
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 198
Diazinon
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 198
First draft prepared by Dr K. Barabás, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1998
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Diazinon
(Environmental health criteria ; 198)
1.Diazinon - toxicity 2.Diazinon - adverse effects
3.Environmental exposure 4.Occupational exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 157198 5 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
PREAMBLE
ABBREVIATIONS
1. SUMMARY
1.1. Identity, physical and chemical properties, analytical
methods
1.2. Production, uses and sources of human and environmental
exposure
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on experimental animals and in vitro test systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory and field
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Primary constituent
2.1.2. Technical product
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.1.1 Manufacturing process
3.2.2. Uses
3.2.3. Formulations
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Volatilization
4.1.2. Movement in soil
4.2. Degradation
4.2.1. Degradation in soil
4.2.2. Degradation in water
4.2.3. Bioconcentration
4.2.3.1 Fish and aquatic invertebrates
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Fruit, vegetables and food
5.1.5. Milk
5.1.6. Meat and fat
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption, distribution and excretion
6.1.1. Oral administration
6.1.1.1 Rats
6.1.1.2 Guinea-pigs
6.1.1.3 Dogs
6.1.1.4 Goats
6.1.1.5 Cow
6.1.1.6 Hens
6.1.2. Dermal application
6.1.2.1 Rats
6.1.2.2 Sheep
6.1.2.3 Humans
6.1.3. Other routes
6.1.3.1 Intraperitoneal administration
6.1.3.2 Subcutaneous administration
6.1.3.3 Intravenous administration
6.2. Metabolism
6.2.1. In vivo metabolic transformations
6.2.1.1 Mice
6.2.1.2 Rats
6.2.1.3 Dogs
6.2.1.4 Sheep
6.2.1.5 Goats
6.2.1.6 Hens
6.2.2. In vitro metabolic transformations
6.3. Metabolic aspects of diazinon toxicity
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Oral
7.1.2. Dermal
7.1.3. Inhalation
7.1.4. Intraperitoneal
7.2. Short-term exposure
7.2.1. Oral
7.2.1.1 Rats
7.2.1.2 Dogs
7.2.1.3 Pigs
7.2.2. Inhalation
7.2.3. Dermal
7.2.3.1 Rabbits
7.3. Long-term exposure
7.3.1. Rats
7.3.2. Dogs
7.3.3. Rhesus monkeys
7.4. Skin and eye irritation; sensitization
7.4.1. Primary skin irritation
7.4.2. Primary eye irritation
7.4.3. Skin sensitization
7.5. Reproduction, embryotoxicity and teratogenicity
7.5.1. Reproduction
7.5.1.1 Rat
7.5.1.2 Cattle
7.5.2. Embryotoxicity and teratogenicity
7.5.2.1 Mice
7.5.2.2 Rats
7.5.2.3 Hamsters
7.5.2.4 Rabbits
7.5.2.5 Chicken
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.7.1. Mice
7.7.2. Rats
7.8. Special studies
7.8.1. Neurotoxicity
7.8.2. Effects on enzymes and transmitters
7.8.3. Effects on the immune system
7.8.4. Effect on pancreas
7.9. Factors that modify toxicity; toxicity of metabolites
7.9.1. Metabolic enzymes
7.9.2. Antidotes
7.9.3. Potentiation
8. EFFECTS ON HUMANS
8.1. Exposure of the general population
8.1.1. Acute toxicity, poisoning incidents
8.1.1.1 Acute pancreatitis
8.1.1.2 Intermediate syndrome
8.1.1.3 Unusual case reports
8.1.2. Controlled human studies
8.2. Occupational exposure
8.2.1. Acute poisoning
8.2.2. Effect of short-term and long-term
exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.2. Aquatic invertebrates
9.3. Fish
9.4. Effects in mesocosms and the field
9.5. Terrestrial invertebrates
9.6. Birds
9.6.1. Field studies
10. EVALUATION OF HUMAN HEALTH RISK AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risk
10.2. Evaluation for effects on the environment
10.2.1. Aquatic organisms
10.2.1.1 Acute risk
10.2.1.2 Chronic risk
10.2.2. Terrestrial organisms
10.2.2.1 Birds
10.2.2.2 Mammals
10.2.2.3 Bees
10.2.2.4 Earthworms
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
11.2. Recommendations for protection of human health and the
environment
11.2.1. Recommendation on regulation of compound
11.2.1.1 Transport and storage
11.2.1.2 Handling
11.2.1.3 Disposal
11.2.1.4 Selection, training and medical
supervision of workers
11.2.1.5 Labelling
11.2.1.6 Residues in food
11.2.2. Prevention of poisoning in man and emergency aid
11.2.2.1 Manufacture and formulation
11.2.2.2 Mixers and applicators
11.2.2.3 Other associated workers
11.2.2.4 Other populations likely to be affected
11.2.3. Entry into treated areas
11.2.4. Emergency aid
11.2.5. Surveillance test
12. FURTHER RESEARCH
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RÉSUMÉ ET ÉVALUATIONS
RESUMEN Y EVALUACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
Financial support for this Task Group meeting was provided by the
United Kingdom Department of Health as part of its contributions to
the IPCS.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
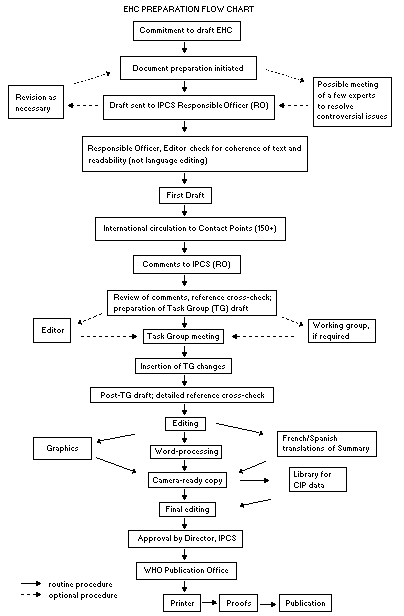
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
Members
Dr P.J. Abbott, Australia and New Zealand Food Authority
(ANZFA), Canberra, Australia
Dr K. Barabás, Department of Public Health, Albert Szent-Gyorgyi,
University Medical School, Szeged, Hungary
Dr A.L. Black, Woden, ACT, Australia
Professor J.F. Borzelleca, Pharmacology and Toxicology,
Richmond, Virginia, USA
Dr P.J. Campbell, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, Kings Pool, York,
United Kingdom
Professor L.G. Costa, Department of Environmental Health,
University of Washington, Seattle, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr I. Dewhurst, Mammalian Toxicology Branch, Pesticides Safety
Directorate, Ministry of Agriculture, Fisheries and Food,
Kings Pool, York, United Kingdom
Dr V. Drevenkar, Institute for Medical Research and Occupational
Health, Zagreb, Croatia
Dr W. Erickson, Environmental Fate and Effects Division,
US Environmental Protection Agency, Washington, D.C., USA
Dr A. Finizio, Group of Ecotoxicology, Institute of Agricultural
Entomology, University of Milan, Milan, Italy
Mr K. Garvey, Office of Pesticide Programs (7501C),
US Environmental Protection Agency, Washington, D.C., USA
Dr A.B. Kocialski, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency,
Washington, D.C., USA
Dr A. Moretto, Institute of Occupational Medicine, University
of Padua, Padua, Italy
Professor O. Pelkonen, Department of Pharmacology and
Toxicology, University of Oulu, Oulu, Finland
Dr D. Ray, Medical Research Council Toxicology Unit, University
of Leicester, Leicester, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Wageningen
Agricultural University, Wageningen, The Netherlands
Observers
Dr J.W. Adcock, AgrEvo UK Limited, Chesterford Park, Saffron,
Waldon, Essex, United Kingdom
Mr D. Arnold, Environmental Sciences, AgrEvo UK Ltd.,
Chesterford Park, Saffron Waldon, Essex, United Kingdom
Dr E. Bellet, CCII, Overland Park, Kansas, USA
Mr Jan Chart, AMVAC Chemical Corporation, Newport Beach,
California, USA
Dr H. Egli, Novartis Crop Protection AG, Basel, Switzerland
Dr P. Harvey, AgrEvo UK Ltd., Chesterford Park, Saffron Walden,
Essex, United Kingdom
Dr G. Krinke, Novartis Crop Protection AG, Basel, Switzerland
Dr A. McReath, DowElanco Limited, Letcombe Regis, Wantage,
Oxford, United Kingdom
Dr H. Scheffler, Novartis Crop Protection AG, Basel, Switzerland
Dr A.E. Smith, Novartis Crop Protection AG, Basel, Switzerland
Secretariat
Dr L. Harrison, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr J.L. Herrman, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr R. Plestina, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met at the Institute for Environment and Health,
Leicester, United Kingdom, from 3 to 8 March 1997. Dr L.L. Smith
welcomed the participants on behalf of the Institute and
Dr R. Plestina on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The CAG reviewed and revised the draft monograph and
made an evaluation of the risks for human health and the environment
from exposure to diazinon.
The first draft of the monograph was prepared by Dr K. Barabás,
Albert Szent-Gyorgyi University Medical School, Szeged, Hungary.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
AChE acetylcholinesterase
ai active ingredient
ChE cholinesterase
CNS central nervous system
DETP diethylthiophosphate
DT degradation time
EDTA ethylenediaminetetraacetic acid
fc field capacity
GABA gamma-aminobutyric acid
ip intraperitoneal
MRL maximum residue limit
NAD nicotinamide adenine dinucleotide
NIOSH National Institute for Occupational Safety and Health (USA)
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OSHA Occupational Safety and Health Administration (USA)
2-PAM pralidoxine (2-pyridine aldoxime methyl) chloride
PEC predicted environmental concentration
TEPP tetraethyl-pyrophosphate
TER toxicity-exposure ratio
TLV threshold limit value
1. SUMMARY
1.1 Identity, physical and chemical properties, analytical methods
The chemical name for diazinon is O, O-diethyl
O-2-isopropyl-6-methylpyrimidinyl-4-yl phosphorothioate. The pure
material forms a colourless liquid with a faint ester-like odour. The
technical active ingredient is a yellow/brown liquid with a slight
compound-specific odour. The boiling point is 83-84°C at 26.6 mPa and
the vapour pressure (volatility) is low (9.7 mPa at 20°C). The
solubility in water at room temperature is 60 mg/litre. Diazinon is
soluble in most organic solvents and has an octanol/water partition
coefficient (log Pow) of 3.40. It is stable in neutral media, but is
slowly hydrolysed in alkaline media and more rapidly in acid media. It
decomposes at temperatures above 120°C.
A large number of sampling and analytical methods have been
developed for the determination of diazinon and its metabolites in
different media. Sensitive methods, such as gas chromatography,
high-performance liquid chromatography, mass spectrometry and
immunoassay methods, are increasingly used.
1.2 Production, uses and sources of human and environmental exposure
Diazinon is a contact organophosphorus insecticide with a wide
range of insecticidal activity. It is effective against adult and
juvenile forms of flying insects, crawling insects, acarians and
spiders. It has been used from the early 1950s. Diazinon is mainly
formulated as wettable powders and emulsifiable concentrates. It is
also available in mixed formulations with other insecticides.
1.3 Environmental transport, distribution and transformation
Volatilization of diazinon from soil is of minor importance.
Diazinon has a tropospheric half-life of 1.5 h.
The movement of diazinon through soil is highly influenced by a
number of factors, particularly by organic matter and calcium
carbonate content. Diazinon is not expected to bind strongly to soil,
owing to its KOC value of 500, and is expected to show moderate
mobility in the soil.
Biological processes appear to be the main factor in the
degradation of diazinon in soil. At 20°C and a soil moisture content
of 60% of field capacity (f.c.) in a silt loam soil, the DT50 was
5 days. Under sterile conditions at 20°C and 60% f.c., the DT50 was
118 days, suggesting that biological activity is mainly responsible
for degradation in soil.
In natural water diazinon has a half-life of the order of 5-15
days. Both chemical and biological processes seem to play a role in
the degradation of diazinon, leading to mineralization within a few
weeks.
Uptake of diazinon by aquatic organisms is rapid. Low
bioconcentration factors have been reported for aquatic organisms,
ranging from 3 for shrimp to 152 for gudgeon, consistent with rapid
metabolism and loss. Depuration half-lives for fish have been reported
to be up to 30 h (muscle).
1.4 Environmental levels and human exposure
Environmental levels of diazinon are generally low. The routes of
exposure for the general population are inhalational and dietary.
Exposure through water is negligible. Occupational exposure is
primarily dermal.
Diazinon uses fall into two major categories: as a pesticide in
agriculture and as a drug in veterinary medicine. Thus, the major
source of diazinon residues in edible crops are from its use as an
agricultural pesticide, while those in meat, offal and other animal
products arise from its use as a veterinary drug containing active
ingredient.
Diazinon residues in vegetables, fruits and animal products are
very low. The results of total-diet studies suggest that diazinon
rapidly breaks down in both plant and animal products. Diazinon has
not been detected in drinking-water samples and its concentrations in
surface water are at the level of ng/litre.
1.5 Kinetics and metabolism
Diazinon may be absorbed from the gastrointestinal tract,
through the intact skin and following inhalation. Transdermal
absorption in humans is low. Diazinon is oxidized by the microsomal
enzymes to cholinesterase-inhibiting metabolites such as diazoxon,
hydroxydiazoxon, and hydroxydiazinon. Only minimal quantities of
metabolites are detectable in milk and eggs. Diazinon and its
metabolites do not accumulate in body tissue; 59-95% of an oral dose
of diazinon is excreted within 24 h and 95-98% is excreted within
7 days, mainly in urine.
The main metabolic pathways of degradation of diazinon are:
a) Cleavage of the ester bond leading to the hydroxypyrimidine
derivatives.
b) Transformation of P-S moiety to the P-O derivate.
c) Oxidation of isopropyl substituent leading to the corresponding
tertiary and primary alcohol derivatives.
d) Oxidation of the methyl substituent leading to the corresponding
alcohol.
e) Glutathione-mediated cleavage of the ester bond leading to a
glutathione conjugate.
The cleavage of the phosphorus ester bond, leading directly, or
via diazoxon, to the pyrimidyl metabolite plays the major role in the
metabolism of diazinon. Metabolites maintaining the phosphorus ester
bond are of transient nature and have been observed only in small
quantities. Yields and rates of production of metabolites vary greatly
between species. The production of diazoxon is not generally
correlated with susceptibility to diazinon poisoning, although it is
lowest in the least susceptible species, the sheep. The extrahepatic
metabolism of diazinon, especially the hydrolysis of diazoxon in
plasma, is more important toxicologically than the metabolism in
the liver, although the liver is probably the most important site
of metabolism in avian species. The metabolites formed, i.e.
diethylphosphoric acid, diethylthiophosphoric acid and the derivates
of the pyrimidinyl ring, are eliminated mainly via the kidneys.
1.6 Effects on experimental animals and in vitro test systems
Improvements in the manufacture of diazinon since 1979 have
significantly reduced the content of highly toxic impurities, e.g.,
tetraethyl-pyrophosphate (TEPP). As a result of these progressive
improvements, the acute oral LD50 of technical grade diazinon has
increased (e.g., from 250 mg/kg to 1250 mg/kg in the rat).
The acute oral, dermal and inhalational toxicity is low.
Short-term and long-term studies in mice, rats, rabbits, dogs and
monkeys have shown that the only effect of concern is dose-related
inhibition of acetyl cholinesterase activity.
Diazinon is slightly irritant to rabbit skin but not to the eye.
Diazinon is not a dermal sensitizer. Reproductive and developmental
studies have revealed no evidence of embryotoxic or teratogenic
potential. There was no effect on reproductive performance at dose
levels that were not toxic to the parent animals. Mutagenicity studies
with various end-points in vivo and in vitro gave no evidence of a
mutagenic potential. There is no evidence of carcinogenicity in rats
or mice. Diazinon does not cause delayed neuropathy in hens. In the
dog and guinea-pig, diazinon has been reported to cause acute
pancreatitis; this is considered to be a species-specific effect.
1.7 Effects on humans
Several cases of accidental or suicidal poisoning by diazinon
have been reported, some of which were fatal. In some of these the
cholinergic syndrome may have been more severe than expected because
of the presence of highly toxic impurities such as TEPP. In certain
cases, acute reversible pancreatitis was associated with a severe
cholinergic syndrome. This occurs also after poisoning with other
cholinesterase inhibitors. In a number of cases, the intermediate
syndrome was also observed. No cases of delayed neuropathy have ever
been reported, as expected from animal data. Reported cases of
poisoning after occupational exposure have always been associated with
the presence of impurities such as TEPP, monothio-TEPP or sulfo-TEPP
in the formulation. These impurities are unlikely to be found in
currently available formulations.
1.8 Effects on other organisms in the laboratory and field
Effects of diazinon on unicellular algae are variable; both
inhibition and stimulation of growth have been reported for different
species at concentrations between 0.01 and 5 mg/litre. Generally,
growth rates are reduced at concentration above 10 mg/litre, although
in certain cases population size can remain unaltered at 100 mg/litre.
Fewer and variable data make effects on other microorganisms difficult
to assess.
Acute LC50 values for aquatic invertebrates range from
0.2 µg/litre for Gammarus fasciatus to 4.0 µg/litre for the shrimp
Hyallela azteca in 96-h tests. Molluscs are substantially less
sensitive according to a single test on the snail Gillia
attilis. Sublethal effects on behaviour have been reported at
concentrations between 0.1 and 0.01 mg/litre.
Acute LC50 values for fish range from 0.09 mg/litre for rainbow
trout (Oncorhynchus mykiss) to 3.1 mg/litre for the catfish
(Channa punctatus). Growth of early life stages of fish was
inhibited at concentrations between 0.01 and 0.2 mg/litre. Brain
acetylcholinesterase activity is suppressed following acute exposure
to diazinon.
The LC50 for the earthworm Eisenia foetida in soil is
130 mg/kg soil.
The acute oral toxicity (LD50) in birds ranges from 1.1 mg/kg
body weight for Japanese quail to 85 mg/kg body weight for cowbirds.
Dietary LC50 values range from 32 mg/kg diet for mallard to 900 mg/kg
diet for Japanese quail (repellency was noted at these high dietary
concentrations). The no-observed-effect concentration in diet for
reproductive effects on birds in laboratory studies was 20 mg/kg
diet for mallard and 40 mg/kg diet for bobwhite quail. Brain
acetylcholinesterase activity is inhibited following ingestion.
Diazinon may also be taken in via the dermal route. There have been
reports of substantial field kills of water fowl following application
of diazinon to turf. Field studies applying liquid formulations to
turf at 4.8 kg ai/ha resulted in no mortality or reproductive effects
on song birds. Application of granules caused a small reduction in
song bird population size compared to that of controls. Ingestion of
small numbers of granules can be fatal for small birds, as
demonstrated in laboratory studies.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Common name: diazinon
Chemical structure:
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
Members
Dr P.J. Abbott, Australia and New Zealand Food Authority
(ANZFA), Canberra, Australia
Dr K. Barabás, Department of Public Health, Albert Szent-Gyorgyi,
University Medical School, Szeged, Hungary
Dr A.L. Black, Woden, ACT, Australia
Professor J.F. Borzelleca, Pharmacology and Toxicology,
Richmond, Virginia, USA
Dr P.J. Campbell, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, Kings Pool, York,
United Kingdom
Professor L.G. Costa, Department of Environmental Health,
University of Washington, Seattle, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr I. Dewhurst, Mammalian Toxicology Branch, Pesticides Safety
Directorate, Ministry of Agriculture, Fisheries and Food,
Kings Pool, York, United Kingdom
Dr V. Drevenkar, Institute for Medical Research and Occupational
Health, Zagreb, Croatia
Dr W. Erickson, Environmental Fate and Effects Division,
US Environmental Protection Agency, Washington, D.C., USA
Dr A. Finizio, Group of Ecotoxicology, Institute of Agricultural
Entomology, University of Milan, Milan, Italy
Mr K. Garvey, Office of Pesticide Programs (7501C),
US Environmental Protection Agency, Washington, D.C., USA
Dr A.B. Kocialski, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency,
Washington, D.C., USA
Dr A. Moretto, Institute of Occupational Medicine, University
of Padua, Padua, Italy
Professor O. Pelkonen, Department of Pharmacology and
Toxicology, University of Oulu, Oulu, Finland
Dr D. Ray, Medical Research Council Toxicology Unit, University
of Leicester, Leicester, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Wageningen
Agricultural University, Wageningen, The Netherlands
Observers
Dr J.W. Adcock, AgrEvo UK Limited, Chesterford Park, Saffron,
Waldon, Essex, United Kingdom
Mr D. Arnold, Environmental Sciences, AgrEvo UK Ltd.,
Chesterford Park, Saffron Waldon, Essex, United Kingdom
Dr E. Bellet, CCII, Overland Park, Kansas, USA
Mr Jan Chart, AMVAC Chemical Corporation, Newport Beach,
California, USA
Dr H. Egli, Novartis Crop Protection AG, Basel, Switzerland
Dr P. Harvey, AgrEvo UK Ltd., Chesterford Park, Saffron Walden,
Essex, United Kingdom
Dr G. Krinke, Novartis Crop Protection AG, Basel, Switzerland
Dr A. McReath, DowElanco Limited, Letcombe Regis, Wantage,
Oxford, United Kingdom
Dr H. Scheffler, Novartis Crop Protection AG, Basel, Switzerland
Dr A.E. Smith, Novartis Crop Protection AG, Basel, Switzerland
Secretariat
Dr L. Harrison, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr J.L. Herrman, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr R. Plestina, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met at the Institute for Environment and Health,
Leicester, United Kingdom, from 3 to 8 March 1997. Dr L.L. Smith
welcomed the participants on behalf of the Institute and
Dr R. Plestina on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The CAG reviewed and revised the draft monograph and
made an evaluation of the risks for human health and the environment
from exposure to diazinon.
The first draft of the monograph was prepared by Dr K. Barabás,
Albert Szent-Gyorgyi University Medical School, Szeged, Hungary.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
AChE acetylcholinesterase
ai active ingredient
ChE cholinesterase
CNS central nervous system
DETP diethylthiophosphate
DT degradation time
EDTA ethylenediaminetetraacetic acid
fc field capacity
GABA gamma-aminobutyric acid
ip intraperitoneal
MRL maximum residue limit
NAD nicotinamide adenine dinucleotide
NIOSH National Institute for Occupational Safety and Health (USA)
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OSHA Occupational Safety and Health Administration (USA)
2-PAM pralidoxine (2-pyridine aldoxime methyl) chloride
PEC predicted environmental concentration
TEPP tetraethyl-pyrophosphate
TER toxicity-exposure ratio
TLV threshold limit value
1. SUMMARY
1.1 Identity, physical and chemical properties, analytical methods
The chemical name for diazinon is O, O-diethyl
O-2-isopropyl-6-methylpyrimidinyl-4-yl phosphorothioate. The pure
material forms a colourless liquid with a faint ester-like odour. The
technical active ingredient is a yellow/brown liquid with a slight
compound-specific odour. The boiling point is 83-84°C at 26.6 mPa and
the vapour pressure (volatility) is low (9.7 mPa at 20°C). The
solubility in water at room temperature is 60 mg/litre. Diazinon is
soluble in most organic solvents and has an octanol/water partition
coefficient (log Pow) of 3.40. It is stable in neutral media, but is
slowly hydrolysed in alkaline media and more rapidly in acid media. It
decomposes at temperatures above 120°C.
A large number of sampling and analytical methods have been
developed for the determination of diazinon and its metabolites in
different media. Sensitive methods, such as gas chromatography,
high-performance liquid chromatography, mass spectrometry and
immunoassay methods, are increasingly used.
1.2 Production, uses and sources of human and environmental exposure
Diazinon is a contact organophosphorus insecticide with a wide
range of insecticidal activity. It is effective against adult and
juvenile forms of flying insects, crawling insects, acarians and
spiders. It has been used from the early 1950s. Diazinon is mainly
formulated as wettable powders and emulsifiable concentrates. It is
also available in mixed formulations with other insecticides.
1.3 Environmental transport, distribution and transformation
Volatilization of diazinon from soil is of minor importance.
Diazinon has a tropospheric half-life of 1.5 h.
The movement of diazinon through soil is highly influenced by a
number of factors, particularly by organic matter and calcium
carbonate content. Diazinon is not expected to bind strongly to soil,
owing to its KOC value of 500, and is expected to show moderate
mobility in the soil.
Biological processes appear to be the main factor in the
degradation of diazinon in soil. At 20°C and a soil moisture content
of 60% of field capacity (f.c.) in a silt loam soil, the DT50 was
5 days. Under sterile conditions at 20°C and 60% f.c., the DT50 was
118 days, suggesting that biological activity is mainly responsible
for degradation in soil.
In natural water diazinon has a half-life of the order of 5-15
days. Both chemical and biological processes seem to play a role in
the degradation of diazinon, leading to mineralization within a few
weeks.
Uptake of diazinon by aquatic organisms is rapid. Low
bioconcentration factors have been reported for aquatic organisms,
ranging from 3 for shrimp to 152 for gudgeon, consistent with rapid
metabolism and loss. Depuration half-lives for fish have been reported
to be up to 30 h (muscle).
1.4 Environmental levels and human exposure
Environmental levels of diazinon are generally low. The routes of
exposure for the general population are inhalational and dietary.
Exposure through water is negligible. Occupational exposure is
primarily dermal.
Diazinon uses fall into two major categories: as a pesticide in
agriculture and as a drug in veterinary medicine. Thus, the major
source of diazinon residues in edible crops are from its use as an
agricultural pesticide, while those in meat, offal and other animal
products arise from its use as a veterinary drug containing active
ingredient.
Diazinon residues in vegetables, fruits and animal products are
very low. The results of total-diet studies suggest that diazinon
rapidly breaks down in both plant and animal products. Diazinon has
not been detected in drinking-water samples and its concentrations in
surface water are at the level of ng/litre.
1.5 Kinetics and metabolism
Diazinon may be absorbed from the gastrointestinal tract,
through the intact skin and following inhalation. Transdermal
absorption in humans is low. Diazinon is oxidized by the microsomal
enzymes to cholinesterase-inhibiting metabolites such as diazoxon,
hydroxydiazoxon, and hydroxydiazinon. Only minimal quantities of
metabolites are detectable in milk and eggs. Diazinon and its
metabolites do not accumulate in body tissue; 59-95% of an oral dose
of diazinon is excreted within 24 h and 95-98% is excreted within
7 days, mainly in urine.
The main metabolic pathways of degradation of diazinon are:
a) Cleavage of the ester bond leading to the hydroxypyrimidine
derivatives.
b) Transformation of P-S moiety to the P-O derivate.
c) Oxidation of isopropyl substituent leading to the corresponding
tertiary and primary alcohol derivatives.
d) Oxidation of the methyl substituent leading to the corresponding
alcohol.
e) Glutathione-mediated cleavage of the ester bond leading to a
glutathione conjugate.
The cleavage of the phosphorus ester bond, leading directly, or
via diazoxon, to the pyrimidyl metabolite plays the major role in the
metabolism of diazinon. Metabolites maintaining the phosphorus ester
bond are of transient nature and have been observed only in small
quantities. Yields and rates of production of metabolites vary greatly
between species. The production of diazoxon is not generally
correlated with susceptibility to diazinon poisoning, although it is
lowest in the least susceptible species, the sheep. The extrahepatic
metabolism of diazinon, especially the hydrolysis of diazoxon in
plasma, is more important toxicologically than the metabolism in
the liver, although the liver is probably the most important site
of metabolism in avian species. The metabolites formed, i.e.
diethylphosphoric acid, diethylthiophosphoric acid and the derivates
of the pyrimidinyl ring, are eliminated mainly via the kidneys.
1.6 Effects on experimental animals and in vitro test systems
Improvements in the manufacture of diazinon since 1979 have
significantly reduced the content of highly toxic impurities, e.g.,
tetraethyl-pyrophosphate (TEPP). As a result of these progressive
improvements, the acute oral LD50 of technical grade diazinon has
increased (e.g., from 250 mg/kg to 1250 mg/kg in the rat).
The acute oral, dermal and inhalational toxicity is low.
Short-term and long-term studies in mice, rats, rabbits, dogs and
monkeys have shown that the only effect of concern is dose-related
inhibition of acetyl cholinesterase activity.
Diazinon is slightly irritant to rabbit skin but not to the eye.
Diazinon is not a dermal sensitizer. Reproductive and developmental
studies have revealed no evidence of embryotoxic or teratogenic
potential. There was no effect on reproductive performance at dose
levels that were not toxic to the parent animals. Mutagenicity studies
with various end-points in vivo and in vitro gave no evidence of a
mutagenic potential. There is no evidence of carcinogenicity in rats
or mice. Diazinon does not cause delayed neuropathy in hens. In the
dog and guinea-pig, diazinon has been reported to cause acute
pancreatitis; this is considered to be a species-specific effect.
1.7 Effects on humans
Several cases of accidental or suicidal poisoning by diazinon
have been reported, some of which were fatal. In some of these the
cholinergic syndrome may have been more severe than expected because
of the presence of highly toxic impurities such as TEPP. In certain
cases, acute reversible pancreatitis was associated with a severe
cholinergic syndrome. This occurs also after poisoning with other
cholinesterase inhibitors. In a number of cases, the intermediate
syndrome was also observed. No cases of delayed neuropathy have ever
been reported, as expected from animal data. Reported cases of
poisoning after occupational exposure have always been associated with
the presence of impurities such as TEPP, monothio-TEPP or sulfo-TEPP
in the formulation. These impurities are unlikely to be found in
currently available formulations.
1.8 Effects on other organisms in the laboratory and field
Effects of diazinon on unicellular algae are variable; both
inhibition and stimulation of growth have been reported for different
species at concentrations between 0.01 and 5 mg/litre. Generally,
growth rates are reduced at concentration above 10 mg/litre, although
in certain cases population size can remain unaltered at 100 mg/litre.
Fewer and variable data make effects on other microorganisms difficult
to assess.
Acute LC50 values for aquatic invertebrates range from
0.2 µg/litre for Gammarus fasciatus to 4.0 µg/litre for the shrimp
Hyallela azteca in 96-h tests. Molluscs are substantially less
sensitive according to a single test on the snail Gillia
attilis. Sublethal effects on behaviour have been reported at
concentrations between 0.1 and 0.01 mg/litre.
Acute LC50 values for fish range from 0.09 mg/litre for rainbow
trout (Oncorhynchus mykiss) to 3.1 mg/litre for the catfish
(Channa punctatus). Growth of early life stages of fish was
inhibited at concentrations between 0.01 and 0.2 mg/litre. Brain
acetylcholinesterase activity is suppressed following acute exposure
to diazinon.
The LC50 for the earthworm Eisenia foetida in soil is
130 mg/kg soil.
The acute oral toxicity (LD50) in birds ranges from 1.1 mg/kg
body weight for Japanese quail to 85 mg/kg body weight for cowbirds.
Dietary LC50 values range from 32 mg/kg diet for mallard to 900 mg/kg
diet for Japanese quail (repellency was noted at these high dietary
concentrations). The no-observed-effect concentration in diet for
reproductive effects on birds in laboratory studies was 20 mg/kg
diet for mallard and 40 mg/kg diet for bobwhite quail. Brain
acetylcholinesterase activity is inhibited following ingestion.
Diazinon may also be taken in via the dermal route. There have been
reports of substantial field kills of water fowl following application
of diazinon to turf. Field studies applying liquid formulations to
turf at 4.8 kg ai/ha resulted in no mortality or reproductive effects
on song birds. Application of granules caused a small reduction in
song bird population size compared to that of controls. Ingestion of
small numbers of granules can be fatal for small birds, as
demonstrated in laboratory studies.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Common name: diazinon
Chemical structure:
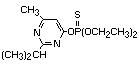 Chemical formula: C12H21N2O3PS
Relative molecular mass: 304.35
IUPAC Chemical names: O,O-diethyl O-2-isopropyl-6-methyl-
pyrimidin-4-yl phosphorothioate
CAS chemical name: O,O-diethyl O-[6-methyl-2-
(1-methylethyl)-4-pirimidinyl]
phosphorothioate
CAS registry number: 333-41-5
RTECS number: TF3325000
Official number: OMS 469; ENT 19 507
Synonyms: dimpylate, diazide, G.24480, Basudin,
Kayazinon, Necidol/Nucidol
2.1.2 Technical product
Trade names: Diazinon (Alpha, Darlingtons Mushroom
Laboratories, Murphy Chemicals and
Rentokil); Basudin (Ciba-Geigy);
Crompest (Cromessel Co. Ltd); Dethlac
(Gerhardt Pharmaceuticals); Isectalac
(Sorex Ltd); Murphy Root Guard (Fisons);
Rentokil Flytrol and Knox out 2FM
(Rentokil); Secto AntSpray and Root
Powder (Secto Ltd); Dazzel, Diagran,
Dianon (Nippon Kayaku); Diazotol
Gardentox, Nipsan (Nippon Kayaku);
Dyzol, Dizion (Nippon Kayaku);
Spectracide (Ciba-Geigy)
2.2 Physical and chemical properties
Diazinon is a clear colourless liquid (technical 95% yellow oil)
with a faint ester-like odour.
Boiling point: 83-84°C at 26.6 mPa; 125°C at 133 mPa
Vapour pressure: 9.7 mPa at 20°C
Density: 1.11 g/cm3 at 20°C
Refractive index: 1.4978-1.4981
Specific gravity: 1.116-1.118 at 20°C
Stability: susceptible to oxidation above 100°C;
stable in neutral media, but slowly
hydrolysed in alkaline media, and more
rapidly in acidic media
Decomposes: above 120°C
Corrosiveness: non-corrosive
Solubility: 60 mg/litre in water at 20°C;
completely miscible with common organic
solvents, e.g., ethers, acetone,
alcohols, benzene, toluene, cyclohexane,
hexane, dichloromethane, petroleum oils
2.3 Analytical methods
Formulated diazinon products are cleaned up by column
chromatography to remove the basic impurities and analysed by
titration with perchloric acid in acetic acid. They are also analysed
by gas-liquid chromatography (Eberle et al., 1974; Allender & Britt,
1994).
Residues in soil, water, air, plants, foods, and animal and human
tissues can be determined using gas chromatography using detectors
selective for phosphorus-containing compounds, and by other
chromatographic techniques. Table 1 outlines various methods for
determination of diazinon in different media.
Farran et al. (1988a) described a method for the determination of
organophosphorus insecticides and their hydrolysis products. The
method involves the analysis of compounds by liquid chromatography in
combination with UV and thermospray-mass spectrometric detection.
An automated identification method has been developed for water-
borne toxicants, including diazinon, using an ion chromatography/
high-performance liquid chromatography system (Fort et al., 1995).
A compendium of analytical methods for organophosphorus compounds
has been issued (NIOSH, 1994).
Table 1. Analytical methods for diazinon
Medium Analytical method References
Air adsorption on XAD-2 resin, gas NIOSH (1994)
chromatography with flame
photometric detector
Soil gas chromatography Singmaster & Acin-
Diaz (1991)
Water extraction with XAD-2 resin, gas Le Bel et al. (1979)
chromatography with nitrogen-
phosphorus detector, gas
chromatography/mass spectrometry
continuous-flow extraction coupled Farran et al. (1988b)
on-line with high-performance liquid
chromatography
liquid-solid extraction, gas Johnson et al. (1991)
chromatography/mass spectrometry
on-line solid-phase extraction, Lacorte & Barcelo
liquid chromatography/thermal spray - (1995)
mass spectrometry
on-line solid-phase extraction, Lacorte & Barcelo
liquid chromatography/atmospheric (1996)
pressure chemical ionization mass
spectrometry
maleic anhydride immunoassay Winnett (1992)
Oil solution gas chromatography Koibuchi et al.
(1975)
Fruit and solvent extraction, gas Ferreira & Silva
vegetables chromatography with thermionic Fernandes (1980)
detector
Apples solvent extraction, gas Asensio et al. (1991)
chromatography with thermionic
detector
Table 1. (con't)
Medium Analytical method References
Oranges matrix solid-phase dispersion Torres et al. (1996)
extraction, gas chromatography with
electron capture detector
Rice solvent extraction, gas Adachi et al. (1984)
chromatography with flame ionization
detector
Spinach preparative thin-layer Gilmore & Cortes
chromatography, autoradiography, (1996)
liquid scintillation counting
solvent extraction, gas Cairns et al. (1985)
chromatography with electrolytic
conductivity detector, gas
chromatography/chemical ionization
mass spectrometry
Milk gas chromatography Toyoda et al. (1990)
Human tissue solvent extraction, thin-layer Kirkbride (1987)
chromatography, gas chromatography
with nitrogen-phosphorus detector
Blood plasma gas chromatography Machin et al. (1975)
solvent extraction, gas Wu et al. (1994)
chromatography with electron capture
detector
Metabolites in urine
DEP, DEPT extraction by anion exchange resin, Lores & Bradway
gas chromatography with flame (1977)
photometric detector Weisskcp & Seiber
(1989)
GW7 550, solvent extraction, gas Lawrence & Iverson
GS 31 144 chromatography with electrolytic (1975)
conductivity detector
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Diazinon does not occur as a natural product.
3.2 Man-made sources
3.2.1 Production levels and processes
3.2.1.1 Manufacturing process
Diazinon is the common name for O, O-diethyl
O-2-isopropyl-6-methylpyrimidin-4-yl phosphorothioate (IUPAC name),
an organophosphate insecticide. Its insecticidal properties were first
described by Gasser (1953) and it was introduced in 1952, by
J. R. Geigy S.A. under the code number G 24480, trade names Basudin,
Diazitol, Neocidol and Nucidol, and the protection of BP 713278; USP
2754243. Meanwhile, improvements in the manufacturing process and the
stabilization of the technical grade diazinon by epoxidized soybean
oil have significantly reduced the content and formation of toxic
by-products and breakdown products and have reduced the acute toxicity
of diazinon products.
3.2.2 Uses
Diazinon is a contact organophosphorus insecticide with a wide
range of insecticidal activity, having long persistence and relatively
low mammalian toxicity. Diazinon is effective against adult and
juvenile forms of insects, but also against acarina. The spectrum of
activity includes the following arthropod groups:
* flying insects: flies and fly maggots, mosquitoes
* crawling insects: cockroaches, bedbugs, lice and ants
* acarina: dog ticks
* arachnideae: spiders
The main applications are rice, fruit, vineyards, sugar-cane,
corn, tobacco, potatoes, horticultural crops, animal dips and sprays.
Diazinon is also used by trained pest control operators in
households and outbuildings to control cockroaches, ants, silverfish,
spiders, carpet beetles and scorpions and in insecticidal collars on
domestic pets.
3.2.3 Formulations
The most important diazinon formulations are: ULV concentrates,
wettable powders 400 g/kg; emulsifiable concentrates 600, 400 and
250 g/litre; dust 20-40 g/kg; granules 30-140 g/kg; aerosols
200 g/litre.
Some typical formulations for agricultural and horticultural use
include: Basudin 5 (50 g a.i./kg); Basudin 10 (100 g a.i./kg) GR;
Basudin 40WP (400 g a.i./kg); Basudin 50SD (500 g a.i./kg); Basudin
60EC (EC 600 g a.i./litre); Diazitol Liquid; Basudin Ulvair 500;
Basudin 20 Mushroom Aerosol, KN; Knox-out (Pennwalt), flowable
microcapsules (230 g a.i./litre); Neocidol 60, Nucidol 60,
EC (600 g a.i./litre) for veterinary use.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Volatilization
It has been shown that diazinon is lost from soil through
volatilization (Harris & Mazurek, 1966), but the rate of loss is
unknown. Results of earlier studies with 14C-labelled insecticide and
the use of capped containers for holding treated soils indicated that
volatility was of minor importance. Under field conditions,
co-distillation, high temperatures and exposed surface areas probably
contribute to a greater loss of the insecticide through
volatilization.
Rate estimations according to the Atkinson incremental method
indicate that diazinon is rapidly degraded by hydroxyl radicals in the
atmosphere. The tropospheric half-life of diazinon lies between 1.3
and 1.5 h (Stamm, 1994).
4.1.2 Movement in soil
A study concerning degradation rate and mobility of diazinon in a
thatch layer of turf grass and in the underlying soil (2.5 cm) was
performed by Sears & Chapman (1979). Immediately following the
pesticide application, 2200 litres of water were applied to the total
treated area of 80 m2. Fourteen days after the application, less than
2% of the compound remained in the grass-thatch layer, and less than
1% in the root zone and in the underlying soil. The authors concluded
that the compound readily disappeared by degradation and/or
volatilization. However, it must be considered that only the top
2.5-cm layer was analysed.
The movement of diazinon and other organophosphorus compounds in
the soil was evaluated by means of soil thin-layer chromatography
(Sharma et al., 1986). The experiment was performed with two types of
soil (silt loam and sandy loam) showing different percentages of
organic matter (1.05 and 0.35%, respectively). The authors found a
generally poorer movement of diazinon in the silt loam soil, probably
due to the higher organic matter content and higher cation exchange
capacity. When natural soils were used as adsorbent and distilled
water as eluent, diazinon showed relatively high mobility. In this
study, the effects of pH and the presence of leachates of alkaline and
saline salts were also evaluated. Diazinon showed a slight decrease of
mobility in both soils at pH 4, whereas at pH 10 there was increase
mobility in the silt loam and slight decrease in mobility in the sandy
loam. The effects of leachate salt were not significant, with the
exception of calcium sulfate, which decreased mobility in the silt
loam soil.
The adsorption and mobility of diazinon in 25 Spanish soils and
the influence of soil properties on both processes were studied
(Arienzo et al., 1994). Adsorption constants of diazinon in the soils
were measured using soil thin-layer chromatography and soil column
leaching. The experiments were conducted with 14C-labelled diazinon.
Adsorption of diazinon was found to follow the Freundlich adsorption
equation. The Freundlich adsorption constant (K) ranged from 0.70 to
25.73. Adsorption was highly significantly correlated (p <0.001) with
the content of organic matter (OM). The median KOM value was 290
corresponding to a KOC value of 500. There was also a significant
correlation (p <0.01) of K and the distribution coefficient Kd with
the silt-plus-clay content in soils with low organic material content
(<2%). On the basis of the soil thin-layer chromatography
experiments, diazinon was found to be slightly mobile in 80% and
immobile in 20% of the soils studied. In the soil column experiment,
the pesticide was quite mobile under saturated flow in soils of light
texture containing little organic matter. Under non-saturated flow
conditions, which are more similar to natural conditions, diazinon
should not be easily leached from the studied soils to groundwater.
4.2 Degradation
4.2.1 Degradation in soil
Seyfried (1994) studied the degradation of diazinon in an
agricultural soil (silt loam, USDA) under various experimental
conditions. At 20°C and a soil moisture of 60% of the field capacity,
the DT50 was 5 days and DT90 22 days. The main metabolite,
2-isopropyl-4-methyl-6-hydroxy pyrimidine, occurred transiently and
degraded with a DT50 of 20 days. Mineralization accounted for 86% of
the applied diazinon within the experimental period of 120 days.
Whereas the application rate did not influence the degradation rate,
there was a dependence on temperature (DT50 of 12 days at 10°C) and
soil moisture (DT50 of 8 days at 30% field capacity). Under sterile
conditions, the DT50 was increased to 118 days at 20°C and 60% field
capacity. This suggests that the main route of soil degradation is
microbial.
Getzin (1968) studied persistence of diazinon in soils and
measured loss in autoclaved and non-autoclaved soil at several
temperatures, moisture contents and pH levels under controlled
laboratory conditions. Microorganisms and non-biological factors
affected the persistence of diazinon in Sultan silt loam. Diazinon was
primarily degraded through non-biological pathways. Although diazinon
was not metabolized to any great extent by microorganisms in Sultan
silt loam, it is known that soil microflora are capable of degrading
the insecticide. Gunner et al. (1966) isolated a bacterium from soil
that utilized diazinon as a source of sulfur, phosphorus, carbon and
nitrogen, but the importance of this microorganism as a contributor to
the metabolism of the insecticide in soil was not determined.
Miles et al. (1978) demonstrated that diazinon can accumulate and
persist in organic soils for more than a year. It was also shown that
diazinon can move from its soil-bound form into the aqueous
environment either via leaching or by direct soil erosion (Miles &
Harris, 1978a). Morganian & Wall (1972) demonstrated that diazinon
treatment of a marine salt marsh led to a build-up of diazinon in salt
marsh sod and mud.
At pH 6.8, the time required for 50% loss of diazinon is 6 weeks
in autoclaved soil and 18 weeks in buffered water. Mortland & Raman
(1967) demonstrated the catalytic hydrolysis of diazinon in CuCl2
solutions and Cu-montmorillonite suspensions. Catalytic reactions of
this nature may occur in soil, but attempts to demonstrate this
phenomenon in Sultan silt loam have so far failed. Moisture variations
from 50 to 100% of the moisture equivalent did not appreciably alter
the degradation rates of diazinon. Variations in soil temperature
between 10 and 30°C resulted in a 4- to 10-fold difference in the time
required for 50% loss of the insecticides in soil. The non-biological
degradation of diazinon increased with increased acidity.
Schoen & Winterlin (1987) have studied the factors affecting the
rate of diazinon degradation in soil. These are pH, soil type, organic
amendments, soil moisture and pesticide concentration. Of the soil
factors investigated, the conditions for diazinon degradation in
pesticide mixtures were optimum when the pesticides were present at
low concentrations in moist soil, amended with peat and acidified to
pH 4. Degradation was least at high pesticide concentration in neutral
or alkaline mineral soil.
Utilization of diazinon by an Arthrobacter species and a
Streptomyces species has been shown to alter the microbial
population by stimulating a selective enrichment of these species.
The Arthrobacter species previously reported to attack the side
chain of the molecule was unable to metabolize completely the ring
portion of the molecule. Similar results demonstrated that the
Streptomyces species, too, could not by itself convert pyrimidinyl
carbon to carbon dioxide. When, however, both the Arthrobacter and
Streptomyces organisms were incubated together, 15-20% of the 14C
appeared as labelled BaCO3 after 18 h, suggesting a synergistic
relationship between these two organisms in attacking the pyrimidinyl
portion of diazinon (Gunner & Zuckerman, 1968).
Barik & Munnecke (1982) demonstrated that a bacterial enzyme can
hydrolyse diazinon in soil. In their research, an enzyme was obtained
from a Pseudomonas sp. that could hydrolyse diazinon and several
other methoxy- or ethoxy-substituted organophosphates. In this
experiment, diazinon, either in 25% EC formulations or as a technical
grade chemical, was enzymatically hydrolysed in an agricultural sandy
soil when present at concentrations up to 1%. The degradation rate was
approximately proportional to enzyme concentration up to 12 units per
20 g soil. This indicates that the initial rate of diazinon
degradation is directly dependent on enzyme activities, and not on
chemical or physical parameters of the soil-pesticide interactions.
Although the enzyme was examined only in one soil, it is expected that
it could also operate on cement or asphalt type surfaces, as well as
on synthetic polymers such as carpet.
Al-Attar & Knowles (1982) studied the uptake, metabolism and
elimination of diazinon in Panagrellus redivivus, a free-living soil
nematode, and Bursaphelenchus xylophilus, a plant parasitic
nematode. Nematodes were exposed to a solution of diazinon labelled
with radiocarbon. Both nematode species metabolized diazinon, although
P. redivivus was more active. Metabolites from B. xylophilus
included O, O-diethyl O-(2-isopropyl-4-methyl-6-pyrimidinyl)
phosphate or diazoxon and pyrimidinol. Radioactivity accumulated to a
greater extent in B. xylophilus than in P. redivivus. Elimination
of radiocarbon was more rapid with P. redivivus than with
B. xylophilus, and this resulted in the presence of high levels
of the polar pyrimidinol metabolite in the incubation medium of
P. redivivus.
4.2.2 Degradation in water
Keller (1983) investigated the degradation of diazinon in samples
of pond and river water, each containing 1% of sediment. Diazinon was
degraded with a DT50 of 7 to 10 days in the pond system and 8 to 15
days in the river water. Mineralization accounted for >60% of the
applied material within 7 weeks in both systems.
In a mesocosm study conducted with 17 treated and 4 untreated
ponds (0.05 hectare each), diazinon degraded rapidly. The
disappearance half-lives averaged 5.2 to 12.2 days (Giddings, 1992).
Kanazawa (1975) found diazinon to be fairly persistent in tap
water in a glass aquarium, degrading to 27% in 30 days.
Ferrando et al. (1992) studied the persistence of diazinon in
natural water from Albufera Lake and in experimental water from their
laboratory. Degradation was faster in lake water, the half-lives being
70 and 79 h for lake and laboratory water, respectively. The
degradation process in both media was comparable until 96 h. The
authors found 43.5 and 49.4% of the applied diazinon in natural and
experimental water, respectively, at 96 h.
4.2.3 Bioconcentration
4.2.3.1 Fish and aquatic invertebrates
The bioconcentration factors (BCF) of diazinon over a 7-day
period were as follows: topmouth gudgeon 152; carp 65; guppy 18;
crayfish 4.9; red snail 17; pond snail 5.9 (Kanazawa, 1978).
Seguchi & Asaka (1981) reported the intake and excretion of
diazinon and its metabolites in freshwater fish, and the relationship
between the BCF of diazinon and fat content of fish. During exposure
to continuous-flow water containing 0.02 mg diazinon/litre the
concentration of diazinon in fish rapidly increased, reaching a
maximum after 3 days. Thereafter, the diazinon concentration slightly
decreased and remained at equilibrium. The BCFs for carp, rainbow
trout, leech and shrimp at equilibrium were 120, 63, 26 and 3,
respectively. As for the metabolites, pyrimidine analogue was found in
all fish species, but diazinon and related compounds were found only
in carp and rainbow trout. The concentration of the metabolites
reached a maximum after 3-7 days exposure to diazinon. Diazinon was
metabolized to diazoxon in the channel catfish liver microsomal enzyme
system, but it was not found in any other fish species. When the fish
were transferred to clean water, diazinon and its metabolites were
rapidly lost from the fish. Seven days after being transferred to
clean water, the diazinon concentration decreased to 0.3-8.0% of the
equilibrium concentration, and the metabolites decreased below the
detection limit.
Similar results have been observed for topmouth gudgeon by
Kanazawa (1975, 1978). A linear relationship was observed between the
bioconcentration ratio and fat content in fish. Seguchi & Asaka (1981)
identified six metabolites of diazinon, and Fujii & Asaka (1982)
identified another three: hydroxydiazinon, hydroxymethyl diazinon and
isopropenyl diazoxon.
The toxicity, accumulation and elimination of diazinon were
investigated in the European eel (Anguilla anguilla). Fish exposed
to sublethal concentration (0.042 mg/litre) accumulated diazinon in
the liver and muscle tissues. The BCFs for diazinon were 1859 in liver
and 775 in muscle over the 96-h exposure period. When removed from
diazinon-containing water, the contaminated fish rapidly eliminated
diazinon. The excretion rate constants were 0.108 per h for liver and
0.016 per h for muscle. Diazinon half-lives were 16.6 and 33.2 h for
liver and muscle, respectively (Sancho et al., 1992).
The freshwater fish Motsugo (Pseudorasbora parva) was reared in
an aquarium tank containing about 1 mg diazinon/litre for 30 days. The
persistence of the insecticide in water and the uptake and excretion
of the insecticide by fish were monitored. Diazinon degraded by 72% in
30 days. The concentration of diazinon in fish reached a maximum level
of 211 mg/kg after 3 days. Afterwards, the concentration of the
insecticide decreased gradually due to metabolism and excretion
(Kanazawa, 1975).
Bioconcentration and excretion of diazinon were studied in the
carp ( Cyprinus carpio L.). The average BCF values for diazinon were
20.9 in muscle, 60.0 in liver, 111.1 in kidney and 32.2 in gall
bladder over a 168-h exposure period. The excretion rate constants of
diazinon (ng/g per h) were 0.002-0.024 for muscle, 0.001-0.020 for
liver, 0.0004-0.004 for kidney and 0.002-0.023 for gall bladder,
respectively (Tsuda et al., 1990).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
The amount of insecticide present in the air of commercial pest
control buildings, service vehicles and food preparation-serving areas
following routine commercial insecticide application has been
measured. Diazinon was measured in the ambient air of storage and
office rooms in six North Carolina (USA) firms in a 4-h period. In the
storage rooms the mean value was 284 (85-837) ng/m3 air and in the
offices 163 (31-572) ng/m3 air. Diazinon was also detected in the
ambient air of vehicles used in commercial pest control activities.
Mean diazinon concentrations (ng/m3 air) in 2-h of sampling from six
vehicles were 88 (7-239) in sedans and 171 (11-543) in vans, the mean
value being 130 (7-543). The highest level of diazinon detected in the
ambient air of offices of pest control building was far below the
allowable limits (TLV : 100 µg/m3) (Wright & Leidy, 1980).
Wright et al. (1982) studied the amount of diazinon in cabs of
stationary pick-up trucks used by the pest control service. Additional
air samples, taken while the same pick-up truck was moving, provided
data for comparison of insecticide levels in individual pick-up trucks
when moving and stationary. Diazinon was present in significantly
greater concentrations than chlorpyrifos. This may be attributable to
the facts that the service technicians kept diazinon in sprayers
during the sampling periods and that they used it in servicing
accounts during the sampling day. It could therefore have contaminated
their clothing and skin and passed into the air when they were in the
pick-ups. The maximum diazinon detected was 5.15 µg/m3 for a 2-h
period or 20.6 µg/m3 for an 8-h period, which is about 1/5 of the
allowable limit. However, the amount of airborne diazinon to which a
technician was actually exposed during a working day was even less
than 20.6 µg/m3, since the maximum time any technician spent in a
pick-up was 3.8 h.
Wachs et al. (1983) reported the concentration of diazinon in
the air of a retail garden store that sold the insecticide. The
concentration found in the air based on the 14-h period pumping
through the polyurethane filters was 3.4 µg/m3. All of the diazinon
was found in the polyurethane plug closest to the air inlet. Diazinon
was not found in the second plug or unused plugs which were similarly
Soxhlet-extracted and analysed. It was concluded that the
concentration of diazinon in air depended on a number of factors,
including the type of formulation, air temperature, type and condition
of containers, prior spills and types of floor covering. The
concentration of diazinon vapour found in this study would not appear
to constitute a hazard to store personnel or customers.
Airborne concentrations of diazinon were measured in rooms for
21 days after crack and crevice application. Residue levels were
greatest in treated rooms (38 µg/m3) followed by adjacent (1 µg/m3)
and upper and lower floor rooms (about 0.4 µg/m3). Low levels of
diazinon were detected in all rooms 21 days after application. Small
amounts of diazinon (corrected to an 8-min application period) were
detected on respirator pads (2.6 µg) and waist pads (2.3 µg) worn by
the applicator (Leidy et al., 1982).
Airborne and surface concentrations of diazinon were measured at
intervals up to 10 days after broadcast spray application onto the
floors of seven offices. Diazinon concentrations peaked 4 h after
application at 163 and 27 µg/m3 of air sampled, respectively.
Airborne concentrations of diazinon indicated that building occupants
should not enter unventilated rooms for at least 2 days after
spraying. Residues on aluminium plates and furniture were examined at
intervals of up to 48 h after spraying, and in many cases the surface
concentrations were higher at 24 or 48 h after spraying than at
one hour. The peak residue concentration of diazinon was 38 ng/cm2 of
surface area sampled at 48 h (Currie et al., 1990).
5.1.2 Water
Insecticide residues on suspended and bottom sediments of streams
of Ontario, Canada, have been studied in a tobacco-growing, vegetable
muck area. Bed load samples contained three to six times higher
concentrations of insecticides than bottom material (Miles, 1976).
From 1985 to 1987, a monitoring survey was conducted to determine
the levels of selected pesticides in farm ditches located in the lower
mainland of British Columbia, Canada. Diazinon was not detected in
ditch water (detection limit = 1 µg/litre). In ditch sediments,
diazinon was sporadically found at concentrations up to 4 µg/kg
(detection limit = 1 µg/kg) (Wan, 1989).
During the first half of 1984, diazinon was not detected in raw
or treated water samples from the Lakeview and Lorne Park Water
Treatment Plants in Toronto, Ontario (detection limit = 10 ng/litre)
(MacLaren Plansearch Inc. & FDC Consultants Inc., 1985).
Detectable concentrations of diazinon occurred in less than 0.1%
of water samples collected from 11 Southern Ontario agricultural
watersheds during 1975-1977. The concentration was mainly below
0.01 µg/litre, the maximum value being 0.15 µg/litre (Frank et al.,
1982).
Sampling performed in 1992 by the United Kingdom National Rivers
Authority showed diazinon at >0.1 µg/litre in 74 out of 2300 fresh
water samples and at > 0.15 µg/litre in 1 out of 12 seawater samples.
5.1.3 Soil
In 1971 hay and soil samples were collected in 9 states in the
USA to determine the incidence and levels of pesticide residues in
hayfields. Residues were detected in 8% of the soil samples and 29% of
the hay samples. Diazinon was detected in four hay samples (Gowen et
al., 1976).
In 1976, soil samples from 28 farms located in six vegetable
growing areas of southwestern Ontario, Canada, contained diazinon
residues from trace amounts (< 0.02 mg/kg) to 0.29 mg/kg (Miles &
Harris, 1978b).
5.1.4 Fruit, vegetables and food
Results of supervised trials and monitoring of diazinon residues
in or on food and feed commodities have been comprehensively reviewed
and summarized (FAO/WHO, 1994a). The following examples indicate that
diazinon residues are generally low.
Ward et al. (1972) performed a study to determine the rate of
decline of diazinon residue on wheat in Texas, USA. There was a steady
decline in the amount of diazinon remaining on foliage samples after
application. Only 0.16 mg/kg and 0.31 mg/kg remained 28 days after
treatment with 0.28 and 0.56 kg a.i./ha, respectively. Harvest samples
showed that less than 0.05 mg/kg remained in either the foliage or
grain.
Between 1978 and 1986, 305 samples of apples were analysed for
residues of a wide range of pesticides used in their production.
Residues of diazinon were found occasionally. They were well below the
maximum residue limit and correlated well with the pattern of use
(Frank et al., 1989).
Between 1986 and 1988, 433 composite vegetable samples
representing 16 commodities, which were treated by various pesticides
including diazinon, were collected from farm deliveries to the
marketplace in Ontario, Canada. All samples were analysed for
insecticides and fungicides. The commodities tested included
asparagus, beans, carrots, celery, cucumbers, lettuce, onions,
peppers, potatoes, radishes, rutabagas and tomatoes. In 64% of
samples, no pesticide residues were identified (the limits of
detection ranged from 0.005 to 0.05 mg/kg). A further 22% had combined
insecticide and fungicide residues below 0.1 mg/kg. Only three samples
(0.7%) had residues that exceeded the Maximum Residue Limit (MRL).
These involved diazinon on celery. While some commodities had no
detectable residues, others had measurable residues of up to three
different pesticides. The highest levels were found on celery, lettuce
and field tomatoes (Frank et al., 1990).
Levels of diazinon permitted in the USA on human food range from
0.1 mg/kg in potatoes to 0.7 mg/kg in most leafy vegetables. During
the course of pesticide surveillance of vegetables, an unknown
analytical response in spinach extract was seen, which was
subsequently identified as diazinon metabolite (2-isopropyl-4-
methyl-pyrimidin-6-ol). Cairns et al. (1985) described an analytical
procedure adapted to confirm both diazinon and its metabolite in
spinach, at very low levels, by methane chemical ionization mass
spectrometry. The presence of this metabolite at the 1 mg/kg level
represents an order of magnitude greater than that found for diazinon
itself.
In a study of diazinon residues in prepared foods, accidentally
exposed during and following treatment, the amounts of diazinon
residues in food left in the room for 30 min after treatment ranged
from 0.02 to 0.05 mg/kg. No detectable residues of diazinon were found
in the potatoes or dinners placed in the rooms 4.5 h after treatment
and removed after 5 h. A person consuming a dinner at the highest
residue found would have ingested 0.0153 mg of diazinon. For a person
weighting 70 kg this would amount to 0.218 µg/kg (Jackson & Wright,
1975).
5.1.5 Milk
Insecticides in polyvinyl chloride pellets were included in a
commercial dairy protein supplement and fed to dairy cows at 1.4, 2.0
and 2.5 mg of diazinon/kg body mass for 2 weeks. No insecticidal
residues were found in milk samples collected at 1, 3, 7, 10 or 14
days. Even 2.5 mg/kg dosage would provide a 5-fold margin of safety
for PVC formulation-diazinon fed to cattle to control face fly larvae
in manure (Lloyd & Matthysse, 1971), and diazinon-PVC was found to
be still a highly effective larvicide if given at the dose of
0.5 mg insecticide/kg per day (Lloyd & Matthysse, 1966, 1970).
Derbyshire & Murphy (1962) reported no diazinon residues in milk
from cows fed 10 mg/kg body weight for 7 days. Robbins et al. (1957)
found only traces of radioactivity in a cow's milk 6-24 h after a
single oral dose of 32P-labelled diazinon (20 mg/kg).
5.1.6 Meat and fat
Tissue residues were determined and toxicity symptoms were noted
after lambs were sprinkled and dipped with 0.06% diazinon emulsion or
sprinkled with 1% diazinon emulsion. The only diazinon residues found
were 1.45-2.30 mg/kg in fat, 1 day after dipping in 0.06% diazinon,
with concurrent 44-47% plasma cholinesterase activity depression. Low
residues were present in blood from these sheep. Most tissues
contained no detectable diazinon at 15 or 26 days after lambs were
dipped in 0.06% diazinon, but fat contained up to 0.52 mg/kg at 15
days and 0.31 mg/kg at 26 days. Sprinkling with 1% diazinon produced
no residues in most tissues. A maximum of 23 mg/kg was found in fat.
The only clinical poisoning involved a 3-day-old lamb dipped in 0.12%
diazinon suspension. Lambs more than 1 week old were not poisoned by
0.06% diazinon nor were lambs more than 1 month old when treated by
0.25% diazinon (Matthysse et al., 1968).
Harrison et al. (1962) found 0.4 mg diazinon/kg in meat of
unshorn sheep 1 day after dipping in 0.05% diazinon emulsion. This
decreased to 0.25 mg/kg and 0.16 mg/kg at 4 and 7 days after dipping,
respectively, and there were negligible amounts 25 days after dipping.
Claborn et al. (1963) found 0.69 mg/kg in beef fat 1 day after
the last of 11 weekly spraying with 0.05% diazinon suspension. The
authors reported a rapid loss of diazinon from beef fat and the amount
of residues were negligible 14 days after spraying.
Samples obtained from retail outlets in the United Kingdom during
1984-1986 generally showed zero or low levels of diazinon residues.
Diazinon was not detected in samples of beef, imported lamb, pork or
veal, but low levels were found in United Kingdom lamb in 1984/1985
(up to 1.7 mg/kg) and 1985/1986 (up to 0.1 mg/kg). Samples of fat
taken in 1986 were analysed and, out of 274, 19% contained diazinon.
In 1987, however, out of 280 samples analysed, 7% contained diazinon
and in four of them residues exceeded the Codex MRL of 0.7 mg/kg fat.
Diazinon was not detected in butter, milk or cheese (MAFF, 1989).
Various pesticides and pollutants were examined in poultry meat
from Israel. The levels of these, which included diazinon in broilers,
turkeys and geese, were said to be extremely low and below the USA
tolerance levels (Kathein, 1986)
5.2 General population exposure
The primary exposure to the general population will be through
intermittent dietary exposure and inhalation exposure. Exposure via
water is negligible. Total-diet studies commenced in the United
Kingdom in 1966. In the second survey (1970-1971) and in the latest
survey (1985-1988), diazinon residues were not detected (Egan &
Weston, 1977; MAFF, 1982, 1986, 1989). Findings similar to those in
the United Kingdom were also made in the USA. Toddler total diets have
also been the subject of investigation in the USA. Diets collected in
ten American cities between 1978 and 1979 were examined. The
components were drinking-water, whole milk, other dairy products and
dairy substitutes, meat/fish/poultry, grain cereals, potatoes,
vegetables, fruit juices, oils and fats, sugars and beverages
(Gartrell et al., 1985a). A similar exercise in the years 1980-1982
was conducted in 13 American cities. The results were similar to those
obtained in 1978-1979, with intake of diazinon being low (Gartrell et
al., 1985a,b).
A total-diet study in New Zealand was performed at 3-monthly
intervals in the period 1974-1975. Of 116 samples analysed, 82 (71%)
had no detectable residues of diazinon. Intakes were well below the
Codex MRLs (Dick et al., 1978).
The overwhelming evidence from residue and total-diet studies
suggests that residues of diazinon are generally within the acceptable
levels set by the Codex Alimentarius Commission. The results suggest
that the compounds are rapidly broken down, whether on plants or in
animals, further reducing the risks to humans (IPCS, 1986).
During a 5-year study period (1981-1986), the US Food and Drug
Administration analysed nearly 20 000 domestic and imported samples of
food and feed commodities for pesticide residues. The results showed
that 29 out of 6391 domestic agricultural commodities and 35 out of
12 044 imported agricultural commodities had diazinon levels greater
than 0.05 mg/kg (Hundley et al., 1988).
Diethyl phosphate (DEP), an organophosphate metabolite, was found
in the urine of symptomatic residents who resided in a household that
had been sprayed with diazinon 4.5 months earlier. Pre- and
post-decontamination data with regard to symptoms and to DEP,
cholinesterase, and surface and air levels underscore the utility of
alkyl phosphate metabolites for monitoring exposure. The data also
emphasize the efficacy of clean-up measures when baseline data are not
available to determine if "within-normal" cholinesterase levels are,
in fact, depressed (Richter et al., 1992).
5.3 Occupational exposure
An occupational exposure study was conducted for a firm employing
22 pest control operators (PCOs) exposed to three organophosphorus
insecticides including diazinon. The 8-h exposure levels were less
than 131.0 µg/m3. Urine samples (24-h) were analysed for alkyl
phosphates and showed the presence of metabolites for these three
insecticides. The effect of this exposure was reflected by a
statistically significant inhibition of plasma cholinesterase activity
among the PCOs, but physical examinations detected no apparent toxic
effects (Hayes et al., 1980).
A behavioural evaluation of pest control workers with short-term
(mean 39 days) low-level exposure to diazinon was conducted in 1985
during the course of a pest control program in California (see section
8.2.2). The diazinon metabolite diethylthiophosphate (DETP) was
measured in pre- and post-shift urine samples and the full-shift
exposure to diazinon was quantified for 19 subjects using personal
air monitoring and passive badges. The median diazinon exposure was
2.1 mg/day (Maizlish et al., 1987).
An investigation was conducted to determine worker exposure to
airborne pesticides during tree and ornamental shrub applications
using hand-held equipment during an entire work shift. Employee
exposure data were collected for 3 consecutive years. The sampling was
performed during the late spring, summer and early autumn when insect
and disease activity was most prevalent. Sampling was conducted at 23
locations. Those applying these chemicals sustained low-level exposure
to acephate, benomyl, carbaryl, chlorothalonil, diazinon and dicofol.
As pesticide label instructions for mixing and applying pesticides
were strictly followed, the tree and ornamental shrub applicators were
able to keep inhalation exposures below the levels recommended by OSHA
and NIOSH. Of the 74 exposures monitored, 67% were below the detection
limit (0.001 mg/m3), while others were 0.001-0.040 mg/m3. This
observation supports the correctness of not including specific
respiratory protection measures on pesticide label directions for
mixing, loading and applying these pesticides (Leonard & Yeary 1990).
Dermal, respiratory and urine measurements were made on workers
applying granular diazinon pesticide formulation. In all, 15 workers
and four control subjects were monitored. The workers applied the
compound in yards and small pastures using hand equipment comparable
to that used in a home environment. Respiratory air samples, ethanol
hand rinse samples, clothing patch samples and urine samples were
collected. The diazinon exposures were correlated with job category,
application duration, application equipment and protective clothing.
The best determinants of diazinon exposure were the job categories and
the use of the belly grinder type of spreader. The rank of exposure
magnitude, from highest to lowest, was the crew using the belly
grinder, the crew not using the belly grinder, the crew chief and the
supervisor. The mean daily dermal and respiratory diazinon exposures
for these four job categories ranged from 0.6 to 11 mg, 0.1 to 1.8 mg,
0.1 to 0.25 mg, and 0.03 to 0.07 mg, respectively. The amount of
urinary diethylthiophosphate increased during the day for all job
levels, but showed variable recovery (Weisskop et al., 1988).
6. KINETICS AND METABOLISM
6.1 Absorption, distribution and excretion
6.1.1 Oral administration
6.1.1.1 Rats
Four male and 2 female Wistar rats were treated with single
oral doses of 0.8 mg [pyrimidine-14C]-diazinon (specific activity
4.0 µCi/mg). An additional group of 4 males received [ethoxy-14C]-
diazinon (3.2 µCi/mg) at the same dose level. During the observation
period of 168 h, both labelled parts of the molecule were excreted
almost completely, 65.4-80.0% of the administered radioactivity being
detected in urine, 16.0-23.5% in the faeces and, with the ethyl-label,
5.6% in the expired air (total recovery 90.2-98.3%). No radioactive
CO2 was detected with the pyrimidine label. The half-life times of
excretion were 7 h with the ethyl label and 12 h for both sexes
treated with the pyrimidine-labelled material. Daily oral
administration of 0.1 mg [pyramidine-14C]-diazinon to male rats for
10 consecutive days resulted in no accumulation of the radioactivity
in any organ investigated (oesophagus, stomach, intestines, liver,
spleen, pancreas, kidneys, lungs, testes, muscles, fat). Six hours
after the last administration, the highest residues were detected in
the muscles (0.77% of the totally applied dose), caecum (0.76%) and
small intestine (0.65%). The residues were below the detectable limit
48 h after the cessation of the treatment (Mücke et al., 1970).
Sprague Dawley rats received [pyrimidine-14C]-diazinon at single
oral doses of 10 mg/kg (specific activity 30.3 µCi/mg) or 100 mg/kg
(specific activity 9.7 µCi/mg). A third group was treated with daily
oral doses of 10 mg/kg technical diazinon (87.7% pure) for 14
consecutive days, followed by a single treatment at the same dose
level with the 14C-labelled compound. The disposition of the
administered 14C was observed for a 7-day period before the animals
were killed and the tissues removed for analysis. The average recovery
of the radioactivity was 99.2%. Elimination of diazinon equivalents
was rapid. In the low-dose group, males and females eliminated 93 and
86%, respectively, of the administered radioactivity in the urine
within 24 h. Faecal elimination amounted to 1.6 and 1.1%,
respectively, in the same time period. In the high-dose group, the
respective values were slightly lower and indicated that the
elimination was more rapid in males (90.8% in urine and 2.2% in
faeces) than in females (58.2% in urine and 0.87% in faeces). The
pre-conditioning of the rats had no influence on absorption and
elimination. Seven days after the administration of the [pyrimidine
14C]-diazinon, the residual radio-activity was generally low. Among
the tissues examined (heart, lung, spleen, kidney, liver, fat, testes,
ovaries, uterus, muscle, brain, blood plasma, blood cells, bone), the
residual radioactivity amounted to approximately 0.01 mg/kg diazinon
equivalents in the low-dose group; only fat (0.02 mg/kg), blood cells
(0.05 mg/kg) and bone (<0.017 mg/kg) contained higher amounts of
radioactivity. In the high-dose group, the residual radioactivity was
8-10 times higher. Pretreatment with technical diazinon for 14 days
led to residues similar to those observed in the low-dose group
(Craine 1989a,b).
6.1.1.2 Guinea-pigs
Male guinea-pigs treated orally with 45 mg/kg [32P]-diazinon
(specific activity 117-197 cpm/mg) in peanut oil, the tissue
distribution was determined at 2, 4, 8 and 16 h after treatment and
the excretion of 32P was investigated over an 8-day period. Following
oral administration, the compound was rapidly absorbed as shown by a
sharp decrease of activity in the stomach and low levels found in the
small intestine. Within 16 h, 46.6% of the administered radioactivity
was eliminated in the urine and 0.34% appeared in the faeces. The
caecum showed a gradual increase of radioactivity, 13-36% of the
administered dose accumulating in the caecum over 16 h after the
administration. Irrespective of this accumulation, within 48 h after
dosing, 80% of the administered radioactivity was eliminated in the
urine while only 8% was eliminated in the faeces (Kaplanis et al.,
1962).
6.1.1.3 Dogs
Two female Beagle dogs were intravenously dosed with 0.2 mg/kg
[ethoxy-14C]-diazinon (specific activity 3.4 µCi/mg) in 0.7 ml
ethanol. Blood samples were drawn at times ranging from 5 min to 7 h
after the injection. The decline of the radioactivity in the blood
was biphasic with a slower second phase. The half-life of elimination
from blood for this second phase was calculated to be 363 min.
Approximately 58% of the administered radioactivity was recovered in
the urine within 24 h after the administration. Another two female
beagle dogs were orally dosed by capsule with 4.0 mg/kg [ethoxy-14C]
diazinon in ethanol. Approximately 85% of the administered
radioactivity was recovered within 24 h after oral administration,
with 53% of it occurring in urine (Iverson et al., 1975).
6.1.1.4 Goats
Two lactating goats were orally treated with [pyrimidine-14C]-
diazinon (specific activity 9.7 µCi/mg) in gelatin capsules for four
consecutive days at a dose level of 4.5 mg/kg per day, corresponding
to a dietary exposure of 100 mg/kg of feed. During the observation
period, in average 64.1% of the administered radioactivity was
excreted with urine, 10.4% with the faeces and 0.31% with the milk.
A plateau of radioactivity in the milk was reached after 3 days of
dosing at a mean level of 0.46 mg/kg diazinon equivalent. At
sacrifice, radioactivity in the blood accounted for 0.2% and the
tissues examined accumulated 0.92% of the administered dose. The
highest residual radioactivity was detected in the kidney (2.0 mg/kg)
and the liver (1.2 mg/kg). The other tissues examined contained
0.23-0.3 mg/kg diazinon equivalents (Simoneaux 1988a,b; Pickles &
Seim, 1988).
6.1.1.5 Cow
A lactating Hereford cow (body weight 268 kg) was orally treated
with a gelatin capsule containing 20 mg/kg 32P-diazinon (specific
activity 518 cpm/µg). Urine and faeces were collected during 36 h
after treatment and further samples were investigated until the study
was terminated after 168 h. In addition, milk and blood samples were
investigated. Within 36 h, approximately 74% of the administered
radioactivity was excreted with the urine, 6.5% appeared in the faeces
and 0.08% was found in the milk. A peak concentration of 2.27 mg/kg
diazinon equivalents was reached 18 h after the administration
(Robbins et al., 1957).
6.1.1.6 Hens
Four laying Leghorn hens were treated with 2-14C-diazinon
(specific activity 30.3 µCi/mg) in gelatine capsules for seven
consecutive days at daily doses of 1.7 mg/kg body weight,
corresponding to a dietary exposure of 25 mg/kg in feed. Excreta and
eggs were collected and, approximately 24 h after the final dose, the
animals were killed and tissue samples of liver, kidney, blood, lean
meat, skin and attached fat, and peritoneal fat were examined.
Elimination of most of the administered radioactivity occurred via the
excreta, with 78.6% of the total dose being excreted during the study
period. Approximately 0.1% of the radioactivity was found in tissues
and blood, less than 0.01% appeared in the egg yolks and 0.07% was
detected in the egg whites. The residual radioactivity in the
tissues amounted to 0.148 mg/kg diazinon equivalents in the kidney,
0.137 mg/kg in blood, 0.11 mg/kg in the liver and 0.01-0.025 mg/kg in
the other tissues examined. The residues in the egg yolks ranged from
0.006 mg/kg diazinon equivalents to 0.065 mg/kg while those in the egg
whites ranged from 0.038 mg/kg to 0.066 mg/kg. On a whole egg basis, a
plateau concentration of 0.047 mg/kg was reached on day 4 of treatment
(Simoneaux 1988c,d; Burgener & Seim, 1988).
6.1.2 Dermal application
6.1.2.1 Rats
The percutaneous absorption of diazinon was investigated in male
and female Sprague Dawley rats dermally exposed to 1 mg/kg (specific
activity 25.2 µCi/mg) and 10 mg/kg (specific activity 2.62 µCi/mg) of
[pyrimidine-14C-diazinon] dissolved in tetrahydrofuran. The dermal
absorption, excretion and tissue residues were determined after 0, 2,
8, 24, 48, 72 and 144 h. At each time point, four rats per sex and
dose group were used. The total recoveries for the balance data
averaged 96.3-101.5%. Calculated t50 absorption rates (i.e. the
amount of time required for 50% of the administered dose to be
absorbed into or penetrate through the skin) in males and females were
11.8 and 5.2 h, respectively, at the low-dose level of 1 mg/kg. At
10 mg/kg the respective t50 absorption rates were 10.2 and 5.3 h,
respectively, indicating that dermal absorption was more rapid in
females and was dose-dependent. The urine was the major route of
excretion in both sexes at both dose levels, 65-78% of the radiolabel
being excreted within 72 h. Times for 50% excretion in males and
females dosed at 1 mg/kg were 28.1 and 26.8 h, respectively. In the
high-dose groups the times for 50% excretion were 24.1 and 20.3 h in
males and females, respectively. The residual radioactivity in tissues
reached a maximum at 8 h after the administration in both dose groups
(plasma, red blood cells, fat, brain, muscle, lung, heart, spleen,
kidney, liver, stomach, small and large intestines, gonads, skin wash
and dissolved skin were assayed). In the low-dose group of males after
8 h, highest values were found in stomach (0.36 mg/kg diazinon
equivalents), small intestines (0.16 mg/kg), kidney (0.15 mg/kg),
liver (0.1 mg/kg) and skin (3.9 mg/kg in the skin wash and 0.86 mg/kg
in the dissolved skin). Reflecting their absorption rate, the females
of the low-dose group showed slightly higher tissue levels and a lower
residual radioactivity in the skin wash. After 144 h, residues were
down to the limit of quantification in most tissues, in both dose
groups and in both sexes (Ballantine, 1984).
6.1.2.2 Sheep
Two sheep were dermally treated with [pyrimidine-14C-diazinon]
(specific activity: 3.7 µCi/mg) dissolved in acetone for three
consecutive days. In order to mimic an extreme maximum exposure in a
dermal treatment of 40 mg/kg, 2270 mg 14C-diazinon was applied daily
to a shaved area of the back that constituted approximately 10% of the
animal's surface area. The area of application was left uncovered. Six
hours after the last administration the animals were killed and heart,
liver, kidney, back fat and leg muscle were analysed. The tissue
extractability was greater than 90% for all tissues. The highest
average residues were detected in kidney (9.4 mg/kg diazinon
equivalents) and back fat (7.3 mg/kg), while levels in heart, liver
and leg muscle amounted to 4-4.4 mg/kg (Capps, 1990; Pickles, 1990).
6.1.2.3 Humans
The dermal absorption of diazinon in humans is much less than in
rats. Six volunteers were dermally treated with [pyrimidine-14C]-
diazinon on the ventral forearm or the abdomen. The test material was
administered in acetone solution (2 µg/cm2) or dissolved in lanoline
wool grease (1.47 µg/cm2) over a 10-cm2 area of the skin without
occlusion. After 24 h, the test substance remaining on the site of
administration was washed off and the renal elimination followed for
seven days. Independent of the vehicle and the site of administration,
only 3-4% of the dose applied was percutaneously absorbed (Wester et
al., 1993).
6.1.3 Other routes
6.1.3.1 Intraperitoneal administration
The tissue distribution of diazinon and the inhibition of
cholinesterase (ChE) activities in plasma and erythrocytes were
investigated using male rats that received a single intraperitoneal
dose of diazinon (100 mg/kg body weight) in olive oil. The blood
diazinon level was estimated to reach a maximum at 1-2 h after
intraperitoneal administration. It was demonstrated that the diazinon
residue levels were highest in the kidney, when comparing the
distribution of diazinon among liver, kidney and brain in the animals
after dosing. Erythrocyte and plasma ChE activities were inhibited
rapidly, but ChE inhibition was greater in the erythrocytes than in
plasma (Tomokuni & Hasegawa, 1985).
The tissue distribution of diazinon and the inhibition of ChE
activities in plasma, erythrocyte and brain was investigated using
male rats and mice that received a single intraperitoneal (i.p.) dose
of diazinon (20 or 100 mg/kg body weight) in olive oil. The blood
diazinon level was estimated to reach a maximum 1-2 h after the i.p.
administration. It was demonstrated that the diazinon residue levels
were highest in the kidney, when comparing the distribution of
diazinon among liver, kidney and brain in the animals after dosing.
The ChE inhibition by diazinon exposure was greater in the plasma than
in the erythrocytes for male mice, while its inhibition was greater in
the erythrocytes for male rats. Brain ChE activity was also inhibited
markedly in the mice after dosing (Tomokuni et al., 1985).
6.1.3.2 Subcutaneous administration
Male guinea-pigs were subcutaneously treated with 45 mg/kg
32P-labelled diazinon (specific activity -117-197 cpm/µg) in peanut
oil. The tissue distribution was determined 2, 4, 8 and 16 h after
treatment, and excretion of 32P was investigated over an 8-day
period. Following subcutaneous administration, urinary elimination
amounted to 20% of the administered dose after 16 h. The levels of
radioactivity found in the gastrointestinal tract were low apart from
the caecum, which accumulated up to 5.5% of the administered dose over
16 h. After 48 h, urinary elimination amounted to about 60%, while
only trace amounts were eliminated with the faeces (Kaplanis et al.,
1962).
6.1.3.3 Intravenous administration
Four female Rhesus monkeys were dosed intravenously with 2.1 µCi
(31.8 µg) [pyrimidine-14C]-diazinon dissolved in propylene glycol.
Within 7 days, average values of 56 and 23% of the dose were
eliminated in urine and faeces, respectively (Wester et al., 1993).
6.2 Metabolism
The metabolic fate of diazinon was studied with different modes
of administration using unlabelled and radiolabelled diazinon in
various species including rat, mouse, guinea-pig, dog, sheep, goat,
cow and chicken. Additional in vitro experiments were conducted
using tissue slices or cell fractions. A comparative summary of the
results available was provided by Hagenbuch & Mücke (1985). In all
species tested, diazinon was rapidly and almost completely absorbed
from the gastrointestinal tract. It was also absorbed from the skin.
The main metabolic pathways of degradation of diazinon are:
a) Cleavage of the ester bond of diazinon or diazinoxon leading to
the hydroxypyrimidine derivatives.
b) Transformation of P-S moiety to the P-O derivative, leading to
the active metabolite, diazoxon.
c) Oxidation of isopropyl substituent leading to the corresponding
tertiary and primary alcohol derivatives.
d) Oxidation of the methyl substituent leading to the corresponding
alcohol.
e) Glutathione-mediated cleavage of the ester bond leading to a
glutathione conjugate.
The hydrolytic and oxidative cleavage of the phosphorus ester
bond, leading directly or via diazoxon to the pyrimidinyl derivative,
play the most prominent role in the metabolism of diazinon.
Glutathione conjugation appears to be of small importance. Metabolites
maintaining the phosphorus ester bond are of transient nature and are
only observed in minor quantities.
The general metabolic pathways of diazinon in mammals are given
in Fig. 1.
Chemical formula: C12H21N2O3PS
Relative molecular mass: 304.35
IUPAC Chemical names: O,O-diethyl O-2-isopropyl-6-methyl-
pyrimidin-4-yl phosphorothioate
CAS chemical name: O,O-diethyl O-[6-methyl-2-
(1-methylethyl)-4-pirimidinyl]
phosphorothioate
CAS registry number: 333-41-5
RTECS number: TF3325000
Official number: OMS 469; ENT 19 507
Synonyms: dimpylate, diazide, G.24480, Basudin,
Kayazinon, Necidol/Nucidol
2.1.2 Technical product
Trade names: Diazinon (Alpha, Darlingtons Mushroom
Laboratories, Murphy Chemicals and
Rentokil); Basudin (Ciba-Geigy);
Crompest (Cromessel Co. Ltd); Dethlac
(Gerhardt Pharmaceuticals); Isectalac
(Sorex Ltd); Murphy Root Guard (Fisons);
Rentokil Flytrol and Knox out 2FM
(Rentokil); Secto AntSpray and Root
Powder (Secto Ltd); Dazzel, Diagran,
Dianon (Nippon Kayaku); Diazotol
Gardentox, Nipsan (Nippon Kayaku);
Dyzol, Dizion (Nippon Kayaku);
Spectracide (Ciba-Geigy)
2.2 Physical and chemical properties
Diazinon is a clear colourless liquid (technical 95% yellow oil)
with a faint ester-like odour.
Boiling point: 83-84°C at 26.6 mPa; 125°C at 133 mPa
Vapour pressure: 9.7 mPa at 20°C
Density: 1.11 g/cm3 at 20°C
Refractive index: 1.4978-1.4981
Specific gravity: 1.116-1.118 at 20°C
Stability: susceptible to oxidation above 100°C;
stable in neutral media, but slowly
hydrolysed in alkaline media, and more
rapidly in acidic media
Decomposes: above 120°C
Corrosiveness: non-corrosive
Solubility: 60 mg/litre in water at 20°C;
completely miscible with common organic
solvents, e.g., ethers, acetone,
alcohols, benzene, toluene, cyclohexane,
hexane, dichloromethane, petroleum oils
2.3 Analytical methods
Formulated diazinon products are cleaned up by column
chromatography to remove the basic impurities and analysed by
titration with perchloric acid in acetic acid. They are also analysed
by gas-liquid chromatography (Eberle et al., 1974; Allender & Britt,
1994).
Residues in soil, water, air, plants, foods, and animal and human
tissues can be determined using gas chromatography using detectors
selective for phosphorus-containing compounds, and by other
chromatographic techniques. Table 1 outlines various methods for
determination of diazinon in different media.
Farran et al. (1988a) described a method for the determination of
organophosphorus insecticides and their hydrolysis products. The
method involves the analysis of compounds by liquid chromatography in
combination with UV and thermospray-mass spectrometric detection.
An automated identification method has been developed for water-
borne toxicants, including diazinon, using an ion chromatography/
high-performance liquid chromatography system (Fort et al., 1995).
A compendium of analytical methods for organophosphorus compounds
has been issued (NIOSH, 1994).
Table 1. Analytical methods for diazinon
Medium Analytical method References
Air adsorption on XAD-2 resin, gas NIOSH (1994)
chromatography with flame
photometric detector
Soil gas chromatography Singmaster & Acin-
Diaz (1991)
Water extraction with XAD-2 resin, gas Le Bel et al. (1979)
chromatography with nitrogen-
phosphorus detector, gas
chromatography/mass spectrometry
continuous-flow extraction coupled Farran et al. (1988b)
on-line with high-performance liquid
chromatography
liquid-solid extraction, gas Johnson et al. (1991)
chromatography/mass spectrometry
on-line solid-phase extraction, Lacorte & Barcelo
liquid chromatography/thermal spray - (1995)
mass spectrometry
on-line solid-phase extraction, Lacorte & Barcelo
liquid chromatography/atmospheric (1996)
pressure chemical ionization mass
spectrometry
maleic anhydride immunoassay Winnett (1992)
Oil solution gas chromatography Koibuchi et al.
(1975)
Fruit and solvent extraction, gas Ferreira & Silva
vegetables chromatography with thermionic Fernandes (1980)
detector
Apples solvent extraction, gas Asensio et al. (1991)
chromatography with thermionic
detector
Table 1. (con't)
Medium Analytical method References
Oranges matrix solid-phase dispersion Torres et al. (1996)
extraction, gas chromatography with
electron capture detector
Rice solvent extraction, gas Adachi et al. (1984)
chromatography with flame ionization
detector
Spinach preparative thin-layer Gilmore & Cortes
chromatography, autoradiography, (1996)
liquid scintillation counting
solvent extraction, gas Cairns et al. (1985)
chromatography with electrolytic
conductivity detector, gas
chromatography/chemical ionization
mass spectrometry
Milk gas chromatography Toyoda et al. (1990)
Human tissue solvent extraction, thin-layer Kirkbride (1987)
chromatography, gas chromatography
with nitrogen-phosphorus detector
Blood plasma gas chromatography Machin et al. (1975)
solvent extraction, gas Wu et al. (1994)
chromatography with electron capture
detector
Metabolites in urine
DEP, DEPT extraction by anion exchange resin, Lores & Bradway
gas chromatography with flame (1977)
photometric detector Weisskcp & Seiber
(1989)
GW7 550, solvent extraction, gas Lawrence & Iverson
GS 31 144 chromatography with electrolytic (1975)
conductivity detector
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Diazinon does not occur as a natural product.
3.2 Man-made sources
3.2.1 Production levels and processes
3.2.1.1 Manufacturing process
Diazinon is the common name for O, O-diethyl
O-2-isopropyl-6-methylpyrimidin-4-yl phosphorothioate (IUPAC name),
an organophosphate insecticide. Its insecticidal properties were first
described by Gasser (1953) and it was introduced in 1952, by
J. R. Geigy S.A. under the code number G 24480, trade names Basudin,
Diazitol, Neocidol and Nucidol, and the protection of BP 713278; USP
2754243. Meanwhile, improvements in the manufacturing process and the
stabilization of the technical grade diazinon by epoxidized soybean
oil have significantly reduced the content and formation of toxic
by-products and breakdown products and have reduced the acute toxicity
of diazinon products.
3.2.2 Uses
Diazinon is a contact organophosphorus insecticide with a wide
range of insecticidal activity, having long persistence and relatively
low mammalian toxicity. Diazinon is effective against adult and
juvenile forms of insects, but also against acarina. The spectrum of
activity includes the following arthropod groups:
* flying insects: flies and fly maggots, mosquitoes
* crawling insects: cockroaches, bedbugs, lice and ants
* acarina: dog ticks
* arachnideae: spiders
The main applications are rice, fruit, vineyards, sugar-cane,
corn, tobacco, potatoes, horticultural crops, animal dips and sprays.
Diazinon is also used by trained pest control operators in
households and outbuildings to control cockroaches, ants, silverfish,
spiders, carpet beetles and scorpions and in insecticidal collars on
domestic pets.
3.2.3 Formulations
The most important diazinon formulations are: ULV concentrates,
wettable powders 400 g/kg; emulsifiable concentrates 600, 400 and
250 g/litre; dust 20-40 g/kg; granules 30-140 g/kg; aerosols
200 g/litre.
Some typical formulations for agricultural and horticultural use
include: Basudin 5 (50 g a.i./kg); Basudin 10 (100 g a.i./kg) GR;
Basudin 40WP (400 g a.i./kg); Basudin 50SD (500 g a.i./kg); Basudin
60EC (EC 600 g a.i./litre); Diazitol Liquid; Basudin Ulvair 500;
Basudin 20 Mushroom Aerosol, KN; Knox-out (Pennwalt), flowable
microcapsules (230 g a.i./litre); Neocidol 60, Nucidol 60,
EC (600 g a.i./litre) for veterinary use.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Volatilization
It has been shown that diazinon is lost from soil through
volatilization (Harris & Mazurek, 1966), but the rate of loss is
unknown. Results of earlier studies with 14C-labelled insecticide and
the use of capped containers for holding treated soils indicated that
volatility was of minor importance. Under field conditions,
co-distillation, high temperatures and exposed surface areas probably
contribute to a greater loss of the insecticide through
volatilization.
Rate estimations according to the Atkinson incremental method
indicate that diazinon is rapidly degraded by hydroxyl radicals in the
atmosphere. The tropospheric half-life of diazinon lies between 1.3
and 1.5 h (Stamm, 1994).
4.1.2 Movement in soil
A study concerning degradation rate and mobility of diazinon in a
thatch layer of turf grass and in the underlying soil (2.5 cm) was
performed by Sears & Chapman (1979). Immediately following the
pesticide application, 2200 litres of water were applied to the total
treated area of 80 m2. Fourteen days after the application, less than
2% of the compound remained in the grass-thatch layer, and less than
1% in the root zone and in the underlying soil. The authors concluded
that the compound readily disappeared by degradation and/or
volatilization. However, it must be considered that only the top
2.5-cm layer was analysed.
The movement of diazinon and other organophosphorus compounds in
the soil was evaluated by means of soil thin-layer chromatography
(Sharma et al., 1986). The experiment was performed with two types of
soil (silt loam and sandy loam) showing different percentages of
organic matter (1.05 and 0.35%, respectively). The authors found a
generally poorer movement of diazinon in the silt loam soil, probably
due to the higher organic matter content and higher cation exchange
capacity. When natural soils were used as adsorbent and distilled
water as eluent, diazinon showed relatively high mobility. In this
study, the effects of pH and the presence of leachates of alkaline and
saline salts were also evaluated. Diazinon showed a slight decrease of
mobility in both soils at pH 4, whereas at pH 10 there was increase
mobility in the silt loam and slight decrease in mobility in the sandy
loam. The effects of leachate salt were not significant, with the
exception of calcium sulfate, which decreased mobility in the silt
loam soil.
The adsorption and mobility of diazinon in 25 Spanish soils and
the influence of soil properties on both processes were studied
(Arienzo et al., 1994). Adsorption constants of diazinon in the soils
were measured using soil thin-layer chromatography and soil column
leaching. The experiments were conducted with 14C-labelled diazinon.
Adsorption of diazinon was found to follow the Freundlich adsorption
equation. The Freundlich adsorption constant (K) ranged from 0.70 to
25.73. Adsorption was highly significantly correlated (p <0.001) with
the content of organic matter (OM). The median KOM value was 290
corresponding to a KOC value of 500. There was also a significant
correlation (p <0.01) of K and the distribution coefficient Kd with
the silt-plus-clay content in soils with low organic material content
(<2%). On the basis of the soil thin-layer chromatography
experiments, diazinon was found to be slightly mobile in 80% and
immobile in 20% of the soils studied. In the soil column experiment,
the pesticide was quite mobile under saturated flow in soils of light
texture containing little organic matter. Under non-saturated flow
conditions, which are more similar to natural conditions, diazinon
should not be easily leached from the studied soils to groundwater.
4.2 Degradation
4.2.1 Degradation in soil
Seyfried (1994) studied the degradation of diazinon in an
agricultural soil (silt loam, USDA) under various experimental
conditions. At 20°C and a soil moisture of 60% of the field capacity,
the DT50 was 5 days and DT90 22 days. The main metabolite,
2-isopropyl-4-methyl-6-hydroxy pyrimidine, occurred transiently and
degraded with a DT50 of 20 days. Mineralization accounted for 86% of
the applied diazinon within the experimental period of 120 days.
Whereas the application rate did not influence the degradation rate,
there was a dependence on temperature (DT50 of 12 days at 10°C) and
soil moisture (DT50 of 8 days at 30% field capacity). Under sterile
conditions, the DT50 was increased to 118 days at 20°C and 60% field
capacity. This suggests that the main route of soil degradation is
microbial.
Getzin (1968) studied persistence of diazinon in soils and
measured loss in autoclaved and non-autoclaved soil at several
temperatures, moisture contents and pH levels under controlled
laboratory conditions. Microorganisms and non-biological factors
affected the persistence of diazinon in Sultan silt loam. Diazinon was
primarily degraded through non-biological pathways. Although diazinon
was not metabolized to any great extent by microorganisms in Sultan
silt loam, it is known that soil microflora are capable of degrading
the insecticide. Gunner et al. (1966) isolated a bacterium from soil
that utilized diazinon as a source of sulfur, phosphorus, carbon and
nitrogen, but the importance of this microorganism as a contributor to
the metabolism of the insecticide in soil was not determined.
Miles et al. (1978) demonstrated that diazinon can accumulate and
persist in organic soils for more than a year. It was also shown that
diazinon can move from its soil-bound form into the aqueous
environment either via leaching or by direct soil erosion (Miles &
Harris, 1978a). Morganian & Wall (1972) demonstrated that diazinon
treatment of a marine salt marsh led to a build-up of diazinon in salt
marsh sod and mud.
At pH 6.8, the time required for 50% loss of diazinon is 6 weeks
in autoclaved soil and 18 weeks in buffered water. Mortland & Raman
(1967) demonstrated the catalytic hydrolysis of diazinon in CuCl2
solutions and Cu-montmorillonite suspensions. Catalytic reactions of
this nature may occur in soil, but attempts to demonstrate this
phenomenon in Sultan silt loam have so far failed. Moisture variations
from 50 to 100% of the moisture equivalent did not appreciably alter
the degradation rates of diazinon. Variations in soil temperature
between 10 and 30°C resulted in a 4- to 10-fold difference in the time
required for 50% loss of the insecticides in soil. The non-biological
degradation of diazinon increased with increased acidity.
Schoen & Winterlin (1987) have studied the factors affecting the
rate of diazinon degradation in soil. These are pH, soil type, organic
amendments, soil moisture and pesticide concentration. Of the soil
factors investigated, the conditions for diazinon degradation in
pesticide mixtures were optimum when the pesticides were present at
low concentrations in moist soil, amended with peat and acidified to
pH 4. Degradation was least at high pesticide concentration in neutral
or alkaline mineral soil.
Utilization of diazinon by an Arthrobacter species and a
Streptomyces species has been shown to alter the microbial
population by stimulating a selective enrichment of these species.
The Arthrobacter species previously reported to attack the side
chain of the molecule was unable to metabolize completely the ring
portion of the molecule. Similar results demonstrated that the
Streptomyces species, too, could not by itself convert pyrimidinyl
carbon to carbon dioxide. When, however, both the Arthrobacter and
Streptomyces organisms were incubated together, 15-20% of the 14C
appeared as labelled BaCO3 after 18 h, suggesting a synergistic
relationship between these two organisms in attacking the pyrimidinyl
portion of diazinon (Gunner & Zuckerman, 1968).
Barik & Munnecke (1982) demonstrated that a bacterial enzyme can
hydrolyse diazinon in soil. In their research, an enzyme was obtained
from a Pseudomonas sp. that could hydrolyse diazinon and several
other methoxy- or ethoxy-substituted organophosphates. In this
experiment, diazinon, either in 25% EC formulations or as a technical
grade chemical, was enzymatically hydrolysed in an agricultural sandy
soil when present at concentrations up to 1%. The degradation rate was
approximately proportional to enzyme concentration up to 12 units per
20 g soil. This indicates that the initial rate of diazinon
degradation is directly dependent on enzyme activities, and not on
chemical or physical parameters of the soil-pesticide interactions.
Although the enzyme was examined only in one soil, it is expected that
it could also operate on cement or asphalt type surfaces, as well as
on synthetic polymers such as carpet.
Al-Attar & Knowles (1982) studied the uptake, metabolism and
elimination of diazinon in Panagrellus redivivus, a free-living soil
nematode, and Bursaphelenchus xylophilus, a plant parasitic
nematode. Nematodes were exposed to a solution of diazinon labelled
with radiocarbon. Both nematode species metabolized diazinon, although
P. redivivus was more active. Metabolites from B. xylophilus
included O, O-diethyl O-(2-isopropyl-4-methyl-6-pyrimidinyl)
phosphate or diazoxon and pyrimidinol. Radioactivity accumulated to a
greater extent in B. xylophilus than in P. redivivus. Elimination
of radiocarbon was more rapid with P. redivivus than with
B. xylophilus, and this resulted in the presence of high levels
of the polar pyrimidinol metabolite in the incubation medium of
P. redivivus.
4.2.2 Degradation in water
Keller (1983) investigated the degradation of diazinon in samples
of pond and river water, each containing 1% of sediment. Diazinon was
degraded with a DT50 of 7 to 10 days in the pond system and 8 to 15
days in the river water. Mineralization accounted for >60% of the
applied material within 7 weeks in both systems.
In a mesocosm study conducted with 17 treated and 4 untreated
ponds (0.05 hectare each), diazinon degraded rapidly. The
disappearance half-lives averaged 5.2 to 12.2 days (Giddings, 1992).
Kanazawa (1975) found diazinon to be fairly persistent in tap
water in a glass aquarium, degrading to 27% in 30 days.
Ferrando et al. (1992) studied the persistence of diazinon in
natural water from Albufera Lake and in experimental water from their
laboratory. Degradation was faster in lake water, the half-lives being
70 and 79 h for lake and laboratory water, respectively. The
degradation process in both media was comparable until 96 h. The
authors found 43.5 and 49.4% of the applied diazinon in natural and
experimental water, respectively, at 96 h.
4.2.3 Bioconcentration
4.2.3.1 Fish and aquatic invertebrates
The bioconcentration factors (BCF) of diazinon over a 7-day
period were as follows: topmouth gudgeon 152; carp 65; guppy 18;
crayfish 4.9; red snail 17; pond snail 5.9 (Kanazawa, 1978).
Seguchi & Asaka (1981) reported the intake and excretion of
diazinon and its metabolites in freshwater fish, and the relationship
between the BCF of diazinon and fat content of fish. During exposure
to continuous-flow water containing 0.02 mg diazinon/litre the
concentration of diazinon in fish rapidly increased, reaching a
maximum after 3 days. Thereafter, the diazinon concentration slightly
decreased and remained at equilibrium. The BCFs for carp, rainbow
trout, leech and shrimp at equilibrium were 120, 63, 26 and 3,
respectively. As for the metabolites, pyrimidine analogue was found in
all fish species, but diazinon and related compounds were found only
in carp and rainbow trout. The concentration of the metabolites
reached a maximum after 3-7 days exposure to diazinon. Diazinon was
metabolized to diazoxon in the channel catfish liver microsomal enzyme
system, but it was not found in any other fish species. When the fish
were transferred to clean water, diazinon and its metabolites were
rapidly lost from the fish. Seven days after being transferred to
clean water, the diazinon concentration decreased to 0.3-8.0% of the
equilibrium concentration, and the metabolites decreased below the
detection limit.
Similar results have been observed for topmouth gudgeon by
Kanazawa (1975, 1978). A linear relationship was observed between the
bioconcentration ratio and fat content in fish. Seguchi & Asaka (1981)
identified six metabolites of diazinon, and Fujii & Asaka (1982)
identified another three: hydroxydiazinon, hydroxymethyl diazinon and
isopropenyl diazoxon.
The toxicity, accumulation and elimination of diazinon were
investigated in the European eel (Anguilla anguilla). Fish exposed
to sublethal concentration (0.042 mg/litre) accumulated diazinon in
the liver and muscle tissues. The BCFs for diazinon were 1859 in liver
and 775 in muscle over the 96-h exposure period. When removed from
diazinon-containing water, the contaminated fish rapidly eliminated
diazinon. The excretion rate constants were 0.108 per h for liver and
0.016 per h for muscle. Diazinon half-lives were 16.6 and 33.2 h for
liver and muscle, respectively (Sancho et al., 1992).
The freshwater fish Motsugo (Pseudorasbora parva) was reared in
an aquarium tank containing about 1 mg diazinon/litre for 30 days. The
persistence of the insecticide in water and the uptake and excretion
of the insecticide by fish were monitored. Diazinon degraded by 72% in
30 days. The concentration of diazinon in fish reached a maximum level
of 211 mg/kg after 3 days. Afterwards, the concentration of the
insecticide decreased gradually due to metabolism and excretion
(Kanazawa, 1975).
Bioconcentration and excretion of diazinon were studied in the
carp ( Cyprinus carpio L.). The average BCF values for diazinon were
20.9 in muscle, 60.0 in liver, 111.1 in kidney and 32.2 in gall
bladder over a 168-h exposure period. The excretion rate constants of
diazinon (ng/g per h) were 0.002-0.024 for muscle, 0.001-0.020 for
liver, 0.0004-0.004 for kidney and 0.002-0.023 for gall bladder,
respectively (Tsuda et al., 1990).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
The amount of insecticide present in the air of commercial pest
control buildings, service vehicles and food preparation-serving areas
following routine commercial insecticide application has been
measured. Diazinon was measured in the ambient air of storage and
office rooms in six North Carolina (USA) firms in a 4-h period. In the
storage rooms the mean value was 284 (85-837) ng/m3 air and in the
offices 163 (31-572) ng/m3 air. Diazinon was also detected in the
ambient air of vehicles used in commercial pest control activities.
Mean diazinon concentrations (ng/m3 air) in 2-h of sampling from six
vehicles were 88 (7-239) in sedans and 171 (11-543) in vans, the mean
value being 130 (7-543). The highest level of diazinon detected in the
ambient air of offices of pest control building was far below the
allowable limits (TLV : 100 µg/m3) (Wright & Leidy, 1980).
Wright et al. (1982) studied the amount of diazinon in cabs of
stationary pick-up trucks used by the pest control service. Additional
air samples, taken while the same pick-up truck was moving, provided
data for comparison of insecticide levels in individual pick-up trucks
when moving and stationary. Diazinon was present in significantly
greater concentrations than chlorpyrifos. This may be attributable to
the facts that the service technicians kept diazinon in sprayers
during the sampling periods and that they used it in servicing
accounts during the sampling day. It could therefore have contaminated
their clothing and skin and passed into the air when they were in the
pick-ups. The maximum diazinon detected was 5.15 µg/m3 for a 2-h
period or 20.6 µg/m3 for an 8-h period, which is about 1/5 of the
allowable limit. However, the amount of airborne diazinon to which a
technician was actually exposed during a working day was even less
than 20.6 µg/m3, since the maximum time any technician spent in a
pick-up was 3.8 h.
Wachs et al. (1983) reported the concentration of diazinon in
the air of a retail garden store that sold the insecticide. The
concentration found in the air based on the 14-h period pumping
through the polyurethane filters was 3.4 µg/m3. All of the diazinon
was found in the polyurethane plug closest to the air inlet. Diazinon
was not found in the second plug or unused plugs which were similarly
Soxhlet-extracted and analysed. It was concluded that the
concentration of diazinon in air depended on a number of factors,
including the type of formulation, air temperature, type and condition
of containers, prior spills and types of floor covering. The
concentration of diazinon vapour found in this study would not appear
to constitute a hazard to store personnel or customers.
Airborne concentrations of diazinon were measured in rooms for
21 days after crack and crevice application. Residue levels were
greatest in treated rooms (38 µg/m3) followed by adjacent (1 µg/m3)
and upper and lower floor rooms (about 0.4 µg/m3). Low levels of
diazinon were detected in all rooms 21 days after application. Small
amounts of diazinon (corrected to an 8-min application period) were
detected on respirator pads (2.6 µg) and waist pads (2.3 µg) worn by
the applicator (Leidy et al., 1982).
Airborne and surface concentrations of diazinon were measured at
intervals up to 10 days after broadcast spray application onto the
floors of seven offices. Diazinon concentrations peaked 4 h after
application at 163 and 27 µg/m3 of air sampled, respectively.
Airborne concentrations of diazinon indicated that building occupants
should not enter unventilated rooms for at least 2 days after
spraying. Residues on aluminium plates and furniture were examined at
intervals of up to 48 h after spraying, and in many cases the surface
concentrations were higher at 24 or 48 h after spraying than at
one hour. The peak residue concentration of diazinon was 38 ng/cm2 of
surface area sampled at 48 h (Currie et al., 1990).
5.1.2 Water
Insecticide residues on suspended and bottom sediments of streams
of Ontario, Canada, have been studied in a tobacco-growing, vegetable
muck area. Bed load samples contained three to six times higher
concentrations of insecticides than bottom material (Miles, 1976).
From 1985 to 1987, a monitoring survey was conducted to determine
the levels of selected pesticides in farm ditches located in the lower
mainland of British Columbia, Canada. Diazinon was not detected in
ditch water (detection limit = 1 µg/litre). In ditch sediments,
diazinon was sporadically found at concentrations up to 4 µg/kg
(detection limit = 1 µg/kg) (Wan, 1989).
During the first half of 1984, diazinon was not detected in raw
or treated water samples from the Lakeview and Lorne Park Water
Treatment Plants in Toronto, Ontario (detection limit = 10 ng/litre)
(MacLaren Plansearch Inc. & FDC Consultants Inc., 1985).
Detectable concentrations of diazinon occurred in less than 0.1%
of water samples collected from 11 Southern Ontario agricultural
watersheds during 1975-1977. The concentration was mainly below
0.01 µg/litre, the maximum value being 0.15 µg/litre (Frank et al.,
1982).
Sampling performed in 1992 by the United Kingdom National Rivers
Authority showed diazinon at >0.1 µg/litre in 74 out of 2300 fresh
water samples and at > 0.15 µg/litre in 1 out of 12 seawater samples.
5.1.3 Soil
In 1971 hay and soil samples were collected in 9 states in the
USA to determine the incidence and levels of pesticide residues in
hayfields. Residues were detected in 8% of the soil samples and 29% of
the hay samples. Diazinon was detected in four hay samples (Gowen et
al., 1976).
In 1976, soil samples from 28 farms located in six vegetable
growing areas of southwestern Ontario, Canada, contained diazinon
residues from trace amounts (< 0.02 mg/kg) to 0.29 mg/kg (Miles &
Harris, 1978b).
5.1.4 Fruit, vegetables and food
Results of supervised trials and monitoring of diazinon residues
in or on food and feed commodities have been comprehensively reviewed
and summarized (FAO/WHO, 1994a). The following examples indicate that
diazinon residues are generally low.
Ward et al. (1972) performed a study to determine the rate of
decline of diazinon residue on wheat in Texas, USA. There was a steady
decline in the amount of diazinon remaining on foliage samples after
application. Only 0.16 mg/kg and 0.31 mg/kg remained 28 days after
treatment with 0.28 and 0.56 kg a.i./ha, respectively. Harvest samples
showed that less than 0.05 mg/kg remained in either the foliage or
grain.
Between 1978 and 1986, 305 samples of apples were analysed for
residues of a wide range of pesticides used in their production.
Residues of diazinon were found occasionally. They were well below the
maximum residue limit and correlated well with the pattern of use
(Frank et al., 1989).
Between 1986 and 1988, 433 composite vegetable samples
representing 16 commodities, which were treated by various pesticides
including diazinon, were collected from farm deliveries to the
marketplace in Ontario, Canada. All samples were analysed for
insecticides and fungicides. The commodities tested included
asparagus, beans, carrots, celery, cucumbers, lettuce, onions,
peppers, potatoes, radishes, rutabagas and tomatoes. In 64% of
samples, no pesticide residues were identified (the limits of
detection ranged from 0.005 to 0.05 mg/kg). A further 22% had combined
insecticide and fungicide residues below 0.1 mg/kg. Only three samples
(0.7%) had residues that exceeded the Maximum Residue Limit (MRL).
These involved diazinon on celery. While some commodities had no
detectable residues, others had measurable residues of up to three
different pesticides. The highest levels were found on celery, lettuce
and field tomatoes (Frank et al., 1990).
Levels of diazinon permitted in the USA on human food range from
0.1 mg/kg in potatoes to 0.7 mg/kg in most leafy vegetables. During
the course of pesticide surveillance of vegetables, an unknown
analytical response in spinach extract was seen, which was
subsequently identified as diazinon metabolite (2-isopropyl-4-
methyl-pyrimidin-6-ol). Cairns et al. (1985) described an analytical
procedure adapted to confirm both diazinon and its metabolite in
spinach, at very low levels, by methane chemical ionization mass
spectrometry. The presence of this metabolite at the 1 mg/kg level
represents an order of magnitude greater than that found for diazinon
itself.
In a study of diazinon residues in prepared foods, accidentally
exposed during and following treatment, the amounts of diazinon
residues in food left in the room for 30 min after treatment ranged
from 0.02 to 0.05 mg/kg. No detectable residues of diazinon were found
in the potatoes or dinners placed in the rooms 4.5 h after treatment
and removed after 5 h. A person consuming a dinner at the highest
residue found would have ingested 0.0153 mg of diazinon. For a person
weighting 70 kg this would amount to 0.218 µg/kg (Jackson & Wright,
1975).
5.1.5 Milk
Insecticides in polyvinyl chloride pellets were included in a
commercial dairy protein supplement and fed to dairy cows at 1.4, 2.0
and 2.5 mg of diazinon/kg body mass for 2 weeks. No insecticidal
residues were found in milk samples collected at 1, 3, 7, 10 or 14
days. Even 2.5 mg/kg dosage would provide a 5-fold margin of safety
for PVC formulation-diazinon fed to cattle to control face fly larvae
in manure (Lloyd & Matthysse, 1971), and diazinon-PVC was found to
be still a highly effective larvicide if given at the dose of
0.5 mg insecticide/kg per day (Lloyd & Matthysse, 1966, 1970).
Derbyshire & Murphy (1962) reported no diazinon residues in milk
from cows fed 10 mg/kg body weight for 7 days. Robbins et al. (1957)
found only traces of radioactivity in a cow's milk 6-24 h after a
single oral dose of 32P-labelled diazinon (20 mg/kg).
5.1.6 Meat and fat
Tissue residues were determined and toxicity symptoms were noted
after lambs were sprinkled and dipped with 0.06% diazinon emulsion or
sprinkled with 1% diazinon emulsion. The only diazinon residues found
were 1.45-2.30 mg/kg in fat, 1 day after dipping in 0.06% diazinon,
with concurrent 44-47% plasma cholinesterase activity depression. Low
residues were present in blood from these sheep. Most tissues
contained no detectable diazinon at 15 or 26 days after lambs were
dipped in 0.06% diazinon, but fat contained up to 0.52 mg/kg at 15
days and 0.31 mg/kg at 26 days. Sprinkling with 1% diazinon produced
no residues in most tissues. A maximum of 23 mg/kg was found in fat.
The only clinical poisoning involved a 3-day-old lamb dipped in 0.12%
diazinon suspension. Lambs more than 1 week old were not poisoned by
0.06% diazinon nor were lambs more than 1 month old when treated by
0.25% diazinon (Matthysse et al., 1968).
Harrison et al. (1962) found 0.4 mg diazinon/kg in meat of
unshorn sheep 1 day after dipping in 0.05% diazinon emulsion. This
decreased to 0.25 mg/kg and 0.16 mg/kg at 4 and 7 days after dipping,
respectively, and there were negligible amounts 25 days after dipping.
Claborn et al. (1963) found 0.69 mg/kg in beef fat 1 day after
the last of 11 weekly spraying with 0.05% diazinon suspension. The
authors reported a rapid loss of diazinon from beef fat and the amount
of residues were negligible 14 days after spraying.
Samples obtained from retail outlets in the United Kingdom during
1984-1986 generally showed zero or low levels of diazinon residues.
Diazinon was not detected in samples of beef, imported lamb, pork or
veal, but low levels were found in United Kingdom lamb in 1984/1985
(up to 1.7 mg/kg) and 1985/1986 (up to 0.1 mg/kg). Samples of fat
taken in 1986 were analysed and, out of 274, 19% contained diazinon.
In 1987, however, out of 280 samples analysed, 7% contained diazinon
and in four of them residues exceeded the Codex MRL of 0.7 mg/kg fat.
Diazinon was not detected in butter, milk or cheese (MAFF, 1989).
Various pesticides and pollutants were examined in poultry meat
from Israel. The levels of these, which included diazinon in broilers,
turkeys and geese, were said to be extremely low and below the USA
tolerance levels (Kathein, 1986)
5.2 General population exposure
The primary exposure to the general population will be through
intermittent dietary exposure and inhalation exposure. Exposure via
water is negligible. Total-diet studies commenced in the United
Kingdom in 1966. In the second survey (1970-1971) and in the latest
survey (1985-1988), diazinon residues were not detected (Egan &
Weston, 1977; MAFF, 1982, 1986, 1989). Findings similar to those in
the United Kingdom were also made in the USA. Toddler total diets have
also been the subject of investigation in the USA. Diets collected in
ten American cities between 1978 and 1979 were examined. The
components were drinking-water, whole milk, other dairy products and
dairy substitutes, meat/fish/poultry, grain cereals, potatoes,
vegetables, fruit juices, oils and fats, sugars and beverages
(Gartrell et al., 1985a). A similar exercise in the years 1980-1982
was conducted in 13 American cities. The results were similar to those
obtained in 1978-1979, with intake of diazinon being low (Gartrell et
al., 1985a,b).
A total-diet study in New Zealand was performed at 3-monthly
intervals in the period 1974-1975. Of 116 samples analysed, 82 (71%)
had no detectable residues of diazinon. Intakes were well below the
Codex MRLs (Dick et al., 1978).
The overwhelming evidence from residue and total-diet studies
suggests that residues of diazinon are generally within the acceptable
levels set by the Codex Alimentarius Commission. The results suggest
that the compounds are rapidly broken down, whether on plants or in
animals, further reducing the risks to humans (IPCS, 1986).
During a 5-year study period (1981-1986), the US Food and Drug
Administration analysed nearly 20 000 domestic and imported samples of
food and feed commodities for pesticide residues. The results showed
that 29 out of 6391 domestic agricultural commodities and 35 out of
12 044 imported agricultural commodities had diazinon levels greater
than 0.05 mg/kg (Hundley et al., 1988).
Diethyl phosphate (DEP), an organophosphate metabolite, was found
in the urine of symptomatic residents who resided in a household that
had been sprayed with diazinon 4.5 months earlier. Pre- and
post-decontamination data with regard to symptoms and to DEP,
cholinesterase, and surface and air levels underscore the utility of
alkyl phosphate metabolites for monitoring exposure. The data also
emphasize the efficacy of clean-up measures when baseline data are not
available to determine if "within-normal" cholinesterase levels are,
in fact, depressed (Richter et al., 1992).
5.3 Occupational exposure
An occupational exposure study was conducted for a firm employing
22 pest control operators (PCOs) exposed to three organophosphorus
insecticides including diazinon. The 8-h exposure levels were less
than 131.0 µg/m3. Urine samples (24-h) were analysed for alkyl
phosphates and showed the presence of metabolites for these three
insecticides. The effect of this exposure was reflected by a
statistically significant inhibition of plasma cholinesterase activity
among the PCOs, but physical examinations detected no apparent toxic
effects (Hayes et al., 1980).
A behavioural evaluation of pest control workers with short-term
(mean 39 days) low-level exposure to diazinon was conducted in 1985
during the course of a pest control program in California (see section
8.2.2). The diazinon metabolite diethylthiophosphate (DETP) was
measured in pre- and post-shift urine samples and the full-shift
exposure to diazinon was quantified for 19 subjects using personal
air monitoring and passive badges. The median diazinon exposure was
2.1 mg/day (Maizlish et al., 1987).
An investigation was conducted to determine worker exposure to
airborne pesticides during tree and ornamental shrub applications
using hand-held equipment during an entire work shift. Employee
exposure data were collected for 3 consecutive years. The sampling was
performed during the late spring, summer and early autumn when insect
and disease activity was most prevalent. Sampling was conducted at 23
locations. Those applying these chemicals sustained low-level exposure
to acephate, benomyl, carbaryl, chlorothalonil, diazinon and dicofol.
As pesticide label instructions for mixing and applying pesticides
were strictly followed, the tree and ornamental shrub applicators were
able to keep inhalation exposures below the levels recommended by OSHA
and NIOSH. Of the 74 exposures monitored, 67% were below the detection
limit (0.001 mg/m3), while others were 0.001-0.040 mg/m3. This
observation supports the correctness of not including specific
respiratory protection measures on pesticide label directions for
mixing, loading and applying these pesticides (Leonard & Yeary 1990).
Dermal, respiratory and urine measurements were made on workers
applying granular diazinon pesticide formulation. In all, 15 workers
and four control subjects were monitored. The workers applied the
compound in yards and small pastures using hand equipment comparable
to that used in a home environment. Respiratory air samples, ethanol
hand rinse samples, clothing patch samples and urine samples were
collected. The diazinon exposures were correlated with job category,
application duration, application equipment and protective clothing.
The best determinants of diazinon exposure were the job categories and
the use of the belly grinder type of spreader. The rank of exposure
magnitude, from highest to lowest, was the crew using the belly
grinder, the crew not using the belly grinder, the crew chief and the
supervisor. The mean daily dermal and respiratory diazinon exposures
for these four job categories ranged from 0.6 to 11 mg, 0.1 to 1.8 mg,
0.1 to 0.25 mg, and 0.03 to 0.07 mg, respectively. The amount of
urinary diethylthiophosphate increased during the day for all job
levels, but showed variable recovery (Weisskop et al., 1988).
6. KINETICS AND METABOLISM
6.1 Absorption, distribution and excretion
6.1.1 Oral administration
6.1.1.1 Rats
Four male and 2 female Wistar rats were treated with single
oral doses of 0.8 mg [pyrimidine-14C]-diazinon (specific activity
4.0 µCi/mg). An additional group of 4 males received [ethoxy-14C]-
diazinon (3.2 µCi/mg) at the same dose level. During the observation
period of 168 h, both labelled parts of the molecule were excreted
almost completely, 65.4-80.0% of the administered radioactivity being
detected in urine, 16.0-23.5% in the faeces and, with the ethyl-label,
5.6% in the expired air (total recovery 90.2-98.3%). No radioactive
CO2 was detected with the pyrimidine label. The half-life times of
excretion were 7 h with the ethyl label and 12 h for both sexes
treated with the pyrimidine-labelled material. Daily oral
administration of 0.1 mg [pyramidine-14C]-diazinon to male rats for
10 consecutive days resulted in no accumulation of the radioactivity
in any organ investigated (oesophagus, stomach, intestines, liver,
spleen, pancreas, kidneys, lungs, testes, muscles, fat). Six hours
after the last administration, the highest residues were detected in
the muscles (0.77% of the totally applied dose), caecum (0.76%) and
small intestine (0.65%). The residues were below the detectable limit
48 h after the cessation of the treatment (Mücke et al., 1970).
Sprague Dawley rats received [pyrimidine-14C]-diazinon at single
oral doses of 10 mg/kg (specific activity 30.3 µCi/mg) or 100 mg/kg
(specific activity 9.7 µCi/mg). A third group was treated with daily
oral doses of 10 mg/kg technical diazinon (87.7% pure) for 14
consecutive days, followed by a single treatment at the same dose
level with the 14C-labelled compound. The disposition of the
administered 14C was observed for a 7-day period before the animals
were killed and the tissues removed for analysis. The average recovery
of the radioactivity was 99.2%. Elimination of diazinon equivalents
was rapid. In the low-dose group, males and females eliminated 93 and
86%, respectively, of the administered radioactivity in the urine
within 24 h. Faecal elimination amounted to 1.6 and 1.1%,
respectively, in the same time period. In the high-dose group, the
respective values were slightly lower and indicated that the
elimination was more rapid in males (90.8% in urine and 2.2% in
faeces) than in females (58.2% in urine and 0.87% in faeces). The
pre-conditioning of the rats had no influence on absorption and
elimination. Seven days after the administration of the [pyrimidine
14C]-diazinon, the residual radio-activity was generally low. Among
the tissues examined (heart, lung, spleen, kidney, liver, fat, testes,
ovaries, uterus, muscle, brain, blood plasma, blood cells, bone), the
residual radioactivity amounted to approximately 0.01 mg/kg diazinon
equivalents in the low-dose group; only fat (0.02 mg/kg), blood cells
(0.05 mg/kg) and bone (<0.017 mg/kg) contained higher amounts of
radioactivity. In the high-dose group, the residual radioactivity was
8-10 times higher. Pretreatment with technical diazinon for 14 days
led to residues similar to those observed in the low-dose group
(Craine 1989a,b).
6.1.1.2 Guinea-pigs
Male guinea-pigs treated orally with 45 mg/kg [32P]-diazinon
(specific activity 117-197 cpm/mg) in peanut oil, the tissue
distribution was determined at 2, 4, 8 and 16 h after treatment and
the excretion of 32P was investigated over an 8-day period. Following
oral administration, the compound was rapidly absorbed as shown by a
sharp decrease of activity in the stomach and low levels found in the
small intestine. Within 16 h, 46.6% of the administered radioactivity
was eliminated in the urine and 0.34% appeared in the faeces. The
caecum showed a gradual increase of radioactivity, 13-36% of the
administered dose accumulating in the caecum over 16 h after the
administration. Irrespective of this accumulation, within 48 h after
dosing, 80% of the administered radioactivity was eliminated in the
urine while only 8% was eliminated in the faeces (Kaplanis et al.,
1962).
6.1.1.3 Dogs
Two female Beagle dogs were intravenously dosed with 0.2 mg/kg
[ethoxy-14C]-diazinon (specific activity 3.4 µCi/mg) in 0.7 ml
ethanol. Blood samples were drawn at times ranging from 5 min to 7 h
after the injection. The decline of the radioactivity in the blood
was biphasic with a slower second phase. The half-life of elimination
from blood for this second phase was calculated to be 363 min.
Approximately 58% of the administered radioactivity was recovered in
the urine within 24 h after the administration. Another two female
beagle dogs were orally dosed by capsule with 4.0 mg/kg [ethoxy-14C]
diazinon in ethanol. Approximately 85% of the administered
radioactivity was recovered within 24 h after oral administration,
with 53% of it occurring in urine (Iverson et al., 1975).
6.1.1.4 Goats
Two lactating goats were orally treated with [pyrimidine-14C]-
diazinon (specific activity 9.7 µCi/mg) in gelatin capsules for four
consecutive days at a dose level of 4.5 mg/kg per day, corresponding
to a dietary exposure of 100 mg/kg of feed. During the observation
period, in average 64.1% of the administered radioactivity was
excreted with urine, 10.4% with the faeces and 0.31% with the milk.
A plateau of radioactivity in the milk was reached after 3 days of
dosing at a mean level of 0.46 mg/kg diazinon equivalent. At
sacrifice, radioactivity in the blood accounted for 0.2% and the
tissues examined accumulated 0.92% of the administered dose. The
highest residual radioactivity was detected in the kidney (2.0 mg/kg)
and the liver (1.2 mg/kg). The other tissues examined contained
0.23-0.3 mg/kg diazinon equivalents (Simoneaux 1988a,b; Pickles &
Seim, 1988).
6.1.1.5 Cow
A lactating Hereford cow (body weight 268 kg) was orally treated
with a gelatin capsule containing 20 mg/kg 32P-diazinon (specific
activity 518 cpm/µg). Urine and faeces were collected during 36 h
after treatment and further samples were investigated until the study
was terminated after 168 h. In addition, milk and blood samples were
investigated. Within 36 h, approximately 74% of the administered
radioactivity was excreted with the urine, 6.5% appeared in the faeces
and 0.08% was found in the milk. A peak concentration of 2.27 mg/kg
diazinon equivalents was reached 18 h after the administration
(Robbins et al., 1957).
6.1.1.6 Hens
Four laying Leghorn hens were treated with 2-14C-diazinon
(specific activity 30.3 µCi/mg) in gelatine capsules for seven
consecutive days at daily doses of 1.7 mg/kg body weight,
corresponding to a dietary exposure of 25 mg/kg in feed. Excreta and
eggs were collected and, approximately 24 h after the final dose, the
animals were killed and tissue samples of liver, kidney, blood, lean
meat, skin and attached fat, and peritoneal fat were examined.
Elimination of most of the administered radioactivity occurred via the
excreta, with 78.6% of the total dose being excreted during the study
period. Approximately 0.1% of the radioactivity was found in tissues
and blood, less than 0.01% appeared in the egg yolks and 0.07% was
detected in the egg whites. The residual radioactivity in the
tissues amounted to 0.148 mg/kg diazinon equivalents in the kidney,
0.137 mg/kg in blood, 0.11 mg/kg in the liver and 0.01-0.025 mg/kg in
the other tissues examined. The residues in the egg yolks ranged from
0.006 mg/kg diazinon equivalents to 0.065 mg/kg while those in the egg
whites ranged from 0.038 mg/kg to 0.066 mg/kg. On a whole egg basis, a
plateau concentration of 0.047 mg/kg was reached on day 4 of treatment
(Simoneaux 1988c,d; Burgener & Seim, 1988).
6.1.2 Dermal application
6.1.2.1 Rats
The percutaneous absorption of diazinon was investigated in male
and female Sprague Dawley rats dermally exposed to 1 mg/kg (specific
activity 25.2 µCi/mg) and 10 mg/kg (specific activity 2.62 µCi/mg) of
[pyrimidine-14C-diazinon] dissolved in tetrahydrofuran. The dermal
absorption, excretion and tissue residues were determined after 0, 2,
8, 24, 48, 72 and 144 h. At each time point, four rats per sex and
dose group were used. The total recoveries for the balance data
averaged 96.3-101.5%. Calculated t50 absorption rates (i.e. the
amount of time required for 50% of the administered dose to be
absorbed into or penetrate through the skin) in males and females were
11.8 and 5.2 h, respectively, at the low-dose level of 1 mg/kg. At
10 mg/kg the respective t50 absorption rates were 10.2 and 5.3 h,
respectively, indicating that dermal absorption was more rapid in
females and was dose-dependent. The urine was the major route of
excretion in both sexes at both dose levels, 65-78% of the radiolabel
being excreted within 72 h. Times for 50% excretion in males and
females dosed at 1 mg/kg were 28.1 and 26.8 h, respectively. In the
high-dose groups the times for 50% excretion were 24.1 and 20.3 h in
males and females, respectively. The residual radioactivity in tissues
reached a maximum at 8 h after the administration in both dose groups
(plasma, red blood cells, fat, brain, muscle, lung, heart, spleen,
kidney, liver, stomach, small and large intestines, gonads, skin wash
and dissolved skin were assayed). In the low-dose group of males after
8 h, highest values were found in stomach (0.36 mg/kg diazinon
equivalents), small intestines (0.16 mg/kg), kidney (0.15 mg/kg),
liver (0.1 mg/kg) and skin (3.9 mg/kg in the skin wash and 0.86 mg/kg
in the dissolved skin). Reflecting their absorption rate, the females
of the low-dose group showed slightly higher tissue levels and a lower
residual radioactivity in the skin wash. After 144 h, residues were
down to the limit of quantification in most tissues, in both dose
groups and in both sexes (Ballantine, 1984).
6.1.2.2 Sheep
Two sheep were dermally treated with [pyrimidine-14C-diazinon]
(specific activity: 3.7 µCi/mg) dissolved in acetone for three
consecutive days. In order to mimic an extreme maximum exposure in a
dermal treatment of 40 mg/kg, 2270 mg 14C-diazinon was applied daily
to a shaved area of the back that constituted approximately 10% of the
animal's surface area. The area of application was left uncovered. Six
hours after the last administration the animals were killed and heart,
liver, kidney, back fat and leg muscle were analysed. The tissue
extractability was greater than 90% for all tissues. The highest
average residues were detected in kidney (9.4 mg/kg diazinon
equivalents) and back fat (7.3 mg/kg), while levels in heart, liver
and leg muscle amounted to 4-4.4 mg/kg (Capps, 1990; Pickles, 1990).
6.1.2.3 Humans
The dermal absorption of diazinon in humans is much less than in
rats. Six volunteers were dermally treated with [pyrimidine-14C]-
diazinon on the ventral forearm or the abdomen. The test material was
administered in acetone solution (2 µg/cm2) or dissolved in lanoline
wool grease (1.47 µg/cm2) over a 10-cm2 area of the skin without
occlusion. After 24 h, the test substance remaining on the site of
administration was washed off and the renal elimination followed for
seven days. Independent of the vehicle and the site of administration,
only 3-4% of the dose applied was percutaneously absorbed (Wester et
al., 1993).
6.1.3 Other routes
6.1.3.1 Intraperitoneal administration
The tissue distribution of diazinon and the inhibition of
cholinesterase (ChE) activities in plasma and erythrocytes were
investigated using male rats that received a single intraperitoneal
dose of diazinon (100 mg/kg body weight) in olive oil. The blood
diazinon level was estimated to reach a maximum at 1-2 h after
intraperitoneal administration. It was demonstrated that the diazinon
residue levels were highest in the kidney, when comparing the
distribution of diazinon among liver, kidney and brain in the animals
after dosing. Erythrocyte and plasma ChE activities were inhibited
rapidly, but ChE inhibition was greater in the erythrocytes than in
plasma (Tomokuni & Hasegawa, 1985).
The tissue distribution of diazinon and the inhibition of ChE
activities in plasma, erythrocyte and brain was investigated using
male rats and mice that received a single intraperitoneal (i.p.) dose
of diazinon (20 or 100 mg/kg body weight) in olive oil. The blood
diazinon level was estimated to reach a maximum 1-2 h after the i.p.
administration. It was demonstrated that the diazinon residue levels
were highest in the kidney, when comparing the distribution of
diazinon among liver, kidney and brain in the animals after dosing.
The ChE inhibition by diazinon exposure was greater in the plasma than
in the erythrocytes for male mice, while its inhibition was greater in
the erythrocytes for male rats. Brain ChE activity was also inhibited
markedly in the mice after dosing (Tomokuni et al., 1985).
6.1.3.2 Subcutaneous administration
Male guinea-pigs were subcutaneously treated with 45 mg/kg
32P-labelled diazinon (specific activity -117-197 cpm/µg) in peanut
oil. The tissue distribution was determined 2, 4, 8 and 16 h after
treatment, and excretion of 32P was investigated over an 8-day
period. Following subcutaneous administration, urinary elimination
amounted to 20% of the administered dose after 16 h. The levels of
radioactivity found in the gastrointestinal tract were low apart from
the caecum, which accumulated up to 5.5% of the administered dose over
16 h. After 48 h, urinary elimination amounted to about 60%, while
only trace amounts were eliminated with the faeces (Kaplanis et al.,
1962).
6.1.3.3 Intravenous administration
Four female Rhesus monkeys were dosed intravenously with 2.1 µCi
(31.8 µg) [pyrimidine-14C]-diazinon dissolved in propylene glycol.
Within 7 days, average values of 56 and 23% of the dose were
eliminated in urine and faeces, respectively (Wester et al., 1993).
6.2 Metabolism
The metabolic fate of diazinon was studied with different modes
of administration using unlabelled and radiolabelled diazinon in
various species including rat, mouse, guinea-pig, dog, sheep, goat,
cow and chicken. Additional in vitro experiments were conducted
using tissue slices or cell fractions. A comparative summary of the
results available was provided by Hagenbuch & Mücke (1985). In all
species tested, diazinon was rapidly and almost completely absorbed
from the gastrointestinal tract. It was also absorbed from the skin.
The main metabolic pathways of degradation of diazinon are:
a) Cleavage of the ester bond of diazinon or diazinoxon leading to
the hydroxypyrimidine derivatives.
b) Transformation of P-S moiety to the P-O derivative, leading to
the active metabolite, diazoxon.
c) Oxidation of isopropyl substituent leading to the corresponding
tertiary and primary alcohol derivatives.
d) Oxidation of the methyl substituent leading to the corresponding
alcohol.
e) Glutathione-mediated cleavage of the ester bond leading to a
glutathione conjugate.
The hydrolytic and oxidative cleavage of the phosphorus ester
bond, leading directly or via diazoxon to the pyrimidinyl derivative,
play the most prominent role in the metabolism of diazinon.
Glutathione conjugation appears to be of small importance. Metabolites
maintaining the phosphorus ester bond are of transient nature and are
only observed in minor quantities.
The general metabolic pathways of diazinon in mammals are given
in Fig. 1.
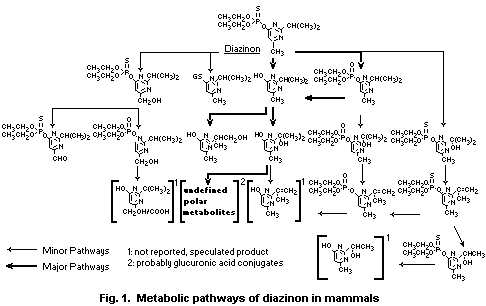 The metabolites formed, i.e. diethylphosphoric acid,
diethylthiophosphoric acid and the derivatives of pyrimidinyl ring,
are eliminated mainly via the kidneys. Only minimal quantities of the
metabolites were detected in milk and eggs.
6.2.1 In vivo metabolic transformations
6.2.1.1 Mice
When male ICR mice (number not stated) were treated orally with
diazinon or [pyrimidine14C] diazinon at 50 or 75 mg/kg body weight,
one half of the high-dose animals died and the rest showed symptoms
(sweating, crouching) (Miyazaki et al., 1970; Sekine, 1972). At the
low dose, no signs of toxicity were observed. Metabolism and excretion
occurred rapidly, and the metabolites diazoxon, O, O-diethyl-
O-[2-(alpha-hydroxyisopropyl)-4-methyl)-6-pyrimidinyl]
phosphorothioate, and O, O-diethyl- O-(2-(2-propenyl)-4-methyl-6-
pyrimidinyl) phosphorothioate were found in the urine 1 h after
treatment. Most of the metabolites were found in urine 6 h after
treatment, but metabolism was not identical in the two dose groups. In
the low-dose group O, O-diethyl- O-(2-isopropyl-4-hydroxymethyl-6-
pyrimidinyl) phosphorothioate and O, O-diethyl- O-(2-isopropyl-4-
formyl-6-pyrimidinyl) phosphorothioate were found, but this was not
observed in the high-dose group. In the high-dose, but not the low-
dose group O, O-diethyl- O-(2-(a-hydroxyethyl)-4-methyl-6-
pyrimidinyl) phosphorothioate was found.
The metabolism of [pyrimidine-14C]-diazinon and [ethoxy-14C]-
diazinon was investigated by Mücke et al. (1970). Four metabolite
fractions were found in urine and faeces, three metabolites
representing approximately 70% of the total radioactivity applied.
Hydrolysis of the ester bond yielded 2-isopropyl-4-methyl-6-
hydroxypyrimidine (22.5% of the applied radioactivity in urine);
oxidation at the primary carbon atom produced 9% of the applied
radioactivity in urine, while oxidation at the tertiary carbon atom of
the isopropyl side chain produced 22%. In addition, trace amounts of
unchanged diazinon were detected in faeces. No cleavage of the
pyrimidine ring with subsequent oxidation of the fragments to CO2
took place (Mücke et al., 1970).
6.2.1.2 Rats
A study by Capps (1989) investigated the diazinon metabolites in
male and female rats orally treated with single doses of 10 and
100 mg/kg [pyrimidine 14C]-diazinon and in rats preconditioned with
14 daily treatments at 10 mg/kg before the final administration of
radiolabelled compound. The metabolite pattern was similar in the
urine and faeces of the rats from all dose groups and from both sexes.
The major urinary metabolites were identified as 2-isopropyl-6-
methyl-4(1 H)- pyrimidinone (average 38.2% of the totally applied
dose), 2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone
(17.3%) and 2-(beta-hydroxyisopropyl-6-methyl-4(1 H)-pyrimidinone
(9.7%). Six unknown aqueous components accounted for an average of
14.9% of the administered dose, and trace amounts of unchanged
diazinon (0.11%), diazoxon (0.14%) and the hydroxy-isopropyl
derivative of diazinon (0.12%) were also detected. The identity of the
metabolites was confirmed by gas chromatography and mass spectrometry
(GC/MS) with synthetic standards.
6.2.1.3 Dogs
The urinary metabolites of Beagle dogs were characterized after
oral administration of 4.0 mg/kg body weight 14C-ring-labelled
diazinon. The metabolite 2-isopropyl-4-methyl-6-hydroxypyrimidine
accounted for 10% of the applied radioactivity in the urine and the
tertiary hydroxy-isopropyl derivative of diazinon represented 23%
(Iverson et al., 1975).
6.2.1.4 Sheep
When two sheep were dermally treated with [pyramidine-14C]-
diazinon, radiolabelled residues were detected in all tissues examined
(heart, liver, kidney, back fat and leg muscle). Unmetabolized
diazinon was the only significant residue in fat, and was a major
residue in heart and leg muscle. The major metabolites in urine and
all tissues except fat were 2-isopropyl-6-methyl-4(1 H)-pyrimidinone
(urine, 10% of the administered radioactivity; liver, 18%; kidney,
23%) and 2-(alpha- hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone
(urine, 22.7%; liver, 10%; kidney, 28%), which were also present in
the form of glucuronide conjugates. The identity of the metabolites
was confirmed by GC/MS with synthetic standards. In addition, several
unidentified polar (urine, 18.6%) and minor amounts of non-polar
(urine, 4.0%) metabolites were detected (Capps, 1990).
6.2.1.5 Goats
Two lactating goats were orally treated with [pyrimidine-14C]-
diazinon in gelatin capsules for four consecutive days. Similarly to
sheep, in urine and faeces the metabolites 2-isopropyl-6-methyl-
4(1 H)-pyrimidinone (urine, 4.5% of the totally administered radio-
activity; faeces, 2.6%) and 2-(alpha-hydroxyisopropyl)-6-methyl-
4(1 H)-pyrimidinone (urine, 12.5%; faeces, 1.7%) were identified.
Approximately 48.6% of the urinary radioactivity consisted of unknown
water-soluble compounds. Characterization of selected tissues showed
the presence of mainly the above-mentioned metabolites. Unchanged
diazinon, its hydroxy-isopropyl derivative and diazoxon accounted for
less than 10% of the radioactivity detected in these tissues.
Metabolites in fat consisted primarily of unchanged diazinon (66%),
its hydroxy-isopropyl derivative (12.5%) and diazoxon (3%). The major
metabolites in the milk were 2-isopropyl-6-methyl-4(1 H)-
pyrimidinone (39.3% of the residual radioactivity) and
2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-pirimidinone (37.3%).
Substantial portions of the polar metabolites in urine, faeces and
tissues were glucuronide conjugates. The identity of the metabolites
was confirmed by GC/MS with synthetic standards (Simoneaux,
1988a,b,e).
6.2.1.6 Hens
Four laying Leghorn hens were treated with [pyrimidine-14C]-
diazinon in gelatin capsules for seven consecutive days at daily doses
of 2.75 mg/kg day. The main metabolites detected in the excreta were
unchanged diazinon (14.9% of the extractable radio-activity),
2-isopropyl-6-methyl-4(1 H)-pyrimidinone (5.9%), 2-(alpha-hydroxy-
isopropyl)-6-methyl-4(1 H)-pyrimidinone (10.8%) and 2-(beta-
hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone (7.2%). Approximately
25% of the radioactivity in the excreta consisted of unknown
water-soluble compounds. The residues in tissues primarily consisted
of 2-isopropyl-6-methyl-4(1 H)-pyrimidinone (0.6-2.6% of the residual
radioactivity), 2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-
pyrimidinone (3.1-6.5%) and 2-(beta-hydroxyisopropyl)-6-methyl-
4(1 H)- pyrimidinone (2.0-5.7%). Unchanged diazinon was detected
primarily in the peritoneal fat (2% of residues). In the eggs,
primarily 2-isopropyl-6-methyl-4(1 H)-pyrimidinone (yolk, 11.1% of
the residual radioactivity; white, 9.4%), 2-(alpha-hydroxyisopropyl)-
6-methyl-4(1 H)-pyrimidinone (yolk, 18.6%; white, 33.3%) and
2-(beta-hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone (yolk, 7.0%;
white, 35.3%) were detected. As in goats, a substantial portion of the
polar metabolites in tissues, eggs and excreta were glucuronide
conjugates. The identity of the metabolites was confirmed by GC/MS
with synthetic standards (Simoneaux, 1988c,e; Simoneaux, 1989).
More information on kinetics and metabolism in other species is
given in chapter 9.
6.2.2 In vitro metabolic transformations
The metabolism of [ethoxy-14C]-diazinon and diazoxon was studied
in vitro using rat liver cell fractions. It was shown that the
degradation by diazinon is catalysed by a microsomal enzyme that
requires NADPH and oxygen, and is inhibited by carbon monoxide. It is
presumably the cytochrome P-450 oxidase system. Diazoxon was shown to
be degraded by enzymes located in the nuclear, mitochondrial,
microsomal and soluble fractions of the liver. The microsomal enzymes
were the most active and were not dependent on NADPH. Reduced
gluthation had little effect. With diazinon, products of the reactions
were diethylphosphorothioic acid and diethylphosphoric acid. Diazoxon
was degraded to diethylphosphoric acid (Yang et al., 1969, 1971;
Nakatsugawa et al., 1969). These results were confirmed by independent
experiments (Dahm, 1970). The oxidation of diazinon was investigated
by using microsomal preparations from rat liver. The major metabolic
products of diazinon were hydroxydiazinon, diazoxon and
hydroxydiazoxon, which are biologically active, and additional
inactive products such as diethylphosphorothioic acid,
diethylphosophoric acid and derivatives of the pyrimidyl moiety. It
was demonstrated that desulfuration, hydroxylation of the ring alkyl
side-chain and cleavage of the aryl phosphate bond may occur,
depending on the presence of NADPH or NADH. EDTA stimulated the
overall metabolism of diazinon (Shishido et al., 1972a).
The enzymatic hydrolysis of diazoxon was investigated using rat
tissue homogenates. The hydrolytic activity of the tissues decreased
in the order liver>blood>lung>heart>kidney>brain. In the liver,
the hydrolytic activity was localized in microsomal preparations.
Diethyl phosphoric acid and 2-isopropyl-4-methyl-6-hydroxypyrimidine
were identified as the products. The reactions were inhibited by EDTA,
heavy and rare earth metal ions, and sulfhydryl reagents (L-cysteine,
2-mercaptoethanol, thioglycolic acid), while calcium ions activated
the hydrolysis (Shishido & Fukami, 1972).
Liver homogenates were prepared from male mice (North Carolina
Department of Health strain) and incubated for 1 h with either
14C-diazinon or 14C-diazoxon. Inhibition of metabolism was
studied by co-incubation with piperonyl butoxide, NIA 16824 or
1-(2-isopropylphenyl) imidazole. Diazoxon formation from diazinon
(thiophosphate to phosphate conversion) was inhibited by 45 to 60% by
the inhibitors studied. All the inhibitors also reduced oxidative
dearylation of diazinon to diethyl phosphoric and diethyl
phosphorothioic acids (Smith et al., 1974).
Conjugation with glutathione forms the third enzymatic mechanism
of the diazinon metabolism in rat tissue preparations (liver, heart,
brain, lung, kidney and blood were investigated). The highest activity
(14-89 times as high as in other tissues) for this reaction was
localized in the cytoplasmatic fractions of the liver. The reaction
products were identified as diethyl phosphorothioic acid and
S-(2-isopropyl-4-methyl-6-hydroxypyrimidinyl) glutathione, which were
formed by conjugation and simultaneous cleavage of the phosphate ester
bond. The enzymatic activity was increased by the addition of
glutathione-SH, and was inhibited by various sulfhydryl reagents,
oxidized glutathione and some chelating agents ( o-phenanthroline,
8-hydroxyquinoline) (Shishido et al., 1972b).
6.3 Metabolic aspects of diazinon toxicity
Diazinon was incubated with liver microsomes and liver slices
from sheep, cow, pig, guinea-pig, rat, turkey, chicken and ducks.
Hydroxydiazinon, isohydroxydiazinon, dehydrodiazinon, their oxons
and diazoxon were identified and determined quantitatively or
semi-quantitatively. It was shown that yields and rates of production
of the metabolites varied greatly between the species. The production
of the oxon was not generally correlated with susceptibility to
diazinon poisoning, although it was lowest in the least susceptible
animal, the sheep. The highly susceptible avian species (acute oral
LD50 of around 2-15 mg/kg) do not produce higher rates of oxons than
rat or pig (acute oral LD50 around 300-600 mg/kg). However, the
mammalian blood hydrolyses diazoxon rapidly, whereas the avian species
have virtually no hydrolytic activity. It was concluded that
extrahepatic metabolism of diazinon, in particular the hydrolysis of
diazoxon in the blood, appears to be the main factor affecting
susceptibility to diazinon poisoning. In mammals the extrahepatic
metabolism of diazinon is more important toxicologically than the
metabolism in the liver, while the liver is probably the most
important site of metabolism in avian species (Machin et al., 1975).
Recently, the hydrolytic metabolism of diazinon by plasma was
investigated in 92 individuals of Hispanic origin (Davies et al.,
1996). Diazoxon is hydrolysed by the enzyme paraoxonase (PON1),
leading to the formation of 2-isopropyl-4-methyl-6-hydroxypyrimidine
and diethylphosphate. An important observation of this study was that
the effect of the PON1 polymorphism for diazoxon hydrolysis relative
to paraoxon hydrolysis was reversed. Thus, RR individuals (Arg192
homozygotes) who displayed high paraoxonase activity had lower
diazonoxonase activity (mean = 7948 U/litre) than QQ homozygotes
(12 318 U/litre).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
Improvements since 1979 in the manufacturing of diazinon have
significantly reduced the content of highly toxic by-products, in
particular tetraethyl-pyrophosphate (TEPP). As a result of these
stepwise improvements, the acute oral LD50 of technical grade
diazinon increased to values around 1000 mg/kg (Piccirillo, 1978;
Bathe & Gfeller, 1980; Schoch & Gfeller, 1985; Kuhn, 1989a). The most
recent study resulted in an oral LD50 in rats of 1250 mg/kg. The LD50
values for different species are summarized in Table 2.
Signs of poisoning after a single dose of diazinon are typical of
organophosphate intoxication and include decrease of spontaneous
activity, sedation, dyspnoea, ataxia, tremors, muscle spasms,
convulsions, lacrimation and diarrhoea. The signs were found to be
reversible in surviving animals.
Diazinon (88% purity) was administered by gavage to 15 rats per
sex and dose at single doses of 0, 2.5, 150, 300 and 600 mg/kg
(Potrepka, 1994). Three, 9 or 24 h after dosing, five animals of each
group were bled for determination of serum and red blood cell
cholinesterase activity and then killed for determination of CNS
cholinesterase activity. There was no mortality. Signs of cholinergic
poisoning, e.g., salivation, diarrhoea and muscle fasciculations, were
observed in animals of both sexes dosed at 300 and 600 mg/kg. Clinical
signs first appeared 3 h after treatment. Maximum observable toxicity
was noted 9 h after treatment in males and 24 h after treatment in
females. Serum cholinesterase activity was significantly decreased at
all time points in all groups treated with 2.5 mg/kg or more. Maximum
inhibition was observed 9 h after dosing, and values remained
depressed at 24 h after dosing. Red blood cell cholinesterase activity
was significantly inhibited at all time intervals in animals of both
sexes treated with >150 mg/kg. Again, maximum inhibition was
observed 9 h after dosing, and activity remained depressed at 24 h
after dosing. In addition, a significant inhibition of red blood cell
cholinesterase activity was noted in females dosed with 2.5 mg/kg
diazinon 9 h after dosing. Cerebellum, cerebral cortex, striatum,
hippocampus and thoracic spinal cord cholinesterase activities were
decreased in female rats dosed with >150 mg/kg at all three time
points. Cerebellum, striatum and hippocampus cholinesterase activities
were decreased in male rats dosed with >150 mg/kg at all three time
intervals, whereas cerebral cortex and thoracic spinal cord
cholinesterase activities were decreased in male rats dosed with
>150 mg/kg at 9 and 24 h after dosing. The NOAEL for inhibition of
brain cholinesterase activity was 2.5 mg/kg for both sexes (Potrepka,
1994).
Table 2. Acute toxicity of diazinona
Species Sex Route of administration LD50 (mg/kg) Reference
Rat male oral 235 Gasser (1953)
Rat male oral 435 Shaffer & West (1960)
Rat male oral 250 Gaines (1969)
Rat female oral 285 Gaines (1969)
Rat both oral 300 Piccirillo (1978)
Rat both oral 422 Bathe & Gfeller (1980)
Rat both oral 1012 Schoch & Gfeller (1985)
Rat both oral 1250 Kuhn (1989a)
Rat male dermal 900 Gaines (1960)
Rat female dermal 455 Gaines (1960)
Rat both dermal >2150 Bathe (1972a)
Rat both inhalation >2300b Holbert (1989)
Mounse male oral 82 Bruce et al. (1955)
Mounse both oral 96 Gasser (1953)
Mounse both oral 187 Bathe (1972b)
Mounse both i.p. 65 Klotzsche (1955)
Guinea-pig oral 320 Gasser (1953)
Rabbit oral 130 Gasser (1953)
Rabbit both dermal >2020 Kuhn (1989b)
Turkey oral 6.8 FAO/WHO (1965)
Chicken oral 40.8 FAO/WHO (1965)
Goose oral 14.7 FAO/WHO (1965)
Gosling oral 2.8 Egyed et al. (1974)
a It should be noted that progressive improvement in the manufacturing process has reduced
the acute toxicity of diazinon.
b This value is the LC50, the units being mg/m3.
Brain cholinesterase activities were determined for white-footed
mice (Peromyscus leucopus) orally dosed with diazinon at 18.8 mg/kg
body weight (Montz & Kirkpatrick, 1985). Following treatment with
diazinon, a latent period of approximately 6 h elapsed during which
time acetylcholinesterase activity was relatively unaffected. After
the latent phase, ChE activity rapidly declined to a minimum 12 h
after dosing. After 48 h, ChE activity recovered to a level only
slightly below that of the controls. The response of both male and
female mouse brain ChE activities declined rapidly at 6 h and reached
a minimum 24 h after dosing. ChE activity of treated animals was
comparable to that of controls 48 h after treatment. Brain ChE
activities of treated female mice were significantly lower (P <0.05)
than those of treated males.
An acute oral toxicity study in rats was conduced in two phases
to determine a NOEL of the test material (87.9% active ingredient) for
clinical, behavioural and body weight effects in phase 1 and a NOEL
for effects on plasma, red blood cell and brain cholinesterase in
phase 2 after single oral gavage application to rats. In phase 1 each
test group consisted of 5 rats. The applied dose levels were 25 and
50 mg/kg body weight for female rats only; levels of 100, 250 and
500 mg/kg body weight were tested in both sexes. One female given
500 mg/kg body weight died. Clinical signs such as miosis,
hypoactivity, absence of pain reflex, red-stained face, yellow-stained
abdomen/urogenital area, soft stool or few faeces were seen in all
animals except 100- mg/kg males and 25-mg/kg females. The NOAEL for
clinical/behavioural/body weight effects was 100 mg/kg body weight in
males and 25 mg/kg body weight in females. In phase 2 each test group
consisted of 5 rats. Males were treated with 0.05, 0.50. 1.0, 10.0,
100 or 500 mg/kg body weight and females with 0.05, 0.12, 0.25, 2.5,
25 and 250 mg/kg body weight. Clinical signs such as miosis,
hypoactivity, absence of pain reflexes, staggered gait, excessive
salivation, red-stained face and yellow-stained and/or wet
ventral/urogenital area were only observed in males at 500 mg/kg body
weight (one male at 100 mg/kg body weight showed miosis) and females
at 250 mg/kg body weight The only findings at necropsy were yellow
staining of the perineum and red paranasal discharge in animals of
these dose groups. Plasma cholinesterase activity was significantly
reduced in males dosed with 10, 100 or 500 mg/kg body weight and in
females dosed with 2.5, 25 or 250 mg/kg body weight. Red blood cell
cholinesterase activity was significantly lower in males given 100 or
500 mg/kg body weight and in females given 25 or 250 mg/kg body
weight. Brain cholinesterase activity was significantly reduced at the
highest dose level for both sexes. Brain cholinesterase activity in
females dosed with 25 mg/kg body weight was inhibited by 37% (without
reaching statistical significance) (Glaza, 1993).
7.1.2 Dermal
Diazinon was dispersed on the shaved back of each of three male
and three female Tif: RAI rats at a level of 2150 mg/kg and covered
with aluminium foil for 24 h. None of the animals died. Neither
clinical signs nor dermal irritation was observed. Autopsy revealed no
substance-related gross organ changes. The acute dermal LD50 in rats
was found to be greater than 2150 mg/kg (Bathe, 1972a). Sherman strain
rats were given one dermal application of diazinon dissolved in
xylene, and no attempt was made to remove the compound during the
observation time of 14 days. The LD50 was 900 mg/kg for males and
455 mg/kg for females (Gaines, 1960).
Dermal LD50 values for diazinon were determined in mice after
application of the solution to hind feet. Values were simultaneously
generated for the ED50 for both cholinesterases (acetylcholinesterase
and pseudocholinesterase). LD50 values were higher than those
reported for mice treated on shaved back skin. Diazinon appeared to
have much more inhibitory potential for blood than for nervous tissue
cholinesterase (Skinner & Kilgore, 1982).
Undiluted diazinon was applied to the clipped dorsal trunk of
each of five male and five female New Zealand White rabbits at a level
of 2020 mg/kg and kept under semi-occlusive dressing for 24 h. Signs
were typical of organophosphate intoxication; two out of ten animals
died. The LD50 was >2020 mg/kg (Kuhn, 1989b,c).
7.1.3 Inhalation
Sprague-Dawley rats were exposed (whole body exposure) to
diazinon for 4 h. The acute inhalation LC50 for diazinon MG 8
FL880045 was greater than 2330 mg/m3 when it was administered
undiluted as an aerosol (Holbert, 1989).
Groups of five male and five female HSD:SD rats were exposed to
an aerosol generated from undiluted liquid diazinon MG87% (88% purity)
for 4 h (nose-only exposure). An exposure concentration of 5540 mg/m3
was obtained, 8.82% of particles being smaller than 1 mm in diameter.
There was no mortality. Prominent in-life observations included
activity decrease, piloerection and polyuria, no longer seen by day 6.
Body weight gain was largely unaffected. Gross necropsy revealed
discoloration of the lungs in all animals. The acute inhalation LC50
for diazinon in the rat was greater than 5440 mg/m3 (Holbert, 1994).
7.1.4 Intraperitoneal
Mild structural and functional changes were observed in the liver
and testes of rats after a single intraperitoneal administration of
diazinon (21.6 mg/kg). Kidney, however, showed no pathological
lesions. Attempts were made to correlate the pathological changes in
these organs with the activity of succinic dehydrogenase, adenosine
triphosphatase and alkaline phosphatase (Dikshith et al., 1975). A
single intraperitoneal dose of diazinon caused hyperglycaemia in rats,
which peaked 2 h after intraperitoneal treatment with 40 mg
diazinon/kg. The brain acetylcholinesterase activity was significantly
reduced. The blood level of pyruvic acid was unchanged while that of
lactic acid was significantly increased. In diazinon-treated
hyperglycaemic animals, the glycogen content of the brain was
depleted, the activities of glycogen phosphorylase, phosphoglucomutase
and hexokinase were significantly increased, and the activity of
glucose-6-phosphatase remained unchanged. Lactate dehydrogenase
activity was increased by treatment with diazinon. The induced changes
may have compensated for the energy requirement of stimulatory effects
caused by the pesticide (Husain & Matin, 1986; Matin & Husain, 1987;
Matin et al.,1989). Changes in energy metabolism are regarded as
secondary, following cholinesterase inhibition.
In an acute intraperitoneal study in rats, an LD50 value of
260 mg/kg body weight was determined for both sexes. Clinical signs
were typical of organophosphate poisoning, e.g., sedation, dyspnoea
and tonic-clonic spasms. Surviving animals had recovered within 3 to 6
days, and no substance-related gross organ changes were observed
(Bathe, 1973).
Diazinon given as an intraperitoneal single dose (40 mg/kg) to
rats produced tremors and convulsions with lactic acidosis. This was
accompanied by depletion of glycogen phosphorylase activity in the
triceps and diaphragm muscles 2 h after the administration (Husain &
Matin, 1986; Husain & Ansari, 1988).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rats
Davies & Holub (1980a,b) found that diazinon (99.2% purity) fed
to rats for 4 weeks at doses up to 25 mg/kg diet produced no visible
toxic manifestations such as tremors or hyperexcitability. Feeding
diazinon 25 mg/kg diet for 30 days produced more significant
reduction of cholinesterase activity in plasma (by 22-30%) and brain
(by 5-9%) among treated females than among males. Erythrocyte
acetylcholinesterase activity was significantly more depressed (by
13-17%) in treated females than in males at days 21-28 of the feeding
trial. The greater degree of cholinesterase inhibition in females was
possibly attributable to the higher amount of diazinon ingested by
females than by males after day 15 of the study.
Female Wistar rats were fed a semi-purified diet containing
either no pesticide or 0.1 to 15 mg/kg diazinon for up to 92 days. At
specified times, blood samples were taken to measure plasma and
erythrocyte cholinesterase activity using a radiometric method.
Additional rats were killed to determine brain cholinesterase
activity. Measurements included body weight gain and feed consumption
during the growing period. Feeding diazinon at the stated levels
produced no visible toxic manifestations. Treated animals showed
weight gain and feed consumption that was comparable to controls.
Feeding trials lasting up to 90 days revealed that rats were highly
sensitive to diazinon after 31 to 35 days exposure, as judged by
reduction of plasma and erythrocyte cholinesterase activities. Brain
acetylcholinesterase was found to be practically insensitive to
dietary intake of diazinon (1.0 to 15 mg/kg), although moderate
reduction (by 6%) of brain enzyme activity was noted among animals fed
10 mg/kg diet at day 92. For all feeding trials, plasma cholinesterase
was a more sensitive indicator of diazinon exposure than erythrocyte
or brain acetylcholinesterase. The NOAEL of diazinon for the rat was
estimated to be 0.1 mg/kg diet, which is equivalent to a daily intake
of 0.009 mg/kg body weight per day (Davies & Holub, 1980a).
In a feeding study, diazinon (87.7% pure) was administered to
groups of 15 male and 15 female Sprague Dawley rats for 13 weeks at
dietary concentrations of 0, 0.5, 5, 250 and 2500 mg/kg, corresponding
to mean daily doses of 0, 0.03, 0.3, 15 and 168 mg/kg body weight in
males and 0, 0.04, 0.4, 19 and 212 mg/kg body weight in females,
respectively. The rats treated at the highest dose level showed
hypersensitivity to touch and sound and some of the males showed
apparent aggressiveness, hyperactivity and soft faeces. Body weight
gain and food consumption were reduced in both sexes. At this dose
level, the haematological examination revealed a decreased haemoglobin
level, a lower haematocrit and an increased number of reticulocytes in
the females. The examination of blood biochemistry revealed a reduced
activity of serum cholinesterase in both sexes treated with 5 mg/kg
diet or more. In addition, the low-dose females showed a 17% reduction
in the erythrocyte cholinesterase activity. At 250 mg/kg diet or more,
the erythrocyte and brain cholinesterase activities were reduced in
both sexes. The absolute and relative liver weights were increased in
both sexes in the highest dose level, and there was microscopic
evidence of centrilobular hepatocellular hypertrophy (Singh et al.,
1988). The NOAEL, based on reduction in brain cholinesterase activity,
was 5 mg/kg diet (equivalent to 0.4 mg/kg body weight per day).
In a second, similar, study (Pettersen & Morrissey, 1994),
Sprague Dawley rats were fed diazinon at 0, 0.3, 30, 300 or 3000 mg/kg
diet for 13 weeks. Serum, erythrocyte and regional brain
cholinesterase inhibition was measured in groups of five males and
five females at weeks 4, 8 and 13. Serum and erythrocyte
cholinesterases were inhibited by 45-86 and 60-75%, respectively, at
30 mg/kg diet, but not consistently at 0.3 mg/kg diet. Brain regional
cholinesterase was inhibited by up to 25% in females at 30 mg/kg diet
and by 55-75% at 300 mg/kg diet. Males were less sensitive, showing no
effect at 30 mg/kg diet and a fall of 62-77% at 3000 mg/kg diet.
Observable neuromuscular deficits were seen only at 3000 mg/kg diet.
Consequently the NOEL and NOAEL for this study was 0.3 mg/kg diet
(equivalent to 0.019 mg/kg body weight per day), based on the
reductions in serum, erythrocyte and brain cholinesterase seen at the
next highest dose of 30 mg/kg diet. However, given the lack of an
intermediate dietary level of 5 mg/kg, which set the NOAEL in the
Singh et al. (1988) study, and the modest fall in brain cholinesterase
seen at 30 mg/kg diet in this second study, it is concluded that the
previous NOAEL of 5 mg/kg diet (0.4 mg/kg body weight per day) should
stand.
The effect of low levels of diazinon treatment on four marker
enzymes in rat heart and skeletal muscles have been investigated by
Wilkinson et al. (1986). Typical differences in succinate
dehydrogenase (SDH), lactate dehydrogenase (LDH), phosphofructokinase
(PFK) and hexokinase (HK) activities were observed between heart and
skeletal muscles. Diazinon feeding had no effect on heart, soleus,
gastrocnemius and plantaris SDH, LDH or PKF enzyme activities after
28 weeks. HK activity was significantly increased in sham-control
soleus and plantaris muscle after 28 weeks. Diazinon feeding inhibited
HK activity in plantaris muscle after 28 weeks treatment. These
results demonstrate that chronic low levels of diazinon have little
effect on the glycolytic and oxidative activity in heart and skeletal
muscles.
Adult Wistar rats were given diazinon by gavage twice weekly at a
dose of 0.5 mg/kg body weight for twenty-eight weeks. Selected control
and experimental animals were killed after 7, 14 and 28 weeks.
Histological examination of the liver revealed that animals repeatedly
treated with sublethal doses of diazinon sustain a form of hepatic
injury characterized by cellular lipid accumulation. The finding of
increased lipid accumulation in the liver following prolonged
treatment with diazinon, however, still does not resolve the question
of whether impaired lipid metabolism and/or storage is the primary
effect (Anthony et al., 1986).
Rats were exposed to diazinon-impregnated strips in a
conventional laboratory animal room. The air in the room was monitored
for the pesticide. Erythrocyte and plasma cholinesterase activities
were determined periodically. Air concentration of the pesticide never
exceeded 1.34 mg/m3. No significant change in enzyme activities were
observed (Hinkle et al., 1980).
7.2.1.2 Dogs
Feeding studies with dogs (Bruce et al., 1955; Williams et al.,
1959) did not differentiate between the sexes and therefore relevant
data from male and female animals were pooled. In another study
(Barnes et al., 1988) diazinon (87% pure) was given to groups of four
male and four female Beagle dogs for 13 weeks at concentrations of 0,
0.1, 0.5, 150 and 300 mg/kg diet, corresponding approximately to a
mean daily intake of 0.0034, 0.02, 5.9 and 10.9 mg/kg body weight
respectively. Vomiting was observed in groups fed a diet containing
150 or 300 mg/kg. Serum cholinesterase activity was reduced at
0.5 mg/kg or more in males and at 150 and 300 mg/kg in females.
Erythrocyte and brain cholinesterases activity was depressed at 150
and 300 mg/kg in both sexes. The histopathological examination
revealed a moderate atrophy of pancreatic acini in one high-dose group
of male dogs. Body weight gain was decreased in females at 150 mg/kg
diet and in both sexes at 300 mg/kg diet. The serum calcium level was
decreased in females at 150 mg/kg diet and in males at 150 and
300 mg/kg diet. The serum albumin level was decreased in both sexes at
300 mg/kg diet. As a slight reduction of serum cholinesterase activity
was the only change observed, 0.5 mg/kg diet, corresponding to a mean
diazinon intake of 0.02 mg/kg body weight per day, is considered to be
the dietary concentration causing no toxicological effect (NOAEL),
based on inhibition of brain and erythrocyte cholinesterase at higher
doses.
7.2.1.3 Pigs
Pigs were orally administered diazinon in capsules at doses of
0, 1.25, 2.5, 5 and 10 mg/kg body weight daily for periods of up to
8 months. In pigs, mortality and cholinergic signs of poisoning were
evident at 2.5 mg/kg body weight per day (FAO/WHO, 1979).
7.2.2 Inhalation
Two short-term inhalation studies were conducted in rats. In the
first study, diazinon (97.1% pure) was administered in an inhalation
chamber to four groups of nine male and female Tif RAIf rats for 6 h
per day, 5 days a week, for three weeks, at concentrations of 0, 151,
245 and 559 mg/m3. Four animals of each sex from the control group
and from the highest concentration group were kept for a 25-day post-
treatment observation period, while the others were sacrificed at day
21 after treatment. No compound-related deaths occurred. Exophthalmus
and diarrhoea were observed at all dose levels. In addition, the high-
dose group animals showed salivation, ruffled fur and tonic-clonic
convulsions during 2 h after each exposure. No toxic signs occurred in
the 25-day follow-up period. Food consumption was reduced during the
first three days of exposure in the high-dose group, plasma
cholinesterase activity was reduced in the intermediate and high-dose
groups, and brain cholinesterase activity was reduced at all dose
levels. Erythrocyte cholinesterase activity was reduced in the high-
dose group. The changes were reversible. There were no macro or
histopathological findings related to the exposure to diazinon (Zak et
al., 1973).
In the second study the main purpose was the definition of a
NOAEL for cholinesterase inhibition. Groups of ten male and ten female
Tif RAlf rats were exposed to diazinon 6 h a day for 5 days per week
for 3 weeks. The effective concentrations at the inhalation site
were 0, 0.05, 0.46, 1.57 and 11.6 mg/m3 air. There were no
compound-related signs, and no changes in body weight or food
consumption. In comparison with the control animals, the number of
erythrocytes, haemoglobin level and packed cell volume were slightly
lower in the highest-dose group. A minor decrease of brain
cholinesterase activity was noted in the highest-dose group of
females. Plasma glucose levels were significantly reduced among males
exposed to 1.57 and 11.6 mg/m3 (Hartmann, 1990). As the exposure to
0.46 mg/m3 inhibited the plasma cholinesterase only, this
concentration is the NOAEL.
7.2.3 Dermal
7.2.3.1 Rabbits
Diazinon (97.1% pure) was suspended in 50% aqueous polyethylene
glycol 300 and topically administered under semi-occlusive dressing to
groups of five male and five female albino rabbits at daily doses of
0, 1, 5 and 100 mg/kg body weight for 5 days per week for 3 weeks. In
the highest-dose group, four males treated at 100 mg/kg died during
the first week of treatment. Consequently, the dose was reduced to
50 mg/kg. Clinical signs were observed in the highest-dose group and
included anorexia, ataxia, fasciculations, tremors, diarrhoea,
hypoactivity, hypotonia and salivation. Most of the signs disappeared
after the dose was reduced. Mild dermal reactions were noted at site
of test substance administration. Body weight gain and food
consumption was similar in all groups, and most laboratory parameters
remained unaffected by the treatment. Reduced cholinesterase
activities were found in serum, red blood cells and the brain in
animals treated at >5 mg/kg. In the highest-dose group, the
reductions were significant for brain, red blood cells and serum
cholinesterase activities, while with 5 mg/kg there was a
statistically significant decrease of activity in the brain
cholinesterase of females only. In the highest-dose animals, the
histopathological examination showed a slight hyperkeratosis of the
skin at the site of treatment (Tai & Katz, 1984). The NOEL was
considered to be 1 mg/kg, based on inhibition of brain cholinesterase.
7.3 Long-term exposure
7.3.1 Rats
Groups of 30 or 40 Sprague Dawley male and female rats received
diazinon (87.7% pure) at dietary concentrations of 0, 0.1, 1.5, 125
and 250 mg/kg (equivalent to a mean daily diazinon intake of 0, 0.004,
0.06, 5 and 10 mg/kg body weight in males and of 0.005, 0.07, 6 and
12 mg/kg body weight in females) for 99 weeks. An additional control
group received 26.5 mg/kg diet epoxidized soybean oil, the stabilizer
used in technical diazinon, at the concentration corresponding to the
application to the test group of animals. Eight to ten randomly
selected animals per sex and dose group were killed after one year of
treatment, and nine or ten animals from the control group and from the
highest-dose group were killed after 4 weeks of recovery at week 56.
There were no compound-related clinical signs. At 250 mg/kg diet, the
mean body weight and the food consumption were slightly, but
significantly, increased in both sexes. Serum cholinesterase
activities were reduced at >1.5 mg/kg diet. Red blood cell and
brain cholinesterase activities were inhibited in groups fed on a diet
containing 125 or 250 mg/kg. Cholinesterase inhibition was at least
partially reversible during the 4-week recovery period after one year
of treatment. No treatment-related changes were seen during pathology
or histology examination (Kirchner et al., 1991). A pathological
re-evaluation of eyes and optic nerves from all control and high-dose
animals of the above study from interim necropsy (week 52), interim
recovery necropsy (week 56) and chronic necropsy (week 98/99) was
conducted. Histopathological lesions like cataracts, inflammatory
changes of the globe and surface of the eye and several different
types of retinal atrophy were found in both control and treated
animals at final sacrifice. None of these findings had an increased
incidence in treated rats compared to controls and they were not
regarded as compound-related. As the dietary concentration of
1.5 mg diazinon/kg, equivalent to a mean intake of 0.06 mg/kg body
weight per day, inhibited serum cholinesterase only, this dose level
was considered to be the NOAEL (Mann, 1993).
7.3.2 Dogs
Groups of Beagle dogs (four males, four females) received
diazinon (87.7% pure) for 52 weeks at dietary concentrations of 0,
0.1, 0.5, 150 and 300 mg/kg (equivalent to a mean daily intake of
0.0032, 0.015, 4.7 and 7.7 mg/kg body weight for males and 0.0037,
0.02, 4.5 and 9.1 mg/kg body weight for females). Owing to a general
lack of body weight gain, the highest dose level was reduced from
300 mg/kg diet to 225 mg/kg diet after 14 weeks of treatment. One male
dog from the highest-dose group showed emaciation and dehydration due
to severely reduced food consumption and weight reduction. In the
other animals from the highest-dose group (300 mg/kg/diet) the body
weight gain was also severely depressed during the initial 14 weeks of
treatment. After the reduction of dose to 225 mg/kg/diet, body weight
gain remained depressed in two of the males, while the females
returned to normal. A slightly reduced body weight gain was also noted
among males at 150 mg/kg diet. Food consumption was reduced in both
sexes fed on a diet containing >150 mg/kg diazinon. Haematology and
urine analysis showed no treatment-related changes. Significantly
decreased cholinesterase activities were noted at >0.5 mg/kg diet.
Serum cholinesterase activity was inhibited at 150 mg/kg diet at
all sampling intervals and at several occasions at 0.5 mg/kg diet. Red
blood cell and brain cholinesterase activities were reduced at
>150 mg/kg diet. In addition, a slight reduction in the mean serum
amylase activity was noted in both sexes fed a diet of >150 mg/kg.
The organ weight analysis showed no treatment-related changes and
macro- and histopathology were unremarkable (Rudzki et al., 1991). A
re-evaluation of eyes and optic nerves from all control and high-dose
animals from the study was conducted. No histopathological findings in
the eyes were noted (Mann, 1993). The dietary diazinon concentration
of 0.5 mg/kg, equivalent to a mean daily diazinon intake of
0.015 mg/kg, inhibited serum cholinesterase activity only, and was
considered to be the NOAEL, based on inhibition of brain and
erythrocyte cholinesterase.
7.3.3 Rhesus monkeys
This study was conducted with a 50% WP diazinon formulation
containing an actual concentration of 48.6% diazinon. The dose levels
given below refer to the active ingredient. Groups of three male and
three female Rhesus monkeys received initial daily doses of 0, 0.1,
1.0 and 10.0 mg diazinon/kg body weight, administered by gastric
intubation. After 34 days the doses were lowered to 0.05, 0.5 and
5.0 mg/kg and after 106 weeks of treatment the study was terminated.
Mortality was similar in all dose groups: the death of one animal
at each dose was of infectious etiology. Clinical signs included
tremor in the highest-dose group animals and an increased incidence
of soft stools was noted at 1.0 and 10.0 mg/kg. In comparison to
the controls, all treated animals gained slightly less weight.
Treatment-related deviations in the laboratory parameters examined
were limited to reductions of cholinesterase activities. At the
0.5 mg/kg dose level, the erythrocyte cholinesterase activity was
occasionally reduced in some animals and plasma cholinesterase
activity was consistently depressed. At the highest-dose level, plasma
and erythrocyte cholinesterase activities were markedly inhibited and
the brain cholinesterase activity was reduced in one monkey. The
postmortem examinations revealed no changes of toxicological
relevance. The daily dose of 0.5 mg diazinon/kg inhibited plasma and
(occasionally slightly) erythrocyte cholinesterase activity. The
toxicologically relevant brain cholinesterase activity remained
unaffected. Therefore, this dose level was considered to be the NOAEL,
based on inhibition of erythrocyte cholinesterase (Cockrell et al.,
1966).
7.4 Skin and eye irritation; sensitization
7.4.1 Primary skin irritation
Diazinon (0.5 ml undiluted, technical material) was applied to
the shaved skin of three male and three female New Zealand white
rabbits and covered with gauze patches and an impermeable material.
The dressing was removed after 4 h. Slight erythema and minimal oedema
were seen in all rabbits, which disappeared within 1 week. In the
report the compound received the descriptive rating as "slightly
irritant" to rabbit skin (Kuhn, 1989b,c).
7.4.2 Primary eye irritation
Diazinon (0.1 ml undiluted technical material) was instilled into
the conjunctival sac of three male and three female New Zealand white
rabbits. In three additional female rabbits, the treated eyes were
washed for 1´ min after the instillation of diazinon. Diazinon
produced mildly irritating reactions of the conjunctivae, which
disappeared within 72 h. It was rated as minimally irritating in
washed and unwashed eyes of rabbits (Kuhn, 1989d).
7.4.3 Skin sensitization
Diazinon was examined for skin sensitizing effects in 10 Hartley
Albino guinea-pigs. Ten animals were initially treated with 0.5 ml
undiluted technical diazinon, applied to the shaved skin under
semi-occlusive dressing for 6 h. After the third treatment one animal
died, most probably due to intoxication. Therefore, further treatments
were conducted with a 10% solution of diazinon in ethanol. After 11
treatments during the 3-week induction period and a 2-week rest phase,
no sensitization resulted after a single challenge administration
(Kuhn, 1989e).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
7.5.1.1 Rat
Diazinon (94.9% pure) was administered in the feed to groups of
30 male and 30 female Sprague Dawley rats for 10 weeks prior to
mating, throughout mating of the F0 animals and during two
generations up to weaning and sacrifice of the F2 pups. The dietary
concentrations used were 0, 10, 100 and 500 mg/kg. Compound-related
clinical signs and mortalities were limited to a few parental females
treated with 500 mg/kg diet. The food consumption was generally
comparable to, or slightly higher than, control values for females of
both parental generations in the diazinon-treated groups, while the
F1 males showed a decreased food consumption during the premating
period at both 100 and 500 mg/kg diet. The body weight increase was
reduced at 500 mg/kg in the F0 females and at 100 and 500 mg/kg for
the F1 animals of both sexes. There were no remarkable gross or
microscopic findings or effects on organ weights in either the F0 or
F1 generation. Reproductive parameters including precoital interval,
gestation duration, mating, fertility and pregnancy indices were
unaffected by the treatment in both generations at the dose levels of
10 and 100 mg/kg diet. At 500 mg/kg there was an increase in the
proportion of dams with prolonged gestation in both generations, and
in the F1 animals there was a trend toward a decrease in the number
of pregnancies and viable newborns and adverse effects on fertility
indices. Mating behaviour was unaffected by treatment. Litter size on
lactation day 0 was decreased in both the F1 and F2 pups at
500 mg/kg, whereas pup weight and sex ratio on day 0 were comparable
to controls for both generations. Decreases in pup survival and
corresponding decreases in pup weight were observed in both
generations at 500 mg/kg and in the F1 pups at 100 mg/kg.
Compound-related clinical and necropsy observations were noted in some
F1 pups at 500 mg/kg (tremor, no milk in stomach). NOAEL was 10 mg/kg
diet for pups and parental animals (Giknis, 1989).
7.5.1.2 Cattle
As a part of a survey to determine causes of abortion in
Wisconsin dairy cattle, the possible role of pesticides was examined.
Of 31 aborted fetuses examined, none contained traces of diazinon. Two
gravid, non-lactating Holstein Friesian cows were orally treated in
their feed with diazinon (Diazinon W 50 wettable powder formulation)
at daily doses of 6.6 mg/kg body weight during their second semester
of gestation until term. The calves were killed after parturition and
samples of perirenal fat, liver and kidney were investigated for
residues of diazinon, as were samples of colostral milk from the cows.
A histopathological examination of the calves, livers and kidneys was
conducted. Both cows treated with diazinon vomited and refused feed on
day 20 of treatment. No abortions occurred and no gross or
histopathological changes were found in the calves. No diazinon
residues were detected in the tissues investigated or in the milk
(Macklin & Ribelin, 1971).
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 Mice
Groups of 19-22 mated female F2 hybrid mice were orally treated
with diazinon (admixed to peanut butter, purity not specified) at
daily doses of 0, 0.18 and 9 mg/kg body weight from the day of mating
until delivery. The dams were weighed daily. After delivery, the
physiological and behavioural development of the pups was examined.
Mothers of all groups gave birth to viable offspring. Litter size and
the birth weights of the pups were similar in treated and untreated
groups. In the group receiving the higher dose of diazinon, the body
weight development of pups was depressed during their first postnatal
week. According to the authors, the testing of physiological and
behavioural development revealed subtle deviations from the normal
development in the offspring from the treated females. After sacrifice
at 101 days of age, the brains of the offspring of mothers treated at
the high-dose level showed ambiguous neuropathological changes in the
forebrains (Spyker & Avery, 1977). This study raises questions that
cannot be answered from the data presented.
7.5.2.2 Rats
Diazinon (purity 95%) was administered orally by gavage to groups
of 28 to 30 pregnant Sprague-Dawley-derived rats on days 6 to 15 of
gestation at dose levels of 0, 15, 50 and 100 mg/kg body weight On day
21 of gestation, all dams were killed and the fetuses delivered by
cesarean section. The dams of the 100 mg/kg group reacted to the
treatment by a marked decrease in food consumption and a body weight
loss in the early administration phase. The dams of the 15 and
50 mg/kg group showed no reaction. The parameters of reproduction
(number of corpora lutea, implantations, resorptions, fetal deaths and
viable fetuses) showed no treatment-related intergroup differences.
The fetal body weights were similar in all groups and the examination
of the offspring did not reveal any teratogenic effects of the
treatment. The NOAEL was 50 mg/kg body weight (Fritz, 1974).
In rats, doses (95.2 mg/kg body weight on day 9) that increased
maternal mortality reduced fetal development, as indicated by reduced
weight of litters and mild "hydronephrosis", but caused no real
teratogenic effect (Dobbins, 1967). A similar result was reported for
an intraperitoneal dosage of 100 and 150 mg/kg on day 11 (Kimbrough &
Gaines, 1968). Repeated administration (40, 50 or 60 mg/kg body weight
per day) on days 7 to 19 of gestation reduced the growth of the dams
but had no effect on the number of resorptions or corpora lutea, on
litter size, or on fetal weight. The cholinesterase activity of the
fetal brain was reduced. A dosage of 75 mg/kg body weight per day was
fatal to dams in 4 to 5 days (Hoberman et al., 1979).
Diazinon (97.4% pure) was administered by gavage to groups of 21
to 25 pregnant Sprague-Dawley rats from day 6 to 15 of gestation at
dose levels of 0, 10, 20 and 100 mg/kg body weight. On day 20 of
gestation, all dams were killed and the fetuses delivered by cesarean
section. In the highest-dose group of, dams a decreased food
consumption was noted during days 6-9 of gestation and the animals
lost weight. The body weight gain of the dams showed some recovery
thereafter, but the overall body weight gain of the highest-dose group
remained significantly below that of the untreated controls. The
numbers of corpora lutea and implantations were similar in all groups.
Resorptions were significantly increased only in the top-dose group
and, accordingly, the number of live fetuses was decreased. The fetal
body weights were higher in the top-dose group than in the untreated
controls. Three single instances of external malformations were
observed at the highest-dose level (one fetus with a filamentous tail,
one fetus with umbilical hernia and one fetus with sublingual
extraneous soft tissue). Since the malformations were not
morphologically related, they were considered to be secondary to
maternal toxicity. An increased incidence of rudimentary 14th ribs was
noted in the fetuses of the highest-dose group, although it remained
within the limits of historical controls. The finding was considered
to be related to fetotoxicity, secondary to severe maternal toxicity.
Other fetal anomalies were comparable between treated and untreated
groups. No evidence of teratogenic effect of diazinon was found
(Infurna & Arthur, 1985).
7.5.2.3 Hamsters
In Golden Syrian hamsters diazinon was administered as individual
oral doses (0.125 and 0.25 mg/kg body weight) during the period of
organogenesis. Technical grade diazinon diluted with corn oil was
used. The dose volume was 10 ml/kg body weight. Control animals
received corn oil on the same days of gestation. The hamsters were
killed on day 14 of gestation. All fetuses were examined for gross
defects when delivered by cesarean section. The viability of hamster
fetuses delivered on day 15 was checked by placing them in a chicken
hatching incubator for 6 h. All fetuses with enough developmental form
for determination of structural defects were counted in determining
the number of fetuses per litter. The total number of fetuses in each
treatment group was divided by the number of mothers surviving to
term. Fetuses dying in late embryonic life and resorption sites were
counted as dead fetuses. No bone defects were seen in four fetuses
chosen at random from each litter for staining and examination of
skeleton. Diazinon did not produce any terata when administered to
hamsters (Robens, 1969).
7.5.2.4 Rabbits
Robens (1969) reported that diazinon had not been found to be
teratogenic in rabbits given 7 or 30 mg/kg body weight, although the
higher dose of diazinon produced cholinergic signs. Diazinon
(89.2% pure) was administered orally by gastric intubation to groups
of 19 to 22 New Zealand white rabbits at daily doses of 7, 25 and
100 mg/kg body weight from day 6 to 18 of gestation. On day 30 of
gestation, all dams were killed and the fetuses delivered by cesarean
section. At the highest-dose level, 9/22 dams died and overt signs of
toxicity included tremors, convulsions, hypoactivity and anorexia.
There were no statistically significant differences among the group
regarding the mean number of implantations and the proportions of
live, dead or resorbed fetuses. The fetal weights were similar in
treated and untreated groups, and neither embryotoxicity nor
teratogenicity was observed (Harris & Holson, 1981).
7.5.2.5 Chicken
Several studies have investigated the teratogenic potential of
diazinon in chick embryos (Khera & Bedok, 1967; Misawa et al., 1981,
1982; Henderson & Kitos, 1982; Byrne & Kitos, 1983; Wyttenbach &
Hwang, 1984; Kushaba-Rugaaju & Kitos, 1985). In view of the lack of
teratogenicity in mammals and the lack of adequate data on
cholinesterase inhibition in ovo, these studies are not considered
relevant in the assessment of mammalian teratogenicity.
7.6 Mutagenicity and related end-points
The summary results of mutagenicity studies conducted with
diazinon are presented in Table 3.
Diazinon did not induce mutations in either Salmonella
typhimurium or Escherichia coli, but produced conflicting results
in mouse lymphoma L5178Y cells at the tk locus. Unscheduled DNA
synthesis was not induced in primary cultures of rat hepatocytes. A
sister chromatid exchange study with human lymphocytes cultured in
whole blood gave equivocal results, while negative results were
obtained in three other in vitro studies and, in vivo, in bone
marrow cells of dosed mice.
Chromosomal aberrations were not induced in cultured human
lymphocytes. In Drosophila melanogaster, diazinon did not induce
either complete or partial chromosome loss.
Table 3. Special studies on the mutagenicity of diazinon
Test system Test object Results Reference
In vitro
Ames Salmonella typhimurium negative Geleick & Arni (1990)
TA98, TA100, TA1535, TA1537,
TA1538; E. coli WP2 uvrA
Ames Salmonella typhimurium negative Marshall et al. (1976)
TA1535, TA1536, TA1537, TA1538
Mouse lymphoma assay Mouse lymphoma cells, negative Dollenmeier & Müller (1986)
L5178Y/tk +/-
Mouse lymphoma assay Mouse lymphoma cells positive McGregor et al. (1988)
L5178Y/tk +/-
Sister chromatid exchange study Chinese hamster cells V79 negative Chen et al. (1981)
Sister chromatid exchange study Human lymphoid cells (LAZ-007) negative Sobti et al. (1982)
Sister chromatid exchange study Chinese hamster V79 cells positive Matsuoka et al. (1979)
Sister chromatid exchange study Whole blood human lymphocytes equivocal Murli & Haworth (1990a)
Sister chromatid exchange study Chinese hamster V79 cells negative Kuroda et al. (1992)
Sister chromatid exchange study Chinese hamster V79 cells negative Nishio & Uyeki (1981)
Table 3. (con't)
Test system Test object Results Reference
Nucleus anomaly Chinese hamster V79 cells negative Hool & Muller (1981c)
Chromosomal aberration Human lymphocytes questionable Lopez & Carrascal (1987)
Chromosomal aberrations Human lymphocytes negative Lopez et al. (1986)
DNA repair test Rat hepatocytes negative Hertner & Arni (1990)
In vivo
Nucleus anomaly Chinese hamster bone marrow cells negative Hool & Müller (1981c)
Micronucleus test Mouse bone marrow cells negative Ceresa & Puri (1988)
Dominant lethal study Mouse, male negative Fritz (1975)
Chromosome aberrations Mouse spermatogonia negative Hool & Müller (1981d)
Chromosome aberrations Mouse spermatocytes negative Hool & Müller (1981b)
Chromosomal loss Drosophila melanogaster negative Woodruff et al. (1983)
Sister chromatid exchange study Mouse bone marrow cells negative Murli & Haworth (1990b)
Nuclear anomaly tests in cultured Chinese hamster V79 cells and
in bone marrow cells of dosed Chinese hamsters gave negative results.
A micronucleus test in bone marrow polychromatic erythrocytes of mice
was also negative.
No chromosomal aberrations were induced in the spermatogonia or
spermatocytids of dosed mice and a dominant lethal test in dosed male
mice was negative. It was concluded that diazinon is not genotoxic.
7.7 Carcinogenicity
7.7.1 Mice
Groups of 50 B6C3F1 mice of each sex were treated with diazinon
(98% pure) at dietary concentrations of 100 and 200 mg/kg diet for
103 weeks. Two additional groups of 25 males and 25 females each
served as untreated controls. Hyperactivity was reported for the dosed
mice but was rare in the control group. The body weight gains for all
dosed male mice were similar to the control group except for the last
20 weeks of the study period. The treated females showed slightly
lower body weights than the controls. The survival at 78 weeks in the
control 100 and 200 mg/kg groups, respectively, was: males, 21/25
(84%), 45/50 (90%), 49/50 (98%); females, 24/25 (96%), 50/50 (100%),
49/50 (98%). Several neoplasms were observed, but apart from lower
neoplasms in male mice none appeared to be treatment-related or had an
incidence in treated groups significantly different from that of
controls. The incidences of hepatocellular carcinomas in the control,
100 and 200 mg/kg groups, respectively, were: 4/21 (19%), 20/46 (43%)
and 10/48 (21%). The corresponding incidences of hepatocellular
carcinomas or adenomas were 5/2 (24%), 20/46 (43%) and 13/48 (27%).
The Cochrane-Armitage test for trend was significant for carcinomas,
but not for liver carcinomas and adenomas combined. Fisher's exact
test for comparison of a dosed group with its matched control groups
was significant (P=0.046) only for hepatocellular carcinomas alone in
the 100 mg/kg group of male mice. In view of the lack of a dose-
related response, the occurrence of liver tumours in male mice could
not be clearly attributed to diazinon (NCI, 1976).
A report submitted to the United Kingdom Ministry of Agriculture,
Fisheries and Food (MAFF, 1991) described a two-year combined chronic
toxicity/carcinogenicity study conducted with male and female B6C3F1
mice (60/dose/sex) administered diets containing diazinon at 0, 100,
200 or 300 mg/kg (males only) or 400 mg/kg (females only). Ten mice of
each sex per group were killed at 12 months. After 24 months, there
were reported to be no treatment-related gross or histopathological
findings. This study provides no evidence for the carcinogenicity of
diazinon in mice. In particular, it does not confirm the observations
described in the above-mentioned study on B6C3F1 mice.
7.7.2 Rats
Groups of 50 male and 50 female Fischer-344 rats received
diazinon (98% purity) at dietary concentrations of 400 and 800 mg/kg
for 103 weeks. All survivors were killed at 105 weeks. An additional
control group of 25 males and 25 females received an untreated diet.
There was no significant effect of treatment upon body weight gain.
Hyperactivity was noted in males and females of the 800 mg/kg group
and males of the 400 mg/kg group. Urine was discoloured in females of
the 800 mg/kg group and in dosed females there was vaginal bleeding
and discharge. Survival at 78 weeks in control, 400 mg/kg and
800 mg/kg groups, respectively, were: males, 24/25 (96%), 49/50 (98%),
49/50 (98%); females, 23/25 (92%), 44/50 (88%), 44/50 (88%). Various
neoplasms were observed, but only the incidence of lymphomas/
leukaemias in dosed groups of males was significantly different from
the controls. The incidences in the control, 400 and 800 mg/kg groups,
respectively, were: males, 5/25 (20%), 25/50 (50%), 12/50 (24%);
females, 2/25 (8%), 6/50 (12%), 6/50 (12%). The Fisher's exact test
for comparison with the matched control was significant (P=0.011) only
in the 400 mg/kg group of males. In view of the lack of a dose-related
response, the occurrence of lymphomas/leukaemias in male rats could
not be clearly attributed to diazinon. The frequency of endometrial
stromal polyps was increased in the dosed groups. This lesion, which
is commonly observed in Fischer-344 rats, occurred at incidences in
the control, 400 mg/kg and 800 mg/kg groups, respectively, of 2/23
(9%), 8/43 (19%) and 11/49 (22%). The carcinogenic status of diazinon
in this study is unclear (NCI, 1979).
The chronic toxicity study of Kirchner et al. (1991) described
earlier (section 7.3.1) included groups of male and female Sprague-
Dawley rats (approximately 20/dose/sex, progressing for longer than 57
weeks) administered diets containing diazinon at concentrations 0,
0.1, 1.5, 125 or 250 mg/kg. No treatment-related increases in
neoplasms were observed. This study does not provide supportive
evidence for a carcinogenic effect of diazinon in rats.
A report submitted to the United Kingdom Ministry of Agriculture,
Fisheries and Food (MAFF, 1991) described a 120-week combined chronic
toxicity/carcinogenicity study conducted with male and female F-344
rats (75/dose/sex) administered diets containing diazinon (0, 0.1, 1.5
or 22.6 mg/kg body weight per day). Rats of both sexes in the highest-
dose group that died late in the study (after week 105) showed
increased ulceration of the forestomach, with associated increased
incidence of acanthosis, hyperkeratosis, submucosal granulation tissue
and hyperplasia of the epithelium. Apparently, no neoplastic lesions
were observed in the study. This study provides no evidence for the
carcinogenicity of diazinon in rats. In particular, it does not
confirm the observations described in the above-mentioned study on
F-344 rats.
It is concluded that diazinon is not carcinogenic in rats or
mice.
7.8 Special studies
7.8.1 Neurotoxicity
In a range-finding experiment, groups of four domestic hens (red
heavy breed) were treated at four dose levels of diazinon (87% pure),
and an acute oral LD50 of 12.5 mg/kg body weight was determined. A
schedule was developed to protect the hens from acute cholinergic
effects. Atropine (10.0 mg/kg intramuscular) was administered prior to
the treatment with diazinon, and 2-PAM was injected at doses of
50 mg/kg at the time of the diazinon administration. In addition, the
hens received post-treatment protection with concurrent doses of
atropine and 2-PAM after approximately 1 and 5 h and as necessary
thereafter. In the main neurotoxicity study, diazinon was given as a
single dose of 28 mg/kg body weight by gastric intubation to a group
of 18 atropine-pretreated hens. All hens were observed for three weeks
and again treated with 13 mg diazinon/kg on day 21. The second
observation period also lasted for three weeks (day 22-42). Additional
groups, which served as negative controls, were given the vehicle
(10 hens treated with 1 ml/kg corn oil), and eight hens, treated with
tri- o-tolyl phosphate (500 mg/kg) served as positive controls. One
diazinon-treated hen died 5 days after the first dose and another one
6 h after the second dose. One hen from a negative control group died
on day 7. There were no neurotoxic signs apparent during either of the
two observation periods in the negative control group or in the
treated groups. No histopathological changes in the brain, spinal cord
or peripheral nerves were detected (Jenkins, 1988).
In a further test for delayed neuropathy Classen (1996) gave 20
hens a single oral dose of 100 mg diazinon/kg body weight. Therapeutic
treatment with physostigmine and atropine for up to 48 h resulted in
survival of 17 hens, despite a peak inhibition of brain cholinesterase
by 83%. No ataxia was seen over a 28-day survival period, and no
inhibition of either brain or spinal cord neuropathy target esterase
(NTE) was seen at either 24 (n=5) or 48 (n=5) h.
Diazinon (88% purity) was administered by gavage to 15 rats per
sex and dose at single doses of 0, 2.5, 150, 300 and 600 mg/kg. A
negative control group of 15 animals per sex received the vehicle
(corn oil) only. A group of 10 animals per sex served as positive
control and was administered a single dose of 150 mg/kg triademefon
60 min prior to the Functional Observational Battery (FOB). Two males
and one female dosed at 600 mg/kg died. A transient reduction in body
weight and body weight gain was observed in males dosed at 300 and
600 mg/kg during week one. Food consumption was reduced in the same
males, and in females dosed at >150 mg/kg. These findings decreased
in incidence and severity with decreasing dose. No treatment-related
effects were observed in the FOB on study days 8 and 15. In males
dosed above 150 mg/kg and in females dosed above 2.5 mg/kg, activity
counts in the figure-8 maze were significantly decreased at the
estimated time of peak effect only. Serum cholinesterase activity was
significantly decreased at the time of peak effect in all diazinon-
treated groups. Full recovery was observed in all groups on study day
15. Red blood cell cholinesterase activity was significantly inhibited
on study day 1 in animals of both sexes treated with >150 mg/kg.
Substantial recovery was observed on study day 15, but significant
inhibition still remained. No inhibition of brain cholinesterase
activity was detected on day 15. No neuropathological effects were
noted for diazinon at necropsy or upon histopathological examination.
It was concluded that the administration of diazinon resulted in
reversible neurotoxicity and cholinergic poisoning without
neuropathological changes. The NOAEL was 2.5 mg/kg body weight for
both sexes (Chow & Richter, 1994).
7.8.2 Effects on enzymes and transmitters
Male rats were treated bi-weekly by gavage with the equivalent of
0.5 mg/kg per day technical diazinon for up to 28 weeks. The animals
were killed at specific time intervals (7, 14 and 28 weeks) and
compared with age-matched controls. Blood and brain tissues were
analysed for cholinesterase activity and for concentrations of
catecholamines and amino acids. Only plasma cholinesterase activity
was significantly reduced. Erythrocyte cholinesterase and brain
cholinesterase were unchanged while during the same period the levels
of several putative brain neurotransmitters, aspartate, glutamate
(excitatory) and taurine as well as GABA (inhibitory) were
significantly reduced in experimental versus control animals. Blood
serotonin level was significantly elevated but no other blood or brain
monoamines were significantly altered. Whilst the authors (Rajendra et
al., 1986) concluded that oral administration of diazinon exerts
effects on brain neurotransmitters, even at the low-dose levels
administered in this study, the biological significance of these
findings is unclear.
Intraperitoneal administration of diazinon diminishes formation
of L-kynurenine by liver slices from 93% at 1 mg/kg dose to 56% at
40 mg/kg. The ratio of critical intermediates in L-tryptophan
biosynthetic pathway, L-kynurenine to N-formyl-L-kynurenine,
decreases and is reversed with increasing dosage of diazinon (Seifert
& Cassida, 1979).
Diazinon altered the formation of several L-tryptophan
metabolites associated with the L-kynurenine pathway in mice. Liver
kynurenine formamidase was inhibited almost completely by diazinon
(10 mg/kg). The enzyme inhibition resulted in reduced L-kynurenine
biosynthesis in livers and a concomitant accumulation of N-formyl-L-
kynurenine. However, in plasma, L-kynurenine level increased up to
5-fold in diazinon-treated mice. Consequently, the urinary excretion
of xanthurenic acid and kynurenic acid was raised 5- to 15-fold. The
revelation of this novel mechanism of diazinon action is an important
piece of information needed for a better understanding of the non-
cholinergic toxicity of organophosphorus acid triesters and
methylcarbamates (Seifert & Pewnim, 1992).
7.8.3 Effects on the immune system
Pregnant F2 dihybrid female mice received either a vehicle or 1
of 2 doses of diazinon (0.18 (n=19) or 9.0 (n=21) mg/kg body weight)
in the diet, daily throughout gestation. All mothers gave birth to
viable, overtly normal offspring at term. However, a significant
number (12%) of pups born to dams who received 9.0 mg/kg died prior to
weaning on day 28; necropsy findings were consistent with death from
respiratory infection. There was no significant difference in
mortality between control and diazinon-treated offspring once they
reached 28 days of age. Determinations of five different classes of
serum immunoglobulin (Ig) concentrations (IgG1, IgG2a, IgG2b, IgA,
IgM) at 101, 400 and 800 days of age indicated transient but
consistent disturbances of 2 Ig classes in offspring as a result of
prenatal diazinon exposure. IgG1 concentrations of male offspring
exposed to 0.18 mg/kg were significantly elevated at 101 days but not
at 400 or 800 days of age. IgG1 concentrations of female offspring
exposed to 9.00 mg/kg were significantly depressed at 101 days but not
different from controls at 400 or 800 days of age. Changes in IgG2b
levels generally were similar to those recorded for IgG1 but of
smaller magnitude. There were no significant effects on serum IgG2b or
IgM concentrations, and only equivocal effects on IgA, as a
consequence of prenatal exposure to either pesticide. This study
offers no conclusive information of an effect of diazinon on the
immune system (Barnett et al., 1980).
7.8.4 Effect on pancreas
Diazinon has been reported to cause acute pancreatitis and
ductal hypertension in dogs, which is due to the absence of
acetylcholinesterase in pancreatic sphincter, duodenal smooth muscle
in dogs and a reliance upon the more readily inhibited
butyrylcholinesterase (Dressel et al., 1980; Frick, 1987).
Kazacos (1991) examined the toxic effects of diazinon on the
guinea-pig pancreas. Guinea-pigs were given single intravenous dose of
diazinon at concentration ranging from 125 to 200 mg/kg body weight.
Examination of pancreatic tissue from animals killed at various time
intervals after dosing revealed acinar cell vacuolization after 24 h.
This cellular injury disappeared within 3 days after the initial toxic
insult. These are considered to be species-specific effects.
7.9 Factors that modify toxicity; toxicity of metabolites
7.9.1 Metabolic enzymes
Abdelsalam & Ford (1986) found that the toxic effects of diazinon
were increased by pretreatment with the hepatic microsomal enzyme
inducers dieldrin and phenobarbitone and that, at the same time, there
was a rise in the liver carboxylesterase activity. In this experiment,
the toxicity of diazinon increased when calves were pretreated with
dieldrin or phenobarbitone despite the increased activity of the liver
carboxylesterase. Apparently carboxylesterase activity was not
sufficient to protect against the toxicity of diazinon in the
pretreated calves. This suggests that either the increased
carboxylesterase activity had only a minor role in the hydrolytic
detoxification of diazinon or active metabolites, or that its effect
might have been overwhelmed by the action of other microsomal oxidase
activity concurrently induced by dieldrin and phenobarbitone leading
to a faster rate of activation than degradation of diazinon.
Abdelsalam & Ford (1987) described the effect of induced liver
and kidney lesions on the toxicity of levamisole and diazinon, two
antihelmintics routinely used in ruminant livestock management, and of
a lung lesion on the toxicity of diazinon in calves. Hepatic or renal
damage such as may occur naturally as a consequence of infection or of
the ingestion of poisonous plants or other toxic substances is,
therefore, likely to affect the metabolism and excretion of drugs
routinely used in antihelmintic control programmes. Liver damage in
calves, induced by the oral administration of the flukecide, carbon
tetrachloride, increased the toxic effect of diazinon but not of
levamisole, whereas the presence of a renal tubular lesion caused by
mercuric chloride enhanced the toxicity of both commonly used
antihelmintic compounds.
7.9.2 Antidotes
The effects of atropine/oxime therapy on diazinon-poisoned
animals was investigated in rats and rabbits. The rats were orally
treated with 235 mg/kg body weight (0.8 LD50) of diazinon (91.9%
pure), while rabbits received 1600 mg diazinon/kg body weight by the
subcutaneous route. In both species, intramuscular administration of
16 mg/kg atropine (10 min post treatment), and 30 mg/kg of pyridine
2-aldoxime methochloride (2-PAM Cl) 24 h later, significantly
increased the reactivation of the diaphragm cholinesterase. The oral
LD50 for diazinon in the rat was increased by a factor of 1.7 when
2-PAM Cl was administered intravenously and 3.1 times when 2-PAM Cl
was given orally. In order to prevent the reappearance of signs the
authors recommended that 2-PAM Cl be administered intravenously at the
same time as atropine followed by repeat oral administrations, as
needed (Harris et al., 1969).
The antidotal efficacy of pralidoxime iodide and obidoxime
dichloride was investigated in goslings poisoned by a supralethal dose
of diazinon. Various doses of both drugs were administered by
intramuscular injection when the poisoned birds were unable to walk.
Pralidoxime at 100 mg/kg induced recovery in 4 out of 6 poisoned
goslings, and 25 mg/kg successfully treated only 1 of 6 birds.
Obidoxime at 25 mg/kg showed no therapeutic properties, whereas 50
and 100 mg/kg delayed the death of some birds by several hours. At
100 mg/kg, all goslings had transient signs of intoxication, which
precluded the use of this compound as an antidote at higher doses
(Shlosberg et al., 1976).
7.9.3 Potentiation
In a study of the potentiation of the acute toxicity of diazinon
by several other pesticides (chlordimeform, iodofenphos, profenphos,
methacrifos), there was no potentiation at equitoxic doses (Sachsse &
Bathe, 1975, 1976, 1977, 1978).
8. EFFECTS ON HUMANS
8.1 Exposure of the general population
8.1.1 Acute toxicity, poisoning incidents
Several cases of intentional (Kabrawala et al., 1965; Banerjee,
1967; Wadia et al., 1974; Klemmer et al., 1978; Poklis, 1980; Wedin et
al., 1984; Hodgson & Smith, 1992) and accidental acute poisoning with
diazinon have been reported. Accidental poisonings were usually due to
ingestion of improperly stored liquid formulation (Hayes, 1963;
Zwiener & Ginsburg, 1988; Weizman & Sofer, 1992; Hodgson & Smith,
1992). Cases of poisoning after cutaneous application of diazinon for
lice treatment have also been described (Muratore et al., 1960; Hayes,
1963; Halle & Sloas, 1987). Several accidents involved children (Mizra
et al., 1972; English et al., 1970; Zwiener & Ginsburg, 1988; Hodgson
& Smith 1992; Weizman & Sofer, 1992; Wagner & Orwick, 1994).
Generally, symptoms and signs were typical of AChE inhibition,
which responded to atropine and oxime treatment. In a case of fatal
suicidal ingestion of diazinon reported by Poklis et al. (1980),
diazinon concentrations in postmortem body fluids and tissues were
found to be 277 mg/litre in blood, 200 mg/litre in the bile and
15 mg/kg in adipose tissue. In the stomach 44 mg were found. Cases
with atypical or unusual features are described in section 8.1.1.3. No
cases of delayed polyneuropathy have been observed, as would be
expected from the negative animal data. The following sections deal
with some specific aspects of acute poisoning by diazinon (i.e., the
acute pancreatitis, the intermediate syndrome, and some unusual case
reports).
8.1.1.1 Acute pancreatitis
Increased levels of serum amylase and glucose have been described
in some severe cases of diazinon poisoning. In certain cases these
enzymatic alterations were accompanied by prominent abdominal symptoms
and signs, including abdominal rigidity. All these were considered
indicative of acute pancreatitis (Dressel et al., 1979; Dagli et al.,
1981; Lee, 1989; Weizman & Sofer, 1992). On one occasion a pancreatic
cyst was observed (Dressel et al., 1979). In all cases, pancreatitis
was mild and patients fully recovered. Severe poisoning with other
organophosphates, e.g., parathion (Weizman & Sofer, 1992), coumaphos
(Lee, 1989) and malathion (Lee, 1989), was also associated with acute
pancreatitis. Apparently, poisoning with an unknown carbamate was also
associated with acute pancreatitis (Weizman & Sofer, 1992).
8.1.1.2 Intermediate syndrome
Senanayake & Karalliedde (1987) described a syndrome caused by
organophosphate pesticides and named it "intermediate", because its
onset was delayed after cholinergic syndrome but appeared earlier than
polyneuropathy. The syndrome, appeared 24-96 h after poisoning during
recovery from the cholinergic crisis. It was characterized by
paralysis of proximal limb muscles, neck flexors, motor cranial nerves
and respiratory muscles. It was resistant to atropine treatment and,
in certain patients, required assisted ventilation. It is noteworthy
that Wadia et al. (1974), reporting the neurological manifestations
seen in 200 consecutive cases of poisoning, mostly with diazinon,
described two different types of signs: type I, characteristic of the
cholinergic syndrome and responsive to atropine, and type II, present
in about 20% of the cases, which appeared at least 24 h later,
resembled those of the intermediate syndrome and were not responsive
to atropine (Wadia et al., 1974).
Other cases of intermediate syndrome due to diazinon poisoning
have been described (Hall & Baker, 1989; Samal & Sahu, 1990).
8.1.1.3 Unusual case reports
Three-week-old twins were hospitalized because of respiratory
distress. One was cyanotic on admission, but both had rapid shallow
breathing, profuse nasal and bronchial secretions, and pinpoint
pupils; muscle fasciculations were not detectable. At 48 h only the
sicker twin had slightly reduced pseudocholinesterase activity.
Treatment was appropriate and recovery uneventful. Investigation
revealed that the babies lived in one side of a two-story house that
had been divided into two apartments by partitions. About 10:30 h on
the previous day the other apartment had been sprayed with 1% diazinon
for cockroach control using approved spot applications directed mainly
at cracks. The twins were the only ones who had remained indoors. The
observed degree of respiratory distress in the presence of little or
no inhibition of cholinesterase was consistent with exclusively
respiratory exposure (English et al.1970).
A 12-week-old infant girl developed persistent hypertonicity of
the extremities without other signs of intoxication. It was discovered
that the organophosphate insecticide diazinon was applied in her
home 5 weeks prior to the onset of signs. Six months after
application, high levels of diazinon residues were found on the floor
(230 ng/cm2), in vacuum cleaner dust (1700 mg/kg), and in the air
(2.8 ng/m3). A diazinon dose of approximately 0.02 mg/kg per day was
calculated and derived from the infant's urine level of diazinon
metabolites determined 6 months after application of diazinon in her
home. Her muscle tone returned to normal shortly after the infant was
removed from the home (Wagner & Orwick, 1994).
According to Hata et al. (1986), atypical ocular bobbing resulted
from an intentional poisoning from diazinon. The authors suggest
acetylcholine as a neurotransmitter substance within the ocular motor
pathway.
A 26-year-old man, who ingested approximately 230 ml of a
solution of an unknown concentration of diazinon in a suicide attempt,
developed a severe cholinergic syndrome which was relived by atropine
and 2-PAM. Diuresis was greatly reduced (22 ml/h) and urine was dark
and cloudy (specific gravity 1.029). It is possible that significant
dehydration may have precipitated this reaction (Wedin et al., 1984;
Albright, 1984).
8.1.2 Controlled human studies
A cumulative toxicity study was conducted with human volunteers.
Four healthy males weighing between 74 and 96 kg and aged between 30
and 45 years ingested diazinon in gelatin capsules (95.4% pure) at a
dose of 0.025 mg/kg per day. Two males received 34 consecutive
treatments (Group B), while the two other volunteers were treated for
4 days, were left untreated for the next 5 days in order to
investigate reversibility of the effects and then received the
capsules again for 32 more days (Group A). The daily dose was split
into three administrations taken after meals around 9, 13 and 19 h.
Parameters of haematology, urine analysis and blood biochemistry
(plasma and erythrocyte cholinesterase, serum alkaline and acidic
phosphatase activities) were determined at regular intervals. A
reversible decrease of plasma cholinesterase activity was observed in
group A during the first 4 days of treatment while the erythrocyte
cholinesterase activity remained unaffected. During the second
treatment period of group A and during the entire treatment of group
B, the cholinesterase activities were similar to those observed at
pretest. No clinical signs or changes in other parameters were noted.
It was concluded that the administered daily dose of 0.025 mg/kg
marginally inhibited the plasma cholinesterase only, and can therefore
be considered as a NOAEL in humans (Payot, 1966).
8.2 Occupational exposure
8.2.1 Acute poisoning
Poisoning following occupational exposure to diazinon was usually
associated with improper or prolonged storage of the commercial
formulations, which, prior to the improvements carried out by the
manufacturer, tended to give rise to more toxic impurities (mainly
TEPP).
Soliman et al. (1982) investigated two spraymen who had a
cholinergic syndrome (with about 90% red blood cell AChE inhibition)
after using a commercial formulation of diazinon which was packaged in
tin-plated cans that had replaced the more expensive aluminium cans.
Analysis of such commercial formulation revealed the presence of TEPP,
sulfo-TEPP, monothiono-TEPP and other impurities, but no diazinon.
Mello et al. (1972) reported an episode where three farmers were
poisoned when using a commercial formulation of diazinon that was
transferred from the original container to another container and then
stored for some time. This formulation contained monothiono-TEPP and
was about 30 times more toxic to rats than a recently prepared and
properly stored formulation. On that occasion, 26 of 40 cows died when
treated against tick infestation with this formulation.
A fatal case of poisoning by diazinon and malathion, possibly by
inhalation, was described by Wecker et al. (1985). The 51-year-old man
was found unconscious in the closed shed where he sprayed three cows
with the pesticides.
Two cases of cutaneous hepatic porphyria of toxic origin were
identified within a short period of time. Both patients were farmers
and recently handled very intensively, without any precaution, some
pesticides containing organophosphorus substances (diazinon). The
symptoms were similar to those of the so-called Turkish porphyria
(Bopp & Kosminsky, 1975).
8.2.2 Effect of short-term and long-term exposure
Neurophysiological investigations and determinations of blood
cholinesterase activities were carried out on 11 Swedish spraymen
exposed to bromophos, diazinon, dursbane and malathion. Plasma
cholinesterase activity was significantly reduced after work, while
erythrocyte cholinesterase activity was unchanged. In none of the
workers with a decreased plasma cholinesterase activity after work
could any related acute neuromuscular disturbance be detected when
the men were tested with repetitive nerve stimulation and with single
fibre electromyography. Signs of subclinical neuropathy were present
as a slight reduction in sensory conduction velocity and increased
fibre density in some workers (Stalberg et al., 1978).
A cohort of 99 workers exposed to diazinon was tested before and
after the work shift with a neurobehavioural test battery, which
included a brief examination, a symptom questionnaire and tests of
concentration, eye-hand coordination, pattern recognition, visual
memory and finger tapping. The median diazinon exposure level was
2.1 mg/day and the mean duration of exposure was 39 days (see section
5.3). There was no correlation between the diethylthiophosphate (DETP)
concentrations or diazinon exposure and pre- or post-shift
neurobehavioural function with linear regression models after
adjusting for age, sex, education and alcohol intake. The study failed
to demonstrate adverse behavioural effects of repeated, low-level
diazinon exposure (Maizlish et al., 1987).
Three groups of agricultural workers with a history of exposure
to organophosphate pesticides were followed up to evaluate the utility
of sequential post-exposure cholinesterase analyses to confirm
organophosphate intoxication in the absence of baseline cholinesterase
values. Three or more cholinergic symptoms were reported by 50 of the
72 patients. Pre-exposure red blood cell cholinesterase activities of
45 workers were above the lower limit of laboratory normal range.
Follow-up examinations, including blood cholinesterase activity
analyses, were conducted in 57 subjects. When final post-exposure
cholinesterase activity determinations were compared with respective
individual normal baseline values, the plasma and red blood cell
activities were shown to be inhibited. The data support the use of
sequential post-exposure blood cholinesterase analyses to confirm the
diagnosis of organophosphate-induced illness in the absence of
baseline values (Coye et al., 1987).
Of 67 described poisoning incidents in California involving 583
people, nineteen involved a pesticide product containing an
organophosphate: most often chlorpyriphos (8), diazinon (3) and
malathion (5). There were also 10 cases that resulted from suicide,
and two cases involved diazinon (Maddy & Edmiston, 1988).
In a study of organophosphate-induced contact dermatitis in 202
patients in Japan, Matsushita et al. (1985) attributed the reactions
mainly to diaoxabenzafos, fenitrothion, leptophos, cyanophos, diazinon
and malathion. The areas affected by dermatitis were fingers (62.4%),
face (39.6%), forearm (31.6%) and neck (29.7%). One quarter of the
cases with dermatitis had symptoms associated with acute
organophosphate poisoning.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Singh (1973) found that three genera of cyanobacteria (blue-green
algae) tolerated diazinon at concentrations of 300 and 400 mg/litre.
Clegg & Koevening (1974) indicated that the population densities of
three species of freshwater algae were relatively unaltered by a
concentration of 100 mg/litre. Butler et al. (1975) demonstrated that
diazinon inhibited growth of numerous species of green algae and
cyanobacteria at concentrations of 0.01 mg/litre and 0.1 mg/litre.
Wong & Chang (1988) studied the effects of diazinon on the growth of
the green alga, Chlamydomonas reinhardtii. They observed a reduction
in growth at concentrations of 5 and 10 mg/litre and complete
inhibition at 20 and 40 mg/litre.
Doggett & Rhodes (1991) determined the effects of diazinon on
phytoplankton population dynamics. The objectives of this study were
to determine the effects of diazinon (1, 5, 10, 20 and 40 mg/litre
concentration) on the growth rates of three widely distributed species
of freshwater algae, and to ascertain the effects of this pesticide on
the diversity of a natural phytoplankton assemblage. Stimulation of
growth was exhibited by Selenastrum capricornutum and Chorella
exposed to diazinon at 1 and 5 mg/litre. The higher concentrations of
10, 20 and 40 mg/litre all inhibited growth. The growth rates of the
cyanobacteria showed a high degree of tolerance; a suppression of
growth was observed only at 40 mg/litre. Two genera of green algae
were stimulated by all concentrations.
The acute toxicity of diazinon to the freshwater rotifer
Brachionus calyciflorus was determined after 24 h of exposure; the
mean LC50 value was 29.2 mg/litre. Based on this result, four
sublethal concentrations were chosen to determine the median lethal
time (LT50) at each concentration of diazinon tested. The
concentrations tested were 1/5, 1/4, ´ and 2/3 of the LC50 (24 h).
The LT50 values ranged from 6.96 to 2.49 days after diazinon
exposure, decreasing with increasing exposure concentration
(Fernandez-Casalderrey et al., 1992).
Several species of soil-borne fungi invade plant roots forming
vascular-arbuscular mycorrhizae which aid in growth and nutrient
element absorption. Pre-plant incorporated treatments at 2 and 4 kg/ha
of trifluralin and diazinon had no significant effect on growth,
phosphorus accumulation or root colonization by mycorrhizal fungi in
soybeans planted in an Andover clay loam. At currently used commercial
rates, diazinon did not affect mycorrhizal development under the
conditions of the experiment (Burpee & Cole, 1978).
Nitrogenase activity of excised soybean nodules was severely
affected by diazinon following application of 3, 1, 0.5 and 0.25
multiples of the amounts stated on the label (Hensley, 1991).
9.2 Aquatic invertebrates
Acute toxicity of diazinon to aquatic invertebrates is summarized
in Table 4.
The effect of diazinon (Basudine, 0.01, 0.1, 1.0 mg/litre) on the
physical and chemical properties of haemolymph of Lymnaea stagnalis
(L.) and Planorbius corneus (L.) infected with trematodes was
studied by Stadnichenko et al. (1987). Haemolymph viscosity and
density decreased after the treatment.
Robertson & Mazzella (1989) determined the toxicity of diazinon
to the freshwater snail Gillia altilis. Based on nominal
calculations, the LC50 values for static 4- and 96-h exposures were
340 mM (93 mg/litre) and 40 mM (11 mg/litre), respectively.
Experiments were conducted to determine the effects of piperonyl
butoxide, a synthetic methylenedioxyphenyl inhibitor of cytochrome(s)
P-450, on the toxicity of organophosphate insecticides to three
cladoceran test species: Ceriodaphnia dubia, Daphnia magna, and
Daphnia pulex. The acute toxicity of diazinon to three tests species
was similar, with LC50 values ranging from 0.50 to 0.80 mg/litre.
Co-administration of piperonyl butoxide effectively reduced the acute
toxicity of metabolically activated diazinon, and piperonyl butoxide
concentrations of 500 or 1000 mg/litre completely blocked the toxicity
of diazinon at concentrations of up to 16 times the 48-h LC50. In the
case of D. pulex, piperonyl butoxide at 500 mg/litre also
significantly reduced the toxicity of diazinon at concentrations of up
to 16 times the 48-h LC50 (Ankley et al., 1991).
9.3 Fish
The acute toxicity of diazinon to fish is summarized in Table 5.
The median lethal concentration (LC50) of diazinon was
determined for Clarias batrachus; the LC50 for 40 days of exposure
to diazinon was 2.4 mg/litre (Tripathi, 1992). The acute toxicities
(24, 48, 72 and 96 h) of eight pesticides to Anguilla anguilla were
determined. The 24- to 96-h LC50 values for diazinon ranged from 0.16
to 0.08 mg/litre (Ferrando et al., 1991).
Bresch (1991) studied the effects of diazinon on early
life-stages in zebrafish. Zebrafish kept at diazinon concentrations of
0.2, 0.04 and 0.008 mg/litre grew alike, and no differences among the
groups were observed. Survival rates of animals of different groups
did not differ either. In no groups were abnormal fish seen.
Table 4. Acute toxicity (LC50) to aquatic invertebrates
Species Duration (h) Concentration (mg/litre) Reference
Snail (Gillia altilis) 96 11 Robertson & Mazzella (1989)
Cladocerans 48 0.0005-0.0008 Ankley et al. (1991)
Cladoceran (Daphnia pulex) 48 0.001 (0.0006-0.0011) Mayer & Ellersieck (1986)
Cladoceran (Simocephalus serrulatus) 48 0.0014 (0.0012-0.0016) at 21°C Mayer & Ellersieck (1986)
0.0018 (0.0014-0.0022) at 15°C
Scud (Gammarus fasciatus) 96 0.0002 (0.00015-0.00028) Mayer & Ellersieck (1986)
Shrimp (Hyallela azteca) 96 0.004-0.006 Collyard et al. (1994)
Desmocaris trispimosa 96 0.0208 (0.0192-0.0224) Ebere & Akintonwa (1992)
Shrimp (Palaemonetes africanus) 96 0.0179 (0.0147-0.020) Ebere & Akintonwa (1992)
Insect larva (Pteronarcys californica) 96 0.025 (0.020-0.030) Mayer & Ellersieck (1986)
Table 5. Acute toxicity (LC50) to fish
Species Duration (h) Concentration (mg/litre) Reference
Bluegill sunfish (Lepomis macrocharis) 96 0.17 (0.012-0.22) Mayer & Ellersieck (1986)
Rainbow trout (Oncorynchus mykiss) 96 0.09 Mayer & Ellersieck (1986)
Cutthroat trout (Salmo clarkii) 96 2.76 (2.28-3.33) in soft water Mayer & Ellersieck (1986)
1.7 (1.39-2.09) in hard water
Walking catfish (Clarias batrachus) 2.41 Tripathi (1992)
Eel (Anguilla anguilla) 96 0.08 (0.06-0.10) Ferrando et al. (1991)
Channel catfish (Channa punctatus) 96 3.1 Sastry & Malik (1982a)
Goby (Gobius) sp. 96 0.25 (0.23-0.28) Ebere & Akintonwa (1992)
Lake trout (Salvelinus namaycush) 96 0.6 (0.4-0.9) Mayer & Ellersieck (1986)
Table 6. Summary of brain acetylcholinesterase activity inhibition by
diazinon in aquatic organisms
Organism Exposure Duration Inhibition Reference
concentration (h) (%)
(mg/litre)
Bluegill 0.1 6 95 Weiss (1961)
Fathead minnow 0.1 18 70 Weiss (1961)
Goldfish 0.1 18 43 Weiss (1961)
Golden shiner 0.1 18 40 Weiss (1961)
Sheepshead minnow 6.5 24 71 Goodman
et al.
(1979)
Whereas growth and survival of fathead minnows were not
influenced at concentrations below 0.2 mg/litre in a test described by
Allison & Hermanutz (1977), the minnows responded more sensitively in
a similar test by another laboratory: effects were observed below
0.08 mg/litre (Jarvinen & Tanner, 1982). Allison (1977) found reduced
larval growth of the flagfish if reared in water containing diazinon
at a concentration of 0.014 mg/litre.
Brain acetylcholinesterase (AchE) activity and changes in
optomotor behaviour were determined in bluegill sunfish, Lepomis
macrochirus, exposed to graded concentrations (0, 15, 30, 5, 60 and
75 µg/litre) of diazinon for a period of 24 h (Dutta et al., 1992). A
significant decrease of AchE activity was observed at and above an
exposure concentration of 45 µg/litre, whereas a decline in the scores
of the "following" responses of the fish was observed at an exposure
concentration of 30 µg/litre.
Weiss (1961) was the first to investigate in vivo brain AChE
activity of fish exposed to organophosphates. He found that the extent
of enzyme inhibition was proportional to the concentration of the
substance and extent of exposure, and suggested that fish brain AChE
activity could be used to detect the presence of anticholinesterases
in the aquatic environment.
Goodman et al. (1979) reported a dose-dependent inhibition of
brain AChE activity in sheepshead minnows for diazinon. For the same
sublethal exposure (6.5 mg/litre), inhibition was independent of the
exposure duration, being 68 and 78% on days 4 and 108, respectively.
The authors found that the brain AChE activity of sheepshead minnows
exposed to diazinon varied and was susceptible to exposure
concentration. AChE was depressed long after no measurable diazinon
was found in exposed sheepshead minnows.
The effect of acute exposure to diazinon (3.1 mg/litre for 96 h)
and chronic exposure to a sublethal concentration (0.31 mg/litre) of
diazinon has been studied in the liver, stomach, intestine and pyloric
caeca of a freshwater teleost fish, Channa punctatus. In acute
exposure, succinate dehydrogenase activity was elevated in intestine
and pyloric caeca. No alteration was noted in lactate dehydrogenase
activity but pyruvate dehydrogenase activity was inhibited in pyloric
caeca. Chronic exposure resulted in inhibition of the activities of
the three dehydrogenases in all four organs at both intervals (Sastry
& Malik, 1982a).
Exposure to diazinon at different concentrations induced
pathological changes in muscle cell organelles of Tilapia nilotica.
The earliest changes in muscles of fish exposed to 10 mg/litre
consisted of swelling of sarcoplasmatic reticulum in many fibres and
the appearance of many cytoplasmatic vacuoles of different sizes. In
muscle of fishes exposed to the LC50 (20 mg/kg) of diazinon,
fragmentation of the myofibrils occurred over the entire length of the
sarcomere with involvement of both the thick myosin filament of the
A-band and the thinner actin filaments of the I-band. It was
speculated that these effects may be attributed to the
anti-cholinesterase activity of these insecticides. (Sakr & Gabr,
1992).
Acute effect of diazinon on the intramembranous particles
(IMPs) of microvilli of the intestinal epithelial cells of Tilapia
nilotica fish was studied using the freeze-fracture technique.
Exposing fish to different repeated concentrations of diazinon
(´ LC50) cause a significant decrease in the population density of
IMPs in P and E faces. IMPs of microvilli found in intestinal
epithelial cells are thought to represent many kinds of protein
including enzymes. It was suggested that diazinon induced a reduction
in enzymatic content of the membrane, which was accompanied by a
decrease in the IMP density of the microvilli (Sakr et al., 1991).
El-Elaimy et al. (1990) reported that exposure of Tilapia nilotica
to increasing concentrations of diazinon induced ultrastructural
alterations in the intestine.
Four-month old adult siblings of zebrafish (Brachydanio rerio)
were exposed to four concentrations of diazinon for up to 168 h. DNA,
RNA, protein and total free amino acid content were measured in the
liver. The DNA, RNA and protein contents were significantly reduced,
whereas the amino acid content was significantly enhanced. All these
changes showed a dose-dependent as well as a time-dependent response
(Ansari & Kumar, 1988).
Anees (1978) presented the results of sub-lethal exposure to
diazinon of an adult freshwater teleost. The concentrations of
insecticide used in the study were given as 0.37 mg/litre per 24 h and
0.28 mg/litre per 96 h. This was a study on the evaluation of
histopathology as a possible indicator of aquatic pollution by
insecticides. Hepatocytes were considerably vacuolated and reduced in
size after 24 h of exposure. However, 96 h of exposure produced less
vacuolization but the liver showed a foamy appearance. Damage to the
hepatic blood supply and the appearance of dark, granular cytoplasmic
inclusions resulted from a 14-day exposure. The same concentrations of
pesticide have already been observed to cause many tissue changes in
the intestine of Channa punctatus and disturbances in the
distribution of serum proteins.
The effect of exposure to the LC50 (12 mg/litre) for 96 h
and to a sublethal concentration (3.3 mg/litre) for 15 and 30 days
was studied in the digestive system of the catfish Heteropneustes
fossilis. The most conspicuous pathological changes in the liver
were vacuolization of the cytoplasm of hepatocytes, enlargement of
nuclei, rupture of cell membrane, liver cord disarray, and damage of
connective tissue. Intercellular spaces were widened. In stomach the
mucosa was eroded. In intestine the nuclei of the columnar epithelial
cells were reduced in volume and the cytoplasm was highly degenerated.
In both acute and chronic exposure alkaline phosphatase and
glucose-6-phosphatase activities were inhibited significantly in the
different portions of the digestive system. Acid phosphatase activity
was elevated in all the portions and in both stages of exposure. In
acute exposure insignificant elevation was observed in the activities
of amylase and maltase, while lactase activity was inhibited. An
inhibition in maltase activity was significant only in the liver.
Lipase activity showed a decrease in all stages of exposures, while
there was no marked alteration in activities of pepsin and trypsin
(Sastry & Malik, 1982b).
9.4 Effects in mesocosms and the field
A mesocosm study was performed with 17 treated and 4 untreated
ponds (each 0.1 acre in surface area and 2.2 metres deep). The ponds
received multiple applications of diazinon with single applications
ranging from 2 to 25 µg/litre and corresponding to total application
rates of 5.7, 11.4, 22.9, 45.8 and 91.5 µg/litre. The following
effects on sediment-dwelling organisms were observed: Trichoptera was
the most sensitive order, with significant reductions at all treatment
levels throughout most of the treatment period. Trichoptera was a
minor component of the macroinvertebrate communities in the mesocosms.
Diptera and Ephermeroptera were intermediate in sensitivity; both were
reduced at 22.9 and 91.5 µg/litre by the end of the treatment period,
though both recovered to control levels shortly after treatment
ended. Odonata was the least sensitive order with effects only at
91.5 µg/litre. Total insect abundance generally followed the trend
of the Diptera, which was the most abundant order; increasingly
severe effects at 22.9 to 91.5 µg/litre during the treatment
period was followed by recovery. The abundance of crustaceans in
macroinvertebrate samples generally followed a similar pattern.
Gastropods were essentially unaffected by diazinon. Though dipterans
as a whole were affected only at 22.9 µg/litre or more and recovered
rapidly, some dipteran sub-taxa were more sensitive or were
significantly reduced in the post-treatment period. Tanypodinae and
its dominant tribe, Pentaneurini, were the most sensitive. These were
significantly reduced in all treated ponds at the end of the treatment
period. Both groups recovered within eight weeks of the last
treatment. The sub-family Chironominae was the next most sensitive
among the family Chironomidae, especially the tribes Tanytarsini and
Chironomini. Tanytarsinids were reduced at 22.9 µg/litre early in the
treatment period and then recovered. Chironomini followed a similar
pattern, including recovery, but were again reduced in number at 22.9
to 91.5 µg/litre. The sub-family Orthocladiinae was the least
sensitive chironomid group with effects only at 91.5 µg/litre and full
recovery within two weeks. With the wide range of sensitivity observed
among the chironomids, the overall impact of diazinon was relatively
minor; statistically significant reductions were observed twice early
in the highest treatment. Two less abundant dipteran families,
Chaoboridae and Ceratopogonidae, were affected at 11.4 µg/litre or
more. The greatest effect on Chaoboridae occurred two months into
treatment and that on Ceratopogonidae occurred during the treatment
period, but sporadic effects persisted beyond (Giddings, 1992).
The application of diazinon to a small stream at 3 mg/litre
resulted in increased drift of benthic organisms and changes in the
composition of benthic fauna. These changes were not persistent,
however, as the communities were restored to normal within 4 weeks of
treatment (Miller et al., 1966).
9.5 Terrestrial invertebrates
Stevenson (1978) reported LD50 values for bees of 0.22 µg/bee
(topical) and 0.20 µg/bee (oral).
When earthworms (Eisenia foetida) were exposed to technical
diazinon in soil, the 14-day LC50 was calculated to be 130 mg/kg
soil. A no-observed-effect concentration of 12.3 mg/kg was reported
(Vial, 1990).
In a field experiment in Connecticut, USA, diazinon was
applied to tobacco fields as a 10% granular formulation at up to
4.48 kg a.i./ha; granules were raked into the soil. Mortality of
earthworms (Lumbricus terrestris) did not differ between treated and
control plots (Kring, 1969).
9.6 Birds
Acute toxicity to birds is summarized in Table 7.
The use of diazinon for controlling flies in sheds used to house
ducks led to the death of an estimated 15 600 young birds (Dougherty,
1957).
Ingestion of a few granules could be lethal to sparrow-sized
birds for diazinon 14G (Hill & Camardese, 1984).
Variability of toxicity among anticholinesterase formulations was
shown with a single dose of diazinon administered orally as technical
grade (TG, 99% a.i.) alone or in corn oil, as granular (GR, 14-15%
a.i.), or as emulsifiable concentrate (EC, 48% a.i.). The rank of the
formulations tested, from most to least toxic, based on statistically
separable (p<0.05) LD50s, was EC > GR=TG > control. The difference
between the least and most toxic form was nearly three-fold (Hill,
1988).
The single acute oral and dermal doses of diazinon were
determined for 8- and 18-week-old broadbreasted white turkeys. The
hazard to turkeys of exposure to soils treated for control of chiggers
was evaluated. Diazinon was lethal for 18-week-old turkeys at 2 mg/kg
orally and toxic at 5 mg/kg dermally. In spite of this high toxicity,
exposing the turkeys to soil treated with 18 kg diazinon per hectare
led to no poisoned birds (Radeleff & Kunz, 1972).
The activity of acetylcholinesterase (AChE) and the density of
muscarinic receptors were measured in brains from normal Japanese
quail (Coturnix coturnix japonica) and from quail after lethal
intoxication with diazinon. The maximum relative loss of activity due
to postmortem decomposition alone during 8 days was 13 and 10% for
AChE and muscarinic receptors, respectively. During postmortem
decomposition, the ratio of AChE and muscarinic receptor activities
remained constant at approximately 1.3:1 in normal brains, while it
was always less than, or equal to, 0.5:1 after intoxication with
diazinon. Normal AChE activity could be estimated from muscarinic
receptor density. Parallel measurement of AChE and muscarinic
receptors may assist in the postmortem diagnosis of death due to acute
poisoning with anti-cholinesterase pesticide when control specimens
are not available (Prijono & Leighton, 1991).
Anticholinesterases do not pass through the mother to the egg in
significant amounts, but they may be deposited on the egg from the
parents feathers or during pesticide application. To simulate topical
exposure, fertile mallard eggs were either immersed for 30 seconds in
an aqueous emulsion or single dose of an anticholinesterase in a
non-toxic oil vehicle pipetted on the shell on day 3 of incubation
which is a critical period with respect to organogenesis in mallards.
Table 7. Acute toxicity to birds
Species Age/weight LD50 LC50 Reference
Japanese quail (Coturnix coturnix japonica) 50-60 days (105-195 g) 4 Sachsse (1973a)
5 days (10-30 g) 1.1 Sachsse & Ullmann (1975a)
50-60 days 900a Sachsse (1972)
Bobwhite quail (Colinus virginianus) 14 days 5.2 Fink (1976)
140-180 g 4.3 Sachsse & Ullmann (1975b)
Peking duck (Anas domesticus) 2.5-3.5 kg 2.7 Sachsse & Ullmann (1976)
5 days (40-125 g) 1.9 Sachsse & Ullmann (1975c)
Domestic hen (Gallus domesticus) 5 days (40-50 g) 14 Sachsse & Ullmann (1975d)
Mallard duck (Anas platyrhynchus) 19 weeks (843-1357 g) 1.44 Fletcher & Pedersen (1988a)
5 days 202a Sachsse (1973b)
9 days (108-120 g) 32 Fletcher & Pedersen (1988c)
Brown-headed cowbird (Molothrus ater) 35-55 g 85 Fletcher & Pedersen (1988b)
a Repellency recorded in all dosage groups.
Organophosphates were shown to be as much as 18 times more toxic when
applied to the shell in an oil compared with following water
immersion. Neither method of egg treatment produced teratogenicity or
developmental effects at realistic pesticide application rates
(Hoffman & Eastin, 1981).
A laboratory reproduction study was conducted with mallard in
which birds were allowed to build their own nests and incubate eggs
(Marselas et al., 1989a). Birds were fed diets containing 0, 5, 10 and
20 mg diazinon/kg diet (20 pairs per dose level). The exposure to 5
and 10 mg/kg did not result in any overt signs of toxicity or effects
on reproductive performance. At 20 mg/kg, there was an increase in the
number of hens that did not incubate and, although egg production was
not affected, there was some reduction in the number of hatchlings and
14-day-old survivors. A companion study (Marselas et al., 1989b) with
bobwhite quail was conducted at dietary concentrations of 10, 20 and
40 mg/kg. Exposures did not result in effects on reproductive
performance and no mortalities or overt signs of toxicity were
observed.
Henderson et al. (1994) described certain oral and dermal
toxicity of diazinon to the domestic pigeon. Dose-dependent gross
symptoms of organophosphorus poisoning usually appear within half an
hour in pigeons dosed orally. There was a 50% inhibition of brain
cholinesterase activity, i.e. ID50 of diazinon was about 2 mg/kg body
weight. Plasma cholinesterase activity in dermally exposed birds was
almost completely inhibited by 24 h after diazinon treatment. The
recovery of plasma cholinesterase after dermal exposure of pigeon was
very slow.
Johnston et al. (1994) studied the interactive effects of
combined treatment of red-legged partridge with diazinon and an
ergosterolbiosynthesis-inhibiting (EBI) fungicide (prochloraz). Birds
were pre-treated with procloraz at 180 mg/kg and then exposed to
diazinon at 4.3 mg/kg 24 h later. There was a tendency to increased
inhibition of cholinesterase in the plasma of treated birds but this
was not significant. Synergism was significant with other
organophosphorus compounds tested.
9.6.1 Field studies
Nine fairways of a golf course located in Bellingham, Washington,
USA, were treated with Diazinon AG500 at a target application rate of
2.2 kg a.i./ha. The chemical application with a boomless sprayer
resulted in a variable distribution of diazinon residues on the turf
that ranged from 1.0 to 6.2 kg a.i./ha. The diazinon-treated turf was
irrigated with 1.3 cm of water immediately following application. The
post-irrigation diazinon residue levels ranged from 100 to 33 mg/kg
(mean=209; SD=88; n=8). These residue levels were higher than expected
based on results of turf studies in other regions of the USA.
Eighty-five American wigeon died after grazing on one treated fairway
on the day of application following irrigation. The brains of all 85
wigeon were analysed for acetylcholinesterase activity. Wigeon that
died on the study area showed 44 to 87% depression of AChE activity
when compared to controls. Upper gastrointestinal tract contents of 15
of the 85 dead wigeons contained 0.96 to 18.1 mg/kg diazinon (Kendall
et al., 1992).
In the USA, use of diazinon has been discontinued on golf courses
since 1988. Waterfowl often congregate near ponds on golf courses and
were exposed to turf treatments. Since this restriction, deaths of
grazing waterfowl (Canada geese, brent geese and American wigeon) have
not occurred. Application rate reduction may also be effective in
reducing risk to Canada geese, the most common grazing bird on turf.
Kendall et al. (1993) closely monitored geese exposed to diazinon
applied twice at 2.2 kg a.i./ha, and found no mortality despite
extensive feeding on treated turf.
Hummell et al. (1992a,b,c) conducted an extensive, multi-plot
assessment of the effects of turf applications of granular and liquid
formulations (4.8 kg a.i./ha) of diazinon on songbirds.
Since the introduction of diazinon, sporadic mortality of
waterfowl feeding on treated turf or on orchard grass has come to
light in Canada. Frank et al. (1991) reported five incidents that
took place in Ontario between 1986 and 1988. Fifty-seven Canada
geese were poisoned by diazinon on turf sites in Ontario 1986-88. It
was determined that median levels of diazinon in turf sprayed and
then properly irrigated ranged from 45.4 to 256 mg/kg for a single
application of 1 kg a.i./ha of the EC formulation. A value of
390 mg/kg was obtained for a single grass sample following spray at
approximately 9 kg a.i./ha. Maximum residue levels for grass recovered
from the oesophagi of geese killed by diazinon on turf, and collected
while still fresh, ranged from 55 to 79 mg/kg.
In trials evaluating applications to large turf areas (an average
of ha), survival, behaviour and reproductive performance were
monitored. Smaller plots (0.09 ha) that simulated large home yards
were established for granular formulations, and survival and plasma
ChE were evaluated. On large plots, the liquid formulation had no
effect on reproductive performance, survival or cholinesterase levels.
Granular applications had a minor impact on bird population size (9.6%
reduction) compared to control plots (4.8% reduction). Reproductive
performance was not affected, although cholinesterase levels were
reduced compared to controls. On the smaller (home-sized) turf plots
there was no apparent effect on bird numbers, and plasma
cholinesterase activity was not affected in any species.
Decarie et al. (1993) sprayed ornamental and other deciduous
trees with diazinon at a rate of 2.2 kg a.i./ha in a suburban area of
Quebec, Canada, where the compound was used to control fruit tree
mites. Twelve nests of the American robin were sprayed and 65 nests
untreated were used as controls. Productivity was compared between
treated and control nests as was the behaviour of the birds. Mean
productivity was not significantly different between the two groups.
Number of feeding flights, number of female feeding flight and number
of faecal sacs removed did not differ between groups but there was a
significant increase in the total time spent sitting on the nest in
diazinon-treated birds. The significance of this is unclear. Plasma
AChE levels were reduced (993 ± 639 µg/litre in treated birds;
3585 ± 1531 µg/litre in controls), but brain AChE activity was
unaffected.
10. EVALUATION OF HUMAN HEALTH RISK AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risk
Diazinon is an organophosphorus pesticide classified by WHO as
"moderately hazardous" Class II (WHO, 1996). It is absorbed from the
gastrointestinal tract, through intact skin, and by inhalation. The
sources of exposure to humans are occupational, accidental or through
diet. Diazinon is used as a pesticide and veterinary drug to control
ectoparasites. The major source of diazinon food residues in edible
crops are from agricultural usage while those in meat, offal and other
animal products arise from veterinary use
The results of total diet studies in the USA, United Kingdom and
New Zealand suggest that levels of exposure are below the recommended
Acceptable Daily Intake (ADI) of 0.002 mg/kg per day (FAO/WHO, 1994b).
Diazinon is rapidly broken down, whether on plants or in animals,
further reducing the risk to humans. Several different application
techniques are used in applying diazinon outside and inside. Residual
spraying and space treatment are often used in controlling pests
indoors. Following recommended use, concentrations of diazinon
detectable in the indoor air are low and do not present a health
hazard. Some studies of agricultural workers have shown cases of
contact dermatitis after exposure to diazinon. Toxicity studies in
humans have shown that the administered daily dose of 0.025 mg/kg body
weight marginally inhibits the plasma cholinesterase activity and it
can be considered as the no-observed-adverse-effect level (NOAEL).
Diazinon is not genotoxic and exhibits no carcinogenic potential in
rats or mice. Several episodes of fatal and non-fatal accidental and
suicidal poisoning have occurred. Acute poisoning causes typical
cholinergic signs and symptoms. Acute pancreatitis can be associated
with severe poisoning.
10.2 Evaluation for effects on the environment
The information on utilization and application rates that has
been employed for this risk assessment is derived from the
agricultural use of diazinon within the European Union. It should be
possible to extrapolate this assessment to other agricultural uses at
similar application rates elsewhere in the world. The application
rates for diazinon can be summarized as follows: arable (tractor-
mounted/drawn hydraulic spray boom applications), 1.0 kg/ha; top fruit
(broadcast air-assisted applications), 1.2 kg/ha.
The following risk assessment is based on the principle of
calculating toxicity-exposure ratios (TERs) (Fig. 2), which follows
the European and Mediterranean Plant Protection Organisation and
Council of Europe (EPPO/CoE) Environmental Risk Assessment Scheme
model and associated trigger values (EPPO/CoE, 1993a,b).
The metabolites formed, i.e. diethylphosphoric acid,
diethylthiophosphoric acid and the derivatives of pyrimidinyl ring,
are eliminated mainly via the kidneys. Only minimal quantities of the
metabolites were detected in milk and eggs.
6.2.1 In vivo metabolic transformations
6.2.1.1 Mice
When male ICR mice (number not stated) were treated orally with
diazinon or [pyrimidine14C] diazinon at 50 or 75 mg/kg body weight,
one half of the high-dose animals died and the rest showed symptoms
(sweating, crouching) (Miyazaki et al., 1970; Sekine, 1972). At the
low dose, no signs of toxicity were observed. Metabolism and excretion
occurred rapidly, and the metabolites diazoxon, O, O-diethyl-
O-[2-(alpha-hydroxyisopropyl)-4-methyl)-6-pyrimidinyl]
phosphorothioate, and O, O-diethyl- O-(2-(2-propenyl)-4-methyl-6-
pyrimidinyl) phosphorothioate were found in the urine 1 h after
treatment. Most of the metabolites were found in urine 6 h after
treatment, but metabolism was not identical in the two dose groups. In
the low-dose group O, O-diethyl- O-(2-isopropyl-4-hydroxymethyl-6-
pyrimidinyl) phosphorothioate and O, O-diethyl- O-(2-isopropyl-4-
formyl-6-pyrimidinyl) phosphorothioate were found, but this was not
observed in the high-dose group. In the high-dose, but not the low-
dose group O, O-diethyl- O-(2-(a-hydroxyethyl)-4-methyl-6-
pyrimidinyl) phosphorothioate was found.
The metabolism of [pyrimidine-14C]-diazinon and [ethoxy-14C]-
diazinon was investigated by Mücke et al. (1970). Four metabolite
fractions were found in urine and faeces, three metabolites
representing approximately 70% of the total radioactivity applied.
Hydrolysis of the ester bond yielded 2-isopropyl-4-methyl-6-
hydroxypyrimidine (22.5% of the applied radioactivity in urine);
oxidation at the primary carbon atom produced 9% of the applied
radioactivity in urine, while oxidation at the tertiary carbon atom of
the isopropyl side chain produced 22%. In addition, trace amounts of
unchanged diazinon were detected in faeces. No cleavage of the
pyrimidine ring with subsequent oxidation of the fragments to CO2
took place (Mücke et al., 1970).
6.2.1.2 Rats
A study by Capps (1989) investigated the diazinon metabolites in
male and female rats orally treated with single doses of 10 and
100 mg/kg [pyrimidine 14C]-diazinon and in rats preconditioned with
14 daily treatments at 10 mg/kg before the final administration of
radiolabelled compound. The metabolite pattern was similar in the
urine and faeces of the rats from all dose groups and from both sexes.
The major urinary metabolites were identified as 2-isopropyl-6-
methyl-4(1 H)- pyrimidinone (average 38.2% of the totally applied
dose), 2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone
(17.3%) and 2-(beta-hydroxyisopropyl-6-methyl-4(1 H)-pyrimidinone
(9.7%). Six unknown aqueous components accounted for an average of
14.9% of the administered dose, and trace amounts of unchanged
diazinon (0.11%), diazoxon (0.14%) and the hydroxy-isopropyl
derivative of diazinon (0.12%) were also detected. The identity of the
metabolites was confirmed by gas chromatography and mass spectrometry
(GC/MS) with synthetic standards.
6.2.1.3 Dogs
The urinary metabolites of Beagle dogs were characterized after
oral administration of 4.0 mg/kg body weight 14C-ring-labelled
diazinon. The metabolite 2-isopropyl-4-methyl-6-hydroxypyrimidine
accounted for 10% of the applied radioactivity in the urine and the
tertiary hydroxy-isopropyl derivative of diazinon represented 23%
(Iverson et al., 1975).
6.2.1.4 Sheep
When two sheep were dermally treated with [pyramidine-14C]-
diazinon, radiolabelled residues were detected in all tissues examined
(heart, liver, kidney, back fat and leg muscle). Unmetabolized
diazinon was the only significant residue in fat, and was a major
residue in heart and leg muscle. The major metabolites in urine and
all tissues except fat were 2-isopropyl-6-methyl-4(1 H)-pyrimidinone
(urine, 10% of the administered radioactivity; liver, 18%; kidney,
23%) and 2-(alpha- hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone
(urine, 22.7%; liver, 10%; kidney, 28%), which were also present in
the form of glucuronide conjugates. The identity of the metabolites
was confirmed by GC/MS with synthetic standards. In addition, several
unidentified polar (urine, 18.6%) and minor amounts of non-polar
(urine, 4.0%) metabolites were detected (Capps, 1990).
6.2.1.5 Goats
Two lactating goats were orally treated with [pyrimidine-14C]-
diazinon in gelatin capsules for four consecutive days. Similarly to
sheep, in urine and faeces the metabolites 2-isopropyl-6-methyl-
4(1 H)-pyrimidinone (urine, 4.5% of the totally administered radio-
activity; faeces, 2.6%) and 2-(alpha-hydroxyisopropyl)-6-methyl-
4(1 H)-pyrimidinone (urine, 12.5%; faeces, 1.7%) were identified.
Approximately 48.6% of the urinary radioactivity consisted of unknown
water-soluble compounds. Characterization of selected tissues showed
the presence of mainly the above-mentioned metabolites. Unchanged
diazinon, its hydroxy-isopropyl derivative and diazoxon accounted for
less than 10% of the radioactivity detected in these tissues.
Metabolites in fat consisted primarily of unchanged diazinon (66%),
its hydroxy-isopropyl derivative (12.5%) and diazoxon (3%). The major
metabolites in the milk were 2-isopropyl-6-methyl-4(1 H)-
pyrimidinone (39.3% of the residual radioactivity) and
2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-pirimidinone (37.3%).
Substantial portions of the polar metabolites in urine, faeces and
tissues were glucuronide conjugates. The identity of the metabolites
was confirmed by GC/MS with synthetic standards (Simoneaux,
1988a,b,e).
6.2.1.6 Hens
Four laying Leghorn hens were treated with [pyrimidine-14C]-
diazinon in gelatin capsules for seven consecutive days at daily doses
of 2.75 mg/kg day. The main metabolites detected in the excreta were
unchanged diazinon (14.9% of the extractable radio-activity),
2-isopropyl-6-methyl-4(1 H)-pyrimidinone (5.9%), 2-(alpha-hydroxy-
isopropyl)-6-methyl-4(1 H)-pyrimidinone (10.8%) and 2-(beta-
hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone (7.2%). Approximately
25% of the radioactivity in the excreta consisted of unknown
water-soluble compounds. The residues in tissues primarily consisted
of 2-isopropyl-6-methyl-4(1 H)-pyrimidinone (0.6-2.6% of the residual
radioactivity), 2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-
pyrimidinone (3.1-6.5%) and 2-(beta-hydroxyisopropyl)-6-methyl-
4(1 H)- pyrimidinone (2.0-5.7%). Unchanged diazinon was detected
primarily in the peritoneal fat (2% of residues). In the eggs,
primarily 2-isopropyl-6-methyl-4(1 H)-pyrimidinone (yolk, 11.1% of
the residual radioactivity; white, 9.4%), 2-(alpha-hydroxyisopropyl)-
6-methyl-4(1 H)-pyrimidinone (yolk, 18.6%; white, 33.3%) and
2-(beta-hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone (yolk, 7.0%;
white, 35.3%) were detected. As in goats, a substantial portion of the
polar metabolites in tissues, eggs and excreta were glucuronide
conjugates. The identity of the metabolites was confirmed by GC/MS
with synthetic standards (Simoneaux, 1988c,e; Simoneaux, 1989).
More information on kinetics and metabolism in other species is
given in chapter 9.
6.2.2 In vitro metabolic transformations
The metabolism of [ethoxy-14C]-diazinon and diazoxon was studied
in vitro using rat liver cell fractions. It was shown that the
degradation by diazinon is catalysed by a microsomal enzyme that
requires NADPH and oxygen, and is inhibited by carbon monoxide. It is
presumably the cytochrome P-450 oxidase system. Diazoxon was shown to
be degraded by enzymes located in the nuclear, mitochondrial,
microsomal and soluble fractions of the liver. The microsomal enzymes
were the most active and were not dependent on NADPH. Reduced
gluthation had little effect. With diazinon, products of the reactions
were diethylphosphorothioic acid and diethylphosphoric acid. Diazoxon
was degraded to diethylphosphoric acid (Yang et al., 1969, 1971;
Nakatsugawa et al., 1969). These results were confirmed by independent
experiments (Dahm, 1970). The oxidation of diazinon was investigated
by using microsomal preparations from rat liver. The major metabolic
products of diazinon were hydroxydiazinon, diazoxon and
hydroxydiazoxon, which are biologically active, and additional
inactive products such as diethylphosphorothioic acid,
diethylphosophoric acid and derivatives of the pyrimidyl moiety. It
was demonstrated that desulfuration, hydroxylation of the ring alkyl
side-chain and cleavage of the aryl phosphate bond may occur,
depending on the presence of NADPH or NADH. EDTA stimulated the
overall metabolism of diazinon (Shishido et al., 1972a).
The enzymatic hydrolysis of diazoxon was investigated using rat
tissue homogenates. The hydrolytic activity of the tissues decreased
in the order liver>blood>lung>heart>kidney>brain. In the liver,
the hydrolytic activity was localized in microsomal preparations.
Diethyl phosphoric acid and 2-isopropyl-4-methyl-6-hydroxypyrimidine
were identified as the products. The reactions were inhibited by EDTA,
heavy and rare earth metal ions, and sulfhydryl reagents (L-cysteine,
2-mercaptoethanol, thioglycolic acid), while calcium ions activated
the hydrolysis (Shishido & Fukami, 1972).
Liver homogenates were prepared from male mice (North Carolina
Department of Health strain) and incubated for 1 h with either
14C-diazinon or 14C-diazoxon. Inhibition of metabolism was
studied by co-incubation with piperonyl butoxide, NIA 16824 or
1-(2-isopropylphenyl) imidazole. Diazoxon formation from diazinon
(thiophosphate to phosphate conversion) was inhibited by 45 to 60% by
the inhibitors studied. All the inhibitors also reduced oxidative
dearylation of diazinon to diethyl phosphoric and diethyl
phosphorothioic acids (Smith et al., 1974).
Conjugation with glutathione forms the third enzymatic mechanism
of the diazinon metabolism in rat tissue preparations (liver, heart,
brain, lung, kidney and blood were investigated). The highest activity
(14-89 times as high as in other tissues) for this reaction was
localized in the cytoplasmatic fractions of the liver. The reaction
products were identified as diethyl phosphorothioic acid and
S-(2-isopropyl-4-methyl-6-hydroxypyrimidinyl) glutathione, which were
formed by conjugation and simultaneous cleavage of the phosphate ester
bond. The enzymatic activity was increased by the addition of
glutathione-SH, and was inhibited by various sulfhydryl reagents,
oxidized glutathione and some chelating agents ( o-phenanthroline,
8-hydroxyquinoline) (Shishido et al., 1972b).
6.3 Metabolic aspects of diazinon toxicity
Diazinon was incubated with liver microsomes and liver slices
from sheep, cow, pig, guinea-pig, rat, turkey, chicken and ducks.
Hydroxydiazinon, isohydroxydiazinon, dehydrodiazinon, their oxons
and diazoxon were identified and determined quantitatively or
semi-quantitatively. It was shown that yields and rates of production
of the metabolites varied greatly between the species. The production
of the oxon was not generally correlated with susceptibility to
diazinon poisoning, although it was lowest in the least susceptible
animal, the sheep. The highly susceptible avian species (acute oral
LD50 of around 2-15 mg/kg) do not produce higher rates of oxons than
rat or pig (acute oral LD50 around 300-600 mg/kg). However, the
mammalian blood hydrolyses diazoxon rapidly, whereas the avian species
have virtually no hydrolytic activity. It was concluded that
extrahepatic metabolism of diazinon, in particular the hydrolysis of
diazoxon in the blood, appears to be the main factor affecting
susceptibility to diazinon poisoning. In mammals the extrahepatic
metabolism of diazinon is more important toxicologically than the
metabolism in the liver, while the liver is probably the most
important site of metabolism in avian species (Machin et al., 1975).
Recently, the hydrolytic metabolism of diazinon by plasma was
investigated in 92 individuals of Hispanic origin (Davies et al.,
1996). Diazoxon is hydrolysed by the enzyme paraoxonase (PON1),
leading to the formation of 2-isopropyl-4-methyl-6-hydroxypyrimidine
and diethylphosphate. An important observation of this study was that
the effect of the PON1 polymorphism for diazoxon hydrolysis relative
to paraoxon hydrolysis was reversed. Thus, RR individuals (Arg192
homozygotes) who displayed high paraoxonase activity had lower
diazonoxonase activity (mean = 7948 U/litre) than QQ homozygotes
(12 318 U/litre).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
Improvements since 1979 in the manufacturing of diazinon have
significantly reduced the content of highly toxic by-products, in
particular tetraethyl-pyrophosphate (TEPP). As a result of these
stepwise improvements, the acute oral LD50 of technical grade
diazinon increased to values around 1000 mg/kg (Piccirillo, 1978;
Bathe & Gfeller, 1980; Schoch & Gfeller, 1985; Kuhn, 1989a). The most
recent study resulted in an oral LD50 in rats of 1250 mg/kg. The LD50
values for different species are summarized in Table 2.
Signs of poisoning after a single dose of diazinon are typical of
organophosphate intoxication and include decrease of spontaneous
activity, sedation, dyspnoea, ataxia, tremors, muscle spasms,
convulsions, lacrimation and diarrhoea. The signs were found to be
reversible in surviving animals.
Diazinon (88% purity) was administered by gavage to 15 rats per
sex and dose at single doses of 0, 2.5, 150, 300 and 600 mg/kg
(Potrepka, 1994). Three, 9 or 24 h after dosing, five animals of each
group were bled for determination of serum and red blood cell
cholinesterase activity and then killed for determination of CNS
cholinesterase activity. There was no mortality. Signs of cholinergic
poisoning, e.g., salivation, diarrhoea and muscle fasciculations, were
observed in animals of both sexes dosed at 300 and 600 mg/kg. Clinical
signs first appeared 3 h after treatment. Maximum observable toxicity
was noted 9 h after treatment in males and 24 h after treatment in
females. Serum cholinesterase activity was significantly decreased at
all time points in all groups treated with 2.5 mg/kg or more. Maximum
inhibition was observed 9 h after dosing, and values remained
depressed at 24 h after dosing. Red blood cell cholinesterase activity
was significantly inhibited at all time intervals in animals of both
sexes treated with >150 mg/kg. Again, maximum inhibition was
observed 9 h after dosing, and activity remained depressed at 24 h
after dosing. In addition, a significant inhibition of red blood cell
cholinesterase activity was noted in females dosed with 2.5 mg/kg
diazinon 9 h after dosing. Cerebellum, cerebral cortex, striatum,
hippocampus and thoracic spinal cord cholinesterase activities were
decreased in female rats dosed with >150 mg/kg at all three time
points. Cerebellum, striatum and hippocampus cholinesterase activities
were decreased in male rats dosed with >150 mg/kg at all three time
intervals, whereas cerebral cortex and thoracic spinal cord
cholinesterase activities were decreased in male rats dosed with
>150 mg/kg at 9 and 24 h after dosing. The NOAEL for inhibition of
brain cholinesterase activity was 2.5 mg/kg for both sexes (Potrepka,
1994).
Table 2. Acute toxicity of diazinona
Species Sex Route of administration LD50 (mg/kg) Reference
Rat male oral 235 Gasser (1953)
Rat male oral 435 Shaffer & West (1960)
Rat male oral 250 Gaines (1969)
Rat female oral 285 Gaines (1969)
Rat both oral 300 Piccirillo (1978)
Rat both oral 422 Bathe & Gfeller (1980)
Rat both oral 1012 Schoch & Gfeller (1985)
Rat both oral 1250 Kuhn (1989a)
Rat male dermal 900 Gaines (1960)
Rat female dermal 455 Gaines (1960)
Rat both dermal >2150 Bathe (1972a)
Rat both inhalation >2300b Holbert (1989)
Mounse male oral 82 Bruce et al. (1955)
Mounse both oral 96 Gasser (1953)
Mounse both oral 187 Bathe (1972b)
Mounse both i.p. 65 Klotzsche (1955)
Guinea-pig oral 320 Gasser (1953)
Rabbit oral 130 Gasser (1953)
Rabbit both dermal >2020 Kuhn (1989b)
Turkey oral 6.8 FAO/WHO (1965)
Chicken oral 40.8 FAO/WHO (1965)
Goose oral 14.7 FAO/WHO (1965)
Gosling oral 2.8 Egyed et al. (1974)
a It should be noted that progressive improvement in the manufacturing process has reduced
the acute toxicity of diazinon.
b This value is the LC50, the units being mg/m3.
Brain cholinesterase activities were determined for white-footed
mice (Peromyscus leucopus) orally dosed with diazinon at 18.8 mg/kg
body weight (Montz & Kirkpatrick, 1985). Following treatment with
diazinon, a latent period of approximately 6 h elapsed during which
time acetylcholinesterase activity was relatively unaffected. After
the latent phase, ChE activity rapidly declined to a minimum 12 h
after dosing. After 48 h, ChE activity recovered to a level only
slightly below that of the controls. The response of both male and
female mouse brain ChE activities declined rapidly at 6 h and reached
a minimum 24 h after dosing. ChE activity of treated animals was
comparable to that of controls 48 h after treatment. Brain ChE
activities of treated female mice were significantly lower (P <0.05)
than those of treated males.
An acute oral toxicity study in rats was conduced in two phases
to determine a NOEL of the test material (87.9% active ingredient) for
clinical, behavioural and body weight effects in phase 1 and a NOEL
for effects on plasma, red blood cell and brain cholinesterase in
phase 2 after single oral gavage application to rats. In phase 1 each
test group consisted of 5 rats. The applied dose levels were 25 and
50 mg/kg body weight for female rats only; levels of 100, 250 and
500 mg/kg body weight were tested in both sexes. One female given
500 mg/kg body weight died. Clinical signs such as miosis,
hypoactivity, absence of pain reflex, red-stained face, yellow-stained
abdomen/urogenital area, soft stool or few faeces were seen in all
animals except 100- mg/kg males and 25-mg/kg females. The NOAEL for
clinical/behavioural/body weight effects was 100 mg/kg body weight in
males and 25 mg/kg body weight in females. In phase 2 each test group
consisted of 5 rats. Males were treated with 0.05, 0.50. 1.0, 10.0,
100 or 500 mg/kg body weight and females with 0.05, 0.12, 0.25, 2.5,
25 and 250 mg/kg body weight. Clinical signs such as miosis,
hypoactivity, absence of pain reflexes, staggered gait, excessive
salivation, red-stained face and yellow-stained and/or wet
ventral/urogenital area were only observed in males at 500 mg/kg body
weight (one male at 100 mg/kg body weight showed miosis) and females
at 250 mg/kg body weight The only findings at necropsy were yellow
staining of the perineum and red paranasal discharge in animals of
these dose groups. Plasma cholinesterase activity was significantly
reduced in males dosed with 10, 100 or 500 mg/kg body weight and in
females dosed with 2.5, 25 or 250 mg/kg body weight. Red blood cell
cholinesterase activity was significantly lower in males given 100 or
500 mg/kg body weight and in females given 25 or 250 mg/kg body
weight. Brain cholinesterase activity was significantly reduced at the
highest dose level for both sexes. Brain cholinesterase activity in
females dosed with 25 mg/kg body weight was inhibited by 37% (without
reaching statistical significance) (Glaza, 1993).
7.1.2 Dermal
Diazinon was dispersed on the shaved back of each of three male
and three female Tif: RAI rats at a level of 2150 mg/kg and covered
with aluminium foil for 24 h. None of the animals died. Neither
clinical signs nor dermal irritation was observed. Autopsy revealed no
substance-related gross organ changes. The acute dermal LD50 in rats
was found to be greater than 2150 mg/kg (Bathe, 1972a). Sherman strain
rats were given one dermal application of diazinon dissolved in
xylene, and no attempt was made to remove the compound during the
observation time of 14 days. The LD50 was 900 mg/kg for males and
455 mg/kg for females (Gaines, 1960).
Dermal LD50 values for diazinon were determined in mice after
application of the solution to hind feet. Values were simultaneously
generated for the ED50 for both cholinesterases (acetylcholinesterase
and pseudocholinesterase). LD50 values were higher than those
reported for mice treated on shaved back skin. Diazinon appeared to
have much more inhibitory potential for blood than for nervous tissue
cholinesterase (Skinner & Kilgore, 1982).
Undiluted diazinon was applied to the clipped dorsal trunk of
each of five male and five female New Zealand White rabbits at a level
of 2020 mg/kg and kept under semi-occlusive dressing for 24 h. Signs
were typical of organophosphate intoxication; two out of ten animals
died. The LD50 was >2020 mg/kg (Kuhn, 1989b,c).
7.1.3 Inhalation
Sprague-Dawley rats were exposed (whole body exposure) to
diazinon for 4 h. The acute inhalation LC50 for diazinon MG 8
FL880045 was greater than 2330 mg/m3 when it was administered
undiluted as an aerosol (Holbert, 1989).
Groups of five male and five female HSD:SD rats were exposed to
an aerosol generated from undiluted liquid diazinon MG87% (88% purity)
for 4 h (nose-only exposure). An exposure concentration of 5540 mg/m3
was obtained, 8.82% of particles being smaller than 1 mm in diameter.
There was no mortality. Prominent in-life observations included
activity decrease, piloerection and polyuria, no longer seen by day 6.
Body weight gain was largely unaffected. Gross necropsy revealed
discoloration of the lungs in all animals. The acute inhalation LC50
for diazinon in the rat was greater than 5440 mg/m3 (Holbert, 1994).
7.1.4 Intraperitoneal
Mild structural and functional changes were observed in the liver
and testes of rats after a single intraperitoneal administration of
diazinon (21.6 mg/kg). Kidney, however, showed no pathological
lesions. Attempts were made to correlate the pathological changes in
these organs with the activity of succinic dehydrogenase, adenosine
triphosphatase and alkaline phosphatase (Dikshith et al., 1975). A
single intraperitoneal dose of diazinon caused hyperglycaemia in rats,
which peaked 2 h after intraperitoneal treatment with 40 mg
diazinon/kg. The brain acetylcholinesterase activity was significantly
reduced. The blood level of pyruvic acid was unchanged while that of
lactic acid was significantly increased. In diazinon-treated
hyperglycaemic animals, the glycogen content of the brain was
depleted, the activities of glycogen phosphorylase, phosphoglucomutase
and hexokinase were significantly increased, and the activity of
glucose-6-phosphatase remained unchanged. Lactate dehydrogenase
activity was increased by treatment with diazinon. The induced changes
may have compensated for the energy requirement of stimulatory effects
caused by the pesticide (Husain & Matin, 1986; Matin & Husain, 1987;
Matin et al.,1989). Changes in energy metabolism are regarded as
secondary, following cholinesterase inhibition.
In an acute intraperitoneal study in rats, an LD50 value of
260 mg/kg body weight was determined for both sexes. Clinical signs
were typical of organophosphate poisoning, e.g., sedation, dyspnoea
and tonic-clonic spasms. Surviving animals had recovered within 3 to 6
days, and no substance-related gross organ changes were observed
(Bathe, 1973).
Diazinon given as an intraperitoneal single dose (40 mg/kg) to
rats produced tremors and convulsions with lactic acidosis. This was
accompanied by depletion of glycogen phosphorylase activity in the
triceps and diaphragm muscles 2 h after the administration (Husain &
Matin, 1986; Husain & Ansari, 1988).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rats
Davies & Holub (1980a,b) found that diazinon (99.2% purity) fed
to rats for 4 weeks at doses up to 25 mg/kg diet produced no visible
toxic manifestations such as tremors or hyperexcitability. Feeding
diazinon 25 mg/kg diet for 30 days produced more significant
reduction of cholinesterase activity in plasma (by 22-30%) and brain
(by 5-9%) among treated females than among males. Erythrocyte
acetylcholinesterase activity was significantly more depressed (by
13-17%) in treated females than in males at days 21-28 of the feeding
trial. The greater degree of cholinesterase inhibition in females was
possibly attributable to the higher amount of diazinon ingested by
females than by males after day 15 of the study.
Female Wistar rats were fed a semi-purified diet containing
either no pesticide or 0.1 to 15 mg/kg diazinon for up to 92 days. At
specified times, blood samples were taken to measure plasma and
erythrocyte cholinesterase activity using a radiometric method.
Additional rats were killed to determine brain cholinesterase
activity. Measurements included body weight gain and feed consumption
during the growing period. Feeding diazinon at the stated levels
produced no visible toxic manifestations. Treated animals showed
weight gain and feed consumption that was comparable to controls.
Feeding trials lasting up to 90 days revealed that rats were highly
sensitive to diazinon after 31 to 35 days exposure, as judged by
reduction of plasma and erythrocyte cholinesterase activities. Brain
acetylcholinesterase was found to be practically insensitive to
dietary intake of diazinon (1.0 to 15 mg/kg), although moderate
reduction (by 6%) of brain enzyme activity was noted among animals fed
10 mg/kg diet at day 92. For all feeding trials, plasma cholinesterase
was a more sensitive indicator of diazinon exposure than erythrocyte
or brain acetylcholinesterase. The NOAEL of diazinon for the rat was
estimated to be 0.1 mg/kg diet, which is equivalent to a daily intake
of 0.009 mg/kg body weight per day (Davies & Holub, 1980a).
In a feeding study, diazinon (87.7% pure) was administered to
groups of 15 male and 15 female Sprague Dawley rats for 13 weeks at
dietary concentrations of 0, 0.5, 5, 250 and 2500 mg/kg, corresponding
to mean daily doses of 0, 0.03, 0.3, 15 and 168 mg/kg body weight in
males and 0, 0.04, 0.4, 19 and 212 mg/kg body weight in females,
respectively. The rats treated at the highest dose level showed
hypersensitivity to touch and sound and some of the males showed
apparent aggressiveness, hyperactivity and soft faeces. Body weight
gain and food consumption were reduced in both sexes. At this dose
level, the haematological examination revealed a decreased haemoglobin
level, a lower haematocrit and an increased number of reticulocytes in
the females. The examination of blood biochemistry revealed a reduced
activity of serum cholinesterase in both sexes treated with 5 mg/kg
diet or more. In addition, the low-dose females showed a 17% reduction
in the erythrocyte cholinesterase activity. At 250 mg/kg diet or more,
the erythrocyte and brain cholinesterase activities were reduced in
both sexes. The absolute and relative liver weights were increased in
both sexes in the highest dose level, and there was microscopic
evidence of centrilobular hepatocellular hypertrophy (Singh et al.,
1988). The NOAEL, based on reduction in brain cholinesterase activity,
was 5 mg/kg diet (equivalent to 0.4 mg/kg body weight per day).
In a second, similar, study (Pettersen & Morrissey, 1994),
Sprague Dawley rats were fed diazinon at 0, 0.3, 30, 300 or 3000 mg/kg
diet for 13 weeks. Serum, erythrocyte and regional brain
cholinesterase inhibition was measured in groups of five males and
five females at weeks 4, 8 and 13. Serum and erythrocyte
cholinesterases were inhibited by 45-86 and 60-75%, respectively, at
30 mg/kg diet, but not consistently at 0.3 mg/kg diet. Brain regional
cholinesterase was inhibited by up to 25% in females at 30 mg/kg diet
and by 55-75% at 300 mg/kg diet. Males were less sensitive, showing no
effect at 30 mg/kg diet and a fall of 62-77% at 3000 mg/kg diet.
Observable neuromuscular deficits were seen only at 3000 mg/kg diet.
Consequently the NOEL and NOAEL for this study was 0.3 mg/kg diet
(equivalent to 0.019 mg/kg body weight per day), based on the
reductions in serum, erythrocyte and brain cholinesterase seen at the
next highest dose of 30 mg/kg diet. However, given the lack of an
intermediate dietary level of 5 mg/kg, which set the NOAEL in the
Singh et al. (1988) study, and the modest fall in brain cholinesterase
seen at 30 mg/kg diet in this second study, it is concluded that the
previous NOAEL of 5 mg/kg diet (0.4 mg/kg body weight per day) should
stand.
The effect of low levels of diazinon treatment on four marker
enzymes in rat heart and skeletal muscles have been investigated by
Wilkinson et al. (1986). Typical differences in succinate
dehydrogenase (SDH), lactate dehydrogenase (LDH), phosphofructokinase
(PFK) and hexokinase (HK) activities were observed between heart and
skeletal muscles. Diazinon feeding had no effect on heart, soleus,
gastrocnemius and plantaris SDH, LDH or PKF enzyme activities after
28 weeks. HK activity was significantly increased in sham-control
soleus and plantaris muscle after 28 weeks. Diazinon feeding inhibited
HK activity in plantaris muscle after 28 weeks treatment. These
results demonstrate that chronic low levels of diazinon have little
effect on the glycolytic and oxidative activity in heart and skeletal
muscles.
Adult Wistar rats were given diazinon by gavage twice weekly at a
dose of 0.5 mg/kg body weight for twenty-eight weeks. Selected control
and experimental animals were killed after 7, 14 and 28 weeks.
Histological examination of the liver revealed that animals repeatedly
treated with sublethal doses of diazinon sustain a form of hepatic
injury characterized by cellular lipid accumulation. The finding of
increased lipid accumulation in the liver following prolonged
treatment with diazinon, however, still does not resolve the question
of whether impaired lipid metabolism and/or storage is the primary
effect (Anthony et al., 1986).
Rats were exposed to diazinon-impregnated strips in a
conventional laboratory animal room. The air in the room was monitored
for the pesticide. Erythrocyte and plasma cholinesterase activities
were determined periodically. Air concentration of the pesticide never
exceeded 1.34 mg/m3. No significant change in enzyme activities were
observed (Hinkle et al., 1980).
7.2.1.2 Dogs
Feeding studies with dogs (Bruce et al., 1955; Williams et al.,
1959) did not differentiate between the sexes and therefore relevant
data from male and female animals were pooled. In another study
(Barnes et al., 1988) diazinon (87% pure) was given to groups of four
male and four female Beagle dogs for 13 weeks at concentrations of 0,
0.1, 0.5, 150 and 300 mg/kg diet, corresponding approximately to a
mean daily intake of 0.0034, 0.02, 5.9 and 10.9 mg/kg body weight
respectively. Vomiting was observed in groups fed a diet containing
150 or 300 mg/kg. Serum cholinesterase activity was reduced at
0.5 mg/kg or more in males and at 150 and 300 mg/kg in females.
Erythrocyte and brain cholinesterases activity was depressed at 150
and 300 mg/kg in both sexes. The histopathological examination
revealed a moderate atrophy of pancreatic acini in one high-dose group
of male dogs. Body weight gain was decreased in females at 150 mg/kg
diet and in both sexes at 300 mg/kg diet. The serum calcium level was
decreased in females at 150 mg/kg diet and in males at 150 and
300 mg/kg diet. The serum albumin level was decreased in both sexes at
300 mg/kg diet. As a slight reduction of serum cholinesterase activity
was the only change observed, 0.5 mg/kg diet, corresponding to a mean
diazinon intake of 0.02 mg/kg body weight per day, is considered to be
the dietary concentration causing no toxicological effect (NOAEL),
based on inhibition of brain and erythrocyte cholinesterase at higher
doses.
7.2.1.3 Pigs
Pigs were orally administered diazinon in capsules at doses of
0, 1.25, 2.5, 5 and 10 mg/kg body weight daily for periods of up to
8 months. In pigs, mortality and cholinergic signs of poisoning were
evident at 2.5 mg/kg body weight per day (FAO/WHO, 1979).
7.2.2 Inhalation
Two short-term inhalation studies were conducted in rats. In the
first study, diazinon (97.1% pure) was administered in an inhalation
chamber to four groups of nine male and female Tif RAIf rats for 6 h
per day, 5 days a week, for three weeks, at concentrations of 0, 151,
245 and 559 mg/m3. Four animals of each sex from the control group
and from the highest concentration group were kept for a 25-day post-
treatment observation period, while the others were sacrificed at day
21 after treatment. No compound-related deaths occurred. Exophthalmus
and diarrhoea were observed at all dose levels. In addition, the high-
dose group animals showed salivation, ruffled fur and tonic-clonic
convulsions during 2 h after each exposure. No toxic signs occurred in
the 25-day follow-up period. Food consumption was reduced during the
first three days of exposure in the high-dose group, plasma
cholinesterase activity was reduced in the intermediate and high-dose
groups, and brain cholinesterase activity was reduced at all dose
levels. Erythrocyte cholinesterase activity was reduced in the high-
dose group. The changes were reversible. There were no macro or
histopathological findings related to the exposure to diazinon (Zak et
al., 1973).
In the second study the main purpose was the definition of a
NOAEL for cholinesterase inhibition. Groups of ten male and ten female
Tif RAlf rats were exposed to diazinon 6 h a day for 5 days per week
for 3 weeks. The effective concentrations at the inhalation site
were 0, 0.05, 0.46, 1.57 and 11.6 mg/m3 air. There were no
compound-related signs, and no changes in body weight or food
consumption. In comparison with the control animals, the number of
erythrocytes, haemoglobin level and packed cell volume were slightly
lower in the highest-dose group. A minor decrease of brain
cholinesterase activity was noted in the highest-dose group of
females. Plasma glucose levels were significantly reduced among males
exposed to 1.57 and 11.6 mg/m3 (Hartmann, 1990). As the exposure to
0.46 mg/m3 inhibited the plasma cholinesterase only, this
concentration is the NOAEL.
7.2.3 Dermal
7.2.3.1 Rabbits
Diazinon (97.1% pure) was suspended in 50% aqueous polyethylene
glycol 300 and topically administered under semi-occlusive dressing to
groups of five male and five female albino rabbits at daily doses of
0, 1, 5 and 100 mg/kg body weight for 5 days per week for 3 weeks. In
the highest-dose group, four males treated at 100 mg/kg died during
the first week of treatment. Consequently, the dose was reduced to
50 mg/kg. Clinical signs were observed in the highest-dose group and
included anorexia, ataxia, fasciculations, tremors, diarrhoea,
hypoactivity, hypotonia and salivation. Most of the signs disappeared
after the dose was reduced. Mild dermal reactions were noted at site
of test substance administration. Body weight gain and food
consumption was similar in all groups, and most laboratory parameters
remained unaffected by the treatment. Reduced cholinesterase
activities were found in serum, red blood cells and the brain in
animals treated at >5 mg/kg. In the highest-dose group, the
reductions were significant for brain, red blood cells and serum
cholinesterase activities, while with 5 mg/kg there was a
statistically significant decrease of activity in the brain
cholinesterase of females only. In the highest-dose animals, the
histopathological examination showed a slight hyperkeratosis of the
skin at the site of treatment (Tai & Katz, 1984). The NOEL was
considered to be 1 mg/kg, based on inhibition of brain cholinesterase.
7.3 Long-term exposure
7.3.1 Rats
Groups of 30 or 40 Sprague Dawley male and female rats received
diazinon (87.7% pure) at dietary concentrations of 0, 0.1, 1.5, 125
and 250 mg/kg (equivalent to a mean daily diazinon intake of 0, 0.004,
0.06, 5 and 10 mg/kg body weight in males and of 0.005, 0.07, 6 and
12 mg/kg body weight in females) for 99 weeks. An additional control
group received 26.5 mg/kg diet epoxidized soybean oil, the stabilizer
used in technical diazinon, at the concentration corresponding to the
application to the test group of animals. Eight to ten randomly
selected animals per sex and dose group were killed after one year of
treatment, and nine or ten animals from the control group and from the
highest-dose group were killed after 4 weeks of recovery at week 56.
There were no compound-related clinical signs. At 250 mg/kg diet, the
mean body weight and the food consumption were slightly, but
significantly, increased in both sexes. Serum cholinesterase
activities were reduced at >1.5 mg/kg diet. Red blood cell and
brain cholinesterase activities were inhibited in groups fed on a diet
containing 125 or 250 mg/kg. Cholinesterase inhibition was at least
partially reversible during the 4-week recovery period after one year
of treatment. No treatment-related changes were seen during pathology
or histology examination (Kirchner et al., 1991). A pathological
re-evaluation of eyes and optic nerves from all control and high-dose
animals of the above study from interim necropsy (week 52), interim
recovery necropsy (week 56) and chronic necropsy (week 98/99) was
conducted. Histopathological lesions like cataracts, inflammatory
changes of the globe and surface of the eye and several different
types of retinal atrophy were found in both control and treated
animals at final sacrifice. None of these findings had an increased
incidence in treated rats compared to controls and they were not
regarded as compound-related. As the dietary concentration of
1.5 mg diazinon/kg, equivalent to a mean intake of 0.06 mg/kg body
weight per day, inhibited serum cholinesterase only, this dose level
was considered to be the NOAEL (Mann, 1993).
7.3.2 Dogs
Groups of Beagle dogs (four males, four females) received
diazinon (87.7% pure) for 52 weeks at dietary concentrations of 0,
0.1, 0.5, 150 and 300 mg/kg (equivalent to a mean daily intake of
0.0032, 0.015, 4.7 and 7.7 mg/kg body weight for males and 0.0037,
0.02, 4.5 and 9.1 mg/kg body weight for females). Owing to a general
lack of body weight gain, the highest dose level was reduced from
300 mg/kg diet to 225 mg/kg diet after 14 weeks of treatment. One male
dog from the highest-dose group showed emaciation and dehydration due
to severely reduced food consumption and weight reduction. In the
other animals from the highest-dose group (300 mg/kg/diet) the body
weight gain was also severely depressed during the initial 14 weeks of
treatment. After the reduction of dose to 225 mg/kg/diet, body weight
gain remained depressed in two of the males, while the females
returned to normal. A slightly reduced body weight gain was also noted
among males at 150 mg/kg diet. Food consumption was reduced in both
sexes fed on a diet containing >150 mg/kg diazinon. Haematology and
urine analysis showed no treatment-related changes. Significantly
decreased cholinesterase activities were noted at >0.5 mg/kg diet.
Serum cholinesterase activity was inhibited at 150 mg/kg diet at
all sampling intervals and at several occasions at 0.5 mg/kg diet. Red
blood cell and brain cholinesterase activities were reduced at
>150 mg/kg diet. In addition, a slight reduction in the mean serum
amylase activity was noted in both sexes fed a diet of >150 mg/kg.
The organ weight analysis showed no treatment-related changes and
macro- and histopathology were unremarkable (Rudzki et al., 1991). A
re-evaluation of eyes and optic nerves from all control and high-dose
animals from the study was conducted. No histopathological findings in
the eyes were noted (Mann, 1993). The dietary diazinon concentration
of 0.5 mg/kg, equivalent to a mean daily diazinon intake of
0.015 mg/kg, inhibited serum cholinesterase activity only, and was
considered to be the NOAEL, based on inhibition of brain and
erythrocyte cholinesterase.
7.3.3 Rhesus monkeys
This study was conducted with a 50% WP diazinon formulation
containing an actual concentration of 48.6% diazinon. The dose levels
given below refer to the active ingredient. Groups of three male and
three female Rhesus monkeys received initial daily doses of 0, 0.1,
1.0 and 10.0 mg diazinon/kg body weight, administered by gastric
intubation. After 34 days the doses were lowered to 0.05, 0.5 and
5.0 mg/kg and after 106 weeks of treatment the study was terminated.
Mortality was similar in all dose groups: the death of one animal
at each dose was of infectious etiology. Clinical signs included
tremor in the highest-dose group animals and an increased incidence
of soft stools was noted at 1.0 and 10.0 mg/kg. In comparison to
the controls, all treated animals gained slightly less weight.
Treatment-related deviations in the laboratory parameters examined
were limited to reductions of cholinesterase activities. At the
0.5 mg/kg dose level, the erythrocyte cholinesterase activity was
occasionally reduced in some animals and plasma cholinesterase
activity was consistently depressed. At the highest-dose level, plasma
and erythrocyte cholinesterase activities were markedly inhibited and
the brain cholinesterase activity was reduced in one monkey. The
postmortem examinations revealed no changes of toxicological
relevance. The daily dose of 0.5 mg diazinon/kg inhibited plasma and
(occasionally slightly) erythrocyte cholinesterase activity. The
toxicologically relevant brain cholinesterase activity remained
unaffected. Therefore, this dose level was considered to be the NOAEL,
based on inhibition of erythrocyte cholinesterase (Cockrell et al.,
1966).
7.4 Skin and eye irritation; sensitization
7.4.1 Primary skin irritation
Diazinon (0.5 ml undiluted, technical material) was applied to
the shaved skin of three male and three female New Zealand white
rabbits and covered with gauze patches and an impermeable material.
The dressing was removed after 4 h. Slight erythema and minimal oedema
were seen in all rabbits, which disappeared within 1 week. In the
report the compound received the descriptive rating as "slightly
irritant" to rabbit skin (Kuhn, 1989b,c).
7.4.2 Primary eye irritation
Diazinon (0.1 ml undiluted technical material) was instilled into
the conjunctival sac of three male and three female New Zealand white
rabbits. In three additional female rabbits, the treated eyes were
washed for 1´ min after the instillation of diazinon. Diazinon
produced mildly irritating reactions of the conjunctivae, which
disappeared within 72 h. It was rated as minimally irritating in
washed and unwashed eyes of rabbits (Kuhn, 1989d).
7.4.3 Skin sensitization
Diazinon was examined for skin sensitizing effects in 10 Hartley
Albino guinea-pigs. Ten animals were initially treated with 0.5 ml
undiluted technical diazinon, applied to the shaved skin under
semi-occlusive dressing for 6 h. After the third treatment one animal
died, most probably due to intoxication. Therefore, further treatments
were conducted with a 10% solution of diazinon in ethanol. After 11
treatments during the 3-week induction period and a 2-week rest phase,
no sensitization resulted after a single challenge administration
(Kuhn, 1989e).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
7.5.1.1 Rat
Diazinon (94.9% pure) was administered in the feed to groups of
30 male and 30 female Sprague Dawley rats for 10 weeks prior to
mating, throughout mating of the F0 animals and during two
generations up to weaning and sacrifice of the F2 pups. The dietary
concentrations used were 0, 10, 100 and 500 mg/kg. Compound-related
clinical signs and mortalities were limited to a few parental females
treated with 500 mg/kg diet. The food consumption was generally
comparable to, or slightly higher than, control values for females of
both parental generations in the diazinon-treated groups, while the
F1 males showed a decreased food consumption during the premating
period at both 100 and 500 mg/kg diet. The body weight increase was
reduced at 500 mg/kg in the F0 females and at 100 and 500 mg/kg for
the F1 animals of both sexes. There were no remarkable gross or
microscopic findings or effects on organ weights in either the F0 or
F1 generation. Reproductive parameters including precoital interval,
gestation duration, mating, fertility and pregnancy indices were
unaffected by the treatment in both generations at the dose levels of
10 and 100 mg/kg diet. At 500 mg/kg there was an increase in the
proportion of dams with prolonged gestation in both generations, and
in the F1 animals there was a trend toward a decrease in the number
of pregnancies and viable newborns and adverse effects on fertility
indices. Mating behaviour was unaffected by treatment. Litter size on
lactation day 0 was decreased in both the F1 and F2 pups at
500 mg/kg, whereas pup weight and sex ratio on day 0 were comparable
to controls for both generations. Decreases in pup survival and
corresponding decreases in pup weight were observed in both
generations at 500 mg/kg and in the F1 pups at 100 mg/kg.
Compound-related clinical and necropsy observations were noted in some
F1 pups at 500 mg/kg (tremor, no milk in stomach). NOAEL was 10 mg/kg
diet for pups and parental animals (Giknis, 1989).
7.5.1.2 Cattle
As a part of a survey to determine causes of abortion in
Wisconsin dairy cattle, the possible role of pesticides was examined.
Of 31 aborted fetuses examined, none contained traces of diazinon. Two
gravid, non-lactating Holstein Friesian cows were orally treated in
their feed with diazinon (Diazinon W 50 wettable powder formulation)
at daily doses of 6.6 mg/kg body weight during their second semester
of gestation until term. The calves were killed after parturition and
samples of perirenal fat, liver and kidney were investigated for
residues of diazinon, as were samples of colostral milk from the cows.
A histopathological examination of the calves, livers and kidneys was
conducted. Both cows treated with diazinon vomited and refused feed on
day 20 of treatment. No abortions occurred and no gross or
histopathological changes were found in the calves. No diazinon
residues were detected in the tissues investigated or in the milk
(Macklin & Ribelin, 1971).
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 Mice
Groups of 19-22 mated female F2 hybrid mice were orally treated
with diazinon (admixed to peanut butter, purity not specified) at
daily doses of 0, 0.18 and 9 mg/kg body weight from the day of mating
until delivery. The dams were weighed daily. After delivery, the
physiological and behavioural development of the pups was examined.
Mothers of all groups gave birth to viable offspring. Litter size and
the birth weights of the pups were similar in treated and untreated
groups. In the group receiving the higher dose of diazinon, the body
weight development of pups was depressed during their first postnatal
week. According to the authors, the testing of physiological and
behavioural development revealed subtle deviations from the normal
development in the offspring from the treated females. After sacrifice
at 101 days of age, the brains of the offspring of mothers treated at
the high-dose level showed ambiguous neuropathological changes in the
forebrains (Spyker & Avery, 1977). This study raises questions that
cannot be answered from the data presented.
7.5.2.2 Rats
Diazinon (purity 95%) was administered orally by gavage to groups
of 28 to 30 pregnant Sprague-Dawley-derived rats on days 6 to 15 of
gestation at dose levels of 0, 15, 50 and 100 mg/kg body weight On day
21 of gestation, all dams were killed and the fetuses delivered by
cesarean section. The dams of the 100 mg/kg group reacted to the
treatment by a marked decrease in food consumption and a body weight
loss in the early administration phase. The dams of the 15 and
50 mg/kg group showed no reaction. The parameters of reproduction
(number of corpora lutea, implantations, resorptions, fetal deaths and
viable fetuses) showed no treatment-related intergroup differences.
The fetal body weights were similar in all groups and the examination
of the offspring did not reveal any teratogenic effects of the
treatment. The NOAEL was 50 mg/kg body weight (Fritz, 1974).
In rats, doses (95.2 mg/kg body weight on day 9) that increased
maternal mortality reduced fetal development, as indicated by reduced
weight of litters and mild "hydronephrosis", but caused no real
teratogenic effect (Dobbins, 1967). A similar result was reported for
an intraperitoneal dosage of 100 and 150 mg/kg on day 11 (Kimbrough &
Gaines, 1968). Repeated administration (40, 50 or 60 mg/kg body weight
per day) on days 7 to 19 of gestation reduced the growth of the dams
but had no effect on the number of resorptions or corpora lutea, on
litter size, or on fetal weight. The cholinesterase activity of the
fetal brain was reduced. A dosage of 75 mg/kg body weight per day was
fatal to dams in 4 to 5 days (Hoberman et al., 1979).
Diazinon (97.4% pure) was administered by gavage to groups of 21
to 25 pregnant Sprague-Dawley rats from day 6 to 15 of gestation at
dose levels of 0, 10, 20 and 100 mg/kg body weight. On day 20 of
gestation, all dams were killed and the fetuses delivered by cesarean
section. In the highest-dose group of, dams a decreased food
consumption was noted during days 6-9 of gestation and the animals
lost weight. The body weight gain of the dams showed some recovery
thereafter, but the overall body weight gain of the highest-dose group
remained significantly below that of the untreated controls. The
numbers of corpora lutea and implantations were similar in all groups.
Resorptions were significantly increased only in the top-dose group
and, accordingly, the number of live fetuses was decreased. The fetal
body weights were higher in the top-dose group than in the untreated
controls. Three single instances of external malformations were
observed at the highest-dose level (one fetus with a filamentous tail,
one fetus with umbilical hernia and one fetus with sublingual
extraneous soft tissue). Since the malformations were not
morphologically related, they were considered to be secondary to
maternal toxicity. An increased incidence of rudimentary 14th ribs was
noted in the fetuses of the highest-dose group, although it remained
within the limits of historical controls. The finding was considered
to be related to fetotoxicity, secondary to severe maternal toxicity.
Other fetal anomalies were comparable between treated and untreated
groups. No evidence of teratogenic effect of diazinon was found
(Infurna & Arthur, 1985).
7.5.2.3 Hamsters
In Golden Syrian hamsters diazinon was administered as individual
oral doses (0.125 and 0.25 mg/kg body weight) during the period of
organogenesis. Technical grade diazinon diluted with corn oil was
used. The dose volume was 10 ml/kg body weight. Control animals
received corn oil on the same days of gestation. The hamsters were
killed on day 14 of gestation. All fetuses were examined for gross
defects when delivered by cesarean section. The viability of hamster
fetuses delivered on day 15 was checked by placing them in a chicken
hatching incubator for 6 h. All fetuses with enough developmental form
for determination of structural defects were counted in determining
the number of fetuses per litter. The total number of fetuses in each
treatment group was divided by the number of mothers surviving to
term. Fetuses dying in late embryonic life and resorption sites were
counted as dead fetuses. No bone defects were seen in four fetuses
chosen at random from each litter for staining and examination of
skeleton. Diazinon did not produce any terata when administered to
hamsters (Robens, 1969).
7.5.2.4 Rabbits
Robens (1969) reported that diazinon had not been found to be
teratogenic in rabbits given 7 or 30 mg/kg body weight, although the
higher dose of diazinon produced cholinergic signs. Diazinon
(89.2% pure) was administered orally by gastric intubation to groups
of 19 to 22 New Zealand white rabbits at daily doses of 7, 25 and
100 mg/kg body weight from day 6 to 18 of gestation. On day 30 of
gestation, all dams were killed and the fetuses delivered by cesarean
section. At the highest-dose level, 9/22 dams died and overt signs of
toxicity included tremors, convulsions, hypoactivity and anorexia.
There were no statistically significant differences among the group
regarding the mean number of implantations and the proportions of
live, dead or resorbed fetuses. The fetal weights were similar in
treated and untreated groups, and neither embryotoxicity nor
teratogenicity was observed (Harris & Holson, 1981).
7.5.2.5 Chicken
Several studies have investigated the teratogenic potential of
diazinon in chick embryos (Khera & Bedok, 1967; Misawa et al., 1981,
1982; Henderson & Kitos, 1982; Byrne & Kitos, 1983; Wyttenbach &
Hwang, 1984; Kushaba-Rugaaju & Kitos, 1985). In view of the lack of
teratogenicity in mammals and the lack of adequate data on
cholinesterase inhibition in ovo, these studies are not considered
relevant in the assessment of mammalian teratogenicity.
7.6 Mutagenicity and related end-points
The summary results of mutagenicity studies conducted with
diazinon are presented in Table 3.
Diazinon did not induce mutations in either Salmonella
typhimurium or Escherichia coli, but produced conflicting results
in mouse lymphoma L5178Y cells at the tk locus. Unscheduled DNA
synthesis was not induced in primary cultures of rat hepatocytes. A
sister chromatid exchange study with human lymphocytes cultured in
whole blood gave equivocal results, while negative results were
obtained in three other in vitro studies and, in vivo, in bone
marrow cells of dosed mice.
Chromosomal aberrations were not induced in cultured human
lymphocytes. In Drosophila melanogaster, diazinon did not induce
either complete or partial chromosome loss.
Table 3. Special studies on the mutagenicity of diazinon
Test system Test object Results Reference
In vitro
Ames Salmonella typhimurium negative Geleick & Arni (1990)
TA98, TA100, TA1535, TA1537,
TA1538; E. coli WP2 uvrA
Ames Salmonella typhimurium negative Marshall et al. (1976)
TA1535, TA1536, TA1537, TA1538
Mouse lymphoma assay Mouse lymphoma cells, negative Dollenmeier & Müller (1986)
L5178Y/tk +/-
Mouse lymphoma assay Mouse lymphoma cells positive McGregor et al. (1988)
L5178Y/tk +/-
Sister chromatid exchange study Chinese hamster cells V79 negative Chen et al. (1981)
Sister chromatid exchange study Human lymphoid cells (LAZ-007) negative Sobti et al. (1982)
Sister chromatid exchange study Chinese hamster V79 cells positive Matsuoka et al. (1979)
Sister chromatid exchange study Whole blood human lymphocytes equivocal Murli & Haworth (1990a)
Sister chromatid exchange study Chinese hamster V79 cells negative Kuroda et al. (1992)
Sister chromatid exchange study Chinese hamster V79 cells negative Nishio & Uyeki (1981)
Table 3. (con't)
Test system Test object Results Reference
Nucleus anomaly Chinese hamster V79 cells negative Hool & Muller (1981c)
Chromosomal aberration Human lymphocytes questionable Lopez & Carrascal (1987)
Chromosomal aberrations Human lymphocytes negative Lopez et al. (1986)
DNA repair test Rat hepatocytes negative Hertner & Arni (1990)
In vivo
Nucleus anomaly Chinese hamster bone marrow cells negative Hool & Müller (1981c)
Micronucleus test Mouse bone marrow cells negative Ceresa & Puri (1988)
Dominant lethal study Mouse, male negative Fritz (1975)
Chromosome aberrations Mouse spermatogonia negative Hool & Müller (1981d)
Chromosome aberrations Mouse spermatocytes negative Hool & Müller (1981b)
Chromosomal loss Drosophila melanogaster negative Woodruff et al. (1983)
Sister chromatid exchange study Mouse bone marrow cells negative Murli & Haworth (1990b)
Nuclear anomaly tests in cultured Chinese hamster V79 cells and
in bone marrow cells of dosed Chinese hamsters gave negative results.
A micronucleus test in bone marrow polychromatic erythrocytes of mice
was also negative.
No chromosomal aberrations were induced in the spermatogonia or
spermatocytids of dosed mice and a dominant lethal test in dosed male
mice was negative. It was concluded that diazinon is not genotoxic.
7.7 Carcinogenicity
7.7.1 Mice
Groups of 50 B6C3F1 mice of each sex were treated with diazinon
(98% pure) at dietary concentrations of 100 and 200 mg/kg diet for
103 weeks. Two additional groups of 25 males and 25 females each
served as untreated controls. Hyperactivity was reported for the dosed
mice but was rare in the control group. The body weight gains for all
dosed male mice were similar to the control group except for the last
20 weeks of the study period. The treated females showed slightly
lower body weights than the controls. The survival at 78 weeks in the
control 100 and 200 mg/kg groups, respectively, was: males, 21/25
(84%), 45/50 (90%), 49/50 (98%); females, 24/25 (96%), 50/50 (100%),
49/50 (98%). Several neoplasms were observed, but apart from lower
neoplasms in male mice none appeared to be treatment-related or had an
incidence in treated groups significantly different from that of
controls. The incidences of hepatocellular carcinomas in the control,
100 and 200 mg/kg groups, respectively, were: 4/21 (19%), 20/46 (43%)
and 10/48 (21%). The corresponding incidences of hepatocellular
carcinomas or adenomas were 5/2 (24%), 20/46 (43%) and 13/48 (27%).
The Cochrane-Armitage test for trend was significant for carcinomas,
but not for liver carcinomas and adenomas combined. Fisher's exact
test for comparison of a dosed group with its matched control groups
was significant (P=0.046) only for hepatocellular carcinomas alone in
the 100 mg/kg group of male mice. In view of the lack of a dose-
related response, the occurrence of liver tumours in male mice could
not be clearly attributed to diazinon (NCI, 1976).
A report submitted to the United Kingdom Ministry of Agriculture,
Fisheries and Food (MAFF, 1991) described a two-year combined chronic
toxicity/carcinogenicity study conducted with male and female B6C3F1
mice (60/dose/sex) administered diets containing diazinon at 0, 100,
200 or 300 mg/kg (males only) or 400 mg/kg (females only). Ten mice of
each sex per group were killed at 12 months. After 24 months, there
were reported to be no treatment-related gross or histopathological
findings. This study provides no evidence for the carcinogenicity of
diazinon in mice. In particular, it does not confirm the observations
described in the above-mentioned study on B6C3F1 mice.
7.7.2 Rats
Groups of 50 male and 50 female Fischer-344 rats received
diazinon (98% purity) at dietary concentrations of 400 and 800 mg/kg
for 103 weeks. All survivors were killed at 105 weeks. An additional
control group of 25 males and 25 females received an untreated diet.
There was no significant effect of treatment upon body weight gain.
Hyperactivity was noted in males and females of the 800 mg/kg group
and males of the 400 mg/kg group. Urine was discoloured in females of
the 800 mg/kg group and in dosed females there was vaginal bleeding
and discharge. Survival at 78 weeks in control, 400 mg/kg and
800 mg/kg groups, respectively, were: males, 24/25 (96%), 49/50 (98%),
49/50 (98%); females, 23/25 (92%), 44/50 (88%), 44/50 (88%). Various
neoplasms were observed, but only the incidence of lymphomas/
leukaemias in dosed groups of males was significantly different from
the controls. The incidences in the control, 400 and 800 mg/kg groups,
respectively, were: males, 5/25 (20%), 25/50 (50%), 12/50 (24%);
females, 2/25 (8%), 6/50 (12%), 6/50 (12%). The Fisher's exact test
for comparison with the matched control was significant (P=0.011) only
in the 400 mg/kg group of males. In view of the lack of a dose-related
response, the occurrence of lymphomas/leukaemias in male rats could
not be clearly attributed to diazinon. The frequency of endometrial
stromal polyps was increased in the dosed groups. This lesion, which
is commonly observed in Fischer-344 rats, occurred at incidences in
the control, 400 mg/kg and 800 mg/kg groups, respectively, of 2/23
(9%), 8/43 (19%) and 11/49 (22%). The carcinogenic status of diazinon
in this study is unclear (NCI, 1979).
The chronic toxicity study of Kirchner et al. (1991) described
earlier (section 7.3.1) included groups of male and female Sprague-
Dawley rats (approximately 20/dose/sex, progressing for longer than 57
weeks) administered diets containing diazinon at concentrations 0,
0.1, 1.5, 125 or 250 mg/kg. No treatment-related increases in
neoplasms were observed. This study does not provide supportive
evidence for a carcinogenic effect of diazinon in rats.
A report submitted to the United Kingdom Ministry of Agriculture,
Fisheries and Food (MAFF, 1991) described a 120-week combined chronic
toxicity/carcinogenicity study conducted with male and female F-344
rats (75/dose/sex) administered diets containing diazinon (0, 0.1, 1.5
or 22.6 mg/kg body weight per day). Rats of both sexes in the highest-
dose group that died late in the study (after week 105) showed
increased ulceration of the forestomach, with associated increased
incidence of acanthosis, hyperkeratosis, submucosal granulation tissue
and hyperplasia of the epithelium. Apparently, no neoplastic lesions
were observed in the study. This study provides no evidence for the
carcinogenicity of diazinon in rats. In particular, it does not
confirm the observations described in the above-mentioned study on
F-344 rats.
It is concluded that diazinon is not carcinogenic in rats or
mice.
7.8 Special studies
7.8.1 Neurotoxicity
In a range-finding experiment, groups of four domestic hens (red
heavy breed) were treated at four dose levels of diazinon (87% pure),
and an acute oral LD50 of 12.5 mg/kg body weight was determined. A
schedule was developed to protect the hens from acute cholinergic
effects. Atropine (10.0 mg/kg intramuscular) was administered prior to
the treatment with diazinon, and 2-PAM was injected at doses of
50 mg/kg at the time of the diazinon administration. In addition, the
hens received post-treatment protection with concurrent doses of
atropine and 2-PAM after approximately 1 and 5 h and as necessary
thereafter. In the main neurotoxicity study, diazinon was given as a
single dose of 28 mg/kg body weight by gastric intubation to a group
of 18 atropine-pretreated hens. All hens were observed for three weeks
and again treated with 13 mg diazinon/kg on day 21. The second
observation period also lasted for three weeks (day 22-42). Additional
groups, which served as negative controls, were given the vehicle
(10 hens treated with 1 ml/kg corn oil), and eight hens, treated with
tri- o-tolyl phosphate (500 mg/kg) served as positive controls. One
diazinon-treated hen died 5 days after the first dose and another one
6 h after the second dose. One hen from a negative control group died
on day 7. There were no neurotoxic signs apparent during either of the
two observation periods in the negative control group or in the
treated groups. No histopathological changes in the brain, spinal cord
or peripheral nerves were detected (Jenkins, 1988).
In a further test for delayed neuropathy Classen (1996) gave 20
hens a single oral dose of 100 mg diazinon/kg body weight. Therapeutic
treatment with physostigmine and atropine for up to 48 h resulted in
survival of 17 hens, despite a peak inhibition of brain cholinesterase
by 83%. No ataxia was seen over a 28-day survival period, and no
inhibition of either brain or spinal cord neuropathy target esterase
(NTE) was seen at either 24 (n=5) or 48 (n=5) h.
Diazinon (88% purity) was administered by gavage to 15 rats per
sex and dose at single doses of 0, 2.5, 150, 300 and 600 mg/kg. A
negative control group of 15 animals per sex received the vehicle
(corn oil) only. A group of 10 animals per sex served as positive
control and was administered a single dose of 150 mg/kg triademefon
60 min prior to the Functional Observational Battery (FOB). Two males
and one female dosed at 600 mg/kg died. A transient reduction in body
weight and body weight gain was observed in males dosed at 300 and
600 mg/kg during week one. Food consumption was reduced in the same
males, and in females dosed at >150 mg/kg. These findings decreased
in incidence and severity with decreasing dose. No treatment-related
effects were observed in the FOB on study days 8 and 15. In males
dosed above 150 mg/kg and in females dosed above 2.5 mg/kg, activity
counts in the figure-8 maze were significantly decreased at the
estimated time of peak effect only. Serum cholinesterase activity was
significantly decreased at the time of peak effect in all diazinon-
treated groups. Full recovery was observed in all groups on study day
15. Red blood cell cholinesterase activity was significantly inhibited
on study day 1 in animals of both sexes treated with >150 mg/kg.
Substantial recovery was observed on study day 15, but significant
inhibition still remained. No inhibition of brain cholinesterase
activity was detected on day 15. No neuropathological effects were
noted for diazinon at necropsy or upon histopathological examination.
It was concluded that the administration of diazinon resulted in
reversible neurotoxicity and cholinergic poisoning without
neuropathological changes. The NOAEL was 2.5 mg/kg body weight for
both sexes (Chow & Richter, 1994).
7.8.2 Effects on enzymes and transmitters
Male rats were treated bi-weekly by gavage with the equivalent of
0.5 mg/kg per day technical diazinon for up to 28 weeks. The animals
were killed at specific time intervals (7, 14 and 28 weeks) and
compared with age-matched controls. Blood and brain tissues were
analysed for cholinesterase activity and for concentrations of
catecholamines and amino acids. Only plasma cholinesterase activity
was significantly reduced. Erythrocyte cholinesterase and brain
cholinesterase were unchanged while during the same period the levels
of several putative brain neurotransmitters, aspartate, glutamate
(excitatory) and taurine as well as GABA (inhibitory) were
significantly reduced in experimental versus control animals. Blood
serotonin level was significantly elevated but no other blood or brain
monoamines were significantly altered. Whilst the authors (Rajendra et
al., 1986) concluded that oral administration of diazinon exerts
effects on brain neurotransmitters, even at the low-dose levels
administered in this study, the biological significance of these
findings is unclear.
Intraperitoneal administration of diazinon diminishes formation
of L-kynurenine by liver slices from 93% at 1 mg/kg dose to 56% at
40 mg/kg. The ratio of critical intermediates in L-tryptophan
biosynthetic pathway, L-kynurenine to N-formyl-L-kynurenine,
decreases and is reversed with increasing dosage of diazinon (Seifert
& Cassida, 1979).
Diazinon altered the formation of several L-tryptophan
metabolites associated with the L-kynurenine pathway in mice. Liver
kynurenine formamidase was inhibited almost completely by diazinon
(10 mg/kg). The enzyme inhibition resulted in reduced L-kynurenine
biosynthesis in livers and a concomitant accumulation of N-formyl-L-
kynurenine. However, in plasma, L-kynurenine level increased up to
5-fold in diazinon-treated mice. Consequently, the urinary excretion
of xanthurenic acid and kynurenic acid was raised 5- to 15-fold. The
revelation of this novel mechanism of diazinon action is an important
piece of information needed for a better understanding of the non-
cholinergic toxicity of organophosphorus acid triesters and
methylcarbamates (Seifert & Pewnim, 1992).
7.8.3 Effects on the immune system
Pregnant F2 dihybrid female mice received either a vehicle or 1
of 2 doses of diazinon (0.18 (n=19) or 9.0 (n=21) mg/kg body weight)
in the diet, daily throughout gestation. All mothers gave birth to
viable, overtly normal offspring at term. However, a significant
number (12%) of pups born to dams who received 9.0 mg/kg died prior to
weaning on day 28; necropsy findings were consistent with death from
respiratory infection. There was no significant difference in
mortality between control and diazinon-treated offspring once they
reached 28 days of age. Determinations of five different classes of
serum immunoglobulin (Ig) concentrations (IgG1, IgG2a, IgG2b, IgA,
IgM) at 101, 400 and 800 days of age indicated transient but
consistent disturbances of 2 Ig classes in offspring as a result of
prenatal diazinon exposure. IgG1 concentrations of male offspring
exposed to 0.18 mg/kg were significantly elevated at 101 days but not
at 400 or 800 days of age. IgG1 concentrations of female offspring
exposed to 9.00 mg/kg were significantly depressed at 101 days but not
different from controls at 400 or 800 days of age. Changes in IgG2b
levels generally were similar to those recorded for IgG1 but of
smaller magnitude. There were no significant effects on serum IgG2b or
IgM concentrations, and only equivocal effects on IgA, as a
consequence of prenatal exposure to either pesticide. This study
offers no conclusive information of an effect of diazinon on the
immune system (Barnett et al., 1980).
7.8.4 Effect on pancreas
Diazinon has been reported to cause acute pancreatitis and
ductal hypertension in dogs, which is due to the absence of
acetylcholinesterase in pancreatic sphincter, duodenal smooth muscle
in dogs and a reliance upon the more readily inhibited
butyrylcholinesterase (Dressel et al., 1980; Frick, 1987).
Kazacos (1991) examined the toxic effects of diazinon on the
guinea-pig pancreas. Guinea-pigs were given single intravenous dose of
diazinon at concentration ranging from 125 to 200 mg/kg body weight.
Examination of pancreatic tissue from animals killed at various time
intervals after dosing revealed acinar cell vacuolization after 24 h.
This cellular injury disappeared within 3 days after the initial toxic
insult. These are considered to be species-specific effects.
7.9 Factors that modify toxicity; toxicity of metabolites
7.9.1 Metabolic enzymes
Abdelsalam & Ford (1986) found that the toxic effects of diazinon
were increased by pretreatment with the hepatic microsomal enzyme
inducers dieldrin and phenobarbitone and that, at the same time, there
was a rise in the liver carboxylesterase activity. In this experiment,
the toxicity of diazinon increased when calves were pretreated with
dieldrin or phenobarbitone despite the increased activity of the liver
carboxylesterase. Apparently carboxylesterase activity was not
sufficient to protect against the toxicity of diazinon in the
pretreated calves. This suggests that either the increased
carboxylesterase activity had only a minor role in the hydrolytic
detoxification of diazinon or active metabolites, or that its effect
might have been overwhelmed by the action of other microsomal oxidase
activity concurrently induced by dieldrin and phenobarbitone leading
to a faster rate of activation than degradation of diazinon.
Abdelsalam & Ford (1987) described the effect of induced liver
and kidney lesions on the toxicity of levamisole and diazinon, two
antihelmintics routinely used in ruminant livestock management, and of
a lung lesion on the toxicity of diazinon in calves. Hepatic or renal
damage such as may occur naturally as a consequence of infection or of
the ingestion of poisonous plants or other toxic substances is,
therefore, likely to affect the metabolism and excretion of drugs
routinely used in antihelmintic control programmes. Liver damage in
calves, induced by the oral administration of the flukecide, carbon
tetrachloride, increased the toxic effect of diazinon but not of
levamisole, whereas the presence of a renal tubular lesion caused by
mercuric chloride enhanced the toxicity of both commonly used
antihelmintic compounds.
7.9.2 Antidotes
The effects of atropine/oxime therapy on diazinon-poisoned
animals was investigated in rats and rabbits. The rats were orally
treated with 235 mg/kg body weight (0.8 LD50) of diazinon (91.9%
pure), while rabbits received 1600 mg diazinon/kg body weight by the
subcutaneous route. In both species, intramuscular administration of
16 mg/kg atropine (10 min post treatment), and 30 mg/kg of pyridine
2-aldoxime methochloride (2-PAM Cl) 24 h later, significantly
increased the reactivation of the diaphragm cholinesterase. The oral
LD50 for diazinon in the rat was increased by a factor of 1.7 when
2-PAM Cl was administered intravenously and 3.1 times when 2-PAM Cl
was given orally. In order to prevent the reappearance of signs the
authors recommended that 2-PAM Cl be administered intravenously at the
same time as atropine followed by repeat oral administrations, as
needed (Harris et al., 1969).
The antidotal efficacy of pralidoxime iodide and obidoxime
dichloride was investigated in goslings poisoned by a supralethal dose
of diazinon. Various doses of both drugs were administered by
intramuscular injection when the poisoned birds were unable to walk.
Pralidoxime at 100 mg/kg induced recovery in 4 out of 6 poisoned
goslings, and 25 mg/kg successfully treated only 1 of 6 birds.
Obidoxime at 25 mg/kg showed no therapeutic properties, whereas 50
and 100 mg/kg delayed the death of some birds by several hours. At
100 mg/kg, all goslings had transient signs of intoxication, which
precluded the use of this compound as an antidote at higher doses
(Shlosberg et al., 1976).
7.9.3 Potentiation
In a study of the potentiation of the acute toxicity of diazinon
by several other pesticides (chlordimeform, iodofenphos, profenphos,
methacrifos), there was no potentiation at equitoxic doses (Sachsse &
Bathe, 1975, 1976, 1977, 1978).
8. EFFECTS ON HUMANS
8.1 Exposure of the general population
8.1.1 Acute toxicity, poisoning incidents
Several cases of intentional (Kabrawala et al., 1965; Banerjee,
1967; Wadia et al., 1974; Klemmer et al., 1978; Poklis, 1980; Wedin et
al., 1984; Hodgson & Smith, 1992) and accidental acute poisoning with
diazinon have been reported. Accidental poisonings were usually due to
ingestion of improperly stored liquid formulation (Hayes, 1963;
Zwiener & Ginsburg, 1988; Weizman & Sofer, 1992; Hodgson & Smith,
1992). Cases of poisoning after cutaneous application of diazinon for
lice treatment have also been described (Muratore et al., 1960; Hayes,
1963; Halle & Sloas, 1987). Several accidents involved children (Mizra
et al., 1972; English et al., 1970; Zwiener & Ginsburg, 1988; Hodgson
& Smith 1992; Weizman & Sofer, 1992; Wagner & Orwick, 1994).
Generally, symptoms and signs were typical of AChE inhibition,
which responded to atropine and oxime treatment. In a case of fatal
suicidal ingestion of diazinon reported by Poklis et al. (1980),
diazinon concentrations in postmortem body fluids and tissues were
found to be 277 mg/litre in blood, 200 mg/litre in the bile and
15 mg/kg in adipose tissue. In the stomach 44 mg were found. Cases
with atypical or unusual features are described in section 8.1.1.3. No
cases of delayed polyneuropathy have been observed, as would be
expected from the negative animal data. The following sections deal
with some specific aspects of acute poisoning by diazinon (i.e., the
acute pancreatitis, the intermediate syndrome, and some unusual case
reports).
8.1.1.1 Acute pancreatitis
Increased levels of serum amylase and glucose have been described
in some severe cases of diazinon poisoning. In certain cases these
enzymatic alterations were accompanied by prominent abdominal symptoms
and signs, including abdominal rigidity. All these were considered
indicative of acute pancreatitis (Dressel et al., 1979; Dagli et al.,
1981; Lee, 1989; Weizman & Sofer, 1992). On one occasion a pancreatic
cyst was observed (Dressel et al., 1979). In all cases, pancreatitis
was mild and patients fully recovered. Severe poisoning with other
organophosphates, e.g., parathion (Weizman & Sofer, 1992), coumaphos
(Lee, 1989) and malathion (Lee, 1989), was also associated with acute
pancreatitis. Apparently, poisoning with an unknown carbamate was also
associated with acute pancreatitis (Weizman & Sofer, 1992).
8.1.1.2 Intermediate syndrome
Senanayake & Karalliedde (1987) described a syndrome caused by
organophosphate pesticides and named it "intermediate", because its
onset was delayed after cholinergic syndrome but appeared earlier than
polyneuropathy. The syndrome, appeared 24-96 h after poisoning during
recovery from the cholinergic crisis. It was characterized by
paralysis of proximal limb muscles, neck flexors, motor cranial nerves
and respiratory muscles. It was resistant to atropine treatment and,
in certain patients, required assisted ventilation. It is noteworthy
that Wadia et al. (1974), reporting the neurological manifestations
seen in 200 consecutive cases of poisoning, mostly with diazinon,
described two different types of signs: type I, characteristic of the
cholinergic syndrome and responsive to atropine, and type II, present
in about 20% of the cases, which appeared at least 24 h later,
resembled those of the intermediate syndrome and were not responsive
to atropine (Wadia et al., 1974).
Other cases of intermediate syndrome due to diazinon poisoning
have been described (Hall & Baker, 1989; Samal & Sahu, 1990).
8.1.1.3 Unusual case reports
Three-week-old twins were hospitalized because of respiratory
distress. One was cyanotic on admission, but both had rapid shallow
breathing, profuse nasal and bronchial secretions, and pinpoint
pupils; muscle fasciculations were not detectable. At 48 h only the
sicker twin had slightly reduced pseudocholinesterase activity.
Treatment was appropriate and recovery uneventful. Investigation
revealed that the babies lived in one side of a two-story house that
had been divided into two apartments by partitions. About 10:30 h on
the previous day the other apartment had been sprayed with 1% diazinon
for cockroach control using approved spot applications directed mainly
at cracks. The twins were the only ones who had remained indoors. The
observed degree of respiratory distress in the presence of little or
no inhibition of cholinesterase was consistent with exclusively
respiratory exposure (English et al.1970).
A 12-week-old infant girl developed persistent hypertonicity of
the extremities without other signs of intoxication. It was discovered
that the organophosphate insecticide diazinon was applied in her
home 5 weeks prior to the onset of signs. Six months after
application, high levels of diazinon residues were found on the floor
(230 ng/cm2), in vacuum cleaner dust (1700 mg/kg), and in the air
(2.8 ng/m3). A diazinon dose of approximately 0.02 mg/kg per day was
calculated and derived from the infant's urine level of diazinon
metabolites determined 6 months after application of diazinon in her
home. Her muscle tone returned to normal shortly after the infant was
removed from the home (Wagner & Orwick, 1994).
According to Hata et al. (1986), atypical ocular bobbing resulted
from an intentional poisoning from diazinon. The authors suggest
acetylcholine as a neurotransmitter substance within the ocular motor
pathway.
A 26-year-old man, who ingested approximately 230 ml of a
solution of an unknown concentration of diazinon in a suicide attempt,
developed a severe cholinergic syndrome which was relived by atropine
and 2-PAM. Diuresis was greatly reduced (22 ml/h) and urine was dark
and cloudy (specific gravity 1.029). It is possible that significant
dehydration may have precipitated this reaction (Wedin et al., 1984;
Albright, 1984).
8.1.2 Controlled human studies
A cumulative toxicity study was conducted with human volunteers.
Four healthy males weighing between 74 and 96 kg and aged between 30
and 45 years ingested diazinon in gelatin capsules (95.4% pure) at a
dose of 0.025 mg/kg per day. Two males received 34 consecutive
treatments (Group B), while the two other volunteers were treated for
4 days, were left untreated for the next 5 days in order to
investigate reversibility of the effects and then received the
capsules again for 32 more days (Group A). The daily dose was split
into three administrations taken after meals around 9, 13 and 19 h.
Parameters of haematology, urine analysis and blood biochemistry
(plasma and erythrocyte cholinesterase, serum alkaline and acidic
phosphatase activities) were determined at regular intervals. A
reversible decrease of plasma cholinesterase activity was observed in
group A during the first 4 days of treatment while the erythrocyte
cholinesterase activity remained unaffected. During the second
treatment period of group A and during the entire treatment of group
B, the cholinesterase activities were similar to those observed at
pretest. No clinical signs or changes in other parameters were noted.
It was concluded that the administered daily dose of 0.025 mg/kg
marginally inhibited the plasma cholinesterase only, and can therefore
be considered as a NOAEL in humans (Payot, 1966).
8.2 Occupational exposure
8.2.1 Acute poisoning
Poisoning following occupational exposure to diazinon was usually
associated with improper or prolonged storage of the commercial
formulations, which, prior to the improvements carried out by the
manufacturer, tended to give rise to more toxic impurities (mainly
TEPP).
Soliman et al. (1982) investigated two spraymen who had a
cholinergic syndrome (with about 90% red blood cell AChE inhibition)
after using a commercial formulation of diazinon which was packaged in
tin-plated cans that had replaced the more expensive aluminium cans.
Analysis of such commercial formulation revealed the presence of TEPP,
sulfo-TEPP, monothiono-TEPP and other impurities, but no diazinon.
Mello et al. (1972) reported an episode where three farmers were
poisoned when using a commercial formulation of diazinon that was
transferred from the original container to another container and then
stored for some time. This formulation contained monothiono-TEPP and
was about 30 times more toxic to rats than a recently prepared and
properly stored formulation. On that occasion, 26 of 40 cows died when
treated against tick infestation with this formulation.
A fatal case of poisoning by diazinon and malathion, possibly by
inhalation, was described by Wecker et al. (1985). The 51-year-old man
was found unconscious in the closed shed where he sprayed three cows
with the pesticides.
Two cases of cutaneous hepatic porphyria of toxic origin were
identified within a short period of time. Both patients were farmers
and recently handled very intensively, without any precaution, some
pesticides containing organophosphorus substances (diazinon). The
symptoms were similar to those of the so-called Turkish porphyria
(Bopp & Kosminsky, 1975).
8.2.2 Effect of short-term and long-term exposure
Neurophysiological investigations and determinations of blood
cholinesterase activities were carried out on 11 Swedish spraymen
exposed to bromophos, diazinon, dursbane and malathion. Plasma
cholinesterase activity was significantly reduced after work, while
erythrocyte cholinesterase activity was unchanged. In none of the
workers with a decreased plasma cholinesterase activity after work
could any related acute neuromuscular disturbance be detected when
the men were tested with repetitive nerve stimulation and with single
fibre electromyography. Signs of subclinical neuropathy were present
as a slight reduction in sensory conduction velocity and increased
fibre density in some workers (Stalberg et al., 1978).
A cohort of 99 workers exposed to diazinon was tested before and
after the work shift with a neurobehavioural test battery, which
included a brief examination, a symptom questionnaire and tests of
concentration, eye-hand coordination, pattern recognition, visual
memory and finger tapping. The median diazinon exposure level was
2.1 mg/day and the mean duration of exposure was 39 days (see section
5.3). There was no correlation between the diethylthiophosphate (DETP)
concentrations or diazinon exposure and pre- or post-shift
neurobehavioural function with linear regression models after
adjusting for age, sex, education and alcohol intake. The study failed
to demonstrate adverse behavioural effects of repeated, low-level
diazinon exposure (Maizlish et al., 1987).
Three groups of agricultural workers with a history of exposure
to organophosphate pesticides were followed up to evaluate the utility
of sequential post-exposure cholinesterase analyses to confirm
organophosphate intoxication in the absence of baseline cholinesterase
values. Three or more cholinergic symptoms were reported by 50 of the
72 patients. Pre-exposure red blood cell cholinesterase activities of
45 workers were above the lower limit of laboratory normal range.
Follow-up examinations, including blood cholinesterase activity
analyses, were conducted in 57 subjects. When final post-exposure
cholinesterase activity determinations were compared with respective
individual normal baseline values, the plasma and red blood cell
activities were shown to be inhibited. The data support the use of
sequential post-exposure blood cholinesterase analyses to confirm the
diagnosis of organophosphate-induced illness in the absence of
baseline values (Coye et al., 1987).
Of 67 described poisoning incidents in California involving 583
people, nineteen involved a pesticide product containing an
organophosphate: most often chlorpyriphos (8), diazinon (3) and
malathion (5). There were also 10 cases that resulted from suicide,
and two cases involved diazinon (Maddy & Edmiston, 1988).
In a study of organophosphate-induced contact dermatitis in 202
patients in Japan, Matsushita et al. (1985) attributed the reactions
mainly to diaoxabenzafos, fenitrothion, leptophos, cyanophos, diazinon
and malathion. The areas affected by dermatitis were fingers (62.4%),
face (39.6%), forearm (31.6%) and neck (29.7%). One quarter of the
cases with dermatitis had symptoms associated with acute
organophosphate poisoning.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Singh (1973) found that three genera of cyanobacteria (blue-green
algae) tolerated diazinon at concentrations of 300 and 400 mg/litre.
Clegg & Koevening (1974) indicated that the population densities of
three species of freshwater algae were relatively unaltered by a
concentration of 100 mg/litre. Butler et al. (1975) demonstrated that
diazinon inhibited growth of numerous species of green algae and
cyanobacteria at concentrations of 0.01 mg/litre and 0.1 mg/litre.
Wong & Chang (1988) studied the effects of diazinon on the growth of
the green alga, Chlamydomonas reinhardtii. They observed a reduction
in growth at concentrations of 5 and 10 mg/litre and complete
inhibition at 20 and 40 mg/litre.
Doggett & Rhodes (1991) determined the effects of diazinon on
phytoplankton population dynamics. The objectives of this study were
to determine the effects of diazinon (1, 5, 10, 20 and 40 mg/litre
concentration) on the growth rates of three widely distributed species
of freshwater algae, and to ascertain the effects of this pesticide on
the diversity of a natural phytoplankton assemblage. Stimulation of
growth was exhibited by Selenastrum capricornutum and Chorella
exposed to diazinon at 1 and 5 mg/litre. The higher concentrations of
10, 20 and 40 mg/litre all inhibited growth. The growth rates of the
cyanobacteria showed a high degree of tolerance; a suppression of
growth was observed only at 40 mg/litre. Two genera of green algae
were stimulated by all concentrations.
The acute toxicity of diazinon to the freshwater rotifer
Brachionus calyciflorus was determined after 24 h of exposure; the
mean LC50 value was 29.2 mg/litre. Based on this result, four
sublethal concentrations were chosen to determine the median lethal
time (LT50) at each concentration of diazinon tested. The
concentrations tested were 1/5, 1/4, ´ and 2/3 of the LC50 (24 h).
The LT50 values ranged from 6.96 to 2.49 days after diazinon
exposure, decreasing with increasing exposure concentration
(Fernandez-Casalderrey et al., 1992).
Several species of soil-borne fungi invade plant roots forming
vascular-arbuscular mycorrhizae which aid in growth and nutrient
element absorption. Pre-plant incorporated treatments at 2 and 4 kg/ha
of trifluralin and diazinon had no significant effect on growth,
phosphorus accumulation or root colonization by mycorrhizal fungi in
soybeans planted in an Andover clay loam. At currently used commercial
rates, diazinon did not affect mycorrhizal development under the
conditions of the experiment (Burpee & Cole, 1978).
Nitrogenase activity of excised soybean nodules was severely
affected by diazinon following application of 3, 1, 0.5 and 0.25
multiples of the amounts stated on the label (Hensley, 1991).
9.2 Aquatic invertebrates
Acute toxicity of diazinon to aquatic invertebrates is summarized
in Table 4.
The effect of diazinon (Basudine, 0.01, 0.1, 1.0 mg/litre) on the
physical and chemical properties of haemolymph of Lymnaea stagnalis
(L.) and Planorbius corneus (L.) infected with trematodes was
studied by Stadnichenko et al. (1987). Haemolymph viscosity and
density decreased after the treatment.
Robertson & Mazzella (1989) determined the toxicity of diazinon
to the freshwater snail Gillia altilis. Based on nominal
calculations, the LC50 values for static 4- and 96-h exposures were
340 mM (93 mg/litre) and 40 mM (11 mg/litre), respectively.
Experiments were conducted to determine the effects of piperonyl
butoxide, a synthetic methylenedioxyphenyl inhibitor of cytochrome(s)
P-450, on the toxicity of organophosphate insecticides to three
cladoceran test species: Ceriodaphnia dubia, Daphnia magna, and
Daphnia pulex. The acute toxicity of diazinon to three tests species
was similar, with LC50 values ranging from 0.50 to 0.80 mg/litre.
Co-administration of piperonyl butoxide effectively reduced the acute
toxicity of metabolically activated diazinon, and piperonyl butoxide
concentrations of 500 or 1000 mg/litre completely blocked the toxicity
of diazinon at concentrations of up to 16 times the 48-h LC50. In the
case of D. pulex, piperonyl butoxide at 500 mg/litre also
significantly reduced the toxicity of diazinon at concentrations of up
to 16 times the 48-h LC50 (Ankley et al., 1991).
9.3 Fish
The acute toxicity of diazinon to fish is summarized in Table 5.
The median lethal concentration (LC50) of diazinon was
determined for Clarias batrachus; the LC50 for 40 days of exposure
to diazinon was 2.4 mg/litre (Tripathi, 1992). The acute toxicities
(24, 48, 72 and 96 h) of eight pesticides to Anguilla anguilla were
determined. The 24- to 96-h LC50 values for diazinon ranged from 0.16
to 0.08 mg/litre (Ferrando et al., 1991).
Bresch (1991) studied the effects of diazinon on early
life-stages in zebrafish. Zebrafish kept at diazinon concentrations of
0.2, 0.04 and 0.008 mg/litre grew alike, and no differences among the
groups were observed. Survival rates of animals of different groups
did not differ either. In no groups were abnormal fish seen.
Table 4. Acute toxicity (LC50) to aquatic invertebrates
Species Duration (h) Concentration (mg/litre) Reference
Snail (Gillia altilis) 96 11 Robertson & Mazzella (1989)
Cladocerans 48 0.0005-0.0008 Ankley et al. (1991)
Cladoceran (Daphnia pulex) 48 0.001 (0.0006-0.0011) Mayer & Ellersieck (1986)
Cladoceran (Simocephalus serrulatus) 48 0.0014 (0.0012-0.0016) at 21°C Mayer & Ellersieck (1986)
0.0018 (0.0014-0.0022) at 15°C
Scud (Gammarus fasciatus) 96 0.0002 (0.00015-0.00028) Mayer & Ellersieck (1986)
Shrimp (Hyallela azteca) 96 0.004-0.006 Collyard et al. (1994)
Desmocaris trispimosa 96 0.0208 (0.0192-0.0224) Ebere & Akintonwa (1992)
Shrimp (Palaemonetes africanus) 96 0.0179 (0.0147-0.020) Ebere & Akintonwa (1992)
Insect larva (Pteronarcys californica) 96 0.025 (0.020-0.030) Mayer & Ellersieck (1986)
Table 5. Acute toxicity (LC50) to fish
Species Duration (h) Concentration (mg/litre) Reference
Bluegill sunfish (Lepomis macrocharis) 96 0.17 (0.012-0.22) Mayer & Ellersieck (1986)
Rainbow trout (Oncorynchus mykiss) 96 0.09 Mayer & Ellersieck (1986)
Cutthroat trout (Salmo clarkii) 96 2.76 (2.28-3.33) in soft water Mayer & Ellersieck (1986)
1.7 (1.39-2.09) in hard water
Walking catfish (Clarias batrachus) 2.41 Tripathi (1992)
Eel (Anguilla anguilla) 96 0.08 (0.06-0.10) Ferrando et al. (1991)
Channel catfish (Channa punctatus) 96 3.1 Sastry & Malik (1982a)
Goby (Gobius) sp. 96 0.25 (0.23-0.28) Ebere & Akintonwa (1992)
Lake trout (Salvelinus namaycush) 96 0.6 (0.4-0.9) Mayer & Ellersieck (1986)
Table 6. Summary of brain acetylcholinesterase activity inhibition by
diazinon in aquatic organisms
Organism Exposure Duration Inhibition Reference
concentration (h) (%)
(mg/litre)
Bluegill 0.1 6 95 Weiss (1961)
Fathead minnow 0.1 18 70 Weiss (1961)
Goldfish 0.1 18 43 Weiss (1961)
Golden shiner 0.1 18 40 Weiss (1961)
Sheepshead minnow 6.5 24 71 Goodman
et al.
(1979)
Whereas growth and survival of fathead minnows were not
influenced at concentrations below 0.2 mg/litre in a test described by
Allison & Hermanutz (1977), the minnows responded more sensitively in
a similar test by another laboratory: effects were observed below
0.08 mg/litre (Jarvinen & Tanner, 1982). Allison (1977) found reduced
larval growth of the flagfish if reared in water containing diazinon
at a concentration of 0.014 mg/litre.
Brain acetylcholinesterase (AchE) activity and changes in
optomotor behaviour were determined in bluegill sunfish, Lepomis
macrochirus, exposed to graded concentrations (0, 15, 30, 5, 60 and
75 µg/litre) of diazinon for a period of 24 h (Dutta et al., 1992). A
significant decrease of AchE activity was observed at and above an
exposure concentration of 45 µg/litre, whereas a decline in the scores
of the "following" responses of the fish was observed at an exposure
concentration of 30 µg/litre.
Weiss (1961) was the first to investigate in vivo brain AChE
activity of fish exposed to organophosphates. He found that the extent
of enzyme inhibition was proportional to the concentration of the
substance and extent of exposure, and suggested that fish brain AChE
activity could be used to detect the presence of anticholinesterases
in the aquatic environment.
Goodman et al. (1979) reported a dose-dependent inhibition of
brain AChE activity in sheepshead minnows for diazinon. For the same
sublethal exposure (6.5 mg/litre), inhibition was independent of the
exposure duration, being 68 and 78% on days 4 and 108, respectively.
The authors found that the brain AChE activity of sheepshead minnows
exposed to diazinon varied and was susceptible to exposure
concentration. AChE was depressed long after no measurable diazinon
was found in exposed sheepshead minnows.
The effect of acute exposure to diazinon (3.1 mg/litre for 96 h)
and chronic exposure to a sublethal concentration (0.31 mg/litre) of
diazinon has been studied in the liver, stomach, intestine and pyloric
caeca of a freshwater teleost fish, Channa punctatus. In acute
exposure, succinate dehydrogenase activity was elevated in intestine
and pyloric caeca. No alteration was noted in lactate dehydrogenase
activity but pyruvate dehydrogenase activity was inhibited in pyloric
caeca. Chronic exposure resulted in inhibition of the activities of
the three dehydrogenases in all four organs at both intervals (Sastry
& Malik, 1982a).
Exposure to diazinon at different concentrations induced
pathological changes in muscle cell organelles of Tilapia nilotica.
The earliest changes in muscles of fish exposed to 10 mg/litre
consisted of swelling of sarcoplasmatic reticulum in many fibres and
the appearance of many cytoplasmatic vacuoles of different sizes. In
muscle of fishes exposed to the LC50 (20 mg/kg) of diazinon,
fragmentation of the myofibrils occurred over the entire length of the
sarcomere with involvement of both the thick myosin filament of the
A-band and the thinner actin filaments of the I-band. It was
speculated that these effects may be attributed to the
anti-cholinesterase activity of these insecticides. (Sakr & Gabr,
1992).
Acute effect of diazinon on the intramembranous particles
(IMPs) of microvilli of the intestinal epithelial cells of Tilapia
nilotica fish was studied using the freeze-fracture technique.
Exposing fish to different repeated concentrations of diazinon
(´ LC50) cause a significant decrease in the population density of
IMPs in P and E faces. IMPs of microvilli found in intestinal
epithelial cells are thought to represent many kinds of protein
including enzymes. It was suggested that diazinon induced a reduction
in enzymatic content of the membrane, which was accompanied by a
decrease in the IMP density of the microvilli (Sakr et al., 1991).
El-Elaimy et al. (1990) reported that exposure of Tilapia nilotica
to increasing concentrations of diazinon induced ultrastructural
alterations in the intestine.
Four-month old adult siblings of zebrafish (Brachydanio rerio)
were exposed to four concentrations of diazinon for up to 168 h. DNA,
RNA, protein and total free amino acid content were measured in the
liver. The DNA, RNA and protein contents were significantly reduced,
whereas the amino acid content was significantly enhanced. All these
changes showed a dose-dependent as well as a time-dependent response
(Ansari & Kumar, 1988).
Anees (1978) presented the results of sub-lethal exposure to
diazinon of an adult freshwater teleost. The concentrations of
insecticide used in the study were given as 0.37 mg/litre per 24 h and
0.28 mg/litre per 96 h. This was a study on the evaluation of
histopathology as a possible indicator of aquatic pollution by
insecticides. Hepatocytes were considerably vacuolated and reduced in
size after 24 h of exposure. However, 96 h of exposure produced less
vacuolization but the liver showed a foamy appearance. Damage to the
hepatic blood supply and the appearance of dark, granular cytoplasmic
inclusions resulted from a 14-day exposure. The same concentrations of
pesticide have already been observed to cause many tissue changes in
the intestine of Channa punctatus and disturbances in the
distribution of serum proteins.
The effect of exposure to the LC50 (12 mg/litre) for 96 h
and to a sublethal concentration (3.3 mg/litre) for 15 and 30 days
was studied in the digestive system of the catfish Heteropneustes
fossilis. The most conspicuous pathological changes in the liver
were vacuolization of the cytoplasm of hepatocytes, enlargement of
nuclei, rupture of cell membrane, liver cord disarray, and damage of
connective tissue. Intercellular spaces were widened. In stomach the
mucosa was eroded. In intestine the nuclei of the columnar epithelial
cells were reduced in volume and the cytoplasm was highly degenerated.
In both acute and chronic exposure alkaline phosphatase and
glucose-6-phosphatase activities were inhibited significantly in the
different portions of the digestive system. Acid phosphatase activity
was elevated in all the portions and in both stages of exposure. In
acute exposure insignificant elevation was observed in the activities
of amylase and maltase, while lactase activity was inhibited. An
inhibition in maltase activity was significant only in the liver.
Lipase activity showed a decrease in all stages of exposures, while
there was no marked alteration in activities of pepsin and trypsin
(Sastry & Malik, 1982b).
9.4 Effects in mesocosms and the field
A mesocosm study was performed with 17 treated and 4 untreated
ponds (each 0.1 acre in surface area and 2.2 metres deep). The ponds
received multiple applications of diazinon with single applications
ranging from 2 to 25 µg/litre and corresponding to total application
rates of 5.7, 11.4, 22.9, 45.8 and 91.5 µg/litre. The following
effects on sediment-dwelling organisms were observed: Trichoptera was
the most sensitive order, with significant reductions at all treatment
levels throughout most of the treatment period. Trichoptera was a
minor component of the macroinvertebrate communities in the mesocosms.
Diptera and Ephermeroptera were intermediate in sensitivity; both were
reduced at 22.9 and 91.5 µg/litre by the end of the treatment period,
though both recovered to control levels shortly after treatment
ended. Odonata was the least sensitive order with effects only at
91.5 µg/litre. Total insect abundance generally followed the trend
of the Diptera, which was the most abundant order; increasingly
severe effects at 22.9 to 91.5 µg/litre during the treatment
period was followed by recovery. The abundance of crustaceans in
macroinvertebrate samples generally followed a similar pattern.
Gastropods were essentially unaffected by diazinon. Though dipterans
as a whole were affected only at 22.9 µg/litre or more and recovered
rapidly, some dipteran sub-taxa were more sensitive or were
significantly reduced in the post-treatment period. Tanypodinae and
its dominant tribe, Pentaneurini, were the most sensitive. These were
significantly reduced in all treated ponds at the end of the treatment
period. Both groups recovered within eight weeks of the last
treatment. The sub-family Chironominae was the next most sensitive
among the family Chironomidae, especially the tribes Tanytarsini and
Chironomini. Tanytarsinids were reduced at 22.9 µg/litre early in the
treatment period and then recovered. Chironomini followed a similar
pattern, including recovery, but were again reduced in number at 22.9
to 91.5 µg/litre. The sub-family Orthocladiinae was the least
sensitive chironomid group with effects only at 91.5 µg/litre and full
recovery within two weeks. With the wide range of sensitivity observed
among the chironomids, the overall impact of diazinon was relatively
minor; statistically significant reductions were observed twice early
in the highest treatment. Two less abundant dipteran families,
Chaoboridae and Ceratopogonidae, were affected at 11.4 µg/litre or
more. The greatest effect on Chaoboridae occurred two months into
treatment and that on Ceratopogonidae occurred during the treatment
period, but sporadic effects persisted beyond (Giddings, 1992).
The application of diazinon to a small stream at 3 mg/litre
resulted in increased drift of benthic organisms and changes in the
composition of benthic fauna. These changes were not persistent,
however, as the communities were restored to normal within 4 weeks of
treatment (Miller et al., 1966).
9.5 Terrestrial invertebrates
Stevenson (1978) reported LD50 values for bees of 0.22 µg/bee
(topical) and 0.20 µg/bee (oral).
When earthworms (Eisenia foetida) were exposed to technical
diazinon in soil, the 14-day LC50 was calculated to be 130 mg/kg
soil. A no-observed-effect concentration of 12.3 mg/kg was reported
(Vial, 1990).
In a field experiment in Connecticut, USA, diazinon was
applied to tobacco fields as a 10% granular formulation at up to
4.48 kg a.i./ha; granules were raked into the soil. Mortality of
earthworms (Lumbricus terrestris) did not differ between treated and
control plots (Kring, 1969).
9.6 Birds
Acute toxicity to birds is summarized in Table 7.
The use of diazinon for controlling flies in sheds used to house
ducks led to the death of an estimated 15 600 young birds (Dougherty,
1957).
Ingestion of a few granules could be lethal to sparrow-sized
birds for diazinon 14G (Hill & Camardese, 1984).
Variability of toxicity among anticholinesterase formulations was
shown with a single dose of diazinon administered orally as technical
grade (TG, 99% a.i.) alone or in corn oil, as granular (GR, 14-15%
a.i.), or as emulsifiable concentrate (EC, 48% a.i.). The rank of the
formulations tested, from most to least toxic, based on statistically
separable (p<0.05) LD50s, was EC > GR=TG > control. The difference
between the least and most toxic form was nearly three-fold (Hill,
1988).
The single acute oral and dermal doses of diazinon were
determined for 8- and 18-week-old broadbreasted white turkeys. The
hazard to turkeys of exposure to soils treated for control of chiggers
was evaluated. Diazinon was lethal for 18-week-old turkeys at 2 mg/kg
orally and toxic at 5 mg/kg dermally. In spite of this high toxicity,
exposing the turkeys to soil treated with 18 kg diazinon per hectare
led to no poisoned birds (Radeleff & Kunz, 1972).
The activity of acetylcholinesterase (AChE) and the density of
muscarinic receptors were measured in brains from normal Japanese
quail (Coturnix coturnix japonica) and from quail after lethal
intoxication with diazinon. The maximum relative loss of activity due
to postmortem decomposition alone during 8 days was 13 and 10% for
AChE and muscarinic receptors, respectively. During postmortem
decomposition, the ratio of AChE and muscarinic receptor activities
remained constant at approximately 1.3:1 in normal brains, while it
was always less than, or equal to, 0.5:1 after intoxication with
diazinon. Normal AChE activity could be estimated from muscarinic
receptor density. Parallel measurement of AChE and muscarinic
receptors may assist in the postmortem diagnosis of death due to acute
poisoning with anti-cholinesterase pesticide when control specimens
are not available (Prijono & Leighton, 1991).
Anticholinesterases do not pass through the mother to the egg in
significant amounts, but they may be deposited on the egg from the
parents feathers or during pesticide application. To simulate topical
exposure, fertile mallard eggs were either immersed for 30 seconds in
an aqueous emulsion or single dose of an anticholinesterase in a
non-toxic oil vehicle pipetted on the shell on day 3 of incubation
which is a critical period with respect to organogenesis in mallards.
Table 7. Acute toxicity to birds
Species Age/weight LD50 LC50 Reference
Japanese quail (Coturnix coturnix japonica) 50-60 days (105-195 g) 4 Sachsse (1973a)
5 days (10-30 g) 1.1 Sachsse & Ullmann (1975a)
50-60 days 900a Sachsse (1972)
Bobwhite quail (Colinus virginianus) 14 days 5.2 Fink (1976)
140-180 g 4.3 Sachsse & Ullmann (1975b)
Peking duck (Anas domesticus) 2.5-3.5 kg 2.7 Sachsse & Ullmann (1976)
5 days (40-125 g) 1.9 Sachsse & Ullmann (1975c)
Domestic hen (Gallus domesticus) 5 days (40-50 g) 14 Sachsse & Ullmann (1975d)
Mallard duck (Anas platyrhynchus) 19 weeks (843-1357 g) 1.44 Fletcher & Pedersen (1988a)
5 days 202a Sachsse (1973b)
9 days (108-120 g) 32 Fletcher & Pedersen (1988c)
Brown-headed cowbird (Molothrus ater) 35-55 g 85 Fletcher & Pedersen (1988b)
a Repellency recorded in all dosage groups.
Organophosphates were shown to be as much as 18 times more toxic when
applied to the shell in an oil compared with following water
immersion. Neither method of egg treatment produced teratogenicity or
developmental effects at realistic pesticide application rates
(Hoffman & Eastin, 1981).
A laboratory reproduction study was conducted with mallard in
which birds were allowed to build their own nests and incubate eggs
(Marselas et al., 1989a). Birds were fed diets containing 0, 5, 10 and
20 mg diazinon/kg diet (20 pairs per dose level). The exposure to 5
and 10 mg/kg did not result in any overt signs of toxicity or effects
on reproductive performance. At 20 mg/kg, there was an increase in the
number of hens that did not incubate and, although egg production was
not affected, there was some reduction in the number of hatchlings and
14-day-old survivors. A companion study (Marselas et al., 1989b) with
bobwhite quail was conducted at dietary concentrations of 10, 20 and
40 mg/kg. Exposures did not result in effects on reproductive
performance and no mortalities or overt signs of toxicity were
observed.
Henderson et al. (1994) described certain oral and dermal
toxicity of diazinon to the domestic pigeon. Dose-dependent gross
symptoms of organophosphorus poisoning usually appear within half an
hour in pigeons dosed orally. There was a 50% inhibition of brain
cholinesterase activity, i.e. ID50 of diazinon was about 2 mg/kg body
weight. Plasma cholinesterase activity in dermally exposed birds was
almost completely inhibited by 24 h after diazinon treatment. The
recovery of plasma cholinesterase after dermal exposure of pigeon was
very slow.
Johnston et al. (1994) studied the interactive effects of
combined treatment of red-legged partridge with diazinon and an
ergosterolbiosynthesis-inhibiting (EBI) fungicide (prochloraz). Birds
were pre-treated with procloraz at 180 mg/kg and then exposed to
diazinon at 4.3 mg/kg 24 h later. There was a tendency to increased
inhibition of cholinesterase in the plasma of treated birds but this
was not significant. Synergism was significant with other
organophosphorus compounds tested.
9.6.1 Field studies
Nine fairways of a golf course located in Bellingham, Washington,
USA, were treated with Diazinon AG500 at a target application rate of
2.2 kg a.i./ha. The chemical application with a boomless sprayer
resulted in a variable distribution of diazinon residues on the turf
that ranged from 1.0 to 6.2 kg a.i./ha. The diazinon-treated turf was
irrigated with 1.3 cm of water immediately following application. The
post-irrigation diazinon residue levels ranged from 100 to 33 mg/kg
(mean=209; SD=88; n=8). These residue levels were higher than expected
based on results of turf studies in other regions of the USA.
Eighty-five American wigeon died after grazing on one treated fairway
on the day of application following irrigation. The brains of all 85
wigeon were analysed for acetylcholinesterase activity. Wigeon that
died on the study area showed 44 to 87% depression of AChE activity
when compared to controls. Upper gastrointestinal tract contents of 15
of the 85 dead wigeons contained 0.96 to 18.1 mg/kg diazinon (Kendall
et al., 1992).
In the USA, use of diazinon has been discontinued on golf courses
since 1988. Waterfowl often congregate near ponds on golf courses and
were exposed to turf treatments. Since this restriction, deaths of
grazing waterfowl (Canada geese, brent geese and American wigeon) have
not occurred. Application rate reduction may also be effective in
reducing risk to Canada geese, the most common grazing bird on turf.
Kendall et al. (1993) closely monitored geese exposed to diazinon
applied twice at 2.2 kg a.i./ha, and found no mortality despite
extensive feeding on treated turf.
Hummell et al. (1992a,b,c) conducted an extensive, multi-plot
assessment of the effects of turf applications of granular and liquid
formulations (4.8 kg a.i./ha) of diazinon on songbirds.
Since the introduction of diazinon, sporadic mortality of
waterfowl feeding on treated turf or on orchard grass has come to
light in Canada. Frank et al. (1991) reported five incidents that
took place in Ontario between 1986 and 1988. Fifty-seven Canada
geese were poisoned by diazinon on turf sites in Ontario 1986-88. It
was determined that median levels of diazinon in turf sprayed and
then properly irrigated ranged from 45.4 to 256 mg/kg for a single
application of 1 kg a.i./ha of the EC formulation. A value of
390 mg/kg was obtained for a single grass sample following spray at
approximately 9 kg a.i./ha. Maximum residue levels for grass recovered
from the oesophagi of geese killed by diazinon on turf, and collected
while still fresh, ranged from 55 to 79 mg/kg.
In trials evaluating applications to large turf areas (an average
of ha), survival, behaviour and reproductive performance were
monitored. Smaller plots (0.09 ha) that simulated large home yards
were established for granular formulations, and survival and plasma
ChE were evaluated. On large plots, the liquid formulation had no
effect on reproductive performance, survival or cholinesterase levels.
Granular applications had a minor impact on bird population size (9.6%
reduction) compared to control plots (4.8% reduction). Reproductive
performance was not affected, although cholinesterase levels were
reduced compared to controls. On the smaller (home-sized) turf plots
there was no apparent effect on bird numbers, and plasma
cholinesterase activity was not affected in any species.
Decarie et al. (1993) sprayed ornamental and other deciduous
trees with diazinon at a rate of 2.2 kg a.i./ha in a suburban area of
Quebec, Canada, where the compound was used to control fruit tree
mites. Twelve nests of the American robin were sprayed and 65 nests
untreated were used as controls. Productivity was compared between
treated and control nests as was the behaviour of the birds. Mean
productivity was not significantly different between the two groups.
Number of feeding flights, number of female feeding flight and number
of faecal sacs removed did not differ between groups but there was a
significant increase in the total time spent sitting on the nest in
diazinon-treated birds. The significance of this is unclear. Plasma
AChE levels were reduced (993 ± 639 µg/litre in treated birds;
3585 ± 1531 µg/litre in controls), but brain AChE activity was
unaffected.
10. EVALUATION OF HUMAN HEALTH RISK AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risk
Diazinon is an organophosphorus pesticide classified by WHO as
"moderately hazardous" Class II (WHO, 1996). It is absorbed from the
gastrointestinal tract, through intact skin, and by inhalation. The
sources of exposure to humans are occupational, accidental or through
diet. Diazinon is used as a pesticide and veterinary drug to control
ectoparasites. The major source of diazinon food residues in edible
crops are from agricultural usage while those in meat, offal and other
animal products arise from veterinary use
The results of total diet studies in the USA, United Kingdom and
New Zealand suggest that levels of exposure are below the recommended
Acceptable Daily Intake (ADI) of 0.002 mg/kg per day (FAO/WHO, 1994b).
Diazinon is rapidly broken down, whether on plants or in animals,
further reducing the risk to humans. Several different application
techniques are used in applying diazinon outside and inside. Residual
spraying and space treatment are often used in controlling pests
indoors. Following recommended use, concentrations of diazinon
detectable in the indoor air are low and do not present a health
hazard. Some studies of agricultural workers have shown cases of
contact dermatitis after exposure to diazinon. Toxicity studies in
humans have shown that the administered daily dose of 0.025 mg/kg body
weight marginally inhibits the plasma cholinesterase activity and it
can be considered as the no-observed-adverse-effect level (NOAEL).
Diazinon is not genotoxic and exhibits no carcinogenic potential in
rats or mice. Several episodes of fatal and non-fatal accidental and
suicidal poisoning have occurred. Acute poisoning causes typical
cholinergic signs and symptoms. Acute pancreatitis can be associated
with severe poisoning.
10.2 Evaluation for effects on the environment
The information on utilization and application rates that has
been employed for this risk assessment is derived from the
agricultural use of diazinon within the European Union. It should be
possible to extrapolate this assessment to other agricultural uses at
similar application rates elsewhere in the world. The application
rates for diazinon can be summarized as follows: arable (tractor-
mounted/drawn hydraulic spray boom applications), 1.0 kg/ha; top fruit
(broadcast air-assisted applications), 1.2 kg/ha.
The following risk assessment is based on the principle of
calculating toxicity-exposure ratios (TERs) (Fig. 2), which follows
the European and Mediterranean Plant Protection Organisation and
Council of Europe (EPPO/CoE) Environmental Risk Assessment Scheme
model and associated trigger values (EPPO/CoE, 1993a,b).
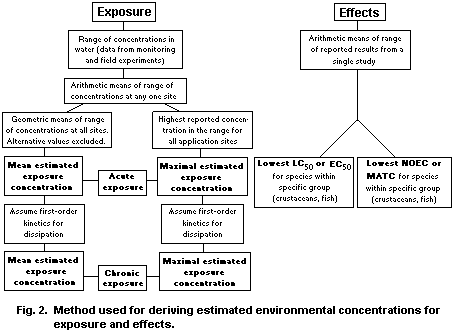 10.2.1 Aquatic organisms
The main risk to aquatic organisms from the use of diazinon is
from spray drift during either arable applications (1.0 kg/ha) or top
fruit air-assisted (1.2 kg/ha). For each of these risk scenarios, the
predicted environmental concentration (PEC) in a 30-cm-deep static
surface water body, arising from either arable-based spray drift at
1 m from the edge of the spray boom or from top fruit air-assisted
spray drift at 3 m from the point of application (both based on
Ganzelmeier et al., 1995), was calculated as follows:
PEC = max application rate (kg/ha) × A (% spray drift)
(mg diazinon/
litre) 300
Where A = 5 for ground-based hydraulic spray application 1 m from edge
of boom
= 30 for air-assisted application 3 m from point of
application
10.2.1.1 Acute risk
The acute EC50 value for the most sensitive fish was
0.09 mg/litre and for the most sensitive aquatic invertebrate
(Gammarus) 0.0002 mg/litre. For the most sensitive algal
species the 14-day NOEC was 1 mg/litre.
(a) Spray drift from ground-based applications: The acute PEC for
spray drift (l m from the edge of the spray boom into a 30-cm-deep
static water body at the maximum application rate (see PEC assumptions
above) is 0.017 mg/litre. Therefore, the TERs, based on this PEC and
the above EC50/NOEC toxicity values, are: fish, 5.4; aquatic
invertebrates, 0.01; and algae, 60. Based on the EPPO/CoE
risk assessment scheme for aquatic organisms, these TERs (i.e. TERs
>10 = low risk; TERs <1 = high risk) indicate a low acute risk to
algae and a high risk to these organisms. The TER for fish was between
1 and 10, indicating an intermediate risk. In such risk situations the
use of a "no-spray" restriction zone next to surface waters may reduce
the risk to such aquatic invertebrates. For example, arable spray
drift at 5 m from the edge of boom is 0.6% (Ganzelmeier et. al.,
1995). Based on this 5-m drift data, the PEC is 0.002 mg/litre and
results in a 5-m TER of 45 for fish. This TER at 5 m indicates that
the use of a 5-m "no-spray" restriction zone next to surface waters
would reduce the acute risk to fish.
(b) Spray drift from broadcast air assisted top fruit
applications: The acute PEC for spray drift (3 m from the point of
application into a 30-cm-deep static water body at the maximum
application rate (see PEC assumptions above) is 0.12 mg/litre.
Therefore, the TERs based on this PEC and the above EC50/NOEC
toxicity values are: fish, 0.75; aquatic invertebrates, 0.0017; and
algae, 8.3. Based on the CoE/EPPO risk assessment scheme for aquatic
organisms, these TERs indicate a high acute risk to fish and
invertebrates and an intermediate risk to algae. Table 8 below
summarizes the acute TERs for aquatic organisms at 1 m for arable and
3 m for broadcast air-assisted applications.
10.2.1.2 Chronic risk
There were no chronic toxicity data available.
10.2.2 Terrestrial organisms
Vertebrates are likely to be exposed to diazinon from either
grazing treated vegetation or consuming contaminated insects. For this
risk assessment, typical application rates of 1 kg/ha are used for
ground spray application on arable crops and 1.2 kg/ha for application
by air-assisted spraying for fruit
10.2.2.1 Birds
The lowest reported acute oral LD50 for birds is 1.1 mg/kg body
weight for the Japanese quail. The dietary LC50 for the mallard duck
is 32 mg/kg diet.
Indicator birds for use in the risk assessment will be:
* Greylag goose (Anser anser), as a grazing species, with a body
weight of 3 kg and total daily food consumption of 900 g
vegetation (dry weight) (Owen, 1975)
* Blue tit (Parus caeruleus), as an insectivorous species, with a
body weight of 11 g and total daily food consumption of 8.23 g
(dry weight) (Kenaga, 1973).
a) Grazing birds
Initial residues on short grass or cereal shoots, arising from
application at 1 kg/ha to arable crops, are estimated to be 112 mg/kg
dry weight (based on 112 × application rate in kg/ha (EPPO/CoE,
1993a,b) and at 134.4 mg/kg from application to fruit at a rate of
1.2 kg/ha. This gives an estimated total oral intake for the goose of
100.8 and 121.0 mg for the two application rates assuming that the
goose ate exclusively food contaminated at this level. This is
equivalent to a daily intake of 33.6 and 40.3 mg/kg body weight,
respectively. TERs can be calculated as follows:
Table 8. Acute toxicity-exposure ratio (TERs) for aquatic organisms at 1 m for arable and 3 m
for broadcast air-assisted applications
Species EC50/NOEC PEC PEC TER TER
(mg/litre) (mg/litre) (mg/litre) (arable) (air-assisted)
(arable) (air-assisted)
Fish (Oncorhynchus mykiss) 0.09 0.017 0.12 5.4 0.75
Aquatic invertebrate (Gammarus) 0.0002 0.017 0.12 0.012 0.0017
Algae (Selenastrum capricornutum) 1.0 0.017 0.12 60.0 8.3
End-point LD50/L50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Bird acute oral 1.1 mg/kg 1 112 0.033
body weight (arable)
Japanese quail
1.2 134.4 0.027
(fruit)
Bird short-term 32 mg/kg diet 1 112 0.29
dietary mallard duck (arable)
1.2 134.4 0.24
(fruit)
The calculated TER values fall well below the EPPO/CoE trigger
values for concern (TER <10) and indicate a high risk to grazing
birds. This potential risk has been confirmed in practice with
reported high incidence of fatalities following application of
diazinon to golf-course turf. This application is no longer
recommended for this reason.
b) Insectivorous birds
Initial residues on small insects arising from application at
1 kg/ha to arable crops are estimated to be 29 mg/kg dry weight (based
on 29 × application rate in kg/ha) (EPPO/CoE, 1993a,b) and 34.8 mg/kg
from application to fruit at a rate of 1.2 kg/ha. This gives an
estimated total oral intake for the blue tit of 0.24 mg and 0.29 mg
for the two application rates assuming that the blue tit ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 21.7 and 26.0 mg/kg body weight, respectively. TERs
can be calculated as follows:
The TERs for acute toxicity to insectivorous birds are
substantially less then the trigger value of <10, indicating high
acute risk to these birds. The risk factors exceed the trigger for
insectivorous birds exposed short-term via the diet.
End-point LD50/LC50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Bird acute oral 1.1 mg/kg 1 29 0.05
body weight (arable)
Japanese quail
1.2 34.8 0.04
(fruit)
Bird short-term 32 mg/kg diet 1 29 1.1
dietary (arable)
1.2 34.8 0.92
(fruit)
10.2.2.2 Mammals
The lowest reported acute oral LD50 for laboratory mammals is
82 mg/kg body weight for the mouse.
Indicator mammals for use in the risk assessment will be:
* Rabbit (Orystolagus cuniculus), as a grazing mammal, with
a body weight of 1200 g and a total daily food consumption
of 500 g vegetation (dry weight) (Ross, personal
communication to the IPCS)
* Shrew (Sorex araneus), as an insectivorous mammal, with a
body weight of 18 g and a total daily food consumption of
18 g (Churchfield, 1986)
a) Grazing mammals
Initial residues on short grass or cereal shoots arising from
application at 1 kg/ha to arable crops are estimated to be 112 mg/kg
dry weight (based on 112 x application rate in kg/ha) (EPPO/CoE,
1993a,b) and at 134.4 mg/kg from an application to fruit at a rate of
1.2 kg/ha. This gives an estimated total oral intake for the rabbit of
56 and 67.2 mg for the two application rates, assuming that the rabbit
ate exclusively food contaminated at this level. This is equivalent to
a daily intake of 46.7 and 56 mg/kg body weight, respectively. TERs
can be calculated as follows:
End-point LD50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Mammal 82 mg/kg 1 56 1.76
acute oral body weight (arable)
mouse
1.2 67.2 1.46
(fruit)
This indicates a high risk to grazing mammals (trigger <10)
comparable to that for grazing birds. Again, the removal of
application to golf-course turf would reduce the likelihood of
exposure to maximum residues of diazinon. However, grazing mammals
consuming short cereal shoots could be killed following recommended
use of the compound.
b) Insectivorous mammals
Initial residues on large insects arising from application at
1 kg/ha to arable crops are estimated to be 2.7 mg/kg dry weight
(based on 2.7 × application rate in kg/ha) (EPPO/CoE, 1993a,b) and
3.24 mg/kg from an application to fruit at a rate of 1.2 kg/ha. This
gives an estimated total oral intake for the shrew of 0.049 mg and
0.058 mg for the two application rates, assuming that the shrew ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 2.7 and 3.2 mg/kg body weight, respectively. TERs can
be calculated as follows:
These TERs fall outside the trigger for high risk for
insectivorous mammals but within the range for medium risk, reflecting
the high acute mammalian toxicity.
End-point LD50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Mammal 82 mg/kg 1 2.7 30.4
acute oral body weight (arable)
mouse
1.2 3.24 25.3
(fruit)
10.2.2.3 Bees
The reported contact and oral toxicity to bees gives LD50 values
of 0.22 and 0.2 µg/bee, respectively. Using application rates of 1000
and 1200 g/ha for cereals and fruit, respectively, hazard quotients
are calculated to be 4545 and 5455 (application in g/ha). The trigger
for concern is >50 (EPPO/CoE, 1993a,b) and, therefore, there is
substantial concern for exposed bees. The compound should not be
applied to flowering plants and exposure of flying bees should be
avoided.
10.2.2.4 Earthworms
Earthworms are likely to be exposed to the highest concentration
of diazinon following use of the granular formulation incorporated in
soil at up to 2000 g/ha. Based on a soil depth of 5 cm and a soil
density of 1.5 g/cm3, the soil PEC would be 2.67 mg/kg, assuming even
distribution in the medium. The reported LC50 for earthworms
(Eisenia foetida) is 130 mg/kg soil, giving a TER of 48.75. As this
is above the trigger value of 10 (EPPO/CoE, 1993a,b), the acute risk
to earthworms should be low. Reported concentrations measured in soil
are at least an order of magnitude lower than the PEC suggesting that
little risk is posed to worms.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
The general population does not face a significant health risk
from diazinon. It may cause acute poisoning in cases of over-exposure
following intentional ingestion or careless handling during its
manufacture and use. The general public is exposed to diazinon in the
form of its residues in food, but the reported intake of diazinon is
far below the acceptable daily intake. Following residual spraying and
space treatment, used to control insects, the general population can
be exposed to residues in the air and on surfaces. With good work
practice and hygiene measures, and if safety precautions and medical
surveillance are enforced, diazinon is unlikely to present a hazard to
those occupationally exposed.
Diazinon does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates, fish, terrestrial birds and mammals, leading to high
risk factors for many of these organisms. Field kills of waterfowl
have been reported following use of the compound on amenity turf.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift and avoid areas where wildfowl are likely to graze).
11.2 Recommendations for protection of human health and the
environment
Certain groups within the population, including agricultural
workers and employees in the chemical industry, have the potential of
being exposed to diazinon. Gardeners and householders may also be
involved.
11.2.1 Recommendation on regulation of compound
Formulations have different classification categories: (FAO/WHO,
1979): liquids over 20% are in category 3; other liquids or solids
over 50% are in category 4; and all other solids are in category 5.
11.2.1.1 Transport and storage
Formulations in categories 3 and 4 should be transported and
stored in clearly labelled rigid and leak-proof containers, away from
containers of food and drink. Storage should be under lock and key and
secure from access by unauthorized people. Formulations in category 5
should be transported and stored in clearly labelled leak-proof
containers, out of reach of children and away from food and drink.
11.2.1.2 Handling
Protective clothing should be used by all handling of the
compound. Adequate washing facilities should be available at all times
during handling and should be close to the site of handling. Eating,
drinking and smoking should be prohibited during handling and before
washing the hands after handling.
11.2.1.3 Disposal
Containers may be decontaminated as recommended by the WHO Expert
Committee on Vector Biology and Control on the Safe Use of Pesticides
(WHO, 1991). Decontaminated containers should not be used for food and
drink. Containers that are not decontaminated should be burned or
crushed and buried below topsoil. Care must taken to avoid subsequent
contamination of water sources.
11.2.1.4 Selection, training and medical supervision of workers
Pre-employment medical examination of workers is desirable.
Workers suffering from active hepatic or renal disease should be
excluded from contact with diazinon. Pre-employment and periodic
cholinesterase test for workers is desirable especially for those
handling concentrates. Training of workers in techniques to avoid
contact is essential. Pilots and loaders should have special training
in application methods and early symptoms of poisoning, and must wear
a suitable respirator. Flagmen, if used, should wear overalls and be
located well away from the dropping zone.
11.2.1.5 Labelling
Diazinon is an organophosphorus compound that inhibits
cholinesterases. It is poisonous if swallowed. It may be absorbed
though the skin. It is important to avoid skin contact, wear hand
protection, clean protective clothing. The material must be kept out
of reach of children and well away from foodstuffs, animal feed and
their containers. If poisoning occurs, a physician should be called.
11.2.1.6 Residues in food
Maximum residue limits have been recommended for diazinon by the
Joint FAO/WHO Meeting on Pesticides Residues (FAO/WHO, 1975).
11.2.2 Prevention of poisoning in man and emergency aid
11.2.2.1 Manufacture and formulation
Closed systems and forced ventilation may be required to reduce
as much as possible the exposure of workers to diazinon.
11.2.2.2 Mixers and applicators
When mixing, protective impermeable boots, rubber apron, clean
overalls and gloves should be worn. When spraying tall crops or during
aerial application, a face mask should be worn, as well as an
impermeable hat, clothing, boots and gloves. The applicator should
avoid working in spray mist and avoid contact with the mouth.
Particular care is needed when equipment is being washed after use.
All protective clothing should be washed immediately after use.
Splashes must be washed immediately from the skin or eyes with large
quantities of water.
11.2.2.3 Other associated workers
People exposed to diazinon and associated with its application
should wear protective clothing and observe the precautions described
in section 11.2.2.2.
11.2.2.4 Other populations likely to be affected
With good application practice, other people should not be
exposed to hazardous amounts of diazinon.
11.2.3 Entry into treated areas
Unprotected people should abide by re-entry periods stated on
product labels.
11.2.4 Emergency aid
If symptoms appear following exposure, the person should stop
work immediately, remove contaminated clothing, wash the affected skin
with soap and water, if available, and flush the area with large
quantities of water. If diazinon has been swallowed and the person is
conscious, vomiting should be induced.
Atropine and oximes are specific antidotes and artificial
respiration may be needed.
11.2.5 Surveillance test
Slight reduction of plasma cholinesterase activity can be
observed. Periodic blood cholinesterase tests for workers is
desirable.
12. FURTHER RESEARCH
1. Studies of exposed worker populations should be continued.
2. Diazinon should be manufactured and formulated in accordance with
international specifications. It should be packed and stored
under conditions that are not conducive to the formation of
acutely toxic impurities.
3. The safe use of diazinon should follow label directions and
precautions for handling, application and disposal.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Diazinon has been evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) on a number of occasions since 1965. An
acceptable daily intake (ADI) of 0.002 mg/kg body weight has been
established and maximal residues levels have been recommended for
diazinon in a wide range of food commodities (FAO/WHO, 1994a,b).
The following NOAELs were established:
Rat (two-year feeding study): 1.5 mg/kg diet
(0.06 mg/kg body weight per day)
Dog (one-year feeding study): 0.5 mg/kg diet
(0.015 mg/kg body weight per day)
Rhesus monkey (two-year study): 0.5 mg/kg body weight per day
Human volunteers (36-day study): 0.025 mg/kg body weight per day
Diazinon has not been evaluated by the International Agency for
research on Cancer (IARC).
REFERENCES
Abdelsalam EB & Ford EJH (1986) Effect of pretreatment with hepatic
microsomal enzyme inducers on the toxicity of diazinon in calves. Res
Vet Sci, 41: 336-339.
Abdelsalam EB & Ford EJH (1987) The effect of induced liver, kidney
and lung lesions on the toxicity of levamisole and diazinon in calves.
J Comp Pathol, 97: 619-627.
Adachi K, Ohokuni N, & Mitsuhashi T (1984) Simple analytical method
for organophosphorus pesticide determination in unpolished rice, using
removal of fats by zinc acetate. J Assoc Off Anal Chem, 67: 798-800.
Al-Attar HJ & Knowles CO (1982) Diazinon uptake, metabolism and
elimination by nematodes. Arch Environ Contam Toxicol, 11: 669-673.
Albright RK (1984) Renal involvement in organophosphate poisoning.
J Am Med Assoc, 252: 1408.
Allender WJ & Britt AG (1994) Analyses of liquid diazinon formulation
and breakdown products: an Australia-wide survey. Bul. Environ Contam
Toxicol, 53: 902-906.
Allison DT (1977) Use of exposure units for estimating aquatic
toxicity of organophosphate pesticides. Washington, DC, US
Environmental Protection Agency (EPA-600/3-77-077).
Allison DT & Hermanutz RO (1977) Toxicity of diazinon to brook trout
and fathead minnows. Washington, DC, US Environmental Protection
Agency (EPA-600/3-77-060).
Anees MA (1978) Hepatic pathology in a fresh-water teleost Channa
punctatus (Bloch) exposed to sub-lethal and chronic levels of
three organophosphorus insecticides. Bull Environ Contam Toxicol,
19: 524-527.
Ankley GT, Dierkes JR, Jensen DA, & Peterson GS (1991) Piperonyl
butoxide as a tool in aquatic toxicological research with
organophosphate insecticides. Ecotoxicol Environ Saf, 21: 266-274.
Ansari BA & Kumar K (1988) Diazinon toxicity: effect on protein and
nucleic acid metabolism in the liver of zebrafish, Brachydanio
rerio (Cyprinidae). Sci Total Environ, 76: 63-68.
Anthony J, Banister E, & Oloffs PC (1986) Effect of sublethal levels
of diazinon: Histopathology of liver. Bull Environ Contam Toxicol,
37: 501-507.
Arienzo M, Crisanto T, Sanchez-Martin T, & Sanchez-Camazano M (1994):
Effect of soil characteristics on adsorption and mobility of (14C)
diazinon. J Agric Food Chem, 42: 1803-1808.
Asensio JS, Barrio CS, Juez MTG, & Bernal JG (1991) Study of the
decay of diazinon and chlorpyriphos in apple samples, using gas
chromatography. Food Chem, 42: 213-224.
Ballantine L (1984) Advanced product chemistry percutaneous absorption
of 2delta-14C-diazinon in rats. Greensboro, North Carolina, Ciba-
Geigy Corporation (Unpublished report No. ABR-84011, submitted to WHO
by Ciba-Geigy Ltd, Basel).
Banerjee D (1967) Pericarditis in acute diazinon poisoning (A case
report). Armed Forces Med J (India), 23: 187-190.
Barik S & Munnecke DM (1982) Enzymatic hydrolysis of concentrated
diazinon in soil. Bull Environ Contam Toxicol, 29: 235-239.
Barnes TB, Hazelette JR, & Arthur AT (1988) Diazinon (mg-8): 13 week
oral toxicity study in dogs (Project No. 882012). Summit, New Jersey,
Ciba-Geigy Corporation (Unpublished report submitted to WHO by Ciba-
Geigy Ltd, Basel).
Barnett JB, Spyker-Cranmer JM, Avery DL, & Hoberman AM (1980)
Immunocompetence over the lifespan of mice exposed in utero to
carbofuran or diazinon: I. Changes in serum immunoglobulin
concentrations. J Environ Pathol Toxicol, 4: 53-63.
Bathe R (1972a) Acute dermal LD50 of technical diazinon (G-24'480)
in the rat (Project No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Bathe R (1972b) G 24`480 tech.: Acute oral LD50 in the mouse
(Project No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Bathe R (1973) Intraperitoneal LD50 of technical diazinon
(G-24480 MG 647) in the rat. Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished project report No. Siss 1679).
Bathe R & Gfeller W (1980) Report on acute oral LD50 in the rat of
technical G24'480 (Project No. 800478). Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Bopp C & Kosminsky B (1975) Toxic hepato-cutaneous porphyria. Med
Cutan Ibero Lat Am, 3: 271-279.
Bresch H (1991) Early life-stage test in zebrafish versus a growth
test in rainbow trout to evaluate toxic effects. Bull Environ Contam
Toxicol, 46: 641-648.
Bruce RB, Howard JW, & Elsea JR (1955) Toxicity of O,O-diethyl
O-(2-isopropyl-6-methyl-4 pyrimidyl) phosphorothioate (diazinon).
J Agric Food Chem, 3: 1017-1021.
Burgener J & Seim V (1988) Biological report for the metabolism of
14C-diazinon in laying hens dosed at 25 ppm ( Project No.
BIOL-88006). Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO Ciba-Geigy Ltd, Basel).
Burpee LL & Cole H Jr (1978) The influence of alachlor, trifluralin
and diazinon on the development of endogenous mycorrhiza in soybeans.
Bull Environ Contam Toxicol, 19: 191-197.
Butler GL, Deason TR, & Kelley JC (1975) The effect of atrazine,
2,4-D, methoxychlor, carbaryl and diazinon on the growth of planktonic
algae. Br Phycol J, 10: 371-372.
Byrne DH & Kitos PA (1983) Teratogenic effects of cholinergic
insecticides in chick embryos: IV. The role of tryptophan in
protecting against limb deformities. Biochem Pharmacol,
32: 2881-2890.
Cairns T, Siegmund EG, & Froberg JE (1985) Identification of
diazinon and its metabolite in spinach by chemical ionization
mass spectrometry. Bull Environ Contam Toxicol, 35: 291-295.
Capps T (1989) Characterization and identification of diazinon
metabolites in rats (Project No. 302211/302925). Greensboro, North
Carolina, Ciba-Geigy Corporation (Unpublished report No. ABR-88164,
submitted to WHO by Ciba-Geigy Ltd, Basel).
Capps TM (1990) Characterization and identification of major
metabolites in tissues of sheep treated dermally with 14C-diazinon
(Project No. 302925) Greensboro, North Carolina, Ciba-Geigy
Corporation (Unpublished report No. ABR-90014, submitted to WHO by
Ciba-Geigy Ltd, Basel).
Ceresa C & Puri E (1988) Micronucleus test, mouse (OECD-conform) G 24
480 tech. (diazinon) (Test No. 871696). Basel, Switzerland, Ciba-Geigy
Ltd ( Unpublished report).
Chen HH, Hsueh JL, Sirianni SR, & Huang CC (1981) Induction of
sister-chromatid exchanges and cell cycle delay in cultured mammalian
cells treated with eight organophosphorus pesticides. Mutat Res,
88: 307-316.
Chow E & Richter AG (1994) Acute neurotoxicity study with D-Z-N
diazinon MG 87% in rats (Project No. F-00175). Farmington,
Connecticut, Ciba-Geigy Corporation, Environmental Health Center
(Unpublished report).
Churchfield S (1986) Shrews. Oswestry, A. Nelson (The Mammal Society
series).
Claborn HV, Mann HD, Younger RL, & Radeleff RD (1963) Diazinon
residues in the fat of sprayed cattle. J Econ Entomol, 56: 858-859.
Classen W (1996) G24.480 tech. - delayed neurotoxicity in hens
following acute exposure (Project No. 952030). Stein, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Clegg TJ & Koevening JL (1974) The effect of four chlorinated
hydrocarbon pesticides and one organophosphate pesticide on ATP levels
in three species of photosynthesizing freshwater algae. Bot Gaz,
135: 368-372.
Cockrell KD, Woodard MW, & Woodard G (1966) Diazinon 50 W: Safety
evaluation by repeated oral administration to monkeys for 106 weeks.
Woodard Research Corporation USA (Unpublished report submitted to WHO
by Ciba-Geigy Ltd, Basel).
Collyard SA, Ankley GT, Hoke RA, & Goldenstein T (1994) Influence of
age on the relative sensitivity of Hyalella azteca to diazinon,
alkylphenol ethoxylates, copper, cadmium and zinc. Arch Environ Contam
Toxicol, 26: 110-113
Coye MJ, Barnett PG, Midtling JE, Velasco AR, Romero P, Clements CL, &
Rose TG (1987) Clinical confirmation of organophosphate poisoning by
serial cholinesterase analyses. Arch Intern Med, 147: 438-442.
Craine EM (1989a) A study of 14C-diazinon disposition in the rat
(biological-phase) (Project No. 302925). Ashland, Ohio, WIL Research
Laboratories Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Craine EM (1989b) A study of 14C-diazinon disposition in the rat
(analytical phase I) (Project No. 302925). Ashald, Ohio, WIL Research
Laboratories Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Currie KL, McDonald EC, Chung LTK, & Higgs AR (1990) Concentration of
diazinon, chlorpyrifos, and bendiocarb after application in offices.
Am Ind Hyg Assoc J, 51(1): 23-27.
Dagli AJ, Moos JS, & Shaikh WA (1981) Acute pancreatitis as a
complication of diazinon poisoning: A case report. J Assoc Physi
India, 29: 794-795.
Dahm PA (1970) Some aspects of the metabolism of parathion and
diazinon. In: O`Brien RD & Yamamoto I ed. Biochemical toxicology of
insecticides. London, New York, Academic Press, pp 51-63.
Davies DB & Holub BJ (1980a) Comparative subacute toxicity of
dietary diazinon in the male and female rat. Toxicol Appl Pharmacol,
54: 359-367.
Davies DB & Holub BJ (1980b) Toxicological evaluation of dietary
diazinon in the rat. Arch Environ Contam Toxicol, 9: 637-650.
Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowall J, & Furlong CE
(1996) The effect of the human serum paraoxonase polymorphism is
reversed with diazoxon, soman and sarin. Nat Genet, 14(3): 334-336.
Decarie R, DesGrange J-L, Lepine C, & Morneau F (1993) Impact of
insecticides on the American robin (Turdus migratorius) in a
suburban environment. Environ Pollut, 80: 231-238
Derbyshire JC & Murphy RT (1962) Diazinon residues in treated
silage and milk of cows fed powdered diazinon. J Agric Food Chem,
10: 384-386.
Dick GL, Heenan MP, Love JL, Udy PB, & Davidson F (1978) Survey of
trace elements and pesticide residue content: 2. Organochlorine and
organophosphorus pesticide residue content. NZ J Sci, 21: 71-78.
Dikshith TS, Behari JR, Datta KK, & Mathur AK (1975) Effect of
diazinon in male rats: Histopathological and biochemical studies.
Environ Physiol Biochem, 5: 293-299.
Dobbins PK (1967) Organic phosphate insecticides as teratogens in the
rat. J Fla Med Assoc, 54: 542-546.
Doggett SM & Rhodes RG (1991) Effects of a diazinon formulation on
unialgal growth rates and phytoplankton diversity. Bull Environ Contam
Toxicol, 47: 36-42.
Dollenmeier P & Müller D (1986) G 24`480 tech.: L5178Y/TK+/- mouse
lymphoma mutagenicity test (Project No. 840396). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Dougherty E III (1957) Thiophosphate poisoning in white Pekin ducks.
Avian Dis, 1: 127-130.
Dressel TD, Goodale RL Jr, Arneson MA, & Borner JW (1979) Pancreatitis
as a complication of anticholinesterase insecticide intoxication. Ann
Surg, 189: 199-204.
Dressel TD, Godale RL, & Borner JW (1980) A study of the
cholinesterases of the canine pancreas and the relationship between
reduced butyrylcholinesterase activity and pancreatic ductal
hypertension. Ann Surg, 192: 614-619.
Dutta H, Marcelino J, & Richmonds Ch (1992) Brain acetylcholinesterase
activity and optomotor behaviour in bluegills, Lepomis macrochirus,
exposed to different concentrations of diazinon. Arch Int Physiol
Biochim Biophys, 100: 331-334.
Ebere AG & Akintonwa A (1992) Acute toxicity of pesticides to
Gobius sp., Palaemonetes africanus, and Desmocaris trispimosa.
Bull Environ Contam Toxicol, 49: 588-592.
Eberle DO, Suter R, Nowak K, & Bosshardt HP (1974) CIPAC collaborative
study of the heat-stability of diazinon formulations using both CUPAC
and AOAC analytical methods. J Assoc Off Anal Chem, 57: 48-52.
Egan H & Weston RE (1977) Pesticide residues: Food surveys in the
United Kingdom. Pestic Sci, 8: 110-116.
Egyed MN, Malkinson M, Eilat A, & Schlosberg A (1974) Basudin
(diazinon) poisoning in goslings. Reffu Vet, 31: 22-26.
El-Elaimy IA, El-Saadany MM, & Gabr SA (1990) Pesticide poisoning to
fresh water teleost: VIII. Ultrastructural alterations of the
intestine of Tilapia nilotica under stress of exposure to diazinon
and neopybuthrin. J Egypt Ger Soc Zool, 1: 223-236.
English T, Ellis EF, & Ackerman J (1970) Organic phosphate poisoning.
Morbid Mortal Wkly Rep (Cent Dis Control), 19: 397-404.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993a) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 23: 1-54.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993b) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 24: 1-87.
FAO/WHO (1965) Diazinon. In: 1965 Evaluation of the toxicity of
pesticide residues in food. Rome, Food and Agriculture Organization of
the United Nations and Geneva, World Health Organization, pp 77-83
(FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27. 65).
FAO/WHO (1975) 1974 Evaluations of some pesticide residues in food.
Geneva, World Health Organization (WHO Pesticide Residues Series,
No 4).
FAO/WHO (1979) Data sheet on pesticides No. 45: Diazinon. Geneva,
World Health Organization (VBC/DS/79.45).
FAO/WHO (1994a) Diazinon. In: Pesticide residues in food - 1993:
Evaluations, Part I - Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 157-294 (FAO Plant Production
and Protection Paper 124).
FAO/WHO (1994b) Diazinon. In: Pesticide residues in food - 1993:
Evaluations, Part II - Toxicology. Geneva, World Health Organization,
pp 57-81 (WHO/PCS/94.4).
Farran A, DePablo J, & Barcelo D (1988a) Identification of
organophosphorus insecticides and their hydrolysis products by
liquid chromatography in combination with UV and thermospray-mass
spectrometric detection. J Chromatogr, 455: 163-172.
Farran A, DePablo J, & Hernandez S (1988b) Continuous-flow extraction
of organophosphorus pesticides coupled on-line with high-performance
liquid chromatography. Anal Chim Acta, 212: 123-131.
Fernandez-Casalderrey A, Ferrando MD, & Andreu-Moliner E (1992)
Endosulfan and diazinon toxicity to the freshwater rotifer
Brachious calcyflorus. J Environ Sci Health, B27: 155-164.
Ferrando MD, Sancho E, & Andreu-Moliner E (1991) Comparative acute
toxicities of selected pesticides to Anguilla anguilla. J Environ
Sci Health, B26(5/6): 491-498.
Ferrando MD, Alarcon V, Fernandez-Casalderrey A, Gamon M, &
Andreu-Moliner E (1992) Persistence of some pesticides in the
aquatic environment. Bull Environ Contam Toxicol, 48: 747-755.
Ferreira JR & Silva Fernandes AM (1980) Gas-liquid chromatographic
determination of organophosphorus insecticide residues in fruits and
vegetables. J Assoc Off Anal Chem, 63: 517-522.
Fink R (1976) Acute oral LD50 to bobwhite quail - diazinon technical.
Basel, Switzerland, Novartis Crop Protection (Unpublished report).
Fletcher DW & Pedersen CA (1988a) Diazinon MG8 technical: 14 day acute
oral LD50 study in mallard ducks. Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Fletcher DW & Pedersen CA (1988b) Diazinon MG8 technical: 14 day acute
oral LD50 study in brown-headed cowbirds. Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Fletcher DW & Pedersen CA (1988c) Diazinon MG8 technical: 8 day acute
dietary LC50 study in mallard ducklings. Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Fort DJ, Delphon J, Powers CR, Helems R, Gonzales R, & Stover EL
(1995) Development of automated methods of identifying toxicants in
the environment. Bull Environ Contam Toxicol, 54: 104-111.
Frank R, Braun HE, Van Hove Holdrinet M, Sirons GJ, & Ripley BD
(1982) Agriculture and water quality in the Canadian Great Lakes
basin: V. Pesticide use in 11 agricultural watersheds and presence in
stream water, 1975-1977. J Environ Qual, 11(3): 497-505.
Frank R, Braun HE, & Ripley BD (1989) Monitoring Ontario-grown apples
for pest control chemicals used in their production. Food Addit
Contam, 6: 227-234.
Frank R, Braun HE, & Ripley BD (1990) Residues of insecticides and
fungicides on Ontario-grown vegetables 1986-1988. Food Addit Contam,
7: 545-554.
Frank R, Mineau P, Braun HE, Barker IK, Kennedy SW, & Trudeau S (1991)
Deaths of Canada geese following spraying of turf with diazinon. Bull
Environ Contam Toxicol, 46: 852-858.
Frick TW, Dalo S, O'Leary JF, Runge W, Borner JW, Baraniewski H,
Dressel T, Shearen JG, & Goodale RL (1987) Effects of insecticide
diazinon on pancreas of dog, cat, and guinea pig. J Environ Pathol
Toxicol Oncol, 7: 1-12.
Fritz H (1974) Reproduction study - G24480 (diazinon techn.) rat -
Segment II: Test for teratogenic or embryotoxic effects. Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Fritz H (1975) Dominant lethal study on G24480 (diazinon techn.)
mouse: Test for cytotoxic or mutagenic effects on male germinal cells
(Project No. 327507) . Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Fujii Y & Asaka S (1982) Metabolism of diazinon and diazoxon in fish
liver preparations. Bull Environ Contam Toxicol, 29: 455-460.
Gaines TB (1960) The acute toxicity of pesticides to rats. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol,
14: 515-534.
Ganzelmeier IH, Rautmann D, Spangenberg R, Streloke M, Herrmann M,
Wenzelburger HJ, & Walter HF (1995) Studies on the spray drift of
plant protection products: Results of a test program carried out
throughout the Federal Republic of Germany. Oxford, London,
Edinburgh, Melbourne, Blackwell Scientific Publications.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1985a)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1978-September 1979. J Assoc Off
Anal Chem, 68: 842-861.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1985b)
Pesticides, selected elements, and other chemicals in adult total
diet samples, October 1978-September 1979. J Assoc Off Anal Chem,
68: 862-875.
Gasser R (1953) [A new insecticide with wide efficacy.] Z Naturforsch,
B8: 225-232 (in German).
Geleick D & Arni P (1990) Salmonella and Escherichia/liver-microsome
test (Project No. 891346) - Test substance: G24480 tech. Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Getzin LW (1968) Persistence of diazinon and zinophos in soil: Effects
of autoclaving, temperature, moisture, and acidity. J Econ Entomol,
61: 1560-1565.
Giddings JM (1992) Aquatic mesocosm test for environmental fate and
ecological effects of diazinon. Wareham, Massachusetts, Springborn
Laboratories, Inc. and Columbia, Missouri, ABC Laboratories, Inc.
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Giknis MLA (1989) Diazinon techn: A two generation reproductive study
in albino rats. Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report).
Gilmore DR & Cortes A (1966) Dual-ban preparative thin-layer
chromatography for the separation of diazinon and related from plant
material. J. Chromatogr, 21: 148-149.
Glaza SM (1993) Acute oral toxicity study of D-Z-N diazinon in rats
(Project No. HWI 6117-221). Madison, Wisconsin, Hazleton Wisconsin,
Inc. (Unpublished report).
Goodman LR, Hansen DJ, Coppage DL, Moore JC, & Matthews E (1979)
Diazinon chronic toxicity to, and brain acethylcholinesterase
inhibition in, the sheepshead minnow, Cyprinodon variegatus. Trans
Am Fish Soc, 108: 479-488.
Gowen JA, Wiersma GB, Tai H, & Mitchell WG (1976) Pesticide levels in
hay and soils from nine states. Pestic Monit J, 10: 114-116.
Gunner HB & Zuckerman BM (1968) Degradation of 'diazinon' by
synergistic microbial action. Nature (Lond), 217: 1183-1184.
Gunner HB, Zuckerman BM, Walker RW, Miller CW, Deubert KH, & Longley
RE (1966) The distribution and persistence of diazinon applied to
plant and soil and its influence on rhizosphere and soil microflora.
Plant Soil, 25: 249-264.
Hagenbuch JP & Mücke W (1985) The fate of diazinon in mammals
(Biochemistry product evaluation 1/85). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Hall SW & Baker BB (1989) Intermediate syndrome from organophosphate
poisoning. Vet Hum Toxicol, 31(4): 355.
Halle A & Sloas DD (1987) Percutaneous organophosphate poisoning .
South Med J, 80: 1179-1181.
Harris SB & Holson JF (1981) G 24`480 tech.: A teratology study of
diazinon (CAS Number 333-41-5) in New Zealand white rabbits (Project
No. 281005). La Jolla, California, Science Applications, Inc.,
Division of Toxicology (Unpublished report submitted to WHO by
Ciba-Geigy Ltd, Basel).
Harris CR & Mazurek JH (1966) Laboratory evaluation of candidate
materials as potential soil insecticides. J Econ Entomol, 59:
1215-1221.
Harris LW, Fleisher HJ, Innerebner TA, Cliff WJ, & Sim VM (1969) The
effects of atropine-oxime therapy on cholinesterase activity and the
survival of animals poisoned with O,O-diethyl-O-(2-isopropyl-6-methyl-
4-pyrimidinyl) phosphorothioate. Toxicol Appl Pharmacol, 15: 216-224.
Harrison DL, Whitten LK, & Maskell PE (1962) Diazinon residues in the
tissues of sheep following dipping. Geigy (Australasia), p 14
(Publication No. 3).
Hartmann HR (1990) G 24`480 tech.: 21-day repeated exposure inhalation
toxicity in the rat - Nose-only exposure (Project No. 891205). Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Hata S, Berstein E, & Davis LE (1986) A typical ocular bobbing in
acute organophosphate poisoning. Ach Neurol, 43: 185-186.
Hayes WJ (1963) Clinical handbook on economic poisons - Emergency
information for treating poisoning. Washington, DC, US Government
Printing Office (Public Health Service Publication No. 476).
Hayes AL, Wise RA. & Weir FW (1980) Assessment of occupational
exposure to organophosphates in pest control operators. Am Ind Hyg
Assoc J, 41: 568-575.
Henderson M & Kitos PA (1982) Do organophosphate insecticides
inhibit the conversion of tryptophan to NAD+ in ovo? Teratology,
26: 173-181.
Henderson JD, Yamamoto JT, Fry DM, Seiber JN, & Wilson BW (1994), Oral
and dermal toxicity of organophosphate pesticides in the domestic
pigeon (Columbia livia). Bull Environ Contam Toxicol, 52: 663-640.
Hensley DL (1991) Pesticide effects on nitrogen fixation in legumes -
Fedrip database. Springfield, Virginia, National Technical
Information Service.
Hertner T & Arni P (1990) G 24`480 tech.: Autoradiographic DNA repair
test in rat hepatocytes (Project No. 891345). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Hill EF (1988) Formulation affects uptake and toxicity of
anticholinesterases in birds. Ann Conf Soc Environ Toxicol Chem,
9: 45.
Hill EF & Camardese MB (1984) Toxicity of anticholinesterase
insecticides to birds: Technical grade versus granular formulations.
Ecotoxicol Environ Saf, 8: 551-563.
Hill EF, Camardese MB, Heinz GH, Spann JW, & DeBevec AB (1984) Acute
toxicity of diazinon is similar for eight stocks of bobwhite. Environ
Toxicol Chem, 3: 61-66.
Hinkle DK, Suggs JE, & Jackson MD (1980) Environmental and biological
effects following application of diazinon impregnated strips within a
laboratory animal room. Lab Anim Sci, 30: 981-983.
Hoberman AM, Cramer JS, Avery DL, & Cranmer MF (1979) Transplacental
inhibition of esterases in fetal brain following exposure to the
organophosphate diazinon. Teratology, 19: 30A-31A.
Hodgson MJ & Smith AD (1992) Commercial and residential poisoning
with anticholinesterases. In: Ballantyne B & Mars TC ed. Clinical
and experimental toxicology of organophosphates and carbamates,
pp 346-363.
Hoffman DJ & Eastin WC Jr (1981) Effects of malathion, diazinon, and
parathion on mallard embryo development and cholinesterase activity.
Environ Res, 26: 472-485.
Holbert MS (1989) Diazinon: Acute inhalation toxicity study in rats
(EPA Guidelines 81-3) (Project No. 5947-89). Houston, Texas,
Stillmeadow, Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Holbert MS (1994): Diazinon: Acute inhalation toxicity study in
rats (Project No. 0067-93. Sugar Land, Texas, Stillmeadow, Inc.
(Unpublished report).
Hool G & Müller D (1981a) G 24`480 tech.: Sister chromatid exchange
study on Chinese hamster (test for mutagenic effects on bone marrow
cells) (Project No. 801504). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report including a supplement report from Strasser
F & Arni P).
Hool G & Müller D (1981b) G 24`480 tech.: Chromosome studies
in male germinal epithelium mouse (test for mutagenic effects on
spermatocytes) (Project No. 801502). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Hool G & Müller D (1981c) G 24`480 tech.: Nucleus anomaly test in
somatic interphase nuclei Chinese hamster (test for mutagenic effects
on bone marrow cells) (Project No. 801503). Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report including a supplement report from
Strasser F & Arni P).
Hool G & Müller D (1981d) G 24`480 tech.: Chromosome studies in
male germinal epithelium mouse (test for mutagenic effects on
spermatogonia) (Project No. 801501). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Hummell R, Brewer LW, Cobb GP III, & Hooper JM (1992a) An assessment
of avian response to and residue chemistry of applications of D-Z-N
diazinon 5G and D-Z-N diazinon AG500 to urban lawns - Part 1: November
1989 application. Pendleton, South Carolina, Clemson University,
Institute of Wildlife and Environmental Toxicology, 77 pp (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Hummell R, Brewer LW, Cobb GP III, & Hooper JM (1992b) An assessment
of avian response to and residue chemistry of applications of D-Z-N
diazinon 5G and D-Z-N diazinon AG500 to urban lawns - Part II: April
1990 application. Pendleton, SC, Clemson University, Institute of
Wildlife and Environmental Toxicology, 100 pp (Unpublished report
submitted to WHO by Ciba-Geigy Ltd, Basel).
Hummell R, Brewer LW, Cobb GP III, & Hooper JM (1992c) An assessment
of avian response to and residue chemistry of applications of D-Z-N
diazinon 5G and D-Z-N diazinon AG500 to urban lawns - Part III: June
1990 application. Pendleton, SC, Clemson University, Institute of
Wildlife and Environmental Toxicology, 133 pp (Unpublished report
submitted to WHO by Ciba-Geigy Ltd, Basel).
Hundley HK, Cairns T, Luke MA, & Matsumoto H (1988) Pesticide residue
findings by the Luke method in domestic and imported foods and
animal feeds for fiscal years 1982-1986. J Assoc Off Anal Chem,
71(5): 875-892.
Husain K & Ansari RA (1988) Influence of cholinergic and adrenergic
blocking drugs on hyperglycemia and brain glycogenolysis in
diazinon-treated animals. Can J Physiol Pharmacol, 66: 1144-1147.
Husain K & Matin MA (1986) Cerebral glycolysis and glycogenolysis in
diazinon treated animals. Arh Hig Rada Toxicol, 37: 29-34.
Infurna RM & Arthur AT (1985) G 24`480 tech.: A teratology study in
Charles River rats (Report No. 52-83). Summit, New Jersey, Ciba-Geigy
Corporation (Unpublished report submitted to WHO by Ciba-Geigy Ltd,
Basel).
IPCS (1986) Environmental health criteria 63: Organophosphorus
insecticides: A general introduction. Geneva, World Health
Organization, International Programme on Chemical Safety, 181 pp.
Iverson F, Grant DL, & Lacroix J (1975) Diazinon metabolism in the
dog. Bull Environ Contam Toxicol, 13: 611-618.
Jackson MD & Wright CG (1975) Diazinon and chlorpyrifos residues in
food after insecticidal treatment in rooms. Bull Environ Contam
Toxicol, 13: 593-595.
Jarvinen AW & Tanner DK (1982) Toxicity of selected controlled release
and corresponding unformulated technical grade pesticides to the
fathead minnow. Environ Pollut, A27: 179-195.
Jenkins LJ (1988) G 24`480 tech.: Acute delayed neurotoxicity of
diazinon MG-8 in domestic fowl (Project No. 5152-87. Houston, Texas,
Stillmeadow, Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Johnson WE, Fendinger NJ, & Plimmer JR (1991) Solid-phase extraction
of pesticides from water: possible interferences from dissolved
organic material. Anal Chem, 63: 1510-1513.
Johnston G, Walker CH, & Dawson AS (1994) Interactive effects between
EBI fungicides (prochloraz, propoconazole and penconazole) and OP
insecticides (dimethoate, chlorpyriphos, diazinon and malathion) in
the hybrid red-legged partridge. Environ Toxicol Chem, 13: 615-620.
Kabrawala VN, Shah RM, & Oza GG (1965) Diazinon poisoning (a study of
25 cases). Indian Pract, 18: 711-717.
Kanazawa J (1975) Uptake and excretion of organophosphorus and
carbamate insecticides by fresh water fish, motsugo, Pseudorasbora
parva. Bull Environ Contam Toxicol, 14: 346-352.
Kanazawa J (1978) Bioconcentration ratio of diazinon by freshwater
fish and snail. Bull Environ Contam Toxicol, 20: 613-617.
Kaplanis JN, Louloudes SJ, & Roan CC (1962) The distribution and
excretion of P32-labeled diazinon in guinea-pigs. Trans Kansas Acad
Sci, 65(1): 70-75.
Kathein R (1986) The development of poultry slaughter and poultry meat
inspection in Israel - a review. Isr J Vet Med, 42: 146-157.
Kazacos EA (1991) Pathogenesis of selected pancreatic diseases of
animals - Fedrip database. Springfield, Virginia, National Technical
Information Service.
Keller A (1983) Degradation of diazinon in aquatic systems (Project
No. 7/83). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished report).
Kenaga E (1973) Factors to be considered in the evaluation of the
toxicity of pesticides to birds in the environment. Environ Qual Saf,
2: 166-181.
Kendall RJ, Brewer LW, Hitchcock RR, & Mayer JR (1992) American
widgeon mortality associated with turf application of diazinon.
J Wildl Dis, 28: 263-267.
Kendall RJ, Brewer LW, & Hitchcock (1993) Response of Canada geese to
a turf application of diazinon AG5000. J Wildl Dis, 29(3): 458-464.
Khera KS & Bedok S (1967) Effects of thiol phosphates on notochordal
and vertebral morphogenesis in chick and duck embryos. Food Cosmet
Toxicol, 5: 359-365.
Kimbrough RD & Gaines TB (1968) Effect of organic phosphorus compounds
and alkylating agents on rat fetus. Arch Environ Health, 16: 805-808.
Kirchner FR (1991) G 24`480 tech.: One/two year oral toxicity study in
rats (Project No. 882018). Summit, New Jersey, Ciba-Geigy Corporation
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kirkbride KP (1987) An estimation of diazinon in omental tissue.
J Anal Toxicol, 11: 6-7.
Klemmer HW, Reichert ER, & Yauger WL (1978), Five cases of intentional
ingestion of 25 percent diazinon with treatment and recovery. Clin
Toxicol, 12(4): 435-444.
Klotzsche C (1955) [On the toxicology of new phosphoric acid ester
insecticides.] Arzneim Forsch, 5: 436-439 (in German).
Koibuchi M, Shibazaki T, & Inoue T (1975) Gas-liquid chromatographic
determination of diazinon in the oil solutions and the emulsifiable
concentrates. Eisei-Shikenjo-Hokoku, 93: 27-30.
Kring JB (1969) Mortality of the earthworm Lumbricus terrestris L.
following soil applications of insecticide to a tobacco field. J Econ
Entomol, 62: 963.
Kuhn JO (1989a) G 24`480 tech.: Acute oral toxicity study in rats
(Project No. 5942-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuhn JO (1989b) Diazinon: Acute dermal toxicity study in rabbits
(Project No. 5943-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuhn JO (1989c) Diazinon: Primary dermal irritation study in rabbits
(Project No. 5945-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuhn JO (1989d) Diazinon: Primary eye irritation study in rabbits
(Project No. 5944-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuhn JO (1989e) Diazinon: Dermal sensitization study in guinea pigs
(Project No. 5946-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuroda K, Yamaguchi Y, & Endo G (1992) Mitotic toxicity, sister
chromatid exchange, and rec assay of pesticides. Arch Environ Contam
Toxicol, 23: 13-18.
Kushaba-Rugaaju S & Kitos PA (1985) Effects of diazinon on nucleotide
and amino acid contents of chick embryos. Teratogen Consid Biochem
Pharmacol, 34(11): 1937-1943.
Lacorte S & Barcelo D (1995) Determination of organophosphorus
pesticides and their transformation products in river water by
automated on-line solid-phase extraction followed by thermospray
liquid chromatography-mass spectrometry. J. Chromatogr, A712: 103-112.
Lacorte S & Barcelo D (1996) Determination of part per trillion levels
of organophosphorus pesticides in groundwater by automated on-line
liquid-solid extraction followed by liquid chromatography/atmospheric
pressure chemical ionization mass spectrometry using positive and
negative ion modes of operation. Anal Chem, 68: 2460-2470.
Lawrence JF & Iverson F (1975) Analysis of the diazinon metabolites G
27550 and GS 31144 by gas-liquid chromatography with nitrogen-specific
detection after derivatization. J Chromatogr, 103: 341-347.
LeBel GL, Williams DT, Griffith G, & Benoit FM (1979) Isolation and
concentration of organophosphorus pesticides from drinking water at
the ng/l level, using macroreticular resin. J Assoc Off Anal Chem,
62: 241-249.
Lee HS (1989) Acute pancreatitis and organophosphate poisoning: a case
report and review. Singap Med J, 30: 599-601.
Leidy RB, Wright CG, & Dupree HE Jr (1982) Concentration and movement
of diazinon in air. J Environ Sci Health, 17: 311-319.
Leonard JA & Yeary RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51(11): 605-609.
Lloyd JE & Matthysse JG (1966) Polymer-insecticide systems for use as
livestock feed additives. J Econ Entomol, 59: 363-367.
Lloyd JE & Matthysse JG (1970) Polyvinyl chloride insecticide pellets
fed to cattle to control face fly larvae in manure. J Econ Entomol,
63: 1271-1281.
Lloyd JE & Matthysse JG (1971) Residues of dichlorvos, diazinon and
dimetilan in milk of cows fed PVC-insecticide feed additives. J Econ
Entomol, 64: 821-822.
Lopez DE & Carrascal E (1987) Sensitivity of human lymphocyte
chromosome to diazinon at different times during cell culture. Bull
Environ Contam Toxicol, 38: 125-130.
Lopez DE, Aleixandre C, Merchan M, & Carrascal E (1986) In vitro
induction of alterations in peripheral blood lymphocytes by different
doses of diazinon. Bull Environ Contam Toxicol, 37: 517-522.
Lores EM & Bradway DE (1977) Extraction and recovery of
organophosphorus metabolites from urine using an anion exchange resin.
J Agric Food Chem, 25: 75-79.
McGregor DB, Brown A, Cattenach P, Edwards I, Mc Bride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma cell
forward mutation assay: III. 72 coded chemicals Environ Mol Mutagen,
12: 85-154 (Published erratum in Environ Mol Mutagen, 12: 345).
Machin AF, Rogers A, Cross AJ, Quick MP, Howells LC, & James NF
(1975) Metabolic aspects of the toxicology of diazinon. Pestic Sci, 6:
461-473.
Macklin AW & Ribelin WE (1971) The relation of pesticides to abortion
in dairy cattle. J Am Vet Med Assoc, 159: 1743-1748.
MacLaren Plansearch Inc. & FDC Consultants Inc. (1985) Drinking water
quality criteria document: Diazinon (Prepared for Ontario Ministry of
the Environment).
Maddy KT & Edmiston S (1988) Selected incidents of illnesses and
injuries related to exposure to pesticides reported by physicians in
California in 1986. Vet Hum Toxicol, 30: 246-254.
MAFF (1982) Report of the Working Party on Pesticide Residues
(1971-1981). The Ninth Report of the Steering Group on Food
Surveillance. London: Her Majesty's Stationery Office (Food
Surveillance Paper No 9).
MAFF (1986) Report of the Working Party on Pesticide Residues
(1982-1985). The Sixteenth Report of the Steering Group on Food
Surveillance. London, Her Majesty's Stationery Office (Food
Surveillance Paper No 16).
MAFF (1989) Report of the Working Party on Pesticide Residues
(1985-1988). The Twenty-Fifth Report of the Steering Group on Food
Surveillance. London, Her Majesty's Stationery Office (Food
Surveillance Paper No 24).
MAFF (1991) Review of diazinon. London, Her Majesty's Stationery
Office (UK Government's Advisory Committee on Pesticides Disclosure
Document No. 35).
Maizlish N, Schenker A, Weisskopf C, Seiber J, & Samuel S (1987) A
behavioural evaluation of pest control workers with short-term,
low-level exposure to the organophosphate diazinon. Am J Ind Med,
12: 153-172.
Mann PC (1993) Histopathological assessment of potential ocular
toxicity of four organophosphate insecticides (Studies Nos. SEF 882018
and SEF 882014). Research Triangle Park, North Carolina, Experimental
Pathology Laboratories, Inc. (Pathology report).
Marselas G, Beavers JB, Smith GJ, & Jaber MJ (1989a) Diazinon: a one
generation reproduction study with the Mallard (Anas platyrhynchos)
using parental incubation. Easton, Maryland, Wildlife International
Ltd (Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Marselas G, Beavers JB, Smith GJ, & Jaber MJ (1989b), Diazinon: a one
generation reproduction study with the Northern bobwhite (Colinus
virginainus) using parental incubation. Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Marshall TC, Dorough HW, & Swim HE (1976) Screening of pesticides for
mutagenic potential using Salmonella typhimurium mutants. J Agric
Food Chem, 24: 560-563.
Matin MA & Husain K (1987) Changes in cerebral glycogenolysis and
related enzymes in diazinon treated hyperglycaemic animals. J Appl
Toxicol, 7(2): 131-134.
Matin MA, Khan SN, Hussain K, & Sattar S (1989) Effect of
adrenalectomy on diazinon-induced changes in carbohydrate
metabolism. Arch Toxicol, 63: 376-380.
Matsuoka A, Hayashi M, & Ishidate M Jr (1979) Chromosomal aberration
tests on 29 chemicals combined with S9 mix in vitro. Mutat Res,
66: 277-290.
Matsushita T, Aoyama K, Yoshimi K, Fujita Y, & Ueda A (1985) Allergic
contact dermatitis from organophosphorus insecticides. Ind Health,
23: 145-154.
Matthysse JG, Gutenmann WH, & Gigger R (1968) Sheep ectoparasite
control: II. Toxicity to sheep, and residues of diazinon and lindane.
J Econ Entomol, 61: 207-209.
Mayer FL.& Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Mello D, Rodrigues Puga F, & Benintendi R (1972) [Intoxications
produced by degradation products of diazinon in its use as a tick
killer.] Biologico, 38: 136-139 (in Portuguese).
Miles JR (1976) Insecticide residues on stream sediments in Ontario,
Canada. Pestic Monit J, 10: 87-91.
Miles JRW & Harris CR (1978a) Insecticide residues in water, sediment,
and fish of the drainage system of the Holland marsh, Ontario, Canada
1972-1975. J Econ Entomol, 71: 125-131.
Miles JRW & Harris CR (1978b) Insecticides residues in organic
soils of six vegetable growing areas in Southwestern Ontario, 1976.
J Environ Health, B13(3): 199-208.
Miles JRW, Harris CR, & Moy P (1978) Insecticide residues in organic
soil of the Holland marsh, Ontario, Canada, 1972-75. J Econ Entomol,
71: 97-101.
Miller CW, Zuckerman BM, & Charig AJ (1966) Water translocation of
diazinon-C14 and parathion-S35 off a model cranberry bog and
subsequent occurrence in fish and mussels. Trans Am Fish Soc,
95: 345-349.
Mirza AM, Schaub N, Samler L, & Reuchelderfer TE (1972)
Organophosphate insecticide diazinon poisoning in children. Med
Ann DC, 41: 559-560.
Misawa W, Doull J, Kitos PA, & Uyeki EM (1981) Teratogenic effects of
cholinergic insecticides in chick embryos: I. Diazinon treatment on
acetylcholinesterase and choline acetyltransferase activities. Toxicol
Appl Pharmacol, 57: 20-29.
Misawa M, Doull J, & Uyeki EM (1982) Teratogenic effects of
cholinergic insecticides in chick embryos: III. Development of
cartilage and bone. J Toxicol Environ Health, 10: 551-563.
Miyazaki H, Tojinbara I, Watanaba Y, Osaka T, & Okui S (1970) Studies
on metabolism of diazinon (O,O-diethyl-O-(2-isopropyl-4-methyl-6-
pyrimidinyl)phosphorothioate) in animals and plants. In: Proceedings
of the First Symposium on Drug Metabolism and Action, Chiba, Japon,
14-15 November 1969, pp 135-138.
Montz WE Jr & Kirkpatrick RL (1985) Temporal patterns of brain
cholinesterase activities of white-footed mice (Peromyscuc
leucopus) following dosing with diazinon or parathion. Arch Environ
Contam Toxicol, 14: 19-24.
Morganian VM & Wall WJ Jr (1972) Dursban and diazinon residues in
biota following treatment of intertidal plots on Cape Cod - 1967-1969.
Pestic Monit J, 6: 160-163.
Mortland MM & Raman KV (1967) Catalytic hydrolysis of some organic
phosphate pesticides by copper. J Agric Food Chem, 15: 163-167.
Mücke W, Alt KO, & Esser HO (1970) Degradation of 14C-labeled
diazinon in the rat. Agric Food Chem, 18: 208-212.
Muratore F, Faiolo H, & Ponzetta G (1960) On four cases of poisoning
by organophosphorus acid esters (antiparasitic) with recovery, with
special reference to hepatic involvement. Minerva Med, 51: 3342-3345.
Murli H & Haworth SR (1990a) Mutagenicity test on diazinon MG8: an
in vitro cytogenetic assay measuring sister chromatid exchange
frequencies in cultured whole blood human lymphocytes (Project No.
12226-0-448).Kensington, Hazleton Laboratories America, Inc.
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Murli H & Haworth SR (1990b) Mutagenicity test on diazinon MG8:
In vivo sister chromatid exchange assay (Project No. 12226-0-458).
Kensington, Maryland, Hazleton Laboratories America, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Nakatsugawa T, Tolman NM, & Dahm PA (1969) Oxidative degradation of
diazinon by rat liver microsomes. Biochem Pharmacol, 18: 685-688.
NCI (National Cancer Institute) (1979) Bioassay of diazinon for
possible carcinogenicity. Washington, DC, US Department of Health,
Education and Welfare (Publication No. (NIH) 79-1392).
NIOSH (1994) Organophosphorous pesticides: Method 5600. In: NIOSH
Manual of Analytical Methods, 4th ed. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, pp 1-20.
Nishio A & Uyeki EM (1981) Induction of sister chromatid exchanges in
Chinese hamster ovary cells by organophosphate insecticides and their
oxygen analogs. J Toxicol Environ Health, 8: 939-946.
Owen, M. (1975) The management of grass swards for captive wildfowl.
Int Zoo Year Book, 16: 135-138.
Payot PH (1966) Subacute oral toxicity study on diazinon AS - Humans.
Basel, Switzerland, J.R. Geigy (Unpublished report submitted to WHO by
Ciba-Geigy Ltd, Basel).
Pettersen JC & Morrissey RL (1994) 90-Day subchronic neurotoxicity
study with diazinon MG87% in rats (Project No. F-00176). Farmington,
Connecticut, Ciba Geigy Corporation, Environmental Health Center.
Piccirillo VJ (1978) G 24`480 tech.: Acute oral toxicity study in rats
(Project No. 483-143). Vienna, Virginia, Hazleton Laboratories
America, Inc. (Unpublished report submitted to WHO by Ciba-Geigy Ltd,
Basel).
Pickles M (1990) Biological report for the metabolism of 14C-diazinon
on sheep (Project No. BIOL-89014). Greensboro, North Carolina, Ciba-
Geigy Corporation (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Pickles M & Seim V (1988) Biological report for the metabolism of
2-pyrimidinyl-14C-diazinon in a lactating goat (Project No.
BIOL-88004). Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Poklis A, Kutz FW, Sperling JF, & Morgan DP (1980) A fatal diazinon
poisoning. Forensic Sci Int, 15: 135-140.
Potrepka RF (1994) Acute cholinesterase inhibition time course study
with D-Z-N Diazinon MG 87% in rats (Project No. F-00185). Farmington,
Connecticut, Ciba-Geigy Corporation, Environmental Health Center
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Prijono WB & Leighton FA (1991) Parallel measurement of brain
acetylcholinesterase and the muscarinic cholinergic receptor in the
diagnosis of acute, lethal poisoning anticholinesterase pesticides.
J. Wildl Dis, 27: 110-115.
Radeleff RD & Kunz SE (1972) Toxicity and hazard of diazinon, ethion,
and supracide to turkeys. J Econ Entomol, 65: 162-165.
Rajendra W, Oloffs PC, & Banister EW (1986) Effects of chronic intake
of diazinon on blood and brain monoamines and amino acids. Drug Chem
Toxicol, 9: 117-131.
Richter ED, Kowalski M, Leventhal A, Grauer F, Marzouk J, Brenner S,
Sholnik I, Lerman S, Zahavi H, & Bashari A (1992) Illness and
excretion of organophosphate metabolites four months after household
pest extermination. Arch Environ Health, 47: 135-138.
Robbins WE, Hopkins TL, & Eddy GW (1957) Metabolism and excretion
of phosphorus-32-labeled diazinon in a cow. J Agric Food Chem,
5: 509-513.
Robens JF (1969)Teratologic studies of carbaryl, diazinon, norea,
disulfiram, and thiram in small laboratory animals. Toxicol Appl
Pharmacol, 15: 152-163.
Robertson JB & Mazzella C (1989) Acute toxicity of the pesticide
diazinon to the freshwater snail Gillia altilis. Bull Environ Contam
Toxicol, 42: 320-324.
Rudzki MW, McCormick GC, & Arthur AT (1991) G 24`480 tech.: 52-week
oral toxicity study in dogs (Project No. 882014). Summit, New Jersey,
Ciba-Geigay Corporation (Unpublished report submitted to WHO by
Ciba-Geigy Ltd, Basel).
Sachsse K (1972) Acute dietary LC50 of technical diazinon in the
Japanese quail. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Sachsse K (1973a) Acute oral LD50 of technical diazinon in the
Japanese quail. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Sachsse K (1973b) 8-Day feeding toxicity of technical diazinon in the
mallard duck. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Bathe R (1975) Potentiation study C 8514 (chlordimeform)
versus 3 insecticides, G 24480 (diazinon), CGA 12223 and CGA 15324 in
the rat. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Bathe R (1976) Potentiation study C 9491 (Jodfenphos)
versus 2 insecticides, G 24480 (diazinon), and C 177 (dichlorovos) in
the rat. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Bathe R (1977) CGA 15324 versus 2 insecticides, GS 13005
(methidathion) and G24480 (diazinon) in the rat. Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Bathe R (1978) Potentiation study CGA 20168 versus
6 insecticides, C 177 (DDVP), C 570 (phosphamidon), GS 13005
(methidathion), G 24480 (diazinon), CGA 15324 and malathion (Project
No. 404478 Siss 6526). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Sachsse K & Ullmann L (1975a) Acute oral LD50 in the "5-day old"
Japanese quail of technical diazinon (G 24480). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Ullmann L (1975b) Acute oral LD50 in the "5-day old"
bobwhite quail of technical diazinon (G 24480). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Ullmann L (1975c) Acute oral LD50 in the "5-day old"
Peking duck of technical diazinon (G 24480). Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Sachsse K & Ullmann L (1975d) Acute oral LD50 in the "5-day old"
domestic hen of technical diazinon (G 24480). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Ullmann L (1976) Acute oral LD50 in the adult Peking
duck of technical G 24480 (diazinon). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Sakr SA & Gabr SA (1992) Ultrastructural changes induced by diazinon
and neopybuthrin in skeletal muscles of Tilapia nilotica. Bull
Environ Contam Toxicol, 48: 467-473.
Sakr SA, Gabr SA, & El Saadany MM (1991) Effect of diazinon on
freeze-fracture images of microvilli of intestinal epithelial cells of
Tilapia nilotica. Z Ernähr.wiss, 30: 268-275.
Samal KK & Sahu CS (1990) Organophosphorus poisoning and intermediate
neurotoxic syndrome. J Assoc Phys India, 38: 181-182.
Sancho E, Ferrando MD, Andreu E, & Gamon M (1992) Acute toxicity,
uptake and clearance of diazinon by the European eel. J Environ Sci
Health, 27: 209-221.
Sastry KV & Malik PV (1982a) Acute and chronic effects of diazinon on
the activities of three dehydrogenases in the digestive system of a
freshwater teleost fish Channa punctatus. Toxicol Lett, 10: 55-59.
Sastry KV & Malik PV (1982b) Histopathological and enzymological
alterations in the digestive system of a freshwater teleost fish,
Heteropneustes fossilis, exposed acutely and chronically to
diazinon. Ecotoxicol Environ Saf, 6: 223-235.
Schoch M & Gfeller W (1985) G 24`480 tech: Acute oral LD50 in the rat
(Project No. 850864). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Schoen SR & Winterlin WL (1987) The effects of various soil factors
and amendments on the degradation of pesticide mixtures. J Environ Sci
Health, B22: 347-377.
Sears MK & Chapman RA (1979) Persistence and movement of four
insecticides applied to turfgrass. J Econ Entomol, 72: 272-274.
Seguchi K & Asaka S (1981) Intake and excretion of diazinon in
freshwater fishes. Bull Environ Contam Toxicol, 27: 244-249.
Seifert J & Cassida JE (1979) Inhibition and reactivation of chicken
kynurin formamidase: in vitro studies with organophosphates. Pestic
Biochem Physiol, 12: 273-279.
Seifert J & Pewnim T (1992) Alteration of mice L-tryptophan metabolism
by the organophosphorous acid triester diazinon. Biochem Pharmacol,
44(11): 2243-2250.
Sekine B (1972) Metabolism of diazinon (O,O-diethyl-O-(2-isopropyl-4-
methyl-6-pyrimidinyl) phosphorothioate. Jpn Pestic Inf, 10: 77-80.
Senanayake N & Karalliedde L (1987) Neurotoxic effects of
organophosphorus insecticides: An intermediate syndrome. N Engl J Med,
316: 761-763.
Seyfried B (1994) Degradation of 14C-diazinon (G 24480) in one soil
incubated under various experimental conditions (Project No. 351358.)
Basel, Switzerland, Novartis Crop Protection (Unpublished report).
Shaffer CB & West B (1960) The acute and subacute toxicity of
technical O,O-diethyl S-2 diethylaminoethyl phosphorthioate hydrogen
oxalate (tetram). Toxicol Appl Pharmacol, 2: 1-13.
Sharma SR, Singh RP, & Ahmed SR (1986) Effect of different salt
leachates on the movement of some phosphorus containing pesticides
in soils using thin layer chromatography. Ecotoxicol Environ Saf,
11: 229-240.
Shishido T & Fukami J (1972) Enzymatic hydrolysis of diazoxon by rat
tissue homogenates. Pestic Biochem Physiol, 2: 39-50.
Shishido T, Usui K, & Fukami J (1972a) Oxidative metabolism of
diazinon by microsomes from rat liver and cockroach fat body. Pestic
Biochem Physiol, 2: 27-38.
Shishido T, Usui K, Sato M, & Fukami J (1972b) Enzymatic conjugation
of diazinon with glutathione in rat and American cockroach. Pestic
Biochem Physiol, 2: 51-63.
Shlosberg A, Egyed MN, Eliat A, Malkinson M, & Preissler E (1976)
Efficacy of pralidoxime iodide and obidoxime dichloride as antidotes
in diazinon-poisoned goslings. Avian Dis, 20: 162-166.
Simoneaux BJ (1988a) Disposition of 14C-diazinon in goats - EPA
Guideline 171-4, 7. Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Simoneaux BJ (1988b) Characterization of 14C-diazinon metabolites in
goats - EPA Guideline 171-4 (Project No. ABR-88118). Greensboro, North
Carolina, Ciba-Geigy Corporation (Unpublished report submitted to WHO
by Ciba-Geigy Ltd, Basel).
Simoneaux BJ (1988c) Disposition of 14C-diazinon in chickens (Project
No. ABR-88116). Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Simoneaux BJ (1988d) Characterization of 14C-diazinon metabolites in
chickens (Project No. ABR-88119). Greensboro, North Carolina, Ciba-
Geigy Corporation (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Simoneaux BJ (1988e) Metabolite identification in hens and goats
treated with 14C-diazinon (Project No. ABR-88135). Greensboro, North
Carolina, Ciba-Geigy Corporation (Unpublished report submitted to WHO
by Ciba-Geigy Ltd, Basel).
Simoneaux BJ (1989) Supplemental report on the nature of residues of
diazinon in hens (Project No. ABR-89040). Greensboro, North Carolina,
Ciba-Geigy Corporation (Unpublished report submitted to WHO by
Ciba-Geigy Ltd, Basel).
Singh PK (1973) Effect of pesticides on blue-green algae. Arch
Mikrobiol, 89: 317-320.
Singh AR, McCormick GC, & Arthur AT (1988) G 24480 tech.: Diazinon
(MG-8) 13-week oral feeding study in rats (Project No. 882011).
Summit, New Jersey, Ciba-Geigy Corporation (Unpublished report
submitted to WHO by Ciba-Geigy Ltd, Basel).
Singmaster J & Acin-Diaz NM (1991) Biochemical and residual properties
of pesticides. Springfield, Virginia, National Technical Information
Service (Fedrip Database).
Skinner CS & Kilgore WW (1982) Acute dermal toxicities of various
organophosphate insecticides in mice. J Toxicol Environ Health,
9: 491-497.
Smith BR, Dauterman WC, & Hodgson E (1974) Selective inhibition of the
metabolism of diazinon and diazoxon in vitro by piperonyl butoxide,
NIA 16824, and 1-(2-isopropylphenyl) imidazole. Pestic Biochem
Physiol, 4: 337-345.
Sobti RC, Krishan A, & Pfaffenberger CD (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human lymphoid
cells in vitro: organophosphates. Mutat Res, 102: 89-102.
Soliman SA, Sovocool GW, Curley A, Ahmed NS, & El-Fiki El-Sabae AK
(1982) Two acute human poisoning cases resulting from exposure to
diazinon transformation products in Egypt. Arch Environ Health,
37: 207-212.
Spyker JM & Avery DL (1977) Neurobehavioral effects of prenatal
exposure to the organophosphate diazinon in mice. J Toxicol Environ
Health, 3: 989-1002.
Stadnichenko AP, Ivanenko LD, & Sitniakovskaia AM (1987) Effect of
phenol and pesticides on the physicochemical properties of the
hemolymph in fresh-water gastropod molluscs infected by trematode
parthenitae. Parazitologiia, 21: 716-720.
Stalberg E, Hilton-Brown P, Kolmodin-Hedman B, Holmstedt B, &
Augustinsson BB (1978) Effect of occupational exposure to
organophosphorus insecticides on neuromuscular function. Scand J
Work Environ Health, 4: 255-261.
Stamm E (1994) Rate estimations of the hydroxyl radical oxidation of
diazinon G 24480 (Project 94SM07.) Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Stevenson JH (1978) The acute toxicity of unformulated pesticides to
worker honey bees (Apis mellifera). Plant Pathol, 27: 38-40
Tai CN & Katz R (1984) G 24`480 tech.: 21-day dermal toxicity study in
rabbits (Project No. 842007). Summit, New Jersey, Ciba-Geigy
Corporation (Unpublished report submitted to WHO by Ciba-Geigy Ltd,
Basel).
Tomokuni K & Hasegawa T (1985), Diazinon concentrations and blood
cholinesterase activities in rats exposed to diazinon. Toxicol Lett,
25: 7-10.
Tomokuni K, Hasegawa T, Hirai Y, & Koga N (1985) The tissue
distribution of diazinon and the inhibition of blood cholinesterase
activities in rats and mice receiving a single intraperitoneal dose of
diazinon. Toxicology, 37: 91-98.
Torres CM, Pico Y, Redondo MJ, & Manes J (1996) Mass solid-phase
dispersion extraction procedure for multiresidue pesticide analysis in
oranges. J. Chromatogr, A719: 95-103.
Toyoda M, Adachi K, Ida T, Noda K, & Minagawa N (1990) Simple
analytical method for organophosphorus pesticide residues in milk.
J Assoc Anal Chem, 73: 770-772.
Tripathi G (1992) Relative toxicity of aldrin, fenvalerate, captan and
diazinon to the freshwater food-fish, Clarias batrachus Biomed
Environ Sci, 5: 33-38.
Tsuda T, Aoki S, Kojima M, & Harada H (1990) Bioconcentration and
excretion of diazinon, IBP, malathion and fenitrothion by carp. Comp
Biochem Physiol, C96: 23-36.
Vial A (1990) Acute toxicity test of G 24480 technical to earthworm
(Eisenia foetida). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report No. 891454).
Wachs T, Gutenmann WH, Buckley EH, & Lisk DJ (1983) Concentration of
diazinon in air of a retail garden store. Bull Environ Contam Toxicol,
31: 582-584.
Wadia RS, Sadagopan C, Amin RB, & Sardesai HV (1974) Neurological
manifestations of organophosphorus insecticide poisoning. J Neurol
Neurosurg Psychiatry, 37: 841-847.
Wagner SL & Orwick DL (1994) Chronic organophosphate exposure
associated with transient hypertonia in an infant. Pediatrics,
94(1): 94-97.
Wan MT (1989) Levels of selected pesticides in farm ditches leading to
rivers in the lower mainland of British Columbia. J Environ Sci
Health, B24(2): 183-203.
Ward CR, Owens JC, & Turner WE (1972) Residues of diazinon remaining
after application to wheat. J Econ Entomol, 65: 899.
Wecker L, Mrak RE, & Dettbran WD (1985) Evidence of necrosis in human
intercostal muscle following inhalation of an organophosphate
insecticide. J Environ Pathol Toxicol Oncol, 6: 171-175.
Wedin GP, Pennente CM, & Sachdev SS (1984) Renal involvement in
organophosphate poisoning. J Am Med Assoc, 252: 1408.
Weiss CM (1961) Physiological effect of organic phosphorus
insecticides on several species of fish. Trans Am Fish Soc,
90: 143-152.
Weisskopf CP, Seiber JN, Maizlish N, & Schenker M (1988) Personnel
exposure to diazinon in a supervised pest eradication programme. Arch
Environ Contam Toxicol, 17: 201-212.
Weisskopf CP & Seiber JN (1989) New approaches to analysis of
organophosphate metabolites in the urine of field workers. In:
Biological monitoring of pesticide exposure. Washington, DC, American
Chemical Society, pp 206-214 (ACS Symposium Series 382).
Weizman Z & Sofer S (1992), Acute pancreatitis in children with
anticholinesterase insecticide intoxication. Pediatrics,
90(2): 204-206.
Wester RC, Sedik L, Melendres J, Logan F, Maibach HI, & Russell I
(1993) Percutaneous absorption of diazinon in humans. Food Chem
Toxicol, 31(8): 569-572.
WHO (1991) Safe Use of Pesticides: Fourteenth report of the Expert
Committee on Vector Biology and Control. Geneva, World Health
Organization (WHO Technical Report Series, No. 813).
WHO (1996) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1996-1997. Geneva, World Health
Organization, International Programme on Chemical Safety
(WHO/PCS/96.3).
Wilkinson JG, Rajendra W, Oloffs PC, & Banister EW (1986) Diazinon
treatment effects on heart and skeletal muscle enzyme activities. J
Environ Sci Health, 21: 103-113.
Williams MW, Fuyat HN, & Fitzhugh OG (1959) Acute and subacute
toxicity of four organic phosphates to dogs. Toxicol Appl Pharmacol,
1: 1-7.
Winnett G (1992) Biochemistry and fate of pesticides in agricultural
use. Springfield, Virginia, National Technical Information Service
(Fedrip Database).
Wong PK & Chang L (1988) The effects of 2,4-D herbicide and
organophosphorus insecticides on growth, photosynthesis, and
chlorophyll a synthesis of Chlamydomonas reinhardtii (mt+).
Environ Pollut, 55: 179-189.
Woodruff RC, Philipps JP, & Irwin D (1983) Pesticide-induced complete
and partial chromosome loss in screens with repair defective females
of Drosophila melanogaster. Environ Mutagen, 5: 835-846.
Wright CG & Leidy RB (1980) Insecticide residues in the air of
buildings and pest control vehicles. Bull Environ Contam Toxicol,
24: 582-589.
Wright CG, Leidy RB, & Dupree HE (1982) Diazinon and chlorpyrifos in
the air of moving and stationary pest control vehicles. Bull Environ
Contam Toxicol, 28: 119-121.
Wu HX, Evreux Gros C, & Descote J (1994) Rapid gas-chromatographic
assay of diazinon: Application to the study of diazinon kinetics in
rats. Biomed Environ Sci, 7: 357-361.
Wyttenbach CR & Hwang JD (1984) Relationship between insecticide-
induced short and wry neck and cervical defects visible histologically
shortly after treatment of chick embryos. J Exp Zool, 229: 437-446.
Yang RS, Dauterman WC, & Hodgson E (1969) Enzymatic degradation of
diazinon by rat liver microsomes. Life Sci, 8: 667-672.
Yang RSH, Hodgson E, & Dauterman WC (1971) Metabolism in vitro of
diazinon and diazoxon in rat liver. J Agric Food Chem, 19: 10-13.
Zak F, Luetkemeier H, Sachsse K, & Hess R (1973) G 24`480 tech.:
21-day inhalation study on the rat with technical diazinon (Project
No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Zwiener RJ & Ginsburg CM (1988) Organophosphate and carbamate
poisoning in infants and children. Pediatrics, 81: 121-126.
RÉSUMÉ ET ÉVALUATIONS
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le diazinon était connu jusqu'à ces dernières années sous le nom
chimique de thiophosphate de di- O-méthyle de d 'O-(isopropyl-2
méthyl-6 pyrimidyl-4). Selon les nouvelles conventions de
nomenclature, sa dénomination chimique est désormais phosphorothioate
de O,O-diéthyle et de O-2-isopropyl-6-méthylpyrimidiny-4-yle. A
l'état pur, il se présente sous la forme d'un liquide incolore
dégageant une légère odeur d'ester. La matière active de qualité
technique est un liquide jaune à brun doté d'une odeur légère mais
caractéristique. Son point d'ébullition est de 83-84°C sous une
pression de 26,6 mPa. Sa tension de vapeur (volatilité) est faible
(9,7 mPa à 20°C). Sa solubilité dans l'eau à la température ambiante
est de 60 mg/litre. Le diazinon est soluble dans la plupart des
solvants organiques et son coefficient de partage entre l'octanol et
l'eau (log Pow) est de 3,40. Il est stable en milieu neutre, mais il
s' hydrolyse lentement en milieu alcalin et plus rapidement en milieu
acide. Il se décompose au-dessus de 120°C.
Il existe de nombreuses méthode d'échantillonnage et d'analyse
pour le recherche et le dosage du diazinon et de ses métabolites dans
différents milieux. On fait de plus en plus appel à des méthodes
sensibles telles que la chromatographie en phase gazeuse, la
chromatographie en phase liquide à haute performance, la spectrométrie
de masse et certaines techniques immunologiques.
2. Production, usages et sources d'exposition humaine et
environnementale
Le diazinon est un insecticide de contact dont le spectre
d'activité est très étendu. Il est efficace contre les formes adultes
et juvéniles d'insectes volants ou rampants, ainsi que contre les
acariens et les arachnides. On l'utilise depuis le début des années
cinquante. Il est principalement commercialisé sous la forme de
concentrés émulsionnables et de poudres mouillables. Il existe
également en formulations dans lesquelles il est associé à d'autres
insecticides.
3. Transport, distribution et transformation dans l'environnement
La volatilisation du diazinon à partir du sol n'a qu'une
importance mineure. Sa demi-vie dans la troposphère est de 1,5 h.
Le mouvement du diazinon dans le sol dépend d'un certain nombre
de facteurs, en particulier de la teneur en matières organiques et en
carbonate de calcium. Le diazinon ne devrait pas être très solide-ment
fixé aux particules du sol, du fait que son Koc est égal à 500 et il
devrait être modérément mobile dans le sol.
La décomposition du diazinon dans le sol s'effectue
principalement par voie biologique. A une température de 20°C et pour
une teneur en eau du sol égale à 60% de la capacité totale, on a
constaté que dans un limon, la DT50 était de 5 jours. Dans des
conditions stériles, et pour la même teneur en eau et la même
température, on a obtenu une DT50 de 118 jours, ce qui donne à penser
que c'est bien l'activité biologique qui est principalement
responsable de la décomposition du diazinon dans le sol.
Dans les eaux naturelles, le diazinon a une demi-vie de l'ordre
de 5 à 15 jours. Des processus chimiques et biologiques semblent
intervenir dans sa décomposition, qui aboutit à une minéralisation en
quelques semaines.
Les organismes aquatiques fixent rapidement le diazinon. On a
fait état de faibles facteurs de bioconcentration chez ces organismes,
allant de 3 pour la crevette à 152 pour le goujon, ce qui correspond
bien à une métabolisation et à une excrétion rapides. Une demi-vie de
dépuration pouvant atteindre 30 h (tissu musculaire) a été mesurée
chez des poissons.
4. Concentrations dans l'environnement et exposition humaine
Le diazinon est généralement présent à faible concentration dans
l'environnement. La population générale peut être exposée par voie
alimentaire ou respiratoire. L'exposition par l'intermédiaire de l'eau
est négligeable. En milieu professionnel, elle est principalement
transcutanée.
On peut diviser les usages du diazinon en deux grandes
catégories: comme pesticide en agriculture et comme médicament en
médecine vétérinaire. Dans ces conditions, les résidus de diazinon
présents dans les cultures vivrières résultent principalement de
l'utilisation de ce composé comme pesticide en agriculture, alors que
ceux qui se retrouvent dans la viande, les abats et autres produits
d'origine animale proviennent de son usage comme principe actif dans
certains médicaments vétérinaires.
Il n'y a que de très petites quantités de résidus dans les
légumes, les fruits et les produits d'origine animale. Des études de
rations totales ont montré que le composé subit une dégradation rapide
dans les produits d'origine animale et végétale. On n'a pas décelé la
présence de diazinon dans les échantillons d'eau de boisson analysés
et sa concentration dans les eaux superficielles est de l'ordre du
nanogramme par litre.
5. Cinétique et métabolisme
Le diazinon peut être résorbé dans les voies digestives, par la
voie transcutanée ou après inhalation. Chez l'homme, l'absorption
transcutanée est faible. Sous l'action des enzymes microsomiennes, le
diazinon est oxydé en métabolites inhibant la cholinestérase comme le
diazoxon, l'hydroxydiazoxon et l'hydroxydiazinon. On ne les retrouve
qu'en quantités infimes dans le lait et les oeufs. Le diazinon et ses
métabolites ne s'accumulent pas dans les tissus de l'organisme: une
dose de diazinon administrée par voie orale est excrétée à 59-95% dans
les 24 h et à 95-98% au bout de 7 jours, principalement dans les
urines.
Les principales voies de dégradation métaboliques sont les
suivantes:
a) Clivage du groupement ester, conduisant à des dérivés de
l'hydroxypyrimidine.
b) Passage de la liaison P=S à la liaison P=O.
c) Oxydation du substituant isopropyl en alcool correspondant
d) Oxydation du substituant méthyl en alcool correspondant
e) Clivage du groupement ester sous l'action du glutathion,
conduisant à un conjugué avec le glutathion.
Le clivage de l'ester phosphorique, qui conduit, directement ou
par l'intermédiaire du diazoxon, aux métabolites à noyau pyrimidine,
constitue l'étape principale du métabolisme du diazinon. Les
métabolites qui conservent le groupement ester phosphorique sont de
nature transitoire et on ne les observe qu'en petite quantité. Les
métabolites sont produits en proportion et à des vitesses très
variables selon les espèces. En règle générale, il n'y a pas de
corrélation entre la sensibilité à l'intoxication par le diazinon et
la production de diazoxon, encore qu'elle soit la plus faible chez le
mouton, qui est précisément l'espèce la moins sensible. Le métabolisme
extrahé-patique du diazinon, en particulier l'hydrolyse du diazoxon
dans le plasma, joue un rôle toxicologique plus important que le
métabolisme intrahépatique, mais il est vrai que chez les oiseaux, le
foie est sans doute l'organe le plus important de ce point de vue. Les
métabolites qui se forment, à savoir l'acide diéthylphosphorique,
l'acide diéthyl-thiophosphorique, et un certain nombre de dérivés
contenant le noyau de la pyrimidine, sont principalement éliminés par
la voie rénale.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
Les améliorations apportées depuis 1979 au procédé de fabrication
du diazinon ont permis de réduire sensiblement sa teneur en impuretés
fortement toxiques, comme le pyrophosphate de tétraéthyle (TEPP).
Grâce à ces améliorations progressives, la DL50 aiguë par voie orale
de diazinon technique est passée, pour le rat, de 250 à 1250 mg/kg.
Quelle que soit la voie d'exposition (orale, transcutanée ou
respiratoire), la toxicité aiguë du diazinon est faible. Des études à
court et à long terme effectuées sur des souris, des rats, des lapins,
des chiens et des singes ont montré que le seul effet préoccupant
était une inhibition, liée à la dose, de l'activité
acétylcholinesterasique.
Le diazinon est légèrement irritant pour la peau chez le lapin
mais il n'irrite pas la muqueuse de l'oeil. Il ne provoque pas de
sensibilisation cutanée. Les études toxicologiques consacrées à ses
effets sur la reproduction et le développement n'ont pas mis en
évidence d'activité embryotoxique ou tératogène. La capacité de
reproduction n'a pas été affectée aux doses non toxiques pour les
animaux de la génération parentale. Des études de mutagénicité
comportant divers points d'aboutissement in vivo et in vitro n'ont
pas non plus indiqué que le diazinon soit doté d'un quelconque pouvoir
mutagène. Aucun signe d'activité cancérogène n'a été relevé chez le
rat et la souris. Le diazinon n'a pas provoqué pas de neuropathie
retardée chez des poules. Chez des chiens et des cobayes, on a observé
des cas de pancréatite aiguë ; on estime que cet effet est propre aux
espèces en cause.
7. Effets sur l'Homme
On a fait état d'un certain nombre d'intoxications accidentelles
ou consécutives à une tentative de suicide, qui, quelquefois, ont eu
une issue mortelle. Dans certaines de ces circonstances, les troubles
de nature cholinergique se sont révélés plus graves que prévu en
raison de la présence d'impuretés très toxiques, comme le
pyrophosphate de tétraéthyle (TEPP). On a pu aussi constater, dans
certains cas, la présence d'une pancréatite aiguë réversible
accompagnant le syndrome cholinergique. Cet effet s'observe également
dans les intoxications par d'autres inhibiteurs de la cholinestérase.
On aussi quelquefois observé un syndrome intermédiaire. Aucun cas de
neuropathie retardée n'a été signalé, conformément aux données
obtenues sur l'animal.. Dans tous les cas d'intoxication
professionnelle dont on a eu connaissance, il y avait présence
d'impuretés telles que le TEPP, le monothio-TEPP ou le sulfo-TEPP dans
la formulation utilisée. Il est peu probable que les formulations
actuelles contiennent encore de ces impuretés.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Le diazinon produit des effets variables sur les algues
unicellulaires; on a constaté des effets d'inhibition et de
stimulation de la croissance chez différentes espèces à des
concentrations de 0,01 et de 5 mg/litre. En général, il y a réduction
de la croissance au-delà de 10 mg/litre, mais dans certains cas,
l'effectif de la population n'a pas été modifié à une concentration de
100 mg/litre. La rareté et la variabilité des données rendent
difficile l'évaluation des effets produits sur les autres
microorganismes.
Les valeurs de la CL50 à 96 h (effets aigus) pour les
invertébrés aquatiques vont de 0,2 dans le cas de Gammarus
fasciatus à 4,0 mg/litre pour la crevette Hyallela azteca. Les
mollusques sont notablement moins sensibles, d'après une épreuve
effectuée sur le gastéropode Gillia attilis. Des effets sublétaux
ont été observés au niveau du comportement à des concentrations
comprises entre 0,1 et 0,01 mg/litre.
Les valeurs de la CL50 (effets aigus) pour les poissons varient
de 0,09 mg/litre chez la truite arc-en-ciel (Oncorhynchus mykiss) à
3,1 mg/litre chez le poisson-chat (Channa punctatus). Chez les
stades juvéniles des poissons, il y a inhibition de la croissance aux
concentrations comprises entre 0,01 et 0,2 mg/litre. Une exposition
aiguë au diazinon provoque la suppression de l'activité acétyl-
cholinestérase cérébrale.
Pour le lombric Eisenia foetida, la CL50 dans le sol est de
130 mg/kg de terre.
Chez les oiseaux, la toxicité aiguë par voie orale (DL50) varie
de 1,1 mg/kg p.c. pour la caille japonaise à 85 mg/kg p.c. pour le
molothre des troupeaux. Les valeurs de la CL50 obtenues lors d'études
d'alimentation, vont de 32 mg/kg de nourriture chez le colvert à
900 mg/kg de nourriture chez la caille japonaise (on observe un effet
répulsif à ces doses élevées). Des études de rations alimentaires en
laboratoire ont montré que la dose sans effet nocif observable sur la
reproduction des oiseaux était égale à 20 mg/kg de nourriture chez le
colvert et à 40 mg/kg de nourriture chez le colin de Virginie. Après
ingestion, il y a inhibition de l'acétylcholinesterase cérébrale. Le
diazinon peut également pénétrer par voie transcutanée. On a signalé
une mortalité importante chez du gibier d'eau à la suite de
pulvérisations de diazinon sur du gazon. Des études de terrain au
cours desquelles on a épandu du diazinon à raison de 4,8 kg de matière
active par hectare, ont montré qu'il n'en résultait aucune mortalité
pour les oiseaux chanteurs. L'épandage de granulés a provoqué une
légère réduction des populations d'oiseaux chanteurs par rapport aux
populations témoins. Les études de laboratoire montrent que
l'ingestion d'un petit nombre de granulés peut être fatale à un oiseau
de petite taille.
RESUMEN Y EVALUACIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El nombre químico del diazinon es O,O-dietilo O-2-isopropil-
6-metilpirimidinil-4-y1 fosforotioato. La sustancia pura forma un
líquido incoloro con un ligero olor a éster. El principio activo de
calidad técnica es un líquido amarillo pardusco con un ligero olor
característico del compuesto. Su punto de ebullición es de 83-84
oC a 26,6 mPa y su presión de vapor (volatilidad) es baja (9,7 mPa
a 20oC). Su solubilidad en agua a temperatura ambiente es de
60 mg/litro. El diazinon es soluble en la mayoría de los disolventes
orgánicos y tiene un coeficiente de reparto octanol/agua (log Pow) de
3,40. Es estable en medios neutros, pero se hidroliza lentamente en
medios alcalinos y más rápidamente en medios ácidos. Se descompone a
temperaturas superiores a los 120oC.
Se ha desarrollado un gran número de métodos de muestreo de
análisis para la determinación del diazinon y sus metabolitos en
distintos medios. Cada vez se utilizan más los métodos sensibles,
tales como la cromatografía de gases, la cromatografía líquida de alta
resolución, la espectrometría de masas y el inmunoensayo.
2. Producción, usos y fuentes de exposición humana y ambiental
El diazinon es un insecticida organofosforado de contacto, con
una actividad insecticida de amplio espectro. Es eficaz contra las
formas adultas y juveniles de insectos, voladores o no, ácaros y
arañas. Se utiliza desde principios del decenio de 1950. El diazinon
se prepara principalmente en forma de polvos humectables y
concentrados emulsionables. También se encuentra en preparaciones
mixtas combinado con otros insecticidas.
3. Transporte, distribución y transformación en el medio ambiente
La volatilización del diazinon en el suelo es de poca magnitud.
El diazinon tiene una semivida troposférica de 1,5 horas.
Su movilidad en el suelo depende en gran medida de una serie de
factores, en particular de la materia orgánica y del contenido en
carbonato de calcio. Su Koc de 500 no da lugar a prever una fijación
fuerte a las partículas del suelo, sino a una movilidad moderada en el
suelo.
Los procesos biológicos parecen ser el factor principal en la
degradación del diazinon en el suelo. A 20 oC y con un contenido de
humedad del suelo del 60% de la capacidad de campo en un suelo franco
limoso, el TD50 fue de 5 días. En condiciones de esterilidad a 20 oC
y con un 60% de capacidad de campo, el TD50 fue de 118 días, lo que
parece indicar que la actividad geológica es la principal responsable
de la degradación en el suelo.
En el agua presente en la naturaleza, el diazinon tiene una
semivida de 5 a 15 días. Al parecer, en la degradación del diazinon
intervienen procesos tanto químicos como biológicos que dan lugar a la
mineralización al cabo de pocas semanas.
La absorción del diazinon por los organismos acuáticos es rápida.
Con respecto a los organismos acuáticos, se han señalado factores de
bioconcentración bajos que oscilan entre 3 en el caso del camarón y
152 en el caso del gobio, lo que parece indicar un metabolismo y una
eliminación rápidos. Se han notificado semividas de depuración en los
peces de hasta 30 horas (mejillón).
4. Niveles medioambientales y exposición humana
Los niveles medioambientales de diazinon son generalmente bajos.
Las vías de exposición de la población general son la inhalación y la
alimentación. La exposición a través del agua es mínima. La exposición
profesional es fundamentalmente cutánea.
Los usos del diazinon se pueden clasificar en dos categorías
principales, a saber, como plaguicida en la agricultura y como fármaco
en la medicina veterinaria. Por consiguiente, la presencia de residuos
de diazinon en los cultivos comestibles obedece principalmente a su
utilización como plaguicida agrícola, mientras que su presencia en la
carne, los despojos y otros productos de origen animal se debe a su
utilización como fármaco de uso veterinario que contiene el principio
activo.
Los residuos de diazinon presentes en verduras, hortalizas,
frutas y productos de origen animal son muy escasos. Los resultados de
estudios realizados sobre dietas totales dan a entender que el
diazinon se degrada rápidamente tanto en los productos de origen
vegetal como en los de origen animal. No se ha detectado la presencia
de diazinon en muestras de agua potable y su concentración en aguas de
superficie se sitúa en niveles de ng/litro.
5. Cinética y metabolismo
El diazinon se puede absorber por el aparato digestivo, a través
de la piel intacta y tras su inhalación. La absorción transcutánea en
el ser humano es baja. Las enzimas microsómices oxidan el diazinon
produciendo metabolitos inhibidores de la colinesterasa, tales como el
diazoxón, el hidroxidiazoxón y el hidroxidiazinon. Sólo se detectan
cantidades mínimas de metabolitos en la leche y los huevos. El
diazinon y sus metabolitos no se acumulan en los tejidos corporales;
el 59-95% de una dosis oral de diazinon se excreta en un plazo de 24
horas y el 95-98% se excreta en un plazo de 7 días, principalmente por
orina.
Las principales vías metabólicas de degradación del diazinon son
las siguientes:
a) Ruptura del enlace de éster, que da lugar a los derivados de la
hidroxipirimidina.
b) Transformación de la fracción P-S en el derivado P-O.
c) Oxidación del sustituyente isopropílico, que da lugar a los
correspondientes derivados alcohólicos terciarios y primarios.
d) Oxidación del sustituyente metílico, que da lugar al alcohol
correspondiente.
e) Ruptura del enlace éster mediada por el glutatión, que da lugar a
un conjugado de glutatión.
La ruptura del enlace éster fosfato, que da lugar, directamente o
por vía del diazoxón, al metabolito pirimidílico, desempeña una
función determinante en el metabolismo del diazinon. Los metabolitos
que mantienen el enlace éster fosfato son de carácter transitorio y
sólo se han detectado en pequeñas cantidades. La cantidad de
metabolitos y su velocidad de producción varían ampliamente de una
especie a otra. En general, la producción de diazoxón no está
relacionada con la sensibilidad a la intoxicación por diazinon, si
bien es más baja en la especie menos vulnerable, los ovinos. El
metabolismo extrahepático del diazinon, en especial la hidrólisis del
diazoxón en el plasma, es toxicológicamente más importante que el
metabolismo hepático, pero este último probablemente sea el más
importante en las especies aviarias. Los metabolitos formados, esto
es, el ácido dietilfosfórico, el ácido dietiltiofosfórico y los
derivados del anillo pirimidinílico, se eliminan principalmente por
los riñones.
6. Efectos en los animales de experimentación y en sistemas de prueba
in vitro
Las mejoras introducidas en la fabricación del diazinon desde
1979 han reducido significativamente su contenido de impurezas
sumamente tóxicas, como por ejemplo el pirofosfato de tetraetilo
(TEPP). Como resultado de estas mejoras progresivas, la DL50 aguda
por vía oral del diazinon de calidad técnica ha aumentado (por
ejemplo, de 250 mg/kg a 1250 mg/kg en la rata).
La toxicidad aguda por vía oral o cutánea o por inhalación es
baja. Los estudios a corto y largo plazo efectuados en ratones, ratas,
conejos, perros y monos han puesto de manifiesto que el único efecto
preocupante es la inhibición de la actividad de la
acetilcolinesterasa, relacionada con la dosis.
En el conejo, el diazinon ocasiona una ligera irritación de la
piel, pero no de los ojos. El diazinon no sensibiliza la piel. Los
estudios de reproducción y desarrollo no han revelado indicios de
potencial embriotóxico ni teratogénico. No se detectaron efectos en el
proceso de reproducción tras la administración de dosis que no eran
tóxicas para los animales progenitores. Los estudios de mutagenicidad
con distintas variables de valoración in vivo y in vitro no
revelaron un potencial mutagénico. No hay indicios de carcinogenicidad
en ratas ni en ratones. El diazinon no causa neuropatía retardada en
las gallinas. Se ha señalado que el diazinon provoca pancreatitis
aguda en perros y cobayos; se considera que se trata de un efecto
específico de determinadas especies.
7. Efectos en el ser humano
Se han señalado varios casos de intoxicación accidental o
suicida con diazinon, algunos de los cuales fueron mortales. En
algunos de ellos, el síndrome colinérgico puede haber sido más grave
de lo previsto debido a la presencia de impurezas sumamente tóxicas,
tales como el TEPP. En algunos casos se produjeron pancreatitis
agudas reversibles asociadas a un síndrome colinérgico grave. Eso se
produce también tras la intoxicación con otros inhibidores de la
colinesterasa. En varios casos también se detectó el síndrome
intermedio. No se ha señalado ningún caso de neuropatía retardada,
como es de prever según los datos dimanantes de estudios realizados en
animales. Los casos notificados de intoxicación tras una exposición
profesional siempre han ido asociados a la presencia de impurezas en
la formulación, tales como el TEPP, el monotio-TEPP o el sulfo-TEPP.
Es poco probable que se encuentren estas impurezas en las
formulaciones disponibles en la actualidad.
8. Efectos en otros organismos en el laboratorio y en el medio
ambiente
Los efectos del diazinon en las algas unicelulares son
variables; se ha señalado tanto la inhibición como la estimulación
del crecimiento en distintas especies, con concentraciones de 0,01 a
5 mg/litro. En términos generales, las tasas de crecimiento disminuyen
con concentraciones superiores a 10 mg/litro, si bien en algunos casos
el tamaño de la población puede permanecer invariable con
concentraciones de 100 mg/litro. La escasez y variabilidad de los
datos dificulta la evaluación de los efectos sobre otros
microorganismos.
En pruebas realizadas a las 96-h, la CL50 aguda para los
invertebrados acuáticos oscilaba entre 0,2 mg/litro en Gammarus
fasciatus y 4,0 mg/litro en camarones (Hyallela azteca). Según una
prueba única a la que se sometió a Gillia attilis, los moluscos son
sustancialmente menos sensibles. Se han señalado efectos subletales en
el comportamiento con concentraciones de 0,1 a 0,01 mg/litro.
La CL50 aguda para los peces oscila entre 0,09 mg/litro para la
trucha arco iris (Oncorhynchus mykiss) y 3,1 mg/litro para el bagre
(Channa punctatus). El crecimiento durante las primeras fases de
la vida de los peces se inhibía con concentraciones de 0,01 a
0,2 mg/litro. La actividad de la acetilcolinesterasa cerebral se
inhibe tras una fuerte exposición al diazinon.
La CL50 en el suelo para la lombriz de tierra (Eisenia
foetida) es de 130 mg/kg de suelo.
La toxicidad aguda por vía oral (DL50) en las aves varía entre
1,1 mg/kg de peso corporal para la codorniz japonesa y 85 mg/kg de
peso corporal para aves del género Molothrus. Los valores de la
CL50 en la alimentación oscilan entre 32 mg/kg de alimentos en
el pato silvestre y 900 mg/kg de alimentos en la codorniz japonesa
(se detectó repelencia con estas altas concentraciones en la
alimentación). En estudios de laboratorio realizados en aves, la
concentración de diazinon en la dieta sin efectos observados en la
reproducción era de 20 mg/kg de alimentos en el pato silvestre y de
40 mg/kg de alimentos en la codorniz (Colinus virginianus). La
actividad de la acetilcolinesterasa cerebral se inhibe tras la
ingestión. El diazinon también se puede absorber por vía cutánea. Se
ha notificado una considerable mortandad de las aves acuáticas en la
naturaleza, tras la aplicación de diazinon al césped. En estudios
sobre el terreno en que se aplicaron formulaciones líquidas al césped
con una concentración de 4,8 kg ai/ha no se registró mortalidad ni se
produjeron efectos sobre la reproducción de las aves canoras. La
aplicación en forma de gránulos provocó una pequeña reducción en el
tamaño poblacional de aves canoras, en comparación con el grupo
testigo. La ingestión de pequeñas cantidades de gránulos puede
resultar mortal para las aves pequeñas, como se ha demostrado en
estudios de laboratorio.
10.2.1 Aquatic organisms
The main risk to aquatic organisms from the use of diazinon is
from spray drift during either arable applications (1.0 kg/ha) or top
fruit air-assisted (1.2 kg/ha). For each of these risk scenarios, the
predicted environmental concentration (PEC) in a 30-cm-deep static
surface water body, arising from either arable-based spray drift at
1 m from the edge of the spray boom or from top fruit air-assisted
spray drift at 3 m from the point of application (both based on
Ganzelmeier et al., 1995), was calculated as follows:
PEC = max application rate (kg/ha) × A (% spray drift)
(mg diazinon/
litre) 300
Where A = 5 for ground-based hydraulic spray application 1 m from edge
of boom
= 30 for air-assisted application 3 m from point of
application
10.2.1.1 Acute risk
The acute EC50 value for the most sensitive fish was
0.09 mg/litre and for the most sensitive aquatic invertebrate
(Gammarus) 0.0002 mg/litre. For the most sensitive algal
species the 14-day NOEC was 1 mg/litre.
(a) Spray drift from ground-based applications: The acute PEC for
spray drift (l m from the edge of the spray boom into a 30-cm-deep
static water body at the maximum application rate (see PEC assumptions
above) is 0.017 mg/litre. Therefore, the TERs, based on this PEC and
the above EC50/NOEC toxicity values, are: fish, 5.4; aquatic
invertebrates, 0.01; and algae, 60. Based on the EPPO/CoE
risk assessment scheme for aquatic organisms, these TERs (i.e. TERs
>10 = low risk; TERs <1 = high risk) indicate a low acute risk to
algae and a high risk to these organisms. The TER for fish was between
1 and 10, indicating an intermediate risk. In such risk situations the
use of a "no-spray" restriction zone next to surface waters may reduce
the risk to such aquatic invertebrates. For example, arable spray
drift at 5 m from the edge of boom is 0.6% (Ganzelmeier et. al.,
1995). Based on this 5-m drift data, the PEC is 0.002 mg/litre and
results in a 5-m TER of 45 for fish. This TER at 5 m indicates that
the use of a 5-m "no-spray" restriction zone next to surface waters
would reduce the acute risk to fish.
(b) Spray drift from broadcast air assisted top fruit
applications: The acute PEC for spray drift (3 m from the point of
application into a 30-cm-deep static water body at the maximum
application rate (see PEC assumptions above) is 0.12 mg/litre.
Therefore, the TERs based on this PEC and the above EC50/NOEC
toxicity values are: fish, 0.75; aquatic invertebrates, 0.0017; and
algae, 8.3. Based on the CoE/EPPO risk assessment scheme for aquatic
organisms, these TERs indicate a high acute risk to fish and
invertebrates and an intermediate risk to algae. Table 8 below
summarizes the acute TERs for aquatic organisms at 1 m for arable and
3 m for broadcast air-assisted applications.
10.2.1.2 Chronic risk
There were no chronic toxicity data available.
10.2.2 Terrestrial organisms
Vertebrates are likely to be exposed to diazinon from either
grazing treated vegetation or consuming contaminated insects. For this
risk assessment, typical application rates of 1 kg/ha are used for
ground spray application on arable crops and 1.2 kg/ha for application
by air-assisted spraying for fruit
10.2.2.1 Birds
The lowest reported acute oral LD50 for birds is 1.1 mg/kg body
weight for the Japanese quail. The dietary LC50 for the mallard duck
is 32 mg/kg diet.
Indicator birds for use in the risk assessment will be:
* Greylag goose (Anser anser), as a grazing species, with a body
weight of 3 kg and total daily food consumption of 900 g
vegetation (dry weight) (Owen, 1975)
* Blue tit (Parus caeruleus), as an insectivorous species, with a
body weight of 11 g and total daily food consumption of 8.23 g
(dry weight) (Kenaga, 1973).
a) Grazing birds
Initial residues on short grass or cereal shoots, arising from
application at 1 kg/ha to arable crops, are estimated to be 112 mg/kg
dry weight (based on 112 × application rate in kg/ha (EPPO/CoE,
1993a,b) and at 134.4 mg/kg from application to fruit at a rate of
1.2 kg/ha. This gives an estimated total oral intake for the goose of
100.8 and 121.0 mg for the two application rates assuming that the
goose ate exclusively food contaminated at this level. This is
equivalent to a daily intake of 33.6 and 40.3 mg/kg body weight,
respectively. TERs can be calculated as follows:
Table 8. Acute toxicity-exposure ratio (TERs) for aquatic organisms at 1 m for arable and 3 m
for broadcast air-assisted applications
Species EC50/NOEC PEC PEC TER TER
(mg/litre) (mg/litre) (mg/litre) (arable) (air-assisted)
(arable) (air-assisted)
Fish (Oncorhynchus mykiss) 0.09 0.017 0.12 5.4 0.75
Aquatic invertebrate (Gammarus) 0.0002 0.017 0.12 0.012 0.0017
Algae (Selenastrum capricornutum) 1.0 0.017 0.12 60.0 8.3
End-point LD50/L50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Bird acute oral 1.1 mg/kg 1 112 0.033
body weight (arable)
Japanese quail
1.2 134.4 0.027
(fruit)
Bird short-term 32 mg/kg diet 1 112 0.29
dietary mallard duck (arable)
1.2 134.4 0.24
(fruit)
The calculated TER values fall well below the EPPO/CoE trigger
values for concern (TER <10) and indicate a high risk to grazing
birds. This potential risk has been confirmed in practice with
reported high incidence of fatalities following application of
diazinon to golf-course turf. This application is no longer
recommended for this reason.
b) Insectivorous birds
Initial residues on small insects arising from application at
1 kg/ha to arable crops are estimated to be 29 mg/kg dry weight (based
on 29 × application rate in kg/ha) (EPPO/CoE, 1993a,b) and 34.8 mg/kg
from application to fruit at a rate of 1.2 kg/ha. This gives an
estimated total oral intake for the blue tit of 0.24 mg and 0.29 mg
for the two application rates assuming that the blue tit ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 21.7 and 26.0 mg/kg body weight, respectively. TERs
can be calculated as follows:
The TERs for acute toxicity to insectivorous birds are
substantially less then the trigger value of <10, indicating high
acute risk to these birds. The risk factors exceed the trigger for
insectivorous birds exposed short-term via the diet.
End-point LD50/LC50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Bird acute oral 1.1 mg/kg 1 29 0.05
body weight (arable)
Japanese quail
1.2 34.8 0.04
(fruit)
Bird short-term 32 mg/kg diet 1 29 1.1
dietary (arable)
1.2 34.8 0.92
(fruit)
10.2.2.2 Mammals
The lowest reported acute oral LD50 for laboratory mammals is
82 mg/kg body weight for the mouse.
Indicator mammals for use in the risk assessment will be:
* Rabbit (Orystolagus cuniculus), as a grazing mammal, with
a body weight of 1200 g and a total daily food consumption
of 500 g vegetation (dry weight) (Ross, personal
communication to the IPCS)
* Shrew (Sorex araneus), as an insectivorous mammal, with a
body weight of 18 g and a total daily food consumption of
18 g (Churchfield, 1986)
a) Grazing mammals
Initial residues on short grass or cereal shoots arising from
application at 1 kg/ha to arable crops are estimated to be 112 mg/kg
dry weight (based on 112 x application rate in kg/ha) (EPPO/CoE,
1993a,b) and at 134.4 mg/kg from an application to fruit at a rate of
1.2 kg/ha. This gives an estimated total oral intake for the rabbit of
56 and 67.2 mg for the two application rates, assuming that the rabbit
ate exclusively food contaminated at this level. This is equivalent to
a daily intake of 46.7 and 56 mg/kg body weight, respectively. TERs
can be calculated as follows:
End-point LD50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Mammal 82 mg/kg 1 56 1.76
acute oral body weight (arable)
mouse
1.2 67.2 1.46
(fruit)
This indicates a high risk to grazing mammals (trigger <10)
comparable to that for grazing birds. Again, the removal of
application to golf-course turf would reduce the likelihood of
exposure to maximum residues of diazinon. However, grazing mammals
consuming short cereal shoots could be killed following recommended
use of the compound.
b) Insectivorous mammals
Initial residues on large insects arising from application at
1 kg/ha to arable crops are estimated to be 2.7 mg/kg dry weight
(based on 2.7 × application rate in kg/ha) (EPPO/CoE, 1993a,b) and
3.24 mg/kg from an application to fruit at a rate of 1.2 kg/ha. This
gives an estimated total oral intake for the shrew of 0.049 mg and
0.058 mg for the two application rates, assuming that the shrew ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 2.7 and 3.2 mg/kg body weight, respectively. TERs can
be calculated as follows:
These TERs fall outside the trigger for high risk for
insectivorous mammals but within the range for medium risk, reflecting
the high acute mammalian toxicity.
End-point LD50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Mammal 82 mg/kg 1 2.7 30.4
acute oral body weight (arable)
mouse
1.2 3.24 25.3
(fruit)
10.2.2.3 Bees
The reported contact and oral toxicity to bees gives LD50 values
of 0.22 and 0.2 µg/bee, respectively. Using application rates of 1000
and 1200 g/ha for cereals and fruit, respectively, hazard quotients
are calculated to be 4545 and 5455 (application in g/ha). The trigger
for concern is >50 (EPPO/CoE, 1993a,b) and, therefore, there is
substantial concern for exposed bees. The compound should not be
applied to flowering plants and exposure of flying bees should be
avoided.
10.2.2.4 Earthworms
Earthworms are likely to be exposed to the highest concentration
of diazinon following use of the granular formulation incorporated in
soil at up to 2000 g/ha. Based on a soil depth of 5 cm and a soil
density of 1.5 g/cm3, the soil PEC would be 2.67 mg/kg, assuming even
distribution in the medium. The reported LC50 for earthworms
(Eisenia foetida) is 130 mg/kg soil, giving a TER of 48.75. As this
is above the trigger value of 10 (EPPO/CoE, 1993a,b), the acute risk
to earthworms should be low. Reported concentrations measured in soil
are at least an order of magnitude lower than the PEC suggesting that
little risk is posed to worms.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
The general population does not face a significant health risk
from diazinon. It may cause acute poisoning in cases of over-exposure
following intentional ingestion or careless handling during its
manufacture and use. The general public is exposed to diazinon in the
form of its residues in food, but the reported intake of diazinon is
far below the acceptable daily intake. Following residual spraying and
space treatment, used to control insects, the general population can
be exposed to residues in the air and on surfaces. With good work
practice and hygiene measures, and if safety precautions and medical
surveillance are enforced, diazinon is unlikely to present a hazard to
those occupationally exposed.
Diazinon does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates, fish, terrestrial birds and mammals, leading to high
risk factors for many of these organisms. Field kills of waterfowl
have been reported following use of the compound on amenity turf.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift and avoid areas where wildfowl are likely to graze).
11.2 Recommendations for protection of human health and the
environment
Certain groups within the population, including agricultural
workers and employees in the chemical industry, have the potential of
being exposed to diazinon. Gardeners and householders may also be
involved.
11.2.1 Recommendation on regulation of compound
Formulations have different classification categories: (FAO/WHO,
1979): liquids over 20% are in category 3; other liquids or solids
over 50% are in category 4; and all other solids are in category 5.
11.2.1.1 Transport and storage
Formulations in categories 3 and 4 should be transported and
stored in clearly labelled rigid and leak-proof containers, away from
containers of food and drink. Storage should be under lock and key and
secure from access by unauthorized people. Formulations in category 5
should be transported and stored in clearly labelled leak-proof
containers, out of reach of children and away from food and drink.
11.2.1.2 Handling
Protective clothing should be used by all handling of the
compound. Adequate washing facilities should be available at all times
during handling and should be close to the site of handling. Eating,
drinking and smoking should be prohibited during handling and before
washing the hands after handling.
11.2.1.3 Disposal
Containers may be decontaminated as recommended by the WHO Expert
Committee on Vector Biology and Control on the Safe Use of Pesticides
(WHO, 1991). Decontaminated containers should not be used for food and
drink. Containers that are not decontaminated should be burned or
crushed and buried below topsoil. Care must taken to avoid subsequent
contamination of water sources.
11.2.1.4 Selection, training and medical supervision of workers
Pre-employment medical examination of workers is desirable.
Workers suffering from active hepatic or renal disease should be
excluded from contact with diazinon. Pre-employment and periodic
cholinesterase test for workers is desirable especially for those
handling concentrates. Training of workers in techniques to avoid
contact is essential. Pilots and loaders should have special training
in application methods and early symptoms of poisoning, and must wear
a suitable respirator. Flagmen, if used, should wear overalls and be
located well away from the dropping zone.
11.2.1.5 Labelling
Diazinon is an organophosphorus compound that inhibits
cholinesterases. It is poisonous if swallowed. It may be absorbed
though the skin. It is important to avoid skin contact, wear hand
protection, clean protective clothing. The material must be kept out
of reach of children and well away from foodstuffs, animal feed and
their containers. If poisoning occurs, a physician should be called.
11.2.1.6 Residues in food
Maximum residue limits have been recommended for diazinon by the
Joint FAO/WHO Meeting on Pesticides Residues (FAO/WHO, 1975).
11.2.2 Prevention of poisoning in man and emergency aid
11.2.2.1 Manufacture and formulation
Closed systems and forced ventilation may be required to reduce
as much as possible the exposure of workers to diazinon.
11.2.2.2 Mixers and applicators
When mixing, protective impermeable boots, rubber apron, clean
overalls and gloves should be worn. When spraying tall crops or during
aerial application, a face mask should be worn, as well as an
impermeable hat, clothing, boots and gloves. The applicator should
avoid working in spray mist and avoid contact with the mouth.
Particular care is needed when equipment is being washed after use.
All protective clothing should be washed immediately after use.
Splashes must be washed immediately from the skin or eyes with large
quantities of water.
11.2.2.3 Other associated workers
People exposed to diazinon and associated with its application
should wear protective clothing and observe the precautions described
in section 11.2.2.2.
11.2.2.4 Other populations likely to be affected
With good application practice, other people should not be
exposed to hazardous amounts of diazinon.
11.2.3 Entry into treated areas
Unprotected people should abide by re-entry periods stated on
product labels.
11.2.4 Emergency aid
If symptoms appear following exposure, the person should stop
work immediately, remove contaminated clothing, wash the affected skin
with soap and water, if available, and flush the area with large
quantities of water. If diazinon has been swallowed and the person is
conscious, vomiting should be induced.
Atropine and oximes are specific antidotes and artificial
respiration may be needed.
11.2.5 Surveillance test
Slight reduction of plasma cholinesterase activity can be
observed. Periodic blood cholinesterase tests for workers is
desirable.
12. FURTHER RESEARCH
1. Studies of exposed worker populations should be continued.
2. Diazinon should be manufactured and formulated in accordance with
international specifications. It should be packed and stored
under conditions that are not conducive to the formation of
acutely toxic impurities.
3. The safe use of diazinon should follow label directions and
precautions for handling, application and disposal.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Diazinon has been evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) on a number of occasions since 1965. An
acceptable daily intake (ADI) of 0.002 mg/kg body weight has been
established and maximal residues levels have been recommended for
diazinon in a wide range of food commodities (FAO/WHO, 1994a,b).
The following NOAELs were established:
Rat (two-year feeding study): 1.5 mg/kg diet
(0.06 mg/kg body weight per day)
Dog (one-year feeding study): 0.5 mg/kg diet
(0.015 mg/kg body weight per day)
Rhesus monkey (two-year study): 0.5 mg/kg body weight per day
Human volunteers (36-day study): 0.025 mg/kg body weight per day
Diazinon has not been evaluated by the International Agency for
research on Cancer (IARC).
REFERENCES
Abdelsalam EB & Ford EJH (1986) Effect of pretreatment with hepatic
microsomal enzyme inducers on the toxicity of diazinon in calves. Res
Vet Sci, 41: 336-339.
Abdelsalam EB & Ford EJH (1987) The effect of induced liver, kidney
and lung lesions on the toxicity of levamisole and diazinon in calves.
J Comp Pathol, 97: 619-627.
Adachi K, Ohokuni N, & Mitsuhashi T (1984) Simple analytical method
for organophosphorus pesticide determination in unpolished rice, using
removal of fats by zinc acetate. J Assoc Off Anal Chem, 67: 798-800.
Al-Attar HJ & Knowles CO (1982) Diazinon uptake, metabolism and
elimination by nematodes. Arch Environ Contam Toxicol, 11: 669-673.
Albright RK (1984) Renal involvement in organophosphate poisoning.
J Am Med Assoc, 252: 1408.
Allender WJ & Britt AG (1994) Analyses of liquid diazinon formulation
and breakdown products: an Australia-wide survey. Bul. Environ Contam
Toxicol, 53: 902-906.
Allison DT (1977) Use of exposure units for estimating aquatic
toxicity of organophosphate pesticides. Washington, DC, US
Environmental Protection Agency (EPA-600/3-77-077).
Allison DT & Hermanutz RO (1977) Toxicity of diazinon to brook trout
and fathead minnows. Washington, DC, US Environmental Protection
Agency (EPA-600/3-77-060).
Anees MA (1978) Hepatic pathology in a fresh-water teleost Channa
punctatus (Bloch) exposed to sub-lethal and chronic levels of
three organophosphorus insecticides. Bull Environ Contam Toxicol,
19: 524-527.
Ankley GT, Dierkes JR, Jensen DA, & Peterson GS (1991) Piperonyl
butoxide as a tool in aquatic toxicological research with
organophosphate insecticides. Ecotoxicol Environ Saf, 21: 266-274.
Ansari BA & Kumar K (1988) Diazinon toxicity: effect on protein and
nucleic acid metabolism in the liver of zebrafish, Brachydanio
rerio (Cyprinidae). Sci Total Environ, 76: 63-68.
Anthony J, Banister E, & Oloffs PC (1986) Effect of sublethal levels
of diazinon: Histopathology of liver. Bull Environ Contam Toxicol,
37: 501-507.
Arienzo M, Crisanto T, Sanchez-Martin T, & Sanchez-Camazano M (1994):
Effect of soil characteristics on adsorption and mobility of (14C)
diazinon. J Agric Food Chem, 42: 1803-1808.
Asensio JS, Barrio CS, Juez MTG, & Bernal JG (1991) Study of the
decay of diazinon and chlorpyriphos in apple samples, using gas
chromatography. Food Chem, 42: 213-224.
Ballantine L (1984) Advanced product chemistry percutaneous absorption
of 2delta-14C-diazinon in rats. Greensboro, North Carolina, Ciba-
Geigy Corporation (Unpublished report No. ABR-84011, submitted to WHO
by Ciba-Geigy Ltd, Basel).
Banerjee D (1967) Pericarditis in acute diazinon poisoning (A case
report). Armed Forces Med J (India), 23: 187-190.
Barik S & Munnecke DM (1982) Enzymatic hydrolysis of concentrated
diazinon in soil. Bull Environ Contam Toxicol, 29: 235-239.
Barnes TB, Hazelette JR, & Arthur AT (1988) Diazinon (mg-8): 13 week
oral toxicity study in dogs (Project No. 882012). Summit, New Jersey,
Ciba-Geigy Corporation (Unpublished report submitted to WHO by Ciba-
Geigy Ltd, Basel).
Barnett JB, Spyker-Cranmer JM, Avery DL, & Hoberman AM (1980)
Immunocompetence over the lifespan of mice exposed in utero to
carbofuran or diazinon: I. Changes in serum immunoglobulin
concentrations. J Environ Pathol Toxicol, 4: 53-63.
Bathe R (1972a) Acute dermal LD50 of technical diazinon (G-24'480)
in the rat (Project No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Bathe R (1972b) G 24`480 tech.: Acute oral LD50 in the mouse
(Project No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Bathe R (1973) Intraperitoneal LD50 of technical diazinon
(G-24480 MG 647) in the rat. Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished project report No. Siss 1679).
Bathe R & Gfeller W (1980) Report on acute oral LD50 in the rat of
technical G24'480 (Project No. 800478). Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Bopp C & Kosminsky B (1975) Toxic hepato-cutaneous porphyria. Med
Cutan Ibero Lat Am, 3: 271-279.
Bresch H (1991) Early life-stage test in zebrafish versus a growth
test in rainbow trout to evaluate toxic effects. Bull Environ Contam
Toxicol, 46: 641-648.
Bruce RB, Howard JW, & Elsea JR (1955) Toxicity of O,O-diethyl
O-(2-isopropyl-6-methyl-4 pyrimidyl) phosphorothioate (diazinon).
J Agric Food Chem, 3: 1017-1021.
Burgener J & Seim V (1988) Biological report for the metabolism of
14C-diazinon in laying hens dosed at 25 ppm ( Project No.
BIOL-88006). Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO Ciba-Geigy Ltd, Basel).
Burpee LL & Cole H Jr (1978) The influence of alachlor, trifluralin
and diazinon on the development of endogenous mycorrhiza in soybeans.
Bull Environ Contam Toxicol, 19: 191-197.
Butler GL, Deason TR, & Kelley JC (1975) The effect of atrazine,
2,4-D, methoxychlor, carbaryl and diazinon on the growth of planktonic
algae. Br Phycol J, 10: 371-372.
Byrne DH & Kitos PA (1983) Teratogenic effects of cholinergic
insecticides in chick embryos: IV. The role of tryptophan in
protecting against limb deformities. Biochem Pharmacol,
32: 2881-2890.
Cairns T, Siegmund EG, & Froberg JE (1985) Identification of
diazinon and its metabolite in spinach by chemical ionization
mass spectrometry. Bull Environ Contam Toxicol, 35: 291-295.
Capps T (1989) Characterization and identification of diazinon
metabolites in rats (Project No. 302211/302925). Greensboro, North
Carolina, Ciba-Geigy Corporation (Unpublished report No. ABR-88164,
submitted to WHO by Ciba-Geigy Ltd, Basel).
Capps TM (1990) Characterization and identification of major
metabolites in tissues of sheep treated dermally with 14C-diazinon
(Project No. 302925) Greensboro, North Carolina, Ciba-Geigy
Corporation (Unpublished report No. ABR-90014, submitted to WHO by
Ciba-Geigy Ltd, Basel).
Ceresa C & Puri E (1988) Micronucleus test, mouse (OECD-conform) G 24
480 tech. (diazinon) (Test No. 871696). Basel, Switzerland, Ciba-Geigy
Ltd ( Unpublished report).
Chen HH, Hsueh JL, Sirianni SR, & Huang CC (1981) Induction of
sister-chromatid exchanges and cell cycle delay in cultured mammalian
cells treated with eight organophosphorus pesticides. Mutat Res,
88: 307-316.
Chow E & Richter AG (1994) Acute neurotoxicity study with D-Z-N
diazinon MG 87% in rats (Project No. F-00175). Farmington,
Connecticut, Ciba-Geigy Corporation, Environmental Health Center
(Unpublished report).
Churchfield S (1986) Shrews. Oswestry, A. Nelson (The Mammal Society
series).
Claborn HV, Mann HD, Younger RL, & Radeleff RD (1963) Diazinon
residues in the fat of sprayed cattle. J Econ Entomol, 56: 858-859.
Classen W (1996) G24.480 tech. - delayed neurotoxicity in hens
following acute exposure (Project No. 952030). Stein, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Clegg TJ & Koevening JL (1974) The effect of four chlorinated
hydrocarbon pesticides and one organophosphate pesticide on ATP levels
in three species of photosynthesizing freshwater algae. Bot Gaz,
135: 368-372.
Cockrell KD, Woodard MW, & Woodard G (1966) Diazinon 50 W: Safety
evaluation by repeated oral administration to monkeys for 106 weeks.
Woodard Research Corporation USA (Unpublished report submitted to WHO
by Ciba-Geigy Ltd, Basel).
Collyard SA, Ankley GT, Hoke RA, & Goldenstein T (1994) Influence of
age on the relative sensitivity of Hyalella azteca to diazinon,
alkylphenol ethoxylates, copper, cadmium and zinc. Arch Environ Contam
Toxicol, 26: 110-113
Coye MJ, Barnett PG, Midtling JE, Velasco AR, Romero P, Clements CL, &
Rose TG (1987) Clinical confirmation of organophosphate poisoning by
serial cholinesterase analyses. Arch Intern Med, 147: 438-442.
Craine EM (1989a) A study of 14C-diazinon disposition in the rat
(biological-phase) (Project No. 302925). Ashland, Ohio, WIL Research
Laboratories Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Craine EM (1989b) A study of 14C-diazinon disposition in the rat
(analytical phase I) (Project No. 302925). Ashald, Ohio, WIL Research
Laboratories Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Currie KL, McDonald EC, Chung LTK, & Higgs AR (1990) Concentration of
diazinon, chlorpyrifos, and bendiocarb after application in offices.
Am Ind Hyg Assoc J, 51(1): 23-27.
Dagli AJ, Moos JS, & Shaikh WA (1981) Acute pancreatitis as a
complication of diazinon poisoning: A case report. J Assoc Physi
India, 29: 794-795.
Dahm PA (1970) Some aspects of the metabolism of parathion and
diazinon. In: O`Brien RD & Yamamoto I ed. Biochemical toxicology of
insecticides. London, New York, Academic Press, pp 51-63.
Davies DB & Holub BJ (1980a) Comparative subacute toxicity of
dietary diazinon in the male and female rat. Toxicol Appl Pharmacol,
54: 359-367.
Davies DB & Holub BJ (1980b) Toxicological evaluation of dietary
diazinon in the rat. Arch Environ Contam Toxicol, 9: 637-650.
Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowall J, & Furlong CE
(1996) The effect of the human serum paraoxonase polymorphism is
reversed with diazoxon, soman and sarin. Nat Genet, 14(3): 334-336.
Decarie R, DesGrange J-L, Lepine C, & Morneau F (1993) Impact of
insecticides on the American robin (Turdus migratorius) in a
suburban environment. Environ Pollut, 80: 231-238
Derbyshire JC & Murphy RT (1962) Diazinon residues in treated
silage and milk of cows fed powdered diazinon. J Agric Food Chem,
10: 384-386.
Dick GL, Heenan MP, Love JL, Udy PB, & Davidson F (1978) Survey of
trace elements and pesticide residue content: 2. Organochlorine and
organophosphorus pesticide residue content. NZ J Sci, 21: 71-78.
Dikshith TS, Behari JR, Datta KK, & Mathur AK (1975) Effect of
diazinon in male rats: Histopathological and biochemical studies.
Environ Physiol Biochem, 5: 293-299.
Dobbins PK (1967) Organic phosphate insecticides as teratogens in the
rat. J Fla Med Assoc, 54: 542-546.
Doggett SM & Rhodes RG (1991) Effects of a diazinon formulation on
unialgal growth rates and phytoplankton diversity. Bull Environ Contam
Toxicol, 47: 36-42.
Dollenmeier P & Müller D (1986) G 24`480 tech.: L5178Y/TK+/- mouse
lymphoma mutagenicity test (Project No. 840396). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Dougherty E III (1957) Thiophosphate poisoning in white Pekin ducks.
Avian Dis, 1: 127-130.
Dressel TD, Goodale RL Jr, Arneson MA, & Borner JW (1979) Pancreatitis
as a complication of anticholinesterase insecticide intoxication. Ann
Surg, 189: 199-204.
Dressel TD, Godale RL, & Borner JW (1980) A study of the
cholinesterases of the canine pancreas and the relationship between
reduced butyrylcholinesterase activity and pancreatic ductal
hypertension. Ann Surg, 192: 614-619.
Dutta H, Marcelino J, & Richmonds Ch (1992) Brain acetylcholinesterase
activity and optomotor behaviour in bluegills, Lepomis macrochirus,
exposed to different concentrations of diazinon. Arch Int Physiol
Biochim Biophys, 100: 331-334.
Ebere AG & Akintonwa A (1992) Acute toxicity of pesticides to
Gobius sp., Palaemonetes africanus, and Desmocaris trispimosa.
Bull Environ Contam Toxicol, 49: 588-592.
Eberle DO, Suter R, Nowak K, & Bosshardt HP (1974) CIPAC collaborative
study of the heat-stability of diazinon formulations using both CUPAC
and AOAC analytical methods. J Assoc Off Anal Chem, 57: 48-52.
Egan H & Weston RE (1977) Pesticide residues: Food surveys in the
United Kingdom. Pestic Sci, 8: 110-116.
Egyed MN, Malkinson M, Eilat A, & Schlosberg A (1974) Basudin
(diazinon) poisoning in goslings. Reffu Vet, 31: 22-26.
El-Elaimy IA, El-Saadany MM, & Gabr SA (1990) Pesticide poisoning to
fresh water teleost: VIII. Ultrastructural alterations of the
intestine of Tilapia nilotica under stress of exposure to diazinon
and neopybuthrin. J Egypt Ger Soc Zool, 1: 223-236.
English T, Ellis EF, & Ackerman J (1970) Organic phosphate poisoning.
Morbid Mortal Wkly Rep (Cent Dis Control), 19: 397-404.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993a) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 23: 1-54.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993b) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 24: 1-87.
FAO/WHO (1965) Diazinon. In: 1965 Evaluation of the toxicity of
pesticide residues in food. Rome, Food and Agriculture Organization of
the United Nations and Geneva, World Health Organization, pp 77-83
(FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27. 65).
FAO/WHO (1975) 1974 Evaluations of some pesticide residues in food.
Geneva, World Health Organization (WHO Pesticide Residues Series,
No 4).
FAO/WHO (1979) Data sheet on pesticides No. 45: Diazinon. Geneva,
World Health Organization (VBC/DS/79.45).
FAO/WHO (1994a) Diazinon. In: Pesticide residues in food - 1993:
Evaluations, Part I - Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 157-294 (FAO Plant Production
and Protection Paper 124).
FAO/WHO (1994b) Diazinon. In: Pesticide residues in food - 1993:
Evaluations, Part II - Toxicology. Geneva, World Health Organization,
pp 57-81 (WHO/PCS/94.4).
Farran A, DePablo J, & Barcelo D (1988a) Identification of
organophosphorus insecticides and their hydrolysis products by
liquid chromatography in combination with UV and thermospray-mass
spectrometric detection. J Chromatogr, 455: 163-172.
Farran A, DePablo J, & Hernandez S (1988b) Continuous-flow extraction
of organophosphorus pesticides coupled on-line with high-performance
liquid chromatography. Anal Chim Acta, 212: 123-131.
Fernandez-Casalderrey A, Ferrando MD, & Andreu-Moliner E (1992)
Endosulfan and diazinon toxicity to the freshwater rotifer
Brachious calcyflorus. J Environ Sci Health, B27: 155-164.
Ferrando MD, Sancho E, & Andreu-Moliner E (1991) Comparative acute
toxicities of selected pesticides to Anguilla anguilla. J Environ
Sci Health, B26(5/6): 491-498.
Ferrando MD, Alarcon V, Fernandez-Casalderrey A, Gamon M, &
Andreu-Moliner E (1992) Persistence of some pesticides in the
aquatic environment. Bull Environ Contam Toxicol, 48: 747-755.
Ferreira JR & Silva Fernandes AM (1980) Gas-liquid chromatographic
determination of organophosphorus insecticide residues in fruits and
vegetables. J Assoc Off Anal Chem, 63: 517-522.
Fink R (1976) Acute oral LD50 to bobwhite quail - diazinon technical.
Basel, Switzerland, Novartis Crop Protection (Unpublished report).
Fletcher DW & Pedersen CA (1988a) Diazinon MG8 technical: 14 day acute
oral LD50 study in mallard ducks. Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Fletcher DW & Pedersen CA (1988b) Diazinon MG8 technical: 14 day acute
oral LD50 study in brown-headed cowbirds. Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Fletcher DW & Pedersen CA (1988c) Diazinon MG8 technical: 8 day acute
dietary LC50 study in mallard ducklings. Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Fort DJ, Delphon J, Powers CR, Helems R, Gonzales R, & Stover EL
(1995) Development of automated methods of identifying toxicants in
the environment. Bull Environ Contam Toxicol, 54: 104-111.
Frank R, Braun HE, Van Hove Holdrinet M, Sirons GJ, & Ripley BD
(1982) Agriculture and water quality in the Canadian Great Lakes
basin: V. Pesticide use in 11 agricultural watersheds and presence in
stream water, 1975-1977. J Environ Qual, 11(3): 497-505.
Frank R, Braun HE, & Ripley BD (1989) Monitoring Ontario-grown apples
for pest control chemicals used in their production. Food Addit
Contam, 6: 227-234.
Frank R, Braun HE, & Ripley BD (1990) Residues of insecticides and
fungicides on Ontario-grown vegetables 1986-1988. Food Addit Contam,
7: 545-554.
Frank R, Mineau P, Braun HE, Barker IK, Kennedy SW, & Trudeau S (1991)
Deaths of Canada geese following spraying of turf with diazinon. Bull
Environ Contam Toxicol, 46: 852-858.
Frick TW, Dalo S, O'Leary JF, Runge W, Borner JW, Baraniewski H,
Dressel T, Shearen JG, & Goodale RL (1987) Effects of insecticide
diazinon on pancreas of dog, cat, and guinea pig. J Environ Pathol
Toxicol Oncol, 7: 1-12.
Fritz H (1974) Reproduction study - G24480 (diazinon techn.) rat -
Segment II: Test for teratogenic or embryotoxic effects. Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Fritz H (1975) Dominant lethal study on G24480 (diazinon techn.)
mouse: Test for cytotoxic or mutagenic effects on male germinal cells
(Project No. 327507) . Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Fujii Y & Asaka S (1982) Metabolism of diazinon and diazoxon in fish
liver preparations. Bull Environ Contam Toxicol, 29: 455-460.
Gaines TB (1960) The acute toxicity of pesticides to rats. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol,
14: 515-534.
Ganzelmeier IH, Rautmann D, Spangenberg R, Streloke M, Herrmann M,
Wenzelburger HJ, & Walter HF (1995) Studies on the spray drift of
plant protection products: Results of a test program carried out
throughout the Federal Republic of Germany. Oxford, London,
Edinburgh, Melbourne, Blackwell Scientific Publications.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1985a)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1978-September 1979. J Assoc Off
Anal Chem, 68: 842-861.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1985b)
Pesticides, selected elements, and other chemicals in adult total
diet samples, October 1978-September 1979. J Assoc Off Anal Chem,
68: 862-875.
Gasser R (1953) [A new insecticide with wide efficacy.] Z Naturforsch,
B8: 225-232 (in German).
Geleick D & Arni P (1990) Salmonella and Escherichia/liver-microsome
test (Project No. 891346) - Test substance: G24480 tech. Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Getzin LW (1968) Persistence of diazinon and zinophos in soil: Effects
of autoclaving, temperature, moisture, and acidity. J Econ Entomol,
61: 1560-1565.
Giddings JM (1992) Aquatic mesocosm test for environmental fate and
ecological effects of diazinon. Wareham, Massachusetts, Springborn
Laboratories, Inc. and Columbia, Missouri, ABC Laboratories, Inc.
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Giknis MLA (1989) Diazinon techn: A two generation reproductive study
in albino rats. Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report).
Gilmore DR & Cortes A (1966) Dual-ban preparative thin-layer
chromatography for the separation of diazinon and related from plant
material. J. Chromatogr, 21: 148-149.
Glaza SM (1993) Acute oral toxicity study of D-Z-N diazinon in rats
(Project No. HWI 6117-221). Madison, Wisconsin, Hazleton Wisconsin,
Inc. (Unpublished report).
Goodman LR, Hansen DJ, Coppage DL, Moore JC, & Matthews E (1979)
Diazinon chronic toxicity to, and brain acethylcholinesterase
inhibition in, the sheepshead minnow, Cyprinodon variegatus. Trans
Am Fish Soc, 108: 479-488.
Gowen JA, Wiersma GB, Tai H, & Mitchell WG (1976) Pesticide levels in
hay and soils from nine states. Pestic Monit J, 10: 114-116.
Gunner HB & Zuckerman BM (1968) Degradation of 'diazinon' by
synergistic microbial action. Nature (Lond), 217: 1183-1184.
Gunner HB, Zuckerman BM, Walker RW, Miller CW, Deubert KH, & Longley
RE (1966) The distribution and persistence of diazinon applied to
plant and soil and its influence on rhizosphere and soil microflora.
Plant Soil, 25: 249-264.
Hagenbuch JP & Mücke W (1985) The fate of diazinon in mammals
(Biochemistry product evaluation 1/85). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Hall SW & Baker BB (1989) Intermediate syndrome from organophosphate
poisoning. Vet Hum Toxicol, 31(4): 355.
Halle A & Sloas DD (1987) Percutaneous organophosphate poisoning .
South Med J, 80: 1179-1181.
Harris SB & Holson JF (1981) G 24`480 tech.: A teratology study of
diazinon (CAS Number 333-41-5) in New Zealand white rabbits (Project
No. 281005). La Jolla, California, Science Applications, Inc.,
Division of Toxicology (Unpublished report submitted to WHO by
Ciba-Geigy Ltd, Basel).
Harris CR & Mazurek JH (1966) Laboratory evaluation of candidate
materials as potential soil insecticides. J Econ Entomol, 59:
1215-1221.
Harris LW, Fleisher HJ, Innerebner TA, Cliff WJ, & Sim VM (1969) The
effects of atropine-oxime therapy on cholinesterase activity and the
survival of animals poisoned with O,O-diethyl-O-(2-isopropyl-6-methyl-
4-pyrimidinyl) phosphorothioate. Toxicol Appl Pharmacol, 15: 216-224.
Harrison DL, Whitten LK, & Maskell PE (1962) Diazinon residues in the
tissues of sheep following dipping. Geigy (Australasia), p 14
(Publication No. 3).
Hartmann HR (1990) G 24`480 tech.: 21-day repeated exposure inhalation
toxicity in the rat - Nose-only exposure (Project No. 891205). Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Hata S, Berstein E, & Davis LE (1986) A typical ocular bobbing in
acute organophosphate poisoning. Ach Neurol, 43: 185-186.
Hayes WJ (1963) Clinical handbook on economic poisons - Emergency
information for treating poisoning. Washington, DC, US Government
Printing Office (Public Health Service Publication No. 476).
Hayes AL, Wise RA. & Weir FW (1980) Assessment of occupational
exposure to organophosphates in pest control operators. Am Ind Hyg
Assoc J, 41: 568-575.
Henderson M & Kitos PA (1982) Do organophosphate insecticides
inhibit the conversion of tryptophan to NAD+ in ovo? Teratology,
26: 173-181.
Henderson JD, Yamamoto JT, Fry DM, Seiber JN, & Wilson BW (1994), Oral
and dermal toxicity of organophosphate pesticides in the domestic
pigeon (Columbia livia). Bull Environ Contam Toxicol, 52: 663-640.
Hensley DL (1991) Pesticide effects on nitrogen fixation in legumes -
Fedrip database. Springfield, Virginia, National Technical
Information Service.
Hertner T & Arni P (1990) G 24`480 tech.: Autoradiographic DNA repair
test in rat hepatocytes (Project No. 891345). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Hill EF (1988) Formulation affects uptake and toxicity of
anticholinesterases in birds. Ann Conf Soc Environ Toxicol Chem,
9: 45.
Hill EF & Camardese MB (1984) Toxicity of anticholinesterase
insecticides to birds: Technical grade versus granular formulations.
Ecotoxicol Environ Saf, 8: 551-563.
Hill EF, Camardese MB, Heinz GH, Spann JW, & DeBevec AB (1984) Acute
toxicity of diazinon is similar for eight stocks of bobwhite. Environ
Toxicol Chem, 3: 61-66.
Hinkle DK, Suggs JE, & Jackson MD (1980) Environmental and biological
effects following application of diazinon impregnated strips within a
laboratory animal room. Lab Anim Sci, 30: 981-983.
Hoberman AM, Cramer JS, Avery DL, & Cranmer MF (1979) Transplacental
inhibition of esterases in fetal brain following exposure to the
organophosphate diazinon. Teratology, 19: 30A-31A.
Hodgson MJ & Smith AD (1992) Commercial and residential poisoning
with anticholinesterases. In: Ballantyne B & Mars TC ed. Clinical
and experimental toxicology of organophosphates and carbamates,
pp 346-363.
Hoffman DJ & Eastin WC Jr (1981) Effects of malathion, diazinon, and
parathion on mallard embryo development and cholinesterase activity.
Environ Res, 26: 472-485.
Holbert MS (1989) Diazinon: Acute inhalation toxicity study in rats
(EPA Guidelines 81-3) (Project No. 5947-89). Houston, Texas,
Stillmeadow, Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Holbert MS (1994): Diazinon: Acute inhalation toxicity study in
rats (Project No. 0067-93. Sugar Land, Texas, Stillmeadow, Inc.
(Unpublished report).
Hool G & Müller D (1981a) G 24`480 tech.: Sister chromatid exchange
study on Chinese hamster (test for mutagenic effects on bone marrow
cells) (Project No. 801504). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report including a supplement report from Strasser
F & Arni P).
Hool G & Müller D (1981b) G 24`480 tech.: Chromosome studies
in male germinal epithelium mouse (test for mutagenic effects on
spermatocytes) (Project No. 801502). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Hool G & Müller D (1981c) G 24`480 tech.: Nucleus anomaly test in
somatic interphase nuclei Chinese hamster (test for mutagenic effects
on bone marrow cells) (Project No. 801503). Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report including a supplement report from
Strasser F & Arni P).
Hool G & Müller D (1981d) G 24`480 tech.: Chromosome studies in
male germinal epithelium mouse (test for mutagenic effects on
spermatogonia) (Project No. 801501). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Hummell R, Brewer LW, Cobb GP III, & Hooper JM (1992a) An assessment
of avian response to and residue chemistry of applications of D-Z-N
diazinon 5G and D-Z-N diazinon AG500 to urban lawns - Part 1: November
1989 application. Pendleton, South Carolina, Clemson University,
Institute of Wildlife and Environmental Toxicology, 77 pp (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Hummell R, Brewer LW, Cobb GP III, & Hooper JM (1992b) An assessment
of avian response to and residue chemistry of applications of D-Z-N
diazinon 5G and D-Z-N diazinon AG500 to urban lawns - Part II: April
1990 application. Pendleton, SC, Clemson University, Institute of
Wildlife and Environmental Toxicology, 100 pp (Unpublished report
submitted to WHO by Ciba-Geigy Ltd, Basel).
Hummell R, Brewer LW, Cobb GP III, & Hooper JM (1992c) An assessment
of avian response to and residue chemistry of applications of D-Z-N
diazinon 5G and D-Z-N diazinon AG500 to urban lawns - Part III: June
1990 application. Pendleton, SC, Clemson University, Institute of
Wildlife and Environmental Toxicology, 133 pp (Unpublished report
submitted to WHO by Ciba-Geigy Ltd, Basel).
Hundley HK, Cairns T, Luke MA, & Matsumoto H (1988) Pesticide residue
findings by the Luke method in domestic and imported foods and
animal feeds for fiscal years 1982-1986. J Assoc Off Anal Chem,
71(5): 875-892.
Husain K & Ansari RA (1988) Influence of cholinergic and adrenergic
blocking drugs on hyperglycemia and brain glycogenolysis in
diazinon-treated animals. Can J Physiol Pharmacol, 66: 1144-1147.
Husain K & Matin MA (1986) Cerebral glycolysis and glycogenolysis in
diazinon treated animals. Arh Hig Rada Toxicol, 37: 29-34.
Infurna RM & Arthur AT (1985) G 24`480 tech.: A teratology study in
Charles River rats (Report No. 52-83). Summit, New Jersey, Ciba-Geigy
Corporation (Unpublished report submitted to WHO by Ciba-Geigy Ltd,
Basel).
IPCS (1986) Environmental health criteria 63: Organophosphorus
insecticides: A general introduction. Geneva, World Health
Organization, International Programme on Chemical Safety, 181 pp.
Iverson F, Grant DL, & Lacroix J (1975) Diazinon metabolism in the
dog. Bull Environ Contam Toxicol, 13: 611-618.
Jackson MD & Wright CG (1975) Diazinon and chlorpyrifos residues in
food after insecticidal treatment in rooms. Bull Environ Contam
Toxicol, 13: 593-595.
Jarvinen AW & Tanner DK (1982) Toxicity of selected controlled release
and corresponding unformulated technical grade pesticides to the
fathead minnow. Environ Pollut, A27: 179-195.
Jenkins LJ (1988) G 24`480 tech.: Acute delayed neurotoxicity of
diazinon MG-8 in domestic fowl (Project No. 5152-87. Houston, Texas,
Stillmeadow, Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Johnson WE, Fendinger NJ, & Plimmer JR (1991) Solid-phase extraction
of pesticides from water: possible interferences from dissolved
organic material. Anal Chem, 63: 1510-1513.
Johnston G, Walker CH, & Dawson AS (1994) Interactive effects between
EBI fungicides (prochloraz, propoconazole and penconazole) and OP
insecticides (dimethoate, chlorpyriphos, diazinon and malathion) in
the hybrid red-legged partridge. Environ Toxicol Chem, 13: 615-620.
Kabrawala VN, Shah RM, & Oza GG (1965) Diazinon poisoning (a study of
25 cases). Indian Pract, 18: 711-717.
Kanazawa J (1975) Uptake and excretion of organophosphorus and
carbamate insecticides by fresh water fish, motsugo, Pseudorasbora
parva. Bull Environ Contam Toxicol, 14: 346-352.
Kanazawa J (1978) Bioconcentration ratio of diazinon by freshwater
fish and snail. Bull Environ Contam Toxicol, 20: 613-617.
Kaplanis JN, Louloudes SJ, & Roan CC (1962) The distribution and
excretion of P32-labeled diazinon in guinea-pigs. Trans Kansas Acad
Sci, 65(1): 70-75.
Kathein R (1986) The development of poultry slaughter and poultry meat
inspection in Israel - a review. Isr J Vet Med, 42: 146-157.
Kazacos EA (1991) Pathogenesis of selected pancreatic diseases of
animals - Fedrip database. Springfield, Virginia, National Technical
Information Service.
Keller A (1983) Degradation of diazinon in aquatic systems (Project
No. 7/83). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished report).
Kenaga E (1973) Factors to be considered in the evaluation of the
toxicity of pesticides to birds in the environment. Environ Qual Saf,
2: 166-181.
Kendall RJ, Brewer LW, Hitchcock RR, & Mayer JR (1992) American
widgeon mortality associated with turf application of diazinon.
J Wildl Dis, 28: 263-267.
Kendall RJ, Brewer LW, & Hitchcock (1993) Response of Canada geese to
a turf application of diazinon AG5000. J Wildl Dis, 29(3): 458-464.
Khera KS & Bedok S (1967) Effects of thiol phosphates on notochordal
and vertebral morphogenesis in chick and duck embryos. Food Cosmet
Toxicol, 5: 359-365.
Kimbrough RD & Gaines TB (1968) Effect of organic phosphorus compounds
and alkylating agents on rat fetus. Arch Environ Health, 16: 805-808.
Kirchner FR (1991) G 24`480 tech.: One/two year oral toxicity study in
rats (Project No. 882018). Summit, New Jersey, Ciba-Geigy Corporation
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kirkbride KP (1987) An estimation of diazinon in omental tissue.
J Anal Toxicol, 11: 6-7.
Klemmer HW, Reichert ER, & Yauger WL (1978), Five cases of intentional
ingestion of 25 percent diazinon with treatment and recovery. Clin
Toxicol, 12(4): 435-444.
Klotzsche C (1955) [On the toxicology of new phosphoric acid ester
insecticides.] Arzneim Forsch, 5: 436-439 (in German).
Koibuchi M, Shibazaki T, & Inoue T (1975) Gas-liquid chromatographic
determination of diazinon in the oil solutions and the emulsifiable
concentrates. Eisei-Shikenjo-Hokoku, 93: 27-30.
Kring JB (1969) Mortality of the earthworm Lumbricus terrestris L.
following soil applications of insecticide to a tobacco field. J Econ
Entomol, 62: 963.
Kuhn JO (1989a) G 24`480 tech.: Acute oral toxicity study in rats
(Project No. 5942-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuhn JO (1989b) Diazinon: Acute dermal toxicity study in rabbits
(Project No. 5943-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuhn JO (1989c) Diazinon: Primary dermal irritation study in rabbits
(Project No. 5945-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuhn JO (1989d) Diazinon: Primary eye irritation study in rabbits
(Project No. 5944-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuhn JO (1989e) Diazinon: Dermal sensitization study in guinea pigs
(Project No. 5946-89). Houston, Texas, Stillmeadow, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Kuroda K, Yamaguchi Y, & Endo G (1992) Mitotic toxicity, sister
chromatid exchange, and rec assay of pesticides. Arch Environ Contam
Toxicol, 23: 13-18.
Kushaba-Rugaaju S & Kitos PA (1985) Effects of diazinon on nucleotide
and amino acid contents of chick embryos. Teratogen Consid Biochem
Pharmacol, 34(11): 1937-1943.
Lacorte S & Barcelo D (1995) Determination of organophosphorus
pesticides and their transformation products in river water by
automated on-line solid-phase extraction followed by thermospray
liquid chromatography-mass spectrometry. J. Chromatogr, A712: 103-112.
Lacorte S & Barcelo D (1996) Determination of part per trillion levels
of organophosphorus pesticides in groundwater by automated on-line
liquid-solid extraction followed by liquid chromatography/atmospheric
pressure chemical ionization mass spectrometry using positive and
negative ion modes of operation. Anal Chem, 68: 2460-2470.
Lawrence JF & Iverson F (1975) Analysis of the diazinon metabolites G
27550 and GS 31144 by gas-liquid chromatography with nitrogen-specific
detection after derivatization. J Chromatogr, 103: 341-347.
LeBel GL, Williams DT, Griffith G, & Benoit FM (1979) Isolation and
concentration of organophosphorus pesticides from drinking water at
the ng/l level, using macroreticular resin. J Assoc Off Anal Chem,
62: 241-249.
Lee HS (1989) Acute pancreatitis and organophosphate poisoning: a case
report and review. Singap Med J, 30: 599-601.
Leidy RB, Wright CG, & Dupree HE Jr (1982) Concentration and movement
of diazinon in air. J Environ Sci Health, 17: 311-319.
Leonard JA & Yeary RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51(11): 605-609.
Lloyd JE & Matthysse JG (1966) Polymer-insecticide systems for use as
livestock feed additives. J Econ Entomol, 59: 363-367.
Lloyd JE & Matthysse JG (1970) Polyvinyl chloride insecticide pellets
fed to cattle to control face fly larvae in manure. J Econ Entomol,
63: 1271-1281.
Lloyd JE & Matthysse JG (1971) Residues of dichlorvos, diazinon and
dimetilan in milk of cows fed PVC-insecticide feed additives. J Econ
Entomol, 64: 821-822.
Lopez DE & Carrascal E (1987) Sensitivity of human lymphocyte
chromosome to diazinon at different times during cell culture. Bull
Environ Contam Toxicol, 38: 125-130.
Lopez DE, Aleixandre C, Merchan M, & Carrascal E (1986) In vitro
induction of alterations in peripheral blood lymphocytes by different
doses of diazinon. Bull Environ Contam Toxicol, 37: 517-522.
Lores EM & Bradway DE (1977) Extraction and recovery of
organophosphorus metabolites from urine using an anion exchange resin.
J Agric Food Chem, 25: 75-79.
McGregor DB, Brown A, Cattenach P, Edwards I, Mc Bride D, Riach C, &
Caspary WJ (1988) Responses of the L5178Y tk+/tk- mouse lymphoma cell
forward mutation assay: III. 72 coded chemicals Environ Mol Mutagen,
12: 85-154 (Published erratum in Environ Mol Mutagen, 12: 345).
Machin AF, Rogers A, Cross AJ, Quick MP, Howells LC, & James NF
(1975) Metabolic aspects of the toxicology of diazinon. Pestic Sci, 6:
461-473.
Macklin AW & Ribelin WE (1971) The relation of pesticides to abortion
in dairy cattle. J Am Vet Med Assoc, 159: 1743-1748.
MacLaren Plansearch Inc. & FDC Consultants Inc. (1985) Drinking water
quality criteria document: Diazinon (Prepared for Ontario Ministry of
the Environment).
Maddy KT & Edmiston S (1988) Selected incidents of illnesses and
injuries related to exposure to pesticides reported by physicians in
California in 1986. Vet Hum Toxicol, 30: 246-254.
MAFF (1982) Report of the Working Party on Pesticide Residues
(1971-1981). The Ninth Report of the Steering Group on Food
Surveillance. London: Her Majesty's Stationery Office (Food
Surveillance Paper No 9).
MAFF (1986) Report of the Working Party on Pesticide Residues
(1982-1985). The Sixteenth Report of the Steering Group on Food
Surveillance. London, Her Majesty's Stationery Office (Food
Surveillance Paper No 16).
MAFF (1989) Report of the Working Party on Pesticide Residues
(1985-1988). The Twenty-Fifth Report of the Steering Group on Food
Surveillance. London, Her Majesty's Stationery Office (Food
Surveillance Paper No 24).
MAFF (1991) Review of diazinon. London, Her Majesty's Stationery
Office (UK Government's Advisory Committee on Pesticides Disclosure
Document No. 35).
Maizlish N, Schenker A, Weisskopf C, Seiber J, & Samuel S (1987) A
behavioural evaluation of pest control workers with short-term,
low-level exposure to the organophosphate diazinon. Am J Ind Med,
12: 153-172.
Mann PC (1993) Histopathological assessment of potential ocular
toxicity of four organophosphate insecticides (Studies Nos. SEF 882018
and SEF 882014). Research Triangle Park, North Carolina, Experimental
Pathology Laboratories, Inc. (Pathology report).
Marselas G, Beavers JB, Smith GJ, & Jaber MJ (1989a) Diazinon: a one
generation reproduction study with the Mallard (Anas platyrhynchos)
using parental incubation. Easton, Maryland, Wildlife International
Ltd (Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Marselas G, Beavers JB, Smith GJ, & Jaber MJ (1989b), Diazinon: a one
generation reproduction study with the Northern bobwhite (Colinus
virginainus) using parental incubation. Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Marshall TC, Dorough HW, & Swim HE (1976) Screening of pesticides for
mutagenic potential using Salmonella typhimurium mutants. J Agric
Food Chem, 24: 560-563.
Matin MA & Husain K (1987) Changes in cerebral glycogenolysis and
related enzymes in diazinon treated hyperglycaemic animals. J Appl
Toxicol, 7(2): 131-134.
Matin MA, Khan SN, Hussain K, & Sattar S (1989) Effect of
adrenalectomy on diazinon-induced changes in carbohydrate
metabolism. Arch Toxicol, 63: 376-380.
Matsuoka A, Hayashi M, & Ishidate M Jr (1979) Chromosomal aberration
tests on 29 chemicals combined with S9 mix in vitro. Mutat Res,
66: 277-290.
Matsushita T, Aoyama K, Yoshimi K, Fujita Y, & Ueda A (1985) Allergic
contact dermatitis from organophosphorus insecticides. Ind Health,
23: 145-154.
Matthysse JG, Gutenmann WH, & Gigger R (1968) Sheep ectoparasite
control: II. Toxicity to sheep, and residues of diazinon and lindane.
J Econ Entomol, 61: 207-209.
Mayer FL.& Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Mello D, Rodrigues Puga F, & Benintendi R (1972) [Intoxications
produced by degradation products of diazinon in its use as a tick
killer.] Biologico, 38: 136-139 (in Portuguese).
Miles JR (1976) Insecticide residues on stream sediments in Ontario,
Canada. Pestic Monit J, 10: 87-91.
Miles JRW & Harris CR (1978a) Insecticide residues in water, sediment,
and fish of the drainage system of the Holland marsh, Ontario, Canada
1972-1975. J Econ Entomol, 71: 125-131.
Miles JRW & Harris CR (1978b) Insecticides residues in organic
soils of six vegetable growing areas in Southwestern Ontario, 1976.
J Environ Health, B13(3): 199-208.
Miles JRW, Harris CR, & Moy P (1978) Insecticide residues in organic
soil of the Holland marsh, Ontario, Canada, 1972-75. J Econ Entomol,
71: 97-101.
Miller CW, Zuckerman BM, & Charig AJ (1966) Water translocation of
diazinon-C14 and parathion-S35 off a model cranberry bog and
subsequent occurrence in fish and mussels. Trans Am Fish Soc,
95: 345-349.
Mirza AM, Schaub N, Samler L, & Reuchelderfer TE (1972)
Organophosphate insecticide diazinon poisoning in children. Med
Ann DC, 41: 559-560.
Misawa W, Doull J, Kitos PA, & Uyeki EM (1981) Teratogenic effects of
cholinergic insecticides in chick embryos: I. Diazinon treatment on
acetylcholinesterase and choline acetyltransferase activities. Toxicol
Appl Pharmacol, 57: 20-29.
Misawa M, Doull J, & Uyeki EM (1982) Teratogenic effects of
cholinergic insecticides in chick embryos: III. Development of
cartilage and bone. J Toxicol Environ Health, 10: 551-563.
Miyazaki H, Tojinbara I, Watanaba Y, Osaka T, & Okui S (1970) Studies
on metabolism of diazinon (O,O-diethyl-O-(2-isopropyl-4-methyl-6-
pyrimidinyl)phosphorothioate) in animals and plants. In: Proceedings
of the First Symposium on Drug Metabolism and Action, Chiba, Japon,
14-15 November 1969, pp 135-138.
Montz WE Jr & Kirkpatrick RL (1985) Temporal patterns of brain
cholinesterase activities of white-footed mice (Peromyscuc
leucopus) following dosing with diazinon or parathion. Arch Environ
Contam Toxicol, 14: 19-24.
Morganian VM & Wall WJ Jr (1972) Dursban and diazinon residues in
biota following treatment of intertidal plots on Cape Cod - 1967-1969.
Pestic Monit J, 6: 160-163.
Mortland MM & Raman KV (1967) Catalytic hydrolysis of some organic
phosphate pesticides by copper. J Agric Food Chem, 15: 163-167.
Mücke W, Alt KO, & Esser HO (1970) Degradation of 14C-labeled
diazinon in the rat. Agric Food Chem, 18: 208-212.
Muratore F, Faiolo H, & Ponzetta G (1960) On four cases of poisoning
by organophosphorus acid esters (antiparasitic) with recovery, with
special reference to hepatic involvement. Minerva Med, 51: 3342-3345.
Murli H & Haworth SR (1990a) Mutagenicity test on diazinon MG8: an
in vitro cytogenetic assay measuring sister chromatid exchange
frequencies in cultured whole blood human lymphocytes (Project No.
12226-0-448).Kensington, Hazleton Laboratories America, Inc.
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Murli H & Haworth SR (1990b) Mutagenicity test on diazinon MG8:
In vivo sister chromatid exchange assay (Project No. 12226-0-458).
Kensington, Maryland, Hazleton Laboratories America, Inc. (Unpublished
report submitted to WHO by Ciba-Geigy Ltd, Basel).
Nakatsugawa T, Tolman NM, & Dahm PA (1969) Oxidative degradation of
diazinon by rat liver microsomes. Biochem Pharmacol, 18: 685-688.
NCI (National Cancer Institute) (1979) Bioassay of diazinon for
possible carcinogenicity. Washington, DC, US Department of Health,
Education and Welfare (Publication No. (NIH) 79-1392).
NIOSH (1994) Organophosphorous pesticides: Method 5600. In: NIOSH
Manual of Analytical Methods, 4th ed. Cincinnati, Ohio, National
Institute for Occupational Safety and Health, pp 1-20.
Nishio A & Uyeki EM (1981) Induction of sister chromatid exchanges in
Chinese hamster ovary cells by organophosphate insecticides and their
oxygen analogs. J Toxicol Environ Health, 8: 939-946.
Owen, M. (1975) The management of grass swards for captive wildfowl.
Int Zoo Year Book, 16: 135-138.
Payot PH (1966) Subacute oral toxicity study on diazinon AS - Humans.
Basel, Switzerland, J.R. Geigy (Unpublished report submitted to WHO by
Ciba-Geigy Ltd, Basel).
Pettersen JC & Morrissey RL (1994) 90-Day subchronic neurotoxicity
study with diazinon MG87% in rats (Project No. F-00176). Farmington,
Connecticut, Ciba Geigy Corporation, Environmental Health Center.
Piccirillo VJ (1978) G 24`480 tech.: Acute oral toxicity study in rats
(Project No. 483-143). Vienna, Virginia, Hazleton Laboratories
America, Inc. (Unpublished report submitted to WHO by Ciba-Geigy Ltd,
Basel).
Pickles M (1990) Biological report for the metabolism of 14C-diazinon
on sheep (Project No. BIOL-89014). Greensboro, North Carolina, Ciba-
Geigy Corporation (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Pickles M & Seim V (1988) Biological report for the metabolism of
2-pyrimidinyl-14C-diazinon in a lactating goat (Project No.
BIOL-88004). Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Poklis A, Kutz FW, Sperling JF, & Morgan DP (1980) A fatal diazinon
poisoning. Forensic Sci Int, 15: 135-140.
Potrepka RF (1994) Acute cholinesterase inhibition time course study
with D-Z-N Diazinon MG 87% in rats (Project No. F-00185). Farmington,
Connecticut, Ciba-Geigy Corporation, Environmental Health Center
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Prijono WB & Leighton FA (1991) Parallel measurement of brain
acetylcholinesterase and the muscarinic cholinergic receptor in the
diagnosis of acute, lethal poisoning anticholinesterase pesticides.
J. Wildl Dis, 27: 110-115.
Radeleff RD & Kunz SE (1972) Toxicity and hazard of diazinon, ethion,
and supracide to turkeys. J Econ Entomol, 65: 162-165.
Rajendra W, Oloffs PC, & Banister EW (1986) Effects of chronic intake
of diazinon on blood and brain monoamines and amino acids. Drug Chem
Toxicol, 9: 117-131.
Richter ED, Kowalski M, Leventhal A, Grauer F, Marzouk J, Brenner S,
Sholnik I, Lerman S, Zahavi H, & Bashari A (1992) Illness and
excretion of organophosphate metabolites four months after household
pest extermination. Arch Environ Health, 47: 135-138.
Robbins WE, Hopkins TL, & Eddy GW (1957) Metabolism and excretion
of phosphorus-32-labeled diazinon in a cow. J Agric Food Chem,
5: 509-513.
Robens JF (1969)Teratologic studies of carbaryl, diazinon, norea,
disulfiram, and thiram in small laboratory animals. Toxicol Appl
Pharmacol, 15: 152-163.
Robertson JB & Mazzella C (1989) Acute toxicity of the pesticide
diazinon to the freshwater snail Gillia altilis. Bull Environ Contam
Toxicol, 42: 320-324.
Rudzki MW, McCormick GC, & Arthur AT (1991) G 24`480 tech.: 52-week
oral toxicity study in dogs (Project No. 882014). Summit, New Jersey,
Ciba-Geigay Corporation (Unpublished report submitted to WHO by
Ciba-Geigy Ltd, Basel).
Sachsse K (1972) Acute dietary LC50 of technical diazinon in the
Japanese quail. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Sachsse K (1973a) Acute oral LD50 of technical diazinon in the
Japanese quail. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Sachsse K (1973b) 8-Day feeding toxicity of technical diazinon in the
mallard duck. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Bathe R (1975) Potentiation study C 8514 (chlordimeform)
versus 3 insecticides, G 24480 (diazinon), CGA 12223 and CGA 15324 in
the rat. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Bathe R (1976) Potentiation study C 9491 (Jodfenphos)
versus 2 insecticides, G 24480 (diazinon), and C 177 (dichlorovos) in
the rat. Basel, Switzerland, Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Bathe R (1977) CGA 15324 versus 2 insecticides, GS 13005
(methidathion) and G24480 (diazinon) in the rat. Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Bathe R (1978) Potentiation study CGA 20168 versus
6 insecticides, C 177 (DDVP), C 570 (phosphamidon), GS 13005
(methidathion), G 24480 (diazinon), CGA 15324 and malathion (Project
No. 404478 Siss 6526). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Sachsse K & Ullmann L (1975a) Acute oral LD50 in the "5-day old"
Japanese quail of technical diazinon (G 24480). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Ullmann L (1975b) Acute oral LD50 in the "5-day old"
bobwhite quail of technical diazinon (G 24480). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Ullmann L (1975c) Acute oral LD50 in the "5-day old"
Peking duck of technical diazinon (G 24480). Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Sachsse K & Ullmann L (1975d) Acute oral LD50 in the "5-day old"
domestic hen of technical diazinon (G 24480). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Sachsse K & Ullmann L (1976) Acute oral LD50 in the adult Peking
duck of technical G 24480 (diazinon). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Sakr SA & Gabr SA (1992) Ultrastructural changes induced by diazinon
and neopybuthrin in skeletal muscles of Tilapia nilotica. Bull
Environ Contam Toxicol, 48: 467-473.
Sakr SA, Gabr SA, & El Saadany MM (1991) Effect of diazinon on
freeze-fracture images of microvilli of intestinal epithelial cells of
Tilapia nilotica. Z Ernähr.wiss, 30: 268-275.
Samal KK & Sahu CS (1990) Organophosphorus poisoning and intermediate
neurotoxic syndrome. J Assoc Phys India, 38: 181-182.
Sancho E, Ferrando MD, Andreu E, & Gamon M (1992) Acute toxicity,
uptake and clearance of diazinon by the European eel. J Environ Sci
Health, 27: 209-221.
Sastry KV & Malik PV (1982a) Acute and chronic effects of diazinon on
the activities of three dehydrogenases in the digestive system of a
freshwater teleost fish Channa punctatus. Toxicol Lett, 10: 55-59.
Sastry KV & Malik PV (1982b) Histopathological and enzymological
alterations in the digestive system of a freshwater teleost fish,
Heteropneustes fossilis, exposed acutely and chronically to
diazinon. Ecotoxicol Environ Saf, 6: 223-235.
Schoch M & Gfeller W (1985) G 24`480 tech: Acute oral LD50 in the rat
(Project No. 850864). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Schoen SR & Winterlin WL (1987) The effects of various soil factors
and amendments on the degradation of pesticide mixtures. J Environ Sci
Health, B22: 347-377.
Sears MK & Chapman RA (1979) Persistence and movement of four
insecticides applied to turfgrass. J Econ Entomol, 72: 272-274.
Seguchi K & Asaka S (1981) Intake and excretion of diazinon in
freshwater fishes. Bull Environ Contam Toxicol, 27: 244-249.
Seifert J & Cassida JE (1979) Inhibition and reactivation of chicken
kynurin formamidase: in vitro studies with organophosphates. Pestic
Biochem Physiol, 12: 273-279.
Seifert J & Pewnim T (1992) Alteration of mice L-tryptophan metabolism
by the organophosphorous acid triester diazinon. Biochem Pharmacol,
44(11): 2243-2250.
Sekine B (1972) Metabolism of diazinon (O,O-diethyl-O-(2-isopropyl-4-
methyl-6-pyrimidinyl) phosphorothioate. Jpn Pestic Inf, 10: 77-80.
Senanayake N & Karalliedde L (1987) Neurotoxic effects of
organophosphorus insecticides: An intermediate syndrome. N Engl J Med,
316: 761-763.
Seyfried B (1994) Degradation of 14C-diazinon (G 24480) in one soil
incubated under various experimental conditions (Project No. 351358.)
Basel, Switzerland, Novartis Crop Protection (Unpublished report).
Shaffer CB & West B (1960) The acute and subacute toxicity of
technical O,O-diethyl S-2 diethylaminoethyl phosphorthioate hydrogen
oxalate (tetram). Toxicol Appl Pharmacol, 2: 1-13.
Sharma SR, Singh RP, & Ahmed SR (1986) Effect of different salt
leachates on the movement of some phosphorus containing pesticides
in soils using thin layer chromatography. Ecotoxicol Environ Saf,
11: 229-240.
Shishido T & Fukami J (1972) Enzymatic hydrolysis of diazoxon by rat
tissue homogenates. Pestic Biochem Physiol, 2: 39-50.
Shishido T, Usui K, & Fukami J (1972a) Oxidative metabolism of
diazinon by microsomes from rat liver and cockroach fat body. Pestic
Biochem Physiol, 2: 27-38.
Shishido T, Usui K, Sato M, & Fukami J (1972b) Enzymatic conjugation
of diazinon with glutathione in rat and American cockroach. Pestic
Biochem Physiol, 2: 51-63.
Shlosberg A, Egyed MN, Eliat A, Malkinson M, & Preissler E (1976)
Efficacy of pralidoxime iodide and obidoxime dichloride as antidotes
in diazinon-poisoned goslings. Avian Dis, 20: 162-166.
Simoneaux BJ (1988a) Disposition of 14C-diazinon in goats - EPA
Guideline 171-4, 7. Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Simoneaux BJ (1988b) Characterization of 14C-diazinon metabolites in
goats - EPA Guideline 171-4 (Project No. ABR-88118). Greensboro, North
Carolina, Ciba-Geigy Corporation (Unpublished report submitted to WHO
by Ciba-Geigy Ltd, Basel).
Simoneaux BJ (1988c) Disposition of 14C-diazinon in chickens (Project
No. ABR-88116). Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Simoneaux BJ (1988d) Characterization of 14C-diazinon metabolites in
chickens (Project No. ABR-88119). Greensboro, North Carolina, Ciba-
Geigy Corporation (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Simoneaux BJ (1988e) Metabolite identification in hens and goats
treated with 14C-diazinon (Project No. ABR-88135). Greensboro, North
Carolina, Ciba-Geigy Corporation (Unpublished report submitted to WHO
by Ciba-Geigy Ltd, Basel).
Simoneaux BJ (1989) Supplemental report on the nature of residues of
diazinon in hens (Project No. ABR-89040). Greensboro, North Carolina,
Ciba-Geigy Corporation (Unpublished report submitted to WHO by
Ciba-Geigy Ltd, Basel).
Singh PK (1973) Effect of pesticides on blue-green algae. Arch
Mikrobiol, 89: 317-320.
Singh AR, McCormick GC, & Arthur AT (1988) G 24480 tech.: Diazinon
(MG-8) 13-week oral feeding study in rats (Project No. 882011).
Summit, New Jersey, Ciba-Geigy Corporation (Unpublished report
submitted to WHO by Ciba-Geigy Ltd, Basel).
Singmaster J & Acin-Diaz NM (1991) Biochemical and residual properties
of pesticides. Springfield, Virginia, National Technical Information
Service (Fedrip Database).
Skinner CS & Kilgore WW (1982) Acute dermal toxicities of various
organophosphate insecticides in mice. J Toxicol Environ Health,
9: 491-497.
Smith BR, Dauterman WC, & Hodgson E (1974) Selective inhibition of the
metabolism of diazinon and diazoxon in vitro by piperonyl butoxide,
NIA 16824, and 1-(2-isopropylphenyl) imidazole. Pestic Biochem
Physiol, 4: 337-345.
Sobti RC, Krishan A, & Pfaffenberger CD (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human lymphoid
cells in vitro: organophosphates. Mutat Res, 102: 89-102.
Soliman SA, Sovocool GW, Curley A, Ahmed NS, & El-Fiki El-Sabae AK
(1982) Two acute human poisoning cases resulting from exposure to
diazinon transformation products in Egypt. Arch Environ Health,
37: 207-212.
Spyker JM & Avery DL (1977) Neurobehavioral effects of prenatal
exposure to the organophosphate diazinon in mice. J Toxicol Environ
Health, 3: 989-1002.
Stadnichenko AP, Ivanenko LD, & Sitniakovskaia AM (1987) Effect of
phenol and pesticides on the physicochemical properties of the
hemolymph in fresh-water gastropod molluscs infected by trematode
parthenitae. Parazitologiia, 21: 716-720.
Stalberg E, Hilton-Brown P, Kolmodin-Hedman B, Holmstedt B, &
Augustinsson BB (1978) Effect of occupational exposure to
organophosphorus insecticides on neuromuscular function. Scand J
Work Environ Health, 4: 255-261.
Stamm E (1994) Rate estimations of the hydroxyl radical oxidation of
diazinon G 24480 (Project 94SM07.) Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Stevenson JH (1978) The acute toxicity of unformulated pesticides to
worker honey bees (Apis mellifera). Plant Pathol, 27: 38-40
Tai CN & Katz R (1984) G 24`480 tech.: 21-day dermal toxicity study in
rabbits (Project No. 842007). Summit, New Jersey, Ciba-Geigy
Corporation (Unpublished report submitted to WHO by Ciba-Geigy Ltd,
Basel).
Tomokuni K & Hasegawa T (1985), Diazinon concentrations and blood
cholinesterase activities in rats exposed to diazinon. Toxicol Lett,
25: 7-10.
Tomokuni K, Hasegawa T, Hirai Y, & Koga N (1985) The tissue
distribution of diazinon and the inhibition of blood cholinesterase
activities in rats and mice receiving a single intraperitoneal dose of
diazinon. Toxicology, 37: 91-98.
Torres CM, Pico Y, Redondo MJ, & Manes J (1996) Mass solid-phase
dispersion extraction procedure for multiresidue pesticide analysis in
oranges. J. Chromatogr, A719: 95-103.
Toyoda M, Adachi K, Ida T, Noda K, & Minagawa N (1990) Simple
analytical method for organophosphorus pesticide residues in milk.
J Assoc Anal Chem, 73: 770-772.
Tripathi G (1992) Relative toxicity of aldrin, fenvalerate, captan and
diazinon to the freshwater food-fish, Clarias batrachus Biomed
Environ Sci, 5: 33-38.
Tsuda T, Aoki S, Kojima M, & Harada H (1990) Bioconcentration and
excretion of diazinon, IBP, malathion and fenitrothion by carp. Comp
Biochem Physiol, C96: 23-36.
Vial A (1990) Acute toxicity test of G 24480 technical to earthworm
(Eisenia foetida). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report No. 891454).
Wachs T, Gutenmann WH, Buckley EH, & Lisk DJ (1983) Concentration of
diazinon in air of a retail garden store. Bull Environ Contam Toxicol,
31: 582-584.
Wadia RS, Sadagopan C, Amin RB, & Sardesai HV (1974) Neurological
manifestations of organophosphorus insecticide poisoning. J Neurol
Neurosurg Psychiatry, 37: 841-847.
Wagner SL & Orwick DL (1994) Chronic organophosphate exposure
associated with transient hypertonia in an infant. Pediatrics,
94(1): 94-97.
Wan MT (1989) Levels of selected pesticides in farm ditches leading to
rivers in the lower mainland of British Columbia. J Environ Sci
Health, B24(2): 183-203.
Ward CR, Owens JC, & Turner WE (1972) Residues of diazinon remaining
after application to wheat. J Econ Entomol, 65: 899.
Wecker L, Mrak RE, & Dettbran WD (1985) Evidence of necrosis in human
intercostal muscle following inhalation of an organophosphate
insecticide. J Environ Pathol Toxicol Oncol, 6: 171-175.
Wedin GP, Pennente CM, & Sachdev SS (1984) Renal involvement in
organophosphate poisoning. J Am Med Assoc, 252: 1408.
Weiss CM (1961) Physiological effect of organic phosphorus
insecticides on several species of fish. Trans Am Fish Soc,
90: 143-152.
Weisskopf CP, Seiber JN, Maizlish N, & Schenker M (1988) Personnel
exposure to diazinon in a supervised pest eradication programme. Arch
Environ Contam Toxicol, 17: 201-212.
Weisskopf CP & Seiber JN (1989) New approaches to analysis of
organophosphate metabolites in the urine of field workers. In:
Biological monitoring of pesticide exposure. Washington, DC, American
Chemical Society, pp 206-214 (ACS Symposium Series 382).
Weizman Z & Sofer S (1992), Acute pancreatitis in children with
anticholinesterase insecticide intoxication. Pediatrics,
90(2): 204-206.
Wester RC, Sedik L, Melendres J, Logan F, Maibach HI, & Russell I
(1993) Percutaneous absorption of diazinon in humans. Food Chem
Toxicol, 31(8): 569-572.
WHO (1991) Safe Use of Pesticides: Fourteenth report of the Expert
Committee on Vector Biology and Control. Geneva, World Health
Organization (WHO Technical Report Series, No. 813).
WHO (1996) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1996-1997. Geneva, World Health
Organization, International Programme on Chemical Safety
(WHO/PCS/96.3).
Wilkinson JG, Rajendra W, Oloffs PC, & Banister EW (1986) Diazinon
treatment effects on heart and skeletal muscle enzyme activities. J
Environ Sci Health, 21: 103-113.
Williams MW, Fuyat HN, & Fitzhugh OG (1959) Acute and subacute
toxicity of four organic phosphates to dogs. Toxicol Appl Pharmacol,
1: 1-7.
Winnett G (1992) Biochemistry and fate of pesticides in agricultural
use. Springfield, Virginia, National Technical Information Service
(Fedrip Database).
Wong PK & Chang L (1988) The effects of 2,4-D herbicide and
organophosphorus insecticides on growth, photosynthesis, and
chlorophyll a synthesis of Chlamydomonas reinhardtii (mt+).
Environ Pollut, 55: 179-189.
Woodruff RC, Philipps JP, & Irwin D (1983) Pesticide-induced complete
and partial chromosome loss in screens with repair defective females
of Drosophila melanogaster. Environ Mutagen, 5: 835-846.
Wright CG & Leidy RB (1980) Insecticide residues in the air of
buildings and pest control vehicles. Bull Environ Contam Toxicol,
24: 582-589.
Wright CG, Leidy RB, & Dupree HE (1982) Diazinon and chlorpyrifos in
the air of moving and stationary pest control vehicles. Bull Environ
Contam Toxicol, 28: 119-121.
Wu HX, Evreux Gros C, & Descote J (1994) Rapid gas-chromatographic
assay of diazinon: Application to the study of diazinon kinetics in
rats. Biomed Environ Sci, 7: 357-361.
Wyttenbach CR & Hwang JD (1984) Relationship between insecticide-
induced short and wry neck and cervical defects visible histologically
shortly after treatment of chick embryos. J Exp Zool, 229: 437-446.
Yang RS, Dauterman WC, & Hodgson E (1969) Enzymatic degradation of
diazinon by rat liver microsomes. Life Sci, 8: 667-672.
Yang RSH, Hodgson E, & Dauterman WC (1971) Metabolism in vitro of
diazinon and diazoxon in rat liver. J Agric Food Chem, 19: 10-13.
Zak F, Luetkemeier H, Sachsse K, & Hess R (1973) G 24`480 tech.:
21-day inhalation study on the rat with technical diazinon (Project
No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Zwiener RJ & Ginsburg CM (1988) Organophosphate and carbamate
poisoning in infants and children. Pediatrics, 81: 121-126.
RÉSUMÉ ET ÉVALUATIONS
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le diazinon était connu jusqu'à ces dernières années sous le nom
chimique de thiophosphate de di- O-méthyle de d 'O-(isopropyl-2
méthyl-6 pyrimidyl-4). Selon les nouvelles conventions de
nomenclature, sa dénomination chimique est désormais phosphorothioate
de O,O-diéthyle et de O-2-isopropyl-6-méthylpyrimidiny-4-yle. A
l'état pur, il se présente sous la forme d'un liquide incolore
dégageant une légère odeur d'ester. La matière active de qualité
technique est un liquide jaune à brun doté d'une odeur légère mais
caractéristique. Son point d'ébullition est de 83-84°C sous une
pression de 26,6 mPa. Sa tension de vapeur (volatilité) est faible
(9,7 mPa à 20°C). Sa solubilité dans l'eau à la température ambiante
est de 60 mg/litre. Le diazinon est soluble dans la plupart des
solvants organiques et son coefficient de partage entre l'octanol et
l'eau (log Pow) est de 3,40. Il est stable en milieu neutre, mais il
s' hydrolyse lentement en milieu alcalin et plus rapidement en milieu
acide. Il se décompose au-dessus de 120°C.
Il existe de nombreuses méthode d'échantillonnage et d'analyse
pour le recherche et le dosage du diazinon et de ses métabolites dans
différents milieux. On fait de plus en plus appel à des méthodes
sensibles telles que la chromatographie en phase gazeuse, la
chromatographie en phase liquide à haute performance, la spectrométrie
de masse et certaines techniques immunologiques.
2. Production, usages et sources d'exposition humaine et
environnementale
Le diazinon est un insecticide de contact dont le spectre
d'activité est très étendu. Il est efficace contre les formes adultes
et juvéniles d'insectes volants ou rampants, ainsi que contre les
acariens et les arachnides. On l'utilise depuis le début des années
cinquante. Il est principalement commercialisé sous la forme de
concentrés émulsionnables et de poudres mouillables. Il existe
également en formulations dans lesquelles il est associé à d'autres
insecticides.
3. Transport, distribution et transformation dans l'environnement
La volatilisation du diazinon à partir du sol n'a qu'une
importance mineure. Sa demi-vie dans la troposphère est de 1,5 h.
Le mouvement du diazinon dans le sol dépend d'un certain nombre
de facteurs, en particulier de la teneur en matières organiques et en
carbonate de calcium. Le diazinon ne devrait pas être très solide-ment
fixé aux particules du sol, du fait que son Koc est égal à 500 et il
devrait être modérément mobile dans le sol.
La décomposition du diazinon dans le sol s'effectue
principalement par voie biologique. A une température de 20°C et pour
une teneur en eau du sol égale à 60% de la capacité totale, on a
constaté que dans un limon, la DT50 était de 5 jours. Dans des
conditions stériles, et pour la même teneur en eau et la même
température, on a obtenu une DT50 de 118 jours, ce qui donne à penser
que c'est bien l'activité biologique qui est principalement
responsable de la décomposition du diazinon dans le sol.
Dans les eaux naturelles, le diazinon a une demi-vie de l'ordre
de 5 à 15 jours. Des processus chimiques et biologiques semblent
intervenir dans sa décomposition, qui aboutit à une minéralisation en
quelques semaines.
Les organismes aquatiques fixent rapidement le diazinon. On a
fait état de faibles facteurs de bioconcentration chez ces organismes,
allant de 3 pour la crevette à 152 pour le goujon, ce qui correspond
bien à une métabolisation et à une excrétion rapides. Une demi-vie de
dépuration pouvant atteindre 30 h (tissu musculaire) a été mesurée
chez des poissons.
4. Concentrations dans l'environnement et exposition humaine
Le diazinon est généralement présent à faible concentration dans
l'environnement. La population générale peut être exposée par voie
alimentaire ou respiratoire. L'exposition par l'intermédiaire de l'eau
est négligeable. En milieu professionnel, elle est principalement
transcutanée.
On peut diviser les usages du diazinon en deux grandes
catégories: comme pesticide en agriculture et comme médicament en
médecine vétérinaire. Dans ces conditions, les résidus de diazinon
présents dans les cultures vivrières résultent principalement de
l'utilisation de ce composé comme pesticide en agriculture, alors que
ceux qui se retrouvent dans la viande, les abats et autres produits
d'origine animale proviennent de son usage comme principe actif dans
certains médicaments vétérinaires.
Il n'y a que de très petites quantités de résidus dans les
légumes, les fruits et les produits d'origine animale. Des études de
rations totales ont montré que le composé subit une dégradation rapide
dans les produits d'origine animale et végétale. On n'a pas décelé la
présence de diazinon dans les échantillons d'eau de boisson analysés
et sa concentration dans les eaux superficielles est de l'ordre du
nanogramme par litre.
5. Cinétique et métabolisme
Le diazinon peut être résorbé dans les voies digestives, par la
voie transcutanée ou après inhalation. Chez l'homme, l'absorption
transcutanée est faible. Sous l'action des enzymes microsomiennes, le
diazinon est oxydé en métabolites inhibant la cholinestérase comme le
diazoxon, l'hydroxydiazoxon et l'hydroxydiazinon. On ne les retrouve
qu'en quantités infimes dans le lait et les oeufs. Le diazinon et ses
métabolites ne s'accumulent pas dans les tissus de l'organisme: une
dose de diazinon administrée par voie orale est excrétée à 59-95% dans
les 24 h et à 95-98% au bout de 7 jours, principalement dans les
urines.
Les principales voies de dégradation métaboliques sont les
suivantes:
a) Clivage du groupement ester, conduisant à des dérivés de
l'hydroxypyrimidine.
b) Passage de la liaison P=S à la liaison P=O.
c) Oxydation du substituant isopropyl en alcool correspondant
d) Oxydation du substituant méthyl en alcool correspondant
e) Clivage du groupement ester sous l'action du glutathion,
conduisant à un conjugué avec le glutathion.
Le clivage de l'ester phosphorique, qui conduit, directement ou
par l'intermédiaire du diazoxon, aux métabolites à noyau pyrimidine,
constitue l'étape principale du métabolisme du diazinon. Les
métabolites qui conservent le groupement ester phosphorique sont de
nature transitoire et on ne les observe qu'en petite quantité. Les
métabolites sont produits en proportion et à des vitesses très
variables selon les espèces. En règle générale, il n'y a pas de
corrélation entre la sensibilité à l'intoxication par le diazinon et
la production de diazoxon, encore qu'elle soit la plus faible chez le
mouton, qui est précisément l'espèce la moins sensible. Le métabolisme
extrahé-patique du diazinon, en particulier l'hydrolyse du diazoxon
dans le plasma, joue un rôle toxicologique plus important que le
métabolisme intrahépatique, mais il est vrai que chez les oiseaux, le
foie est sans doute l'organe le plus important de ce point de vue. Les
métabolites qui se forment, à savoir l'acide diéthylphosphorique,
l'acide diéthyl-thiophosphorique, et un certain nombre de dérivés
contenant le noyau de la pyrimidine, sont principalement éliminés par
la voie rénale.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
Les améliorations apportées depuis 1979 au procédé de fabrication
du diazinon ont permis de réduire sensiblement sa teneur en impuretés
fortement toxiques, comme le pyrophosphate de tétraéthyle (TEPP).
Grâce à ces améliorations progressives, la DL50 aiguë par voie orale
de diazinon technique est passée, pour le rat, de 250 à 1250 mg/kg.
Quelle que soit la voie d'exposition (orale, transcutanée ou
respiratoire), la toxicité aiguë du diazinon est faible. Des études à
court et à long terme effectuées sur des souris, des rats, des lapins,
des chiens et des singes ont montré que le seul effet préoccupant
était une inhibition, liée à la dose, de l'activité
acétylcholinesterasique.
Le diazinon est légèrement irritant pour la peau chez le lapin
mais il n'irrite pas la muqueuse de l'oeil. Il ne provoque pas de
sensibilisation cutanée. Les études toxicologiques consacrées à ses
effets sur la reproduction et le développement n'ont pas mis en
évidence d'activité embryotoxique ou tératogène. La capacité de
reproduction n'a pas été affectée aux doses non toxiques pour les
animaux de la génération parentale. Des études de mutagénicité
comportant divers points d'aboutissement in vivo et in vitro n'ont
pas non plus indiqué que le diazinon soit doté d'un quelconque pouvoir
mutagène. Aucun signe d'activité cancérogène n'a été relevé chez le
rat et la souris. Le diazinon n'a pas provoqué pas de neuropathie
retardée chez des poules. Chez des chiens et des cobayes, on a observé
des cas de pancréatite aiguë ; on estime que cet effet est propre aux
espèces en cause.
7. Effets sur l'Homme
On a fait état d'un certain nombre d'intoxications accidentelles
ou consécutives à une tentative de suicide, qui, quelquefois, ont eu
une issue mortelle. Dans certaines de ces circonstances, les troubles
de nature cholinergique se sont révélés plus graves que prévu en
raison de la présence d'impuretés très toxiques, comme le
pyrophosphate de tétraéthyle (TEPP). On a pu aussi constater, dans
certains cas, la présence d'une pancréatite aiguë réversible
accompagnant le syndrome cholinergique. Cet effet s'observe également
dans les intoxications par d'autres inhibiteurs de la cholinestérase.
On aussi quelquefois observé un syndrome intermédiaire. Aucun cas de
neuropathie retardée n'a été signalé, conformément aux données
obtenues sur l'animal.. Dans tous les cas d'intoxication
professionnelle dont on a eu connaissance, il y avait présence
d'impuretés telles que le TEPP, le monothio-TEPP ou le sulfo-TEPP dans
la formulation utilisée. Il est peu probable que les formulations
actuelles contiennent encore de ces impuretés.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Le diazinon produit des effets variables sur les algues
unicellulaires; on a constaté des effets d'inhibition et de
stimulation de la croissance chez différentes espèces à des
concentrations de 0,01 et de 5 mg/litre. En général, il y a réduction
de la croissance au-delà de 10 mg/litre, mais dans certains cas,
l'effectif de la population n'a pas été modifié à une concentration de
100 mg/litre. La rareté et la variabilité des données rendent
difficile l'évaluation des effets produits sur les autres
microorganismes.
Les valeurs de la CL50 à 96 h (effets aigus) pour les
invertébrés aquatiques vont de 0,2 dans le cas de Gammarus
fasciatus à 4,0 mg/litre pour la crevette Hyallela azteca. Les
mollusques sont notablement moins sensibles, d'après une épreuve
effectuée sur le gastéropode Gillia attilis. Des effets sublétaux
ont été observés au niveau du comportement à des concentrations
comprises entre 0,1 et 0,01 mg/litre.
Les valeurs de la CL50 (effets aigus) pour les poissons varient
de 0,09 mg/litre chez la truite arc-en-ciel (Oncorhynchus mykiss) à
3,1 mg/litre chez le poisson-chat (Channa punctatus). Chez les
stades juvéniles des poissons, il y a inhibition de la croissance aux
concentrations comprises entre 0,01 et 0,2 mg/litre. Une exposition
aiguë au diazinon provoque la suppression de l'activité acétyl-
cholinestérase cérébrale.
Pour le lombric Eisenia foetida, la CL50 dans le sol est de
130 mg/kg de terre.
Chez les oiseaux, la toxicité aiguë par voie orale (DL50) varie
de 1,1 mg/kg p.c. pour la caille japonaise à 85 mg/kg p.c. pour le
molothre des troupeaux. Les valeurs de la CL50 obtenues lors d'études
d'alimentation, vont de 32 mg/kg de nourriture chez le colvert à
900 mg/kg de nourriture chez la caille japonaise (on observe un effet
répulsif à ces doses élevées). Des études de rations alimentaires en
laboratoire ont montré que la dose sans effet nocif observable sur la
reproduction des oiseaux était égale à 20 mg/kg de nourriture chez le
colvert et à 40 mg/kg de nourriture chez le colin de Virginie. Après
ingestion, il y a inhibition de l'acétylcholinesterase cérébrale. Le
diazinon peut également pénétrer par voie transcutanée. On a signalé
une mortalité importante chez du gibier d'eau à la suite de
pulvérisations de diazinon sur du gazon. Des études de terrain au
cours desquelles on a épandu du diazinon à raison de 4,8 kg de matière
active par hectare, ont montré qu'il n'en résultait aucune mortalité
pour les oiseaux chanteurs. L'épandage de granulés a provoqué une
légère réduction des populations d'oiseaux chanteurs par rapport aux
populations témoins. Les études de laboratoire montrent que
l'ingestion d'un petit nombre de granulés peut être fatale à un oiseau
de petite taille.
RESUMEN Y EVALUACIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El nombre químico del diazinon es O,O-dietilo O-2-isopropil-
6-metilpirimidinil-4-y1 fosforotioato. La sustancia pura forma un
líquido incoloro con un ligero olor a éster. El principio activo de
calidad técnica es un líquido amarillo pardusco con un ligero olor
característico del compuesto. Su punto de ebullición es de 83-84
oC a 26,6 mPa y su presión de vapor (volatilidad) es baja (9,7 mPa
a 20oC). Su solubilidad en agua a temperatura ambiente es de
60 mg/litro. El diazinon es soluble en la mayoría de los disolventes
orgánicos y tiene un coeficiente de reparto octanol/agua (log Pow) de
3,40. Es estable en medios neutros, pero se hidroliza lentamente en
medios alcalinos y más rápidamente en medios ácidos. Se descompone a
temperaturas superiores a los 120oC.
Se ha desarrollado un gran número de métodos de muestreo de
análisis para la determinación del diazinon y sus metabolitos en
distintos medios. Cada vez se utilizan más los métodos sensibles,
tales como la cromatografía de gases, la cromatografía líquida de alta
resolución, la espectrometría de masas y el inmunoensayo.
2. Producción, usos y fuentes de exposición humana y ambiental
El diazinon es un insecticida organofosforado de contacto, con
una actividad insecticida de amplio espectro. Es eficaz contra las
formas adultas y juveniles de insectos, voladores o no, ácaros y
arañas. Se utiliza desde principios del decenio de 1950. El diazinon
se prepara principalmente en forma de polvos humectables y
concentrados emulsionables. También se encuentra en preparaciones
mixtas combinado con otros insecticidas.
3. Transporte, distribución y transformación en el medio ambiente
La volatilización del diazinon en el suelo es de poca magnitud.
El diazinon tiene una semivida troposférica de 1,5 horas.
Su movilidad en el suelo depende en gran medida de una serie de
factores, en particular de la materia orgánica y del contenido en
carbonato de calcio. Su Koc de 500 no da lugar a prever una fijación
fuerte a las partículas del suelo, sino a una movilidad moderada en el
suelo.
Los procesos biológicos parecen ser el factor principal en la
degradación del diazinon en el suelo. A 20 oC y con un contenido de
humedad del suelo del 60% de la capacidad de campo en un suelo franco
limoso, el TD50 fue de 5 días. En condiciones de esterilidad a 20 oC
y con un 60% de capacidad de campo, el TD50 fue de 118 días, lo que
parece indicar que la actividad geológica es la principal responsable
de la degradación en el suelo.
En el agua presente en la naturaleza, el diazinon tiene una
semivida de 5 a 15 días. Al parecer, en la degradación del diazinon
intervienen procesos tanto químicos como biológicos que dan lugar a la
mineralización al cabo de pocas semanas.
La absorción del diazinon por los organismos acuáticos es rápida.
Con respecto a los organismos acuáticos, se han señalado factores de
bioconcentración bajos que oscilan entre 3 en el caso del camarón y
152 en el caso del gobio, lo que parece indicar un metabolismo y una
eliminación rápidos. Se han notificado semividas de depuración en los
peces de hasta 30 horas (mejillón).
4. Niveles medioambientales y exposición humana
Los niveles medioambientales de diazinon son generalmente bajos.
Las vías de exposición de la población general son la inhalación y la
alimentación. La exposición a través del agua es mínima. La exposición
profesional es fundamentalmente cutánea.
Los usos del diazinon se pueden clasificar en dos categorías
principales, a saber, como plaguicida en la agricultura y como fármaco
en la medicina veterinaria. Por consiguiente, la presencia de residuos
de diazinon en los cultivos comestibles obedece principalmente a su
utilización como plaguicida agrícola, mientras que su presencia en la
carne, los despojos y otros productos de origen animal se debe a su
utilización como fármaco de uso veterinario que contiene el principio
activo.
Los residuos de diazinon presentes en verduras, hortalizas,
frutas y productos de origen animal son muy escasos. Los resultados de
estudios realizados sobre dietas totales dan a entender que el
diazinon se degrada rápidamente tanto en los productos de origen
vegetal como en los de origen animal. No se ha detectado la presencia
de diazinon en muestras de agua potable y su concentración en aguas de
superficie se sitúa en niveles de ng/litro.
5. Cinética y metabolismo
El diazinon se puede absorber por el aparato digestivo, a través
de la piel intacta y tras su inhalación. La absorción transcutánea en
el ser humano es baja. Las enzimas microsómices oxidan el diazinon
produciendo metabolitos inhibidores de la colinesterasa, tales como el
diazoxón, el hidroxidiazoxón y el hidroxidiazinon. Sólo se detectan
cantidades mínimas de metabolitos en la leche y los huevos. El
diazinon y sus metabolitos no se acumulan en los tejidos corporales;
el 59-95% de una dosis oral de diazinon se excreta en un plazo de 24
horas y el 95-98% se excreta en un plazo de 7 días, principalmente por
orina.
Las principales vías metabólicas de degradación del diazinon son
las siguientes:
a) Ruptura del enlace de éster, que da lugar a los derivados de la
hidroxipirimidina.
b) Transformación de la fracción P-S en el derivado P-O.
c) Oxidación del sustituyente isopropílico, que da lugar a los
correspondientes derivados alcohólicos terciarios y primarios.
d) Oxidación del sustituyente metílico, que da lugar al alcohol
correspondiente.
e) Ruptura del enlace éster mediada por el glutatión, que da lugar a
un conjugado de glutatión.
La ruptura del enlace éster fosfato, que da lugar, directamente o
por vía del diazoxón, al metabolito pirimidílico, desempeña una
función determinante en el metabolismo del diazinon. Los metabolitos
que mantienen el enlace éster fosfato son de carácter transitorio y
sólo se han detectado en pequeñas cantidades. La cantidad de
metabolitos y su velocidad de producción varían ampliamente de una
especie a otra. En general, la producción de diazoxón no está
relacionada con la sensibilidad a la intoxicación por diazinon, si
bien es más baja en la especie menos vulnerable, los ovinos. El
metabolismo extrahepático del diazinon, en especial la hidrólisis del
diazoxón en el plasma, es toxicológicamente más importante que el
metabolismo hepático, pero este último probablemente sea el más
importante en las especies aviarias. Los metabolitos formados, esto
es, el ácido dietilfosfórico, el ácido dietiltiofosfórico y los
derivados del anillo pirimidinílico, se eliminan principalmente por
los riñones.
6. Efectos en los animales de experimentación y en sistemas de prueba
in vitro
Las mejoras introducidas en la fabricación del diazinon desde
1979 han reducido significativamente su contenido de impurezas
sumamente tóxicas, como por ejemplo el pirofosfato de tetraetilo
(TEPP). Como resultado de estas mejoras progresivas, la DL50 aguda
por vía oral del diazinon de calidad técnica ha aumentado (por
ejemplo, de 250 mg/kg a 1250 mg/kg en la rata).
La toxicidad aguda por vía oral o cutánea o por inhalación es
baja. Los estudios a corto y largo plazo efectuados en ratones, ratas,
conejos, perros y monos han puesto de manifiesto que el único efecto
preocupante es la inhibición de la actividad de la
acetilcolinesterasa, relacionada con la dosis.
En el conejo, el diazinon ocasiona una ligera irritación de la
piel, pero no de los ojos. El diazinon no sensibiliza la piel. Los
estudios de reproducción y desarrollo no han revelado indicios de
potencial embriotóxico ni teratogénico. No se detectaron efectos en el
proceso de reproducción tras la administración de dosis que no eran
tóxicas para los animales progenitores. Los estudios de mutagenicidad
con distintas variables de valoración in vivo y in vitro no
revelaron un potencial mutagénico. No hay indicios de carcinogenicidad
en ratas ni en ratones. El diazinon no causa neuropatía retardada en
las gallinas. Se ha señalado que el diazinon provoca pancreatitis
aguda en perros y cobayos; se considera que se trata de un efecto
específico de determinadas especies.
7. Efectos en el ser humano
Se han señalado varios casos de intoxicación accidental o
suicida con diazinon, algunos de los cuales fueron mortales. En
algunos de ellos, el síndrome colinérgico puede haber sido más grave
de lo previsto debido a la presencia de impurezas sumamente tóxicas,
tales como el TEPP. En algunos casos se produjeron pancreatitis
agudas reversibles asociadas a un síndrome colinérgico grave. Eso se
produce también tras la intoxicación con otros inhibidores de la
colinesterasa. En varios casos también se detectó el síndrome
intermedio. No se ha señalado ningún caso de neuropatía retardada,
como es de prever según los datos dimanantes de estudios realizados en
animales. Los casos notificados de intoxicación tras una exposición
profesional siempre han ido asociados a la presencia de impurezas en
la formulación, tales como el TEPP, el monotio-TEPP o el sulfo-TEPP.
Es poco probable que se encuentren estas impurezas en las
formulaciones disponibles en la actualidad.
8. Efectos en otros organismos en el laboratorio y en el medio
ambiente
Los efectos del diazinon en las algas unicelulares son
variables; se ha señalado tanto la inhibición como la estimulación
del crecimiento en distintas especies, con concentraciones de 0,01 a
5 mg/litro. En términos generales, las tasas de crecimiento disminuyen
con concentraciones superiores a 10 mg/litro, si bien en algunos casos
el tamaño de la población puede permanecer invariable con
concentraciones de 100 mg/litro. La escasez y variabilidad de los
datos dificulta la evaluación de los efectos sobre otros
microorganismos.
En pruebas realizadas a las 96-h, la CL50 aguda para los
invertebrados acuáticos oscilaba entre 0,2 mg/litro en Gammarus
fasciatus y 4,0 mg/litro en camarones (Hyallela azteca). Según una
prueba única a la que se sometió a Gillia attilis, los moluscos son
sustancialmente menos sensibles. Se han señalado efectos subletales en
el comportamiento con concentraciones de 0,1 a 0,01 mg/litro.
La CL50 aguda para los peces oscila entre 0,09 mg/litro para la
trucha arco iris (Oncorhynchus mykiss) y 3,1 mg/litro para el bagre
(Channa punctatus). El crecimiento durante las primeras fases de
la vida de los peces se inhibía con concentraciones de 0,01 a
0,2 mg/litro. La actividad de la acetilcolinesterasa cerebral se
inhibe tras una fuerte exposición al diazinon.
La CL50 en el suelo para la lombriz de tierra (Eisenia
foetida) es de 130 mg/kg de suelo.
La toxicidad aguda por vía oral (DL50) en las aves varía entre
1,1 mg/kg de peso corporal para la codorniz japonesa y 85 mg/kg de
peso corporal para aves del género Molothrus. Los valores de la
CL50 en la alimentación oscilan entre 32 mg/kg de alimentos en
el pato silvestre y 900 mg/kg de alimentos en la codorniz japonesa
(se detectó repelencia con estas altas concentraciones en la
alimentación). En estudios de laboratorio realizados en aves, la
concentración de diazinon en la dieta sin efectos observados en la
reproducción era de 20 mg/kg de alimentos en el pato silvestre y de
40 mg/kg de alimentos en la codorniz (Colinus virginianus). La
actividad de la acetilcolinesterasa cerebral se inhibe tras la
ingestión. El diazinon también se puede absorber por vía cutánea. Se
ha notificado una considerable mortandad de las aves acuáticas en la
naturaleza, tras la aplicación de diazinon al césped. En estudios
sobre el terreno en que se aplicaron formulaciones líquidas al césped
con una concentración de 4,8 kg ai/ha no se registró mortalidad ni se
produjeron efectos sobre la reproducción de las aves canoras. La
aplicación en forma de gránulos provocó una pequeña reducción en el
tamaño poblacional de aves canoras, en comparación con el grupo
testigo. La ingestión de pequeñas cantidades de gránulos puede
resultar mortal para las aves pequeñas, como se ha demostrado en
estudios de laboratorio.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
Members
Dr P.J. Abbott, Australia and New Zealand Food Authority
(ANZFA), Canberra, Australia
Dr K. Barabás, Department of Public Health, Albert Szent-Gyorgyi,
University Medical School, Szeged, Hungary
Dr A.L. Black, Woden, ACT, Australia
Professor J.F. Borzelleca, Pharmacology and Toxicology,
Richmond, Virginia, USA
Dr P.J. Campbell, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, Kings Pool, York,
United Kingdom
Professor L.G. Costa, Department of Environmental Health,
University of Washington, Seattle, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr I. Dewhurst, Mammalian Toxicology Branch, Pesticides Safety
Directorate, Ministry of Agriculture, Fisheries and Food,
Kings Pool, York, United Kingdom
Dr V. Drevenkar, Institute for Medical Research and Occupational
Health, Zagreb, Croatia
Dr W. Erickson, Environmental Fate and Effects Division,
US Environmental Protection Agency, Washington, D.C., USA
Dr A. Finizio, Group of Ecotoxicology, Institute of Agricultural
Entomology, University of Milan, Milan, Italy
Mr K. Garvey, Office of Pesticide Programs (7501C),
US Environmental Protection Agency, Washington, D.C., USA
Dr A.B. Kocialski, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency,
Washington, D.C., USA
Dr A. Moretto, Institute of Occupational Medicine, University
of Padua, Padua, Italy
Professor O. Pelkonen, Department of Pharmacology and
Toxicology, University of Oulu, Oulu, Finland
Dr D. Ray, Medical Research Council Toxicology Unit, University
of Leicester, Leicester, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Wageningen
Agricultural University, Wageningen, The Netherlands
Observers
Dr J.W. Adcock, AgrEvo UK Limited, Chesterford Park, Saffron,
Waldon, Essex, United Kingdom
Mr D. Arnold, Environmental Sciences, AgrEvo UK Ltd.,
Chesterford Park, Saffron Waldon, Essex, United Kingdom
Dr E. Bellet, CCII, Overland Park, Kansas, USA
Mr Jan Chart, AMVAC Chemical Corporation, Newport Beach,
California, USA
Dr H. Egli, Novartis Crop Protection AG, Basel, Switzerland
Dr P. Harvey, AgrEvo UK Ltd., Chesterford Park, Saffron Walden,
Essex, United Kingdom
Dr G. Krinke, Novartis Crop Protection AG, Basel, Switzerland
Dr A. McReath, DowElanco Limited, Letcombe Regis, Wantage,
Oxford, United Kingdom
Dr H. Scheffler, Novartis Crop Protection AG, Basel, Switzerland
Dr A.E. Smith, Novartis Crop Protection AG, Basel, Switzerland
Secretariat
Dr L. Harrison, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr J.L. Herrman, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr R. Plestina, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met at the Institute for Environment and Health,
Leicester, United Kingdom, from 3 to 8 March 1997. Dr L.L. Smith
welcomed the participants on behalf of the Institute and
Dr R. Plestina on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The CAG reviewed and revised the draft monograph and
made an evaluation of the risks for human health and the environment
from exposure to diazinon.
The first draft of the monograph was prepared by Dr K. Barabás,
Albert Szent-Gyorgyi University Medical School, Szeged, Hungary.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
AChE acetylcholinesterase
ai active ingredient
ChE cholinesterase
CNS central nervous system
DETP diethylthiophosphate
DT degradation time
EDTA ethylenediaminetetraacetic acid
fc field capacity
GABA gamma-aminobutyric acid
ip intraperitoneal
MRL maximum residue limit
NAD nicotinamide adenine dinucleotide
NIOSH National Institute for Occupational Safety and Health (USA)
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OSHA Occupational Safety and Health Administration (USA)
2-PAM pralidoxine (2-pyridine aldoxime methyl) chloride
PEC predicted environmental concentration
TEPP tetraethyl-pyrophosphate
TER toxicity-exposure ratio
TLV threshold limit value
1. SUMMARY
1.1 Identity, physical and chemical properties, analytical methods
The chemical name for diazinon is O, O-diethyl
O-2-isopropyl-6-methylpyrimidinyl-4-yl phosphorothioate. The pure
material forms a colourless liquid with a faint ester-like odour. The
technical active ingredient is a yellow/brown liquid with a slight
compound-specific odour. The boiling point is 83-84°C at 26.6 mPa and
the vapour pressure (volatility) is low (9.7 mPa at 20°C). The
solubility in water at room temperature is 60 mg/litre. Diazinon is
soluble in most organic solvents and has an octanol/water partition
coefficient (log Pow) of 3.40. It is stable in neutral media, but is
slowly hydrolysed in alkaline media and more rapidly in acid media. It
decomposes at temperatures above 120°C.
A large number of sampling and analytical methods have been
developed for the determination of diazinon and its metabolites in
different media. Sensitive methods, such as gas chromatography,
high-performance liquid chromatography, mass spectrometry and
immunoassay methods, are increasingly used.
1.2 Production, uses and sources of human and environmental exposure
Diazinon is a contact organophosphorus insecticide with a wide
range of insecticidal activity. It is effective against adult and
juvenile forms of flying insects, crawling insects, acarians and
spiders. It has been used from the early 1950s. Diazinon is mainly
formulated as wettable powders and emulsifiable concentrates. It is
also available in mixed formulations with other insecticides.
1.3 Environmental transport, distribution and transformation
Volatilization of diazinon from soil is of minor importance.
Diazinon has a tropospheric half-life of 1.5 h.
The movement of diazinon through soil is highly influenced by a
number of factors, particularly by organic matter and calcium
carbonate content. Diazinon is not expected to bind strongly to soil,
owing to its KOC value of 500, and is expected to show moderate
mobility in the soil.
Biological processes appear to be the main factor in the
degradation of diazinon in soil. At 20°C and a soil moisture content
of 60% of field capacity (f.c.) in a silt loam soil, the DT50 was
5 days. Under sterile conditions at 20°C and 60% f.c., the DT50 was
118 days, suggesting that biological activity is mainly responsible
for degradation in soil.
In natural water diazinon has a half-life of the order of 5-15
days. Both chemical and biological processes seem to play a role in
the degradation of diazinon, leading to mineralization within a few
weeks.
Uptake of diazinon by aquatic organisms is rapid. Low
bioconcentration factors have been reported for aquatic organisms,
ranging from 3 for shrimp to 152 for gudgeon, consistent with rapid
metabolism and loss. Depuration half-lives for fish have been reported
to be up to 30 h (muscle).
1.4 Environmental levels and human exposure
Environmental levels of diazinon are generally low. The routes of
exposure for the general population are inhalational and dietary.
Exposure through water is negligible. Occupational exposure is
primarily dermal.
Diazinon uses fall into two major categories: as a pesticide in
agriculture and as a drug in veterinary medicine. Thus, the major
source of diazinon residues in edible crops are from its use as an
agricultural pesticide, while those in meat, offal and other animal
products arise from its use as a veterinary drug containing active
ingredient.
Diazinon residues in vegetables, fruits and animal products are
very low. The results of total-diet studies suggest that diazinon
rapidly breaks down in both plant and animal products. Diazinon has
not been detected in drinking-water samples and its concentrations in
surface water are at the level of ng/litre.
1.5 Kinetics and metabolism
Diazinon may be absorbed from the gastrointestinal tract,
through the intact skin and following inhalation. Transdermal
absorption in humans is low. Diazinon is oxidized by the microsomal
enzymes to cholinesterase-inhibiting metabolites such as diazoxon,
hydroxydiazoxon, and hydroxydiazinon. Only minimal quantities of
metabolites are detectable in milk and eggs. Diazinon and its
metabolites do not accumulate in body tissue; 59-95% of an oral dose
of diazinon is excreted within 24 h and 95-98% is excreted within
7 days, mainly in urine.
The main metabolic pathways of degradation of diazinon are:
a) Cleavage of the ester bond leading to the hydroxypyrimidine
derivatives.
b) Transformation of P-S moiety to the P-O derivate.
c) Oxidation of isopropyl substituent leading to the corresponding
tertiary and primary alcohol derivatives.
d) Oxidation of the methyl substituent leading to the corresponding
alcohol.
e) Glutathione-mediated cleavage of the ester bond leading to a
glutathione conjugate.
The cleavage of the phosphorus ester bond, leading directly, or
via diazoxon, to the pyrimidyl metabolite plays the major role in the
metabolism of diazinon. Metabolites maintaining the phosphorus ester
bond are of transient nature and have been observed only in small
quantities. Yields and rates of production of metabolites vary greatly
between species. The production of diazoxon is not generally
correlated with susceptibility to diazinon poisoning, although it is
lowest in the least susceptible species, the sheep. The extrahepatic
metabolism of diazinon, especially the hydrolysis of diazoxon in
plasma, is more important toxicologically than the metabolism in
the liver, although the liver is probably the most important site
of metabolism in avian species. The metabolites formed, i.e.
diethylphosphoric acid, diethylthiophosphoric acid and the derivates
of the pyrimidinyl ring, are eliminated mainly via the kidneys.
1.6 Effects on experimental animals and in vitro test systems
Improvements in the manufacture of diazinon since 1979 have
significantly reduced the content of highly toxic impurities, e.g.,
tetraethyl-pyrophosphate (TEPP). As a result of these progressive
improvements, the acute oral LD50 of technical grade diazinon has
increased (e.g., from 250 mg/kg to 1250 mg/kg in the rat).
The acute oral, dermal and inhalational toxicity is low.
Short-term and long-term studies in mice, rats, rabbits, dogs and
monkeys have shown that the only effect of concern is dose-related
inhibition of acetyl cholinesterase activity.
Diazinon is slightly irritant to rabbit skin but not to the eye.
Diazinon is not a dermal sensitizer. Reproductive and developmental
studies have revealed no evidence of embryotoxic or teratogenic
potential. There was no effect on reproductive performance at dose
levels that were not toxic to the parent animals. Mutagenicity studies
with various end-points in vivo and in vitro gave no evidence of a
mutagenic potential. There is no evidence of carcinogenicity in rats
or mice. Diazinon does not cause delayed neuropathy in hens. In the
dog and guinea-pig, diazinon has been reported to cause acute
pancreatitis; this is considered to be a species-specific effect.
1.7 Effects on humans
Several cases of accidental or suicidal poisoning by diazinon
have been reported, some of which were fatal. In some of these the
cholinergic syndrome may have been more severe than expected because
of the presence of highly toxic impurities such as TEPP. In certain
cases, acute reversible pancreatitis was associated with a severe
cholinergic syndrome. This occurs also after poisoning with other
cholinesterase inhibitors. In a number of cases, the intermediate
syndrome was also observed. No cases of delayed neuropathy have ever
been reported, as expected from animal data. Reported cases of
poisoning after occupational exposure have always been associated with
the presence of impurities such as TEPP, monothio-TEPP or sulfo-TEPP
in the formulation. These impurities are unlikely to be found in
currently available formulations.
1.8 Effects on other organisms in the laboratory and field
Effects of diazinon on unicellular algae are variable; both
inhibition and stimulation of growth have been reported for different
species at concentrations between 0.01 and 5 mg/litre. Generally,
growth rates are reduced at concentration above 10 mg/litre, although
in certain cases population size can remain unaltered at 100 mg/litre.
Fewer and variable data make effects on other microorganisms difficult
to assess.
Acute LC50 values for aquatic invertebrates range from
0.2 µg/litre for Gammarus fasciatus to 4.0 µg/litre for the shrimp
Hyallela azteca in 96-h tests. Molluscs are substantially less
sensitive according to a single test on the snail Gillia
attilis. Sublethal effects on behaviour have been reported at
concentrations between 0.1 and 0.01 mg/litre.
Acute LC50 values for fish range from 0.09 mg/litre for rainbow
trout (Oncorhynchus mykiss) to 3.1 mg/litre for the catfish
(Channa punctatus). Growth of early life stages of fish was
inhibited at concentrations between 0.01 and 0.2 mg/litre. Brain
acetylcholinesterase activity is suppressed following acute exposure
to diazinon.
The LC50 for the earthworm Eisenia foetida in soil is
130 mg/kg soil.
The acute oral toxicity (LD50) in birds ranges from 1.1 mg/kg
body weight for Japanese quail to 85 mg/kg body weight for cowbirds.
Dietary LC50 values range from 32 mg/kg diet for mallard to 900 mg/kg
diet for Japanese quail (repellency was noted at these high dietary
concentrations). The no-observed-effect concentration in diet for
reproductive effects on birds in laboratory studies was 20 mg/kg
diet for mallard and 40 mg/kg diet for bobwhite quail. Brain
acetylcholinesterase activity is inhibited following ingestion.
Diazinon may also be taken in via the dermal route. There have been
reports of substantial field kills of water fowl following application
of diazinon to turf. Field studies applying liquid formulations to
turf at 4.8 kg ai/ha resulted in no mortality or reproductive effects
on song birds. Application of granules caused a small reduction in
song bird population size compared to that of controls. Ingestion of
small numbers of granules can be fatal for small birds, as
demonstrated in laboratory studies.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Common name: diazinon
Chemical structure:
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
Members
Dr P.J. Abbott, Australia and New Zealand Food Authority
(ANZFA), Canberra, Australia
Dr K. Barabás, Department of Public Health, Albert Szent-Gyorgyi,
University Medical School, Szeged, Hungary
Dr A.L. Black, Woden, ACT, Australia
Professor J.F. Borzelleca, Pharmacology and Toxicology,
Richmond, Virginia, USA
Dr P.J. Campbell, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, Kings Pool, York,
United Kingdom
Professor L.G. Costa, Department of Environmental Health,
University of Washington, Seattle, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr I. Dewhurst, Mammalian Toxicology Branch, Pesticides Safety
Directorate, Ministry of Agriculture, Fisheries and Food,
Kings Pool, York, United Kingdom
Dr V. Drevenkar, Institute for Medical Research and Occupational
Health, Zagreb, Croatia
Dr W. Erickson, Environmental Fate and Effects Division,
US Environmental Protection Agency, Washington, D.C., USA
Dr A. Finizio, Group of Ecotoxicology, Institute of Agricultural
Entomology, University of Milan, Milan, Italy
Mr K. Garvey, Office of Pesticide Programs (7501C),
US Environmental Protection Agency, Washington, D.C., USA
Dr A.B. Kocialski, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency,
Washington, D.C., USA
Dr A. Moretto, Institute of Occupational Medicine, University
of Padua, Padua, Italy
Professor O. Pelkonen, Department of Pharmacology and
Toxicology, University of Oulu, Oulu, Finland
Dr D. Ray, Medical Research Council Toxicology Unit, University
of Leicester, Leicester, United Kingdom
Dr J.H.M. Temmink, Department of Toxicology, Wageningen
Agricultural University, Wageningen, The Netherlands
Observers
Dr J.W. Adcock, AgrEvo UK Limited, Chesterford Park, Saffron,
Waldon, Essex, United Kingdom
Mr D. Arnold, Environmental Sciences, AgrEvo UK Ltd.,
Chesterford Park, Saffron Waldon, Essex, United Kingdom
Dr E. Bellet, CCII, Overland Park, Kansas, USA
Mr Jan Chart, AMVAC Chemical Corporation, Newport Beach,
California, USA
Dr H. Egli, Novartis Crop Protection AG, Basel, Switzerland
Dr P. Harvey, AgrEvo UK Ltd., Chesterford Park, Saffron Walden,
Essex, United Kingdom
Dr G. Krinke, Novartis Crop Protection AG, Basel, Switzerland
Dr A. McReath, DowElanco Limited, Letcombe Regis, Wantage,
Oxford, United Kingdom
Dr H. Scheffler, Novartis Crop Protection AG, Basel, Switzerland
Dr A.E. Smith, Novartis Crop Protection AG, Basel, Switzerland
Secretariat
Dr L. Harrison, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr J.L. Herrman, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P.G. Jenkins, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Dr R. Plestina, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA FOR DIAZINON
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides (JMP) met at the Institute for Environment and Health,
Leicester, United Kingdom, from 3 to 8 March 1997. Dr L.L. Smith
welcomed the participants on behalf of the Institute and
Dr R. Plestina on behalf of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The CAG reviewed and revised the draft monograph and
made an evaluation of the risks for human health and the environment
from exposure to diazinon.
The first draft of the monograph was prepared by Dr K. Barabás,
Albert Szent-Gyorgyi University Medical School, Szeged, Hungary.
Extensive scientific comments were received following circulation of
the first draft to the IPCS contact points for Environmental Health
Criteria monographs and these comments were incorporated into the
second draft by the Secretariat.
Dr R. Plestina and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
AChE acetylcholinesterase
ai active ingredient
ChE cholinesterase
CNS central nervous system
DETP diethylthiophosphate
DT degradation time
EDTA ethylenediaminetetraacetic acid
fc field capacity
GABA gamma-aminobutyric acid
ip intraperitoneal
MRL maximum residue limit
NAD nicotinamide adenine dinucleotide
NIOSH National Institute for Occupational Safety and Health (USA)
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OSHA Occupational Safety and Health Administration (USA)
2-PAM pralidoxine (2-pyridine aldoxime methyl) chloride
PEC predicted environmental concentration
TEPP tetraethyl-pyrophosphate
TER toxicity-exposure ratio
TLV threshold limit value
1. SUMMARY
1.1 Identity, physical and chemical properties, analytical methods
The chemical name for diazinon is O, O-diethyl
O-2-isopropyl-6-methylpyrimidinyl-4-yl phosphorothioate. The pure
material forms a colourless liquid with a faint ester-like odour. The
technical active ingredient is a yellow/brown liquid with a slight
compound-specific odour. The boiling point is 83-84°C at 26.6 mPa and
the vapour pressure (volatility) is low (9.7 mPa at 20°C). The
solubility in water at room temperature is 60 mg/litre. Diazinon is
soluble in most organic solvents and has an octanol/water partition
coefficient (log Pow) of 3.40. It is stable in neutral media, but is
slowly hydrolysed in alkaline media and more rapidly in acid media. It
decomposes at temperatures above 120°C.
A large number of sampling and analytical methods have been
developed for the determination of diazinon and its metabolites in
different media. Sensitive methods, such as gas chromatography,
high-performance liquid chromatography, mass spectrometry and
immunoassay methods, are increasingly used.
1.2 Production, uses and sources of human and environmental exposure
Diazinon is a contact organophosphorus insecticide with a wide
range of insecticidal activity. It is effective against adult and
juvenile forms of flying insects, crawling insects, acarians and
spiders. It has been used from the early 1950s. Diazinon is mainly
formulated as wettable powders and emulsifiable concentrates. It is
also available in mixed formulations with other insecticides.
1.3 Environmental transport, distribution and transformation
Volatilization of diazinon from soil is of minor importance.
Diazinon has a tropospheric half-life of 1.5 h.
The movement of diazinon through soil is highly influenced by a
number of factors, particularly by organic matter and calcium
carbonate content. Diazinon is not expected to bind strongly to soil,
owing to its KOC value of 500, and is expected to show moderate
mobility in the soil.
Biological processes appear to be the main factor in the
degradation of diazinon in soil. At 20°C and a soil moisture content
of 60% of field capacity (f.c.) in a silt loam soil, the DT50 was
5 days. Under sterile conditions at 20°C and 60% f.c., the DT50 was
118 days, suggesting that biological activity is mainly responsible
for degradation in soil.
In natural water diazinon has a half-life of the order of 5-15
days. Both chemical and biological processes seem to play a role in
the degradation of diazinon, leading to mineralization within a few
weeks.
Uptake of diazinon by aquatic organisms is rapid. Low
bioconcentration factors have been reported for aquatic organisms,
ranging from 3 for shrimp to 152 for gudgeon, consistent with rapid
metabolism and loss. Depuration half-lives for fish have been reported
to be up to 30 h (muscle).
1.4 Environmental levels and human exposure
Environmental levels of diazinon are generally low. The routes of
exposure for the general population are inhalational and dietary.
Exposure through water is negligible. Occupational exposure is
primarily dermal.
Diazinon uses fall into two major categories: as a pesticide in
agriculture and as a drug in veterinary medicine. Thus, the major
source of diazinon residues in edible crops are from its use as an
agricultural pesticide, while those in meat, offal and other animal
products arise from its use as a veterinary drug containing active
ingredient.
Diazinon residues in vegetables, fruits and animal products are
very low. The results of total-diet studies suggest that diazinon
rapidly breaks down in both plant and animal products. Diazinon has
not been detected in drinking-water samples and its concentrations in
surface water are at the level of ng/litre.
1.5 Kinetics and metabolism
Diazinon may be absorbed from the gastrointestinal tract,
through the intact skin and following inhalation. Transdermal
absorption in humans is low. Diazinon is oxidized by the microsomal
enzymes to cholinesterase-inhibiting metabolites such as diazoxon,
hydroxydiazoxon, and hydroxydiazinon. Only minimal quantities of
metabolites are detectable in milk and eggs. Diazinon and its
metabolites do not accumulate in body tissue; 59-95% of an oral dose
of diazinon is excreted within 24 h and 95-98% is excreted within
7 days, mainly in urine.
The main metabolic pathways of degradation of diazinon are:
a) Cleavage of the ester bond leading to the hydroxypyrimidine
derivatives.
b) Transformation of P-S moiety to the P-O derivate.
c) Oxidation of isopropyl substituent leading to the corresponding
tertiary and primary alcohol derivatives.
d) Oxidation of the methyl substituent leading to the corresponding
alcohol.
e) Glutathione-mediated cleavage of the ester bond leading to a
glutathione conjugate.
The cleavage of the phosphorus ester bond, leading directly, or
via diazoxon, to the pyrimidyl metabolite plays the major role in the
metabolism of diazinon. Metabolites maintaining the phosphorus ester
bond are of transient nature and have been observed only in small
quantities. Yields and rates of production of metabolites vary greatly
between species. The production of diazoxon is not generally
correlated with susceptibility to diazinon poisoning, although it is
lowest in the least susceptible species, the sheep. The extrahepatic
metabolism of diazinon, especially the hydrolysis of diazoxon in
plasma, is more important toxicologically than the metabolism in
the liver, although the liver is probably the most important site
of metabolism in avian species. The metabolites formed, i.e.
diethylphosphoric acid, diethylthiophosphoric acid and the derivates
of the pyrimidinyl ring, are eliminated mainly via the kidneys.
1.6 Effects on experimental animals and in vitro test systems
Improvements in the manufacture of diazinon since 1979 have
significantly reduced the content of highly toxic impurities, e.g.,
tetraethyl-pyrophosphate (TEPP). As a result of these progressive
improvements, the acute oral LD50 of technical grade diazinon has
increased (e.g., from 250 mg/kg to 1250 mg/kg in the rat).
The acute oral, dermal and inhalational toxicity is low.
Short-term and long-term studies in mice, rats, rabbits, dogs and
monkeys have shown that the only effect of concern is dose-related
inhibition of acetyl cholinesterase activity.
Diazinon is slightly irritant to rabbit skin but not to the eye.
Diazinon is not a dermal sensitizer. Reproductive and developmental
studies have revealed no evidence of embryotoxic or teratogenic
potential. There was no effect on reproductive performance at dose
levels that were not toxic to the parent animals. Mutagenicity studies
with various end-points in vivo and in vitro gave no evidence of a
mutagenic potential. There is no evidence of carcinogenicity in rats
or mice. Diazinon does not cause delayed neuropathy in hens. In the
dog and guinea-pig, diazinon has been reported to cause acute
pancreatitis; this is considered to be a species-specific effect.
1.7 Effects on humans
Several cases of accidental or suicidal poisoning by diazinon
have been reported, some of which were fatal. In some of these the
cholinergic syndrome may have been more severe than expected because
of the presence of highly toxic impurities such as TEPP. In certain
cases, acute reversible pancreatitis was associated with a severe
cholinergic syndrome. This occurs also after poisoning with other
cholinesterase inhibitors. In a number of cases, the intermediate
syndrome was also observed. No cases of delayed neuropathy have ever
been reported, as expected from animal data. Reported cases of
poisoning after occupational exposure have always been associated with
the presence of impurities such as TEPP, monothio-TEPP or sulfo-TEPP
in the formulation. These impurities are unlikely to be found in
currently available formulations.
1.8 Effects on other organisms in the laboratory and field
Effects of diazinon on unicellular algae are variable; both
inhibition and stimulation of growth have been reported for different
species at concentrations between 0.01 and 5 mg/litre. Generally,
growth rates are reduced at concentration above 10 mg/litre, although
in certain cases population size can remain unaltered at 100 mg/litre.
Fewer and variable data make effects on other microorganisms difficult
to assess.
Acute LC50 values for aquatic invertebrates range from
0.2 µg/litre for Gammarus fasciatus to 4.0 µg/litre for the shrimp
Hyallela azteca in 96-h tests. Molluscs are substantially less
sensitive according to a single test on the snail Gillia
attilis. Sublethal effects on behaviour have been reported at
concentrations between 0.1 and 0.01 mg/litre.
Acute LC50 values for fish range from 0.09 mg/litre for rainbow
trout (Oncorhynchus mykiss) to 3.1 mg/litre for the catfish
(Channa punctatus). Growth of early life stages of fish was
inhibited at concentrations between 0.01 and 0.2 mg/litre. Brain
acetylcholinesterase activity is suppressed following acute exposure
to diazinon.
The LC50 for the earthworm Eisenia foetida in soil is
130 mg/kg soil.
The acute oral toxicity (LD50) in birds ranges from 1.1 mg/kg
body weight for Japanese quail to 85 mg/kg body weight for cowbirds.
Dietary LC50 values range from 32 mg/kg diet for mallard to 900 mg/kg
diet for Japanese quail (repellency was noted at these high dietary
concentrations). The no-observed-effect concentration in diet for
reproductive effects on birds in laboratory studies was 20 mg/kg
diet for mallard and 40 mg/kg diet for bobwhite quail. Brain
acetylcholinesterase activity is inhibited following ingestion.
Diazinon may also be taken in via the dermal route. There have been
reports of substantial field kills of water fowl following application
of diazinon to turf. Field studies applying liquid formulations to
turf at 4.8 kg ai/ha resulted in no mortality or reproductive effects
on song birds. Application of granules caused a small reduction in
song bird population size compared to that of controls. Ingestion of
small numbers of granules can be fatal for small birds, as
demonstrated in laboratory studies.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Common name: diazinon
Chemical structure:
 Chemical formula: C12H21N2O3PS
Relative molecular mass: 304.35
IUPAC Chemical names: O,O-diethyl O-2-isopropyl-6-methyl-
pyrimidin-4-yl phosphorothioate
CAS chemical name: O,O-diethyl O-[6-methyl-2-
(1-methylethyl)-4-pirimidinyl]
phosphorothioate
CAS registry number:
Chemical formula: C12H21N2O3PS
Relative molecular mass: 304.35
IUPAC Chemical names: O,O-diethyl O-2-isopropyl-6-methyl-
pyrimidin-4-yl phosphorothioate
CAS chemical name: O,O-diethyl O-[6-methyl-2-
(1-methylethyl)-4-pirimidinyl]
phosphorothioate
CAS registry number:  The metabolites formed, i.e. diethylphosphoric acid,
diethylthiophosphoric acid and the derivatives of pyrimidinyl ring,
are eliminated mainly via the kidneys. Only minimal quantities of the
metabolites were detected in milk and eggs.
6.2.1 In vivo metabolic transformations
6.2.1.1 Mice
When male ICR mice (number not stated) were treated orally with
diazinon or [pyrimidine14C] diazinon at 50 or 75 mg/kg body weight,
one half of the high-dose animals died and the rest showed symptoms
(sweating, crouching) (Miyazaki et al., 1970; Sekine, 1972). At the
low dose, no signs of toxicity were observed. Metabolism and excretion
occurred rapidly, and the metabolites diazoxon, O, O-diethyl-
O-[2-(alpha-hydroxyisopropyl)-4-methyl)-6-pyrimidinyl]
phosphorothioate, and O, O-diethyl- O-(2-(2-propenyl)-4-methyl-6-
pyrimidinyl) phosphorothioate were found in the urine 1 h after
treatment. Most of the metabolites were found in urine 6 h after
treatment, but metabolism was not identical in the two dose groups. In
the low-dose group O, O-diethyl- O-(2-isopropyl-4-hydroxymethyl-6-
pyrimidinyl) phosphorothioate and O, O-diethyl- O-(2-isopropyl-4-
formyl-6-pyrimidinyl) phosphorothioate were found, but this was not
observed in the high-dose group. In the high-dose, but not the low-
dose group O, O-diethyl- O-(2-(a-hydroxyethyl)-4-methyl-6-
pyrimidinyl) phosphorothioate was found.
The metabolism of [pyrimidine-14C]-diazinon and [ethoxy-14C]-
diazinon was investigated by Mücke et al. (1970). Four metabolite
fractions were found in urine and faeces, three metabolites
representing approximately 70% of the total radioactivity applied.
Hydrolysis of the ester bond yielded 2-isopropyl-4-methyl-6-
hydroxypyrimidine (22.5% of the applied radioactivity in urine);
oxidation at the primary carbon atom produced 9% of the applied
radioactivity in urine, while oxidation at the tertiary carbon atom of
the isopropyl side chain produced 22%. In addition, trace amounts of
unchanged diazinon were detected in faeces. No cleavage of the
pyrimidine ring with subsequent oxidation of the fragments to CO2
took place (Mücke et al., 1970).
6.2.1.2 Rats
A study by Capps (1989) investigated the diazinon metabolites in
male and female rats orally treated with single doses of 10 and
100 mg/kg [pyrimidine 14C]-diazinon and in rats preconditioned with
14 daily treatments at 10 mg/kg before the final administration of
radiolabelled compound. The metabolite pattern was similar in the
urine and faeces of the rats from all dose groups and from both sexes.
The major urinary metabolites were identified as 2-isopropyl-6-
methyl-4(1 H)- pyrimidinone (average 38.2% of the totally applied
dose), 2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone
(17.3%) and 2-(beta-hydroxyisopropyl-6-methyl-4(1 H)-pyrimidinone
(9.7%). Six unknown aqueous components accounted for an average of
14.9% of the administered dose, and trace amounts of unchanged
diazinon (0.11%), diazoxon (0.14%) and the hydroxy-isopropyl
derivative of diazinon (0.12%) were also detected. The identity of the
metabolites was confirmed by gas chromatography and mass spectrometry
(GC/MS) with synthetic standards.
6.2.1.3 Dogs
The urinary metabolites of Beagle dogs were characterized after
oral administration of 4.0 mg/kg body weight 14C-ring-labelled
diazinon. The metabolite 2-isopropyl-4-methyl-6-hydroxypyrimidine
accounted for 10% of the applied radioactivity in the urine and the
tertiary hydroxy-isopropyl derivative of diazinon represented 23%
(Iverson et al., 1975).
6.2.1.4 Sheep
When two sheep were dermally treated with [pyramidine-14C]-
diazinon, radiolabelled residues were detected in all tissues examined
(heart, liver, kidney, back fat and leg muscle). Unmetabolized
diazinon was the only significant residue in fat, and was a major
residue in heart and leg muscle. The major metabolites in urine and
all tissues except fat were 2-isopropyl-6-methyl-4(1 H)-pyrimidinone
(urine, 10% of the administered radioactivity; liver, 18%; kidney,
23%) and 2-(alpha- hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone
(urine, 22.7%; liver, 10%; kidney, 28%), which were also present in
the form of glucuronide conjugates. The identity of the metabolites
was confirmed by GC/MS with synthetic standards. In addition, several
unidentified polar (urine, 18.6%) and minor amounts of non-polar
(urine, 4.0%) metabolites were detected (Capps, 1990).
6.2.1.5 Goats
Two lactating goats were orally treated with [pyrimidine-14C]-
diazinon in gelatin capsules for four consecutive days. Similarly to
sheep, in urine and faeces the metabolites 2-isopropyl-6-methyl-
4(1 H)-pyrimidinone (urine, 4.5% of the totally administered radio-
activity; faeces, 2.6%) and 2-(alpha-hydroxyisopropyl)-6-methyl-
4(1 H)-pyrimidinone (urine, 12.5%; faeces, 1.7%) were identified.
Approximately 48.6% of the urinary radioactivity consisted of unknown
water-soluble compounds. Characterization of selected tissues showed
the presence of mainly the above-mentioned metabolites. Unchanged
diazinon, its hydroxy-isopropyl derivative and diazoxon accounted for
less than 10% of the radioactivity detected in these tissues.
Metabolites in fat consisted primarily of unchanged diazinon (66%),
its hydroxy-isopropyl derivative (12.5%) and diazoxon (3%). The major
metabolites in the milk were 2-isopropyl-6-methyl-4(1 H)-
pyrimidinone (39.3% of the residual radioactivity) and
2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-pirimidinone (37.3%).
Substantial portions of the polar metabolites in urine, faeces and
tissues were glucuronide conjugates. The identity of the metabolites
was confirmed by GC/MS with synthetic standards (Simoneaux,
1988a,b,e).
6.2.1.6 Hens
Four laying Leghorn hens were treated with [pyrimidine-14C]-
diazinon in gelatin capsules for seven consecutive days at daily doses
of 2.75 mg/kg day. The main metabolites detected in the excreta were
unchanged diazinon (14.9% of the extractable radio-activity),
2-isopropyl-6-methyl-4(1 H)-pyrimidinone (5.9%), 2-(alpha-hydroxy-
isopropyl)-6-methyl-4(1 H)-pyrimidinone (10.8%) and 2-(beta-
hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone (7.2%). Approximately
25% of the radioactivity in the excreta consisted of unknown
water-soluble compounds. The residues in tissues primarily consisted
of 2-isopropyl-6-methyl-4(1 H)-pyrimidinone (0.6-2.6% of the residual
radioactivity), 2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-
pyrimidinone (3.1-6.5%) and 2-(beta-hydroxyisopropyl)-6-methyl-
4(1 H)- pyrimidinone (2.0-5.7%). Unchanged diazinon was detected
primarily in the peritoneal fat (2% of residues). In the eggs,
primarily 2-isopropyl-6-methyl-4(1 H)-pyrimidinone (yolk, 11.1% of
the residual radioactivity; white, 9.4%), 2-(alpha-hydroxyisopropyl)-
6-methyl-4(1 H)-pyrimidinone (yolk, 18.6%; white, 33.3%) and
2-(beta-hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone (yolk, 7.0%;
white, 35.3%) were detected. As in goats, a substantial portion of the
polar metabolites in tissues, eggs and excreta were glucuronide
conjugates. The identity of the metabolites was confirmed by GC/MS
with synthetic standards (Simoneaux, 1988c,e; Simoneaux, 1989).
More information on kinetics and metabolism in other species is
given in chapter 9.
6.2.2 In vitro metabolic transformations
The metabolism of [ethoxy-14C]-diazinon and diazoxon was studied
in vitro using rat liver cell fractions. It was shown that the
degradation by diazinon is catalysed by a microsomal enzyme that
requires NADPH and oxygen, and is inhibited by carbon monoxide. It is
presumably the cytochrome P-450 oxidase system. Diazoxon was shown to
be degraded by enzymes located in the nuclear, mitochondrial,
microsomal and soluble fractions of the liver. The microsomal enzymes
were the most active and were not dependent on NADPH. Reduced
gluthation had little effect. With diazinon, products of the reactions
were diethylphosphorothioic acid and diethylphosphoric acid. Diazoxon
was degraded to diethylphosphoric acid (Yang et al., 1969, 1971;
Nakatsugawa et al., 1969). These results were confirmed by independent
experiments (Dahm, 1970). The oxidation of diazinon was investigated
by using microsomal preparations from rat liver. The major metabolic
products of diazinon were hydroxydiazinon, diazoxon and
hydroxydiazoxon, which are biologically active, and additional
inactive products such as diethylphosphorothioic acid,
diethylphosophoric acid and derivatives of the pyrimidyl moiety. It
was demonstrated that desulfuration, hydroxylation of the ring alkyl
side-chain and cleavage of the aryl phosphate bond may occur,
depending on the presence of NADPH or NADH. EDTA stimulated the
overall metabolism of diazinon (Shishido et al., 1972a).
The enzymatic hydrolysis of diazoxon was investigated using rat
tissue homogenates. The hydrolytic activity of the tissues decreased
in the order liver>blood>lung>heart>kidney>brain. In the liver,
the hydrolytic activity was localized in microsomal preparations.
Diethyl phosphoric acid and 2-isopropyl-4-methyl-6-hydroxypyrimidine
were identified as the products. The reactions were inhibited by EDTA,
heavy and rare earth metal ions, and sulfhydryl reagents (L-cysteine,
2-mercaptoethanol, thioglycolic acid), while calcium ions activated
the hydrolysis (Shishido & Fukami, 1972).
Liver homogenates were prepared from male mice (North Carolina
Department of Health strain) and incubated for 1 h with either
14C-diazinon or 14C-diazoxon. Inhibition of metabolism was
studied by co-incubation with piperonyl butoxide, NIA 16824 or
1-(2-isopropylphenyl) imidazole. Diazoxon formation from diazinon
(thiophosphate to phosphate conversion) was inhibited by 45 to 60% by
the inhibitors studied. All the inhibitors also reduced oxidative
dearylation of diazinon to diethyl phosphoric and diethyl
phosphorothioic acids (Smith et al., 1974).
Conjugation with glutathione forms the third enzymatic mechanism
of the diazinon metabolism in rat tissue preparations (liver, heart,
brain, lung, kidney and blood were investigated). The highest activity
(14-89 times as high as in other tissues) for this reaction was
localized in the cytoplasmatic fractions of the liver. The reaction
products were identified as diethyl phosphorothioic acid and
S-(2-isopropyl-4-methyl-6-hydroxypyrimidinyl) glutathione, which were
formed by conjugation and simultaneous cleavage of the phosphate ester
bond. The enzymatic activity was increased by the addition of
glutathione-SH, and was inhibited by various sulfhydryl reagents,
oxidized glutathione and some chelating agents ( o-phenanthroline,
8-hydroxyquinoline) (Shishido et al., 1972b).
6.3 Metabolic aspects of diazinon toxicity
Diazinon was incubated with liver microsomes and liver slices
from sheep, cow, pig, guinea-pig, rat, turkey, chicken and ducks.
Hydroxydiazinon, isohydroxydiazinon, dehydrodiazinon, their oxons
and diazoxon were identified and determined quantitatively or
semi-quantitatively. It was shown that yields and rates of production
of the metabolites varied greatly between the species. The production
of the oxon was not generally correlated with susceptibility to
diazinon poisoning, although it was lowest in the least susceptible
animal, the sheep. The highly susceptible avian species (acute oral
LD50 of around 2-15 mg/kg) do not produce higher rates of oxons than
rat or pig (acute oral LD50 around 300-600 mg/kg). However, the
mammalian blood hydrolyses diazoxon rapidly, whereas the avian species
have virtually no hydrolytic activity. It was concluded that
extrahepatic metabolism of diazinon, in particular the hydrolysis of
diazoxon in the blood, appears to be the main factor affecting
susceptibility to diazinon poisoning. In mammals the extrahepatic
metabolism of diazinon is more important toxicologically than the
metabolism in the liver, while the liver is probably the most
important site of metabolism in avian species (Machin et al., 1975).
Recently, the hydrolytic metabolism of diazinon by plasma was
investigated in 92 individuals of Hispanic origin (Davies et al.,
1996). Diazoxon is hydrolysed by the enzyme paraoxonase (PON1),
leading to the formation of 2-isopropyl-4-methyl-6-hydroxypyrimidine
and diethylphosphate. An important observation of this study was that
the effect of the PON1 polymorphism for diazoxon hydrolysis relative
to paraoxon hydrolysis was reversed. Thus, RR individuals (Arg192
homozygotes) who displayed high paraoxonase activity had lower
diazonoxonase activity (mean = 7948 U/litre) than QQ homozygotes
(12 318 U/litre).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
Improvements since 1979 in the manufacturing of diazinon have
significantly reduced the content of highly toxic by-products, in
particular tetraethyl-pyrophosphate (TEPP). As a result of these
stepwise improvements, the acute oral LD50 of technical grade
diazinon increased to values around 1000 mg/kg (Piccirillo, 1978;
Bathe & Gfeller, 1980; Schoch & Gfeller, 1985; Kuhn, 1989a). The most
recent study resulted in an oral LD50 in rats of 1250 mg/kg. The LD50
values for different species are summarized in Table 2.
Signs of poisoning after a single dose of diazinon are typical of
organophosphate intoxication and include decrease of spontaneous
activity, sedation, dyspnoea, ataxia, tremors, muscle spasms,
convulsions, lacrimation and diarrhoea. The signs were found to be
reversible in surviving animals.
Diazinon (88% purity) was administered by gavage to 15 rats per
sex and dose at single doses of 0, 2.5, 150, 300 and 600 mg/kg
(Potrepka, 1994). Three, 9 or 24 h after dosing, five animals of each
group were bled for determination of serum and red blood cell
cholinesterase activity and then killed for determination of CNS
cholinesterase activity. There was no mortality. Signs of cholinergic
poisoning, e.g., salivation, diarrhoea and muscle fasciculations, were
observed in animals of both sexes dosed at 300 and 600 mg/kg. Clinical
signs first appeared 3 h after treatment. Maximum observable toxicity
was noted 9 h after treatment in males and 24 h after treatment in
females. Serum cholinesterase activity was significantly decreased at
all time points in all groups treated with 2.5 mg/kg or more. Maximum
inhibition was observed 9 h after dosing, and values remained
depressed at 24 h after dosing. Red blood cell cholinesterase activity
was significantly inhibited at all time intervals in animals of both
sexes treated with >150 mg/kg. Again, maximum inhibition was
observed 9 h after dosing, and activity remained depressed at 24 h
after dosing. In addition, a significant inhibition of red blood cell
cholinesterase activity was noted in females dosed with 2.5 mg/kg
diazinon 9 h after dosing. Cerebellum, cerebral cortex, striatum,
hippocampus and thoracic spinal cord cholinesterase activities were
decreased in female rats dosed with >150 mg/kg at all three time
points. Cerebellum, striatum and hippocampus cholinesterase activities
were decreased in male rats dosed with >150 mg/kg at all three time
intervals, whereas cerebral cortex and thoracic spinal cord
cholinesterase activities were decreased in male rats dosed with
>150 mg/kg at 9 and 24 h after dosing. The NOAEL for inhibition of
brain cholinesterase activity was 2.5 mg/kg for both sexes (Potrepka,
1994).
Table 2. Acute toxicity of diazinona
Species Sex Route of administration LD50 (mg/kg) Reference
Rat male oral 235 Gasser (1953)
Rat male oral 435 Shaffer & West (1960)
Rat male oral 250 Gaines (1969)
Rat female oral 285 Gaines (1969)
Rat both oral 300 Piccirillo (1978)
Rat both oral 422 Bathe & Gfeller (1980)
Rat both oral 1012 Schoch & Gfeller (1985)
Rat both oral 1250 Kuhn (1989a)
Rat male dermal 900 Gaines (1960)
Rat female dermal 455 Gaines (1960)
Rat both dermal >2150 Bathe (1972a)
Rat both inhalation >2300b Holbert (1989)
Mounse male oral 82 Bruce et al. (1955)
Mounse both oral 96 Gasser (1953)
Mounse both oral 187 Bathe (1972b)
Mounse both i.p. 65 Klotzsche (1955)
Guinea-pig oral 320 Gasser (1953)
Rabbit oral 130 Gasser (1953)
Rabbit both dermal >2020 Kuhn (1989b)
Turkey oral 6.8 FAO/WHO (1965)
Chicken oral 40.8 FAO/WHO (1965)
Goose oral 14.7 FAO/WHO (1965)
Gosling oral 2.8 Egyed et al. (1974)
a It should be noted that progressive improvement in the manufacturing process has reduced
the acute toxicity of diazinon.
b This value is the LC50, the units being mg/m3.
Brain cholinesterase activities were determined for white-footed
mice (Peromyscus leucopus) orally dosed with diazinon at 18.8 mg/kg
body weight (Montz & Kirkpatrick, 1985). Following treatment with
diazinon, a latent period of approximately 6 h elapsed during which
time acetylcholinesterase activity was relatively unaffected. After
the latent phase, ChE activity rapidly declined to a minimum 12 h
after dosing. After 48 h, ChE activity recovered to a level only
slightly below that of the controls. The response of both male and
female mouse brain ChE activities declined rapidly at 6 h and reached
a minimum 24 h after dosing. ChE activity of treated animals was
comparable to that of controls 48 h after treatment. Brain ChE
activities of treated female mice were significantly lower (P <0.05)
than those of treated males.
An acute oral toxicity study in rats was conduced in two phases
to determine a NOEL of the test material (87.9% active ingredient) for
clinical, behavioural and body weight effects in phase 1 and a NOEL
for effects on plasma, red blood cell and brain cholinesterase in
phase 2 after single oral gavage application to rats. In phase 1 each
test group consisted of 5 rats. The applied dose levels were 25 and
50 mg/kg body weight for female rats only; levels of 100, 250 and
500 mg/kg body weight were tested in both sexes. One female given
500 mg/kg body weight died. Clinical signs such as miosis,
hypoactivity, absence of pain reflex, red-stained face, yellow-stained
abdomen/urogenital area, soft stool or few faeces were seen in all
animals except 100- mg/kg males and 25-mg/kg females. The NOAEL for
clinical/behavioural/body weight effects was 100 mg/kg body weight in
males and 25 mg/kg body weight in females. In phase 2 each test group
consisted of 5 rats. Males were treated with 0.05, 0.50. 1.0, 10.0,
100 or 500 mg/kg body weight and females with 0.05, 0.12, 0.25, 2.5,
25 and 250 mg/kg body weight. Clinical signs such as miosis,
hypoactivity, absence of pain reflexes, staggered gait, excessive
salivation, red-stained face and yellow-stained and/or wet
ventral/urogenital area were only observed in males at 500 mg/kg body
weight (one male at 100 mg/kg body weight showed miosis) and females
at 250 mg/kg body weight The only findings at necropsy were yellow
staining of the perineum and red paranasal discharge in animals of
these dose groups. Plasma cholinesterase activity was significantly
reduced in males dosed with 10, 100 or 500 mg/kg body weight and in
females dosed with 2.5, 25 or 250 mg/kg body weight. Red blood cell
cholinesterase activity was significantly lower in males given 100 or
500 mg/kg body weight and in females given 25 or 250 mg/kg body
weight. Brain cholinesterase activity was significantly reduced at the
highest dose level for both sexes. Brain cholinesterase activity in
females dosed with 25 mg/kg body weight was inhibited by 37% (without
reaching statistical significance) (Glaza, 1993).
7.1.2 Dermal
Diazinon was dispersed on the shaved back of each of three male
and three female Tif: RAI rats at a level of 2150 mg/kg and covered
with aluminium foil for 24 h. None of the animals died. Neither
clinical signs nor dermal irritation was observed. Autopsy revealed no
substance-related gross organ changes. The acute dermal LD50 in rats
was found to be greater than 2150 mg/kg (Bathe, 1972a). Sherman strain
rats were given one dermal application of diazinon dissolved in
xylene, and no attempt was made to remove the compound during the
observation time of 14 days. The LD50 was 900 mg/kg for males and
455 mg/kg for females (Gaines, 1960).
Dermal LD50 values for diazinon were determined in mice after
application of the solution to hind feet. Values were simultaneously
generated for the ED50 for both cholinesterases (acetylcholinesterase
and pseudocholinesterase). LD50 values were higher than those
reported for mice treated on shaved back skin. Diazinon appeared to
have much more inhibitory potential for blood than for nervous tissue
cholinesterase (Skinner & Kilgore, 1982).
Undiluted diazinon was applied to the clipped dorsal trunk of
each of five male and five female New Zealand White rabbits at a level
of 2020 mg/kg and kept under semi-occlusive dressing for 24 h. Signs
were typical of organophosphate intoxication; two out of ten animals
died. The LD50 was >2020 mg/kg (Kuhn, 1989b,c).
7.1.3 Inhalation
Sprague-Dawley rats were exposed (whole body exposure) to
diazinon for 4 h. The acute inhalation LC50 for diazinon MG 8
FL880045 was greater than 2330 mg/m3 when it was administered
undiluted as an aerosol (Holbert, 1989).
Groups of five male and five female HSD:SD rats were exposed to
an aerosol generated from undiluted liquid diazinon MG87% (88% purity)
for 4 h (nose-only exposure). An exposure concentration of 5540 mg/m3
was obtained, 8.82% of particles being smaller than 1 mm in diameter.
There was no mortality. Prominent in-life observations included
activity decrease, piloerection and polyuria, no longer seen by day 6.
Body weight gain was largely unaffected. Gross necropsy revealed
discoloration of the lungs in all animals. The acute inhalation LC50
for diazinon in the rat was greater than 5440 mg/m3 (Holbert, 1994).
7.1.4 Intraperitoneal
Mild structural and functional changes were observed in the liver
and testes of rats after a single intraperitoneal administration of
diazinon (21.6 mg/kg). Kidney, however, showed no pathological
lesions. Attempts were made to correlate the pathological changes in
these organs with the activity of succinic dehydrogenase, adenosine
triphosphatase and alkaline phosphatase (Dikshith et al., 1975). A
single intraperitoneal dose of diazinon caused hyperglycaemia in rats,
which peaked 2 h after intraperitoneal treatment with 40 mg
diazinon/kg. The brain acetylcholinesterase activity was significantly
reduced. The blood level of pyruvic acid was unchanged while that of
lactic acid was significantly increased. In diazinon-treated
hyperglycaemic animals, the glycogen content of the brain was
depleted, the activities of glycogen phosphorylase, phosphoglucomutase
and hexokinase were significantly increased, and the activity of
glucose-6-phosphatase remained unchanged. Lactate dehydrogenase
activity was increased by treatment with diazinon. The induced changes
may have compensated for the energy requirement of stimulatory effects
caused by the pesticide (Husain & Matin, 1986; Matin & Husain, 1987;
Matin et al.,1989). Changes in energy metabolism are regarded as
secondary, following cholinesterase inhibition.
In an acute intraperitoneal study in rats, an LD50 value of
260 mg/kg body weight was determined for both sexes. Clinical signs
were typical of organophosphate poisoning, e.g., sedation, dyspnoea
and tonic-clonic spasms. Surviving animals had recovered within 3 to 6
days, and no substance-related gross organ changes were observed
(Bathe, 1973).
Diazinon given as an intraperitoneal single dose (40 mg/kg) to
rats produced tremors and convulsions with lactic acidosis. This was
accompanied by depletion of glycogen phosphorylase activity in the
triceps and diaphragm muscles 2 h after the administration (Husain &
Matin, 1986; Husain & Ansari, 1988).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rats
Davies & Holub (1980a,b) found that diazinon (99.2% purity) fed
to rats for 4 weeks at doses up to 25 mg/kg diet produced no visible
toxic manifestations such as tremors or hyperexcitability. Feeding
diazinon 25 mg/kg diet for 30 days produced more significant
reduction of cholinesterase activity in plasma (by 22-30%) and brain
(by 5-9%) among treated females than among males. Erythrocyte
acetylcholinesterase activity was significantly more depressed (by
13-17%) in treated females than in males at days 21-28 of the feeding
trial. The greater degree of cholinesterase inhibition in females was
possibly attributable to the higher amount of diazinon ingested by
females than by males after day 15 of the study.
Female Wistar rats were fed a semi-purified diet containing
either no pesticide or 0.1 to 15 mg/kg diazinon for up to 92 days. At
specified times, blood samples were taken to measure plasma and
erythrocyte cholinesterase activity using a radiometric method.
Additional rats were killed to determine brain cholinesterase
activity. Measurements included body weight gain and feed consumption
during the growing period. Feeding diazinon at the stated levels
produced no visible toxic manifestations. Treated animals showed
weight gain and feed consumption that was comparable to controls.
Feeding trials lasting up to 90 days revealed that rats were highly
sensitive to diazinon after 31 to 35 days exposure, as judged by
reduction of plasma and erythrocyte cholinesterase activities. Brain
acetylcholinesterase was found to be practically insensitive to
dietary intake of diazinon (1.0 to 15 mg/kg), although moderate
reduction (by 6%) of brain enzyme activity was noted among animals fed
10 mg/kg diet at day 92. For all feeding trials, plasma cholinesterase
was a more sensitive indicator of diazinon exposure than erythrocyte
or brain acetylcholinesterase. The NOAEL of diazinon for the rat was
estimated to be 0.1 mg/kg diet, which is equivalent to a daily intake
of 0.009 mg/kg body weight per day (Davies & Holub, 1980a).
In a feeding study, diazinon (87.7% pure) was administered to
groups of 15 male and 15 female Sprague Dawley rats for 13 weeks at
dietary concentrations of 0, 0.5, 5, 250 and 2500 mg/kg, corresponding
to mean daily doses of 0, 0.03, 0.3, 15 and 168 mg/kg body weight in
males and 0, 0.04, 0.4, 19 and 212 mg/kg body weight in females,
respectively. The rats treated at the highest dose level showed
hypersensitivity to touch and sound and some of the males showed
apparent aggressiveness, hyperactivity and soft faeces. Body weight
gain and food consumption were reduced in both sexes. At this dose
level, the haematological examination revealed a decreased haemoglobin
level, a lower haematocrit and an increased number of reticulocytes in
the females. The examination of blood biochemistry revealed a reduced
activity of serum cholinesterase in both sexes treated with 5 mg/kg
diet or more. In addition, the low-dose females showed a 17% reduction
in the erythrocyte cholinesterase activity. At 250 mg/kg diet or more,
the erythrocyte and brain cholinesterase activities were reduced in
both sexes. The absolute and relative liver weights were increased in
both sexes in the highest dose level, and there was microscopic
evidence of centrilobular hepatocellular hypertrophy (Singh et al.,
1988). The NOAEL, based on reduction in brain cholinesterase activity,
was 5 mg/kg diet (equivalent to 0.4 mg/kg body weight per day).
In a second, similar, study (Pettersen & Morrissey, 1994),
Sprague Dawley rats were fed diazinon at 0, 0.3, 30, 300 or 3000 mg/kg
diet for 13 weeks. Serum, erythrocyte and regional brain
cholinesterase inhibition was measured in groups of five males and
five females at weeks 4, 8 and 13. Serum and erythrocyte
cholinesterases were inhibited by 45-86 and 60-75%, respectively, at
30 mg/kg diet, but not consistently at 0.3 mg/kg diet. Brain regional
cholinesterase was inhibited by up to 25% in females at 30 mg/kg diet
and by 55-75% at 300 mg/kg diet. Males were less sensitive, showing no
effect at 30 mg/kg diet and a fall of 62-77% at 3000 mg/kg diet.
Observable neuromuscular deficits were seen only at 3000 mg/kg diet.
Consequently the NOEL and NOAEL for this study was 0.3 mg/kg diet
(equivalent to 0.019 mg/kg body weight per day), based on the
reductions in serum, erythrocyte and brain cholinesterase seen at the
next highest dose of 30 mg/kg diet. However, given the lack of an
intermediate dietary level of 5 mg/kg, which set the NOAEL in the
Singh et al. (1988) study, and the modest fall in brain cholinesterase
seen at 30 mg/kg diet in this second study, it is concluded that the
previous NOAEL of 5 mg/kg diet (0.4 mg/kg body weight per day) should
stand.
The effect of low levels of diazinon treatment on four marker
enzymes in rat heart and skeletal muscles have been investigated by
Wilkinson et al. (1986). Typical differences in succinate
dehydrogenase (SDH), lactate dehydrogenase (LDH), phosphofructokinase
(PFK) and hexokinase (HK) activities were observed between heart and
skeletal muscles. Diazinon feeding had no effect on heart, soleus,
gastrocnemius and plantaris SDH, LDH or PKF enzyme activities after
28 weeks. HK activity was significantly increased in sham-control
soleus and plantaris muscle after 28 weeks. Diazinon feeding inhibited
HK activity in plantaris muscle after 28 weeks treatment. These
results demonstrate that chronic low levels of diazinon have little
effect on the glycolytic and oxidative activity in heart and skeletal
muscles.
Adult Wistar rats were given diazinon by gavage twice weekly at a
dose of 0.5 mg/kg body weight for twenty-eight weeks. Selected control
and experimental animals were killed after 7, 14 and 28 weeks.
Histological examination of the liver revealed that animals repeatedly
treated with sublethal doses of diazinon sustain a form of hepatic
injury characterized by cellular lipid accumulation. The finding of
increased lipid accumulation in the liver following prolonged
treatment with diazinon, however, still does not resolve the question
of whether impaired lipid metabolism and/or storage is the primary
effect (Anthony et al., 1986).
Rats were exposed to diazinon-impregnated strips in a
conventional laboratory animal room. The air in the room was monitored
for the pesticide. Erythrocyte and plasma cholinesterase activities
were determined periodically. Air concentration of the pesticide never
exceeded 1.34 mg/m3. No significant change in enzyme activities were
observed (Hinkle et al., 1980).
7.2.1.2 Dogs
Feeding studies with dogs (Bruce et al., 1955; Williams et al.,
1959) did not differentiate between the sexes and therefore relevant
data from male and female animals were pooled. In another study
(Barnes et al., 1988) diazinon (87% pure) was given to groups of four
male and four female Beagle dogs for 13 weeks at concentrations of 0,
0.1, 0.5, 150 and 300 mg/kg diet, corresponding approximately to a
mean daily intake of 0.0034, 0.02, 5.9 and 10.9 mg/kg body weight
respectively. Vomiting was observed in groups fed a diet containing
150 or 300 mg/kg. Serum cholinesterase activity was reduced at
0.5 mg/kg or more in males and at 150 and 300 mg/kg in females.
Erythrocyte and brain cholinesterases activity was depressed at 150
and 300 mg/kg in both sexes. The histopathological examination
revealed a moderate atrophy of pancreatic acini in one high-dose group
of male dogs. Body weight gain was decreased in females at 150 mg/kg
diet and in both sexes at 300 mg/kg diet. The serum calcium level was
decreased in females at 150 mg/kg diet and in males at 150 and
300 mg/kg diet. The serum albumin level was decreased in both sexes at
300 mg/kg diet. As a slight reduction of serum cholinesterase activity
was the only change observed, 0.5 mg/kg diet, corresponding to a mean
diazinon intake of 0.02 mg/kg body weight per day, is considered to be
the dietary concentration causing no toxicological effect (NOAEL),
based on inhibition of brain and erythrocyte cholinesterase at higher
doses.
7.2.1.3 Pigs
Pigs were orally administered diazinon in capsules at doses of
0, 1.25, 2.5, 5 and 10 mg/kg body weight daily for periods of up to
8 months. In pigs, mortality and cholinergic signs of poisoning were
evident at 2.5 mg/kg body weight per day (FAO/WHO, 1979).
7.2.2 Inhalation
Two short-term inhalation studies were conducted in rats. In the
first study, diazinon (97.1% pure) was administered in an inhalation
chamber to four groups of nine male and female Tif RAIf rats for 6 h
per day, 5 days a week, for three weeks, at concentrations of 0, 151,
245 and 559 mg/m3. Four animals of each sex from the control group
and from the highest concentration group were kept for a 25-day post-
treatment observation period, while the others were sacrificed at day
21 after treatment. No compound-related deaths occurred. Exophthalmus
and diarrhoea were observed at all dose levels. In addition, the high-
dose group animals showed salivation, ruffled fur and tonic-clonic
convulsions during 2 h after each exposure. No toxic signs occurred in
the 25-day follow-up period. Food consumption was reduced during the
first three days of exposure in the high-dose group, plasma
cholinesterase activity was reduced in the intermediate and high-dose
groups, and brain cholinesterase activity was reduced at all dose
levels. Erythrocyte cholinesterase activity was reduced in the high-
dose group. The changes were reversible. There were no macro or
histopathological findings related to the exposure to diazinon (Zak et
al., 1973).
In the second study the main purpose was the definition of a
NOAEL for cholinesterase inhibition. Groups of ten male and ten female
Tif RAlf rats were exposed to diazinon 6 h a day for 5 days per week
for 3 weeks. The effective concentrations at the inhalation site
were 0, 0.05, 0.46, 1.57 and 11.6 mg/m3 air. There were no
compound-related signs, and no changes in body weight or food
consumption. In comparison with the control animals, the number of
erythrocytes, haemoglobin level and packed cell volume were slightly
lower in the highest-dose group. A minor decrease of brain
cholinesterase activity was noted in the highest-dose group of
females. Plasma glucose levels were significantly reduced among males
exposed to 1.57 and 11.6 mg/m3 (Hartmann, 1990). As the exposure to
0.46 mg/m3 inhibited the plasma cholinesterase only, this
concentration is the NOAEL.
7.2.3 Dermal
7.2.3.1 Rabbits
Diazinon (97.1% pure) was suspended in 50% aqueous polyethylene
glycol 300 and topically administered under semi-occlusive dressing to
groups of five male and five female albino rabbits at daily doses of
0, 1, 5 and 100 mg/kg body weight for 5 days per week for 3 weeks. In
the highest-dose group, four males treated at 100 mg/kg died during
the first week of treatment. Consequently, the dose was reduced to
50 mg/kg. Clinical signs were observed in the highest-dose group and
included anorexia, ataxia, fasciculations, tremors, diarrhoea,
hypoactivity, hypotonia and salivation. Most of the signs disappeared
after the dose was reduced. Mild dermal reactions were noted at site
of test substance administration. Body weight gain and food
consumption was similar in all groups, and most laboratory parameters
remained unaffected by the treatment. Reduced cholinesterase
activities were found in serum, red blood cells and the brain in
animals treated at >5 mg/kg. In the highest-dose group, the
reductions were significant for brain, red blood cells and serum
cholinesterase activities, while with 5 mg/kg there was a
statistically significant decrease of activity in the brain
cholinesterase of females only. In the highest-dose animals, the
histopathological examination showed a slight hyperkeratosis of the
skin at the site of treatment (Tai & Katz, 1984). The NOEL was
considered to be 1 mg/kg, based on inhibition of brain cholinesterase.
7.3 Long-term exposure
7.3.1 Rats
Groups of 30 or 40 Sprague Dawley male and female rats received
diazinon (87.7% pure) at dietary concentrations of 0, 0.1, 1.5, 125
and 250 mg/kg (equivalent to a mean daily diazinon intake of 0, 0.004,
0.06, 5 and 10 mg/kg body weight in males and of 0.005, 0.07, 6 and
12 mg/kg body weight in females) for 99 weeks. An additional control
group received 26.5 mg/kg diet epoxidized soybean oil, the stabilizer
used in technical diazinon, at the concentration corresponding to the
application to the test group of animals. Eight to ten randomly
selected animals per sex and dose group were killed after one year of
treatment, and nine or ten animals from the control group and from the
highest-dose group were killed after 4 weeks of recovery at week 56.
There were no compound-related clinical signs. At 250 mg/kg diet, the
mean body weight and the food consumption were slightly, but
significantly, increased in both sexes. Serum cholinesterase
activities were reduced at >1.5 mg/kg diet. Red blood cell and
brain cholinesterase activities were inhibited in groups fed on a diet
containing 125 or 250 mg/kg. Cholinesterase inhibition was at least
partially reversible during the 4-week recovery period after one year
of treatment. No treatment-related changes were seen during pathology
or histology examination (Kirchner et al., 1991). A pathological
re-evaluation of eyes and optic nerves from all control and high-dose
animals of the above study from interim necropsy (week 52), interim
recovery necropsy (week 56) and chronic necropsy (week 98/99) was
conducted. Histopathological lesions like cataracts, inflammatory
changes of the globe and surface of the eye and several different
types of retinal atrophy were found in both control and treated
animals at final sacrifice. None of these findings had an increased
incidence in treated rats compared to controls and they were not
regarded as compound-related. As the dietary concentration of
1.5 mg diazinon/kg, equivalent to a mean intake of 0.06 mg/kg body
weight per day, inhibited serum cholinesterase only, this dose level
was considered to be the NOAEL (Mann, 1993).
7.3.2 Dogs
Groups of Beagle dogs (four males, four females) received
diazinon (87.7% pure) for 52 weeks at dietary concentrations of 0,
0.1, 0.5, 150 and 300 mg/kg (equivalent to a mean daily intake of
0.0032, 0.015, 4.7 and 7.7 mg/kg body weight for males and 0.0037,
0.02, 4.5 and 9.1 mg/kg body weight for females). Owing to a general
lack of body weight gain, the highest dose level was reduced from
300 mg/kg diet to 225 mg/kg diet after 14 weeks of treatment. One male
dog from the highest-dose group showed emaciation and dehydration due
to severely reduced food consumption and weight reduction. In the
other animals from the highest-dose group (300 mg/kg/diet) the body
weight gain was also severely depressed during the initial 14 weeks of
treatment. After the reduction of dose to 225 mg/kg/diet, body weight
gain remained depressed in two of the males, while the females
returned to normal. A slightly reduced body weight gain was also noted
among males at 150 mg/kg diet. Food consumption was reduced in both
sexes fed on a diet containing >150 mg/kg diazinon. Haematology and
urine analysis showed no treatment-related changes. Significantly
decreased cholinesterase activities were noted at >0.5 mg/kg diet.
Serum cholinesterase activity was inhibited at 150 mg/kg diet at
all sampling intervals and at several occasions at 0.5 mg/kg diet. Red
blood cell and brain cholinesterase activities were reduced at
>150 mg/kg diet. In addition, a slight reduction in the mean serum
amylase activity was noted in both sexes fed a diet of >150 mg/kg.
The organ weight analysis showed no treatment-related changes and
macro- and histopathology were unremarkable (Rudzki et al., 1991). A
re-evaluation of eyes and optic nerves from all control and high-dose
animals from the study was conducted. No histopathological findings in
the eyes were noted (Mann, 1993). The dietary diazinon concentration
of 0.5 mg/kg, equivalent to a mean daily diazinon intake of
0.015 mg/kg, inhibited serum cholinesterase activity only, and was
considered to be the NOAEL, based on inhibition of brain and
erythrocyte cholinesterase.
7.3.3 Rhesus monkeys
This study was conducted with a 50% WP diazinon formulation
containing an actual concentration of 48.6% diazinon. The dose levels
given below refer to the active ingredient. Groups of three male and
three female Rhesus monkeys received initial daily doses of 0, 0.1,
1.0 and 10.0 mg diazinon/kg body weight, administered by gastric
intubation. After 34 days the doses were lowered to 0.05, 0.5 and
5.0 mg/kg and after 106 weeks of treatment the study was terminated.
Mortality was similar in all dose groups: the death of one animal
at each dose was of infectious etiology. Clinical signs included
tremor in the highest-dose group animals and an increased incidence
of soft stools was noted at 1.0 and 10.0 mg/kg. In comparison to
the controls, all treated animals gained slightly less weight.
Treatment-related deviations in the laboratory parameters examined
were limited to reductions of cholinesterase activities. At the
0.5 mg/kg dose level, the erythrocyte cholinesterase activity was
occasionally reduced in some animals and plasma cholinesterase
activity was consistently depressed. At the highest-dose level, plasma
and erythrocyte cholinesterase activities were markedly inhibited and
the brain cholinesterase activity was reduced in one monkey. The
postmortem examinations revealed no changes of toxicological
relevance. The daily dose of 0.5 mg diazinon/kg inhibited plasma and
(occasionally slightly) erythrocyte cholinesterase activity. The
toxicologically relevant brain cholinesterase activity remained
unaffected. Therefore, this dose level was considered to be the NOAEL,
based on inhibition of erythrocyte cholinesterase (Cockrell et al.,
1966).
7.4 Skin and eye irritation; sensitization
7.4.1 Primary skin irritation
Diazinon (0.5 ml undiluted, technical material) was applied to
the shaved skin of three male and three female New Zealand white
rabbits and covered with gauze patches and an impermeable material.
The dressing was removed after 4 h. Slight erythema and minimal oedema
were seen in all rabbits, which disappeared within 1 week. In the
report the compound received the descriptive rating as "slightly
irritant" to rabbit skin (Kuhn, 1989b,c).
7.4.2 Primary eye irritation
Diazinon (0.1 ml undiluted technical material) was instilled into
the conjunctival sac of three male and three female New Zealand white
rabbits. In three additional female rabbits, the treated eyes were
washed for 1´ min after the instillation of diazinon. Diazinon
produced mildly irritating reactions of the conjunctivae, which
disappeared within 72 h. It was rated as minimally irritating in
washed and unwashed eyes of rabbits (Kuhn, 1989d).
7.4.3 Skin sensitization
Diazinon was examined for skin sensitizing effects in 10 Hartley
Albino guinea-pigs. Ten animals were initially treated with 0.5 ml
undiluted technical diazinon, applied to the shaved skin under
semi-occlusive dressing for 6 h. After the third treatment one animal
died, most probably due to intoxication. Therefore, further treatments
were conducted with a 10% solution of diazinon in ethanol. After 11
treatments during the 3-week induction period and a 2-week rest phase,
no sensitization resulted after a single challenge administration
(Kuhn, 1989e).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
7.5.1.1 Rat
Diazinon (94.9% pure) was administered in the feed to groups of
30 male and 30 female Sprague Dawley rats for 10 weeks prior to
mating, throughout mating of the F0 animals and during two
generations up to weaning and sacrifice of the F2 pups. The dietary
concentrations used were 0, 10, 100 and 500 mg/kg. Compound-related
clinical signs and mortalities were limited to a few parental females
treated with 500 mg/kg diet. The food consumption was generally
comparable to, or slightly higher than, control values for females of
both parental generations in the diazinon-treated groups, while the
F1 males showed a decreased food consumption during the premating
period at both 100 and 500 mg/kg diet. The body weight increase was
reduced at 500 mg/kg in the F0 females and at 100 and 500 mg/kg for
the F1 animals of both sexes. There were no remarkable gross or
microscopic findings or effects on organ weights in either the F0 or
F1 generation. Reproductive parameters including precoital interval,
gestation duration, mating, fertility and pregnancy indices were
unaffected by the treatment in both generations at the dose levels of
10 and 100 mg/kg diet. At 500 mg/kg there was an increase in the
proportion of dams with prolonged gestation in both generations, and
in the F1 animals there was a trend toward a decrease in the number
of pregnancies and viable newborns and adverse effects on fertility
indices. Mating behaviour was unaffected by treatment. Litter size on
lactation day 0 was decreased in both the F1 and F2 pups at
500 mg/kg, whereas pup weight and sex ratio on day 0 were comparable
to controls for both generations. Decreases in pup survival and
corresponding decreases in pup weight were observed in both
generations at 500 mg/kg and in the F1 pups at 100 mg/kg.
Compound-related clinical and necropsy observations were noted in some
F1 pups at 500 mg/kg (tremor, no milk in stomach). NOAEL was 10 mg/kg
diet for pups and parental animals (Giknis, 1989).
7.5.1.2 Cattle
As a part of a survey to determine causes of abortion in
Wisconsin dairy cattle, the possible role of pesticides was examined.
Of 31 aborted fetuses examined, none contained traces of diazinon. Two
gravid, non-lactating Holstein Friesian cows were orally treated in
their feed with diazinon (Diazinon W 50 wettable powder formulation)
at daily doses of 6.6 mg/kg body weight during their second semester
of gestation until term. The calves were killed after parturition and
samples of perirenal fat, liver and kidney were investigated for
residues of diazinon, as were samples of colostral milk from the cows.
A histopathological examination of the calves, livers and kidneys was
conducted. Both cows treated with diazinon vomited and refused feed on
day 20 of treatment. No abortions occurred and no gross or
histopathological changes were found in the calves. No diazinon
residues were detected in the tissues investigated or in the milk
(Macklin & Ribelin, 1971).
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 Mice
Groups of 19-22 mated female F2 hybrid mice were orally treated
with diazinon (admixed to peanut butter, purity not specified) at
daily doses of 0, 0.18 and 9 mg/kg body weight from the day of mating
until delivery. The dams were weighed daily. After delivery, the
physiological and behavioural development of the pups was examined.
Mothers of all groups gave birth to viable offspring. Litter size and
the birth weights of the pups were similar in treated and untreated
groups. In the group receiving the higher dose of diazinon, the body
weight development of pups was depressed during their first postnatal
week. According to the authors, the testing of physiological and
behavioural development revealed subtle deviations from the normal
development in the offspring from the treated females. After sacrifice
at 101 days of age, the brains of the offspring of mothers treated at
the high-dose level showed ambiguous neuropathological changes in the
forebrains (Spyker & Avery, 1977). This study raises questions that
cannot be answered from the data presented.
7.5.2.2 Rats
Diazinon (purity 95%) was administered orally by gavage to groups
of 28 to 30 pregnant Sprague-Dawley-derived rats on days 6 to 15 of
gestation at dose levels of 0, 15, 50 and 100 mg/kg body weight On day
21 of gestation, all dams were killed and the fetuses delivered by
cesarean section. The dams of the 100 mg/kg group reacted to the
treatment by a marked decrease in food consumption and a body weight
loss in the early administration phase. The dams of the 15 and
50 mg/kg group showed no reaction. The parameters of reproduction
(number of corpora lutea, implantations, resorptions, fetal deaths and
viable fetuses) showed no treatment-related intergroup differences.
The fetal body weights were similar in all groups and the examination
of the offspring did not reveal any teratogenic effects of the
treatment. The NOAEL was 50 mg/kg body weight (Fritz, 1974).
In rats, doses (95.2 mg/kg body weight on day 9) that increased
maternal mortality reduced fetal development, as indicated by reduced
weight of litters and mild "hydronephrosis", but caused no real
teratogenic effect (Dobbins, 1967). A similar result was reported for
an intraperitoneal dosage of 100 and 150 mg/kg on day 11 (Kimbrough &
Gaines, 1968). Repeated administration (40, 50 or 60 mg/kg body weight
per day) on days 7 to 19 of gestation reduced the growth of the dams
but had no effect on the number of resorptions or corpora lutea, on
litter size, or on fetal weight. The cholinesterase activity of the
fetal brain was reduced. A dosage of 75 mg/kg body weight per day was
fatal to dams in 4 to 5 days (Hoberman et al., 1979).
Diazinon (97.4% pure) was administered by gavage to groups of 21
to 25 pregnant Sprague-Dawley rats from day 6 to 15 of gestation at
dose levels of 0, 10, 20 and 100 mg/kg body weight. On day 20 of
gestation, all dams were killed and the fetuses delivered by cesarean
section. In the highest-dose group of, dams a decreased food
consumption was noted during days 6-9 of gestation and the animals
lost weight. The body weight gain of the dams showed some recovery
thereafter, but the overall body weight gain of the highest-dose group
remained significantly below that of the untreated controls. The
numbers of corpora lutea and implantations were similar in all groups.
Resorptions were significantly increased only in the top-dose group
and, accordingly, the number of live fetuses was decreased. The fetal
body weights were higher in the top-dose group than in the untreated
controls. Three single instances of external malformations were
observed at the highest-dose level (one fetus with a filamentous tail,
one fetus with umbilical hernia and one fetus with sublingual
extraneous soft tissue). Since the malformations were not
morphologically related, they were considered to be secondary to
maternal toxicity. An increased incidence of rudimentary 14th ribs was
noted in the fetuses of the highest-dose group, although it remained
within the limits of historical controls. The finding was considered
to be related to fetotoxicity, secondary to severe maternal toxicity.
Other fetal anomalies were comparable between treated and untreated
groups. No evidence of teratogenic effect of diazinon was found
(Infurna & Arthur, 1985).
7.5.2.3 Hamsters
In Golden Syrian hamsters diazinon was administered as individual
oral doses (0.125 and 0.25 mg/kg body weight) during the period of
organogenesis. Technical grade diazinon diluted with corn oil was
used. The dose volume was 10 ml/kg body weight. Control animals
received corn oil on the same days of gestation. The hamsters were
killed on day 14 of gestation. All fetuses were examined for gross
defects when delivered by cesarean section. The viability of hamster
fetuses delivered on day 15 was checked by placing them in a chicken
hatching incubator for 6 h. All fetuses with enough developmental form
for determination of structural defects were counted in determining
the number of fetuses per litter. The total number of fetuses in each
treatment group was divided by the number of mothers surviving to
term. Fetuses dying in late embryonic life and resorption sites were
counted as dead fetuses. No bone defects were seen in four fetuses
chosen at random from each litter for staining and examination of
skeleton. Diazinon did not produce any terata when administered to
hamsters (Robens, 1969).
7.5.2.4 Rabbits
Robens (1969) reported that diazinon had not been found to be
teratogenic in rabbits given 7 or 30 mg/kg body weight, although the
higher dose of diazinon produced cholinergic signs. Diazinon
(89.2% pure) was administered orally by gastric intubation to groups
of 19 to 22 New Zealand white rabbits at daily doses of 7, 25 and
100 mg/kg body weight from day 6 to 18 of gestation. On day 30 of
gestation, all dams were killed and the fetuses delivered by cesarean
section. At the highest-dose level, 9/22 dams died and overt signs of
toxicity included tremors, convulsions, hypoactivity and anorexia.
There were no statistically significant differences among the group
regarding the mean number of implantations and the proportions of
live, dead or resorbed fetuses. The fetal weights were similar in
treated and untreated groups, and neither embryotoxicity nor
teratogenicity was observed (Harris & Holson, 1981).
7.5.2.5 Chicken
Several studies have investigated the teratogenic potential of
diazinon in chick embryos (Khera & Bedok, 1967; Misawa et al., 1981,
1982; Henderson & Kitos, 1982; Byrne & Kitos, 1983; Wyttenbach &
Hwang, 1984; Kushaba-Rugaaju & Kitos, 1985). In view of the lack of
teratogenicity in mammals and the lack of adequate data on
cholinesterase inhibition in ovo, these studies are not considered
relevant in the assessment of mammalian teratogenicity.
7.6 Mutagenicity and related end-points
The summary results of mutagenicity studies conducted with
diazinon are presented in Table 3.
Diazinon did not induce mutations in either Salmonella
typhimurium or Escherichia coli, but produced conflicting results
in mouse lymphoma L5178Y cells at the tk locus. Unscheduled DNA
synthesis was not induced in primary cultures of rat hepatocytes. A
sister chromatid exchange study with human lymphocytes cultured in
whole blood gave equivocal results, while negative results were
obtained in three other in vitro studies and, in vivo, in bone
marrow cells of dosed mice.
Chromosomal aberrations were not induced in cultured human
lymphocytes. In Drosophila melanogaster, diazinon did not induce
either complete or partial chromosome loss.
Table 3. Special studies on the mutagenicity of diazinon
Test system Test object Results Reference
In vitro
Ames Salmonella typhimurium negative Geleick & Arni (1990)
TA98, TA100, TA1535, TA1537,
TA1538; E. coli WP2 uvrA
Ames Salmonella typhimurium negative Marshall et al. (1976)
TA1535, TA1536, TA1537, TA1538
Mouse lymphoma assay Mouse lymphoma cells, negative Dollenmeier & Müller (1986)
L5178Y/tk +/-
Mouse lymphoma assay Mouse lymphoma cells positive McGregor et al. (1988)
L5178Y/tk +/-
Sister chromatid exchange study Chinese hamster cells V79 negative Chen et al. (1981)
Sister chromatid exchange study Human lymphoid cells (LAZ-007) negative Sobti et al. (1982)
Sister chromatid exchange study Chinese hamster V79 cells positive Matsuoka et al. (1979)
Sister chromatid exchange study Whole blood human lymphocytes equivocal Murli & Haworth (1990a)
Sister chromatid exchange study Chinese hamster V79 cells negative Kuroda et al. (1992)
Sister chromatid exchange study Chinese hamster V79 cells negative Nishio & Uyeki (1981)
Table 3. (con't)
Test system Test object Results Reference
Nucleus anomaly Chinese hamster V79 cells negative Hool & Muller (1981c)
Chromosomal aberration Human lymphocytes questionable Lopez & Carrascal (1987)
Chromosomal aberrations Human lymphocytes negative Lopez et al. (1986)
DNA repair test Rat hepatocytes negative Hertner & Arni (1990)
In vivo
Nucleus anomaly Chinese hamster bone marrow cells negative Hool & Müller (1981c)
Micronucleus test Mouse bone marrow cells negative Ceresa & Puri (1988)
Dominant lethal study Mouse, male negative Fritz (1975)
Chromosome aberrations Mouse spermatogonia negative Hool & Müller (1981d)
Chromosome aberrations Mouse spermatocytes negative Hool & Müller (1981b)
Chromosomal loss Drosophila melanogaster negative Woodruff et al. (1983)
Sister chromatid exchange study Mouse bone marrow cells negative Murli & Haworth (1990b)
Nuclear anomaly tests in cultured Chinese hamster V79 cells and
in bone marrow cells of dosed Chinese hamsters gave negative results.
A micronucleus test in bone marrow polychromatic erythrocytes of mice
was also negative.
No chromosomal aberrations were induced in the spermatogonia or
spermatocytids of dosed mice and a dominant lethal test in dosed male
mice was negative. It was concluded that diazinon is not genotoxic.
7.7 Carcinogenicity
7.7.1 Mice
Groups of 50 B6C3F1 mice of each sex were treated with diazinon
(98% pure) at dietary concentrations of 100 and 200 mg/kg diet for
103 weeks. Two additional groups of 25 males and 25 females each
served as untreated controls. Hyperactivity was reported for the dosed
mice but was rare in the control group. The body weight gains for all
dosed male mice were similar to the control group except for the last
20 weeks of the study period. The treated females showed slightly
lower body weights than the controls. The survival at 78 weeks in the
control 100 and 200 mg/kg groups, respectively, was: males, 21/25
(84%), 45/50 (90%), 49/50 (98%); females, 24/25 (96%), 50/50 (100%),
49/50 (98%). Several neoplasms were observed, but apart from lower
neoplasms in male mice none appeared to be treatment-related or had an
incidence in treated groups significantly different from that of
controls. The incidences of hepatocellular carcinomas in the control,
100 and 200 mg/kg groups, respectively, were: 4/21 (19%), 20/46 (43%)
and 10/48 (21%). The corresponding incidences of hepatocellular
carcinomas or adenomas were 5/2 (24%), 20/46 (43%) and 13/48 (27%).
The Cochrane-Armitage test for trend was significant for carcinomas,
but not for liver carcinomas and adenomas combined. Fisher's exact
test for comparison of a dosed group with its matched control groups
was significant (P=0.046) only for hepatocellular carcinomas alone in
the 100 mg/kg group of male mice. In view of the lack of a dose-
related response, the occurrence of liver tumours in male mice could
not be clearly attributed to diazinon (NCI, 1976).
A report submitted to the United Kingdom Ministry of Agriculture,
Fisheries and Food (MAFF, 1991) described a two-year combined chronic
toxicity/carcinogenicity study conducted with male and female B6C3F1
mice (60/dose/sex) administered diets containing diazinon at 0, 100,
200 or 300 mg/kg (males only) or 400 mg/kg (females only). Ten mice of
each sex per group were killed at 12 months. After 24 months, there
were reported to be no treatment-related gross or histopathological
findings. This study provides no evidence for the carcinogenicity of
diazinon in mice. In particular, it does not confirm the observations
described in the above-mentioned study on B6C3F1 mice.
7.7.2 Rats
Groups of 50 male and 50 female Fischer-344 rats received
diazinon (98% purity) at dietary concentrations of 400 and 800 mg/kg
for 103 weeks. All survivors were killed at 105 weeks. An additional
control group of 25 males and 25 females received an untreated diet.
There was no significant effect of treatment upon body weight gain.
Hyperactivity was noted in males and females of the 800 mg/kg group
and males of the 400 mg/kg group. Urine was discoloured in females of
the 800 mg/kg group and in dosed females there was vaginal bleeding
and discharge. Survival at 78 weeks in control, 400 mg/kg and
800 mg/kg groups, respectively, were: males, 24/25 (96%), 49/50 (98%),
49/50 (98%); females, 23/25 (92%), 44/50 (88%), 44/50 (88%). Various
neoplasms were observed, but only the incidence of lymphomas/
leukaemias in dosed groups of males was significantly different from
the controls. The incidences in the control, 400 and 800 mg/kg groups,
respectively, were: males, 5/25 (20%), 25/50 (50%), 12/50 (24%);
females, 2/25 (8%), 6/50 (12%), 6/50 (12%). The Fisher's exact test
for comparison with the matched control was significant (P=0.011) only
in the 400 mg/kg group of males. In view of the lack of a dose-related
response, the occurrence of lymphomas/leukaemias in male rats could
not be clearly attributed to diazinon. The frequency of endometrial
stromal polyps was increased in the dosed groups. This lesion, which
is commonly observed in Fischer-344 rats, occurred at incidences in
the control, 400 mg/kg and 800 mg/kg groups, respectively, of 2/23
(9%), 8/43 (19%) and 11/49 (22%). The carcinogenic status of diazinon
in this study is unclear (NCI, 1979).
The chronic toxicity study of Kirchner et al. (1991) described
earlier (section 7.3.1) included groups of male and female Sprague-
Dawley rats (approximately 20/dose/sex, progressing for longer than 57
weeks) administered diets containing diazinon at concentrations 0,
0.1, 1.5, 125 or 250 mg/kg. No treatment-related increases in
neoplasms were observed. This study does not provide supportive
evidence for a carcinogenic effect of diazinon in rats.
A report submitted to the United Kingdom Ministry of Agriculture,
Fisheries and Food (MAFF, 1991) described a 120-week combined chronic
toxicity/carcinogenicity study conducted with male and female F-344
rats (75/dose/sex) administered diets containing diazinon (0, 0.1, 1.5
or 22.6 mg/kg body weight per day). Rats of both sexes in the highest-
dose group that died late in the study (after week 105) showed
increased ulceration of the forestomach, with associated increased
incidence of acanthosis, hyperkeratosis, submucosal granulation tissue
and hyperplasia of the epithelium. Apparently, no neoplastic lesions
were observed in the study. This study provides no evidence for the
carcinogenicity of diazinon in rats. In particular, it does not
confirm the observations described in the above-mentioned study on
F-344 rats.
It is concluded that diazinon is not carcinogenic in rats or
mice.
7.8 Special studies
7.8.1 Neurotoxicity
In a range-finding experiment, groups of four domestic hens (red
heavy breed) were treated at four dose levels of diazinon (87% pure),
and an acute oral LD50 of 12.5 mg/kg body weight was determined. A
schedule was developed to protect the hens from acute cholinergic
effects. Atropine (10.0 mg/kg intramuscular) was administered prior to
the treatment with diazinon, and 2-PAM was injected at doses of
50 mg/kg at the time of the diazinon administration. In addition, the
hens received post-treatment protection with concurrent doses of
atropine and 2-PAM after approximately 1 and 5 h and as necessary
thereafter. In the main neurotoxicity study, diazinon was given as a
single dose of 28 mg/kg body weight by gastric intubation to a group
of 18 atropine-pretreated hens. All hens were observed for three weeks
and again treated with 13 mg diazinon/kg on day 21. The second
observation period also lasted for three weeks (day 22-42). Additional
groups, which served as negative controls, were given the vehicle
(10 hens treated with 1 ml/kg corn oil), and eight hens, treated with
tri- o-tolyl phosphate (500 mg/kg) served as positive controls. One
diazinon-treated hen died 5 days after the first dose and another one
6 h after the second dose. One hen from a negative control group died
on day 7. There were no neurotoxic signs apparent during either of the
two observation periods in the negative control group or in the
treated groups. No histopathological changes in the brain, spinal cord
or peripheral nerves were detected (Jenkins, 1988).
In a further test for delayed neuropathy Classen (1996) gave 20
hens a single oral dose of 100 mg diazinon/kg body weight. Therapeutic
treatment with physostigmine and atropine for up to 48 h resulted in
survival of 17 hens, despite a peak inhibition of brain cholinesterase
by 83%. No ataxia was seen over a 28-day survival period, and no
inhibition of either brain or spinal cord neuropathy target esterase
(NTE) was seen at either 24 (n=5) or 48 (n=5) h.
Diazinon (88% purity) was administered by gavage to 15 rats per
sex and dose at single doses of 0, 2.5, 150, 300 and 600 mg/kg. A
negative control group of 15 animals per sex received the vehicle
(corn oil) only. A group of 10 animals per sex served as positive
control and was administered a single dose of 150 mg/kg triademefon
60 min prior to the Functional Observational Battery (FOB). Two males
and one female dosed at 600 mg/kg died. A transient reduction in body
weight and body weight gain was observed in males dosed at 300 and
600 mg/kg during week one. Food consumption was reduced in the same
males, and in females dosed at >150 mg/kg. These findings decreased
in incidence and severity with decreasing dose. No treatment-related
effects were observed in the FOB on study days 8 and 15. In males
dosed above 150 mg/kg and in females dosed above 2.5 mg/kg, activity
counts in the figure-8 maze were significantly decreased at the
estimated time of peak effect only. Serum cholinesterase activity was
significantly decreased at the time of peak effect in all diazinon-
treated groups. Full recovery was observed in all groups on study day
15. Red blood cell cholinesterase activity was significantly inhibited
on study day 1 in animals of both sexes treated with >150 mg/kg.
Substantial recovery was observed on study day 15, but significant
inhibition still remained. No inhibition of brain cholinesterase
activity was detected on day 15. No neuropathological effects were
noted for diazinon at necropsy or upon histopathological examination.
It was concluded that the administration of diazinon resulted in
reversible neurotoxicity and cholinergic poisoning without
neuropathological changes. The NOAEL was 2.5 mg/kg body weight for
both sexes (Chow & Richter, 1994).
7.8.2 Effects on enzymes and transmitters
Male rats were treated bi-weekly by gavage with the equivalent of
0.5 mg/kg per day technical diazinon for up to 28 weeks. The animals
were killed at specific time intervals (7, 14 and 28 weeks) and
compared with age-matched controls. Blood and brain tissues were
analysed for cholinesterase activity and for concentrations of
catecholamines and amino acids. Only plasma cholinesterase activity
was significantly reduced. Erythrocyte cholinesterase and brain
cholinesterase were unchanged while during the same period the levels
of several putative brain neurotransmitters, aspartate, glutamate
(excitatory) and taurine as well as GABA (inhibitory) were
significantly reduced in experimental versus control animals. Blood
serotonin level was significantly elevated but no other blood or brain
monoamines were significantly altered. Whilst the authors (Rajendra et
al., 1986) concluded that oral administration of diazinon exerts
effects on brain neurotransmitters, even at the low-dose levels
administered in this study, the biological significance of these
findings is unclear.
Intraperitoneal administration of diazinon diminishes formation
of L-kynurenine by liver slices from 93% at 1 mg/kg dose to 56% at
40 mg/kg. The ratio of critical intermediates in L-tryptophan
biosynthetic pathway, L-kynurenine to N-formyl-L-kynurenine,
decreases and is reversed with increasing dosage of diazinon (Seifert
& Cassida, 1979).
Diazinon altered the formation of several L-tryptophan
metabolites associated with the L-kynurenine pathway in mice. Liver
kynurenine formamidase was inhibited almost completely by diazinon
(10 mg/kg). The enzyme inhibition resulted in reduced L-kynurenine
biosynthesis in livers and a concomitant accumulation of N-formyl-L-
kynurenine. However, in plasma, L-kynurenine level increased up to
5-fold in diazinon-treated mice. Consequently, the urinary excretion
of xanthurenic acid and kynurenic acid was raised 5- to 15-fold. The
revelation of this novel mechanism of diazinon action is an important
piece of information needed for a better understanding of the non-
cholinergic toxicity of organophosphorus acid triesters and
methylcarbamates (Seifert & Pewnim, 1992).
7.8.3 Effects on the immune system
Pregnant F2 dihybrid female mice received either a vehicle or 1
of 2 doses of diazinon (0.18 (n=19) or 9.0 (n=21) mg/kg body weight)
in the diet, daily throughout gestation. All mothers gave birth to
viable, overtly normal offspring at term. However, a significant
number (12%) of pups born to dams who received 9.0 mg/kg died prior to
weaning on day 28; necropsy findings were consistent with death from
respiratory infection. There was no significant difference in
mortality between control and diazinon-treated offspring once they
reached 28 days of age. Determinations of five different classes of
serum immunoglobulin (Ig) concentrations (IgG1, IgG2a, IgG2b, IgA,
IgM) at 101, 400 and 800 days of age indicated transient but
consistent disturbances of 2 Ig classes in offspring as a result of
prenatal diazinon exposure. IgG1 concentrations of male offspring
exposed to 0.18 mg/kg were significantly elevated at 101 days but not
at 400 or 800 days of age. IgG1 concentrations of female offspring
exposed to 9.00 mg/kg were significantly depressed at 101 days but not
different from controls at 400 or 800 days of age. Changes in IgG2b
levels generally were similar to those recorded for IgG1 but of
smaller magnitude. There were no significant effects on serum IgG2b or
IgM concentrations, and only equivocal effects on IgA, as a
consequence of prenatal exposure to either pesticide. This study
offers no conclusive information of an effect of diazinon on the
immune system (Barnett et al., 1980).
7.8.4 Effect on pancreas
Diazinon has been reported to cause acute pancreatitis and
ductal hypertension in dogs, which is due to the absence of
acetylcholinesterase in pancreatic sphincter, duodenal smooth muscle
in dogs and a reliance upon the more readily inhibited
butyrylcholinesterase (Dressel et al., 1980; Frick, 1987).
Kazacos (1991) examined the toxic effects of diazinon on the
guinea-pig pancreas. Guinea-pigs were given single intravenous dose of
diazinon at concentration ranging from 125 to 200 mg/kg body weight.
Examination of pancreatic tissue from animals killed at various time
intervals after dosing revealed acinar cell vacuolization after 24 h.
This cellular injury disappeared within 3 days after the initial toxic
insult. These are considered to be species-specific effects.
7.9 Factors that modify toxicity; toxicity of metabolites
7.9.1 Metabolic enzymes
Abdelsalam & Ford (1986) found that the toxic effects of diazinon
were increased by pretreatment with the hepatic microsomal enzyme
inducers dieldrin and phenobarbitone and that, at the same time, there
was a rise in the liver carboxylesterase activity. In this experiment,
the toxicity of diazinon increased when calves were pretreated with
dieldrin or phenobarbitone despite the increased activity of the liver
carboxylesterase. Apparently carboxylesterase activity was not
sufficient to protect against the toxicity of diazinon in the
pretreated calves. This suggests that either the increased
carboxylesterase activity had only a minor role in the hydrolytic
detoxification of diazinon or active metabolites, or that its effect
might have been overwhelmed by the action of other microsomal oxidase
activity concurrently induced by dieldrin and phenobarbitone leading
to a faster rate of activation than degradation of diazinon.
Abdelsalam & Ford (1987) described the effect of induced liver
and kidney lesions on the toxicity of levamisole and diazinon, two
antihelmintics routinely used in ruminant livestock management, and of
a lung lesion on the toxicity of diazinon in calves. Hepatic or renal
damage such as may occur naturally as a consequence of infection or of
the ingestion of poisonous plants or other toxic substances is,
therefore, likely to affect the metabolism and excretion of drugs
routinely used in antihelmintic control programmes. Liver damage in
calves, induced by the oral administration of the flukecide, carbon
tetrachloride, increased the toxic effect of diazinon but not of
levamisole, whereas the presence of a renal tubular lesion caused by
mercuric chloride enhanced the toxicity of both commonly used
antihelmintic compounds.
7.9.2 Antidotes
The effects of atropine/oxime therapy on diazinon-poisoned
animals was investigated in rats and rabbits. The rats were orally
treated with 235 mg/kg body weight (0.8 LD50) of diazinon (91.9%
pure), while rabbits received 1600 mg diazinon/kg body weight by the
subcutaneous route. In both species, intramuscular administration of
16 mg/kg atropine (10 min post treatment), and 30 mg/kg of pyridine
2-aldoxime methochloride (2-PAM Cl) 24 h later, significantly
increased the reactivation of the diaphragm cholinesterase. The oral
LD50 for diazinon in the rat was increased by a factor of 1.7 when
2-PAM Cl was administered intravenously and 3.1 times when 2-PAM Cl
was given orally. In order to prevent the reappearance of signs the
authors recommended that 2-PAM Cl be administered intravenously at the
same time as atropine followed by repeat oral administrations, as
needed (Harris et al., 1969).
The antidotal efficacy of pralidoxime iodide and obidoxime
dichloride was investigated in goslings poisoned by a supralethal dose
of diazinon. Various doses of both drugs were administered by
intramuscular injection when the poisoned birds were unable to walk.
Pralidoxime at 100 mg/kg induced recovery in 4 out of 6 poisoned
goslings, and 25 mg/kg successfully treated only 1 of 6 birds.
Obidoxime at 25 mg/kg showed no therapeutic properties, whereas 50
and 100 mg/kg delayed the death of some birds by several hours. At
100 mg/kg, all goslings had transient signs of intoxication, which
precluded the use of this compound as an antidote at higher doses
(Shlosberg et al., 1976).
7.9.3 Potentiation
In a study of the potentiation of the acute toxicity of diazinon
by several other pesticides (chlordimeform, iodofenphos, profenphos,
methacrifos), there was no potentiation at equitoxic doses (Sachsse &
Bathe, 1975, 1976, 1977, 1978).
8. EFFECTS ON HUMANS
8.1 Exposure of the general population
8.1.1 Acute toxicity, poisoning incidents
Several cases of intentional (Kabrawala et al., 1965; Banerjee,
1967; Wadia et al., 1974; Klemmer et al., 1978; Poklis, 1980; Wedin et
al., 1984; Hodgson & Smith, 1992) and accidental acute poisoning with
diazinon have been reported. Accidental poisonings were usually due to
ingestion of improperly stored liquid formulation (Hayes, 1963;
Zwiener & Ginsburg, 1988; Weizman & Sofer, 1992; Hodgson & Smith,
1992). Cases of poisoning after cutaneous application of diazinon for
lice treatment have also been described (Muratore et al., 1960; Hayes,
1963; Halle & Sloas, 1987). Several accidents involved children (Mizra
et al., 1972; English et al., 1970; Zwiener & Ginsburg, 1988; Hodgson
& Smith 1992; Weizman & Sofer, 1992; Wagner & Orwick, 1994).
Generally, symptoms and signs were typical of AChE inhibition,
which responded to atropine and oxime treatment. In a case of fatal
suicidal ingestion of diazinon reported by Poklis et al. (1980),
diazinon concentrations in postmortem body fluids and tissues were
found to be 277 mg/litre in blood, 200 mg/litre in the bile and
15 mg/kg in adipose tissue. In the stomach 44 mg were found. Cases
with atypical or unusual features are described in section 8.1.1.3. No
cases of delayed polyneuropathy have been observed, as would be
expected from the negative animal data. The following sections deal
with some specific aspects of acute poisoning by diazinon (i.e., the
acute pancreatitis, the intermediate syndrome, and some unusual case
reports).
8.1.1.1 Acute pancreatitis
Increased levels of serum amylase and glucose have been described
in some severe cases of diazinon poisoning. In certain cases these
enzymatic alterations were accompanied by prominent abdominal symptoms
and signs, including abdominal rigidity. All these were considered
indicative of acute pancreatitis (Dressel et al., 1979; Dagli et al.,
1981; Lee, 1989; Weizman & Sofer, 1992). On one occasion a pancreatic
cyst was observed (Dressel et al., 1979). In all cases, pancreatitis
was mild and patients fully recovered. Severe poisoning with other
organophosphates, e.g., parathion (Weizman & Sofer, 1992), coumaphos
(Lee, 1989) and malathion (Lee, 1989), was also associated with acute
pancreatitis. Apparently, poisoning with an unknown carbamate was also
associated with acute pancreatitis (Weizman & Sofer, 1992).
8.1.1.2 Intermediate syndrome
Senanayake & Karalliedde (1987) described a syndrome caused by
organophosphate pesticides and named it "intermediate", because its
onset was delayed after cholinergic syndrome but appeared earlier than
polyneuropathy. The syndrome, appeared 24-96 h after poisoning during
recovery from the cholinergic crisis. It was characterized by
paralysis of proximal limb muscles, neck flexors, motor cranial nerves
and respiratory muscles. It was resistant to atropine treatment and,
in certain patients, required assisted ventilation. It is noteworthy
that Wadia et al. (1974), reporting the neurological manifestations
seen in 200 consecutive cases of poisoning, mostly with diazinon,
described two different types of signs: type I, characteristic of the
cholinergic syndrome and responsive to atropine, and type II, present
in about 20% of the cases, which appeared at least 24 h later,
resembled those of the intermediate syndrome and were not responsive
to atropine (Wadia et al., 1974).
Other cases of intermediate syndrome due to diazinon poisoning
have been described (Hall & Baker, 1989; Samal & Sahu, 1990).
8.1.1.3 Unusual case reports
Three-week-old twins were hospitalized because of respiratory
distress. One was cyanotic on admission, but both had rapid shallow
breathing, profuse nasal and bronchial secretions, and pinpoint
pupils; muscle fasciculations were not detectable. At 48 h only the
sicker twin had slightly reduced pseudocholinesterase activity.
Treatment was appropriate and recovery uneventful. Investigation
revealed that the babies lived in one side of a two-story house that
had been divided into two apartments by partitions. About 10:30 h on
the previous day the other apartment had been sprayed with 1% diazinon
for cockroach control using approved spot applications directed mainly
at cracks. The twins were the only ones who had remained indoors. The
observed degree of respiratory distress in the presence of little or
no inhibition of cholinesterase was consistent with exclusively
respiratory exposure (English et al.1970).
A 12-week-old infant girl developed persistent hypertonicity of
the extremities without other signs of intoxication. It was discovered
that the organophosphate insecticide diazinon was applied in her
home 5 weeks prior to the onset of signs. Six months after
application, high levels of diazinon residues were found on the floor
(230 ng/cm2), in vacuum cleaner dust (1700 mg/kg), and in the air
(2.8 ng/m3). A diazinon dose of approximately 0.02 mg/kg per day was
calculated and derived from the infant's urine level of diazinon
metabolites determined 6 months after application of diazinon in her
home. Her muscle tone returned to normal shortly after the infant was
removed from the home (Wagner & Orwick, 1994).
According to Hata et al. (1986), atypical ocular bobbing resulted
from an intentional poisoning from diazinon. The authors suggest
acetylcholine as a neurotransmitter substance within the ocular motor
pathway.
A 26-year-old man, who ingested approximately 230 ml of a
solution of an unknown concentration of diazinon in a suicide attempt,
developed a severe cholinergic syndrome which was relived by atropine
and 2-PAM. Diuresis was greatly reduced (22 ml/h) and urine was dark
and cloudy (specific gravity 1.029). It is possible that significant
dehydration may have precipitated this reaction (Wedin et al., 1984;
Albright, 1984).
8.1.2 Controlled human studies
A cumulative toxicity study was conducted with human volunteers.
Four healthy males weighing between 74 and 96 kg and aged between 30
and 45 years ingested diazinon in gelatin capsules (95.4% pure) at a
dose of 0.025 mg/kg per day. Two males received 34 consecutive
treatments (Group B), while the two other volunteers were treated for
4 days, were left untreated for the next 5 days in order to
investigate reversibility of the effects and then received the
capsules again for 32 more days (Group A). The daily dose was split
into three administrations taken after meals around 9, 13 and 19 h.
Parameters of haematology, urine analysis and blood biochemistry
(plasma and erythrocyte cholinesterase, serum alkaline and acidic
phosphatase activities) were determined at regular intervals. A
reversible decrease of plasma cholinesterase activity was observed in
group A during the first 4 days of treatment while the erythrocyte
cholinesterase activity remained unaffected. During the second
treatment period of group A and during the entire treatment of group
B, the cholinesterase activities were similar to those observed at
pretest. No clinical signs or changes in other parameters were noted.
It was concluded that the administered daily dose of 0.025 mg/kg
marginally inhibited the plasma cholinesterase only, and can therefore
be considered as a NOAEL in humans (Payot, 1966).
8.2 Occupational exposure
8.2.1 Acute poisoning
Poisoning following occupational exposure to diazinon was usually
associated with improper or prolonged storage of the commercial
formulations, which, prior to the improvements carried out by the
manufacturer, tended to give rise to more toxic impurities (mainly
TEPP).
Soliman et al. (1982) investigated two spraymen who had a
cholinergic syndrome (with about 90% red blood cell AChE inhibition)
after using a commercial formulation of diazinon which was packaged in
tin-plated cans that had replaced the more expensive aluminium cans.
Analysis of such commercial formulation revealed the presence of TEPP,
sulfo-TEPP, monothiono-TEPP and other impurities, but no diazinon.
Mello et al. (1972) reported an episode where three farmers were
poisoned when using a commercial formulation of diazinon that was
transferred from the original container to another container and then
stored for some time. This formulation contained monothiono-TEPP and
was about 30 times more toxic to rats than a recently prepared and
properly stored formulation. On that occasion, 26 of 40 cows died when
treated against tick infestation with this formulation.
A fatal case of poisoning by diazinon and malathion, possibly by
inhalation, was described by Wecker et al. (1985). The 51-year-old man
was found unconscious in the closed shed where he sprayed three cows
with the pesticides.
Two cases of cutaneous hepatic porphyria of toxic origin were
identified within a short period of time. Both patients were farmers
and recently handled very intensively, without any precaution, some
pesticides containing organophosphorus substances (diazinon). The
symptoms were similar to those of the so-called Turkish porphyria
(Bopp & Kosminsky, 1975).
8.2.2 Effect of short-term and long-term exposure
Neurophysiological investigations and determinations of blood
cholinesterase activities were carried out on 11 Swedish spraymen
exposed to bromophos, diazinon, dursbane and malathion. Plasma
cholinesterase activity was significantly reduced after work, while
erythrocyte cholinesterase activity was unchanged. In none of the
workers with a decreased plasma cholinesterase activity after work
could any related acute neuromuscular disturbance be detected when
the men were tested with repetitive nerve stimulation and with single
fibre electromyography. Signs of subclinical neuropathy were present
as a slight reduction in sensory conduction velocity and increased
fibre density in some workers (Stalberg et al., 1978).
A cohort of 99 workers exposed to diazinon was tested before and
after the work shift with a neurobehavioural test battery, which
included a brief examination, a symptom questionnaire and tests of
concentration, eye-hand coordination, pattern recognition, visual
memory and finger tapping. The median diazinon exposure level was
2.1 mg/day and the mean duration of exposure was 39 days (see section
5.3). There was no correlation between the diethylthiophosphate (DETP)
concentrations or diazinon exposure and pre- or post-shift
neurobehavioural function with linear regression models after
adjusting for age, sex, education and alcohol intake. The study failed
to demonstrate adverse behavioural effects of repeated, low-level
diazinon exposure (Maizlish et al., 1987).
Three groups of agricultural workers with a history of exposure
to organophosphate pesticides were followed up to evaluate the utility
of sequential post-exposure cholinesterase analyses to confirm
organophosphate intoxication in the absence of baseline cholinesterase
values. Three or more cholinergic symptoms were reported by 50 of the
72 patients. Pre-exposure red blood cell cholinesterase activities of
45 workers were above the lower limit of laboratory normal range.
Follow-up examinations, including blood cholinesterase activity
analyses, were conducted in 57 subjects. When final post-exposure
cholinesterase activity determinations were compared with respective
individual normal baseline values, the plasma and red blood cell
activities were shown to be inhibited. The data support the use of
sequential post-exposure blood cholinesterase analyses to confirm the
diagnosis of organophosphate-induced illness in the absence of
baseline values (Coye et al., 1987).
Of 67 described poisoning incidents in California involving 583
people, nineteen involved a pesticide product containing an
organophosphate: most often chlorpyriphos (8), diazinon (3) and
malathion (5). There were also 10 cases that resulted from suicide,
and two cases involved diazinon (Maddy & Edmiston, 1988).
In a study of organophosphate-induced contact dermatitis in 202
patients in Japan, Matsushita et al. (1985) attributed the reactions
mainly to diaoxabenzafos, fenitrothion, leptophos, cyanophos, diazinon
and malathion. The areas affected by dermatitis were fingers (62.4%),
face (39.6%), forearm (31.6%) and neck (29.7%). One quarter of the
cases with dermatitis had symptoms associated with acute
organophosphate poisoning.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Singh (1973) found that three genera of cyanobacteria (blue-green
algae) tolerated diazinon at concentrations of 300 and 400 mg/litre.
Clegg & Koevening (1974) indicated that the population densities of
three species of freshwater algae were relatively unaltered by a
concentration of 100 mg/litre. Butler et al. (1975) demonstrated that
diazinon inhibited growth of numerous species of green algae and
cyanobacteria at concentrations of 0.01 mg/litre and 0.1 mg/litre.
Wong & Chang (1988) studied the effects of diazinon on the growth of
the green alga, Chlamydomonas reinhardtii. They observed a reduction
in growth at concentrations of 5 and 10 mg/litre and complete
inhibition at 20 and 40 mg/litre.
Doggett & Rhodes (1991) determined the effects of diazinon on
phytoplankton population dynamics. The objectives of this study were
to determine the effects of diazinon (1, 5, 10, 20 and 40 mg/litre
concentration) on the growth rates of three widely distributed species
of freshwater algae, and to ascertain the effects of this pesticide on
the diversity of a natural phytoplankton assemblage. Stimulation of
growth was exhibited by Selenastrum capricornutum and Chorella
exposed to diazinon at 1 and 5 mg/litre. The higher concentrations of
10, 20 and 40 mg/litre all inhibited growth. The growth rates of the
cyanobacteria showed a high degree of tolerance; a suppression of
growth was observed only at 40 mg/litre. Two genera of green algae
were stimulated by all concentrations.
The acute toxicity of diazinon to the freshwater rotifer
Brachionus calyciflorus was determined after 24 h of exposure; the
mean LC50 value was 29.2 mg/litre. Based on this result, four
sublethal concentrations were chosen to determine the median lethal
time (LT50) at each concentration of diazinon tested. The
concentrations tested were 1/5, 1/4, ´ and 2/3 of the LC50 (24 h).
The LT50 values ranged from 6.96 to 2.49 days after diazinon
exposure, decreasing with increasing exposure concentration
(Fernandez-Casalderrey et al., 1992).
Several species of soil-borne fungi invade plant roots forming
vascular-arbuscular mycorrhizae which aid in growth and nutrient
element absorption. Pre-plant incorporated treatments at 2 and 4 kg/ha
of trifluralin and diazinon had no significant effect on growth,
phosphorus accumulation or root colonization by mycorrhizal fungi in
soybeans planted in an Andover clay loam. At currently used commercial
rates, diazinon did not affect mycorrhizal development under the
conditions of the experiment (Burpee & Cole, 1978).
Nitrogenase activity of excised soybean nodules was severely
affected by diazinon following application of 3, 1, 0.5 and 0.25
multiples of the amounts stated on the label (Hensley, 1991).
9.2 Aquatic invertebrates
Acute toxicity of diazinon to aquatic invertebrates is summarized
in Table 4.
The effect of diazinon (Basudine, 0.01, 0.1, 1.0 mg/litre) on the
physical and chemical properties of haemolymph of Lymnaea stagnalis
(L.) and Planorbius corneus (L.) infected with trematodes was
studied by Stadnichenko et al. (1987). Haemolymph viscosity and
density decreased after the treatment.
Robertson & Mazzella (1989) determined the toxicity of diazinon
to the freshwater snail Gillia altilis. Based on nominal
calculations, the LC50 values for static 4- and 96-h exposures were
340 mM (93 mg/litre) and 40 mM (11 mg/litre), respectively.
Experiments were conducted to determine the effects of piperonyl
butoxide, a synthetic methylenedioxyphenyl inhibitor of cytochrome(s)
P-450, on the toxicity of organophosphate insecticides to three
cladoceran test species: Ceriodaphnia dubia, Daphnia magna, and
Daphnia pulex. The acute toxicity of diazinon to three tests species
was similar, with LC50 values ranging from 0.50 to 0.80 mg/litre.
Co-administration of piperonyl butoxide effectively reduced the acute
toxicity of metabolically activated diazinon, and piperonyl butoxide
concentrations of 500 or 1000 mg/litre completely blocked the toxicity
of diazinon at concentrations of up to 16 times the 48-h LC50. In the
case of D. pulex, piperonyl butoxide at 500 mg/litre also
significantly reduced the toxicity of diazinon at concentrations of up
to 16 times the 48-h LC50 (Ankley et al., 1991).
9.3 Fish
The acute toxicity of diazinon to fish is summarized in Table 5.
The median lethal concentration (LC50) of diazinon was
determined for Clarias batrachus; the LC50 for 40 days of exposure
to diazinon was 2.4 mg/litre (Tripathi, 1992). The acute toxicities
(24, 48, 72 and 96 h) of eight pesticides to Anguilla anguilla were
determined. The 24- to 96-h LC50 values for diazinon ranged from 0.16
to 0.08 mg/litre (Ferrando et al., 1991).
Bresch (1991) studied the effects of diazinon on early
life-stages in zebrafish. Zebrafish kept at diazinon concentrations of
0.2, 0.04 and 0.008 mg/litre grew alike, and no differences among the
groups were observed. Survival rates of animals of different groups
did not differ either. In no groups were abnormal fish seen.
Table 4. Acute toxicity (LC50) to aquatic invertebrates
Species Duration (h) Concentration (mg/litre) Reference
Snail (Gillia altilis) 96 11 Robertson & Mazzella (1989)
Cladocerans 48 0.0005-0.0008 Ankley et al. (1991)
Cladoceran (Daphnia pulex) 48 0.001 (0.0006-0.0011) Mayer & Ellersieck (1986)
Cladoceran (Simocephalus serrulatus) 48 0.0014 (0.0012-0.0016) at 21°C Mayer & Ellersieck (1986)
0.0018 (0.0014-0.0022) at 15°C
Scud (Gammarus fasciatus) 96 0.0002 (0.00015-0.00028) Mayer & Ellersieck (1986)
Shrimp (Hyallela azteca) 96 0.004-0.006 Collyard et al. (1994)
Desmocaris trispimosa 96 0.0208 (0.0192-0.0224) Ebere & Akintonwa (1992)
Shrimp (Palaemonetes africanus) 96 0.0179 (0.0147-0.020) Ebere & Akintonwa (1992)
Insect larva (Pteronarcys californica) 96 0.025 (0.020-0.030) Mayer & Ellersieck (1986)
Table 5. Acute toxicity (LC50) to fish
Species Duration (h) Concentration (mg/litre) Reference
Bluegill sunfish (Lepomis macrocharis) 96 0.17 (0.012-0.22) Mayer & Ellersieck (1986)
Rainbow trout (Oncorynchus mykiss) 96 0.09 Mayer & Ellersieck (1986)
Cutthroat trout (Salmo clarkii) 96 2.76 (2.28-3.33) in soft water Mayer & Ellersieck (1986)
1.7 (1.39-2.09) in hard water
Walking catfish (Clarias batrachus) 2.41 Tripathi (1992)
Eel (Anguilla anguilla) 96 0.08 (0.06-0.10) Ferrando et al. (1991)
Channel catfish (Channa punctatus) 96 3.1 Sastry & Malik (1982a)
Goby (Gobius) sp. 96 0.25 (0.23-0.28) Ebere & Akintonwa (1992)
Lake trout (Salvelinus namaycush) 96 0.6 (0.4-0.9) Mayer & Ellersieck (1986)
Table 6. Summary of brain acetylcholinesterase activity inhibition by
diazinon in aquatic organisms
Organism Exposure Duration Inhibition Reference
concentration (h) (%)
(mg/litre)
Bluegill 0.1 6 95 Weiss (1961)
Fathead minnow 0.1 18 70 Weiss (1961)
Goldfish 0.1 18 43 Weiss (1961)
Golden shiner 0.1 18 40 Weiss (1961)
Sheepshead minnow 6.5 24 71 Goodman
et al.
(1979)
Whereas growth and survival of fathead minnows were not
influenced at concentrations below 0.2 mg/litre in a test described by
Allison & Hermanutz (1977), the minnows responded more sensitively in
a similar test by another laboratory: effects were observed below
0.08 mg/litre (Jarvinen & Tanner, 1982). Allison (1977) found reduced
larval growth of the flagfish if reared in water containing diazinon
at a concentration of 0.014 mg/litre.
Brain acetylcholinesterase (AchE) activity and changes in
optomotor behaviour were determined in bluegill sunfish, Lepomis
macrochirus, exposed to graded concentrations (0, 15, 30, 5, 60 and
75 µg/litre) of diazinon for a period of 24 h (Dutta et al., 1992). A
significant decrease of AchE activity was observed at and above an
exposure concentration of 45 µg/litre, whereas a decline in the scores
of the "following" responses of the fish was observed at an exposure
concentration of 30 µg/litre.
Weiss (1961) was the first to investigate in vivo brain AChE
activity of fish exposed to organophosphates. He found that the extent
of enzyme inhibition was proportional to the concentration of the
substance and extent of exposure, and suggested that fish brain AChE
activity could be used to detect the presence of anticholinesterases
in the aquatic environment.
Goodman et al. (1979) reported a dose-dependent inhibition of
brain AChE activity in sheepshead minnows for diazinon. For the same
sublethal exposure (6.5 mg/litre), inhibition was independent of the
exposure duration, being 68 and 78% on days 4 and 108, respectively.
The authors found that the brain AChE activity of sheepshead minnows
exposed to diazinon varied and was susceptible to exposure
concentration. AChE was depressed long after no measurable diazinon
was found in exposed sheepshead minnows.
The effect of acute exposure to diazinon (3.1 mg/litre for 96 h)
and chronic exposure to a sublethal concentration (0.31 mg/litre) of
diazinon has been studied in the liver, stomach, intestine and pyloric
caeca of a freshwater teleost fish, Channa punctatus. In acute
exposure, succinate dehydrogenase activity was elevated in intestine
and pyloric caeca. No alteration was noted in lactate dehydrogenase
activity but pyruvate dehydrogenase activity was inhibited in pyloric
caeca. Chronic exposure resulted in inhibition of the activities of
the three dehydrogenases in all four organs at both intervals (Sastry
& Malik, 1982a).
Exposure to diazinon at different concentrations induced
pathological changes in muscle cell organelles of Tilapia nilotica.
The earliest changes in muscles of fish exposed to 10 mg/litre
consisted of swelling of sarcoplasmatic reticulum in many fibres and
the appearance of many cytoplasmatic vacuoles of different sizes. In
muscle of fishes exposed to the LC50 (20 mg/kg) of diazinon,
fragmentation of the myofibrils occurred over the entire length of the
sarcomere with involvement of both the thick myosin filament of the
A-band and the thinner actin filaments of the I-band. It was
speculated that these effects may be attributed to the
anti-cholinesterase activity of these insecticides. (Sakr & Gabr,
1992).
Acute effect of diazinon on the intramembranous particles
(IMPs) of microvilli of the intestinal epithelial cells of Tilapia
nilotica fish was studied using the freeze-fracture technique.
Exposing fish to different repeated concentrations of diazinon
(´ LC50) cause a significant decrease in the population density of
IMPs in P and E faces. IMPs of microvilli found in intestinal
epithelial cells are thought to represent many kinds of protein
including enzymes. It was suggested that diazinon induced a reduction
in enzymatic content of the membrane, which was accompanied by a
decrease in the IMP density of the microvilli (Sakr et al., 1991).
El-Elaimy et al. (1990) reported that exposure of Tilapia nilotica
to increasing concentrations of diazinon induced ultrastructural
alterations in the intestine.
Four-month old adult siblings of zebrafish (Brachydanio rerio)
were exposed to four concentrations of diazinon for up to 168 h. DNA,
RNA, protein and total free amino acid content were measured in the
liver. The DNA, RNA and protein contents were significantly reduced,
whereas the amino acid content was significantly enhanced. All these
changes showed a dose-dependent as well as a time-dependent response
(Ansari & Kumar, 1988).
Anees (1978) presented the results of sub-lethal exposure to
diazinon of an adult freshwater teleost. The concentrations of
insecticide used in the study were given as 0.37 mg/litre per 24 h and
0.28 mg/litre per 96 h. This was a study on the evaluation of
histopathology as a possible indicator of aquatic pollution by
insecticides. Hepatocytes were considerably vacuolated and reduced in
size after 24 h of exposure. However, 96 h of exposure produced less
vacuolization but the liver showed a foamy appearance. Damage to the
hepatic blood supply and the appearance of dark, granular cytoplasmic
inclusions resulted from a 14-day exposure. The same concentrations of
pesticide have already been observed to cause many tissue changes in
the intestine of Channa punctatus and disturbances in the
distribution of serum proteins.
The effect of exposure to the LC50 (12 mg/litre) for 96 h
and to a sublethal concentration (3.3 mg/litre) for 15 and 30 days
was studied in the digestive system of the catfish Heteropneustes
fossilis. The most conspicuous pathological changes in the liver
were vacuolization of the cytoplasm of hepatocytes, enlargement of
nuclei, rupture of cell membrane, liver cord disarray, and damage of
connective tissue. Intercellular spaces were widened. In stomach the
mucosa was eroded. In intestine the nuclei of the columnar epithelial
cells were reduced in volume and the cytoplasm was highly degenerated.
In both acute and chronic exposure alkaline phosphatase and
glucose-6-phosphatase activities were inhibited significantly in the
different portions of the digestive system. Acid phosphatase activity
was elevated in all the portions and in both stages of exposure. In
acute exposure insignificant elevation was observed in the activities
of amylase and maltase, while lactase activity was inhibited. An
inhibition in maltase activity was significant only in the liver.
Lipase activity showed a decrease in all stages of exposures, while
there was no marked alteration in activities of pepsin and trypsin
(Sastry & Malik, 1982b).
9.4 Effects in mesocosms and the field
A mesocosm study was performed with 17 treated and 4 untreated
ponds (each 0.1 acre in surface area and 2.2 metres deep). The ponds
received multiple applications of diazinon with single applications
ranging from 2 to 25 µg/litre and corresponding to total application
rates of 5.7, 11.4, 22.9, 45.8 and 91.5 µg/litre. The following
effects on sediment-dwelling organisms were observed: Trichoptera was
the most sensitive order, with significant reductions at all treatment
levels throughout most of the treatment period. Trichoptera was a
minor component of the macroinvertebrate communities in the mesocosms.
Diptera and Ephermeroptera were intermediate in sensitivity; both were
reduced at 22.9 and 91.5 µg/litre by the end of the treatment period,
though both recovered to control levels shortly after treatment
ended. Odonata was the least sensitive order with effects only at
91.5 µg/litre. Total insect abundance generally followed the trend
of the Diptera, which was the most abundant order; increasingly
severe effects at 22.9 to 91.5 µg/litre during the treatment
period was followed by recovery. The abundance of crustaceans in
macroinvertebrate samples generally followed a similar pattern.
Gastropods were essentially unaffected by diazinon. Though dipterans
as a whole were affected only at 22.9 µg/litre or more and recovered
rapidly, some dipteran sub-taxa were more sensitive or were
significantly reduced in the post-treatment period. Tanypodinae and
its dominant tribe, Pentaneurini, were the most sensitive. These were
significantly reduced in all treated ponds at the end of the treatment
period. Both groups recovered within eight weeks of the last
treatment. The sub-family Chironominae was the next most sensitive
among the family Chironomidae, especially the tribes Tanytarsini and
Chironomini. Tanytarsinids were reduced at 22.9 µg/litre early in the
treatment period and then recovered. Chironomini followed a similar
pattern, including recovery, but were again reduced in number at 22.9
to 91.5 µg/litre. The sub-family Orthocladiinae was the least
sensitive chironomid group with effects only at 91.5 µg/litre and full
recovery within two weeks. With the wide range of sensitivity observed
among the chironomids, the overall impact of diazinon was relatively
minor; statistically significant reductions were observed twice early
in the highest treatment. Two less abundant dipteran families,
Chaoboridae and Ceratopogonidae, were affected at 11.4 µg/litre or
more. The greatest effect on Chaoboridae occurred two months into
treatment and that on Ceratopogonidae occurred during the treatment
period, but sporadic effects persisted beyond (Giddings, 1992).
The application of diazinon to a small stream at 3 mg/litre
resulted in increased drift of benthic organisms and changes in the
composition of benthic fauna. These changes were not persistent,
however, as the communities were restored to normal within 4 weeks of
treatment (Miller et al., 1966).
9.5 Terrestrial invertebrates
Stevenson (1978) reported LD50 values for bees of 0.22 µg/bee
(topical) and 0.20 µg/bee (oral).
When earthworms (Eisenia foetida) were exposed to technical
diazinon in soil, the 14-day LC50 was calculated to be 130 mg/kg
soil. A no-observed-effect concentration of 12.3 mg/kg was reported
(Vial, 1990).
In a field experiment in Connecticut, USA, diazinon was
applied to tobacco fields as a 10% granular formulation at up to
4.48 kg a.i./ha; granules were raked into the soil. Mortality of
earthworms (Lumbricus terrestris) did not differ between treated and
control plots (Kring, 1969).
9.6 Birds
Acute toxicity to birds is summarized in Table 7.
The use of diazinon for controlling flies in sheds used to house
ducks led to the death of an estimated 15 600 young birds (Dougherty,
1957).
Ingestion of a few granules could be lethal to sparrow-sized
birds for diazinon 14G (Hill & Camardese, 1984).
Variability of toxicity among anticholinesterase formulations was
shown with a single dose of diazinon administered orally as technical
grade (TG, 99% a.i.) alone or in corn oil, as granular (GR, 14-15%
a.i.), or as emulsifiable concentrate (EC, 48% a.i.). The rank of the
formulations tested, from most to least toxic, based on statistically
separable (p<0.05) LD50s, was EC > GR=TG > control. The difference
between the least and most toxic form was nearly three-fold (Hill,
1988).
The single acute oral and dermal doses of diazinon were
determined for 8- and 18-week-old broadbreasted white turkeys. The
hazard to turkeys of exposure to soils treated for control of chiggers
was evaluated. Diazinon was lethal for 18-week-old turkeys at 2 mg/kg
orally and toxic at 5 mg/kg dermally. In spite of this high toxicity,
exposing the turkeys to soil treated with 18 kg diazinon per hectare
led to no poisoned birds (Radeleff & Kunz, 1972).
The activity of acetylcholinesterase (AChE) and the density of
muscarinic receptors were measured in brains from normal Japanese
quail (Coturnix coturnix japonica) and from quail after lethal
intoxication with diazinon. The maximum relative loss of activity due
to postmortem decomposition alone during 8 days was 13 and 10% for
AChE and muscarinic receptors, respectively. During postmortem
decomposition, the ratio of AChE and muscarinic receptor activities
remained constant at approximately 1.3:1 in normal brains, while it
was always less than, or equal to, 0.5:1 after intoxication with
diazinon. Normal AChE activity could be estimated from muscarinic
receptor density. Parallel measurement of AChE and muscarinic
receptors may assist in the postmortem diagnosis of death due to acute
poisoning with anti-cholinesterase pesticide when control specimens
are not available (Prijono & Leighton, 1991).
Anticholinesterases do not pass through the mother to the egg in
significant amounts, but they may be deposited on the egg from the
parents feathers or during pesticide application. To simulate topical
exposure, fertile mallard eggs were either immersed for 30 seconds in
an aqueous emulsion or single dose of an anticholinesterase in a
non-toxic oil vehicle pipetted on the shell on day 3 of incubation
which is a critical period with respect to organogenesis in mallards.
Table 7. Acute toxicity to birds
Species Age/weight LD50 LC50 Reference
Japanese quail (Coturnix coturnix japonica) 50-60 days (105-195 g) 4 Sachsse (1973a)
5 days (10-30 g) 1.1 Sachsse & Ullmann (1975a)
50-60 days 900a Sachsse (1972)
Bobwhite quail (Colinus virginianus) 14 days 5.2 Fink (1976)
140-180 g 4.3 Sachsse & Ullmann (1975b)
Peking duck (Anas domesticus) 2.5-3.5 kg 2.7 Sachsse & Ullmann (1976)
5 days (40-125 g) 1.9 Sachsse & Ullmann (1975c)
Domestic hen (Gallus domesticus) 5 days (40-50 g) 14 Sachsse & Ullmann (1975d)
Mallard duck (Anas platyrhynchus) 19 weeks (843-1357 g) 1.44 Fletcher & Pedersen (1988a)
5 days 202a Sachsse (1973b)
9 days (108-120 g) 32 Fletcher & Pedersen (1988c)
Brown-headed cowbird (Molothrus ater) 35-55 g 85 Fletcher & Pedersen (1988b)
a Repellency recorded in all dosage groups.
Organophosphates were shown to be as much as 18 times more toxic when
applied to the shell in an oil compared with following water
immersion. Neither method of egg treatment produced teratogenicity or
developmental effects at realistic pesticide application rates
(Hoffman & Eastin, 1981).
A laboratory reproduction study was conducted with mallard in
which birds were allowed to build their own nests and incubate eggs
(Marselas et al., 1989a). Birds were fed diets containing 0, 5, 10 and
20 mg diazinon/kg diet (20 pairs per dose level). The exposure to 5
and 10 mg/kg did not result in any overt signs of toxicity or effects
on reproductive performance. At 20 mg/kg, there was an increase in the
number of hens that did not incubate and, although egg production was
not affected, there was some reduction in the number of hatchlings and
14-day-old survivors. A companion study (Marselas et al., 1989b) with
bobwhite quail was conducted at dietary concentrations of 10, 20 and
40 mg/kg. Exposures did not result in effects on reproductive
performance and no mortalities or overt signs of toxicity were
observed.
Henderson et al. (1994) described certain oral and dermal
toxicity of diazinon to the domestic pigeon. Dose-dependent gross
symptoms of organophosphorus poisoning usually appear within half an
hour in pigeons dosed orally. There was a 50% inhibition of brain
cholinesterase activity, i.e. ID50 of diazinon was about 2 mg/kg body
weight. Plasma cholinesterase activity in dermally exposed birds was
almost completely inhibited by 24 h after diazinon treatment. The
recovery of plasma cholinesterase after dermal exposure of pigeon was
very slow.
Johnston et al. (1994) studied the interactive effects of
combined treatment of red-legged partridge with diazinon and an
ergosterolbiosynthesis-inhibiting (EBI) fungicide (prochloraz). Birds
were pre-treated with procloraz at 180 mg/kg and then exposed to
diazinon at 4.3 mg/kg 24 h later. There was a tendency to increased
inhibition of cholinesterase in the plasma of treated birds but this
was not significant. Synergism was significant with other
organophosphorus compounds tested.
9.6.1 Field studies
Nine fairways of a golf course located in Bellingham, Washington,
USA, were treated with Diazinon AG500 at a target application rate of
2.2 kg a.i./ha. The chemical application with a boomless sprayer
resulted in a variable distribution of diazinon residues on the turf
that ranged from 1.0 to 6.2 kg a.i./ha. The diazinon-treated turf was
irrigated with 1.3 cm of water immediately following application. The
post-irrigation diazinon residue levels ranged from 100 to 33 mg/kg
(mean=209; SD=88; n=8). These residue levels were higher than expected
based on results of turf studies in other regions of the USA.
Eighty-five American wigeon died after grazing on one treated fairway
on the day of application following irrigation. The brains of all 85
wigeon were analysed for acetylcholinesterase activity. Wigeon that
died on the study area showed 44 to 87% depression of AChE activity
when compared to controls. Upper gastrointestinal tract contents of 15
of the 85 dead wigeons contained 0.96 to 18.1 mg/kg diazinon (Kendall
et al., 1992).
In the USA, use of diazinon has been discontinued on golf courses
since 1988. Waterfowl often congregate near ponds on golf courses and
were exposed to turf treatments. Since this restriction, deaths of
grazing waterfowl (Canada geese, brent geese and American wigeon) have
not occurred. Application rate reduction may also be effective in
reducing risk to Canada geese, the most common grazing bird on turf.
Kendall et al. (1993) closely monitored geese exposed to diazinon
applied twice at 2.2 kg a.i./ha, and found no mortality despite
extensive feeding on treated turf.
Hummell et al. (1992a,b,c) conducted an extensive, multi-plot
assessment of the effects of turf applications of granular and liquid
formulations (4.8 kg a.i./ha) of diazinon on songbirds.
Since the introduction of diazinon, sporadic mortality of
waterfowl feeding on treated turf or on orchard grass has come to
light in Canada. Frank et al. (1991) reported five incidents that
took place in Ontario between 1986 and 1988. Fifty-seven Canada
geese were poisoned by diazinon on turf sites in Ontario 1986-88. It
was determined that median levels of diazinon in turf sprayed and
then properly irrigated ranged from 45.4 to 256 mg/kg for a single
application of 1 kg a.i./ha of the EC formulation. A value of
390 mg/kg was obtained for a single grass sample following spray at
approximately 9 kg a.i./ha. Maximum residue levels for grass recovered
from the oesophagi of geese killed by diazinon on turf, and collected
while still fresh, ranged from 55 to 79 mg/kg.
In trials evaluating applications to large turf areas (an average
of ha), survival, behaviour and reproductive performance were
monitored. Smaller plots (0.09 ha) that simulated large home yards
were established for granular formulations, and survival and plasma
ChE were evaluated. On large plots, the liquid formulation had no
effect on reproductive performance, survival or cholinesterase levels.
Granular applications had a minor impact on bird population size (9.6%
reduction) compared to control plots (4.8% reduction). Reproductive
performance was not affected, although cholinesterase levels were
reduced compared to controls. On the smaller (home-sized) turf plots
there was no apparent effect on bird numbers, and plasma
cholinesterase activity was not affected in any species.
Decarie et al. (1993) sprayed ornamental and other deciduous
trees with diazinon at a rate of 2.2 kg a.i./ha in a suburban area of
Quebec, Canada, where the compound was used to control fruit tree
mites. Twelve nests of the American robin were sprayed and 65 nests
untreated were used as controls. Productivity was compared between
treated and control nests as was the behaviour of the birds. Mean
productivity was not significantly different between the two groups.
Number of feeding flights, number of female feeding flight and number
of faecal sacs removed did not differ between groups but there was a
significant increase in the total time spent sitting on the nest in
diazinon-treated birds. The significance of this is unclear. Plasma
AChE levels were reduced (993 ± 639 µg/litre in treated birds;
3585 ± 1531 µg/litre in controls), but brain AChE activity was
unaffected.
10. EVALUATION OF HUMAN HEALTH RISK AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risk
Diazinon is an organophosphorus pesticide classified by WHO as
"moderately hazardous" Class II (WHO, 1996). It is absorbed from the
gastrointestinal tract, through intact skin, and by inhalation. The
sources of exposure to humans are occupational, accidental or through
diet. Diazinon is used as a pesticide and veterinary drug to control
ectoparasites. The major source of diazinon food residues in edible
crops are from agricultural usage while those in meat, offal and other
animal products arise from veterinary use
The results of total diet studies in the USA, United Kingdom and
New Zealand suggest that levels of exposure are below the recommended
Acceptable Daily Intake (ADI) of 0.002 mg/kg per day (FAO/WHO, 1994b).
Diazinon is rapidly broken down, whether on plants or in animals,
further reducing the risk to humans. Several different application
techniques are used in applying diazinon outside and inside. Residual
spraying and space treatment are often used in controlling pests
indoors. Following recommended use, concentrations of diazinon
detectable in the indoor air are low and do not present a health
hazard. Some studies of agricultural workers have shown cases of
contact dermatitis after exposure to diazinon. Toxicity studies in
humans have shown that the administered daily dose of 0.025 mg/kg body
weight marginally inhibits the plasma cholinesterase activity and it
can be considered as the no-observed-adverse-effect level (NOAEL).
Diazinon is not genotoxic and exhibits no carcinogenic potential in
rats or mice. Several episodes of fatal and non-fatal accidental and
suicidal poisoning have occurred. Acute poisoning causes typical
cholinergic signs and symptoms. Acute pancreatitis can be associated
with severe poisoning.
10.2 Evaluation for effects on the environment
The information on utilization and application rates that has
been employed for this risk assessment is derived from the
agricultural use of diazinon within the European Union. It should be
possible to extrapolate this assessment to other agricultural uses at
similar application rates elsewhere in the world. The application
rates for diazinon can be summarized as follows: arable (tractor-
mounted/drawn hydraulic spray boom applications), 1.0 kg/ha; top fruit
(broadcast air-assisted applications), 1.2 kg/ha.
The following risk assessment is based on the principle of
calculating toxicity-exposure ratios (TERs) (Fig. 2), which follows
the European and Mediterranean Plant Protection Organisation and
Council of Europe (EPPO/CoE) Environmental Risk Assessment Scheme
model and associated trigger values (EPPO/CoE, 1993a,b).
The metabolites formed, i.e. diethylphosphoric acid,
diethylthiophosphoric acid and the derivatives of pyrimidinyl ring,
are eliminated mainly via the kidneys. Only minimal quantities of the
metabolites were detected in milk and eggs.
6.2.1 In vivo metabolic transformations
6.2.1.1 Mice
When male ICR mice (number not stated) were treated orally with
diazinon or [pyrimidine14C] diazinon at 50 or 75 mg/kg body weight,
one half of the high-dose animals died and the rest showed symptoms
(sweating, crouching) (Miyazaki et al., 1970; Sekine, 1972). At the
low dose, no signs of toxicity were observed. Metabolism and excretion
occurred rapidly, and the metabolites diazoxon, O, O-diethyl-
O-[2-(alpha-hydroxyisopropyl)-4-methyl)-6-pyrimidinyl]
phosphorothioate, and O, O-diethyl- O-(2-(2-propenyl)-4-methyl-6-
pyrimidinyl) phosphorothioate were found in the urine 1 h after
treatment. Most of the metabolites were found in urine 6 h after
treatment, but metabolism was not identical in the two dose groups. In
the low-dose group O, O-diethyl- O-(2-isopropyl-4-hydroxymethyl-6-
pyrimidinyl) phosphorothioate and O, O-diethyl- O-(2-isopropyl-4-
formyl-6-pyrimidinyl) phosphorothioate were found, but this was not
observed in the high-dose group. In the high-dose, but not the low-
dose group O, O-diethyl- O-(2-(a-hydroxyethyl)-4-methyl-6-
pyrimidinyl) phosphorothioate was found.
The metabolism of [pyrimidine-14C]-diazinon and [ethoxy-14C]-
diazinon was investigated by Mücke et al. (1970). Four metabolite
fractions were found in urine and faeces, three metabolites
representing approximately 70% of the total radioactivity applied.
Hydrolysis of the ester bond yielded 2-isopropyl-4-methyl-6-
hydroxypyrimidine (22.5% of the applied radioactivity in urine);
oxidation at the primary carbon atom produced 9% of the applied
radioactivity in urine, while oxidation at the tertiary carbon atom of
the isopropyl side chain produced 22%. In addition, trace amounts of
unchanged diazinon were detected in faeces. No cleavage of the
pyrimidine ring with subsequent oxidation of the fragments to CO2
took place (Mücke et al., 1970).
6.2.1.2 Rats
A study by Capps (1989) investigated the diazinon metabolites in
male and female rats orally treated with single doses of 10 and
100 mg/kg [pyrimidine 14C]-diazinon and in rats preconditioned with
14 daily treatments at 10 mg/kg before the final administration of
radiolabelled compound. The metabolite pattern was similar in the
urine and faeces of the rats from all dose groups and from both sexes.
The major urinary metabolites were identified as 2-isopropyl-6-
methyl-4(1 H)- pyrimidinone (average 38.2% of the totally applied
dose), 2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone
(17.3%) and 2-(beta-hydroxyisopropyl-6-methyl-4(1 H)-pyrimidinone
(9.7%). Six unknown aqueous components accounted for an average of
14.9% of the administered dose, and trace amounts of unchanged
diazinon (0.11%), diazoxon (0.14%) and the hydroxy-isopropyl
derivative of diazinon (0.12%) were also detected. The identity of the
metabolites was confirmed by gas chromatography and mass spectrometry
(GC/MS) with synthetic standards.
6.2.1.3 Dogs
The urinary metabolites of Beagle dogs were characterized after
oral administration of 4.0 mg/kg body weight 14C-ring-labelled
diazinon. The metabolite 2-isopropyl-4-methyl-6-hydroxypyrimidine
accounted for 10% of the applied radioactivity in the urine and the
tertiary hydroxy-isopropyl derivative of diazinon represented 23%
(Iverson et al., 1975).
6.2.1.4 Sheep
When two sheep were dermally treated with [pyramidine-14C]-
diazinon, radiolabelled residues were detected in all tissues examined
(heart, liver, kidney, back fat and leg muscle). Unmetabolized
diazinon was the only significant residue in fat, and was a major
residue in heart and leg muscle. The major metabolites in urine and
all tissues except fat were 2-isopropyl-6-methyl-4(1 H)-pyrimidinone
(urine, 10% of the administered radioactivity; liver, 18%; kidney,
23%) and 2-(alpha- hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone
(urine, 22.7%; liver, 10%; kidney, 28%), which were also present in
the form of glucuronide conjugates. The identity of the metabolites
was confirmed by GC/MS with synthetic standards. In addition, several
unidentified polar (urine, 18.6%) and minor amounts of non-polar
(urine, 4.0%) metabolites were detected (Capps, 1990).
6.2.1.5 Goats
Two lactating goats were orally treated with [pyrimidine-14C]-
diazinon in gelatin capsules for four consecutive days. Similarly to
sheep, in urine and faeces the metabolites 2-isopropyl-6-methyl-
4(1 H)-pyrimidinone (urine, 4.5% of the totally administered radio-
activity; faeces, 2.6%) and 2-(alpha-hydroxyisopropyl)-6-methyl-
4(1 H)-pyrimidinone (urine, 12.5%; faeces, 1.7%) were identified.
Approximately 48.6% of the urinary radioactivity consisted of unknown
water-soluble compounds. Characterization of selected tissues showed
the presence of mainly the above-mentioned metabolites. Unchanged
diazinon, its hydroxy-isopropyl derivative and diazoxon accounted for
less than 10% of the radioactivity detected in these tissues.
Metabolites in fat consisted primarily of unchanged diazinon (66%),
its hydroxy-isopropyl derivative (12.5%) and diazoxon (3%). The major
metabolites in the milk were 2-isopropyl-6-methyl-4(1 H)-
pyrimidinone (39.3% of the residual radioactivity) and
2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-pirimidinone (37.3%).
Substantial portions of the polar metabolites in urine, faeces and
tissues were glucuronide conjugates. The identity of the metabolites
was confirmed by GC/MS with synthetic standards (Simoneaux,
1988a,b,e).
6.2.1.6 Hens
Four laying Leghorn hens were treated with [pyrimidine-14C]-
diazinon in gelatin capsules for seven consecutive days at daily doses
of 2.75 mg/kg day. The main metabolites detected in the excreta were
unchanged diazinon (14.9% of the extractable radio-activity),
2-isopropyl-6-methyl-4(1 H)-pyrimidinone (5.9%), 2-(alpha-hydroxy-
isopropyl)-6-methyl-4(1 H)-pyrimidinone (10.8%) and 2-(beta-
hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone (7.2%). Approximately
25% of the radioactivity in the excreta consisted of unknown
water-soluble compounds. The residues in tissues primarily consisted
of 2-isopropyl-6-methyl-4(1 H)-pyrimidinone (0.6-2.6% of the residual
radioactivity), 2-(alpha-hydroxyisopropyl)-6-methyl-4(1 H)-
pyrimidinone (3.1-6.5%) and 2-(beta-hydroxyisopropyl)-6-methyl-
4(1 H)- pyrimidinone (2.0-5.7%). Unchanged diazinon was detected
primarily in the peritoneal fat (2% of residues). In the eggs,
primarily 2-isopropyl-6-methyl-4(1 H)-pyrimidinone (yolk, 11.1% of
the residual radioactivity; white, 9.4%), 2-(alpha-hydroxyisopropyl)-
6-methyl-4(1 H)-pyrimidinone (yolk, 18.6%; white, 33.3%) and
2-(beta-hydroxyisopropyl)-6-methyl-4(1 H)-pyrimidinone (yolk, 7.0%;
white, 35.3%) were detected. As in goats, a substantial portion of the
polar metabolites in tissues, eggs and excreta were glucuronide
conjugates. The identity of the metabolites was confirmed by GC/MS
with synthetic standards (Simoneaux, 1988c,e; Simoneaux, 1989).
More information on kinetics and metabolism in other species is
given in chapter 9.
6.2.2 In vitro metabolic transformations
The metabolism of [ethoxy-14C]-diazinon and diazoxon was studied
in vitro using rat liver cell fractions. It was shown that the
degradation by diazinon is catalysed by a microsomal enzyme that
requires NADPH and oxygen, and is inhibited by carbon monoxide. It is
presumably the cytochrome P-450 oxidase system. Diazoxon was shown to
be degraded by enzymes located in the nuclear, mitochondrial,
microsomal and soluble fractions of the liver. The microsomal enzymes
were the most active and were not dependent on NADPH. Reduced
gluthation had little effect. With diazinon, products of the reactions
were diethylphosphorothioic acid and diethylphosphoric acid. Diazoxon
was degraded to diethylphosphoric acid (Yang et al., 1969, 1971;
Nakatsugawa et al., 1969). These results were confirmed by independent
experiments (Dahm, 1970). The oxidation of diazinon was investigated
by using microsomal preparations from rat liver. The major metabolic
products of diazinon were hydroxydiazinon, diazoxon and
hydroxydiazoxon, which are biologically active, and additional
inactive products such as diethylphosphorothioic acid,
diethylphosophoric acid and derivatives of the pyrimidyl moiety. It
was demonstrated that desulfuration, hydroxylation of the ring alkyl
side-chain and cleavage of the aryl phosphate bond may occur,
depending on the presence of NADPH or NADH. EDTA stimulated the
overall metabolism of diazinon (Shishido et al., 1972a).
The enzymatic hydrolysis of diazoxon was investigated using rat
tissue homogenates. The hydrolytic activity of the tissues decreased
in the order liver>blood>lung>heart>kidney>brain. In the liver,
the hydrolytic activity was localized in microsomal preparations.
Diethyl phosphoric acid and 2-isopropyl-4-methyl-6-hydroxypyrimidine
were identified as the products. The reactions were inhibited by EDTA,
heavy and rare earth metal ions, and sulfhydryl reagents (L-cysteine,
2-mercaptoethanol, thioglycolic acid), while calcium ions activated
the hydrolysis (Shishido & Fukami, 1972).
Liver homogenates were prepared from male mice (North Carolina
Department of Health strain) and incubated for 1 h with either
14C-diazinon or 14C-diazoxon. Inhibition of metabolism was
studied by co-incubation with piperonyl butoxide, NIA 16824 or
1-(2-isopropylphenyl) imidazole. Diazoxon formation from diazinon
(thiophosphate to phosphate conversion) was inhibited by 45 to 60% by
the inhibitors studied. All the inhibitors also reduced oxidative
dearylation of diazinon to diethyl phosphoric and diethyl
phosphorothioic acids (Smith et al., 1974).
Conjugation with glutathione forms the third enzymatic mechanism
of the diazinon metabolism in rat tissue preparations (liver, heart,
brain, lung, kidney and blood were investigated). The highest activity
(14-89 times as high as in other tissues) for this reaction was
localized in the cytoplasmatic fractions of the liver. The reaction
products were identified as diethyl phosphorothioic acid and
S-(2-isopropyl-4-methyl-6-hydroxypyrimidinyl) glutathione, which were
formed by conjugation and simultaneous cleavage of the phosphate ester
bond. The enzymatic activity was increased by the addition of
glutathione-SH, and was inhibited by various sulfhydryl reagents,
oxidized glutathione and some chelating agents ( o-phenanthroline,
8-hydroxyquinoline) (Shishido et al., 1972b).
6.3 Metabolic aspects of diazinon toxicity
Diazinon was incubated with liver microsomes and liver slices
from sheep, cow, pig, guinea-pig, rat, turkey, chicken and ducks.
Hydroxydiazinon, isohydroxydiazinon, dehydrodiazinon, their oxons
and diazoxon were identified and determined quantitatively or
semi-quantitatively. It was shown that yields and rates of production
of the metabolites varied greatly between the species. The production
of the oxon was not generally correlated with susceptibility to
diazinon poisoning, although it was lowest in the least susceptible
animal, the sheep. The highly susceptible avian species (acute oral
LD50 of around 2-15 mg/kg) do not produce higher rates of oxons than
rat or pig (acute oral LD50 around 300-600 mg/kg). However, the
mammalian blood hydrolyses diazoxon rapidly, whereas the avian species
have virtually no hydrolytic activity. It was concluded that
extrahepatic metabolism of diazinon, in particular the hydrolysis of
diazoxon in the blood, appears to be the main factor affecting
susceptibility to diazinon poisoning. In mammals the extrahepatic
metabolism of diazinon is more important toxicologically than the
metabolism in the liver, while the liver is probably the most
important site of metabolism in avian species (Machin et al., 1975).
Recently, the hydrolytic metabolism of diazinon by plasma was
investigated in 92 individuals of Hispanic origin (Davies et al.,
1996). Diazoxon is hydrolysed by the enzyme paraoxonase (PON1),
leading to the formation of 2-isopropyl-4-methyl-6-hydroxypyrimidine
and diethylphosphate. An important observation of this study was that
the effect of the PON1 polymorphism for diazoxon hydrolysis relative
to paraoxon hydrolysis was reversed. Thus, RR individuals (Arg192
homozygotes) who displayed high paraoxonase activity had lower
diazonoxonase activity (mean = 7948 U/litre) than QQ homozygotes
(12 318 U/litre).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
Improvements since 1979 in the manufacturing of diazinon have
significantly reduced the content of highly toxic by-products, in
particular tetraethyl-pyrophosphate (TEPP). As a result of these
stepwise improvements, the acute oral LD50 of technical grade
diazinon increased to values around 1000 mg/kg (Piccirillo, 1978;
Bathe & Gfeller, 1980; Schoch & Gfeller, 1985; Kuhn, 1989a). The most
recent study resulted in an oral LD50 in rats of 1250 mg/kg. The LD50
values for different species are summarized in Table 2.
Signs of poisoning after a single dose of diazinon are typical of
organophosphate intoxication and include decrease of spontaneous
activity, sedation, dyspnoea, ataxia, tremors, muscle spasms,
convulsions, lacrimation and diarrhoea. The signs were found to be
reversible in surviving animals.
Diazinon (88% purity) was administered by gavage to 15 rats per
sex and dose at single doses of 0, 2.5, 150, 300 and 600 mg/kg
(Potrepka, 1994). Three, 9 or 24 h after dosing, five animals of each
group were bled for determination of serum and red blood cell
cholinesterase activity and then killed for determination of CNS
cholinesterase activity. There was no mortality. Signs of cholinergic
poisoning, e.g., salivation, diarrhoea and muscle fasciculations, were
observed in animals of both sexes dosed at 300 and 600 mg/kg. Clinical
signs first appeared 3 h after treatment. Maximum observable toxicity
was noted 9 h after treatment in males and 24 h after treatment in
females. Serum cholinesterase activity was significantly decreased at
all time points in all groups treated with 2.5 mg/kg or more. Maximum
inhibition was observed 9 h after dosing, and values remained
depressed at 24 h after dosing. Red blood cell cholinesterase activity
was significantly inhibited at all time intervals in animals of both
sexes treated with >150 mg/kg. Again, maximum inhibition was
observed 9 h after dosing, and activity remained depressed at 24 h
after dosing. In addition, a significant inhibition of red blood cell
cholinesterase activity was noted in females dosed with 2.5 mg/kg
diazinon 9 h after dosing. Cerebellum, cerebral cortex, striatum,
hippocampus and thoracic spinal cord cholinesterase activities were
decreased in female rats dosed with >150 mg/kg at all three time
points. Cerebellum, striatum and hippocampus cholinesterase activities
were decreased in male rats dosed with >150 mg/kg at all three time
intervals, whereas cerebral cortex and thoracic spinal cord
cholinesterase activities were decreased in male rats dosed with
>150 mg/kg at 9 and 24 h after dosing. The NOAEL for inhibition of
brain cholinesterase activity was 2.5 mg/kg for both sexes (Potrepka,
1994).
Table 2. Acute toxicity of diazinona
Species Sex Route of administration LD50 (mg/kg) Reference
Rat male oral 235 Gasser (1953)
Rat male oral 435 Shaffer & West (1960)
Rat male oral 250 Gaines (1969)
Rat female oral 285 Gaines (1969)
Rat both oral 300 Piccirillo (1978)
Rat both oral 422 Bathe & Gfeller (1980)
Rat both oral 1012 Schoch & Gfeller (1985)
Rat both oral 1250 Kuhn (1989a)
Rat male dermal 900 Gaines (1960)
Rat female dermal 455 Gaines (1960)
Rat both dermal >2150 Bathe (1972a)
Rat both inhalation >2300b Holbert (1989)
Mounse male oral 82 Bruce et al. (1955)
Mounse both oral 96 Gasser (1953)
Mounse both oral 187 Bathe (1972b)
Mounse both i.p. 65 Klotzsche (1955)
Guinea-pig oral 320 Gasser (1953)
Rabbit oral 130 Gasser (1953)
Rabbit both dermal >2020 Kuhn (1989b)
Turkey oral 6.8 FAO/WHO (1965)
Chicken oral 40.8 FAO/WHO (1965)
Goose oral 14.7 FAO/WHO (1965)
Gosling oral 2.8 Egyed et al. (1974)
a It should be noted that progressive improvement in the manufacturing process has reduced
the acute toxicity of diazinon.
b This value is the LC50, the units being mg/m3.
Brain cholinesterase activities were determined for white-footed
mice (Peromyscus leucopus) orally dosed with diazinon at 18.8 mg/kg
body weight (Montz & Kirkpatrick, 1985). Following treatment with
diazinon, a latent period of approximately 6 h elapsed during which
time acetylcholinesterase activity was relatively unaffected. After
the latent phase, ChE activity rapidly declined to a minimum 12 h
after dosing. After 48 h, ChE activity recovered to a level only
slightly below that of the controls. The response of both male and
female mouse brain ChE activities declined rapidly at 6 h and reached
a minimum 24 h after dosing. ChE activity of treated animals was
comparable to that of controls 48 h after treatment. Brain ChE
activities of treated female mice were significantly lower (P <0.05)
than those of treated males.
An acute oral toxicity study in rats was conduced in two phases
to determine a NOEL of the test material (87.9% active ingredient) for
clinical, behavioural and body weight effects in phase 1 and a NOEL
for effects on plasma, red blood cell and brain cholinesterase in
phase 2 after single oral gavage application to rats. In phase 1 each
test group consisted of 5 rats. The applied dose levels were 25 and
50 mg/kg body weight for female rats only; levels of 100, 250 and
500 mg/kg body weight were tested in both sexes. One female given
500 mg/kg body weight died. Clinical signs such as miosis,
hypoactivity, absence of pain reflex, red-stained face, yellow-stained
abdomen/urogenital area, soft stool or few faeces were seen in all
animals except 100- mg/kg males and 25-mg/kg females. The NOAEL for
clinical/behavioural/body weight effects was 100 mg/kg body weight in
males and 25 mg/kg body weight in females. In phase 2 each test group
consisted of 5 rats. Males were treated with 0.05, 0.50. 1.0, 10.0,
100 or 500 mg/kg body weight and females with 0.05, 0.12, 0.25, 2.5,
25 and 250 mg/kg body weight. Clinical signs such as miosis,
hypoactivity, absence of pain reflexes, staggered gait, excessive
salivation, red-stained face and yellow-stained and/or wet
ventral/urogenital area were only observed in males at 500 mg/kg body
weight (one male at 100 mg/kg body weight showed miosis) and females
at 250 mg/kg body weight The only findings at necropsy were yellow
staining of the perineum and red paranasal discharge in animals of
these dose groups. Plasma cholinesterase activity was significantly
reduced in males dosed with 10, 100 or 500 mg/kg body weight and in
females dosed with 2.5, 25 or 250 mg/kg body weight. Red blood cell
cholinesterase activity was significantly lower in males given 100 or
500 mg/kg body weight and in females given 25 or 250 mg/kg body
weight. Brain cholinesterase activity was significantly reduced at the
highest dose level for both sexes. Brain cholinesterase activity in
females dosed with 25 mg/kg body weight was inhibited by 37% (without
reaching statistical significance) (Glaza, 1993).
7.1.2 Dermal
Diazinon was dispersed on the shaved back of each of three male
and three female Tif: RAI rats at a level of 2150 mg/kg and covered
with aluminium foil for 24 h. None of the animals died. Neither
clinical signs nor dermal irritation was observed. Autopsy revealed no
substance-related gross organ changes. The acute dermal LD50 in rats
was found to be greater than 2150 mg/kg (Bathe, 1972a). Sherman strain
rats were given one dermal application of diazinon dissolved in
xylene, and no attempt was made to remove the compound during the
observation time of 14 days. The LD50 was 900 mg/kg for males and
455 mg/kg for females (Gaines, 1960).
Dermal LD50 values for diazinon were determined in mice after
application of the solution to hind feet. Values were simultaneously
generated for the ED50 for both cholinesterases (acetylcholinesterase
and pseudocholinesterase). LD50 values were higher than those
reported for mice treated on shaved back skin. Diazinon appeared to
have much more inhibitory potential for blood than for nervous tissue
cholinesterase (Skinner & Kilgore, 1982).
Undiluted diazinon was applied to the clipped dorsal trunk of
each of five male and five female New Zealand White rabbits at a level
of 2020 mg/kg and kept under semi-occlusive dressing for 24 h. Signs
were typical of organophosphate intoxication; two out of ten animals
died. The LD50 was >2020 mg/kg (Kuhn, 1989b,c).
7.1.3 Inhalation
Sprague-Dawley rats were exposed (whole body exposure) to
diazinon for 4 h. The acute inhalation LC50 for diazinon MG 8
FL880045 was greater than 2330 mg/m3 when it was administered
undiluted as an aerosol (Holbert, 1989).
Groups of five male and five female HSD:SD rats were exposed to
an aerosol generated from undiluted liquid diazinon MG87% (88% purity)
for 4 h (nose-only exposure). An exposure concentration of 5540 mg/m3
was obtained, 8.82% of particles being smaller than 1 mm in diameter.
There was no mortality. Prominent in-life observations included
activity decrease, piloerection and polyuria, no longer seen by day 6.
Body weight gain was largely unaffected. Gross necropsy revealed
discoloration of the lungs in all animals. The acute inhalation LC50
for diazinon in the rat was greater than 5440 mg/m3 (Holbert, 1994).
7.1.4 Intraperitoneal
Mild structural and functional changes were observed in the liver
and testes of rats after a single intraperitoneal administration of
diazinon (21.6 mg/kg). Kidney, however, showed no pathological
lesions. Attempts were made to correlate the pathological changes in
these organs with the activity of succinic dehydrogenase, adenosine
triphosphatase and alkaline phosphatase (Dikshith et al., 1975). A
single intraperitoneal dose of diazinon caused hyperglycaemia in rats,
which peaked 2 h after intraperitoneal treatment with 40 mg
diazinon/kg. The brain acetylcholinesterase activity was significantly
reduced. The blood level of pyruvic acid was unchanged while that of
lactic acid was significantly increased. In diazinon-treated
hyperglycaemic animals, the glycogen content of the brain was
depleted, the activities of glycogen phosphorylase, phosphoglucomutase
and hexokinase were significantly increased, and the activity of
glucose-6-phosphatase remained unchanged. Lactate dehydrogenase
activity was increased by treatment with diazinon. The induced changes
may have compensated for the energy requirement of stimulatory effects
caused by the pesticide (Husain & Matin, 1986; Matin & Husain, 1987;
Matin et al.,1989). Changes in energy metabolism are regarded as
secondary, following cholinesterase inhibition.
In an acute intraperitoneal study in rats, an LD50 value of
260 mg/kg body weight was determined for both sexes. Clinical signs
were typical of organophosphate poisoning, e.g., sedation, dyspnoea
and tonic-clonic spasms. Surviving animals had recovered within 3 to 6
days, and no substance-related gross organ changes were observed
(Bathe, 1973).
Diazinon given as an intraperitoneal single dose (40 mg/kg) to
rats produced tremors and convulsions with lactic acidosis. This was
accompanied by depletion of glycogen phosphorylase activity in the
triceps and diaphragm muscles 2 h after the administration (Husain &
Matin, 1986; Husain & Ansari, 1988).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rats
Davies & Holub (1980a,b) found that diazinon (99.2% purity) fed
to rats for 4 weeks at doses up to 25 mg/kg diet produced no visible
toxic manifestations such as tremors or hyperexcitability. Feeding
diazinon 25 mg/kg diet for 30 days produced more significant
reduction of cholinesterase activity in plasma (by 22-30%) and brain
(by 5-9%) among treated females than among males. Erythrocyte
acetylcholinesterase activity was significantly more depressed (by
13-17%) in treated females than in males at days 21-28 of the feeding
trial. The greater degree of cholinesterase inhibition in females was
possibly attributable to the higher amount of diazinon ingested by
females than by males after day 15 of the study.
Female Wistar rats were fed a semi-purified diet containing
either no pesticide or 0.1 to 15 mg/kg diazinon for up to 92 days. At
specified times, blood samples were taken to measure plasma and
erythrocyte cholinesterase activity using a radiometric method.
Additional rats were killed to determine brain cholinesterase
activity. Measurements included body weight gain and feed consumption
during the growing period. Feeding diazinon at the stated levels
produced no visible toxic manifestations. Treated animals showed
weight gain and feed consumption that was comparable to controls.
Feeding trials lasting up to 90 days revealed that rats were highly
sensitive to diazinon after 31 to 35 days exposure, as judged by
reduction of plasma and erythrocyte cholinesterase activities. Brain
acetylcholinesterase was found to be practically insensitive to
dietary intake of diazinon (1.0 to 15 mg/kg), although moderate
reduction (by 6%) of brain enzyme activity was noted among animals fed
10 mg/kg diet at day 92. For all feeding trials, plasma cholinesterase
was a more sensitive indicator of diazinon exposure than erythrocyte
or brain acetylcholinesterase. The NOAEL of diazinon for the rat was
estimated to be 0.1 mg/kg diet, which is equivalent to a daily intake
of 0.009 mg/kg body weight per day (Davies & Holub, 1980a).
In a feeding study, diazinon (87.7% pure) was administered to
groups of 15 male and 15 female Sprague Dawley rats for 13 weeks at
dietary concentrations of 0, 0.5, 5, 250 and 2500 mg/kg, corresponding
to mean daily doses of 0, 0.03, 0.3, 15 and 168 mg/kg body weight in
males and 0, 0.04, 0.4, 19 and 212 mg/kg body weight in females,
respectively. The rats treated at the highest dose level showed
hypersensitivity to touch and sound and some of the males showed
apparent aggressiveness, hyperactivity and soft faeces. Body weight
gain and food consumption were reduced in both sexes. At this dose
level, the haematological examination revealed a decreased haemoglobin
level, a lower haematocrit and an increased number of reticulocytes in
the females. The examination of blood biochemistry revealed a reduced
activity of serum cholinesterase in both sexes treated with 5 mg/kg
diet or more. In addition, the low-dose females showed a 17% reduction
in the erythrocyte cholinesterase activity. At 250 mg/kg diet or more,
the erythrocyte and brain cholinesterase activities were reduced in
both sexes. The absolute and relative liver weights were increased in
both sexes in the highest dose level, and there was microscopic
evidence of centrilobular hepatocellular hypertrophy (Singh et al.,
1988). The NOAEL, based on reduction in brain cholinesterase activity,
was 5 mg/kg diet (equivalent to 0.4 mg/kg body weight per day).
In a second, similar, study (Pettersen & Morrissey, 1994),
Sprague Dawley rats were fed diazinon at 0, 0.3, 30, 300 or 3000 mg/kg
diet for 13 weeks. Serum, erythrocyte and regional brain
cholinesterase inhibition was measured in groups of five males and
five females at weeks 4, 8 and 13. Serum and erythrocyte
cholinesterases were inhibited by 45-86 and 60-75%, respectively, at
30 mg/kg diet, but not consistently at 0.3 mg/kg diet. Brain regional
cholinesterase was inhibited by up to 25% in females at 30 mg/kg diet
and by 55-75% at 300 mg/kg diet. Males were less sensitive, showing no
effect at 30 mg/kg diet and a fall of 62-77% at 3000 mg/kg diet.
Observable neuromuscular deficits were seen only at 3000 mg/kg diet.
Consequently the NOEL and NOAEL for this study was 0.3 mg/kg diet
(equivalent to 0.019 mg/kg body weight per day), based on the
reductions in serum, erythrocyte and brain cholinesterase seen at the
next highest dose of 30 mg/kg diet. However, given the lack of an
intermediate dietary level of 5 mg/kg, which set the NOAEL in the
Singh et al. (1988) study, and the modest fall in brain cholinesterase
seen at 30 mg/kg diet in this second study, it is concluded that the
previous NOAEL of 5 mg/kg diet (0.4 mg/kg body weight per day) should
stand.
The effect of low levels of diazinon treatment on four marker
enzymes in rat heart and skeletal muscles have been investigated by
Wilkinson et al. (1986). Typical differences in succinate
dehydrogenase (SDH), lactate dehydrogenase (LDH), phosphofructokinase
(PFK) and hexokinase (HK) activities were observed between heart and
skeletal muscles. Diazinon feeding had no effect on heart, soleus,
gastrocnemius and plantaris SDH, LDH or PKF enzyme activities after
28 weeks. HK activity was significantly increased in sham-control
soleus and plantaris muscle after 28 weeks. Diazinon feeding inhibited
HK activity in plantaris muscle after 28 weeks treatment. These
results demonstrate that chronic low levels of diazinon have little
effect on the glycolytic and oxidative activity in heart and skeletal
muscles.
Adult Wistar rats were given diazinon by gavage twice weekly at a
dose of 0.5 mg/kg body weight for twenty-eight weeks. Selected control
and experimental animals were killed after 7, 14 and 28 weeks.
Histological examination of the liver revealed that animals repeatedly
treated with sublethal doses of diazinon sustain a form of hepatic
injury characterized by cellular lipid accumulation. The finding of
increased lipid accumulation in the liver following prolonged
treatment with diazinon, however, still does not resolve the question
of whether impaired lipid metabolism and/or storage is the primary
effect (Anthony et al., 1986).
Rats were exposed to diazinon-impregnated strips in a
conventional laboratory animal room. The air in the room was monitored
for the pesticide. Erythrocyte and plasma cholinesterase activities
were determined periodically. Air concentration of the pesticide never
exceeded 1.34 mg/m3. No significant change in enzyme activities were
observed (Hinkle et al., 1980).
7.2.1.2 Dogs
Feeding studies with dogs (Bruce et al., 1955; Williams et al.,
1959) did not differentiate between the sexes and therefore relevant
data from male and female animals were pooled. In another study
(Barnes et al., 1988) diazinon (87% pure) was given to groups of four
male and four female Beagle dogs for 13 weeks at concentrations of 0,
0.1, 0.5, 150 and 300 mg/kg diet, corresponding approximately to a
mean daily intake of 0.0034, 0.02, 5.9 and 10.9 mg/kg body weight
respectively. Vomiting was observed in groups fed a diet containing
150 or 300 mg/kg. Serum cholinesterase activity was reduced at
0.5 mg/kg or more in males and at 150 and 300 mg/kg in females.
Erythrocyte and brain cholinesterases activity was depressed at 150
and 300 mg/kg in both sexes. The histopathological examination
revealed a moderate atrophy of pancreatic acini in one high-dose group
of male dogs. Body weight gain was decreased in females at 150 mg/kg
diet and in both sexes at 300 mg/kg diet. The serum calcium level was
decreased in females at 150 mg/kg diet and in males at 150 and
300 mg/kg diet. The serum albumin level was decreased in both sexes at
300 mg/kg diet. As a slight reduction of serum cholinesterase activity
was the only change observed, 0.5 mg/kg diet, corresponding to a mean
diazinon intake of 0.02 mg/kg body weight per day, is considered to be
the dietary concentration causing no toxicological effect (NOAEL),
based on inhibition of brain and erythrocyte cholinesterase at higher
doses.
7.2.1.3 Pigs
Pigs were orally administered diazinon in capsules at doses of
0, 1.25, 2.5, 5 and 10 mg/kg body weight daily for periods of up to
8 months. In pigs, mortality and cholinergic signs of poisoning were
evident at 2.5 mg/kg body weight per day (FAO/WHO, 1979).
7.2.2 Inhalation
Two short-term inhalation studies were conducted in rats. In the
first study, diazinon (97.1% pure) was administered in an inhalation
chamber to four groups of nine male and female Tif RAIf rats for 6 h
per day, 5 days a week, for three weeks, at concentrations of 0, 151,
245 and 559 mg/m3. Four animals of each sex from the control group
and from the highest concentration group were kept for a 25-day post-
treatment observation period, while the others were sacrificed at day
21 after treatment. No compound-related deaths occurred. Exophthalmus
and diarrhoea were observed at all dose levels. In addition, the high-
dose group animals showed salivation, ruffled fur and tonic-clonic
convulsions during 2 h after each exposure. No toxic signs occurred in
the 25-day follow-up period. Food consumption was reduced during the
first three days of exposure in the high-dose group, plasma
cholinesterase activity was reduced in the intermediate and high-dose
groups, and brain cholinesterase activity was reduced at all dose
levels. Erythrocyte cholinesterase activity was reduced in the high-
dose group. The changes were reversible. There were no macro or
histopathological findings related to the exposure to diazinon (Zak et
al., 1973).
In the second study the main purpose was the definition of a
NOAEL for cholinesterase inhibition. Groups of ten male and ten female
Tif RAlf rats were exposed to diazinon 6 h a day for 5 days per week
for 3 weeks. The effective concentrations at the inhalation site
were 0, 0.05, 0.46, 1.57 and 11.6 mg/m3 air. There were no
compound-related signs, and no changes in body weight or food
consumption. In comparison with the control animals, the number of
erythrocytes, haemoglobin level and packed cell volume were slightly
lower in the highest-dose group. A minor decrease of brain
cholinesterase activity was noted in the highest-dose group of
females. Plasma glucose levels were significantly reduced among males
exposed to 1.57 and 11.6 mg/m3 (Hartmann, 1990). As the exposure to
0.46 mg/m3 inhibited the plasma cholinesterase only, this
concentration is the NOAEL.
7.2.3 Dermal
7.2.3.1 Rabbits
Diazinon (97.1% pure) was suspended in 50% aqueous polyethylene
glycol 300 and topically administered under semi-occlusive dressing to
groups of five male and five female albino rabbits at daily doses of
0, 1, 5 and 100 mg/kg body weight for 5 days per week for 3 weeks. In
the highest-dose group, four males treated at 100 mg/kg died during
the first week of treatment. Consequently, the dose was reduced to
50 mg/kg. Clinical signs were observed in the highest-dose group and
included anorexia, ataxia, fasciculations, tremors, diarrhoea,
hypoactivity, hypotonia and salivation. Most of the signs disappeared
after the dose was reduced. Mild dermal reactions were noted at site
of test substance administration. Body weight gain and food
consumption was similar in all groups, and most laboratory parameters
remained unaffected by the treatment. Reduced cholinesterase
activities were found in serum, red blood cells and the brain in
animals treated at >5 mg/kg. In the highest-dose group, the
reductions were significant for brain, red blood cells and serum
cholinesterase activities, while with 5 mg/kg there was a
statistically significant decrease of activity in the brain
cholinesterase of females only. In the highest-dose animals, the
histopathological examination showed a slight hyperkeratosis of the
skin at the site of treatment (Tai & Katz, 1984). The NOEL was
considered to be 1 mg/kg, based on inhibition of brain cholinesterase.
7.3 Long-term exposure
7.3.1 Rats
Groups of 30 or 40 Sprague Dawley male and female rats received
diazinon (87.7% pure) at dietary concentrations of 0, 0.1, 1.5, 125
and 250 mg/kg (equivalent to a mean daily diazinon intake of 0, 0.004,
0.06, 5 and 10 mg/kg body weight in males and of 0.005, 0.07, 6 and
12 mg/kg body weight in females) for 99 weeks. An additional control
group received 26.5 mg/kg diet epoxidized soybean oil, the stabilizer
used in technical diazinon, at the concentration corresponding to the
application to the test group of animals. Eight to ten randomly
selected animals per sex and dose group were killed after one year of
treatment, and nine or ten animals from the control group and from the
highest-dose group were killed after 4 weeks of recovery at week 56.
There were no compound-related clinical signs. At 250 mg/kg diet, the
mean body weight and the food consumption were slightly, but
significantly, increased in both sexes. Serum cholinesterase
activities were reduced at >1.5 mg/kg diet. Red blood cell and
brain cholinesterase activities were inhibited in groups fed on a diet
containing 125 or 250 mg/kg. Cholinesterase inhibition was at least
partially reversible during the 4-week recovery period after one year
of treatment. No treatment-related changes were seen during pathology
or histology examination (Kirchner et al., 1991). A pathological
re-evaluation of eyes and optic nerves from all control and high-dose
animals of the above study from interim necropsy (week 52), interim
recovery necropsy (week 56) and chronic necropsy (week 98/99) was
conducted. Histopathological lesions like cataracts, inflammatory
changes of the globe and surface of the eye and several different
types of retinal atrophy were found in both control and treated
animals at final sacrifice. None of these findings had an increased
incidence in treated rats compared to controls and they were not
regarded as compound-related. As the dietary concentration of
1.5 mg diazinon/kg, equivalent to a mean intake of 0.06 mg/kg body
weight per day, inhibited serum cholinesterase only, this dose level
was considered to be the NOAEL (Mann, 1993).
7.3.2 Dogs
Groups of Beagle dogs (four males, four females) received
diazinon (87.7% pure) for 52 weeks at dietary concentrations of 0,
0.1, 0.5, 150 and 300 mg/kg (equivalent to a mean daily intake of
0.0032, 0.015, 4.7 and 7.7 mg/kg body weight for males and 0.0037,
0.02, 4.5 and 9.1 mg/kg body weight for females). Owing to a general
lack of body weight gain, the highest dose level was reduced from
300 mg/kg diet to 225 mg/kg diet after 14 weeks of treatment. One male
dog from the highest-dose group showed emaciation and dehydration due
to severely reduced food consumption and weight reduction. In the
other animals from the highest-dose group (300 mg/kg/diet) the body
weight gain was also severely depressed during the initial 14 weeks of
treatment. After the reduction of dose to 225 mg/kg/diet, body weight
gain remained depressed in two of the males, while the females
returned to normal. A slightly reduced body weight gain was also noted
among males at 150 mg/kg diet. Food consumption was reduced in both
sexes fed on a diet containing >150 mg/kg diazinon. Haematology and
urine analysis showed no treatment-related changes. Significantly
decreased cholinesterase activities were noted at >0.5 mg/kg diet.
Serum cholinesterase activity was inhibited at 150 mg/kg diet at
all sampling intervals and at several occasions at 0.5 mg/kg diet. Red
blood cell and brain cholinesterase activities were reduced at
>150 mg/kg diet. In addition, a slight reduction in the mean serum
amylase activity was noted in both sexes fed a diet of >150 mg/kg.
The organ weight analysis showed no treatment-related changes and
macro- and histopathology were unremarkable (Rudzki et al., 1991). A
re-evaluation of eyes and optic nerves from all control and high-dose
animals from the study was conducted. No histopathological findings in
the eyes were noted (Mann, 1993). The dietary diazinon concentration
of 0.5 mg/kg, equivalent to a mean daily diazinon intake of
0.015 mg/kg, inhibited serum cholinesterase activity only, and was
considered to be the NOAEL, based on inhibition of brain and
erythrocyte cholinesterase.
7.3.3 Rhesus monkeys
This study was conducted with a 50% WP diazinon formulation
containing an actual concentration of 48.6% diazinon. The dose levels
given below refer to the active ingredient. Groups of three male and
three female Rhesus monkeys received initial daily doses of 0, 0.1,
1.0 and 10.0 mg diazinon/kg body weight, administered by gastric
intubation. After 34 days the doses were lowered to 0.05, 0.5 and
5.0 mg/kg and after 106 weeks of treatment the study was terminated.
Mortality was similar in all dose groups: the death of one animal
at each dose was of infectious etiology. Clinical signs included
tremor in the highest-dose group animals and an increased incidence
of soft stools was noted at 1.0 and 10.0 mg/kg. In comparison to
the controls, all treated animals gained slightly less weight.
Treatment-related deviations in the laboratory parameters examined
were limited to reductions of cholinesterase activities. At the
0.5 mg/kg dose level, the erythrocyte cholinesterase activity was
occasionally reduced in some animals and plasma cholinesterase
activity was consistently depressed. At the highest-dose level, plasma
and erythrocyte cholinesterase activities were markedly inhibited and
the brain cholinesterase activity was reduced in one monkey. The
postmortem examinations revealed no changes of toxicological
relevance. The daily dose of 0.5 mg diazinon/kg inhibited plasma and
(occasionally slightly) erythrocyte cholinesterase activity. The
toxicologically relevant brain cholinesterase activity remained
unaffected. Therefore, this dose level was considered to be the NOAEL,
based on inhibition of erythrocyte cholinesterase (Cockrell et al.,
1966).
7.4 Skin and eye irritation; sensitization
7.4.1 Primary skin irritation
Diazinon (0.5 ml undiluted, technical material) was applied to
the shaved skin of three male and three female New Zealand white
rabbits and covered with gauze patches and an impermeable material.
The dressing was removed after 4 h. Slight erythema and minimal oedema
were seen in all rabbits, which disappeared within 1 week. In the
report the compound received the descriptive rating as "slightly
irritant" to rabbit skin (Kuhn, 1989b,c).
7.4.2 Primary eye irritation
Diazinon (0.1 ml undiluted technical material) was instilled into
the conjunctival sac of three male and three female New Zealand white
rabbits. In three additional female rabbits, the treated eyes were
washed for 1´ min after the instillation of diazinon. Diazinon
produced mildly irritating reactions of the conjunctivae, which
disappeared within 72 h. It was rated as minimally irritating in
washed and unwashed eyes of rabbits (Kuhn, 1989d).
7.4.3 Skin sensitization
Diazinon was examined for skin sensitizing effects in 10 Hartley
Albino guinea-pigs. Ten animals were initially treated with 0.5 ml
undiluted technical diazinon, applied to the shaved skin under
semi-occlusive dressing for 6 h. After the third treatment one animal
died, most probably due to intoxication. Therefore, further treatments
were conducted with a 10% solution of diazinon in ethanol. After 11
treatments during the 3-week induction period and a 2-week rest phase,
no sensitization resulted after a single challenge administration
(Kuhn, 1989e).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
7.5.1.1 Rat
Diazinon (94.9% pure) was administered in the feed to groups of
30 male and 30 female Sprague Dawley rats for 10 weeks prior to
mating, throughout mating of the F0 animals and during two
generations up to weaning and sacrifice of the F2 pups. The dietary
concentrations used were 0, 10, 100 and 500 mg/kg. Compound-related
clinical signs and mortalities were limited to a few parental females
treated with 500 mg/kg diet. The food consumption was generally
comparable to, or slightly higher than, control values for females of
both parental generations in the diazinon-treated groups, while the
F1 males showed a decreased food consumption during the premating
period at both 100 and 500 mg/kg diet. The body weight increase was
reduced at 500 mg/kg in the F0 females and at 100 and 500 mg/kg for
the F1 animals of both sexes. There were no remarkable gross or
microscopic findings or effects on organ weights in either the F0 or
F1 generation. Reproductive parameters including precoital interval,
gestation duration, mating, fertility and pregnancy indices were
unaffected by the treatment in both generations at the dose levels of
10 and 100 mg/kg diet. At 500 mg/kg there was an increase in the
proportion of dams with prolonged gestation in both generations, and
in the F1 animals there was a trend toward a decrease in the number
of pregnancies and viable newborns and adverse effects on fertility
indices. Mating behaviour was unaffected by treatment. Litter size on
lactation day 0 was decreased in both the F1 and F2 pups at
500 mg/kg, whereas pup weight and sex ratio on day 0 were comparable
to controls for both generations. Decreases in pup survival and
corresponding decreases in pup weight were observed in both
generations at 500 mg/kg and in the F1 pups at 100 mg/kg.
Compound-related clinical and necropsy observations were noted in some
F1 pups at 500 mg/kg (tremor, no milk in stomach). NOAEL was 10 mg/kg
diet for pups and parental animals (Giknis, 1989).
7.5.1.2 Cattle
As a part of a survey to determine causes of abortion in
Wisconsin dairy cattle, the possible role of pesticides was examined.
Of 31 aborted fetuses examined, none contained traces of diazinon. Two
gravid, non-lactating Holstein Friesian cows were orally treated in
their feed with diazinon (Diazinon W 50 wettable powder formulation)
at daily doses of 6.6 mg/kg body weight during their second semester
of gestation until term. The calves were killed after parturition and
samples of perirenal fat, liver and kidney were investigated for
residues of diazinon, as were samples of colostral milk from the cows.
A histopathological examination of the calves, livers and kidneys was
conducted. Both cows treated with diazinon vomited and refused feed on
day 20 of treatment. No abortions occurred and no gross or
histopathological changes were found in the calves. No diazinon
residues were detected in the tissues investigated or in the milk
(Macklin & Ribelin, 1971).
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 Mice
Groups of 19-22 mated female F2 hybrid mice were orally treated
with diazinon (admixed to peanut butter, purity not specified) at
daily doses of 0, 0.18 and 9 mg/kg body weight from the day of mating
until delivery. The dams were weighed daily. After delivery, the
physiological and behavioural development of the pups was examined.
Mothers of all groups gave birth to viable offspring. Litter size and
the birth weights of the pups were similar in treated and untreated
groups. In the group receiving the higher dose of diazinon, the body
weight development of pups was depressed during their first postnatal
week. According to the authors, the testing of physiological and
behavioural development revealed subtle deviations from the normal
development in the offspring from the treated females. After sacrifice
at 101 days of age, the brains of the offspring of mothers treated at
the high-dose level showed ambiguous neuropathological changes in the
forebrains (Spyker & Avery, 1977). This study raises questions that
cannot be answered from the data presented.
7.5.2.2 Rats
Diazinon (purity 95%) was administered orally by gavage to groups
of 28 to 30 pregnant Sprague-Dawley-derived rats on days 6 to 15 of
gestation at dose levels of 0, 15, 50 and 100 mg/kg body weight On day
21 of gestation, all dams were killed and the fetuses delivered by
cesarean section. The dams of the 100 mg/kg group reacted to the
treatment by a marked decrease in food consumption and a body weight
loss in the early administration phase. The dams of the 15 and
50 mg/kg group showed no reaction. The parameters of reproduction
(number of corpora lutea, implantations, resorptions, fetal deaths and
viable fetuses) showed no treatment-related intergroup differences.
The fetal body weights were similar in all groups and the examination
of the offspring did not reveal any teratogenic effects of the
treatment. The NOAEL was 50 mg/kg body weight (Fritz, 1974).
In rats, doses (95.2 mg/kg body weight on day 9) that increased
maternal mortality reduced fetal development, as indicated by reduced
weight of litters and mild "hydronephrosis", but caused no real
teratogenic effect (Dobbins, 1967). A similar result was reported for
an intraperitoneal dosage of 100 and 150 mg/kg on day 11 (Kimbrough &
Gaines, 1968). Repeated administration (40, 50 or 60 mg/kg body weight
per day) on days 7 to 19 of gestation reduced the growth of the dams
but had no effect on the number of resorptions or corpora lutea, on
litter size, or on fetal weight. The cholinesterase activity of the
fetal brain was reduced. A dosage of 75 mg/kg body weight per day was
fatal to dams in 4 to 5 days (Hoberman et al., 1979).
Diazinon (97.4% pure) was administered by gavage to groups of 21
to 25 pregnant Sprague-Dawley rats from day 6 to 15 of gestation at
dose levels of 0, 10, 20 and 100 mg/kg body weight. On day 20 of
gestation, all dams were killed and the fetuses delivered by cesarean
section. In the highest-dose group of, dams a decreased food
consumption was noted during days 6-9 of gestation and the animals
lost weight. The body weight gain of the dams showed some recovery
thereafter, but the overall body weight gain of the highest-dose group
remained significantly below that of the untreated controls. The
numbers of corpora lutea and implantations were similar in all groups.
Resorptions were significantly increased only in the top-dose group
and, accordingly, the number of live fetuses was decreased. The fetal
body weights were higher in the top-dose group than in the untreated
controls. Three single instances of external malformations were
observed at the highest-dose level (one fetus with a filamentous tail,
one fetus with umbilical hernia and one fetus with sublingual
extraneous soft tissue). Since the malformations were not
morphologically related, they were considered to be secondary to
maternal toxicity. An increased incidence of rudimentary 14th ribs was
noted in the fetuses of the highest-dose group, although it remained
within the limits of historical controls. The finding was considered
to be related to fetotoxicity, secondary to severe maternal toxicity.
Other fetal anomalies were comparable between treated and untreated
groups. No evidence of teratogenic effect of diazinon was found
(Infurna & Arthur, 1985).
7.5.2.3 Hamsters
In Golden Syrian hamsters diazinon was administered as individual
oral doses (0.125 and 0.25 mg/kg body weight) during the period of
organogenesis. Technical grade diazinon diluted with corn oil was
used. The dose volume was 10 ml/kg body weight. Control animals
received corn oil on the same days of gestation. The hamsters were
killed on day 14 of gestation. All fetuses were examined for gross
defects when delivered by cesarean section. The viability of hamster
fetuses delivered on day 15 was checked by placing them in a chicken
hatching incubator for 6 h. All fetuses with enough developmental form
for determination of structural defects were counted in determining
the number of fetuses per litter. The total number of fetuses in each
treatment group was divided by the number of mothers surviving to
term. Fetuses dying in late embryonic life and resorption sites were
counted as dead fetuses. No bone defects were seen in four fetuses
chosen at random from each litter for staining and examination of
skeleton. Diazinon did not produce any terata when administered to
hamsters (Robens, 1969).
7.5.2.4 Rabbits
Robens (1969) reported that diazinon had not been found to be
teratogenic in rabbits given 7 or 30 mg/kg body weight, although the
higher dose of diazinon produced cholinergic signs. Diazinon
(89.2% pure) was administered orally by gastric intubation to groups
of 19 to 22 New Zealand white rabbits at daily doses of 7, 25 and
100 mg/kg body weight from day 6 to 18 of gestation. On day 30 of
gestation, all dams were killed and the fetuses delivered by cesarean
section. At the highest-dose level, 9/22 dams died and overt signs of
toxicity included tremors, convulsions, hypoactivity and anorexia.
There were no statistically significant differences among the group
regarding the mean number of implantations and the proportions of
live, dead or resorbed fetuses. The fetal weights were similar in
treated and untreated groups, and neither embryotoxicity nor
teratogenicity was observed (Harris & Holson, 1981).
7.5.2.5 Chicken
Several studies have investigated the teratogenic potential of
diazinon in chick embryos (Khera & Bedok, 1967; Misawa et al., 1981,
1982; Henderson & Kitos, 1982; Byrne & Kitos, 1983; Wyttenbach &
Hwang, 1984; Kushaba-Rugaaju & Kitos, 1985). In view of the lack of
teratogenicity in mammals and the lack of adequate data on
cholinesterase inhibition in ovo, these studies are not considered
relevant in the assessment of mammalian teratogenicity.
7.6 Mutagenicity and related end-points
The summary results of mutagenicity studies conducted with
diazinon are presented in Table 3.
Diazinon did not induce mutations in either Salmonella
typhimurium or Escherichia coli, but produced conflicting results
in mouse lymphoma L5178Y cells at the tk locus. Unscheduled DNA
synthesis was not induced in primary cultures of rat hepatocytes. A
sister chromatid exchange study with human lymphocytes cultured in
whole blood gave equivocal results, while negative results were
obtained in three other in vitro studies and, in vivo, in bone
marrow cells of dosed mice.
Chromosomal aberrations were not induced in cultured human
lymphocytes. In Drosophila melanogaster, diazinon did not induce
either complete or partial chromosome loss.
Table 3. Special studies on the mutagenicity of diazinon
Test system Test object Results Reference
In vitro
Ames Salmonella typhimurium negative Geleick & Arni (1990)
TA98, TA100, TA1535, TA1537,
TA1538; E. coli WP2 uvrA
Ames Salmonella typhimurium negative Marshall et al. (1976)
TA1535, TA1536, TA1537, TA1538
Mouse lymphoma assay Mouse lymphoma cells, negative Dollenmeier & Müller (1986)
L5178Y/tk +/-
Mouse lymphoma assay Mouse lymphoma cells positive McGregor et al. (1988)
L5178Y/tk +/-
Sister chromatid exchange study Chinese hamster cells V79 negative Chen et al. (1981)
Sister chromatid exchange study Human lymphoid cells (LAZ-007) negative Sobti et al. (1982)
Sister chromatid exchange study Chinese hamster V79 cells positive Matsuoka et al. (1979)
Sister chromatid exchange study Whole blood human lymphocytes equivocal Murli & Haworth (1990a)
Sister chromatid exchange study Chinese hamster V79 cells negative Kuroda et al. (1992)
Sister chromatid exchange study Chinese hamster V79 cells negative Nishio & Uyeki (1981)
Table 3. (con't)
Test system Test object Results Reference
Nucleus anomaly Chinese hamster V79 cells negative Hool & Muller (1981c)
Chromosomal aberration Human lymphocytes questionable Lopez & Carrascal (1987)
Chromosomal aberrations Human lymphocytes negative Lopez et al. (1986)
DNA repair test Rat hepatocytes negative Hertner & Arni (1990)
In vivo
Nucleus anomaly Chinese hamster bone marrow cells negative Hool & Müller (1981c)
Micronucleus test Mouse bone marrow cells negative Ceresa & Puri (1988)
Dominant lethal study Mouse, male negative Fritz (1975)
Chromosome aberrations Mouse spermatogonia negative Hool & Müller (1981d)
Chromosome aberrations Mouse spermatocytes negative Hool & Müller (1981b)
Chromosomal loss Drosophila melanogaster negative Woodruff et al. (1983)
Sister chromatid exchange study Mouse bone marrow cells negative Murli & Haworth (1990b)
Nuclear anomaly tests in cultured Chinese hamster V79 cells and
in bone marrow cells of dosed Chinese hamsters gave negative results.
A micronucleus test in bone marrow polychromatic erythrocytes of mice
was also negative.
No chromosomal aberrations were induced in the spermatogonia or
spermatocytids of dosed mice and a dominant lethal test in dosed male
mice was negative. It was concluded that diazinon is not genotoxic.
7.7 Carcinogenicity
7.7.1 Mice
Groups of 50 B6C3F1 mice of each sex were treated with diazinon
(98% pure) at dietary concentrations of 100 and 200 mg/kg diet for
103 weeks. Two additional groups of 25 males and 25 females each
served as untreated controls. Hyperactivity was reported for the dosed
mice but was rare in the control group. The body weight gains for all
dosed male mice were similar to the control group except for the last
20 weeks of the study period. The treated females showed slightly
lower body weights than the controls. The survival at 78 weeks in the
control 100 and 200 mg/kg groups, respectively, was: males, 21/25
(84%), 45/50 (90%), 49/50 (98%); females, 24/25 (96%), 50/50 (100%),
49/50 (98%). Several neoplasms were observed, but apart from lower
neoplasms in male mice none appeared to be treatment-related or had an
incidence in treated groups significantly different from that of
controls. The incidences of hepatocellular carcinomas in the control,
100 and 200 mg/kg groups, respectively, were: 4/21 (19%), 20/46 (43%)
and 10/48 (21%). The corresponding incidences of hepatocellular
carcinomas or adenomas were 5/2 (24%), 20/46 (43%) and 13/48 (27%).
The Cochrane-Armitage test for trend was significant for carcinomas,
but not for liver carcinomas and adenomas combined. Fisher's exact
test for comparison of a dosed group with its matched control groups
was significant (P=0.046) only for hepatocellular carcinomas alone in
the 100 mg/kg group of male mice. In view of the lack of a dose-
related response, the occurrence of liver tumours in male mice could
not be clearly attributed to diazinon (NCI, 1976).
A report submitted to the United Kingdom Ministry of Agriculture,
Fisheries and Food (MAFF, 1991) described a two-year combined chronic
toxicity/carcinogenicity study conducted with male and female B6C3F1
mice (60/dose/sex) administered diets containing diazinon at 0, 100,
200 or 300 mg/kg (males only) or 400 mg/kg (females only). Ten mice of
each sex per group were killed at 12 months. After 24 months, there
were reported to be no treatment-related gross or histopathological
findings. This study provides no evidence for the carcinogenicity of
diazinon in mice. In particular, it does not confirm the observations
described in the above-mentioned study on B6C3F1 mice.
7.7.2 Rats
Groups of 50 male and 50 female Fischer-344 rats received
diazinon (98% purity) at dietary concentrations of 400 and 800 mg/kg
for 103 weeks. All survivors were killed at 105 weeks. An additional
control group of 25 males and 25 females received an untreated diet.
There was no significant effect of treatment upon body weight gain.
Hyperactivity was noted in males and females of the 800 mg/kg group
and males of the 400 mg/kg group. Urine was discoloured in females of
the 800 mg/kg group and in dosed females there was vaginal bleeding
and discharge. Survival at 78 weeks in control, 400 mg/kg and
800 mg/kg groups, respectively, were: males, 24/25 (96%), 49/50 (98%),
49/50 (98%); females, 23/25 (92%), 44/50 (88%), 44/50 (88%). Various
neoplasms were observed, but only the incidence of lymphomas/
leukaemias in dosed groups of males was significantly different from
the controls. The incidences in the control, 400 and 800 mg/kg groups,
respectively, were: males, 5/25 (20%), 25/50 (50%), 12/50 (24%);
females, 2/25 (8%), 6/50 (12%), 6/50 (12%). The Fisher's exact test
for comparison with the matched control was significant (P=0.011) only
in the 400 mg/kg group of males. In view of the lack of a dose-related
response, the occurrence of lymphomas/leukaemias in male rats could
not be clearly attributed to diazinon. The frequency of endometrial
stromal polyps was increased in the dosed groups. This lesion, which
is commonly observed in Fischer-344 rats, occurred at incidences in
the control, 400 mg/kg and 800 mg/kg groups, respectively, of 2/23
(9%), 8/43 (19%) and 11/49 (22%). The carcinogenic status of diazinon
in this study is unclear (NCI, 1979).
The chronic toxicity study of Kirchner et al. (1991) described
earlier (section 7.3.1) included groups of male and female Sprague-
Dawley rats (approximately 20/dose/sex, progressing for longer than 57
weeks) administered diets containing diazinon at concentrations 0,
0.1, 1.5, 125 or 250 mg/kg. No treatment-related increases in
neoplasms were observed. This study does not provide supportive
evidence for a carcinogenic effect of diazinon in rats.
A report submitted to the United Kingdom Ministry of Agriculture,
Fisheries and Food (MAFF, 1991) described a 120-week combined chronic
toxicity/carcinogenicity study conducted with male and female F-344
rats (75/dose/sex) administered diets containing diazinon (0, 0.1, 1.5
or 22.6 mg/kg body weight per day). Rats of both sexes in the highest-
dose group that died late in the study (after week 105) showed
increased ulceration of the forestomach, with associated increased
incidence of acanthosis, hyperkeratosis, submucosal granulation tissue
and hyperplasia of the epithelium. Apparently, no neoplastic lesions
were observed in the study. This study provides no evidence for the
carcinogenicity of diazinon in rats. In particular, it does not
confirm the observations described in the above-mentioned study on
F-344 rats.
It is concluded that diazinon is not carcinogenic in rats or
mice.
7.8 Special studies
7.8.1 Neurotoxicity
In a range-finding experiment, groups of four domestic hens (red
heavy breed) were treated at four dose levels of diazinon (87% pure),
and an acute oral LD50 of 12.5 mg/kg body weight was determined. A
schedule was developed to protect the hens from acute cholinergic
effects. Atropine (10.0 mg/kg intramuscular) was administered prior to
the treatment with diazinon, and 2-PAM was injected at doses of
50 mg/kg at the time of the diazinon administration. In addition, the
hens received post-treatment protection with concurrent doses of
atropine and 2-PAM after approximately 1 and 5 h and as necessary
thereafter. In the main neurotoxicity study, diazinon was given as a
single dose of 28 mg/kg body weight by gastric intubation to a group
of 18 atropine-pretreated hens. All hens were observed for three weeks
and again treated with 13 mg diazinon/kg on day 21. The second
observation period also lasted for three weeks (day 22-42). Additional
groups, which served as negative controls, were given the vehicle
(10 hens treated with 1 ml/kg corn oil), and eight hens, treated with
tri- o-tolyl phosphate (500 mg/kg) served as positive controls. One
diazinon-treated hen died 5 days after the first dose and another one
6 h after the second dose. One hen from a negative control group died
on day 7. There were no neurotoxic signs apparent during either of the
two observation periods in the negative control group or in the
treated groups. No histopathological changes in the brain, spinal cord
or peripheral nerves were detected (Jenkins, 1988).
In a further test for delayed neuropathy Classen (1996) gave 20
hens a single oral dose of 100 mg diazinon/kg body weight. Therapeutic
treatment with physostigmine and atropine for up to 48 h resulted in
survival of 17 hens, despite a peak inhibition of brain cholinesterase
by 83%. No ataxia was seen over a 28-day survival period, and no
inhibition of either brain or spinal cord neuropathy target esterase
(NTE) was seen at either 24 (n=5) or 48 (n=5) h.
Diazinon (88% purity) was administered by gavage to 15 rats per
sex and dose at single doses of 0, 2.5, 150, 300 and 600 mg/kg. A
negative control group of 15 animals per sex received the vehicle
(corn oil) only. A group of 10 animals per sex served as positive
control and was administered a single dose of 150 mg/kg triademefon
60 min prior to the Functional Observational Battery (FOB). Two males
and one female dosed at 600 mg/kg died. A transient reduction in body
weight and body weight gain was observed in males dosed at 300 and
600 mg/kg during week one. Food consumption was reduced in the same
males, and in females dosed at >150 mg/kg. These findings decreased
in incidence and severity with decreasing dose. No treatment-related
effects were observed in the FOB on study days 8 and 15. In males
dosed above 150 mg/kg and in females dosed above 2.5 mg/kg, activity
counts in the figure-8 maze were significantly decreased at the
estimated time of peak effect only. Serum cholinesterase activity was
significantly decreased at the time of peak effect in all diazinon-
treated groups. Full recovery was observed in all groups on study day
15. Red blood cell cholinesterase activity was significantly inhibited
on study day 1 in animals of both sexes treated with >150 mg/kg.
Substantial recovery was observed on study day 15, but significant
inhibition still remained. No inhibition of brain cholinesterase
activity was detected on day 15. No neuropathological effects were
noted for diazinon at necropsy or upon histopathological examination.
It was concluded that the administration of diazinon resulted in
reversible neurotoxicity and cholinergic poisoning without
neuropathological changes. The NOAEL was 2.5 mg/kg body weight for
both sexes (Chow & Richter, 1994).
7.8.2 Effects on enzymes and transmitters
Male rats were treated bi-weekly by gavage with the equivalent of
0.5 mg/kg per day technical diazinon for up to 28 weeks. The animals
were killed at specific time intervals (7, 14 and 28 weeks) and
compared with age-matched controls. Blood and brain tissues were
analysed for cholinesterase activity and for concentrations of
catecholamines and amino acids. Only plasma cholinesterase activity
was significantly reduced. Erythrocyte cholinesterase and brain
cholinesterase were unchanged while during the same period the levels
of several putative brain neurotransmitters, aspartate, glutamate
(excitatory) and taurine as well as GABA (inhibitory) were
significantly reduced in experimental versus control animals. Blood
serotonin level was significantly elevated but no other blood or brain
monoamines were significantly altered. Whilst the authors (Rajendra et
al., 1986) concluded that oral administration of diazinon exerts
effects on brain neurotransmitters, even at the low-dose levels
administered in this study, the biological significance of these
findings is unclear.
Intraperitoneal administration of diazinon diminishes formation
of L-kynurenine by liver slices from 93% at 1 mg/kg dose to 56% at
40 mg/kg. The ratio of critical intermediates in L-tryptophan
biosynthetic pathway, L-kynurenine to N-formyl-L-kynurenine,
decreases and is reversed with increasing dosage of diazinon (Seifert
& Cassida, 1979).
Diazinon altered the formation of several L-tryptophan
metabolites associated with the L-kynurenine pathway in mice. Liver
kynurenine formamidase was inhibited almost completely by diazinon
(10 mg/kg). The enzyme inhibition resulted in reduced L-kynurenine
biosynthesis in livers and a concomitant accumulation of N-formyl-L-
kynurenine. However, in plasma, L-kynurenine level increased up to
5-fold in diazinon-treated mice. Consequently, the urinary excretion
of xanthurenic acid and kynurenic acid was raised 5- to 15-fold. The
revelation of this novel mechanism of diazinon action is an important
piece of information needed for a better understanding of the non-
cholinergic toxicity of organophosphorus acid triesters and
methylcarbamates (Seifert & Pewnim, 1992).
7.8.3 Effects on the immune system
Pregnant F2 dihybrid female mice received either a vehicle or 1
of 2 doses of diazinon (0.18 (n=19) or 9.0 (n=21) mg/kg body weight)
in the diet, daily throughout gestation. All mothers gave birth to
viable, overtly normal offspring at term. However, a significant
number (12%) of pups born to dams who received 9.0 mg/kg died prior to
weaning on day 28; necropsy findings were consistent with death from
respiratory infection. There was no significant difference in
mortality between control and diazinon-treated offspring once they
reached 28 days of age. Determinations of five different classes of
serum immunoglobulin (Ig) concentrations (IgG1, IgG2a, IgG2b, IgA,
IgM) at 101, 400 and 800 days of age indicated transient but
consistent disturbances of 2 Ig classes in offspring as a result of
prenatal diazinon exposure. IgG1 concentrations of male offspring
exposed to 0.18 mg/kg were significantly elevated at 101 days but not
at 400 or 800 days of age. IgG1 concentrations of female offspring
exposed to 9.00 mg/kg were significantly depressed at 101 days but not
different from controls at 400 or 800 days of age. Changes in IgG2b
levels generally were similar to those recorded for IgG1 but of
smaller magnitude. There were no significant effects on serum IgG2b or
IgM concentrations, and only equivocal effects on IgA, as a
consequence of prenatal exposure to either pesticide. This study
offers no conclusive information of an effect of diazinon on the
immune system (Barnett et al., 1980).
7.8.4 Effect on pancreas
Diazinon has been reported to cause acute pancreatitis and
ductal hypertension in dogs, which is due to the absence of
acetylcholinesterase in pancreatic sphincter, duodenal smooth muscle
in dogs and a reliance upon the more readily inhibited
butyrylcholinesterase (Dressel et al., 1980; Frick, 1987).
Kazacos (1991) examined the toxic effects of diazinon on the
guinea-pig pancreas. Guinea-pigs were given single intravenous dose of
diazinon at concentration ranging from 125 to 200 mg/kg body weight.
Examination of pancreatic tissue from animals killed at various time
intervals after dosing revealed acinar cell vacuolization after 24 h.
This cellular injury disappeared within 3 days after the initial toxic
insult. These are considered to be species-specific effects.
7.9 Factors that modify toxicity; toxicity of metabolites
7.9.1 Metabolic enzymes
Abdelsalam & Ford (1986) found that the toxic effects of diazinon
were increased by pretreatment with the hepatic microsomal enzyme
inducers dieldrin and phenobarbitone and that, at the same time, there
was a rise in the liver carboxylesterase activity. In this experiment,
the toxicity of diazinon increased when calves were pretreated with
dieldrin or phenobarbitone despite the increased activity of the liver
carboxylesterase. Apparently carboxylesterase activity was not
sufficient to protect against the toxicity of diazinon in the
pretreated calves. This suggests that either the increased
carboxylesterase activity had only a minor role in the hydrolytic
detoxification of diazinon or active metabolites, or that its effect
might have been overwhelmed by the action of other microsomal oxidase
activity concurrently induced by dieldrin and phenobarbitone leading
to a faster rate of activation than degradation of diazinon.
Abdelsalam & Ford (1987) described the effect of induced liver
and kidney lesions on the toxicity of levamisole and diazinon, two
antihelmintics routinely used in ruminant livestock management, and of
a lung lesion on the toxicity of diazinon in calves. Hepatic or renal
damage such as may occur naturally as a consequence of infection or of
the ingestion of poisonous plants or other toxic substances is,
therefore, likely to affect the metabolism and excretion of drugs
routinely used in antihelmintic control programmes. Liver damage in
calves, induced by the oral administration of the flukecide, carbon
tetrachloride, increased the toxic effect of diazinon but not of
levamisole, whereas the presence of a renal tubular lesion caused by
mercuric chloride enhanced the toxicity of both commonly used
antihelmintic compounds.
7.9.2 Antidotes
The effects of atropine/oxime therapy on diazinon-poisoned
animals was investigated in rats and rabbits. The rats were orally
treated with 235 mg/kg body weight (0.8 LD50) of diazinon (91.9%
pure), while rabbits received 1600 mg diazinon/kg body weight by the
subcutaneous route. In both species, intramuscular administration of
16 mg/kg atropine (10 min post treatment), and 30 mg/kg of pyridine
2-aldoxime methochloride (2-PAM Cl) 24 h later, significantly
increased the reactivation of the diaphragm cholinesterase. The oral
LD50 for diazinon in the rat was increased by a factor of 1.7 when
2-PAM Cl was administered intravenously and 3.1 times when 2-PAM Cl
was given orally. In order to prevent the reappearance of signs the
authors recommended that 2-PAM Cl be administered intravenously at the
same time as atropine followed by repeat oral administrations, as
needed (Harris et al., 1969).
The antidotal efficacy of pralidoxime iodide and obidoxime
dichloride was investigated in goslings poisoned by a supralethal dose
of diazinon. Various doses of both drugs were administered by
intramuscular injection when the poisoned birds were unable to walk.
Pralidoxime at 100 mg/kg induced recovery in 4 out of 6 poisoned
goslings, and 25 mg/kg successfully treated only 1 of 6 birds.
Obidoxime at 25 mg/kg showed no therapeutic properties, whereas 50
and 100 mg/kg delayed the death of some birds by several hours. At
100 mg/kg, all goslings had transient signs of intoxication, which
precluded the use of this compound as an antidote at higher doses
(Shlosberg et al., 1976).
7.9.3 Potentiation
In a study of the potentiation of the acute toxicity of diazinon
by several other pesticides (chlordimeform, iodofenphos, profenphos,
methacrifos), there was no potentiation at equitoxic doses (Sachsse &
Bathe, 1975, 1976, 1977, 1978).
8. EFFECTS ON HUMANS
8.1 Exposure of the general population
8.1.1 Acute toxicity, poisoning incidents
Several cases of intentional (Kabrawala et al., 1965; Banerjee,
1967; Wadia et al., 1974; Klemmer et al., 1978; Poklis, 1980; Wedin et
al., 1984; Hodgson & Smith, 1992) and accidental acute poisoning with
diazinon have been reported. Accidental poisonings were usually due to
ingestion of improperly stored liquid formulation (Hayes, 1963;
Zwiener & Ginsburg, 1988; Weizman & Sofer, 1992; Hodgson & Smith,
1992). Cases of poisoning after cutaneous application of diazinon for
lice treatment have also been described (Muratore et al., 1960; Hayes,
1963; Halle & Sloas, 1987). Several accidents involved children (Mizra
et al., 1972; English et al., 1970; Zwiener & Ginsburg, 1988; Hodgson
& Smith 1992; Weizman & Sofer, 1992; Wagner & Orwick, 1994).
Generally, symptoms and signs were typical of AChE inhibition,
which responded to atropine and oxime treatment. In a case of fatal
suicidal ingestion of diazinon reported by Poklis et al. (1980),
diazinon concentrations in postmortem body fluids and tissues were
found to be 277 mg/litre in blood, 200 mg/litre in the bile and
15 mg/kg in adipose tissue. In the stomach 44 mg were found. Cases
with atypical or unusual features are described in section 8.1.1.3. No
cases of delayed polyneuropathy have been observed, as would be
expected from the negative animal data. The following sections deal
with some specific aspects of acute poisoning by diazinon (i.e., the
acute pancreatitis, the intermediate syndrome, and some unusual case
reports).
8.1.1.1 Acute pancreatitis
Increased levels of serum amylase and glucose have been described
in some severe cases of diazinon poisoning. In certain cases these
enzymatic alterations were accompanied by prominent abdominal symptoms
and signs, including abdominal rigidity. All these were considered
indicative of acute pancreatitis (Dressel et al., 1979; Dagli et al.,
1981; Lee, 1989; Weizman & Sofer, 1992). On one occasion a pancreatic
cyst was observed (Dressel et al., 1979). In all cases, pancreatitis
was mild and patients fully recovered. Severe poisoning with other
organophosphates, e.g., parathion (Weizman & Sofer, 1992), coumaphos
(Lee, 1989) and malathion (Lee, 1989), was also associated with acute
pancreatitis. Apparently, poisoning with an unknown carbamate was also
associated with acute pancreatitis (Weizman & Sofer, 1992).
8.1.1.2 Intermediate syndrome
Senanayake & Karalliedde (1987) described a syndrome caused by
organophosphate pesticides and named it "intermediate", because its
onset was delayed after cholinergic syndrome but appeared earlier than
polyneuropathy. The syndrome, appeared 24-96 h after poisoning during
recovery from the cholinergic crisis. It was characterized by
paralysis of proximal limb muscles, neck flexors, motor cranial nerves
and respiratory muscles. It was resistant to atropine treatment and,
in certain patients, required assisted ventilation. It is noteworthy
that Wadia et al. (1974), reporting the neurological manifestations
seen in 200 consecutive cases of poisoning, mostly with diazinon,
described two different types of signs: type I, characteristic of the
cholinergic syndrome and responsive to atropine, and type II, present
in about 20% of the cases, which appeared at least 24 h later,
resembled those of the intermediate syndrome and were not responsive
to atropine (Wadia et al., 1974).
Other cases of intermediate syndrome due to diazinon poisoning
have been described (Hall & Baker, 1989; Samal & Sahu, 1990).
8.1.1.3 Unusual case reports
Three-week-old twins were hospitalized because of respiratory
distress. One was cyanotic on admission, but both had rapid shallow
breathing, profuse nasal and bronchial secretions, and pinpoint
pupils; muscle fasciculations were not detectable. At 48 h only the
sicker twin had slightly reduced pseudocholinesterase activity.
Treatment was appropriate and recovery uneventful. Investigation
revealed that the babies lived in one side of a two-story house that
had been divided into two apartments by partitions. About 10:30 h on
the previous day the other apartment had been sprayed with 1% diazinon
for cockroach control using approved spot applications directed mainly
at cracks. The twins were the only ones who had remained indoors. The
observed degree of respiratory distress in the presence of little or
no inhibition of cholinesterase was consistent with exclusively
respiratory exposure (English et al.1970).
A 12-week-old infant girl developed persistent hypertonicity of
the extremities without other signs of intoxication. It was discovered
that the organophosphate insecticide diazinon was applied in her
home 5 weeks prior to the onset of signs. Six months after
application, high levels of diazinon residues were found on the floor
(230 ng/cm2), in vacuum cleaner dust (1700 mg/kg), and in the air
(2.8 ng/m3). A diazinon dose of approximately 0.02 mg/kg per day was
calculated and derived from the infant's urine level of diazinon
metabolites determined 6 months after application of diazinon in her
home. Her muscle tone returned to normal shortly after the infant was
removed from the home (Wagner & Orwick, 1994).
According to Hata et al. (1986), atypical ocular bobbing resulted
from an intentional poisoning from diazinon. The authors suggest
acetylcholine as a neurotransmitter substance within the ocular motor
pathway.
A 26-year-old man, who ingested approximately 230 ml of a
solution of an unknown concentration of diazinon in a suicide attempt,
developed a severe cholinergic syndrome which was relived by atropine
and 2-PAM. Diuresis was greatly reduced (22 ml/h) and urine was dark
and cloudy (specific gravity 1.029). It is possible that significant
dehydration may have precipitated this reaction (Wedin et al., 1984;
Albright, 1984).
8.1.2 Controlled human studies
A cumulative toxicity study was conducted with human volunteers.
Four healthy males weighing between 74 and 96 kg and aged between 30
and 45 years ingested diazinon in gelatin capsules (95.4% pure) at a
dose of 0.025 mg/kg per day. Two males received 34 consecutive
treatments (Group B), while the two other volunteers were treated for
4 days, were left untreated for the next 5 days in order to
investigate reversibility of the effects and then received the
capsules again for 32 more days (Group A). The daily dose was split
into three administrations taken after meals around 9, 13 and 19 h.
Parameters of haematology, urine analysis and blood biochemistry
(plasma and erythrocyte cholinesterase, serum alkaline and acidic
phosphatase activities) were determined at regular intervals. A
reversible decrease of plasma cholinesterase activity was observed in
group A during the first 4 days of treatment while the erythrocyte
cholinesterase activity remained unaffected. During the second
treatment period of group A and during the entire treatment of group
B, the cholinesterase activities were similar to those observed at
pretest. No clinical signs or changes in other parameters were noted.
It was concluded that the administered daily dose of 0.025 mg/kg
marginally inhibited the plasma cholinesterase only, and can therefore
be considered as a NOAEL in humans (Payot, 1966).
8.2 Occupational exposure
8.2.1 Acute poisoning
Poisoning following occupational exposure to diazinon was usually
associated with improper or prolonged storage of the commercial
formulations, which, prior to the improvements carried out by the
manufacturer, tended to give rise to more toxic impurities (mainly
TEPP).
Soliman et al. (1982) investigated two spraymen who had a
cholinergic syndrome (with about 90% red blood cell AChE inhibition)
after using a commercial formulation of diazinon which was packaged in
tin-plated cans that had replaced the more expensive aluminium cans.
Analysis of such commercial formulation revealed the presence of TEPP,
sulfo-TEPP, monothiono-TEPP and other impurities, but no diazinon.
Mello et al. (1972) reported an episode where three farmers were
poisoned when using a commercial formulation of diazinon that was
transferred from the original container to another container and then
stored for some time. This formulation contained monothiono-TEPP and
was about 30 times more toxic to rats than a recently prepared and
properly stored formulation. On that occasion, 26 of 40 cows died when
treated against tick infestation with this formulation.
A fatal case of poisoning by diazinon and malathion, possibly by
inhalation, was described by Wecker et al. (1985). The 51-year-old man
was found unconscious in the closed shed where he sprayed three cows
with the pesticides.
Two cases of cutaneous hepatic porphyria of toxic origin were
identified within a short period of time. Both patients were farmers
and recently handled very intensively, without any precaution, some
pesticides containing organophosphorus substances (diazinon). The
symptoms were similar to those of the so-called Turkish porphyria
(Bopp & Kosminsky, 1975).
8.2.2 Effect of short-term and long-term exposure
Neurophysiological investigations and determinations of blood
cholinesterase activities were carried out on 11 Swedish spraymen
exposed to bromophos, diazinon, dursbane and malathion. Plasma
cholinesterase activity was significantly reduced after work, while
erythrocyte cholinesterase activity was unchanged. In none of the
workers with a decreased plasma cholinesterase activity after work
could any related acute neuromuscular disturbance be detected when
the men were tested with repetitive nerve stimulation and with single
fibre electromyography. Signs of subclinical neuropathy were present
as a slight reduction in sensory conduction velocity and increased
fibre density in some workers (Stalberg et al., 1978).
A cohort of 99 workers exposed to diazinon was tested before and
after the work shift with a neurobehavioural test battery, which
included a brief examination, a symptom questionnaire and tests of
concentration, eye-hand coordination, pattern recognition, visual
memory and finger tapping. The median diazinon exposure level was
2.1 mg/day and the mean duration of exposure was 39 days (see section
5.3). There was no correlation between the diethylthiophosphate (DETP)
concentrations or diazinon exposure and pre- or post-shift
neurobehavioural function with linear regression models after
adjusting for age, sex, education and alcohol intake. The study failed
to demonstrate adverse behavioural effects of repeated, low-level
diazinon exposure (Maizlish et al., 1987).
Three groups of agricultural workers with a history of exposure
to organophosphate pesticides were followed up to evaluate the utility
of sequential post-exposure cholinesterase analyses to confirm
organophosphate intoxication in the absence of baseline cholinesterase
values. Three or more cholinergic symptoms were reported by 50 of the
72 patients. Pre-exposure red blood cell cholinesterase activities of
45 workers were above the lower limit of laboratory normal range.
Follow-up examinations, including blood cholinesterase activity
analyses, were conducted in 57 subjects. When final post-exposure
cholinesterase activity determinations were compared with respective
individual normal baseline values, the plasma and red blood cell
activities were shown to be inhibited. The data support the use of
sequential post-exposure blood cholinesterase analyses to confirm the
diagnosis of organophosphate-induced illness in the absence of
baseline values (Coye et al., 1987).
Of 67 described poisoning incidents in California involving 583
people, nineteen involved a pesticide product containing an
organophosphate: most often chlorpyriphos (8), diazinon (3) and
malathion (5). There were also 10 cases that resulted from suicide,
and two cases involved diazinon (Maddy & Edmiston, 1988).
In a study of organophosphate-induced contact dermatitis in 202
patients in Japan, Matsushita et al. (1985) attributed the reactions
mainly to diaoxabenzafos, fenitrothion, leptophos, cyanophos, diazinon
and malathion. The areas affected by dermatitis were fingers (62.4%),
face (39.6%), forearm (31.6%) and neck (29.7%). One quarter of the
cases with dermatitis had symptoms associated with acute
organophosphate poisoning.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Singh (1973) found that three genera of cyanobacteria (blue-green
algae) tolerated diazinon at concentrations of 300 and 400 mg/litre.
Clegg & Koevening (1974) indicated that the population densities of
three species of freshwater algae were relatively unaltered by a
concentration of 100 mg/litre. Butler et al. (1975) demonstrated that
diazinon inhibited growth of numerous species of green algae and
cyanobacteria at concentrations of 0.01 mg/litre and 0.1 mg/litre.
Wong & Chang (1988) studied the effects of diazinon on the growth of
the green alga, Chlamydomonas reinhardtii. They observed a reduction
in growth at concentrations of 5 and 10 mg/litre and complete
inhibition at 20 and 40 mg/litre.
Doggett & Rhodes (1991) determined the effects of diazinon on
phytoplankton population dynamics. The objectives of this study were
to determine the effects of diazinon (1, 5, 10, 20 and 40 mg/litre
concentration) on the growth rates of three widely distributed species
of freshwater algae, and to ascertain the effects of this pesticide on
the diversity of a natural phytoplankton assemblage. Stimulation of
growth was exhibited by Selenastrum capricornutum and Chorella
exposed to diazinon at 1 and 5 mg/litre. The higher concentrations of
10, 20 and 40 mg/litre all inhibited growth. The growth rates of the
cyanobacteria showed a high degree of tolerance; a suppression of
growth was observed only at 40 mg/litre. Two genera of green algae
were stimulated by all concentrations.
The acute toxicity of diazinon to the freshwater rotifer
Brachionus calyciflorus was determined after 24 h of exposure; the
mean LC50 value was 29.2 mg/litre. Based on this result, four
sublethal concentrations were chosen to determine the median lethal
time (LT50) at each concentration of diazinon tested. The
concentrations tested were 1/5, 1/4, ´ and 2/3 of the LC50 (24 h).
The LT50 values ranged from 6.96 to 2.49 days after diazinon
exposure, decreasing with increasing exposure concentration
(Fernandez-Casalderrey et al., 1992).
Several species of soil-borne fungi invade plant roots forming
vascular-arbuscular mycorrhizae which aid in growth and nutrient
element absorption. Pre-plant incorporated treatments at 2 and 4 kg/ha
of trifluralin and diazinon had no significant effect on growth,
phosphorus accumulation or root colonization by mycorrhizal fungi in
soybeans planted in an Andover clay loam. At currently used commercial
rates, diazinon did not affect mycorrhizal development under the
conditions of the experiment (Burpee & Cole, 1978).
Nitrogenase activity of excised soybean nodules was severely
affected by diazinon following application of 3, 1, 0.5 and 0.25
multiples of the amounts stated on the label (Hensley, 1991).
9.2 Aquatic invertebrates
Acute toxicity of diazinon to aquatic invertebrates is summarized
in Table 4.
The effect of diazinon (Basudine, 0.01, 0.1, 1.0 mg/litre) on the
physical and chemical properties of haemolymph of Lymnaea stagnalis
(L.) and Planorbius corneus (L.) infected with trematodes was
studied by Stadnichenko et al. (1987). Haemolymph viscosity and
density decreased after the treatment.
Robertson & Mazzella (1989) determined the toxicity of diazinon
to the freshwater snail Gillia altilis. Based on nominal
calculations, the LC50 values for static 4- and 96-h exposures were
340 mM (93 mg/litre) and 40 mM (11 mg/litre), respectively.
Experiments were conducted to determine the effects of piperonyl
butoxide, a synthetic methylenedioxyphenyl inhibitor of cytochrome(s)
P-450, on the toxicity of organophosphate insecticides to three
cladoceran test species: Ceriodaphnia dubia, Daphnia magna, and
Daphnia pulex. The acute toxicity of diazinon to three tests species
was similar, with LC50 values ranging from 0.50 to 0.80 mg/litre.
Co-administration of piperonyl butoxide effectively reduced the acute
toxicity of metabolically activated diazinon, and piperonyl butoxide
concentrations of 500 or 1000 mg/litre completely blocked the toxicity
of diazinon at concentrations of up to 16 times the 48-h LC50. In the
case of D. pulex, piperonyl butoxide at 500 mg/litre also
significantly reduced the toxicity of diazinon at concentrations of up
to 16 times the 48-h LC50 (Ankley et al., 1991).
9.3 Fish
The acute toxicity of diazinon to fish is summarized in Table 5.
The median lethal concentration (LC50) of diazinon was
determined for Clarias batrachus; the LC50 for 40 days of exposure
to diazinon was 2.4 mg/litre (Tripathi, 1992). The acute toxicities
(24, 48, 72 and 96 h) of eight pesticides to Anguilla anguilla were
determined. The 24- to 96-h LC50 values for diazinon ranged from 0.16
to 0.08 mg/litre (Ferrando et al., 1991).
Bresch (1991) studied the effects of diazinon on early
life-stages in zebrafish. Zebrafish kept at diazinon concentrations of
0.2, 0.04 and 0.008 mg/litre grew alike, and no differences among the
groups were observed. Survival rates of animals of different groups
did not differ either. In no groups were abnormal fish seen.
Table 4. Acute toxicity (LC50) to aquatic invertebrates
Species Duration (h) Concentration (mg/litre) Reference
Snail (Gillia altilis) 96 11 Robertson & Mazzella (1989)
Cladocerans 48 0.0005-0.0008 Ankley et al. (1991)
Cladoceran (Daphnia pulex) 48 0.001 (0.0006-0.0011) Mayer & Ellersieck (1986)
Cladoceran (Simocephalus serrulatus) 48 0.0014 (0.0012-0.0016) at 21°C Mayer & Ellersieck (1986)
0.0018 (0.0014-0.0022) at 15°C
Scud (Gammarus fasciatus) 96 0.0002 (0.00015-0.00028) Mayer & Ellersieck (1986)
Shrimp (Hyallela azteca) 96 0.004-0.006 Collyard et al. (1994)
Desmocaris trispimosa 96 0.0208 (0.0192-0.0224) Ebere & Akintonwa (1992)
Shrimp (Palaemonetes africanus) 96 0.0179 (0.0147-0.020) Ebere & Akintonwa (1992)
Insect larva (Pteronarcys californica) 96 0.025 (0.020-0.030) Mayer & Ellersieck (1986)
Table 5. Acute toxicity (LC50) to fish
Species Duration (h) Concentration (mg/litre) Reference
Bluegill sunfish (Lepomis macrocharis) 96 0.17 (0.012-0.22) Mayer & Ellersieck (1986)
Rainbow trout (Oncorynchus mykiss) 96 0.09 Mayer & Ellersieck (1986)
Cutthroat trout (Salmo clarkii) 96 2.76 (2.28-3.33) in soft water Mayer & Ellersieck (1986)
1.7 (1.39-2.09) in hard water
Walking catfish (Clarias batrachus) 2.41 Tripathi (1992)
Eel (Anguilla anguilla) 96 0.08 (0.06-0.10) Ferrando et al. (1991)
Channel catfish (Channa punctatus) 96 3.1 Sastry & Malik (1982a)
Goby (Gobius) sp. 96 0.25 (0.23-0.28) Ebere & Akintonwa (1992)
Lake trout (Salvelinus namaycush) 96 0.6 (0.4-0.9) Mayer & Ellersieck (1986)
Table 6. Summary of brain acetylcholinesterase activity inhibition by
diazinon in aquatic organisms
Organism Exposure Duration Inhibition Reference
concentration (h) (%)
(mg/litre)
Bluegill 0.1 6 95 Weiss (1961)
Fathead minnow 0.1 18 70 Weiss (1961)
Goldfish 0.1 18 43 Weiss (1961)
Golden shiner 0.1 18 40 Weiss (1961)
Sheepshead minnow 6.5 24 71 Goodman
et al.
(1979)
Whereas growth and survival of fathead minnows were not
influenced at concentrations below 0.2 mg/litre in a test described by
Allison & Hermanutz (1977), the minnows responded more sensitively in
a similar test by another laboratory: effects were observed below
0.08 mg/litre (Jarvinen & Tanner, 1982). Allison (1977) found reduced
larval growth of the flagfish if reared in water containing diazinon
at a concentration of 0.014 mg/litre.
Brain acetylcholinesterase (AchE) activity and changes in
optomotor behaviour were determined in bluegill sunfish, Lepomis
macrochirus, exposed to graded concentrations (0, 15, 30, 5, 60 and
75 µg/litre) of diazinon for a period of 24 h (Dutta et al., 1992). A
significant decrease of AchE activity was observed at and above an
exposure concentration of 45 µg/litre, whereas a decline in the scores
of the "following" responses of the fish was observed at an exposure
concentration of 30 µg/litre.
Weiss (1961) was the first to investigate in vivo brain AChE
activity of fish exposed to organophosphates. He found that the extent
of enzyme inhibition was proportional to the concentration of the
substance and extent of exposure, and suggested that fish brain AChE
activity could be used to detect the presence of anticholinesterases
in the aquatic environment.
Goodman et al. (1979) reported a dose-dependent inhibition of
brain AChE activity in sheepshead minnows for diazinon. For the same
sublethal exposure (6.5 mg/litre), inhibition was independent of the
exposure duration, being 68 and 78% on days 4 and 108, respectively.
The authors found that the brain AChE activity of sheepshead minnows
exposed to diazinon varied and was susceptible to exposure
concentration. AChE was depressed long after no measurable diazinon
was found in exposed sheepshead minnows.
The effect of acute exposure to diazinon (3.1 mg/litre for 96 h)
and chronic exposure to a sublethal concentration (0.31 mg/litre) of
diazinon has been studied in the liver, stomach, intestine and pyloric
caeca of a freshwater teleost fish, Channa punctatus. In acute
exposure, succinate dehydrogenase activity was elevated in intestine
and pyloric caeca. No alteration was noted in lactate dehydrogenase
activity but pyruvate dehydrogenase activity was inhibited in pyloric
caeca. Chronic exposure resulted in inhibition of the activities of
the three dehydrogenases in all four organs at both intervals (Sastry
& Malik, 1982a).
Exposure to diazinon at different concentrations induced
pathological changes in muscle cell organelles of Tilapia nilotica.
The earliest changes in muscles of fish exposed to 10 mg/litre
consisted of swelling of sarcoplasmatic reticulum in many fibres and
the appearance of many cytoplasmatic vacuoles of different sizes. In
muscle of fishes exposed to the LC50 (20 mg/kg) of diazinon,
fragmentation of the myofibrils occurred over the entire length of the
sarcomere with involvement of both the thick myosin filament of the
A-band and the thinner actin filaments of the I-band. It was
speculated that these effects may be attributed to the
anti-cholinesterase activity of these insecticides. (Sakr & Gabr,
1992).
Acute effect of diazinon on the intramembranous particles
(IMPs) of microvilli of the intestinal epithelial cells of Tilapia
nilotica fish was studied using the freeze-fracture technique.
Exposing fish to different repeated concentrations of diazinon
(´ LC50) cause a significant decrease in the population density of
IMPs in P and E faces. IMPs of microvilli found in intestinal
epithelial cells are thought to represent many kinds of protein
including enzymes. It was suggested that diazinon induced a reduction
in enzymatic content of the membrane, which was accompanied by a
decrease in the IMP density of the microvilli (Sakr et al., 1991).
El-Elaimy et al. (1990) reported that exposure of Tilapia nilotica
to increasing concentrations of diazinon induced ultrastructural
alterations in the intestine.
Four-month old adult siblings of zebrafish (Brachydanio rerio)
were exposed to four concentrations of diazinon for up to 168 h. DNA,
RNA, protein and total free amino acid content were measured in the
liver. The DNA, RNA and protein contents were significantly reduced,
whereas the amino acid content was significantly enhanced. All these
changes showed a dose-dependent as well as a time-dependent response
(Ansari & Kumar, 1988).
Anees (1978) presented the results of sub-lethal exposure to
diazinon of an adult freshwater teleost. The concentrations of
insecticide used in the study were given as 0.37 mg/litre per 24 h and
0.28 mg/litre per 96 h. This was a study on the evaluation of
histopathology as a possible indicator of aquatic pollution by
insecticides. Hepatocytes were considerably vacuolated and reduced in
size after 24 h of exposure. However, 96 h of exposure produced less
vacuolization but the liver showed a foamy appearance. Damage to the
hepatic blood supply and the appearance of dark, granular cytoplasmic
inclusions resulted from a 14-day exposure. The same concentrations of
pesticide have already been observed to cause many tissue changes in
the intestine of Channa punctatus and disturbances in the
distribution of serum proteins.
The effect of exposure to the LC50 (12 mg/litre) for 96 h
and to a sublethal concentration (3.3 mg/litre) for 15 and 30 days
was studied in the digestive system of the catfish Heteropneustes
fossilis. The most conspicuous pathological changes in the liver
were vacuolization of the cytoplasm of hepatocytes, enlargement of
nuclei, rupture of cell membrane, liver cord disarray, and damage of
connective tissue. Intercellular spaces were widened. In stomach the
mucosa was eroded. In intestine the nuclei of the columnar epithelial
cells were reduced in volume and the cytoplasm was highly degenerated.
In both acute and chronic exposure alkaline phosphatase and
glucose-6-phosphatase activities were inhibited significantly in the
different portions of the digestive system. Acid phosphatase activity
was elevated in all the portions and in both stages of exposure. In
acute exposure insignificant elevation was observed in the activities
of amylase and maltase, while lactase activity was inhibited. An
inhibition in maltase activity was significant only in the liver.
Lipase activity showed a decrease in all stages of exposures, while
there was no marked alteration in activities of pepsin and trypsin
(Sastry & Malik, 1982b).
9.4 Effects in mesocosms and the field
A mesocosm study was performed with 17 treated and 4 untreated
ponds (each 0.1 acre in surface area and 2.2 metres deep). The ponds
received multiple applications of diazinon with single applications
ranging from 2 to 25 µg/litre and corresponding to total application
rates of 5.7, 11.4, 22.9, 45.8 and 91.5 µg/litre. The following
effects on sediment-dwelling organisms were observed: Trichoptera was
the most sensitive order, with significant reductions at all treatment
levels throughout most of the treatment period. Trichoptera was a
minor component of the macroinvertebrate communities in the mesocosms.
Diptera and Ephermeroptera were intermediate in sensitivity; both were
reduced at 22.9 and 91.5 µg/litre by the end of the treatment period,
though both recovered to control levels shortly after treatment
ended. Odonata was the least sensitive order with effects only at
91.5 µg/litre. Total insect abundance generally followed the trend
of the Diptera, which was the most abundant order; increasingly
severe effects at 22.9 to 91.5 µg/litre during the treatment
period was followed by recovery. The abundance of crustaceans in
macroinvertebrate samples generally followed a similar pattern.
Gastropods were essentially unaffected by diazinon. Though dipterans
as a whole were affected only at 22.9 µg/litre or more and recovered
rapidly, some dipteran sub-taxa were more sensitive or were
significantly reduced in the post-treatment period. Tanypodinae and
its dominant tribe, Pentaneurini, were the most sensitive. These were
significantly reduced in all treated ponds at the end of the treatment
period. Both groups recovered within eight weeks of the last
treatment. The sub-family Chironominae was the next most sensitive
among the family Chironomidae, especially the tribes Tanytarsini and
Chironomini. Tanytarsinids were reduced at 22.9 µg/litre early in the
treatment period and then recovered. Chironomini followed a similar
pattern, including recovery, but were again reduced in number at 22.9
to 91.5 µg/litre. The sub-family Orthocladiinae was the least
sensitive chironomid group with effects only at 91.5 µg/litre and full
recovery within two weeks. With the wide range of sensitivity observed
among the chironomids, the overall impact of diazinon was relatively
minor; statistically significant reductions were observed twice early
in the highest treatment. Two less abundant dipteran families,
Chaoboridae and Ceratopogonidae, were affected at 11.4 µg/litre or
more. The greatest effect on Chaoboridae occurred two months into
treatment and that on Ceratopogonidae occurred during the treatment
period, but sporadic effects persisted beyond (Giddings, 1992).
The application of diazinon to a small stream at 3 mg/litre
resulted in increased drift of benthic organisms and changes in the
composition of benthic fauna. These changes were not persistent,
however, as the communities were restored to normal within 4 weeks of
treatment (Miller et al., 1966).
9.5 Terrestrial invertebrates
Stevenson (1978) reported LD50 values for bees of 0.22 µg/bee
(topical) and 0.20 µg/bee (oral).
When earthworms (Eisenia foetida) were exposed to technical
diazinon in soil, the 14-day LC50 was calculated to be 130 mg/kg
soil. A no-observed-effect concentration of 12.3 mg/kg was reported
(Vial, 1990).
In a field experiment in Connecticut, USA, diazinon was
applied to tobacco fields as a 10% granular formulation at up to
4.48 kg a.i./ha; granules were raked into the soil. Mortality of
earthworms (Lumbricus terrestris) did not differ between treated and
control plots (Kring, 1969).
9.6 Birds
Acute toxicity to birds is summarized in Table 7.
The use of diazinon for controlling flies in sheds used to house
ducks led to the death of an estimated 15 600 young birds (Dougherty,
1957).
Ingestion of a few granules could be lethal to sparrow-sized
birds for diazinon 14G (Hill & Camardese, 1984).
Variability of toxicity among anticholinesterase formulations was
shown with a single dose of diazinon administered orally as technical
grade (TG, 99% a.i.) alone or in corn oil, as granular (GR, 14-15%
a.i.), or as emulsifiable concentrate (EC, 48% a.i.). The rank of the
formulations tested, from most to least toxic, based on statistically
separable (p<0.05) LD50s, was EC > GR=TG > control. The difference
between the least and most toxic form was nearly three-fold (Hill,
1988).
The single acute oral and dermal doses of diazinon were
determined for 8- and 18-week-old broadbreasted white turkeys. The
hazard to turkeys of exposure to soils treated for control of chiggers
was evaluated. Diazinon was lethal for 18-week-old turkeys at 2 mg/kg
orally and toxic at 5 mg/kg dermally. In spite of this high toxicity,
exposing the turkeys to soil treated with 18 kg diazinon per hectare
led to no poisoned birds (Radeleff & Kunz, 1972).
The activity of acetylcholinesterase (AChE) and the density of
muscarinic receptors were measured in brains from normal Japanese
quail (Coturnix coturnix japonica) and from quail after lethal
intoxication with diazinon. The maximum relative loss of activity due
to postmortem decomposition alone during 8 days was 13 and 10% for
AChE and muscarinic receptors, respectively. During postmortem
decomposition, the ratio of AChE and muscarinic receptor activities
remained constant at approximately 1.3:1 in normal brains, while it
was always less than, or equal to, 0.5:1 after intoxication with
diazinon. Normal AChE activity could be estimated from muscarinic
receptor density. Parallel measurement of AChE and muscarinic
receptors may assist in the postmortem diagnosis of death due to acute
poisoning with anti-cholinesterase pesticide when control specimens
are not available (Prijono & Leighton, 1991).
Anticholinesterases do not pass through the mother to the egg in
significant amounts, but they may be deposited on the egg from the
parents feathers or during pesticide application. To simulate topical
exposure, fertile mallard eggs were either immersed for 30 seconds in
an aqueous emulsion or single dose of an anticholinesterase in a
non-toxic oil vehicle pipetted on the shell on day 3 of incubation
which is a critical period with respect to organogenesis in mallards.
Table 7. Acute toxicity to birds
Species Age/weight LD50 LC50 Reference
Japanese quail (Coturnix coturnix japonica) 50-60 days (105-195 g) 4 Sachsse (1973a)
5 days (10-30 g) 1.1 Sachsse & Ullmann (1975a)
50-60 days 900a Sachsse (1972)
Bobwhite quail (Colinus virginianus) 14 days 5.2 Fink (1976)
140-180 g 4.3 Sachsse & Ullmann (1975b)
Peking duck (Anas domesticus) 2.5-3.5 kg 2.7 Sachsse & Ullmann (1976)
5 days (40-125 g) 1.9 Sachsse & Ullmann (1975c)
Domestic hen (Gallus domesticus) 5 days (40-50 g) 14 Sachsse & Ullmann (1975d)
Mallard duck (Anas platyrhynchus) 19 weeks (843-1357 g) 1.44 Fletcher & Pedersen (1988a)
5 days 202a Sachsse (1973b)
9 days (108-120 g) 32 Fletcher & Pedersen (1988c)
Brown-headed cowbird (Molothrus ater) 35-55 g 85 Fletcher & Pedersen (1988b)
a Repellency recorded in all dosage groups.
Organophosphates were shown to be as much as 18 times more toxic when
applied to the shell in an oil compared with following water
immersion. Neither method of egg treatment produced teratogenicity or
developmental effects at realistic pesticide application rates
(Hoffman & Eastin, 1981).
A laboratory reproduction study was conducted with mallard in
which birds were allowed to build their own nests and incubate eggs
(Marselas et al., 1989a). Birds were fed diets containing 0, 5, 10 and
20 mg diazinon/kg diet (20 pairs per dose level). The exposure to 5
and 10 mg/kg did not result in any overt signs of toxicity or effects
on reproductive performance. At 20 mg/kg, there was an increase in the
number of hens that did not incubate and, although egg production was
not affected, there was some reduction in the number of hatchlings and
14-day-old survivors. A companion study (Marselas et al., 1989b) with
bobwhite quail was conducted at dietary concentrations of 10, 20 and
40 mg/kg. Exposures did not result in effects on reproductive
performance and no mortalities or overt signs of toxicity were
observed.
Henderson et al. (1994) described certain oral and dermal
toxicity of diazinon to the domestic pigeon. Dose-dependent gross
symptoms of organophosphorus poisoning usually appear within half an
hour in pigeons dosed orally. There was a 50% inhibition of brain
cholinesterase activity, i.e. ID50 of diazinon was about 2 mg/kg body
weight. Plasma cholinesterase activity in dermally exposed birds was
almost completely inhibited by 24 h after diazinon treatment. The
recovery of plasma cholinesterase after dermal exposure of pigeon was
very slow.
Johnston et al. (1994) studied the interactive effects of
combined treatment of red-legged partridge with diazinon and an
ergosterolbiosynthesis-inhibiting (EBI) fungicide (prochloraz). Birds
were pre-treated with procloraz at 180 mg/kg and then exposed to
diazinon at 4.3 mg/kg 24 h later. There was a tendency to increased
inhibition of cholinesterase in the plasma of treated birds but this
was not significant. Synergism was significant with other
organophosphorus compounds tested.
9.6.1 Field studies
Nine fairways of a golf course located in Bellingham, Washington,
USA, were treated with Diazinon AG500 at a target application rate of
2.2 kg a.i./ha. The chemical application with a boomless sprayer
resulted in a variable distribution of diazinon residues on the turf
that ranged from 1.0 to 6.2 kg a.i./ha. The diazinon-treated turf was
irrigated with 1.3 cm of water immediately following application. The
post-irrigation diazinon residue levels ranged from 100 to 33 mg/kg
(mean=209; SD=88; n=8). These residue levels were higher than expected
based on results of turf studies in other regions of the USA.
Eighty-five American wigeon died after grazing on one treated fairway
on the day of application following irrigation. The brains of all 85
wigeon were analysed for acetylcholinesterase activity. Wigeon that
died on the study area showed 44 to 87% depression of AChE activity
when compared to controls. Upper gastrointestinal tract contents of 15
of the 85 dead wigeons contained 0.96 to 18.1 mg/kg diazinon (Kendall
et al., 1992).
In the USA, use of diazinon has been discontinued on golf courses
since 1988. Waterfowl often congregate near ponds on golf courses and
were exposed to turf treatments. Since this restriction, deaths of
grazing waterfowl (Canada geese, brent geese and American wigeon) have
not occurred. Application rate reduction may also be effective in
reducing risk to Canada geese, the most common grazing bird on turf.
Kendall et al. (1993) closely monitored geese exposed to diazinon
applied twice at 2.2 kg a.i./ha, and found no mortality despite
extensive feeding on treated turf.
Hummell et al. (1992a,b,c) conducted an extensive, multi-plot
assessment of the effects of turf applications of granular and liquid
formulations (4.8 kg a.i./ha) of diazinon on songbirds.
Since the introduction of diazinon, sporadic mortality of
waterfowl feeding on treated turf or on orchard grass has come to
light in Canada. Frank et al. (1991) reported five incidents that
took place in Ontario between 1986 and 1988. Fifty-seven Canada
geese were poisoned by diazinon on turf sites in Ontario 1986-88. It
was determined that median levels of diazinon in turf sprayed and
then properly irrigated ranged from 45.4 to 256 mg/kg for a single
application of 1 kg a.i./ha of the EC formulation. A value of
390 mg/kg was obtained for a single grass sample following spray at
approximately 9 kg a.i./ha. Maximum residue levels for grass recovered
from the oesophagi of geese killed by diazinon on turf, and collected
while still fresh, ranged from 55 to 79 mg/kg.
In trials evaluating applications to large turf areas (an average
of ha), survival, behaviour and reproductive performance were
monitored. Smaller plots (0.09 ha) that simulated large home yards
were established for granular formulations, and survival and plasma
ChE were evaluated. On large plots, the liquid formulation had no
effect on reproductive performance, survival or cholinesterase levels.
Granular applications had a minor impact on bird population size (9.6%
reduction) compared to control plots (4.8% reduction). Reproductive
performance was not affected, although cholinesterase levels were
reduced compared to controls. On the smaller (home-sized) turf plots
there was no apparent effect on bird numbers, and plasma
cholinesterase activity was not affected in any species.
Decarie et al. (1993) sprayed ornamental and other deciduous
trees with diazinon at a rate of 2.2 kg a.i./ha in a suburban area of
Quebec, Canada, where the compound was used to control fruit tree
mites. Twelve nests of the American robin were sprayed and 65 nests
untreated were used as controls. Productivity was compared between
treated and control nests as was the behaviour of the birds. Mean
productivity was not significantly different between the two groups.
Number of feeding flights, number of female feeding flight and number
of faecal sacs removed did not differ between groups but there was a
significant increase in the total time spent sitting on the nest in
diazinon-treated birds. The significance of this is unclear. Plasma
AChE levels were reduced (993 ± 639 µg/litre in treated birds;
3585 ± 1531 µg/litre in controls), but brain AChE activity was
unaffected.
10. EVALUATION OF HUMAN HEALTH RISK AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risk
Diazinon is an organophosphorus pesticide classified by WHO as
"moderately hazardous" Class II (WHO, 1996). It is absorbed from the
gastrointestinal tract, through intact skin, and by inhalation. The
sources of exposure to humans are occupational, accidental or through
diet. Diazinon is used as a pesticide and veterinary drug to control
ectoparasites. The major source of diazinon food residues in edible
crops are from agricultural usage while those in meat, offal and other
animal products arise from veterinary use
The results of total diet studies in the USA, United Kingdom and
New Zealand suggest that levels of exposure are below the recommended
Acceptable Daily Intake (ADI) of 0.002 mg/kg per day (FAO/WHO, 1994b).
Diazinon is rapidly broken down, whether on plants or in animals,
further reducing the risk to humans. Several different application
techniques are used in applying diazinon outside and inside. Residual
spraying and space treatment are often used in controlling pests
indoors. Following recommended use, concentrations of diazinon
detectable in the indoor air are low and do not present a health
hazard. Some studies of agricultural workers have shown cases of
contact dermatitis after exposure to diazinon. Toxicity studies in
humans have shown that the administered daily dose of 0.025 mg/kg body
weight marginally inhibits the plasma cholinesterase activity and it
can be considered as the no-observed-adverse-effect level (NOAEL).
Diazinon is not genotoxic and exhibits no carcinogenic potential in
rats or mice. Several episodes of fatal and non-fatal accidental and
suicidal poisoning have occurred. Acute poisoning causes typical
cholinergic signs and symptoms. Acute pancreatitis can be associated
with severe poisoning.
10.2 Evaluation for effects on the environment
The information on utilization and application rates that has
been employed for this risk assessment is derived from the
agricultural use of diazinon within the European Union. It should be
possible to extrapolate this assessment to other agricultural uses at
similar application rates elsewhere in the world. The application
rates for diazinon can be summarized as follows: arable (tractor-
mounted/drawn hydraulic spray boom applications), 1.0 kg/ha; top fruit
(broadcast air-assisted applications), 1.2 kg/ha.
The following risk assessment is based on the principle of
calculating toxicity-exposure ratios (TERs) (Fig. 2), which follows
the European and Mediterranean Plant Protection Organisation and
Council of Europe (EPPO/CoE) Environmental Risk Assessment Scheme
model and associated trigger values (EPPO/CoE, 1993a,b).
 10.2.1 Aquatic organisms
The main risk to aquatic organisms from the use of diazinon is
from spray drift during either arable applications (1.0 kg/ha) or top
fruit air-assisted (1.2 kg/ha). For each of these risk scenarios, the
predicted environmental concentration (PEC) in a 30-cm-deep static
surface water body, arising from either arable-based spray drift at
1 m from the edge of the spray boom or from top fruit air-assisted
spray drift at 3 m from the point of application (both based on
Ganzelmeier et al., 1995), was calculated as follows:
PEC = max application rate (kg/ha) × A (% spray drift)
(mg diazinon/
litre) 300
Where A = 5 for ground-based hydraulic spray application 1 m from edge
of boom
= 30 for air-assisted application 3 m from point of
application
10.2.1.1 Acute risk
The acute EC50 value for the most sensitive fish was
0.09 mg/litre and for the most sensitive aquatic invertebrate
(Gammarus) 0.0002 mg/litre. For the most sensitive algal
species the 14-day NOEC was 1 mg/litre.
(a) Spray drift from ground-based applications: The acute PEC for
spray drift (l m from the edge of the spray boom into a 30-cm-deep
static water body at the maximum application rate (see PEC assumptions
above) is 0.017 mg/litre. Therefore, the TERs, based on this PEC and
the above EC50/NOEC toxicity values, are: fish, 5.4; aquatic
invertebrates, 0.01; and algae, 60. Based on the EPPO/CoE
risk assessment scheme for aquatic organisms, these TERs (i.e. TERs
>10 = low risk; TERs <1 = high risk) indicate a low acute risk to
algae and a high risk to these organisms. The TER for fish was between
1 and 10, indicating an intermediate risk. In such risk situations the
use of a "no-spray" restriction zone next to surface waters may reduce
the risk to such aquatic invertebrates. For example, arable spray
drift at 5 m from the edge of boom is 0.6% (Ganzelmeier et. al.,
1995). Based on this 5-m drift data, the PEC is 0.002 mg/litre and
results in a 5-m TER of 45 for fish. This TER at 5 m indicates that
the use of a 5-m "no-spray" restriction zone next to surface waters
would reduce the acute risk to fish.
(b) Spray drift from broadcast air assisted top fruit
applications: The acute PEC for spray drift (3 m from the point of
application into a 30-cm-deep static water body at the maximum
application rate (see PEC assumptions above) is 0.12 mg/litre.
Therefore, the TERs based on this PEC and the above EC50/NOEC
toxicity values are: fish, 0.75; aquatic invertebrates, 0.0017; and
algae, 8.3. Based on the CoE/EPPO risk assessment scheme for aquatic
organisms, these TERs indicate a high acute risk to fish and
invertebrates and an intermediate risk to algae. Table 8 below
summarizes the acute TERs for aquatic organisms at 1 m for arable and
3 m for broadcast air-assisted applications.
10.2.1.2 Chronic risk
There were no chronic toxicity data available.
10.2.2 Terrestrial organisms
Vertebrates are likely to be exposed to diazinon from either
grazing treated vegetation or consuming contaminated insects. For this
risk assessment, typical application rates of 1 kg/ha are used for
ground spray application on arable crops and 1.2 kg/ha for application
by air-assisted spraying for fruit
10.2.2.1 Birds
The lowest reported acute oral LD50 for birds is 1.1 mg/kg body
weight for the Japanese quail. The dietary LC50 for the mallard duck
is 32 mg/kg diet.
Indicator birds for use in the risk assessment will be:
* Greylag goose (Anser anser), as a grazing species, with a body
weight of 3 kg and total daily food consumption of 900 g
vegetation (dry weight) (Owen, 1975)
* Blue tit (Parus caeruleus), as an insectivorous species, with a
body weight of 11 g and total daily food consumption of 8.23 g
(dry weight) (Kenaga, 1973).
a) Grazing birds
Initial residues on short grass or cereal shoots, arising from
application at 1 kg/ha to arable crops, are estimated to be 112 mg/kg
dry weight (based on 112 × application rate in kg/ha (EPPO/CoE,
1993a,b) and at 134.4 mg/kg from application to fruit at a rate of
1.2 kg/ha. This gives an estimated total oral intake for the goose of
100.8 and 121.0 mg for the two application rates assuming that the
goose ate exclusively food contaminated at this level. This is
equivalent to a daily intake of 33.6 and 40.3 mg/kg body weight,
respectively. TERs can be calculated as follows:
Table 8. Acute toxicity-exposure ratio (TERs) for aquatic organisms at 1 m for arable and 3 m
for broadcast air-assisted applications
Species EC50/NOEC PEC PEC TER TER
(mg/litre) (mg/litre) (mg/litre) (arable) (air-assisted)
(arable) (air-assisted)
Fish (Oncorhynchus mykiss) 0.09 0.017 0.12 5.4 0.75
Aquatic invertebrate (Gammarus) 0.0002 0.017 0.12 0.012 0.0017
Algae (Selenastrum capricornutum) 1.0 0.017 0.12 60.0 8.3
End-point LD50/L50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Bird acute oral 1.1 mg/kg 1 112 0.033
body weight (arable)
Japanese quail
1.2 134.4 0.027
(fruit)
Bird short-term 32 mg/kg diet 1 112 0.29
dietary mallard duck (arable)
1.2 134.4 0.24
(fruit)
The calculated TER values fall well below the EPPO/CoE trigger
values for concern (TER <10) and indicate a high risk to grazing
birds. This potential risk has been confirmed in practice with
reported high incidence of fatalities following application of
diazinon to golf-course turf. This application is no longer
recommended for this reason.
b) Insectivorous birds
Initial residues on small insects arising from application at
1 kg/ha to arable crops are estimated to be 29 mg/kg dry weight (based
on 29 × application rate in kg/ha) (EPPO/CoE, 1993a,b) and 34.8 mg/kg
from application to fruit at a rate of 1.2 kg/ha. This gives an
estimated total oral intake for the blue tit of 0.24 mg and 0.29 mg
for the two application rates assuming that the blue tit ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 21.7 and 26.0 mg/kg body weight, respectively. TERs
can be calculated as follows:
The TERs for acute toxicity to insectivorous birds are
substantially less then the trigger value of <10, indicating high
acute risk to these birds. The risk factors exceed the trigger for
insectivorous birds exposed short-term via the diet.
End-point LD50/LC50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Bird acute oral 1.1 mg/kg 1 29 0.05
body weight (arable)
Japanese quail
1.2 34.8 0.04
(fruit)
Bird short-term 32 mg/kg diet 1 29 1.1
dietary (arable)
1.2 34.8 0.92
(fruit)
10.2.2.2 Mammals
The lowest reported acute oral LD50 for laboratory mammals is
82 mg/kg body weight for the mouse.
Indicator mammals for use in the risk assessment will be:
* Rabbit (Orystolagus cuniculus), as a grazing mammal, with
a body weight of 1200 g and a total daily food consumption
of 500 g vegetation (dry weight) (Ross, personal
communication to the IPCS)
* Shrew (Sorex araneus), as an insectivorous mammal, with a
body weight of 18 g and a total daily food consumption of
18 g (Churchfield, 1986)
a) Grazing mammals
Initial residues on short grass or cereal shoots arising from
application at 1 kg/ha to arable crops are estimated to be 112 mg/kg
dry weight (based on 112 x application rate in kg/ha) (EPPO/CoE,
1993a,b) and at 134.4 mg/kg from an application to fruit at a rate of
1.2 kg/ha. This gives an estimated total oral intake for the rabbit of
56 and 67.2 mg for the two application rates, assuming that the rabbit
ate exclusively food contaminated at this level. This is equivalent to
a daily intake of 46.7 and 56 mg/kg body weight, respectively. TERs
can be calculated as follows:
End-point LD50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Mammal 82 mg/kg 1 56 1.76
acute oral body weight (arable)
mouse
1.2 67.2 1.46
(fruit)
This indicates a high risk to grazing mammals (trigger <10)
comparable to that for grazing birds. Again, the removal of
application to golf-course turf would reduce the likelihood of
exposure to maximum residues of diazinon. However, grazing mammals
consuming short cereal shoots could be killed following recommended
use of the compound.
b) Insectivorous mammals
Initial residues on large insects arising from application at
1 kg/ha to arable crops are estimated to be 2.7 mg/kg dry weight
(based on 2.7 × application rate in kg/ha) (EPPO/CoE, 1993a,b) and
3.24 mg/kg from an application to fruit at a rate of 1.2 kg/ha. This
gives an estimated total oral intake for the shrew of 0.049 mg and
0.058 mg for the two application rates, assuming that the shrew ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 2.7 and 3.2 mg/kg body weight, respectively. TERs can
be calculated as follows:
These TERs fall outside the trigger for high risk for
insectivorous mammals but within the range for medium risk, reflecting
the high acute mammalian toxicity.
End-point LD50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Mammal 82 mg/kg 1 2.7 30.4
acute oral body weight (arable)
mouse
1.2 3.24 25.3
(fruit)
10.2.2.3 Bees
The reported contact and oral toxicity to bees gives LD50 values
of 0.22 and 0.2 µg/bee, respectively. Using application rates of 1000
and 1200 g/ha for cereals and fruit, respectively, hazard quotients
are calculated to be 4545 and 5455 (application in g/ha). The trigger
for concern is >50 (EPPO/CoE, 1993a,b) and, therefore, there is
substantial concern for exposed bees. The compound should not be
applied to flowering plants and exposure of flying bees should be
avoided.
10.2.2.4 Earthworms
Earthworms are likely to be exposed to the highest concentration
of diazinon following use of the granular formulation incorporated in
soil at up to 2000 g/ha. Based on a soil depth of 5 cm and a soil
density of 1.5 g/cm3, the soil PEC would be 2.67 mg/kg, assuming even
distribution in the medium. The reported LC50 for earthworms
(Eisenia foetida) is 130 mg/kg soil, giving a TER of 48.75. As this
is above the trigger value of 10 (EPPO/CoE, 1993a,b), the acute risk
to earthworms should be low. Reported concentrations measured in soil
are at least an order of magnitude lower than the PEC suggesting that
little risk is posed to worms.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
The general population does not face a significant health risk
from diazinon. It may cause acute poisoning in cases of over-exposure
following intentional ingestion or careless handling during its
manufacture and use. The general public is exposed to diazinon in the
form of its residues in food, but the reported intake of diazinon is
far below the acceptable daily intake. Following residual spraying and
space treatment, used to control insects, the general population can
be exposed to residues in the air and on surfaces. With good work
practice and hygiene measures, and if safety precautions and medical
surveillance are enforced, diazinon is unlikely to present a hazard to
those occupationally exposed.
Diazinon does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates, fish, terrestrial birds and mammals, leading to high
risk factors for many of these organisms. Field kills of waterfowl
have been reported following use of the compound on amenity turf.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift and avoid areas where wildfowl are likely to graze).
11.2 Recommendations for protection of human health and the
environment
Certain groups within the population, including agricultural
workers and employees in the chemical industry, have the potential of
being exposed to diazinon. Gardeners and householders may also be
involved.
11.2.1 Recommendation on regulation of compound
Formulations have different classification categories: (FAO/WHO,
1979): liquids over 20% are in category 3; other liquids or solids
over 50% are in category 4; and all other solids are in category 5.
11.2.1.1 Transport and storage
Formulations in categories 3 and 4 should be transported and
stored in clearly labelled rigid and leak-proof containers, away from
containers of food and drink. Storage should be under lock and key and
secure from access by unauthorized people. Formulations in category 5
should be transported and stored in clearly labelled leak-proof
containers, out of reach of children and away from food and drink.
11.2.1.2 Handling
Protective clothing should be used by all handling of the
compound. Adequate washing facilities should be available at all times
during handling and should be close to the site of handling. Eating,
drinking and smoking should be prohibited during handling and before
washing the hands after handling.
11.2.1.3 Disposal
Containers may be decontaminated as recommended by the WHO Expert
Committee on Vector Biology and Control on the Safe Use of Pesticides
(WHO, 1991). Decontaminated containers should not be used for food and
drink. Containers that are not decontaminated should be burned or
crushed and buried below topsoil. Care must taken to avoid subsequent
contamination of water sources.
11.2.1.4 Selection, training and medical supervision of workers
Pre-employment medical examination of workers is desirable.
Workers suffering from active hepatic or renal disease should be
excluded from contact with diazinon. Pre-employment and periodic
cholinesterase test for workers is desirable especially for those
handling concentrates. Training of workers in techniques to avoid
contact is essential. Pilots and loaders should have special training
in application methods and early symptoms of poisoning, and must wear
a suitable respirator. Flagmen, if used, should wear overalls and be
located well away from the dropping zone.
11.2.1.5 Labelling
Diazinon is an organophosphorus compound that inhibits
cholinesterases. It is poisonous if swallowed. It may be absorbed
though the skin. It is important to avoid skin contact, wear hand
protection, clean protective clothing. The material must be kept out
of reach of children and well away from foodstuffs, animal feed and
their containers. If poisoning occurs, a physician should be called.
11.2.1.6 Residues in food
Maximum residue limits have been recommended for diazinon by the
Joint FAO/WHO Meeting on Pesticides Residues (FAO/WHO, 1975).
11.2.2 Prevention of poisoning in man and emergency aid
11.2.2.1 Manufacture and formulation
Closed systems and forced ventilation may be required to reduce
as much as possible the exposure of workers to diazinon.
11.2.2.2 Mixers and applicators
When mixing, protective impermeable boots, rubber apron, clean
overalls and gloves should be worn. When spraying tall crops or during
aerial application, a face mask should be worn, as well as an
impermeable hat, clothing, boots and gloves. The applicator should
avoid working in spray mist and avoid contact with the mouth.
Particular care is needed when equipment is being washed after use.
All protective clothing should be washed immediately after use.
Splashes must be washed immediately from the skin or eyes with large
quantities of water.
11.2.2.3 Other associated workers
People exposed to diazinon and associated with its application
should wear protective clothing and observe the precautions described
in section 11.2.2.2.
11.2.2.4 Other populations likely to be affected
With good application practice, other people should not be
exposed to hazardous amounts of diazinon.
11.2.3 Entry into treated areas
Unprotected people should abide by re-entry periods stated on
product labels.
11.2.4 Emergency aid
If symptoms appear following exposure, the person should stop
work immediately, remove contaminated clothing, wash the affected skin
with soap and water, if available, and flush the area with large
quantities of water. If diazinon has been swallowed and the person is
conscious, vomiting should be induced.
Atropine and oximes are specific antidotes and artificial
respiration may be needed.
11.2.5 Surveillance test
Slight reduction of plasma cholinesterase activity can be
observed. Periodic blood cholinesterase tests for workers is
desirable.
12. FURTHER RESEARCH
1. Studies of exposed worker populations should be continued.
2. Diazinon should be manufactured and formulated in accordance with
international specifications. It should be packed and stored
under conditions that are not conducive to the formation of
acutely toxic impurities.
3. The safe use of diazinon should follow label directions and
precautions for handling, application and disposal.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Diazinon has been evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) on a number of occasions since 1965. An
acceptable daily intake (ADI) of 0.002 mg/kg body weight has been
established and maximal residues levels have been recommended for
diazinon in a wide range of food commodities (FAO/WHO, 1994a,b).
The following NOAELs were established:
Rat (two-year feeding study): 1.5 mg/kg diet
(0.06 mg/kg body weight per day)
Dog (one-year feeding study): 0.5 mg/kg diet
(0.015 mg/kg body weight per day)
Rhesus monkey (two-year study): 0.5 mg/kg body weight per day
Human volunteers (36-day study): 0.025 mg/kg body weight per day
Diazinon has not been evaluated by the International Agency for
research on Cancer (IARC).
REFERENCES
Abdelsalam EB & Ford EJH (1986) Effect of pretreatment with hepatic
microsomal enzyme inducers on the toxicity of diazinon in calves. Res
Vet Sci, 41: 336-339.
Abdelsalam EB & Ford EJH (1987) The effect of induced liver, kidney
and lung lesions on the toxicity of levamisole and diazinon in calves.
J Comp Pathol, 97: 619-627.
Adachi K, Ohokuni N, & Mitsuhashi T (1984) Simple analytical method
for organophosphorus pesticide determination in unpolished rice, using
removal of fats by zinc acetate. J Assoc Off Anal Chem, 67: 798-800.
Al-Attar HJ & Knowles CO (1982) Diazinon uptake, metabolism and
elimination by nematodes. Arch Environ Contam Toxicol, 11: 669-673.
Albright RK (1984) Renal involvement in organophosphate poisoning.
J Am Med Assoc, 252: 1408.
Allender WJ & Britt AG (1994) Analyses of liquid diazinon formulation
and breakdown products: an Australia-wide survey. Bul. Environ Contam
Toxicol, 53: 902-906.
Allison DT (1977) Use of exposure units for estimating aquatic
toxicity of organophosphate pesticides. Washington, DC, US
Environmental Protection Agency (EPA-600/3-77-077).
Allison DT & Hermanutz RO (1977) Toxicity of diazinon to brook trout
and fathead minnows. Washington, DC, US Environmental Protection
Agency (EPA-600/3-77-060).
Anees MA (1978) Hepatic pathology in a fresh-water teleost Channa
punctatus (Bloch) exposed to sub-lethal and chronic levels of
three organophosphorus insecticides. Bull Environ Contam Toxicol,
19: 524-527.
Ankley GT, Dierkes JR, Jensen DA, & Peterson GS (1991) Piperonyl
butoxide as a tool in aquatic toxicological research with
organophosphate insecticides. Ecotoxicol Environ Saf, 21: 266-274.
Ansari BA & Kumar K (1988) Diazinon toxicity: effect on protein and
nucleic acid metabolism in the liver of zebrafish, Brachydanio
rerio (Cyprinidae). Sci Total Environ, 76: 63-68.
Anthony J, Banister E, & Oloffs PC (1986) Effect of sublethal levels
of diazinon: Histopathology of liver. Bull Environ Contam Toxicol,
37: 501-507.
Arienzo M, Crisanto T, Sanchez-Martin T, & Sanchez-Camazano M (1994):
Effect of soil characteristics on adsorption and mobility of (14C)
diazinon. J Agric Food Chem, 42: 1803-1808.
Asensio JS, Barrio CS, Juez MTG, & Bernal JG (1991) Study of the
decay of diazinon and chlorpyriphos in apple samples, using gas
chromatography. Food Chem, 42: 213-224.
Ballantine L (1984) Advanced product chemistry percutaneous absorption
of 2delta-14C-diazinon in rats. Greensboro, North Carolina, Ciba-
Geigy Corporation (Unpublished report No. ABR-84011, submitted to WHO
by Ciba-Geigy Ltd, Basel).
Banerjee D (1967) Pericarditis in acute diazinon poisoning (A case
report). Armed Forces Med J (India), 23: 187-190.
Barik S & Munnecke DM (1982) Enzymatic hydrolysis of concentrated
diazinon in soil. Bull Environ Contam Toxicol, 29: 235-239.
Barnes TB, Hazelette JR, & Arthur AT (1988) Diazinon (mg-8): 13 week
oral toxicity study in dogs (Project No. 882012). Summit, New Jersey,
Ciba-Geigy Corporation (Unpublished report submitted to WHO by Ciba-
Geigy Ltd, Basel).
Barnett JB, Spyker-Cranmer JM, Avery DL, & Hoberman AM (1980)
Immunocompetence over the lifespan of mice exposed in utero to
carbofuran or diazinon: I. Changes in serum immunoglobulin
concentrations. J Environ Pathol Toxicol, 4: 53-63.
Bathe R (1972a) Acute dermal LD50 of technical diazinon (G-24'480)
in the rat (Project No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Bathe R (1972b) G 24`480 tech.: Acute oral LD50 in the mouse
(Project No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Bathe R (1973) Intraperitoneal LD50 of technical diazinon
(G-24480 MG 647) in the rat. Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished project report No. Siss 1679).
Bathe R & Gfeller W (1980) Report on acute oral LD50 in the rat of
technical G24'480 (Project No. 800478). Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Bopp C & Kosminsky B (1975) Toxic hepato-cutaneous porphyria. Med
Cutan Ibero Lat Am, 3: 271-279.
Bresch H (1991) Early life-stage test in zebrafish versus a growth
test in rainbow trout to evaluate toxic effects. Bull Environ Contam
Toxicol, 46: 641-648.
Bruce RB, Howard JW, & Elsea JR (1955) Toxicity of O,O-diethyl
O-(2-isopropyl-6-methyl-4 pyrimidyl) phosphorothioate (diazinon).
J Agric Food Chem, 3: 1017-1021.
Burgener J & Seim V (1988) Biological report for the metabolism of
14C-diazinon in laying hens dosed at 25 ppm ( Project No.
BIOL-88006). Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO Ciba-Geigy Ltd, Basel).
Burpee LL & Cole H Jr (1978) The influence of alachlor, trifluralin
and diazinon on the development of endogenous mycorrhiza in soybeans.
Bull Environ Contam Toxicol, 19: 191-197.
Butler GL, Deason TR, & Kelley JC (1975) The effect of atrazine,
2,4-D, methoxychlor, carbaryl and diazinon on the growth of planktonic
algae. Br Phycol J, 10: 371-372.
Byrne DH & Kitos PA (1983) Teratogenic effects of cholinergic
insecticides in chick embryos: IV. The role of tryptophan in
protecting against limb deformities. Biochem Pharmacol,
32: 2881-2890.
Cairns T, Siegmund EG, & Froberg JE (1985) Identification of
diazinon and its metabolite in spinach by chemical ionization
mass spectrometry. Bull Environ Contam Toxicol, 35: 291-295.
Capps T (1989) Characterization and identification of diazinon
metabolites in rats (Project No. 302211/302925). Greensboro, North
Carolina, Ciba-Geigy Corporation (Unpublished report No. ABR-88164,
submitted to WHO by Ciba-Geigy Ltd, Basel).
Capps TM (1990) Characterization and identification of major
metabolites in tissues of sheep treated dermally with 14C-diazinon
(Project No. 302925) Greensboro, North Carolina, Ciba-Geigy
Corporation (Unpublished report No. ABR-90014, submitted to WHO by
Ciba-Geigy Ltd, Basel).
Ceresa C & Puri E (1988) Micronucleus test, mouse (OECD-conform) G 24
480 tech. (diazinon) (Test No. 871696). Basel, Switzerland, Ciba-Geigy
Ltd ( Unpublished report).
Chen HH, Hsueh JL, Sirianni SR, & Huang CC (1981) Induction of
sister-chromatid exchanges and cell cycle delay in cultured mammalian
cells treated with eight organophosphorus pesticides. Mutat Res,
88: 307-316.
Chow E & Richter AG (1994) Acute neurotoxicity study with D-Z-N
diazinon MG 87% in rats (Project No. F-00175). Farmington,
Connecticut, Ciba-Geigy Corporation, Environmental Health Center
(Unpublished report).
Churchfield S (1986) Shrews. Oswestry, A. Nelson (The Mammal Society
series).
Claborn HV, Mann HD, Younger RL, & Radeleff RD (1963) Diazinon
residues in the fat of sprayed cattle. J Econ Entomol, 56: 858-859.
Classen W (1996) G24.480 tech. - delayed neurotoxicity in hens
following acute exposure (Project No. 952030). Stein, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Clegg TJ & Koevening JL (1974) The effect of four chlorinated
hydrocarbon pesticides and one organophosphate pesticide on ATP levels
in three species of photosynthesizing freshwater algae. Bot Gaz,
135: 368-372.
Cockrell KD, Woodard MW, & Woodard G (1966) Diazinon 50 W: Safety
evaluation by repeated oral administration to monkeys for 106 weeks.
Woodard Research Corporation USA (Unpublished report submitted to WHO
by Ciba-Geigy Ltd, Basel).
Collyard SA, Ankley GT, Hoke RA, & Goldenstein T (1994) Influence of
age on the relative sensitivity of Hyalella azteca to diazinon,
alkylphenol ethoxylates, copper, cadmium and zinc. Arch Environ Contam
Toxicol, 26: 110-113
Coye MJ, Barnett PG, Midtling JE, Velasco AR, Romero P, Clements CL, &
Rose TG (1987) Clinical confirmation of organophosphate poisoning by
serial cholinesterase analyses. Arch Intern Med, 147: 438-442.
Craine EM (1989a) A study of 14C-diazinon disposition in the rat
(biological-phase) (Project No. 302925). Ashland, Ohio, WIL Research
Laboratories Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Craine EM (1989b) A study of 14C-diazinon disposition in the rat
(analytical phase I) (Project No. 302925). Ashald, Ohio, WIL Research
Laboratories Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Currie KL, McDonald EC, Chung LTK, & Higgs AR (1990) Concentration of
diazinon, chlorpyrifos, and bendiocarb after application in offices.
Am Ind Hyg Assoc J, 51(1): 23-27.
Dagli AJ, Moos JS, & Shaikh WA (1981) Acute pancreatitis as a
complication of diazinon poisoning: A case report. J Assoc Physi
India, 29: 794-795.
Dahm PA (1970) Some aspects of the metabolism of parathion and
diazinon. In: O`Brien RD & Yamamoto I ed. Biochemical toxicology of
insecticides. London, New York, Academic Press, pp 51-63.
Davies DB & Holub BJ (1980a) Comparative subacute toxicity of
dietary diazinon in the male and female rat. Toxicol Appl Pharmacol,
54: 359-367.
Davies DB & Holub BJ (1980b) Toxicological evaluation of dietary
diazinon in the rat. Arch Environ Contam Toxicol, 9: 637-650.
Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowall J, & Furlong CE
(1996) The effect of the human serum paraoxonase polymorphism is
reversed with diazoxon, soman and sarin. Nat Genet, 14(3): 334-336.
Decarie R, DesGrange J-L, Lepine C, & Morneau F (1993) Impact of
insecticides on the American robin (Turdus migratorius) in a
suburban environment. Environ Pollut, 80: 231-238
Derbyshire JC & Murphy RT (1962) Diazinon residues in treated
silage and milk of cows fed powdered diazinon. J Agric Food Chem,
10: 384-386.
Dick GL, Heenan MP, Love JL, Udy PB, & Davidson F (1978) Survey of
trace elements and pesticide residue content: 2. Organochlorine and
organophosphorus pesticide residue content. NZ J Sci, 21: 71-78.
Dikshith TS, Behari JR, Datta KK, & Mathur AK (1975) Effect of
diazinon in male rats: Histopathological and biochemical studies.
Environ Physiol Biochem, 5: 293-299.
Dobbins PK (1967) Organic phosphate insecticides as teratogens in the
rat. J Fla Med Assoc, 54: 542-546.
Doggett SM & Rhodes RG (1991) Effects of a diazinon formulation on
unialgal growth rates and phytoplankton diversity. Bull Environ Contam
Toxicol, 47: 36-42.
Dollenmeier P & Müller D (1986) G 24`480 tech.: L5178Y/TK+/- mouse
lymphoma mutagenicity test (Project No. 840396). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Dougherty E III (1957) Thiophosphate poisoning in white Pekin ducks.
Avian Dis, 1: 127-130.
Dressel TD, Goodale RL Jr, Arneson MA, & Borner JW (1979) Pancreatitis
as a complication of anticholinesterase insecticide intoxication. Ann
Surg, 189: 199-204.
Dressel TD, Godale RL, & Borner JW (1980) A study of the
cholinesterases of the canine pancreas and the relationship between
reduced butyrylcholinesterase activity and pancreatic ductal
hypertension. Ann Surg, 192: 614-619.
Dutta H, Marcelino J, & Richmonds Ch (1992) Brain acetylcholinesterase
activity and optomotor behaviour in bluegills, Lepomis macrochirus,
exposed to different concentrations of diazinon. Arch Int Physiol
Biochim Biophys, 100: 331-334.
Ebere AG & Akintonwa A (1992) Acute toxicity of pesticides to
Gobius sp., Palaemonetes africanus, and Desmocaris trispimosa.
Bull Environ Contam Toxicol, 49: 588-592.
Eberle DO, Suter R, Nowak K, & Bosshardt HP (1974) CIPAC collaborative
study of the heat-stability of diazinon formulations using both CUPAC
and AOAC analytical methods. J Assoc Off Anal Chem, 57: 48-52.
Egan H & Weston RE (1977) Pesticide residues: Food surveys in the
United Kingdom. Pestic Sci, 8: 110-116.
Egyed MN, Malkinson M, Eilat A, & Schlosberg A (1974) Basudin
(diazinon) poisoning in goslings. Reffu Vet, 31: 22-26.
El-Elaimy IA, El-Saadany MM, & Gabr SA (1990) Pesticide poisoning to
fresh water teleost: VIII. Ultrastructural alterations of the
intestine of Tilapia nilotica under stress of exposure to diazinon
and neopybuthrin. J Egypt Ger Soc Zool, 1: 223-236.
English T, Ellis EF, & Ackerman J (1970) Organic phosphate poisoning.
Morbid Mortal Wkly Rep (Cent Dis Control), 19: 397-404.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993a) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 23: 1-54.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993b) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 24: 1-87.
FAO/WHO (1965) Diazinon. In: 1965 Evaluation of the toxicity of
pesticide residues in food. Rome, Food and Agriculture Organization of
the United Nations and Geneva, World Health Organization, pp 77-83
(FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27. 65).
FAO/WHO (1975) 1974 Evaluations of some pesticide residues in food.
Geneva, World Health Organization (WHO Pesticide Residues Series,
No 4).
FAO/WHO (1979) Data sheet on pesticides No. 45: Diazinon. Geneva,
World Health Organization (VBC/DS/79.45).
FAO/WHO (1994a) Diazinon. In: Pesticide residues in food - 1993:
Evaluations, Part I - Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 157-294 (FAO Plant Production
and Protection Paper 124).
FAO/WHO (1994b) Diazinon. In: Pesticide residues in food - 1993:
Evaluations, Part II - Toxicology. Geneva, World Health Organization,
pp 57-81 (WHO/PCS/94.4).
Farran A, DePablo J, & Barcelo D (1988a) Identification of
organophosphorus insecticides and their hydrolysis products by
liquid chromatography in combination with UV and thermospray-mass
spectrometric detection. J Chromatogr, 455: 163-172.
Farran A, DePablo J, & Hernandez S (1988b) Continuous-flow extraction
of organophosphorus pesticides coupled on-line with high-performance
liquid chromatography. Anal Chim Acta, 212: 123-131.
Fernandez-Casalderrey A, Ferrando MD, & Andreu-Moliner E (1992)
Endosulfan and diazinon toxicity to the freshwater rotifer
Brachious calcyflorus. J Environ Sci Health, B27: 155-164.
Ferrando MD, Sancho E, & Andreu-Moliner E (1991) Comparative acute
toxicities of selected pesticides to Anguilla anguilla. J Environ
Sci Health, B26(5/6): 491-498.
Ferrando MD, Alarcon V, Fernandez-Casalderrey A, Gamon M, &
Andreu-Moliner E (1992) Persistence of some pesticides in the
aquatic environment. Bull Environ Contam Toxicol, 48: 747-755.
Ferreira JR & Silva Fernandes AM (1980) Gas-liquid chromatographic
determination of organophosphorus insecticide residues in fruits and
vegetables. J Assoc Off Anal Chem, 63: 517-522.
Fink R (1976) Acute oral LD50 to bobwhite quail - diazinon technical.
Basel, Switzerland, Novartis Crop Protection (Unpublished report).
Fletcher DW & Pedersen CA (1988a) Diazinon MG8 technical: 14 day acute
oral LD50 study in mallard ducks. Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Fletcher DW & Pedersen CA (1988b) Diazinon MG8 technical: 14 day acute
oral LD50 study in brown-headed cowbirds. Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Fletcher DW & Pedersen CA (1988c) Diazinon MG8 technical: 8 day acute
dietary LC50 study in mallard ducklings. Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Fort DJ, Delphon J, Powers CR, Helems R, Gonzales R, & Stover EL
(1995) Development of automated methods of identifying toxicants in
the environment. Bull Environ Contam Toxicol, 54: 104-111.
Frank R, Braun HE, Van Hove Holdrinet M, Sirons GJ, & Ripley BD
(1982) Agriculture and water quality in the Canadian Great Lakes
basin: V. Pesticide use in 11 agricultural watersheds and presence in
stream water, 1975-1977. J Environ Qual, 11(3): 497-505.
Frank R, Braun HE, & Ripley BD (1989) Monitoring Ontario-grown apples
for pest control chemicals used in their production. Food Addit
Contam, 6: 227-234.
Frank R, Braun HE, & Ripley BD (1990) Residues of insecticides and
fungicides on Ontario-grown vegetables 1986-1988. Food Addit Contam,
7: 545-554.
Frank R, Mineau P, Braun HE, Barker IK, Kennedy SW, & Trudeau S (1991)
Deaths of Canada geese following spraying of turf with diazinon. Bull
Environ Contam Toxicol, 46: 852-858.
Frick TW, Dalo S, O'Leary JF, Runge W, Borner JW, Baraniewski H,
Dressel T, Shearen JG, & Goodale RL (1987) Effects of insecticide
diazinon on pancreas of dog, cat, and guinea pig. J Environ Pathol
Toxicol Oncol, 7: 1-12.
Fritz H (1974) Reproduction study - G24480 (diazinon techn.) rat -
Segment II: Test for teratogenic or embryotoxic effects. Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Fritz H (1975) Dominant lethal study on G24480 (diazinon techn.)
mouse: Test for cytotoxic or mutagenic effects on male germinal cells
(Project No. 327507) . Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Fujii Y & Asaka S (1982) Metabolism of diazinon and diazoxon in fish
liver preparations. Bull Environ Contam Toxicol, 29: 455-460.
Gaines TB (1960) The acute toxicity of pesticides to rats. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol,
14: 515-534.
Ganzelmeier IH, Rautmann D, Spangenberg R, Streloke M, Herrmann M,
Wenzelburger HJ, & Walter HF (1995) Studies on the spray drift of
plant protection products: Results of a test program carried out
throughout the Federal Republic of Germany. Oxford, London,
Edinburgh, Melbourne, Blackwell Scientific Publications.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1985a)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1978-September 1979. J Assoc Off
Anal Chem, 68: 842-861.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1985b)
Pesticides, selected elements, and other chemicals in adult total
diet samples, October 1978-September 1979. J Assoc Off Anal Chem,
68: 862-875.
Gasser R (1953) [A new insecticide with wide efficacy.] Z Naturforsch,
B8: 225-232 (in German).
Geleick D & Arni P (1990) Salmonella and Escherichia/liver-microsome
test (Project No. 891346) - Test substance: G24480 tech. Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Getzin LW (1968) Persistence of diazinon and zinophos in soil: Effects
of autoclaving, temperature, moisture, and acidity. J Econ Entomol,
61: 1560-1565.
Giddings JM (1992) Aquatic mesocosm test for environmental fate and
ecological effects of diazinon. Wareham, Massachusetts, Springborn
Laboratories, Inc. and Columbia, Missouri, ABC Laboratories, Inc.
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Giknis MLA (1989) Diazinon techn: A two generation reproductive study
in albino rats. Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report).
Gilmore DR & Cortes A (1966) Dual-ban preparative thin-layer
chromatography for the separation of diazinon and related from plant
material. J. Chromatogr, 21: 148-149.
Glaza SM (1993) Acute oral toxicity study of D-Z-N diazinon in rats
(Project No. HWI 6117-221). Madison, Wisconsin, Hazleton Wisconsin,
Inc. (Unpublished report).
Goodman LR, Hansen DJ, Coppage DL, Moore JC, & Matthews E (1979)
Diazinon chronic toxicity to, and brain acethylcholinesterase
inhibition in, the sheepshead minnow, Cyprinodon variegatus. Trans
Am Fish Soc, 108: 479-488.
Gowen JA, Wiersma GB, Tai H, & Mitchell WG (1976) Pesticide levels in
hay and soils from nine states. Pestic Monit J, 10: 114-116.
Gunner HB & Zuckerman BM (1968) Degradation of 'diazinon' by
synergistic microbial action. Nature (Lond), 217: 1183-1184.
Gunner HB, Zuckerman BM, Walker RW, Miller CW, Deubert KH, & Longley
RE (1966) The distribution and persistence of diazinon applied to
plant and soil and its influence on rhizosphere and soil microflora.
Plant Soil, 25: 249-264.
Hagenbuch JP & Mücke W (1985) The fate of diazinon in mammals
(Biochemistry product evaluation 1/85). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Hall SW & Baker BB (1989) Intermediate syndrome from organophosphate
poisoning. Vet Hum Toxicol, 31(4): 355.
Halle A & Sloas DD (1987) Percutaneous organophosphate poisoning .
South Med J, 80: 1179-1181.
Harris SB & Holson JF (1981) G 24`480 tech.: A teratology study of
diazinon (CAS Number
10.2.1 Aquatic organisms
The main risk to aquatic organisms from the use of diazinon is
from spray drift during either arable applications (1.0 kg/ha) or top
fruit air-assisted (1.2 kg/ha). For each of these risk scenarios, the
predicted environmental concentration (PEC) in a 30-cm-deep static
surface water body, arising from either arable-based spray drift at
1 m from the edge of the spray boom or from top fruit air-assisted
spray drift at 3 m from the point of application (both based on
Ganzelmeier et al., 1995), was calculated as follows:
PEC = max application rate (kg/ha) × A (% spray drift)
(mg diazinon/
litre) 300
Where A = 5 for ground-based hydraulic spray application 1 m from edge
of boom
= 30 for air-assisted application 3 m from point of
application
10.2.1.1 Acute risk
The acute EC50 value for the most sensitive fish was
0.09 mg/litre and for the most sensitive aquatic invertebrate
(Gammarus) 0.0002 mg/litre. For the most sensitive algal
species the 14-day NOEC was 1 mg/litre.
(a) Spray drift from ground-based applications: The acute PEC for
spray drift (l m from the edge of the spray boom into a 30-cm-deep
static water body at the maximum application rate (see PEC assumptions
above) is 0.017 mg/litre. Therefore, the TERs, based on this PEC and
the above EC50/NOEC toxicity values, are: fish, 5.4; aquatic
invertebrates, 0.01; and algae, 60. Based on the EPPO/CoE
risk assessment scheme for aquatic organisms, these TERs (i.e. TERs
>10 = low risk; TERs <1 = high risk) indicate a low acute risk to
algae and a high risk to these organisms. The TER for fish was between
1 and 10, indicating an intermediate risk. In such risk situations the
use of a "no-spray" restriction zone next to surface waters may reduce
the risk to such aquatic invertebrates. For example, arable spray
drift at 5 m from the edge of boom is 0.6% (Ganzelmeier et. al.,
1995). Based on this 5-m drift data, the PEC is 0.002 mg/litre and
results in a 5-m TER of 45 for fish. This TER at 5 m indicates that
the use of a 5-m "no-spray" restriction zone next to surface waters
would reduce the acute risk to fish.
(b) Spray drift from broadcast air assisted top fruit
applications: The acute PEC for spray drift (3 m from the point of
application into a 30-cm-deep static water body at the maximum
application rate (see PEC assumptions above) is 0.12 mg/litre.
Therefore, the TERs based on this PEC and the above EC50/NOEC
toxicity values are: fish, 0.75; aquatic invertebrates, 0.0017; and
algae, 8.3. Based on the CoE/EPPO risk assessment scheme for aquatic
organisms, these TERs indicate a high acute risk to fish and
invertebrates and an intermediate risk to algae. Table 8 below
summarizes the acute TERs for aquatic organisms at 1 m for arable and
3 m for broadcast air-assisted applications.
10.2.1.2 Chronic risk
There were no chronic toxicity data available.
10.2.2 Terrestrial organisms
Vertebrates are likely to be exposed to diazinon from either
grazing treated vegetation or consuming contaminated insects. For this
risk assessment, typical application rates of 1 kg/ha are used for
ground spray application on arable crops and 1.2 kg/ha for application
by air-assisted spraying for fruit
10.2.2.1 Birds
The lowest reported acute oral LD50 for birds is 1.1 mg/kg body
weight for the Japanese quail. The dietary LC50 for the mallard duck
is 32 mg/kg diet.
Indicator birds for use in the risk assessment will be:
* Greylag goose (Anser anser), as a grazing species, with a body
weight of 3 kg and total daily food consumption of 900 g
vegetation (dry weight) (Owen, 1975)
* Blue tit (Parus caeruleus), as an insectivorous species, with a
body weight of 11 g and total daily food consumption of 8.23 g
(dry weight) (Kenaga, 1973).
a) Grazing birds
Initial residues on short grass or cereal shoots, arising from
application at 1 kg/ha to arable crops, are estimated to be 112 mg/kg
dry weight (based on 112 × application rate in kg/ha (EPPO/CoE,
1993a,b) and at 134.4 mg/kg from application to fruit at a rate of
1.2 kg/ha. This gives an estimated total oral intake for the goose of
100.8 and 121.0 mg for the two application rates assuming that the
goose ate exclusively food contaminated at this level. This is
equivalent to a daily intake of 33.6 and 40.3 mg/kg body weight,
respectively. TERs can be calculated as follows:
Table 8. Acute toxicity-exposure ratio (TERs) for aquatic organisms at 1 m for arable and 3 m
for broadcast air-assisted applications
Species EC50/NOEC PEC PEC TER TER
(mg/litre) (mg/litre) (mg/litre) (arable) (air-assisted)
(arable) (air-assisted)
Fish (Oncorhynchus mykiss) 0.09 0.017 0.12 5.4 0.75
Aquatic invertebrate (Gammarus) 0.0002 0.017 0.12 0.012 0.0017
Algae (Selenastrum capricornutum) 1.0 0.017 0.12 60.0 8.3
End-point LD50/L50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Bird acute oral 1.1 mg/kg 1 112 0.033
body weight (arable)
Japanese quail
1.2 134.4 0.027
(fruit)
Bird short-term 32 mg/kg diet 1 112 0.29
dietary mallard duck (arable)
1.2 134.4 0.24
(fruit)
The calculated TER values fall well below the EPPO/CoE trigger
values for concern (TER <10) and indicate a high risk to grazing
birds. This potential risk has been confirmed in practice with
reported high incidence of fatalities following application of
diazinon to golf-course turf. This application is no longer
recommended for this reason.
b) Insectivorous birds
Initial residues on small insects arising from application at
1 kg/ha to arable crops are estimated to be 29 mg/kg dry weight (based
on 29 × application rate in kg/ha) (EPPO/CoE, 1993a,b) and 34.8 mg/kg
from application to fruit at a rate of 1.2 kg/ha. This gives an
estimated total oral intake for the blue tit of 0.24 mg and 0.29 mg
for the two application rates assuming that the blue tit ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 21.7 and 26.0 mg/kg body weight, respectively. TERs
can be calculated as follows:
The TERs for acute toxicity to insectivorous birds are
substantially less then the trigger value of <10, indicating high
acute risk to these birds. The risk factors exceed the trigger for
insectivorous birds exposed short-term via the diet.
End-point LD50/LC50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Bird acute oral 1.1 mg/kg 1 29 0.05
body weight (arable)
Japanese quail
1.2 34.8 0.04
(fruit)
Bird short-term 32 mg/kg diet 1 29 1.1
dietary (arable)
1.2 34.8 0.92
(fruit)
10.2.2.2 Mammals
The lowest reported acute oral LD50 for laboratory mammals is
82 mg/kg body weight for the mouse.
Indicator mammals for use in the risk assessment will be:
* Rabbit (Orystolagus cuniculus), as a grazing mammal, with
a body weight of 1200 g and a total daily food consumption
of 500 g vegetation (dry weight) (Ross, personal
communication to the IPCS)
* Shrew (Sorex araneus), as an insectivorous mammal, with a
body weight of 18 g and a total daily food consumption of
18 g (Churchfield, 1986)
a) Grazing mammals
Initial residues on short grass or cereal shoots arising from
application at 1 kg/ha to arable crops are estimated to be 112 mg/kg
dry weight (based on 112 x application rate in kg/ha) (EPPO/CoE,
1993a,b) and at 134.4 mg/kg from an application to fruit at a rate of
1.2 kg/ha. This gives an estimated total oral intake for the rabbit of
56 and 67.2 mg for the two application rates, assuming that the rabbit
ate exclusively food contaminated at this level. This is equivalent to
a daily intake of 46.7 and 56 mg/kg body weight, respectively. TERs
can be calculated as follows:
End-point LD50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Mammal 82 mg/kg 1 56 1.76
acute oral body weight (arable)
mouse
1.2 67.2 1.46
(fruit)
This indicates a high risk to grazing mammals (trigger <10)
comparable to that for grazing birds. Again, the removal of
application to golf-course turf would reduce the likelihood of
exposure to maximum residues of diazinon. However, grazing mammals
consuming short cereal shoots could be killed following recommended
use of the compound.
b) Insectivorous mammals
Initial residues on large insects arising from application at
1 kg/ha to arable crops are estimated to be 2.7 mg/kg dry weight
(based on 2.7 × application rate in kg/ha) (EPPO/CoE, 1993a,b) and
3.24 mg/kg from an application to fruit at a rate of 1.2 kg/ha. This
gives an estimated total oral intake for the shrew of 0.049 mg and
0.058 mg for the two application rates, assuming that the shrew ate
exclusively food contaminated at this level. This is equivalent to a
daily intake of 2.7 and 3.2 mg/kg body weight, respectively. TERs can
be calculated as follows:
These TERs fall outside the trigger for high risk for
insectivorous mammals but within the range for medium risk, reflecting
the high acute mammalian toxicity.
End-point LD50 Application Predicted TER
rate concentration
(kg/ha) in food
(mg/kg)
Mammal 82 mg/kg 1 2.7 30.4
acute oral body weight (arable)
mouse
1.2 3.24 25.3
(fruit)
10.2.2.3 Bees
The reported contact and oral toxicity to bees gives LD50 values
of 0.22 and 0.2 µg/bee, respectively. Using application rates of 1000
and 1200 g/ha for cereals and fruit, respectively, hazard quotients
are calculated to be 4545 and 5455 (application in g/ha). The trigger
for concern is >50 (EPPO/CoE, 1993a,b) and, therefore, there is
substantial concern for exposed bees. The compound should not be
applied to flowering plants and exposure of flying bees should be
avoided.
10.2.2.4 Earthworms
Earthworms are likely to be exposed to the highest concentration
of diazinon following use of the granular formulation incorporated in
soil at up to 2000 g/ha. Based on a soil depth of 5 cm and a soil
density of 1.5 g/cm3, the soil PEC would be 2.67 mg/kg, assuming even
distribution in the medium. The reported LC50 for earthworms
(Eisenia foetida) is 130 mg/kg soil, giving a TER of 48.75. As this
is above the trigger value of 10 (EPPO/CoE, 1993a,b), the acute risk
to earthworms should be low. Reported concentrations measured in soil
are at least an order of magnitude lower than the PEC suggesting that
little risk is posed to worms.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
The general population does not face a significant health risk
from diazinon. It may cause acute poisoning in cases of over-exposure
following intentional ingestion or careless handling during its
manufacture and use. The general public is exposed to diazinon in the
form of its residues in food, but the reported intake of diazinon is
far below the acceptable daily intake. Following residual spraying and
space treatment, used to control insects, the general population can
be exposed to residues in the air and on surfaces. With good work
practice and hygiene measures, and if safety precautions and medical
surveillance are enforced, diazinon is unlikely to present a hazard to
those occupationally exposed.
Diazinon does not persist in the environment and is not
accumulated by organisms. It has high acute toxicity to aquatic
invertebrates, fish, terrestrial birds and mammals, leading to high
risk factors for many of these organisms. Field kills of waterfowl
have been reported following use of the compound on amenity turf.
Precautions should be taken to minimize exposure of non-target
organisms (e.g., do not spray over water bodies, minimize exposure by
spray drift and avoid areas where wildfowl are likely to graze).
11.2 Recommendations for protection of human health and the
environment
Certain groups within the population, including agricultural
workers and employees in the chemical industry, have the potential of
being exposed to diazinon. Gardeners and householders may also be
involved.
11.2.1 Recommendation on regulation of compound
Formulations have different classification categories: (FAO/WHO,
1979): liquids over 20% are in category 3; other liquids or solids
over 50% are in category 4; and all other solids are in category 5.
11.2.1.1 Transport and storage
Formulations in categories 3 and 4 should be transported and
stored in clearly labelled rigid and leak-proof containers, away from
containers of food and drink. Storage should be under lock and key and
secure from access by unauthorized people. Formulations in category 5
should be transported and stored in clearly labelled leak-proof
containers, out of reach of children and away from food and drink.
11.2.1.2 Handling
Protective clothing should be used by all handling of the
compound. Adequate washing facilities should be available at all times
during handling and should be close to the site of handling. Eating,
drinking and smoking should be prohibited during handling and before
washing the hands after handling.
11.2.1.3 Disposal
Containers may be decontaminated as recommended by the WHO Expert
Committee on Vector Biology and Control on the Safe Use of Pesticides
(WHO, 1991). Decontaminated containers should not be used for food and
drink. Containers that are not decontaminated should be burned or
crushed and buried below topsoil. Care must taken to avoid subsequent
contamination of water sources.
11.2.1.4 Selection, training and medical supervision of workers
Pre-employment medical examination of workers is desirable.
Workers suffering from active hepatic or renal disease should be
excluded from contact with diazinon. Pre-employment and periodic
cholinesterase test for workers is desirable especially for those
handling concentrates. Training of workers in techniques to avoid
contact is essential. Pilots and loaders should have special training
in application methods and early symptoms of poisoning, and must wear
a suitable respirator. Flagmen, if used, should wear overalls and be
located well away from the dropping zone.
11.2.1.5 Labelling
Diazinon is an organophosphorus compound that inhibits
cholinesterases. It is poisonous if swallowed. It may be absorbed
though the skin. It is important to avoid skin contact, wear hand
protection, clean protective clothing. The material must be kept out
of reach of children and well away from foodstuffs, animal feed and
their containers. If poisoning occurs, a physician should be called.
11.2.1.6 Residues in food
Maximum residue limits have been recommended for diazinon by the
Joint FAO/WHO Meeting on Pesticides Residues (FAO/WHO, 1975).
11.2.2 Prevention of poisoning in man and emergency aid
11.2.2.1 Manufacture and formulation
Closed systems and forced ventilation may be required to reduce
as much as possible the exposure of workers to diazinon.
11.2.2.2 Mixers and applicators
When mixing, protective impermeable boots, rubber apron, clean
overalls and gloves should be worn. When spraying tall crops or during
aerial application, a face mask should be worn, as well as an
impermeable hat, clothing, boots and gloves. The applicator should
avoid working in spray mist and avoid contact with the mouth.
Particular care is needed when equipment is being washed after use.
All protective clothing should be washed immediately after use.
Splashes must be washed immediately from the skin or eyes with large
quantities of water.
11.2.2.3 Other associated workers
People exposed to diazinon and associated with its application
should wear protective clothing and observe the precautions described
in section 11.2.2.2.
11.2.2.4 Other populations likely to be affected
With good application practice, other people should not be
exposed to hazardous amounts of diazinon.
11.2.3 Entry into treated areas
Unprotected people should abide by re-entry periods stated on
product labels.
11.2.4 Emergency aid
If symptoms appear following exposure, the person should stop
work immediately, remove contaminated clothing, wash the affected skin
with soap and water, if available, and flush the area with large
quantities of water. If diazinon has been swallowed and the person is
conscious, vomiting should be induced.
Atropine and oximes are specific antidotes and artificial
respiration may be needed.
11.2.5 Surveillance test
Slight reduction of plasma cholinesterase activity can be
observed. Periodic blood cholinesterase tests for workers is
desirable.
12. FURTHER RESEARCH
1. Studies of exposed worker populations should be continued.
2. Diazinon should be manufactured and formulated in accordance with
international specifications. It should be packed and stored
under conditions that are not conducive to the formation of
acutely toxic impurities.
3. The safe use of diazinon should follow label directions and
precautions for handling, application and disposal.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Diazinon has been evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) on a number of occasions since 1965. An
acceptable daily intake (ADI) of 0.002 mg/kg body weight has been
established and maximal residues levels have been recommended for
diazinon in a wide range of food commodities (FAO/WHO, 1994a,b).
The following NOAELs were established:
Rat (two-year feeding study): 1.5 mg/kg diet
(0.06 mg/kg body weight per day)
Dog (one-year feeding study): 0.5 mg/kg diet
(0.015 mg/kg body weight per day)
Rhesus monkey (two-year study): 0.5 mg/kg body weight per day
Human volunteers (36-day study): 0.025 mg/kg body weight per day
Diazinon has not been evaluated by the International Agency for
research on Cancer (IARC).
REFERENCES
Abdelsalam EB & Ford EJH (1986) Effect of pretreatment with hepatic
microsomal enzyme inducers on the toxicity of diazinon in calves. Res
Vet Sci, 41: 336-339.
Abdelsalam EB & Ford EJH (1987) The effect of induced liver, kidney
and lung lesions on the toxicity of levamisole and diazinon in calves.
J Comp Pathol, 97: 619-627.
Adachi K, Ohokuni N, & Mitsuhashi T (1984) Simple analytical method
for organophosphorus pesticide determination in unpolished rice, using
removal of fats by zinc acetate. J Assoc Off Anal Chem, 67: 798-800.
Al-Attar HJ & Knowles CO (1982) Diazinon uptake, metabolism and
elimination by nematodes. Arch Environ Contam Toxicol, 11: 669-673.
Albright RK (1984) Renal involvement in organophosphate poisoning.
J Am Med Assoc, 252: 1408.
Allender WJ & Britt AG (1994) Analyses of liquid diazinon formulation
and breakdown products: an Australia-wide survey. Bul. Environ Contam
Toxicol, 53: 902-906.
Allison DT (1977) Use of exposure units for estimating aquatic
toxicity of organophosphate pesticides. Washington, DC, US
Environmental Protection Agency (EPA-600/3-77-077).
Allison DT & Hermanutz RO (1977) Toxicity of diazinon to brook trout
and fathead minnows. Washington, DC, US Environmental Protection
Agency (EPA-600/3-77-060).
Anees MA (1978) Hepatic pathology in a fresh-water teleost Channa
punctatus (Bloch) exposed to sub-lethal and chronic levels of
three organophosphorus insecticides. Bull Environ Contam Toxicol,
19: 524-527.
Ankley GT, Dierkes JR, Jensen DA, & Peterson GS (1991) Piperonyl
butoxide as a tool in aquatic toxicological research with
organophosphate insecticides. Ecotoxicol Environ Saf, 21: 266-274.
Ansari BA & Kumar K (1988) Diazinon toxicity: effect on protein and
nucleic acid metabolism in the liver of zebrafish, Brachydanio
rerio (Cyprinidae). Sci Total Environ, 76: 63-68.
Anthony J, Banister E, & Oloffs PC (1986) Effect of sublethal levels
of diazinon: Histopathology of liver. Bull Environ Contam Toxicol,
37: 501-507.
Arienzo M, Crisanto T, Sanchez-Martin T, & Sanchez-Camazano M (1994):
Effect of soil characteristics on adsorption and mobility of (14C)
diazinon. J Agric Food Chem, 42: 1803-1808.
Asensio JS, Barrio CS, Juez MTG, & Bernal JG (1991) Study of the
decay of diazinon and chlorpyriphos in apple samples, using gas
chromatography. Food Chem, 42: 213-224.
Ballantine L (1984) Advanced product chemistry percutaneous absorption
of 2delta-14C-diazinon in rats. Greensboro, North Carolina, Ciba-
Geigy Corporation (Unpublished report No. ABR-84011, submitted to WHO
by Ciba-Geigy Ltd, Basel).
Banerjee D (1967) Pericarditis in acute diazinon poisoning (A case
report). Armed Forces Med J (India), 23: 187-190.
Barik S & Munnecke DM (1982) Enzymatic hydrolysis of concentrated
diazinon in soil. Bull Environ Contam Toxicol, 29: 235-239.
Barnes TB, Hazelette JR, & Arthur AT (1988) Diazinon (mg-8): 13 week
oral toxicity study in dogs (Project No. 882012). Summit, New Jersey,
Ciba-Geigy Corporation (Unpublished report submitted to WHO by Ciba-
Geigy Ltd, Basel).
Barnett JB, Spyker-Cranmer JM, Avery DL, & Hoberman AM (1980)
Immunocompetence over the lifespan of mice exposed in utero to
carbofuran or diazinon: I. Changes in serum immunoglobulin
concentrations. J Environ Pathol Toxicol, 4: 53-63.
Bathe R (1972a) Acute dermal LD50 of technical diazinon (G-24'480)
in the rat (Project No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Bathe R (1972b) G 24`480 tech.: Acute oral LD50 in the mouse
(Project No. Siss 1679). Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Bathe R (1973) Intraperitoneal LD50 of technical diazinon
(G-24480 MG 647) in the rat. Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished project report No. Siss 1679).
Bathe R & Gfeller W (1980) Report on acute oral LD50 in the rat of
technical G24'480 (Project No. 800478). Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Bopp C & Kosminsky B (1975) Toxic hepato-cutaneous porphyria. Med
Cutan Ibero Lat Am, 3: 271-279.
Bresch H (1991) Early life-stage test in zebrafish versus a growth
test in rainbow trout to evaluate toxic effects. Bull Environ Contam
Toxicol, 46: 641-648.
Bruce RB, Howard JW, & Elsea JR (1955) Toxicity of O,O-diethyl
O-(2-isopropyl-6-methyl-4 pyrimidyl) phosphorothioate (diazinon).
J Agric Food Chem, 3: 1017-1021.
Burgener J & Seim V (1988) Biological report for the metabolism of
14C-diazinon in laying hens dosed at 25 ppm ( Project No.
BIOL-88006). Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report submitted to WHO Ciba-Geigy Ltd, Basel).
Burpee LL & Cole H Jr (1978) The influence of alachlor, trifluralin
and diazinon on the development of endogenous mycorrhiza in soybeans.
Bull Environ Contam Toxicol, 19: 191-197.
Butler GL, Deason TR, & Kelley JC (1975) The effect of atrazine,
2,4-D, methoxychlor, carbaryl and diazinon on the growth of planktonic
algae. Br Phycol J, 10: 371-372.
Byrne DH & Kitos PA (1983) Teratogenic effects of cholinergic
insecticides in chick embryos: IV. The role of tryptophan in
protecting against limb deformities. Biochem Pharmacol,
32: 2881-2890.
Cairns T, Siegmund EG, & Froberg JE (1985) Identification of
diazinon and its metabolite in spinach by chemical ionization
mass spectrometry. Bull Environ Contam Toxicol, 35: 291-295.
Capps T (1989) Characterization and identification of diazinon
metabolites in rats (Project No. 302211/302925). Greensboro, North
Carolina, Ciba-Geigy Corporation (Unpublished report No. ABR-88164,
submitted to WHO by Ciba-Geigy Ltd, Basel).
Capps TM (1990) Characterization and identification of major
metabolites in tissues of sheep treated dermally with 14C-diazinon
(Project No. 302925) Greensboro, North Carolina, Ciba-Geigy
Corporation (Unpublished report No. ABR-90014, submitted to WHO by
Ciba-Geigy Ltd, Basel).
Ceresa C & Puri E (1988) Micronucleus test, mouse (OECD-conform) G 24
480 tech. (diazinon) (Test No. 871696). Basel, Switzerland, Ciba-Geigy
Ltd ( Unpublished report).
Chen HH, Hsueh JL, Sirianni SR, & Huang CC (1981) Induction of
sister-chromatid exchanges and cell cycle delay in cultured mammalian
cells treated with eight organophosphorus pesticides. Mutat Res,
88: 307-316.
Chow E & Richter AG (1994) Acute neurotoxicity study with D-Z-N
diazinon MG 87% in rats (Project No. F-00175). Farmington,
Connecticut, Ciba-Geigy Corporation, Environmental Health Center
(Unpublished report).
Churchfield S (1986) Shrews. Oswestry, A. Nelson (The Mammal Society
series).
Claborn HV, Mann HD, Younger RL, & Radeleff RD (1963) Diazinon
residues in the fat of sprayed cattle. J Econ Entomol, 56: 858-859.
Classen W (1996) G24.480 tech. - delayed neurotoxicity in hens
following acute exposure (Project No. 952030). Stein, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Clegg TJ & Koevening JL (1974) The effect of four chlorinated
hydrocarbon pesticides and one organophosphate pesticide on ATP levels
in three species of photosynthesizing freshwater algae. Bot Gaz,
135: 368-372.
Cockrell KD, Woodard MW, & Woodard G (1966) Diazinon 50 W: Safety
evaluation by repeated oral administration to monkeys for 106 weeks.
Woodard Research Corporation USA (Unpublished report submitted to WHO
by Ciba-Geigy Ltd, Basel).
Collyard SA, Ankley GT, Hoke RA, & Goldenstein T (1994) Influence of
age on the relative sensitivity of Hyalella azteca to diazinon,
alkylphenol ethoxylates, copper, cadmium and zinc. Arch Environ Contam
Toxicol, 26: 110-113
Coye MJ, Barnett PG, Midtling JE, Velasco AR, Romero P, Clements CL, &
Rose TG (1987) Clinical confirmation of organophosphate poisoning by
serial cholinesterase analyses. Arch Intern Med, 147: 438-442.
Craine EM (1989a) A study of 14C-diazinon disposition in the rat
(biological-phase) (Project No. 302925). Ashland, Ohio, WIL Research
Laboratories Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Craine EM (1989b) A study of 14C-diazinon disposition in the rat
(analytical phase I) (Project No. 302925). Ashald, Ohio, WIL Research
Laboratories Inc. (Unpublished report submitted to WHO by Ciba-Geigy
Ltd, Basel).
Currie KL, McDonald EC, Chung LTK, & Higgs AR (1990) Concentration of
diazinon, chlorpyrifos, and bendiocarb after application in offices.
Am Ind Hyg Assoc J, 51(1): 23-27.
Dagli AJ, Moos JS, & Shaikh WA (1981) Acute pancreatitis as a
complication of diazinon poisoning: A case report. J Assoc Physi
India, 29: 794-795.
Dahm PA (1970) Some aspects of the metabolism of parathion and
diazinon. In: O`Brien RD & Yamamoto I ed. Biochemical toxicology of
insecticides. London, New York, Academic Press, pp 51-63.
Davies DB & Holub BJ (1980a) Comparative subacute toxicity of
dietary diazinon in the male and female rat. Toxicol Appl Pharmacol,
54: 359-367.
Davies DB & Holub BJ (1980b) Toxicological evaluation of dietary
diazinon in the rat. Arch Environ Contam Toxicol, 9: 637-650.
Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowall J, & Furlong CE
(1996) The effect of the human serum paraoxonase polymorphism is
reversed with diazoxon, soman and sarin. Nat Genet, 14(3): 334-336.
Decarie R, DesGrange J-L, Lepine C, & Morneau F (1993) Impact of
insecticides on the American robin (Turdus migratorius) in a
suburban environment. Environ Pollut, 80: 231-238
Derbyshire JC & Murphy RT (1962) Diazinon residues in treated
silage and milk of cows fed powdered diazinon. J Agric Food Chem,
10: 384-386.
Dick GL, Heenan MP, Love JL, Udy PB, & Davidson F (1978) Survey of
trace elements and pesticide residue content: 2. Organochlorine and
organophosphorus pesticide residue content. NZ J Sci, 21: 71-78.
Dikshith TS, Behari JR, Datta KK, & Mathur AK (1975) Effect of
diazinon in male rats: Histopathological and biochemical studies.
Environ Physiol Biochem, 5: 293-299.
Dobbins PK (1967) Organic phosphate insecticides as teratogens in the
rat. J Fla Med Assoc, 54: 542-546.
Doggett SM & Rhodes RG (1991) Effects of a diazinon formulation on
unialgal growth rates and phytoplankton diversity. Bull Environ Contam
Toxicol, 47: 36-42.
Dollenmeier P & Müller D (1986) G 24`480 tech.: L5178Y/TK+/- mouse
lymphoma mutagenicity test (Project No. 840396). Basel, Switzerland,
Ciba-Geigy Ltd (Unpublished report).
Dougherty E III (1957) Thiophosphate poisoning in white Pekin ducks.
Avian Dis, 1: 127-130.
Dressel TD, Goodale RL Jr, Arneson MA, & Borner JW (1979) Pancreatitis
as a complication of anticholinesterase insecticide intoxication. Ann
Surg, 189: 199-204.
Dressel TD, Godale RL, & Borner JW (1980) A study of the
cholinesterases of the canine pancreas and the relationship between
reduced butyrylcholinesterase activity and pancreatic ductal
hypertension. Ann Surg, 192: 614-619.
Dutta H, Marcelino J, & Richmonds Ch (1992) Brain acetylcholinesterase
activity and optomotor behaviour in bluegills, Lepomis macrochirus,
exposed to different concentrations of diazinon. Arch Int Physiol
Biochim Biophys, 100: 331-334.
Ebere AG & Akintonwa A (1992) Acute toxicity of pesticides to
Gobius sp., Palaemonetes africanus, and Desmocaris trispimosa.
Bull Environ Contam Toxicol, 49: 588-592.
Eberle DO, Suter R, Nowak K, & Bosshardt HP (1974) CIPAC collaborative
study of the heat-stability of diazinon formulations using both CUPAC
and AOAC analytical methods. J Assoc Off Anal Chem, 57: 48-52.
Egan H & Weston RE (1977) Pesticide residues: Food surveys in the
United Kingdom. Pestic Sci, 8: 110-116.
Egyed MN, Malkinson M, Eilat A, & Schlosberg A (1974) Basudin
(diazinon) poisoning in goslings. Reffu Vet, 31: 22-26.
El-Elaimy IA, El-Saadany MM, & Gabr SA (1990) Pesticide poisoning to
fresh water teleost: VIII. Ultrastructural alterations of the
intestine of Tilapia nilotica under stress of exposure to diazinon
and neopybuthrin. J Egypt Ger Soc Zool, 1: 223-236.
English T, Ellis EF, & Ackerman J (1970) Organic phosphate poisoning.
Morbid Mortal Wkly Rep (Cent Dis Control), 19: 397-404.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993a) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 23: 1-54.
EPPO/CoE (European & Mediterranean Plant Protection Organisation/
Council of Europe) (1993b) Decision making schemes for the
environmental risk assessment of plant protection products.
OEPP/EPPO Bull, 24: 1-87.
FAO/WHO (1965) Diazinon. In: 1965 Evaluation of the toxicity of
pesticide residues in food. Rome, Food and Agriculture Organization of
the United Nations and Geneva, World Health Organization, pp 77-83
(FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27. 65).
FAO/WHO (1975) 1974 Evaluations of some pesticide residues in food.
Geneva, World Health Organization (WHO Pesticide Residues Series,
No 4).
FAO/WHO (1979) Data sheet on pesticides No. 45: Diazinon. Geneva,
World Health Organization (VBC/DS/79.45).
FAO/WHO (1994a) Diazinon. In: Pesticide residues in food - 1993:
Evaluations, Part I - Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 157-294 (FAO Plant Production
and Protection Paper 124).
FAO/WHO (1994b) Diazinon. In: Pesticide residues in food - 1993:
Evaluations, Part II - Toxicology. Geneva, World Health Organization,
pp 57-81 (WHO/PCS/94.4).
Farran A, DePablo J, & Barcelo D (1988a) Identification of
organophosphorus insecticides and their hydrolysis products by
liquid chromatography in combination with UV and thermospray-mass
spectrometric detection. J Chromatogr, 455: 163-172.
Farran A, DePablo J, & Hernandez S (1988b) Continuous-flow extraction
of organophosphorus pesticides coupled on-line with high-performance
liquid chromatography. Anal Chim Acta, 212: 123-131.
Fernandez-Casalderrey A, Ferrando MD, & Andreu-Moliner E (1992)
Endosulfan and diazinon toxicity to the freshwater rotifer
Brachious calcyflorus. J Environ Sci Health, B27: 155-164.
Ferrando MD, Sancho E, & Andreu-Moliner E (1991) Comparative acute
toxicities of selected pesticides to Anguilla anguilla. J Environ
Sci Health, B26(5/6): 491-498.
Ferrando MD, Alarcon V, Fernandez-Casalderrey A, Gamon M, &
Andreu-Moliner E (1992) Persistence of some pesticides in the
aquatic environment. Bull Environ Contam Toxicol, 48: 747-755.
Ferreira JR & Silva Fernandes AM (1980) Gas-liquid chromatographic
determination of organophosphorus insecticide residues in fruits and
vegetables. J Assoc Off Anal Chem, 63: 517-522.
Fink R (1976) Acute oral LD50 to bobwhite quail - diazinon technical.
Basel, Switzerland, Novartis Crop Protection (Unpublished report).
Fletcher DW & Pedersen CA (1988a) Diazinon MG8 technical: 14 day acute
oral LD50 study in mallard ducks. Basel, Switzerland, Ciba-Geigy Ltd
(Unpublished report).
Fletcher DW & Pedersen CA (1988b) Diazinon MG8 technical: 14 day acute
oral LD50 study in brown-headed cowbirds. Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Fletcher DW & Pedersen CA (1988c) Diazinon MG8 technical: 8 day acute
dietary LC50 study in mallard ducklings. Basel, Switzerland, Ciba-
Geigy Ltd (Unpublished report).
Fort DJ, Delphon J, Powers CR, Helems R, Gonzales R, & Stover EL
(1995) Development of automated methods of identifying toxicants in
the environment. Bull Environ Contam Toxicol, 54: 104-111.
Frank R, Braun HE, Van Hove Holdrinet M, Sirons GJ, & Ripley BD
(1982) Agriculture and water quality in the Canadian Great Lakes
basin: V. Pesticide use in 11 agricultural watersheds and presence in
stream water, 1975-1977. J Environ Qual, 11(3): 497-505.
Frank R, Braun HE, & Ripley BD (1989) Monitoring Ontario-grown apples
for pest control chemicals used in their production. Food Addit
Contam, 6: 227-234.
Frank R, Braun HE, & Ripley BD (1990) Residues of insecticides and
fungicides on Ontario-grown vegetables 1986-1988. Food Addit Contam,
7: 545-554.
Frank R, Mineau P, Braun HE, Barker IK, Kennedy SW, & Trudeau S (1991)
Deaths of Canada geese following spraying of turf with diazinon. Bull
Environ Contam Toxicol, 46: 852-858.
Frick TW, Dalo S, O'Leary JF, Runge W, Borner JW, Baraniewski H,
Dressel T, Shearen JG, & Goodale RL (1987) Effects of insecticide
diazinon on pancreas of dog, cat, and guinea pig. J Environ Pathol
Toxicol Oncol, 7: 1-12.
Fritz H (1974) Reproduction study - G24480 (diazinon techn.) rat -
Segment II: Test for teratogenic or embryotoxic effects. Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Fritz H (1975) Dominant lethal study on G24480 (diazinon techn.)
mouse: Test for cytotoxic or mutagenic effects on male germinal cells
(Project No. 327507) . Basel, Switzerland, Ciba-Geigy Ltd (Unpublished
report).
Fujii Y & Asaka S (1982) Metabolism of diazinon and diazoxon in fish
liver preparations. Bull Environ Contam Toxicol, 29: 455-460.
Gaines TB (1960) The acute toxicity of pesticides to rats. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides. Toxicol Appl Pharmacol,
14: 515-534.
Ganzelmeier IH, Rautmann D, Spangenberg R, Streloke M, Herrmann M,
Wenzelburger HJ, & Walter HF (1995) Studies on the spray drift of
plant protection products: Results of a test program carried out
throughout the Federal Republic of Germany. Oxford, London,
Edinburgh, Melbourne, Blackwell Scientific Publications.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1985a)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1978-September 1979. J Assoc Off
Anal Chem, 68: 842-861.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1985b)
Pesticides, selected elements, and other chemicals in adult total
diet samples, October 1978-September 1979. J Assoc Off Anal Chem,
68: 862-875.
Gasser R (1953) [A new insecticide with wide efficacy.] Z Naturforsch,
B8: 225-232 (in German).
Geleick D & Arni P (1990) Salmonella and Escherichia/liver-microsome
test (Project No. 891346) - Test substance: G24480 tech. Basel,
Switzerland, Ciba-Geigy Ltd (Unpublished report).
Getzin LW (1968) Persistence of diazinon and zinophos in soil: Effects
of autoclaving, temperature, moisture, and acidity. J Econ Entomol,
61: 1560-1565.
Giddings JM (1992) Aquatic mesocosm test for environmental fate and
ecological effects of diazinon. Wareham, Massachusetts, Springborn
Laboratories, Inc. and Columbia, Missouri, ABC Laboratories, Inc.
(Unpublished report submitted to WHO by Ciba-Geigy Ltd, Basel).
Giknis MLA (1989) Diazinon techn: A two generation reproductive study
in albino rats. Greensboro, North Carolina, Ciba-Geigy Corporation
(Unpublished report).
Gilmore DR & Cortes A (1966) Dual-ban preparative thin-layer
chromatography for the separation of diazinon and related from plant
material. J. Chromatogr, 21: 148-149.
Glaza SM (1993) Acute oral toxicity study of D-Z-N diazinon in rats
(Project No. HWI 6117-221). Madison, Wisconsin, Hazleton Wisconsin,
Inc. (Unpublished report).
Goodman LR, Hansen DJ, Coppage DL, Moore JC, & Matthews E (1979)
Diazinon chronic toxicity to, and brain acethylcholinesterase
inhibition in, the sheepshead minnow, Cyprinodon variegatus. Trans
Am Fish Soc, 108: 479-488.
Gowen JA, Wiersma GB, Tai H, & Mitchell WG (1976) Pesticide levels in
hay and soils from nine states. Pestic Monit J, 10: 114-116.
Gunner HB & Zuckerman BM (1968) Degradation of 'diazinon' by
synergistic microbial action. Nature (Lond), 217: 1183-1184.
Gunner HB, Zuckerman BM, Walker RW, Miller CW, Deubert KH, & Longley
RE (1966) The distribution and persistence of diazinon applied to
plant and soil and its influence on rhizosphere and soil microflora.
Plant Soil, 25: 249-264.
Hagenbuch JP & Mücke W (1985) The fate of diazinon in mammals
(Biochemistry product evaluation 1/85). Basel, Switzerland, Ciba-Geigy
Ltd (Unpublished report).
Hall SW & Baker BB (1989) Intermediate syndrome from organophosphate
poisoning. Vet Hum Toxicol, 31(4): 355.
Halle A & Sloas DD (1987) Percutaneous organophosphate poisoning .
South Med J, 80: 1179-1181.
Harris SB & Holson JF (1981) G 24`480 tech.: A teratology study of
diazinon (CAS Number 