
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 201
SELECTED CHLOROALKYL ETHERS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. R. Liteplo and Ms R. Gomes, Health Canada,
Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer-review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Selected chloroalkyl ethers.
(Environmental health criteria ; 201)
1. Bis(Chloromethyl) ether - toxicity
2. Bis(Chloromethyl) ether - adverse effects
3. Environmental exposure 4. Occupational exposure
I. International Programme on Chemical Safety II.Series
ISBN 92 4 157201 9 (NLM Classification: QZ 202)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED CHLOROALKYL ETHERS
PREAMBLE
ABBREVIATIONS
1. SUMMARY AND CONCLUSIONS
1.1. Identity, physical and chemical properties, analytical
methods
1.2. Sources of human exposure
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on laboratory animals and in vitro test systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory and field
1.9. Conclusions
1.9.1. BCEE
1.9.2. BCME and CMME
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. BCEE
2.4.2. BCME
2.4.3. CMME
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production
3.2.1.1 BCEE
3.2.1.2 BCME
3.2.1.3 CMME
3.2.2. Uses
3.2.2.1 BCEE
3.2.2.2 BCME
3.2.2.3 CMME
3.2.3. Sources in the environment
3.2.3.1 BCEE
3.2.3.2 BCME and CMME
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. BCEE
4.1.2. BCME and CMME
4.2. Abiotic degradation
4.2.1. BCEE
4.2.2. BCME and CMME
4.3. Biodegradation, biotransformation and bioaccumulation
4.3.1. BCEE
4.3.2. BCME and CMME
4.4. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. BCEE
5.1.2. BCME and CMME
5.2. General population exposure
5.3. Occupational exposure
5.3.1. BCEE
5.3.2. BCME and CMME
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
6.1. Absorption and distribution
6.2. Metabolism
6.3. Elimination
7. EFFECTS ON EXPERIMENTAL MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. BCEE
7.1.2. BCME and CMME
7.2. Short-term exposure
7.2.1. BCEE
7.2.2. BCME
7.2.3. CMME
7.3. Long-term exposure/carcinogenicity
7.3.1. BCEE
7.3.2. BCME
7.3.3. CMME
7.4. Mutagenicity and related end-points
7.4.1. BCEE
7.4.2. BCME
7.4.3. CMME
7.5. Other toxicity studies
8. EFFECTS ON HUMANS
8.1. General population exposure
8.1.1. Human exposure studies
8.2. Occupational exposure
8.2.1. Case reports
8.2.2. Epidemiological studies
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. BCEE
10.1.2. BCME and CMME
10.1.3. Guidance values
10.2. Evaluation of effects on the environment
10.2.1. BCEE
10.2.2. BCME and CMME
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
12. FURTHER RESEARCH
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RÉSUMÉ ET CONCLUSIONS
RESUMEN Y CONCLUSIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number 5 U01 ES02617-
15 from the National Institute of Environmental Health Sciences,
National Institutes of Health, USA, and by financial support from the
European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and epidemiological
methods in order to have internationally comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
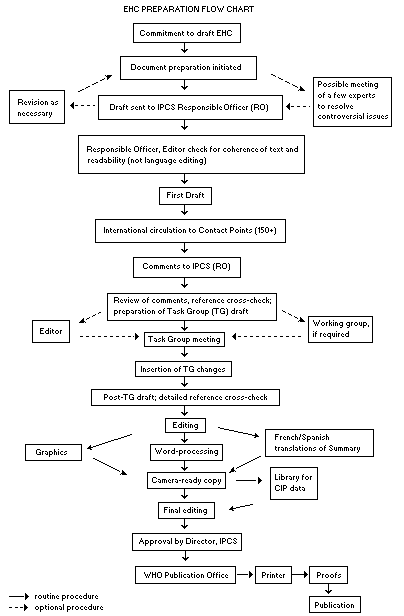
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED
CHLOROALKYL ETHERS
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton,
Surrey, United Kingdom
Dr R. Chhabra, Division of Intramural Research, Environmental
Toxicology Program, Toxicology Branch, National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA (Chairman)
Dr H. Ellisa, Epidemiology Department, Rohm & Haas, Bristol,
Pennsylvania, USA
Dr B. Gilbert, FarManguinhos, FIOCRUZ, Institute of
Pharmaceutical Technology, Ministry of Health, Rio de Janeiro,
Brazil
Professor M. Jakubowski, Occupational and Environmental
Hygiene Division, Nofer Institute of Occupational Medicine, Lodz,
Poland
Dr S.K. Kashyap, National Institute of Occupational Health,
Meghani Nagar, Ahmedabad, India (Vice-chairman)
Dr R. Liteplo, Environmental Health Directorate, Health Protection
Branch, Environmental Health Centre, Ottawa, Ontario, Canada
(Co-rapporteur)
Dr E.E. McConnell, Laurdane Estates, Raleigh, North Carolina,
USA
Dr H. Naito, Ibaraki Prefecture University of Health Sciences,
Amimachi, Inashikigun, Japan
Dr W. Popp, Universitätsklinikum Essen, Institut für Hygiene und
Arbeitsmedizin, Essen, Germany
Dr R. Sram, Laboratory of Genetic Ecotoxicology, c/o Institute of
Experimental Medicine, Prague, Czech Republic
a Invited, but unable to attend.
Dr Shou-Zheng Xue, Toxicology Programme, Shanghai Medical
University, Shanghai, China
Secretariat
Dr G.C. Becking, IPCS/IRRU, World Health Organization,
Research Triangle Park, North Carolina, USA
Ms R. Gomes, Health Canada, Environmental Health Directorate,
Tunney's Pasture, Ottawa, Ontario, Canada (Co-rapporteur)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED
CHLOROALKYL ETHERS
A WHO Task Group on Environmental Health Criteria for Selected
Chloroalkyl Ethers met at the British Industrial Biological Research
Association (BIBRA) Toxicology International, Carshalton, Surrey,
United Kingdom, from 18 to 23 March 1996. Dr D. Anderson opened the
meeting and welcomed the participants on behalf of the host institute.
Dr G.C. Becking, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS and the three cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks to human health
and the environment from exposure to selected chloroalkyl ethers.
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contribution to the IPCS.
The first and second drafts of this monograph were prepared by Dr
R. Liteplo and Ms R. Gomes, Health Canada, Ottawa. The second draft
incorporated the comments received following circulation of the first
draft to the IPCS contact points for environmental health criteria
monographs.
Dr G.C. Becking (IPCS Central Unit, Interregional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation of the document
are gratefully acknowledged.
ABBREVIATIONS
BCEE bis(2-chloroethyl) ether
BCME bis(chloromethyl) ether
CMME chloromethyl methyl ether
MTD maximum tolerated dose
PMA phorbol myristate acetate
TDGA thiodiglycolic acid
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, analytical methods
Bis(2-chloroethyl) ether (BCEE), bis(chloromethyl) ether (BCME)
and chloromethyl methyl ether (CMME) are chemicals from a large class
known as chloroalkyl ethers. The three ethers are colourless volatile
liquids at room temperature having characteristic odours. The vapour
pressure of these three compounds is high. The solubility of BCEE is
1.7% in water and its octanol/water partition coefficient is 1.46. The
alpha-chloroalkyl ethers BCME and CMME are reactive compounds,
hydrolysing rapidly in aqueous media (with half-lives of approximately
38 seconds and <0.007 seconds, respectively); hydrolysis of the more
stable ß-chloroether BCEE is slower (with a half-life in water of
about 20 years).
Sampling and analytical methods have been described for BCEE in
water and for BCME and CMME in air. Typically, determination is by
gas chromatography (GC-electron capture) or GC mass spectrometry.
1.2 Sources of human exposure
Natural sources of BCEE, BCME or CMME in the environment have not
been identified. The recent production data available are limited and
confined to the USA and Canada. Approximately 104 tonnes of BCEE were
produced in the USA in 1986 for use as a solvent and in the production
of polymers and several industrial processes. Industrial uses of BCME
are currently restricted in the USA to specific intermediate chemical
reactions. BCME has also been produced for use in the production of
ion exchange resins, manufacture of other polymers, and as a solvent
in polymerization reactions. In China, some 200 tonnes of BCME are
produced annually as an intermediate in the manufacture of the
insecticide synergist, octachlorodipropyl ether. Technical grade CMME
contains from 1 to 8% BCME.
1.3 Environmental transport, distribution and transformation
The mobility and distribution of the selected chloroalkyl ethers
is influenced by the high reactivity of BCME and CMME and the water
solubility and stability of BCEE. The alpha-chloroalkyl ethers BCME
and CMME are hydrolysed rapidly in aqueous media and degraded quickly
by photolysis. In aqueous media, the hydrolytic products of BCME and
CMME are formaldehyde and hydrochloric acid, and methanol,
formaldehyde and hydrochloric acid, respectively. The decomposition
products of BCME and CMME in air include hydrogen chloride,
formaldehyde and chloromethylformate, and chloromethyl and methyl
formate, respectively. BCEE is soluble in water; rainfall removes it
from the atmosphere and it tends to remain in water with very slow
hydrolysis. BCEE evaporates from surface water within a week and is
degraded in a little more than a day in the atmosphere by abiotic
processes.
Owing to the highly reactive nature of the alpha-chloroalkyl
ethers in water and air, CMME and BCME are not expected to be present
in the environment; however BCEE may be persistent due to the relative
stability of ß-chloroalkyl ethers.
1.4 Environmental levels and human exposure
Only limited data on levels of BCEE in environmental media are
available. It has been identified in air but not quantified; levels up
to 0.42 µg/litre have been found in drinking-water in the USA.
Reported levels of BCEE in groundwater have ranged from 0.001 µg/litre
at an industrial gypsum waste disposal site in Belgium to 840 µg/litre
near a waste disposal site in the USA. Higher concentrations have been
measured in landfill leachates. Information on levels of BCEE in
foodstuffs is not available, but bioaccumulation is not expected to
occur.
Quantitative data on levels of BCME or CMME in environmental
media are not available.
Based on the maximum reported level of BCEE in drinking-water,
i.e., 0.42 µg/litre, the average human (64 kg) consuming 1.4
litres/day would have an intake of about 0.01 µg/kg body weight per
day from this source, with unknown amounts from other environmental
sources. No estimates can be made on the daily intake of BCME and CMME
from environmental sources. However, based upon the lack of
persistence of BCME and CMME in the environment, average human
exposure to these compounds is likely to be very low.
Based on limited older data, workers in industries related to
plastics and textile production could have been exposed to between 1.2
and 72.9 µg BCME/m3 in workroom air. However, a recent study of a
resin-manufacturing plant reported average occupational exposures
ranging from 2.4 to 20.6 µg/m3. Data from other studies reported
levels of BCME as low as 0.01 µg/m3. Higher occupational exposure to
BCME occurred in China up until 1975 and still occurs on a lower level
in the manufacture of octachlorodipropyl ether. General population
exposure to BCME and CMME occurs where they are produced by the
widespread burning of this synergist in mosquito coils.
The highest reported concentrations of BCEE in the USA for
industrial effluents are 8 to 170 µg/litre and for municipal and
industrial waste landfill leachates 12 400 µg/litre.
1.5 Kinetics and metabolism
Quantitative information on the kinetics and metabolism of BCEE,
BCME and CMME in humans is not available. However, it is anticipated
that although in vivo BCME and CMME would be rapidly hydrolysed in
tissues to formaldehyde and hydrogen chloride, and methanol,
formaldehyde and hydrogen chloride, respectively, there should be
alkylation activity.
Limited data show that radioactive BCEE administered to rats by
inhalation or gavage is rapidly absorbed. Less than 3% of the
radioactivity was retained 48 h after gavage dosing.
BCEE is readily metabolized in rats. The principal metabolite is
thiodiglycolic acid (TDGA). After rats were given a single gavage dose
of [14C]-BCEE, approximately 12% of the administered radioactivity
was present as 14CO2.
BCEE is eliminated quickly in both rats and rhesus monkeys. Less
than 2% of the radioactivity was recovered in the faeces of monkeys 72
h after oral administration of [14C]-BCEE; approximately 2.3% of the
administered radioactivity was found in rat tissues or faeces 48 h
after dosing. Over 50% of the radioactivity was recovered in the urine
and exhaled air 12 h after a gavage dose of [14C]-BCEE was
administered to rats. Less than 2% of the radioactivity expired
through the lungs was exhaled as the parent compound.
1.6 Effects on laboratory animals and in vitro test systems
BCEE is acutely toxic by the oral, inhalation or dermal routes of
exposure. Reported LD50 values for the oral exposure of animal
species to BCEE range from 75 to 215 mg/kg body weight. BCME and CMME
are acutely toxic by inhalation or ingestion. Reported LC50 values
for the inhalation exposure of laboratory animals to BCME or CMME
range from 25 to 48 mg/m3, and from 182 to 215 mg/m3, respectively.
Exposure of laboratory animals by inhalation to high single
concentrations of BCEE (>320 mg/m3) resulted in eye irritation as
well as congestion, oedema, and haemorrhage in the lungs. During
inhalation of BCME, irritation of the eyes and respiratory tract were
noted as well as necrotizing bronchitis. Skin application resulted in
erythema and necrosis, and application to the eye resulted in corneal
necrosis. Similar effects were noted after exposure to CMME.
Increased mortality and tracheal hyperplasia were observed in
rats and hamsters following multiple inhalation exposure to 4.7 mg
BCME/m3. Similar results were observed in rats repeatedly exposed by
inhalation to 3.3 or 33 mg CMME/m3.
In general, positive results were obtained when BCEE, BCME and
CMME were tested for mutagenicity in vitro. However, interpretation
of the results is difficult given the lack of details in the reports
available. BCME and CMME have been reported to increase unscheduled
DNA synthesis in vitro, and BCME increased the level of transformed
cells in in vitro assays.
In small groups of males from two strains of hybrid F1 mice (and
in females from one F1 strain) treated orally with BCEE
(time-weighted dose 41.3 mg/kg body weight over 18 months), there was
a significant increase in the incidence of hepatomas (combined benign
hepatomas and malignant tumours) compared to unexposed controls. Four
other limited studies in rats and mice using oral gavage, subcutaneous
or intraperitoneal injection and skin painting failed to confirm these
findings.
Carcinogenicity studies in experimental animals (mice and rats)
exposed to BCME showed significantly elevated incidence of pulmonary
adenomas and respiratory tumours. In mice, inhalation exposure also
showed evidence of an elevated incidence of lung tumours.
Studies with CMME have shown an increased incidence of tracheal
metaplasia and bronchial hyperplasia in a dose-dependent manner in
rats. However, results of carcinogenicity bioassays are inconclusive
in animal studies.
Information regarding the reproductive, developmental,
immunological or neurological toxicity of BCEE, BCME or CMME is not
available.
1.7 Effects on humans
BCEE was found to be irritating to the eyes and nasal passages of
humans at levels >150 mg/m3 following short-term exposure.
No epidemiological studies on the effects of long-term exposure
to BCEE have been reported.
In eight epidemiological studies, exposure of workers to BCME
(CMME) was associated with increased risk of lung cancer. Workers
exposed to commercial grade CMME were probably also exposed to BCME.
The predominant tumours in exposed workers were small cell carcinomas,
quite distinct from the chiefly squamous cell carcinomas usually found
in smokers. The association between exposure to BCME (CMME) and lung
cancer was strong, with standardized mortality ratios ranging up to
21. The type of lung cancer, latency period and average age of
appearance of lung tumours in workers exposed to BCME (CMME) have been
remarkably consistent. For CMME, there is also evidence of a positive
relationship between a qualitative measure of exposure and mortality
due to lung cancer.
Even concentrations of 0.01 µg BCME/m3 and 20 µg CMME/m3, in
the course of occupational exposure, increased the frequency of
chromosomal aberrations in the peripheral lymphocytes of exposed
workers.
Information has not been reported regarding the neurological,
immunological, developmental or reproductive effects of BCME or CMME
in humans.
1.8 Effects on other organisms in the laboratory and field
There have been few studies on the effects of BCEE on
environmental organisms; most are restricted to aquatic species. For
BCEE a 7-day LC50 concentration in the guppy of 56.9 mg/litre, a 96-h
LC50 in fish of 600 mg/litre and a 48-h LC50 in Daphnia magna of
240 mg/litre have been reported.
Anaerobic microbial activity was not inhibited at concentrations
of BCEE up to 100 mg/litre and an LC10 of 600 µg/litre has been
reported for microbes indigenous to waste stabilization ponds.
No information on the toxicological effects of BCME and CMME on
environmental organisms has been reported.
1.9 Conclusions
1.9.1 BCEE
- Exposure of terrestrial organisms to BCEE is considered to be
negligible because of the low rate of release and its short
persistence in the atmosphere.
- Although it is more persistent in water, the highest reported
concentration of BCEE in surface water is approximately five
orders of magnitude lower than the concentration found to induce
adverse effects in the guppy, the most sensitive aquatic species
identified among existing toxicity studies.
- Owing to the lack of available information on concentrations of
BCEE in several environmental media to which humans are exposed,
it is not possible to estimate quantitatively the total daily
intake of BCEE.
- Available data on the toxicity of BCEE in humans are limited.
Information on the developmental and reproductive effects of BCEE
in laboratory animals has not been identified, and none of the
long-term studies in laboratory animals is of sufficient quality
to provide quantitative information on either the potential of
BCEE to cause cancer or the toxicological effects produced by
long-term exposure to this substance.
- In the absence of adequate toxicological and carcinogenicity
data, it is prudent to minimize human exposure to BCEE.
1.9.2 BCME and CMME
- If these substances were to enter the environment, they would
both be rapidly broken down by hydrolysis and photo-oxidation.
Data concerning concentrations of BCME and CMME in the ambient
environment have not been reported.
- BCME and technical grade CMME (which contains BCME) are proven
human carcinogens. In addition, both of these chemicals are
carcinogens in laboratory animals. Both chemicals cause
chromosomal aberrations in occupationally exposed workers.
Occupational and general population exposure to these compounds
should be eliminated.
- Based on the fate of these substances in the environment and the
lack of exposure, there is no reason to suspect that adverse
effects on aquatic and terrestrial organisms would occur.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Bis(2-chloroethyl) ether (BCEE), bis(chloromethyl) ether (BCME)
and chloromethyl methyl ether (CMME) are included in a large class of
chemical substances known as the chloroalkyl ethers. Identifying
features of BCEE, BCME and CMME are summarized in Table 1.
2.2 Physical and chemical properties
BCEE, a ß-chloroalkyl ether, is a colourless, volatile liquid
with a "chlorinated solvent-like" odour (Sittig, 1981). BCME and CMME,
both alpha-chloroalkyl ethers, are also colourless, volatile liquids
with characteristic odours. The odour of BCME has been described as
"suffocating" (Sittig, 1981; Verschueren, 1983), while that of CMME
has been described as "irritating" (Verschueren, 1983). Technical
grade CMME contains from 1 to 8% BCME (Travenius, 1982) and, unless
otherwise indicated in this monograph, CMME refers to the technical
grade material. In general, the vapour pressure and water solubility
of these compounds are high, and the log octanol/water partition
coefficients (log Kow) are low. The ß-chloroalkylethers like BCEE are
only slightly reactive towards water, but the alpha-chloroalkyl ethers
like BCME and CMME are rapidly hydrolysed by water, and their
solubility, Kow, Koc and Henry's Law constant cannot be
experimentally determined. The physical and chemical properties of the
selected chloroalkyl ethers are presented in Table 2.
2.3 Conversion factors
At 25°C and 101.3 kPa, the conversion factors for BCEE, BCME and
CMME in air are as follows:
BCEE: 1 ppm (v/v) = 5.85 mg/m3; 1 mg/m3 = 0.17 ppm
BCME: 1 ppm (v/v) = 4.7 mg/m3; 1 mg/m3 = 0.21 ppm
CMME: 1 ppm (v/v) = 3.3 mg/m3; 1 mg/m3 = 0.30 ppm
2.4 Analytical methods
2.4.1 BCEE
One method for the analysis of BCEE in water involves solvent
extraction (using diethyl ether in pentane, methylene chloride, or
ethyl ether in hexane), concentration with a Kuderna-Danish (K-D)
apparatus, and separation and analysis by gas chromatography with
electron capture detection (GC/EC) or gas chromatography mass
spectrometry (GC/MS) (Dressman et al., 1977; Quaghebeur et al., 1986).
This method has been expanded to include clean-up with Florisil and
K-D concentration of the sorbed fraction prior to analysis by GC/EC
(McMillin et al., 1984). Vapour stripping using helium or nitrogen gas
has also been used to extract BCEE from samples of ground and surface
Table 1. Information on the identity of BCEE, BCME and CMME (US NLM, 1996)
Compound Identification Molecular Chemical structure Relative Synonyms
(CAS number)a formula molecular mass
Bis(2-chloroethyl) ether BCEE C4H8Cl2O Cl-(CH2)2-O-(CH2)2-Cl 143.02 dichloroethyl ether,
(111-44-4) dichloroethyl oxide,
bis (ß-chloroethyl) ether,
dichloroether,
1,1'-oxybis(2-chloro)ethane,
1,5-dichloro-3-oxapentane,
1-chloro-2-(ß-chloroethoxy)-
ethane,
2,2'-dichloroethyl ether,
ß,ß'-dichlorodiethyl ether,
bis(chloro-2-ethyl) oxide,
di(ß-chloroethyl) ether,
di(2-chloroethyl) ether,
ether, bis(2-chloroethyl),
sym-dichloroethyl ether,
diethylene glycol dichloride.
Bis(chloromethyl) ether BCME C2H4Cl2O Cl-CH2-O-CH2-Cl 114.97 chloro(chloromethoxy) methane,
(542-88-1) sym-dichloro-dimethyl ether,
oxybis(chloromethane),
dichloromethyl ether,
bichloromethyl ether,
dichlorodimethyl ether,
1,1'-dichlorodimethyl ether.
Table 1. (continued)
Compound Identification Molecular Chemical structure Relative Synonyms
(CAS number)a formula molecular mass
Chloromethyl methyl ether CMME C2H5ClO Cl-CH2-O-CH3 80.52 chloromethoxymethane,
(107-30-2) monochlorodimethyl ether,
methoxymethyl chloride,
chlorodimethyl ether,
methyl chloromethyl ether,
monochloromethyl methyl ether.
a Chemical Abstracts Services registry number.
Table 2. Physical and chemical properties of BCEE, BCME and CMME
Physical/chemical property BCEE BCME CMME
Melting point (°C) -50a -41.5a -103.5b
Boiling point (°C) 178.67a 104a 59.5b
Vapour pressure (mmHg) 0.71 at 20°Cb 30 at 22°Cc 122 at 20°Cd
Vapour density 4.93b 3.97b 2.8d
Water solubility (mg/litre) 10 200b NA NA
Log octanol/water partition
coefficient (log Kow) 1.46c NA NA
Henry's Law constant
(atm.m3/mol) 1.31 x 10-5c NA NA
Soil sorption coefficient
(log Koc) 1.1c NA NA
Hydrolysis rate constant
in water 4 x 10-6 h-1 at 25°Cc 0.05 sec-1h >90 sec-1 at 25°Ce
in air not available 1.7 x 10-1 sec-1 at 45°Ci 0.0018 min-1 at 29°Ck
Photolysis rate constant
in water 24 to <360 mol-1.h-1c 3 to <360 mol-1.h-1c not available
in air 1.79 x 10-11 cm3.mol-1.sec-1f not available 1.0 x 10-10 mol-1.sec-1e
Half-life
in water 20 years at 25°C (hydrolysis)c 38 sec at 20°C (hydrolysis)j <0.007 sec at 25°Ce
in air 13.44 h at 25°C (indirect photolysis)f >25 h at 25°C (hydrolysis)k 3.5 to 6 min at 25°C
(hydrolysis)i
in soil 1 to 6 months (estimate)g not available not available
Table 2 (continued)
a Weast & Astle (1985) g Howard et al. (1991)
b Verschueren (1983) h Tou & Kallos (1974a)
c Mabey et al. (1982) i Nichols & Merritt (1973)
d CCINFO (1991) j US EPA (1980)
e Radding et al. (1977) k Tou & Kallos (1974b)
f US EPA (1987b)
NA = not applicable. Due to the extremely rapid hydrolysis of this substance in water, it is not possible to obtain an experimental
value, and calculated values are meaningless.
water. Typically, this step is followed by concentration of the
extract with a cold or lipophilic vapour trap, and analysis by GC/MS
(Hites et al., 1979; DeWalle & Chian, 1981). An additional technique
has been described by Kleopfer & Fairless (1972), in which samples of
water are passed through an activated carbon filter, followed by
Soxhlet extraction of the carbon, drying of the extract with sodium
sulfate, K-D concentration, Shriner-Fuson separation of the acidic,
basic and neutral fractions, and analysis of the last by GC/MS.
Determination of BCEE in air involves passing air samples through a
sorbent, followed by elution and analysis by gas chromatography
(NIOSH, 1984).
Reported detection limits for these methodologies differ by up to
two orders of magnitude. Detection limits for the procedure described
by Dressman et al. (1977) and Quaghebeur et al. (1986) range from
0.005 to 0.04 µg/litre, respectively. Limits of detection for the
methods described by McMillin et al. (1984) and Kleopfer & Fairless
(1972) are 0.3 and 0.2 µg/litre, respectively.
2.4.2 BCME
While information concerning the sampling and analysis of BCME in
water, soil or foodstuffs was not available, considerable data on
techniques for the analysis of low levels (µg/m3) of BCME in air have
been identified (Collier, 1972; Evans et al., 1975; Frankel & Black,
1976; Parkes et al., 1976; Kallos, 1981; Muller et al., 1981; Galvin &
House, 1988; Blease et al., 1989). Typically, air samples are drawn
into a (Poropak or Tenax) sorption tube, thermally eluted, and
analysed by GC/MS or GC/EC. Two additional methods have been described
which involve the direct derivatization of BCME (with
2,4,6-trichlorophenol or sodium pentafluorophenolate), and subsequent
analysis by GC/EC (Sawicki et al., 1976; Langelaan & Nielen, 1989).
Norpoth et al. (1981) reported a spectrophotometric method for the
determination of BCME.
Collier (1972), Frankel & Black (1976) and Galvin & House (1988)
reported a detection limit of 470 ng/m3 for BCME in air, while Evans
et al. (1975) and Langelaan & Nielen (1989) achieved detection limits
as low as 50 and 14 ng/m3, respectively. Muller et al. (1981) did not
report a detection limit, but quantified BCME at a concentration of
2.35 µg/m3 in air. A detection limit of 0.94 µg/m3 was reported for
the spectrophotometric quantification method described by Norpoth et
al. (1981). The methods described by Sawicki et al. (1976) and Parkes
et al. (1976) have a detection limit of 2.35 µg/m3, while a detection
limit of approximately 4.7 ng/m3 was established for the method
described by Blease et al. (1989), in which high resolution was
achieved with the combined use of gas chromatography and tandem mass
spectrometry (GC/MS/MS).
2.4.3 CMME
Identified methods for the sampling and analysis of CMME in
environmental media are limited to techniques developed for monitoring
low levels (µg/m3) in air. Several methods have been described which
involve the derivatization of CMME (with 2,4,6-trichlorophenol or
sodium pentafluorophenolate) and subsequent analysis by GC/EC (Sawicki
et al., 1976; Kallos et al., 1977; Langhorst et al., 1981; Langhorst,
1985; Langelaan & Nielen, 1989). The limits of detection for these
methodologies are 49 ng/m3 (Langelaan & Nielen, 1989), 1.65 µg/m3
(Sawicki et al., 1976; Langhorst et al., 1981) and 3.29 µg/m3 (Kallos
et al., 1977).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Natural sources of BCEE, BCME or CMME in the environment have not
been identified. While BCME could be formed spontaneously from the
reaction of formaldehyde and chloride ions in an acidic atmosphere,
this reaction is unlikely in the general environment, although it may
be important in occupational settings (Durkin et al., 1975; Tou &
Kallos, 1976; Kallos & Tou, 1977; Travenius, 1982).
3.2 Anthropogenic sources
3.2.1 Production
Only limited information on the production of BCEE, BCME or CMME
has been reported.
3.2.1.1 BCEE
BCEE used to be prepared commercially in the USA as a by-product
in the manufacture of ethylene oxide by the chlorohydrin process, but
this process went out of use in the USA in 1973 (IARC, 1975). Other
methods of production also involving ethylene glycol or ethylene,
ethylene chlorohydrin and chlorine as reagents have been mentioned
(Durkin et al., 1975; IARC, 1975; ATSDR, 1989a). In 1975, two US
companies, one German and one Japanese company manufactured BCEE for
captive use as a solvent or chemical intermediate (IARC, 1975).
3.2.1.2 BCME
BCME is formed when formaldehyde reacts with chloride ions in an
acidic medium (Travenius, 1982). In China, BCME is produced by the
reaction of paraformaldehyde and hydrogen chloride gas as an
intermediate in the synthesis of the insecticide synergist S-2,
octachlorodipropyl ether [bis(1,2,3,3-tetrachloropropyl)ether] to
which it is converted in a one-part process. The scale of S-2
production is believed to be around 700 tonnes/year, which would
require over 200 tonnes of BCME. Specific synthesis reactions include
the reaction between paraformaldehyde and chlorosulfonic acid (Durkin
et al., 1975) and the saturation of a paraformaldehyde solution in
cold sulfuric acid with hydrogen chloride (US EPA, 1980). Small
amounts (several percent) of BCME are also produced during the
synthesis of CMME from gaseous hydrogen chloride and heated methanol
and formaldehyde (Durkin et al., 1975). In addition, the decomposition
products of commercial forms of CMME can combine to produce 1 to 8%
BCME as an impurity (Travenius, 1982). While BCME is not produced in
commercial quantities in Canada or the USA, it has been produced in
small quantities for use as a chemical intermediate in laboratory
applications (IARC, 1974).
3.2.1.3 CMME
CMME is produced by the reaction of anhydrous hydrogen chloride,
methanol and formaldehyde (Fishbein, 1979) or by the direct
chlorination of dimethyl ether (Durkin et al., 1975). An additional
method, which is designed to produce CMME that is free of BCME
impurities, involves the addition of actinium chloride to a slight
excess of anhydrous dimethoxymethane at room temperature (CCINFO,
1991). Production of CMME in the USA was estimated to be at least 4590
tonnes in 1977 and about 2.27 tonnes in 1982 (HSDB, 1996).
3.2.2 Uses
Only information concerning the use of BCEE, BCME or CMME in
Canada and the USA is available.
3.2.2.1 BCEE
In the USA, BCEE was formerly used in the process for the
manufacture of methyldithiocarbamic acid fungicide commonly known as
metham-sodium. Besides this use, approximately 20% of the BCEE sold in
the USA was used in the production of polymers, and 7% was either used
to synthesize a derivative of diquat or recycled for use as a
co-solvent (S. Helmhout, personal communication to the IPCS, 1992).
Other applications have included its use as a solvent for fats, waxes,
greases and esters; as a constituent of paints, varnishes and
lacquers; as a solvent for the removal of fatty substances from
various textiles, and as a penetrant and wetting agent in the textile
industry. It has also been used in the purification of oils and
gasoline, as a soil fumigant, insecticide and acaricide, and as an
intermediate in the manufacture of pharmaceuticals and other chemicals
(Durkin et al., 1975; IARC, 1975; US EPA, 1987a; ATSDR, 1989a).
3.2.2.2 BCME
In the USA, industrial use of BCME has been restricted since the
early 1980s to specific intermediate chemical reactions (Travenius,
1982). In China, BCME is an intermediate in the production of the
insecticide synergist S-2, octachlorodipropylether (see section
3.2.1.2). In the past, BCME has been used as a chloromethylating agent
in the production of ion exchange resins, water repellents and other
textile-treating agents, the manufacture of polymers, and a solvent
for polymerization reactions (Fishbein, 1979). Specific minor uses of
BCME have included the crosslinking of cellulose, the preparation of
three-block styrene-butadiene-styrene polymers, and the surface
treatment of vulcanized rubber to increase adhesion of epoxy resin and
polyurethane elastomers (Durkin et al., 1975).
Available data indicate that there is currently no commercial
activity involving more than one kilogram of BCME in Canada
(Government of Canada, 1993b).
3.2.2.3 CMME
In the USA, industrial use of CMME has been restricted since the
early 1980s to specific intermediate chemical reactions (Travenius,
1982). Based on available data, there is currently no commercial
activity in Canada involving more than one kilogram of CMME
(Government of Canada, 1993b).
In the past, CMME has been used as a chloromethylating agent in
many synthetic processes, most notably in the production of anion
exchange resins (Durkin et al., 1975). It has also been used as a
solvent for polymerization reactions (Fishbein, 1979), in the
synthesis of methoxymethyl ethers of phenols, the crosslinking of
polystyrene, and the surface treatment of vulcanized rubber (Durkin et
al., 1975).
3.2.3 Sources in the environment
Information on the release of BCEE, BCME and CMME in countries
other than the USA and Canada has not been reported.
3.2.3.1 BCEE
BCEE may enter the environment as a by-product from the
chlorination of waste streams containing ethylene or propylene, and as
a contaminant in the fungicide metam-sodium. It has been estimated,
based on the quantities imported and the known level of contamination,
that less than 100 g of BCEE would have been released into the
Canadian environment in 1990 from metam-sodium (Government of Canada,
1993a). In the USA, a total of 2700 kg/year was estimated to be
released into the environment from chemical plants in 1989. Seventy
percent of this amount was reported to be emitted to the air, while
the remaining 30% was released in water (US EPA, 1990). The
chlorination of drinking-water containing diethyl ether can result in
the formation of BCEE (NRC, 1977); however, quantitative data have not
been identified.
3.2.3.2 BCME and CMME
It was reported in the Toxic Release Inventory Database (US EPA,
1990) that less than 1 kg of BCME and 50 kg of CMME were released into
the atmosphere in the USA from industrial producers and users during
1989. However, release occurred in the two-step production of
octachlorodipropyl ether in China (Chen et al., 1996). This process
ceased in 1975, but manufacture of octachlorodipropyl ether was
revived in 1987 using a one-step process, from which gas releases and
accidental liquid spills occur. There is no information on the amount
of BCME that may remain as a contaminant of the product, which
contains formaldehyde and hydrogen chloride (BCME's precursors). There
is, however, gas-chromatographic evidence that CMME and BCME are
released into the air by the burning of octachlorodipropyl ether in
mosquito coils. No information is available from these sources
concerning the release of BCME or CMME into other media (water, soil,
underground injection), but, owing to their rapid rate of hydrolysis,
these compounds are not expected to remain as such for prolonged
periods in waste streams from plants where they are produced or used
(IARC, 1974).
The spontaneous formation of BCME or CMME in drinking-water from
the chlorination of ethers has not been investigated. However, in view
of their rapid rate of hydrolysis (see section 4.2.2), it is unlikely
that BCME or CMME would be present as contaminants in drinking-water
(Durkin et al., 1975).
No information has been identified concerning the quantities of
BCME or CMME released into the environment during storage or
transportation. However, these amounts are likely to be insignificant
since BCME and CMME have been usually produced and used in "closed
system" operations where containment prevents the release of these
chemicals into the environment (Durkin et al., 1975).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 BCEE
Based on the low-to-moderate Henry's Law constant (1.3 × 10-5
atm.m3/mol), BCEE would tend to remain in water. The air/water ratio,
as well as the Henry's Law constant, will determine the amounts of
BCEE distributed between the two compartments. Rainfall would probably
result in the removal of BCEE from the atmosphere (Durkin et al.,
1975). Using the approach of Mackay & Wolkoff (1973), Durkin et al.
(1975) calculated the half-life with respect to volatilization of BCEE
from a body of water to be 5.78 days at 25°C. Similarly, a
volatilization half-life of 3.4 days (from water) was calculated by
the US EPA (1987b). Thus the removal of BCEE from surface water will
probably occur within a week, although it will persist in bottom
water. Based upon its low log Koc (organic carbon partition
coefficient) and high water solubility, BCEE is not expected to adsorb
to soil or sediment and is therefore considered to be mobile in these
media (US EPA, 1987b). The US EPA (1987b) reported that, because of
its vapour pressure, BCEE should volatilize relatively rapidly from
dry surfaces. In the only study dealing with soil volatilization (a
7-day microcosm study by Piwoni et al. (1986) in which the soil was
kept moist), an insignificant amount (3%) of applied BCEE was
calculated to have volatilized.
4.1.2 BCME and CMME
Information regarding the mobility and distribution of BCME and
CMME in environmental media is limited. Callahan et al. (1979)
suggested that BCME could volatilize rapidly from an aquatic system
only if it were discharged in a water-immiscible solvent with a high
vapour pressure. Once in the atmosphere, these substances would be
rapidly degraded by photo-oxidation or hydrolysis. Very little
information was identified concerning the behaviour of BCME or CMME in
soil. It is unlikely that BCME and CMME are mobile in soil as both
compounds hydrolyse rapidly in an aqueous environment.
4.2 Abiotic degradation
4.2.1 BCEE
At a temperature of 20°C in water, a hydrolysis half-life of 20
to 22 years was estimated for BCEE (Mabey et al., 1982; Milano et al.,
1989). The US EPA estimated the half-life for the reaction of BCEE
with hydroxyl radicals in the atmosphere to be approximately 2.8 days
(A. Leifer, Office of Toxic Substances, US EPA, personal
communication, 1992). A half-life of 13.4 h has been reported for the
indirect photolysis of BCEE in the gaseous phase (US EPA, 1987b).
Photolysis products of BCEE include 2-chloroethanol, ethyl alcohol,
methyl alcohol, 2-chloroethyl ethyl ether, peracetic acetic acid,
1-(2-chloroethoxy)-1,2-epoxyethane, acetaldehyde and chloracetaldehyde
(Milano et al., 1989).
4.2.2 BCME and CMME
BCME and CMME are removed from environmental media via abiotic
processes. In the atmosphere, these substances are degraded by
photo-oxidation or hydrolysis. Cuppit (1980) reported atmospheric
half-lives of < 2.9 days for BCME and < 3.9 days for CMME. Tou &
Kallos (1974a) reported half-lives for atmospheric hydrolysis of
> 1 day for BCME and between 0.0024 (Nichols & Merritt, 1973) and
0.27 days for CMME, in humid air. At low humidity levels, however,
BCME may be degraded by oxidative as well as hydrolytic pathways. In
air, the decomposition products for BCME include hydrogen chloride,
formaldehyde and chloromethylformate, while those of CMME include
chloromethyl and methyl formate (Cupitt, 1980).
BCME and CMME hydrolyse rapidly in water. At 20°C, half-lives in
water of 38 seconds for BCME and < 1 second for CMME have been
reported (Tou et al., 1974; Radding et al., 1977; US EPA, 1980).
Although BCME may be degraded by oxidation, the extremely rapid
hydrolysis of BCME in aqueous media precludes any significant
oxidative degradation of this substance in aquatic systems (Callahan
et al., 1979). BCME is hydrolysed to formaldehyde and hydrogen
chloride (ATSDR, 1989b), while CMME is hydrolysed to hydrogen
chloride, methanol and formaldehyde (Travenius, 1982).
4.3 Biodegradation, biotransformation and bioaccumulation
4.3.1 BCEE
In the only study identified, Tabak et al. (1981) reported that
BCEE was completely biodegraded within 7 days in an aqueous medium
inoculated with sewage sludge. Although data on the biodegradation of
BCEE in soil are limited, this process may play some role in the fate
of this substance in soil. Kincannon & Lin (1986) reported a half-life
of BCEE in soil of approximately 16.7 days, based on the results of a
97-day soil column study in which the degradation of BCEE mixed with
hexachloroethane (as a constituent of a hazardous waste sludge) was
quantified.
For biota, Barrows et al. (1978) reported a bioconcentration
factor (BCF) of 11 and a biological half-life of between 4 and 7 days
for BCEE in bluegill sunfish (Lepomis marochirus) based on the
results of a study in which the fish were exposed to BCEE (under
flow-through conditions) for 14 days at a mean water concentration of
10 µg/litre.
4.3.2 BCME and CMME
No information on the biodegradation of either BCME or CMME in
soil was identified. However, their high rates of hydrolysis in
aqueous media preclude any possibility of BCME or CMME bioaccumulating
in organisms.
4.4 Ultimate fate following use
Owing to the highly reactive nature of the
alpha-chloroalkylethers in water and air, CMME and BCME are not
expected to be present in the general environment (Durkin et al.,
1975). However, owing to the relative stability of ß-chloroalkylethers
in environmental media, BCEE may be persistent in the general
environment (Durkin et al., 1975).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 BCEE
Quantitative information on the levels of BCEE in air is limited
to a single study in the USA in which this substance was detected (but
not quantified) in the atmosphere above two landfill sites in New
Jersey (US NLM, 1996).
Available data concerning the levels of BCEE detected in surface
water and drinking-water are summarized in Tables 3 and 4,
respectively. BCEE has been detected in samples of municipal
drinking-water at mean concentrations of up to 0.42 µg/litre in the
USA (Kraybill, 1977). The highest concentration reported for selected
surface waters was 58 µg/litre in Belgium, in the vicinity of
industrial discharges (Quaghebeur et al., 1986).
Identified studies concerning the levels of BCEE in groundwater
were limited to surveys conducted in the vicinity of contaminated
areas; concentrations of BCEE ranged from 0.001 µg/litre in samples
collected at an industrial gypsum waste disposal site in Belgium
(Quaghebeur et al., 1986) to 840 µg/litre in samples collected near a
municipal and industrial waste landfill site in the USA (DeWalle &
Chian, 1981).
Identified studies on the levels of BCEE in soil were limited to
two investigations in which this compound was detected in samples
collected from contaminated areas in the USA. BCEE was monitored (but
not quantified) in samples of soil collected at Love Canal, New York
(Hauser & Bromberg, 1982), and measured at a mean concentration of 140
mg/kg in samples of soil from waste disposal sites in the USA (ATSDR,
1989a).
No information is available on the levels of BCEE in foodstuffs.
Based on its high water solubility and low Kow, BCEE is not expected
to bioaccumulate in fish or other aquatic species (ATSDR, 1989a).
The concentration of BCEE in in-plant effluents in Canada has
been reported to range from 6.1 to 1057 µg/litre (Government of
Canada, 1993a). These effluents are diluted with cooling water before
being discharged to the environment and, although levels of BCEE at
the outflow pipe were not monitored, they were probably below the
limit of detection.
The highest concentrations of BCEE in the USA were reported for
industrial effluents (8 to 170 µg/litre), and municipal and industrial
waste landfill leachates (12 400 µg/litre) (DeWalle & Chian, 1981).
Table 3. Bis(2-chloroethyl) ether levels in surface water
Location Number of Concentrationb Remarks Reference
samplesa mean (range)
(µg/litre)
Philadelphia, USA NR ND samples collected from April 1975 to July 1975 Manwaring et al. (1977)
from the Delaware River, upstream from a
water treatment plant
NR trace samples collected in April 1975 from the
Delaware River, upstream from a chemical plant Manwaring et al. (1977)
2 trace samples collected in October 1976 from the
Delaware River Sheldon & Hites (1978)
5 (ND - trace) samples collected in March 1977 from the
Delaware River Sheldon & Hites (1978);
US EPA (1980)
New Orleans and
Baton Rouge, USA 3 0.11 (0.04 - 0.16) Pellizzari et al. (1979)
Houston, USA 1 (1) 1.4 Pellizzari et al. (1979)
Nitro, USA NR 0.041 samples collected from the Kanawha River Rosen et al. (1963);
Durkin et al. (1975)
USAc 808 (3) < 10.0 median limit of detection, 10.0 µg/litre Staples et al. (1985)
Belgiumc NR (7 - 58) samples collected from Haine River adjacent to
industrial discharges Quaghebeur et al. (1986)
Belgiumc NR (trace - 7.9) samples collected from Durme River, Scheldt
River and Gheut-Terneuzen Channel
downstream from industrial discharges Quaghebeur et al. (1986)
a Value in parenthesis indicates the number of samples with detectable levels of bis(2-chloroethyl) ether.
b Mean and/or (range) of concentrations, unless otherwise indicated; detection limits were reported, when possible.
c Locations were not specified.
NR = not reported; ND = not detected
Table 4. Bis(2-chloroethyl) ether levels in drinking water
Location Number of Concentrationb Detection Remarks Reference
samplesa mean (range) limit
(µg/litre) (µg/litre)
Toronto, Canada 50 (0) ND 0.00003 finished drinking-water Kendall (1990)
Toronto, Canada 8 (0) ND 0.001 bottled spring water Kendall (1990)
Alberta, Canadac 1512 (1) ND (ND - trace) 1 samples of treated (from 215 Alberta Ministry of the
sites) and raw (from 14 sites) Environment (1991)
drinking-water
collected from January 1986
to June 1991
Nitro, USA 1 (1) 0.2 NR tap water DeWalle & Chian (1981)
Evansville, USA 1 NQ NR finished drinking-water Kleopfer & Fairless (1972)
Philadelphia, USA NR NQ NR finished drinking-water Suffet et al. (1980)
collected between 1975
and 1977
Philadelphia, USA NR < 0.1 (0.04 - 0.6) NR finished drinking-water Manwaring et al. (1977)
collected between February
1975 and July 1975
New Orleans, USA NR (0.04 - 0.16) NR finished drinking-water Keith et al. (1976)
collected in August 1974
Philadelphia, USA NR (0.03 - < 1) NR raw drinking-water collected Manwaring et al. (1977)
between April 1975 and
July 1975
Philadelphia, USA NR (0.4 - 0.5) NR raw drinking-water Durkin et al. (1975)
Table 4. (continued)
Location Number of Concentrationb Detection Remarks Reference
samplesa mean (range) limit
(µg/litre) (µg/litre)
USAc NR 0.42 NR finished drinking-water Kraybill (1977)
NR ND 5 finished drinking-water US EPA (1980)
collected (between March
1976 and April 1976) from
112 cities during the
National Organics Monitoring
Survey (NOMS) (Phase I)
NR 0.0115 (ND - 0.36) 0.005 finished drinking-water Dressman et al. (1977)
collected (between May 1976
and June 1976) from 113
cities during the NOMS
(Phase II); BCEE was detected
in drinking-water from 13
cities at a mean concentration
of 0.10 µg/litre
USAc NR 0.0017 NR finished drinking-water US EPA (1980)
collected (between November
1976 and June 1977) from 110
cities during the NOMS (Phase
III); BCEE was detected in
drinking-water from 8 cities
at a mean concentration of
0.024 µg/litre
NR (0.02 - 0.12) NR drinking-water from 80 cities Fishbein (1979)
Netherlandsc NR 0.1 maximum NR Kraybill (1977)
Table 4 (continued)
a Values in parenthesis indicate the number of samples with detectable levels of bis(2-chloroethyl) ether.
b Mean and/or (range) of concentrations, unless otherwise indicated.
c Locations were not specified
ND = not detected
NR = not reported
NQ = not quantified
5.1.2 BCME and CMME
No information has been reported on levels of BCME or CMME in
ambient air or the indoor air of homes or offices. In a small survey
of outdoor air in the Netherlands, BCME and CMME were not detected
(detection limits, 14.1 µg/m3 and 49.5 µg/m3, respectively) in
samples collected in the neighbourhood of a potential emission source
(distance and source were not specified) (Langelaan & Nielen, 1989).
Available data on the levels of BCME or CMME in drinking-water,
surface water or ground water are limited to one investigation in
which BCME was not detected (detection limit, 10 µg/litre) in a total
of 317 samples of surface and groundwater from unspecified locations
in the USA (Staples et al., 1985).
Quantitative data concerning the levels of BCME or CMME in soil
have not been reported. However, in view of their rapid rate of
hydrolysis, these compounds are not expected to persist as
contaminants in moist soil (US NLM, 1996). Similarly, while no studies
on the levels of BCME or CMME in foodstuffs have been reported, the
high rates of hydrolysis reduce the likelihood of BCME or CMME
bioaccumulating in the food chain (US NLM, 1996).
No reliable data on levels of either BCME or CMME in industrial
effluents have been reported.
5.2 General population exposure
Quantitative data concerning the levels of BCEE in the general
environment are restricted to the results of studies in which the
levels of this substance in surface water and drinking-water have been
assessed. Based on a daily volume of ingestion for adults of 1.4
litres, a mean body weight for males and females of 64 kg (IPCS,
1994), and the highest mean concentration of BCEE in drinking-water
presented in Table 4 (0.42 µg/litre), the estimated intake of BCEE
from drinking-water for adults would be approximately 0.01 µg/kg body
weight per day.
Adequate information on the concentrations of BCME and CMME in
air, drinking-water, soil, or foodstuffs have not been reported, and
therefore it is not possible to estimate the intake of these
substances. No quantitative data are available for the exposure of
populations that use mosquito coils containing octachlorodipropyl
ether (see section 3.2.3.2), but the number of users of such coils is
of the order of millions in China.
5.3 Occupational exposure
5.3.1 BCEE
Occupational exposure to BCEE (via inhalation or dermal contact)
may occur in individuals involved in the dry cleaning and textile
industries, or in the processing of gum, lacquer, oil, paint, soap and
tar (Tabershaw et al., 1977). However, no investigations concerning
quantitative levels of exposure to BCEE in the workplace have been
reported.
5.3.2 BCME and CMME
Occupational exposure to BCME or CMME may occur in laboratory and
textile workers, and in individuals involved in the production of
anion-exchange resins, organic chemicals and polymers (Lemen et al.,
1976; US EPA, 1980). In China, occupational exposure to BCME occurs in
the manufacture of octachlorodipropyl ether. Under conditions where
vapours of formaldehyde and hydrochloric acid co-exist, BCME may form
spontaneously in air. Available quantitative data concerning
occupational exposure to either BCME or CMME are limited to
investigations of the levels of BCME in workroom air (Table 5).
BCME may be produced in solution from a variety of sources of
formaldehyde and chloride ions, and has been detected in the vapours
above these solutions (Frankel et al., 1974). In one study, the
concentration of BCME in the headspace above formalin slurries
containing Freidel-Crafts (chloride) salts ranged from 0.99 to
7.1 mg/m3 (210 to 1500 ppb) (Frankel et al., 1974).
While no recent studies have been identified where levels of
occupational exposure to CMME have been reported, it has been
estimated that in the past, concentrations of CMME in workroom air may
have ranged from 4.7 to 47 mg/m3 (1-10 ppm) (Travenius, 1982).
Table 5. Concentrations of bis(chloromethyl) ether in workroom air
Industry Sampling period Concentrationa Detection limit Reference
(µg/m3) (µg/m3)
Dye auxiliaries (resin) production; Jan. 1976 - Aug. 1976 ND 0.5 or 0.9 Yao & Miller (1979)
dye manufacture; fertilizer
production; textile finishing on
woven goods; hospital procedures;
foundry products (research plant);
foundry products (full-scale plant)
(USA)
Plastics industryb (USA) Jan. 1973 <4.7 - 72.9 NR Eisner (1974)
Textile finishing plants (4) (USA) Nov. 1974 - Dec. 1974 <0.5 - 37.6 0.5 Marceleno (1974)
Chemical plant (UK) 1978 <4.7 NR Travenius (1982)
Chemical plant (Netherlands) NR 1.2 - 3.8 0.5 van der Ven & Venema
(1979)
Resin manufacturing plantd (France) 1979 - 1984 2.8 - 20.6 NR Gowers et al. (1993)
a Concentrations of bis(chloromethyl) ether measured in workroom air
b Samples of air collected at the Diamond Shamrock Chemical Company in California, in the vicinity of reactors used to condense
phenol and formaldehyde
c Unspecified industrial operations; location of sample acquisition was not reported
d Range of average concentrations from various areas in the plant
ND - not detected
NR - not reported
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
Quantitative information on the absorption, distribution,
elimination and metabolism of BCEE, BCME or CMME in humans is not
available.
6.1 Absorption and distribution
Gwinner et al. (1983) reported that more than 95% of the total
[14C]-BCEE vapour (calculated to be approximately 75 mg) introduced
into an inhalation chamber containing three male Wistar rats was
absorbed by the animals after an 18-h exposure. When the tissue
(protein)-associated radioactivity (per gram of tissue) was examined
after this exposure period, approximately 0.32% of the administered
radioactivity was present in the liver, while 0.17 and 0.12% were
found in the kidney and small intestine, respectively. Only 0.07% of
the administered radioactivity was present in the lungs. Lingg et al.
(1982) administered by gavage a single dose of [14C]-BCEE (40 mg/kg
body weight, dissolved in corn oil) to male Sprague-Dawley rats and
monitored the amount of radioactivity present in a limited number of
tissues during the subsequent 48-h period. After 48 h, the percentage
of administered 14C was found to be 11.5 in expired CO2, 64.7 in
urine, 2.4 in faeces, and 2.3 in organs and tissues. In tissues,
approximately 1, 0.56, 0.49 and 0.19% of the radioactivity was
retained in muscle, kidney, blood and liver, respectively.
Quantitative data on the absorption and distribution of BCME or CMME
in animal species have not been reported.
6.2 Metabolism
BCEE is readily metabolized following absorption. Thiodiglycolic
acid (TDGA) was the principal metabolic product (representing 50 to
80% of the total metabolites) in the urine of rats administered BCEE
either orally, by intraperitoneal injection or by inhalation (Lingg et
al., 1979, 1982; Muller et al., 1979; Norpoth et al., 1986).
2-Chloroethoxy-acetic acid, N-acetyl-S-[2-(2-chloroethoxy)-ethyl]-L-
cysteine, 1-(2-chloroethyl)-ß-D-glucopyranosiduronic acid and
S-carboxymethyl-L-cysteine have been reported to be minor metabolites
(each comprising less than 10% of the total) in the urine of rats
administered BCEE (Lingg et al., 1979, 1982; Muller et al., 1979).
Lingg et al. (1982) reported that in male Sprague-Dawley rats
administered (by gavage) a single dose of [14C]-BCEE (40 mg/kg body
weight, dissolved in corn oil), approximately 12% of the radioactivity
was metabolized to 14CO2.
The formation of TDGA from BCEE involves a number of steps (Lingg
et al., 1979, 1982; Muller et al., 1979; Gwinner et al., 1983; Norpoth
et al., 1986). BCEE is believed to undergo oxidative degradation
(involving ether cleavage) to produce chloroacetaldehyde and
chloroethanol (which itself is rapidly converted to
chloroacetaldehyde) (Gwinner et al., 1983). It is believed that
chloroacetaldehyde is subsequently converted to chloroacetic acid,
which after conjugation with glutathione and further modification,
produces TDGA. The formation of N-acetyl-S-[2-(2-chloroethoxy)ethyl]-
L-cysteine is believed to involve the direct substitution of one of
the chlorine atoms in BCEE with cysteine (Lingg et al., 1982).
S-Carboxymethyl-L-cysteine, although not detected in all studies in
which the metabolism of BCEE was examined, has been postulated to be
an intermediate in the synthesis of TDGA (Lingg et al., 1982).
1-(2-Chloroethyl)-ß-D-glucopyranosiduronic acid is evidence of the
occurence of 2-chloroethanol among metabolic products, while
S-carboxymethyl- n-cysteine may be produced by alkylation of
glutathione by chloroacetaldehyde (Lingg et al., 1982), and
2-chloroethoxy-acetic acid is believed to be produced via the
oxidative dehalogenation of BCEE (Lingg et al., 1982).
Information on the metabolism of BCME or CMME in laboratory
animals has not been reported; however it is anticipated that BCME and
CMME would be rapidly hydrolysed in the aqueous environment of
tissues, forming formaldehyde and hydrogen chloride, and methanol,
formaldehyde and hydrogen chloride, respectively. However, the effects
of BCME (CMME) are most likely attributable to their direct alkylating
activity (van Duuren, 1989).
6.3 Elimination
Although quantitative information on the elimination of BCME or
CMME in laboratory animals is not available, limited quantitative data
concerning the elimination of BCEE (administered orally) in laboratory
animals have been reported. Lingg et al. (1982) administered (by
gavage) a single dose of [14C]-BCEE (40 mg/kg body weight, dissolved
in corn oil) to male Sprague-Dawley rats and monitored the amount of
radioactivity appearing in the faeces, urine and expired air during
the subsequent 48-h period. Twelve hours after the administration of
[14C]-BCEE, 50% of the radioactivity had been lost in the urine and
exhaled air (as 14CO2). Lingg et al. (1979) estimated that less than
2% of the administered radioactivity that was expired through the
lungs was exhaled as the parent compound. Forty-eight hours after the
oral administration of [14C]-BCEE, approximately 65% of the
radioactivity was excreted in the urine and 11.5% exhaled from the
lungs (total loss of 76%); approximately 2.3 and 2.4% of the
administered radioactivity remained in the organs (and tissues) and
faeces, respectively.
Smith et al. (1985) reported that 24, 48 and 72 h after the oral
administration (by gavage) of [14C]-BCEE (10 mg/kg body weight, in a
solution containing ethanol, Emulphor and distilled water) to two
female Rhesus monkeys, approximately 43, 56 and 58% of the
administered radioactivity had been eliminated in the urine.
Seventy-two hours after the administration of [14C]-BCEE, less than
2% of the radioactivity was recovered in the faeces.
7. EFFECTS ON EXPERIMENTAL MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Information on the acute toxicity of BCEE, BCME and CMME is
summarized in Table 6.
7.1.1 BCEE
Although the acute toxicity of BCEE has been examined in a number
of studies, complete experimental details were not always provided.
Reported LD50 values for the oral exposure of animal species to BCEE
range from 75 to 215 mg/kg body weight. An LC50 of 5850 mg/m3 (1000
ppm) was estimated from studies in which Sherman strain rats were
exposed to BCEE for 0.75 h (Smyth & Carpenter, 1948). The exposure of
guinea-pigs to 5850 mg/m3 for 3.8 to 5.5 h resulted in the death of
the animals (Schrenk et al., 1933). Exposure to 1521 mg/m3 (260 ppm)
resulted in the death of the animals after 7.5 to 12.3 h of continuous
exposure. No deaths were observed after exposure to 205 mg/m3 (35
ppm) for up to 13.5 h, although slight nasal irritation was observed
within 3 to 10 min of exposure to this concentration. Acute exposure
of guinea-pigs to BCEE vapour (320 mg/m3) caused eye irritation (as
indicated by squinting and lacrimation) as well as congestion, oedema
and haemorrhage in the lungs; liver, kidney and brain congestion was
also noted (Schrenk et al., 1933). The severity of the toxicological
effects produced by exposure to the higher concentrations of BCEE was
also related to the length of the exposure period. Effects in
Sprague-Dawley rats or CD-1 mice administered a single oral dose of
BCEE (dissolved in cottonseed oil) included ptosis, increased
salivation, diarrhoea, decreased activity and ataxia (Drake & Myer,
1992).
Smyth & Carpenter (1948) reported that the dermal exposure of
guinea-pigs to BCEE caused skin irritation; the LD50 was 366 mg/kg
body weight.
7.1.2 BCME and CMME
Reported LC50 values for the exposure (by inhalation) of
laboratory animals to BCME range from 25 to 48 mg/m3 (5.3 to 10.3
ppm). The acute exposure (by inhalation) of animals to BCME produced
severe irritation of the eyes and respiratory tract (congestion,
oedema and haemorrhage (mainly of the lungs) and acute necrotizing
bronchitis (Union Carbide, 1968; Drew et al., 1975). The median life
span of rats exposed (by inhalation) to 0, 3.3, 9.9, 32.4 or 44.7
mg/m3 (0, 0.7, 2.1, 6.9 or 9.5 ppm) was 462, 420, 36, 2 and 2 days,
respectively. For hamsters exposed (by inhalation) to these
concentrations of BCME, the median life span was 675, 657, 68, 16 and
4 days, respectively (Drew et al., 1975). Exposure to 9.9 mg/m3
(2.1 ppm) for 7 h increased the incidence of tracheal and bronchial
hyperplasia 2- to 3-fold in rats and 4- to 5-fold in hamsters,
compared to unexposed controls (Drew et al., 1975).
Table 6. Acute toxicity of BCEE, BCME and CMME
Speciesa Route LC50 or LD50 Reference
(duration)
BCEE
Rat (Sherman) inhalation (0.75 h) LC50: 5850 mg/m3 (1000 ppm) Smyth & Carpenter (1948)
Rat (Sherman) oral LD50: 75 mg/kg bw Smyth & Carpenter (1948)
Rat oral LD50: 105 mg/kg bw Spector (1956)
Rat (Sprague-Dawley) oral LD50: 175 mg/kg bw Drake & Myer (1992)
Mouse oral LD50: 136 mg/kg bw Spector (1956)
Mouse (CD-1) oral LD50: 215 mg/kg bw Drake & Myer (1992)
Rabbit oral LD50: 126 mg/kg bw Spector (1956)
Guinea-pig dermal (poultice; 24 h) LD50: 366 mg/kg bw Smyth & Carpenter (1948)
BCME
Rat (Sprague-Dawley) inhalation (7 h) LC50: 33 mg/m3 (7 ppm) Drew et al. (1975)
Rat inhalationb LC50: 48 mg/m3 (10.3 ppm) Union Carbide (1968)
Mouse (A/Heston) inhalation (6 h) LC50: 25 mg/m3 (5.3 ppm) Leong et al. (1971)
Hamster (Syrian) inhalation (7 h) LC50: 33 mg/m3 (7 ppm) Drew et al. (1975)
Rat (Wistar) oral (undiluted) LD50: 0.21 ml/kg bw (278 mg/kg bw) Union Carbide (1968)
Rabbit (New Zealand) dermal (undiluted; 24 h) LD50: 0.28 ml/kg bw (370 mg/kg bw) Union Carbide (1968)
CMMEc
Rat inhalation (7 h) LC50: 182 mg/m3 (55 ppm) Drew et al. (1975)
Hamster inhalation (7 h) LC50: 215 mg/m3 (65 ppm) Drew et al. (1975)
Rat oral LD50: 817 mg/kg bw NIOSH (1974)
a Data on strain presented if reported in study.
b Duration not specified.
c Containing BCME.
Reported LC50 values for the exposure (by inhalation) of
laboratory animals to CMME range from 182 to 215 mg/m3 (55 to 65
ppm). Exposure to CMME produced pulmonary congestion, oedema,
haemorrhage and acute necrotizing bronchitis (Drew et al., 1975);
however the toxic effects produced by CMME may be due, at least in
part, to contaminating BCME.
Application of BCME to the skin of rabbits produced erythema and
necrosis, while exposure of the eye to this substance produced severe
corneal necrosis (Union Carbide, 1968).
7.2 Short-term exposure
7.2.1 BCEE
Information on the effects of short-term or subchronic exposure
of animals to BCEE is limited primarily to range-finding studies for
carcinogenicity bioassays. Theiss et al. (1977) reported that the
maximum tolerated dose (MTD) of BCEE in A/St male mice (receiving 6
intraperitoneal injections over a 2-week period) was 40 mg/kg body
weight. The administration (route not clearly specified) of 19 daily
doses (100 mg/kg body weight) of BCEE (deemed to be the MTD) to two
strains of hybrid F1 mice [strain (C57BL/6 × C3H/Anf)F1 and strain
(C57BL/6 × AKR)F1] had no effect on mortality, although other
toxicological effects were not reported (Innes et al., 1969).
7.2.2 BCME
In one study (Drew et al., 1975) on the short-term toxicity of
BCME, groups of 50 male Sprague-Dawley rats and Syrian hamsters were
exposed by inhalation to 0 or 4.7 mg/m3 (0 or 1 ppm) for 1, 3, 10 or
30 multiple 6-h exposures (duration between exposures not specified),
after which time the animals were observed for their entire life span
and the trachea and bronchi examined histopathologically. In groups of
rats exposed to BCME for 0, 1, 3, 10 or 30 occasions, 50% mortality
was observed after 66, 66, 20, 4 and 4 weeks, respectively. The
incidence of tracheal hyperplasia, with and without atypias, increased
from 27% after 1 exposure to 89% after 30 exposures to BCME. The
incidence of tracheal squamous metaplasia increased after 3 to 30
exposures. The incidence of bronchial hyperplasia and squamous
metaplasia increased with greater exposure to BCME. In hamsters
subjected to 0, 1, 3, 10 or 30 exposures (6-h) to BCME, 50% mortality
was observed after 95, 95, 70, 22 and 8 weeks, respectively. The
incidence of tracheal hyperplasia, with and without atypias, tracheal
squamous metaplasia and alveolar metaplasia with atypia increased with
more frequent exposure to BCME. Exposure to BCME also produced
bronchoalveolar metaplasia, squamous metaplasia with atypia and
atypical alveolar epithelium. Evidence of subarachnoid haemorrhage was
observed in 24% of the rats and 8% of the hamsters that received 30
exposures (6-h) to 4.7 mg/m3 (1 ppm) (Drew et al., 1975).
7.2.3 CMME
In one study on the short-term toxicity of CMME, groups of 25
male Sprague-Dawley rats were exposed (by inhalation) to 3.3 or 33
mg/m3 (1 or 10 ppm) for 30 days (duration and frequency of exposure
not specified) (Drew et al., 1975). Exposure to 3.3 mg/m3 resulted in
8% mortality, but no effect on body weight, within 30 days (data for
unexposed controls were not presented). Regenerative hyperplasia and
squamous metaplasia in bronchial epithelium were observed in rats
killed 2 weeks after the last exposure. Exposure to 33 mg/m3 resulted
in 88% mortality within 30 days (data for controls not presented);
marked (not quantified) weight decrease was observed with some
recovery towards the end of exposure. Significant (not quantified)
increases in lung/body weight ratios were observed in rats that died
after exposure to CMME; regenerative hyperplasia of bronchial
epithelium was also observed.
7.3 Long-term exposure/carcinogenicity
Studies on long-term exposure and carcinogenicity are given in
Table 7.
7.3.1 BCEE
Studies on the toxicological effects produced by the long-term
exposure of laboratory animals to BCEE have focused on its
carcinogenic potential. However there are numerous deficiencies in all
of these studies, compared to the more stringent protocols used in
current carcinogenicity bioassays.
Innes et al. (1969) assessed the carcinogenicity of BCEE in mice
following ingestion. Groups of 18 males and 18 females from two
strains of hybrid F1 mice [(C57BL/6 × C3H/Anf) and (C57BL/6 × AKR)]
were administered by stomach tube approximately 100 mg/kg body weight
BCEE (dissolved in distilled water) from the age of 7 to 28 days
(although the amount of BCEE was not adjusted during this period to
account for weight gain). Once the mice had reached four weeks of age,
the BCEE was then provided in the diet at a concentration of 300 mg/kg
diet until the mice were 18 months of age, after which time they were
killed and necropsied. The time-weighted average dose for these
studies was calculated to be 41.3 mg/kg body weight per day (US EPA,
1987a). There were multiple groups of controls consisting of animals
of both strains and sexes. "Hepatomas" (representing benign hepatomas
and malignant tumours), tumours of the pulmonary system (adenomas and
adenocarcinomas) and lymphomas (Type-B reticulum cell sarcomas and
leukaemias) were the predominant types of tumours observed in these
animals. Compared to unexposed controls, the incidence of "hepatomas"
was significantly (p = 0.01) increased in the treated (C57BL/6 ×
C3H/Anf)F1 mice (in males, 8/79 versus 14/16; in females, 0/87 versus
4/18; in control and exposed animals, respectively) and in (C57BL/6 ×
AKR)F1 males (5/90 versus 9/17 in control and exposed animals,
respectively). However the incidence of pulmonary tumours or lymphomas
was not significantly increased in the BCEE-exposed animals of either
Table 7. Long-term exposure/carcinogenicity of BCEE, BCME and CMME
Protocol Result Comments Reference
BCEE
Groups of 18 males and 18 females from The incidence of "hepatomas" (benign Evidence of Innes et al.
two strains of F1 hybrid mice [(C57BL/6 x and malignant tumours), "pulmonary increased incidence (1969)
C3H/Anf) and (C57BL/6 x AKR)] were tumours" and lymphomas in the male of liver tumours.
given (by gavage) approximately 100 control and BCEE-exposed (C57BL/6 x However, study
mg/kg bw BCEE (dissolved in distilled C3H/Anf)F1 mice was 8/79 and 14/16 limited owing to
water) from the age of 7 to 28 days. Once (p = 0.01), 5/79 and 0/16 and 5/79 small number of
the mice had reached four weeks of age, and 2/16, respectively; the incidence BCEE-exposed
BCEE was then provided in the diet at a of these tumours in the female control animals, use of
concentration of 300 mg/kg until the mice and (C57BL/6 x C3H/Anf)F1 mice was single dose level
were 18 months of age, after which time 0/87 and 4/18 (p = 0.01), 3/87 and and inadequate
they were sacrificed and necropsied. The 0/18 and 4/87 and 0/18, respectively. reporting of tumour
time-weighted-average dose for these The incidence of "hepatomas" (benign pathology. Amount
studies was calculated to be 41.3 mg/kg and malignant tumours), "pulmonary of BCEE was not
bw/day (US EPA, 1987a). Controls tumours" and lymphomas in the male adjusted during
consisted of multiple groups of animals of control and BCEE-exposed (C57BL/6 x initial period to
both strains and sexes. C3H/AKR)F1 mice was 5/90 and 9/17 account for weight
(p = 0.01), 10/90 and 2/17 and 1/90 gain.
and 0/17, respectively; the incidence
of these tumours in the female control
and BCEE-exposed mice was 1/82 and
0/18, 3/82 and 0/18 and 4/82 and 1/18,
respectively.
Table 7. (continued)
Protocol Result Comments Reference
BCME (dissolved in a solution containing The authors reported that BCEE was not No reported Weisburger et
sodium chloride, Polysorbate 80, carcinogenic in these male or female evidence of al., 1981
carboxy-methylcellulose and benzyl rats; however, there was a "substantial carcinogenicity.
alcohol) was administered by gavage to difference" between the mean weight of However, study
groups of 26 male and 26 female Charles the females administered BCEE and limited due to
River CD rats (at doses of 50 and corresponding controls, as well as "a small number of
25 mg/kg bw) twice weekly for 78 weeks, reduction" in the mean weight of the BCEE-exposed
after which time the animals were high-dose male rats, compared to the animals and
observed for a further 26-week period. controls. Notably, survival after 52 relatively short
The animals were necropsied and tissues weeks on the study was only 65% for the exposure period.
examined histopathologically, either at high-dose females and 96-100% for the The size of
the end of the study or when the animals other BCEE-exposed animals. The survival control groups
became moribund. Groups of controls of for the control animals at 52 weeks was was not clearly
each sex were administered vehicle alone. 97% and 99% for males and females, stated, and
respectively. quantitative data
on tumour
incidence were
not presented.
Groups of 20 male A/St mice were injected The incidence of lung tumours (expressed No evidence of Theiss et al.,
intraperitoneally three times a week with as the number of lung tumours/mouse) in carcinogenicity 1977
8, 20 or 40 mg/kg bw BCEE (dissolved in the BCEE-exposed animals (approximately in a limited
tricaprylin). Mice injected with 8 and 0.13 lung tumours/mouse) was lower than study of
20 mg/kg bw BCEE received a total of 24 that observed in animals injected with carcinogenic
injections while animals administered vehicle alone (0.39 lung tumours/mouse). potential.
40 mg/kg bw BCEE only received 4 injections.
Controls (n = 20) were injected with
vehicle alone. The mice were sacrificed
24 weeks after the initial injection and
the number of surface lung tumours
(adenomas) determined.
Table 7. (continued)
Protocol Result Comments Reference
Thirty female ICR/Ha Swiss mice were Compared to animals injected with vehicle Inconclusive van Duuren et
injected subcutaneously with 1 mg BCEE alone, where no tumours developed at the evidence of al., 1972
(suspended in 0.05 ml mineral oil) once site of injection, 2/30 animals injected carcinogenicity
a week for life (median survival time with BCEE developed sarcomas at the site in a limited study
of animals was 656 days). Controls of injection. involving small
(n = 30) were administered vehicle alone. numbers of animals
administered one
dose-level with
inadequate
reporting of data
on other effects.
Groups of 50 male and 50 female The incidence of all malignant and No evidence of Norpoth et al.,
Sprague-Dawley rats were injected benign tumours (e.g., mesenchymal, carcinogenicity 1986
subcutaneously with either 4.36 µmole epithelial, sarcomas, carcinomas, in a study
(0.62 mg) or 13.1 µmole(1.87 mg) BCEE unclassified) in the untreated controls, involving limited
(dissolved in 0.25 ml DMSO) once a vehicle-treated controls, low- and exposure to
week for two years. Controls were high-dose males and females was 2/35, BCEE with limited
administered DMSO or left untreated. 4/35, 4/50 and 6/50, and 24/50, 24/50, reporting of
23/50 and 22/50, respectively. The toxicological
median survival time of the untreated effects.
control, vehicle-treated control, low-
and high-dose groups was 696, 605, 590
and 643 (for males), and 639, 668, 629
and 654 days (for females), respectively.
Table 7. (continued)
Protocol Result Comments Reference
One milligram of BCEE (in 0.1 ml The incidence of skin papillomas at No evidence of Van Duuren et
benzene) was applied to the skin of 20 the site of application was 2/20 and skin tumour al., 1972
female ICR/Ha Swiss mice. Two weeks 3/20 in the control and BCEE-initiated initiating
later the secondary (promotion) animals, respectively. activity by
treatment (2.5 µg phorbol myristate BCEE.
acetate (PMA) in 0.1 ml acetone, three
times weekly) commenced and was
maintained for the lifespan of the
animals. Controls were administered
PMA alone.
BCME
Fifty A/Heston male mice were exposed Exposure to BCME produced loss of body Limited evidence Leong et al.,
(by inhalation) to 0 or 5 mg/m3 BCME weight, respiratory distress and death. of increased 1971
(industrial grade) for 6 h/day, Survival of control and BCME-exposed pulmonary tumour
5 days/week for 82 days, after which mice was 90% and 28%, respectively. burden in mice
exposure was terminated and survivors The incidence of pulmonary adenomas was exposed to one
observed for a further 10 weeks. The 20/49 and 26/47 in control and concentration of
animals were necropsied and lungs BCME-exposed mice respectively BCME for a
examined pathologically. (statistical significance not specified). relatively short
The average number of pulmonary period.
adenomas/animal among tumour-bearing
mice was 2.2 for controls and 5.2 for
the BCME-exposed group.
Table 7. (continued)
Protocol Result Comments Reference
Groups of 120 male Sprague-Dawley rats Six-month survival was greater than Increased incidence Leong et
were exposed (by inhalation) to 0, 1, 10 97% for control and BCME-exposed rats. of respiratory al., 1981
or 100 ppb (0, 0.0047, 0.047, 0.47 mg/m3) Nineteen-month survival for animals tract tumours in
BCME 6 h/day, 5 days/week for 6 months, exposed to 0, 0.0047 or 0.047 mg/m3 was rats exposed to
after which exposure was terminated and approximately 45%, while no animals BCME.
the rats observed for a further 22 months. exposed to 0.47 mg/m3 survived. After 6
Eight rats from each group were sacrificed months there was no significant
after 6 months for haematological, difference in the weights of the total
cytological, cytogenetic and body, liver, kidneys, brain, heart and
histopathological analyses. testes; exposure to BCME produced no
adverse haematological or cytogenetic
effects. The incidence of "respiratory
tract" tumours was 0/112, 0/113, 0/111
and 102/111, respectively; in the
highest-concentration group, there were
96 esthesioneuroepitheliomas
(significantly different [p < 0.05]
than controls), four pulmonary adenomas,
one carcinoma of the nasal passage and
an esthesio-neuroepithelioma metastasis
in the lung.
Table 7. (continued)
Protocol Result Comments Reference
Groups of 20 to 50 male Sprague-Dawley Sixty exposures to BCME had no effect Increased incidence Kuschner et
rats were exposed (by inhalation) to 0 or upon mortality, although the time at of respiratory al., 1975
0.1 ppm (0 or 0.47 mg/m3) BCME 6 h/day, which 50% mortality was reached was tract tumours
5 days/week for 2, 4, 8, 12, 16 and 20 reduced by approximately 24% in animals in rats exposed
weeks (10, 20, 40, 60, 80 or 100 exposures), receiving 80 or 100 exposures to BCME. to BCME.
after which the rats were necropsied and Animals surviving 30 weeks had
examined histopathologically. "respiratory tract cancers" (26 in
the nasal cavity and 13 in the lung).
The incidence of "respiratory tract
cancer" in animals surviving for more
than 210 days and receiving 10, 20,
40, 60, 80 or 100 exposures of BCME
was, 1/41 (2.4%), 3/46 (6.5%), 4/18
(22.2%), 4/18 (22.2%), 15/34 (44.1%)
and 12/20 (60.0%), respectively
(statistical significance not specified).
No lung tumours were observed following
up to 40 exposures to BCME. The
incidence of squamous cell carcinomas
of the lung was 2/20, 3/50 and 8/30,
after 60, 80 and 100 exposures,
respectively.
Groups of 20 female Sprague-Dawley rats The incidence of malignant tumours at Limited evidence van Duuren
were injected subcutaneously once per the site of injection (i.e., fibrosarcoma) of carcinogenicity et al., 1969
week with 3 mg BCME (dissolved in 0.1 ml and in the breast (i.e., adenocarcinoma) in rats injected
mineral oil) or vehicle alone for in the control and BCME-exposed animals subcutaneously
approximately 300 days. (Because of the was 0/20 and 1/20, and 5/20 and 0/20, with BCME.
corrosive effects produced by BCME, after respectively.
114 days the dose was reduced to 1 mg,
and injections performed only three times
per month; however because of severe
weight loss of the animals, the injections
were terminated after 300 days).
Table 7. (continued)
Protocol Result Comments Reference
Fifty female and 50 male newborn ICR Exposure to BCME had no effect upon growth Increased incidence Gargus et
Swiss mice received a single subcutaneous or survival of the mice. The incidence of of lung tumours al., 1969
injection of 12.5 µl/kg bw (16.6 mg/kg bw) pulmonary adenomas in the control and in mice injected
BCME (dissolved in peanut oil) and the BCME-exposed males was 2/30 and 25/50, subcutaneously
animals were observed for a period of six respectively; 5/20 controls and 20/50 of with BCME.
months, after which the survivors were the BCME-exposed females had lung adenomas.
necropsied and the number of lung tumours
(adenomas, based on histopathological
analysis) quantified. Controls (20 females
and 30 males) received a single
subcutaneous injection of vehicle alone.
Thirty male and 30 female XVIInc/Z mice Approximately 0%, 44% and 42% of the male Evidence of Zajdela et
received 32 subcutaneous injections of controls and male and female BCME-exposed carcinogenicity al., 1980
0.3 mg BCME (dissolved in mineral oil) mice, respectively, had tumours (e.g., in mice injected
over a period of 42 weeks. Controls fibrosarcomas and squamous carcinomas) subcutaneously
consisted of 30 XVIInc/Z male mice injected at the site of injection. with BCME.
with vehicle alone.
Groups of 20 female ICR/Ha mice which The incidence of squamous cell carcinomas Evidence of van Duuren
received 2 mg BCME (applied dermally) or of the skin in the controls and carcinogenicity et al., 1969
solvent (i.e., benzene) alone (controls) BCME-exposed animals was 0/20 and 12/20, in mice exposed
thrice weekly for 325 days. respectively. dermally to BCME.
Table 7. (continued)
Protocol Result Comments Reference
CMME
Fifty A/Heston male mice were exposed The incidence of pulmonary tumours in Suggestion of Leong et
(by inhalation) to 0 or 2 ppm (0 or CMME-exposed mice (25/50) was not increased al., 1971
6.6 mg/m3) (industrial grade) CMME for significantly (i.e., p > 0.05) different pulmonary tumour
6 h/day, 5 days/week for 101 days, after from that in the unexposed controls burden in mice
which time exposure was terminated and (20/49). The average number of pulmonary exposed to one
survivors observed for a further 7 weeks. tumours/animal among tumour-bearing mice concentration
The animals were necropsied and lungs was 2.2 and 3.1 for the control and of CMME for a
examined histopathologically. CMME-exposed group, respectively. relatively short
period.
Seventy-four male Sprague-Dawley rats Exposure to CMME had no effect upon No clear Laskin et
were exposed (by inhalation) to 0 or mortality or body weight gain. The evidence of al., 1975
1 ppm (0 or 3.3 mg/m3) (industrial grade) incidence of tracheal squamous metaplasia carcinogenicity
CMME for 6 h/day, 5 days/week for their and bronchial hyperplasia was 3% and 10%, in a limited
entire lifespan (up to 852 days). The and 35% and 59%, in the control and study.
rats were necropsied and tissues examined BCME-exposed animals respectively
histopathologically. (statistical significance not stated).
Two respiratory tract cancers (lung
squamous cell carcinoma and an
esthesioneuroepithelioma of olfactory
epithelium) were found in animals
exposed to CMME (but presumably not
in unexposed controls).
Table 7. (continued)
Protocol Result Comments Reference
Ninety male Syrian hamsters were exposed Exposure to CMME had no effect upon No clear Laskin et
(by inhalation) to 1 ppm (3.3 mg/m3) mortality or body weight gain. The evidence of al., 1975
(industrial grade) CMME for 6 h/day, incidence of tracheal squamous metaplasia carcinogenicity
5 days/week for their entire lifespan was 0% and 2%, and incidence of bronchial in a limited
(up to 852 days). The hamsters were hyperplasia was 5% and 8%, in the control study.
necropsied and tissues examined and BCME-exposed animals respectively
histopathologically. Eighty-eight unexposed (statistical significance not stated).
animals served as controls. One lung adenocarcinoma and a tracheal
squamous papilloma were observed in two
animals exposed to CMME.
Groups of 20 female Sprague-Dawley rats The incidence of malignant tumours at No evidence of van Duuren
were injected subcutaneously once per the site of injection (i.e., fibrosarcoma) carcinogenicity et al., 1969
week with 3 mg laboratory purified CMME and in the breast (adenocarcinoma) in the in rats injected
(dissolved in 0.1 ml mineral oil) or control and CMME-exposed animals subcutaneously
vehicle alone for approximately 300 days. was 0/20 and 1/20, and 1/20 and 0/20, with laboratory
Because of moderate corrosive effects, respectively. purified CMME.
the injections were terminated after
this time.
Thirty female ICR/Ha Swiss mice were Compared to the animals injected with Evidence of van Duuren
injected (subcutaneously) with 0.3 mg of vehicle alone, where no tumours developed carcinogenicity et al., 1972
laboratory purified CMME (suspended in at the site of injection, 10/30 animals in mice
0.05 ml mineral oil) once a week for injected with CMME developed sarcomas at injected
life. Controls (n = 30) received the site of injection. subcutaneously
vehicle alone. with CMME.
Table 7. (continued)
Protocol Result Comments Reference
Forty-eight female and 51 male newborn The numbers of female mice with lung No increase in Gargus et
ICR Swiss mice received a single adenomas in the control (vehicle) and the incidence al., 1969
subcutaneous injection of 125 µl/kg bw CMME-exposed groups were 5/20 and 8/48, of lung
(132.5 mg/kg bw) CMME dissolved in peanut respectively. The numbers of male mice tumours in
oil; the animals were observed for a with lung adenomas in the control mice injected
period of six months, after which time (vehicle) and CMME-exposed groups were subcutaneously
the survivors were necropsied and the 2/30 and 9/51, respectively. with CMME.
number of lung tumours (adenomas, based
on histopathological analysis) quantified.
Controls (20 females and 30 males)
received a single subcutaneous injection
of vehicle alone.
Groups of 20 female ICR/Ha mice received No squamous cell carcinomas of the No evidence of van Duuren
2 mg CMME (applied dermally) or solvent skin were observed in either the carcinogenicity et al., 1969
(i.e., benzene) alone (controls) thrice control or CMME-exposed animals. in mice exposed
weekly for 325 days. dermally to
CMME.
sex. Clinical, biochemical or haematological effects were not
addressed in the published account of this study.
Weisburger et al. (1981) reported that the oral administration of
BCEE to rats had no significant carcinogenic effect. BCEE (dissolved
in a solution containing sodium chloride, Polysorbate 80,
carboxy-methylcellulose and benzyl alcohol) was administered (by
gavage) to groups of 26 male and 26 female Charles River CD rats (at
doses of 50 and 25 mg/kg body weight) twice weekly for 78 weeks, after
which time the animals were observed for a further 26-week period.
Control groups of each sex (the size of which was not clearly stated)
were administered vehicle alone. The authors reported (although no
data on tumour incidence were presented) that BCEE was not
carcinogenic in these male or female rats. However, the authors
indicated (but results were not quantified) that there was a
"substantial difference" between the mean body weight of the females
administered both doses of BCEE and that of the corresponding
controls, as well as "a reduction" in the mean body weight of the
high-dose male rats, compared to controls. Notably, survival after 52
weeks on the study was only 65% for the high-dose females and 96 to
100% for the other BCEE-exposed animals. The survival for the control
animals at 52 weeks was 97 and 99% for males and females,
respectively. Clinical, biochemical or haematological effects were not
addressed in the published account of this study.
Theiss et al. (1977) assessed the potential of BCEE to produce
lung tumours in groups of 20 male A/St mice injected intraperitoneally
three times per week with 8, 20 or 40 mg/kg body weight BCEE
(dissolved in tricaprylin). Mice injected with 8 and 20 mg/kg received
a total of 24 injections while animals administered 40 mg/kg only
received 4 injections. Controls (n = 20) were injected with vehicle
(tricaprylin) alone. The mice were killed 24 weeks after the initial
injection and the number of surface lung tumours (adenomas)
determined. The incidence of lung tumours (expressed as the number of
lung tumours/mouse) in the BCEE-exposed animals was less than that
observed in animals injected with vehicle alone.
The potential of BCEE to induce tumours was also investigated in
a study in which groups of 30 female ICR/Ha Swiss mice were injected
subcutaneously with 1 mg BCEE (suspended in 0.05 ml mineral oil) once
per week for life (the median survival time of animals was 656 days)
(van Duuren et al., 1972). Compared to animals injected with vehicle
alone, where no tumours developed at the site of injection, 2/30
animals injected with BCEE developed sarcomas at the site of
injection. Norpoth et al. (1986) also examined the carcinogenicity of
BCEE in a study in which groups of 50 male and 50 female
Sprague-Dawley rats were injected subcutaneously with either 4.36
µmole (0.62 mg) or 13.1 µmole BCEE (1.87 mg) (dissolved in 0.25 ml
DMSO) once per week over a 2-year period. Controls were injected with
DMSO (alone) or left untreated. The incidence of all malignant and
benign tumours (e.g., mesenchymal, epithelial, sarcomas, carcinomas
and unclassified) in the BCEE-exposed animals was not significantly
different from that in the controls. The median survival time of the
untreated control, vehicle-treated control, and low- and high-dose
groups was 696, 605, 590 and 643 (for males), and 639, 668, 629 and
654 days (for females), respectively.
Van Duuren et al. (1972) assessed the skin-tumour-initiating
potential of BCEE. One milligram BCEE (in 0.1 ml benzene) was applied
to the skin of 20 female ICR/Ha Swiss mice. Two weeks later the
secondary (promotion) treatment (2.5 µg PMA in 0.1 ml acetone, three
times weekly) commenced and was maintained for the life span of the
animals. Compared to controls (administered PMA alone) where 2/20
animals developed papillomas, 3/20 of the BCEE-initiated animals
developed papillomas at the site of application.
7.3.2 BCME
Studies on the toxicological effects produced by long-term
exposure (by inhalation) to BCME have been restricted primarily to
limited carcinogenesis bioassays. The exposure (by inhalation) of male
A/Heston mice to 5 mg/m3 for 6 h/day, 5 days/week for a period of 82
days, followed by a 10-week observation period, produced a marked
reduction in survival (90 and 28% in control and BCME-exposed mice,
respectively) and an increase in the number of pulmonary adenomas
(20/49 and 26/47 in control and BCME-exposed mice, respectively),
although the statistical significance was not specified (Leong et al.,
1971). The average number of pulmonary adenomas per animal among
tumour-bearing mice was 2.2 for controls and 5.2 for the BCME-exposed
group.
The exposure (by inhalation) of groups of 144 to 157 male ICR/Ha
mice to concentrations of BCME from 0.0047 to 0.47 mg/m3 (1 to 100
ppb) for 6 h/day, 5 days/week for a period of 6 months, followed by an
18-month observation period, produced a reduction in survival (55, 35,
25 and 18% in mice exposed to 0, 0.0047, 0.047 or 0.47 mg/m3 [0, 1,
10 or 100 ppb], respectively), although no difference in survival
(> 90%) was observed between the control and BCME-exposed groups
after 24 months (Leong et al., 1981). All mice developed an ascending
urinary tract infection. After 6 months, the incidence of pulmonary
adenomas in surviving mice exposed to 0, 0.0047, 0.047 and 0.47 mg/m3
was 9/86, 5/45, 3/37 and 8/27 (p < 0.05), respectively. Exposure to
these concentrations of BCME had no adverse effect on body weight and
produced no nasal or eye irritation.
Groups of 120 male Sprague-Dawley rats were exposed (by
inhalation) to BCME concentrations of 0, 0.0047, 0.047 or 0.47 mg/m3
(0, 1, 10 or 100 ppb) 6 h/day, 5 days/week for 6 months, after which
time exposure was terminated and the animals observed for a further 22
months (Leong et al., 1981). Although 6-month survival was greater
than 97% for control and BCME-exposed rats, 19-month survival for
animals exposed to 0, 0.0047 or 0.047 mg/m3 was approximately 45%,
while none of the animals exposed to 0.47 mg/m3 survived. After 6
months there was no significant difference in the weights of the total
body, liver, kidneys, brain, heart and testes, and no adverse
haematological or cytogenetic effects were observed. The incidence of
"respiratory tract" tumours in animals exposed to 0, 0.0047, 0.047 or
0.47 mg/m3 was 0/112, 0/113, 0/111 and 102/111, respectively; in the
highest-concentration group, there were 96 esthesioneuroepitheliomas
(significantly different [p < 0.05] from controls), 1 carcinoma of
the nasal passage and an esthesioneuroepithelioma metastasis in the
lung, and 4 pulmonary adenomas.
Kuschner et al. (1975) exposed (by inhalation) groups of 20 to 50
male Sprague-Dawley rats to 0.47 mg BCME/m3 (0.1 ppm) for 6 h/day,
5 days/week for 2, 4, 8, 12, 16 or 20 weeks (10, 20, 40, 60, 80 or 100
exposures). Sixty exposures to BCME had no effect on mortality,
although the time at which 50% mortality was reached was reduced by
approximately 24% in animals receiving 80 or 100 exposures to BCME.
The incidence of "respiratory tract cancer" in animals surviving for
more than 210 days and receiving 10, 20, 40, 60, 80 or 100 exposures
of BCME was 1/41 (2.4%), 3/46 (6.5%), 4/18 (22.2%), 4/18 (22.2%),
15/34 (44.1%) and 12/20 (60.0%), respectively. No lung tumours were
observed following up to 40 exposures to BCME; however, the incidence
of squamous cell carcinomas of the lung was 2/20, 3/50 and 8/30 after
60, 80 and 100 exposures, respectively.
The carcinogenicity of BCME has also been examined following
subcutaneous injection in rats and mice. Groups of 20 female
Sprague-Dawley rats (weighing between 120 and 125 g) were injected
subcutaneously once per week with 3 mg BCME (dissolved in 0.1 ml
mineral oil) or vehicle alone for approximately 300 days (van Duuren
et al., 1969). Because of the corrosive effects produced by BCME,
after 114 days the dose was reduced to 1 mg, and injections were
performed only three times per month; however, because of severe
weight loss of the animals, the injections were terminated after 300
days. In the controls administered vehicle alone, no tumours were
observed at the site of injection; however a fibroadenoma and an
adenocarcinoma (of the breast) were observed elsewhere. In the group
of animals administered BCME, two fibromas and five fibrosarcomas were
observed at the site of injection, and one fibroadenoma (of the
breast) was found elsewhere (van Duuren et al., 1969).
The potential of BCME to increase the incidence of spontaneous
lung tumours in mice was assessed by Gargus et al. (1969). A group of
50 female and 50 male newborn ICR Swiss mice received a single
subcutaneous injection of 12.5 µl/kg body weight (16.6 mg/kg body
weight) BCME (dissolved in peanut oil) and the animals were observed
for a period of 6 months, after which time the survivors were
necropsied and the number of lung tumours (adenomas, based on
histopathological analysis) quantified. A group of control animals (20
females and 30 males) received a single subcutaneous injection of
vehicle alone. The numbers of female mice with pulmonary adenomas in
the control (vehicle) and BCME-exposed groups were 5/20 and 20/50,
respectively. The numbers of male mice with pulmonary adenomas in the
control (vehicle) and BCME-exposed groups were 2/30 and 25/50,
respectively. The administration of BCME had no effect upon the growth
or survival of the mice.
Zajdela et al. (1980) assessed the carcinogenicity of BCME
following repeated subcutaneous injection in male and female XVIInc/Z
mice. Groups of 30 males and 30 females received 32 injections of 0.3
mg BCME (dissolved in mineral oil) over a period of 42 weeks. The
control group consisted of 30 male mice injected with vehicle alone.
After 110 days (when the first sarcoma was observed), survival in the
control and male and female BCME-exposed groups was 100, 90 and 80%,
respectively. The number of animals with tumours (mainly
fibrosarcomas) at the site of injection was 0/30, 12/27 and 10/24,
respectively (p < 0.0001). The incidence of tumours at locations
other than the site of injection was the same in the control and
BCME-exposed groups. The incidence of pulmonary adenomas in the
control and BCME-exposed groups was 2/30 and 7/30, respectively; this
difference was not statistically significant (Zajdela et al., 1980).
The incidence of squamous cell carcinomas of the skin in female
ICR/Ha mice that received 2 mg BCME (applied dermally) or solvent
(i.e., benzene) alone thrice weekly for 325 days was 12/20 and 0/20,
respectively (van Duuren et al., 1969). A two-stage skin tumour
carcinogenesis bioassay was conducted in which the primary treatment
involved the application of 1 mg BCME (dissolved in 80 µl benzene) to
the dorsal skin of 28 male XVIInc/Z mice and the secondary treatment,
commencing 14 days later, involved the application three times per
week of 2 µg PMA (dissolved in acetone) to the dorsal skin of these
animals for 42 weeks. The incidence of mice with squamous cell
carcinomas was 0/28 and 3/28 in unexposed and BCME-initiated animals,
respectively (Zajdela et al., 1980).
7.3.3 CMME
Studies on the toxicological effects produced by long-term
inhalational exposure to CMME have been restricted primarily to
limited carcinogenesis bioassays in mice, rats and hamsters.
A study was conducted in which 50 A/Heston male mice were exposed
(by inhalation) to 0 or 6.6 mg CMME/m3 (0 or 2 ppm) for 6 h/day,
5 days/week for 101 days, followed by an observation period of
7 weeks. Although there was no significant effect upon the incidence
of pulmonary tumours, the average number of pulmonary tumours per
animal among tumour-bearing mice was 3.1 and 2.2 for the CMME-exposed
and control groups, respectively (Leong et al., 1971).
In a study in which 74 male Sprague-Dawley rats were exposed (by
inhalation) to 0 or 3.3 mg CMME/m3 (0 or 1 ppm) for 6 h/day,
5 days/week for their entire life span (up to 852 days), the incidence
of tracheal squamous metaplasia and bronchial hyperplasia was 3 and
10%, and 35 and 59%, in the control and CMME-exposed animals,
respectively. Two respiratory tract cancers (lung squamous cell
carcinoma and an esthesioneuroepithelioma of the olfactory epithelium)
were found in animals exposed to CMME (but presumably not in unexposed
controls) (Laskin et al., 1975). Exposure to CMME had no effect upon
mortality or body weight gain.
The exposure (6 h/day, 5 days/week) of 90 male hamsters to 3.3 mg
CMME/m3 (1 ppm) for virtually their entire lives increased the
incidence of tracheal metaplasia and bronchial hyperplasia compared to
88 unexposed controls. The incidence of tracheal squamous meta-plasia
was 0 and 2%, and the incidence of bronchial hyperplasia was 5 and 8%,
in the control and CMME-exposed animals, respectively (statistical
significance not stated). One lung adenocarcinoma and a tracheal
squamous papilloma were observed in two animals exposed to CMME;
presumably none was found in unexposed controls (Laskin et al., 1975).
The carcinogenicity of purified CMME has also been examined
following subcutaneous injection of this substance in rats and mice.
Groups of 20 female Sprague-Dawley rats (weighing between 120 and 125
g) were injected once per week with 3 mg (laboratory purified) CMME
(dissolved in 0.1 ml mineral oil) or vehicle alone for approximately
300 days; because of moderate corrosive effects, the injections were
terminated after this time (van Duuren et al., 1969). In controls
administered the vehicle alone, no tumours were observed at the site
of injection; however a fibroadenoma and an adenocarcinoma (of the
breast) were found elsewhere. In animals administered (laboratory
purified) CMME, a fibrosarcoma (at the site of injection) in one
animal was the only tumour described.
Van Duuren et al. (1972) subcutaneously injected (laboratory
purified) CMME (dissolved in 0.05 ml mineral oil, 300 µg/animal, once
per week) to a group of 30 female ICR/Ha Swiss mice for their entire
lives, and a similarly sized group of controls received vehicle alone.
Median survival time was 643 days and 496 days, and the numbers of
mice with sarcomas at the site of injection were 0 and 10 in the
control and purified CMME-exposed groups, respectively.
The potential of CMME to increase the incidence of spontaneous
lung tumours in mice was assessed by Gargus et al. (1969). A group of
48 female and 51 male newborn ICR Swiss mice received a single
subcutaneous injection of 125 µl/kg body weight (132.5 mg/kg body
weight) CMME dissolved in peanut oil, and subsequently observed for a
period of 6 months, after which time the survivors were necropsied and
the number of lung tumours (adenomas, based on histopathological
analysis) quantified. Controls (20 females and 30 males) received a
single subcutaneous injection of vehicle alone. The numbers of female
mice with adenomas in the control (vehicle) and CMME-exposed groups
were 5/20 and 8/48, respectively. The numbers of male mice with
adenomas in the control (vehicle) and CMME-exposed groups were 2/30
and 9/51, respectively.
Purified CMME was not carcinogenic when applied thrice weekly
(2 mg/animal for 325 days) to the skin of female ICR/Ha Swiss mice
(van Duuren et al., 1969).
7.4 Mutagenicity and related end-points
7.4.1 BCEE
A small number of investigations have been performed to assess
the genotoxic potential of BCEE. The in vitro studies have yielded
somewhat equivocal results. However it should be noted that, in
general, detailed descriptions of the laboratory conditions were not
provided, making interpretation of the findings difficult. The
mutagenic activity of BCEE in bacteria has been examined in a number
of strains, in the presence and absence of metabolic activating
systems. Simmon et al. (1977a,b) reported BCEE (vapour) to be strongly
mutagenic in Salmonella typhimurium TA100 in the absence of a
metabolic activating system, with the number of revertants increasing
with the duration of exposure. Simmon et al. (1977a,b) also reported
that in suspension assays BCEE was mutagenic in S. typhimurium
strains TA1535 and TA100, as well as in Saccharomyces cerevisiae
D3. Norpoth et al. (1986) reported "weak" mutagenic activity in
S. typhimurium TA100 (in the presence of a metabolic activating
system) when BCEE (up to 40 µg/dish) was added directly to culture
plates. Mortelmans et al. (1986) reported BCEE (up to 10 mg/plate) had
"weak" mutagenic activity in a number of S. typhimurium strains
(TA100, TA1535, TA1537, TA98), either in the presence or absence of a
metabolic activating system. Shirasu et al. (1975) reported that BCEE
was mutagenic in various strains of Escherichia coli, Bacillus
subtilis and S. typhimurium, although experimental details were
not provided in the published account of this study. In contrast,
Quinto & Radman (1987) reported that BCEE was not mutagenic in the MT
103, MT 119 and MT 126 tester strains of E. coli, although complete
experimental details were not provided in this published account.
Foureman et al. (1994) considered BCEE mutagenic, based upon the
results of a sex-linked recessive lethal assay in which male
Drosophila were injected with the compound. However the response was
negative when the males were fed BCEE. To examine the genotoxicity of
BCEE in mammals, Jorgenson et al. (1977) performed heritable
translocation assays in mice administered BCEE orally. These authors
concluded that BCEE did not promote heritable translocations. However,
few experimental details were provided in this published account.
Gwinner et al. (1983) did not detect radioactivity bound to liver DNA
or RNA isolated from male Sprague-Dawley rats exposed (by inhalation)
to [1-14C]-BCEE (amount not clearly specified) for 18 h.
7.4.2 BCME
The genotoxicity of BCME has been examined in a variety of
limited and generally poorly documented studies. BCME (at a maximum
concentration of 20 µg/plate) was mutagenic in the presence of an
exogenous metabolic activating system in S. typhimurium strain
TA100, based on a 3-fold increase in the frequency of revertants above
control levels. However, similar results were not observed in
S. typhimurium strains TA1535, TA1538 and TA98 (Anderson & Styles,
1978). BCME was also reported to be mutagenic in various strains of
E. coli and S. typhimurium, but experimental details and results
were not provided (Mukai & Hawryluk, 1973).
BCME (at concentrations as low as 0.16 µg/ml) was reported to
increase DNA repair (unscheduled DNA synthesis) in human skin
fibroblasts, although quantitative results were not provided (Agrelo &
Severin, 1981). In in vitro assays with BHK-21 and human lung WI-38
cells, concentrations of BCME between 0.008 and 25 mg/ml (in the
presence of an exogenous metabolic activating system) increased the
frequency of transformed cells approximately 6.6- and 11-fold,
respectively (Styles, 1978). The exposure (in vitro) of human
neonatal foreskin fibroblasts to concentrations of BCME between 0.1
and 8 µg/ml produced a 3- to 14-fold increase in the frequency of
anchorage-independent cells (Kurian et al., 1990).
BCME was reported to directly alkylate DNA (at guanine and
adenine residues) when the two substances were incubated together in
an in vitro assay (Goldschmidt et al., 1975). It was reported to
damage RNA within bacteriophage R17 (Shooter, 1975).
7.4.3 CMME
CMME was reported to be mutagenic in various strains of E. coli
and S. typhimurium. However, experimental details or results were
not provided in this published account (Mukai & Hawryluk, 1973). In
the presence of an exogenous metabolic activating system, CMME (1 and
10 mmol/litre) increased unscheduled DNA synthesis in human
lymphocytes approximately 30 and 100%, respectively (Perocco et al.,
1983).
7.5 Other toxicity studies
The exposure (by inhalation) of male Sprague-Dawley rats to 0.47
mg BCME/m3 (100 ppb) for 6 h/day, 5 days/week, for a period of six
months had no observable effect upon the nervous or reproductive
systems, based on gross and microscopic analysis (Leong et al., 1981).
No other relevant information regarding the reproductive,
developmental, immunological or neurological toxicity of BCEE, BCME or
CMME was identified.
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Human exposure studies
Schrenk et al. (1933) reported that the "brief" (time not stated)
exposure of men to concentrations of BCEE ranging from 3218 to 5850
mg/m3 (550 to 1000 ppm) caused irritation to the eyes (lacrimation)
and nasal passages, such that exposure was considered intolerable.
Inhalation of BCEE also caused nausea. The intensity of such effects
gradually declined as the concentration of BCEE was lowered from 1521
mg/m3 (260 ppm) to 585 mg/m3 (100 ppm); exposure to 205 mg/m3 (35
ppm) was reported to be "only slightly offensive and practically free
from irritation". No clinical studies on BCME and CMME were
identified.
8.2 Occupational exposure
8.2.1 Case reports
The death of a worker in a fulling mill (textile factory) was
attributed to the inhalation of BCEE, but details were not provided
(Elkins, 1959). One anecdotal report on the occurrence of dermatitis
in textile workers exposed to resins containing BCEE was identified
(Kirwin & Sandmeyer, 1981).
Sakabe (1973) reported that 5 out of 32 Japanese males employed
in dyestuffs factories who had been exposed to BCME died of "lung
cancer" (between 1963 and 1969). Small (oat) cell carcinoma was
identified in one of the cases. Quantitative information on exposure
was not provided in this published account and these individuals were
exposed to a number of chemical substances in addition to BCME
(smoking habits could not be confirmed). However, because a large
proportion (approximately 16%) of the individuals exposed to BCME
developed lung cancer, and those exposed to chemicals other than BCME
did not, the authors attributed the occurrence of these pulmonary
tumours to exposure to BCME.
Reznick et al. (1977) reported the case of a 45-year-old male
chemist who had died of a slightly differentiated adenocarcinoma of
the lung. Twelve years earlier this individual had been exposed to
BCME and CMME over a period of two years. Although quantitative
information on exposure was not presented (and the individual was also
exposed to vinyl chloride), the lung adenocarcinoma was attributed to
his exposure to BCME and CMME.
Roe (1985) reported the case of three males (between 35 and 40
years of age) who had died of lung cancer (small (oat) cell and
squamous cell carcinomas) after having been occupationally exposed to
BCME. Although quantitative or qualitative information on exposure was
not provided and the individuals had been smokers, the relatively
young age at which these individuals died was attributed to their
exposure to BCME.
8.2.2 Epidemiological studies
Data on the effects of long-term exposure to BCEE on human health
were not identified. In a number of epidemiological studies, mortality
and morbidity due to cancer in workers occupationally exposed to BCME
and CMME have been examined. Lemen et al. (1976) examined the
incidence of lung cancer in a group of workers employed at a chemical
plant in California, USA, where BCME was used in the production of
ion-exchange resins. The authors identified 136 individuals who had
been employed for at least 5 years between 1955 and 1972. The number
of cases of lung cancer (5) was significantly greater (p < 0.01) than
the number expected (0.54), based on age-respiratory cancer-specific
incidence rates for white males in the state of Connecticut in
1960-1962. Notably, 80% of the tumours were small cell
undifferentiated cancers. Importantly, lung tumours in persons
occupationally exposed to BCME and CMME are predominantly small (oat)
cell carcinomas (Weiss, 1976; Pasternack et al., 1977). The occurrence
of this type of lung cancer in these individuals is quite distinct
from that caused by tobacco, one of the potential confounders in such
studies, where the lung tumours are predominantly squamous cell
carcinomas (Weiss, 1976; Pasternack et al., 1977). Individuals (80%
were smokers) with cancer averaged 47 years of age, and the average
latency period was approximately 10 years. Quantitative or qualitative
information on exposure was not provided in this published account.
The incidence of metaplastic and atypical cells in the sputum of
workers exposed to BCME was greater than in controls (uranium miners),
based on cytological analysis.
Nishimura et al. (1990) examined the incidence of lung cancer in
a group of Japanese workers employed in two dyestuff factories where
BCME was used. The study group consisted of 35 males employed at these
plants between 1955 and 1970. The number of cases (13) of lung cancer
(11 cases were in smokers) was significantly (p < 0.001) higher than
the number expected (0.62). Tumours from eight of the individuals were
examined histopathologically; four were diagnosed as small cell
undifferentiated carcinomas, two were adenocarcinomas and one a large
cell carcinoma; in one individual, both a small cell carcinoma and an
adenocarcinoma were found. The average age at which individuals with
lung cancer died was 46 years, and the latency period was
approximately 13.5 years. The average duration of exposure to BCME was
approximately 7.2 years, although no other quantitative or qualitative
information on exposure was provided.
Since technical grade CMME contains between 1 and 8% BCME
(Travenius, 1982), in epidemiological studies in which mortality and
the incidence of cancer in workers exposed to CMME were examined, the
effects may have been due (at least in part) to BCME. Weiss (1976)
reported the results of a 10-year prospective study (1963-1973) in
which 125 male employees of a chemical plant in the USA who had been
occupationally exposed to CMME(BCME), were examined with respect to
the "incidence" of pulmonary cancer. No quantitative or qualitative
information on exposure was provided. However, an exposure index (low,
medium and high) based on type and duration of job associated with
potential exposure to CMME(BCME) was developed. Eleven cases of lung
cancer were reported in 49 individuals with medium or high exposure to
CMME(BCME); no "incidence" of lung cancer was reported in 76 workers
with no or low exposure to CMME(BCME). The number of deaths (16)
during this period was 2.7-fold greater than the number expected
(5.9), based on a comparison with death rates for white males in the
USA. All of the excess deaths (10) were attributable to lung cancer,
100% of which were small cell carcinomas that developed in individuals
less than 55 years of age. The latency period for these cancers ranged
from 10 to 24 years. Among individuals exposed to CMME(BCME) the
"incidence" of pulmonary tumours was inversely related to their use of
tobacco (Weiss, 1980). In a follow-up study of these workers, the
number of deaths (13) due to lung cancer (which were attributable to
either moderate or heavy exposure to CMME[BCME]) was 19.5-fold greater
than the number (0.66) expected, based on lung cancer mortality rates
in the surrounding municipality (Philadelphia) (Weiss, 1982). The
standardized mortality ratio for deaths due to lung cancer which
peaked 15 to 19 years from the onset of exposure, declined during the
subsequent 20- to 29-year period. Subsequently, Weiss (1989) indicated
that over-representation of workers with moderate to high exposure
within the cohort led to some over-estimation of the risk of lung
cancer. However, even when such selection bias was accounted for, an
increased risk of lung cancer remained associated with exposure to
CMME(BCME).
Maher & DeFonso (1987) examined mortality in a group of workers
exposed to CMME(BCME). [This report represented an update and
extension of a previous investigation on death due to lung cancer
performed by these authors (DeFonso & Kelton, 1976)]. The study
population consisted of a group of 737 "exposed" and 2120 "unexposed"
white male workers (who comprised 97% of the labour force) employed
for any length of time at a chemical plant in the USA between 1948 and
1971. The vital status of 90% of the group was determined up to 1982.
No quantitative information on exposure was provided, but an exposure
rating (from 0-6) was developed based on the type of work, proximity
of exposure to CMME(BCME) and production methods. Cumulative exposure
was calculated based on the exposure rating and duration of employment
at a particular job. The expected number of deaths for each type of
cancer was calculated using cause-specific death rates for white males
residing in the surrounding municipality (Philadelphia). Information
on smoking habits was incomplete but "no marked differences between
smoking habits of exposed and unexposed workers were noted" (Maher &
DeFonso, 1987). Among the workers exposed to CMME(BCME), the number of
deaths (32) due to cancer of the "respiratory tract" was significantly
(P < 0.01) higher than the number expected (11.5). For individuals
not exposed to CMME(BCME), the number of deaths (25) due to
respiratory tract cancer was similar to those expected (23.8). In the
CMME(BCME)-exposed group, the number of deaths due to cancer of the
digestive, genito-urinary, haematopoietic, lymphatic and central
nervous systems was not significantly greater than expected. The
greatest increase in deaths due to cancer of the respiratory tract
occurred approximately 10 to 20 years after the first exposure to
CMME(BCME). Among workers exposed to CMME(BCME), the ratio of
observed/expected number of deaths due to lung cancer was lower
between 1975 and 1981 than for the period between 1960 and 1974, this
being attributed to a reduction in the level of exposure to CMME(BCME)
in 1971 as a result of the implementation of stringent engineering
controls on the use of this substance (Maher & DeFonso, 1987).
Collingwood et al. (1987) assessed mortality due to respiratory
cancer in a group of workers employed at seven industrial facilities
in which CMME(BCME) was produced or utilized. This report represented
a follow-up and extension of a previous study by two of these authors
(Pasternack et al., 1977). The study group (97% white, 96% male)
comprised 2460 CMME(BCME)-exposed and 3692 unexposed workers employed
between 1948 and 1980. Only limited information on smoking habits was
available. No quantitative or qualitative information on exposure was
provided, but an exposure index (taking into account type of job,
frequency of work and potential exposure to CMME(BCME)) was developed.
Cumulative exposure was calculated on the basis of the exposure index
and duration of employment at a particular job. The number of expected
deaths was calculated from death rates in the USA specific for age,
cause, sex, race and calendar year. Among workers exposed to
CMME(BCME), the standardized mortality ratio for death due to
respiratory cancer was significantly increased (SMR = 3.01;
95% CI = 2.24-3.98); this was attributed to excess deaths at two
companies (where the ratio of observed to expected deaths due to lung
cancer among exposed workers was 32/7.4 and 9/1.5). In the entire
study group, there were 90 deaths due to respiratory cancer, 52 and 38
in the CMME(BCME)-exposed and unexposed groups, respectively. In those
cases with verifiable histology, 12/32 (38%) cases in the exposed
group had small (oat) cell carcinomas while 6/20 (30%) cases in the
unexposed group had adenocarcinomas. The relative risk of death due to
lung cancer was found to be related to total cumulative exposure based
on a regression model.
Gowers et al. (1993) examined the incidence of lung cancer among
1203 males employed at an ion-exchange resin manufacturing plant in
France between 1958 and 1986; of the total study cohort, 258 were
exposed to technical grade CMME (i.e., containing BCME), the remainder
were considered to be unexposed. Data on the incidence of lung cancer
among these workers was obtained from a local registry; the expected
number of lung cancer cases was calculated, based upon incidence rates
for males provided by a registry serving an area some 250 km distant
from the plant (i.e., external population). There were 8 (one small
cell carcinoma) and 11 (10 small cell carcinomas) cases of lung cancer
among the unexposed and CMME(BCME)-exposed workers, respectively.
Reductions in occupational exposure to CMME(BCME) were instituted in
1972 and 1984. Potential exposure to CMME(BCME) was rated according to
employment experience; cumulative exposure was based upon the job
exposure rating and length of employment at a particular job. Based
upon limited monitoring studies, conducted between 1979 and 1984, the
average level of BCME in the plant ranged from 3.3 to 20.7 µg/m3
(0.7 to 4.4 ppb); after 1984 the average level in the plant was
reportedly < 2.4 µg/m3 (0.5 ppb). The rate ratio for lung cancer
among the CMME(BCME)-exposed workers, compared to the rate for the
unexposed workers or external population, was 5.5 (95% CI = 2.0-12.3)
and 7.6 (95% CI = 4.3-13.5), respectively. The rate ratio for lung
cancer among the unexposed workers, compared to the rate for the
external population, was 1.6 (95% CI = 0.8-3.12). Linear regression
analysis revealed an increased rate of lung cancer with increasing
cumulative exposure. The ratio of observed/expected cases of lung
cancer for the CMME(BCME)-exposed workers (based upon comparison with
the external population) generally increased with increasing
cumulative exposure. The mean age at diagnosis for the unexposed and
CMME(BCME)-exposed workers was 56.5 and 46 years, respectively; the
mean induction period for lung cancer among the CMME(BCME)-exposed
workers was approximately 13 years.
Excess deaths due to lung cancer have also been reported for
Chinese (Xue et al., 1988) chemical workers exposed to
"chloromethylether"; latency as low as 2 years was reported. In a
follow-up study of one of the cohorts examined by Xue et al. (1988),
exposed to both CMME and BCME, there was an increased rate of lung
markings similar to asbestosis as well as evidence of reduced
pulmonary function (Xue et al., 1996). The magnitude of these changes
was higher among workers exposed before 1975 to CMME levels of 0.096
µg/m3 than to those exposed after 1981 to approximately 0.013 µg/m3.
Sram et al. (1983, 1985) reported the increased frequency of
chromosomal aberrations in peripheral lymphocytes of workers (1.64 to
3.75% in controls, and 5.06 to 5.49% in subjects employed from 1-10
years) exposed in the production of ion exchange resins to levels of
0.01 to 0.1 µg BCME/m3 and 20 to > 200 µg CMME/m3.
No relevant studies are available concerning the neurological,
immunological, developmental or reproductive effects of BCME or CMME
in humans.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
For aquatic species, the 7-day LC50 for exposure of the guppy
(Poecilia reticulata) to BCEE was 56.9 mg/litre (Konemann, 1981).
Buccafusco et al. (1981) reported a 96-h LC50 of 600 mg BCEE/litre
for the bluegill sunfish (Lepomis macrochirus). LeBlanc (1980)
reported a 48-h LC50 of 240 mg BCEE/litre for Daphnia magna. In all
three of the above studies, organisms were exposed to nominal
concentrations of BCEE in closed containers, under static or
static-with-renewal conditions.
Anaerobic activity was not inhibited when microorganisms were
exposed to BCEE at concentrations up to 100 mg/litre in a nutrient
buffer solution (Johnson & Young, 1983). Cho et al. (1989) reported an
LC50 and an LC10 of 2160 and 600 µg BCEE/litre, respectively, for
microbes indigenous to industrial waste stabilization ponds and that
required a supply of organic material for food.
No relevant data are available to assess toxicity of BCEE to
species of wildlife, and no studies have been identified in which the
toxicity of either BCME or CMME to aquatic or terrestrial organisms
was investigated.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 BCEE
The lack of available information on concentrations of BCEE in
several environmental media to which humans are exposed precludes
quantitative estimation of the total daily intake of this substance
from the general environment. Based upon extremely limited data, the
estimated intake of BCEE from drinking-water for adults would be
approximately 0.01 µg/kg body weight per day. Quantitative information
on the extent of potential workplace exposure to this substance was
not identified.
Available data on the toxicity of BCEE in humans are extremely
limited. Irritation to the eyes, nasal passages and respiratory tract
could result from acute inhalation exposure to moderate levels of
BCEE. Data were considered inadequate to assess the human health risks
of non-neoplastic effects arising from longer-term exposure to this
substance.
Studies on the toxicological effects produced by the long-term
exposure of laboratory animals to BCEE have focused on its
carcinogenic potential. Some very limited evidence of carcinogenicity
in hybrid F1 mice was reported in one study (Innes et al., 1969).
However, none of the long-term (subchronic or chronic/
carcinogenicity) studies in laboratory animals is considered to be of
sufficient quality to provide useful quantitative information on the
carcinogenic potential of BCEE or the toxicological effects produced
by long-term exposure to this substance. Moreover, studies of
developmental and reproductive effects of BCEE in laboratory animals
have not been identified. Available data were, therefore, considered
inadequate to assess the risks to human health associated with
exposure to BCEE in the general or occupational environments.
10.1.2 BCME and CMME
Information on the concentrations of BCME and CMME in air,
drinking-water, soil or foodstuffs were not identified, and therefore
it was not possible to estimate the intake of these substances by the
general population. However, owing to the extremely rapid hydrolysis
of these compounds in aqueous media, exposure of non-occupationally
exposed individuals is likely to be negligible. However, in some
countries exposure of the general population to chloromethyl ethers
may occur through the use of mosquito coils. Information on
occupational exposure to BCME and CMME is also limited, although a
recent study of a resin manufacturing plant reported lower levels of
BCME than had been observed in previous, older investigations of
plastics, textile and chemical manufacturing plants.
Based upon the results of studies conducted with animals,
inhalation of BCME or CMME may produce severe irritation of the eyes
and respiratory tract as well as necrotizing bronchitis. Dermal
exposure to BCME and CMME can result in erythema and necrosis.
In all of the cohort studies of occupationally exposed workers
conducted to date, an association between lung cancer and exposure to
either BCME or CMME has been observed. The type of lung cancer, the
standardized mortality ratios, the latency periods and average age of
appearance of lung cancer in groups of workers exposed to either BCME
or CMME have been remarkably consistent. The type and incidence of
lung cancer in individuals exposed to BCME or CMME, predominantly
small (oat) cell carcinomas, occurring in relatively young individuals
after short latency periods (as low as 2 years), is distinct from that
caused by tobacco, one of the potential confounders in such studies,
where lung tumours are predominantly squamous cell carcinomas,
occurring after long latency periods in individuals greater than 60
years of age. The association between exposure to either BCME or CMME
and lung cancer is strong, with standardized mortality ratios ranging
up to 21.
For CMME, there is also evidence of a positive relationship
between a qualitative measure of exposure and mortality due to lung
cancer. In two studies of occupationally exposed individuals, the
standardized mortality ratios for deaths due to lung cancer peaked 10
to 20 years following the onset of exposure. Furthermore, observation
of an association between occupational exposure to BCME or CMME and
the development of lung cancer is plausible. This observation is based
on the results of early, rather limited carcinogenesis bioassays in
exposed animal species, in which increases in the incidence of
tumours, predominantly of the respiratory tract, have been observed,
as well as on available data on the genotoxicity of BCME and CMME.
The observed association of lung cancer and occupational exposure
to either BCME or CMME fulfil traditional criteria for assessment of
causality in epidemiological studies, i.e. consistency, strength,
specificity, temporal relationship, exposure-response relationship,
plausibility and supporting data on chromosomal effects in workers
exposed to 0.01 µg BCME/m3 and 20 µg CMME/m3. Clearly, BCME and
technical grade CMME are carcinogenic to humans, and, therefore,
exposure to these substances should be eliminated.
10.1.3 Guidance values
Available data on BCEE were considered inadequate to derive a
meaningful guidance value for this substance.
Inhalation is the principal route of exposure to these
substances. Data available in epidemiological studies of workers
exposed to BCME and CMME are inadequate to characterize quantitatively
the exposure-response relationship for carcinogenicity. There is
evidence that the general population may be exposed to BCME and CMME
through the use of mosquito coils, but there are no quantitative
exposure data available. However, in humans there is an increase in
cancer incidence (latency period as short as 2 years) with cumulative
exposure to BCME and an increase in chromosomal aberrations in workers
at levels as low as 0.01 µg BCME/m3 and 20 µg CMME/m3. Based on
multistage modelling of the incidence of esthesioneuro-epitheliomas in
rats exposed to BCME (Leong et al., 1981), the estimated concentration
of this substance associated with a 5% increase in tumour incidence
(TD05), corrected for intermittent (6 of 24 h, 5 days/week) versus
continuous exposure, is 6 µg/m3. Data on CMME were insufficient to
derive a TD50. Limitations of the critical study including the
relatively short period of exposure (6 months followed by 22-month
observation period) and sharp increase in the incidence of these
tumours between the mid- and high-concentration groups, should,
however, be borne in mind in the interpretation of this value.
The above analysis of data further strengthens the recommendation
to eliminate human exposure to BCME and CMME.
10.2 Evaluation of effects on the environment
10.2.1 BCEE
BCEE is highly soluble in water and tends to remain there,
although some volatilization from soil and water to the atmosphere
occurs. Owing to lack of adsorption, BCEE is mobile in soils,
especially those with low organic carbon content, and therefore it has
the potential to reach groundwater. BCEE does not bioaccumulate or
biomagnify to any significant extent.
Exposure of terrestrial organisms to BCEE is considered to be
negligible because of its extremely low rate of release and short
persistence in the atmosphere. For aquatic biota, a 7-day LC50 of
56.9 mg/litre (nominal concentration) for the guppy (Poecilia
reticulata) has been reported. The lowest LC50 reported for acute
toxicity (48-h) is 240 mg/litre (nominal concentration) for Daphnia
magna. The highest concentration of BCEE reported for surface water
in the USA (1.4 µg/litre) is approximately 40 000 times lower than the
reported 7-day LC50 for the guppy (Poecilia reticulata).
Although it is relatively persistent in water, the highest
reported concentration of BCEE in surface water is approximately five
orders of magnitude lower than the concentration found to induce
adverse effects in the guppy, the most sensitive aquatic species
identified among existing toxicity studies. Therefore, BCEE is not
expected to pose a significant risk to environmental organisms.
10.2.2 BCME and CMME
Both substances are readily hydrolysed in aqueous media or
photo-oxidized in the atmosphere and, therefore, are not likely to
accumulate. Because of their extremely short residence times, levels
in the environment (if any) are likely to be extremely low. Thus,
despite the lack of data concerning the environmental toxicity of BCME
and CMME, there is no reason to suspect that adverse effects could
occur to organisms living in the ambient environment.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
a) Exposure to BCME and technical CMME should be eliminated.
b) Levels of BCEE in environmental media to which humans are exposed
should be determined.
12. FURTHER RESEARCH
a) Workers previously exposed to BCME and technical grade CMME
should be followed using all available methods, including markers
of biological effect for the detection of lung cancer at an early
stage.
b) The degree of exposure of the general public to BCME and CMME
through the use of mosquito coils containing the S-2 synergist
octachlorodipropyl ether should be measured. In this study the
degree of possible contamination of the S-2 synergist by BCME and
CMME should also be taken into account.
c) If it is still used, the toxicological profile for BCEE should be
determined in well-designed toxicological studies.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
BCEE has been evaluated by the International Agency for Research
on Cancer (IARC) and placed in Group 3 - "not classifiable as to its
carcinogenicity in humans" (IARC, 1987). BCME and CMME are considered
by IARC to be carcinogenic to humans (Group 1) (IARC, 1987).
REFERENCES
Agrelo CE & Severin BJ (1981) A simplified method for measuring
scheduled and unscheduled DNA synthesis in human fibroblasts.
Toxicology, 21: 151-158.
Albert RE, Sellakumar AR, Laskin S, Kuschner M, Nelson N, & Snyder CA
(1982) Gaseous formaldehyde and hydrogen chloride induction of nasal
cancer in the rat. J Natl Cancer Inst, 68: 597-603.
Anderson D & Styles JA (1978) The bacterial mutation test. Br J
Cancer, 37: 924-930.
ATSDR (1989a) Toxicological profile for bis(2-chloroethyl)ether.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry
(Report PB90-168683).
ATSDR (1989b) Toxicological profile for bis(chloromethyl) ether.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry
(Report PB90-168691).
Barrows M, Petrocelli S, Macek K, & Carrol J (1978) Bioconcentration
and elimination of selected water pollutants by bluegill sunfish
(Leptomis macrochirus). In: Haque R ed. Dynamics, exposure and
hazard assessment of toxic chemicals. Ann Arbor, Michigan, Ann Arbor
Science Publishers, Inc., pp 379-392.
Blease T, Scrivens J, & Morden W (1989) The determination of
atmospheric bis(chloromethyl) ether by gas chromatography/ tandem mass
spectrometry. Biomed Environ Mass Spectrom, 18: 775-779.
Buccafusco RJ, Ells SJ, & LeBlanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Callahan M, Slimak M, Gabel N, May I, Fowler C, Freed J, Durfee R,
Whitmore F, Maestri B, Mabey W, Holt B, & Gould C (1979) Water-related
environmental fate of 129 priority pollutants: Volume II - Halogenated
aliphatic hydrocarbons, halogenated ethers, monocyclic aromatics,
phthalate esters, polycyclic aromatic hydrocarbons, nitrosamines, and
miscellaneous compounds. Washington, DC, US Environmental Protection
Agency, Office of Water Planning and Standards, Office of Water and
Waste Management (EPA-440/4-79-029b, PB80-204381).
CCINFO (1991) Canadian Centre for Occupational Health and Safety
database: Data for chloromethyl methyl ether. Hamilton, Ontario,
Canadian Centre for Occupational Health and Safety.
Chen BI, Sun FG, & Xue SZ (1997)The occurrence of a cluster of lung
cancer among the workers in the synthesis of octachloropropanyl ether.
Chin J Ind Med, 10(5): 305-306.
Cho Y-H, Davis EM, & Ramey GD (1989) Assessing microbial toxicity of
2-ethoxyethanol and bis(2-chloroethyl)ether by a modified spread plate
method. Environ Technol Lett, 10: 875-886.
Collier L (1972) Determination of bis(chloromethyl) ether at the ppb
level in air samples by high-resolution mass spectroscopy. Environ Sci
Technol, 6: 930-932.
Collingwood KW, Pasternack BS, & Shore RE (1987) An industry-wide
survey of respiratory cancer in chemical workers exposed to
chloromethyl ethers. J Natl Cancer Inst, 78: 1127-1136.
Cuppit L (1980) Fate of toxic and hazardous materials in the air
environment. Research Triangle Park, US Environmental Protection
Agency, Atmospheric Chemistry and Physics Laboratory
(EPA-600/3-80-084, PB80-221948).
DeFonso LR & Kelton SC (1976) Lung cancer following exposure to
chloromethyl methyl ether. Arch Environ Health, 31: 125-130.
DeWalle FB & Chian ES (1981) Detection of trace organics in well water
near a solid waste landfill. J Am Water Works Assoc, 73: 206-211.
Drake KD & Myer JR (1992) Acute oral toxicity of DCEE (dichloroethyl
ether) in rats and mice. Acute Toxicol Data, 1: 163-164.
Dressman RC, Fair J, & McFarren EF (1977) Determinative method of
analysis of aqueous sample extracts for bis(2-chloro) ethers and
dichlorobenzenes. Environ Sci Technol, 11: 719-721.
Drew RT, Laskin S, Kuschner M, & Nelson N (1975) Inhalation
carcinogenicity of haloethers: I. The acute inhalation toxicity of
chloromethyl methyl ether and bis(chloromethyl)ether. Arch Environ
Health, 30: 61-69.
Durkin P, Howard P, & Saxena J (1975) Investigation of selected
environmental contaminants: Haloethers. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances
(EPA 68-1-2996, PB-246 356).
Eisner D (1974) Formation of bis-chloromethylether from hydrogen
chloride and formaldehyde. Redwood City, California, Diamond Shamrock
Chemical Co., Nopco Chemical Division (Report No. EQR-7412).
Elkins HB (1959) Chemistry of industrial toxicology. New York, John
Wiley and Sons.
Evans K, Mathias A, Mellor N, Silvester R, & Williams A (1975)
Detection and estimation of bis(chloromethyl) ether in air by
gas-chromatography high-resolution mass spectrometry. Anal Chem, 47:
821-824.
Fishbein L (1979) Potential halogenated industrial carcinogenic and
mutagenic chemicals: III. Alkane halides, alkanols and ethers. Sci
Total Environ, 11: 223-257.
Foureman P, Mason JM, Valencia R, & Zimmering S (1994) Chemical
mutagenesis testing in Drosophila: IX. Results of 50 coded compounds
tested for the National Toxicology Program. Environ Mol Mutagen, 23:
51-63.
Frankel L & Black R (1976) Automatic gas chromatographic monitor for
the determination of parts-per-billion levels of bis(chloromethyl)
ether. Anal Chem, 48: 732-737.
Frankel LS, McCallum K, & Collier L (1974) Formation of
bis(chloromethyl) ether from formaldehyde and hydrogen chloride.
Environ Sci Technol, 8: 356-359.
Galvin R & House M (1988) Atmospheric monitoring of bis(chloromethyl)
ether at low ppb levels using an automated system. Environ Technol
Lett, 9: 563-570.
Gargus JL, Reese WH, & Rutter HA (1969) Induction of lung adenomas in
newborn mice by bis(chloromethyl)ether. Toxicol Appl Pharmacol, 15:
92-96.
Goldschmidt BM, van Duuren BL, & Frenkel K (1975) The reaction of
14C-labelled bis(chloromethyl)ether with DNA. Proc Am Assoc Cancer
Res, 16: 66.
Government of Canada (1993a) Canadian Environmental Protection Act -
Priority substances list assessment report: Bis(2-chloroethyl) ether.
Ottawa, Government of Canada.
Government of Canada (1993b) Canadian Environmental Protection Act -
Priority substances list assessment report: Bis(chloromethyl)ether and
chloromethyl methyl ether. Ottawa, Government of Canada.
Gowers DS, DeFonso LR, Schaffer P, Karli A, Monroe CB, Bernabeu L, &
Renshaw FM (1993) Incidence of respiratory cancer among workers
exposed to chloromethyl-ethers. Am J Epidemiol, 137: 31-42.
Gwinner LM, Laib RJ, Filser JG, & Bolt HM (1983) Evidence of
chloroethylene oxide being the reactive metabolite of vinyl chloride
toward DNA: Comparative studies with 2,2'-dichloro-diethylether.
Carcinogenesis, 4: 1483-1486.
Hauser T & Bromberg S (1982) EPA's program at Love Canal. Environ
Monit Assess, 2: 249-272.
Hites RA, Jungclaus GA, & Lopez-Avila V (1979) Potentially toxic
organic compounds in industrial waste-waters and river systems: Two
case studies. In: Monitoring toxic substances. Washington, DC,
American Chemical Society, pp 63-90 (American Chemical Symposium
Series 94).
Howard P, Boething R, Jarvis W, Meylan W, & Michalenko E (1991)
Handbook of environmental degradation rates. Chelsea, Michigan, Lewis
Publishers, Inc.
IARC (1974) Bis(chloromethyl)ether. Some aromatic amines, hydrazines,
and related substances, N-nitroso compounds and miscellaneous
alkylating agents. Lyon, International Agency for Research on Cancer,
pp 231-245 (Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, Volume 4).
IARC (1975) Bis(2-chloroethyl) ether. In: Some aziridines, N-, S- and
O-mustards and selenium. Lyon, France, International Agency for
Research on Cancer, pp 117-123 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, Volume 9).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volums 1 to 42. Lyon, International Agency for
Research on Cancer, p 58 (Monographs on the Evaluation of Carcinogenic
Risks to Humans, Supplement 7).
Innes JRM, Ulland BM, Valerio MG, Petrucelli L, Fishbein L, Pallotta
AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, & Peters J
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J Natl Cancer Inst,
42: 1101-1114.
IPCS (1994) Environmental health criteria 170: Assessing human health
risks of chemicals: Derivation of guidance values for health-based
exposure limits. Geneva, World Health Organization, International
Programme on Chemical Safety, 71 pp.
Johnson LD & Young JC (1983) Inhibition of anaerobic digestion by
organic priority pollutants. J Water Pollut Control Fed,
55: 1441-1449.
Jorgenson TA, Rushbrook CJ, Newell GW, & Tardiff RG (1977) Study of
the mutagenic potential of bis(2-chloroethyl) and
bis(2-chloroisopropyl) ethers in mice by the heritable translocation
test. Toxicol Appl Pharmacol, 41: 196-197.
Kallos G (1981) Oxygen induced response enhancement of determination
of bis(chloromethyl) ether by gas chromatography with nickel-63
electron capture detection. Anal Chem, 53: 963-965.
Kallos G & Tou J (1977) Study of photolytic oxidation and chlorination
reactions of dimethyl ether and chlorine in ambient air. Environ Sci
Technol, 11: 1101-1105.
Kallos G, Albe W, & Solomon R (1977) On-column reaction gas
chromatography for determination of chloromethyl methyl ether at the
one part-per-billion level in ambient air. Anal Chem, 49: 1817-1820.
Keith L, Garrison A, Allen F, Carter M, Floyd T, Pope J, & Thruston A
(1976) Identification of organic compounds in drinking-water from
thirteen U.S. cities. In: Keith LH ed. Identification and analysis
of organic pollutants in water. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., p 356.
Kendall PR (1990) The quality of drinking-water in Toronto: A review
of tapwater, bottled water and water treated by a point-of-use device
- Summary report. Toronto, Canada, Department of Public Health,
Environmental Protection Office, 41 pp.
Kincannon D & Lin Y (1986) Microbial degradation of hazardous wastes
by land treatment. In: Proceedings of the 40th Industrial Waste
Conference, Purdue University, West Lafayette, IN, 14-16 May 1985.
Boston, Ann Arbor Science Publishers, Inc., pp 607-619.
Kirwin CJ & Sandmeyer EE (1981) Ethers. In: Clayton GD & Clayton FE
ed. Patty's industrial hygiene and toxicology: Volume IIA, 3rd ed.
New York, John Wiley and Sons, pp 2491-2565.
Kleopfer RD & Fairless BJ (1972) Characterization of organic
components in a municipal water supply. Environ Sci Technol,
6: 1036-1037.
Konemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies - Part 1: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Kraybill HF (1977) Global distribution of carcinogenic pollutants in
water. Ann NY Acad Sci, 298: 80-89.
Kurian P, Nesnow S, & Milo GE (1990) Quantitative evaluation of the
effects of human carcinogens and related chemicals on human foreskin
fibroblasts. Cell Biol Toxicol, 6: 171-184.
Kuschner M, Laskin S, Drew RT, Cappiello V, & Nelson N (1975)
Inhalation carcinogenicity of alpha haloethers: III. Lifetime and
limited period inhalation studies with bis(chloromethyl)ether at 0.1
ppm. Arch Environ Health, 30: 73-77.
Langelaan F & Nielen M (1989) Determination of trace levels of
chloromethyl methyl ether and bis(chloromethyl) ether in air. Int J
Environ Anal Chem, 36: 27-34.
Langhorst M (1985) Monitoring chloromethyl methyl ether in air. In:
Fishbein L & O'Neill KI ed. Environmental carcinogens. Selected
methods of analysis - Volume 7: Some volatile halogenated
hydrocarbons. Lyon, International Agency for Research on Cancer, pp
235-246 (IARC Scientific Publications No. 68).
Langhorst M, Melcher R, & Kallos G (1981) Reactive adsorbent
derivative collection and gas chromatographic determination of
chloromethyl methyl ether in air. Am Ind Hyg Assoc J, 42: 47-55.
Laskin S, Drew RT, Cappiello V, Kuschner M, & Nelson N (1975)
Inhalation carcinogenicity of alpha halo ethers: II. Chronic
inhalation studies with chloromethyl methyl ether. Arch Environ
Health, 30: 70-73.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water
fleas (Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lemen R, Johnson W, Wagoner J, Archer V, & Saccomanno G (1976)
Cytologic observations and incidence following exposure to BCME. Ann
NY Acad Sci, 271: 71-80.
Leong BKJ, MacFarland HN, & Reese WH (1971) Induction of lung adenomas
by chronic inhalation of bis (chloromethyl) ether. Arch Environ
Health, 22: 663-666.
Leong BKJ, Kociba RJ, & Jersey GC (1981) A lifetime study of rats and
mice exposed to vapors of bis(chloromethyl)ether. Toxicol Appl
Pharmacol, 58: 269-281.
Lingg RD, Kaylor WH, Pyle SM, & Tardiff RG (1979) Thiodiglycolic
acid: a major metabolite of bis(2-chloroethyl) ether. Toxicol Appl
Pharmacol, 47: 23-34.
Lingg RD, Kaylor WH, Pyle SM, & Domino MM (1982) Metabolism of
bis(2-chloroethyl)ether and bis(2-chloroisopropyl)ether in the rat.
Arch Environ Contam Toxicol, 11: 173-183.
Mabey W, Smith J, Podoll R, Johnson H, Mil T, Chou T, Gates J,
Partridge I, Jaber H, & Vandenberg D (1982) Aquatic fate processes
data for organic priority pollutants. Washington, DC, US Environmental
Protection Agency, Office of Water Regulations and Standards,
Monitoring and Data Support Division (EPA 440/4-81-014).
Mackay D & Wolkoff A (1973) Rate of evaporation of low-solubility
contaminants from water bodies to the atmosphere. Environ Sci Technol,
7: 611-614.
McMillin CR, Gable RC, Kyne JM, Quill RP, Snyder AD, & Thomas JA
(1984) EPA method study 21, method 611: Haloethers. Cincinnati, Ohio,
US Environmental Protection Agency, Environmental and Support
Laboratory, Office of Research and Development (EPA-600/4-84-052,
PB84-205939).
Maher KV & DeFonso LR (1987) Respiratory cancer among
chloromethylether workers. J Natl Cancer Inst, 78: 839-843.
Manwaring J, Blankenship W, Miller L, & Voigt F (1977)
Bis(2-chloroethyl) ether removal from drinking-water by source
protection. In: Pojasek RB ed. Drinking-water quality enhancement
through source protection. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., pp 417-429.
Marceleno T (1974) Survey of Burlington Industries, Inc., Burlington
House Finishing Plant, Form Fabrics Plant, Durham Domestics Plant,
Brookneal Finishing Plant. Cincinnati,Ohio, National Institute for
Occupational Safety and Health, Environmental Investigations Branch,
Division of Field Studies and Clinical Investigations (Report
PB82-151077).
Milano J, Bernat-Escallon C, & Vernet J (1989) Dégradations dans l'eau
par hydrolyse et photolyse du bis-2 chloroéthyl éther. Environ Technol
Lett, 10: 291-300.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 7: 1-119.
Mukai FH & Hawryluk I (1973) Mutagenicity of some halo-ethers and
halo-ketones. Mutat Res, 21: 228.
Muller G, Norpoth K, & Eckard R (1979) Identification of
S-(carboxymethyl)-L-cysteine and thiodiglycollic acid, urinary
metabolites of 2,2'-bis-(chloroethyl)-ether in the rat. Cancer Lett,
7: 299-305.
Muller G, Norpoth K, & Travenius S (1981) Quantitative determination
of bis(chloromethyl) ether (BCME) in the ppb range by using portable
air sample collectors. Int Arch Occup Environ Health, 48: 325-329.
National Research Council (NRC) (1977) Drinking-water and health -
Volume 1. Washington, DC, National Academy Press, p 711.
Nichols RW & Merritt RF (1973) Brief communication: relative
solvolytic reactivities of chloromethyl ether and bis(chloromethyl)
ether. J Natl Cancer Inst, 50: 1373-1374.
NIOSH (1974) The toxic substances list, 1974 edition. Cincinnati,
Ohio, National Institute for Occupational Safety and Health, p 134 (US
Department of Health, Education and Welfare Publication No. 74).
NIOSH (1984) Method 1004: Dichloroethyl ether. In: NIOSH manual of
analytical methods, 3rd ed. Cincinnati, Ohio, National Institute for
Occupational Health and Safety, pp 1004/1-1004/3.
Nishimura K, Miyashita K, Yoshida Y, Kuroda M, Matsumoto M, Matsumoto
K, Takeda S, & Hara I (1990) An epidemiological study of lung cancer
among workers exposed to bis(chloromethyl)ether. Jpn J Ind Health, 32:
448-453.
Norpoth K, Muller G, Zilius Z, Ulynec U, & Travenius SZM (1981)
Sensitive spectrophotometric determination of carcinogenic alpha
chloro ethers. Int Arch Occup Environ Health, 49: 151-155.
Norpoth K, Heger M, Muller G, Mohtashamipur E, Kemena A, & Witting C
(1986) Investigations of metabolism, genotoxic effects, and
carcinogenicity of 2,2-dichlorodiethyl ether. J Cancer Res Clin
Oncol, 112: 125-130.
Parkes D, Ganz C, Polinsky A, & Schultze J (1976) A simple gas
chromatographic method for the analysis of trace organics in ambient
air. Am Ind Hyg Assoc J, 37: 165-173.
Pasternack BS, Shore RE, & Albert RE (1977) Occupational exposure to
chloromethylethers. J Occup Med, 19: 741-746.
Pellizzari E, Erickson M, & Zweidinger R (1979) Formulation of a
preliminary assessment of halogenated compounds in man and
environmental media. Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances (EPA-560/13-79-006, PB 80-112170).
Perocco P, Bolognesi S, & Alberghini W (1983) Toxic activity of 17
industrial compounds on human lymphocytes cultured in vitro. Toxicol
Lett, 16: 69-76.
Piwoni M, Wilson J, Walters D, Wilson B, & Enfield C (1986) Behaviour
of organic pollutants during rapid infiltration of wastewater into
soil: I. Processes, definition, and characterization using a
microcosm. Hazard Waste Hazard Mater, 3: 43-55.
Quaghebeur DG, Hierneaux G, & DeWulf E (1986) Tracing a source of
pollution by determination of specific pollutants in surface- and
groundwater. Proceedings of the 4th European Symposium, Vienna,
Austria, 1985, pp 142-146.
Quinto I & Radman M (1987) Carcinogenic potency in rodents versus
genotoxic potency in E. coli: a correlation analysis for
bifunctional alkylating agents. Mutat Res, 181: 235-242.
Radding S, Holt B, Jones J, Jiu D, Mill T, & Hendry D (1977) Review of
the environmental fate of selected chemicals. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (EPA
560/5-77/003).
Reznick G, Wagner WW, & Atay Z (1977) Lung cancer following exposure
to bis(chloromethyl)ether: A case report. J Environ Pathol Toxicol, 1:
105-111.
Roe FJC (1985) Chloromethylation: Three lung cancer deaths in young
men. Lancet, 2: 268.
Rosen A, Skeel R, & Ettinger M (1963) Relationship of river water
odour to specific organic contaminants. J Water Pollut Control Fed,
35(6): 777-782.
Sakabe H (1973) Lung cancer due to exposure to
bis(chloromethyl)ether. Ind Health, 11: 145-148.
Sawicki E, Belsky T, Friedel R, Hyde D, Monkman J, Rasmussen R,
Ripperton L, & White L (1976) Analytical method for chloromethyl
methyl ether (CMME) and bis(chloromethyl) ether (BCME) in air. Health
Lab Sci, 13: 78-81.
Schrenk HH, Patty FA, & Yant WP (1933) Acute response of guinea pigs
to vapors of some new commercial organic compounds. Public Health Rep,
48: 1389-1398.
Sellakumar AR, Snyder CA, Soloman JJ, & Albert RE (1985)
Carcinogenicity of formaldehyde and hydrogen chloride in rats. Toxicol
Appl Pharmacol, 81: 401-406.
Sheldon L & Hites R (1978) Organic compounds in the Delaware River.
Environ Sci Technol, 12(10): 1188-1194.
Shirasu Y, Moriya M, Kato K, & Kada T (1975) Mutagenicity screening of
pesticides in microbial systems: II. Mutat Res, 31: 268-269.
Shooter KV (1975) Assays for phosphotriester formation in the reaction
of bacteriophage R17 with a group of alkylating agents. Chem-Biol
Interact, 11: 575-588.
Simmon VF, Kauhanen K, & Tardiff RG (1977a) Mutagenic activity of
chemicals identified in drinking water. In: Scott D, Bridges BA, &
Sobels FH ed. Progress in genetic toxicology. Amsterdam,
Elsevier/North-Holland, pp 249-258.
Simmon VF, Kauhanen K, & Tardiff RG (1977b) Mutagenic assays with
bis-(2-chloroethyl) ether. In : Plaa GL & Duncan WAM ed. Proceedings
of the First International Congress on Toxicology. New York, London,
San Francisco, Academic Press, p 31.
Sittig M (1981) Handbook of toxic and hazardous chemicals. Park
Ridge, New Jersey, Noyes Publications, 729 pp.
Smith CC, Cragg ST, Wolfe GF, & Weigel WW (1985) Investigation of the
metabolism of chlorinated hydrocarbons in sub-human species. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Health Effects Research Laboratory (PB85-152387).
Smyth HF Jr & Carpenter CP (1948) Further experience with the range
finding test in the industrial toxicology laboratory. J Ind Hyg
Toxicol, 30: 63-68.
Spector WS ed. (1956) Handbook of toxicology. Philadelphia,
Pennsylvania, W.B. Saunders Co., 671 pp.
Sram RJ, Samkova I, & Hola N (1983) High-dose ascorbic acid
prophylaxis in workers occupationally exposed to halogenated ethers. J
Hyg Epidemiol Microbiol Immunol, 27: 305-318.
Sram RJ, Landa K, & Roznickova I (1985) The use of cytogenetic
analysis of peripheral lymphocytes as a method for checking the level
of MAC in Czechoslovakia. Mutat Res, 147: 322.
Staples CA, Werner A, & Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environ Toxicol Chem, 4: 131-142.
Styles JA (1978) Mammalian cell transformation in vitro. Br J
Cancer, 37: 931-936.
Suffet I, Brenner L, & Cairo P (1980) GC/MS identification of trace
organics in Philadelphia drinking-water during a 2-year period. Water
Res, 14: 853-867.
Tabak H, Quave S, Mashni C, & Barth E (1981) Biodegradability studies
with organic priority pollutant compounds. J Water Pollut Control Fed,
53: 1503-1518.
Tabershaw IR, Utidjian HMD, & Kawahara BL (1977) Chemical hazards. In:
Occupational diseases: A guide to their recognition. Washington, DC,
United States Department of Health, Education and Welfare, p 131
(Publication DHEW (NIOSH) 77-181).
Theiss WC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States
drinking-waters by pulmonary tumor response in strain A mice. Cancer
Res, 37: 2717-2720.
Tou J & Kallos G (1974a) Study of aqueous HCl and formaldehyde
mixtures for formation of bis(chloromethyl) ether. Am Ind Hyg Assoc J,
35: 419-422.
Tou J & Kallos G (1974b) Kinetic study of the stabilities of
chloromethyl methyl ether and bis(chloromethyl) ether in humid air.
Anal Chem, 46: 1866-1869.
Tou J & Kallos G (1976) Possible formation of bis(chloromethyl) ether
from the reactions of formaldehyde and chloride ion. Anal Chem, 48:
958-963.
Tou J, Westover L, & Sonnabend L (1974) Kinetic studies of
bis(chloromethyl) ether hydrolysis by mass spectrometry. J Phys Chem,
78: 1096-1098.
Travenius S (1982) Formation and occurrence of bis(chloromethyl)
ether and its prevention in the chemical industry. Scand J Work
Environ Health, 8(suppl 3): 1-86.
Union Carbide (1968) Bis(chloromethyl)ether: Range finding toxicity
studies. Danbury, Connecticut, Union Carbide Corporation, 5 pp (Report
No. 31-85).
US EPA (1980) Ambient water quality criteria for chloroalkyl ethers.
Washington, DC, US Environmental Protection Agency, Environmental
Criteria Assessment Office, Office of Water Regulations and Standards,
Criteria and Standards Division (EPA-440/5-80-03, PB81-117418).
US EPA (1987a) Health and environmental effects document for
haloethers. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (ECAO-CIN-G014).
US EPA (1987b) Health effects assessment for bis(2-chloroethyl)
ether. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (EPA/600/8-88023,
PB88-179486).
US EPA (1990) Toxic chemical release inventory database for 1989.
Washington, DC, US Environmental Protection Agency and National
Library of Medicine
US NLM (1996) Hazardous substances database: Records for
bis(chloromethyl)ether, bis(2-chloroethyl)ether and chloromethyl
methyl ether. Bethesda, Maryland, National Library of Medicine.
van der Ven LGJ & Venema A (1979) Determination of
bis(chloromethyl)ether in air. Anal Chem, 51: 1016-1019.
van Duuren BL (1989) Comparison of potency of human carcinogens: vinyl
chloride, chloromethylmethyl ether and bis(chloromethyl)ether. Environ
Res, 49: 143-151.
van Duuren BL, Sivak A, Goldschmidt BM, Katz C, & Melchionne S (1969)
Carcinogenicity of halo-ethers. J Natl Cancer Inst, 43: 481-486.
van Duuren BL, Katz C, Goldschmidt BM, Frenkel K, & Sivak A (1972)
Carcinogenicity of halo-ethers: II. Structure-activity relationships
of analogues of bis(chloromethyl)ether. J Natl Cancer Inst, 48:
1431-1439.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. Toronto, Canada, Van Nostrand Reinhold Co., 1310
pp.
Weast RC & Astle M ed. (1985) CRC handbook of data on organic
compounds - Volume 1. Boca Raton, Florida, CRC Press.
Weisburger EK, Ulland BM, Nam J-M, Gart JJ, & Weisburger JH (1981)
Carcinogenicity tests of certain environmental and industrial
chemicals. J Natl Cancer Inst, 67: 75-88.
Weiss W (1976) Chloromethyl ethers, cigarettes, cough and cancer. J
Occup Med, 18: 194-199.
Weiss W (1980) The cigarette factor in lung cancer due to chloromethyl
ethers. J Occup Med, 22: 527-529.
Weiss W (1982) Epidemic curve of respiratory cancer due to
chloromethylethers. J Natl Cancer Inst, 69: 1265-1270.
Weiss W (1989) Lung cancer due to chloromethyl ethers: bias in cohort
definition. J Occup Med, 31: 102-105.
Xue SZ, Quian C, Tang GF, Wang ZQ, Zhou DH, Deng J, Zhao JZ, & He YP
(1988) Epidemiological investigations on the lung cancer among
chloro-methyl-ether exposures. In: Xue SZ & Liang Y. ed. Occupational
health in industrialization and modernization. Shanghai, Shanghai
Medical University Press, pp 75-80 (Bulletin of WHO Collaborating
Centre for Occupational Health, Shanghai, Volume 2).
Xue MI, Tang GF, Diao WX, & Cai MD (1996) Follow-up study on the
cohorts exposed to chloro-methyl ethers. Chin J Ind Med, 9(4) 225-227.
Yao CC & Miller GC (1979) Research study on bis(chloromethyl) ether
formation and detection in selected work environments. Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations and Field Studies (Report
PB83-174102).
Zajdela F, Croisy A, Barbin A, Malaveille C, Tomatis L, & Bartsch H
(1980) Carcinogenicity of chloroethylene oxide, an ultimate reactive
metabolite of vinyl chloride, and bis(chloromethyl)ether after
subcutaneous administration and in initiation promotion experiments in
mice. Cancer Res, 40: 352-356.
RÉSUMÉ ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le bis (2-chloroéthyl) éther (BCEE), le bis (chlorométhyl) éther
(BCME) et le chlorométhylméthyléther (CMME) sont des composés
chimiques qui appartiennent à un vaste groupe de produits connus sous
le nom de chloroalkyléthers. A la température ambiante, ces trois
éthers se présentent sous la forme de liquides volatils incolores à
l'odeur caractéristique. Ils sont dotés d'une forte tension de vapeur.
La solubilité dans l'eau du BCEE est de 1,7% et son coefficient de
partage entre l'octanol et l'eau est égal à 1,46. Le BCME et le CMME,
qui sont des alpha-chloroalkyléthers, sont des composés réactifs. Ils
subissent une hydrolyse rapide en milieu aqueux (avec une demi-vie ou
temps de demi-hydrolyse respectivement égale à 38 secondes et < 0,007
secondes); le BCEE, qui est un ß-chloroéthyléther, s'hydrolyse plus
lentement (demi-vie dans l'eau approximativement égale à 20 ans).
Les méthodes d'échantillonnage et d'analyse applicables au BCEE
dans l'eau et au CMME dans l'air sont décrites dans la littérature. On
peut citer comme exemples caractéristiques la chromatographie en phase
gazeuse (détection par capture d'électrons) ou le couplage
chromatographie en phase gazeuse-spectrométrie de masse.
2. Sources d'exposition humaine
On n'a pas trouvé dans l'environnement de BCEE, de BCME ou de
CMME qui soient d'origine naturelle. Les données de production
récentes se limitent aux Etats-Unis et au Canada. On a produit environ
10 000 tonnes de BCEE aux Etats-Unis en 1986 en vue d'une utilisation
comme solvant, pour la production de polymères ou encore dans un
certain nombre de processus industriels. Dans ce même pays, l'usage du
BCME est actuellement limité à certaines réactions chimiques
intermédiaires bien déterminées. On produit également du BCME en vue
de la fabrication de résines échangeuses d'ions ou autres types de
polymères ou encore comme solvant dans les réactions de
polymérisation. En Chine, on produit chaque année, environ 200 tonnes
de BCME comme intermédiaire dans la préparation d'un synergisant
d'insecticide, l'octachlorodipropyléther. Le CMME de qualité technique
contient de 1 à 8% de BCME.
3. Transport, distribution et transformation dans l'environnement
La mobilité et la distribution de ces chloroalkyléthers sont,
dans le cas du BCME et du CMME, déterminées par la grande réactivité
de ces composés et, dans le cas du BCEE, par la grande solubilité et
stabilité dans l'eau de cet éther. Le BCME et le CMME, des éthers
alpha-chloroalkylés, subissent une hydrolyse rapide en milieu aqueux
et sont rapidement décomposés par photolyse. En milieu aqueux, les
produits d'hydrolyse du BCME et du CMME sont constitués de
formaldéhyde et d'acide chlorhydrique dans le cas du premier et de
méthanol, de formaldéhyde et d'acide chlorhydrique dans le cas du
second. Parmi les produits de décomposition du BCME et du CMME,
figurent le chlorure d'hydrogène, le formaldéhyde et le
chlorométhylformiate, pour le premier, et le chlorométhyl- ainsi que
le méthylformiate, pour le second. Le BCEE est soluble dans l'eau; les
précipitations l'éliminent de l'atmosphère et il a tendance à rester
dans l'eau où il subit une très lente hydrolyse. En l'espace d'une
semaine, il s'évapore de la surface et se décompose en un peu moins
d'une journée sous l'action de processus abiotiques.
En raison du caractère extrêmement réactif des
alpha-chloroalkyléthers dans l'eau et dans l'air, on ne peut guère
s'attendre à trouver du CMME et du BCME dans l'environnement.
Toutefois, le BCEE peut présenter une plus grande persistance en
raison de la meilleure stabilité relative des ß-chloroalkyléthers.
4. Niveaux d'exposition dans l'environnement et exposition humaine
On ne dispose que de données limitées sur la concentration du
BCEE dans les divers compartiments de l'environnement. On l'a mis en
évidence dans l'air, mais sans procéder à un dosage; aux Etats-Unis,
on en a trouvé jusqu'à 42 µg/litre dans de l'eau de boisson. Les
concentrations rapportées dans la littérature en ce qui concerne les
eaux de surface vont de 0,001 µg/litre dans une décharge industrielle
de gypse en Belgique, à 840 µg/litre dans une autre décharge située
aux Etats-Unis. On a mesuré des concentrations encore plus élevées
dans les eaux de lessivage d'une décharge contrôlée. On n'a pas
connaissance de la teneur des denrées alimentaires en BCEE, mais on ne
pense pas qu'il puisse y avoir bioaccumulation.
On ne dispose d'aucune donnée sur la concentration du BCME et du
CMME dans les divers compartiments de l'environnement.
Si l'on se base sur la concentration maximale de BCEE rapportée
pour l'eau de boisson, soit 42 µg/litre, un être humain moyen pesant
64 kg et consommant 1,4 litres d'eau par jour ingérerait
quotidiennement environ 0,01 µg de ce produit par kg de poids
corporel, plus une quantité indéterminée provenant de sources
inconnues. Il est impossible d'évaluer la dose quotidienne de BCME et
de CMME ingérée à partir de sources environnementales. Toutefois,
comme ces deux composés ne persistent pas dans l'environnement, il est
probable que l'exposition humaine à ces produits est très faible.
En s'appuyant sur des données limitées et assez anciennes, on
pense que les ouvriers travaillant à la production de plastiques et de
fibres textiles ont pu être exposés, dans l'air des lieux de travail,
à des concentrations de BCME comprises entre 1,2 et 72,9 µg/m3.
Cependant, une récente étude, effectuée dans une usine de production
de résines plastiques, fait état d'une exposition professionnelle
moyenne allant de 2,4 à 20,6 µg/m3. Selon d'autres travaux, la
concentration de BCME ne dépasserait pas 0,01 µg/m3.En Chine,
l'exposition au BCME a été plus élevée que ces chiffres jusqu'en 1975
et alle persiste encore, quoiqu'à un niveau moindre, dans les usines
qui produisent de l'octachlorodipropyléther. Il y exposition de la
population générale au BCME et au CMME là où l'on fait beaucoup brûler
de serpentins anti-moustiques qui en contiennent comme synergisants.
La concentration la plus élevée de BCEE signalée aux Etats-Unis
dans les effluents industriels se situe entre 8 et 170 µg/litre; dans
le cas d'eaux provenant du lessivage de décharges contrôlées
industrielles et municipales, la concentration était de 12 400
µg/litre.
5. Cinétique et métabolisme
On ne dispose pas de données quantitatives sur la cinétique et le
métabolisme du BCEE, du BCME et du CMME chez l'homme. On estime
toutefois que, même si le BCME et le CMME doivent, en principe, être
rapidement hydrolysés in vivo, pour donner, le premier, du
formaldéhyde et de l'acide chlorhydrique et le second, du
formaldéhyde, du méthanol et de l'acide chlorhydrique, il se produit
sans doute une alkylation.
D'après des données limitées, du BCEE radiomarqué administré à
des rats par inhalation ou gavage subit une résorption rapide. Après
administration par gavage, on a constaté que l'organisme des rats
n'avait retenu que moins de 3% de la dose initiale au bout de 24
heures.
Chez le rat, le BCEE est rapidement métabolisé. Son principal
métabolite est l'acide thiodiglycolique (TDGA). Chez des rats qui
avaient reçu par gavage une dose unique de 14C-BCEE, on a constaté
que 12% environ de la radioactivité absorbée se trouvait sous la forme
de 14CO2.
Chez le rhésus comme chez le rat, le BCEE est rapidement éliminé.
Chez des rhésus auxquels on avait administré du 14C-BCEE par la voie
orale, on a retrouvé moins de 2% de la radioactivité initiale dans les
matières fécales 72 h après l'administration. Chez des rats, c'est
approximativement 2,3% de la radioactivité initiale qui ont été
retrouvés dans les tissus et les matières fécales, 48 h après
l'administration. Après avoir administré par gavage du 14C-BCEE à des
rats, on a retrouvé plus de 50% de la radioactivité dans les urines et
dans l'air expiré 12 heures après l'administration. Moins de 2% de la
radioactivité présente dans l'air expiré correspondaient au composé
initial.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
Absorbé par la voie orale, par inhalation ou par voie
transcutanée, le BCEE peut provoquer des intoxications aiguës. Les
valeurs de la DL50 dont il est fait état dans la littérature en cas
d'exposition d'animaux par la voie orale, vont de 75 à 215 mg/kg de
poids corporel. Le BCME et le CMME provoquent également des
intoxications aiguës lorsqu'ils sont absorbés par voie orale ou par
inhalation. Les valeurs de la CL50 pour des animaux de laboratoire
exposés par la voie respiratoire à du BCME ou à du CMME, vont de 25 à
48 mg/m3 dans le cas du premier composé et de 182 à 215 mg/m3 dans
le cas du second.
L'exposition d'animaux de laboratoire par la voie respiratoire à
une seule mais forte concentration de BCEE (>320 mg/m3), a provoqué
une irritation oculaire ainsi qu'une congestion, un oedème et des
hémorragies pulmonaires. Pendant l'inhalation de BCME, on a noté une
irritation des yeux et des voies respiratoires ainsi qu'une bronchite
nécrosante. L'application du produit sur la peau a donné lieu à un
érythème et à une nécrose. L'instillation dans les yeux provoque une
nécrose cornéenne. On a observé des effets analogues après exposition
au CMME.
On a constaté un accroissement de la mortalité et une hyperplasie
trachéenne chez des rats et des hamsters exposés par la voie
respiratoire à du BCME à la dose de 4,7 mg/m3. Des résultats
analogues ont été obtenus à plusieurs reprises chez des rats exposés à
du CMME par la voie respiratoire à raison de 3,3 ou de 33 mg de
composé par m3.
En général, les épreuves de mutagénicité in vitro ont donné des
résultats positifs avec le BCEE, le BCME et le CMME. Toutefois, les
résultats sont difficiles à interpréter en raison de l'absence de
détails dans les comptes rendus de ces expériences. Selon la
littérature, le BCME et le CMME provoquent in vitro un accroissement
de la synthèse non programmée de l'ADN et le BCME augmente la
proportion de cellules transformées, également in vitro. Dans de
petits groupes de souris mâles appartenant à deux souches de souris
hybrides F1 (de même que chez les femelles d'une des souches F1),
qui avaient reçu du BCEE par voie orale (dose pondérée par rapport au
temps égale à 41,3 mg/kg p.c. sur une période de 18 mois), on a
observé une augmentation significative de l'incidence des tumeurs
hépatiques (bénignes et malignes) par rapport aux animaux témoins non
exposés. Quatre autres études de portée plus limitée effectuées sur
des rats et des souris qui recevaient le composé par gavage, en
injections sous-cutanées ou intrapéritonéales ou par badigeonnage
cutané, n'ont pas permis de confirmer ces résultats.
Les études de cancérogénicité effectuées sur des animaux de
laboratoire (souris ou rats) exposés à du BCME, ont révélé un
accroissement significatif de l'incidence des adénomes pulmonaires et
autres tumeurs des voies respiratoires. Chez la souris, on a également
obtenu des indices d'une élévation de l'incidence des tumeurs
pulmonaires.
Les études effectuées sur le CMME ont révélé, chez le rat, un
accroissement de l'incidence des métaplasies trachéennes et des
hyperplasies bronchiques qui dépendait de la dose. Toutefois, les
résultats des épreuves de cancérogénicité menées sur l'animal n'ont
pas donné de résultats concluants.
On ne dispose d'aucun renseignement concernant les effets
toxiques éventuels du BCEE, du BCME et du CMME sur la fonction de
reproduction, le développement, le système immunitaire et le système
nerveux.
7. Effets sur l'homme
On a constaté que le BCEE avait un effet irritant sur l'oeil et
les fosses nasales aux concentrations supérieures à 150 mg/m3 après
exposition de courte durée.
On n'a pas trace d'études épidémiologiques sur les effets à long
terme d'une exposition au BCEE.
Une association entre l'exposition d'ouvriers à du BCME ou du
CMME et un risque accru de cancer du poumon a été mise en évidence
dans 8 études épidémiologiques. Les travailleurs exposés à du CMME de
qualité technique l'étaient probablement aussi à du BCME. Les tumeurs
prédominantes observées chez les ouvriers exposés étaient des
carcinomes à petites cellules, tout à fait distincts des carcinomes
essentiellement spinocellulaires qui s'observent. chez les fumeurs. Il
s'agissait d'une forte association, avec des rapports comparatifs de
mortalité qui allaient jusqu'à 2,1. Le type de cancer pulmonaire, la
période de latence et l'âge moyen d'apparition de la tumeur chez les
ouvriers exposés au BCME ou au CMME présentaient une cohérence
remarquable. Dans le cas du CMME, on est également fondé à penser
qu'il y a une relation positive entre l'expression qualitative de
l'exposition et la mortalité par cancer du poumon.
Lors d'une exposition professionnelle, et même à des
concentrations de 0,01 µg/m3 de BCME ou de 20 µg/m3 de CMME, on a
constaté une augmentation de la fréquence des aberrations
chromosomiques dans les lymphocytes du sang périphérique des ouvriers
exposés.
On ne dispose d'aucun renseignement concernant les effets du BCME
ou du CMME sur la fonction de reproduction, le développement, le
système nerveux et le système immunitaire chez l'homme.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Peu d'études ont été consacrées aux effets du BCEE sur les êtres
vivants dans leur milieu naturel; la plupart des travaux se limitent
aux espèces aquatiques. Ainsi la CL50 à 7 jours pour le guppy est
égale à 56,9 mg/litre; pour d'autres poissons on a trouvé une CL50 à
96 h de 600 mg/litre et en ce qui concerne les invertébrés, on a fait
état d'une CL50 à 48 h égale à 240 mg/litre pour Daphnia magna.
Il n'y a pas eu d'inhibition de l'activité microbienne anaérobie
en présence de BCEE à des concentrations allant jusqu'à 100 mg/litre
et on a trouvé une CL10 de 600 µg/litre pour des microorganismes
colonisant des bassins de stabilisation.
On ne dispose d'aucune donnée concernant les effets toxiques que
le BCME ou le CMME pourraient exercer sur les êtres vivants dans leur
milieu naturel.
9. Conclusions
9.1 BCEE
- L'exposition des organismes terrestres au BCEE est jugée
négligeable du fait que ce composé n'est que lentement libéré
dans l'environnement et qu'il ne subsiste que peu de temps dans
l'atmosphère.
- Le BCEE persiste davantage dans l'eau, mais la concentration la
plus élevée mesurée dans les eaux de surface est beaucoup plus
faible (environ cinq ordres de grandeur) que celle qui se révèle
toxique pour le guppy, l'espèce la plus sensible selon les études
toxicologiques.
- En raison de l'absence de données concernant la concentration du
BCEE dans un certain nombre de compartiments de l'environnement
auxquels l'homme est exposé, il n'est pas possible de donner une
estimation quantitative de la dose totale de ce composé absorbée
au cours d'une journée.
- On ne dispose que de données limitées sur la toxicité du BCEE
pour l'homme. On ne trouve pas de relation des effets sur la
reproduction et le développement qui auraient pu être observés
chez des animaux de laboratoire. Par ailleurs, aucune des études
à long terme effectuées sur les animaux de laboratoire n'est
d'une qualité suffisante pour que l'on puisse en tirer des
données quantitatives sur la cancérogénicité du BCEE ou sur les
effets toxiques à longue échéance que ce composé pourrait
produire.
- Faute de données toxicologiques et cancérogénétiques suffisantes,
la prudence commande de faire en sorte que l'exposition humaine
soit réduite au minimum.
9.2 BCME et CMME
- Au cas ou ces composés pénétreraient dans l'environnement, ils
subiraient rapidement une hydrolyse et une photo-oxydation. On ne
possède pas de données sur la concentration du BCME et du CMME
dans l'environnement.
- Le BCME et le CMME de qualité technique (qui contient du BCME)
sont des substances dont la cancérogénicité pour l'homme est
prouvée. Ils se sont d'ailleurs tous les deux révélés
cancérogènes pour les animaux de laboratoire. Ces deux composés
provoquent des aberrations chromosomiques chez les travailleurs
qui leur sont exposés de par leur profession. Il faut éviter
toute exposition professionnelle et toute exposition de la
population générale à ces composés.
- Compte tenu de la destinée de ces composés dans l'environnement
et de l'absence d'exposition, il n'y aucune raison de craindre
des effets nocifs sur les organismes terrestres ou aquatiques.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El bis(2-cloroetil)éter (BCEE), el bis(clorometil)éter (BCME) y
el clorometilmetiléter (CMME) son sustancias químicas de una clase
amplia conocida como cloroalquiléteres. Los tres éteres son líquidos
volátiles incoloros a temperatura ambiente con olores característicos.
La presión de vapor de estos tres compuestos es alta. La solubilidad
del BCEE es del 1,7% en agua, y su coeficiente de reparto octanol/
agua es de 1,46. Los alpha-cloroalquiléteres BCME y CMME son
compuestos reactivos, que se hidrolizan con rapidez en medios acuosos
(con semividas de alrededor de 38 segundos y <0,007 segundos,
respectivamente); la hidrólisis del ß-cloroéter BCEE, más estable, es
más lenta (con una semivida en agua de unos 20 años).
Se han descrito métodos de muestreo y analíticos para el BCEE en
el agua y para el BCME y el CMME en el aire. Normalmente la
determinación se efectúa por cromatografía de gases (CG-captura de
electrones) o CG-espectrometría de masas.
2. Fuentes de exposición humana
No se han identificado fuentes naturales de BCEE, BCME o CMME en
el medio ambiente. Los datos recientes de producción disponible son
limitados y corresponden solamente a los Estados Unidos y el Canadá.
En 1986 se produjeron en los Estados Unidos alrededor de 104
toneladas de BCEE para utilizarlo como disolvente y en la producción
de polímeros y en varios procesos industriales. Las aplicaciones
industriales del BCME están actualmente limitadas en los Estados
Unidos a reacciones químicas intermedias específicas. También se ha
producido BCME para utilizarlo en la obtención de resinas de
intercambio iónico, en la fabricación de otros polímeros y como
disolvente en reacciones de polimerización. En China se producen unas
200 toneladas al año de BCME como producto intermedio en la
fabricación de octaclorodipropiléter, un insecticida sinérgico. El
CMME de calidad técnica contiene del 1% al 8% de BCME.
3. Transporte, distribución y transformación en el medio ambiente
En la movilidad y distribución de los cloroalquiléteres
seleccionados influyen tanto la elevada radiactividad del BCME y del
CMME como la solubilidad en agua y la estabilidad del BCEE. Los
alpha-cloroalquiléteres BCME y CMME se hidrolizan con rapidez en
medios acuosos y se degradan en poco tiempo por fotolisis. En medios
acuosos, los productos hidrolíticos del BCME y del CMME son
formaldehído y ácido clorhídrico, y metanol, formaldehído y ácido
clorhídrico, respectivamente. Los productos de descomposición del BCME
y el CMME en el aire son ácido clorhídrico, formaldehído y formato de
clorometilo, y formato de clorometilo y de metilo, respectivamente. El
BCEE es soluble en agua; la lluvia lo elimina de la atmósfera y tiende
a mantenerse en el agua, con una hidrólisis muy lenta. El BCEE se
evapora del agua superficial en una semana y se degrada en poco más de
un día en la atmósfera mediante procesos abióticos.
Debido al carácter muy reactivo de los alpha-cloroalquiléteres en
el agua y en el aire, no es previsible la presencia de CMME y BCME en
el medio ambiente; sin embargo, el BCEE puede ser persistente, debido
a la estabilidad relativa de los ß-cloroalquiléteres.
4. Niveles ambientales y exposición humana
Los datos disponibles sobre los niveles de BCEE en medios
ambientales son limitados. Se ha identificado en el aire, pero sin
determinación cuantitativa; se han encontrado niveles de hasta 0,42
µg/litro en el agua potable en los Estados Unidos. Los niveles
notificados de BCEE en el agua fréatica han oscilado entre 0,001
µg/litro en un vertedero de yeso industrial en Bélgica y 840 µg/litro
cerca de un vertedero en los Estados Unidos. Se han medido
concentraciones más elevadas en productos de lixiviación de
vertederos. No se dispone de información sobre los niveles de BCEE en
los productos alimenticios, pero se supone que no se produce
bioacumulación.
No se dispone de datos cuantitativos sobre los niveles de BCME o
CMME en el medio ambiente.
Tomando como base el nivel máximo notificado de BCEE en el agua
potable, es decir, 0,42 µg/litro, la persona de tipo medio (64 kg) que
consuma 1,4 litros/día tendrá una ingesta aproximada de 0,01 µg/kg de
peso corporal al día de esta procedencia, con cantidades desconocidas
de otras fuentes del medio ambiente. No se puede hacer ninguna
estimación de la ingesta diaria de BCME y CMME a partir de fuentes del
medio ambiente. Sin embargo, considerando la falta de persistencia del
BCME y del CMME en el medio ambiente es probable que la exposición
humana media a estos compuestos sea muy baja.
En función de datos limitados más antiguos, los trabajadores de
industrias relacionadas con los plásticos y la producción textil
podrían haber estado expuestos a cantidades comprendidas entre 1,2 y
72,9 µg de BCME/m3 en el aire del lugar de trabajo. Sin embargo, en
un estudio reciente de una fábrica de resina se señalaron exposiciones
medias en el trabajo comprendidas entre 2,4 y 20,6 µg/m3. En los
datos de otros estudios se indicaron niveles de BCME muy bajos de 0,01
µg/m3. En China se producía hasta 1975 una exposición más alta en el
trabajo al BCME, y sigue existiendo con un nivel menor en la
fabricación de octaclorodipropiléter. La población general está
expuesta al BCME y al CMME cuando se producen en la quema generalizada
de este producto sinérgico en serpentines fumigantes de mosquitos.
Las concentraciones más elevadas notificadas de BCEE en los
Estados Unidos en efluentes industriales son de 8 a 170 µg/litro, y en
los productos de lixiviación de vertederos municipales e industriales
de 12 400 µg/litro.
5. Cinética y metabolismo
No se dispone de información cuantitativa sobre la cinética y el
metabolismo del BCEE, del BCME y del CMME en el ser humano. Sin
embargo, se supone que, aunque el BCME y el CMME se hidrolicen con
rapidez in vivo en los tejidos a formaldehído y ácido clorhídrico, y
a metanol, formaldehído y ácido clorhídrico respectivamente, debe
haber actividad de alquilación.
Los datos limitados que se conocen indican que el BCEE radiactivo
administrado a ratas por inhalación o con sonda se absorbe con
rapidez. A las 48 horas de la administración por sonda se retenía
menos del 3% de la radiactividad.
El BCEE se metaboliza fácilmente en ratas. El principal
metabolito es el ácido tiodiglicólico. Después de administrar una
dosis única por sonda de [14C]-BCEE, alrededor del 12% de la
radiactividad administrada estaba presente en forma de 14CO2.
El BCEE se elimina con rapidez tanto en ratas como en monos
resus. A las 72 horas de la administración oral de [14C]-BCEE se
recuperó menos del 2% de la radiactividad en las heces de los monos; a
las 48 horas de la administración, se encontró alrededor del 2,3% de
la radiactividad administrada en los tejidos o las heces de ratas; más
del 50% de la radiactividad se recuperó en la orina y en el aire
exhalado a las 12 horas de la administración a ratas con sonda de una
dosis de [14C]-BCEE. De la radiactividad expirada a través de los
pulmones, correspondió al compuesto original menos del 2%.
6. Efectos en animales de laboratorio y en sistemas de prueba
in vitro
El BCEE tiene toxicidad aguda por vías de exposición oral,
inhalación o cutánea. Los valores notificados de la DL50 para la
exposición oral de especies animales al BCEE oscilan entre 75 y 215
mg/kg de peso corporal. El BCME y el CMME tienen toxicidad aguda por
inhalación o ingestión. Los valores de la CL50 notificados para la
exposición de animales de laboratorio por inhalación al BCME o al CMME
oscila entre 25 y 48 mg/m3 y entre 182 y 215 mg/m3, respectivamente.
La exposición de animales de laboratorio por inhalación a una
dosis elevada única de BCEE (>320 mg/m3) provocó irritación ocular,
además de congestión, edema y hemorragia pulmonar. Durante la
inhalación de BCME, se observó irritación ocular y de las vías
respiratorias, así como bronquitis necrosante. La aplicación cutánea
dio lugar a la aparición de eritema y necrosis, y la aplicación en el
ojo indujo necrosis corneal. Tras la exposición al CMME se observaron
efectos análogos.
En ratas y hámsteres se observó un aumento de la mortalidad y la
hiperplasia traqueal después de la exposición por inhalación múltiple
a 4,7 mg de BCME/m3. Se observaron efectos análogos en ratas
expuestas repetidamente por inhalación a 3,3 o 33 mg de CMME/m3.
En general, se obtuvieron resultados positivos en las pruebas de
mutagenicidad del BCEE, del BCME y del CMME in vitro. Sin embargo,
la interpretación de los resultados resulta difícil, debido a la falta
de detalles en los informes disponibles. Se ha notificado que el BCME
y el CMME aumentan la síntesis no programada de ADN in vitro, y el
BCME elevó el nivel de células transformadas en pruebas in vitro.
En pequeños grupos de machos pertenecientes a dos estirpes de
ratones F1 híbridos (y en hembras de una estirpe F1) tratados por
vía oral con BCEE (dosis media ponderada por el tiempo de 41,3 mg/kg
de peso corporal durante 18 meses), se registró un aumento
significativo de la incidencia de hepatomas (hepatomas benignos y
tumores malignos combinados) en comparación con los testigos no
tratados. En otros cuatro estudios limitados con ratas y ratones en
los que se utilizó la administración oral con sonda, la inyección
subcutánea o intraperitoneal y la aplicación sobre la piel no se
confirmaron esos resultados.
Los estudios de carcinogenicidad en animales experimentales
(ratones y ratas) expuestos a BCME pusieron de manifiesto una
incidencia significativamente elevada de adenomas pulmonares y tumores
de las vías respiratorias. En ratones, tras la exposición por
inhalación también se observaron pruebas de una incidencia elevada de
tumores pulmonares.
Los estudios realizados con CMME han puesto de manifiesto una
mayor incidencia de metaplasia traqueal e hiperplasia bronquial
dependiente de la dosis en ratas. Sin embargo, los resultados de las
biovaloraciones de carcinogenicidad en estudios con animales no han
sido concluyentes.
No hay información disponible relativa a la toxicidad
reproductiva, en el desarrollo, inmunológica o neurológica del BCEE,
del BCME o del CMME.
7. Efectos en el ser humano
Se ha comprobado que el BCEE irrita los ojos y los orificios
nasales de las personas en concentraciones >150 mg/m3 tras una
exposición breve.
No se tienen noticias de estudios epidemiológicos sobre los
efectos de la exposición prolongada al BCEE.
En ocho estudios epidemiológicos, la exposición de los
trabajadores al BCME (CMME) se relacionó con un aumento del riesgo de
cáncer de pulmón. Los trabajadores expuestos al CMME de calidad
comercial probablemente también estuvieron expuestos al BCME. Los
tumores predominantes en los trabajadores expuestos fueron carcinomas
de células pequeñas, bastante distintos de los que son principalmente
de células escamosas y que suelen aparecer en los fumadores. Hubo una
relación clara entre la exposición al BCME (CMME) y el cáncer de
pulmón, con unas razones de mortalidad normalizada que llegaban hasta
21. El tipo de cáncer de pulmón, el período de latencia y la edad
media de aparición de los tumores de pulmón en los trabajadores
expuestos al BCME (CMME) han sido básicamente invariables. Para el
CMME hay también pruebas de una relación positiva entre una medida
cualitativa de la exposición y la mortalidad debida a cáncer de
pulmón.
En el curso de una exposición en el trabajo, incluso
concentraciones de 0,01 µg de BCME/m3 y de 20 µg de CMME/m3
aumentaron la frecuencia de aberraciones cromosómicas en los
linfocitos periféricos de los trabajadores expuestos.
No se dispone de información relativa a los efectos neurológicos,
inmunológicos, en el desarrollo o reproductivos del BCME o del CMME en
el ser humano.
8. Efectos en otros organismos en el laboratorio y en condiciones
naturales
Son pocos los estudios que se han realizado sobre los efectos del
BCEE en los organismos del medio ambiente; la mayoría se limitan a
especies acuáticas. Para el BCEE, se ha notificado un valor de la
CL50 en siete días en Lebistes reticulatus de 56,9 mg/litro, una
CL50 en peces en 96 horas de 600 mg/litro y una CL50 en 48 horas en
Daphnia magna de 240 mg/litro.
La actividad microbiana anaerobia no se vio inhibida en
concentraciones de BCEE de hasta 100 mg/litro, y se ha notificado una
CL10 de 600 µg/litro en el caso de microorganismos indígenas en
estanques de estabilización de desechos.
No hay información sobre los efectos toxicológicos del BCME y del
CMME en los organismos del medio ambiente.
9. Conclusiones
9.1 BCEE
- Se considera que la exposición de los organismos terrestres al
BCEE es insignificante, debido a la escasa tasa de liberación y
su breve persistencia en la atmósfera.
- Aunque es más persistente en el agua, la concentración más
elevada notificada de BCEE en agua superficial es aproximadamente
cinco veces inferior a la concentración con la que se ha
comprobado que induce efectos adversos en Lebistes reticulatus,
que es la especie acuática más sensible identificada en los
estudios de toxicidad realizados.
- Debido a la falta de información disponible sobre las
concentraciones de BCEE en varios tipos de medios a los cuales
está expuesto el ser humano, no es posible estimar
cuantitativamente la ingesta diaria total de BCEE.
- Los datos disponibles sobre la toxicidad del BCEE en el ser
humano son limitados. No se ha encontrado información sobre los
efectos del BCEE en el desarrollo y la reproducción en animales
de laboratorio, y ninguno de los estudios de larga duración en
animales de laboratorio tiene suficiente calidad para
proporcionar información cuantitativa acerca del potencial del
BCEE para provocar cáncer o sobre los efectos toxicológicos
producidos por la exposición prolongada a esta sustancia.
- En ausencia de datos toxicológicos y de carcinogenicidad
adecuados, es conveniente reducir al mínimo la exposición humana
al BCEE.
9.2 BCME y CMME
- En el caso de que estas sustancias se incorporaran al medio
ambiente, se degradarían con rapidez por hidrólisis y
fotooxidación. No se han identificado datos relativos a las
concentraciones de BCME y CMME en el medio ambiente natural.
- Está demostrado que el BCME y el CMME de calidad técnica (que
contiene BCME) son carcinógenos para el ser humano. Además, ambos
productos químicos son carcinógenos en animales de laboratorio.
Los dos provocan aberraciones cromosómicas en los trabajadores
expuestos en el trabajo. Se debe eliminar la exposición en el
trabajo y de la población general a estos compuestos.
- Tomando como base el destino de estas sustancias en el medio
ambiente y la falta de exposición, no hay motivo para suponer que
se produzcan efectos adversos en organismos acuáticos y
terrestres.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED
CHLOROALKYL ETHERS
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton,
Surrey, United Kingdom
Dr R. Chhabra, Division of Intramural Research, Environmental
Toxicology Program, Toxicology Branch, National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA (Chairman)
Dr H. Ellisa, Epidemiology Department, Rohm & Haas, Bristol,
Pennsylvania, USA
Dr B. Gilbert, FarManguinhos, FIOCRUZ, Institute of
Pharmaceutical Technology, Ministry of Health, Rio de Janeiro,
Brazil
Professor M. Jakubowski, Occupational and Environmental
Hygiene Division, Nofer Institute of Occupational Medicine, Lodz,
Poland
Dr S.K. Kashyap, National Institute of Occupational Health,
Meghani Nagar, Ahmedabad, India (Vice-chairman)
Dr R. Liteplo, Environmental Health Directorate, Health Protection
Branch, Environmental Health Centre, Ottawa, Ontario, Canada
(Co-rapporteur)
Dr E.E. McConnell, Laurdane Estates, Raleigh, North Carolina,
USA
Dr H. Naito, Ibaraki Prefecture University of Health Sciences,
Amimachi, Inashikigun, Japan
Dr W. Popp, Universitätsklinikum Essen, Institut für Hygiene und
Arbeitsmedizin, Essen, Germany
Dr R. Sram, Laboratory of Genetic Ecotoxicology, c/o Institute of
Experimental Medicine, Prague, Czech Republic
a Invited, but unable to attend.
Dr Shou-Zheng Xue, Toxicology Programme, Shanghai Medical
University, Shanghai, China
Secretariat
Dr G.C. Becking, IPCS/IRRU, World Health Organization,
Research Triangle Park, North Carolina, USA
Ms R. Gomes, Health Canada, Environmental Health Directorate,
Tunney's Pasture, Ottawa, Ontario, Canada (Co-rapporteur)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED
CHLOROALKYL ETHERS
A WHO Task Group on Environmental Health Criteria for Selected
Chloroalkyl Ethers met at the British Industrial Biological Research
Association (BIBRA) Toxicology International, Carshalton, Surrey,
United Kingdom, from 18 to 23 March 1996. Dr D. Anderson opened the
meeting and welcomed the participants on behalf of the host institute.
Dr G.C. Becking, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS and the three cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks to human health
and the environment from exposure to selected chloroalkyl ethers.
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contribution to the IPCS.
The first and second drafts of this monograph were prepared by Dr
R. Liteplo and Ms R. Gomes, Health Canada, Ottawa. The second draft
incorporated the comments received following circulation of the first
draft to the IPCS contact points for environmental health criteria
monographs.
Dr G.C. Becking (IPCS Central Unit, Interregional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation of the document
are gratefully acknowledged.
ABBREVIATIONS
BCEE bis(2-chloroethyl) ether
BCME bis(chloromethyl) ether
CMME chloromethyl methyl ether
MTD maximum tolerated dose
PMA phorbol myristate acetate
TDGA thiodiglycolic acid
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, analytical methods
Bis(2-chloroethyl) ether (BCEE), bis(chloromethyl) ether (BCME)
and chloromethyl methyl ether (CMME) are chemicals from a large class
known as chloroalkyl ethers. The three ethers are colourless volatile
liquids at room temperature having characteristic odours. The vapour
pressure of these three compounds is high. The solubility of BCEE is
1.7% in water and its octanol/water partition coefficient is 1.46. The
alpha-chloroalkyl ethers BCME and CMME are reactive compounds,
hydrolysing rapidly in aqueous media (with half-lives of approximately
38 seconds and <0.007 seconds, respectively); hydrolysis of the more
stable ß-chloroether BCEE is slower (with a half-life in water of
about 20 years).
Sampling and analytical methods have been described for BCEE in
water and for BCME and CMME in air. Typically, determination is by
gas chromatography (GC-electron capture) or GC mass spectrometry.
1.2 Sources of human exposure
Natural sources of BCEE, BCME or CMME in the environment have not
been identified. The recent production data available are limited and
confined to the USA and Canada. Approximately 104 tonnes of BCEE were
produced in the USA in 1986 for use as a solvent and in the production
of polymers and several industrial processes. Industrial uses of BCME
are currently restricted in the USA to specific intermediate chemical
reactions. BCME has also been produced for use in the production of
ion exchange resins, manufacture of other polymers, and as a solvent
in polymerization reactions. In China, some 200 tonnes of BCME are
produced annually as an intermediate in the manufacture of the
insecticide synergist, octachlorodipropyl ether. Technical grade CMME
contains from 1 to 8% BCME.
1.3 Environmental transport, distribution and transformation
The mobility and distribution of the selected chloroalkyl ethers
is influenced by the high reactivity of BCME and CMME and the water
solubility and stability of BCEE. The alpha-chloroalkyl ethers BCME
and CMME are hydrolysed rapidly in aqueous media and degraded quickly
by photolysis. In aqueous media, the hydrolytic products of BCME and
CMME are formaldehyde and hydrochloric acid, and methanol,
formaldehyde and hydrochloric acid, respectively. The decomposition
products of BCME and CMME in air include hydrogen chloride,
formaldehyde and chloromethylformate, and chloromethyl and methyl
formate, respectively. BCEE is soluble in water; rainfall removes it
from the atmosphere and it tends to remain in water with very slow
hydrolysis. BCEE evaporates from surface water within a week and is
degraded in a little more than a day in the atmosphere by abiotic
processes.
Owing to the highly reactive nature of the alpha-chloroalkyl
ethers in water and air, CMME and BCME are not expected to be present
in the environment; however BCEE may be persistent due to the relative
stability of ß-chloroalkyl ethers.
1.4 Environmental levels and human exposure
Only limited data on levels of BCEE in environmental media are
available. It has been identified in air but not quantified; levels up
to 0.42 µg/litre have been found in drinking-water in the USA.
Reported levels of BCEE in groundwater have ranged from 0.001 µg/litre
at an industrial gypsum waste disposal site in Belgium to 840 µg/litre
near a waste disposal site in the USA. Higher concentrations have been
measured in landfill leachates. Information on levels of BCEE in
foodstuffs is not available, but bioaccumulation is not expected to
occur.
Quantitative data on levels of BCME or CMME in environmental
media are not available.
Based on the maximum reported level of BCEE in drinking-water,
i.e., 0.42 µg/litre, the average human (64 kg) consuming 1.4
litres/day would have an intake of about 0.01 µg/kg body weight per
day from this source, with unknown amounts from other environmental
sources. No estimates can be made on the daily intake of BCME and CMME
from environmental sources. However, based upon the lack of
persistence of BCME and CMME in the environment, average human
exposure to these compounds is likely to be very low.
Based on limited older data, workers in industries related to
plastics and textile production could have been exposed to between 1.2
and 72.9 µg BCME/m3 in workroom air. However, a recent study of a
resin-manufacturing plant reported average occupational exposures
ranging from 2.4 to 20.6 µg/m3. Data from other studies reported
levels of BCME as low as 0.01 µg/m3. Higher occupational exposure to
BCME occurred in China up until 1975 and still occurs on a lower level
in the manufacture of octachlorodipropyl ether. General population
exposure to BCME and CMME occurs where they are produced by the
widespread burning of this synergist in mosquito coils.
The highest reported concentrations of BCEE in the USA for
industrial effluents are 8 to 170 µg/litre and for municipal and
industrial waste landfill leachates 12 400 µg/litre.
1.5 Kinetics and metabolism
Quantitative information on the kinetics and metabolism of BCEE,
BCME and CMME in humans is not available. However, it is anticipated
that although in vivo BCME and CMME would be rapidly hydrolysed in
tissues to formaldehyde and hydrogen chloride, and methanol,
formaldehyde and hydrogen chloride, respectively, there should be
alkylation activity.
Limited data show that radioactive BCEE administered to rats by
inhalation or gavage is rapidly absorbed. Less than 3% of the
radioactivity was retained 48 h after gavage dosing.
BCEE is readily metabolized in rats. The principal metabolite is
thiodiglycolic acid (TDGA). After rats were given a single gavage dose
of [14C]-BCEE, approximately 12% of the administered radioactivity
was present as 14CO2.
BCEE is eliminated quickly in both rats and rhesus monkeys. Less
than 2% of the radioactivity was recovered in the faeces of monkeys 72
h after oral administration of [14C]-BCEE; approximately 2.3% of the
administered radioactivity was found in rat tissues or faeces 48 h
after dosing. Over 50% of the radioactivity was recovered in the urine
and exhaled air 12 h after a gavage dose of [14C]-BCEE was
administered to rats. Less than 2% of the radioactivity expired
through the lungs was exhaled as the parent compound.
1.6 Effects on laboratory animals and in vitro test systems
BCEE is acutely toxic by the oral, inhalation or dermal routes of
exposure. Reported LD50 values for the oral exposure of animal
species to BCEE range from 75 to 215 mg/kg body weight. BCME and CMME
are acutely toxic by inhalation or ingestion. Reported LC50 values
for the inhalation exposure of laboratory animals to BCME or CMME
range from 25 to 48 mg/m3, and from 182 to 215 mg/m3, respectively.
Exposure of laboratory animals by inhalation to high single
concentrations of BCEE (>320 mg/m3) resulted in eye irritation as
well as congestion, oedema, and haemorrhage in the lungs. During
inhalation of BCME, irritation of the eyes and respiratory tract were
noted as well as necrotizing bronchitis. Skin application resulted in
erythema and necrosis, and application to the eye resulted in corneal
necrosis. Similar effects were noted after exposure to CMME.
Increased mortality and tracheal hyperplasia were observed in
rats and hamsters following multiple inhalation exposure to 4.7 mg
BCME/m3. Similar results were observed in rats repeatedly exposed by
inhalation to 3.3 or 33 mg CMME/m3.
In general, positive results were obtained when BCEE, BCME and
CMME were tested for mutagenicity in vitro. However, interpretation
of the results is difficult given the lack of details in the reports
available. BCME and CMME have been reported to increase unscheduled
DNA synthesis in vitro, and BCME increased the level of transformed
cells in in vitro assays.
In small groups of males from two strains of hybrid F1 mice (and
in females from one F1 strain) treated orally with BCEE
(time-weighted dose 41.3 mg/kg body weight over 18 months), there was
a significant increase in the incidence of hepatomas (combined benign
hepatomas and malignant tumours) compared to unexposed controls. Four
other limited studies in rats and mice using oral gavage, subcutaneous
or intraperitoneal injection and skin painting failed to confirm these
findings.
Carcinogenicity studies in experimental animals (mice and rats)
exposed to BCME showed significantly elevated incidence of pulmonary
adenomas and respiratory tumours. In mice, inhalation exposure also
showed evidence of an elevated incidence of lung tumours.
Studies with CMME have shown an increased incidence of tracheal
metaplasia and bronchial hyperplasia in a dose-dependent manner in
rats. However, results of carcinogenicity bioassays are inconclusive
in animal studies.
Information regarding the reproductive, developmental,
immunological or neurological toxicity of BCEE, BCME or CMME is not
available.
1.7 Effects on humans
BCEE was found to be irritating to the eyes and nasal passages of
humans at levels >150 mg/m3 following short-term exposure.
No epidemiological studies on the effects of long-term exposure
to BCEE have been reported.
In eight epidemiological studies, exposure of workers to BCME
(CMME) was associated with increased risk of lung cancer. Workers
exposed to commercial grade CMME were probably also exposed to BCME.
The predominant tumours in exposed workers were small cell carcinomas,
quite distinct from the chiefly squamous cell carcinomas usually found
in smokers. The association between exposure to BCME (CMME) and lung
cancer was strong, with standardized mortality ratios ranging up to
21. The type of lung cancer, latency period and average age of
appearance of lung tumours in workers exposed to BCME (CMME) have been
remarkably consistent. For CMME, there is also evidence of a positive
relationship between a qualitative measure of exposure and mortality
due to lung cancer.
Even concentrations of 0.01 µg BCME/m3 and 20 µg CMME/m3, in
the course of occupational exposure, increased the frequency of
chromosomal aberrations in the peripheral lymphocytes of exposed
workers.
Information has not been reported regarding the neurological,
immunological, developmental or reproductive effects of BCME or CMME
in humans.
1.8 Effects on other organisms in the laboratory and field
There have been few studies on the effects of BCEE on
environmental organisms; most are restricted to aquatic species. For
BCEE a 7-day LC50 concentration in the guppy of 56.9 mg/litre, a 96-h
LC50 in fish of 600 mg/litre and a 48-h LC50 in Daphnia magna of
240 mg/litre have been reported.
Anaerobic microbial activity was not inhibited at concentrations
of BCEE up to 100 mg/litre and an LC10 of 600 µg/litre has been
reported for microbes indigenous to waste stabilization ponds.
No information on the toxicological effects of BCME and CMME on
environmental organisms has been reported.
1.9 Conclusions
1.9.1 BCEE
- Exposure of terrestrial organisms to BCEE is considered to be
negligible because of the low rate of release and its short
persistence in the atmosphere.
- Although it is more persistent in water, the highest reported
concentration of BCEE in surface water is approximately five
orders of magnitude lower than the concentration found to induce
adverse effects in the guppy, the most sensitive aquatic species
identified among existing toxicity studies.
- Owing to the lack of available information on concentrations of
BCEE in several environmental media to which humans are exposed,
it is not possible to estimate quantitatively the total daily
intake of BCEE.
- Available data on the toxicity of BCEE in humans are limited.
Information on the developmental and reproductive effects of BCEE
in laboratory animals has not been identified, and none of the
long-term studies in laboratory animals is of sufficient quality
to provide quantitative information on either the potential of
BCEE to cause cancer or the toxicological effects produced by
long-term exposure to this substance.
- In the absence of adequate toxicological and carcinogenicity
data, it is prudent to minimize human exposure to BCEE.
1.9.2 BCME and CMME
- If these substances were to enter the environment, they would
both be rapidly broken down by hydrolysis and photo-oxidation.
Data concerning concentrations of BCME and CMME in the ambient
environment have not been reported.
- BCME and technical grade CMME (which contains BCME) are proven
human carcinogens. In addition, both of these chemicals are
carcinogens in laboratory animals. Both chemicals cause
chromosomal aberrations in occupationally exposed workers.
Occupational and general population exposure to these compounds
should be eliminated.
- Based on the fate of these substances in the environment and the
lack of exposure, there is no reason to suspect that adverse
effects on aquatic and terrestrial organisms would occur.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Bis(2-chloroethyl) ether (BCEE), bis(chloromethyl) ether (BCME)
and chloromethyl methyl ether (CMME) are included in a large class of
chemical substances known as the chloroalkyl ethers. Identifying
features of BCEE, BCME and CMME are summarized in Table 1.
2.2 Physical and chemical properties
BCEE, a ß-chloroalkyl ether, is a colourless, volatile liquid
with a "chlorinated solvent-like" odour (Sittig, 1981). BCME and CMME,
both alpha-chloroalkyl ethers, are also colourless, volatile liquids
with characteristic odours. The odour of BCME has been described as
"suffocating" (Sittig, 1981; Verschueren, 1983), while that of CMME
has been described as "irritating" (Verschueren, 1983). Technical
grade CMME contains from 1 to 8% BCME (Travenius, 1982) and, unless
otherwise indicated in this monograph, CMME refers to the technical
grade material. In general, the vapour pressure and water solubility
of these compounds are high, and the log octanol/water partition
coefficients (log Kow) are low. The ß-chloroalkylethers like BCEE are
only slightly reactive towards water, but the alpha-chloroalkyl ethers
like BCME and CMME are rapidly hydrolysed by water, and their
solubility, Kow, Koc and Henry's Law constant cannot be
experimentally determined. The physical and chemical properties of the
selected chloroalkyl ethers are presented in Table 2.
2.3 Conversion factors
At 25°C and 101.3 kPa, the conversion factors for BCEE, BCME and
CMME in air are as follows:
BCEE: 1 ppm (v/v) = 5.85 mg/m3; 1 mg/m3 = 0.17 ppm
BCME: 1 ppm (v/v) = 4.7 mg/m3; 1 mg/m3 = 0.21 ppm
CMME: 1 ppm (v/v) = 3.3 mg/m3; 1 mg/m3 = 0.30 ppm
2.4 Analytical methods
2.4.1 BCEE
One method for the analysis of BCEE in water involves solvent
extraction (using diethyl ether in pentane, methylene chloride, or
ethyl ether in hexane), concentration with a Kuderna-Danish (K-D)
apparatus, and separation and analysis by gas chromatography with
electron capture detection (GC/EC) or gas chromatography mass
spectrometry (GC/MS) (Dressman et al., 1977; Quaghebeur et al., 1986).
This method has been expanded to include clean-up with Florisil and
K-D concentration of the sorbed fraction prior to analysis by GC/EC
(McMillin et al., 1984). Vapour stripping using helium or nitrogen gas
has also been used to extract BCEE from samples of ground and surface
Table 1. Information on the identity of BCEE, BCME and CMME (US NLM, 1996)
Compound Identification Molecular Chemical structure Relative Synonyms
(CAS number)a formula molecular mass
Bis(2-chloroethyl) ether BCEE C4H8Cl2O Cl-(CH2)2-O-(CH2)2-Cl 143.02 dichloroethyl ether,
(111-44-4) dichloroethyl oxide,
bis (ß-chloroethyl) ether,
dichloroether,
1,1'-oxybis(2-chloro)ethane,
1,5-dichloro-3-oxapentane,
1-chloro-2-(ß-chloroethoxy)-
ethane,
2,2'-dichloroethyl ether,
ß,ß'-dichlorodiethyl ether,
bis(chloro-2-ethyl) oxide,
di(ß-chloroethyl) ether,
di(2-chloroethyl) ether,
ether, bis(2-chloroethyl),
sym-dichloroethyl ether,
diethylene glycol dichloride.
Bis(chloromethyl) ether BCME C2H4Cl2O Cl-CH2-O-CH2-Cl 114.97 chloro(chloromethoxy) methane,
(542-88-1) sym-dichloro-dimethyl ether,
oxybis(chloromethane),
dichloromethyl ether,
bichloromethyl ether,
dichlorodimethyl ether,
1,1'-dichlorodimethyl ether.
Table 1. (continued)
Compound Identification Molecular Chemical structure Relative Synonyms
(CAS number)a formula molecular mass
Chloromethyl methyl ether CMME C2H5ClO Cl-CH2-O-CH3 80.52 chloromethoxymethane,
(107-30-2) monochlorodimethyl ether,
methoxymethyl chloride,
chlorodimethyl ether,
methyl chloromethyl ether,
monochloromethyl methyl ether.
a Chemical Abstracts Services registry number.
Table 2. Physical and chemical properties of BCEE, BCME and CMME
Physical/chemical property BCEE BCME CMME
Melting point (°C) -50a -41.5a -103.5b
Boiling point (°C) 178.67a 104a 59.5b
Vapour pressure (mmHg) 0.71 at 20°Cb 30 at 22°Cc 122 at 20°Cd
Vapour density 4.93b 3.97b 2.8d
Water solubility (mg/litre) 10 200b NA NA
Log octanol/water partition
coefficient (log Kow) 1.46c NA NA
Henry's Law constant
(atm.m3/mol) 1.31 x 10-5c NA NA
Soil sorption coefficient
(log Koc) 1.1c NA NA
Hydrolysis rate constant
in water 4 x 10-6 h-1 at 25°Cc 0.05 sec-1h >90 sec-1 at 25°Ce
in air not available 1.7 x 10-1 sec-1 at 45°Ci 0.0018 min-1 at 29°Ck
Photolysis rate constant
in water 24 to <360 mol-1.h-1c 3 to <360 mol-1.h-1c not available
in air 1.79 x 10-11 cm3.mol-1.sec-1f not available 1.0 x 10-10 mol-1.sec-1e
Half-life
in water 20 years at 25°C (hydrolysis)c 38 sec at 20°C (hydrolysis)j <0.007 sec at 25°Ce
in air 13.44 h at 25°C (indirect photolysis)f >25 h at 25°C (hydrolysis)k 3.5 to 6 min at 25°C
(hydrolysis)i
in soil 1 to 6 months (estimate)g not available not available
Table 2 (continued)
a Weast & Astle (1985) g Howard et al. (1991)
b Verschueren (1983) h Tou & Kallos (1974a)
c Mabey et al. (1982) i Nichols & Merritt (1973)
d CCINFO (1991) j US EPA (1980)
e Radding et al. (1977) k Tou & Kallos (1974b)
f US EPA (1987b)
NA = not applicable. Due to the extremely rapid hydrolysis of this substance in water, it is not possible to obtain an experimental
value, and calculated values are meaningless.
water. Typically, this step is followed by concentration of the
extract with a cold or lipophilic vapour trap, and analysis by GC/MS
(Hites et al., 1979; DeWalle & Chian, 1981). An additional technique
has been described by Kleopfer & Fairless (1972), in which samples of
water are passed through an activated carbon filter, followed by
Soxhlet extraction of the carbon, drying of the extract with sodium
sulfate, K-D concentration, Shriner-Fuson separation of the acidic,
basic and neutral fractions, and analysis of the last by GC/MS.
Determination of BCEE in air involves passing air samples through a
sorbent, followed by elution and analysis by gas chromatography
(NIOSH, 1984).
Reported detection limits for these methodologies differ by up to
two orders of magnitude. Detection limits for the procedure described
by Dressman et al. (1977) and Quaghebeur et al. (1986) range from
0.005 to 0.04 µg/litre, respectively. Limits of detection for the
methods described by McMillin et al. (1984) and Kleopfer & Fairless
(1972) are 0.3 and 0.2 µg/litre, respectively.
2.4.2 BCME
While information concerning the sampling and analysis of BCME in
water, soil or foodstuffs was not available, considerable data on
techniques for the analysis of low levels (µg/m3) of BCME in air have
been identified (Collier, 1972; Evans et al., 1975; Frankel & Black,
1976; Parkes et al., 1976; Kallos, 1981; Muller et al., 1981; Galvin &
House, 1988; Blease et al., 1989). Typically, air samples are drawn
into a (Poropak or Tenax) sorption tube, thermally eluted, and
analysed by GC/MS or GC/EC. Two additional methods have been described
which involve the direct derivatization of BCME (with
2,4,6-trichlorophenol or sodium pentafluorophenolate), and subsequent
analysis by GC/EC (Sawicki et al., 1976; Langelaan & Nielen, 1989).
Norpoth et al. (1981) reported a spectrophotometric method for the
determination of BCME.
Collier (1972), Frankel & Black (1976) and Galvin & House (1988)
reported a detection limit of 470 ng/m3 for BCME in air, while Evans
et al. (1975) and Langelaan & Nielen (1989) achieved detection limits
as low as 50 and 14 ng/m3, respectively. Muller et al. (1981) did not
report a detection limit, but quantified BCME at a concentration of
2.35 µg/m3 in air. A detection limit of 0.94 µg/m3 was reported for
the spectrophotometric quantification method described by Norpoth et
al. (1981). The methods described by Sawicki et al. (1976) and Parkes
et al. (1976) have a detection limit of 2.35 µg/m3, while a detection
limit of approximately 4.7 ng/m3 was established for the method
described by Blease et al. (1989), in which high resolution was
achieved with the combined use of gas chromatography and tandem mass
spectrometry (GC/MS/MS).
2.4.3 CMME
Identified methods for the sampling and analysis of CMME in
environmental media are limited to techniques developed for monitoring
low levels (µg/m3) in air. Several methods have been described which
involve the derivatization of CMME (with 2,4,6-trichlorophenol or
sodium pentafluorophenolate) and subsequent analysis by GC/EC (Sawicki
et al., 1976; Kallos et al., 1977; Langhorst et al., 1981; Langhorst,
1985; Langelaan & Nielen, 1989). The limits of detection for these
methodologies are 49 ng/m3 (Langelaan & Nielen, 1989), 1.65 µg/m3
(Sawicki et al., 1976; Langhorst et al., 1981) and 3.29 µg/m3 (Kallos
et al., 1977).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Natural sources of BCEE, BCME or CMME in the environment have not
been identified. While BCME could be formed spontaneously from the
reaction of formaldehyde and chloride ions in an acidic atmosphere,
this reaction is unlikely in the general environment, although it may
be important in occupational settings (Durkin et al., 1975; Tou &
Kallos, 1976; Kallos & Tou, 1977; Travenius, 1982).
3.2 Anthropogenic sources
3.2.1 Production
Only limited information on the production of BCEE, BCME or CMME
has been reported.
3.2.1.1 BCEE
BCEE used to be prepared commercially in the USA as a by-product
in the manufacture of ethylene oxide by the chlorohydrin process, but
this process went out of use in the USA in 1973 (IARC, 1975). Other
methods of production also involving ethylene glycol or ethylene,
ethylene chlorohydrin and chlorine as reagents have been mentioned
(Durkin et al., 1975; IARC, 1975; ATSDR, 1989a). In 1975, two US
companies, one German and one Japanese company manufactured BCEE for
captive use as a solvent or chemical intermediate (IARC, 1975).
3.2.1.2 BCME
BCME is formed when formaldehyde reacts with chloride ions in an
acidic medium (Travenius, 1982). In China, BCME is produced by the
reaction of paraformaldehyde and hydrogen chloride gas as an
intermediate in the synthesis of the insecticide synergist S-2,
octachlorodipropyl ether [bis(1,2,3,3-tetrachloropropyl)ether] to
which it is converted in a one-part process. The scale of S-2
production is believed to be around 700 tonnes/year, which would
require over 200 tonnes of BCME. Specific synthesis reactions include
the reaction between paraformaldehyde and chlorosulfonic acid (Durkin
et al., 1975) and the saturation of a paraformaldehyde solution in
cold sulfuric acid with hydrogen chloride (US EPA, 1980). Small
amounts (several percent) of BCME are also produced during the
synthesis of CMME from gaseous hydrogen chloride and heated methanol
and formaldehyde (Durkin et al., 1975). In addition, the decomposition
products of commercial forms of CMME can combine to produce 1 to 8%
BCME as an impurity (Travenius, 1982). While BCME is not produced in
commercial quantities in Canada or the USA, it has been produced in
small quantities for use as a chemical intermediate in laboratory
applications (IARC, 1974).
3.2.1.3 CMME
CMME is produced by the reaction of anhydrous hydrogen chloride,
methanol and formaldehyde (Fishbein, 1979) or by the direct
chlorination of dimethyl ether (Durkin et al., 1975). An additional
method, which is designed to produce CMME that is free of BCME
impurities, involves the addition of actinium chloride to a slight
excess of anhydrous dimethoxymethane at room temperature (CCINFO,
1991). Production of CMME in the USA was estimated to be at least 4590
tonnes in 1977 and about 2.27 tonnes in 1982 (HSDB, 1996).
3.2.2 Uses
Only information concerning the use of BCEE, BCME or CMME in
Canada and the USA is available.
3.2.2.1 BCEE
In the USA, BCEE was formerly used in the process for the
manufacture of methyldithiocarbamic acid fungicide commonly known as
metham-sodium. Besides this use, approximately 20% of the BCEE sold in
the USA was used in the production of polymers, and 7% was either used
to synthesize a derivative of diquat or recycled for use as a
co-solvent (S. Helmhout, personal communication to the IPCS, 1992).
Other applications have included its use as a solvent for fats, waxes,
greases and esters; as a constituent of paints, varnishes and
lacquers; as a solvent for the removal of fatty substances from
various textiles, and as a penetrant and wetting agent in the textile
industry. It has also been used in the purification of oils and
gasoline, as a soil fumigant, insecticide and acaricide, and as an
intermediate in the manufacture of pharmaceuticals and other chemicals
(Durkin et al., 1975; IARC, 1975; US EPA, 1987a; ATSDR, 1989a).
3.2.2.2 BCME
In the USA, industrial use of BCME has been restricted since the
early 1980s to specific intermediate chemical reactions (Travenius,
1982). In China, BCME is an intermediate in the production of the
insecticide synergist S-2, octachlorodipropylether (see section
3.2.1.2). In the past, BCME has been used as a chloromethylating agent
in the production of ion exchange resins, water repellents and other
textile-treating agents, the manufacture of polymers, and a solvent
for polymerization reactions (Fishbein, 1979). Specific minor uses of
BCME have included the crosslinking of cellulose, the preparation of
three-block styrene-butadiene-styrene polymers, and the surface
treatment of vulcanized rubber to increase adhesion of epoxy resin and
polyurethane elastomers (Durkin et al., 1975).
Available data indicate that there is currently no commercial
activity involving more than one kilogram of BCME in Canada
(Government of Canada, 1993b).
3.2.2.3 CMME
In the USA, industrial use of CMME has been restricted since the
early 1980s to specific intermediate chemical reactions (Travenius,
1982). Based on available data, there is currently no commercial
activity in Canada involving more than one kilogram of CMME
(Government of Canada, 1993b).
In the past, CMME has been used as a chloromethylating agent in
many synthetic processes, most notably in the production of anion
exchange resins (Durkin et al., 1975). It has also been used as a
solvent for polymerization reactions (Fishbein, 1979), in the
synthesis of methoxymethyl ethers of phenols, the crosslinking of
polystyrene, and the surface treatment of vulcanized rubber (Durkin et
al., 1975).
3.2.3 Sources in the environment
Information on the release of BCEE, BCME and CMME in countries
other than the USA and Canada has not been reported.
3.2.3.1 BCEE
BCEE may enter the environment as a by-product from the
chlorination of waste streams containing ethylene or propylene, and as
a contaminant in the fungicide metam-sodium. It has been estimated,
based on the quantities imported and the known level of contamination,
that less than 100 g of BCEE would have been released into the
Canadian environment in 1990 from metam-sodium (Government of Canada,
1993a). In the USA, a total of 2700 kg/year was estimated to be
released into the environment from chemical plants in 1989. Seventy
percent of this amount was reported to be emitted to the air, while
the remaining 30% was released in water (US EPA, 1990). The
chlorination of drinking-water containing diethyl ether can result in
the formation of BCEE (NRC, 1977); however, quantitative data have not
been identified.
3.2.3.2 BCME and CMME
It was reported in the Toxic Release Inventory Database (US EPA,
1990) that less than 1 kg of BCME and 50 kg of CMME were released into
the atmosphere in the USA from industrial producers and users during
1989. However, release occurred in the two-step production of
octachlorodipropyl ether in China (Chen et al., 1996). This process
ceased in 1975, but manufacture of octachlorodipropyl ether was
revived in 1987 using a one-step process, from which gas releases and
accidental liquid spills occur. There is no information on the amount
of BCME that may remain as a contaminant of the product, which
contains formaldehyde and hydrogen chloride (BCME's precursors). There
is, however, gas-chromatographic evidence that CMME and BCME are
released into the air by the burning of octachlorodipropyl ether in
mosquito coils. No information is available from these sources
concerning the release of BCME or CMME into other media (water, soil,
underground injection), but, owing to their rapid rate of hydrolysis,
these compounds are not expected to remain as such for prolonged
periods in waste streams from plants where they are produced or used
(IARC, 1974).
The spontaneous formation of BCME or CMME in drinking-water from
the chlorination of ethers has not been investigated. However, in view
of their rapid rate of hydrolysis (see section 4.2.2), it is unlikely
that BCME or CMME would be present as contaminants in drinking-water
(Durkin et al., 1975).
No information has been identified concerning the quantities of
BCME or CMME released into the environment during storage or
transportation. However, these amounts are likely to be insignificant
since BCME and CMME have been usually produced and used in "closed
system" operations where containment prevents the release of these
chemicals into the environment (Durkin et al., 1975).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 BCEE
Based on the low-to-moderate Henry's Law constant (1.3 × 10-5
atm.m3/mol), BCEE would tend to remain in water. The air/water ratio,
as well as the Henry's Law constant, will determine the amounts of
BCEE distributed between the two compartments. Rainfall would probably
result in the removal of BCEE from the atmosphere (Durkin et al.,
1975). Using the approach of Mackay & Wolkoff (1973), Durkin et al.
(1975) calculated the half-life with respect to volatilization of BCEE
from a body of water to be 5.78 days at 25°C. Similarly, a
volatilization half-life of 3.4 days (from water) was calculated by
the US EPA (1987b). Thus the removal of BCEE from surface water will
probably occur within a week, although it will persist in bottom
water. Based upon its low log Koc (organic carbon partition
coefficient) and high water solubility, BCEE is not expected to adsorb
to soil or sediment and is therefore considered to be mobile in these
media (US EPA, 1987b). The US EPA (1987b) reported that, because of
its vapour pressure, BCEE should volatilize relatively rapidly from
dry surfaces. In the only study dealing with soil volatilization (a
7-day microcosm study by Piwoni et al. (1986) in which the soil was
kept moist), an insignificant amount (3%) of applied BCEE was
calculated to have volatilized.
4.1.2 BCME and CMME
Information regarding the mobility and distribution of BCME and
CMME in environmental media is limited. Callahan et al. (1979)
suggested that BCME could volatilize rapidly from an aquatic system
only if it were discharged in a water-immiscible solvent with a high
vapour pressure. Once in the atmosphere, these substances would be
rapidly degraded by photo-oxidation or hydrolysis. Very little
information was identified concerning the behaviour of BCME or CMME in
soil. It is unlikely that BCME and CMME are mobile in soil as both
compounds hydrolyse rapidly in an aqueous environment.
4.2 Abiotic degradation
4.2.1 BCEE
At a temperature of 20°C in water, a hydrolysis half-life of 20
to 22 years was estimated for BCEE (Mabey et al., 1982; Milano et al.,
1989). The US EPA estimated the half-life for the reaction of BCEE
with hydroxyl radicals in the atmosphere to be approximately 2.8 days
(A. Leifer, Office of Toxic Substances, US EPA, personal
communication, 1992). A half-life of 13.4 h has been reported for the
indirect photolysis of BCEE in the gaseous phase (US EPA, 1987b).
Photolysis products of BCEE include 2-chloroethanol, ethyl alcohol,
methyl alcohol, 2-chloroethyl ethyl ether, peracetic acetic acid,
1-(2-chloroethoxy)-1,2-epoxyethane, acetaldehyde and chloracetaldehyde
(Milano et al., 1989).
4.2.2 BCME and CMME
BCME and CMME are removed from environmental media via abiotic
processes. In the atmosphere, these substances are degraded by
photo-oxidation or hydrolysis. Cuppit (1980) reported atmospheric
half-lives of < 2.9 days for BCME and < 3.9 days for CMME. Tou &
Kallos (1974a) reported half-lives for atmospheric hydrolysis of
> 1 day for BCME and between 0.0024 (Nichols & Merritt, 1973) and
0.27 days for CMME, in humid air. At low humidity levels, however,
BCME may be degraded by oxidative as well as hydrolytic pathways. In
air, the decomposition products for BCME include hydrogen chloride,
formaldehyde and chloromethylformate, while those of CMME include
chloromethyl and methyl formate (Cupitt, 1980).
BCME and CMME hydrolyse rapidly in water. At 20°C, half-lives in
water of 38 seconds for BCME and < 1 second for CMME have been
reported (Tou et al., 1974; Radding et al., 1977; US EPA, 1980).
Although BCME may be degraded by oxidation, the extremely rapid
hydrolysis of BCME in aqueous media precludes any significant
oxidative degradation of this substance in aquatic systems (Callahan
et al., 1979). BCME is hydrolysed to formaldehyde and hydrogen
chloride (ATSDR, 1989b), while CMME is hydrolysed to hydrogen
chloride, methanol and formaldehyde (Travenius, 1982).
4.3 Biodegradation, biotransformation and bioaccumulation
4.3.1 BCEE
In the only study identified, Tabak et al. (1981) reported that
BCEE was completely biodegraded within 7 days in an aqueous medium
inoculated with sewage sludge. Although data on the biodegradation of
BCEE in soil are limited, this process may play some role in the fate
of this substance in soil. Kincannon & Lin (1986) reported a half-life
of BCEE in soil of approximately 16.7 days, based on the results of a
97-day soil column study in which the degradation of BCEE mixed with
hexachloroethane (as a constituent of a hazardous waste sludge) was
quantified.
For biota, Barrows et al. (1978) reported a bioconcentration
factor (BCF) of 11 and a biological half-life of between 4 and 7 days
for BCEE in bluegill sunfish (Lepomis marochirus) based on the
results of a study in which the fish were exposed to BCEE (under
flow-through conditions) for 14 days at a mean water concentration of
10 µg/litre.
4.3.2 BCME and CMME
No information on the biodegradation of either BCME or CMME in
soil was identified. However, their high rates of hydrolysis in
aqueous media preclude any possibility of BCME or CMME bioaccumulating
in organisms.
4.4 Ultimate fate following use
Owing to the highly reactive nature of the
alpha-chloroalkylethers in water and air, CMME and BCME are not
expected to be present in the general environment (Durkin et al.,
1975). However, owing to the relative stability of ß-chloroalkylethers
in environmental media, BCEE may be persistent in the general
environment (Durkin et al., 1975).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 BCEE
Quantitative information on the levels of BCEE in air is limited
to a single study in the USA in which this substance was detected (but
not quantified) in the atmosphere above two landfill sites in New
Jersey (US NLM, 1996).
Available data concerning the levels of BCEE detected in surface
water and drinking-water are summarized in Tables 3 and 4,
respectively. BCEE has been detected in samples of municipal
drinking-water at mean concentrations of up to 0.42 µg/litre in the
USA (Kraybill, 1977). The highest concentration reported for selected
surface waters was 58 µg/litre in Belgium, in the vicinity of
industrial discharges (Quaghebeur et al., 1986).
Identified studies concerning the levels of BCEE in groundwater
were limited to surveys conducted in the vicinity of contaminated
areas; concentrations of BCEE ranged from 0.001 µg/litre in samples
collected at an industrial gypsum waste disposal site in Belgium
(Quaghebeur et al., 1986) to 840 µg/litre in samples collected near a
municipal and industrial waste landfill site in the USA (DeWalle &
Chian, 1981).
Identified studies on the levels of BCEE in soil were limited to
two investigations in which this compound was detected in samples
collected from contaminated areas in the USA. BCEE was monitored (but
not quantified) in samples of soil collected at Love Canal, New York
(Hauser & Bromberg, 1982), and measured at a mean concentration of 140
mg/kg in samples of soil from waste disposal sites in the USA (ATSDR,
1989a).
No information is available on the levels of BCEE in foodstuffs.
Based on its high water solubility and low Kow, BCEE is not expected
to bioaccumulate in fish or other aquatic species (ATSDR, 1989a).
The concentration of BCEE in in-plant effluents in Canada has
been reported to range from 6.1 to 1057 µg/litre (Government of
Canada, 1993a). These effluents are diluted with cooling water before
being discharged to the environment and, although levels of BCEE at
the outflow pipe were not monitored, they were probably below the
limit of detection.
The highest concentrations of BCEE in the USA were reported for
industrial effluents (8 to 170 µg/litre), and municipal and industrial
waste landfill leachates (12 400 µg/litre) (DeWalle & Chian, 1981).
Table 3. Bis(2-chloroethyl) ether levels in surface water
Location Number of Concentrationb Remarks Reference
samplesa mean (range)
(µg/litre)
Philadelphia, USA NR ND samples collected from April 1975 to July 1975 Manwaring et al. (1977)
from the Delaware River, upstream from a
water treatment plant
NR trace samples collected in April 1975 from the
Delaware River, upstream from a chemical plant Manwaring et al. (1977)
2 trace samples collected in October 1976 from the
Delaware River Sheldon & Hites (1978)
5 (ND - trace) samples collected in March 1977 from the
Delaware River Sheldon & Hites (1978);
US EPA (1980)
New Orleans and
Baton Rouge, USA 3 0.11 (0.04 - 0.16) Pellizzari et al. (1979)
Houston, USA 1 (1) 1.4 Pellizzari et al. (1979)
Nitro, USA NR 0.041 samples collected from the Kanawha River Rosen et al. (1963);
Durkin et al. (1975)
USAc 808 (3) < 10.0 median limit of detection, 10.0 µg/litre Staples et al. (1985)
Belgiumc NR (7 - 58) samples collected from Haine River adjacent to
industrial discharges Quaghebeur et al. (1986)
Belgiumc NR (trace - 7.9) samples collected from Durme River, Scheldt
River and Gheut-Terneuzen Channel
downstream from industrial discharges Quaghebeur et al. (1986)
a Value in parenthesis indicates the number of samples with detectable levels of bis(2-chloroethyl) ether.
b Mean and/or (range) of concentrations, unless otherwise indicated; detection limits were reported, when possible.
c Locations were not specified.
NR = not reported; ND = not detected
Table 4. Bis(2-chloroethyl) ether levels in drinking water
Location Number of Concentrationb Detection Remarks Reference
samplesa mean (range) limit
(µg/litre) (µg/litre)
Toronto, Canada 50 (0) ND 0.00003 finished drinking-water Kendall (1990)
Toronto, Canada 8 (0) ND 0.001 bottled spring water Kendall (1990)
Alberta, Canadac 1512 (1) ND (ND - trace) 1 samples of treated (from 215 Alberta Ministry of the
sites) and raw (from 14 sites) Environment (1991)
drinking-water
collected from January 1986
to June 1991
Nitro, USA 1 (1) 0.2 NR tap water DeWalle & Chian (1981)
Evansville, USA 1 NQ NR finished drinking-water Kleopfer & Fairless (1972)
Philadelphia, USA NR NQ NR finished drinking-water Suffet et al. (1980)
collected between 1975
and 1977
Philadelphia, USA NR < 0.1 (0.04 - 0.6) NR finished drinking-water Manwaring et al. (1977)
collected between February
1975 and July 1975
New Orleans, USA NR (0.04 - 0.16) NR finished drinking-water Keith et al. (1976)
collected in August 1974
Philadelphia, USA NR (0.03 - < 1) NR raw drinking-water collected Manwaring et al. (1977)
between April 1975 and
July 1975
Philadelphia, USA NR (0.4 - 0.5) NR raw drinking-water Durkin et al. (1975)
Table 4. (continued)
Location Number of Concentrationb Detection Remarks Reference
samplesa mean (range) limit
(µg/litre) (µg/litre)
USAc NR 0.42 NR finished drinking-water Kraybill (1977)
NR ND 5 finished drinking-water US EPA (1980)
collected (between March
1976 and April 1976) from
112 cities during the
National Organics Monitoring
Survey (NOMS) (Phase I)
NR 0.0115 (ND - 0.36) 0.005 finished drinking-water Dressman et al. (1977)
collected (between May 1976
and June 1976) from 113
cities during the NOMS
(Phase II); BCEE was detected
in drinking-water from 13
cities at a mean concentration
of 0.10 µg/litre
USAc NR 0.0017 NR finished drinking-water US EPA (1980)
collected (between November
1976 and June 1977) from 110
cities during the NOMS (Phase
III); BCEE was detected in
drinking-water from 8 cities
at a mean concentration of
0.024 µg/litre
NR (0.02 - 0.12) NR drinking-water from 80 cities Fishbein (1979)
Netherlandsc NR 0.1 maximum NR Kraybill (1977)
Table 4 (continued)
a Values in parenthesis indicate the number of samples with detectable levels of bis(2-chloroethyl) ether.
b Mean and/or (range) of concentrations, unless otherwise indicated.
c Locations were not specified
ND = not detected
NR = not reported
NQ = not quantified
5.1.2 BCME and CMME
No information has been reported on levels of BCME or CMME in
ambient air or the indoor air of homes or offices. In a small survey
of outdoor air in the Netherlands, BCME and CMME were not detected
(detection limits, 14.1 µg/m3 and 49.5 µg/m3, respectively) in
samples collected in the neighbourhood of a potential emission source
(distance and source were not specified) (Langelaan & Nielen, 1989).
Available data on the levels of BCME or CMME in drinking-water,
surface water or ground water are limited to one investigation in
which BCME was not detected (detection limit, 10 µg/litre) in a total
of 317 samples of surface and groundwater from unspecified locations
in the USA (Staples et al., 1985).
Quantitative data concerning the levels of BCME or CMME in soil
have not been reported. However, in view of their rapid rate of
hydrolysis, these compounds are not expected to persist as
contaminants in moist soil (US NLM, 1996). Similarly, while no studies
on the levels of BCME or CMME in foodstuffs have been reported, the
high rates of hydrolysis reduce the likelihood of BCME or CMME
bioaccumulating in the food chain (US NLM, 1996).
No reliable data on levels of either BCME or CMME in industrial
effluents have been reported.
5.2 General population exposure
Quantitative data concerning the levels of BCEE in the general
environment are restricted to the results of studies in which the
levels of this substance in surface water and drinking-water have been
assessed. Based on a daily volume of ingestion for adults of 1.4
litres, a mean body weight for males and females of 64 kg (IPCS,
1994), and the highest mean concentration of BCEE in drinking-water
presented in Table 4 (0.42 µg/litre), the estimated intake of BCEE
from drinking-water for adults would be approximately 0.01 µg/kg body
weight per day.
Adequate information on the concentrations of BCME and CMME in
air, drinking-water, soil, or foodstuffs have not been reported, and
therefore it is not possible to estimate the intake of these
substances. No quantitative data are available for the exposure of
populations that use mosquito coils containing octachlorodipropyl
ether (see section 3.2.3.2), but the number of users of such coils is
of the order of millions in China.
5.3 Occupational exposure
5.3.1 BCEE
Occupational exposure to BCEE (via inhalation or dermal contact)
may occur in individuals involved in the dry cleaning and textile
industries, or in the processing of gum, lacquer, oil, paint, soap and
tar (Tabershaw et al., 1977). However, no investigations concerning
quantitative levels of exposure to BCEE in the workplace have been
reported.
5.3.2 BCME and CMME
Occupational exposure to BCME or CMME may occur in laboratory and
textile workers, and in individuals involved in the production of
anion-exchange resins, organic chemicals and polymers (Lemen et al.,
1976; US EPA, 1980). In China, occupational exposure to BCME occurs in
the manufacture of octachlorodipropyl ether. Under conditions where
vapours of formaldehyde and hydrochloric acid co-exist, BCME may form
spontaneously in air. Available quantitative data concerning
occupational exposure to either BCME or CMME are limited to
investigations of the levels of BCME in workroom air (Table 5).
BCME may be produced in solution from a variety of sources of
formaldehyde and chloride ions, and has been detected in the vapours
above these solutions (Frankel et al., 1974). In one study, the
concentration of BCME in the headspace above formalin slurries
containing Freidel-Crafts (chloride) salts ranged from 0.99 to
7.1 mg/m3 (210 to 1500 ppb) (Frankel et al., 1974).
While no recent studies have been identified where levels of
occupational exposure to CMME have been reported, it has been
estimated that in the past, concentrations of CMME in workroom air may
have ranged from 4.7 to 47 mg/m3 (1-10 ppm) (Travenius, 1982).
Table 5. Concentrations of bis(chloromethyl) ether in workroom air
Industry Sampling period Concentrationa Detection limit Reference
(µg/m3) (µg/m3)
Dye auxiliaries (resin) production; Jan. 1976 - Aug. 1976 ND 0.5 or 0.9 Yao & Miller (1979)
dye manufacture; fertilizer
production; textile finishing on
woven goods; hospital procedures;
foundry products (research plant);
foundry products (full-scale plant)
(USA)
Plastics industryb (USA) Jan. 1973 <4.7 - 72.9 NR Eisner (1974)
Textile finishing plants (4) (USA) Nov. 1974 - Dec. 1974 <0.5 - 37.6 0.5 Marceleno (1974)
Chemical plant (UK) 1978 <4.7 NR Travenius (1982)
Chemical plant (Netherlands) NR 1.2 - 3.8 0.5 van der Ven & Venema
(1979)
Resin manufacturing plantd (France) 1979 - 1984 2.8 - 20.6 NR Gowers et al. (1993)
a Concentrations of bis(chloromethyl) ether measured in workroom air
b Samples of air collected at the Diamond Shamrock Chemical Company in California, in the vicinity of reactors used to condense
phenol and formaldehyde
c Unspecified industrial operations; location of sample acquisition was not reported
d Range of average concentrations from various areas in the plant
ND - not detected
NR - not reported
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
Quantitative information on the absorption, distribution,
elimination and metabolism of BCEE, BCME or CMME in humans is not
available.
6.1 Absorption and distribution
Gwinner et al. (1983) reported that more than 95% of the total
[14C]-BCEE vapour (calculated to be approximately 75 mg) introduced
into an inhalation chamber containing three male Wistar rats was
absorbed by the animals after an 18-h exposure. When the tissue
(protein)-associated radioactivity (per gram of tissue) was examined
after this exposure period, approximately 0.32% of the administered
radioactivity was present in the liver, while 0.17 and 0.12% were
found in the kidney and small intestine, respectively. Only 0.07% of
the administered radioactivity was present in the lungs. Lingg et al.
(1982) administered by gavage a single dose of [14C]-BCEE (40 mg/kg
body weight, dissolved in corn oil) to male Sprague-Dawley rats and
monitored the amount of radioactivity present in a limited number of
tissues during the subsequent 48-h period. After 48 h, the percentage
of administered 14C was found to be 11.5 in expired CO2, 64.7 in
urine, 2.4 in faeces, and 2.3 in organs and tissues. In tissues,
approximately 1, 0.56, 0.49 and 0.19% of the radioactivity was
retained in muscle, kidney, blood and liver, respectively.
Quantitative data on the absorption and distribution of BCME or CMME
in animal species have not been reported.
6.2 Metabolism
BCEE is readily metabolized following absorption. Thiodiglycolic
acid (TDGA) was the principal metabolic product (representing 50 to
80% of the total metabolites) in the urine of rats administered BCEE
either orally, by intraperitoneal injection or by inhalation (Lingg et
al., 1979, 1982; Muller et al., 1979; Norpoth et al., 1986).
2-Chloroethoxy-acetic acid, N-acetyl-S-[2-(2-chloroethoxy)-ethyl]-L-
cysteine, 1-(2-chloroethyl)-ß-D-glucopyranosiduronic acid and
S-carboxymethyl-L-cysteine have been reported to be minor metabolites
(each comprising less than 10% of the total) in the urine of rats
administered BCEE (Lingg et al., 1979, 1982; Muller et al., 1979).
Lingg et al. (1982) reported that in male Sprague-Dawley rats
administered (by gavage) a single dose of [14C]-BCEE (40 mg/kg body
weight, dissolved in corn oil), approximately 12% of the radioactivity
was metabolized to 14CO2.
The formation of TDGA from BCEE involves a number of steps (Lingg
et al., 1979, 1982; Muller et al., 1979; Gwinner et al., 1983; Norpoth
et al., 1986). BCEE is believed to undergo oxidative degradation
(involving ether cleavage) to produce chloroacetaldehyde and
chloroethanol (which itself is rapidly converted to
chloroacetaldehyde) (Gwinner et al., 1983). It is believed that
chloroacetaldehyde is subsequently converted to chloroacetic acid,
which after conjugation with glutathione and further modification,
produces TDGA. The formation of N-acetyl-S-[2-(2-chloroethoxy)ethyl]-
L-cysteine is believed to involve the direct substitution of one of
the chlorine atoms in BCEE with cysteine (Lingg et al., 1982).
S-Carboxymethyl-L-cysteine, although not detected in all studies in
which the metabolism of BCEE was examined, has been postulated to be
an intermediate in the synthesis of TDGA (Lingg et al., 1982).
1-(2-Chloroethyl)-ß-D-glucopyranosiduronic acid is evidence of the
occurence of 2-chloroethanol among metabolic products, while
S-carboxymethyl- n-cysteine may be produced by alkylation of
glutathione by chloroacetaldehyde (Lingg et al., 1982), and
2-chloroethoxy-acetic acid is believed to be produced via the
oxidative dehalogenation of BCEE (Lingg et al., 1982).
Information on the metabolism of BCME or CMME in laboratory
animals has not been reported; however it is anticipated that BCME and
CMME would be rapidly hydrolysed in the aqueous environment of
tissues, forming formaldehyde and hydrogen chloride, and methanol,
formaldehyde and hydrogen chloride, respectively. However, the effects
of BCME (CMME) are most likely attributable to their direct alkylating
activity (van Duuren, 1989).
6.3 Elimination
Although quantitative information on the elimination of BCME or
CMME in laboratory animals is not available, limited quantitative data
concerning the elimination of BCEE (administered orally) in laboratory
animals have been reported. Lingg et al. (1982) administered (by
gavage) a single dose of [14C]-BCEE (40 mg/kg body weight, dissolved
in corn oil) to male Sprague-Dawley rats and monitored the amount of
radioactivity appearing in the faeces, urine and expired air during
the subsequent 48-h period. Twelve hours after the administration of
[14C]-BCEE, 50% of the radioactivity had been lost in the urine and
exhaled air (as 14CO2). Lingg et al. (1979) estimated that less than
2% of the administered radioactivity that was expired through the
lungs was exhaled as the parent compound. Forty-eight hours after the
oral administration of [14C]-BCEE, approximately 65% of the
radioactivity was excreted in the urine and 11.5% exhaled from the
lungs (total loss of 76%); approximately 2.3 and 2.4% of the
administered radioactivity remained in the organs (and tissues) and
faeces, respectively.
Smith et al. (1985) reported that 24, 48 and 72 h after the oral
administration (by gavage) of [14C]-BCEE (10 mg/kg body weight, in a
solution containing ethanol, Emulphor and distilled water) to two
female Rhesus monkeys, approximately 43, 56 and 58% of the
administered radioactivity had been eliminated in the urine.
Seventy-two hours after the administration of [14C]-BCEE, less than
2% of the radioactivity was recovered in the faeces.
7. EFFECTS ON EXPERIMENTAL MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
Information on the acute toxicity of BCEE, BCME and CMME is
summarized in Table 6.
7.1.1 BCEE
Although the acute toxicity of BCEE has been examined in a number
of studies, complete experimental details were not always provided.
Reported LD50 values for the oral exposure of animal species to BCEE
range from 75 to 215 mg/kg body weight. An LC50 of 5850 mg/m3 (1000
ppm) was estimated from studies in which Sherman strain rats were
exposed to BCEE for 0.75 h (Smyth & Carpenter, 1948). The exposure of
guinea-pigs to 5850 mg/m3 for 3.8 to 5.5 h resulted in the death of
the animals (Schrenk et al., 1933). Exposure to 1521 mg/m3 (260 ppm)
resulted in the death of the animals after 7.5 to 12.3 h of continuous
exposure. No deaths were observed after exposure to 205 mg/m3 (35
ppm) for up to 13.5 h, although slight nasal irritation was observed
within 3 to 10 min of exposure to this concentration. Acute exposure
of guinea-pigs to BCEE vapour (320 mg/m3) caused eye irritation (as
indicated by squinting and lacrimation) as well as congestion, oedema
and haemorrhage in the lungs; liver, kidney and brain congestion was
also noted (Schrenk et al., 1933). The severity of the toxicological
effects produced by exposure to the higher concentrations of BCEE was
also related to the length of the exposure period. Effects in
Sprague-Dawley rats or CD-1 mice administered a single oral dose of
BCEE (dissolved in cottonseed oil) included ptosis, increased
salivation, diarrhoea, decreased activity and ataxia (Drake & Myer,
1992).
Smyth & Carpenter (1948) reported that the dermal exposure of
guinea-pigs to BCEE caused skin irritation; the LD50 was 366 mg/kg
body weight.
7.1.2 BCME and CMME
Reported LC50 values for the exposure (by inhalation) of
laboratory animals to BCME range from 25 to 48 mg/m3 (5.3 to 10.3
ppm). The acute exposure (by inhalation) of animals to BCME produced
severe irritation of the eyes and respiratory tract (congestion,
oedema and haemorrhage (mainly of the lungs) and acute necrotizing
bronchitis (Union Carbide, 1968; Drew et al., 1975). The median life
span of rats exposed (by inhalation) to 0, 3.3, 9.9, 32.4 or 44.7
mg/m3 (0, 0.7, 2.1, 6.9 or 9.5 ppm) was 462, 420, 36, 2 and 2 days,
respectively. For hamsters exposed (by inhalation) to these
concentrations of BCME, the median life span was 675, 657, 68, 16 and
4 days, respectively (Drew et al., 1975). Exposure to 9.9 mg/m3
(2.1 ppm) for 7 h increased the incidence of tracheal and bronchial
hyperplasia 2- to 3-fold in rats and 4- to 5-fold in hamsters,
compared to unexposed controls (Drew et al., 1975).
Table 6. Acute toxicity of BCEE, BCME and CMME
Speciesa Route LC50 or LD50 Reference
(duration)
BCEE
Rat (Sherman) inhalation (0.75 h) LC50: 5850 mg/m3 (1000 ppm) Smyth & Carpenter (1948)
Rat (Sherman) oral LD50: 75 mg/kg bw Smyth & Carpenter (1948)
Rat oral LD50: 105 mg/kg bw Spector (1956)
Rat (Sprague-Dawley) oral LD50: 175 mg/kg bw Drake & Myer (1992)
Mouse oral LD50: 136 mg/kg bw Spector (1956)
Mouse (CD-1) oral LD50: 215 mg/kg bw Drake & Myer (1992)
Rabbit oral LD50: 126 mg/kg bw Spector (1956)
Guinea-pig dermal (poultice; 24 h) LD50: 366 mg/kg bw Smyth & Carpenter (1948)
BCME
Rat (Sprague-Dawley) inhalation (7 h) LC50: 33 mg/m3 (7 ppm) Drew et al. (1975)
Rat inhalationb LC50: 48 mg/m3 (10.3 ppm) Union Carbide (1968)
Mouse (A/Heston) inhalation (6 h) LC50: 25 mg/m3 (5.3 ppm) Leong et al. (1971)
Hamster (Syrian) inhalation (7 h) LC50: 33 mg/m3 (7 ppm) Drew et al. (1975)
Rat (Wistar) oral (undiluted) LD50: 0.21 ml/kg bw (278 mg/kg bw) Union Carbide (1968)
Rabbit (New Zealand) dermal (undiluted; 24 h) LD50: 0.28 ml/kg bw (370 mg/kg bw) Union Carbide (1968)
CMMEc
Rat inhalation (7 h) LC50: 182 mg/m3 (55 ppm) Drew et al. (1975)
Hamster inhalation (7 h) LC50: 215 mg/m3 (65 ppm) Drew et al. (1975)
Rat oral LD50: 817 mg/kg bw NIOSH (1974)
a Data on strain presented if reported in study.
b Duration not specified.
c Containing BCME.
Reported LC50 values for the exposure (by inhalation) of
laboratory animals to CMME range from 182 to 215 mg/m3 (55 to 65
ppm). Exposure to CMME produced pulmonary congestion, oedema,
haemorrhage and acute necrotizing bronchitis (Drew et al., 1975);
however the toxic effects produced by CMME may be due, at least in
part, to contaminating BCME.
Application of BCME to the skin of rabbits produced erythema and
necrosis, while exposure of the eye to this substance produced severe
corneal necrosis (Union Carbide, 1968).
7.2 Short-term exposure
7.2.1 BCEE
Information on the effects of short-term or subchronic exposure
of animals to BCEE is limited primarily to range-finding studies for
carcinogenicity bioassays. Theiss et al. (1977) reported that the
maximum tolerated dose (MTD) of BCEE in A/St male mice (receiving 6
intraperitoneal injections over a 2-week period) was 40 mg/kg body
weight. The administration (route not clearly specified) of 19 daily
doses (100 mg/kg body weight) of BCEE (deemed to be the MTD) to two
strains of hybrid F1 mice [strain (C57BL/6 × C3H/Anf)F1 and strain
(C57BL/6 × AKR)F1] had no effect on mortality, although other
toxicological effects were not reported (Innes et al., 1969).
7.2.2 BCME
In one study (Drew et al., 1975) on the short-term toxicity of
BCME, groups of 50 male Sprague-Dawley rats and Syrian hamsters were
exposed by inhalation to 0 or 4.7 mg/m3 (0 or 1 ppm) for 1, 3, 10 or
30 multiple 6-h exposures (duration between exposures not specified),
after which time the animals were observed for their entire life span
and the trachea and bronchi examined histopathologically. In groups of
rats exposed to BCME for 0, 1, 3, 10 or 30 occasions, 50% mortality
was observed after 66, 66, 20, 4 and 4 weeks, respectively. The
incidence of tracheal hyperplasia, with and without atypias, increased
from 27% after 1 exposure to 89% after 30 exposures to BCME. The
incidence of tracheal squamous metaplasia increased after 3 to 30
exposures. The incidence of bronchial hyperplasia and squamous
metaplasia increased with greater exposure to BCME. In hamsters
subjected to 0, 1, 3, 10 or 30 exposures (6-h) to BCME, 50% mortality
was observed after 95, 95, 70, 22 and 8 weeks, respectively. The
incidence of tracheal hyperplasia, with and without atypias, tracheal
squamous metaplasia and alveolar metaplasia with atypia increased with
more frequent exposure to BCME. Exposure to BCME also produced
bronchoalveolar metaplasia, squamous metaplasia with atypia and
atypical alveolar epithelium. Evidence of subarachnoid haemorrhage was
observed in 24% of the rats and 8% of the hamsters that received 30
exposures (6-h) to 4.7 mg/m3 (1 ppm) (Drew et al., 1975).
7.2.3 CMME
In one study on the short-term toxicity of CMME, groups of 25
male Sprague-Dawley rats were exposed (by inhalation) to 3.3 or 33
mg/m3 (1 or 10 ppm) for 30 days (duration and frequency of exposure
not specified) (Drew et al., 1975). Exposure to 3.3 mg/m3 resulted in
8% mortality, but no effect on body weight, within 30 days (data for
unexposed controls were not presented). Regenerative hyperplasia and
squamous metaplasia in bronchial epithelium were observed in rats
killed 2 weeks after the last exposure. Exposure to 33 mg/m3 resulted
in 88% mortality within 30 days (data for controls not presented);
marked (not quantified) weight decrease was observed with some
recovery towards the end of exposure. Significant (not quantified)
increases in lung/body weight ratios were observed in rats that died
after exposure to CMME; regenerative hyperplasia of bronchial
epithelium was also observed.
7.3 Long-term exposure/carcinogenicity
Studies on long-term exposure and carcinogenicity are given in
Table 7.
7.3.1 BCEE
Studies on the toxicological effects produced by the long-term
exposure of laboratory animals to BCEE have focused on its
carcinogenic potential. However there are numerous deficiencies in all
of these studies, compared to the more stringent protocols used in
current carcinogenicity bioassays.
Innes et al. (1969) assessed the carcinogenicity of BCEE in mice
following ingestion. Groups of 18 males and 18 females from two
strains of hybrid F1 mice [(C57BL/6 × C3H/Anf) and (C57BL/6 × AKR)]
were administered by stomach tube approximately 100 mg/kg body weight
BCEE (dissolved in distilled water) from the age of 7 to 28 days
(although the amount of BCEE was not adjusted during this period to
account for weight gain). Once the mice had reached four weeks of age,
the BCEE was then provided in the diet at a concentration of 300 mg/kg
diet until the mice were 18 months of age, after which time they were
killed and necropsied. The time-weighted average dose for these
studies was calculated to be 41.3 mg/kg body weight per day (US EPA,
1987a). There were multiple groups of controls consisting of animals
of both strains and sexes. "Hepatomas" (representing benign hepatomas
and malignant tumours), tumours of the pulmonary system (adenomas and
adenocarcinomas) and lymphomas (Type-B reticulum cell sarcomas and
leukaemias) were the predominant types of tumours observed in these
animals. Compared to unexposed controls, the incidence of "hepatomas"
was significantly (p = 0.01) increased in the treated (C57BL/6 ×
C3H/Anf)F1 mice (in males, 8/79 versus 14/16; in females, 0/87 versus
4/18; in control and exposed animals, respectively) and in (C57BL/6 ×
AKR)F1 males (5/90 versus 9/17 in control and exposed animals,
respectively). However the incidence of pulmonary tumours or lymphomas
was not significantly increased in the BCEE-exposed animals of either
Table 7. Long-term exposure/carcinogenicity of BCEE, BCME and CMME
Protocol Result Comments Reference
BCEE
Groups of 18 males and 18 females from The incidence of "hepatomas" (benign Evidence of Innes et al.
two strains of F1 hybrid mice [(C57BL/6 x and malignant tumours), "pulmonary increased incidence (1969)
C3H/Anf) and (C57BL/6 x AKR)] were tumours" and lymphomas in the male of liver tumours.
given (by gavage) approximately 100 control and BCEE-exposed (C57BL/6 x However, study
mg/kg bw BCEE (dissolved in distilled C3H/Anf)F1 mice was 8/79 and 14/16 limited owing to
water) from the age of 7 to 28 days. Once (p = 0.01), 5/79 and 0/16 and 5/79 small number of
the mice had reached four weeks of age, and 2/16, respectively; the incidence BCEE-exposed
BCEE was then provided in the diet at a of these tumours in the female control animals, use of
concentration of 300 mg/kg until the mice and (C57BL/6 x C3H/Anf)F1 mice was single dose level
were 18 months of age, after which time 0/87 and 4/18 (p = 0.01), 3/87 and and inadequate
they were sacrificed and necropsied. The 0/18 and 4/87 and 0/18, respectively. reporting of tumour
time-weighted-average dose for these The incidence of "hepatomas" (benign pathology. Amount
studies was calculated to be 41.3 mg/kg and malignant tumours), "pulmonary of BCEE was not
bw/day (US EPA, 1987a). Controls tumours" and lymphomas in the male adjusted during
consisted of multiple groups of animals of control and BCEE-exposed (C57BL/6 x initial period to
both strains and sexes. C3H/AKR)F1 mice was 5/90 and 9/17 account for weight
(p = 0.01), 10/90 and 2/17 and 1/90 gain.
and 0/17, respectively; the incidence
of these tumours in the female control
and BCEE-exposed mice was 1/82 and
0/18, 3/82 and 0/18 and 4/82 and 1/18,
respectively.
Table 7. (continued)
Protocol Result Comments Reference
BCME (dissolved in a solution containing The authors reported that BCEE was not No reported Weisburger et
sodium chloride, Polysorbate 80, carcinogenic in these male or female evidence of al., 1981
carboxy-methylcellulose and benzyl rats; however, there was a "substantial carcinogenicity.
alcohol) was administered by gavage to difference" between the mean weight of However, study
groups of 26 male and 26 female Charles the females administered BCEE and limited due to
River CD rats (at doses of 50 and corresponding controls, as well as "a small number of
25 mg/kg bw) twice weekly for 78 weeks, reduction" in the mean weight of the BCEE-exposed
after which time the animals were high-dose male rats, compared to the animals and
observed for a further 26-week period. controls. Notably, survival after 52 relatively short
The animals were necropsied and tissues weeks on the study was only 65% for the exposure period.
examined histopathologically, either at high-dose females and 96-100% for the The size of
the end of the study or when the animals other BCEE-exposed animals. The survival control groups
became moribund. Groups of controls of for the control animals at 52 weeks was was not clearly
each sex were administered vehicle alone. 97% and 99% for males and females, stated, and
respectively. quantitative data
on tumour
incidence were
not presented.
Groups of 20 male A/St mice were injected The incidence of lung tumours (expressed No evidence of Theiss et al.,
intraperitoneally three times a week with as the number of lung tumours/mouse) in carcinogenicity 1977
8, 20 or 40 mg/kg bw BCEE (dissolved in the BCEE-exposed animals (approximately in a limited
tricaprylin). Mice injected with 8 and 0.13 lung tumours/mouse) was lower than study of
20 mg/kg bw BCEE received a total of 24 that observed in animals injected with carcinogenic
injections while animals administered vehicle alone (0.39 lung tumours/mouse). potential.
40 mg/kg bw BCEE only received 4 injections.
Controls (n = 20) were injected with
vehicle alone. The mice were sacrificed
24 weeks after the initial injection and
the number of surface lung tumours
(adenomas) determined.
Table 7. (continued)
Protocol Result Comments Reference
Thirty female ICR/Ha Swiss mice were Compared to animals injected with vehicle Inconclusive van Duuren et
injected subcutaneously with 1 mg BCEE alone, where no tumours developed at the evidence of al., 1972
(suspended in 0.05 ml mineral oil) once site of injection, 2/30 animals injected carcinogenicity
a week for life (median survival time with BCEE developed sarcomas at the site in a limited study
of animals was 656 days). Controls of injection. involving small
(n = 30) were administered vehicle alone. numbers of animals
administered one
dose-level with
inadequate
reporting of data
on other effects.
Groups of 50 male and 50 female The incidence of all malignant and No evidence of Norpoth et al.,
Sprague-Dawley rats were injected benign tumours (e.g., mesenchymal, carcinogenicity 1986
subcutaneously with either 4.36 µmole epithelial, sarcomas, carcinomas, in a study
(0.62 mg) or 13.1 µmole(1.87 mg) BCEE unclassified) in the untreated controls, involving limited
(dissolved in 0.25 ml DMSO) once a vehicle-treated controls, low- and exposure to
week for two years. Controls were high-dose males and females was 2/35, BCEE with limited
administered DMSO or left untreated. 4/35, 4/50 and 6/50, and 24/50, 24/50, reporting of
23/50 and 22/50, respectively. The toxicological
median survival time of the untreated effects.
control, vehicle-treated control, low-
and high-dose groups was 696, 605, 590
and 643 (for males), and 639, 668, 629
and 654 days (for females), respectively.
Table 7. (continued)
Protocol Result Comments Reference
One milligram of BCEE (in 0.1 ml The incidence of skin papillomas at No evidence of Van Duuren et
benzene) was applied to the skin of 20 the site of application was 2/20 and skin tumour al., 1972
female ICR/Ha Swiss mice. Two weeks 3/20 in the control and BCEE-initiated initiating
later the secondary (promotion) animals, respectively. activity by
treatment (2.5 µg phorbol myristate BCEE.
acetate (PMA) in 0.1 ml acetone, three
times weekly) commenced and was
maintained for the lifespan of the
animals. Controls were administered
PMA alone.
BCME
Fifty A/Heston male mice were exposed Exposure to BCME produced loss of body Limited evidence Leong et al.,
(by inhalation) to 0 or 5 mg/m3 BCME weight, respiratory distress and death. of increased 1971
(industrial grade) for 6 h/day, Survival of control and BCME-exposed pulmonary tumour
5 days/week for 82 days, after which mice was 90% and 28%, respectively. burden in mice
exposure was terminated and survivors The incidence of pulmonary adenomas was exposed to one
observed for a further 10 weeks. The 20/49 and 26/47 in control and concentration of
animals were necropsied and lungs BCME-exposed mice respectively BCME for a
examined pathologically. (statistical significance not specified). relatively short
The average number of pulmonary period.
adenomas/animal among tumour-bearing
mice was 2.2 for controls and 5.2 for
the BCME-exposed group.
Table 7. (continued)
Protocol Result Comments Reference
Groups of 120 male Sprague-Dawley rats Six-month survival was greater than Increased incidence Leong et
were exposed (by inhalation) to 0, 1, 10 97% for control and BCME-exposed rats. of respiratory al., 1981
or 100 ppb (0, 0.0047, 0.047, 0.47 mg/m3) Nineteen-month survival for animals tract tumours in
BCME 6 h/day, 5 days/week for 6 months, exposed to 0, 0.0047 or 0.047 mg/m3 was rats exposed to
after which exposure was terminated and approximately 45%, while no animals BCME.
the rats observed for a further 22 months. exposed to 0.47 mg/m3 survived. After 6
Eight rats from each group were sacrificed months there was no significant
after 6 months for haematological, difference in the weights of the total
cytological, cytogenetic and body, liver, kidneys, brain, heart and
histopathological analyses. testes; exposure to BCME produced no
adverse haematological or cytogenetic
effects. The incidence of "respiratory
tract" tumours was 0/112, 0/113, 0/111
and 102/111, respectively; in the
highest-concentration group, there were
96 esthesioneuroepitheliomas
(significantly different [p < 0.05]
than controls), four pulmonary adenomas,
one carcinoma of the nasal passage and
an esthesio-neuroepithelioma metastasis
in the lung.
Table 7. (continued)
Protocol Result Comments Reference
Groups of 20 to 50 male Sprague-Dawley Sixty exposures to BCME had no effect Increased incidence Kuschner et
rats were exposed (by inhalation) to 0 or upon mortality, although the time at of respiratory al., 1975
0.1 ppm (0 or 0.47 mg/m3) BCME 6 h/day, which 50% mortality was reached was tract tumours
5 days/week for 2, 4, 8, 12, 16 and 20 reduced by approximately 24% in animals in rats exposed
weeks (10, 20, 40, 60, 80 or 100 exposures), receiving 80 or 100 exposures to BCME. to BCME.
after which the rats were necropsied and Animals surviving 30 weeks had
examined histopathologically. "respiratory tract cancers" (26 in
the nasal cavity and 13 in the lung).
The incidence of "respiratory tract
cancer" in animals surviving for more
than 210 days and receiving 10, 20,
40, 60, 80 or 100 exposures of BCME
was, 1/41 (2.4%), 3/46 (6.5%), 4/18
(22.2%), 4/18 (22.2%), 15/34 (44.1%)
and 12/20 (60.0%), respectively
(statistical significance not specified).
No lung tumours were observed following
up to 40 exposures to BCME. The
incidence of squamous cell carcinomas
of the lung was 2/20, 3/50 and 8/30,
after 60, 80 and 100 exposures,
respectively.
Groups of 20 female Sprague-Dawley rats The incidence of malignant tumours at Limited evidence van Duuren
were injected subcutaneously once per the site of injection (i.e., fibrosarcoma) of carcinogenicity et al., 1969
week with 3 mg BCME (dissolved in 0.1 ml and in the breast (i.e., adenocarcinoma) in rats injected
mineral oil) or vehicle alone for in the control and BCME-exposed animals subcutaneously
approximately 300 days. (Because of the was 0/20 and 1/20, and 5/20 and 0/20, with BCME.
corrosive effects produced by BCME, after respectively.
114 days the dose was reduced to 1 mg,
and injections performed only three times
per month; however because of severe
weight loss of the animals, the injections
were terminated after 300 days).
Table 7. (continued)
Protocol Result Comments Reference
Fifty female and 50 male newborn ICR Exposure to BCME had no effect upon growth Increased incidence Gargus et
Swiss mice received a single subcutaneous or survival of the mice. The incidence of of lung tumours al., 1969
injection of 12.5 µl/kg bw (16.6 mg/kg bw) pulmonary adenomas in the control and in mice injected
BCME (dissolved in peanut oil) and the BCME-exposed males was 2/30 and 25/50, subcutaneously
animals were observed for a period of six respectively; 5/20 controls and 20/50 of with BCME.
months, after which the survivors were the BCME-exposed females had lung adenomas.
necropsied and the number of lung tumours
(adenomas, based on histopathological
analysis) quantified. Controls (20 females
and 30 males) received a single
subcutaneous injection of vehicle alone.
Thirty male and 30 female XVIInc/Z mice Approximately 0%, 44% and 42% of the male Evidence of Zajdela et
received 32 subcutaneous injections of controls and male and female BCME-exposed carcinogenicity al., 1980
0.3 mg BCME (dissolved in mineral oil) mice, respectively, had tumours (e.g., in mice injected
over a period of 42 weeks. Controls fibrosarcomas and squamous carcinomas) subcutaneously
consisted of 30 XVIInc/Z male mice injected at the site of injection. with BCME.
with vehicle alone.
Groups of 20 female ICR/Ha mice which The incidence of squamous cell carcinomas Evidence of van Duuren
received 2 mg BCME (applied dermally) or of the skin in the controls and carcinogenicity et al., 1969
solvent (i.e., benzene) alone (controls) BCME-exposed animals was 0/20 and 12/20, in mice exposed
thrice weekly for 325 days. respectively. dermally to BCME.
Table 7. (continued)
Protocol Result Comments Reference
CMME
Fifty A/Heston male mice were exposed The incidence of pulmonary tumours in Suggestion of Leong et
(by inhalation) to 0 or 2 ppm (0 or CMME-exposed mice (25/50) was not increased al., 1971
6.6 mg/m3) (industrial grade) CMME for significantly (i.e., p > 0.05) different pulmonary tumour
6 h/day, 5 days/week for 101 days, after from that in the unexposed controls burden in mice
which time exposure was terminated and (20/49). The average number of pulmonary exposed to one
survivors observed for a further 7 weeks. tumours/animal among tumour-bearing mice concentration
The animals were necropsied and lungs was 2.2 and 3.1 for the control and of CMME for a
examined histopathologically. CMME-exposed group, respectively. relatively short
period.
Seventy-four male Sprague-Dawley rats Exposure to CMME had no effect upon No clear Laskin et
were exposed (by inhalation) to 0 or mortality or body weight gain. The evidence of al., 1975
1 ppm (0 or 3.3 mg/m3) (industrial grade) incidence of tracheal squamous metaplasia carcinogenicity
CMME for 6 h/day, 5 days/week for their and bronchial hyperplasia was 3% and 10%, in a limited
entire lifespan (up to 852 days). The and 35% and 59%, in the control and study.
rats were necropsied and tissues examined BCME-exposed animals respectively
histopathologically. (statistical significance not stated).
Two respiratory tract cancers (lung
squamous cell carcinoma and an
esthesioneuroepithelioma of olfactory
epithelium) were found in animals
exposed to CMME (but presumably not
in unexposed controls).
Table 7. (continued)
Protocol Result Comments Reference
Ninety male Syrian hamsters were exposed Exposure to CMME had no effect upon No clear Laskin et
(by inhalation) to 1 ppm (3.3 mg/m3) mortality or body weight gain. The evidence of al., 1975
(industrial grade) CMME for 6 h/day, incidence of tracheal squamous metaplasia carcinogenicity
5 days/week for their entire lifespan was 0% and 2%, and incidence of bronchial in a limited
(up to 852 days). The hamsters were hyperplasia was 5% and 8%, in the control study.
necropsied and tissues examined and BCME-exposed animals respectively
histopathologically. Eighty-eight unexposed (statistical significance not stated).
animals served as controls. One lung adenocarcinoma and a tracheal
squamous papilloma were observed in two
animals exposed to CMME.
Groups of 20 female Sprague-Dawley rats The incidence of malignant tumours at No evidence of van Duuren
were injected subcutaneously once per the site of injection (i.e., fibrosarcoma) carcinogenicity et al., 1969
week with 3 mg laboratory purified CMME and in the breast (adenocarcinoma) in the in rats injected
(dissolved in 0.1 ml mineral oil) or control and CMME-exposed animals subcutaneously
vehicle alone for approximately 300 days. was 0/20 and 1/20, and 1/20 and 0/20, with laboratory
Because of moderate corrosive effects, respectively. purified CMME.
the injections were terminated after
this time.
Thirty female ICR/Ha Swiss mice were Compared to the animals injected with Evidence of van Duuren
injected (subcutaneously) with 0.3 mg of vehicle alone, where no tumours developed carcinogenicity et al., 1972
laboratory purified CMME (suspended in at the site of injection, 10/30 animals in mice
0.05 ml mineral oil) once a week for injected with CMME developed sarcomas at injected
life. Controls (n = 30) received the site of injection. subcutaneously
vehicle alone. with CMME.
Table 7. (continued)
Protocol Result Comments Reference
Forty-eight female and 51 male newborn The numbers of female mice with lung No increase in Gargus et
ICR Swiss mice received a single adenomas in the control (vehicle) and the incidence al., 1969
subcutaneous injection of 125 µl/kg bw CMME-exposed groups were 5/20 and 8/48, of lung
(132.5 mg/kg bw) CMME dissolved in peanut respectively. The numbers of male mice tumours in
oil; the animals were observed for a with lung adenomas in the control mice injected
period of six months, after which time (vehicle) and CMME-exposed groups were subcutaneously
the survivors were necropsied and the 2/30 and 9/51, respectively. with CMME.
number of lung tumours (adenomas, based
on histopathological analysis) quantified.
Controls (20 females and 30 males)
received a single subcutaneous injection
of vehicle alone.
Groups of 20 female ICR/Ha mice received No squamous cell carcinomas of the No evidence of van Duuren
2 mg CMME (applied dermally) or solvent skin were observed in either the carcinogenicity et al., 1969
(i.e., benzene) alone (controls) thrice control or CMME-exposed animals. in mice exposed
weekly for 325 days. dermally to
CMME.
sex. Clinical, biochemical or haematological effects were not
addressed in the published account of this study.
Weisburger et al. (1981) reported that the oral administration of
BCEE to rats had no significant carcinogenic effect. BCEE (dissolved
in a solution containing sodium chloride, Polysorbate 80,
carboxy-methylcellulose and benzyl alcohol) was administered (by
gavage) to groups of 26 male and 26 female Charles River CD rats (at
doses of 50 and 25 mg/kg body weight) twice weekly for 78 weeks, after
which time the animals were observed for a further 26-week period.
Control groups of each sex (the size of which was not clearly stated)
were administered vehicle alone. The authors reported (although no
data on tumour incidence were presented) that BCEE was not
carcinogenic in these male or female rats. However, the authors
indicated (but results were not quantified) that there was a
"substantial difference" between the mean body weight of the females
administered both doses of BCEE and that of the corresponding
controls, as well as "a reduction" in the mean body weight of the
high-dose male rats, compared to controls. Notably, survival after 52
weeks on the study was only 65% for the high-dose females and 96 to
100% for the other BCEE-exposed animals. The survival for the control
animals at 52 weeks was 97 and 99% for males and females,
respectively. Clinical, biochemical or haematological effects were not
addressed in the published account of this study.
Theiss et al. (1977) assessed the potential of BCEE to produce
lung tumours in groups of 20 male A/St mice injected intraperitoneally
three times per week with 8, 20 or 40 mg/kg body weight BCEE
(dissolved in tricaprylin). Mice injected with 8 and 20 mg/kg received
a total of 24 injections while animals administered 40 mg/kg only
received 4 injections. Controls (n = 20) were injected with vehicle
(tricaprylin) alone. The mice were killed 24 weeks after the initial
injection and the number of surface lung tumours (adenomas)
determined. The incidence of lung tumours (expressed as the number of
lung tumours/mouse) in the BCEE-exposed animals was less than that
observed in animals injected with vehicle alone.
The potential of BCEE to induce tumours was also investigated in
a study in which groups of 30 female ICR/Ha Swiss mice were injected
subcutaneously with 1 mg BCEE (suspended in 0.05 ml mineral oil) once
per week for life (the median survival time of animals was 656 days)
(van Duuren et al., 1972). Compared to animals injected with vehicle
alone, where no tumours developed at the site of injection, 2/30
animals injected with BCEE developed sarcomas at the site of
injection. Norpoth et al. (1986) also examined the carcinogenicity of
BCEE in a study in which groups of 50 male and 50 female
Sprague-Dawley rats were injected subcutaneously with either 4.36
µmole (0.62 mg) or 13.1 µmole BCEE (1.87 mg) (dissolved in 0.25 ml
DMSO) once per week over a 2-year period. Controls were injected with
DMSO (alone) or left untreated. The incidence of all malignant and
benign tumours (e.g., mesenchymal, epithelial, sarcomas, carcinomas
and unclassified) in the BCEE-exposed animals was not significantly
different from that in the controls. The median survival time of the
untreated control, vehicle-treated control, and low- and high-dose
groups was 696, 605, 590 and 643 (for males), and 639, 668, 629 and
654 days (for females), respectively.
Van Duuren et al. (1972) assessed the skin-tumour-initiating
potential of BCEE. One milligram BCEE (in 0.1 ml benzene) was applied
to the skin of 20 female ICR/Ha Swiss mice. Two weeks later the
secondary (promotion) treatment (2.5 µg PMA in 0.1 ml acetone, three
times weekly) commenced and was maintained for the life span of the
animals. Compared to controls (administered PMA alone) where 2/20
animals developed papillomas, 3/20 of the BCEE-initiated animals
developed papillomas at the site of application.
7.3.2 BCME
Studies on the toxicological effects produced by long-term
exposure (by inhalation) to BCME have been restricted primarily to
limited carcinogenesis bioassays. The exposure (by inhalation) of male
A/Heston mice to 5 mg/m3 for 6 h/day, 5 days/week for a period of 82
days, followed by a 10-week observation period, produced a marked
reduction in survival (90 and 28% in control and BCME-exposed mice,
respectively) and an increase in the number of pulmonary adenomas
(20/49 and 26/47 in control and BCME-exposed mice, respectively),
although the statistical significance was not specified (Leong et al.,
1971). The average number of pulmonary adenomas per animal among
tumour-bearing mice was 2.2 for controls and 5.2 for the BCME-exposed
group.
The exposure (by inhalation) of groups of 144 to 157 male ICR/Ha
mice to concentrations of BCME from 0.0047 to 0.47 mg/m3 (1 to 100
ppb) for 6 h/day, 5 days/week for a period of 6 months, followed by an
18-month observation period, produced a reduction in survival (55, 35,
25 and 18% in mice exposed to 0, 0.0047, 0.047 or 0.47 mg/m3 [0, 1,
10 or 100 ppb], respectively), although no difference in survival
(> 90%) was observed between the control and BCME-exposed groups
after 24 months (Leong et al., 1981). All mice developed an ascending
urinary tract infection. After 6 months, the incidence of pulmonary
adenomas in surviving mice exposed to 0, 0.0047, 0.047 and 0.47 mg/m3
was 9/86, 5/45, 3/37 and 8/27 (p < 0.05), respectively. Exposure to
these concentrations of BCME had no adverse effect on body weight and
produced no nasal or eye irritation.
Groups of 120 male Sprague-Dawley rats were exposed (by
inhalation) to BCME concentrations of 0, 0.0047, 0.047 or 0.47 mg/m3
(0, 1, 10 or 100 ppb) 6 h/day, 5 days/week for 6 months, after which
time exposure was terminated and the animals observed for a further 22
months (Leong et al., 1981). Although 6-month survival was greater
than 97% for control and BCME-exposed rats, 19-month survival for
animals exposed to 0, 0.0047 or 0.047 mg/m3 was approximately 45%,
while none of the animals exposed to 0.47 mg/m3 survived. After 6
months there was no significant difference in the weights of the total
body, liver, kidneys, brain, heart and testes, and no adverse
haematological or cytogenetic effects were observed. The incidence of
"respiratory tract" tumours in animals exposed to 0, 0.0047, 0.047 or
0.47 mg/m3 was 0/112, 0/113, 0/111 and 102/111, respectively; in the
highest-concentration group, there were 96 esthesioneuroepitheliomas
(significantly different [p < 0.05] from controls), 1 carcinoma of
the nasal passage and an esthesioneuroepithelioma metastasis in the
lung, and 4 pulmonary adenomas.
Kuschner et al. (1975) exposed (by inhalation) groups of 20 to 50
male Sprague-Dawley rats to 0.47 mg BCME/m3 (0.1 ppm) for 6 h/day,
5 days/week for 2, 4, 8, 12, 16 or 20 weeks (10, 20, 40, 60, 80 or 100
exposures). Sixty exposures to BCME had no effect on mortality,
although the time at which 50% mortality was reached was reduced by
approximately 24% in animals receiving 80 or 100 exposures to BCME.
The incidence of "respiratory tract cancer" in animals surviving for
more than 210 days and receiving 10, 20, 40, 60, 80 or 100 exposures
of BCME was 1/41 (2.4%), 3/46 (6.5%), 4/18 (22.2%), 4/18 (22.2%),
15/34 (44.1%) and 12/20 (60.0%), respectively. No lung tumours were
observed following up to 40 exposures to BCME; however, the incidence
of squamous cell carcinomas of the lung was 2/20, 3/50 and 8/30 after
60, 80 and 100 exposures, respectively.
The carcinogenicity of BCME has also been examined following
subcutaneous injection in rats and mice. Groups of 20 female
Sprague-Dawley rats (weighing between 120 and 125 g) were injected
subcutaneously once per week with 3 mg BCME (dissolved in 0.1 ml
mineral oil) or vehicle alone for approximately 300 days (van Duuren
et al., 1969). Because of the corrosive effects produced by BCME,
after 114 days the dose was reduced to 1 mg, and injections were
performed only three times per month; however, because of severe
weight loss of the animals, the injections were terminated after 300
days. In the controls administered vehicle alone, no tumours were
observed at the site of injection; however a fibroadenoma and an
adenocarcinoma (of the breast) were observed elsewhere. In the group
of animals administered BCME, two fibromas and five fibrosarcomas were
observed at the site of injection, and one fibroadenoma (of the
breast) was found elsewhere (van Duuren et al., 1969).
The potential of BCME to increase the incidence of spontaneous
lung tumours in mice was assessed by Gargus et al. (1969). A group of
50 female and 50 male newborn ICR Swiss mice received a single
subcutaneous injection of 12.5 µl/kg body weight (16.6 mg/kg body
weight) BCME (dissolved in peanut oil) and the animals were observed
for a period of 6 months, after which time the survivors were
necropsied and the number of lung tumours (adenomas, based on
histopathological analysis) quantified. A group of control animals (20
females and 30 males) received a single subcutaneous injection of
vehicle alone. The numbers of female mice with pulmonary adenomas in
the control (vehicle) and BCME-exposed groups were 5/20 and 20/50,
respectively. The numbers of male mice with pulmonary adenomas in the
control (vehicle) and BCME-exposed groups were 2/30 and 25/50,
respectively. The administration of BCME had no effect upon the growth
or survival of the mice.
Zajdela et al. (1980) assessed the carcinogenicity of BCME
following repeated subcutaneous injection in male and female XVIInc/Z
mice. Groups of 30 males and 30 females received 32 injections of 0.3
mg BCME (dissolved in mineral oil) over a period of 42 weeks. The
control group consisted of 30 male mice injected with vehicle alone.
After 110 days (when the first sarcoma was observed), survival in the
control and male and female BCME-exposed groups was 100, 90 and 80%,
respectively. The number of animals with tumours (mainly
fibrosarcomas) at the site of injection was 0/30, 12/27 and 10/24,
respectively (p < 0.0001). The incidence of tumours at locations
other than the site of injection was the same in the control and
BCME-exposed groups. The incidence of pulmonary adenomas in the
control and BCME-exposed groups was 2/30 and 7/30, respectively; this
difference was not statistically significant (Zajdela et al., 1980).
The incidence of squamous cell carcinomas of the skin in female
ICR/Ha mice that received 2 mg BCME (applied dermally) or solvent
(i.e., benzene) alone thrice weekly for 325 days was 12/20 and 0/20,
respectively (van Duuren et al., 1969). A two-stage skin tumour
carcinogenesis bioassay was conducted in which the primary treatment
involved the application of 1 mg BCME (dissolved in 80 µl benzene) to
the dorsal skin of 28 male XVIInc/Z mice and the secondary treatment,
commencing 14 days later, involved the application three times per
week of 2 µg PMA (dissolved in acetone) to the dorsal skin of these
animals for 42 weeks. The incidence of mice with squamous cell
carcinomas was 0/28 and 3/28 in unexposed and BCME-initiated animals,
respectively (Zajdela et al., 1980).
7.3.3 CMME
Studies on the toxicological effects produced by long-term
inhalational exposure to CMME have been restricted primarily to
limited carcinogenesis bioassays in mice, rats and hamsters.
A study was conducted in which 50 A/Heston male mice were exposed
(by inhalation) to 0 or 6.6 mg CMME/m3 (0 or 2 ppm) for 6 h/day,
5 days/week for 101 days, followed by an observation period of
7 weeks. Although there was no significant effect upon the incidence
of pulmonary tumours, the average number of pulmonary tumours per
animal among tumour-bearing mice was 3.1 and 2.2 for the CMME-exposed
and control groups, respectively (Leong et al., 1971).
In a study in which 74 male Sprague-Dawley rats were exposed (by
inhalation) to 0 or 3.3 mg CMME/m3 (0 or 1 ppm) for 6 h/day,
5 days/week for their entire life span (up to 852 days), the incidence
of tracheal squamous metaplasia and bronchial hyperplasia was 3 and
10%, and 35 and 59%, in the control and CMME-exposed animals,
respectively. Two respiratory tract cancers (lung squamous cell
carcinoma and an esthesioneuroepithelioma of the olfactory epithelium)
were found in animals exposed to CMME (but presumably not in unexposed
controls) (Laskin et al., 1975). Exposure to CMME had no effect upon
mortality or body weight gain.
The exposure (6 h/day, 5 days/week) of 90 male hamsters to 3.3 mg
CMME/m3 (1 ppm) for virtually their entire lives increased the
incidence of tracheal metaplasia and bronchial hyperplasia compared to
88 unexposed controls. The incidence of tracheal squamous meta-plasia
was 0 and 2%, and the incidence of bronchial hyperplasia was 5 and 8%,
in the control and CMME-exposed animals, respectively (statistical
significance not stated). One lung adenocarcinoma and a tracheal
squamous papilloma were observed in two animals exposed to CMME;
presumably none was found in unexposed controls (Laskin et al., 1975).
The carcinogenicity of purified CMME has also been examined
following subcutaneous injection of this substance in rats and mice.
Groups of 20 female Sprague-Dawley rats (weighing between 120 and 125
g) were injected once per week with 3 mg (laboratory purified) CMME
(dissolved in 0.1 ml mineral oil) or vehicle alone for approximately
300 days; because of moderate corrosive effects, the injections were
terminated after this time (van Duuren et al., 1969). In controls
administered the vehicle alone, no tumours were observed at the site
of injection; however a fibroadenoma and an adenocarcinoma (of the
breast) were found elsewhere. In animals administered (laboratory
purified) CMME, a fibrosarcoma (at the site of injection) in one
animal was the only tumour described.
Van Duuren et al. (1972) subcutaneously injected (laboratory
purified) CMME (dissolved in 0.05 ml mineral oil, 300 µg/animal, once
per week) to a group of 30 female ICR/Ha Swiss mice for their entire
lives, and a similarly sized group of controls received vehicle alone.
Median survival time was 643 days and 496 days, and the numbers of
mice with sarcomas at the site of injection were 0 and 10 in the
control and purified CMME-exposed groups, respectively.
The potential of CMME to increase the incidence of spontaneous
lung tumours in mice was assessed by Gargus et al. (1969). A group of
48 female and 51 male newborn ICR Swiss mice received a single
subcutaneous injection of 125 µl/kg body weight (132.5 mg/kg body
weight) CMME dissolved in peanut oil, and subsequently observed for a
period of 6 months, after which time the survivors were necropsied and
the number of lung tumours (adenomas, based on histopathological
analysis) quantified. Controls (20 females and 30 males) received a
single subcutaneous injection of vehicle alone. The numbers of female
mice with adenomas in the control (vehicle) and CMME-exposed groups
were 5/20 and 8/48, respectively. The numbers of male mice with
adenomas in the control (vehicle) and CMME-exposed groups were 2/30
and 9/51, respectively.
Purified CMME was not carcinogenic when applied thrice weekly
(2 mg/animal for 325 days) to the skin of female ICR/Ha Swiss mice
(van Duuren et al., 1969).
7.4 Mutagenicity and related end-points
7.4.1 BCEE
A small number of investigations have been performed to assess
the genotoxic potential of BCEE. The in vitro studies have yielded
somewhat equivocal results. However it should be noted that, in
general, detailed descriptions of the laboratory conditions were not
provided, making interpretation of the findings difficult. The
mutagenic activity of BCEE in bacteria has been examined in a number
of strains, in the presence and absence of metabolic activating
systems. Simmon et al. (1977a,b) reported BCEE (vapour) to be strongly
mutagenic in Salmonella typhimurium TA100 in the absence of a
metabolic activating system, with the number of revertants increasing
with the duration of exposure. Simmon et al. (1977a,b) also reported
that in suspension assays BCEE was mutagenic in S. typhimurium
strains TA1535 and TA100, as well as in Saccharomyces cerevisiae
D3. Norpoth et al. (1986) reported "weak" mutagenic activity in
S. typhimurium TA100 (in the presence of a metabolic activating
system) when BCEE (up to 40 µg/dish) was added directly to culture
plates. Mortelmans et al. (1986) reported BCEE (up to 10 mg/plate) had
"weak" mutagenic activity in a number of S. typhimurium strains
(TA100, TA1535, TA1537, TA98), either in the presence or absence of a
metabolic activating system. Shirasu et al. (1975) reported that BCEE
was mutagenic in various strains of Escherichia coli, Bacillus
subtilis and S. typhimurium, although experimental details were
not provided in the published account of this study. In contrast,
Quinto & Radman (1987) reported that BCEE was not mutagenic in the MT
103, MT 119 and MT 126 tester strains of E. coli, although complete
experimental details were not provided in this published account.
Foureman et al. (1994) considered BCEE mutagenic, based upon the
results of a sex-linked recessive lethal assay in which male
Drosophila were injected with the compound. However the response was
negative when the males were fed BCEE. To examine the genotoxicity of
BCEE in mammals, Jorgenson et al. (1977) performed heritable
translocation assays in mice administered BCEE orally. These authors
concluded that BCEE did not promote heritable translocations. However,
few experimental details were provided in this published account.
Gwinner et al. (1983) did not detect radioactivity bound to liver DNA
or RNA isolated from male Sprague-Dawley rats exposed (by inhalation)
to [1-14C]-BCEE (amount not clearly specified) for 18 h.
7.4.2 BCME
The genotoxicity of BCME has been examined in a variety of
limited and generally poorly documented studies. BCME (at a maximum
concentration of 20 µg/plate) was mutagenic in the presence of an
exogenous metabolic activating system in S. typhimurium strain
TA100, based on a 3-fold increase in the frequency of revertants above
control levels. However, similar results were not observed in
S. typhimurium strains TA1535, TA1538 and TA98 (Anderson & Styles,
1978). BCME was also reported to be mutagenic in various strains of
E. coli and S. typhimurium, but experimental details and results
were not provided (Mukai & Hawryluk, 1973).
BCME (at concentrations as low as 0.16 µg/ml) was reported to
increase DNA repair (unscheduled DNA synthesis) in human skin
fibroblasts, although quantitative results were not provided (Agrelo &
Severin, 1981). In in vitro assays with BHK-21 and human lung WI-38
cells, concentrations of BCME between 0.008 and 25 mg/ml (in the
presence of an exogenous metabolic activating system) increased the
frequency of transformed cells approximately 6.6- and 11-fold,
respectively (Styles, 1978). The exposure (in vitro) of human
neonatal foreskin fibroblasts to concentrations of BCME between 0.1
and 8 µg/ml produced a 3- to 14-fold increase in the frequency of
anchorage-independent cells (Kurian et al., 1990).
BCME was reported to directly alkylate DNA (at guanine and
adenine residues) when the two substances were incubated together in
an in vitro assay (Goldschmidt et al., 1975). It was reported to
damage RNA within bacteriophage R17 (Shooter, 1975).
7.4.3 CMME
CMME was reported to be mutagenic in various strains of E. coli
and S. typhimurium. However, experimental details or results were
not provided in this published account (Mukai & Hawryluk, 1973). In
the presence of an exogenous metabolic activating system, CMME (1 and
10 mmol/litre) increased unscheduled DNA synthesis in human
lymphocytes approximately 30 and 100%, respectively (Perocco et al.,
1983).
7.5 Other toxicity studies
The exposure (by inhalation) of male Sprague-Dawley rats to 0.47
mg BCME/m3 (100 ppb) for 6 h/day, 5 days/week, for a period of six
months had no observable effect upon the nervous or reproductive
systems, based on gross and microscopic analysis (Leong et al., 1981).
No other relevant information regarding the reproductive,
developmental, immunological or neurological toxicity of BCEE, BCME or
CMME was identified.
8. EFFECTS ON HUMANS
8.1 General population exposure
8.1.1 Human exposure studies
Schrenk et al. (1933) reported that the "brief" (time not stated)
exposure of men to concentrations of BCEE ranging from 3218 to 5850
mg/m3 (550 to 1000 ppm) caused irritation to the eyes (lacrimation)
and nasal passages, such that exposure was considered intolerable.
Inhalation of BCEE also caused nausea. The intensity of such effects
gradually declined as the concentration of BCEE was lowered from 1521
mg/m3 (260 ppm) to 585 mg/m3 (100 ppm); exposure to 205 mg/m3 (35
ppm) was reported to be "only slightly offensive and practically free
from irritation". No clinical studies on BCME and CMME were
identified.
8.2 Occupational exposure
8.2.1 Case reports
The death of a worker in a fulling mill (textile factory) was
attributed to the inhalation of BCEE, but details were not provided
(Elkins, 1959). One anecdotal report on the occurrence of dermatitis
in textile workers exposed to resins containing BCEE was identified
(Kirwin & Sandmeyer, 1981).
Sakabe (1973) reported that 5 out of 32 Japanese males employed
in dyestuffs factories who had been exposed to BCME died of "lung
cancer" (between 1963 and 1969). Small (oat) cell carcinoma was
identified in one of the cases. Quantitative information on exposure
was not provided in this published account and these individuals were
exposed to a number of chemical substances in addition to BCME
(smoking habits could not be confirmed). However, because a large
proportion (approximately 16%) of the individuals exposed to BCME
developed lung cancer, and those exposed to chemicals other than BCME
did not, the authors attributed the occurrence of these pulmonary
tumours to exposure to BCME.
Reznick et al. (1977) reported the case of a 45-year-old male
chemist who had died of a slightly differentiated adenocarcinoma of
the lung. Twelve years earlier this individual had been exposed to
BCME and CMME over a period of two years. Although quantitative
information on exposure was not presented (and the individual was also
exposed to vinyl chloride), the lung adenocarcinoma was attributed to
his exposure to BCME and CMME.
Roe (1985) reported the case of three males (between 35 and 40
years of age) who had died of lung cancer (small (oat) cell and
squamous cell carcinomas) after having been occupationally exposed to
BCME. Although quantitative or qualitative information on exposure was
not provided and the individuals had been smokers, the relatively
young age at which these individuals died was attributed to their
exposure to BCME.
8.2.2 Epidemiological studies
Data on the effects of long-term exposure to BCEE on human health
were not identified. In a number of epidemiological studies, mortality
and morbidity due to cancer in workers occupationally exposed to BCME
and CMME have been examined. Lemen et al. (1976) examined the
incidence of lung cancer in a group of workers employed at a chemical
plant in California, USA, where BCME was used in the production of
ion-exchange resins. The authors identified 136 individuals who had
been employed for at least 5 years between 1955 and 1972. The number
of cases of lung cancer (5) was significantly greater (p < 0.01) than
the number expected (0.54), based on age-respiratory cancer-specific
incidence rates for white males in the state of Connecticut in
1960-1962. Notably, 80% of the tumours were small cell
undifferentiated cancers. Importantly, lung tumours in persons
occupationally exposed to BCME and CMME are predominantly small (oat)
cell carcinomas (Weiss, 1976; Pasternack et al., 1977). The occurrence
of this type of lung cancer in these individuals is quite distinct
from that caused by tobacco, one of the potential confounders in such
studies, where the lung tumours are predominantly squamous cell
carcinomas (Weiss, 1976; Pasternack et al., 1977). Individuals (80%
were smokers) with cancer averaged 47 years of age, and the average
latency period was approximately 10 years. Quantitative or qualitative
information on exposure was not provided in this published account.
The incidence of metaplastic and atypical cells in the sputum of
workers exposed to BCME was greater than in controls (uranium miners),
based on cytological analysis.
Nishimura et al. (1990) examined the incidence of lung cancer in
a group of Japanese workers employed in two dyestuff factories where
BCME was used. The study group consisted of 35 males employed at these
plants between 1955 and 1970. The number of cases (13) of lung cancer
(11 cases were in smokers) was significantly (p < 0.001) higher than
the number expected (0.62). Tumours from eight of the individuals were
examined histopathologically; four were diagnosed as small cell
undifferentiated carcinomas, two were adenocarcinomas and one a large
cell carcinoma; in one individual, both a small cell carcinoma and an
adenocarcinoma were found. The average age at which individuals with
lung cancer died was 46 years, and the latency period was
approximately 13.5 years. The average duration of exposure to BCME was
approximately 7.2 years, although no other quantitative or qualitative
information on exposure was provided.
Since technical grade CMME contains between 1 and 8% BCME
(Travenius, 1982), in epidemiological studies in which mortality and
the incidence of cancer in workers exposed to CMME were examined, the
effects may have been due (at least in part) to BCME. Weiss (1976)
reported the results of a 10-year prospective study (1963-1973) in
which 125 male employees of a chemical plant in the USA who had been
occupationally exposed to CMME(BCME), were examined with respect to
the "incidence" of pulmonary cancer. No quantitative or qualitative
information on exposure was provided. However, an exposure index (low,
medium and high) based on type and duration of job associated with
potential exposure to CMME(BCME) was developed. Eleven cases of lung
cancer were reported in 49 individuals with medium or high exposure to
CMME(BCME); no "incidence" of lung cancer was reported in 76 workers
with no or low exposure to CMME(BCME). The number of deaths (16)
during this period was 2.7-fold greater than the number expected
(5.9), based on a comparison with death rates for white males in the
USA. All of the excess deaths (10) were attributable to lung cancer,
100% of which were small cell carcinomas that developed in individuals
less than 55 years of age. The latency period for these cancers ranged
from 10 to 24 years. Among individuals exposed to CMME(BCME) the
"incidence" of pulmonary tumours was inversely related to their use of
tobacco (Weiss, 1980). In a follow-up study of these workers, the
number of deaths (13) due to lung cancer (which were attributable to
either moderate or heavy exposure to CMME[BCME]) was 19.5-fold greater
than the number (0.66) expected, based on lung cancer mortality rates
in the surrounding municipality (Philadelphia) (Weiss, 1982). The
standardized mortality ratio for deaths due to lung cancer which
peaked 15 to 19 years from the onset of exposure, declined during the
subsequent 20- to 29-year period. Subsequently, Weiss (1989) indicated
that over-representation of workers with moderate to high exposure
within the cohort led to some over-estimation of the risk of lung
cancer. However, even when such selection bias was accounted for, an
increased risk of lung cancer remained associated with exposure to
CMME(BCME).
Maher & DeFonso (1987) examined mortality in a group of workers
exposed to CMME(BCME). [This report represented an update and
extension of a previous investigation on death due to lung cancer
performed by these authors (DeFonso & Kelton, 1976)]. The study
population consisted of a group of 737 "exposed" and 2120 "unexposed"
white male workers (who comprised 97% of the labour force) employed
for any length of time at a chemical plant in the USA between 1948 and
1971. The vital status of 90% of the group was determined up to 1982.
No quantitative information on exposure was provided, but an exposure
rating (from 0-6) was developed based on the type of work, proximity
of exposure to CMME(BCME) and production methods. Cumulative exposure
was calculated based on the exposure rating and duration of employment
at a particular job. The expected number of deaths for each type of
cancer was calculated using cause-specific death rates for white males
residing in the surrounding municipality (Philadelphia). Information
on smoking habits was incomplete but "no marked differences between
smoking habits of exposed and unexposed workers were noted" (Maher &
DeFonso, 1987). Among the workers exposed to CMME(BCME), the number of
deaths (32) due to cancer of the "respiratory tract" was significantly
(P < 0.01) higher than the number expected (11.5). For individuals
not exposed to CMME(BCME), the number of deaths (25) due to
respiratory tract cancer was similar to those expected (23.8). In the
CMME(BCME)-exposed group, the number of deaths due to cancer of the
digestive, genito-urinary, haematopoietic, lymphatic and central
nervous systems was not significantly greater than expected. The
greatest increase in deaths due to cancer of the respiratory tract
occurred approximately 10 to 20 years after the first exposure to
CMME(BCME). Among workers exposed to CMME(BCME), the ratio of
observed/expected number of deaths due to lung cancer was lower
between 1975 and 1981 than for the period between 1960 and 1974, this
being attributed to a reduction in the level of exposure to CMME(BCME)
in 1971 as a result of the implementation of stringent engineering
controls on the use of this substance (Maher & DeFonso, 1987).
Collingwood et al. (1987) assessed mortality due to respiratory
cancer in a group of workers employed at seven industrial facilities
in which CMME(BCME) was produced or utilized. This report represented
a follow-up and extension of a previous study by two of these authors
(Pasternack et al., 1977). The study group (97% white, 96% male)
comprised 2460 CMME(BCME)-exposed and 3692 unexposed workers employed
between 1948 and 1980. Only limited information on smoking habits was
available. No quantitative or qualitative information on exposure was
provided, but an exposure index (taking into account type of job,
frequency of work and potential exposure to CMME(BCME)) was developed.
Cumulative exposure was calculated on the basis of the exposure index
and duration of employment at a particular job. The number of expected
deaths was calculated from death rates in the USA specific for age,
cause, sex, race and calendar year. Among workers exposed to
CMME(BCME), the standardized mortality ratio for death due to
respiratory cancer was significantly increased (SMR = 3.01;
95% CI = 2.24-3.98); this was attributed to excess deaths at two
companies (where the ratio of observed to expected deaths due to lung
cancer among exposed workers was 32/7.4 and 9/1.5). In the entire
study group, there were 90 deaths due to respiratory cancer, 52 and 38
in the CMME(BCME)-exposed and unexposed groups, respectively. In those
cases with verifiable histology, 12/32 (38%) cases in the exposed
group had small (oat) cell carcinomas while 6/20 (30%) cases in the
unexposed group had adenocarcinomas. The relative risk of death due to
lung cancer was found to be related to total cumulative exposure based
on a regression model.
Gowers et al. (1993) examined the incidence of lung cancer among
1203 males employed at an ion-exchange resin manufacturing plant in
France between 1958 and 1986; of the total study cohort, 258 were
exposed to technical grade CMME (i.e., containing BCME), the remainder
were considered to be unexposed. Data on the incidence of lung cancer
among these workers was obtained from a local registry; the expected
number of lung cancer cases was calculated, based upon incidence rates
for males provided by a registry serving an area some 250 km distant
from the plant (i.e., external population). There were 8 (one small
cell carcinoma) and 11 (10 small cell carcinomas) cases of lung cancer
among the unexposed and CMME(BCME)-exposed workers, respectively.
Reductions in occupational exposure to CMME(BCME) were instituted in
1972 and 1984. Potential exposure to CMME(BCME) was rated according to
employment experience; cumulative exposure was based upon the job
exposure rating and length of employment at a particular job. Based
upon limited monitoring studies, conducted between 1979 and 1984, the
average level of BCME in the plant ranged from 3.3 to 20.7 µg/m3
(0.7 to 4.4 ppb); after 1984 the average level in the plant was
reportedly < 2.4 µg/m3 (0.5 ppb). The rate ratio for lung cancer
among the CMME(BCME)-exposed workers, compared to the rate for the
unexposed workers or external population, was 5.5 (95% CI = 2.0-12.3)
and 7.6 (95% CI = 4.3-13.5), respectively. The rate ratio for lung
cancer among the unexposed workers, compared to the rate for the
external population, was 1.6 (95% CI = 0.8-3.12). Linear regression
analysis revealed an increased rate of lung cancer with increasing
cumulative exposure. The ratio of observed/expected cases of lung
cancer for the CMME(BCME)-exposed workers (based upon comparison with
the external population) generally increased with increasing
cumulative exposure. The mean age at diagnosis for the unexposed and
CMME(BCME)-exposed workers was 56.5 and 46 years, respectively; the
mean induction period for lung cancer among the CMME(BCME)-exposed
workers was approximately 13 years.
Excess deaths due to lung cancer have also been reported for
Chinese (Xue et al., 1988) chemical workers exposed to
"chloromethylether"; latency as low as 2 years was reported. In a
follow-up study of one of the cohorts examined by Xue et al. (1988),
exposed to both CMME and BCME, there was an increased rate of lung
markings similar to asbestosis as well as evidence of reduced
pulmonary function (Xue et al., 1996). The magnitude of these changes
was higher among workers exposed before 1975 to CMME levels of 0.096
µg/m3 than to those exposed after 1981 to approximately 0.013 µg/m3.
Sram et al. (1983, 1985) reported the increased frequency of
chromosomal aberrations in peripheral lymphocytes of workers (1.64 to
3.75% in controls, and 5.06 to 5.49% in subjects employed from 1-10
years) exposed in the production of ion exchange resins to levels of
0.01 to 0.1 µg BCME/m3 and 20 to > 200 µg CMME/m3.
No relevant studies are available concerning the neurological,
immunological, developmental or reproductive effects of BCME or CMME
in humans.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
For aquatic species, the 7-day LC50 for exposure of the guppy
(Poecilia reticulata) to BCEE was 56.9 mg/litre (Konemann, 1981).
Buccafusco et al. (1981) reported a 96-h LC50 of 600 mg BCEE/litre
for the bluegill sunfish (Lepomis macrochirus). LeBlanc (1980)
reported a 48-h LC50 of 240 mg BCEE/litre for Daphnia magna. In all
three of the above studies, organisms were exposed to nominal
concentrations of BCEE in closed containers, under static or
static-with-renewal conditions.
Anaerobic activity was not inhibited when microorganisms were
exposed to BCEE at concentrations up to 100 mg/litre in a nutrient
buffer solution (Johnson & Young, 1983). Cho et al. (1989) reported an
LC50 and an LC10 of 2160 and 600 µg BCEE/litre, respectively, for
microbes indigenous to industrial waste stabilization ponds and that
required a supply of organic material for food.
No relevant data are available to assess toxicity of BCEE to
species of wildlife, and no studies have been identified in which the
toxicity of either BCME or CMME to aquatic or terrestrial organisms
was investigated.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 BCEE
The lack of available information on concentrations of BCEE in
several environmental media to which humans are exposed precludes
quantitative estimation of the total daily intake of this substance
from the general environment. Based upon extremely limited data, the
estimated intake of BCEE from drinking-water for adults would be
approximately 0.01 µg/kg body weight per day. Quantitative information
on the extent of potential workplace exposure to this substance was
not identified.
Available data on the toxicity of BCEE in humans are extremely
limited. Irritation to the eyes, nasal passages and respiratory tract
could result from acute inhalation exposure to moderate levels of
BCEE. Data were considered inadequate to assess the human health risks
of non-neoplastic effects arising from longer-term exposure to this
substance.
Studies on the toxicological effects produced by the long-term
exposure of laboratory animals to BCEE have focused on its
carcinogenic potential. Some very limited evidence of carcinogenicity
in hybrid F1 mice was reported in one study (Innes et al., 1969).
However, none of the long-term (subchronic or chronic/
carcinogenicity) studies in laboratory animals is considered to be of
sufficient quality to provide useful quantitative information on the
carcinogenic potential of BCEE or the toxicological effects produced
by long-term exposure to this substance. Moreover, studies of
developmental and reproductive effects of BCEE in laboratory animals
have not been identified. Available data were, therefore, considered
inadequate to assess the risks to human health associated with
exposure to BCEE in the general or occupational environments.
10.1.2 BCME and CMME
Information on the concentrations of BCME and CMME in air,
drinking-water, soil or foodstuffs were not identified, and therefore
it was not possible to estimate the intake of these substances by the
general population. However, owing to the extremely rapid hydrolysis
of these compounds in aqueous media, exposure of non-occupationally
exposed individuals is likely to be negligible. However, in some
countries exposure of the general population to chloromethyl ethers
may occur through the use of mosquito coils. Information on
occupational exposure to BCME and CMME is also limited, although a
recent study of a resin manufacturing plant reported lower levels of
BCME than had been observed in previous, older investigations of
plastics, textile and chemical manufacturing plants.
Based upon the results of studies conducted with animals,
inhalation of BCME or CMME may produce severe irritation of the eyes
and respiratory tract as well as necrotizing bronchitis. Dermal
exposure to BCME and CMME can result in erythema and necrosis.
In all of the cohort studies of occupationally exposed workers
conducted to date, an association between lung cancer and exposure to
either BCME or CMME has been observed. The type of lung cancer, the
standardized mortality ratios, the latency periods and average age of
appearance of lung cancer in groups of workers exposed to either BCME
or CMME have been remarkably consistent. The type and incidence of
lung cancer in individuals exposed to BCME or CMME, predominantly
small (oat) cell carcinomas, occurring in relatively young individuals
after short latency periods (as low as 2 years), is distinct from that
caused by tobacco, one of the potential confounders in such studies,
where lung tumours are predominantly squamous cell carcinomas,
occurring after long latency periods in individuals greater than 60
years of age. The association between exposure to either BCME or CMME
and lung cancer is strong, with standardized mortality ratios ranging
up to 21.
For CMME, there is also evidence of a positive relationship
between a qualitative measure of exposure and mortality due to lung
cancer. In two studies of occupationally exposed individuals, the
standardized mortality ratios for deaths due to lung cancer peaked 10
to 20 years following the onset of exposure. Furthermore, observation
of an association between occupational exposure to BCME or CMME and
the development of lung cancer is plausible. This observation is based
on the results of early, rather limited carcinogenesis bioassays in
exposed animal species, in which increases in the incidence of
tumours, predominantly of the respiratory tract, have been observed,
as well as on available data on the genotoxicity of BCME and CMME.
The observed association of lung cancer and occupational exposure
to either BCME or CMME fulfil traditional criteria for assessment of
causality in epidemiological studies, i.e. consistency, strength,
specificity, temporal relationship, exposure-response relationship,
plausibility and supporting data on chromosomal effects in workers
exposed to 0.01 µg BCME/m3 and 20 µg CMME/m3. Clearly, BCME and
technical grade CMME are carcinogenic to humans, and, therefore,
exposure to these substances should be eliminated.
10.1.3 Guidance values
Available data on BCEE were considered inadequate to derive a
meaningful guidance value for this substance.
Inhalation is the principal route of exposure to these
substances. Data available in epidemiological studies of workers
exposed to BCME and CMME are inadequate to characterize quantitatively
the exposure-response relationship for carcinogenicity. There is
evidence that the general population may be exposed to BCME and CMME
through the use of mosquito coils, but there are no quantitative
exposure data available. However, in humans there is an increase in
cancer incidence (latency period as short as 2 years) with cumulative
exposure to BCME and an increase in chromosomal aberrations in workers
at levels as low as 0.01 µg BCME/m3 and 20 µg CMME/m3. Based on
multistage modelling of the incidence of esthesioneuro-epitheliomas in
rats exposed to BCME (Leong et al., 1981), the estimated concentration
of this substance associated with a 5% increase in tumour incidence
(TD05), corrected for intermittent (6 of 24 h, 5 days/week) versus
continuous exposure, is 6 µg/m3. Data on CMME were insufficient to
derive a TD50. Limitations of the critical study including the
relatively short period of exposure (6 months followed by 22-month
observation period) and sharp increase in the incidence of these
tumours between the mid- and high-concentration groups, should,
however, be borne in mind in the interpretation of this value.
The above analysis of data further strengthens the recommendation
to eliminate human exposure to BCME and CMME.
10.2 Evaluation of effects on the environment
10.2.1 BCEE
BCEE is highly soluble in water and tends to remain there,
although some volatilization from soil and water to the atmosphere
occurs. Owing to lack of adsorption, BCEE is mobile in soils,
especially those with low organic carbon content, and therefore it has
the potential to reach groundwater. BCEE does not bioaccumulate or
biomagnify to any significant extent.
Exposure of terrestrial organisms to BCEE is considered to be
negligible because of its extremely low rate of release and short
persistence in the atmosphere. For aquatic biota, a 7-day LC50 of
56.9 mg/litre (nominal concentration) for the guppy (Poecilia
reticulata) has been reported. The lowest LC50 reported for acute
toxicity (48-h) is 240 mg/litre (nominal concentration) for Daphnia
magna. The highest concentration of BCEE reported for surface water
in the USA (1.4 µg/litre) is approximately 40 000 times lower than the
reported 7-day LC50 for the guppy (Poecilia reticulata).
Although it is relatively persistent in water, the highest
reported concentration of BCEE in surface water is approximately five
orders of magnitude lower than the concentration found to induce
adverse effects in the guppy, the most sensitive aquatic species
identified among existing toxicity studies. Therefore, BCEE is not
expected to pose a significant risk to environmental organisms.
10.2.2 BCME and CMME
Both substances are readily hydrolysed in aqueous media or
photo-oxidized in the atmosphere and, therefore, are not likely to
accumulate. Because of their extremely short residence times, levels
in the environment (if any) are likely to be extremely low. Thus,
despite the lack of data concerning the environmental toxicity of BCME
and CMME, there is no reason to suspect that adverse effects could
occur to organisms living in the ambient environment.
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
a) Exposure to BCME and technical CMME should be eliminated.
b) Levels of BCEE in environmental media to which humans are exposed
should be determined.
12. FURTHER RESEARCH
a) Workers previously exposed to BCME and technical grade CMME
should be followed using all available methods, including markers
of biological effect for the detection of lung cancer at an early
stage.
b) The degree of exposure of the general public to BCME and CMME
through the use of mosquito coils containing the S-2 synergist
octachlorodipropyl ether should be measured. In this study the
degree of possible contamination of the S-2 synergist by BCME and
CMME should also be taken into account.
c) If it is still used, the toxicological profile for BCEE should be
determined in well-designed toxicological studies.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
BCEE has been evaluated by the International Agency for Research
on Cancer (IARC) and placed in Group 3 - "not classifiable as to its
carcinogenicity in humans" (IARC, 1987). BCME and CMME are considered
by IARC to be carcinogenic to humans (Group 1) (IARC, 1987).
REFERENCES
Agrelo CE & Severin BJ (1981) A simplified method for measuring
scheduled and unscheduled DNA synthesis in human fibroblasts.
Toxicology, 21: 151-158.
Albert RE, Sellakumar AR, Laskin S, Kuschner M, Nelson N, & Snyder CA
(1982) Gaseous formaldehyde and hydrogen chloride induction of nasal
cancer in the rat. J Natl Cancer Inst, 68: 597-603.
Anderson D & Styles JA (1978) The bacterial mutation test. Br J
Cancer, 37: 924-930.
ATSDR (1989a) Toxicological profile for bis(2-chloroethyl)ether.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry
(Report PB90-168683).
ATSDR (1989b) Toxicological profile for bis(chloromethyl) ether.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry
(Report PB90-168691).
Barrows M, Petrocelli S, Macek K, & Carrol J (1978) Bioconcentration
and elimination of selected water pollutants by bluegill sunfish
(Leptomis macrochirus). In: Haque R ed. Dynamics, exposure and
hazard assessment of toxic chemicals. Ann Arbor, Michigan, Ann Arbor
Science Publishers, Inc., pp 379-392.
Blease T, Scrivens J, & Morden W (1989) The determination of
atmospheric bis(chloromethyl) ether by gas chromatography/ tandem mass
spectrometry. Biomed Environ Mass Spectrom, 18: 775-779.
Buccafusco RJ, Ells SJ, & LeBlanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Callahan M, Slimak M, Gabel N, May I, Fowler C, Freed J, Durfee R,
Whitmore F, Maestri B, Mabey W, Holt B, & Gould C (1979) Water-related
environmental fate of 129 priority pollutants: Volume II - Halogenated
aliphatic hydrocarbons, halogenated ethers, monocyclic aromatics,
phthalate esters, polycyclic aromatic hydrocarbons, nitrosamines, and
miscellaneous compounds. Washington, DC, US Environmental Protection
Agency, Office of Water Planning and Standards, Office of Water and
Waste Management (EPA-440/4-79-029b, PB80-204381).
CCINFO (1991) Canadian Centre for Occupational Health and Safety
database: Data for chloromethyl methyl ether. Hamilton, Ontario,
Canadian Centre for Occupational Health and Safety.
Chen BI, Sun FG, & Xue SZ (1997)The occurrence of a cluster of lung
cancer among the workers in the synthesis of octachloropropanyl ether.
Chin J Ind Med, 10(5): 305-306.
Cho Y-H, Davis EM, & Ramey GD (1989) Assessing microbial toxicity of
2-ethoxyethanol and bis(2-chloroethyl)ether by a modified spread plate
method. Environ Technol Lett, 10: 875-886.
Collier L (1972) Determination of bis(chloromethyl) ether at the ppb
level in air samples by high-resolution mass spectroscopy. Environ Sci
Technol, 6: 930-932.
Collingwood KW, Pasternack BS, & Shore RE (1987) An industry-wide
survey of respiratory cancer in chemical workers exposed to
chloromethyl ethers. J Natl Cancer Inst, 78: 1127-1136.
Cuppit L (1980) Fate of toxic and hazardous materials in the air
environment. Research Triangle Park, US Environmental Protection
Agency, Atmospheric Chemistry and Physics Laboratory
(EPA-600/3-80-084, PB80-221948).
DeFonso LR & Kelton SC (1976) Lung cancer following exposure to
chloromethyl methyl ether. Arch Environ Health, 31: 125-130.
DeWalle FB & Chian ES (1981) Detection of trace organics in well water
near a solid waste landfill. J Am Water Works Assoc, 73: 206-211.
Drake KD & Myer JR (1992) Acute oral toxicity of DCEE (dichloroethyl
ether) in rats and mice. Acute Toxicol Data, 1: 163-164.
Dressman RC, Fair J, & McFarren EF (1977) Determinative method of
analysis of aqueous sample extracts for bis(2-chloro) ethers and
dichlorobenzenes. Environ Sci Technol, 11: 719-721.
Drew RT, Laskin S, Kuschner M, & Nelson N (1975) Inhalation
carcinogenicity of haloethers: I. The acute inhalation toxicity of
chloromethyl methyl ether and bis(chloromethyl)ether. Arch Environ
Health, 30: 61-69.
Durkin P, Howard P, & Saxena J (1975) Investigation of selected
environmental contaminants: Haloethers. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances
(EPA 68-1-2996, PB-246 356).
Eisner D (1974) Formation of bis-chloromethylether from hydrogen
chloride and formaldehyde. Redwood City, California, Diamond Shamrock
Chemical Co., Nopco Chemical Division (Report No. EQR-7412).
Elkins HB (1959) Chemistry of industrial toxicology. New York, John
Wiley and Sons.
Evans K, Mathias A, Mellor N, Silvester R, & Williams A (1975)
Detection and estimation of bis(chloromethyl) ether in air by
gas-chromatography high-resolution mass spectrometry. Anal Chem, 47:
821-824.
Fishbein L (1979) Potential halogenated industrial carcinogenic and
mutagenic chemicals: III. Alkane halides, alkanols and ethers. Sci
Total Environ, 11: 223-257.
Foureman P, Mason JM, Valencia R, & Zimmering S (1994) Chemical
mutagenesis testing in Drosophila: IX. Results of 50 coded compounds
tested for the National Toxicology Program. Environ Mol Mutagen, 23:
51-63.
Frankel L & Black R (1976) Automatic gas chromatographic monitor for
the determination of parts-per-billion levels of bis(chloromethyl)
ether. Anal Chem, 48: 732-737.
Frankel LS, McCallum K, & Collier L (1974) Formation of
bis(chloromethyl) ether from formaldehyde and hydrogen chloride.
Environ Sci Technol, 8: 356-359.
Galvin R & House M (1988) Atmospheric monitoring of bis(chloromethyl)
ether at low ppb levels using an automated system. Environ Technol
Lett, 9: 563-570.
Gargus JL, Reese WH, & Rutter HA (1969) Induction of lung adenomas in
newborn mice by bis(chloromethyl)ether. Toxicol Appl Pharmacol, 15:
92-96.
Goldschmidt BM, van Duuren BL, & Frenkel K (1975) The reaction of
14C-labelled bis(chloromethyl)ether with DNA. Proc Am Assoc Cancer
Res, 16: 66.
Government of Canada (1993a) Canadian Environmental Protection Act -
Priority substances list assessment report: Bis(2-chloroethyl) ether.
Ottawa, Government of Canada.
Government of Canada (1993b) Canadian Environmental Protection Act -
Priority substances list assessment report: Bis(chloromethyl)ether and
chloromethyl methyl ether. Ottawa, Government of Canada.
Gowers DS, DeFonso LR, Schaffer P, Karli A, Monroe CB, Bernabeu L, &
Renshaw FM (1993) Incidence of respiratory cancer among workers
exposed to chloromethyl-ethers. Am J Epidemiol, 137: 31-42.
Gwinner LM, Laib RJ, Filser JG, & Bolt HM (1983) Evidence of
chloroethylene oxide being the reactive metabolite of vinyl chloride
toward DNA: Comparative studies with 2,2'-dichloro-diethylether.
Carcinogenesis, 4: 1483-1486.
Hauser T & Bromberg S (1982) EPA's program at Love Canal. Environ
Monit Assess, 2: 249-272.
Hites RA, Jungclaus GA, & Lopez-Avila V (1979) Potentially toxic
organic compounds in industrial waste-waters and river systems: Two
case studies. In: Monitoring toxic substances. Washington, DC,
American Chemical Society, pp 63-90 (American Chemical Symposium
Series 94).
Howard P, Boething R, Jarvis W, Meylan W, & Michalenko E (1991)
Handbook of environmental degradation rates. Chelsea, Michigan, Lewis
Publishers, Inc.
IARC (1974) Bis(chloromethyl)ether. Some aromatic amines, hydrazines,
and related substances, N-nitroso compounds and miscellaneous
alkylating agents. Lyon, International Agency for Research on Cancer,
pp 231-245 (Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, Volume 4).
IARC (1975) Bis(2-chloroethyl) ether. In: Some aziridines, N-, S- and
O-mustards and selenium. Lyon, France, International Agency for
Research on Cancer, pp 117-123 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Man, Volume 9).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volums 1 to 42. Lyon, International Agency for
Research on Cancer, p 58 (Monographs on the Evaluation of Carcinogenic
Risks to Humans, Supplement 7).
Innes JRM, Ulland BM, Valerio MG, Petrucelli L, Fishbein L, Pallotta
AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, & Peters J
(1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J Natl Cancer Inst,
42: 1101-1114.
IPCS (1994) Environmental health criteria 170: Assessing human health
risks of chemicals: Derivation of guidance values for health-based
exposure limits. Geneva, World Health Organization, International
Programme on Chemical Safety, 71 pp.
Johnson LD & Young JC (1983) Inhibition of anaerobic digestion by
organic priority pollutants. J Water Pollut Control Fed,
55: 1441-1449.
Jorgenson TA, Rushbrook CJ, Newell GW, & Tardiff RG (1977) Study of
the mutagenic potential of bis(2-chloroethyl) and
bis(2-chloroisopropyl) ethers in mice by the heritable translocation
test. Toxicol Appl Pharmacol, 41: 196-197.
Kallos G (1981) Oxygen induced response enhancement of determination
of bis(chloromethyl) ether by gas chromatography with nickel-63
electron capture detection. Anal Chem, 53: 963-965.
Kallos G & Tou J (1977) Study of photolytic oxidation and chlorination
reactions of dimethyl ether and chlorine in ambient air. Environ Sci
Technol, 11: 1101-1105.
Kallos G, Albe W, & Solomon R (1977) On-column reaction gas
chromatography for determination of chloromethyl methyl ether at the
one part-per-billion level in ambient air. Anal Chem, 49: 1817-1820.
Keith L, Garrison A, Allen F, Carter M, Floyd T, Pope J, & Thruston A
(1976) Identification of organic compounds in drinking-water from
thirteen U.S. cities. In: Keith LH ed. Identification and analysis
of organic pollutants in water. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., p 356.
Kendall PR (1990) The quality of drinking-water in Toronto: A review
of tapwater, bottled water and water treated by a point-of-use device
- Summary report. Toronto, Canada, Department of Public Health,
Environmental Protection Office, 41 pp.
Kincannon D & Lin Y (1986) Microbial degradation of hazardous wastes
by land treatment. In: Proceedings of the 40th Industrial Waste
Conference, Purdue University, West Lafayette, IN, 14-16 May 1985.
Boston, Ann Arbor Science Publishers, Inc., pp 607-619.
Kirwin CJ & Sandmeyer EE (1981) Ethers. In: Clayton GD & Clayton FE
ed. Patty's industrial hygiene and toxicology: Volume IIA, 3rd ed.
New York, John Wiley and Sons, pp 2491-2565.
Kleopfer RD & Fairless BJ (1972) Characterization of organic
components in a municipal water supply. Environ Sci Technol,
6: 1036-1037.
Konemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies - Part 1: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-221.
Kraybill HF (1977) Global distribution of carcinogenic pollutants in
water. Ann NY Acad Sci, 298: 80-89.
Kurian P, Nesnow S, & Milo GE (1990) Quantitative evaluation of the
effects of human carcinogens and related chemicals on human foreskin
fibroblasts. Cell Biol Toxicol, 6: 171-184.
Kuschner M, Laskin S, Drew RT, Cappiello V, & Nelson N (1975)
Inhalation carcinogenicity of alpha haloethers: III. Lifetime and
limited period inhalation studies with bis(chloromethyl)ether at 0.1
ppm. Arch Environ Health, 30: 73-77.
Langelaan F & Nielen M (1989) Determination of trace levels of
chloromethyl methyl ether and bis(chloromethyl) ether in air. Int J
Environ Anal Chem, 36: 27-34.
Langhorst M (1985) Monitoring chloromethyl methyl ether in air. In:
Fishbein L & O'Neill KI ed. Environmental carcinogens. Selected
methods of analysis - Volume 7: Some volatile halogenated
hydrocarbons. Lyon, International Agency for Research on Cancer, pp
235-246 (IARC Scientific Publications No. 68).
Langhorst M, Melcher R, & Kallos G (1981) Reactive adsorbent
derivative collection and gas chromatographic determination of
chloromethyl methyl ether in air. Am Ind Hyg Assoc J, 42: 47-55.
Laskin S, Drew RT, Cappiello V, Kuschner M, & Nelson N (1975)
Inhalation carcinogenicity of alpha halo ethers: II. Chronic
inhalation studies with chloromethyl methyl ether. Arch Environ
Health, 30: 70-73.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water
fleas (Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lemen R, Johnson W, Wagoner J, Archer V, & Saccomanno G (1976)
Cytologic observations and incidence following exposure to BCME. Ann
NY Acad Sci, 271: 71-80.
Leong BKJ, MacFarland HN, & Reese WH (1971) Induction of lung adenomas
by chronic inhalation of bis (chloromethyl) ether. Arch Environ
Health, 22: 663-666.
Leong BKJ, Kociba RJ, & Jersey GC (1981) A lifetime study of rats and
mice exposed to vapors of bis(chloromethyl)ether. Toxicol Appl
Pharmacol, 58: 269-281.
Lingg RD, Kaylor WH, Pyle SM, & Tardiff RG (1979) Thiodiglycolic
acid: a major metabolite of bis(2-chloroethyl) ether. Toxicol Appl
Pharmacol, 47: 23-34.
Lingg RD, Kaylor WH, Pyle SM, & Domino MM (1982) Metabolism of
bis(2-chloroethyl)ether and bis(2-chloroisopropyl)ether in the rat.
Arch Environ Contam Toxicol, 11: 173-183.
Mabey W, Smith J, Podoll R, Johnson H, Mil T, Chou T, Gates J,
Partridge I, Jaber H, & Vandenberg D (1982) Aquatic fate processes
data for organic priority pollutants. Washington, DC, US Environmental
Protection Agency, Office of Water Regulations and Standards,
Monitoring and Data Support Division (EPA 440/4-81-014).
Mackay D & Wolkoff A (1973) Rate of evaporation of low-solubility
contaminants from water bodies to the atmosphere. Environ Sci Technol,
7: 611-614.
McMillin CR, Gable RC, Kyne JM, Quill RP, Snyder AD, & Thomas JA
(1984) EPA method study 21, method 611: Haloethers. Cincinnati, Ohio,
US Environmental Protection Agency, Environmental and Support
Laboratory, Office of Research and Development (EPA-600/4-84-052,
PB84-205939).
Maher KV & DeFonso LR (1987) Respiratory cancer among
chloromethylether workers. J Natl Cancer Inst, 78: 839-843.
Manwaring J, Blankenship W, Miller L, & Voigt F (1977)
Bis(2-chloroethyl) ether removal from drinking-water by source
protection. In: Pojasek RB ed. Drinking-water quality enhancement
through source protection. Ann Arbor, Michigan, Ann Arbor Science
Publishers, Inc., pp 417-429.
Marceleno T (1974) Survey of Burlington Industries, Inc., Burlington
House Finishing Plant, Form Fabrics Plant, Durham Domestics Plant,
Brookneal Finishing Plant. Cincinnati,Ohio, National Institute for
Occupational Safety and Health, Environmental Investigations Branch,
Division of Field Studies and Clinical Investigations (Report
PB82-151077).
Milano J, Bernat-Escallon C, & Vernet J (1989) Dégradations dans l'eau
par hydrolyse et photolyse du bis-2 chloroéthyl éther. Environ Technol
Lett, 10: 291-300.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 7: 1-119.
Mukai FH & Hawryluk I (1973) Mutagenicity of some halo-ethers and
halo-ketones. Mutat Res, 21: 228.
Muller G, Norpoth K, & Eckard R (1979) Identification of
S-(carboxymethyl)-L-cysteine and thiodiglycollic acid, urinary
metabolites of 2,2'-bis-(chloroethyl)-ether in the rat. Cancer Lett,
7: 299-305.
Muller G, Norpoth K, & Travenius S (1981) Quantitative determination
of bis(chloromethyl) ether (BCME) in the ppb range by using portable
air sample collectors. Int Arch Occup Environ Health, 48: 325-329.
National Research Council (NRC) (1977) Drinking-water and health -
Volume 1. Washington, DC, National Academy Press, p 711.
Nichols RW & Merritt RF (1973) Brief communication: relative
solvolytic reactivities of chloromethyl ether and bis(chloromethyl)
ether. J Natl Cancer Inst, 50: 1373-1374.
NIOSH (1974) The toxic substances list, 1974 edition. Cincinnati,
Ohio, National Institute for Occupational Safety and Health, p 134 (US
Department of Health, Education and Welfare Publication No. 74).
NIOSH (1984) Method 1004: Dichloroethyl ether. In: NIOSH manual of
analytical methods, 3rd ed. Cincinnati, Ohio, National Institute for
Occupational Health and Safety, pp 1004/1-1004/3.
Nishimura K, Miyashita K, Yoshida Y, Kuroda M, Matsumoto M, Matsumoto
K, Takeda S, & Hara I (1990) An epidemiological study of lung cancer
among workers exposed to bis(chloromethyl)ether. Jpn J Ind Health, 32:
448-453.
Norpoth K, Muller G, Zilius Z, Ulynec U, & Travenius SZM (1981)
Sensitive spectrophotometric determination of carcinogenic alpha
chloro ethers. Int Arch Occup Environ Health, 49: 151-155.
Norpoth K, Heger M, Muller G, Mohtashamipur E, Kemena A, & Witting C
(1986) Investigations of metabolism, genotoxic effects, and
carcinogenicity of 2,2-dichlorodiethyl ether. J Cancer Res Clin
Oncol, 112: 125-130.
Parkes D, Ganz C, Polinsky A, & Schultze J (1976) A simple gas
chromatographic method for the analysis of trace organics in ambient
air. Am Ind Hyg Assoc J, 37: 165-173.
Pasternack BS, Shore RE, & Albert RE (1977) Occupational exposure to
chloromethylethers. J Occup Med, 19: 741-746.
Pellizzari E, Erickson M, & Zweidinger R (1979) Formulation of a
preliminary assessment of halogenated compounds in man and
environmental media. Washington, DC, US Environmental Protection
Agency, Office of Toxic Substances (EPA-560/13-79-006, PB 80-112170).
Perocco P, Bolognesi S, & Alberghini W (1983) Toxic activity of 17
industrial compounds on human lymphocytes cultured in vitro. Toxicol
Lett, 16: 69-76.
Piwoni M, Wilson J, Walters D, Wilson B, & Enfield C (1986) Behaviour
of organic pollutants during rapid infiltration of wastewater into
soil: I. Processes, definition, and characterization using a
microcosm. Hazard Waste Hazard Mater, 3: 43-55.
Quaghebeur DG, Hierneaux G, & DeWulf E (1986) Tracing a source of
pollution by determination of specific pollutants in surface- and
groundwater. Proceedings of the 4th European Symposium, Vienna,
Austria, 1985, pp 142-146.
Quinto I & Radman M (1987) Carcinogenic potency in rodents versus
genotoxic potency in E. coli: a correlation analysis for
bifunctional alkylating agents. Mutat Res, 181: 235-242.
Radding S, Holt B, Jones J, Jiu D, Mill T, & Hendry D (1977) Review of
the environmental fate of selected chemicals. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (EPA
560/5-77/003).
Reznick G, Wagner WW, & Atay Z (1977) Lung cancer following exposure
to bis(chloromethyl)ether: A case report. J Environ Pathol Toxicol, 1:
105-111.
Roe FJC (1985) Chloromethylation: Three lung cancer deaths in young
men. Lancet, 2: 268.
Rosen A, Skeel R, & Ettinger M (1963) Relationship of river water
odour to specific organic contaminants. J Water Pollut Control Fed,
35(6): 777-782.
Sakabe H (1973) Lung cancer due to exposure to
bis(chloromethyl)ether. Ind Health, 11: 145-148.
Sawicki E, Belsky T, Friedel R, Hyde D, Monkman J, Rasmussen R,
Ripperton L, & White L (1976) Analytical method for chloromethyl
methyl ether (CMME) and bis(chloromethyl) ether (BCME) in air. Health
Lab Sci, 13: 78-81.
Schrenk HH, Patty FA, & Yant WP (1933) Acute response of guinea pigs
to vapors of some new commercial organic compounds. Public Health Rep,
48: 1389-1398.
Sellakumar AR, Snyder CA, Soloman JJ, & Albert RE (1985)
Carcinogenicity of formaldehyde and hydrogen chloride in rats. Toxicol
Appl Pharmacol, 81: 401-406.
Sheldon L & Hites R (1978) Organic compounds in the Delaware River.
Environ Sci Technol, 12(10): 1188-1194.
Shirasu Y, Moriya M, Kato K, & Kada T (1975) Mutagenicity screening of
pesticides in microbial systems: II. Mutat Res, 31: 268-269.
Shooter KV (1975) Assays for phosphotriester formation in the reaction
of bacteriophage R17 with a group of alkylating agents. Chem-Biol
Interact, 11: 575-588.
Simmon VF, Kauhanen K, & Tardiff RG (1977a) Mutagenic activity of
chemicals identified in drinking water. In: Scott D, Bridges BA, &
Sobels FH ed. Progress in genetic toxicology. Amsterdam,
Elsevier/North-Holland, pp 249-258.
Simmon VF, Kauhanen K, & Tardiff RG (1977b) Mutagenic assays with
bis-(2-chloroethyl) ether. In : Plaa GL & Duncan WAM ed. Proceedings
of the First International Congress on Toxicology. New York, London,
San Francisco, Academic Press, p 31.
Sittig M (1981) Handbook of toxic and hazardous chemicals. Park
Ridge, New Jersey, Noyes Publications, 729 pp.
Smith CC, Cragg ST, Wolfe GF, & Weigel WW (1985) Investigation of the
metabolism of chlorinated hydrocarbons in sub-human species. Research
Triangle Park, North Carolina, US Environmental Protection Agency,
Health Effects Research Laboratory (PB85-152387).
Smyth HF Jr & Carpenter CP (1948) Further experience with the range
finding test in the industrial toxicology laboratory. J Ind Hyg
Toxicol, 30: 63-68.
Spector WS ed. (1956) Handbook of toxicology. Philadelphia,
Pennsylvania, W.B. Saunders Co., 671 pp.
Sram RJ, Samkova I, & Hola N (1983) High-dose ascorbic acid
prophylaxis in workers occupationally exposed to halogenated ethers. J
Hyg Epidemiol Microbiol Immunol, 27: 305-318.
Sram RJ, Landa K, & Roznickova I (1985) The use of cytogenetic
analysis of peripheral lymphocytes as a method for checking the level
of MAC in Czechoslovakia. Mutat Res, 147: 322.
Staples CA, Werner A, & Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environ Toxicol Chem, 4: 131-142.
Styles JA (1978) Mammalian cell transformation in vitro. Br J
Cancer, 37: 931-936.
Suffet I, Brenner L, & Cairo P (1980) GC/MS identification of trace
organics in Philadelphia drinking-water during a 2-year period. Water
Res, 14: 853-867.
Tabak H, Quave S, Mashni C, & Barth E (1981) Biodegradability studies
with organic priority pollutant compounds. J Water Pollut Control Fed,
53: 1503-1518.
Tabershaw IR, Utidjian HMD, & Kawahara BL (1977) Chemical hazards. In:
Occupational diseases: A guide to their recognition. Washington, DC,
United States Department of Health, Education and Welfare, p 131
(Publication DHEW (NIOSH) 77-181).
Theiss WC, Stoner GD, Shimkin MB, & Weisburger EK (1977) Test for
carcinogenicity of organic contaminants of United States
drinking-waters by pulmonary tumor response in strain A mice. Cancer
Res, 37: 2717-2720.
Tou J & Kallos G (1974a) Study of aqueous HCl and formaldehyde
mixtures for formation of bis(chloromethyl) ether. Am Ind Hyg Assoc J,
35: 419-422.
Tou J & Kallos G (1974b) Kinetic study of the stabilities of
chloromethyl methyl ether and bis(chloromethyl) ether in humid air.
Anal Chem, 46: 1866-1869.
Tou J & Kallos G (1976) Possible formation of bis(chloromethyl) ether
from the reactions of formaldehyde and chloride ion. Anal Chem, 48:
958-963.
Tou J, Westover L, & Sonnabend L (1974) Kinetic studies of
bis(chloromethyl) ether hydrolysis by mass spectrometry. J Phys Chem,
78: 1096-1098.
Travenius S (1982) Formation and occurrence of bis(chloromethyl)
ether and its prevention in the chemical industry. Scand J Work
Environ Health, 8(suppl 3): 1-86.
Union Carbide (1968) Bis(chloromethyl)ether: Range finding toxicity
studies. Danbury, Connecticut, Union Carbide Corporation, 5 pp (Report
No. 31-85).
US EPA (1980) Ambient water quality criteria for chloroalkyl ethers.
Washington, DC, US Environmental Protection Agency, Environmental
Criteria Assessment Office, Office of Water Regulations and Standards,
Criteria and Standards Division (EPA-440/5-80-03, PB81-117418).
US EPA (1987a) Health and environmental effects document for
haloethers. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (ECAO-CIN-G014).
US EPA (1987b) Health effects assessment for bis(2-chloroethyl)
ether. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Criteria and Assessment Office (EPA/600/8-88023,
PB88-179486).
US EPA (1990) Toxic chemical release inventory database for 1989.
Washington, DC, US Environmental Protection Agency and National
Library of Medicine
US NLM (1996) Hazardous substances database: Records for
bis(chloromethyl)ether, bis(2-chloroethyl)ether and chloromethyl
methyl ether. Bethesda, Maryland, National Library of Medicine.
van der Ven LGJ & Venema A (1979) Determination of
bis(chloromethyl)ether in air. Anal Chem, 51: 1016-1019.
van Duuren BL (1989) Comparison of potency of human carcinogens: vinyl
chloride, chloromethylmethyl ether and bis(chloromethyl)ether. Environ
Res, 49: 143-151.
van Duuren BL, Sivak A, Goldschmidt BM, Katz C, & Melchionne S (1969)
Carcinogenicity of halo-ethers. J Natl Cancer Inst, 43: 481-486.
van Duuren BL, Katz C, Goldschmidt BM, Frenkel K, & Sivak A (1972)
Carcinogenicity of halo-ethers: II. Structure-activity relationships
of analogues of bis(chloromethyl)ether. J Natl Cancer Inst, 48:
1431-1439.
Verschueren K (1983) Handbook of environmental data on organic
chemicals, 2nd ed. Toronto, Canada, Van Nostrand Reinhold Co., 1310
pp.
Weast RC & Astle M ed. (1985) CRC handbook of data on organic
compounds - Volume 1. Boca Raton, Florida, CRC Press.
Weisburger EK, Ulland BM, Nam J-M, Gart JJ, & Weisburger JH (1981)
Carcinogenicity tests of certain environmental and industrial
chemicals. J Natl Cancer Inst, 67: 75-88.
Weiss W (1976) Chloromethyl ethers, cigarettes, cough and cancer. J
Occup Med, 18: 194-199.
Weiss W (1980) The cigarette factor in lung cancer due to chloromethyl
ethers. J Occup Med, 22: 527-529.
Weiss W (1982) Epidemic curve of respiratory cancer due to
chloromethylethers. J Natl Cancer Inst, 69: 1265-1270.
Weiss W (1989) Lung cancer due to chloromethyl ethers: bias in cohort
definition. J Occup Med, 31: 102-105.
Xue SZ, Quian C, Tang GF, Wang ZQ, Zhou DH, Deng J, Zhao JZ, & He YP
(1988) Epidemiological investigations on the lung cancer among
chloro-methyl-ether exposures. In: Xue SZ & Liang Y. ed. Occupational
health in industrialization and modernization. Shanghai, Shanghai
Medical University Press, pp 75-80 (Bulletin of WHO Collaborating
Centre for Occupational Health, Shanghai, Volume 2).
Xue MI, Tang GF, Diao WX, & Cai MD (1996) Follow-up study on the
cohorts exposed to chloro-methyl ethers. Chin J Ind Med, 9(4) 225-227.
Yao CC & Miller GC (1979) Research study on bis(chloromethyl) ether
formation and detection in selected work environments. Cincinnati,
Ohio, National Institute for Occupational Safety and Health, Division
of Surveillance, Hazard Evaluations and Field Studies (Report
PB83-174102).
Zajdela F, Croisy A, Barbin A, Malaveille C, Tomatis L, & Bartsch H
(1980) Carcinogenicity of chloroethylene oxide, an ultimate reactive
metabolite of vinyl chloride, and bis(chloromethyl)ether after
subcutaneous administration and in initiation promotion experiments in
mice. Cancer Res, 40: 352-356.
RÉSUMÉ ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le bis (2-chloroéthyl) éther (BCEE), le bis (chlorométhyl) éther
(BCME) et le chlorométhylméthyléther (CMME) sont des composés
chimiques qui appartiennent à un vaste groupe de produits connus sous
le nom de chloroalkyléthers. A la température ambiante, ces trois
éthers se présentent sous la forme de liquides volatils incolores à
l'odeur caractéristique. Ils sont dotés d'une forte tension de vapeur.
La solubilité dans l'eau du BCEE est de 1,7% et son coefficient de
partage entre l'octanol et l'eau est égal à 1,46. Le BCME et le CMME,
qui sont des alpha-chloroalkyléthers, sont des composés réactifs. Ils
subissent une hydrolyse rapide en milieu aqueux (avec une demi-vie ou
temps de demi-hydrolyse respectivement égale à 38 secondes et < 0,007
secondes); le BCEE, qui est un ß-chloroéthyléther, s'hydrolyse plus
lentement (demi-vie dans l'eau approximativement égale à 20 ans).
Les méthodes d'échantillonnage et d'analyse applicables au BCEE
dans l'eau et au CMME dans l'air sont décrites dans la littérature. On
peut citer comme exemples caractéristiques la chromatographie en phase
gazeuse (détection par capture d'électrons) ou le couplage
chromatographie en phase gazeuse-spectrométrie de masse.
2. Sources d'exposition humaine
On n'a pas trouvé dans l'environnement de BCEE, de BCME ou de
CMME qui soient d'origine naturelle. Les données de production
récentes se limitent aux Etats-Unis et au Canada. On a produit environ
10 000 tonnes de BCEE aux Etats-Unis en 1986 en vue d'une utilisation
comme solvant, pour la production de polymères ou encore dans un
certain nombre de processus industriels. Dans ce même pays, l'usage du
BCME est actuellement limité à certaines réactions chimiques
intermédiaires bien déterminées. On produit également du BCME en vue
de la fabrication de résines échangeuses d'ions ou autres types de
polymères ou encore comme solvant dans les réactions de
polymérisation. En Chine, on produit chaque année, environ 200 tonnes
de BCME comme intermédiaire dans la préparation d'un synergisant
d'insecticide, l'octachlorodipropyléther. Le CMME de qualité technique
contient de 1 à 8% de BCME.
3. Transport, distribution et transformation dans l'environnement
La mobilité et la distribution de ces chloroalkyléthers sont,
dans le cas du BCME et du CMME, déterminées par la grande réactivité
de ces composés et, dans le cas du BCEE, par la grande solubilité et
stabilité dans l'eau de cet éther. Le BCME et le CMME, des éthers
alpha-chloroalkylés, subissent une hydrolyse rapide en milieu aqueux
et sont rapidement décomposés par photolyse. En milieu aqueux, les
produits d'hydrolyse du BCME et du CMME sont constitués de
formaldéhyde et d'acide chlorhydrique dans le cas du premier et de
méthanol, de formaldéhyde et d'acide chlorhydrique dans le cas du
second. Parmi les produits de décomposition du BCME et du CMME,
figurent le chlorure d'hydrogène, le formaldéhyde et le
chlorométhylformiate, pour le premier, et le chlorométhyl- ainsi que
le méthylformiate, pour le second. Le BCEE est soluble dans l'eau; les
précipitations l'éliminent de l'atmosphère et il a tendance à rester
dans l'eau où il subit une très lente hydrolyse. En l'espace d'une
semaine, il s'évapore de la surface et se décompose en un peu moins
d'une journée sous l'action de processus abiotiques.
En raison du caractère extrêmement réactif des
alpha-chloroalkyléthers dans l'eau et dans l'air, on ne peut guère
s'attendre à trouver du CMME et du BCME dans l'environnement.
Toutefois, le BCEE peut présenter une plus grande persistance en
raison de la meilleure stabilité relative des ß-chloroalkyléthers.
4. Niveaux d'exposition dans l'environnement et exposition humaine
On ne dispose que de données limitées sur la concentration du
BCEE dans les divers compartiments de l'environnement. On l'a mis en
évidence dans l'air, mais sans procéder à un dosage; aux Etats-Unis,
on en a trouvé jusqu'à 42 µg/litre dans de l'eau de boisson. Les
concentrations rapportées dans la littérature en ce qui concerne les
eaux de surface vont de 0,001 µg/litre dans une décharge industrielle
de gypse en Belgique, à 840 µg/litre dans une autre décharge située
aux Etats-Unis. On a mesuré des concentrations encore plus élevées
dans les eaux de lessivage d'une décharge contrôlée. On n'a pas
connaissance de la teneur des denrées alimentaires en BCEE, mais on ne
pense pas qu'il puisse y avoir bioaccumulation.
On ne dispose d'aucune donnée sur la concentration du BCME et du
CMME dans les divers compartiments de l'environnement.
Si l'on se base sur la concentration maximale de BCEE rapportée
pour l'eau de boisson, soit 42 µg/litre, un être humain moyen pesant
64 kg et consommant 1,4 litres d'eau par jour ingérerait
quotidiennement environ 0,01 µg de ce produit par kg de poids
corporel, plus une quantité indéterminée provenant de sources
inconnues. Il est impossible d'évaluer la dose quotidienne de BCME et
de CMME ingérée à partir de sources environnementales. Toutefois,
comme ces deux composés ne persistent pas dans l'environnement, il est
probable que l'exposition humaine à ces produits est très faible.
En s'appuyant sur des données limitées et assez anciennes, on
pense que les ouvriers travaillant à la production de plastiques et de
fibres textiles ont pu être exposés, dans l'air des lieux de travail,
à des concentrations de BCME comprises entre 1,2 et 72,9 µg/m3.
Cependant, une récente étude, effectuée dans une usine de production
de résines plastiques, fait état d'une exposition professionnelle
moyenne allant de 2,4 à 20,6 µg/m3. Selon d'autres travaux, la
concentration de BCME ne dépasserait pas 0,01 µg/m3.En Chine,
l'exposition au BCME a été plus élevée que ces chiffres jusqu'en 1975
et alle persiste encore, quoiqu'à un niveau moindre, dans les usines
qui produisent de l'octachlorodipropyléther. Il y exposition de la
population générale au BCME et au CMME là où l'on fait beaucoup brûler
de serpentins anti-moustiques qui en contiennent comme synergisants.
La concentration la plus élevée de BCEE signalée aux Etats-Unis
dans les effluents industriels se situe entre 8 et 170 µg/litre; dans
le cas d'eaux provenant du lessivage de décharges contrôlées
industrielles et municipales, la concentration était de 12 400
µg/litre.
5. Cinétique et métabolisme
On ne dispose pas de données quantitatives sur la cinétique et le
métabolisme du BCEE, du BCME et du CMME chez l'homme. On estime
toutefois que, même si le BCME et le CMME doivent, en principe, être
rapidement hydrolysés in vivo, pour donner, le premier, du
formaldéhyde et de l'acide chlorhydrique et le second, du
formaldéhyde, du méthanol et de l'acide chlorhydrique, il se produit
sans doute une alkylation.
D'après des données limitées, du BCEE radiomarqué administré à
des rats par inhalation ou gavage subit une résorption rapide. Après
administration par gavage, on a constaté que l'organisme des rats
n'avait retenu que moins de 3% de la dose initiale au bout de 24
heures.
Chez le rat, le BCEE est rapidement métabolisé. Son principal
métabolite est l'acide thiodiglycolique (TDGA). Chez des rats qui
avaient reçu par gavage une dose unique de 14C-BCEE, on a constaté
que 12% environ de la radioactivité absorbée se trouvait sous la forme
de 14CO2.
Chez le rhésus comme chez le rat, le BCEE est rapidement éliminé.
Chez des rhésus auxquels on avait administré du 14C-BCEE par la voie
orale, on a retrouvé moins de 2% de la radioactivité initiale dans les
matières fécales 72 h après l'administration. Chez des rats, c'est
approximativement 2,3% de la radioactivité initiale qui ont été
retrouvés dans les tissus et les matières fécales, 48 h après
l'administration. Après avoir administré par gavage du 14C-BCEE à des
rats, on a retrouvé plus de 50% de la radioactivité dans les urines et
dans l'air expiré 12 heures après l'administration. Moins de 2% de la
radioactivité présente dans l'air expiré correspondaient au composé
initial.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
Absorbé par la voie orale, par inhalation ou par voie
transcutanée, le BCEE peut provoquer des intoxications aiguës. Les
valeurs de la DL50 dont il est fait état dans la littérature en cas
d'exposition d'animaux par la voie orale, vont de 75 à 215 mg/kg de
poids corporel. Le BCME et le CMME provoquent également des
intoxications aiguës lorsqu'ils sont absorbés par voie orale ou par
inhalation. Les valeurs de la CL50 pour des animaux de laboratoire
exposés par la voie respiratoire à du BCME ou à du CMME, vont de 25 à
48 mg/m3 dans le cas du premier composé et de 182 à 215 mg/m3 dans
le cas du second.
L'exposition d'animaux de laboratoire par la voie respiratoire à
une seule mais forte concentration de BCEE (>320 mg/m3), a provoqué
une irritation oculaire ainsi qu'une congestion, un oedème et des
hémorragies pulmonaires. Pendant l'inhalation de BCME, on a noté une
irritation des yeux et des voies respiratoires ainsi qu'une bronchite
nécrosante. L'application du produit sur la peau a donné lieu à un
érythème et à une nécrose. L'instillation dans les yeux provoque une
nécrose cornéenne. On a observé des effets analogues après exposition
au CMME.
On a constaté un accroissement de la mortalité et une hyperplasie
trachéenne chez des rats et des hamsters exposés par la voie
respiratoire à du BCME à la dose de 4,7 mg/m3. Des résultats
analogues ont été obtenus à plusieurs reprises chez des rats exposés à
du CMME par la voie respiratoire à raison de 3,3 ou de 33 mg de
composé par m3.
En général, les épreuves de mutagénicité in vitro ont donné des
résultats positifs avec le BCEE, le BCME et le CMME. Toutefois, les
résultats sont difficiles à interpréter en raison de l'absence de
détails dans les comptes rendus de ces expériences. Selon la
littérature, le BCME et le CMME provoquent in vitro un accroissement
de la synthèse non programmée de l'ADN et le BCME augmente la
proportion de cellules transformées, également in vitro. Dans de
petits groupes de souris mâles appartenant à deux souches de souris
hybrides F1 (de même que chez les femelles d'une des souches F1),
qui avaient reçu du BCEE par voie orale (dose pondérée par rapport au
temps égale à 41,3 mg/kg p.c. sur une période de 18 mois), on a
observé une augmentation significative de l'incidence des tumeurs
hépatiques (bénignes et malignes) par rapport aux animaux témoins non
exposés. Quatre autres études de portée plus limitée effectuées sur
des rats et des souris qui recevaient le composé par gavage, en
injections sous-cutanées ou intrapéritonéales ou par badigeonnage
cutané, n'ont pas permis de confirmer ces résultats.
Les études de cancérogénicité effectuées sur des animaux de
laboratoire (souris ou rats) exposés à du BCME, ont révélé un
accroissement significatif de l'incidence des adénomes pulmonaires et
autres tumeurs des voies respiratoires. Chez la souris, on a également
obtenu des indices d'une élévation de l'incidence des tumeurs
pulmonaires.
Les études effectuées sur le CMME ont révélé, chez le rat, un
accroissement de l'incidence des métaplasies trachéennes et des
hyperplasies bronchiques qui dépendait de la dose. Toutefois, les
résultats des épreuves de cancérogénicité menées sur l'animal n'ont
pas donné de résultats concluants.
On ne dispose d'aucun renseignement concernant les effets
toxiques éventuels du BCEE, du BCME et du CMME sur la fonction de
reproduction, le développement, le système immunitaire et le système
nerveux.
7. Effets sur l'homme
On a constaté que le BCEE avait un effet irritant sur l'oeil et
les fosses nasales aux concentrations supérieures à 150 mg/m3 après
exposition de courte durée.
On n'a pas trace d'études épidémiologiques sur les effets à long
terme d'une exposition au BCEE.
Une association entre l'exposition d'ouvriers à du BCME ou du
CMME et un risque accru de cancer du poumon a été mise en évidence
dans 8 études épidémiologiques. Les travailleurs exposés à du CMME de
qualité technique l'étaient probablement aussi à du BCME. Les tumeurs
prédominantes observées chez les ouvriers exposés étaient des
carcinomes à petites cellules, tout à fait distincts des carcinomes
essentiellement spinocellulaires qui s'observent. chez les fumeurs. Il
s'agissait d'une forte association, avec des rapports comparatifs de
mortalité qui allaient jusqu'à 2,1. Le type de cancer pulmonaire, la
période de latence et l'âge moyen d'apparition de la tumeur chez les
ouvriers exposés au BCME ou au CMME présentaient une cohérence
remarquable. Dans le cas du CMME, on est également fondé à penser
qu'il y a une relation positive entre l'expression qualitative de
l'exposition et la mortalité par cancer du poumon.
Lors d'une exposition professionnelle, et même à des
concentrations de 0,01 µg/m3 de BCME ou de 20 µg/m3 de CMME, on a
constaté une augmentation de la fréquence des aberrations
chromosomiques dans les lymphocytes du sang périphérique des ouvriers
exposés.
On ne dispose d'aucun renseignement concernant les effets du BCME
ou du CMME sur la fonction de reproduction, le développement, le
système nerveux et le système immunitaire chez l'homme.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Peu d'études ont été consacrées aux effets du BCEE sur les êtres
vivants dans leur milieu naturel; la plupart des travaux se limitent
aux espèces aquatiques. Ainsi la CL50 à 7 jours pour le guppy est
égale à 56,9 mg/litre; pour d'autres poissons on a trouvé une CL50 à
96 h de 600 mg/litre et en ce qui concerne les invertébrés, on a fait
état d'une CL50 à 48 h égale à 240 mg/litre pour Daphnia magna.
Il n'y a pas eu d'inhibition de l'activité microbienne anaérobie
en présence de BCEE à des concentrations allant jusqu'à 100 mg/litre
et on a trouvé une CL10 de 600 µg/litre pour des microorganismes
colonisant des bassins de stabilisation.
On ne dispose d'aucune donnée concernant les effets toxiques que
le BCME ou le CMME pourraient exercer sur les êtres vivants dans leur
milieu naturel.
9. Conclusions
9.1 BCEE
- L'exposition des organismes terrestres au BCEE est jugée
négligeable du fait que ce composé n'est que lentement libéré
dans l'environnement et qu'il ne subsiste que peu de temps dans
l'atmosphère.
- Le BCEE persiste davantage dans l'eau, mais la concentration la
plus élevée mesurée dans les eaux de surface est beaucoup plus
faible (environ cinq ordres de grandeur) que celle qui se révèle
toxique pour le guppy, l'espèce la plus sensible selon les études
toxicologiques.
- En raison de l'absence de données concernant la concentration du
BCEE dans un certain nombre de compartiments de l'environnement
auxquels l'homme est exposé, il n'est pas possible de donner une
estimation quantitative de la dose totale de ce composé absorbée
au cours d'une journée.
- On ne dispose que de données limitées sur la toxicité du BCEE
pour l'homme. On ne trouve pas de relation des effets sur la
reproduction et le développement qui auraient pu être observés
chez des animaux de laboratoire. Par ailleurs, aucune des études
à long terme effectuées sur les animaux de laboratoire n'est
d'une qualité suffisante pour que l'on puisse en tirer des
données quantitatives sur la cancérogénicité du BCEE ou sur les
effets toxiques à longue échéance que ce composé pourrait
produire.
- Faute de données toxicologiques et cancérogénétiques suffisantes,
la prudence commande de faire en sorte que l'exposition humaine
soit réduite au minimum.
9.2 BCME et CMME
- Au cas ou ces composés pénétreraient dans l'environnement, ils
subiraient rapidement une hydrolyse et une photo-oxydation. On ne
possède pas de données sur la concentration du BCME et du CMME
dans l'environnement.
- Le BCME et le CMME de qualité technique (qui contient du BCME)
sont des substances dont la cancérogénicité pour l'homme est
prouvée. Ils se sont d'ailleurs tous les deux révélés
cancérogènes pour les animaux de laboratoire. Ces deux composés
provoquent des aberrations chromosomiques chez les travailleurs
qui leur sont exposés de par leur profession. Il faut éviter
toute exposition professionnelle et toute exposition de la
population générale à ces composés.
- Compte tenu de la destinée de ces composés dans l'environnement
et de l'absence d'exposition, il n'y aucune raison de craindre
des effets nocifs sur les organismes terrestres ou aquatiques.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El bis(2-cloroetil)éter (BCEE), el bis(clorometil)éter (BCME) y
el clorometilmetiléter (CMME) son sustancias químicas de una clase
amplia conocida como cloroalquiléteres. Los tres éteres son líquidos
volátiles incoloros a temperatura ambiente con olores característicos.
La presión de vapor de estos tres compuestos es alta. La solubilidad
del BCEE es del 1,7% en agua, y su coeficiente de reparto octanol/
agua es de 1,46. Los alpha-cloroalquiléteres BCME y CMME son
compuestos reactivos, que se hidrolizan con rapidez en medios acuosos
(con semividas de alrededor de 38 segundos y <0,007 segundos,
respectivamente); la hidrólisis del ß-cloroéter BCEE, más estable, es
más lenta (con una semivida en agua de unos 20 años).
Se han descrito métodos de muestreo y analíticos para el BCEE en
el agua y para el BCME y el CMME en el aire. Normalmente la
determinación se efectúa por cromatografía de gases (CG-captura de
electrones) o CG-espectrometría de masas.
2. Fuentes de exposición humana
No se han identificado fuentes naturales de BCEE, BCME o CMME en
el medio ambiente. Los datos recientes de producción disponible son
limitados y corresponden solamente a los Estados Unidos y el Canadá.
En 1986 se produjeron en los Estados Unidos alrededor de 104
toneladas de BCEE para utilizarlo como disolvente y en la producción
de polímeros y en varios procesos industriales. Las aplicaciones
industriales del BCME están actualmente limitadas en los Estados
Unidos a reacciones químicas intermedias específicas. También se ha
producido BCME para utilizarlo en la obtención de resinas de
intercambio iónico, en la fabricación de otros polímeros y como
disolvente en reacciones de polimerización. En China se producen unas
200 toneladas al año de BCME como producto intermedio en la
fabricación de octaclorodipropiléter, un insecticida sinérgico. El
CMME de calidad técnica contiene del 1% al 8% de BCME.
3. Transporte, distribución y transformación en el medio ambiente
En la movilidad y distribución de los cloroalquiléteres
seleccionados influyen tanto la elevada radiactividad del BCME y del
CMME como la solubilidad en agua y la estabilidad del BCEE. Los
alpha-cloroalquiléteres BCME y CMME se hidrolizan con rapidez en
medios acuosos y se degradan en poco tiempo por fotolisis. En medios
acuosos, los productos hidrolíticos del BCME y del CMME son
formaldehído y ácido clorhídrico, y metanol, formaldehído y ácido
clorhídrico, respectivamente. Los productos de descomposición del BCME
y el CMME en el aire son ácido clorhídrico, formaldehído y formato de
clorometilo, y formato de clorometilo y de metilo, respectivamente. El
BCEE es soluble en agua; la lluvia lo elimina de la atmósfera y tiende
a mantenerse en el agua, con una hidrólisis muy lenta. El BCEE se
evapora del agua superficial en una semana y se degrada en poco más de
un día en la atmósfera mediante procesos abióticos.
Debido al carácter muy reactivo de los alpha-cloroalquiléteres en
el agua y en el aire, no es previsible la presencia de CMME y BCME en
el medio ambiente; sin embargo, el BCEE puede ser persistente, debido
a la estabilidad relativa de los ß-cloroalquiléteres.
4. Niveles ambientales y exposición humana
Los datos disponibles sobre los niveles de BCEE en medios
ambientales son limitados. Se ha identificado en el aire, pero sin
determinación cuantitativa; se han encontrado niveles de hasta 0,42
µg/litro en el agua potable en los Estados Unidos. Los niveles
notificados de BCEE en el agua fréatica han oscilado entre 0,001
µg/litro en un vertedero de yeso industrial en Bélgica y 840 µg/litro
cerca de un vertedero en los Estados Unidos. Se han medido
concentraciones más elevadas en productos de lixiviación de
vertederos. No se dispone de información sobre los niveles de BCEE en
los productos alimenticios, pero se supone que no se produce
bioacumulación.
No se dispone de datos cuantitativos sobre los niveles de BCME o
CMME en el medio ambiente.
Tomando como base el nivel máximo notificado de BCEE en el agua
potable, es decir, 0,42 µg/litro, la persona de tipo medio (64 kg) que
consuma 1,4 litros/día tendrá una ingesta aproximada de 0,01 µg/kg de
peso corporal al día de esta procedencia, con cantidades desconocidas
de otras fuentes del medio ambiente. No se puede hacer ninguna
estimación de la ingesta diaria de BCME y CMME a partir de fuentes del
medio ambiente. Sin embargo, considerando la falta de persistencia del
BCME y del CMME en el medio ambiente es probable que la exposición
humana media a estos compuestos sea muy baja.
En función de datos limitados más antiguos, los trabajadores de
industrias relacionadas con los plásticos y la producción textil
podrían haber estado expuestos a cantidades comprendidas entre 1,2 y
72,9 µg de BCME/m3 en el aire del lugar de trabajo. Sin embargo, en
un estudio reciente de una fábrica de resina se señalaron exposiciones
medias en el trabajo comprendidas entre 2,4 y 20,6 µg/m3. En los
datos de otros estudios se indicaron niveles de BCME muy bajos de 0,01
µg/m3. En China se producía hasta 1975 una exposición más alta en el
trabajo al BCME, y sigue existiendo con un nivel menor en la
fabricación de octaclorodipropiléter. La población general está
expuesta al BCME y al CMME cuando se producen en la quema generalizada
de este producto sinérgico en serpentines fumigantes de mosquitos.
Las concentraciones más elevadas notificadas de BCEE en los
Estados Unidos en efluentes industriales son de 8 a 170 µg/litro, y en
los productos de lixiviación de vertederos municipales e industriales
de 12 400 µg/litro.
5. Cinética y metabolismo
No se dispone de información cuantitativa sobre la cinética y el
metabolismo del BCEE, del BCME y del CMME en el ser humano. Sin
embargo, se supone que, aunque el BCME y el CMME se hidrolicen con
rapidez in vivo en los tejidos a formaldehído y ácido clorhídrico, y
a metanol, formaldehído y ácido clorhídrico respectivamente, debe
haber actividad de alquilación.
Los datos limitados que se conocen indican que el BCEE radiactivo
administrado a ratas por inhalación o con sonda se absorbe con
rapidez. A las 48 horas de la administración por sonda se retenía
menos del 3% de la radiactividad.
El BCEE se metaboliza fácilmente en ratas. El principal
metabolito es el ácido tiodiglicólico. Después de administrar una
dosis única por sonda de [14C]-BCEE, alrededor del 12% de la
radiactividad administrada estaba presente en forma de 14CO2.
El BCEE se elimina con rapidez tanto en ratas como en monos
resus. A las 72 horas de la administración oral de [14C]-BCEE se
recuperó menos del 2% de la radiactividad en las heces de los monos; a
las 48 horas de la administración, se encontró alrededor del 2,3% de
la radiactividad administrada en los tejidos o las heces de ratas; más
del 50% de la radiactividad se recuperó en la orina y en el aire
exhalado a las 12 horas de la administración a ratas con sonda de una
dosis de [14C]-BCEE. De la radiactividad expirada a través de los
pulmones, correspondió al compuesto original menos del 2%.
6. Efectos en animales de laboratorio y en sistemas de prueba
in vitro
El BCEE tiene toxicidad aguda por vías de exposición oral,
inhalación o cutánea. Los valores notificados de la DL50 para la
exposición oral de especies animales al BCEE oscilan entre 75 y 215
mg/kg de peso corporal. El BCME y el CMME tienen toxicidad aguda por
inhalación o ingestión. Los valores de la CL50 notificados para la
exposición de animales de laboratorio por inhalación al BCME o al CMME
oscila entre 25 y 48 mg/m3 y entre 182 y 215 mg/m3, respectivamente.
La exposición de animales de laboratorio por inhalación a una
dosis elevada única de BCEE (>320 mg/m3) provocó irritación ocular,
además de congestión, edema y hemorragia pulmonar. Durante la
inhalación de BCME, se observó irritación ocular y de las vías
respiratorias, así como bronquitis necrosante. La aplicación cutánea
dio lugar a la aparición de eritema y necrosis, y la aplicación en el
ojo indujo necrosis corneal. Tras la exposición al CMME se observaron
efectos análogos.
En ratas y hámsteres se observó un aumento de la mortalidad y la
hiperplasia traqueal después de la exposición por inhalación múltiple
a 4,7 mg de BCME/m3. Se observaron efectos análogos en ratas
expuestas repetidamente por inhalación a 3,3 o 33 mg de CMME/m3.
En general, se obtuvieron resultados positivos en las pruebas de
mutagenicidad del BCEE, del BCME y del CMME in vitro. Sin embargo,
la interpretación de los resultados resulta difícil, debido a la falta
de detalles en los informes disponibles. Se ha notificado que el BCME
y el CMME aumentan la síntesis no programada de ADN in vitro, y el
BCME elevó el nivel de células transformadas en pruebas in vitro.
En pequeños grupos de machos pertenecientes a dos estirpes de
ratones F1 híbridos (y en hembras de una estirpe F1) tratados por
vía oral con BCEE (dosis media ponderada por el tiempo de 41,3 mg/kg
de peso corporal durante 18 meses), se registró un aumento
significativo de la incidencia de hepatomas (hepatomas benignos y
tumores malignos combinados) en comparación con los testigos no
tratados. En otros cuatro estudios limitados con ratas y ratones en
los que se utilizó la administración oral con sonda, la inyección
subcutánea o intraperitoneal y la aplicación sobre la piel no se
confirmaron esos resultados.
Los estudios de carcinogenicidad en animales experimentales
(ratones y ratas) expuestos a BCME pusieron de manifiesto una
incidencia significativamente elevada de adenomas pulmonares y tumores
de las vías respiratorias. En ratones, tras la exposición por
inhalación también se observaron pruebas de una incidencia elevada de
tumores pulmonares.
Los estudios realizados con CMME han puesto de manifiesto una
mayor incidencia de metaplasia traqueal e hiperplasia bronquial
dependiente de la dosis en ratas. Sin embargo, los resultados de las
biovaloraciones de carcinogenicidad en estudios con animales no han
sido concluyentes.
No hay información disponible relativa a la toxicidad
reproductiva, en el desarrollo, inmunológica o neurológica del BCEE,
del BCME o del CMME.
7. Efectos en el ser humano
Se ha comprobado que el BCEE irrita los ojos y los orificios
nasales de las personas en concentraciones >150 mg/m3 tras una
exposición breve.
No se tienen noticias de estudios epidemiológicos sobre los
efectos de la exposición prolongada al BCEE.
En ocho estudios epidemiológicos, la exposición de los
trabajadores al BCME (CMME) se relacionó con un aumento del riesgo de
cáncer de pulmón. Los trabajadores expuestos al CMME de calidad
comercial probablemente también estuvieron expuestos al BCME. Los
tumores predominantes en los trabajadores expuestos fueron carcinomas
de células pequeñas, bastante distintos de los que son principalmente
de células escamosas y que suelen aparecer en los fumadores. Hubo una
relación clara entre la exposición al BCME (CMME) y el cáncer de
pulmón, con unas razones de mortalidad normalizada que llegaban hasta
21. El tipo de cáncer de pulmón, el período de latencia y la edad
media de aparición de los tumores de pulmón en los trabajadores
expuestos al BCME (CMME) han sido básicamente invariables. Para el
CMME hay también pruebas de una relación positiva entre una medida
cualitativa de la exposición y la mortalidad debida a cáncer de
pulmón.
En el curso de una exposición en el trabajo, incluso
concentraciones de 0,01 µg de BCME/m3 y de 20 µg de CMME/m3
aumentaron la frecuencia de aberraciones cromosómicas en los
linfocitos periféricos de los trabajadores expuestos.
No se dispone de información relativa a los efectos neurológicos,
inmunológicos, en el desarrollo o reproductivos del BCME o del CMME en
el ser humano.
8. Efectos en otros organismos en el laboratorio y en condiciones
naturales
Son pocos los estudios que se han realizado sobre los efectos del
BCEE en los organismos del medio ambiente; la mayoría se limitan a
especies acuáticas. Para el BCEE, se ha notificado un valor de la
CL50 en siete días en Lebistes reticulatus de 56,9 mg/litro, una
CL50 en peces en 96 horas de 600 mg/litro y una CL50 en 48 horas en
Daphnia magna de 240 mg/litro.
La actividad microbiana anaerobia no se vio inhibida en
concentraciones de BCEE de hasta 100 mg/litro, y se ha notificado una
CL10 de 600 µg/litro en el caso de microorganismos indígenas en
estanques de estabilización de desechos.
No hay información sobre los efectos toxicológicos del BCME y del
CMME en los organismos del medio ambiente.
9. Conclusiones
9.1 BCEE
- Se considera que la exposición de los organismos terrestres al
BCEE es insignificante, debido a la escasa tasa de liberación y
su breve persistencia en la atmósfera.
- Aunque es más persistente en el agua, la concentración más
elevada notificada de BCEE en agua superficial es aproximadamente
cinco veces inferior a la concentración con la que se ha
comprobado que induce efectos adversos en Lebistes reticulatus,
que es la especie acuática más sensible identificada en los
estudios de toxicidad realizados.
- Debido a la falta de información disponible sobre las
concentraciones de BCEE en varios tipos de medios a los cuales
está expuesto el ser humano, no es posible estimar
cuantitativamente la ingesta diaria total de BCEE.
- Los datos disponibles sobre la toxicidad del BCEE en el ser
humano son limitados. No se ha encontrado información sobre los
efectos del BCEE en el desarrollo y la reproducción en animales
de laboratorio, y ninguno de los estudios de larga duración en
animales de laboratorio tiene suficiente calidad para
proporcionar información cuantitativa acerca del potencial del
BCEE para provocar cáncer o sobre los efectos toxicológicos
producidos por la exposición prolongada a esta sustancia.
- En ausencia de datos toxicológicos y de carcinogenicidad
adecuados, es conveniente reducir al mínimo la exposición humana
al BCEE.
9.2 BCME y CMME
- En el caso de que estas sustancias se incorporaran al medio
ambiente, se degradarían con rapidez por hidrólisis y
fotooxidación. No se han identificado datos relativos a las
concentraciones de BCME y CMME en el medio ambiente natural.
- Está demostrado que el BCME y el CMME de calidad técnica (que
contiene BCME) son carcinógenos para el ser humano. Además, ambos
productos químicos son carcinógenos en animales de laboratorio.
Los dos provocan aberraciones cromosómicas en los trabajadores
expuestos en el trabajo. Se debe eliminar la exposición en el
trabajo y de la población general a estos compuestos.
- Tomando como base el destino de estas sustancias en el medio
ambiente y la falta de exposición, no hay motivo para suponer que
se produzcan efectos adversos en organismos acuáticos y
terrestres.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED
CHLOROALKYL ETHERS
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton,
Surrey, United Kingdom
Dr R. Chhabra, Division of Intramural Research, Environmental
Toxicology Program, Toxicology Branch, National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA (Chairman)
Dr H. Ellisa, Epidemiology Department, Rohm & Haas, Bristol,
Pennsylvania, USA
Dr B. Gilbert, FarManguinhos, FIOCRUZ, Institute of
Pharmaceutical Technology, Ministry of Health, Rio de Janeiro,
Brazil
Professor M. Jakubowski, Occupational and Environmental
Hygiene Division, Nofer Institute of Occupational Medicine, Lodz,
Poland
Dr S.K. Kashyap, National Institute of Occupational Health,
Meghani Nagar, Ahmedabad, India (Vice-chairman)
Dr R. Liteplo, Environmental Health Directorate, Health Protection
Branch, Environmental Health Centre, Ottawa, Ontario, Canada
(Co-rapporteur)
Dr E.E. McConnell, Laurdane Estates, Raleigh, North Carolina,
USA
Dr H. Naito, Ibaraki Prefecture University of Health Sciences,
Amimachi, Inashikigun, Japan
Dr W. Popp, Universitätsklinikum Essen, Institut für Hygiene und
Arbeitsmedizin, Essen, Germany
Dr R. Sram, Laboratory of Genetic Ecotoxicology, c/o Institute of
Experimental Medicine, Prague, Czech Republic
a Invited, but unable to attend.
Dr Shou-Zheng Xue, Toxicology Programme, Shanghai Medical
University, Shanghai, China
Secretariat
Dr G.C. Becking, IPCS/IRRU, World Health Organization,
Research Triangle Park, North Carolina, USA
Ms R. Gomes, Health Canada, Environmental Health Directorate,
Tunney's Pasture, Ottawa, Ontario, Canada (Co-rapporteur)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED
CHLOROALKYL ETHERS
A WHO Task Group on Environmental Health Criteria for Selected
Chloroalkyl Ethers met at the British Industrial Biological Research
Association (BIBRA) Toxicology International, Carshalton, Surrey,
United Kingdom, from 18 to 23 March 1996. Dr D. Anderson opened the
meeting and welcomed the participants on behalf of the host institute.
Dr G.C. Becking, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS and the three cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks to human health
and the environment from exposure to selected chloroalkyl ethers.
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contribution to the IPCS.
The first and second drafts of this monograph were prepared by Dr
R. Liteplo and Ms R. Gomes, Health Canada, Ottawa. The second draft
incorporated the comments received following circulation of the first
draft to the IPCS contact points for environmental health criteria
monographs.
Dr G.C. Becking (IPCS Central Unit, Interregional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation of the document
are gratefully acknowledged.
ABBREVIATIONS
BCEE bis(2-chloroethyl) ether
BCME bis(chloromethyl) ether
CMME chloromethyl methyl ether
MTD maximum tolerated dose
PMA phorbol myristate acetate
TDGA thiodiglycolic acid
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, analytical methods
Bis(2-chloroethyl) ether (BCEE), bis(chloromethyl) ether (BCME)
and chloromethyl methyl ether (CMME) are chemicals from a large class
known as chloroalkyl ethers. The three ethers are colourless volatile
liquids at room temperature having characteristic odours. The vapour
pressure of these three compounds is high. The solubility of BCEE is
1.7% in water and its octanol/water partition coefficient is 1.46. The
alpha-chloroalkyl ethers BCME and CMME are reactive compounds,
hydrolysing rapidly in aqueous media (with half-lives of approximately
38 seconds and <0.007 seconds, respectively); hydrolysis of the more
stable ß-chloroether BCEE is slower (with a half-life in water of
about 20 years).
Sampling and analytical methods have been described for BCEE in
water and for BCME and CMME in air. Typically, determination is by
gas chromatography (GC-electron capture) or GC mass spectrometry.
1.2 Sources of human exposure
Natural sources of BCEE, BCME or CMME in the environment have not
been identified. The recent production data available are limited and
confined to the USA and Canada. Approximately 104 tonnes of BCEE were
produced in the USA in 1986 for use as a solvent and in the production
of polymers and several industrial processes. Industrial uses of BCME
are currently restricted in the USA to specific intermediate chemical
reactions. BCME has also been produced for use in the production of
ion exchange resins, manufacture of other polymers, and as a solvent
in polymerization reactions. In China, some 200 tonnes of BCME are
produced annually as an intermediate in the manufacture of the
insecticide synergist, octachlorodipropyl ether. Technical grade CMME
contains from 1 to 8% BCME.
1.3 Environmental transport, distribution and transformation
The mobility and distribution of the selected chloroalkyl ethers
is influenced by the high reactivity of BCME and CMME and the water
solubility and stability of BCEE. The alpha-chloroalkyl ethers BCME
and CMME are hydrolysed rapidly in aqueous media and degraded quickly
by photolysis. In aqueous media, the hydrolytic products of BCME and
CMME are formaldehyde and hydrochloric acid, and methanol,
formaldehyde and hydrochloric acid, respectively. The decomposition
products of BCME and CMME in air include hydrogen chloride,
formaldehyde and chloromethylformate, and chloromethyl and methyl
formate, respectively. BCEE is soluble in water; rainfall removes it
from the atmosphere and it tends to remain in water with very slow
hydrolysis. BCEE evaporates from surface water within a week and is
degraded in a little more than a day in the atmosphere by abiotic
processes.
Owing to the highly reactive nature of the alpha-chloroalkyl
ethers in water and air, CMME and BCME are not expected to be present
in the environment; however BCEE may be persistent due to the relative
stability of ß-chloroalkyl ethers.
1.4 Environmental levels and human exposure
Only limited data on levels of BCEE in environmental media are
available. It has been identified in air but not quantified; levels up
to 0.42 µg/litre have been found in drinking-water in the USA.
Reported levels of BCEE in groundwater have ranged from 0.001 µg/litre
at an industrial gypsum waste disposal site in Belgium to 840 µg/litre
near a waste disposal site in the USA. Higher concentrations have been
measured in landfill leachates. Information on levels of BCEE in
foodstuffs is not available, but bioaccumulation is not expected to
occur.
Quantitative data on levels of BCME or CMME in environmental
media are not available.
Based on the maximum reported level of BCEE in drinking-water,
i.e., 0.42 µg/litre, the average human (64 kg) consuming 1.4
litres/day would have an intake of about 0.01 µg/kg body weight per
day from this source, with unknown amounts from other environmental
sources. No estimates can be made on the daily intake of BCME and CMME
from environmental sources. However, based upon the lack of
persistence of BCME and CMME in the environment, average human
exposure to these compounds is likely to be very low.
Based on limited older data, workers in industries related to
plastics and textile production could have been exposed to between 1.2
and 72.9 µg BCME/m3 in workroom air. However, a recent study of a
resin-manufacturing plant reported average occupational exposures
ranging from 2.4 to 20.6 µg/m3. Data from other studies reported
levels of BCME as low as 0.01 µg/m3. Higher occupational exposure to
BCME occurred in China up until 1975 and still occurs on a lower level
in the manufacture of octachlorodipropyl ether. General population
exposure to BCME and CMME occurs where they are produced by the
widespread burning of this synergist in mosquito coils.
The highest reported concentrations of BCEE in the USA for
industrial effluents are 8 to 170 µg/litre and for municipal and
industrial waste landfill leachates 12 400 µg/litre.
1.5 Kinetics and metabolism
Quantitative information on the kinetics and metabolism of BCEE,
BCME and CMME in humans is not available. However, it is anticipated
that although in vivo BCME and CMME would be rapidly hydrolysed in
tissues to formaldehyde and hydrogen chloride, and methanol,
formaldehyde and hydrogen chloride, respectively, there should be
alkylation activity.
Limited data show that radioactive BCEE administered to rats by
inhalation or gavage is rapidly absorbed. Less than 3% of the
radioactivity was retained 48 h after gavage dosing.
BCEE is readily metabolized in rats. The principal metabolite is
thiodiglycolic acid (TDGA). After rats were given a single gavage dose
of [14C]-BCEE, approximately 12% of the administered radioactivity
was present as 14CO2.
BCEE is eliminated quickly in both rats and rhesus monkeys. Less
than 2% of the radioactivity was recovered in the faeces of monkeys 72
h after oral administration of [14C]-BCEE; approximately 2.3% of the
administered radioactivity was found in rat tissues or faeces 48 h
after dosing. Over 50% of the radioactivity was recovered in the urine
and exhaled air 12 h after a gavage dose of [14C]-BCEE was
administered to rats. Less than 2% of the radioactivity expired
through the lungs was exhaled as the parent compound.
1.6 Effects on laboratory animals and in vitro test systems
BCEE is acutely toxic by the oral, inhalation or dermal routes of
exposure. Reported LD50 values for the oral exposure of animal
species to BCEE range from 75 to 215 mg/kg body weight. BCME and CMME
are acutely toxic by inhalation or ingestion. Reported LC50 values
for the inhalation exposure of laboratory animals to BCME or CMME
range from 25 to 48 mg/m3, and from 182 to 215 mg/m3, respectively.
Exposure of laboratory animals by inhalation to high single
concentrations of BCEE (>320 mg/m3) resulted in eye irritation as
well as congestion, oedema, and haemorrhage in the lungs. During
inhalation of BCME, irritation of the eyes and respiratory tract were
noted as well as necrotizing bronchitis. Skin application resulted in
erythema and necrosis, and application to the eye resulted in corneal
necrosis. Similar effects were noted after exposure to CMME.
Increased mortality and tracheal hyperplasia were observed in
rats and hamsters following multiple inhalation exposure to 4.7 mg
BCME/m3. Similar results were observed in rats repeatedly exposed by
inhalation to 3.3 or 33 mg CMME/m3.
In general, positive results were obtained when BCEE, BCME and
CMME were tested for mutagenicity in vitro. However, interpretation
of the results is difficult given the lack of details in the reports
available. BCME and CMME have been reported to increase unscheduled
DNA synthesis in vitro, and BCME increased the level of transformed
cells in in vitro assays.
In small groups of males from two strains of hybrid F1 mice (and
in females from one F1 strain) treated orally with BCEE
(time-weighted dose 41.3 mg/kg body weight over 18 months), there was
a significant increase in the incidence of hepatomas (combined benign
hepatomas and malignant tumours) compared to unexposed controls. Four
other limited studies in rats and mice using oral gavage, subcutaneous
or intraperitoneal injection and skin painting failed to confirm these
findings.
Carcinogenicity studies in experimental animals (mice and rats)
exposed to BCME showed significantly elevated incidence of pulmonary
adenomas and respiratory tumours. In mice, inhalation exposure also
showed evidence of an elevated incidence of lung tumours.
Studies with CMME have shown an increased incidence of tracheal
metaplasia and bronchial hyperplasia in a dose-dependent manner in
rats. However, results of carcinogenicity bioassays are inconclusive
in animal studies.
Information regarding the reproductive, developmental,
immunological or neurological toxicity of BCEE, BCME or CMME is not
available.
1.7 Effects on humans
BCEE was found to be irritating to the eyes and nasal passages of
humans at levels >150 mg/m3 following short-term exposure.
No epidemiological studies on the effects of long-term exposure
to BCEE have been reported.
In eight epidemiological studies, exposure of workers to BCME
(CMME) was associated with increased risk of lung cancer. Workers
exposed to commercial grade CMME were probably also exposed to BCME.
The predominant tumours in exposed workers were small cell carcinomas,
quite distinct from the chiefly squamous cell carcinomas usually found
in smokers. The association between exposure to BCME (CMME) and lung
cancer was strong, with standardized mortality ratios ranging up to
21. The type of lung cancer, latency period and average age of
appearance of lung tumours in workers exposed to BCME (CMME) have been
remarkably consistent. For CMME, there is also evidence of a positive
relationship between a qualitative measure of exposure and mortality
due to lung cancer.
Even concentrations of 0.01 µg BCME/m3 and 20 µg CMME/m3, in
the course of occupational exposure, increased the frequency of
chromosomal aberrations in the peripheral lymphocytes of exposed
workers.
Information has not been reported regarding the neurological,
immunological, developmental or reproductive effects of BCME or CMME
in humans.
1.8 Effects on other organisms in the laboratory and field
There have been few studies on the effects of BCEE on
environmental organisms; most are restricted to aquatic species. For
BCEE a 7-day LC50 concentration in the guppy of 56.9 mg/litre, a 96-h
LC50 in fish of 600 mg/litre and a 48-h LC50 in Daphnia magna of
240 mg/litre have been reported.
Anaerobic microbial activity was not inhibited at concentrations
of BCEE up to 100 mg/litre and an LC10 of 600 µg/litre has been
reported for microbes indigenous to waste stabilization ponds.
No information on the toxicological effects of BCME and CMME on
environmental organisms has been reported.
1.9 Conclusions
1.9.1 BCEE
- Exposure of terrestrial organisms to BCEE is considered to be
negligible because of the low rate of release and its short
persistence in the atmosphere.
- Although it is more persistent in water, the highest reported
concentration of BCEE in surface water is approximately five
orders of magnitude lower than the concentration found to induce
adverse effects in the guppy, the most sensitive aquatic species
identified among existing toxicity studies.
- Owing to the lack of available information on concentrations of
BCEE in several environmental media to which humans are exposed,
it is not possible to estimate quantitatively the total daily
intake of BCEE.
- Available data on the toxicity of BCEE in humans are limited.
Information on the developmental and reproductive effects of BCEE
in laboratory animals has not been identified, and none of the
long-term studies in laboratory animals is of sufficient quality
to provide quantitative information on either the potential of
BCEE to cause cancer or the toxicological effects produced by
long-term exposure to this substance.
- In the absence of adequate toxicological and carcinogenicity
data, it is prudent to minimize human exposure to BCEE.
1.9.2 BCME and CMME
- If these substances were to enter the environment, they would
both be rapidly broken down by hydrolysis and photo-oxidation.
Data concerning concentrations of BCME and CMME in the ambient
environment have not been reported.
- BCME and technical grade CMME (which contains BCME) are proven
human carcinogens. In addition, both of these chemicals are
carcinogens in laboratory animals. Both chemicals cause
chromosomal aberrations in occupationally exposed workers.
Occupational and general population exposure to these compounds
should be eliminated.
- Based on the fate of these substances in the environment and the
lack of exposure, there is no reason to suspect that adverse
effects on aquatic and terrestrial organisms would occur.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Bis(2-chloroethyl) ether (BCEE), bis(chloromethyl) ether (BCME)
and chloromethyl methyl ether (CMME) are included in a large class of
chemical substances known as the chloroalkyl ethers. Identifying
features of BCEE, BCME and CMME are summarized in Table 1.
2.2 Physical and chemical properties
BCEE, a ß-chloroalkyl ether, is a colourless, volatile liquid
with a "chlorinated solvent-like" odour (Sittig, 1981). BCME and CMME,
both alpha-chloroalkyl ethers, are also colourless, volatile liquids
with characteristic odours. The odour of BCME has been described as
"suffocating" (Sittig, 1981; Verschueren, 1983), while that of CMME
has been described as "irritating" (Verschueren, 1983). Technical
grade CMME contains from 1 to 8% BCME (Travenius, 1982) and, unless
otherwise indicated in this monograph, CMME refers to the technical
grade material. In general, the vapour pressure and water solubility
of these compounds are high, and the log octanol/water partition
coefficients (log Kow) are low. The ß-chloroalkylethers like BCEE are
only slightly reactive towards water, but the alpha-chloroalkyl ethers
like BCME and CMME are rapidly hydrolysed by water, and their
solubility, Kow, Koc and Henry's Law constant cannot be
experimentally determined. The physical and chemical properties of the
selected chloroalkyl ethers are presented in Table 2.
2.3 Conversion factors
At 25°C and 101.3 kPa, the conversion factors for BCEE, BCME and
CMME in air are as follows:
BCEE: 1 ppm (v/v) = 5.85 mg/m3; 1 mg/m3 = 0.17 ppm
BCME: 1 ppm (v/v) = 4.7 mg/m3; 1 mg/m3 = 0.21 ppm
CMME: 1 ppm (v/v) = 3.3 mg/m3; 1 mg/m3 = 0.30 ppm
2.4 Analytical methods
2.4.1 BCEE
One method for the analysis of BCEE in water involves solvent
extraction (using diethyl ether in pentane, methylene chloride, or
ethyl ether in hexane), concentration with a Kuderna-Danish (K-D)
apparatus, and separation and analysis by gas chromatography with
electron capture detection (GC/EC) or gas chromatography mass
spectrometry (GC/MS) (Dressman et al., 1977; Quaghebeur et al., 1986).
This method has been expanded to include clean-up with Florisil and
K-D concentration of the sorbed fraction prior to analysis by GC/EC
(McMillin et al., 1984). Vapour stripping using helium or nitrogen gas
has also been used to extract BCEE from samples of ground and surface
Table 1. Information on the identity of BCEE, BCME and CMME (US NLM, 1996)
Compound Identification Molecular Chemical structure Relative Synonyms
(CAS number)a formula molecular mass
Bis(2-chloroethyl) ether BCEE C4H8Cl2O Cl-(CH2)2-O-(CH2)2-Cl 143.02 dichloroethyl ether,
(
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED
CHLOROALKYL ETHERS
Members
Dr D. Anderson, BIBRA Toxicology International, Carshalton,
Surrey, United Kingdom
Dr R. Chhabra, Division of Intramural Research, Environmental
Toxicology Program, Toxicology Branch, National Institute of
Environmental Health Sciences, Research Triangle Park, North
Carolina, USA (Chairman)
Dr H. Ellisa, Epidemiology Department, Rohm & Haas, Bristol,
Pennsylvania, USA
Dr B. Gilbert, FarManguinhos, FIOCRUZ, Institute of
Pharmaceutical Technology, Ministry of Health, Rio de Janeiro,
Brazil
Professor M. Jakubowski, Occupational and Environmental
Hygiene Division, Nofer Institute of Occupational Medicine, Lodz,
Poland
Dr S.K. Kashyap, National Institute of Occupational Health,
Meghani Nagar, Ahmedabad, India (Vice-chairman)
Dr R. Liteplo, Environmental Health Directorate, Health Protection
Branch, Environmental Health Centre, Ottawa, Ontario, Canada
(Co-rapporteur)
Dr E.E. McConnell, Laurdane Estates, Raleigh, North Carolina,
USA
Dr H. Naito, Ibaraki Prefecture University of Health Sciences,
Amimachi, Inashikigun, Japan
Dr W. Popp, Universitätsklinikum Essen, Institut für Hygiene und
Arbeitsmedizin, Essen, Germany
Dr R. Sram, Laboratory of Genetic Ecotoxicology, c/o Institute of
Experimental Medicine, Prague, Czech Republic
a Invited, but unable to attend.
Dr Shou-Zheng Xue, Toxicology Programme, Shanghai Medical
University, Shanghai, China
Secretariat
Dr G.C. Becking, IPCS/IRRU, World Health Organization,
Research Triangle Park, North Carolina, USA
Ms R. Gomes, Health Canada, Environmental Health Directorate,
Tunney's Pasture, Ottawa, Ontario, Canada (Co-rapporteur)
IPCS TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR SELECTED
CHLOROALKYL ETHERS
A WHO Task Group on Environmental Health Criteria for Selected
Chloroalkyl Ethers met at the British Industrial Biological Research
Association (BIBRA) Toxicology International, Carshalton, Surrey,
United Kingdom, from 18 to 23 March 1996. Dr D. Anderson opened the
meeting and welcomed the participants on behalf of the host institute.
Dr G.C. Becking, IPCS, welcomed the participants on behalf of Dr M.
Mercier, Director of the IPCS and the three cooperating organizations
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
criteria monograph and made an evaluation of the risks to human health
and the environment from exposure to selected chloroalkyl ethers.
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contribution to the IPCS.
The first and second drafts of this monograph were prepared by Dr
R. Liteplo and Ms R. Gomes, Health Canada, Ottawa. The second draft
incorporated the comments received following circulation of the first
draft to the IPCS contact points for environmental health criteria
monographs.
Dr G.C. Becking (IPCS Central Unit, Interregional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively.
The efforts of all who helped in the preparation of the document
are gratefully acknowledged.
ABBREVIATIONS
BCEE bis(2-chloroethyl) ether
BCME bis(chloromethyl) ether
CMME chloromethyl methyl ether
MTD maximum tolerated dose
PMA phorbol myristate acetate
TDGA thiodiglycolic acid
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, analytical methods
Bis(2-chloroethyl) ether (BCEE), bis(chloromethyl) ether (BCME)
and chloromethyl methyl ether (CMME) are chemicals from a large class
known as chloroalkyl ethers. The three ethers are colourless volatile
liquids at room temperature having characteristic odours. The vapour
pressure of these three compounds is high. The solubility of BCEE is
1.7% in water and its octanol/water partition coefficient is 1.46. The
alpha-chloroalkyl ethers BCME and CMME are reactive compounds,
hydrolysing rapidly in aqueous media (with half-lives of approximately
38 seconds and <0.007 seconds, respectively); hydrolysis of the more
stable ß-chloroether BCEE is slower (with a half-life in water of
about 20 years).
Sampling and analytical methods have been described for BCEE in
water and for BCME and CMME in air. Typically, determination is by
gas chromatography (GC-electron capture) or GC mass spectrometry.
1.2 Sources of human exposure
Natural sources of BCEE, BCME or CMME in the environment have not
been identified. The recent production data available are limited and
confined to the USA and Canada. Approximately 104 tonnes of BCEE were
produced in the USA in 1986 for use as a solvent and in the production
of polymers and several industrial processes. Industrial uses of BCME
are currently restricted in the USA to specific intermediate chemical
reactions. BCME has also been produced for use in the production of
ion exchange resins, manufacture of other polymers, and as a solvent
in polymerization reactions. In China, some 200 tonnes of BCME are
produced annually as an intermediate in the manufacture of the
insecticide synergist, octachlorodipropyl ether. Technical grade CMME
contains from 1 to 8% BCME.
1.3 Environmental transport, distribution and transformation
The mobility and distribution of the selected chloroalkyl ethers
is influenced by the high reactivity of BCME and CMME and the water
solubility and stability of BCEE. The alpha-chloroalkyl ethers BCME
and CMME are hydrolysed rapidly in aqueous media and degraded quickly
by photolysis. In aqueous media, the hydrolytic products of BCME and
CMME are formaldehyde and hydrochloric acid, and methanol,
formaldehyde and hydrochloric acid, respectively. The decomposition
products of BCME and CMME in air include hydrogen chloride,
formaldehyde and chloromethylformate, and chloromethyl and methyl
formate, respectively. BCEE is soluble in water; rainfall removes it
from the atmosphere and it tends to remain in water with very slow
hydrolysis. BCEE evaporates from surface water within a week and is
degraded in a little more than a day in the atmosphere by abiotic
processes.
Owing to the highly reactive nature of the alpha-chloroalkyl
ethers in water and air, CMME and BCME are not expected to be present
in the environment; however BCEE may be persistent due to the relative
stability of ß-chloroalkyl ethers.
1.4 Environmental levels and human exposure
Only limited data on levels of BCEE in environmental media are
available. It has been identified in air but not quantified; levels up
to 0.42 µg/litre have been found in drinking-water in the USA.
Reported levels of BCEE in groundwater have ranged from 0.001 µg/litre
at an industrial gypsum waste disposal site in Belgium to 840 µg/litre
near a waste disposal site in the USA. Higher concentrations have been
measured in landfill leachates. Information on levels of BCEE in
foodstuffs is not available, but bioaccumulation is not expected to
occur.
Quantitative data on levels of BCME or CMME in environmental
media are not available.
Based on the maximum reported level of BCEE in drinking-water,
i.e., 0.42 µg/litre, the average human (64 kg) consuming 1.4
litres/day would have an intake of about 0.01 µg/kg body weight per
day from this source, with unknown amounts from other environmental
sources. No estimates can be made on the daily intake of BCME and CMME
from environmental sources. However, based upon the lack of
persistence of BCME and CMME in the environment, average human
exposure to these compounds is likely to be very low.
Based on limited older data, workers in industries related to
plastics and textile production could have been exposed to between 1.2
and 72.9 µg BCME/m3 in workroom air. However, a recent study of a
resin-manufacturing plant reported average occupational exposures
ranging from 2.4 to 20.6 µg/m3. Data from other studies reported
levels of BCME as low as 0.01 µg/m3. Higher occupational exposure to
BCME occurred in China up until 1975 and still occurs on a lower level
in the manufacture of octachlorodipropyl ether. General population
exposure to BCME and CMME occurs where they are produced by the
widespread burning of this synergist in mosquito coils.
The highest reported concentrations of BCEE in the USA for
industrial effluents are 8 to 170 µg/litre and for municipal and
industrial waste landfill leachates 12 400 µg/litre.
1.5 Kinetics and metabolism
Quantitative information on the kinetics and metabolism of BCEE,
BCME and CMME in humans is not available. However, it is anticipated
that although in vivo BCME and CMME would be rapidly hydrolysed in
tissues to formaldehyde and hydrogen chloride, and methanol,
formaldehyde and hydrogen chloride, respectively, there should be
alkylation activity.
Limited data show that radioactive BCEE administered to rats by
inhalation or gavage is rapidly absorbed. Less than 3% of the
radioactivity was retained 48 h after gavage dosing.
BCEE is readily metabolized in rats. The principal metabolite is
thiodiglycolic acid (TDGA). After rats were given a single gavage dose
of [14C]-BCEE, approximately 12% of the administered radioactivity
was present as 14CO2.
BCEE is eliminated quickly in both rats and rhesus monkeys. Less
than 2% of the radioactivity was recovered in the faeces of monkeys 72
h after oral administration of [14C]-BCEE; approximately 2.3% of the
administered radioactivity was found in rat tissues or faeces 48 h
after dosing. Over 50% of the radioactivity was recovered in the urine
and exhaled air 12 h after a gavage dose of [14C]-BCEE was
administered to rats. Less than 2% of the radioactivity expired
through the lungs was exhaled as the parent compound.
1.6 Effects on laboratory animals and in vitro test systems
BCEE is acutely toxic by the oral, inhalation or dermal routes of
exposure. Reported LD50 values for the oral exposure of animal
species to BCEE range from 75 to 215 mg/kg body weight. BCME and CMME
are acutely toxic by inhalation or ingestion. Reported LC50 values
for the inhalation exposure of laboratory animals to BCME or CMME
range from 25 to 48 mg/m3, and from 182 to 215 mg/m3, respectively.
Exposure of laboratory animals by inhalation to high single
concentrations of BCEE (>320 mg/m3) resulted in eye irritation as
well as congestion, oedema, and haemorrhage in the lungs. During
inhalation of BCME, irritation of the eyes and respiratory tract were
noted as well as necrotizing bronchitis. Skin application resulted in
erythema and necrosis, and application to the eye resulted in corneal
necrosis. Similar effects were noted after exposure to CMME.
Increased mortality and tracheal hyperplasia were observed in
rats and hamsters following multiple inhalation exposure to 4.7 mg
BCME/m3. Similar results were observed in rats repeatedly exposed by
inhalation to 3.3 or 33 mg CMME/m3.
In general, positive results were obtained when BCEE, BCME and
CMME were tested for mutagenicity in vitro. However, interpretation
of the results is difficult given the lack of details in the reports
available. BCME and CMME have been reported to increase unscheduled
DNA synthesis in vitro, and BCME increased the level of transformed
cells in in vitro assays.
In small groups of males from two strains of hybrid F1 mice (and
in females from one F1 strain) treated orally with BCEE
(time-weighted dose 41.3 mg/kg body weight over 18 months), there was
a significant increase in the incidence of hepatomas (combined benign
hepatomas and malignant tumours) compared to unexposed controls. Four
other limited studies in rats and mice using oral gavage, subcutaneous
or intraperitoneal injection and skin painting failed to confirm these
findings.
Carcinogenicity studies in experimental animals (mice and rats)
exposed to BCME showed significantly elevated incidence of pulmonary
adenomas and respiratory tumours. In mice, inhalation exposure also
showed evidence of an elevated incidence of lung tumours.
Studies with CMME have shown an increased incidence of tracheal
metaplasia and bronchial hyperplasia in a dose-dependent manner in
rats. However, results of carcinogenicity bioassays are inconclusive
in animal studies.
Information regarding the reproductive, developmental,
immunological or neurological toxicity of BCEE, BCME or CMME is not
available.
1.7 Effects on humans
BCEE was found to be irritating to the eyes and nasal passages of
humans at levels >150 mg/m3 following short-term exposure.
No epidemiological studies on the effects of long-term exposure
to BCEE have been reported.
In eight epidemiological studies, exposure of workers to BCME
(CMME) was associated with increased risk of lung cancer. Workers
exposed to commercial grade CMME were probably also exposed to BCME.
The predominant tumours in exposed workers were small cell carcinomas,
quite distinct from the chiefly squamous cell carcinomas usually found
in smokers. The association between exposure to BCME (CMME) and lung
cancer was strong, with standardized mortality ratios ranging up to
21. The type of lung cancer, latency period and average age of
appearance of lung tumours in workers exposed to BCME (CMME) have been
remarkably consistent. For CMME, there is also evidence of a positive
relationship between a qualitative measure of exposure and mortality
due to lung cancer.
Even concentrations of 0.01 µg BCME/m3 and 20 µg CMME/m3, in
the course of occupational exposure, increased the frequency of
chromosomal aberrations in the peripheral lymphocytes of exposed
workers.
Information has not been reported regarding the neurological,
immunological, developmental or reproductive effects of BCME or CMME
in humans.
1.8 Effects on other organisms in the laboratory and field
There have been few studies on the effects of BCEE on
environmental organisms; most are restricted to aquatic species. For
BCEE a 7-day LC50 concentration in the guppy of 56.9 mg/litre, a 96-h
LC50 in fish of 600 mg/litre and a 48-h LC50 in Daphnia magna of
240 mg/litre have been reported.
Anaerobic microbial activity was not inhibited at concentrations
of BCEE up to 100 mg/litre and an LC10 of 600 µg/litre has been
reported for microbes indigenous to waste stabilization ponds.
No information on the toxicological effects of BCME and CMME on
environmental organisms has been reported.
1.9 Conclusions
1.9.1 BCEE
- Exposure of terrestrial organisms to BCEE is considered to be
negligible because of the low rate of release and its short
persistence in the atmosphere.
- Although it is more persistent in water, the highest reported
concentration of BCEE in surface water is approximately five
orders of magnitude lower than the concentration found to induce
adverse effects in the guppy, the most sensitive aquatic species
identified among existing toxicity studies.
- Owing to the lack of available information on concentrations of
BCEE in several environmental media to which humans are exposed,
it is not possible to estimate quantitatively the total daily
intake of BCEE.
- Available data on the toxicity of BCEE in humans are limited.
Information on the developmental and reproductive effects of BCEE
in laboratory animals has not been identified, and none of the
long-term studies in laboratory animals is of sufficient quality
to provide quantitative information on either the potential of
BCEE to cause cancer or the toxicological effects produced by
long-term exposure to this substance.
- In the absence of adequate toxicological and carcinogenicity
data, it is prudent to minimize human exposure to BCEE.
1.9.2 BCME and CMME
- If these substances were to enter the environment, they would
both be rapidly broken down by hydrolysis and photo-oxidation.
Data concerning concentrations of BCME and CMME in the ambient
environment have not been reported.
- BCME and technical grade CMME (which contains BCME) are proven
human carcinogens. In addition, both of these chemicals are
carcinogens in laboratory animals. Both chemicals cause
chromosomal aberrations in occupationally exposed workers.
Occupational and general population exposure to these compounds
should be eliminated.
- Based on the fate of these substances in the environment and the
lack of exposure, there is no reason to suspect that adverse
effects on aquatic and terrestrial organisms would occur.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Bis(2-chloroethyl) ether (BCEE), bis(chloromethyl) ether (BCME)
and chloromethyl methyl ether (CMME) are included in a large class of
chemical substances known as the chloroalkyl ethers. Identifying
features of BCEE, BCME and CMME are summarized in Table 1.
2.2 Physical and chemical properties
BCEE, a ß-chloroalkyl ether, is a colourless, volatile liquid
with a "chlorinated solvent-like" odour (Sittig, 1981). BCME and CMME,
both alpha-chloroalkyl ethers, are also colourless, volatile liquids
with characteristic odours. The odour of BCME has been described as
"suffocating" (Sittig, 1981; Verschueren, 1983), while that of CMME
has been described as "irritating" (Verschueren, 1983). Technical
grade CMME contains from 1 to 8% BCME (Travenius, 1982) and, unless
otherwise indicated in this monograph, CMME refers to the technical
grade material. In general, the vapour pressure and water solubility
of these compounds are high, and the log octanol/water partition
coefficients (log Kow) are low. The ß-chloroalkylethers like BCEE are
only slightly reactive towards water, but the alpha-chloroalkyl ethers
like BCME and CMME are rapidly hydrolysed by water, and their
solubility, Kow, Koc and Henry's Law constant cannot be
experimentally determined. The physical and chemical properties of the
selected chloroalkyl ethers are presented in Table 2.
2.3 Conversion factors
At 25°C and 101.3 kPa, the conversion factors for BCEE, BCME and
CMME in air are as follows:
BCEE: 1 ppm (v/v) = 5.85 mg/m3; 1 mg/m3 = 0.17 ppm
BCME: 1 ppm (v/v) = 4.7 mg/m3; 1 mg/m3 = 0.21 ppm
CMME: 1 ppm (v/v) = 3.3 mg/m3; 1 mg/m3 = 0.30 ppm
2.4 Analytical methods
2.4.1 BCEE
One method for the analysis of BCEE in water involves solvent
extraction (using diethyl ether in pentane, methylene chloride, or
ethyl ether in hexane), concentration with a Kuderna-Danish (K-D)
apparatus, and separation and analysis by gas chromatography with
electron capture detection (GC/EC) or gas chromatography mass
spectrometry (GC/MS) (Dressman et al., 1977; Quaghebeur et al., 1986).
This method has been expanded to include clean-up with Florisil and
K-D concentration of the sorbed fraction prior to analysis by GC/EC
(McMillin et al., 1984). Vapour stripping using helium or nitrogen gas
has also been used to extract BCEE from samples of ground and surface
Table 1. Information on the identity of BCEE, BCME and CMME (US NLM, 1996)
Compound Identification Molecular Chemical structure Relative Synonyms
(CAS number)a formula molecular mass
Bis(2-chloroethyl) ether BCEE C4H8Cl2O Cl-(CH2)2-O-(CH2)2-Cl 143.02 dichloroethyl ether,
(