spitz MR (1991) Ethyl alcohol as a co-carcinogen
with special reference to the aerodigestive tract: a cytogenetic
study. Anticancer Res, 11: 1097-1102.
Hughes JM & Weill H (1991) Asbestos as a precursor of asbestos related
lung cancer: Results of a prospective mortality study. Br J Ind Med,
48: 229-233.
IARC (1984) Polynuclear aromatic compounds: Part 3. Industrial
exposures in aluminum production, coal gasification, coke production,
and iron and steel founding. Lyon, International Agency for Research
on Cancer, pp 37-190 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 34).
IARC (1985a) Tobacco habits other than smoking: Betel-quid and
areca-nut chewing and some related nitrosamines. Lyon, International
Agency for Research on Cancer, 291 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
37).
IARC (1985b) Polynuclear aromatic compounds: Part 4. Bitumens,
coal-tars and derived products, shale-oils and soots. Lyon,
International Agency for Research on Cancer, pp 83-241 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
35).
IARC (1986) Tobacco smoking. Lyon, International Agency for Research
on Cancer, 421 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 38).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, 440 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IARC (1988) Man-made mineral fibres and radon. Lyon, International
Agency for Research on Cancer, 300 pp (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Volume 43).
IARC (1989a) Antimony trioxide and antimony trisulfide. In: Some
organic solvents, resin monomers and related compounds, pigments and
occupational exposures in paint manufacture and painting. Lyon,
International Agency for Research on Cancer, pp 291-305 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
47).
IARC (1989b) Occupational exposures in petroleum refining; crude oil
and major petroleum fuels. Lyon, International Agency for Research on
Cancer, pp 109-110 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 45).
IARC (1989c) Diesel and gasoline engine exhaust and some nitroarenes.
Lyon, International Agency for Research on Cancer, pp 41-185 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
46)..
IARC (1990) Chromium, nickel and welding. Lyon, International Agency
for Research on Cancer, pp 257-445 (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Volume 49).
IARC (1991) Tenth International Meeting on N-Nitroso Compounds,
Mycotoxins and Tobacco Smoke: Relevance to Human Cancer. Lyon,
International Agency for Research on Cancer, 594 pp (IARC Scientific
Publications No. 105).
IARC (1993) Beryllium, cadmium, mercury, and exposures in the glass
manufacturing industry. Lyon, International Agency for Research on
Cancer, 444 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 58).
IARC (1994a) Ethylene oxide. In: Some industrial chemicals. Lyon,
International Agency for Research on Cancer, pp 73-159 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
60).
IARC (1994b) Infection with schistosomes ( Schistosoma haematobium,
Schistosoma mansoni and Schistosoma japonicul). In: Schistosomes,
liver flukes and Helicobacter pylori. Lyon, International Agency for
Research on Cancer, Volume 61).
IARC (1995) Wood dust and formaldehyde. Lyon, International Agency for
Research on Cancer, pp 191-194 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 62).
ICRP (International Commission on Radiological Protection) (1994)
Human respiratory tract model for radiological protection. Amsterdam,
Oxford, New York, Elsevier Science Publishers (ICRP Publication No.
66).
Idris AM, Nair J, Oshima H, Friesen M, Brouet I, Faustman EM, &
Bartsch H (1991) Unusual high levels of carcinogenic tobacco-specific
nitrosamines in Sudan snuff (toombak). Carcinogenesis, 12: 1115-1118.
Idris AM, Prokopczyk B, & Hoffmann D (1994) Toombak: a major risk
factor for cancer of the oral cavity in Sudan. Prev Med, 23: 832-839.
ILO (1991) Smoking contributes to noise-induced hearing loss: ILO
contribution to WHO -- World no tobacco day, 1992. Geneva,
International Labour Organisation.
Inskip PD & Boice JD (1994) Radiotherapy-induced lung cancer among
women who smoke. Cancer, 73(6): 1541-1543.
IPCS (1990) Environmental health criteria 106: Beryllium. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 40-53.
IPCS (1991a) Environmental health criteria 108: Nickel. Geneva, World
Health Organization, International Programme on Chemical Safety, pp
48-67.
IPCS (1991b) Environmental health criteria 125: Platinum. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 79-80.
IPCS (1992) Environmental health criteria 134: Cadmium. Geneva, World
Health Organization, International Programme on Chemical Safety, 280
pp.
IPCS (1995) Environmental health criteria 165: Inorganic lead. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 93-98.
IPCS (1996) Environmental health criteria 171: Diesel fuel and exhaust
emissions. Geneva, World Health Organization, International Programme
on Chemical Safety, 389 pp.
ISAAC (1998) International Study of Asthma and Allergies in Childhood
(ISAAC) Steering Committee: Worldwide variation in the prevalence of
symptoms of asthma, allergic rhino conjunctivitis, and atopic eczema.
Lancet, 351: 1225-1232.
Iscovich J, Castelletto R, Esteve J, Muñoz N, Colanzi R, Coronel A,
Deamezola I, Tassi V, & Arslan A (1987) Tobacco smoking, occupational
exposure and bladder cancer in Argentina. Int J Cancer, 40: 734-740.
Istvan J & Matarazzo JD (1984) Tobacco, alcohol and caffeine use: a
review of their interrelationships. Psychol Bull, 95: 301-326.
Iwata F, Zhang XY, & Leung FW (1995) Aggravation of gastric mucosal
lesions in rat stomach by tobacco cigarette smoke. Dig Dis Sci, 40:
1118-1124.
Izard C & Liberman C (1978) Acrolein. Mutat Res, 47(2): 115-138.
Jarup L & Pershagen G (1991) Arsenic exposure, smoking and lung cancer
in smelter workers -- A case-control study. Am J Epidemiol, 134(6):
545-551.
Järvholm B, Larsson S, Hagberg S, Olling S, Ryd W, & Torén K (1993)
Quantitative importance of asbestos as a cause of lung cancer in a
Swedish industrial city: a case-referent study. Eur Respir J, 6(9):
1271-1275.
Jayant K & Pakhale SS (1985) Toxic constituents in bidi smoke. In:
Sanghvi LD & Notami P ed. Tobacco and health: The Indian scene.
Bombay, Tata Memorial Center, pp 101-110.
Jindal RM & Patel SM (1992) Buerger's disease and cigarette smoking in
Bangladesh. Ann R Coll Surg Engl, 74(6): 436-437.
Johansson SL, Hirsch JM, Larsson PA, Saidi J, & Oesterdahl BG (1989)
Snuff-induced carcinogenesis: effect of snuff in rats initiated with
4-nitroquinoline- N-oxide. Cancer Res, 49: 3063-3069.
JOM (1984) Vibration syndrome in chipping and grinding workers. J
Occup Med, 26(10): 765-788.
Jones RD (1994) Survey of antimony workers: mortality 1961-92. Occup
Environ Med, 51(11): 772-776.
Jörgensen HS (1984) Lung cancer among underground workers in the iron
ore mine of Kiruna based on thirty years of observation. Ann Acad Med
Singap, 13(suppl 2): 371-377.
Jörgensen HS, Kolmodin-Hedman B, & Stjernberg N (1988) Follow-up study
of pulmonary function and respiratory tract symptoms in workers in a
Swedish iron ore mine. J Occup Med, 30(12): 953-957.
Jorquera R, Castonguay A, & Schüller HM (1992) Effects of pregnancy
and ethanol treatment on the metabolism of 4-(methyl
nitrosamino)-1-(pyridyl)-1-butanone by hamster liver and lung
microsomes. Drug Metab Dispos, 20: 510-517.
Jusko WJ (1978) Role of tobacco smoking in pharmacokinetics. J
Pharmacokinet Biopharm, 6: 7-39.
Jussawalla DJ & Deshpande VA (1971) Evaluation of cancer risk in
tobacco chewers and smokers: an epidemiological assessment. Cancer,
28(1): 244-252.
Kaldor JM & L'Abbe KA (1990) Interaction between human carcinogens.
In: Vainio H, Sorsa M, & McMichael AJ ed Complex mixtures and cancer
risk. Lyon, International Agency for Research on Cancer, pp 35-43
(IARC Scientific Publications No. 104).
Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, & Fraumeni
JF (1984) Urinary tract infection and risk of bladder cancer. Am J
Epidemiol, 119(4): 510-515.
Kawajiri K & Fujii-Kuriyama Y (1991) P450 and human cancer. Jpn J
Cancer Res, 82(12): 1325-1335.
Kawajiri K, Nakachi K, Imai K, Hyashi S, & Watanabe J (1990)
Individual differences in lung cancer susceptibility in relation to
polymorphysms of P-450IA1 gene and cigarette dose. Princess Takamatsu
Int Symp Ser, 21: 55-61.
Kazantzis G & Armstrong BG (1984) Renal function in relation to low
levels of cadmium exposure in a group of smelter workers. Environ
Health Perspect, 54: 193-199.
Kazantzis G, Blanks RG, & Sullivan KR (1992) Is cadmium a human
carcinogen? In: Nordberg GF, Herber REM, & Alessio L ed. Cadmium in
the human environment: Toxicity and carcinogenicity. Lyon,
International Agency for Research on Cancer, pp 435-445.
Keller AZ & Terris M (1965) The association of alcohol and tobacco
with cancer of the mouth and pharynx. Am J Publ Health, 55: 1578-1585.
Keen P, de Moor NG, Shapiro MP, & Cohen L (1955) The etiology of
respiratory tract cancer in the South African Bantu. Br J Cancer, 9:
528-538.
Kibelstis JA, Morgan EJ, Reger R, Lapp NL, Seaton A, & Morgan WK
(1973) Prevalence of bronchitis and airway obstruction in American
bituminous coal miners. Am Rev Respir Dis, 108(4): 886-893.
Kilburn KH (1989) Byssinosis, emphysema, and mortality of American
textile workers: [Editorial]. Arch Environ Health, 38(5): 277.
Kilpikari MD (1982) Mortality among male rubber workers in Finland.
Arch Environ Health, 37(5): 295-299.
Kimura N (1977) Epidemiological studies in Japan on smoking and
cardiovascular disease. In: Proceedings of 3rd World Conference on
Smoking and Health, New York City, 2-5 June 1975. Volume II: Health
consequence, education, cessation and social actions. Washington, DC,
US Department of Health, Education and Welfare, pp 185-198 (DHEW
Publication (NIH) 77:1413).
King M, Wight A, DeSanctis GT, el-Azab J, Phillips DM, Angus GE, &
Cosio MG (1989) Mucus hypersecretion and viscoelasticity changes in
cigarette smoking dogs. Exp Lung Res, 15(3): 375-389.
Kleinfeld M, Messite J, & Langer AM (1973) A study of workers exposed
to asbestiform minerals in commercial talc manufacture. Environ Res,
6: 132-143.
Kobayashi W, Hoffmann D, & Wynder EL (1974) A study of tobacco
carcinogenesis: XII. Epithelial changes induced in the upper
respiratory tracts of Syrian golden hamsters by cigarette smoke. J
Natl Cancer Inst, 534: 1085-1089.
Kobusch AB, Simard A, Feldstein M, Vauclair R, Gibbs GW, Bergeron F,
Morissette N, & Davis R (1984) Pulmonary cytology in chrysotile
asbestos workers. J Chron Dis, 37(8): 599-607.
Kodell RL & Pounds JG (1991) Assessing the toxicity of chemical
mixtures. In: Krewski D & Franklin CA ed. Statistics in toxicology.
New York, Gordan & Breach, pp 557-589.
Kodell RL, Krewski D, & Zielinski J (1991) Additive and multiplicative
relative risk in the two-stage clonal expansion model of
carcinogenesis. Risk Anal, 11: 483-490.
Kohlmeier L, Arminger G, Bartolomeycik S, Bellach B, Rehm J, & Thamm M
(1992) Pet birds as an independent risk factor for lung cancer:
case-control study. Br Med J, 305: 986-989.
Koppang N, Rivenson A, Reith A, Dahle HK, Evenson O, & Hoffman D
(1992) A study of tobacco carcinogenesis: XLVIII. Carcinogenicity of
N'-nitrosonornicotine in mink ( Mustela vison). Carcinogenesis,
13(11): 1957-1960.
Koppang N, Rivenson A, Dahle HK, & Hoffman D (1997) A study of tobacco
carcinogenesis: LIII. Carcinogenicity of N'-nitrosonornicotine (NNN)
and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in mink
( Mustela vison). Cancer Lett, 111: 167-171.
Kotin P (1994) The epidemiological evidence on the carcinogenicity of
beryllium, by MacMahon: [Editorial]. J Occup Med, 36(1): 25-26.
Kreiss K, Wassermann S, Mroz MM, & Newman LS (1993) Beryllium disease
screening in the ceramics industry. J Occup Med, 35: 16-25.
Kreiss K, Mroz MM, Newman LS, Martyny J, & Zhen B (1996) Machining
risk of beryllium disease and sensitization with median exposures
below 2 µg/m3. Am J Ind Med, 30: 16-25.
Kremer AM, Pal TM, Boleij JSM, Schouten JP, & Rijcken B (1994) Airway
hyperresponsiveness, prevalence of chronic respiratory symptoms, and
lung function in workers exposed to irritants. Occup Environ Med, 51:
3-13.
Krishna K (1978) Tobacco chewing in pregnancy. Br J Obstet Gynaecol,
85: 726-728.
Kristensen TS (1989) Cardiovascular disease and the work environment:
A critical review of the epidemiological literature on chemical
factors. Scand J Work Environ Health, 15: 245-264.
Krivan V, Schneider G, Baumann H, & Reus (1994) Multi-element
characterization of tobacco smoke condensate. Fresenius J Anal Chem,
348: 218-225.
Kuntz WD & McCord CP (1974) Polymer-fume fever. J Occup Med, 16(7):
480-482.
Kusiak RA, Springer J, Ritchie AC, & Muller J (1991) Carcinoma of the
lung in Ontario gold miners: possible aetiological factors. Br J Ind
Med, 48: 808-817.
Kusiak RA, Ritchie AC, Muller J, & Springer J (1993) Mortality from
lung cancer in Ontario uranium miners. Br J Ind Med, 50(10): 920-928.
Lai YL & Diamond L (1992) Cigarette smoke exposure does not prevent
cadmium-induced alterations in rat lungs. J Toxicol Environ Health,
35(1): 63-76.
LGC (Laboratory of the Government Chemist) (1982) Report of the
Government chemist, 1981. London, Her Majesty's Stationery Office
(HMSO), p 109.
Lakier JB (1992) Smoking and cardiovascular disease. Am J Med,
93(suppl 1A): 8-12.
Lamm SH, Parkinson M, Anderson M, & Taylor W (1992) Determinants of
lung cancer risk among cadmium-exposed workers. Ann Epidemiol, 2(3):
195-211.
Lancet (1985) Smoking, occupation, and allergic lung disease. Lancet,
1(8435): 965.
Landrigan PJ & Straub WE (1985) Occupational lead exposure aboard a
tall ship. Am J Ind Med, 8(3): 233-239.
Langård S & Norseth T (1975) A cohort study of bronchial carcinomas in
workers producing chlorinated pigments. Br J. Ind Med, 32: 62-65.
Langård S & Vigander T (1983) Occurrence of lung cancer in workers
producing chromium pigments. Br J Ind Med, 40(1): 71-74.
Larsson PA, Johansson SL, Vahlne A, & Hirsch JM (1989) Snuff
carcinogenesis: effects of long-term snuff administration after
initiated with 4-nitroquinoline- N-oxide and Herpes simplex virus
type 1. J Oral Pathol Med, 18: 187-192.
La Vecchia C, Negri E, D'Avanzo B, Savoldelli R, & Franceschi S (1991)
Genital and urinary tract diseases and bladder cancer. Cancer Res,
51(2): 629-631.
La Voie EJ, Adams JD, Reinhardt J, Rivenson A, & Hoffmann D (1986)
Toxicity studies on clove cigarette smoke and constituents of clove:
Determination of the LD50 of eugenol by intratracheal installation in
rats and hamsters. Arch Toxicol, 59: 78-81.
Leanderson P & Tagesson C (1989) Cigarette smoke potentiates the
DNA-damaging effect of manmade mineral fibers. Am J Ind Med, 16:
697-706.
Lednar WM, Tyroler HA, McMichael AJ, & Shy CM (1977) The occupational
determinants of chronic disabling pulmonary disease in rubber workers.
J Occup Med, 19: 263-268.
Leduc D, de Francquen P, Jacobovitz D, Vanderweyer R, Lauwerys R, &
De Vuyst P (1993) Association of cadmium exposure with rapidly
progressive emphysema in a smoker. Thorax, 48: 570-571.
Lehtovirta P & Forss M (1978) The acute effect of smoking on
intervillous blood flow of the placenta. Br J Obstet Gynaecol, 85:
729-731.
Leigh J, Outhred KG, McKenzie HI, Glick M, & Wiles AN (1983)
Quantified pathology of emphysema, pneumoconiosis, and bronchitis in
coal workers. Br J Ind Med, 40: 258-263.
Letourneau EG, Krewski D, Choi NW, Goddard MJ, McGregor MJ, Zielinsky
JM, & Du J (1994) Case-control study of residential radon and lung
cancer in Winnipeg, Manitoba, Canada. Am J Epidemiol, 140(4): 310-322.
Liddell FD, Thomas DC, Gibbs GW, & McDonald JC (1984) Fibre exposure
and mortality from pneumoconiosis, respiratory, and abdominal
malignancies in chrysotile production in Quebec, 1926-1975. Ann Acad
Med Singap, 13(suppl): 340-344.
Lidegaard O (1993) Oral contraceptive use and risk of a cerebral
thromboembolic attack: Results of a case-control study. Br Med J, 306:
956-963.
Lilis R, Valciukas JA, Weber JP, Malkin J, & Selikoff IJ (1984a)
Epidemiological study of renal function in copper smelter workers.
Environ Health Perspect, 54: 181-192.
Lilis R, Valciukas JA, Weber JP, Fischbein A, Nicholson WJ, Campbell
C, Malkin J, & Selikoff IJ (1984b) Distribution of blood lead, blood
cadmium, urinary cadmium, and urinary arsenic levels in employees of a
copper smelter. Environ Res, 33: 76-95.
Lindberg E & Hedenstierna G (1983) Chrome plating: symptoms, findings
in the upper airways and effects on lung function. Arch Environ
Health, 38(6): 364-374.
Ljungman AG, Lindahl M, & Tagesson C (1994) Asbestos fibres and man
made mineral fibres: induction and release of tumour necrosis factor
from rat alveolar macrophages. Occup Environ Med, 51: 777-783.
Lourenco RV, Klimek MF, & Borowski CJ (1971) Deposition and clearance
of 2 micron particles in the tracheobronchial tree of normal subjects
-- smokers and nonsmokers. J Clin Invest, 50: 65-83.
Lubin JH & Boice JD (1997) Lung cancer risk from residential radon:
Meta-analysis of eight epidemiologic studies. J Natl Cancer Inst,
89(1): 49-57.
Lubin JH, Boice JD, Edling C, Hornung RW, Howe GR, Kunz E, Kusiak RA,
Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, Yao SX, &
Pierce DA (1995) Lung cancer in radon-exposed miners and estimation of
risk from indoor exposure. J Natl Cancer Inst, 87(11): 817-827.
Lubin JH, Tomasek L, Edling C, Hornung RW, Howe G, Kunz E, Kusiak RA,
Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, & Yao SX
(1997) Estimating lung cancer mortality from residential radon using
data for low exposures of miners. Radiat Res, 147(2): 126-134.
McCoy GD, Hecht SS, Katayama S, & Wynder EL (1981) Differential effect
of chronic ethanol consumption on the carcinogenicity of
N-nitrosopyrolidine and N-nitrosonornicotine in male Syrian golden
hamsters. Cancer Res, 41: 2849-2854.
McDuffie HH, Klaassen DJ, & Dosman JA (1990) Is pesticide use related
to the risk of primary lung cancer in Saskatchewan? J Occup Med,
32(10): 996-1002.
Mackenney RP (1983) A population study of Dupuytren's contracture.
Hand, 15(2): 155-161.
McLaughlin RF & Tueller EF (1971) Anatomic and histologic changes of
early emphysema. Chest, 59: 592-599.
McLintock JC (1971) The selection of juvenile entrants to mining. Br J
Ind Med, 28: 45-51.
MacMahon B (1994) The epidemiological evidence on the carcinogenicity
of beryllium in humans. J Occup Med, 36(1): 15-24.
Magnus K, Andersen A, & Hegetveit AC (1982) Cancer of respiratory
organs among workers at a nickel refinery in Norway. Int J Cancer, 30:
681-685.
Maheswaren R, Gill JS, & Beevers DG (1993) Blood pressure and
industrial lead exposure. Am J Epidemiol, 137(6): 645-653.
Makhyoun NA (1974) Smoking and bladder cancer in Egypt. Br J Cancer,
30: 577-581.
Mann JI, Vessey MP, Thorogood M, & Doll R (1975) Myocardial infarction
in young women with special reference to oral contraceptive practice.
Br Med J, 2: 241-245.
Mann JI, Doll R, Thorogood M, Vessey MP, & Waters WE (1976) Risk
factors for myocardial infarction in young women. Br J Prev Soc Med,
30: 94-100.
Marine WM, Gurr D, & Jacobsen M (1988) Clinically important
respiratory effects of dust exposure and smoking in British coal
miners. Am Rev Respir Dis, 137: 106-112.
Martell EA (1974) Radioactivity of tobacco trichomes and insoluble
smoke particles. Nature (Lond), 249: 215-217.
Martischnig KM, Newell DJ, Barnsley WC, Cowan WK, Feinmann EL, &
Oliver E (1977) Unsuspected exposure to asbestos and bronchogenic
carcinoma. Br Med J, 1(6063): 746-749.
Mayberry RM (1985) Cigarette smoking, herpes simplex virus type 2
infection, and cervical abnormalities. Am J Pub1 Health, 75(6):
676-678.
Mehta FS, Pindborg JJ, Hamner JE, Gupta PC, Daftary DK, Sahiar BE,
Shroff BC, Sanghvi LD, Bhonsle RB, Choksi SK, Dandekar VV, Mehta YN,
Pitkar VK, Sinor PN, Shah NC, Turner PS, & Upadhyay SA (1971) Report
on investigations of oral cancer and precancerous lesions in Indian
rural populations, 1966-1969. Copenhagen, Munksgaard, pp 48-120.
Merchant JA, Kilburn KH, O'Fallon WM, Hamilton JD, & Lumsden JC (1972)
Byssinosis and chronic bronchitis among cotton textile workers. Ann
Intern Med, 76: 423-433.
Merchant JA, Lumsden JC, Kilburn KH, O'Fallon WM, Ujida JR, & Germino
VH (1973) An industrial study of the biological effects of cotton dust
and cigarette smoke exposure. J Occup Med, 15(3): 212-221.
Meurman LO, Kiviluoto R, & Hakama M (1979) Combined effects of
asbestos exposure and tobacco smoking on Finnish anthophyllite miners
and millers. Ann NY Acad Sci, 330: 491-495.
Michaylov MA, Pressyanov DS, & Kalinov KB (1995) Bronchial dysplasia
induced by radiation in miners exposed to 222Rn progeny. Occup Environ
Med, 52: 82-85.
Mikkelsen OA (1978) Dupuytren's disease-the influence of occupation
and previous hand injuries. Hand, 10(1): 1-8.
Miller LG (1990) Cigarettes and drug therapy: Pharmacokinetic and
pharmacodynamic considerations. Clin Pharmacol, 9: 125-135.
Miller A (1993) Pulmonary function in asbestosis and asbestos related
pleural disease. Environ Res, 61(1): 1-18.
Miller BG & Jacobsen M (1985) Dust exposure, pneumoconiosis, and
mortality of coal miners. Br J Ind Med, 42(11): 723-733.
Miller FJ & Kimbell JS (1995) Regional dosimetry of inhaled reactive
gases. In: McClellan RO & Henderson RF ed. Concepts in inhalation
toxicology, 2nd ed. Washington, DC, Taylor & Francis, chapter 10, pp
257-288.
Misiewicz A, Radwan K, Karmolinski M, & Dziewit T (1992) [Degree of
ventilation disturbances in persons with occupational exposure to
manganese.] Wiad Lek, 45(23-24): 890-893 (in Polish).
Misiewicz A, Radwan K, Karmolinski M, Dziewit T, & Matysek A (1994)
[Chronic bronchitis in workers producing iron-manganese alloys.] Wiad
Lek, 47(7-8): 257-261 (in Polish).
Mitacek EJ, Brunnemann KD, & Polednak XP (1990) "Tar", nicotine and
carbon monoxide content of Thai cigarettes and implication for cancer
prevention in Thailand. Cancer Detect Prev, 14: 515-520.
Mitacek EJ, Brunnemann KD, Polednak AP, Hoffmann D, & Suttajet M
(1991) Composition of popular tobacco products in Thailand and its
relevance to disease prevention. Prev Med, 20: 764-773.
Mitacek EJ, Brunnemann KD, Hoffmann D, Limsila T, Suttajet M, Martin
N, & Kaplan LS (1999) Volatile N-nitrosamines and tobacco specific
N-nitrosamines in the smoke of Thai cigarettes: a suspected risk
factor liver cancer in Thailand. Carcinogenesis, 20(1): 133-137.
Mitchell CA & Gandevia B (1971) Respiratory symptoms and skin
reactivity in workers exposed to proteolytic enzymes in the detergent
industry. Am Rev Respir Dis, 104: 1-12.
Modigh C, Axelsson G, Alavanja M, Andersson L, & Rylander P (1996) Pet
birds and risk of lung cancer in Sweden: a case-control study. Br Med
J, 313: 1236-1238.
Molyneux MKB & Tombleson JBL (1970) An epidemiological study of
respiratory symptoms in Lancashire mills, 1963-66. Br J Ind Med,
27(3): 225-234.
Monson RR & Fine LJ (1978) Cancer mortality and morbidity among rubber
workers. J Natl Cancer Inst, 61(4): 1047-1053.
Monson RR & Nakano KK (1976a) Mortality among rubber workers: 1. White
male union employees in Akron, Ohio. Am J Epidemiol, 103(3): 284-296.
Monson RR & Nakano KK (1976b) Mortality among rubber workers: 2. Other
employees. Am J Epidemiol, 103(3): 297-303.
Moolgavkar SH, Dewanji A, & Luebeck G (1989) Cigarette smoking and
lung cancer: reanalysis of the British doctors' data. J Natl Cancer
Inst, 81: 415-420.
Moolgavkar SH, Luebeck EG, Krewski D, & Zielinski JM (1993) Radon,
cigarette smoke, and lung cancer: A re-analysis of the Colorado
Plateau uranium miners' data. Epidemiology, 4: 204-217.
Morabia A, Stellman S, Lumey LH & Wynder EL (1998) Parakeets,
canaries, finches, parrots, and lung cancer: no association. Br J
Cancer, 77: 501-504.
Morgan DC, Smyth JT, Lister RW, Pethybridge RJ, Gilson JC, Callaghan
P, & Thomas GO (1975) Chest symptoms in farming communities with
special reference to farmer's lung. Br J Ind Med, 32: 228-234.
Morgan WKC (1982) Occupational bronchitis. Eur J Respir Dis, 63(suppl
23): 117-127.
Morgan KT (1998) Commentary on Coggins CRE (1998): a review of chronic
inhalation studies with mainstream cigarette smoke in rats and mice.
Toxicol Pathol, 26(3): 315.
Mori K (1964) Acceleration of experimental lung cancers in rats by
inhalation of cigarette smoke. Gann, 55: 175-181.
Morimoto Y, Kido M, Tanaka I, Fujino A, Higashi T, & Yokosaki Y (1993)
Synergistic effects of mineral fibres and cigarette smoke on the
production of tumour necrosis factor by alveolar macrophages of rats.
Br J Ind Med, 50: 955-960.
Mougne C, MacLennan R, & Atsana S (1982) Smoking, chewing and drinking
in Ban Pong, Northern Thailand. Soc Sci Med, 16(1): 99-106.
MRC (Medical Research Council) (1965) Special Committee on the
Aetiology of Chronic Bronchitis: Definition and classification of
chronic bronchitis for clinical and epidemiological purposes. Lancet,
1: 775-779.
Muir CS & McKinney PA (1992) Cancer of the oesophagus: A global
overview. Eur J Cancer Prev, 1: 259-264.
Murai Y, Kitagawa M, Matsui K, Koizumi F, & Miwa A (1994) Asbestos
fibre analysis in seven asbestosis cases. Arch Environ Health, 49(1):
67-72.
Murphy SE & Heiblum R (1990) Effect of nicotine and tobacco-specific
nitrosamines on the metabolism of N'-nitrosonornicotine and
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 11:
1663-1666.
Muscat JE & Wynder EL (1991) Cigarette smoking, asbestos exposure, and
malignant mesothelioma. Cancer Res, 51(9): 2263-2267.
Nair J, Pakhale SS, & Bhide SV (1989) Carcinogenic tobacco-specific
nitrosamines in Indian tobacco products. Food Chem Toxicol, 27(11):
751-753.
Najem GR, Louria DB, Seebode JJ, Thind IS, Prusakowski JM, Ambrose RB,
& Fernicola AR (1982) Life time occupation, smoking, caffeine,
saccharin, hair dyes and bladder carcinogenesis. Int J Epidemiol, 11:
212-217.
Nakachi K, Imai K, Hayashi S, Watanabe J, & Kawajiri K (1991) Genetic
susceptibility to squamous cell carcinoma of the lung in relation to
cigarette smoking dose. Cancer Res, 51(19): 5177-5180.
Nanda PK & Sharma MM (1988) Immediate effect of tobacco chewing in the
form of "paan" on certain cardio-respiratory parameters. Indian J
Physiol Pharmacol, 32(2): 105-113.
Nandakumar A, Thimmasetty KT, Sreeramereddy NM, Venugopal TC, Rajanna
N, Vinutha AT, Srinivas N, & Bhargava MK (1990) A population-based
case-control investigation on cancer of the oral cavity in Bangalore,
India. Br J Cancer, 62: 847-851.
Naus A, Engler V, Hetychova M, & Vavrechova O (1966) Work injuries and
smoking. Ind Med Surg, 35: 880-881.
Negri E, LaVecchia C, Franceschi S, Decarli A, & Bruzzi P (1992)
Attributable risks for oesophageal cancer in Northern Italy. Eur J
Cancer, 28A: 1167-1171.
Nery LE, Sandoval PR, Jardim JR, Bagatin E, & Alonso G (1988) The
effects of smoking and silica exposure on pulmonary epithelial
permeability: a radioaerosol study with 99mTc-DTPA. Brazilian J Med
Biol Res, 21(2): 223-232.
Nery LE, Florencio RT, Sandoval PR, Rodrigues RT, Alonso G, & Mason GR
(1993) Additive effects of exposure to silica dust and smoking on
pulmonary epithelial permeability: a radioaerosol study with
technetium-99m labelled DTPA. Thorax, 48(3): 264-268.
Neugut AI, Robinson E, Lee WC, Murray T, Karwoski K, & Kutcher G
(1993) Lung cancer after radiation therapy for breast cancer. Cancer,
71: 3054-3057.
Neugut AI, Murray T, Santos J, Amols H, Hayes MK, Flannery JT, &
Robinson E (1994) Increased risk of lung cancer following breast
cancer radiation therapy in cigarette smokers. Cancer, 73: 1615-1620.
Neurath G & Ehmke H (1964) [Studies on the nitrate content of
tobacco.] Beitr Tabakforsch, 2: 333-344 (in German).
Nevius E, Bennett R, & Sobel S (1982) Critique of FM Sturtevant paper
"Smoking, oral contraceptives, and thromboembolic disease". Int J
Fertil, 27: 16-20.
Newhouse ML & Berry G (1976) Predictions of mortality from mesothelial
tumours in asbestos factory workers. Br J Ind Med, 33: 147-151.
Newhouse ML & Berry G (1979) Patterns of mortality in asbestos factory
workers in London. Ann NY Acad Sci, 330: 53-60.
Ng TP, Chan SL, & Lam KP (1987) Radiological progression and lung
function in silicosis: A ten year follow up study. Br Med J,
295(6591): 164-167.
Ng TP, Chan SL, & Lee J (1990) Mortality of a cohort of men in a
silicosis register: Further evidence of an association with lung
cancer. Am J Ind Med, 17(2): 163-171.
Ng TP, Chan SL, & Lee J (1992) Predictors of mortality in silicosis.
Respir Med, 86(2): 115-119.
Niewoehner DE (1991) What lies ahead? Future research and treatment
for chronic obstructive pulmonary disease. Am J Med, 91(4A): 41S-46S.
Niewoehner DE, Kleinerman J, & Rice DB (1974) Pathologic changes in
the peripheral airways of young cigarette smokers. N Engl J Med, 291:
755-758.
NIH (National Institutes of Health) (1996) The FTC cigarette test
method for determining tar, nicotine, and carbon monoxide yields of US
cigarette smoking. Washington, DC, US Department of Health and Human
Services, 275 pp (Smoking and Tobacco Control Monograph 7).
NIH (National Institutes of Health) (1998) Cigars: Health effects and
trends. Washington, DC, US Department of Health and Human Services,
232 pp (Smoking and Tobacco Control Monograph 9).
Nilsson K, Hendriksson R, Cai YQ, Hellstrom S, Hornqvist-Bylunds S, &
Bjermer L (1992) Effects of tobacco smoke on radiation-induced
pneumonitis in rats. Int J Radiat Biol, 62: 719-727.
NIOSH (1995) Criteria for a recommended standard: Occupational
exposure to respirable coal mine dust. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, 336 pp.
Nishikawa M, Ikeda H, Nishiyama H, Yamakawa H, Suzuki S, & Okubo T
(1992) Combined effects of ozone and cigarette smoke on airway
responsiveness and vascular permeability in guinea pigs. Lung, 170:
311-322.
Nishiyama H, Yano H, Nishiwaki Y, Kitaya T, Matsuyama T, Kodama T,
Suemasu K, Tamai S, & Takemoto K (1985) Lung cancer in chromate
workers: Analysis of 11 cases. Jpn J Clin Oncol, 15(3): 489-497.
Norman V, Ghrig AM, Lassa TM, & Moss BL (1983) The effect of
nitrogenous blend compounds on NO/NO2 and HCl levels in mainstream
and sidestream smoke. Bertr Tabakforsch, 12: 55-62.
NRC (National Research Council) (1988) Committee on the Biological
Effects of Ionizing Radiations: BEIR-IV: Health risks of radon and
other internally deposited alpha-emitters. Washington, DC, National
Academy Press, 602 pp.
O'Dell JR, Linder J, Markin RS, & Moore GF (1987) Thromboangiitis
obliterans (Buerger's disease) and smokeless tobacco. Arthritis-Rheum,
30(9): 1054-1056.
Olin JW (1994) Thromboangiitis obliterans. Curr Opin Rheumatol, 6(1):
44-49.
Oxman AD, Muir DC, Shannon HS, Stock SR, Hnizdo E, & Lange HJ (1993)
Occupational dust exposure and chronic obstructive pulmonary disease:
A systematic overview of the evidence. Am Rev Respir Dis, 148(1):
38-48.
Park NH, Dokko H, Li SL, & Cherrick HM (1991a) Synergism of herpes
symplex virus and tobacco-specific N'-nitrosamines in cell
transformation. J Oral Maxillofac Surg, 49(3): 276-281.
Park NH, Sapp JP, & Herbosa EG (1991b) Oral cancer induced in hamsters
with Herpes simplex virus infection and simulated snuff dipping. Oral
Surg Oral Med Oral Pathol, 62: 164-168.
Parka KR (1983) Smoking as a moderator of the relationship between
affective state and absence from work. J Appl Psychol, 68: 698-708.
Patrianakos C & Hoffmann D (1979) Chemical studies on tobacco smoke:
LXIV. On the analysis of aromatic amines. Anal Toxicol, 3: 150-154.
Pedersen E, Hogetveit AC, & Andersen A (1973) Cancer of respiratory
organs among workers at a nickel refinery in Norway. Int J Cancer, 12:
32-41.
Penner M & Penner S (1990) Excess insured health care costs from
tobacco-using employees in a large group plan. J Occup Med, 32:
521-523.
Pepys J, Pickering CAC, & Hughes EG (1972) Asthma due to inhaled
chemical agents -- complex salts of platinum. Clin Allergy, 2:
391-396.
Pershagen G (1985) Lung cancer mortality among men living near an
arsenic-emitting smelter. Am J Epidemiol, 122: 684-694.
Pershagen G, Wall S, Taube A, & Linnman L (1981) On the interaction
between occupational arsenic exposure and smoking and its relationship
to lung cancer. Scand J Work Environ Health, 7: 302-309.
Pershagen G, Bergman F, Klominek J, Damber L, & Wall S (1987)
Histological types of lung cancer among smelter workers exposed to
arsenic. Br J Ind Med, 44: 454-458.
Pershagen G, Akerblom G, Axelson O, Clavensjo B, Damber L, Desai G,
Enflo A, Legarde F, Mellander H, Svartengren M, & Swedjemark GU (1994)
Residential radon exposure and lung cancer in Sweden. N Engl J Med,
330(3): 159-164.
Petersen R, Andersen M, Mikkelsen S, & Nielsen SL (1995) Prognosis of
vibration induced white finger: a follow-up study. Occup Environ Med,
52(2): 110-115.
Peterson KL, Heninger RW, & Seegmiller RE (1981) Fetotoxicity
following chronic prenatal treatment of mice with tobacco smoke and
ethanol. Bull Environ Contam Toxicol, 26: 813-819.
Philipson K, Falk R, Gustafsson J, & Camner P (1996) Long-term lung
clearance of 195Au-labeled Teflon particles in humans. Exp Lung Res,
22: 65-83.
Pieraccini G, Luceri, F & Moneti, G (1992) New
gas-chromatographic/mass spectrometric method for the quantitative
analysis of primary aromatic amines in main and sidestream cigarette
smoke. I. Rapid Commun Mass Spectrom, 6: 406-409.
Pindborg JJ, Mehta PS, Gupta PC, Daftary DK, & Smith CJ (1971) Reverse
smoking in Andhra Pradesh, India: A study of palatal lesions among
10,169 villagers. Br J Cancer, 25: 10-20.
Plamenac P, Santic Z, Nikulin A, & Serdarevic H (1985) Cytologic
changes of the respiratory tract in vineyard spraying workers. Eur J
Respir Dis, 67: 50-55.
Post WK, Burdorf A, & Bruggeling TG (1994) Relations between
respiratory symptoms and sickness among workers in the animal feed
industry. Occup Environ Med, 51: 440-446.
Pratt PC, Vollmer RT, & Miller JA (1980) Epidemiology of pulmonary
lesions in nontextile and cotton textile workers: a retrospective
autopsy analysis. Arch Environ Health, 35: 133-138.
Prentice RL, Yoshimoto Y, & Mason MW (1983) Relationship of cigarette
smoking and radiation exposure to cancer mortality in Hiroshima and
Nagasaki. J Natl Cancer Inst, 70: 611-622.
Pyykko I, Pekkarinen J, & Stark J (1987) Sensory-neural hearing loss
during combined noise and vibration exposure. Int Arch Occup Environ
Health, 59: 439-454.
Ramakrishnan S, Sulochana KN, Selvaraj T, Abdul-Rahim A, Lakshmi M, &
Arunagiri K (1995) Smoking of beedies and cataract: cadmium and
vitamin C in the lens and blood. Br J Ophthalmol, 79: 202-206.
Rang EH & Acheson ED (1981) Cancer in furniture workers. Int J
Epidemiol, 10(3): 253-261.
RCP (Royal College of Physicians) (1962) Smoking and health. London,
Pitman, pp 2-8.
Reddy CR (1974) Carcinoma of hard palate in India in relation to
reverse smoking of chuttas. J Natl Cancer Inst, 53(3): 615-619.
Reddy DJ & Rao KV (1957) Cancer of the palate in coastal Andhra due to
smoking of cigars with burning end inside the mouth. Indian J Med Sci,
11: 791-798.
Reddy DJ, Reddy BD, & Rao PR (1960) Experimental production of cancer
with tobacco tar and heat. Cancer, 13: 263-269.
Reith AK, Reichborn-Kjennerud S, Aubele M, Jutting U, Gais P, & Burger
G (1994) Biological monitoring of chemical exposure in nickel workers
by imaging cytometry (ICM) of nasal smears. Anal Cell Pathol, 6(1):
9-21.
Rivenson A, Hecht SS, & Hoffmann D (1991) Carcinogenicity of
tobacco-specific N-nitrosamines: the role of the vascular network in
the selection of target organs. Crit Rev Toxicol, 21: 255-264.
Roberts DL (1988) Natural tobacco flavor. Recent Adv Tob, 14: 45-81.
Robertson PB, Walsh MM, & Greene JC (1997) Oral effects of smokeless
tobacco used by professional baseball players. Adv Dental Res, 11:
307-312.
Rodenhuis S & Slebos RJC (1992) Clinical significance of ras oncogene
activation in human lung cancer. Cancer Res, 52(suppl): S2665-S2669.
Roels H, Sarhan MJ, Hanotiau I, de Fays M, Genet P, Bernard A, Buchet
JP, & Lauwerys R (1985) Preclinical toxic effects of manganese in
workers in a Mn salts and oxides producing plant. Sci Total Environ,
42: 201-206.
Roth J, Mahrlein W, Kummer G, & Krause M (1985) [Results of an
epidemiological analysis of the pathogenesis of chronic obstructive
bronchitis in miners.] Z Erkrank Atmungsorg, 164(3): 277-279 (in
German).
Rothman K & Keller A (1972) The effect of joint exposure to alcohol
and tobacco on risk of cancer of the mouth and pharynx. J Chronic Dis,
25: 711-716.
Roush GC (1996) Cancer of the nasal cavity and paranasal sinuses. In:
Schuttenfeld D & Franinini JF ed. Cancer epidemiology and prevention.
New York, Oxford, Oxford University Press, pp 587-602.
Ruckley VA, Gauld SJ, Chapman JS, Davis JM, Douglas AN, Fernie JM,
Jacobsen M, & Lamb D (1984) Emphysema and dust exposure in a group of
coal workers. Am Rev Respir Dis, 129: 528-532.
Russell MAH (1976) Tobacco smoking and nicotine dependence. In:
Gibbons RJ, Israel Y, Kalent H, Papham RE, Schmidt W, & Smart RG ed.
Recent advances in alcohol and drug problems. New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp 1-47.
Ryan J, Zwerling C, & John E (1992) Occupational risks associated with
cigarette smoking: A prospective study. Am J Pub Health, 82: 29-32.
Rylander R, Sjostrand M, & Bergstrom R (1979) Free lung cell response
after combined exposure to cigarette smoke and industrial dusts.
Toxicology, 12: 211-220.
Sanden A & Jarvholm B (1991) A study of predictors of mesothelioma in
shipyard workers exposed to asbestos. J Occup Med, 33(7): 770-773.
Sanden A, Jarvholm B, Larsson S, & Thiringer G (1992) The risk of lung
cancer and mesothelioma after cessation of asbestos exposure: a
prospective cohort study of shipyard workers. Eur Respir J, 5:
281-285.
Sankaranarayanan R, Duffy SW, Day NE, Nair MK, Padmakumary G, & Day NE
(1989a) A case control investigation of cancer of the oral tongue and
floor of the mouth in southern India. Int J Cancer, 44(4): 617-621.
Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, & Padmanabhan TK
(1989b) Tobacco chewing, alcohol and nasal snuff in cancer of the
gingiva in Kerala, India. Br J Cancer, 60(4): 638-643.
Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, & Nair MK (1990)
Risk factors for cancer of the buccal and labial mucosa in Kerala,
southern India. J Epidemiol Community Health, 44(4): 286-292.
Cerik R (1987) The interactions of tobacco smoking and other agents in
cancer etiology. Epidemiol Rev, 9: 175-193.
Saris M & Lucic-Palaic S (1977) Possible synergism of exposure to
airborne manganese and smoking habit in occurrence of respiratory
symptoms. In: Walton WH & McGovern B ed. Inhaled particles -- IV.
Oxford, New York, Pergamon Press, pp 773-779.
Saumet JL, Leftheriotis G, Dittmar A, & Delhomme G (1986) Relationship
between pulse amplitude and thermal exchange in the finger: the effect
of smoking. Psychopharmacology (Berlin), 92(1): 118-121.
Schlueter DP, Banaszaak EF, Fink JN, & Barboriak J (1978) Occupational
asthma due to tetrachlorophthalic anhydride. J Occup Med, 20: 183-188
Schneider G & Krivan V (1993) Multi-element analysis of tobacco and
tobacco smoke condensate by instrumental neutron activation analysis
and atomic absorption spectrometry. Int J Environ Anal Chem, 53:
87-100.
Schnorr TM, Steenland K, Thun MJ, & Rinsky RA (1995) Mortality in a
cohort of antimony smelter workers. Am J Ind Med, 27(5): 759-770.
Schottenfeld D, Grant RC, & Wynder WL (1974) The role of alcohol and
tobacco in multiple primary cancers of the upper digestive system,
larynx and lung: A prospective study. Prev Med, 3: 277-293.
Schüller HM, Jorquera R, Reichert A, & Castonguay A (1993)
Transplacental induction of pancreas tumors in hamsters by ethanol and
the tobacco-specific nitrosamine 4-(methyl
nitrosamino)-1-(pyridyl)-1-butanone. Cancer Res, 53: 2498-2501.
Seaton A, Lamb D, Brown WR, Sclare G, & Middleton WG (1981)
Pneumoconiosis of shale miners. Thorax, 36: 412-418.
Seaton A, Godden DJ, & Brown K (1994) Increase in asthma: a more toxic
environment or a more susceptible population? Thorax, 49: 171-174.
Selig R & Nestler K (1985) [Epidemiology of chronic bronchitis from
the viewpoint of occupational medicine.] Z Erkrank Atmungsorg, 164(3):
273-276 (in German).
Selikoff IJ & Frank AL (1983) Keynote address. Potential of in vitro
tests in asbestos-related problems. Environ Health Perspect, 51: 1-4.
Selikoff IJ, Hammond EC, & Churg J (1968) Asbestos exposure, smoking
and neoplasia. J Am Med Assoc, 204(2): 106-112.
Selikoff IJ, Seidman H, & Hammond EC (1980) Mortality effects of
cigarette smoking among amosite asbestos factory workers. J Natl
Cancer Inst, 65: 507-513.
Sherrill DL, Halonen M, & Burrows B (1994) Relationships between total
serum IgE, atopy and smoking: a twenty-year follow-up analysis. J
Allergy Clin Immunol, 94: 954-962.
Sherson D, Sigsgaard T, Overgaard E, Loft S, Poulsen HE, & Jongeneelen
FJ (1992) Interaction of smoking, uptake of polycyclic hydrocarbons,
and cytochrome P450IA2 activity among foundry workers. Br J Ind Med,
49(3): 197-202.
Shim C & Williams MH (1986) Effect of odors in asthma. Am J Med,
80(1): 18-22.
Shirakawa T, Kusaka Y, & Morimoto K (1992) Combined effects of smoking
habits and occupational exposure to hard metal on total IgE
antibodies. Chest, 101(6): 1569-1576.
Shopland DR, Eyre HJ, & Pechacek TF (1991) Smoking attributable cancer
mortality in 1991: is lung cancer now the leading cause of death among
smokers in the United States? J Natl Cancer Inst, 83(16): 1142-1148.
Sidney S, Tekawa I, & Friedman GD (1989) Mentholated cigarette use
among multiphasic examinees, 1979-86. Am J Pub Health, 79(10):
1415-1416.
Simarak S, de Jong UW, Breslow N, Dahl CJ, Ruckphaopunt K, Scheelings
P, & Maclennan R (1977) Cancer of the oral cavity, pharynx/larynx and
lung in Northern Thailand: case-control study and analysis of cigar
smoke. Br J Cancer, 36: 130-140.
Simecek C, Sefrna F, & Simecková B (1986) [Evaluation of the role of
smoking in the development of chronic bronchitis after elimination of
other supporting factors.] Stud Pneumol Phtiseologica Czech, 46(3):
168-170 (in Czech).
Sluis-Cremer GK, Walters LG, & Sichel HS (1967) Chronic bronchitis in
miners and non-miners: an epidemiologic survey of a community in the
gold mining area in the Transvaal. Br J Ind Med, 24: 1-12.
Smith IE, Coles CD, Lancaster J, Fernhoff PM, & Falek A (1986) The
effect of volume and duration of prenatal ethanol exposure on neonatal
physical and behavioral development. Neurobehav Toxicol Teratol, 8:
375-381.
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I,
Duggan HM, Wood R, Kosnett MJ, & Smith MT (1992) Cancer risks from
arsenic in drinking water. Environ Health Perspect, 97: 259-267.
Sopori ML, Goud NS, & Kaplan AM (1994) Effects of tobacco smoke on the
immune system. In: Dean JH, Luster MI, Munson AE, & Kimber I ed.
Immunotoxicology and immunopharmacology. New York, Raven Press, pp
413-434.
Sopori ML, Kozak W, Savage SM, Geng Y, & Kluger MJ (1998)
Nicotine-induced modulation of T cell function: Implications for
inflammation and infection. Adv Exp Med Biol, 437: 279-289.
Sprince NL, Oliver LC, Eisen EA, Greene RE, & Chamberlin RI (1988)
Cobalt exposure and lung disease in tungsten carbide production: A
cross-sectional study of current workers. Am Rev Respir Dis, 138:
1220-1226.
Stanton MF, Layard M, Tegeris A, Miller E, May M, & Kent E (1977)
Carcinogenicity of fibrous glass: pleural response in the rat in
relation to fiber dimension. J Natl Cancer Inst, 58: 587-603.
Steenland K (1994) Age specific interactions between smoking and radon
among United States uranium miners. Occup Environ Med, 51(3): 192-194.
Steenland K & Thun M (1986) Interaction between tobacco smoking and
occupational exposures in the causation of lung cancer. J Occup Med,
28(2): 110-118.
Steenland K & Ward E (1991) Lung cancer incidence among patients with
beryllium disease: a cohort mortality study. J Natl Cancer Inst,
83(19): 1380-1385.
Steenland K & Ward E (1993) Passive smoking and the risk of heart
disease. J Natl Cancer Inst, 85(2): 1698-1699.
Steindorf K, Lubin J, Wichman HE, & Becher H (1995) Lung cancer deaths
attributable to indoor radon exposure in West Germany. Int J
Epidemiol, 24(3): 485-492.
Stellman SD, Boffetta P, & Garfinkel L (1988) Smoking habits of
800,000 American men and women in relation to their occupations. Am J
Ind Med, 13: 43-58.
Stellman SD, Muscat JE, Thompson S, Hoffmann D, & Wynder EL (1997)
Risk of squamous cell carcinoma and adenocarcinoma of the lung in
relation to lifetime filter cigarette smoking. Cancer, 80: 382-388.
Stöber W & Abel WR (1996) Lung cancer due to diesel soot particles in
ambient air? Int Arch Occup Environ Health, 68(suppl): S3-S61.
Stohrer G (1991) Arsenic: opportunity for risk assessment. Toxicology,
65: 525-531.
Sturtevant FM (1982) Smoking, oral contraceptives, and thromboembolic
disease. Int J Fertil, 27: 2-13.
Sunderman FW & Sunderman FW Jr (1961) Nickel poisoning: XI.
Implications of nickel as a pulmonary carcinogen in tobacco smoke. Am
J Clin Pathol, 35: 203-205.
Talbot RJ, Morgan A, Moores SR, & Matulionis DH (1987) Preliminary
studies of the interaction between 239PuO2 and cigarette smoke in the
mouse lung. Int J Radiat Biol, 51: 1101-1110.
Tawfik HN (1987) Carcinoma of the urinary bladder associated with
schistosomiasis in Egypt: the possible causal relationship. Princess
Takamatsu Int Symp Ser, 18: 197-209.
Taylor PR, Qiao YL, Schatzkin A, Yao SX, Lubin J, Mao BL, Rao JY,
McAdams M, Xuan XZ, & Li JY (1989) Relation of arsenic exposure to
lung cancer among tin miners in Yunan Province, China. Br J Ind Med,
46: 881-886.
Thériault G, de Guire L, Gingras S, & Laroche G (1982) Raynaud's
phenomenon in forestry workers in Quebec. Can Med Assoc J, 126:
1404-1408.
Thériault G, Tremblay C, Cordier S, & Gingras S (1984) Bladder cancer
in the aluminium industry. Lancet, 1(8383): 947-950.
Thomas TL & Stewart PA (1986) Mortality from lung cancer and
respiratory disease among pottery workers exposed to silica and talc.
Am J Epidemiol, 124: 530.
Thomas DC & Whittemore AS (1988) Methods for testing interactions,
with applications to occupational exposures, smoking and lung cancer.
Am J Ind Med, 13: 131-147.
Thomas GB, Williams CE, & Hoger NG (1981) Some non-auditory correlates
of the hearing threshold levels of an aviation noise-exposed
population. Aviation Space Environ Med, 52(9): 531-536.
Thun MJ, Lally CA, Flanney JT, Calle EE, Flander WD, & Heath CW Jr
(1997) Cigarette smoking and changes in the histopathology of the
lung. J Natl Cancer Inst, 89: 1580-1586.
Tokarskaya ZB, Okladnikova ND, Belyaeva ZD, & Drozhko EG (1995) The
influence of radiation and nonradiation factors on the lung cancer
incidence among the workers of the nuclear enterprise Mayak. Health
Phys, 69: 356-366.
Townsend MC, Sussman NB, Enterline PE, Morgan WK, Belk HD, & Dinman BD
(1988) Radiographic abnormalities in relation to total dust exposure
at a bauxite refinery and alumina-based chemical products plant. Am
Rev Respir Dis, 138: 90-95.
Trethowen WN, Burge PS, Rossiter CE, Harrington JM, & Calvert IA
(1995) Study of the respiratory health of employees of seven European
plants that manufacture ceramic fibres. Occup Environ Med, 52(2):
97-104.
Tricker AR & Preussmann R (1992) Volatile N-nitrosamines in
mainstream cigarette smoke: occurrence and formation. Clin Invest,
70(3-4): 283-289.
Tseng WP, Chu HM, How SW, Fong JM, Lin CS, & Yeh S (1968) Prevalence
of skin cancer in an endemic area of chronic arsenicism in Taiwan. J
Natl Cancer Inst, 40: 453-463.
Tso TC (1990) Production physiology and biochemistry of tobacco
plants. Belsville, Maryland, IDEALS, p 753.
Tso TC, Harley N, & Alexander LT (1966) Source of lead-210 and
polonium-210 in tobacco. Science, 153: 880-882.
Tsuda M & Kurashima Y (1991) Tobacco smoking, chewing, and snuff
dipping: factors contributing to the endogenous formation of
N-nitroso compounds. Crit Rev Toxicol, 21(4): 243-253.
Tsuda T, Nagira T, Yamamoto M, & Kume Y (1990) An epidemiological
study on cancer in certified arsenic poisoning patients in Toroku. Ind
Health, 28: 53-62.
Tsuda T, Babazono A, Yamamoto E, Kurumatani N, Mino Y, Ogawa T, Kishi
Y, & Aoyama H (1995) Ingested arsenic and internal cancer: a
historical cohort followed for 33 years. Am J Epidemiol, 141(3):
198-209.
Tuyns AJ (1983) Oesophageal cancer in non-smoking drinkers and
non-drinking smokers. Int J Cancer, 32: 443-444.
Tuyns AJ (1991) Aetiology of head and neck cancer: tobacco, alcohol
and diet. Otorhynolaryngology, 46: 15-24.
Tuyns AJ, Péquignot G, & Jensen OM (1977) Le cancer de l'oesophage en
Ille-et-Vilaine en fonction des niveaux de consommation d'alcool et de
tabac: Des risques qui se multiplient. Bull Cancer, 64: 45-60.
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic
Radiation) (1988) Sources and effects of ionizing radiation. New York,
United Nations.
US ATSDR (1995) Toxicological profile for asbestos (update). Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry, 224 pp.
US DA (1990) World tobacco situation. Washington, DC, US Department of
Agriculture, Foreign Agricultural Service (Circular Series FT 4-90).
US DA (1997) Tobacco situation and outlook yearbook -- US output,
removal and consumption 1950-1997: Cigarettes, cigars, smoking tobacco
and specific tobacco products. Washington, DC, US Department of
Agriculture, Economic Research Service, pp 14-17 (TBS-240).
US EPA (1988) Technical support document on risk assessment of
chemical mixtures. Washington, DC, US Environmental Protection Agency,
32 pp (EPA/600/8-90/064).
US Surgeon General (1979) Smoking and health: A report of the Surgeon
General. Washington, DC, US Department of Health, Education and
Welfare (DHEW Publication No. (PHS) 79-50066).
US Surgeon General (1982) The health consequences of smoking: Cancer
-- A report of the Surgeon General. Rockville, Maryland, US Department
of Health and Human Services, Public Health Service, 332 pp.
US Surgeon General (1983) The health consequences of smoking:
Cardiovascular disease -- A report of the Surgeon General. Rockville,
Maryland, US Department of Health and Human Services, Public Health
Service, pp 6-8.
US Surgeon General (1984) The health consequences of smoking: Chronic
obstructive lung disease -- A report of the Surgeon General.
Rockville, Maryland, US Department of Health and Human Service (DHHS
Publication No. (PHS) 84-50205).
US Surgeon General (1986) The health consequences of using smokeless
tobacco -- A report of the Advisory Committee to the Surgeon General.
Bethesda, Maryland, US Department of Health and Human Services, Public
Health Service (Publication No. (NIH) 86-2874).
US Surgeon General (1988) The health consequences of smoking: Nicotine
addiction -- A report of the Surgeon General. Rockville, Maryland, US
Department of Health and Human Services, Centers for Disease Control,
pp 21-560.
US Surgeon General (1989) Reducing the health consequences of smoking:
25 years of progress -- A report of the Surgeon General. Rockville,
Maryland, US Department of Health and Human Services, Public Health
Service (DHHS Publication No. (CDC) 89-8411).
Vainio H & Boffetta P (1994) Mechanisms of the combined effect of
asbestos and smoking in the etiology of lung cancer [Review]. Scand J
Work Environ Health, 20(4): 235-242.
Vainio H, Husgafvel-Pursiainen K, Anttila S, Hackman P, & Partanen T
(1993) Interaction between smoking and asbestos in human lung
adenocarcinoma: role of K-ras mutations. Environ Health Perspect,
101(suppl 3): 189-192.
Vallyathan V, Shi XL, Dalal NS, Irr W, & Castranova V (1988)
Generation of free radicals from freshly fractured silica dust.
Potential role in acute silica-induced lung injury. Am Rev Respir Dis,
138: 1213-1219.
Van Leeuwen FE, Klokman WJ, Stoval M, Hagwenbeek A, van den
Belt-Dusebout AW, Noyon R, Boice JD, Burgers MV, & Somers R (1995)
Roles of radiotherapy and smoking in lung cancer following Hodgkin's
disease. J Natl Cancer Inst, 87(20): 1530-1537.
Van Peenen PFD, Blanchard AG, Wolkonsky PM, & Gill TM (1986) Health
insurance claims of petrochemical company employees. J Occup Med, 28:
237-240.
Van Tuinen M & Land G (1986) Smoking and excess sick leave in a
department of health. J Occup Med, 28: 33-35.
Van Schooten FJ, van Leeuwen FE, Hillebrand MJ, de Rijke ME, Hart AA,
van Veen HG, Oosterink S, & Kriek E (1990) Determination of
benzo(a)pyrene diol epoxide-DNA adducts in white blood cell DNA from
coke-oven workers: the impact of smoking. J Natl Cancer Inst, 82(11):
927-933.
Venables KM, Topping MD, Howe W, Luczynska CM, Hawkins R, & Taylor AJ
(1985) Interaction of smoking and atopy in producing specific IgE
antibody against a hapten protein conjugate. Br Med J, 290: 201-204.
Venables KM, Tee RD, Hawkins ER, Gordon DJ, Wale CJ, Farrer NM, Lam
TH, Baxter PJ, & Newman-Taylor A (1988a) Laboratory animal allergy in
a pharmaceutical company. Br J Ind Med, 45: 667-671.
Venables KM, Upton KM, Hawkins ER, Tee RD, Longbottom JL, &
Newman-Taylor AJ (1988b) Smoking, atopy, and laboratory animal
allergy. Br J Ind Med, 45: 667-671.
Venables KM, Dally MB, Nunn AJ, Stevens JF, Stephens R, Farrer N,
Hunter JV, Stewart M, Hughes EG, & Newman-Taylor AJ (1989) Smoking and
occupational allergy in workers in a platinum refinery. Br Med J, 299:
939-942.
Vessey MP & Doll R (1969) Investigation of relationship between the
use of oral contraceptives and thromboembolic disease: a further
report. Br Med J, 2: 651-657.
Viikari-Juntura E, Riihimaki H, Tola S, Videman T, & Mutanen P (1994)
Neck trouble in machine operating, dynamic physical work and sedentary
work: a prospective study on occupational and individual risk factors.
J Clin Epidemiol, 47(12): 1411-1422.
Vineis P (1992) Epidemiological models of carcinogenesis: the example
of bladder cancer. Cancer Epidemiol Biomarkers Prev, 1(2): 149-153.
Vineis P, Esteve J, & Terracini B (1984) Bladder cancer and smoking in
males: types of cigarettes, age of start, effect of stopping and
interaction with occupation. Int J Cancer, 34: 165-170.
Voges E ed. (1984) Tobacco encyclopedia. Mainz, Germany, Tabilk
Journal International, 467 pp.
Wagner JC, Pooley FD, Gibbs A, Lyons J, Sheers G, & Moncrieff CB
(1986) Inhalation of china stone and china clay dusts: relationships
between the mineralogy of dust retained in the lungs and pathological
changes. Thorax, 41: 190-196.
Wald NJ, Dall R, Darby S, Kinjlnk S, Petri R, & Pike M ed. (1985)
Trends in smoking related diseases in Britain. Oxford, Oxford
University Press.
Warren CPW (1977) Extrinsic allergic alveolitis: a disease commoner in
non-smokers. Thorax, 32: 567-569.
Weiland SK, Mundt KA, Keil U, Kraemer B, Birk T, Person M, Bucher AM,
Straif K, Schumann J, & Chambless L (1996) Cancer mortality among
workers in the German rubber industry: 1981-91. Occup Environ Med, 53:
289-298.
Weiss W (1971) Cigarette smoking, asbestos and pulmonary fibrosis. Am
Rev Respir Dis, 104: 223-226.
Weiss W (1976) Chloromethyl ethers, cigarettes, cough and cancer. J
Occup Med, 18(3): 194-199.
Weiss W (1980) The cigarette factor in lung cancer due to chloromethyl
ethers. J Occup Med, 22(8): 527-529.
Weiss W (1982) Epidemic curve of respiratory cancer due to
chloromethyl ethers. J Natl Cancer Inst, 69(6): 1265-1270.
Weiss W (1984) Cigarette smoke, asbestos and small irregular
opacities. Am Rev Respir Dis, 130(2): 293-301.
Weiss W & Boucot KR (1975) The respiratory effects of chloromethyl
methyl ether. J Am Med Assoc, 234(11): 1139-1142.
Weiss W & Nash D (1997) An epidemic of lung cancer due to chloromethyl
ethers: Thirty years of observation. J Occup Environ Med, 39:
1003-1009.
Welch K, Higgins I, Oh M, & Burchfiel C (1982) Arsenic exposure,
smoking and respiratory cancer in copper smelter workers. Arch Environ
Health, 37: 325-335.
WHO (1980) Recommended health-based limits in occupational exposure to
heavy metals. Geneva, World Health Organization, pp 21-35 (WHO
Technical Report Series 647).
WHO (1985) Regional Workshop on the Control of Tobacco-related
Diseases, New Delhi, 22-26 July 1985. New Delhi, World Health
Organization Regional Office for South-East Asia.
WHO (1997) Tobacco or health: A global status report. Geneva, World
Health Organization, pp 10-18.
Wicklund KG, Daling JR, Allard J, & Weiss NS (1988) Respiratory cancer
among orchardists in Washington State, 1968 to 1980. J Occup Med,
30(7): 561-564.
Wilbert W (1987) Tobacco and shamanism in South America (Psychoactive
plants of the world). New Haven, London, Yale University Press, 294
pp.
Wilhelmsen L (1977) Recent studies on smoking and CVD epidemiology:
Scandinavia and some other Western European countries. In: Proceedings
of 3rd World Conference on Smoking and Health -- Volume II: Health
consequences, education, cessation, and social action. Washington, DC,
Department of Health, Education and Welfare, pp 171-177 (Publication
No. (NIH) 77-1413).
Williams MK, Walford J, & King E (1983) Blood lead and the symptoms of
lead absorption. Br J Ind Med, 40(3): 285-292.
Winkelstein W (1990) Smoking and cervical cancer current status: a
review. Am J Epidem, 131: 945-957.
Winkelstein W, Shillitoe EJ, Brand R, & Johnson KK (1984) Further
comments on cancer of the uterine cervix, smoking and herpes virus
infection. Am J Epidem, 119(1): 1-8.
Winn DM (1997) Epidemiology of cancer and other systemic effects
associated with the use of smokeless tobacco. Adv Dent Res, 11(3):
313-321.
Wise MB & Guerin MR (1986) Chemical analysis of the major constituents
in clove cigarette smoke. In: Hoffmann D & Harris CC ed. Mechanisms in
tobacco carcinogenesis. Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 151-162 (Banbury Report No. 23).
Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE
(1997) The carcinogenicity of environmental tobacco smoke.
Carcinogenesis, 18(3): 575-586.
Wong SH, Ogle CW, & Cho CH (1986) Influence of chronic or acute
nicotine pretreatment on ethanol-induced gastric ulceration in the
rat. J Pharm Pharmacol, 38: 537-540.
Wong O, Harris F, & Smith TJ (1993) Health effects of gasoline
exposure: II. Mortality patterns of distribution workers in the United
States. Environ Health Perspect, 101(suppl 6): 63-76.
Wright JT, Waterson EJ, Barrison IG, Toplis PJ, Lewis IG, Gordon MG,
Macrae KD, Morris NF, & Murray-Lyon IM (1983) Alcohol consumption,
pregnancy and low birth weight. Lancet, 1: 663-665.
Wright JT, Macrae KD, Barrison IG, & Waterson EJ (1984) Effects of
moderate alcohol consumption and smoking on fetal outcome. London,
Pitman, pp 240-253 (Ciba Foundation Symposium Series No. 105).
Wynder EL & Bross IJ (1961) A study of etiological factors in cancer
of the oesophagus. Cancer, 14: 389-413.
Wynder EL & Gori GB (1977) Contribution of the environment on cancer
incidence: an epidemiologic exercise. J Natl Cancer Inst, 58: 825-832.
Wynder EL & Graham EG (1950) Tobacco smoking as a possible etiological
factor in bronchiogenic carcinoma. J Am Med Assoc, 37: 4608-4622.
Wynder El & Hoffmann D (1967) Tobacco and tobacco smoke: Studies in
experimental carcinogenesis. New York, London, Academic Press.
Wynder El & Hoffmann D (1994) Smoking and lung cancer: challenges and
opportunities. Cancer Res, 54: 5284-5295.
Wynder EL, Covey LS, Mabuchi K, & Mushinki M (1976) Environmental
factors in cancers of the larynx: A second look. Cancer, 38:
1591-1601.
Yazicioglu S, Ilcayto R, Balci K, Sayli BS, & Yorulmaz B (1980)
Pleural calcification, pleural mesotheliomas and bronchial cancers
caused by tremolite dust. Thorax, 35: 564-569.
Yorukoglu Y, Ilgit E, Zengin M, Nazliel K, Salman E, & Yucel E (1993)
Thromboangiitis obliterans (Buerger's disease) in women (a
reevaluation). Angiology, 44(7): 527-532.
Yoshizawa K (1984) [Clinicopathological analysis of lung cancer and
epithelial changes of the bronchus in chromate workers.] Shikoku Acta
Med, 40(1): 123-134 (in Japanese).
Yue L (1992) Cadmium in tobacco. Biomed Environ Sci, 5(1): 53-56.
Zacny JP (1990) Behavioral aspects of alcohol-tobacco interactions.
Recent Dev Alcohol, 8: 205-219.
Zahran FM, Ardawi MSM, & Al-Fayez SF (1985) Carboxyhaemoglobin
concentrations in smokers of sheesha and cigarettes in Saudi Arabia.
Br Med J, 291: 1768-1770.
Zelman S (1973) Correlation of smoking history with hearing loss. J Am
Med Assoc, 223(8): 920.
Zetterstrom O, Osterman K, Machado L, & Johansson SGO (1981) Another
smoking hazard: raised IgE concentration and increased risk of
occupational allergy. Br Med J, 283: 1215-1217.
Zhang Z-F, Yu S-Z, Li W-X, & Choi BCK (1989) Smoking, occupational
exposure to rubber and lung cancer. Br J Ind Med, 46: 12-15.
Zhu H & Wang Z (1993) Study of occupational cancer in asbestos
factories in China. Br J Ind Med, 50(11): 1039-1042.
Zuskin E, Zagar Z, Schacter EN, Mustajbego J, & Kern J (1992)
Respiratory symptoms and ventilatory capacity in swine confinement
workers. Br J Ind Med, 49(6): 435-440.
SYNOPSIS
1. Introduction
Le tabac, notamment lorsqu'il est fumé, exerce divers effets nuisibles
à la santé; il est directement en cause dans un certain nombre de
maladies graves et peut accentuer l'action nocive d'autres agents
chimiques, physiques ou biologiques. Les produits chimiques et autres
agents présents sur les lieux de travail peuvent, si l'on n'y veille pas
suffisamment, provoquer diverses pathologies, des invalidités et des
décès prématurés. Il est clair que sur les lieux de travail, des effets
indésirables peuvent résulter de la synergie entre le tabagisme et
d'autres facteurs de risque. La plupart des interactions entre les
constituants nocifs de la fumée de tabac et certaines substances
chimiques toxiques se produisent lorsque celles-ci sont présentes dans
l'atmosphère, mais le tabagisme peut également, comme on l'a observé,
interagir avec des agents toxiques absorbés par voie buccale ou autre.
La consommation de tabac est universelle, des pays économiquement
défavorisés aux nations industrialisées les plus riches. Le tabac est
consommé par les hommes et les femmes, les enfants et les adultes et des
millions de gens sont exposés malgré eux à la fumée de tabac présente
dans l'environnement. Il y a de nombreuses explications au tabagisme,
mais la raison principale de son universalité tient à la présence, dans
les feuilles de toutes les variétés de tabac, de nicotine, une substance
qui engendre la dépendance. Cette dernière pénètre dans l'organisme du
consommateur en quantité variable, selon le type de tabagisme auquel il
s'adonne (Chapitre 2). L'apparition de la cigarette, qui peut être
produite en quantités industrielles, qu'il est facile de se procurer à
un prix relativement bas et que sa légèreté permet de tenir entre les
lèvres en gardant les mains libres, a profondément modifié le
comportement des fumeurs, que ce soit dans la vie en général ou sur les
lieux de travail.
Il y a beaucoup de pays où fumer est considéré comme très dangereux pour
la santé et comme un facteur qui contribue de manière importante à la
mortalité résultant d'un certain nombre d'affections courantes. Ces pays
ont adopté une législation qui vise à alerter les consommateurs des
risques qu'ils courent et ils ont également pris des mesures pour
freiner la consommation, soit par la taxation, soit par la mise en
oeuvre de campagnes d'information à destination du grand public qui ont
pour but d'attirer l'attention sur les dangers du tabagisme et les
avantages qu'il y a à cesser de fumer ou à ne pas commencer du tout.
Certains pays n'ont toutefois pas encore pris de mesures décisives pour
traiter le problème du tabagisme.
L'activité professionnelle comporte souvent une part de risque. Elle
peut être de nature à mettre la santé en danger et à contaminer
l'environnement. La culture du tabac elle-même implique l'utilisation de
pesticides, la récolte des feuilles peut provoquer des intoxications
dues à l'absorption percutanée de nicotine et les diverses opérations
auxquelles elles sont ensuite soumises expose les travailleurs à
respirer les poussières et les spores de champignons présentes dans
l'atmosphère. Dans les zones où sont implantées des manufactures de
tabac, on observe une incidence élevée de cancers chez les sujets de
sexe masculin. L'air des exploitations minières est chargé de poussières
minérales et dans l'agriculture ou les industries qui utilisent des
matières premières d'origine biologique, les travailleurs sont exposés
à des poussières de même origine. Le soudage donne également lieu à la
production de vapeurs, et les gaz, fumées, brouillards etc. produits
dans de nombreuses industries sont aussi source de dangers. Une chaleur
excessive ou l'exposition aux ultraviolets peuvent nuire au bien-être
des travailleurs. On admet désormais que les rayonnements ionisants émis
dans les mines ou par certains appareils modernes constituent un risque
professionnel. Nombreuses sont les activités professionnelles qui
entraînent une exposition à des niveaux de bruit excessifs ou à des
vibrations nocives. Toutes ces conditions de travail ont des effets
indésirables sur la santé, mais qui peuvent être plus graves pour les
fumeurs que pour les non fumeurs. Dans un grand nombre de pays, il est
interdit de fumer sur les lieux de travail, essentiellement d'ailleurs
en raison des risques d'incendie ou d'explosion. Dans d'autres, cette
réglementation n'est pas toujours appliquée. Dans certains pays
nouvellement industrialisés, les problèmes sanitaires liés à l'activité
professionnelle ne sont pas encore totalement pris en compte et de
nombreux employés et ouvriers ne sont pas conscients des risques de leur
métier sur le plan sanitaire. En outre, il existe un vaste secteur
industriel "non officiel", en particulier dans les pays en
développement, où le travail, qui se fait à domicile, peut comporter
l'utilisation de produits chimiques (solvants, résines, colorants de
synthèse etc.) auxquels toute la famille va donc se trouver exposée. En
outre, il n'existe pas de réglementation qui restreigne l'exposition ou
interdise de fumer dans ces circonstances.
Beaucoup moins bien défini est le cas d'effets nocifs résultant de
l'action combinée d'une exposition à la fumée de tabac, provenant du
courant principal ou de l'environnement, et à des agents présents dans
le milieu domestique. On sait toutefois qu'en ce qui concerne
l'incidence du cancer du poumon, la courbe dose-réponse obtenue dans le
cas d'une exposition domestique au radon est analogue à celle qu'on
obtient chez des mineurs exposés à ce gaz, avec un risque plus important
chez les fumeurs.
2. Exemples d'effets combinés d'une exposition à la fumée de tabac et
à d'autres agents
On est fondé à penser que dans le cas de certains effets toxiques (en
l'occurrence le cancer) il existe une synergie entre le fait de fumer et
l'exposition à l'arsenic, à l'amiante, à l'éthanol, à la silice et aux
rayonnements (radon, bombe atomique, rayons X). D'un autre côté, il y a
également lieu de croire à l'existence d'un antagonisme entre le
tabagisme et les chlorométhyléthers cancérogènes comme le
chlorométhyl-méthyléther (CMME) et le bis (chlorométhyléther) (BCME)
(Hoffmann & Winder, 1976; CIRC, 1986), entre le tabagisme et l'alvéolite
allergique ou encore entre le tabagisme et la bérylliose chronique.
Fumer accentue les effets nocifs d'une exposition à la poussière chez
les mineurs de charbon, ou aux pesticides chez ceux qui en manipulent,
et cette accentuation du risque s'observe également dans l'industrie du
caoutchouc et du pétrole. Les mineurs de charbon qui fument risquent
davantage de contracter une bronchite chronique ou une pneumopathie
obstructive, mais pas un emphysème. Les cancers du poumon observés chez
les mineurs de charbon sont attribués en totalité au tabagisme. Fumer
peut accroître les effets d'une exposition aux poussières végétales qui
engendrent des affections respiratoires chroniques, comme la byssinose
produite par la poussière de coton et le cancer des fosses nasales
provoqué par la poussière de bois.
3. Composition des feuilles et de la fumée de tabac
On a isolé plus de 3040 composés chimiques des feuilles de tabac après
transformation (Roberts, 1988). La plupart d'entre eux sont des
constituants de la feuille, mais d'autres résultent des conditions de
culture (sol et atmosphère de la région) ou encore des produits
agrochimiques utilisés et des traitements subis (sauçage,
humidification, aromatisation et séchage). On constate des différences
selon la région d'origine du tabac, les variétés et les diverses
méthodes de séchage et de transformation utilisées. Ces différences
peuvent affecter la proportion des divers constituants mais la
composition globale ne varie pas. Parmi les importants composés toxiques
que l'on a mis en évidence, on trouve, à côté de la nicotine, des
nitrosamines cancérogènes qui proviennent de l'action des nitrites, des
amines, des protéines et des alcaloïdes d'origine foliaire, des
hydrocarbures aromatiques polycycliques formés au cours du séchage, des
éléments radioactifs captés dans le sol et dans l'air ainsi que du
cadmium dans le cas de tabacs cultivés sur des sols riches en cadmium.
Lorsque l'on fume, la combustion du tabac conduit à la formation de
nombreux produits de pyrolyse ou résultant d'autres types de réactions.
4. Fumée du courant principal
La fumée de tabac est un aérosol consistant en une phase particulaire
constituée de gouttelettes de liquide dispersées dans une phase gazeuse
ou vapeur. Lorsque l'on fume une cigarette, il se forme de nombreux
composés qui résultent de la pyrolyse du tabac. Ceux-ci peuvent, soit
traverser la cigarette dans la fumée constituant le courant
principal-certains d'entre eux se condensant légèrement en arrière du
cône incandescent-, soit passé dans l'air à partir de l'extrémité
incandescente, dans la fumée qui constitue le courant latéral. A chaque
bouffée, ces composés se concentrent dans la fumée car les produits qui
s'étaient déjà condensés viennent s'y ajouter -- la zone de condensation
se réduisant à mesure que la cigarette se raccourcit. La nature
physicochimique de la fumée dépend du traitement subi par le tabac et de
sa combustion, de la porosité et du traitement du papier et du type de
bout-filtre (Hoffmann & Hoffmann, 1997). Dans le cas d'une cigarette ou
de ce que l'on appelle un "bidi" en Asie (du tabac roulé dans la feuille
d'une plante), la composition chimique de la fumée dépend de facteurs
tels que les dimensions et la porosité de l'enveloppe ainsi que des
paramètres du fumage : volume, fréquence et durée des bouffées (NIH,
1998). Les variations de composition chimique concernent davantage la
proportion des différents constituants que la présence ou l'absence de
tel ou tel composé.
La fumée du courant principal est produite à l'intérieur du cône
incandescent dans une atmosphère relativement pauvre en oxygène à une
température de combustion de 850-950°C. Au départ, les particules de
cette fumée ont un diamètre aérodynamique massique médian (DAMM) de 0,2
à 0,3 µm; toutefois, dès qu'elles pénètrent dans les voies
respiratoires, où le degré d'humidité est de 100%, elles s'agrègent pour
former des particules de plus grande taille et se comportent alors comme
si leur DAMM était de l'ordre du micromètre. Environ 50 à 90% des
particules inhalées peuvent être retenues dans les voies respiratoires
(Wynder & Hoffmann, 1967; Hinds et al., 1983). Pour des raisons d'ordre
dimensionnel, les particules présentes dans l'aérosol, les constituants
de la phase gazeuse et les gaz permanents sont capables d'atteindre les
alvéoles lors de l'inhalation de la fumée. Le comportement des
constituants hydrophiles en présence d'une forte humidité fait que le
dépôt dans l'arbre trachéobronchique revêt un caractère complexe, mais
de toute manière, la fumée s'insinue dans la totalité des voies
respiratoires.
Près de 4000 constituants ont été répertoriés dans la fumée du courant
principal à côté d'un nombre indéterminés de substances non identifiées
(Roberts, 1988). La fumée du courant principal comporte une phase
particulaire et une phase gazeuse. La phase particulaire contient de la
nicotine, des nitrosamines telles que la
4-(méthylnitrosamino)-1-(3-pyridyl)-1-butanone (NKK) et la
N-nitrosonornicotine (NNN), des métaux comme le cadmium, le nickel, le
zinc et le polonium-210, des hydrocarbures polycycliques et des amines
cancérogènes comme le 4-aminobiphényle. La phase gazeuse renferme du
monoxyde et du dioxyde de carbone, du benzène, de l'ammoniac, du
formaldéhyde, du cyanure d'hydrogène, de la N-nitrosodiméthylamine, de
la N-nitrosodiéthylamine et un certain nombre d'autres composés. Les
composés présents dans la fumée de tabac peuvent, selon leurs effets
biologiques, être classés en asphyxiants, irritants, ciliatoxines,
mutagènes, cancérogènes, inhibiteurs d'enzymes, neurotoxines ou dérivés
dotés d'action pharmaceutique. C'est principalement par les voies
respiratoires que la fumée de tabac pénètre dans l'organisme mais de
nombreux constituants, présents en particulier dans la fumée de pipe et
de cigare, se dissolvent dans la salive et sont soit avalés, soit
absorbés au niveau de la cavité buccale. Les fumeurs de pipe et de
cigare n'inhalent généralement pas la fumée, qui demeure dans la cavité
buccale où, on vient de le voir, elle se dissout dans la salive et peut
être soit absorbée par passage à travers la muqueuse buccale, soit être
directement avalée (NIH, 1998). Les boissons alcoolisées, par leur effet
solvant sur les constituants de la fumée, en facilitent la résorption.
5. Fumée du courant latéral
La fumée du courant latéral est généralement produite à une température
de combustion plus faible (500-600°C) dans une atmosphère réductrice.
Les particules de cette fumée ont, lorsqu'elles sont fraîchement émises,
une taille à peu près équivalente à celle des particules du courant
principal, avec un diamètre aérodynamique massique médian (DAMM)
d'environ 0,2 µm. Quantitativement, la composition de la fumée du
courant latéral est analogue à celle de la fumée du courant principal.
Certaines substances du courant latéral sont émises à une concentration
(rapportée à 1 g de tabac brûlé) plus élevée que les constituants du
courant principal. C'est notamment le cas de composés cancérogènes comme
la N-nitrosodiméthylamine et la N-nitrosodiéthylamine ou encore de
métaux comme le nickel et le cadmium. Beaucoup de dérivés cancérogènes
sont plus concentrés dans la fumée du courant latéral que dans celle du
courant principal. Des épreuves biologiques consistant à badigeonner la
peau de souris avec un condensant de fumée du courant latéral ont montré
que ce dernier est plus cancérogène que celui de la fumée du courant
principal (Wynder & Hoffmann, 1967; US Surgeon General, 1986; NIH,
1998).
6. La manière de fumer la cigarette et ses effets sur la toxicité de la
fumée
Les cigarettes n'ont pas toutes la même teneur en nicotine et le fumeur
"tire" plus ou moins fort en inhalant plus ou moins profondément pour
satisfaire son besoin de nicotine. Il s'ensuit qu'en fumant une
cigarette à bout-filtre pauvre en nicotine (< 1,2 mg) le fumeur va
tirer plus intensément, ce qui ne sera pas sans effet sur la toxicité
(NIH, 1998).
7. Résumé des conclusions et des recommandations
La consommation de tabac, notamment en le fumant, constitue un problème
de santé publique d'une extrême importance en raison de la morbidité et
de la mortalité qui en résultent. Outre les effets nocifs causés par une
utilisation active du tabac, on a montré qu'il en existe aussi qui
résultent de l'exposition passive à la fumée présente dans
l'environnement. Les dangers du tabagisme sont également accrus par la
possibilité d'interactions avec certains agents chimiques, physiques ou
biologiques présents sur les lieux de travail ou dans l'environnement en
général. On connaît certes quelques cas d'interactions antagonistes,
mais les risques inhérents au tabagisme l'emportent de très loin sur ses
effets protecteurs apparents. Tout doit être mis en oeuvre pour faire
cesser la consommation de tabac et en particulier l'habitude de fumer.
Il faut s'opposer très vigoureusement au tabagisme sur les lieux
publics. En outre, pour éviter des interactions avec d'autres types
d'exposition tout en éliminant le risque d'exposition passive à la fumée
de tabac, il faut interdire de fumer sur les lieux de travail.
Il faut aussi vivement inciter les gens à ne pas fumer à la maison afin
de protéger la santé de la famille et notamment celle des enfants. On
évitera ainsi également des interactions potentiellement dangereuses
avec d'autres types d'exposition pouvant survenir dans l'environnement
domestique. Il est nécessaire d'établir sans attendre des programmes
éducatifs sur les dangers du tabagisme pour la santé. Les professionnels
de la santé doivent prêter assistance aux personnes qui désirent cesser
de fumer. Comme le tabagisme peut entraîner une modification des
réactions aux médicaments ou autres formes de traitement, voire susciter
à leur encontre des réactions indésirables, les médecins doivent ajuster
les doses de leurs patients en conséquence et surveiller leurs
réactions.
PANORAMA GENERAL
1. Introducción
El consumo de tabaco, particularmente el hábito de fumar, provoca una
serie de efectos nocivos para salud, está directamente relacionado con
varias enfermedades graves y puede aumentar los efectos adversos de
otros agentes químicos, físicos y biológicos. Si no se controlan, los
agentes químicos y de otro tipo pueden producir en el puesto trabajo
enfermedades, discapacidades y la muerte prematura. Es evidente que en
el lugar de trabajo los efectos adversos pueden deberse a la interacción
sinérgica del humo de tabaco con otros peligros. La mayor parte de las
interacciones de los constituyentes nocivos del humo de tabaco con
sustancias químicas tóxicas se producen cuando estas últimas están en el
aire, aunque se han notificado asimismo interacciones del humo con
agentes perjudiciales ingeridos y/o absorbidos.
El consumo de tabaco está generalizado en todo el mundo, desde los
países de bajos ingresos hasta los industrializados más ricos. Lo
utilizan hombres y mujeres, niños y adultos, y millones de personas
están involuntariamente expuestas al humo de tabaco en su entorno. Si
bien hay numerosas explicaciones del hábito de fumar, la razón principal
de su ubicuidad es el efecto adictivo de la nicotina, droga presente en
todas las formas de la hoja de tabaco y que llega al consumidor en
cantidades variables según los distintos tipos de consumo (capítulo 2).
La aparición del cigarrillo, de producción masiva, fácil de obtener,
relativamente económico y ligero de peso, de manera que se puede llevar
en la boca dejando las manos libres, ha tenido repercusiones importantes
en el hábito de fumar, tanto en general como en el puesto de trabajo.
En muchos países se reconoce que el humo de tabaco constituye un peligro
grave para la salud y es un factor que contribuye de manera importante
a la muerte causada por diversas enfermedades comunes. En esos países se
ha aplicado una legislación de alerta sanitaria y medidas impositivas
para el control de su consumo, así como programas de educación del
público sobre los peligros del tabaco y las ventajas de no comenzar a
fumar o de interrumpir el consumo. Sin embargo, todavía hay países donde
no se han puesto en marcha medidas decisivas para abordar el problema
del consumo de tabaco.
Son muchas las situaciones laborales que conllevan un elemento de
riesgo. El tipo de trabajo puede generar efectos nocivos para salud y
las actividades laborales pueden provocar la contaminación de medio
ambiente. El propio cultivo del tabaco requiere el uso de plaguicidas,
la recolección de la hoja puede ocasionar trastornos debido a la
absorción de nicotina a través de la piel y su elaboración expone a los
trabajadores a peligros para la salud provocados por el polvo y las
esporas de hongos presentes en el aire. Se ha notificado una elevada
incidencia de cáncer en el sexo masculino en zonas con industrias
tabaqueras. En la minería existe polvo de minerales en el aire y en la
agricultura y la industria basadas en materias primas producidas
biológicamente hay polvo biológico. Durante las actividades de soldadura
se produce humo y en muchas industrias crean peligro los gases, humos,
neblinas y vapores cargados de sustancias orgánicas y/o inorgánicas
tóxicas. Un calor excesivo o la exposición a luz ultravioleta pueden
ser perjudiciales para el bienestar de los trabajadores. Está admitido
que las radiaciones ionizantes en la minería y en la tecnología moderna
son un peligro en el lugar de trabajo. En numerosas actividades, los
trabajadores están expuestos a un ruido excesivo o a vibraciones
mecánicas peligrosas. Estas condiciones laborales pueden afectar más
negativamente a la salud de las personas fumadoras que a la de las no
fumadoras. Son muchos los países en los que está prohibido fumar en el
trabajo, fundamentalmente por razones de seguridad en cuanto
incendios/explosiones. Sin embargo, en algunos países no siempre se
cumple la reglamentación. En varios países recientemente
industrializados no se han abordado todavía plenamente los problemas de
salud asociados con el trabajo y muchos empleadores y trabajadores
desconocen los peligros para la salud de sus actividades. Además, existe
un amplio "sector extraoficial" de la industria, particularmente en los
países en desarrollo, en que se trabaja en el hogar y se utilizan
sustancias químicas (en particular disolventes, resinas y colorantes
sintéticos), estando expuesta toda la familia, y no hay restricciones
sobre la exposición a los peligros en el trabajo o al humo.
Está mucho menos definida la situación en relación con los efectos
adversos en la salud derivados de la exposición combinada al humo de
tabaco -- de la corriente principal o del medio ambiente -- y a los
agentes del entorno doméstico. Sin embargo, la incidencia de cáncer de
pulmón y la concentración de radón en los hogares tiene una relación
dosis-respuesta similar a la que se produce entre el cáncer de pulmón y
la concentración de radón en las minas, y el riesgo es más elevado para
los fumadores.
2. Ejemplos de efectos combinados de la exposición al humo de
tabaco y a otras sustancias
Está demostrada la existencia de sinergia en la producción de efectos
nocivos (cáncer) entre el humo de tabaco y la exposición al arsénico, el
amianto, el etanol, el silicio y las radiaciones (radón, bomba atómica,
rayos X). Por otra parte, hay pruebas de antagonismo en el caso del humo
de tabaco y los clorometiléteres carcinogénicos, es decir, el
clorometilmetiléter (CMME) y el bis(clorometil)éter (BCME) (Hoffmann y
Wynder, 1976; CIIC, 1986), el humo de tabaco y la alveolitis alérgica y
el humo de tabaco y la beriliosis crónica. El humo de tabaco influye en
el riesgo para la salud de la exposición en la extracción de carbón, el
manejo de plaguicidas y las industrias del caucho y el petróleo. Los
trabajadores de las minas de carbón que fuman tienen un riesgo más
elevado de contraer bronquitis crónica y enfermedades obstructivas de
las vías respiratorias, pero no enfisema. El cáncer de pulmón de los
mineros del carbón se ha atribuido totalmente al humo de tabaco. El humo
de tabaco puede aumentar el riesgo para salud de la exposición a polvos
vegetales que producen trastornos respiratorios crónicos, como la
bisinosis debida al polvo del algodón y el cáncer nasal provocado por el
polvo de la madera.
3. Composición de la hoja del tabaco y del humo de tabaco
De las hojas de tabaco elaboradas se han aislado más de 3040 compuestos
químicos (Roberts, 1988). La mayoría son constituyentes de la hoja, pero
la presencia de algunos depende de las condiciones de cultivo, como el
suelo y la atmósfera de la zona, mientras que otros se derivan del uso
de productos químicos agrícolas, de revestimientos, humectantes y
aromatizantes añadidos a las hojas y de los métodos de curado. Las
diferentes variedades de tabaco que se cultivan en los distintos países,
así como las distintas formas de curado y elaboración, ponen de
manifiesto diversas diferencias. Las proporciones de los distintos
constituyentes pueden ser diferentes, pero no la composición general.
Entre los compuestos tóxicos importantes identificados, aparte de la
nicotina, figuran nitrosaminas carcinogénicas, derivadas de nitritos,
aminas, proteínas y alcaloides presentes en la hoja, hidro-carburos
aromáticos policíclicos procedentes del proceso de curado, elementos
radiactivos absorbidos del suelo y el aire, y cadmio en el tabaco
cultivado en suelos ricos en este elemento. Cuando se quema tabaco al
fumar, se forman numerosos productos derivados de la pirólisis y de
otras reacciones.
4. Corriente principal del humo de tabaco
El humo del tabaco es un aerosol formado por una fase particulada de
gotitas de líquido dispersas en una fase de gas/vapor. Al fumar un
cigarrillo se forman numerosos compuestos por la pirólisis del tabaco.
Éstos pasan a través del cigarrillo en la corriente principal del humo,
condensándose algunos a corta distancia detrás del cono de combustión,
o bien se emiten en el aire a partir del extremo que se quema como humo
lateral. En cada bocanada el humo se hace progresivamente más fuerte,
porque se le añade material previamente condensado y porque la longitud
del cigarrillo disponible para la ulterior condensación disminuye. Las
características fisicoquímicas del humo dependen de la elaboración y la
combustión del tabaco, la porosidad y el tratamiento del papel en el que
está envuelto y del tipo de filtro (Hoffman y Hoffman, 1997). En el caso
de un cigarrillo o "bidi" asiático (tabaco envuelto en hoja vegetal), la
química del humo se ve afectada por factores tales como las dimensiones,
la porosidad de la envoltura y los parámetros del volumen, la frecuencia
y la duración de la bocanada de humo (NIH, 1998). Las variaciones en la
química del humo se dan fundamentalmente en la proporción entre sus
constituyentes, más que en la presencia o ausencia de compuestos
concretos.
El humo de la corriente principal se genera en una atmósfera con un
contenido comparativamente bajo de oxígeno a una temperatura de
combustión de 850-950°C en el cono de combustión. Al principio, las
partículas presentes en la corriente principal tienen un diámetro
aerodinámico medio de la masa (MMAD) de 0,2 a 0,3 micras; sin embargo,
en cuanto llegan al tracto respiratorio, con una humedad del 100%, se
unen para formar partículas mayores y se comportan como si su MMAD fuera
del orden de micras. En el tracto respiratorio se puede retener
alrededor del 50-90% de todas las partículas inhaladas (Wynder y
Hoffmann, 1967; Hinds et al., 1983). Por lo que se refiere al tamaño, al
inhalar el humo pueden llegar a los alvéolos las partículas en aerosol,
los constituyentes de la fase de vapor y los gases permanentes. La
deposición en el árbol traqueobronquial se ve complicada por el
comportamiento de los constituyentes hidrofílicos en condiciones de
humedad elevada, pero el humo llega a todas las partes de las vías
respiratorias.
El humo de la corriente principal contiene cerca de 4000 sustancias
químicas identificadas y un número desconocido de sustancias químicas
sin identificar (Roberts, 1988). El humo de la corriente principal se
puede dividir en una fase de partículas y otra de gas. La fase de
partículas contiene nicotina, nitrosaminas como la
4-(metilnitrosamino)-1-(3-piridil)-1-butanona (NNK) y la
N-nitrosonornicotina (NNN), metales como el cadmio, el níquel, el zinc
y el polonio-210, hidrocarburos policíclicos y aminas carcinogénicas,
como el 4-aminobifenilo. La fase de vapor contiene monóxido de carbono,
anhídrido carbónico, benceno, amoníaco, formaldehído, cianuro de
hidrógeno, N-nitrosodimetilamina, N-nitrosodietilamina y otros
compuestos. Los compuestos del humo de tabaco se pueden clasificar por
su actividad biológica como asfixiantes, irritantes, ciliatoxinas,
mutágenos, carcinógenos, inhibidores de las enzimas, neurotoxinas o
compuestos farmacológicamente activos. El principal punto de entrada del
humo del cigarrillo en el organismo es por las vías respiratorias, pero
muchos constituyentes, en particular del humo de pipa y de cigarro, se
disuelven en la saliva y se absorben en la cavidad bucal o se ingieren.
Los fumadores de cigarros y de pipa no suelen inhalar el humo, que
permanece en la cavidad bucal, se disuelve en la saliva y se absorbe a
través de las membranas mucosas o se ingiere (NIH, 1998). Las bebidas
alcohólicas tienen un efecto disolvente de los constituyentes del humo,
facilitando su absorción.
5. Humo de tabaco lateral
El humo lateral se forma con una temperatura de combustión más baja
(500-600°C) en una atmósfera reductora. Las partículas del humo lateral
fresco son prácticamente del mismo tamaño que las de la corriente
principal, con un diámetro aerodinámico medio de la masa de alrededor de
0,2 micras. Desde el punto de vista cualitativo, la composición del humo
lateral es semejante a la del humo de la corriente principal. Algunas
sustancias químicas se emiten en el humo lateral con una concentración
mayor por gramo de tabaco quemado que en el humo de la corriente
principal. Esto es particularmente aplicable a carcinógenos como la
N-nitrosodimetilamina y la N-nitrosodietilamina y a metales como el
níquel o el cadmio. Muchos compuestos carcinógenos están más
concentrados en el humo lateral que en el principal. En biovaloraciones
con aplicación a la piel de ratones se ha demostrado que el humo lateral
condensado es más carcinogénico que el principal (Wynder y Hoffmann,
1967; US Surgeon General, 1986; NIH, 1998).
6. Efectos de la manera de fumar los cigarrillos en la
toxicidad del humo
El contenido de nicotina de los distintos cigarrillos varía, y el
fumador, para satisfacer la necesidad adquirida de nicotina, la ajusta
mediante la intensidad con la que fuma y la profundidad de la
inhalación. Por consiguiente, el fumador de cigarrillos con filtro y de
contenido bajo en nicotina (< 1,2 mg) fuma con mayor intensidad, y esto
influye en la toxicidad (NIH, 1998).
7. Resumen de las conclusiones y recomendaciones
El consumo de tabaco, en particular el hábito de fumar, representa un
peligro para la salud pública de la máxima importancia y es una causa
prevenible importante de morbilidad y mortalidad. Además de los efectos
adversos del consumo activo de tabaco para la salud, se han demostrado
efectos adversos derivados de la exposición al humo de tabaco presente
en el medio ambiente. Los riesgos del hábito de fumar también aumentan
como consecuencia de las interacciones con ciertos peligros químicos,
físicos y biológicos existentes en el lugar de trabajo y en el medio
ambiente general. Hay un pequeño número de casos de interacciones
antagonistas, pero los riesgos del humo de tabaco para la salud son muy
superiores a cualquier efecto protector aparente.
Se deben adoptar todas las medidas posibles para eliminar el consumo de
tabaco, en particular el hábito de fumar, y se ha de disuadir con
firmeza de fumar en lugares públicos. A fin de evitar la interacción con
otros tipos de exposición ocupacional y de eliminar el riesgo de
exposición al humo de tabaco del medio ambiente, debería prohibirse
fumar en el lugar de trabajo.
Con objeto de proteger la salud, en particular la de los niños, se
debería desalentar con firmeza el hábito de fumar en el hogar. De esta
manera se previenen posibles interacciones perjudiciales entre el humo
de tabaco y la exposición a otros peligros en la vivienda. Hay una
necesidad imperiosa de programas educativos sobre los peligros del
hábito de fumar para la salud. Los profesionales de la salud deberían
prestar asistencia para ayudar a los fumadores a dejar este hábito.
Debido a que el humo puede provocar una alteración de la respuesta a los
medicamentos y otros tratamientos o una reacción adversa a éstos, los
médicos deberían estudiar la posibilidad de introducir ajustes
apropiados de la dosificación y vigilar a los pacientes.
2.1.2 Tobacco smoking
Smoking prevalence data and most studies into the effects of smoking
have concentrated on cigarette smoking, yet worldwide only about 55%
of tobacco is used for cigarettes. The rest, along with significant
amounts traded in "farm gate" and "local market" sales or "home
grown", is smoked in "bidis" or other hand-rolled devices, or in some
form of pipe (IARC, 1986). Tobacco consumption data give an insight
into tobacco-related disease distribution, as can information on ways
of smoking.
Table 1. Estimated smoking prevalence by WHO Region, early 1990s
(from WHO, 1997)
Men Women
(%) (%)
WHO Regions:
African Regiona 29 4
Region of the Americas 35 22
Eastern Mediterranean Region 35 4
European Region 46 26
South-East Asia Region 44 4
Western Pacific Region 60 8
More developed countries 42 24
Less developed countries 48 7
World 47 12
a Smoking prevalence estimates for African Region are based on
very limited information
Differences in smoke chemistry occur with different tobacco cultivars
and curing methods. Curing removes moisture so that the leaf can be
stored without fermenting or rotting, and the time and temperature of
curing influences enzyme reactions such as deamination and oxidation,
and the content of oils and resins. Air-, flue-, sun-, and fire-curing
methods are employed and each produces distinctive tobaccos which are
intended for smoking in a specific way or to suit a particular
preference.
Between 1933 and the late 1940s, the yields from an average cigarette
varied from 33 to 49 mg tar and from < 1 to 3 mg nicotine (Creek et
al., 1994). However, in the 1960s and 1970s, the average yield from
cigarettes in Western Europe and the USA was around 16 mg tar and 1.5
mg nicotine per cigarette. Current average levels are lower. Changes
in the levels of tar and nicotine have resulted in the characteristic
more intense puffing and smoke inhalation pattern of smokers of
low-yield cigarettes (Djordjevic et al., 1995; NIH, 1996). The
high tar yields measured in the smoke of cigarettes in developed
countries before 1950 are comparable with current tar yields of
"bidis", which range from 23 to 48 mg per cigarette (Hoffmann et al.,
1974; Jayant & Pakhale, 1985; Ball & Simpson, 1987.) The "kretek" (a
cigarette of strong tobacco and cloves) of Indonesia yields up to
71 mg tar (WHO, 1985). Smoking materials in northern Thailand
(cigarette or cigar of strong tobacco plus various vegetable
materials) produce high levels of tar and nicotine (Simarak et al.,
1977).
For smoking, tobacco leaf can be flue-cured, light air-cured,
fire-cured or sun-cured, and can be of dark, oriental or N. rustica
type. Flue-cured tobacco is used for cigarettes and pipe tobacco.
Air-cured tobacco is used in blended cigarettes. Dark tobaccos are
widely grown, usually air-cured, and mostly used in their countries of
origin for dark cigarettes, "bidis", cigars, in pipes and "sheeshas",
and for chewing tobaccos and snuff (Voges, 1984). Some tobaccos have
low nitrate levels ranging up to 0.9% (Neurath & Ehmke, 1964; Wynder &
Hoffmann, 1967) and their smoke contains low nitrogen oxide levels in
the range of 50-200 mg NOx/cigarette (Norman et al., 1983). In the
reducing atmosphere of the burning cone, NOx gives rise to amino
radicals, which react with benzene, biphenyl, naphthalene and other
ring hydrocarbons to form aromatic amines. Aniline, alkylated
anilines, aminobiphenyls, and naphthylamines are therefore found in
higher quantities in the smoke of tobaccos with a high nitrate content
(Patrianakos & Hoffmann, 1979; Pieraccini et al., 1992; Grimmer et
al., 1995). Smokers of these tobaccos can inhale greater quantities of
aromatic amines, such as the human bladder carcinogens
2-naphthyl-amine and 4-aminobiphenyl, and have higher concentrations
of haemoglobin adducts in their blood (Bartsch et al., 1993). This is
the basis for the higher risk of bladder cancer among the smokers of
cigarettes made from dark tobaccos (Vineis et al., 1984; D'Avanzo et
al., 1990; Vineis, 1992).
In the Indian subcontinent, the "bidi" is the common smoking device.
It consists of tobacco flakes, loosely packed and hand-rolled in a
tendu or temburni leaf ( Diospyros melanoxylon). It contains less
tobacco (0.223 g) than a cigarette (0.782 g) (Ramakrishnan et al.,
1995) but up to 8.2% nicotine compared with up to 3.7% in cigarette
tobacco. "Bidi" smoke contains 23 to 48 mg tar and 1.7 to 2.9 mg
nicotine per cigarette (Hoffmann et al., 1974; Jayant & Pakhale,
1985). In India, where around 7% of world tobacco is consumed (US DA,
1990), around 30% of tobacco is smoked as cigarettes, 50% as "bidis",
10% in other ways, and 10% is used for chewing. "Bidis" need to be
puffed frequently to ensure even burning and can generate up to 70 mg
of carbon monoxide, while a US non-filter cigarette smoked under
identical conditions generated 25 mg carbon monoxide (Hoffmann et al.,
1974; Jayant & Pakhale, 1985).
Table 2. Estimated smoking prevalence, ranked in order of male smoking prevalencea
Rank Countryb Men Women Rank Country Men Women
(%) (%) (%) (%)
1 Republic of Korea (1989) 68.2 6.7 21 Seychelles (1989) 50.9 10.3
2 Latvia (1993) 67 12 22 Bolivia (1992) 50 21.4
2 Russian Federation (1993) 67 30 23 Albania (1990) 49.8 7.9
4 Dominican Republic (1990) 66.3 13.6 24 Cuba (1990) 49.3 24.5
5 Tonga (1991) 65 14 25 Bulgaria (1989) 49 17
6 Turkey (1988) 63 24 25 Thailand (1995) 49 4
7 China (1984)c 61 7 27 Spain (1993) 48 25
8 Bangladesh (1990) 60 15 28 Mauritius (1992) 47.2 3.7
9 Fiji (1988) 59.3 30.6 29 Greece (1994) 46 28
10 Japan (1994) 59 14.8 29 Papua New Guinea (1990) 46 28
11 Sri Lanka (1988) 54.8 0.8 31 Israel (1989) 45 30
12 Algeria (1980) 53 10 32 Cook Islands (1988) 44 26
12 Indonesia (1986) 53 4 33 Czech Republic (1994) 43 31
12 Samoa (1994) 53 18.6 33 Jamaica (1990) 43 13
15 Saudi Arabia (1990) 52.7 N/Ad 33 Philippines (1987) 43 8
16 Estonia (1994) 52 24 33 Slovakia (1992) 43 26
16 Kuwait (1991) 52 12 37 Cyprus (1990) 42.5 7.2
16 Lithuania (1992) 52 10 38 Austria (1992) 42 27
16 South Africa (1995) 52 17 39 Malaysia (1986) 41 4
20 Poland (1993) 51 29 39 Peru (1989) 41 13
41 Uruguay (1990) 40.9 26.6 66 Colombia (1992) 35.1 19.1
42 Argentina (1992) 40 23 67 Costa Rica (1988) 35 20
42 France (1993) 40 27 67 Slovenia (1994) 35 23
42 Hungary 40 27 69 Swaziland (1989) 33 8
42 India (1980s) 40 3 70 Luxembourg (1993) 32 26
42 Iraq (1990) 40 5 71 Singapore (1995) 31.9 2.7
42 Malta (1992) 40 18 72 Belgium (1993) 31 19
50 Brazil (1989) 39.9 25.4 72 Canada (1991) 31 29
51 Egypt (1986) 39.8 1 72 Iceland (1994) 31 28
52 Morocco (1990) 39.6 9.1 75 Australia (1993) 29 21
53 Lesotho (1989) 38.3 1 75 Ireland (1993) 29 28
53 Mexico (1990) 38.3 14.4 77 UK (1994) 28 26
55 El Salvador (1988) 38 12 78 USA (1993) 27.7 22.5
Table 2. (cont'd)
Rank Countryb Men Women Rank Country Men Women
(%) (%) (%) (%)
55 Italy (1994) 38 26 79 Pakistan (1980) 27.4 4.4
55 Portugal (1994) 38 15 80 Finland (1994) 27 19
58 Chile (1990) 37.9 25.1 81 Turkmenistan (1992) 26.6 0.5
59 Guatemala (1989) 37.8 17.7 82 Nigeria (1990) 24.4 6.7
60 Denmark (1993) 37 37 83 Paraguay (1990) 24.1 5.5
61 Germany (1992) 36.8 21.5 84 Bahrain (1991) 24 6
62 Norway (1994) 36.4 35.5 84 New Zealand (1992) 24 22
63 Honduras (1988) 36 11 86 Sweden (1994) 22 24
63 Netherlands (1994) 36 29 87 Bahamas (1989) 19.3 3.8
63 Switzerland (1992) 36 26
a Adapted from: WHO (1997).
b The year given in parentheses is the latest available year for data.
c Some 1991 data suggest that there has been little change in smoking prevalence since 1984.
d Data not available.
Table 3. Trends in adult consumption of cigarettes from 1970-1972 to 1990-1992 (Adapted from WHO, 1997)
Annual % change
1970-1972 to 1990-1992
WHO Regions:
African Region +1.2
Region of the Americas -1.5
Eastern Mediterranean Region +1.4
European Region 0
South-East Asia Region +1.8
Western Pacific Region +3
More developed countries -0.5
Less developed countries +2.5
World -0.8
Tar yields for Russian cigarettes were found to be high (21.6-29.2
mg) and cigarettes containing N. rustica produced high smoke
concentrations of tobacco-specific nitrosamines (TSNA) (up to 620 ng
total TSNA/cigarette) (Djordjevic et al., 1991). Indigenous cigarettes
and cigars in Thailand, containing tobacco and other vegetable
materials, have up to 41 mg and 200 mg tar, up to 5.5 mg and 11.4 mg
nicotine, and 41 mg and 820 mg carbon monoxide in cigarette and cigar
smoke, respectively. Indonesian cigarette smoke contains up to 100 ng
of carcinogenic volatile N-nitrosamines and up to 1580 ng of
carcinogenic tobacco-specific N-nitrosamines (Mitacek et al., 1990,
1991), and high tar cigarettes contain up to 28.1 mg tobacco-specific
N-nitrosamines per cigarette (Brunnemann et al., 1996).
Many cigar-like devices are smoked throughout Asia. The tobacco can be
rolled in a tobacco leaf or in the leaves of the jackfruit tree
( Artocarpus integrifolia), banana ( Musa paradisiaca) or hansali
( Grewia microcos) (Bhonsle et al., 1976). They may be smoked
conventionally or in the reverse manner with the burning end inside
the mouth (Reddy, 1974). In Thailand they can contain strong tobacco
and a mixture of koi bark ( Streblus asper), dry tamarind pod
( Tamarindus indica), khai bark ( Homonoia riparia, Euphorbiaceae),
Areca palm bark ( Areca catechu) or other tree bark; they can be
rolled in a banana leaf or have fragrant additives such as sandalwood
Mougne et al. (1982). They contain high levels of tar and nicotine
(Simarak et al., 1977; Mitacek et al., 1999).
Additives are used to enhance the fragrance or taste of smoke
(Hoffmann & Hoffmann, 1997). "Casing sauces", consisting of sugars,
aromatic substances and compounds such as glycerol, propylene glycol,
ethylene glycol and diethylene glycol, which resist changes in
moisture content, are sprayed on the leaf before it is cut to
condition it for processing. Flavouring and dressing compounds are
added to cut tobacco after drying and include licorice, menthol,
cocoa, chocolate, ginger, cinnamon, vanilla, molasses, angelica,
honey, essential oils from anise, clove and juniper, resins and plant
extracts and organic compounds such as coumarins. Additives have been
widely used in pipe and chewing tobaccos. They have also become
important in cigarette tobacco with the development of low-tar and
low-nicotine tobaccos and the use of stem, midrib or reconstituted
leaf (which lacks the aromas and flavours of natural tobacco leaf
lamina) and of tobacco dust requiring additives to ensure its
adherence to cut tobacco.
Added glycerol is transferred to mainstream smoke: 3-6% in cigarette
smoke and 35-43% in pipe smoke (IARC, 1986) and one of its pyrolysis
products is acrolein. Levels of acrolein ranging from 69 mg to 230 mg
per cigarette have been reported and air concentrations as high as
0.46 mg/m3 have been found in smoke-filled rooms (Izard & Liberman,
1978). Acrolein is extremely irritating to the eyes and nasal mucosa,
it affects mitotic and ciliary activity, at the cellular level it has
cytotoxic and cilia-depressant effects, and it can act as a mutagen
(Izard & Liberman, 1978).
In the USA, additives used in cigarette manufacture are food additive
compounds that are "generally recognized as safe (GRAS)" and,
therefore, also considered "safe" as additives to tobacco. However,
the non-volatile additives are to some extent pyrolized during smoking
and can give rise to toxic and/or carcinogenic agents in the smoke. A
major group of compounds formed during pyrolysis is that of the
polynuclear aromatic hydrocarbons. Ethylene glycol is pyrolytically
converted to the human carcinogen ethylene oxide (IARC, 1994a).
Another example of carcinogen formation during tobacco curing and
smoking is the case of MH-30, a sucker growth control agent formulated
from maleic hydrazide in diethanolamine. Residual MH-30 on tobacco
leads to the formation of N-nitrosodiethanolamine (NDELA) in the
smoke (Brunnemann & Hoffmann, 1981). The use of MH-30 on tobacco has
been forbidden in the USA since 1981, and NDELA levels in tobacco and
its smoke have declined (Brunnemann & Hoffmann, 1991).
The use of cloves as a tobacco additive can have health effects. In
Indonesia, the smoke of "kreteks", a blend of ground cloves with
60-65% of tobacco, contains between 41 and 113 mg of tar, and between
1.2 and 4.5 mg nicotine (WHO, 1985; Wise & Guerin, 1986). Mainstream
smoke of kreteks without filter tips contains 19-23 mg of eugenol
released from the cloves, while filter-tipped kretek smoke contains up
to 15 mg (LGC, 1982; Wise & Guerin, 1986). Inhalation of eugenol in
the smoke of kreteks can lead to interstitial haemorrhaging and
congestion of the lung, acute emphysema and acute pulmonary oedema.
These effects were also seen in Syrian golden hamsters exposed to the
smoke of kreteks (LaVoie et al., 1986).
Menthol and other additives that produce a sensation of coolness but
without a mint flavour have also been used in cigarettes. There is no
evidence that these additives result in a higher risk (Cummings et
al., 1987; Sidney et al., 1989).
Many shapes and sizes of smoking pipes are found worldwide (Voges,
1984). Various types of tobacco are used and the smoke can range from
mild to very strong. In the sheesha water pipe the tobacco is kept
alight by pieces of glowing charcoal and the smoke is drawn through
water before being inhaled. Sheesha smoke is mild and low in
particulate matter, benzo( a) pyrene and volatile phenols (Hoffmann
et al., 1961), but it has a high level of carbon monoxide, in part
from the charcoal that keeps the tobacco burning, and smokers have
high carboxyhaemoglobin levels and a reduced FEV1 (8.5% in women, 45%
in men) (Zahran et al., 1985; Al-Fayez et al., 1988).
2.1.3 Tobacco chewing and snuff
The popularity of tobacco chewing and snuff (finely powdered tobacco
leaf) has varied. Nasal inhalation of snuff has given way to the oral
application of snuff and other tobacco-containing mixtures between the
gum and lip, or gum and cheeks, or under the tongue. Chewing tobacco
comes in several forms and may have flavour added from syrups,
liquorice and brandy. Tobacco chewing has retained its popularity in
heavy industries, such as steel and coal mining, woodworking and the
petroleum industry, where the flammability hazard precludes smoking.
In Sweden, 17% of the population uses oral snuff. In the USA, sales of
chewing tobacco have declined but sales of oral snuff have increased
by 61% (US DA, 1997) owing to the popularity of the latter with
teenagers and young adult men. Oral tobacco use in India is very
common but, generally, in developing countries it is declining because
urban populations and younger age groups are smoking cigarettes.
"Betel-quid" chewing is common in Asia and Africa. The basic contents
of a betel-quid are slices of areca nut ( Areca catechu), lime and
tobacco, wrapped in a betel pepper leaf ( Piper betle), but it may
also contain dried dates, menthol and spices such as cardamom, cloves,
coriander, mace and cinnamon.
Other tobacco preparations for oral use often contain lime, calcium
carbonate, sodium carbonate, some form of ash or flavouring materials.
The lime and other agents assist the release of nicotine (Voges, 1984;
Idris et al., 1991).
2.2 Responses to mainstream smoke
Tobacco use has direct effects on health and is responsible for a
variety of diseases. These form a basis for studying interactions
between tobacco use and chemical, physical and biological agents, and
associated effects on health.
2.2.1 Acute responses
Inhaled irritant chemicals cause inflammation of the upper respiratory
tract (URT) and paranasal sinuses, sore throat and bronchial oedema.
Pulmonary oedema may follow if irritants penetrate the lower
respiratory tract. Acute irritation of the URT usually follows
inhalation of highly soluble gases. Slightly less soluble gases cause
URT and bronchial irritation, while relatively insoluble gases
penetrate deeply into the lung and can have delayed effects including
pulmonary oedema (Miller & Kimbell, 1995). Cigarette smoke is a
complex mixture of organic and inorganic constituents with particulate
and vapour phases and highly reactive free radicals (Hocking & Golde,
1979). It contains many compounds with irritant properties, which can
affect all parts of the lung. Kremer et al. (1994) examined the
association between occupational exposures to a variety of airway
irritants and respiratory system effects, and whether the association
was modified by smoking, airway hyperresponsiveness and allergy. The
irritants were SO2, HCl, SO42-, polyester vapour, polyamide vapour,
and oil mist and vapour. Current smoking, airway hyperresponsiveness
and allergy were significantly associated with a higher prevalence of
chronic respiratory symptoms, independent of each other and of
irritant exposure. The association between exposure and the prevalence
of chronic respiratory symptoms was greater in smokers than in non- or
ex-smokers.
2.2.1.1 Acute bronchitis
Acute bronchitis is an inflammation of the bronchial mucous membrane,
initially accompanied by a dry painful cough and followed by
mucopurulent sputum. The cause may be infectious agents or chemical
agents such as tobacco smoke, dusts, fumes, vapours or gases.
2.2.1.2 Asthma
Asthma is a chronic pulmonary inflammatory disease associated with
bronchial hyperreactivity causing paroxysmal dyspnoea due to spasm of
the bronchial musculature, swelling of the mucous membranes and the
production of viscid mucus. In the majority of asthma cases a clear
association exists with atopic IgE-mediated hypersensitivity. Allergic
asthma is a response to a specific agent. Many chemical agents,
including mixtures such as tobacco smoke, can induce asthma. Smoking
enhances the effect of other agents and can reduce the latent period
from first exposure to onset of sensitization.
There has been an increase in the prevalence of asthma in many
countries, and roles for environmental factors, increased
susceptibility and tobacco smoking have been suggested (ISAAC, 1998).
Cigarette smoking together, with atopic status, age, URT infection and
genetic factors, has been considered to increase susceptibility
(Venables et al., 1985; Seaton et al., 1993). Studies have shown a
relationship between cigarette smoking and serum IgE levels. Smokers
have higher levels of IgE with increasing age, compared to non-smoking
controls. A relationship has also been observed between IgE level and
the number of cigarettes smoked (Sherrill et al., 1994).
2.2.2 Chronic responses
2.2.2.1 Chronic obstructive lung diseases
The chronic obstructive lung diseases (COLD) are chronic bronchitis,
small airways disease, toxic bronchiolitis obliterans, emphysema and
fibrosis (Niewoehner et al., 1974; Niewoehner, 1991).
These diseases form an important group of pulmonary diseases caused by
smoking (and by chemical atmospheric pollution) (US Surgeon General,
1984). In a study by Simecek et al. (1986) of 215 229 adults in a
region of former-Czechoslovakia, smoking was the most important risk
factor in COLD. Risks for male non-smokers and light smokers under 30
years of age were 1.18% and 2.28%, respectively; for men aged 50
years, smoking more than 20 cigarettes a day, the risk was 20.36%
compared with 3.31% for non-smokers of the same age. In the USA,
80-90% of the mortality from COLD has been attributed to cigarette
smoking. Cigar and pipe smokers who inhale the smoke also have an
increased death rate from COLD (US Surgeon General, 1984, 1989; NIH,
1998). Between 1979 and 1993, the age-adjusted annual death rate from
COLD in women increased by 122% to 17.1 per 100 000, while in men it
increased by 14% to 27.8 per 100 000. The greater increase of COLD
among women during this period reflected the increase in smoking by
women.
2.2.2.2 Chronic bronchitis
Chronic bronchitis has been variously defined. The United Kingdom
Medical Research Council (MRC, 1965) considered it to be a condition
with persistent production of sputum, which might be associated with
cough, occurring on most days for at least three months in the year
for at least two successive years. The Council recommended a
classification of simple chronic bronchitis, chronic or recurrent
mucopurulent bronchitis or chronic obstructive bronchitis. In a
workplace context, Morgan (1982) defined it as "a condition
characterized by cough and sputum for at least three months of the
year, which may or may not be accompanied by airways obstruction, and
which is a consequence of prolonged inhalation of dust or irritant
gases at the workplace". Fletcher & Pride (1984) suggested an improved
terminology with the term chronic bronchitis meaning chronic or
recurrent bronchial hypersecretion only, abandoning the term chronic
obstructive bronchitis because this implies a causal connection
between mucus hypersecretion and airflow obstruction. In smokers'
lungs the irritant constituents of tobacco smoke cause hypersecretion
of mucus, alter its physical properties and chemical structure, and
impair the mucociliary clearance mechanism. Removal of major
ciliatoxins (hydrogen cyanide and volatile aldehydes) from the smoke
stream by charcoal filter tips reduces the effects on the lung
epithelium (Friedman et al., 1972). Mineral dusts, particularly those
encountered in mining, many biological dusts, irritant vapours and
gases, inorganic and organic chemical dusts and sprays can all cause
chronic bronchitis.
2.2.2.3 Small airways disease
Small airways disease (SAD) is a widespread narrowing of membranous
bronchioli. It is inflammatory in origin and is often associated with
excess mucus and an accumulation of macrophages in the respiratory
bronchioli. SAD is mainly caused by smoking, but can be associated
with environmental and industrial pollutants (Cosio et al., 1980).
2.2.2.4 Emphysema
Emphysema has been defined as a condition of the lung characterized by
an abnormal enlargement of the airspaces distal to the terminal
non-respiratory bronchioli, accompanied by destructive changes in the
alveolar walls, and without obvious fibrosis. It tends to be prevalent
in older age groups and follows SAD. For purposes of postmortem
examination of lung slices of miners, Ruckley et al. (1984) defined
emphysema as the presence of air spaces of 1 mm or more in size.
Macrophages that have engulfed foreign particles, including smoke
particulate matter in the lungs of smokers, and which have been found
accumulated in the bronchioli (Niewoehner et al., 1974) and in the
lung parenchyma (McLaughlin & Tueller, 1971) have been implicated in
the pathogenesis of emphysema. There are different forms of emphysema,
which vary with the nature of the insult to the tissues. One
hypothesis is that tobacco smoke causes increased production and
release of proteolytic enzymes, such as elastase, and interferes with
normal antiproteolytic mechanisms. Emphysema has been induced
experimentally in animals by endotracheal installation of elastase or
homogenates of alveolar macrophages or polymorphonuclear leukocytes.
Chronic exposure to tobacco smoke has a number of effects on alveolar
macrophages, including changes in metabolism, alteration of the enzyme
content and impairment of RNA and protein synthesis (Hocking & Golde,
1979). The function of alveolar macrophages is to remove inhaled
foreign material from the alveoli and respiratory bronchioles, and
their numbers increase when the lungs are exposed to particles and
gases. It has been demonstrated that the macrophage count is higher in
people exposed to cigarette smoke than in non-exposed people (Harris
et al., 1974; Rylander et al., 1979). Alveolar macrophages from
smokers are more active than those from non-smokers, numbers are
increased, there are morphological changes with an increased cell
diameter, crystalline inclusions and surface membrane alteration
(Sopori et al., 1994).
2.2.2.5 Pulmonary fibrosis
Pulmonary fibrosis is the abnormal formation of fibrous or scar tissue
and is the response of bronchiolar tissue to the deposition of an
inhaled inciting agent. Mineral and other dusts are causes of
pulmonary fibrosis. The radiological changes seen in early stages of
dust fibroses are associated with relatively minor lung function
impairment, but continuous exposure leads to a greater degree of
fibrosis and to progressive massive fibrosis in some subjects. On
histological, animal experimental and radiographic evidence, Weiss
(1984) concluded that cigarette smoking could cause diffuse fibrosis.
2.2.2.6 Effects on the immune system
Tobacco smoking and exposure to environmental tobacco smoke increase
susceptibility to pulmonary infections, and changes in immune
processes may be involved. Tobacco smoking affects humoral and
cellular immunity in humans and experimental animals, but the
magnitude of the changes vary widely among studies. In humans,
cigarette smoke has marked effects on alveolar macrophage morphology
and physiology, it decreases serum immunoglobulin (IgA, IgG, IgM) but
increases IgE, and has a range of effects on B- and T-lymphocytes.
Similar effects are found in experimental animals (Sopori et al.,
1994). Studies in rats and mice show that cigarette smoke or nicotine
induces impaired responses of systemically distributed B- and
T-lymphocytes to antigen-induced signalling (Geng et al., 1995, 1996).
T-lymphocyte unresponsiveness, with decreased antibody response to
T-dependent antigens, is important in response to infection (Sopori et
al., 1998).
2.2.3 Cancer
Many forms of cancer have been associated with inhaled particulate
matter, vapours, fumes and gases. Examples are lung cancer associated
with tobacco smoking and inhalation of asbestos, fumes from metal
moulding and coking plants, particles and gases inhaled by motor
vehicle drivers, dusts in several types of mines where there is an
accumulation of alpha-emitting radioisotopes, and contact with
materials such as arsenic, chromates, nickel, chloromethyl ethers,
mustard gas and polycyclic aromatic hydrocarbons. Pleural mesothelioma
has been associated with asbestos, nasal and sinus cancer with nickel
refining and wood dust exposure, leukaemia with ionizing radiations
and benzene, bladder cancer with the manufacture and use of dyes and
in the rubber industry, and liver cancer with the use of vinyl
chloride (IARC, 1987).
Cigarette smoke contains many toxic chemicals, including chemicals
that are DNA reactive and cytotoxic or become DNA reactive upon
metabolic activation. Consequently, cigarette smoke has the potential
to initiate genetic lesions. Moolgavkar et al. (1989) postulated
mechanisms by which cigarette smoke induces lung cancer by fitting the
two-stage clonal expansion model of carcinogenesis to lung cancer
mortality data derived from a large cohort of British doctors who
smoked. This analysis suggested that tobacco smoke affected both the
rates of mutation and cell proliferation involved in the model,
supporting the hypothesis that tobacco smoke acts as a complete
carcinogen.
Cigarette smoking is causally associated with cancer of the lung,
larynx, pharynx, oesophagus, pancreas, kidney and urinary bladder. It
is also associated with cancer of the nasal cavity, liver, uterine
cervix and myeloid leukaemia (RCP, 1962; US Surgeon General, 1982,
1989; IARC, 1986; Winkelstein, 1990; Brownson et al., 1993; Roush,
1996).
In 1991, in the USA, it was estimated that 90.3% of the lung cancer
deaths in men and 78.5% of lung cancer deaths in women were
attributable to cigarette smoking. Deaths from oesophageal cancer were
linked to smoking in 78.2% of the cases in men and 74.3% of the cases
in women. Smoking was held responsible for 81.2% of deaths from
laryngeal cancer in men and 86.7% in women, for 91.5% and 61.2%,
respectively, of deaths from oral cancer, and 46.5% and 36.7%,
respectively, of deaths from bladder cancer. Smoking-attributable
deaths from cancer of the kidney in men and women were 47.6 and 12.3%,
respectively, and for pancreatic cancer the figures were 28.6% and
33.3% respectively. In women, 32.4% of uterine cervical cancer deaths
were attributed to cigarette smoking (Shopland et al., 1991).
There has been a change in the rates of different types of lung cancer
among smokers. In 1950, squamous cell carcinoma (SCC) occurred 17
times more often than adenocarcinoma (AC) (Wynder & Graham, 1950) in
cigarette smokers. In 1991, Devesa et al. (1991) reported that in male
cigarette smokers the ratio of SCC to AC was 2.4:1 between 1969 and
1971, and changed to 1.4:1 between 1984 and 1986; in cigarette-smoking
women, the SCC to AC ratio changed from 3.6:1 in 1950 to 0.57:1 in
1984-1986. Between 1970 and 1980 some studies showed a 20-50%
reduction in risk of lung cancer for long-term smokers of filter
cigarettes as compared to smokers of non-filter cigarettes (IARC,
1986) but later studies indicated a similar risk for lung cancer in
smokers of filter and non-filter cigarettes (Stellman et al., 1997;
Thun et al., 1997). The changes in the ratio of SCC to AC, and the
disappearance of an advantage of filter cigarette smoking in terms of
lung cancer risk, have been related to changes in smoke yields, which
have caused smokers to modify patterns of puff drawing and smoke
inhalation. Smokers regulate the speed and the quantity of their
nicotine uptake to achieve the desired pharmacological effects
(Benowitz et al., 1988; Djordjevic et al., 1995). Smokers of lower
nicotine cigarettes draw puffs of greater volume, at a higher
frequency, and inhale more deeply; this is governed by the amount of
nicotine in the smoke (Wynder & Hoffmann, 1994).
Smoking is a major risk factor for the early stage development of
oesophageal and gastric adenocarcinomas, accounting for 40% of cases,
and may have contributed to the increase in the incidence of these
cancers, especially in older people (Gammon et al., 1997).
Cigar smoking is causally associated with cancer of the oral cavity,
the pharynx and the lung, even though for cigar smokers, who do not or
only minimally inhale the smoke, the lung cancer risk is considerably
lower than that for cigarette smokers. Cigar smoking is also
associated with cancer of the pancreas and of the urinary bladder
(NIH, 1998). Oral snuff users have an increased risk of cancer of the
oral cavity and, possibly, cancer of the oesophagus, pancreas and
urinary bladder (US Surgeon General, 1986).
It has been suggested that around 4-5% of all lung cancer is related
to occupational exposure (Wynder & Gori, 1977; Doll & Peto, 1981;
Morgan, 1982 ). Occupational and any other forms of exposure to
chemical compounds are of limited importance in the etiology of lung
cancer whereas tobacco smoking is the cause of approximately 85-90% of
cases (Shopland et al., 1991).
"Reverse smoking", in which rolled tobacco leaves are smoked with the
burning end inside the mouth, has been linked to carcinoma of the hard
palate (Reddy & Rao, 1957; Mehta et al., 1971; Pindborg et al., 1971;
Reddy, 1974; Bhonsle et al., 1976). Reddy et al. (1960) simulated the
effect of reverse smoking experimentally by painting the skin of male
and female mice on alternate days with the tar from Indian cigars and
exposing the painted skin to a temperature of 58°C for 3 min; the heat
treatment enhanced the dermal tumour response.
2.2.4 Cardiovascular effects
Cigarette smoking is a major independent risk factor for
cardiovascular disease (CVD). Cigarette smoking acts synergistically
with other risk factors, such as elevated cholesterol levels and
hypertension (Wilhelmsen, 1977; Gibinski, 1977; US Surgeon General,
1983). Prospective studies indicated that elevated cholesterol and
hypertension appear to be prerequisites for CVD in cigarette smokers
(Kimura, 1977). It has been estimated that in countries with a long
history of cigarette smoking the tobacco habit is responsible for
26-30% of early deaths from CVD (US Surgeon General, 1983; Wald et
al., 1985). Those who smoke several cigars a day and inhale the smoke
also face an increased risk of CVD (NIH, 1998).
The major contributors to the cardiovascular effects of tobacco smoke
are carbon monoxide and nicotine (Lakier, 1992), as well as nitrogen
oxides (NOx), hydrogen cyanide and tar; minor contributors are
cadmium, zinc and carbon disulfide (US Surgeon General, 1983). The
smoke of cigarettes can be slightly acidic (pH 5.6-5.9) or weakly
alkaline (pH 6.7-7.9) depending on the type of tobacco and the blend
(Brunnemann & Hoffmann, 1974). In the slightly acidic smoke, nicotine
is protonated, i.e., bound to a salt or acid moiety and is part of the
particulate phase. Weakly alkaline smoke contains a small percentage
of protonated nicotine (often up to 50% of the nicotine is
unprotonated) in the vapour phase. In contrast to the protonated
variety, unprotonated nicotine is rapidly absorbed through the oral
mucosa and this is why smokers of cigarettes with weakly acidic smoke
and smokers of cigars do not need to inhale into the lung to
experience the pharmacological effects of nicotine. Protonated
nicotine in the particulate matter of slightly acidic cigarette smoke
is not or is only minimally absorbed through the oral mucosa, so that
smokers inhale the smoke and absorption into the bloodstream takes
place in the lung (Armitage & Turner, 1970; Russell, 1976; Benowitz et
al., 1988).
Smoking has been associated with a two-to-fourfold increased risk of
coronary heart disease (CHD), a greater than 70% excess rate of death
from CHD, and an elevated risk of sudden death (Lakier, 1992).
Nicotine causes increases in heart rate and blood pressure, stimulates
nerve endings that are activated by acetylcholine, causes increased
mobilization of free fatty acids in the serum and enhances platelet
adhesiveness. These effects increase cardiac load (which for
individuals with some forms of heart disease will not be met by
increased coronary blood flow) and interfere with metabolic exchange
across capillary walls, leading to ischaemic episodes and thrombosis.
Carbon monoxide increases carboxyhaemoglobin concentrations in the
blood and lowers its oxygen-carrying capacity: increased oxygen debt
after exercise and impairment of endurance performance are evident in
smokers, compared to non-smokers. Carbon monoxide also has an affinity
for myoglobin and interferes with oxygen uptake by the myocardium.
"Bidi" smoke contains a higher concentration of carbon monoxide than
cigarette smoke (Hoffmann et al., 1974; Jayant & Pakhale, 1985; Ball &
Simpson, 1987). Most of the Asian smoking devices with their
non-porous wrappers and dark tobacco contain higher levels of nicotine
(Simarak et al., 1977; WHO, 1985). High nicotine and carbon monoxide
are also obtained from many dark tobacco cigarettes and from cigars.
"Sheesha" water pipe smoke contains small amounts of nicotine but is
rich in carbon monoxide; the blood carboxyhaemoglobin concentration in
sheesha smokers is higher than in cigarette smokers (Zahran et al.,
1985).
Cigarette smoking has been considered to be the primary cause of
Buerger's disease (thromboangiitis obliterans), an inflammatory
obliterative, non-atherosclerotic, vascular disease. The disease
usually becomes quiescent if the patient stops smoking cigarettes
(Olin, 1994). It was rare in women but an increasing number of cases
has been observed and ascribed to the increased use of tobacco by
young women (Yorukoglu et al., 1993).
Cardiovascular disease has been associated with exposure to other
factors, which can be classified as physical, chemical and biological,
and with occupation or life-style. The combination of smoking with any
of these factors will predispose to an increased detrimental
cardiovascular effect.
In women who smoke, peripheral vasoconstriction (PV) leading to acute
intervillous placental blood flow was measured during smoking and
attributed to nicotine, which simultaneously caused increases in heart
rate and blood pressure. It was suggested that PV explained fetal
growth retardation and other complications of pregnancy (Lehtovirta &
Forss, 1978).
2.2.5 Smoking and occupational accidents, injuries and
absenteeism
A survey of public employees found that cigarette smokers took 23%
more sick leave than non-smokers (Van Tuinen & Land, 1986). In another
study among 2537 postal workers in the USA, cigarette smokers had a
1.29 times higher accident rate (CI 1.07-1.55), and a 1.55 times
higher injury rate (CI 1.11-1.77) than non-smokers (Ryan et al.,
1992). Other studies confirm the higher rates of injury, occupational
accidents and absenteeism in smokers (Naus et al., 1966; Parka, 1983;
US Surgeon General, 1985; Hawker & Holtby, 1988). There are higher
costs of illness for cigarette smoking employees compared to
non-smokers (Van Peenen et al., 1986; Penner & Penner, 1990.
2.3 Health risks from smokeless tobacco use
2.3.1 Introduction
Oral cancers have been linked with oral tobacco use. The consequences
of chewing tobacco can be reactional keratosis, irreversible gingival
recession, periodontitis, oral dysplasia and leukoplakia, cancer and
cardiovascular effects (Chakrabarti et al., 1991; Guggenheimer, 1991).
In India, chewing material containing tobacco has been shown to be a
primary cause of oral cancer (Jussawalla & Deshpande, 1971).
2.3.2 Cancer
Leukoplakia and oral cancer are common results of oral tobacco use,
particularly in the countries of Asia (IARC, 1985a). Simarak et al.
(1977) reported a strong association between betel chewing and oral
cancer in northern Thailand. Sankaranarayanan et al. (1989a,b, 1990)
associated oral cancers in southern India with tobacco chewing.
Chakrabarti et al. (1991) reported much higher levels of pre-malignant
and malignant lesions of the oral cavity in tobacco chewers;
Nandakumar et al. (1990) found the relative risk to be elevated in
both sexes, but appreciably higher in females. From chemical analysis
of Indian tobacco products, it was concluded that their use may lead
to high exposures to carcinogenic tobacco nitrosamines (Nair et al.,
1989).
Case-control studies reported a higher incidence of oral cancer in
oral snuff users than in those not using any form of tobacco. There
was up to a 50-fold excess risk of cancer of the cheek and gum in
long-term oral snuff users (Axéll et al., 1978; US Surgeon General,
1986a; Winn, 1997). Idris et al. (1991, 1994), reported a high
incidence rate of oral cancer in men in northern Sudan who used an
oral snuff with relatively high concentrations of nicotine (0.8-3.2%)
and nornicotine. In a study of baseball players who chewed or (the
majority) used snuff orally, there was higher prevalence of
leukoplakia, plaque formation, gingivitis and dental disorders
compared to non-users (Robertson et al., 1997).
A case-control study on nasal cavity and paranasal sinus cancer and
snuff use showed that snuff users have a significantly increased risk
for adenocarcinoma and squamous cell carcinoma in the nasal cavity
(Brinton et al., 1984). N'-nitrosonornicotine is present and is an
organ-specific carcinogen that induces benign and malignant tumours of
the nasal cavity in rats, hamsters and mink (Rivenson et al., 1991;
Koppang et al., 1992, 1997; Hoffmann et al., 1994). Studies conducted
in South Africa showed that people using local snuff made from tobacco
and aloe plant ash as nasal applications have an increased risk for
tumours of the maxillary antrum (Keen et al., 1955). It was thought
that this snuff mixture contained high levels of nickel and chromium,
which may be associated with the induction of these tumours (Baumslag
et al., 1971).
Instillation of snuff into the lips of rats induced benign and
malignant tumours in the oral cavity (Hirsch & Thilander, 1981; Hecht
et al., 1986; Johansson et al., 1989; Larsson et al., 1989).
Instillation of snuff into the buccal pouch of hamsters, which were
repeatedly infected with Herpes simplex virus type I or II, led to
oral tumours, although no tumours occurred when either the virus or
tobacco was applied alone (Park et al., 1991b).
More than 3050 chemical compounds have been identified as tobacco
constituents (Roberts, 1988) and 50 are known carcinogens (Brunnemann
& Hoffmann, 1992). Nitrosamines, especially the tobacco-specific
nitrosamines (TSNA), must be regarded as major oral carcinogens. Oral
tumours were elicited when an aqueous solution of N-nitrosonornicotine
(NNN) and 4(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was
applied to the oral surfaces of rats (Hecht et al., 1993).
2.3.3 Cardiovascular disease
The effect of tobacco chewing on cardiovascular disease has received
less attention than the effect of cancer. Benowitz et al. (1988)
compared the cardiovascular effects of smokeless tobacco, cigarettes
and nicotine gum and reported that all tobacco use increased heart
rate and blood pressure. Nanda & Sharma (1988) recorded incremental
increases in heart rate and blood pressure following tobacco chewing.
However, Eliasson et al. (1991) found no significant elevation of
diastolic blood pressure in young snuff users. Bolinder et al. (1994)
found an excess risk of death from cardiovascular and cerebrovascular
disease among smokeless tobacco users. Tobacco chewing has detrimental
effects on pregnancy (Krishna, 1978) which, in the absence of any
anoxia due to carbon monoxide found in smokers, could result from
nicotine-induced vasoconstriction.
O'Dell et al. (1987) reported a case of Buerger's disease in a
38-year-old man that was associated with the use of smokeless tobacco.
A regimen which included complete abstinence from tobacco resulted in
a resolution of the symptoms. Buerger's disease is the commonest
vascular disease in the Indian subcontinent where tobacco consumption
is high (Jindal & Patel, 1992). In a study of the disease in
Bangladesh, 39 patients (38 males, 1 female) were investigated. All
but two were current long-term smokers, one male had given up smoking
6 months previously and the one woman with the disease (it is uncommon
in women) was a tobacco chewer (Grove & Stansby, 1992).
3. EFFECTS ON HEALTH OF TOBACCO USE AND EXPOSURE TO OTHER CHEMICALS
3.1 Introduction
3.1.1 Interaction
To discuss the interaction between tobacco and other agents as risk
factors for cancer and other adverse health effects, it is necessary
to define what is meant by the term "interaction". In general terms,
interaction represents a departure from additivity, in which the
combined effect of exposure to two agents is in some sense the sum of
the effects of the individual agents (US EPA, 1988). Synergism occurs
when the combined effect is greater than the sum of the component
effects; antagonism occurs when the effect of the combination is less
than would be suggested by summing the effects of the components.
3.1.2 Measuring interaction
To make these concepts precise, the scale in which risk is measured
and the manner in which risks are summed must be specified (Kaldor &
L'Abbe, 1990). In epidemiological investigations, the age-adjusted
relative risk is often used to characterize risk. In risk assessment
applications involving chronic health effects such as cancer, the
cumulative risk over a period of time may be of more direct interest.
In laboratory studies of carcinogenicity, for example, the lifetime
probability of tumour induction is often used to describe risk. In
order to take into account the age at which an adverse effect is
induced, the expected loss in life expectancy has also been used to
evaluate risk (UNSCEAR, 1988).
Kodell & Pounds (1991) discuss interaction in terms of departures from
response additivity and dose additivity. Dose additivity occurs
when the combined effect of two agents can be expressed in terms of a
total dose of the two agents, taking into account their relative
potencies. Dose additivity presumes that the two agents act by the
same biological mechanism, and that the effective dose of one agent is
simply a dilution of the dose of the other agent. Response additivity
occurs when the two agents act independently of each other. In this
case, the probabilities of an adverse effect due to each of the agents
can be treated as statistically independent and can be combined
accordingly.
The quantification of interaction may be illustrated using the
age-specific relative risk (A,B) associated with exposure to two
agents A and B. The relative risk is formally defined as:
relative risk (A,B) = I(A,B)/I(0,0)
where I(A,B) denotes the incidence rate of the disease of
interest at a specified age in the presence of exposure and
I(0,0) is the incidence rate in the absence of exposure. Lack of
interaction then corresponds to additivity of the excess relative
risk (ERR = relative risk - 1).
Specifically,
ERR(A,B) = ERR(A,0) + ERR(0,B)
where ERR(A,0) and ERR(0,B) denote the excess relative risks for
A and B alone, respectively. In terms of relative risk,
additivity is equivalent to:
relative risk (A,B) = relative risk (A,0) + relative risk
(0,B) - 1
For a supra-additive relative risk relationship, such as the
multiplicative relative risk model:
relative risk (A,B) = relative risk (A,0)RR(0,B)
and reflects a synergistic effect.
It is of interest to note that as the relative risk (A,0) and relative
risk (0,B) become small, the multiplicative relative risk model
approximates the additive relative risk model. Under this
multiplicative model, a strong synergistic relationship may be
apparent at high exposures, yet become negligible at low exposures.
This, along with the realization that relative risks of less than
about two are difficult to detect epidemiologically, suggests that
relatively high exposures are likely to be required to evaluate
interactive effects reliably.
Brown & Chu (1989) and Kodell et al. (1991) investigated the type of
interactions that might be expected under both the Armitage-Doll
multi-stage model and the Moolgavkar-Venzon-Knudson two-stage model of
carcinogenesis following exposure to two carcinogens that may affect
different stages in the model. These theoretical results indicate that
a variety of synergistic interactions are possible, including
supra-additive, multiplicative, and supra-multiplicative.
Although the additive excess relative risk model described above
provides a useful baseline for evaluating interaction, it is not the
only model that has been proposed for this purpose. Kaldor & L'Abbe
(1990) pointed out that the multiplicative relative risk model will
become the baseline model for evaluating interactions following a
logarithmic transformation of the relative risk. Thomas & Whittemore
(1988) review arguments in favour of the additive and multiplicative
models as the basis for evaluating interaction. Steenland & Thun
(1986) illustrate how these two models can be applied in evaluating
tobacco/occupation interactions in the causation of lung cancer.
This brief overview illustrates that interaction can be measured in
different ways, with the most appropriate depending on the nature of
the problem at hand. In general, however, all of the commonly
encountered measures of synergism indicate that the risk associated
with combined exposure to two agents is in some sense greater than
would be expected based on the risks of individual exposures. Although
no attempt is made throughout this monograph to systematically specify
the precise nature of the interactive effects reported in the
literature, most interactive effects documented in this monograph have
been identified through epidemiological investigations, in which the
additive age-specific relative risk model is the predominant approach
to describing interaction (Saracci, 1987; Greenland, 1993).
There are four principal ways in which tobacco smoke can interact with
other chemicals to impair the health of the smoker. They are not
mutually exclusive and in fact there are many situations in which they
may occur together, particularly in the workplace or the environs of
industry.
a) Modification of effects
Cigarette smoke can modify the harmful effects associated with other
toxic agents, in some cases causing a highly elevated risk, e.g., the
effects of smoking on diseases related to asbestos, alpha-radiation,
arsenic and some organic compounds.
b) Increased concentration effects
Chemical compounds hazardous to health are often found in both tobacco
smoke and the working environment and each source can augment the dose
obtained from the other, e.g., carbon monoxide, acrolein, benzene and
heavy metal elements.
c) Vector effects
Materials used in the workplace that produce harmful chemical agents
when they are burnt or vaporized can contaminate smoking materials and
cause the smoke to be far more injurious when the tobacco is smoked,
e.g., polytetrafluoroethylene and methylparathion.
d) Other interactions
Tobacco smoke can affect a physiological process and increase the
impairment of physical or physiological functions caused by another
activity. For instance, impaired lung clearance will affect the
residence time of inhaled toxic materials, the effect of smoking on
the peripheral vascular system can enhance the detrimental effects of
vibration and noise, and smoking may alter the effect of drugs taken
for other purposes.
3.1.3 Effects of tobacco smoking on lung deposition and clearance of
particles
Pulmonary deposition of inspired particles depends on their
physicochemical properties and on airway structure and geometry.
Mathematical models describing the deposition of particles in the
various airway sections show that in compromised airways, as is the
case in patients suffering from asthma and chronic obstructive lung
diseases, particle deposition is enhanced several-fold (ICRP, 1994).
Tobacco smoking can be an indirect cause of enhanced deposition of
inspirable particles. In addition, during tobacco smoking the
breathing pattern is changed to more frequent and deeper inhalation,
especially in the case of low-nicotine cigarettes, which can result in
an increased inhaled dose and dose rate of inspirable compounds (IARC,
1990).
Clearance of particles deposited in the lung is a complex
physiological process involving relatively rapid tracheobronchial
clearance, in which mucus is moved upward by ciliary action to the
pharynx and swallowed, and slower deep-lung clearance, in which
phagocytic cells remove inhaled particles. These processes are
balanced by the solubility of the inhaled particles, with relatively
insoluble particles having a longer residence time in the alveolar
portions of the lungs. A longer residence time in the lung would be
accompanied by a greater possibility that harmful effects could occur.
Both rapid and slow clearance phases are reduced by smoking, although
probably by different mechanisms. Cigarette smoke contains significant
concentrations of ciliatoxic agents, such as hydrogen cyanide,
formaldehyde, acetaldehyde, acrolein and nitrogen oxides, which
greatly contribute to retarded clearance of inhaled particles by
inhibiting lung clearance mechanisms (Battista, 1976). Retardation of
clearance has been seen for airway-deposited particles, in which
decreased mucus transport velocities slow this normally relatively
rapid phase of clearance (Lourenco et al., 1971; Chopra et al., 1979).
The deep-lung clearance of relatively insoluble particles is retarded
in smokers. Cohen et al. (1979) found that 1 year after a tracer
particle exposure, some 50% and 10% of the original lung burden
remained in the lungs of smokers and non-smokers, respectively.
Bohning et al. (1982) and Philipson et al. (1996) found that smoking
retarded long-term particle clearance from the lungs. The mechanism(s)
for interference with this longer-term phase of clearance has not been
shown definitively, but may be related to impairment of phagocyte
function and/or smoke-induced lung damage.
3.2 Interactions between tobacco smoke and other agents
3.2.1 Asbestos
Asbestos is a generic name for a group of fibrous silicates, differing
in colour, fibre arrangement and length. Recognition of the health
risks of asbestos has led to major reductions in production and uses.
Asbestos types are classified according to their physical
characteristics as serpentine or amphibole and differ in their
relative carcinogenic potential. Amosite and crocidolite are
amphiboles and have short and straight needle-like fibres. Chrysotile
is a serpentine and consists of long, pliable white fibres. The longer
fibre varieties of asbestos can be spun into yarn which can be woven
into fabric; short fibre varieties can be incorporated into cement,
asbestos board and tiles. Asbestos products have been used in a
variety of applications including electrical and thermal insulation in
buildings, fire and safety equipment, brake linings of motor vehicles,
and shipbuilding. Workers in asbestos mining and processing and a wide
range of manufacturing industries are exposed to various forms of
asbestos, while others are exposed in maintenance work, demolition and
recycling operations.
Occupational exposure to asbestos is associated with asbestosis and
cancers at various sites, notably pleural mesothelioma and lung
cancer. Differences between the effect of asbestos on the health of
smokers and non-smokers have been reported, and studies have been
conducted aimed specifically at elucidating the combined effects of
smoking and asbestos exposure. Perioccupational exposure to asbestos
is a hazard to household contacts of asbestos workers, who bring home
dust on their clothes, and to people living in areas where there is
environmental contamination by asbestos dust from industry (Anderson
et al., 1979).
The amphibole varieties of asbestos (crocidolite and amosite) have the
highest carcinogenic risk. Crocidolite presents a greater risk than
amosite, which in turn is more dangerous than chrysotile, a serpentine
variety. Erionite and tremolite are non-asbestos fibrous minerals used
in building in some parts of the world and there is a high prevalence
of mesothelioma in these regions (Baris et al., 1979; Yazicioglu et
al., 1980).
Because there are many different occupations and environmental
situations in which asbestos exposure might occur, along with a wide
range of possible levels of exposure and variety of types of asbestos
in use, it is difficult to define clearly asbestos exposure or the
smoking habits of those exposed. The smoking history of the population
sampled is important, because there have been changes in smoking
materials and prevalences of smoking in many countries (Cheng & Kong,
1992). In many studies, only the number of smokers within sub-groups
of workers with asbestos-related disease have been reported, rather
than the detailed smoking habits of the exposed population. A widely
used assumption is that the smoking habits of asbestos-exposed workers
reflect those of blue collar workers and are thus higher than national
average figures. Table 5 gives examples of smoking prevalence in
different groups of asbestos-exposed workers.
3.2.1.1 Asbestos and lung cancer
Exposure to asbestos dust carries a risk of parenchymal and pleural
fibrosis, mesothelioma and lung cancer. Selikoff et al. (1968) and
Berry et al. (1972) showed that cigarette smoking was an added hazard
among asbestos workers. In combination, the two hazards are associated
with very high lung cancer rates. Studies were carried out (e.g.,
Hammond & Selikoff, 1973; Martischnig et al., 1977; Hammond et al.,
1979; Selikoff et al., 1980; Acheson et al., 1984; Berry et al., 1985)
to determine whether cigarette smoke and asbestos act independently,
Table 5. Smoking prevalence in asbestos-exposed workers
Exposure Smoking habits References
Asbestos textile workers 75% smokers Weiss (1971)
46% cigarette smokers
36% ex-cigarette smokers
5.5% pipe/cigar smokers
Electrochemical plant 84% to 87% were smokers
(two areas) or ex-smokers Kobusch et al. (1984)
Population in Telemark, Asbestos exposed: Hilt (1986)
Norway 44.6% smokers
36.0% ex-smokers
Not exposed:
40.95% smokers
28.6% ex-smokers
Survey of 800 000 American Asbestos exposed: Stellman et al. (1988)
men and women in 1982 33.6% smokers
47.3% ex-smokers
Lung cancer case-referent Men: 95% smokers Järvholm (1993)
study; Swedish industrial city Women: 78% smokers
Shipyard workers in 46% smokers Sanden et al. (1992)
Gothenburg, Sweden 31% ex-smokers
December 1987 21% non-smokers
2% not known
Asbestos factory workers Men: 74% smokers Newhouse & Berry (1979)
(male population average 66%)
Women: 49% smokers
(female population average 40%)
their combined effect being the sum of the individual effects, or
there is an interaction with the ultimate effect being a product of
the two risk factors. In some studies, the effects of smoke and
asbestos appeared to be additive, in others multiplicative and in
others somewhere between the two. Reasons for the lack of consistency
among the studies may relate to the size of the population sampled,
its average age, social class and residential area, the type of
asbestos involved, the time scale covered and the intensity of
exposure to asbestos. The weight of evidence favours a synergistic or
multiplicative model for the interaction of asbestos and smoking.
While the differences may be partly linked to the carcinogenic
potential of different types of asbestos and to different smoking
materials and ways of smoking, including passive smoking (Cheng &
Kong, 1992), they also reflect the complex nature of tobacco smoke,
which contains complete carcinogens, tumour promoters and
co-carcinogens and other compounds that can influence the multistage
carcinogenic process. However, whatever the type of smoking/asbestos
interaction influencing the incidence of lung cancer, there is a
greatly increased risk for the asbestos-exposed worker who smokes
(Table 6).
Hammond et al. (1979) found a very strong synergistic effect and this
was supported by studies of shipyard workers in Italy (Bovenzi et al.,
1993), asbestos factory workers in London (Newhouse & Berry, 1979),
Finnish anthophyllite miners and millers (Meurman et al., 1979),
chrysotile workers in China (Cheng & Kong, 1992; Zhu & Wang, 1993) and
workers exposed to crocidolite in Western Australia (de Klerk et al.,
1991). Cheng & Kong (1992) reported a lower ratio of non-smoking to
smoking lung cancer death rates and suggested that this reflected
passive smoking among non-smokers and the use by most smokers of
hand-rolled cigarettes. Liddell et al. (1984) found that their data
fitted both an additive model and a multiplicative model and concluded
that the combined relative risk lay somewhere between the two.
Selikoff et al. (1980), from a study of amosite factory workers, and
Berry et al. (1985), from a study of asbestos factory workers,
favoured an additive model. However, caution is required because of
the definitions of additive and multiplicative used by different
authors and the overlap between these terms and such words as
synergism and promoter.
Molecular biology studies of autopsy specimens of lung tumour tissue
from of cigarette smokers have revealed that cigarette smoking induces
K-ras mutation (Rodenhuis & Slebos, 1992). It has been suggested that
such cigarette-smoke-induced K-ras oncogene mutations are promoted by
the presence of asbestos, which creates selective growth conditions
for the mutated cells (Vainio et al., 1993). Vainio & Boffetta (1994)
concluded that both tobacco smoke and asbestos fibres can be genotoxic
and cytotoxic, and cause proliferative lesions in the lungs. Tobacco
smoke contains carcinogens that bind to critical genes and cause
mutations. Asbestos fibres cause chronic inflammation of the lungs,
which releases various cytokines and growth factors, and may provide a
selective growth advantage for mutated cells.
Table 6. Age-standardized lung cancer death ratesa for cigarette smoking and/or
occupational exposure to asbestos dust compared with no smoking and no occupational
exposure to asbestos dust (from: Hammond et al., 1979)
Group Exposure to History cigarette Death Mortality Mortality
asbestos? smoking? rate difference ratio
Control No No 1.3 0.0 1.00
Asbestos workers Yes No 58.4 +47.1 5.17
Control No Yes 122.6 +111.3 10.85
Asbestos workers Yes Yes 601.6 +590.3 53.24
a Rate per 100 000 man-years standardized for age on the distribution of the
man-years of all the asbestos workers; number of lung cancer deaths based on
death certificate information
3.2.1.2 Asbestos and pleural mesothelioma
There is an established relationship between exposure to asbestos
- crocidolite, amosite, chrysotile - and pleural mesothelioma
(Stellman, 1988). In shipyard workers mainly exposed to chrysotile,
Sanden et al. (1992) found an increase in pleural mesotheliomas up to
15 years after cessation of exposure. Asbestos is also linked with
peritoneal mesothelioma (Newhouse & Berry, 1976). The risk of lung
cancer was found to fall after exposure ceased, suggesting that
asbestos acted as a lung cancer promoter, but the risk of mesothelioma
long after cessation of exposure indicated that asbestos acted as a
complete carcinogen. Mesothelioma can have an extremely long latent
period, with cases presenting even 30 years or more after first
exposure (Newhouse & Berry, 1976). Up to 90% of cases of pleural
mesothelioma have been attributed to asbestos but there is no evidence
directly associating smoking with the disease, or showing that smoking
has any influence on the incidence of asbestos-related pleural
mesothelioma (Berry et al., 1985; Hughes & Weill, 1991; Sanden &
Jarvholm, 1991; Muscat & Wynder, 1991).
3.2.1.3 Asbestos and other forms of cancer
Asbestos fibres have been found in many tissues, other than the lungs,
of asbestos workers. There is evidence that an asbestos/smoking
interaction increases the incidence of cancer of the oesophagus,
pharynx, buccal cavity and larynx but not of pleural or peritoneal
mesothelioma, or of cancer of the stomach, colon-rectum or kidney, for
which smoking and non-smoking asbestos workers are at equal risk
(Hammond et al., 1979; Selikoff & Frank, 1983; US ATSDR, 1995).
3.2.1.4 Asbestosis
Asbestosis is a fibrotic reaction to asbestos in the lungs. In a
review of histological, animal experimental and radiological evidence,
Weiss (1984) concluded that cigarette smoking could result in diffuse
fibrosis similar to that caused by asbestos, and the fibrosis showed a
dose-response to the duration and degree of smoking. Prevalence
studies are consistent in showing a higher frequency of diffuse small
irregular opacities in asbestos workers who are smokers than in those
who are non-smokers. It has been suggested that the effects may be
additive. Tobacco smoke affects lung clearance and hence the retention
of asbestos fibres in the lungs. In asbestosis the intensity of
fibrosis correlates with the number of asbestos bodies in the lungs,
and Murai et al. (1994) concluded that reduction of lung clearance by
tobacco smoke could increase the intensity of fibrosis. Crocidolite
fibres are the most fibrogenic of the various types of asbestos but
De Klerk et al. (1991) concluded that smoking had no measurable effect
on crocidolite asbestosis.
An interaction between asbestos and smoking causing a greater
frequency of obstructive airways disease in asbestos workers who smoke
was found in a study of pulmonary function changes caused by
asbestosis (Selikoff & Frank, 1983). Miller (1993) presented similar
results suggesting an interaction between asbestos and smoking. In a
prospective mortality study, Hughes & Weill (1991) concluded that
asbestosis is a precursor of asbestos-related lung cancer, but they
were unable to assess an interaction between tobacco smoking and
asbestosis because all the cases were in smokers and there were no
non-smokers.
In rats, asbestos fibres stimulate alveolar macrophages to generate
the inflammatory and fibrogenic mediators, tumour necrosis
factor-alpha (TNF-alpha), and this may be the cause of inflammation
and lung fibrosis due to asbestos (Ljungman et al., 1994). In in vitro
studies Morimoto et al. (1993) found synergism between chrysotile
fibres and cigarette smoke in the stimulation of the formation of
TNF-alpha by rat alveolar macrophages.
3.3 Non-asbestos fibres
3.3.1 Glass fibre
IARC (1988) classified glasswool as possibly carcinogenic to humans
(Group 2B) and glass filaments as not classifiable as to their
carcinogenicity to humans (Group 3), based on sufficient evidence for
the carcinogenicity of glasswool and inadequate evidence for the
carcinogenicity of glass filaments in experimental animals and
inadequate evidence for the carcinogenicity of glasswool and glass
filaments in humans. There are data on exposure to glass fibre and
tobacco smoke. Enterline et al. (1987a) carried out a case control
study of 7586 glasswool workers in four plants producing small
diameter fibres, less than 3 µm in diameter. Smoking histories were
obtained for 75% of the workers. Analysis of data by logistic
regression showed that smoking was a powerful variable and multiplied
the effect of fibre exposure. In a case-control study of the influence
of non-workplace factors on respiratory disease in employees of a
glass fibre manufacturing facility, Chiazze et al. (1992, 1995)
concluded that smoking, and not exposure to glass fibre, was the most
important risk factor for the increased lung cancer risk but was not
as important for non-malignant respiratory disease. In a further
analysis, using data not previously available, Chiazze et al. (1995)
estimated the extent of confounding by cigarette smoking, and
suggested that adjusting for the confounding effect could reduce the
lung cancer standardized mortality ratio to a non-statistically
significant level.
3.3.2 Rockwool, slagwool and ceramic fibres
IARC (1988) concluded that there was limited evidence for the
carcinogenicity of rockwool and inadequate evidence for the
carcinogenicity of slagwool in experimental animals, with limited
evidence for the carcinogenicity of rock-/slagwool in humans: the
overall evaluation for both was Group 2B, possibly carcinogenic to
humans. For ceramic fibres there was sufficient evidence for their
carcinogenicity in experimental animals, with no data on their
carcinogenicity in humans: the overall evaluation for ceramic fibres
was also Group 2B, possibly carcinogenic to humans.
In a study of insulation workers using rock and glass wool, (Clausen
et al., 1993) concluded that exposure was associated with an increased
risk of developing obstructive lung disease. In a study of respiratory
health in 628 workers in seven European plants manufacturing ceramic
fibres, skin, eye and nasal irritation, breathlessness and wheezing
were common findings (Trethowan et al., 1995). Respiratory symptoms
were more frequent in smokers and increased with the amount smoked.
The authors concluded that exposure caused irritation, similar to that
caused by other man-made fibres, and that cumulative exposure could
cause airways obstruction by promoting the effects of cigarette smoke.
Ljungman et al. (1994) demonstrated in rats that rock wool, slag wool,
kaolin ceramic fibre and silicon carbide fibre stimulated alveolar
macrophages to generate tumour necrosis factor-alpha (TNF-alpha), a
potent inflammatory and fibrogenic mediator. In in vitro studies
Morimoto et al. (1993) found synergism between mineral fibres
(chrysotile and alumina silicate ceramic fibres) and cigarette smoke
in the stimulation of the formation of TNF-alpha by rat alveolar
macrophages. Leanderson & Tagesson (1989) found that cigarette smoke
potentiated the DNA-damaging effect of man-made mineral fibres
(rockwool, glasswool and ceramic fibres).
3.4 Inorganic chemicals
3.4.1 Arsenic
Compounds of arsenic have been used as pesticides and as preservatives
of wood and leather. Arsenic is present in many metal ores and is
released during smelting Radon progeny are frequently encountered as a
contaminant of arsenic. In some parts of the world arsenic is found in
drinking-water in relatively high concentrations.
Arsenic and its compounds are carcinogenic (WHO, 1980; IARC, 1987;
Tsuda et al., 1990, 1995). Skin cancer can occur after ingestion of
arsenic (Tseng et al., 1968; Smith et al., 1992), and lung cancer
after inhalation of arsenic by smelter workers or by people living
nearby (Welch et al., 1982; Pershagen, 1985; Pershagen et al., 1987)
or by agricultural workers exposed to the pesticide lead arsenate
(Wicklund et al., 1988). IARC (1987) classed arsenic and arsenic
compounds as Group 1, carcinogenic to humans. It has been suggested
that arsenic in drinking-water may also cause liver, lung, kidney and
bladder cancer (Smith et al., 1992).
A study of the lung cancer risk among cadmium-exposed workers
suggested that exposure to arsenic and tobacco smoke may have been the
cause of an increased rate of lung cancer, rather than exposure to
cadmium particulates (Lamm et al., 1992). Tsuda et al. (1990)
suggested an interaction between arsenic and smoking in exposed
workers in a small Japanese village where arsenic was mined and
refined. However, the village water and air were highly polluted by
emissions from the smelter and from slag disposal, making interaction
between arsenic and smoking difficult to assess. A study of copper
smelter workers in the USA indicated that the effect of arsenic was
probably more important in lung cancer than that of tobacco smoke
(Welch et al., 1982). Studies in Sweden showed increased lung cancer
risks from arsenic exposure at a copper smelter; a multiplicative
effect for smoking and arsenic was found and age-standardized rate
ratios for lung cancer mortality were 3.0 for arsenic-exposed workers,
4.9 for smokers with no arsenic exposure and 14.6 for arsenic-exposed
smokers (Pershagen et al., 1981). In a later study, Pershagen (1985)
reported an additive effect for smoking and arsenic exposure on lung
cancer incidence in situations where the arsenic exposure was lower.
In a cohort of 3916 Swedish copper smelter workers, the risk of
developing lung cancer from the interaction between arsenic and
smoking was intermediate between additive and multiplicative and
appeared to be less pronounced among heavy smokers (Jarup & Pershagen,
1991).
There was no evidence of synergism between arsenic and tobacco smoke
in tin miners in Yunan Province, China. The lung cancer risk was
greater for arsenic than for smoking, and simultaneous assessment of
arsenic and radon exposure revealed radon to be the greater risk
(Taylor et al., 1989). In Ontario it was concluded that the excess
lung cancer mortality of gold miners and uranium miners was probably
due to exposure to arsenic and short-lived radon decay products
(Kusiak et al., 1991). This was consistent with the hypothesis that
the risk of lung cancer from exposure to arsenic is enhanced by
exposure to other carcinogens (Kusiak et al., 1993).
Hertz-Picciotto et al. (1992) assembled data from several studies to
examine possible synergism between smoking and exposure to arsenic and
an increased risk of lung cancer. The joint effect from both exposures
consistently exceeded the sum of the separate effects: a minimum of
30% to 54% of lung cancer cases among those with both exposures could
not be attributed to either one or the other exposure alone. The
conclusion was that arsenic and smoking acted synergistically to cause
lung cancer. Arsenic-induced lung cancer was not limited to exposure
to inhaled arsenic because there was evidence of synergism between
ingested arsenic and smoking (Tsuda et al., 1995). An association of
arsenic exposures with bladder cancer was confined to subjects who had
been smokers Bates et al. (1995).
In a Swedish study of lung cancer in arsenic workers, it was found
that cases among smelter workers who had never smoked showed a
histological distribution resembling that of smokers, probably
reflecting an exposure to carcinogenic agents at the smelter which
influence the risk of different histological types in the same way as
smoking (Pershagen et al., 1987). Tobacco smoking primarily induces
epidermoid and small cell carcinomas but there are also increased
risks for other cell types. The proportion of small cell carcinomas
was greater in uranium miners than in the general population (Kusiak
et al., 1993). In smokers, there were no pronounced differences in the
histological type of lung carcinomas between arsenic exposed smelter
workers and controls (Pershagen et al., 1987).
It has been suggested that the potentiation of the carcinogenic
properties of arsenic by smoking could be due to inorganic arsenic
requiring a strong co-carcinogen to manifest a carcinogenic effect, or
that arsenic itself might be acting as co-carcinogen rather than as a
direct carcinogen (Stohrer, 1991; Tsuda et al., 1995).
3.4.2 Beryllium
Beryllium is a metal with a number of uses including alloys, nuclear
energy applications, and in the rocket and aerospace industry (IPCS,
1990; IARC, 1993). Fine dusts and fumes of the metal and some of its
salts are hazardous and when inhaled are deposited in the lungs from
where beryllium may be widely distributed throughout the body.
Beryllium metal, oxide and some salts give rise to acute inflammation
on skin contact, particularly when accompanied by friction or
perspiration. Short exposure to dusts and fumes can cause acute
inflammation of mucous membranes: conjunctivitis, bronchitis,
pneumonitis. Granulomatous reaction can follow chronic inflammation of
the skin, and lesions may appear in the liver and elsewhere after long
periods of absorption from the lungs. Beryllium and its compounds are
a cause of delayed pneumonitis and pulmonary granulomas. IARC (1993)
classified beryllium and beryllium compounds as Group 1, carcinogenic
to humans, on the basis of sufficient evidence in humans and in
experimental animals. However, in epidemiological studies the
information on smoking was incomplete and the data did not rule out
the possibility that the few excess deaths observed could have been
due to smoking rather than to any other cause (Steenland & Ward, 1991,
1993; Eisenbud, 1993; MacMahon, 1994; Kotin, 1994).
The prevalence of chronic beryllium disease (CBD) is reduced in
smokers compared with non-smokers. The disease is preceded by the
development of beryllium-specific sensitization. In two studies
examining different worker populations, the prevalence of smoking in
those with clinically diagnosed CBD was lower than in those that were
sensitized but did not have the disease (Kreiss et al., 1993; Kreiss
et al., 1996).
3.4.3 Chromium
Chromium and its compounds are used in metallurgical, chemical,
electroplating and leather tanning industries (IARC, 1990). The
principal route of entry to the body is through the lungs. Chromium
ulcers of the skin and dermatitis can result from handling chromium
products and deposition of chromates on mucous membranes can also
cause ulceration which, in the nasal septum, can lead to perforation
(Lindberg & Hedenstierna, 1983; IARC, 1990). Chromium and some
chromium compounds are respiratory tract sensitizers and a cause
asthma. Hexavalent chromium salts have been associated with lung
cancer both in experimental animals and in epidemiological studies.
IARC (1990) concluded that there is sufficient evidence in humans for
the carcinogenicity of hexavalent chromium compounds as encountered in
chromate production, chromate pigment production and chromium plating
industries.
Langård & Norseth (1975) suggested that cigarette smoking increases
the risk for lung cancer in workers exposed to chromate dust. Other
studies (Abe et al., 1982; Langård & Vigander, 1983; Yoshizawa, 1984;
Nishiyama et al., 1985) have suggested that workers with exposure to
chromium compounds who are also smokers may be at greater risk than
non-smokers. However, the numbers were too small for conclusions on
interactions to be drawn.
3.4.4 Nickel
Exposure to nickel or its compounds occurs in mining, refining,
smelting and alloying the metal, in nickel plating and in welding. It
is used in battery manufacture, electroplating, enamelling, ceramics,
the chemical and petroleum industries and in dyestuffs and ink making.
Exposure may be by skin contact or inhalation of dusts, fumes, mists
or gaseous nickel carbonyl (IPCS, 1991a). In occupational exposure the
daily intake and absorption/retention vary widely between industries
(IARC, 1990).
Nickel is absorbed from the soil by the tobacco plant. During smoking,
up to 20% of the nickel in the tobacco is transferred to mainstream
smoke. This high transfer rate, compared to the much lower transfer
rates of other metals, has been explained by the formation of the
volatile nickel carbonyl (Sunderman & Sunderman, 1961). Nickel
carbonyl is a strong lung carcinogen in rats (IARC, 1990).
IARC (1990) evaluated the carcinogenicity of nickel and nickel
compounds and classified nickel compounds as carcinogenic to humans
(Group 1) and nickel as possibly carcinogenic to humans (Group 2B). In
an epidemiological study on a cohort of 916 workers in a Norwegian
nickel refinery, four work categories were defined: i) roasting and
smelting; ii) electrolysis; iii) other processes; and iv) other work
groups. All groups showed an excess risk of respiratory cancer. In the
roasting and smelting department there were excess risks for lung
cancer (O/E = 12/2.5) and nasal cavity cancer (O/E = 5/0.1). In the
electrolysis department there were also excess risks of lung cancer
(O/E = 26/3.6) and nasal cavity cancer (0/E = 6/0.2) (Pedersen et al.,
1973). Magnus et al. (1982) updated this study and found evidence of
an additive effect of smoking and nickel exposure in the induction of
respiratory cancer. Histological examination of nasal biopsy specimens
from 59 retired nickel workers, 21 of whom were smokers and snuff
users, showed a higher score of nasal epithelial dysplasia in smokers
than in non-smokers, and 4 workers with nasal carcinoma were smokers
(Boysen et al., 1984). In monitoring nickel exposure by imaging
cytometry of nasal smears (Reith et al., 1994), it was possible to
distinguish between workers who were exposed to different nickel
compounds and to distinguish between smoking and non-smoking nickel
workers.
3.4.5 Manganese
There is occupational exposure to manganese in its mining, the
ferromanganese and iron and steel industries, the production of dry
cell batteries, and the manufacture and use of welding rods. Manganese
is released by the combustion of the gasoline additive,
methylcyclopentadienyl manganese tricarbonyl (MMT), used in some
countries. However, the amount in gasoline (approximately 10 mg/litre)
and emitted in vehicle exhausts is small and does not to lead to human
exposure (Health Canada, 1994). The principal route of entry of
manganese is through the lungs but, because most of the compounds are
insoluble, only the smallest particles, as contained in furnace and
welding fume, are capable of reaching the alveoli and being
phagocytosed and absorbed (IARC, 1986).
Manganese is present in tobacco leaves and it has been reported (IARC,
1986) that 0.003 µg of manganese appears in the mainstream smoke from
one cigarette and thus contributes to a smoker's manganese intake in
the form of small and dangerous particles.
Manganese is neurotoxic and long-term occupational exposure can cause
a condition resembling Parkinson's disease. It causes lung damage
leading to an increased incidence of pneumonia and a higher rate of
acute and chronic bronchitis. In studies of manganese alloy production
workers and chronic lung disease, smokers were more affected than
non-smokers and the relationship between the number of cigarettes
smoked and the prevalence of respiratory tract symptoms suggested that
smoking acted synergistically with manganese (Saris & Lucic-Palaic,
1977). In a study of workers producing manganese salts and oxide
(Roels et al., 1985) smoking and manganese exposure were additive in
producing preclinical toxic effects. In studies on workers producing
iron-manganese alloys, one concerned with chronic bronchitis
(Misiewicz et al., 1994) and the other with pulmonary ventilatory
disturbance (Misiewicz et al., 1992), there was no relationship
between the occupational exposure or its duration and health effects.
The chronic bronchitis and ventilatory disturbance were attributed to
cigarette smoking.
3.4.6 Platinum
Platinum is used as a catalyst in many chemical processes and in motor
vehicle exhausts. Chloroplatinic acid is an intermediate in the
preparation of a large number of complex salts, which are used in
platinum refining, the chemical industry and, therapeutically, in
cancer chemotherapy.
Platinum salt sensitivity is an IgE-mediated immune response (IPCS,
1991b). Ammonium hexachloroplatinate is used as an intermediate in
platinum refining, and its inhalation provokes asthmatic responses and
elicits immediate skin test responses in sensitized individuals (Pepys
et al., 1972). In a cohort study of 91 workers in a platinum refinery
(Venables et al., 1989), 22 developed respiratory symptoms and an
immediate skin test response to ammonium hexachloroplatinate. The risk
was greatest in the first year of employment and smokers had an
increased risk of becoming sensitized. In another study, out of 78
workers at a platinum refinery, 32 (41%) had developed platinum salts
sensitivity after 24 months of exposure and the risk of sensitization
was about 8 times greater for smokers (Calverley et al., 1995).
Baker et al. (1990) conducted a cross-sectional study of respiratory
and dermatological effects of platinum salt sensitization in 136
workers (107 current employees and 29 former employees) at a precious
metal refinery. Twenty three workers (22%) had become sensitized.
Platinum salts sensitivity was not associated with atopicity but was
strongly associated with cigarette smoking status.
3.4.7 Silica
Silicosis (lung fibrosis caused by silica) is not only a hazard of
mining. It is also found in bricklayers, cement makers, workers in
pottery, porcelain and ceramics, rock drillers, workers chipping,
grinding or polishing stone, in sandblasting, using grinding stones to
smooth or polish precious stones, metals or optical glass, and in the
manufacture of polishing materials such as metal polishes and
toothpaste. The number of industries generating silica dust is large.
The amount of respirable dust varies from one to another and, because
silica is an active adsorbent, it can become contaminated and have its
toxic potential changed. Furthermore, freshly fractured silica dust
may exhibit a different surface reactivity and cytotoxicity from that
of aged silica (Vallyathan et al., 1988).
Hnizdo & Sluis-Cremer (1991), in a study of gold miners, linked high
exposure to silica dust with lung cancer and found a combined effect
of dust and smoking that fitted a multiplicative model for lung
cancer. Amandus et al. (1992) studied lung cancer in men with
diagnosed silicosis and suggested that there was an association
between the two diseases. In a study of iron foundry workers
(Andjelkovich et al., 1994), cigarette smoking was a strong predictor
of lung cancer whereas silica exposure showed no association with the
disease.
Chronic silicosis develops after 20 to 40 years of exposure to silica
dust. There are also other types of pneumoconiosis related to the
nature of the dust, and chronic bronchitis and airways obstruction
have been associated with silica dust exposure. A hypothesis for the
pathogenesis of chronic silicosis is that silica particles are
phagocytosed by the alveolar macrophages for which they have a marked
selective toxicity. Permanent macrophage activation initiates
inflammatory reactions leading to the formation of collagenous fibres.
Acute silicosis arises from the inhalation of more highly reactive
silica (Vallyathan et al., 1988). The link between silicosis and
smoking was examined in a study of smoking and silica exposure on
pulmonary epithelial permeability. Faster clearance of a radioaerosol
from the upper lung regions was found for smokers (Nery et al., 1988,
1993). The question of silica clearance was considered in an analysis
of an association between silicosis and smoking: differences in
collagenization for smokers and non-smokers were attributed to
differences in the interception of silica particulate matter by mucus
(Hessel et al., 1991). In studies by Ng et al. (1987, 1992), smoking
was not considered to affect the progression of silicosis in granite
quarry workers. However, examining the association of silicosis with
lung cancer, Ng et al. (1990) concluded that the excess lung cancer
risk in silicosis is attributable to smoking, and there appeared to be
a synergistic effect between smoking and silica/silicosis regarding
the risk of developing lung cancer.
In a study of 562 South African gold miners exposed to low levels of
dust with a high (50-70%) silica content, the incidence of chronic
bronchitis was higher than in non-dust-exposed controls. Although the
percentage of smokers was higher in those with chronic bronchitis in
both groups, there was a significant excess in the dust-exposed
smokers (Sluis-Cremer et al., 1967). The authors concluded that there
was a factor in dust-exposed smokers that increased the incidence of
chronic bronchitis above that expected from smoking alone. In a study
of 2209 South African gold miners and 483 non-miners on the effect of
silica dust and tobacco smoking on mortality from chronic obstructive
lung disease, it was found that miners who smoked and were exposed to
silica dust were at higher risk of dying from chronic obstructive lung
disease than smokers not exposed to silica dust. In South African gold
mines about 30% of the respirable dust is free silica. It was
concluded that tobacco smoking and silica dust acted synergistically
in causing chronic obstructive lung disease (Hnizdo, 1990). Hnizdo et
al. (1990) applied additive and multiplicative relative risk models to
the same sample and found that departure from additivity increased
progressively with the severity of obstructive impairment. They
concluded that tobacco smoking potentiated the effect of dust in
causing respiratory impairment and that severe respiratory impairment
could have been prevented through elimination of tobacco smoking
(Hnizdo et al., 1990). Oxman et al., (1993) analysed the relationship
between occupational dust exposure and chronic obstructive lung
disease in both gold and coal miners and found a significant
association between loss of lung function and cumulative respirable
dust exposure, which was greater in gold miners. In this study there
was no evidence of interaction with tobacco smoking for gold miners,
and the authors suggested that the increased risk of lung function
loss was due to exposure to dust having a higher silica content than
coal dust. Among iron ore miners in Sweden, Jörgensen et al. (1988)
found a strong relationship between chronic bronchitis and smoking,
but not with working underground. The two risk factors, silica dust
and smoking, appeared to be additive but the smoking effect was far
greater than that of silica dust. In a study of small airway disease
in patients with silica dust exposure, with and without radiographic
evidence of silicosis, and smoking, Avolio et al. (1986) found no
differences in lung function and prevalence of small airways disease
with silicosis. However, in both groups small airway disease was
significantly related to tobacco smoking, indicating that this had a
more powerful effect than silicosis.
3.5 Organic chemical agents
Many organic compounds with properties covering a wide spectrum of
molecular structure and biological activity are encountered in a
variety of industries. The effects of a few compounds, some of which
are encountered in specific industries, and smoking have been studied.
Where organic compounds occur in both tobacco smoke and the workplace,
the effect of smoking becomes one of dose augmentation, although
modification of effect can also occur. Some organic compounds would
normally not be found in tobacco smoke but are present because
workplace materials have contaminated smoking materials and they are
then pyrolysed or volatilized during smoking.
3.5.1 Chloromethyl ethers
Chloromethyl methyl ether (CMME) and its contaminant bis(chloromethyl)
ether (BCME) are used in the synthetic chemical industry, in the
manufacture of ion exchange resins and in polymer production. They are
carcinogenic when inhaled, BCME more so than CMME. In a long-term
study of chemical workers, 93 had exposure to chloromethyl ethers and
22 died from lung cancer. Of 32 workers who had no exposure, 3 died
from lung cancer (Weiss & Boucot, 1975; Weiss, 1976, 1980, 1982). For
the 22 cases in of lung cancer in the exposed workers, a dose-response
relationship was established. In the groups with heavy occupational
exposure, there were fewer heavy smokers (>20 cigarettes per day)
than in the groups with lower occupational exposure. This
statistically significant shift might be explained by self-selection
of heavy smokers out of the high occupational exposure groups because
of the bronchial irritation that is caused by the exposure to
chloromethyl ethers, or cigarette smoking might have an antagonistic
activity (Steenland & Thun, 1986; Thomas & Whittemore, 1988; Weiss &
Nash, 1997).
3.5.2 Tetrachlorophthalic anhydride
Tetrachlorophthalic anhydride (TCPA) is used as an epoxy resin curing
agent. It is respiratory tract sensitizer and causes asthma (Schlueter
et al., 1978). In a study using a radio allergosorbent test with a
TCPA human serum albumin conjugate, specific IgE antibody was detected
in serum from 24 out of 300 factory floor workers exposed to TCPA. Of
these 24, 20 (83%) were current smokers, compared with 133 (48%) of
276 without antibody (p<0.01), and there was a weaker association
with atopy, defined by skin tests with common allergens. Smoking and
atopy interacted, the prevalence of antibody being 16% in atopic
smokers, 12% in non-atopic smokers, 8% in atopic non-smokers and none
in the non-atopic non-smokers. It was concluded that smoking may
predispose to, and interact with atopy in the production of specific
IgE antibody to this hapten protein conjugate.
3.5.3 Dyestuffs
There is an established relationship between bladder cancer and
exposure to certain aromatic amines encountered in the dyestuffs
industry, e.g., benzidine, 4-aminobiphenyl and 2-naphthylamine (IARC,
1987), and smoking is causally associated with bladder cancer (IARC,
1986; US Surgeon General, 1989). From an analysis of 991 cases by
Cartwright (1982), a significant risk of bladder cancer was associated
with cigarette smoking, and a dose-response relationship, based on
years of employment, was found in workers in dyestuffs manufacturing.
The risks were considered to be additive. Overall, there was a
significant risk of bladder cancer associated with cigarette smoking,
a risk ratio of 1.8 for males, and there were significant overall
risks associated with occupations such as those of process workers in
the dye manufacturing industry who had a risk of 2.9 for males. When
dye manufacturing process workers who were smokers were compared with
non-smoking workers, the risk for smokers was 4.6, while for
non-smokers the risk was 1.9.
Boyko et al. (1985) concluded that arylamines in the dyestuffs
industry posed a major threat of bladder cancer. However, there was
little evidence to support an effect due to smoking or an interaction
between smoking and occupational exposure.
In an area of Spain where 44% of the adult population worked in dyeing
and printing textile fabrics, there was an increased risk of bladder
cancer for smokers (OR 2.3) (Gonzalez et al., 1985). The estimated
risks for occupation and for smoking and occupational exposure were OR
5.5 and 11.7, respectively. The observed effect was multiplicative.
Tobacco smoke contains many amines, including the bladder carcinogens
4-aminobiphenyl (>9 ng/cigarette) and 2-naphthylamine (54
ng/cigarette) (Patrianakos & Hoffmann, 1979; Pieraccini et al., 1992;
Grimmer et al., 1995).
In a study of risk factors for bladder cancer in Spain (Bravo et al.,
1987), the results were considered to corroborate previous data that
bladder cancer does not have a single cause. Cigarette smoking was
considered an important cause but one which was additional to
urological disease or occupational exposure, among other factors. In a
study of men in Spain (Gonzalez et al., 1989), increased risks of
bladder cancer were found for textile workers (OR 1.97), mechanics and
maintenance workers (OR 1.86), and workers in the printing industry
(OR 2.06). The highest risk was in those who were employed in the
textile industry before the age of 25 and prior to 1960. Among
mechanics the highest risk was for those who started after the age of
25 and after 1960. The OR for smokers who had also been employed in
one of the high-risk occupations was 7.82, which is compatible with a
multiplicative effect of joint exposure to tobacco smoke and
occupational hazards. In an Italian study (D'Avanzo et al., 1990),
risk additivity was found for the interaction between tobacco smoke
and several occupations associated with bladder cancer but the
occupations were not specified. The bladder cancer risk for smokers of
black tobacco was higher (OR = 3.7) compared with smokers of blond
tobacco cigarettes (OR = 2.6). A higher risk for black tobacco than
for blond varieties and a protective effect for smokers of tipped
cigarettes was also reported in a study in Northern Italy where a
multiplicative effect for smoking and high risk occupations was also
found (Vineis et al., 1984).
Bartsch et al. (1993) correlated the higher incidence of bladder
cancer among smokers of black tobacco with high yield aromatic amines,
particularly 4-aminobiphenyl from black tobacco (5 times greater than
from blond tobacco). The concentrations of urinary mutagens and of
4-aminobiphenyl adducts in the blood were also higher in smokers of
black tobacco.
A Chinese study of bladder cancer incidence and mortality in workers
with benzidine exposure found a marked dose response and an elevated
risk for both producers and users of benzidine. Workers exposed to
benzidine who were smokers had a 31-fold risk, while the risk for
exposed workers who were non-smokers was 11-fold, and a multiplicative
interaction was suggested (Bi et al., 1992).
3.5.4 Polycyclic aromatic hydrocarbons
Tobacco smoke contains many polycyclic aromatic hydrocarbons (PAHs)
(IARC, 1986), a number of which, such as benz( a)pyrene and
dibenz( a,h)anthracene, are known to be carcinogenic (IARC, 1987;
Hoffmann & Hoffmann, 1997). PAHs are generated by incomplete
combustion of organic matter in many industrial processes and
constitute a hazard not only in occupations but also as environmental
pollutants, representing primary risk factors as lung and bladder
carcinogens. A tobacco smoker can obtain one dose of PAHs from tobacco
smoke and another from the industrial or environmental source.
Furthermore, an interaction of tobacco smoke and an occupational
hazard is a possibility. PAHs in the workplace are often accompanied
by many other toxic compounds, particularly irritants, and, in
addition to carcinogenic PAHs, tobacco smoke contains co-carcinogens
and tumour promoters as well as ciliatoxic agents, irritants and other
biologically active species.
PAHs occur in coal gas manufacture, coking oven fumes, aluminium
smelting, in the use of tar and asphalt, in oil refining and the
exhaust from internal combustion engines. They are frequently
accompanied by irritant fumes or aerosols and potentially harmful
particulate matter. There is a lack of smoking data for workers in
many of these industries, but it has been assumed that the smoking
prevalence is at least as high as the average for blue collar workers.
At a Norwegian smelter 69% of workers were smokers when the expected
prevalence of smoking was 52% (Abramson et al., 1989). The percentage
of smokers and ex-smokers among workers exposed to chemicals and coal
tar pitch in a 1982 survey of 800 000 men and women in the USA was
49.9% against 46.1% for the average worker (Stellman et al., 1988).
A Canadian study found a high prevalence of bladder cancer in
aluminium smelter workers, particularly among those employed in
Söderberg potrooms where carbon electrodes made from a mixture of
petroleum pitch and coal pitch are used and PAH levels are high
(Thériault et al., 1984). Changing electrodes, breaking the crust that
forms on top of the molten metal and cleaning out the "pots" are
activities that create air pollution by tar volatiles including
carcinogenic PAHs which, measured as benzo( a)pyrene, could reach a
concentration of 800 µg/m3/8 h (Bjorseth et al., 1978). High levels
of PAHs were found in urine samples from aluminium plant workers
(Haugen et al., 1986). Lung cancer rates among aluminium reduction
plant workers are also high (Gibbs & Horowitz, 1979). Tobacco smoke
appears to increase the risk. In a study by Thériault et al. (1984),
the numbers were too small to determine whether the interaction was
additive or multiplicative, but in another study (Bjorseth et al.,
1978) there was suggestive, but not conclusive, evidence that the
relative risks from combined exposure to tar volatiles and cigarette
smoke were multiplicative. In a study in which the preceding data were
augmented (Armstrong et al., 1986), the tar volatiles were confirmed
as the cause of bladder cancer and the results suggested that a
multiplicative risk arose from a combined exposure to tar volatiles
and cigarette smoke.
Gullvåg et al. (1985) found that the alveolar macrophage count for
workers in the potrooms of an aluminium reduction plant was elevated
and for workers who were also smokers the count was further elevated.
The conclusion was that smoking and workplace pollution act
synergistically in increasing the number of alveolar macrophages.
Workers in coke oven plants have a higher incidence of lung cancer
than the general population and a measurable concentration of PAHs in
urine, which is higher in smokers than non-smokers (Haugen et al.,
1986). Van Schooten et al. (1990) analysed blood samples from coke
oven workers for PAH-DNA adducts and urine for 1-hydroxypyrene and
compared the results with those of non-exposed workers. Levels were
elevated in coke oven workers and in both exposed and control groups
the PAH-DNA adduct levels were higher among smokers than among
non-smokers.
Professional drivers are exposed to benzene and carcinogenic PAHs and
nitroarenes through the exhaust of petrol (gasoline) and,
particularly, diesel engines. An excess of lung cancer has been found
in this occupational group, with a suggestion of a synergistic
interaction between smoking and occupational exposure (Damber &
Larsson, 1985a).
Diesel exhaust contains large quantities of carbonaceous particulates
with adsorbed PAHs. The association between lung cancer and diesel
exhaust and the contributing role of cigarette smoking has been
considered to be problematic (Garshick et al., 1987, 1988; Boffetta et
al., 1988; Stöber & Abel, 1996; IPCS, 1996). In its evaluation, IARC
(IARC, 1989c) considered diesel engine exhaust to be probably
carcinogenic to humans (group 2A) and gasoline engine exhaust as being
possibly carcinogenic to humans (group 2B).
In most of the workplaces where PAHs contaminate the atmosphere, there
are also gases, fumes and aerosols that contain other hazardous
materials that act as irritants; they may play a role in the etiology
of chronic obstructive lung disease. It is important to include
smoking in epidemiological studies. In a study of lung cancer
mortality rates and smoking patterns in workers in the motor vehicle
industry, proportionate mortality rates were considerably reduced when
smoking rates were taken into account. An increased lung cancer risk
has been described among foundry workers; PAHs and silica were
considered to be possible etiological factors (Sherson et al., 1992).
IARC (IARC, 1984, 1985b) considers the following technical products as
carcinogenic to humans: coal tar and coal tar pitches, shale oils,
soots, effluent aerosols from coal gasification, and emissions from
coke ovens. Exposure occurring in the production of aluminium is
classified as probably carcinogenic to humans, whereas the exposures
to aerosol emissions from iron and steel foundries are classified as
possibly carcinogenic to humans.
3.5.5 Ethanol
Clinical and epidemiological studies have established a strong
relationship between smoking and drinking (Istvan & Matarazzo, 1984;
Bien & Burge, 1990; Zacny, 1990).
Elevated tobacco and alcohol consumption are regarded as the major
risk factors for oropharyngeal and oesophageal cancer in many
developed countries (Herity et al., 1982; Tuyns, 1983, 1991; Boyle et
al., 1990; Muir & McKinney, 1992; Negri et al., 1992).
It has been difficult to distinguish the separate effects of these
agents since many smokers tend to consume alcoholic beverages and
vice versa. In addition, the consumption of one substance may have
an effect on the use of the other substance. The possible interactions
(e.g., multiplicative effect) of tobacco smoking and alcohol
consumption for cancers of the oral cavity, pharynx and larynx have
been evaluated by IARC (1986). IARC (1986) concluded that tobacco
smoking was an important cause of oral, oropharyngeal, hypopharyngeal,
laryngeal and oesophageal cancers and combined ethanol consumption
increased the risk substantially. In a case control study of 1114
patients with oropharyngeal cancer, Blot et al. (1988) showed that the
risk to consumers of tobacco and alcohol was multiplicative rather
than additive and increased 35-fold in those who consumed two or more
packs of cigarettes and more than four alcoholic drinks per day. The
risk was higher in those consuming spirits or beer than in those
consuming wine and was lower in lifetime smokers of filter cigarettes.
Alcohol consumption and smoking affect fetal outcome, leading to
infants with low birth weight (Wright et al., 1983, 1984; Smith et
al., 1986).
In contrast to the many studies in laboratory animals of the
interactions of ethanol with tobacco-smoke condensate (TSC) and
specific tobacco constituents, e.g., nicotine (receptor studies)
(Collins, 1990), (gastric-mucosal damage) (Wong et al., 1986; Cho et
al., 1990), tobacco-smoke-specific nitrosamines, e.g.,
N-nitrosonornicotine (metabolism and carcinogenicity) (McCoy et al.,
1981; Castonguay et al., 1983, 1984) and
4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK) (Jorquera et al.,
1992; Schüller et al., 1993), there has been a relative paucity of
studies involving ethanol and tobacco smoke per se. These latter
studies have included fetotoxicity in mice (Peterson et al., 1981),
gastric mucosal damage (Iwata et al., 1995; Chow et al., 1996), and
mechanisms underlying behavioural association between alcohol and
tobacco consumption (Zacny, 1990).
In in vitro studies on the effect of tobacco smoke condensate on rat
buccal mucosa cells following exposure to ethanol, the level of
adducts was higher than in controls, suggesting an increased uptake of
carcinogens in the condensate (Autrup et al., 1992). Hsu et al. (1991)
studied in vitro genotoxicity of tobacco smoke condensate in
conjunction with 2% and 4% ethanol in human lymphoid cell lines.
Ethanol potentiated clastogenicity, measured by frequency of
chromosome breaks per cell, in a dose-dependent manner, and the
results indicated that ethanol at relatively high doses inhibited DNA
and chromosome repair systems.
Swiss Albino mice fetuses prenatally exposed to both tobacco smoke and
ethanol had a high resorption frequency, a significant reduction in
fetal weight and length, and neonatal growth retardation, indicating
that ethanol and tobacco smoke may interact to produce fetotoxicity
(Peterson et al., 1981).
Both cigarette smoking and ethanol consumption individually have been
associated with gastric and duodenal ulcers in humans and animals.
Exposure to cigarette smoke significantly potentiated ethanol-induced
gastric mucosal damage in Sprague-Dawley rats (Iwata et al., 1995;
Chow et al., 1996).
The effect of smoking on the incidence of cancers of the oral cavity,
oropharynx, hypopharynx and larynx is often combined with other
factors, principally alcohol, in the Western world. The possibility of
interaction between cigarette smoking and alcohol consumption is
complex (Burch et al., 1981).
Rothman & Keller (1972) reviewed the effect of joint exposure to
tobacco and alcohol with regard to oral cancers alone (based on data
published earlier by Keller & Terris, 1965) and concluded that a
single multiplicative function of the relative risks associated with
alcohol and tobacco separately provided an adequate summary of their
joint effect.
Wynder & Bross (1961) studied etiological factors in cancer of the
oesophagus, considered the consumption and effects of tobacco and
alcohol separately and together, and considered that the combined
effect was multiplicative. Tuyns et al. (1977) reported a similar
pattern of joint effect of tobacco and alcohol in a retrospective
study of oesophageal cancer in Brittany, France. The relative risk of
developing oesophageal cancer increased linearly with daily
consumption of alcohol and tobacco independently. The combined effect
fitted a multiplicative model.
Wynder et al. (1976) analysed environmental factors in cancer of the
larynx and showed a combined effect of tobacco and alcohol. In the
presence of smoking, heavy drinking increased the risk of cancer of
the larynx, especially for cancer of the supraglottic portion of the
larynx. Similar findings were reported by Burch et al. (1981).
In a prospective epidemiological study, the relative risk of incurring
a single primary carcinoma of the oral cavity, pharynx, larynx and
oesophagus in any one of these sites was increased independently by
the duration and intensity of exposure to tobacco or alcohol and
sustained exposure enhanced the risk in a multiplicative or
synergistic fashion (Schottenfeld et al., 1974) The relative risk of
multiple primary cancers in the sub-group with combined exposures to
high levels of tobacco and alcohol was 3.9 times that of patients
exposed previously to low levels of alcohol and tobacco.
3.5.6 Other organic compounds
Exposure situations involving compounds and mixtures of organic
compounds for which no definite smoking interactions have been
established but which are known to present serious health hazards are
summarized in chapter 4.
3.6 Physical agents
3.6.1 Radiation
The harmful forms of ionizing radiation that are of concern are
alpha- and beta-particles and gamma- and X-rays. All cause cellular
damage and have been implicated in carcinogenesis. IARC (1988)
classified radon and its decay products as Group 1 (carcinogenic to
humans) on the basis of sufficient evidence in humans and in
laboratory animals. The interaction of the effects of these radiations
with the effects of tobacco smoke has been studied. Radium is present
in uranium and other minerals and in all rocks and soils. It emits
alpha- and beta-particles and gamma-rays and decays to form the
chemically inert radioactive gas radon, which is released in tiny
amounts into the atmosphere where its concentration is extremely small
because of dilution. It can, however, become more concentrated in some
locations, particularly in uranium and other mines and in residential
buildings. Radon is an inspirable gas and its radioactive decay
products are ionized metal atoms, which adhere to inspirable dust
particles. These atoms are themselves undergoing radioactive decay and
emitting damaging alpha- and beta-particles and gamma-rays. In
addition to interactions of tobacco smoke with radon in mines and
residential situations, other effects of tobacco smoke and radiation
interactions have been studied in atom-bomb survivors and in the low
energy transfer radiation involved in the use of therapeutic radiation
(X-rays).
3.6.1.1 Radon in mines (high linear energy transfer (LET)
alpha-radiation)
Unless mines are well ventilated, the atmospheric concentration of
radon becomes significant. The gas and its radioactive decay products,
the radon daughters, can be inhaled. The daughters have short
half-lives and their decay is proceeding while the particles to which
they adhere are resident in the lungs and before they can be removed
by normal lung clearance. Thus radiation is delivered directly to the
delicate lung tissues where it causes an excess of lung cancer among
some miners.
Observations in several mining communities, e.g., among uranium miners
in the USA, Czechoslovakia, Canada and France, workers in a niobium
mine in Norway, iron ore miners in Sweden, tin miners in China and the
United Kingdom, and fluorspar miners in Newfoundland, showed a
significant dose-related increase in lung cancer risk with exposure to
radon and radon daughter elements (Archer, 1988). In miners who were
cigarette smokers, there was an interaction between the radiation
exposure and the smoke exposure leading to more than the expected
number of cases of cancer. The latent period for induction of lung
cancer was longer when the exposure to radioactivity started at a
younger age, it was shortened by high exposure rates and by cigarette
smoking, and lung cancers developed at lower levels of exposure to
radioactivity in miners who smoked than in those who were non-smokers.
In Bulgaria, Michaylov et al. (1995) used sputum cytology to study
bronchial cell dysplasia in 334 miners (uranium and metal mines)
exposed to 222Rn progeny, and 100 control miners from a metal mine
where radon was virtually absent. The dust and silica concentrations
and exposure to diesel exhaust and explosion gases were similar. The
frequency of bronchial cell dysplasia was significantly higher in
radon-exposed miners than in controls and the frequency of dysplasia
in smokers was significantly greater than in non-smokers.
The lower prevalence of lung cancer among coal miners than among other
underground workers is probably because coal mines are well ventilated
to reduce fire and explosion risk, and no build up of radioactivity
occurs. Attempts to reduce silicosis by ventilation have achieved a
similar effect. In some Swedish mines, because freezing occurred when
outside air was used for ventilation, filtration was achieved in the
1920s by circulating the air through old underground mine workings,
with the result that the potential for silicosis was reduced. However,
an increase in lung cancer was found because radioactive materials
built up, a fact that only became evident many years later (Archer,
1988).
The nature of the interaction between radon and cigarette smoke is not
clear. In a study by Edling (1982), the effects of smoking and radon
were considered to be additive, whereas in another by Damber & Larsson
(1985b) the effect was multiplicative. From a long-term study on
Swedish iron ore miners (Jörgensen, 1984), it was concluded that
tobacco smoke acts as a tumour promoter, an effect that has been
demonstrated in almost all animal studies. The concept that radon
serves as a tumour initiator and tobacco smoke as the tumour promoter
for the induction and development of lung cancer is supported by a
sequence of studies. Tobacco contains small amounts of polonium-210
(210Po), which primarily originates from phosphate fertilizers (Tso et
al., 1966) and, to a minor extent, from airborne 210Po trapped by the
glandular hairs (trichomes) found on the soil-facing surfaces of
tobacco leaves (Martell, 1974). 210Po is a decay product of radon -222
and an emitter of alpha-particles. It is present in cigarette smoke,
and the bronchial epithelium of smokers contains 2-10 times more 210Po
than is found in these tissues in non-smokers (Harley et al., 1980).
The alpha-radiation of 210Po damages DNA in the bronchial airways and
serves as a tumour inhibitor, and the tar in the tobacco smoke acts as
a tumour promoter.
The frequencies of different histological types of lung cancer among
miners have varied with working conditions and follow-up time. It has
also been shown (Archer, 1988) that the age range of the population
under observation can influence the conclusion. Thus, the
smoking-radon relationship appears to be multiplicative only for the
group aged 35-65 years. Steenland (1994) found the death rates from
lung cancer in smoking uranium miners to be intermediate between
additive and multiplicative for the two exposures, but, when
stratified for age, the multiplicative model fitted well for the
youngest and oldest categories but poorly for the middle range. In a
comprehensive analysis of data from 11 studies of radon-induced health
risks (Lubin et al., 1995), it was concluded that the joint effect of
radon progeny exposure and smoking is greater than the sum of the
individual effects and for smokers is higher by a factor of at least
three. The tobacco of cigarettes contains 0.1-1.0 pCi of 210Po
(Cohen et al., 1985; Hoffmann et al., 1986).
The conclusion reached by the US Surgeon General (1985) was that the
smoking-radon interaction consists of two parts: an additive effect of
the contribution of the two agents on the number of tumours produced
and an accelerating effect due to tumour promoters in cigarette smoke.
Thus for a miner who smokes, not only is the chance of lung cancer
greater but the latent period is shorter and therefore the cancer
appears sooner in smokers.
3.6.1.2 Environmental radon (high linear energy transfer (LET)
alpha-radiation)
Alpha-radiation from radon daughters in the home or in other
situations where there are enclosed spaces with poor ventilation,
e.g., where strict energy conservation measures have been adopted,
presents an elevated health hazard to occupants, particularly smokers,
and is a matter of public health concern. The ease with which ionized
radon daughters could be attracted to environmental tobacco smoke
particles and the possibility of a higher than additive combined
effect of radon progeny and smoke clearly indicate the importance of
residential contamination by radon.
The relative risk in the range of exposure experienced by miners has
been found to be linear, and it has been suggested from extrapolation
that exposures at the lower levels found in homes would carry some
risk (Lubin et al., 1995). Steindorf et al. (1995) calculated that 7%
of all lung cancer deaths in the western part of Germany may be due to
residential radon.
Axelson (1995) reviewed cancer risks from exposure to radon in the
home and suggested that cancers other than lung cancer may also be
related to indoor radon, especially leukaemia, kidney cancer and
malignant melanoma. However, it was acknowledged that studies of radon
and miners gave no clear support for this. Alavanja et al. (1995)
listed other risk factors as being responsible for lung cancer in
lifetime non-smokers and found a small non-significant risk for
subjects exposed to domestic radon at median concentrations. In a
case-control study of lung cancer in relation to exposure to radon in
homes (Letourneau et al., 1994), no increase in the relative risk for
any of the histological types of lung cancer was detected in relation
to cumulative exposure to radon. On the other hand, Biberman et al.
(1993) found an increased risk for small cell lung cancer following
residential long-term exposure to radon at a low-dose level. In a
large case-control study in Sweden, Pershagen et al. (1994) reported
an increased relative risk of lung cancer within the highest exposure
group. In an attempt to resolve the conflicting epidemiological data,
Lubin & Boice (1997) conducted a meta-analysis of eight large-scale
case-control studies of residential radon and lung cancer. This
analysis was consistent with an excess lung cancer risk. Furthermore,
the slope of the exposure- response curve derived from this
meta-analysis was comparable with that obtained from a combined
analysis of eleven miner cohorts exposed to radon (Lubin et al.,
1997).
The combined analysis of the miner data also confirmed the strong
synergistic relationship between radon and tobacco at high levels of
exposure to these two agents (Lubin et al., 1997), although it is
difficult to determine whether the interaction is closer to additive
or multiplicative (Chaffey & Bowie, 1994). When extrapolated to lower
levels of exposure, however, the magnitude of this interaction is
substantially diminished (Moolgavkar et al., 1993). However, Pershagen
et al. (1994) reported some evidence of a synergistic effect between
tobacco and residential radon exposure, with the relative risk of
radon-induced lung cancer being highest among heavy smokers. In a
case-control study of 982 subjects with lung cancer and 3185 hospital
or population control subjects, lung cancer risk was examined in
relation to residential radon concentration and length of time that
subjects were resident, and adjusted for age, sex and smoking (Darby
et al., 1998). The relative risk increased in an exposure-related
manner with time-weighted residential radon concentration and fitted
the data from studies in miners and the effect of smoking. Regardless
of the magnitude of any interaction between tobacco smoking and
residential radon, the lung cancer risks due to smoking exceed the
risk associated with radon in homes.
3.6.1.3 Atomic bomb site radiation (low linear energy transfer (LET)
radiation)
In tobacco-smoking survivors of atomic bombing in Hiroshima and
Nagasaki, Japan, elevated levels of cancer of several sites have been
reported. In the case of lung cancer both additive and multiplicative
models fit the data (Prentice et al., 1983; US NRC, 1988).
3.6.1.4 Therapeutic X-rays (low linear energy transfer (LET)
radiation)
Lung cancer as a possible side effect of the radiation therapy used to
treat breast cancer has been studied by Neugut et al. (1993, 1994) and
discussed by Inskip & Boice (1994). Neugut et al. (1993) reported that
the risk was greater in the ipsilateral than in the contralateral
lung. In a second study (Neugut et al., 1994), a three-fold relative
risk was found for the effect of radiation therapy among 10-year
survivors, a 14-fold risk was associated with smoking alone, and a
33-fold risk was found among irradiated smokers; in each case the
effect was most pronounced for ipsilateral lung cancer. A
multiplicative interaction was proposed and the implications of the
results for the design of treatment of breast cancer in smokers was
considered. The increased risk of lung cancer among survivors of
Hodgkin's disease(HD) was studied by van Leeuwen et al. (1995). Their
overall conclusions were that the risk of lung cancer increased more
with increasing radiation dose in HD patients who smoked than among
those who did not smoke. Thus, smokers were at greater risk from the
radiotherapy than non-smokers. The interaction between the
carcinogenic effects of smoking and radiation was significantly
stronger than multiplicative, and the low lung cancer rate found among
women with HD was attributable to the delayed popularity of smoking
among Dutch women, a fact shown by the male/female lung cancer ratio
(13:5) in the Netherlands in 1980.
3.6.1.5 Nuclear plant
Ongoing epidemiological studies are being conducted on the workforce
exposed to radiation at the Mayak plant in Russia. Of 500 workers
examined in a case-control study (Tokarskaya et al., 1995), 162
workers had contracted lung cancer, and the remaining 338 served as
radiation-exposed, non-tumour-bearing controls. Both the incidence and
duration of smoking was significantly higher in workers contracting
tumours compared to combined male and female controls. The strongest
smoking-related effect was for squamous cell carcinomas, followed by
adenocarcinomas, then small cell carcinomas. However, the findings are
complicated by the fact that the great majority of the workforce was
male, and there was only one of the 148 "never smokers" among the male
lung cancer cases.
3.6.1.6 Summary
In summary, miners subjected to chronic exposure throughout a working
lifetime to high-LET radiation show a radiation/tobacco smoke
interaction greater than additive and sometimes multiplicative. Atomic
bomb survivors exposed instantaneously to low-LET radiation show in
some cases an additive and in others a multiplicative interaction. The
results for residentially exposed smokers, subjected to a lifetime of
very low dose exposure, tend to show similar interactions to those for
miners. Tobacco-smoking patients subjected to therapeutic radiation
(low-LET) show a multiplicative interaction.
3.6.2 Vibration
Raynaud's phenomenon is an episodic disorder that produces
intermittent attacks of blanching in the extremities and there may be
numbness or tingling in the hands and fingers. There are several
causes (the term "Raynaud's disease" is applied when the cause is not
known). It was first associated with the use of vibrating tools among
Italian miners in 1911, and the association has since been reported
for a wide range of hand-held vibrating tools such as impact hammers,
chipping hammers, grinders, riveters and the motor-driven chain-saws
used in forestry. The terms vibration white finger (VWF), vibration
syndrome, traumatic vasospastic disease and dead finger have been used
for this condition that begins with numbness and tingling, followed by
blanching and can include intermittent episodes of hand and finger
pain and flushing. With continuing exposure to vibration the symptoms
may become more severe and continue after the cessation of exposure.
Damage to digital arteries and narrowing of the lumen has been
associated with vibration syndrome (JOM, 1984) and, because nicotine
acts as a vasoconstrictor, it has been suggested (JOM, 1984) that
limiting smoking could aid blood flow to the extremities and thus
reduce the condition. In a survey of forestry workers in Quebec in
1977-1978 (Thériault et al., 1982), a prevalence of Raynaud's
phenomenon among 1540 woodcutters was found in 30.5% of chain-saw
users and there was a strong association between this and cigarette
smoking; the relative risks were 3.60 for non-smokers, 6.55 for
smokers and 1.72 for smokers who had not used a chain-saw:
corresponding to an additive effect for the two risk factors. From
another study of the effect of tobacco use on a cohort of men with
VWF, in which the extent of tobacco use was confirmed by blood
nicotine and cotinine measurements (Ekenvall & Lindblad, 1989), it was
shown that tobacco aggravates the symptoms of VWF. Patients with
advanced symptoms were found to use tobacco more frequently and to
have higher blood cotinine levels than patients with less advanced
disease. In a study of the prognosis of VWF (Petersen et al., 1995),
an improvement in the condition occurred when there was no exposure to
either vibration or smoking, whereas an aggravation of the condition
was most notable in smokers. Whole-body vibration has been associated
with persistent severe neck trouble, and smoking was an added
predictor for this condition (Viikari-Juntura et al., 1994). Finger
temperature changes have been measured after smoking a cigarette; in
all cases a reduction in temperature was recorded (Saumet et al.,1986;
Bornmyr & Svensson, 1991).
3.6.3 Noise
In a study of aviators in 1963 at the US Naval Aerospace Medical
Research Laboratory (Thomas et al., 1981), two hearing level groups
were identified, one with normal and the other with impaired hearing.
The impaired hearing group had smoked more cigarettes for a longer
period of time than had those in the normal hearing group. In another
study the relationship between cigarette smoking and hearing loss was
studied in 2348 noise-exposed workers at an aerospace company and it
was found that smoking was a clear risk factor in noise-induced
hearing loss: the OR was 1.27 for "ever smokers" and 1.39 for present
smokers, compared with non-smokers (Barone et al., 1987). Vascular
insufficiency of the cochlear organ has been cited as the predominant
cause of progressive hearing loss that occurs with age. It was
suggested that smoking reduces the cochlear blood supply by:
a) vasospasm induced by nicotine; b) atherosclerotic narrowing of
vessels; and c) thrombotic occlusions (Zelman, 1973). In a study of
1000 subjects at a Veterans Hospital, Zelman (1973) found that whilst
age and sex were the most important variables, at all measured
frequencies the percentage of loss was greater for smokers, the
differences being greater at higher frequencies. From a retrospective
analysis of audiograms taken between 1984 and 1990 of a cohort of 119
workers, 78.8% of smokers compared with 25.7% of non-smokers had
noise-induced hearing loss (ILO, 1991). Although these studies
demonstrated a positive correlation between smoking and hearing loss,
Friedman et al. (1969) and Pyykko et al. (1987) were not able to show
that smoking was a significant risk factor to hearing loss.
3.6.4 Dupuytren's contracture
Dupuytren's contracture is a contracture of some muscle membranes of
the palm of the hand that causes the little finger and ring finger to
be drawn into the palm from where they cannot be extended. It is
characterized by a retractile sclerosis of the palmar aponeurosis,
which may progress to an irreducible flexion of the fingers. Opinions
differ on the influence of occupation, handedness and hand injury on
Dupuytrens contracture which Dupuytren himself attributed to chronic
occupational injury. Some people assert that heavy work always causes
the disease, while others claim that it is not responsible. Mikkelsen
(1978) studied 901 cases in an epidemiological study of 15 950 and
concluded, after isolating hand trauma, that Dupuytren's contracture
is caused by heavy work. The contribution made by tobacco has been as
contentious as has the cause of Dupuytren's contracture. Hand
thermography of affected fingers in Dupuytren's contracture shows a
drop in temperature of up to 3 degrees; and hand temperature falls by
a similar amount during smoking. In a study of 84 men and 16 women
with Dupuytren's contracture, Fraser-Moodie (1976) found no evidence
that smoking was connected with the condition, a conclusion also
reached by Mackenney (1983). However, cigarette smoking was listed
among the responsible factors for Dupuytren's contracture by Attali et
al. (1987), as well as by An et al. (1988), who found that cigarette
smoking was linked statistically to Dupuytren's contracture and
suggested that it may be involved in its pathogenesis by producing
microvascular occlusion and subsequent fibrosis and contracture. An et
al. (1988) concluded that cigarette smoking was one of the most
significant factors in the development of peripheral vasculopathy
Abelin et al. (1990) showed a significant association between
Dupuytren's contracture and smoking habits.
3.7 Biological agents
3.7.1 Biological (vegetable) dusts
Uncontrolled exposure to airborne vegetable dusts can affect health
and occurs worldwide in many workplaces, e.g., in agricultural
operations, textile industries, construction, carpentry and the
furniture industry. The population exposed is large, particularly in
developing countries where whole families, from young children to the
elderly, may engage in agricultural activities and small scale
manufacturing operations using vegetable products. These agents can
take the form of vegetable dusts, airborne fungal spores and
microorganisms, animal danders and feathers, herbicides and pesticides
and their residues. Processing of agricultural products, such as
cotton, flax, hemp, grain, tobacco, paprika and tea, and the milling
of certain varieties of wood are occupations where vegetable dust
exposures have been associated with detrimental health effects.
In addition to the irritation and bronchitis that is associated with
exposure to almost any dust, biological dusts can cause byssinosis,
allergic and immunological responses and, in some cases, nasal and
paranasal cancer. All these conditions can be affected in different
measure by tobacco smoking.
3.7.1.1 Cotton dust
Byssinosis is a respiratory disease of textile workers. The disease is
found in many cotton processing countries. It is more prevalent in the
dusty stages of cotton processing, such as carding, than in weaving.
Byssinosis, or similar symptoms, and bronchitis have also been found
in flax, hemp, jute and sisal workers. The characteristic symptoms are
tightness of the chest and shortness of breath on returning to work
after a period of absence. There is the possibility of progression to
permanent respiratory disability.
In a study of textile workers in South Carolina, USA, in 1973, the
smoking prevalence was almost the same for workers as for controls
(Beck et al., 1984). In another study of cotton workers in 1963-1966
(Molyneux & Tombleson, 1970), the percentage of male current smokers
was 62.5% and of ex-smokers 16.4%; among females the figures were
33.9% and 6.1%, respectively. Among flax scutchers in Normandy in
1986-1987, 56% were smokers and 18% were ex-smokers; compared with 45%
and 15%, respectively, for the controls (Cinkotai et al., 1988a). In
31 Lancashire textile factories (1988) 47.5% of the 4656 workers
interviewed were smokers (Cinkotai et al., 1988b). Of 800 000 American
workers surveyed (Stellman et al., 1988) for smoking habits in 1982,
5.9% were exposed to textile fibres or dust: 28.5% of these were
regular cigarette smokers and 44.9% were former cigarette smokers;
compared with 23.5% and 43.5%, respectively, in other occupations not
exposed to textiles.
Increases in both byssinosis and bronchitis were attributed to cotton
dust exposure and smoking in the cotton industry (Molyneux &
Tombleson, 1970; Merchant et al., 1973). From an industrial study of
the effects of cotton dust and cigarette smoke, Merchant et al. (1972)
concluded that smokers showed an increase in both the prevalence and
severity of cotton dust-induced byssinosis and that cigarette smoke
also increased the detrimental effect of cotton dust on ventilatory
capacity. It was suggested that the impairment of lung clearance
mechanisms by cigarette smoke could be responsible for the deleterious
effect of cotton dust and that smoking might lower the threshold of
susceptibility to the effects of inhaled cotton dust. Additivity and
the equal importance of the effects of smoke and cotton dust have been
suggested (Beck et al., 1984) but since different lung function
parameters are affected it would seem that the two factors affect
different sites. The fact that workers who stopped smoking, whilst
remaining in the same job, lost their byssinotic symptoms was
significant. A survey by Cinkotai et al. (1988a) of workers in 31
textile factories in Lancashire, United Kingdom, showed that
byssinotic symptoms (in decreasing order) were related to years in the
industry, degree of dust exposure, quality of cotton in use, ethnic
origin of workers and smoking habits. Symptoms of chronic bronchitis
were related primarily to smoking habits and then to factors connected
with the occupation. In a study of hemp workers (Bouhuys & Zuskin,
1976), decline in ventilatory function was more pronounced in smokers.
It was suggested (Cinkotai et al., 1988b) that a surprisingly low
prevalence of byssinotic symptoms in 12 flax scutching mills in
Normandy may have been due to either self selection of the workforce
or to an absence of the causative agent in the dust. Persistent cough
and phlegm production were associated with tobacco use.
In textile-related pulmonary disease, smoking as a primary causative
factor was reported by Pratt et al. (1980), and a similar conclusion
was arrived at from lung function tests carried out over a 3-year
period on 153 women (103 smokers, 50 non-smokers) with grades 2 and 3
byssinosis by Honeybourne & Pickering (1986). Cancer deaths in general
and lung cancer in particular were lower in workers exposed to cotton
dust than in others (Enterline et al., 1985). Kilburn (1989) suggested
that the effect of byssinosis on mortality of textile workers from
pulmonary disease needed more comprehensive study.
3.7.1.2 Wood dust
IARC (1995) evaluated the carcinogenic risk of wood dust and
classified it as carcinogenic to humans (Group 1), based on sufficient
evidence in humans and inadequate evidence in animals. The risk of
developing cancer of the nasal cavity among workers manufacturing
wooden furniture has been shown to be up to 100 times greater than for
the general population (Rang & Acheson, 1981). The effect is worst in
the most dusty areas (Rang & Acheson, 1981; Hayes et al., 1986). The
association of risk with certain hardwoods and the finishing of fine
furniture, rather than with woodworking in general, suggests that it
may be allied to both the chemical and physical nature of the dust. In
the study where the nasal cancer incidence was 100 times greater than
for the general population, it did not appear to be affected by
smoking habits. A similar conclusion was reached in a study in an area
of Italy with a large number of cases of nasal cancer among wood and
leather workers (Cecchi et al., 1980). An association with smoking was
established in other studies, and current and past smoking habits were
shown to be a risk factor for developing squamous cell cancer of the
sinus in men (Fukuda et al., 1987). A case-control study of 121 male
woodworkers who were examined for cancer of the nasal cavity or
paranasal sinus, in British Columbia in Canada between 1939 and 1977
(Elwood, 1981), showed increased relative risks associated with
occupations involving exposure to wood (relative risk 2.5) and with
smoking (relative risk 4.9). In a study in North Carolina and Virginia
in the USA between 1970 and 1980 (Brinton et al., 1984), a major
finding was the elevated risk of nasal cavity and sinus cancer among
cigarette smokers. However, the nature of any interaction of wood dust
and tobacco smoking needs further study because adenocarcinomata
appear to be the tumour type associated with wood dust, whereas the
relative risks for squamous-cell and small-cell cancers tend to be
higher for smokers. The available data do not permit an assessment of
the degree of interaction between smoking and wood dust exposure.
3.7.1.3 Allergic responses
This type of response can occur in the upper airways, where it is
manifest as hay fever, in response to certain types of pollen, or in
the bronchi as asthma, or it may appear in both. Some of the dusts
that cause allergic airways responses (occupational asthma) are grain
dusts from various cereals and their products, wood dusts particularly
from red cedar and iroko, and dusts from teas and tobacco. Among
asthmatics, environmental cigarette smoke makes the effect of the
asthma worse (Shim & Williams, 1986), and smoking effects appear to be
additive to that of asthma from other causes (Conolly et al., 1988).
Grain dust exposure and smoking have been found to cause increases in
the prevalence of respiratory symptoms and reductions in pulmonary
function of grain elevator workers. The effect of smoking was slightly
more pronounced; the combined effect of grain dust and smoking
appeared to be additive, except in the least exposed workers (5 years
or less) where a synergistic effect was observed in tests for
peripheral airways dysfunction (Cotton et al., 1983). Chan-Yeung et
al. (1985) stated that the effect of grain dust and smoking was
additive and not synergistic in causing a decrease in lung function.
In 303 workers in the animal feed industry exposed to dusts of grains
and cassava, 61 (20%) showed respiratory symptoms; in office staff in
the same plant the prevalence was similar. The plant workers had a
higher prevalence of smoking than office staff. Current smoking was
strongly associated with respiratory complaints in both groups (Post
et al., 1994).
Occupational asthma also occurs in flour, tea, coffee and rice
handlers. In Italian bakers and pastry makers De Zotti et al. (1994)
found that 54 (23%) of the 226 subjects were atopic. Forty (18%) were
skin-test-positive to storage mites, 27 (12%) to wheat flour and 17
(8%) to alpha-amylase. Skin sensitization to these occupational
allergens was significantly associated with atopy, smoking and
duration of exposure. In a study of 401 workers in bakeries or flour
mills, Cullinan et al. (1994a) found that work-related symptoms of
allergy were more common in smokers, with little difference between
atopic and non-atopic workers. However, smoking was not independently
related to either symptoms or positive skin test.
Zetterstrom et al. (1981) skin-prick-tested 129 workers in a coffee
roastery with green coffee bean and castor bean extracts. There was a
significantly increased prevalence of positive skin prick tests in
smokers. In enzyme detergent workers with occupational asthma, it was
found that twice as many smokers as non-smokers exhibited asthmatic
symptoms (Greenberg et al., 1970; Mitchell & Gandevia, 1971).
Smokers are more likely to show higher specific antibody production
and correspondingly be more susceptible to asthma.
3.7.2 Other biological agents
Although many biological dusts are known to have detrimental health
effects, there have been few studies of any interaction of smoking
with these agents. Extrinsic allergic alveolitis may be caused by
spores from a number of fungi which are small enough to reach the
pulmonary compartment. There are several forms of allergic alveolitis,
of which farmer's lung, bagasse pneumonitis, and bird fancier's lung
are examples. These are caused by fungal spores in mouldy hay or
mouldy sugar cane or an agent in bird feathers, respectively, and are
immunologically mediated (Lancet, 1985). Extrinsic allergic alveolitis
is a rare example of a respiratory disease which is more prevalent in
non-smokers than in smokers. In one study, 14 of 18 patients (11 with
farmer's lung and 7 with bird fancier's lung) were non-smokers, twice
the proportion of non-smokers in patients with cryptogenic fibrosing
alveolitis or in the local population (Warren, 1977). Carrillo et al.
(1991) investigated IgG response to pigeon serum and its relation to
tobacco smoking in 160 pigeon fanciers. The sensitization rate was
31.9%. Pigeon fanciers who were current smokers had significantly
lower levels of IgG antibodies to pigeon serum (P<0.001).
Precipitating antibodies to Micropolyspora faeni, a common cause of
farmer's lung, were found to be twice as common in the area of
non-smokers in farming communities compared with smokers (Morgan et
al., 1975). Alveolar macrophage phagocytosis has been shown to be
depressed by cigarette smoke and it has been suggested this may
explain its apparent protective effect (Hocking & Golde, 1979).
3.7.3 Agents found in factory farming (animal
confinement effects)
Factory farming of pigs, where the animals are kept in confined
conditions, is common practice in livestock production in many
developed countries and it has been found to be accompanied by adverse
respiratory symptoms in workers. Donham et al. (1984) summarized the
effects as: acute toxicosis and inflammation of the respiratory tract
from inhaling hydrogen sulfide; acute asthma-like symptoms;
bronchitis; and delayed or hypersensitivity pneumonitis-like symptoms.
They found that smoking interacted additively with the bronchitis and
obstructive symptoms of the condition. In a study of workers in swine
confinement areas, Zuskin et al. (1992) reported similar effects. They
also found that smoking aggravated acute and chronic respiratory
symptoms and impairment of lung function.
3.7.4 Laboratory animals
Venables et al. (1988b) examined data from three cross-sectional
surveys of 296 laboratory workers around 30 years of age exposed to
small mammals. Two populations were of pharmaceutical research workers
(N = 133 and 140) and one of research workers in a tobacco company
(N = 23). One of the pharmaceutical research worker populations had a
laboratory animal allergy (LAA) prevalence rate of over 40% (Venables
et al., 1988a). The tobacco company research workers were exposed only
to rats while the other two populations were exposed to rats, mice,
guinea-pigs and rabbits. Atopy was determined by skin prick test to
non-animal aeroallergens. Sensitization to laboratory animals was
determined by response to skin prick tests using urinary extracts from
the species used in each laboratory. Radioallergoabsorbent tests
(RASTs) were used to measure serum IgE antibody concentration to the
urinary extracts in two of the populations.
Atopy in the three populations ranged from 30% to 44%, and positive
skin response to urinary extract ranged from 13% to 48%. Pooled data
from the three surveys showed an association between smoking and
positive skin response to urinary extract. Associations with smoking
persisted after stratifying by atopic status, suggesting that smoking
was a risk factor for developing laboratory animal allergy.
In other studies of 238 laboratory workers without previous
occupational exposure to rats in three institutions specializing in
small animal research, atopy was again determined by skin prick test
response to non-animal aeroallergens and sensitization to laboratory
rats by response to urinary extract. Exposure to total dust and rat
urinary aeroallergen was also measured. Allergy to rats was positively
related to exposure intensity and this was stronger in atopic
subjects. Positive responses to skin prick testing with rat urinary
extract were strongly related to atopy and to smoking at all levels of
exposure (Cullinan et al., 1994b).
Several studies from Europe have shown an association between
ownership of pet birds or pigeons and lung cancer (Holst et al., 1988;
Kohlmeier et al., 1992; Gardiner et al., 1992). The relative risk
adjusted for smoking was 6.7 (2.2-20.0). However, two community-based
case-control studies, one from the USA and one from Sweden, could not
confirm an association between pet birds and lung cancer (Alavanja et
al., 1996; Modigh et al., 1996). In 1998, a hospital-based
case-control study conducted in New York City and Washington, DC, with
887 cases and 1350 controls, did not show an association of keeping
pet birds with lung cancer in non-smokers. There was a ten-fold
increase of lung cancer among smokers who were not bird keepers over
non-smokers, but there was no indication of synergism between smoking
and keeping a pet bird (Morabia et al., 1998).
3.7.5 Schistosomiasis
In a study carried out in Spain, risk factors for urinary bladder
cancer were identified (Bravo et al., 1987). The factors were listed
in order of importance and the first three were total number of
cigarettes smoked, history of urological disease and exposure to an
occupational risk. Vineis (1992) summarized epidemiological,
biochemical and molecular evidence that clearly linked smoking with an
increased risk of bladder cancer. Cohen & Johansson (1992) considered
smoking to be the most important etiological factor for bladder
cancer. They also implicated a variety of occupational exposures and,
in some parts of the world, an association with various endemic
diseases including schistosomiasis. Schistosomiasis is a waterborne
parasitic disease found in many developing countries in Africa, Asia
and South America. It is a widespread occupational disease for
agricultural workers, and also affects members of the general
population.
A phase in the life-cycle of the trematodes responsible for the
disease lives in the blood vessels of visceral organs and their eggs
are discharged through the bladder or intestine in urine and faeces.
Some species live in the mesenteric veins and the eggs are discharged
in the faeces but the eggs of Schistosoma haematobium mature in the
veins of the bladder and are discharged in the urine. The eggs mature
in water and the resultant larvae infect freshwater snails. Within the
snail the parasites multiply to produce free swimming cercaria larvae
which can infect humans via skin penetration and repeat the cycle. S.
haematobium is found in nearly all countries in the African continent
and it has been found that the incidence of bladder cancer is higher
in areas with a high prevalence of infection than in areas with a low
prevalence. IARC classifies infection with S. haematobium as
carcinogenic to humans (Group 1) (IARC, 1994b). In Egypt, the most
common form of cancer is bladder cancer, accounting for 27.6% of all
malignancies encountered (38.5% of cancers in males and 11.3% in
females), and these high levels have been attributed to underlying
schistosomiasis (Tawfik, 1987). Makhyoun (1974) carried out a
case-control study of smoking among Egyptian males with and without a
previous history of S. haematobium infection. A smoking index was
calculated (average number of cigarettes per day × duration of smoking
in years) to categorize subjects. The smoking index (intensity and
duration of smoking) was higher in all the patients with bladder
cancer. In the patients with a previous schistosomiasis infection,
22.7% were moderate or heavy smokers compared with 79.3% of the
non-schistosomiasis patients. In the latter there was a good
correlation with the smoking index but in the bladder cancer patients
with previous schistosomiasis there was no significant difference in
smoking index between patients and matched controls. It was not
possible to identify an interaction between smoking and
schistosomiasis in the production of bladder cancer.
However, in a review of the role of S. haematobium in human bladder
cancer, Badawi et al. (1995) referred to several major studies that
implicated this infection with the subsequent development of bladder
cancer. Badawi et al. (1995) listed examples of a co-carcinogenic
effect of parasitic infection in the presence of chemical carcinogens
and it has been suggested (Hicks et al., 1980; Hicks, 1982) that
schistosomiasis could supply the necessary proliferative stimulus to
accelerate cancer growth from latent tumour foci on exposure to
carcinogenic nitrosamines. Nitrosamines have been implicated as
carcinogens among tobacco chewers and oral snuff users (Hecht &
Hoffmann, 1988, 1991; Hoffmann et al., 1991a), nitrosamines have been
demonstrated in smoke (Tricker & Preussmann, 1992; Hoffmann et al.,
1991b) and nicotine-derived N-nitrosamines cause cancer (Hoffmann &
Hoffmann, 1991; (IARC, 1991). Some N-nitrosamines are excreted as
esters via the urinary tract, e.g., N-nitroso-di- n-butanol or NNK
as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. They may hydrolyse
in the presence of infectious agents in the bladder, as is the case in
schistosomiasis, and thus appear in their precarcinogenic form,
thereby increasing the risk for cancer in the urinary tract.
Endogenous nitrosation, which is increased by tobacco smoking, chewing
and oral snuff use has been demonstrated by monitoring urinary
N-nitrosamino acids (Tsuda & Kurashima, 1991). Badawi & Mostafa
(1993) and Badawi et al. (1995) suggested that the evidence for an
association between urinary schistosomiasis and bladder cancer
development is sufficient to justify the conclusion that S.
haematobium infection is a factor in inducing preneoplastic changes
in the bladder. Infection can reduce detoxification mechanisms (and
thus prolong retention of carcinogens in the bladder), stimulate
nitrosamine synthesis, and alter the activity of
carcinogen-metabolizing enzymes.
The conclusion is that an interaction is likely between tobacco use
and schistosomiasis infection.
3.7.6 Other urinary tract infections
Kantor et al. (1984) found that the joint effect of urinary tract
infection and cigarette smoking on bladder cancer was slightly beyond
that expected under an additive model and suggested that patients with
cystitis may be especially prone to tobacco-derived carcinogens in the
urine. La Vecchia et al. (1991) studied cystitis, gonorrhoea and
condylomata acuminata and found an interaction between urinary tract
infection and tobacco that appeared to be multiplicative, with
relative risk 2.4 for "ever smoking", 3.2 for cystitis alone, and 10.3
for combined exposure. It was concluded that a relationship between
urinary tract infection (and possibly some genital infections) and
bladder cancer indicated a cancer promotion role of infection and a
multiplicative smoking interaction. There have been reports of
synergism between herpes simplex virus and tobacco-specific
N-nitrosamines in cell transformation (Park et al., 1991a); an
additive interaction between smoking and herpes simplex virus type 2
in the promotion of cervical abnormalities (Mayberry, 1985), and the
possibility of an interaction between cigarette smoking and herpes
virus infection leading to cancer of the uterine cervix (Winkelstein
et al., 1984).
3.7.7 Sarcoidosis
Bresnitz & Strom (1983) reviewed sarcoidosis and concluded that
tobacco smoking might have a protective effect for the pathogenesis of
sarcoidosis. Sarcoidosis is a generalized granulomatous disease
involving the reticulo-endothelial system with lesions predominantly
in the lymphatic system. Harf et al. (1986) investigated the smoking
habits of 113 cases of histologically confirmed sarcoidosis in a
case-control study in Lyon, France. Smoking habits were defined in 101
cases and these were compared with a control group of healthy
volunteers. There was a highly statistically significant negative
association between sarcoidosis and smoking (OR = 3.8; 95% confidence
interval: 2.4, 6.5) in both cases.
3.8 Vector effects
Toxic chemicals, as well as harmless materials that produce harmful
chemical agents when they are burnt or vaporized, can be inadvertently
transferred to cigarettes or other smoking materials and cause the
smoke to be more injurious when it is inhaled.
3.8.1 Polytetrafluoroethylene
Polytetrafluoroethylene (Teflon(R)) is used in coatings for cooking
utensils, for making chemical vessels, gaskets and bearings and in
sprays as a mould release agent. Polytetrafluoroethylene and polyvinyl
fluoride are inert materials but their thermal decomposition products
can be very biologically reactive. Cigarettes can be easily
contaminated in the workplace and, when smoked, the polymer burns to
form fumes which cause "polymer-fume fever": severe gripping chest
pain giving rise to difficulty in breathing; trembling and shaking;
elevated temperature; and severe diaphoresis. The symptoms pass after
a day or two, but recur on again smoking a contaminated cigarette.
Before the cause was recognized a case was recorded of a person, who
used the polymer in a mould release spray, having some 40 attacks
(Kuntz & McCord, 1974). Another case was a person who referred to the
disease as "mould machine pneumonia" (Kuntz & McCord, 1974). Other
cases have been reported (Albrecht & Bryant, 1987) and better
occupational hygiene and a ban on smoking in the workplace resulted in
the disappearance of symptoms in those previously affected.
3.8.2 Mercury
Inorganic mercury occurs in many industries, as elemental mercury in
scientific and electrical instruments, as amalgams with many other
metals, in paints and pigments and in the chemical industry, as well
as in mining and extraction of the metal. Organic mercury compounds
are used as antiseptics, disinfectants, fungicides, bactericides and
herbicides. Contamination of smoking materials can lead to the
inhalation of mercury vapour. Mercury has been detected by neutron
activation analysis as a naturally occurring trace element in tobacco
(<1.0 ng/g) and in the smoke of cigarettes (4 ng/cigarette)
(Schneider & Krivan, 1993; Krivan et al., 1994).
3.9 Effects of tobacco smoking and metabolism of drugs and other
chemicals
3.9.1 Oral contraceptive use
In the 1970s it was postulated that an interaction between tobacco use
and oral contraceptives increased the risk of myocardial infarction in
women. In a study of women less than 45 years of age, a greater
proportion of moderate to heavy smokers were found in oral
contraceptive users experiencing myocardial infarction, compared
against a control population (Mann et al., 1975, 1976). Sturtevant
(1982), however, found no "convincing evidence" for an interaction or
synergism between the factors smoking and oral contraceptive use for
the major classes of cardiovascular disease. Lidegaard (1993) reported
no difference in the proportion of smokers between users and non-users
of oral contraceptives in Danish women experiencing a cerebral
thromboembolic attack and age-matched controls. Regarding thrombosis,
the data of Vessey & Doll (1969) suggest an increased thromboembolism
risk from oral contraceptive use for heavy smokers compared to
non-smokers. However, other studies reviewed by Sturtevant (1982) and
Nevius et al. (1982) are said to be ambiguous regarding a possible
interaction between smoking and oral contraceptive use. On balance,
there is evidence for certain interactions between smoking and oral
contraceptive use: the US Surgeon General (1983a), concluded that
"women who use oral contraceptives and who smoke increase their risk
of a myocardial infarction by an approximately tenfold factor,
compared with women who neither use oral contraceptives nor smoke",
and "the use of both cigarettes and oral contraceptives greatly
increases the risk for subarachnoid haemorrhage among women."
3.9.2 Drug and chemical metabolism
A number of studies have demonstrated that the metabolism of various
drugs and other chemicals is influenced by the smoking status of the
individual. This effect is sufficiently noteworthy that the US Surgeon
General (1979) report concluded that it is "apparent that cigarette
smoking is one of the primary causes of drug interactions in humans".
The extensive review of the literature at that time led to the
conclusions that, with respect to the influence of smoking on the
disposition/metabolism of other compounds: (a) the dominant effect of
smoking is enhanced drug disposition caused by the induction of
hepatic enzymes; (b) tobacco smoke contains many enzyme inducers,
notably polynuclear aromatic hydrocarbons; and (c) smoking can induce
the metabolism of various therapeutic agents and their pharmacological
and/or clinical effects. The metabolism of chemical carcinogens
involves various isozymes of cytochromes P450 and differences in their
genotypes or phenotypes may be a main factor responsible for
differences among individuals in susceptibility to carcinogens.
Metabolic activation of the procarcinogens such as benzo( a)pyrene to
the ultimate form is accomplished by cytochrome P450IA1 (Kawajiri et
al., 1990; Kawajiri & Fujii-Kuriyama, 1991; Nakachi et al., 1991).
Although the most noteworthy effects of smoking cited were related to
enzyme induction, it should also be noted that other components of the
smoke, such as carbon monoxide, nicotine, cadmium, some pesticides,
cyanide and acrolein, may serve to inhibit the function of some
enzymes (Jusko, 1978). An example for this phenomenon is the
inhibition by nicotine of the P450 isozymes that are involved in the
metabolic activation of NNN and NNK (Murphy & Heiblum, 1990). Nicotine
levels exceed those of NNN and NNK by more than 500 times. Therefore,
it was not surprising that even increasing NNN and NNK levels in snuff
ten-fold by adding the synthetic compounds did not alter the
carcinogenic potency of snuff in the oral cavity of rats (Hecht et
al., 1986).
Miller (1990) reviewed how cigarette smoking affects the
pharmacokinetic and pharmacodynamic properties of various drugs. The
drugs pentazocine, phenylbutazone and heparin show increased
metabolism in smokers. Also in smokers, the metabolism of oestrogen
and theophylline is increased. In addition, although smoking does not
pharmacokinetically affect the drugs propranolol and pindolol, the
nicotine in smoke is associated with elevations in blood pressure, and
thus smoking might serve to inhibit the antihypertensive effects of
these beta-adrenergic receptor blockers. On the other hand, smoking
had no effect on the drug disposition and/or pharmacological effects
of various other drugs examined (Jusko, 1978; Miller, 1990).
3.10 Animal studies of the interactions between cigarette smoke
exposure and other agents
Fourteen chronic inhalation studies (whole-body or nose-only exposure)
with mainstream cigarette smoke in rats and mice were reviewed by
Coggins (1998) and the results and histopathological changes
contrasted with epidemiological studies in humans. In most of the
studies there were epithelial changes in conducting airways and
increased numbers of alveolar macrophages, occasionally associated
with alveolar metaplasia. Lung adenomas and adenocarcinomas were seen
in some of the studies but no statistically significant increase in
the incidence of malignant lung tumours was found in either rats or
mice. This contrasts with human epidemiology where there is an
increased lung cancer risk. In an invited commentary, Morgan (1998)
stated that, based on these differences, rodents may be ineffective
models for predicting human health risk, at least for certain inhaled
materials, but stressed the interspecies differences in respiratory
anatomy and physiology and the need for consideration of the many
factors that may lead to differences in the effects of tobacco smoke
exposure.
The existing animal toxicology studies regarding interactions between
tobacco smoke and other materials do not form a comprehensive body of
work on the topic. A number of combinations of exposures between smoke
and other agents have been studied. Results are at times inconsistent
or contradictory, and the mechanisms by which interactions occur are
often not understood. This section provides a brief review of the
literature. In general, the existing work: (a) was generally performed
using rodents; (b) usually (but not always) examined the effects of
cigarette smoking (or components of the smoke) combined with either
with specific chemical components of the smoke or with radiation; and
(c) usually examined cancer as the biological response end-point of
interest. Other studies have focused on the use of cigarette smoke
components or condensates, and have used models such as in vitro
cell systems or mouse skin; a discussion of these studies is beyond
the scope of this section.
3.10.1 Non-cancer end-points
Several studies in experimental animals have examined the effects of
cigarette smoke administered over short periods of time (from hours to
daily exposures over several weeks). The cigarette smoke-induced
increase in the number of pulmonary macrophages and leukocytes noted
in humans has also been seen in animals such as the guinea-pig, even
after short exposures (Rylander et al., 1979). In addition, Morimoto
et al. (1993) observed a synergistic increase between mineral fibres
and exposure to cigarette smoke in the production of tumour necrosis
factor by rat alveolar macrophages. On the other hand, a 10-week
tobacco smoke exposure in rats suppressed radiation- induced pulmonary
inflammation (Nilsson et al., 1992), and a 12-week exposure of rats to
smoke did not influence the lung damage caused by an intratracheal
instillation of cadmium (Lai & Diamond, 1992).
Nishikawa et al. (1992) studied in guinea-pigs the effects of combined
exposure and single exposure to ozone and cigarette smoke on airway
responsiveness and tracheal vascular permeability and found that the
combined exposure increased airway responsiveness and vascular
permeability to a greater extent in terms of magnitude, but not in
duration, than a single exposure. This indicated that combined
exposure was more harmful than exposure to either agent alone.
3.10.2 Cancer studies: tobacco (cigarette) smoke plus other chemicals
Mori (1964) studied rats receiving multiple subcutaneous injections of
the carcinogen 4-nitroquinoline-1-oxide (NQO) with or without 6-7
month inhalation of cigarette smoke. Six of eight rats had lung
carcinomas in the combined exposure group compared to 3 of 9 rats in
the NQO-only group, and tumours occurred earlier. Davis et al. (1975)
studied Wistar rats receiving single intratracheal instillations of
benzo( a)pyrene with or without cigarette smoke inhalation for most
of the lifespan. Slight elevations of pulmonary squamous neoplasia
were noted in the combined exposure group compared with the individual
agents alone. However, the effects were not statistically significant.
In mice, inhaled cigarette smoke did not influence the occurrence of
lung tumours in (a) B6C3F1 mice pretreated with 3-methylcholanthrene
or benzo( a)pyrene (Henry & Kouri, 1984), (b) C57BL mice receiving
benzo( a)pyrene or influenza virus(Harris & Negroni, 1967), (c) A/J
mice receiving intraperitoneal injections of the tobacco specific
nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and a
6-month inhalation of smoke using a whole-body mode of exposure (Finch
et al., 1996), or (d) A/J mice exposed to sidestream smoke plus the
carcinogen 3-methylcholanthrene or urethane (Witschi et al., 1997). On
the other hand, a carcinogenic synergism in lung adenoma formation was
reported in Strain A mice some 6 months after initiation of treatment
with intraperitoneally injected urethane and skin-painted cigarette
smoke tar (DiPaolo & Sheehe, 1962). However, the non- pulmonary route
of exposure makes it difficult to interpret the relevance of this
result.
Several studies in hamsters have combined cigarette smoke exposure
with other agents. Dontenwill et al. (1973) combined smoke exposure
with a single intratracheal instillation of asbestos, but found no
significant differences in laryngeal lesions or tumours in the group
exposed to both materials, compared with the group receiving smoke
alone; the asbestos alone was non-tumorigenic. The combination of a
single intratracheal administration of dimethylbenz( a)anthrene
(DMBA) and smoke was found to increase the tumour incidence in the
oral cavity, pharynx, trachea and non-pulmonary tissues, compared to
the individual agents alone (Dontenwill et al., 1973). A similar
synergism between DMBA and smoke in producing laryngeal papillomas and
other oral and laryngeal lesions was reported by Hoffmann et al.
(1979) and by Kobayashi et al. (1974), and a synergism was also noted
between the nitrosamine DENA and smoke (Dontenwill et al., 1973).
Hamsters pretreated with a single dose of either 1.0, 3.3 or 10.0 mg
of NNK, and subsequently exposed twice daily for up to 72 weeks to
diluted cigarette smoke developed significantly more tumours in the
respiratory tract than hamsters receiving the identical dosage of NNK
and subjected to sham-smoking (Hecht et al., 1983). This supports the
concept that tobacco smoke has tumour-promoting activity. A
combination of smoke plus benzo( a)-pyrene adsorbed onto haematite
produced tracheal and laryngeal hyperplasia, whereas either agent
alone did not (Hoffmann et al., 1979), but no tumours were produced.
3.10.3 Cancer studies: cigarette smoke plus radiation
Dogs were used to study the potential interactions between radon and
cigarette smoke inhalation in producing lung cancer (Cross et al.,
1982). The lung tumour incidence in dogs exposed to radon plus
cigarette smoke for 4-5 years was decreased compared to that of dogs
receiving a mixture of radon, radon daughters, and uranium ore dust.
Studies have examined the potential interactions between tobacco smoke
and the alpha-emitters radon or plutonium-239 in rats or mice. In a
series of studies, Chameaud et al. (1982) examined the effects of
combined exposures to radon and cigarette smoke in Sprague-Dawley
rats, as well as the effects of pre-treatment with either radon or
smoke exposure. Smoke exposures begun before radon exposure did not
influence the radon-induced lung tumour incidence, but this increased
by 2- to 3-fold in the combined exposure group receiving radon before
cigarette smoke compared to the group receiving radon alone.
Finch et al. (1995a) examined F-344 rats receiving a single inhalation
exposure to 239PuO2, combined with chronic (up to 30 months)
cigarette smoke exposure, and found a synergistic crude tumour
incidence in rats receiving both agents, compared with animals
receiving the radiation alone. Most of these effects could be
explained by a cigarette-smoke-induced retardation of lung clearance
of the 239PuO2 particles (Finch et al., 1995b). This group is
continuing to study the potential combined effects of smoke and X-ray
exposure in rats, and the potential effects of smoke combined with
either X- or alpha-irradiation in mice (Finch et al., 1995a). Talbot
et al. (1987) reported results of a study in which mice inhaled
cigarette smoke, 239PuO2 or both materials. As in the case of rats,
cigarette smoke exposure caused retarded lung clearance and thus led
to greater radiation doses in animals receiving both agents.
4. EFFECTS OF EXPOSURE TO TOBACCO SMOKE AND OTHER AGENTS: SEPARATE
EFFECTS OR POSSIBLE INTERACTIONS
4.1 Coal mining
4.1.1 Coal dust
Coal miners can suffer from chronic bronchitis, coal workers
pneumoconiosis, progressive massive fibrosis, and emphysema. The
respiratory impairment appears as radiologically visible and
functional changes in the lungs, but although some of these are
associated solely with coal dust, some are also closely associated
with smoking. In the past it has been difficult to apportion
attributable risk to the two causes due to the fact that coalface
workers, the group subjected to the highest dust exposure, have a
different smoking pattern and perhaps a different daily consumption of
smoking materials than non-coalface workers because smoking in the
mine is forbidden. With the limitation of dust in modern mining, the
effect of the two factors, dust and smoking, is becoming clearer.
However, difficulties arise in interpreting data because non-smokers
may accumulate more dust, as they have less absenteeism, a different
pattern of lung clearance and a longer life (NIOSH, 1995).
In most of the countries surveyed, the prevalence of smoking by miners
tends to have been somewhat higher than in either the male population
as a whole, or among most other occupational groups, although a
distinction is seldom drawn between miners and coal miners except in
studies centred on coal mining populations. Between 1963 and 1975 in
the United Kingdom, the smoking prevalence fell for the male
population (and for miners) from 54% (miners 77%) to 47% (miners 49%)
(Lee, 1976). The figures for 1988 and 1990 were 33% and 31% (Bennett
et al., 1996) and for face-trained coalminers 35.7% in 1989 (Elliott,
1995). In a study of 8555 American miners from 29 bituminous coal
mines (Kibelstis et al., 1973), over 50% were smokers and 25% were
ex-smokers. In a United Kingdom study (Love & Miller, 1982), only 13%
of 1677 coal miners from 5 British collieries, who were examined in a
lung function study, were non-smokers; 66% were regular smokers and
the remainder were intermittent or ex-smokers. In a 20 year follow-up
study of a population of coal British miners and others (Cochrane &
Moore, 1980), 69% of the coal miners were smokers. These examples
typify the smoking prevalence of coal miners prior to the early
1980's. A 1982 survey of 800 000 American men and women in relation to
their occupations (Stellman et al., 1988) found that among miners
(type unspecified) 29.4% had never smoked regularly, 31.5% were
current smokers and 39.1% were former cigarette smokers. This may
reflect a general trend in the countries with higher income economies
where smoking has been decreasing in many sections of the population.
4.1.2 Bronchitis in coal miners
Kibelstis et al. (1973) found that the prevalence of bronchitis in
coal miners who smoked was higher than in non-smoking coal miners.
Coalface workers had more bronchitis and more airway obstruction than
surface workers and the difference between smokers working at the
coalface and non-smoking surface workers showed that the effect of
smoking was five times greater than that of coal dust. A German
epidemiological evaluation of chronic obstructive bronchitis in 5605
miners, 1276 ex-miners and 3898 individuals who had never worked in a
mine showed that smoking has a more serious effect on miners than on
other groups (Roth et al., 1985). It is possible that, within a mining
community, the other two groups may have a predisposition to the
disease and have achieved their status by job selection or job escape.
In a study of new entrants into coal mining McLintock (1971) found
that those who drop out of mining tend to be less physically fit and
more prone to chest problems than those who remain.
Morgan (1982) analysed the effects of cigarette smoking, dust exposure
and environmental factors on respiratory disease, and concluded that
bronchitis and airways obstruction were two separate responses to
cigarette smoking. The airflow obstruction found in smokers is due to
small airways disease and an involvement of respiratory bronchioles
leading to the development of emphysema. In coal miners, the
prevalence of bronchitis among non-smokers is related to the degree of
dust exposure. Marine et al. (1988) analysed data from studies on 53
382 coal miners in the United Kingdom and found that smoking miners
were at greater risk of developing chronic bronchitis. In a study by
Selig & Nestler (1985) of the relationship between chronic bronchitis,
smoking and dust (unspecified source), heavy smoking was equated with
20 years of dust exposure. From postmortem examinations of coal mine
workers, a correlation was reported between clinical chronic
bronchitis and smoking (Selig & Nestler, 1985).
The chronic bronchitis of coal miners is probably a combination of (a)
mucus hypersecretion caused by dust; (b) mucus hypersecretion, mucus
modification and clearance impairment caused by tobacco smoke; (c)
small airways disease caused by tobacco smoke; and (d) the effect of
dust on small airways tissue already inflamed by smoking. Bronchitis
due to smoking causes mucus hypersecretion which is much greater than
that due to coal dust (Morgan, 1982). Cigarette smoke impairs lung
clearance by changing the physical and chemical properties of mucus
and causing ciliastasis. Rheological measurement show changes in the
viscoelastic properties of mucus; chemically the mucus glycoprotein
structure is changed (King et al., 1989) and the irritant gases in
smoke cause abnormal mucus secretion and ciliastasis (Holbrook, 1977).
Small airways disease and bronchiolitis, leading to emphysema, are due
to smoking rather than to dust (Morgan, 1982).
4.1.3 Emphysema and pneumoconiosis in coal miners
Dust in coal mining is considered to be the primary cause of coal
workers pneumoconiosis. In a study of coalworkers and non-coalworkers,
Cockroft et al. (1982) concluded, after taking any effect of smoking
into account, that there was a 7-fold excess of emphysema in
coalworkers. Results of postmortem examinations of 866 Australian
miners (Leigh et al., 1983) showed a positive correlation between dust
exposure and emphysema and pneumoconiosis, with the severity highest
in non-smokers. However, smoking and non-smoking coalface workers were
not compared. From a postmortem comparison of lungs from 450 coal
miners, Rockley et al. (1984) found that emphysema occurred more
frequently in smokers (72%) than in ex-smokers (65%) or in non-smokers
(42%) and the relative frequency increased with age at death. The
study considered the possibility that coal dust might cause emphysema
which inhibits clearance and, in turn, promotes fibrosis, or
alternatively that fibrosis caused by dust increases the chance of
emphysema. However, the findings of a study of South Wales coal miners
(Fletcher., 1972) militated against dust-induced emphysema. It has
been suggested that differences in emphysema between coalworkers and
non-coalworkers can be accounted for by taking into account current
smokers in the two groups. Morgan (1982) concluded that the evidence
militates against obstructive emphysema occurring more commonly in
coal miners than in the general population, or that more dust
inhalation leads to a greater likelihood of emphysema developing.
Reviews of small airways disease (SAD) suggested that emphysema
proceeds from smoking-induced SAD. Cosio et al. (1980) considered that
their observations supported the hypothesis that SAD is causally
related to centrilobular emphysema, but not necessarily to panlobular
emphysema.
4.1.4 Lung cancer in coal miners
Perhaps as a result of failure to control confounding factors, there
has been a lack of consistency among reports on the relationship
between coal mining and lung cancer incidence in miners. In a direct
evaluation of the relationship between lung cancer mortality and coal
mine dust exposure, controlling for smoking status, Ames et al. (1983)
found no evidence of a link between coal mine dust exposure and lung
cancer risk, nor of an interaction effect, although the expected lung
cancer risk in cigarette smokers was observed. From a study of dust
exposure, pneumoconiosis and mortality of coal miners (Miller &
Jacobsen, 1985) it was found that lung cancer mortality among miners
who smoked was 5.5 times higher than in "never smokers" but that the
effect was entirely due to smoking.
Radon and radon daughter contamination of the dust in coal mines might
be expected to be as prevalent as in all other mines, and thus the
apparent very low lung cancer risk in coal mining may seem unexpected.
However, because of the explosion risk in coal mines, the ventilation
is usually efficient and a build-up of radioactivity is probably less
likely than in other types of mine.
4.2 Other mineral dusts
4.2.1 Talc
Talc is a hydrated magnesium silicate, often contaminated with free
silica or fibrous asbestos-like minerals such as tremolite and
anthophyllite. The only significant difference in the effects on
exposed and non-exposed was in the number and severity of cases of
dyspnoea in the talc workers, and smoking was considered to be an
aggravating factor (Kleinfeld et al., 1973).
4.2.2 Kaolin
Kaolin (pure China clay) is a hydrated aluminium silicate used for
ceramics and as a filler in the paper, rubber and paint industries.
The dry powder can give rise to fibrotic nodules in the lungs (Seaton
et al., 1981; Wagner et al., 1986). Characteristic smoker's inclusions
have been seen in transmission electron micrographs of pulmonary
alveolar macrophages obtained from cigarette smokers. The contents of
these inclusions are heterogeneous and include electron-dense areas,
lipid material and needle-like structures. These have properties
consistent with the composition of kaolinite. Kaolinite is present in
cigarette smoke from different brands, and pulmonary alveolar
macrophages are able to ingest this material in vivo (Hocking &
Golde, 1979).
4.2.3 Alumina
Alumina (aluminium oxide) is extremely hard and is used as an abrasive
(corundum). A cross-sectional study of 788 employees of an aluminium
production company examined the relationship of radiographic
abnormalities to smoking and dust exposure during bauxite and alumina
mining and refining (Townsend et al., 1988). Chest radiographs showed
a moderate time trend of increasing prevalence of small opacities in
non-smokers with high cumulative dust exposures. In most exposure
categories, smokers had a higher prevalence of opacities than
non-smokers. For cumulative exposures of less than 100 mg/m3-years,
increasing trends with duration of exposure were accentuated in
smokers as compared to non-smokers. The stronger effects observed in
smokers were attributed to the joint effects of duration of smoking
and duration of occupational exposure (Townsend et al., 1988).
4.3 Fibrous minerals
Fibrous minerals have been implicated in pleural thickening, pulmonary
fibrosis, mesothelioma and lung cancer in some villages in the
Anatolian region of Turkey (Artvinli & Baris, 1979), where fibrous
zeolite minerals (chabazite and erionite) are present in volcanic
deposits and used in buildings. Erionite has been shown to induce
mesothelioma. The symptoms and pathology of the respiratory disorders
and malignant disease were similar to those of asbestos. Asbestos-type
diseases have also been described in communities exposed to zeolite
minerals and tremolite dust in other similar regions by Baris et al.
(1979) and Yazicioglu et al. (1980). Non-asbestos fibrous materials
have been associated with pulmonary fibrosis (Stanton et al., 1977).
There are no data on any interaction of these minerals with smoking
but there appears to be a potential for interaction.
Wollastonite is a fibrous monocalcium silicate, which has been used as
a substitute for asbestos, as a filler and flux in ceramics, in
grinding wheels, refractory products, building blocks and acoustic
tiles. It is weakly fibrogenic. Hanke et al. (1984) studied a small
population of workers exposed to wollastonite and attributed
significant levels of chronic cough, phlegm and bronchitis to smoking
and not to exposure to wollastonite.
4.4 Metals
4.4.1 Antimony
Antimony is chemically similar to arsenic. Arsenic is metalloid,
antimony is a metal, both have volatile hydrides and form halogen,
oxygen and sulfur derivatives. Compounds of both frequently occur
together, particularly in smelter fume. Biological effects are similar
to those of arsenic (De Wolff & Edelbroek, 1994; De Wolff, 1995).
The concentrations of antimony, arsenic, cadmium, chromium, cobalt,
lanthanum, lead, selenium and zinc measured in lung tissues of
deceased smelter workers suggested that lung cancer risk was
multifactorial, involving carcinogenic and anti-carcinogenic factors
(Gerhardsson & Nordberg, 1993). A 30-year study at an antimony smelter
did not specifically implicate antimony as the cause of excess lung
cancer because of concurrent exposure to other carcinogens (Jones,
1994). In another study of smelters, the data suggested an increase in
lung cancer and non-malignant respiratory and heart disease (Schnorr
et al., 1995).
Antimony can have harmful effects on lung tissues, including
pneumoconiosis. IARC (1989a) evaluated antimony trioxide and antimony
trisulfide and concluded that the trioxide was possibly carcinogenic
to humans (Group 2B) and the trisulfide was not classifiable (Group
3). It is likely that the effects of inhalation of antimony fume/dust
and tobacco smoke would be worse than inhaling either separately. The
similarities of antimony to arsenic, both chemically and in some
biological effects, leads to the conclusion that tobacco smoke and
antimony could interact like tobacco smoke and arsenic in producing
toxicity.
4.4.2 Cadmium
Most zinc and lead-zinc ores contain small amounts of cadmium. It is
used in electroplating, in metal alloys (with copper for overhead
wires, and aluminium for casting), in nickel-cadmium dry cells, for
pigment manufacture and use, added to silver to prevent staining, and
it is a hazard of welding. The main route of exposure for the
non-smoking general population is via food, while for exposed workers
it enters the body mainly by inhalation. Tobacco is an important
source of cadmium in smokers (IPCS, 1992), and the tobacco source
affects the level of cadmium exposure (Yue, 1992). Any intake is
important because cadmium has an extremely long biological half-life.
It was suggested (Hassler et al., 1983) that higher levels of cadmium
in the blood and urine of exposed workers could arise both from
workplace contamination of cigarettes and transfer as fume during
smoking.
Cadmium has various toxic effects, the earliest being impairment of
renal tubular function leading to failure of resorption and excretion
of low molecular weight protein, glycosuria, aminoaciduria and
hypercalciuria (IPCS, 1992; IARC, 1993). It has been associated with
some types of lung disorder (emphysema, obstructive pulmonary disease
and diffuse fibrosis) (IPCS, 1992) Exposure to cadmium compounds has
been associated with cancer of the lung. There is some evidence for an
association with prostatic cancer (IARC, 1993). Other epidemiological
studies did not confirm an increased risk of prostatic cancer
(Kazantzis et al., 1992). The evaluation by IARC (1993) concluded that
cadmium and cadmium compounds are carcinogenic to humans (Group 1).
The supposition that cadmium in cigarette tobacco or in the workplace
may cause lung cancer has been questioned (Lamm et al., 1992;
Hertz-Picciotto & Hu, 1994). In a cohort mortality study of cadmium
workers in England, an observed increase lung cancer risk could not be
attributed strictly to cadmium due to the presence of multiple
confounding factors, particularly arsenic (Kazantzis et al., 1992).
Lamm et al. (1992) also suggested that arsenic may be responsible for
the observed lung cancer increased. The many elements in the lung
tissues of deceased smelter workers (Gerhardsson & Nordberg, 1993)
illustrates the difficulty in apportioning a role to one material in a
multifactorial environment.
Cadmium affects the myocardium and produces hypertension in animal
studies but the induction of cardiovascular disease and hypertension
in humans has not been demonstrated in epidemiological studies
(Kristensen, 1989; IPCS, 1992)). Blood and urine cadmium levels are
higher in smokers than in non-smokers and were found to be
considerably elevated in smokers working in an alkaline battery
factory (Hassler et al., 1983) and in smelter workers who were also
smokers (Kazantzis & Armstrong, 1984; Lilis et al., 1984a,b).
Davison et al. (1988) reported a cadmium dose/effect relationship in
functional and radiological evidence of emphysema in 101 subjects.
Leduc et al. (1993) described the very rapid development of emphysema
in a smoker after exposure to very high levels of cadmium. Smoking by
cumulatively increasing the body burden and hindering the lung
clearance may have provided an additional cause for the emphysema.
However, an additive or synergistic mechanism for the cadmium plus
smoking effect could not be inferred in this case because of the high
cadmium dose.
4.4.3 Cobalt
Cobalt is used in the production of alloys, tungsten carbide tools,
permanent magnets, and in the electrical industry.
Cobalt has toxic effects (Beliles, 1994). Cobalt exposure has been
linked to various allergic reactions (Shirakawa et al., 1992); hard
metal exposure and smoking together arithmetically increased total IgE
levels. Interstitial lung disease has been associated with cobalt in
susceptible individuals (Sprince et al., 1988), although in a study
involving the manufacture of permanent magnets (Deng et al., 1991)
abnormalities in pulmonary function and respiratory symptoms were no
higher than those of a reference population, except for 4 subjects out
of 362, who showed diffuse patches consistent with pneumoconiosis.
4.4.4 Lead
Lead is used in batteries, paint, glass, ceramics, fuel additives and
other industrial applications. In lead-using industries the main route
of exposure is by inhalation, mainly as dust and fume. Lead has a
range of toxic effects on blood, and the renal and nervous systems
(IPCS, 1995).
Levels of lead in blood vary from one area to another, between urban,
rural and occupationally exposed populations, and between men and
women (IPCS, 1995). The tobacco plant absorbs lead from the soil and
around 5-6% of that in cigarettes is inhaled in the smoke. Lead
concentrations in the smoke from one cigarette were found to range
from 0.017 to 0.98 µg (IARC, 1986). Higher blood lead and erythrocyte
protoporphyrin levels have been demonstrated in heavy smokers exposed
to lead (Williams et al., 1983; Landrigan & Straub, 1985); these could
have been partly due to contaminated cigarettes acting as vectors.
Other studies on occupationally exposed workers showed a progressive
increase in blood lead with an increase in the number of cigarettes
smoked (Maheswaren et al., 1993).
The association between lead exposure, tobacco smoke exposure and
blood pressure was examined in a cross-sectional study on 809 men
occupationally exposed to lead in a battery factory but only a small
increase in systolic blood pressure was found (Maheswaren et al.,
1993). There was no evidence of interactive effects between smoking
and lead exposure, but the absorbed lead from cigarettes added to the
body burden.
4.5 Rubber industry
Some of the principal hazards in the industry are fumes, talc, carbon
black, chemical additives and organic solvents but the components of
the hazard mixture differ between different areas of work. A high risk
of pulmonary disease has been reported in the rubber industry. It was
elevated for smokers, particularly those employed in areas where there
were respirable particulates and/or solvents (Lednar et al., 1977).
The data suggested an interaction between smoking and hazards
encountered in mixing (particulates), extrusion (solvent sprays and
mould release agents), and curing (solvents and rubber reaction
products). A problem in epidemiological studies in the industry arises
because of movement of workers between jobs. Some high-risk workers
who were also smokers were involved in finishing and inspection but
they tended to be older employees who had worked in other areas before
moving to this particular job. Emphysema was the principal pulmonary
condition leading to premature termination of employment (Lednar et
al., 1977).
IARC (1987) classified the rubber industry as Group 1, based on
sufficient evidence for carcinogenicity to humans. Excess mortality
from cancers of various sites, the site usually being associated with
the nature of the work and types of exposure, has been reported with
bladder cancer being associated with exposure to aromatic amines (Fox
et al., 1974; Monson & Nakano, 1976a,b; Monson & Fine, 1978;
Kilpikari, 1982; IARC, 1987; Zhang et al., 1989; Weiland et al.,
1996). Lung cancer was associated with curing and inner tube
manufacture. The use of talc in the rubber industry has been
associated with pulmonary disease (Kleinfeld et al., 1973). In the
rubber industry the relative risk of lung cancer for talc-exposed
workers was 3.2 for men and 4.4 for women (Zhang et al., 1989),
compared with 2.5 times excess lung cancer risk in talc-using
industries not associated with rubber manufacture (Thomas & Stewart,
1986). In jobs where very high lung cancer rates were found, smoking
levels were also very high, but any possible interaction effect from
the two exposures could not be assessed.
Gastrointestinal cancer, bladder cancer and leukaemia were found to be
associated with jobs in the rubber industry (Monson & Fine, 1978;
IARC, 1987). Possible causes, such as exposure to carbon black,
plasticisers, antioxidants, arylamino compounds and benzene have been
suggested. Urine samples from rubber workers showed a higher mutagenic
activity in the middle of a working week than at the beginning of a
week in both smokers and non-smokers, indicating the presence of
mutagens in the work environment. In a study involving the analysis of
urine from rubber workers for mutagenic factors, a possible
synergistic effect of smoking and occupational exposure was found in
smokers (Wicklund et al., 1988). There was a relationship between skin
contamination and urinary mutagenicity (Bos et al., 1989). Other
studies involving the analysis of urine from rubber workers for
mutagenic factors also suggested a possible synergistic effect of
smoking and occupational exposure among smokers (Falk et al., 1980;
Crebelli et al., 1985).
Andjelkovich et al. (1988) carried out a case-control study of lung
cancer in workers at a rubber manufacturing plant. There was an
association between lung cancer mortality risk and work in certain
areas for smokers and non-smokers, and the risk was greater in
smokers. Zhang et al. (1989) studied a cohort of 957 men and 667 women
employed in a rubber factory. The relative risk of lung cancer for
smokers was 8.5 for men and 11.4 for women, and for those exposed to
curing agents or talc the relative risk was 3.2 for men and 4.6 for
women. Additive and multiplicative models were used to evaluate the
interaction between smoking and occupational exposure on lung cancer.
The additive interaction term was not statistically significant and
the multiplicative interaction was negative. Weiland et al. (1996)
were not able to find interactions between smoking and exposure in the
rubber industry.
4.6 Petroleum industry
Petroleum refining involves exposure of workers to a large number of
chemical compounds occurring in crude oil or encountered in production
processes as intermediates, catalysts, additives or in the final
products. Because of fire risk, there are sections of the industry
where smoking is not permitted. However, in a study of 10 923 male and
624 female employees of the Australian petroleum industry between 1981
and 1984, it was found that the smoking habits did not differ
substantially from those of the general population (Christie et al.,
1986), and continued surveillance of these workers showed that
mortality rates were lower than for the general population (Christie
et al., 1987).
IARC (1989b) concluded that there is limited evidence that working in
petroleum refineries entails a carcinogenic risk. This limited
evidence applies to skin cancer and leukaemia; for all other cancer
sites on which information was available, the evidence was inadequate.
The overall evaluation, taking account of sufficient or limited
evidence in experimental animals for the carcinogenicity of various
distillates produced during petroleum refining, was that occupational
exposures in petroleum refining are probably carcinogenic to humans
(Group 2A).
In a study of 92 men with histologically confirmed renal cell
carcinoma (Domiano et al., 1985), it was concluded that there could be
an interaction between long-term gasoline exposure and heavy smoking.
In a case-control study of bladder cancer in New Jersey, USA, Najem et
al. (1982) found a significantly elevated risk of bladder cancer in
patients who had worked in the petroleum industry (OR, 2.5, CI,
1.2-5.5). While the risk was increased in current smokers (OR, 2.6),
it was higher in "never smokers" (OR, 5.6) and only slightly elevated
in ex-smokers (OR, 1.4). In another hospital-based study in Argentina
an association was found between bladder cancer and oil refinery work,
along with an elevated risk of lung cancer in smokers, but the numbers
were too few for any evaluation of the interaction (Iscovich et al.,
1987).
There was no increased mortality from either kidney cancer or
leukaemia in employees exposed to gasoline (Wong et al., 1993). A low
frequency of bladder cancer among refinery workers was attributed to
an assumed lower level of smoking among the group of workers studied
(Higginson et al., 1984).
In the study of workers occupationally exposed to gasoline vapour, the
sister chromatid exchange (SCE) frequency in peripheral blood
lymphocytes was used as an indicator of genotoxic response. Workers
employed in gasoline retail outlets were classified according to
cigarette smoking habits. The SCE frequency in lymphocytes was
unaffected by cigarette smoke or gasoline exposure alone, but
increased with combined exposure. The increased SCE frequency observed
in smokers occupationally exposed to gasoline vapour could be due to
the activation of hepatic enzymes by cigarette smoke, leading to a
greater formation of reactive metabolites of gasoline vapour (Edwards
& Priestly, 1993).
4.7 Pesticides
A variety of chemical compounds, natural and synthetic, are used as
pesticides. The number of individuals exposed during manufacture is
relatively small, and processes are usually contained, but the
worldwide population of pesticide users is very large. Most of the
world's population is exposed to pesticide residues in food and
drinking-water (Ecobichon, 1995). There is some information concerning
effects on health from interaction of pesticide exposure and smoking.
"Vineyard sprayer's lung", is an occupational disease associated with
the spraying of copper-sulfate-based Bordeaux Mixture. In a study of
the cytological changes in the respiratory tract of vineyard workers
spraying Bordeaux Mixture (Plamenac et al., 1985), the macrophages of
control subjects contained no copper whereas copper was detected in
64% of the sprayers. Abnormal cytological changes in the sputum were
found in smokers, in sprayers and controls, and atypical squamous
metaplasia of the respiratory epithelium was observed in 29% of the
sprayers who were smokers.
In a study to evaluate the hypothesis that exposure to lead arsenate
resulted in an excess mortality from lung cancer, Wicklund et al.
(1988) compared 155 male orchard workers who had died of respiratory
cancer with 155 orchard workers who had died of other causes. Two
groups of non-orchard workers (620) were used as matched controls.
There was no difference in lead arsenate exposure between the orchard
worker group. Although cigarette smoking was common among the orchard
workers, their smoking habits were similar to the non-orchard worker
control groups. In both groups of orchard workers mortality from
respiratory cancer was higher in smokers than in non-smokers.
McDuffie et al. (1990) examined the possibility that pesticide use was
related to the risk of primary lung cancer. In a case-control study
using data from a population-based cancer registry, they interviewed
273 men and 103 women with diagnosed primary lung cancer and compared
their occupational exposures, medical history and working
characteristics with 187 male community control subjects. There was no
correlation of lung cancer risk with exposure to pesticides and
adjusting for smoking did not alter this.
5. CONCLUSIONS AND RECOMMENDATIONS
5.1 Conclusions
1. Tobacco use, particularly smoking, is the single most important
public health hazard in the world today, and a major preventable
cause of morbidity and mortality.
2. In addition to the adverse health effects of active tobacco use,
adverse health effects have also been demonstrated as a result of
exposure to environmental tobacco smoke.
3. The risks from tobacco smoke are demonstrably increased through
interactions with certain chemical, physical and biological
hazards found in the workplace and general environment.
4. Based on this review of the scientific literature, additional
interactive effects, not yet identified, may exist.
5. In addition to synergistic interactions between tobacco smoke and
other agents, a few instances of antagonistic interactions were
also noted. However, even in these cases, the health risks of
tobacco smoke far outweigh any apparent protective effects.
5.2 Recommendations for protection of human health
1. All possible measures should be taken to eliminate tobacco use,
particularly smoking.
2. In order to avoid interaction with occupational exposures, and
eliminate the risks of exposure to environmental tobacco smoke,
smoking in the workplace should be prohibited.
3. Smoking in public places should be strongly discouraged.
4. In order to reduce the risks of exposure to environmental tobacco
smoke, particularly for children, smoking in domestic
environments should be strongly discouraged. Such action will also
avoid possible deleterious interactions between tobacco smoke and
residential exposures to other hazards.
5. Awareness-building through active educational programmes on the
health hazards of smoking should be undertaken in both developed
and developing countries. This should include enhanced
communication about deleterious interactions between tobacco and
other agents. Governments, industry, health and educational
professionals, and the general public should share in this
responsibility.
6. Since smoking may result in altered response or adverse reactions
to drugs and other therapeutic treatments, appropriate dose
adjustments and patient surveillance should be taken into
consideration by clinicians.
7. Health professionals should provide assistance to smokers to stop
smoking. This may necessitate the allocation of additional
resources for this purpose.
6. FURTHER RESEARCH
1. A number of different methodological approaches to investigating
interactions currently exist. The feasibility of harmonizing
methodologies for the assessment of potential interactions
between two or more health hazards should be explored. In the
interim, investigators should make every effort to state clearly
which methodologies have been used when reporting their results.
2. In many of the observational studies included in this review,
exposure data were limited. In order to improve future
investigations of interactions among human health hazards, more
complete and accurate exposure assessments should be performed.
3. Further epidemiological investigations of potential interactions
between tobacco smoke and other hazards in both the occupational
and general environments are needed to identify additional
populations at risk. Such interactive effects can lead to risks
higher than would be predicted by separate analyses of the risks
associated with individual hazards.
Epidemiological investigations in countries where the health
effects of tobacco have not been extensively studied previously
are of particular importance. This information would be of value
in characterizing the morbidity and mortality due to tobacco use
in those countries.
4. Additional research is needed to clarify the toxicological
mechanisms by which tobacco smoke leads to adverse health
effects, and by which tobacco smoke and other agents interact.
This information will be of use in the design and interpretation
of epidemiological studies on the health effects of tobacco,
including interactive effects.
5. Additional epidemiological research on the health effects of
passive smoking, particularly carcinogenic, cardiorespiratory and
allergic effects, would be of value in characterizing the effects
of low levels of exposure to tobacco smoke. Interactions between
environmental tobacco smoke and other health hazards also warrant
further investigation.
6. Established biomarker methods should be applied to the monitoring
of workers for the early detection of harmful exposures to toxic
or carcinogenic agents, and, in many areas, new biomarker methods
should be developed to improve hazard identification.
Consideration should be given to evaluating the ongoing exposure
of workers to active or passive tobacco smoke by monitoring
salivary or urinary cotinine, one of the major nicotine
metabolites.
7. A comprehensive review of the adverse health effects of
environmental tobacco smoke should be undertaken. This review
would provide an authoritative summary of the health impacts of
environmental tobacco smoke, as well as interactions between
environmental tobacco smoke and other health hazards.
REFERENCES
Abe S, Ohsaki Y, Kimura K, Tsuneta Y, Mikami H, & Murao M (1982)
Chromate lung cancer with special reference to its cell type and
relation to the manufacturing process. Cancer, 49(4): 783-787.
Abelin T, Segmüller H, & Segmüller G (1990) Tobacco and alcohol
consumption as risk factors of Dupuytren's contracture. In: Tobacco
and health 1990 -- The global war: Proceedings of the 7th World
Conference on Tobacco and Health, Perth, Western Australia, 1-5 April
1990. Perth, Department of Health of Western Australia, pp 449-450.
Abramson MJ, Wlodarczyk JH, Saunders NA, & Hensley MJ (1989) Does
aluminium smelting cause lung disease. Am Rev Respir Dis, 139:
1042-1057.
Acheson ED, Gardner MJ, Winter PD, & Bennett C (1984) Cancer in a
factory using amosite asbestos. Int J Epidemiol, 13(1): 3-10.
Alavanja MC, Brownson RC, Benichou J, Swanson C, & Boice JD (1995)
Attributable risk of lung cancer in lifetime nonsmokers and long-term
ex-smokers (Missouri, United States). Cancer Causes Control, 6(3):
209-216.
Alavanja MC, Brownson RC, Berger E, Lubin J, & Modigh C (1996) Avian
exposure and risk of lung cancer in women in Missouri: population
based case-control study. Br Med J, 313(7067): 1233-1235.
Albrecht WN & Bryant CJ (1987) Polymer-fume fever associated with
smoking and use of a mold-release spray containing
polytetrafluoroethylene. J Occup Med, 29(10): 817-819.
Al-Fayez SF, Salleh M, Ardawi M, & Zahran FM (1988) Effects of sheesha
and cigarette smoking on pulmonary function of Saudi males and
females. Trop Geogr Med, 40(2): 115-123.
Amandus HE, Castellan RM, Shy C, Heineman EF, & Blair A (1992)
Reevaluation of silicosis and lung cancer in North Carolina dusty
trades workers. Am J Ind Med, 22(2): 147-153.
Ames RG, Amandus H, Attfield M, Green FY, & Vallyathan V (1983) Does
coal mine dust present a risk for lung cancer? A case-control study of
US coal miners. Arch Environ Health, 38(6): 331-333.
An HS, Southworth SR, Jackson WT, & Russ B (1988) Cigarette smoking
and Dupuytren's contracture of the hand. J Hand Surg, 13A: 872-874
Anderson HA, Lilis R, Daum SM, & Selikoff IJ (1979) Asbestosis among
household contacts of asbestos factory workers. Ann NY Acad Sci, 330:
387-399.
Andjelkovich DA, Abdelghany N, Mathew RM, & Blum S (1988) Lung cancer
case-control study in a rubber manufacturing plant. Am J Ind Med,
14(5): 559-574.
Andjelkovich DA, Shy CM, Brown MH, Janszen DB, Levine RJ, & Richardson
RB (1994) Mortality of iron foundry workers: III. Lung cancer
case-control study. J Occup Med, 36(12): 1301-1309.
Archer VE (1988) Lung cancer risk in underground miners: cohort
case-control studies. Yale J Biol Med, 61: 183-193.
Armitage AK & Turner DM (1970) Absorption of nicotine in cigarette and
cigar smoke through the oral mucosa. Nature (Lond), 226: 1231-1232.
Armstrong BG, Tremblay CG, Cyr D, & Theriault GP (1986) Estimating the
relationship between exposure to tar volatiles and the incidence of
bladder cancer in aluminium smelter workers. Scand J Work Environ
Health, 12: 486-493.
Artvinli M & Baris YI (1979) Malignant mesotheliomas in a small
village in the Anatolian Region of Turkey: An epidemiologic study. J
Natl Cancer Inst, 63(1): 17-20.
Attali P, Ink O, Pelletier G, Vernier C, Jean F, Moulton L, & Etienne
JP (1987) Dupuytren's contracture, alcohol consumption, and chronic
liver disease. Arch Int Med, 147: 1065-1067.
Autrup JI, Hansen C, & Autrup H (1992) Detection of tobacco smoke
carcinogen-DNA adducts in cultured rat buccal mucosa cells following
exposure to ethanol and total cigarette smoke condensate or chewing
tobacco. Chem-Biol Interact, 85: 141-150
Avolio G, Cacciabue M, Rolla G, Caria E, Oliaro A, Carlino F, Pepino
E, Polizzi S, & Bucca C (1986) [Functional compromise of the small
airways in subjects exposed to SiO2.] Minerva Med, 77(45-46):
2183-2185 (in Italian).
Axéll T, Mörnstad H, & Sundström B (1978) [Snuff and cancer of the
oral cavity.] Lakartidningen, 75: 2224-2226 (in Swedish).
Axelson O (1995) Cancer risks from exposure to radon in homes. Environ
Health Perspect, 103(suppl 2): 37-43.
Badawi AF & Mostafa MH (1993) Possible mechanisms of alteration in the
capacities of carcinogen metabolizing enzymes during schistosomiasis
and their role in bladder cancer induction. J Int Med Res, 21(6):
281-305.
Badawi AF, Mostafa MH, Probert A, & O'Connor PJ (1995) Role of
schistosomiasis in human bladder cancer: evidence of association,
aetiological factors, and basic mechanisms of carcinogenesis. Eur J
Cancer Prev, 4: 45-59.
Baker DB, Gann PH, Brooks SM, Gallagher J, & Bernstein IL (1990)
Cross-sectional study of platinum salts sensitization among precious
metals refinery workers. Am J Ind Med, 18: 653-664.
Ball J & Simpson D (1987) Round the world: India -- Tobacco and ill
health. Lancet, 18: 149.
Baris Y, Artvilini M, & Sahin AA (1979) Environmental mesothelioma in
Turkey. Ann NY Acad Sci, 330: 423-432.
Barone JA, Peters JM, Garabrant DH, Bernstein L, & Krebsbach R (1987)
Smoking as a risk factor in noise-induced hearing loss. J Occup Med,
29(9): 741-745
Bartsch H, Malaveille C, Friesen M, Kadlubar FF, & Vineis P (1993)
Black (air-cured) and blond (flue-cured) tobacco cancer risk. IV:
Molecular dosimetry studies implicate aromatic amines as bladder
carcinogens. Eur J Cancer, 29A(8): 1199-1207
Bates MN, Smith AH, & Cantor P (1995) Case-control study of bladder
cancer and arsenic in drinking water. Am J Epidemiol, 141(6): 523-530.
Battista SP (1976) Ciliatoxic components in cigarette smoke. In:
Proceedings of 3rd World Conference on Smoking and Health, New York
City, 2-5 June 1975 -- Volume 1: Modifying the risk for the smoker.
Washington, DC, US Department of Health, Education and Welfare, pp
517-534 (DHEW Publication No. (NIH) 76-1221).
Baumslag N, Keen P, & Petering HG (1971) Carcinoma of the maxillary
antrum and its relationship to trace metal content of snuff. Arch
Environ Health, 23: 1-5.
Beck GJ, Maunder LR, & Schachter EN (1984) Cotton dust and smoking
effects on lung function in cotton textile workers. Am J Epidemiol,
119(1): 33-43.
Beliles RP (1994) Cobalt. In: Clayton GD & Clayton FE ed. The metals
-- Patty's industrial hygiene and toxicology. New York,
Wiley-Interscience, chapter 27, pp 1986-1999.
Bennett N, Jarvis L, Rowlands O, Singleton N, & Haselden L (1996)
Office of Population Censuses and Surveys, Social Survey Division:
Results from the 1994 general household survey. London, Her Majesty's
Stationery Office (HMSO), pp 92-102.
Benowitz NL, Porchet H, Sheiner L, & Jacob P (1988) Nicotine
absorption and cardiovascular effects with smokeless tobacco use:
comparison with cigarettes and nicotine gum. Clin Pharmacol Ther,
44(1): 23-28.
Berry G, Newhouse ML, & Turok M (1972) Combined effects of asbestos
exposure and smoking on mortality from lung cancer in factory workers.
Lancet, 2: 476-479.
Berry G, Newhouse ML, & Antonis P (1985) Combined effect of asbestos
and smoking on mortality from lung cancer and mesothelioma in factory
workers. Br J Ind Med, 42: 12-18.
Bhonsle RB, Murti PR, Gupta PC, & Mehta FS (1976) Reverse dhumti
smoking in Goa: an epidemiologic study of 5,499 villagers for oral
precancerous lesions. Indian J Cancer, 13(4): 301-305.
Bi W, Hayes RB, Feng P, Qi Y, You X, Zhen J, Zhang M, Qu B, Fu Z, Chen
M, Chien HTC, & Blot WJ (1992) Mortality and incidence of bladder
cancer in benzidine-exposed workers in China. Am J Ind Med, 21(4):
481-489.
Biberman R, Lusky A, Schlesinger T, Margaloit M, Neeman E, & Modan B
(1993) Increased risk for small cell lung cancer following residential
exposure to low dose radon: a pilot study. Arch Environ Health, 48(4):
209-212.
Bien TH & Burge R (1990) Smoking and drinking: A review of the
literature. Int J Addict, 25: 1429-1454.
Bjorseth A, Bjorseth O, & Feldstad PE (1978) Polycyclic aromatic
hydrocarbons in the work atmosphere; I. Determination in an aluminium
reduction plant. Scand J Work Environ Health, 4: 212-223.
Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS,
Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, & Fraumeni
JF Jr (1988) Smoking and drinking in relation to oral and pharyngeal
cancer. Cancer Res, 48: 3282-3287.
Boffetta P, Stellman SD, & Garfinkel L (1988) Diesel exhaust exposure
and mortality among males in the American Cancer Society prospective
study. Am J Ind Med, 14: 403-415.
Bohning DE, Atkins HL, & Cohn SN (1982) Long-term particle clearance
in man: normal and impaired. Ann Occup Hyg, 26: 259-271.
Bolinder G, Alfredsson L, Englund A, & de Faire U (1994) Smokeless
tobacco use and increased cardiovascular mortality among Swedish
construction workers. Am J Public Health, 84(3): 399-404.
Bornmyr S & Svensson H (1991) Thermography and laser-Doppler flowmetry
for monitoring changes in finger skin blood flow upon cigarette
smoking. Clin Physiol, 11(2): 135-141.
Bos RP, Kromhout H, Ikink H, de Haan W, Koppejan J, & Theuws JLG
(1989) Mutagens in urine of non-smoking and smoking workers in an
aircraft tyre retreading plant: Skin exposure as a causal factor.
Mutat Res, 223(1): 41-48.
Bouhuys A & Zuskin E (1976) Chronic respiratory disease in hemp
workers. Ann Intern Med, 84: 398-405.
Bovenzi M, Stanta G, Antiga G, Perruzzo P, & Cavallieri F (1993)
Occupational exposure and lung cancer risk in a coastal area of
northeastern Italy. Int Arch Occup Environ Health, 65(1): 35-41.
Boyko RW, Cartwright RA, & Glashan RW (1985) Bladder cancer in dye
manufacturing workers. J Occup Med, 27(11): 799-803.
Boyle P, Macfarlane GJ, Maisonneuve P, Zheng T, Scully C, & Tedesco B
(1990) Epidemiology of mouth cancer in 1989: a review. J R Soc Med,
83: 724-730.
Boysen M, Solberg LA, Torjussen W, Poppe S, & Hogetveit AC (1984)
Histological changes, rhinoscopical findings and nickel concentration
in plasma and urine in retired nickel workers. Acta Oto-Laryngol,
97(´): 105-115.
Bravo MP, Del Rey Calero J, & Conde M (1987) Risk factors of bladder
cancer in Spain. Neoplasma, 34(5): 633-637.
Bresnitz EA & Strom BL (1983) Epidemiology of sarcoidosis. Epidemiol
Rev, 5: 124-156.
Brinton LA, Blot WJ, Becker JA, Winn DM, Browder JP, & Farmer JC Jr
(1984) A case-control study of cancers of the nasal cavity and
paranasal sinuses. Am J Epidemiol, 119(6): 896-906.
Brown CC & Chu KC (1989) Additive and multiplicative models and
multistage carcinogenesis theory. Risk Anal, 9: 9-105.
Brownson RC, Novotny TE, & Perry MC (1993) Cigarette smoking and adult
leukemia: A meta analysis. Arch Intern Med, 153: 469-475.
Brunnemann KD & Hoffmann D (1974) The pH of tobacco smoke. Food Cosmet
Toxicol, 12: 115-124.
Brunnemann KD & Hoffmann D (1981) Assessment of the carcinogenic
N-nitrosodiethanolamine in tobacco products and tobacco smoke.
Carcinogenesis, 2: 1123-1127.
Brunnemann KD & Hoffmann D (1991) Chemical studies on tobacco smoke
XCC: Decreased concentrations of N-nitrosodiethanilamine and
N-nitrosomorpholine in commercial tobacco products. J Agric Food
Chem, 39: 207-208.
Brunnemann KD & Hoffmann D (1992) Chemical composition of smokeless
tobacco products. Bethesda, Maryland, National Cancer Institute, pp
96-108 (Smoking and Tobacco Control Monographs, No. 2).
Brunnemann KD, Mitacek EJ, Liu Y, Limsila T, & Suttajit M (1996)
Assessment of major carcinogenic tobacco-specific N-nitrosoamines in
Thai cigarettes. Cancer Detect Prev, 20(2): 114-121.
Burch JD, Howe GR, Miller AB, & Semenciw R (1981) Tobacco, alcohol,
asbestos, and nickel in the etiology of cancer of the larynx: A
case-control study. J Natl Cancer Inst, 67: 1219-1224.
Calverley AE, Rees D, Dowdeswell RJ, Linnett PJ, & Kielkowski D (1995)
Platinum salt sensitivity in refinery workers: incidence and effects
of smoking and exposure. Occup Environ Med, 52: 661-666.
Carrillo T, de Castro R, Cuevas M, Diaz F, & Cabrera P (1991) Effect
of cigarette smoking on the humoral immune response in pigeon
fanciers. Allergy, 46: 241-244.
Cartwright R (1982) Occupational bladder cancer and cigarette smoking
in West Yorkshire. Scand J Work Environ Health, 8(suppl 1): 79-82.
Castonguay A, Tjalve H, & Hecht SS (1983) Tissue distribution of the
tobacco-specific carcinogen
4-(methyl-nitrosamino)-1-(pyridyl)-1-butanone and its metabolites in
F344 rats. Cancer Res, 43: 631-635.
Castonguay A, Rivenson A, Trushin N, Reinhardt J, Spathopoulos S,
Weiss CJ, Reiss B, & Hecht SS (1984) Effects of chronic ethanol
consumption on the metabolism and carcinogenicity of
N-nitrosonornicotine in F344 rats. Cancer Res, 44: 2285-2290.
Cecchi F, Buiatti E, Kriebel D, Nastasi L, & Santucci M (1980)
Adenocarcinoma of the nose and paranasal sinuses in shoemakers and
woodworkers in the province of Florence, Italy (1963-77). Br J Ind
Med, 37: 222-225.
Chaffey CM & Bowie C (1994) Radon and health -- an update [Review]. J
Public Health Med, 16(4): 465-470.
Chakrabarti RN, Dutta K, Sikdar S, & Ghosh K (1991) Smokeless tobacco
and premalignant and malignant lesions of the oral cavity. Indian J
Med Sci, 45(10): 273-275.
Chameaud J, Perraud R, Chretien J, Masse R, & Lafuma J (1982) Combined
effects of inhalation of radon daughter products and tobacco smoke.
In: Sanders CL ed. Pulmonary toxicology of respirable particles.
Washington, DC, US Department of Energy, Technical Information Center,
pp 551-557 (CONF-791002).
Chan-Yeung M, Enarson D, & Grzybowski S (1985) Grain dust and
respiratory health. Can Med Assoc J, 133(10): 969-973.
Cheng WN & Kong J (1992) Retrospective mortality cohort study of
chrysotile asbestos products workers in Tianjin 1972-1987. Environ
Res, 59(1): 271-278.
Chiazze L Jr, Watkins DK, & Fryar C (1992) A case-control study of
malignant and non-malignant respiratory disease among employees of a
fibreglass manufacturing facility. Br J Ind Med, 49(5): 326-331.
Chiazze L Jr, Watkins DK, & Fryar C (1995) Adjustment for the
confounding effect of cigarette smoking in an historical cohort
mortality study of workers in a fiberglass manufacturing facility. J
Occup Environ Med, 37(6): 744-748.
Chopra SK, Taplin GV, Elam D, Carson SA, & Golde D (1979) Measurement
of tracheal mucociliary transport velocity in humans -- smokers versus
non-smokers. Am Rev Resp Dis, 119(4): 205 (Abstract).
Cho CH, Chen BW, Hui WM, & Lam SL (1990) The influence of acute or
chronic nicotine treatment on ethanol-induced gastric mucosal damage
in rats. Dig Dis Sci, 35: 106-112.
Chow JYC, Ma L, & Cho CH (1996) An experimental model for studying
passive cigarette smoking effects on gastric ulceration. Life Sci, 58:
2415-2422.
Christie D, Gordon I, & Robinson K (1986) Smoking in an industrial
population: An analysis by birth cohort. Med J Aust, 145: 11-14.
Christie D, Robinson K, Gordon I, Webley C, & Bisby J (1987) Current
mortality in the Australian petroleum industry: the healthy worker
effect and the influence of life-style factors. Med J Aust, 147:
222-225.
Cinkotai FF, Rigby A, Pickering CAC, Seaborn D, & Faragher E (1988a)
Recent trends in the prevalence of byssinotic symptoms in the
Lancashire textile industry. Br J Ind Med, 45: 782-789.
Cinkotai FF, Emo P, Gibbs ACC, & Jouany JM (1988b) Low prevalence of
byssinotic symptoms in 12 flax scutching mills in Normandy, France. Br
J Ind Med, 45: 325-328.
Clausen J, Netterstrom B, & Wolff C (1993) Lung function in insulation
workers. Br J Ind Med, 50(3): 252-256.
Cochrane AL & Moore F (1980) A 20-year follow-up of a population
sample (aged 25-34) including coal-miners and foundry workers in
Stavely, Derbyshire. Br J Ind Med, 37: 230-233.
Cockroft A, Seal RME, Wagner JC, Lyons JP, Ryder R, & Andersson N
(1982) Post-mortem study of emphysema in coalworkers and
non-coalworkers. Lancet, 2(8298): 600-603.
Coggins CRE (1998) A review of chronic inhalation studies with
mainstream cigarette smoke in rats and mice. Toxicol Pathol, 26(3):
307-314.
Cohen SM & Johansson SL (1992) Epidemiology and etiology of bladder
cancer. Urol Clin North Am, 19(3): 421-428.
Cohen D, Arai SF, & Brain JD (1979) Smoking impairs long-term
clearance from the lung. Science, 204: 514-517.
Cohen BS, Harley NH, & Tso TC (1985) Clearance of polonium-210
enriched cigarette smoke from rat trachea and lung. Toxicol Appl
Pharmacol, 79: 314-322.
Collins AC (1990) Interactions of ethanol and nicotine at the receptor
level. Recent Dev Alcohol, 8: 221-231.
Conolly CK, Chan NS, & Prescott RJ (1988) The relationship between age
and duration of asthma and the presence of persistent obstruction in
asthma. Postgrad Med J, 64(752): 422-425.
Cosio MG, Hale KA, & Niewoehner DE (1980) Morphologic and morphometric
effects of prolonged cigarette smoking on the small airways. Am Rev
Respir Dis, 122: 265-271.
Cotton DJ, Graham BL, Li KYR, Froh F, Barnett GD, & Dosman JA (1983)
Effects of grain dust exposure and smoking on respiratory symptoms and
lung function. J Occup Med, 25(2): 131-141.
Crebelli R, Paoletti A, Falcone E, Aquilana G, Fabri G, & Carere A
(1985) Mutagenicity studies in a tyre plant: in vitro activity of
workers' urinary concentrates and raw materials. Br J Ind Med, 42(7):
481-487.
Creek L, Capehart T, & Griese V (1994) US tobacco statistics,
1935-1992. US Dep Agric Stat Bull, 869: 14.
Cromwell BT (1955) Modern methods of plant analysis -- Volume IV: The
alkaloids. Berlin, Gottingen, Heidelberg, Springer-Verlag, pp 367-516.
Cross FT, Palmer RF, Filipy RE, Dagle GE, & Stuart BO (1982)
Carcinogenic effects of radon daughters, uranium ore dust and
cigarette smoke in beagle dogs. Health Phys, 42(1): 33-52.
Cullinan P, Lowson D, Nieuwenhuijsen MJ, Sandiford C, Tee RD, Venables
KM, McDonald JC, & Newman-Taylor AJ (1994a) Work related symptoms,
sensitisation, and estimated exposure in workers not previously
exposed to flour. Occup Environ Med, 51: 579-583.
Cullinan P, Lowson D, Nieuwenhuijsen MJ, Gordon S, Tee RD, Venables
KM, McDonald JC, & Newman-Taylor AJ (1994b) Work related symptoms,
sensitisation, and estimated exposure in workers not previously
exposed to laboratory rats. Occup Environ Med, 51: 589-592.
Cummings KM, Giovino G, & Mendicino AJ (1987) Cigarette advertizing
and black white differences in brand preference. Public Health Rep,
102(6): 698-701.
Damber L & Larsson LG (1985a) Professional driving, smoking and lung
cancer: a case referent study. Br J Ind Med, 42: 246-252.
Damber L & Larsson LG (1985b) Underground mining, smoking and lung
cancer: a case-control study in the iron ore municipalities in
Northern Sweden. J Natl Cancer Inst, 74(6): 1207-1213.
Darby S, Whitley E, Silcocks P, Thakrar B, Green M, Lomas P, Miles J,
Reeves G, Fearn T, & Doll R (1998) Risk of lung cancer associated with
residential radon exposure in south-west England: a case control
study. Br J Cancer, 78(3): 394-408.
D'Avanzo B, Negri E, La Vecchia C, Gramenzi A, Bianchi C, Franceschi
S, & Boyle P (1990) Cigarette smoking and bladder cancer. Eur J
Cancer, 26(6): 714-718.
Davis BR, Whitehead JK, Gill ME, Lee PN, Butterworth AD, & Roe FJC
(1975) Response of rat lung to inhaled tobacco smoke with or without
prior exposure to 3,4-benzpyrene (BP) given by intratracheal
instillation. Br J Cancer, 31: 469-484.
Davison AG, Fayers PM, Newman Taylor AJ, Venables KM, Darbyshire J,
Pickering CA, Chettle DR, Franklin D, Guthrie CJ, Scott MC, O'Malley
D, Holden H, Mason HJ, Wright AL, & Gompertz D (1988) Cadmium fume
inhalation and emphysema. Lancet, 8587: 663-667.
De Klerk NH, Musk AW, Armstrong BK, & Hobbs MS (1991) Smoking,
exposure to crocidolite, and the incidence of lung cancer and
asbestosis. Br J Ind Med, 48(6): 412-417.
Deng JF, Sinks T, Elliot L, Smith D, Singal M, & Fine L (1991)
Characterisation of respiratory health and exposures at a sintered
permanent magnet manufacturer. Br J Ind Med, 48: 609-615.
Devesa SS, Shaw GL, & Blot WJ (1991) Changing patterns of lung cancer
incidence by histological type. Can Epi Bio Prev, 1: 29-43.
De Wolff FA & Edelbroek PM (1994) Neurotoxicity of arsenic and its
compounds. In: de Wolff FA ed. Vinken and Bruyn's handbook of clinical
neurology. Part I. Intoxications of the nervous system. Amsterdam,
Elsevier, pp 283-291.
De Wolff FA (1995) Antimony and health. Br Med J, 310: 1216-1217.
De Zotti R, Larese F, Bovenzi M, Negro C, & Molinari S (1994) Allergic
airway disease in Italian bakers and pastry makers. Occup Environ Med,
51: 548-552.
DiPaolo JA & Sheehe PR (1962) The combined carcinogenic effect of
cigarette smoke condensate and urethan. Cancer Res, 22: 1058-1060.
Djordjevic MV, Sigountos CW, Hoffmann D, Brunnemann KD, Kagan MR, Bush
LP, Safaev RD, Belitsky GA, & Zaridze D (1991) Assessment of major
carcinogens and alkaloids in the tobacco and mainstream smoke of USSR
cigarettes. Int J Cancer, 47: 348-351.
Djordjevic MV, Fan J, Ferguson S, & Hoffmann D (1995) Self-regulation
of smoking intensity: Smoke yields of the low-nicotine, "low-tar"
cigarettes. Carcinogenesis, 16: 2015-2021.
Doll R & Peto R (1981) The causes of cancer: Quantitative estimates of
avoidable risk of cancer in the United States today. Natl Cancer Inst,
66: 1191-1308.
Domiano SF, Vena JE, & Swanson MK (1985) Gasoline exposure, smoking
and kidney cancer. J Occup Med, 27(6): 398-399.
Donham KJ, Zavala DC, & Merchant JA (1984) Respiratory symptoms and
lung function among workers in swine confinement buildings: a cross
sectional epidemiological study. Arch Environ Health, 39(2): 96-101.
Dontenwill W, Chavalier H-J, Harke H-P, Lafrenz U, Rechzeh G, &
Schneider B (1973) Investigations on the effects of chronic
cigarette-smoke inhalation in Syrian Golden Hamsters. J Natl Cancer
Inst, 51(6): 1781-1832.
Ecobichon DJ (1995) Toxic effects of pesticides. In: Klaassen CD ed.
Casarett and Doull's toxicology, 5th ed. New York, McGraw-Hill,
chapter 22, pp 643-689.
Edling C (1982) Lung cancer and smoking in a group of iron ore miners.
Am J Ind Med, 3(2): 191-199.
Edwards JW & Priestly BG (1993) Sister chromatid exchanges in
lymphocytes of petroleum retailers. Br J Ind Med, 50: 149-154.
Eisenbud M (1993) Lung cancer incidence among patients with beryllium
disease. J Natl Cancer Inst, 85(2): 1697-1698.
Ekenvall L & Lindblad LE (1989) Effect of tobacco use on vibration
white finger disease. J Occup Med, 31(1): 13-16.
Eliasson M, Lundblad D, & Hägg E (1991) Cardiovascular risk factors in
young snuff-users and cigarette smokers. J Intern Med, 230(1): 17-22.
Elliott R (1995) Smoking drinking and occupation: Occupational Health.
In: Drever F ed. Office of Population Censuses and Surveys, Health and
Safety Executive: The registrar general's decennial supplement for
England and Wales. London, Her Majesty's Stationery Office (HMSO), pp
195-201.
Elwood MJ (1981) Wood exposure and smoking: Association with cancer of
the nasal cavity and paranasal sinuses in British Columbia. Can Med
Assoc J, 124: 1573-1577.
Enterline PE, Sykora JL, Keleti G, & Lange JH (1985) Endotoxins,
cotton dust, and cancer. Lancet, 2(8461): 934-935.
Enterline PE, Marsh GM, Henderson V, & Callahan C (1987a) Mortality
update of a cohort of US man-made mineral fibre workers. Ann Occup
Hyg, 31: 625-656.
Enterline PE, Marsh GM, Esmen NA, Henderson VL, Callahan CM, & Paik M
(1987b) Some effects of cigarette smoking, arsenic, and SO2 on
mortality among US copper smelter workers. J Occup Med, 29(10):
831-838.
Falk K, Sorsa M, Vainio H, & Kilpikari I (1980) Mutagenicity in urine
of workers in rubber industry. Mutat Res, 79(1): 45-52.
Finch GL, Nikula KJ, Barr EB, Bechtold WE, Chen BT, Griffith WC, Hahn
FF, Hobbs CH, Hoover MD, Lundgren DL, & Mauderly JL (1995a) Effects of
combined exposure of F344 rats to radiation and chronically inhaled
cigarette smoke: Annual report of the Inhalation Toxicology Research
Institute. Washington, DC, Department of Energy (ITRI-146).
Finch GL, Nikula KJ, Chen BT, Barr EB, Chang I-Y, & Hobbs CH (1995b)
Effect of chronic cigarette smoke exposure on lung clearance of tracer
particles inhaled by rats. Fundam Appl Toxicol, 24: 76-85.
Finch GL, Nikula K, Belinsky S, Barr EB, Stoner G, & Lechner J (1996)
Failure of cigarette smoke to induce or promote lung cancer in the A/J
mouse. Cancer Lett, 99: 161-167.
Fletcher CM (1972) Coal workers pneumoconiosis [letter]. Br Med J, 3:
116.
Fletcher CM & Pride NB (1984) Definitions of emphysema, chronic
bronchitis, asthma, and airflow obstruction. Thorax, 39: 81-85.
Fox AJ, Lindars DC, & Owen R (1974) A survey of occupational cancer in
the rubber and cable making industries: Results of five-year analysis,
1967-71. Br J Ind Med, 31: 140-151.
Fraser-Moodie A (1976) Dupuytren's contracture and cigarette smoking.
Br J Plastic Surg, 29: 214-215.
Friedman GD, Siegeland AB, & Seltzer CC (1969) Cigarette smoking and
exposure to occupational hazards. Am J Epidemiol, 98: 175-183.
Friedman S, Fletcher CM, & Field GB (1972) Effects of smoking modified
cigarettes on respiratory symptoms and ventilatory capacity. J Natl
Cancer Inst, 48: 1805-1810.
Fukuda K, Shibata A, & Harada K (1987) Squamous cell cancer of the
maxillary sinus in Hokkaido, Japan: A case-control study. Br J Ind
Med, 44: 263-266.
Gammon MD, Schoenberg JB, Ahian H, Risch HA, Vaughan TL, Chow WH,
Rotterdam H, West AB, Dubrow R, & Stanford JL (1997) Tobacco, alcohol,
and socioeconomic status and adenocarcinoma of the esophagus and
gastric cardia. J Natl Cancer Inst, 89(17): 1277-1284.
Gardiner AJS, Forey BA, & Lee PN (1992) Avian exposure and
bronchogenic carcinoma. Br Med J, 305: 989-992.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1987) A case-control study of lung cancer
and diesel exhaust exposure in railroad workers. Am Rev Respir Dis,
135: 1242-1248.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1988) A retrospective cohort study of lung
cancer and diesel exhaust exposure in railroad workers. Am Rev Respir
Dis, 137: 820-825.
Geng Y, Savage SM, Johnson LJ, Seagrave J, & Sopori ML (1995) Effects
of nicotine on the immune system: I. Chronic exposure to nicotine
impairs antigen receptor-mediated signal transduction in lymphocytes.
Toxicol Appl Pharmacol, 135: 268-278.
Geng Y, Savage SM, Razanai-Boroujerdi S, & Sopori ML (1996) Effects of
nicotine on the immune system: II. Chronic nicotine treatment induces
T cell anergy. J Immunol, 156(7): 2384-2390.
Gerhardsson L & Nordberg GF (1993) Lung cancer in smelter workers
- interactions of metals as indicated by tissue levels. Scand J Work
Environ Health, 19(suppl 1): 90-94.
Gibbs GW & Horowitz I (1979) Lung cancer mortality in aluminium
reduction plant workers. J Occup Med, 21(5): 347-353.
Gibinski K (1977) Studies on cardiovascular effects of smoking in
Poland and other Eastern European countries. In: Proceedings of 3rd
World Conference on Smoking and Health, New York City, 2-5 June 1975
- Volume II: Health consequence, education, cessation and social
actions. Washington, DC, US Department of Health, Education and
Welfare, pp 179-184 (DHEW Publication No. (NIH) 77:1413).
Gonzalez CA, Lopez-Abente G, Errezola M, Castejon J, Estrada A,
Garcia-Mila M, Gili P, Huguet M, Serrat M, Soler F, & Rodriguez C
(1985) Occupation, tobacco use, coffee and bladder cancer in the
county of Mataro (Spain). Cancer, 55: 2031-2034.
Gonzalez CA, Lopez-Abente G, Errezola M, Escolar A, Riboli E,
Izarzugaza I, & Nebot M (1989) Occupation and bladder cancer in Spain:
a multi-centre case control study. Int J Epidemiol, 18(3): 569-577.
Greenberg M, Milne JF, & Watt A (1970) A survey of workers exposed to
dusts containing derivatives of Bacillus subtilis. Br Med J, ii:
629-633.
Greenland S (1993) Basic problems in interaction assessment. Environ
Health Perspect, 101(suppl 4): 59-65.
Grimmer G, Schneider D, Nanjack KW, Deltbarn G, & Jacob J (1995)
Intercept-reactant method for the determination of aromatic amines in
mainstream tobacco smoke. Beitr Tabilk-Forsch, 16: 141-156.
Grove WJ & Stansby GP (1992) Buerger's disease and cigarette smoking
in Bangladesh. Ann R Coll Surg Engl, 74: 115-118.
Guggenheimer J (1991) Implications of smokeless tobacco use in
athletes [Review]. Dent Clin North Am, 35(4): 797-808.
Gullvåg B, Mylius E, & Nilsen A (1985) Counting alveolar macrophages
from expectorate samples of exposed workers as a test for lung
irritation from occupational exposure. Bull Environ Contam Toxicol,
34: 702-708.
Hammond EC & Selikoff IJ (1973) Relation of cigarette smoking to risk
of death of asbestos- associated disease among insulation workers in
the United States. In: Bogovski P, Gilson JC, Timbrell V, & Wagner JC
Biological effects of asbestos. Lyon, International Agency for
Research on Cancer, pp 312-317 (IARC Scientific Publication No. 8).
Hammond EC, Selikoff IJ, & Seidman H (1979) Asbestos exposure,
cigarette smoking and death rates. Ann NY Acad Sci, 333: 473-489.
Hanke W, Sepulveda MJ, Watson A, & Jankovic J (1984) Respiratory
morbidity in wollastonite workers. Br J Ind Med, 41: 474-479.
Harf RA, Ethevenaux C, Gleize J, Perrin-Fayolle M, Guerin JG, &
Ollagnier C (1986) Reduced prevalence of smokers in sarcoidosis:
Results of a case-control study. Ann NY Acad Sci, 465: 625-631.
Harley NH, Cohen BS, & Tso TC (1980) Polonium-210: A questionable risk
factor in smoking-related carcinogenesis. In: Gori GB & Bock FG ed. A
safe cigarette? Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp 93-104 (Banbury Report No. 3).
Harris RJC & Negroni G (1967) Production of lung carcinomas in C57BL
mice exposed to a cigarette smoke and air mixture. Br Med J, 4:
637-641.
Harris RJC, Negroni G, Ludgate S, Pick CR, Chesterman FC, & Maidment
BJ (1974) The incidence of lung tumours in C57BL mice exposed to
cigarette smoke: air mixtures for prolonged periods. Int J Cancer, 14:
130-136.
Hassler E, Lind B, & Piscator M (1983) Cadmium in blood and urine
related to present and past exposure: A study of workers in an
alkaline battery factory. Br J Ind Med, 40: 420-425.
Haugen A, Becher G, Benestad C, Vahakangas K, Trivers GE, Newman MJ, &
Harris CC (1986) Determination of polycyclic aromatic hydrocarbons in
the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA,
and antibodies to the adducts in sera from coke oven workers exposed
to measured amounts of polycyclic aromatic hydrocarbons in the work
atmosphere. Cancer Res, 46(8): 4178-4183.
Hawker R & Holtby I (1988) Smoking and absence from work in a
population of student nurses. Public Health (Lond), 102: 161-167.
Hayes RB, Gerin M, Raatgever JW, & de Bruyn A (1986) Wood-related
occupations, wood dust exposure, and sinonasal cancer. Am J Epidemiol,
124(4): 569-577.
Health Canada (1994) Risk assessment for the combustion products of
methylcyclo-pentadienyl manganese tricarbonyl (MMT) in gasoline.
Ottawa, Ontario, Health Canada, Environmental Health Directorate, 113
pp.
Hecht SS & Hoffmann D (1988) Tobacco specific nitrosamines, an
important group of carcinogens in tobacco and tobacco smoke.
Carcinogenesis, 9(6): 875-884.
Hecht SS & Hoffmann D (1991) N-nitroso compounds and tobacco-induced
cancers in man. In: O'Neill IK, Chen J, & Bartsch H ed. Relevance to
human cancer of N-nitroso compounds, tobacco smoke and mycotoxins.
Lyon, International Agency for Research on Cancer, pp 54-61 (IARC
Scientific Publications No. 105).
Hecht SS, Adams JD, Numoto S, & Hoffmann D (1983) Induction of
respiratory tract tumors in Syrian golden hamsters by a single dose of
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 4:
1287-1290.
Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, & Hoffmann D
(1986) Induction of oral cavity tumors in F344 rats by
tobacco-specific N-nitrosamines and snuff. Cancer Res, 46:
4162-4166.
Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, & Hoffmann
D (1993) A tobacco-specific lung carcinogen in the urine of men
exposed to cigarette smoke. N Engl J Med, 329: 1543-1546.
Henry CJ & Kouri RE (1984) Chronic exposure of mice to cigarette smoke
-- Final Report to the Council for Tobacco Research -- USA, Inc.
(Contract CTR-0030). New York, Field, Rich and Associates, Inc.
Herity B, Moriarity M, Daly L, Dunn J, & Bourke GJ (1982) The role of
tobacco and alcohol in the aetiology of lung and larynx cancer. Br J
Cancer, 46: 961-964.
Hertz-Picciotto I, & Hu SW (1994) Contribution of cadmium in
cigarettes to lung cancer: an evaluation of risk assessment
methodologies. Arch Environ Health, 49(4): 297-302.
Hertz-Picciotto I, Smith AH, Holtzman D, Lipsett M, & Alexeef G (1992)
Synergism between occupational arsenic exposure and smoking in the
induction of lung cancer. Epidemiology, 3(1): 23-31.
Hessel PA, Sluis-Cremer GK, & Lee SL (1991) Distribution of silicotic
collagenization in relation to smoking habits. Am Rev Respir Dis,
144(2): 297-301.
Hicks RM (1982) Nitrosamines as possible etiological agents in
bilharzial bladder cancer. In: Magee PM ed. Nitrosamines and human
cancer. Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp 455-471 (Banbury Report No. 12).
Hicks RM, James C, & Webbe G (1980) Effect of Schistosoma
haematobium and N-butyl- N-(4-hydroxybutyl)nitrosamine on the
development of urothelial neoplasia in baboon. Br J Cancer, 42:
730-755.
Higginson J, Muir C, & Buffler PA (1984) The epidemiology of renal
carcinoma in humans with a note on the effect of exposure to gasoline.
In: Mehlman MA, Hemstreet GP III, Thorp JJ, & Weaver NK ed. Advances
in modern environmental toxicology -- Volume VII: Renal effects of
petroleum hydrocarbons. Princeton, New Jersey, Princeton Scientific
Publishers, pp 203-226.
Hilt B, Langard S, Lund-Larsen PG, & Lien JT (1986) Previous asbestos
exposure and smoking habits in the county of Telemark, Norway -- A
cross-sectional population study. Scand J Work Environ Health, 12:
561-566.
Hinds WC, First MW, Huber GI, & Shea JW (1983) A method for measuring
respiratory deposition of cigarette smoke during smoking. Am Ind Hyg
J, 44: 113-118.
Hirsch JM & Thilander H (1981) Snuff-induced lesions of the oral
mucosa: an experimental model in the rat. J Oral Pathol, 10: 342-353.
Hnizdo E (1990) Combined effect of silica dust and tobacco smoking on
mortality from chronic obstructive lung disease in gold miners. Br J
Ind Med, 47: 656-664.
Hnizdo E & Sluis-Cremer GK (1991) Silica exposure, silicosis, and lung
cancer: a mortality study of South African gold miners. Br J Ind Med,
48(1): 53-60.
Hnizdo E, Baskind E, & Sluis-Cremer GK (1990) Combined effects of
silica dust exposure and tobacco smoking on the prevalence of
respiratory impairments among gold miners. Scand J Work Environ
Health, 16: 411-422.
Hocking WG & Golde DW (1979) The pulmonary-alveolar macrophage. N Engl
J Med, 301: 580-587, 639-645.
Hoffmann D & Hoffmann I (1991) Recent developments in smoking related
cancer research. Smok Relat Dis, 5(2): 77-93.
Hoffmann D & Hoffmann I (1997) The changing cigarette: 1950-1995. J
Toxicol Environ Health, 50: 307-364.
Hoffmann D & Wynder EL (1976) Smoking and occupational cancer. Prev
Med, 5: 245-261.
Hoffmann D, Rathkamp G, & Wynder EL (1961) Comparison of the yields of
several selected compounds in the smoke of different tobacco products.
J Natl Cancer Inst, 31: 627-637.
Hoffmann D, Sanghvi LD, & Wynder EL (1974) Chemical studies on tobacco
smoke: XXVIII. Comparative chemical analysis of Indian bidi and
American cigarette smoke. Int J Cancer, 14: 49-53.
Hoffmann D, Rivenson A, Hecht SS, Hilfrich J, Kobayashi N, & Wynder EL
(1979) Model studies in tobacco carcinogenesis with the Syrian Golden
Hamster. Prog Exp Tumor Res, 24: 370-390.
Hoffmann D, Harley NH, Fisenne I, Adams JD, & Brunnemann KD (1986)
Carcinogenic agents in snuff. J Natl Cancer Inst, 76: 435-437.
Hoffmann D, Rivenson A, Chung FL, & Hecht SS (1991a) Nicotine-derived
nitrosamines and their relevance in tobacco carcinogenesis. Crit Rev
Toxicol, 21(4): 305-311.
Hoffmann D, Melikian AA, & Brunnemann KD (1991b) Studies in tobacco
carcinogenesis. Lyon, International Agency for Research on Cancer, pp
482-484 (IARC Scientific Publications No. 105).
Hoffmann D, Brunnemann KD, Prokopczyk B, & Djordjevic MV (1994)
Tobacco-specific N-nitrosamines and areca-derived N-nitrosamines:
chemistry, biochemistry, carcinogenicity, and relevance to humans. J
Toxicol Environ Health, 41: 1-52.
Holbrook JH (1977) Tobacco and health. CA-Cancer J Clin, 27: 344-353.
Holst PA, Kromhout D, & Brand R (1988) For debate: pet birds as an
independent risk factor for lung cancer. Br Med J, 297: 1319-1324.
Honeybourne D & Pickering CAC (1986) Physiological evidence that
emphysema is not a feature of byssinosis. Thorax, 41(1): 6-11.
Hsu TC, Furlong C, & Spitz MR (1991) Ethyl alcohol as a co-carcinogen
with special reference to the aerodigestive tract: a cytogenetic
study. Anticancer Res, 11: 1097-1102.
Hughes JM & Weill H (1991) Asbestos as a precursor of asbestos related
lung cancer: Results of a prospective mortality study. Br J Ind Med,
48: 229-233.
IARC (1984) Polynuclear aromatic compounds: Part 3. Industrial
exposures in aluminum production, coal gasification, coke production,
and iron and steel founding. Lyon, International Agency for Research
on Cancer, pp 37-190 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 34).
IARC (1985a) Tobacco habits other than smoking: Betel-quid and
areca-nut chewing and some related nitrosamines. Lyon, International
Agency for Research on Cancer, 291 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
37).
IARC (1985b) Polynuclear aromatic compounds: Part 4. Bitumens,
coal-tars and derived products, shale-oils and soots. Lyon,
International Agency for Research on Cancer, pp 83-241 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
35).
IARC (1986) Tobacco smoking. Lyon, International Agency for Research
on Cancer, 421 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 38).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, 440 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IARC (1988) Man-made mineral fibres and radon. Lyon, International
Agency for Research on Cancer, 300 pp (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Volume 43).
IARC (1989a) Antimony trioxide and antimony trisulfide. In: Some
organic solvents, resin monomers and related compounds, pigments and
occupational exposures in paint manufacture and painting. Lyon,
International Agency for Research on Cancer, pp 291-305 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
47).
IARC (1989b) Occupational exposures in petroleum refining; crude oil
and major petroleum fuels. Lyon, International Agency for Research on
Cancer, pp 109-110 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 45).
IARC (1989c) Diesel and gasoline engine exhaust and some nitroarenes.
Lyon, International Agency for Research on Cancer, pp 41-185 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
46)..
IARC (1990) Chromium, nickel and welding. Lyon, International Agency
for Research on Cancer, pp 257-445 (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Volume 49).
IARC (1991) Tenth International Meeting on N-Nitroso Compounds,
Mycotoxins and Tobacco Smoke: Relevance to Human Cancer. Lyon,
International Agency for Research on Cancer, 594 pp (IARC Scientific
Publications No. 105).
IARC (1993) Beryllium, cadmium, mercury, and exposures in the glass
manufacturing industry. Lyon, International Agency for Research on
Cancer, 444 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 58).
IARC (1994a) Ethylene oxide. In: Some industrial chemicals. Lyon,
International Agency for Research on Cancer, pp 73-159 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
60).
IARC (1994b) Infection with schistosomes ( Schistosoma haematobium,
Schistosoma mansoni and Schistosoma japonicul). In: Schistosomes,
liver flukes and Helicobacter pylori. Lyon, International Agency for
Research on Cancer, Volume 61).
IARC (1995) Wood dust and formaldehyde. Lyon, International Agency for
Research on Cancer, pp 191-194 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 62).
ICRP (International Commission on Radiological Protection) (1994)
Human respiratory tract model for radiological protection. Amsterdam,
Oxford, New York, Elsevier Science Publishers (ICRP Publication No.
66).
Idris AM, Nair J, Oshima H, Friesen M, Brouet I, Faustman EM, &
Bartsch H (1991) Unusual high levels of carcinogenic tobacco-specific
nitrosamines in Sudan snuff (toombak). Carcinogenesis, 12: 1115-1118.
Idris AM, Prokopczyk B, & Hoffmann D (1994) Toombak: a major risk
factor for cancer of the oral cavity in Sudan. Prev Med, 23: 832-839.
ILO (1991) Smoking contributes to noise-induced hearing loss: ILO
contribution to WHO -- World no tobacco day, 1992. Geneva,
International Labour Organisation.
Inskip PD & Boice JD (1994) Radiotherapy-induced lung cancer among
women who smoke. Cancer, 73(6): 1541-1543.
IPCS (1990) Environmental health criteria 106: Beryllium. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 40-53.
IPCS (1991a) Environmental health criteria 108: Nickel. Geneva, World
Health Organization, International Programme on Chemical Safety, pp
48-67.
IPCS (1991b) Environmental health criteria 125: Platinum. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 79-80.
IPCS (1992) Environmental health criteria 134: Cadmium. Geneva, World
Health Organization, International Programme on Chemical Safety, 280
pp.
IPCS (1995) Environmental health criteria 165: Inorganic lead. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 93-98.
IPCS (1996) Environmental health criteria 171: Diesel fuel and exhaust
emissions. Geneva, World Health Organization, International Programme
on Chemical Safety, 389 pp.
ISAAC (1998) International Study of Asthma and Allergies in Childhood
(ISAAC) Steering Committee: Worldwide variation in the prevalence of
symptoms of asthma, allergic rhino conjunctivitis, and atopic eczema.
Lancet, 351: 1225-1232.
Iscovich J, Castelletto R, Esteve J, Muñoz N, Colanzi R, Coronel A,
Deamezola I, Tassi V, & Arslan A (1987) Tobacco smoking, occupational
exposure and bladder cancer in Argentina. Int J Cancer, 40: 734-740.
Istvan J & Matarazzo JD (1984) Tobacco, alcohol and caffeine use: a
review of their interrelationships. Psychol Bull, 95: 301-326.
Iwata F, Zhang XY, & Leung FW (1995) Aggravation of gastric mucosal
lesions in rat stomach by tobacco cigarette smoke. Dig Dis Sci, 40:
1118-1124.
Izard C & Liberman C (1978) Acrolein. Mutat Res, 47(2): 115-138.
Jarup L & Pershagen G (1991) Arsenic exposure, smoking and lung cancer
in smelter workers -- A case-control study. Am J Epidemiol, 134(6):
545-551.
Järvholm B, Larsson S, Hagberg S, Olling S, Ryd W, & Torén K (1993)
Quantitative importance of asbestos as a cause of lung cancer in a
Swedish industrial city: a case-referent study. Eur Respir J, 6(9):
1271-1275.
Jayant K & Pakhale SS (1985) Toxic constituents in bidi smoke. In:
Sanghvi LD & Notami P ed. Tobacco and health: The Indian scene.
Bombay, Tata Memorial Center, pp 101-110.
Jindal RM & Patel SM (1992) Buerger's disease and cigarette smoking in
Bangladesh. Ann R Coll Surg Engl, 74(6): 436-437.
Johansson SL, Hirsch JM, Larsson PA, Saidi J, & Oesterdahl BG (1989)
Snuff-induced carcinogenesis: effect of snuff in rats initiated with
4-nitroquinoline- N-oxide. Cancer Res, 49: 3063-3069.
JOM (1984) Vibration syndrome in chipping and grinding workers. J
Occup Med, 26(10): 765-788.
Jones RD (1994) Survey of antimony workers: mortality 1961-92. Occup
Environ Med, 51(11): 772-776.
Jörgensen HS (1984) Lung cancer among underground workers in the iron
ore mine of Kiruna based on thirty years of observation. Ann Acad Med
Singap, 13(suppl 2): 371-377.
Jörgensen HS, Kolmodin-Hedman B, & Stjernberg N (1988) Follow-up study
of pulmonary function and respiratory tract symptoms in workers in a
Swedish iron ore mine. J Occup Med, 30(12): 953-957.
Jorquera R, Castonguay A, & Schüller HM (1992) Effects of pregnancy
and ethanol treatment on the metabolism of 4-(methyl
nitrosamino)-1-(pyridyl)-1-butanone by hamster liver and lung
microsomes. Drug Metab Dispos, 20: 510-517.
Jusko WJ (1978) Role of tobacco smoking in pharmacokinetics. J
Pharmacokinet Biopharm, 6: 7-39.
Jussawalla DJ & Deshpande VA (1971) Evaluation of cancer risk in
tobacco chewers and smokers: an epidemiological assessment. Cancer,
28(1): 244-252.
Kaldor JM & L'Abbe KA (1990) Interaction between human carcinogens.
In: Vainio H, Sorsa M, & McMichael AJ ed Complex mixtures and cancer
risk. Lyon, International Agency for Research on Cancer, pp 35-43
(IARC Scientific Publications No. 104).
Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, & Fraumeni
JF (1984) Urinary tract infection and risk of bladder cancer. Am J
Epidemiol, 119(4): 510-515.
Kawajiri K & Fujii-Kuriyama Y (1991) P450 and human cancer. Jpn J
Cancer Res, 82(12): 1325-1335.
Kawajiri K, Nakachi K, Imai K, Hyashi S, & Watanabe J (1990)
Individual differences in lung cancer susceptibility in relation to
polymorphysms of P-450IA1 gene and cigarette dose. Princess Takamatsu
Int Symp Ser, 21: 55-61.
Kazantzis G & Armstrong BG (1984) Renal function in relation to low
levels of cadmium exposure in a group of smelter workers. Environ
Health Perspect, 54: 193-199.
Kazantzis G, Blanks RG, & Sullivan KR (1992) Is cadmium a human
carcinogen? In: Nordberg GF, Herber REM, & Alessio L ed. Cadmium in
the human environment: Toxicity and carcinogenicity. Lyon,
International Agency for Research on Cancer, pp 435-445.
Keller AZ & Terris M (1965) The association of alcohol and tobacco
with cancer of the mouth and pharynx. Am J Publ Health, 55: 1578-1585.
Keen P, de Moor NG, Shapiro MP, & Cohen L (1955) The etiology of
respiratory tract cancer in the South African Bantu. Br J Cancer, 9:
528-538.
Kibelstis JA, Morgan EJ, Reger R, Lapp NL, Seaton A, & Morgan WK
(1973) Prevalence of bronchitis and airway obstruction in American
bituminous coal miners. Am Rev Respir Dis, 108(4): 886-893.
Kilburn KH (1989) Byssinosis, emphysema, and mortality of American
textile workers: [Editorial]. Arch Environ Health, 38(5): 277.
Kilpikari MD (1982) Mortality among male rubber workers in Finland.
Arch Environ Health, 37(5): 295-299.
Kimura N (1977) Epidemiological studies in Japan on smoking and
cardiovascular disease. In: Proceedings of 3rd World Conference on
Smoking and Health, New York City, 2-5 June 1975. Volume II: Health
consequence, education, cessation and social actions. Washington, DC,
US Department of Health, Education and Welfare, pp 185-198 (DHEW
Publication (NIH) 77:1413).
King M, Wight A, DeSanctis GT, el-Azab J, Phillips DM, Angus GE, &
Cosio MG (1989) Mucus hypersecretion and viscoelasticity changes in
cigarette smoking dogs. Exp Lung Res, 15(3): 375-389.
Kleinfeld M, Messite J, & Langer AM (1973) A study of workers exposed
to asbestiform minerals in commercial talc manufacture. Environ Res,
6: 132-143.
Kobayashi W, Hoffmann D, & Wynder EL (1974) A study of tobacco
carcinogenesis: XII. Epithelial changes induced in the upper
respiratory tracts of Syrian golden hamsters by cigarette smoke. J
Natl Cancer Inst, 534: 1085-1089.
Kobusch AB, Simard A, Feldstein M, Vauclair R, Gibbs GW, Bergeron F,
Morissette N, & Davis R (1984) Pulmonary cytology in chrysotile
asbestos workers. J Chron Dis, 37(8): 599-607.
Kodell RL & Pounds JG (1991) Assessing the toxicity of chemical
mixtures. In: Krewski D & Franklin CA ed. Statistics in toxicology.
New York, Gordan & Breach, pp 557-589.
Kodell RL, Krewski D, & Zielinski J (1991) Additive and multiplicative
relative risk in the two-stage clonal expansion model of
carcinogenesis. Risk Anal, 11: 483-490.
Kohlmeier L, Arminger G, Bartolomeycik S, Bellach B, Rehm J, & Thamm M
(1992) Pet birds as an independent risk factor for lung cancer:
case-control study. Br Med J, 305: 986-989.
Koppang N, Rivenson A, Reith A, Dahle HK, Evenson O, & Hoffman D
(1992) A study of tobacco carcinogenesis: XLVIII. Carcinogenicity of
N'-nitrosonornicotine in mink ( Mustela vison). Carcinogenesis,
13(11): 1957-1960.
Koppang N, Rivenson A, Dahle HK, & Hoffman D (1997) A study of tobacco
carcinogenesis: LIII. Carcinogenicity of N'-nitrosonornicotine (NNN)
and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in mink
( Mustela vison). Cancer Lett, 111: 167-171.
Kotin P (1994) The epidemiological evidence on the carcinogenicity of
beryllium, by MacMahon: [Editorial]. J Occup Med, 36(1): 25-26.
Kreiss K, Wassermann S, Mroz MM, & Newman LS (1993) Beryllium disease
screening in the ceramics industry. J Occup Med, 35: 16-25.
Kreiss K, Mroz MM, Newman LS, Martyny J, & Zhen B (1996) Machining
risk of beryllium disease and sensitization with median exposures
below 2 µg/m3. Am J Ind Med, 30: 16-25.
Kremer AM, Pal TM, Boleij JSM, Schouten JP, & Rijcken B (1994) Airway
hyperresponsiveness, prevalence of chronic respiratory symptoms, and
lung function in workers exposed to irritants. Occup Environ Med, 51:
3-13.
Krishna K (1978) Tobacco chewing in pregnancy. Br J Obstet Gynaecol,
85: 726-728.
Kristensen TS (1989) Cardiovascular disease and the work environment:
A critical review of the epidemiological literature on chemical
factors. Scand J Work Environ Health, 15: 245-264.
Krivan V, Schneider G, Baumann H, & Reus (1994) Multi-element
characterization of tobacco smoke condensate. Fresenius J Anal Chem,
348: 218-225.
Kuntz WD & McCord CP (1974) Polymer-fume fever. J Occup Med, 16(7):
480-482.
Kusiak RA, Springer J, Ritchie AC, & Muller J (1991) Carcinoma of the
lung in Ontario gold miners: possible aetiological factors. Br J Ind
Med, 48: 808-817.
Kusiak RA, Ritchie AC, Muller J, & Springer J (1993) Mortality from
lung cancer in Ontario uranium miners. Br J Ind Med, 50(10): 920-928.
Lai YL & Diamond L (1992) Cigarette smoke exposure does not prevent
cadmium-induced alterations in rat lungs. J Toxicol Environ Health,
35(1): 63-76.
LGC (Laboratory of the Government Chemist) (1982) Report of the
Government chemist, 1981. London, Her Majesty's Stationery Office
(HMSO), p 109.
Lakier JB (1992) Smoking and cardiovascular disease. Am J Med,
93(suppl 1A): 8-12.
Lamm SH, Parkinson M, Anderson M, & Taylor W (1992) Determinants of
lung cancer risk among cadmium-exposed workers. Ann Epidemiol, 2(3):
195-211.
Lancet (1985) Smoking, occupation, and allergic lung disease. Lancet,
1(8435): 965.
Landrigan PJ & Straub WE (1985) Occupational lead exposure aboard a
tall ship. Am J Ind Med, 8(3): 233-239.
Langård S & Norseth T (1975) A cohort study of bronchial carcinomas in
workers producing chlorinated pigments. Br J. Ind Med, 32: 62-65.
Langård S & Vigander T (1983) Occurrence of lung cancer in workers
producing chromium pigments. Br J Ind Med, 40(1): 71-74.
Larsson PA, Johansson SL, Vahlne A, & Hirsch JM (1989) Snuff
carcinogenesis: effects of long-term snuff administration after
initiated with 4-nitroquinoline- N-oxide and Herpes simplex virus
type 1. J Oral Pathol Med, 18: 187-192.
La Vecchia C, Negri E, D'Avanzo B, Savoldelli R, & Franceschi S (1991)
Genital and urinary tract diseases and bladder cancer. Cancer Res,
51(2): 629-631.
La Voie EJ, Adams JD, Reinhardt J, Rivenson A, & Hoffmann D (1986)
Toxicity studies on clove cigarette smoke and constituents of clove:
Determination of the LD50 of eugenol by intratracheal installation in
rats and hamsters. Arch Toxicol, 59: 78-81.
Leanderson P & Tagesson C (1989) Cigarette smoke potentiates the
DNA-damaging effect of manmade mineral fibers. Am J Ind Med, 16:
697-706.
Lednar WM, Tyroler HA, McMichael AJ, & Shy CM (1977) The occupational
determinants of chronic disabling pulmonary disease in rubber workers.
J Occup Med, 19: 263-268.
Leduc D, de Francquen P, Jacobovitz D, Vanderweyer R, Lauwerys R, &
De Vuyst P (1993) Association of cadmium exposure with rapidly
progressive emphysema in a smoker. Thorax, 48: 570-571.
Lehtovirta P & Forss M (1978) The acute effect of smoking on
intervillous blood flow of the placenta. Br J Obstet Gynaecol, 85:
729-731.
Leigh J, Outhred KG, McKenzie HI, Glick M, & Wiles AN (1983)
Quantified pathology of emphysema, pneumoconiosis, and bronchitis in
coal workers. Br J Ind Med, 40: 258-263.
Letourneau EG, Krewski D, Choi NW, Goddard MJ, McGregor MJ, Zielinsky
JM, & Du J (1994) Case-control study of residential radon and lung
cancer in Winnipeg, Manitoba, Canada. Am J Epidemiol, 140(4): 310-322.
Liddell FD, Thomas DC, Gibbs GW, & McDonald JC (1984) Fibre exposure
and mortality from pneumoconiosis, respiratory, and abdominal
malignancies in chrysotile production in Quebec, 1926-1975. Ann Acad
Med Singap, 13(suppl): 340-344.
Lidegaard O (1993) Oral contraceptive use and risk of a cerebral
thromboembolic attack: Results of a case-control study. Br Med J, 306:
956-963.
Lilis R, Valciukas JA, Weber JP, Malkin J, & Selikoff IJ (1984a)
Epidemiological study of renal function in copper smelter workers.
Environ Health Perspect, 54: 181-192.
Lilis R, Valciukas JA, Weber JP, Fischbein A, Nicholson WJ, Campbell
C, Malkin J, & Selikoff IJ (1984b) Distribution of blood lead, blood
cadmium, urinary cadmium, and urinary arsenic levels in employees of a
copper smelter. Environ Res, 33: 76-95.
Lindberg E & Hedenstierna G (1983) Chrome plating: symptoms, findings
in the upper airways and effects on lung function. Arch Environ
Health, 38(6): 364-374.
Ljungman AG, Lindahl M, & Tagesson C (1994) Asbestos fibres and man
made mineral fibres: induction and release of tumour necrosis factor
from rat alveolar macrophages. Occup Environ Med, 51: 777-783.
Lourenco RV, Klimek MF, & Borowski CJ (1971) Deposition and clearance
of 2 micron particles in the tracheobronchial tree of normal subjects
-- smokers and nonsmokers. J Clin Invest, 50: 65-83.
Lubin JH & Boice JD (1997) Lung cancer risk from residential radon:
Meta-analysis of eight epidemiologic studies. J Natl Cancer Inst,
89(1): 49-57.
Lubin JH, Boice JD, Edling C, Hornung RW, Howe GR, Kunz E, Kusiak RA,
Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, Yao SX, &
Pierce DA (1995) Lung cancer in radon-exposed miners and estimation of
risk from indoor exposure. J Natl Cancer Inst, 87(11): 817-827.
Lubin JH, Tomasek L, Edling C, Hornung RW, Howe G, Kunz E, Kusiak RA,
Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, & Yao SX
(1997) Estimating lung cancer mortality from residential radon using
data for low exposures of miners. Radiat Res, 147(2): 126-134.
McCoy GD, Hecht SS, Katayama S, & Wynder EL (1981) Differential effect
of chronic ethanol consumption on the carcinogenicity of
N-nitrosopyrolidine and N-nitrosonornicotine in male Syrian golden
hamsters. Cancer Res, 41: 2849-2854.
McDuffie HH, Klaassen DJ, & Dosman JA (1990) Is pesticide use related
to the risk of primary lung cancer in Saskatchewan? J Occup Med,
32(10): 996-1002.
Mackenney RP (1983) A population study of Dupuytren's contracture.
Hand, 15(2): 155-161.
McLaughlin RF & Tueller EF (1971) Anatomic and histologic changes of
early emphysema. Chest, 59: 592-599.
McLintock JC (1971) The selection of juvenile entrants to mining. Br J
Ind Med, 28: 45-51.
MacMahon B (1994) The epidemiological evidence on the carcinogenicity
of beryllium in humans. J Occup Med, 36(1): 15-24.
Magnus K, Andersen A, & Hegetveit AC (1982) Cancer of respiratory
organs among workers at a nickel refinery in Norway. Int J Cancer, 30:
681-685.
Maheswaren R, Gill JS, & Beevers DG (1993) Blood pressure and
industrial lead exposure. Am J Epidemiol, 137(6): 645-653.
Makhyoun NA (1974) Smoking and bladder cancer in Egypt. Br J Cancer,
30: 577-581.
Mann JI, Vessey MP, Thorogood M, & Doll R (1975) Myocardial infarction
in young women with special reference to oral contraceptive practice.
Br Med J, 2: 241-245.
Mann JI, Doll R, Thorogood M, Vessey MP, & Waters WE (1976) Risk
factors for myocardial infarction in young women. Br J Prev Soc Med,
30: 94-100.
Marine WM, Gurr D, & Jacobsen M (1988) Clinically important
respiratory effects of dust exposure and smoking in British coal
miners. Am Rev Respir Dis, 137: 106-112.
Martell EA (1974) Radioactivity of tobacco trichomes and insoluble
smoke particles. Nature (Lond), 249: 215-217.
Martischnig KM, Newell DJ, Barnsley WC, Cowan WK, Feinmann EL, &
Oliver E (1977) Unsuspected exposure to asbestos and bronchogenic
carcinoma. Br Med J, 1(6063): 746-749.
Mayberry RM (1985) Cigarette smoking, herpes simplex virus type 2
infection, and cervical abnormalities. Am J Pub1 Health, 75(6):
676-678.
Mehta FS, Pindborg JJ, Hamner JE, Gupta PC, Daftary DK, Sahiar BE,
Shroff BC, Sanghvi LD, Bhonsle RB, Choksi SK, Dandekar VV, Mehta YN,
Pitkar VK, Sinor PN, Shah NC, Turner PS, & Upadhyay SA (1971) Report
on investigations of oral cancer and precancerous lesions in Indian
rural populations, 1966-1969. Copenhagen, Munksgaard, pp 48-120.
Merchant JA, Kilburn KH, O'Fallon WM, Hamilton JD, & Lumsden JC (1972)
Byssinosis and chronic bronchitis among cotton textile workers. Ann
Intern Med, 76: 423-433.
Merchant JA, Lumsden JC, Kilburn KH, O'Fallon WM, Ujida JR, & Germino
VH (1973) An industrial study of the biological effects of cotton dust
and cigarette smoke exposure. J Occup Med, 15(3): 212-221.
Meurman LO, Kiviluoto R, & Hakama M (1979) Combined effects of
asbestos exposure and tobacco smoking on Finnish anthophyllite miners
and millers. Ann NY Acad Sci, 330: 491-495.
Michaylov MA, Pressyanov DS, & Kalinov KB (1995) Bronchial dysplasia
induced by radiation in miners exposed to 222Rn progeny. Occup Environ
Med, 52: 82-85.
Mikkelsen OA (1978) Dupuytren's disease-the influence of occupation
and previous hand injuries. Hand, 10(1): 1-8.
Miller LG (1990) Cigarettes and drug therapy: Pharmacokinetic and
pharmacodynamic considerations. Clin Pharmacol, 9: 125-135.
Miller A (1993) Pulmonary function in asbestosis and asbestos related
pleural disease. Environ Res, 61(1): 1-18.
Miller BG & Jacobsen M (1985) Dust exposure, pneumoconiosis, and
mortality of coal miners. Br J Ind Med, 42(11): 723-733.
Miller FJ & Kimbell JS (1995) Regional dosimetry of inhaled reactive
gases. In: McClellan RO & Henderson RF ed. Concepts in inhalation
toxicology, 2nd ed. Washington, DC, Taylor & Francis, chapter 10, pp
257-288.
Misiewicz A, Radwan K, Karmolinski M, & Dziewit T (1992) [Degree of
ventilation disturbances in persons with occupational exposure to
manganese.] Wiad Lek, 45(23-24): 890-893 (in Polish).
Misiewicz A, Radwan K, Karmolinski M, Dziewit T, & Matysek A (1994)
[Chronic bronchitis in workers producing iron-manganese alloys.] Wiad
Lek, 47(7-8): 257-261 (in Polish).
Mitacek EJ, Brunnemann KD, & Polednak XP (1990) "Tar", nicotine and
carbon monoxide content of Thai cigarettes and implication for cancer
prevention in Thailand. Cancer Detect Prev, 14: 515-520.
Mitacek EJ, Brunnemann KD, Polednak AP, Hoffmann D, & Suttajet M
(1991) Composition of popular tobacco products in Thailand and its
relevance to disease prevention. Prev Med, 20: 764-773.
Mitacek EJ, Brunnemann KD, Hoffmann D, Limsila T, Suttajet M, Martin
N, & Kaplan LS (1999) Volatile N-nitrosamines and tobacco specific
N-nitrosamines in the smoke of Thai cigarettes: a suspected risk
factor liver cancer in Thailand. Carcinogenesis, 20(1): 133-137.
Mitchell CA & Gandevia B (1971) Respiratory symptoms and skin
reactivity in workers exposed to proteolytic enzymes in the detergent
industry. Am Rev Respir Dis, 104: 1-12.
Modigh C, Axelsson G, Alavanja M, Andersson L, & Rylander P (1996) Pet
birds and risk of lung cancer in Sweden: a case-control study. Br Med
J, 313: 1236-1238.
Molyneux MKB & Tombleson JBL (1970) An epidemiological study of
respiratory symptoms in Lancashire mills, 1963-66. Br J Ind Med,
27(3): 225-234.
Monson RR & Fine LJ (1978) Cancer mortality and morbidity among rubber
workers. J Natl Cancer Inst, 61(4): 1047-1053.
Monson RR & Nakano KK (1976a) Mortality among rubber workers: 1. White
male union employees in Akron, Ohio. Am J Epidemiol, 103(3): 284-296.
Monson RR & Nakano KK (1976b) Mortality among rubber workers: 2. Other
employees. Am J Epidemiol, 103(3): 297-303.
Moolgavkar SH, Dewanji A, & Luebeck G (1989) Cigarette smoking and
lung cancer: reanalysis of the British doctors' data. J Natl Cancer
Inst, 81: 415-420.
Moolgavkar SH, Luebeck EG, Krewski D, & Zielinski JM (1993) Radon,
cigarette smoke, and lung cancer: A re-analysis of the Colorado
Plateau uranium miners' data. Epidemiology, 4: 204-217.
Morabia A, Stellman S, Lumey LH & Wynder EL (1998) Parakeets,
canaries, finches, parrots, and lung cancer: no association. Br J
Cancer, 77: 501-504.
Morgan DC, Smyth JT, Lister RW, Pethybridge RJ, Gilson JC, Callaghan
P, & Thomas GO (1975) Chest symptoms in farming communities with
special reference to farmer's lung. Br J Ind Med, 32: 228-234.
Morgan WKC (1982) Occupational bronchitis. Eur J Respir Dis, 63(suppl
23): 117-127.
Morgan KT (1998) Commentary on Coggins CRE (1998): a review of chronic
inhalation studies with mainstream cigarette smoke in rats and mice.
Toxicol Pathol, 26(3): 315.
Mori K (1964) Acceleration of experimental lung cancers in rats by
inhalation of cigarette smoke. Gann, 55: 175-181.
Morimoto Y, Kido M, Tanaka I, Fujino A, Higashi T, & Yokosaki Y (1993)
Synergistic effects of mineral fibres and cigarette smoke on the
production of tumour necrosis factor by alveolar macrophages of rats.
Br J Ind Med, 50: 955-960.
Mougne C, MacLennan R, & Atsana S (1982) Smoking, chewing and drinking
in Ban Pong, Northern Thailand. Soc Sci Med, 16(1): 99-106.
MRC (Medical Research Council) (1965) Special Committee on the
Aetiology of Chronic Bronchitis: Definition and classification of
chronic bronchitis for clinical and epidemiological purposes. Lancet,
1: 775-779.
Muir CS & McKinney PA (1992) Cancer of the oesophagus: A global
overview. Eur J Cancer Prev, 1: 259-264.
Murai Y, Kitagawa M, Matsui K, Koizumi F, & Miwa A (1994) Asbestos
fibre analysis in seven asbestosis cases. Arch Environ Health, 49(1):
67-72.
Murphy SE & Heiblum R (1990) Effect of nicotine and tobacco-specific
nitrosamines on the metabolism of N'-nitrosonornicotine and
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 11:
1663-1666.
Muscat JE & Wynder EL (1991) Cigarette smoking, asbestos exposure, and
malignant mesothelioma. Cancer Res, 51(9): 2263-2267.
Nair J, Pakhale SS, & Bhide SV (1989) Carcinogenic tobacco-specific
nitrosamines in Indian tobacco products. Food Chem Toxicol, 27(11):
751-753.
Najem GR, Louria DB, Seebode JJ, Thind IS, Prusakowski JM, Ambrose RB,
& Fernicola AR (1982) Life time occupation, smoking, caffeine,
saccharin, hair dyes and bladder carcinogenesis. Int J Epidemiol, 11:
212-217.
Nakachi K, Imai K, Hayashi S, Watanabe J, & Kawajiri K (1991) Genetic
susceptibility to squamous cell carcinoma of the lung in relation to
cigarette smoking dose. Cancer Res, 51(19): 5177-5180.
Nanda PK & Sharma MM (1988) Immediate effect of tobacco chewing in the
form of "paan" on certain cardio-respiratory parameters. Indian J
Physiol Pharmacol, 32(2): 105-113.
Nandakumar A, Thimmasetty KT, Sreeramereddy NM, Venugopal TC, Rajanna
N, Vinutha AT, Srinivas N, & Bhargava MK (1990) A population-based
case-control investigation on cancer of the oral cavity in Bangalore,
India. Br J Cancer, 62: 847-851.
Naus A, Engler V, Hetychova M, & Vavrechova O (1966) Work injuries and
smoking. Ind Med Surg, 35: 880-881.
Negri E, LaVecchia C, Franceschi S, Decarli A, & Bruzzi P (1992)
Attributable risks for oesophageal cancer in Northern Italy. Eur J
Cancer, 28A: 1167-1171.
Nery LE, Sandoval PR, Jardim JR, Bagatin E, & Alonso G (1988) The
effects of smoking and silica exposure on pulmonary epithelial
permeability: a radioaerosol study with 99mTc-DTPA. Brazilian J Med
Biol Res, 21(2): 223-232.
Nery LE, Florencio RT, Sandoval PR, Rodrigues RT, Alonso G, & Mason GR
(1993) Additive effects of exposure to silica dust and smoking on
pulmonary epithelial permeability: a radioaerosol study with
technetium-99m labelled DTPA. Thorax, 48(3): 264-268.
Neugut AI, Robinson E, Lee WC, Murray T, Karwoski K, & Kutcher G
(1993) Lung cancer after radiation therapy for breast cancer. Cancer,
71: 3054-3057.
Neugut AI, Murray T, Santos J, Amols H, Hayes MK, Flannery JT, &
Robinson E (1994) Increased risk of lung cancer following breast
cancer radiation therapy in cigarette smokers. Cancer, 73: 1615-1620.
Neurath G & Ehmke H (1964) [Studies on the nitrate content of
tobacco.] Beitr Tabakforsch, 2: 333-344 (in German).
Nevius E, Bennett R, & Sobel S (1982) Critique of FM Sturtevant paper
"Smoking, oral contraceptives, and thromboembolic disease". Int J
Fertil, 27: 16-20.
Newhouse ML & Berry G (1976) Predictions of mortality from mesothelial
tumours in asbestos factory workers. Br J Ind Med, 33: 147-151.
Newhouse ML & Berry G (1979) Patterns of mortality in asbestos factory
workers in London. Ann NY Acad Sci, 330: 53-60.
Ng TP, Chan SL, & Lam KP (1987) Radiological progression and lung
function in silicosis: A ten year follow up study. Br Med J,
295(6591): 164-167.
Ng TP, Chan SL, & Lee J (1990) Mortality of a cohort of men in a
silicosis register: Further evidence of an association with lung
cancer. Am J Ind Med, 17(2): 163-171.
Ng TP, Chan SL, & Lee J (1992) Predictors of mortality in silicosis.
Respir Med, 86(2): 115-119.
Niewoehner DE (1991) What lies ahead? Future research and treatment
for chronic obstructive pulmonary disease. Am J Med, 91(4A): 41S-46S.
Niewoehner DE, Kleinerman J, & Rice DB (1974) Pathologic changes in
the peripheral airways of young cigarette smokers. N Engl J Med, 291:
755-758.
NIH (National Institutes of Health) (1996) The FTC cigarette test
method for determining tar, nicotine, and carbon monoxide yields of US
cigarette smoking. Washington, DC, US Department of Health and Human
Services, 275 pp (Smoking and Tobacco Control Monograph 7).
NIH (National Institutes of Health) (1998) Cigars: Health effects and
trends. Washington, DC, US Department of Health and Human Services,
232 pp (Smoking and Tobacco Control Monograph 9).
Nilsson K, Hendriksson R, Cai YQ, Hellstrom S, Hornqvist-Bylunds S, &
Bjermer L (1992) Effects of tobacco smoke on radiation-induced
pneumonitis in rats. Int J Radiat Biol, 62: 719-727.
NIOSH (1995) Criteria for a recommended standard: Occupational
exposure to respirable coal mine dust. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, 336 pp.
Nishikawa M, Ikeda H, Nishiyama H, Yamakawa H, Suzuki S, & Okubo T
(1992) Combined effects of ozone and cigarette smoke on airway
responsiveness and vascular permeability in guinea pigs. Lung, 170:
311-322.
Nishiyama H, Yano H, Nishiwaki Y, Kitaya T, Matsuyama T, Kodama T,
Suemasu K, Tamai S, & Takemoto K (1985) Lung cancer in chromate
workers: Analysis of 11 cases. Jpn J Clin Oncol, 15(3): 489-497.
Norman V, Ghrig AM, Lassa TM, & Moss BL (1983) The effect of
nitrogenous blend compounds on NO/NO2 and HCl levels in mainstream
and sidestream smoke. Bertr Tabakforsch, 12: 55-62.
NRC (National Research Council) (1988) Committee on the Biological
Effects of Ionizing Radiations: BEIR-IV: Health risks of radon and
other internally deposited alpha-emitters. Washington, DC, National
Academy Press, 602 pp.
O'Dell JR, Linder J, Markin RS, & Moore GF (1987) Thromboangiitis
obliterans (Buerger's disease) and smokeless tobacco. Arthritis-Rheum,
30(9): 1054-1056.
Olin JW (1994) Thromboangiitis obliterans. Curr Opin Rheumatol, 6(1):
44-49.
Oxman AD, Muir DC, Shannon HS, Stock SR, Hnizdo E, & Lange HJ (1993)
Occupational dust exposure and chronic obstructive pulmonary disease:
A systematic overview of the evidence. Am Rev Respir Dis, 148(1):
38-48.
Park NH, Dokko H, Li SL, & Cherrick HM (1991a) Synergism of herpes
symplex virus and tobacco-specific N'-nitrosamines in cell
transformation. J Oral Maxillofac Surg, 49(3): 276-281.
Park NH, Sapp JP, & Herbosa EG (1991b) Oral cancer induced in hamsters
with Herpes simplex virus infection and simulated snuff dipping. Oral
Surg Oral Med Oral Pathol, 62: 164-168.
Parka KR (1983) Smoking as a moderator of the relationship between
affective state and absence from work. J Appl Psychol, 68: 698-708.
Patrianakos C & Hoffmann D (1979) Chemical studies on tobacco smoke:
LXIV. On the analysis of aromatic amines. Anal Toxicol, 3: 150-154.
Pedersen E, Hogetveit AC, & Andersen A (1973) Cancer of respiratory
organs among workers at a nickel refinery in Norway. Int J Cancer, 12:
32-41.
Penner M & Penner S (1990) Excess insured health care costs from
tobacco-using employees in a large group plan. J Occup Med, 32:
521-523.
Pepys J, Pickering CAC, & Hughes EG (1972) Asthma due to inhaled
chemical agents -- complex salts of platinum. Clin Allergy, 2:
391-396.
Pershagen G (1985) Lung cancer mortality among men living near an
arsenic-emitting smelter. Am J Epidemiol, 122: 684-694.
Pershagen G, Wall S, Taube A, & Linnman L (1981) On the interaction
between occupational arsenic exposure and smoking and its relationship
to lung cancer. Scand J Work Environ Health, 7: 302-309.
Pershagen G, Bergman F, Klominek J, Damber L, & Wall S (1987)
Histological types of lung cancer among smelter workers exposed to
arsenic. Br J Ind Med, 44: 454-458.
Pershagen G, Akerblom G, Axelson O, Clavensjo B, Damber L, Desai G,
Enflo A, Legarde F, Mellander H, Svartengren M, & Swedjemark GU (1994)
Residential radon exposure and lung cancer in Sweden. N Engl J Med,
330(3): 159-164.
Petersen R, Andersen M, Mikkelsen S, & Nielsen SL (1995) Prognosis of
vibration induced white finger: a follow-up study. Occup Environ Med,
52(2): 110-115.
Peterson KL, Heninger RW, & Seegmiller RE (1981) Fetotoxicity
following chronic prenatal treatment of mice with tobacco smoke and
ethanol. Bull Environ Contam Toxicol, 26: 813-819.
Philipson K, Falk R, Gustafsson J, & Camner P (1996) Long-term lung
clearance of 195Au-labeled Teflon particles in humans. Exp Lung Res,
22: 65-83.
Pieraccini G, Luceri, F & Moneti, G (1992) New
gas-chromatographic/mass spectrometric method for the quantitative
analysis of primary aromatic amines in main and sidestream cigarette
smoke. I. Rapid Commun Mass Spectrom, 6: 406-409.
Pindborg JJ, Mehta PS, Gupta PC, Daftary DK, & Smith CJ (1971) Reverse
smoking in Andhra Pradesh, India: A study of palatal lesions among
10,169 villagers. Br J Cancer, 25: 10-20.
Plamenac P, Santic Z, Nikulin A, & Serdarevic H (1985) Cytologic
changes of the respiratory tract in vineyard spraying workers. Eur J
Respir Dis, 67: 50-55.
Post WK, Burdorf A, & Bruggeling TG (1994) Relations between
respiratory symptoms and sickness among workers in the animal feed
industry. Occup Environ Med, 51: 440-446.
Pratt PC, Vollmer RT, & Miller JA (1980) Epidemiology of pulmonary
lesions in nontextile and cotton textile workers: a retrospective
autopsy analysis. Arch Environ Health, 35: 133-138.
Prentice RL, Yoshimoto Y, & Mason MW (1983) Relationship of cigarette
smoking and radiation exposure to cancer mortality in Hiroshima and
Nagasaki. J Natl Cancer Inst, 70: 611-622.
Pyykko I, Pekkarinen J, & Stark J (1987) Sensory-neural hearing loss
during combined noise and vibration exposure. Int Arch Occup Environ
Health, 59: 439-454.
Ramakrishnan S, Sulochana KN, Selvaraj T, Abdul-Rahim A, Lakshmi M, &
Arunagiri K (1995) Smoking of beedies and cataract: cadmium and
vitamin C in the lens and blood. Br J Ophthalmol, 79: 202-206.
Rang EH & Acheson ED (1981) Cancer in furniture workers. Int J
Epidemiol, 10(3): 253-261.
RCP (Royal College of Physicians) (1962) Smoking and health. London,
Pitman, pp 2-8.
Reddy CR (1974) Carcinoma of hard palate in India in relation to
reverse smoking of chuttas. J Natl Cancer Inst, 53(3): 615-619.
Reddy DJ & Rao KV (1957) Cancer of the palate in coastal Andhra due to
smoking of cigars with burning end inside the mouth. Indian J Med Sci,
11: 791-798.
Reddy DJ, Reddy BD, & Rao PR (1960) Experimental production of cancer
with tobacco tar and heat. Cancer, 13: 263-269.
Reith AK, Reichborn-Kjennerud S, Aubele M, Jutting U, Gais P, & Burger
G (1994) Biological monitoring of chemical exposure in nickel workers
by imaging cytometry (ICM) of nasal smears. Anal Cell Pathol, 6(1):
9-21.
Rivenson A, Hecht SS, & Hoffmann D (1991) Carcinogenicity of
tobacco-specific N-nitrosamines: the role of the vascular network in
the selection of target organs. Crit Rev Toxicol, 21: 255-264.
Roberts DL (1988) Natural tobacco flavor. Recent Adv Tob, 14: 45-81.
Robertson PB, Walsh MM, & Greene JC (1997) Oral effects of smokeless
tobacco used by professional baseball players. Adv Dental Res, 11:
307-312.
Rodenhuis S & Slebos RJC (1992) Clinical significance of ras oncogene
activation in human lung cancer. Cancer Res, 52(suppl): S2665-S2669.
Roels H, Sarhan MJ, Hanotiau I, de Fays M, Genet P, Bernard A, Buchet
JP, & Lauwerys R (1985) Preclinical toxic effects of manganese in
workers in a Mn salts and oxides producing plant. Sci Total Environ,
42: 201-206.
Roth J, Mahrlein W, Kummer G, & Krause M (1985) [Results of an
epidemiological analysis of the pathogenesis of chronic obstructive
bronchitis in miners.] Z Erkrank Atmungsorg, 164(3): 277-279 (in
German).
Rothman K & Keller A (1972) The effect of joint exposure to alcohol
and tobacco on risk of cancer of the mouth and pharynx. J Chronic Dis,
25: 711-716.
Roush GC (1996) Cancer of the nasal cavity and paranasal sinuses. In:
Schuttenfeld D & Franinini JF ed. Cancer epidemiology and prevention.
New York, Oxford, Oxford University Press, pp 587-602.
Ruckley VA, Gauld SJ, Chapman JS, Davis JM, Douglas AN, Fernie JM,
Jacobsen M, & Lamb D (1984) Emphysema and dust exposure in a group of
coal workers. Am Rev Respir Dis, 129: 528-532.
Russell MAH (1976) Tobacco smoking and nicotine dependence. In:
Gibbons RJ, Israel Y, Kalent H, Papham RE, Schmidt W, & Smart RG ed.
Recent advances in alcohol and drug problems. New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp 1-47.
Ryan J, Zwerling C, & John E (1992) Occupational risks associated with
cigarette smoking: A prospective study. Am J Pub Health, 82: 29-32.
Rylander R, Sjostrand M, & Bergstrom R (1979) Free lung cell response
after combined exposure to cigarette smoke and industrial dusts.
Toxicology, 12: 211-220.
Sanden A & Jarvholm B (1991) A study of predictors of mesothelioma in
shipyard workers exposed to asbestos. J Occup Med, 33(7): 770-773.
Sanden A, Jarvholm B, Larsson S, & Thiringer G (1992) The risk of lung
cancer and mesothelioma after cessation of asbestos exposure: a
prospective cohort study of shipyard workers. Eur Respir J, 5:
281-285.
Sankaranarayanan R, Duffy SW, Day NE, Nair MK, Padmakumary G, & Day NE
(1989a) A case control investigation of cancer of the oral tongue and
floor of the mouth in southern India. Int J Cancer, 44(4): 617-621.
Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, & Padmanabhan TK
(1989b) Tobacco chewing, alcohol and nasal snuff in cancer of the
gingiva in Kerala, India. Br J Cancer, 60(4): 638-643.
Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, & Nair MK (1990)
Risk factors for cancer of the buccal and labial mucosa in Kerala,
southern India. J Epidemiol Community Health, 44(4): 286-292.
Cerik R (1987) The interactions of tobacco smoking and other agents in
cancer etiology. Epidemiol Rev, 9: 175-193.
Saris M & Lucic-Palaic S (1977) Possible synergism of exposure to
airborne manganese and smoking habit in occurrence of respiratory
symptoms. In: Walton WH & McGovern B ed. Inhaled particles -- IV.
Oxford, New York, Pergamon Press, pp 773-779.
Saumet JL, Leftheriotis G, Dittmar A, & Delhomme G (1986) Relationship
between pulse amplitude and thermal exchange in the finger: the effect
of smoking. Psychopharmacology (Berlin), 92(1): 118-121.
Schlueter DP, Banaszaak EF, Fink JN, & Barboriak J (1978) Occupational
asthma due to tetrachlorophthalic anhydride. J Occup Med, 20: 183-188
Schneider G & Krivan V (1993) Multi-element analysis of tobacco and
tobacco smoke condensate by instrumental neutron activation analysis
and atomic absorption spectrometry. Int J Environ Anal Chem, 53:
87-100.
Schnorr TM, Steenland K, Thun MJ, & Rinsky RA (1995) Mortality in a
cohort of antimony smelter workers. Am J Ind Med, 27(5): 759-770.
Schottenfeld D, Grant RC, & Wynder WL (1974) The role of alcohol and
tobacco in multiple primary cancers of the upper digestive system,
larynx and lung: A prospective study. Prev Med, 3: 277-293.
Schüller HM, Jorquera R, Reichert A, & Castonguay A (1993)
Transplacental induction of pancreas tumors in hamsters by ethanol and
the tobacco-specific nitrosamine 4-(methyl
nitrosamino)-1-(pyridyl)-1-butanone. Cancer Res, 53: 2498-2501.
Seaton A, Lamb D, Brown WR, Sclare G, & Middleton WG (1981)
Pneumoconiosis of shale miners. Thorax, 36: 412-418.
Seaton A, Godden DJ, & Brown K (1994) Increase in asthma: a more toxic
environment or a more susceptible population? Thorax, 49: 171-174.
Selig R & Nestler K (1985) [Epidemiology of chronic bronchitis from
the viewpoint of occupational medicine.] Z Erkrank Atmungsorg, 164(3):
273-276 (in German).
Selikoff IJ & Frank AL (1983) Keynote address. Potential of in vitro
tests in asbestos-related problems. Environ Health Perspect, 51: 1-4.
Selikoff IJ, Hammond EC, & Churg J (1968) Asbestos exposure, smoking
and neoplasia. J Am Med Assoc, 204(2): 106-112.
Selikoff IJ, Seidman H, & Hammond EC (1980) Mortality effects of
cigarette smoking among amosite asbestos factory workers. J Natl
Cancer Inst, 65: 507-513.
Sherrill DL, Halonen M, & Burrows B (1994) Relationships between total
serum IgE, atopy and smoking: a twenty-year follow-up analysis. J
Allergy Clin Immunol, 94: 954-962.
Sherson D, Sigsgaard T, Overgaard E, Loft S, Poulsen HE, & Jongeneelen
FJ (1992) Interaction of smoking, uptake of polycyclic hydrocarbons,
and cytochrome P450IA2 activity among foundry workers. Br J Ind Med,
49(3): 197-202.
Shim C & Williams MH (1986) Effect of odors in asthma. Am J Med,
80(1): 18-22.
Shirakawa T, Kusaka Y, & Morimoto K (1992) Combined effects of smoking
habits and occupational exposure to hard metal on total IgE
antibodies. Chest, 101(6): 1569-1576.
Shopland DR, Eyre HJ, & Pechacek TF (1991) Smoking attributable cancer
mortality in 1991: is lung cancer now the leading cause of death among
smokers in the United States? J Natl Cancer Inst, 83(16): 1142-1148.
Sidney S, Tekawa I, & Friedman GD (1989) Mentholated cigarette use
among multiphasic examinees, 1979-86. Am J Pub Health, 79(10):
1415-1416.
Simarak S, de Jong UW, Breslow N, Dahl CJ, Ruckphaopunt K, Scheelings
P, & Maclennan R (1977) Cancer of the oral cavity, pharynx/larynx and
lung in Northern Thailand: case-control study and analysis of cigar
smoke. Br J Cancer, 36: 130-140.
Simecek C, Sefrna F, & Simecková B (1986) [Evaluation of the role of
smoking in the development of chronic bronchitis after elimination of
other supporting factors.] Stud Pneumol Phtiseologica Czech, 46(3):
168-170 (in Czech).
Sluis-Cremer GK, Walters LG, & Sichel HS (1967) Chronic bronchitis in
miners and non-miners: an epidemiologic survey of a community in the
gold mining area in the Transvaal. Br J Ind Med, 24: 1-12.
Smith IE, Coles CD, Lancaster J, Fernhoff PM, & Falek A (1986) The
effect of volume and duration of prenatal ethanol exposure on neonatal
physical and behavioral development. Neurobehav Toxicol Teratol, 8:
375-381.
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I,
Duggan HM, Wood R, Kosnett MJ, & Smith MT (1992) Cancer risks from
arsenic in drinking water. Environ Health Perspect, 97: 259-267.
Sopori ML, Goud NS, & Kaplan AM (1994) Effects of tobacco smoke on the
immune system. In: Dean JH, Luster MI, Munson AE, & Kimber I ed.
Immunotoxicology and immunopharmacology. New York, Raven Press, pp
413-434.
Sopori ML, Kozak W, Savage SM, Geng Y, & Kluger MJ (1998)
Nicotine-induced modulation of T cell function: Implications for
inflammation and infection. Adv Exp Med Biol, 437: 279-289.
Sprince NL, Oliver LC, Eisen EA, Greene RE, & Chamberlin RI (1988)
Cobalt exposure and lung disease in tungsten carbide production: A
cross-sectional study of current workers. Am Rev Respir Dis, 138:
1220-1226.
Stanton MF, Layard M, Tegeris A, Miller E, May M, & Kent E (1977)
Carcinogenicity of fibrous glass: pleural response in the rat in
relation to fiber dimension. J Natl Cancer Inst, 58: 587-603.
Steenland K (1994) Age specific interactions between smoking and radon
among United States uranium miners. Occup Environ Med, 51(3): 192-194.
Steenland K & Thun M (1986) Interaction between tobacco smoking and
occupational exposures in the causation of lung cancer. J Occup Med,
28(2): 110-118.
Steenland K & Ward E (1991) Lung cancer incidence among patients with
beryllium disease: a cohort mortality study. J Natl Cancer Inst,
83(19): 1380-1385.
Steenland K & Ward E (1993) Passive smoking and the risk of heart
disease. J Natl Cancer Inst, 85(2): 1698-1699.
Steindorf K, Lubin J, Wichman HE, & Becher H (1995) Lung cancer deaths
attributable to indoor radon exposure in West Germany. Int J
Epidemiol, 24(3): 485-492.
Stellman SD, Boffetta P, & Garfinkel L (1988) Smoking habits of
800,000 American men and women in relation to their occupations. Am J
Ind Med, 13: 43-58.
Stellman SD, Muscat JE, Thompson S, Hoffmann D, & Wynder EL (1997)
Risk of squamous cell carcinoma and adenocarcinoma of the lung in
relation to lifetime filter cigarette smoking. Cancer, 80: 382-388.
Stöber W & Abel WR (1996) Lung cancer due to diesel soot particles in
ambient air? Int Arch Occup Environ Health, 68(suppl): S3-S61.
Stohrer G (1991) Arsenic: opportunity for risk assessment. Toxicology,
65: 525-531.
Sturtevant FM (1982) Smoking, oral contraceptives, and thromboembolic
disease. Int J Fertil, 27: 2-13.
Sunderman FW & Sunderman FW Jr (1961) Nickel poisoning: XI.
Implications of nickel as a pulmonary carcinogen in tobacco smoke. Am
J Clin Pathol, 35: 203-205.
Talbot RJ, Morgan A, Moores SR, & Matulionis DH (1987) Preliminary
studies of the interaction between 239PuO2 and cigarette smoke in the
mouse lung. Int J Radiat Biol, 51: 1101-1110.
Tawfik HN (1987) Carcinoma of the urinary bladder associated with
schistosomiasis in Egypt: the possible causal relationship. Princess
Takamatsu Int Symp Ser, 18: 197-209.
Taylor PR, Qiao YL, Schatzkin A, Yao SX, Lubin J, Mao BL, Rao JY,
McAdams M, Xuan XZ, & Li JY (1989) Relation of arsenic exposure to
lung cancer among tin miners in Yunan Province, China. Br J Ind Med,
46: 881-886.
Thériault G, de Guire L, Gingras S, & Laroche G (1982) Raynaud's
phenomenon in forestry workers in Quebec. Can Med Assoc J, 126:
1404-1408.
Thériault G, Tremblay C, Cordier S, & Gingras S (1984) Bladder cancer
in the aluminium industry. Lancet, 1(8383): 947-950.
Thomas TL & Stewart PA (1986) Mortality from lung cancer and
respiratory disease among pottery workers exposed to silica and talc.
Am J Epidemiol, 124: 530.
Thomas DC & Whittemore AS (1988) Methods for testing interactions,
with applications to occupational exposures, smoking and lung cancer.
Am J Ind Med, 13: 131-147.
Thomas GB, Williams CE, & Hoger NG (1981) Some non-auditory correlates
of the hearing threshold levels of an aviation noise-exposed
population. Aviation Space Environ Med, 52(9): 531-536.
Thun MJ, Lally CA, Flanney JT, Calle EE, Flander WD, & Heath CW Jr
(1997) Cigarette smoking and changes in the histopathology of the
lung. J Natl Cancer Inst, 89: 1580-1586.
Tokarskaya ZB, Okladnikova ND, Belyaeva ZD, & Drozhko EG (1995) The
influence of radiation and nonradiation factors on the lung cancer
incidence among the workers of the nuclear enterprise Mayak. Health
Phys, 69: 356-366.
Townsend MC, Sussman NB, Enterline PE, Morgan WK, Belk HD, & Dinman BD
(1988) Radiographic abnormalities in relation to total dust exposure
at a bauxite refinery and alumina-based chemical products plant. Am
Rev Respir Dis, 138: 90-95.
Trethowen WN, Burge PS, Rossiter CE, Harrington JM, & Calvert IA
(1995) Study of the respiratory health of employees of seven European
plants that manufacture ceramic fibres. Occup Environ Med, 52(2):
97-104.
Tricker AR & Preussmann R (1992) Volatile N-nitrosamines in
mainstream cigarette smoke: occurrence and formation. Clin Invest,
70(3-4): 283-289.
Tseng WP, Chu HM, How SW, Fong JM, Lin CS, & Yeh S (1968) Prevalence
of skin cancer in an endemic area of chronic arsenicism in Taiwan. J
Natl Cancer Inst, 40: 453-463.
Tso TC (1990) Production physiology and biochemistry of tobacco
plants. Belsville, Maryland, IDEALS, p 753.
Tso TC, Harley N, & Alexander LT (1966) Source of lead-210 and
polonium-210 in tobacco. Science, 153: 880-882.
Tsuda M & Kurashima Y (1991) Tobacco smoking, chewing, and snuff
dipping: factors contributing to the endogenous formation of
N-nitroso compounds. Crit Rev Toxicol, 21(4): 243-253.
Tsuda T, Nagira T, Yamamoto M, & Kume Y (1990) An epidemiological
study on cancer in certified arsenic poisoning patients in Toroku. Ind
Health, 28: 53-62.
Tsuda T, Babazono A, Yamamoto E, Kurumatani N, Mino Y, Ogawa T, Kishi
Y, & Aoyama H (1995) Ingested arsenic and internal cancer: a
historical cohort followed for 33 years. Am J Epidemiol, 141(3):
198-209.
Tuyns AJ (1983) Oesophageal cancer in non-smoking drinkers and
non-drinking smokers. Int J Cancer, 32: 443-444.
Tuyns AJ (1991) Aetiology of head and neck cancer: tobacco, alcohol
and diet. Otorhynolaryngology, 46: 15-24.
Tuyns AJ, Péquignot G, & Jensen OM (1977) Le cancer de l'oesophage en
Ille-et-Vilaine en fonction des niveaux de consommation d'alcool et de
tabac: Des risques qui se multiplient. Bull Cancer, 64: 45-60.
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic
Radiation) (1988) Sources and effects of ionizing radiation. New York,
United Nations.
US ATSDR (1995) Toxicological profile for asbestos (update). Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry, 224 pp.
US DA (1990) World tobacco situation. Washington, DC, US Department of
Agriculture, Foreign Agricultural Service (Circular Series FT 4-90).
US DA (1997) Tobacco situation and outlook yearbook -- US output,
removal and consumption 1950-1997: Cigarettes, cigars, smoking tobacco
and specific tobacco products. Washington, DC, US Department of
Agriculture, Economic Research Service, pp 14-17 (TBS-240).
US EPA (1988) Technical support document on risk assessment of
chemical mixtures. Washington, DC, US Environmental Protection Agency,
32 pp (EPA/600/8-90/064).
US Surgeon General (1979) Smoking and health: A report of the Surgeon
General. Washington, DC, US Department of Health, Education and
Welfare (DHEW Publication No. (PHS) 79-50066).
US Surgeon General (1982) The health consequences of smoking: Cancer
-- A report of the Surgeon General. Rockville, Maryland, US Department
of Health and Human Services, Public Health Service, 332 pp.
US Surgeon General (1983) The health consequences of smoking:
Cardiovascular disease -- A report of the Surgeon General. Rockville,
Maryland, US Department of Health and Human Services, Public Health
Service, pp 6-8.
US Surgeon General (1984) The health consequences of smoking: Chronic
obstructive lung disease -- A report of the Surgeon General.
Rockville, Maryland, US Department of Health and Human Service (DHHS
Publication No. (PHS) 84-50205).
US Surgeon General (1986) The health consequences of using smokeless
tobacco -- A report of the Advisory Committee to the Surgeon General.
Bethesda, Maryland, US Department of Health and Human Services, Public
Health Service (Publication No. (NIH) 86-2874).
US Surgeon General (1988) The health consequences of smoking: Nicotine
addiction -- A report of the Surgeon General. Rockville, Maryland, US
Department of Health and Human Services, Centers for Disease Control,
pp 21-560.
US Surgeon General (1989) Reducing the health consequences of smoking:
25 years of progress -- A report of the Surgeon General. Rockville,
Maryland, US Department of Health and Human Services, Public Health
Service (DHHS Publication No. (CDC) 89-8411).
Vainio H & Boffetta P (1994) Mechanisms of the combined effect of
asbestos and smoking in the etiology of lung cancer [Review]. Scand J
Work Environ Health, 20(4): 235-242.
Vainio H, Husgafvel-Pursiainen K, Anttila S, Hackman P, & Partanen T
(1993) Interaction between smoking and asbestos in human lung
adenocarcinoma: role of K-ras mutations. Environ Health Perspect,
101(suppl 3): 189-192.
Vallyathan V, Shi XL, Dalal NS, Irr W, & Castranova V (1988)
Generation of free radicals from freshly fractured silica dust.
Potential role in acute silica-induced lung injury. Am Rev Respir Dis,
138: 1213-1219.
Van Leeuwen FE, Klokman WJ, Stoval M, Hagwenbeek A, van den
Belt-Dusebout AW, Noyon R, Boice JD, Burgers MV, & Somers R (1995)
Roles of radiotherapy and smoking in lung cancer following Hodgkin's
disease. J Natl Cancer Inst, 87(20): 1530-1537.
Van Peenen PFD, Blanchard AG, Wolkonsky PM, & Gill TM (1986) Health
insurance claims of petrochemical company employees. J Occup Med, 28:
237-240.
Van Tuinen M & Land G (1986) Smoking and excess sick leave in a
department of health. J Occup Med, 28: 33-35.
Van Schooten FJ, van Leeuwen FE, Hillebrand MJ, de Rijke ME, Hart AA,
van Veen HG, Oosterink S, & Kriek E (1990) Determination of
benzo(a)pyrene diol epoxide-DNA adducts in white blood cell DNA from
coke-oven workers: the impact of smoking. J Natl Cancer Inst, 82(11):
927-933.
Venables KM, Topping MD, Howe W, Luczynska CM, Hawkins R, & Taylor AJ
(1985) Interaction of smoking and atopy in producing specific IgE
antibody against a hapten protein conjugate. Br Med J, 290: 201-204.
Venables KM, Tee RD, Hawkins ER, Gordon DJ, Wale CJ, Farrer NM, Lam
TH, Baxter PJ, & Newman-Taylor A (1988a) Laboratory animal allergy in
a pharmaceutical company. Br J Ind Med, 45: 667-671.
Venables KM, Upton KM, Hawkins ER, Tee RD, Longbottom JL, &
Newman-Taylor AJ (1988b) Smoking, atopy, and laboratory animal
allergy. Br J Ind Med, 45: 667-671.
Venables KM, Dally MB, Nunn AJ, Stevens JF, Stephens R, Farrer N,
Hunter JV, Stewart M, Hughes EG, & Newman-Taylor AJ (1989) Smoking and
occupational allergy in workers in a platinum refinery. Br Med J, 299:
939-942.
Vessey MP & Doll R (1969) Investigation of relationship between the
use of oral contraceptives and thromboembolic disease: a further
report. Br Med J, 2: 651-657.
Viikari-Juntura E, Riihimaki H, Tola S, Videman T, & Mutanen P (1994)
Neck trouble in machine operating, dynamic physical work and sedentary
work: a prospective study on occupational and individual risk factors.
J Clin Epidemiol, 47(12): 1411-1422.
Vineis P (1992) Epidemiological models of carcinogenesis: the example
of bladder cancer. Cancer Epidemiol Biomarkers Prev, 1(2): 149-153.
Vineis P, Esteve J, & Terracini B (1984) Bladder cancer and smoking in
males: types of cigarettes, age of start, effect of stopping and
interaction with occupation. Int J Cancer, 34: 165-170.
Voges E ed. (1984) Tobacco encyclopedia. Mainz, Germany, Tabilk
Journal International, 467 pp.
Wagner JC, Pooley FD, Gibbs A, Lyons J, Sheers G, & Moncrieff CB
(1986) Inhalation of china stone and china clay dusts: relationships
between the mineralogy of dust retained in the lungs and pathological
changes. Thorax, 41: 190-196.
Wald NJ, Dall R, Darby S, Kinjlnk S, Petri R, & Pike M ed. (1985)
Trends in smoking related diseases in Britain. Oxford, Oxford
University Press.
Warren CPW (1977) Extrinsic allergic alveolitis: a disease commoner in
non-smokers. Thorax, 32: 567-569.
Weiland SK, Mundt KA, Keil U, Kraemer B, Birk T, Person M, Bucher AM,
Straif K, Schumann J, & Chambless L (1996) Cancer mortality among
workers in the German rubber industry: 1981-91. Occup Environ Med, 53:
289-298.
Weiss W (1971) Cigarette smoking, asbestos and pulmonary fibrosis. Am
Rev Respir Dis, 104: 223-226.
Weiss W (1976) Chloromethyl ethers, cigarettes, cough and cancer. J
Occup Med, 18(3): 194-199.
Weiss W (1980) The cigarette factor in lung cancer due to chloromethyl
ethers. J Occup Med, 22(8): 527-529.
Weiss W (1982) Epidemic curve of respiratory cancer due to
chloromethyl ethers. J Natl Cancer Inst, 69(6): 1265-1270.
Weiss W (1984) Cigarette smoke, asbestos and small irregular
opacities. Am Rev Respir Dis, 130(2): 293-301.
Weiss W & Boucot KR (1975) The respiratory effects of chloromethyl
methyl ether. J Am Med Assoc, 234(11): 1139-1142.
Weiss W & Nash D (1997) An epidemic of lung cancer due to chloromethyl
ethers: Thirty years of observation. J Occup Environ Med, 39:
1003-1009.
Welch K, Higgins I, Oh M, & Burchfiel C (1982) Arsenic exposure,
smoking and respiratory cancer in copper smelter workers. Arch Environ
Health, 37: 325-335.
WHO (1980) Recommended health-based limits in occupational exposure to
heavy metals. Geneva, World Health Organization, pp 21-35 (WHO
Technical Report Series 647).
WHO (1985) Regional Workshop on the Control of Tobacco-related
Diseases, New Delhi, 22-26 July 1985. New Delhi, World Health
Organization Regional Office for South-East Asia.
WHO (1997) Tobacco or health: A global status report. Geneva, World
Health Organization, pp 10-18.
Wicklund KG, Daling JR, Allard J, & Weiss NS (1988) Respiratory cancer
among orchardists in Washington State, 1968 to 1980. J Occup Med,
30(7): 561-564.
Wilbert W (1987) Tobacco and shamanism in South America (Psychoactive
plants of the world). New Haven, London, Yale University Press, 294
pp.
Wilhelmsen L (1977) Recent studies on smoking and CVD epidemiology:
Scandinavia and some other Western European countries. In: Proceedings
of 3rd World Conference on Smoking and Health -- Volume II: Health
consequences, education, cessation, and social action. Washington, DC,
Department of Health, Education and Welfare, pp 171-177 (Publication
No. (NIH) 77-1413).
Williams MK, Walford J, & King E (1983) Blood lead and the symptoms of
lead absorption. Br J Ind Med, 40(3): 285-292.
Winkelstein W (1990) Smoking and cervical cancer current status: a
review. Am J Epidem, 131: 945-957.
Winkelstein W, Shillitoe EJ, Brand R, & Johnson KK (1984) Further
comments on cancer of the uterine cervix, smoking and herpes virus
infection. Am J Epidem, 119(1): 1-8.
Winn DM (1997) Epidemiology of cancer and other systemic effects
associated with the use of smokeless tobacco. Adv Dent Res, 11(3):
313-321.
Wise MB & Guerin MR (1986) Chemical analysis of the major constituents
in clove cigarette smoke. In: Hoffmann D & Harris CC ed. Mechanisms in
tobacco carcinogenesis. Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 151-162 (Banbury Report No. 23).
Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE
(1997) The carcinogenicity of environmental tobacco smoke.
Carcinogenesis, 18(3): 575-586.
Wong SH, Ogle CW, & Cho CH (1986) Influence of chronic or acute
nicotine pretreatment on ethanol-induced gastric ulceration in the
rat. J Pharm Pharmacol, 38: 537-540.
Wong O, Harris F, & Smith TJ (1993) Health effects of gasoline
exposure: II. Mortality patterns of distribution workers in the United
States. Environ Health Perspect, 101(suppl 6): 63-76.
Wright JT, Waterson EJ, Barrison IG, Toplis PJ, Lewis IG, Gordon MG,
Macrae KD, Morris NF, & Murray-Lyon IM (1983) Alcohol consumption,
pregnancy and low birth weight. Lancet, 1: 663-665.
Wright JT, Macrae KD, Barrison IG, & Waterson EJ (1984) Effects of
moderate alcohol consumption and smoking on fetal outcome. London,
Pitman, pp 240-253 (Ciba Foundation Symposium Series No. 105).
Wynder EL & Bross IJ (1961) A study of etiological factors in cancer
of the oesophagus. Cancer, 14: 389-413.
Wynder EL & Gori GB (1977) Contribution of the environment on cancer
incidence: an epidemiologic exercise. J Natl Cancer Inst, 58: 825-832.
Wynder EL & Graham EG (1950) Tobacco smoking as a possible etiological
factor in bronchiogenic carcinoma. J Am Med Assoc, 37: 4608-4622.
Wynder El & Hoffmann D (1967) Tobacco and tobacco smoke: Studies in
experimental carcinogenesis. New York, London, Academic Press.
Wynder El & Hoffmann D (1994) Smoking and lung cancer: challenges and
opportunities. Cancer Res, 54: 5284-5295.
Wynder EL, Covey LS, Mabuchi K, & Mushinki M (1976) Environmental
factors in cancers of the larynx: A second look. Cancer, 38:
1591-1601.
Yazicioglu S, Ilcayto R, Balci K, Sayli BS, & Yorulmaz B (1980)
Pleural calcification, pleural mesotheliomas and bronchial cancers
caused by tremolite dust. Thorax, 35: 564-569.
Yorukoglu Y, Ilgit E, Zengin M, Nazliel K, Salman E, & Yucel E (1993)
Thromboangiitis obliterans (Buerger's disease) in women (a
reevaluation). Angiology, 44(7): 527-532.
Yoshizawa K (1984) [Clinicopathological analysis of lung cancer and
epithelial changes of the bronchus in chromate workers.] Shikoku Acta
Med, 40(1): 123-134 (in Japanese).
Yue L (1992) Cadmium in tobacco. Biomed Environ Sci, 5(1): 53-56.
Zacny JP (1990) Behavioral aspects of alcohol-tobacco interactions.
Recent Dev Alcohol, 8: 205-219.
Zahran FM, Ardawi MSM, & Al-Fayez SF (1985) Carboxyhaemoglobin
concentrations in smokers of sheesha and cigarettes in Saudi Arabia.
Br Med J, 291: 1768-1770.
Zelman S (1973) Correlation of smoking history with hearing loss. J Am
Med Assoc, 223(8): 920.
Zetterstrom O, Osterman K, Machado L, & Johansson SGO (1981) Another
smoking hazard: raised IgE concentration and increased risk of
occupational allergy. Br Med J, 283: 1215-1217.
Zhang Z-F, Yu S-Z, Li W-X, & Choi BCK (1989) Smoking, occupational
exposure to rubber and lung cancer. Br J Ind Med, 46: 12-15.
Zhu H & Wang Z (1993) Study of occupational cancer in asbestos
factories in China. Br J Ind Med, 50(11): 1039-1042.
Zuskin E, Zagar Z, Schacter EN, Mustajbego J, & Kern J (1992)
Respiratory symptoms and ventilatory capacity in swine confinement
workers. Br J Ind Med, 49(6): 435-440.
SYNOPSIS
1. Introduction
Le tabac, notamment lorsqu'il est fumé, exerce divers effets nuisibles
à la santé; il est directement en cause dans un certain nombre de
maladies graves et peut accentuer l'action nocive d'autres agents
chimiques, physiques ou biologiques. Les produits chimiques et autres
agents présents sur les lieux de travail peuvent, si l'on n'y veille pas
suffisamment, provoquer diverses pathologies, des invalidités et des
décès prématurés. Il est clair que sur les lieux de travail, des effets
indésirables peuvent résulter de la synergie entre le tabagisme et
d'autres facteurs de risque. La plupart des interactions entre les
constituants nocifs de la fumée de tabac et certaines substances
chimiques toxiques se produisent lorsque celles-ci sont présentes dans
l'atmosphère, mais le tabagisme peut également, comme on l'a observé,
interagir avec des agents toxiques absorbés par voie buccale ou autre.
La consommation de tabac est universelle, des pays économiquement
défavorisés aux nations industrialisées les plus riches. Le tabac est
consommé par les hommes et les femmes, les enfants et les adultes et des
millions de gens sont exposés malgré eux à la fumée de tabac présente
dans l'environnement. Il y a de nombreuses explications au tabagisme,
mais la raison principale de son universalité tient à la présence, dans
les feuilles de toutes les variétés de tabac, de nicotine, une substance
qui engendre la dépendance. Cette dernière pénètre dans l'organisme du
consommateur en quantité variable, selon le type de tabagisme auquel il
s'adonne (Chapitre 2). L'apparition de la cigarette, qui peut être
produite en quantités industrielles, qu'il est facile de se procurer à
un prix relativement bas et que sa légèreté permet de tenir entre les
lèvres en gardant les mains libres, a profondément modifié le
comportement des fumeurs, que ce soit dans la vie en général ou sur les
lieux de travail.
Il y a beaucoup de pays où fumer est considéré comme très dangereux pour
la santé et comme un facteur qui contribue de manière importante à la
mortalité résultant d'un certain nombre d'affections courantes. Ces pays
ont adopté une législation qui vise à alerter les consommateurs des
risques qu'ils courent et ils ont également pris des mesures pour
freiner la consommation, soit par la taxation, soit par la mise en
oeuvre de campagnes d'information à destination du grand public qui ont
pour but d'attirer l'attention sur les dangers du tabagisme et les
avantages qu'il y a à cesser de fumer ou à ne pas commencer du tout.
Certains pays n'ont toutefois pas encore pris de mesures décisives pour
traiter le problème du tabagisme.
L'activité professionnelle comporte souvent une part de risque. Elle
peut être de nature à mettre la santé en danger et à contaminer
l'environnement. La culture du tabac elle-même implique l'utilisation de
pesticides, la récolte des feuilles peut provoquer des intoxications
dues à l'absorption percutanée de nicotine et les diverses opérations
auxquelles elles sont ensuite soumises expose les travailleurs à
respirer les poussières et les spores de champignons présentes dans
l'atmosphère. Dans les zones où sont implantées des manufactures de
tabac, on observe une incidence élevée de cancers chez les sujets de
sexe masculin. L'air des exploitations minières est chargé de poussières
minérales et dans l'agriculture ou les industries qui utilisent des
matières premières d'origine biologique, les travailleurs sont exposés
à des poussières de même origine. Le soudage donne également lieu à la
production de vapeurs, et les gaz, fumées, brouillards etc. produits
dans de nombreuses industries sont aussi source de dangers. Une chaleur
excessive ou l'exposition aux ultraviolets peuvent nuire au bien-être
des travailleurs. On admet désormais que les rayonnements ionisants émis
dans les mines ou par certains appareils modernes constituent un risque
professionnel. Nombreuses sont les activités professionnelles qui
entraînent une exposition à des niveaux de bruit excessifs ou à des
vibrations nocives. Toutes ces conditions de travail ont des effets
indésirables sur la santé, mais qui peuvent être plus graves pour les
fumeurs que pour les non fumeurs. Dans un grand nombre de pays, il est
interdit de fumer sur les lieux de travail, essentiellement d'ailleurs
en raison des risques d'incendie ou d'explosion. Dans d'autres, cette
réglementation n'est pas toujours appliquée. Dans certains pays
nouvellement industrialisés, les problèmes sanitaires liés à l'activité
professionnelle ne sont pas encore totalement pris en compte et de
nombreux employés et ouvriers ne sont pas conscients des risques de leur
métier sur le plan sanitaire. En outre, il existe un vaste secteur
industriel "non officiel", en particulier dans les pays en
développement, où le travail, qui se fait à domicile, peut comporter
l'utilisation de produits chimiques (solvants, résines, colorants de
synthèse etc.) auxquels toute la famille va donc se trouver exposée. En
outre, il n'existe pas de réglementation qui restreigne l'exposition ou
interdise de fumer dans ces circonstances.
Beaucoup moins bien défini est le cas d'effets nocifs résultant de
l'action combinée d'une exposition à la fumée de tabac, provenant du
courant principal ou de l'environnement, et à des agents présents dans
le milieu domestique. On sait toutefois qu'en ce qui concerne
l'incidence du cancer du poumon, la courbe dose-réponse obtenue dans le
cas d'une exposition domestique au radon est analogue à celle qu'on
obtient chez des mineurs exposés à ce gaz, avec un risque plus important
chez les fumeurs.
2. Exemples d'effets combinés d'une exposition à la fumée de tabac et
à d'autres agents
On est fondé à penser que dans le cas de certains effets toxiques (en
l'occurrence le cancer) il existe une synergie entre le fait de fumer et
l'exposition à l'arsenic, à l'amiante, à l'éthanol, à la silice et aux
rayonnements (radon, bombe atomique, rayons X). D'un autre côté, il y a
également lieu de croire à l'existence d'un antagonisme entre le
tabagisme et les chlorométhyléthers cancérogènes comme le
chlorométhyl-méthyléther (CMME) et le bis (chlorométhyléther) (BCME)
(Hoffmann & Winder, 1976; CIRC, 1986), entre le tabagisme et l'alvéolite
allergique ou encore entre le tabagisme et la bérylliose chronique.
Fumer accentue les effets nocifs d'une exposition à la poussière chez
les mineurs de charbon, ou aux pesticides chez ceux qui en manipulent,
et cette accentuation du risque s'observe également dans l'industrie du
caoutchouc et du pétrole. Les mineurs de charbon qui fument risquent
davantage de contracter une bronchite chronique ou une pneumopathie
obstructive, mais pas un emphysème. Les cancers du poumon observés chez
les mineurs de charbon sont attribués en totalité au tabagisme. Fumer
peut accroître les effets d'une exposition aux poussières végétales qui
engendrent des affections respiratoires chroniques, comme la byssinose
produite par la poussière de coton et le cancer des fosses nasales
provoqué par la poussière de bois.
3. Composition des feuilles et de la fumée de tabac
On a isolé plus de 3040 composés chimiques des feuilles de tabac après
transformation (Roberts, 1988). La plupart d'entre eux sont des
constituants de la feuille, mais d'autres résultent des conditions de
culture (sol et atmosphère de la région) ou encore des produits
agrochimiques utilisés et des traitements subis (sauçage,
humidification, aromatisation et séchage). On constate des différences
selon la région d'origine du tabac, les variétés et les diverses
méthodes de séchage et de transformation utilisées. Ces différences
peuvent affecter la proportion des divers constituants mais la
composition globale ne varie pas. Parmi les importants composés toxiques
que l'on a mis en évidence, on trouve, à côté de la nicotine, des
nitrosamines cancérogènes qui proviennent de l'action des nitrites, des
amines, des protéines et des alcaloïdes d'origine foliaire, des
hydrocarbures aromatiques polycycliques formés au cours du séchage, des
éléments radioactifs captés dans le sol et dans l'air ainsi que du
cadmium dans le cas de tabacs cultivés sur des sols riches en cadmium.
Lorsque l'on fume, la combustion du tabac conduit à la formation de
nombreux produits de pyrolyse ou résultant d'autres types de réactions.
4. Fumée du courant principal
La fumée de tabac est un aérosol consistant en une phase particulaire
constituée de gouttelettes de liquide dispersées dans une phase gazeuse
ou vapeur. Lorsque l'on fume une cigarette, il se forme de nombreux
composés qui résultent de la pyrolyse du tabac. Ceux-ci peuvent, soit
traverser la cigarette dans la fumée constituant le courant
principal-certains d'entre eux se condensant légèrement en arrière du
cône incandescent-, soit passé dans l'air à partir de l'extrémité
incandescente, dans la fumée qui constitue le courant latéral. A chaque
bouffée, ces composés se concentrent dans la fumée car les produits qui
s'étaient déjà condensés viennent s'y ajouter -- la zone de condensation
se réduisant à mesure que la cigarette se raccourcit. La nature
physicochimique de la fumée dépend du traitement subi par le tabac et de
sa combustion, de la porosité et du traitement du papier et du type de
bout-filtre (Hoffmann & Hoffmann, 1997). Dans le cas d'une cigarette ou
de ce que l'on appelle un "bidi" en Asie (du tabac roulé dans la feuille
d'une plante), la composition chimique de la fumée dépend de facteurs
tels que les dimensions et la porosité de l'enveloppe ainsi que des
paramètres du fumage : volume, fréquence et durée des bouffées (NIH,
1998). Les variations de composition chimique concernent davantage la
proportion des différents constituants que la présence ou l'absence de
tel ou tel composé.
La fumée du courant principal est produite à l'intérieur du cône
incandescent dans une atmosphère relativement pauvre en oxygène à une
température de combustion de 850-950°C. Au départ, les particules de
cette fumée ont un diamètre aérodynamique massique médian (DAMM) de 0,2
à 0,3 µm; toutefois, dès qu'elles pénètrent dans les voies
respiratoires, où le degré d'humidité est de 100%, elles s'agrègent pour
former des particules de plus grande taille et se comportent alors comme
si leur DAMM était de l'ordre du micromètre. Environ 50 à 90% des
particules inhalées peuvent être retenues dans les voies respiratoires
(Wynder & Hoffmann, 1967; Hinds et al., 1983). Pour des raisons d'ordre
dimensionnel, les particules présentes dans l'aérosol, les constituants
de la phase gazeuse et les gaz permanents sont capables d'atteindre les
alvéoles lors de l'inhalation de la fumée. Le comportement des
constituants hydrophiles en présence d'une forte humidité fait que le
dépôt dans l'arbre trachéobronchique revêt un caractère complexe, mais
de toute manière, la fumée s'insinue dans la totalité des voies
respiratoires.
Près de 4000 constituants ont été répertoriés dans la fumée du courant
principal à côté d'un nombre indéterminés de substances non identifiées
(Roberts, 1988). La fumée du courant principal comporte une phase
particulaire et une phase gazeuse. La phase particulaire contient de la
nicotine, des nitrosamines telles que la
4-(méthylnitrosamino)-1-(3-pyridyl)-1-butanone (NKK) et la
N-nitrosonornicotine (NNN), des métaux comme le cadmium, le nickel, le
zinc et le polonium-210, des hydrocarbures polycycliques et des amines
cancérogènes comme le 4-aminobiphényle. La phase gazeuse renferme du
monoxyde et du dioxyde de carbone, du benzène, de l'ammoniac, du
formaldéhyde, du cyanure d'hydrogène, de la N-nitrosodiméthylamine, de
la N-nitrosodiéthylamine et un certain nombre d'autres composés. Les
composés présents dans la fumée de tabac peuvent, selon leurs effets
biologiques, être classés en asphyxiants, irritants, ciliatoxines,
mutagènes, cancérogènes, inhibiteurs d'enzymes, neurotoxines ou dérivés
dotés d'action pharmaceutique. C'est principalement par les voies
respiratoires que la fumée de tabac pénètre dans l'organisme mais de
nombreux constituants, présents en particulier dans la fumée de pipe et
de cigare, se dissolvent dans la salive et sont soit avalés, soit
absorbés au niveau de la cavité buccale. Les fumeurs de pipe et de
cigare n'inhalent généralement pas la fumée, qui demeure dans la cavité
buccale où, on vient de le voir, elle se dissout dans la salive et peut
être soit absorbée par passage à travers la muqueuse buccale, soit être
directement avalée (NIH, 1998). Les boissons alcoolisées, par leur effet
solvant sur les constituants de la fumée, en facilitent la résorption.
5. Fumée du courant latéral
La fumée du courant latéral est généralement produite à une température
de combustion plus faible (500-600°C) dans une atmosphère réductrice.
Les particules de cette fumée ont, lorsqu'elles sont fraîchement émises,
une taille à peu près équivalente à celle des particules du courant
principal, avec un diamètre aérodynamique massique médian (DAMM)
d'environ 0,2 µm. Quantitativement, la composition de la fumée du
courant latéral est analogue à celle de la fumée du courant principal.
Certaines substances du courant latéral sont émises à une concentration
(rapportée à 1 g de tabac brûlé) plus élevée que les constituants du
courant principal. C'est notamment le cas de composés cancérogènes comme
la N-nitrosodiméthylamine et la N-nitrosodiéthylamine ou encore de
métaux comme le nickel et le cadmium. Beaucoup de dérivés cancérogènes
sont plus concentrés dans la fumée du courant latéral que dans celle du
courant principal. Des épreuves biologiques consistant à badigeonner la
peau de souris avec un condensant de fumée du courant latéral ont montré
que ce dernier est plus cancérogène que celui de la fumée du courant
principal (Wynder & Hoffmann, 1967; US Surgeon General, 1986; NIH,
1998).
6. La manière de fumer la cigarette et ses effets sur la toxicité de la
fumée
Les cigarettes n'ont pas toutes la même teneur en nicotine et le fumeur
"tire" plus ou moins fort en inhalant plus ou moins profondément pour
satisfaire son besoin de nicotine. Il s'ensuit qu'en fumant une
cigarette à bout-filtre pauvre en nicotine (< 1,2 mg) le fumeur va
tirer plus intensément, ce qui ne sera pas sans effet sur la toxicité
(NIH, 1998).
7. Résumé des conclusions et des recommandations
La consommation de tabac, notamment en le fumant, constitue un problème
de santé publique d'une extrême importance en raison de la morbidité et
de la mortalité qui en résultent. Outre les effets nocifs causés par une
utilisation active du tabac, on a montré qu'il en existe aussi qui
résultent de l'exposition passive à la fumée présente dans
l'environnement. Les dangers du tabagisme sont également accrus par la
possibilité d'interactions avec certains agents chimiques, physiques ou
biologiques présents sur les lieux de travail ou dans l'environnement en
général. On connaît certes quelques cas d'interactions antagonistes,
mais les risques inhérents au tabagisme l'emportent de très loin sur ses
effets protecteurs apparents. Tout doit être mis en oeuvre pour faire
cesser la consommation de tabac et en particulier l'habitude de fumer.
Il faut s'opposer très vigoureusement au tabagisme sur les lieux
publics. En outre, pour éviter des interactions avec d'autres types
d'exposition tout en éliminant le risque d'exposition passive à la fumée
de tabac, il faut interdire de fumer sur les lieux de travail.
Il faut aussi vivement inciter les gens à ne pas fumer à la maison afin
de protéger la santé de la famille et notamment celle des enfants. On
évitera ainsi également des interactions potentiellement dangereuses
avec d'autres types d'exposition pouvant survenir dans l'environnement
domestique. Il est nécessaire d'établir sans attendre des programmes
éducatifs sur les dangers du tabagisme pour la santé. Les professionnels
de la santé doivent prêter assistance aux personnes qui désirent cesser
de fumer. Comme le tabagisme peut entraîner une modification des
réactions aux médicaments ou autres formes de traitement, voire susciter
à leur encontre des réactions indésirables, les médecins doivent ajuster
les doses de leurs patients en conséquence et surveiller leurs
réactions.
PANORAMA GENERAL
1. Introducción
El consumo de tabaco, particularmente el hábito de fumar, provoca una
serie de efectos nocivos para salud, está directamente relacionado con
varias enfermedades graves y puede aumentar los efectos adversos de
otros agentes químicos, físicos y biológicos. Si no se controlan, los
agentes químicos y de otro tipo pueden producir en el puesto trabajo
enfermedades, discapacidades y la muerte prematura. Es evidente que en
el lugar de trabajo los efectos adversos pueden deberse a la interacción
sinérgica del humo de tabaco con otros peligros. La mayor parte de las
interacciones de los constituyentes nocivos del humo de tabaco con
sustancias químicas tóxicas se producen cuando estas últimas están en el
aire, aunque se han notificado asimismo interacciones del humo con
agentes perjudiciales ingeridos y/o absorbidos.
El consumo de tabaco está generalizado en todo el mundo, desde los
países de bajos ingresos hasta los industrializados más ricos. Lo
utilizan hombres y mujeres, niños y adultos, y millones de personas
están involuntariamente expuestas al humo de tabaco en su entorno. Si
bien hay numerosas explicaciones del hábito de fumar, la razón principal
de su ubicuidad es el efecto adictivo de la nicotina, droga presente en
todas las formas de la hoja de tabaco y que llega al consumidor en
cantidades variables según los distintos tipos de consumo (capítulo 2).
La aparición del cigarrillo, de producción masiva, fácil de obtener,
relativamente económico y ligero de peso, de manera que se puede llevar
en la boca dejando las manos libres, ha tenido repercusiones importantes
en el hábito de fumar, tanto en general como en el puesto de trabajo.
En muchos países se reconoce que el humo de tabaco constituye un peligro
grave para la salud y es un factor que contribuye de manera importante
a la muerte causada por diversas enfermedades comunes. En esos países se
ha aplicado una legislación de alerta sanitaria y medidas impositivas
para el control de su consumo, así como programas de educación del
público sobre los peligros del tabaco y las ventajas de no comenzar a
fumar o de interrumpir el consumo. Sin embargo, todavía hay países donde
no se han puesto en marcha medidas decisivas para abordar el problema
del consumo de tabaco.
Son muchas las situaciones laborales que conllevan un elemento de
riesgo. El tipo de trabajo puede generar efectos nocivos para salud y
las actividades laborales pueden provocar la contaminación de medio
ambiente. El propio cultivo del tabaco requiere el uso de plaguicidas,
la recolección de la hoja puede ocasionar trastornos debido a la
absorción de nicotina a través de la piel y su elaboración expone a los
trabajadores a peligros para la salud provocados por el polvo y las
esporas de hongos presentes en el aire. Se ha notificado una elevada
incidencia de cáncer en el sexo masculino en zonas con industrias
tabaqueras. En la minería existe polvo de minerales en el aire y en la
agricultura y la industria basadas en materias primas producidas
biológicamente hay polvo biológico. Durante las actividades de soldadura
se produce humo y en muchas industrias crean peligro los gases, humos,
neblinas y vapores cargados de sustancias orgánicas y/o inorgánicas
tóxicas. Un calor excesivo o la exposición a luz ultravioleta pueden
ser perjudiciales para el bienestar de los trabajadores. Está admitido
que las radiaciones ionizantes en la minería y en la tecnología moderna
son un peligro en el lugar de trabajo. En numerosas actividades, los
trabajadores están expuestos a un ruido excesivo o a vibraciones
mecánicas peligrosas. Estas condiciones laborales pueden afectar más
negativamente a la salud de las personas fumadoras que a la de las no
fumadoras. Son muchos los países en los que está prohibido fumar en el
trabajo, fundamentalmente por razones de seguridad en cuanto
incendios/explosiones. Sin embargo, en algunos países no siempre se
cumple la reglamentación. En varios países recientemente
industrializados no se han abordado todavía plenamente los problemas de
salud asociados con el trabajo y muchos empleadores y trabajadores
desconocen los peligros para la salud de sus actividades. Además, existe
un amplio "sector extraoficial" de la industria, particularmente en los
países en desarrollo, en que se trabaja en el hogar y se utilizan
sustancias químicas (en particular disolventes, resinas y colorantes
sintéticos), estando expuesta toda la familia, y no hay restricciones
sobre la exposición a los peligros en el trabajo o al humo.
Está mucho menos definida la situación en relación con los efectos
adversos en la salud derivados de la exposición combinada al humo de
tabaco -- de la corriente principal o del medio ambiente -- y a los
agentes del entorno doméstico. Sin embargo, la incidencia de cáncer de
pulmón y la concentración de radón en los hogares tiene una relación
dosis-respuesta similar a la que se produce entre el cáncer de pulmón y
la concentración de radón en las minas, y el riesgo es más elevado para
los fumadores.
2. Ejemplos de efectos combinados de la exposición al humo de
tabaco y a otras sustancias
Está demostrada la existencia de sinergia en la producción de efectos
nocivos (cáncer) entre el humo de tabaco y la exposición al arsénico, el
amianto, el etanol, el silicio y las radiaciones (radón, bomba atómica,
rayos X). Por otra parte, hay pruebas de antagonismo en el caso del humo
de tabaco y los clorometiléteres carcinogénicos, es decir, el
clorometilmetiléter (CMME) y el bis(clorometil)éter (BCME) (Hoffmann y
Wynder, 1976; CIIC, 1986), el humo de tabaco y la alveolitis alérgica y
el humo de tabaco y la beriliosis crónica. El humo de tabaco influye en
el riesgo para la salud de la exposición en la extracción de carbón, el
manejo de plaguicidas y las industrias del caucho y el petróleo. Los
trabajadores de las minas de carbón que fuman tienen un riesgo más
elevado de contraer bronquitis crónica y enfermedades obstructivas de
las vías respiratorias, pero no enfisema. El cáncer de pulmón de los
mineros del carbón se ha atribuido totalmente al humo de tabaco. El humo
de tabaco puede aumentar el riesgo para salud de la exposición a polvos
vegetales que producen trastornos respiratorios crónicos, como la
bisinosis debida al polvo del algodón y el cáncer nasal provocado por el
polvo de la madera.
3. Composición de la hoja del tabaco y del humo de tabaco
De las hojas de tabaco elaboradas se han aislado más de 3040 compuestos
químicos (Roberts, 1988). La mayoría son constituyentes de la hoja, pero
la presencia de algunos depende de las condiciones de cultivo, como el
suelo y la atmósfera de la zona, mientras que otros se derivan del uso
de productos químicos agrícolas, de revestimientos, humectantes y
aromatizantes añadidos a las hojas y de los métodos de curado. Las
diferentes variedades de tabaco que se cultivan en los distintos países,
así como las distintas formas de curado y elaboración, ponen de
manifiesto diversas diferencias. Las proporciones de los distintos
constituyentes pueden ser diferentes, pero no la composición general.
Entre los compuestos tóxicos importantes identificados, aparte de la
nicotina, figuran nitrosaminas carcinogénicas, derivadas de nitritos,
aminas, proteínas y alcaloides presentes en la hoja, hidro-carburos
aromáticos policíclicos procedentes del proceso de curado, elementos
radiactivos absorbidos del suelo y el aire, y cadmio en el tabaco
cultivado en suelos ricos en este elemento. Cuando se quema tabaco al
fumar, se forman numerosos productos derivados de la pirólisis y de
otras reacciones.
4. Corriente principal del humo de tabaco
El humo del tabaco es un aerosol formado por una fase particulada de
gotitas de líquido dispersas en una fase de gas/vapor. Al fumar un
cigarrillo se forman numerosos compuestos por la pirólisis del tabaco.
Éstos pasan a través del cigarrillo en la corriente principal del humo,
condensándose algunos a corta distancia detrás del cono de combustión,
o bien se emiten en el aire a partir del extremo que se quema como humo
lateral. En cada bocanada el humo se hace progresivamente más fuerte,
porque se le añade material previamente condensado y porque la longitud
del cigarrillo disponible para la ulterior condensación disminuye. Las
características fisicoquímicas del humo dependen de la elaboración y la
combustión del tabaco, la porosidad y el tratamiento del papel en el que
está envuelto y del tipo de filtro (Hoffman y Hoffman, 1997). En el caso
de un cigarrillo o "bidi" asiático (tabaco envuelto en hoja vegetal), la
química del humo se ve afectada por factores tales como las dimensiones,
la porosidad de la envoltura y los parámetros del volumen, la frecuencia
y la duración de la bocanada de humo (NIH, 1998). Las variaciones en la
química del humo se dan fundamentalmente en la proporción entre sus
constituyentes, más que en la presencia o ausencia de compuestos
concretos.
El humo de la corriente principal se genera en una atmósfera con un
contenido comparativamente bajo de oxígeno a una temperatura de
combustión de 850-950°C en el cono de combustión. Al principio, las
partículas presentes en la corriente principal tienen un diámetro
aerodinámico medio de la masa (MMAD) de 0,2 a 0,3 micras; sin embargo,
en cuanto llegan al tracto respiratorio, con una humedad del 100%, se
unen para formar partículas mayores y se comportan como si su MMAD fuera
del orden de micras. En el tracto respiratorio se puede retener
alrededor del 50-90% de todas las partículas inhaladas (Wynder y
Hoffmann, 1967; Hinds et al., 1983). Por lo que se refiere al tamaño, al
inhalar el humo pueden llegar a los alvéolos las partículas en aerosol,
los constituyentes de la fase de vapor y los gases permanentes. La
deposición en el árbol traqueobronquial se ve complicada por el
comportamiento de los constituyentes hidrofílicos en condiciones de
humedad elevada, pero el humo llega a todas las partes de las vías
respiratorias.
El humo de la corriente principal contiene cerca de 4000 sustancias
químicas identificadas y un número desconocido de sustancias químicas
sin identificar (Roberts, 1988). El humo de la corriente principal se
puede dividir en una fase de partículas y otra de gas. La fase de
partículas contiene nicotina, nitrosaminas como la
4-(metilnitrosamino)-1-(3-piridil)-1-butanona (NNK) y la
N-nitrosonornicotina (NNN), metales como el cadmio, el níquel, el zinc
y el polonio-210, hidrocarburos policíclicos y aminas carcinogénicas,
como el 4-aminobifenilo. La fase de vapor contiene monóxido de carbono,
anhídrido carbónico, benceno, amoníaco, formaldehído, cianuro de
hidrógeno, N-nitrosodimetilamina, N-nitrosodietilamina y otros
compuestos. Los compuestos del humo de tabaco se pueden clasificar por
su actividad biológica como asfixiantes, irritantes, ciliatoxinas,
mutágenos, carcinógenos, inhibidores de las enzimas, neurotoxinas o
compuestos farmacológicamente activos. El principal punto de entrada del
humo del cigarrillo en el organismo es por las vías respiratorias, pero
muchos constituyentes, en particular del humo de pipa y de cigarro, se
disuelven en la saliva y se absorben en la cavidad bucal o se ingieren.
Los fumadores de cigarros y de pipa no suelen inhalar el humo, que
permanece en la cavidad bucal, se disuelve en la saliva y se absorbe a
través de las membranas mucosas o se ingiere (NIH, 1998). Las bebidas
alcohólicas tienen un efecto disolvente de los constituyentes del humo,
facilitando su absorción.
5. Humo de tabaco lateral
El humo lateral se forma con una temperatura de combustión más baja
(500-600°C) en una atmósfera reductora. Las partículas del humo lateral
fresco son prácticamente del mismo tamaño que las de la corriente
principal, con un diámetro aerodinámico medio de la masa de alrededor de
0,2 micras. Desde el punto de vista cualitativo, la composición del humo
lateral es semejante a la del humo de la corriente principal. Algunas
sustancias químicas se emiten en el humo lateral con una concentración
mayor por gramo de tabaco quemado que en el humo de la corriente
principal. Esto es particularmente aplicable a carcinógenos como la
N-nitrosodimetilamina y la N-nitrosodietilamina y a metales como el
níquel o el cadmio. Muchos compuestos carcinógenos están más
concentrados en el humo lateral que en el principal. En biovaloraciones
con aplicación a la piel de ratones se ha demostrado que el humo lateral
condensado es más carcinogénico que el principal (Wynder y Hoffmann,
1967; US Surgeon General, 1986; NIH, 1998).
6. Efectos de la manera de fumar los cigarrillos en la
toxicidad del humo
El contenido de nicotina de los distintos cigarrillos varía, y el
fumador, para satisfacer la necesidad adquirida de nicotina, la ajusta
mediante la intensidad con la que fuma y la profundidad de la
inhalación. Por consiguiente, el fumador de cigarrillos con filtro y de
contenido bajo en nicotina (< 1,2 mg) fuma con mayor intensidad, y esto
influye en la toxicidad (NIH, 1998).
7. Resumen de las conclusiones y recomendaciones
El consumo de tabaco, en particular el hábito de fumar, representa un
peligro para la salud pública de la máxima importancia y es una causa
prevenible importante de morbilidad y mortalidad. Además de los efectos
adversos del consumo activo de tabaco para la salud, se han demostrado
efectos adversos derivados de la exposición al humo de tabaco presente
en el medio ambiente. Los riesgos del hábito de fumar también aumentan
como consecuencia de las interacciones con ciertos peligros químicos,
físicos y biológicos existentes en el lugar de trabajo y en el medio
ambiente general. Hay un pequeño número de casos de interacciones
antagonistas, pero los riesgos del humo de tabaco para la salud son muy
superiores a cualquier efecto protector aparente.
Se deben adoptar todas las medidas posibles para eliminar el consumo de
tabaco, en particular el hábito de fumar, y se ha de disuadir con
firmeza de fumar en lugares públicos. A fin de evitar la interacción con
otros tipos de exposición ocupacional y de eliminar el riesgo de
exposición al humo de tabaco del medio ambiente, debería prohibirse
fumar en el lugar de trabajo.
Con objeto de proteger la salud, en particular la de los niños, se
debería desalentar con firmeza el hábito de fumar en el hogar. De esta
manera se previenen posibles interacciones perjudiciales entre el humo
de tabaco y la exposición a otros peligros en la vivienda. Hay una
necesidad imperiosa de programas educativos sobre los peligros del
hábito de fumar para la salud. Los profesionales de la salud deberían
prestar asistencia para ayudar a los fumadores a dejar este hábito.
Debido a que el humo puede provocar una alteración de la respuesta a los
medicamentos y otros tratamientos o una reacción adversa a éstos, los
médicos deberían estudiar la posibilidad de introducir ajustes
apropiados de la dosificación y vigilar a los pacientes.
 WHO WORKING GROUP ON HEALTH RISKS FROM THE COMBINED EFFECTS OF TOBACCO
SMOKING AND EXPOSURE TO OTHER CHEMICALS
(Geneva, 25-26 April 1966)
Members
Dr G. Finch, Inhalation Toxicology Research Institute, Lovelace
Biomedical and Environmental Research Institute, Albuquerque, New
Mexico, USA
Professor G. Pershagen, Division of Epidemiology, Institute of
Environmental Medicine, Karolinska Institute, Stockholm, Sweden
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
Professor H.-P. Witschi, Institute of Toxicology and Environmental
Health, University of California, Davis, California, USA
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr N.F. Collishaw, Tobacco or Health, Programme on Substance
Abuse, World Health Organization, Geneva, Switzerland
WHO TASK GROUP ON HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE
AND EXPOSURE TO OTHER AGENTS
(Geneva, 18-21 February 1997)
Members
Dr G. Finch, Lovelace Respiratory Research Institute, Inhalation
Toxicology Research Institute, Albuquerque, New Mexico, USA
( Chairman)
Dr L. Fishbein, Fairfax, VA, USA ( Joint Rapporteur)
Dr Dorota Jarosinska, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Professor G. Kazantzis, Royal School of Mines, London, United
Kingdom ( Joint Rapporteur)
Professor U. Keil, Institute for Epidemiology and Social Medicine,
Münster, Germany
Dr D. Krewski, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
( Vice-Chairman)
Dr L. van Bree, Laboratory of Health Effects Research, National
Institute of Public Health and the Environment, Bilthoven, The
Netherlands
Observers
Dr G. Minotti, Catholic University of the Sacred Heart, Rome, Italy
( representing the International Union of Pharmaceutical
Societies)
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
Mrs B.F. Goelzer, Occupational Health, World Health Organization,
Geneva, Switzerland
Mr N.E. Collishaw, Programme on Substance Abuse, World Health
Organization, Geneva, Switzerland
HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE AND EXPOSURE TO
OTHER AGENTS
A WHO Task Group on Health Effects of Interactions Between Tobacco Use
and Exposure to Other Agents met at the World Health Organization,
Geneva, from 18 to 21 February 1997. Dr E. Smith, IPCS, welcomed the
participants on behalf of Dr M. Mercier, Director IPCS, and the
cooperating organizations. The Task Group reviewed and revised the
draft monograph and developed a new text.
The first draft of the monograph was prepared by Dr K. Rothwell,
Knaresborough, Yorkshire, United Kingdom. This draft was further
developed by a Working Group held in WHO, Geneva, 25-26 April 1996,
and then circulated for international comment to IPCS contact points
for Environmental Health Criteria monographs. Comments were
incorporated in a second draft prepared by Dr K. Rothwell. This draft
was reviewed at the Task Group meeting and a text given further
limited circulation to Task Group members and a number of other
experts, including the US EPA National Center for Environmental
Assessment under the coordination of Dr D. Mukerjee, for final
comment. In the development of the monograph, contributions were made
by a number of authors listed below.
Dr E. Smith (IPCS Unit for the Assessment of Risk and Methods) was
responsible for the scientific content of the monograph and Dr P.G.
Jenkins (IPCS Central Unit) for the technical editing.
The efforts of all who helped in the preparation and finalization of
the monograph are gratefully acknowledged.
The authors were:
Main authors
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
( Coordinating author)
Dr G. Finch, Lovelace Respiratory Research Institute, Albuquerque,
New Mexico, USA
Dr L. Fishbein, Fairfax, Virginia, USA
Dr D. Krewski, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Professor G. Pershagen, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden
Professor H-P. Witschi, Institute of Toxicology and Environmental
Health, University of California, Davis, California, USA
Contributing authors
Dr D. Jarosinska, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Professor G. Kazantzis, Royal School of Mines, London, United
Kingdom
Professor U. Keil, Institute for Epidemiology and Social Medicine,
Munster, Germany
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr L. van Bree, National Institute of Public Health and the
Environment, Bilthoven, the Netherlands
* * *
The IPCS expresses its gratitude to the external reviewers who
provided comments and other relevant material, in particular the
United Kingdom Department of Health, London, and the US Environmental
Protection Agency's Office of Research and Development, National
Center for Environmental Assessment, Cincinnati, Ohio, USA.
The funds for the preparation, review and publication of this
monograph were generously provided by Health Canada.
ABBREVIATIONS
BCME bis(chloromethyl) ether
CMME chloromethyl methyl ether
COLD chronic obstructive lung disease
CHD coronary heart disease
CVD cardiovascular disease
ERR excess relative risk
FIV1 forced expiratory volume (1 second)
IARC International Agency for Research on Cancer
LET linear energy transfer
MMAD mass median aerodynamic diameter
NOx nitrogen oxides
SAD small airways disease
TSNA tobacco-specific nitroasamines
URT upper respiratory tract
1. OVERVIEW
1.1 Introduction
Tobacco use, particularly smoking, causes a range of adverse health
effects, is directly implicated in a number of serious diseases, and
can increase adverse effects of other chemical, physical and
biological agents. Chemicals and other agents in workplaces can cause,
if not controlled, disease, incapacity and early death. In the
workplace it is clear that adverse effects can be produced by the
synergistic interaction of tobacco smoking and other hazards. The
majority of interactions of harmful tobacco smoke constituents with
toxic chemicals occur when the latter are airborne, although
interactions of smoking with ingested and/or absorbed harmful agents
have also been reported.
Tobacco use is widespread throughout the world, from countries with
low income economies to the most affluent industrialized nations.
Tobacco is used by men and women, by children and adults, and millions
of people are involuntarily subjected to environmental tobacco smoke.
There are numerous explanations for the tobacco habit but the main
reason for its ubiquity is the addictive drug nicotine present in all
forms of tobacco leaf and delivered in varying amounts to the user by
the various methods of tobacco use (chapter 2). The advent of the
cigarette, mass produced, easily obtainable, relatively cheap and
light in weight, so it can be held in the mouth leaving the hands
free, has had a major impact on smoking habits, in general and in
workplaces.
In many countries tobacco smoking is recognized as a serious health
hazard and a major contributing factor to deaths from a number of
common diseases. In these countries health warning legislation and
measures to control consumption by taxation have been implemented, as
well as public education programmes on the dangers of smoking and the
benefits to be gained from not starting or from stopping. However,
there are still countries where decisive action has yet to be taken to
deal with the problem of tobacco use.
Many work situations involve an element of risk. The nature of the
work may generate harmful effects on health, and working activities
may cause environmental contamination. Tobacco growing itself involves
the use of pesticides, harvesting of tobacco leaf can cause sickness
due to skin absorption of nicotine, and processing exposes workers to
health hazards from airborne dust and fungal spores. A high male
cancer incidence has been reported in areas with tobacco industries.
In mining there are airborne mineral dusts and, in farming and
industries using biologically produced raw materials, biological dusts
are found. Fumes are produced during welding, and gases, smokes, mists
and vapours containing inorganic and/or organic toxic substances
present hazards in many industries. Excessive heat or exposure to
ultraviolet light can be detrimental to the well-being of workers.
Ionizing radiation in mining and modern technology is recognized as a
workplace hazard. In many occupations workers are subjected to
excessive noise or harmful mechanical vibration. Working conditions
can impact adversely on health to a greater extent in smokers than
non-smokers. In many countries, smoking at the workplace is
prohibited, primarily for reasons of fire/explosion safety. However,
in some countries, regulations are not always enforced. In some newly
industrializing countries health problems associated with work have
not yet been fully addressed and many employers and workers are
ignorant of the dangers to health of their occupations. In addition,
there is the large "informal sector" of industry, particularly in
developing countries, where the home is the workplace, chemicals are
used (including solvents, resins, and synthetic dyestuffs), the whole
family is exposed, and there are no restrictions on exposure to work
hazards or smoking.
The situation for adverse health effects resulting from combined
exposure to tobacco smoke, mainstream or environmental, and agents in
the domestic environment is much less defined. However, the incidence
of lung cancer and the concentration of radon in homes has a similar
dose-response to lung cancer and radon in mines, and the risk is
higher in smokers.
1.2 Examples of combined effects of tobacco smoking and other
exposures
There is evidence for synergism in the production of adverse effects
(cancer) between tobacco smoking and exposure to arsenic, asbestos,
ethanol, silica and radiation (radon, atomic bomb, X-ray). On the
other hand there is evidence for antagonism in the case of tobacco
smoking and the carcinogenic chloromethyl ethers, i.e. chloromethyl
methyl ether (CMME) and bis(chloromethyl) ether (BCME) (Hoffmann &
Wynder, 1976; IARC, 1986), tobacco smoking and allergic alveolitis,
and tobacco smoking and chronic beryllium disease. Tobacco smoking
affects the health risks of exposures in coal mining, pesticide
handling, and in the rubber and petroleum industries. Coal miners who
smoke are at greater risk of developing chronic bronchitis and
obstructive airway disease but not emphysema. Lung cancer in coal
miners has been attributed entirely to tobacco smoking. Tobacco
smoking can increase the health risks of exposure to vegetable dusts
that produce chronic respiratory conditions, such as byssinosis
produced by cotton dust, and nasal cancer caused by wood dusts.
1.3 Composition of tobacco leaf and tobacco smoke
More than 3040 chemical compounds have been isolated from processed
tobacco leaf (Roberts, 1988). Most are leaf constituents, but some
arise from growing conditions such as the soil and atmosphere in an
area, while others originate from the use of agricultural chemicals,
from casings, humectants and flavourings added to the leaves, and from
curing methods. Different tobacco varieties grown in different
countries, and cured and processed in various ways show differences.
The proportions of individual constituents may differ but not the
overall composition. Among important toxic compounds identified, other
than nicotine, are carcinogenic nitrosamines, derived from nitrites,
amines, proteins and alkaloids present in the leaf, polycyclic
aromatic hydrocarbons resulting from the curing process, radioactive
elements absorbed from the soil and the air, and cadmium in tobacco
grown on cadmium-rich soils. When tobacco is burned in the course of
smoking, many pyrolysis and other reaction products are formed.
1.4 Mainstream tobacco smoke
Tobacco smoke is an aerosol consisting of a particulate phase of
liquid droplets dispersed in a gas/vapour phase. When a cigarette is
smoked, many compounds are formed by pyrolysis of the tobacco. These
either pass through the cigarette as mainstream smoke, some being
condensed a short distance behind the burning cone, or they are
emitted into the air from the burning end as sidestream smoke. With
each puff the smoke becomes progressively stronger because previously
condensed material is added to the smoke and the length of cigarette
available for further condensation is decreasing. The physicochemical
nature of the smoke depends on the processing and burning of the
tobacco, the porosity and treatment of the paper wrapper, and on the
type of filter tip (Hoffmann & Hoffmann, 1997). In the case of a
cigarette or Asian "bidi" (tobacco wrapped in vegetable leaf), the
smoke chemistry is affected by such factors as dimensions, wrapper
porosity and the smoking parameters of puff volume, frequency and
duration (NIH, 1998). Variations in smoke chemistry are mainly in the
balance of smoke constituents rather than the presence or absence of
particular compounds.
Mainstream smoke is generated in a comparatively low-oxygen atmosphere
at a burning temperature of 850-950°C in the fire cone. Initially,
mainstream smoke particles have a mass median aerodynamic diameter
(MMAD) of 0.2 to 0.3 µm; however, as soon as they encounter the 100%
humidity of the respiratory tract, they coalesce into larger particles
and behave as if their MMAD was in the micrometre range. Between 50
and 90% of all inhaled particulate matter may be retained in the
respiratory tract (Wynder & Hoffmann, 1967; Hinds et al., 1983). From
size considerations, the aerosol particulate matter, the vapour phase
constituents and the permanent gases are capable of reaching the
alveoli when smoke is inhaled. Deposition in the tracheobronchial tree
is complicated by the behaviour of hydrophilic constituents in the
high humidity conditions, but smoke reaches every part of the airways.
Mainstream smoke contains nearly 4000 identified chemicals and an
unknown number of unidentified chemicals (Roberts, 1988). Mainstream
smoke can be divided into particulate and gas phases. Mainstream smoke
particulate phase contains nicotine, nitrosamines such as
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and
N-nitrosonornicotine (NNN), metals such as cadmium, nickel, zinc and
polonium-210, polycyclic hydrocarbons, and carcinogenic amines, such
as 4-aminobiphenyl. The vapour phase contains carbon monoxide, carbon
dioxide, benzene, ammonia, formaldehyde, hydrogen cyanide,
N-nitrosodimethylamine, N-nitrosodiethylamine and other compounds.
Compounds in tobacco smoke can be classified by their biological
activity as asphyxiants, irritants, ciliatoxins, mutagens,
carcinogens, enzyme inhibitors, neurotoxins or pharmacologically
active compounds. The main point of entry of cigarette smoke into the
body is via the airways, but many constituents, particularly from pipe
and cigar smoke, dissolve in saliva and are absorbed in the buccal
cavity or are swallowed. Cigar and pipe smokers generally do not
inhale the smoke and it remains in the oral cavity, is dissolved in
the saliva and absorbed through the mucous membranes or swallowed
(NIH, 1998). Alcoholic drinks have a solvent effect for the smoke
constituents facilitating their absorption.
1.5 Sidestream tobacco smoke
Sidestream smoke is generated at lower burning temperature (500-600°C)
in a reducing atmosphere. Fresh sidestream smoke particles are about
the same size as mainstream smoke particles with a mass median
aerodynamic diameter (MMAD) of approximately 0.2 µm. Qualitatively,
sidestream smoke composition is similar to the composition of
mainstream smoke. Some chemicals in sidestream smoke are emitted at
higher concentrations per gram of tobacco burned than in mainstream
smoke. This is particularly so for carcinogens such as
N-nitrosodimethylamine and N-nitrosodiethyl-amine, and for metals
such as nickel or cadmium. Many carcinogenic compounds are more
concentrated in sidestream than in mainstream smoke. Mouse
skin-painting bioassays have shown that condensate of sidestream smoke
is more carcinogenic than that of mainstream smoke (Wynder & Hoffmann,
1967; US Surgeon General, 1986; NIH, 1998).
1.6 Effects of ways of cigarette smoking on smoke toxicity
The nicotine content of different cigarettes varies, and the smoker
adjusts the smoking intensity and depth of inhalation to satisfy the
acquired nicotine need. Consequently, the smoker of a filter cigarette
with a low nicotine yield (<1.2 mg) smokes more intensively and this
influences toxicity (NIH, 1998).
1.7 Summary of conclusions and recommendations
Tobacco use, particularly smoking, is a most important public health
hazard and a major preventable cause of morbidity and mortality. In
addition to the adverse health effects of active tobacco use, adverse
health effects have been demonstrated to result from exposure to
environmental tobacco smoke. The risks from tobacco smoking are also
increased through interactions with certain chemical, physical and
biological hazards found in the workplace and general environment.
There are a few instances of antagonistic interactions, but the health
risks of tobacco smoke far outweigh any apparent protective effects.
All possible measures should be taken to eliminate tobacco use,
particularly smoking, and smoking in public places should be strongly
discouraged. To avoid interaction with occupational exposures and to
eliminate the risk of exposure to environmental tobacco smoke, smoking
in the workplace should be prohibited.
To protect health, in particular that of children, smoking in domestic
environments should be strongly discouraged. This will prevent
possible harmful interactions between tobacco smoke and residential
exposures to other hazards. There is a pressing need for educational
programmes on the health hazards of smoking. Health professionals
should provide assistance to help smokers quit. Since smoking may
result in altered response or adverse reactions to drugs and other
treatments, appropriate dose adjustments and patient surveillance
should be taken into consideration by clinicians.
2. EXPOSURE TO TOBACCO PRODUCTS AND HEALTH RISKS FROM TOBACCO USE
2.1 Tobacco and its uses
2.1.1 Introduction
The genus Nicotiana, a member of the plant family Solanaceae, is
represented by about 100 species and sub-species (Cromwell, 1955; Tso,
1990) widely distributed throughout the world. The species N.
tabacum and N. rustica are the principal sources of tobacco. The
primary intention in using tobacco is to obtain the alkaloid nicotine
and, once the habit has been established, nicotine appears to fulfil
both a pharmacological and psychological need (US Surgeon General,
1988; NIH, 1998).
Tobacco and its uses were unknown outside America before its discovery
by Columbus. In a description of tobacco use in South America at that
period, Wilbert (1987) used information from European explorers.
There were six types of tobacco use: chewing, drinking, licking,
rectal insertion, nasal and oral snuffing, and smoking. Smoking was by
far the most common, while rectal application was only used
occasionally. Tobacco smoke was inhaled via the mouth through tubes or
rolls of tobacco leaf, or through the nostrils using a Y-shaped tube.
Alternatively, it was swallowed and belched back, blown from one
person to another, or blown into the eyes. Leaves were chewed, alone
or mixed with ash, powdered shells or honey, or they were held in the
mouth and sucked. Infusions were drunk or a concentrated infusion was
licked or even used as an enema. Many ways of preparing and taking
snuff among different tribes were reported. Tobacco use was linked to
religious rituals.
The rest of the world adopted tobacco use in the forms of smoking,
chewing and snuffing. Smoking has remained the most popular. Tobacco
smoking consists of burning the cured leaf, perhaps mixed with
fragrant additives, in a pipe or tube. Cigarette smoking has largely
replaced other methods in many countries. Reasons for the popularity
of cigarettes include their ready availability resulting from
large-scale manufacture and their greater convenience over other forms
of tobacco use (IARC, 1986). In developed countries, cigarettes
account for at least 80% of overall tobacco consumption, but in most
developing countries other ways of using tobacco predominate, although
cigarette smoking is increasing. Fig. 1 shows estimated daily
cigarette consumption on a regional basis, Tables 1 and 2 show daily
smoking prevalences on a regional and national basis and Table 3 shows
trends in cigarette consumption.
WHO WORKING GROUP ON HEALTH RISKS FROM THE COMBINED EFFECTS OF TOBACCO
SMOKING AND EXPOSURE TO OTHER CHEMICALS
(Geneva, 25-26 April 1966)
Members
Dr G. Finch, Inhalation Toxicology Research Institute, Lovelace
Biomedical and Environmental Research Institute, Albuquerque, New
Mexico, USA
Professor G. Pershagen, Division of Epidemiology, Institute of
Environmental Medicine, Karolinska Institute, Stockholm, Sweden
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
Professor H.-P. Witschi, Institute of Toxicology and Environmental
Health, University of California, Davis, California, USA
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr N.F. Collishaw, Tobacco or Health, Programme on Substance
Abuse, World Health Organization, Geneva, Switzerland
WHO TASK GROUP ON HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE
AND EXPOSURE TO OTHER AGENTS
(Geneva, 18-21 February 1997)
Members
Dr G. Finch, Lovelace Respiratory Research Institute, Inhalation
Toxicology Research Institute, Albuquerque, New Mexico, USA
( Chairman)
Dr L. Fishbein, Fairfax, VA, USA ( Joint Rapporteur)
Dr Dorota Jarosinska, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Professor G. Kazantzis, Royal School of Mines, London, United
Kingdom ( Joint Rapporteur)
Professor U. Keil, Institute for Epidemiology and Social Medicine,
Münster, Germany
Dr D. Krewski, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
( Vice-Chairman)
Dr L. van Bree, Laboratory of Health Effects Research, National
Institute of Public Health and the Environment, Bilthoven, The
Netherlands
Observers
Dr G. Minotti, Catholic University of the Sacred Heart, Rome, Italy
( representing the International Union of Pharmaceutical
Societies)
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
Mrs B.F. Goelzer, Occupational Health, World Health Organization,
Geneva, Switzerland
Mr N.E. Collishaw, Programme on Substance Abuse, World Health
Organization, Geneva, Switzerland
HEALTH EFFECTS OF INTERACTIONS BETWEEN TOBACCO USE AND EXPOSURE TO
OTHER AGENTS
A WHO Task Group on Health Effects of Interactions Between Tobacco Use
and Exposure to Other Agents met at the World Health Organization,
Geneva, from 18 to 21 February 1997. Dr E. Smith, IPCS, welcomed the
participants on behalf of Dr M. Mercier, Director IPCS, and the
cooperating organizations. The Task Group reviewed and revised the
draft monograph and developed a new text.
The first draft of the monograph was prepared by Dr K. Rothwell,
Knaresborough, Yorkshire, United Kingdom. This draft was further
developed by a Working Group held in WHO, Geneva, 25-26 April 1996,
and then circulated for international comment to IPCS contact points
for Environmental Health Criteria monographs. Comments were
incorporated in a second draft prepared by Dr K. Rothwell. This draft
was reviewed at the Task Group meeting and a text given further
limited circulation to Task Group members and a number of other
experts, including the US EPA National Center for Environmental
Assessment under the coordination of Dr D. Mukerjee, for final
comment. In the development of the monograph, contributions were made
by a number of authors listed below.
Dr E. Smith (IPCS Unit for the Assessment of Risk and Methods) was
responsible for the scientific content of the monograph and Dr P.G.
Jenkins (IPCS Central Unit) for the technical editing.
The efforts of all who helped in the preparation and finalization of
the monograph are gratefully acknowledged.
The authors were:
Main authors
Dr K. Rothwell, Knaresborough, Yorkshire, United Kingdom
( Coordinating author)
Dr G. Finch, Lovelace Respiratory Research Institute, Albuquerque,
New Mexico, USA
Dr L. Fishbein, Fairfax, Virginia, USA
Dr D. Krewski, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Professor G. Pershagen, Institute of Environmental Medicine,
Karolinska Institute, Stockholm, Sweden
Professor H-P. Witschi, Institute of Toxicology and Environmental
Health, University of California, Davis, California, USA
Contributing authors
Dr D. Jarosinska, Institute of Occupational Medicine and
Environmental Health, Sosnowiec, Poland
Professor G. Kazantzis, Royal School of Mines, London, United
Kingdom
Professor U. Keil, Institute for Epidemiology and Social Medicine,
Munster, Germany
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr L. van Bree, National Institute of Public Health and the
Environment, Bilthoven, the Netherlands
* * *
The IPCS expresses its gratitude to the external reviewers who
provided comments and other relevant material, in particular the
United Kingdom Department of Health, London, and the US Environmental
Protection Agency's Office of Research and Development, National
Center for Environmental Assessment, Cincinnati, Ohio, USA.
The funds for the preparation, review and publication of this
monograph were generously provided by Health Canada.
ABBREVIATIONS
BCME bis(chloromethyl) ether
CMME chloromethyl methyl ether
COLD chronic obstructive lung disease
CHD coronary heart disease
CVD cardiovascular disease
ERR excess relative risk
FIV1 forced expiratory volume (1 second)
IARC International Agency for Research on Cancer
LET linear energy transfer
MMAD mass median aerodynamic diameter
NOx nitrogen oxides
SAD small airways disease
TSNA tobacco-specific nitroasamines
URT upper respiratory tract
1. OVERVIEW
1.1 Introduction
Tobacco use, particularly smoking, causes a range of adverse health
effects, is directly implicated in a number of serious diseases, and
can increase adverse effects of other chemical, physical and
biological agents. Chemicals and other agents in workplaces can cause,
if not controlled, disease, incapacity and early death. In the
workplace it is clear that adverse effects can be produced by the
synergistic interaction of tobacco smoking and other hazards. The
majority of interactions of harmful tobacco smoke constituents with
toxic chemicals occur when the latter are airborne, although
interactions of smoking with ingested and/or absorbed harmful agents
have also been reported.
Tobacco use is widespread throughout the world, from countries with
low income economies to the most affluent industrialized nations.
Tobacco is used by men and women, by children and adults, and millions
of people are involuntarily subjected to environmental tobacco smoke.
There are numerous explanations for the tobacco habit but the main
reason for its ubiquity is the addictive drug nicotine present in all
forms of tobacco leaf and delivered in varying amounts to the user by
the various methods of tobacco use (chapter 2). The advent of the
cigarette, mass produced, easily obtainable, relatively cheap and
light in weight, so it can be held in the mouth leaving the hands
free, has had a major impact on smoking habits, in general and in
workplaces.
In many countries tobacco smoking is recognized as a serious health
hazard and a major contributing factor to deaths from a number of
common diseases. In these countries health warning legislation and
measures to control consumption by taxation have been implemented, as
well as public education programmes on the dangers of smoking and the
benefits to be gained from not starting or from stopping. However,
there are still countries where decisive action has yet to be taken to
deal with the problem of tobacco use.
Many work situations involve an element of risk. The nature of the
work may generate harmful effects on health, and working activities
may cause environmental contamination. Tobacco growing itself involves
the use of pesticides, harvesting of tobacco leaf can cause sickness
due to skin absorption of nicotine, and processing exposes workers to
health hazards from airborne dust and fungal spores. A high male
cancer incidence has been reported in areas with tobacco industries.
In mining there are airborne mineral dusts and, in farming and
industries using biologically produced raw materials, biological dusts
are found. Fumes are produced during welding, and gases, smokes, mists
and vapours containing inorganic and/or organic toxic substances
present hazards in many industries. Excessive heat or exposure to
ultraviolet light can be detrimental to the well-being of workers.
Ionizing radiation in mining and modern technology is recognized as a
workplace hazard. In many occupations workers are subjected to
excessive noise or harmful mechanical vibration. Working conditions
can impact adversely on health to a greater extent in smokers than
non-smokers. In many countries, smoking at the workplace is
prohibited, primarily for reasons of fire/explosion safety. However,
in some countries, regulations are not always enforced. In some newly
industrializing countries health problems associated with work have
not yet been fully addressed and many employers and workers are
ignorant of the dangers to health of their occupations. In addition,
there is the large "informal sector" of industry, particularly in
developing countries, where the home is the workplace, chemicals are
used (including solvents, resins, and synthetic dyestuffs), the whole
family is exposed, and there are no restrictions on exposure to work
hazards or smoking.
The situation for adverse health effects resulting from combined
exposure to tobacco smoke, mainstream or environmental, and agents in
the domestic environment is much less defined. However, the incidence
of lung cancer and the concentration of radon in homes has a similar
dose-response to lung cancer and radon in mines, and the risk is
higher in smokers.
1.2 Examples of combined effects of tobacco smoking and other
exposures
There is evidence for synergism in the production of adverse effects
(cancer) between tobacco smoking and exposure to arsenic, asbestos,
ethanol, silica and radiation (radon, atomic bomb, X-ray). On the
other hand there is evidence for antagonism in the case of tobacco
smoking and the carcinogenic chloromethyl ethers, i.e. chloromethyl
methyl ether (CMME) and bis(chloromethyl) ether (BCME) (Hoffmann &
Wynder, 1976; IARC, 1986), tobacco smoking and allergic alveolitis,
and tobacco smoking and chronic beryllium disease. Tobacco smoking
affects the health risks of exposures in coal mining, pesticide
handling, and in the rubber and petroleum industries. Coal miners who
smoke are at greater risk of developing chronic bronchitis and
obstructive airway disease but not emphysema. Lung cancer in coal
miners has been attributed entirely to tobacco smoking. Tobacco
smoking can increase the health risks of exposure to vegetable dusts
that produce chronic respiratory conditions, such as byssinosis
produced by cotton dust, and nasal cancer caused by wood dusts.
1.3 Composition of tobacco leaf and tobacco smoke
More than 3040 chemical compounds have been isolated from processed
tobacco leaf (Roberts, 1988). Most are leaf constituents, but some
arise from growing conditions such as the soil and atmosphere in an
area, while others originate from the use of agricultural chemicals,
from casings, humectants and flavourings added to the leaves, and from
curing methods. Different tobacco varieties grown in different
countries, and cured and processed in various ways show differences.
The proportions of individual constituents may differ but not the
overall composition. Among important toxic compounds identified, other
than nicotine, are carcinogenic nitrosamines, derived from nitrites,
amines, proteins and alkaloids present in the leaf, polycyclic
aromatic hydrocarbons resulting from the curing process, radioactive
elements absorbed from the soil and the air, and cadmium in tobacco
grown on cadmium-rich soils. When tobacco is burned in the course of
smoking, many pyrolysis and other reaction products are formed.
1.4 Mainstream tobacco smoke
Tobacco smoke is an aerosol consisting of a particulate phase of
liquid droplets dispersed in a gas/vapour phase. When a cigarette is
smoked, many compounds are formed by pyrolysis of the tobacco. These
either pass through the cigarette as mainstream smoke, some being
condensed a short distance behind the burning cone, or they are
emitted into the air from the burning end as sidestream smoke. With
each puff the smoke becomes progressively stronger because previously
condensed material is added to the smoke and the length of cigarette
available for further condensation is decreasing. The physicochemical
nature of the smoke depends on the processing and burning of the
tobacco, the porosity and treatment of the paper wrapper, and on the
type of filter tip (Hoffmann & Hoffmann, 1997). In the case of a
cigarette or Asian "bidi" (tobacco wrapped in vegetable leaf), the
smoke chemistry is affected by such factors as dimensions, wrapper
porosity and the smoking parameters of puff volume, frequency and
duration (NIH, 1998). Variations in smoke chemistry are mainly in the
balance of smoke constituents rather than the presence or absence of
particular compounds.
Mainstream smoke is generated in a comparatively low-oxygen atmosphere
at a burning temperature of 850-950°C in the fire cone. Initially,
mainstream smoke particles have a mass median aerodynamic diameter
(MMAD) of 0.2 to 0.3 µm; however, as soon as they encounter the 100%
humidity of the respiratory tract, they coalesce into larger particles
and behave as if their MMAD was in the micrometre range. Between 50
and 90% of all inhaled particulate matter may be retained in the
respiratory tract (Wynder & Hoffmann, 1967; Hinds et al., 1983). From
size considerations, the aerosol particulate matter, the vapour phase
constituents and the permanent gases are capable of reaching the
alveoli when smoke is inhaled. Deposition in the tracheobronchial tree
is complicated by the behaviour of hydrophilic constituents in the
high humidity conditions, but smoke reaches every part of the airways.
Mainstream smoke contains nearly 4000 identified chemicals and an
unknown number of unidentified chemicals (Roberts, 1988). Mainstream
smoke can be divided into particulate and gas phases. Mainstream smoke
particulate phase contains nicotine, nitrosamines such as
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and
N-nitrosonornicotine (NNN), metals such as cadmium, nickel, zinc and
polonium-210, polycyclic hydrocarbons, and carcinogenic amines, such
as 4-aminobiphenyl. The vapour phase contains carbon monoxide, carbon
dioxide, benzene, ammonia, formaldehyde, hydrogen cyanide,
N-nitrosodimethylamine, N-nitrosodiethylamine and other compounds.
Compounds in tobacco smoke can be classified by their biological
activity as asphyxiants, irritants, ciliatoxins, mutagens,
carcinogens, enzyme inhibitors, neurotoxins or pharmacologically
active compounds. The main point of entry of cigarette smoke into the
body is via the airways, but many constituents, particularly from pipe
and cigar smoke, dissolve in saliva and are absorbed in the buccal
cavity or are swallowed. Cigar and pipe smokers generally do not
inhale the smoke and it remains in the oral cavity, is dissolved in
the saliva and absorbed through the mucous membranes or swallowed
(NIH, 1998). Alcoholic drinks have a solvent effect for the smoke
constituents facilitating their absorption.
1.5 Sidestream tobacco smoke
Sidestream smoke is generated at lower burning temperature (500-600°C)
in a reducing atmosphere. Fresh sidestream smoke particles are about
the same size as mainstream smoke particles with a mass median
aerodynamic diameter (MMAD) of approximately 0.2 µm. Qualitatively,
sidestream smoke composition is similar to the composition of
mainstream smoke. Some chemicals in sidestream smoke are emitted at
higher concentrations per gram of tobacco burned than in mainstream
smoke. This is particularly so for carcinogens such as
N-nitrosodimethylamine and N-nitrosodiethyl-amine, and for metals
such as nickel or cadmium. Many carcinogenic compounds are more
concentrated in sidestream than in mainstream smoke. Mouse
skin-painting bioassays have shown that condensate of sidestream smoke
is more carcinogenic than that of mainstream smoke (Wynder & Hoffmann,
1967; US Surgeon General, 1986; NIH, 1998).
1.6 Effects of ways of cigarette smoking on smoke toxicity
The nicotine content of different cigarettes varies, and the smoker
adjusts the smoking intensity and depth of inhalation to satisfy the
acquired nicotine need. Consequently, the smoker of a filter cigarette
with a low nicotine yield (<1.2 mg) smokes more intensively and this
influences toxicity (NIH, 1998).
1.7 Summary of conclusions and recommendations
Tobacco use, particularly smoking, is a most important public health
hazard and a major preventable cause of morbidity and mortality. In
addition to the adverse health effects of active tobacco use, adverse
health effects have been demonstrated to result from exposure to
environmental tobacco smoke. The risks from tobacco smoking are also
increased through interactions with certain chemical, physical and
biological hazards found in the workplace and general environment.
There are a few instances of antagonistic interactions, but the health
risks of tobacco smoke far outweigh any apparent protective effects.
All possible measures should be taken to eliminate tobacco use,
particularly smoking, and smoking in public places should be strongly
discouraged. To avoid interaction with occupational exposures and to
eliminate the risk of exposure to environmental tobacco smoke, smoking
in the workplace should be prohibited.
To protect health, in particular that of children, smoking in domestic
environments should be strongly discouraged. This will prevent
possible harmful interactions between tobacco smoke and residential
exposures to other hazards. There is a pressing need for educational
programmes on the health hazards of smoking. Health professionals
should provide assistance to help smokers quit. Since smoking may
result in altered response or adverse reactions to drugs and other
treatments, appropriate dose adjustments and patient surveillance
should be taken into consideration by clinicians.
2. EXPOSURE TO TOBACCO PRODUCTS AND HEALTH RISKS FROM TOBACCO USE
2.1 Tobacco and its uses
2.1.1 Introduction
The genus Nicotiana, a member of the plant family Solanaceae, is
represented by about 100 species and sub-species (Cromwell, 1955; Tso,
1990) widely distributed throughout the world. The species N.
tabacum and N. rustica are the principal sources of tobacco. The
primary intention in using tobacco is to obtain the alkaloid nicotine
and, once the habit has been established, nicotine appears to fulfil
both a pharmacological and psychological need (US Surgeon General,
1988; NIH, 1998).
Tobacco and its uses were unknown outside America before its discovery
by Columbus. In a description of tobacco use in South America at that
period, Wilbert (1987) used information from European explorers.
There were six types of tobacco use: chewing, drinking, licking,
rectal insertion, nasal and oral snuffing, and smoking. Smoking was by
far the most common, while rectal application was only used
occasionally. Tobacco smoke was inhaled via the mouth through tubes or
rolls of tobacco leaf, or through the nostrils using a Y-shaped tube.
Alternatively, it was swallowed and belched back, blown from one
person to another, or blown into the eyes. Leaves were chewed, alone
or mixed with ash, powdered shells or honey, or they were held in the
mouth and sucked. Infusions were drunk or a concentrated infusion was
licked or even used as an enema. Many ways of preparing and taking
snuff among different tribes were reported. Tobacco use was linked to
religious rituals.
The rest of the world adopted tobacco use in the forms of smoking,
chewing and snuffing. Smoking has remained the most popular. Tobacco
smoking consists of burning the cured leaf, perhaps mixed with
fragrant additives, in a pipe or tube. Cigarette smoking has largely
replaced other methods in many countries. Reasons for the popularity
of cigarettes include their ready availability resulting from
large-scale manufacture and their greater convenience over other forms
of tobacco use (IARC, 1986). In developed countries, cigarettes
account for at least 80% of overall tobacco consumption, but in most
developing countries other ways of using tobacco predominate, although
cigarette smoking is increasing. Fig. 1 shows estimated daily
cigarette consumption on a regional basis, Tables 1 and 2 show daily
smoking prevalences on a regional and national basis and Table 3 shows
trends in cigarette consumption.
 2.1.2 Tobacco smoking
Smoking prevalence data and most studies into the effects of smoking
have concentrated on cigarette smoking, yet worldwide only about 55%
of tobacco is used for cigarettes. The rest, along with significant
amounts traded in "farm gate" and "local market" sales or "home
grown", is smoked in "bidis" or other hand-rolled devices, or in some
form of pipe (IARC, 1986). Tobacco consumption data give an insight
into tobacco-related disease distribution, as can information on ways
of smoking.
Table 1. Estimated smoking prevalence by WHO Region, early 1990s
(from WHO, 1997)
Men Women
(%) (%)
WHO Regions:
African Regiona 29 4
Region of the Americas 35 22
Eastern Mediterranean Region 35 4
European Region 46 26
South-East Asia Region 44 4
Western Pacific Region 60 8
More developed countries 42 24
Less developed countries 48 7
World 47 12
a Smoking prevalence estimates for African Region are based on
very limited information
Differences in smoke chemistry occur with different tobacco cultivars
and curing methods. Curing removes moisture so that the leaf can be
stored without fermenting or rotting, and the time and temperature of
curing influences enzyme reactions such as deamination and oxidation,
and the content of oils and resins. Air-, flue-, sun-, and fire-curing
methods are employed and each produces distinctive tobaccos which are
intended for smoking in a specific way or to suit a particular
preference.
Between 1933 and the late 1940s, the yields from an average cigarette
varied from 33 to 49 mg tar and from < 1 to 3 mg nicotine (Creek et
al., 1994). However, in the 1960s and 1970s, the average yield from
cigarettes in Western Europe and the USA was around 16 mg tar and 1.5
mg nicotine per cigarette. Current average levels are lower. Changes
in the levels of tar and nicotine have resulted in the characteristic
more intense puffing and smoke inhalation pattern of smokers of
low-yield cigarettes (Djordjevic et al., 1995; NIH, 1996). The
high tar yields measured in the smoke of cigarettes in developed
countries before 1950 are comparable with current tar yields of
"bidis", which range from 23 to 48 mg per cigarette (Hoffmann et al.,
1974; Jayant & Pakhale, 1985; Ball & Simpson, 1987.) The "kretek" (a
cigarette of strong tobacco and cloves) of Indonesia yields up to
71 mg tar (WHO, 1985). Smoking materials in northern Thailand
(cigarette or cigar of strong tobacco plus various vegetable
materials) produce high levels of tar and nicotine (Simarak et al.,
1977).
For smoking, tobacco leaf can be flue-cured, light air-cured,
fire-cured or sun-cured, and can be of dark, oriental or N. rustica
type. Flue-cured tobacco is used for cigarettes and pipe tobacco.
Air-cured tobacco is used in blended cigarettes. Dark tobaccos are
widely grown, usually air-cured, and mostly used in their countries of
origin for dark cigarettes, "bidis", cigars, in pipes and "sheeshas",
and for chewing tobaccos and snuff (Voges, 1984). Some tobaccos have
low nitrate levels ranging up to 0.9% (Neurath & Ehmke, 1964; Wynder &
Hoffmann, 1967) and their smoke contains low nitrogen oxide levels in
the range of 50-200 mg NOx/cigarette (Norman et al., 1983). In the
reducing atmosphere of the burning cone, NOx gives rise to amino
radicals, which react with benzene, biphenyl, naphthalene and other
ring hydrocarbons to form aromatic amines. Aniline, alkylated
anilines, aminobiphenyls, and naphthylamines are therefore found in
higher quantities in the smoke of tobaccos with a high nitrate content
(Patrianakos & Hoffmann, 1979; Pieraccini et al., 1992; Grimmer et
al., 1995). Smokers of these tobaccos can inhale greater quantities of
aromatic amines, such as the human bladder carcinogens
2-naphthyl-amine and 4-aminobiphenyl, and have higher concentrations
of haemoglobin adducts in their blood (Bartsch et al., 1993). This is
the basis for the higher risk of bladder cancer among the smokers of
cigarettes made from dark tobaccos (Vineis et al., 1984; D'Avanzo et
al., 1990; Vineis, 1992).
In the Indian subcontinent, the "bidi" is the common smoking device.
It consists of tobacco flakes, loosely packed and hand-rolled in a
tendu or temburni leaf ( Diospyros melanoxylon). It contains less
tobacco (0.223 g) than a cigarette (0.782 g) (Ramakrishnan et al.,
1995) but up to 8.2% nicotine compared with up to 3.7% in cigarette
tobacco. "Bidi" smoke contains 23 to 48 mg tar and 1.7 to 2.9 mg
nicotine per cigarette (Hoffmann et al., 1974; Jayant & Pakhale,
1985). In India, where around 7% of world tobacco is consumed (US DA,
1990), around 30% of tobacco is smoked as cigarettes, 50% as "bidis",
10% in other ways, and 10% is used for chewing. "Bidis" need to be
puffed frequently to ensure even burning and can generate up to 70 mg
of carbon monoxide, while a US non-filter cigarette smoked under
identical conditions generated 25 mg carbon monoxide (Hoffmann et al.,
1974; Jayant & Pakhale, 1985).
Table 2. Estimated smoking prevalence, ranked in order of male smoking prevalencea
Rank Countryb Men Women Rank Country Men Women
(%) (%) (%) (%)
1 Republic of Korea (1989) 68.2 6.7 21 Seychelles (1989) 50.9 10.3
2 Latvia (1993) 67 12 22 Bolivia (1992) 50 21.4
2 Russian Federation (1993) 67 30 23 Albania (1990) 49.8 7.9
4 Dominican Republic (1990) 66.3 13.6 24 Cuba (1990) 49.3 24.5
5 Tonga (1991) 65 14 25 Bulgaria (1989) 49 17
6 Turkey (1988) 63 24 25 Thailand (1995) 49 4
7 China (1984)c 61 7 27 Spain (1993) 48 25
8 Bangladesh (1990) 60 15 28 Mauritius (1992) 47.2 3.7
9 Fiji (1988) 59.3 30.6 29 Greece (1994) 46 28
10 Japan (1994) 59 14.8 29 Papua New Guinea (1990) 46 28
11 Sri Lanka (1988) 54.8 0.8 31 Israel (1989) 45 30
12 Algeria (1980) 53 10 32 Cook Islands (1988) 44 26
12 Indonesia (1986) 53 4 33 Czech Republic (1994) 43 31
12 Samoa (1994) 53 18.6 33 Jamaica (1990) 43 13
15 Saudi Arabia (1990) 52.7 N/Ad 33 Philippines (1987) 43 8
16 Estonia (1994) 52 24 33 Slovakia (1992) 43 26
16 Kuwait (1991) 52 12 37 Cyprus (1990) 42.5 7.2
16 Lithuania (1992) 52 10 38 Austria (1992) 42 27
16 South Africa (1995) 52 17 39 Malaysia (1986) 41 4
20 Poland (1993) 51 29 39 Peru (1989) 41 13
41 Uruguay (1990) 40.9 26.6 66 Colombia (1992) 35.1 19.1
42 Argentina (1992) 40 23 67 Costa Rica (1988) 35 20
42 France (1993) 40 27 67 Slovenia (1994) 35 23
42 Hungary 40 27 69 Swaziland (1989) 33 8
42 India (1980s) 40 3 70 Luxembourg (1993) 32 26
42 Iraq (1990) 40 5 71 Singapore (1995) 31.9 2.7
42 Malta (1992) 40 18 72 Belgium (1993) 31 19
50 Brazil (1989) 39.9 25.4 72 Canada (1991) 31 29
51 Egypt (1986) 39.8 1 72 Iceland (1994) 31 28
52 Morocco (1990) 39.6 9.1 75 Australia (1993) 29 21
53 Lesotho (1989) 38.3 1 75 Ireland (1993) 29 28
53 Mexico (1990) 38.3 14.4 77 UK (1994) 28 26
55 El Salvador (1988) 38 12 78 USA (1993) 27.7 22.5
Table 2. (cont'd)
Rank Countryb Men Women Rank Country Men Women
(%) (%) (%) (%)
55 Italy (1994) 38 26 79 Pakistan (1980) 27.4 4.4
55 Portugal (1994) 38 15 80 Finland (1994) 27 19
58 Chile (1990) 37.9 25.1 81 Turkmenistan (1992) 26.6 0.5
59 Guatemala (1989) 37.8 17.7 82 Nigeria (1990) 24.4 6.7
60 Denmark (1993) 37 37 83 Paraguay (1990) 24.1 5.5
61 Germany (1992) 36.8 21.5 84 Bahrain (1991) 24 6
62 Norway (1994) 36.4 35.5 84 New Zealand (1992) 24 22
63 Honduras (1988) 36 11 86 Sweden (1994) 22 24
63 Netherlands (1994) 36 29 87 Bahamas (1989) 19.3 3.8
63 Switzerland (1992) 36 26
a Adapted from: WHO (1997).
b The year given in parentheses is the latest available year for data.
c Some 1991 data suggest that there has been little change in smoking prevalence since 1984.
d Data not available.
Table 3. Trends in adult consumption of cigarettes from 1970-1972 to 1990-1992 (Adapted from WHO, 1997)
Annual % change
1970-1972 to 1990-1992
WHO Regions:
African Region +1.2
Region of the Americas -1.5
Eastern Mediterranean Region +1.4
European Region 0
South-East Asia Region +1.8
Western Pacific Region +3
More developed countries -0.5
Less developed countries +2.5
World -0.8
Tar yields for Russian cigarettes were found to be high (21.6-29.2
mg) and cigarettes containing N. rustica produced high smoke
concentrations of tobacco-specific nitrosamines (TSNA) (up to 620 ng
total TSNA/cigarette) (Djordjevic et al., 1991). Indigenous cigarettes
and cigars in Thailand, containing tobacco and other vegetable
materials, have up to 41 mg and 200 mg tar, up to 5.5 mg and 11.4 mg
nicotine, and 41 mg and 820 mg carbon monoxide in cigarette and cigar
smoke, respectively. Indonesian cigarette smoke contains up to 100 ng
of carcinogenic volatile N-nitrosamines and up to 1580 ng of
carcinogenic tobacco-specific N-nitrosamines (Mitacek et al., 1990,
1991), and high tar cigarettes contain up to 28.1 mg tobacco-specific
N-nitrosamines per cigarette (Brunnemann et al., 1996).
Many cigar-like devices are smoked throughout Asia. The tobacco can be
rolled in a tobacco leaf or in the leaves of the jackfruit tree
( Artocarpus integrifolia), banana ( Musa paradisiaca) or hansali
( Grewia microcos) (Bhonsle et al., 1976). They may be smoked
conventionally or in the reverse manner with the burning end inside
the mouth (Reddy, 1974). In Thailand they can contain strong tobacco
and a mixture of koi bark ( Streblus asper), dry tamarind pod
( Tamarindus indica), khai bark ( Homonoia riparia, Euphorbiaceae),
Areca palm bark ( Areca catechu) or other tree bark; they can be
rolled in a banana leaf or have fragrant additives such as sandalwood
Mougne et al. (1982). They contain high levels of tar and nicotine
(Simarak et al., 1977; Mitacek et al., 1999).
Additives are used to enhance the fragrance or taste of smoke
(Hoffmann & Hoffmann, 1997). "Casing sauces", consisting of sugars,
aromatic substances and compounds such as glycerol, propylene glycol,
ethylene glycol and diethylene glycol, which resist changes in
moisture content, are sprayed on the leaf before it is cut to
condition it for processing. Flavouring and dressing compounds are
added to cut tobacco after drying and include licorice, menthol,
cocoa, chocolate, ginger, cinnamon, vanilla, molasses, angelica,
honey, essential oils from anise, clove and juniper, resins and plant
extracts and organic compounds such as coumarins. Additives have been
widely used in pipe and chewing tobaccos. They have also become
important in cigarette tobacco with the development of low-tar and
low-nicotine tobaccos and the use of stem, midrib or reconstituted
leaf (which lacks the aromas and flavours of natural tobacco leaf
lamina) and of tobacco dust requiring additives to ensure its
adherence to cut tobacco.
Added glycerol is transferred to mainstream smoke: 3-6% in cigarette
smoke and 35-43% in pipe smoke (IARC, 1986) and one of its pyrolysis
products is acrolein. Levels of acrolein ranging from 69 mg to 230 mg
per cigarette have been reported and air concentrations as high as
0.46 mg/m3 have been found in smoke-filled rooms (Izard & Liberman,
1978). Acrolein is extremely irritating to the eyes and nasal mucosa,
it affects mitotic and ciliary activity, at the cellular level it has
cytotoxic and cilia-depressant effects, and it can act as a mutagen
(Izard & Liberman, 1978).
In the USA, additives used in cigarette manufacture are food additive
compounds that are "generally recognized as safe (GRAS)" and,
therefore, also considered "safe" as additives to tobacco. However,
the non-volatile additives are to some extent pyrolized during smoking
and can give rise to toxic and/or carcinogenic agents in the smoke. A
major group of compounds formed during pyrolysis is that of the
polynuclear aromatic hydrocarbons. Ethylene glycol is pyrolytically
converted to the human carcinogen ethylene oxide (IARC, 1994a).
Another example of carcinogen formation during tobacco curing and
smoking is the case of MH-30, a sucker growth control agent formulated
from maleic hydrazide in diethanolamine. Residual MH-30 on tobacco
leads to the formation of N-nitrosodiethanolamine (NDELA) in the
smoke (Brunnemann & Hoffmann, 1981). The use of MH-30 on tobacco has
been forbidden in the USA since 1981, and NDELA levels in tobacco and
its smoke have declined (Brunnemann & Hoffmann, 1991).
The use of cloves as a tobacco additive can have health effects. In
Indonesia, the smoke of "kreteks", a blend of ground cloves with
60-65% of tobacco, contains between 41 and 113 mg of tar, and between
1.2 and 4.5 mg nicotine (WHO, 1985; Wise & Guerin, 1986). Mainstream
smoke of kreteks without filter tips contains 19-23 mg of eugenol
released from the cloves, while filter-tipped kretek smoke contains up
to 15 mg (LGC, 1982; Wise & Guerin, 1986). Inhalation of eugenol in
the smoke of kreteks can lead to interstitial haemorrhaging and
congestion of the lung, acute emphysema and acute pulmonary oedema.
These effects were also seen in Syrian golden hamsters exposed to the
smoke of kreteks (LaVoie et al., 1986).
Menthol and other additives that produce a sensation of coolness but
without a mint flavour have also been used in cigarettes. There is no
evidence that these additives result in a higher risk (Cummings et
al., 1987; Sidney et al., 1989).
Many shapes and sizes of smoking pipes are found worldwide (Voges,
1984). Various types of tobacco are used and the smoke can range from
mild to very strong. In the sheesha water pipe the tobacco is kept
alight by pieces of glowing charcoal and the smoke is drawn through
water before being inhaled. Sheesha smoke is mild and low in
particulate matter, benzo( a) pyrene and volatile phenols (Hoffmann
et al., 1961), but it has a high level of carbon monoxide, in part
from the charcoal that keeps the tobacco burning, and smokers have
high carboxyhaemoglobin levels and a reduced FEV1 (8.5% in women, 45%
in men) (Zahran et al., 1985; Al-Fayez et al., 1988).
2.1.3 Tobacco chewing and snuff
The popularity of tobacco chewing and snuff (finely powdered tobacco
leaf) has varied. Nasal inhalation of snuff has given way to the oral
application of snuff and other tobacco-containing mixtures between the
gum and lip, or gum and cheeks, or under the tongue. Chewing tobacco
comes in several forms and may have flavour added from syrups,
liquorice and brandy. Tobacco chewing has retained its popularity in
heavy industries, such as steel and coal mining, woodworking and the
petroleum industry, where the flammability hazard precludes smoking.
In Sweden, 17% of the population uses oral snuff. In the USA, sales of
chewing tobacco have declined but sales of oral snuff have increased
by 61% (US DA, 1997) owing to the popularity of the latter with
teenagers and young adult men. Oral tobacco use in India is very
common but, generally, in developing countries it is declining because
urban populations and younger age groups are smoking cigarettes.
"Betel-quid" chewing is common in Asia and Africa. The basic contents
of a betel-quid are slices of areca nut ( Areca catechu), lime and
tobacco, wrapped in a betel pepper leaf ( Piper betle), but it may
also contain dried dates, menthol and spices such as cardamom, cloves,
coriander, mace and cinnamon.
Other tobacco preparations for oral use often contain lime, calcium
carbonate, sodium carbonate, some form of ash or flavouring materials.
The lime and other agents assist the release of nicotine (Voges, 1984;
Idris et al., 1991).
2.2 Responses to mainstream smoke
Tobacco use has direct effects on health and is responsible for a
variety of diseases. These form a basis for studying interactions
between tobacco use and chemical, physical and biological agents, and
associated effects on health.
2.2.1 Acute responses
Inhaled irritant chemicals cause inflammation of the upper respiratory
tract (URT) and paranasal sinuses, sore throat and bronchial oedema.
Pulmonary oedema may follow if irritants penetrate the lower
respiratory tract. Acute irritation of the URT usually follows
inhalation of highly soluble gases. Slightly less soluble gases cause
URT and bronchial irritation, while relatively insoluble gases
penetrate deeply into the lung and can have delayed effects including
pulmonary oedema (Miller & Kimbell, 1995). Cigarette smoke is a
complex mixture of organic and inorganic constituents with particulate
and vapour phases and highly reactive free radicals (Hocking & Golde,
1979). It contains many compounds with irritant properties, which can
affect all parts of the lung. Kremer et al. (1994) examined the
association between occupational exposures to a variety of airway
irritants and respiratory system effects, and whether the association
was modified by smoking, airway hyperresponsiveness and allergy. The
irritants were SO2, HCl, SO42-, polyester vapour, polyamide vapour,
and oil mist and vapour. Current smoking, airway hyperresponsiveness
and allergy were significantly associated with a higher prevalence of
chronic respiratory symptoms, independent of each other and of
irritant exposure. The association between exposure and the prevalence
of chronic respiratory symptoms was greater in smokers than in non- or
ex-smokers.
2.2.1.1 Acute bronchitis
Acute bronchitis is an inflammation of the bronchial mucous membrane,
initially accompanied by a dry painful cough and followed by
mucopurulent sputum. The cause may be infectious agents or chemical
agents such as tobacco smoke, dusts, fumes, vapours or gases.
2.2.1.2 Asthma
Asthma is a chronic pulmonary inflammatory disease associated with
bronchial hyperreactivity causing paroxysmal dyspnoea due to spasm of
the bronchial musculature, swelling of the mucous membranes and the
production of viscid mucus. In the majority of asthma cases a clear
association exists with atopic IgE-mediated hypersensitivity. Allergic
asthma is a response to a specific agent. Many chemical agents,
including mixtures such as tobacco smoke, can induce asthma. Smoking
enhances the effect of other agents and can reduce the latent period
from first exposure to onset of sensitization.
There has been an increase in the prevalence of asthma in many
countries, and roles for environmental factors, increased
susceptibility and tobacco smoking have been suggested (ISAAC, 1998).
Cigarette smoking together, with atopic status, age, URT infection and
genetic factors, has been considered to increase susceptibility
(Venables et al., 1985; Seaton et al., 1993). Studies have shown a
relationship between cigarette smoking and serum IgE levels. Smokers
have higher levels of IgE with increasing age, compared to non-smoking
controls. A relationship has also been observed between IgE level and
the number of cigarettes smoked (Sherrill et al., 1994).
2.2.2 Chronic responses
2.2.2.1 Chronic obstructive lung diseases
The chronic obstructive lung diseases (COLD) are chronic bronchitis,
small airways disease, toxic bronchiolitis obliterans, emphysema and
fibrosis (Niewoehner et al., 1974; Niewoehner, 1991).
These diseases form an important group of pulmonary diseases caused by
smoking (and by chemical atmospheric pollution) (US Surgeon General,
1984). In a study by Simecek et al. (1986) of 215 229 adults in a
region of former-Czechoslovakia, smoking was the most important risk
factor in COLD. Risks for male non-smokers and light smokers under 30
years of age were 1.18% and 2.28%, respectively; for men aged 50
years, smoking more than 20 cigarettes a day, the risk was 20.36%
compared with 3.31% for non-smokers of the same age. In the USA,
80-90% of the mortality from COLD has been attributed to cigarette
smoking. Cigar and pipe smokers who inhale the smoke also have an
increased death rate from COLD (US Surgeon General, 1984, 1989; NIH,
1998). Between 1979 and 1993, the age-adjusted annual death rate from
COLD in women increased by 122% to 17.1 per 100 000, while in men it
increased by 14% to 27.8 per 100 000. The greater increase of COLD
among women during this period reflected the increase in smoking by
women.
2.2.2.2 Chronic bronchitis
Chronic bronchitis has been variously defined. The United Kingdom
Medical Research Council (MRC, 1965) considered it to be a condition
with persistent production of sputum, which might be associated with
cough, occurring on most days for at least three months in the year
for at least two successive years. The Council recommended a
classification of simple chronic bronchitis, chronic or recurrent
mucopurulent bronchitis or chronic obstructive bronchitis. In a
workplace context, Morgan (1982) defined it as "a condition
characterized by cough and sputum for at least three months of the
year, which may or may not be accompanied by airways obstruction, and
which is a consequence of prolonged inhalation of dust or irritant
gases at the workplace". Fletcher & Pride (1984) suggested an improved
terminology with the term chronic bronchitis meaning chronic or
recurrent bronchial hypersecretion only, abandoning the term chronic
obstructive bronchitis because this implies a causal connection
between mucus hypersecretion and airflow obstruction. In smokers'
lungs the irritant constituents of tobacco smoke cause hypersecretion
of mucus, alter its physical properties and chemical structure, and
impair the mucociliary clearance mechanism. Removal of major
ciliatoxins (hydrogen cyanide and volatile aldehydes) from the smoke
stream by charcoal filter tips reduces the effects on the lung
epithelium (Friedman et al., 1972). Mineral dusts, particularly those
encountered in mining, many biological dusts, irritant vapours and
gases, inorganic and organic chemical dusts and sprays can all cause
chronic bronchitis.
2.2.2.3 Small airways disease
Small airways disease (SAD) is a widespread narrowing of membranous
bronchioli. It is inflammatory in origin and is often associated with
excess mucus and an accumulation of macrophages in the respiratory
bronchioli. SAD is mainly caused by smoking, but can be associated
with environmental and industrial pollutants (Cosio et al., 1980).
2.2.2.4 Emphysema
Emphysema has been defined as a condition of the lung characterized by
an abnormal enlargement of the airspaces distal to the terminal
non-respiratory bronchioli, accompanied by destructive changes in the
alveolar walls, and without obvious fibrosis. It tends to be prevalent
in older age groups and follows SAD. For purposes of postmortem
examination of lung slices of miners, Ruckley et al. (1984) defined
emphysema as the presence of air spaces of 1 mm or more in size.
Macrophages that have engulfed foreign particles, including smoke
particulate matter in the lungs of smokers, and which have been found
accumulated in the bronchioli (Niewoehner et al., 1974) and in the
lung parenchyma (McLaughlin & Tueller, 1971) have been implicated in
the pathogenesis of emphysema. There are different forms of emphysema,
which vary with the nature of the insult to the tissues. One
hypothesis is that tobacco smoke causes increased production and
release of proteolytic enzymes, such as elastase, and interferes with
normal antiproteolytic mechanisms. Emphysema has been induced
experimentally in animals by endotracheal installation of elastase or
homogenates of alveolar macrophages or polymorphonuclear leukocytes.
Chronic exposure to tobacco smoke has a number of effects on alveolar
macrophages, including changes in metabolism, alteration of the enzyme
content and impairment of RNA and protein synthesis (Hocking & Golde,
1979). The function of alveolar macrophages is to remove inhaled
foreign material from the alveoli and respiratory bronchioles, and
their numbers increase when the lungs are exposed to particles and
gases. It has been demonstrated that the macrophage count is higher in
people exposed to cigarette smoke than in non-exposed people (Harris
et al., 1974; Rylander et al., 1979). Alveolar macrophages from
smokers are more active than those from non-smokers, numbers are
increased, there are morphological changes with an increased cell
diameter, crystalline inclusions and surface membrane alteration
(Sopori et al., 1994).
2.2.2.5 Pulmonary fibrosis
Pulmonary fibrosis is the abnormal formation of fibrous or scar tissue
and is the response of bronchiolar tissue to the deposition of an
inhaled inciting agent. Mineral and other dusts are causes of
pulmonary fibrosis. The radiological changes seen in early stages of
dust fibroses are associated with relatively minor lung function
impairment, but continuous exposure leads to a greater degree of
fibrosis and to progressive massive fibrosis in some subjects. On
histological, animal experimental and radiographic evidence, Weiss
(1984) concluded that cigarette smoking could cause diffuse fibrosis.
2.2.2.6 Effects on the immune system
Tobacco smoking and exposure to environmental tobacco smoke increase
susceptibility to pulmonary infections, and changes in immune
processes may be involved. Tobacco smoking affects humoral and
cellular immunity in humans and experimental animals, but the
magnitude of the changes vary widely among studies. In humans,
cigarette smoke has marked effects on alveolar macrophage morphology
and physiology, it decreases serum immunoglobulin (IgA, IgG, IgM) but
increases IgE, and has a range of effects on B- and T-lymphocytes.
Similar effects are found in experimental animals (Sopori et al.,
1994). Studies in rats and mice show that cigarette smoke or nicotine
induces impaired responses of systemically distributed B- and
T-lymphocytes to antigen-induced signalling (Geng et al., 1995, 1996).
T-lymphocyte unresponsiveness, with decreased antibody response to
T-dependent antigens, is important in response to infection (Sopori et
al., 1998).
2.2.3 Cancer
Many forms of cancer have been associated with inhaled particulate
matter, vapours, fumes and gases. Examples are lung cancer associated
with tobacco smoking and inhalation of asbestos, fumes from metal
moulding and coking plants, particles and gases inhaled by motor
vehicle drivers, dusts in several types of mines where there is an
accumulation of alpha-emitting radioisotopes, and contact with
materials such as arsenic, chromates, nickel, chloromethyl ethers,
mustard gas and polycyclic aromatic hydrocarbons. Pleural mesothelioma
has been associated with asbestos, nasal and sinus cancer with nickel
refining and wood dust exposure, leukaemia with ionizing radiations
and benzene, bladder cancer with the manufacture and use of dyes and
in the rubber industry, and liver cancer with the use of vinyl
chloride (IARC, 1987).
Cigarette smoke contains many toxic chemicals, including chemicals
that are DNA reactive and cytotoxic or become DNA reactive upon
metabolic activation. Consequently, cigarette smoke has the potential
to initiate genetic lesions. Moolgavkar et al. (1989) postulated
mechanisms by which cigarette smoke induces lung cancer by fitting the
two-stage clonal expansion model of carcinogenesis to lung cancer
mortality data derived from a large cohort of British doctors who
smoked. This analysis suggested that tobacco smoke affected both the
rates of mutation and cell proliferation involved in the model,
supporting the hypothesis that tobacco smoke acts as a complete
carcinogen.
Cigarette smoking is causally associated with cancer of the lung,
larynx, pharynx, oesophagus, pancreas, kidney and urinary bladder. It
is also associated with cancer of the nasal cavity, liver, uterine
cervix and myeloid leukaemia (RCP, 1962; US Surgeon General, 1982,
1989; IARC, 1986; Winkelstein, 1990; Brownson et al., 1993; Roush,
1996).
In 1991, in the USA, it was estimated that 90.3% of the lung cancer
deaths in men and 78.5% of lung cancer deaths in women were
attributable to cigarette smoking. Deaths from oesophageal cancer were
linked to smoking in 78.2% of the cases in men and 74.3% of the cases
in women. Smoking was held responsible for 81.2% of deaths from
laryngeal cancer in men and 86.7% in women, for 91.5% and 61.2%,
respectively, of deaths from oral cancer, and 46.5% and 36.7%,
respectively, of deaths from bladder cancer. Smoking-attributable
deaths from cancer of the kidney in men and women were 47.6 and 12.3%,
respectively, and for pancreatic cancer the figures were 28.6% and
33.3% respectively. In women, 32.4% of uterine cervical cancer deaths
were attributed to cigarette smoking (Shopland et al., 1991).
There has been a change in the rates of different types of lung cancer
among smokers. In 1950, squamous cell carcinoma (SCC) occurred 17
times more often than adenocarcinoma (AC) (Wynder & Graham, 1950) in
cigarette smokers. In 1991, Devesa et al. (1991) reported that in male
cigarette smokers the ratio of SCC to AC was 2.4:1 between 1969 and
1971, and changed to 1.4:1 between 1984 and 1986; in cigarette-smoking
women, the SCC to AC ratio changed from 3.6:1 in 1950 to 0.57:1 in
1984-1986. Between 1970 and 1980 some studies showed a 20-50%
reduction in risk of lung cancer for long-term smokers of filter
cigarettes as compared to smokers of non-filter cigarettes (IARC,
1986) but later studies indicated a similar risk for lung cancer in
smokers of filter and non-filter cigarettes (Stellman et al., 1997;
Thun et al., 1997). The changes in the ratio of SCC to AC, and the
disappearance of an advantage of filter cigarette smoking in terms of
lung cancer risk, have been related to changes in smoke yields, which
have caused smokers to modify patterns of puff drawing and smoke
inhalation. Smokers regulate the speed and the quantity of their
nicotine uptake to achieve the desired pharmacological effects
(Benowitz et al., 1988; Djordjevic et al., 1995). Smokers of lower
nicotine cigarettes draw puffs of greater volume, at a higher
frequency, and inhale more deeply; this is governed by the amount of
nicotine in the smoke (Wynder & Hoffmann, 1994).
Smoking is a major risk factor for the early stage development of
oesophageal and gastric adenocarcinomas, accounting for 40% of cases,
and may have contributed to the increase in the incidence of these
cancers, especially in older people (Gammon et al., 1997).
Cigar smoking is causally associated with cancer of the oral cavity,
the pharynx and the lung, even though for cigar smokers, who do not or
only minimally inhale the smoke, the lung cancer risk is considerably
lower than that for cigarette smokers. Cigar smoking is also
associated with cancer of the pancreas and of the urinary bladder
(NIH, 1998). Oral snuff users have an increased risk of cancer of the
oral cavity and, possibly, cancer of the oesophagus, pancreas and
urinary bladder (US Surgeon General, 1986).
It has been suggested that around 4-5% of all lung cancer is related
to occupational exposure (Wynder & Gori, 1977; Doll & Peto, 1981;
Morgan, 1982 ). Occupational and any other forms of exposure to
chemical compounds are of limited importance in the etiology of lung
cancer whereas tobacco smoking is the cause of approximately 85-90% of
cases (Shopland et al., 1991).
"Reverse smoking", in which rolled tobacco leaves are smoked with the
burning end inside the mouth, has been linked to carcinoma of the hard
palate (Reddy & Rao, 1957; Mehta et al., 1971; Pindborg et al., 1971;
Reddy, 1974; Bhonsle et al., 1976). Reddy et al. (1960) simulated the
effect of reverse smoking experimentally by painting the skin of male
and female mice on alternate days with the tar from Indian cigars and
exposing the painted skin to a temperature of 58°C for 3 min; the heat
treatment enhanced the dermal tumour response.
2.2.4 Cardiovascular effects
Cigarette smoking is a major independent risk factor for
cardiovascular disease (CVD). Cigarette smoking acts synergistically
with other risk factors, such as elevated cholesterol levels and
hypertension (Wilhelmsen, 1977; Gibinski, 1977; US Surgeon General,
1983). Prospective studies indicated that elevated cholesterol and
hypertension appear to be prerequisites for CVD in cigarette smokers
(Kimura, 1977). It has been estimated that in countries with a long
history of cigarette smoking the tobacco habit is responsible for
26-30% of early deaths from CVD (US Surgeon General, 1983; Wald et
al., 1985). Those who smoke several cigars a day and inhale the smoke
also face an increased risk of CVD (NIH, 1998).
The major contributors to the cardiovascular effects of tobacco smoke
are carbon monoxide and nicotine (Lakier, 1992), as well as nitrogen
oxides (NOx), hydrogen cyanide and tar; minor contributors are
cadmium, zinc and carbon disulfide (US Surgeon General, 1983). The
smoke of cigarettes can be slightly acidic (pH 5.6-5.9) or weakly
alkaline (pH 6.7-7.9) depending on the type of tobacco and the blend
(Brunnemann & Hoffmann, 1974). In the slightly acidic smoke, nicotine
is protonated, i.e., bound to a salt or acid moiety and is part of the
particulate phase. Weakly alkaline smoke contains a small percentage
of protonated nicotine (often up to 50% of the nicotine is
unprotonated) in the vapour phase. In contrast to the protonated
variety, unprotonated nicotine is rapidly absorbed through the oral
mucosa and this is why smokers of cigarettes with weakly acidic smoke
and smokers of cigars do not need to inhale into the lung to
experience the pharmacological effects of nicotine. Protonated
nicotine in the particulate matter of slightly acidic cigarette smoke
is not or is only minimally absorbed through the oral mucosa, so that
smokers inhale the smoke and absorption into the bloodstream takes
place in the lung (Armitage & Turner, 1970; Russell, 1976; Benowitz et
al., 1988).
Smoking has been associated with a two-to-fourfold increased risk of
coronary heart disease (CHD), a greater than 70% excess rate of death
from CHD, and an elevated risk of sudden death (Lakier, 1992).
Nicotine causes increases in heart rate and blood pressure, stimulates
nerve endings that are activated by acetylcholine, causes increased
mobilization of free fatty acids in the serum and enhances platelet
adhesiveness. These effects increase cardiac load (which for
individuals with some forms of heart disease will not be met by
increased coronary blood flow) and interfere with metabolic exchange
across capillary walls, leading to ischaemic episodes and thrombosis.
Carbon monoxide increases carboxyhaemoglobin concentrations in the
blood and lowers its oxygen-carrying capacity: increased oxygen debt
after exercise and impairment of endurance performance are evident in
smokers, compared to non-smokers. Carbon monoxide also has an affinity
for myoglobin and interferes with oxygen uptake by the myocardium.
"Bidi" smoke contains a higher concentration of carbon monoxide than
cigarette smoke (Hoffmann et al., 1974; Jayant & Pakhale, 1985; Ball &
Simpson, 1987). Most of the Asian smoking devices with their
non-porous wrappers and dark tobacco contain higher levels of nicotine
(Simarak et al., 1977; WHO, 1985). High nicotine and carbon monoxide
are also obtained from many dark tobacco cigarettes and from cigars.
"Sheesha" water pipe smoke contains small amounts of nicotine but is
rich in carbon monoxide; the blood carboxyhaemoglobin concentration in
sheesha smokers is higher than in cigarette smokers (Zahran et al.,
1985).
Cigarette smoking has been considered to be the primary cause of
Buerger's disease (thromboangiitis obliterans), an inflammatory
obliterative, non-atherosclerotic, vascular disease. The disease
usually becomes quiescent if the patient stops smoking cigarettes
(Olin, 1994). It was rare in women but an increasing number of cases
has been observed and ascribed to the increased use of tobacco by
young women (Yorukoglu et al., 1993).
Cardiovascular disease has been associated with exposure to other
factors, which can be classified as physical, chemical and biological,
and with occupation or life-style. The combination of smoking with any
of these factors will predispose to an increased detrimental
cardiovascular effect.
In women who smoke, peripheral vasoconstriction (PV) leading to acute
intervillous placental blood flow was measured during smoking and
attributed to nicotine, which simultaneously caused increases in heart
rate and blood pressure. It was suggested that PV explained fetal
growth retardation and other complications of pregnancy (Lehtovirta &
Forss, 1978).
2.2.5 Smoking and occupational accidents, injuries and
absenteeism
A survey of public employees found that cigarette smokers took 23%
more sick leave than non-smokers (Van Tuinen & Land, 1986). In another
study among 2537 postal workers in the USA, cigarette smokers had a
1.29 times higher accident rate (CI 1.07-1.55), and a 1.55 times
higher injury rate (CI 1.11-1.77) than non-smokers (Ryan et al.,
1992). Other studies confirm the higher rates of injury, occupational
accidents and absenteeism in smokers (Naus et al., 1966; Parka, 1983;
US Surgeon General, 1985; Hawker & Holtby, 1988). There are higher
costs of illness for cigarette smoking employees compared to
non-smokers (Van Peenen et al., 1986; Penner & Penner, 1990.
2.3 Health risks from smokeless tobacco use
2.3.1 Introduction
Oral cancers have been linked with oral tobacco use. The consequences
of chewing tobacco can be reactional keratosis, irreversible gingival
recession, periodontitis, oral dysplasia and leukoplakia, cancer and
cardiovascular effects (Chakrabarti et al., 1991; Guggenheimer, 1991).
In India, chewing material containing tobacco has been shown to be a
primary cause of oral cancer (Jussawalla & Deshpande, 1971).
2.3.2 Cancer
Leukoplakia and oral cancer are common results of oral tobacco use,
particularly in the countries of Asia (IARC, 1985a). Simarak et al.
(1977) reported a strong association between betel chewing and oral
cancer in northern Thailand. Sankaranarayanan et al. (1989a,b, 1990)
associated oral cancers in southern India with tobacco chewing.
Chakrabarti et al. (1991) reported much higher levels of pre-malignant
and malignant lesions of the oral cavity in tobacco chewers;
Nandakumar et al. (1990) found the relative risk to be elevated in
both sexes, but appreciably higher in females. From chemical analysis
of Indian tobacco products, it was concluded that their use may lead
to high exposures to carcinogenic tobacco nitrosamines (Nair et al.,
1989).
Case-control studies reported a higher incidence of oral cancer in
oral snuff users than in those not using any form of tobacco. There
was up to a 50-fold excess risk of cancer of the cheek and gum in
long-term oral snuff users (Axéll et al., 1978; US Surgeon General,
1986a; Winn, 1997). Idris et al. (1991, 1994), reported a high
incidence rate of oral cancer in men in northern Sudan who used an
oral snuff with relatively high concentrations of nicotine (0.8-3.2%)
and nornicotine. In a study of baseball players who chewed or (the
majority) used snuff orally, there was higher prevalence of
leukoplakia, plaque formation, gingivitis and dental disorders
compared to non-users (Robertson et al., 1997).
A case-control study on nasal cavity and paranasal sinus cancer and
snuff use showed that snuff users have a significantly increased risk
for adenocarcinoma and squamous cell carcinoma in the nasal cavity
(Brinton et al., 1984). N'-nitrosonornicotine is present and is an
organ-specific carcinogen that induces benign and malignant tumours of
the nasal cavity in rats, hamsters and mink (Rivenson et al., 1991;
Koppang et al., 1992, 1997; Hoffmann et al., 1994). Studies conducted
in South Africa showed that people using local snuff made from tobacco
and aloe plant ash as nasal applications have an increased risk for
tumours of the maxillary antrum (Keen et al., 1955). It was thought
that this snuff mixture contained high levels of nickel and chromium,
which may be associated with the induction of these tumours (Baumslag
et al., 1971).
Instillation of snuff into the lips of rats induced benign and
malignant tumours in the oral cavity (Hirsch & Thilander, 1981; Hecht
et al., 1986; Johansson et al., 1989; Larsson et al., 1989).
Instillation of snuff into the buccal pouch of hamsters, which were
repeatedly infected with Herpes simplex virus type I or II, led to
oral tumours, although no tumours occurred when either the virus or
tobacco was applied alone (Park et al., 1991b).
More than 3050 chemical compounds have been identified as tobacco
constituents (Roberts, 1988) and 50 are known carcinogens (Brunnemann
& Hoffmann, 1992). Nitrosamines, especially the tobacco-specific
nitrosamines (TSNA), must be regarded as major oral carcinogens. Oral
tumours were elicited when an aqueous solution of N-nitrosonornicotine
(NNN) and 4(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was
applied to the oral surfaces of rats (Hecht et al., 1993).
2.3.3 Cardiovascular disease
The effect of tobacco chewing on cardiovascular disease has received
less attention than the effect of cancer. Benowitz et al. (1988)
compared the cardiovascular effects of smokeless tobacco, cigarettes
and nicotine gum and reported that all tobacco use increased heart
rate and blood pressure. Nanda & Sharma (1988) recorded incremental
increases in heart rate and blood pressure following tobacco chewing.
However, Eliasson et al. (1991) found no significant elevation of
diastolic blood pressure in young snuff users. Bolinder et al. (1994)
found an excess risk of death from cardiovascular and cerebrovascular
disease among smokeless tobacco users. Tobacco chewing has detrimental
effects on pregnancy (Krishna, 1978) which, in the absence of any
anoxia due to carbon monoxide found in smokers, could result from
nicotine-induced vasoconstriction.
O'Dell et al. (1987) reported a case of Buerger's disease in a
38-year-old man that was associated with the use of smokeless tobacco.
A regimen which included complete abstinence from tobacco resulted in
a resolution of the symptoms. Buerger's disease is the commonest
vascular disease in the Indian subcontinent where tobacco consumption
is high (Jindal & Patel, 1992). In a study of the disease in
Bangladesh, 39 patients (38 males, 1 female) were investigated. All
but two were current long-term smokers, one male had given up smoking
6 months previously and the one woman with the disease (it is uncommon
in women) was a tobacco chewer (Grove & Stansby, 1992).
3. EFFECTS ON HEALTH OF TOBACCO USE AND EXPOSURE TO OTHER CHEMICALS
3.1 Introduction
3.1.1 Interaction
To discuss the interaction between tobacco and other agents as risk
factors for cancer and other adverse health effects, it is necessary
to define what is meant by the term "interaction". In general terms,
interaction represents a departure from additivity, in which the
combined effect of exposure to two agents is in some sense the sum of
the effects of the individual agents (US EPA, 1988). Synergism occurs
when the combined effect is greater than the sum of the component
effects; antagonism occurs when the effect of the combination is less
than would be suggested by summing the effects of the components.
3.1.2 Measuring interaction
To make these concepts precise, the scale in which risk is measured
and the manner in which risks are summed must be specified (Kaldor &
L'Abbe, 1990). In epidemiological investigations, the age-adjusted
relative risk is often used to characterize risk. In risk assessment
applications involving chronic health effects such as cancer, the
cumulative risk over a period of time may be of more direct interest.
In laboratory studies of carcinogenicity, for example, the lifetime
probability of tumour induction is often used to describe risk. In
order to take into account the age at which an adverse effect is
induced, the expected loss in life expectancy has also been used to
evaluate risk (UNSCEAR, 1988).
Kodell & Pounds (1991) discuss interaction in terms of departures from
response additivity and dose additivity. Dose additivity occurs
when the combined effect of two agents can be expressed in terms of a
total dose of the two agents, taking into account their relative
potencies. Dose additivity presumes that the two agents act by the
same biological mechanism, and that the effective dose of one agent is
simply a dilution of the dose of the other agent. Response additivity
occurs when the two agents act independently of each other. In this
case, the probabilities of an adverse effect due to each of the agents
can be treated as statistically independent and can be combined
accordingly.
The quantification of interaction may be illustrated using the
age-specific relative risk (A,B) associated with exposure to two
agents A and B. The relative risk is formally defined as:
relative risk (A,B) = I(A,B)/I(0,0)
where I(A,B) denotes the incidence rate of the disease of
interest at a specified age in the presence of exposure and
I(0,0) is the incidence rate in the absence of exposure. Lack of
interaction then corresponds to additivity of the excess relative
risk (ERR = relative risk - 1).
Specifically,
ERR(A,B) = ERR(A,0) + ERR(0,B)
where ERR(A,0) and ERR(0,B) denote the excess relative risks for
A and B alone, respectively. In terms of relative risk,
additivity is equivalent to:
relative risk (A,B) = relative risk (A,0) + relative risk
(0,B) - 1
For a supra-additive relative risk relationship, such as the
multiplicative relative risk model:
relative risk (A,B) = relative risk (A,0)RR(0,B)
and reflects a synergistic effect.
It is of interest to note that as the relative risk (A,0) and relative
risk (0,B) become small, the multiplicative relative risk model
approximates the additive relative risk model. Under this
multiplicative model, a strong synergistic relationship may be
apparent at high exposures, yet become negligible at low exposures.
This, along with the realization that relative risks of less than
about two are difficult to detect epidemiologically, suggests that
relatively high exposures are likely to be required to evaluate
interactive effects reliably.
Brown & Chu (1989) and Kodell et al. (1991) investigated the type of
interactions that might be expected under both the Armitage-Doll
multi-stage model and the Moolgavkar-Venzon-Knudson two-stage model of
carcinogenesis following exposure to two carcinogens that may affect
different stages in the model. These theoretical results indicate that
a variety of synergistic interactions are possible, including
supra-additive, multiplicative, and supra-multiplicative.
Although the additive excess relative risk model described above
provides a useful baseline for evaluating interaction, it is not the
only model that has been proposed for this purpose. Kaldor & L'Abbe
(1990) pointed out that the multiplicative relative risk model will
become the baseline model for evaluating interactions following a
logarithmic transformation of the relative risk. Thomas & Whittemore
(1988) review arguments in favour of the additive and multiplicative
models as the basis for evaluating interaction. Steenland & Thun
(1986) illustrate how these two models can be applied in evaluating
tobacco/occupation interactions in the causation of lung cancer.
This brief overview illustrates that interaction can be measured in
different ways, with the most appropriate depending on the nature of
the problem at hand. In general, however, all of the commonly
encountered measures of synergism indicate that the risk associated
with combined exposure to two agents is in some sense greater than
would be expected based on the risks of individual exposures. Although
no attempt is made throughout this monograph to systematically specify
the precise nature of the interactive effects reported in the
literature, most interactive effects documented in this monograph have
been identified through epidemiological investigations, in which the
additive age-specific relative risk model is the predominant approach
to describing interaction (Saracci, 1987; Greenland, 1993).
There are four principal ways in which tobacco smoke can interact with
other chemicals to impair the health of the smoker. They are not
mutually exclusive and in fact there are many situations in which they
may occur together, particularly in the workplace or the environs of
industry.
a) Modification of effects
Cigarette smoke can modify the harmful effects associated with other
toxic agents, in some cases causing a highly elevated risk, e.g., the
effects of smoking on diseases related to asbestos, alpha-radiation,
arsenic and some organic compounds.
b) Increased concentration effects
Chemical compounds hazardous to health are often found in both tobacco
smoke and the working environment and each source can augment the dose
obtained from the other, e.g., carbon monoxide, acrolein, benzene and
heavy metal elements.
c) Vector effects
Materials used in the workplace that produce harmful chemical agents
when they are burnt or vaporized can contaminate smoking materials and
cause the smoke to be far more injurious when the tobacco is smoked,
e.g., polytetrafluoroethylene and methylparathion.
d) Other interactions
Tobacco smoke can affect a physiological process and increase the
impairment of physical or physiological functions caused by another
activity. For instance, impaired lung clearance will affect the
residence time of inhaled toxic materials, the effect of smoking on
the peripheral vascular system can enhance the detrimental effects of
vibration and noise, and smoking may alter the effect of drugs taken
for other purposes.
3.1.3 Effects of tobacco smoking on lung deposition and clearance of
particles
Pulmonary deposition of inspired particles depends on their
physicochemical properties and on airway structure and geometry.
Mathematical models describing the deposition of particles in the
various airway sections show that in compromised airways, as is the
case in patients suffering from asthma and chronic obstructive lung
diseases, particle deposition is enhanced several-fold (ICRP, 1994).
Tobacco smoking can be an indirect cause of enhanced deposition of
inspirable particles. In addition, during tobacco smoking the
breathing pattern is changed to more frequent and deeper inhalation,
especially in the case of low-nicotine cigarettes, which can result in
an increased inhaled dose and dose rate of inspirable compounds (IARC,
1990).
Clearance of particles deposited in the lung is a complex
physiological process involving relatively rapid tracheobronchial
clearance, in which mucus is moved upward by ciliary action to the
pharynx and swallowed, and slower deep-lung clearance, in which
phagocytic cells remove inhaled particles. These processes are
balanced by the solubility of the inhaled particles, with relatively
insoluble particles having a longer residence time in the alveolar
portions of the lungs. A longer residence time in the lung would be
accompanied by a greater possibility that harmful effects could occur.
Both rapid and slow clearance phases are reduced by smoking, although
probably by different mechanisms. Cigarette smoke contains significant
concentrations of ciliatoxic agents, such as hydrogen cyanide,
formaldehyde, acetaldehyde, acrolein and nitrogen oxides, which
greatly contribute to retarded clearance of inhaled particles by
inhibiting lung clearance mechanisms (Battista, 1976). Retardation of
clearance has been seen for airway-deposited particles, in which
decreased mucus transport velocities slow this normally relatively
rapid phase of clearance (Lourenco et al., 1971; Chopra et al., 1979).
The deep-lung clearance of relatively insoluble particles is retarded
in smokers. Cohen et al. (1979) found that 1 year after a tracer
particle exposure, some 50% and 10% of the original lung burden
remained in the lungs of smokers and non-smokers, respectively.
Bohning et al. (1982) and Philipson et al. (1996) found that smoking
retarded long-term particle clearance from the lungs. The mechanism(s)
for interference with this longer-term phase of clearance has not been
shown definitively, but may be related to impairment of phagocyte
function and/or smoke-induced lung damage.
3.2 Interactions between tobacco smoke and other agents
3.2.1 Asbestos
Asbestos is a generic name for a group of fibrous silicates, differing
in colour, fibre arrangement and length. Recognition of the health
risks of asbestos has led to major reductions in production and uses.
Asbestos types are classified according to their physical
characteristics as serpentine or amphibole and differ in their
relative carcinogenic potential. Amosite and crocidolite are
amphiboles and have short and straight needle-like fibres. Chrysotile
is a serpentine and consists of long, pliable white fibres. The longer
fibre varieties of asbestos can be spun into yarn which can be woven
into fabric; short fibre varieties can be incorporated into cement,
asbestos board and tiles. Asbestos products have been used in a
variety of applications including electrical and thermal insulation in
buildings, fire and safety equipment, brake linings of motor vehicles,
and shipbuilding. Workers in asbestos mining and processing and a wide
range of manufacturing industries are exposed to various forms of
asbestos, while others are exposed in maintenance work, demolition and
recycling operations.
Occupational exposure to asbestos is associated with asbestosis and
cancers at various sites, notably pleural mesothelioma and lung
cancer. Differences between the effect of asbestos on the health of
smokers and non-smokers have been reported, and studies have been
conducted aimed specifically at elucidating the combined effects of
smoking and asbestos exposure. Perioccupational exposure to asbestos
is a hazard to household contacts of asbestos workers, who bring home
dust on their clothes, and to people living in areas where there is
environmental contamination by asbestos dust from industry (Anderson
et al., 1979).
The amphibole varieties of asbestos (crocidolite and amosite) have the
highest carcinogenic risk. Crocidolite presents a greater risk than
amosite, which in turn is more dangerous than chrysotile, a serpentine
variety. Erionite and tremolite are non-asbestos fibrous minerals used
in building in some parts of the world and there is a high prevalence
of mesothelioma in these regions (Baris et al., 1979; Yazicioglu et
al., 1980).
Because there are many different occupations and environmental
situations in which asbestos exposure might occur, along with a wide
range of possible levels of exposure and variety of types of asbestos
in use, it is difficult to define clearly asbestos exposure or the
smoking habits of those exposed. The smoking history of the population
sampled is important, because there have been changes in smoking
materials and prevalences of smoking in many countries (Cheng & Kong,
1992). In many studies, only the number of smokers within sub-groups
of workers with asbestos-related disease have been reported, rather
than the detailed smoking habits of the exposed population. A widely
used assumption is that the smoking habits of asbestos-exposed workers
reflect those of blue collar workers and are thus higher than national
average figures. Table 5 gives examples of smoking prevalence in
different groups of asbestos-exposed workers.
3.2.1.1 Asbestos and lung cancer
Exposure to asbestos dust carries a risk of parenchymal and pleural
fibrosis, mesothelioma and lung cancer. Selikoff et al. (1968) and
Berry et al. (1972) showed that cigarette smoking was an added hazard
among asbestos workers. In combination, the two hazards are associated
with very high lung cancer rates. Studies were carried out (e.g.,
Hammond & Selikoff, 1973; Martischnig et al., 1977; Hammond et al.,
1979; Selikoff et al., 1980; Acheson et al., 1984; Berry et al., 1985)
to determine whether cigarette smoke and asbestos act independently,
Table 5. Smoking prevalence in asbestos-exposed workers
Exposure Smoking habits References
Asbestos textile workers 75% smokers Weiss (1971)
46% cigarette smokers
36% ex-cigarette smokers
5.5% pipe/cigar smokers
Electrochemical plant 84% to 87% were smokers
(two areas) or ex-smokers Kobusch et al. (1984)
Population in Telemark, Asbestos exposed: Hilt (1986)
Norway 44.6% smokers
36.0% ex-smokers
Not exposed:
40.95% smokers
28.6% ex-smokers
Survey of 800 000 American Asbestos exposed: Stellman et al. (1988)
men and women in 1982 33.6% smokers
47.3% ex-smokers
Lung cancer case-referent Men: 95% smokers Järvholm (1993)
study; Swedish industrial city Women: 78% smokers
Shipyard workers in 46% smokers Sanden et al. (1992)
Gothenburg, Sweden 31% ex-smokers
December 1987 21% non-smokers
2% not known
Asbestos factory workers Men: 74% smokers Newhouse & Berry (1979)
(male population average 66%)
Women: 49% smokers
(female population average 40%)
their combined effect being the sum of the individual effects, or
there is an interaction with the ultimate effect being a product of
the two risk factors. In some studies, the effects of smoke and
asbestos appeared to be additive, in others multiplicative and in
others somewhere between the two. Reasons for the lack of consistency
among the studies may relate to the size of the population sampled,
its average age, social class and residential area, the type of
asbestos involved, the time scale covered and the intensity of
exposure to asbestos. The weight of evidence favours a synergistic or
multiplicative model for the interaction of asbestos and smoking.
While the differences may be partly linked to the carcinogenic
potential of different types of asbestos and to different smoking
materials and ways of smoking, including passive smoking (Cheng &
Kong, 1992), they also reflect the complex nature of tobacco smoke,
which contains complete carcinogens, tumour promoters and
co-carcinogens and other compounds that can influence the multistage
carcinogenic process. However, whatever the type of smoking/asbestos
interaction influencing the incidence of lung cancer, there is a
greatly increased risk for the asbestos-exposed worker who smokes
(Table 6).
Hammond et al. (1979) found a very strong synergistic effect and this
was supported by studies of shipyard workers in Italy (Bovenzi et al.,
1993), asbestos factory workers in London (Newhouse & Berry, 1979),
Finnish anthophyllite miners and millers (Meurman et al., 1979),
chrysotile workers in China (Cheng & Kong, 1992; Zhu & Wang, 1993) and
workers exposed to crocidolite in Western Australia (de Klerk et al.,
1991). Cheng & Kong (1992) reported a lower ratio of non-smoking to
smoking lung cancer death rates and suggested that this reflected
passive smoking among non-smokers and the use by most smokers of
hand-rolled cigarettes. Liddell et al. (1984) found that their data
fitted both an additive model and a multiplicative model and concluded
that the combined relative risk lay somewhere between the two.
Selikoff et al. (1980), from a study of amosite factory workers, and
Berry et al. (1985), from a study of asbestos factory workers,
favoured an additive model. However, caution is required because of
the definitions of additive and multiplicative used by different
authors and the overlap between these terms and such words as
synergism and promoter.
Molecular biology studies of autopsy specimens of lung tumour tissue
from of cigarette smokers have revealed that cigarette smoking induces
K-ras mutation (Rodenhuis & Slebos, 1992). It has been suggested that
such cigarette-smoke-induced K-ras oncogene mutations are promoted by
the presence of asbestos, which creates selective growth conditions
for the mutated cells (Vainio et al., 1993). Vainio & Boffetta (1994)
concluded that both tobacco smoke and asbestos fibres can be genotoxic
and cytotoxic, and cause proliferative lesions in the lungs. Tobacco
smoke contains carcinogens that bind to critical genes and cause
mutations. Asbestos fibres cause chronic inflammation of the lungs,
which releases various cytokines and growth factors, and may provide a
selective growth advantage for mutated cells.
Table 6. Age-standardized lung cancer death ratesa for cigarette smoking and/or
occupational exposure to asbestos dust compared with no smoking and no occupational
exposure to asbestos dust (from: Hammond et al., 1979)
Group Exposure to History cigarette Death Mortality Mortality
asbestos? smoking? rate difference ratio
Control No No 1.3 0.0 1.00
Asbestos workers Yes No 58.4 +47.1 5.17
Control No Yes 122.6 +111.3 10.85
Asbestos workers Yes Yes 601.6 +590.3 53.24
a Rate per 100 000 man-years standardized for age on the distribution of the
man-years of all the asbestos workers; number of lung cancer deaths based on
death certificate information
3.2.1.2 Asbestos and pleural mesothelioma
There is an established relationship between exposure to asbestos
- crocidolite, amosite, chrysotile - and pleural mesothelioma
(Stellman, 1988). In shipyard workers mainly exposed to chrysotile,
Sanden et al. (1992) found an increase in pleural mesotheliomas up to
15 years after cessation of exposure. Asbestos is also linked with
peritoneal mesothelioma (Newhouse & Berry, 1976). The risk of lung
cancer was found to fall after exposure ceased, suggesting that
asbestos acted as a lung cancer promoter, but the risk of mesothelioma
long after cessation of exposure indicated that asbestos acted as a
complete carcinogen. Mesothelioma can have an extremely long latent
period, with cases presenting even 30 years or more after first
exposure (Newhouse & Berry, 1976). Up to 90% of cases of pleural
mesothelioma have been attributed to asbestos but there is no evidence
directly associating smoking with the disease, or showing that smoking
has any influence on the incidence of asbestos-related pleural
mesothelioma (Berry et al., 1985; Hughes & Weill, 1991; Sanden &
Jarvholm, 1991; Muscat & Wynder, 1991).
3.2.1.3 Asbestos and other forms of cancer
Asbestos fibres have been found in many tissues, other than the lungs,
of asbestos workers. There is evidence that an asbestos/smoking
interaction increases the incidence of cancer of the oesophagus,
pharynx, buccal cavity and larynx but not of pleural or peritoneal
mesothelioma, or of cancer of the stomach, colon-rectum or kidney, for
which smoking and non-smoking asbestos workers are at equal risk
(Hammond et al., 1979; Selikoff & Frank, 1983; US ATSDR, 1995).
3.2.1.4 Asbestosis
Asbestosis is a fibrotic reaction to asbestos in the lungs. In a
review of histological, animal experimental and radiological evidence,
Weiss (1984) concluded that cigarette smoking could result in diffuse
fibrosis similar to that caused by asbestos, and the fibrosis showed a
dose-response to the duration and degree of smoking. Prevalence
studies are consistent in showing a higher frequency of diffuse small
irregular opacities in asbestos workers who are smokers than in those
who are non-smokers. It has been suggested that the effects may be
additive. Tobacco smoke affects lung clearance and hence the retention
of asbestos fibres in the lungs. In asbestosis the intensity of
fibrosis correlates with the number of asbestos bodies in the lungs,
and Murai et al. (1994) concluded that reduction of lung clearance by
tobacco smoke could increase the intensity of fibrosis. Crocidolite
fibres are the most fibrogenic of the various types of asbestos but
De Klerk et al. (1991) concluded that smoking had no measurable effect
on crocidolite asbestosis.
An interaction between asbestos and smoking causing a greater
frequency of obstructive airways disease in asbestos workers who smoke
was found in a study of pulmonary function changes caused by
asbestosis (Selikoff & Frank, 1983). Miller (1993) presented similar
results suggesting an interaction between asbestos and smoking. In a
prospective mortality study, Hughes & Weill (1991) concluded that
asbestosis is a precursor of asbestos-related lung cancer, but they
were unable to assess an interaction between tobacco smoking and
asbestosis because all the cases were in smokers and there were no
non-smokers.
In rats, asbestos fibres stimulate alveolar macrophages to generate
the inflammatory and fibrogenic mediators, tumour necrosis
factor-alpha (TNF-alpha), and this may be the cause of inflammation
and lung fibrosis due to asbestos (Ljungman et al., 1994). In in vitro
studies Morimoto et al. (1993) found synergism between chrysotile
fibres and cigarette smoke in the stimulation of the formation of
TNF-alpha by rat alveolar macrophages.
3.3 Non-asbestos fibres
3.3.1 Glass fibre
IARC (1988) classified glasswool as possibly carcinogenic to humans
(Group 2B) and glass filaments as not classifiable as to their
carcinogenicity to humans (Group 3), based on sufficient evidence for
the carcinogenicity of glasswool and inadequate evidence for the
carcinogenicity of glass filaments in experimental animals and
inadequate evidence for the carcinogenicity of glasswool and glass
filaments in humans. There are data on exposure to glass fibre and
tobacco smoke. Enterline et al. (1987a) carried out a case control
study of 7586 glasswool workers in four plants producing small
diameter fibres, less than 3 µm in diameter. Smoking histories were
obtained for 75% of the workers. Analysis of data by logistic
regression showed that smoking was a powerful variable and multiplied
the effect of fibre exposure. In a case-control study of the influence
of non-workplace factors on respiratory disease in employees of a
glass fibre manufacturing facility, Chiazze et al. (1992, 1995)
concluded that smoking, and not exposure to glass fibre, was the most
important risk factor for the increased lung cancer risk but was not
as important for non-malignant respiratory disease. In a further
analysis, using data not previously available, Chiazze et al. (1995)
estimated the extent of confounding by cigarette smoking, and
suggested that adjusting for the confounding effect could reduce the
lung cancer standardized mortality ratio to a non-statistically
significant level.
3.3.2 Rockwool, slagwool and ceramic fibres
IARC (1988) concluded that there was limited evidence for the
carcinogenicity of rockwool and inadequate evidence for the
carcinogenicity of slagwool in experimental animals, with limited
evidence for the carcinogenicity of rock-/slagwool in humans: the
overall evaluation for both was Group 2B, possibly carcinogenic to
humans. For ceramic fibres there was sufficient evidence for their
carcinogenicity in experimental animals, with no data on their
carcinogenicity in humans: the overall evaluation for ceramic fibres
was also Group 2B, possibly carcinogenic to humans.
In a study of insulation workers using rock and glass wool, (Clausen
et al., 1993) concluded that exposure was associated with an increased
risk of developing obstructive lung disease. In a study of respiratory
health in 628 workers in seven European plants manufacturing ceramic
fibres, skin, eye and nasal irritation, breathlessness and wheezing
were common findings (Trethowan et al., 1995). Respiratory symptoms
were more frequent in smokers and increased with the amount smoked.
The authors concluded that exposure caused irritation, similar to that
caused by other man-made fibres, and that cumulative exposure could
cause airways obstruction by promoting the effects of cigarette smoke.
Ljungman et al. (1994) demonstrated in rats that rock wool, slag wool,
kaolin ceramic fibre and silicon carbide fibre stimulated alveolar
macrophages to generate tumour necrosis factor-alpha (TNF-alpha), a
potent inflammatory and fibrogenic mediator. In in vitro studies
Morimoto et al. (1993) found synergism between mineral fibres
(chrysotile and alumina silicate ceramic fibres) and cigarette smoke
in the stimulation of the formation of TNF-alpha by rat alveolar
macrophages. Leanderson & Tagesson (1989) found that cigarette smoke
potentiated the DNA-damaging effect of man-made mineral fibres
(rockwool, glasswool and ceramic fibres).
3.4 Inorganic chemicals
3.4.1 Arsenic
Compounds of arsenic have been used as pesticides and as preservatives
of wood and leather. Arsenic is present in many metal ores and is
released during smelting Radon progeny are frequently encountered as a
contaminant of arsenic. In some parts of the world arsenic is found in
drinking-water in relatively high concentrations.
Arsenic and its compounds are carcinogenic (WHO, 1980; IARC, 1987;
Tsuda et al., 1990, 1995). Skin cancer can occur after ingestion of
arsenic (Tseng et al., 1968; Smith et al., 1992), and lung cancer
after inhalation of arsenic by smelter workers or by people living
nearby (Welch et al., 1982; Pershagen, 1985; Pershagen et al., 1987)
or by agricultural workers exposed to the pesticide lead arsenate
(Wicklund et al., 1988). IARC (1987) classed arsenic and arsenic
compounds as Group 1, carcinogenic to humans. It has been suggested
that arsenic in drinking-water may also cause liver, lung, kidney and
bladder cancer (Smith et al., 1992).
A study of the lung cancer risk among cadmium-exposed workers
suggested that exposure to arsenic and tobacco smoke may have been the
cause of an increased rate of lung cancer, rather than exposure to
cadmium particulates (Lamm et al., 1992). Tsuda et al. (1990)
suggested an interaction between arsenic and smoking in exposed
workers in a small Japanese village where arsenic was mined and
refined. However, the village water and air were highly polluted by
emissions from the smelter and from slag disposal, making interaction
between arsenic and smoking difficult to assess. A study of copper
smelter workers in the USA indicated that the effect of arsenic was
probably more important in lung cancer than that of tobacco smoke
(Welch et al., 1982). Studies in Sweden showed increased lung cancer
risks from arsenic exposure at a copper smelter; a multiplicative
effect for smoking and arsenic was found and age-standardized rate
ratios for lung cancer mortality were 3.0 for arsenic-exposed workers,
4.9 for smokers with no arsenic exposure and 14.6 for arsenic-exposed
smokers (Pershagen et al., 1981). In a later study, Pershagen (1985)
reported an additive effect for smoking and arsenic exposure on lung
cancer incidence in situations where the arsenic exposure was lower.
In a cohort of 3916 Swedish copper smelter workers, the risk of
developing lung cancer from the interaction between arsenic and
smoking was intermediate between additive and multiplicative and
appeared to be less pronounced among heavy smokers (Jarup & Pershagen,
1991).
There was no evidence of synergism between arsenic and tobacco smoke
in tin miners in Yunan Province, China. The lung cancer risk was
greater for arsenic than for smoking, and simultaneous assessment of
arsenic and radon exposure revealed radon to be the greater risk
(Taylor et al., 1989). In Ontario it was concluded that the excess
lung cancer mortality of gold miners and uranium miners was probably
due to exposure to arsenic and short-lived radon decay products
(Kusiak et al., 1991). This was consistent with the hypothesis that
the risk of lung cancer from exposure to arsenic is enhanced by
exposure to other carcinogens (Kusiak et al., 1993).
Hertz-Picciotto et al. (1992) assembled data from several studies to
examine possible synergism between smoking and exposure to arsenic and
an increased risk of lung cancer. The joint effect from both exposures
consistently exceeded the sum of the separate effects: a minimum of
30% to 54% of lung cancer cases among those with both exposures could
not be attributed to either one or the other exposure alone. The
conclusion was that arsenic and smoking acted synergistically to cause
lung cancer. Arsenic-induced lung cancer was not limited to exposure
to inhaled arsenic because there was evidence of synergism between
ingested arsenic and smoking (Tsuda et al., 1995). An association of
arsenic exposures with bladder cancer was confined to subjects who had
been smokers Bates et al. (1995).
In a Swedish study of lung cancer in arsenic workers, it was found
that cases among smelter workers who had never smoked showed a
histological distribution resembling that of smokers, probably
reflecting an exposure to carcinogenic agents at the smelter which
influence the risk of different histological types in the same way as
smoking (Pershagen et al., 1987). Tobacco smoking primarily induces
epidermoid and small cell carcinomas but there are also increased
risks for other cell types. The proportion of small cell carcinomas
was greater in uranium miners than in the general population (Kusiak
et al., 1993). In smokers, there were no pronounced differences in the
histological type of lung carcinomas between arsenic exposed smelter
workers and controls (Pershagen et al., 1987).
It has been suggested that the potentiation of the carcinogenic
properties of arsenic by smoking could be due to inorganic arsenic
requiring a strong co-carcinogen to manifest a carcinogenic effect, or
that arsenic itself might be acting as co-carcinogen rather than as a
direct carcinogen (Stohrer, 1991; Tsuda et al., 1995).
3.4.2 Beryllium
Beryllium is a metal with a number of uses including alloys, nuclear
energy applications, and in the rocket and aerospace industry (IPCS,
1990; IARC, 1993). Fine dusts and fumes of the metal and some of its
salts are hazardous and when inhaled are deposited in the lungs from
where beryllium may be widely distributed throughout the body.
Beryllium metal, oxide and some salts give rise to acute inflammation
on skin contact, particularly when accompanied by friction or
perspiration. Short exposure to dusts and fumes can cause acute
inflammation of mucous membranes: conjunctivitis, bronchitis,
pneumonitis. Granulomatous reaction can follow chronic inflammation of
the skin, and lesions may appear in the liver and elsewhere after long
periods of absorption from the lungs. Beryllium and its compounds are
a cause of delayed pneumonitis and pulmonary granulomas. IARC (1993)
classified beryllium and beryllium compounds as Group 1, carcinogenic
to humans, on the basis of sufficient evidence in humans and in
experimental animals. However, in epidemiological studies the
information on smoking was incomplete and the data did not rule out
the possibility that the few excess deaths observed could have been
due to smoking rather than to any other cause (Steenland & Ward, 1991,
1993; Eisenbud, 1993; MacMahon, 1994; Kotin, 1994).
The prevalence of chronic beryllium disease (CBD) is reduced in
smokers compared with non-smokers. The disease is preceded by the
development of beryllium-specific sensitization. In two studies
examining different worker populations, the prevalence of smoking in
those with clinically diagnosed CBD was lower than in those that were
sensitized but did not have the disease (Kreiss et al., 1993; Kreiss
et al., 1996).
3.4.3 Chromium
Chromium and its compounds are used in metallurgical, chemical,
electroplating and leather tanning industries (IARC, 1990). The
principal route of entry to the body is through the lungs. Chromium
ulcers of the skin and dermatitis can result from handling chromium
products and deposition of chromates on mucous membranes can also
cause ulceration which, in the nasal septum, can lead to perforation
(Lindberg & Hedenstierna, 1983; IARC, 1990). Chromium and some
chromium compounds are respiratory tract sensitizers and a cause
asthma. Hexavalent chromium salts have been associated with lung
cancer both in experimental animals and in epidemiological studies.
IARC (1990) concluded that there is sufficient evidence in humans for
the carcinogenicity of hexavalent chromium compounds as encountered in
chromate production, chromate pigment production and chromium plating
industries.
Langård & Norseth (1975) suggested that cigarette smoking increases
the risk for lung cancer in workers exposed to chromate dust. Other
studies (Abe et al., 1982; Langård & Vigander, 1983; Yoshizawa, 1984;
Nishiyama et al., 1985) have suggested that workers with exposure to
chromium compounds who are also smokers may be at greater risk than
non-smokers. However, the numbers were too small for conclusions on
interactions to be drawn.
3.4.4 Nickel
Exposure to nickel or its compounds occurs in mining, refining,
smelting and alloying the metal, in nickel plating and in welding. It
is used in battery manufacture, electroplating, enamelling, ceramics,
the chemical and petroleum industries and in dyestuffs and ink making.
Exposure may be by skin contact or inhalation of dusts, fumes, mists
or gaseous nickel carbonyl (IPCS, 1991a). In occupational exposure the
daily intake and absorption/retention vary widely between industries
(IARC, 1990).
Nickel is absorbed from the soil by the tobacco plant. During smoking,
up to 20% of the nickel in the tobacco is transferred to mainstream
smoke. This high transfer rate, compared to the much lower transfer
rates of other metals, has been explained by the formation of the
volatile nickel carbonyl (Sunderman & Sunderman, 1961). Nickel
carbonyl is a strong lung carcinogen in rats (IARC, 1990).
IARC (1990) evaluated the carcinogenicity of nickel and nickel
compounds and classified nickel compounds as carcinogenic to humans
(Group 1) and nickel as possibly carcinogenic to humans (Group 2B). In
an epidemiological study on a cohort of 916 workers in a Norwegian
nickel refinery, four work categories were defined: i) roasting and
smelting; ii) electrolysis; iii) other processes; and iv) other work
groups. All groups showed an excess risk of respiratory cancer. In the
roasting and smelting department there were excess risks for lung
cancer (O/E = 12/2.5) and nasal cavity cancer (O/E = 5/0.1). In the
electrolysis department there were also excess risks of lung cancer
(O/E = 26/3.6) and nasal cavity cancer (0/E = 6/0.2) (Pedersen et al.,
1973). Magnus et al. (1982) updated this study and found evidence of
an additive effect of smoking and nickel exposure in the induction of
respiratory cancer. Histological examination of nasal biopsy specimens
from 59 retired nickel workers, 21 of whom were smokers and snuff
users, showed a higher score of nasal epithelial dysplasia in smokers
than in non-smokers, and 4 workers with nasal carcinoma were smokers
(Boysen et al., 1984). In monitoring nickel exposure by imaging
cytometry of nasal smears (Reith et al., 1994), it was possible to
distinguish between workers who were exposed to different nickel
compounds and to distinguish between smoking and non-smoking nickel
workers.
3.4.5 Manganese
There is occupational exposure to manganese in its mining, the
ferromanganese and iron and steel industries, the production of dry
cell batteries, and the manufacture and use of welding rods. Manganese
is released by the combustion of the gasoline additive,
methylcyclopentadienyl manganese tricarbonyl (MMT), used in some
countries. However, the amount in gasoline (approximately 10 mg/litre)
and emitted in vehicle exhausts is small and does not to lead to human
exposure (Health Canada, 1994). The principal route of entry of
manganese is through the lungs but, because most of the compounds are
insoluble, only the smallest particles, as contained in furnace and
welding fume, are capable of reaching the alveoli and being
phagocytosed and absorbed (IARC, 1986).
Manganese is present in tobacco leaves and it has been reported (IARC,
1986) that 0.003 µg of manganese appears in the mainstream smoke from
one cigarette and thus contributes to a smoker's manganese intake in
the form of small and dangerous particles.
Manganese is neurotoxic and long-term occupational exposure can cause
a condition resembling Parkinson's disease. It causes lung damage
leading to an increased incidence of pneumonia and a higher rate of
acute and chronic bronchitis. In studies of manganese alloy production
workers and chronic lung disease, smokers were more affected than
non-smokers and the relationship between the number of cigarettes
smoked and the prevalence of respiratory tract symptoms suggested that
smoking acted synergistically with manganese (Saris & Lucic-Palaic,
1977). In a study of workers producing manganese salts and oxide
(Roels et al., 1985) smoking and manganese exposure were additive in
producing preclinical toxic effects. In studies on workers producing
iron-manganese alloys, one concerned with chronic bronchitis
(Misiewicz et al., 1994) and the other with pulmonary ventilatory
disturbance (Misiewicz et al., 1992), there was no relationship
between the occupational exposure or its duration and health effects.
The chronic bronchitis and ventilatory disturbance were attributed to
cigarette smoking.
3.4.6 Platinum
Platinum is used as a catalyst in many chemical processes and in motor
vehicle exhausts. Chloroplatinic acid is an intermediate in the
preparation of a large number of complex salts, which are used in
platinum refining, the chemical industry and, therapeutically, in
cancer chemotherapy.
Platinum salt sensitivity is an IgE-mediated immune response (IPCS,
1991b). Ammonium hexachloroplatinate is used as an intermediate in
platinum refining, and its inhalation provokes asthmatic responses and
elicits immediate skin test responses in sensitized individuals (Pepys
et al., 1972). In a cohort study of 91 workers in a platinum refinery
(Venables et al., 1989), 22 developed respiratory symptoms and an
immediate skin test response to ammonium hexachloroplatinate. The risk
was greatest in the first year of employment and smokers had an
increased risk of becoming sensitized. In another study, out of 78
workers at a platinum refinery, 32 (41%) had developed platinum salts
sensitivity after 24 months of exposure and the risk of sensitization
was about 8 times greater for smokers (Calverley et al., 1995).
Baker et al. (1990) conducted a cross-sectional study of respiratory
and dermatological effects of platinum salt sensitization in 136
workers (107 current employees and 29 former employees) at a precious
metal refinery. Twenty three workers (22%) had become sensitized.
Platinum salts sensitivity was not associated with atopicity but was
strongly associated with cigarette smoking status.
3.4.7 Silica
Silicosis (lung fibrosis caused by silica) is not only a hazard of
mining. It is also found in bricklayers, cement makers, workers in
pottery, porcelain and ceramics, rock drillers, workers chipping,
grinding or polishing stone, in sandblasting, using grinding stones to
smooth or polish precious stones, metals or optical glass, and in the
manufacture of polishing materials such as metal polishes and
toothpaste. The number of industries generating silica dust is large.
The amount of respirable dust varies from one to another and, because
silica is an active adsorbent, it can become contaminated and have its
toxic potential changed. Furthermore, freshly fractured silica dust
may exhibit a different surface reactivity and cytotoxicity from that
of aged silica (Vallyathan et al., 1988).
Hnizdo & Sluis-Cremer (1991), in a study of gold miners, linked high
exposure to silica dust with lung cancer and found a combined effect
of dust and smoking that fitted a multiplicative model for lung
cancer. Amandus et al. (1992) studied lung cancer in men with
diagnosed silicosis and suggested that there was an association
between the two diseases. In a study of iron foundry workers
(Andjelkovich et al., 1994), cigarette smoking was a strong predictor
of lung cancer whereas silica exposure showed no association with the
disease.
Chronic silicosis develops after 20 to 40 years of exposure to silica
dust. There are also other types of pneumoconiosis related to the
nature of the dust, and chronic bronchitis and airways obstruction
have been associated with silica dust exposure. A hypothesis for the
pathogenesis of chronic silicosis is that silica particles are
phagocytosed by the alveolar macrophages for which they have a marked
selective toxicity. Permanent macrophage activation initiates
inflammatory reactions leading to the formation of collagenous fibres.
Acute silicosis arises from the inhalation of more highly reactive
silica (Vallyathan et al., 1988). The link between silicosis and
smoking was examined in a study of smoking and silica exposure on
pulmonary epithelial permeability. Faster clearance of a radioaerosol
from the upper lung regions was found for smokers (Nery et al., 1988,
1993). The question of silica clearance was considered in an analysis
of an association between silicosis and smoking: differences in
collagenization for smokers and non-smokers were attributed to
differences in the interception of silica particulate matter by mucus
(Hessel et al., 1991). In studies by Ng et al. (1987, 1992), smoking
was not considered to affect the progression of silicosis in granite
quarry workers. However, examining the association of silicosis with
lung cancer, Ng et al. (1990) concluded that the excess lung cancer
risk in silicosis is attributable to smoking, and there appeared to be
a synergistic effect between smoking and silica/silicosis regarding
the risk of developing lung cancer.
In a study of 562 South African gold miners exposed to low levels of
dust with a high (50-70%) silica content, the incidence of chronic
bronchitis was higher than in non-dust-exposed controls. Although the
percentage of smokers was higher in those with chronic bronchitis in
both groups, there was a significant excess in the dust-exposed
smokers (Sluis-Cremer et al., 1967). The authors concluded that there
was a factor in dust-exposed smokers that increased the incidence of
chronic bronchitis above that expected from smoking alone. In a study
of 2209 South African gold miners and 483 non-miners on the effect of
silica dust and tobacco smoking on mortality from chronic obstructive
lung disease, it was found that miners who smoked and were exposed to
silica dust were at higher risk of dying from chronic obstructive lung
disease than smokers not exposed to silica dust. In South African gold
mines about 30% of the respirable dust is free silica. It was
concluded that tobacco smoking and silica dust acted synergistically
in causing chronic obstructive lung disease (Hnizdo, 1990). Hnizdo et
al. (1990) applied additive and multiplicative relative risk models to
the same sample and found that departure from additivity increased
progressively with the severity of obstructive impairment. They
concluded that tobacco smoking potentiated the effect of dust in
causing respiratory impairment and that severe respiratory impairment
could have been prevented through elimination of tobacco smoking
(Hnizdo et al., 1990). Oxman et al., (1993) analysed the relationship
between occupational dust exposure and chronic obstructive lung
disease in both gold and coal miners and found a significant
association between loss of lung function and cumulative respirable
dust exposure, which was greater in gold miners. In this study there
was no evidence of interaction with tobacco smoking for gold miners,
and the authors suggested that the increased risk of lung function
loss was due to exposure to dust having a higher silica content than
coal dust. Among iron ore miners in Sweden, Jörgensen et al. (1988)
found a strong relationship between chronic bronchitis and smoking,
but not with working underground. The two risk factors, silica dust
and smoking, appeared to be additive but the smoking effect was far
greater than that of silica dust. In a study of small airway disease
in patients with silica dust exposure, with and without radiographic
evidence of silicosis, and smoking, Avolio et al. (1986) found no
differences in lung function and prevalence of small airways disease
with silicosis. However, in both groups small airway disease was
significantly related to tobacco smoking, indicating that this had a
more powerful effect than silicosis.
3.5 Organic chemical agents
Many organic compounds with properties covering a wide spectrum of
molecular structure and biological activity are encountered in a
variety of industries. The effects of a few compounds, some of which
are encountered in specific industries, and smoking have been studied.
Where organic compounds occur in both tobacco smoke and the workplace,
the effect of smoking becomes one of dose augmentation, although
modification of effect can also occur. Some organic compounds would
normally not be found in tobacco smoke but are present because
workplace materials have contaminated smoking materials and they are
then pyrolysed or volatilized during smoking.
3.5.1 Chloromethyl ethers
Chloromethyl methyl ether (CMME) and its contaminant bis(chloromethyl)
ether (BCME) are used in the synthetic chemical industry, in the
manufacture of ion exchange resins and in polymer production. They are
carcinogenic when inhaled, BCME more so than CMME. In a long-term
study of chemical workers, 93 had exposure to chloromethyl ethers and
22 died from lung cancer. Of 32 workers who had no exposure, 3 died
from lung cancer (Weiss & Boucot, 1975; Weiss, 1976, 1980, 1982). For
the 22 cases in of lung cancer in the exposed workers, a dose-response
relationship was established. In the groups with heavy occupational
exposure, there were fewer heavy smokers (>20 cigarettes per day)
than in the groups with lower occupational exposure. This
statistically significant shift might be explained by self-selection
of heavy smokers out of the high occupational exposure groups because
of the bronchial irritation that is caused by the exposure to
chloromethyl ethers, or cigarette smoking might have an antagonistic
activity (Steenland & Thun, 1986; Thomas & Whittemore, 1988; Weiss &
Nash, 1997).
3.5.2 Tetrachlorophthalic anhydride
Tetrachlorophthalic anhydride (TCPA) is used as an epoxy resin curing
agent. It is respiratory tract sensitizer and causes asthma (Schlueter
et al., 1978). In a study using a radio allergosorbent test with a
TCPA human serum albumin conjugate, specific IgE antibody was detected
in serum from 24 out of 300 factory floor workers exposed to TCPA. Of
these 24, 20 (83%) were current smokers, compared with 133 (48%) of
276 without antibody (p<0.01), and there was a weaker association
with atopy, defined by skin tests with common allergens. Smoking and
atopy interacted, the prevalence of antibody being 16% in atopic
smokers, 12% in non-atopic smokers, 8% in atopic non-smokers and none
in the non-atopic non-smokers. It was concluded that smoking may
predispose to, and interact with atopy in the production of specific
IgE antibody to this hapten protein conjugate.
3.5.3 Dyestuffs
There is an established relationship between bladder cancer and
exposure to certain aromatic amines encountered in the dyestuffs
industry, e.g., benzidine, 4-aminobiphenyl and 2-naphthylamine (IARC,
1987), and smoking is causally associated with bladder cancer (IARC,
1986; US Surgeon General, 1989). From an analysis of 991 cases by
Cartwright (1982), a significant risk of bladder cancer was associated
with cigarette smoking, and a dose-response relationship, based on
years of employment, was found in workers in dyestuffs manufacturing.
The risks were considered to be additive. Overall, there was a
significant risk of bladder cancer associated with cigarette smoking,
a risk ratio of 1.8 for males, and there were significant overall
risks associated with occupations such as those of process workers in
the dye manufacturing industry who had a risk of 2.9 for males. When
dye manufacturing process workers who were smokers were compared with
non-smoking workers, the risk for smokers was 4.6, while for
non-smokers the risk was 1.9.
Boyko et al. (1985) concluded that arylamines in the dyestuffs
industry posed a major threat of bladder cancer. However, there was
little evidence to support an effect due to smoking or an interaction
between smoking and occupational exposure.
In an area of Spain where 44% of the adult population worked in dyeing
and printing textile fabrics, there was an increased risk of bladder
cancer for smokers (OR 2.3) (Gonzalez et al., 1985). The estimated
risks for occupation and for smoking and occupational exposure were OR
5.5 and 11.7, respectively. The observed effect was multiplicative.
Tobacco smoke contains many amines, including the bladder carcinogens
4-aminobiphenyl (>9 ng/cigarette) and 2-naphthylamine (54
ng/cigarette) (Patrianakos & Hoffmann, 1979; Pieraccini et al., 1992;
Grimmer et al., 1995).
In a study of risk factors for bladder cancer in Spain (Bravo et al.,
1987), the results were considered to corroborate previous data that
bladder cancer does not have a single cause. Cigarette smoking was
considered an important cause but one which was additional to
urological disease or occupational exposure, among other factors. In a
study of men in Spain (Gonzalez et al., 1989), increased risks of
bladder cancer were found for textile workers (OR 1.97), mechanics and
maintenance workers (OR 1.86), and workers in the printing industry
(OR 2.06). The highest risk was in those who were employed in the
textile industry before the age of 25 and prior to 1960. Among
mechanics the highest risk was for those who started after the age of
25 and after 1960. The OR for smokers who had also been employed in
one of the high-risk occupations was 7.82, which is compatible with a
multiplicative effect of joint exposure to tobacco smoke and
occupational hazards. In an Italian study (D'Avanzo et al., 1990),
risk additivity was found for the interaction between tobacco smoke
and several occupations associated with bladder cancer but the
occupations were not specified. The bladder cancer risk for smokers of
black tobacco was higher (OR = 3.7) compared with smokers of blond
tobacco cigarettes (OR = 2.6). A higher risk for black tobacco than
for blond varieties and a protective effect for smokers of tipped
cigarettes was also reported in a study in Northern Italy where a
multiplicative effect for smoking and high risk occupations was also
found (Vineis et al., 1984).
Bartsch et al. (1993) correlated the higher incidence of bladder
cancer among smokers of black tobacco with high yield aromatic amines,
particularly 4-aminobiphenyl from black tobacco (5 times greater than
from blond tobacco). The concentrations of urinary mutagens and of
4-aminobiphenyl adducts in the blood were also higher in smokers of
black tobacco.
A Chinese study of bladder cancer incidence and mortality in workers
with benzidine exposure found a marked dose response and an elevated
risk for both producers and users of benzidine. Workers exposed to
benzidine who were smokers had a 31-fold risk, while the risk for
exposed workers who were non-smokers was 11-fold, and a multiplicative
interaction was suggested (Bi et al., 1992).
3.5.4 Polycyclic aromatic hydrocarbons
Tobacco smoke contains many polycyclic aromatic hydrocarbons (PAHs)
(IARC, 1986), a number of which, such as benz( a)pyrene and
dibenz( a,h)anthracene, are known to be carcinogenic (IARC, 1987;
Hoffmann & Hoffmann, 1997). PAHs are generated by incomplete
combustion of organic matter in many industrial processes and
constitute a hazard not only in occupations but also as environmental
pollutants, representing primary risk factors as lung and bladder
carcinogens. A tobacco smoker can obtain one dose of PAHs from tobacco
smoke and another from the industrial or environmental source.
Furthermore, an interaction of tobacco smoke and an occupational
hazard is a possibility. PAHs in the workplace are often accompanied
by many other toxic compounds, particularly irritants, and, in
addition to carcinogenic PAHs, tobacco smoke contains co-carcinogens
and tumour promoters as well as ciliatoxic agents, irritants and other
biologically active species.
PAHs occur in coal gas manufacture, coking oven fumes, aluminium
smelting, in the use of tar and asphalt, in oil refining and the
exhaust from internal combustion engines. They are frequently
accompanied by irritant fumes or aerosols and potentially harmful
particulate matter. There is a lack of smoking data for workers in
many of these industries, but it has been assumed that the smoking
prevalence is at least as high as the average for blue collar workers.
At a Norwegian smelter 69% of workers were smokers when the expected
prevalence of smoking was 52% (Abramson et al., 1989). The percentage
of smokers and ex-smokers among workers exposed to chemicals and coal
tar pitch in a 1982 survey of 800 000 men and women in the USA was
49.9% against 46.1% for the average worker (Stellman et al., 1988).
A Canadian study found a high prevalence of bladder cancer in
aluminium smelter workers, particularly among those employed in
Söderberg potrooms where carbon electrodes made from a mixture of
petroleum pitch and coal pitch are used and PAH levels are high
(Thériault et al., 1984). Changing electrodes, breaking the crust that
forms on top of the molten metal and cleaning out the "pots" are
activities that create air pollution by tar volatiles including
carcinogenic PAHs which, measured as benzo( a)pyrene, could reach a
concentration of 800 µg/m3/8 h (Bjorseth et al., 1978). High levels
of PAHs were found in urine samples from aluminium plant workers
(Haugen et al., 1986). Lung cancer rates among aluminium reduction
plant workers are also high (Gibbs & Horowitz, 1979). Tobacco smoke
appears to increase the risk. In a study by Thériault et al. (1984),
the numbers were too small to determine whether the interaction was
additive or multiplicative, but in another study (Bjorseth et al.,
1978) there was suggestive, but not conclusive, evidence that the
relative risks from combined exposure to tar volatiles and cigarette
smoke were multiplicative. In a study in which the preceding data were
augmented (Armstrong et al., 1986), the tar volatiles were confirmed
as the cause of bladder cancer and the results suggested that a
multiplicative risk arose from a combined exposure to tar volatiles
and cigarette smoke.
Gullvåg et al. (1985) found that the alveolar macrophage count for
workers in the potrooms of an aluminium reduction plant was elevated
and for workers who were also smokers the count was further elevated.
The conclusion was that smoking and workplace pollution act
synergistically in increasing the number of alveolar macrophages.
Workers in coke oven plants have a higher incidence of lung cancer
than the general population and a measurable concentration of PAHs in
urine, which is higher in smokers than non-smokers (Haugen et al.,
1986). Van Schooten et al. (1990) analysed blood samples from coke
oven workers for PAH-DNA adducts and urine for 1-hydroxypyrene and
compared the results with those of non-exposed workers. Levels were
elevated in coke oven workers and in both exposed and control groups
the PAH-DNA adduct levels were higher among smokers than among
non-smokers.
Professional drivers are exposed to benzene and carcinogenic PAHs and
nitroarenes through the exhaust of petrol (gasoline) and,
particularly, diesel engines. An excess of lung cancer has been found
in this occupational group, with a suggestion of a synergistic
interaction between smoking and occupational exposure (Damber &
Larsson, 1985a).
Diesel exhaust contains large quantities of carbonaceous particulates
with adsorbed PAHs. The association between lung cancer and diesel
exhaust and the contributing role of cigarette smoking has been
considered to be problematic (Garshick et al., 1987, 1988; Boffetta et
al., 1988; Stöber & Abel, 1996; IPCS, 1996). In its evaluation, IARC
(IARC, 1989c) considered diesel engine exhaust to be probably
carcinogenic to humans (group 2A) and gasoline engine exhaust as being
possibly carcinogenic to humans (group 2B).
In most of the workplaces where PAHs contaminate the atmosphere, there
are also gases, fumes and aerosols that contain other hazardous
materials that act as irritants; they may play a role in the etiology
of chronic obstructive lung disease. It is important to include
smoking in epidemiological studies. In a study of lung cancer
mortality rates and smoking patterns in workers in the motor vehicle
industry, proportionate mortality rates were considerably reduced when
smoking rates were taken into account. An increased lung cancer risk
has been described among foundry workers; PAHs and silica were
considered to be possible etiological factors (Sherson et al., 1992).
IARC (IARC, 1984, 1985b) considers the following technical products as
carcinogenic to humans: coal tar and coal tar pitches, shale oils,
soots, effluent aerosols from coal gasification, and emissions from
coke ovens. Exposure occurring in the production of aluminium is
classified as probably carcinogenic to humans, whereas the exposures
to aerosol emissions from iron and steel foundries are classified as
possibly carcinogenic to humans.
3.5.5 Ethanol
Clinical and epidemiological studies have established a strong
relationship between smoking and drinking (Istvan & Matarazzo, 1984;
Bien & Burge, 1990; Zacny, 1990).
Elevated tobacco and alcohol consumption are regarded as the major
risk factors for oropharyngeal and oesophageal cancer in many
developed countries (Herity et al., 1982; Tuyns, 1983, 1991; Boyle et
al., 1990; Muir & McKinney, 1992; Negri et al., 1992).
It has been difficult to distinguish the separate effects of these
agents since many smokers tend to consume alcoholic beverages and
vice versa. In addition, the consumption of one substance may have
an effect on the use of the other substance. The possible interactions
(e.g., multiplicative effect) of tobacco smoking and alcohol
consumption for cancers of the oral cavity, pharynx and larynx have
been evaluated by IARC (1986). IARC (1986) concluded that tobacco
smoking was an important cause of oral, oropharyngeal, hypopharyngeal,
laryngeal and oesophageal cancers and combined ethanol consumption
increased the risk substantially. In a case control study of 1114
patients with oropharyngeal cancer, Blot et al. (1988) showed that the
risk to consumers of tobacco and alcohol was multiplicative rather
than additive and increased 35-fold in those who consumed two or more
packs of cigarettes and more than four alcoholic drinks per day. The
risk was higher in those consuming spirits or beer than in those
consuming wine and was lower in lifetime smokers of filter cigarettes.
Alcohol consumption and smoking affect fetal outcome, leading to
infants with low birth weight (Wright et al., 1983, 1984; Smith et
al., 1986).
In contrast to the many studies in laboratory animals of the
interactions of ethanol with tobacco-smoke condensate (TSC) and
specific tobacco constituents, e.g., nicotine (receptor studies)
(Collins, 1990), (gastric-mucosal damage) (Wong et al., 1986; Cho et
al., 1990), tobacco-smoke-specific nitrosamines, e.g.,
N-nitrosonornicotine (metabolism and carcinogenicity) (McCoy et al.,
1981; Castonguay et al., 1983, 1984) and
4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK) (Jorquera et al.,
1992; Schüller et al., 1993), there has been a relative paucity of
studies involving ethanol and tobacco smoke per se. These latter
studies have included fetotoxicity in mice (Peterson et al., 1981),
gastric mucosal damage (Iwata et al., 1995; Chow et al., 1996), and
mechanisms underlying behavioural association between alcohol and
tobacco consumption (Zacny, 1990).
In in vitro studies on the effect of tobacco smoke condensate on rat
buccal mucosa cells following exposure to ethanol, the level of
adducts was higher than in controls, suggesting an increased uptake of
carcinogens in the condensate (Autrup et al., 1992). Hsu et al. (1991)
studied in vitro genotoxicity of tobacco smoke condensate in
conjunction with 2% and 4% ethanol in human lymphoid cell lines.
Ethanol potentiated clastogenicity, measured by frequency of
chromosome breaks per cell, in a dose-dependent manner, and the
results indicated that ethanol at relatively high doses inhibited DNA
and chromosome repair systems.
Swiss Albino mice fetuses prenatally exposed to both tobacco smoke and
ethanol had a high resorption frequency, a significant reduction in
fetal weight and length, and neonatal growth retardation, indicating
that ethanol and tobacco smoke may interact to produce fetotoxicity
(Peterson et al., 1981).
Both cigarette smoking and ethanol consumption individually have been
associated with gastric and duodenal ulcers in humans and animals.
Exposure to cigarette smoke significantly potentiated ethanol-induced
gastric mucosal damage in Sprague-Dawley rats (Iwata et al., 1995;
Chow et al., 1996).
The effect of smoking on the incidence of cancers of the oral cavity,
oropharynx, hypopharynx and larynx is often combined with other
factors, principally alcohol, in the Western world. The possibility of
interaction between cigarette smoking and alcohol consumption is
complex (Burch et al., 1981).
Rothman & Keller (1972) reviewed the effect of joint exposure to
tobacco and alcohol with regard to oral cancers alone (based on data
published earlier by Keller & Terris, 1965) and concluded that a
single multiplicative function of the relative risks associated with
alcohol and tobacco separately provided an adequate summary of their
joint effect.
Wynder & Bross (1961) studied etiological factors in cancer of the
oesophagus, considered the consumption and effects of tobacco and
alcohol separately and together, and considered that the combined
effect was multiplicative. Tuyns et al. (1977) reported a similar
pattern of joint effect of tobacco and alcohol in a retrospective
study of oesophageal cancer in Brittany, France. The relative risk of
developing oesophageal cancer increased linearly with daily
consumption of alcohol and tobacco independently. The combined effect
fitted a multiplicative model.
Wynder et al. (1976) analysed environmental factors in cancer of the
larynx and showed a combined effect of tobacco and alcohol. In the
presence of smoking, heavy drinking increased the risk of cancer of
the larynx, especially for cancer of the supraglottic portion of the
larynx. Similar findings were reported by Burch et al. (1981).
In a prospective epidemiological study, the relative risk of incurring
a single primary carcinoma of the oral cavity, pharynx, larynx and
oesophagus in any one of these sites was increased independently by
the duration and intensity of exposure to tobacco or alcohol and
sustained exposure enhanced the risk in a multiplicative or
synergistic fashion (Schottenfeld et al., 1974) The relative risk of
multiple primary cancers in the sub-group with combined exposures to
high levels of tobacco and alcohol was 3.9 times that of patients
exposed previously to low levels of alcohol and tobacco.
3.5.6 Other organic compounds
Exposure situations involving compounds and mixtures of organic
compounds for which no definite smoking interactions have been
established but which are known to present serious health hazards are
summarized in chapter 4.
3.6 Physical agents
3.6.1 Radiation
The harmful forms of ionizing radiation that are of concern are
alpha- and beta-particles and gamma- and X-rays. All cause cellular
damage and have been implicated in carcinogenesis. IARC (1988)
classified radon and its decay products as Group 1 (carcinogenic to
humans) on the basis of sufficient evidence in humans and in
laboratory animals. The interaction of the effects of these radiations
with the effects of tobacco smoke has been studied. Radium is present
in uranium and other minerals and in all rocks and soils. It emits
alpha- and beta-particles and gamma-rays and decays to form the
chemically inert radioactive gas radon, which is released in tiny
amounts into the atmosphere where its concentration is extremely small
because of dilution. It can, however, become more concentrated in some
locations, particularly in uranium and other mines and in residential
buildings. Radon is an inspirable gas and its radioactive decay
products are ionized metal atoms, which adhere to inspirable dust
particles. These atoms are themselves undergoing radioactive decay and
emitting damaging alpha- and beta-particles and gamma-rays. In
addition to interactions of tobacco smoke with radon in mines and
residential situations, other effects of tobacco smoke and radiation
interactions have been studied in atom-bomb survivors and in the low
energy transfer radiation involved in the use of therapeutic radiation
(X-rays).
3.6.1.1 Radon in mines (high linear energy transfer (LET)
alpha-radiation)
Unless mines are well ventilated, the atmospheric concentration of
radon becomes significant. The gas and its radioactive decay products,
the radon daughters, can be inhaled. The daughters have short
half-lives and their decay is proceeding while the particles to which
they adhere are resident in the lungs and before they can be removed
by normal lung clearance. Thus radiation is delivered directly to the
delicate lung tissues where it causes an excess of lung cancer among
some miners.
Observations in several mining communities, e.g., among uranium miners
in the USA, Czechoslovakia, Canada and France, workers in a niobium
mine in Norway, iron ore miners in Sweden, tin miners in China and the
United Kingdom, and fluorspar miners in Newfoundland, showed a
significant dose-related increase in lung cancer risk with exposure to
radon and radon daughter elements (Archer, 1988). In miners who were
cigarette smokers, there was an interaction between the radiation
exposure and the smoke exposure leading to more than the expected
number of cases of cancer. The latent period for induction of lung
cancer was longer when the exposure to radioactivity started at a
younger age, it was shortened by high exposure rates and by cigarette
smoking, and lung cancers developed at lower levels of exposure to
radioactivity in miners who smoked than in those who were non-smokers.
In Bulgaria, Michaylov et al. (1995) used sputum cytology to study
bronchial cell dysplasia in 334 miners (uranium and metal mines)
exposed to 222Rn progeny, and 100 control miners from a metal mine
where radon was virtually absent. The dust and silica concentrations
and exposure to diesel exhaust and explosion gases were similar. The
frequency of bronchial cell dysplasia was significantly higher in
radon-exposed miners than in controls and the frequency of dysplasia
in smokers was significantly greater than in non-smokers.
The lower prevalence of lung cancer among coal miners than among other
underground workers is probably because coal mines are well ventilated
to reduce fire and explosion risk, and no build up of radioactivity
occurs. Attempts to reduce silicosis by ventilation have achieved a
similar effect. In some Swedish mines, because freezing occurred when
outside air was used for ventilation, filtration was achieved in the
1920s by circulating the air through old underground mine workings,
with the result that the potential for silicosis was reduced. However,
an increase in lung cancer was found because radioactive materials
built up, a fact that only became evident many years later (Archer,
1988).
The nature of the interaction between radon and cigarette smoke is not
clear. In a study by Edling (1982), the effects of smoking and radon
were considered to be additive, whereas in another by Damber & Larsson
(1985b) the effect was multiplicative. From a long-term study on
Swedish iron ore miners (Jörgensen, 1984), it was concluded that
tobacco smoke acts as a tumour promoter, an effect that has been
demonstrated in almost all animal studies. The concept that radon
serves as a tumour initiator and tobacco smoke as the tumour promoter
for the induction and development of lung cancer is supported by a
sequence of studies. Tobacco contains small amounts of polonium-210
(210Po), which primarily originates from phosphate fertilizers (Tso et
al., 1966) and, to a minor extent, from airborne 210Po trapped by the
glandular hairs (trichomes) found on the soil-facing surfaces of
tobacco leaves (Martell, 1974). 210Po is a decay product of radon -222
and an emitter of alpha-particles. It is present in cigarette smoke,
and the bronchial epithelium of smokers contains 2-10 times more 210Po
than is found in these tissues in non-smokers (Harley et al., 1980).
The alpha-radiation of 210Po damages DNA in the bronchial airways and
serves as a tumour inhibitor, and the tar in the tobacco smoke acts as
a tumour promoter.
The frequencies of different histological types of lung cancer among
miners have varied with working conditions and follow-up time. It has
also been shown (Archer, 1988) that the age range of the population
under observation can influence the conclusion. Thus, the
smoking-radon relationship appears to be multiplicative only for the
group aged 35-65 years. Steenland (1994) found the death rates from
lung cancer in smoking uranium miners to be intermediate between
additive and multiplicative for the two exposures, but, when
stratified for age, the multiplicative model fitted well for the
youngest and oldest categories but poorly for the middle range. In a
comprehensive analysis of data from 11 studies of radon-induced health
risks (Lubin et al., 1995), it was concluded that the joint effect of
radon progeny exposure and smoking is greater than the sum of the
individual effects and for smokers is higher by a factor of at least
three. The tobacco of cigarettes contains 0.1-1.0 pCi of 210Po
(Cohen et al., 1985; Hoffmann et al., 1986).
The conclusion reached by the US Surgeon General (1985) was that the
smoking-radon interaction consists of two parts: an additive effect of
the contribution of the two agents on the number of tumours produced
and an accelerating effect due to tumour promoters in cigarette smoke.
Thus for a miner who smokes, not only is the chance of lung cancer
greater but the latent period is shorter and therefore the cancer
appears sooner in smokers.
3.6.1.2 Environmental radon (high linear energy transfer (LET)
alpha-radiation)
Alpha-radiation from radon daughters in the home or in other
situations where there are enclosed spaces with poor ventilation,
e.g., where strict energy conservation measures have been adopted,
presents an elevated health hazard to occupants, particularly smokers,
and is a matter of public health concern. The ease with which ionized
radon daughters could be attracted to environmental tobacco smoke
particles and the possibility of a higher than additive combined
effect of radon progeny and smoke clearly indicate the importance of
residential contamination by radon.
The relative risk in the range of exposure experienced by miners has
been found to be linear, and it has been suggested from extrapolation
that exposures at the lower levels found in homes would carry some
risk (Lubin et al., 1995). Steindorf et al. (1995) calculated that 7%
of all lung cancer deaths in the western part of Germany may be due to
residential radon.
Axelson (1995) reviewed cancer risks from exposure to radon in the
home and suggested that cancers other than lung cancer may also be
related to indoor radon, especially leukaemia, kidney cancer and
malignant melanoma. However, it was acknowledged that studies of radon
and miners gave no clear support for this. Alavanja et al. (1995)
listed other risk factors as being responsible for lung cancer in
lifetime non-smokers and found a small non-significant risk for
subjects exposed to domestic radon at median concentrations. In a
case-control study of lung cancer in relation to exposure to radon in
homes (Letourneau et al., 1994), no increase in the relative risk for
any of the histological types of lung cancer was detected in relation
to cumulative exposure to radon. On the other hand, Biberman et al.
(1993) found an increased risk for small cell lung cancer following
residential long-term exposure to radon at a low-dose level. In a
large case-control study in Sweden, Pershagen et al. (1994) reported
an increased relative risk of lung cancer within the highest exposure
group. In an attempt to resolve the conflicting epidemiological data,
Lubin & Boice (1997) conducted a meta-analysis of eight large-scale
case-control studies of residential radon and lung cancer. This
analysis was consistent with an excess lung cancer risk. Furthermore,
the slope of the exposure- response curve derived from this
meta-analysis was comparable with that obtained from a combined
analysis of eleven miner cohorts exposed to radon (Lubin et al.,
1997).
The combined analysis of the miner data also confirmed the strong
synergistic relationship between radon and tobacco at high levels of
exposure to these two agents (Lubin et al., 1997), although it is
difficult to determine whether the interaction is closer to additive
or multiplicative (Chaffey & Bowie, 1994). When extrapolated to lower
levels of exposure, however, the magnitude of this interaction is
substantially diminished (Moolgavkar et al., 1993). However, Pershagen
et al. (1994) reported some evidence of a synergistic effect between
tobacco and residential radon exposure, with the relative risk of
radon-induced lung cancer being highest among heavy smokers. In a
case-control study of 982 subjects with lung cancer and 3185 hospital
or population control subjects, lung cancer risk was examined in
relation to residential radon concentration and length of time that
subjects were resident, and adjusted for age, sex and smoking (Darby
et al., 1998). The relative risk increased in an exposure-related
manner with time-weighted residential radon concentration and fitted
the data from studies in miners and the effect of smoking. Regardless
of the magnitude of any interaction between tobacco smoking and
residential radon, the lung cancer risks due to smoking exceed the
risk associated with radon in homes.
3.6.1.3 Atomic bomb site radiation (low linear energy transfer (LET)
radiation)
In tobacco-smoking survivors of atomic bombing in Hiroshima and
Nagasaki, Japan, elevated levels of cancer of several sites have been
reported. In the case of lung cancer both additive and multiplicative
models fit the data (Prentice et al., 1983; US NRC, 1988).
3.6.1.4 Therapeutic X-rays (low linear energy transfer (LET)
radiation)
Lung cancer as a possible side effect of the radiation therapy used to
treat breast cancer has been studied by Neugut et al. (1993, 1994) and
discussed by Inskip & Boice (1994). Neugut et al. (1993) reported that
the risk was greater in the ipsilateral than in the contralateral
lung. In a second study (Neugut et al., 1994), a three-fold relative
risk was found for the effect of radiation therapy among 10-year
survivors, a 14-fold risk was associated with smoking alone, and a
33-fold risk was found among irradiated smokers; in each case the
effect was most pronounced for ipsilateral lung cancer. A
multiplicative interaction was proposed and the implications of the
results for the design of treatment of breast cancer in smokers was
considered. The increased risk of lung cancer among survivors of
Hodgkin's disease(HD) was studied by van Leeuwen et al. (1995). Their
overall conclusions were that the risk of lung cancer increased more
with increasing radiation dose in HD patients who smoked than among
those who did not smoke. Thus, smokers were at greater risk from the
radiotherapy than non-smokers. The interaction between the
carcinogenic effects of smoking and radiation was significantly
stronger than multiplicative, and the low lung cancer rate found among
women with HD was attributable to the delayed popularity of smoking
among Dutch women, a fact shown by the male/female lung cancer ratio
(13:5) in the Netherlands in 1980.
3.6.1.5 Nuclear plant
Ongoing epidemiological studies are being conducted on the workforce
exposed to radiation at the Mayak plant in Russia. Of 500 workers
examined in a case-control study (Tokarskaya et al., 1995), 162
workers had contracted lung cancer, and the remaining 338 served as
radiation-exposed, non-tumour-bearing controls. Both the incidence and
duration of smoking was significantly higher in workers contracting
tumours compared to combined male and female controls. The strongest
smoking-related effect was for squamous cell carcinomas, followed by
adenocarcinomas, then small cell carcinomas. However, the findings are
complicated by the fact that the great majority of the workforce was
male, and there was only one of the 148 "never smokers" among the male
lung cancer cases.
3.6.1.6 Summary
In summary, miners subjected to chronic exposure throughout a working
lifetime to high-LET radiation show a radiation/tobacco smoke
interaction greater than additive and sometimes multiplicative. Atomic
bomb survivors exposed instantaneously to low-LET radiation show in
some cases an additive and in others a multiplicative interaction. The
results for residentially exposed smokers, subjected to a lifetime of
very low dose exposure, tend to show similar interactions to those for
miners. Tobacco-smoking patients subjected to therapeutic radiation
(low-LET) show a multiplicative interaction.
3.6.2 Vibration
Raynaud's phenomenon is an episodic disorder that produces
intermittent attacks of blanching in the extremities and there may be
numbness or tingling in the hands and fingers. There are several
causes (the term "Raynaud's disease" is applied when the cause is not
known). It was first associated with the use of vibrating tools among
Italian miners in 1911, and the association has since been reported
for a wide range of hand-held vibrating tools such as impact hammers,
chipping hammers, grinders, riveters and the motor-driven chain-saws
used in forestry. The terms vibration white finger (VWF), vibration
syndrome, traumatic vasospastic disease and dead finger have been used
for this condition that begins with numbness and tingling, followed by
blanching and can include intermittent episodes of hand and finger
pain and flushing. With continuing exposure to vibration the symptoms
may become more severe and continue after the cessation of exposure.
Damage to digital arteries and narrowing of the lumen has been
associated with vibration syndrome (JOM, 1984) and, because nicotine
acts as a vasoconstrictor, it has been suggested (JOM, 1984) that
limiting smoking could aid blood flow to the extremities and thus
reduce the condition. In a survey of forestry workers in Quebec in
1977-1978 (Thériault et al., 1982), a prevalence of Raynaud's
phenomenon among 1540 woodcutters was found in 30.5% of chain-saw
users and there was a strong association between this and cigarette
smoking; the relative risks were 3.60 for non-smokers, 6.55 for
smokers and 1.72 for smokers who had not used a chain-saw:
corresponding to an additive effect for the two risk factors. From
another study of the effect of tobacco use on a cohort of men with
VWF, in which the extent of tobacco use was confirmed by blood
nicotine and cotinine measurements (Ekenvall & Lindblad, 1989), it was
shown that tobacco aggravates the symptoms of VWF. Patients with
advanced symptoms were found to use tobacco more frequently and to
have higher blood cotinine levels than patients with less advanced
disease. In a study of the prognosis of VWF (Petersen et al., 1995),
an improvement in the condition occurred when there was no exposure to
either vibration or smoking, whereas an aggravation of the condition
was most notable in smokers. Whole-body vibration has been associated
with persistent severe neck trouble, and smoking was an added
predictor for this condition (Viikari-Juntura et al., 1994). Finger
temperature changes have been measured after smoking a cigarette; in
all cases a reduction in temperature was recorded (Saumet et al.,1986;
Bornmyr & Svensson, 1991).
3.6.3 Noise
In a study of aviators in 1963 at the US Naval Aerospace Medical
Research Laboratory (Thomas et al., 1981), two hearing level groups
were identified, one with normal and the other with impaired hearing.
The impaired hearing group had smoked more cigarettes for a longer
period of time than had those in the normal hearing group. In another
study the relationship between cigarette smoking and hearing loss was
studied in 2348 noise-exposed workers at an aerospace company and it
was found that smoking was a clear risk factor in noise-induced
hearing loss: the OR was 1.27 for "ever smokers" and 1.39 for present
smokers, compared with non-smokers (Barone et al., 1987). Vascular
insufficiency of the cochlear organ has been cited as the predominant
cause of progressive hearing loss that occurs with age. It was
suggested that smoking reduces the cochlear blood supply by:
a) vasospasm induced by nicotine; b) atherosclerotic narrowing of
vessels; and c) thrombotic occlusions (Zelman, 1973). In a study of
1000 subjects at a Veterans Hospital, Zelman (1973) found that whilst
age and sex were the most important variables, at all measured
frequencies the percentage of loss was greater for smokers, the
differences being greater at higher frequencies. From a retrospective
analysis of audiograms taken between 1984 and 1990 of a cohort of 119
workers, 78.8% of smokers compared with 25.7% of non-smokers had
noise-induced hearing loss (ILO, 1991). Although these studies
demonstrated a positive correlation between smoking and hearing loss,
Friedman et al. (1969) and Pyykko et al. (1987) were not able to show
that smoking was a significant risk factor to hearing loss.
3.6.4 Dupuytren's contracture
Dupuytren's contracture is a contracture of some muscle membranes of
the palm of the hand that causes the little finger and ring finger to
be drawn into the palm from where they cannot be extended. It is
characterized by a retractile sclerosis of the palmar aponeurosis,
which may progress to an irreducible flexion of the fingers. Opinions
differ on the influence of occupation, handedness and hand injury on
Dupuytrens contracture which Dupuytren himself attributed to chronic
occupational injury. Some people assert that heavy work always causes
the disease, while others claim that it is not responsible. Mikkelsen
(1978) studied 901 cases in an epidemiological study of 15 950 and
concluded, after isolating hand trauma, that Dupuytren's contracture
is caused by heavy work. The contribution made by tobacco has been as
contentious as has the cause of Dupuytren's contracture. Hand
thermography of affected fingers in Dupuytren's contracture shows a
drop in temperature of up to 3 degrees; and hand temperature falls by
a similar amount during smoking. In a study of 84 men and 16 women
with Dupuytren's contracture, Fraser-Moodie (1976) found no evidence
that smoking was connected with the condition, a conclusion also
reached by Mackenney (1983). However, cigarette smoking was listed
among the responsible factors for Dupuytren's contracture by Attali et
al. (1987), as well as by An et al. (1988), who found that cigarette
smoking was linked statistically to Dupuytren's contracture and
suggested that it may be involved in its pathogenesis by producing
microvascular occlusion and subsequent fibrosis and contracture. An et
al. (1988) concluded that cigarette smoking was one of the most
significant factors in the development of peripheral vasculopathy
Abelin et al. (1990) showed a significant association between
Dupuytren's contracture and smoking habits.
3.7 Biological agents
3.7.1 Biological (vegetable) dusts
Uncontrolled exposure to airborne vegetable dusts can affect health
and occurs worldwide in many workplaces, e.g., in agricultural
operations, textile industries, construction, carpentry and the
furniture industry. The population exposed is large, particularly in
developing countries where whole families, from young children to the
elderly, may engage in agricultural activities and small scale
manufacturing operations using vegetable products. These agents can
take the form of vegetable dusts, airborne fungal spores and
microorganisms, animal danders and feathers, herbicides and pesticides
and their residues. Processing of agricultural products, such as
cotton, flax, hemp, grain, tobacco, paprika and tea, and the milling
of certain varieties of wood are occupations where vegetable dust
exposures have been associated with detrimental health effects.
In addition to the irritation and bronchitis that is associated with
exposure to almost any dust, biological dusts can cause byssinosis,
allergic and immunological responses and, in some cases, nasal and
paranasal cancer. All these conditions can be affected in different
measure by tobacco smoking.
3.7.1.1 Cotton dust
Byssinosis is a respiratory disease of textile workers. The disease is
found in many cotton processing countries. It is more prevalent in the
dusty stages of cotton processing, such as carding, than in weaving.
Byssinosis, or similar symptoms, and bronchitis have also been found
in flax, hemp, jute and sisal workers. The characteristic symptoms are
tightness of the chest and shortness of breath on returning to work
after a period of absence. There is the possibility of progression to
permanent respiratory disability.
In a study of textile workers in South Carolina, USA, in 1973, the
smoking prevalence was almost the same for workers as for controls
(Beck et al., 1984). In another study of cotton workers in 1963-1966
(Molyneux & Tombleson, 1970), the percentage of male current smokers
was 62.5% and of ex-smokers 16.4%; among females the figures were
33.9% and 6.1%, respectively. Among flax scutchers in Normandy in
1986-1987, 56% were smokers and 18% were ex-smokers; compared with 45%
and 15%, respectively, for the controls (Cinkotai et al., 1988a). In
31 Lancashire textile factories (1988) 47.5% of the 4656 workers
interviewed were smokers (Cinkotai et al., 1988b). Of 800 000 American
workers surveyed (Stellman et al., 1988) for smoking habits in 1982,
5.9% were exposed to textile fibres or dust: 28.5% of these were
regular cigarette smokers and 44.9% were former cigarette smokers;
compared with 23.5% and 43.5%, respectively, in other occupations not
exposed to textiles.
Increases in both byssinosis and bronchitis were attributed to cotton
dust exposure and smoking in the cotton industry (Molyneux &
Tombleson, 1970; Merchant et al., 1973). From an industrial study of
the effects of cotton dust and cigarette smoke, Merchant et al. (1972)
concluded that smokers showed an increase in both the prevalence and
severity of cotton dust-induced byssinosis and that cigarette smoke
also increased the detrimental effect of cotton dust on ventilatory
capacity. It was suggested that the impairment of lung clearance
mechanisms by cigarette smoke could be responsible for the deleterious
effect of cotton dust and that smoking might lower the threshold of
susceptibility to the effects of inhaled cotton dust. Additivity and
the equal importance of the effects of smoke and cotton dust have been
suggested (Beck et al., 1984) but since different lung function
parameters are affected it would seem that the two factors affect
different sites. The fact that workers who stopped smoking, whilst
remaining in the same job, lost their byssinotic symptoms was
significant. A survey by Cinkotai et al. (1988a) of workers in 31
textile factories in Lancashire, United Kingdom, showed that
byssinotic symptoms (in decreasing order) were related to years in the
industry, degree of dust exposure, quality of cotton in use, ethnic
origin of workers and smoking habits. Symptoms of chronic bronchitis
were related primarily to smoking habits and then to factors connected
with the occupation. In a study of hemp workers (Bouhuys & Zuskin,
1976), decline in ventilatory function was more pronounced in smokers.
It was suggested (Cinkotai et al., 1988b) that a surprisingly low
prevalence of byssinotic symptoms in 12 flax scutching mills in
Normandy may have been due to either self selection of the workforce
or to an absence of the causative agent in the dust. Persistent cough
and phlegm production were associated with tobacco use.
In textile-related pulmonary disease, smoking as a primary causative
factor was reported by Pratt et al. (1980), and a similar conclusion
was arrived at from lung function tests carried out over a 3-year
period on 153 women (103 smokers, 50 non-smokers) with grades 2 and 3
byssinosis by Honeybourne & Pickering (1986). Cancer deaths in general
and lung cancer in particular were lower in workers exposed to cotton
dust than in others (Enterline et al., 1985). Kilburn (1989) suggested
that the effect of byssinosis on mortality of textile workers from
pulmonary disease needed more comprehensive study.
3.7.1.2 Wood dust
IARC (1995) evaluated the carcinogenic risk of wood dust and
classified it as carcinogenic to humans (Group 1), based on sufficient
evidence in humans and inadequate evidence in animals. The risk of
developing cancer of the nasal cavity among workers manufacturing
wooden furniture has been shown to be up to 100 times greater than for
the general population (Rang & Acheson, 1981). The effect is worst in
the most dusty areas (Rang & Acheson, 1981; Hayes et al., 1986). The
association of risk with certain hardwoods and the finishing of fine
furniture, rather than with woodworking in general, suggests that it
may be allied to both the chemical and physical nature of the dust. In
the study where the nasal cancer incidence was 100 times greater than
for the general population, it did not appear to be affected by
smoking habits. A similar conclusion was reached in a study in an area
of Italy with a large number of cases of nasal cancer among wood and
leather workers (Cecchi et al., 1980). An association with smoking was
established in other studies, and current and past smoking habits were
shown to be a risk factor for developing squamous cell cancer of the
sinus in men (Fukuda et al., 1987). A case-control study of 121 male
woodworkers who were examined for cancer of the nasal cavity or
paranasal sinus, in British Columbia in Canada between 1939 and 1977
(Elwood, 1981), showed increased relative risks associated with
occupations involving exposure to wood (relative risk 2.5) and with
smoking (relative risk 4.9). In a study in North Carolina and Virginia
in the USA between 1970 and 1980 (Brinton et al., 1984), a major
finding was the elevated risk of nasal cavity and sinus cancer among
cigarette smokers. However, the nature of any interaction of wood dust
and tobacco smoking needs further study because adenocarcinomata
appear to be the tumour type associated with wood dust, whereas the
relative risks for squamous-cell and small-cell cancers tend to be
higher for smokers. The available data do not permit an assessment of
the degree of interaction between smoking and wood dust exposure.
3.7.1.3 Allergic responses
This type of response can occur in the upper airways, where it is
manifest as hay fever, in response to certain types of pollen, or in
the bronchi as asthma, or it may appear in both. Some of the dusts
that cause allergic airways responses (occupational asthma) are grain
dusts from various cereals and their products, wood dusts particularly
from red cedar and iroko, and dusts from teas and tobacco. Among
asthmatics, environmental cigarette smoke makes the effect of the
asthma worse (Shim & Williams, 1986), and smoking effects appear to be
additive to that of asthma from other causes (Conolly et al., 1988).
Grain dust exposure and smoking have been found to cause increases in
the prevalence of respiratory symptoms and reductions in pulmonary
function of grain elevator workers. The effect of smoking was slightly
more pronounced; the combined effect of grain dust and smoking
appeared to be additive, except in the least exposed workers (5 years
or less) where a synergistic effect was observed in tests for
peripheral airways dysfunction (Cotton et al., 1983). Chan-Yeung et
al. (1985) stated that the effect of grain dust and smoking was
additive and not synergistic in causing a decrease in lung function.
In 303 workers in the animal feed industry exposed to dusts of grains
and cassava, 61 (20%) showed respiratory symptoms; in office staff in
the same plant the prevalence was similar. The plant workers had a
higher prevalence of smoking than office staff. Current smoking was
strongly associated with respiratory complaints in both groups (Post
et al., 1994).
Occupational asthma also occurs in flour, tea, coffee and rice
handlers. In Italian bakers and pastry makers De Zotti et al. (1994)
found that 54 (23%) of the 226 subjects were atopic. Forty (18%) were
skin-test-positive to storage mites, 27 (12%) to wheat flour and 17
(8%) to alpha-amylase. Skin sensitization to these occupational
allergens was significantly associated with atopy, smoking and
duration of exposure. In a study of 401 workers in bakeries or flour
mills, Cullinan et al. (1994a) found that work-related symptoms of
allergy were more common in smokers, with little difference between
atopic and non-atopic workers. However, smoking was not independently
related to either symptoms or positive skin test.
Zetterstrom et al. (1981) skin-prick-tested 129 workers in a coffee
roastery with green coffee bean and castor bean extracts. There was a
significantly increased prevalence of positive skin prick tests in
smokers. In enzyme detergent workers with occupational asthma, it was
found that twice as many smokers as non-smokers exhibited asthmatic
symptoms (Greenberg et al., 1970; Mitchell & Gandevia, 1971).
Smokers are more likely to show higher specific antibody production
and correspondingly be more susceptible to asthma.
3.7.2 Other biological agents
Although many biological dusts are known to have detrimental health
effects, there have been few studies of any interaction of smoking
with these agents. Extrinsic allergic alveolitis may be caused by
spores from a number of fungi which are small enough to reach the
pulmonary compartment. There are several forms of allergic alveolitis,
of which farmer's lung, bagasse pneumonitis, and bird fancier's lung
are examples. These are caused by fungal spores in mouldy hay or
mouldy sugar cane or an agent in bird feathers, respectively, and are
immunologically mediated (Lancet, 1985). Extrinsic allergic alveolitis
is a rare example of a respiratory disease which is more prevalent in
non-smokers than in smokers. In one study, 14 of 18 patients (11 with
farmer's lung and 7 with bird fancier's lung) were non-smokers, twice
the proportion of non-smokers in patients with cryptogenic fibrosing
alveolitis or in the local population (Warren, 1977). Carrillo et al.
(1991) investigated IgG response to pigeon serum and its relation to
tobacco smoking in 160 pigeon fanciers. The sensitization rate was
31.9%. Pigeon fanciers who were current smokers had significantly
lower levels of IgG antibodies to pigeon serum (P<0.001).
Precipitating antibodies to Micropolyspora faeni, a common cause of
farmer's lung, were found to be twice as common in the area of
non-smokers in farming communities compared with smokers (Morgan et
al., 1975). Alveolar macrophage phagocytosis has been shown to be
depressed by cigarette smoke and it has been suggested this may
explain its apparent protective effect (Hocking & Golde, 1979).
3.7.3 Agents found in factory farming (animal
confinement effects)
Factory farming of pigs, where the animals are kept in confined
conditions, is common practice in livestock production in many
developed countries and it has been found to be accompanied by adverse
respiratory symptoms in workers. Donham et al. (1984) summarized the
effects as: acute toxicosis and inflammation of the respiratory tract
from inhaling hydrogen sulfide; acute asthma-like symptoms;
bronchitis; and delayed or hypersensitivity pneumonitis-like symptoms.
They found that smoking interacted additively with the bronchitis and
obstructive symptoms of the condition. In a study of workers in swine
confinement areas, Zuskin et al. (1992) reported similar effects. They
also found that smoking aggravated acute and chronic respiratory
symptoms and impairment of lung function.
3.7.4 Laboratory animals
Venables et al. (1988b) examined data from three cross-sectional
surveys of 296 laboratory workers around 30 years of age exposed to
small mammals. Two populations were of pharmaceutical research workers
(N = 133 and 140) and one of research workers in a tobacco company
(N = 23). One of the pharmaceutical research worker populations had a
laboratory animal allergy (LAA) prevalence rate of over 40% (Venables
et al., 1988a). The tobacco company research workers were exposed only
to rats while the other two populations were exposed to rats, mice,
guinea-pigs and rabbits. Atopy was determined by skin prick test to
non-animal aeroallergens. Sensitization to laboratory animals was
determined by response to skin prick tests using urinary extracts from
the species used in each laboratory. Radioallergoabsorbent tests
(RASTs) were used to measure serum IgE antibody concentration to the
urinary extracts in two of the populations.
Atopy in the three populations ranged from 30% to 44%, and positive
skin response to urinary extract ranged from 13% to 48%. Pooled data
from the three surveys showed an association between smoking and
positive skin response to urinary extract. Associations with smoking
persisted after stratifying by atopic status, suggesting that smoking
was a risk factor for developing laboratory animal allergy.
In other studies of 238 laboratory workers without previous
occupational exposure to rats in three institutions specializing in
small animal research, atopy was again determined by skin prick test
response to non-animal aeroallergens and sensitization to laboratory
rats by response to urinary extract. Exposure to total dust and rat
urinary aeroallergen was also measured. Allergy to rats was positively
related to exposure intensity and this was stronger in atopic
subjects. Positive responses to skin prick testing with rat urinary
extract were strongly related to atopy and to smoking at all levels of
exposure (Cullinan et al., 1994b).
Several studies from Europe have shown an association between
ownership of pet birds or pigeons and lung cancer (Holst et al., 1988;
Kohlmeier et al., 1992; Gardiner et al., 1992). The relative risk
adjusted for smoking was 6.7 (2.2-20.0). However, two community-based
case-control studies, one from the USA and one from Sweden, could not
confirm an association between pet birds and lung cancer (Alavanja et
al., 1996; Modigh et al., 1996). In 1998, a hospital-based
case-control study conducted in New York City and Washington, DC, with
887 cases and 1350 controls, did not show an association of keeping
pet birds with lung cancer in non-smokers. There was a ten-fold
increase of lung cancer among smokers who were not bird keepers over
non-smokers, but there was no indication of synergism between smoking
and keeping a pet bird (Morabia et al., 1998).
3.7.5 Schistosomiasis
In a study carried out in Spain, risk factors for urinary bladder
cancer were identified (Bravo et al., 1987). The factors were listed
in order of importance and the first three were total number of
cigarettes smoked, history of urological disease and exposure to an
occupational risk. Vineis (1992) summarized epidemiological,
biochemical and molecular evidence that clearly linked smoking with an
increased risk of bladder cancer. Cohen & Johansson (1992) considered
smoking to be the most important etiological factor for bladder
cancer. They also implicated a variety of occupational exposures and,
in some parts of the world, an association with various endemic
diseases including schistosomiasis. Schistosomiasis is a waterborne
parasitic disease found in many developing countries in Africa, Asia
and South America. It is a widespread occupational disease for
agricultural workers, and also affects members of the general
population.
A phase in the life-cycle of the trematodes responsible for the
disease lives in the blood vessels of visceral organs and their eggs
are discharged through the bladder or intestine in urine and faeces.
Some species live in the mesenteric veins and the eggs are discharged
in the faeces but the eggs of Schistosoma haematobium mature in the
veins of the bladder and are discharged in the urine. The eggs mature
in water and the resultant larvae infect freshwater snails. Within the
snail the parasites multiply to produce free swimming cercaria larvae
which can infect humans via skin penetration and repeat the cycle. S.
haematobium is found in nearly all countries in the African continent
and it has been found that the incidence of bladder cancer is higher
in areas with a high prevalence of infection than in areas with a low
prevalence. IARC classifies infection with S. haematobium as
carcinogenic to humans (Group 1) (IARC, 1994b). In Egypt, the most
common form of cancer is bladder cancer, accounting for 27.6% of all
malignancies encountered (38.5% of cancers in males and 11.3% in
females), and these high levels have been attributed to underlying
schistosomiasis (Tawfik, 1987). Makhyoun (1974) carried out a
case-control study of smoking among Egyptian males with and without a
previous history of S. haematobium infection. A smoking index was
calculated (average number of cigarettes per day × duration of smoking
in years) to categorize subjects. The smoking index (intensity and
duration of smoking) was higher in all the patients with bladder
cancer. In the patients with a previous schistosomiasis infection,
22.7% were moderate or heavy smokers compared with 79.3% of the
non-schistosomiasis patients. In the latter there was a good
correlation with the smoking index but in the bladder cancer patients
with previous schistosomiasis there was no significant difference in
smoking index between patients and matched controls. It was not
possible to identify an interaction between smoking and
schistosomiasis in the production of bladder cancer.
However, in a review of the role of S. haematobium in human bladder
cancer, Badawi et al. (1995) referred to several major studies that
implicated this infection with the subsequent development of bladder
cancer. Badawi et al. (1995) listed examples of a co-carcinogenic
effect of parasitic infection in the presence of chemical carcinogens
and it has been suggested (Hicks et al., 1980; Hicks, 1982) that
schistosomiasis could supply the necessary proliferative stimulus to
accelerate cancer growth from latent tumour foci on exposure to
carcinogenic nitrosamines. Nitrosamines have been implicated as
carcinogens among tobacco chewers and oral snuff users (Hecht &
Hoffmann, 1988, 1991; Hoffmann et al., 1991a), nitrosamines have been
demonstrated in smoke (Tricker & Preussmann, 1992; Hoffmann et al.,
1991b) and nicotine-derived N-nitrosamines cause cancer (Hoffmann &
Hoffmann, 1991; (IARC, 1991). Some N-nitrosamines are excreted as
esters via the urinary tract, e.g., N-nitroso-di- n-butanol or NNK
as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. They may hydrolyse
in the presence of infectious agents in the bladder, as is the case in
schistosomiasis, and thus appear in their precarcinogenic form,
thereby increasing the risk for cancer in the urinary tract.
Endogenous nitrosation, which is increased by tobacco smoking, chewing
and oral snuff use has been demonstrated by monitoring urinary
N-nitrosamino acids (Tsuda & Kurashima, 1991). Badawi & Mostafa
(1993) and Badawi et al. (1995) suggested that the evidence for an
association between urinary schistosomiasis and bladder cancer
development is sufficient to justify the conclusion that S.
haematobium infection is a factor in inducing preneoplastic changes
in the bladder. Infection can reduce detoxification mechanisms (and
thus prolong retention of carcinogens in the bladder), stimulate
nitrosamine synthesis, and alter the activity of
carcinogen-metabolizing enzymes.
The conclusion is that an interaction is likely between tobacco use
and schistosomiasis infection.
3.7.6 Other urinary tract infections
Kantor et al. (1984) found that the joint effect of urinary tract
infection and cigarette smoking on bladder cancer was slightly beyond
that expected under an additive model and suggested that patients with
cystitis may be especially prone to tobacco-derived carcinogens in the
urine. La Vecchia et al. (1991) studied cystitis, gonorrhoea and
condylomata acuminata and found an interaction between urinary tract
infection and tobacco that appeared to be multiplicative, with
relative risk 2.4 for "ever smoking", 3.2 for cystitis alone, and 10.3
for combined exposure. It was concluded that a relationship between
urinary tract infection (and possibly some genital infections) and
bladder cancer indicated a cancer promotion role of infection and a
multiplicative smoking interaction. There have been reports of
synergism between herpes simplex virus and tobacco-specific
N-nitrosamines in cell transformation (Park et al., 1991a); an
additive interaction between smoking and herpes simplex virus type 2
in the promotion of cervical abnormalities (Mayberry, 1985), and the
possibility of an interaction between cigarette smoking and herpes
virus infection leading to cancer of the uterine cervix (Winkelstein
et al., 1984).
3.7.7 Sarcoidosis
Bresnitz & Strom (1983) reviewed sarcoidosis and concluded that
tobacco smoking might have a protective effect for the pathogenesis of
sarcoidosis. Sarcoidosis is a generalized granulomatous disease
involving the reticulo-endothelial system with lesions predominantly
in the lymphatic system. Harf et al. (1986) investigated the smoking
habits of 113 cases of histologically confirmed sarcoidosis in a
case-control study in Lyon, France. Smoking habits were defined in 101
cases and these were compared with a control group of healthy
volunteers. There was a highly statistically significant negative
association between sarcoidosis and smoking (OR = 3.8; 95% confidence
interval: 2.4, 6.5) in both cases.
3.8 Vector effects
Toxic chemicals, as well as harmless materials that produce harmful
chemical agents when they are burnt or vaporized, can be inadvertently
transferred to cigarettes or other smoking materials and cause the
smoke to be more injurious when it is inhaled.
3.8.1 Polytetrafluoroethylene
Polytetrafluoroethylene (Teflon(R)) is used in coatings for cooking
utensils, for making chemical vessels, gaskets and bearings and in
sprays as a mould release agent. Polytetrafluoroethylene and polyvinyl
fluoride are inert materials but their thermal decomposition products
can be very biologically reactive. Cigarettes can be easily
contaminated in the workplace and, when smoked, the polymer burns to
form fumes which cause "polymer-fume fever": severe gripping chest
pain giving rise to difficulty in breathing; trembling and shaking;
elevated temperature; and severe diaphoresis. The symptoms pass after
a day or two, but recur on again smoking a contaminated cigarette.
Before the cause was recognized a case was recorded of a person, who
used the polymer in a mould release spray, having some 40 attacks
(Kuntz & McCord, 1974). Another case was a person who referred to the
disease as "mould machine pneumonia" (Kuntz & McCord, 1974). Other
cases have been reported (Albrecht & Bryant, 1987) and better
occupational hygiene and a ban on smoking in the workplace resulted in
the disappearance of symptoms in those previously affected.
3.8.2 Mercury
Inorganic mercury occurs in many industries, as elemental mercury in
scientific and electrical instruments, as amalgams with many other
metals, in paints and pigments and in the chemical industry, as well
as in mining and extraction of the metal. Organic mercury compounds
are used as antiseptics, disinfectants, fungicides, bactericides and
herbicides. Contamination of smoking materials can lead to the
inhalation of mercury vapour. Mercury has been detected by neutron
activation analysis as a naturally occurring trace element in tobacco
(<1.0 ng/g) and in the smoke of cigarettes (4 ng/cigarette)
(Schneider & Krivan, 1993; Krivan et al., 1994).
3.9 Effects of tobacco smoking and metabolism of drugs and other
chemicals
3.9.1 Oral contraceptive use
In the 1970s it was postulated that an interaction between tobacco use
and oral contraceptives increased the risk of myocardial infarction in
women. In a study of women less than 45 years of age, a greater
proportion of moderate to heavy smokers were found in oral
contraceptive users experiencing myocardial infarction, compared
against a control population (Mann et al., 1975, 1976). Sturtevant
(1982), however, found no "convincing evidence" for an interaction or
synergism between the factors smoking and oral contraceptive use for
the major classes of cardiovascular disease. Lidegaard (1993) reported
no difference in the proportion of smokers between users and non-users
of oral contraceptives in Danish women experiencing a cerebral
thromboembolic attack and age-matched controls. Regarding thrombosis,
the data of Vessey & Doll (1969) suggest an increased thromboembolism
risk from oral contraceptive use for heavy smokers compared to
non-smokers. However, other studies reviewed by Sturtevant (1982) and
Nevius et al. (1982) are said to be ambiguous regarding a possible
interaction between smoking and oral contraceptive use. On balance,
there is evidence for certain interactions between smoking and oral
contraceptive use: the US Surgeon General (1983a), concluded that
"women who use oral contraceptives and who smoke increase their risk
of a myocardial infarction by an approximately tenfold factor,
compared with women who neither use oral contraceptives nor smoke",
and "the use of both cigarettes and oral contraceptives greatly
increases the risk for subarachnoid haemorrhage among women."
3.9.2 Drug and chemical metabolism
A number of studies have demonstrated that the metabolism of various
drugs and other chemicals is influenced by the smoking status of the
individual. This effect is sufficiently noteworthy that the US Surgeon
General (1979) report concluded that it is "apparent that cigarette
smoking is one of the primary causes of drug interactions in humans".
The extensive review of the literature at that time led to the
conclusions that, with respect to the influence of smoking on the
disposition/metabolism of other compounds: (a) the dominant effect of
smoking is enhanced drug disposition caused by the induction of
hepatic enzymes; (b) tobacco smoke contains many enzyme inducers,
notably polynuclear aromatic hydrocarbons; and (c) smoking can induce
the metabolism of various therapeutic agents and their pharmacological
and/or clinical effects. The metabolism of chemical carcinogens
involves various isozymes of cytochromes P450 and differences in their
genotypes or phenotypes may be a main factor responsible for
differences among individuals in susceptibility to carcinogens.
Metabolic activation of the procarcinogens such as benzo( a)pyrene to
the ultimate form is accomplished by cytochrome P450IA1 (Kawajiri et
al., 1990; Kawajiri & Fujii-Kuriyama, 1991; Nakachi et al., 1991).
Although the most noteworthy effects of smoking cited were related to
enzyme induction, it should also be noted that other components of the
smoke, such as carbon monoxide, nicotine, cadmium, some pesticides,
cyanide and acrolein, may serve to inhibit the function of some
enzymes (Jusko, 1978). An example for this phenomenon is the
inhibition by nicotine of the P450 isozymes that are involved in the
metabolic activation of NNN and NNK (Murphy & Heiblum, 1990). Nicotine
levels exceed those of NNN and NNK by more than 500 times. Therefore,
it was not surprising that even increasing NNN and NNK levels in snuff
ten-fold by adding the synthetic compounds did not alter the
carcinogenic potency of snuff in the oral cavity of rats (Hecht et
al., 1986).
Miller (1990) reviewed how cigarette smoking affects the
pharmacokinetic and pharmacodynamic properties of various drugs. The
drugs pentazocine, phenylbutazone and heparin show increased
metabolism in smokers. Also in smokers, the metabolism of oestrogen
and theophylline is increased. In addition, although smoking does not
pharmacokinetically affect the drugs propranolol and pindolol, the
nicotine in smoke is associated with elevations in blood pressure, and
thus smoking might serve to inhibit the antihypertensive effects of
these beta-adrenergic receptor blockers. On the other hand, smoking
had no effect on the drug disposition and/or pharmacological effects
of various other drugs examined (Jusko, 1978; Miller, 1990).
3.10 Animal studies of the interactions between cigarette smoke
exposure and other agents
Fourteen chronic inhalation studies (whole-body or nose-only exposure)
with mainstream cigarette smoke in rats and mice were reviewed by
Coggins (1998) and the results and histopathological changes
contrasted with epidemiological studies in humans. In most of the
studies there were epithelial changes in conducting airways and
increased numbers of alveolar macrophages, occasionally associated
with alveolar metaplasia. Lung adenomas and adenocarcinomas were seen
in some of the studies but no statistically significant increase in
the incidence of malignant lung tumours was found in either rats or
mice. This contrasts with human epidemiology where there is an
increased lung cancer risk. In an invited commentary, Morgan (1998)
stated that, based on these differences, rodents may be ineffective
models for predicting human health risk, at least for certain inhaled
materials, but stressed the interspecies differences in respiratory
anatomy and physiology and the need for consideration of the many
factors that may lead to differences in the effects of tobacco smoke
exposure.
The existing animal toxicology studies regarding interactions between
tobacco smoke and other materials do not form a comprehensive body of
work on the topic. A number of combinations of exposures between smoke
and other agents have been studied. Results are at times inconsistent
or contradictory, and the mechanisms by which interactions occur are
often not understood. This section provides a brief review of the
literature. In general, the existing work: (a) was generally performed
using rodents; (b) usually (but not always) examined the effects of
cigarette smoking (or components of the smoke) combined with either
with specific chemical components of the smoke or with radiation; and
(c) usually examined cancer as the biological response end-point of
interest. Other studies have focused on the use of cigarette smoke
components or condensates, and have used models such as in vitro
cell systems or mouse skin; a discussion of these studies is beyond
the scope of this section.
3.10.1 Non-cancer end-points
Several studies in experimental animals have examined the effects of
cigarette smoke administered over short periods of time (from hours to
daily exposures over several weeks). The cigarette smoke-induced
increase in the number of pulmonary macrophages and leukocytes noted
in humans has also been seen in animals such as the guinea-pig, even
after short exposures (Rylander et al., 1979). In addition, Morimoto
et al. (1993) observed a synergistic increase between mineral fibres
and exposure to cigarette smoke in the production of tumour necrosis
factor by rat alveolar macrophages. On the other hand, a 10-week
tobacco smoke exposure in rats suppressed radiation- induced pulmonary
inflammation (Nilsson et al., 1992), and a 12-week exposure of rats to
smoke did not influence the lung damage caused by an intratracheal
instillation of cadmium (Lai & Diamond, 1992).
Nishikawa et al. (1992) studied in guinea-pigs the effects of combined
exposure and single exposure to ozone and cigarette smoke on airway
responsiveness and tracheal vascular permeability and found that the
combined exposure increased airway responsiveness and vascular
permeability to a greater extent in terms of magnitude, but not in
duration, than a single exposure. This indicated that combined
exposure was more harmful than exposure to either agent alone.
3.10.2 Cancer studies: tobacco (cigarette) smoke plus other chemicals
Mori (1964) studied rats receiving multiple subcutaneous injections of
the carcinogen 4-nitroquinoline-1-oxide (NQO) with or without 6-7
month inhalation of cigarette smoke. Six of eight rats had lung
carcinomas in the combined exposure group compared to 3 of 9 rats in
the NQO-only group, and tumours occurred earlier. Davis et al. (1975)
studied Wistar rats receiving single intratracheal instillations of
benzo( a)pyrene with or without cigarette smoke inhalation for most
of the lifespan. Slight elevations of pulmonary squamous neoplasia
were noted in the combined exposure group compared with the individual
agents alone. However, the effects were not statistically significant.
In mice, inhaled cigarette smoke did not influence the occurrence of
lung tumours in (a) B6C3F1 mice pretreated with 3-methylcholanthrene
or benzo( a)pyrene (Henry & Kouri, 1984), (b) C57BL mice receiving
benzo( a)pyrene or influenza virus(Harris & Negroni, 1967), (c) A/J
mice receiving intraperitoneal injections of the tobacco specific
nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and a
6-month inhalation of smoke using a whole-body mode of exposure (Finch
et al., 1996), or (d) A/J mice exposed to sidestream smoke plus the
carcinogen 3-methylcholanthrene or urethane (Witschi et al., 1997). On
the other hand, a carcinogenic synergism in lung adenoma formation was
reported in Strain A mice some 6 months after initiation of treatment
with intraperitoneally injected urethane and skin-painted cigarette
smoke tar (DiPaolo & Sheehe, 1962). However, the non- pulmonary route
of exposure makes it difficult to interpret the relevance of this
result.
Several studies in hamsters have combined cigarette smoke exposure
with other agents. Dontenwill et al. (1973) combined smoke exposure
with a single intratracheal instillation of asbestos, but found no
significant differences in laryngeal lesions or tumours in the group
exposed to both materials, compared with the group receiving smoke
alone; the asbestos alone was non-tumorigenic. The combination of a
single intratracheal administration of dimethylbenz( a)anthrene
(DMBA) and smoke was found to increase the tumour incidence in the
oral cavity, pharynx, trachea and non-pulmonary tissues, compared to
the individual agents alone (Dontenwill et al., 1973). A similar
synergism between DMBA and smoke in producing laryngeal papillomas and
other oral and laryngeal lesions was reported by Hoffmann et al.
(1979) and by Kobayashi et al. (1974), and a synergism was also noted
between the nitrosamine DENA and smoke (Dontenwill et al., 1973).
Hamsters pretreated with a single dose of either 1.0, 3.3 or 10.0 mg
of NNK, and subsequently exposed twice daily for up to 72 weeks to
diluted cigarette smoke developed significantly more tumours in the
respiratory tract than hamsters receiving the identical dosage of NNK
and subjected to sham-smoking (Hecht et al., 1983). This supports the
concept that tobacco smoke has tumour-promoting activity. A
combination of smoke plus benzo( a)-pyrene adsorbed onto haematite
produced tracheal and laryngeal hyperplasia, whereas either agent
alone did not (Hoffmann et al., 1979), but no tumours were produced.
3.10.3 Cancer studies: cigarette smoke plus radiation
Dogs were used to study the potential interactions between radon and
cigarette smoke inhalation in producing lung cancer (Cross et al.,
1982). The lung tumour incidence in dogs exposed to radon plus
cigarette smoke for 4-5 years was decreased compared to that of dogs
receiving a mixture of radon, radon daughters, and uranium ore dust.
Studies have examined the potential interactions between tobacco smoke
and the alpha-emitters radon or plutonium-239 in rats or mice. In a
series of studies, Chameaud et al. (1982) examined the effects of
combined exposures to radon and cigarette smoke in Sprague-Dawley
rats, as well as the effects of pre-treatment with either radon or
smoke exposure. Smoke exposures begun before radon exposure did not
influence the radon-induced lung tumour incidence, but this increased
by 2- to 3-fold in the combined exposure group receiving radon before
cigarette smoke compared to the group receiving radon alone.
Finch et al. (1995a) examined F-344 rats receiving a single inhalation
exposure to 239PuO2, combined with chronic (up to 30 months)
cigarette smoke exposure, and found a synergistic crude tumour
incidence in rats receiving both agents, compared with animals
receiving the radiation alone. Most of these effects could be
explained by a cigarette-smoke-induced retardation of lung clearance
of the 239PuO2 particles (Finch et al., 1995b). This group is
continuing to study the potential combined effects of smoke and X-ray
exposure in rats, and the potential effects of smoke combined with
either X- or alpha-irradiation in mice (Finch et al., 1995a). Talbot
et al. (1987) reported results of a study in which mice inhaled
cigarette smoke, 239PuO2 or both materials. As in the case of rats,
cigarette smoke exposure caused retarded lung clearance and thus led
to greater radiation doses in animals receiving both agents.
4. EFFECTS OF EXPOSURE TO TOBACCO SMOKE AND OTHER AGENTS: SEPARATE
EFFECTS OR POSSIBLE INTERACTIONS
4.1 Coal mining
4.1.1 Coal dust
Coal miners can suffer from chronic bronchitis, coal workers
pneumoconiosis, progressive massive fibrosis, and emphysema. The
respiratory impairment appears as radiologically visible and
functional changes in the lungs, but although some of these are
associated solely with coal dust, some are also closely associated
with smoking. In the past it has been difficult to apportion
attributable risk to the two causes due to the fact that coalface
workers, the group subjected to the highest dust exposure, have a
different smoking pattern and perhaps a different daily consumption of
smoking materials than non-coalface workers because smoking in the
mine is forbidden. With the limitation of dust in modern mining, the
effect of the two factors, dust and smoking, is becoming clearer.
However, difficulties arise in interpreting data because non-smokers
may accumulate more dust, as they have less absenteeism, a different
pattern of lung clearance and a longer life (NIOSH, 1995).
In most of the countries surveyed, the prevalence of smoking by miners
tends to have been somewhat higher than in either the male population
as a whole, or among most other occupational groups, although a
distinction is seldom drawn between miners and coal miners except in
studies centred on coal mining populations. Between 1963 and 1975 in
the United Kingdom, the smoking prevalence fell for the male
population (and for miners) from 54% (miners 77%) to 47% (miners 49%)
(Lee, 1976). The figures for 1988 and 1990 were 33% and 31% (Bennett
et al., 1996) and for face-trained coalminers 35.7% in 1989 (Elliott,
1995). In a study of 8555 American miners from 29 bituminous coal
mines (Kibelstis et al., 1973), over 50% were smokers and 25% were
ex-smokers. In a United Kingdom study (Love & Miller, 1982), only 13%
of 1677 coal miners from 5 British collieries, who were examined in a
lung function study, were non-smokers; 66% were regular smokers and
the remainder were intermittent or ex-smokers. In a 20 year follow-up
study of a population of coal British miners and others (Cochrane &
Moore, 1980), 69% of the coal miners were smokers. These examples
typify the smoking prevalence of coal miners prior to the early
1980's. A 1982 survey of 800 000 American men and women in relation to
their occupations (Stellman et al., 1988) found that among miners
(type unspecified) 29.4% had never smoked regularly, 31.5% were
current smokers and 39.1% were former cigarette smokers. This may
reflect a general trend in the countries with higher income economies
where smoking has been decreasing in many sections of the population.
4.1.2 Bronchitis in coal miners
Kibelstis et al. (1973) found that the prevalence of bronchitis in
coal miners who smoked was higher than in non-smoking coal miners.
Coalface workers had more bronchitis and more airway obstruction than
surface workers and the difference between smokers working at the
coalface and non-smoking surface workers showed that the effect of
smoking was five times greater than that of coal dust. A German
epidemiological evaluation of chronic obstructive bronchitis in 5605
miners, 1276 ex-miners and 3898 individuals who had never worked in a
mine showed that smoking has a more serious effect on miners than on
other groups (Roth et al., 1985). It is possible that, within a mining
community, the other two groups may have a predisposition to the
disease and have achieved their status by job selection or job escape.
In a study of new entrants into coal mining McLintock (1971) found
that those who drop out of mining tend to be less physically fit and
more prone to chest problems than those who remain.
Morgan (1982) analysed the effects of cigarette smoking, dust exposure
and environmental factors on respiratory disease, and concluded that
bronchitis and airways obstruction were two separate responses to
cigarette smoking. The airflow obstruction found in smokers is due to
small airways disease and an involvement of respiratory bronchioles
leading to the development of emphysema. In coal miners, the
prevalence of bronchitis among non-smokers is related to the degree of
dust exposure. Marine et al. (1988) analysed data from studies on 53
382 coal miners in the United Kingdom and found that smoking miners
were at greater risk of developing chronic bronchitis. In a study by
Selig & Nestler (1985) of the relationship between chronic bronchitis,
smoking and dust (unspecified source), heavy smoking was equated with
20 years of dust exposure. From postmortem examinations of coal mine
workers, a correlation was reported between clinical chronic
bronchitis and smoking (Selig & Nestler, 1985).
The chronic bronchitis of coal miners is probably a combination of (a)
mucus hypersecretion caused by dust; (b) mucus hypersecretion, mucus
modification and clearance impairment caused by tobacco smoke; (c)
small airways disease caused by tobacco smoke; and (d) the effect of
dust on small airways tissue already inflamed by smoking. Bronchitis
due to smoking causes mucus hypersecretion which is much greater than
that due to coal dust (Morgan, 1982). Cigarette smoke impairs lung
clearance by changing the physical and chemical properties of mucus
and causing ciliastasis. Rheological measurement show changes in the
viscoelastic properties of mucus; chemically the mucus glycoprotein
structure is changed (King et al., 1989) and the irritant gases in
smoke cause abnormal mucus secretion and ciliastasis (Holbrook, 1977).
Small airways disease and bronchiolitis, leading to emphysema, are due
to smoking rather than to dust (Morgan, 1982).
4.1.3 Emphysema and pneumoconiosis in coal miners
Dust in coal mining is considered to be the primary cause of coal
workers pneumoconiosis. In a study of coalworkers and non-coalworkers,
Cockroft et al. (1982) concluded, after taking any effect of smoking
into account, that there was a 7-fold excess of emphysema in
coalworkers. Results of postmortem examinations of 866 Australian
miners (Leigh et al., 1983) showed a positive correlation between dust
exposure and emphysema and pneumoconiosis, with the severity highest
in non-smokers. However, smoking and non-smoking coalface workers were
not compared. From a postmortem comparison of lungs from 450 coal
miners, Rockley et al. (1984) found that emphysema occurred more
frequently in smokers (72%) than in ex-smokers (65%) or in non-smokers
(42%) and the relative frequency increased with age at death. The
study considered the possibility that coal dust might cause emphysema
which inhibits clearance and, in turn, promotes fibrosis, or
alternatively that fibrosis caused by dust increases the chance of
emphysema. However, the findings of a study of South Wales coal miners
(Fletcher., 1972) militated against dust-induced emphysema. It has
been suggested that differences in emphysema between coalworkers and
non-coalworkers can be accounted for by taking into account current
smokers in the two groups. Morgan (1982) concluded that the evidence
militates against obstructive emphysema occurring more commonly in
coal miners than in the general population, or that more dust
inhalation leads to a greater likelihood of emphysema developing.
Reviews of small airways disease (SAD) suggested that emphysema
proceeds from smoking-induced SAD. Cosio et al. (1980) considered that
their observations supported the hypothesis that SAD is causally
related to centrilobular emphysema, but not necessarily to panlobular
emphysema.
4.1.4 Lung cancer in coal miners
Perhaps as a result of failure to control confounding factors, there
has been a lack of consistency among reports on the relationship
between coal mining and lung cancer incidence in miners. In a direct
evaluation of the relationship between lung cancer mortality and coal
mine dust exposure, controlling for smoking status, Ames et al. (1983)
found no evidence of a link between coal mine dust exposure and lung
cancer risk, nor of an interaction effect, although the expected lung
cancer risk in cigarette smokers was observed. From a study of dust
exposure, pneumoconiosis and mortality of coal miners (Miller &
Jacobsen, 1985) it was found that lung cancer mortality among miners
who smoked was 5.5 times higher than in "never smokers" but that the
effect was entirely due to smoking.
Radon and radon daughter contamination of the dust in coal mines might
be expected to be as prevalent as in all other mines, and thus the
apparent very low lung cancer risk in coal mining may seem unexpected.
However, because of the explosion risk in coal mines, the ventilation
is usually efficient and a build-up of radioactivity is probably less
likely than in other types of mine.
4.2 Other mineral dusts
4.2.1 Talc
Talc is a hydrated magnesium silicate, often contaminated with free
silica or fibrous asbestos-like minerals such as tremolite and
anthophyllite. The only significant difference in the effects on
exposed and non-exposed was in the number and severity of cases of
dyspnoea in the talc workers, and smoking was considered to be an
aggravating factor (Kleinfeld et al., 1973).
4.2.2 Kaolin
Kaolin (pure China clay) is a hydrated aluminium silicate used for
ceramics and as a filler in the paper, rubber and paint industries.
The dry powder can give rise to fibrotic nodules in the lungs (Seaton
et al., 1981; Wagner et al., 1986). Characteristic smoker's inclusions
have been seen in transmission electron micrographs of pulmonary
alveolar macrophages obtained from cigarette smokers. The contents of
these inclusions are heterogeneous and include electron-dense areas,
lipid material and needle-like structures. These have properties
consistent with the composition of kaolinite. Kaolinite is present in
cigarette smoke from different brands, and pulmonary alveolar
macrophages are able to ingest this material in vivo (Hocking &
Golde, 1979).
4.2.3 Alumina
Alumina (aluminium oxide) is extremely hard and is used as an abrasive
(corundum). A cross-sectional study of 788 employees of an aluminium
production company examined the relationship of radiographic
abnormalities to smoking and dust exposure during bauxite and alumina
mining and refining (Townsend et al., 1988). Chest radiographs showed
a moderate time trend of increasing prevalence of small opacities in
non-smokers with high cumulative dust exposures. In most exposure
categories, smokers had a higher prevalence of opacities than
non-smokers. For cumulative exposures of less than 100 mg/m3-years,
increasing trends with duration of exposure were accentuated in
smokers as compared to non-smokers. The stronger effects observed in
smokers were attributed to the joint effects of duration of smoking
and duration of occupational exposure (Townsend et al., 1988).
4.3 Fibrous minerals
Fibrous minerals have been implicated in pleural thickening, pulmonary
fibrosis, mesothelioma and lung cancer in some villages in the
Anatolian region of Turkey (Artvinli & Baris, 1979), where fibrous
zeolite minerals (chabazite and erionite) are present in volcanic
deposits and used in buildings. Erionite has been shown to induce
mesothelioma. The symptoms and pathology of the respiratory disorders
and malignant disease were similar to those of asbestos. Asbestos-type
diseases have also been described in communities exposed to zeolite
minerals and tremolite dust in other similar regions by Baris et al.
(1979) and Yazicioglu et al. (1980). Non-asbestos fibrous materials
have been associated with pulmonary fibrosis (Stanton et al., 1977).
There are no data on any interaction of these minerals with smoking
but there appears to be a potential for interaction.
Wollastonite is a fibrous monocalcium silicate, which has been used as
a substitute for asbestos, as a filler and flux in ceramics, in
grinding wheels, refractory products, building blocks and acoustic
tiles. It is weakly fibrogenic. Hanke et al. (1984) studied a small
population of workers exposed to wollastonite and attributed
significant levels of chronic cough, phlegm and bronchitis to smoking
and not to exposure to wollastonite.
4.4 Metals
4.4.1 Antimony
Antimony is chemically similar to arsenic. Arsenic is metalloid,
antimony is a metal, both have volatile hydrides and form halogen,
oxygen and sulfur derivatives. Compounds of both frequently occur
together, particularly in smelter fume. Biological effects are similar
to those of arsenic (De Wolff & Edelbroek, 1994; De Wolff, 1995).
The concentrations of antimony, arsenic, cadmium, chromium, cobalt,
lanthanum, lead, selenium and zinc measured in lung tissues of
deceased smelter workers suggested that lung cancer risk was
multifactorial, involving carcinogenic and anti-carcinogenic factors
(Gerhardsson & Nordberg, 1993). A 30-year study at an antimony smelter
did not specifically implicate antimony as the cause of excess lung
cancer because of concurrent exposure to other carcinogens (Jones,
1994). In another study of smelters, the data suggested an increase in
lung cancer and non-malignant respiratory and heart disease (Schnorr
et al., 1995).
Antimony can have harmful effects on lung tissues, including
pneumoconiosis. IARC (1989a) evaluated antimony trioxide and antimony
trisulfide and concluded that the trioxide was possibly carcinogenic
to humans (Group 2B) and the trisulfide was not classifiable (Group
3). It is likely that the effects of inhalation of antimony fume/dust
and tobacco smoke would be worse than inhaling either separately. The
similarities of antimony to arsenic, both chemically and in some
biological effects, leads to the conclusion that tobacco smoke and
antimony could interact like tobacco smoke and arsenic in producing
toxicity.
4.4.2 Cadmium
Most zinc and lead-zinc ores contain small amounts of cadmium. It is
used in electroplating, in metal alloys (with copper for overhead
wires, and aluminium for casting), in nickel-cadmium dry cells, for
pigment manufacture and use, added to silver to prevent staining, and
it is a hazard of welding. The main route of exposure for the
non-smoking general population is via food, while for exposed workers
it enters the body mainly by inhalation. Tobacco is an important
source of cadmium in smokers (IPCS, 1992), and the tobacco source
affects the level of cadmium exposure (Yue, 1992). Any intake is
important because cadmium has an extremely long biological half-life.
It was suggested (Hassler et al., 1983) that higher levels of cadmium
in the blood and urine of exposed workers could arise both from
workplace contamination of cigarettes and transfer as fume during
smoking.
Cadmium has various toxic effects, the earliest being impairment of
renal tubular function leading to failure of resorption and excretion
of low molecular weight protein, glycosuria, aminoaciduria and
hypercalciuria (IPCS, 1992; IARC, 1993). It has been associated with
some types of lung disorder (emphysema, obstructive pulmonary disease
and diffuse fibrosis) (IPCS, 1992) Exposure to cadmium compounds has
been associated with cancer of the lung. There is some evidence for an
association with prostatic cancer (IARC, 1993). Other epidemiological
studies did not confirm an increased risk of prostatic cancer
(Kazantzis et al., 1992). The evaluation by IARC (1993) concluded that
cadmium and cadmium compounds are carcinogenic to humans (Group 1).
The supposition that cadmium in cigarette tobacco or in the workplace
may cause lung cancer has been questioned (Lamm et al., 1992;
Hertz-Picciotto & Hu, 1994). In a cohort mortality study of cadmium
workers in England, an observed increase lung cancer risk could not be
attributed strictly to cadmium due to the presence of multiple
confounding factors, particularly arsenic (Kazantzis et al., 1992).
Lamm et al. (1992) also suggested that arsenic may be responsible for
the observed lung cancer increased. The many elements in the lung
tissues of deceased smelter workers (Gerhardsson & Nordberg, 1993)
illustrates the difficulty in apportioning a role to one material in a
multifactorial environment.
Cadmium affects the myocardium and produces hypertension in animal
studies but the induction of cardiovascular disease and hypertension
in humans has not been demonstrated in epidemiological studies
(Kristensen, 1989; IPCS, 1992)). Blood and urine cadmium levels are
higher in smokers than in non-smokers and were found to be
considerably elevated in smokers working in an alkaline battery
factory (Hassler et al., 1983) and in smelter workers who were also
smokers (Kazantzis & Armstrong, 1984; Lilis et al., 1984a,b).
Davison et al. (1988) reported a cadmium dose/effect relationship in
functional and radiological evidence of emphysema in 101 subjects.
Leduc et al. (1993) described the very rapid development of emphysema
in a smoker after exposure to very high levels of cadmium. Smoking by
cumulatively increasing the body burden and hindering the lung
clearance may have provided an additional cause for the emphysema.
However, an additive or synergistic mechanism for the cadmium plus
smoking effect could not be inferred in this case because of the high
cadmium dose.
4.4.3 Cobalt
Cobalt is used in the production of alloys, tungsten carbide tools,
permanent magnets, and in the electrical industry.
Cobalt has toxic effects (Beliles, 1994). Cobalt exposure has been
linked to various allergic reactions (Shirakawa et al., 1992); hard
metal exposure and smoking together arithmetically increased total IgE
levels. Interstitial lung disease has been associated with cobalt in
susceptible individuals (Sprince et al., 1988), although in a study
involving the manufacture of permanent magnets (Deng et al., 1991)
abnormalities in pulmonary function and respiratory symptoms were no
higher than those of a reference population, except for 4 subjects out
of 362, who showed diffuse patches consistent with pneumoconiosis.
4.4.4 Lead
Lead is used in batteries, paint, glass, ceramics, fuel additives and
other industrial applications. In lead-using industries the main route
of exposure is by inhalation, mainly as dust and fume. Lead has a
range of toxic effects on blood, and the renal and nervous systems
(IPCS, 1995).
Levels of lead in blood vary from one area to another, between urban,
rural and occupationally exposed populations, and between men and
women (IPCS, 1995). The tobacco plant absorbs lead from the soil and
around 5-6% of that in cigarettes is inhaled in the smoke. Lead
concentrations in the smoke from one cigarette were found to range
from 0.017 to 0.98 µg (IARC, 1986). Higher blood lead and erythrocyte
protoporphyrin levels have been demonstrated in heavy smokers exposed
to lead (Williams et al., 1983; Landrigan & Straub, 1985); these could
have been partly due to contaminated cigarettes acting as vectors.
Other studies on occupationally exposed workers showed a progressive
increase in blood lead with an increase in the number of cigarettes
smoked (Maheswaren et al., 1993).
The association between lead exposure, tobacco smoke exposure and
blood pressure was examined in a cross-sectional study on 809 men
occupationally exposed to lead in a battery factory but only a small
increase in systolic blood pressure was found (Maheswaren et al.,
1993). There was no evidence of interactive effects between smoking
and lead exposure, but the absorbed lead from cigarettes added to the
body burden.
4.5 Rubber industry
Some of the principal hazards in the industry are fumes, talc, carbon
black, chemical additives and organic solvents but the components of
the hazard mixture differ between different areas of work. A high risk
of pulmonary disease has been reported in the rubber industry. It was
elevated for smokers, particularly those employed in areas where there
were respirable particulates and/or solvents (Lednar et al., 1977).
The data suggested an interaction between smoking and hazards
encountered in mixing (particulates), extrusion (solvent sprays and
mould release agents), and curing (solvents and rubber reaction
products). A problem in epidemiological studies in the industry arises
because of movement of workers between jobs. Some high-risk workers
who were also smokers were involved in finishing and inspection but
they tended to be older employees who had worked in other areas before
moving to this particular job. Emphysema was the principal pulmonary
condition leading to premature termination of employment (Lednar et
al., 1977).
IARC (1987) classified the rubber industry as Group 1, based on
sufficient evidence for carcinogenicity to humans. Excess mortality
from cancers of various sites, the site usually being associated with
the nature of the work and types of exposure, has been reported with
bladder cancer being associated with exposure to aromatic amines (Fox
et al., 1974; Monson & Nakano, 1976a,b; Monson & Fine, 1978;
Kilpikari, 1982; IARC, 1987; Zhang et al., 1989; Weiland et al.,
1996). Lung cancer was associated with curing and inner tube
manufacture. The use of talc in the rubber industry has been
associated with pulmonary disease (Kleinfeld et al., 1973). In the
rubber industry the relative risk of lung cancer for talc-exposed
workers was 3.2 for men and 4.4 for women (Zhang et al., 1989),
compared with 2.5 times excess lung cancer risk in talc-using
industries not associated with rubber manufacture (Thomas & Stewart,
1986). In jobs where very high lung cancer rates were found, smoking
levels were also very high, but any possible interaction effect from
the two exposures could not be assessed.
Gastrointestinal cancer, bladder cancer and leukaemia were found to be
associated with jobs in the rubber industry (Monson & Fine, 1978;
IARC, 1987). Possible causes, such as exposure to carbon black,
plasticisers, antioxidants, arylamino compounds and benzene have been
suggested. Urine samples from rubber workers showed a higher mutagenic
activity in the middle of a working week than at the beginning of a
week in both smokers and non-smokers, indicating the presence of
mutagens in the work environment. In a study involving the analysis of
urine from rubber workers for mutagenic factors, a possible
synergistic effect of smoking and occupational exposure was found in
smokers (Wicklund et al., 1988). There was a relationship between skin
contamination and urinary mutagenicity (Bos et al., 1989). Other
studies involving the analysis of urine from rubber workers for
mutagenic factors also suggested a possible synergistic effect of
smoking and occupational exposure among smokers (Falk et al., 1980;
Crebelli et al., 1985).
Andjelkovich et al. (1988) carried out a case-control study of lung
cancer in workers at a rubber manufacturing plant. There was an
association between lung cancer mortality risk and work in certain
areas for smokers and non-smokers, and the risk was greater in
smokers. Zhang et al. (1989) studied a cohort of 957 men and 667 women
employed in a rubber factory. The relative risk of lung cancer for
smokers was 8.5 for men and 11.4 for women, and for those exposed to
curing agents or talc the relative risk was 3.2 for men and 4.6 for
women. Additive and multiplicative models were used to evaluate the
interaction between smoking and occupational exposure on lung cancer.
The additive interaction term was not statistically significant and
the multiplicative interaction was negative. Weiland et al. (1996)
were not able to find interactions between smoking and exposure in the
rubber industry.
4.6 Petroleum industry
Petroleum refining involves exposure of workers to a large number of
chemical compounds occurring in crude oil or encountered in production
processes as intermediates, catalysts, additives or in the final
products. Because of fire risk, there are sections of the industry
where smoking is not permitted. However, in a study of 10 923 male and
624 female employees of the Australian petroleum industry between 1981
and 1984, it was found that the smoking habits did not differ
substantially from those of the general population (Christie et al.,
1986), and continued surveillance of these workers showed that
mortality rates were lower than for the general population (Christie
et al., 1987).
IARC (1989b) concluded that there is limited evidence that working in
petroleum refineries entails a carcinogenic risk. This limited
evidence applies to skin cancer and leukaemia; for all other cancer
sites on which information was available, the evidence was inadequate.
The overall evaluation, taking account of sufficient or limited
evidence in experimental animals for the carcinogenicity of various
distillates produced during petroleum refining, was that occupational
exposures in petroleum refining are probably carcinogenic to humans
(Group 2A).
In a study of 92 men with histologically confirmed renal cell
carcinoma (Domiano et al., 1985), it was concluded that there could be
an interaction between long-term gasoline exposure and heavy smoking.
In a case-control study of bladder cancer in New Jersey, USA, Najem et
al. (1982) found a significantly elevated risk of bladder cancer in
patients who had worked in the petroleum industry (OR, 2.5, CI,
1.2-5.5). While the risk was increased in current smokers (OR, 2.6),
it was higher in "never smokers" (OR, 5.6) and only slightly elevated
in ex-smokers (OR, 1.4). In another hospital-based study in Argentina
an association was found between bladder cancer and oil refinery work,
along with an elevated risk of lung cancer in smokers, but the numbers
were too few for any evaluation of the interaction (Iscovich et al.,
1987).
There was no increased mortality from either kidney cancer or
leukaemia in employees exposed to gasoline (Wong et al., 1993). A low
frequency of bladder cancer among refinery workers was attributed to
an assumed lower level of smoking among the group of workers studied
(Higginson et al., 1984).
In the study of workers occupationally exposed to gasoline vapour, the
sister chromatid exchange (SCE) frequency in peripheral blood
lymphocytes was used as an indicator of genotoxic response. Workers
employed in gasoline retail outlets were classified according to
cigarette smoking habits. The SCE frequency in lymphocytes was
unaffected by cigarette smoke or gasoline exposure alone, but
increased with combined exposure. The increased SCE frequency observed
in smokers occupationally exposed to gasoline vapour could be due to
the activation of hepatic enzymes by cigarette smoke, leading to a
greater formation of reactive metabolites of gasoline vapour (Edwards
& Priestly, 1993).
4.7 Pesticides
A variety of chemical compounds, natural and synthetic, are used as
pesticides. The number of individuals exposed during manufacture is
relatively small, and processes are usually contained, but the
worldwide population of pesticide users is very large. Most of the
world's population is exposed to pesticide residues in food and
drinking-water (Ecobichon, 1995). There is some information concerning
effects on health from interaction of pesticide exposure and smoking.
"Vineyard sprayer's lung", is an occupational disease associated with
the spraying of copper-sulfate-based Bordeaux Mixture. In a study of
the cytological changes in the respiratory tract of vineyard workers
spraying Bordeaux Mixture (Plamenac et al., 1985), the macrophages of
control subjects contained no copper whereas copper was detected in
64% of the sprayers. Abnormal cytological changes in the sputum were
found in smokers, in sprayers and controls, and atypical squamous
metaplasia of the respiratory epithelium was observed in 29% of the
sprayers who were smokers.
In a study to evaluate the hypothesis that exposure to lead arsenate
resulted in an excess mortality from lung cancer, Wicklund et al.
(1988) compared 155 male orchard workers who had died of respiratory
cancer with 155 orchard workers who had died of other causes. Two
groups of non-orchard workers (620) were used as matched controls.
There was no difference in lead arsenate exposure between the orchard
worker group. Although cigarette smoking was common among the orchard
workers, their smoking habits were similar to the non-orchard worker
control groups. In both groups of orchard workers mortality from
respiratory cancer was higher in smokers than in non-smokers.
McDuffie et al. (1990) examined the possibility that pesticide use was
related to the risk of primary lung cancer. In a case-control study
using data from a population-based cancer registry, they interviewed
273 men and 103 women with diagnosed primary lung cancer and compared
their occupational exposures, medical history and working
characteristics with 187 male community control subjects. There was no
correlation of lung cancer risk with exposure to pesticides and
adjusting for smoking did not alter this.
5. CONCLUSIONS AND RECOMMENDATIONS
5.1 Conclusions
1. Tobacco use, particularly smoking, is the single most important
public health hazard in the world today, and a major preventable
cause of morbidity and mortality.
2. In addition to the adverse health effects of active tobacco use,
adverse health effects have also been demonstrated as a result of
exposure to environmental tobacco smoke.
3. The risks from tobacco smoke are demonstrably increased through
interactions with certain chemical, physical and biological
hazards found in the workplace and general environment.
4. Based on this review of the scientific literature, additional
interactive effects, not yet identified, may exist.
5. In addition to synergistic interactions between tobacco smoke and
other agents, a few instances of antagonistic interactions were
also noted. However, even in these cases, the health risks of
tobacco smoke far outweigh any apparent protective effects.
5.2 Recommendations for protection of human health
1. All possible measures should be taken to eliminate tobacco use,
particularly smoking.
2. In order to avoid interaction with occupational exposures, and
eliminate the risks of exposure to environmental tobacco smoke,
smoking in the workplace should be prohibited.
3. Smoking in public places should be strongly discouraged.
4. In order to reduce the risks of exposure to environmental tobacco
smoke, particularly for children, smoking in domestic
environments should be strongly discouraged. Such action will also
avoid possible deleterious interactions between tobacco smoke and
residential exposures to other hazards.
5. Awareness-building through active educational programmes on the
health hazards of smoking should be undertaken in both developed
and developing countries. This should include enhanced
communication about deleterious interactions between tobacco and
other agents. Governments, industry, health and educational
professionals, and the general public should share in this
responsibility.
6. Since smoking may result in altered response or adverse reactions
to drugs and other therapeutic treatments, appropriate dose
adjustments and patient surveillance should be taken into
consideration by clinicians.
7. Health professionals should provide assistance to smokers to stop
smoking. This may necessitate the allocation of additional
resources for this purpose.
6. FURTHER RESEARCH
1. A number of different methodological approaches to investigating
interactions currently exist. The feasibility of harmonizing
methodologies for the assessment of potential interactions
between two or more health hazards should be explored. In the
interim, investigators should make every effort to state clearly
which methodologies have been used when reporting their results.
2. In many of the observational studies included in this review,
exposure data were limited. In order to improve future
investigations of interactions among human health hazards, more
complete and accurate exposure assessments should be performed.
3. Further epidemiological investigations of potential interactions
between tobacco smoke and other hazards in both the occupational
and general environments are needed to identify additional
populations at risk. Such interactive effects can lead to risks
higher than would be predicted by separate analyses of the risks
associated with individual hazards.
Epidemiological investigations in countries where the health
effects of tobacco have not been extensively studied previously
are of particular importance. This information would be of value
in characterizing the morbidity and mortality due to tobacco use
in those countries.
4. Additional research is needed to clarify the toxicological
mechanisms by which tobacco smoke leads to adverse health
effects, and by which tobacco smoke and other agents interact.
This information will be of use in the design and interpretation
of epidemiological studies on the health effects of tobacco,
including interactive effects.
5. Additional epidemiological research on the health effects of
passive smoking, particularly carcinogenic, cardiorespiratory and
allergic effects, would be of value in characterizing the effects
of low levels of exposure to tobacco smoke. Interactions between
environmental tobacco smoke and other health hazards also warrant
further investigation.
6. Established biomarker methods should be applied to the monitoring
of workers for the early detection of harmful exposures to toxic
or carcinogenic agents, and, in many areas, new biomarker methods
should be developed to improve hazard identification.
Consideration should be given to evaluating the ongoing exposure
of workers to active or passive tobacco smoke by monitoring
salivary or urinary cotinine, one of the major nicotine
metabolites.
7. A comprehensive review of the adverse health effects of
environmental tobacco smoke should be undertaken. This review
would provide an authoritative summary of the health impacts of
environmental tobacco smoke, as well as interactions between
environmental tobacco smoke and other health hazards.
REFERENCES
Abe S, Ohsaki Y, Kimura K, Tsuneta Y, Mikami H, & Murao M (1982)
Chromate lung cancer with special reference to its cell type and
relation to the manufacturing process. Cancer, 49(4): 783-787.
Abelin T, Segmüller H, & Segmüller G (1990) Tobacco and alcohol
consumption as risk factors of Dupuytren's contracture. In: Tobacco
and health 1990 -- The global war: Proceedings of the 7th World
Conference on Tobacco and Health, Perth, Western Australia, 1-5 April
1990. Perth, Department of Health of Western Australia, pp 449-450.
Abramson MJ, Wlodarczyk JH, Saunders NA, & Hensley MJ (1989) Does
aluminium smelting cause lung disease. Am Rev Respir Dis, 139:
1042-1057.
Acheson ED, Gardner MJ, Winter PD, & Bennett C (1984) Cancer in a
factory using amosite asbestos. Int J Epidemiol, 13(1): 3-10.
Alavanja MC, Brownson RC, Benichou J, Swanson C, & Boice JD (1995)
Attributable risk of lung cancer in lifetime nonsmokers and long-term
ex-smokers (Missouri, United States). Cancer Causes Control, 6(3):
209-216.
Alavanja MC, Brownson RC, Berger E, Lubin J, & Modigh C (1996) Avian
exposure and risk of lung cancer in women in Missouri: population
based case-control study. Br Med J, 313(7067): 1233-1235.
Albrecht WN & Bryant CJ (1987) Polymer-fume fever associated with
smoking and use of a mold-release spray containing
polytetrafluoroethylene. J Occup Med, 29(10): 817-819.
Al-Fayez SF, Salleh M, Ardawi M, & Zahran FM (1988) Effects of sheesha
and cigarette smoking on pulmonary function of Saudi males and
females. Trop Geogr Med, 40(2): 115-123.
Amandus HE, Castellan RM, Shy C, Heineman EF, & Blair A (1992)
Reevaluation of silicosis and lung cancer in North Carolina dusty
trades workers. Am J Ind Med, 22(2): 147-153.
Ames RG, Amandus H, Attfield M, Green FY, & Vallyathan V (1983) Does
coal mine dust present a risk for lung cancer? A case-control study of
US coal miners. Arch Environ Health, 38(6): 331-333.
An HS, Southworth SR, Jackson WT, & Russ B (1988) Cigarette smoking
and Dupuytren's contracture of the hand. J Hand Surg, 13A: 872-874
Anderson HA, Lilis R, Daum SM, & Selikoff IJ (1979) Asbestosis among
household contacts of asbestos factory workers. Ann NY Acad Sci, 330:
387-399.
Andjelkovich DA, Abdelghany N, Mathew RM, & Blum S (1988) Lung cancer
case-control study in a rubber manufacturing plant. Am J Ind Med,
14(5): 559-574.
Andjelkovich DA, Shy CM, Brown MH, Janszen DB, Levine RJ, & Richardson
RB (1994) Mortality of iron foundry workers: III. Lung cancer
case-control study. J Occup Med, 36(12): 1301-1309.
Archer VE (1988) Lung cancer risk in underground miners: cohort
case-control studies. Yale J Biol Med, 61: 183-193.
Armitage AK & Turner DM (1970) Absorption of nicotine in cigarette and
cigar smoke through the oral mucosa. Nature (Lond), 226: 1231-1232.
Armstrong BG, Tremblay CG, Cyr D, & Theriault GP (1986) Estimating the
relationship between exposure to tar volatiles and the incidence of
bladder cancer in aluminium smelter workers. Scand J Work Environ
Health, 12: 486-493.
Artvinli M & Baris YI (1979) Malignant mesotheliomas in a small
village in the Anatolian Region of Turkey: An epidemiologic study. J
Natl Cancer Inst, 63(1): 17-20.
Attali P, Ink O, Pelletier G, Vernier C, Jean F, Moulton L, & Etienne
JP (1987) Dupuytren's contracture, alcohol consumption, and chronic
liver disease. Arch Int Med, 147: 1065-1067.
Autrup JI, Hansen C, & Autrup H (1992) Detection of tobacco smoke
carcinogen-DNA adducts in cultured rat buccal mucosa cells following
exposure to ethanol and total cigarette smoke condensate or chewing
tobacco. Chem-Biol Interact, 85: 141-150
Avolio G, Cacciabue M, Rolla G, Caria E, Oliaro A, Carlino F, Pepino
E, Polizzi S, & Bucca C (1986) [Functional compromise of the small
airways in subjects exposed to SiO2.] Minerva Med, 77(45-46):
2183-2185 (in Italian).
Axéll T, Mörnstad H, & Sundström B (1978) [Snuff and cancer of the
oral cavity.] Lakartidningen, 75: 2224-2226 (in Swedish).
Axelson O (1995) Cancer risks from exposure to radon in homes. Environ
Health Perspect, 103(suppl 2): 37-43.
Badawi AF & Mostafa MH (1993) Possible mechanisms of alteration in the
capacities of carcinogen metabolizing enzymes during schistosomiasis
and their role in bladder cancer induction. J Int Med Res, 21(6):
281-305.
Badawi AF, Mostafa MH, Probert A, & O'Connor PJ (1995) Role of
schistosomiasis in human bladder cancer: evidence of association,
aetiological factors, and basic mechanisms of carcinogenesis. Eur J
Cancer Prev, 4: 45-59.
Baker DB, Gann PH, Brooks SM, Gallagher J, & Bernstein IL (1990)
Cross-sectional study of platinum salts sensitization among precious
metals refinery workers. Am J Ind Med, 18: 653-664.
Ball J & Simpson D (1987) Round the world: India -- Tobacco and ill
health. Lancet, 18: 149.
Baris Y, Artvilini M, & Sahin AA (1979) Environmental mesothelioma in
Turkey. Ann NY Acad Sci, 330: 423-432.
Barone JA, Peters JM, Garabrant DH, Bernstein L, & Krebsbach R (1987)
Smoking as a risk factor in noise-induced hearing loss. J Occup Med,
29(9): 741-745
Bartsch H, Malaveille C, Friesen M, Kadlubar FF, & Vineis P (1993)
Black (air-cured) and blond (flue-cured) tobacco cancer risk. IV:
Molecular dosimetry studies implicate aromatic amines as bladder
carcinogens. Eur J Cancer, 29A(8): 1199-1207
Bates MN, Smith AH, & Cantor P (1995) Case-control study of bladder
cancer and arsenic in drinking water. Am J Epidemiol, 141(6): 523-530.
Battista SP (1976) Ciliatoxic components in cigarette smoke. In:
Proceedings of 3rd World Conference on Smoking and Health, New York
City, 2-5 June 1975 -- Volume 1: Modifying the risk for the smoker.
Washington, DC, US Department of Health, Education and Welfare, pp
517-534 (DHEW Publication No. (NIH) 76-1221).
Baumslag N, Keen P, & Petering HG (1971) Carcinoma of the maxillary
antrum and its relationship to trace metal content of snuff. Arch
Environ Health, 23: 1-5.
Beck GJ, Maunder LR, & Schachter EN (1984) Cotton dust and smoking
effects on lung function in cotton textile workers. Am J Epidemiol,
119(1): 33-43.
Beliles RP (1994) Cobalt. In: Clayton GD & Clayton FE ed. The metals
-- Patty's industrial hygiene and toxicology. New York,
Wiley-Interscience, chapter 27, pp 1986-1999.
Bennett N, Jarvis L, Rowlands O, Singleton N, & Haselden L (1996)
Office of Population Censuses and Surveys, Social Survey Division:
Results from the 1994 general household survey. London, Her Majesty's
Stationery Office (HMSO), pp 92-102.
Benowitz NL, Porchet H, Sheiner L, & Jacob P (1988) Nicotine
absorption and cardiovascular effects with smokeless tobacco use:
comparison with cigarettes and nicotine gum. Clin Pharmacol Ther,
44(1): 23-28.
Berry G, Newhouse ML, & Turok M (1972) Combined effects of asbestos
exposure and smoking on mortality from lung cancer in factory workers.
Lancet, 2: 476-479.
Berry G, Newhouse ML, & Antonis P (1985) Combined effect of asbestos
and smoking on mortality from lung cancer and mesothelioma in factory
workers. Br J Ind Med, 42: 12-18.
Bhonsle RB, Murti PR, Gupta PC, & Mehta FS (1976) Reverse dhumti
smoking in Goa: an epidemiologic study of 5,499 villagers for oral
precancerous lesions. Indian J Cancer, 13(4): 301-305.
Bi W, Hayes RB, Feng P, Qi Y, You X, Zhen J, Zhang M, Qu B, Fu Z, Chen
M, Chien HTC, & Blot WJ (1992) Mortality and incidence of bladder
cancer in benzidine-exposed workers in China. Am J Ind Med, 21(4):
481-489.
Biberman R, Lusky A, Schlesinger T, Margaloit M, Neeman E, & Modan B
(1993) Increased risk for small cell lung cancer following residential
exposure to low dose radon: a pilot study. Arch Environ Health, 48(4):
209-212.
Bien TH & Burge R (1990) Smoking and drinking: A review of the
literature. Int J Addict, 25: 1429-1454.
Bjorseth A, Bjorseth O, & Feldstad PE (1978) Polycyclic aromatic
hydrocarbons in the work atmosphere; I. Determination in an aluminium
reduction plant. Scand J Work Environ Health, 4: 212-223.
Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS,
Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, & Fraumeni
JF Jr (1988) Smoking and drinking in relation to oral and pharyngeal
cancer. Cancer Res, 48: 3282-3287.
Boffetta P, Stellman SD, & Garfinkel L (1988) Diesel exhaust exposure
and mortality among males in the American Cancer Society prospective
study. Am J Ind Med, 14: 403-415.
Bohning DE, Atkins HL, & Cohn SN (1982) Long-term particle clearance
in man: normal and impaired. Ann Occup Hyg, 26: 259-271.
Bolinder G, Alfredsson L, Englund A, & de Faire U (1994) Smokeless
tobacco use and increased cardiovascular mortality among Swedish
construction workers. Am J Public Health, 84(3): 399-404.
Bornmyr S & Svensson H (1991) Thermography and laser-Doppler flowmetry
for monitoring changes in finger skin blood flow upon cigarette
smoking. Clin Physiol, 11(2): 135-141.
Bos RP, Kromhout H, Ikink H, de Haan W, Koppejan J, & Theuws JLG
(1989) Mutagens in urine of non-smoking and smoking workers in an
aircraft tyre retreading plant: Skin exposure as a causal factor.
Mutat Res, 223(1): 41-48.
Bouhuys A & Zuskin E (1976) Chronic respiratory disease in hemp
workers. Ann Intern Med, 84: 398-405.
Bovenzi M, Stanta G, Antiga G, Perruzzo P, & Cavallieri F (1993)
Occupational exposure and lung cancer risk in a coastal area of
northeastern Italy. Int Arch Occup Environ Health, 65(1): 35-41.
Boyko RW, Cartwright RA, & Glashan RW (1985) Bladder cancer in dye
manufacturing workers. J Occup Med, 27(11): 799-803.
Boyle P, Macfarlane GJ, Maisonneuve P, Zheng T, Scully C, & Tedesco B
(1990) Epidemiology of mouth cancer in 1989: a review. J R Soc Med,
83: 724-730.
Boysen M, Solberg LA, Torjussen W, Poppe S, & Hogetveit AC (1984)
Histological changes, rhinoscopical findings and nickel concentration
in plasma and urine in retired nickel workers. Acta Oto-Laryngol,
97(´): 105-115.
Bravo MP, Del Rey Calero J, & Conde M (1987) Risk factors of bladder
cancer in Spain. Neoplasma, 34(5): 633-637.
Bresnitz EA & Strom BL (1983) Epidemiology of sarcoidosis. Epidemiol
Rev, 5: 124-156.
Brinton LA, Blot WJ, Becker JA, Winn DM, Browder JP, & Farmer JC Jr
(1984) A case-control study of cancers of the nasal cavity and
paranasal sinuses. Am J Epidemiol, 119(6): 896-906.
Brown CC & Chu KC (1989) Additive and multiplicative models and
multistage carcinogenesis theory. Risk Anal, 9: 9-105.
Brownson RC, Novotny TE, & Perry MC (1993) Cigarette smoking and adult
leukemia: A meta analysis. Arch Intern Med, 153: 469-475.
Brunnemann KD & Hoffmann D (1974) The pH of tobacco smoke. Food Cosmet
Toxicol, 12: 115-124.
Brunnemann KD & Hoffmann D (1981) Assessment of the carcinogenic
N-nitrosodiethanolamine in tobacco products and tobacco smoke.
Carcinogenesis, 2: 1123-1127.
Brunnemann KD & Hoffmann D (1991) Chemical studies on tobacco smoke
XCC: Decreased concentrations of N-nitrosodiethanilamine and
N-nitrosomorpholine in commercial tobacco products. J Agric Food
Chem, 39: 207-208.
Brunnemann KD & Hoffmann D (1992) Chemical composition of smokeless
tobacco products. Bethesda, Maryland, National Cancer Institute, pp
96-108 (Smoking and Tobacco Control Monographs, No. 2).
Brunnemann KD, Mitacek EJ, Liu Y, Limsila T, & Suttajit M (1996)
Assessment of major carcinogenic tobacco-specific N-nitrosoamines in
Thai cigarettes. Cancer Detect Prev, 20(2): 114-121.
Burch JD, Howe GR, Miller AB, & Semenciw R (1981) Tobacco, alcohol,
asbestos, and nickel in the etiology of cancer of the larynx: A
case-control study. J Natl Cancer Inst, 67: 1219-1224.
Calverley AE, Rees D, Dowdeswell RJ, Linnett PJ, & Kielkowski D (1995)
Platinum salt sensitivity in refinery workers: incidence and effects
of smoking and exposure. Occup Environ Med, 52: 661-666.
Carrillo T, de Castro R, Cuevas M, Diaz F, & Cabrera P (1991) Effect
of cigarette smoking on the humoral immune response in pigeon
fanciers. Allergy, 46: 241-244.
Cartwright R (1982) Occupational bladder cancer and cigarette smoking
in West Yorkshire. Scand J Work Environ Health, 8(suppl 1): 79-82.
Castonguay A, Tjalve H, & Hecht SS (1983) Tissue distribution of the
tobacco-specific carcinogen
4-(methyl-nitrosamino)-1-(pyridyl)-1-butanone and its metabolites in
F344 rats. Cancer Res, 43: 631-635.
Castonguay A, Rivenson A, Trushin N, Reinhardt J, Spathopoulos S,
Weiss CJ, Reiss B, & Hecht SS (1984) Effects of chronic ethanol
consumption on the metabolism and carcinogenicity of
N-nitrosonornicotine in F344 rats. Cancer Res, 44: 2285-2290.
Cecchi F, Buiatti E, Kriebel D, Nastasi L, & Santucci M (1980)
Adenocarcinoma of the nose and paranasal sinuses in shoemakers and
woodworkers in the province of Florence, Italy (1963-77). Br J Ind
Med, 37: 222-225.
Chaffey CM & Bowie C (1994) Radon and health -- an update [Review]. J
Public Health Med, 16(4): 465-470.
Chakrabarti RN, Dutta K, Sikdar S, & Ghosh K (1991) Smokeless tobacco
and premalignant and malignant lesions of the oral cavity. Indian J
Med Sci, 45(10): 273-275.
Chameaud J, Perraud R, Chretien J, Masse R, & Lafuma J (1982) Combined
effects of inhalation of radon daughter products and tobacco smoke.
In: Sanders CL ed. Pulmonary toxicology of respirable particles.
Washington, DC, US Department of Energy, Technical Information Center,
pp 551-557 (CONF-791002).
Chan-Yeung M, Enarson D, & Grzybowski S (1985) Grain dust and
respiratory health. Can Med Assoc J, 133(10): 969-973.
Cheng WN & Kong J (1992) Retrospective mortality cohort study of
chrysotile asbestos products workers in Tianjin 1972-1987. Environ
Res, 59(1): 271-278.
Chiazze L Jr, Watkins DK, & Fryar C (1992) A case-control study of
malignant and non-malignant respiratory disease among employees of a
fibreglass manufacturing facility. Br J Ind Med, 49(5): 326-331.
Chiazze L Jr, Watkins DK, & Fryar C (1995) Adjustment for the
confounding effect of cigarette smoking in an historical cohort
mortality study of workers in a fiberglass manufacturing facility. J
Occup Environ Med, 37(6): 744-748.
Chopra SK, Taplin GV, Elam D, Carson SA, & Golde D (1979) Measurement
of tracheal mucociliary transport velocity in humans -- smokers versus
non-smokers. Am Rev Resp Dis, 119(4): 205 (Abstract).
Cho CH, Chen BW, Hui WM, & Lam SL (1990) The influence of acute or
chronic nicotine treatment on ethanol-induced gastric mucosal damage
in rats. Dig Dis Sci, 35: 106-112.
Chow JYC, Ma L, & Cho CH (1996) An experimental model for studying
passive cigarette smoking effects on gastric ulceration. Life Sci, 58:
2415-2422.
Christie D, Gordon I, & Robinson K (1986) Smoking in an industrial
population: An analysis by birth cohort. Med J Aust, 145: 11-14.
Christie D, Robinson K, Gordon I, Webley C, & Bisby J (1987) Current
mortality in the Australian petroleum industry: the healthy worker
effect and the influence of life-style factors. Med J Aust, 147:
222-225.
Cinkotai FF, Rigby A, Pickering CAC, Seaborn D, & Faragher E (1988a)
Recent trends in the prevalence of byssinotic symptoms in the
Lancashire textile industry. Br J Ind Med, 45: 782-789.
Cinkotai FF, Emo P, Gibbs ACC, & Jouany JM (1988b) Low prevalence of
byssinotic symptoms in 12 flax scutching mills in Normandy, France. Br
J Ind Med, 45: 325-328.
Clausen J, Netterstrom B, & Wolff C (1993) Lung function in insulation
workers. Br J Ind Med, 50(3): 252-256.
Cochrane AL & Moore F (1980) A 20-year follow-up of a population
sample (aged 25-34) including coal-miners and foundry workers in
Stavely, Derbyshire. Br J Ind Med, 37: 230-233.
Cockroft A, Seal RME, Wagner JC, Lyons JP, Ryder R, & Andersson N
(1982) Post-mortem study of emphysema in coalworkers and
non-coalworkers. Lancet, 2(8298): 600-603.
Coggins CRE (1998) A review of chronic inhalation studies with
mainstream cigarette smoke in rats and mice. Toxicol Pathol, 26(3):
307-314.
Cohen SM & Johansson SL (1992) Epidemiology and etiology of bladder
cancer. Urol Clin North Am, 19(3): 421-428.
Cohen D, Arai SF, & Brain JD (1979) Smoking impairs long-term
clearance from the lung. Science, 204: 514-517.
Cohen BS, Harley NH, & Tso TC (1985) Clearance of polonium-210
enriched cigarette smoke from rat trachea and lung. Toxicol Appl
Pharmacol, 79: 314-322.
Collins AC (1990) Interactions of ethanol and nicotine at the receptor
level. Recent Dev Alcohol, 8: 221-231.
Conolly CK, Chan NS, & Prescott RJ (1988) The relationship between age
and duration of asthma and the presence of persistent obstruction in
asthma. Postgrad Med J, 64(752): 422-425.
Cosio MG, Hale KA, & Niewoehner DE (1980) Morphologic and morphometric
effects of prolonged cigarette smoking on the small airways. Am Rev
Respir Dis, 122: 265-271.
Cotton DJ, Graham BL, Li KYR, Froh F, Barnett GD, & Dosman JA (1983)
Effects of grain dust exposure and smoking on respiratory symptoms and
lung function. J Occup Med, 25(2): 131-141.
Crebelli R, Paoletti A, Falcone E, Aquilana G, Fabri G, & Carere A
(1985) Mutagenicity studies in a tyre plant: in vitro activity of
workers' urinary concentrates and raw materials. Br J Ind Med, 42(7):
481-487.
Creek L, Capehart T, & Griese V (1994) US tobacco statistics,
1935-1992. US Dep Agric Stat Bull, 869: 14.
Cromwell BT (1955) Modern methods of plant analysis -- Volume IV: The
alkaloids. Berlin, Gottingen, Heidelberg, Springer-Verlag, pp 367-516.
Cross FT, Palmer RF, Filipy RE, Dagle GE, & Stuart BO (1982)
Carcinogenic effects of radon daughters, uranium ore dust and
cigarette smoke in beagle dogs. Health Phys, 42(1): 33-52.
Cullinan P, Lowson D, Nieuwenhuijsen MJ, Sandiford C, Tee RD, Venables
KM, McDonald JC, & Newman-Taylor AJ (1994a) Work related symptoms,
sensitisation, and estimated exposure in workers not previously
exposed to flour. Occup Environ Med, 51: 579-583.
Cullinan P, Lowson D, Nieuwenhuijsen MJ, Gordon S, Tee RD, Venables
KM, McDonald JC, & Newman-Taylor AJ (1994b) Work related symptoms,
sensitisation, and estimated exposure in workers not previously
exposed to laboratory rats. Occup Environ Med, 51: 589-592.
Cummings KM, Giovino G, & Mendicino AJ (1987) Cigarette advertizing
and black white differences in brand preference. Public Health Rep,
102(6): 698-701.
Damber L & Larsson LG (1985a) Professional driving, smoking and lung
cancer: a case referent study. Br J Ind Med, 42: 246-252.
Damber L & Larsson LG (1985b) Underground mining, smoking and lung
cancer: a case-control study in the iron ore municipalities in
Northern Sweden. J Natl Cancer Inst, 74(6): 1207-1213.
Darby S, Whitley E, Silcocks P, Thakrar B, Green M, Lomas P, Miles J,
Reeves G, Fearn T, & Doll R (1998) Risk of lung cancer associated with
residential radon exposure in south-west England: a case control
study. Br J Cancer, 78(3): 394-408.
D'Avanzo B, Negri E, La Vecchia C, Gramenzi A, Bianchi C, Franceschi
S, & Boyle P (1990) Cigarette smoking and bladder cancer. Eur J
Cancer, 26(6): 714-718.
Davis BR, Whitehead JK, Gill ME, Lee PN, Butterworth AD, & Roe FJC
(1975) Response of rat lung to inhaled tobacco smoke with or without
prior exposure to 3,4-benzpyrene (BP) given by intratracheal
instillation. Br J Cancer, 31: 469-484.
Davison AG, Fayers PM, Newman Taylor AJ, Venables KM, Darbyshire J,
Pickering CA, Chettle DR, Franklin D, Guthrie CJ, Scott MC, O'Malley
D, Holden H, Mason HJ, Wright AL, & Gompertz D (1988) Cadmium fume
inhalation and emphysema. Lancet, 8587: 663-667.
De Klerk NH, Musk AW, Armstrong BK, & Hobbs MS (1991) Smoking,
exposure to crocidolite, and the incidence of lung cancer and
asbestosis. Br J Ind Med, 48(6): 412-417.
Deng JF, Sinks T, Elliot L, Smith D, Singal M, & Fine L (1991)
Characterisation of respiratory health and exposures at a sintered
permanent magnet manufacturer. Br J Ind Med, 48: 609-615.
Devesa SS, Shaw GL, & Blot WJ (1991) Changing patterns of lung cancer
incidence by histological type. Can Epi Bio Prev, 1: 29-43.
De Wolff FA & Edelbroek PM (1994) Neurotoxicity of arsenic and its
compounds. In: de Wolff FA ed. Vinken and Bruyn's handbook of clinical
neurology. Part I. Intoxications of the nervous system. Amsterdam,
Elsevier, pp 283-291.
De Wolff FA (1995) Antimony and health. Br Med J, 310: 1216-1217.
De Zotti R, Larese F, Bovenzi M, Negro C, & Molinari S (1994) Allergic
airway disease in Italian bakers and pastry makers. Occup Environ Med,
51: 548-552.
DiPaolo JA & Sheehe PR (1962) The combined carcinogenic effect of
cigarette smoke condensate and urethan. Cancer Res, 22: 1058-1060.
Djordjevic MV, Sigountos CW, Hoffmann D, Brunnemann KD, Kagan MR, Bush
LP, Safaev RD, Belitsky GA, & Zaridze D (1991) Assessment of major
carcinogens and alkaloids in the tobacco and mainstream smoke of USSR
cigarettes. Int J Cancer, 47: 348-351.
Djordjevic MV, Fan J, Ferguson S, & Hoffmann D (1995) Self-regulation
of smoking intensity: Smoke yields of the low-nicotine, "low-tar"
cigarettes. Carcinogenesis, 16: 2015-2021.
Doll R & Peto R (1981) The causes of cancer: Quantitative estimates of
avoidable risk of cancer in the United States today. Natl Cancer Inst,
66: 1191-1308.
Domiano SF, Vena JE, & Swanson MK (1985) Gasoline exposure, smoking
and kidney cancer. J Occup Med, 27(6): 398-399.
Donham KJ, Zavala DC, & Merchant JA (1984) Respiratory symptoms and
lung function among workers in swine confinement buildings: a cross
sectional epidemiological study. Arch Environ Health, 39(2): 96-101.
Dontenwill W, Chavalier H-J, Harke H-P, Lafrenz U, Rechzeh G, &
Schneider B (1973) Investigations on the effects of chronic
cigarette-smoke inhalation in Syrian Golden Hamsters. J Natl Cancer
Inst, 51(6): 1781-1832.
Ecobichon DJ (1995) Toxic effects of pesticides. In: Klaassen CD ed.
Casarett and Doull's toxicology, 5th ed. New York, McGraw-Hill,
chapter 22, pp 643-689.
Edling C (1982) Lung cancer and smoking in a group of iron ore miners.
Am J Ind Med, 3(2): 191-199.
Edwards JW & Priestly BG (1993) Sister chromatid exchanges in
lymphocytes of petroleum retailers. Br J Ind Med, 50: 149-154.
Eisenbud M (1993) Lung cancer incidence among patients with beryllium
disease. J Natl Cancer Inst, 85(2): 1697-1698.
Ekenvall L & Lindblad LE (1989) Effect of tobacco use on vibration
white finger disease. J Occup Med, 31(1): 13-16.
Eliasson M, Lundblad D, & Hägg E (1991) Cardiovascular risk factors in
young snuff-users and cigarette smokers. J Intern Med, 230(1): 17-22.
Elliott R (1995) Smoking drinking and occupation: Occupational Health.
In: Drever F ed. Office of Population Censuses and Surveys, Health and
Safety Executive: The registrar general's decennial supplement for
England and Wales. London, Her Majesty's Stationery Office (HMSO), pp
195-201.
Elwood MJ (1981) Wood exposure and smoking: Association with cancer of
the nasal cavity and paranasal sinuses in British Columbia. Can Med
Assoc J, 124: 1573-1577.
Enterline PE, Sykora JL, Keleti G, & Lange JH (1985) Endotoxins,
cotton dust, and cancer. Lancet, 2(8461): 934-935.
Enterline PE, Marsh GM, Henderson V, & Callahan C (1987a) Mortality
update of a cohort of US man-made mineral fibre workers. Ann Occup
Hyg, 31: 625-656.
Enterline PE, Marsh GM, Esmen NA, Henderson VL, Callahan CM, & Paik M
(1987b) Some effects of cigarette smoking, arsenic, and SO2 on
mortality among US copper smelter workers. J Occup Med, 29(10):
831-838.
Falk K, Sorsa M, Vainio H, & Kilpikari I (1980) Mutagenicity in urine
of workers in rubber industry. Mutat Res, 79(1): 45-52.
Finch GL, Nikula KJ, Barr EB, Bechtold WE, Chen BT, Griffith WC, Hahn
FF, Hobbs CH, Hoover MD, Lundgren DL, & Mauderly JL (1995a) Effects of
combined exposure of F344 rats to radiation and chronically inhaled
cigarette smoke: Annual report of the Inhalation Toxicology Research
Institute. Washington, DC, Department of Energy (ITRI-146).
Finch GL, Nikula KJ, Chen BT, Barr EB, Chang I-Y, & Hobbs CH (1995b)
Effect of chronic cigarette smoke exposure on lung clearance of tracer
particles inhaled by rats. Fundam Appl Toxicol, 24: 76-85.
Finch GL, Nikula K, Belinsky S, Barr EB, Stoner G, & Lechner J (1996)
Failure of cigarette smoke to induce or promote lung cancer in the A/J
mouse. Cancer Lett, 99: 161-167.
Fletcher CM (1972) Coal workers pneumoconiosis [letter]. Br Med J, 3:
116.
Fletcher CM & Pride NB (1984) Definitions of emphysema, chronic
bronchitis, asthma, and airflow obstruction. Thorax, 39: 81-85.
Fox AJ, Lindars DC, & Owen R (1974) A survey of occupational cancer in
the rubber and cable making industries: Results of five-year analysis,
1967-71. Br J Ind Med, 31: 140-151.
Fraser-Moodie A (1976) Dupuytren's contracture and cigarette smoking.
Br J Plastic Surg, 29: 214-215.
Friedman GD, Siegeland AB, & Seltzer CC (1969) Cigarette smoking and
exposure to occupational hazards. Am J Epidemiol, 98: 175-183.
Friedman S, Fletcher CM, & Field GB (1972) Effects of smoking modified
cigarettes on respiratory symptoms and ventilatory capacity. J Natl
Cancer Inst, 48: 1805-1810.
Fukuda K, Shibata A, & Harada K (1987) Squamous cell cancer of the
maxillary sinus in Hokkaido, Japan: A case-control study. Br J Ind
Med, 44: 263-266.
Gammon MD, Schoenberg JB, Ahian H, Risch HA, Vaughan TL, Chow WH,
Rotterdam H, West AB, Dubrow R, & Stanford JL (1997) Tobacco, alcohol,
and socioeconomic status and adenocarcinoma of the esophagus and
gastric cardia. J Natl Cancer Inst, 89(17): 1277-1284.
Gardiner AJS, Forey BA, & Lee PN (1992) Avian exposure and
bronchogenic carcinoma. Br Med J, 305: 989-992.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1987) A case-control study of lung cancer
and diesel exhaust exposure in railroad workers. Am Rev Respir Dis,
135: 1242-1248.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1988) A retrospective cohort study of lung
cancer and diesel exhaust exposure in railroad workers. Am Rev Respir
Dis, 137: 820-825.
Geng Y, Savage SM, Johnson LJ, Seagrave J, & Sopori ML (1995) Effects
of nicotine on the immune system: I. Chronic exposure to nicotine
impairs antigen receptor-mediated signal transduction in lymphocytes.
Toxicol Appl Pharmacol, 135: 268-278.
Geng Y, Savage SM, Razanai-Boroujerdi S, & Sopori ML (1996) Effects of
nicotine on the immune system: II. Chronic nicotine treatment induces
T cell anergy. J Immunol, 156(7): 2384-2390.
Gerhardsson L & Nordberg GF (1993) Lung cancer in smelter workers
- interactions of metals as indicated by tissue levels. Scand J Work
Environ Health, 19(suppl 1): 90-94.
Gibbs GW & Horowitz I (1979) Lung cancer mortality in aluminium
reduction plant workers. J Occup Med, 21(5): 347-353.
Gibinski K (1977) Studies on cardiovascular effects of smoking in
Poland and other Eastern European countries. In: Proceedings of 3rd
World Conference on Smoking and Health, New York City, 2-5 June 1975
- Volume II: Health consequence, education, cessation and social
actions. Washington, DC, US Department of Health, Education and
Welfare, pp 179-184 (DHEW Publication No. (NIH) 77:1413).
Gonzalez CA, Lopez-Abente G, Errezola M, Castejon J, Estrada A,
Garcia-Mila M, Gili P, Huguet M, Serrat M, Soler F, & Rodriguez C
(1985) Occupation, tobacco use, coffee and bladder cancer in the
county of Mataro (Spain). Cancer, 55: 2031-2034.
Gonzalez CA, Lopez-Abente G, Errezola M, Escolar A, Riboli E,
Izarzugaza I, & Nebot M (1989) Occupation and bladder cancer in Spain:
a multi-centre case control study. Int J Epidemiol, 18(3): 569-577.
Greenberg M, Milne JF, & Watt A (1970) A survey of workers exposed to
dusts containing derivatives of Bacillus subtilis. Br Med J, ii:
629-633.
Greenland S (1993) Basic problems in interaction assessment. Environ
Health Perspect, 101(suppl 4): 59-65.
Grimmer G, Schneider D, Nanjack KW, Deltbarn G, & Jacob J (1995)
Intercept-reactant method for the determination of aromatic amines in
mainstream tobacco smoke. Beitr Tabilk-Forsch, 16: 141-156.
Grove WJ & Stansby GP (1992) Buerger's disease and cigarette smoking
in Bangladesh. Ann R Coll Surg Engl, 74: 115-118.
Guggenheimer J (1991) Implications of smokeless tobacco use in
athletes [Review]. Dent Clin North Am, 35(4): 797-808.
Gullvåg B, Mylius E, & Nilsen A (1985) Counting alveolar macrophages
from expectorate samples of exposed workers as a test for lung
irritation from occupational exposure. Bull Environ Contam Toxicol,
34: 702-708.
Hammond EC & Selikoff IJ (1973) Relation of cigarette smoking to risk
of death of asbestos- associated disease among insulation workers in
the United States. In: Bogovski P, Gilson JC, Timbrell V, & Wagner JC
Biological effects of asbestos. Lyon, International Agency for
Research on Cancer, pp 312-317 (IARC Scientific Publication No. 8).
Hammond EC, Selikoff IJ, & Seidman H (1979) Asbestos exposure,
cigarette smoking and death rates. Ann NY Acad Sci, 333: 473-489.
Hanke W, Sepulveda MJ, Watson A, & Jankovic J (1984) Respiratory
morbidity in wollastonite workers. Br J Ind Med, 41: 474-479.
Harf RA, Ethevenaux C, Gleize J, Perrin-Fayolle M, Guerin JG, &
Ollagnier C (1986) Reduced prevalence of smokers in sarcoidosis:
Results of a case-control study. Ann NY Acad Sci, 465: 625-631.
Harley NH, Cohen BS, & Tso TC (1980) Polonium-210: A questionable risk
factor in smoking-related carcinogenesis. In: Gori GB & Bock FG ed. A
safe cigarette? Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp 93-104 (Banbury Report No. 3).
Harris RJC & Negroni G (1967) Production of lung carcinomas in C57BL
mice exposed to a cigarette smoke and air mixture. Br Med J, 4:
637-641.
Harris RJC, Negroni G, Ludgate S, Pick CR, Chesterman FC, & Maidment
BJ (1974) The incidence of lung tumours in C57BL mice exposed to
cigarette smoke: air mixtures for prolonged periods. Int J Cancer, 14:
130-136.
Hassler E, Lind B, & Piscator M (1983) Cadmium in blood and urine
related to present and past exposure: A study of workers in an
alkaline battery factory. Br J Ind Med, 40: 420-425.
Haugen A, Becher G, Benestad C, Vahakangas K, Trivers GE, Newman MJ, &
Harris CC (1986) Determination of polycyclic aromatic hydrocarbons in
the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA,
and antibodies to the adducts in sera from coke oven workers exposed
to measured amounts of polycyclic aromatic hydrocarbons in the work
atmosphere. Cancer Res, 46(8): 4178-4183.
Hawker R & Holtby I (1988) Smoking and absence from work in a
population of student nurses. Public Health (Lond), 102: 161-167.
Hayes RB, Gerin M, Raatgever JW, & de Bruyn A (1986) Wood-related
occupations, wood dust exposure, and sinonasal cancer. Am J Epidemiol,
124(4): 569-577.
Health Canada (1994) Risk assessment for the combustion products of
methylcyclo-pentadienyl manganese tricarbonyl (MMT) in gasoline.
Ottawa, Ontario, Health Canada, Environmental Health Directorate, 113
pp.
Hecht SS & Hoffmann D (1988) Tobacco specific nitrosamines, an
important group of carcinogens in tobacco and tobacco smoke.
Carcinogenesis, 9(6): 875-884.
Hecht SS & Hoffmann D (1991) N-nitroso compounds and tobacco-induced
cancers in man. In: O'Neill IK, Chen J, & Bartsch H ed. Relevance to
human cancer of N-nitroso compounds, tobacco smoke and mycotoxins.
Lyon, International Agency for Research on Cancer, pp 54-61 (IARC
Scientific Publications No. 105).
Hecht SS, Adams JD, Numoto S, & Hoffmann D (1983) Induction of
respiratory tract tumors in Syrian golden hamsters by a single dose of
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 4:
1287-1290.
Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, & Hoffmann D
(1986) Induction of oral cavity tumors in F344 rats by
tobacco-specific N-nitrosamines and snuff. Cancer Res, 46:
4162-4166.
Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, & Hoffmann
D (1993) A tobacco-specific lung carcinogen in the urine of men
exposed to cigarette smoke. N Engl J Med, 329: 1543-1546.
Henry CJ & Kouri RE (1984) Chronic exposure of mice to cigarette smoke
-- Final Report to the Council for Tobacco Research -- USA, Inc.
(Contract CTR-0030). New York, Field, Rich and Associates, Inc.
Herity B, Moriarity M, Daly L, Dunn J, & Bourke GJ (1982) The role of
tobacco and alcohol in the aetiology of lung and larynx cancer. Br J
Cancer, 46: 961-964.
Hertz-Picciotto I, & Hu SW (1994) Contribution of cadmium in
cigarettes to lung cancer: an evaluation of risk assessment
methodologies. Arch Environ Health, 49(4): 297-302.
Hertz-Picciotto I, Smith AH, Holtzman D, Lipsett M, & Alexeef G (1992)
Synergism between occupational arsenic exposure and smoking in the
induction of lung cancer. Epidemiology, 3(1): 23-31.
Hessel PA, Sluis-Cremer GK, & Lee SL (1991) Distribution of silicotic
collagenization in relation to smoking habits. Am Rev Respir Dis,
144(2): 297-301.
Hicks RM (1982) Nitrosamines as possible etiological agents in
bilharzial bladder cancer. In: Magee PM ed. Nitrosamines and human
cancer. Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp 455-471 (Banbury Report No. 12).
Hicks RM, James C, & Webbe G (1980) Effect of Schistosoma
haematobium and N-butyl- N-(4-hydroxybutyl)nitrosamine on the
development of urothelial neoplasia in baboon. Br J Cancer, 42:
730-755.
Higginson J, Muir C, & Buffler PA (1984) The epidemiology of renal
carcinoma in humans with a note on the effect of exposure to gasoline.
In: Mehlman MA, Hemstreet GP III, Thorp JJ, & Weaver NK ed. Advances
in modern environmental toxicology -- Volume VII: Renal effects of
petroleum hydrocarbons. Princeton, New Jersey, Princeton Scientific
Publishers, pp 203-226.
Hilt B, Langard S, Lund-Larsen PG, & Lien JT (1986) Previous asbestos
exposure and smoking habits in the county of Telemark, Norway -- A
cross-sectional population study. Scand J Work Environ Health, 12:
561-566.
Hinds WC, First MW, Huber GI, & Shea JW (1983) A method for measuring
respiratory deposition of cigarette smoke during smoking. Am Ind Hyg
J, 44: 113-118.
Hirsch JM & Thilander H (1981) Snuff-induced lesions of the oral
mucosa: an experimental model in the rat. J Oral Pathol, 10: 342-353.
Hnizdo E (1990) Combined effect of silica dust and tobacco smoking on
mortality from chronic obstructive lung disease in gold miners. Br J
Ind Med, 47: 656-664.
Hnizdo E & Sluis-Cremer GK (1991) Silica exposure, silicosis, and lung
cancer: a mortality study of South African gold miners. Br J Ind Med,
48(1): 53-60.
Hnizdo E, Baskind E, & Sluis-Cremer GK (1990) Combined effects of
silica dust exposure and tobacco smoking on the prevalence of
respiratory impairments among gold miners. Scand J Work Environ
Health, 16: 411-422.
Hocking WG & Golde DW (1979) The pulmonary-alveolar macrophage. N Engl
J Med, 301: 580-587, 639-645.
Hoffmann D & Hoffmann I (1991) Recent developments in smoking related
cancer research. Smok Relat Dis, 5(2): 77-93.
Hoffmann D & Hoffmann I (1997) The changing cigarette: 1950-1995. J
Toxicol Environ Health, 50: 307-364.
Hoffmann D & Wynder EL (1976) Smoking and occupational cancer. Prev
Med, 5: 245-261.
Hoffmann D, Rathkamp G, & Wynder EL (1961) Comparison of the yields of
several selected compounds in the smoke of different tobacco products.
J Natl Cancer Inst, 31: 627-637.
Hoffmann D, Sanghvi LD, & Wynder EL (1974) Chemical studies on tobacco
smoke: XXVIII. Comparative chemical analysis of Indian bidi and
American cigarette smoke. Int J Cancer, 14: 49-53.
Hoffmann D, Rivenson A, Hecht SS, Hilfrich J, Kobayashi N, & Wynder EL
(1979) Model studies in tobacco carcinogenesis with the Syrian Golden
Hamster. Prog Exp Tumor Res, 24: 370-390.
Hoffmann D, Harley NH, Fisenne I, Adams JD, & Brunnemann KD (1986)
Carcinogenic agents in snuff. J Natl Cancer Inst, 76: 435-437.
Hoffmann D, Rivenson A, Chung FL, & Hecht SS (1991a) Nicotine-derived
nitrosamines and their relevance in tobacco carcinogenesis. Crit Rev
Toxicol, 21(4): 305-311.
Hoffmann D, Melikian AA, & Brunnemann KD (1991b) Studies in tobacco
carcinogenesis. Lyon, International Agency for Research on Cancer, pp
482-484 (IARC Scientific Publications No. 105).
Hoffmann D, Brunnemann KD, Prokopczyk B, & Djordjevic MV (1994)
Tobacco-specific N-nitrosamines and areca-derived N-nitrosamines:
chemistry, biochemistry, carcinogenicity, and relevance to humans. J
Toxicol Environ Health, 41: 1-52.
Holbrook JH (1977) Tobacco and health. CA-Cancer J Clin, 27: 344-353.
Holst PA, Kromhout D, & Brand R (1988) For debate: pet birds as an
independent risk factor for lung cancer. Br Med J, 297: 1319-1324.
Honeybourne D & Pickering CAC (1986) Physiological evidence that
emphysema is not a feature of byssinosis. Thorax, 41(1): 6-11.
Hsu TC, Furlong C, & Spitz MR (1991) Ethyl alcohol as a co-carcinogen
with special reference to the aerodigestive tract: a cytogenetic
study. Anticancer Res, 11: 1097-1102.
Hughes JM & Weill H (1991) Asbestos as a precursor of asbestos related
lung cancer: Results of a prospective mortality study. Br J Ind Med,
48: 229-233.
IARC (1984) Polynuclear aromatic compounds: Part 3. Industrial
exposures in aluminum production, coal gasification, coke production,
and iron and steel founding. Lyon, International Agency for Research
on Cancer, pp 37-190 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 34).
IARC (1985a) Tobacco habits other than smoking: Betel-quid and
areca-nut chewing and some related nitrosamines. Lyon, International
Agency for Research on Cancer, 291 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
37).
IARC (1985b) Polynuclear aromatic compounds: Part 4. Bitumens,
coal-tars and derived products, shale-oils and soots. Lyon,
International Agency for Research on Cancer, pp 83-241 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
35).
IARC (1986) Tobacco smoking. Lyon, International Agency for Research
on Cancer, 421 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 38).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, 440 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IARC (1988) Man-made mineral fibres and radon. Lyon, International
Agency for Research on Cancer, 300 pp (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Volume 43).
IARC (1989a) Antimony trioxide and antimony trisulfide. In: Some
organic solvents, resin monomers and related compounds, pigments and
occupational exposures in paint manufacture and painting. Lyon,
International Agency for Research on Cancer, pp 291-305 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
47).
IARC (1989b) Occupational exposures in petroleum refining; crude oil
and major petroleum fuels. Lyon, International Agency for Research on
Cancer, pp 109-110 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 45).
IARC (1989c) Diesel and gasoline engine exhaust and some nitroarenes.
Lyon, International Agency for Research on Cancer, pp 41-185 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
46)..
IARC (1990) Chromium, nickel and welding. Lyon, International Agency
for Research on Cancer, pp 257-445 (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Volume 49).
IARC (1991) Tenth International Meeting on N-Nitroso Compounds,
Mycotoxins and Tobacco Smoke: Relevance to Human Cancer. Lyon,
International Agency for Research on Cancer, 594 pp (IARC Scientific
Publications No. 105).
IARC (1993) Beryllium, cadmium, mercury, and exposures in the glass
manufacturing industry. Lyon, International Agency for Research on
Cancer, 444 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 58).
IARC (1994a) Ethylene oxide. In: Some industrial chemicals. Lyon,
International Agency for Research on Cancer, pp 73-159 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
60).
IARC (1994b) Infection with schistosomes ( Schistosoma haematobium,
Schistosoma mansoni and Schistosoma japonicul). In: Schistosomes,
liver flukes and Helicobacter pylori. Lyon, International Agency for
Research on Cancer, Volume 61).
IARC (1995) Wood dust and formaldehyde. Lyon, International Agency for
Research on Cancer, pp 191-194 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 62).
ICRP (International Commission on Radiological Protection) (1994)
Human respiratory tract model for radiological protection. Amsterdam,
Oxford, New York, Elsevier Science Publishers (ICRP Publication No.
66).
Idris AM, Nair J, Oshima H, Friesen M, Brouet I, Faustman EM, &
Bartsch H (1991) Unusual high levels of carcinogenic tobacco-specific
nitrosamines in Sudan snuff (toombak). Carcinogenesis, 12: 1115-1118.
Idris AM, Prokopczyk B, & Hoffmann D (1994) Toombak: a major risk
factor for cancer of the oral cavity in Sudan. Prev Med, 23: 832-839.
ILO (1991) Smoking contributes to noise-induced hearing loss: ILO
contribution to WHO -- World no tobacco day, 1992. Geneva,
International Labour Organisation.
Inskip PD & Boice JD (1994) Radiotherapy-induced lung cancer among
women who smoke. Cancer, 73(6): 1541-1543.
IPCS (1990) Environmental health criteria 106: Beryllium. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 40-53.
IPCS (1991a) Environmental health criteria 108: Nickel. Geneva, World
Health Organization, International Programme on Chemical Safety, pp
48-67.
IPCS (1991b) Environmental health criteria 125: Platinum. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 79-80.
IPCS (1992) Environmental health criteria 134: Cadmium. Geneva, World
Health Organization, International Programme on Chemical Safety, 280
pp.
IPCS (1995) Environmental health criteria 165: Inorganic lead. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 93-98.
IPCS (1996) Environmental health criteria 171: Diesel fuel and exhaust
emissions. Geneva, World Health Organization, International Programme
on Chemical Safety, 389 pp.
ISAAC (1998) International Study of Asthma and Allergies in Childhood
(ISAAC) Steering Committee: Worldwide variation in the prevalence of
symptoms of asthma, allergic rhino conjunctivitis, and atopic eczema.
Lancet, 351: 1225-1232.
Iscovich J, Castelletto R, Esteve J, Muñoz N, Colanzi R, Coronel A,
Deamezola I, Tassi V, & Arslan A (1987) Tobacco smoking, occupational
exposure and bladder cancer in Argentina. Int J Cancer, 40: 734-740.
Istvan J & Matarazzo JD (1984) Tobacco, alcohol and caffeine use: a
review of their interrelationships. Psychol Bull, 95: 301-326.
Iwata F, Zhang XY, & Leung FW (1995) Aggravation of gastric mucosal
lesions in rat stomach by tobacco cigarette smoke. Dig Dis Sci, 40:
1118-1124.
Izard C & Liberman C (1978) Acrolein. Mutat Res, 47(2): 115-138.
Jarup L & Pershagen G (1991) Arsenic exposure, smoking and lung cancer
in smelter workers -- A case-control study. Am J Epidemiol, 134(6):
545-551.
Järvholm B, Larsson S, Hagberg S, Olling S, Ryd W, & Torén K (1993)
Quantitative importance of asbestos as a cause of lung cancer in a
Swedish industrial city: a case-referent study. Eur Respir J, 6(9):
1271-1275.
Jayant K & Pakhale SS (1985) Toxic constituents in bidi smoke. In:
Sanghvi LD & Notami P ed. Tobacco and health: The Indian scene.
Bombay, Tata Memorial Center, pp 101-110.
Jindal RM & Patel SM (1992) Buerger's disease and cigarette smoking in
Bangladesh. Ann R Coll Surg Engl, 74(6): 436-437.
Johansson SL, Hirsch JM, Larsson PA, Saidi J, & Oesterdahl BG (1989)
Snuff-induced carcinogenesis: effect of snuff in rats initiated with
4-nitroquinoline- N-oxide. Cancer Res, 49: 3063-3069.
JOM (1984) Vibration syndrome in chipping and grinding workers. J
Occup Med, 26(10): 765-788.
Jones RD (1994) Survey of antimony workers: mortality 1961-92. Occup
Environ Med, 51(11): 772-776.
Jörgensen HS (1984) Lung cancer among underground workers in the iron
ore mine of Kiruna based on thirty years of observation. Ann Acad Med
Singap, 13(suppl 2): 371-377.
Jörgensen HS, Kolmodin-Hedman B, & Stjernberg N (1988) Follow-up study
of pulmonary function and respiratory tract symptoms in workers in a
Swedish iron ore mine. J Occup Med, 30(12): 953-957.
Jorquera R, Castonguay A, & Schüller HM (1992) Effects of pregnancy
and ethanol treatment on the metabolism of 4-(methyl
nitrosamino)-1-(pyridyl)-1-butanone by hamster liver and lung
microsomes. Drug Metab Dispos, 20: 510-517.
Jusko WJ (1978) Role of tobacco smoking in pharmacokinetics. J
Pharmacokinet Biopharm, 6: 7-39.
Jussawalla DJ & Deshpande VA (1971) Evaluation of cancer risk in
tobacco chewers and smokers: an epidemiological assessment. Cancer,
28(1): 244-252.
Kaldor JM & L'Abbe KA (1990) Interaction between human carcinogens.
In: Vainio H, Sorsa M, & McMichael AJ ed Complex mixtures and cancer
risk. Lyon, International Agency for Research on Cancer, pp 35-43
(IARC Scientific Publications No. 104).
Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, & Fraumeni
JF (1984) Urinary tract infection and risk of bladder cancer. Am J
Epidemiol, 119(4): 510-515.
Kawajiri K & Fujii-Kuriyama Y (1991) P450 and human cancer. Jpn J
Cancer Res, 82(12): 1325-1335.
Kawajiri K, Nakachi K, Imai K, Hyashi S, & Watanabe J (1990)
Individual differences in lung cancer susceptibility in relation to
polymorphysms of P-450IA1 gene and cigarette dose. Princess Takamatsu
Int Symp Ser, 21: 55-61.
Kazantzis G & Armstrong BG (1984) Renal function in relation to low
levels of cadmium exposure in a group of smelter workers. Environ
Health Perspect, 54: 193-199.
Kazantzis G, Blanks RG, & Sullivan KR (1992) Is cadmium a human
carcinogen? In: Nordberg GF, Herber REM, & Alessio L ed. Cadmium in
the human environment: Toxicity and carcinogenicity. Lyon,
International Agency for Research on Cancer, pp 435-445.
Keller AZ & Terris M (1965) The association of alcohol and tobacco
with cancer of the mouth and pharynx. Am J Publ Health, 55: 1578-1585.
Keen P, de Moor NG, Shapiro MP, & Cohen L (1955) The etiology of
respiratory tract cancer in the South African Bantu. Br J Cancer, 9:
528-538.
Kibelstis JA, Morgan EJ, Reger R, Lapp NL, Seaton A, & Morgan WK
(1973) Prevalence of bronchitis and airway obstruction in American
bituminous coal miners. Am Rev Respir Dis, 108(4): 886-893.
Kilburn KH (1989) Byssinosis, emphysema, and mortality of American
textile workers: [Editorial]. Arch Environ Health, 38(5): 277.
Kilpikari MD (1982) Mortality among male rubber workers in Finland.
Arch Environ Health, 37(5): 295-299.
Kimura N (1977) Epidemiological studies in Japan on smoking and
cardiovascular disease. In: Proceedings of 3rd World Conference on
Smoking and Health, New York City, 2-5 June 1975. Volume II: Health
consequence, education, cessation and social actions. Washington, DC,
US Department of Health, Education and Welfare, pp 185-198 (DHEW
Publication (NIH) 77:1413).
King M, Wight A, DeSanctis GT, el-Azab J, Phillips DM, Angus GE, &
Cosio MG (1989) Mucus hypersecretion and viscoelasticity changes in
cigarette smoking dogs. Exp Lung Res, 15(3): 375-389.
Kleinfeld M, Messite J, & Langer AM (1973) A study of workers exposed
to asbestiform minerals in commercial talc manufacture. Environ Res,
6: 132-143.
Kobayashi W, Hoffmann D, & Wynder EL (1974) A study of tobacco
carcinogenesis: XII. Epithelial changes induced in the upper
respiratory tracts of Syrian golden hamsters by cigarette smoke. J
Natl Cancer Inst, 534: 1085-1089.
Kobusch AB, Simard A, Feldstein M, Vauclair R, Gibbs GW, Bergeron F,
Morissette N, & Davis R (1984) Pulmonary cytology in chrysotile
asbestos workers. J Chron Dis, 37(8): 599-607.
Kodell RL & Pounds JG (1991) Assessing the toxicity of chemical
mixtures. In: Krewski D & Franklin CA ed. Statistics in toxicology.
New York, Gordan & Breach, pp 557-589.
Kodell RL, Krewski D, & Zielinski J (1991) Additive and multiplicative
relative risk in the two-stage clonal expansion model of
carcinogenesis. Risk Anal, 11: 483-490.
Kohlmeier L, Arminger G, Bartolomeycik S, Bellach B, Rehm J, & Thamm M
(1992) Pet birds as an independent risk factor for lung cancer:
case-control study. Br Med J, 305: 986-989.
Koppang N, Rivenson A, Reith A, Dahle HK, Evenson O, & Hoffman D
(1992) A study of tobacco carcinogenesis: XLVIII. Carcinogenicity of
N'-nitrosonornicotine in mink ( Mustela vison). Carcinogenesis,
13(11): 1957-1960.
Koppang N, Rivenson A, Dahle HK, & Hoffman D (1997) A study of tobacco
carcinogenesis: LIII. Carcinogenicity of N'-nitrosonornicotine (NNN)
and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in mink
( Mustela vison). Cancer Lett, 111: 167-171.
Kotin P (1994) The epidemiological evidence on the carcinogenicity of
beryllium, by MacMahon: [Editorial]. J Occup Med, 36(1): 25-26.
Kreiss K, Wassermann S, Mroz MM, & Newman LS (1993) Beryllium disease
screening in the ceramics industry. J Occup Med, 35: 16-25.
Kreiss K, Mroz MM, Newman LS, Martyny J, & Zhen B (1996) Machining
risk of beryllium disease and sensitization with median exposures
below 2 µg/m3. Am J Ind Med, 30: 16-25.
Kremer AM, Pal TM, Boleij JSM, Schouten JP, & Rijcken B (1994) Airway
hyperresponsiveness, prevalence of chronic respiratory symptoms, and
lung function in workers exposed to irritants. Occup Environ Med, 51:
3-13.
Krishna K (1978) Tobacco chewing in pregnancy. Br J Obstet Gynaecol,
85: 726-728.
Kristensen TS (1989) Cardiovascular disease and the work environment:
A critical review of the epidemiological literature on chemical
factors. Scand J Work Environ Health, 15: 245-264.
Krivan V, Schneider G, Baumann H, & Reus (1994) Multi-element
characterization of tobacco smoke condensate. Fresenius J Anal Chem,
348: 218-225.
Kuntz WD & McCord CP (1974) Polymer-fume fever. J Occup Med, 16(7):
480-482.
Kusiak RA, Springer J, Ritchie AC, & Muller J (1991) Carcinoma of the
lung in Ontario gold miners: possible aetiological factors. Br J Ind
Med, 48: 808-817.
Kusiak RA, Ritchie AC, Muller J, & Springer J (1993) Mortality from
lung cancer in Ontario uranium miners. Br J Ind Med, 50(10): 920-928.
Lai YL & Diamond L (1992) Cigarette smoke exposure does not prevent
cadmium-induced alterations in rat lungs. J Toxicol Environ Health,
35(1): 63-76.
LGC (Laboratory of the Government Chemist) (1982) Report of the
Government chemist, 1981. London, Her Majesty's Stationery Office
(HMSO), p 109.
Lakier JB (1992) Smoking and cardiovascular disease. Am J Med,
93(suppl 1A): 8-12.
Lamm SH, Parkinson M, Anderson M, & Taylor W (1992) Determinants of
lung cancer risk among cadmium-exposed workers. Ann Epidemiol, 2(3):
195-211.
Lancet (1985) Smoking, occupation, and allergic lung disease. Lancet,
1(8435): 965.
Landrigan PJ & Straub WE (1985) Occupational lead exposure aboard a
tall ship. Am J Ind Med, 8(3): 233-239.
Langård S & Norseth T (1975) A cohort study of bronchial carcinomas in
workers producing chlorinated pigments. Br J. Ind Med, 32: 62-65.
Langård S & Vigander T (1983) Occurrence of lung cancer in workers
producing chromium pigments. Br J Ind Med, 40(1): 71-74.
Larsson PA, Johansson SL, Vahlne A, & Hirsch JM (1989) Snuff
carcinogenesis: effects of long-term snuff administration after
initiated with 4-nitroquinoline- N-oxide and Herpes simplex virus
type 1. J Oral Pathol Med, 18: 187-192.
La Vecchia C, Negri E, D'Avanzo B, Savoldelli R, & Franceschi S (1991)
Genital and urinary tract diseases and bladder cancer. Cancer Res,
51(2): 629-631.
La Voie EJ, Adams JD, Reinhardt J, Rivenson A, & Hoffmann D (1986)
Toxicity studies on clove cigarette smoke and constituents of clove:
Determination of the LD50 of eugenol by intratracheal installation in
rats and hamsters. Arch Toxicol, 59: 78-81.
Leanderson P & Tagesson C (1989) Cigarette smoke potentiates the
DNA-damaging effect of manmade mineral fibers. Am J Ind Med, 16:
697-706.
Lednar WM, Tyroler HA, McMichael AJ, & Shy CM (1977) The occupational
determinants of chronic disabling pulmonary disease in rubber workers.
J Occup Med, 19: 263-268.
Leduc D, de Francquen P, Jacobovitz D, Vanderweyer R, Lauwerys R, &
De Vuyst P (1993) Association of cadmium exposure with rapidly
progressive emphysema in a smoker. Thorax, 48: 570-571.
Lehtovirta P & Forss M (1978) The acute effect of smoking on
intervillous blood flow of the placenta. Br J Obstet Gynaecol, 85:
729-731.
Leigh J, Outhred KG, McKenzie HI, Glick M, & Wiles AN (1983)
Quantified pathology of emphysema, pneumoconiosis, and bronchitis in
coal workers. Br J Ind Med, 40: 258-263.
Letourneau EG, Krewski D, Choi NW, Goddard MJ, McGregor MJ, Zielinsky
JM, & Du J (1994) Case-control study of residential radon and lung
cancer in Winnipeg, Manitoba, Canada. Am J Epidemiol, 140(4): 310-322.
Liddell FD, Thomas DC, Gibbs GW, & McDonald JC (1984) Fibre exposure
and mortality from pneumoconiosis, respiratory, and abdominal
malignancies in chrysotile production in Quebec, 1926-1975. Ann Acad
Med Singap, 13(suppl): 340-344.
Lidegaard O (1993) Oral contraceptive use and risk of a cerebral
thromboembolic attack: Results of a case-control study. Br Med J, 306:
956-963.
Lilis R, Valciukas JA, Weber JP, Malkin J, & Selikoff IJ (1984a)
Epidemiological study of renal function in copper smelter workers.
Environ Health Perspect, 54: 181-192.
Lilis R, Valciukas JA, Weber JP, Fischbein A, Nicholson WJ, Campbell
C, Malkin J, & Selikoff IJ (1984b) Distribution of blood lead, blood
cadmium, urinary cadmium, and urinary arsenic levels in employees of a
copper smelter. Environ Res, 33: 76-95.
Lindberg E & Hedenstierna G (1983) Chrome plating: symptoms, findings
in the upper airways and effects on lung function. Arch Environ
Health, 38(6): 364-374.
Ljungman AG, Lindahl M, & Tagesson C (1994) Asbestos fibres and man
made mineral fibres: induction and release of tumour necrosis factor
from rat alveolar macrophages. Occup Environ Med, 51: 777-783.
Lourenco RV, Klimek MF, & Borowski CJ (1971) Deposition and clearance
of 2 micron particles in the tracheobronchial tree of normal subjects
-- smokers and nonsmokers. J Clin Invest, 50: 65-83.
Lubin JH & Boice JD (1997) Lung cancer risk from residential radon:
Meta-analysis of eight epidemiologic studies. J Natl Cancer Inst,
89(1): 49-57.
Lubin JH, Boice JD, Edling C, Hornung RW, Howe GR, Kunz E, Kusiak RA,
Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, Yao SX, &
Pierce DA (1995) Lung cancer in radon-exposed miners and estimation of
risk from indoor exposure. J Natl Cancer Inst, 87(11): 817-827.
Lubin JH, Tomasek L, Edling C, Hornung RW, Howe G, Kunz E, Kusiak RA,
Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, & Yao SX
(1997) Estimating lung cancer mortality from residential radon using
data for low exposures of miners. Radiat Res, 147(2): 126-134.
McCoy GD, Hecht SS, Katayama S, & Wynder EL (1981) Differential effect
of chronic ethanol consumption on the carcinogenicity of
N-nitrosopyrolidine and N-nitrosonornicotine in male Syrian golden
hamsters. Cancer Res, 41: 2849-2854.
McDuffie HH, Klaassen DJ, & Dosman JA (1990) Is pesticide use related
to the risk of primary lung cancer in Saskatchewan? J Occup Med,
32(10): 996-1002.
Mackenney RP (1983) A population study of Dupuytren's contracture.
Hand, 15(2): 155-161.
McLaughlin RF & Tueller EF (1971) Anatomic and histologic changes of
early emphysema. Chest, 59: 592-599.
McLintock JC (1971) The selection of juvenile entrants to mining. Br J
Ind Med, 28: 45-51.
MacMahon B (1994) The epidemiological evidence on the carcinogenicity
of beryllium in humans. J Occup Med, 36(1): 15-24.
Magnus K, Andersen A, & Hegetveit AC (1982) Cancer of respiratory
organs among workers at a nickel refinery in Norway. Int J Cancer, 30:
681-685.
Maheswaren R, Gill JS, & Beevers DG (1993) Blood pressure and
industrial lead exposure. Am J Epidemiol, 137(6): 645-653.
Makhyoun NA (1974) Smoking and bladder cancer in Egypt. Br J Cancer,
30: 577-581.
Mann JI, Vessey MP, Thorogood M, & Doll R (1975) Myocardial infarction
in young women with special reference to oral contraceptive practice.
Br Med J, 2: 241-245.
Mann JI, Doll R, Thorogood M, Vessey MP, & Waters WE (1976) Risk
factors for myocardial infarction in young women. Br J Prev Soc Med,
30: 94-100.
Marine WM, Gurr D, & Jacobsen M (1988) Clinically important
respiratory effects of dust exposure and smoking in British coal
miners. Am Rev Respir Dis, 137: 106-112.
Martell EA (1974) Radioactivity of tobacco trichomes and insoluble
smoke particles. Nature (Lond), 249: 215-217.
Martischnig KM, Newell DJ, Barnsley WC, Cowan WK, Feinmann EL, &
Oliver E (1977) Unsuspected exposure to asbestos and bronchogenic
carcinoma. Br Med J, 1(6063): 746-749.
Mayberry RM (1985) Cigarette smoking, herpes simplex virus type 2
infection, and cervical abnormalities. Am J Pub1 Health, 75(6):
676-678.
Mehta FS, Pindborg JJ, Hamner JE, Gupta PC, Daftary DK, Sahiar BE,
Shroff BC, Sanghvi LD, Bhonsle RB, Choksi SK, Dandekar VV, Mehta YN,
Pitkar VK, Sinor PN, Shah NC, Turner PS, & Upadhyay SA (1971) Report
on investigations of oral cancer and precancerous lesions in Indian
rural populations, 1966-1969. Copenhagen, Munksgaard, pp 48-120.
Merchant JA, Kilburn KH, O'Fallon WM, Hamilton JD, & Lumsden JC (1972)
Byssinosis and chronic bronchitis among cotton textile workers. Ann
Intern Med, 76: 423-433.
Merchant JA, Lumsden JC, Kilburn KH, O'Fallon WM, Ujida JR, & Germino
VH (1973) An industrial study of the biological effects of cotton dust
and cigarette smoke exposure. J Occup Med, 15(3): 212-221.
Meurman LO, Kiviluoto R, & Hakama M (1979) Combined effects of
asbestos exposure and tobacco smoking on Finnish anthophyllite miners
and millers. Ann NY Acad Sci, 330: 491-495.
Michaylov MA, Pressyanov DS, & Kalinov KB (1995) Bronchial dysplasia
induced by radiation in miners exposed to 222Rn progeny. Occup Environ
Med, 52: 82-85.
Mikkelsen OA (1978) Dupuytren's disease-the influence of occupation
and previous hand injuries. Hand, 10(1): 1-8.
Miller LG (1990) Cigarettes and drug therapy: Pharmacokinetic and
pharmacodynamic considerations. Clin Pharmacol, 9: 125-135.
Miller A (1993) Pulmonary function in asbestosis and asbestos related
pleural disease. Environ Res, 61(1): 1-18.
Miller BG & Jacobsen M (1985) Dust exposure, pneumoconiosis, and
mortality of coal miners. Br J Ind Med, 42(11): 723-733.
Miller FJ & Kimbell JS (1995) Regional dosimetry of inhaled reactive
gases. In: McClellan RO & Henderson RF ed. Concepts in inhalation
toxicology, 2nd ed. Washington, DC, Taylor & Francis, chapter 10, pp
257-288.
Misiewicz A, Radwan K, Karmolinski M, & Dziewit T (1992) [Degree of
ventilation disturbances in persons with occupational exposure to
manganese.] Wiad Lek, 45(23-24): 890-893 (in Polish).
Misiewicz A, Radwan K, Karmolinski M, Dziewit T, & Matysek A (1994)
[Chronic bronchitis in workers producing iron-manganese alloys.] Wiad
Lek, 47(7-8): 257-261 (in Polish).
Mitacek EJ, Brunnemann KD, & Polednak XP (1990) "Tar", nicotine and
carbon monoxide content of Thai cigarettes and implication for cancer
prevention in Thailand. Cancer Detect Prev, 14: 515-520.
Mitacek EJ, Brunnemann KD, Polednak AP, Hoffmann D, & Suttajet M
(1991) Composition of popular tobacco products in Thailand and its
relevance to disease prevention. Prev Med, 20: 764-773.
Mitacek EJ, Brunnemann KD, Hoffmann D, Limsila T, Suttajet M, Martin
N, & Kaplan LS (1999) Volatile N-nitrosamines and tobacco specific
N-nitrosamines in the smoke of Thai cigarettes: a suspected risk
factor liver cancer in Thailand. Carcinogenesis, 20(1): 133-137.
Mitchell CA & Gandevia B (1971) Respiratory symptoms and skin
reactivity in workers exposed to proteolytic enzymes in the detergent
industry. Am Rev Respir Dis, 104: 1-12.
Modigh C, Axelsson G, Alavanja M, Andersson L, & Rylander P (1996) Pet
birds and risk of lung cancer in Sweden: a case-control study. Br Med
J, 313: 1236-1238.
Molyneux MKB & Tombleson JBL (1970) An epidemiological study of
respiratory symptoms in Lancashire mills, 1963-66. Br J Ind Med,
27(3): 225-234.
Monson RR & Fine LJ (1978) Cancer mortality and morbidity among rubber
workers. J Natl Cancer Inst, 61(4): 1047-1053.
Monson RR & Nakano KK (1976a) Mortality among rubber workers: 1. White
male union employees in Akron, Ohio. Am J Epidemiol, 103(3): 284-296.
Monson RR & Nakano KK (1976b) Mortality among rubber workers: 2. Other
employees. Am J Epidemiol, 103(3): 297-303.
Moolgavkar SH, Dewanji A, & Luebeck G (1989) Cigarette smoking and
lung cancer: reanalysis of the British doctors' data. J Natl Cancer
Inst, 81: 415-420.
Moolgavkar SH, Luebeck EG, Krewski D, & Zielinski JM (1993) Radon,
cigarette smoke, and lung cancer: A re-analysis of the Colorado
Plateau uranium miners' data. Epidemiology, 4: 204-217.
Morabia A, Stellman S, Lumey LH & Wynder EL (1998) Parakeets,
canaries, finches, parrots, and lung cancer: no association. Br J
Cancer, 77: 501-504.
Morgan DC, Smyth JT, Lister RW, Pethybridge RJ, Gilson JC, Callaghan
P, & Thomas GO (1975) Chest symptoms in farming communities with
special reference to farmer's lung. Br J Ind Med, 32: 228-234.
Morgan WKC (1982) Occupational bronchitis. Eur J Respir Dis, 63(suppl
23): 117-127.
Morgan KT (1998) Commentary on Coggins CRE (1998): a review of chronic
inhalation studies with mainstream cigarette smoke in rats and mice.
Toxicol Pathol, 26(3): 315.
Mori K (1964) Acceleration of experimental lung cancers in rats by
inhalation of cigarette smoke. Gann, 55: 175-181.
Morimoto Y, Kido M, Tanaka I, Fujino A, Higashi T, & Yokosaki Y (1993)
Synergistic effects of mineral fibres and cigarette smoke on the
production of tumour necrosis factor by alveolar macrophages of rats.
Br J Ind Med, 50: 955-960.
Mougne C, MacLennan R, & Atsana S (1982) Smoking, chewing and drinking
in Ban Pong, Northern Thailand. Soc Sci Med, 16(1): 99-106.
MRC (Medical Research Council) (1965) Special Committee on the
Aetiology of Chronic Bronchitis: Definition and classification of
chronic bronchitis for clinical and epidemiological purposes. Lancet,
1: 775-779.
Muir CS & McKinney PA (1992) Cancer of the oesophagus: A global
overview. Eur J Cancer Prev, 1: 259-264.
Murai Y, Kitagawa M, Matsui K, Koizumi F, & Miwa A (1994) Asbestos
fibre analysis in seven asbestosis cases. Arch Environ Health, 49(1):
67-72.
Murphy SE & Heiblum R (1990) Effect of nicotine and tobacco-specific
nitrosamines on the metabolism of N'-nitrosonornicotine and
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 11:
1663-1666.
Muscat JE & Wynder EL (1991) Cigarette smoking, asbestos exposure, and
malignant mesothelioma. Cancer Res, 51(9): 2263-2267.
Nair J, Pakhale SS, & Bhide SV (1989) Carcinogenic tobacco-specific
nitrosamines in Indian tobacco products. Food Chem Toxicol, 27(11):
751-753.
Najem GR, Louria DB, Seebode JJ, Thind IS, Prusakowski JM, Ambrose RB,
& Fernicola AR (1982) Life time occupation, smoking, caffeine,
saccharin, hair dyes and bladder carcinogenesis. Int J Epidemiol, 11:
212-217.
Nakachi K, Imai K, Hayashi S, Watanabe J, & Kawajiri K (1991) Genetic
susceptibility to squamous cell carcinoma of the lung in relation to
cigarette smoking dose. Cancer Res, 51(19): 5177-5180.
Nanda PK & Sharma MM (1988) Immediate effect of tobacco chewing in the
form of "paan" on certain cardio-respiratory parameters. Indian J
Physiol Pharmacol, 32(2): 105-113.
Nandakumar A, Thimmasetty KT, Sreeramereddy NM, Venugopal TC, Rajanna
N, Vinutha AT, Srinivas N, & Bhargava MK (1990) A population-based
case-control investigation on cancer of the oral cavity in Bangalore,
India. Br J Cancer, 62: 847-851.
Naus A, Engler V, Hetychova M, & Vavrechova O (1966) Work injuries and
smoking. Ind Med Surg, 35: 880-881.
Negri E, LaVecchia C, Franceschi S, Decarli A, & Bruzzi P (1992)
Attributable risks for oesophageal cancer in Northern Italy. Eur J
Cancer, 28A: 1167-1171.
Nery LE, Sandoval PR, Jardim JR, Bagatin E, & Alonso G (1988) The
effects of smoking and silica exposure on pulmonary epithelial
permeability: a radioaerosol study with 99mTc-DTPA. Brazilian J Med
Biol Res, 21(2): 223-232.
Nery LE, Florencio RT, Sandoval PR, Rodrigues RT, Alonso G, & Mason GR
(1993) Additive effects of exposure to silica dust and smoking on
pulmonary epithelial permeability: a radioaerosol study with
technetium-99m labelled DTPA. Thorax, 48(3): 264-268.
Neugut AI, Robinson E, Lee WC, Murray T, Karwoski K, & Kutcher G
(1993) Lung cancer after radiation therapy for breast cancer. Cancer,
71: 3054-3057.
Neugut AI, Murray T, Santos J, Amols H, Hayes MK, Flannery JT, &
Robinson E (1994) Increased risk of lung cancer following breast
cancer radiation therapy in cigarette smokers. Cancer, 73: 1615-1620.
Neurath G & Ehmke H (1964) [Studies on the nitrate content of
tobacco.] Beitr Tabakforsch, 2: 333-344 (in German).
Nevius E, Bennett R, & Sobel S (1982) Critique of FM Sturtevant paper
"Smoking, oral contraceptives, and thromboembolic disease". Int J
Fertil, 27: 16-20.
Newhouse ML & Berry G (1976) Predictions of mortality from mesothelial
tumours in asbestos factory workers. Br J Ind Med, 33: 147-151.
Newhouse ML & Berry G (1979) Patterns of mortality in asbestos factory
workers in London. Ann NY Acad Sci, 330: 53-60.
Ng TP, Chan SL, & Lam KP (1987) Radiological progression and lung
function in silicosis: A ten year follow up study. Br Med J,
295(6591): 164-167.
Ng TP, Chan SL, & Lee J (1990) Mortality of a cohort of men in a
silicosis register: Further evidence of an association with lung
cancer. Am J Ind Med, 17(2): 163-171.
Ng TP, Chan SL, & Lee J (1992) Predictors of mortality in silicosis.
Respir Med, 86(2): 115-119.
Niewoehner DE (1991) What lies ahead? Future research and treatment
for chronic obstructive pulmonary disease. Am J Med, 91(4A): 41S-46S.
Niewoehner DE, Kleinerman J, & Rice DB (1974) Pathologic changes in
the peripheral airways of young cigarette smokers. N Engl J Med, 291:
755-758.
NIH (National Institutes of Health) (1996) The FTC cigarette test
method for determining tar, nicotine, and carbon monoxide yields of US
cigarette smoking. Washington, DC, US Department of Health and Human
Services, 275 pp (Smoking and Tobacco Control Monograph 7).
NIH (National Institutes of Health) (1998) Cigars: Health effects and
trends. Washington, DC, US Department of Health and Human Services,
232 pp (Smoking and Tobacco Control Monograph 9).
Nilsson K, Hendriksson R, Cai YQ, Hellstrom S, Hornqvist-Bylunds S, &
Bjermer L (1992) Effects of tobacco smoke on radiation-induced
pneumonitis in rats. Int J Radiat Biol, 62: 719-727.
NIOSH (1995) Criteria for a recommended standard: Occupational
exposure to respirable coal mine dust. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, 336 pp.
Nishikawa M, Ikeda H, Nishiyama H, Yamakawa H, Suzuki S, & Okubo T
(1992) Combined effects of ozone and cigarette smoke on airway
responsiveness and vascular permeability in guinea pigs. Lung, 170:
311-322.
Nishiyama H, Yano H, Nishiwaki Y, Kitaya T, Matsuyama T, Kodama T,
Suemasu K, Tamai S, & Takemoto K (1985) Lung cancer in chromate
workers: Analysis of 11 cases. Jpn J Clin Oncol, 15(3): 489-497.
Norman V, Ghrig AM, Lassa TM, & Moss BL (1983) The effect of
nitrogenous blend compounds on NO/NO2 and HCl levels in mainstream
and sidestream smoke. Bertr Tabakforsch, 12: 55-62.
NRC (National Research Council) (1988) Committee on the Biological
Effects of Ionizing Radiations: BEIR-IV: Health risks of radon and
other internally deposited alpha-emitters. Washington, DC, National
Academy Press, 602 pp.
O'Dell JR, Linder J, Markin RS, & Moore GF (1987) Thromboangiitis
obliterans (Buerger's disease) and smokeless tobacco. Arthritis-Rheum,
30(9): 1054-1056.
Olin JW (1994) Thromboangiitis obliterans. Curr Opin Rheumatol, 6(1):
44-49.
Oxman AD, Muir DC, Shannon HS, Stock SR, Hnizdo E, & Lange HJ (1993)
Occupational dust exposure and chronic obstructive pulmonary disease:
A systematic overview of the evidence. Am Rev Respir Dis, 148(1):
38-48.
Park NH, Dokko H, Li SL, & Cherrick HM (1991a) Synergism of herpes
symplex virus and tobacco-specific N'-nitrosamines in cell
transformation. J Oral Maxillofac Surg, 49(3): 276-281.
Park NH, Sapp JP, & Herbosa EG (1991b) Oral cancer induced in hamsters
with Herpes simplex virus infection and simulated snuff dipping. Oral
Surg Oral Med Oral Pathol, 62: 164-168.
Parka KR (1983) Smoking as a moderator of the relationship between
affective state and absence from work. J Appl Psychol, 68: 698-708.
Patrianakos C & Hoffmann D (1979) Chemical studies on tobacco smoke:
LXIV. On the analysis of aromatic amines. Anal Toxicol, 3: 150-154.
Pedersen E, Hogetveit AC, & Andersen A (1973) Cancer of respiratory
organs among workers at a nickel refinery in Norway. Int J Cancer, 12:
32-41.
Penner M & Penner S (1990) Excess insured health care costs from
tobacco-using employees in a large group plan. J Occup Med, 32:
521-523.
Pepys J, Pickering CAC, & Hughes EG (1972) Asthma due to inhaled
chemical agents -- complex salts of platinum. Clin Allergy, 2:
391-396.
Pershagen G (1985) Lung cancer mortality among men living near an
arsenic-emitting smelter. Am J Epidemiol, 122: 684-694.
Pershagen G, Wall S, Taube A, & Linnman L (1981) On the interaction
between occupational arsenic exposure and smoking and its relationship
to lung cancer. Scand J Work Environ Health, 7: 302-309.
Pershagen G, Bergman F, Klominek J, Damber L, & Wall S (1987)
Histological types of lung cancer among smelter workers exposed to
arsenic. Br J Ind Med, 44: 454-458.
Pershagen G, Akerblom G, Axelson O, Clavensjo B, Damber L, Desai G,
Enflo A, Legarde F, Mellander H, Svartengren M, & Swedjemark GU (1994)
Residential radon exposure and lung cancer in Sweden. N Engl J Med,
330(3): 159-164.
Petersen R, Andersen M, Mikkelsen S, & Nielsen SL (1995) Prognosis of
vibration induced white finger: a follow-up study. Occup Environ Med,
52(2): 110-115.
Peterson KL, Heninger RW, & Seegmiller RE (1981) Fetotoxicity
following chronic prenatal treatment of mice with tobacco smoke and
ethanol. Bull Environ Contam Toxicol, 26: 813-819.
Philipson K, Falk R, Gustafsson J, & Camner P (1996) Long-term lung
clearance of 195Au-labeled Teflon particles in humans. Exp Lung Res,
22: 65-83.
Pieraccini G, Luceri, F & Moneti, G (1992) New
gas-chromatographic/mass spectrometric method for the quantitative
analysis of primary aromatic amines in main and sidestream cigarette
smoke. I. Rapid Commun Mass Spectrom, 6: 406-409.
Pindborg JJ, Mehta PS, Gupta PC, Daftary DK, & Smith CJ (1971) Reverse
smoking in Andhra Pradesh, India: A study of palatal lesions among
10,169 villagers. Br J Cancer, 25: 10-20.
Plamenac P, Santic Z, Nikulin A, & Serdarevic H (1985) Cytologic
changes of the respiratory tract in vineyard spraying workers. Eur J
Respir Dis, 67: 50-55.
Post WK, Burdorf A, & Bruggeling TG (1994) Relations between
respiratory symptoms and sickness among workers in the animal feed
industry. Occup Environ Med, 51: 440-446.
Pratt PC, Vollmer RT, & Miller JA (1980) Epidemiology of pulmonary
lesions in nontextile and cotton textile workers: a retrospective
autopsy analysis. Arch Environ Health, 35: 133-138.
Prentice RL, Yoshimoto Y, & Mason MW (1983) Relationship of cigarette
smoking and radiation exposure to cancer mortality in Hiroshima and
Nagasaki. J Natl Cancer Inst, 70: 611-622.
Pyykko I, Pekkarinen J, & Stark J (1987) Sensory-neural hearing loss
during combined noise and vibration exposure. Int Arch Occup Environ
Health, 59: 439-454.
Ramakrishnan S, Sulochana KN, Selvaraj T, Abdul-Rahim A, Lakshmi M, &
Arunagiri K (1995) Smoking of beedies and cataract: cadmium and
vitamin C in the lens and blood. Br J Ophthalmol, 79: 202-206.
Rang EH & Acheson ED (1981) Cancer in furniture workers. Int J
Epidemiol, 10(3): 253-261.
RCP (Royal College of Physicians) (1962) Smoking and health. London,
Pitman, pp 2-8.
Reddy CR (1974) Carcinoma of hard palate in India in relation to
reverse smoking of chuttas. J Natl Cancer Inst, 53(3): 615-619.
Reddy DJ & Rao KV (1957) Cancer of the palate in coastal Andhra due to
smoking of cigars with burning end inside the mouth. Indian J Med Sci,
11: 791-798.
Reddy DJ, Reddy BD, & Rao PR (1960) Experimental production of cancer
with tobacco tar and heat. Cancer, 13: 263-269.
Reith AK, Reichborn-Kjennerud S, Aubele M, Jutting U, Gais P, & Burger
G (1994) Biological monitoring of chemical exposure in nickel workers
by imaging cytometry (ICM) of nasal smears. Anal Cell Pathol, 6(1):
9-21.
Rivenson A, Hecht SS, & Hoffmann D (1991) Carcinogenicity of
tobacco-specific N-nitrosamines: the role of the vascular network in
the selection of target organs. Crit Rev Toxicol, 21: 255-264.
Roberts DL (1988) Natural tobacco flavor. Recent Adv Tob, 14: 45-81.
Robertson PB, Walsh MM, & Greene JC (1997) Oral effects of smokeless
tobacco used by professional baseball players. Adv Dental Res, 11:
307-312.
Rodenhuis S & Slebos RJC (1992) Clinical significance of ras oncogene
activation in human lung cancer. Cancer Res, 52(suppl): S2665-S2669.
Roels H, Sarhan MJ, Hanotiau I, de Fays M, Genet P, Bernard A, Buchet
JP, & Lauwerys R (1985) Preclinical toxic effects of manganese in
workers in a Mn salts and oxides producing plant. Sci Total Environ,
42: 201-206.
Roth J, Mahrlein W, Kummer G, & Krause M (1985) [Results of an
epidemiological analysis of the pathogenesis of chronic obstructive
bronchitis in miners.] Z Erkrank Atmungsorg, 164(3): 277-279 (in
German).
Rothman K & Keller A (1972) The effect of joint exposure to alcohol
and tobacco on risk of cancer of the mouth and pharynx. J Chronic Dis,
25: 711-716.
Roush GC (1996) Cancer of the nasal cavity and paranasal sinuses. In:
Schuttenfeld D & Franinini JF ed. Cancer epidemiology and prevention.
New York, Oxford, Oxford University Press, pp 587-602.
Ruckley VA, Gauld SJ, Chapman JS, Davis JM, Douglas AN, Fernie JM,
Jacobsen M, & Lamb D (1984) Emphysema and dust exposure in a group of
coal workers. Am Rev Respir Dis, 129: 528-532.
Russell MAH (1976) Tobacco smoking and nicotine dependence. In:
Gibbons RJ, Israel Y, Kalent H, Papham RE, Schmidt W, & Smart RG ed.
Recent advances in alcohol and drug problems. New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp 1-47.
Ryan J, Zwerling C, & John E (1992) Occupational risks associated with
cigarette smoking: A prospective study. Am J Pub Health, 82: 29-32.
Rylander R, Sjostrand M, & Bergstrom R (1979) Free lung cell response
after combined exposure to cigarette smoke and industrial dusts.
Toxicology, 12: 211-220.
Sanden A & Jarvholm B (1991) A study of predictors of mesothelioma in
shipyard workers exposed to asbestos. J Occup Med, 33(7): 770-773.
Sanden A, Jarvholm B, Larsson S, & Thiringer G (1992) The risk of lung
cancer and mesothelioma after cessation of asbestos exposure: a
prospective cohort study of shipyard workers. Eur Respir J, 5:
281-285.
Sankaranarayanan R, Duffy SW, Day NE, Nair MK, Padmakumary G, & Day NE
(1989a) A case control investigation of cancer of the oral tongue and
floor of the mouth in southern India. Int J Cancer, 44(4): 617-621.
Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, & Padmanabhan TK
(1989b) Tobacco chewing, alcohol and nasal snuff in cancer of the
gingiva in Kerala, India. Br J Cancer, 60(4): 638-643.
Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, & Nair MK (1990)
Risk factors for cancer of the buccal and labial mucosa in Kerala,
southern India. J Epidemiol Community Health, 44(4): 286-292.
Cerik R (1987) The interactions of tobacco smoking and other agents in
cancer etiology. Epidemiol Rev, 9: 175-193.
Saris M & Lucic-Palaic S (1977) Possible synergism of exposure to
airborne manganese and smoking habit in occurrence of respiratory
symptoms. In: Walton WH & McGovern B ed. Inhaled particles -- IV.
Oxford, New York, Pergamon Press, pp 773-779.
Saumet JL, Leftheriotis G, Dittmar A, & Delhomme G (1986) Relationship
between pulse amplitude and thermal exchange in the finger: the effect
of smoking. Psychopharmacology (Berlin), 92(1): 118-121.
Schlueter DP, Banaszaak EF, Fink JN, & Barboriak J (1978) Occupational
asthma due to tetrachlorophthalic anhydride. J Occup Med, 20: 183-188
Schneider G & Krivan V (1993) Multi-element analysis of tobacco and
tobacco smoke condensate by instrumental neutron activation analysis
and atomic absorption spectrometry. Int J Environ Anal Chem, 53:
87-100.
Schnorr TM, Steenland K, Thun MJ, & Rinsky RA (1995) Mortality in a
cohort of antimony smelter workers. Am J Ind Med, 27(5): 759-770.
Schottenfeld D, Grant RC, & Wynder WL (1974) The role of alcohol and
tobacco in multiple primary cancers of the upper digestive system,
larynx and lung: A prospective study. Prev Med, 3: 277-293.
Schüller HM, Jorquera R, Reichert A, & Castonguay A (1993)
Transplacental induction of pancreas tumors in hamsters by ethanol and
the tobacco-specific nitrosamine 4-(methyl
nitrosamino)-1-(pyridyl)-1-butanone. Cancer Res, 53: 2498-2501.
Seaton A, Lamb D, Brown WR, Sclare G, & Middleton WG (1981)
Pneumoconiosis of shale miners. Thorax, 36: 412-418.
Seaton A, Godden DJ, & Brown K (1994) Increase in asthma: a more toxic
environment or a more susceptible population? Thorax, 49: 171-174.
Selig R & Nestler K (1985) [Epidemiology of chronic bronchitis from
the viewpoint of occupational medicine.] Z Erkrank Atmungsorg, 164(3):
273-276 (in German).
Selikoff IJ & Frank AL (1983) Keynote address. Potential of in vitro
tests in asbestos-related problems. Environ Health Perspect, 51: 1-4.
Selikoff IJ, Hammond EC, & Churg J (1968) Asbestos exposure, smoking
and neoplasia. J Am Med Assoc, 204(2): 106-112.
Selikoff IJ, Seidman H, & Hammond EC (1980) Mortality effects of
cigarette smoking among amosite asbestos factory workers. J Natl
Cancer Inst, 65: 507-513.
Sherrill DL, Halonen M, & Burrows B (1994) Relationships between total
serum IgE, atopy and smoking: a twenty-year follow-up analysis. J
Allergy Clin Immunol, 94: 954-962.
Sherson D, Sigsgaard T, Overgaard E, Loft S, Poulsen HE, & Jongeneelen
FJ (1992) Interaction of smoking, uptake of polycyclic hydrocarbons,
and cytochrome P450IA2 activity among foundry workers. Br J Ind Med,
49(3): 197-202.
Shim C & Williams MH (1986) Effect of odors in asthma. Am J Med,
80(1): 18-22.
Shirakawa T, Kusaka Y, & Morimoto K (1992) Combined effects of smoking
habits and occupational exposure to hard metal on total IgE
antibodies. Chest, 101(6): 1569-1576.
Shopland DR, Eyre HJ, & Pechacek TF (1991) Smoking attributable cancer
mortality in 1991: is lung cancer now the leading cause of death among
smokers in the United States? J Natl Cancer Inst, 83(16): 1142-1148.
Sidney S, Tekawa I, & Friedman GD (1989) Mentholated cigarette use
among multiphasic examinees, 1979-86. Am J Pub Health, 79(10):
1415-1416.
Simarak S, de Jong UW, Breslow N, Dahl CJ, Ruckphaopunt K, Scheelings
P, & Maclennan R (1977) Cancer of the oral cavity, pharynx/larynx and
lung in Northern Thailand: case-control study and analysis of cigar
smoke. Br J Cancer, 36: 130-140.
Simecek C, Sefrna F, & Simecková B (1986) [Evaluation of the role of
smoking in the development of chronic bronchitis after elimination of
other supporting factors.] Stud Pneumol Phtiseologica Czech, 46(3):
168-170 (in Czech).
Sluis-Cremer GK, Walters LG, & Sichel HS (1967) Chronic bronchitis in
miners and non-miners: an epidemiologic survey of a community in the
gold mining area in the Transvaal. Br J Ind Med, 24: 1-12.
Smith IE, Coles CD, Lancaster J, Fernhoff PM, & Falek A (1986) The
effect of volume and duration of prenatal ethanol exposure on neonatal
physical and behavioral development. Neurobehav Toxicol Teratol, 8:
375-381.
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I,
Duggan HM, Wood R, Kosnett MJ, & Smith MT (1992) Cancer risks from
arsenic in drinking water. Environ Health Perspect, 97: 259-267.
Sopori ML, Goud NS, & Kaplan AM (1994) Effects of tobacco smoke on the
immune system. In: Dean JH, Luster MI, Munson AE, & Kimber I ed.
Immunotoxicology and immunopharmacology. New York, Raven Press, pp
413-434.
Sopori ML, Kozak W, Savage SM, Geng Y, & Kluger MJ (1998)
Nicotine-induced modulation of T cell function: Implications for
inflammation and infection. Adv Exp Med Biol, 437: 279-289.
Sprince NL, Oliver LC, Eisen EA, Greene RE, & Chamberlin RI (1988)
Cobalt exposure and lung disease in tungsten carbide production: A
cross-sectional study of current workers. Am Rev Respir Dis, 138:
1220-1226.
Stanton MF, Layard M, Tegeris A, Miller E, May M, & Kent E (1977)
Carcinogenicity of fibrous glass: pleural response in the rat in
relation to fiber dimension. J Natl Cancer Inst, 58: 587-603.
Steenland K (1994) Age specific interactions between smoking and radon
among United States uranium miners. Occup Environ Med, 51(3): 192-194.
Steenland K & Thun M (1986) Interaction between tobacco smoking and
occupational exposures in the causation of lung cancer. J Occup Med,
28(2): 110-118.
Steenland K & Ward E (1991) Lung cancer incidence among patients with
beryllium disease: a cohort mortality study. J Natl Cancer Inst,
83(19): 1380-1385.
Steenland K & Ward E (1993) Passive smoking and the risk of heart
disease. J Natl Cancer Inst, 85(2): 1698-1699.
Steindorf K, Lubin J, Wichman HE, & Becher H (1995) Lung cancer deaths
attributable to indoor radon exposure in West Germany. Int J
Epidemiol, 24(3): 485-492.
Stellman SD, Boffetta P, & Garfinkel L (1988) Smoking habits of
800,000 American men and women in relation to their occupations. Am J
Ind Med, 13: 43-58.
Stellman SD, Muscat JE, Thompson S, Hoffmann D, & Wynder EL (1997)
Risk of squamous cell carcinoma and adenocarcinoma of the lung in
relation to lifetime filter cigarette smoking. Cancer, 80: 382-388.
Stöber W & Abel WR (1996) Lung cancer due to diesel soot particles in
ambient air? Int Arch Occup Environ Health, 68(suppl): S3-S61.
Stohrer G (1991) Arsenic: opportunity for risk assessment. Toxicology,
65: 525-531.
Sturtevant FM (1982) Smoking, oral contraceptives, and thromboembolic
disease. Int J Fertil, 27: 2-13.
Sunderman FW & Sunderman FW Jr (1961) Nickel poisoning: XI.
Implications of nickel as a pulmonary carcinogen in tobacco smoke. Am
J Clin Pathol, 35: 203-205.
Talbot RJ, Morgan A, Moores SR, & Matulionis DH (1987) Preliminary
studies of the interaction between 239PuO2 and cigarette smoke in the
mouse lung. Int J Radiat Biol, 51: 1101-1110.
Tawfik HN (1987) Carcinoma of the urinary bladder associated with
schistosomiasis in Egypt: the possible causal relationship. Princess
Takamatsu Int Symp Ser, 18: 197-209.
Taylor PR, Qiao YL, Schatzkin A, Yao SX, Lubin J, Mao BL, Rao JY,
McAdams M, Xuan XZ, & Li JY (1989) Relation of arsenic exposure to
lung cancer among tin miners in Yunan Province, China. Br J Ind Med,
46: 881-886.
Thériault G, de Guire L, Gingras S, & Laroche G (1982) Raynaud's
phenomenon in forestry workers in Quebec. Can Med Assoc J, 126:
1404-1408.
Thériault G, Tremblay C, Cordier S, & Gingras S (1984) Bladder cancer
in the aluminium industry. Lancet, 1(8383): 947-950.
Thomas TL & Stewart PA (1986) Mortality from lung cancer and
respiratory disease among pottery workers exposed to silica and talc.
Am J Epidemiol, 124: 530.
Thomas DC & Whittemore AS (1988) Methods for testing interactions,
with applications to occupational exposures, smoking and lung cancer.
Am J Ind Med, 13: 131-147.
Thomas GB, Williams CE, & Hoger NG (1981) Some non-auditory correlates
of the hearing threshold levels of an aviation noise-exposed
population. Aviation Space Environ Med, 52(9): 531-536.
Thun MJ, Lally CA, Flanney JT, Calle EE, Flander WD, & Heath CW Jr
(1997) Cigarette smoking and changes in the histopathology of the
lung. J Natl Cancer Inst, 89: 1580-1586.
Tokarskaya ZB, Okladnikova ND, Belyaeva ZD, & Drozhko EG (1995) The
influence of radiation and nonradiation factors on the lung cancer
incidence among the workers of the nuclear enterprise Mayak. Health
Phys, 69: 356-366.
Townsend MC, Sussman NB, Enterline PE, Morgan WK, Belk HD, & Dinman BD
(1988) Radiographic abnormalities in relation to total dust exposure
at a bauxite refinery and alumina-based chemical products plant. Am
Rev Respir Dis, 138: 90-95.
Trethowen WN, Burge PS, Rossiter CE, Harrington JM, & Calvert IA
(1995) Study of the respiratory health of employees of seven European
plants that manufacture ceramic fibres. Occup Environ Med, 52(2):
97-104.
Tricker AR & Preussmann R (1992) Volatile N-nitrosamines in
mainstream cigarette smoke: occurrence and formation. Clin Invest,
70(3-4): 283-289.
Tseng WP, Chu HM, How SW, Fong JM, Lin CS, & Yeh S (1968) Prevalence
of skin cancer in an endemic area of chronic arsenicism in Taiwan. J
Natl Cancer Inst, 40: 453-463.
Tso TC (1990) Production physiology and biochemistry of tobacco
plants. Belsville, Maryland, IDEALS, p 753.
Tso TC, Harley N, & Alexander LT (1966) Source of lead-210 and
polonium-210 in tobacco. Science, 153: 880-882.
Tsuda M & Kurashima Y (1991) Tobacco smoking, chewing, and snuff
dipping: factors contributing to the endogenous formation of
N-nitroso compounds. Crit Rev Toxicol, 21(4): 243-253.
Tsuda T, Nagira T, Yamamoto M, & Kume Y (1990) An epidemiological
study on cancer in certified arsenic poisoning patients in Toroku. Ind
Health, 28: 53-62.
Tsuda T, Babazono A, Yamamoto E, Kurumatani N, Mino Y, Ogawa T, Kishi
Y, & Aoyama H (1995) Ingested arsenic and internal cancer: a
historical cohort followed for 33 years. Am J Epidemiol, 141(3):
198-209.
Tuyns AJ (1983) Oesophageal cancer in non-smoking drinkers and
non-drinking smokers. Int J Cancer, 32: 443-444.
Tuyns AJ (1991) Aetiology of head and neck cancer: tobacco, alcohol
and diet. Otorhynolaryngology, 46: 15-24.
Tuyns AJ, Péquignot G, & Jensen OM (1977) Le cancer de l'oesophage en
Ille-et-Vilaine en fonction des niveaux de consommation d'alcool et de
tabac: Des risques qui se multiplient. Bull Cancer, 64: 45-60.
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic
Radiation) (1988) Sources and effects of ionizing radiation. New York,
United Nations.
US ATSDR (1995) Toxicological profile for asbestos (update). Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry, 224 pp.
US DA (1990) World tobacco situation. Washington, DC, US Department of
Agriculture, Foreign Agricultural Service (Circular Series FT 4-90).
US DA (1997) Tobacco situation and outlook yearbook -- US output,
removal and consumption 1950-1997: Cigarettes, cigars, smoking tobacco
and specific tobacco products. Washington, DC, US Department of
Agriculture, Economic Research Service, pp 14-17 (TBS-240).
US EPA (1988) Technical support document on risk assessment of
chemical mixtures. Washington, DC, US Environmental Protection Agency,
32 pp (EPA/600/8-90/064).
US Surgeon General (1979) Smoking and health: A report of the Surgeon
General. Washington, DC, US Department of Health, Education and
Welfare (DHEW Publication No. (PHS) 79-50066).
US Surgeon General (1982) The health consequences of smoking: Cancer
-- A report of the Surgeon General. Rockville, Maryland, US Department
of Health and Human Services, Public Health Service, 332 pp.
US Surgeon General (1983) The health consequences of smoking:
Cardiovascular disease -- A report of the Surgeon General. Rockville,
Maryland, US Department of Health and Human Services, Public Health
Service, pp 6-8.
US Surgeon General (1984) The health consequences of smoking: Chronic
obstructive lung disease -- A report of the Surgeon General.
Rockville, Maryland, US Department of Health and Human Service (DHHS
Publication No. (PHS) 84-50205).
US Surgeon General (1986) The health consequences of using smokeless
tobacco -- A report of the Advisory Committee to the Surgeon General.
Bethesda, Maryland, US Department of Health and Human Services, Public
Health Service (Publication No. (NIH) 86-2874).
US Surgeon General (1988) The health consequences of smoking: Nicotine
addiction -- A report of the Surgeon General. Rockville, Maryland, US
Department of Health and Human Services, Centers for Disease Control,
pp 21-560.
US Surgeon General (1989) Reducing the health consequences of smoking:
25 years of progress -- A report of the Surgeon General. Rockville,
Maryland, US Department of Health and Human Services, Public Health
Service (DHHS Publication No. (CDC) 89-8411).
Vainio H & Boffetta P (1994) Mechanisms of the combined effect of
asbestos and smoking in the etiology of lung cancer [Review]. Scand J
Work Environ Health, 20(4): 235-242.
Vainio H, Husgafvel-Pursiainen K, Anttila S, Hackman P, & Partanen T
(1993) Interaction between smoking and asbestos in human lung
adenocarcinoma: role of K-ras mutations. Environ Health Perspect,
101(suppl 3): 189-192.
Vallyathan V, Shi XL, Dalal NS, Irr W, & Castranova V (1988)
Generation of free radicals from freshly fractured silica dust.
Potential role in acute silica-induced lung injury. Am Rev Respir Dis,
138: 1213-1219.
Van Leeuwen FE, Klokman WJ, Stoval M, Hagwenbeek A, van den
Belt-Dusebout AW, Noyon R, Boice JD, Burgers MV, & Somers R (1995)
Roles of radiotherapy and smoking in lung cancer following Hodgkin's
disease. J Natl Cancer Inst, 87(20): 1530-1537.
Van Peenen PFD, Blanchard AG, Wolkonsky PM, & Gill TM (1986) Health
insurance claims of petrochemical company employees. J Occup Med, 28:
237-240.
Van Tuinen M & Land G (1986) Smoking and excess sick leave in a
department of health. J Occup Med, 28: 33-35.
Van Schooten FJ, van Leeuwen FE, Hillebrand MJ, de Rijke ME, Hart AA,
van Veen HG, Oosterink S, & Kriek E (1990) Determination of
benzo(a)pyrene diol epoxide-DNA adducts in white blood cell DNA from
coke-oven workers: the impact of smoking. J Natl Cancer Inst, 82(11):
927-933.
Venables KM, Topping MD, Howe W, Luczynska CM, Hawkins R, & Taylor AJ
(1985) Interaction of smoking and atopy in producing specific IgE
antibody against a hapten protein conjugate. Br Med J, 290: 201-204.
Venables KM, Tee RD, Hawkins ER, Gordon DJ, Wale CJ, Farrer NM, Lam
TH, Baxter PJ, & Newman-Taylor A (1988a) Laboratory animal allergy in
a pharmaceutical company. Br J Ind Med, 45: 667-671.
Venables KM, Upton KM, Hawkins ER, Tee RD, Longbottom JL, &
Newman-Taylor AJ (1988b) Smoking, atopy, and laboratory animal
allergy. Br J Ind Med, 45: 667-671.
Venables KM, Dally MB, Nunn AJ, Stevens JF, Stephens R, Farrer N,
Hunter JV, Stewart M, Hughes EG, & Newman-Taylor AJ (1989) Smoking and
occupational allergy in workers in a platinum refinery. Br Med J, 299:
939-942.
Vessey MP & Doll R (1969) Investigation of relationship between the
use of oral contraceptives and thromboembolic disease: a further
report. Br Med J, 2: 651-657.
Viikari-Juntura E, Riihimaki H, Tola S, Videman T, & Mutanen P (1994)
Neck trouble in machine operating, dynamic physical work and sedentary
work: a prospective study on occupational and individual risk factors.
J Clin Epidemiol, 47(12): 1411-1422.
Vineis P (1992) Epidemiological models of carcinogenesis: the example
of bladder cancer. Cancer Epidemiol Biomarkers Prev, 1(2): 149-153.
Vineis P, Esteve J, & Terracini B (1984) Bladder cancer and smoking in
males: types of cigarettes, age of start, effect of stopping and
interaction with occupation. Int J Cancer, 34: 165-170.
Voges E ed. (1984) Tobacco encyclopedia. Mainz, Germany, Tabilk
Journal International, 467 pp.
Wagner JC, Pooley FD, Gibbs A, Lyons J, Sheers G, & Moncrieff CB
(1986) Inhalation of china stone and china clay dusts: relationships
between the mineralogy of dust retained in the lungs and pathological
changes. Thorax, 41: 190-196.
Wald NJ, Dall R, Darby S, Kinjlnk S, Petri R, & Pike M ed. (1985)
Trends in smoking related diseases in Britain. Oxford, Oxford
University Press.
Warren CPW (1977) Extrinsic allergic alveolitis: a disease commoner in
non-smokers. Thorax, 32: 567-569.
Weiland SK, Mundt KA, Keil U, Kraemer B, Birk T, Person M, Bucher AM,
Straif K, Schumann J, & Chambless L (1996) Cancer mortality among
workers in the German rubber industry: 1981-91. Occup Environ Med, 53:
289-298.
Weiss W (1971) Cigarette smoking, asbestos and pulmonary fibrosis. Am
Rev Respir Dis, 104: 223-226.
Weiss W (1976) Chloromethyl ethers, cigarettes, cough and cancer. J
Occup Med, 18(3): 194-199.
Weiss W (1980) The cigarette factor in lung cancer due to chloromethyl
ethers. J Occup Med, 22(8): 527-529.
Weiss W (1982) Epidemic curve of respiratory cancer due to
chloromethyl ethers. J Natl Cancer Inst, 69(6): 1265-1270.
Weiss W (1984) Cigarette smoke, asbestos and small irregular
opacities. Am Rev Respir Dis, 130(2): 293-301.
Weiss W & Boucot KR (1975) The respiratory effects of chloromethyl
methyl ether. J Am Med Assoc, 234(11): 1139-1142.
Weiss W & Nash D (1997) An epidemic of lung cancer due to chloromethyl
ethers: Thirty years of observation. J Occup Environ Med, 39:
1003-1009.
Welch K, Higgins I, Oh M, & Burchfiel C (1982) Arsenic exposure,
smoking and respiratory cancer in copper smelter workers. Arch Environ
Health, 37: 325-335.
WHO (1980) Recommended health-based limits in occupational exposure to
heavy metals. Geneva, World Health Organization, pp 21-35 (WHO
Technical Report Series 647).
WHO (1985) Regional Workshop on the Control of Tobacco-related
Diseases, New Delhi, 22-26 July 1985. New Delhi, World Health
Organization Regional Office for South-East Asia.
WHO (1997) Tobacco or health: A global status report. Geneva, World
Health Organization, pp 10-18.
Wicklund KG, Daling JR, Allard J, & Weiss NS (1988) Respiratory cancer
among orchardists in Washington State, 1968 to 1980. J Occup Med,
30(7): 561-564.
Wilbert W (1987) Tobacco and shamanism in South America (Psychoactive
plants of the world). New Haven, London, Yale University Press, 294
pp.
Wilhelmsen L (1977) Recent studies on smoking and CVD epidemiology:
Scandinavia and some other Western European countries. In: Proceedings
of 3rd World Conference on Smoking and Health -- Volume II: Health
consequences, education, cessation, and social action. Washington, DC,
Department of Health, Education and Welfare, pp 171-177 (Publication
No. (NIH) 77-1413).
Williams MK, Walford J, & King E (1983) Blood lead and the symptoms of
lead absorption. Br J Ind Med, 40(3): 285-292.
Winkelstein W (1990) Smoking and cervical cancer current status: a
review. Am J Epidem, 131: 945-957.
Winkelstein W, Shillitoe EJ, Brand R, & Johnson KK (1984) Further
comments on cancer of the uterine cervix, smoking and herpes virus
infection. Am J Epidem, 119(1): 1-8.
Winn DM (1997) Epidemiology of cancer and other systemic effects
associated with the use of smokeless tobacco. Adv Dent Res, 11(3):
313-321.
Wise MB & Guerin MR (1986) Chemical analysis of the major constituents
in clove cigarette smoke. In: Hoffmann D & Harris CC ed. Mechanisms in
tobacco carcinogenesis. Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 151-162 (Banbury Report No. 23).
Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE
(1997) The carcinogenicity of environmental tobacco smoke.
Carcinogenesis, 18(3): 575-586.
Wong SH, Ogle CW, & Cho CH (1986) Influence of chronic or acute
nicotine pretreatment on ethanol-induced gastric ulceration in the
rat. J Pharm Pharmacol, 38: 537-540.
Wong O, Harris F, & Smith TJ (1993) Health effects of gasoline
exposure: II. Mortality patterns of distribution workers in the United
States. Environ Health Perspect, 101(suppl 6): 63-76.
Wright JT, Waterson EJ, Barrison IG, Toplis PJ, Lewis IG, Gordon MG,
Macrae KD, Morris NF, & Murray-Lyon IM (1983) Alcohol consumption,
pregnancy and low birth weight. Lancet, 1: 663-665.
Wright JT, Macrae KD, Barrison IG, & Waterson EJ (1984) Effects of
moderate alcohol consumption and smoking on fetal outcome. London,
Pitman, pp 240-253 (Ciba Foundation Symposium Series No. 105).
Wynder EL & Bross IJ (1961) A study of etiological factors in cancer
of the oesophagus. Cancer, 14: 389-413.
Wynder EL & Gori GB (1977) Contribution of the environment on cancer
incidence: an epidemiologic exercise. J Natl Cancer Inst, 58: 825-832.
Wynder EL & Graham EG (1950) Tobacco smoking as a possible etiological
factor in bronchiogenic carcinoma. J Am Med Assoc, 37: 4608-4622.
Wynder El & Hoffmann D (1967) Tobacco and tobacco smoke: Studies in
experimental carcinogenesis. New York, London, Academic Press.
Wynder El & Hoffmann D (1994) Smoking and lung cancer: challenges and
opportunities. Cancer Res, 54: 5284-5295.
Wynder EL, Covey LS, Mabuchi K, & Mushinki M (1976) Environmental
factors in cancers of the larynx: A second look. Cancer, 38:
1591-1601.
Yazicioglu S, Ilcayto R, Balci K, Sayli BS, & Yorulmaz B (1980)
Pleural calcification, pleural mesotheliomas and bronchial cancers
caused by tremolite dust. Thorax, 35: 564-569.
Yorukoglu Y, Ilgit E, Zengin M, Nazliel K, Salman E, & Yucel E (1993)
Thromboangiitis obliterans (Buerger's disease) in women (a
reevaluation). Angiology, 44(7): 527-532.
Yoshizawa K (1984) [Clinicopathological analysis of lung cancer and
epithelial changes of the bronchus in chromate workers.] Shikoku Acta
Med, 40(1): 123-134 (in Japanese).
Yue L (1992) Cadmium in tobacco. Biomed Environ Sci, 5(1): 53-56.
Zacny JP (1990) Behavioral aspects of alcohol-tobacco interactions.
Recent Dev Alcohol, 8: 205-219.
Zahran FM, Ardawi MSM, & Al-Fayez SF (1985) Carboxyhaemoglobin
concentrations in smokers of sheesha and cigarettes in Saudi Arabia.
Br Med J, 291: 1768-1770.
Zelman S (1973) Correlation of smoking history with hearing loss. J Am
Med Assoc, 223(8): 920.
Zetterstrom O, Osterman K, Machado L, & Johansson SGO (1981) Another
smoking hazard: raised IgE concentration and increased risk of
occupational allergy. Br Med J, 283: 1215-1217.
Zhang Z-F, Yu S-Z, Li W-X, & Choi BCK (1989) Smoking, occupational
exposure to rubber and lung cancer. Br J Ind Med, 46: 12-15.
Zhu H & Wang Z (1993) Study of occupational cancer in asbestos
factories in China. Br J Ind Med, 50(11): 1039-1042.
Zuskin E, Zagar Z, Schacter EN, Mustajbego J, & Kern J (1992)
Respiratory symptoms and ventilatory capacity in swine confinement
workers. Br J Ind Med, 49(6): 435-440.
SYNOPSIS
1. Introduction
Le tabac, notamment lorsqu'il est fumé, exerce divers effets nuisibles
à la santé; il est directement en cause dans un certain nombre de
maladies graves et peut accentuer l'action nocive d'autres agents
chimiques, physiques ou biologiques. Les produits chimiques et autres
agents présents sur les lieux de travail peuvent, si l'on n'y veille pas
suffisamment, provoquer diverses pathologies, des invalidités et des
décès prématurés. Il est clair que sur les lieux de travail, des effets
indésirables peuvent résulter de la synergie entre le tabagisme et
d'autres facteurs de risque. La plupart des interactions entre les
constituants nocifs de la fumée de tabac et certaines substances
chimiques toxiques se produisent lorsque celles-ci sont présentes dans
l'atmosphère, mais le tabagisme peut également, comme on l'a observé,
interagir avec des agents toxiques absorbés par voie buccale ou autre.
La consommation de tabac est universelle, des pays économiquement
défavorisés aux nations industrialisées les plus riches. Le tabac est
consommé par les hommes et les femmes, les enfants et les adultes et des
millions de gens sont exposés malgré eux à la fumée de tabac présente
dans l'environnement. Il y a de nombreuses explications au tabagisme,
mais la raison principale de son universalité tient à la présence, dans
les feuilles de toutes les variétés de tabac, de nicotine, une substance
qui engendre la dépendance. Cette dernière pénètre dans l'organisme du
consommateur en quantité variable, selon le type de tabagisme auquel il
s'adonne (Chapitre 2). L'apparition de la cigarette, qui peut être
produite en quantités industrielles, qu'il est facile de se procurer à
un prix relativement bas et que sa légèreté permet de tenir entre les
lèvres en gardant les mains libres, a profondément modifié le
comportement des fumeurs, que ce soit dans la vie en général ou sur les
lieux de travail.
Il y a beaucoup de pays où fumer est considéré comme très dangereux pour
la santé et comme un facteur qui contribue de manière importante à la
mortalité résultant d'un certain nombre d'affections courantes. Ces pays
ont adopté une législation qui vise à alerter les consommateurs des
risques qu'ils courent et ils ont également pris des mesures pour
freiner la consommation, soit par la taxation, soit par la mise en
oeuvre de campagnes d'information à destination du grand public qui ont
pour but d'attirer l'attention sur les dangers du tabagisme et les
avantages qu'il y a à cesser de fumer ou à ne pas commencer du tout.
Certains pays n'ont toutefois pas encore pris de mesures décisives pour
traiter le problème du tabagisme.
L'activité professionnelle comporte souvent une part de risque. Elle
peut être de nature à mettre la santé en danger et à contaminer
l'environnement. La culture du tabac elle-même implique l'utilisation de
pesticides, la récolte des feuilles peut provoquer des intoxications
dues à l'absorption percutanée de nicotine et les diverses opérations
auxquelles elles sont ensuite soumises expose les travailleurs à
respirer les poussières et les spores de champignons présentes dans
l'atmosphère. Dans les zones où sont implantées des manufactures de
tabac, on observe une incidence élevée de cancers chez les sujets de
sexe masculin. L'air des exploitations minières est chargé de poussières
minérales et dans l'agriculture ou les industries qui utilisent des
matières premières d'origine biologique, les travailleurs sont exposés
à des poussières de même origine. Le soudage donne également lieu à la
production de vapeurs, et les gaz, fumées, brouillards etc. produits
dans de nombreuses industries sont aussi source de dangers. Une chaleur
excessive ou l'exposition aux ultraviolets peuvent nuire au bien-être
des travailleurs. On admet désormais que les rayonnements ionisants émis
dans les mines ou par certains appareils modernes constituent un risque
professionnel. Nombreuses sont les activités professionnelles qui
entraînent une exposition à des niveaux de bruit excessifs ou à des
vibrations nocives. Toutes ces conditions de travail ont des effets
indésirables sur la santé, mais qui peuvent être plus graves pour les
fumeurs que pour les non fumeurs. Dans un grand nombre de pays, il est
interdit de fumer sur les lieux de travail, essentiellement d'ailleurs
en raison des risques d'incendie ou d'explosion. Dans d'autres, cette
réglementation n'est pas toujours appliquée. Dans certains pays
nouvellement industrialisés, les problèmes sanitaires liés à l'activité
professionnelle ne sont pas encore totalement pris en compte et de
nombreux employés et ouvriers ne sont pas conscients des risques de leur
métier sur le plan sanitaire. En outre, il existe un vaste secteur
industriel "non officiel", en particulier dans les pays en
développement, où le travail, qui se fait à domicile, peut comporter
l'utilisation de produits chimiques (solvants, résines, colorants de
synthèse etc.) auxquels toute la famille va donc se trouver exposée. En
outre, il n'existe pas de réglementation qui restreigne l'exposition ou
interdise de fumer dans ces circonstances.
Beaucoup moins bien défini est le cas d'effets nocifs résultant de
l'action combinée d'une exposition à la fumée de tabac, provenant du
courant principal ou de l'environnement, et à des agents présents dans
le milieu domestique. On sait toutefois qu'en ce qui concerne
l'incidence du cancer du poumon, la courbe dose-réponse obtenue dans le
cas d'une exposition domestique au radon est analogue à celle qu'on
obtient chez des mineurs exposés à ce gaz, avec un risque plus important
chez les fumeurs.
2. Exemples d'effets combinés d'une exposition à la fumée de tabac et
à d'autres agents
On est fondé à penser que dans le cas de certains effets toxiques (en
l'occurrence le cancer) il existe une synergie entre le fait de fumer et
l'exposition à l'arsenic, à l'amiante, à l'éthanol, à la silice et aux
rayonnements (radon, bombe atomique, rayons X). D'un autre côté, il y a
également lieu de croire à l'existence d'un antagonisme entre le
tabagisme et les chlorométhyléthers cancérogènes comme le
chlorométhyl-méthyléther (CMME) et le bis (chlorométhyléther) (BCME)
(Hoffmann & Winder, 1976; CIRC, 1986), entre le tabagisme et l'alvéolite
allergique ou encore entre le tabagisme et la bérylliose chronique.
Fumer accentue les effets nocifs d'une exposition à la poussière chez
les mineurs de charbon, ou aux pesticides chez ceux qui en manipulent,
et cette accentuation du risque s'observe également dans l'industrie du
caoutchouc et du pétrole. Les mineurs de charbon qui fument risquent
davantage de contracter une bronchite chronique ou une pneumopathie
obstructive, mais pas un emphysème. Les cancers du poumon observés chez
les mineurs de charbon sont attribués en totalité au tabagisme. Fumer
peut accroître les effets d'une exposition aux poussières végétales qui
engendrent des affections respiratoires chroniques, comme la byssinose
produite par la poussière de coton et le cancer des fosses nasales
provoqué par la poussière de bois.
3. Composition des feuilles et de la fumée de tabac
On a isolé plus de 3040 composés chimiques des feuilles de tabac après
transformation (Roberts, 1988). La plupart d'entre eux sont des
constituants de la feuille, mais d'autres résultent des conditions de
culture (sol et atmosphère de la région) ou encore des produits
agrochimiques utilisés et des traitements subis (sauçage,
humidification, aromatisation et séchage). On constate des différences
selon la région d'origine du tabac, les variétés et les diverses
méthodes de séchage et de transformation utilisées. Ces différences
peuvent affecter la proportion des divers constituants mais la
composition globale ne varie pas. Parmi les importants composés toxiques
que l'on a mis en évidence, on trouve, à côté de la nicotine, des
nitrosamines cancérogènes qui proviennent de l'action des nitrites, des
amines, des protéines et des alcaloïdes d'origine foliaire, des
hydrocarbures aromatiques polycycliques formés au cours du séchage, des
éléments radioactifs captés dans le sol et dans l'air ainsi que du
cadmium dans le cas de tabacs cultivés sur des sols riches en cadmium.
Lorsque l'on fume, la combustion du tabac conduit à la formation de
nombreux produits de pyrolyse ou résultant d'autres types de réactions.
4. Fumée du courant principal
La fumée de tabac est un aérosol consistant en une phase particulaire
constituée de gouttelettes de liquide dispersées dans une phase gazeuse
ou vapeur. Lorsque l'on fume une cigarette, il se forme de nombreux
composés qui résultent de la pyrolyse du tabac. Ceux-ci peuvent, soit
traverser la cigarette dans la fumée constituant le courant
principal-certains d'entre eux se condensant légèrement en arrière du
cône incandescent-, soit passé dans l'air à partir de l'extrémité
incandescente, dans la fumée qui constitue le courant latéral. A chaque
bouffée, ces composés se concentrent dans la fumée car les produits qui
s'étaient déjà condensés viennent s'y ajouter -- la zone de condensation
se réduisant à mesure que la cigarette se raccourcit. La nature
physicochimique de la fumée dépend du traitement subi par le tabac et de
sa combustion, de la porosité et du traitement du papier et du type de
bout-filtre (Hoffmann & Hoffmann, 1997). Dans le cas d'une cigarette ou
de ce que l'on appelle un "bidi" en Asie (du tabac roulé dans la feuille
d'une plante), la composition chimique de la fumée dépend de facteurs
tels que les dimensions et la porosité de l'enveloppe ainsi que des
paramètres du fumage : volume, fréquence et durée des bouffées (NIH,
1998). Les variations de composition chimique concernent davantage la
proportion des différents constituants que la présence ou l'absence de
tel ou tel composé.
La fumée du courant principal est produite à l'intérieur du cône
incandescent dans une atmosphère relativement pauvre en oxygène à une
température de combustion de 850-950°C. Au départ, les particules de
cette fumée ont un diamètre aérodynamique massique médian (DAMM) de 0,2
à 0,3 µm; toutefois, dès qu'elles pénètrent dans les voies
respiratoires, où le degré d'humidité est de 100%, elles s'agrègent pour
former des particules de plus grande taille et se comportent alors comme
si leur DAMM était de l'ordre du micromètre. Environ 50 à 90% des
particules inhalées peuvent être retenues dans les voies respiratoires
(Wynder & Hoffmann, 1967; Hinds et al., 1983). Pour des raisons d'ordre
dimensionnel, les particules présentes dans l'aérosol, les constituants
de la phase gazeuse et les gaz permanents sont capables d'atteindre les
alvéoles lors de l'inhalation de la fumée. Le comportement des
constituants hydrophiles en présence d'une forte humidité fait que le
dépôt dans l'arbre trachéobronchique revêt un caractère complexe, mais
de toute manière, la fumée s'insinue dans la totalité des voies
respiratoires.
Près de 4000 constituants ont été répertoriés dans la fumée du courant
principal à côté d'un nombre indéterminés de substances non identifiées
(Roberts, 1988). La fumée du courant principal comporte une phase
particulaire et une phase gazeuse. La phase particulaire contient de la
nicotine, des nitrosamines telles que la
4-(méthylnitrosamino)-1-(3-pyridyl)-1-butanone (NKK) et la
N-nitrosonornicotine (NNN), des métaux comme le cadmium, le nickel, le
zinc et le polonium-210, des hydrocarbures polycycliques et des amines
cancérogènes comme le 4-aminobiphényle. La phase gazeuse renferme du
monoxyde et du dioxyde de carbone, du benzène, de l'ammoniac, du
formaldéhyde, du cyanure d'hydrogène, de la N-nitrosodiméthylamine, de
la N-nitrosodiéthylamine et un certain nombre d'autres composés. Les
composés présents dans la fumée de tabac peuvent, selon leurs effets
biologiques, être classés en asphyxiants, irritants, ciliatoxines,
mutagènes, cancérogènes, inhibiteurs d'enzymes, neurotoxines ou dérivés
dotés d'action pharmaceutique. C'est principalement par les voies
respiratoires que la fumée de tabac pénètre dans l'organisme mais de
nombreux constituants, présents en particulier dans la fumée de pipe et
de cigare, se dissolvent dans la salive et sont soit avalés, soit
absorbés au niveau de la cavité buccale. Les fumeurs de pipe et de
cigare n'inhalent généralement pas la fumée, qui demeure dans la cavité
buccale où, on vient de le voir, elle se dissout dans la salive et peut
être soit absorbée par passage à travers la muqueuse buccale, soit être
directement avalée (NIH, 1998). Les boissons alcoolisées, par leur effet
solvant sur les constituants de la fumée, en facilitent la résorption.
5. Fumée du courant latéral
La fumée du courant latéral est généralement produite à une température
de combustion plus faible (500-600°C) dans une atmosphère réductrice.
Les particules de cette fumée ont, lorsqu'elles sont fraîchement émises,
une taille à peu près équivalente à celle des particules du courant
principal, avec un diamètre aérodynamique massique médian (DAMM)
d'environ 0,2 µm. Quantitativement, la composition de la fumée du
courant latéral est analogue à celle de la fumée du courant principal.
Certaines substances du courant latéral sont émises à une concentration
(rapportée à 1 g de tabac brûlé) plus élevée que les constituants du
courant principal. C'est notamment le cas de composés cancérogènes comme
la N-nitrosodiméthylamine et la N-nitrosodiéthylamine ou encore de
métaux comme le nickel et le cadmium. Beaucoup de dérivés cancérogènes
sont plus concentrés dans la fumée du courant latéral que dans celle du
courant principal. Des épreuves biologiques consistant à badigeonner la
peau de souris avec un condensant de fumée du courant latéral ont montré
que ce dernier est plus cancérogène que celui de la fumée du courant
principal (Wynder & Hoffmann, 1967; US Surgeon General, 1986; NIH,
1998).
6. La manière de fumer la cigarette et ses effets sur la toxicité de la
fumée
Les cigarettes n'ont pas toutes la même teneur en nicotine et le fumeur
"tire" plus ou moins fort en inhalant plus ou moins profondément pour
satisfaire son besoin de nicotine. Il s'ensuit qu'en fumant une
cigarette à bout-filtre pauvre en nicotine (< 1,2 mg) le fumeur va
tirer plus intensément, ce qui ne sera pas sans effet sur la toxicité
(NIH, 1998).
7. Résumé des conclusions et des recommandations
La consommation de tabac, notamment en le fumant, constitue un problème
de santé publique d'une extrême importance en raison de la morbidité et
de la mortalité qui en résultent. Outre les effets nocifs causés par une
utilisation active du tabac, on a montré qu'il en existe aussi qui
résultent de l'exposition passive à la fumée présente dans
l'environnement. Les dangers du tabagisme sont également accrus par la
possibilité d'interactions avec certains agents chimiques, physiques ou
biologiques présents sur les lieux de travail ou dans l'environnement en
général. On connaît certes quelques cas d'interactions antagonistes,
mais les risques inhérents au tabagisme l'emportent de très loin sur ses
effets protecteurs apparents. Tout doit être mis en oeuvre pour faire
cesser la consommation de tabac et en particulier l'habitude de fumer.
Il faut s'opposer très vigoureusement au tabagisme sur les lieux
publics. En outre, pour éviter des interactions avec d'autres types
d'exposition tout en éliminant le risque d'exposition passive à la fumée
de tabac, il faut interdire de fumer sur les lieux de travail.
Il faut aussi vivement inciter les gens à ne pas fumer à la maison afin
de protéger la santé de la famille et notamment celle des enfants. On
évitera ainsi également des interactions potentiellement dangereuses
avec d'autres types d'exposition pouvant survenir dans l'environnement
domestique. Il est nécessaire d'établir sans attendre des programmes
éducatifs sur les dangers du tabagisme pour la santé. Les professionnels
de la santé doivent prêter assistance aux personnes qui désirent cesser
de fumer. Comme le tabagisme peut entraîner une modification des
réactions aux médicaments ou autres formes de traitement, voire susciter
à leur encontre des réactions indésirables, les médecins doivent ajuster
les doses de leurs patients en conséquence et surveiller leurs
réactions.
PANORAMA GENERAL
1. Introducción
El consumo de tabaco, particularmente el hábito de fumar, provoca una
serie de efectos nocivos para salud, está directamente relacionado con
varias enfermedades graves y puede aumentar los efectos adversos de
otros agentes químicos, físicos y biológicos. Si no se controlan, los
agentes químicos y de otro tipo pueden producir en el puesto trabajo
enfermedades, discapacidades y la muerte prematura. Es evidente que en
el lugar de trabajo los efectos adversos pueden deberse a la interacción
sinérgica del humo de tabaco con otros peligros. La mayor parte de las
interacciones de los constituyentes nocivos del humo de tabaco con
sustancias químicas tóxicas se producen cuando estas últimas están en el
aire, aunque se han notificado asimismo interacciones del humo con
agentes perjudiciales ingeridos y/o absorbidos.
El consumo de tabaco está generalizado en todo el mundo, desde los
países de bajos ingresos hasta los industrializados más ricos. Lo
utilizan hombres y mujeres, niños y adultos, y millones de personas
están involuntariamente expuestas al humo de tabaco en su entorno. Si
bien hay numerosas explicaciones del hábito de fumar, la razón principal
de su ubicuidad es el efecto adictivo de la nicotina, droga presente en
todas las formas de la hoja de tabaco y que llega al consumidor en
cantidades variables según los distintos tipos de consumo (capítulo 2).
La aparición del cigarrillo, de producción masiva, fácil de obtener,
relativamente económico y ligero de peso, de manera que se puede llevar
en la boca dejando las manos libres, ha tenido repercusiones importantes
en el hábito de fumar, tanto en general como en el puesto de trabajo.
En muchos países se reconoce que el humo de tabaco constituye un peligro
grave para la salud y es un factor que contribuye de manera importante
a la muerte causada por diversas enfermedades comunes. En esos países se
ha aplicado una legislación de alerta sanitaria y medidas impositivas
para el control de su consumo, así como programas de educación del
público sobre los peligros del tabaco y las ventajas de no comenzar a
fumar o de interrumpir el consumo. Sin embargo, todavía hay países donde
no se han puesto en marcha medidas decisivas para abordar el problema
del consumo de tabaco.
Son muchas las situaciones laborales que conllevan un elemento de
riesgo. El tipo de trabajo puede generar efectos nocivos para salud y
las actividades laborales pueden provocar la contaminación de medio
ambiente. El propio cultivo del tabaco requiere el uso de plaguicidas,
la recolección de la hoja puede ocasionar trastornos debido a la
absorción de nicotina a través de la piel y su elaboración expone a los
trabajadores a peligros para la salud provocados por el polvo y las
esporas de hongos presentes en el aire. Se ha notificado una elevada
incidencia de cáncer en el sexo masculino en zonas con industrias
tabaqueras. En la minería existe polvo de minerales en el aire y en la
agricultura y la industria basadas en materias primas producidas
biológicamente hay polvo biológico. Durante las actividades de soldadura
se produce humo y en muchas industrias crean peligro los gases, humos,
neblinas y vapores cargados de sustancias orgánicas y/o inorgánicas
tóxicas. Un calor excesivo o la exposición a luz ultravioleta pueden
ser perjudiciales para el bienestar de los trabajadores. Está admitido
que las radiaciones ionizantes en la minería y en la tecnología moderna
son un peligro en el lugar de trabajo. En numerosas actividades, los
trabajadores están expuestos a un ruido excesivo o a vibraciones
mecánicas peligrosas. Estas condiciones laborales pueden afectar más
negativamente a la salud de las personas fumadoras que a la de las no
fumadoras. Son muchos los países en los que está prohibido fumar en el
trabajo, fundamentalmente por razones de seguridad en cuanto
incendios/explosiones. Sin embargo, en algunos países no siempre se
cumple la reglamentación. En varios países recientemente
industrializados no se han abordado todavía plenamente los problemas de
salud asociados con el trabajo y muchos empleadores y trabajadores
desconocen los peligros para la salud de sus actividades. Además, existe
un amplio "sector extraoficial" de la industria, particularmente en los
países en desarrollo, en que se trabaja en el hogar y se utilizan
sustancias químicas (en particular disolventes, resinas y colorantes
sintéticos), estando expuesta toda la familia, y no hay restricciones
sobre la exposición a los peligros en el trabajo o al humo.
Está mucho menos definida la situación en relación con los efectos
adversos en la salud derivados de la exposición combinada al humo de
tabaco -- de la corriente principal o del medio ambiente -- y a los
agentes del entorno doméstico. Sin embargo, la incidencia de cáncer de
pulmón y la concentración de radón en los hogares tiene una relación
dosis-respuesta similar a la que se produce entre el cáncer de pulmón y
la concentración de radón en las minas, y el riesgo es más elevado para
los fumadores.
2. Ejemplos de efectos combinados de la exposición al humo de
tabaco y a otras sustancias
Está demostrada la existencia de sinergia en la producción de efectos
nocivos (cáncer) entre el humo de tabaco y la exposición al arsénico, el
amianto, el etanol, el silicio y las radiaciones (radón, bomba atómica,
rayos X). Por otra parte, hay pruebas de antagonismo en el caso del humo
de tabaco y los clorometiléteres carcinogénicos, es decir, el
clorometilmetiléter (CMME) y el bis(clorometil)éter (BCME) (Hoffmann y
Wynder, 1976; CIIC, 1986), el humo de tabaco y la alveolitis alérgica y
el humo de tabaco y la beriliosis crónica. El humo de tabaco influye en
el riesgo para la salud de la exposición en la extracción de carbón, el
manejo de plaguicidas y las industrias del caucho y el petróleo. Los
trabajadores de las minas de carbón que fuman tienen un riesgo más
elevado de contraer bronquitis crónica y enfermedades obstructivas de
las vías respiratorias, pero no enfisema. El cáncer de pulmón de los
mineros del carbón se ha atribuido totalmente al humo de tabaco. El humo
de tabaco puede aumentar el riesgo para salud de la exposición a polvos
vegetales que producen trastornos respiratorios crónicos, como la
bisinosis debida al polvo del algodón y el cáncer nasal provocado por el
polvo de la madera.
3. Composición de la hoja del tabaco y del humo de tabaco
De las hojas de tabaco elaboradas se han aislado más de 3040 compuestos
químicos (Roberts, 1988). La mayoría son constituyentes de la hoja, pero
la presencia de algunos depende de las condiciones de cultivo, como el
suelo y la atmósfera de la zona, mientras que otros se derivan del uso
de productos químicos agrícolas, de revestimientos, humectantes y
aromatizantes añadidos a las hojas y de los métodos de curado. Las
diferentes variedades de tabaco que se cultivan en los distintos países,
así como las distintas formas de curado y elaboración, ponen de
manifiesto diversas diferencias. Las proporciones de los distintos
constituyentes pueden ser diferentes, pero no la composición general.
Entre los compuestos tóxicos importantes identificados, aparte de la
nicotina, figuran nitrosaminas carcinogénicas, derivadas de nitritos,
aminas, proteínas y alcaloides presentes en la hoja, hidro-carburos
aromáticos policíclicos procedentes del proceso de curado, elementos
radiactivos absorbidos del suelo y el aire, y cadmio en el tabaco
cultivado en suelos ricos en este elemento. Cuando se quema tabaco al
fumar, se forman numerosos productos derivados de la pirólisis y de
otras reacciones.
4. Corriente principal del humo de tabaco
El humo del tabaco es un aerosol formado por una fase particulada de
gotitas de líquido dispersas en una fase de gas/vapor. Al fumar un
cigarrillo se forman numerosos compuestos por la pirólisis del tabaco.
Éstos pasan a través del cigarrillo en la corriente principal del humo,
condensándose algunos a corta distancia detrás del cono de combustión,
o bien se emiten en el aire a partir del extremo que se quema como humo
lateral. En cada bocanada el humo se hace progresivamente más fuerte,
porque se le añade material previamente condensado y porque la longitud
del cigarrillo disponible para la ulterior condensación disminuye. Las
características fisicoquímicas del humo dependen de la elaboración y la
combustión del tabaco, la porosidad y el tratamiento del papel en el que
está envuelto y del tipo de filtro (Hoffman y Hoffman, 1997). En el caso
de un cigarrillo o "bidi" asiático (tabaco envuelto en hoja vegetal), la
química del humo se ve afectada por factores tales como las dimensiones,
la porosidad de la envoltura y los parámetros del volumen, la frecuencia
y la duración de la bocanada de humo (NIH, 1998). Las variaciones en la
química del humo se dan fundamentalmente en la proporción entre sus
constituyentes, más que en la presencia o ausencia de compuestos
concretos.
El humo de la corriente principal se genera en una atmósfera con un
contenido comparativamente bajo de oxígeno a una temperatura de
combustión de 850-950°C en el cono de combustión. Al principio, las
partículas presentes en la corriente principal tienen un diámetro
aerodinámico medio de la masa (MMAD) de 0,2 a 0,3 micras; sin embargo,
en cuanto llegan al tracto respiratorio, con una humedad del 100%, se
unen para formar partículas mayores y se comportan como si su MMAD fuera
del orden de micras. En el tracto respiratorio se puede retener
alrededor del 50-90% de todas las partículas inhaladas (Wynder y
Hoffmann, 1967; Hinds et al., 1983). Por lo que se refiere al tamaño, al
inhalar el humo pueden llegar a los alvéolos las partículas en aerosol,
los constituyentes de la fase de vapor y los gases permanentes. La
deposición en el árbol traqueobronquial se ve complicada por el
comportamiento de los constituyentes hidrofílicos en condiciones de
humedad elevada, pero el humo llega a todas las partes de las vías
respiratorias.
El humo de la corriente principal contiene cerca de 4000 sustancias
químicas identificadas y un número desconocido de sustancias químicas
sin identificar (Roberts, 1988). El humo de la corriente principal se
puede dividir en una fase de partículas y otra de gas. La fase de
partículas contiene nicotina, nitrosaminas como la
4-(metilnitrosamino)-1-(3-piridil)-1-butanona (NNK) y la
N-nitrosonornicotina (NNN), metales como el cadmio, el níquel, el zinc
y el polonio-210, hidrocarburos policíclicos y aminas carcinogénicas,
como el 4-aminobifenilo. La fase de vapor contiene monóxido de carbono,
anhídrido carbónico, benceno, amoníaco, formaldehído, cianuro de
hidrógeno, N-nitrosodimetilamina, N-nitrosodietilamina y otros
compuestos. Los compuestos del humo de tabaco se pueden clasificar por
su actividad biológica como asfixiantes, irritantes, ciliatoxinas,
mutágenos, carcinógenos, inhibidores de las enzimas, neurotoxinas o
compuestos farmacológicamente activos. El principal punto de entrada del
humo del cigarrillo en el organismo es por las vías respiratorias, pero
muchos constituyentes, en particular del humo de pipa y de cigarro, se
disuelven en la saliva y se absorben en la cavidad bucal o se ingieren.
Los fumadores de cigarros y de pipa no suelen inhalar el humo, que
permanece en la cavidad bucal, se disuelve en la saliva y se absorbe a
través de las membranas mucosas o se ingiere (NIH, 1998). Las bebidas
alcohólicas tienen un efecto disolvente de los constituyentes del humo,
facilitando su absorción.
5. Humo de tabaco lateral
El humo lateral se forma con una temperatura de combustión más baja
(500-600°C) en una atmósfera reductora. Las partículas del humo lateral
fresco son prácticamente del mismo tamaño que las de la corriente
principal, con un diámetro aerodinámico medio de la masa de alrededor de
0,2 micras. Desde el punto de vista cualitativo, la composición del humo
lateral es semejante a la del humo de la corriente principal. Algunas
sustancias químicas se emiten en el humo lateral con una concentración
mayor por gramo de tabaco quemado que en el humo de la corriente
principal. Esto es particularmente aplicable a carcinógenos como la
N-nitrosodimetilamina y la N-nitrosodietilamina y a metales como el
níquel o el cadmio. Muchos compuestos carcinógenos están más
concentrados en el humo lateral que en el principal. En biovaloraciones
con aplicación a la piel de ratones se ha demostrado que el humo lateral
condensado es más carcinogénico que el principal (Wynder y Hoffmann,
1967; US Surgeon General, 1986; NIH, 1998).
6. Efectos de la manera de fumar los cigarrillos en la
toxicidad del humo
El contenido de nicotina de los distintos cigarrillos varía, y el
fumador, para satisfacer la necesidad adquirida de nicotina, la ajusta
mediante la intensidad con la que fuma y la profundidad de la
inhalación. Por consiguiente, el fumador de cigarrillos con filtro y de
contenido bajo en nicotina (< 1,2 mg) fuma con mayor intensidad, y esto
influye en la toxicidad (NIH, 1998).
7. Resumen de las conclusiones y recomendaciones
El consumo de tabaco, en particular el hábito de fumar, representa un
peligro para la salud pública de la máxima importancia y es una causa
prevenible importante de morbilidad y mortalidad. Además de los efectos
adversos del consumo activo de tabaco para la salud, se han demostrado
efectos adversos derivados de la exposición al humo de tabaco presente
en el medio ambiente. Los riesgos del hábito de fumar también aumentan
como consecuencia de las interacciones con ciertos peligros químicos,
físicos y biológicos existentes en el lugar de trabajo y en el medio
ambiente general. Hay un pequeño número de casos de interacciones
antagonistas, pero los riesgos del humo de tabaco para la salud son muy
superiores a cualquier efecto protector aparente.
Se deben adoptar todas las medidas posibles para eliminar el consumo de
tabaco, en particular el hábito de fumar, y se ha de disuadir con
firmeza de fumar en lugares públicos. A fin de evitar la interacción con
otros tipos de exposición ocupacional y de eliminar el riesgo de
exposición al humo de tabaco del medio ambiente, debería prohibirse
fumar en el lugar de trabajo.
Con objeto de proteger la salud, en particular la de los niños, se
debería desalentar con firmeza el hábito de fumar en el hogar. De esta
manera se previenen posibles interacciones perjudiciales entre el humo
de tabaco y la exposición a otros peligros en la vivienda. Hay una
necesidad imperiosa de programas educativos sobre los peligros del
hábito de fumar para la salud. Los profesionales de la salud deberían
prestar asistencia para ayudar a los fumadores a dejar este hábito.
Debido a que el humo puede provocar una alteración de la respuesta a los
medicamentos y otros tratamientos o una reacción adversa a éstos, los
médicos deberían estudiar la posibilidad de introducir ajustes
apropiados de la dosificación y vigilar a los pacientes.
2.1.2 Tobacco smoking
Smoking prevalence data and most studies into the effects of smoking
have concentrated on cigarette smoking, yet worldwide only about 55%
of tobacco is used for cigarettes. The rest, along with significant
amounts traded in "farm gate" and "local market" sales or "home
grown", is smoked in "bidis" or other hand-rolled devices, or in some
form of pipe (IARC, 1986). Tobacco consumption data give an insight
into tobacco-related disease distribution, as can information on ways
of smoking.
Table 1. Estimated smoking prevalence by WHO Region, early 1990s
(from WHO, 1997)
Men Women
(%) (%)
WHO Regions:
African Regiona 29 4
Region of the Americas 35 22
Eastern Mediterranean Region 35 4
European Region 46 26
South-East Asia Region 44 4
Western Pacific Region 60 8
More developed countries 42 24
Less developed countries 48 7
World 47 12
a Smoking prevalence estimates for African Region are based on
very limited information
Differences in smoke chemistry occur with different tobacco cultivars
and curing methods. Curing removes moisture so that the leaf can be
stored without fermenting or rotting, and the time and temperature of
curing influences enzyme reactions such as deamination and oxidation,
and the content of oils and resins. Air-, flue-, sun-, and fire-curing
methods are employed and each produces distinctive tobaccos which are
intended for smoking in a specific way or to suit a particular
preference.
Between 1933 and the late 1940s, the yields from an average cigarette
varied from 33 to 49 mg tar and from < 1 to 3 mg nicotine (Creek et
al., 1994). However, in the 1960s and 1970s, the average yield from
cigarettes in Western Europe and the USA was around 16 mg tar and 1.5
mg nicotine per cigarette. Current average levels are lower. Changes
in the levels of tar and nicotine have resulted in the characteristic
more intense puffing and smoke inhalation pattern of smokers of
low-yield cigarettes (Djordjevic et al., 1995; NIH, 1996). The
high tar yields measured in the smoke of cigarettes in developed
countries before 1950 are comparable with current tar yields of
"bidis", which range from 23 to 48 mg per cigarette (Hoffmann et al.,
1974; Jayant & Pakhale, 1985; Ball & Simpson, 1987.) The "kretek" (a
cigarette of strong tobacco and cloves) of Indonesia yields up to
71 mg tar (WHO, 1985). Smoking materials in northern Thailand
(cigarette or cigar of strong tobacco plus various vegetable
materials) produce high levels of tar and nicotine (Simarak et al.,
1977).
For smoking, tobacco leaf can be flue-cured, light air-cured,
fire-cured or sun-cured, and can be of dark, oriental or N. rustica
type. Flue-cured tobacco is used for cigarettes and pipe tobacco.
Air-cured tobacco is used in blended cigarettes. Dark tobaccos are
widely grown, usually air-cured, and mostly used in their countries of
origin for dark cigarettes, "bidis", cigars, in pipes and "sheeshas",
and for chewing tobaccos and snuff (Voges, 1984). Some tobaccos have
low nitrate levels ranging up to 0.9% (Neurath & Ehmke, 1964; Wynder &
Hoffmann, 1967) and their smoke contains low nitrogen oxide levels in
the range of 50-200 mg NOx/cigarette (Norman et al., 1983). In the
reducing atmosphere of the burning cone, NOx gives rise to amino
radicals, which react with benzene, biphenyl, naphthalene and other
ring hydrocarbons to form aromatic amines. Aniline, alkylated
anilines, aminobiphenyls, and naphthylamines are therefore found in
higher quantities in the smoke of tobaccos with a high nitrate content
(Patrianakos & Hoffmann, 1979; Pieraccini et al., 1992; Grimmer et
al., 1995). Smokers of these tobaccos can inhale greater quantities of
aromatic amines, such as the human bladder carcinogens
2-naphthyl-amine and 4-aminobiphenyl, and have higher concentrations
of haemoglobin adducts in their blood (Bartsch et al., 1993). This is
the basis for the higher risk of bladder cancer among the smokers of
cigarettes made from dark tobaccos (Vineis et al., 1984; D'Avanzo et
al., 1990; Vineis, 1992).
In the Indian subcontinent, the "bidi" is the common smoking device.
It consists of tobacco flakes, loosely packed and hand-rolled in a
tendu or temburni leaf ( Diospyros melanoxylon). It contains less
tobacco (0.223 g) than a cigarette (0.782 g) (Ramakrishnan et al.,
1995) but up to 8.2% nicotine compared with up to 3.7% in cigarette
tobacco. "Bidi" smoke contains 23 to 48 mg tar and 1.7 to 2.9 mg
nicotine per cigarette (Hoffmann et al., 1974; Jayant & Pakhale,
1985). In India, where around 7% of world tobacco is consumed (US DA,
1990), around 30% of tobacco is smoked as cigarettes, 50% as "bidis",
10% in other ways, and 10% is used for chewing. "Bidis" need to be
puffed frequently to ensure even burning and can generate up to 70 mg
of carbon monoxide, while a US non-filter cigarette smoked under
identical conditions generated 25 mg carbon monoxide (Hoffmann et al.,
1974; Jayant & Pakhale, 1985).
Table 2. Estimated smoking prevalence, ranked in order of male smoking prevalencea
Rank Countryb Men Women Rank Country Men Women
(%) (%) (%) (%)
1 Republic of Korea (1989) 68.2 6.7 21 Seychelles (1989) 50.9 10.3
2 Latvia (1993) 67 12 22 Bolivia (1992) 50 21.4
2 Russian Federation (1993) 67 30 23 Albania (1990) 49.8 7.9
4 Dominican Republic (1990) 66.3 13.6 24 Cuba (1990) 49.3 24.5
5 Tonga (1991) 65 14 25 Bulgaria (1989) 49 17
6 Turkey (1988) 63 24 25 Thailand (1995) 49 4
7 China (1984)c 61 7 27 Spain (1993) 48 25
8 Bangladesh (1990) 60 15 28 Mauritius (1992) 47.2 3.7
9 Fiji (1988) 59.3 30.6 29 Greece (1994) 46 28
10 Japan (1994) 59 14.8 29 Papua New Guinea (1990) 46 28
11 Sri Lanka (1988) 54.8 0.8 31 Israel (1989) 45 30
12 Algeria (1980) 53 10 32 Cook Islands (1988) 44 26
12 Indonesia (1986) 53 4 33 Czech Republic (1994) 43 31
12 Samoa (1994) 53 18.6 33 Jamaica (1990) 43 13
15 Saudi Arabia (1990) 52.7 N/Ad 33 Philippines (1987) 43 8
16 Estonia (1994) 52 24 33 Slovakia (1992) 43 26
16 Kuwait (1991) 52 12 37 Cyprus (1990) 42.5 7.2
16 Lithuania (1992) 52 10 38 Austria (1992) 42 27
16 South Africa (1995) 52 17 39 Malaysia (1986) 41 4
20 Poland (1993) 51 29 39 Peru (1989) 41 13
41 Uruguay (1990) 40.9 26.6 66 Colombia (1992) 35.1 19.1
42 Argentina (1992) 40 23 67 Costa Rica (1988) 35 20
42 France (1993) 40 27 67 Slovenia (1994) 35 23
42 Hungary 40 27 69 Swaziland (1989) 33 8
42 India (1980s) 40 3 70 Luxembourg (1993) 32 26
42 Iraq (1990) 40 5 71 Singapore (1995) 31.9 2.7
42 Malta (1992) 40 18 72 Belgium (1993) 31 19
50 Brazil (1989) 39.9 25.4 72 Canada (1991) 31 29
51 Egypt (1986) 39.8 1 72 Iceland (1994) 31 28
52 Morocco (1990) 39.6 9.1 75 Australia (1993) 29 21
53 Lesotho (1989) 38.3 1 75 Ireland (1993) 29 28
53 Mexico (1990) 38.3 14.4 77 UK (1994) 28 26
55 El Salvador (1988) 38 12 78 USA (1993) 27.7 22.5
Table 2. (cont'd)
Rank Countryb Men Women Rank Country Men Women
(%) (%) (%) (%)
55 Italy (1994) 38 26 79 Pakistan (1980) 27.4 4.4
55 Portugal (1994) 38 15 80 Finland (1994) 27 19
58 Chile (1990) 37.9 25.1 81 Turkmenistan (1992) 26.6 0.5
59 Guatemala (1989) 37.8 17.7 82 Nigeria (1990) 24.4 6.7
60 Denmark (1993) 37 37 83 Paraguay (1990) 24.1 5.5
61 Germany (1992) 36.8 21.5 84 Bahrain (1991) 24 6
62 Norway (1994) 36.4 35.5 84 New Zealand (1992) 24 22
63 Honduras (1988) 36 11 86 Sweden (1994) 22 24
63 Netherlands (1994) 36 29 87 Bahamas (1989) 19.3 3.8
63 Switzerland (1992) 36 26
a Adapted from: WHO (1997).
b The year given in parentheses is the latest available year for data.
c Some 1991 data suggest that there has been little change in smoking prevalence since 1984.
d Data not available.
Table 3. Trends in adult consumption of cigarettes from 1970-1972 to 1990-1992 (Adapted from WHO, 1997)
Annual % change
1970-1972 to 1990-1992
WHO Regions:
African Region +1.2
Region of the Americas -1.5
Eastern Mediterranean Region +1.4
European Region 0
South-East Asia Region +1.8
Western Pacific Region +3
More developed countries -0.5
Less developed countries +2.5
World -0.8
Tar yields for Russian cigarettes were found to be high (21.6-29.2
mg) and cigarettes containing N. rustica produced high smoke
concentrations of tobacco-specific nitrosamines (TSNA) (up to 620 ng
total TSNA/cigarette) (Djordjevic et al., 1991). Indigenous cigarettes
and cigars in Thailand, containing tobacco and other vegetable
materials, have up to 41 mg and 200 mg tar, up to 5.5 mg and 11.4 mg
nicotine, and 41 mg and 820 mg carbon monoxide in cigarette and cigar
smoke, respectively. Indonesian cigarette smoke contains up to 100 ng
of carcinogenic volatile N-nitrosamines and up to 1580 ng of
carcinogenic tobacco-specific N-nitrosamines (Mitacek et al., 1990,
1991), and high tar cigarettes contain up to 28.1 mg tobacco-specific
N-nitrosamines per cigarette (Brunnemann et al., 1996).
Many cigar-like devices are smoked throughout Asia. The tobacco can be
rolled in a tobacco leaf or in the leaves of the jackfruit tree
( Artocarpus integrifolia), banana ( Musa paradisiaca) or hansali
( Grewia microcos) (Bhonsle et al., 1976). They may be smoked
conventionally or in the reverse manner with the burning end inside
the mouth (Reddy, 1974). In Thailand they can contain strong tobacco
and a mixture of koi bark ( Streblus asper), dry tamarind pod
( Tamarindus indica), khai bark ( Homonoia riparia, Euphorbiaceae),
Areca palm bark ( Areca catechu) or other tree bark; they can be
rolled in a banana leaf or have fragrant additives such as sandalwood
Mougne et al. (1982). They contain high levels of tar and nicotine
(Simarak et al., 1977; Mitacek et al., 1999).
Additives are used to enhance the fragrance or taste of smoke
(Hoffmann & Hoffmann, 1997). "Casing sauces", consisting of sugars,
aromatic substances and compounds such as glycerol, propylene glycol,
ethylene glycol and diethylene glycol, which resist changes in
moisture content, are sprayed on the leaf before it is cut to
condition it for processing. Flavouring and dressing compounds are
added to cut tobacco after drying and include licorice, menthol,
cocoa, chocolate, ginger, cinnamon, vanilla, molasses, angelica,
honey, essential oils from anise, clove and juniper, resins and plant
extracts and organic compounds such as coumarins. Additives have been
widely used in pipe and chewing tobaccos. They have also become
important in cigarette tobacco with the development of low-tar and
low-nicotine tobaccos and the use of stem, midrib or reconstituted
leaf (which lacks the aromas and flavours of natural tobacco leaf
lamina) and of tobacco dust requiring additives to ensure its
adherence to cut tobacco.
Added glycerol is transferred to mainstream smoke: 3-6% in cigarette
smoke and 35-43% in pipe smoke (IARC, 1986) and one of its pyrolysis
products is acrolein. Levels of acrolein ranging from 69 mg to 230 mg
per cigarette have been reported and air concentrations as high as
0.46 mg/m3 have been found in smoke-filled rooms (Izard & Liberman,
1978). Acrolein is extremely irritating to the eyes and nasal mucosa,
it affects mitotic and ciliary activity, at the cellular level it has
cytotoxic and cilia-depressant effects, and it can act as a mutagen
(Izard & Liberman, 1978).
In the USA, additives used in cigarette manufacture are food additive
compounds that are "generally recognized as safe (GRAS)" and,
therefore, also considered "safe" as additives to tobacco. However,
the non-volatile additives are to some extent pyrolized during smoking
and can give rise to toxic and/or carcinogenic agents in the smoke. A
major group of compounds formed during pyrolysis is that of the
polynuclear aromatic hydrocarbons. Ethylene glycol is pyrolytically
converted to the human carcinogen ethylene oxide (IARC, 1994a).
Another example of carcinogen formation during tobacco curing and
smoking is the case of MH-30, a sucker growth control agent formulated
from maleic hydrazide in diethanolamine. Residual MH-30 on tobacco
leads to the formation of N-nitrosodiethanolamine (NDELA) in the
smoke (Brunnemann & Hoffmann, 1981). The use of MH-30 on tobacco has
been forbidden in the USA since 1981, and NDELA levels in tobacco and
its smoke have declined (Brunnemann & Hoffmann, 1991).
The use of cloves as a tobacco additive can have health effects. In
Indonesia, the smoke of "kreteks", a blend of ground cloves with
60-65% of tobacco, contains between 41 and 113 mg of tar, and between
1.2 and 4.5 mg nicotine (WHO, 1985; Wise & Guerin, 1986). Mainstream
smoke of kreteks without filter tips contains 19-23 mg of eugenol
released from the cloves, while filter-tipped kretek smoke contains up
to 15 mg (LGC, 1982; Wise & Guerin, 1986). Inhalation of eugenol in
the smoke of kreteks can lead to interstitial haemorrhaging and
congestion of the lung, acute emphysema and acute pulmonary oedema.
These effects were also seen in Syrian golden hamsters exposed to the
smoke of kreteks (LaVoie et al., 1986).
Menthol and other additives that produce a sensation of coolness but
without a mint flavour have also been used in cigarettes. There is no
evidence that these additives result in a higher risk (Cummings et
al., 1987; Sidney et al., 1989).
Many shapes and sizes of smoking pipes are found worldwide (Voges,
1984). Various types of tobacco are used and the smoke can range from
mild to very strong. In the sheesha water pipe the tobacco is kept
alight by pieces of glowing charcoal and the smoke is drawn through
water before being inhaled. Sheesha smoke is mild and low in
particulate matter, benzo( a) pyrene and volatile phenols (Hoffmann
et al., 1961), but it has a high level of carbon monoxide, in part
from the charcoal that keeps the tobacco burning, and smokers have
high carboxyhaemoglobin levels and a reduced FEV1 (8.5% in women, 45%
in men) (Zahran et al., 1985; Al-Fayez et al., 1988).
2.1.3 Tobacco chewing and snuff
The popularity of tobacco chewing and snuff (finely powdered tobacco
leaf) has varied. Nasal inhalation of snuff has given way to the oral
application of snuff and other tobacco-containing mixtures between the
gum and lip, or gum and cheeks, or under the tongue. Chewing tobacco
comes in several forms and may have flavour added from syrups,
liquorice and brandy. Tobacco chewing has retained its popularity in
heavy industries, such as steel and coal mining, woodworking and the
petroleum industry, where the flammability hazard precludes smoking.
In Sweden, 17% of the population uses oral snuff. In the USA, sales of
chewing tobacco have declined but sales of oral snuff have increased
by 61% (US DA, 1997) owing to the popularity of the latter with
teenagers and young adult men. Oral tobacco use in India is very
common but, generally, in developing countries it is declining because
urban populations and younger age groups are smoking cigarettes.
"Betel-quid" chewing is common in Asia and Africa. The basic contents
of a betel-quid are slices of areca nut ( Areca catechu), lime and
tobacco, wrapped in a betel pepper leaf ( Piper betle), but it may
also contain dried dates, menthol and spices such as cardamom, cloves,
coriander, mace and cinnamon.
Other tobacco preparations for oral use often contain lime, calcium
carbonate, sodium carbonate, some form of ash or flavouring materials.
The lime and other agents assist the release of nicotine (Voges, 1984;
Idris et al., 1991).
2.2 Responses to mainstream smoke
Tobacco use has direct effects on health and is responsible for a
variety of diseases. These form a basis for studying interactions
between tobacco use and chemical, physical and biological agents, and
associated effects on health.
2.2.1 Acute responses
Inhaled irritant chemicals cause inflammation of the upper respiratory
tract (URT) and paranasal sinuses, sore throat and bronchial oedema.
Pulmonary oedema may follow if irritants penetrate the lower
respiratory tract. Acute irritation of the URT usually follows
inhalation of highly soluble gases. Slightly less soluble gases cause
URT and bronchial irritation, while relatively insoluble gases
penetrate deeply into the lung and can have delayed effects including
pulmonary oedema (Miller & Kimbell, 1995). Cigarette smoke is a
complex mixture of organic and inorganic constituents with particulate
and vapour phases and highly reactive free radicals (Hocking & Golde,
1979). It contains many compounds with irritant properties, which can
affect all parts of the lung. Kremer et al. (1994) examined the
association between occupational exposures to a variety of airway
irritants and respiratory system effects, and whether the association
was modified by smoking, airway hyperresponsiveness and allergy. The
irritants were SO2, HCl, SO42-, polyester vapour, polyamide vapour,
and oil mist and vapour. Current smoking, airway hyperresponsiveness
and allergy were significantly associated with a higher prevalence of
chronic respiratory symptoms, independent of each other and of
irritant exposure. The association between exposure and the prevalence
of chronic respiratory symptoms was greater in smokers than in non- or
ex-smokers.
2.2.1.1 Acute bronchitis
Acute bronchitis is an inflammation of the bronchial mucous membrane,
initially accompanied by a dry painful cough and followed by
mucopurulent sputum. The cause may be infectious agents or chemical
agents such as tobacco smoke, dusts, fumes, vapours or gases.
2.2.1.2 Asthma
Asthma is a chronic pulmonary inflammatory disease associated with
bronchial hyperreactivity causing paroxysmal dyspnoea due to spasm of
the bronchial musculature, swelling of the mucous membranes and the
production of viscid mucus. In the majority of asthma cases a clear
association exists with atopic IgE-mediated hypersensitivity. Allergic
asthma is a response to a specific agent. Many chemical agents,
including mixtures such as tobacco smoke, can induce asthma. Smoking
enhances the effect of other agents and can reduce the latent period
from first exposure to onset of sensitization.
There has been an increase in the prevalence of asthma in many
countries, and roles for environmental factors, increased
susceptibility and tobacco smoking have been suggested (ISAAC, 1998).
Cigarette smoking together, with atopic status, age, URT infection and
genetic factors, has been considered to increase susceptibility
(Venables et al., 1985; Seaton et al., 1993). Studies have shown a
relationship between cigarette smoking and serum IgE levels. Smokers
have higher levels of IgE with increasing age, compared to non-smoking
controls. A relationship has also been observed between IgE level and
the number of cigarettes smoked (Sherrill et al., 1994).
2.2.2 Chronic responses
2.2.2.1 Chronic obstructive lung diseases
The chronic obstructive lung diseases (COLD) are chronic bronchitis,
small airways disease, toxic bronchiolitis obliterans, emphysema and
fibrosis (Niewoehner et al., 1974; Niewoehner, 1991).
These diseases form an important group of pulmonary diseases caused by
smoking (and by chemical atmospheric pollution) (US Surgeon General,
1984). In a study by Simecek et al. (1986) of 215 229 adults in a
region of former-Czechoslovakia, smoking was the most important risk
factor in COLD. Risks for male non-smokers and light smokers under 30
years of age were 1.18% and 2.28%, respectively; for men aged 50
years, smoking more than 20 cigarettes a day, the risk was 20.36%
compared with 3.31% for non-smokers of the same age. In the USA,
80-90% of the mortality from COLD has been attributed to cigarette
smoking. Cigar and pipe smokers who inhale the smoke also have an
increased death rate from COLD (US Surgeon General, 1984, 1989; NIH,
1998). Between 1979 and 1993, the age-adjusted annual death rate from
COLD in women increased by 122% to 17.1 per 100 000, while in men it
increased by 14% to 27.8 per 100 000. The greater increase of COLD
among women during this period reflected the increase in smoking by
women.
2.2.2.2 Chronic bronchitis
Chronic bronchitis has been variously defined. The United Kingdom
Medical Research Council (MRC, 1965) considered it to be a condition
with persistent production of sputum, which might be associated with
cough, occurring on most days for at least three months in the year
for at least two successive years. The Council recommended a
classification of simple chronic bronchitis, chronic or recurrent
mucopurulent bronchitis or chronic obstructive bronchitis. In a
workplace context, Morgan (1982) defined it as "a condition
characterized by cough and sputum for at least three months of the
year, which may or may not be accompanied by airways obstruction, and
which is a consequence of prolonged inhalation of dust or irritant
gases at the workplace". Fletcher & Pride (1984) suggested an improved
terminology with the term chronic bronchitis meaning chronic or
recurrent bronchial hypersecretion only, abandoning the term chronic
obstructive bronchitis because this implies a causal connection
between mucus hypersecretion and airflow obstruction. In smokers'
lungs the irritant constituents of tobacco smoke cause hypersecretion
of mucus, alter its physical properties and chemical structure, and
impair the mucociliary clearance mechanism. Removal of major
ciliatoxins (hydrogen cyanide and volatile aldehydes) from the smoke
stream by charcoal filter tips reduces the effects on the lung
epithelium (Friedman et al., 1972). Mineral dusts, particularly those
encountered in mining, many biological dusts, irritant vapours and
gases, inorganic and organic chemical dusts and sprays can all cause
chronic bronchitis.
2.2.2.3 Small airways disease
Small airways disease (SAD) is a widespread narrowing of membranous
bronchioli. It is inflammatory in origin and is often associated with
excess mucus and an accumulation of macrophages in the respiratory
bronchioli. SAD is mainly caused by smoking, but can be associated
with environmental and industrial pollutants (Cosio et al., 1980).
2.2.2.4 Emphysema
Emphysema has been defined as a condition of the lung characterized by
an abnormal enlargement of the airspaces distal to the terminal
non-respiratory bronchioli, accompanied by destructive changes in the
alveolar walls, and without obvious fibrosis. It tends to be prevalent
in older age groups and follows SAD. For purposes of postmortem
examination of lung slices of miners, Ruckley et al. (1984) defined
emphysema as the presence of air spaces of 1 mm or more in size.
Macrophages that have engulfed foreign particles, including smoke
particulate matter in the lungs of smokers, and which have been found
accumulated in the bronchioli (Niewoehner et al., 1974) and in the
lung parenchyma (McLaughlin & Tueller, 1971) have been implicated in
the pathogenesis of emphysema. There are different forms of emphysema,
which vary with the nature of the insult to the tissues. One
hypothesis is that tobacco smoke causes increased production and
release of proteolytic enzymes, such as elastase, and interferes with
normal antiproteolytic mechanisms. Emphysema has been induced
experimentally in animals by endotracheal installation of elastase or
homogenates of alveolar macrophages or polymorphonuclear leukocytes.
Chronic exposure to tobacco smoke has a number of effects on alveolar
macrophages, including changes in metabolism, alteration of the enzyme
content and impairment of RNA and protein synthesis (Hocking & Golde,
1979). The function of alveolar macrophages is to remove inhaled
foreign material from the alveoli and respiratory bronchioles, and
their numbers increase when the lungs are exposed to particles and
gases. It has been demonstrated that the macrophage count is higher in
people exposed to cigarette smoke than in non-exposed people (Harris
et al., 1974; Rylander et al., 1979). Alveolar macrophages from
smokers are more active than those from non-smokers, numbers are
increased, there are morphological changes with an increased cell
diameter, crystalline inclusions and surface membrane alteration
(Sopori et al., 1994).
2.2.2.5 Pulmonary fibrosis
Pulmonary fibrosis is the abnormal formation of fibrous or scar tissue
and is the response of bronchiolar tissue to the deposition of an
inhaled inciting agent. Mineral and other dusts are causes of
pulmonary fibrosis. The radiological changes seen in early stages of
dust fibroses are associated with relatively minor lung function
impairment, but continuous exposure leads to a greater degree of
fibrosis and to progressive massive fibrosis in some subjects. On
histological, animal experimental and radiographic evidence, Weiss
(1984) concluded that cigarette smoking could cause diffuse fibrosis.
2.2.2.6 Effects on the immune system
Tobacco smoking and exposure to environmental tobacco smoke increase
susceptibility to pulmonary infections, and changes in immune
processes may be involved. Tobacco smoking affects humoral and
cellular immunity in humans and experimental animals, but the
magnitude of the changes vary widely among studies. In humans,
cigarette smoke has marked effects on alveolar macrophage morphology
and physiology, it decreases serum immunoglobulin (IgA, IgG, IgM) but
increases IgE, and has a range of effects on B- and T-lymphocytes.
Similar effects are found in experimental animals (Sopori et al.,
1994). Studies in rats and mice show that cigarette smoke or nicotine
induces impaired responses of systemically distributed B- and
T-lymphocytes to antigen-induced signalling (Geng et al., 1995, 1996).
T-lymphocyte unresponsiveness, with decreased antibody response to
T-dependent antigens, is important in response to infection (Sopori et
al., 1998).
2.2.3 Cancer
Many forms of cancer have been associated with inhaled particulate
matter, vapours, fumes and gases. Examples are lung cancer associated
with tobacco smoking and inhalation of asbestos, fumes from metal
moulding and coking plants, particles and gases inhaled by motor
vehicle drivers, dusts in several types of mines where there is an
accumulation of alpha-emitting radioisotopes, and contact with
materials such as arsenic, chromates, nickel, chloromethyl ethers,
mustard gas and polycyclic aromatic hydrocarbons. Pleural mesothelioma
has been associated with asbestos, nasal and sinus cancer with nickel
refining and wood dust exposure, leukaemia with ionizing radiations
and benzene, bladder cancer with the manufacture and use of dyes and
in the rubber industry, and liver cancer with the use of vinyl
chloride (IARC, 1987).
Cigarette smoke contains many toxic chemicals, including chemicals
that are DNA reactive and cytotoxic or become DNA reactive upon
metabolic activation. Consequently, cigarette smoke has the potential
to initiate genetic lesions. Moolgavkar et al. (1989) postulated
mechanisms by which cigarette smoke induces lung cancer by fitting the
two-stage clonal expansion model of carcinogenesis to lung cancer
mortality data derived from a large cohort of British doctors who
smoked. This analysis suggested that tobacco smoke affected both the
rates of mutation and cell proliferation involved in the model,
supporting the hypothesis that tobacco smoke acts as a complete
carcinogen.
Cigarette smoking is causally associated with cancer of the lung,
larynx, pharynx, oesophagus, pancreas, kidney and urinary bladder. It
is also associated with cancer of the nasal cavity, liver, uterine
cervix and myeloid leukaemia (RCP, 1962; US Surgeon General, 1982,
1989; IARC, 1986; Winkelstein, 1990; Brownson et al., 1993; Roush,
1996).
In 1991, in the USA, it was estimated that 90.3% of the lung cancer
deaths in men and 78.5% of lung cancer deaths in women were
attributable to cigarette smoking. Deaths from oesophageal cancer were
linked to smoking in 78.2% of the cases in men and 74.3% of the cases
in women. Smoking was held responsible for 81.2% of deaths from
laryngeal cancer in men and 86.7% in women, for 91.5% and 61.2%,
respectively, of deaths from oral cancer, and 46.5% and 36.7%,
respectively, of deaths from bladder cancer. Smoking-attributable
deaths from cancer of the kidney in men and women were 47.6 and 12.3%,
respectively, and for pancreatic cancer the figures were 28.6% and
33.3% respectively. In women, 32.4% of uterine cervical cancer deaths
were attributed to cigarette smoking (Shopland et al., 1991).
There has been a change in the rates of different types of lung cancer
among smokers. In 1950, squamous cell carcinoma (SCC) occurred 17
times more often than adenocarcinoma (AC) (Wynder & Graham, 1950) in
cigarette smokers. In 1991, Devesa et al. (1991) reported that in male
cigarette smokers the ratio of SCC to AC was 2.4:1 between 1969 and
1971, and changed to 1.4:1 between 1984 and 1986; in cigarette-smoking
women, the SCC to AC ratio changed from 3.6:1 in 1950 to 0.57:1 in
1984-1986. Between 1970 and 1980 some studies showed a 20-50%
reduction in risk of lung cancer for long-term smokers of filter
cigarettes as compared to smokers of non-filter cigarettes (IARC,
1986) but later studies indicated a similar risk for lung cancer in
smokers of filter and non-filter cigarettes (Stellman et al., 1997;
Thun et al., 1997). The changes in the ratio of SCC to AC, and the
disappearance of an advantage of filter cigarette smoking in terms of
lung cancer risk, have been related to changes in smoke yields, which
have caused smokers to modify patterns of puff drawing and smoke
inhalation. Smokers regulate the speed and the quantity of their
nicotine uptake to achieve the desired pharmacological effects
(Benowitz et al., 1988; Djordjevic et al., 1995). Smokers of lower
nicotine cigarettes draw puffs of greater volume, at a higher
frequency, and inhale more deeply; this is governed by the amount of
nicotine in the smoke (Wynder & Hoffmann, 1994).
Smoking is a major risk factor for the early stage development of
oesophageal and gastric adenocarcinomas, accounting for 40% of cases,
and may have contributed to the increase in the incidence of these
cancers, especially in older people (Gammon et al., 1997).
Cigar smoking is causally associated with cancer of the oral cavity,
the pharynx and the lung, even though for cigar smokers, who do not or
only minimally inhale the smoke, the lung cancer risk is considerably
lower than that for cigarette smokers. Cigar smoking is also
associated with cancer of the pancreas and of the urinary bladder
(NIH, 1998). Oral snuff users have an increased risk of cancer of the
oral cavity and, possibly, cancer of the oesophagus, pancreas and
urinary bladder (US Surgeon General, 1986).
It has been suggested that around 4-5% of all lung cancer is related
to occupational exposure (Wynder & Gori, 1977; Doll & Peto, 1981;
Morgan, 1982 ). Occupational and any other forms of exposure to
chemical compounds are of limited importance in the etiology of lung
cancer whereas tobacco smoking is the cause of approximately 85-90% of
cases (Shopland et al., 1991).
"Reverse smoking", in which rolled tobacco leaves are smoked with the
burning end inside the mouth, has been linked to carcinoma of the hard
palate (Reddy & Rao, 1957; Mehta et al., 1971; Pindborg et al., 1971;
Reddy, 1974; Bhonsle et al., 1976). Reddy et al. (1960) simulated the
effect of reverse smoking experimentally by painting the skin of male
and female mice on alternate days with the tar from Indian cigars and
exposing the painted skin to a temperature of 58°C for 3 min; the heat
treatment enhanced the dermal tumour response.
2.2.4 Cardiovascular effects
Cigarette smoking is a major independent risk factor for
cardiovascular disease (CVD). Cigarette smoking acts synergistically
with other risk factors, such as elevated cholesterol levels and
hypertension (Wilhelmsen, 1977; Gibinski, 1977; US Surgeon General,
1983). Prospective studies indicated that elevated cholesterol and
hypertension appear to be prerequisites for CVD in cigarette smokers
(Kimura, 1977). It has been estimated that in countries with a long
history of cigarette smoking the tobacco habit is responsible for
26-30% of early deaths from CVD (US Surgeon General, 1983; Wald et
al., 1985). Those who smoke several cigars a day and inhale the smoke
also face an increased risk of CVD (NIH, 1998).
The major contributors to the cardiovascular effects of tobacco smoke
are carbon monoxide and nicotine (Lakier, 1992), as well as nitrogen
oxides (NOx), hydrogen cyanide and tar; minor contributors are
cadmium, zinc and carbon disulfide (US Surgeon General, 1983). The
smoke of cigarettes can be slightly acidic (pH 5.6-5.9) or weakly
alkaline (pH 6.7-7.9) depending on the type of tobacco and the blend
(Brunnemann & Hoffmann, 1974). In the slightly acidic smoke, nicotine
is protonated, i.e., bound to a salt or acid moiety and is part of the
particulate phase. Weakly alkaline smoke contains a small percentage
of protonated nicotine (often up to 50% of the nicotine is
unprotonated) in the vapour phase. In contrast to the protonated
variety, unprotonated nicotine is rapidly absorbed through the oral
mucosa and this is why smokers of cigarettes with weakly acidic smoke
and smokers of cigars do not need to inhale into the lung to
experience the pharmacological effects of nicotine. Protonated
nicotine in the particulate matter of slightly acidic cigarette smoke
is not or is only minimally absorbed through the oral mucosa, so that
smokers inhale the smoke and absorption into the bloodstream takes
place in the lung (Armitage & Turner, 1970; Russell, 1976; Benowitz et
al., 1988).
Smoking has been associated with a two-to-fourfold increased risk of
coronary heart disease (CHD), a greater than 70% excess rate of death
from CHD, and an elevated risk of sudden death (Lakier, 1992).
Nicotine causes increases in heart rate and blood pressure, stimulates
nerve endings that are activated by acetylcholine, causes increased
mobilization of free fatty acids in the serum and enhances platelet
adhesiveness. These effects increase cardiac load (which for
individuals with some forms of heart disease will not be met by
increased coronary blood flow) and interfere with metabolic exchange
across capillary walls, leading to ischaemic episodes and thrombosis.
Carbon monoxide increases carboxyhaemoglobin concentrations in the
blood and lowers its oxygen-carrying capacity: increased oxygen debt
after exercise and impairment of endurance performance are evident in
smokers, compared to non-smokers. Carbon monoxide also has an affinity
for myoglobin and interferes with oxygen uptake by the myocardium.
"Bidi" smoke contains a higher concentration of carbon monoxide than
cigarette smoke (Hoffmann et al., 1974; Jayant & Pakhale, 1985; Ball &
Simpson, 1987). Most of the Asian smoking devices with their
non-porous wrappers and dark tobacco contain higher levels of nicotine
(Simarak et al., 1977; WHO, 1985). High nicotine and carbon monoxide
are also obtained from many dark tobacco cigarettes and from cigars.
"Sheesha" water pipe smoke contains small amounts of nicotine but is
rich in carbon monoxide; the blood carboxyhaemoglobin concentration in
sheesha smokers is higher than in cigarette smokers (Zahran et al.,
1985).
Cigarette smoking has been considered to be the primary cause of
Buerger's disease (thromboangiitis obliterans), an inflammatory
obliterative, non-atherosclerotic, vascular disease. The disease
usually becomes quiescent if the patient stops smoking cigarettes
(Olin, 1994). It was rare in women but an increasing number of cases
has been observed and ascribed to the increased use of tobacco by
young women (Yorukoglu et al., 1993).
Cardiovascular disease has been associated with exposure to other
factors, which can be classified as physical, chemical and biological,
and with occupation or life-style. The combination of smoking with any
of these factors will predispose to an increased detrimental
cardiovascular effect.
In women who smoke, peripheral vasoconstriction (PV) leading to acute
intervillous placental blood flow was measured during smoking and
attributed to nicotine, which simultaneously caused increases in heart
rate and blood pressure. It was suggested that PV explained fetal
growth retardation and other complications of pregnancy (Lehtovirta &
Forss, 1978).
2.2.5 Smoking and occupational accidents, injuries and
absenteeism
A survey of public employees found that cigarette smokers took 23%
more sick leave than non-smokers (Van Tuinen & Land, 1986). In another
study among 2537 postal workers in the USA, cigarette smokers had a
1.29 times higher accident rate (CI 1.07-1.55), and a 1.55 times
higher injury rate (CI 1.11-1.77) than non-smokers (Ryan et al.,
1992). Other studies confirm the higher rates of injury, occupational
accidents and absenteeism in smokers (Naus et al., 1966; Parka, 1983;
US Surgeon General, 1985; Hawker & Holtby, 1988). There are higher
costs of illness for cigarette smoking employees compared to
non-smokers (Van Peenen et al., 1986; Penner & Penner, 1990.
2.3 Health risks from smokeless tobacco use
2.3.1 Introduction
Oral cancers have been linked with oral tobacco use. The consequences
of chewing tobacco can be reactional keratosis, irreversible gingival
recession, periodontitis, oral dysplasia and leukoplakia, cancer and
cardiovascular effects (Chakrabarti et al., 1991; Guggenheimer, 1991).
In India, chewing material containing tobacco has been shown to be a
primary cause of oral cancer (Jussawalla & Deshpande, 1971).
2.3.2 Cancer
Leukoplakia and oral cancer are common results of oral tobacco use,
particularly in the countries of Asia (IARC, 1985a). Simarak et al.
(1977) reported a strong association between betel chewing and oral
cancer in northern Thailand. Sankaranarayanan et al. (1989a,b, 1990)
associated oral cancers in southern India with tobacco chewing.
Chakrabarti et al. (1991) reported much higher levels of pre-malignant
and malignant lesions of the oral cavity in tobacco chewers;
Nandakumar et al. (1990) found the relative risk to be elevated in
both sexes, but appreciably higher in females. From chemical analysis
of Indian tobacco products, it was concluded that their use may lead
to high exposures to carcinogenic tobacco nitrosamines (Nair et al.,
1989).
Case-control studies reported a higher incidence of oral cancer in
oral snuff users than in those not using any form of tobacco. There
was up to a 50-fold excess risk of cancer of the cheek and gum in
long-term oral snuff users (Axéll et al., 1978; US Surgeon General,
1986a; Winn, 1997). Idris et al. (1991, 1994), reported a high
incidence rate of oral cancer in men in northern Sudan who used an
oral snuff with relatively high concentrations of nicotine (0.8-3.2%)
and nornicotine. In a study of baseball players who chewed or (the
majority) used snuff orally, there was higher prevalence of
leukoplakia, plaque formation, gingivitis and dental disorders
compared to non-users (Robertson et al., 1997).
A case-control study on nasal cavity and paranasal sinus cancer and
snuff use showed that snuff users have a significantly increased risk
for adenocarcinoma and squamous cell carcinoma in the nasal cavity
(Brinton et al., 1984). N'-nitrosonornicotine is present and is an
organ-specific carcinogen that induces benign and malignant tumours of
the nasal cavity in rats, hamsters and mink (Rivenson et al., 1991;
Koppang et al., 1992, 1997; Hoffmann et al., 1994). Studies conducted
in South Africa showed that people using local snuff made from tobacco
and aloe plant ash as nasal applications have an increased risk for
tumours of the maxillary antrum (Keen et al., 1955). It was thought
that this snuff mixture contained high levels of nickel and chromium,
which may be associated with the induction of these tumours (Baumslag
et al., 1971).
Instillation of snuff into the lips of rats induced benign and
malignant tumours in the oral cavity (Hirsch & Thilander, 1981; Hecht
et al., 1986; Johansson et al., 1989; Larsson et al., 1989).
Instillation of snuff into the buccal pouch of hamsters, which were
repeatedly infected with Herpes simplex virus type I or II, led to
oral tumours, although no tumours occurred when either the virus or
tobacco was applied alone (Park et al., 1991b).
More than 3050 chemical compounds have been identified as tobacco
constituents (Roberts, 1988) and 50 are known carcinogens (Brunnemann
& Hoffmann, 1992). Nitrosamines, especially the tobacco-specific
nitrosamines (TSNA), must be regarded as major oral carcinogens. Oral
tumours were elicited when an aqueous solution of N-nitrosonornicotine
(NNN) and 4(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) was
applied to the oral surfaces of rats (Hecht et al., 1993).
2.3.3 Cardiovascular disease
The effect of tobacco chewing on cardiovascular disease has received
less attention than the effect of cancer. Benowitz et al. (1988)
compared the cardiovascular effects of smokeless tobacco, cigarettes
and nicotine gum and reported that all tobacco use increased heart
rate and blood pressure. Nanda & Sharma (1988) recorded incremental
increases in heart rate and blood pressure following tobacco chewing.
However, Eliasson et al. (1991) found no significant elevation of
diastolic blood pressure in young snuff users. Bolinder et al. (1994)
found an excess risk of death from cardiovascular and cerebrovascular
disease among smokeless tobacco users. Tobacco chewing has detrimental
effects on pregnancy (Krishna, 1978) which, in the absence of any
anoxia due to carbon monoxide found in smokers, could result from
nicotine-induced vasoconstriction.
O'Dell et al. (1987) reported a case of Buerger's disease in a
38-year-old man that was associated with the use of smokeless tobacco.
A regimen which included complete abstinence from tobacco resulted in
a resolution of the symptoms. Buerger's disease is the commonest
vascular disease in the Indian subcontinent where tobacco consumption
is high (Jindal & Patel, 1992). In a study of the disease in
Bangladesh, 39 patients (38 males, 1 female) were investigated. All
but two were current long-term smokers, one male had given up smoking
6 months previously and the one woman with the disease (it is uncommon
in women) was a tobacco chewer (Grove & Stansby, 1992).
3. EFFECTS ON HEALTH OF TOBACCO USE AND EXPOSURE TO OTHER CHEMICALS
3.1 Introduction
3.1.1 Interaction
To discuss the interaction between tobacco and other agents as risk
factors for cancer and other adverse health effects, it is necessary
to define what is meant by the term "interaction". In general terms,
interaction represents a departure from additivity, in which the
combined effect of exposure to two agents is in some sense the sum of
the effects of the individual agents (US EPA, 1988). Synergism occurs
when the combined effect is greater than the sum of the component
effects; antagonism occurs when the effect of the combination is less
than would be suggested by summing the effects of the components.
3.1.2 Measuring interaction
To make these concepts precise, the scale in which risk is measured
and the manner in which risks are summed must be specified (Kaldor &
L'Abbe, 1990). In epidemiological investigations, the age-adjusted
relative risk is often used to characterize risk. In risk assessment
applications involving chronic health effects such as cancer, the
cumulative risk over a period of time may be of more direct interest.
In laboratory studies of carcinogenicity, for example, the lifetime
probability of tumour induction is often used to describe risk. In
order to take into account the age at which an adverse effect is
induced, the expected loss in life expectancy has also been used to
evaluate risk (UNSCEAR, 1988).
Kodell & Pounds (1991) discuss interaction in terms of departures from
response additivity and dose additivity. Dose additivity occurs
when the combined effect of two agents can be expressed in terms of a
total dose of the two agents, taking into account their relative
potencies. Dose additivity presumes that the two agents act by the
same biological mechanism, and that the effective dose of one agent is
simply a dilution of the dose of the other agent. Response additivity
occurs when the two agents act independently of each other. In this
case, the probabilities of an adverse effect due to each of the agents
can be treated as statistically independent and can be combined
accordingly.
The quantification of interaction may be illustrated using the
age-specific relative risk (A,B) associated with exposure to two
agents A and B. The relative risk is formally defined as:
relative risk (A,B) = I(A,B)/I(0,0)
where I(A,B) denotes the incidence rate of the disease of
interest at a specified age in the presence of exposure and
I(0,0) is the incidence rate in the absence of exposure. Lack of
interaction then corresponds to additivity of the excess relative
risk (ERR = relative risk - 1).
Specifically,
ERR(A,B) = ERR(A,0) + ERR(0,B)
where ERR(A,0) and ERR(0,B) denote the excess relative risks for
A and B alone, respectively. In terms of relative risk,
additivity is equivalent to:
relative risk (A,B) = relative risk (A,0) + relative risk
(0,B) - 1
For a supra-additive relative risk relationship, such as the
multiplicative relative risk model:
relative risk (A,B) = relative risk (A,0)RR(0,B)
and reflects a synergistic effect.
It is of interest to note that as the relative risk (A,0) and relative
risk (0,B) become small, the multiplicative relative risk model
approximates the additive relative risk model. Under this
multiplicative model, a strong synergistic relationship may be
apparent at high exposures, yet become negligible at low exposures.
This, along with the realization that relative risks of less than
about two are difficult to detect epidemiologically, suggests that
relatively high exposures are likely to be required to evaluate
interactive effects reliably.
Brown & Chu (1989) and Kodell et al. (1991) investigated the type of
interactions that might be expected under both the Armitage-Doll
multi-stage model and the Moolgavkar-Venzon-Knudson two-stage model of
carcinogenesis following exposure to two carcinogens that may affect
different stages in the model. These theoretical results indicate that
a variety of synergistic interactions are possible, including
supra-additive, multiplicative, and supra-multiplicative.
Although the additive excess relative risk model described above
provides a useful baseline for evaluating interaction, it is not the
only model that has been proposed for this purpose. Kaldor & L'Abbe
(1990) pointed out that the multiplicative relative risk model will
become the baseline model for evaluating interactions following a
logarithmic transformation of the relative risk. Thomas & Whittemore
(1988) review arguments in favour of the additive and multiplicative
models as the basis for evaluating interaction. Steenland & Thun
(1986) illustrate how these two models can be applied in evaluating
tobacco/occupation interactions in the causation of lung cancer.
This brief overview illustrates that interaction can be measured in
different ways, with the most appropriate depending on the nature of
the problem at hand. In general, however, all of the commonly
encountered measures of synergism indicate that the risk associated
with combined exposure to two agents is in some sense greater than
would be expected based on the risks of individual exposures. Although
no attempt is made throughout this monograph to systematically specify
the precise nature of the interactive effects reported in the
literature, most interactive effects documented in this monograph have
been identified through epidemiological investigations, in which the
additive age-specific relative risk model is the predominant approach
to describing interaction (Saracci, 1987; Greenland, 1993).
There are four principal ways in which tobacco smoke can interact with
other chemicals to impair the health of the smoker. They are not
mutually exclusive and in fact there are many situations in which they
may occur together, particularly in the workplace or the environs of
industry.
a) Modification of effects
Cigarette smoke can modify the harmful effects associated with other
toxic agents, in some cases causing a highly elevated risk, e.g., the
effects of smoking on diseases related to asbestos, alpha-radiation,
arsenic and some organic compounds.
b) Increased concentration effects
Chemical compounds hazardous to health are often found in both tobacco
smoke and the working environment and each source can augment the dose
obtained from the other, e.g., carbon monoxide, acrolein, benzene and
heavy metal elements.
c) Vector effects
Materials used in the workplace that produce harmful chemical agents
when they are burnt or vaporized can contaminate smoking materials and
cause the smoke to be far more injurious when the tobacco is smoked,
e.g., polytetrafluoroethylene and methylparathion.
d) Other interactions
Tobacco smoke can affect a physiological process and increase the
impairment of physical or physiological functions caused by another
activity. For instance, impaired lung clearance will affect the
residence time of inhaled toxic materials, the effect of smoking on
the peripheral vascular system can enhance the detrimental effects of
vibration and noise, and smoking may alter the effect of drugs taken
for other purposes.
3.1.3 Effects of tobacco smoking on lung deposition and clearance of
particles
Pulmonary deposition of inspired particles depends on their
physicochemical properties and on airway structure and geometry.
Mathematical models describing the deposition of particles in the
various airway sections show that in compromised airways, as is the
case in patients suffering from asthma and chronic obstructive lung
diseases, particle deposition is enhanced several-fold (ICRP, 1994).
Tobacco smoking can be an indirect cause of enhanced deposition of
inspirable particles. In addition, during tobacco smoking the
breathing pattern is changed to more frequent and deeper inhalation,
especially in the case of low-nicotine cigarettes, which can result in
an increased inhaled dose and dose rate of inspirable compounds (IARC,
1990).
Clearance of particles deposited in the lung is a complex
physiological process involving relatively rapid tracheobronchial
clearance, in which mucus is moved upward by ciliary action to the
pharynx and swallowed, and slower deep-lung clearance, in which
phagocytic cells remove inhaled particles. These processes are
balanced by the solubility of the inhaled particles, with relatively
insoluble particles having a longer residence time in the alveolar
portions of the lungs. A longer residence time in the lung would be
accompanied by a greater possibility that harmful effects could occur.
Both rapid and slow clearance phases are reduced by smoking, although
probably by different mechanisms. Cigarette smoke contains significant
concentrations of ciliatoxic agents, such as hydrogen cyanide,
formaldehyde, acetaldehyde, acrolein and nitrogen oxides, which
greatly contribute to retarded clearance of inhaled particles by
inhibiting lung clearance mechanisms (Battista, 1976). Retardation of
clearance has been seen for airway-deposited particles, in which
decreased mucus transport velocities slow this normally relatively
rapid phase of clearance (Lourenco et al., 1971; Chopra et al., 1979).
The deep-lung clearance of relatively insoluble particles is retarded
in smokers. Cohen et al. (1979) found that 1 year after a tracer
particle exposure, some 50% and 10% of the original lung burden
remained in the lungs of smokers and non-smokers, respectively.
Bohning et al. (1982) and Philipson et al. (1996) found that smoking
retarded long-term particle clearance from the lungs. The mechanism(s)
for interference with this longer-term phase of clearance has not been
shown definitively, but may be related to impairment of phagocyte
function and/or smoke-induced lung damage.
3.2 Interactions between tobacco smoke and other agents
3.2.1 Asbestos
Asbestos is a generic name for a group of fibrous silicates, differing
in colour, fibre arrangement and length. Recognition of the health
risks of asbestos has led to major reductions in production and uses.
Asbestos types are classified according to their physical
characteristics as serpentine or amphibole and differ in their
relative carcinogenic potential. Amosite and crocidolite are
amphiboles and have short and straight needle-like fibres. Chrysotile
is a serpentine and consists of long, pliable white fibres. The longer
fibre varieties of asbestos can be spun into yarn which can be woven
into fabric; short fibre varieties can be incorporated into cement,
asbestos board and tiles. Asbestos products have been used in a
variety of applications including electrical and thermal insulation in
buildings, fire and safety equipment, brake linings of motor vehicles,
and shipbuilding. Workers in asbestos mining and processing and a wide
range of manufacturing industries are exposed to various forms of
asbestos, while others are exposed in maintenance work, demolition and
recycling operations.
Occupational exposure to asbestos is associated with asbestosis and
cancers at various sites, notably pleural mesothelioma and lung
cancer. Differences between the effect of asbestos on the health of
smokers and non-smokers have been reported, and studies have been
conducted aimed specifically at elucidating the combined effects of
smoking and asbestos exposure. Perioccupational exposure to asbestos
is a hazard to household contacts of asbestos workers, who bring home
dust on their clothes, and to people living in areas where there is
environmental contamination by asbestos dust from industry (Anderson
et al., 1979).
The amphibole varieties of asbestos (crocidolite and amosite) have the
highest carcinogenic risk. Crocidolite presents a greater risk than
amosite, which in turn is more dangerous than chrysotile, a serpentine
variety. Erionite and tremolite are non-asbestos fibrous minerals used
in building in some parts of the world and there is a high prevalence
of mesothelioma in these regions (Baris et al., 1979; Yazicioglu et
al., 1980).
Because there are many different occupations and environmental
situations in which asbestos exposure might occur, along with a wide
range of possible levels of exposure and variety of types of asbestos
in use, it is difficult to define clearly asbestos exposure or the
smoking habits of those exposed. The smoking history of the population
sampled is important, because there have been changes in smoking
materials and prevalences of smoking in many countries (Cheng & Kong,
1992). In many studies, only the number of smokers within sub-groups
of workers with asbestos-related disease have been reported, rather
than the detailed smoking habits of the exposed population. A widely
used assumption is that the smoking habits of asbestos-exposed workers
reflect those of blue collar workers and are thus higher than national
average figures. Table 5 gives examples of smoking prevalence in
different groups of asbestos-exposed workers.
3.2.1.1 Asbestos and lung cancer
Exposure to asbestos dust carries a risk of parenchymal and pleural
fibrosis, mesothelioma and lung cancer. Selikoff et al. (1968) and
Berry et al. (1972) showed that cigarette smoking was an added hazard
among asbestos workers. In combination, the two hazards are associated
with very high lung cancer rates. Studies were carried out (e.g.,
Hammond & Selikoff, 1973; Martischnig et al., 1977; Hammond et al.,
1979; Selikoff et al., 1980; Acheson et al., 1984; Berry et al., 1985)
to determine whether cigarette smoke and asbestos act independently,
Table 5. Smoking prevalence in asbestos-exposed workers
Exposure Smoking habits References
Asbestos textile workers 75% smokers Weiss (1971)
46% cigarette smokers
36% ex-cigarette smokers
5.5% pipe/cigar smokers
Electrochemical plant 84% to 87% were smokers
(two areas) or ex-smokers Kobusch et al. (1984)
Population in Telemark, Asbestos exposed: Hilt (1986)
Norway 44.6% smokers
36.0% ex-smokers
Not exposed:
40.95% smokers
28.6% ex-smokers
Survey of 800 000 American Asbestos exposed: Stellman et al. (1988)
men and women in 1982 33.6% smokers
47.3% ex-smokers
Lung cancer case-referent Men: 95% smokers Järvholm (1993)
study; Swedish industrial city Women: 78% smokers
Shipyard workers in 46% smokers Sanden et al. (1992)
Gothenburg, Sweden 31% ex-smokers
December 1987 21% non-smokers
2% not known
Asbestos factory workers Men: 74% smokers Newhouse & Berry (1979)
(male population average 66%)
Women: 49% smokers
(female population average 40%)
their combined effect being the sum of the individual effects, or
there is an interaction with the ultimate effect being a product of
the two risk factors. In some studies, the effects of smoke and
asbestos appeared to be additive, in others multiplicative and in
others somewhere between the two. Reasons for the lack of consistency
among the studies may relate to the size of the population sampled,
its average age, social class and residential area, the type of
asbestos involved, the time scale covered and the intensity of
exposure to asbestos. The weight of evidence favours a synergistic or
multiplicative model for the interaction of asbestos and smoking.
While the differences may be partly linked to the carcinogenic
potential of different types of asbestos and to different smoking
materials and ways of smoking, including passive smoking (Cheng &
Kong, 1992), they also reflect the complex nature of tobacco smoke,
which contains complete carcinogens, tumour promoters and
co-carcinogens and other compounds that can influence the multistage
carcinogenic process. However, whatever the type of smoking/asbestos
interaction influencing the incidence of lung cancer, there is a
greatly increased risk for the asbestos-exposed worker who smokes
(Table 6).
Hammond et al. (1979) found a very strong synergistic effect and this
was supported by studies of shipyard workers in Italy (Bovenzi et al.,
1993), asbestos factory workers in London (Newhouse & Berry, 1979),
Finnish anthophyllite miners and millers (Meurman et al., 1979),
chrysotile workers in China (Cheng & Kong, 1992; Zhu & Wang, 1993) and
workers exposed to crocidolite in Western Australia (de Klerk et al.,
1991). Cheng & Kong (1992) reported a lower ratio of non-smoking to
smoking lung cancer death rates and suggested that this reflected
passive smoking among non-smokers and the use by most smokers of
hand-rolled cigarettes. Liddell et al. (1984) found that their data
fitted both an additive model and a multiplicative model and concluded
that the combined relative risk lay somewhere between the two.
Selikoff et al. (1980), from a study of amosite factory workers, and
Berry et al. (1985), from a study of asbestos factory workers,
favoured an additive model. However, caution is required because of
the definitions of additive and multiplicative used by different
authors and the overlap between these terms and such words as
synergism and promoter.
Molecular biology studies of autopsy specimens of lung tumour tissue
from of cigarette smokers have revealed that cigarette smoking induces
K-ras mutation (Rodenhuis & Slebos, 1992). It has been suggested that
such cigarette-smoke-induced K-ras oncogene mutations are promoted by
the presence of asbestos, which creates selective growth conditions
for the mutated cells (Vainio et al., 1993). Vainio & Boffetta (1994)
concluded that both tobacco smoke and asbestos fibres can be genotoxic
and cytotoxic, and cause proliferative lesions in the lungs. Tobacco
smoke contains carcinogens that bind to critical genes and cause
mutations. Asbestos fibres cause chronic inflammation of the lungs,
which releases various cytokines and growth factors, and may provide a
selective growth advantage for mutated cells.
Table 6. Age-standardized lung cancer death ratesa for cigarette smoking and/or
occupational exposure to asbestos dust compared with no smoking and no occupational
exposure to asbestos dust (from: Hammond et al., 1979)
Group Exposure to History cigarette Death Mortality Mortality
asbestos? smoking? rate difference ratio
Control No No 1.3 0.0 1.00
Asbestos workers Yes No 58.4 +47.1 5.17
Control No Yes 122.6 +111.3 10.85
Asbestos workers Yes Yes 601.6 +590.3 53.24
a Rate per 100 000 man-years standardized for age on the distribution of the
man-years of all the asbestos workers; number of lung cancer deaths based on
death certificate information
3.2.1.2 Asbestos and pleural mesothelioma
There is an established relationship between exposure to asbestos
- crocidolite, amosite, chrysotile - and pleural mesothelioma
(Stellman, 1988). In shipyard workers mainly exposed to chrysotile,
Sanden et al. (1992) found an increase in pleural mesotheliomas up to
15 years after cessation of exposure. Asbestos is also linked with
peritoneal mesothelioma (Newhouse & Berry, 1976). The risk of lung
cancer was found to fall after exposure ceased, suggesting that
asbestos acted as a lung cancer promoter, but the risk of mesothelioma
long after cessation of exposure indicated that asbestos acted as a
complete carcinogen. Mesothelioma can have an extremely long latent
period, with cases presenting even 30 years or more after first
exposure (Newhouse & Berry, 1976). Up to 90% of cases of pleural
mesothelioma have been attributed to asbestos but there is no evidence
directly associating smoking with the disease, or showing that smoking
has any influence on the incidence of asbestos-related pleural
mesothelioma (Berry et al., 1985; Hughes & Weill, 1991; Sanden &
Jarvholm, 1991; Muscat & Wynder, 1991).
3.2.1.3 Asbestos and other forms of cancer
Asbestos fibres have been found in many tissues, other than the lungs,
of asbestos workers. There is evidence that an asbestos/smoking
interaction increases the incidence of cancer of the oesophagus,
pharynx, buccal cavity and larynx but not of pleural or peritoneal
mesothelioma, or of cancer of the stomach, colon-rectum or kidney, for
which smoking and non-smoking asbestos workers are at equal risk
(Hammond et al., 1979; Selikoff & Frank, 1983; US ATSDR, 1995).
3.2.1.4 Asbestosis
Asbestosis is a fibrotic reaction to asbestos in the lungs. In a
review of histological, animal experimental and radiological evidence,
Weiss (1984) concluded that cigarette smoking could result in diffuse
fibrosis similar to that caused by asbestos, and the fibrosis showed a
dose-response to the duration and degree of smoking. Prevalence
studies are consistent in showing a higher frequency of diffuse small
irregular opacities in asbestos workers who are smokers than in those
who are non-smokers. It has been suggested that the effects may be
additive. Tobacco smoke affects lung clearance and hence the retention
of asbestos fibres in the lungs. In asbestosis the intensity of
fibrosis correlates with the number of asbestos bodies in the lungs,
and Murai et al. (1994) concluded that reduction of lung clearance by
tobacco smoke could increase the intensity of fibrosis. Crocidolite
fibres are the most fibrogenic of the various types of asbestos but
De Klerk et al. (1991) concluded that smoking had no measurable effect
on crocidolite asbestosis.
An interaction between asbestos and smoking causing a greater
frequency of obstructive airways disease in asbestos workers who smoke
was found in a study of pulmonary function changes caused by
asbestosis (Selikoff & Frank, 1983). Miller (1993) presented similar
results suggesting an interaction between asbestos and smoking. In a
prospective mortality study, Hughes & Weill (1991) concluded that
asbestosis is a precursor of asbestos-related lung cancer, but they
were unable to assess an interaction between tobacco smoking and
asbestosis because all the cases were in smokers and there were no
non-smokers.
In rats, asbestos fibres stimulate alveolar macrophages to generate
the inflammatory and fibrogenic mediators, tumour necrosis
factor-alpha (TNF-alpha), and this may be the cause of inflammation
and lung fibrosis due to asbestos (Ljungman et al., 1994). In in vitro
studies Morimoto et al. (1993) found synergism between chrysotile
fibres and cigarette smoke in the stimulation of the formation of
TNF-alpha by rat alveolar macrophages.
3.3 Non-asbestos fibres
3.3.1 Glass fibre
IARC (1988) classified glasswool as possibly carcinogenic to humans
(Group 2B) and glass filaments as not classifiable as to their
carcinogenicity to humans (Group 3), based on sufficient evidence for
the carcinogenicity of glasswool and inadequate evidence for the
carcinogenicity of glass filaments in experimental animals and
inadequate evidence for the carcinogenicity of glasswool and glass
filaments in humans. There are data on exposure to glass fibre and
tobacco smoke. Enterline et al. (1987a) carried out a case control
study of 7586 glasswool workers in four plants producing small
diameter fibres, less than 3 µm in diameter. Smoking histories were
obtained for 75% of the workers. Analysis of data by logistic
regression showed that smoking was a powerful variable and multiplied
the effect of fibre exposure. In a case-control study of the influence
of non-workplace factors on respiratory disease in employees of a
glass fibre manufacturing facility, Chiazze et al. (1992, 1995)
concluded that smoking, and not exposure to glass fibre, was the most
important risk factor for the increased lung cancer risk but was not
as important for non-malignant respiratory disease. In a further
analysis, using data not previously available, Chiazze et al. (1995)
estimated the extent of confounding by cigarette smoking, and
suggested that adjusting for the confounding effect could reduce the
lung cancer standardized mortality ratio to a non-statistically
significant level.
3.3.2 Rockwool, slagwool and ceramic fibres
IARC (1988) concluded that there was limited evidence for the
carcinogenicity of rockwool and inadequate evidence for the
carcinogenicity of slagwool in experimental animals, with limited
evidence for the carcinogenicity of rock-/slagwool in humans: the
overall evaluation for both was Group 2B, possibly carcinogenic to
humans. For ceramic fibres there was sufficient evidence for their
carcinogenicity in experimental animals, with no data on their
carcinogenicity in humans: the overall evaluation for ceramic fibres
was also Group 2B, possibly carcinogenic to humans.
In a study of insulation workers using rock and glass wool, (Clausen
et al., 1993) concluded that exposure was associated with an increased
risk of developing obstructive lung disease. In a study of respiratory
health in 628 workers in seven European plants manufacturing ceramic
fibres, skin, eye and nasal irritation, breathlessness and wheezing
were common findings (Trethowan et al., 1995). Respiratory symptoms
were more frequent in smokers and increased with the amount smoked.
The authors concluded that exposure caused irritation, similar to that
caused by other man-made fibres, and that cumulative exposure could
cause airways obstruction by promoting the effects of cigarette smoke.
Ljungman et al. (1994) demonstrated in rats that rock wool, slag wool,
kaolin ceramic fibre and silicon carbide fibre stimulated alveolar
macrophages to generate tumour necrosis factor-alpha (TNF-alpha), a
potent inflammatory and fibrogenic mediator. In in vitro studies
Morimoto et al. (1993) found synergism between mineral fibres
(chrysotile and alumina silicate ceramic fibres) and cigarette smoke
in the stimulation of the formation of TNF-alpha by rat alveolar
macrophages. Leanderson & Tagesson (1989) found that cigarette smoke
potentiated the DNA-damaging effect of man-made mineral fibres
(rockwool, glasswool and ceramic fibres).
3.4 Inorganic chemicals
3.4.1 Arsenic
Compounds of arsenic have been used as pesticides and as preservatives
of wood and leather. Arsenic is present in many metal ores and is
released during smelting Radon progeny are frequently encountered as a
contaminant of arsenic. In some parts of the world arsenic is found in
drinking-water in relatively high concentrations.
Arsenic and its compounds are carcinogenic (WHO, 1980; IARC, 1987;
Tsuda et al., 1990, 1995). Skin cancer can occur after ingestion of
arsenic (Tseng et al., 1968; Smith et al., 1992), and lung cancer
after inhalation of arsenic by smelter workers or by people living
nearby (Welch et al., 1982; Pershagen, 1985; Pershagen et al., 1987)
or by agricultural workers exposed to the pesticide lead arsenate
(Wicklund et al., 1988). IARC (1987) classed arsenic and arsenic
compounds as Group 1, carcinogenic to humans. It has been suggested
that arsenic in drinking-water may also cause liver, lung, kidney and
bladder cancer (Smith et al., 1992).
A study of the lung cancer risk among cadmium-exposed workers
suggested that exposure to arsenic and tobacco smoke may have been the
cause of an increased rate of lung cancer, rather than exposure to
cadmium particulates (Lamm et al., 1992). Tsuda et al. (1990)
suggested an interaction between arsenic and smoking in exposed
workers in a small Japanese village where arsenic was mined and
refined. However, the village water and air were highly polluted by
emissions from the smelter and from slag disposal, making interaction
between arsenic and smoking difficult to assess. A study of copper
smelter workers in the USA indicated that the effect of arsenic was
probably more important in lung cancer than that of tobacco smoke
(Welch et al., 1982). Studies in Sweden showed increased lung cancer
risks from arsenic exposure at a copper smelter; a multiplicative
effect for smoking and arsenic was found and age-standardized rate
ratios for lung cancer mortality were 3.0 for arsenic-exposed workers,
4.9 for smokers with no arsenic exposure and 14.6 for arsenic-exposed
smokers (Pershagen et al., 1981). In a later study, Pershagen (1985)
reported an additive effect for smoking and arsenic exposure on lung
cancer incidence in situations where the arsenic exposure was lower.
In a cohort of 3916 Swedish copper smelter workers, the risk of
developing lung cancer from the interaction between arsenic and
smoking was intermediate between additive and multiplicative and
appeared to be less pronounced among heavy smokers (Jarup & Pershagen,
1991).
There was no evidence of synergism between arsenic and tobacco smoke
in tin miners in Yunan Province, China. The lung cancer risk was
greater for arsenic than for smoking, and simultaneous assessment of
arsenic and radon exposure revealed radon to be the greater risk
(Taylor et al., 1989). In Ontario it was concluded that the excess
lung cancer mortality of gold miners and uranium miners was probably
due to exposure to arsenic and short-lived radon decay products
(Kusiak et al., 1991). This was consistent with the hypothesis that
the risk of lung cancer from exposure to arsenic is enhanced by
exposure to other carcinogens (Kusiak et al., 1993).
Hertz-Picciotto et al. (1992) assembled data from several studies to
examine possible synergism between smoking and exposure to arsenic and
an increased risk of lung cancer. The joint effect from both exposures
consistently exceeded the sum of the separate effects: a minimum of
30% to 54% of lung cancer cases among those with both exposures could
not be attributed to either one or the other exposure alone. The
conclusion was that arsenic and smoking acted synergistically to cause
lung cancer. Arsenic-induced lung cancer was not limited to exposure
to inhaled arsenic because there was evidence of synergism between
ingested arsenic and smoking (Tsuda et al., 1995). An association of
arsenic exposures with bladder cancer was confined to subjects who had
been smokers Bates et al. (1995).
In a Swedish study of lung cancer in arsenic workers, it was found
that cases among smelter workers who had never smoked showed a
histological distribution resembling that of smokers, probably
reflecting an exposure to carcinogenic agents at the smelter which
influence the risk of different histological types in the same way as
smoking (Pershagen et al., 1987). Tobacco smoking primarily induces
epidermoid and small cell carcinomas but there are also increased
risks for other cell types. The proportion of small cell carcinomas
was greater in uranium miners than in the general population (Kusiak
et al., 1993). In smokers, there were no pronounced differences in the
histological type of lung carcinomas between arsenic exposed smelter
workers and controls (Pershagen et al., 1987).
It has been suggested that the potentiation of the carcinogenic
properties of arsenic by smoking could be due to inorganic arsenic
requiring a strong co-carcinogen to manifest a carcinogenic effect, or
that arsenic itself might be acting as co-carcinogen rather than as a
direct carcinogen (Stohrer, 1991; Tsuda et al., 1995).
3.4.2 Beryllium
Beryllium is a metal with a number of uses including alloys, nuclear
energy applications, and in the rocket and aerospace industry (IPCS,
1990; IARC, 1993). Fine dusts and fumes of the metal and some of its
salts are hazardous and when inhaled are deposited in the lungs from
where beryllium may be widely distributed throughout the body.
Beryllium metal, oxide and some salts give rise to acute inflammation
on skin contact, particularly when accompanied by friction or
perspiration. Short exposure to dusts and fumes can cause acute
inflammation of mucous membranes: conjunctivitis, bronchitis,
pneumonitis. Granulomatous reaction can follow chronic inflammation of
the skin, and lesions may appear in the liver and elsewhere after long
periods of absorption from the lungs. Beryllium and its compounds are
a cause of delayed pneumonitis and pulmonary granulomas. IARC (1993)
classified beryllium and beryllium compounds as Group 1, carcinogenic
to humans, on the basis of sufficient evidence in humans and in
experimental animals. However, in epidemiological studies the
information on smoking was incomplete and the data did not rule out
the possibility that the few excess deaths observed could have been
due to smoking rather than to any other cause (Steenland & Ward, 1991,
1993; Eisenbud, 1993; MacMahon, 1994; Kotin, 1994).
The prevalence of chronic beryllium disease (CBD) is reduced in
smokers compared with non-smokers. The disease is preceded by the
development of beryllium-specific sensitization. In two studies
examining different worker populations, the prevalence of smoking in
those with clinically diagnosed CBD was lower than in those that were
sensitized but did not have the disease (Kreiss et al., 1993; Kreiss
et al., 1996).
3.4.3 Chromium
Chromium and its compounds are used in metallurgical, chemical,
electroplating and leather tanning industries (IARC, 1990). The
principal route of entry to the body is through the lungs. Chromium
ulcers of the skin and dermatitis can result from handling chromium
products and deposition of chromates on mucous membranes can also
cause ulceration which, in the nasal septum, can lead to perforation
(Lindberg & Hedenstierna, 1983; IARC, 1990). Chromium and some
chromium compounds are respiratory tract sensitizers and a cause
asthma. Hexavalent chromium salts have been associated with lung
cancer both in experimental animals and in epidemiological studies.
IARC (1990) concluded that there is sufficient evidence in humans for
the carcinogenicity of hexavalent chromium compounds as encountered in
chromate production, chromate pigment production and chromium plating
industries.
Langård & Norseth (1975) suggested that cigarette smoking increases
the risk for lung cancer in workers exposed to chromate dust. Other
studies (Abe et al., 1982; Langård & Vigander, 1983; Yoshizawa, 1984;
Nishiyama et al., 1985) have suggested that workers with exposure to
chromium compounds who are also smokers may be at greater risk than
non-smokers. However, the numbers were too small for conclusions on
interactions to be drawn.
3.4.4 Nickel
Exposure to nickel or its compounds occurs in mining, refining,
smelting and alloying the metal, in nickel plating and in welding. It
is used in battery manufacture, electroplating, enamelling, ceramics,
the chemical and petroleum industries and in dyestuffs and ink making.
Exposure may be by skin contact or inhalation of dusts, fumes, mists
or gaseous nickel carbonyl (IPCS, 1991a). In occupational exposure the
daily intake and absorption/retention vary widely between industries
(IARC, 1990).
Nickel is absorbed from the soil by the tobacco plant. During smoking,
up to 20% of the nickel in the tobacco is transferred to mainstream
smoke. This high transfer rate, compared to the much lower transfer
rates of other metals, has been explained by the formation of the
volatile nickel carbonyl (Sunderman & Sunderman, 1961). Nickel
carbonyl is a strong lung carcinogen in rats (IARC, 1990).
IARC (1990) evaluated the carcinogenicity of nickel and nickel
compounds and classified nickel compounds as carcinogenic to humans
(Group 1) and nickel as possibly carcinogenic to humans (Group 2B). In
an epidemiological study on a cohort of 916 workers in a Norwegian
nickel refinery, four work categories were defined: i) roasting and
smelting; ii) electrolysis; iii) other processes; and iv) other work
groups. All groups showed an excess risk of respiratory cancer. In the
roasting and smelting department there were excess risks for lung
cancer (O/E = 12/2.5) and nasal cavity cancer (O/E = 5/0.1). In the
electrolysis department there were also excess risks of lung cancer
(O/E = 26/3.6) and nasal cavity cancer (0/E = 6/0.2) (Pedersen et al.,
1973). Magnus et al. (1982) updated this study and found evidence of
an additive effect of smoking and nickel exposure in the induction of
respiratory cancer. Histological examination of nasal biopsy specimens
from 59 retired nickel workers, 21 of whom were smokers and snuff
users, showed a higher score of nasal epithelial dysplasia in smokers
than in non-smokers, and 4 workers with nasal carcinoma were smokers
(Boysen et al., 1984). In monitoring nickel exposure by imaging
cytometry of nasal smears (Reith et al., 1994), it was possible to
distinguish between workers who were exposed to different nickel
compounds and to distinguish between smoking and non-smoking nickel
workers.
3.4.5 Manganese
There is occupational exposure to manganese in its mining, the
ferromanganese and iron and steel industries, the production of dry
cell batteries, and the manufacture and use of welding rods. Manganese
is released by the combustion of the gasoline additive,
methylcyclopentadienyl manganese tricarbonyl (MMT), used in some
countries. However, the amount in gasoline (approximately 10 mg/litre)
and emitted in vehicle exhausts is small and does not to lead to human
exposure (Health Canada, 1994). The principal route of entry of
manganese is through the lungs but, because most of the compounds are
insoluble, only the smallest particles, as contained in furnace and
welding fume, are capable of reaching the alveoli and being
phagocytosed and absorbed (IARC, 1986).
Manganese is present in tobacco leaves and it has been reported (IARC,
1986) that 0.003 µg of manganese appears in the mainstream smoke from
one cigarette and thus contributes to a smoker's manganese intake in
the form of small and dangerous particles.
Manganese is neurotoxic and long-term occupational exposure can cause
a condition resembling Parkinson's disease. It causes lung damage
leading to an increased incidence of pneumonia and a higher rate of
acute and chronic bronchitis. In studies of manganese alloy production
workers and chronic lung disease, smokers were more affected than
non-smokers and the relationship between the number of cigarettes
smoked and the prevalence of respiratory tract symptoms suggested that
smoking acted synergistically with manganese (Saris & Lucic-Palaic,
1977). In a study of workers producing manganese salts and oxide
(Roels et al., 1985) smoking and manganese exposure were additive in
producing preclinical toxic effects. In studies on workers producing
iron-manganese alloys, one concerned with chronic bronchitis
(Misiewicz et al., 1994) and the other with pulmonary ventilatory
disturbance (Misiewicz et al., 1992), there was no relationship
between the occupational exposure or its duration and health effects.
The chronic bronchitis and ventilatory disturbance were attributed to
cigarette smoking.
3.4.6 Platinum
Platinum is used as a catalyst in many chemical processes and in motor
vehicle exhausts. Chloroplatinic acid is an intermediate in the
preparation of a large number of complex salts, which are used in
platinum refining, the chemical industry and, therapeutically, in
cancer chemotherapy.
Platinum salt sensitivity is an IgE-mediated immune response (IPCS,
1991b). Ammonium hexachloroplatinate is used as an intermediate in
platinum refining, and its inhalation provokes asthmatic responses and
elicits immediate skin test responses in sensitized individuals (Pepys
et al., 1972). In a cohort study of 91 workers in a platinum refinery
(Venables et al., 1989), 22 developed respiratory symptoms and an
immediate skin test response to ammonium hexachloroplatinate. The risk
was greatest in the first year of employment and smokers had an
increased risk of becoming sensitized. In another study, out of 78
workers at a platinum refinery, 32 (41%) had developed platinum salts
sensitivity after 24 months of exposure and the risk of sensitization
was about 8 times greater for smokers (Calverley et al., 1995).
Baker et al. (1990) conducted a cross-sectional study of respiratory
and dermatological effects of platinum salt sensitization in 136
workers (107 current employees and 29 former employees) at a precious
metal refinery. Twenty three workers (22%) had become sensitized.
Platinum salts sensitivity was not associated with atopicity but was
strongly associated with cigarette smoking status.
3.4.7 Silica
Silicosis (lung fibrosis caused by silica) is not only a hazard of
mining. It is also found in bricklayers, cement makers, workers in
pottery, porcelain and ceramics, rock drillers, workers chipping,
grinding or polishing stone, in sandblasting, using grinding stones to
smooth or polish precious stones, metals or optical glass, and in the
manufacture of polishing materials such as metal polishes and
toothpaste. The number of industries generating silica dust is large.
The amount of respirable dust varies from one to another and, because
silica is an active adsorbent, it can become contaminated and have its
toxic potential changed. Furthermore, freshly fractured silica dust
may exhibit a different surface reactivity and cytotoxicity from that
of aged silica (Vallyathan et al., 1988).
Hnizdo & Sluis-Cremer (1991), in a study of gold miners, linked high
exposure to silica dust with lung cancer and found a combined effect
of dust and smoking that fitted a multiplicative model for lung
cancer. Amandus et al. (1992) studied lung cancer in men with
diagnosed silicosis and suggested that there was an association
between the two diseases. In a study of iron foundry workers
(Andjelkovich et al., 1994), cigarette smoking was a strong predictor
of lung cancer whereas silica exposure showed no association with the
disease.
Chronic silicosis develops after 20 to 40 years of exposure to silica
dust. There are also other types of pneumoconiosis related to the
nature of the dust, and chronic bronchitis and airways obstruction
have been associated with silica dust exposure. A hypothesis for the
pathogenesis of chronic silicosis is that silica particles are
phagocytosed by the alveolar macrophages for which they have a marked
selective toxicity. Permanent macrophage activation initiates
inflammatory reactions leading to the formation of collagenous fibres.
Acute silicosis arises from the inhalation of more highly reactive
silica (Vallyathan et al., 1988). The link between silicosis and
smoking was examined in a study of smoking and silica exposure on
pulmonary epithelial permeability. Faster clearance of a radioaerosol
from the upper lung regions was found for smokers (Nery et al., 1988,
1993). The question of silica clearance was considered in an analysis
of an association between silicosis and smoking: differences in
collagenization for smokers and non-smokers were attributed to
differences in the interception of silica particulate matter by mucus
(Hessel et al., 1991). In studies by Ng et al. (1987, 1992), smoking
was not considered to affect the progression of silicosis in granite
quarry workers. However, examining the association of silicosis with
lung cancer, Ng et al. (1990) concluded that the excess lung cancer
risk in silicosis is attributable to smoking, and there appeared to be
a synergistic effect between smoking and silica/silicosis regarding
the risk of developing lung cancer.
In a study of 562 South African gold miners exposed to low levels of
dust with a high (50-70%) silica content, the incidence of chronic
bronchitis was higher than in non-dust-exposed controls. Although the
percentage of smokers was higher in those with chronic bronchitis in
both groups, there was a significant excess in the dust-exposed
smokers (Sluis-Cremer et al., 1967). The authors concluded that there
was a factor in dust-exposed smokers that increased the incidence of
chronic bronchitis above that expected from smoking alone. In a study
of 2209 South African gold miners and 483 non-miners on the effect of
silica dust and tobacco smoking on mortality from chronic obstructive
lung disease, it was found that miners who smoked and were exposed to
silica dust were at higher risk of dying from chronic obstructive lung
disease than smokers not exposed to silica dust. In South African gold
mines about 30% of the respirable dust is free silica. It was
concluded that tobacco smoking and silica dust acted synergistically
in causing chronic obstructive lung disease (Hnizdo, 1990). Hnizdo et
al. (1990) applied additive and multiplicative relative risk models to
the same sample and found that departure from additivity increased
progressively with the severity of obstructive impairment. They
concluded that tobacco smoking potentiated the effect of dust in
causing respiratory impairment and that severe respiratory impairment
could have been prevented through elimination of tobacco smoking
(Hnizdo et al., 1990). Oxman et al., (1993) analysed the relationship
between occupational dust exposure and chronic obstructive lung
disease in both gold and coal miners and found a significant
association between loss of lung function and cumulative respirable
dust exposure, which was greater in gold miners. In this study there
was no evidence of interaction with tobacco smoking for gold miners,
and the authors suggested that the increased risk of lung function
loss was due to exposure to dust having a higher silica content than
coal dust. Among iron ore miners in Sweden, Jörgensen et al. (1988)
found a strong relationship between chronic bronchitis and smoking,
but not with working underground. The two risk factors, silica dust
and smoking, appeared to be additive but the smoking effect was far
greater than that of silica dust. In a study of small airway disease
in patients with silica dust exposure, with and without radiographic
evidence of silicosis, and smoking, Avolio et al. (1986) found no
differences in lung function and prevalence of small airways disease
with silicosis. However, in both groups small airway disease was
significantly related to tobacco smoking, indicating that this had a
more powerful effect than silicosis.
3.5 Organic chemical agents
Many organic compounds with properties covering a wide spectrum of
molecular structure and biological activity are encountered in a
variety of industries. The effects of a few compounds, some of which
are encountered in specific industries, and smoking have been studied.
Where organic compounds occur in both tobacco smoke and the workplace,
the effect of smoking becomes one of dose augmentation, although
modification of effect can also occur. Some organic compounds would
normally not be found in tobacco smoke but are present because
workplace materials have contaminated smoking materials and they are
then pyrolysed or volatilized during smoking.
3.5.1 Chloromethyl ethers
Chloromethyl methyl ether (CMME) and its contaminant bis(chloromethyl)
ether (BCME) are used in the synthetic chemical industry, in the
manufacture of ion exchange resins and in polymer production. They are
carcinogenic when inhaled, BCME more so than CMME. In a long-term
study of chemical workers, 93 had exposure to chloromethyl ethers and
22 died from lung cancer. Of 32 workers who had no exposure, 3 died
from lung cancer (Weiss & Boucot, 1975; Weiss, 1976, 1980, 1982). For
the 22 cases in of lung cancer in the exposed workers, a dose-response
relationship was established. In the groups with heavy occupational
exposure, there were fewer heavy smokers (>20 cigarettes per day)
than in the groups with lower occupational exposure. This
statistically significant shift might be explained by self-selection
of heavy smokers out of the high occupational exposure groups because
of the bronchial irritation that is caused by the exposure to
chloromethyl ethers, or cigarette smoking might have an antagonistic
activity (Steenland & Thun, 1986; Thomas & Whittemore, 1988; Weiss &
Nash, 1997).
3.5.2 Tetrachlorophthalic anhydride
Tetrachlorophthalic anhydride (TCPA) is used as an epoxy resin curing
agent. It is respiratory tract sensitizer and causes asthma (Schlueter
et al., 1978). In a study using a radio allergosorbent test with a
TCPA human serum albumin conjugate, specific IgE antibody was detected
in serum from 24 out of 300 factory floor workers exposed to TCPA. Of
these 24, 20 (83%) were current smokers, compared with 133 (48%) of
276 without antibody (p<0.01), and there was a weaker association
with atopy, defined by skin tests with common allergens. Smoking and
atopy interacted, the prevalence of antibody being 16% in atopic
smokers, 12% in non-atopic smokers, 8% in atopic non-smokers and none
in the non-atopic non-smokers. It was concluded that smoking may
predispose to, and interact with atopy in the production of specific
IgE antibody to this hapten protein conjugate.
3.5.3 Dyestuffs
There is an established relationship between bladder cancer and
exposure to certain aromatic amines encountered in the dyestuffs
industry, e.g., benzidine, 4-aminobiphenyl and 2-naphthylamine (IARC,
1987), and smoking is causally associated with bladder cancer (IARC,
1986; US Surgeon General, 1989). From an analysis of 991 cases by
Cartwright (1982), a significant risk of bladder cancer was associated
with cigarette smoking, and a dose-response relationship, based on
years of employment, was found in workers in dyestuffs manufacturing.
The risks were considered to be additive. Overall, there was a
significant risk of bladder cancer associated with cigarette smoking,
a risk ratio of 1.8 for males, and there were significant overall
risks associated with occupations such as those of process workers in
the dye manufacturing industry who had a risk of 2.9 for males. When
dye manufacturing process workers who were smokers were compared with
non-smoking workers, the risk for smokers was 4.6, while for
non-smokers the risk was 1.9.
Boyko et al. (1985) concluded that arylamines in the dyestuffs
industry posed a major threat of bladder cancer. However, there was
little evidence to support an effect due to smoking or an interaction
between smoking and occupational exposure.
In an area of Spain where 44% of the adult population worked in dyeing
and printing textile fabrics, there was an increased risk of bladder
cancer for smokers (OR 2.3) (Gonzalez et al., 1985). The estimated
risks for occupation and for smoking and occupational exposure were OR
5.5 and 11.7, respectively. The observed effect was multiplicative.
Tobacco smoke contains many amines, including the bladder carcinogens
4-aminobiphenyl (>9 ng/cigarette) and 2-naphthylamine (54
ng/cigarette) (Patrianakos & Hoffmann, 1979; Pieraccini et al., 1992;
Grimmer et al., 1995).
In a study of risk factors for bladder cancer in Spain (Bravo et al.,
1987), the results were considered to corroborate previous data that
bladder cancer does not have a single cause. Cigarette smoking was
considered an important cause but one which was additional to
urological disease or occupational exposure, among other factors. In a
study of men in Spain (Gonzalez et al., 1989), increased risks of
bladder cancer were found for textile workers (OR 1.97), mechanics and
maintenance workers (OR 1.86), and workers in the printing industry
(OR 2.06). The highest risk was in those who were employed in the
textile industry before the age of 25 and prior to 1960. Among
mechanics the highest risk was for those who started after the age of
25 and after 1960. The OR for smokers who had also been employed in
one of the high-risk occupations was 7.82, which is compatible with a
multiplicative effect of joint exposure to tobacco smoke and
occupational hazards. In an Italian study (D'Avanzo et al., 1990),
risk additivity was found for the interaction between tobacco smoke
and several occupations associated with bladder cancer but the
occupations were not specified. The bladder cancer risk for smokers of
black tobacco was higher (OR = 3.7) compared with smokers of blond
tobacco cigarettes (OR = 2.6). A higher risk for black tobacco than
for blond varieties and a protective effect for smokers of tipped
cigarettes was also reported in a study in Northern Italy where a
multiplicative effect for smoking and high risk occupations was also
found (Vineis et al., 1984).
Bartsch et al. (1993) correlated the higher incidence of bladder
cancer among smokers of black tobacco with high yield aromatic amines,
particularly 4-aminobiphenyl from black tobacco (5 times greater than
from blond tobacco). The concentrations of urinary mutagens and of
4-aminobiphenyl adducts in the blood were also higher in smokers of
black tobacco.
A Chinese study of bladder cancer incidence and mortality in workers
with benzidine exposure found a marked dose response and an elevated
risk for both producers and users of benzidine. Workers exposed to
benzidine who were smokers had a 31-fold risk, while the risk for
exposed workers who were non-smokers was 11-fold, and a multiplicative
interaction was suggested (Bi et al., 1992).
3.5.4 Polycyclic aromatic hydrocarbons
Tobacco smoke contains many polycyclic aromatic hydrocarbons (PAHs)
(IARC, 1986), a number of which, such as benz( a)pyrene and
dibenz( a,h)anthracene, are known to be carcinogenic (IARC, 1987;
Hoffmann & Hoffmann, 1997). PAHs are generated by incomplete
combustion of organic matter in many industrial processes and
constitute a hazard not only in occupations but also as environmental
pollutants, representing primary risk factors as lung and bladder
carcinogens. A tobacco smoker can obtain one dose of PAHs from tobacco
smoke and another from the industrial or environmental source.
Furthermore, an interaction of tobacco smoke and an occupational
hazard is a possibility. PAHs in the workplace are often accompanied
by many other toxic compounds, particularly irritants, and, in
addition to carcinogenic PAHs, tobacco smoke contains co-carcinogens
and tumour promoters as well as ciliatoxic agents, irritants and other
biologically active species.
PAHs occur in coal gas manufacture, coking oven fumes, aluminium
smelting, in the use of tar and asphalt, in oil refining and the
exhaust from internal combustion engines. They are frequently
accompanied by irritant fumes or aerosols and potentially harmful
particulate matter. There is a lack of smoking data for workers in
many of these industries, but it has been assumed that the smoking
prevalence is at least as high as the average for blue collar workers.
At a Norwegian smelter 69% of workers were smokers when the expected
prevalence of smoking was 52% (Abramson et al., 1989). The percentage
of smokers and ex-smokers among workers exposed to chemicals and coal
tar pitch in a 1982 survey of 800 000 men and women in the USA was
49.9% against 46.1% for the average worker (Stellman et al., 1988).
A Canadian study found a high prevalence of bladder cancer in
aluminium smelter workers, particularly among those employed in
Söderberg potrooms where carbon electrodes made from a mixture of
petroleum pitch and coal pitch are used and PAH levels are high
(Thériault et al., 1984). Changing electrodes, breaking the crust that
forms on top of the molten metal and cleaning out the "pots" are
activities that create air pollution by tar volatiles including
carcinogenic PAHs which, measured as benzo( a)pyrene, could reach a
concentration of 800 µg/m3/8 h (Bjorseth et al., 1978). High levels
of PAHs were found in urine samples from aluminium plant workers
(Haugen et al., 1986). Lung cancer rates among aluminium reduction
plant workers are also high (Gibbs & Horowitz, 1979). Tobacco smoke
appears to increase the risk. In a study by Thériault et al. (1984),
the numbers were too small to determine whether the interaction was
additive or multiplicative, but in another study (Bjorseth et al.,
1978) there was suggestive, but not conclusive, evidence that the
relative risks from combined exposure to tar volatiles and cigarette
smoke were multiplicative. In a study in which the preceding data were
augmented (Armstrong et al., 1986), the tar volatiles were confirmed
as the cause of bladder cancer and the results suggested that a
multiplicative risk arose from a combined exposure to tar volatiles
and cigarette smoke.
Gullvåg et al. (1985) found that the alveolar macrophage count for
workers in the potrooms of an aluminium reduction plant was elevated
and for workers who were also smokers the count was further elevated.
The conclusion was that smoking and workplace pollution act
synergistically in increasing the number of alveolar macrophages.
Workers in coke oven plants have a higher incidence of lung cancer
than the general population and a measurable concentration of PAHs in
urine, which is higher in smokers than non-smokers (Haugen et al.,
1986). Van Schooten et al. (1990) analysed blood samples from coke
oven workers for PAH-DNA adducts and urine for 1-hydroxypyrene and
compared the results with those of non-exposed workers. Levels were
elevated in coke oven workers and in both exposed and control groups
the PAH-DNA adduct levels were higher among smokers than among
non-smokers.
Professional drivers are exposed to benzene and carcinogenic PAHs and
nitroarenes through the exhaust of petrol (gasoline) and,
particularly, diesel engines. An excess of lung cancer has been found
in this occupational group, with a suggestion of a synergistic
interaction between smoking and occupational exposure (Damber &
Larsson, 1985a).
Diesel exhaust contains large quantities of carbonaceous particulates
with adsorbed PAHs. The association between lung cancer and diesel
exhaust and the contributing role of cigarette smoking has been
considered to be problematic (Garshick et al., 1987, 1988; Boffetta et
al., 1988; Stöber & Abel, 1996; IPCS, 1996). In its evaluation, IARC
(IARC, 1989c) considered diesel engine exhaust to be probably
carcinogenic to humans (group 2A) and gasoline engine exhaust as being
possibly carcinogenic to humans (group 2B).
In most of the workplaces where PAHs contaminate the atmosphere, there
are also gases, fumes and aerosols that contain other hazardous
materials that act as irritants; they may play a role in the etiology
of chronic obstructive lung disease. It is important to include
smoking in epidemiological studies. In a study of lung cancer
mortality rates and smoking patterns in workers in the motor vehicle
industry, proportionate mortality rates were considerably reduced when
smoking rates were taken into account. An increased lung cancer risk
has been described among foundry workers; PAHs and silica were
considered to be possible etiological factors (Sherson et al., 1992).
IARC (IARC, 1984, 1985b) considers the following technical products as
carcinogenic to humans: coal tar and coal tar pitches, shale oils,
soots, effluent aerosols from coal gasification, and emissions from
coke ovens. Exposure occurring in the production of aluminium is
classified as probably carcinogenic to humans, whereas the exposures
to aerosol emissions from iron and steel foundries are classified as
possibly carcinogenic to humans.
3.5.5 Ethanol
Clinical and epidemiological studies have established a strong
relationship between smoking and drinking (Istvan & Matarazzo, 1984;
Bien & Burge, 1990; Zacny, 1990).
Elevated tobacco and alcohol consumption are regarded as the major
risk factors for oropharyngeal and oesophageal cancer in many
developed countries (Herity et al., 1982; Tuyns, 1983, 1991; Boyle et
al., 1990; Muir & McKinney, 1992; Negri et al., 1992).
It has been difficult to distinguish the separate effects of these
agents since many smokers tend to consume alcoholic beverages and
vice versa. In addition, the consumption of one substance may have
an effect on the use of the other substance. The possible interactions
(e.g., multiplicative effect) of tobacco smoking and alcohol
consumption for cancers of the oral cavity, pharynx and larynx have
been evaluated by IARC (1986). IARC (1986) concluded that tobacco
smoking was an important cause of oral, oropharyngeal, hypopharyngeal,
laryngeal and oesophageal cancers and combined ethanol consumption
increased the risk substantially. In a case control study of 1114
patients with oropharyngeal cancer, Blot et al. (1988) showed that the
risk to consumers of tobacco and alcohol was multiplicative rather
than additive and increased 35-fold in those who consumed two or more
packs of cigarettes and more than four alcoholic drinks per day. The
risk was higher in those consuming spirits or beer than in those
consuming wine and was lower in lifetime smokers of filter cigarettes.
Alcohol consumption and smoking affect fetal outcome, leading to
infants with low birth weight (Wright et al., 1983, 1984; Smith et
al., 1986).
In contrast to the many studies in laboratory animals of the
interactions of ethanol with tobacco-smoke condensate (TSC) and
specific tobacco constituents, e.g., nicotine (receptor studies)
(Collins, 1990), (gastric-mucosal damage) (Wong et al., 1986; Cho et
al., 1990), tobacco-smoke-specific nitrosamines, e.g.,
N-nitrosonornicotine (metabolism and carcinogenicity) (McCoy et al.,
1981; Castonguay et al., 1983, 1984) and
4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK) (Jorquera et al.,
1992; Schüller et al., 1993), there has been a relative paucity of
studies involving ethanol and tobacco smoke per se. These latter
studies have included fetotoxicity in mice (Peterson et al., 1981),
gastric mucosal damage (Iwata et al., 1995; Chow et al., 1996), and
mechanisms underlying behavioural association between alcohol and
tobacco consumption (Zacny, 1990).
In in vitro studies on the effect of tobacco smoke condensate on rat
buccal mucosa cells following exposure to ethanol, the level of
adducts was higher than in controls, suggesting an increased uptake of
carcinogens in the condensate (Autrup et al., 1992). Hsu et al. (1991)
studied in vitro genotoxicity of tobacco smoke condensate in
conjunction with 2% and 4% ethanol in human lymphoid cell lines.
Ethanol potentiated clastogenicity, measured by frequency of
chromosome breaks per cell, in a dose-dependent manner, and the
results indicated that ethanol at relatively high doses inhibited DNA
and chromosome repair systems.
Swiss Albino mice fetuses prenatally exposed to both tobacco smoke and
ethanol had a high resorption frequency, a significant reduction in
fetal weight and length, and neonatal growth retardation, indicating
that ethanol and tobacco smoke may interact to produce fetotoxicity
(Peterson et al., 1981).
Both cigarette smoking and ethanol consumption individually have been
associated with gastric and duodenal ulcers in humans and animals.
Exposure to cigarette smoke significantly potentiated ethanol-induced
gastric mucosal damage in Sprague-Dawley rats (Iwata et al., 1995;
Chow et al., 1996).
The effect of smoking on the incidence of cancers of the oral cavity,
oropharynx, hypopharynx and larynx is often combined with other
factors, principally alcohol, in the Western world. The possibility of
interaction between cigarette smoking and alcohol consumption is
complex (Burch et al., 1981).
Rothman & Keller (1972) reviewed the effect of joint exposure to
tobacco and alcohol with regard to oral cancers alone (based on data
published earlier by Keller & Terris, 1965) and concluded that a
single multiplicative function of the relative risks associated with
alcohol and tobacco separately provided an adequate summary of their
joint effect.
Wynder & Bross (1961) studied etiological factors in cancer of the
oesophagus, considered the consumption and effects of tobacco and
alcohol separately and together, and considered that the combined
effect was multiplicative. Tuyns et al. (1977) reported a similar
pattern of joint effect of tobacco and alcohol in a retrospective
study of oesophageal cancer in Brittany, France. The relative risk of
developing oesophageal cancer increased linearly with daily
consumption of alcohol and tobacco independently. The combined effect
fitted a multiplicative model.
Wynder et al. (1976) analysed environmental factors in cancer of the
larynx and showed a combined effect of tobacco and alcohol. In the
presence of smoking, heavy drinking increased the risk of cancer of
the larynx, especially for cancer of the supraglottic portion of the
larynx. Similar findings were reported by Burch et al. (1981).
In a prospective epidemiological study, the relative risk of incurring
a single primary carcinoma of the oral cavity, pharynx, larynx and
oesophagus in any one of these sites was increased independently by
the duration and intensity of exposure to tobacco or alcohol and
sustained exposure enhanced the risk in a multiplicative or
synergistic fashion (Schottenfeld et al., 1974) The relative risk of
multiple primary cancers in the sub-group with combined exposures to
high levels of tobacco and alcohol was 3.9 times that of patients
exposed previously to low levels of alcohol and tobacco.
3.5.6 Other organic compounds
Exposure situations involving compounds and mixtures of organic
compounds for which no definite smoking interactions have been
established but which are known to present serious health hazards are
summarized in chapter 4.
3.6 Physical agents
3.6.1 Radiation
The harmful forms of ionizing radiation that are of concern are
alpha- and beta-particles and gamma- and X-rays. All cause cellular
damage and have been implicated in carcinogenesis. IARC (1988)
classified radon and its decay products as Group 1 (carcinogenic to
humans) on the basis of sufficient evidence in humans and in
laboratory animals. The interaction of the effects of these radiations
with the effects of tobacco smoke has been studied. Radium is present
in uranium and other minerals and in all rocks and soils. It emits
alpha- and beta-particles and gamma-rays and decays to form the
chemically inert radioactive gas radon, which is released in tiny
amounts into the atmosphere where its concentration is extremely small
because of dilution. It can, however, become more concentrated in some
locations, particularly in uranium and other mines and in residential
buildings. Radon is an inspirable gas and its radioactive decay
products are ionized metal atoms, which adhere to inspirable dust
particles. These atoms are themselves undergoing radioactive decay and
emitting damaging alpha- and beta-particles and gamma-rays. In
addition to interactions of tobacco smoke with radon in mines and
residential situations, other effects of tobacco smoke and radiation
interactions have been studied in atom-bomb survivors and in the low
energy transfer radiation involved in the use of therapeutic radiation
(X-rays).
3.6.1.1 Radon in mines (high linear energy transfer (LET)
alpha-radiation)
Unless mines are well ventilated, the atmospheric concentration of
radon becomes significant. The gas and its radioactive decay products,
the radon daughters, can be inhaled. The daughters have short
half-lives and their decay is proceeding while the particles to which
they adhere are resident in the lungs and before they can be removed
by normal lung clearance. Thus radiation is delivered directly to the
delicate lung tissues where it causes an excess of lung cancer among
some miners.
Observations in several mining communities, e.g., among uranium miners
in the USA, Czechoslovakia, Canada and France, workers in a niobium
mine in Norway, iron ore miners in Sweden, tin miners in China and the
United Kingdom, and fluorspar miners in Newfoundland, showed a
significant dose-related increase in lung cancer risk with exposure to
radon and radon daughter elements (Archer, 1988). In miners who were
cigarette smokers, there was an interaction between the radiation
exposure and the smoke exposure leading to more than the expected
number of cases of cancer. The latent period for induction of lung
cancer was longer when the exposure to radioactivity started at a
younger age, it was shortened by high exposure rates and by cigarette
smoking, and lung cancers developed at lower levels of exposure to
radioactivity in miners who smoked than in those who were non-smokers.
In Bulgaria, Michaylov et al. (1995) used sputum cytology to study
bronchial cell dysplasia in 334 miners (uranium and metal mines)
exposed to 222Rn progeny, and 100 control miners from a metal mine
where radon was virtually absent. The dust and silica concentrations
and exposure to diesel exhaust and explosion gases were similar. The
frequency of bronchial cell dysplasia was significantly higher in
radon-exposed miners than in controls and the frequency of dysplasia
in smokers was significantly greater than in non-smokers.
The lower prevalence of lung cancer among coal miners than among other
underground workers is probably because coal mines are well ventilated
to reduce fire and explosion risk, and no build up of radioactivity
occurs. Attempts to reduce silicosis by ventilation have achieved a
similar effect. In some Swedish mines, because freezing occurred when
outside air was used for ventilation, filtration was achieved in the
1920s by circulating the air through old underground mine workings,
with the result that the potential for silicosis was reduced. However,
an increase in lung cancer was found because radioactive materials
built up, a fact that only became evident many years later (Archer,
1988).
The nature of the interaction between radon and cigarette smoke is not
clear. In a study by Edling (1982), the effects of smoking and radon
were considered to be additive, whereas in another by Damber & Larsson
(1985b) the effect was multiplicative. From a long-term study on
Swedish iron ore miners (Jörgensen, 1984), it was concluded that
tobacco smoke acts as a tumour promoter, an effect that has been
demonstrated in almost all animal studies. The concept that radon
serves as a tumour initiator and tobacco smoke as the tumour promoter
for the induction and development of lung cancer is supported by a
sequence of studies. Tobacco contains small amounts of polonium-210
(210Po), which primarily originates from phosphate fertilizers (Tso et
al., 1966) and, to a minor extent, from airborne 210Po trapped by the
glandular hairs (trichomes) found on the soil-facing surfaces of
tobacco leaves (Martell, 1974). 210Po is a decay product of radon -222
and an emitter of alpha-particles. It is present in cigarette smoke,
and the bronchial epithelium of smokers contains 2-10 times more 210Po
than is found in these tissues in non-smokers (Harley et al., 1980).
The alpha-radiation of 210Po damages DNA in the bronchial airways and
serves as a tumour inhibitor, and the tar in the tobacco smoke acts as
a tumour promoter.
The frequencies of different histological types of lung cancer among
miners have varied with working conditions and follow-up time. It has
also been shown (Archer, 1988) that the age range of the population
under observation can influence the conclusion. Thus, the
smoking-radon relationship appears to be multiplicative only for the
group aged 35-65 years. Steenland (1994) found the death rates from
lung cancer in smoking uranium miners to be intermediate between
additive and multiplicative for the two exposures, but, when
stratified for age, the multiplicative model fitted well for the
youngest and oldest categories but poorly for the middle range. In a
comprehensive analysis of data from 11 studies of radon-induced health
risks (Lubin et al., 1995), it was concluded that the joint effect of
radon progeny exposure and smoking is greater than the sum of the
individual effects and for smokers is higher by a factor of at least
three. The tobacco of cigarettes contains 0.1-1.0 pCi of 210Po
(Cohen et al., 1985; Hoffmann et al., 1986).
The conclusion reached by the US Surgeon General (1985) was that the
smoking-radon interaction consists of two parts: an additive effect of
the contribution of the two agents on the number of tumours produced
and an accelerating effect due to tumour promoters in cigarette smoke.
Thus for a miner who smokes, not only is the chance of lung cancer
greater but the latent period is shorter and therefore the cancer
appears sooner in smokers.
3.6.1.2 Environmental radon (high linear energy transfer (LET)
alpha-radiation)
Alpha-radiation from radon daughters in the home or in other
situations where there are enclosed spaces with poor ventilation,
e.g., where strict energy conservation measures have been adopted,
presents an elevated health hazard to occupants, particularly smokers,
and is a matter of public health concern. The ease with which ionized
radon daughters could be attracted to environmental tobacco smoke
particles and the possibility of a higher than additive combined
effect of radon progeny and smoke clearly indicate the importance of
residential contamination by radon.
The relative risk in the range of exposure experienced by miners has
been found to be linear, and it has been suggested from extrapolation
that exposures at the lower levels found in homes would carry some
risk (Lubin et al., 1995). Steindorf et al. (1995) calculated that 7%
of all lung cancer deaths in the western part of Germany may be due to
residential radon.
Axelson (1995) reviewed cancer risks from exposure to radon in the
home and suggested that cancers other than lung cancer may also be
related to indoor radon, especially leukaemia, kidney cancer and
malignant melanoma. However, it was acknowledged that studies of radon
and miners gave no clear support for this. Alavanja et al. (1995)
listed other risk factors as being responsible for lung cancer in
lifetime non-smokers and found a small non-significant risk for
subjects exposed to domestic radon at median concentrations. In a
case-control study of lung cancer in relation to exposure to radon in
homes (Letourneau et al., 1994), no increase in the relative risk for
any of the histological types of lung cancer was detected in relation
to cumulative exposure to radon. On the other hand, Biberman et al.
(1993) found an increased risk for small cell lung cancer following
residential long-term exposure to radon at a low-dose level. In a
large case-control study in Sweden, Pershagen et al. (1994) reported
an increased relative risk of lung cancer within the highest exposure
group. In an attempt to resolve the conflicting epidemiological data,
Lubin & Boice (1997) conducted a meta-analysis of eight large-scale
case-control studies of residential radon and lung cancer. This
analysis was consistent with an excess lung cancer risk. Furthermore,
the slope of the exposure- response curve derived from this
meta-analysis was comparable with that obtained from a combined
analysis of eleven miner cohorts exposed to radon (Lubin et al.,
1997).
The combined analysis of the miner data also confirmed the strong
synergistic relationship between radon and tobacco at high levels of
exposure to these two agents (Lubin et al., 1997), although it is
difficult to determine whether the interaction is closer to additive
or multiplicative (Chaffey & Bowie, 1994). When extrapolated to lower
levels of exposure, however, the magnitude of this interaction is
substantially diminished (Moolgavkar et al., 1993). However, Pershagen
et al. (1994) reported some evidence of a synergistic effect between
tobacco and residential radon exposure, with the relative risk of
radon-induced lung cancer being highest among heavy smokers. In a
case-control study of 982 subjects with lung cancer and 3185 hospital
or population control subjects, lung cancer risk was examined in
relation to residential radon concentration and length of time that
subjects were resident, and adjusted for age, sex and smoking (Darby
et al., 1998). The relative risk increased in an exposure-related
manner with time-weighted residential radon concentration and fitted
the data from studies in miners and the effect of smoking. Regardless
of the magnitude of any interaction between tobacco smoking and
residential radon, the lung cancer risks due to smoking exceed the
risk associated with radon in homes.
3.6.1.3 Atomic bomb site radiation (low linear energy transfer (LET)
radiation)
In tobacco-smoking survivors of atomic bombing in Hiroshima and
Nagasaki, Japan, elevated levels of cancer of several sites have been
reported. In the case of lung cancer both additive and multiplicative
models fit the data (Prentice et al., 1983; US NRC, 1988).
3.6.1.4 Therapeutic X-rays (low linear energy transfer (LET)
radiation)
Lung cancer as a possible side effect of the radiation therapy used to
treat breast cancer has been studied by Neugut et al. (1993, 1994) and
discussed by Inskip & Boice (1994). Neugut et al. (1993) reported that
the risk was greater in the ipsilateral than in the contralateral
lung. In a second study (Neugut et al., 1994), a three-fold relative
risk was found for the effect of radiation therapy among 10-year
survivors, a 14-fold risk was associated with smoking alone, and a
33-fold risk was found among irradiated smokers; in each case the
effect was most pronounced for ipsilateral lung cancer. A
multiplicative interaction was proposed and the implications of the
results for the design of treatment of breast cancer in smokers was
considered. The increased risk of lung cancer among survivors of
Hodgkin's disease(HD) was studied by van Leeuwen et al. (1995). Their
overall conclusions were that the risk of lung cancer increased more
with increasing radiation dose in HD patients who smoked than among
those who did not smoke. Thus, smokers were at greater risk from the
radiotherapy than non-smokers. The interaction between the
carcinogenic effects of smoking and radiation was significantly
stronger than multiplicative, and the low lung cancer rate found among
women with HD was attributable to the delayed popularity of smoking
among Dutch women, a fact shown by the male/female lung cancer ratio
(13:5) in the Netherlands in 1980.
3.6.1.5 Nuclear plant
Ongoing epidemiological studies are being conducted on the workforce
exposed to radiation at the Mayak plant in Russia. Of 500 workers
examined in a case-control study (Tokarskaya et al., 1995), 162
workers had contracted lung cancer, and the remaining 338 served as
radiation-exposed, non-tumour-bearing controls. Both the incidence and
duration of smoking was significantly higher in workers contracting
tumours compared to combined male and female controls. The strongest
smoking-related effect was for squamous cell carcinomas, followed by
adenocarcinomas, then small cell carcinomas. However, the findings are
complicated by the fact that the great majority of the workforce was
male, and there was only one of the 148 "never smokers" among the male
lung cancer cases.
3.6.1.6 Summary
In summary, miners subjected to chronic exposure throughout a working
lifetime to high-LET radiation show a radiation/tobacco smoke
interaction greater than additive and sometimes multiplicative. Atomic
bomb survivors exposed instantaneously to low-LET radiation show in
some cases an additive and in others a multiplicative interaction. The
results for residentially exposed smokers, subjected to a lifetime of
very low dose exposure, tend to show similar interactions to those for
miners. Tobacco-smoking patients subjected to therapeutic radiation
(low-LET) show a multiplicative interaction.
3.6.2 Vibration
Raynaud's phenomenon is an episodic disorder that produces
intermittent attacks of blanching in the extremities and there may be
numbness or tingling in the hands and fingers. There are several
causes (the term "Raynaud's disease" is applied when the cause is not
known). It was first associated with the use of vibrating tools among
Italian miners in 1911, and the association has since been reported
for a wide range of hand-held vibrating tools such as impact hammers,
chipping hammers, grinders, riveters and the motor-driven chain-saws
used in forestry. The terms vibration white finger (VWF), vibration
syndrome, traumatic vasospastic disease and dead finger have been used
for this condition that begins with numbness and tingling, followed by
blanching and can include intermittent episodes of hand and finger
pain and flushing. With continuing exposure to vibration the symptoms
may become more severe and continue after the cessation of exposure.
Damage to digital arteries and narrowing of the lumen has been
associated with vibration syndrome (JOM, 1984) and, because nicotine
acts as a vasoconstrictor, it has been suggested (JOM, 1984) that
limiting smoking could aid blood flow to the extremities and thus
reduce the condition. In a survey of forestry workers in Quebec in
1977-1978 (Thériault et al., 1982), a prevalence of Raynaud's
phenomenon among 1540 woodcutters was found in 30.5% of chain-saw
users and there was a strong association between this and cigarette
smoking; the relative risks were 3.60 for non-smokers, 6.55 for
smokers and 1.72 for smokers who had not used a chain-saw:
corresponding to an additive effect for the two risk factors. From
another study of the effect of tobacco use on a cohort of men with
VWF, in which the extent of tobacco use was confirmed by blood
nicotine and cotinine measurements (Ekenvall & Lindblad, 1989), it was
shown that tobacco aggravates the symptoms of VWF. Patients with
advanced symptoms were found to use tobacco more frequently and to
have higher blood cotinine levels than patients with less advanced
disease. In a study of the prognosis of VWF (Petersen et al., 1995),
an improvement in the condition occurred when there was no exposure to
either vibration or smoking, whereas an aggravation of the condition
was most notable in smokers. Whole-body vibration has been associated
with persistent severe neck trouble, and smoking was an added
predictor for this condition (Viikari-Juntura et al., 1994). Finger
temperature changes have been measured after smoking a cigarette; in
all cases a reduction in temperature was recorded (Saumet et al.,1986;
Bornmyr & Svensson, 1991).
3.6.3 Noise
In a study of aviators in 1963 at the US Naval Aerospace Medical
Research Laboratory (Thomas et al., 1981), two hearing level groups
were identified, one with normal and the other with impaired hearing.
The impaired hearing group had smoked more cigarettes for a longer
period of time than had those in the normal hearing group. In another
study the relationship between cigarette smoking and hearing loss was
studied in 2348 noise-exposed workers at an aerospace company and it
was found that smoking was a clear risk factor in noise-induced
hearing loss: the OR was 1.27 for "ever smokers" and 1.39 for present
smokers, compared with non-smokers (Barone et al., 1987). Vascular
insufficiency of the cochlear organ has been cited as the predominant
cause of progressive hearing loss that occurs with age. It was
suggested that smoking reduces the cochlear blood supply by:
a) vasospasm induced by nicotine; b) atherosclerotic narrowing of
vessels; and c) thrombotic occlusions (Zelman, 1973). In a study of
1000 subjects at a Veterans Hospital, Zelman (1973) found that whilst
age and sex were the most important variables, at all measured
frequencies the percentage of loss was greater for smokers, the
differences being greater at higher frequencies. From a retrospective
analysis of audiograms taken between 1984 and 1990 of a cohort of 119
workers, 78.8% of smokers compared with 25.7% of non-smokers had
noise-induced hearing loss (ILO, 1991). Although these studies
demonstrated a positive correlation between smoking and hearing loss,
Friedman et al. (1969) and Pyykko et al. (1987) were not able to show
that smoking was a significant risk factor to hearing loss.
3.6.4 Dupuytren's contracture
Dupuytren's contracture is a contracture of some muscle membranes of
the palm of the hand that causes the little finger and ring finger to
be drawn into the palm from where they cannot be extended. It is
characterized by a retractile sclerosis of the palmar aponeurosis,
which may progress to an irreducible flexion of the fingers. Opinions
differ on the influence of occupation, handedness and hand injury on
Dupuytrens contracture which Dupuytren himself attributed to chronic
occupational injury. Some people assert that heavy work always causes
the disease, while others claim that it is not responsible. Mikkelsen
(1978) studied 901 cases in an epidemiological study of 15 950 and
concluded, after isolating hand trauma, that Dupuytren's contracture
is caused by heavy work. The contribution made by tobacco has been as
contentious as has the cause of Dupuytren's contracture. Hand
thermography of affected fingers in Dupuytren's contracture shows a
drop in temperature of up to 3 degrees; and hand temperature falls by
a similar amount during smoking. In a study of 84 men and 16 women
with Dupuytren's contracture, Fraser-Moodie (1976) found no evidence
that smoking was connected with the condition, a conclusion also
reached by Mackenney (1983). However, cigarette smoking was listed
among the responsible factors for Dupuytren's contracture by Attali et
al. (1987), as well as by An et al. (1988), who found that cigarette
smoking was linked statistically to Dupuytren's contracture and
suggested that it may be involved in its pathogenesis by producing
microvascular occlusion and subsequent fibrosis and contracture. An et
al. (1988) concluded that cigarette smoking was one of the most
significant factors in the development of peripheral vasculopathy
Abelin et al. (1990) showed a significant association between
Dupuytren's contracture and smoking habits.
3.7 Biological agents
3.7.1 Biological (vegetable) dusts
Uncontrolled exposure to airborne vegetable dusts can affect health
and occurs worldwide in many workplaces, e.g., in agricultural
operations, textile industries, construction, carpentry and the
furniture industry. The population exposed is large, particularly in
developing countries where whole families, from young children to the
elderly, may engage in agricultural activities and small scale
manufacturing operations using vegetable products. These agents can
take the form of vegetable dusts, airborne fungal spores and
microorganisms, animal danders and feathers, herbicides and pesticides
and their residues. Processing of agricultural products, such as
cotton, flax, hemp, grain, tobacco, paprika and tea, and the milling
of certain varieties of wood are occupations where vegetable dust
exposures have been associated with detrimental health effects.
In addition to the irritation and bronchitis that is associated with
exposure to almost any dust, biological dusts can cause byssinosis,
allergic and immunological responses and, in some cases, nasal and
paranasal cancer. All these conditions can be affected in different
measure by tobacco smoking.
3.7.1.1 Cotton dust
Byssinosis is a respiratory disease of textile workers. The disease is
found in many cotton processing countries. It is more prevalent in the
dusty stages of cotton processing, such as carding, than in weaving.
Byssinosis, or similar symptoms, and bronchitis have also been found
in flax, hemp, jute and sisal workers. The characteristic symptoms are
tightness of the chest and shortness of breath on returning to work
after a period of absence. There is the possibility of progression to
permanent respiratory disability.
In a study of textile workers in South Carolina, USA, in 1973, the
smoking prevalence was almost the same for workers as for controls
(Beck et al., 1984). In another study of cotton workers in 1963-1966
(Molyneux & Tombleson, 1970), the percentage of male current smokers
was 62.5% and of ex-smokers 16.4%; among females the figures were
33.9% and 6.1%, respectively. Among flax scutchers in Normandy in
1986-1987, 56% were smokers and 18% were ex-smokers; compared with 45%
and 15%, respectively, for the controls (Cinkotai et al., 1988a). In
31 Lancashire textile factories (1988) 47.5% of the 4656 workers
interviewed were smokers (Cinkotai et al., 1988b). Of 800 000 American
workers surveyed (Stellman et al., 1988) for smoking habits in 1982,
5.9% were exposed to textile fibres or dust: 28.5% of these were
regular cigarette smokers and 44.9% were former cigarette smokers;
compared with 23.5% and 43.5%, respectively, in other occupations not
exposed to textiles.
Increases in both byssinosis and bronchitis were attributed to cotton
dust exposure and smoking in the cotton industry (Molyneux &
Tombleson, 1970; Merchant et al., 1973). From an industrial study of
the effects of cotton dust and cigarette smoke, Merchant et al. (1972)
concluded that smokers showed an increase in both the prevalence and
severity of cotton dust-induced byssinosis and that cigarette smoke
also increased the detrimental effect of cotton dust on ventilatory
capacity. It was suggested that the impairment of lung clearance
mechanisms by cigarette smoke could be responsible for the deleterious
effect of cotton dust and that smoking might lower the threshold of
susceptibility to the effects of inhaled cotton dust. Additivity and
the equal importance of the effects of smoke and cotton dust have been
suggested (Beck et al., 1984) but since different lung function
parameters are affected it would seem that the two factors affect
different sites. The fact that workers who stopped smoking, whilst
remaining in the same job, lost their byssinotic symptoms was
significant. A survey by Cinkotai et al. (1988a) of workers in 31
textile factories in Lancashire, United Kingdom, showed that
byssinotic symptoms (in decreasing order) were related to years in the
industry, degree of dust exposure, quality of cotton in use, ethnic
origin of workers and smoking habits. Symptoms of chronic bronchitis
were related primarily to smoking habits and then to factors connected
with the occupation. In a study of hemp workers (Bouhuys & Zuskin,
1976), decline in ventilatory function was more pronounced in smokers.
It was suggested (Cinkotai et al., 1988b) that a surprisingly low
prevalence of byssinotic symptoms in 12 flax scutching mills in
Normandy may have been due to either self selection of the workforce
or to an absence of the causative agent in the dust. Persistent cough
and phlegm production were associated with tobacco use.
In textile-related pulmonary disease, smoking as a primary causative
factor was reported by Pratt et al. (1980), and a similar conclusion
was arrived at from lung function tests carried out over a 3-year
period on 153 women (103 smokers, 50 non-smokers) with grades 2 and 3
byssinosis by Honeybourne & Pickering (1986). Cancer deaths in general
and lung cancer in particular were lower in workers exposed to cotton
dust than in others (Enterline et al., 1985). Kilburn (1989) suggested
that the effect of byssinosis on mortality of textile workers from
pulmonary disease needed more comprehensive study.
3.7.1.2 Wood dust
IARC (1995) evaluated the carcinogenic risk of wood dust and
classified it as carcinogenic to humans (Group 1), based on sufficient
evidence in humans and inadequate evidence in animals. The risk of
developing cancer of the nasal cavity among workers manufacturing
wooden furniture has been shown to be up to 100 times greater than for
the general population (Rang & Acheson, 1981). The effect is worst in
the most dusty areas (Rang & Acheson, 1981; Hayes et al., 1986). The
association of risk with certain hardwoods and the finishing of fine
furniture, rather than with woodworking in general, suggests that it
may be allied to both the chemical and physical nature of the dust. In
the study where the nasal cancer incidence was 100 times greater than
for the general population, it did not appear to be affected by
smoking habits. A similar conclusion was reached in a study in an area
of Italy with a large number of cases of nasal cancer among wood and
leather workers (Cecchi et al., 1980). An association with smoking was
established in other studies, and current and past smoking habits were
shown to be a risk factor for developing squamous cell cancer of the
sinus in men (Fukuda et al., 1987). A case-control study of 121 male
woodworkers who were examined for cancer of the nasal cavity or
paranasal sinus, in British Columbia in Canada between 1939 and 1977
(Elwood, 1981), showed increased relative risks associated with
occupations involving exposure to wood (relative risk 2.5) and with
smoking (relative risk 4.9). In a study in North Carolina and Virginia
in the USA between 1970 and 1980 (Brinton et al., 1984), a major
finding was the elevated risk of nasal cavity and sinus cancer among
cigarette smokers. However, the nature of any interaction of wood dust
and tobacco smoking needs further study because adenocarcinomata
appear to be the tumour type associated with wood dust, whereas the
relative risks for squamous-cell and small-cell cancers tend to be
higher for smokers. The available data do not permit an assessment of
the degree of interaction between smoking and wood dust exposure.
3.7.1.3 Allergic responses
This type of response can occur in the upper airways, where it is
manifest as hay fever, in response to certain types of pollen, or in
the bronchi as asthma, or it may appear in both. Some of the dusts
that cause allergic airways responses (occupational asthma) are grain
dusts from various cereals and their products, wood dusts particularly
from red cedar and iroko, and dusts from teas and tobacco. Among
asthmatics, environmental cigarette smoke makes the effect of the
asthma worse (Shim & Williams, 1986), and smoking effects appear to be
additive to that of asthma from other causes (Conolly et al., 1988).
Grain dust exposure and smoking have been found to cause increases in
the prevalence of respiratory symptoms and reductions in pulmonary
function of grain elevator workers. The effect of smoking was slightly
more pronounced; the combined effect of grain dust and smoking
appeared to be additive, except in the least exposed workers (5 years
or less) where a synergistic effect was observed in tests for
peripheral airways dysfunction (Cotton et al., 1983). Chan-Yeung et
al. (1985) stated that the effect of grain dust and smoking was
additive and not synergistic in causing a decrease in lung function.
In 303 workers in the animal feed industry exposed to dusts of grains
and cassava, 61 (20%) showed respiratory symptoms; in office staff in
the same plant the prevalence was similar. The plant workers had a
higher prevalence of smoking than office staff. Current smoking was
strongly associated with respiratory complaints in both groups (Post
et al., 1994).
Occupational asthma also occurs in flour, tea, coffee and rice
handlers. In Italian bakers and pastry makers De Zotti et al. (1994)
found that 54 (23%) of the 226 subjects were atopic. Forty (18%) were
skin-test-positive to storage mites, 27 (12%) to wheat flour and 17
(8%) to alpha-amylase. Skin sensitization to these occupational
allergens was significantly associated with atopy, smoking and
duration of exposure. In a study of 401 workers in bakeries or flour
mills, Cullinan et al. (1994a) found that work-related symptoms of
allergy were more common in smokers, with little difference between
atopic and non-atopic workers. However, smoking was not independently
related to either symptoms or positive skin test.
Zetterstrom et al. (1981) skin-prick-tested 129 workers in a coffee
roastery with green coffee bean and castor bean extracts. There was a
significantly increased prevalence of positive skin prick tests in
smokers. In enzyme detergent workers with occupational asthma, it was
found that twice as many smokers as non-smokers exhibited asthmatic
symptoms (Greenberg et al., 1970; Mitchell & Gandevia, 1971).
Smokers are more likely to show higher specific antibody production
and correspondingly be more susceptible to asthma.
3.7.2 Other biological agents
Although many biological dusts are known to have detrimental health
effects, there have been few studies of any interaction of smoking
with these agents. Extrinsic allergic alveolitis may be caused by
spores from a number of fungi which are small enough to reach the
pulmonary compartment. There are several forms of allergic alveolitis,
of which farmer's lung, bagasse pneumonitis, and bird fancier's lung
are examples. These are caused by fungal spores in mouldy hay or
mouldy sugar cane or an agent in bird feathers, respectively, and are
immunologically mediated (Lancet, 1985). Extrinsic allergic alveolitis
is a rare example of a respiratory disease which is more prevalent in
non-smokers than in smokers. In one study, 14 of 18 patients (11 with
farmer's lung and 7 with bird fancier's lung) were non-smokers, twice
the proportion of non-smokers in patients with cryptogenic fibrosing
alveolitis or in the local population (Warren, 1977). Carrillo et al.
(1991) investigated IgG response to pigeon serum and its relation to
tobacco smoking in 160 pigeon fanciers. The sensitization rate was
31.9%. Pigeon fanciers who were current smokers had significantly
lower levels of IgG antibodies to pigeon serum (P<0.001).
Precipitating antibodies to Micropolyspora faeni, a common cause of
farmer's lung, were found to be twice as common in the area of
non-smokers in farming communities compared with smokers (Morgan et
al., 1975). Alveolar macrophage phagocytosis has been shown to be
depressed by cigarette smoke and it has been suggested this may
explain its apparent protective effect (Hocking & Golde, 1979).
3.7.3 Agents found in factory farming (animal
confinement effects)
Factory farming of pigs, where the animals are kept in confined
conditions, is common practice in livestock production in many
developed countries and it has been found to be accompanied by adverse
respiratory symptoms in workers. Donham et al. (1984) summarized the
effects as: acute toxicosis and inflammation of the respiratory tract
from inhaling hydrogen sulfide; acute asthma-like symptoms;
bronchitis; and delayed or hypersensitivity pneumonitis-like symptoms.
They found that smoking interacted additively with the bronchitis and
obstructive symptoms of the condition. In a study of workers in swine
confinement areas, Zuskin et al. (1992) reported similar effects. They
also found that smoking aggravated acute and chronic respiratory
symptoms and impairment of lung function.
3.7.4 Laboratory animals
Venables et al. (1988b) examined data from three cross-sectional
surveys of 296 laboratory workers around 30 years of age exposed to
small mammals. Two populations were of pharmaceutical research workers
(N = 133 and 140) and one of research workers in a tobacco company
(N = 23). One of the pharmaceutical research worker populations had a
laboratory animal allergy (LAA) prevalence rate of over 40% (Venables
et al., 1988a). The tobacco company research workers were exposed only
to rats while the other two populations were exposed to rats, mice,
guinea-pigs and rabbits. Atopy was determined by skin prick test to
non-animal aeroallergens. Sensitization to laboratory animals was
determined by response to skin prick tests using urinary extracts from
the species used in each laboratory. Radioallergoabsorbent tests
(RASTs) were used to measure serum IgE antibody concentration to the
urinary extracts in two of the populations.
Atopy in the three populations ranged from 30% to 44%, and positive
skin response to urinary extract ranged from 13% to 48%. Pooled data
from the three surveys showed an association between smoking and
positive skin response to urinary extract. Associations with smoking
persisted after stratifying by atopic status, suggesting that smoking
was a risk factor for developing laboratory animal allergy.
In other studies of 238 laboratory workers without previous
occupational exposure to rats in three institutions specializing in
small animal research, atopy was again determined by skin prick test
response to non-animal aeroallergens and sensitization to laboratory
rats by response to urinary extract. Exposure to total dust and rat
urinary aeroallergen was also measured. Allergy to rats was positively
related to exposure intensity and this was stronger in atopic
subjects. Positive responses to skin prick testing with rat urinary
extract were strongly related to atopy and to smoking at all levels of
exposure (Cullinan et al., 1994b).
Several studies from Europe have shown an association between
ownership of pet birds or pigeons and lung cancer (Holst et al., 1988;
Kohlmeier et al., 1992; Gardiner et al., 1992). The relative risk
adjusted for smoking was 6.7 (2.2-20.0). However, two community-based
case-control studies, one from the USA and one from Sweden, could not
confirm an association between pet birds and lung cancer (Alavanja et
al., 1996; Modigh et al., 1996). In 1998, a hospital-based
case-control study conducted in New York City and Washington, DC, with
887 cases and 1350 controls, did not show an association of keeping
pet birds with lung cancer in non-smokers. There was a ten-fold
increase of lung cancer among smokers who were not bird keepers over
non-smokers, but there was no indication of synergism between smoking
and keeping a pet bird (Morabia et al., 1998).
3.7.5 Schistosomiasis
In a study carried out in Spain, risk factors for urinary bladder
cancer were identified (Bravo et al., 1987). The factors were listed
in order of importance and the first three were total number of
cigarettes smoked, history of urological disease and exposure to an
occupational risk. Vineis (1992) summarized epidemiological,
biochemical and molecular evidence that clearly linked smoking with an
increased risk of bladder cancer. Cohen & Johansson (1992) considered
smoking to be the most important etiological factor for bladder
cancer. They also implicated a variety of occupational exposures and,
in some parts of the world, an association with various endemic
diseases including schistosomiasis. Schistosomiasis is a waterborne
parasitic disease found in many developing countries in Africa, Asia
and South America. It is a widespread occupational disease for
agricultural workers, and also affects members of the general
population.
A phase in the life-cycle of the trematodes responsible for the
disease lives in the blood vessels of visceral organs and their eggs
are discharged through the bladder or intestine in urine and faeces.
Some species live in the mesenteric veins and the eggs are discharged
in the faeces but the eggs of Schistosoma haematobium mature in the
veins of the bladder and are discharged in the urine. The eggs mature
in water and the resultant larvae infect freshwater snails. Within the
snail the parasites multiply to produce free swimming cercaria larvae
which can infect humans via skin penetration and repeat the cycle. S.
haematobium is found in nearly all countries in the African continent
and it has been found that the incidence of bladder cancer is higher
in areas with a high prevalence of infection than in areas with a low
prevalence. IARC classifies infection with S. haematobium as
carcinogenic to humans (Group 1) (IARC, 1994b). In Egypt, the most
common form of cancer is bladder cancer, accounting for 27.6% of all
malignancies encountered (38.5% of cancers in males and 11.3% in
females), and these high levels have been attributed to underlying
schistosomiasis (Tawfik, 1987). Makhyoun (1974) carried out a
case-control study of smoking among Egyptian males with and without a
previous history of S. haematobium infection. A smoking index was
calculated (average number of cigarettes per day × duration of smoking
in years) to categorize subjects. The smoking index (intensity and
duration of smoking) was higher in all the patients with bladder
cancer. In the patients with a previous schistosomiasis infection,
22.7% were moderate or heavy smokers compared with 79.3% of the
non-schistosomiasis patients. In the latter there was a good
correlation with the smoking index but in the bladder cancer patients
with previous schistosomiasis there was no significant difference in
smoking index between patients and matched controls. It was not
possible to identify an interaction between smoking and
schistosomiasis in the production of bladder cancer.
However, in a review of the role of S. haematobium in human bladder
cancer, Badawi et al. (1995) referred to several major studies that
implicated this infection with the subsequent development of bladder
cancer. Badawi et al. (1995) listed examples of a co-carcinogenic
effect of parasitic infection in the presence of chemical carcinogens
and it has been suggested (Hicks et al., 1980; Hicks, 1982) that
schistosomiasis could supply the necessary proliferative stimulus to
accelerate cancer growth from latent tumour foci on exposure to
carcinogenic nitrosamines. Nitrosamines have been implicated as
carcinogens among tobacco chewers and oral snuff users (Hecht &
Hoffmann, 1988, 1991; Hoffmann et al., 1991a), nitrosamines have been
demonstrated in smoke (Tricker & Preussmann, 1992; Hoffmann et al.,
1991b) and nicotine-derived N-nitrosamines cause cancer (Hoffmann &
Hoffmann, 1991; (IARC, 1991). Some N-nitrosamines are excreted as
esters via the urinary tract, e.g., N-nitroso-di- n-butanol or NNK
as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. They may hydrolyse
in the presence of infectious agents in the bladder, as is the case in
schistosomiasis, and thus appear in their precarcinogenic form,
thereby increasing the risk for cancer in the urinary tract.
Endogenous nitrosation, which is increased by tobacco smoking, chewing
and oral snuff use has been demonstrated by monitoring urinary
N-nitrosamino acids (Tsuda & Kurashima, 1991). Badawi & Mostafa
(1993) and Badawi et al. (1995) suggested that the evidence for an
association between urinary schistosomiasis and bladder cancer
development is sufficient to justify the conclusion that S.
haematobium infection is a factor in inducing preneoplastic changes
in the bladder. Infection can reduce detoxification mechanisms (and
thus prolong retention of carcinogens in the bladder), stimulate
nitrosamine synthesis, and alter the activity of
carcinogen-metabolizing enzymes.
The conclusion is that an interaction is likely between tobacco use
and schistosomiasis infection.
3.7.6 Other urinary tract infections
Kantor et al. (1984) found that the joint effect of urinary tract
infection and cigarette smoking on bladder cancer was slightly beyond
that expected under an additive model and suggested that patients with
cystitis may be especially prone to tobacco-derived carcinogens in the
urine. La Vecchia et al. (1991) studied cystitis, gonorrhoea and
condylomata acuminata and found an interaction between urinary tract
infection and tobacco that appeared to be multiplicative, with
relative risk 2.4 for "ever smoking", 3.2 for cystitis alone, and 10.3
for combined exposure. It was concluded that a relationship between
urinary tract infection (and possibly some genital infections) and
bladder cancer indicated a cancer promotion role of infection and a
multiplicative smoking interaction. There have been reports of
synergism between herpes simplex virus and tobacco-specific
N-nitrosamines in cell transformation (Park et al., 1991a); an
additive interaction between smoking and herpes simplex virus type 2
in the promotion of cervical abnormalities (Mayberry, 1985), and the
possibility of an interaction between cigarette smoking and herpes
virus infection leading to cancer of the uterine cervix (Winkelstein
et al., 1984).
3.7.7 Sarcoidosis
Bresnitz & Strom (1983) reviewed sarcoidosis and concluded that
tobacco smoking might have a protective effect for the pathogenesis of
sarcoidosis. Sarcoidosis is a generalized granulomatous disease
involving the reticulo-endothelial system with lesions predominantly
in the lymphatic system. Harf et al. (1986) investigated the smoking
habits of 113 cases of histologically confirmed sarcoidosis in a
case-control study in Lyon, France. Smoking habits were defined in 101
cases and these were compared with a control group of healthy
volunteers. There was a highly statistically significant negative
association between sarcoidosis and smoking (OR = 3.8; 95% confidence
interval: 2.4, 6.5) in both cases.
3.8 Vector effects
Toxic chemicals, as well as harmless materials that produce harmful
chemical agents when they are burnt or vaporized, can be inadvertently
transferred to cigarettes or other smoking materials and cause the
smoke to be more injurious when it is inhaled.
3.8.1 Polytetrafluoroethylene
Polytetrafluoroethylene (Teflon(R)) is used in coatings for cooking
utensils, for making chemical vessels, gaskets and bearings and in
sprays as a mould release agent. Polytetrafluoroethylene and polyvinyl
fluoride are inert materials but their thermal decomposition products
can be very biologically reactive. Cigarettes can be easily
contaminated in the workplace and, when smoked, the polymer burns to
form fumes which cause "polymer-fume fever": severe gripping chest
pain giving rise to difficulty in breathing; trembling and shaking;
elevated temperature; and severe diaphoresis. The symptoms pass after
a day or two, but recur on again smoking a contaminated cigarette.
Before the cause was recognized a case was recorded of a person, who
used the polymer in a mould release spray, having some 40 attacks
(Kuntz & McCord, 1974). Another case was a person who referred to the
disease as "mould machine pneumonia" (Kuntz & McCord, 1974). Other
cases have been reported (Albrecht & Bryant, 1987) and better
occupational hygiene and a ban on smoking in the workplace resulted in
the disappearance of symptoms in those previously affected.
3.8.2 Mercury
Inorganic mercury occurs in many industries, as elemental mercury in
scientific and electrical instruments, as amalgams with many other
metals, in paints and pigments and in the chemical industry, as well
as in mining and extraction of the metal. Organic mercury compounds
are used as antiseptics, disinfectants, fungicides, bactericides and
herbicides. Contamination of smoking materials can lead to the
inhalation of mercury vapour. Mercury has been detected by neutron
activation analysis as a naturally occurring trace element in tobacco
(<1.0 ng/g) and in the smoke of cigarettes (4 ng/cigarette)
(Schneider & Krivan, 1993; Krivan et al., 1994).
3.9 Effects of tobacco smoking and metabolism of drugs and other
chemicals
3.9.1 Oral contraceptive use
In the 1970s it was postulated that an interaction between tobacco use
and oral contraceptives increased the risk of myocardial infarction in
women. In a study of women less than 45 years of age, a greater
proportion of moderate to heavy smokers were found in oral
contraceptive users experiencing myocardial infarction, compared
against a control population (Mann et al., 1975, 1976). Sturtevant
(1982), however, found no "convincing evidence" for an interaction or
synergism between the factors smoking and oral contraceptive use for
the major classes of cardiovascular disease. Lidegaard (1993) reported
no difference in the proportion of smokers between users and non-users
of oral contraceptives in Danish women experiencing a cerebral
thromboembolic attack and age-matched controls. Regarding thrombosis,
the data of Vessey & Doll (1969) suggest an increased thromboembolism
risk from oral contraceptive use for heavy smokers compared to
non-smokers. However, other studies reviewed by Sturtevant (1982) and
Nevius et al. (1982) are said to be ambiguous regarding a possible
interaction between smoking and oral contraceptive use. On balance,
there is evidence for certain interactions between smoking and oral
contraceptive use: the US Surgeon General (1983a), concluded that
"women who use oral contraceptives and who smoke increase their risk
of a myocardial infarction by an approximately tenfold factor,
compared with women who neither use oral contraceptives nor smoke",
and "the use of both cigarettes and oral contraceptives greatly
increases the risk for subarachnoid haemorrhage among women."
3.9.2 Drug and chemical metabolism
A number of studies have demonstrated that the metabolism of various
drugs and other chemicals is influenced by the smoking status of the
individual. This effect is sufficiently noteworthy that the US Surgeon
General (1979) report concluded that it is "apparent that cigarette
smoking is one of the primary causes of drug interactions in humans".
The extensive review of the literature at that time led to the
conclusions that, with respect to the influence of smoking on the
disposition/metabolism of other compounds: (a) the dominant effect of
smoking is enhanced drug disposition caused by the induction of
hepatic enzymes; (b) tobacco smoke contains many enzyme inducers,
notably polynuclear aromatic hydrocarbons; and (c) smoking can induce
the metabolism of various therapeutic agents and their pharmacological
and/or clinical effects. The metabolism of chemical carcinogens
involves various isozymes of cytochromes P450 and differences in their
genotypes or phenotypes may be a main factor responsible for
differences among individuals in susceptibility to carcinogens.
Metabolic activation of the procarcinogens such as benzo( a)pyrene to
the ultimate form is accomplished by cytochrome P450IA1 (Kawajiri et
al., 1990; Kawajiri & Fujii-Kuriyama, 1991; Nakachi et al., 1991).
Although the most noteworthy effects of smoking cited were related to
enzyme induction, it should also be noted that other components of the
smoke, such as carbon monoxide, nicotine, cadmium, some pesticides,
cyanide and acrolein, may serve to inhibit the function of some
enzymes (Jusko, 1978). An example for this phenomenon is the
inhibition by nicotine of the P450 isozymes that are involved in the
metabolic activation of NNN and NNK (Murphy & Heiblum, 1990). Nicotine
levels exceed those of NNN and NNK by more than 500 times. Therefore,
it was not surprising that even increasing NNN and NNK levels in snuff
ten-fold by adding the synthetic compounds did not alter the
carcinogenic potency of snuff in the oral cavity of rats (Hecht et
al., 1986).
Miller (1990) reviewed how cigarette smoking affects the
pharmacokinetic and pharmacodynamic properties of various drugs. The
drugs pentazocine, phenylbutazone and heparin show increased
metabolism in smokers. Also in smokers, the metabolism of oestrogen
and theophylline is increased. In addition, although smoking does not
pharmacokinetically affect the drugs propranolol and pindolol, the
nicotine in smoke is associated with elevations in blood pressure, and
thus smoking might serve to inhibit the antihypertensive effects of
these beta-adrenergic receptor blockers. On the other hand, smoking
had no effect on the drug disposition and/or pharmacological effects
of various other drugs examined (Jusko, 1978; Miller, 1990).
3.10 Animal studies of the interactions between cigarette smoke
exposure and other agents
Fourteen chronic inhalation studies (whole-body or nose-only exposure)
with mainstream cigarette smoke in rats and mice were reviewed by
Coggins (1998) and the results and histopathological changes
contrasted with epidemiological studies in humans. In most of the
studies there were epithelial changes in conducting airways and
increased numbers of alveolar macrophages, occasionally associated
with alveolar metaplasia. Lung adenomas and adenocarcinomas were seen
in some of the studies but no statistically significant increase in
the incidence of malignant lung tumours was found in either rats or
mice. This contrasts with human epidemiology where there is an
increased lung cancer risk. In an invited commentary, Morgan (1998)
stated that, based on these differences, rodents may be ineffective
models for predicting human health risk, at least for certain inhaled
materials, but stressed the interspecies differences in respiratory
anatomy and physiology and the need for consideration of the many
factors that may lead to differences in the effects of tobacco smoke
exposure.
The existing animal toxicology studies regarding interactions between
tobacco smoke and other materials do not form a comprehensive body of
work on the topic. A number of combinations of exposures between smoke
and other agents have been studied. Results are at times inconsistent
or contradictory, and the mechanisms by which interactions occur are
often not understood. This section provides a brief review of the
literature. In general, the existing work: (a) was generally performed
using rodents; (b) usually (but not always) examined the effects of
cigarette smoking (or components of the smoke) combined with either
with specific chemical components of the smoke or with radiation; and
(c) usually examined cancer as the biological response end-point of
interest. Other studies have focused on the use of cigarette smoke
components or condensates, and have used models such as in vitro
cell systems or mouse skin; a discussion of these studies is beyond
the scope of this section.
3.10.1 Non-cancer end-points
Several studies in experimental animals have examined the effects of
cigarette smoke administered over short periods of time (from hours to
daily exposures over several weeks). The cigarette smoke-induced
increase in the number of pulmonary macrophages and leukocytes noted
in humans has also been seen in animals such as the guinea-pig, even
after short exposures (Rylander et al., 1979). In addition, Morimoto
et al. (1993) observed a synergistic increase between mineral fibres
and exposure to cigarette smoke in the production of tumour necrosis
factor by rat alveolar macrophages. On the other hand, a 10-week
tobacco smoke exposure in rats suppressed radiation- induced pulmonary
inflammation (Nilsson et al., 1992), and a 12-week exposure of rats to
smoke did not influence the lung damage caused by an intratracheal
instillation of cadmium (Lai & Diamond, 1992).
Nishikawa et al. (1992) studied in guinea-pigs the effects of combined
exposure and single exposure to ozone and cigarette smoke on airway
responsiveness and tracheal vascular permeability and found that the
combined exposure increased airway responsiveness and vascular
permeability to a greater extent in terms of magnitude, but not in
duration, than a single exposure. This indicated that combined
exposure was more harmful than exposure to either agent alone.
3.10.2 Cancer studies: tobacco (cigarette) smoke plus other chemicals
Mori (1964) studied rats receiving multiple subcutaneous injections of
the carcinogen 4-nitroquinoline-1-oxide (NQO) with or without 6-7
month inhalation of cigarette smoke. Six of eight rats had lung
carcinomas in the combined exposure group compared to 3 of 9 rats in
the NQO-only group, and tumours occurred earlier. Davis et al. (1975)
studied Wistar rats receiving single intratracheal instillations of
benzo( a)pyrene with or without cigarette smoke inhalation for most
of the lifespan. Slight elevations of pulmonary squamous neoplasia
were noted in the combined exposure group compared with the individual
agents alone. However, the effects were not statistically significant.
In mice, inhaled cigarette smoke did not influence the occurrence of
lung tumours in (a) B6C3F1 mice pretreated with 3-methylcholanthrene
or benzo( a)pyrene (Henry & Kouri, 1984), (b) C57BL mice receiving
benzo( a)pyrene or influenza virus(Harris & Negroni, 1967), (c) A/J
mice receiving intraperitoneal injections of the tobacco specific
nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and a
6-month inhalation of smoke using a whole-body mode of exposure (Finch
et al., 1996), or (d) A/J mice exposed to sidestream smoke plus the
carcinogen 3-methylcholanthrene or urethane (Witschi et al., 1997). On
the other hand, a carcinogenic synergism in lung adenoma formation was
reported in Strain A mice some 6 months after initiation of treatment
with intraperitoneally injected urethane and skin-painted cigarette
smoke tar (DiPaolo & Sheehe, 1962). However, the non- pulmonary route
of exposure makes it difficult to interpret the relevance of this
result.
Several studies in hamsters have combined cigarette smoke exposure
with other agents. Dontenwill et al. (1973) combined smoke exposure
with a single intratracheal instillation of asbestos, but found no
significant differences in laryngeal lesions or tumours in the group
exposed to both materials, compared with the group receiving smoke
alone; the asbestos alone was non-tumorigenic. The combination of a
single intratracheal administration of dimethylbenz( a)anthrene
(DMBA) and smoke was found to increase the tumour incidence in the
oral cavity, pharynx, trachea and non-pulmonary tissues, compared to
the individual agents alone (Dontenwill et al., 1973). A similar
synergism between DMBA and smoke in producing laryngeal papillomas and
other oral and laryngeal lesions was reported by Hoffmann et al.
(1979) and by Kobayashi et al. (1974), and a synergism was also noted
between the nitrosamine DENA and smoke (Dontenwill et al., 1973).
Hamsters pretreated with a single dose of either 1.0, 3.3 or 10.0 mg
of NNK, and subsequently exposed twice daily for up to 72 weeks to
diluted cigarette smoke developed significantly more tumours in the
respiratory tract than hamsters receiving the identical dosage of NNK
and subjected to sham-smoking (Hecht et al., 1983). This supports the
concept that tobacco smoke has tumour-promoting activity. A
combination of smoke plus benzo( a)-pyrene adsorbed onto haematite
produced tracheal and laryngeal hyperplasia, whereas either agent
alone did not (Hoffmann et al., 1979), but no tumours were produced.
3.10.3 Cancer studies: cigarette smoke plus radiation
Dogs were used to study the potential interactions between radon and
cigarette smoke inhalation in producing lung cancer (Cross et al.,
1982). The lung tumour incidence in dogs exposed to radon plus
cigarette smoke for 4-5 years was decreased compared to that of dogs
receiving a mixture of radon, radon daughters, and uranium ore dust.
Studies have examined the potential interactions between tobacco smoke
and the alpha-emitters radon or plutonium-239 in rats or mice. In a
series of studies, Chameaud et al. (1982) examined the effects of
combined exposures to radon and cigarette smoke in Sprague-Dawley
rats, as well as the effects of pre-treatment with either radon or
smoke exposure. Smoke exposures begun before radon exposure did not
influence the radon-induced lung tumour incidence, but this increased
by 2- to 3-fold in the combined exposure group receiving radon before
cigarette smoke compared to the group receiving radon alone.
Finch et al. (1995a) examined F-344 rats receiving a single inhalation
exposure to 239PuO2, combined with chronic (up to 30 months)
cigarette smoke exposure, and found a synergistic crude tumour
incidence in rats receiving both agents, compared with animals
receiving the radiation alone. Most of these effects could be
explained by a cigarette-smoke-induced retardation of lung clearance
of the 239PuO2 particles (Finch et al., 1995b). This group is
continuing to study the potential combined effects of smoke and X-ray
exposure in rats, and the potential effects of smoke combined with
either X- or alpha-irradiation in mice (Finch et al., 1995a). Talbot
et al. (1987) reported results of a study in which mice inhaled
cigarette smoke, 239PuO2 or both materials. As in the case of rats,
cigarette smoke exposure caused retarded lung clearance and thus led
to greater radiation doses in animals receiving both agents.
4. EFFECTS OF EXPOSURE TO TOBACCO SMOKE AND OTHER AGENTS: SEPARATE
EFFECTS OR POSSIBLE INTERACTIONS
4.1 Coal mining
4.1.1 Coal dust
Coal miners can suffer from chronic bronchitis, coal workers
pneumoconiosis, progressive massive fibrosis, and emphysema. The
respiratory impairment appears as radiologically visible and
functional changes in the lungs, but although some of these are
associated solely with coal dust, some are also closely associated
with smoking. In the past it has been difficult to apportion
attributable risk to the two causes due to the fact that coalface
workers, the group subjected to the highest dust exposure, have a
different smoking pattern and perhaps a different daily consumption of
smoking materials than non-coalface workers because smoking in the
mine is forbidden. With the limitation of dust in modern mining, the
effect of the two factors, dust and smoking, is becoming clearer.
However, difficulties arise in interpreting data because non-smokers
may accumulate more dust, as they have less absenteeism, a different
pattern of lung clearance and a longer life (NIOSH, 1995).
In most of the countries surveyed, the prevalence of smoking by miners
tends to have been somewhat higher than in either the male population
as a whole, or among most other occupational groups, although a
distinction is seldom drawn between miners and coal miners except in
studies centred on coal mining populations. Between 1963 and 1975 in
the United Kingdom, the smoking prevalence fell for the male
population (and for miners) from 54% (miners 77%) to 47% (miners 49%)
(Lee, 1976). The figures for 1988 and 1990 were 33% and 31% (Bennett
et al., 1996) and for face-trained coalminers 35.7% in 1989 (Elliott,
1995). In a study of 8555 American miners from 29 bituminous coal
mines (Kibelstis et al., 1973), over 50% were smokers and 25% were
ex-smokers. In a United Kingdom study (Love & Miller, 1982), only 13%
of 1677 coal miners from 5 British collieries, who were examined in a
lung function study, were non-smokers; 66% were regular smokers and
the remainder were intermittent or ex-smokers. In a 20 year follow-up
study of a population of coal British miners and others (Cochrane &
Moore, 1980), 69% of the coal miners were smokers. These examples
typify the smoking prevalence of coal miners prior to the early
1980's. A 1982 survey of 800 000 American men and women in relation to
their occupations (Stellman et al., 1988) found that among miners
(type unspecified) 29.4% had never smoked regularly, 31.5% were
current smokers and 39.1% were former cigarette smokers. This may
reflect a general trend in the countries with higher income economies
where smoking has been decreasing in many sections of the population.
4.1.2 Bronchitis in coal miners
Kibelstis et al. (1973) found that the prevalence of bronchitis in
coal miners who smoked was higher than in non-smoking coal miners.
Coalface workers had more bronchitis and more airway obstruction than
surface workers and the difference between smokers working at the
coalface and non-smoking surface workers showed that the effect of
smoking was five times greater than that of coal dust. A German
epidemiological evaluation of chronic obstructive bronchitis in 5605
miners, 1276 ex-miners and 3898 individuals who had never worked in a
mine showed that smoking has a more serious effect on miners than on
other groups (Roth et al., 1985). It is possible that, within a mining
community, the other two groups may have a predisposition to the
disease and have achieved their status by job selection or job escape.
In a study of new entrants into coal mining McLintock (1971) found
that those who drop out of mining tend to be less physically fit and
more prone to chest problems than those who remain.
Morgan (1982) analysed the effects of cigarette smoking, dust exposure
and environmental factors on respiratory disease, and concluded that
bronchitis and airways obstruction were two separate responses to
cigarette smoking. The airflow obstruction found in smokers is due to
small airways disease and an involvement of respiratory bronchioles
leading to the development of emphysema. In coal miners, the
prevalence of bronchitis among non-smokers is related to the degree of
dust exposure. Marine et al. (1988) analysed data from studies on 53
382 coal miners in the United Kingdom and found that smoking miners
were at greater risk of developing chronic bronchitis. In a study by
Selig & Nestler (1985) of the relationship between chronic bronchitis,
smoking and dust (unspecified source), heavy smoking was equated with
20 years of dust exposure. From postmortem examinations of coal mine
workers, a correlation was reported between clinical chronic
bronchitis and smoking (Selig & Nestler, 1985).
The chronic bronchitis of coal miners is probably a combination of (a)
mucus hypersecretion caused by dust; (b) mucus hypersecretion, mucus
modification and clearance impairment caused by tobacco smoke; (c)
small airways disease caused by tobacco smoke; and (d) the effect of
dust on small airways tissue already inflamed by smoking. Bronchitis
due to smoking causes mucus hypersecretion which is much greater than
that due to coal dust (Morgan, 1982). Cigarette smoke impairs lung
clearance by changing the physical and chemical properties of mucus
and causing ciliastasis. Rheological measurement show changes in the
viscoelastic properties of mucus; chemically the mucus glycoprotein
structure is changed (King et al., 1989) and the irritant gases in
smoke cause abnormal mucus secretion and ciliastasis (Holbrook, 1977).
Small airways disease and bronchiolitis, leading to emphysema, are due
to smoking rather than to dust (Morgan, 1982).
4.1.3 Emphysema and pneumoconiosis in coal miners
Dust in coal mining is considered to be the primary cause of coal
workers pneumoconiosis. In a study of coalworkers and non-coalworkers,
Cockroft et al. (1982) concluded, after taking any effect of smoking
into account, that there was a 7-fold excess of emphysema in
coalworkers. Results of postmortem examinations of 866 Australian
miners (Leigh et al., 1983) showed a positive correlation between dust
exposure and emphysema and pneumoconiosis, with the severity highest
in non-smokers. However, smoking and non-smoking coalface workers were
not compared. From a postmortem comparison of lungs from 450 coal
miners, Rockley et al. (1984) found that emphysema occurred more
frequently in smokers (72%) than in ex-smokers (65%) or in non-smokers
(42%) and the relative frequency increased with age at death. The
study considered the possibility that coal dust might cause emphysema
which inhibits clearance and, in turn, promotes fibrosis, or
alternatively that fibrosis caused by dust increases the chance of
emphysema. However, the findings of a study of South Wales coal miners
(Fletcher., 1972) militated against dust-induced emphysema. It has
been suggested that differences in emphysema between coalworkers and
non-coalworkers can be accounted for by taking into account current
smokers in the two groups. Morgan (1982) concluded that the evidence
militates against obstructive emphysema occurring more commonly in
coal miners than in the general population, or that more dust
inhalation leads to a greater likelihood of emphysema developing.
Reviews of small airways disease (SAD) suggested that emphysema
proceeds from smoking-induced SAD. Cosio et al. (1980) considered that
their observations supported the hypothesis that SAD is causally
related to centrilobular emphysema, but not necessarily to panlobular
emphysema.
4.1.4 Lung cancer in coal miners
Perhaps as a result of failure to control confounding factors, there
has been a lack of consistency among reports on the relationship
between coal mining and lung cancer incidence in miners. In a direct
evaluation of the relationship between lung cancer mortality and coal
mine dust exposure, controlling for smoking status, Ames et al. (1983)
found no evidence of a link between coal mine dust exposure and lung
cancer risk, nor of an interaction effect, although the expected lung
cancer risk in cigarette smokers was observed. From a study of dust
exposure, pneumoconiosis and mortality of coal miners (Miller &
Jacobsen, 1985) it was found that lung cancer mortality among miners
who smoked was 5.5 times higher than in "never smokers" but that the
effect was entirely due to smoking.
Radon and radon daughter contamination of the dust in coal mines might
be expected to be as prevalent as in all other mines, and thus the
apparent very low lung cancer risk in coal mining may seem unexpected.
However, because of the explosion risk in coal mines, the ventilation
is usually efficient and a build-up of radioactivity is probably less
likely than in other types of mine.
4.2 Other mineral dusts
4.2.1 Talc
Talc is a hydrated magnesium silicate, often contaminated with free
silica or fibrous asbestos-like minerals such as tremolite and
anthophyllite. The only significant difference in the effects on
exposed and non-exposed was in the number and severity of cases of
dyspnoea in the talc workers, and smoking was considered to be an
aggravating factor (Kleinfeld et al., 1973).
4.2.2 Kaolin
Kaolin (pure China clay) is a hydrated aluminium silicate used for
ceramics and as a filler in the paper, rubber and paint industries.
The dry powder can give rise to fibrotic nodules in the lungs (Seaton
et al., 1981; Wagner et al., 1986). Characteristic smoker's inclusions
have been seen in transmission electron micrographs of pulmonary
alveolar macrophages obtained from cigarette smokers. The contents of
these inclusions are heterogeneous and include electron-dense areas,
lipid material and needle-like structures. These have properties
consistent with the composition of kaolinite. Kaolinite is present in
cigarette smoke from different brands, and pulmonary alveolar
macrophages are able to ingest this material in vivo (Hocking &
Golde, 1979).
4.2.3 Alumina
Alumina (aluminium oxide) is extremely hard and is used as an abrasive
(corundum). A cross-sectional study of 788 employees of an aluminium
production company examined the relationship of radiographic
abnormalities to smoking and dust exposure during bauxite and alumina
mining and refining (Townsend et al., 1988). Chest radiographs showed
a moderate time trend of increasing prevalence of small opacities in
non-smokers with high cumulative dust exposures. In most exposure
categories, smokers had a higher prevalence of opacities than
non-smokers. For cumulative exposures of less than 100 mg/m3-years,
increasing trends with duration of exposure were accentuated in
smokers as compared to non-smokers. The stronger effects observed in
smokers were attributed to the joint effects of duration of smoking
and duration of occupational exposure (Townsend et al., 1988).
4.3 Fibrous minerals
Fibrous minerals have been implicated in pleural thickening, pulmonary
fibrosis, mesothelioma and lung cancer in some villages in the
Anatolian region of Turkey (Artvinli & Baris, 1979), where fibrous
zeolite minerals (chabazite and erionite) are present in volcanic
deposits and used in buildings. Erionite has been shown to induce
mesothelioma. The symptoms and pathology of the respiratory disorders
and malignant disease were similar to those of asbestos. Asbestos-type
diseases have also been described in communities exposed to zeolite
minerals and tremolite dust in other similar regions by Baris et al.
(1979) and Yazicioglu et al. (1980). Non-asbestos fibrous materials
have been associated with pulmonary fibrosis (Stanton et al., 1977).
There are no data on any interaction of these minerals with smoking
but there appears to be a potential for interaction.
Wollastonite is a fibrous monocalcium silicate, which has been used as
a substitute for asbestos, as a filler and flux in ceramics, in
grinding wheels, refractory products, building blocks and acoustic
tiles. It is weakly fibrogenic. Hanke et al. (1984) studied a small
population of workers exposed to wollastonite and attributed
significant levels of chronic cough, phlegm and bronchitis to smoking
and not to exposure to wollastonite.
4.4 Metals
4.4.1 Antimony
Antimony is chemically similar to arsenic. Arsenic is metalloid,
antimony is a metal, both have volatile hydrides and form halogen,
oxygen and sulfur derivatives. Compounds of both frequently occur
together, particularly in smelter fume. Biological effects are similar
to those of arsenic (De Wolff & Edelbroek, 1994; De Wolff, 1995).
The concentrations of antimony, arsenic, cadmium, chromium, cobalt,
lanthanum, lead, selenium and zinc measured in lung tissues of
deceased smelter workers suggested that lung cancer risk was
multifactorial, involving carcinogenic and anti-carcinogenic factors
(Gerhardsson & Nordberg, 1993). A 30-year study at an antimony smelter
did not specifically implicate antimony as the cause of excess lung
cancer because of concurrent exposure to other carcinogens (Jones,
1994). In another study of smelters, the data suggested an increase in
lung cancer and non-malignant respiratory and heart disease (Schnorr
et al., 1995).
Antimony can have harmful effects on lung tissues, including
pneumoconiosis. IARC (1989a) evaluated antimony trioxide and antimony
trisulfide and concluded that the trioxide was possibly carcinogenic
to humans (Group 2B) and the trisulfide was not classifiable (Group
3). It is likely that the effects of inhalation of antimony fume/dust
and tobacco smoke would be worse than inhaling either separately. The
similarities of antimony to arsenic, both chemically and in some
biological effects, leads to the conclusion that tobacco smoke and
antimony could interact like tobacco smoke and arsenic in producing
toxicity.
4.4.2 Cadmium
Most zinc and lead-zinc ores contain small amounts of cadmium. It is
used in electroplating, in metal alloys (with copper for overhead
wires, and aluminium for casting), in nickel-cadmium dry cells, for
pigment manufacture and use, added to silver to prevent staining, and
it is a hazard of welding. The main route of exposure for the
non-smoking general population is via food, while for exposed workers
it enters the body mainly by inhalation. Tobacco is an important
source of cadmium in smokers (IPCS, 1992), and the tobacco source
affects the level of cadmium exposure (Yue, 1992). Any intake is
important because cadmium has an extremely long biological half-life.
It was suggested (Hassler et al., 1983) that higher levels of cadmium
in the blood and urine of exposed workers could arise both from
workplace contamination of cigarettes and transfer as fume during
smoking.
Cadmium has various toxic effects, the earliest being impairment of
renal tubular function leading to failure of resorption and excretion
of low molecular weight protein, glycosuria, aminoaciduria and
hypercalciuria (IPCS, 1992; IARC, 1993). It has been associated with
some types of lung disorder (emphysema, obstructive pulmonary disease
and diffuse fibrosis) (IPCS, 1992) Exposure to cadmium compounds has
been associated with cancer of the lung. There is some evidence for an
association with prostatic cancer (IARC, 1993). Other epidemiological
studies did not confirm an increased risk of prostatic cancer
(Kazantzis et al., 1992). The evaluation by IARC (1993) concluded that
cadmium and cadmium compounds are carcinogenic to humans (Group 1).
The supposition that cadmium in cigarette tobacco or in the workplace
may cause lung cancer has been questioned (Lamm et al., 1992;
Hertz-Picciotto & Hu, 1994). In a cohort mortality study of cadmium
workers in England, an observed increase lung cancer risk could not be
attributed strictly to cadmium due to the presence of multiple
confounding factors, particularly arsenic (Kazantzis et al., 1992).
Lamm et al. (1992) also suggested that arsenic may be responsible for
the observed lung cancer increased. The many elements in the lung
tissues of deceased smelter workers (Gerhardsson & Nordberg, 1993)
illustrates the difficulty in apportioning a role to one material in a
multifactorial environment.
Cadmium affects the myocardium and produces hypertension in animal
studies but the induction of cardiovascular disease and hypertension
in humans has not been demonstrated in epidemiological studies
(Kristensen, 1989; IPCS, 1992)). Blood and urine cadmium levels are
higher in smokers than in non-smokers and were found to be
considerably elevated in smokers working in an alkaline battery
factory (Hassler et al., 1983) and in smelter workers who were also
smokers (Kazantzis & Armstrong, 1984; Lilis et al., 1984a,b).
Davison et al. (1988) reported a cadmium dose/effect relationship in
functional and radiological evidence of emphysema in 101 subjects.
Leduc et al. (1993) described the very rapid development of emphysema
in a smoker after exposure to very high levels of cadmium. Smoking by
cumulatively increasing the body burden and hindering the lung
clearance may have provided an additional cause for the emphysema.
However, an additive or synergistic mechanism for the cadmium plus
smoking effect could not be inferred in this case because of the high
cadmium dose.
4.4.3 Cobalt
Cobalt is used in the production of alloys, tungsten carbide tools,
permanent magnets, and in the electrical industry.
Cobalt has toxic effects (Beliles, 1994). Cobalt exposure has been
linked to various allergic reactions (Shirakawa et al., 1992); hard
metal exposure and smoking together arithmetically increased total IgE
levels. Interstitial lung disease has been associated with cobalt in
susceptible individuals (Sprince et al., 1988), although in a study
involving the manufacture of permanent magnets (Deng et al., 1991)
abnormalities in pulmonary function and respiratory symptoms were no
higher than those of a reference population, except for 4 subjects out
of 362, who showed diffuse patches consistent with pneumoconiosis.
4.4.4 Lead
Lead is used in batteries, paint, glass, ceramics, fuel additives and
other industrial applications. In lead-using industries the main route
of exposure is by inhalation, mainly as dust and fume. Lead has a
range of toxic effects on blood, and the renal and nervous systems
(IPCS, 1995).
Levels of lead in blood vary from one area to another, between urban,
rural and occupationally exposed populations, and between men and
women (IPCS, 1995). The tobacco plant absorbs lead from the soil and
around 5-6% of that in cigarettes is inhaled in the smoke. Lead
concentrations in the smoke from one cigarette were found to range
from 0.017 to 0.98 µg (IARC, 1986). Higher blood lead and erythrocyte
protoporphyrin levels have been demonstrated in heavy smokers exposed
to lead (Williams et al., 1983; Landrigan & Straub, 1985); these could
have been partly due to contaminated cigarettes acting as vectors.
Other studies on occupationally exposed workers showed a progressive
increase in blood lead with an increase in the number of cigarettes
smoked (Maheswaren et al., 1993).
The association between lead exposure, tobacco smoke exposure and
blood pressure was examined in a cross-sectional study on 809 men
occupationally exposed to lead in a battery factory but only a small
increase in systolic blood pressure was found (Maheswaren et al.,
1993). There was no evidence of interactive effects between smoking
and lead exposure, but the absorbed lead from cigarettes added to the
body burden.
4.5 Rubber industry
Some of the principal hazards in the industry are fumes, talc, carbon
black, chemical additives and organic solvents but the components of
the hazard mixture differ between different areas of work. A high risk
of pulmonary disease has been reported in the rubber industry. It was
elevated for smokers, particularly those employed in areas where there
were respirable particulates and/or solvents (Lednar et al., 1977).
The data suggested an interaction between smoking and hazards
encountered in mixing (particulates), extrusion (solvent sprays and
mould release agents), and curing (solvents and rubber reaction
products). A problem in epidemiological studies in the industry arises
because of movement of workers between jobs. Some high-risk workers
who were also smokers were involved in finishing and inspection but
they tended to be older employees who had worked in other areas before
moving to this particular job. Emphysema was the principal pulmonary
condition leading to premature termination of employment (Lednar et
al., 1977).
IARC (1987) classified the rubber industry as Group 1, based on
sufficient evidence for carcinogenicity to humans. Excess mortality
from cancers of various sites, the site usually being associated with
the nature of the work and types of exposure, has been reported with
bladder cancer being associated with exposure to aromatic amines (Fox
et al., 1974; Monson & Nakano, 1976a,b; Monson & Fine, 1978;
Kilpikari, 1982; IARC, 1987; Zhang et al., 1989; Weiland et al.,
1996). Lung cancer was associated with curing and inner tube
manufacture. The use of talc in the rubber industry has been
associated with pulmonary disease (Kleinfeld et al., 1973). In the
rubber industry the relative risk of lung cancer for talc-exposed
workers was 3.2 for men and 4.4 for women (Zhang et al., 1989),
compared with 2.5 times excess lung cancer risk in talc-using
industries not associated with rubber manufacture (Thomas & Stewart,
1986). In jobs where very high lung cancer rates were found, smoking
levels were also very high, but any possible interaction effect from
the two exposures could not be assessed.
Gastrointestinal cancer, bladder cancer and leukaemia were found to be
associated with jobs in the rubber industry (Monson & Fine, 1978;
IARC, 1987). Possible causes, such as exposure to carbon black,
plasticisers, antioxidants, arylamino compounds and benzene have been
suggested. Urine samples from rubber workers showed a higher mutagenic
activity in the middle of a working week than at the beginning of a
week in both smokers and non-smokers, indicating the presence of
mutagens in the work environment. In a study involving the analysis of
urine from rubber workers for mutagenic factors, a possible
synergistic effect of smoking and occupational exposure was found in
smokers (Wicklund et al., 1988). There was a relationship between skin
contamination and urinary mutagenicity (Bos et al., 1989). Other
studies involving the analysis of urine from rubber workers for
mutagenic factors also suggested a possible synergistic effect of
smoking and occupational exposure among smokers (Falk et al., 1980;
Crebelli et al., 1985).
Andjelkovich et al. (1988) carried out a case-control study of lung
cancer in workers at a rubber manufacturing plant. There was an
association between lung cancer mortality risk and work in certain
areas for smokers and non-smokers, and the risk was greater in
smokers. Zhang et al. (1989) studied a cohort of 957 men and 667 women
employed in a rubber factory. The relative risk of lung cancer for
smokers was 8.5 for men and 11.4 for women, and for those exposed to
curing agents or talc the relative risk was 3.2 for men and 4.6 for
women. Additive and multiplicative models were used to evaluate the
interaction between smoking and occupational exposure on lung cancer.
The additive interaction term was not statistically significant and
the multiplicative interaction was negative. Weiland et al. (1996)
were not able to find interactions between smoking and exposure in the
rubber industry.
4.6 Petroleum industry
Petroleum refining involves exposure of workers to a large number of
chemical compounds occurring in crude oil or encountered in production
processes as intermediates, catalysts, additives or in the final
products. Because of fire risk, there are sections of the industry
where smoking is not permitted. However, in a study of 10 923 male and
624 female employees of the Australian petroleum industry between 1981
and 1984, it was found that the smoking habits did not differ
substantially from those of the general population (Christie et al.,
1986), and continued surveillance of these workers showed that
mortality rates were lower than for the general population (Christie
et al., 1987).
IARC (1989b) concluded that there is limited evidence that working in
petroleum refineries entails a carcinogenic risk. This limited
evidence applies to skin cancer and leukaemia; for all other cancer
sites on which information was available, the evidence was inadequate.
The overall evaluation, taking account of sufficient or limited
evidence in experimental animals for the carcinogenicity of various
distillates produced during petroleum refining, was that occupational
exposures in petroleum refining are probably carcinogenic to humans
(Group 2A).
In a study of 92 men with histologically confirmed renal cell
carcinoma (Domiano et al., 1985), it was concluded that there could be
an interaction between long-term gasoline exposure and heavy smoking.
In a case-control study of bladder cancer in New Jersey, USA, Najem et
al. (1982) found a significantly elevated risk of bladder cancer in
patients who had worked in the petroleum industry (OR, 2.5, CI,
1.2-5.5). While the risk was increased in current smokers (OR, 2.6),
it was higher in "never smokers" (OR, 5.6) and only slightly elevated
in ex-smokers (OR, 1.4). In another hospital-based study in Argentina
an association was found between bladder cancer and oil refinery work,
along with an elevated risk of lung cancer in smokers, but the numbers
were too few for any evaluation of the interaction (Iscovich et al.,
1987).
There was no increased mortality from either kidney cancer or
leukaemia in employees exposed to gasoline (Wong et al., 1993). A low
frequency of bladder cancer among refinery workers was attributed to
an assumed lower level of smoking among the group of workers studied
(Higginson et al., 1984).
In the study of workers occupationally exposed to gasoline vapour, the
sister chromatid exchange (SCE) frequency in peripheral blood
lymphocytes was used as an indicator of genotoxic response. Workers
employed in gasoline retail outlets were classified according to
cigarette smoking habits. The SCE frequency in lymphocytes was
unaffected by cigarette smoke or gasoline exposure alone, but
increased with combined exposure. The increased SCE frequency observed
in smokers occupationally exposed to gasoline vapour could be due to
the activation of hepatic enzymes by cigarette smoke, leading to a
greater formation of reactive metabolites of gasoline vapour (Edwards
& Priestly, 1993).
4.7 Pesticides
A variety of chemical compounds, natural and synthetic, are used as
pesticides. The number of individuals exposed during manufacture is
relatively small, and processes are usually contained, but the
worldwide population of pesticide users is very large. Most of the
world's population is exposed to pesticide residues in food and
drinking-water (Ecobichon, 1995). There is some information concerning
effects on health from interaction of pesticide exposure and smoking.
"Vineyard sprayer's lung", is an occupational disease associated with
the spraying of copper-sulfate-based Bordeaux Mixture. In a study of
the cytological changes in the respiratory tract of vineyard workers
spraying Bordeaux Mixture (Plamenac et al., 1985), the macrophages of
control subjects contained no copper whereas copper was detected in
64% of the sprayers. Abnormal cytological changes in the sputum were
found in smokers, in sprayers and controls, and atypical squamous
metaplasia of the respiratory epithelium was observed in 29% of the
sprayers who were smokers.
In a study to evaluate the hypothesis that exposure to lead arsenate
resulted in an excess mortality from lung cancer, Wicklund et al.
(1988) compared 155 male orchard workers who had died of respiratory
cancer with 155 orchard workers who had died of other causes. Two
groups of non-orchard workers (620) were used as matched controls.
There was no difference in lead arsenate exposure between the orchard
worker group. Although cigarette smoking was common among the orchard
workers, their smoking habits were similar to the non-orchard worker
control groups. In both groups of orchard workers mortality from
respiratory cancer was higher in smokers than in non-smokers.
McDuffie et al. (1990) examined the possibility that pesticide use was
related to the risk of primary lung cancer. In a case-control study
using data from a population-based cancer registry, they interviewed
273 men and 103 women with diagnosed primary lung cancer and compared
their occupational exposures, medical history and working
characteristics with 187 male community control subjects. There was no
correlation of lung cancer risk with exposure to pesticides and
adjusting for smoking did not alter this.
5. CONCLUSIONS AND RECOMMENDATIONS
5.1 Conclusions
1. Tobacco use, particularly smoking, is the single most important
public health hazard in the world today, and a major preventable
cause of morbidity and mortality.
2. In addition to the adverse health effects of active tobacco use,
adverse health effects have also been demonstrated as a result of
exposure to environmental tobacco smoke.
3. The risks from tobacco smoke are demonstrably increased through
interactions with certain chemical, physical and biological
hazards found in the workplace and general environment.
4. Based on this review of the scientific literature, additional
interactive effects, not yet identified, may exist.
5. In addition to synergistic interactions between tobacco smoke and
other agents, a few instances of antagonistic interactions were
also noted. However, even in these cases, the health risks of
tobacco smoke far outweigh any apparent protective effects.
5.2 Recommendations for protection of human health
1. All possible measures should be taken to eliminate tobacco use,
particularly smoking.
2. In order to avoid interaction with occupational exposures, and
eliminate the risks of exposure to environmental tobacco smoke,
smoking in the workplace should be prohibited.
3. Smoking in public places should be strongly discouraged.
4. In order to reduce the risks of exposure to environmental tobacco
smoke, particularly for children, smoking in domestic
environments should be strongly discouraged. Such action will also
avoid possible deleterious interactions between tobacco smoke and
residential exposures to other hazards.
5. Awareness-building through active educational programmes on the
health hazards of smoking should be undertaken in both developed
and developing countries. This should include enhanced
communication about deleterious interactions between tobacco and
other agents. Governments, industry, health and educational
professionals, and the general public should share in this
responsibility.
6. Since smoking may result in altered response or adverse reactions
to drugs and other therapeutic treatments, appropriate dose
adjustments and patient surveillance should be taken into
consideration by clinicians.
7. Health professionals should provide assistance to smokers to stop
smoking. This may necessitate the allocation of additional
resources for this purpose.
6. FURTHER RESEARCH
1. A number of different methodological approaches to investigating
interactions currently exist. The feasibility of harmonizing
methodologies for the assessment of potential interactions
between two or more health hazards should be explored. In the
interim, investigators should make every effort to state clearly
which methodologies have been used when reporting their results.
2. In many of the observational studies included in this review,
exposure data were limited. In order to improve future
investigations of interactions among human health hazards, more
complete and accurate exposure assessments should be performed.
3. Further epidemiological investigations of potential interactions
between tobacco smoke and other hazards in both the occupational
and general environments are needed to identify additional
populations at risk. Such interactive effects can lead to risks
higher than would be predicted by separate analyses of the risks
associated with individual hazards.
Epidemiological investigations in countries where the health
effects of tobacco have not been extensively studied previously
are of particular importance. This information would be of value
in characterizing the morbidity and mortality due to tobacco use
in those countries.
4. Additional research is needed to clarify the toxicological
mechanisms by which tobacco smoke leads to adverse health
effects, and by which tobacco smoke and other agents interact.
This information will be of use in the design and interpretation
of epidemiological studies on the health effects of tobacco,
including interactive effects.
5. Additional epidemiological research on the health effects of
passive smoking, particularly carcinogenic, cardiorespiratory and
allergic effects, would be of value in characterizing the effects
of low levels of exposure to tobacco smoke. Interactions between
environmental tobacco smoke and other health hazards also warrant
further investigation.
6. Established biomarker methods should be applied to the monitoring
of workers for the early detection of harmful exposures to toxic
or carcinogenic agents, and, in many areas, new biomarker methods
should be developed to improve hazard identification.
Consideration should be given to evaluating the ongoing exposure
of workers to active or passive tobacco smoke by monitoring
salivary or urinary cotinine, one of the major nicotine
metabolites.
7. A comprehensive review of the adverse health effects of
environmental tobacco smoke should be undertaken. This review
would provide an authoritative summary of the health impacts of
environmental tobacco smoke, as well as interactions between
environmental tobacco smoke and other health hazards.
REFERENCES
Abe S, Ohsaki Y, Kimura K, Tsuneta Y, Mikami H, & Murao M (1982)
Chromate lung cancer with special reference to its cell type and
relation to the manufacturing process. Cancer, 49(4): 783-787.
Abelin T, Segmüller H, & Segmüller G (1990) Tobacco and alcohol
consumption as risk factors of Dupuytren's contracture. In: Tobacco
and health 1990 -- The global war: Proceedings of the 7th World
Conference on Tobacco and Health, Perth, Western Australia, 1-5 April
1990. Perth, Department of Health of Western Australia, pp 449-450.
Abramson MJ, Wlodarczyk JH, Saunders NA, & Hensley MJ (1989) Does
aluminium smelting cause lung disease. Am Rev Respir Dis, 139:
1042-1057.
Acheson ED, Gardner MJ, Winter PD, & Bennett C (1984) Cancer in a
factory using amosite asbestos. Int J Epidemiol, 13(1): 3-10.
Alavanja MC, Brownson RC, Benichou J, Swanson C, & Boice JD (1995)
Attributable risk of lung cancer in lifetime nonsmokers and long-term
ex-smokers (Missouri, United States). Cancer Causes Control, 6(3):
209-216.
Alavanja MC, Brownson RC, Berger E, Lubin J, & Modigh C (1996) Avian
exposure and risk of lung cancer in women in Missouri: population
based case-control study. Br Med J, 313(7067): 1233-1235.
Albrecht WN & Bryant CJ (1987) Polymer-fume fever associated with
smoking and use of a mold-release spray containing
polytetrafluoroethylene. J Occup Med, 29(10): 817-819.
Al-Fayez SF, Salleh M, Ardawi M, & Zahran FM (1988) Effects of sheesha
and cigarette smoking on pulmonary function of Saudi males and
females. Trop Geogr Med, 40(2): 115-123.
Amandus HE, Castellan RM, Shy C, Heineman EF, & Blair A (1992)
Reevaluation of silicosis and lung cancer in North Carolina dusty
trades workers. Am J Ind Med, 22(2): 147-153.
Ames RG, Amandus H, Attfield M, Green FY, & Vallyathan V (1983) Does
coal mine dust present a risk for lung cancer? A case-control study of
US coal miners. Arch Environ Health, 38(6): 331-333.
An HS, Southworth SR, Jackson WT, & Russ B (1988) Cigarette smoking
and Dupuytren's contracture of the hand. J Hand Surg, 13A: 872-874
Anderson HA, Lilis R, Daum SM, & Selikoff IJ (1979) Asbestosis among
household contacts of asbestos factory workers. Ann NY Acad Sci, 330:
387-399.
Andjelkovich DA, Abdelghany N, Mathew RM, & Blum S (1988) Lung cancer
case-control study in a rubber manufacturing plant. Am J Ind Med,
14(5): 559-574.
Andjelkovich DA, Shy CM, Brown MH, Janszen DB, Levine RJ, & Richardson
RB (1994) Mortality of iron foundry workers: III. Lung cancer
case-control study. J Occup Med, 36(12): 1301-1309.
Archer VE (1988) Lung cancer risk in underground miners: cohort
case-control studies. Yale J Biol Med, 61: 183-193.
Armitage AK & Turner DM (1970) Absorption of nicotine in cigarette and
cigar smoke through the oral mucosa. Nature (Lond), 226: 1231-1232.
Armstrong BG, Tremblay CG, Cyr D, & Theriault GP (1986) Estimating the
relationship between exposure to tar volatiles and the incidence of
bladder cancer in aluminium smelter workers. Scand J Work Environ
Health, 12: 486-493.
Artvinli M & Baris YI (1979) Malignant mesotheliomas in a small
village in the Anatolian Region of Turkey: An epidemiologic study. J
Natl Cancer Inst, 63(1): 17-20.
Attali P, Ink O, Pelletier G, Vernier C, Jean F, Moulton L, & Etienne
JP (1987) Dupuytren's contracture, alcohol consumption, and chronic
liver disease. Arch Int Med, 147: 1065-1067.
Autrup JI, Hansen C, & Autrup H (1992) Detection of tobacco smoke
carcinogen-DNA adducts in cultured rat buccal mucosa cells following
exposure to ethanol and total cigarette smoke condensate or chewing
tobacco. Chem-Biol Interact, 85: 141-150
Avolio G, Cacciabue M, Rolla G, Caria E, Oliaro A, Carlino F, Pepino
E, Polizzi S, & Bucca C (1986) [Functional compromise of the small
airways in subjects exposed to SiO2.] Minerva Med, 77(45-46):
2183-2185 (in Italian).
Axéll T, Mörnstad H, & Sundström B (1978) [Snuff and cancer of the
oral cavity.] Lakartidningen, 75: 2224-2226 (in Swedish).
Axelson O (1995) Cancer risks from exposure to radon in homes. Environ
Health Perspect, 103(suppl 2): 37-43.
Badawi AF & Mostafa MH (1993) Possible mechanisms of alteration in the
capacities of carcinogen metabolizing enzymes during schistosomiasis
and their role in bladder cancer induction. J Int Med Res, 21(6):
281-305.
Badawi AF, Mostafa MH, Probert A, & O'Connor PJ (1995) Role of
schistosomiasis in human bladder cancer: evidence of association,
aetiological factors, and basic mechanisms of carcinogenesis. Eur J
Cancer Prev, 4: 45-59.
Baker DB, Gann PH, Brooks SM, Gallagher J, & Bernstein IL (1990)
Cross-sectional study of platinum salts sensitization among precious
metals refinery workers. Am J Ind Med, 18: 653-664.
Ball J & Simpson D (1987) Round the world: India -- Tobacco and ill
health. Lancet, 18: 149.
Baris Y, Artvilini M, & Sahin AA (1979) Environmental mesothelioma in
Turkey. Ann NY Acad Sci, 330: 423-432.
Barone JA, Peters JM, Garabrant DH, Bernstein L, & Krebsbach R (1987)
Smoking as a risk factor in noise-induced hearing loss. J Occup Med,
29(9): 741-745
Bartsch H, Malaveille C, Friesen M, Kadlubar FF, & Vineis P (1993)
Black (air-cured) and blond (flue-cured) tobacco cancer risk. IV:
Molecular dosimetry studies implicate aromatic amines as bladder
carcinogens. Eur J Cancer, 29A(8): 1199-1207
Bates MN, Smith AH, & Cantor P (1995) Case-control study of bladder
cancer and arsenic in drinking water. Am J Epidemiol, 141(6): 523-530.
Battista SP (1976) Ciliatoxic components in cigarette smoke. In:
Proceedings of 3rd World Conference on Smoking and Health, New York
City, 2-5 June 1975 -- Volume 1: Modifying the risk for the smoker.
Washington, DC, US Department of Health, Education and Welfare, pp
517-534 (DHEW Publication No. (NIH) 76-1221).
Baumslag N, Keen P, & Petering HG (1971) Carcinoma of the maxillary
antrum and its relationship to trace metal content of snuff. Arch
Environ Health, 23: 1-5.
Beck GJ, Maunder LR, & Schachter EN (1984) Cotton dust and smoking
effects on lung function in cotton textile workers. Am J Epidemiol,
119(1): 33-43.
Beliles RP (1994) Cobalt. In: Clayton GD & Clayton FE ed. The metals
-- Patty's industrial hygiene and toxicology. New York,
Wiley-Interscience, chapter 27, pp 1986-1999.
Bennett N, Jarvis L, Rowlands O, Singleton N, & Haselden L (1996)
Office of Population Censuses and Surveys, Social Survey Division:
Results from the 1994 general household survey. London, Her Majesty's
Stationery Office (HMSO), pp 92-102.
Benowitz NL, Porchet H, Sheiner L, & Jacob P (1988) Nicotine
absorption and cardiovascular effects with smokeless tobacco use:
comparison with cigarettes and nicotine gum. Clin Pharmacol Ther,
44(1): 23-28.
Berry G, Newhouse ML, & Turok M (1972) Combined effects of asbestos
exposure and smoking on mortality from lung cancer in factory workers.
Lancet, 2: 476-479.
Berry G, Newhouse ML, & Antonis P (1985) Combined effect of asbestos
and smoking on mortality from lung cancer and mesothelioma in factory
workers. Br J Ind Med, 42: 12-18.
Bhonsle RB, Murti PR, Gupta PC, & Mehta FS (1976) Reverse dhumti
smoking in Goa: an epidemiologic study of 5,499 villagers for oral
precancerous lesions. Indian J Cancer, 13(4): 301-305.
Bi W, Hayes RB, Feng P, Qi Y, You X, Zhen J, Zhang M, Qu B, Fu Z, Chen
M, Chien HTC, & Blot WJ (1992) Mortality and incidence of bladder
cancer in benzidine-exposed workers in China. Am J Ind Med, 21(4):
481-489.
Biberman R, Lusky A, Schlesinger T, Margaloit M, Neeman E, & Modan B
(1993) Increased risk for small cell lung cancer following residential
exposure to low dose radon: a pilot study. Arch Environ Health, 48(4):
209-212.
Bien TH & Burge R (1990) Smoking and drinking: A review of the
literature. Int J Addict, 25: 1429-1454.
Bjorseth A, Bjorseth O, & Feldstad PE (1978) Polycyclic aromatic
hydrocarbons in the work atmosphere; I. Determination in an aluminium
reduction plant. Scand J Work Environ Health, 4: 212-223.
Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS,
Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, & Fraumeni
JF Jr (1988) Smoking and drinking in relation to oral and pharyngeal
cancer. Cancer Res, 48: 3282-3287.
Boffetta P, Stellman SD, & Garfinkel L (1988) Diesel exhaust exposure
and mortality among males in the American Cancer Society prospective
study. Am J Ind Med, 14: 403-415.
Bohning DE, Atkins HL, & Cohn SN (1982) Long-term particle clearance
in man: normal and impaired. Ann Occup Hyg, 26: 259-271.
Bolinder G, Alfredsson L, Englund A, & de Faire U (1994) Smokeless
tobacco use and increased cardiovascular mortality among Swedish
construction workers. Am J Public Health, 84(3): 399-404.
Bornmyr S & Svensson H (1991) Thermography and laser-Doppler flowmetry
for monitoring changes in finger skin blood flow upon cigarette
smoking. Clin Physiol, 11(2): 135-141.
Bos RP, Kromhout H, Ikink H, de Haan W, Koppejan J, & Theuws JLG
(1989) Mutagens in urine of non-smoking and smoking workers in an
aircraft tyre retreading plant: Skin exposure as a causal factor.
Mutat Res, 223(1): 41-48.
Bouhuys A & Zuskin E (1976) Chronic respiratory disease in hemp
workers. Ann Intern Med, 84: 398-405.
Bovenzi M, Stanta G, Antiga G, Perruzzo P, & Cavallieri F (1993)
Occupational exposure and lung cancer risk in a coastal area of
northeastern Italy. Int Arch Occup Environ Health, 65(1): 35-41.
Boyko RW, Cartwright RA, & Glashan RW (1985) Bladder cancer in dye
manufacturing workers. J Occup Med, 27(11): 799-803.
Boyle P, Macfarlane GJ, Maisonneuve P, Zheng T, Scully C, & Tedesco B
(1990) Epidemiology of mouth cancer in 1989: a review. J R Soc Med,
83: 724-730.
Boysen M, Solberg LA, Torjussen W, Poppe S, & Hogetveit AC (1984)
Histological changes, rhinoscopical findings and nickel concentration
in plasma and urine in retired nickel workers. Acta Oto-Laryngol,
97(´): 105-115.
Bravo MP, Del Rey Calero J, & Conde M (1987) Risk factors of bladder
cancer in Spain. Neoplasma, 34(5): 633-637.
Bresnitz EA & Strom BL (1983) Epidemiology of sarcoidosis. Epidemiol
Rev, 5: 124-156.
Brinton LA, Blot WJ, Becker JA, Winn DM, Browder JP, & Farmer JC Jr
(1984) A case-control study of cancers of the nasal cavity and
paranasal sinuses. Am J Epidemiol, 119(6): 896-906.
Brown CC & Chu KC (1989) Additive and multiplicative models and
multistage carcinogenesis theory. Risk Anal, 9: 9-105.
Brownson RC, Novotny TE, & Perry MC (1993) Cigarette smoking and adult
leukemia: A meta analysis. Arch Intern Med, 153: 469-475.
Brunnemann KD & Hoffmann D (1974) The pH of tobacco smoke. Food Cosmet
Toxicol, 12: 115-124.
Brunnemann KD & Hoffmann D (1981) Assessment of the carcinogenic
N-nitrosodiethanolamine in tobacco products and tobacco smoke.
Carcinogenesis, 2: 1123-1127.
Brunnemann KD & Hoffmann D (1991) Chemical studies on tobacco smoke
XCC: Decreased concentrations of N-nitrosodiethanilamine and
N-nitrosomorpholine in commercial tobacco products. J Agric Food
Chem, 39: 207-208.
Brunnemann KD & Hoffmann D (1992) Chemical composition of smokeless
tobacco products. Bethesda, Maryland, National Cancer Institute, pp
96-108 (Smoking and Tobacco Control Monographs, No. 2).
Brunnemann KD, Mitacek EJ, Liu Y, Limsila T, & Suttajit M (1996)
Assessment of major carcinogenic tobacco-specific N-nitrosoamines in
Thai cigarettes. Cancer Detect Prev, 20(2): 114-121.
Burch JD, Howe GR, Miller AB, & Semenciw R (1981) Tobacco, alcohol,
asbestos, and nickel in the etiology of cancer of the larynx: A
case-control study. J Natl Cancer Inst, 67: 1219-1224.
Calverley AE, Rees D, Dowdeswell RJ, Linnett PJ, & Kielkowski D (1995)
Platinum salt sensitivity in refinery workers: incidence and effects
of smoking and exposure. Occup Environ Med, 52: 661-666.
Carrillo T, de Castro R, Cuevas M, Diaz F, & Cabrera P (1991) Effect
of cigarette smoking on the humoral immune response in pigeon
fanciers. Allergy, 46: 241-244.
Cartwright R (1982) Occupational bladder cancer and cigarette smoking
in West Yorkshire. Scand J Work Environ Health, 8(suppl 1): 79-82.
Castonguay A, Tjalve H, & Hecht SS (1983) Tissue distribution of the
tobacco-specific carcinogen
4-(methyl-nitrosamino)-1-(pyridyl)-1-butanone and its metabolites in
F344 rats. Cancer Res, 43: 631-635.
Castonguay A, Rivenson A, Trushin N, Reinhardt J, Spathopoulos S,
Weiss CJ, Reiss B, & Hecht SS (1984) Effects of chronic ethanol
consumption on the metabolism and carcinogenicity of
N-nitrosonornicotine in F344 rats. Cancer Res, 44: 2285-2290.
Cecchi F, Buiatti E, Kriebel D, Nastasi L, & Santucci M (1980)
Adenocarcinoma of the nose and paranasal sinuses in shoemakers and
woodworkers in the province of Florence, Italy (1963-77). Br J Ind
Med, 37: 222-225.
Chaffey CM & Bowie C (1994) Radon and health -- an update [Review]. J
Public Health Med, 16(4): 465-470.
Chakrabarti RN, Dutta K, Sikdar S, & Ghosh K (1991) Smokeless tobacco
and premalignant and malignant lesions of the oral cavity. Indian J
Med Sci, 45(10): 273-275.
Chameaud J, Perraud R, Chretien J, Masse R, & Lafuma J (1982) Combined
effects of inhalation of radon daughter products and tobacco smoke.
In: Sanders CL ed. Pulmonary toxicology of respirable particles.
Washington, DC, US Department of Energy, Technical Information Center,
pp 551-557 (CONF-791002).
Chan-Yeung M, Enarson D, & Grzybowski S (1985) Grain dust and
respiratory health. Can Med Assoc J, 133(10): 969-973.
Cheng WN & Kong J (1992) Retrospective mortality cohort study of
chrysotile asbestos products workers in Tianjin 1972-1987. Environ
Res, 59(1): 271-278.
Chiazze L Jr, Watkins DK, & Fryar C (1992) A case-control study of
malignant and non-malignant respiratory disease among employees of a
fibreglass manufacturing facility. Br J Ind Med, 49(5): 326-331.
Chiazze L Jr, Watkins DK, & Fryar C (1995) Adjustment for the
confounding effect of cigarette smoking in an historical cohort
mortality study of workers in a fiberglass manufacturing facility. J
Occup Environ Med, 37(6): 744-748.
Chopra SK, Taplin GV, Elam D, Carson SA, & Golde D (1979) Measurement
of tracheal mucociliary transport velocity in humans -- smokers versus
non-smokers. Am Rev Resp Dis, 119(4): 205 (Abstract).
Cho CH, Chen BW, Hui WM, & Lam SL (1990) The influence of acute or
chronic nicotine treatment on ethanol-induced gastric mucosal damage
in rats. Dig Dis Sci, 35: 106-112.
Chow JYC, Ma L, & Cho CH (1996) An experimental model for studying
passive cigarette smoking effects on gastric ulceration. Life Sci, 58:
2415-2422.
Christie D, Gordon I, & Robinson K (1986) Smoking in an industrial
population: An analysis by birth cohort. Med J Aust, 145: 11-14.
Christie D, Robinson K, Gordon I, Webley C, & Bisby J (1987) Current
mortality in the Australian petroleum industry: the healthy worker
effect and the influence of life-style factors. Med J Aust, 147:
222-225.
Cinkotai FF, Rigby A, Pickering CAC, Seaborn D, & Faragher E (1988a)
Recent trends in the prevalence of byssinotic symptoms in the
Lancashire textile industry. Br J Ind Med, 45: 782-789.
Cinkotai FF, Emo P, Gibbs ACC, & Jouany JM (1988b) Low prevalence of
byssinotic symptoms in 12 flax scutching mills in Normandy, France. Br
J Ind Med, 45: 325-328.
Clausen J, Netterstrom B, & Wolff C (1993) Lung function in insulation
workers. Br J Ind Med, 50(3): 252-256.
Cochrane AL & Moore F (1980) A 20-year follow-up of a population
sample (aged 25-34) including coal-miners and foundry workers in
Stavely, Derbyshire. Br J Ind Med, 37: 230-233.
Cockroft A, Seal RME, Wagner JC, Lyons JP, Ryder R, & Andersson N
(1982) Post-mortem study of emphysema in coalworkers and
non-coalworkers. Lancet, 2(8298): 600-603.
Coggins CRE (1998) A review of chronic inhalation studies with
mainstream cigarette smoke in rats and mice. Toxicol Pathol, 26(3):
307-314.
Cohen SM & Johansson SL (1992) Epidemiology and etiology of bladder
cancer. Urol Clin North Am, 19(3): 421-428.
Cohen D, Arai SF, & Brain JD (1979) Smoking impairs long-term
clearance from the lung. Science, 204: 514-517.
Cohen BS, Harley NH, & Tso TC (1985) Clearance of polonium-210
enriched cigarette smoke from rat trachea and lung. Toxicol Appl
Pharmacol, 79: 314-322.
Collins AC (1990) Interactions of ethanol and nicotine at the receptor
level. Recent Dev Alcohol, 8: 221-231.
Conolly CK, Chan NS, & Prescott RJ (1988) The relationship between age
and duration of asthma and the presence of persistent obstruction in
asthma. Postgrad Med J, 64(752): 422-425.
Cosio MG, Hale KA, & Niewoehner DE (1980) Morphologic and morphometric
effects of prolonged cigarette smoking on the small airways. Am Rev
Respir Dis, 122: 265-271.
Cotton DJ, Graham BL, Li KYR, Froh F, Barnett GD, & Dosman JA (1983)
Effects of grain dust exposure and smoking on respiratory symptoms and
lung function. J Occup Med, 25(2): 131-141.
Crebelli R, Paoletti A, Falcone E, Aquilana G, Fabri G, & Carere A
(1985) Mutagenicity studies in a tyre plant: in vitro activity of
workers' urinary concentrates and raw materials. Br J Ind Med, 42(7):
481-487.
Creek L, Capehart T, & Griese V (1994) US tobacco statistics,
1935-1992. US Dep Agric Stat Bull, 869: 14.
Cromwell BT (1955) Modern methods of plant analysis -- Volume IV: The
alkaloids. Berlin, Gottingen, Heidelberg, Springer-Verlag, pp 367-516.
Cross FT, Palmer RF, Filipy RE, Dagle GE, & Stuart BO (1982)
Carcinogenic effects of radon daughters, uranium ore dust and
cigarette smoke in beagle dogs. Health Phys, 42(1): 33-52.
Cullinan P, Lowson D, Nieuwenhuijsen MJ, Sandiford C, Tee RD, Venables
KM, McDonald JC, & Newman-Taylor AJ (1994a) Work related symptoms,
sensitisation, and estimated exposure in workers not previously
exposed to flour. Occup Environ Med, 51: 579-583.
Cullinan P, Lowson D, Nieuwenhuijsen MJ, Gordon S, Tee RD, Venables
KM, McDonald JC, & Newman-Taylor AJ (1994b) Work related symptoms,
sensitisation, and estimated exposure in workers not previously
exposed to laboratory rats. Occup Environ Med, 51: 589-592.
Cummings KM, Giovino G, & Mendicino AJ (1987) Cigarette advertizing
and black white differences in brand preference. Public Health Rep,
102(6): 698-701.
Damber L & Larsson LG (1985a) Professional driving, smoking and lung
cancer: a case referent study. Br J Ind Med, 42: 246-252.
Damber L & Larsson LG (1985b) Underground mining, smoking and lung
cancer: a case-control study in the iron ore municipalities in
Northern Sweden. J Natl Cancer Inst, 74(6): 1207-1213.
Darby S, Whitley E, Silcocks P, Thakrar B, Green M, Lomas P, Miles J,
Reeves G, Fearn T, & Doll R (1998) Risk of lung cancer associated with
residential radon exposure in south-west England: a case control
study. Br J Cancer, 78(3): 394-408.
D'Avanzo B, Negri E, La Vecchia C, Gramenzi A, Bianchi C, Franceschi
S, & Boyle P (1990) Cigarette smoking and bladder cancer. Eur J
Cancer, 26(6): 714-718.
Davis BR, Whitehead JK, Gill ME, Lee PN, Butterworth AD, & Roe FJC
(1975) Response of rat lung to inhaled tobacco smoke with or without
prior exposure to 3,4-benzpyrene (BP) given by intratracheal
instillation. Br J Cancer, 31: 469-484.
Davison AG, Fayers PM, Newman Taylor AJ, Venables KM, Darbyshire J,
Pickering CA, Chettle DR, Franklin D, Guthrie CJ, Scott MC, O'Malley
D, Holden H, Mason HJ, Wright AL, & Gompertz D (1988) Cadmium fume
inhalation and emphysema. Lancet, 8587: 663-667.
De Klerk NH, Musk AW, Armstrong BK, & Hobbs MS (1991) Smoking,
exposure to crocidolite, and the incidence of lung cancer and
asbestosis. Br J Ind Med, 48(6): 412-417.
Deng JF, Sinks T, Elliot L, Smith D, Singal M, & Fine L (1991)
Characterisation of respiratory health and exposures at a sintered
permanent magnet manufacturer. Br J Ind Med, 48: 609-615.
Devesa SS, Shaw GL, & Blot WJ (1991) Changing patterns of lung cancer
incidence by histological type. Can Epi Bio Prev, 1: 29-43.
De Wolff FA & Edelbroek PM (1994) Neurotoxicity of arsenic and its
compounds. In: de Wolff FA ed. Vinken and Bruyn's handbook of clinical
neurology. Part I. Intoxications of the nervous system. Amsterdam,
Elsevier, pp 283-291.
De Wolff FA (1995) Antimony and health. Br Med J, 310: 1216-1217.
De Zotti R, Larese F, Bovenzi M, Negro C, & Molinari S (1994) Allergic
airway disease in Italian bakers and pastry makers. Occup Environ Med,
51: 548-552.
DiPaolo JA & Sheehe PR (1962) The combined carcinogenic effect of
cigarette smoke condensate and urethan. Cancer Res, 22: 1058-1060.
Djordjevic MV, Sigountos CW, Hoffmann D, Brunnemann KD, Kagan MR, Bush
LP, Safaev RD, Belitsky GA, & Zaridze D (1991) Assessment of major
carcinogens and alkaloids in the tobacco and mainstream smoke of USSR
cigarettes. Int J Cancer, 47: 348-351.
Djordjevic MV, Fan J, Ferguson S, & Hoffmann D (1995) Self-regulation
of smoking intensity: Smoke yields of the low-nicotine, "low-tar"
cigarettes. Carcinogenesis, 16: 2015-2021.
Doll R & Peto R (1981) The causes of cancer: Quantitative estimates of
avoidable risk of cancer in the United States today. Natl Cancer Inst,
66: 1191-1308.
Domiano SF, Vena JE, & Swanson MK (1985) Gasoline exposure, smoking
and kidney cancer. J Occup Med, 27(6): 398-399.
Donham KJ, Zavala DC, & Merchant JA (1984) Respiratory symptoms and
lung function among workers in swine confinement buildings: a cross
sectional epidemiological study. Arch Environ Health, 39(2): 96-101.
Dontenwill W, Chavalier H-J, Harke H-P, Lafrenz U, Rechzeh G, &
Schneider B (1973) Investigations on the effects of chronic
cigarette-smoke inhalation in Syrian Golden Hamsters. J Natl Cancer
Inst, 51(6): 1781-1832.
Ecobichon DJ (1995) Toxic effects of pesticides. In: Klaassen CD ed.
Casarett and Doull's toxicology, 5th ed. New York, McGraw-Hill,
chapter 22, pp 643-689.
Edling C (1982) Lung cancer and smoking in a group of iron ore miners.
Am J Ind Med, 3(2): 191-199.
Edwards JW & Priestly BG (1993) Sister chromatid exchanges in
lymphocytes of petroleum retailers. Br J Ind Med, 50: 149-154.
Eisenbud M (1993) Lung cancer incidence among patients with beryllium
disease. J Natl Cancer Inst, 85(2): 1697-1698.
Ekenvall L & Lindblad LE (1989) Effect of tobacco use on vibration
white finger disease. J Occup Med, 31(1): 13-16.
Eliasson M, Lundblad D, & Hägg E (1991) Cardiovascular risk factors in
young snuff-users and cigarette smokers. J Intern Med, 230(1): 17-22.
Elliott R (1995) Smoking drinking and occupation: Occupational Health.
In: Drever F ed. Office of Population Censuses and Surveys, Health and
Safety Executive: The registrar general's decennial supplement for
England and Wales. London, Her Majesty's Stationery Office (HMSO), pp
195-201.
Elwood MJ (1981) Wood exposure and smoking: Association with cancer of
the nasal cavity and paranasal sinuses in British Columbia. Can Med
Assoc J, 124: 1573-1577.
Enterline PE, Sykora JL, Keleti G, & Lange JH (1985) Endotoxins,
cotton dust, and cancer. Lancet, 2(8461): 934-935.
Enterline PE, Marsh GM, Henderson V, & Callahan C (1987a) Mortality
update of a cohort of US man-made mineral fibre workers. Ann Occup
Hyg, 31: 625-656.
Enterline PE, Marsh GM, Esmen NA, Henderson VL, Callahan CM, & Paik M
(1987b) Some effects of cigarette smoking, arsenic, and SO2 on
mortality among US copper smelter workers. J Occup Med, 29(10):
831-838.
Falk K, Sorsa M, Vainio H, & Kilpikari I (1980) Mutagenicity in urine
of workers in rubber industry. Mutat Res, 79(1): 45-52.
Finch GL, Nikula KJ, Barr EB, Bechtold WE, Chen BT, Griffith WC, Hahn
FF, Hobbs CH, Hoover MD, Lundgren DL, & Mauderly JL (1995a) Effects of
combined exposure of F344 rats to radiation and chronically inhaled
cigarette smoke: Annual report of the Inhalation Toxicology Research
Institute. Washington, DC, Department of Energy (ITRI-146).
Finch GL, Nikula KJ, Chen BT, Barr EB, Chang I-Y, & Hobbs CH (1995b)
Effect of chronic cigarette smoke exposure on lung clearance of tracer
particles inhaled by rats. Fundam Appl Toxicol, 24: 76-85.
Finch GL, Nikula K, Belinsky S, Barr EB, Stoner G, & Lechner J (1996)
Failure of cigarette smoke to induce or promote lung cancer in the A/J
mouse. Cancer Lett, 99: 161-167.
Fletcher CM (1972) Coal workers pneumoconiosis [letter]. Br Med J, 3:
116.
Fletcher CM & Pride NB (1984) Definitions of emphysema, chronic
bronchitis, asthma, and airflow obstruction. Thorax, 39: 81-85.
Fox AJ, Lindars DC, & Owen R (1974) A survey of occupational cancer in
the rubber and cable making industries: Results of five-year analysis,
1967-71. Br J Ind Med, 31: 140-151.
Fraser-Moodie A (1976) Dupuytren's contracture and cigarette smoking.
Br J Plastic Surg, 29: 214-215.
Friedman GD, Siegeland AB, & Seltzer CC (1969) Cigarette smoking and
exposure to occupational hazards. Am J Epidemiol, 98: 175-183.
Friedman S, Fletcher CM, & Field GB (1972) Effects of smoking modified
cigarettes on respiratory symptoms and ventilatory capacity. J Natl
Cancer Inst, 48: 1805-1810.
Fukuda K, Shibata A, & Harada K (1987) Squamous cell cancer of the
maxillary sinus in Hokkaido, Japan: A case-control study. Br J Ind
Med, 44: 263-266.
Gammon MD, Schoenberg JB, Ahian H, Risch HA, Vaughan TL, Chow WH,
Rotterdam H, West AB, Dubrow R, & Stanford JL (1997) Tobacco, alcohol,
and socioeconomic status and adenocarcinoma of the esophagus and
gastric cardia. J Natl Cancer Inst, 89(17): 1277-1284.
Gardiner AJS, Forey BA, & Lee PN (1992) Avian exposure and
bronchogenic carcinoma. Br Med J, 305: 989-992.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1987) A case-control study of lung cancer
and diesel exhaust exposure in railroad workers. Am Rev Respir Dis,
135: 1242-1248.
Garshick E, Schenker MB, Muñoz A, Segal M, Smith TJ, Woskie SR,
Hammond SK, & Speizer FE (1988) A retrospective cohort study of lung
cancer and diesel exhaust exposure in railroad workers. Am Rev Respir
Dis, 137: 820-825.
Geng Y, Savage SM, Johnson LJ, Seagrave J, & Sopori ML (1995) Effects
of nicotine on the immune system: I. Chronic exposure to nicotine
impairs antigen receptor-mediated signal transduction in lymphocytes.
Toxicol Appl Pharmacol, 135: 268-278.
Geng Y, Savage SM, Razanai-Boroujerdi S, & Sopori ML (1996) Effects of
nicotine on the immune system: II. Chronic nicotine treatment induces
T cell anergy. J Immunol, 156(7): 2384-2390.
Gerhardsson L & Nordberg GF (1993) Lung cancer in smelter workers
- interactions of metals as indicated by tissue levels. Scand J Work
Environ Health, 19(suppl 1): 90-94.
Gibbs GW & Horowitz I (1979) Lung cancer mortality in aluminium
reduction plant workers. J Occup Med, 21(5): 347-353.
Gibinski K (1977) Studies on cardiovascular effects of smoking in
Poland and other Eastern European countries. In: Proceedings of 3rd
World Conference on Smoking and Health, New York City, 2-5 June 1975
- Volume II: Health consequence, education, cessation and social
actions. Washington, DC, US Department of Health, Education and
Welfare, pp 179-184 (DHEW Publication No. (NIH) 77:1413).
Gonzalez CA, Lopez-Abente G, Errezola M, Castejon J, Estrada A,
Garcia-Mila M, Gili P, Huguet M, Serrat M, Soler F, & Rodriguez C
(1985) Occupation, tobacco use, coffee and bladder cancer in the
county of Mataro (Spain). Cancer, 55: 2031-2034.
Gonzalez CA, Lopez-Abente G, Errezola M, Escolar A, Riboli E,
Izarzugaza I, & Nebot M (1989) Occupation and bladder cancer in Spain:
a multi-centre case control study. Int J Epidemiol, 18(3): 569-577.
Greenberg M, Milne JF, & Watt A (1970) A survey of workers exposed to
dusts containing derivatives of Bacillus subtilis. Br Med J, ii:
629-633.
Greenland S (1993) Basic problems in interaction assessment. Environ
Health Perspect, 101(suppl 4): 59-65.
Grimmer G, Schneider D, Nanjack KW, Deltbarn G, & Jacob J (1995)
Intercept-reactant method for the determination of aromatic amines in
mainstream tobacco smoke. Beitr Tabilk-Forsch, 16: 141-156.
Grove WJ & Stansby GP (1992) Buerger's disease and cigarette smoking
in Bangladesh. Ann R Coll Surg Engl, 74: 115-118.
Guggenheimer J (1991) Implications of smokeless tobacco use in
athletes [Review]. Dent Clin North Am, 35(4): 797-808.
Gullvåg B, Mylius E, & Nilsen A (1985) Counting alveolar macrophages
from expectorate samples of exposed workers as a test for lung
irritation from occupational exposure. Bull Environ Contam Toxicol,
34: 702-708.
Hammond EC & Selikoff IJ (1973) Relation of cigarette smoking to risk
of death of asbestos- associated disease among insulation workers in
the United States. In: Bogovski P, Gilson JC, Timbrell V, & Wagner JC
Biological effects of asbestos. Lyon, International Agency for
Research on Cancer, pp 312-317 (IARC Scientific Publication No. 8).
Hammond EC, Selikoff IJ, & Seidman H (1979) Asbestos exposure,
cigarette smoking and death rates. Ann NY Acad Sci, 333: 473-489.
Hanke W, Sepulveda MJ, Watson A, & Jankovic J (1984) Respiratory
morbidity in wollastonite workers. Br J Ind Med, 41: 474-479.
Harf RA, Ethevenaux C, Gleize J, Perrin-Fayolle M, Guerin JG, &
Ollagnier C (1986) Reduced prevalence of smokers in sarcoidosis:
Results of a case-control study. Ann NY Acad Sci, 465: 625-631.
Harley NH, Cohen BS, & Tso TC (1980) Polonium-210: A questionable risk
factor in smoking-related carcinogenesis. In: Gori GB & Bock FG ed. A
safe cigarette? Cold Spring Harbor, New York, Cold Spring Harbor
Laboratory, pp 93-104 (Banbury Report No. 3).
Harris RJC & Negroni G (1967) Production of lung carcinomas in C57BL
mice exposed to a cigarette smoke and air mixture. Br Med J, 4:
637-641.
Harris RJC, Negroni G, Ludgate S, Pick CR, Chesterman FC, & Maidment
BJ (1974) The incidence of lung tumours in C57BL mice exposed to
cigarette smoke: air mixtures for prolonged periods. Int J Cancer, 14:
130-136.
Hassler E, Lind B, & Piscator M (1983) Cadmium in blood and urine
related to present and past exposure: A study of workers in an
alkaline battery factory. Br J Ind Med, 40: 420-425.
Haugen A, Becher G, Benestad C, Vahakangas K, Trivers GE, Newman MJ, &
Harris CC (1986) Determination of polycyclic aromatic hydrocarbons in
the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA,
and antibodies to the adducts in sera from coke oven workers exposed
to measured amounts of polycyclic aromatic hydrocarbons in the work
atmosphere. Cancer Res, 46(8): 4178-4183.
Hawker R & Holtby I (1988) Smoking and absence from work in a
population of student nurses. Public Health (Lond), 102: 161-167.
Hayes RB, Gerin M, Raatgever JW, & de Bruyn A (1986) Wood-related
occupations, wood dust exposure, and sinonasal cancer. Am J Epidemiol,
124(4): 569-577.
Health Canada (1994) Risk assessment for the combustion products of
methylcyclo-pentadienyl manganese tricarbonyl (MMT) in gasoline.
Ottawa, Ontario, Health Canada, Environmental Health Directorate, 113
pp.
Hecht SS & Hoffmann D (1988) Tobacco specific nitrosamines, an
important group of carcinogens in tobacco and tobacco smoke.
Carcinogenesis, 9(6): 875-884.
Hecht SS & Hoffmann D (1991) N-nitroso compounds and tobacco-induced
cancers in man. In: O'Neill IK, Chen J, & Bartsch H ed. Relevance to
human cancer of N-nitroso compounds, tobacco smoke and mycotoxins.
Lyon, International Agency for Research on Cancer, pp 54-61 (IARC
Scientific Publications No. 105).
Hecht SS, Adams JD, Numoto S, & Hoffmann D (1983) Induction of
respiratory tract tumors in Syrian golden hamsters by a single dose of
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 4:
1287-1290.
Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, & Hoffmann D
(1986) Induction of oral cavity tumors in F344 rats by
tobacco-specific N-nitrosamines and snuff. Cancer Res, 46:
4162-4166.
Hecht SS, Carmella SG, Murphy SE, Akerkar S, Brunnemann KD, & Hoffmann
D (1993) A tobacco-specific lung carcinogen in the urine of men
exposed to cigarette smoke. N Engl J Med, 329: 1543-1546.
Henry CJ & Kouri RE (1984) Chronic exposure of mice to cigarette smoke
-- Final Report to the Council for Tobacco Research -- USA, Inc.
(Contract CTR-0030). New York, Field, Rich and Associates, Inc.
Herity B, Moriarity M, Daly L, Dunn J, & Bourke GJ (1982) The role of
tobacco and alcohol in the aetiology of lung and larynx cancer. Br J
Cancer, 46: 961-964.
Hertz-Picciotto I, & Hu SW (1994) Contribution of cadmium in
cigarettes to lung cancer: an evaluation of risk assessment
methodologies. Arch Environ Health, 49(4): 297-302.
Hertz-Picciotto I, Smith AH, Holtzman D, Lipsett M, & Alexeef G (1992)
Synergism between occupational arsenic exposure and smoking in the
induction of lung cancer. Epidemiology, 3(1): 23-31.
Hessel PA, Sluis-Cremer GK, & Lee SL (1991) Distribution of silicotic
collagenization in relation to smoking habits. Am Rev Respir Dis,
144(2): 297-301.
Hicks RM (1982) Nitrosamines as possible etiological agents in
bilharzial bladder cancer. In: Magee PM ed. Nitrosamines and human
cancer. Cold Spring Harbor, New York, Cold Spring Harbor Laboratory,
pp 455-471 (Banbury Report No. 12).
Hicks RM, James C, & Webbe G (1980) Effect of Schistosoma
haematobium and N-butyl- N-(4-hydroxybutyl)nitrosamine on the
development of urothelial neoplasia in baboon. Br J Cancer, 42:
730-755.
Higginson J, Muir C, & Buffler PA (1984) The epidemiology of renal
carcinoma in humans with a note on the effect of exposure to gasoline.
In: Mehlman MA, Hemstreet GP III, Thorp JJ, & Weaver NK ed. Advances
in modern environmental toxicology -- Volume VII: Renal effects of
petroleum hydrocarbons. Princeton, New Jersey, Princeton Scientific
Publishers, pp 203-226.
Hilt B, Langard S, Lund-Larsen PG, & Lien JT (1986) Previous asbestos
exposure and smoking habits in the county of Telemark, Norway -- A
cross-sectional population study. Scand J Work Environ Health, 12:
561-566.
Hinds WC, First MW, Huber GI, & Shea JW (1983) A method for measuring
respiratory deposition of cigarette smoke during smoking. Am Ind Hyg
J, 44: 113-118.
Hirsch JM & Thilander H (1981) Snuff-induced lesions of the oral
mucosa: an experimental model in the rat. J Oral Pathol, 10: 342-353.
Hnizdo E (1990) Combined effect of silica dust and tobacco smoking on
mortality from chronic obstructive lung disease in gold miners. Br J
Ind Med, 47: 656-664.
Hnizdo E & Sluis-Cremer GK (1991) Silica exposure, silicosis, and lung
cancer: a mortality study of South African gold miners. Br J Ind Med,
48(1): 53-60.
Hnizdo E, Baskind E, & Sluis-Cremer GK (1990) Combined effects of
silica dust exposure and tobacco smoking on the prevalence of
respiratory impairments among gold miners. Scand J Work Environ
Health, 16: 411-422.
Hocking WG & Golde DW (1979) The pulmonary-alveolar macrophage. N Engl
J Med, 301: 580-587, 639-645.
Hoffmann D & Hoffmann I (1991) Recent developments in smoking related
cancer research. Smok Relat Dis, 5(2): 77-93.
Hoffmann D & Hoffmann I (1997) The changing cigarette: 1950-1995. J
Toxicol Environ Health, 50: 307-364.
Hoffmann D & Wynder EL (1976) Smoking and occupational cancer. Prev
Med, 5: 245-261.
Hoffmann D, Rathkamp G, & Wynder EL (1961) Comparison of the yields of
several selected compounds in the smoke of different tobacco products.
J Natl Cancer Inst, 31: 627-637.
Hoffmann D, Sanghvi LD, & Wynder EL (1974) Chemical studies on tobacco
smoke: XXVIII. Comparative chemical analysis of Indian bidi and
American cigarette smoke. Int J Cancer, 14: 49-53.
Hoffmann D, Rivenson A, Hecht SS, Hilfrich J, Kobayashi N, & Wynder EL
(1979) Model studies in tobacco carcinogenesis with the Syrian Golden
Hamster. Prog Exp Tumor Res, 24: 370-390.
Hoffmann D, Harley NH, Fisenne I, Adams JD, & Brunnemann KD (1986)
Carcinogenic agents in snuff. J Natl Cancer Inst, 76: 435-437.
Hoffmann D, Rivenson A, Chung FL, & Hecht SS (1991a) Nicotine-derived
nitrosamines and their relevance in tobacco carcinogenesis. Crit Rev
Toxicol, 21(4): 305-311.
Hoffmann D, Melikian AA, & Brunnemann KD (1991b) Studies in tobacco
carcinogenesis. Lyon, International Agency for Research on Cancer, pp
482-484 (IARC Scientific Publications No. 105).
Hoffmann D, Brunnemann KD, Prokopczyk B, & Djordjevic MV (1994)
Tobacco-specific N-nitrosamines and areca-derived N-nitrosamines:
chemistry, biochemistry, carcinogenicity, and relevance to humans. J
Toxicol Environ Health, 41: 1-52.
Holbrook JH (1977) Tobacco and health. CA-Cancer J Clin, 27: 344-353.
Holst PA, Kromhout D, & Brand R (1988) For debate: pet birds as an
independent risk factor for lung cancer. Br Med J, 297: 1319-1324.
Honeybourne D & Pickering CAC (1986) Physiological evidence that
emphysema is not a feature of byssinosis. Thorax, 41(1): 6-11.
Hsu TC, Furlong C, & Spitz MR (1991) Ethyl alcohol as a co-carcinogen
with special reference to the aerodigestive tract: a cytogenetic
study. Anticancer Res, 11: 1097-1102.
Hughes JM & Weill H (1991) Asbestos as a precursor of asbestos related
lung cancer: Results of a prospective mortality study. Br J Ind Med,
48: 229-233.
IARC (1984) Polynuclear aromatic compounds: Part 3. Industrial
exposures in aluminum production, coal gasification, coke production,
and iron and steel founding. Lyon, International Agency for Research
on Cancer, pp 37-190 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 34).
IARC (1985a) Tobacco habits other than smoking: Betel-quid and
areca-nut chewing and some related nitrosamines. Lyon, International
Agency for Research on Cancer, 291 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
37).
IARC (1985b) Polynuclear aromatic compounds: Part 4. Bitumens,
coal-tars and derived products, shale-oils and soots. Lyon,
International Agency for Research on Cancer, pp 83-241 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
35).
IARC (1986) Tobacco smoking. Lyon, International Agency for Research
on Cancer, 421 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 38).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, 440 pp (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IARC (1988) Man-made mineral fibres and radon. Lyon, International
Agency for Research on Cancer, 300 pp (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Volume 43).
IARC (1989a) Antimony trioxide and antimony trisulfide. In: Some
organic solvents, resin monomers and related compounds, pigments and
occupational exposures in paint manufacture and painting. Lyon,
International Agency for Research on Cancer, pp 291-305 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
47).
IARC (1989b) Occupational exposures in petroleum refining; crude oil
and major petroleum fuels. Lyon, International Agency for Research on
Cancer, pp 109-110 (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 45).
IARC (1989c) Diesel and gasoline engine exhaust and some nitroarenes.
Lyon, International Agency for Research on Cancer, pp 41-185 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
46)..
IARC (1990) Chromium, nickel and welding. Lyon, International Agency
for Research on Cancer, pp 257-445 (IARC Monographs on the Evaluation
of Carcinogenic Risks to Humans, Volume 49).
IARC (1991) Tenth International Meeting on N-Nitroso Compounds,
Mycotoxins and Tobacco Smoke: Relevance to Human Cancer. Lyon,
International Agency for Research on Cancer, 594 pp (IARC Scientific
Publications No. 105).
IARC (1993) Beryllium, cadmium, mercury, and exposures in the glass
manufacturing industry. Lyon, International Agency for Research on
Cancer, 444 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 58).
IARC (1994a) Ethylene oxide. In: Some industrial chemicals. Lyon,
International Agency for Research on Cancer, pp 73-159 (IARC
Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume
60).
IARC (1994b) Infection with schistosomes ( Schistosoma haematobium,
Schistosoma mansoni and Schistosoma japonicul). In: Schistosomes,
liver flukes and Helicobacter pylori. Lyon, International Agency for
Research on Cancer, Volume 61).
IARC (1995) Wood dust and formaldehyde. Lyon, International Agency for
Research on Cancer, pp 191-194 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 62).
ICRP (International Commission on Radiological Protection) (1994)
Human respiratory tract model for radiological protection. Amsterdam,
Oxford, New York, Elsevier Science Publishers (ICRP Publication No.
66).
Idris AM, Nair J, Oshima H, Friesen M, Brouet I, Faustman EM, &
Bartsch H (1991) Unusual high levels of carcinogenic tobacco-specific
nitrosamines in Sudan snuff (toombak). Carcinogenesis, 12: 1115-1118.
Idris AM, Prokopczyk B, & Hoffmann D (1994) Toombak: a major risk
factor for cancer of the oral cavity in Sudan. Prev Med, 23: 832-839.
ILO (1991) Smoking contributes to noise-induced hearing loss: ILO
contribution to WHO -- World no tobacco day, 1992. Geneva,
International Labour Organisation.
Inskip PD & Boice JD (1994) Radiotherapy-induced lung cancer among
women who smoke. Cancer, 73(6): 1541-1543.
IPCS (1990) Environmental health criteria 106: Beryllium. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 40-53.
IPCS (1991a) Environmental health criteria 108: Nickel. Geneva, World
Health Organization, International Programme on Chemical Safety, pp
48-67.
IPCS (1991b) Environmental health criteria 125: Platinum. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 79-80.
IPCS (1992) Environmental health criteria 134: Cadmium. Geneva, World
Health Organization, International Programme on Chemical Safety, 280
pp.
IPCS (1995) Environmental health criteria 165: Inorganic lead. Geneva,
World Health Organization, International Programme on Chemical Safety,
pp 93-98.
IPCS (1996) Environmental health criteria 171: Diesel fuel and exhaust
emissions. Geneva, World Health Organization, International Programme
on Chemical Safety, 389 pp.
ISAAC (1998) International Study of Asthma and Allergies in Childhood
(ISAAC) Steering Committee: Worldwide variation in the prevalence of
symptoms of asthma, allergic rhino conjunctivitis, and atopic eczema.
Lancet, 351: 1225-1232.
Iscovich J, Castelletto R, Esteve J, Muñoz N, Colanzi R, Coronel A,
Deamezola I, Tassi V, & Arslan A (1987) Tobacco smoking, occupational
exposure and bladder cancer in Argentina. Int J Cancer, 40: 734-740.
Istvan J & Matarazzo JD (1984) Tobacco, alcohol and caffeine use: a
review of their interrelationships. Psychol Bull, 95: 301-326.
Iwata F, Zhang XY, & Leung FW (1995) Aggravation of gastric mucosal
lesions in rat stomach by tobacco cigarette smoke. Dig Dis Sci, 40:
1118-1124.
Izard C & Liberman C (1978) Acrolein. Mutat Res, 47(2): 115-138.
Jarup L & Pershagen G (1991) Arsenic exposure, smoking and lung cancer
in smelter workers -- A case-control study. Am J Epidemiol, 134(6):
545-551.
Järvholm B, Larsson S, Hagberg S, Olling S, Ryd W, & Torén K (1993)
Quantitative importance of asbestos as a cause of lung cancer in a
Swedish industrial city: a case-referent study. Eur Respir J, 6(9):
1271-1275.
Jayant K & Pakhale SS (1985) Toxic constituents in bidi smoke. In:
Sanghvi LD & Notami P ed. Tobacco and health: The Indian scene.
Bombay, Tata Memorial Center, pp 101-110.
Jindal RM & Patel SM (1992) Buerger's disease and cigarette smoking in
Bangladesh. Ann R Coll Surg Engl, 74(6): 436-437.
Johansson SL, Hirsch JM, Larsson PA, Saidi J, & Oesterdahl BG (1989)
Snuff-induced carcinogenesis: effect of snuff in rats initiated with
4-nitroquinoline- N-oxide. Cancer Res, 49: 3063-3069.
JOM (1984) Vibration syndrome in chipping and grinding workers. J
Occup Med, 26(10): 765-788.
Jones RD (1994) Survey of antimony workers: mortality 1961-92. Occup
Environ Med, 51(11): 772-776.
Jörgensen HS (1984) Lung cancer among underground workers in the iron
ore mine of Kiruna based on thirty years of observation. Ann Acad Med
Singap, 13(suppl 2): 371-377.
Jörgensen HS, Kolmodin-Hedman B, & Stjernberg N (1988) Follow-up study
of pulmonary function and respiratory tract symptoms in workers in a
Swedish iron ore mine. J Occup Med, 30(12): 953-957.
Jorquera R, Castonguay A, & Schüller HM (1992) Effects of pregnancy
and ethanol treatment on the metabolism of 4-(methyl
nitrosamino)-1-(pyridyl)-1-butanone by hamster liver and lung
microsomes. Drug Metab Dispos, 20: 510-517.
Jusko WJ (1978) Role of tobacco smoking in pharmacokinetics. J
Pharmacokinet Biopharm, 6: 7-39.
Jussawalla DJ & Deshpande VA (1971) Evaluation of cancer risk in
tobacco chewers and smokers: an epidemiological assessment. Cancer,
28(1): 244-252.
Kaldor JM & L'Abbe KA (1990) Interaction between human carcinogens.
In: Vainio H, Sorsa M, & McMichael AJ ed Complex mixtures and cancer
risk. Lyon, International Agency for Research on Cancer, pp 35-43
(IARC Scientific Publications No. 104).
Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, & Fraumeni
JF (1984) Urinary tract infection and risk of bladder cancer. Am J
Epidemiol, 119(4): 510-515.
Kawajiri K & Fujii-Kuriyama Y (1991) P450 and human cancer. Jpn J
Cancer Res, 82(12): 1325-1335.
Kawajiri K, Nakachi K, Imai K, Hyashi S, & Watanabe J (1990)
Individual differences in lung cancer susceptibility in relation to
polymorphysms of P-450IA1 gene and cigarette dose. Princess Takamatsu
Int Symp Ser, 21: 55-61.
Kazantzis G & Armstrong BG (1984) Renal function in relation to low
levels of cadmium exposure in a group of smelter workers. Environ
Health Perspect, 54: 193-199.
Kazantzis G, Blanks RG, & Sullivan KR (1992) Is cadmium a human
carcinogen? In: Nordberg GF, Herber REM, & Alessio L ed. Cadmium in
the human environment: Toxicity and carcinogenicity. Lyon,
International Agency for Research on Cancer, pp 435-445.
Keller AZ & Terris M (1965) The association of alcohol and tobacco
with cancer of the mouth and pharynx. Am J Publ Health, 55: 1578-1585.
Keen P, de Moor NG, Shapiro MP, & Cohen L (1955) The etiology of
respiratory tract cancer in the South African Bantu. Br J Cancer, 9:
528-538.
Kibelstis JA, Morgan EJ, Reger R, Lapp NL, Seaton A, & Morgan WK
(1973) Prevalence of bronchitis and airway obstruction in American
bituminous coal miners. Am Rev Respir Dis, 108(4): 886-893.
Kilburn KH (1989) Byssinosis, emphysema, and mortality of American
textile workers: [Editorial]. Arch Environ Health, 38(5): 277.
Kilpikari MD (1982) Mortality among male rubber workers in Finland.
Arch Environ Health, 37(5): 295-299.
Kimura N (1977) Epidemiological studies in Japan on smoking and
cardiovascular disease. In: Proceedings of 3rd World Conference on
Smoking and Health, New York City, 2-5 June 1975. Volume II: Health
consequence, education, cessation and social actions. Washington, DC,
US Department of Health, Education and Welfare, pp 185-198 (DHEW
Publication (NIH) 77:1413).
King M, Wight A, DeSanctis GT, el-Azab J, Phillips DM, Angus GE, &
Cosio MG (1989) Mucus hypersecretion and viscoelasticity changes in
cigarette smoking dogs. Exp Lung Res, 15(3): 375-389.
Kleinfeld M, Messite J, & Langer AM (1973) A study of workers exposed
to asbestiform minerals in commercial talc manufacture. Environ Res,
6: 132-143.
Kobayashi W, Hoffmann D, & Wynder EL (1974) A study of tobacco
carcinogenesis: XII. Epithelial changes induced in the upper
respiratory tracts of Syrian golden hamsters by cigarette smoke. J
Natl Cancer Inst, 534: 1085-1089.
Kobusch AB, Simard A, Feldstein M, Vauclair R, Gibbs GW, Bergeron F,
Morissette N, & Davis R (1984) Pulmonary cytology in chrysotile
asbestos workers. J Chron Dis, 37(8): 599-607.
Kodell RL & Pounds JG (1991) Assessing the toxicity of chemical
mixtures. In: Krewski D & Franklin CA ed. Statistics in toxicology.
New York, Gordan & Breach, pp 557-589.
Kodell RL, Krewski D, & Zielinski J (1991) Additive and multiplicative
relative risk in the two-stage clonal expansion model of
carcinogenesis. Risk Anal, 11: 483-490.
Kohlmeier L, Arminger G, Bartolomeycik S, Bellach B, Rehm J, & Thamm M
(1992) Pet birds as an independent risk factor for lung cancer:
case-control study. Br Med J, 305: 986-989.
Koppang N, Rivenson A, Reith A, Dahle HK, Evenson O, & Hoffman D
(1992) A study of tobacco carcinogenesis: XLVIII. Carcinogenicity of
N'-nitrosonornicotine in mink ( Mustela vison). Carcinogenesis,
13(11): 1957-1960.
Koppang N, Rivenson A, Dahle HK, & Hoffman D (1997) A study of tobacco
carcinogenesis: LIII. Carcinogenicity of N'-nitrosonornicotine (NNN)
and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in mink
( Mustela vison). Cancer Lett, 111: 167-171.
Kotin P (1994) The epidemiological evidence on the carcinogenicity of
beryllium, by MacMahon: [Editorial]. J Occup Med, 36(1): 25-26.
Kreiss K, Wassermann S, Mroz MM, & Newman LS (1993) Beryllium disease
screening in the ceramics industry. J Occup Med, 35: 16-25.
Kreiss K, Mroz MM, Newman LS, Martyny J, & Zhen B (1996) Machining
risk of beryllium disease and sensitization with median exposures
below 2 µg/m3. Am J Ind Med, 30: 16-25.
Kremer AM, Pal TM, Boleij JSM, Schouten JP, & Rijcken B (1994) Airway
hyperresponsiveness, prevalence of chronic respiratory symptoms, and
lung function in workers exposed to irritants. Occup Environ Med, 51:
3-13.
Krishna K (1978) Tobacco chewing in pregnancy. Br J Obstet Gynaecol,
85: 726-728.
Kristensen TS (1989) Cardiovascular disease and the work environment:
A critical review of the epidemiological literature on chemical
factors. Scand J Work Environ Health, 15: 245-264.
Krivan V, Schneider G, Baumann H, & Reus (1994) Multi-element
characterization of tobacco smoke condensate. Fresenius J Anal Chem,
348: 218-225.
Kuntz WD & McCord CP (1974) Polymer-fume fever. J Occup Med, 16(7):
480-482.
Kusiak RA, Springer J, Ritchie AC, & Muller J (1991) Carcinoma of the
lung in Ontario gold miners: possible aetiological factors. Br J Ind
Med, 48: 808-817.
Kusiak RA, Ritchie AC, Muller J, & Springer J (1993) Mortality from
lung cancer in Ontario uranium miners. Br J Ind Med, 50(10): 920-928.
Lai YL & Diamond L (1992) Cigarette smoke exposure does not prevent
cadmium-induced alterations in rat lungs. J Toxicol Environ Health,
35(1): 63-76.
LGC (Laboratory of the Government Chemist) (1982) Report of the
Government chemist, 1981. London, Her Majesty's Stationery Office
(HMSO), p 109.
Lakier JB (1992) Smoking and cardiovascular disease. Am J Med,
93(suppl 1A): 8-12.
Lamm SH, Parkinson M, Anderson M, & Taylor W (1992) Determinants of
lung cancer risk among cadmium-exposed workers. Ann Epidemiol, 2(3):
195-211.
Lancet (1985) Smoking, occupation, and allergic lung disease. Lancet,
1(8435): 965.
Landrigan PJ & Straub WE (1985) Occupational lead exposure aboard a
tall ship. Am J Ind Med, 8(3): 233-239.
Langård S & Norseth T (1975) A cohort study of bronchial carcinomas in
workers producing chlorinated pigments. Br J. Ind Med, 32: 62-65.
Langård S & Vigander T (1983) Occurrence of lung cancer in workers
producing chromium pigments. Br J Ind Med, 40(1): 71-74.
Larsson PA, Johansson SL, Vahlne A, & Hirsch JM (1989) Snuff
carcinogenesis: effects of long-term snuff administration after
initiated with 4-nitroquinoline- N-oxide and Herpes simplex virus
type 1. J Oral Pathol Med, 18: 187-192.
La Vecchia C, Negri E, D'Avanzo B, Savoldelli R, & Franceschi S (1991)
Genital and urinary tract diseases and bladder cancer. Cancer Res,
51(2): 629-631.
La Voie EJ, Adams JD, Reinhardt J, Rivenson A, & Hoffmann D (1986)
Toxicity studies on clove cigarette smoke and constituents of clove:
Determination of the LD50 of eugenol by intratracheal installation in
rats and hamsters. Arch Toxicol, 59: 78-81.
Leanderson P & Tagesson C (1989) Cigarette smoke potentiates the
DNA-damaging effect of manmade mineral fibers. Am J Ind Med, 16:
697-706.
Lednar WM, Tyroler HA, McMichael AJ, & Shy CM (1977) The occupational
determinants of chronic disabling pulmonary disease in rubber workers.
J Occup Med, 19: 263-268.
Leduc D, de Francquen P, Jacobovitz D, Vanderweyer R, Lauwerys R, &
De Vuyst P (1993) Association of cadmium exposure with rapidly
progressive emphysema in a smoker. Thorax, 48: 570-571.
Lehtovirta P & Forss M (1978) The acute effect of smoking on
intervillous blood flow of the placenta. Br J Obstet Gynaecol, 85:
729-731.
Leigh J, Outhred KG, McKenzie HI, Glick M, & Wiles AN (1983)
Quantified pathology of emphysema, pneumoconiosis, and bronchitis in
coal workers. Br J Ind Med, 40: 258-263.
Letourneau EG, Krewski D, Choi NW, Goddard MJ, McGregor MJ, Zielinsky
JM, & Du J (1994) Case-control study of residential radon and lung
cancer in Winnipeg, Manitoba, Canada. Am J Epidemiol, 140(4): 310-322.
Liddell FD, Thomas DC, Gibbs GW, & McDonald JC (1984) Fibre exposure
and mortality from pneumoconiosis, respiratory, and abdominal
malignancies in chrysotile production in Quebec, 1926-1975. Ann Acad
Med Singap, 13(suppl): 340-344.
Lidegaard O (1993) Oral contraceptive use and risk of a cerebral
thromboembolic attack: Results of a case-control study. Br Med J, 306:
956-963.
Lilis R, Valciukas JA, Weber JP, Malkin J, & Selikoff IJ (1984a)
Epidemiological study of renal function in copper smelter workers.
Environ Health Perspect, 54: 181-192.
Lilis R, Valciukas JA, Weber JP, Fischbein A, Nicholson WJ, Campbell
C, Malkin J, & Selikoff IJ (1984b) Distribution of blood lead, blood
cadmium, urinary cadmium, and urinary arsenic levels in employees of a
copper smelter. Environ Res, 33: 76-95.
Lindberg E & Hedenstierna G (1983) Chrome plating: symptoms, findings
in the upper airways and effects on lung function. Arch Environ
Health, 38(6): 364-374.
Ljungman AG, Lindahl M, & Tagesson C (1994) Asbestos fibres and man
made mineral fibres: induction and release of tumour necrosis factor
from rat alveolar macrophages. Occup Environ Med, 51: 777-783.
Lourenco RV, Klimek MF, & Borowski CJ (1971) Deposition and clearance
of 2 micron particles in the tracheobronchial tree of normal subjects
-- smokers and nonsmokers. J Clin Invest, 50: 65-83.
Lubin JH & Boice JD (1997) Lung cancer risk from residential radon:
Meta-analysis of eight epidemiologic studies. J Natl Cancer Inst,
89(1): 49-57.
Lubin JH, Boice JD, Edling C, Hornung RW, Howe GR, Kunz E, Kusiak RA,
Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, Yao SX, &
Pierce DA (1995) Lung cancer in radon-exposed miners and estimation of
risk from indoor exposure. J Natl Cancer Inst, 87(11): 817-827.
Lubin JH, Tomasek L, Edling C, Hornung RW, Howe G, Kunz E, Kusiak RA,
Morrison HI, Radford EP, Samet JM, Tirmarche M, Woodward A, & Yao SX
(1997) Estimating lung cancer mortality from residential radon using
data for low exposures of miners. Radiat Res, 147(2): 126-134.
McCoy GD, Hecht SS, Katayama S, & Wynder EL (1981) Differential effect
of chronic ethanol consumption on the carcinogenicity of
N-nitrosopyrolidine and N-nitrosonornicotine in male Syrian golden
hamsters. Cancer Res, 41: 2849-2854.
McDuffie HH, Klaassen DJ, & Dosman JA (1990) Is pesticide use related
to the risk of primary lung cancer in Saskatchewan? J Occup Med,
32(10): 996-1002.
Mackenney RP (1983) A population study of Dupuytren's contracture.
Hand, 15(2): 155-161.
McLaughlin RF & Tueller EF (1971) Anatomic and histologic changes of
early emphysema. Chest, 59: 592-599.
McLintock JC (1971) The selection of juvenile entrants to mining. Br J
Ind Med, 28: 45-51.
MacMahon B (1994) The epidemiological evidence on the carcinogenicity
of beryllium in humans. J Occup Med, 36(1): 15-24.
Magnus K, Andersen A, & Hegetveit AC (1982) Cancer of respiratory
organs among workers at a nickel refinery in Norway. Int J Cancer, 30:
681-685.
Maheswaren R, Gill JS, & Beevers DG (1993) Blood pressure and
industrial lead exposure. Am J Epidemiol, 137(6): 645-653.
Makhyoun NA (1974) Smoking and bladder cancer in Egypt. Br J Cancer,
30: 577-581.
Mann JI, Vessey MP, Thorogood M, & Doll R (1975) Myocardial infarction
in young women with special reference to oral contraceptive practice.
Br Med J, 2: 241-245.
Mann JI, Doll R, Thorogood M, Vessey MP, & Waters WE (1976) Risk
factors for myocardial infarction in young women. Br J Prev Soc Med,
30: 94-100.
Marine WM, Gurr D, & Jacobsen M (1988) Clinically important
respiratory effects of dust exposure and smoking in British coal
miners. Am Rev Respir Dis, 137: 106-112.
Martell EA (1974) Radioactivity of tobacco trichomes and insoluble
smoke particles. Nature (Lond), 249: 215-217.
Martischnig KM, Newell DJ, Barnsley WC, Cowan WK, Feinmann EL, &
Oliver E (1977) Unsuspected exposure to asbestos and bronchogenic
carcinoma. Br Med J, 1(6063): 746-749.
Mayberry RM (1985) Cigarette smoking, herpes simplex virus type 2
infection, and cervical abnormalities. Am J Pub1 Health, 75(6):
676-678.
Mehta FS, Pindborg JJ, Hamner JE, Gupta PC, Daftary DK, Sahiar BE,
Shroff BC, Sanghvi LD, Bhonsle RB, Choksi SK, Dandekar VV, Mehta YN,
Pitkar VK, Sinor PN, Shah NC, Turner PS, & Upadhyay SA (1971) Report
on investigations of oral cancer and precancerous lesions in Indian
rural populations, 1966-1969. Copenhagen, Munksgaard, pp 48-120.
Merchant JA, Kilburn KH, O'Fallon WM, Hamilton JD, & Lumsden JC (1972)
Byssinosis and chronic bronchitis among cotton textile workers. Ann
Intern Med, 76: 423-433.
Merchant JA, Lumsden JC, Kilburn KH, O'Fallon WM, Ujida JR, & Germino
VH (1973) An industrial study of the biological effects of cotton dust
and cigarette smoke exposure. J Occup Med, 15(3): 212-221.
Meurman LO, Kiviluoto R, & Hakama M (1979) Combined effects of
asbestos exposure and tobacco smoking on Finnish anthophyllite miners
and millers. Ann NY Acad Sci, 330: 491-495.
Michaylov MA, Pressyanov DS, & Kalinov KB (1995) Bronchial dysplasia
induced by radiation in miners exposed to 222Rn progeny. Occup Environ
Med, 52: 82-85.
Mikkelsen OA (1978) Dupuytren's disease-the influence of occupation
and previous hand injuries. Hand, 10(1): 1-8.
Miller LG (1990) Cigarettes and drug therapy: Pharmacokinetic and
pharmacodynamic considerations. Clin Pharmacol, 9: 125-135.
Miller A (1993) Pulmonary function in asbestosis and asbestos related
pleural disease. Environ Res, 61(1): 1-18.
Miller BG & Jacobsen M (1985) Dust exposure, pneumoconiosis, and
mortality of coal miners. Br J Ind Med, 42(11): 723-733.
Miller FJ & Kimbell JS (1995) Regional dosimetry of inhaled reactive
gases. In: McClellan RO & Henderson RF ed. Concepts in inhalation
toxicology, 2nd ed. Washington, DC, Taylor & Francis, chapter 10, pp
257-288.
Misiewicz A, Radwan K, Karmolinski M, & Dziewit T (1992) [Degree of
ventilation disturbances in persons with occupational exposure to
manganese.] Wiad Lek, 45(23-24): 890-893 (in Polish).
Misiewicz A, Radwan K, Karmolinski M, Dziewit T, & Matysek A (1994)
[Chronic bronchitis in workers producing iron-manganese alloys.] Wiad
Lek, 47(7-8): 257-261 (in Polish).
Mitacek EJ, Brunnemann KD, & Polednak XP (1990) "Tar", nicotine and
carbon monoxide content of Thai cigarettes and implication for cancer
prevention in Thailand. Cancer Detect Prev, 14: 515-520.
Mitacek EJ, Brunnemann KD, Polednak AP, Hoffmann D, & Suttajet M
(1991) Composition of popular tobacco products in Thailand and its
relevance to disease prevention. Prev Med, 20: 764-773.
Mitacek EJ, Brunnemann KD, Hoffmann D, Limsila T, Suttajet M, Martin
N, & Kaplan LS (1999) Volatile N-nitrosamines and tobacco specific
N-nitrosamines in the smoke of Thai cigarettes: a suspected risk
factor liver cancer in Thailand. Carcinogenesis, 20(1): 133-137.
Mitchell CA & Gandevia B (1971) Respiratory symptoms and skin
reactivity in workers exposed to proteolytic enzymes in the detergent
industry. Am Rev Respir Dis, 104: 1-12.
Modigh C, Axelsson G, Alavanja M, Andersson L, & Rylander P (1996) Pet
birds and risk of lung cancer in Sweden: a case-control study. Br Med
J, 313: 1236-1238.
Molyneux MKB & Tombleson JBL (1970) An epidemiological study of
respiratory symptoms in Lancashire mills, 1963-66. Br J Ind Med,
27(3): 225-234.
Monson RR & Fine LJ (1978) Cancer mortality and morbidity among rubber
workers. J Natl Cancer Inst, 61(4): 1047-1053.
Monson RR & Nakano KK (1976a) Mortality among rubber workers: 1. White
male union employees in Akron, Ohio. Am J Epidemiol, 103(3): 284-296.
Monson RR & Nakano KK (1976b) Mortality among rubber workers: 2. Other
employees. Am J Epidemiol, 103(3): 297-303.
Moolgavkar SH, Dewanji A, & Luebeck G (1989) Cigarette smoking and
lung cancer: reanalysis of the British doctors' data. J Natl Cancer
Inst, 81: 415-420.
Moolgavkar SH, Luebeck EG, Krewski D, & Zielinski JM (1993) Radon,
cigarette smoke, and lung cancer: A re-analysis of the Colorado
Plateau uranium miners' data. Epidemiology, 4: 204-217.
Morabia A, Stellman S, Lumey LH & Wynder EL (1998) Parakeets,
canaries, finches, parrots, and lung cancer: no association. Br J
Cancer, 77: 501-504.
Morgan DC, Smyth JT, Lister RW, Pethybridge RJ, Gilson JC, Callaghan
P, & Thomas GO (1975) Chest symptoms in farming communities with
special reference to farmer's lung. Br J Ind Med, 32: 228-234.
Morgan WKC (1982) Occupational bronchitis. Eur J Respir Dis, 63(suppl
23): 117-127.
Morgan KT (1998) Commentary on Coggins CRE (1998): a review of chronic
inhalation studies with mainstream cigarette smoke in rats and mice.
Toxicol Pathol, 26(3): 315.
Mori K (1964) Acceleration of experimental lung cancers in rats by
inhalation of cigarette smoke. Gann, 55: 175-181.
Morimoto Y, Kido M, Tanaka I, Fujino A, Higashi T, & Yokosaki Y (1993)
Synergistic effects of mineral fibres and cigarette smoke on the
production of tumour necrosis factor by alveolar macrophages of rats.
Br J Ind Med, 50: 955-960.
Mougne C, MacLennan R, & Atsana S (1982) Smoking, chewing and drinking
in Ban Pong, Northern Thailand. Soc Sci Med, 16(1): 99-106.
MRC (Medical Research Council) (1965) Special Committee on the
Aetiology of Chronic Bronchitis: Definition and classification of
chronic bronchitis for clinical and epidemiological purposes. Lancet,
1: 775-779.
Muir CS & McKinney PA (1992) Cancer of the oesophagus: A global
overview. Eur J Cancer Prev, 1: 259-264.
Murai Y, Kitagawa M, Matsui K, Koizumi F, & Miwa A (1994) Asbestos
fibre analysis in seven asbestosis cases. Arch Environ Health, 49(1):
67-72.
Murphy SE & Heiblum R (1990) Effect of nicotine and tobacco-specific
nitrosamines on the metabolism of N'-nitrosonornicotine and
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis, 11:
1663-1666.
Muscat JE & Wynder EL (1991) Cigarette smoking, asbestos exposure, and
malignant mesothelioma. Cancer Res, 51(9): 2263-2267.
Nair J, Pakhale SS, & Bhide SV (1989) Carcinogenic tobacco-specific
nitrosamines in Indian tobacco products. Food Chem Toxicol, 27(11):
751-753.
Najem GR, Louria DB, Seebode JJ, Thind IS, Prusakowski JM, Ambrose RB,
& Fernicola AR (1982) Life time occupation, smoking, caffeine,
saccharin, hair dyes and bladder carcinogenesis. Int J Epidemiol, 11:
212-217.
Nakachi K, Imai K, Hayashi S, Watanabe J, & Kawajiri K (1991) Genetic
susceptibility to squamous cell carcinoma of the lung in relation to
cigarette smoking dose. Cancer Res, 51(19): 5177-5180.
Nanda PK & Sharma MM (1988) Immediate effect of tobacco chewing in the
form of "paan" on certain cardio-respiratory parameters. Indian J
Physiol Pharmacol, 32(2): 105-113.
Nandakumar A, Thimmasetty KT, Sreeramereddy NM, Venugopal TC, Rajanna
N, Vinutha AT, Srinivas N, & Bhargava MK (1990) A population-based
case-control investigation on cancer of the oral cavity in Bangalore,
India. Br J Cancer, 62: 847-851.
Naus A, Engler V, Hetychova M, & Vavrechova O (1966) Work injuries and
smoking. Ind Med Surg, 35: 880-881.
Negri E, LaVecchia C, Franceschi S, Decarli A, & Bruzzi P (1992)
Attributable risks for oesophageal cancer in Northern Italy. Eur J
Cancer, 28A: 1167-1171.
Nery LE, Sandoval PR, Jardim JR, Bagatin E, & Alonso G (1988) The
effects of smoking and silica exposure on pulmonary epithelial
permeability: a radioaerosol study with 99mTc-DTPA. Brazilian J Med
Biol Res, 21(2): 223-232.
Nery LE, Florencio RT, Sandoval PR, Rodrigues RT, Alonso G, & Mason GR
(1993) Additive effects of exposure to silica dust and smoking on
pulmonary epithelial permeability: a radioaerosol study with
technetium-99m labelled DTPA. Thorax, 48(3): 264-268.
Neugut AI, Robinson E, Lee WC, Murray T, Karwoski K, & Kutcher G
(1993) Lung cancer after radiation therapy for breast cancer. Cancer,
71: 3054-3057.
Neugut AI, Murray T, Santos J, Amols H, Hayes MK, Flannery JT, &
Robinson E (1994) Increased risk of lung cancer following breast
cancer radiation therapy in cigarette smokers. Cancer, 73: 1615-1620.
Neurath G & Ehmke H (1964) [Studies on the nitrate content of
tobacco.] Beitr Tabakforsch, 2: 333-344 (in German).
Nevius E, Bennett R, & Sobel S (1982) Critique of FM Sturtevant paper
"Smoking, oral contraceptives, and thromboembolic disease". Int J
Fertil, 27: 16-20.
Newhouse ML & Berry G (1976) Predictions of mortality from mesothelial
tumours in asbestos factory workers. Br J Ind Med, 33: 147-151.
Newhouse ML & Berry G (1979) Patterns of mortality in asbestos factory
workers in London. Ann NY Acad Sci, 330: 53-60.
Ng TP, Chan SL, & Lam KP (1987) Radiological progression and lung
function in silicosis: A ten year follow up study. Br Med J,
295(6591): 164-167.
Ng TP, Chan SL, & Lee J (1990) Mortality of a cohort of men in a
silicosis register: Further evidence of an association with lung
cancer. Am J Ind Med, 17(2): 163-171.
Ng TP, Chan SL, & Lee J (1992) Predictors of mortality in silicosis.
Respir Med, 86(2): 115-119.
Niewoehner DE (1991) What lies ahead? Future research and treatment
for chronic obstructive pulmonary disease. Am J Med, 91(4A): 41S-46S.
Niewoehner DE, Kleinerman J, & Rice DB (1974) Pathologic changes in
the peripheral airways of young cigarette smokers. N Engl J Med, 291:
755-758.
NIH (National Institutes of Health) (1996) The FTC cigarette test
method for determining tar, nicotine, and carbon monoxide yields of US
cigarette smoking. Washington, DC, US Department of Health and Human
Services, 275 pp (Smoking and Tobacco Control Monograph 7).
NIH (National Institutes of Health) (1998) Cigars: Health effects and
trends. Washington, DC, US Department of Health and Human Services,
232 pp (Smoking and Tobacco Control Monograph 9).
Nilsson K, Hendriksson R, Cai YQ, Hellstrom S, Hornqvist-Bylunds S, &
Bjermer L (1992) Effects of tobacco smoke on radiation-induced
pneumonitis in rats. Int J Radiat Biol, 62: 719-727.
NIOSH (1995) Criteria for a recommended standard: Occupational
exposure to respirable coal mine dust. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, 336 pp.
Nishikawa M, Ikeda H, Nishiyama H, Yamakawa H, Suzuki S, & Okubo T
(1992) Combined effects of ozone and cigarette smoke on airway
responsiveness and vascular permeability in guinea pigs. Lung, 170:
311-322.
Nishiyama H, Yano H, Nishiwaki Y, Kitaya T, Matsuyama T, Kodama T,
Suemasu K, Tamai S, & Takemoto K (1985) Lung cancer in chromate
workers: Analysis of 11 cases. Jpn J Clin Oncol, 15(3): 489-497.
Norman V, Ghrig AM, Lassa TM, & Moss BL (1983) The effect of
nitrogenous blend compounds on NO/NO2 and HCl levels in mainstream
and sidestream smoke. Bertr Tabakforsch, 12: 55-62.
NRC (National Research Council) (1988) Committee on the Biological
Effects of Ionizing Radiations: BEIR-IV: Health risks of radon and
other internally deposited alpha-emitters. Washington, DC, National
Academy Press, 602 pp.
O'Dell JR, Linder J, Markin RS, & Moore GF (1987) Thromboangiitis
obliterans (Buerger's disease) and smokeless tobacco. Arthritis-Rheum,
30(9): 1054-1056.
Olin JW (1994) Thromboangiitis obliterans. Curr Opin Rheumatol, 6(1):
44-49.
Oxman AD, Muir DC, Shannon HS, Stock SR, Hnizdo E, & Lange HJ (1993)
Occupational dust exposure and chronic obstructive pulmonary disease:
A systematic overview of the evidence. Am Rev Respir Dis, 148(1):
38-48.
Park NH, Dokko H, Li SL, & Cherrick HM (1991a) Synergism of herpes
symplex virus and tobacco-specific N'-nitrosamines in cell
transformation. J Oral Maxillofac Surg, 49(3): 276-281.
Park NH, Sapp JP, & Herbosa EG (1991b) Oral cancer induced in hamsters
with Herpes simplex virus infection and simulated snuff dipping. Oral
Surg Oral Med Oral Pathol, 62: 164-168.
Parka KR (1983) Smoking as a moderator of the relationship between
affective state and absence from work. J Appl Psychol, 68: 698-708.
Patrianakos C & Hoffmann D (1979) Chemical studies on tobacco smoke:
LXIV. On the analysis of aromatic amines. Anal Toxicol, 3: 150-154.
Pedersen E, Hogetveit AC, & Andersen A (1973) Cancer of respiratory
organs among workers at a nickel refinery in Norway. Int J Cancer, 12:
32-41.
Penner M & Penner S (1990) Excess insured health care costs from
tobacco-using employees in a large group plan. J Occup Med, 32:
521-523.
Pepys J, Pickering CAC, & Hughes EG (1972) Asthma due to inhaled
chemical agents -- complex salts of platinum. Clin Allergy, 2:
391-396.
Pershagen G (1985) Lung cancer mortality among men living near an
arsenic-emitting smelter. Am J Epidemiol, 122: 684-694.
Pershagen G, Wall S, Taube A, & Linnman L (1981) On the interaction
between occupational arsenic exposure and smoking and its relationship
to lung cancer. Scand J Work Environ Health, 7: 302-309.
Pershagen G, Bergman F, Klominek J, Damber L, & Wall S (1987)
Histological types of lung cancer among smelter workers exposed to
arsenic. Br J Ind Med, 44: 454-458.
Pershagen G, Akerblom G, Axelson O, Clavensjo B, Damber L, Desai G,
Enflo A, Legarde F, Mellander H, Svartengren M, & Swedjemark GU (1994)
Residential radon exposure and lung cancer in Sweden. N Engl J Med,
330(3): 159-164.
Petersen R, Andersen M, Mikkelsen S, & Nielsen SL (1995) Prognosis of
vibration induced white finger: a follow-up study. Occup Environ Med,
52(2): 110-115.
Peterson KL, Heninger RW, & Seegmiller RE (1981) Fetotoxicity
following chronic prenatal treatment of mice with tobacco smoke and
ethanol. Bull Environ Contam Toxicol, 26: 813-819.
Philipson K, Falk R, Gustafsson J, & Camner P (1996) Long-term lung
clearance of 195Au-labeled Teflon particles in humans. Exp Lung Res,
22: 65-83.
Pieraccini G, Luceri, F & Moneti, G (1992) New
gas-chromatographic/mass spectrometric method for the quantitative
analysis of primary aromatic amines in main and sidestream cigarette
smoke. I. Rapid Commun Mass Spectrom, 6: 406-409.
Pindborg JJ, Mehta PS, Gupta PC, Daftary DK, & Smith CJ (1971) Reverse
smoking in Andhra Pradesh, India: A study of palatal lesions among
10,169 villagers. Br J Cancer, 25: 10-20.
Plamenac P, Santic Z, Nikulin A, & Serdarevic H (1985) Cytologic
changes of the respiratory tract in vineyard spraying workers. Eur J
Respir Dis, 67: 50-55.
Post WK, Burdorf A, & Bruggeling TG (1994) Relations between
respiratory symptoms and sickness among workers in the animal feed
industry. Occup Environ Med, 51: 440-446.
Pratt PC, Vollmer RT, & Miller JA (1980) Epidemiology of pulmonary
lesions in nontextile and cotton textile workers: a retrospective
autopsy analysis. Arch Environ Health, 35: 133-138.
Prentice RL, Yoshimoto Y, & Mason MW (1983) Relationship of cigarette
smoking and radiation exposure to cancer mortality in Hiroshima and
Nagasaki. J Natl Cancer Inst, 70: 611-622.
Pyykko I, Pekkarinen J, & Stark J (1987) Sensory-neural hearing loss
during combined noise and vibration exposure. Int Arch Occup Environ
Health, 59: 439-454.
Ramakrishnan S, Sulochana KN, Selvaraj T, Abdul-Rahim A, Lakshmi M, &
Arunagiri K (1995) Smoking of beedies and cataract: cadmium and
vitamin C in the lens and blood. Br J Ophthalmol, 79: 202-206.
Rang EH & Acheson ED (1981) Cancer in furniture workers. Int J
Epidemiol, 10(3): 253-261.
RCP (Royal College of Physicians) (1962) Smoking and health. London,
Pitman, pp 2-8.
Reddy CR (1974) Carcinoma of hard palate in India in relation to
reverse smoking of chuttas. J Natl Cancer Inst, 53(3): 615-619.
Reddy DJ & Rao KV (1957) Cancer of the palate in coastal Andhra due to
smoking of cigars with burning end inside the mouth. Indian J Med Sci,
11: 791-798.
Reddy DJ, Reddy BD, & Rao PR (1960) Experimental production of cancer
with tobacco tar and heat. Cancer, 13: 263-269.
Reith AK, Reichborn-Kjennerud S, Aubele M, Jutting U, Gais P, & Burger
G (1994) Biological monitoring of chemical exposure in nickel workers
by imaging cytometry (ICM) of nasal smears. Anal Cell Pathol, 6(1):
9-21.
Rivenson A, Hecht SS, & Hoffmann D (1991) Carcinogenicity of
tobacco-specific N-nitrosamines: the role of the vascular network in
the selection of target organs. Crit Rev Toxicol, 21: 255-264.
Roberts DL (1988) Natural tobacco flavor. Recent Adv Tob, 14: 45-81.
Robertson PB, Walsh MM, & Greene JC (1997) Oral effects of smokeless
tobacco used by professional baseball players. Adv Dental Res, 11:
307-312.
Rodenhuis S & Slebos RJC (1992) Clinical significance of ras oncogene
activation in human lung cancer. Cancer Res, 52(suppl): S2665-S2669.
Roels H, Sarhan MJ, Hanotiau I, de Fays M, Genet P, Bernard A, Buchet
JP, & Lauwerys R (1985) Preclinical toxic effects of manganese in
workers in a Mn salts and oxides producing plant. Sci Total Environ,
42: 201-206.
Roth J, Mahrlein W, Kummer G, & Krause M (1985) [Results of an
epidemiological analysis of the pathogenesis of chronic obstructive
bronchitis in miners.] Z Erkrank Atmungsorg, 164(3): 277-279 (in
German).
Rothman K & Keller A (1972) The effect of joint exposure to alcohol
and tobacco on risk of cancer of the mouth and pharynx. J Chronic Dis,
25: 711-716.
Roush GC (1996) Cancer of the nasal cavity and paranasal sinuses. In:
Schuttenfeld D & Franinini JF ed. Cancer epidemiology and prevention.
New York, Oxford, Oxford University Press, pp 587-602.
Ruckley VA, Gauld SJ, Chapman JS, Davis JM, Douglas AN, Fernie JM,
Jacobsen M, & Lamb D (1984) Emphysema and dust exposure in a group of
coal workers. Am Rev Respir Dis, 129: 528-532.
Russell MAH (1976) Tobacco smoking and nicotine dependence. In:
Gibbons RJ, Israel Y, Kalent H, Papham RE, Schmidt W, & Smart RG ed.
Recent advances in alcohol and drug problems. New York, Chichester,
Brisbane, Toronto, John Wiley & Sons, pp 1-47.
Ryan J, Zwerling C, & John E (1992) Occupational risks associated with
cigarette smoking: A prospective study. Am J Pub Health, 82: 29-32.
Rylander R, Sjostrand M, & Bergstrom R (1979) Free lung cell response
after combined exposure to cigarette smoke and industrial dusts.
Toxicology, 12: 211-220.
Sanden A & Jarvholm B (1991) A study of predictors of mesothelioma in
shipyard workers exposed to asbestos. J Occup Med, 33(7): 770-773.
Sanden A, Jarvholm B, Larsson S, & Thiringer G (1992) The risk of lung
cancer and mesothelioma after cessation of asbestos exposure: a
prospective cohort study of shipyard workers. Eur Respir J, 5:
281-285.
Sankaranarayanan R, Duffy SW, Day NE, Nair MK, Padmakumary G, & Day NE
(1989a) A case control investigation of cancer of the oral tongue and
floor of the mouth in southern India. Int J Cancer, 44(4): 617-621.
Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, & Padmanabhan TK
(1989b) Tobacco chewing, alcohol and nasal snuff in cancer of the
gingiva in Kerala, India. Br J Cancer, 60(4): 638-643.
Sankaranarayanan R, Duffy SW, Padmakumary G, Day NE, & Nair MK (1990)
Risk factors for cancer of the buccal and labial mucosa in Kerala,
southern India. J Epidemiol Community Health, 44(4): 286-292.
Cerik R (1987) The interactions of tobacco smoking and other agents in
cancer etiology. Epidemiol Rev, 9: 175-193.
Saris M & Lucic-Palaic S (1977) Possible synergism of exposure to
airborne manganese and smoking habit in occurrence of respiratory
symptoms. In: Walton WH & McGovern B ed. Inhaled particles -- IV.
Oxford, New York, Pergamon Press, pp 773-779.
Saumet JL, Leftheriotis G, Dittmar A, & Delhomme G (1986) Relationship
between pulse amplitude and thermal exchange in the finger: the effect
of smoking. Psychopharmacology (Berlin), 92(1): 118-121.
Schlueter DP, Banaszaak EF, Fink JN, & Barboriak J (1978) Occupational
asthma due to tetrachlorophthalic anhydride. J Occup Med, 20: 183-188
Schneider G & Krivan V (1993) Multi-element analysis of tobacco and
tobacco smoke condensate by instrumental neutron activation analysis
and atomic absorption spectrometry. Int J Environ Anal Chem, 53:
87-100.
Schnorr TM, Steenland K, Thun MJ, & Rinsky RA (1995) Mortality in a
cohort of antimony smelter workers. Am J Ind Med, 27(5): 759-770.
Schottenfeld D, Grant RC, & Wynder WL (1974) The role of alcohol and
tobacco in multiple primary cancers of the upper digestive system,
larynx and lung: A prospective study. Prev Med, 3: 277-293.
Schüller HM, Jorquera R, Reichert A, & Castonguay A (1993)
Transplacental induction of pancreas tumors in hamsters by ethanol and
the tobacco-specific nitrosamine 4-(methyl
nitrosamino)-1-(pyridyl)-1-butanone. Cancer Res, 53: 2498-2501.
Seaton A, Lamb D, Brown WR, Sclare G, & Middleton WG (1981)
Pneumoconiosis of shale miners. Thorax, 36: 412-418.
Seaton A, Godden DJ, & Brown K (1994) Increase in asthma: a more toxic
environment or a more susceptible population? Thorax, 49: 171-174.
Selig R & Nestler K (1985) [Epidemiology of chronic bronchitis from
the viewpoint of occupational medicine.] Z Erkrank Atmungsorg, 164(3):
273-276 (in German).
Selikoff IJ & Frank AL (1983) Keynote address. Potential of in vitro
tests in asbestos-related problems. Environ Health Perspect, 51: 1-4.
Selikoff IJ, Hammond EC, & Churg J (1968) Asbestos exposure, smoking
and neoplasia. J Am Med Assoc, 204(2): 106-112.
Selikoff IJ, Seidman H, & Hammond EC (1980) Mortality effects of
cigarette smoking among amosite asbestos factory workers. J Natl
Cancer Inst, 65: 507-513.
Sherrill DL, Halonen M, & Burrows B (1994) Relationships between total
serum IgE, atopy and smoking: a twenty-year follow-up analysis. J
Allergy Clin Immunol, 94: 954-962.
Sherson D, Sigsgaard T, Overgaard E, Loft S, Poulsen HE, & Jongeneelen
FJ (1992) Interaction of smoking, uptake of polycyclic hydrocarbons,
and cytochrome P450IA2 activity among foundry workers. Br J Ind Med,
49(3): 197-202.
Shim C & Williams MH (1986) Effect of odors in asthma. Am J Med,
80(1): 18-22.
Shirakawa T, Kusaka Y, & Morimoto K (1992) Combined effects of smoking
habits and occupational exposure to hard metal on total IgE
antibodies. Chest, 101(6): 1569-1576.
Shopland DR, Eyre HJ, & Pechacek TF (1991) Smoking attributable cancer
mortality in 1991: is lung cancer now the leading cause of death among
smokers in the United States? J Natl Cancer Inst, 83(16): 1142-1148.
Sidney S, Tekawa I, & Friedman GD (1989) Mentholated cigarette use
among multiphasic examinees, 1979-86. Am J Pub Health, 79(10):
1415-1416.
Simarak S, de Jong UW, Breslow N, Dahl CJ, Ruckphaopunt K, Scheelings
P, & Maclennan R (1977) Cancer of the oral cavity, pharynx/larynx and
lung in Northern Thailand: case-control study and analysis of cigar
smoke. Br J Cancer, 36: 130-140.
Simecek C, Sefrna F, & Simecková B (1986) [Evaluation of the role of
smoking in the development of chronic bronchitis after elimination of
other supporting factors.] Stud Pneumol Phtiseologica Czech, 46(3):
168-170 (in Czech).
Sluis-Cremer GK, Walters LG, & Sichel HS (1967) Chronic bronchitis in
miners and non-miners: an epidemiologic survey of a community in the
gold mining area in the Transvaal. Br J Ind Med, 24: 1-12.
Smith IE, Coles CD, Lancaster J, Fernhoff PM, & Falek A (1986) The
effect of volume and duration of prenatal ethanol exposure on neonatal
physical and behavioral development. Neurobehav Toxicol Teratol, 8:
375-381.
Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I,
Duggan HM, Wood R, Kosnett MJ, & Smith MT (1992) Cancer risks from
arsenic in drinking water. Environ Health Perspect, 97: 259-267.
Sopori ML, Goud NS, & Kaplan AM (1994) Effects of tobacco smoke on the
immune system. In: Dean JH, Luster MI, Munson AE, & Kimber I ed.
Immunotoxicology and immunopharmacology. New York, Raven Press, pp
413-434.
Sopori ML, Kozak W, Savage SM, Geng Y, & Kluger MJ (1998)
Nicotine-induced modulation of T cell function: Implications for
inflammation and infection. Adv Exp Med Biol, 437: 279-289.
Sprince NL, Oliver LC, Eisen EA, Greene RE, & Chamberlin RI (1988)
Cobalt exposure and lung disease in tungsten carbide production: A
cross-sectional study of current workers. Am Rev Respir Dis, 138:
1220-1226.
Stanton MF, Layard M, Tegeris A, Miller E, May M, & Kent E (1977)
Carcinogenicity of fibrous glass: pleural response in the rat in
relation to fiber dimension. J Natl Cancer Inst, 58: 587-603.
Steenland K (1994) Age specific interactions between smoking and radon
among United States uranium miners. Occup Environ Med, 51(3): 192-194.
Steenland K & Thun M (1986) Interaction between tobacco smoking and
occupational exposures in the causation of lung cancer. J Occup Med,
28(2): 110-118.
Steenland K & Ward E (1991) Lung cancer incidence among patients with
beryllium disease: a cohort mortality study. J Natl Cancer Inst,
83(19): 1380-1385.
Steenland K & Ward E (1993) Passive smoking and the risk of heart
disease. J Natl Cancer Inst, 85(2): 1698-1699.
Steindorf K, Lubin J, Wichman HE, & Becher H (1995) Lung cancer deaths
attributable to indoor radon exposure in West Germany. Int J
Epidemiol, 24(3): 485-492.
Stellman SD, Boffetta P, & Garfinkel L (1988) Smoking habits of
800,000 American men and women in relation to their occupations. Am J
Ind Med, 13: 43-58.
Stellman SD, Muscat JE, Thompson S, Hoffmann D, & Wynder EL (1997)
Risk of squamous cell carcinoma and adenocarcinoma of the lung in
relation to lifetime filter cigarette smoking. Cancer, 80: 382-388.
Stöber W & Abel WR (1996) Lung cancer due to diesel soot particles in
ambient air? Int Arch Occup Environ Health, 68(suppl): S3-S61.
Stohrer G (1991) Arsenic: opportunity for risk assessment. Toxicology,
65: 525-531.
Sturtevant FM (1982) Smoking, oral contraceptives, and thromboembolic
disease. Int J Fertil, 27: 2-13.
Sunderman FW & Sunderman FW Jr (1961) Nickel poisoning: XI.
Implications of nickel as a pulmonary carcinogen in tobacco smoke. Am
J Clin Pathol, 35: 203-205.
Talbot RJ, Morgan A, Moores SR, & Matulionis DH (1987) Preliminary
studies of the interaction between 239PuO2 and cigarette smoke in the
mouse lung. Int J Radiat Biol, 51: 1101-1110.
Tawfik HN (1987) Carcinoma of the urinary bladder associated with
schistosomiasis in Egypt: the possible causal relationship. Princess
Takamatsu Int Symp Ser, 18: 197-209.
Taylor PR, Qiao YL, Schatzkin A, Yao SX, Lubin J, Mao BL, Rao JY,
McAdams M, Xuan XZ, & Li JY (1989) Relation of arsenic exposure to
lung cancer among tin miners in Yunan Province, China. Br J Ind Med,
46: 881-886.
Thériault G, de Guire L, Gingras S, & Laroche G (1982) Raynaud's
phenomenon in forestry workers in Quebec. Can Med Assoc J, 126:
1404-1408.
Thériault G, Tremblay C, Cordier S, & Gingras S (1984) Bladder cancer
in the aluminium industry. Lancet, 1(8383): 947-950.
Thomas TL & Stewart PA (1986) Mortality from lung cancer and
respiratory disease among pottery workers exposed to silica and talc.
Am J Epidemiol, 124: 530.
Thomas DC & Whittemore AS (1988) Methods for testing interactions,
with applications to occupational exposures, smoking and lung cancer.
Am J Ind Med, 13: 131-147.
Thomas GB, Williams CE, & Hoger NG (1981) Some non-auditory correlates
of the hearing threshold levels of an aviation noise-exposed
population. Aviation Space Environ Med, 52(9): 531-536.
Thun MJ, Lally CA, Flanney JT, Calle EE, Flander WD, & Heath CW Jr
(1997) Cigarette smoking and changes in the histopathology of the
lung. J Natl Cancer Inst, 89: 1580-1586.
Tokarskaya ZB, Okladnikova ND, Belyaeva ZD, & Drozhko EG (1995) The
influence of radiation and nonradiation factors on the lung cancer
incidence among the workers of the nuclear enterprise Mayak. Health
Phys, 69: 356-366.
Townsend MC, Sussman NB, Enterline PE, Morgan WK, Belk HD, & Dinman BD
(1988) Radiographic abnormalities in relation to total dust exposure
at a bauxite refinery and alumina-based chemical products plant. Am
Rev Respir Dis, 138: 90-95.
Trethowen WN, Burge PS, Rossiter CE, Harrington JM, & Calvert IA
(1995) Study of the respiratory health of employees of seven European
plants that manufacture ceramic fibres. Occup Environ Med, 52(2):
97-104.
Tricker AR & Preussmann R (1992) Volatile N-nitrosamines in
mainstream cigarette smoke: occurrence and formation. Clin Invest,
70(3-4): 283-289.
Tseng WP, Chu HM, How SW, Fong JM, Lin CS, & Yeh S (1968) Prevalence
of skin cancer in an endemic area of chronic arsenicism in Taiwan. J
Natl Cancer Inst, 40: 453-463.
Tso TC (1990) Production physiology and biochemistry of tobacco
plants. Belsville, Maryland, IDEALS, p 753.
Tso TC, Harley N, & Alexander LT (1966) Source of lead-210 and
polonium-210 in tobacco. Science, 153: 880-882.
Tsuda M & Kurashima Y (1991) Tobacco smoking, chewing, and snuff
dipping: factors contributing to the endogenous formation of
N-nitroso compounds. Crit Rev Toxicol, 21(4): 243-253.
Tsuda T, Nagira T, Yamamoto M, & Kume Y (1990) An epidemiological
study on cancer in certified arsenic poisoning patients in Toroku. Ind
Health, 28: 53-62.
Tsuda T, Babazono A, Yamamoto E, Kurumatani N, Mino Y, Ogawa T, Kishi
Y, & Aoyama H (1995) Ingested arsenic and internal cancer: a
historical cohort followed for 33 years. Am J Epidemiol, 141(3):
198-209.
Tuyns AJ (1983) Oesophageal cancer in non-smoking drinkers and
non-drinking smokers. Int J Cancer, 32: 443-444.
Tuyns AJ (1991) Aetiology of head and neck cancer: tobacco, alcohol
and diet. Otorhynolaryngology, 46: 15-24.
Tuyns AJ, Péquignot G, & Jensen OM (1977) Le cancer de l'oesophage en
Ille-et-Vilaine en fonction des niveaux de consommation d'alcool et de
tabac: Des risques qui se multiplient. Bull Cancer, 64: 45-60.
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic
Radiation) (1988) Sources and effects of ionizing radiation. New York,
United Nations.
US ATSDR (1995) Toxicological profile for asbestos (update). Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry, 224 pp.
US DA (1990) World tobacco situation. Washington, DC, US Department of
Agriculture, Foreign Agricultural Service (Circular Series FT 4-90).
US DA (1997) Tobacco situation and outlook yearbook -- US output,
removal and consumption 1950-1997: Cigarettes, cigars, smoking tobacco
and specific tobacco products. Washington, DC, US Department of
Agriculture, Economic Research Service, pp 14-17 (TBS-240).
US EPA (1988) Technical support document on risk assessment of
chemical mixtures. Washington, DC, US Environmental Protection Agency,
32 pp (EPA/600/8-90/064).
US Surgeon General (1979) Smoking and health: A report of the Surgeon
General. Washington, DC, US Department of Health, Education and
Welfare (DHEW Publication No. (PHS) 79-50066).
US Surgeon General (1982) The health consequences of smoking: Cancer
-- A report of the Surgeon General. Rockville, Maryland, US Department
of Health and Human Services, Public Health Service, 332 pp.
US Surgeon General (1983) The health consequences of smoking:
Cardiovascular disease -- A report of the Surgeon General. Rockville,
Maryland, US Department of Health and Human Services, Public Health
Service, pp 6-8.
US Surgeon General (1984) The health consequences of smoking: Chronic
obstructive lung disease -- A report of the Surgeon General.
Rockville, Maryland, US Department of Health and Human Service (DHHS
Publication No. (PHS) 84-50205).
US Surgeon General (1986) The health consequences of using smokeless
tobacco -- A report of the Advisory Committee to the Surgeon General.
Bethesda, Maryland, US Department of Health and Human Services, Public
Health Service (Publication No. (NIH) 86-2874).
US Surgeon General (1988) The health consequences of smoking: Nicotine
addiction -- A report of the Surgeon General. Rockville, Maryland, US
Department of Health and Human Services, Centers for Disease Control,
pp 21-560.
US Surgeon General (1989) Reducing the health consequences of smoking:
25 years of progress -- A report of the Surgeon General. Rockville,
Maryland, US Department of Health and Human Services, Public Health
Service (DHHS Publication No. (CDC) 89-8411).
Vainio H & Boffetta P (1994) Mechanisms of the combined effect of
asbestos and smoking in the etiology of lung cancer [Review]. Scand J
Work Environ Health, 20(4): 235-242.
Vainio H, Husgafvel-Pursiainen K, Anttila S, Hackman P, & Partanen T
(1993) Interaction between smoking and asbestos in human lung
adenocarcinoma: role of K-ras mutations. Environ Health Perspect,
101(suppl 3): 189-192.
Vallyathan V, Shi XL, Dalal NS, Irr W, & Castranova V (1988)
Generation of free radicals from freshly fractured silica dust.
Potential role in acute silica-induced lung injury. Am Rev Respir Dis,
138: 1213-1219.
Van Leeuwen FE, Klokman WJ, Stoval M, Hagwenbeek A, van den
Belt-Dusebout AW, Noyon R, Boice JD, Burgers MV, & Somers R (1995)
Roles of radiotherapy and smoking in lung cancer following Hodgkin's
disease. J Natl Cancer Inst, 87(20): 1530-1537.
Van Peenen PFD, Blanchard AG, Wolkonsky PM, & Gill TM (1986) Health
insurance claims of petrochemical company employees. J Occup Med, 28:
237-240.
Van Tuinen M & Land G (1986) Smoking and excess sick leave in a
department of health. J Occup Med, 28: 33-35.
Van Schooten FJ, van Leeuwen FE, Hillebrand MJ, de Rijke ME, Hart AA,
van Veen HG, Oosterink S, & Kriek E (1990) Determination of
benzo(a)pyrene diol epoxide-DNA adducts in white blood cell DNA from
coke-oven workers: the impact of smoking. J Natl Cancer Inst, 82(11):
927-933.
Venables KM, Topping MD, Howe W, Luczynska CM, Hawkins R, & Taylor AJ
(1985) Interaction of smoking and atopy in producing specific IgE
antibody against a hapten protein conjugate. Br Med J, 290: 201-204.
Venables KM, Tee RD, Hawkins ER, Gordon DJ, Wale CJ, Farrer NM, Lam
TH, Baxter PJ, & Newman-Taylor A (1988a) Laboratory animal allergy in
a pharmaceutical company. Br J Ind Med, 45: 667-671.
Venables KM, Upton KM, Hawkins ER, Tee RD, Longbottom JL, &
Newman-Taylor AJ (1988b) Smoking, atopy, and laboratory animal
allergy. Br J Ind Med, 45: 667-671.
Venables KM, Dally MB, Nunn AJ, Stevens JF, Stephens R, Farrer N,
Hunter JV, Stewart M, Hughes EG, & Newman-Taylor AJ (1989) Smoking and
occupational allergy in workers in a platinum refinery. Br Med J, 299:
939-942.
Vessey MP & Doll R (1969) Investigation of relationship between the
use of oral contraceptives and thromboembolic disease: a further
report. Br Med J, 2: 651-657.
Viikari-Juntura E, Riihimaki H, Tola S, Videman T, & Mutanen P (1994)
Neck trouble in machine operating, dynamic physical work and sedentary
work: a prospective study on occupational and individual risk factors.
J Clin Epidemiol, 47(12): 1411-1422.
Vineis P (1992) Epidemiological models of carcinogenesis: the example
of bladder cancer. Cancer Epidemiol Biomarkers Prev, 1(2): 149-153.
Vineis P, Esteve J, & Terracini B (1984) Bladder cancer and smoking in
males: types of cigarettes, age of start, effect of stopping and
interaction with occupation. Int J Cancer, 34: 165-170.
Voges E ed. (1984) Tobacco encyclopedia. Mainz, Germany, Tabilk
Journal International, 467 pp.
Wagner JC, Pooley FD, Gibbs A, Lyons J, Sheers G, & Moncrieff CB
(1986) Inhalation of china stone and china clay dusts: relationships
between the mineralogy of dust retained in the lungs and pathological
changes. Thorax, 41: 190-196.
Wald NJ, Dall R, Darby S, Kinjlnk S, Petri R, & Pike M ed. (1985)
Trends in smoking related diseases in Britain. Oxford, Oxford
University Press.
Warren CPW (1977) Extrinsic allergic alveolitis: a disease commoner in
non-smokers. Thorax, 32: 567-569.
Weiland SK, Mundt KA, Keil U, Kraemer B, Birk T, Person M, Bucher AM,
Straif K, Schumann J, & Chambless L (1996) Cancer mortality among
workers in the German rubber industry: 1981-91. Occup Environ Med, 53:
289-298.
Weiss W (1971) Cigarette smoking, asbestos and pulmonary fibrosis. Am
Rev Respir Dis, 104: 223-226.
Weiss W (1976) Chloromethyl ethers, cigarettes, cough and cancer. J
Occup Med, 18(3): 194-199.
Weiss W (1980) The cigarette factor in lung cancer due to chloromethyl
ethers. J Occup Med, 22(8): 527-529.
Weiss W (1982) Epidemic curve of respiratory cancer due to
chloromethyl ethers. J Natl Cancer Inst, 69(6): 1265-1270.
Weiss W (1984) Cigarette smoke, asbestos and small irregular
opacities. Am Rev Respir Dis, 130(2): 293-301.
Weiss W & Boucot KR (1975) The respiratory effects of chloromethyl
methyl ether. J Am Med Assoc, 234(11): 1139-1142.
Weiss W & Nash D (1997) An epidemic of lung cancer due to chloromethyl
ethers: Thirty years of observation. J Occup Environ Med, 39:
1003-1009.
Welch K, Higgins I, Oh M, & Burchfiel C (1982) Arsenic exposure,
smoking and respiratory cancer in copper smelter workers. Arch Environ
Health, 37: 325-335.
WHO (1980) Recommended health-based limits in occupational exposure to
heavy metals. Geneva, World Health Organization, pp 21-35 (WHO
Technical Report Series 647).
WHO (1985) Regional Workshop on the Control of Tobacco-related
Diseases, New Delhi, 22-26 July 1985. New Delhi, World Health
Organization Regional Office for South-East Asia.
WHO (1997) Tobacco or health: A global status report. Geneva, World
Health Organization, pp 10-18.
Wicklund KG, Daling JR, Allard J, & Weiss NS (1988) Respiratory cancer
among orchardists in Washington State, 1968 to 1980. J Occup Med,
30(7): 561-564.
Wilbert W (1987) Tobacco and shamanism in South America (Psychoactive
plants of the world). New Haven, London, Yale University Press, 294
pp.
Wilhelmsen L (1977) Recent studies on smoking and CVD epidemiology:
Scandinavia and some other Western European countries. In: Proceedings
of 3rd World Conference on Smoking and Health -- Volume II: Health
consequences, education, cessation, and social action. Washington, DC,
Department of Health, Education and Welfare, pp 171-177 (Publication
No. (NIH) 77-1413).
Williams MK, Walford J, & King E (1983) Blood lead and the symptoms of
lead absorption. Br J Ind Med, 40(3): 285-292.
Winkelstein W (1990) Smoking and cervical cancer current status: a
review. Am J Epidem, 131: 945-957.
Winkelstein W, Shillitoe EJ, Brand R, & Johnson KK (1984) Further
comments on cancer of the uterine cervix, smoking and herpes virus
infection. Am J Epidem, 119(1): 1-8.
Winn DM (1997) Epidemiology of cancer and other systemic effects
associated with the use of smokeless tobacco. Adv Dent Res, 11(3):
313-321.
Wise MB & Guerin MR (1986) Chemical analysis of the major constituents
in clove cigarette smoke. In: Hoffmann D & Harris CC ed. Mechanisms in
tobacco carcinogenesis. Cold Spring Harbor, New York, Cold Spring
Harbor Laboratory, pp 151-162 (Banbury Report No. 23).
Witschi H, Espiritu I, Peake JL, Wu K, Maronpot RR, Pinkerton KE
(1997) The carcinogenicity of environmental tobacco smoke.
Carcinogenesis, 18(3): 575-586.
Wong SH, Ogle CW, & Cho CH (1986) Influence of chronic or acute
nicotine pretreatment on ethanol-induced gastric ulceration in the
rat. J Pharm Pharmacol, 38: 537-540.
Wong O, Harris F, & Smith TJ (1993) Health effects of gasoline
exposure: II. Mortality patterns of distribution workers in the United
States. Environ Health Perspect, 101(suppl 6): 63-76.
Wright JT, Waterson EJ, Barrison IG, Toplis PJ, Lewis IG, Gordon MG,
Macrae KD, Morris NF, & Murray-Lyon IM (1983) Alcohol consumption,
pregnancy and low birth weight. Lancet, 1: 663-665.
Wright JT, Macrae KD, Barrison IG, & Waterson EJ (1984) Effects of
moderate alcohol consumption and smoking on fetal outcome. London,
Pitman, pp 240-253 (Ciba Foundation Symposium Series No. 105).
Wynder EL & Bross IJ (1961) A study of etiological factors in cancer
of the oesophagus. Cancer, 14: 389-413.
Wynder EL & Gori GB (1977) Contribution of the environment on cancer
incidence: an epidemiologic exercise. J Natl Cancer Inst, 58: 825-832.
Wynder EL & Graham EG (1950) Tobacco smoking as a possible etiological
factor in bronchiogenic carcinoma. J Am Med Assoc, 37: 4608-4622.
Wynder El & Hoffmann D (1967) Tobacco and tobacco smoke: Studies in
experimental carcinogenesis. New York, London, Academic Press.
Wynder El & Hoffmann D (1994) Smoking and lung cancer: challenges and
opportunities. Cancer Res, 54: 5284-5295.
Wynder EL, Covey LS, Mabuchi K, & Mushinki M (1976) Environmental
factors in cancers of the larynx: A second look. Cancer, 38:
1591-1601.
Yazicioglu S, Ilcayto R, Balci K, Sayli BS, & Yorulmaz B (1980)
Pleural calcification, pleural mesotheliomas and bronchial cancers
caused by tremolite dust. Thorax, 35: 564-569.
Yorukoglu Y, Ilgit E, Zengin M, Nazliel K, Salman E, & Yucel E (1993)
Thromboangiitis obliterans (Buerger's disease) in women (a
reevaluation). Angiology, 44(7): 527-532.
Yoshizawa K (1984) [Clinicopathological analysis of lung cancer and
epithelial changes of the bronchus in chromate workers.] Shikoku Acta
Med, 40(1): 123-134 (in Japanese).
Yue L (1992) Cadmium in tobacco. Biomed Environ Sci, 5(1): 53-56.
Zacny JP (1990) Behavioral aspects of alcohol-tobacco interactions.
Recent Dev Alcohol, 8: 205-219.
Zahran FM, Ardawi MSM, & Al-Fayez SF (1985) Carboxyhaemoglobin
concentrations in smokers of sheesha and cigarettes in Saudi Arabia.
Br Med J, 291: 1768-1770.
Zelman S (1973) Correlation of smoking history with hearing loss. J Am
Med Assoc, 223(8): 920.
Zetterstrom O, Osterman K, Machado L, & Johansson SGO (1981) Another
smoking hazard: raised IgE concentration and increased risk of
occupational allergy. Br Med J, 283: 1215-1217.
Zhang Z-F, Yu S-Z, Li W-X, & Choi BCK (1989) Smoking, occupational
exposure to rubber and lung cancer. Br J Ind Med, 46: 12-15.
Zhu H & Wang Z (1993) Study of occupational cancer in asbestos
factories in China. Br J Ind Med, 50(11): 1039-1042.
Zuskin E, Zagar Z, Schacter EN, Mustajbego J, & Kern J (1992)
Respiratory symptoms and ventilatory capacity in swine confinement
workers. Br J Ind Med, 49(6): 435-440.
SYNOPSIS
1. Introduction
Le tabac, notamment lorsqu'il est fumé, exerce divers effets nuisibles
à la santé; il est directement en cause dans un certain nombre de
maladies graves et peut accentuer l'action nocive d'autres agents
chimiques, physiques ou biologiques. Les produits chimiques et autres
agents présents sur les lieux de travail peuvent, si l'on n'y veille pas
suffisamment, provoquer diverses pathologies, des invalidités et des
décès prématurés. Il est clair que sur les lieux de travail, des effets
indésirables peuvent résulter de la synergie entre le tabagisme et
d'autres facteurs de risque. La plupart des interactions entre les
constituants nocifs de la fumée de tabac et certaines substances
chimiques toxiques se produisent lorsque celles-ci sont présentes dans
l'atmosphère, mais le tabagisme peut également, comme on l'a observé,
interagir avec des agents toxiques absorbés par voie buccale ou autre.
La consommation de tabac est universelle, des pays économiquement
défavorisés aux nations industrialisées les plus riches. Le tabac est
consommé par les hommes et les femmes, les enfants et les adultes et des
millions de gens sont exposés malgré eux à la fumée de tabac présente
dans l'environnement. Il y a de nombreuses explications au tabagisme,
mais la raison principale de son universalité tient à la présence, dans
les feuilles de toutes les variétés de tabac, de nicotine, une substance
qui engendre la dépendance. Cette dernière pénètre dans l'organisme du
consommateur en quantité variable, selon le type de tabagisme auquel il
s'adonne (Chapitre 2). L'apparition de la cigarette, qui peut être
produite en quantités industrielles, qu'il est facile de se procurer à
un prix relativement bas et que sa légèreté permet de tenir entre les
lèvres en gardant les mains libres, a profondément modifié le
comportement des fumeurs, que ce soit dans la vie en général ou sur les
lieux de travail.
Il y a beaucoup de pays où fumer est considéré comme très dangereux pour
la santé et comme un facteur qui contribue de manière importante à la
mortalité résultant d'un certain nombre d'affections courantes. Ces pays
ont adopté une législation qui vise à alerter les consommateurs des
risques qu'ils courent et ils ont également pris des mesures pour
freiner la consommation, soit par la taxation, soit par la mise en
oeuvre de campagnes d'information à destination du grand public qui ont
pour but d'attirer l'attention sur les dangers du tabagisme et les
avantages qu'il y a à cesser de fumer ou à ne pas commencer du tout.
Certains pays n'ont toutefois pas encore pris de mesures décisives pour
traiter le problème du tabagisme.
L'activité professionnelle comporte souvent une part de risque. Elle
peut être de nature à mettre la santé en danger et à contaminer
l'environnement. La culture du tabac elle-même implique l'utilisation de
pesticides, la récolte des feuilles peut provoquer des intoxications
dues à l'absorption percutanée de nicotine et les diverses opérations
auxquelles elles sont ensuite soumises expose les travailleurs à
respirer les poussières et les spores de champignons présentes dans
l'atmosphère. Dans les zones où sont implantées des manufactures de
tabac, on observe une incidence élevée de cancers chez les sujets de
sexe masculin. L'air des exploitations minières est chargé de poussières
minérales et dans l'agriculture ou les industries qui utilisent des
matières premières d'origine biologique, les travailleurs sont exposés
à des poussières de même origine. Le soudage donne également lieu à la
production de vapeurs, et les gaz, fumées, brouillards etc. produits
dans de nombreuses industries sont aussi source de dangers. Une chaleur
excessive ou l'exposition aux ultraviolets peuvent nuire au bien-être
des travailleurs. On admet désormais que les rayonnements ionisants émis
dans les mines ou par certains appareils modernes constituent un risque
professionnel. Nombreuses sont les activités professionnelles qui
entraînent une exposition à des niveaux de bruit excessifs ou à des
vibrations nocives. Toutes ces conditions de travail ont des effets
indésirables sur la santé, mais qui peuvent être plus graves pour les
fumeurs que pour les non fumeurs. Dans un grand nombre de pays, il est
interdit de fumer sur les lieux de travail, essentiellement d'ailleurs
en raison des risques d'incendie ou d'explosion. Dans d'autres, cette
réglementation n'est pas toujours appliquée. Dans certains pays
nouvellement industrialisés, les problèmes sanitaires liés à l'activité
professionnelle ne sont pas encore totalement pris en compte et de
nombreux employés et ouvriers ne sont pas conscients des risques de leur
métier sur le plan sanitaire. En outre, il existe un vaste secteur
industriel "non officiel", en particulier dans les pays en
développement, où le travail, qui se fait à domicile, peut comporter
l'utilisation de produits chimiques (solvants, résines, colorants de
synthèse etc.) auxquels toute la famille va donc se trouver exposée. En
outre, il n'existe pas de réglementation qui restreigne l'exposition ou
interdise de fumer dans ces circonstances.
Beaucoup moins bien défini est le cas d'effets nocifs résultant de
l'action combinée d'une exposition à la fumée de tabac, provenant du
courant principal ou de l'environnement, et à des agents présents dans
le milieu domestique. On sait toutefois qu'en ce qui concerne
l'incidence du cancer du poumon, la courbe dose-réponse obtenue dans le
cas d'une exposition domestique au radon est analogue à celle qu'on
obtient chez des mineurs exposés à ce gaz, avec un risque plus important
chez les fumeurs.
2. Exemples d'effets combinés d'une exposition à la fumée de tabac et
à d'autres agents
On est fondé à penser que dans le cas de certains effets toxiques (en
l'occurrence le cancer) il existe une synergie entre le fait de fumer et
l'exposition à l'arsenic, à l'amiante, à l'éthanol, à la silice et aux
rayonnements (radon, bombe atomique, rayons X). D'un autre côté, il y a
également lieu de croire à l'existence d'un antagonisme entre le
tabagisme et les chlorométhyléthers cancérogènes comme le
chlorométhyl-méthyléther (CMME) et le bis (chlorométhyléther) (BCME)
(Hoffmann & Winder, 1976; CIRC, 1986), entre le tabagisme et l'alvéolite
allergique ou encore entre le tabagisme et la bérylliose chronique.
Fumer accentue les effets nocifs d'une exposition à la poussière chez
les mineurs de charbon, ou aux pesticides chez ceux qui en manipulent,
et cette accentuation du risque s'observe également dans l'industrie du
caoutchouc et du pétrole. Les mineurs de charbon qui fument risquent
davantage de contracter une bronchite chronique ou une pneumopathie
obstructive, mais pas un emphysème. Les cancers du poumon observés chez
les mineurs de charbon sont attribués en totalité au tabagisme. Fumer
peut accroître les effets d'une exposition aux poussières végétales qui
engendrent des affections respiratoires chroniques, comme la byssinose
produite par la poussière de coton et le cancer des fosses nasales
provoqué par la poussière de bois.
3. Composition des feuilles et de la fumée de tabac
On a isolé plus de 3040 composés chimiques des feuilles de tabac après
transformation (Roberts, 1988). La plupart d'entre eux sont des
constituants de la feuille, mais d'autres résultent des conditions de
culture (sol et atmosphère de la région) ou encore des produits
agrochimiques utilisés et des traitements subis (sauçage,
humidification, aromatisation et séchage). On constate des différences
selon la région d'origine du tabac, les variétés et les diverses
méthodes de séchage et de transformation utilisées. Ces différences
peuvent affecter la proportion des divers constituants mais la
composition globale ne varie pas. Parmi les importants composés toxiques
que l'on a mis en évidence, on trouve, à côté de la nicotine, des
nitrosamines cancérogènes qui proviennent de l'action des nitrites, des
amines, des protéines et des alcaloïdes d'origine foliaire, des
hydrocarbures aromatiques polycycliques formés au cours du séchage, des
éléments radioactifs captés dans le sol et dans l'air ainsi que du
cadmium dans le cas de tabacs cultivés sur des sols riches en cadmium.
Lorsque l'on fume, la combustion du tabac conduit à la formation de
nombreux produits de pyrolyse ou résultant d'autres types de réactions.
4. Fumée du courant principal
La fumée de tabac est un aérosol consistant en une phase particulaire
constituée de gouttelettes de liquide dispersées dans une phase gazeuse
ou vapeur. Lorsque l'on fume une cigarette, il se forme de nombreux
composés qui résultent de la pyrolyse du tabac. Ceux-ci peuvent, soit
traverser la cigarette dans la fumée constituant le courant
principal-certains d'entre eux se condensant légèrement en arrière du
cône incandescent-, soit passé dans l'air à partir de l'extrémité
incandescente, dans la fumée qui constitue le courant latéral. A chaque
bouffée, ces composés se concentrent dans la fumée car les produits qui
s'étaient déjà condensés viennent s'y ajouter -- la zone de condensation
se réduisant à mesure que la cigarette se raccourcit. La nature
physicochimique de la fumée dépend du traitement subi par le tabac et de
sa combustion, de la porosité et du traitement du papier et du type de
bout-filtre (Hoffmann & Hoffmann, 1997). Dans le cas d'une cigarette ou
de ce que l'on appelle un "bidi" en Asie (du tabac roulé dans la feuille
d'une plante), la composition chimique de la fumée dépend de facteurs
tels que les dimensions et la porosité de l'enveloppe ainsi que des
paramètres du fumage : volume, fréquence et durée des bouffées (NIH,
1998). Les variations de composition chimique concernent davantage la
proportion des différents constituants que la présence ou l'absence de
tel ou tel composé.
La fumée du courant principal est produite à l'intérieur du cône
incandescent dans une atmosphère relativement pauvre en oxygène à une
température de combustion de 850-950°C. Au départ, les particules de
cette fumée ont un diamètre aérodynamique massique médian (DAMM) de 0,2
à 0,3 µm; toutefois, dès qu'elles pénètrent dans les voies
respiratoires, où le degré d'humidité est de 100%, elles s'agrègent pour
former des particules de plus grande taille et se comportent alors comme
si leur DAMM était de l'ordre du micromètre. Environ 50 à 90% des
particules inhalées peuvent être retenues dans les voies respiratoires
(Wynder & Hoffmann, 1967; Hinds et al., 1983). Pour des raisons d'ordre
dimensionnel, les particules présentes dans l'aérosol, les constituants
de la phase gazeuse et les gaz permanents sont capables d'atteindre les
alvéoles lors de l'inhalation de la fumée. Le comportement des
constituants hydrophiles en présence d'une forte humidité fait que le
dépôt dans l'arbre trachéobronchique revêt un caractère complexe, mais
de toute manière, la fumée s'insinue dans la totalité des voies
respiratoires.
Près de 4000 constituants ont été répertoriés dans la fumée du courant
principal à côté d'un nombre indéterminés de substances non identifiées
(Roberts, 1988). La fumée du courant principal comporte une phase
particulaire et une phase gazeuse. La phase particulaire contient de la
nicotine, des nitrosamines telles que la
4-(méthylnitrosamino)-1-(3-pyridyl)-1-butanone (NKK) et la
N-nitrosonornicotine (NNN), des métaux comme le cadmium, le nickel, le
zinc et le polonium-210, des hydrocarbures polycycliques et des amines
cancérogènes comme le 4-aminobiphényle. La phase gazeuse renferme du
monoxyde et du dioxyde de carbone, du benzène, de l'ammoniac, du
formaldéhyde, du cyanure d'hydrogène, de la N-nitrosodiméthylamine, de
la N-nitrosodiéthylamine et un certain nombre d'autres composés. Les
composés présents dans la fumée de tabac peuvent, selon leurs effets
biologiques, être classés en asphyxiants, irritants, ciliatoxines,
mutagènes, cancérogènes, inhibiteurs d'enzymes, neurotoxines ou dérivés
dotés d'action pharmaceutique. C'est principalement par les voies
respiratoires que la fumée de tabac pénètre dans l'organisme mais de
nombreux constituants, présents en particulier dans la fumée de pipe et
de cigare, se dissolvent dans la salive et sont soit avalés, soit
absorbés au niveau de la cavité buccale. Les fumeurs de pipe et de
cigare n'inhalent généralement pas la fumée, qui demeure dans la cavité
buccale où, on vient de le voir, elle se dissout dans la salive et peut
être soit absorbée par passage à travers la muqueuse buccale, soit être
directement avalée (NIH, 1998). Les boissons alcoolisées, par leur effet
solvant sur les constituants de la fumée, en facilitent la résorption.
5. Fumée du courant latéral
La fumée du courant latéral est généralement produite à une température
de combustion plus faible (500-600°C) dans une atmosphère réductrice.
Les particules de cette fumée ont, lorsqu'elles sont fraîchement émises,
une taille à peu près équivalente à celle des particules du courant
principal, avec un diamètre aérodynamique massique médian (DAMM)
d'environ 0,2 µm. Quantitativement, la composition de la fumée du
courant latéral est analogue à celle de la fumée du courant principal.
Certaines substances du courant latéral sont émises à une concentration
(rapportée à 1 g de tabac brûlé) plus élevée que les constituants du
courant principal. C'est notamment le cas de composés cancérogènes comme
la N-nitrosodiméthylamine et la N-nitrosodiéthylamine ou encore de
métaux comme le nickel et le cadmium. Beaucoup de dérivés cancérogènes
sont plus concentrés dans la fumée du courant latéral que dans celle du
courant principal. Des épreuves biologiques consistant à badigeonner la
peau de souris avec un condensant de fumée du courant latéral ont montré
que ce dernier est plus cancérogène que celui de la fumée du courant
principal (Wynder & Hoffmann, 1967; US Surgeon General, 1986; NIH,
1998).
6. La manière de fumer la cigarette et ses effets sur la toxicité de la
fumée
Les cigarettes n'ont pas toutes la même teneur en nicotine et le fumeur
"tire" plus ou moins fort en inhalant plus ou moins profondément pour
satisfaire son besoin de nicotine. Il s'ensuit qu'en fumant une
cigarette à bout-filtre pauvre en nicotine (< 1,2 mg) le fumeur va
tirer plus intensément, ce qui ne sera pas sans effet sur la toxicité
(NIH, 1998).
7. Résumé des conclusions et des recommandations
La consommation de tabac, notamment en le fumant, constitue un problème
de santé publique d'une extrême importance en raison de la morbidité et
de la mortalité qui en résultent. Outre les effets nocifs causés par une
utilisation active du tabac, on a montré qu'il en existe aussi qui
résultent de l'exposition passive à la fumée présente dans
l'environnement. Les dangers du tabagisme sont également accrus par la
possibilité d'interactions avec certains agents chimiques, physiques ou
biologiques présents sur les lieux de travail ou dans l'environnement en
général. On connaît certes quelques cas d'interactions antagonistes,
mais les risques inhérents au tabagisme l'emportent de très loin sur ses
effets protecteurs apparents. Tout doit être mis en oeuvre pour faire
cesser la consommation de tabac et en particulier l'habitude de fumer.
Il faut s'opposer très vigoureusement au tabagisme sur les lieux
publics. En outre, pour éviter des interactions avec d'autres types
d'exposition tout en éliminant le risque d'exposition passive à la fumée
de tabac, il faut interdire de fumer sur les lieux de travail.
Il faut aussi vivement inciter les gens à ne pas fumer à la maison afin
de protéger la santé de la famille et notamment celle des enfants. On
évitera ainsi également des interactions potentiellement dangereuses
avec d'autres types d'exposition pouvant survenir dans l'environnement
domestique. Il est nécessaire d'établir sans attendre des programmes
éducatifs sur les dangers du tabagisme pour la santé. Les professionnels
de la santé doivent prêter assistance aux personnes qui désirent cesser
de fumer. Comme le tabagisme peut entraîner une modification des
réactions aux médicaments ou autres formes de traitement, voire susciter
à leur encontre des réactions indésirables, les médecins doivent ajuster
les doses de leurs patients en conséquence et surveiller leurs
réactions.
PANORAMA GENERAL
1. Introducción
El consumo de tabaco, particularmente el hábito de fumar, provoca una
serie de efectos nocivos para salud, está directamente relacionado con
varias enfermedades graves y puede aumentar los efectos adversos de
otros agentes químicos, físicos y biológicos. Si no se controlan, los
agentes químicos y de otro tipo pueden producir en el puesto trabajo
enfermedades, discapacidades y la muerte prematura. Es evidente que en
el lugar de trabajo los efectos adversos pueden deberse a la interacción
sinérgica del humo de tabaco con otros peligros. La mayor parte de las
interacciones de los constituyentes nocivos del humo de tabaco con
sustancias químicas tóxicas se producen cuando estas últimas están en el
aire, aunque se han notificado asimismo interacciones del humo con
agentes perjudiciales ingeridos y/o absorbidos.
El consumo de tabaco está generalizado en todo el mundo, desde los
países de bajos ingresos hasta los industrializados más ricos. Lo
utilizan hombres y mujeres, niños y adultos, y millones de personas
están involuntariamente expuestas al humo de tabaco en su entorno. Si
bien hay numerosas explicaciones del hábito de fumar, la razón principal
de su ubicuidad es el efecto adictivo de la nicotina, droga presente en
todas las formas de la hoja de tabaco y que llega al consumidor en
cantidades variables según los distintos tipos de consumo (capítulo 2).
La aparición del cigarrillo, de producción masiva, fácil de obtener,
relativamente económico y ligero de peso, de manera que se puede llevar
en la boca dejando las manos libres, ha tenido repercusiones importantes
en el hábito de fumar, tanto en general como en el puesto de trabajo.
En muchos países se reconoce que el humo de tabaco constituye un peligro
grave para la salud y es un factor que contribuye de manera importante
a la muerte causada por diversas enfermedades comunes. En esos países se
ha aplicado una legislación de alerta sanitaria y medidas impositivas
para el control de su consumo, así como programas de educación del
público sobre los peligros del tabaco y las ventajas de no comenzar a
fumar o de interrumpir el consumo. Sin embargo, todavía hay países donde
no se han puesto en marcha medidas decisivas para abordar el problema
del consumo de tabaco.
Son muchas las situaciones laborales que conllevan un elemento de
riesgo. El tipo de trabajo puede generar efectos nocivos para salud y
las actividades laborales pueden provocar la contaminación de medio
ambiente. El propio cultivo del tabaco requiere el uso de plaguicidas,
la recolección de la hoja puede ocasionar trastornos debido a la
absorción de nicotina a través de la piel y su elaboración expone a los
trabajadores a peligros para la salud provocados por el polvo y las
esporas de hongos presentes en el aire. Se ha notificado una elevada
incidencia de cáncer en el sexo masculino en zonas con industrias
tabaqueras. En la minería existe polvo de minerales en el aire y en la
agricultura y la industria basadas en materias primas producidas
biológicamente hay polvo biológico. Durante las actividades de soldadura
se produce humo y en muchas industrias crean peligro los gases, humos,
neblinas y vapores cargados de sustancias orgánicas y/o inorgánicas
tóxicas. Un calor excesivo o la exposición a luz ultravioleta pueden
ser perjudiciales para el bienestar de los trabajadores. Está admitido
que las radiaciones ionizantes en la minería y en la tecnología moderna
son un peligro en el lugar de trabajo. En numerosas actividades, los
trabajadores están expuestos a un ruido excesivo o a vibraciones
mecánicas peligrosas. Estas condiciones laborales pueden afectar más
negativamente a la salud de las personas fumadoras que a la de las no
fumadoras. Son muchos los países en los que está prohibido fumar en el
trabajo, fundamentalmente por razones de seguridad en cuanto
incendios/explosiones. Sin embargo, en algunos países no siempre se
cumple la reglamentación. En varios países recientemente
industrializados no se han abordado todavía plenamente los problemas de
salud asociados con el trabajo y muchos empleadores y trabajadores
desconocen los peligros para la salud de sus actividades. Además, existe
un amplio "sector extraoficial" de la industria, particularmente en los
países en desarrollo, en que se trabaja en el hogar y se utilizan
sustancias químicas (en particular disolventes, resinas y colorantes
sintéticos), estando expuesta toda la familia, y no hay restricciones
sobre la exposición a los peligros en el trabajo o al humo.
Está mucho menos definida la situación en relación con los efectos
adversos en la salud derivados de la exposición combinada al humo de
tabaco -- de la corriente principal o del medio ambiente -- y a los
agentes del entorno doméstico. Sin embargo, la incidencia de cáncer de
pulmón y la concentración de radón en los hogares tiene una relación
dosis-respuesta similar a la que se produce entre el cáncer de pulmón y
la concentración de radón en las minas, y el riesgo es más elevado para
los fumadores.
2. Ejemplos de efectos combinados de la exposición al humo de
tabaco y a otras sustancias
Está demostrada la existencia de sinergia en la producción de efectos
nocivos (cáncer) entre el humo de tabaco y la exposición al arsénico, el
amianto, el etanol, el silicio y las radiaciones (radón, bomba atómica,
rayos X). Por otra parte, hay pruebas de antagonismo en el caso del humo
de tabaco y los clorometiléteres carcinogénicos, es decir, el
clorometilmetiléter (CMME) y el bis(clorometil)éter (BCME) (Hoffmann y
Wynder, 1976; CIIC, 1986), el humo de tabaco y la alveolitis alérgica y
el humo de tabaco y la beriliosis crónica. El humo de tabaco influye en
el riesgo para la salud de la exposición en la extracción de carbón, el
manejo de plaguicidas y las industrias del caucho y el petróleo. Los
trabajadores de las minas de carbón que fuman tienen un riesgo más
elevado de contraer bronquitis crónica y enfermedades obstructivas de
las vías respiratorias, pero no enfisema. El cáncer de pulmón de los
mineros del carbón se ha atribuido totalmente al humo de tabaco. El humo
de tabaco puede aumentar el riesgo para salud de la exposición a polvos
vegetales que producen trastornos respiratorios crónicos, como la
bisinosis debida al polvo del algodón y el cáncer nasal provocado por el
polvo de la madera.
3. Composición de la hoja del tabaco y del humo de tabaco
De las hojas de tabaco elaboradas se han aislado más de 3040 compuestos
químicos (Roberts, 1988). La mayoría son constituyentes de la hoja, pero
la presencia de algunos depende de las condiciones de cultivo, como el
suelo y la atmósfera de la zona, mientras que otros se derivan del uso
de productos químicos agrícolas, de revestimientos, humectantes y
aromatizantes añadidos a las hojas y de los métodos de curado. Las
diferentes variedades de tabaco que se cultivan en los distintos países,
así como las distintas formas de curado y elaboración, ponen de
manifiesto diversas diferencias. Las proporciones de los distintos
constituyentes pueden ser diferentes, pero no la composición general.
Entre los compuestos tóxicos importantes identificados, aparte de la
nicotina, figuran nitrosaminas carcinogénicas, derivadas de nitritos,
aminas, proteínas y alcaloides presentes en la hoja, hidro-carburos
aromáticos policíclicos procedentes del proceso de curado, elementos
radiactivos absorbidos del suelo y el aire, y cadmio en el tabaco
cultivado en suelos ricos en este elemento. Cuando se quema tabaco al
fumar, se forman numerosos productos derivados de la pirólisis y de
otras reacciones.
4. Corriente principal del humo de tabaco
El humo del tabaco es un aerosol formado por una fase particulada de
gotitas de líquido dispersas en una fase de gas/vapor. Al fumar un
cigarrillo se forman numerosos compuestos por la pirólisis del tabaco.
Éstos pasan a través del cigarrillo en la corriente principal del humo,
condensándose algunos a corta distancia detrás del cono de combustión,
o bien se emiten en el aire a partir del extremo que se quema como humo
lateral. En cada bocanada el humo se hace progresivamente más fuerte,
porque se le añade material previamente condensado y porque la longitud
del cigarrillo disponible para la ulterior condensación disminuye. Las
características fisicoquímicas del humo dependen de la elaboración y la
combustión del tabaco, la porosidad y el tratamiento del papel en el que
está envuelto y del tipo de filtro (Hoffman y Hoffman, 1997). En el caso
de un cigarrillo o "bidi" asiático (tabaco envuelto en hoja vegetal), la
química del humo se ve afectada por factores tales como las dimensiones,
la porosidad de la envoltura y los parámetros del volumen, la frecuencia
y la duración de la bocanada de humo (NIH, 1998). Las variaciones en la
química del humo se dan fundamentalmente en la proporción entre sus
constituyentes, más que en la presencia o ausencia de compuestos
concretos.
El humo de la corriente principal se genera en una atmósfera con un
contenido comparativamente bajo de oxígeno a una temperatura de
combustión de 850-950°C en el cono de combustión. Al principio, las
partículas presentes en la corriente principal tienen un diámetro
aerodinámico medio de la masa (MMAD) de 0,2 a 0,3 micras; sin embargo,
en cuanto llegan al tracto respiratorio, con una humedad del 100%, se
unen para formar partículas mayores y se comportan como si su MMAD fuera
del orden de micras. En el tracto respiratorio se puede retener
alrededor del 50-90% de todas las partículas inhaladas (Wynder y
Hoffmann, 1967; Hinds et al., 1983). Por lo que se refiere al tamaño, al
inhalar el humo pueden llegar a los alvéolos las partículas en aerosol,
los constituyentes de la fase de vapor y los gases permanentes. La
deposición en el árbol traqueobronquial se ve complicada por el
comportamiento de los constituyentes hidrofílicos en condiciones de
humedad elevada, pero el humo llega a todas las partes de las vías
respiratorias.
El humo de la corriente principal contiene cerca de 4000 sustancias
químicas identificadas y un número desconocido de sustancias químicas
sin identificar (Roberts, 1988). El humo de la corriente principal se
puede dividir en una fase de partículas y otra de gas. La fase de
partículas contiene nicotina, nitrosaminas como la
4-(metilnitrosamino)-1-(3-piridil)-1-butanona (NNK) y la
N-nitrosonornicotina (NNN), metales como el cadmio, el níquel, el zinc
y el polonio-210, hidrocarburos policíclicos y aminas carcinogénicas,
como el 4-aminobifenilo. La fase de vapor contiene monóxido de carbono,
anhídrido carbónico, benceno, amoníaco, formaldehído, cianuro de
hidrógeno, N-nitrosodimetilamina, N-nitrosodietilamina y otros
compuestos. Los compuestos del humo de tabaco se pueden clasificar por
su actividad biológica como asfixiantes, irritantes, ciliatoxinas,
mutágenos, carcinógenos, inhibidores de las enzimas, neurotoxinas o
compuestos farmacológicamente activos. El principal punto de entrada del
humo del cigarrillo en el organismo es por las vías respiratorias, pero
muchos constituyentes, en particular del humo de pipa y de cigarro, se
disuelven en la saliva y se absorben en la cavidad bucal o se ingieren.
Los fumadores de cigarros y de pipa no suelen inhalar el humo, que
permanece en la cavidad bucal, se disuelve en la saliva y se absorbe a
través de las membranas mucosas o se ingiere (NIH, 1998). Las bebidas
alcohólicas tienen un efecto disolvente de los constituyentes del humo,
facilitando su absorción.
5. Humo de tabaco lateral
El humo lateral se forma con una temperatura de combustión más baja
(500-600°C) en una atmósfera reductora. Las partículas del humo lateral
fresco son prácticamente del mismo tamaño que las de la corriente
principal, con un diámetro aerodinámico medio de la masa de alrededor de
0,2 micras. Desde el punto de vista cualitativo, la composición del humo
lateral es semejante a la del humo de la corriente principal. Algunas
sustancias químicas se emiten en el humo lateral con una concentración
mayor por gramo de tabaco quemado que en el humo de la corriente
principal. Esto es particularmente aplicable a carcinógenos como la
N-nitrosodimetilamina y la N-nitrosodietilamina y a metales como el
níquel o el cadmio. Muchos compuestos carcinógenos están más
concentrados en el humo lateral que en el principal. En biovaloraciones
con aplicación a la piel de ratones se ha demostrado que el humo lateral
condensado es más carcinogénico que el principal (Wynder y Hoffmann,
1967; US Surgeon General, 1986; NIH, 1998).
6. Efectos de la manera de fumar los cigarrillos en la
toxicidad del humo
El contenido de nicotina de los distintos cigarrillos varía, y el
fumador, para satisfacer la necesidad adquirida de nicotina, la ajusta
mediante la intensidad con la que fuma y la profundidad de la
inhalación. Por consiguiente, el fumador de cigarrillos con filtro y de
contenido bajo en nicotina (< 1,2 mg) fuma con mayor intensidad, y esto
influye en la toxicidad (NIH, 1998).
7. Resumen de las conclusiones y recomendaciones
El consumo de tabaco, en particular el hábito de fumar, representa un
peligro para la salud pública de la máxima importancia y es una causa
prevenible importante de morbilidad y mortalidad. Además de los efectos
adversos del consumo activo de tabaco para la salud, se han demostrado
efectos adversos derivados de la exposición al humo de tabaco presente
en el medio ambiente. Los riesgos del hábito de fumar también aumentan
como consecuencia de las interacciones con ciertos peligros químicos,
físicos y biológicos existentes en el lugar de trabajo y en el medio
ambiente general. Hay un pequeño número de casos de interacciones
antagonistas, pero los riesgos del humo de tabaco para la salud son muy
superiores a cualquier efecto protector aparente.
Se deben adoptar todas las medidas posibles para eliminar el consumo de
tabaco, en particular el hábito de fumar, y se ha de disuadir con
firmeza de fumar en lugares públicos. A fin de evitar la interacción con
otros tipos de exposición ocupacional y de eliminar el riesgo de
exposición al humo de tabaco del medio ambiente, debería prohibirse
fumar en el lugar de trabajo.
Con objeto de proteger la salud, en particular la de los niños, se
debería desalentar con firmeza el hábito de fumar en el hogar. De esta
manera se previenen posibles interacciones perjudiciales entre el humo
de tabaco y la exposición a otros peligros en la vivienda. Hay una
necesidad imperiosa de programas educativos sobre los peligros del
hábito de fumar para la salud. Los profesionales de la salud deberían
prestar asistencia para ayudar a los fumadores a dejar este hábito.
Debido a que el humo puede provocar una alteración de la respuesta a los
medicamentos y otros tratamientos o una reacción adversa a éstos, los
médicos deberían estudiar la posibilidad de introducir ajustes
apropiados de la dosificación y vigilar a los pacientes.
