
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 194
Aluminium
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 194
First draft prepared by Dr H. Habs, Dr B. Simon and Professor K.U.
Thiedemann (Fraunhofer Institute, Hanover, Germany) and Mr P. Howe
(Institute of Terrestrial Ecology, Monks Wood, United Kingdom)
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1997
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Aluminium
(Environmental health criteria ; 194)
1.Aluminium - toxicity 2.Aluminium - adverse effects
3.Environmental exposure I.Series
ISBN 92 4 157194 2 (NLM Classification: QV 65)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1997
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
1. SUMMARY AND CONCLUSIONS
1.1. Identity, physical and chemical properties
1.2. Analytical methods
1.3. Sources of human and environmental exposure
1.4. Environmental transport, distribution and transformation
1.5. Environmental levels and human exposure
1.6. Kinetics and metabolism
1.6.1. Humans
1.6.2. Animals
1.7. Effects on laboratory mammals and in vitro test systems
1.8. Effects on humans
1.9. Effects on other organisms in the laboratory and field
1.10. Conclusions
1.10.1. General population
1.10.2. Subpopulations at special risk
1.10.3. Occupationally exposed populations
1.10.4. Environmental effects
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.2.1. Aluminium metal
2.2.2. Aluminium compounds
2.3. Analytical methods
2.3.1. Sampling and sample preparation
2.3.2. Separation and concentration
2.3.3. Detection and measurement
2.3.4. Speciation analysis of aluminium in water
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Freshwater
4.1.2.1 Dissolved aluminium
4.1.2.2 Aluminium adsorbed on particles
4.1.2.3 Aluminium in acidified waters
4.1.3. Seawater
4.1.4. Soil
4.1.5. Vegetation and wildlife
4.2. Biotransformation
4.2.1. Biodegradation and abiotic degradation
4.2.2. Bioaccumulation
4.2.2.1 Plants
4.2.2.2 Invertebrates
4.2.2.3 Fish
4.2.2.4 Birds
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Precipitation
5.1.3. Water
5.1.3.1 Freshwater
5.1.3.2 Seawater
5.1.4. Soil and sediment
5.1.5. Terrestrial and aquatic organisms
5.2. Occupational exposure
5.3. General population exposures
5.3.1. Air
5.3.2. Food and beverages
5.3.3. Drinking-water
5.3.4. Miscellaneous exposures
5.3.5. Total human intake of aluminium from
all environmental pathways
5.3.6. Aluminium uptake
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
6.1. Absorption
6.1.1. Animal studies
6.1.1.1 Inhalation exposure
6.1.1.2 Oral administration
6.1.1.3 Dermal
6.1.2. Studies in humans
6.1.2.1 Inhalation exposures
6.1.2.2 Oral administration
6.1.2.3 Dermal exposure
6.2. Distribution
6.2.1. Animal studies
6.2.2. Human studies
6.2.2.1 Transport in blood
6.2.2.2 Plasma aluminium concentrations in humans
6.2.2.3 Tissue aluminium concentrations in humans
6.3. Elimination and excretion
6.3.1. Animal studies
6.3.2. Human studies
6.3.2.1 Urinary excretion
6.3.2.2 Biliary excretion
6.4. Biological indices of exposure, body burden and organ
concentration
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short- and long-term exposure
7.2.1. Oral administration
7.2.2. Inhalation exposure
7.2.3. Parenteral administration
7.3. Reproductive and developmental toxicity
7.3.1. Reproductive effects
7.3.2. Developmental effects
7.4. Mutagenicity and related end-points
7.4.1. Interactions with DNA
7.4.2. Mutations
7.4.3. Chromosomal effects
7.5. Carcinogenicity
7.6. Neurotoxicity
7.6.1. Impairments of cognitive and motor function
7.6.2. Alterations in electrophysiological properties
7.6.3. Metabolic effects in the nervous system
7.7. Effects on bone
7.7.1. Toxic effects of aluminium in the skeleton
7.7.2. Dose response
7.8. Effects on mineral metabolism
8. EFFECTS ON HUMANS
8.1. General population exposure
8.1.1. Acute toxicity
8.1.2. Effects of short-term exposure
8.1.3. Neurotoxic effects
8.1.3.1 Aluminium and Alzheimer's disease (AD)
8.1.3.2 Epidemiological studies on AD and
environmental aluminium levels
8.1.3.3 Epidemiological studies relating
aluminium concentrations in water to
cognitive dysfunction
8.1.3.4 Other neurological conditions in the
general population
8.1.3.5 Conclusions regarding neurological
effects of aluminium
8.1.4. Allergic effects
8.2. Occupational exposure
8.2.1. Respiratory tract effects
8.2.1.1 Restrictive pulmonary disease
8.2.1.2 Obstructive pulmonary disease
8.2.2. Central nervous system effects
8.3. Cancer
8.4. Genotoxicity
8.5. Reproductive toxicity
8.6. Subpopulations at special risk
8.6.1. Encephalopathy
8.6.2. Osteomalacia
8.6.3. Microcytic anaemia
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.1.1 Water
9.1.1.2 Soil
9.1.2. Aquatic organisms
9.1.2.1 Plants
9.1.2.2 Invertebrates
9.1.2.3 Fish
9.1.2.4 Amphibians
9.1.3. Terrestrial organisms
9.1.3.1 Plants
9.1.3.2 Invertebrates
9.1.3.3 Birds
9.2. Field observations
9.2.1. Microorganisms
9.2.2. Aquatic organisms
9.2.2.1 Plants
9.2.2.2 Invertebrates
9.2.2.3 Vertebrates
9.2.3. Terrestrial organisms
9.2.3.1 Plants
9.2.3.2 Invertebrates
9.2.3.3 Vertebrates
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Health effects
10.1.1. Exposure assessment
10.1.2. Evaluation of animal data
10.1.3. Evaluation of human data
10.2. Evaluation of effects on the environment
10.2.1. Exposure
10.2.2. Effects
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
11.1.1. Healthy general population
11.1.2. Subpopulations at special risk
11.1.3. Occupationally exposed populations
11.1.4. Environmental risk
11.2. Recommendations
11.2.1. Public health protection
11.2.2. Recommendations for protection of the environment
12. FURTHER RESEARCH
12.1. Bioavailability and kinetics
12.2. Toxicological data
12.3. Research on the relationship between aluminium exposure and
Alzheimer's disease
12.4. Occupational exposure
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCE
RESUME ET CONCLUSIONS
RESUMEN Y CONCLUSIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Ch�telaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined
below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
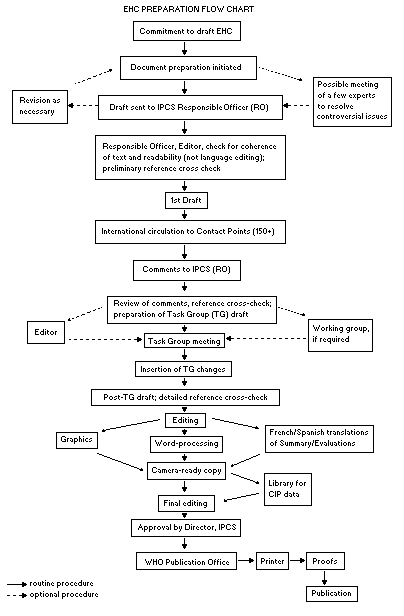
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
 All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
Members
Dr T.M. Florence, Centre for Environmental Health Sciences, Oyster
Bay, New South Wales, Australia
Dr M. Golub, California Regional Primate Research Center, University
of California, Davis, California, USA
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
( Co-Rapporteur)
Professor D.R. McLachlan ( Retired from: Centre for Research in
Neurogenerative Diseases, University of Toronto, Toronto,
Ontario, Canada
Dr M. Moore, National Health and Medical Research Council, National
Research Centre for Environmental Toxicology, Coopers Plains,
Brisbane, Australia ( Chairman)
Dr T.V. O'Donnell, University of Otago, Wellington South, New Zealand
( Vice-Chairman)
Professor B. Rosseland, Norwegian Institute of Water Research (NIVA),
Oslo, Norway
Dr B. Simon, Fraunhofer Institute, Hanover, Germany ( Co-Rapporteur)
Dr B. Sjogren, Department of Occupational Medicine, Swedish National
Institute for Working Life, Solna, Sweden
Dr L. Smith, Disease Control Service, Public Health Branch, Ontario
Ministry of Health, North York, Ontario, Canada
Dr E. Storey, Royal Melbourne Hospital, Department of Pathology,
University of Melbourne, Parkville, Victoria, Australia
Dr H. Temmink, Department of Toxicology, Agricultural University,
Wageningen, The Netherlands ( Vice-Chairman)
Dr M.K. Ward, Department of Renal Medicine, Royal Victoria Infirmary,
Newcastle-upon-Tyne, United Kingdom
Dr M. Wilhelm, Health Institute, University of Dusseldorf, Dusseldorf,
Germany
Professor H.M. Wisniewski, New York State Institute for Basic
Research in Developmental Disabilities, Staten Island, New York,
USA
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute
of Occupational Medicine, Ministry of Health, Beijing, China
Observers
Dr K. Bentley, Environmental Health Assessment and Criteria, Human
Services and Health, Woden, Australia
Dr O.C. B�ckman, Norsk Hydro, Porsgrunn Research Centre, Porsgrunn,
Norway
Dr J. Borak, Occupational and Environmental Health, Jonathan Borak &
Co., New Haven, Connecticut, USA
Dr I. Calder, Occupational and Environmental Health, South Australian
Health Commission, Adelaide, Australia
Dr J.N. Fisher, ALCOA of Australia Ltd, Point Henry Works, Geelong,
Victoria, Australia
Mr D. Hughes, Environment, Mount Isa Mine Holdings, Brisbane,
Australia
Dr P. Imray, Environmental Health Branch, Queensland Health, Brisbane,
Australia
Ms M.E. Meek, Environmental Health Directorate, Health Canada,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr N. Priest, AEA Technology, Harwell, Didcot, Oxfordshire, United
Kingdom
Dr D. Wilcox, Medical Section, Health Services, Sydney Water, Sydney,
Australia
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety
Inter-regional Research Unit, World Health Organization, Research
Triangle Park, North Carolina, USA ( Secretary)
Dr D. Johns, DPIE, Coal and Mineral Division, Canberra, Australia
( Temporary Adviser)
Mr D. Wagner, Chemicals Safety Unit, Human Services and Health,
Canberra, Australia ( Temporary Adviser)
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
A WHO Task Group on Environmental Health Criteria for Aluminium
met in Brisbane, Australia, from 24 to 28 April 1995. The meeting
was sponsored by a consortium of Australian Commonwealth and State
Governments through a national steering committee chaired by
Dr K. Bentley, Director, Health and Environmental Policy, Department
of Health and Family Services, Canberra. The meeting was hosted and
organized by the NHMRC National Research Centre for Environmental
Toxicology (NRCET), Dr M. Moore, Director, being responsible for
the arrangements. Dr D. Lange, Chief Health Officer, welcomed
participants on behalf of Queensland Health, and Professor L. Roy
Webb, Vice-chancellor, Griffith University, welcomed them on behalf of
NRCET. Dr G.C. Becking, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS and the three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft criteria monograph and made an evaluation of the risks to human
health and the environment from exposure to aluminium.
The first draft was prepared under the coordination of Dr G.
Rosner, Fraunhofer Institute of Toxicology and Aerosol Research,
Germany, and Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood,
United Kingdom. The draft reviewed by the Task Group, incorporating
the comments received following review by the IPCS Contact Points, was
prepared through the cooperative effort of the Fraunhofer Institute,
Institute of Terrestrial Ecology and the Secretariat.
Dr G.C. Becking (IPCS Central Unit, Inter-regional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively, of
this monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
ABBREVIATIONS
AD Alzheimer's disease
AIBD aluminium-induced bone disease
cAMP cyclic adenosine monophosphate
CI confidence interval
1,25-(OH)2-D3 1,25-dihydroxy-vitamin D3
DOC dissolved organic carbon
EDTA ethylenediaminetetraacetic acid
i.p. intraperitoneal
i.v. intravenous
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
LTP long-term potentiation
NFT neurofibrillary tangle
NIOSH National Institute for Occupational Safety and
Health (USA)
NOEC no-observed-effect concentration
NOEL no-observed-effect level
NTA nitrilotriacetic acid
OR odds ratio
PHF paired helical filaments
Pt platinum unit (1 unit equals the colour produced by
lung chloroplatinate in 1 litre of water)
PTH parathyroid hormone
s.c. subcutaneous
WAIS Weschler Adult Intelligence Scale
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties
Aluminium is a silvery-white, ductile and malleable metal. It
belongs to group IIIA of the Periodic Table, and in compounds it is
usually found as AlIII. It forms about 8% of the earth's crust and is
one of the most reactive of the common metals. Exposure to water,
oxygen or other oxidants leads to the formation of a superficial
coating of aluminium oxide, which provides the metal with a high
resistance to corrosion. Aluminium oxide is soluble in mineral acids
and strong alkalis but insoluble in water, whereas aluminium chloride,
nitrate and sulfate are water soluble. Aluminium halogenides, hydride
and lower aluminium alkyls react violently with water.
Aluminium possesses high electrical and thermal conductivity, low
density and great resistance to corrosion. It is often alloyed with
other metals. Aluminium alloys are light, strong and readily machined
into shapes.
1.2 Analytical methods
Various analytical methods have been developed to determine
aluminium in biological and environmental samples. Graphite furnace
- atomic-absorption spectrometry (GF-AAS) and inductively coupled
plasma - atomic-emission spectrometry (ICP-AES) are the most
frequently used methods. Contamination of the samples with aluminium
from air, vessels or reagents during sampling and preparation is the
main source of analytical error. Depending on sample pretreatment,
separation and concentration procedures, detection limits are
1.9-4 �g/litre in biological fluids and 0.005-0.5 �g/g dry weight in
tissues using GF-AAS, and 5 �g/m3 in air and 3 �g/litre in water
using ICP-AES.
1.3 Sources of human and environmental exposure
Aluminium is released to the environment both by natural
processes and from anthropogenic sources. It is highly concentrated in
soil-derived dusts from such activities as mining and agriculture, and
in particulate matter from coal combustion. Aluminium silicates
(clays), a major component of soils, contribute to the aluminium
levels of dust. Natural processes far outweigh direct anthropogenic
contributions to the environment. Mobilization of aluminium through
human actions is mostly indirect and occurs as a result of emission of
acidifying substances. In general, decreasing pH results in an
increase in mobility and bioavailability for monomeric forms of
aluminium. The most important raw material for the production of
aluminium is bauxite, which contains up to 55% alumina (aluminium
oxide). World bauxite production was 106 million tonnes in 1992.
Aluminium metal has a vide variety of uses, including structural
materials in construction, automobiles and aircraft, and the
production of metal alloys. Aluminium compounds and materials also
have a wide variety of uses, including production of glass, ceramics,
rubber, wood preservatives, pharmaceuticals and waterproofing
textiles. Natural aluminium minerals, especially bentonite and
zeolite, are used in water purification, sugar refining, brewing and
paper industries.
1.4 Environmental transport, distribution and transformation
Aluminium occurs ubiquitously in the environment in the form of
silicates, oxides and hydroxides, combined with other elements such as
sodium and fluorine and as complexes with organic matter. It is not
found as a free metal because of its reactivity. It has only one
oxidation state (+3) in nature; therefore, its transport and
distribution in the environment depend only upon its coordination
chemistry and the chemical-physical characteristics of the local
environmental system. At pH values greater than 5.5, naturally
occurring aluminium compounds exist predominantly in an undissolved
form such as gibbsite (Al(OH)3) or as aluminosilicates, except in the
presence of high amounts of dissolved organic material, which binds
with aluminium and can lead to increased concentrations of dissolved
aluminium in streams and lakes. Several factors influence aluminium
mobility and subsequent transport within the environment. These
include chemical speciation, hydrological flow paths, soil-water
interactions, and the composition of the underlying geological
materials. The solubility of aluminium in equilibrium with solid phase
Al(OH)3 is highly dependent on pH and on complexing agents such as
fluoride, silicate, phosphate and organic matter. The chemistry of
inorganic aluminium in acid soil and stream water can be considered in
terms of mineral solubility, ion exchange and water mixing processes.
Upon acidification of soils, aluminium can be released into
solution for transport to streams. Mobilization of aluminium by acid
precipitation results in more aluminium being available for plant
uptake.
1.5 Environmental levels and human exposure
Aluminium is a major constituent of a number of atmospheric
components particularly in soil-derived dusts (both from natural
sources and human activity) and particulates from coal combustion. In
urban areas aluminium levels in street dust range from 3.7 to
11.6 �g/kg. Airborne aluminium levels vary from 0.5 ng/m3 over
Antarctica to more than 1000 ng/m3 in industrialized areas.
Surface freshwater and soil water aluminium concentrations can
vary substantially, being dependent on physico-chemical and geological
factors. Aluminium can be suspended or dissolved. It can be bound with
organic or inorganic ligands, or it can exist as a free aluminium ion.
In natural waters aluminium exists in both monomeric and polymeric
forms. Aluminium speciation is determined by pH and the concentrations
of dissolved organic carbon (DOC), fluoride, sulfate, phosphate and
suspended particulates. Dissolved aluminium concentrations for water
in the circumneutral pH range are usually quite low, ranging from
1.0 to 50 �g/litre. This rises to 500-1000 �g/litre in more acidic
water. At the extreme acidity of water affected by acid mine drainage,
dissolved aluminium concentrations of up to 90 mg/litre have been
measured.
Non-occupational human exposure to aluminium in the environment
is primarily through ingestion of food and water. Of these, food is
the principal contributor. The daily intake of aluminium from food and
beverages in adults ranges between 2.5 and 13 mg. This is between 90
and 95% of total intake. Drinking-water may contribute around 0.4 mg
daily at present international guideline values, but is more likely
to be around 0.2 mg/day. Pulmonary exposure may contribute up to
0.04 mg/day. In some circumstances, such as occupational exposure and
antacid use, the levels of exposure will be much greater. For example,
> 500 mg of aluminium may be consumed in two average-sized antacid
tablets. There are some difficulties in assessing uptake from these
exposures because of analytical and sampling difficulties. Isotopic
investigations with Al26 indicate that one of the most bioavailable
forms of aluminium is the citrate and that there could be as much as
1% absorption when aluminium is in this form. However, humans would
absorb only 3% of their total daily uptake of aluminium from drinking-
water, a relatively minor source compared to food.
1.6 Kinetics and metabolism
1.6.1 Humans
Aluminium and its compounds appear to be poorly absorbed in
humans, although the rate and extent of absorption have not been
adequately studied. Concentrations of aluminium in blood and urine
have been used as a readily available measure of aluminium uptake,
increased urine levels having been observed among aluminium welders
and aluminium flake-powder producers.
The mechanism of gastrointestinal absorption of aluminium has not
yet been fully elucidated. Variability results from the chemical
properties of the element and the formation of various chemical
species, which is dependent upon the pH, ionic strength, presence of
competing elements (silicon), and the presence of complexing agents
within the gastrointestinal tract (e.g., citrate).
The biological behaviour and gastrointestinal absorption of
aluminium in humans ingesting aluminium compounds has been studied by
using the radioactive isotope Al26. Significant intersubject
variability has been demonstrated. Measured fractional uptakes of 5 �
10-3 for aluminium as citrate, 1.04 � 10-4 for aluminium hydroxide
and 1.36 � 10-3 for the hydroxide given with citrate were reported. A
study of the fractional uptake of aluminium from drinking-water showed
an uptake fraction of 2.35 � 10-3. It was concluded that members of
the general population consuming 1.5 litres/day of drinking-water
containing 100 �g aluminium/litre would absorb about 3% of their total
daily intake of aluminium from this source depending upon the levels
found in food and the frequency of antacid use.
The proportion of plasma Al3+ normally bound to protein in
humans may be as high as 70-90% in haemodialysis patients with
moderately increased plasma aluminium. The highest levels of aluminium
may be found in the lungs, where it may be present as inhaled
insoluble particles.
The urine is the most important route of aluminium excretion.
After peroral administration of a single dose of aluminium, 83% was
excreted in urine after 13 days and 1.8% in the faeces. The half-life
of urinary concentration among welders exposed for more than 10 years
was 6 months or longer. Among retired workers exposed to aluminium
flake powders, the calculated half-lives were between 0.7 and 8 years.
1.6.2 Animals
Absorption via the gastrointestinal tract is usually less than
1%. The main factors influencing absorption are solubility, pH and
chemical species. Organic complexing compounds, notably citrate,
increase absorption. The aluminium absorption may interact with
calcium and iron transport systems. Dermal and inhalation absorption
has not been studied in detail. Aluminium is distributed in most
organs within the body with accumulation occurring mainly in bone at
high dose levels. To a limited but as yet undetermined extent,
aluminium passes the blood-brain barrier and is also distributed to
the fetus. Aluminium is eliminated effectively by urine. Plasma half-
life is about 1 h in rodents.
1.7 Effects on laboratory mammals and in vitro test systems
The acute toxicity of metallic aluminium and aluminium compounds
is low, the reported oral LD50 values being in the range of several
hundred to 1000 mg aluminium/kg body weight per day. However, the
LC50 values for inhalation have not been identified.
In short-term studies in which an adequate range of end-points
was examined following exposure of rats, mice or dogs to various
aluminium compounds (sodium aluminium phosphate, aluminium hydroxide,
aluminium nitrate) in the diet or drinking-water, only minimal effects
(decreases in body weight gain generally associated with decreases in
food consumption or mild histopathological effects) have been observed
at the highest administered doses (70 to 300 mg aluminium/kg body
weight per day). Systemic effects following parenteral administration
also included kidney dysfunction.
Adequate inhalation studies were not identified. Following
intratracheal administration of aluminium oxide, particle-associated
fibrosis was observed, similar to that found in other studies on
silica and coal dust.
No overt fetotoxicity was noted, nor were general reproductive
parameters noted after gavage treatment of rats with 13, 26 or 52 mg
aluminium/kg body weight per day (as aluminium nitrate). However, a
dose-dependent delay in the growth of offspring was noted with females
administered 13 mg/kg and in male offspring at 26 mg/kg. The lowest-
observed-adverse-effect level (LOAEL) for developmental effects
(decreased ossification, increased incidence of vertebral and
sternebrae terata and reduced fetal weight) was 13 mg/kg (aluminium
nitrate). These effects were not observed at much higher doses of
aluminium hydroxide. There were reductions in postnatal growth at
13 mg/kg (aluminium nitrate), although maternal toxicity was not
examined. In studies on brain development, grip strength was impaired
in offspring of dams fed 100 mg aluminium/kg body weight as aluminium
lactate in the diet, in the absence of maternal toxicity.
There is no indication that aluminium is carcinogenic. It can
form complexes with DNA and cross-link chromosomal proteins and DNA,
but it has not been shown to be mutagenic in bacteria or induce
mutation or transformation in mammalian cells in vitro. Chromosomal
aberrations have been observed in bone marrow cells of exposed mice
and rats.
There is considerable evidence that aluminium is neurotoxic in
experimental animals, although there is considerable variation among
species. In susceptible species, toxicity following parenteral
administration is characterized by progressive neurological
impairment, resulting in death with status epilepticus (LD50 =
6 �g Al/g dry weight of brain). Morphologically, the progressive
encephalopathy is associated with neurofibrillary pathology in
large and medium size neurons predominantly in the spinal cord,
brainstem and selected areas of the hippocampus. These tangles are
morphologically and biochemically different from those that occur in
Alzheimer's disease (AD). Behavioural impairment has been observed in
the absence of overt encephalopathy or neurohistopathology in
experimental animals exposed to soluble aluminium salts (e.g.,
lactate, chloride) in the diet or drinking-water at doses of 50 mg
aluminium/kg body weight per day or more.
Osteomalacia, as it presents in man, is observed consistently in
larger species (e.g., dogs and pigs) exposed to aluminium; a similar
condition is observed in rodents. These effects appear to occur in all
species, including humans, at aluminium levels of 100 to 200 �g/g bone
ash.
1.8 Effects on humans
No acute pathogenic effects in the general population have been
described after exposure to aluminium.
In England, a population of about 20 000 individuals was exposed
for at least 5 days to increased levels of aluminium sulfate,
accidentally placed in a drinking-water facility. Case reports of
nausea, vomiting, diarrhoea, mouth ulcers, skin ulcers, skin rashes
and arthritic pain were noted. It was concluded that the symptoms were
mostly mild and short-lived. No lasting effects on health could be
attributed to the known exposures from aluminium in the drinking-
water.
It has been hypothesized that aluminium in the drinking-water is
a risk factor for the development or acceleration of AD as well as for
impaired cognitive function in the elderly. It has also been suggested
that stamped fine aluminium powder and fume may be risk factors for
impaired cognitive function and pulmonary disease in certain
occupations.
Some 20 epidemiological studies have been carried out to test the
hypothesis that aluminium in drinking-water is a risk factor for AD,
and two studies have evaluated the association between aluminiun in
drinking-water and impaired cognitive function. Study designs ranged
from ecological to case control. Eight studies in populations in
Norway, Canada, France, Switzerland and England were considered
of sufficiently high quality to meet the general criteria for
exposure and outcome assessment and the adjustment for at least
some confounding variables. Of the six studies that examined the
relationship between aluminium in drinking-water and dementia or AD,
three found a positive relationship but three did not. However, each
of the studies had some deficiencies in the study design (e.g.,
ecological exposure assessment, failure to consider aluminium exposure
from all sources and to control for important confounders such as
education, socioeconomic status and family history, the use of
surrogate outcome measures for AD, and selection bias). In general,
the relative rists determined were less than 2, with large confidence
intervals, when the total aluminium concentration in drinking-water
was 100 �g/litre or higher. Based on current knowledge on the
pathogenesis of AD and the totality of evidence from these
epidemiological studies, it was concluded that the present
epidemiological evidence does not support a causal association between
AD and aluminium in drinking-water.
In addition to the epidemiological studies that examined the
relationship between AD and aluminium in drinking-water, two studies
examined cognitive dysfunction and AD in elderly populations in
relation to the levels of aluminium in drinking-water. The results
were again conflicting. One study of 800 male octogenarians consuming
drinking-water with aluminium concentrations up to 98 �g/litre found
no relationship. The second study used "any evidence of mental
impairment" as an outcome measure and found a relative risk of 1.72 at
aluminium concentrations greater than 85 �g/litre in 250 males. Such
data are insufficient to show that aluminium is a cause of cognitive
impairment in the elderly.
Reports of impaired cognitive function related to aluminium
exposure are conflicting. Most studies are on small populations, and
the methodology used in these studies is open to question with respect
to magnitude of effect reported, exposure assessment and confounding
factors. In a comparative study of cognitive impairment in miners
exposed to a powder containing 85% finely ground aluminium and 15%
aluminium oxide (as prophylaxis against silica) and unexposed miners,
the cognitive test scores and the proportion impaired in at least one
test indicated a disadvantage for the exposed miners. A positive
exposure-related trend of increased risk was noted.
In all occupational studies reported, the magnitude of effects
found, presence of confounding factors, problems with exposure
assessment and the probability of mixed exposures all make the data
insufficient to conclude that aluminium is a cause of cognitive
impairment in workers exposed occupationally to aluminium.
Neurological syndromes including impairment of cognitive
function, motor dysfunction and peripheral neuropathy have been
reported in limited studies of workers exposed to aluminium fume. A
small population of aluminium welders who were compared with iron
welders were reported to show a small decrement in repetitive motor
function. When a questionnaire methodology was used in another study,
an increase in neuropsychiatric symptoms was reported.
Iatrogenic exposure in patients with chronic renal failure,
exposed to aluminium-containing dialysis fluids and pharmaceutical
products, may cause encephalopathy, vitamin-D-resistant osteomalacia
and microcytic anaemia. These clinical syndromes can be prevented by
reduction in exposure to aluminium.
Premature infants, even where kidney impairment is not severe
enough to cause raised blood creatinine levels, may develop increased
tissue loading of aluminium, particularly in bone, when exposed to
iatrogenic sources of aluminium. Where there is kidney failure,
seizures and encephalopathy may occur.
Although human exposure to aluminium is widespread, in only a few
cases has hypersensitivity been reported following exposure to some
aluminium compounds after dermal application or parenteral
administration.
Pulmonary fibrosis was reported in some workers exposed to very
fine stamped aluminium powder in the manufacture of explosives and
fireworks. Nearly all cases involved exposure to aluminium particles
coated with mineral oil. That process is no longer used. Other cases
of pulmonary fibrosis have related to mineral exposures to other
agents such as silica and asbestos and cannot be attributed solely to
aluminium.
Irritant-induced asthma has been associated with inhalation
of aluminium sulfate, aluminium fluoride, potassium aluminium
tetrafluoride and with the complex environment of the potrooms during
aluminium production.
There is insufficient information to allow for classification of
the cancer risk from human exposures to aluminium and its compounds.
Animal studies do not indicate that aluminium or aluminium compounds
are carcinogenic.
1.9 Effects on other organisms in the laboratory and field
Aquatic unicellular algae showed increased toxic effect at low
pH, where bioavailability of aluminium is increased. They are more
sensitive than other microorganisms, the majority of 19 lake species
showing complete growth inhibition at 200 �g/litre total aluminium
(pH 5.5). Selection of aluminium-tolerant strains is possible; green
algae capable of growing in the presence of 48 mg/litre at pH 4.6 have
been isolated.
For aquatic invertebrates, LC50 values range from 0.48 mg/litre
(polychaete) to 59.6 mg/litre (daphnid). For fish, 96-h LC50 values
range from 0.095 mg/litre (American flagfish) to 235 mg/litre
(mosquito fish). However, care must be taken when interpreting the
results because of the significant effects of pH on the availability
of aluminium. The wide range of LC50 values probably reflects
variable availability. The addition of chelating agents, such as NTA
and EDTA, reduces the acute toxicity of aluminium to fish.
Responses to aluminium by macroinvertebrates are variable. In the
normal pH range aluminium toxicity increases with decreasing pH;
however, in very acidic waters aluminium can reduce the effects of
acid stress. Some invertebrates are very resistant to acid stress and
can be very numerous in acidic waters. Increased drift rate of
invertebrates has been reported in streams suffering either pH or
pH/aluminium stress; this is a common response to a variety of
stressors. Lake invertebrates generally survived field exposure to
aluminium but suffered as a result of phosphate reduction in
oligotrophic conditions induced by precipitation with aluminium.
Short- and long-term toxicity tests on fish have been carried out
under a variety of conditions and, most importantly, at a range of pH
values. The data show that significant effects have been observed at
monomeric inorganic aluminium levels as low as 25 �g/litre. However,
the complex relationship between acidity and aluminium bioavailability
makes interpretation of the toxicity data more difficult. At very
low pH (not normally found in natural waters) the hydrogen ion
concentration appears to be the toxic factor, with the addition of
aluminium tending to reduce toxicity. In the pH range 4.5 to 6.0
aluminium in equilibrium exerts its maximum toxic effect. Toxicity has
also been shown to increase with increasing pH levels in the alkaline
pH region. The mechanism of aluminium toxicity to fish has been
attributed to the inability of fish to maintain their osmoregulatory
balance, as well as respiratory problems associated with precipitation
of aluminium on the gill mucus. The former effect is associated with
lower pH levels. These laboratory findings have been confirmed by
field studies especially in areas under acid stress.
Amphibian eggs and larvae are affected by acidity and aluminium,
with interaction between the two factors. Reduced hatching, delayed
hatching, delayed metamorphosis, metamorphosis at small size, and
mortality have been reported in various species and at aluminium
concentrations below 1 mg/litre.
Exposure of roots of terrestrial plants to aluminium can cause
diminished root growth, reduced uptake of plant nutrients and stunted
plant development. Tolerance to aluminium has been demonstrated both
in the laboratory and the field.
1.10 Conclusions
1.10.1 General population
Hazards to neurological development and brain function from
exposure to aluminium have been identified through animal studies.
However, aluminium has not been demonstrated to pose a health risk to
healthy, non-occupationally exposed humans.
There is no evidence to support a primary causative role of
aluminium in Alzheimer's disease (AD), and aluminium does not induce
AD pathology in vivo in any species, including humans.
The hypothesis that exposure of the elderly population in some
regions to elevated levels of aluminium in drinking-water may
exacerbate or accelerate AD lacks adequate supporting data.
The data in support of the hypothesis that particular exposures,
either occupational or via drinking-water, may be associated with non-
specific impaired cognitive function are also inadequate.
There is insufficient health-related evidence to justify
revisions to existing WHO Guidelines for aluminium exposure in
healthy, non-occupationally exposed humans. As an example, there is an
inadequate scientific basis for setting a health-based standard for
aluminium in drinking-water.
1.10.2 Subpopulations at special risk
In people of all ages with impaired renal function, aluminium
accumulation has been shown to cause the clinical syndrome of
encephalopathy, vitamin-D-resistant osteomalacia and microcytic
anaemia. The sources of aluminium are haemodialysis fluid and
aluminium-containing pharmaceutical agents (e.g., phosphate binders).
Intestinal absorption can be exacerbated by the use of citrate-
containing products. Patients with renal failure are thus at risk of
neurotoxicity from aluminium.
Iatrogenic aluminium exposure poses a hazard to patients with
chronic renal failure. Premature infants have higher body burdens of
aluminium than other infants. Every effort should be made to limit
such exposure in these groups.
1.10.3 Occupationally exposed populations
Workers having long-term, high-level exposure to fine aluminium
particulates may be at increased risk of adverse health effects.
However, there are insufficient data from which to develop with any
degree of certainty occupational exposure limits with regards to the
adverse effects of aluminium.
Exposure to stamped pyrotechnic aluminium powder most often
coated with mineral oil lubricants has caused pulmonary fibrosis
(aluminosis), whereas exposure to other forms of aluminium has not
been proven to cause pulmonary fibrosis. Most reported cases had
exposure to other potentially fibrogenic agents.
Irritant-induced asthma has been associated with inhalation of
aluminium sulfate, aluminium fluoride or potassium aluminium
tetrafluoride, and with the complex environment within the potrooms
during aluminium production.
1.10.4 Environmental effects
Aluminium-bearing solid phases in the environment are relatively
insoluble, particularly at circumneutral pH values, resulting in low
concentrations of dissolved aluminium in most natural water.
In acidic or poorly buffered environments subjected to strong
acidifying inputs, concentrations of aluminium can increase to levels
resulting in adverse effects on both aquatic organisms and terrestrial
plants. However, there exist large species, strain and life history
stage differences in sensitivity to this metal.
The detrimental biological effects from elevated concentrations
of inorganic monomeric aluminium can be mitigated in the presence of
organic acids, fluorides, silicate and high levels of calcium and
magnesium.
There is a substantial reduction in species richness associated
with the mobilization of the more toxic forms of aluminium in acid-
stressed waters. This loss of species diversity is observed at all
trophic levels.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
The element aluminium (Al) was first obtained in an impure form
by Oersted in 1825, and pure aluminium was prepared by Woehler two
years later. The name aluminium is derived from alum, which the
ancient Greeks used as an astringent in medicine (Lide, 1991).
Aluminium is the most abundant metallic element and constitutes
8.13% of the earth's crust. Owing to its high reactivity, it is always
found combined with other elements and does not occur in its pure
state. Combined with oxygen, silicon, the alkali and alkaline-earth
metals, and fluorine, and as hydroxides, sulfates and phosphates,
aluminium appears in a wide variety of minerals (Frank et al., 1985;
Hudson et al., 1985; Lide, 1991).
Some aluminium compounds, synonyms and molecular formulae are
listed in Table 1. The most abundant natural aluminium ores are shown
in Table 2.
2.1 Identity
Pure aluminium is a silvery-white, malleable, ductile metal with
the atomic number of 13 and the relative atomic mass of 26.98. With
few exceptions aluminium is found in chemical compounds as AlIII.
Aluminium occurs naturally as 27Al; eight radioactive isotopes are
known, of which 26Al is the most stable with a half-life of 7.4 �
105 years (Frank et al., 1985).
2.2 Physical and chemical properties
2.2.1 Aluminium metal
Elemental aluminium possesses many desirable characteristics and
is therefore widely used in commerce (Sax & Lewis, 1987; Lide, 1991).
Aluminium crystallizes in a face-centered cubic lattice that is stable
from 4 K to melting point; the coordination number is 12, it is light
and malleable, and thus is easily formed into a variety of shapes
(Frank et al., 1985).
Owing to the high charge/radius ratio of Al3+ in aqueous
solutions, the ion proteolyses part of the water envelope and forms
hydroxo complexes. It can also complex with electron-rich species,
such as fluoride and chloride. The chemical properties of aluminium
resemble those of beryllium and silicon. Because of its amphoteric
character, it reacts with mineral acids and strong alkalis (Sax &
Lewis, 1987). Although aluminium is one of the most reactive of the
common metals used commercially, it has excellent resistance to
Table 1. Chemical names, synonyms and molecular formulae of elemental aluminium and aluminium compoundsa
Chemical name CAS registry number Synonyms Formula
Aluminium 7429-90-5 Aluminium, metana Al
Aluminium chloride 7446-70-0 Aluminium trichloride AlCl3
Aluminium chlorohydrate 1327-41-9 Aluminium chlorohydroxide, AlCl(OH)5
11097-68-0 aluminium chloride, basic,
84861-98-3 chlorhydrol, polyaluminium chlorideb Al2Cl(OH)52H2O
Aluminium fluoride 7784-18-1 Aluminium trifluoride AlF3
Aluminium lactate 18917-91-4 Aluctyl Al(C3H5O3)3
Aluminium oxidec 1302-74-5 alpha-Alumina, corundum Al2O3
Aluminium oxide hydroxidec 14457-84-2 Diaspore alpha-AlO(OH) or alpha-Al2O3H2O
Aluminium oxide hydroxidec 1318-23-6 Boehmite gamma-AlO(OH) or gamma-Al2O3H2O
Aluminium oxide, trihydratec 20257-20-9 Bayerite, alpha-aluminium trihydroxide alpha-Al(OH)3 or alpha-Al2O33H2O
Aluminium oxide, trihydratec 13840-05-6 Nordstrandite, �-aluminium trihydroxide �-Al(OH)3 or �-Al2O33H2O
Aluminium oxide, trihydratec 14762-49-3 Gibbsite, hydrargillite, gamma-aluminium gamma-Al(OH)3 or gamma-Al2O33H2O
trihydroxide
Nitric acid, aluminium salt 13473-90-0 Aluminium trinitrate, aluminium nitrate Al(NO3)3
Table 1. (Con't)
Chemical name CAS registry number Synonyms Formula
Phosphoric acid,
aluminium salt 7784-30-7 Aluminium orthophosphate AlPO4
Sodium aluminate 1302-42-7 NaAlO2, Na2OAl2O3 or
Na2Al2O4
Sulfuric acid, aluminium salt 10043-01-3 Alum, aluminium trisulfate, cake alum Al2(SO4)3
Trimethylaluminiumb 75-24-1 Al(CH3)3
2-Propanol, aluminium saltb 555-31-7 Aluminium isopropoxide, aluminium Al(OCH(CH3)2)3
isopropylate
2-Butanol, aluminium saltb 2269-22-9 Aluminium sec-butoxide, aluminium Al(OC4H9)3
butylate
a adapted from ATSDR (1992)
b Zietz (1985)
c Hudson et al. (1985)
Table 2. CAS chemical names and registry numbers, synonyms, trade names, content and molecular formula of aluminium oresa
Chemical name CAS registry Synonyms and trade Composition Formula
number names
Aluminium magnesium - Magnesium aluminium 48.8% O MgAl2(SiO4)2
silicate silicate 21.4% Si
20.6% Al
9.3% Mg
Aluminium silicate, hydrate - Kaolinite 40% Al2O3b Al2Si2O5(OH)4 or
46% SiO2 Al2O3SiO2H2O
14% H2O
Aluminium silicofluoride - Topaz 71.2% F 2Al2O32Al(F,OH)33SiO2
17.6% Si
11.2% Al
Ammonium aluminium 7784-26-1 Ammonium alum, - NH4Al(SO4)212H2O or
sulfate, hydrate ammonium Al2O3(NH4)2O24HOH
aluminium sulfate
Bauxite 1318-16-7 - 30-75% Al2O3 -
3-25% Fe2O3
9-31% H2O
2-9% SiO2
1-3% TiO2
Table 2. (Con't)
Chemical name CAS registry Synonyms and trade Composition Formula
number names
Potassium aluminium 7784-24-9 Potash alum, potassium 37% Al2O3 K(AlO)3(SO4)212H2O or
sulfate, hydrate aluminium sulfate 11% K2O Al2(SO4)3K2SO424HOH
39% SO3
13% H2O
Sodium aluminium 15096-52-3 Cryolite, greenland - Na3AlF6 or 3NaFAlF3
fluoride spar, isestone
Sodium aluminium 7784-28-3 Sodium alum, sodium - NaAl(SO4)212H2O or
sulfate, hydrate aluminium sulfate Al2(SO4)2Na2SO424HOH
Sodium calcium - Anorthosite, soda-lime 26-35% Al2O3b Na2OAl2O36SiO2 &
silicoaluminate feldspar 46-59% SiO2 CaOAl2O32SiO2
8-18% CaO
1-7% Na2O
a From: Sax & Lewis (1987)
b US Bureau of Mines (1967)
corrosion. Exposed to oxygen, water or other oxidants, a continuous
film of aluminium oxide (Al2O3) grows rapidly on the nascent
aluminium surface, providing the metal with a high resistance to
corrosion. The oxide film dissolves in alkaline solutions with
evolution of hydrogen and formation of soluble alkali-metal aluminates
(Sax & Lewis, 1987).
The oxide film on the solid metal is resistant to some acids
(e.g., nitric acid), and prevents further chemical attack on the
metal. However, the protective oxide film dissolves in some acids
(e.g., hydrochloric or hot sulfuric acids) and also in alkaline
solutions, exposing the metal to further reactions. At elevated
temperatures, aluminium metal reacts with water (above 180�C),
producing Al(OH)3 and H2, and with many metal oxides producing
Al2O3 and the metal. This reaction is used to produce certain
metals, for example, manganese and alloys (e.g., ferro-titanium).
Finely divided aluminium dust can ignite and cause explosions
(Wade & Banister, 1973; Frank et al., 1985).
Many applications of aluminium and its alloys are based upon its
inherent properties of high electrical and thermal conductivity, low
density, and great resistance to corrosion. Pure aluminium is soft and
lacks strength, but it can be alloyed with small amounts of Cu, Mg,
Si, Mn and other elements to impart greater strength and a variety of
other useful properties. Aluminium alloys are light, strong and
readily worked into a variety of shapes (Frank et al., 1985; Lide,
1991).
2.2.2 Aluminium compounds
The aluminium compounds of the greatest industrial importance are
aluminium oxide, aluminium sulfate and aluminium silicate. Some
physical and chemical data of aluminium and selected aluminium
compounds are summarized in Table 3.
Aluminium oxide is a white powder that is found as balls or
lump of various mesh sizes. Owing to its amphoteric character, it is
soluble in mineral acids and strong alkali. Aluminium oxide is
found in different modifications. The hexagonally closest-packed
alpha-modification "corundum" (alpha-Al2O3) is the most stable
oxide. Emery is an abrasive containing corundum, and ruby and sapphire
are impure crystalline varieties of gem quality (Hudson et al., 1985).
Formation of aluminium oxide by dehydration of the hydroxides produces
a series of alumina types still containing a small proportion of
hydroxyl groups and retaining some chemical reactivity. All oxides
produced at low temperatures are collectively referred to as
transitional oxides. Those formed by dehydration below 600�C are known
Table 3. Physical and chemical properties of aluminium and some of its compoundsa
Chemical name Relative atomic/ Melting Boiling Relative density Crystalline Solubilityd
molecular mass point (�C) point (�C) (g/cm3)b form
Aluminium 26.98 660 2450c 2.708 silver-white cubic sol alkali, HCl, H2SO4;
insol H2O, HNO3e
alpha-Aluminium 77.99 300 (-H2O) 2.420 monoclinic, powder sol acid; insol H2O,
hydroxide (bayerite) alcohole
Aluminium nitrate 213.00 74 135 - rhombic delinq. sol H2O, alkali,
(decomposes) acetone, HNO3
Aluminium oxide 101.94 2072 2980 3.965 (25) hexagonal very sl sol benzene;
insol H2O
gamma-Aluminium oxide 59.99 - - 3.440 orthorhomic sol acid; sl sol
hydroxide (boehmite) alkali; insol H2O,
alcohole
Aluminium phosphate 121.95 1500 - 2.566 rhombic platelets sol acid, alkali; insol H2O
Aluminium sulfate, 342.14 700 - 2.710 powder sol H2O, dil acid; sl
anhydrate (decomposes) sol alkali
Aluminium sulfate, 666.41 87 - 1.690 (17) monoclinic sol H2O, dil. acid; sl
hydrate (decomposes) sol alkali
Aluminium 204.25 119 141 1.035 (20) crystals sol alcohol, benzene,
isopropoxidee chloroform
a Compiled from ATSDR (1992)
b Temperature is given in parentheses
c Sax & Lewis (1987)
d Sol = soluble; insol = insoluble; sl = slightly
e Lide (1991)
as gamma-aluminas or activated aluminas, while the aluminas formed by
dehydration at higher temperatures (900-1000�C), the rho-aluminas, are
nearly anhydrous Al2O3 (Wade & Banister, 1973). At 1400�C all
transitional alumina converts to alpha-alumina (Hudson et al., 1985).
The structural and compositional differences among various forms of
alumina are associated with differing particulate size, particulate
surface area, surface reactivity and catalytic activity.
Various forms of aluminium hydroxides are known. The best defined
forms are the trihydroxides (Al(OH)3) and the oxide-hydroxides
(AlO(OH)). Besides these well-defined crystalline forms, several other
hydroxides have been described in the literature (Wefers & Bell,
1972). The aluminium hydroxides found abundantly in nature are
gibbsite (Al(OH)3), diaspore �-(AlO(OH)), and boehmite
alpha-(AlO(OH)). They all convert to aluminium oxide when heated
(Hudson et al., 1985).
Aluminium sulfate can exist with varying proportions of water,
the common form being Al2(SO4)3�18H2O. It is almost insoluble
in anhydrous alcohol, but readily soluble in water. Above 770�C
decomposition to aluminium oxide is observed. Aluminium sulfate is
mainly used in water treatment, dyeing, leather tanning and in the
production of other aluminium compounds. Alums are crystalline double
salts composed of aluminium, sulfate and a monovalent cation, such
as potassium, sodium or ammonium, and have the general formula
M+Al3+(SO4)2�12H2O. In aqueous solution, alums show all the
chemical properties that their components show separately (Helmboldt
et al., 1985).
Clays are aluminium silicates. They have cation-exchange capacity
and the amounts and types of clay minerals in a soil largely determine
its physical properties and suitability for agriculture (Wild, 1988).
Aluminium halogenides, hydrides and lower aluminium alkyls react
violently with molecular oxygen, and are spontaneously inflammable in
air and explosive with water. Industrially these compounds are used as
co-catalysts for organometallic and organic synthesis, and as
intermediates in various production processes (Stokinger, 1987;
Budavari, 1989).
Further compounds of industrial interest are aluminium antimonide
(AlSb) and selenide (AlSe), which are employed in the semiconductor
technology industry (Budavari, 1989). Aluminium phosphide (AlP) is
used as a rodenticide and pesticide, but it is not discussed in this
monograph since its biocidal activity is due to the phosphide moiety
and not to the aluminium.
2.3 Analytical methods
Various methods for sampling, sample preparation and
determination of aluminium in biological and environmental samples
have been developed and described. An overview of standard methods is
given in Table 4.
2.3.1 Sampling and sample preparation
Because of the ubiquitous distribution of aluminium in nature,
care must be taken during sampling and sample preparation to avoid
contamination. Most analytical errors are due to contamination of the
sample with aluminium from air, vessels and reagents during sampling
and preparation for analysis. To prevent aluminium contamination, the
use of aluminium-free polyethylene, polypropylene, teflon or quartz
materials is recommended. Containers and laboratory materials have to
be washed with warm, dilute nitric acid and subsequently rinsed with
de-ionized water prior to use (Andersen, 1987).
Air is sampled with high volume samplers using low-ash cellulose
or cellulose ester filters for particulate aluminium (NIOSH, 1984).
Biological samples need to be preserved by cooling, freezing or
lyophilization. Preservation with 10% formalin is not recommended
because of a high risk of aluminium contamination (Bouman et al.,
1986).
Homogeneity of the samples is an absolute prerequisite for
accurate analysis. To prepare samples for analysis, inorganic samples
are usually dissolved in nitric acid or extracted with water.
Solutions are filtered with a membrane filter and the particulate
residue is analysed separately (Dunemann & Schwedt, 1984).
Water (DIN, 1993) and urine should be acidified with HNO3 or HCl
to pH < 2 to prevent adsorption effects and the precipitation of
salts. This ensures that aluminium remains in solution. Water samples
for speciation analysis should be stored, without acidification, in
high-density polyethylene bottles (Berden et al., 1994; Fairman et
al., 1994). Prior to analysis biological tissues must be homogenized
and separated or extracted. Blood and urine samples may be separated
by centrifugation and diluted, or, if appropriate, analysed directly
without pretreatment.
Table 4. Analytical methods for aluminium and aluminium compoundsa
Medium Sample preparation Analytical method Detection limit Recovery Reference
Environmental
samples
Air Sample collection on cellulose FAAS 500 �g/m3 (100-litre n.g. NIOSH (1984)
filter, ashing with HNO3 sample)
Sample collection on cellulose ICP-AES 5 �g/m3 (500-litre n.g. NIOSH (1984)
filter, ashing with HNO3 sample)
Water Reaction with sulfonitrazo DAF Spectrophotometry 4 �g/litre n.g. Ermolenko &
Dedkov (1988)
Reaction with Chromazurol S Spectrophotometry 0.0005 �g/0.5 ml- n.g. Schwedt (1989)
sample
Reaction with alizarin S Spectrophotometry 10 �g/litre; 50 �g/litre n.g. DIN (1993)
(after digestion)
Digestion with HNO3 and ICP-AES 100 �g/litre n.g. DIN (1993)
H2O2
Filtration, digestion with HNO3, Spectrophotometry 6-13 �g/litre range 98-100% van Benschoten &
reaction with 8-hydroxyquinoline ICP-AES 3 �g/litre limit detection Edzwald (1990)
Soil Extraction with H2O, filtration, GF-AAS n.g. Gardinier et
high-performance size exclusion al. (1987)
chromatography
Soil Extraction with H2O, filtration, Spectrophotometry 0.005 �g (absolute) n.g. Dunemann &
gel chromatography, reaction Schwedt (1984)
with Chromazurol
Fly ash Vacuum dried NAA n.g. Fleming &
Lindstrom (1987)
Table 4. (Con't)
Medium Sample preparation Analytical method Detection limit Recovery Reference
Rock, soil, Dried, digestion with ICP-AES 1-5 �g/litre > 57% Que Hee &
paint, HNO3/HCl Boyle (1988)
citrus leaves
Biological
samples
Serum Centrifugation, dilution with GF-AAS 14.3-150 �g/litre 97-102% Bettinelli et
Mg(NO3)2 (analytical range) al. (1985)
Plasma, Centrifugation, dilution with GF-AAS 4 �g/litre 90-102% Gardinier et
serum water al. (1981)
Whole blood, Dilution with Triton X-100 GF-AAS 1.9 �g/litre (serum), n.g. van der Voet
plasma, serum 1.8 �g/litre (plasma), et al. (1985)
2.3 �g/litre (blood)
Biological Wet-digestion, complexation NAA 2.1 �g/litre (liver) n.g. Blotcky et
tissue, urine with Tiron, anion-exchange 0.18 �g/ml (urine) al. (1992)
chromatography
Urine, blood Dilution with water ICP-AES 6 �g/litre n.g. Sanz-Medel et
al. (1987)
Dilution with water ICP-AES 0.3 �g/litre n.g. Mauras &
Allain (1985)
Table 4. (Con't)
Medium Sample preparation Analytical method Detection limit Recovery Reference
Biological Freeze dry, grind NAA n.g. Yukawa et
tissues al. (1980)
Dried, digestion with HNO3, GF-AAS 0.5 �g/g dry tissue 80-117% Bouman et
dilution with water al. (1986)
Dried, digestion with HNO3, AMS 106 atoms 26Al n.g. Kobayashi et
cation-exchange al. (1990)
chromatography
Digestion, high-performance Spectrophotometry 7 �g/litre 87-94% Dean (1989)
ion-exchange chromatography,
reaction with Tiron
Hair Washed with 2-propanol, GF-AAS 0.65 �g/g dry weight 84-105% Chappuis et
digestion with HNO3 al. (1988)
Body fluids Dilution with HNO3/HCl ICP-AES 1-5 �g/litre > 57% Que Hee &
Boyle (1988)
Haemodialysis Dilution with HNO3 and GF-AAS 3 �g/litre 93-108% Andersen (1987)
concentrates Triton X-100 (Zeeman-corrected)
Haemodialysis Reaction with ferron in Phosphorimetry 5.4 �g/litre n.g. De La Campa
fluids CTAB et al. (1988)
a AMS = accelerator mass spectrometry; CTAB = cetyltriammonium bromide; EDTA = ethylenediaminetetraacetic acid; FAAS = flame
atomic-absorption spectrophotometry; ferron = 7-iodo-8-quinolinol-5-sulfonic acid; GF-AAS = graphite furnace - atomic-absorption
spectrophotometry; ICP-AES = inductively coupled plasma - atomic-emission spectrophotometry; NAA = neutron activation analysis;
n.g. = not given; Tiron = 4,5-dihydroxy-1,3-benzenedisulfonic acid
Free aluminium may be determined directly from the samples or the
sample extracts. To determine insoluble aluminium compounds and
organically bound species, the samples (organic matter, air-filters,
water, soil, etc.) need to be subjected to wet ashing (digestion) or
dry ashing. Wet ashing, i.e. heating with nitric acid under reflux, is
suitable for most organic and biological samples. The residues are
dissolved in acids before analysis (NIOSH, 1984; Kobayashi et al.,
1990; DIN, 1993). After digestion, differentiation between free metal
species and kinetically labile and stable complexes is not possible.
2.3.2 Separation and concentration
A fractionation procedure for aluminium species in water using an
0.22 �m size filter has been proposed by van Benschoten & Edzwald
(1990). Total reactive aluminium is determined in the unfiltered,
acidified sample. Dissolved monomeric aluminium is analysed in the
unfiltered sample without acidification. Analysis of total dissolved
aluminium is performed after filtration and acidification of the
sample. Dissolved organically bound aluminium is analysed after
separation of the filtered sample on a column of cation exchange
resin. The eluate is acidified and analysed colorimetrically. For the
determination of dissolved organic monomeric aluminium, samples are
passed through a cation exchange column and are analysed with no
acidification.
In order to carry out long-term characterization of the highly
acute toxicity during the initial phase of aluminium polymerization in
"mixing zones" (Rosseland et al., 1992), in situ fractionation
techniques such as ultrafiltration (Lydersen et al., 1987) are
recommended (see section 9.1.2.3).
For the extraction of aluminium bound to fulvic acids, soil
samples may be extracted with copper chloride solution (Gardinier et
al., 1987). The clean-up of aqueous extracts of soil samples can be
performed by gel chromatography (Dunemann & Schwedt, 1984) or by size
exclusion chromatography. These methods are very mild and thus
suitable for the determination of labile aluminium species (Gardinier
et al., 1987).
Water samples may be concentrated by careful evaporation (DIN,
1993). Macro quantities of aluminium can be separated from small
amounts of interfering elements by precipitation of aluminium as
its hydroxide or phosphate. Chelating agents, such as EDTA,
8-hydroxyquinoline, and 2,2'-dihydroxyazobenzene, can be used to
extract aluminium into an organic solvent (Alderman & Gitelman, 1980).
Biological materials contain a variety of compounds that
can severely interfere with aluminium determinations. Hence,
chromatographic methods are often employed for sample purification.
Biological tissue samples may be cleaned-up by cation-exchange
chromatography after acid digestion (Dean, 1989; Kobayashi et al.,
1990). Blotcky et al. (1992) proposed the chelating of aluminium prior
to anion-exchange chromatography. Precolumn derivatization coupled
with reversed-phase high performance liquid chromatography (RP-HPLC)
is an effective method for the separation of the chelates of
different interfering metal ions (Nagaosa et al., 1991). Solvent
extraction of aluminium chelate complexes, e.g., 2,4-pentanedione and
4-methyl-2-pentanone, has been described as a separation and pre-
concentration step in the analysis of body fluids (Buratti et al.,
1984).
2.3.3 Detection and measurement
Spectrophotometric methods for aluminium analysis are simple
and quick, and are most often used for the determination of aluminium
in water. Samples are treated with inorganic or organic reagents to
form coloured soluble complexes that can be measured by absorption
spectrometry. Disadvantages of these methods are the narrowness of
the pH range of the reaction, the instability of the complexes, the
low selectivity, and the low sensitivity (Bettinelli et al., 1985).
The working range for the aluminium determination with chromazurol C
is 25-1000 �g/litre (Schwedt, 1989), with alizarin S it is 10-500
�g/litre (DIN, 1993), and with Tiron it is 7-5000 �g/litre (Dean,
1989). Detection limits of 1 �g/litre can be achieved. Chromatographic
separation of chelates of interfering metals increases the selectivity
of spectrophotometric methods.
De La Campa et al. (1988) and Garc�a et al. (1991) reported a
room temperature phosphorimetric method for aluminium analysis.
Aluminium reacts with 7-iodo-8-quinolinol-5-sulfonic acid (ferron) in
cetyltrimethylammonium bromide micelles to form a highly
phosphorescent complex. The method is used to determine aluminium in
water and dialysis fluids. The given detection limits are 5.4 �g/litre
and 2 �g/litre, respectively.
Instrumental methods applied to the determination of aluminium
include neutron activation, X-ray fluorescence, flame atomic-
absorption spectrophotometry, inductively coupled plasma - atomic-
emission spectrophotometry (ICP-AES) and graphite furnace - atomic-
absorption spectrophotometry (GF-AAS). However, neither X-ray
fluorescence nor flame absorption methods are sensitive enough to
measure trace levels in biological samples (Bettinelli et al., 1985).
The NIOSH procedure for aluminium analysis in air is applicable over a
working range of 50-5000 �g per sample or 0.5-10 mg/m3 for a
100-litre sample (NIOSH, 1984).
Neutron activation analysis produces excellent results but the
methods are time consuming and the facilities are not always readily
available. The method is used for determining aluminium in fly ash
(Fleming & Lindstrom, 1987) and biological tissues (Yukawa et al.,
1980; Blotcky et al., 1992). After digestion and concentration of the
biological samples, a detection limit of 2.1 �g/g was found for bovine
liver (Blotcky et al. 1992).
GF-AAS is the most frequently used technique to determine
aluminium at low concentrations. Detection limits between 0.5 and 4
�g/litre or �g/g are achieved with the analysis of various
environmental and biological samples (Gardinier et al., 1981; van der
Voet et al., 1985; Bettinelli et al., 1985; Andersen, 1987). Most
liquid samples can be injected directly after dilution into GF-AAS.
Dilution is necessary because most biological fluids have high salt
contents (in the order of 30%) (Andersen, 1987). To prevent
precipitation of aluminium and the formation of carbon residues, EDTA
or Triton X can serve as diluents. Ammonia may be added to convert
aluminium to aluminate and thus avoid loss of aluminium as its
chloride (Gardinier et al., 1981). Triton X-100 is used to reduce the
viscosity of the samples, and MgNO3 is added as a matrix modifier to
improve the volatility of aluminium (Bettinelli et al., 1985).
ICP-AES is used for the determination of aluminium in various
biological and environmental samples, allowing the simultaneous
determination of different elements at low levels of interference
(Mauras & Allain, 1985; Sanz-Medel et al., 1987). The NIOSH method for
aluminium determination in air samples is recommended for a working
range of 5-2000 �g/m3 for a 500-litre sample (NIOSH, 1984). A
detection limit of 1 �g/litre in biological and environmental samples
has been reported by Que Hee & Boyle (1988). ICP can also be combined
with a mass spectrometer to further increase the sensitivity of the
method. As a multi-element detector for reversed-phase liquid
chromatography, ICP-MS offers the ability to measure isotope ratios on
eluting peaks and to remove troublesome matrices on-line (Thompson &
Houk, 1986).
For 26Al tracer experiments (Kobayashi et al. 1990), the
application of accelerator mass spectrometry (AMS) has been described.
The limit of detection is 106 atoms; thus the sensitivity of AMS is
105 times greater than that of gamma-ray counting techniques.
Aluminium concentrations in human brain can be investigated by
laser multipoint microprobe mass analysis (LAMMA) using focussed laser
ionization with time-of-flight mass spectrometry (Stern et al., 1986).
27Al nuclear magnetic resonance (NMR) may be used to ascertain the
coordination of aluminium in soil solutions (Schierl, 1985).
Aluminium in natural water samples has been determined using
reversed-phase liquid chromatography of the 8-quinolinol complex using
spectrophotometric detection. A detection limit of 2 �g/litre was
reported (Nagaose et al., 1991).
2.3.4 Speciation analysis of aluminium in water
Speciation analysis aims to distinguish and determine
quantitatively different groups of physico-chemical species present
in a water sample. All speciation methods, with the exception of
potentiometric techniques and direct spectroscopic methods (e.g.,
NMR), will alter the speciation of the sample during measurement. This
may not be a disadvantage, particularly if, as is usual, the
speciation analysis is being carried out in order to estimate the
toxicity of the sample to aquatic biota. Toxicity itself is a dynamic
process, and the interaction of aluminium species in water with a
biomembrane (e.g., a fish gill) will change the aluminium species
distribution in the solution close to the biomembrane. The best
speciation probe is one that reacts with aluminium in a water sample
to a similar extent and at a similar rate to the reaction of a
biomembrane with the aluminium in the samples.
Speciation analysis of aluminium in a water sample is usually
carried out after first filtering the sample through a 0.45 �m
membrane filter to remove particulate matter. The filtrate can then be
analysed for groups of species by several different techniques,
including kinetic spectrophotometry (Parker & Bertsch, 1992a,b), ion
exchange (Driscoll, 1984) and ion chromatography (Jones, 1991).
Combinations of methods such as sample acidification, kinetic
spectrophotometry and ion exchange are frequently used to determine a
variety of species (Driscoll, 1984; Courtijn et al., 1990; van
Benschoten & Edzwald, 1990). These speciation schemes provide
information on various speciation groups, including total dissolved
aluminium, acid-soluble aluminium, total monomeric aluminium, reactive
monomeric aluminium, non-reactive monomeric aluminium, aluminium
fluoride complexes, organic monomeric aluminium and inorganic
monomeric aluminium. The terms "reactive" and "labile", as applied to
aluminium species, are operationally defined and refer to species that
react rapidly with an analytical probe such as a cation exchange resin
or a chromogenic reagent.
The aluminium species that are most toxic to aquatic organisms
are believed to reside in the reactive monomeric inorganic aluminium
fraction and to consist principally of aluminium hydroxy complexes
(Helliwell et al., 1983; Fairman et al., 1994; Parent & Cambell,
1994). Although the fluoro complex is toxic, it is less so than the
aluminium hydroxy complexes (Helliwell et al., 1983).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Aluminium is released to the environment both by natural
processes and from anthropogenic sources. Natural processes far
outweigh the contribution of anthropogenic sources because aluminium
is a major constituent of the earth's crust, making up about 8% of the
earth's surface (Lantzy & Mackenzie, 1979). Anthropogenic releases
are mostly indirect, for example, through emission of acidifying
substances such as sulfur dioxide and nitrogen oxides to the
atmosphere. These acidify rain and soil and contribute to dissolution
of aluminium from the soil. The largest anthropogenic impact on
aluminium movement in the environment is through enhanced wind and
water erosion from cultivated land, notably when fallow. Aluminium is
the third most abundant element. It does not occur naturally in the
metallic, elemental state, but is widely distributed in the earth's
crust in combination with oxygen, fluorine, silicon and other
constituents. Aluminium occurs ubiquitously in silicates such as
feldspars and micas, complexed with sodium and fluoride as cryolite,
and in bauxite rock, which is composed of hydrous aluminium oxides,
aluminium hydroxides and impurities such as free silica. In general,
decreasing pH as a result of acid rain or the release of acid mine
drainage results in increased mobility of the monomeric forms of
aluminium (ATSDR, 1992). Chemical speciation in soil and water
affecting the bioavailability of aluminium to organisms is discussed
in chapter 4.
3.2 Anthropogenic sources
Direct anthropogenic releases of aluminium compounds are
primarily to the atmosphere and are associated with industrial
processes such as smelting. However, the use of aluminium and
aluminium compounds in processing, packaging, storage of food products
and as flocculants in the treatment of drinking-water may contribute
to its presence in drinking-water and food stuffs (ATSDR, 1992).
3.2.1 Production levels and processes
The most important raw material for the production of aluminium
is bauxite, which contains up to 55% alumina (aluminium oxide). The
commercial deposits of bauxite are mainly gibbsite (Al2O3�3H2O) and
boehmite (Al2O3�H2O). The bauxite is extracted by open-cast mining
(Dinman, 1983).
The production of the metal comprises two basic steps: refining
and reduction. Refining involves the production of alumina from
bauxite by the Bayer process in which bauxite is digested at high
temperature and pressure in a strong solution of caustic soda. The
resultant hydrate is crystallized and calcined to the oxide. Reduction
involves the reduction of alumina to virgin aluminium metal by the
Hall-Heroult electrolytic process using carbon electrodes and a
cryolite flux (Dinman, 1983).
World bauxite production was 106 million tonnes in 1992. A
comparison of the quarterly average figures for 1993 and 1994 with
this figure shows that production in major producing countries is
remaining fairly constant (World Bureau of Metal Statistics, 1994).
The total primary aluminium production for 1992 is summarized in Table
5. The amount of aluminium recovered from purchased or tolled scrap in
1992 was 14% of the total primary production figure. The total alumina
production for 1992 is summarized in Table 6. The total alumina
production figure includes 30 million tonnes for metallurgical uses
and 3 million tonnes for non-metallurgical uses. The total figures for
primary aluminium and alumina production have not changed greatly
since 1988.
3.2.2 Uses
Aluminium metal has a wide variety of uses including structural
material for construction, automobiles and aircraft, and the
production of metal alloys. Other uses include die-cast motor parts,
cooking utensils, decorations, road signs, fencing, beverage cans,
food packaging, foil, corrosion-resistant chemical equipment, solid
fuel rocket propellents and explosives, dental crowns, and denture
materials. In the electrical industry aluminium is used for power
lines, electrical conductors, insulated cables and wiring (ATSDR,
1992).
Table 5. Primary aluminium production in 1992 (from: IPAI, 1993)
Geographical area Thousands of tonnes
Africa 617
North America 6016
Latin America 1949
East and South Asia 1379
Europe 3319
Oceania 1483
Total 14 763
Table 6. Alumina production in 1992 (from: IPAI, 1993)
Geographical area Thousands of tonnes
Africa 604
North America 5812
Latin America 7627
East and South Asia 2360
Europe 5565
Oceania 11 803
Total 33 771
Aluminium compounds and materials also have a wide variety of
uses, some of which are listed in Table 7. Aluminium powder is used in
paints, protective coatings and fireworks. Natural aluminium minerals
especially bentonite and zeolite are used in water purification, sugar
refining, brewing and paper industries. Aluminium sulfate is used for
water purification, as a mordant in dyeing, and in paper production.
Other aluminium compounds are used as tanning agents in the leather
industry, and as components of human and veterinary medicines, glues,
disinfectants, and in toothpaste, styptic pencils, deodorants,
antacids and food additives. Clays (aluminium silicates) are used as
industrial raw materials (e.g., production of ceramics), and
aluminates are constituents of cement. Alkyl aluminium products are
used as catalysts for the production of low pressure polyethylene
(ATSDR, 1992).
Table 7. Main uses of aluminium compoundsa
Aluminium compounds Uses
alums hardening agent and setting accelerator for gypsum
plaster, in tanning and dyeing, and (formerly) in styptic
pencils
aluminas in water treatment and as accelerator for concrete
solidification (high alumina cements)
alkoxides in varnishes, for textile impregnation, in cosmetics and
as an intermediate in pharmaceutical production
borate production of glass and ceramics
carbonate antacid
chlorides production of rubber, lubricants and wood preservatives,
and in cosmetics as an astringent; the anhydrous
product is used as a catalyst and raw material in the
chemical and petrochemical industries; active ingredient
in antiperspirants
hydroxide stomach antacid, other pharmaceuticals
isopropoxide used in the soap and paint industries; waterproofing
textiles
phosphate antacid
silicate component of dental cement; antacid, food additives
sulfate used in water purification as a flocculent, in paper
production, as a mordant in dyeing, and as a starting
material for the production of other aluminium
compounds
trioxide used as an absorbent, abrasive and refractory material
sodium aluminium food additives
phosphate
a From: Helmbolt et al. (1985); ATSDR (1992)
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Aluminium occurs ubiquitously in silicates such as feldspars and
micas, complexed with sodium and fluoride as cryolite, and in bauxite
rock composed of hydrous aluminium oxides, aluminium hydroxides, and
impurities such as free silica (ATSDR, 1992). Aluminium is not
found as a free metal because of its reactivity. It has only one
oxidation state (+3); therefore, its transport and distribution in
the environment depend upon its coordination chemistry and the
characteristics of the local environmental system. Aluminium
partitions between solid and liquid phases by reacting and complexing
with water molecules and electron-rich anions, such as chloride,
fluoride, sulfate, nitrate, phosphate and negatively charged
functional groups on humic materials and clay.
At a pH greater than 5.5, naturally occurring aluminium compounds
exist predominantly in an undissolved form such as gibbsite (Al(OH)3)
or as aluminosilicates, except in the presence of high amounts of
dissolved organic material such as fulvic acid, which binds with
aluminium and can cause an increase in dissolved aluminium
concentrations in streams and lakes (ATSDR, 1992). Several processes
influence aluminium mobility and its subsequent transport within the
environment; these include chemical speciation, hydrological flow
paths, other spatial and temporal factors related to soil-water
interactions, and the composition of the underlying geological
materials (Grant et al., 1990). Watersheds with shallow, acidic soils
and poorly buffered surface waters mobilize aluminium when exposed to
acidic deposition (Driscoll et al., 1988).
4.1.1 Air
Aluminium enters the atmosphere as a major constituent of a
number of atmospheric particulates, such as soil-derived dusts from
erosion and particulates from coal combustion (Grant et al., 1990).
Eisenreich (1980) studied the atmospheric loading of aluminium to Lake
Michigan, USA. It was found that aluminium was generally associated
with large particles (> 2 �m diameter) and that these were deposited
near the source. The total atmospheric loading of aluminium to the
lake was calculated to be 0.86 kg/ha per year. The more industrialized
area south of the lake contributes 75% of this total loading. Cambray
et al. (1975) calculated the dry deposition flux of aluminium to the
North Sea to be 51 000 tonnes/year. Ottley & Harrison (1993)
calculated the flux to be 7 300 tonnes/year; they suggest that the
lower estimate is due to more spatially appropriate and extensive air
monitoring since 1975. Rahn (1981) calculated the input of aluminium
from the atmosphere to the Arctic Ocean at 30 000 tonnes/year. The
input was significantly less than those of oceanic and riverine inputs
(140 000 and 110 000 tonnes/year, respectively).
Guieu et al. (1991) compared atmospheric inputs with river inputs
of aluminium for the Golfe du Lion, France. Atmospheric inputs were
found to be 11% of total inputs of aluminium. Rainwater was analysed
for aluminium and only 19% was found in the dissolved fraction
(< 0.4 �m). Losno et al. (1993) monitored rainwater and snow for
aluminium and found large variations in the solubility of aluminium.
The variations seem to be largely due to pH, lower pH values
increasing the solubility of aluminium. Thermodynamic calculations
reveal that, at pH values higher than 5, equilibrium with gibbsite or
an insoluble trivalent alkaline form of aluminium acts to limit
solubility, whereas, at lower pH values, aluminium could be in
equilibrium with a hydroxysulfate salt.
4.1.2 Freshwater
4.1.2.1 Dissolved aluminium
In groundwater or surface water systems an equilibrium is formed
that controls the extent to which aluminium dissolution can occur. The
solubility of aluminium in equilibrium with solid phase Al(OH)3 is
highly pH-dependent. Aquo complex Al(H2O)63+ predominates at low
pH values (e.g., pH < 4), but as the pH of the solution increases
(e.g., pH 4-6) and/or the temperature rises, the positive charge
of aluminium forces hydrolysis of a water ligand producing the
Al(OH)(H2O)52+ ion. The degree of hydrolysis increases as the
solution pH increases, resulting in a series of Al-OH complexes such
as Al(OH)2+, Al(OH)2+, Al(OH) 4 - (Schecher & Driscoll, 1987).
Fluoride ions, being similar in size to hydroxyl ions, will readily
substitute in these complexes. At pH < 5.5, molar concentrations of
aluminium in certain areas exceed concentrations of fluoride ions and
form low ligand number complexes. The concentration of Al-F complexes
under those conditions is limited by the total concentration of
fluoride ions. At pH > 7.0, Al-OH complexes predominate in waters
that are low in dissolved organic matter and silicate. Under acidic
conditions sulfate also forms complexes with aluminium. Even though
sulfate concentrations are typically higher than those of fluoride in
surface waters, Al-SO42- complexes are significant only at high
sulfate concentrations and low pH values (Courtijn et al., 1987).
The chemical speciation of aluminium in natural water regulates
its mobility, bioavailability and toxicity. The concentration of
aluminium in some natural water as a function of pH can be estimated
by thermodynamic calculations. The actual geochemical mobility of
aluminium is very complex and difficult to predict in areas affected
by acid deposition, as non-equilibrium processes usually predominate
during episodic events associated with aluminium release and transport
(Bull & Hall, 1986; Lawrence et al., 1988; Seip et al., 1989).
The chemistry of inorganic aluminium in acid soil and stream
water can be considered in terms of mineral solubility, ion exchange
and water mixing processes (Neal et al., 1990). The minerals that
determine stream water Al3+ activity in acidic and acid-sensitive
systems are kaoline (Al2Si2O5(OH)4), various forms of aluminium
hydroxide (Al(OH)3), aluminium hydroxy sulfate (Al(OH)SO 4) and
aluminium hydroxy silicate (Seip et al., 1984; Neal & Williams, 1988;
Nealet al., 1990).
Goenaga & Williams (1988) found that the concentrations of Al-F
and Al-SO4 complexes in Welsh upland water were < 40 �g/litre and
accounted for less than 20% of the inorganic aluminium at pH < 5.0.
Organic complexes were significant (16 to 49 �g/litre) even in samples
with a low total organic carbon content. Organic monomeric aluminium
increased during high flows as total organic carbon (TOC) and free
ionic aluminium concentrations increased. LaZerte (1984) analysed
streams and lakes during spring snow melt in acidified catchments in
Ontario, Canada (pH 4.1-6.9, and TOC 1-24 mg/litre). Very little
polymeric and amorphous aluminium was found, and most of the inorganic
monomeric fraction was in the fluoride complexes.
Neal (1988) found that Plynlimon (Wales) stream water was
saturated or over-saturated with respect to some crystalline form of
Al(OH)3, and saturated or under-saturated with respect to amorphous
Al(OH)3: the level of saturation became less as pH decreased. The
solubility relationship for the kaolin mineral group is that at low pH
the waters are saturated with respect to crystalline kaolin
(kaolinite) and under-saturated with respect to poorly crystalline
kaolin (low crystallinity hallyosite). At higher pH the waters become
progressively more over-saturated with respect to crystalline forms
and near to saturation with respect to poorly crystalline forms. It
appears that at high pH the waters are in approximate equilibrium with
amorphous Al(OH)3 whilst at low pH values the waters are in
equilibrium with an aluminium hydroxy sulfate phase (equilibrium
constant 10-4.9).
Aluminium begins to polymerize when the pH of an acidic solution
increases notably over pH 4.5. Polymerization implies that in the
first step, two hydroxyls are shared by two aluminium atoms, e.g.,
2 Al(OH)(H2O)52+ -> Al2(OH)2(H2O)84+ + 2 H2O
Polymerization gradually proceeds to larger structures, eventually
leading to the formation of the Al13 "polycation" (Hem & Robertson,
1967; Parker & Bertsch, 1992a,b). As polymers coalesce, they increase
in relative molecular mass, eventually becoming large enough to
precipitate aluminium hydroxide from solution. As the precipitate ages
the solubility decreases (Chow, 1992). Tipping et al. (1988b) found
that if precipitation occurs at pH 4 to 6 it involves the formation of
aluminium (oxy)hydroxide and not aluminosilicates or basic aluminium
sulfates. The solubility of the (oxy)hydroxide was highly temperature
dependent and decreased in the presence of sulfate and more
particularly humate. L�kewille & van Breemen (1992) analysed
precipitates from stream bottoms in the Senne area of northern Germany
and found them to consist of amorphous aluminium hydroxide,
co-precipitated with minor amounts of sulfate, phosphate and silica.
Aluminium is predominantly cationic under acidic conditions and
strongly binds with negatively charged organic functional groups such
as fulvic acid and humic acid (Chow, 1992). Aluminium-humate complex
formation has been modelled by several researchers. For example,
Tipping et al. (1988a,c) developed a model using data collected from
acidified streams in northern England and Scotland. This model
describes the equilibria between humic acid substances, aluminium
species, calcium ions and hydrogen ions under acidic conditions (pH <
6) (Tipping et al., 1989a). Tipping & Backes (1988) reported on two
models of aluminium-humic acid complexation. Reasonable results were
obtained only at pH 4.0 to 5.0. Plankey & Patterson (1987) used a
fluorescence technique to study the complex formation kinetics of
aluminium with a single metal-free fulvic acid isolated from an
Adirondack mountain soil (USA). At pH 3.0 to 4.5 two types of
aluminium binding sites were identified.
4.1.2.2 Aluminium adsorbed on particles
Goenaga et al. (1987) collected stream water from tributaries of
Llyn Brianne reservoir, Wales. Analysis of the freshwater samples
showed total aluminium and monomeric aluminium concentrations to be
positively correlated with suspended solid content. The levels of
total (acid digestible) aluminium that were detected in filtrates of
freshwater samples were affected by the pore diameter of the membrane
filter used. Between 13% and 50% of this form of aluminium initially
present in the sample could be removed by a 0.4 �m pore diameter
membrane, depending on the level of suspended solid originally
present. Goenaga & Williams (1988) calculated the amount of aluminium
adsorbed onto suspended solids by measuring monomeric aluminium before
and after separation of suspended solids by filtration (0.015 �m pore
diameter). Adsorbed aluminium was low (< 20 �g/litre) during dry
weather periods; however, the adsorbed fraction was very significant
(> 40 �g/litre) for samples with high suspended solids (>
20 mg/litre) collected from a Welsh upland area during a storm
episode. Goenaga & Williams (1990) found that aluminium associated
with particles > 0.015 �m was negligible (< 20 �g/litre) in spot
samples with a suspended solid content of < 5 mg/litre but
significant (< 200 �g/litre) for episode samples with high suspended
solids (> 20 mg/litre); between 40% and 60% of the adsorbed aluminium
was found to be associated with particles > 0.4 �m. Tipping et al.
(1989b) studied the adsorption of aluminium in water from various
acidified streams in northern England. The adsorption of aluminium by
particles was found to increase with total aluminium, pH and particle
concentration. Calculations using an adsorption equation, and taking
competition by dissolved humic substances into account, suggest that
adsorbed aluminium may commonly account for a significant proportion
(> 10%) of total monomeric aluminium in such water.
4.1.2.3 Aluminium in acidified waters
Fisher et al. (1968) monitored aluminium concentrations in
acidified streams of the Hubbard Brook Experimental Forest, New
Hampshire, USA from 1964 to 1966. Annual loads of aluminium were
calculated to be between 0.9 and 2.4 kg/ha for three streams. The
acidic deposition at the Hubbard Brook ecosystem has induced a series
of geochemical responses. Firstly, hydrogen ion acidity is neutralized
by the dissolution of alumina primarily found in the soil zone.
Secondly, hydrogen ion acidity and aluminium acidity are neutralized
by the chemical weathering of silicate materials (Johnson et al.,
1981). Hall et al. (1980) acidified a stream in the Hubbard Brook
Experimental Forest. The pH of the control stream ranged from 5.7 to
6.4 and in the acidified stream from 3.9 to 4.5. Dissolved aluminium
increased significantly in the acidified stream water by an average of
181%compared to the control stream. Lawrence et al. (1988) reported
that at high elevations the increased stream flow was associated with
reduced surface water acidity and decreased inorganic aluminium
concentrations. At low elevations, increased stream flow was
associated with increases in stream acidity and concentrations of
inorganic aluminium. The contributions of flow from the more acidic
upper region of the watershed during high-flow conditions appear to be
the major hydrological influence on stream chemistry. In the acid-
affected Experimental Forest system, acidity of low-order stream water
was high due to elevated inputs of strong acids (sulfuric and nitric)
relative to the releases of basic cations from soil. Concentrations of
aluminium were also high and predominantly in labile (inorganic)
monomeric form. For comparison, samples were collected from the
Jamieson Creek watershed, British Columbia, Canada. For this watershed
the low-order stream water was acidic due to low concentrations of
basic cations coupled with the presence of organic acids.
Concentrations of aluminium were relatively low and largely associated
with organic solutes (Driscoll et al., 1988).
Large temporal and spatial variations in aluminium concentration
occur in Welsh stream water due to hydrological and land use controls.
The most acidic streams have the highest aluminium concentration.
Variations in stream and soil water aluminium concentrations in the
order semi-natural moorland < conifer forested moorland < recently
harvested forest can be explained by ion exchange reactions related to
changes in the anion concentrations passing through the system and
weathering (Neal et al., 1990). Lawrence et al. (1986) found that
observed altitude trends in stream aluminium chemistry may be related
to spatial variations in vegetation type and mineral soil depth.
Mobilization of aluminium was studied in streams at the Hubbard Brook
Experimental Forest, New Hampshire, USA. At the highest altitudes
maximum densities of spruce and fir vegetation occur, and aluminium
appears to be mobilized by transformations involving dissolved organic
matter. At mid-altitudes hardwood vegetation predominates and the
mechanism of aluminium mobilization shifts to dissolution by strong
acids within the mineral soil. At the lowest altitudes relatively
thick mineral soil seems to limit aluminium mobility resulting in low
concentrations in stream water. During 1983 and 1984 an experimental
watershed at Hubbard Brook was commercially whole-tree harvested.
Whole-tree harvesting resulted in a large increase in stream nitrate
concentrations, followed by a decrease in pH and concomitant increase
in inorganic aluminium (Lawrence et al., 1987). Ormerod et al. (1989)
studied the spatial patterns in aluminium and pH data from 113 Welsh
catchments of contrasting land use. It was found that pH declined and
aluminium increased significantly with increasing forest cover. The
percentage contribution of labile aluminium to the total filterable
concentration ranged from 39% to 90%, the highest levels being
associated with streams draining forest. Neal et al. (1992) found that
felling conifers led to decreases in pH and increases in aluminium
concentrations in streams and soils at Plynlimon, Wales, for the first
2 years. The major changes were found to occur during the winter storm
flow periods. The trends were reversed after the first two years. The
short-term effects (2-3 years) of forest harvesting on soil and stream
water inorganic aluminium chemistry were predominantly controlled by
the nitrogen dynamics of the site. A reduction in inorganic aluminium
was observed concomitant with declines in nitrate and total inorganic
anions. However, 4-5 years after harvesting, inorganic aluminium
concentration in soil and stream water of Welsh and Cumbrian study
sites was still greater than that expected in moorland catchments
(Reynolds et al., 1992).
Bird et al. (1990) compared the effects of a winter "rain on
snow" episode with a summer storm episode on the pH and dissolved
aluminium levels in a moorland stream and a conifer forest stream. The
pre-episode conditions were broadly similar in both streams. In
winter, following the snow melt, both moorland and forest stream
showed reduced pH accompanied by increases in dissolved aluminium
concentrations as buffering capacity provided by the calcium was
exceeded by anions such as sulfate. The timing of flow changes was
similar, but flush of solutes to the moorland stream was more rapid.
Both streams had a similar buffering capacity, but the changes in the
forest stream were much greater (pH reduction of 2 units and dissolved
aluminium levels exceeding 1 mg/litre), reflecting a greater flux of
anions. The surface run-off and reduced buffering capacity from frozen
soils led to the changes in both streams. During the summer episode,
again the forest stream showed a greater reduction in pH and higher
increase in dissolved aluminium; however, the concentrations of
sulfate were much lower in both streams and less aluminium was
mobilized.
Mach & Brezonik (1989) studied the biogeochemical cycling of
aluminium in acidified and reference basins of Little Rock Lake,
Wisconsin, USA. Background dissolved lake water aluminium
concentrations were 7 �g/litre (0.4 �m pore-size). The acidified basin
was acidified in a step-wise manner. Acidification to pH 5.1 resulted
in a 45% elevation of dissolved aluminium levels (15.5 �g/litre) over
reference basin levels (10.7 �g/litre). Analysis of suspended
particulate matter collected from both basins revealed lower levels of
particulate aluminium in the acidified basin, demonstrating that there
is reduced affinity for particulate matter at the lower pH. Brezonik
et al. (1990) studied the effects of acidification of Little Rock Lake
on the dissolved concentrations of aluminium. Aluminium desorbed from
sediments of the lake basin at pH 4 and below in laboratory studies.
In littoral enclosures, dissolved aluminium was elevated above control
levels at pH 4.5, and elevated levels were observed in pelagic
enclosures at both pH 5.0 and 4.5. Dissolved aluminium levels remained
constant in the acidified basin at pH 5.6 and 5.1 (16 �g/litre, while
concentrations in the reference basin declined to 11 �g/litre during
the study period. The authors stated that these levels of aluminium
were low compared with other reported values because the lake was
hydrologically isolated.
Cosby et al. (1985) presented a mathematical model (MAGIC; Model
Acidification of Groundwater In Catchments) that uses quantitative
descriptions of soil chemical processes to estimate the long-term
chemical changes that occur in soil, soil water and surface waters of
catchments in response to changes in atmospheric deposition. The model
is based on soil base cation exchange, dissolution of aluminium
hydroxide and solution of carbon dioxide. The model uses "average" or
lumped representations of these spatially distributed catchment
processes. The long-term responses of the model are controlled by
sulfate adsorption and primary weathering of base cations in the
catchment soils. The model was applied to the Shenandoah National
Park, Virginia, USA, and indicated that the alkalinity of surface
waters had been reduced by as much as 50% over the last 140 years.
Cosby et al. (1986) applied the MAGIC model to a sub-catchment in
southwestern Scotland. Assuming that deposition rates are maintained
in the future at 1984 levels, the model indicated that stream pH was
likely to decline. Neal et al. (1986) reported that the model predicts
increases in pH (reductions in acidity) with conifer deforestation.
In the Welsh uplands the model simulates quite accurately the
acidification of catchments (Whitehead et al., 1990). The model shows
that atmospheric deposition is the primary cause of stream
acidification with conifer afforestation enhancing stream acidity.
Historical trends determined by the model indicate that acidification
has been present since the turn of the century (Whitehead et al.,
1988). Ormerod et al. (1990) compared the predicted effects of reduced
acidic deposition and liming on stream acidification with actual
treatments. The results indicate that liming and 90% reduction in
sulfate deposition reduce concentrations of soluble aluminium to
similar levels. However, calcium concentrations and pH were increased
by liming to values that were high by comparison with conditions
simulated under low acid deposition.
A dynamic model has been developed that reproduces major trends
in chemical and hydrological behaviour in Norwegian catchments.
Christophersen & Seip (1982) reported that a simple two-reservoir
model incorporating a small number of physically realistic processes
accounts for the major short-term variations in stream water chemistry
during the snow-free season at a 0.41 km2 catchment in coniferous
forest on granite bedrock at Birkenes, Norway. The model incorporates
both hydrolytic and sulfate sub-models, and a cation sub-model that
includes hydrogen, aluminium, calcium and magnesium ions. Typical
characteristics predicted by the model include positive correlations
between hydrogen ions and aluminium concentrations and discharge, and
negative correlations between these factors and the calcium and
magnesium concentrations. Seip et al. (1989) attempted to model
episodic changes in stream water chemistry of hydrogen and aluminium
ions. However, only partial success was achieved. Trends were correct
for hydrogen ions but there were discrepancies at peak heights. There
were correct predictions for aluminium concentrations in the autumn
but not in the spring.
4.1.3 Seawater
In contrast to fresh water, seawater (salinity > 32%) has a
constant pH of approximately 8.2. Hydes (1977) found that bottom and
suspended clay sediments probably act as a source of dissolved
aluminium to seawater. However, removal below predictions of clay
solubility is probably the result of biological activity.
Hydes & Liss (1977) reported that approximately 30% of the
dissolved aluminium entering the Conwy Estuary, Wales, appears to be
removed during mixing with seawater. The removal occurs during the
early stages of mixing and is virtually complete by the time the
salinity reaches 8%. The authors concluded that the most likely
mechanism involves the trapping of aluminium adsorbed onto the surface
of fine clay particles entering with the freshwater as the particles
are irreversibly coagulated on mixing with saline water.
Mackenzie et al. (1978) measured the concentration of aluminium
in a vertical hydrographic profile of the Mediterranean Sea. They
found that the concentrations did not correspond to seasonal
thermocline, nitrate minimum and an oxygen maximum, thus supporting
the hypothesis that aluminium cycles in the oceans are associated with
the activity of diatoms. Stoffyn (1979) stated that experimental
evidence using the diatom Skeletonema costatum supports the
hypothesis that the concentration and distribution of dissolved
aluminium in ocean water is controlled by biological activity in the
surface waters. However, Hydes (1979) reported that the distribution
of dissolved aluminium in open ocean waters is probably controlled by
the solution of aluminium from atmospherically derived particles and
bottom sediments balanced against scavenging by siliceous shells of
dead organisms. Chou & Wollast (1989) studied eight vertical profiles
of dissolved aluminium in the Mediterranean Sea. They found that
dissolved aluminium is depleted in surface waters as compared with
deep waters. The high concentrations of aluminium in deep water may
result from fluxes from pore water in sediments to the overlying
water. These authors suggested that aluminium is possibly removed by
biological processes in the euphotic zone.
4.1.4 Soil
In soil, aluminium is released into solution for transport to
streams upon acidification. The chemistry of inorganic aluminium in
acid soil and, in particular, the solubility controls are very similar
to those given in section 4.1.2 for fresh water (Furrer, 1993).
However, the extrapolation of stream water chemistry to soil is
difficult because of the complexity of hydrological pathways and
chemical reactivity during water mixing (Neal, 1988).
Water mixing processes may be important in determining the
relationship between aluminium (inorganic) and hydrogen ions.
Consequently there is a need to model how these species vary as a
function of mixing so that comparisons can be made with field
observation. In order to resolve the effects of mixing, a basic
calculation is made to allow soil to mix and degas carbon dioxide in
the stream. To do this it is assumed that pCO2 in soil/groundwater
and the stream is 25 and 2 times the atmospheric value, respectively,
and that bicarbonate principally controls the acid buffering (Neal et
al., 1990).
In soils, solid-phase aluminium occurs in the lattice structure
of minerals, in inter-layer sites of expanding clay minerals, and in
poorly ordered minerals (allophane and hydrous oxides) of variable
composition. Natural acidification processes result in increasing
solubility of aluminium. At moderately acidic levels (pH 5.5)
aluminium appears as the exchangeable cation that dominates in the
lower mineral horizons initially as poly-nuclear hydroxy ions but
subsequently as the simple mono-nuclear ions. Aluminium ions displace
calcium at permanent-charge exchange sites (Bache, 1980). The cation
exchange system of acid soils provides a large reserve of ionic
aluminium, which can be brought into solution when soluble salts
percolate through soil. Ligands, such as fluoride and organic anions,
which form aluminium complexes, combine with aluminium and maintain
higher concentrations of aluminium than might be expected, especially
at pH 5-7 (Bache, 1986). In soil the most soluble form of aluminium
under acidic conditions is non-silicate organically bound aluminium,
while the amorphous aluminium hydroxy forms are more soluble than the
crystalline forms (ATSDR, 1992; Sj�str�m, 1994).
Bloom et al. (1979) found that hydrolysis of organically bound
aluminium is a major source of buffering in the pH range 4 to 5 for
dilute salt suspensions of acid soils. The exchange of aluminium ions
from organic matter exchange sites controls the relationship between
pH and Al3+ activity in acid soils that have a low amount of
permanent-charge cation exchange capacity relative to the quantity of
organic matter. Walker et al. (1990) studied the influence of organic
matter on the solubility of aluminium in organic soil horizons from
different geographical regions of North America. The equilibrium
solubility of aluminium was dependent on pH and the degree to which
soil organic matter was saturated with aluminium. Soluble aluminium
increased with decreasing pH and increased with increasing surface-
bound aluminium at each pH level. Temperature dependence and rate
studies suggested that aluminium solubility was governed by an ion-
exchange reaction between H+ and aluminium and the organic matter.
Litaor (1987) studied the aluminium chemistry in an alpine
watershed, Front Range, Colorado, USA. It was found that the aluminium
solubility in the interstitial water is complex and controlled by
organic solutes, H4SiO4 and pH. However, neither pH nor sulfate
concentrations correlated with aluminium concentrations. The chemical
equilibrium of aluminium was controlled by amorphous aluminosilicate.
The most important inorganic aluminium complexes are the
mononuclear and to a lesser extent the polynuclear hydroxo species.
The formation of these complexes is directly coupled to pH and also,
to a lesser extent, to ionic strength. The hexa aqua-aluminium cation
(Al(H2O)63+) predominates at low pH, whereas the mononuclear
(Al(OH)2+) and dihydroxo-mononuclear species (Al(OH)2+) become
important in the circumneutral pH range. The tetrahydroxo aluminium
anion (aluminate, Al(OH)4-) is the predominant species at higher pH,
and is responsible for the increasing solubility of aluminium above pH
6.2. Polynuclear aluminium complexes can be the predominant species in
solution over a wide pH range, although, being formed under non-
equilibrium conditions, they are difficult to predict (Grant et al.,
1990). Dahlgren & Ugolini (1989) collected leachates from subalpine
spodosol located in the Cascade Range, Washington, USA. The ability of
organic acids to complex aluminium in these subalpine soils increases
from pH 3.8 to 5.0. Walker et al. (1988) found that adsorption of
aluminium by aluminosilicate clay minerals, such as montmorillonite,
kaolinite and vermiculite, is controlled by a simple electrostatic
cation exchange involving outer sphere complexes. Adsorption to
vermiculite may also be controlled by internal ion diffusion.
Equilibrium constants (Ka) for the formation of an adsorbed
aluminium clay complex were high (approx. 105) for the three minerals,
suggesting that they play a significant role in controlling aluminium
concentrations in soil solutions.
Blume & Br�mmer (1991) studied the influence of soil acidity on
aluminium binding in sandy soils with a low humus content (< 2%).
Binding was found to decrease steadily from very strong at pH 5.5-7.0
to very weak at pH 2.5. The binding of aluminium was found to be
increased by increasing the organic matter content. In loam or clay
soils binding was increased compared with sandy soils at all levels of
pH.
Driscoll et al. (1989) studied the chemistry and transfer of
aluminium in a forested watershed of the Adirondack region, New York,
USA. The drainage waters from the watershed were highly acidic due to
elevated inputs of both sulfuric and nitric acids compared with the
release of basic cations. The conditions facilitated the mobilization
of aluminium. Alumino-organic solutes were mainly released from the
soil organic horizon with inorganic monomeric aluminium derived
predominantly from the mineral soil and to a lesser extent from the
soil organic horizon. Inorganic monomeric aluminium predominated in
the drainage water. The deposition of organic monomeric aluminium in
the stream bed coincided with the dissolved organic content retention
whilst the deposition of inorganic monomeric aluminium appeared to be
facilitated by nitrate retention.
Nilsson & Bergkvist (1983) studied soil acidification and
aluminium chemistry in three adjacent catchments on the Swedish west
coast. The concentration of organic aluminium was linearly correlated
with concentrations of carbon. The percentage of organic species in
the dissolved aluminium decreased with increasing depth from > 90% in
the upper layers to < 10% below 55 cm. The average concentration
of total aluminium increased with increasing depth from 3.3 to
9.8 �mole/litre at 5 cm to 95.3 to 115 �mole/litre below 55 cm.
Atmospheric acid inputs have a strong impact on the aluminium
chemistry of acidic sandy soils with low concentrations of basic
cations. The base saturation of such soils is low and the rate of
basic cation weathering does not increase with rate of acid input. Any
additional acidic deposition is neutralized by aluminium. Dissolution
of aluminium is the major acid sink in such soils, neutralizing 30% to
95% of the total acid load (Mulder et al., 1989).
Bergkvist (1987) studied the leaching of aluminium in a brown
forest soil and a podzol with adjacent stands of spruce, beech and an
open regeneration area in South Sweden using lysimeter techniques. The
leaching of aluminium was greatest from the podzol. The solubility of
aluminium increased suddenly within a small pH range (4.5-4.0) in the
B horizon (15 to 55 cm). The concentration of aluminium in soil
solution increased from 2 to 10 mg/litre when the pH decreased from
4.4 to 4.2. Berggren et al. (1990) reported that forest soils of south
Sweden are losing base cations, such as aluminium, owing to increased
leaching rates following soil acidification. The processes controlling
the mobilization of aluminium in podzols and cambisols of southern
Sweden were investigated by Berggren (1992). Podzols in spruce and
beech stands had a high release of organic compounds from the upper 5
to 7 cm (O/Ah horizons), which resulted in high organic complexation
of aluminium in soil solution at a depth of 15 cm (E horizon). Organic
complexes were mainly adsorbed or precipitated at 20 to 40 cm (upper
Bh horizon) and the overall transport of aluminium at 50 cm was
governed by a pH-dependent dissolution of solid-phase aluminium. In
the cambisols inorganic aluminium predominated at both 15 cm and 50 cm
with solubility being closely related to solution pH. The results
indicate that the relatively large organically bound solid-phase
aluminium pools in both soil types give rise to the measured solution
aluminium activities. The authors also found that aluminium in
solution efficiently competed for exchange sites and played an
important role in the mobilization of cadmium in these soils.
In laboratory studies simulating snow melt leaching of forest
soils, nitric acid leached more aluminium than did sulfuric acid
from soil columns representative of high elevation forest soils
and watersheds thought to be sensitive to acidification by acid
precipitation. Increasing the nitric acid concentration 100-fold
(pH 5 to 3) increased the total aluminium concentration in the
leachate from 0.70 to 0.85 mmol/litre, while increasing the sulfuric
acid had no effect. Similar experiments with albic and ochric mineral
soil horizons revealed no difference between the acids and no effect
of increasing acid concentrations (James & Riha, 1989).
The acidity produced by soil nitrification is buffered by
exchange with base cations. In acidified soils aluminium will be
dissolved or the pH of the soil will decrease. Aluminium release from
soil is also dependent on the sulfate supply and the capacity of the
soil to provide aluminium hydroxides. The molar ratio between
aluminium and nitrate is important in evaluating the effects of
nitrification. During dry summers the release of aluminium ions may be
entirely controlled by the acidification caused by nitrification
(Gundersen & Rasmussen, 1990).
4.1.5 Vegetation and wildlife
Mobilization of aluminium by acid rain results in more aluminium
being available to plants (ATSDR, 1992).
Henriksen et al. (1988a) found during acidification experiments
in Norwegian streams that the buffering capacity was 20 times higher
than that associated with the water alone and a reduction of the pH to
5 resulted in large releases of aluminium (up to 2500 �g/litre).
The source of the buffering and the reservoir of aluminium was
hypothesized to be dense growths of liverwort. A second experiment
confirmed that liverworts are involved in the ion exchange of base
cations and aluminium during acid episodes.
Vogt et al. (1987) studied the aluminium concentrations of above-
and below-ground tissues of a white fir ( Abies amabilis) stand in
the Cascade mountains, Washington, USA. It was found that 97% of the
total detrital cycling of aluminium was below ground. When compared
with the other elements analysed, aluminium showed the highest
proportion of total annual element pool circulated (82%). The large
root biomasses of these stands allows large amounts of aluminium to be
accumulated and immobilized. The high root turnover observed for these
stands appears to be due to the root senescence occurring in response
to high aluminium accumulation. However, there is little impact on
short-term elemental cycling because the roots decay very slowly
(99% decay = 456 years).
4.2 Biotransformation
4.2.1 Biodegradation and abiotic degradation
Elemental aluminium does not degrade in the environment. In the
trivalent oxidation state, it can complex with electron-rich species
(ATSDR, 1992).
4.2.1 Bioaccumulation
4.2.2.1 Plants
Plants differ in their ability to take up aluminium; some
accumulate aluminium whereas others are able to immobilize it at the
root surface (Roy et al., 1988). Exposure to aluminium in nutrient
solution leads to accumulation, especially in the roots (Lee, 1972;
Boxman et al., 1991).
Aluminium taken up by roots is mainly found in the mucilage layer
on the root tip surface (Horst et al., 1982) and in the walls of the
epidermis and cortex cells (Huett & Menary, 1980). In the cell wall
pectins, aluminium ions compete with calcium ions for the same
absorption sites (Wagatsuma, 1983). Some aluminium is taken up in the
cytoplasm and bound to nucleic acids and acid-soluble phosphates
(Wagatsuma, 1983). Aluminium is translocated only to a small extent to
shoots.
The concentrations of aluminium in leaf tissue of a variety of
plants growing on limestone soils in Sweden (pH around 8) were found
to be similar to those in plants growing on an acid silicate (granite;
pH 4.1-4.9) site, although the aluminium concentration in the topsoil
solution was at least one order of magnitude lower in the limestone
than in the acid silicate soils (Tyler, 1994).
4.2.2.2 Invertebrates
Ryther et al. (1979) cultured soft shell clams ( Mya arenaria),
hard shell clams ( Mercenaria mercenaria), American oysters
( Crassostrea virginica) and sand worms ( Nereis virens) in tanks
containing fly ash from a coal-burning power station. The fly ash
contained a wide range of elements including aluminium at 105.8 g/kg.
After a period of 4 months the sand worms and the edible parts of the
clams and oyster were analysed. Aluminium concentrations were
9645 mg/kg (dry weight) for the sand worms and 8218, 268 and
1373 mg/kg for soft shell clams, hard shell clams and oysters,
respectively.
Crayfish ( Orconectes virilis) from a lake with an average total
aluminium concentration of 36 �g/litre were placed in caged tubes
spiked with 40 �g/litre total aluminium and transferred to a lake with
background levels of 8 �g/litre total aluminium. Half of the tubes
were acidified to pH 5.3 and the others remained at pH 6.7. None of
the crayfish accumulated aluminium. Controls had lower aluminium
concentrations in the hepatopancreas and abdominal muscle after 25 to
27 days. Crayfish under acidified conditions retained aluminium in the
hepatopancreas and not in the muscle whereas those at pH 6.7 retained
aluminium in the muscle and not the hepatopancreas. The same authors
also carried out a laboratory experiment with crayfish obtained
from the original lake. Crayfish were maintained in a solution of
500 �g/litre for 14 days. No tissues showed an increase in aluminium
levels. However, crayfish transferred back to the original lake water
for 16 days retained aluminium only in the carapace and gills (Malley
et al., 1987).
Havas (1985) exposed water fleas ( Daphnia magna) to total
aluminium concentrations of 0.02, 0.32 and 1.02 mg/litre for 24 h.
Bioconcentration was related to pH, the highest concentration factors
occurring at pH 6.5 and the lowest at pH 4.5; there was no effect of
increasing the calcium concentration from 2.5 to 12.5 mg/litre. Mean
bioaccumulation factors ranged from 11 000 to 18 000 at pH 6.5, 3000
to 9000 at pH 5.0, and 1200 to 4300 at pH 4.5.
Frick & Herrmann (1990) studied the accumulation of aluminium
by nymphs of the mayfly ( Heptagenia sulphurea) exposed to
concentrations of 0.2 and 2 mg inorganic monomeric aluminium/litre at
pH 4.5 for up to 4 weeks. The highest mean concentrations found in
mayflies were 1.24 and 2.34 mg/g aluminium (dry weight) for the higher
treatment groups, which did not undergo moulting. The major part of
the aluminium was deposited on/in the exuviae of the nymphs, as
aluminium determinations revealed a 70% decrease in content after
moulting.
4.2.2.3 Fish
Cleveland et al. (1986) exposed brook trout ( Salvelinus
fontinalis) eggs, larvae and juveniles to 300 �g/litre total
aluminium at three pH levels. At 30 days post-hatch for larvae and
for an exposure period of 30 days for juveniles (37 to 67 days),
significantly more aluminium was accumulated at pH 5.28 than at either
pH 7.24 or 4.44. Aluminium levels at pH 5.28 were 398 and 112 mg/kg
for the larvae and juveniles, respectively. At pH 7.24 residues were
12 and 33 mg/kg, and at pH 4.44, 71 and 17 mg/kg, respectively.
Cleveland et al. (1991) maintained brook trout in water containing
200 �g/litre total aluminium at pH values of 5.0, 6.0 and 7.2 for 56
days. Estimated steady state bioconcentration factors for aluminium,
which were inversely related to pH, were 215 at pH 5.3, 123 at pH 6.1
and 36 at pH 7.2. The estimated time to 90% steady state was 1.5 days
at pH 5.3, 4.2 days at pH 6.1 and 1.7 days at pH 7.2. Elimination
during the 28-day depuration phase was more rapid at pH 5.3 than at pH
6.1 or 7.2. Karlsson-Norrgren et al. (1986b) found that brown trout
( Salmo trutta) accumulated significantly more aluminium in gill
tissue at pH 5.5 than at pH 7.0 (60-160 �g/kg and 10-40 �g/kg dry
weight, respectively) when exposed to 200-500 �g total
aluminium/litre. Skogheim et al. (1984) found a gill aluminium
accumulation of 70 to 341 �g/g fresh weight in dying Atlantic salmon
( Salmo salar) during an episodic fish kill in the river Ogna,
Norway, at pH 5.4-5.5 and total aluminium and labile aluminium
concentrations of 160 and 130 �g/litre, respectively.
Segner et al. (1988) exposed young brown trout ( Salmo trutta)
to total aluminium (230 �g/litre) at pH 5.0 in high calcium water at a
temperature of 12�C for 5 days. Whole body aluminium concentrations
were 230 mg/kg dry weight in aluminium-exposed fish, as compared to
75 mg/kg (pH 5.0) and 44 mg/kg (pH 7.2) for fish in aluminium-free
water.
Wicklund Glynn et al. (1992) exposed minnows ( Phoxinus
phoxinus) to acidic water (pH 5.0) with and without total aluminium
(150 �g/litre) at varying calcium (0, 0.07 and 2 mmol/litre) and humus
(5 and 25 Pt) concentrations for 15 days. Aluminium concentrations in
the gills were highest in the lower calcium level groups with or
without humus. In the absence of calcium the median aluminium level in
the gills was 109 mg/kg wet weight, and at 2 mmol/litre calcium the
aluminium level was 50 mg/kg.
4.2.2.4 Birds
Carri�re et al. (1986) fed ring doves ( Streptopelia risoria) on
a diet containing 0.1% aluminium sulfate with reduced calcium and
phosphorus (0.9% Ca; 0.5% P) for a period of 4 months. Analysis of
the tissues of breeding adult doves revealed that there was no
accumulation of aluminium in kidney, brain or male femurae; however,
the femurae of female doves showed a significant increase from a mean
of 7.42 mg/kg dry weight in controls to 15.87 mg/kg in treated birds.
Juvenile doves fed on diets containing 500, 1000 and 1500 mg/kg
aluminium sulfate did not accumulate aluminium in leg and wing bones
but did show a significant tendency to accumulate in the sternum.
Sparling (1991) fed black ducks ( Anas rubripes) and mallard
( Anas platyrhynchos) on a diet containing 200, 1000 or 5000 mg
aluminium/kg with varying amounts of calcium (3600 and 15 100 mg/kg)
and phosphorus (6200, 13 500 and 21 500 mg/kg) for a period of 10
weeks. All of the diets produced a dose-related and significant
increase in the aluminium content of the femur. Black ducks maintained
on normal calcium and phosphorus levels showed femur aluminium
concentrations of 5.42, 13.6 and 19.5 mg/kg after 10 weeks at the
three dose levels, respectively. Mallard femurs contained aluminium
levels of 9.49, 12.1 and 18 mg/kg, respectively.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Aluminium is ubiquitous in the environment and its chemistry is
controlled by pH, mineralogical composition, and the quantity and
qualitative nature of the organic constituents present. It is,
therefore, difficult to provide generalized estimates of natural
background concentrations (Grant et al., 1990). Aluminium is released
to the environment by both natural processes and anthropogenic
sources. It is a major constituent of the earth's crust, and natural
mobilization of aluminium far outweighs the direct contribution from
anthropogenic sources (Lantzy & Mackenzie, 1979). The concentrations
of aluminium in the different environmental compartments are dependent
on its speciation and mobilization (see section 4.1). Jones & Bennett
(1985) summarized the data on aluminium concentrations in the
environment and produced a list of representative values as follows:
urban air 1000 ng/m3 (160-7000 ng/m3), rural air 200 ng/m3
(150-325 ng/m3), agricultural soil 70 000 mg/kg (10 000-300 000
mg/kg), fresh water (dissolved) 50 �g/litre (1-2250 �g/litre), ocean
(dissolved) 2 �g/litre (1-5 �g/litre), and terrestrial plants
100 mg/kg (50-600 mg/kg).
5.1.1 Air
Aluminium is a major constituent of a number of atmospheric
components, being highly concentrated in soil-derived dusts and in
particulates from coal combustion (Grant et al., 1990). The sources of
soil-derived dust are both natural (Sorenson et al., 1974) and from
human activity such as mining and agriculture (Eisenreich, 1980).
Leharne et al. (1992) monitored street dust from the inner London
area, United Kingdom, and found aluminium levels ranging from 3.7 to
11.6 �g/kg. The largest sources of particle-borne aluminium are the
flux of dust from soil and rock materials in the earth's crust and
from volcanic eruptions (Lee & von Lehmden, 1973; Sorenson et al.,
1974; Lantzy & Mackenzie, 1979). Atmospheric aluminium concentrations
show widespread temporal and spatial variations. The concentrations of
aluminium in air are summarized in Table 8 and range from 0.5 ng/m3
over Antarctica to > 1000 ng/m3 in industrialized areas.
Table 8. Concentrations of aluminium in air
Area Year Particle Aluminium Reference
size concentration
(�m) a (ng/m3)
Antarctic 1970 NR 0.57 Zoller et al.
(0.32-0.81) (1974)
Arctic 1976-1978 NR 25 Rahn (1981)
(Barrow, Alaska)
Hawaii 1967 > 0.15 2-40 Hoffman et al.
(1969)
Atlantic Ocean NR 8-370 Duce et al.
(1975)
NR 95 (41-160) Windom (1981)
Atlantic Ocean NR 102-184 Windom (1981)
near coast of USA
North Sea 1988-1989 NR 294.5 Chester &
(21-887) Bradshaw (1991)
1988-1989 NR 197 Ottley &
(17-903) Harrison (1993)
1985-1986 NR 210 Kersten et al.
(64-600) (1988)
Baltic Sea 1985 NR 218 H�s�nen et al.
(47-800) (1990)
Kiel Bight, 1981-1983 NR 394 Schneider (1987)
Germany (68-720)
USA cities & 1975-1977 < 3.5 48-1983 Stevens et al.
industrial areas (1978)
1975-1977 > 3.5 331-8678 Stevens et al.
(1978)
Buffalo, 1968-1969 NR 1000-8000 Pillay & Thomas
New York, USA (1971)
Southern Arizona 1974 NR 5700 Moyers et al.
(urban) (1977)
Table 8. (Con't)
Area Year Particle Aluminium Reference
size concentration
(�m) a (ng/m3)
Southern Arizona 1974 NR 1200 Moyers et al.
(rural) (1977)
Charleston, 1976 < 3.5 74 Lewis & Macias
West Virginia (1980)
1976 > 3.5 1100 Lewis & Macias
(1980)
UK (non-urban 1972-1973 NR 27-640 ng/kg Cawse (1974)
sites)
Birkenes, 1978-1979 NR 80 Amundsen et al.
S. Norway (1992)
1985-1986 NR 73 Amundsen et al.
(1992)
a NR = not reported
Amundsen et al. (1992) analysed air samples from Birkenes,
southern Norway, for aluminium and found concentrations to be
highest in the spring period from March to May. The authors
concluded that this was due to soil dust from wind erosion and
agricultural activities because soils in Europe are likely to be dry
during this period. Windom (1981) measured aluminium concentrations of
28 000 ng/m3 during a dust storm.
Aluminium was found to be concentrated up to 2650 ng/m3 in the
Baltimore harbour tunnel, a two-fold increase on the air intake levels
(Ondov et al., 1982).
5.1.2 Precipitation
Aluminium has been measured in atmospheric precipitation in the
USA at concentrations of up to 1200 �g/litre (Feth et al., 1964;
Fisher et al., 1968; Norton, 1971).
Feth et al. (1964) analysed snow samples from the northern Sierra
Nevada, USA, in 1959. Aluminium was detected in 7 out of 8 samples at
a mean concentration of 30 �g/litre. Ecker et al. (1990) measured
aluminium in wet-deposited snow at several sites in Japan, both
urban and rural. Mean aluminium concentrations ranged from 9.6 to
25.8 �g/litre. Average aluminium concentrations of freshly deposited
snow at Shiramine (a mountain site) were 2.4 �g/litre in the insoluble
fraction (> 0.45 �m) and 15.0 �g/litre in the soluble fraction (<
0.45 �m).
Rainwater collected in southern central Florida, USA, between
1967 and 1969 contained aluminium concentrations ranging from not
detectable to 900 �g/litre (Dantzman & Breland, 1969). Guieu et al.
(1991) monitored rainfall at 45 sites in the south of France and found
mean aluminium concentrations of 487 �g/litre and 55 �g/litre in the
particulate (> 0.4 �m) and dissolved fractions (< 0.4 �m),
respectively. Cawse (1974) measured aluminium in rainfall and dry
deposition from seven non-urban United Kingdom sites in 1972 and 1973.
Aluminium concentrations ranged from 56 to 14 800 �g/litre for
rainfall and from 3.9 to 42 �g/cm2 per year for dry deposition.
Aluminium concentrations in rainfall, total and dry deposition were
measured for Bagauda, Nigeria, in 1976. Aluminium concentrations were
1700 �g/litre for rainfall and 220 and 145 �g/cm2 per year for total
and dry deposition, respectively (Beavington & Cawse, 1979).
5.1.3 Water
5.1.3.1 Freshwater
Surface freshwater aluminium concentrations can vary
significantly, being dependent on the various physicochemical and
mineralogical factors described in section 4.1. Aluminium can occur in
a number of different forms in freshwater. It can be suspended or
dissolved. It can be bound with organic or inorganic ligands, or it
can exist as a free aluminium ion. It can exist as a monomer in
natural water, but tends to polymerize with time (see section 2.3.4).
Aluminium speciation is determined by pH, dissolved organic carbon
(DOC), fluoride, sulfate, phosphate, silicate and suspended
particulate matter. Dissolved aluminium concentrations for water in
the circumneutral pH range are usually quite low, ranging from 1.0 to
50 �g/litre, and rise to 500 to 1000 �g/litre in more acidic waters.
At the extreme acidity of waters affected by acid mine drainage,
dissolved aluminium concentrations of up to 90 mg/litre can be
measured (Filipek et al., 1987). Aluminium can also be leached from
landfill containing coal combustion ash and aluminium smelting wastes
(Sorenson et al., 1974). The concentrations of aluminium in freshwater
are summarized in Table 9.
Table 9. Concentrations of aluminium in freshwater
Area Year pH Particle Aluminium Detection Reference
size concentration limit
(�m) (�g/litre) (�g/litre)
Lake Gardsjon catchment, 1981 4.0-6.4 < 0.45 300-2500 Lee (1985)
Sweden
Swedish lakes 1980 5.7-8.8 69 (10-243) 10 Borg (1987)
Loch Ard Forest and 1979-1980 4.63 < 1.0 400 Caines et al. (1985)
Galloway streams, Scotland 6.62 < 1.0 25 Caines et al. (1985)
Llyn Brianne catchment, Wales 1984-1985 4.6-5.3 < 0.45 120-430 Goenaga & Williams (1990)
1984-1985 4.87 42 �Eq/litre Whitehead et al. (1988)
1984-1985 5.2 18 �Eq/litre Whitehead et al. (1988)
1984-1985 6.9 7 �Eq/litre Whitehead et al. (1988)
Rivers Esk and Duddon,
Cumbria, UK
(moderate flow) 1983-1984 4.3-7.2 20-940 Bull & Hall (1986)
(low flow) 1983-1984 4.8-7.5 5-245 Bull & Hall (1986)
Vosges mountain streams, 1990 6.96 64 Mersch et al. (1993)
France 1990 4.64-5.74 185-351 Mersch et al. (1993)
Boglakes, NE Belgium 1984-1986 3.4-3.9 < 0.4 150-3770 20 Courtijn et al. (1987)
1984-1985 3.4-3.9 > 0.4 3200-65 000 Courtijn et al. (1987)
1984-1986 6.0 < 0.4 < 20 20 Courtijn et al. (1987)
1984-1985 6.0 > 0.4 78 100-145 100 Courtijn et al. (1987)
Table 9. (Con't)
Area Year pH Particle Aluminium Detection Reference
size concentration limit
(�m) (�g/litre) (�g/litre)
Stream water, California, USA < 0.45 15 7 Silvey (1967)
St Lawrence river, USA 1974-1976 7.6-8.0 < 0.4 64 Yeats & Bewers (1982)
1974-1976 7.6-8.0 > 0.4 964 Yeats & Bewers (1982)
South central Florida, streams 1969 200-300 Dantzman & Breland
(1969)
Highway drains, Louisiana 1990 412 (250-1270) Madigosky et al. (1992)
Northern California streams 1972 5-10 Jones et al. (1974)
Shield lakes, Ontario and 1982 4.4-7.1 46-372 Stokes et al. (1985)
Quebec
Zaire river 1976 6.8 < 0.45 28-44 van Bennekom & Jager
(1978)
Niger river 1976 6.7 < 0.45 3-6 van Bennekom & Jager
(1978)
Reservoirs, Madras, India 1991 14 (5-210) 2 Pitchai et al. (1992)
Orange river, Vioolsdrif, 1958-1959 6.4-8.1 36-1080 de Villiers (1962)
South Africa
River Yodo, Japan 1981-1990 10-1150 Yagi et al. (1992)
Aluminium occurs ubiquitously in natural waters. Aluminium levels
in surface waters can be increased by intense urban and industrial
activity (Eisenreich, 1980). Kopp & Kroner (1970) monitored rivers and
lakes in the USA from 1962 to 1967 and detected aluminium in 31%
of samples. Mean dissolved aluminium levels ranged from 11 to
333 �g/litre, the highest levels of 2760 �g/litre being measured in
the Missouri river. Aluminium was found to be predominantly in the
suspended sediment fraction (> 0.45) with a mean concentration of
3860 �g/litre (compared with the dissolved phase at 74 �g/litre).
Filipek et al. (1987) reported that weathering of sulfide ores
exposed to the atmosphere in inactive mines and tailings dumps
released large quantities of sulfuric acid and metals such as
aluminium (up to 90 mg/litre). Boult et al. (1994) measured aluminium
in the Afon Goch, Anglesey, Wales, a stream polluted by mine drainage.
Sampling sites with pH 2.40, 5.99 and 6.49 gave mean soluble aluminium
concentrations (< 0.45 �m) of 55.56, 1.14 and 0.12 mg/litre,
respectively; mean aluminium concentrations in the particulate phase
(> 0.45 �m) were 0.31, 2.15 and 0.69 mg/litre, respectively. Koga
(1967) sampled water discharged from Wairakei drill holes in 1965.
Total aluminium concentrations in filtered water ranged from 0.023 to
0.05 mg/litre. Zelenov (1965) found elevated levels (4913 mg/litre) of
aluminium in water of a volcanic crater lake in Indonesia.
The concentrations of dissolved aluminium in water vary with pH
levels and the humic-derived acid content of the water (ATSDR, 1992).
Watt et al. (1983) compared Nova Scotian river water samples from
1954-1955 with those from 1980-1981 and found that significant
decreases in the pH corresponded to significant increases in dissolved
aluminium. High aluminium concentrations occur in surface waters when
the pH is less than 5 (Sorenson et al., 1974; Filipek et al., 1987).
In general, aluminium levels in surface waters at pH levels above 5.5
will be less than 0.1 mg/litre (Sorenson et al., 1974). However, even
at neutral pHs, higher aluminium levels have been detected where the
humic acid content is high (ATSDR, 1992).
In the Thousand Lake Survey in Norway (Henriksen et al., 1988b),
90% of the lakes with pH below 5.4 had a concentration of inorganic
monomeric aluminium above 60 �g/litre. Lakes with a pH of 4.6-4.8
and 4.8-5.0 had concentrations of 146-170 and 101-135 �g/litre,
respectively.
Generally, the data indicate that total aluminium concentrations
in surface waters are elevated during periods of high flow, following
episodic storm events, and/or during spring snow melt. Many studies
also reported corresponding increases in the labile or inorganic
aluminium fraction during these periods (LaZerte, 1984; Bull & Hall,
1986; Henriksen et al., 1988c; Lawrence et al., 1988).
Jones et al. (1974) monitored streams in Northern California and
found that samples collected during low flow periods contained
aluminium concentrations of between 1 and 3 �g/litre, whereas those
collected during moderate flow periods contained 10 �g/litre. Values
of aluminium that were higher than expected were associated with storm
run-off.
Caines et al. (1985) monitored streams in the Loch Ard Forest
and Galloway areas of Scotland during 1978, 1979 and 1980. The
concentrations of aluminium were found to be very closely dependent on
the hydrogen ion concentrations in the stream water. The maximum
concentration of aluminium in a stream with an average pH of 6.62 was
25 �g/litre, compared with almost 400 �g/litre in a stream with an
average pH of 4.63. The seasonal variation that occurred in the most
acid stream had a maximum of 394 �g/litre in February, which declined
rapidly to 35 �g/litre in May, during 1980. The authors stated that
the sharp decrease coincided with a period of low rainfall which
resulted in a rise in stream pH and greatly reduced leaching of
aluminium from the catchment.
Bull & Hall (1986) measured aluminium in the rivers Esk and
Duddon, Cumbria, United Kingdom, and their tributaries. The findings
showed a relationship between inorganic aluminium and pH, while
organic aluminium was generally low in these rivers. In general, lower
pHs and higher aluminium concentrations occurred at higher river flow
rates.
Ecker et al. (1990) monitored aluminium in the first and last
meltwater run-off from snowfields at Shiramine, a mountainous area in
Japan. Average aluminium concentrations were non-detectable and
445.6 �g/litre in the insoluble fraction (> 0 45 �m) of the first and
last run-offs, respectively, and 25.0 and 19.9 �g/litre, respectively,
in the soluble fraction (< 0.45 �m).
5.1.3.2 Seawater
The concentrations of aluminium in seawater are summarized in
Table 10. The concentration of aluminium is dependent on the salinity
of the water. Concentrations in open seawater are typically around
1 to 2 �g/litre in the dissolved fraction (< 0.45 �m). Bruland (1983)
stated that the concentration of aluminium in surface seawater in the
open ocean reflects atmospheric input and scavenging processes; the
concentration is low at high latitudes in the North Atlantic because
of a low atmospheric input and a higher scavenging rate resulting from
intensified biological activity in these waters. Aluminium is higher
in surface waters of mid-latitudes due to the higher atmospheric input
and lower scavenging rate in these oligotrophic waters.
Table 10. Concentrations of aluminium in seawater
Area Year Depth Salinity Particle Aluminium Reference
(m) (%) size concentration
(�m) (�g/litre)
Open Atlantic Ocean 1980-1982 15.2 and Kremling (1985)
25.4 nmol/kg
North-East Atlantic 1982 < 150 35-37 16-32 nmol/litre Hydes (1983)
Ocean 1982 > 1000 35-37 6-11 nmol/litre
Atlantic Ocean near 1974 300-1100 0.3-4.26 Alberts et al. (1976)
Carribean islands 1977-1978 0-2730 33-37 0.51-2.41 Stoffyn & Mackenzie (1982)
Atlantic Ocean near 1951-1952 0-10 Simons et al. (1953)
USA coast
Gulf of Mexico > 0.45 2.0 (0.2-10.2) Feely et al. (1971)
1951-1952 2-5 Simons et al. (1953)
Pacific Ocean near < 0.45 1 Sackett & Arrhenius (1962)
USA coast > 0.45 0.2-27 Sackett & Arrhenius (1962)
0.3 < 0.45 9.8 (3.7-166) Silvey (1967)
Weddel Sea, Antarctica < 0.45 1 Sackett & Arrhenius (1962)
> 0.45 4-120 Sackett & Arrhenius (1962)
North Sea 1988 6 34-35 < 0.4 10.2-49.2 Hydes & Kremling (1993)
nmol/litre
Mediterranean Sea 1976-1977 0-1500 35-38 1.0-4.8 Stoffyn & Mackenzie (1982)
5.1.4 Soil and sediment
Aluminium partitions from water to sediment and particulate
matter especially at circumneutral pH. The concentrations of aluminium
in sediment are summarized in Table 11. Mean aluminium concentrations
range from 20 000 to 80 000 mg/kg. Subramanian et al. (1988) measured
heavy metals in the bed sediments and particulate matter of the Ganges
Estuary, India. Average aluminium concentrations were 56 526 mg/kg for
bed sediments and 70 222 mg/kg for suspended sediments. Sanin et al.
(1992) measured elements in the sediments of the river Goksu and the
Tasucu Delta, Turkey. Mean aluminium concentrations ranged from
20 700 to 26 800 mg/kg for the river and from 30 150 to 42 875 mg/kg
for the delta. Benninger & Wells (1993) sampled sediment from the
Neuse river estuary, North Carolina, USA, between 1982 and 1990.
Aluminium concentrations ranged from 2.1 to 4.9 mmol/g, equivalent to
10.7% to 25.0% aluminium oxide. Fileman et al. (1991) found a mean
aluminium level of 2490 mg/kg in suspended particulate material from
the Dogger Bank region of the central North Sea.
Aluminium is one of the most abundant elements in soil and
concentrations vary widely. Shacklette & Boerngen (1984) collated the
aluminium concentrations measured by the US Geological Survey; levels
ranged from 700 to 100 000 mg/kg with an average of 72 000 mg/kg.
Beavington & Cawse (1979) analysed soil from Baguada, Nigeria, and
found aluminium levels of 24 000 �g/g (dry weight).
Table 11. Concentrations of aluminium in sediment
Area Yeara Aluminium Reference
concentration (mg/kg)
River Goksu, NR 20 800 to 26 600 Sanin et al.
Turkey (1992)
Tasucu Delta, NR 30 150 to 42 875 Sanin et al.
Turkey (1992)
Tadenac Lake, 1979 31 000 to 64 800 Wren et al.
Ontario (1983)
Turkey Lakes, 1981-1982 31 000 to 56 300 Johnson et al.
Ontario (1986)
Hamilton, 1986-1987 25 941 to 68 870 Irvine et al.
Ontario (1992)
(urban run-off)
Fontana Lake, 1978 36 400 to 84 600 Abernathy et al.
North Carolina (1984)
a NR = not reported
Aluminium is found in soil interstitial water at levels similar
to those reported for freshwater. Litaor (1987) measured a mean
aluminium concentration of 24.8 �mole/litre for the interstitial water
(pH 6.0) of soil from the Green Lakes Valley Front Range in Colorado,
USA.
5.1.5 Terrestrial and aquatic organisms
Mason & MacDonald (1988) monitored aluminium levels in aquatic
moss ( Fontinalis squamosa) from the River Mawddach catchment, Wales
(polluted with drainage water from disused mines) in 1984 and 1985.
Mean aluminium concentrations ranged from 1970 to 26 800 mg/kg (dry
weight). Mersch et al. (1993) transplanted aquatic moss ( Amblystegium
riparium) from a non-acidified stream to streams with pH values
ranging from 4.64 to 5.74. The moss accumulated aluminium, those
exposed to acidified streams containing aluminium ranging from 10 390
to 12 700 �g/g (dry weight) and those in a control stream (pH 6.96)
containing 7750 �g/g. Caines et al. (1985) collected aquatic
liverworts ( Nardia compressa and Scapania undulata) from streams
in the Loch Ard and Galloway areas of Scotland. Mean liverwort
aluminium concentrations were 3148, 6166 and 8532 mg/kg (dry weight)
from streams containing 195, 71 and 24 �g/litre, respectively.
Bioconcentration of aluminium occurred in all streams; however,
increased hydrogen ion concentrations were associated with decreased
liverwort aluminium concentrations. Albers & Camardese (1993a)
monitored aquatic plants from acidified and non-acidified constructed
wetlands. Aluminium concentrations for bur-reed ( Sparganium
americnum) and bladderwort ( Utricularia spp.) were 167 and 533 �g/g
(dry weight), respectively, for acidified wetlands and 104 and
487 �g/g for non-acidified wetlands. Duckweed ( Lemna spp.) and green
algae ( Oedogonium spp.) contained 998 �g aluminium/g at the non-
acidified sites; acidified wetlands did not contain duckweed or green
algae. Bur-reed (Sparganium spp.), bladderwort and pondweed collected
from sites in Maryland and Maine contained aluminium concentrations of
74.6, 1740 and 296 �g/g, respectively. The accumulation of aluminium
by these aquatic plants correlated poorly with the water concentration
(Albers & Camardese, 1993b).
Leinonen (1989) collected leaves of Vaccinium myrtillus from
untreated forest, clear-cut untilled forest and clear-cut tilled land
in Kuru, southern Finland in 1987. Aluminium levels were significantly
higher in the tilled area, with levels of approximately 140 mg/kg dry
weight in untilled areas and 185 mg/kg in the tilled area. Moomaw et
al. (1959) collected a wide selection of Hawaiian plant species from
highly leached latosol soils of low pH and high aluminium content.
Aluminium concentrations ranged from 59 to 16 000 mg/kg dry weight.
Thirteen of the 23 species contained aluminium levels in excess of
1000 mg/kg; the highest levels were found in the pteridophyte
Polypodium phymatoides and the dicotyledon Melastoma malahathricum.
Beavington & Cawse (1979) analysed sorghum grain from Bagauda,
Nigeria, and found aluminium levels of 90 �g/g dry weight.
Wyttenbach et al. (1985) collected needles of Picea abies from
around the city of Winterthur, Switzerland. The washed needles were
analysed for a wide range of elements including aluminium. The mean
aluminium content of the needles was 19 mg/kg (10-64 mg/kg). It was
found that washing the needles had removed more than 80% of the
aluminium residue. Landolt et al. (1989) sampled spruce needles from
locations throughout Switzerland in 1983 to study the distribution of
elements. The mean aluminium concentration was found to be 61.4 mg/kg
with a range of 12.88 to 344.5 mg/kg. H�s�nen & Huttunen (1989)
measured the aluminium content of the annual rings of pine trees
( Pinus sylvestris). The mean concentration for the period 1920 to
1980 was 4.2 mg/kg (3.4-5.1 mg/kg). In the areas associated with
higher sulfur deposition there had been increases in aluminium uptake
since 1950.
Malley et al. (1987) collected crayfish ( Orconectes virilis)
from a lake in northwestern Ontario containing a total aluminium
concentration of 36 �g/litre. Mean aluminium concentrations in the
crayfish were highest in the gut tissue (774 mg/kg) and there were
levels of 65.2, 84.4 and 50.4 mg/kg in the carapace, green gland and
ovary, respectively. Madigosky et al. (1991) monitored red swamp
crayfish ( Procambarus clarkii) from roadside drainage ditches
in Louisiana, USA. Aluminium concentrations ranged from 1.75
to 981.50 mg/kg dry weight in the order abdominal muscle
< hepatopancreas < exoskeleton < alimentary canal tissue. The
crayfish contained significantly higher levels of aluminium than those
found in control crayfish sampled from a commercial crayfish farm.
Madigosky et al. (1992) collected crayfish during 1990 from a site
near to a Louisiana highway intersection. Aluminium concentrations
were 2409 and 2342 mg/kg for intestinal tissue and contents,
respectively, while concentrations of 527 and 27 388 mg/kg were found
for stomach tissue and contents, respectively. It was found that
purging the crayfish in 1.5% sodium chloride for 6 h did not
significantly reduce aluminium in the gut tissue. However, there was
an increase in the water concentration of aluminium probably caused by
its release from exterior tissue sites.
Albers & Camardese (1993a) collected aquatic insects from both
acidified and non-acidified constructed wetlands; aluminium
concentrations were 94.3 and 158 �g/g (dry weight) for the two types
of wetland, respectively. Albers & Camardese (1993b) analysed aquatic
insects from sites in Maryland (224 �g/g) and in Maine (102 �g/g). The
same authors analysed crayfish and snails from sites in Maryland and
Maine in 1987. Whole body aluminium concentrations were found to be 66
to 542 �g/g (dry weight) and 27 to 398 �g/g for the two species,
respectively.
Brumbaugh & Kane (1985) collected smallmouth bass ( Micropterus
dolomieui) from the Chatuge reservoir on the border between Georgia
and North Carolina, USA. The reservoir receives run-off from poorly
buffered, forested watersheds, and the average pH of the reservoir was
6.3. Mean aluminium concentrations were 58 �g/g wet weight for gills
and 3.0, 2.5, 1.5 and < 1.0 �g/g for the carcass, gut, liver, and
kidney, respectively. Fish collected from the vicinity of a liquid
waste site in North Carolina, USA contained mean aluminium levels
ranging from 10.9 to 18.2 mg/kg (wet weight - based on whole gutted
fish) (Loehle & Paller, 1990). Buergel & Soltero (1983) analysed
plankton and fish ( Oncorhynchus mykiss) from a lake in Washington
State, USA, that had been treated with aluminium sulfate to reduce
high phosphorus concentrations. Total and dissolved aluminium in the
lake water ranged from 0.16 to 0.75 mg/litre, and from 0.09 to
0.42 mg/litre, respectively. Aluminium concentrations in plankton
ranged from 6.53 to 49.81 mg/kg, while those in various fish tissues
ranged from 0.07 to 6.25 mg/kg with the highest levels concentrated in
the gills. The aluminium concentrations measured in the fish were not
significantly different from those analysed in fish from untreated
lakes. Berg & Burns (1985) compared the aluminium concentrations in
fish tissues from a lake receiving water treatment plant sludge
containing aluminium hydroxide with a control lake. Both lakes had pH
values in the range 7.0 to 8.0. Dissolved aluminium was 0.1 mg/litre
in the treated lake and < 0.1 mg/litre in the control lake. Aluminium
was found in all tissues of all fish analysed. Liver, kidney and gill
samples from channel catfish ( Ictalurus punctatus) taken from the
polluted lake contained significantly more aluminium than those from
the control lake. For catfish brain and muscle, and for all tissues
from largemouth bass ( Micropterus salmoides) and gizzard shad
( Dorosoma cepedianum) there were no significant differences.
Aluminium concentrations ranged from 60.8 to 1808.9 mg/kg; the highest
concentrations were found in the liver and brain.
Karlsson-Norrgren et al. (1986a) collected and analysed brown
trout ( Salmo trutta) from two fish farms within acid-susceptible
areas in Sweden using lime-treated waters. Preliming, the water had a
pH of 4.6 to 4.7, with total and labile aluminium concentrations of
390-516 and 270-300 �g/litre, respectively. The post-liming water
quality (to the hatchery) was 208-261 �g/litre as total aluminium and
12-80 �g/litre as labile aluminium. Trout from a third non-acidified
location (pH 6.9; total aluminium levels in water 35 �g/litre) were
also analysed. Aluminium concentrations ranged from 89.3 mg/kg (wet
weight) for gills to 0.8 mg/kg for muscle in fish from acid-
susceptible areas. Fish from the control area contained aluminium
ranging from 2.6 mg/kg in the intestine to 0.6 mg/kg in muscle, while
levels in the gills were 1.9 mg/kg.
Hellou et al. (1992a) analysed muscle samples from the bluefin
tuna ( Thunnus thynnus) collected off the cost of Newfoundland,
Canada, in 1990. Aluminium concentrations ranged from 0.4 to 1.9 �g/g
dry weight with a mean value of 1.0 �g/g. Hellou et al. (1992b) found
aluminium concentrations of < 1 to 8 �g/g dry weight in muscle, liver
and ovaries of cod ( Gadus morhua) sampled from several sites off the
coast of Newfoundland during 1990 and 1991.
Wren et al. (1983) analysed fish, bird and mammal muscle from
Tadenac Lake (a Precambrian Shield lake), Ontario, Canada, and its
surrounding area. The lake had a pH of 7.1 and contained 47 400 mg
aluminium/kg in the sediment. Mean aluminium concentrations ranged
from 1.7 to 2.8 mg/kg (wet weight) for fish and from 2.5 to 5.2 mg/kg
for birds and mammals.
5.2 Occupational exposure
The levels of aluminium to which workers are exposed vary greatly
according to the type of industry and whether adequate industrial
hygiene practices are adhered to. Most studies have dealt with
inhalation of aluminium-containing dust particles rather than
aluminium per se. Some, however, have utilized urinary aluminium
determinations as an indicator of exposure (Sj�gren et al., 1983;
Gitelman et al., 1995). Utilizing such a technique for exposure is
essential, since it is rare for a worker to be exposed solely to
aluminium but rather to a mixture of aluminium-containing dusts and
chemicals.
Occupational exposure limits for aluminium fumes and dust have
been developed in many countries. Time-weighted averages of 5 mg/m3
(respirable dust) and 10 mg/m3 (total dust) have generally been
accepted. However, an occupational exposure limit of 1 mg/m3
calculated as aluminium has been proposed in Sweden regarding
aluminium-containing respirable fumes (Sj�gren & Ekinder, 1992).
Given the minimal amount of data on actual aluminium levels in
workplace air, it is difficult to estimate a daily exposure from the
occupational setting. Based on a recent publication, aluminium process
and production workers are generally exposed to less than 1 mg per 8-h
shift, assuming 10 m3 inhaled per shift (Gitelman et al., 1995). It
should be noted that, in some occupations and under less than optimal
industrial hygiene practices, occupational exposures to aluminium
could be higher. Welders performing metal-inert gas welding have been
exposed to 4 mg/m3 (calculated as aluminium) and these particles are
generally less than 1 �m (Sj�gren & Ulfvarson, 1985; Sj�gren et al.,
1985). Assuming 10 m3 inhaled per shift, this implies an exposure of
40 mg per shift.
Occupational exposures have been reported as total dust or
particulate matter: e.g., potroom workers, 1.67 mg/m3 (Kongerud &
Samuelsen, 1991); production of abrasives, 0.2 to 44.6 mg aluminium
oxide/m3 (Jederlinic et al., 1990); MIG welders, 10 mg/m3; TIG
welders, 1 mg/m3; respirable particles with a mean aluminium content
of 39% (Ulfvarson, 1981; Sj�gren et al., 1985) and aluminium soldering
of aluminium cables, 1.1 mg/m3 respirable dust decreasing to
0.7 mg/m3 after installation of a vacuum collection system
(Hjortsbert, 1994).
5.3 General population exposures
5.3.1 Air
Pulmonary exposure to aluminium is determined by air
concentration, particulate size and ventilatory volume. Air
concentrations vary between low levels in rural settings
(20-500 ng/m3) and higher levels in urban settings
(1000-6000 ng/m3) (see Table 8). Particles larger than 5-10 �m
diameter tend to be removed from inhaled air and penetrate poorly into
the lungs. Humans living in an urban area with ambient aluminium
concentrations of about 2000 ng/m3, particle size < 5 �m and a
ventilatory volume of 20 m3/day would be exposed to 40 �g
aluminium/day by inhalation.
5.3.2 Food and beverages
Since aluminium is a major component of the earth's crust, it is
naturally present in varying amounts in most food-stuffs consumed. The
actual concentration in food and beverages from various countries will
vary widely depending upon the food product, the type of processing
used and, in particular, the levels of aluminium-containing food
additives permitted and the geographical area in which food crops are
grown. In general, the foods highest in aluminium are those than
contain aluminium additives (e.g., grain products (flour), processed
dairy products, infant formulae, etc.). Foods naturally high in
aluminium include baked potato (skin on), spinach, prune juice and tea
(Pennington & Schoen, 1995).
The preparation and storage of food in aluminium vessels, foil or
cans, may increase the aluminium content, particularly in the case of
foods that are acidic, salty or alkaline (Greger et al., 1985b; Nagy &
Nikdel, 1986; Baxter et al., 1988). Preparing acidic foods such as
tomatoes and rhubarb in aluminium pans was found to lead to a
significant increase in the level of aluminium in the food (0.5 mg/kg
wet weight raw tomatoes to 3.3 mg/kg wet weight cooked), whereas only
a slight increase was noted in similarly prepared rice or potatoes
(Greger et al., 1985b). Although individual foodstuffs may leach
aluminium from the vessel, there are indications that aluminium from
cookware represents only a small fraction of the total dietary intake
(Kupchella & Syty, 1980; Savory et al., 1987).
The total intake of aluminium from food and beverages (excluding
drinking-water) in several countries is given in Table 12. All
estimates are less than 15 mg/day, with the lower values probably
reflecting a lower use of aluminium additives in the preparation of
cereal grain products (bread, etc.) (UK MAFF, 1993).
Table 12. Estimated average dietary intake of aluminium in various countries
Country Method of Estimated intake of Reference
samplinga aluminium
(mg/day)
Australia MB 2.4 (male) NFA (1993)
1.9 (female)
Canada MB 0.08-0.69b (infants) Dabeka &
McKenzie (1992)
Finland TD 6.7 Varo &
Koivistoinen (1980)
Germany MB 11.0 (males) Treptow &
MB 8.0 (females) Askar (1987)
DD 0.78 (5-8 years old) Wilhelm et al.
(1995)
Japan TD 4.5 Teraoka et al.
(1981)
Netherlands DD 3.1 (mean male and female) Ellen et al. (1990)
Sweden DD 13.0 (female) Jorhem &
Haegglund (1992)
Switzerland DD 4.4 Knutti &
Zimmerli (1985)
UK TD 0.03-0.05 (4-month infant)c UK MAFF (1993)
0.27-0.53 (4-month infant)d
TD 3.9
USA TD 0.7 (6-11 month old infant) Pennington &
6.5 (6 years old) Schoen (1995)
11.5 (14-16 year old male)
7.1 (adult female)
8.2 (adult male)
a MB = Market basket survey; TD = Total diet study; DD = Duplicate diet study
b Range represents intake of an infant (0-1 month old) fed cow's milk to that
for an infant (1-3 month old) fed exclusively soya-based formulae
c Range for infants fed cow's milk-based formulae
d Range for infants fed soya-based formulae
5.3.3 Drinking-water
Aluminium levels in drinking-water, whether distributed through
household plumbing or as bottled water, vary according to the natural
levels found in the source and whether aluminium flocculants were used
during the purification process. An international drinking-water
guideline for aluminium was based on aesthetic rather than health
grounds (WHO, 1993).
Results from extensive monitoring of drinking-water supplies have
been obtained from Germany (Wilhelm & Idel, 1995), Ontario Canada
(OMEE, 1995), and the United Kingdom (UK MAFF, 1993).
In Germany, levels of aluminium in public water supplies averaged
(median) 10 �g/litre in the western region while 2.7% of public
supplies in the eastern region exceeded 200 �g/litre. It was estimated
that 500 000 people were exposed to these high levels. Aluminium
levels of up to 10 000 �g/litre were reported in drinking-water from
private wells in areas where the soil had low buffering capacity and
was subjected to high acidic stress (M�hlenberg, 1990; Wilhelm & Idel,
1995).
In a province-wide survey of the aluminium content of public
water supplies in Ontario, Canada, approximately 75% of all average
levels in 1993 and 1994 were less than 100 �g/litre, the present
operational guideline for Ontario (OMEE, 1995). The range of average
values was 40 to 851 �g/litre.
A large monitoring programme in 1991 by the water companies in
the United Kingdom (75 305 samples) reported that only 553 (0.7%)
exceeded the United Kingdom aluminium standard of 200 �g/litre (UK
MAFF, 1993). Drinking-water would add about 400 �g aluminium to the
daily intake, assuming a consumption of 2 litres water daily at the
aesthetic guideline value of 200 �g/litre (WHO, 1993). From the
monitoring data discussed above and normal intakes of water, a more
realistic intake would be at or below 200 �g/day from monitored
municipal supplies.
5.3.4 Miscellaneous exposures
The use of antacids and buffered analgesics may result in large
intakes of aluminium, far in excess of that normally consumed in food
(Shore & Wyatt, 1983; Lione, 1983; Schenck et al., 1989). It has been
estimated that daily doses of aluminium in antacids and buffered
analgesics range from 840 to 5000 mg and 130 to 730 mg per day,
respectively (Lione, 1983). These are approximately two to three
orders of magnitude greater than normal dietary intakes (see Table 12)
and well in excess of the recommended provisional tolerable weekly
intake (PTWI) of 420 mg for a 60-kg adult (FAO/WHO, 1989).
Aluminium compounds are widely used in the preparation of
cosmetics, particularly in antiperspirants (Sorenson et al., 1974).
However, there are no reliable data supporting dermal absorption from
such products.
5.3.5 Total human intake of aluminium from all environmental pathways
In calculating total human exposures one must be aware of the
quality of the sampling and analytical procedures, particularly when
using data from earlier studies. Total intake of aluminium must
consider all routes of exposure, i.e. inhalation, oral and dermal.
For humans, non-occupationally exposed to aluminium, oral intake
of aluminium represents the major route of exposure. As shown in Table
12 the total daily intake of aluminium in adults ranges from 2.5 to
13 mg/day, depending upon the country of origin as well as the age and
sex of the subject. The variation reflects different dietary habits as
well as the level of additives used in food processing. For infants
(under 6 months) daily intakes range from 0.27 to 0.53 mg/day for
those consuming soya-based formulae and 0.03 to 0.05 mg/day for
infants consuming cow's milk formulae (UK MAFF, 1993). Similar values
were reported from Canada (respectively, 0.08 and 0.69) (Dabeka &
McKenzie, 1990). Aluminium intake from breast milk has been calculated
to be < 0.04 mg/day (UK MAFF, 1993).
In conclusion, the total intake of aluminium by the general
population varies between 2.5 and 13 mg/day. In most countries over
95% of this comes from food and less than 1% from airborne aluminium.
As noted in section 5.3.4, these intakes can be increased greatly
(10 to 100 times) through the use of aluminium-containing antacids and
buffered analgesics. Total daily exposure to aluminium from all
sources, other than medicines, and for all age groups has been shown
to be less than the PTWI of 1 mg/kg per day (WHO, 1993).
5.3.6 Aluminium uptake
In view of the fact that over 95% of the normal daily intake of
aluminium comes from food and water, uptake from the gastrointestinal
tract will play a major role in determining tissue levels of the
metal. The ratio of intake to uptake will be a major determinant in
the risk of orally ingested aluminium to humans. Factors affecting
gastrointestinal absorption of aluminium are discussed in section
6.1.2.
Recent studies of the bioavailability and uptake of aluminium in
human volunteers have employed the radioactive isotope 26Al, which
may be detected at very low masses, i.e. 5 � 10-15 g using accelerator
mass spectrometry (AMS). The first of these was a study by Day et al.
(1991), who measured the uptake of aluminium in one volunteer
following the ingestion of 1.1 �g of the isotope in sodium citrate.
For this study aluminium uptake was assessed by extrapolation from a
single measurement of 26Al in blood plasma 6 h after administration.
The fraction of absorbed aluminium was estimated to be 1%. Later, the
same technique was employed by Day et al. (1994) to estimate aluminium
uptake from orange juice (with or without added silicate) in control
subjects and Down�s syndrome patients. For the normal subjects uptake
factors ranging from 0.04 to 1.5 � 10-4 were calculated. The addition
of silica reduced the uptake by a factor of about 7. In the Down�s
syndrome patients, many of whom develop AD, uptake was approximately 5
times higher than in controls (4.7 � 10-4 compared with 0.91 � 10-4).
Most recently, human bioavailability studies have been undertaken
by Priest (1994) using a more vigorous methodology, employing the
collection of blood samples and total excreta for a period of up to a
week after a single administration of the aluminium compound. The
results obtained showed significant intersubject variability in the
extent and timing of aluminium absorption and indicated that the
method employed by Day et al. (1994) was of limited utility. Two main
studies were undertaken. The first was a study of the uptake of
aluminium, as aluminium citrate, aluminium hydroxide and aluminium
hydroxide in the presence of citrate, from the gut following the
administration of 100 mg aluminium by gastric tube (Priest, 1994). The
measured fractional uptakes were as follows: 5 � 10-3 for aluminium
as citrate; 1.04 � 10-4 for aluminium hydroxide; 1.36 � 10-3 for
hydroxide in the presence of sodium citrate. This study demonstrated
the greater bioavailability of the citrate complex and the ability of
citrate to enhance the uptake of aluminium taken in another chemical
form. The second study measured the fractional uptake of aluminium
from drinking-water using a similar technique, but different
volunteers (Priest et al., 1995a,b,c). The measured uptake fraction
was 2.2 � 10-4. It was concluded that members of the public, drinking
1.5 litres per day of water containing 100 �g aluminium/litre, would
absorb from this source about 3% of their total daily aluminium
uptake. This result suggests that drinking-water, under most
circumstances, is likely to be a minor source of aluminium for humans.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
Investigations into the kinetics of aluminium include estimation
of typical toxicokinetic parameters as well as issues specifically
related to the chemistry of aluminium and its compounds. Many studies
have been performed at high-dose levels. Since there are indications
that the toxicokinetics of aluminium are dose-dependent, these results
should be interpreted cautiously with respect to their relevance to
humans (Wilhelm et al., 1990). In addition, owing to large variations
in experimental protocols employed, many data-sets are not comparable,
making the interpretation of these data very difficult.
6.1 Absorption
6.1.1 Animal studies
6.1.1.1 Inhalation exposure
Reports of systematic studies of the pulmonary absorption of
aluminium in experimental animals have not been identified. However,
aluminium has been detected in organs other than the lung following
some inhalation experiments.
In rats and guinea-pigs exposed for 24 months to 0.25-25 mg/m3
aluminium chlorohydrate, aluminium was present primarily in the lungs.
The only other organs with significant concentrations of aluminium
were the peribronchial lymph nodes in guinea-pigs and the adrenal
glands in rats (Stone et al., 1979).
In New Zealand rabbits exposed to 0.56 mg aluminium/m3 for
5 months, there was a 2.5 fold increase in the aluminium content of
the brain (10.1 mg/kg dry weight) compared to control (4.1 mg/kg dry
weight) animals, while the concentration of aluminium in serum was
only slightly increased (R�llin et al., 1991a).
6.1.1.2 Oral administration
The gastrointestinal tract is the most important port of entry.
In addition, inhaled aluminium aerosols that are cleared from the
surface of the mucous membranes of the respiratory tract by action of
the mucociliary escalator are swallowed and thus may be absorbed from
the gastrointestinal tract.
Based on available data, absorption via the gastrointestinal
tract in experimental animals is generally less than 1%. However,
estimates of the proportion absorbed vary considerably, in part, as a
result of the different conditions of exposure (i.e., use of citrate
versus hydroxide salts, etc.) to various compounds (see Table 13).
Values from balance studies are probably overestimates, since the
amount of aluminium retained in the gut was probably calculated as
absorbed aluminium. The
rather high value obtained by Gupta et al. (1986) has not been
confirmed. Some studies on aluminium uptake after oral administration
of various compounds are summarized in Table 14 and Table 15, where
uptake has been measured by blood aluminium levels or tissue levels.
Results from studies on isolated intestinal organ systems
support the findings of low absorption rates of aluminium from the
gastrointestinal tract (J�ger et al., 1991). Also, although not
directly applicable to the human situation, experiments where
aluminium salts have been given to rats and mice by interperitoneal
injection further support the low amount of aluminium absorbed from
the gastrointestinal tract (Leblondel & Allain, 1980; Muller et al.,
1992; Greger & Powers, 1992). For example, blood aluminium levels in
rats given 10 mg aluminium chloride/kg body weight per day for 11 days
were about 15 times greater than controls (20 �g/litre compared to
300 �g/litre) (Muller et al., 1992). In contrast, in rats fed 0.1%
aluminium chloride in the diet for up to 25 days there was only a 22%
increase in blood aluminium levels (0.91 mg/litre compared to
1.11 mg/litre) (Mayor et al., 1977).
The mechanism of intestinal absorption of aluminium is fairly
complex and not yet fully elucidated (van der Voet, 1992). This
complexity results from the very particular chemical properties of the
element, i.e. (1) great variability of solubility at different pH
values, amphoteric character, and formation of various chemical
species depending on the pH, the ionic strength and the presence of
complexing agents in the intestine (Martin, 1992), and (2) the complex
organisation of the mammalian digestive tract where the chyme passes
through a sequence of chemical environments differing in pH, presence
of secretory products, etc. In addition, the different parts of the
intestine may be distinct with regard to their resorptive properties
and may be influenced by variation in physiological conditions. There
are indications that aluminium interacts with the gastrointestinal
calcium transport system (Adler & Berlyne, 1985; Provan & Yokel, 1988)
and with transferrin-mediated iron uptake (van der Voet & de Wolff,
1987; J�ger et al., 1991). There is consistent evidence that
absorption of aluminium increases in the presence of citrate (Slanina
et al., 1986; Froment et al., 1989a,b). There are some data suggesting
that uptake increases after fasting (Walton et al., 1994).
6.1.1.3 Dermal
Aluminium absorption via the skin in animals has not been
studied.
Table 13. Gastrointestinal absorption of aluminium compoundsa
Species Dose Form f (%)b Methodc Remarks References
Rat 8.1 mg/kg AlCl3 27 3 Gupta et al. (1986)
Rat 1; 12 mg Al/kg lactate 0.18 2 Wilhelm et al. (1992)
Rat 1; 12 mg Al/kg lactate 0.02 3 Wilhelm et al. (1992)
Rat 35 mg Al/kg sucralfate, lactate 0.015 2 Froment et al. (1989a)
Rat 35 mg Al/kg AlCl3 0.037 2 Froment et al. (1989a)
Rat 1.20 mmol Al/kg lactate 0.037 2 Froment et al. (1989a)
Rat 3.8 ng 26Al and 0.02 2 Jouhanneau et al.
63 ng 27Al in citrate (1993)
and citrate-free 0.02 2
solutions
Rat 1; 12 mg Al/kg lactate 0.18 2 Wilhelm et al. (1992)
Rat 1; 12 mg Al/kg lactate 0.02 3 Wilhelm et al. (1992)
Rat 35 mg Al/kg sucralfate 0.015 2 Froment et al. (1989a,b)
Rat 35 mg Al/kg Al(OH)3 0.015 2 Froment et al. (1989a,b)
Rat 35 mg Al/kg AlCl3 0.037 2 Froment et al. (1989a,b)
Table 13. (Con't)
Species Dose Form f (%)b Methodc Remarks References
Rabbit 10.8, 540 mg lactate 0.70-1.9 3 no significant influence Yokel & McNamara
Al/kg of dose (1985)
Rabbit 2.5-10 mmol/kg various 0.3-2.2 3 absorption: soluble>insoluble; Yokel & McNamara
minor differences between (1989)
organic and inorganic forms;
best bioavailability, citrate;
minor influence of renal
impairment
Sheep 1-2 g/day Al2(SO4)3; 2-15 1 order of absorption: Allen & Fontenot
Al-citrate; Al2(SO4)3>Al citrate>AlCl3 (1984)
AlCl3
a Modified from: Wilhelm et al. (1990)
b f = mass Al absorbed + mass Al ingested
c 1 = balance study; 2 = estimation based on urinary excretion; 3 = comparison of areas under plasma aluminium concentration after oral
and intravenous application
Table 14. Tissue aluminium concentrations in experimental animals administered aluminium compounds orallya
Species Treatment Bone Brain Reference
Mouse (BALB/c, AlCl3, gavage n.d. n.d. Cranmer et al. (1986)
5-10/group) 200 mg/kg per day
300 mg/kg per day
Mouse (Swiss, 6/group) Al lactate, 25 mg Al/kg (5.3 mg/kg w.w.) 35.3 mg/kg w.w. Golub et al. (1989)
(= control),
500 mg Al/kg diet 5.0 mg/kg w.w. 38.3 mg/kg w.w.
1000 mg Al/kg diet 6.5 mg/kg w.w. 108.7 mg/kg w.w.
Rat (8 Wistar) 2835 mg Al/kg in feed, femur Ondreicka et al. (1966)
(as Al2(SO4)3), 24 days (702 mg/kg w.w.) (7.1 mg/kg w.w.)
912 mg/kg w.w. 10.8 mg/kg w.w.
Rat (juvenile, aluminium in water n.d. n.d. Cann et al. (1979)
male SD, 8/group) (1) control
(2) 0.32 g Al/litre, 29 days
(3) low Ca2+ + Al
Rat (male SD, 8/group) 100 mg Al/kg b.w. cortex: Slanina et al. (1984)
6 d/w; by gavage (0.36 mg/kg w.w.) (0.013 mg/kg w.w.)
Al(OH)3 (9 weeks); 0.41 mg/kg w.w. 0.013 mg/kg w.w.
Al citrate (4 weeks); x 40 increased 0.057 mg/kg w.w.
citric acid (4 weeks) x 20 increased 0.028 mg/kg w.w.
Rat (male SD, 7/group) gavage; 3 d/w; 11 week (0.22 mg/kg w.w.) (0.016 mg/kg w.w.) Slanina et al. (1985)
Al(OH)3 0.89 mg/kg w.w. 0.012 mg/kg w.w.
Al citrate 10.7 mg/kg w.w. 0.048 mg/kg w.w.
Al(OH)3 + citrate 26.6 mg/kg w.w. 0.092 mg/kg w.w.
Table 14. (Con't)
Species Treatment Bone Brain Reference
Rat (weanling, male 270 mg Al/kg diet, 18 days tibia: (1.9 mg/kg w.w.) (0.0 mg/kg w.w.) Greger et al. (1985a)
SD, 6/group) Al(OH)3 15.6 mg/kg w.w. 2.2 mg/kg w.w.
Al palmitate 15.0 mg/kg w.w. 0.6 mg/kg w.w.
Al lactate 13.0 mg/kg w.w. 1.6 mg/kg w.w.
AlPO4 14.5 mg/kg w.w. 1.3 mg/kg w.w.
Rat (weanling, male Al(OH)3 in diet, 67 days tibia: (4.04 mg/kg) n.d. Greger et al. (1986)
SD, 9/group) 257 mg Al/kg diet 11.3 mg/kg (3.13 mg/kg)
1075 mg Al/kg diet 10.4 mg/kg
Rat (male SD, 10/group) Al(NO3)3, in water, 4 w (5.75 mg/kg w.w.) (1.4 mg/kg w.w.) G�mez et al. (1986)
375 mg/kg/d 11.4 mg/kg w.w. 7.7 mg/kg w.w.
750 mg/kg/d 8.5 mg/kg w.w. 10.1 mg/kg w.w.
1500 mg/kg/d 17.7 mg/kg w.w. 7.9 mg/kg w.w.
Rat (female SD, Al(NO3)3, oral, 100 d (17.15 mg/kg w.w.) (< 0.5 mg/kg w.w.) Domingo et al. (1987b)
10/group) 360 mg/kg w.w. 75.08 mg/kg w.w. 4.93 mg/kg w.w.
720 mg/kg w.w. 79.18 mg/kg w.w. 2.09 mg/kg w.w.
3600 mg/kg w.w. 56.39 mg/kg w.w. 4.28 mg/kg w.w.
Rat (SD, weanling Al(OH)3, in feed, 10 d femur: (6.8 mg/kg d.w.) n.d. Chan et al. (1988)
6/group) 50-60 mg/kg b.w. 8.4 mg/kg d.w.
Rat (male, weanling SD) Al(OH)3, 28 d tibia: n.d. Ecelbarger & Greger
13 mg Al/kg diet 36 mmol/kg w.w. (1991)
+ 5 mmol/kg citrate 36 mmol/kg w.w.
41 mg Al/kg diet 50 mmol/kg w.w.
+ 5 mmol/kg citrate 69 mmol/kg w.w.
Table 14. (Con't)
Species Treatment Bone Brain Reference
Rat (18 male, Al(OH)3 in feed, 29 days tibia: (28.9 mmol/kg w.w.) n.d. Greger & Powers
weanling SD) 0.39 �mol Al/g diet 52.6 mmol/kg w.w. (1992)
aluminium + 4% citrate 74.4 mmol/kg w.w.
100 �mol Al/g diet 79.6 mmol/kg w.w.
+ 4% citrate
Rabbit (female NZ, inhalation exposure (18.2 mg/kg d.w.) (4.1 mg/kg d.w.) R�llin et al. (1991a)
8/group) 0.56 mg Al/m3 as Al2O3 22.2 mg/kg d.w. 10.1 mg/kg d.w.
8 h/d; 5 d/w; 5 months
Rabbit (male NZ, 50 g/kg AlCl3, in feed (n.d.) cortex, gray matter: Thornton et al.
3-4/group) 1 month (n.d.) 3.1 mg/kg d.w. (1983)
ethanol 1.3 mg/kg d.w.
aluminium + ethanol 3.0 mg/kg d.w.
Dog Al(OH)3 in feed, 3 g/d; n.d. cerebral cortex: Arieff et al. (1979)
5 months 0.77 mg/kg d.w.
2.4 mg/kg d.w.
Cattle (steer, 6/group) AlCl3 in feed, 84 d (n.d.) (6.4 mg/kg w.w.) Valdivia et al.
300 mg/kg 7.6 mg/kg w.w. (1978)
600 mg/kg 5.5 mg/kg w.w.
1200 mg/kg 7.7 mg/kg w.w.
Values in parentheses are normal control values in unexposed animals; b.w. = body weight; d = day; d.w. = dry weight;
n.d. = not detected; NZ = New Zealand; SD = Sprague-Dawley; w = week; w.w. = wet weight
Table 15. Blood aluminium concentrations in experimental animals exposed orally to aluminium compoundsa
Species Sample Dose Duration Compound Aluminium concentration Reference
(control value)
Rat (8 Wistar) blood 2835 mg Al/kg feed 24 days Al2(SO4)3 (6.5 mg/kg w.w.) Ondreicka et al.
10.8 mg/kg w.w. (1966)
Rat (male albino) serum Al(OH)3 (0.24 mg/litre) Berlyne et
150 mg Al/kg/d, gavage 0.99 mg/litre al. (1972)
Rat (male SD, serum AlCl3 (0.91 mg/litre) Mayor et al.
8/group) 0.1% aluminium in feed day 10: 1.12 mg/litre, (1977)
day 25: 1.09 mg/litre
Rat (male SD, blood 11 weeks (0.005 mg/kg w.w.) Slanina et al.
7/group) 3 d/w, gavage Al(OH)3 0.009 mg/kg w.w. (1985)
Al citrate 0.014 mg/kg w.w.
Al(OH)3 + citrate 0.039 mg/kg w.w.
Rat (male SD, blood Al(NO3)3 (3.7 mg/kg w.w.) G�mez et al.
10/group) 375 mg/kg/d 3.1 mg/kg w.w. (1986)
750 mg/kg/d 2.5 mg/kg w.w.
1500 mg/kg/d, in water 3.0 mg/kg w.w.
Rat (female SD, blood 100 days Al(NO3)3 (< 0.5 mg/kg) Domingo et al.
10/group) 360 mg/kg w.w. < 0.5 mg/kg (1987b)
720 mg/kg w.w. < 0.5 mg/kg
3600 mg/kg w.w., oral < 0.5 mg/kg
Rat (18 male, serum 29 days Al(OH)3 (0.28 �mol/litre) Greger &
weanling SD) 0.39 mmol Al/kg diet 0.98 �mol/litre Powers (1992)
aluminium + 4% citrate 1.15 �mol/litre
100 mmol Al/kg diet
+ 4% citrate, in feed 1.09 �mol/litre
Table 15. (Con't)
Species Sample Dose Duration Compound Aluminium concentration Reference
(control value)
Rat (weanling serum 160 mg Al/kg d, gavage, 10 days (18.8 �g/litre) Santos et al.
SD, 4/group) 1,25-(OH)2-D3 24.3 �g/litre (1987)
1,25-(OH)2-D3 + Al(OH)3 29.5 �g/litre
1,25-(OH)2-D3 + Al citrate 16.3 �g/litre
Rabbit (male serum 1 month (5 �g/litre) Thornton et al.
NZ, 3-4/group) 50 g/kg in feed AlCl3 14 �g/litre (1983)
Al+ethanol 24 �g/litre
Cattle (steer, blood 84 days AlCl3 (0.103 mg/litre) Valdivia et al.
6/group) 300 mg/kg 0.118 mg/litre (1978)
600 mg/kg 0.100 mg/litre
1200 mg/kg, in feed 0.120 mg/litre
a 1,25-(OH)2-D3 = 1,25-dihydroxy-vitamin D3; d = day; NZ = New Zealand; SD = Sprague-Dawley; w = week; w.w. = wet weight
6.1.2 Studies in humans
6.1.2.1 Inhalation exposures
Studies dealing with the absorption of aluminium compounds in
humans usually use the blood aluminium concentration or the urinary
aluminium excretion as a marker of uptake (Schaller & Valentin, 1984;
Ganrot, 1986). However, part of the aluminium-containing particulates
deposited in the respiratory tract is cleared from the organ by
mucociliary action and, when swallowed, enters the digestive tract.
This means that after inhalation exposure to aluminium compounds not
all aluminium appearing in the systemic circulation or in the urine
necessarily arises solely from absorption in the respiratory tract.
"Insoluble" particulates may be slowly dissolved and thus enter
the blood circulation. Owing to the chemical properties of aluminium,
the absorption of aluminium metal or its compounds by the respiratory
system depends on the aluminium species inhaled and the biological
environment in the tissue compartment where they are deposited
(Martin, 1992).
There is evidence from a number of reports that even aluminium
compounds that are almost insoluble in water are bioavailable when
introduced into the respiratory system. For example, increased urinary
concentrations have been observed in aluminium welders and aluminium
flake and powder producers after exposure to relatively insoluble
particulate matter and metallic fumes and dusts. The levels of tissue
aluminium after inhalation exposures are given in Table 16. When
comparing the analytical results given in these tables, one has to
keep in mind, however, that the analytical precision of the aluminium
determination has been hampered by the potential for contamination
during sampling and processing in view of the ubiquitous presence of
the element (Steinegger et al., 1990). Analytical techniques for
determination of aluminium have been improved considerably during
recent years (see Chapter 2).
6.1.2.2 Oral administration
In view of the fact that over 95% of the normal daily intake of
aluminium comes from food and water, uptake from the gastrointestinal
tract will play a major role in determining tissue levels of the
metal.
The mechanism of gastrointestinal absorption of aluminium is
fairly complex and has not yet been fully elucidated (van der Voet,
1992). This complexity results from (1) the unique chemical properties
of the element, particularly its amphoteric character, leading to
marked variability in solubility at different pH values and the
formation of various chemical species in the gut depending on the pH,
the ionic
strength and the presence of complexing agents (Martin, 1992), and (2)
the complex organization of the mammalian digestive tract where the
chyme passes through a sequence of chemical environments differing
greatly in pH, presence of secretory products, etc. In addition, the
different parts of the intestine may be distinct with regard to their
absorptive and resorptive properties with respect to aluminium.
Aluminium species may be modified in the gut prior to absorption
(Skalsky & Carchman, 1983; Ganrot, 1986; Martin, 1986, 1992).
Quantitatively the intraluminal absorption depends upon the amount of
the chemical species present in the gut lumen, in the blood, and in
the interstitial fluid. Absorption is influenced by the presence of
other complexing ligands (citrate, lactate, etc.) and competing ions
(e.g., iron, silicon). Other factors proposed to influence absorption
include: age; renal function; and iron and calcium status (Birchall,
1991; van der Voet, 1992; Edwardson, 1993).
To date, research concerning the intestinal absorption of
aluminium in humans has been mainly guided by clinical problems and
has used a variety of physiological states and chemical conditions.
Owing to the large variations in experimental conditions, many results
are not comparable and interpretation of their relevance to the health
population becomes very difficult or impossible.
Gastrointestinal absorption of aluminium in humans ingesting
antacids or phosphate binders is well documented. Variable quantities
of Al(OH)3 or Al2(CO3)3 given to volunteers or patients for
different periods of time resulted in significant increases in plasma
and/or urinary aluminium concentrations (Cam et al., 1976; Kaehny et
al., 1977a; Recker et al., 1977; Gorsky et al., 1979; Mauras et al.,
1982; Herzog et al., 1982; Greger & Baier, 1983a). Administration of
the insoluble AlPO4 did not significantly alter blood and urinary
aluminium levels (Kaehny et al., 1977a). The results of these studies
are summarized in Tables 16 and 17. It must be emphasized, however,
where compounds are poorly bioavailable, the expected incremental
increase in the plasma aluminium level after exposure may be lower
than can be detected against normal plasma aluminium levels.
Recent studies of the bioavailability and uptake of aluminium in
human volunteers have employed the radioactive isotope 26Al, which
may be detected at very low concentrations (5 � 10-15 g) using
accelerator mass spectrometry (AMS). The first of these was a study by
Day et al. (1991) who measured the uptake of aluminium in one
volunteer following the ingestion of 1.1 �g of the isotope in sodium
citrate. For this study aluminium uptake was assessed by extrapolation
from a single measurement of 26Al in blood plasma 6 h after
Table 16. Blood and urine aluminium concentrations in humans after oral ingestion of aluminium compoundsa
Subjects and Aluminium concentration Aluminium concentration Remarks Reference
treatment in blood (�g/litre) in urine (�g/litre)
5 normal subjects, not specified not specified Al absorption Cam et al.(1976)
2 patients with CRF;
Al(OH)3 antacids, normal: 0.3-3.6 mmol/day
86-91 mmol/day CRF: 3.3-9.1 mmol/day
Normal subjects, not detected (85.8 �g/day) Recker et al. (1977)
Al(OH)3, oral, 3.8 g increased by 4-10 times
Al/day for 3 days
Normal subjects, plasma: (8-16) cumulative increase in Kaehny et al. (1977a)
2.2 g Al for 3 days (6-7) excretion (�g)
Al(OH)3 17 176-325 730 � 487
Al2(CO3)3 14 51-355 567 � 437
Al(OH)2-aminoacetate 17 243-726 1430 � 1157
AlPO4 9 52-60 123 � 77
Normal subjects 6.2 (serum) not detected Marsden et al.
CRF 13.4 (serum) (1979)
CRF + Al 34.1
Normal subjects, antacids plasma Al: urinary Al excretion: Al balance positive during Gorsky et al. (1979)
taken orally, 23-313 2-fold increase 2- to 6-fold increase Al administration
mg/day for 18-30 days
Al-hydrocarbonate (before Al: 8.35) (before Al: 6.35) serum aluminium in dialysed Mauras et al.
(Lithiagel), oral, 1.84 g 3-day Al: 15.9 3-day Al: 430.8 patients treated with Aludrox: (1982)
Al/day; 5 days 5-day Al: 14.8 5-day Al: 262.5 6-254 �g/litre
after 1 week Al: 8.0 after 1 week Al: 12.2
Table 16. (Con't)
Subjects and Aluminium concentration Aluminium concentration Remarks Reference
treatment in blood (�g/litre) in urine (�g/litre)
Al-supplemented diet serum (before: 4) urinary excretion: normalized to creatinine Greger & Baier
control: 4.6 mg Al/day control: 4 control: 35-36 �g/day control: 20 mg/kg (1983a)
test: 125 mg Al/day test: 7 test: 105-129 �g/day test: 57-72 mg/kg
12 subjects serum Al Herzog et al.
(normal young) given placebo: 0.3-0.9 placebo: 1.0-3.0 (1982)
280 nM Mg + 190 nM test: 0.8-1.1 test: 3.6-20.2
Al/day for 4 weeks
CRF, (3.4 plasma) no correlation with Al Wilhelm et al.
on home dialysis 37.7-68.7 concentration in hair (1989)
on CAPD 33.9-45.0
a control values are given in parentheses
CAPD = continuous ambulatory peritoneal dialysis; CRF = chronic renal failure
Table 17. Tissue aluminium concentrations (mg/kg) in humans exposed to aluminium compoundsa
Subjects and Bone Muscle Kidney Liver Lung Brain Remarks Reference
treatment
Normal n.d. 1.55 d.w. 2.02 d.w. 2.4 d.w. 122.5 d.w. 1.4 d.w. adrenal: 4.8 d.w. Tipton &
adult spleen: 3.7 d.w. Cook
duod.: 4.56 d.w. (1963)
jejun.: 2.84 d.w.
ileum: 9.86 d.w.
Healthy hard water area: 0.5 w.w. whole 2.6 w.w. 18.2 w.w. whole brain: Hamilton
human 73.4 w.w. kidney: 0.5 w.w. et al.
controls soft water area: 0.4 w.w. frontal lobe: (1973)
60 w.w. cortex: 0.05 w.w.
0.4 w.w. basal ganglia:
medulla: 0.07 w.w.
0.3 w.w.
Normal males < 15 d.w. n.d. 11 d.w. 19 d.w. 230 d.w. n.d. spleen: (22 d.w.) Teraoka
Stonemason n.d. n.d. 16 d.w. 130 d.w. 2000 d.w. 520 d.w. (1981)
heart: (11 d.w.)
2.0 d.w.
adrenal: (37 d.w.)
n.d.
Ball-mill room 30 w.w. n.d. n.d. 90 w.w. upper lobe: 5 w.w. McLaughlin
worker in 430 w.w. et al.
aluminium powder lower lobe: (1962)
factory 340 w.w.
Surgical and n.d. no Al n.d. n.d. n.d. n.d. no Al intake: Cann et
autopsy specimen intake: PT: 13 d.w. al. (1979)
(hyperparathyroidism) 2.0 d.w. thy: 3.5 d.w.
Al intake: Al intake:
7.6 d.w. PT: 78 d.w.
thy: 8.8 d.w.
Table 17. (Con't)
Subjects and Bone Muscle Kidney Liver Lung Brain Remarks Reference
treatment
Normal controls: 10.6 23.6 17.5 15.8 97.2 11.9 spleen: 17.2 Flendrig
uraemia, non-dial: 6.4 24.7 33.8 19.7 142.3 n.d. 25.1 et al.
uraemia, dial: 23.5 39.6 44.1 32.9 127.1 12.1 37.9 (1976)
DES: 272.7 13.8 156.5 610.2 99.6 66.1 454.5
DES cortical bone: n.d. n.d. n.d. grey matter: brain white Alfrey et
normal control (3.88) (1.22) (2.18) matter: (2.00) al.
uraemia/dial 46.83 DES: 23.6 non-DES: 6.5 non-DES: 3.81 (1976)b
uraemia/non-dial 8.4 non-DES: DES: 24.98 DES: 5.59
trabecular 10.24
bone: (2.39)
98.48
37.4
Iliac bone n.d. n.d. n.d. n.d. n.d. correlation of Ellis et
(biopsy or duration of al.
autopsy spec.) dialysis and (1979)
control 5.7 ash bone Al
uraemia, non-dial 13.6 ash
dial 151.8 ash
dial + transpl 92 ash
Patients n.d. grey matter: spleen: Alfrey
healthy controls 3.3 d.w. 1.2 d.w. 4.0 d.w. 56 d.w. 2.2 d.w. 3.8 d.w. (1980)
uraemia, non-dial 27 d.w. 2.6 d.w. 25.5 d.w. 75 d.w. 4.1 d.w. 35 d.w.
uraemia, dial 115 d.w. 9.1 d.w. 160 d.w. 89 d.w. 8.5 d.w. 243 d.w.
DES 281 d.w. 15 d.w. 301 d.w. 215 d.w. 24.5 d.w. 493 d.w.
Table 17. (Con't)
Subjects and Bone Muscle Kidney Liver Lung Brain Remarks Reference
treatment
Uraemia + dial n.d. n.d. n.d. n.d. n.d. Hodsman
normal control 2.4 d.w. et al.
osteomalacia 175 d.w. (1982)
osteitis fibrosa 46 d.w.
mixed lesions 81 d.w.
mild lesions 67 d.w.
Normal, necropsy n.d. n.d. n.d. n.d. n.d. cortex: Crapper
0.23-2.7 d.w. et al.
white matter: (1973)
0.6-1.1 d.w.
Normal adult n.d. n.d. n.d. n.d. n.d. 1.9 d.w. Crapper
infant 0.7 d.w. et al.
fetus 0.7 d.w. (1976)
Normal controls n.d. n.d. n.d. n.d. n.d. 2.5 d.w. whole brain McDermott
5.6 d.w. hippocampus et al.
2.4 d.w. frontal cortex (1979)
1.4 d.w. temporal cortex
2.9 d.w. parietal cortex
2.9 d.w. occipital cortex
2.6 d.w. cerebellum
1.5 d.w. corpus callosum
4.1 d.w. mininges
1.3 d.w. isolated neurons
Normal adult n.d. n.d. n.d. n.d. n.d. 0.467 w.w. Markesbery
normal infant 0.298 d.w. et al.
(1981)
Table 17. (Con't)
Subjects and Bone Muscle Kidney Liver Lung Brain Remarks Reference
treatment
Al welders (2) (0.6-5 d.w.) n.d. n.d. n.d. n.d. n.d. also increased: Elinder et
welding fumes 18-29 d.w. blood and al. (1991)
urinary Al
concentration
CRF, no correlation Wilhelm
cumulative (median values) with Al et al.
oral Al intake: concentration (1989)
0 kg 5.3 in hair:
< 0.25 kg 47.5 control: 2.6
0.25 to 0.5 kg 56.7 dial: 1.6-5.5
0.5 to 1.0 kg 62.6
1.0 to 5.0 kg 133.4
Controls 18.8 w.w. n.d. n.d. n.d. n.d. n.d. no differences Burnel et
(surgical between cortical al. (1982)
specimens) and medullary
CRF 6-130 w.w. bone;
(biopsies; no correlation
intake of with age
variable
amounts of Al)
a Normal control values are given in parentheses
DES = dialysis encephalopathy syndrome; dial= on haemodialysis;
w.w. = wet weight; non-dial= not on haemodialysis;
d.w. = dry weight; transpl= transplantation;
n.d. = no data; PT= parathyroid gland;
ARF = acute renal failure; thy= thyroid gland
CRF = chronic renal failure;
b All concentrations expressed as mg Al/kg fat-free solid
administration. Day estimated the fraction of absorbed aluminium to be
1%. Later, the same technique was employed by Day et al. (1994) to
estimate aluminium uptake from orange juice (with or without added
silicate) in control subjects and patients with Down�s syndrome.
Results were expressed as the gastrointestinal absorption factor
(F1), defined as the ratio of mass of aluminium absorbed to mass of
aluminium ingested. For the normal subjects uptake factors ranging
from 0.04 to 1.5 � 10-4 were calculated. The addition of silica
reduced the uptake by a factor of about 7. In the Down�s syndrome
patients uptake was apparently 5 times higher than in controls (4.7 �
10-4 compared to an average of 0.91 � 10-4 in controls).
Most recently, human bioavailability studies have been undertaken
by Priest and his co-workers using a methodology employing the
collection of blood samples and total excreta for a period of up to a
week after a single administration of aluminium compound. The results
of these experiments showed significant intersubject variability in
the extent and timing of aluminium absorption, indicating the
shortcomings of the methods employed by Day et al. (1991, 1994). Two
main studies were undertaken. The first was a study of the uptake from
the gut of aluminium, as aluminium citrate, aluminium hydroxide, and
aluminium hydroxide in the presence of citrate, following the
administration of 100 mg aluminium by gastric tube (Priest, 1994). The
absorption fractions obtained were as follows: 5 � 10-3 for aluminium
as citrate; 1.04 � 10-4 for aluminium hydroxide; and 1.36 � 10-3 for
hydroxide in the presence of sodium citrate. This study demonstrated
the greater bioavailability of the citrate complex and the ability of
citrate to enhance the uptake of aluminium taken in another chemical
form. The second study measured the fractional uptake of aluminium
from drinking-water using a similar technique, but different
volunteers (Priest et al., 1995a). The measured uptake fraction was
2.2 � 10-4. It was concluded that members of the public, drinking
1.5 litres/day of water containing 100 �g of aluminium/litre, would
absorb about 3% of their total daily aluminium uptake from this
source. This result suggests that drinking-water, under most
circumstances, is likely to be a minor source of aluminium for humans.
6.1.2.3 Dermal exposure
There is no direct evidence that aluminium is absorbed through
the intact skin of humans.
6.2 Distribution
6.2.1 Animal studies
After absorption aluminium is bound in the plasma primarily to
transferrin and, to a lesser extent, also to albumin (Trapp, 1983;
Bertholf et al., 1984; Martin, 1986). There are indications that
aluminium binding to protein is dose-dependent, with low binding rates
at unexposed plasma levels (H�hr et al., 1989; Wilhelm et al., 1990).
Aluminium distribution depends on the animal species used, route of
administration and the aluminium compound administered. Volumes of
distribution for aluminium have been estimated only following
parenteral administration (Wilhelm et al., 1990) and are thus not
relevant to the exposure of the general population. After a single
dose of aluminium lactate (1 mg/kg body weight) in rats, no tissue
uptake could be detected, whereas at 12 000 mg/kg body weight the only
significant increase in tissue aluminium occurred in bone (Wilhelm et
al., 1992). In other oral studies summarized in Table 17, more or less
significant increases in tissue levels after aluminium ingestion were
found, these increases generally being dose-dependent. In animals
receiving aluminium, increases in tissue levels were most marked in
bone.
It has to be considered that at high-dose levels aluminium is
toxic to the tissue of the gastrointestinal tract, thus inducing
pathological changes that might be followed by increased uptake (J�ger
et al., 1991). Gut tissue pathology has not been investigated in most
distribution studies.
Two studies have reported on aluminium accumulation following
administration in drinking-water. Fulton et al. (1989) administered 0,
0.1, 2.0 or 100 mg/litre Al(OH)3 or AlCl3 (equivalent to 0, 0.01,
0.2 or 5.5 mg Al/kg body weight per day) in drinking-water containing
acetate or citrate at various pH levels to Sprague-Dawley rats
(6 rats per group) for 10 weeks. In the highest-dose group, aluminium
accumulated in intestinal cells but not in other tissues investigated.
The effect was more pronounced when citrate was added and when water
with an acidic pH was used.
In a recent study, Walton et al. (1995) administered to eight
fasted adult rats by gavage 4 ml of aluminium-free drinking-water
containing 1.0 �g 27Al and 70 becquerel (0.1 �g) 26Al. Two
experimental animals had brain 26Al/27Al ratios similar to the two
controls while the remaining six animals had ratios of 26Al/27Al in
brain tissue that were substantially higher than background. However,
the variation between animals was marked (148 � 19 to 5220 � 208).
Studies in mice (Golub et al., 1993), rats (Muller et al., 1992)
and rabbits (Yokel, 1984) indicate that aluminium is not readily
transferred from the dam to offspring via nursing.
Studies in mice (Golub et al., 1993) and rabbits (Yokel, 1985)
indicate that aluminium compounds that are bioavailable and therefore
available to the dam also reach the fetus. However, a study in rats
reported elevated levels of aluminium in maternal tissue, but not in
the fetus, after exposure to aluminium lactate in the diet during
gestation (Muller et al., 1993). Limited data in mice (Cranmer et al.,
1986) and rabbits (Yokel, 1985) show higher concentrations of
aluminium in the placenta than in maternal or fetal tissues after
administration of aluminium to dams.
6.2.2 Human studies
6.2.2.1 Transport in blood
The extent to which plasma aluminium is normally bound to
proteins may be as high as 70 to 90% in haemodialysis patients with
moderately increased plasma aluminium levels (25 to 200 �g/litre).
Studies using 26Al (Day et al., 1994) have shown that one day
after injection, 99% of aluminium in blood is present in the plasma
fraction and 1% in the erythrocytes. By contrast, 880 days after
injection, 14% was associated with the erythrocyte fraction,
indicating the probable incorporation of aluminium into the
erythrocytes during erythropoiesis. One day after injection, 80% of
the plasma fraction was found to be associated with transferrin, 10%
with albumin, 5% with low molecular weight proteins (5000-50 000
molecular weight) and about 4% with a lower molecular weight fraction.
No details of the plasma speciation at 880 days were given. Priest et
al. (1996) found that only about 50% of injected aluminium was
recoverable from blood at 15 min after injection. It was suggested
that aluminium may pass through blood vessel walls to establish an
equilibrium between aluminium in blood and aluminium in the
extravascular tissue fluids. In contrast, the same study showed that
gallium remained in the blood. This and other observations suggested
that gallium is an inappropriate surrogate for aluminium in
bioavailability and kinetic studies (Priest et al., 1991).
6.2.2.2 Plasma aluminium concentrations in humans
The role of aluminium in the etiology of dialysis-related
disorders such as encephalopathy, vitamin-D-resistant osteomalacia,
and normochromic microcytic anaemia has drawn attention to the
possible mechanisms of aluminium uptake through the parenteral and
intestinal routes.
Normal human plasma or serum aluminium values reported vary
largely, mainly due to methodological problems in the analytical
technique and with sample contamination. Ganrot (1986) emphasized this
in his extensive review.
As methods have improved, suggested reference values for plasma
levels have been revised downwards, and it was suggested by Nieboer et
al. (1995) that the actual value in normal subjects lies in the range
of 0.04 to 0.07 �mol/litre (1.1 to 1.9 �g/litre).
Seasonal variations in serum aluminium concentrations in patients
with moderate chronic renal failure were observed by Nordal et al.
(1988), with peak levels occurring in the autumn. These variations
were presumed to be related to an increased gastrointestinal
absorption due to waterborne factors.
The data summarized in Tables 16 and 17 indicate that, as is the
case with experimental animals, the aluminium concentrations in human
blood and selected tissues are increased after ingestion or inhalation
of aluminium compounds.
6.2.2.3 Tissue aluminium concentrations in humans
(a) Normal concentrations
The available data relating to the aluminium concentrations found
in various human tissues are summarized in Table 17. It should be
noted that the measurement of tissue concentrations is difficult. In
particular, where the average concentrations for a tissue have been
reconstructed, by extrapolation, from the analysis of small samples of
the tissues concerned, they may be significantly affected by lack of
homogeneity in the distribution of the metal in the organ and will
also amplify errors due to sample contamination. In this respect the
reconstruction of average bone concentrations is particularly
difficult, given that small samples of bone collected from disparate
skeletal sites will contain very different levels of aluminium as a
result of the different amounts of bone surface to which the metal
binds. Owing to the above difficulties it is commonly prudent to
ignore measurements that indicate very high levels of aluminium in a
particular tissue without additional biological data to explain the
findings. This approach has been taken by a Canadian group (Nieboer et
al., 1995), which based its conclusions on the lowest levels
consistently reported for the tissues considered (bone and brain). It
was concluded that normal levels of aluminium in bone are in the order
of 1-3 �g/g (wet weight) and that background levels in brain tissue
(mostly in the grey matter) are around 1-3 �g/g (dry weight) or <
0.5 �g/g (wet weight), based on the lowest levels consistently
reported. The authors also showed that the bone and brain aluminium
levels are significantly elevated in patients with renal failure who
are treated with either aluminium-containing phosphate scavengers or
who received total parenteral nutrition with aluminium-contaminated
intravenous nutrient solutions.
With respect to other tissues, measurements suggest that the
highest levels of aluminium are found in the lung (56-215 mg/kg dry
weight) (Alfrey et al., 1980), presumably as inhaled, undissolved,
aluminium-containing particles. Similarly, inhaled particles may
relocate in the regional (hilar) lymph nodes and in the organs
comprising the reticulo-endothelial system, i.e. liver, spleen and
bone marrow. This may explain some or, in the case of the lymph nodes,
most of the aluminium present in these organs. For example, Teraoka
(1981) reported the levels of aluminium in the lungs and reticulo-
endothelial organs of a stone mason dying from silicosis and
corpulmonale: lungs (2 g/kg dry weight); hilar lymph nodes (3.2 g/kg
dry weight); spleen (520 mg/kg dry weight) and liver (130 mg/kg dry
weight). Excluding particulate aluminium, it is likely that of all the
extrapulmonary tissues only the skeleton contains significant levels
of aluminium. This is the conclusion of Priest (1993), who based his
conclusion on a theoretical consideration of ion size of Al3+,
supported by measurements of the distribution of the sources of 26Al
gamma-emission in a human volunteer at earlier times after injection
(using the same techniques gallium uptake in the liver was estimated
to be about 30%). The possibility that at later times liver levels
build up would be consistent with rather high levels for this tissue
(Flendrig et al., 1976) and with the observation of Day et al. (1994)
that some aluminium is taken up by red blood cells - considering the
role of the liver in the breakdown of old red blood cells.
(b) Concentrations after aluminium exposure
Data on the tissue burden of aluminium-exposed humans are mostly
derived from patients with chronic uraemia as well from occupationally
exposed workers. In these patients, the aluminium intake may come from
more than one source and is difficult to follow.
More than 40 years after exposure, the cerebrospinal fluid of an
aluminium powder worker exposed heavily during 1944-1946 contained 250
�g aluminium/litre. Aluminium-associated pulmonary fibrosis
(aluminosis) was diagnosed in 1946 (Sj�gren et al., 1994a,b). The
normal value for cerebrospinal fluid is less than 10 �g/litre.
In two heavily exposed aluminium welders, bone aluminium
concentrations were 18 mg/kg dry weight and 29 mg/kg dry weight,
clearly above the reference range for the investigators (0.6-5 mg/kg
dry weight) (Elinder et al., 1991). In a worker producing fine
aluminium powder, who developed encephalopathy and pulmonary fibrosis
after 13.5 years of work, the aluminium concentration in lung and
brain was increased 20-fold and in the liver 122-fold (McLaughlin et
al., 1962).
In patients with impaired renal function, a wide range of tissue
aluminium concentrations have been reported (bone, 8.4-281 mg/kg;
muscle, 2-23.6 mg/kg; brain, 2.2-25 mg/kg; Table 16). This wide range
reflects the variable exposure to aluminium-containing phosphate-
binding pharmaceutical agents and the duration of haemodialysis. The
increased tissue concentrations are associated with the clinical
syndromes of encephalopathy, osteomalacia and microcytic anaemia.
Channon et al.
(1988) reported a positive correlation between the dose of aluminium-
containing phosphate-binding pharmaceutical agent prescribed and bone
aluminium content, but no correlation with the serum aluminium
concentration for the 6 months preceding bone biopsy.
In a worker exposed to aluminium dust and powder in a ball-mill
room for 13.5 years, the aluminium concentration in lung and brain was
increased 20 times and that of the liver 122 times over the normal
value (Table 16). Teraoka (1981) published data indicating that
aluminium concentrations were increased in the lungs (2000 mg/kg dry
weight), hilar lymph nodes (3200 mg/kg dry weight), spleen (520 mg/kg
dry weight), and liver (130 mg/kg dry weight) in a stone-mason dying
from silicosis. The average organ concentrations in normal unexposed
control males were 230, 2000, 22 and 19 mg/kg, respectively
(Table 16).
6.3 Elimination and excretion
6.3.1 Animal studies
In animals aluminium is eliminated effectively by urine.
Following single intravenous doses of up to 100 �g/kg body weight in
rats, aluminium was quantitatively recovered from urine (Wilhelm et
al., 1992). It is difficult to obtain an accurate half-life for low
oral doses, since the rate of aluminium absorption is low. Data on
plasma half-life and on renal clearance have been mainly obtained from
parenteral administration generally using high doses of aluminium
(Wilhelm et al., 1990). It seems that at doses comparable with human
exposure the plasma half-life is less than 1 h.
Based on stop-flow experiments conducted in pigs, Monteagudo et
al. (1988) concluded that aluminium excretion occurs in the distal
tubule of the kidney and is situated close to the sites of maximal
calcium and sodium ion reabsorption.
6.3.2 Human studies
6.3.2.1 Urinary excretion
The biokinetics of aluminium in man has been evaluated by Priest
and his co-workers (Priest et al., 1991, 1995b, 1996; Talbot et al.,
1995) using 26Al injected into human volunteers. These authors
described the pattern of urinary excretion of the isotope following
its intravenous injection as citrate, the effect of excretion on body
retention and the relationship between aluminium levels in blood and
in urine/faeces. In a first study using a single volunteer (Priest et
al., 1991, 1995b, 1996), the authors confirmed that aluminium is
overwhelmingly excreted by the urinary route and that most blood
aluminium is cleared to excretion. More than half of the 26Al had
left the blood within 15 min and the decline continued, leaving < 1%
in the blood after 2 days. Total excretion up to 13 days was 83%
(urine) and 1.8% (faeces), leaving 15% in the body. After 4 months
more than 90% was excreted. With increasing time the rate of urinary
excretion, as indicated by the fraction of retained aluminium (Rt),
decreased with time (t) according to the power function:
Rt = 35.4 t-0.32(t > 1)
At 1178 days after injection about 4% remained in the body; an
estimated 94% had been excreted by the urinary route and 2% in the
faeces. Faecal excretion most likely represented aluminium that had
entered the gastrointestinal tract in bile. The power function
calculated for the fraction of aluminium excreted by the urinary route
(Ut) at time (t) after intake was:
Ut = 0.47 t-1.36
The excretory clearance rate (ECR) from whole blood (in kg/day)
during the first two weeks after injection was expressed as:
ECR = 42 t-0.14
In a second study (Talbot et al., 1995) of shorter duration, but
using six male volunteers, inter-subject variability was examined.
This showed significant inter-subject variation in the pattern of
aluminium excretion. For example, after 5 days an average of
71.8% � 7.3% (SD) of the injected activity had been excreted in urine
(range 62.4-82.9%). Of this total, an average of 59.1% was excreted in
the first day, 7.2% in day two, 2.6% in day three, 1.7% in day four
and 1.1% in the fifth day. In the same period an average of 1.2% was
excreted in the faeces. With respect to blood clearance to urine, the
study showed a gradual decrease in the fraction of blood aluminium
excreted per unit time, indicating a changing speciation of aluminium
in the blood. Overall, the results of the study were wholly consistent
with those generated in the single volunteer study, with the first
volunteer showing aluminium biokinetics in the middle of the range of
results generated by the multi-volunteer study.
Sj�gren et al. (1985) studied previously unexposed volunteers and
individuals previously exposed to welding fumes containing aluminium
at their workplaces for different periods of time. All subjects were
exposed to welding fumes containing aluminium during a working day for
about 8 h. In previously unexposed individuals urinary aluminium
concentration after exposure was increased but decreased to pre-
exposure levels after a few days. The half-life of the first phase of
excretion was approximately 8 h. In welders exposed for less than 2
years in the aluminium concentration decreased during the weekend, the
half-life being about 9 days. In welders exposed for more than 10
years urinary concentration did not change despite cessation of
exposure. In these welders, the urine half-life was more than 6 months
(Sj�gren et al., 1988).
Elinder et al. (1991) analysed urinary, blood and bone aluminium
content in two aluminium welders exposed to welding fumes for more
than 20 years. The urinary values varied from 107 to 351 �g
aluminium/litre. The daily urinary aluminium elimination was estimated
to be 0.06% of the estimated body burden (on the basis of bone
aluminium content), which corresponds to a half-life of about 3 years.
Ljunggren et al. (1991) studied workers occupationally exposed to
aluminium flake powder after acute exposure and after periods of non-
exposure of varying length. The calculated half-life during 4-5 weeks
of exposure-free vacations was 6.8 weeks. In workers retired (for 6
months to 14 years) after exposure periods of 9 to 50 years, the
calculated half-life varied between 0.7 and 7.9 years, depending on
the length of the exposure-free period.
6.3.2.2 Biliary excretion
There is insufficient information to comment on biliary excretion
of aluminium in humans.
6.4 Biological indices of exposure, body burden and organ
concentration
Aluminium levels in blood and urine have been used to determine
exposure levels. However, this relation is relatively weak. Blood
aluminium levels are a poor indicator of tissue stores, rather
indicating acute intake or rate of tissue store mobilization. In
patients with impaired renal function, blood and urine levels are poor
indicators of tissue levels.
High occupational exposure levels seem to be reflected better by
urine levels than by blood levels, but the quantitative relation is
not well established. Balance studies by current methodologies are not
possible. Increased blood and urine concentrations have been observed
in several groups of occupationally exposed workers and a quantitative
relationship between the amount inhaled and the urinary aluminium
concentration has been suggested (Sj�gren et al., 1983).
A linear relationship has been observed between the air levels of
aluminium exposure, the number of exposure years and the post-shift
urine level in aluminium welders (Sj�gren et al., 1988):
UAl = 41.7 � AAl + 6.7 � E � 4.6
where UAl = urine aluminium level (�g/litre)
AAl = air aluminium level (mg/m3)
E = years of exposure
Owing to intra-individual variation, this equation can only be
used for groups, not individuals. There have been no adequate
investigations of the relation between air levels of exposure and
urine or blood levels in workers apart from welders.
No information exists regarding the use of biological exposure
indices in general populations.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity is influenced by the solubility and
bioavailability of the aluminium compounds administered. Aluminium
compounds are only poorly absorbed after exposure by the
gastrointestinal, respiratory and dermal routes. The acute toxicity of
aluminium metal and aluminium compounds is relatively low. No LC50
has been identified in inhalation studies.
Lethal doses of soluble aluminium compounds, such as AlCl3,
Al(NO3)3 and Al2(SO4)3, have been determined by the oral or
parenteral routes. Some LD50 values are given in Table 18.
Table 18. LD50 values for various aluminium compounds
Compound Species Route of LD50 Reference
administration (mg Al/kg b.w.)
AlCl3 mouse (male oral (gavage) 770 Ondreicka
Al2(SO4)3 Dobr� Voda) 980 et al. (1966)
Al(NO3)3 mouse (Swiss; oral (gavage) 286 Llobet et al.
20/sex) i.p. 133 (1987)
AlCl3 oral (gavage) 222
i.p. 105
Al2(SO4)3 oral (gavage) > 730
i.p. 40
AlBr3 oral (gavage) 164
i.p. 108
Al(NO3)3 rat (Sprague- oral (gavage) 261 Llobet et al.
Dawley, 20/sex) i.p. 65 (1987)
AlCl3 oral (gavage) 370
i.p. 81
Al2(SO4)3 oral (gavage) > 730
i.p. 25
AlBr3 oral (gavage) 162
i.p. 82
7.2 Short- and long-term exposure
7.2.1 Oral administration
Available data on the toxicity of aluminium compounds following
repeated oral administration are presented in Table 19. Few of
the studies reviewed are adequate to serve as a basis for the
determination of effect levels, since many were designed to address
principally aspects such as effect of citrate and decrements in kidney
function on the body burden of aluminium; a very limited range of
toxicological end-points was also examined in these studies
(Ecelbarger & Greger, 1991; Greger & Powers, 1992).
There have been several repeated dose toxicity studies in which a
wide range of end-points, including clinical signs, food and water
consumption, growth, haematological and serum analyses, tissue and
plasma concentrations of aluminium, histopathology, has been examined
following oral exposure to various aluminium compounds. There were no
treatment-related effects in rats fed up to 288 mg Al/kg body weight
per day as sodium aluminium phosphate or 302 mg Al/kg body weight per
day as aluminium hydroxide in the diet for 28 days (Hicks et al.,
1987). In a subchronic study in which aluminium nitrate was
administered in drinking-water to rats, the only effect observed was a
significant decrease in body weight gain associated with a decrease in
food consumption at 261 mg Al/kg body weight per day. (NOEL = 52 mg
Al/kg body weight per day) (Domingo et al., 1987b).
When small groups of Beagle dogs were given sodium aluminium
phosphate for 6 months in the diet, there were no treatment-related
effects except for a decrease in food consumption not associated with
a decrease in body weight (NOEL = approximately 70 mg Al/kg body
weight) (Katz et al., 1984). Similarly, in small groups of Beagle dogs
administered up to 80 mg Al/kg body weight per day as sodium aluminium
phosphate for 26 weeks, the only treatment-related effect was a sharp,
transient decrease in food consumption and concomitant decrease in
body weight in males (LOEL = 75 to 80 mg Al/kg body weight per day;
Pettersen et al., 1990).
Table 19. Toxicity of aluminium compounds after repeated oral administration
Protocol descriptiona End-points examined Results Reference
5-10 male rats (Weizman strain) clinical signs, histopathology periorbital bleeding in 3 of 5 animals at 350 Berlyne
per group; (appears to have been mg/kg body weight per day Al2(SO4); tissue and et al.
Al2(SO4)3 (200 or 350 mg Al/kg limited to animals that serum levels highest in nephrectomized animals; (1972)
body weight per day in drinking died during study), tissue clinical signs of toxicity and death in all groups
water), AlC13 (250 mg Al/kg concentrations of nephrectomized animals; comments: protocol and
body weight per day in drinking results poorly documented; inadequate for
water), Al(OH3) (150 mg Al/kg establishment of effect levels
body weight per day by gavage)
for an unspecified period; groups
of animals that were 5/6
nephrectomized similarly exposed
Controls: 56 male Sprague-Dawley aluminium concentrations in five of these measures (concentrations in tibia, Greger &
rats 10.5 mg Al/kg exposed groups tibia, liver, kidney and serum; liver, and serum and urinary excretion with and Powers
16 animals, ingesting: aluminium serum aluminium concentrations without DFO treatment); were highly correlated (1992)
hydroxide, 1079 mg Al/kg diet after DFO; urinary with oral exposure; changes induced by DFO were
(29 days) or 1012 mg Al/kg diet aluminium excretion with and very small; ingestion of citrate had small but
plus 4% citrate (29 days), 2688 without DFO treatment; body significant effects on aluminium retention; rats
mg/kg and 4% citrate (12 or 29 and organ weight gain and fed citrate weighed less (not associated with
days); 24 h prior to sacrifice, haematological status differences in intake of feed) and had significantly
all animals injected i.p. with enlarged kidneys and livers and significantly smaller
desferrioxamine (DFO) or buffer tibias; haematocrits were inversely correlated to
tissue concentrations of aluminium - more evident
with oral than parenteral exposure; hepatic iron
was elevated with the citrate-containing diets but
was only weakly correlated with hepatic aluminium
concentrations;
Table 19. (Con't)
Protocol descriptiona End-points examined Results Reference
comments: comparisons of aluminium exposure in Greger &
tibias and sera of rats exposed parenterally and Powers
orally indicated that 0.01 to 0.04% of dietary (1992)
aluminium was absorbed; inadequate for establishment
of effect levels owing to limited range of end-points
examined; primarily an investigation to monitor
aluminium body burdens
Administration to groups of 6 rats tissue concentrations of ingestion of citrate increased retention of aluminium Ecelbarger
exposed in a 2x2x2x2 factorial aluminium; body and in bone of rats fed 1000 mg Al/kg diet and increased & Greger
design of diets containing 13 or organ weight changes apparent absorption of zinc; when dietary calcium (1991)
1112 mg Al/kg as aluminium intake was increased from 67 to 250 mmol/kg diet,
hydroxide, citrate (0 or 5.2 mmol/kg aluminium concentrations in bone were reduced without
diet) and calcium (2.7 or 10.0 g/kg a change in growth of rats; reduction in kidney
diet) for 30 days; groups of 6 rats function (by removal of one kidney), which was
exposed in a 4x2 factorial design insufficient to alter growth, increased aluminium
to 14 or 904 mg Al/kg diet and one retention in bone by 13% in rats fed aluminium; rats
of 4 levels of citrate (0, 10, 21 or fed aluminium retained only 0.01 to 0.5% as much as
31 mmol/kg diet) for 28 days; those injected; comments:authors concluded that
groups of 7 rats exposed in 2x2x2 tissue concentrations and, presumably, toxicity can
factorial design to 9 or 1044 mg be altered by moderate changes in diet and kidney
Al/kg diet and citrate (0 or function even though overall retention of orally
21 mmole/kg diet) sham-operated administered aluminium is low, inadequate for
or with one kidney removed for establishment of effect levels owing to limited
28 days range of end-points examined; designed primarily to
investigate effect of dietary citrate, calcium
and decreases in kidney function on absorption
of aluminium
Table 19. (Con't)
Protocol descriptiona End-points examined Results Reference
Groups of 10 female Sprague- clinical signs, food and water no effects on any end-points examined except for G�mez
Dawley receiving 1, 26, 52 or consumption, growth, mild histopathological changes in the spleen and et al.
104 mg Al/kg body weight per haematological and serum liver of high dose group (hyperaemia in the red pulp (1986)
day as Al(NO3)3 in drinking analyses, tissue and plasma of the spleen and liver; periportal lymphomonocytic
water for 28 days concentrations of aluminium, infiltrate in the liver); dose-dependent accumulation
histopathology of aluminium in spleen, heart and gastrointestinal
tract; comments: well-conducted repeated dose
toxicity in which a wide range of end-points was
examined, though short term; NOEL = 52 mg Al/kg
body weight per day; LOEL = 104 mg Al/kg body
weight per day (aluminium nitrate)
Groups of female Sprague-Dawley clinical signs, food and water significant decrease in body weight gain at highest Domingo
rats receiving 0, 26, 52 or 261 mg consumption, organ and body dose associated with decrease in food consumption; et al.
Al/kg body weight per day as weights, haematological and no dose-dependent accumulation of aluminium in (1987b)
Al(NO3)3 in drinking-water for serum analyses, tissue and tissues; comments: well-conducted repeated dose
100 days plasma concentrations of toxicity in which a wide range of end-points;
aluminium, histopathology LOEL = 261 mg Al/kg body weight per day; NOEL =
52 mg Al/kg body weight per day for subchronic
period of exposure
Groups of Beagle dogs (4/sex) clinical signs, food and water statistically significant decreases in food Katz et
administered 0, 0.3, 1.0 or 3.0% consumption, organ and body consumption in females but no associated decrease al.
sodium aluminium phosphate in weights, haematological and in body weight; no other treatment-related effects; (1984)
the diet for 6 months (118, 317 serum analyses, urinalysis, comments: range of end-points examined; subchronic
and 1034 mg/kg body weight per ophthalmological examinations, period of exposure for dogs; small group sizes;
day in males and 112, 361 and tissue and plasma NOEL = 1087 mg/kg body weight per day (approximately
1087 mg/kg body weight per day concentrations of aluminium, 70 mg Al/kg body weight per day)
in females histopathology
Table 19. (Con't)
Protocol descriptiona End-points examined Results Reference
Groups of Beagle dogs (4/sex) clinical signs, food and water sharp, transient decrease in food consumption and Pettersen
administered 0, 10, 22 to 27 or consumption, organ and body concomitant decrease in body weight in high-dose et al.
75 to 80 mg Al/kg body weight weights, haematological and males; in same group, decrease in testes weight and (1990)
per day as sodium aluminium serum analyses, urinalysis, histopathological changes in the liver and kidney
phosphate in the diet for ophthalmological examinations, considered to be secondary to decreased food
26 weeks tissue and plasma consumption; slight increase in concentration of
concentrations of aluminium, aluminium in the brain in high-dose females but not
histopathology males; comments: wide range of end-points examined;
subchronic period of exposure for dogs; small group
sizes; minimal toxicity at the highest dose;
LOEL = 75 to 80 mg Al/kg body weight per day
Groups of 25 male Sprague-Dawley
rats fed control diets or
30 000 mg/kg
KASAL I (6% aluminium-sodium clinical signs, food and water no treatment-related effects or significant Hicks et
aluminium phosphate), 7000 or consumption, organ and body deposition of aluminium in bone; comments: wide al.
30 000 mg/kg KASAL II (13% weights, haematology and range of end-points examined; short-term exposure; (1987)
aluminium-sodium aluminium clinical chemistry urinalysis, no effects at 141 mg Al/kg body weight per day
phosphate) or 14 470 mg/kg ophthalmological examinations, KASAL I; NOEL for KASAL II = 288 mg Al/kg body
aluminium hydroxide for 28 days concentrations of weight per day; no effects at 302 mg Al/kg body
(5, 141, 67, 288, 302 mg Al/kg aluminium in the femur, weight per day aluminium hydroxide
body weight per day, respectively) histopathology
Table 19. (Con't)
Protocol descriptiona End-points examined Results Reference
Groups of 15 albino male rats histopathological examination dose-related cytotoxic effect in the liver - Roy et
administered aluminium sulfate of heart, liver, kidney, cytoplasmic degeneration at 17 to 29 mg Al/kg body al.
(0, 17, 22, 29, 43, 86 and brain, testes, stomach and weight with multifocal degeneration and fibrous (1991b)
172 mg Al/kg body weight) and femur tissue proliferation at higher doses. Dose-related
potassium aluminium sulfate effects in the kidney at 17 mg Al/kg body weight
(43 mg Al/kg body weight, 29 mg per day as aluminium sulfate, slight swelling of
Al/kg body weight) in deionized the tubules. With increased dose, increased swelling
water for 21 days; 5 rats killed and degeneration of the cortical tubules.
weekly Degeneration of the nerve cell at 29 and 43 mg Al/kg
body weight per day aluminium sulfate and
potassium aluminium sulfate, respectively, which
was more severe at higher doses. Some evidence of
spermatological cell decrease at doses of 43 mg Al/kg
body weight per day and above. Multifocal degeneration
and decalcification at 43 mg Al/kg body weight
per day and above for both salts, which increased with
increasing dose; degeneration of calcified bone and
irregularity of osteoblasts in animals exposed to 86
and 171 mg Al/kg body weight as aluminium sulfate.
Hyperplasia and ulceration of stomach at highest doses.
Comments: difficult to verify reported effect levels
based on limited information presented in paper.
a Strain, number of animals/group and vehicle specified, where available; doses reported as mg/kg body weight, unless specified
In a study that was not well reported, mild histopathological
effects on the kidney and liver of rats, which increased in severity
with dose, were reported at doses as low as 17.2 mg Al/kg body weight
(as aluminium sulfate) administered by gavage for 21 days (Roy et al.,
1991b). Data presented in the report were inadequate to verify the
reported effect levels.
7.2.2 Inhalation exposure
Many inhalation/intra-tracheal instillation studies have
been conducted using aluminium compounds, including chloride,
chlorohydrate, oxyhydrate and oxides (Stacy et al., 1959; Corrin,
1963; Christie et al., 1963; Gross et al., 1973; Drew et al., 1974;
Steinhagen et al., 1978; Stone et al., 1979; Finelli et al., 1981;
Thompson et al., 1986). However, in the case of the inhalation
studies, little data is available concerning exposure conditions and
the size of the ambient aerosol, and some studies were of relatively
short duration compared with the life-span of the animal employed.
Consequently, although no toxic effects were reported in nearly all
cases, it is not possible to assess how much, if any, of the compound
was deposited in the lungs of the experimental animal, and the time-
span of the experiment may have been too short to demonstrate delayed
effects. The only inhalation study that demonstrated an effect was
that of Finelli et al. (1981) who described increased lung size. Where
aluminium oxide particles were administered by intra-tracheal
instillation, fibrosis has been consistently reported (Stacy et al.,
1959; Corrin, 1963). Dinman (1988) described this disease as "alumina-
related pulmonary disease". However, such fibrosis is a common
consequence of the inhalation of many particles, including silica and
coal; it is unlikely to be related to aluminium per se, but rather
to the physical properties of the particle inhaled.
7.2.3 Parenteral administration
Aluminium compounds were found to possess an increased toxicity
when administered parenterally rather than orally. The effect depends
on the dose, the aluminium compound used and the particular animal
model. It can vary from death to behavioural alteration (loss of
memory), loss of weight or minor changes in aluminium accumulation in
bone. A LOAEL of approximately 1 mg/kg body weight per day can be
obtained by this route for osteomalacia or for deterioration of renal
function (Chan et al., 1983; Henry et al., 1984; Quarles et al., 1985;
Br�unlich et al., 1986). Partially nephrectomized animals exhibited
greater susceptibility to aluminium (Ittel et al., 1992).
7.3 Reproductive and developmental toxicity
7.3.1 Reproductive effects
The limited number of studies are not able to provide adequate
information on reproductive toxicity (Domingo, 1995). Of these, few
provide direct and complete evaluations of reproduction.
Domingo et al. (1987a) administered aluminium nitrate at 0, 13,
26 or 52 mg Al/kg body weight per day by intubation to male rats for
60 days prior to mating and to virgin females treated for 14 days
prior to mating. The same doses were administered by gavage to
pregnant animals from 14 days gestation to 21 days of lactation in a
separate study (Domingo et al., 1987c). Domingo et al. (1987a)
reported no reproductive effects on fertility (number of litters
produced), litter size, or intrauterine or postnatal offspring
mortality. Numbers of corpus lutea on day 13 of gestation were
significantly lower at 52 mg Al/kg body weight per day. However a
dose-dependant delay in the growth of the pups was observed in all
treatment groups; female offspring were affected at 13 mg Al/kg body
weight per day and males at 26 and 52 mg Al/kg body weight per day.
Because of the design of the study by Domingo et al. (1987a) it is not
clear whether the postnatal growth effects in offspring represented
general toxicity to male or female parents, or represented specific
effects on reproduction or development. However, the reported LOAEL
for adverse effects in females in this study was 13 mg Al/kg body
weight per day.
Dosing of pregnant animals from 14 days gestation to 21 days
lactation with 13, 26 or 52 mg Al/kg body weight per day did not
produce overt fetotoxicity (Domingo et al., 1987c), but growth of
offspring was significantly delayed (body weight, body length and tail
length) from birth to weaning.
7.3.2 Developmental effects
Details of the study designs of oral or gavage dosing studies of
developmental toxicity are given in Table 20. Aluminium chloride
administered i.p. to rats (Benett et al., 1975) or i.v. to mice (Wide,
1984) during embryogenesis produced a syndrome characterized by
delayed and incomplete ossification of skull and vertebrae, skeletal
variations and malformations, internal haemorrhage and reduced fetal
growth. Abnormal digits were also noted in rats. Administration of
aluminium chloride in the diet with accompanying parathyroid hormone
(PTH) injection (McCormack et al., 1979) also produced reduced
skeletal ossification and increased incidence of skeletal variations.
Maternal toxicity in the study of Benett et al. (1975) included
reduced weight gain, hepatic granulomas and necrosis and maternal
death at the highest dose levels (100 and 200 mg Al/kg per day).
Resorption and embryolethality were seen primarily at highly
maternally toxic doses (200 mg Al/kg per day). Maternal monitoring and
reduced gestational weight gain were not seen at the LOAEL for
developmental toxicity (75 mg Al/kg per day; day 9-13 of gestation).
At this dose mean fetal weight and crown rump were reduced, and the
number of resorptions increased. The use of i.p. administration makes
it difficult to interpret these results with regards to human health
effects.
The severity of developmental aluminium toxicity by the oral
route is highly dependent on the form of aluminium and the presence of
organic chelators that influence bioavailability, as demonstrated in a
series of studies by Domingo (1995) using gavage administration during
embryogenesis. Aluminium nitrate (nonahydrate) produced developmental
effects in rats (Paternain et al., 1988) similar to the effects seen
after i.p. injections (Benett et al., 1975), including skeletal
variations, poor ossification, haemorrhage, oligodactyly and some soft
tissue malformations. Aluminium hydroxide did not produce either
maternal or developmental toxicity when it was administered by gavage
during embryogenesis to rats (G�mez et al., 1990) or mice (Domingo et
al., 1989). When aluminium hydroxide was administered with ascorbate
(Colomina et al., 1994), no maternal or developmental toxicity was
seen in mice in spite of elevated maternal tissue concentrations of
aluminium, whereas aluminium hydroxide given with citrate produced
maternal and fetal toxicity in rats (G�mez et al., 1991). Aluminium
hydroxide given with lactate was not toxic, but aluminium lactate
administration produced developmental toxicity, in mice including poor
ossification, skeletal variations and cleft palate (Colomina et al.,
1992).
In summary, developmental toxicology syndromes described above
commonly included growth retardation, such as lower fetal weights and
length (Bennet et al., 1975; Paternain et al., 1988; G�mez et al.,
1990; Colomina et al., 1992). A study using s.c. administration of
aluminium lactate did not demonstrate any developmental effects other
than lower fetal length (Golub et al., 1987). Postnatal growth
retardation has also been demonstrated in rats exposed in late
gestation to aluminium nitrate (Domingo, 1987c) and in rabbits treated
postnatally with aluminium lactate s.c. (Yokel, 1984). Reduced bone
formation demonstrated when postnatal aluminium lactate was
administered subcutaneously in rabbits (Yokel, 1987) supports the
importance of bone as a target organ for developmental effects of
aluminium.
Table 20. Developmental toxicity after oral administration of aluminium saltsa
Species Route of application Compound Dose Duration Reference
Mouse (Swiss, oral (gavage) Al(OH)3 66.5, 133, 266 mg/kg gest. day 6-15 Domingo et al.
20/group) b.w./day (1989)
Mouse (BALB/c, oral (gavage) AlCl3 oral: 200, 300 mg/kg gest. day 7-16 Cranmer et al.
6-7 mice/group) b.w./day (1986)
Mouse (Swiss oral (feed) Al lactate 500, 1000 mg Al/kg diet gest. day 0 to day Golub et al.
Webster, 15/group) 21 p.p. (1987)
Mouse (16 Swiss oral (feed) Al lactate 25, 500, 1000 mg Al/kg diet gest. day 0, until Donald et al.
Webster) weaning (1989)
Mouse (Swiss albino oral (gavage) Al(OH)3 57.5 mg/kg b.w./day gest. day 6-15 Colomina et
CD-1, 10-13/group) Al lactate 166 mg/kg/day al. (1992)
Al(OH)3 + lactic acid 627 mg/kg/day
Mouse (Swiss oral (feed) Al lactate 25, 1000 mg/kg diet/day gest. day 0 - day Golub et al.
Webster) 20 p.p. (1992)
Mouse (CBA) oral (water) Al2(SO4)3 750 mg/litre gest. day 10-17 Clayton et al.
(1992)
Rat (Sprague-Dawley) oral (feed) AlCl3 50 mg/kg b.w. gest. day 6-19 McCormack
et al. (1979)
Rat (Wistar, 14/group) oral (feed) Al lactate 100, 200, 300 mg Al/kg b.w. gest. day 8 to Bernuzzi et al.
parturition (1986, 1989a,b)
Rat (Sprague-Dawley) oral (gavage) Al(NO3)3 180, 360, 720 mg/kg day gest. day 14-21 Domingo et al.
(1987a)
Table 20. (Con't)
Species Route of application Compound Dose Duration Reference
Rat (Sprague-Dawley, oral (gavage) Al(NO3)3 180, 360, 720 mg/kg b.w. gest. day 6-14 Paternain et
10/group) al. (1988)
Rat (Sprague-Dawley, oral (gavage) Al(OH)3 384 mg/kg/day gest. day 6-15 G�mez et al.
15-19/group) Al citrate 1064 mg/kg/day (1991)
Al(OH)3 + citric acid
Rat (Wistar, oral (gavage) Al(OH)3 192, 384, 768 mg/kg/day gest. day 6-15 G�mez et al.
18-19/group) (1990)
Rat (Wistar, oral (feed) Al lactate 400 mg Al/kg diet gest. day 1-7 Muller et al.
6-9/group) gest. day 1-14 (1990)
gest. day 1-20
a b.w. = body weight; gest. = gestational; p.p. = postpartum
Effects of aluminium exposures on brain development have also
been studied in mice (Donald et al., 1989; Golub et al., 1992, 1994,
1995; Clayton et al., 1992) and rats (Bernuzzi et al., 1986, 1989a,b;
Muller et al., 1990; Cherroret et al., 1992) by the oral route, and in
rabbits (Yokel, 1984, 1985, 1987) using subcutaneous injections.
Effects recorded in immature animals in more than one study in rats
and mice included impaired performance of reflexes and simple
behaviours (e.g., righting reflex, grasping, negative geotaxis, rod
climbing). Other effects included footsplay and temperature
sensitivity (tail withdrawal); (Donald et al., 1989; Golub et al.,
1992) and auditory startle (Golub et al., 1994). Postnatal mortality
and growth were also affected at the higher doses in only some of
these studies. Studies of cognitive parameters in immature animals are
limited to evaluation of classical conditioning of the nictating
membrane response in rabbits. No effect of aluminium was seen after
postnatal subcutaneous injection of aluminium lactate (Yokel, 1987);
both enhancement and impairment of conditioning were seen after
exposure during gestation to aluminium lactate, depending on the dose
(Yokel, 1985).
Adult rats and mice have also been evaluated for brain function
after developmental exposures. Reduced grip strength and startle
responsiveness were found to persist up to 150 days of age in mice
(Golub et al., 1995). No effect was found on a light avoidance task in
rats after gestational (Bernuzzi et al., 1989b) or postnatal exposure
(Cherroret et al., 1992). Radial maze learning/performance was also
unaffected by postnatal exposure (Cherroret et al., 1992). No effects
on delayed alternation or discrimination reversals were recorded in
mice after dietary exposure during gestation and lactation (Golub et
al., 1995).
The lowest-observed-adverse-effect level (LOAEL) for
developmental effects after oral dosing was 13 mg Al/kg body weight
per day by oral gavage of aluminium nitrate (Paternian et al., 1988).
A dose-response relationship was noted with the highest dose of this
extremely soluble aluminium salts (52 mg Al/kg body weight)
representing 1/5 the LD50 (see Table 18). At 13 mg Al/kg per day,
decreased ossification of skull bones, increased incidence of
vertebral and sternebrae, and reduced fetal weight and tail length
were reported with higher incidence of these effects at the higher
doses (26 and 52 mg Al/kg body weight). Maternal toxicity (reduced
weight gain during pregnancy) was reported to occur in a dose-
dependent manner. No developmental toxicity was noted using much
higher doses of aluminium hydroxide (266 mg Al/kg per day) in a
similarly designed study (G�mez et al., 1990).
After administration of aluminium lactate in the diet of mice and
rats a LOAEL of 100 mg Al/kg body weight per day was reported (Donald
et al., 1989; Bernuzzi et al., 1989a,b).
The effect reported at 100 mg Al/kg per day by Bernuzzi et al.
(1989a,b) in rats was impaired grip strength in the 6-day-old
offspring of dams fed aluminium lactate in diet throughout gestation.
Dose response was indicated in this study; at higher doses (200 and
400 mg Al/kg per day) impaired grip strength was reported along with
impaired righting reflex and locomotor coordination. No effects on
maternal or offspring weight were reported at 100 mg Al/kg although
they occurred at the higher doses. Litters were not culled to a
standard size at birth in this study. The litter was used as the unit
of statistical analysis to avoid litter effect.
The effects reported at 100 mg Al/kg per day by Donald et al.
(1989) in mice were increased landing foot splay, increased hindlimb
grip strength and decreased temperature sensitivity in 21-day-old
offspring of mice fed aluminium lactate in the diet throughout
gestation and lactation. There was no effect on negative geotaxis or
startle reflexes in the offspring. Dose response was not indicated in
this study; similar effects were reported at a higher dose (200 mg
Al/kg). No effects on maternal or offspring weights were found at
either dose. It is not stated whether litters were culled to a
standard number at birth. To address litter effects, litter was nested
under treatment group in the statistical analysis. In this study,
daily aluminium intake was estimated based on food intake at the
beginning of pregnancy.
7.4 Mutagenicity and related end-points
7.4.1 Interactions with DNA
A number of observations indicate that aluminium is able to form
complexes with DNA and can cross-link chromosomal proteins and DNA. In
thermal denaturation, circular dichroism and fluorescence studies,
Karlik et al. (1980) found that aluminium had a stabilizing effect
upon the DNA double helix at a pH > 6, while at lower pH levels,
binding of aluminium de-stabilized the DNA double helix. A recent
investigation of NMR spectra and circular dichroism of DNA-aluminium
complexes indicated that Al3+ binds to the phosphate oxygen while
hydroxylated aluminium-species probably prefer other sites such as DNA
bases (Rao & Divakar, 1993).
Cross-linking of various cytoplasmic proteins to DNA was
investigated in live Novikoff ascites hepatoma cells exposed to
aluminium in vitro (Wedrychowski et al., 1986). Cross-linking agents
frequently produce clastogenic effects, owing to conformational
distortions that prohibit proper replication of the DNA. More recently
it was shown that AlF4- stimulates the glycation of the histone H1
in the proximity of its nucleotide-binding site, thus interfering with
nucleoside triphosphate hydrolysis by H1 and with nucleotide
modulation of H1 DNA binding (Tarkka et al., 1993).
In addition, it has been shown that micro-molar levels of
aluminium reduce 3H-thymidine incorporation in a transformed cell
line (UMR 106-01) by impeding the cell cycle progression (Blair et
al., 1989). More specifically, aluminium was shown to inhibit the
ADP-ribosylation, a mechanism important for DNA repair, in vivo and
in vitro (Crapper McLachlan et al., 1983).
7.4.2 Mutations
The rec-assay using Bacillus subtilis strains failed to show
mutagenic activity for Al2O3, AlCl3 or Al2(SO4)3 at
concentrations of 1-10 mM (Nishioka, 1975; Kada et al., 1980;
Kanematsu et al., 1980; L�onard & Gerber, 1988; Bhamra & Costa,
1992). No reverse mutations were observed in the Ames test using
Salmonella typhimurium strain TA102 with AlCl3 (concentration
range 10-100 nM per plate; Marzin & Phi, 1985). No morphological
transformations were seen in Syrian hamster embryo cells after
application of aluminium salts (no further specification) (Di Paolo &
Casto, 1979). No induction of forward mutations were observed at the
thymidine kinase locus in L5178Y mouse lymphoma assay with AlCl3 when
tested at concentrations up to 625 �g AlCl3/ml (Oberly et al., 1982).
7.4.3 Chromosomal effects
A significant increase of chromatid-type aberrations (including
gaps, breaks, translocation and ring formations), with non-random
distribution over the chromosome complement, was found in bone marrow
cells from mice that were dosed interperitoneally with AlCl3 (Manna &
Das, 1972). Prolonged treatment of rats with Al2(SO4)3 or
KAl(SO4)2 caused a dose-dependent inhibition of dividing cells (bone
marrow) and an increase in chromosomal aberrations (Roy et al.,
1991a). Chromosomal aberrations were also induced in peritoneal cells
from rats, mice and Chinese hamsters (Bhamra & Costa, 1992) as well as
in human leukocyte cultures (Roy et al., 1990). Aluminium caused a
concentration-dependent bimodal change in the number of sister
chromatid exchange in cultured human lymphocytes and increased the
unscheduled DNA synthesis in cultured human astrocytes (De Boni et
al., 1980).
7.5 Carcinogenicity
Based on limited early studies, there is little indication that
aluminium is carcinogenic (Leonard & Gerber, 1988; Bhamra & Costa,
1992). Some studies indicated that inhalation of aluminium-containing
fibres and particles may induce carcinomas in the lung. However, in
these cases it is likely that the toxicity reflects the physical
properties of the particles/fibres (3.5 �m median diameter).
Similarly, aluminium implanted subcutaneously has induced soft tissue
carcinomas at the site of implantation, but in these cases also the
effects are probably related to a chronic foreign body reaction rather
than to the aluminium ion itself (Stanton, 1974; Pigott & Ishmael,
1981; Pigott et al., 1981; Krueger et al., 1984).
7.6 Neurotoxicity
Considerable evidence indicates that aluminium is neurotoxic to
experimental animals, but species variation exists. In susceptible
animals, the toxicity is characterized by progressive neurological
impairment resulting in death associated with status epilepticus.
Morphologically, the progressive encephalopathy is associated with
neurofibrillary pathology in large and medium size neurons
predominantly in the spinal cord, brain stem, and selected areas of
cortex (hippocampus and cingulate gyrus). However the nature of the
accumulated fibrils at the light microscopic level and under the
electron microscope differ from those found in AD. The tangles are not
birefringent and are composed of 10 nm neurofilaments. The proteins
involved in the aluminium-induced neurofibrillary tangles also differ
from those found in the human diseases (see section 8.1.3.1). However,
aluminium is the only known trace element capable of inducing this
type of mylo-encephalopathy in susceptible animals (rabbit, cat,
guinea-pig, ferret). The epileptogenic property of aluminium, in
contrast to the progressive encephalopathy, occurs in all species
studied (e.g., primates, rodents, fish). Routes of administration of
aluminium sufficient to induce the encephalopathy include intrathecal
intracerebral and subcutaneous injections. There have been no reports
of progressive encephalopathy or epilepsy when aluminium compounds
were given orally.
The brain aluminium concentration necessary to achieve LD50 in
rabbits is about 6 �g aluminium/g dry weight (Crapper McLachlan et
al., 1989; McLachlan & Massiah, 1992). The normal brain aluminium
concentration in healthy rabbits is approximately 1.1 �g/dry weight.
7.6.1 Impairments of cognitive and motor function
Cats and adult or infant rabbits given intracerebral injections
of soluble aluminium compounds revealed a progressive impairment in
learning and memory performance after an asymptomatic period of 8 to
10 days (Crapper & Dalton, 1973a,b; Petit et al., 1980; Rabe et al.,
1982; Solomon et al., 1990). Repeated subcutaneous injections of
aluminium in rabbits affected classical conditioning (Yokel, 1983).
The intracisternal injection of a single or repeated low doses of
metallic aluminium in rabbits resulted in altered motor function
(Wisniewski et al., 1982; Bugiani & Ghetti, 1982; Strong et al., 1991;
Strong & Garruto, 1991b). The animals developed progressive myelopathy
and topographically specific motor neuron degeneration. They exhibited
myoclonic jerks and muscular weakness. Histopathologically a
neurofibrillary degeneration with swelling of the proximal axonal
processes of anterior horn neurons was present. The authors proposed
these preparations as possible models of human amyotrophic lateral
sclerosis.
Behavioural impairment has been reported in laboratory animals
exposed to aluminium in the diet or drinking-water in the absence of
overt encephalopathy or neurohistopathology. Both rats (Commissaris et
al., 1982; Thorne et al., 1987; Connor et al., 1988) and mice
(Yen-Koo, 1992) have demonstrated such impairments at doses exceeding
200 mg Al/kg body weight. While significant alterations in acquisition
and retention of learned behaviour were documented, the possible role
of organ damage (kidney, liver, immunological) due to aluminium was
incompletely evaluated.
7.6.2 Alterations in electrophysiological properties
The progressive encephalopathy and morphological alterations are
also associated with electrophysiological changes. The epileptic
seizures are associated with slowing of the EEG and epileptic
activity. The mechanisms that evoke the neuronal hyperexcitability
have not yet been completely elucidated but may involve altered
membrane electrotonic properties, K+ conductance, and synaptic
processes (Franceschetti et al., 1990). Associative long-term
potentiation (LTP), describes strengthening of a previously weak
synaptic input by concomitant activation of a strong synaptic input.
LTP can last up to several weeks and has been used as a model for the
hippocampal contribution to memory. LTP was not sustained normally in
hippocampal slices from rabbits exposed to intracranial injections of
aluminium about 7 days prior to sacrifice. The occurrence of this
electrophysiological alteration corresponds to the onset of
behavioural changes but is not necessarily accompanied by
neurofibrillary pathology in the hippocampal neurons exhibiting
impaired LTP. The loss of LTP can be partially reversed by an increase
in the calcium concentration in the bath (Farnell et al., 1985;
Crapper McLachlan & Farnell, 1986).
In summary, aluminium exposure has been used as an animal model
for the study of epilepsy, information processing, cognitive
dysfunction and motor neuron disease.
7.6.3 Metabolic effects in the nervous system
Considerable experimental evidence implicates aluminium in
alterations in the second messenger systems of cAMP and G proteins
(Steinweis & Gilman, 1982; Johnson & Jope, 1987; Johnson et al., 1990,
1992). An increased cAMP concentration in brain tissue is a
prerequisite for an increase in the phosphorylation of proteins. An
elevation of protein kinase C activity and in the basal activity of
cAMP-dependent protein kinase resulted in hyperphosphorylation of 12
proteins in rats chronically treated with aluminium (Johnson et al.,
1990). In rats chronically treated with low oral doses of aluminium,
hyperphosphorylation of MAP-2 was increased by 150% and the
neurofilament H subunit by 150-200%, while the phosphorylation of
several other proteins including tau was not different from that of
control rats (Johnson et al., 1990; Jope & Johnson, 1992). It was
suggested that abnormal phosphorylation may impair the axonal
transport of cytoskeletal proteins.
Ohtawa et al. (1983) showed that Al3+ binding to ferritin
reduced the binding of Fe2+ in rats fed aluminium. The free
intracellular Fe 2+ augmented the peroxidation of membrane lipids.
Lipid peroxidation in kidney, lung, liver and spleen were not
affected. The increased lipid peroxidation is at least, in part, due
to inhibition of superoxide dismutase in the brain but not in other
organ systems. An increase in lipid peroxidation was also shown in
chickens fed with aluminium sulfate (Chainy et al., 1993). It is
presumed that an increase in lipid peroxidation may be part of the
mechanisms underlying aluminium neurotoxicity.
Aluminium is not equally distributed among chromatin fractions
within the nucleus in control and aluminium-treated preparations.
Concentrations of aluminium on highly condensed, non-transcribed
chromatin are 15 to 20 times higher than those on active, decondensed
chromatin (Crapper et al., 1980). Several experimental models have
demonstrated that aluminium inhibits RNA synthesis and therefore may
have an effect on gene expression.
Transient change in the blood-brain barrier to [14C]sucrose have
been observed following low-dose intraperitoneal injection of
aluminium compounds (Kim et al., 1986). Intraperitoneal administration
of aluminium compounds increased the permeability of the blood-brain
barrier for a number of peptide and steroid hormones, such as
prolactin, growth hormone, luteinizing hormone, thyroxine and
cortisol. The greatest increase in penetration was observed for
thyroxine, which is transported by a carrier-mediated mechanism (Banks
& Kastin, 1985). From further studies on other transport systems, it
was suggested that aluminium selectively alters the transport systems
of the blood-brain barrier (Banks et al., 1988a; Vorbrodt et al.,
1994).
7.7 Effects on bone
The skeleton is the principal site of aluminium deposition in the
body. Aluminium deposited in this organ is important both because of
its toxic effects on bone tissues and because the deposits act as a
reservoir. Aluminium continues to be released from this reservoir to
the blood stream for a long time after intake as a consequence of bone
turnover, commonly referred to as bone remodelling.
Within the skeleton aluminium, in common with most other
polyvalent metal ions, deposits on bone surfaces within a very thin
layer (van de Vyver & Visser, 1990). How metal ions deposit in this
way is unclear, but three modes of uptake have been suggested.
Firstly, the metal may become trapped within the hydration shell of
the bone mineral crystal, secondly, it may become incorporated into
new bone crystals as they form at sites of bone accretion, and,
finally, they may become bound by acidic organic components of the
bone matrix, such as phosphoproteins (Priest, 1990). Subsequently,
much of the aluminium may remain on surfaces until it back-exchanges
into tissue fluids or may become locked into the bone matrix. "Locked
in" ions may then become buried to form volume deposits below bone
surfaces, as a result of bone accretion, or may be released by the
bone resorption process. Resorbed aluminium first enters osteoclasts
and macrophages and then returns to tissue fluids, including blood,
for recycling or excretion. The burial and resorption processes take
many years in man, where only a minority of surfaces show remodelling
activity, but occur rapidly in most experimental animals. As the rate
of bone turnover is largely under hormonal control, it follows that
hormones also regulate the retention by and release from the skeleton
of the more permanent deposits of aluminium. In this respect the most
important hormones are calcitonin and parathyroid hormone (PTH), which
act to increase either the rate of bone formation or the rate of bone
resorption in response to serum levels of calcium.
7.7.1 Toxic effects of aluminium in the skeleton
Excess deposits of aluminium in the skeleton may result in a
syndrome commonly referred to as "aluminium-induced bone disease"
(AIBD). This has been reviewed by Goodman (1986, 1990), van de Vyver &
Visser (1990) and Quarles (1991). A summary of some of the animal
models used to study osteomalacia induced by aluminium is given in
Table 21. It should be noted that all models utilize intraperitoneal
or intravenous routes of administration, making it impossible to
extrapolate to the risk in humans exposed to aluminium primarily by
the oral route. AIBD presents as a moderate to severe low bone
turnover osteomalacia, which is often insensitive to the vitamin D
complexes that reverse the osteomalacia of rickets. It may also result
in the de-coupling of the bone resorption and bone accretion processes
producing an excess volume of structurally incompetent bone (neo-
osteogenesis) and in disturbances in the normal processes of
endochondral ossification in the long-bone metaphyses. In osteomalacic
bones, osteoid (the unmineralized bone matrix) fails to mineralize or
is increased (Goodman et al., 1984a,b; Sedman et al., 1987; Quarles et
al., 1988), tetracycline markers of bone mineralization are not
incorporated (Ellis et al., 1979) and bone resorption is reduced,
resulting in an increase in the volume of unmineralized bone.
Associated with these changes is a reduction in the number of
osteoblasts (bone-forming cells) (Robertson et al., 1983) and a
reduction in the level of circulatory osteocalcin produced by these
cells. If osteomalacic bones are stained for aluminium then the metal
may be demonstrated as being present at the mineralized bone/osteoid
interface. Where present it would seem that the aluminium inhibits the
mineralization of osteoid, this amounting to a complete block in
severe cases. However, the mechanism for this block is unknown.
Goodman (1990) has suggested that it results from an impairment of the
movement of calcium and phosphate ions from the tissue fluids to the
face of the forming hydroxyapatite bone-mineral crystals.
Aluminium, like some other metals, e.g., strontium, when present
in bone crystals reduces the ability of the osteoclast (the bone
resorbing cells) to resorb the mineral. As expected, reduced bone
turnover may result in a reduced level of calcium in blood
(hypocalcaemia), which, in turn, might be expected to produce changes
in the levels of bone-active hormones in the circulation. In
particular aluminium-induced hypocalcaemia would be expected to result
in increased production of parathyroid hormone (PTH), in an attempt to
stimulate bone resorption and restore normal blood calcium levels.
However, the available evidence suggests that this does not occur and
that the levels of circulating PTH are normal or even reduced (Goodman
et al., 1984a). Rodriguez et al. (1990) found that PTH is able to
stimulate osteoblasts in the presence of aluminium, but that it cannot
improve mineralization. Similarly, there is also much evidence to
suggest that fluoride (a potent stimulator of osteoblast numbers)
interacts with aluminium in the skeleton by antagonizing the
aluminium-induced reduction in osteoblast numbers, but does not
ameliorate the aluminium-induced decrease in mineralization (Ittel et
al., 1992).
Of the available animal models, only the larger species, e.g.,
dogs and pigs, consistently show osteomalacia as it presents in man
(Goodman, 1990). In rodents bone remodelling is an unimportant feature
of normal bone turnover (bone growth continues throughout life), and
in these animals aluminium does not produce classical osteomalacia,
although the changes in bone accretion, mineralization and resorption
seen in the larger species are also seen in rats and mice. In these
species, the disturbances seen in the ossification and resorption of
bone under the growth cartilages may indicate a risk of similar
occurrences in aluminium-contaminated children. Animal studies have
shown that osteomalacia in trabecular bone is induced faster than in
cortical bone, a result of the lower bone turnover in the latter
(Goodman et al., 1984a,b).
Table 21. Experimental animal models of aluminium-induced osteomalaciaa
Species Route of Compound Dose Duration of Al concentration Histomorphometry Reference
application treatment in boneb
Rat (20 i.p. AlCl3 0.27-2.7 mg once daily; 5 days/ (15.4 �g/g), n.d. Ellis et
Wistar) Al/day week; 48-85 days 109.3-176 �g/g al. (1979)
Rat i.p. AlCl3 N (control), once daily; 5 days/ n.d. % osteoid: N: 6.6; Robertson
week; 90-120 days LD: 7.8; HD: 1.8; et al.
LD: 0.1 mg osteoid width (�m): N: 3.3; (1983)
Al/day; LD: 3.7; HD: 14.9;
HD: 1.0 mg tetracycline uptake:
Al/day irregular, attenuated in HD
Rat (74 i.p. AlCl3 1.5 mg Al/kg 5 days/week; 55 mg/kg d.w. Alfrey et
male SD) b.w. per day 35-79 days al. (1985)
Rat i.p. Al, 2 mg/day 5 days/week, not specified decreased bone formation Goodman
(weanling, elemental 4 weeks and osteoid maturation et al.
male H, in Al-treated animals (1984b)
10/group)
Rat i.p. Al, 2 mg/day 5 days/week, not specified formation of periosteal bone Goodman
(weanling elemental 44 days and matrix reduced (1984)
male H,
10/group)
Rat (male i.p. AlCl3 1.5 mg Al/kg 5 days/week, control: osteoid width (�m): normal: Chan et al.
W, 23) day; 9 weeks 1.8 mg/kg d.w.; 3.5; Al-treated: 3.0 (1983)
Al-treated:
47.0 mg/kg d.w.
Table 21. (Con't)
Species Route of Compound Dose Duration of Al concentration Histomorphometry Reference
application treatment in boneb
Rat (male i.p. AlCl3 10 mg/kg 5 days/week 3, 6, 9 weeks, 9 w: trabecular and Ott et al.
H, 18/group) per day 3 weeks recovery endosteal bone formation (1987)
weanling, decreased;
adult periosteal bone formation
normal, recovery to
normal level
Dog i.v. AlCl3 1 mg/kg b.w. 3 times weekly control: 10.5 % osteoid surface: Quarles
(Beagle, 3 weeks mg/kg d.w. control: 35.6% et al.
6/group) Al-treated: 73.6 Al-treated: 35.8% (1985)
mg/kg d.w.
Dog (6 i.v. AlCl3 1 mg Al/kg 3-5 weeks (1.3 mg/kg), % osteoid: (2.8)b 7.0 Goodman
female b.w. per day 94 mg/kg d.w. osteoid width: (5.7)b 8.0 et al.
mongrel) poor tetracycline uptake (1984a)
Dog i.v. AlCl3 0.75 mg Al/kg 5 days/week, (7.4 mg/kg d.w.) number of osteoblasts Galceran
(female b.w. 3 months 202.6 mg/kg d.w. 8-fold decreased et al.
mongrel, (1987)
7/group)
Dog i.v. AlCl3 0.75 mg Al/kg 3 days/week, (2.2 �g/g) low dose, 8 weeks; reduced Quarles
(18 male b.w. 16 weeks low dose, bone resorption and et al.
Beagles) 8 weeks: osteoblastic surfaces; low (1988)
1.2 mg Al/kg 65.8 mg/kg low turnover: low dose, 16 weeks;
b.w. dose, 16 weeks: increased trabecular number;
161.7 mg/kg uncoupled bone formation
Table 21. (Con't)
Species Route of Compound Dose Duration of Al concentration Histomorphometry Reference
application treatment in boneb
high dose, high dose, 8 and 16 weeks:
8 weeks: uncoupled bone formation
125.2 mg/kg
high dose,
16 weeks:
152.2 mg/kg
Dog i.v. AlCl3 1.25 mg/kg 3 days/week: (4.2 ng/litre) sham op. + Al bone volume, Quarles
(8 male b.w. per day 8 weeks sham op. + Al: tabecular number increased et al.
Beagles), 147 ng/litre (1989)
normal
Piglets i.v. AlCl3 1.5 mg/kg 8 weeks (1.6 mg/kg d.w.) osteoid seam width, osteoid Sedman
(8 Yorkshire) per day 241 mg/kg d.w. volume, mineralization lag et al.
time: increased; reduction of (1987)
active bone forming surface
a b.w = body weight; d.w. = dry weight; H = Holtzman; i.p. = intraperitoneally; i.v. = intravenous; n.d. = no data; SD = Sprague-Dawley;
W = Wistar; N = control; LD = low dose; HD = high dose; op = operation
b Control values are given in parentheses
Neo-osteogenesis, resulting in large increases in the volume of
trabecular bone, following aluminium administration has been observed
in a number of animal studies (Galceran et al., 1987; Quarles et al.,
1988, 1990) and has even been suggested as a possible treatment for
post-menopausal osteoporosis in women. In these studies a dose and
time-dependent effect was seen, lower levels of aluminium suppressing
bone formation but higher doses for long periods of time resulting in
an increased bone volume. Under such conditions it is likely that bone
apposition has become uncoupled from bone resorption processes - a
pathological change.
7.7.2 Dose response
As indicated above, the extent of pathological changes in bone
produced by aluminium after parenteral administration is dose-
dependent (Goodman, 1986), being barely perceptible at low doses, but
marked or severe at high doses. However, dose response will be much
affected by the period of aluminium accumulation, so that a single
high intake of aluminium may produce transient changes that are more
marked than in the case of higher accumulations over a long period. In
general, the available evidence suggests that, as in humans, bone
aluminium levels in the order of 100-200 �g/kg bone ash are required
to produce these changes. The study of Ellis et al. (1979) showed that
levels of aluminium in bone in the order of 100 �g/g bone ash produced
few bone changes in rats, but that higher levels produced marked
changes in the rat metaphysis and osteomalacic changes, these being
most marked in rats with excess aluminium levels of about 200 �g/g
bone ash. The authors found that osteotoxic doses of aluminium were
similar in humans and rats. Longer term studies in dogs (see Table 21)
show that low exposures to aluminium for short times produced few
morphological changes in bone, but that similar or higher dose levels
for a longer period did result in observable changes. Uncoupled bone
formation (neo-osteogenesis) was produced after 16 weeks at a final
bone aluminium level of 170 �g/g dry weight (Quarles et al., 1988). An
earlier study by the same authors showed no observable bone changes
after 9 weeks at a final bone aluminium content of 70 �g/g dry weight
(Quarles et al., 1985). Similarly, human aluminium levels of this
order are unlikely to result in osteomalacia.
7.8 Effects on mineral metabolism
Oral application of AlCl3 (300 to 1200 mg/kg diet) to cattle for
84 days did not change plasma or tissue levels of calcium, phosphorus,
magnesium or iron, although increased levels of zinc were seen in
liver and kidney (Valdivia et al., 1978). In sheep (20 lambs) fed a
diet supplemented with AlCl3 (2000 mg/kg for 56 days), Valdivia et
al. (1982) observed a reduction of the apparent calcium absorption and
lower plasma/serum phosphate levels. The ingestion of moderate
concentrations of aluminium (aluminium lactate, aluminium palmitate,
aluminium phosphate, aluminium hydroxide; 5-272 �g Al/g diet) also had
no effect on tissue calcium, magnesium or iron levels in male Sprague-
Dawley rats (Greger et al., 1985a). Small effects were seen on tissue
levels of phosphorus, zinc and copper. In rats receiving high
intraperitoneal doses of aluminium (AlCl3, 2.7 mg Al/day) repeatedly
for 10 days, the calcium concentration was increased in brain, liver
and spleen, but not in the heart, and serum calcium levels were not
significantly affected (Burnatowska-Hledin & Mayor, 1984). No changes
in plasma magnesium levels were seen in this study.
Reduced plasma magnesium concentrations were observed in cows
(Kappel et al., 1983; Allen et al., 1986) and sheep (Valdivia et al.,
1982) after feeding a diet supplemented with various amounts of
aluminium chloride, sulfate or citrate. In sheep (24 lambs) fed an
aluminium-rich diet (1450 �g/g), the magnesium content of kidney and
bone was reduced (Rosa et al., 1982).
From their experiments in dogs (female mongrel) receiving i.v.
injections of AlCl3 (1 mg Al/kg body weight, 5 days/week, for 3-5
weeks), Henry et al. (1984) concluded that the increased serum calcium
concentration was due to an increased liberation from bone.
In cows fed a diet supplemented with aluminium citrate
(1730 mg Al/kg dry weight for 56 days), serum and urinary calcium
concentrations were increased (Allen et al., 1986).
The effect of vitamin D and its metabolites on calcium metabolism
following aluminium intoxication was studied by Hodsman et al. (1984)
and by Henry & Norman (1985). In vitamin-D-deficient rats receiving
repeated intraperitoneal injections of AlCl3 (9.25 mg aluminium for
33 days), serum calcium levels were increased regardless of the
vitamin D status of the animals (Hodsman et al., 1984). Treatment of
chickens with AlCl3 (5 mg Al/kg body weight intraperitoneally for 5
days) partially blocked the intestinal calcium absorption response to
vitamin D in vitamin-D-deficient animals, although serum calcium
levels were elevated (Henry & Norman, 1985). No consistent effects of
aluminium on the bone calcium mobilization response to vitamin D or
1,25(OH)2D3 were noted. The authors concluded that the ability of
the intestine to respond normally to 1,25(OH)2D3 may be compromised
by the aluminium application.
A depression of the serum phosphate level was also observed in
vitamin-D-deficient rats receiving repeated intraperitoneal injections
of AlCl3 (9.25 mg aluminium for 33 days; Hodsman et al., 1984).
Aluminium forms complexes with fluoride, which are considerably
more stable than the respective Fe3+ complexes. Ingestion of
relatively small amounts of aluminium decreases the fluoride
concentration available in the intestinal lumen by complexation and
thus fluoride absorption from the intestine. Fluoride and AlF4-
stimulate the enzyme adenylate cyclase (Sternweis & Gilman, 1982).
This effect presumably proceeds by complexation of Al3+ that is
present as a contaminant of the substrate ATP, thus raising the
effective ATP concentration (Martin, 1986).
Aluminium exerts its protective effect from fluorine toxicosis,
which has been reported in hens (Hahn & Guenter, 1986), turkeys (Cakir
et al., 1977) and sheep (Saia et al., 1977), also by formation of a
stable complex between Al3+ and F-, thus increasing the fecal
fluoride excretion.
8. EFFECTS ON HUMANS
8.1 General population exposure
Aluminium is a potential neurotoxic agent in humans (Steinegger
et al., 1990). Humans have highly efficient natural barriers to limit
aluminium concentration within the central nervous system except under
specific conditions such as renal failure. Encephalopathy attributed
to aluminium intoxication in patients receiving treatment for chronic
renal failure is discussed in section 8.3.1.
8.1.1 Acute toxicity
There is little indication that aluminium is acutely toxic by
oral exposure despite its widespread occurrence in foods, drinking-
water and many antacid preparations.
8.1.2 Effects of short-term exposure
In 1988 a population of perhaps 20 000 local residents and
numerous tourists were exposed for 5 days or more to unknown levels of
aluminium sulfate, subsequent to 20 tonnes of concentrated aluminium
sulfate being accidentally placed in the Lowermoor water treatment
plants in Camelford, England. The drinking-water also contained
elevated concentrations of lead and copper, which leached from the
plumbing systems due to increased water acidity. In view of the
anecdotal reports of nausea, mouth ulcers, skin rashes and increased
arthritic pain, some lasting for months after the exposure, the
Cornwall District Health Authority convened a Health Advisory Group
which prepared an official report on the incident (Clayton, 1989). The
report described the exposure scenario and made conclusions based on
expert knowledge of aluminium toxicity and interviews with local
residences and tourists claiming long-lasting adverse health effects.
No one was exposed to levels of aluminium over 100 mg/litre since such
water is unpalatable. Levels between 10 and 50 mg/litre were found for
only 1-3 days and water levels for the next month were above 0.2 mg/
litre but below 1 mg/litre. The report concluded that such exposures
should not pose a hazard to human health. Furthermore, the report
concluded that there is no evidence to support the causation by the
levels of aluminium, zinc, lead and sulfate of joint and muscle pain,
memory loss, hypersensitivity or gastrointestinal disorders reported
by residents and tourists some months after the incident. The report
stated: "In our view it is not possible to attribute the very real
current health complaints to the toxic effects of the incident, except
insofar as they are the consequence of the sustained anxiety naturally
felt by many people". Other reports, such as that of McMillan et al.
(1993), despite containing major scientific deficiencies, do not
provide evidence contrary to this conclusion.
8.1.3 Neurotoxic effects
8.1.3.1 Aluminium and Alzheimer's disease (AD)
It has been suggested that aluminium exposure is a risk factor
for the development or acceleration of onset of Alzheimer's disease
(AD) in humans (Crapper McLachlan, 1986; Crapper McLachlan et al.,
1989). The precise pathogenic role of aluminium in AD is judged
controversial and remains to be defined (Wisniewski & Wen, 1992;
Wischik et al., 1992; Edwardson, 1992).
The purported association is based on six points:
1. The experimental induction of neurofibrillary changes in the
neurons of certain species of animals, which suffer a unique
progressive neurological impairment after parenteral administration of
aluminium salts (see chapter 7). However, these neurofibrillary
changes differ from those seen in AD in staining properties and
ultrastructural and biochemical composition (Wisniewski & Wen, 1992).
2. The presence of elevated aluminium levels in bulk grey matter of
AD-affected brains, which have been found by most investigators using
various techniques, including graphite furnace atomic absorption
spectroscopy (Crapper et al., 1973, 1976), neutron activation
techniques, and inductively coupled mass spectroscopy. However, some
investigators using the same techniques have failed to find elevated
aluminium levels in the brains of patients with AD (McDermott et al.,
1979; Jacobs et al., 1989).
3. The reported detection of aluminium in the amyloid core of
classical plaques, neurofibrillary tangles and neuronal nuclei
affected by neurofibrillary pathology in AD. Increased aluminium
levels have been found within the neurofibrillary tangles of AD using
laser microprobe mass spectrometry (LMMS) (Good et al., 1992).
However, using the same technology, others have been unable to
replicate these results (Lovell et al., 1993). These techniques are
difficult. There may be technical reasons for the disparity in the
results, or some of the differences may relate to aluminium
contamination of fixatives and stains (Landsberg et al., 1992). AD
patients may have an altered blood-brain barrier that allows excess
aluminium to accumulate in the brain (Wisniewski & Kozlowski, 1982;
Liss & Thornton, 1986; Banks et al., 1988b), although this may in turn
be secondary to deposition of amyloid fibrils in the walls of large
and small cerebral vessels (amyloid angiopathy) (Wisniewski et al.,
1992). If it is accepted that aluminium levels are raised in classical
plaques and neurofibrillary tangles in AD, it has been proposed that
this may be a secondary phenomenon rather than the primary etiological
agent.
4. Epidemiological studies showing an association between aluminium
intake in drinking-water and an increase in the prevalence of AD. The
methodology and interpretation of these studies is still under debate,
and is considered further in section 8.1.3.2.
5. The reported decrease in the rate of disease progression in
clinically diagnosed AD patients in one, single-blind, oral-versus-
placebo controlled trial of desferrioxamine, a trivalent ion chelator
administered by intramuscular injection (McLachlan et al., 1992).
However, this study has been criticized on three grounds: a)
desferrioxamine also chelates iron, which has been linked to free
radical damage in AD (Andorn et al., 1990; Crapper McLachlan et al.,
1991); b) desferrioxamine may have acted through an anti-inflammatory
effect in this (at least partly) inflammatory disease (Crapper
McLachlan et al., 1991); c) the absence of a double-blind placebo
controlled design may have resulted in differential treatment of
patients in the active arm (Wisniewski & Rabe, 1992).
6. The reported interactions of aluminium with �-amyloid protein
(the major component of AD plaques) and with purified paired helical
filament tau protein (the major component of neurofibrillary tangles,
NFTs). The neurotoxicity of �-amyloid and the formation of plaque
deposits is dependent on its aggregation, which has been found to be
promoted by low millimolar concentrations of aluminium, iron and zinc
(Mantyh et al., 1993). However, this pattern of results has been
attributed to iodination-induced alteration of the �-protein structure
by Bush et al. (1994), who reported that zinc is a much more potent
metallic ion aggregator of native �-protein than aluminium, being
active at low micromolar concentrations. In vitro studies have
failed to induce Alzheimer-type paired helical filaments (PHFs) in any
cellular system. However, human neuroblastoma cells in tissue culture,
exposed to aluminium, exhibit epitopes found in AD tangles. Mesco et
al. (1991) reported aluminium induction of the well-known Alz50
epitope-recognizing NFT, and Guy et al. (1991) reported the
development of an epitope, recognized by an antibody staining for NFT
and neuropil threads. Alz50 expression is also observed in
experimental aluminium encephalopathy. Shin et al. (1994) have found
that aluminium binds to and stabilizes paired helical filament tau,
both in vitro and in vivo. Although aluminium-stabilized PHF tau
induced co-deposition of �-protein in vivo, the relevance of these
recent unconfirmed findings to AD is as yet unclear.
It seems likely that the causation and pathogenesis of AD is
multi-factorial (i.e. it may be regarded as a syndrome rather than a
disease) and that genetic factors and environmental factors each
contribute to a greater or lesser extent in the individual case.
Recent genetic studies show that in a small proportion of dominant
familial cases, a single point mutation near or in the �-protein
segment of the amyloid precursor protein is necessary to cause the
disease - a situation of great theoretical importance despite its
rarity (Goate et al., 1991).
Other familial AD pedigrees have been linked to a locus on
chromosome 14 (St. George-Hyslop et al., 1992; van Broeckhoven et al.,
1992). Apo-E allele status is also a major risk factor, the presence
of one E4 allele conveying a relative risk of about 3 (Saunders et
al., 1993). Other environmental risk factors include low educational
and socio-economic status, and head injury (van Duijin et al., 1991).
It is against this knowledge base that the possible contribution of
aluminium to AD must be evaluated.
8.1.3.2 Epidemiological studies on AD and environmental aluminium
levels
Many studies have examined risk factors for AD. Among the many
case control studies that have been carried out, head trauma, family
history, thyroid status, maternal age, child with Down syndrome all
stand out as important risk factors (van Duijn et al., 1991).
Aluminium exposure as a single risk factor began to be examined in the
early 1980s, when reports of the increased level of aluminium in
brains of AD patients suggested that this might also be a factor. The
availability of water-borne aluminium measurements in many public
water supplies and of readily available vital statistics made the
study of this exposure relatively accessible.
Studies that examine the relationship between aluminium in
drinking-water and AD have been carried out in five separate
populations: Norway (Flaten, 1990), Ontario, Canada (Neri & Hewitt,
1991; McLachlan, 1996), France (Michel et al., 1991; Jaqmin et al.,
1994), Switzerland (Wettstein et al., 1991) and England (Martyn et
al., 1989). These are summarized in Table 22. Several studies examined
the water aluminium-AD relationship in the course of investigating
other associations (Wood et al., 1988; Frecker, 1991). In addition,
exposure from aluminium-containing antiperspirants (Graves et al.,
1990) and aluminium-containing antacids (Flaten et al., 1991) have
also been explored as risk factors for dementia and/or AD.
Each of the studies that relate aluminium in drinking-water to
AD can be assessed systematically for comparability of exposed and
control groups, precision of exposure assessment and outcome
definition. Ideally, exposed and control groups should be controlled
for age, sex, socio-economic status and other variables that can
confound results (e.g., education, family history, etc.). Exposure
should include concentration and duration for each member of the study
group and include a dose range which can be used to assess dose-
response relationships. The outcome should be measured preferably by
standard criteria, not by surrogates (e.g., dementia for AD), and
latency should be incorporated into the analysis (Smith, 1995).
Table 22. Summary of epidemiological studies of aluminium in drinking-water and dementia or Alzheimer's disease (AD)a
Type of study Exposure measure of Outcome measure/ Results Reference
aluminum intake data source RR
Ecological aluminum in drinking-water mention of dementia AD only Flaten
(concurrent) ICD9 290, 290.1 (dementia) (1990)
4 seasonal samples 342.0 (Parkinson's disease) Al Males Females
348.0 (ALS); sex-adjusted < 0.05 1.00 1.00
death certificate 0.05-0.2 1.15 1.19
> 0.2 1.32 1.42
PD and ALS - no gradient
Morbidity aluminum in finished drinking- dementia by diagnostic category all males and females Martyn et
prevalence water; historical (not standard) RR 1.3-1.5, no dose-response al. (1989)
CT scan center records < 65 males and females
age-sex-adjusted 1.4-1.7b, dose response
Morbidity finished drinking-water "cases" were hospital discharges RR from OR, gradient for AD Neri &
prevalence aluminum; historical with Dx of AD (ICD9 331.0), Hewitt
case control presenile dementia (ICD9 290). Al RR from OR (1991)
age/sex/residence-matched < 0.01 1.00
controls with other Dx 0.01-0.099 1.13
0.10-0.199 1.26
HMRI data base - Ontario > 0.2 1.46
Morbidity aluminum in finished drinking- mnemic skills in octogenerarians no difference in mean scores of Wettstein
prevalence water; residence >15 years urinary and serum Al tests for cognitive function et al.
(1991)
Table 22. (Con't)
Type of study Exposure measure of Outcome measure/ Results Reference
aluminum intake data source RR
Morbidity urinary aluminum and serum sample of 800 residents in high slightly higher serum aluminium Wettstein
aluminum; historical and & low aluminum areas 10 AD in AD in low aluminium areas; et al.
concurrent patients & controls in each area similar urinary excretion in AD (1991)
and controls
age, sex, education
hypothesis of association not
population based supported
Morbidity aluminum in drinking-water; cognitive function in sample of probable AD - gradient-adjusted Michel
prevalence historical > 65 years by test battery for age, education, residence et al.
(DSM III) (1991)
RR 4.53/100 �g/litre aluminium
population-based (2792); age, sex, (NSS); RR corrected to NS with
education, ses, Al in water - many current aluminium measurement
sources for the data
Case control aluminium in drinking-water; pathological; confirmation of RR 1.7 aluminium > 100 �g/litre McLachlan
residence-weighted diagnosis in all cases and controls RR 2.5 aluminium > 100 �g/litre et al.
historical no age-sex-education adjustment based adjustment for 10-year (1996)
weighted exposure history
Morbidity aluminum in water, pH, cognitive function calcium protective Jacqmin
prevalence calcium RR = 1.2 with pH < 7.3 et al.
case control NS /all other pH values (1994)
a AD = Alzheimer's disease; PD = Parkinson's disease; RR = relative; OR = odds ratio; NS = not significant; ses = socioeconomic status
b Significant (P < 0.05) of highest exposure only
In addition, a variety of study designs from least powerful to
most powerful will allow a progressive assessment of the relationship
under study (e.g., ecological, cross-sectional, case control, cohort).
Nearly 20 studies that examine the relationship between AD and
drinking-water aluminium levels have been published.
Studies in five populations using different design are of
sufficient quality and meet the general criteria for exposure and
outcome assessment and for the adjustment of at least some confounding
factors (e.g., age and sex) in order to be used here to evaluate the
relationship between water-borne aluminium and AD.
Results of four studies are consistent for a positive
relationship between water-borne aluminium and AD (Martyn et al.,
1989; Neri & Hewitt, 1990; Flaten et al., 1991; McLachlan et al.,
1996). Three found a "dose-response" relationship (McLachlan, 1989;
Flaten, 1990; Neri & Hewitt, 1991) and one found a significant
relationship between high and low aluminium exposure (Martyn et al.,
1989). Adjustment for sex was performed in two of these studies
(Martyn et al., 1989; Flaten, 1990). One study (McLachlan et al.,
1996) did not adjust for age, sex or any other confounding factor but
did correct for "during life" exposure.
With regard to exposure assessment, all of the positive studies
used ecological assessment of exposure but from only one source, the
public water supply. Total exposure to aluminium was not determined.
It is therefore impossible to determine if the relationship observed
is due to water aluminium alone without explicit adjustment for, and
information about, other sources of aluminium intake.
Studies that showed no association between water aluminium and AD
(Wettstein et al., 1991; Michel et al., 1991) were more precise in
their outcome measure. However, the water aluminium levels were very
low and corresponded to the water aluminium levels found as the lowest
concentrations of the studies that showed a positive association.
Initial results reported by Michel et al. (1991) in the Bordeaux
cohort study showed a high, though not significant, risk (4.5) for
exposure to water aluminium levels greater than 0.10 mg/litre when
10- to 15-year historical analyses of water were used. When current
analyses of water were used, the relationship disappeared (Jacqmin et
al., 1994). However, other relationships appeared, such as the
increase in risk of cognitive impairment when the pH was below 7.3, a
decrease in risk with a pH greater than 7.3, and no elevated risk when
pH was not considered. An inverse relationship was found between
cognitive impairment and calcium concentration (Jacqmin et al., 1994).
The initial observation of elevated risk for cognitive impairment
with a set of numbers that were possibly random (Flaten, 1990) and the
changes in risk level with other water quality parameters such as
calcium and pH are difficult to interpret and require further
evaluation.
Studies with more precise outcome and exposure measures would be
expected to show the highest relative risks or odds ratios.
McLachlan et al. (1996) examined the relationship between
autopsy-confirmed AD and aluminium in drinking-water. The odds ratio
of exposure to water above 100 �g/litre was calculated for confirmed
cases of AD and a combination of AD and other neuropathology, compared
to controls. Cases and controls were obtained from brains donated to a
tissue bank supported by lay organizations. Control neuropathology
included normal brains and brains with non-AD neuropathology. The
aluminium level in water was obtained from a data bank of water
measurements for public water supplies. A next-of-kin interview was
used to refine exposure by weighing for length of residence by
assigning exposure to residence not to place of death.
The odds ratio of exposure to water with an aluminium
concentration of > 100 �g/litre for AD alone compared to controls
was 1.7 (1.2-2.5). The use of weighted exposure measures (residential
group levels of aluminium in water) increased the odds ratios for the
same comparison groups to 2.5 or more.
The use of pathologically confirmed outcome measures and of
accurate exposure measures brings added strength to the studies
examining the relationship between aluminium and AD. Notwithstanding,
this study is subject to selection bias of the sample (voluntary
donation to a tissue bank), the possibility of misclassification of
the ecological measure of exposure and a failure to account for
confounding factors.
The study of McLachlan et al. (1996) does show the highest
relative risks of any of these studies. However, this study suffers
from lack of adjustment for very important known risk factors, such as
age, sex, education and socioeconomic status.
8.1.3.3 Epidemiological studies relating aluminium concentrations in
water to cognitive dysfunction
Wettstein et al. (1991) looked for a relationship between water
aluminium levels up to 98 �g/litre and cognitive function in a male
population (800 men) of octogenarians but found none (OR = 0.92)
(CI = 0.66-1.29).
Forbes et al. (1994) examined a cohort of 2000 men followed since
1959 in a longitudinal study of ageing. The outcome measure examined
was "any evidence of mental impairment" as measured by skills of daily
living assessment. In the survivors of the cohort for whom information
was available (290 individuals), exposure was linked to the level of
aluminium in their drinking-water obtained from the Ontario Canada
Drinking Water Surveillance database. In an analysis that considered
fluoride and aluminium in drinking-water, the odds ratio after
exposure to water with high aluminium and low fluoride levels was 3.98
[CI = 1.72-9.19]. In a multi-variate analysis, which adjusted for a
variety of confounding factors, the adjusted odds ratio for high
aluminium level (> 85 �g/litre) was 1.72 (1.08-2.75).
8.1.3.4 Other neurological conditions in the general population
Other severe neurological diseases, such as amyotrophic lateral
sclerosis, Parkinsonism and the dementia complex of Guam, have been
related to aluminium accumulation in the brain (Gajdusek & Salazar,
1982; Perl et al., 1982; Garruto et al., 1984). However, the role of
aluminium in these conditions is still under considerable scientific
debate.
8.1.3.5 Conclusions regarding neurological effects of aluminium
The positive relationship between aluminium in drinking-water and
AD, which has been demonstrated in several epidemiological studies,
cannot be totally dismissed. However, strong reservations about
inferring a causal relationship are warranted in view of the failure
of studies to account for demonstrated confounding factors and for
aluminium intake from all sources.
Taken together, the relative risks for AD from exposure to
aluminium in drinking-water at levels above 100 �g/litre as determined
in these studies are low. But, because the risk estimates are
imprecise for a variety of methodological reasons, a population-
attributable risk cannot be calculated with precision. Such
predictions may, however, be useful in making decisions about the need
to control the exposure to aluminium in the general population.
In light of the above studies, which consider water-borne
aluminium as the sole risk factor, and the recent findings that water
accounts for less than 5% of daily uptake of aluminium, it is
difficult to reconcile a presumable impact on cognition. Several lines
of investigation should be pursued to elucidate further the nature of
the relationship found in these studies (see Chapter 12).
8.1.4 Allergic effects
Although human exposure to aluminium is widespread,
hypersensitivity has been reported following exposure to some
aluminium compounds in only a few cases, either after dermal
application or parenteral administration.
A case of contact sensitivity to aluminium was reported in
Sweden. The patient had regularly been using an aluminium chloride
roll-on antiperspirant and developed an itchy dermatitis in the
axillae. Patch-tests with aluminium chloride were positive (Fischer &
Rystedt, 1982). Contact allergy to aluminium also occurred in a
patient hyposensitized with aluminium-precipitated grass pollen
(Clemmensen & Knudsen, 1980). Two cases of contact allergy to
aluminium after use of topical medications containing aluminium
acetotartrate have been reported (Meding et al., 1984).
Childhood immunization with an aluminium-bound vaccine can lead
to delayed hypersensitivity to aluminium. Children who had had
previous injections with these vaccines showed positive patch-tests to
aluminium chloride (B�hler-Sommeregger & Lindemayr, 1986; Veien et
al., 1986).
In Denmark a follow-up study was made of 202 children (age
6-15 years) who had received hyposensitization therapy with various
aluminium-containing extracts (subcutaneous application) for
an average of 3 years. One to three years after cessation of
hyposensitization, 4% (13 children) still had severely pruriginous
treatment-resistant subcutaneous nodules in their forearm (application
site). Six of these 13 children were patch-tested and four reacted
positively on aluminium chloride administration (Frost et al., 1985).
8.2 Occupational exposure
This section deals with the effects observed in occupations where
workers are exposed to aluminium metal and aluminium compounds. Where
exposures are to mixed dusts and/or chemical mixtures, one cannot
infer causality between aluminium exposure and effects from studies on
such workers.
8.2.1 Respiratory tract effects
Respiratory disorders among workers in the aluminium industry
have been reviewed in detail (Dinman, 1988b; Abramson et al., 1989).
8.2.1.1 Restrictive pulmonary disease
Historically, pulmonary fibrosis has been associated with various
jobs within the aluminium industry. Shaver's disease (described in the
1940s) was a form of silicosis associated with the production of
corundum abrasives (Shaver & Riddell, 1947). Another historically
important occupational exposure associated with pulmonary fibrosis was
experienced by "pyro powder" workers, who were exposed to very fine
stamped aluminium powder (generally < 1 �m), including that used in
the manufacture of explosives and fireworks (Doese, 1938; Meyer &
Kasper, 1942; Mitchell et al., 1961; Jordan, 1961; McLaughlin et al.,
1962; Gross et al., 1970). In that process, oils and solvents were
used to coat particles to prevent naturally occurring oxidation, and
nearly all cases of fibrosis were reported in workers exposed to
mineral-oil-coated particles. That process is no longer used (Dinman,
1988a) and only one case has been reported since 1960 (McLaughlin et
al., 1962). This syndrome indicates the potential pulmonary effect of
non-oxidized aluminium metal, but such exposures do not occur in
nature.
In a report of nine cases of workers exposed to aluminium oxide
(mean duration of exposure 25 years), abnormal chest roentgenograms
were described, as well as pathological lung functions in three of the
cases (Jederlinic et al., 1990). Biopsies were taken from these three
patients and analysed by electron microscopy and microprobe analysis.
Interstitial fibrosis was the main histological finding. Metals
occurred in amounts several orders of magnitude above background
levels and the majority was aluminium oxide. The authors stated that
aluminium oxide was the most likely cause for the development of
interstitial fibrosis in these workers and that asbestos could be
ruled out. Exposure to a "mixed dust", including free silica, also
seemed to be a possible explanation.
With the exception of this exposure, pathological findings
associated with aluminium exposure listed in Table 23 refers to mixed
exposures, and cannot be solely attributed to aluminium. Other
exposures, such as to silica or other metals, must be considered.
8.2.1.2 Obstructive pulmonary disease
a) Asthma
A potentially persistent form of occupational asthma related to
primary aluminium smelting (pot room asthma) has been reported over
the past 35 years; reversible symptoms, airflow limitation and
increased bronchial responsiveness have been described (O'Donnell et
al., 1989). The likely causes are irritant airborne particulate and
fumes contributed by cryolite (sodium aluminium fluoride), gaseous
hydrogen fluoride and other agents that may be adsorbed onto
aluminium. A close relationship in aluminium potroom workers between
levels of exposure to fluoride, which may be one of a number of
Table 23. Clinical and pathological pulmonary findings in aluminium-exposed workersa
Exposure Clinical effects Pathological changes Confounding exposures Reference
Aluminium powder cough, DOE, abnormal pulmonary alveolar proteinosis iron, kaolinite, mica, rutile, Miller et al.
grinder for 6 years X-ray, restrictive PFTs calcium at biopsy (1984)
Polisher for cough, DOE bronchogenic cancer; diffuse stainless steel, chromium, de Vuyst et
24 years interstitial fibrosis nickel, cigarettes (45 packet- al. (1986)
years), silica (biopsy)
Catalyst fabrication cough, DOE, mild restriction non-caseating granuloma; iron, copper, zinc, nickel, de Vuyst et
no clinical evidence T-lymphocyte alveolitis chromium, manganese, cobalt, al. (1987)
of sarcoid molybdenum, vanadium,
palladium, silica, nobellium
on biopsy; cigarettes
Welding fumes mild ventilatory restriction diffuse and focal fibrosis; iron, cigarettes Vallyathan
pigmented content of macrophages et al. (1982)
Welding fumes, X-ray interstitial pattern; interstitial granuloma, smoker, no TB Chen et
intermittent welder dyspnoea macrophages, foreign body giant al. (1978)
(1965-1970) cells, crystals, EDA indicated
aluminium crystals
Welder for 16 years dyspnoea, X-ray bilateral, lung biopsy, diffuse chronic ex-smoker Herbert et
hazy basal infiltrates, interstitial pneumonia, al. (1982)
reduced TLC (3.5/6.8) predominantly desquamative
DOE = dyspnoea on exertion; TLC = total lung capacity (measured/predicted (6.8 litres)); EDA = energy dispersive analysis;
PFT = pulmonary function test; TB = tuberculosis
general inhalant irritants, and the work-related asthmatic symptoms
has been shown (Kongerud et al., 1990; Kongerud, 1991). A positive
association between plasma levels of fluoride and increased bronchial
responsiveness has also been reported (S�yseth et al., 1994).
A similar occupational asthma ascribed to irritant particulate
has also been described among workers following technical failure in
plants producing aluminium fluoride and aluminium sulfate (Simonsson
et al., 1985) and in solderers working with potassium aluminium
tetrafluoride flux (Hjortsbert et al., 1994).
b) Chronic bronchitis
Aluminium production and processing may lead to high levels of
workplace exposure to dusts and particulate.
In Italy the possible association of aluminium exposure and
pneumoconiosis was investigated (Saia et al., 1981). Chronic
bronchitis symptoms were found in 39% of the 119 exposed workers and
in 13% of the 119 control subjects. The X-ray findings showed one kind
of pneumoconiosis with small irregular opacities or accentuation of
broncopulmonary markings in 29% of the exposed workers and in 15% of
the controls.
A case study of 2086 employees at the Arkansas operations of a
large aluminium production company was performed (Townsend et al.,
1985). The study indicated that long-term high accumulative dust
exposure was associated with decreased levels of pulmonary function in
active workers at a bauxite refinery and aluminium-based chemical
products plant. A follow-up study of this cohort (Townsend et al.,
1985) supported the conclusion regarding respiratory effects of dust
in the workplace related to lung function.
In a cross-sectional study (Sj�gren & Ulfvarson, 1985) on 64
aluminium welders and 64 age-matched controls (non-welding industrial
workers), an increased prevalence of chronic bronchitis was observed
but there were no effects on pulmonary function. The prevalence of
chronic bronchitis among aluminium welders was similar to that of
welders working with stainless steel or iron.
8.2.2 Central nervous system effects
A number of neurological effects have been associated with
occupational exposure to aluminium, including impairment of cognitive
function, motor dysfunction and peripheral neuropathy.
Welders exposed to aluminium fumes for about 13 years had
significantly more neuropsychiatric symptoms (ascertained from
positive answers in a questionnaire) than railway track welders not
exposed to aluminium (Sj�gren et al., 1990). Despite the potential
bias associated with the questionnaire methodology used in this study,
a dose-response effect was seen.
In a further study (Sj�gren et al., 1994b) 38 aluminium-exposed
welders (median urinary aluminium level, 22 �g/litre; median exposure
time, 4.5 years) were compared with a group of 39 iron-exposed
welders. Small decrements in the speed of repetitive motor functions
were found, but there were no differences in other neurophysiological
or neuropsychological parameters.
A mixture of finely ground aluminium (85%) and aluminium oxide
(15%) powder was used between 1944 and 1979 as a prophylactic against
silicosis. Underground gold and uranium miners were exposed to an
aluminium dust concentration of 20 000-34 000 particles/ml air
(approximately 30 mg/m3) in their changing room before each shift for
10 min (Rifat et al., 1990). Exposure to aluminium powder in the
cohort ranged from 6 months to 36 years. A yearly deposition in each
miner of about 375 mg of aluminium powder has been calculated.
From the 29 000 underground miners examined in provincial chest
clinics between 1955 and 1979, a sampling frame was constructed
containing a cohort of 6604. Two samples were drawn from this cohort.
One sample consisted of 369 exposed and 369 unexposed matched miners
adjusted for age and year of their first mining experience in Ontario,
Canada, and total mining time. The second sample consisted of 678
randomly drawn miners in equal numbers from the exposed and unexposed
populations. Between 1988 and 1989, miners who could be traced were
interviewed and psychometric testing was performed. Cognitive test
scores and proportions impaired in at least one test indicated a
disadvantage for exposed miners. A positive exposure-related trend in
increased risk was described.
A group of 87 workers (average age 40.7 years) from an aluminium
foundry exposed to workplace aluminium concentrations ranging between
4.6 and 11.5 mg/m3 air, with an exposure time of at least 6 years,
was studied by Hosovski et al. (1990). Sixty non-exposed workers
matched for age, job, seniority and social status served as control.
Psychomotor and psychometric tests were performed, except on workers
who consumed alcohol or who had taken psychotropic drugs within a
month prior to the test. A significant difference in complex reaction
time, oculomotor coordination and the sum of manipulative tests was
noted in exposed workers compared to controls. In the Weschler Adult
Intelligence Scale (WAIS), the most significant differences were found
in the memory subtest.
In contrast, Bast-Petersen et al. (1994) did not find any
impairments in small groups of foundry workers (8) or potroom workers
(14) in a broad battery of psychometric tests. A cluster of aluminium
potroom workers exposed to unhooded pots for 4 or more years displayed
an increased incidence of impairment of cognitive function and/or
defects in motor control. However, insufficient biochemical
investigations were undertaken to determine whether aluminium or other
potential neurotoxins were the causative agent (Longstreth et al.,
1985; White et al., 1992).
In a case where a man was exposed to ultrafine aluminium powder
for 13.5 years in the ballmill area of an aluminium factory, the
individual died following a rapidly progressive encephalopathy, and
his brain was found to contain elevated aluminium levels (McLaughlin
et al., 1962).
8.3 Cancer
There is insufficient information to allow for the classification
of the cancer risk from human exposures to aluminium and its
compounds.
8.4 Genotoxicity
In an abstract, Haugen et al. (1983) reported no increase in the
number of sister chromatid exchanges in peripheral blood lymphocytes
of workers employed in an aluminium factory. There have been no
reports concerning genetic effects of aluminium in humans following
oral exposure to aluminium.
8.5 Reproductive toxicity
There is no information regarding reproductive toxicity in humans
following exposure to aluminium.
8.6 Subpopulations at special risk
Aluminium intoxication developed over weeks or months in patients
with chronic renal failure when dialysis fluids or parenteral
solutions contained aluminium (Alfrey et al., 1972; Klein, 1991), or
when the main source was aluminium-containing oral phosphate binders.
In patients suffering from renal failure, increases in serum and
tissue aluminium concentration were observed. The increased aluminium
content in brains of patients with renal failure seems to be the major
etiological factor in the development of the neurological syndrome
termed either dialysis encephalopathy or dialysis dementia. The
development of a specific form of osteomalacia and of microcytic,
hypochromic anaemia is also attributed to aluminium (Ward, 1991).
Aluminium intoxication is caused by using haemodialysis fluids
made from tap water without removal of the aluminium (Elliot et al.,
1978). After the introduction of water treatment with a combination of
filtration, softening, carbon absorption, reverse osmosis and
de-ionization, these clinical syndromes were prevented. Nephrologists
limit the exposure to aluminium from dialysis fluids and drugs. This
follows the introduction of guidelines in the USA, Canada, Japan and
the EEC. As a consequence, in most dialysis centres the dialysis
fluids are monitored and the aluminium level is kept below
0.4 �mol/litre (10 �g/litre). Aluminium-free phosphate-binding agents
such as calcium carbonate are preferably used for oral medication. The
same clinical syndromes have been described in patients with renal
impairments, including premature infants who have not been dialyzed,
and are a consequence of aluminium accumulation from aluminium-
containing pharmaceutical products and parenteral solutions (Finberg
et al., 1986).
8.6.1 Encephalopathy
Dialysis encephalopathy is a complication of prolonged
haemodialysis first described in 1972 (Alfrey et al., 1972). The main
symptoms are speech disorder followed by the development of dementia,
convulsions and myoclonus. The mean duration of dialysis was 48 months
and the dialysis fluids were made with untreated tap water. Elevated
aluminium contents were found in the brain, muscle and bone tissues
of the affected patients. The same findings were reported from
other dialysis centres in Europe and the USA. Many outbreaks of
encephalopathy have been described in association with the use of
dialysis fluids containing a high concentration of aluminium, usually
above 200 �g/litre (Flendrig et al., 1976; McDermott et al., 1978;
Alfrey, 1978).
In a study with 55 patients suffering from dialysis
encephalopathy in six dialysis centres using a uniform clinical
classification, the incidence of dialysis encephalopathy rose
significantly with increasing cumulative exposure to aluminium via the
dialysate (Schreeder et al., 1983).
Epidemiological studies of dialysis centres in England showed
that encephalopathy was almost non-existent in those centres using
water with aluminium concentrations less than 50 �g/litre to prepare
dialysis fluids. The incidence of encephalopathy rose progressively
with higher water concentrations of aluminium. The Registration
Committee of the European Dialysis and Transplant Association made a
European survey, which showed clusters of encephalopathy in certain
areas of Britain, Spain, Greece and Scandinavia. In Britain, 92% of
the patients in these areas had been treated with dialysis fluids made
from softened tap water (Kerr & Ward, 1988). No signs of overt
aluminium toxicity were observed in 27 long-term haemodialysis
patients on dialysis fluids containing low aluminium concentrations
(Altmann et al., 1989) and these subjects had only mildly elevated
serum aluminium levels. However, defects in several tests of
psychomotor function, including digit coding, were found.
8.6.2 Osteomalacia
Osteomalacia has also been observed in patients with chronic
renal failure, exposed to aluminium in dialysis fluids, or in infants
with renal failure treated with aluminium hydroxide to control
hyperphosphataemia (Ward et al., 1978; Andreoli et al., 1984). Bone
pain, myopathy, pathological fractures and poor response to vitamin D
therapy are the characteristic symptoms of osteomalacia, accompanied
by radiological changes, including partial and complete non-healing
fractures, osteopenia, and reduction in calcified bone area (Simpson
et al., 1973). When aluminium was removed from fluids used for
dialysis, the incidence of osteomalacia diminished. The level without
undue risk was estimated to be 30 �g/litre or less (Platts et al.,
1984). The aluminium content of the bone is increased in patients with
renal disease, treated by haemodialysis, and this aluminium may remain
in the bone even after successful renal transplantation (Ellis et al.,
1979). The aluminium in patients with osteomalacia was found to be
mainly localized at the interface between the osteoid and the
calcified matrix (Cournot-Witmer et al., 1981). Vitamin-D-resistant
osteomalacia due to aluminium is a progressive metabolic bone disease.
The mechanism for the disordered bone formation remains to be
clarified.
8.6.3 Microcytic anaemia
In a study of ten aluminium-intoxicated dialysis patients,
microcytic anaemia was observed. The disease was reversible after
deionization of the dialysis water (Touam et al., 1983). The mechanism
by which an excess of aluminium induces microcytic anaemia remains to
be clarified (Wills & Savory, 1983, 1989).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Den Dooren de Jong (1971) inoculated a medium containing
aluminium chloride (10-4 mol/litre) with three Azobacter strains.
After incubation for 1 to 2 days, the aluminium had produced an
inhibition zone of 14 mm with increased pigmentation at the edges. The
inhibition zone, when compared with that produced by other metals, was
found to indicate intermediate toxicity.
9.1.1.1 Water
Sheets (1957) studied the effect of shock loadings of aluminium
sulfate on the biochemical oxygen demand of sewage sludge and found
that a concentration of 18 mg/litre caused a 50% reduction.
Panasenkov (1987) found that aluminium sulfate at concentrations
of 0.1 and 1 mg/litre had no effect on the heterotrophic fixation of
14CO2 or the number of saprophytes of a natural bacterial coenosis.
However, at 10 mg/litre aluminium sulfate reduced these two parameters
by 50% and 60%, respectively.
Dobbs et al. (1989) studied the effect of complexation on the
toxicity of aluminium using the Microtox system based on measurement
of the light output of a luminescent bacterium. Aluminium citrate
complexes were essentially non-toxic up to levels of at least
25 mg Al/litre. The one-hour EC50, defined as a 50% reduction in
light output, was 300 �g/litre in the absence of citrate. Complexation
by fluoride was complicated by a toxic response to fluoride itself.
Correction for this effect still revealed an appreciable toxicity of
aluminium fluoride complexes. The toxic response in the presence of 9
and 36 mg/litre fulvic acid was reduced by 24% and 61%, respectively.
Hoke et al. (1992) reported 15-min EC50 values (Microtox tests) for
aluminium chloride (as aluminium) of 5.57 and 3.31 mg/litre for
solutions osmotically adjusted with 22% sodium chloride and 20.4%
sucrose, respectively.
9.1.1.2 Soil
Zwarun et al. (1971) found the soil bacterium Bacillus sp. to
be resistant to aluminium. Increasing acidity from pH 6.6 to pH 4.5
reduced the number of surviving cells suspended for 3 h in an acetate
buffer. Addition of 80 mg Al/litre produced no further reduction, even
though the cell walls were saturated with aluminium. Zwarun & Thomas
(1973) exposed the soil bacterium Pseudomonas stutzeri to acidic
conditions at aluminium concentrations of 1, 10 and 80 mg/litre for
3 h. Increasing the acidity reduced the number of surviving cells. The
addition of aluminium (10 mg/litre) at pH 4.5 significantly reduced
the survival rate of cells, and 80 mg/litre was lethal.
Thompson & Medve (1984) studied the effects of aluminium
(0-500 �g/ml) on the growth of the ectomycorrhizal fungi Cenococcum
graniforme, Suillus luteus, Thelephora terrestris, and three
isolates of Pisolithus tinctorius (all four species are commonly
found on spoil tips). T. terrestris was the most sensitive species
showing no growth at aluminium concentrations > 150 �g/ml, whereas
S. luteus was the most tolerant, being unaffected at aluminium
concentrations < 350 �g/ml. The other species showed reduced growth
at all aluminium concentrations. However, the results obtained were
not consistent with field observations.
The severity of the incidence of the fungal disease potato scab
was reduced by the addition of aluminium (Mizuno & Yoshida, 1993).
9.1.2 Aquatic organisms
9.1.2.1 Plants
Algal assays are often carried out in culture media containing
high concentrations of nutrients, including phosphate. These nutrients
ameliorate the toxicity of aluminium, and limit the application of the
results to natural waters. For example, in culture medium the green
alga Chlorella pyrenoidosa successfully grew at concentrations of
up to 12 mg total aluminium/litre at pH 4.6, but an aluminium
concentration of 24 mg/litre was toxic. By selecting those algae which
tolerated high levels of aluminium, the authors were able to develop
algal cultures that grew at up to 48 mg/litre (Foy & Gerloff, 1972).
However, Helliwell et al. (1983) found that maximum toxicity of
aluminium to algal growth was achieved at pH 5.8 to 6.2, 5 �g labile
Al/litre significantly inhibiting the growth of the alga Chlorella
pyrenoidosa in a synthetic hard water. The assay showed that
toxicity is a function of the labile, rather than the total,
aluminium. It was found that Al (OH)2+ was the aluminium species
most toxic to the alga. Similar results were reported by Parent &
Campbell (1994), although they found that polymerized aluminium
species were also toxic.
Bringmann & K�hn (1959) found a toxic threshold of 1.5 to
2 mg/litre aluminium chloride for the green alga Scenedesmus
quadricauda. Rueter et al. (1987) found that a concentration of
60 �mol aluminium/litre at pH 5.7 is required to produce any
inhibitory effect on the growth rate of the S. quadricauda. In an
experiment studying the effect of both copper and aluminium on the
growth of Scenedesmus it was found that the majority of the toxic
action was due to indirect chemical interactions that result in higher
cupric ion activities.
H�rnstr�m et al. (1984) studied the effects of aluminium on 19
freshwater algal species in lake water at pH 5.5. The biotests showed
that 13 of the species, including all of the desmoids and diatoms,
experienced complete growth inhibition at 200 �g/litre. In fact, two
of the four diatom species ( Nitschia actinastroides and Synedra
nana) showed 47% inhibition at 100 �g/litre. The most insensitive
group was the Chrysophyceae, where three out of five species were
unaffected at 400 �g/litre.
Lindemann et al. (1990) studied the impact of aluminium
(4-220 �mol/litre) on green algae ( Scenedesmus sp. and Chlorella
sp.) isolated from acidic and alkaline headwater streams in a semi-
continuous culture media at pH 5. The threshold for inhibition was
found to be less than 4 �mol aluminium/litre when the aluminium was
added to the medium abruptly. When the aluminium was added gradually
there was a lag period, which can be explained by the formation of
polymeric aluminium hydroxy compounds and the precipitation of
aluminium hydroxides and phosphates; this reduces the amount of
aluminium bioavailable to the algae. Folsom et al. (1986) studied
the toxicity of aluminium (0, 10, 25 and 50 mg/litre) to the acid-
tolerant green alga Chlorella saccarophila at pH 3.0 and found a
concentration-dependent growth inhibition. Addition of the fast-
exchange ions Ca2+, Mg2+, Na+ and K+ (150 mg/litre) caused some
reversal of the toxic effects of aluminium.
Exley et al. (1993) studied the effect of aluminium on the
freshwater diatom Navicula pelliculosa and the amelioration of this
toxicity with silicon. An aluminium concentration of 10 �mol/litre
significantly inhibited diatom growth at all but the highest silicic
acid concentration (100 �mol/litre). The mechanism of action was found
to be independent of a direct effect on cell biomass, chlorophyll
a content per cell or the protein content of each cell. The toxicity
of aluminium was mitigated by increasing the nominal phosphorus
concentration from 1 to 10 �mol/litre. Further growth experiments
were carried out on the non-silicon-requiring green alga Chlorella
vulgaris. The rate of growth was significantly inhibited at
48 �mol/litre. The inhibitory effects were again removed at the
highest silicic acid concentration. The authors concluded that the
mechanism of aluminium toxicity was a reduction in the bioavailability
of phosphorus.
Genter & Amyot (1994) exposed freshwater benthic algal
populations to aluminium concentrations of 50, 100 and 500 �g Al/litre
(as aluminium sulfate) at pH 4.8 in artificial streams. During the
28-day test, aluminium in acidified water inhibited the abundance of
diatoms and cyanobacteria (blue-green algae) more than the acidity
alone. Aluminium decreased chlorophyll abundance beyond the effects of
acidity alone at 500 �g/litre.
Nalewajko & Paul (1985) studied the effect of aluminium on
phytoplankton collected from a circumneutral and an acid-stressed
lake. Addition of aluminium (50 �g/litre) caused significant decreases
in microbial phosphate uptake and photosynthesis. The effects were
more pronounced at pH 5.2-6.9 (lake water pH range) than at pH 4.5,
there being larger decreases in phytoplankton from the acid-stressed
lake. The authors reported that the toxicity was due to both the
precipitation of phosphate as particles after addition of aluminium
and the direct effect of aluminium.
Stanley (1974) grew the aquatic angiosperm Eurasian milfoil
( Myriophyllum spicatum) in solutions of aluminium for 32 days. There
was a 50% inhibition of root dry weight, shoot dry weight, root length
and shoot length at aluminium concentrations of 2.5, 7.6, 5.1 and
12.7 mg/litre, respectively.
9.1.2.2 Invertebrates
a) Acute toxicity
The acute toxicity of aluminium to aquatic invertebrates is
summarized in Table 24; 48-h and 96-h LC50 values range from
0.48 mg/litre (polychaete) to 59.6 mg/litre (daphnid). However, care
must be taken when interpreting the results because of the significant
effects of pH on the availability of aluminium.
Bringmann & K�hn (1959) found no effect on Daphnia magna
immobilization at aluminium chloride concentrations of up to
1000 mg/litre over a 48 h exposure period at pH 7.5.
Havens (1990) exposed two acid-sensitive cladocerans ( Daphnia
galeata and D. retrocurva) and one acid-tolerant cladoceran
( Bosmina longirostris) to aluminium concentrations of 200 �g/litre
at pH 5.0 for 24 h. The exposure consistently resulted in nearly 100%
mortality for D. galeata and D. retrocurva, but mortality rates
for B. longirostris were not significantly different from those of
controls (2-6%). Haematoxylin staining procedures revealed that the
daphnids showed marked aluminium binding at the maxillary glands (the
site of ion exchange), whereas B. longirostris showed no noticeable
aluminium binding.
Havas & Hutchinson (1982) studied the tolerance of crustaceans,
collected from acid (pH 2.8) and alkaline (pH 8.2) tundra ponds, to
low pH and elevated levels of aluminium. When adjusted to pH 4.5,
water from acid ponds was more toxic than water from alkaline ponds,
probably due to elevated concentrations of aluminium (up to 20 mg
Al/litre). Removal of heavy metals and aluminium by co-precipitation
significantly reduced the toxicity of adjusted pond
Table 24. Toxicity of aluminium (LC50) to aquatic invertebrates
Organism Size/age Stat/flowa Temperature Hardnessb pH Salt Duration LC50c Reference
(�C) (mg/litre) (h) (mg/litre)
Bivalve stat 20-25 3.5 96 > 1.0 m Mackie
Pisidium stat 20-25 4.5 96 > 0.4 m (1989)
casertanum
Bivalve stat 20-25 3.5 96 > 1.0 m Mackie
Pisidium stat 20-25 4.5 96 > 0.4 m (1989)
compressum
Gastropod stat 20-25 3.5 96 > 1.0 m Mackie
Amnicola stat 20-25 4.5 96 > 0.4 m (1989)
limosa
Hyallela stat 20-25 3.5 96 > 1.0 m Mackie
azteca stat 20-25 4.5 96 > 0.4 m (1989)
Enallagma sp. stat 20-25 3.5 96 > 1.0 m Mackie
stat 20-25 4.5 96 > 0.4 m (1989)
Polychaete stat 20-25 7.6-8.0 chloride 96 > 2.0 n Petrich &
Neanthes Reish
arenaceodentata (1979)
Polychaete stat 7.6-8.0 chloride 96 2.0 n Petrich &
Polychaete stat 7.6-8.0 chloride 96 0.48 n Reish (1979)
Ctenodrilus
serratus
Copepod adult stat 20 7d 8.0 chloride 96 10 Bengtsson
Nitocra (7.5-13.4) (1978)
spinipes
Table 24. (Con't)
Organism Size/age Stat/flowa Temperature Hardnessb pH Salt Duration LC50c Reference
(�C) (mg/litre) (h) (mg/litre)
Water flea < 24 h stat 17-19 44-53 7.4-8.2 chloride 48 3.9 n Biesinger &
Daphnia Christensen
magna (1972)
stat 12-15 240 7.2-7.8 ammonium 48 59.6 Khangarot
sulfate (45.8-73.3) ne & Ray
(1989)
a Stat = static conditions (water unchanged for duration of test)
b Hardness expressed as mg CaCO3/litre
c n = based on nominal concentrations; m = based on measured concentrations
d Salinity (%)
e EC50 based on immobilization
water to crustaceans. The subsequent addition of 20 mg/litre aluminium
resulted in 100% mortality of Daphnia within 20 h, whereas the
addition of other metals (iron, nickel and zinc) did not restore the
toxicity.
Havas (1985) studied the effect of aluminium chloride (0.02, 0.32
and 1.02 mg Al/litre), pH (6.5, 5.0 and 4.5) and calcium (2.5 and
12.5 mg/litre) on the survival of Daphnia magna during a 48-h
exposure period. Maximum aluminium toxicity was observed at pH 6.5 and
a calcium concentration of 2.5 mg/litre. A pH of 5.0 was toxic to
D. magna in soft water, 50% of the daphnids being immobilized within
24 h. Aluminium marginally increased the toxicity of water at pH 5.0.
At pH 4.5, high concentrations of aluminium significantly reduced the
hydrogen ion toxicity. However, this amelioration was short lived and
all of the Daphnia had died within 24 h. Havas & Likens (1985)
exposed the crustaceans Daphnia catawba and Holopedium gibberum,
and the insect larvae Chaoborus punctipennis and Chironomus
anthrocinus to the same aluminium concentrations (0.02, 0.32 and
1.02 mg Al/litre) at pH levels of 3.5, 4.0, 4.5, 5.0 and 6.5. The
crustaceans were exposed for 72 h and the insect larvae for 168 h.
D. catawba was the most acid-sensitive species, mortality being
significantly increased at and below pH 5.0; high concentrations
of aluminium significantly increased mortality only at pH 6.5.
H. gibberum was less sensitive to both hydrogen ions and aluminium
than D. catawba. The highest aluminium concentration was moderately
toxic at pH 6.5; however, as with D. catawba, the effect of
aluminium at lower pH was completely masked by hydrogen ion toxicity.
Neither aluminium nor hydrogen ions affected the mortality of
C. punctipennis or C. anthrocinus.
Lamb & Bailey (1981) studied the effects of aluminium sulfate on
larvae of the midge Tanytarsus dissimilis at pH 7.8. There was no
apparent effect of aluminium on either second or third instar larvae
at aluminium sulfate doses of between 80 and 960 mg/litre after 96 h.
Owing to the polymeric, coagulant nature of aluminium sulfate, a white
grey precipitate (up to 3-4 mm) formed in all solutions.
Six common macro-invertebrates were exposed to 200 �g/litre
aluminium sulfate at pH 4.5 and a calcium concentration of
2.45 mg/litre. The order of acid sensitivity (mean 48-h survival is
given in parentheses) for the species tested was: Caenis sp. (2%)
> Hyalella azteca (12%) > Enallagma sp. (20%) > Gyraulus sp.
(55%) > Chironomidae (94%) > Hydracarina (99%). Aluminium
significantly reduced survival still further in H. azteca, Gyraulus
sp. and Chironomidae. However, the addition of aluminium significantly
increased survival for Enallagma sp. and Caenis sp. when compared
with the acid-only group (Havens, 1993).
b) Long-term toxicity
France & Stokes (1987) studied the effect of nominal aluminium
concentrations of 0.05 to 0.70 mg/litre on the hydrogen ion toxicity
to the amphipod Hyalella azteca over an 8-day period. Aluminium
concentrations of 0.25 and 0.40 mg/litre at pH 4.8 and 0.40 mg/litre
at pH 4.3 significantly increased the mortality of H. azteca
compared with that in reference aluminium concentrations of
0.05 mg/litre. Mortality rates remained unchanged with the addition of
0.25 mg Al/litre at pH 4.3 or 5.3 and with either 0.40 mg Al/litre or
0.70 mg Al/litre at pH 4.0. The authors predicted from these results
that mortality of this amphipod from springmelt pulses will be
determined primarily by hydrogen ions and only secondarily by
aluminium in the pH range 4.3 to 5.3. Berrill et al. (1985) found no
effect of aluminium (up to 200 �Eq/litre) on the accumulated 10-day
mortality caused by hydrogen ions in the crayfish Orconectes
rusticus, O. propinquus and Cambarus robustus.
Biesinger & Christensen (1972) exposed water fleas ( Daphnia
magna) to aluminium chloride for a period of three weeks in Lake
Superior water (pH 7.4-8.2). An LC50 of 1.4 mg total aluminium/litre
was calculated and the EC50, based on reproductive impairment, was
found to be 0.68 mg/litre.
Burton & Allan (1986) exposed three species of stream
invertebrates ( Nemoura, Asellus and Physella) to aluminium
concentrations of 250 or 500 �g/litre at pH 4, 5 and 7 for 28 days in
experimental streams. Survival of all species was significantly
decreased at pH 4; the addition of aluminium at 15�C did not cause
additional mortality. However, at 2�C or with low organic matter the
addition of 500 �g/litre caused a significant additional mortality for
both Nemoura and Asellus. Addition of citrate reduced the effect
of aluminium in low-organic treatments.
Petrich & Reish (1979) studied the effect of aluminium chloride
(pH 7.6-8.0) on the polychaetes Neanthes arenaceodenata, Capitella
capitata and Ctenodrilus serratus. Neither Capitella nor Neanthes
were affected by a 7-day exposure to 2 mg/litre aluminium chloride
(the maximum concentration that could be used without precipitation in
seawater). Ctenodrilus showed significant reproductive suppression
during a 28-day exposure to aluminium chloride concentrations of
0.5 mg/litre or more.
c) Physiological and biochemical effects
Herrmann & Andersson (1986) exposed the nymphs of three mayfly
species Heptagenia fuscogrisea, H. sulphurea and Ephemera danica
to total inorganic monomeric aluminium levels of 500 and 2000 �g/litre
at pH 4.0 and 4.8 for 10 days. The oxygen consumption rate of nymphs
was monitored. The rate showed a tendency to increase at 500 �g/litre
for H. sulphurea and E. danica. At 2000 �g/litre there were
significant increases in the oxygen consumption rate for all three
species at both pH levels. E. danica, which is restricted to less
heavily acidified regions, was the most severely affected by the
aluminium treatments. Exposure of E. danica and H. sulphurea to
the same aluminium and pH regimes for 14 days caused significant
aluminium-related decreases in sodium levels (Herrmann, 1987).
Malley & Chang (1985) studied calcium-45 uptake by postmoult
crayfish ( Orconectes virilis) exposed to aluminium chloride
concentrations of 200, 500 and 1000 �g Al/litre for 2 to 3 h at pH 5.3
to 7.2. An aluminium concentration of 200 �g/litre had no effect on
calcium uptake at neutral pH. However, reducing the pH to 5.5 caused
an inhibition of calcium uptake. Exposure of crayfish to 500 �g/litre,
under acidic conditions, also caused a significant reduction in
calcium uptake. However, exposure to acidic conditions alone revealed
that most of the reduction was due to acidic conditions rather than
aluminium. In fact, transferring the crayfish from 500 �g Al/litre to
1000 �g Al/litre, under acidic conditions, had no significant effect.
Witters et al. (1984) maintained the air-breathing water bugs
( Corixa punctata) at aluminium chloride concentrations of 0.15, 0.3
(the natural level), 2.5, 5, 10 and 50 mg Al/litre at pH 3 and 4. A
dose-related decrease in sodium-influx was observed and there was a
significant 50% decrease when comparing the lowest concentration with
10 mg/litre.
d) Population studies
Havens (1991) studied the effect of aluminium on the survival
of littoral zooplankton species collected from alkaline lakes.
Toxicity tests were performed at pH 4.5 with or without aluminium
(500 �g/litre) for 24 h. The four cladocerans Simocephalus
serrulatus, Diaphanosoma birgii, Acantholeberis curvirostris and
Chydorus sphaericus were unaffected by either the acidic conditions
or aluminium. The cladoceran Eurycercus lamellatus and the copepod
Acanthocyclops vernalis suffered 100% mortality at pH 4.5 with or
without aluminium. The cladocerans Camptocercus rectirostris, Alona
costata and Pleuroxus denticulatus and the copepod Mesocyclops
edax showed decreased survival at pH 4.5 and a significantly greater
decrease in survival under acid conditions and aluminium exposure.
Havens & Heath (1989) carried out an in situ mesocosm study of
zooplankton responses to acidification and aluminium. Large plastic
enclosures were acidified (pH 4.5) with or without the addition of
aluminium, giving an inorganic monomeric aluminium concentration of
180 �g/litre. The populations of acid-sensitive species declined more
rapidly in the acid-plus-aluminium treatment than in the acid-alone
treatment. Two cladocerans ( Bosmina longirostris and Chydorus
sphaericus) were tolerant to acidity and aluminium. Havens & Decosta
(1987) performed bioassays using in situ enclosures to expose
zooplankton to acidified waters (pH 4.7) with and without the addition
of aluminium (300 �g/litre) for up to 49 days. Acidification did not
affect abundance of zooplankton or succession because all species were
acid-tolerant. However, addition of aluminium resulted in a reduction
in zooplankton abundance.
9.1.2.3 Fish
The bioavailability and toxicity of aluminium varies with its
chemical speciation. In the case of fish, higher polymers are less
toxic than monomers and polymers of low relative molecular mass.
Polymerization is a slow process, hence the biological activity of
aluminium in water depends not only on aluminium concentration and
conditions such as pH, temperature and the presence of complexing
ions, but can also depend on the pre-history of the water. The various
aluminium species differ in their effects on fish gills, either
disturbing the ion balance or interfering with respiration. The
toxicity diminishes if the aluminium is inactivated by complexation
with organic ligands, fluoride or silicate, or by extensive
polymerization to large molecules in the water (Rosseland & Staurnes,
1994).
a) Acute toxicity
The acute toxicity of aluminium to fish is summarized in Table
25. The 96-h LC50 values range from 0.095 mg/litre (American
flagfish) to 235 mg/litre (mosquito fish). However, care must be taken
when interpreting these results because of the significant effects of
pH on the availability of aluminium. The wide range of LC50 values
probably reflects this variable availability. LT50 values for
salmonids are also summarized in Table 25. Muramoto (1981) found that
addition of the complexans NTA and EDTA reduced the acute (48-h)
toxicity of aluminium to carp ( Cyprinus carpio).
Rosseland & Skogheim (1984) exposed three salmonid species,
Atlantic salmon ( Salmo salar), brown trout ( Salmo trutta) and
brook trout ( Salvelinus fontinalis), to inorganic monomeric
aluminium concentrations of 120, 225 and 415 �g/litre (as aluminium
sulfate) under flow-through conditions. Owing to the acidity of the
aluminium sulfate the pH decreased from 6.6 to 4.9. Pre-smolt salmon
were the most sensitive, showing 100% mortality within 48 h at
245 �g/litre. Brook trout were the least sensitive, mortalities only
Table 25. Toxicity of aluminium to fish (laboratory studies)
Organism Size/ Stat/flowa Temperature Hardnessb Calcium pH Salt LT50 (mg 96-h LC50c Reference
age (�C) (mg/litre) concentration inorganic (mg Al/litre)
(mg/litre) monomeric
(Al/litre)
Atlantic 1 + flow 5.2 5 2.0 4.95 sulfate 59 0.245 m Rosseland &
salmon 2 + 4.95 33 0.245 m Skogheim
(Salmo salar) 1 + flow 5.2 5 2.0 4.94 sulfate 57 0.313 m (1984)
2 + 4.94 sulfate 22 0.313 m
1 + 4.90 27 0.463 m
2 + 4.90 15 0.463 m
Brown trout 2 + flow 5.2 5 2.0 4.94 sulfate 40 0.313 m
(Salmo trutta) 1 + 4.90 57 0.463
2 + 4.80 30 0.463
Atlantic 2 + stat 3.7 1.3 5.06 chloride 108 0.075 Skogheim &
salmon (38 g) 1.3 4.92 38 0.137 m Rosseland
(Salmo salar) 1.3 4.90 32 0.177 m (1986)
Mummichog 2.7 g stat 20 6.6d ammonium 3.6 n Dorfman
(Fundulus sulfate (1977)
heteroclitus) 2.7 g stat 20 17d ammonium 27.5 n
sulfate
2.7 g stat 20 7.9d chloride 3.6 n Dorfman
2.7 g stat 20 18.8d chloride 31.5 n (1977)
Mosquito fish stat 20-21 4.3-7.2 chloride 133 n Wallen et al.
(Gambusia stat 19-22 4.4-7.7 sulfate 235 n (1957)
affinis)
Table 25. (Con't)
Organism Size/ Stat/flowa Temperature Hardnessb Calcium pH Salt LT50 (mg 96-h LC50c Reference
age (�C) (mg/litre) concentration inorganic (mg Al/litre)
(mg/litre) monomeric
(Al/litre)
Fathead 0.45 g stat 22 38 7.4 nitratee 4.25 Mayer &
minnow (3.3-5.5) Ellersieck
(Pimephales 0.45 g stat 22 38 7.4 sulfatef 4.4 (1986)
promelas) (3.4-5.6)
American 2-3 stat 25 6.0 5.8 0.095 m Hutchinson &
flagfish days Sprague
(Jordanella (1986)
floridae)
a Stat = static conditions (water unchanged for duration of test) d Salinity (%)
b Hardness expressed as mg CaCO3/litre e 7.2% technical material
c n = based on nominal concentrations; m = based on measured concentrations f 8.1% technical material
occurring at 463 �g/litre (less than 25% over the 64-h exposure). The
authors reported that whenever aluminium sulfate was added excessive
mucus was observed between the gill lamellae; or all species mucus
clogging increased with an increased addition of aluminium. However,
there was no excessive mucus on gills of fish that died in acid brook
water with naturally occurring aluminium concentrations.
Schofield & Trojnar (1980) exposed brook trout ( Salvelinus
fontinalis) fry to aluminium (0.1-0.5 mg/litre) at various pH levels
(4.0-5.2). At pH 4.0 survival of fish was not related to aluminium
concentration, the LT50 values ranging from 2.8 to 5.2 days. However,
at pH levels of > 4.4, mortality increased with increasing
aluminium concentration. At pH 4.9 and 5.2 neither acidity nor 0.1 mg
Al/litre affected fish mortality; 0.5 mg Al/litre produced LT50
values ranging from 1.6 to 3.3 days. Symptoms of stress were darkening
of skin coloration and cessation of feeding. All fish at pH 4.0 and
4.4 showed these symptoms, although they took longer to develop at pH
4.4 with 0 or 0.1 mg/litre aluminium. No symptoms were observed at pH
4.9 and 5.2 for aluminium concentrations of 0.1 mg/litre; however,
stress symptoms were seen in all groups exposed to > 0.25 mg/litre
aluminium at any pH level. Heavy accumulations of mucous and cellular
debris on the gills were found in trout exposed to > 0.25 mg/litre
aluminium at pH levels of > 4.4. Histopathological changes
observed in sections of gills from fish exposed to aluminium levels
> 0.5 mg/litre included cell proliferation at the distal ends of
gill filaments, lamellar oedema and fusion, epithelial desquamation,
filament collapse, and general loss of gill structure.
Gundersen et al. (1994) exposed rainbow trout ( Oncorhynchus
mykiss) to aluminium at pH values ranging from 7.97 to 8.56 in 96-h
tests. No significant mortality was observed at pH 8.33 or less and
filterable aluminium concentrations of 0.52 mg/litre or less. However,
100% mortality was found at pH 8.58 and a filterable aluminium
concentration of 1 mg/litre. The 96-h LC50 values ranged from 0.36 to
0.79 mg filterable aluminium/litre at weakly alkaline pH levels.
Young brown trout ( Salmo trutta) exposed for 5 days to pH 5 in
high calcium water at temperatures of 4 and 12�C showed no alterations
in growth or in mucous cell concentration and volume. However,
exposure to aluminium (230 �g/litre) under the same testing regime
resulted in significant growth depression but no changes to mucous
cell morphometrics (Segner et al., 1988).
Freeman & Everhart (1971) found that the toxicity of aluminium
hydroxide complexes (5.2 mg/litre) to rainbow trout ( Oncorhynchus
mykiss) increased with the amount of aluminium dissolved. At pH 6.8,
8.0, 8.5 and 9.0 the amounts of aluminium dissolved were 1%, 10%, 31%
and 97%, respectively, and the respective LT50 values were 38.90,
31.96, 7.46 and 2.98 days. Surviving fish recovered rapidly in all
groups, except those exposed at pH 8.0, with normal growth being
resumed within 2 weeks (Freeman, 1973).
b) Long-term toxicity
Hickie et al. (1993) exposed rainbow trout ( Oncorhynchus
mykiss) to aluminium for 23-26 days after hatching at pH 5.8 and
4.9. The 144-h LC50 for total aluminium was found to be > 1050
and 91 �g/litre at the two pH levels, respectively. An LC50 of
1.17 �g/litre was calculated for fish exposed from 16 to 19 days after
hatching at pH 4.9.
Neville & Campbell (1988) exposed juvenile rainbow trout
( Oncorhynchus mykiss) to aluminium (2.8 �mol/litre nominal
concentration) in a flow-through system over a pH range of 4.0 to 6.5
for up to 11 days. The response of trout to aluminium was most severe
at pH 4.5 (electrolyte loss) and 6.1 (asphyxia). At pH 4.0 there was
competition between hydrogen ions and aluminium for binding at the
gill surface which reduced toxicity. However, the toxic response at pH
6.1 appeared to be more complex being either a bimodal response to two
different aluminium species or a physical response to precipitation on
the gill surface.
Driscoll et al. (1980) studied the toxic effect of aluminium on
brook trout ( Salvelinus fontinalis) fry. At pH values of 4.4 and 5.2
there was no effect on survival during the 14-day exposure period. The
addition of inorganic monomeric aluminium (0.42-0.48 mg/litre)
produced an LT50 of 115 h at pH 5.2 and 256 h at pH 4.4. Treatment
with excess fluoride or citrate reduced the toxicity of aluminium.
Skogheim & Rosseland (1986) exposed Atlantic salmon ( Salmo salar) to
aluminium at varying pH levels. No mortality occurred during a 20-day
exposure to pH 5.07 alone. At pH 5.06 and 75 �g Al/litre the LT50 was
108 h, at pH 4.92 and 137 �g Al/litre the LT50 was 38 h, and at pH
4.9 and 177 �g Al/litre the LT50 was 32 h. Brown (1983) exposed brown
trout ( Salmo trutta) to aluminium (0, 0.25 and 0.5 mg/litre) at pH
values ranging from 4.5 to 5.4 and calcium concentrations ranging from
0.5 to 2.0 mg/litre for 16 days. Survival was relatively unaffected by
pH except at a calcium level of 0.25 mg/litre and a pH of 4.5. High
mortality was observed at both aluminium exposure levels at calcium
levels of 0.25 and 0.5 mg/litre. At calcium levels of 1.0 and
2.0 mg/litre there was increased mortality at the highest aluminium
concentration.
Gundersen et al. (1994) studied the effects of aluminium on
rainbow trout ( Oncorhynchus mykiss) in 16-day tests. Growth rates
were higher at weakly alkaline pH (7.97-8.10) than at near-neutral pH
(7.30-7.35). The authors concluded that polymeric and colloidal forms
of aluminium are more potent than soluble forms in restricting growth.
Trout exposed to aluminium at 0.53 to 2.56 mg/litre and humic acid at
4.31 to 5.23 mg/litre had higher specific growth rates and lower
mortality than those exposed to aluminium and no humic acid at all the
pH values tested. When exposed to sub-lethal concentrations of
aluminium (38 �g/litre nominal concentration) in a synthetic soft
water of pH 5.2, rainbow trout became acclimated to aluminium and
showed increased resistance when exposed to lethal levels of aluminium
(162 �g/litre nominal concentration) in the same soft water.
Acclimation was associated with reduced disturbances of ionoregulation
and respiration (Wilson et al., 1994a). Acclimation to 38 �g/litre
(nominal concentration) also caused a 4-fold increase in gill mucous
density and a reduction in apparent lamellar surface area (Wilson et
al., 1994b). Acclimation to sub-lethal levels of aluminium could
explain the continued presence of fish populations in acidified lakes
and rivers containing more than 100 �g Al/litre. Wicklund Glynn et al.
(1992) exposed minnows ( Phoxinus phoxinus) to acidic water (pH 5.0)
with and without aluminium (150 �g/litre) at various calcium (0, 0.07
and 2 mmol/litre) and humus (5 and 25 Pt) concentrations for 15 days.
Mortality among fish exposed to aluminium was higher than among
unexposed fish but was less at the highest calcium level. At 0.07 mmol
calcium/litre, the aluminium-induced mortality was reduced by the
presence of humus. Gill morphology was altered after exposure to
aluminium at pH 5.0, but was not affected by different concentrations
of calcium or humus.
Juvenile brook trout ( Salvelinus fontinalis) were
intermittently or continuously exposed to aluminium (0.2 to
1.2 mg/litre) at pH 4.4 or 4.9 for 24 days. There was 100% survival of
fish at both pH levels in the absence of aluminium, regardless of
exposure regime. Aluminium significantly reduced survival at
0.2 mg/litre or more for all exposure regimes except the intermittent
exposure at pH 4.4 where significant mortality was observed at
0.4 mg/litre or more. When aluminium concentration was expressed as
the 24-day mean, it was shown that intermittent exposure was more
toxic than continuous exposure (Siddens et al., 1986). Ingersoll et
al. (1990) exposed 1-year-old brook trout ( Salvelinus fontinalis) to
combinations of aluminium, pH and calcium during a 28-day experiment.
Survival was reduced at inorganic monomeric aluminium concentrations
of 29 �g/litre at pH 5.2 and > 228 �g/litre at pH 4.4 or 4.8. Fish
weight was reduced at an aluminium concentration of > 34 �g/litre
and pH < 4.8. The gills sampled from low pH groups showed lifting of
the outer epithelium and hypertrophy of chloride and epithelial cells.
These effects were more pronounced at low pH with elevated aluminium
concentrations. Effects such as vacuolation and degeneration of
epithelial and chloride cells and the presence of dense cells were
also observed at low pH and elevated aluminium concentration.
No mortality of lake trout ( Salvelinus namaycush) embryos
occurred during 5-day exposures to aluminium sulfate (0, 100 and
200 �g Al/litre) at pH 5.0 or during 21- and 32-day recovery periods.
None of the embryos or later alevins displayed erratic swimming
behaviour or mucus accumulation around the mouth or gills. After
21-day (late embryos) and 32-day (early embryos) recovery periods,
fish at the highest aluminium concentrations were significantly
smaller in length, had reduced whole body concentrations of calcium
and potassium, and were significantly less successful as predators on
Daphnia magna (Gunn & Noakes, 1987).
Cleveland et al. (1991) exposed brook trout ( Salvelinus
fontinalis) to a nominal aluminium concentration of 200 �g/litre
for 56 days under flow-through conditions at pH 5.3, 6.1 and 7.2.
The weights of trout exposed to pH 5.3 and 6.1 did not differ
significantly throughout the study. After day 3 fish exposed to pH 7.2
weighed significantly more than those at pH 5.3 and 6.1. Mortality was
significantly higher in brook trout exposed to pH 5.3 than in those
exposed to pH 6.1 (except on day 56) or 7.2.
c) Lifestage effects
Not only do species differences in response to a given pH and
aluminium concentration exist but great differences in sensitivity
also exist between strains of the same species as well as between
different life-history stages (Rosseland et al., 1990; Rosseland &
Staurnes, 1994).
Fivelstad & Leivestad (1984) studied the toxicity of aluminium to
different life-stages of Atlantic salmon ( Salmo salar) and brown
trout ( Salmo trutta). To study the effect of acidity citrate was
added. Only 1 of 200 swim-up salmon larvae died during a 108-h
exposure at pH 4.9; no behavioural responses were observed. However,
when exposed to aluminium concentrations ranging from 110 to
300 �g/litre, salmon swim-up larvae were more sensitive than the
postlarval stage. Toxicity was found to be most significantly
correlated with inorganic monomeric aluminium concentration, survival
time decreasing with increasing aluminium concentration. The LT50 at
an inorganic monomeric aluminium concentration of 148 �g/litre was
26 h for swim-up larvae. Exposure of salmon parr to natural aluminium
variations (50-180 �g/litre) at pH 5.3 induced hyperventilatory
responses together with increases in haematocrit and small decreases
in chloride. The authors concluded that coughing, hyperventilation and
excessive mucous clogging on the gill surface was due to an irritant
effect of aluminium. Brown trout exposed at pH 5 to the same aluminium
regime showed no sublethal stress symptoms.
Rosseland & Skogheim (1984) demonstrated the increased
sensitivity of Atlantic salmon undergoing smoltification compared to
younger year classes. In a laboratory study at pH 4.9-5.0 and
inorganic monomeric Al concentrations of 130-463 �g/litre, pre-smolt
(age 2 years) were more sensitive than parr (age 1 year) in all
combinations of pH and aluminium. For instance, at pH 4.95 and 245 �g
Al/litre, the LT50 for pre-smolt was 35 h whereas the LT50 for parr
was 60 h.
Early life-stage (fertilized eggs, alevins and swim-up fry)
golden trout ( Oncorhynchus aguabonita aguabonita) were exposed to
low pH (4.5-6.5) and aluminium (50-300 �g Al/litre, nominal
concentration) for 7 days. Significant mortality occurred at pH 4.5
in the absence of aluminium, at pH 5.5 in the presence of 100 �g
aluminium/litre for larvae and at pH 5.0 with 300 �g aluminium/litre
for alevins. The duration of swimming and feeding activity was
unaffected by treatment in golden trout exposed as eggs. Locomotory
behaviour of alevins was severely inhibited at both pH 5.0 and 5.5
irrespective of treatment and at pH 4.5 and 6.0 in aluminium-exposed
fish. Feeding activity was reduced at pH 4.5, at pH 5.0 with
> 50 �g aluminium/litre and at pH 5.5 with 100 �g/litre. Swimming
activity was not greatly affected among fish exposed as swim-up
larvae. Feeding activity was greatly inhibited at all aluminium
concentrations and at pH 4.5 (DeLonay et al., 1993).
Farag et al. (1993) studied the effect of aluminium (50-300 �g
Al/litre, nominal concentration) on eggs, eyed embryos, alevins and
swim-up larvae of cutthroat trout ( Oncorhynchus clarki bouvieri) at
pH values ranging from 4.5 to 6.5 for either 7 days or during
continuous exposure until 40 days after hatching. Fish survival
decreased when pH was lowered to 5.0 or 4.5 for 7 days during the egg
stage. Alevin and swim-up larval stages were less sensitive to low
pH and more sensitive to aluminium, with 100 �g/litre at pH 5.0
significantly decreasing survival. The eyed embryo stage was the most
resistant; there was > 90% survival in all groups except in the
presence of > 100 �g/litre at both pH 5.0 and 5.5. During continuous
exposure, survival decreased with time and individuals died earlier in
each life-stage when exposed to combinations of pH and aluminium than
did those exposed to pH alone. Swim-up larvae were the most sensitive
group with regard to growth and all larvae exposed to 50 �g/litre
showed significantly reduced growth.
Buckler et al. (1987) exposed striped bass ( Morone saxatilis)
of different ages to total aluminium (up to 400 �g/litre) at various
pH levels (pH 5.0-7.2) in a flow-through diluter system for 7 days.
Eleven-day-old fish showed significant mortality at pH 6.0
irrespective of aluminium exposure; significant mortality was observed
at 25 �g/litre for pH 6.5 and at 400 �g/litre for pH 7.2. Older fish
(160 days) were less sensitive, showing significant mortality at 50 �g
Al/litre for pH 6.0, 200 �g/litre for pH 6.5, and 400 �g/litre for pH
7.2. In a similar test, 300 �g/litre was lethal to 100% of both 159-
and 195-day-old bass at pH 5.5, but produced no observable adverse
effects at pH 6.5 or 7.2. Mortality among 159-day-old fish held in
water at pH 5.5 without aluminium was 22% after 7 days, there being no
deaths among control fish. No mortality was observed among 195-day-old
fish exposed to pH 5.5 alone.
Eggs, larvae and post-larvae of white suckers ( Catostomus
commersoni) and brook trout ( Salvelinus fontinalis) were exposed
to pH levels of 4.2 to 5.6 and inorganic monomeric aluminium
concentrations ranging from 0 to 0.5 mg/litre. White sucker embryos
were very sensitive to low pH levels, with none surviving to the eyed
stage at pH levels of 5.0 or less. The addition of aluminium increased
embryo survival through the eyed stage but did not increase hatching.
At pH levels of 5.4 and 5.6 survival to eyed stage increased to 38% to
69% and was 74% to 81% in controls. At pH levels above 5.2 the
presence of aluminium resulted in embryo deformities. Survival of
trout eggs through the eyed stage was unaffected at pH 4.6 or more
irrespective of aluminium treatment. However, in the absence of
aluminium at pH levels of 4.4 survival decreased and at pH 4.2 no
embryos survived. The addition of aluminium to low pH groups
significantly increased survival through the eyed stage to hatching.
All white sucker larvae died within 146 h at pH levels below 5.0 with
or without aluminium. In the absence of aluminium at pH levels greater
than 5.0, more than 80% survived the 13-day experiment; however, the
addition of aluminium further decreased the survival of larvae. At pH
levels of 4.4 or more > 97% of trout larvae survived without
aluminium. The addition of more than 0.1 mg Al/litre at all pH levels
decreased the survival of larvae. White sucker post-larvae were
sensitive to low pH levels, only 16 to 68% surviving at pH 4.6. The
addition of aluminium to acidic solutions further decreased survival.
In the presence of high levels of aluminium (0.3 or 0.5 mg/litre) at
all pH levels, all post-larvae died within 75 h. At aluminium levels
of 0.1 and 0.2 mg/litre at low pH levels all post-larvae died within
145 h. Brook trout post-larvae were tolerant of low pH levels. All
post-larvae survived at pH levels ranging from 6.99 to 4.22 without
aluminium. At aluminium levels of 0.2 mg/litre or more survival was
decreased at all pH levels (Baker & Schofield, 1982).
Cleveland et al. (1986) carried out a partial life-cycle toxicity
study on brook trout ( Salvelinus fontinalis) for 60 days in a flow-
through proportional diluter. Eyed brook trout eggs and the resultant
larvae were exposed in water containing 3 mg calcium/litre at
nominal pH values of 7.2, 5.5 and 4.5 with and without aluminium
(300 �g Al/litre, nominal concentration) until 30 days after hatching.
Mortality of trout eggs was not influenced by aluminium but was
significantly increased by low pH. Larval growth and mortality was
unaffected by aluminium at pH 7.2 and 4.5, but mortality was
significantly increased and growth decreased by aluminium at pH 5.5.
DNA and RNA content was significantly reduced by aluminium at pH 5.5.
In general swimming and feeding behaviour were unaffected by aluminium
at pH 7.2 and significantly reduced by aluminium at pH 5.5. At pH 4.5
behaviour was inhibited to such an extent that possible effects of
aluminium were masked. In a second experiment 37-day-old trout were
exposed to similar conditions. Mortality was significantly increased
by aluminium at pH 5.5 and 4.5. Aluminium significantly reduced growth
at pH 7.2 and 5.5. DNA and RNA content was significantly increased by
aluminium at pH 5.5. Juvenile trout behaviour was less affected by
acid conditions than in the case of larvae; there were significant
decreases in behaviour in the presence of aluminium at pH 5.5 and 4.5.
Hunn et al. (1987) utilizing a similar experimental set-up found that
embryo mortality exceeded 80% at pH 4.5, averaged 15% to 18% at pH 5.5
and was less than 2% at pH 7.5. Aluminium significantly increased
mortality at pH 4.5 but did not affect mortality at pH 5.5 or 7.5.
Hatching success was pH-dependent and was not influenced by aluminium
exposure. Brook trout larvae suffered 100% mortality at pH 4.5, 20%
mortality at pH 7.5 with or without aluminium, 69% mortality at pH 5.5
without aluminium and 100% mortality within 15 days with aluminium.
Cleveland et al. (1989) reported that 60-day no-observed-effect
nominal concentrations for aluminium at pH 5.6 to 5.7 were 29 �g/litre
for swimming capacity, 68 �g/litre for weight and 142 �g/litre for
frequency of movement (2 min period), strike frequency (directed at
prey), fry mortality and length. At pH 6.5 to 6.6 no-observed-effect
concentration (NOEC) values were 88 �g/litre for length and weight,
169 �g/litre for fry mortality and > 350 �g/litre for movement,
strike frequency and swimming capacity.
d) Physiological and biochemical effects
Physiological and biochemical effects have recently been reviewed
by Rosseland & Staurnes (1994).
Witters (1986) studied the effect of total aluminium
(350 �g/litre), pH (4.1 and 6.1) and calcium concentration (38 and
190 �Eq/litre) on ion balance and haematology in rainbow trout
( Oncorhynchus mykiss) exposed for 3.5 h. None of the treatment
combinations affected the number of erythrocytes or the haemoglobin
content. However, exposure to aluminium under acidic conditions
significantly reduced plasma osmolarity and increased plasma potassium
levels. Plasma sodium and chloride levels were significantly reduced
under acidic conditions with or without aluminium. The haematocrit
value was significantly increased and plasma ammonium decreased by
aluminium only under acidic conditions and at low calcium
concentration.
Muniz & Leivestad (1980) reported that brown trout ( Salmo
trutta) exposed to acidic conditions (pH 4.3 to 5.5) showed plasma
losses of both chloride and sodium. These losses were enhanced by the
addition of total aluminium at 900 �g/litre. Fish showing signs of
stress exhibited hyperventilation, coughing and excessive mucus
clogging of the gills. The authors reported that the changes in blood
electrolytes indicate that aluminium toxicity is similar to that seen
with hydrogen ion stress. However, aluminium can cause such effects at
pH levels that are not physiologically harmful.
Wood & McDonald (1987) studied the physiology of brook trout and
rainbow trout exposed to inorganic monomeric aluminium concentrations
ranging from 111 to 1000 �g/litre (as aluminium chloride) at pHs
ranging from 4.4 to 6.5. Acid stress alone for 10 days was not lethal
to adult brook trout, but there was a net loss of sodium and chloride
ions. The addition of aluminium resulted in an increased loss of ions
and severe mortality. At pH 4.8, low calcium level (25 �Eq/litre) and
an aluminium concentration of 333 �g/litre, the LT50 was found to be
39 h. At a lower pH (4.4) the average survival time was twice as long.
The cause of death was ionoregulatory failure. However, increasing the
calcium levels (400 �Eq/litre) still killed fish almost as quickly but
the cause of death was respiratory disturbance. Rainbow trout were
more sensitive to both acid and acid/aluminium than brook trout.
Respiratory disturbances were found to be the cause of death in both
high and low calcium groups exposed to acid/aluminium conditions. For
both species there was a correlation between toxic effects and
aluminium accumulation in gills.
Dalziel et al. (1986) exposed brown trout to nominal aluminium
concentrations of 8 �mol/litre at pH levels ranging from 7.0 to 4.0
and calcium levels of 10 or 50 �mol/litre. Low pH had little effect on
the influx of sodium, but the addition of aluminium significantly
reduced influx at pH 4.5 and 4.0. Efflux of sodium tended to be
increased by low pH, but no further effect was caused by aluminium.
Aluminium at higher pH values appeared to have no effect on sodium
fluxes. Dalziel et al. (1987) found that reduced pH levels had no
effect on the sodium influx in brown trout ( Salmo trutta). However,
the presence of aluminium at concentrations of 2 �mol/litre at pH 4.5
and 4.0 significantly decreased sodium influx. At pH 5.4 there was no
effect of aluminium on influx. Sodium efflux was significantly
increased at low pH. Increasing the aluminium concentration at pH 5.4,
and to a smaller extent at pH 4.5, tended to increase efflux. There
was no effect of aluminium on sodium efflux at pH 4.0.
Booth et al. (1988) studied the effects of total aluminium at
concentrations of 333 and 1000 �g/litre and at low pH (5.2-4.4) on
net ion fluxes and ion balance in the brook trout ( Salvelinus
fontinalis) over a period of 11 days. Low pH caused a pH-dependent
net loss of sodium and chloride ions and the addition of aluminium
increased this loss. The authors reported that any fish losing more
than 4% of total sodium ions during the initial 24 h of aluminium
exposure was 90% more likely to die. All fish exposed to aluminium
accumulated it on gill surfaces; fish that died accumulated more
aluminium than survivors.
Leivestad et al. (1987) studied the effect of inorganic monomeric
aluminium on Atlantic salmon ( Salmo salar) exposed for 28 weeks at
pH levels of 4.8-6.5 and aluminium concentrations of 50-350 �g/litre.
The authors found that failure in ionic regulation was the primary
cause for mortality and that Na-K-ATPase activity was reduced at toxic
aluminium levels. The symptoms were correlated with ion-exchangeable
aluminium, precipitating aluminium hydroxide having low toxicity.
Staurnes et al. (1993) maintained smolting Atlantic salmon in soft
water at pH 5 both with and without 50 �g/litre total aluminium.
Exposure to acid water resulted in osmoregulatory failure and high
mortality, and aluminium greatly enhanced the toxicity. Sensitivity to
acid and acid-aluminium increased when fish had developed to seawater-
tolerant smolts. Gill carbonic anhydrase activity was reduced by
aluminium exposure. Fish in both treatment groups had low seawater
tolerance and this was related to a decline in Na+/K+-ATPase
activity.
Hutchinson et al. (1987) studied the effects of total aluminium
(0-1000 �g/litre) at pH levels ranging from 3.8 to 6.0 on the early
lifestages of lake trout ( Salvelinus namaycush), brook trout
( Salvelinus fontinalis) and pumpkin seed sunfish ( Lepomis
gibbosus). Three different responses were observed: a) aluminium
toxicity at pH < 5.0 represented joint action with hydrogen ions
producing ionoregulatory failure; b) at pH 5.0-6.0 aluminium toxicity
required concentrations of inorganic forms that greatly exceeded
theoretical gibbsite solubility; c) at acutely lethal levels of pH and
ionic strength, aluminium increased the resistance time of eggs, fry
and adults.
Ogilvie & Stechey (1983) studied the respiratory responses of
rainbow trout ( Oncorhynchus mykiss) to aluminium exposure (50 to
500 �g/litre) at a pH of 6.0 and an exposure period of 26 h. Mean
opercular rate was significantly increased at 500 �g aluminium/litre
and mean cough rate was significantly increased at both 200 and
500 �g/litre. Spontaneous locomotion and mean activity levels were
variable in all groups.
Neurotoxic effects on the olfactory organ of rainbow trout were
demonstrated by Klaprat et al. (1988), who exposed the fish to pH 7.7
and pH 4.7 both with (5.0, 9.5 and 20.0 �mol total Al/litre) and
without aluminium. At pH 4.7 alone, increased mucous was observed over
parts of the olfactory epithelium. After Al additions, however, loss
of receptor cell cilia, irregular shaped olfactory knobs, changed
microvilli and swellings of microridge cells were observed. Electrical
response from the olfactory nerve to L-serin was not changed by pH
alone, but was depressed by aluminium additives. Since sensory organs
play a very important role in the behavioural ecology of fish
populations (feeding, alarm signals, pheromones, imprinting, spawning,
etc), neurotoxic effects on the olfactory organ can have great adverse
effects in nature (Rosseland & Staurnes, 1994).
e) Pathological effects
Hunter et al. (1980) noted that rainbow trout ( Oncorhynchus
mykiss) which survived exposure to 50 mg/litre aluminium at pH 8.0
to 9.0 showed several pathological signs of toxicity. These included
proliferative changes in the gills and congestion of the secondary
lamellae, slight demyelination of the brain, extensive necrosis of the
liver, severe inflammatory glomerular necrosis of the kidney and some
evidence of skin hyperplasia. Karlsson-Norrgren et al. (1986b) exposed
brown trout ( Salmo trutta) to aluminium sulfate (50, 200 and 500 �g
total aluminium/litre) at pH 5.5 and 7.0 for up to 6 weeks. Advanced
gill lesions (enlargement of secondary lamellae due to the increased
number of chloride cells in the epithelia) were observed in fish
exposed to aluminium at pH 5.5 and a temperature of 2.5�C. The lesions
contained cytoplasmic aluminium precipitates. The addition of humus or
increasing the pH to 7.0 reduced or inhibited the effects of
aluminium. A water temperature of 15�C reduced the gill lesions
observed at 2.5�C. However, prolonged exposure to higher water
temperatures produced gill alterations even in controls. Eggs and the
resulting fry of Atlantic salmon ( Salmo salar) exposed to aluminium
(38-300 �g/litre) at pH 5.5 were investigated. Scanning electron
microscopy revealed gill abnormalities, which included poorly
developed or absent secondary lamellae, fused primary lamellae,
proliferation of epithelial cells and increased numbers of surface
pits. These effects were not noted when fish were raised in aluminium-
free water at pH values ranging from 4.5 to 7.2 (Jagoe et al., 1987).
9.1.2.4 Amphibians
Clark & LaZerte (1985) exposed eggs and tadpoles of the American
toad ( Bufo americanus) and the wood frog ( Rana sylvatica) to total
aluminium concentrations of 10, 20, 50, 100 and 200 �g/litre at pH
ranging from 4.14 to 5.75. A nominal pH of 4.14 significantly reduced
hatching in the absence of aluminium. Aluminium had no effect on
hatching at pH 4.75 or pH 5.75. However, at pH 4.14 there was a
significant reduction in hatching for eggs exposed to any aluminium
concentration compared with eggs without aluminium at the same pH
level. Tadpoles that hatched out were not affected by any aluminium
concentration or pH level.
Clark & Hall (1985) studied the effects of total aluminium
(7-210 �g/litre), pH (pH 4.41-6.29) and dissolved organic content
(2.2-9.9 mg/litre) at calcium concentrations of 2 mg/litre on
B. americanus, R. sylvatica and the spotted salamander ( Ambystoma
maculatum). High aluminium, low pH and high dissolved organic
content (DOC) significantly reduced hatching success of B.
americanus. For R. sylvatica neither aluminium nor pH correlated
significantly with hatching success over the range of pH tested.
Hatching success for A. maculatum ranged from 41% at pH 4.4 to 68%
at pH 6.1. Although pH was not significantly correlated with hatching
success, a greater number of eggs hatched above pH 5.0 than below, the
difference being significant. Decreased hatching success was
correlated with high aluminium and high DOC. In a second experiment
the authors studied the effects of aluminium (total aluminium
54-75 �g/litre) and pH (pH 4.23-5.8) at calcium concentrations of
2 mg/litre. Hatching success of B. americanus and R. sylvatica was
unaffected at pH 4.8 and 5.8 with total aluminium concentrations of 54
to 63 �g/litre. However, there were significant reductions in hatching
at a pH of 4.3 and total aluminium concentration of 75 �g/litre.
Hatching success of A. maculatum was not correlated significantly
with pH. However, hatching success was only 57% at the highest pH
value and lowest aluminium concentration.
Gascon et al. (1987) studied the affects of total dissolved
aluminium (7.4 �mol/litre), pH (4.5 and 6.2) and calcium (25 and
500 �Eq/litre) on the eggs and tadpoles of Rana sylvatica. There was
no egg mortality in any group. Hatching was significantly delayed in
groups exposed to aluminium under acidic conditions. Tadpoles exposed
to aluminium at pH 4.5 and calcium at 25 �Eq/litre suffered 100%
mortality. In a similar group where the pH was allowed to drift up to
5.3, mortality was not significant. Metamorphosis in surviving
tadpoles was significantly delayed by acidic conditions and aluminium
exposure. Growth, as measured by dry weight, was significantly
depressed in the group suffering 100% mortality. In the groups exposed
to aluminium with either pH rising to 5.3 or calcium at 500 �Eq/litre,
growth was significantly increased over controls.
Freda & McDonald (1990) exposed embryos (4 to 5 days) and
tadpoles (96 h) of the leopard frog ( Rana pipiens) to a range of
total aluminium concentrations (250-1000 �g/litre) and pHs (4.2-6.5).
The pH and the aluminium concentration had a significant effect on the
survival of embryos. All control embryos hatched whereas 94% of
embryos at pH 4.2 failed to hatch. At pH 4.2 and 4.4 the addition
of aluminium ameliorated the effects of low pH, increasing
hatchability to 78-99%. At pH 4.6 and 4.8 aluminium was found to be
toxic. The LC50 values for aluminium at pH 4.6 and 4.8 were 811 and
403 �g/litre, respectively. Tadpoles (pre-stage 25) were less
sensitive to low pH than embryos, showing 20% mortality at pH 4.2.
However, they were much more sensitive to aluminium, all the tadpoles
dying at aluminium concentrations > 500 �g/litre and pH 4.4 or 4.6,
and at > 250 �g/litre and pH 4.8. Three-week-old tadpoles were less
sensitive to lowered pH and elevated aluminium than embryos or newly
hatched tadpoles. Low pH (4.2) had no effect on the survival of
tadpoles, and aluminium was only toxic at pH 4.8 with 40% mortality at
1000 �g/litre.
Common frog ( Rana temporaria) tadpoles were raised to
metamorphosis at total aluminium concentrations of 800 and
1600 �g/litre and at pH 4.4. Decreasing pH reduced maximum body size
and delayed metamorphosis. Growth was depressed and metamorphosis
delayed at 800 �g Al/litre; at 1600 �g/litre small tadpoles had
arrested growth and development and subsequently died, whereas large
tadpoles metamorphosed at a very small size (Cummins, 1986).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Numerous studies exist of plants exposed to aluminium in nutrient
solution or sand culture. They show that exposure causes diminished
root growth and development, reduced uptake of plant nutrients
(notably phosphorus, calcium and magnesium) and stunted plant growth
(Bartlett & Riego, 1972a,b; G�ransson & Eldhuset, 1987; Boxman et al.,
1991; Keltjens & Tan (1993). The effect of aluminium on plants is
complex. It can act directly on plant cell processes (Taylor, 1991) or
indirectly by interfering with plant nutrition (Roy et al., 1988;
Taylor, 1991).
Plant species vary in their response to aluminium (Roy et al.,
1988; Taylor, 1995). Even within species (e.g., wheat, Triticum
aestivum), aluminium sensitive and tolerant varieties exist (Taylor
& Foy, 1985; Kinraide et al., 1992; Huang et al., 1992a; Wheeler et
al., 1993). There are reports that aluminium can benefit plants
(Hackett, 1962, 1964, 1967). Various proposed mechanisms are listed in
the review by Roy et al. (1988). However, it seems that exposure to
excessive concentrations of aluminium is detrimental to plants, but
the level that is excessive is highly variable.
9.1.3.2 Invertebrates
No data have been reported on the effects of aluminium on
terrestrial invertebrates.
9.1.3.3 Birds
Hussein et al. (1988) fed Japanese quail ( Coturnix coturnix
japonica) on diets containing 0.05, 0.1, 0.15 and 0.3% aluminium (as
aluminium sulfate) for 4 weeks. Egg production was significantly
decreased at 0.1% and body weight gain at 0.15%. Feed intake was
significantly depressed temporarily at 0.1 and 0.15% and permanently
at 0.3%. Eggshell breaking strength was temporarily reduced (after 1
week only) at 0.1, 0.15 and 0.3%.
Hussein et al. (1989a) fed white leghorn laying hens on a diet
containing 0.05, 0.1 or 0.15% aluminium (as aluminium sulfate) for 28
days. Feed intake, body weight, tibia breaking strength and plasma
inorganic phosphorus were significantly reduced at 0.15%. Egg
production was only significantly depressed after 21 days at 0.15%.
Eggshell breaking strength was unaffected by the treatment. In a
second experiment hens were exposed to diets containing up to 0.3%
aluminium for 42 days. Feeding 0.3% aluminium significantly decreased
plasma inorganic phosphorus in samples collected immediately following
oviposition (10 to 42 days). Plasma calcium, tibia weight and tibia
breaking strength were unaffected. Egg production and feed intake were
significantly reduced during days 1 to 21 but not during days 22 to
42. The effects of 0.3% aluminium on the egg production and shell
quality of laying hens are similar to those obtained with conventional
force-moulting procedures using feed restriction (Hussein et al.,
1989b). White leghorn laying hens were maintained on a diet containing
0, 0.15 or 0.3% aluminium for 17 weeks. Hatchability of eggs was
unaffected while fertility and body weight of chicks were
significantly depressed at both aluminium treatments. Total egg
production and feed consumption both were significantly reduced at the
highest aluminium dose (Wisser et al., 1990).
Carri�re et al. (1986) fed ring doves ( Streptopelia risoria)
on a diet containing 0.1% aluminium sulfate with reduced calcium
and phosphorus levels (0.9% Ca; 0.5% P) for a period of 4 months.
There were no significant effects on egg production, fertility,
hatchability, growth or final weight of chicks. Egg permeability was
initially decreased but subsequently recovered to normal levels. The
diet had no effect on plasma calcium, phosphorus or magnesium. There
was no effect on weight or growth rate in juvenile doves fed diets
containing 500, 1000 or 1500 mg/kg aluminium sulfate from day 21 to
day 63.
9.2 Field observations
9.2.1 Microorganisms
No data have been reported regarding effects in the field of
aluminium on microorganisms.
9.2.2 Aquatic organisms
9.2.2.1 Plants
H�rnstr�m et al. (1984) studied the effects of pH and different
levels of aluminium on lake phytoplankton from the Swedish west coast
area. They concluded that the absence of several phytoplankton species
in acid lakes was not caused by the low pH but rather a raised
aluminium supply from the surrounding land, which produced
oligotrophic waters through precipitation of phosphorus. Comparing
phytoplankton communities in strongly acid lakes with low and high
levels of aluminium revealed that aluminium toxicity alone contributed
significantly to the reduced numbers of phytoplankton species.
9.2.2.2 Invertebrates
Aston et al. (1987) found that for streams in Wales and the Peak
district, United Kingdom, the population density and biomass of
freshwater invertebrates were generally lowest in streams with low pH
and high aluminium content. H�rnstr�m et al. (1984) reported that most
zooplankton species found in acidic lakes of the Swedish west coast
were relatively resistant to acidity but were more susceptible to the
oligotrophication process caused by the precipitation of phosphorus by
aluminium. There was also an inverse correlation between the number of
invertebrates and the aluminium concentration.
Hall et al. (1985) added aluminium chloride (0.28-3.8 mg total
Al/litre) to a stream to simulate episodic release during acidic
snowmelt. Significant decreases in pH and dissolved oxygen content
accompanied increases in aluminium. There was an increased drift of
invertebrates with increasing aluminium concentration. The gradually
increasing drift rate of benthic macro-invertebrates appeared to be a
stress response to the aluminium/hydrogen ion concentrations. Drift of
terrestrial insects feeding at the water surface appeared to be due to
the reduction in surface tension of the aluminium-treated portion of
the stream. Hall et al. (1987) found that during second-order stream
experiments, overall more aquatic invertebrates drifted at pH 5.0
during aluminium chloride addition (> 0.28 mg total Al/litre) than at
pH 5.0 during hydrochloric acid addition (0.012 mg total Al/litre).
McCahon et al. (1987) subdivided a stream into sections of low pH
(4.3) and low aluminium concentration (0.052 mg total Al/litre) by
adding sulfuric acid and high aluminium concentration (0.35 mg total
Al/litre; pH 5.0) by adding aluminium sulfate. In the acid zone,
mayfly mortalities of 20% and 5.3% after 24 h were observed for
Baetis rhodani and Ecdyonurus venosus, respectively. Mortality
rose to 52.6% after 48 h for E. venosus. Mayflies killed during the
exposure did not stain for aluminium or mucus. Similar mortalities
were observed for both species in the acid/aluminium zone. However,
both species gave an aluminium-positive reaction for all parts of the
body examined, aluminium being concentrated in the gut, within and
surrounding the gill plates, and on the outer surface of the abdomen.
Ormerod et al. (1987a) created simultaneous episodes of low pH
(4.28), and low pH (5.02) with increased aluminium content (347 �g
Al/litre) in a soft-water stream in upland Wales . In situ toxicity
tests were performed, and Chironomus riparius, Hydropsyche
angustipennis and Dinocras cephalotes were found to have suffered
no mortality. Ecdyonurus venosus, Baetis rhodani and Gammarus
pulex showed up to 25% mortality in both treatment zones. Drift
densities increased, especially in the aluminium-treated zone,
Baetis rhodani showing an increase of 8 times. Baetis rhodani was
the only invertebrate to show a significant decline in benthic density
(in the aluminium zone), which was due mostly to drift. Weatherley et
al. (1988) created 24-h experimental episodes by adding acid,
aluminium and citric acid to different treatment zones of an upland
stream. Drift density was only observed for the ephemerophteran Baetis
rhodani, and was found to be unaffected by flow at pH 7 or
organically-bound aluminium. Both acidity (pH 4.9) and labile
aluminium (0.11 mg/litre) increased drift density. Benthic density was
significantly decreased by both labile and organically bound
aluminium.
9.2.2.3 Vertebrates
Grahn (1980) reported two fish kills in lakes Ransj�n and �mten,
two pristine acid lakes in Sweden, during 1978 and 1979. In 1978 the
main part of the ciscoe ( Coregonus albula) population was wiped out.
One week later the pH was found to be 5.4 at the surface and 4.9 at
the bottom, with maximum aluminium levels of 0.91 mg/litre in
groundwater and 0.31 mg/litre in stream water. One year later a
similar incident occurred when the pH was 6.0 in surface water and 5.4
in bottom water. Total aluminium concentrations ranged from 0.36 to
0.52 mg/litre. Analysis of fish gills revealed that aluminium
concentrations were at least 6 to 7 times higher than those found in
fish gills from reference lakes in the area. The authors conclude that
weather patterns caused an increase in phytoplankton, which in turn
increased pH levels near the surface. Aluminium hydroxide was
precipitated out in the epilimnion water. Ciscoes migrate to the
surface to feed where the flocculated aluminium hydroxide caused fish
gills to clog and fish to die of suffocation.
Hunter et al. (1980) investigated Black Cart water, a tributary
of the River Clyde, Scotland, following sporadic occurrences of fish
kills (brown trout Salmo trutta) during 1974 and 1975. Aluminium
levels were 475 mg/kg (dry weight) in the lateral muscle of dead fish
and 358 mg/kg in live fish. Effluent waters from an anodizing factory
(pH 6.3-7.3; total aluminium 12-530 �g/litre and soluble aluminium
20-360 �g/litre) were mixed with waters of pH 8.35-8.70 and total and
soluble aluminium concentrations of 190-220 �g/litre and < 20
�g/litre, respectively. Downstream of the confluent, which was the
area of fish kills, the waters varied in pH between 8.1 and 8.5, and
in total (200-1300 �g/litre) and soluble (20-33 �g/litre) aluminium.
The authors suggested that when a high pH river receives high loadings
of aluminium from water-treatment works soluble aluminium
concentrations lethal to fish can occur.
Schofield & Trojnar (1980) reported that lakes in the Adirondack
mountains, USA, in which stocked brook trout ( Salvelinus fontinalis)
were not recovered in netting surveys during 1975, were those with
significantly lower mean pH (4.79 versus 5.25), calcium level (1.62
versus 1.87 mg/litre) and magnesium level (0.30 versus 0.39 mg/litre),
and higher mean aluminium concentrations (0.29 versus 0.11 mg/litre).
The covariance analysis of water quality suggested that aluminium
intoxication may have been a primary factor in determining the success
or failure of fish stocking, whereas both pH and calcium level showed
a lack of significance.
Henriksen et al. (1984) showed the acid-aluminium-rich (pH 5.1;
inorganic monomeric aluminium 30 �g/litre) waters of the river
Vikedal, in southwestern Norway, during a period of increased water
flow, to be toxic to pre-smolt Atlantic salmon ( Salmo salar).
Skogheim et al. (1984) reported massive deaths of adult Atlantic
salmon in the River Ogna in south-west Norway in 1982. Newly run
migrating spawners were killed when acid tributary water (pH 4.7;
inorganic monomeric Al 340-360 �g/litre) was mixed into the falling
main stream flow of better water quality (pH 5.96-6.18; inorganic
monomeric Al 12-13 �g/litre). A near total mortality of salmon
occurred for several km downstream of the confluent (pH 4.76-5.41;
inorganic monomeric Al 110-307 �g/litre). Blood samples from highly
stressed fish (plasma chloride 103-113 mEq/litre) demonstrated
osmoregulative failure (normal values 120-135 mEq/litre), and gill
aluminium accumulation (70-341 �g Al/g fresh weight) was around 10
times higher than normal values.
Harriman et al. (1987) studied the long-term fish population
changes in streams and lochs of south-west Scotland during 1978, 1979
and 1984. Trout were not caught in areas known to have contained them
in the past. Fish-less lochs and streams contained the levels of
acidity (pH 4-5) and aluminium (up to 300 �g labile monoesic Al/litre)
known to be toxic to fish. The authors concluded that acidic
deposition was probably the major cause of the change in the fish
population in this area.
Eaton et al. (1992) compared laboratory exposure of four embryo-
larval fish species to aluminium (< 6.0-70 �g/litre) and pH levels of
4.5 to 7.0 with field observations. Laboratory results showed that
monomeric aluminium concentrations of approximately 50 �g/litre, which
were comparable to aluminium concentrations in the acidified half of a
lake, increased low pH toxicity. However, the laboratory tests
for largemouth bass ( Micropterus salmoides) and rock bass
( Ambloplites rupestris) underestimated field toxicity, those for
black crappie ( Pomoxis nigromaculatus) overestimated field toxicity
and those for yellow perch ( Perca flavescens) were similar to those
observed in the field.
Karlsson-Norrgren et al. (1986a) collected and examined brown
trout ( Salmo trutta) from two fish farms within acid-susceptible
areas in Sweden. The total aluminium concentrations in the water were
200 to 300 �g/litre. Trout from a third non-acidified location
(aluminium levels in water 35 �g/litre) were also examined. Light and
electron microscopic examination of fish from the acid-susceptible
areas revealed two major types of gill lesion characterized by
chloride cell hyperplasia, and enlargement of the intercellular
spaces, in the secondary lamellar epithelium. The gills from the
control area appeared normal, exhibiting features characteristic of
salmonids.
Norrgren & Degerman (1993) transferred eyed eggs of Atlantic
salmon ( Salmo salar) and brown trout ( Salmo trutta) in plastic
boxes to various locations on the Morrum river, Sweden. The pH levels
ranged from 5.1 to 6.7 and total mean aluminium levels ranged from 933
to 569 �g/litre (mean reactive aluminium concentrations ranged from
8 to 203 �g/litre). No significant effects were observed on hatching
(13 days) or fry survival (77 days) at pH > 5.7 and reactive
aluminium concentrations of up to 49 �g/litre. At pH 5.1 and a total
aluminium concentration of 569 �g/litre (reactive aluminium =
203 �g/litre), there was a significant reduction in the salmon hatch
but not the trout hatch; total fry mortality after 77 days was 100%
for salmon and 94% for trout. Histochemical studies revealed aluminium
precipitates in the gills of dead fish; precipitates were associated
with the apical plasma membrane.
Stoner et al. (1984) carried out 17-day survival studies of caged
brown trout ( Salmo trutta) in streams of the river Tywi catchment,
Wales. All streams where fish died had concurrent mean total aluminium
concentrations exceeding 21 �Eq/litre and mean pH levels of less than
6. There was a high positive correlation between fish mortality
(expressed as the inverse of LT50) and mean filterable aluminium
concentration. Differences in LT50 values reflected differences in
aluminium at similar pH levels. There were considerable differences in
gill aluminium concentrations between "control" fish (61 �g/g) and
those that died most rapidly (3505 �g/g). There was also a positive
correlation between mortality rate and gill aluminium concentration.
Weatherley et al. (1990) studied the survival of eggs, alevins and
parr of brown trout ( Salmo trutta) in streams of different acidity
(pH 4.7-6.9). Egg survival from fertilization to hatching was
independent of the mean total monomeric aluminium concentration, which
ranged from 3 to 397 �g/litre. However, the survival of alevins was
strongly related to both aluminium and pH; LC50 values for 28- and
42-day exposures were approximately 19 and 15 �g total monomeric
aluminium/litre, respectively (equivalent to 79 and 72 �g/litre of
0.45 �m filterable aluminium). The 21-day LC50 for parr (3 months)
was between 84 and 105 �g/litre filterable aluminium.
Ormerod et al. (1987a) created an acidic episode in a softwater
stream in upland Wales. Three stretches were identified: (a) upper
reach with natural waters of pH 7.0 (40 �g total Al/litre), (b)
addition of sulfuric acid to pH 4.2-4.5 (52 �g total Al/litre), and
(c) pH 5.2 (347 �g total Al/litre). Caged salmon ( Salmo salar) and
brown trout ( Salmo trutta) showed 7-10% mortality in the acid zone
(b) and 50-87% in the aluminium zone (c). Salmon (14.7 h) had a
significantly shorter LT50 than trout (23.5 h) in the aluminium zone
(c). Ormerod et al. (1987b) reported that brown trout ( Salmo trutta)
exposed to similar episodes showed reduced plasma sodium at low pH
(4.2), which appeared to be decreased by the addition of aluminium;
however, at pH 4.8 to 5.1 aluminium increased the effect. At pH
4.8-5.1 with the addition of aluminium, fish showed an increased
frequency of ventilation. At lower pH effects on ventilation were less
consistent.
McCahon et al. (1987) reported that trout and salmon exposed to
acid conditions (pH 4.3) alone and low aluminium concentration
(0.052 mg/litre) did not stain for aluminium, although increased mucus
production was demonstrated in the gills. Fish exposed to aluminium
(0.35 mg/litre) at low pH (5.0) exhibited extensive aluminium and
mucus coating of the secondary gill lamellae. Fish that died in the
acid/aluminium zone showed large significant increases in gill
aluminium content compared with controls or with fish from the acid-
only zone. Mean aluminium concentrations in fish gills from the
acid/aluminium zone were 2950 and 3050 �g/g (dry weight) for trout and
salmon, respectively.
Rosseland et al. (1992) exposed in situ caged smolts of
Atlantic salmon ( Salmo salar) and sea trout ( Salmo trutta) to
waters in an acid tributary (pH 4.8; inorganic monomeric aluminium
236 �g/litre) and the mixing zone with the limed river Audna (Norway)
(pH 7.0; inorganic monomeric aluminium 17 �g/litre). In the acid
tributary LT50 values were 22 h and 40 h for the two species,
respectively. However, in the mixing zone, between 1 and 3 min after
mixing (pH 5.8-5.9; inorganic monomeric Al 78-111 �g/litre), both
species gave an LT50 of 7 h. Fish showed osmoregulatory failure (loss
of plasma ions and reduced gill Na/K-ATPase) and gill lesions. No
mortality occurred 5 min after mixing or in the limed water. The
authors concluded that the increased toxicity in the mixing zone was
due to the transformation of aluminium from a low to a high molecular
weight precipitating species. The ecological importance of these
mixing zones was pointed out (especially being related to seasonal
changes) as low temperatures (longer polymerization period) and high
flows both would increase the river stretch being affected by these
processes.
Pol�o et al. (1994) used the same water sources to a channel
experiment with control mixing of the waters. Caged Atlantic salmon
were exposed to acid stream water (pH 4.89; inorganic monomeric
Al 139 �g/litre), limed stream water (pH 6.41; inorganic monomeric
Al 14 �g/litre) and the mixture of the two (pH 5.64; inorganic
monomeric Al 70 �g/litre). No mortality occurred in the limed water,
but there was a 27% mortality in the acid stream water after 72 h
exposure. In the mixed water, mortality of up to 90% (mean of 75%)
occurred in the first two metres and up to 1 min after mixing, the
LT50 being 60 h. Within the next 3 min of reaction a total mortality
of 16% was found. Fish in the most toxic zone lost plasma ions at a
slower rate than in the acid, less toxic zone, apparently
demonstrating a different mechanism of toxicity. Although no mortality
occurred towards the end of the channel (4-7 min after mixing), loss
of plasma ions and increased haematocrit were found, indicating
suboptimal conditions.
Lydersen et al. (1994) exposed caged one-year-old parr of brown
trout to mixing zone conditions in a channel, using as input water
from an acid lake (pH 5.3; total Al 379 �g/litre and inorganic
monomeric Al 350 �g/litre) and a neutral lake of pH 7.0, total Al
38 �g/litre. After mixing, a constant pH of 6.1-6.2 was achieved all
along the channel. Total mortality in the lake water was 15% after
24 h. Downstream of the confluent (9 seconds of mixing) mortality
started after 5 h, reaching 100% after 17 h. The LT50 values after 9,
18, 45, 136, 180 and 225 seconds of mixing were 7.5, 9, 12, 17, 23 and
26 h, respectively. Through the use of improved in situ aluminium-
separation techniques, the authors could for the first time
demonstrate the increase in high molecular Al forms (polymerization)
at stations along the channel and relate this directly to toxicity.
Freda & McDonald (1993) transplanted embryos and tadpoles of the
wood frog ( Rana sylvatica), the American toad ( Bufo americanus)
and the spotted salamander ( Ambystoma maculatum) to acidified ponds.
Statistical analysis revealed that pH was the only significant
parameter in determining mortality; the concentrations of total and
labile aluminium were not correlated with the survival of any of these
species.
9.2.3 Terrestrial organisms
9.2.3.1 Plants
Aluminium toxicity to plants is well known in agriculture and
forestry (Wild, 1988). Direct and indirect effects of enhanced
aluminium availability in soil due to soil acidification may be a
cause of current problems in some European forests (Abrahamsen et al.,
1994).
Reich et al. (1994) monitored the light-saturated net
photosynthesis, dark respiration and foliar nutrient content of Scots
pine ( Pinus sylvestris) growing in polluted and non-polluted sites.
At the polluted site soil pH and calcium and magnesium contents were
10 times lower and aluminium content 10 times higher than at the
control site. The rate of photosynthesis was lower and that of dark
respiration was higher at the polluted site. Photosynthesis was found
to be negatively correlated to the aluminium:calcium ratio across both
sites. Dark respiration rate increased with nitrogen and aluminium
contents. Based on the data, the authors concluded that at the
polluted site there were excessive soil aluminium levels and deficient
levels of magnesium. Low needle magnesium level and high aluminium
concentrations and aluminium:calcium ratios resulted in reduced
photosynthetic capacity and increased respiration.
Cronan et al. (1987) reported that observed field total
monomeric aluminium concentrations and aluminium ion activities were
considerably lower than laboratory-based toxic thresholds for several
tree species. However, analysis suggests that calcium/aluminium ratios
for fine roots were near to critical levels. Aluminium toxicity could
possibly have been acting as a stress factor either directly or
indirectly by nutritional interference.
Joslin et al. (1988) sampled roots and foliage of spruce trees
from five sites in North America and Europe. Aluminium concentrations
in fine roots from B horizons were highly correlated with soil
solution monomeric aluminium. Sites with higher levels of plant-
available aluminium had spruce trees with lower foliage levels of
calcium and magnesium. The authors concluded that this may be evidence
of aluminium interference in calcium and magnesium uptake and
transport. There was no obvious interference of aluminium in the
uptake and translocation of phosphorus.
Godbold et al. (1988b) studied the effects of aluminium on
nutrient fluxes in Picea abies. The authors reported that, using
X-ray microanalysis, the distribution of aluminium, magnesium, calcium
and potassium was found to be similar in roots to those collected from
declining spruce stands in Solling, Germany.
Cuenca & Herrera (1987) reported that trees analysed from a
tropical cloud forest growing in acid/aluminium-rich soil were found
to have two distinct strategies for survival: accumulator plants, with
aluminium levels of > 1000 mg/kg, and non-accumulators. Both types
contained large amounts of aluminium in the roots, but in accumulators
the plasmalemma in endodermic cells was permeable to aluminium. The
authors found that both groups were able to accumulate aluminium in
cell walls, alkalinize the surrounding rhizosphere and produce
chelating agents, but all at a high ecological cost and with low
growth rates as a result.
Most cultivated plants are non-accumulators and efficiently
exclude aluminium from the harvested parts. A notable exception is tea
( Camellia sinensis) where aluminium concentration in mature leaves
can reach 1.7% Al/dry weight (Chenery, 1955; Coriat & Gillard, 1986).
Clarkson (1967) studied the aluminium tolerance of four species
of Agrostris from different sites in the United Kingdom. The most
sensitive species was A. stolanifera from a site with no detectable
soil aluminium (as exchangeable ions) and the most tolerant was
A. setacea from a site with soil levels of 3.6 mEq Al/kg. Growing
the species in soils from each of the different sites revealed that
peak growth was achieved in soil from the site where the plant was
collected.
9.2.3.2 Invertebrates
No information has been reported regarding the field effects of
aluminium on terrestrial invertebrates.
9.2.3.3 Vertebrates
Nyholm (1981) found aluminium in the bone marrow tissue of the
humeri of pied flycatchers ( Ficedula hypoleuca) with impaired
breeding from the shores of Lake Tjultr�sk, Sweden. The impairments
included production of small clutches, defective eggshell formation
and intrauterine bleeding. The authors stated that these impairments
compare with the symptoms of aluminium intoxication in mammals.
However, the effects may be indirect, the result of phosphate binding
in the intestinal tract rather than direct toxicity of aluminium.
Ormerod et al. (1986) reported that sites without breeding
dippers ( Cinclus cinclus) had significantly higher mean
concentrations of filterable aluminium, lower mean pH, fewer
trichopteran larvae and ephemeropteran nymphs, and were on rivers with
more conifer afforestation on their catchments than sites where
dippers were present. Ormerod et al. (1988) found no evidence that
aluminium (0.47-2.13 mg/g; dry weight) in the invertebrate prey of
dippers ( Cinclus cinclus) collected from streams of different pH
from Wales and Scotland adversely affected shell thickness or mass of
eggs.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Health effects
The overall assessment of the risks of aluminium exposure to
healthy, non-occupationally exposed humans is rendered uncertain by
several deficiencies in the database, namely:
i) the questionable relevance to humans of animal experiments
designed for hazard identification, in which massive doses were
administered by parenteral routes, and the lack of data based on
oral dosing at relevant exposure levels;
ii) the dubious relevance to humans of the many biochemical findings
from cell culture experiments and other in vitro models;
iii) the uncertainty in the analytical and estimated cumulative
exposure data in epidemiological studies of the relationship of
aluminium in drinking-water to Alzheimer's disease (AD), and the
less than robust dose-response data obtained from such studies;
iv) the complete lack of epidemiological studies relating to total
aluminium exposure, when it is considered that aluminium derived
from drinking-water is responsible for only a small fraction of
such exposure.
It should be noted, however, that animal models employing oral
aluminium administration provide some limited support for cognitive
and motor impairment in the absence of neuropathological changes.
Adverse health effects from aluminium exposure have been
definitely established in one special group, i.e. patients with
severely impaired renal function. These patients are at risk from
severe neurological dysfunction and are also at risk of vitamin-D-
resistant osteomalacia and microcytic anaemia from iatrogenic exposure
to aluminium-containing preparations. The toxicity of oral aluminium-
containing preparations is exacerbated by concurrent use of oral
citrate-containing preparations under these circumstances.
An increased tissue loading of aluminium, especially in bone, has
been shown in premature infants without azotaemia. Current paediatric
practice is to warn about the potential risk that may be associated
with increased tissue levels of aluminium following parenteral
administration or oral intake of products with elevated aluminium
content.
10.1.1 Exposure assessment
Human exposure to aluminium can vary greatly depending on diet,
use of specific medications, sources of drinking-water and exposure to
other ambient and occupational sources of aluminium. In many countries
non-occupationally exposed adults are exposed to between 2.5 and 13 mg
aluminium/day from air, water and food (0.08-0.18 mg/kg body weight
per day for a 60-kg individual). However, large variations in daily
intake can occur as a consequence of differing intakes of foods
containing commonly encountered food additives. Infants consuming
formulae based on cow's milk have very low aluminium intakes
(0.1-0.8 mg/litre), whereas those fed soya-based formulae have much
greater aluminium intakes (3.1-4.3 mg/litre). Moreover, use of
aluminium containing antacids and/or buffered analgesics can increase
daily intake between 10 and 1000 times. Actual exposures will vary
from country to country, but the above figures can be used as a first
approximation for assessing human health risks from aluminium
exposure.
Occupationally exposed populations have background exposure
levels similar to those of non-occupationally exposed populations, but
their work can lead to significantly increased exposure. Actual levels
of exposure depends on the specific work tasks performed, the type and
form of aluminium compound encountered, and the adequacy of workplace
hygiene practices. Inhalation is the most important route of
occupational exposure, but the extent of pulmonary uptake and
retention has not been determined in most occupational settings. Based
on limited data, daily occupational aluminium exposure can range from
<1 mg to 40 mg per 8-h shift.
10.1.2 Evaluation of animal data
Information from toxicokinetic studies in animals is consistent
with and complementary to that obtained in humans. Available studies
indicate that oral bioavailability is low (< 1%) and they provide
valuable information on dose-related concentrations and effects in
target tissues.
In general, the animal studies evaluated were not designed for
risk assessment purposes. However, these studies provide information
relevant to hazard identification and some data on dose-response.
The target tissues in animals are similar to those in humans and
include the bone and nervous system.
In developmental studies in rodents, reduced intrauterine
weight gains were noted in the female offspring of dams gavaged with
aluminium nitrate at doses as low as 13 mg Al/kg body weight per
day, whereas male offspring were not affected at this dose level.
Neurobehavioural and motor development were affected at 100 and
200 mg Al/kg body weight per day when aluminium lactate was
administered to mice in the feed.
There is no indication that aluminium is carcinogenic.
10.1.3 Evaluation of human data
There is no indication that aluminium is acutely toxic after oral
intake. A causal relationship between short-term exposure to high
levels of aluminium in drinking-water and adverse health effects is
not supported by available data.
The information available is insufficient to classify the
carcinogenic risk to humans from exposure to aluminium and aluminium
compounds.
No information is available associating aluminium exposures with
adverse reproductive effects.
Although human exposure to aluminium is widespread, only a few
reports of hypersensitivity exist. In all cases the hypersensitivity
followed dermal or parenteral administration.
In the healthy general population the major health concern
relates to the purported association between the intake of aluminium
and the development and/or acceleration of the onset of AD and other
neurotoxic effects. After an in-depth analysis of the available
epidemiological data (section 8.1.3 - Table 22), considering study
design, exposure measurements and relative risks reported, it was
concluded that:
* On the whole, the positive relationship between aluminium in
drinking-water and AD, which was demonstrated in several
epidemiological studies, cannot be totally dismissed. However,
strong reservations about inferring a causal relationship are
warranted in view of the failure of these studies to account for
demonstrated confounding factors and for total aluminium intake
from all sources.
* Taken together, the relative risks for AD from exposure to
aluminium in drinking-water above 100 �g/litre, as determined in
these studies, are low (less than 2.0). But, because the risk
estimates are imprecise for a variety of methodological reasons,
a population-attributable risk cannot be calculated with
precision. Such imprecise predictions may, however, be useful in
making decisions about the need to control exposures to aluminium
in the general population.
* In light of the above studies, which consider water-borne
aluminium as the sole risk factor, and the recent findings that
water accounts for less than 5% of daily intake of aluminium, it
is difficult to reconcile this with the presumed impact on
cognition. Several lines of investigation should be pursued to
further elucidate the nature of the relationship found in these
studies.
Data from many clinical studies have firmly established the
causal relationship between aluminium exposure in patients with
chronic renal failure (including premature infants with kidney
failure) and the development of encephalopathy (dementia), vitamin-D-
resistant osteomalacia, and microcytic anaemia. The major sources of
aluminium exposure have been indicated as the level of aluminium in
the water used to prepare the dialysis fluids and treatment with
aluminium-containing phosphate binders. Dialysis encephalopathy was
found to be rare when the aluminium level in the dialysis fluid was
maintained below 50 �g/litre. The use of aluminium-free phosphate-
binding agents will also decrease the risk of developing
encephalopathy in patients treated for kidney failure.
In many occupational settings workers are exposed not simply to
aluminium fumes and dust, but rather to a complex mixture of chemicals
and dusts, of which only some contain aluminium. Therefore, it is
essential that urinary aluminium levels be determined as a measure of
exposure (dose), in addition to workplace levels of particulates, if a
causal association between aluminium and effects on the respiratory
and nervous systems is to be developed with any degree of certainty.
Only a few of the published surveys contain such information.
In the past, exposure to stamped pyrotechnic aluminium powder,
usually coated with mineral oil lubricants, has caused pulmonary
fibrosis. However, exposure to other forms of aluminium has not proved
to be a cause of pulmonary fibrosis.
There is no evidence of specific toxicity of any immunological
reaction to the element aluminium as a cause of occupational asthma.
This disorder is considered to be irritant-induced following exposure
during the production of compounds such as aluminium sulfate,
aluminium fluoride and potassium aluminium tetrafluoride, or in
production areas of primary aluminium smelting. Exposure to dust and
particulate in workers fabricating (welding) aluminium have been
associated with an increased level of chronic bronchitis or decreased
lung function. A causative role of non-aluminium fume or dusts, e.g.,
fluorides, cannot be ruled out. It was reported that the chronic
bronchitis noted in aluminium welders was similar to that in welders
working with stainless steel or iron.
There is some evidence that aluminium exposure in welders, miners
and foundry workers can lead to an impairment of cognitive and motor
functions. However, owing to the small cohorts used in some studies,
methodological deficiencies in most studies, and conflicting results
from different studies, there is considerable uncertainty in ascribing
a causative role for aluminium in the neurological effects reported.
In one large study of miners exposed to aluminium powder, the
difficulties in adequately controlling for confounding factors also
lead to much uncertainty in accepting the positive effects observed on
cognition. There is a need for additional studies to clarify the
uncertainties in these data.
10.2 Evaluation of effects on the environment
10.2.1 Exposure
Aluminium is among the least mobile of the major elements of the
geological sedimentary cycle. In air it is present mainly as wind-
blown soil particles. Aluminium-bearing solid phases are relatively
insoluble, particularly in the circumneutral pH range, and so most
natural waters contain low concentrations of dissolved aluminium.
However, the solubility of aluminium is highly dependent on pH,
increasing at both low and high pH values, which reflects the
amphoteric nature of the element. This fact, coupled with the large
reservoir of aluminium in soils and sediments, means that aluminium
concentrations can be substantially enhanced in acidic or poorly
buffered environments subjected to sustained or periodic exposure to
strong acidifying inputs. Under such conditions aluminium can be
transported from soil to surface waters. Afforestation, the cessation
of liming, and sulfide oxidation all contribute to acidification and
thus to the release of previously bound aluminium. However, a major
cause of acidification is acidifying deposition from the atmosphere.
10.2.2 Effects
Laboratory and field studies have revealed that the inorganic
mononuclear aluminium complexes are more toxic to both aquatic
organisms and terrestrial plant species than the organically complexed
forms. Aluminium has been shown to affect adversely both aquatic
organisms and terrestrial plants at concentrations found in acidic or
poorly buffered environments. However, there exist large species,
strain and life-history stage differences in sensitivity. Elevated
concentrations of inorganic monomeric aluminium have detrimental
biological effects that are mitigated in the presence of organic
acids, fluoride, silicate, and high concentrations of calcium and
magnesium. There is a substantial reduction in species richness
associated with the mobilization of the more toxic forms of aluminium
in acid-stressed waters; this loss of species diversity is reflected
at all trophic levels. In waters with non-steady-state aluminium
chemistry (ongoing polymerization), toxicity can become extreme in pH
ranges not normally associated with toxicity.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
In reaching the following conclusions with regards to the risks
from aluminium exposure the Task Group felt it was appropriate to
consider separately the human populations at risk as well as the
environment. The conclusions are, therefore, grouped as to the risk to
the healthy general population, healthy workers and individuals with
impaired kidney function. This is followed by conclusions regarding
the risk to the environment from aluminium.
11.1 Conclusions
11.1.1 Healthy general population
Hazards posed by aluminium to intrauterine and neurological
development and brain function have been identified through animal
studies. However, aluminium has not been shown to pose a health risk
to healthy, non-occupationally exposed humans.
There is no evidence to support a primary causative role of
aluminium in Alzheimer's disease (AD). Aluminium does not induce AD
pathology in vivo in any species, including humans.
The hypothesis that exposure of the elderly population in some
regions to high levels of aluminium in drinking-water may exacerbate
or accelerate AD is not supported by available data.
It has also been hypothesized that particular exposures, either
occupational or via drinking-water, may be associated with non-
specific impaired cognitive function. The data in support of this
hypothesis are currently inadequate.
There is insufficient health-related evidence to justify
revisions to existing WHO Guidelines for aluminium exposure in
healthy, non-occupationally exposed humans. As an example, there is an
inadequate scientific basis for setting a health-based standard for
aluminium in drinking-water.
11.1.2 Subpopulations at special risk
In people of all ages with impaired renal function, aluminium
accumulation has been shown to cause the clinical syndrome of
encephalopathy, vitamin-D-resistant osteomalacia and microcytic
anaemia. The sources of aluminium are haemodialysis fluid and
aluminium-containing pharmaceutical agents (e.g., phosphate binders).
Intestinal absorption can be exacerbated by the use of citrate-
containing products. Patients with renal failure are thus at risk of
neurotoxicity from aluminium.
Iatrogenic aluminium exposure poses a hazard to patients with
chronic renal failure and to premature infants. Every effort should be
made to limit such exposure in these groups.
11.1.3 Occupationally exposed populations
Workers having long-term, high-level exposure to fine aluminium
particulates may be at increased risk of adverse health effects.
However, there are insufficient data from which to develop, with any
degree of certainty, occupational exposure limits with regards to the
adverse effects of aluminium.
Exposure to stamped pyrotechnic aluminium powder, usually
coated with mineral oil lubricants, has caused pulmonary fibrosis
(aluminosis), whereas exposure to other forms of aluminium has not
been proved to cause pulmonary fibrosis. Most reported cases involved
exposure to other potentially fibrogenic agents.
Irritant-induced asthma has been shown to be associated with
inhalation of aluminium sulfate, aluminium fluoride and potassium
aluminium tetrafluoride, and found to occur within the complex
environment of primary aluminium production, especially in potrooms.
11.1.4 Environmental risk
Aluminium-bearing solid phases in the environment are relatively
insoluble, particularly at circumneutral pH values, resulting in low
concentrations of dissolved aluminium in most natural waters.
In acidic or poorly buffered environments subjected to strong
acidifying inputs, concentrations of aluminium can increase to levels
resulting in adverse effects on both aquatic organisms and terrestrial
plants. However, there exist large species, strain and life-history
stage differences in sensitivity to this metal.
The detrimental biological effects from elevated concentrations
of inorganic monomeric aluminium can be mitigated in the presence of
organic acids, fluorides, silicate and high levels of calcium and
magnesium.
A substantial reduction in species richness is associated with
the mobilization of the more toxic forms of aluminium in acid-stressed
waters. This loss of species diversity is reflected at all trophic
levels.
11.2 Recommendations
11.2.1 Public health protection
a) Strategies should be developed to limit exposure for patients
with severly impaired renal function who are exposed to aluminium
from pharmaceutical products.
b) For patients with end-stage renal failure treated by dialysis,
exposure to aluminium should be limited by treatment of the water
and elimination of aluminium contamination of the chemicals used
to prepare dialysis fluid. It is suggested that dialysis fluids
should contain less than 15 �g aluminium/litre.
11.2.2 Recommendations for protection of the environment
a) Soil and water acidification can mobilize aluminium and have
detrimental effects on aquatic and terrestrial lifeforms. Actions
that cause enhanced acidification, e.g., emissions of SO2, NOx
and NH3, should continue to be restricted.
b) When waters are limed to mitigate aluminium toxicity to fish, it
should be done in such a manner that sufficient time is available
for establishment of equilibrium between the aluminium species
before the water reaches the most biologically sensitive or
valuable parts of the system.
12. FURTHER RESEARCH
In order to improve the scientific database on aluminium for the
assessment of the risks to human health, thus decreasing the present
level of uncertainty, the Task Group considered that additional
research in several areas was essential.
12.1 Bioavailability and kinetics
(a) Future studies should address the issue of whether aluminium in
drinking-water is more bioavailable than that from other sources
such as food, consumer products and food additives paying
particular attention to the study of factors that can modify
aluminium uptake by the body. This question is best answered by
studies in humans using 26Al although appropriately designed
animal experiments would provide useful data.
(b) Once aluminium has gained access to the brain, at what rate is it
mobilized or excreted? Is the brain a "one-way sink for
aluminium"? These questions can only be addressed successfully in
an animal model where serial cortical biopsies can be performed.
(c) In view of the importance of achieving international concordance
of accurate chemical analysis for aluminium in environmental and
biological samples, it is recommended that the development of
expert reference laboratories and systems of quality control
should be facilitated and supported.
12.2 Toxicological data
(a) It is thought unlikely that further studies of parenteral
aluminium exposure to normal animals will improve the database
significantly. There is, however, a need for well-designed animal
studies focusing on neurotoxicity and reproductive and
developmental end-points using protocols developed for the
assessment of dose-response relationships for exposures by either
the oral or inhalation routes.
(b) Research in experimental animals on the factors related to the
development of Alzheimer's disease (AD) is hampered by the lack
of a suitable model. However, aged primates or transgenic mice
bearing the human APP gene with familial AD point mutation could
potentially be used for this purpose (with some reservations).
The advantage of these models is that total aluminium intake can
be controlled during the relevant portion of the animal's life
span (about 3-10 years and 1-2 years for primates and mice,
respectively).
(c) There is a requirement for further studies to determine the
effects of diminished renal function on aluminium toxicokinetics.
Such studies should be designed to assess the impact of early-
stage renal disease (varying degrees) and age-related decreases
in renal function on aluminium retention and toxicity.
12.3 Research on the relationship between aluminium exposure and
Alzheimer's disease
(a) Further studies of aluminium levels in bulk tissues from brains
of patients with AD are unlikely to be of value in resolving the
current controversy or enable the distinction to be made between
a pathogenic factor and an epiphenomenon.
(b) The knowledge base is unlikely to be advanced significantly by
further ecological retrospective studies of exposure to aluminium
in drinking-water and AD. Rather, prospective studies of matched
cohorts of elderly people from stable populations living in areas
with high and low levels of aluminium in drinking-water are
required, with special attention to known confounding factors.
Exposure should preferably be assessed through monitoring of
aluminium in the tap water of all cohort members. Although as an
outcome measure it is preferable to compare the pathological
configuration of AD to reference populations, this may be
impractical, and standardized clinical assessments have to be
relied on using carefully considered criteria for the diagnosis
of Alzheimer-type dementia.
(c) As Alzheimer-type neuropathology is almost universal in older
Down's syndrome subjects, and as decline in cognitive function,
as assessed by decline in activities of daily living skills, is
relatively common (25-35), a point study of rates of cognitive
impairment in Down's syndrome patients over 40 years of age from
high and low water aluminium areas might be performed relatively
quickly and with small numbers of subjects. Unbiased subject
recruitment would, however, present problems.
(d) If the hypothesis that higher drinking-water aluminium levels are
an accelerating or exacerbating factor for AD is correct, a
neuropathological assessment of elderly subjects dying of an
unrelated cause requiring routine postmortem examination (e.g.,
passengers in road traffic accident) ought to reveal a greater
burden of Alzheimer's pathology in those from areas with high
aluminium levels in drinking-water than those from areas with low
aluminium levels.
12.4 Occupational exposure
The possible adverse effects of occupational aluminium exposure
on the health of workers should be a specific priority for future
research.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
At the Thirty-third Meeting of the Joint FAO/WHO Expert Committee
on Food Additives and Food Contaminants, a provisional tolerable
weekly intake (PTWI) of 7.0 mg/kg body weight was recommended. This
value included the intake of aluminium from its use as a food additive
(FAO/WHO, 1989).
On the basis of non-health-related criteria, a WHO Drinking-Water
Guideline of 0.2 mg/litre has been proposed (WHO, 1993). No health-
based guideline was recommended.
The carcinogenic risk from aluminium and its compounds has not
been evaluated by the International Agency for Research on Cancer
(IARC). However, that there is sufficient evidence that certain
exposures occurring during aluminium production cause cancer in
humans. Most epidemiological studies suggest pitch volatiles to be
the causative agent (IARC, 1987).
Regulatory standards for aluminium and some aluminium compounds
established by national bodies in several countries and the European
Union are summarized in the legal file of the International Register
of Potentially Toxic Chemicals (IRPTC, 1993).
REFERENCES
Abernathy AR, Larson GL, & Mathews RC (1984) Heavy metals in the
surficial sediments of Fontana Lake, North Carolina. Water Res,
18(3): 351-354.
Abrahamsen G, Stuanes AO, & Tveite B (1994) Long-term experiments with
acid rain in Norwegian forest ecosystems. Berlin, Heidelberg, New
York, Springer-Verlag, pp 297-331.
Abramson MJ, Wlodarczyk JH, Saunders NA, & Hensley MJ (1989) Does
aluminum smelting cause lung disease? Am Rev Respir Dis, 139:
1042-1057.
Adler AJ & Berlyne GM (1985) Duodenal aluminum absorption in the rat:
effect of vitamin D. Am J Physiol, 249: G/209-G/213.
Albers PH & Camardese MB (1993a) Effects of acidification on metal
accumulation by aquatic plants and invertebrates. 1. Constructed
wetlands. Environ Toxicol Chem, 12: 959-967.
Albers PH & Camardese MB (1993b) Effects of acidification on metal
accumulation by aquatic plants and invertebrates. 2. Wetlands, ponds
and small lakes. Environ Toxicol Chem, 12: 969-976.
Alberts JJ, Leyden DE, & Patterson TA (1976) Distribution of total Al,
Cd, Co, Cu, Ni and Zn in the tongue of the ocean and the northwestern
Atlantic Ocean. Mar Chem, 4: 51-56.
Alderman FR & Gitelman HJ (1980) Improved electrothermal determination
of aluminum in serum by atomic absorption spectroscopy. Clin Chem,
26: 258-260.
Alfrey AC (1978) Dialysis encephalopathy syndrome. Annu Rev Med,
29: 93-98.
Alfrey AC (1980) Aluminum metabolism in uremia. Neurotoxicology,
1: 43-53.
Alfrey AC, Mishell JM, Burks J, Contiguglia SR, Rudolph H, Lewin E, &
Holmes JH (1972) Syndrome of dyspraxia and multifocal seizures
associated with chronic hemodialysis. Trans Am Soc Artif Intern
Organs, 18: 257-261.
Alfrey AC, Legendre GR, & Kaehny WD (1976) The dialysis encephalopathy
syndrome: Possible aluminum intoxication. N Engl J Med, 294:
184-188.
Alfrey AC, Hegg A, & Craswell P (1980) Metabolism and toxicity of
aluminium in renal failure. Am J Clin Nutr, 33: 1509-1516.
Alfrey AC, Sedman A, & Chan YL (1985) The compartmentalization and
metabolism of aluminum in uremic rats. J Lab Clin Med, 105: 227-233.
Allen VG & Fontenot JP (1984) Influence of aluminium as sulfate,
chloride and citrate on magnesium and calcium metabolism in sheep.
J Anim Sci, 59: 798-804.
Allen VG, Horn FP, & Fontenot JP (1986) Influence of ingestion of
aluminium, citric acid and soil on mineral metabolism of lactating
beef cows. J Anim Sci, 62: 1396-1403.
Altmann P, Dhanesha U, Hamon C, Cunningham J, Blair J, & Marsh F
(1989) Disturbance of cerebral function by aluminium in haemodialysis
patients without overt aluminium toxicity. Lancet, 2: 7-12.
Amundsen CE, Hanssen JE, Semb A, & Steinnes E (1992) Long-range
atmospheric transport of trace elements to southern Norway. Atmos
Environ, A26(7): 1309-1324.
Andersen JR (1987) Graphite furnace atomic absorption spectrometric
screening method for the determination of aluminium in haemodialysis
concentrates. J Anal Atmos Spectrom, 2: 257-259.
Andorn MC, Britton RS, & Bacon BR (1990) Evidence that lipid
peroxidation and total iron are increased in Alzheimer's brain.
Neurobiol Aging, 11: 316.
Andreoli SP, Bergstein JM, & Sherrard DJ (1984) Aluminum intoxication
from aluminum-containing phosphate binders in children with azotemia
not undergoing dialysis. N Engl J Med, 310: 1079-1084.
Arieff AI, Cooper JD, Armstrong D, & Lazarowitz VC (1979) Dementia,
renal failure, and brain aluminum. Ann Int Med, 90: 741-747.
Aston RJ, Sadler K, Milner AGP, & Lynam S (1987) The effects of pH and
related factors on stream invertebrates. Leatherhead, Central
Electricity Generating Board, Technology Planning and Research
Division, Central Electricity Research Laboratories (Report No.
TPRD/L/2792/N84).
ATSDR (1992) Toxicological profile for aluminum and compounds.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry,
136 pp (TP-91/01).
Bache BW (1980) The acidification of soils. In: Hutchinson TC & Havas
M ed. Effects of acid precipitation on terrestrial ecosystems. New
York, London, Plenum Press, pp 183-202.
Bache BW (1986) Aluminium mobilization in soils and waters. J Geol Soc
(Lond), 143: 699-706.
Baker JP & Schofield CL (1982) Aluminum toxicity to fish in acidic
waters. Water Air Soil Pollut, 18: 289-309.
Banks WA & Kastin AJ (1985) Aluminum alters the permeability of the
blood-brain barrier to some non-peptides. Neuropharmacology, 24:
407-412.
Banks WA, Kastin AJ, Fasold MB, Barrera CM, & Augereau G (1988a)
Studies of the slow bidirectional transport of iron and transferrin
across the blood-brain barrier. Brain Res Bull, 21: 881-885.
Banks WA, Kastin AJ, & Fasold MB (1988b) Differential effect of
aluminum on the blood-brain barrier transport of peptides, technetium
and albumin. J Pharmacol Exp Ther, 244: 579-585.
Bartlett RJ & Riego DC (1972a) Toxicity of hydroxy aluminum in
relation to pH and phosphorus. Soil Sci, 114(3): 194-200.
Bartlett RJ & Riego DC (1972b) Effect of chelation on the toxicity of
aluminum. Plant Soil, 37: 419-423.
Bast-Pettersen R, Drablos PA, Goffeng LO, Thomassen Y, & Torres CG
(1994) Neuropsychological deficit among elderly workers in aluminium
production. Am J Ind Med, 25: 649-662.
Baxter MJ, Burrell JA, Crews HM, & Massey RC (1988) The effects of
fluoride on the leaching of aluminium saucepans during cooking. Food
Addit Contam, 5: 651-656.
Beavington F & Cawse PA (1979) The deposition of trace elements and
major nutrients in dust and rainwater in northern Nigeria. Sci Total
Environ, 13: 263-274.
Benett RW, Persaud TVN, & Moore KL (1975) Experimental studies on the
effects of aluminum on pregnancy and fetal development. Anat Anz,
138: 365-378.
Bengtsson B-E (1978) Use of a harpacticoid copepod in toxicity tests.
Mar Pollut Bull, 9(9): 238-241.
Benninger LK & Wells JT (1993) Sources of sediment to the Neuse River
estuary, North Carolina. Mar Chem, 43: 137-156.
Berd�n M, Clarke N, Danielsson LG, & Spar�n A (1994) Aluminium
speciation: variations caused by the choice of analytical method and
by sample storage. Water Air Soil Pollut, 72: 213-233.
Berg DJ & Burns TA (1985) The distribution of aluminum in the tissues
of three fish species. J Freshw Ecol, 3: 113-120.
Berggren D (1992) Speciation and mobilization of aluminium and cadmium
in podzols and cambisols of S. Sweden. Water Air Soil Pollut,
62: 125-156.
Bergkvist B (1987) Leaching of metals from forest soils as influenced
by tree species and management. For Ecol Manage, 22: 29-56.
Berlyne GM, Yagil R, Ben-Ari J, Weinberger G, Knopf E, & Danovitch GM
(1972) Aluminium toxicity in rats. Lancet, 1: 564-567.
Bernuzzi V, Desor D, & Lehr PR (1986) Effects of prenatal aluminium
exposure on neuromotor maturation in the rat. Neurobehav Toxicol
Teratol, 8: 115-119.
Bernuzzi V, Desor D, & Lehr PR (1989a) Effects of postnatal aluminium
lactate exposure on neuromotor maturation in the rat. Bull Environ
Contam Toxicol, 42: 451-455.
Bernuzzi V, Desor D, & Lehr PR (1989b) Developmental alterations in
offspring of female rats orally intoxicated by aluminum chloride or
lactate during gestation. Teratology, 40: 21-27.
Berrill M, Hollett L, Margosian A, & Hudson J (1985) Variation in
tolerance to low environmental pH by the crayfish Orconectes
rusticus, O. propinquus, and Cambarus robustus. Can J Zool, 63:
2586-2589.
Bertholf RL, Wills MR, & Savory J (1984) Quantitative study of
aluminum binding to human serum albumin and transferrin by chelex
competitive binding assay. Biochem Biophys Res Commun, 125: 1020-
1024.
Bettinelli M, Baroni U, Fontana F, & Poisetti P (1985) Evaluation of
the L'vov platform and matric modification for the determination of
aluminium in serum. Analyst, 110: 19-22.
Bhamra RK & Costa M (1992) Trace elements aluminum, arsenic, cadmium,
mercury, and nickel. In: Lippmann M ed. Environmental toxicants: Human
exposures and their health effects. New York, Van Nostrand Reinhold
Company, pp 575-632.
Biesinger KE & Christensen GM (1972) Effects of various metals on
survival, growth, reproduction, and metabolism of Daphnia magna.
J Fish Res Board Can, 29: 1691-1700.
Birchall JD (1991) The role of silicon in aluminium toxicity. In: Lord
Walton of Detchant ed. Alzheimer's disease and the environment.
London, Royal Society of Medicine Services Ltd, pp 70-77 (Round Table
Series No. 26).
Bird SC, Brown SJ, & Vaughan E (1990) The influence of land management
on stream water chemistry. In: Edwards RW, Gee AS, & Stoner JH ed.
Acid waters in Wales. Dordrecht, Boston, London, Kluwer Academic
Publishers, pp 241-253.
Blotcky AJ, Claassen JP, Roman FR, Rack EP, & Badakhsh S (1992)
Determination of aluminum by chemical and instrumental neutron
activation analysis in biological standard reference material and
human brain tissue. Anal Chem, 64: 2910-2913.
Blume H-P & Br�mmer G (1991) Prediction of heavy metal behavior in
soil by means of simple field tests. Ecotoxicol Environ Saf, 22:
164-174.
B�hler-Sommeregger K & Lindemayr H (1986) Contact sensitivity to
aluminium. Contact Dermatitis, 15: 278-281.
Booth CE, McDonald DG, Simons BP, & Wood CM (1988) Effects of aluminum
and low pH on net ion fluxes and ion balance in the brook trout
( Salvelinus fontinalis). Can J Fish Aquat Sci, 45: 1563-1574.
Borg H (1987) Trace metals and water chemistry of forest lakes in
northern Sweden. Water Res, 21(1): 65-72.
Boult S, Collins DN, White KN, & Curtis CD (1994) Metal transport in a
stream polluted by acid mine drainage - the Afon Goch, Anglesey, UK.
Environ Pollut, 84: 279-284.
Bouman AA, Platenkamp AJ, & Posma FD (1986) Determination of aluminium
in human tissues by flameless atomic absorption spectroscopy and
comparison of reference values. Ann Clin Biochem, 23: 97-101.
Boxman AW, Krabbendam H, Bellemakers MJS, & Roelofs JGM (1991) Effects
of ammonium and aluminium on the development and nutrition of Pinus
nigra in hydroculture. Environ Pollut, 73: 119-136.
Brady DJ, Edwards DG, Asher CJ, & Blamey FPC (1993) Calcium
amelioration of aluminium toxicity effects on root hair development in
soybean [ Glycine max (L.) Merr.]. New Phytol, 123: 531-538.
Br�unlich H, Fleck C, Kersten L, Stein G, Laske V, M�ller A, & Keil E
(1986) Renal effects of aluminium in uraemic rats and rats with intact
kidney function. J Appl Toxicol, 6: 55-59.
Brezonik PL, Mach CE, Downing G, Richardson N, & Brighman M (1990)
Effects of acidification on minor and trace metal chemistry in Little
Rock Lake, Wisconsin. Environ Toxicol Chem, 9: 871-885.
Bringmann G & K�hn R (1959) [The toxic effects of wastewater on
aquatic bacteria, algae, and small crustacea.] Gesundheits-Ingenieur,
80(4): 115-120 (in German).
Brown DJA (1983) Effect of calcium and aluminum concentrations on the
survival of brown trout ( Salmo trutta) at low pH. Bull Environ
Contam Toxicol, 30: 582-587.
Bruland KW (1983) Trace elements in sea-water. In: Riley JP & Chester
R ed. Chemical oceanography. New York, London, Academic Press, vol 8,
pp 157-220.
Brumbaugh WG & Kane DA (1985) Variability of aluminum concentrations
in organs and whole bodies of smallmouth bass ( Micropterus
dolimieui). Environ Sci Technol, 19: 828-831.
Buckler DR, Mehrle PM, Cleveland L, & Dwyer FJ (1987) Influence of pH
on the toxicity of aluminium and other inorganic contaminants to east
coast striped bass. Water Air Soil Pollut, 35: 97-106.
Budavari S ed. (1989) The Merck index: An encyclopedia of chemicals,
drugs and biologicals, 11th ed. Rahway, New Jersey, Merck & Co.,
pp 54-60.
Buergel PM & Soltero RA (1983) The distribution and accumulation of
aluminum in rainbow trout following a whole-lake alum treatment.
J Freshw Ecol, 2(1): 37-44.
Bugiani O & Ghetti B (1982) Progressing encephalomyelopathy with
muscular athropy, induced by aluminum powder. Neurobiol Aging,
3: 209-222.
Bull KR & Hall JR (1986) Aluminium in the rivers Esk and Duddon,
Cumbria, and their tributaries. Environ Pollut, B12: 165-193.
Buratti M, Caravelli G, Calzaferri G, & Colombi A (1984) Determination
of aluminium in body fluids by solvent extraction and atomic
absorption spectroscopy with electro-thermal atomization. Clin Chim
Acta, 141: 253-259.
Burnatowska-Hledin MA & Mayor GH (1984) The effects of aluminum
loading on selected tissue calcium and magnesium concentrations in
rats. Biol Trace Elem Res, 6: 531-535.
Burnel D, Hutin MF, Netter P, Kessler M, Huriet C, & Gaucher A (1982)
Int�r�t du dosage de l'aluminium osseux par polarographie
impulsionelle. Pathol Biol, 30: 27-32.
Burton TM & Allan JW (1986) Influence of pH, aluminum, and organic
matter on stream invertebrates. Can J Fish Aquat Sci, 43: 1285-1289.
Bush AI, Pettingell WH, Multhaup G, Paradis MD, Vonsattel J-P, Gusella
JF, Beyreuther K, Masters CL, & Tanzi RE (1994) Rapid induction of
Alzheimer A� amyloid formation by zinc. Science, 265: 1464-1467.
Caines LA, Watt AW, & Wells DE (1985) The uptake and release of some
trace metals by aquatic bryophytes in acidified waters in Scotland.
Environ Pollut, B10: 1-18.
Cakir A, Sullivan TW, & Struwe FJ (1977) Effect of supplemental
aluminum on the value of fertilizer phosphates in turkey diets. Poult
Sci, 56: 544-549.
Cam JM, Luck VA, Eastwood JB, & De Wardener HE (1976) The effect of
aluminium hydroxide orally on calcium, phosphorus and aluminium in
normal subjects. Clin Sci Mol Med, 51: 407-414.
Cann CE, Prussin SG, & Gordan GS (1979) Aluminum uptake by the
parathyroid glands. J Clin Endocrinol Metab, 49: 543-545.
Carri�re D, Fischer KL, Peakall DB, & Anghern P (1986) Effects of
dietary aluminum sulphate on reproductive success and growth of ringed
turtle doves ( Streptopelia risoria). Can J Zool, 64: 1500-1505.
Cawse PA (1974) A survey of atmospheric trace elements in the U.K.
(1972-73). Harwell, United Kingdom Atomic Energy Authority, 84 pp
(Report No. AERE-R 7669).
Cawse PA (1987) Trace and major elements in the atmosphere at rural
locations in Great Britain, 1972-1981. In: Coughtrey PJ, Martin MH, &
Unsworth MH ed. Pollutant transport and fate in ecosystems. Oxford,
London, Blackwell Scientific Publications, pp 89-112 (British
Ecological Society Special Publication No. 6).
Chadwick DJ & Whelan J (1992) Aluminium in biology and medicine. New
York, Chichester, Brisbane, Toronto, John Wiley and Sons, 138 pp
(Ciba Foundation Symposium No. 169).
Chainy GBN, Sahoo A, & Swain C (1993) Effect of aluminum on lipid
peroxidation of cerebral hemisphere of chick. Bull Environ Contam
Toxicol, 50: 85-91.
Chan YL, Alfrey AC, Posen S, Lissner D, Hills E, Dunstan CR, & Evans
RA (1983) Effect of aluminum on normal and uremic rats: tissue
distribution, vitamin D metabolites, and quantitative bone histology.
Calcif Tissue Int, 35: 344-351.
Chan JC, Jacob M, Brown S, Savory J, & Wills MR (1988) Aluminum
metabolism in rats: effects of vitamin D, dihydrotachysterol,
1,25-dihydroxyvitamin D and phosphate binders. Nephron, 48: 61-64.
Channon SM, Arfeen S, & Ward MK (1988) Long-term accumulation of
aluminium in patients with renal failure. Trace Elem Med, 5:
154-157.
Chappuis P, Duhaux L, Paolaggi F, De Vernejoul MC, & Rousselet F
(1988) Analytical problems encountered in determining aluminum status
from hair in controls and hemodialyzed patients. Clin Chem, 34:
2253-2255.
Chen W, Monnat RJ, Chen M, & Mottet K (1978) Aluminum induced
pulmonary granulomatosis. Hum Pathol, 9: 705-712.
Chenery EM (1955) A preliminary study of aluminium and the tea bush.
Plant Soil, VI(2): 174-200.
Cherroret G, Bernuzzi V, Desor D, Hutin M-F, Burnel D, & Lehr PR
(1992) Effects of postnatal aluminum exposure on choline
acetyltransferase activity and learning abilities in the rat.
Neurotoxicol Teratol, 14: 259-264.
Chester R & Bradshaw GF (1991) Source control on the distribution of
particulate trace metals in the North Sea atmosphere. Mar Pollut Bull,
22(1): 30-36.
Chou L & Wollast R (1989) Distribution and geochemistry of aluminium
in the Mediterranean Sea. Water Pollut Res Rep, 13: 232-240.
Chow WM (1992) Behaviour of aluminium and its ecological significance
in natural waters. In: Schulhof P ed. IWSA International Workshop on
Aluminium in Drinking Water, Hong Kong, 15-17 January 1992. Oxford,
London, Blackwell Scientific Publications, pp 1-10.
Christie H, Mackay RJ, & Fisher AM (1963) Pulmonary effects of
inhalation of aluminum by rats and hamsters. Ind Hyg J, 24: 47-56.
Christophersen N & Seip HM (1982) A model for streamwater chemistry at
Birkenes, Norway. Water Resour Res, 18(4): 977-996.
Clark KL & Hall RJ (1985) Effects of elevated hydrogen ion and
aluminum concentrations on the survival of amphibian embryos and
larvae. Can J Zool, 63: 116-123.
Clark KL & Lazerte BD (1985) A laboratory study of the effects of
aluminum and pH on amphibian eggs and tadpoles. Can J Fish Aquat Sci,
42: 1544-1551.
Clarkson DT (1967) Aluminium tolerance in species within the genus
Agrostis. J Ecol, 54: 167-178.
Clayton DB (1989) Water polution at Lowermoore North Cornwall: Report
of the Lowermoor incident health advisory committee. Truro, United
Kingdom, Cornwall District Health Authority, 22 pp.
Clayton RM, Sedowofia SKA, Rankin JM, & Manning A (1992) Long-term
effects of aluminium on the fetal mouse brain. Life Sci, 51:
1921-1928.
Clemmensen O & Knudsen HE (1980) Contact sensitivity to aluminium in a
patient hyposensitized with aluminium precipitated grass pollen.
Contact Dermatitis, 6: 305-308.
Cleveland L, Little EE, Hamilton SJ, Buckler DR, & Huhn JB (1986)
Interactive toxicity of aluminum and acidity to early life stages of
brook trout. Trans Am Fish Soc, 115: 610-620.
Cleveland L, Little EE, Wiedmeyer RH, & Buckler DR (1989) Chronic
no-observed-effect concentrations of aluminum for brook trout exposed
in low-calcium, dilute acidic water. In: Lewis TE ed. Environmental
chemistry and toxicology of aluminum. Proceedings of the 194th Annual
Meeting of the American Chemical Society, New Orleans (LA), 30
August-4 September 1987. Chelsea, Michigan, Lewis Publishers Inc.,
pp 229-246.
Cleveland L, Buckler DR, & Brumbaugh WG (1991) Residue dynamics and
effects of aluminum on growth and mortality in brook trout. Environ
Toxicol Chem, 10: 243-248.
Colomina MT, Gomez M, Domingo JL, Llobet JM, & Corbella J (1992)
Concurrent ingestion of lactate and aluminum can result in development
toxicity in mice. Res Commun Chem Pathol Pharmacol, 77: 95-106.
Colomina MT, Gomez M, Domingo JL, Llobet JM, & Corbella J (1994) Lack
of maternal and developmental toxicity in mice given high doses of
aluminium hydroxide and ascorbic acid during gestation. Pharmacol
Toxicol, 74: 236-239.
Commisaris RL, Cordon JJ, Sprague S, Keiser J, Mayov G, & Rich R
(1982) Behavioral changes in rats after chronic aluminium and
parathyroid hormone administration. Neurobehav Toxicol Teratol,
4: 403-410.
Connor DJ, Jope RS, & Harrell LE (1988) Chronic, oral aluminum
administration to rats: Cognition and cholinergic parameters.
Pharmacol Biochem Behav, 31: 467-474.
Coriat A-M & Gillard RD (1986) Beware the cups that cheer. Nature
(Lond), 321: 570.
Corrin B (1963) Aluminium pneumoconiosis. II. Effect on the rat lung
of intratracheal injections of stamped aluminium powders containing
different lubricating agents and of a granular aluminium powder.
Br J Ind Med, 20: 268-276.
Cosby BJ, Wright RF, Hornberger GM, & Galloway JN (1985) Modeling the
effects of acid deposition: estimation of long-term water quality
responses in a small forested catchment. Water Resour Res, 21(11):
1591-1601.
Cosby BJ, Whitehead PG, & Neale R (1986) A preliminary model of
long-term changes in stream acidity in southwestern Scotland. J
Hydrol, 84: 381-401.
Cournot-Witmer G, Zinngraff J, Plachot JJ, Escaig F, Lefevre R,
Boumati P, Bourdeau A, Garab�dian M, Galle P, Bourdon R, Dr�eke T, &
Balsan S (1981) Aluminium localization in bone from hemodialyzed
patients: Relationship to matrix mineralization. Kidney Int, 20:
375-385.
Courtijn E, Vandecasteele C, Yu ZQ, Nagels M, & Dams R (1987)
Determination and speciation of aluminium in acidified surface waters.
In: Witters H & Vanderborght O ed. Ecophysiology of acid stress in
aquatic organisms: Proceedings of an International Symposium, Antwerp,
13-16 January 1987. Antwerp, Belgian Royal Zoological Society,
pp 33-44.
Courtijn E, Vandecasteele C, & Dams R (1990) Speciation of aluminium
in surface water. Sci Total Environ, 90: 191-202.
Cranmer JM, Wilkins JD, Cannon DJ, & Smith L (1986) Fetal-placental-
maternal uptake of aluminum in mice following gestational exposure:
Effect of dose and route of administration. Neurotoxicology, 7:
601-608.
Crapper DR & Dalton AJ (1973a) Alterations in short term retention,
conditioned avoidance response acquisition and motivation following
aluminum induced neurofibrillary degeneration. Physiol Behav, 10:
925-933.
Crapper DR & Dalton AJ (1973b) Aluminum induced neurofibrillary
degeneration, brain electrical activity and alterations in acquisition
and retention. Physiol Behav, 10: 935-945.
Crapper DR, Krishnan SS, & Dalton AJ (1973) Brain aluminum
distribution in Alzheimer's disease and experimental neurofibrillary
degeneration. Science, 180: 511-513.
Crapper DR, Krishnan SS, & Quittkat S (1976) Aluminum, neurofibrillar
degeneration and Alzheimer's disease. Brain, 99: 67-80.
Crapper DR, Quittkat S, Krishnan SS, Dalton AJ, & De Boni U (1980)
Intranuclear aluminum content in Alzheimer's disease, dialysis
encephalopathy and experimental aluminum encephalopathy. Acta
Neuropathol, 50: 19-24.
Crapper McLachlan DR (1986) Aluminum and Alzheimer's disease.
Neurobiol Aging, 7: 525-532.
Crapper McLachlan DR & Farnell BJ (1986) Cellular mechanisms of
aluminium toxicity. Ann Ist Super Sanita, 22: 697-702.
Crapper McLachlan DR, Dam TV, Farnell BJ, & Lewis PN (1983) Aluminum
inhibition of ADP-ribosylation in vivo and in vitro. Neurobehav
Toxicol Teratol, 5: 645-647.
Crapper McLachlan DR, Lukiw WJ, & Kruck TPA (1989) New evidence for
an active role of aluminum in Alzheimer's disease. Can J Neurol Sci,
16: 490-497.
Crapper McLachlan DR, Dalton AJ, Kruck TPA, Bell MY, Smith WL, Kalow
W, & Andrews DF (1991) Intramuscular desferrioxamine in patients with
Alzheimer's disease. Lancet, 337: 1304-1308.
Cronan CS, Kelly JM, Schofield CL, & Goldstein RA (1987) Aluminium
geochemistry and tree toxicity in forests exposed to acidic
deposition. In: Perry R ed. Acid rain: Scientific and technical
advances. London, Selper, pp 649-656.
Cuenca G & Herrera R (1987) Ecophysiology of aluminium in terrestrial
plants, growing in acid and aluminium-rich tropical soils. In: Witters
H & Vanderborght O ed. Ecophysiology of acid stress in aquatic
organisms: Proceedings of an International Symposium, Antwerp, 13-16
January 1987. Antwerp, Belgian Royal Zoological Society, pp 57-73.
Cummins CP (1986) Effects of aluminium and low pH on growth and
development in Rana temporaria tadpoles. Oecologia, 69: 248-252.
Dabeka RW & McKenzie AD (1990) Aluminium levels in Canadian infant
formulae and estimation of aluminium intakes from formulae by infants
0-3 months old. Food Addit Contam, 7(2): 275-282.
Dahlgren RA & Ugolini FC (1989) Aluminum fractionation of soil
solutions from unperturbed and tephra-treated spodosols, Cascade
Range, Washington, USA. Soil Sci Soc Am J, 53: 559-566.
Dalziel TRK, Morris R, & Brown DJA (1986) The effects of low pH, low
calcium concentrations and elevated aluminium concentrations on sodium
fluxes in brown trout, Salmo trutta L. Water Air Soil Pollut, 30:
569-577.
Dalziel TRK, Morris R, & Brown DJA (1987) Sodium uptake inhibition
in brown trout, Salmo trutta exposed to elevated aluminium
concentrations at low pH. In: Witters H & Vanderborght O ed.
Ecophysiology of acid stress in aquatic organisms: Proceedings of
an International Conference, Antwerp, 13-16 January 1987. Antwerp,
Belgian Royal Zoological Society, pp 421-434.
Dantzman CL & Breland HL (1969) Chemical status of some water sources
in south central Florida. Soil Crop Sci Soc Fla Proc, 29: 18-28.
Day JP, Barker J, Evans LJA, Perks J, Seabright PJ, Ackrill P, Lilley
JS, Drumm PV, & Newton GWA (1991) Aluminium absorption studied by
26Al tracer. Lancet, 337: 1345.
Day JP, Barker J, King SJ, Miller RV, Templar J, Lilley JS, Drumm PV,
Newton GWA, Fifield LK, Stone JOH, Allan GL, Edwardson JA, Moore PB,
Ferrier IN, Priest ND, Newton D, Talbot RJ, Brock JH, Sanchez L,
Dobson CB, Itzhaki RF, Radunovic A, & Bradbury MWB (1994) Biological
chemistry of aluminium studied using 26Al and accelerator mass
spectrometry. Nucl Instrum Methods Phys Res, B92: 463-468.
Dean JR (1989) Ion chromatographic determination of aluminium with
ultraviolet spectrophotometric detection. Analyst, 114: 165-168.
De Boni U, Seger M, & Crapper McLachlan DR (1980) Functional
consequences of chromatin bound aluminum in cultured human cells.
In: Liss L ed. Aluminum neurotoxicity. Park Forest, South Illinois,
Pathotox Publishers, Inc., pp 65-81.
De La Campa MRF, Garcia MED, & Sanz-Medel A (1988) Room-temperature
liquid phosphorimetry of the aluminium-ferron chelate in micellar
media. Determination of aluminium. Anal Chim Acta, 212: 235-243.
Delonay AJ, Little EE, Woodward DF, Brumbauch WG, Farag AM, & Rabeni
CF (1993) Sensitivity of early-life-stage golden trout to low pH and
elevated aluminum. Environ Toxicol Chem, 12: 1223-1232.
Den Dooren De Jong LE (1971) Tolerance of Azotobacter for metallic
and non-metallic ions. Antonie van Leeuwenhoek, 37: 119-124.
De Villiers PR (1962) The chemical composition of the water of the
Orange River at Vioolsdrif, Cape Province. Ann Geol Opname Repub
S Afr, 1: 197-208.
De Vuyst P, Dumortier P, Rickaert F, Van De Weyer R, Lenclud C, &
Yernault JC (1986) Occupational lung fibrosis in an aluminium
polisher. Eur J Respir Dis, 68: 131-140.
De Vuyst P, Dumortier P, Schanden� L, Estenne M, Verhest A, & Yernault
JC (1987) Sarcoid-like lung granulomatosis induced by aluminum dust.
Am Rev Respir Dis, 135: 493-497.
DIN (1993) [German standard procedures for testing water, sewage water
and sludge: Physical, chemical, biological and bacterial procedures.]
Society of German Chemists, Expert Group on Water Chemistry and German
Institute for Standardization (DIN), Standardization Committee for
Water (NAW), vol II, pp E9/1-11, E22/1-21 (DIN 38 406, Sections 9
and 22) (in German).
Dinman BD (1983) Aluminium, alloys and compounds. In: Parmeggiani
L ed. Encyclopedia of occupational health and safety. Geneva,
International Labour Organisation, vol 1, pp 131-135.
Dinman BD (1988a) Aluminium in the lung: the pyropowder conundrum.
J Occup Med, 29(11): 869-876.
Dinman BD (1988b) Alumina-related pulmonary disease. J Occup Med,
30: 328-335.
Di Paolo JA & Casto BC (1979) Quantitative studies of in vitro
morphological transformation of Syrian hamster cells by inorganic
metal salts. Cancer Res, 39: 1008-1013.
Dobbs AJ, French P, Gunn AM, Hunt DTE, & Winnard DA (1989) Aluminum
speciation and toxicity in upland waters. In: Lewis TE ed.
Environmental chemistry and toxicology of aluminum. Proceedings of the
194th Annual Meeting of the American Chemical Society, New Orleans,
LA, 30 August-4 September 1987. Chelsea, Michigan, Lewis Publishers
Inc., pp 209-228.
Doese M (1938) [Industrial medicine studies on injury to health caused
by aluminium, in particular lung disease from aluminium dust.] Arch
Gewerbepathol, 8: 501-531 (in German).
Domingo JL (1995) Reproductive and developmental toxicity of
aluminium: A review. Neurotoxicol Teratol, 17(4): 515-521.
Domingo JL, Paternain JL, Llobet JM, & Corbella J (1987a) Effects of
oral aluminum administration on perinatal and postnatal development in
rats. Res Commun Chem Pathol Pharmacol, 57: 129-132.
Domingo JL, Llobet JM, Gomez M, Tomas JM, & Corbella J (1987b)
Nutritional and toxicological effects of short-term ingestion of
aluminum by the rat. Res Commun Chem Pathol Pharmacol, 56: 409-419.
Domingo JL, Paternain JL, Llobet JM, & Corbella J (1987c) The effects
of aluminium ingestion on reproduction and postnatal survival in rats.
Life Sci, 41: 1127-1131.
Domingo JL, Gomez M, Bosque MA, & Corbella J (1989) Lack of
teratogenicity of aluminum hydroxide in rats. Life Sci, 45: 243-247.
Donald JM, Golub MS, Gershwin ME, & Keen CL (1989) Neurobehavioral
effects in offspring of mice given excess aluminum in diet during
gestation and lactation. Neurotoxicol Teratol, 11: 345-351.
Dorfman D (1977) Tolerance of Fundulus heteroclitus to different
metals in salt waters. Bull NJ Acad Sci, 22(2): 21-23.
Drew RT, Gupta BN, Bend JR, & Hook GER (1974) Inhalation studies with
a glycol complex of aluminum-chloride-hydroxide. Arch Environ Health,
28: 321-326.
Driscoll CT (1984) A procedure for the fractionation of aqueous
aluminum in dilute acidic waters. Int J Environ Anal Chem, 16:
267-283.
Driscoll CT, Baker JP, Bisogni JJ, & Schofield CL (1980) Effect of
aluminium speciation on fish in dilute acidified waters. Nature
(Lond), 284: 161-164.
Driscoll CT, Johnson NM, Likens GE, & Feller MC (1988) Effects of
acidic deposition on the chemistry of headwater streams: a comparison
between Hubbard Brook, New Hampshire, and Jamieson Creek, British
Columbia. Water Resour Res, 24(2): 195-200.
Driscoll CT, Wyskowski BJ, Destaffan P, & Newton RM (1989) Chemistry
and transfer of aluminum in a forested watershed in the Adirondack
region of New York, USA. In: Lewis TE ed. Environmental chemistry and
toxicology of aluminum. Proceedings of the 194th Annual Meeting of the
American Chemical Society, New Orleans, LA, 30 August-4 September
1987. Chelsea, Michigan, Lewis Publishers Inc., pp 83-105.
Duce RA, Hoffman GL, & Zoller WH (1975) Atmospheric trace metals at
remote northern and southern hemisphere sites: pollution or natural?
Science, 187: 59-61.
Dunemann L & Schwedt G (1984) [Element species analysis in soil
solutions by gel chromatography and chemical reaction detectors.]
Fresenius Z Anal Chem, 317: 394-399 (in German).
Eaton JG, Swenson WA, McCormick JH, Simonson TD, & Jensen KM (1992) A
field and laboratory investigation of acid effects on largemouth bass,
rock bass, black crappie, and yellow perch. Trans Am Fish Soc, 121:
644-658.
Ecelbarger CA & Greger JL (1991) Dietary citrate and kidney function
affect aluminum, zinc, and iron utilization in rats. J Nutr, 121:
1755-1762.
Ecker F-J, Hirai E, & Chohji T (1990) The release of heavy metals from
snowpacks on the Japan Sea side of Japan. Environ Pollut, 65:
141-153.
Edwardson JA (1992) Alzheimer's disease and brain mineral metabolism.
In: Munro H & Schlierf G ed. Nutrition of the elderly. New York,
Raven Press, pp 193-202 (Nestl� Nutrition Workshop Series, Volume 29).
Edwardson JA, Candy JM, Ince PG, McArthur FK, Morris CM, Oakley AE,
Taylor GA, & Bjertness E (1992) Aluminium accumulation, beta-amyloid
deposition and neurofibrillary changes in the central nervous system.
In: Aluminium in biology and medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 165-185 (Ciba Foundation Symposium
No. 169).
Edwardson JA, Moore PB, Ferrier IN, Lilley JS, Newton GWA, Barker J,
Templar J, & Day JP (1993) Effect of silicon on gastrointestinal
absorption of aluminium. Lancet, 342(7): 211-212.
Eisenreich SJ (1980) Atmospheric input of trace metals to Lake
Michigan. Water Air Soil Pollut, 13: 287-301.
Elinder CG, Ahrengart L, Lidums V, Pettersson E, & Sj�gren B (1991)
Evidence of aluminium accumulation in aluminium welders. Br J Ind Med,
48: 735-738.
Ellen G, Egmond E, Van Loon JW, Sahertian ET, & Tolsma K (1990)
Dietary intakes of some essential and non-essential trace elements,
nitrate, nitrite and N-nitrosamines by Dutch adults estimated in a
24-hour duplicate portion study. Food Addit Contam, 7: 207-221.
Elliot HL, Dryburgh F, Fell GS, Sabet S, & MacDougall AI (1978)
Aluminium toxicity during regular haemodialysis. Br Med J,
1: 1101-1103.
Ellis HA, McCarthy JH, & Herrington J (1979) Bone aluminium in
haemodialysed patients and in rats injected with aluminium chloride:
relationship to impaired bone mineralisation. J Clin Pathol,
32: 832-844.
Ermolenko LV & Dedkov YM (1988) Photometric determination of aluminum
in water with the sulfonitrazo DAF reagent. J Anal Chem (USSR),
43: 815-820.
Exley C, Tollervey A, Gray G, Roberts S, & Birchall JD (1993) Silicon,
aluminium and the biological availability of phosphorus in algae. Proc
R Soc (Lond), B253: 93-99.
Fairman B, Sanz-Medel A, Gallego M, Quintela MJ, Jones P, & Benson R
(1994) Method comparison for the determination of labile aluminium
species in natural waters. Anal Chim Acta, 286: 401-409.
FAO/WHO (1989) Aluminium. In: Evaluation of certain food additives and
contaminants. Thirty-third report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
pp 28-31 (WHO Technical Report Series No. 776).
Farag AM, Woodward DF, Little EE, Steadman B, & Vertucci FA (1993) The
effects of low pH and elevated aluminum on yellowstone cutthroat trout
( Oncorhynchus clarki bouvieri). Environ Toxicol Chem, 12: 719-731.
Farnell BJ, Crapper McLachlan DR, Baimbridge K, De Boni U, Wong L,
& Wood PL (1985) Calcium metabolism in aluminum encephalopathy. Exp
Neurol, 88: 68-83.
Feely RA, Sackett WM, & Harris JE (1971) Distribution of particulate
aluminum in the Gulf of Mexico. J Geophys Res, 76(24): 5893-5902.
Feth JH, Rogers SM, & Roberson CE (1964) Chemical composition of snow
in the northern Sierra Nevada and other areas. Washington, DC, US
Department of Interior, Geological Survey, 39 pp (Geological Survey-
Water Supply Paper No. 1535-J).
Fileman CF, Althaus M, Law RJ, & Haslam I (1991) Dissolved and
particulate trace metals in surface waters over the Dogger Bank,
Central North Sea. Mar Pollut Bull, 22(5): 241-244.
Filipek LH, Nordstrom DK, & Ficklin WH (1987) Interaction of acid
mine drainage with waters and sediments of West Squaw Creek in the
West Shasta mining district, California. Environ Sci Technol,
21: 388-396.
Finberg L, Dweck HS, Holmes F, Kretchmer N, Mauer AM, Reynolds JW,
& Suskind RM (1986) Aluminum toxicity in infants and children.
Pediatrics, 78: 1150-1154.
Finelli VN, Que Hee SS, & Niemeier RW (1981) Influence of exposure to
aluminium chloride and fluoride dusts on some biochemical and
physiological parameters in rats. In: Brown SS & Davies DS ed. Organ-
directed toxicity chemical indices and mechanisms (IUPAC). New York,
Pergamon Press, pp 291-295.
Fischer T & Rystedt I (1982) A case of contact sensitivity to
aluminium. Contact Dermatitis, 8: 343.
Fisher DW, Gambell AW, Likens GE, & Bormann FH (1968) Atmospheric
contributions to water quality of streams in the Hubbard Brook
Experimental Forest, New Hampshire. Water Resour Res, 4: 1115-1126.
Fivelstad S & Leivestad H (1984) Aluminium toxicity to Atlantic salmon
( Salmo salar L.) and brown trout ( Salmo trutta L.): mortality and
physiological response. Oslo, Norway, Institute of Fresh Water
Ecology, pp 69-77 (Research Report No. 61).
Flaten TP (1990) Geographical associations between aluminium in
drinking water and death rates with dementia (including Alzheimer's
disease), Parkinson's disease and amyotrophic lateral sclerosis in
Norway. Environ Geochem Health, 12: 152-167.
Flaten TP, Glattre E, Viste A, & S�reide O (1991) Mortality from
dementia among gastro-duodenal ulcer patients. J Epidemiol Community
Health, 45: 203-206.
Fleming RF & Lindstrom RM (1987) Precise determination of aluminum by
instrumental neutron activation. J Radioanal Nucl Chem, 113: 35-42.
Flendrig JA, Kruis H, & Das HA (1976) Aluminium and dialysis dementia.
Lancet, 1: 1235.
Flora SJS, Dhawan M, & Tandon SK (1991) Effects of combined exposure
to aluminium and ethanol on aluminium body burden and some neuronal,
hepatic and haematopoietic biochemical variables in the rat. Hum Exp
Toxicol, 10: 45-48.
Folsom BR, Popescu NA, & Wood JM (1986) Comparative study of aluminum
and copper transport and toxicity in an acid-tolerant freshwater green
alga. Environ Sci Technol, 20: 616-620.
Forbes WF, McAiney CA, Hayward LM, & Agwani N (1994) Geochemical risk
factors for mental functioning, based on the Ontario longitudinal
study of aging (LSA): II. The role of pH. Can J Aging, 13: 249-267.
Foy CD & Gerloff GC (1972) Response of Chlorella pyrenoidosa to
aluminum and low pH. J Phycol, 8: 268-271.
Fraga CG, Oteiza PI, Golub MS, Gershwin ME, & Keen CL (1990) Effects
of aluminum on brain lipid peroxidation. Toxicol Lett, 51: 213-219.
France RL & Stokes PM (1987) Influence of manganese, calcium, and
aluminum on hydrogen ion toxicity to the amphipod Hyalella azteca.
Can J Zool, 65: 3071-3078.
Franceschetti S, Bugiani O, Panzica F, Tagliavini F, & Avanzini G
(1990) Changes in excitability of CA1 pyramidal neurons in slices
prepared from AlCl3-treated rabbits. Epilepsy Res, 6: 39-48.
Frank WB, Haupin WE, Dawless RK, Granger DA, Wei MW, Calhoun KJ, &
Bonney TB (1985) Aluminum. In: Gerhartz W, Yamamoto YS, Campbell FT,
Pfefferkorn R, & Rounsaville JF ed. Ullmann's encyclopedia of
industrial chemistry - Volume A1: Abrasives to aluminium oxide, 5th
revised ed. Weinheim, Verlag Chemie, pp 459-480.
Frecker MF (1991) Dementia in Newfoundlands: Identification of a
geographical isolate? J Epidemiol Community Health, 45: 307-311.
Freda J & McDonald DG (1990) Effects of aluminum on the leopard
frog, Rana pipiens: life stage comparisons and aluminum uptake. Can
J Fish Aquat Sci, 47: 210-216.
Freda J & McDonald DG (1993) Toxicity of amphibian breeding ponds in
the Sudbury region. Can J Fish Aquat Sci, 50: 1497-1503.
Freeman RA (1973) Recovery of rainbow trout from aluminum poisoning.
Trans Am Fish Soc, 102(1): 152-154.
Freeman RA & EverharT WH (1971) Toxicity of aluminum hydroxide
complexes in neutral and basic media to rainbow trout. Trans Am Fish
Soc, 100(4): 644-658.
Frick KG & Herrmann J (1990) Aluminum accumulation in a lotic mayfly
at low pH - a laboratory study. Ecotoxicol Environ Saf, 19: 81-88.
Froment DH, Buddington B, Miller NL, & Alfrey AC (1989a) Effect of
solubility on the gastrointestinal absorption of aluminum from various
aluminum compounds in the rat. J Lab Clin Med, 114: 237-242.
Froment DPH, Molitoris BA, Buddington B, Miller N, & Alfrey AC (1989b)
Site and mechanism of enhanced gastrointestinal absorption of aluminum
by citrate. Kidney Int, 36: 978-984.
Frost L, Johansen P, Pedersen S, Veien N, Aabel �stergaard P, &
Nielsen MH (1985) Persistant subcutaneous nodules in children
hyposensitized with aluminium-containing allergen extracts. Allergy,
40: 368-372.
Fulton B, Jaw S, & Jeffery EH (1989) Bioavailability of aluminum from
drinking water. Fundam Appl Toxicol, 12: 144-150.
Furrer G (1993) New aspects on the chemistry of aluminum in soils.
Aquat Sci, 55(4): 281-290.
Gajdusek DC & Salazar AM (1982) Amyotrophic lateral sclerosis and
parkinsonian syndromes in high incidence among the Auyu and Jakai
people of West New Guinea. Neurology, 32: 107-126.
Galceran T, Finch J, Bergfeld M, Coburn JW, Martin K, Teitelbaum S, &
Slatopolsky E (1987) Biological effects of aluminum on normal dogs:
studies on the isolated perfused bone. Endocrinology (Baltimore),
121: 406-413.
Ganrot PO (1986) Metabolism and possible health effects of aluminum.
Environ Health Perspect, 65: 363-441.
Garcia RP, Liu YM, Garcia MED, & Sanz-Medel A (1991) Solid-surface
room-temperature phosphorescence optosensing in continuous flow
systems: an approach for ultratrace metal ion determination. Anal
Chem, 63: 1759-1763.
Gardinier PE, Ottaway JM, Fell GS, & Halls DJ (1981) Determination of
aluminium in blood plasma or serum by electrothermal atomic absorption
spectrometry. Anal Chim Acta, 128: 57-66.
Gardinier PE, Schierl R, & Kreutzer K (1987) Aluminium speciation in
soil solutions as studied by size exclusion chromatography. Plant
Soil, 103: 151-154.
Garruto RM, Fukatsu R, Yanagihara R, Gajdusek DC, Hook G, & Fiori CE
(1984) Imaging of calcium and aluminum in neurofibrillary tangle-
bearing neurons in parkinsonism-dementia of Guam. Proc Natl Acad Sci
(USA), 81: 1875-1879.
Gascon C, Planas D, & Moreau G (1987) The interaction of pH, calcium
and aluminium concentrations on the survival and development of
wood frog ( Rana sylvatica) eggs and tadpoles. In: Witters H &
Vanderborght O ed. Ecophysiology of acid stress in aquatic organisms:
Proceedings of an International Symposium, Antwerp, 13-16 January
1987. Antwerp, Belgian Royal Zoological Society, pp 189-199.
Genter RB & Amyot DJ (1994) Freshwater benthic algal population and
community changes due to acidity and aluminum-acid mixtures in
artificial streams. Environ Toxicol Chem, 13(3): 369-380.
Gitelman HJ, Alderman FR, Kurs-Lasky M, & Rockette HE (1995) Serum and
urinary aluminium levels of workers in the aluminium industry. Ann
Occup Hyg, 39: 181-191.
Goate AM, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L,
Guiffral L, Haynes A, & Hardy JA (1991) Segregation of a mis-sense
mutation in the amyloid precursor protein gene with familial Alzheimer
disease. Nature (Lond), 349: 704-706.
Godbold DL, Dictus K, & H�ttermann A (1988a) Influence of aluminium
and nitrate on root growth and mineral nutrition of Norway spruce
( Picea abies) seedlings. Can J For Res, 18: 1167-1171.
Godbold DL, Fritz E, & H�ttermann A (1988b) Aluminum toxicity and
forest decline. Proc Natl Acad Sci (USA), 85: 3888-3892.
Goenaga X & Williams DJA (1988) Aluminium speciation in surface
waters from a Welsh upland area. Environ Pollut, 52: 131-149.
Goenaga X & Williams DJA (1990) Determination of aluminium speciation
in acid waters. In: Edwards RW, Gee AS, & Stoner JH ed. Acid waters in
Wales. Dordrecht, Boston, London, Kluwer Academic Publishers,
pp 189-201.
Goenaga X, Bryant R, & Williams DJA (1987) Influence of sorption
processes on aluminum determinations in acidic waters. Anal Chem,
59: 2673-2678.
Golub MS, Gershwin ME, Donald JM, Negri S, & Keen CL (1987) Maternal
and developmental toxicity of chronic aluminum exposure in mice.
Fundam Appl Toxicol, 8: 346-357.
Golub MS, Donald JM, Gershwin ME, & Keen CL (1989) Effects of aluminum
ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol,
11: 231-235.
Golub MS, Keen CL, & Gershwin ME (1992) Neurodevelopmental effect
of aluminum in mice: Fostering studies. Neurotoxicol Teratol,
14: 177-182.
Golub MS, Han B, Keen CL, & Gershwin ME (1993) Developmental patterns
of aluminium in mouse brain and interactions between dietary aluminium
excess and manganese deficiency. Toxicology, 81: 33-47.
Golub MS, Han B, Keen CL, & Gershwin ME (1994) Auditory startle in
Swiss Webster mice fed excess aluminium in diet. Neurotoxicol Teratol,
16: 423-425.
Golub MS, Han B, Keen CL, Gershwin ME, & Tarara RP (1995) Behavioral
performance of Swiss Webster mice exposed to excess dietary aluminum
during development or during development and as adults. Toxicol Appl
Pharmacol, 133: 64-72.
Gomez M, Domingo JL, Llobet JM, Tomas JM, & Corbella J (1986) Short-
term oral toxicity study of aluminium in rats. Arch Pharmacol Toxicol,
12: 145-151.
Gomez M, Bosque MA, Domingo JL, Llobet JM, & Corbella J (1990)
Evaluation of the maternal and developmental toxicity of aluminum
from high doses of aluminum hydroxide in rats. Vet Hum Toxicol,
32: 545-548.
Gomez M, Domingo JL, & Llobet JM (1991) Developmental toxicity
evaluation of oral aluminum in rats: Influence of citrate.
Neurotoxicol Teratol, 13: 323-328.
Good PF, Perl DP, Bierer LM, & Schmeidler J (1992) Selective
accumulation of aluminium and iron in the neurofibrillary tangles of
Alzheimer's disease: a laser microprobe (LAMMA) study. Ann Neurol,
31: 286-292.
Goodman WG (1984) Short-term aluminum administration in the rat:
reductions in formation without osteomalacia. J Lab Clin Med,
103: 749-757.
Goodman WG (1986) Experimental aluminum-induced bone disease: Studies
in vivo. Kidney Int, 18(suppl): S32-S36.
Goodman WG (1990) Pathophysiologic mechanisms of aluminum toxicity:
Aluminum-induced bone disease. In: de Broe ME & Coburn JW ed. Aluminum
and renal failure. Dordrecht, Boston, London, Kluwer Academic
Publishers, pp 87-108.
Goodman WG, Henry DA, Horst R, Nudelman RK, Alfrey AC, & Coburn JW
(1984a) Parenteral aluminum administration in the dog: II. Induction
osteomalacia and effects on vitamin D metabolism. Kidney Int,
25: 370-375.
Goodman WG, Gilligan J, & Horst R (1984b) Short-term aluminum
administration in the rat: effects on bone formation and relationship
with renal osteomalacia. J Clin Invest, 73: 171-181.
G�ransson A & Eldhuset TD (1987) Effects of aluminium on growth and
nutrient uptake of Betula pendula seedlings. Physiol Plant,
69: 193-199.
Grahn O (1980) Fishkills in two moderately acid lakes due to high
aluminum concentration. In: Drabl�s D & Tollan A ed. Ecological impact
of acid precipitation: Proceedings of an International Conference.
Sandefjord, Norway, 11-14 March 1980. Oslo-�s, SNSF, pp 310-311.
Grant LD, Elias R, Goyer R, Nicholson W, & Olem H (1990) Indirect
health effects associated with acidic deposition. Washington, DC,
National Acid Precipitation Assessment Program, 173 pp (NAPAP Report
No. 23).
Graves AB, White E, Koepsell TD, Reifler BV, Van Belle G, & Larson EB
(1990) The association between aluminum-containing products and
Alzheimer's disease. J Clin Epidemiol, 43: 35-44.
Greger JL & Baier MJ (1983a) Excretion and retention of low or
moderate levels of aluminium by human subjects. Food Chem Toxicol,
21: 473-477.
Greger JL & Baier MJ (1983b) Effect of dietary aluminum on mineral
metabolism of adult males. Am J Clin Nutr, 38: 411-419.
Greger JL & Powers CF (1992) Assessment of exposure to parenteral and
oral aluminum with and without citrate using a desferrioxamine test in
rats. Toxicology, 76: 119-132.
Greger JL, Bula EN, & Gum ET (1985a) Mineral metabolism of rats fed
moderate levels of various aluminum compounds for short periods of
time. J Nutr, 115: 1708-1716.
Greger JL, Goetz W, & Sullivan D (1985b) Aluminium levels in foods
cooked and stored in aluminium pans, trays and foil. J Food Prot,
48: 772-777.
Greger JL, Gum ET, & Bula EN (1986) Mineral metabolism of rats fed
various levels of aluminum hydroxide. Biol Trace Elem Res, 9: 67-77.
Gross P, De Treville RTP, Cralley LJ, Grandquist WT, & Pundsack FL
(1970) The pulmonary response to fibrous dusts of diverse
compositions. Am Ind Hyg Assoc J, 31: 125-132.
Gross P, Harley RA, & Detreville RTP (1973) Pulmonary reaction to
metallic aluminum powders. Arch Environ Health, 26: 227-236.
Guieu C, Martin JM, Thomas AJ, & Elbaz-Poulichet F (1991) Atmospheric
versus river inputs of metals to the Gulf of Lions: Total
concentrations, partitioning and fluxes. Mar Pollut Bull, 22(4):
176-183.
Gundersen P & Rasmussen L (1990) Nitrification in forest soils:
effects from nitrogen deposition on soil acidification and aluminum
release. Rev Environ Contam Toxicol, 113: 1-45.
Gundersen DT, Bustaman S, Seim WK, & Curtis LR (1994) pH, hardness,
and humic acid influence aluminum toxicity to rainbow trout
( Oncorhynchus mykiss) in weakly alkaline waters. Can J Fish Aquat
Sci, 51: 1345-1355.
Gunn JM & Noakes DLG (1987) Latent effects of pulse exposure to
aluminum and low pH on size, ionic composition, and feeding efficiency
of lake trout ( Salvelinus namaycush) alevins. Can J Fish Aquat Sci,
44: 1418-1424.
Gupta SK, Waters DH, & Gwilt PR (1986) Absorption and disposition of
aluminium in the rat. J Pharm Sci, 75: 586-589.
Guy SP, Jones D, Mann DMA, & Itzhaki RF (1991) Human neuroblastoma
cells treated with aluminium express an epitope associated with
Alzheimer's disease neurofibrillary tangles. Neurosci Lett,
121: 166-168.
Hackett C (1962) Stimulative effects of aluminium on plant growth.
Nature (Lond), 195(4840): 471-472.
Hackett C (1964) Ecological aspects of the nutrition of Deschampsia
flexuosa (L.) Trin: I. The effect of aluminium, manganese and pH on
germination. J Ecol, 52: 159-167.
Hackett C (1967) Ecological aspects of the nutrition of Deschampsia
flexuosa (L.) Trin: III. Investigation of phosphorus requirement and
response to aluminium in water culture, and a study of growth in soil.
J Ecol, 55: 831-840.
Hahn PHB & Guenter W (1986) Effect of dietary fluoride and aluminum on
laying hen performance and fluoride concentration in blood, soft
tissue, bone, and egg. Poult Sci, 65: 1343-1349.
Hall RJ, Likens GE, Fiance SB, & Hendrey GR (1980) Experimental
acidification of a stream in the Hubbard Brook Experimental Forest,
New Hampshire. Ecology, 61(4): 976-989.
Hall RJ, Driscoll CT, Likens GE, & Pratt JM (1985) Physical, chemical,
and biological consequences of episodic aluminum additions to a
stream. Limnol Oceanogr, 30(1): 212-220.
Hall RJ, Driscoll CT, & Likens GE (1987) Importance of hydrogen ions
and aluminium in regulating the structure and function of stream
ecosystems: an experimental test. Freshw Biol, 18: 17-43.
Harriman R, Morrison BRS, Caines LA, Collen P, & Watt AW (1987)
Long-term changes in fish populations of acid streams and lochs in
Galloway, south west Scotland. Water Air Soil Pollut, 32: 89-112.
H�s�nen E & Huttunen S (1989) Acid deposition and the element
composition of pine tree rings. Chemosphere, 18(9/10): 1913-1920.
H�s�nen E, Lipponen M, Minkkinen P, Kattainen R, Markkanen K, &
Brjukhanov P (1990) Elemental concentrations of aerosol samples from
the Baltic Sea area. Chemosphere, 21(3): 339-347.
Haugen AA, Becher G, & Bj�rseth A (1983) Biological monitoring of
occupational exposure to polycyclic aromatic hydrocarbons (PAHs) in an
aluminium plant (Abstract). Eur J Cancer Clin Oncol, 19: 1287.
Havas M (1985) Aluminum bioaccumulation and toxicity to Daphnia
magna in soft water at low pH. Can J Fish Aquat Sci, 42:
1741-1748.
Havas M & Hutchinson TC (1982) Aquatic invertebrates from the Smoking
Hills, N.W.T.: effect of pH and metals on mortality. Can J Fish Aquat
Sci, 39: 890-903.
Havas M & Likens GE (1985) Toxicity of aluminum and hydrogen ions to
Daphnia catawba, Holopedium gibberum, Chaoborus punctipennis, and
Chironomus anthrocinus from Mirror Lake, New Hampshire. Can J Zool,
63: 1114-1119.
Havens KE (1990) Aluminum binding to ion exchange sites in
acid-sensitive versus acid-tolerant cladocerans. Environ Pollut,
64: 133-141.
Havens KE (1991) Littoral zooplankton responses to acid and aluminum
stress during short-term laboratory bioassays. Environ Pollut,
73: 71-84.
Havens KE (1993) Acid and aluminum effects on the survival of
littoral macro-invertebrates during acute bioassays. Environ Pollut,
80: 95-100.
Havens KE & Heath RT (1989) Acid and aluminum effects on freshwater
zooplankton: an in situ mesocosm study. Environ Pollut,
62: 195-211.
Helliwell S, Batley GE, Florence TM, & Lumsden BG (1983) Speciation
and toxicity of aluminium in a model fresh water. Environ Technol
Lett, 4: 141-144.
Hellou J, Fancey LL, & Payne JF (1992a) Concentrations of twenty-four
elements in bluefin tuna Thunnus thynnus from the northwest
Atlantic. Chemosphere, 24(2): 211-218.
Hellou J, Warren WG, Payne JF, Belkhode S, & Lobel P (1992b) Heavy
metals and other elements in three tissues of cod, Gadus morhua from
the northwest Atlantic. Mar Pollut Bull, 24(9): 452-458.
Helmboldt O, Hudson LK, Misra C, Wefers K, Stark H, & Danner M (1985)
Aluminum compounds, inorganic. In: Gerhartz W, Yamamoto YS, Campbell
FT, Pfefferkorn R, & Rounsaville JF ed. Ullmann's encyclopedia of
industrial chemistry - Volume A1: Abrasives to aluminum oxide, 5th
revised ed. Weinheim, Verlag Chemie, pp 527-541.
Hem JD & Roberson CE (1967) Form and stability of aluminum hydroxide
complexes in dilute solution. In: Chemistry of aluminum in natural
water. Washington, DC, US Department of Interior, Geological Survey
(Geological Survey-Water Supply Paper No. 1827-A).
Henriksen A, Skogheim OK, & Rosseland BO (1984) Episodic changes in pH
and aluminium-speciation kill fish in a Norwegian salmon river.
Vatten, 40: 255-260.
Henriksen A, Lien L, Traaen TS, Sevaldrud IS, & Brakke DF (1988a) Lake
acidification in Norway: present and predicted fish status. Ambio,
18(6): 314-321.
Henriksen A, Lien L, Traaen TS, Sevaldrud IS, & Brakke DF (1988b) Lake
acidification in Norway - present and predicted chemical status.
Ambio, 17(4): 259-266.
Henriksen A, Wathne BM, R�geberg EJS, Norton SA, & Brakke DF (1988c)
The role of stream substrates in aluminium mobility and acid
neutralization. Water Res, 22(8): 1069-1073.
Henry HL & Norman AW (1985) Interactions between aluminium and the
actions and metabolism of vitamin D3 in the chick. Calcif Tissue Int,
37: 484-490.
Henry DA, Goodman WG, Nudelman RK, Didomenico NC, Alfrey AC,
Slatopolsky E, Stanley TM, & Coburn JW (1984) Parenteral aluminum
administration in the dog: I. Plasma kinetics, tissue levels, calcium
metabolism, and para-thyroid hormone. Kidney Int, 25: 362-369.
Herbert A, Sterling G, Abraham J, & Corrin B (1982) Desquamative
interstitial pneumonia in an aluminum welder. Hum Pathol,
13: 694-699.
Herrmann J (1987) Sodium levels of lotic mayfly nymphs being exposed
to aluminium at low pH. A preliminary report. Ann Soc R Zool Belg,
117: 181-188.
Herrmann J & Andersson KG (1986) Aluminium impact on respiration of
lotic mayflies at low pH. Water Air Soil Pollut, 30: 703-709.
Herzog P, Schmitt KF, Grendhal T, Van der Linden J, & Holterm�ller KH
(1982) VI.5. Evaluation of serum and urine electrolyte changes during
therapy with a magnesium-aluminum containing antacid: results of a
prospective study. In: Halter F ed. Antacids in the eighties:
Symposium on Antacids, Hamburg, June 1980. M�nchen, Urban &
Schwarzenberg, pp 123-135.
Hickie BE, Hutchinson NJ, Dixon DG, & Hodson PV (1993) Toxicity of
trace metal mixtures to alevin rainbow trout ( Oncorhynchus mykiss)
and larval fathead minnow ( Pimephales promelas) in soft, acidic
water. Can J Fish Aquat Sci, 50: 1348-1355.
Hicks JS, Hackett DS, & Sprague GL (1987) Toxicity and aluminium
concentration in bone following dietary administration of two
sodium aluminium phosphate formulations in rats. Food Chem Toxicol,
25(7): 533-538.
Hjortsbert U, Orbaek P, Arborelius M Jr, & Karlsson J-E (1994) Upper
airway irritation and small airways hyperreactivity due to exposure to
potassium aluminium tetrafluoride flux: an extended case report.
Occup Environ Med, 51: 706-709.
Hodsman AB, Sherrard DJ, Alfrey AC, Ott S, Brickman AS, Miller NL,
Maloney NA, & Coburn JW (1982) Bone aluminum and histomorphometric
features of renal osteodystrophy. J Clin Endocrinol Metab,
54: 539-546.
Hodsman AB, Anderson C, & Leung FY (1984) Accelerated accumulation of
aluminum by osteoid matrix in vitamin D deficiency. Miner Electrolyte
Metab, 10: 309-315.
H�hr D, Abel J, & Wilhelm M (1989) Renal clearance of aluminium:
studies in the isolated perfused rat kidney. Toxicol Lett,
45: 165-174.
Hoke RA, Giesy JP, & Kreis RG (1992) Sediment pore water toxicity
identification in the lower Fox River and Green Bay, Wisconsin, using
the microtox assay. Ecotoxicol Environ Saf, 23: 343-354.
H�rnstr�m E, Ekstr�m C, & Duraini MO (1984) Effects of pH and
different levels of aluminium on lake plankton in the Swedish west
coast area. Oslo, Norway, Institute of Fresh Water Ecology, pp 115-127
(Research Report No. 61).
Horst WJ, Wagner A, & Marschner H (1982) Mucilage protects root
meristems from aluminium injury. Z Pflanzenphysiol Bd, 105: 435-444.
Hosovski E, Mastelica Z, Sunderic D, & Radulovic D (1990) Mental
abilities of workers exposed to aluminium. Med Lav, 81: 119-123.
Huang JW, Grunes DL, & Kochian LV (1992a) Aluminum effects on the
kinetics of calcium uptake into cells of the wheat root apex:
Quantification of calcium fluxes using a calcium-selective vibrating
microelectrode. Planta, 188: 414-421.
Huang JW, Shaff JE, Grunes DL, & Kochian LV (1992b) Aluminum effects
on calcium fluxes at the root apex of aluminum-tolerant and
aluminum-sensitive wheat cultivars. Plant Physiol, 98: 230-237.
Hudson LK, Misra C, & Wefers K (1985) Aluminum oxide. In: Gerhartz W,
Yamamoto YS, Campbell FT, Pfefferkorn R, & Rounsaville JF ed.
Ullmann's encyclopedia of industrial chemistry - Volume A1: Abrasives
to aluminum oxide, 5th revised ed. Weinheim, Verlag Chemie,
pp 557-594.
Huett DO & Menary RC (1980) Aluminium distribution in freeze-dried
roots of cabbage, lettuce and kikuyu grass by energy-dispersive x-ray
analysis. Aust J Plant Physiol, 7: 101-111.
Hunn JB, Cleveland L, & Little EE (1987) Influence of pH and aluminum
on developing brook trout in a low calcium water. Environ Pollut,
43: 63-73.
Hunter JB, Ross SL, & Tannahill J (1980) Aluminium pollution and fish
toxicity. J Water Pollut Control Fed, 79: 413-420.
Hussein AS, Cantor AH, & Johnson TH (1988) Use of high levels of
dietary aluminum and zinc for inducing pauses in egg production of
Japanese quail. Poult Sci, 67: 1157-1165.
Hussein AS, Cantor AH, & Johnson TH (1989a) Effect of dietary aluminum
on calcium and phosphorus metabolism and performance of laying hens.
Poult Sci, 68: 706-714.
Hussein AS, Cantor AH, & Johnson TH (1989b) Comparison of the use of
dietary aluminum with the use of feed restriction for force-molting
laying hens. Poult Sci, 68: 891-896.
Hutchinson NJ & Sprague JB (1986) Toxicity of trace metal mixtures
to American flagfish ( Jordanella floridae) in soft, acidic water and
implications for cultural acidification. Can J Fish Aquat Sci, 43:
647-655.
Hydes DJ (1977) Dissolved aluminium concentration in sea water. Nature
(Lond), 268: 136-137.
Hydes DJ (1979) Aluminum in seawater: control by inorganic processes.
Science, 205: 1260-1262.
Hydes DJ (1983) Distribution of aluminium in waters of the north east
Atlantic 250N to 350N. Geochim Cosmochim Acta, 47: 967-973.
Hydes DJ & Kremling K (1993) Patchiness in dissolved metals (Al, Cd,
Co, Cu, Mn, Ni) in North Sea surface waters: seasonal differences and
influence of suspended sediment. Cont Shelf Res, 13(10): 1083-1101.
Hydes DJ & Liss PS (1977) The behaviour of dissolved aluminium in
estuarine and coastal waters. Estuar Coast Mar Sci, 5: 755-769.
IARC (1987) Cadmium and cadmium compounds. In: Overall evaluations of
carcinogenicity: An updating of IARC Monographs, Volumes 1 to 42.
Lyon, International Agency for Research on Cancer, pp 139-142 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Supplement 7).
Ingersoll CG, Gulley DD, Mount DR, Mueller ME, Fernandez JD, Hockett
JR, & Bergman HL (1990) Aluminum and acid toxicity to two strains
of brook trout ( Salvelinus fontinalis). Can J Fish Aquat Sci,
47: 1641-1648.
IPAI (1993) Statistical summary - Volume 5. London, International
Primary Aluminium Institute, 36 pp.
IRPTC (1993) IRPTC legal file 1986. Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
Irvine KN, Drake JJ, & James W (1992) Particulate trace element
concentrations and loadings associated with erosion of pervious urban
land. Environ Technol, 13: 231-237.
Ittel TH, Gruber E, Heinrichs A, Handt S, Hofst�dter F, & Sieberth HG
(1992) Effect of fluoride on aluminum-induced bone disease in rats
with renal failure. Kidney Int, 41: 1340-1348.
Jacobs RW, Duong T, Jones RE, Trapp GA, & Scheibel AB (1989) A
re-examination of aluminium in Alzheimer's disease: Analysis by
energy dispersive x-ray microprobe and flameless atomic absorbtion
spectrophotometry. Can J Neurol Sci, 16: 498-503.
Jacqmin H, Commenges D, Letenneur L, Barberger-Gateau P, & Dartigues
JF (1994) Components of drinking water and risk of cognitive
impairment in the elderly. Am J. Epidemiol, 139: 48-57.
J�ger DE, Wilhelm M, Witte G, & Ohnesorge FK (1991) Intestinal
absorption of aluminium: studies in the isolated perfused rat
intestinal preparation. J Trace Elem Electrolytes Health Dis,
5(2): 81-85.
Jagoe CH, Haines TA, & Buckler DR (1987) Abnormal gill development in
Atlantic salmon ( Salmo salar) fry exposed to aluminium at low pH.
In: Witters H & Vanderborght O ed. Ecophysiology of acid stress in
aquatic organisms: Proceedings of an International Symposium, Antwerp,
13-16 January 1987. Antwerp, Belgian Royal Zoological Society,
pp 375-386.
James BR & Riha SJ (1989) Aluminum leaching by mineral acids in
forest soils: I. Nitric-sulfuric acid differences. Soil Sci Soc Am J,
53: 259-264.
Jederlinic PJ, Abraham JL, Churg A, Himmelstein JS, Epler GR, &
Gaensler EA (1990) Pulmonary fibrosis in aluminum oxide workers. Am
Rev Respir Dis, 142: 1179-1184.
Johnson GVM & Jope RS (1987) Aluminum alters cyclic AMP and cyclic
GMP levels but not presynaptic cholinergic markers in rat brain
in vivo. Brain Res, 403: 1-6.
Johnson NM, Driscoll CT, Eaton JS, Likens GE, & McDowell WH (1981)
'Acid rain' dissolved aluminum and chemical weathering at the Hubbard
Brook Experimental Forest, New Hampshire. Geochim Cosmochim Acta,
45: 1421-1437.
Johnson MG, Culp LR, & George SE (1986) Temporal and spatial trends in
metal loadings to sediments of the Turkey Lakes, Ontario. Can J Fish
Aquat Sci, 43: 754-762.
Johnson GVW, Cogdill KW, & Jope RS (1990) Oral aluminum alters
in vitro protein phosphorylation and kinase activities in rat brain.
Neurobiol Aging, 11: 209-216.
Johnson GVW, Watson AL Jr, Lartius R, Uemura E, & Jope RS (1992)
Dietary aluminum selectively decreases MAP-2 in brains of developing
and adult rats. Neurotoxicology, 13: 463-474.
Jones P (1991) The investigation of aluminium speciation in natural
and potable waters using short-column ion chromatography. Int J
Environ Anal Chem, 44: 1-10.
Jones KC & Bennett BG (1985) Exposure commitment assessments of
environmental pollutants. London, University of London, King's
College, Monitoring and Assessment Research Centre, 33 pp (MARC
Technical Report 33).
Jones BF, Kennedy VC, & Zellweger GW (1974) Comparison of observed and
calculated concentrations of dissolved Al and Fe in stream water.
Water Resour Res, 10(4): 791-793.
Jope RS & Johnson GVW (1992) Neurotoxic effects of dietary aluminium.
In: Aluminium in biology and medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 254-267 (Ciba Foundation Symposium
No. 169.
Jorhem L & Haegglund G (1992) Aluminium in foodstuffs and diets in
Sweden. Z Lebensm.unters Forsch, 194: 38-42.
Joslin JD, Kelly JM, Wolfe MH, & Rustad LE (1988) Elemental patterns
in roots and foliage of mature spruce across a gradient of soil
aluminium. Water Air Soil Pollut, 40: 375-390.
Jouhanneau P, Lacour B, Raisbeck G, Yiou F, Banide H, Brown E, &
Dr�eke T (1993) Gastrointestinal absorption of aluminium in rats
using 26Al and accelerator mass spectrometry. Clin Nephrol,
40(4): 244-248.
Kabra SG & Narayanan R (1988) Aluminium phosphide: worse than Bhopal.
Lancet, 1: 1333.
Kada T, Hirano K, & Shirasu Y (1980) Screening of environmental
chemical mutagens by the rec(-) assay system with Bacillus
subtilis. In: Hollaender A & de Serres FJ ed. Chemical mutagens:
Principles and methods for their detection. New York, London, Plenum
Press, pp 149-173.
Kaehny WD, Hegg AP, & Alfrey AC (1977a) Gastrointestinal absorption
of aluminum from aluminum- containing antacids. N Engl J Med,
296: 1389-1390.
Kaehny WD, Alfrey AC, Holman RE, & Shorr WJ (1977b) Aluminium transfer
during hemodialysis. Kidney Int, 12: 361-365.
Kanematsu N, Hara M, & Kada T (1980) Rec assay and mutagenicity
studies on metal compounds. Mutat Res, 77: 109-116.
Kappel LC, Youngberg H, Ingraham RH, Hembry FG, Robinson DL, & Cherney
JH (1983) Effects of dietary aluminum on magnesium status of cows. Am
J Vet Res, 44: 770-773.
Karlik SJ, Eichhorn GL, & Crapper McLachlan DR (1980) Molecular
interactions of aluminum with DNA. Neurotoxicology, 1: 83-88.
Karlsson-Norrgren L, Dickson W, Ljungberg O, & Runn P (1986a) Acid
water and aluminium exposure: gill lesions and aluminium accumulation
in farmed brown trout, Salmo trutta L. J Fish Dis, 9: 1-9.
Karlsson-Norrgren L, Bj�rklund I, Ljungberg O, & Runn P (1986b) Acid
water and aluminium exposure: experimentally induced gill lesions in
brown trout, Salmo trutta L. J Fish Dis, 9: 11-25.
Katz AC, Frank DW, Sauerhoff MW, Zwicker GM, & Freudenthal RI (1984) A
6-month dietary toxicity study of acidic sodium aluminium phosphate in
beagle dogs. Food Chem Toxicol, 22: 7-9.
Kerr DNS & Ward MK (1988) Aluminum intoxication: History of its
clinical recognition and management. In: Sigel H & Sigel A ed. Metal
ions in biological systems - Volume 24: Aluminum and its role in
biology. New York, Basel, Marcel Dekker, Inc., pp 217-258.
Kersten M, Dicke M, Kriews M, Naumann K, Schmidt D, Schulz M,
Schwikowski M, & Steiger M (1988) Distribution and fate of heavy
metals in the North Sea. In: Salomons W ed. Pollution of the North
Sea: An assessment. Berlin, Heidelberg, New York, Springer Verlag,
pp 300-347.
Khangarot BS & Ray PK (1989) Investigation of correlation between
physicochemical properties of metals and their toxicity to the water
flea Daphnia magna Straus. Ecotoxicol Environ Saf, 18: 109-120.
Kim YS, Lee MH, & Wisniewski HM (1986) Aluminum induced reversible
change in permeability of the blood-brain barrier to [14C]sucrose.
Brain Res, 377: 286-291.
King RG, Sharp JA, & Boura ALA (1983) The effects of Al3+, Cd2+ and
Mn2+ on human erythrocyte choline transport. Biochem Pharmacol,
32: 3611-3617.
Kinraide TB, Ryan PR, & Kochian LV (1992) Interactive effects of
Al3+, H+, and other cations on root elongation considered in terms
of cell-surface electrical potential. Plant Physiol, 99: 1461-1468.
Klaprat DA, Brown SB, & Hara TJ (1988) The effect of low pH and
aluminum on the olfactory organ of rainbow trout, Salmo gairdneri.
Environ Biol Fish, 22(1): 69-77.
Klein GL (1991) The aluminum content of parenteral solutions: Current
status. Nutr Rev, 49: 74-79.
Knutti R & Zimmerli B (1985) [Investigation of the daily portions of
Swiss food establishments: III. Lead, cadmium, mercury, nickel and
aluminium. Mitt Geb Lebensm.hyg, 76: 206-232 (in German).
Kobayashi K, Yumoto S, Nagai H, Hosoyama Y, Imamura M, Masuzawa S,
Koizumi Y, & Yamashita H (1990) 26Al tracer experiment by accelerator
mass spectrometry and its application to the studies for amyotrophic
lateral sclerosis and Alzheimer's disease. I. Proc Jpn Acad, B66:
189-192.
Koga A (1967) Iron, aluminium and manganese concentrations in waters
discharged from Wairakei drillholes. N Z J Sci, 10: 979-987.
Kongerud J (1991) Occupational exposure and asthma: An epidemioligic
study of aluminium potroom workers. Nor J Occup Med, 2(suppl):
1-192.
Kongerud J & Samuelsen SO (1991) A longitudinal study of respiratory
symptoms in aluminium potroom workers. Am Rev Respir Dis, 144:
10-16.
Kongerud J, Groennesby JK, & Magnus P (1990) Respiratory symptoms and
lung function of aluminium potroom workers. Scand J Work Environ
Health, 16: 270-277.
Kopp JF & Kroner RC (1970) Trace metals in the waters of the United
States. Washington, DC, US Department of Interior, Federal Water
Pollution Control Administration (Report No. 9208-4).
Kremling K (1985) The distribution of cadmium, copper, nickel,
manganese, and aluminium in surface waters of the open Atlantic and
European shelf area. Deep-Sea Res, 32(5): 531-555.
Krueger GL, Morris TK, Suskind RR, & Widner EM (1984) The health
effects of aluminum compounds in mammals. CRC Crit Rev Toxicol,
13: 1-24.
Kupchella L & Syty A (1980) Determination of nickel, manganese, copper
and aluminium in chewing gum by nonflame atomic absorption
spectrometry. J Agric Food Chem, 28: 1035-1036.
Lamb DS & Bailey GC (1981) Acute and chronic effects of alum to midge
larva ( Diptera: Chironomidae). Bull Environ Contam Toxicol,
27: 59-67.
Landolt W, Guecheva M, & Bucher JB (1989) The spatial distribution of
different elements in and on the foliage of Norway spruce growing in
Switzerland. Environ Pollut, 56: 155-167.
Landsberg JP, McDonald B, & Watt F (1992) Absence of aluminium in
neuritic plaque cores in Alzheimer's disease. Nature (Lond),
360: 65-68.
Lantzy RJ & MacKenzie FT (1979) Atmospheric trace metals: global
cycles and assessment of man's impact. Geochim Cosmochim Acta,
43: 511-525.
Lawrence GB, Fuller RD, & Driscoll CT (1986) Spatial relationships of
aluminum chemistry in the streams of the Hubbard Brook Experimental
Forest, New Hampshire. Biogeochemistry, 2: 115-135.
Lawrence GB, Fuller RD, & Driscoll CT (1987) Release of aluminum
following whole-tree harvesting at the Hubbard Brook Experimental
Forest, New Hampshire. J Environ Qual, 16(4): 383-390.
Lawrence GB, Driscoll CT, & Fuller RD (1988) Hydrologic control of
aluminum chemistry in an acidic headwater stream. Water Resour Res,
24(5): 659-669.
Lazerte BD (1984) Forms of aqueous aluminum in acidified catchments of
central Ontario: a methodological analysis. Can J Fish Aquat Sci,
41: 766-776.
Leblondel G & Allain P (1980) Blood and brain aluminium concentrations
in mice after intraperitoneal injection of different aluminium
compounds. Res Commun Chem Pathol Pharmacol, 27: 579-586.
Lee CR (1972) Interrelationships of aluminum and manganese on the
potato plant. Agron J, 64: 546-549.
Lee YH (1985) Aluminium speciation in different water types. Ecol
Bull, 37: 109-119.
Lee RE & Von Lehmden DJ (1973) Trace metal pollution in the
environment. J Air Pollut Control Assoc, 23(10): 853-857.
Leharne S, Charlesworth D, & Chowdhry B (1992) A survey of metal
levels in street dusts in an inner London neighbourhood. Environ Int,
18: 263-270.
Leinonen KP (1989) The influence of soil preparation on the levels of
aluminium, manganese, iron, copper, zinc, cadmium and mercury in
Vaccinium myrtillus. Chemosphere, 18(7/8): 1581-1587.
Leivestad H, Jensen E, Kjartansson H, & Xingfu L (1987) Aqueous
speciation of aluminium and toxic effects on Atlantic salmon. In:
Witters H & Vanderborght O ed. Ecophysiology of acid stress in aquatic
organisms: Proceedings of an International Symposium, Antwerp, 13-16
January 1987. Antwerp, Belgian Royal Zoological Society, pp 387-398.
L�onard A & Gerber GB (1988) Mutagenicity, carcinogenicity, and
teratogenicity of aluminum. Mutat Res, 196: 247-257.
Lewis CW & Macias ES (1980) Composition of size-fractionated aerosol
in Charleston, West Virginia. Atmos Environ, 14: 185-194.
Lide DR (1991) CRC handboook of chemistry and physics: A ready-
reference book of chemical and physical data, 71st ed. Boca Raton,
Florida, CRC Press, pp 4/3, 4/41-4/42.
Lindemann J, Holtkamp E, & Herrmann R (1990) The impact of aluminium
on green algae isolated from two hydrochemically different headwater
streams, Bavaria, Germany. Environ Pollut, 67: 61-77.
Lione A (1983) The prophylactic reduction of aluminium intake. Food
Chem Toxicol, 21: 103-109.
Liss L & Thornton DJ (1986) The rationale for aluminum absorption
control in early stages of Alzheimer disease. Neurobiol Aging,
7: 552-554.
Litaor MI (1987) Aluminum chemistry: fractionation, speciation, and
mineral equilibria of soil interstitial waters of an alpine watershed,
Front Range, Colorado. Geochim Cosmochim Acta, 51: 1285-1295.
Ljunggren KG, Lidums V, & Sj�gren B (1991) Blood and urine levels of
aluminium among workers exposed to aluminium flakes. Br J Ind Med,
48: 106-109.
Llobet JM, Domingo JL, Gomez M, Tomas JM, & Corbella J (1987) Acute
toxicity studies of aluminium compounds: antidotal efficacy of several
chelating agents. Pharmacol Toxicol, 60: 280-283.
Longstreth WT, Rosenstock L, & Heyer NJ (1985) Potroom palsy?
Neurologic disorder in three aluminum smelter workers. Arch Int Med,
145: 1972-1975.
Losno R, Colin JL, Le Bris N, Bergametti G, Jickells T, & Lim B (1993)
Aluminium solubility in rainwater and molten snow. J Atmos Chem,
17: 29-43.
Lovell MA, Ehmann WD, & Markesbery WR (1993) Laser microprobe analysis
of brain aluminium in Alzheimer's disease. Ann Neurol, 33: 36-42.
L�kewille A & Van Breemen N (1992) Aluminium precipitates from
groundwater of an aquifer affected by acid atmospheric deposition in
the Senne, northern Germany. Water Air Soil Pollut, 63: 411-416.
Lydersen E, Bj�rnstad HE, Salbu B, & Pappas AC (1987) Trace element
speciation in natural waters using hollow-fiber ultrafiltration. In:
Ladner L ed. Speciation of metals in water, sediments and soil
systems: Lecture notes in earth sciences. Berlin, Heidelberg, New
York, Springer Verlag, vol 11, pp 58-97.
Lydersen E, Salbu B, Poleo ABS, & Muniz IP (1990) The influence of
temperature on aqueous aluminium chemistry. Water Air Soil Pollut,
51: 203-215.
Lydersen E, Poleo ABS, Nandrup Pettersen M, Riise G, Salbu B, Kroglund
F, & Rosseland BO (1994) The importance of "in situ" measurements to
relate toxicity and chemistry in dynamic aluminium freshwater systems.
J Ecol Chem, 3: 357-365.
McCahon CP, Pascoe D, & McKavanagh C (1987) Histochemical observations
on the salmonids Salmo salar L. and Salmo trutta L. and the
ephemeropterans Baetis rhodani(Pict.) and Ecdyonurus venosus
(Fabr.) following a simulated episode of acidity in an upland stream.
Hydrobiologia, 153: 3-12.
McCormack KM, Ottosen LD, Sanger VL, Sprague S, Mayor GH, & Hook JB
(1979) Effect on prenatal administration of aluminum and parathyroid
hormone on fetal development in the rat. Proc Soc Exp Biol Med,
161: 74-77.
McDermott JR, Smith A, Ward MK, Parkinson IS, & Kerr DNS (1978)
Brain-aluminium concentration in dialyses encephalopathy. Lancet,
1: 901-904.
McDermott JR, Smith I, Iqbal K, & Wisniewski HM (1979) Brain aluminum
in aging and Alzheimer disease. Neurology, 29: 809-814.
Mach CE & Brezonik PL (1989) Trace metal research at Little Rock Lake,
Wisconsin: background data, enclosure experiments, and the first three
years of acidification. Sci Total Environ, 87/88: 269-285.
MacKenzie FT, Stoffyn M, & Wollast R (1978) Aluminum in seawater:
control by biological activity. Science, 199: 680-682.
Mackie GL (1989) Tolerances of five benthic invertebrates to hydrogen
ions and metals (Cd, Pb, Al). Arch Environ Contam Toxicol,
18: 215-223.
McLachlan DRC (1989) Aluminium neurotoxicity: Criteria for assigning a
role in Alzheimer's disease. In: Lewis TE ed. Environmental chemistry
and toxicology of aluminium. Chelsea, Michigan, Lewis Publishers Inc.,
pp 299-315.
McLachlan DRC (in press) Aluminium and the risk for Alzheimer's
disease. Environmetrics.
McLachlan DR & Massiah J (1992) Aluminum ingestion: A risk factor for
Alzheimer's disease? Chapter 4. In: Isaacson RL & Jensen KF ed. The
vulnerable brain and environmental risks - Volume 2. Toxins in food.
New York, London, Plenum Press, chapter 4, pp 49-60.
McLachlan DR, Bergeron C, Smith JE, Boomer D, & Rifat SL (1996) Risk
for neuropathologically confirmed Alzheimer's disease and residual
aluminium in municipal drinking water employing weighted residential
histories. Neurology, 46(2): 401-405.
McLaughlin AIG, Kazantzis G, King E, Teare D, Porter RJ, & Owen R
(1962) Pulmonary fibrosis and encephalopathy associated with the
inhalation of aluminium dust. Br J Ind Med, 19: 253-263.
McMillan TM, Freemont AJ, Herxheimer A, Denton J, Taylor AP, Pazianas
M, Cummin ARC, & Eastwood JB (1993) Camelford water poisoning
accident: serial neuropsychological assessments and further
observations on bone aluminium. Hum Exp Toxicol, 12: 37-42.
Madigosky SR, Alvarez-Hernandez X, & Glass J (1991) Lead, cadmium, and
aluminum accumulation in the red swamp crayfish Procambarus clarkii
G. collected from roadside drainage ditches in Louisiana. Arch Environ
Contam Toxicol, 20: 253-258.
Madigosky SR, Alvarez-Hernandez X, & Glass J (1992) Concentrations of
aluminum in gut tissue of crayfish ( Procambarus clarkii), purged in
sodium chloride. Bull Environ Contam Toxicol, 49: 626-632.
Malley DF & Chang PSS (1985) Effects of aluminum and acid on calcium
uptake by the crayfish Orconectes virilis. Arch Environ Contam
Toxicol, 14: 739-747.
Malley DF, Chang PSS, & Moore CM (1987) Changes in the aluminum
content of tissues of crayfish held in the laboratory and in
experimental field enclosures. Can Tech Rep Fish Aquat Sci,
1480: 54-68.
Manna GK & Das RK (1972) Chromosome aberration in mice induced by
aluminum chloride. Nucleus, 15: 180-186.
Mantyh PW, Ghilardi JR, Rogers S, Demaster E, Clark JA, Stimson ER, &
Maggio JE (1993) Aluminum, iron, and zinc ions promote aggregation of
physiological concentrations of �-amyloid peptide. J Neurochem,
61: 1171-1174.
Markesbery WR, Ehmann WD, Hossain TIM, Alauddin M, & Goodin DT (1981)
Instrumental neutron activation analysis of brain aluminum in
Alzheimer disease and aging. Ann Neurol, 10: 511-516.
Marsden SNE, Parkinson IS, Ward MK, Ellsi HA, & Kerr DNS (1979)
Evidence for aluminium accumulation in renal failure. Proc Eur Dial
Transplant Assoc, 16: 588-596.
Martin RB (1986) The chemistry of aluminium as related to biology and
medicine. Clin Chem, 32: 1797-1806.
Martin RB (1992) Aluminium speciation in biology. In: Aluminium in
biology and medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, pp 5-25 (Ciba Foundation Symposium No. 169).
Martyn CN, Osmond C, Edwardson JA, Barker DJP, Harris EC, & Lacey RF
(1989) Geographical relation between Alzheimer's disease and aluminium
in drinking water. Lancet, 1: 59-62.
Marzin DR & Phi HV (1985) Study of the mutagenicity of metal
derivatives with Salmonella typhimurium TA102. Mutat Res,
155: 49-51.
Mason CF & MacDonald SM (1988) Metal contamination in mosses and otter
distribution in a rural Welsh river receiving mine drainage.
Chemosphere, 17(6): 1159-1166.
Mauras Y & Allain P (1985) Automatic determination of aluminum in
biological samples by inductively coupled plasma emission
spectrometry. Anal Chem, 57: 1706.
Mauras Y, Allain P, & Riberi P (1982) Etude de l'absorption digestive
de l'hydrocarbonate d'aluminium chez l'individu sain. Th�rapie,
37: 598-600.
Mayor GH, Keiser JA, Makdani D, & Ku PK (1977) Aluminum absorption
and distribution: Effect of parathyroid hormone. Science,
197: 1187-1189.
Meding B, Augustsson A, & Hansson C (1984) Short communications. Patch
test reactions to aluminium. Contact Dermatitis, 10: 107.
Mersch J, Gu�rold F, Rousselle P, & Pihan J-C (1993) Transplanted
aquatic mosses for monitoring trace metal mobilization in acidified
streams of the Vosges Mountains, France. Bull Environ Contam Toxicol,
51: 255-259.
Mesco ER, Kachen C, & Timiras PS (1991) Effects of aluminium on tau
proteins in human neuroblastoma cells. Mol Chem Neuropathol,
14(3): 199-212.
Meyer FA & Kasper W (1942) [Examination of the effects of aluminium on
the lung.] Dtsch Arch Klin Med, 189: 471-495 (in German).
Michel P, Commenges D, Dartigues JF, Gagnon M, Barberger-Gateau P,
Letenneur L, & Paquid Research Group (1991) Study of the relationship
between aluminium concentration in drinking water and risk of
Alzheimer's disease. In: Iqbal K, McLachlan DRC, Winblad B, &
Wisniewski HM ed. Alzheimer's disease: Basic mechanisms, diagnosis and
therapeutic strategies. New York, John Wiley, pp 387-391.
Miller RR, Churg AM, Hutcheon M, & Lam S (1984) Pulmonary alveolar
proteinosis and aluminum dust exposure. Am Rev Respir Dis,
130: 312-315.
Mitchell J, Manning GB, Molyneux M, & Lane RE (1961) Pulmonary
fibrosis in workers exposed to finely powdered aluminium. Br J Ind
Med, 18: 10-20.
Mizuno N & Yoshida H (1993) Effects of exchangeable aluminium on the
reduction of potato scab. Plant Soil, 155/156: 505-508.
Monteagudo FSE, Isaacson LC, Wilson G, Hickman R, & Folb PI (1988)
Aluminium exretion by the distal tubule of the pig kidney. Nephron,
49: 245-250.
Moomaw JC, Nakamura MT, & Sherman GD (1959) Aluminium in some Hawaiian
plants. Pac Sci, 13: 335-341.
M�hlenberg W (1990) [High aluminium levels in wells of the southern
sandy health-land of lower Saxony by acid precipitation:
Interpretation from hygienic and ecological points of view.]
�ffentl Gesundh.wes, 52: 1-8 (in German with English summary).
Mulder J, Van Breemen N, Rasmussen L, & Driscoll CT (1989) Aluminum
chemistry of acidic sandy soils with various inputs of acidic
deposition in the Netherlands and in Denmark In: Lewis TE ed.
Environmental chemistry and toxicology of aluminum. Proceedings of the
194th Annual Meeting of the American Chemical Society, New Orleans,
LA, 30 August-4 September 1987. Chelsea, Michigan, Lewis Publishers
Inc., pp 171-169.
Muller G, Bernuzzi V, Desor D, Hutin M-F, Burnel D, & Lehr PR (1990)
Developmental alterations in offspring of female rats orally
intoxicated by aluminum lactate at different gestation periods.
Teratology, 42: 253-261.
Muller G, Hutin MF, Burnel D, & Lehr PR (1992) Aluminum transfer
through milk in female rats intoxicated by aluminum chloride. Biol
Trace Elem Res, 34: 79-87.
Muller G, Burnel D, Gery A, & Lehr PR (1993) Element variations in
pregnant and nonpregnant female rats orally intoxicated by aluminium
lactate. Biol Trace Elem Res, 39: 211-219.
Muniz IP & Leivestad H (1980) Toxic effects of aluminium on the brown
trout, Salmo trutta L. In: Drabl�s D & Tollan A ed. Ecological
impact of acid precipitation: Proceedings of an International
Conference, Sandefjord, Norway, 11-14 March 1980. Oslo-�s, SNSF,
pp 320-321.
Muramoto S (1981) Influence of complexans (NTA, EDTA) on the toxicity
of aluminum chloride and sulfate to fish at high concentrations. Bull
Environ Contam Toxicol, 27: 221-225.
Nagy S & Nikdel S (1986) Tin, iron and aluminum contents of
commercially canned single-strength grapefruit juice stored at
varying temperatures. J Agric Food Chem, 34: 588-593.
Nalewajko C & Paul B (1985) Effects of manipulations of aluminum
concentrations and pH on phosphate uptake and photosynthesis of
planktonic communities in two Precambrian shield lakes. Can J Fish
Aquat Sci, 42: 1946-1953.
Neal C (1988) Aluminium solubility relationships in acid waters: a
practical example of the need for a radical reappraisal. J Hydrol,
104: 141-159.
Neal C & Williams RJ (1988) Towards establishing aluminium hydroxy
silicate solubility relationships for natural waters. J Hydrol,
97: 347-352.
Neal C, Whitehead P, Neale R, & Cosby J (1986) Modelling the effects
of acidic deposition and conifer afforestation on stream acidity in
the British uplands. J Hydrol, 86: 15-26.
Neal C, Reynolds B, Stevens PA, Hornung M, & Brown SJ (1990) Dissolved
inorganic aluminium in acidic stream and soil waters in Wales. In:
Edwards RW, Gee AS, & Stoner JH ed. Acid waters in Wales. Dordrecht,
Boston, London, Kluwer Academic Publishers, pp 173-188.
Neal C, Reynolds B, Smith CJ, Hill S, Neal M, Conway T, Ryland GP,
Jeffrey H, Robson AJ, & Fisher R (1992) The impact of conifer
harvesting on stream water pH, alkalinity and aluminium concentrations
for the British uplands: an example for an acidic and acid sensitive
catchment in mid-Wales. Sci Total Environ, 126: 75-87.
Neri LC & Hewitt D (1991) Aluminium, Alzheimer`s disease, and drinking
water. Lancet, 338: 390.
Neville CM & Campbell PGC (1988) Possible mechanisms of aluminum
toxicity in a dilute, acidic environment to fingerlings and older life
stages of salmonids. Water Air Soil Pollut, 42: 311-327.
NFA (1993) The 1992 Australian market basket survey - A total diet
survey of pesticides and contaminants. Canberra, Australian National
Food Authority, 96 pp.
Nieboer E, Gibson BL, Oxman AD, & Kramer JR (1995) Health effects of
aluminium: A Critical review with emphasis in drinking water. Environ
Rev, 3: 29-85.
Nilsson SI & Bergkvist B (1983) Aluminium chemistry and acidification
processes in a shallow podzol on the Swedish westcoast. Water Air Soil
Pollut, 20: 311-329.
NIOSH (1984) Aluminium and compounds: Method 7300. In: NIOSH manual.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 5, p 173/1.
Nishioka H (1975) Mutagenic activities of metal compounds in bacteria.
Mutat Res, 31: 185-189.
Nordal KP, Dahl E, Thomassen Y, Brodwall EK, & Halse J (1988)
Seasonal variations in serum aluminium concentrations. Pharmacol
Toxicol, 62: 80-83.
Norrgren L & Degerman E (1993) Effects of different water qualities
on the early development of Atlantic salmon and brown trout exposed
in situ. Ambio, 22(4): 213-218.
Norton SA (1971) Geochemical cycles involving flora, lake water and
bottom sediments. Orono, Maine, University of Maine, Water Resources
Research Center (NTIS PB-206 197).
Nyholm NEI (1981) Evidence of involvement of aluminum in causation of
defective formation of eggshells and of impaired breeding in wild
passerine birds. Environ Res, 26: 363-371.
Oberly TJ, Piper CE, & McDonald DS (1982) Mutagenicity of metal
salts in the L5178Y mouse lymphoma assay. J Toxicol Environ Health,
9: 367-376.
O'Donnell TV, Welford B, & Coleman ED (1989) Potroom asthma: New
Zealand experience and follow-up. Am J Ind Med, 15: 43-49.
Ogilvie DM & Stechey DM (1983) Effects of aluminum on respiratory
responses and spontaneous activity of rainbow trout, Salmo
gairdneri. Environ Toxicol Chem, 2: 43-48.
Ohtawa M, Seko M, & Takayama F (1983) Effect of aluminum ingestion on
lipid peroxidation in rats. Chem Pharm Bull, 31: 1415-1418.
OMEE (1995) Drinking water surveillance programme - Province of
Ontario. Toronto, Canada, Ontario Ministry of Environment and Energy.
Ondov JM, Zoller WH, & Gordon GE (1982) Trace element emissions on
aerosols from motor vehicles. Environ Sci Technol, 16(6): 318-328.
Ondreicka R, Ginter E, & Kortus J (1966) Chronic toxicity of aluminium
in rats and mice and its effects on phosphorus metabolism. Br J Ind
Med, 23: 305-312.
Ormerod SJ, Allinson N, Hudson D, & Tyler SJ (1986) The distribution
of breeding dippers ( Cinclus cinclus (L.); Aves) in relation to
stream acidity in upland Wales. Freshw Biol, 16: 501-507.
Ormerod SJ, Boole P, McCahon CP, Weatherley NS, Pascoe D, & Edwards RW
(1987a) Short-term experimental acidification of a Welsh stream:
comparing the biological effects of hydrogen ions and aluminium.
Freshw Biol, 17: 341-356.
Ormerod SJ, Weatherly NS, French P, Blake S, & Jones WM (1987b) The
physiological response of brown trout Salmo trutta to induced
episodes of low pH and elevated aluminium in a Welsh hill-stream In:
Witters H & Vanderborght O ed. Ecophysiology of acid stress in aquatic
organisms: Proceedings of an International Symposium, Antwerp, 13-16
January 1987. Antwerp, Belgian Royal Zoological Society, pp 435-447.
Ormerod SJ, Bull KR, Cummins CP, Tyler SJ, & Vickery JA (1988) Egg
mass and shell thickness in dippers Cinclus cinclus in relation to
stream acidity in Wales and Scotland. Environ Pollut, 55: 107-121.
Ormerod SJ, Donald AP, & Brown SJ (1989) The influence of plantation
forestry on the pH and aluminium concentration of upland Welsh
streams: a re-examination. Environ Pollut, 62: 47-62.
Ormerod SJ, Weatherley NS, Merrett WJ, Gee AS, & Whitehead PG (1990)
Restoring acidified streams in upland Wales: a modelling comparison of
the chemical and biological effects of liming and reduced sulphate
deposition. Environ Pollut, 64: 67-85.
Ott SM, Feist E, Andress DL, Liu CC, Sherrard DJ, Alfrey AC,
Slatopolsky E, & Howard GA (1987) Development and reversibility
of aluminum-induced bone lesions in the rat. J Lab Clin Med,
109: 40-47.
Ottley CJ & Harrison RM (1993) Atmospheric dry deposition flux of
metallic species to the North Sea. Atmos Environ, 27A(5): 685-695.
Panasenkov YV (1987) Determination of the effect of aluminium sulphate
on natural microbial coenoses in experiment. J Hyg Epidemiol Microbiol
Immunol, 31(3): 279-286.
Parent L & Campbell PGC (1994) Aluminum bioavailability to the green
alga Chlorella pyrenoidosa in acidified synthetic soft water.
Environ Toxicol Chem, 13(4): 587-598.
Parker DR & Bertsch PM (1992a) Identification and quantification of
the "Al13" tridecameric polycation using ferron. Environ Sci Technol,
26(5): 908-914.
Parker DR & Bertsch PM (1992b) Formation of the "Al13" tridecameric
polycation under diverse synthesis conditions. Environ Sci Technol,
26(5): 914-916.
Paternain JL, Domingo JL, Llobet JM, & Corbella J (1988) Embryotoxic
and teratogenic effects of aluminum nitrate in rats upon oral
administration. Teratology, 38: 253-257.
Pennington JAT & Schoen SA (1995) Estimates of dietary exposure to
aluminium. Food Addit Contam, 12(1): 119-128.
Perl DP, Gajdusek DC, Garruto RM, Yanagihara RT, & Gibbs CJ (1982)
Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis
and parkinsonism-dementia of Guam. Science, 217: 1053-1055.
Petit TL, Biedermann GB, & McMullen PA (1980) Neurofibrillary
degeneration, dying back and learning-memory deficits after aluminum
administration, implications for brain aging. Exp Neurol,
67: 152-162.
Petrich SM & Reish DJ (1979) Effects of aluminium and nickel on
survival and reproduction in polychaetous annelids. Bull Environ
Contam Toxicol, 23: 698-702.
Pettersen JC, Hackett DS, & Zwicker GM (1990) Twenty-six week toxicity
study with kasal (basic sodium aluminum phosphate) in beagle dogs.
Environ Geochem Health, 12: 121-123.
Pigott GH & Ishmael J (1981) An assessment of the fibrogenic potential
of two refractory fibres by intraperitoneal injection in rats. Toxicol
Lett, 8: 153.
Pigott GH, Gaskell BA, & Ishmael J (1981) Effects of long term
inhalation of alumina fibres in rats. Br J Exp Pathol, 62: 323-331.
Pillay KKS & Thomas CC (1971) Determination of the trace element
levels in atmospheric pollutants by neutron activation analysis.
J Radioanal Chem, 7: 107-118.
Pitchai R, Subramanian R, Selvapathy P, & Elangovan R (1992) Aluminium
content of drinking water in Madras. Water Supply, 10(4): 83-86.
Plankey BJ & Patterson HH (1987) Kinetics of aluminum-fulvic acid
complexation in acidic waters. Environ Sci Technol, 21: 595-601.
Platts MM, Owen G, & Smith S (1984) Water purification and the
incidence of fractures in patients receiving home haemodialysis
supervised by a single centre: evidence for "safe" upper limit of
aluminium in water. Br Med J, 288: 969-972.
Pol�o ABS, Lydersen E, Rosseland BO, Kroglund F, Salbu B, Vogt RD, &
Kvellestad A (1994) Increased mortality of fish due to changing
Al-chemistry of mixing zones between limed streams and acidic
tributaries. Water Air Soil Pollut, 75: 339-351.
Priest ND (1990) The distribution and behaviour of heavy metals in the
skeleton and body: studies with bone-seeking radionuclides. In: Priest
ND & Van de Vyver F ed. Trace metals and fluoride in bones and teeth.
Boca Raton, Florida, CRC Press, pp 83-139.
Priest ND (1993) The bioavailability and metabolism of aluminium
compounds in man. Proc Nutr Soc, 52: 231-240.
Priest ND (1994) A human volunteer feeding study using aluminium-26
labelled aluminium citrate, aluminium hydroxide and aluminium
hydroxide in the presence of citrate. Harwell, Oxfordshire, AEA
Technology (Report No. AEA-TPD-268).
Priest ND, Newton, D, & Talbot RJ (1991) Metabolism of aluminium-26
and gallium-67 in a volunteer following their injections as citrates.
Harwell, Oxfordshire, AEA Technology (Report No. AEA-EE-0106).
Priest N, Newton D, & Talbot R (1992) Metabolism of Al-26 and Ga-67
following injection as citrates. In: Gitelman HJ ed. Proceedings of
the Second International Conference on Aluminium and Health.
Washington, DC, Alumunium Association, pp 7-10.
Priest ND, Talbot RJ, & Austin JG (1995a) The bioavailability in young
adults of aluminium ingested in drinking water. Harwell, Oxfordshire,
AEA Technology (Report No. AEA-TPD-269).
Priest ND, Newton D, Day JP, Talbot RJ, & Warner AJ (1995b) Human
metabolism of aluminium-26 and gallium-67 injected as citrates. Hum
Exp Toxicol, 14(3): 287-293.
Priest N, Newton D, & Talbot R (1996) Studies on the metabolism of
injected and ingested aluminium using 26Al. In: Proceedings of the
Third International Conference on Aluminium and Health, Miami,
Florida. Washington, DC, Aluminium Association, pp 30-34.
Provan SD & Yokel RA (1988) Aluminum uptake by the in situ rat gut
preparation. J Pharmacol Exp Ther, 245: 928-931.
Quarles LD (1991) Paradoxical toxic and trophic osseous actions
of aluminum: Potential explanations. Miner Electrolyte Metab,
17: 233-239.
Quarles LD, Dennis VW, Gitelman HJ, Harrelson JM, & Drezner MK (1985)
Aluminum deposition at the osteoid-bone interface. J Clin Invest,
75: 1441-1447.
Quarles LD, Gitelman HJ, & Drezner MK (1988) Induction of bone
formation in the beagle: a novel effect of aluminum. J Clin Invest,
81: 1056-1066.
Quarles LD, Gitelman HJ, & Drezner MK (1989) Aluminium-induced de novo
bone formation in the beagle: a parathyroid hormone-dependent event.
J Clin Invest, 83: 1644-1650.
Quarles LD, Murphy G, Vogler JB, & Drezner MK (1990) Aluminum-induced
neo-osteogenesis: a generalized process affecting trabecular
networking in the axial skeleton. J Bone Miner Metab, 5: 625-635.
Que Hee SS & Boyle JR (1988) Simultaneous multielemental analysis of
some environmental and biological samples by inductively coupled
plasma atomic emission spectrometry. Anal Chem, 60: 1033-1042.
Rabe A, Lee MH, Shek J, & Wisniewski HM (1982) Learning deficit in
immature rabbits with aluminum-induced neurofibrillary changes. Exp
Neurol, 76: 441-446.
Rahn KA (1981) Atmospheric, riverine and oceanic sources of
seven trace constituents to the Arctic Ocean. Atmos Environ,
15(8): 1507-1516.
Rao KSJ & Divakar S (1993) Spectroscopic studies on the effects of
aluminum ion on calf-thymus DNA. Bull Environ Contam Toxicol,
50: 92-99.
Recker RR, Blotcky AJ, Leffler JA, & Rack EP (1977) Evidence for
aluminum absorption from the gastrointestinal tract and bone
deposition by aluminum carbonate ingestion with normal renal function.
J Lab Clin Med, 90: 810-815.
Reich PB, Oleskyn J, & Tjoelker MG (1994) Relationship of aluminium
and calcium to net CO2 exchange among diverse Scots pine provenances
under pollution stress in Poland. Oecologia, 97: 82-92.
Reynolds B, Stevens PA, Adamson JK, Hughes S, & Roberts JD (1992)
Effects of clearfelling on stream and soil water aluminium chemistry
in three UK forests. Environ Pollut, 77: 157-165.
Rifat SL, Eastwood MR, Crapper McLachlan DR, & Corey PN (1990) Effect
of exposure of miners to aluminium powder. Lancet, 336: 1162-1165.
Robertson JA, Felsenfeld AJ, Haygood CC, Wilson P, Clarke C, & Llach F
(1983) Animal model of aluminum-induced osteomalacia: Role of chronic
renal failure. Kidney Int, 23: 327-335.
Rodriguez M, Felsenfeld AJ, & Llach F (1990) Aluminum administration
in the rat separately affects the osteoblast and bone mineralization.
J Bone Miner Res, 5: 59-68.
R�llin HB, Theodorou P, & Kilroe-Smith TA (1991a) Deposition of
aluminium in tissues of rabbits exposed to inhalations of low
concentration of Al2O3 dust. Br J Ind Med, 48: 389-391.
R�llin HB, Theodorou P, & Kilroe-Smith TA (1991b) The effect of
exposure to aluminium on concentrations of essential metals in serum
of foundry workers. Br J Ind Med, 48: 243-246.
Ronneberg A & Langmark F (1992) Epidemiologic evidence of cancer in
aluminium reduction plant workers. Am J Ind Med, 22: 573-590.
Rosa IV, Henry PR, & Ammermann CB (1982) Interrelationship of dietary
phosphorus, aluminium and iron on performance and tissue mineral
composition in lambs. J Anim Sci, 1982: 1231-1240.
Rosseland BO & Skogheim OK (1984) A comparative study on salmonid fish
species in acid aluminium-rich water. II. Physiological stress and
mortality of one- and two-year-old fish. Oslo, Norway, Institute of
Fresh Water Ecology, pp 186-194 (Research Report No. 61).
Rosseland BO & Staurnes M (1994) Physiological mechanisms for toxic
effects and resistance to acidic water: an ecophysiological and
ecotoxicological approach. In: Steinberg CEW & wright RF ed.
Acidification of freshwater ecosystems: Implications for the future.
New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
pp 228-246.
Rosseland BO, Eldhuset TD, & Staurnes M (1990) Environmental effects
of aluminium. Environ Geochem Health, 12(1-2): 17-27.
Rosseland BO, Blakar IA, Bulger A, Kroglund F, Kvellstad A, Lydersen
E, Oughton DH, Salbu B, Staurnes M, & Vogt R (1992) The mixing zone
between limed and acidic river waters: complex aluminium chemistry and
extreme toxicity for salmonids. Environ Pollut, 78(1/3): 3-8.
Roy AK, Sharma A, & Talukder G (1988) Some aspects of aluminum
toxicity in plants. Bot Rev, 54(2): 145-178.
Roy AK, Talukder G, & Sharma A (1990) Effects of aluminium sulphate on
human leukocyte chromosomes in vitro. Mutat Res, 244: 179-184.
Roy AK, Sharma A, & Talukder G (1991a) Effects of aluminum salts on
bone marrow chromosomes in rats in vivo. Cytobios, 66: 105-112.
Roy AK, Talukder G, & Sharma A (1991b) Similar effects in vivo of
two aluminum salts on the liver, kidney, bone, and brain of
Rattus norvegicus. Bull Environ Contam Toxicol, 47: 288-295.
Rueter JG, O'Reilly KT, & Petersen RR (1987) Indirect aluminum
toxicity to the green alga Scenedesmus through increased cupric ion
activity. Environ Sci Technol, 21(5): 435-438.
Ryther J, Losordo TM, Furr AK, Parkinson TF, Gutenman WH, Pakkala IS,
& Lisk DJ (1979) Concentration of elements in marine organisms
cultured in seawater flowing through coal-fly ash. Bull Environ Contam
Toxicol, 23: 207-210.
Sackett W & Arrhenius G (1962) Distribution of aluminum species in
the hydrosphere. I. Aluminum in the ocean. Geochim Cosmochim Acta,
26: 955-968.
Saia AN, Slagsvold P, Bergh H, & Laksesvela B (1977) High fluorine
water to weather sheep maintained in pens. Nord Vet Med,
29: 172-180.
Saia B, Cortese S, Piazza G, Camposampietro A, & Clonfero E (1981)
Chest x-ray findings among aluminium production plant workers. Med
Lav, 4: 323-329.
St George-Hyslop P, Haines J, Rogaev E, Mortilla M, Vaula G, Pericak-
Vance M, Foncin JF, Montesi M, Bruni A, Sorbi S, Raincro I, Pinessi I,
Pollen D, Polinski R, Nee I, Kennedy J, Macciardi F, Rogaeva E, Liang
Y, Alexandrova N, Lukiw W, Schlumpf K, Tanzi R, Tsuda T, Farrer L,
Cantu J-M, Duara R, Amaducci L, Bergamini I, Gusella J, Roses A, &
Crapper McLachlan D (1992) Genetic evidence for a novel familial
Alzheimer disease gene on chromosome 14. Nature (Genet), 2: 330-334.
Sanin S, Tuncel G, Gaines AF, & Balkas TI (1992) Concentrations and
distributions of some major and minor elements in the sediments of
the River G�ksu and Tasucu Delta, Turkey. Mar Pollut Bull,
24(3): 167-169.
Santos F, Chan JCM, Yang MS, Savory J, & Wills MR (1987) Aluminum
deposition in the central nervous system: Preferential accumulation in
the hippocampus in weenling rats. Med Biol, 65: 53-55.
Sanz-Medel A, Roza RR, Alonson RG, Vallina AN, & Cannata J (1987)
Atomic spectrometric methods (atomic absorption and inductively
coupled plasma atomic emission) for the determination of aluminium at
the parts per billion level in biological fluids. J Anal Atmos Spec,
2: 177-184.
Saunders A, Strittmatter WJ, Schmechel St George-Hyslop P, Pericak-
Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper MacLachlan DR, Alberts
MJ, Hulette C, Crain B, Goldgaber D, & Roses AD (1993) Association of
apolipoprotein E allelle E4 with late onset familial and sporadic
Alzheimer disease. Neurology, 43: 1467-1472.
Savory J, Nicholson JR, & Wills MR (1987) Is aluminium leaching
enhanced by fluoride? Nature (Lond), 327: 107-108.
Sax NI & Lewis RJSR (1987) Hawley's condensed chemical dictionary,
11th ed. New York, Van Nostrand Reinhold Company, pp 42-51.
Schaller KH & Valentin H (1984) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Commission of the European Communities, pp 21-29.
Schecher WD & Driscoll CT (1987) An evaluation of uncertainty
associated with aluminum equilibrium calculations. Water Resour Res,
23(4): 525-534.
Schenck RU, Bjorksten J, & Yaeger L (1989) Composition and
consequences of aluminum in water, beverages, and other ingestibles.
In: Lewis TE ed. Environmental chemistry and toxicology of aluminum.
Chelsa, Michigan, Lewis Publishers Inc., pp 247-269.
Schofield CL & Trojnar JR (1980) Aluminum toxicity to brook trout
( Salvelinus fontinalis) in acidified waters. In: Toribara TY, Miller
MW, & Marrow PE ed. Polluted rain: Proceedings of the 12th Rochester
International Conference on Environmental Toxicity, Rochester, May
1979. New York, London, Plenum Press, pp 341-366.
Schreeder MT, Favero MS, Hughes JR, Petersen NJ, Bennett PH, & Maynard
JE (1983) Dialysis encephalopathy and aluminum exposure: An
epidemiologic analysis. J Chronic Dis, 36: 581-593.
Schwedt G (1989) [Photometric determination of aluminium.] Stuttgart,
Wissenschaftliche Verlagsgesellschaft mbH, pp IV/A1/1-4, II/A1/1-3 (in
German).
Sedman AB, Alfrey AC, Miller NL, & Goodman WG (1987) Tissue and
cellular basis for impaired bone formation in aluminum-related
osteomalacia in the pig. J Clin Invest, 79: 86-92.
Segner H, Marthaler R, & Linnenbach M (1988) Growth, aluminium uptake
and mucous cell morphometrics of early life stages of brown trout,
Salmo trutta, in low pH water. Environ Biol Fish, 21(2): 153-159.
Seip HM, M�ller L, & Naas A (1984) Aluminium speciation: comparison of
two spectrophotometric analytical methods and observed concentrations
in some acidic aquatic systems in southern Norway. Water Air Soil
Pollut, 23: 81-95.
Seip HM, Christophersen N, & Sullivan TJ (1989) Episodic variations in
streamwater aluminum chemistry at Birkenes, southernmost Norway. In:
Lewis TE ed. Environmental chemistry and toxicology of aluminum.
Proceedings of the 194th Annual Meeting of the American Chemical
Society, New Orleans, LA, 30 August-4 September 1987. Chelsea,
Michigan, Lewis Publishers Inc., pp 159-169.
Shacklette HT & Boerngen JG (1984) Element concentrations in soils and
other surficial materials of the conterminous United States.
Washington, DC, US Department of Interior, Geological Survey, 105 pp
(Geological Survey Professional Paper No. 1270).
Shaver CG & Riddell AR (1947) Lung changes associated with the
manufacture of alumina abrasives. J Ind Hyg Toxicol, 29: 145-157.
Sheets WD (1957) Toxicity studies of metal-finishing wastes. Sew Ind
Wastes, 29(12): 1380-1384.
Shin R-W, Lee VM-Y, & Trojanowski JQ (1994) Aluminum modifies the
properties of Alzheimer's disease PHF(tau) proteins in vivo and
in vitro. J Neurosci, 14(11): 7221-7233.
Shore D & Wyatt RJ (1983) Aluminum and Alzheimer's disease. J Nerv
Ment Dis, 71: 553-558.
Siddens LK, Seim WK, Curtis LR, & Chapman GA (1986) Comparison of
continuous and episodic exposure to acidic, aluminum-contaminated
waters of brook trout ( Salvelinus fontinalis). Can J Fish Aquat Sci,
43: 2036-2040.
Silvey WD (1967) Occurrence of selected minor elements in the waters
of California. Washington, DC, US Department of Interior, Geological
Survey, 25 pp (Geological Survey-Water Supply Paper No. 1535-L).
Simons LH, Monaghan PH, & Taggart MS (1953) Aluminum and iron in
Atlantic and Gulf of Mexico surface waters. Anal Chem, 25: 989-990.
Simonsson Bg, Sj�berg A, Rolf C, & Haeger-Aronsen B (1985) Acute and
long-term airway hyperreactivity in aluminium-salt exposed workers
with nocturnal asthma. Eur J Respir Dis, 66: 105-118.
Simpson W, Kerr DNS, Hill AVL, & Siddiqui JY (1973) Skeletal changes
in patients on regular hemodialysis. Diagn Radiol, 107: 313-320.
Sj�gren B & Elinder CG (1992) Proposal of a dose-response relationship
between aluminium welding fume exposure and effect on the central
nervous system. Med Lav, 83(5): 484-488.
Sj�gren B & Ulfvarson U (1985) Respiratory symtoms and pulmonary
function among welders working with aluminum, stainless steel and
railroad tracks. Scand J Work Environ Health, 11: 27-32.
Sj�gren B, Lundberg I, & Lidums V (1983) Aluminium in the blood and
urine of industrially exposed workers. Br J Ind Med, 40: 301-304.
Sj�gren B, Lidums V, Hakansson M, & Hedstrom L (1985) Exposure and
urinary excretion of aluminum during welding. Scand J Work Environ
Health, 11: 39-43.
Sj�gren B, Elinder CG, Lidums V, & Chang G (1988) Uptake and urinary
excretion of aluminum among welders. Int Arch Occup Environ Health,
60: 77-79.
Sj�gren B, Gustavsson P, & Hogstedt C (1990) Neuropsychiatric
symptoms among welders exposed to neurotoxic metals. Br J Ind Med,
47: 704-707.
Sj�gren B, Ljunggren KG, Almkvist O, Frech W, & Basun H (1994a)
Aluminosis and dementia. Lancet, 344(8930): 1154.
Sj�gren B, Iregren A, Frech W, Hagman M, Johansson L, Tesarz M, &
Wennberg A (1994b) [Effects on the nervous system among welders
exposed to aluminum or manganese.] Solna, Sweden, National Institute
of Occupational Health, 27 pp (Work and Health Scientific Publication
Series) (in Swedish with English summary).
Sj�str�m J (1994) Aluminium and sulphate in acidic soils and
groundwaters on the Swedish west coast. Water Air Soil Pollut,
75: 127-139.
Skalsky HL & Carchman RA (1983) Aluminum homeostasis in man. J Am Coll
Toxicol, 2: 405-423.
Skogheim OK & Rosseland BO (1986) Mortality of smolt of Atlantic
salmon, Salmo salar L., at low levels of aluminium in acidic
softwater. Bull Environ Contam Toxicol, 37: 258-265.
Skogheim OK, Rosseland BO, & Sevaldrud IH (1984) Deaths of spawners of
Atlantic salmon ( Salmo salar L.) in River Ogna, SW Norway, caused by
acidified aluminium-rich water. Oslo, Norway, Institute of Fresh Water
Ecology, pp 195-202 (Research Report No. 61).
Slanina P, Falkeborn Y, Frech W, & Cedergren A (1984) Aluminium
concentrations in the brain and bone of rats fed citric acid,
aluminium citrate or aluminium hydroxide. Food Chem Toxicol,
22: 391-397.
Slanina P, Frech W, Bernhardson A, Cedergren A, & Mattsson P (1985)
Influence of dietary factors on aluminium absorption and retention in
the brain and bone rats. Acta Pharmacol Toxicol, 56: 331-336.
Slanina P, Frech W, Ekstr�m L-G, L��f L, Slorach S, & Cedergren A
(1986) Dietary citric acid enhances absorption of aluminium in
antacids. Clin Chem, 32: 539-541.
Smith LF (1995) Public health role, aluminium and Alzheimer's disease.
Environmetrics, 6: 277-286.
Solomon PR, Koota D, & Pendlebury WW (1990) Disrupted retention of the
classically conditioned nictitating membrane response in the aluminum-
intoxicated rabbit using electrical stimulation of the brain as a
conditioned stimulus. Neurobiol Aging, 11: 523-528.
Sorenson JRJ, Campbell IR, Tepper LB, & Lingg RD (1974) Aluminum in
the environment and human health. Environ Health Perspect, 8: 3-95.
S�yseth V, Kingerud J, Ekstrand J, & Boe J (1994) Relation between
exposure to fluoride and bronchial responsiveness in aluminium potroom
workers with work-related asthma-like symptoms. Thorax, 49: 984-989.
Sparling DW (1991) Acid precipitation and food quality: effects of
dietary Al, Ca, and P on bone and liver characteristics in American
black ducks and mallards. Arch Environ Contam Toxicol, 21: 281-288.
Stacy BD, King EJ, Harrison CV, Nagelschmidt G, & Nelson S (1959)
Tissue changes in rats' lungs caused by hydroxides, oxides and
phosphates of aluminium and iron. Pathol Bacteriol, 77: 417-426.
Stanley RA (1974) Toxicity of heavy metals and salts to eurasian
watermilfoil ( Myriophyllum spicatum L.). Arch Environ Contam
Toxicol, 2(4): 331-341.
Stanton MF (1974) Fiber carcinogenesis: is asbestos the only hazard?
(editorial). J Natl Cancer Inst, 52: 633.
Staurnes M, Blix P, & Reite OB (1993) Effects of acid water and
aluminum on parr-smolt transformation and seawater tolerance in
Atlantic salmon, Salmo salar. Can J Fish Aquat Sci, 50: 1816-1827.
Steinegger A, Rickenbacher U, & Schlatter C (1990) Aluminium. In:
Hutzinger O ed. The handbook of environmental chemistry. Berlin,
Heidelberg, New York, Springer Verlag, vol 3, part E, pp 155-184.
Steinhagen WH, Cavender FL, & Cockrell BY (1978) Six month inhalation
exposures of rats and guinea pigs to aluminum chlorhydrate. J Environ
Pathol Toxicol, 1: 267-277.
Stern AJ, Perl DP, Monoz-Garcia D, Good PF, Abraham C, & Selkoe DJ
(1986) Investigation of silicon and aluminum content in isolated
senile plaque cores by laser microprobe mass analysis (LAMMA).
J Neuropathol Exp Neurol, 45: 361.
Sternweis PC & Gilman AG (1982) Aluminum: A requirement for activation
of the regulatory component of adenylate cyclase by fluoride. Proc
Natl Acad Sci (USA), 79: 4888-4891.
Stoffyn M (1979) Biological control of dissolved aluminum in seawater:
experimental evidence. Science, 203: 651-653.
Stoffyn M & MacKenzie FT (1982) Fate of dissolved aluminum in the
oceans. Mar Chem, 11: 105-127.
Stokinger HE (1987) The metals. In: Clayton GD & Clayton FE ed.
Patty's industrial hygiene and toxicology, 3rd revised ed. - Volume
2A: Toxicology. New York, Chichester, Brisbane, Toronto, John Wiley
and Sons, pp 1493-1505.
Stone CJ, McLaurin DA, Steinhagen WH, Cavender FL, & Haseman JK (1979)
Tissue deposition patterns after chronic inhalation exposures of rats
and guinea pigs to aluminum chlorhydrate. Toxicol Appl Pharmacol,
49: 71-76.
Stoner JH, Gee AS, & Wade KR (1984) The effects of acidification on
the ecology of streams in the Upper Tywi catchment in west Wales.
Environ Pollut, A35: 125-157.
Strong MJ & Garruto RM (1991a) Neuron-specific thresholds of aluminum
toxicity in vitro: A comparative analysis of dissociated fetal
rabbit hippocampal and motor neuron-enriched cultures. Lab Invest,
65: 243-249.
Strong MJ & Garruto RM (1991b) Chronic aluminum-induced motor neuron
degeneration: Clinical, neuropathological and molecular biological
aspects. Can J Neurol Sci, 18: 428-431.
Strong MJ, Wolff AV, Wakayama I, & Garruto RM (1991) Aluminum-induced
chronic myelopathy in rabbits. Neurotoxicology, 12: 9-22.
Subramanian V, Jha PK, & Van Grieken R (1988) Heavy metals in the
Ganges estuary. Mar Pollut Bull, 19(6): 290-293.
Talbot RJ, Newton D, Priest ND, Austin JG, & Day JP (1995)
Inter-subject variability in the metabolism of aluminium following
intravenous injection as citrate. Hum Exp Toxicol, 14(7): 595-599.
Tarkka T, Yli-M�yry N, Mannermaa RM, Majamaa K, & Oikarinen J (1993)
Specific non-enzymatic glycation of the rat histone H1 nucleotide
binding site in vitro in the presence of AlF4-. A putative
mechanism for impaired chromatin function. Biochim Biophys Acta,
1180: 294-298.
Taylor GJ (1991) Current views of the aluminum stress response: the
physiological basis of tolerance. Curr Top Plant Biochem Physiol,
10: 57-93.
Taylor GJ (1995) Overcoming barriers to understanding the cellular
basis of aluminium resistance. Plant Soil, 171(1): 89-103.
Taylor GJ & Foy CD (1985) Mechanisms of aluminum tolerance in
Triticum aestivum L. (wheat): I. Differential pH induced by winter
cultivars in nutrient solutions. Am J Bot, 72(5): 695-701.
Teraoka H (1981) Distribution of 24 elements in the internal organs
of normal males and metallic workers in Japan. Arch Environ Health,
36: 155-165.
Teraoka H, Morii F, & Kobayashi J (1981) The concentrations of 24
elements in foodstuffs and the estimate of their daily intake. J Jpn
Soc Food Nutr, 34: 221-239.
Thompson JJ & Houk RS (1986) Inductively coupled plasma mass
spectrometric detection for multielement flow injection analysis and
elemental speciation by reversed-phase liquid chromatography. Anal
Chem, 58: 2541-2548.
Thompson GW & Medve RJ (1984) Effects of aluminum and manganese
on the growth of ectomycorrhizal fungi. Appl Environ Microbiol,
48(3): 556-560.
Thomson SM, Burnett DC, Bergmann JD, & Hixson CJ (1986) Comparative
inhalation hazards of aluminum and brass powders using
bronchopulmonary lavage as an indicator of lung damage. J Appl
Toxicol, 6: 197-209.
Thorne BM, Cook A, Donohoe T, Lyon S, Medeiros DM, & Moutzoukis C
(1987) Aluminum toxicity and behavior in the weanling Long-Evans rat.
Bull Psychon Soc, 25: 129-132.
Thornton DJ, Liss L, & Lott JA (1983) Effect of ethanol on the uptake
and distribution of aluminum in rats. Clin Chem, 29: 1281.
Tipping E & Backes CA (1988) Organic complexation of Al in acid
waters: model-testing by titration of a streamwater sample. Water Res,
22(5): 593-595.
Tipping E, Woof C, Backes CA, & Ohnstad M (1988a) Aluminium speciation
in acidic natural waters: testing of a model for Al-humic
complexation. Water Res, 22(3): 321-326.
Tipping E, Woof C, Walters PB, & Ohnstad M (1988b) Conditions required
for the precipitation of aluminium in acidic natural waters. Water
Res, 22(5): 585-592.
Tipping E, Backes CA, & Hurley MA (1988c) The complexation of protons,
aluminium and calcium by aquatic humic substances: a model
incorporating binding-site heterogeneity and macroionic effects. Water
Res, 22(5): 597-611.
Tipping E, Backes CA, & Hurley MA (1989a) Modeling the interactions of
Al species, protons and Ca2+ with humic substances in acid waters and
soils In: Lewis TE ed. Environmental chemistry and toxicology of
aluminum. Proceedings of the 194th Annual Meeting of the American
Chemical Society, New Orleans, LA, 30 August-4 September 1987.
Chelsea, Michigan, Lewis Publishers Inc., pp 59-82.
Tipping E, Ohnstad M, & Woof C (1989b) Adsorption of aluminium by
stream particulates. Environ Pollut, 57: 85-96.
Tipton IH & Cook MJ (1963) Trace elements in human tissue: Part II.
Adult subjects from the United States. Health Phys, 9: 103-145.
Townsend MC, Enterline PE, Sussman NB, Bonney TB, & Rippey LL (1985)
Pulmonary function in relation to total dust exposure at a bauxite
refinery and alumina-based chemical products plant. Am Rev Respir Dis,
132: 1174-1180.
Trapp GA (1983) Plasma aluminum is bound to transferrin. Life Sci,
33: 311.
Tyler G (1994) Plant uptake of aluminium from calcareous soils.
Experientia (Basel), 50: 701-703.
Ulfvarson U (1981) Survey of air contaminants from welding. Scand J
Work Environ Health, 7(suppl 2): 1-28.
UK MAFF (1993) Aluminium in food - 39th report of the Steering Group
on Chemical Aspects of Food Surveillance. London, UK Ministry of
Agriculture, Fisheries and Food, 52 pp.
US Bureau of Mines (1967) Potential sources of aluminium. Washington,
DC, US Government Printing Office, pp 3-4.
Valdivia R, Ammerman CB, Wilcox CJ, & Henry PR (1978) Effect of
dietary aluminum on animal performance and tissue mineral levels in
growing steers. J Anim Sci, 47: 1351-1356.
Valdivia R, Ammermann CB, Henry PR, Feaster JP, & Wilcox CJ (1982)
Effect of dietary aluminium and phosphorus on performance, phosphorus
utilization and tissue mineral composition in sheep. J Anim Sci,
55: 402-410.
Vallyathan V, Bergeron WN, Robichaux PA, & Craighead JE (1982)
Pulmonary fibrosis in an aluminum arc welder. Chest, 81: 372-374.
Van Bennekom AJ & Jager JE (1978) Dissolved aluminium in the Zaire
river plume. Neth J Sea Res, 12(3/4): 358-367.
Van Benschoten JE & Edzwald JK (1990) Measuring aluminum during
water treatment: methodology and application. J Am Water Works Assoc,
82: 71-78.
Van Broeckhoven C, Backhovens H, Cruts M, De Winter G, Bruyland M,
Cras P, Martin J-J (1992) Mapping of a gene predisposing to early
onset Alzheimer's disease to chromosome 14q24.3. Nature (Genet),
2: 335-339.
Van de Vyver F & Visser WJ (1990) Aluminium accumulation in bone.
In: Priest ND & Van de Vyver F Trace metals and fluoride in bones and
teeth. Boca Raton, Florida, CRC Press, pp 41-81.
Van der Voet GB (1992) Intestinal absorption of aluminium. In:
Aluminium in biology and medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 109-122 (Ciba Foundation Symposium
No. 169).
Van der Voet GB & De Wolff FA (1984) A method of studying the
intestinal absorption of aluminium in the rat. Arch Toxicol,
55: 168-172.
Van der Voet GB, De Haas EJM, & De Wolff FA (1985) Monitoring of
aluminum in whole blood, plasma, serum, and water by a single
procedure using flameless atomic absorption spectrophotometry. J Anal
Toxicol, 9: 97-100.
Van Duijn CM, Stijnen T, & Hofman A (1991) Risk factors for
Alzheimer's disease: overview of the EURODEM collaborative re-analysis
of case-control studies. Int J Epidemiol, 20(2): S/4-S/12.
Varo P & Koivistoinen P (1980) Mineral element composition of Finnish
foods: XII. General discussion and nutritional evaluation. Acta Agric
Scand, 22(suppl): 165-171.
Veien NK, Hattel T, Justesen O, & Noerholm A (1986) Aluminium allergy.
Contact Dermatitis, 15: 295-297.
Vogt KA, Dahlgren R, Ugolini F, Zabowski D, Moore EE, & Zasoski R
(1987) Aluminum, Fe, Ca, Mg, K, Mn, Cu, Zn, and P in above- and
below-ground biomass. II. Pools and circulation in a subalpine
Abies amabilis stand. Biogeochemistry, 4: 295-311.
Vorbrodt AW, Dobrogowska DH, & Lossinsky AS (1994) Ultracytochemical
studies of the effects of aluminum on the blood-brain barrier of mice.
J Microchem Cytochem, 42(2): 203-212.
Wade K & Banister AJ (1973) The chemistry of aluminium, gallium,
indium and thallium: Comprehensive inorganic chemistry. Oxford, New
York, Pergamon Press, chapter 12, pp 993-1036.
Wagatsuma T (1983) Characterization of absorption sites for aluminum
in the roots. Soil Sci Plant Nutr, 29(4): 499-515.
Walker WJ, Cronan CS, & Patterson HH (1988) A kinetic study of
aluminum adsorption by aluminosilicate clay minerals. Geochim
Cosmochim Acta, 52: 55-62.
Walker WJ, Cronan CS, & Bloom PR (1990) Aluminum solubility in organic
soil horizons from northern and southern forested watersheds. Soil Sci
Soc Am J, 54: 369-374.
Wallen IE, Greer WC, & Lasater R (1957) Toxicity to Gambusia
affinis of certain pure chemicals in turbid waters. Sew Ind Wastes,
29: 695-711.
Walton J, Hams G, & Wilcox D (1994) Bioavailability of aluminium from
drinking water: Co-exposure with foods and beverages. Melbourne,
Australia, Urban Water Research Association of Australia, Melbourne
Water Corporation, 38 pp (Research Report No. 83).
Walton J, Tuniz C, Fink D, Jacobsen G, & Wilcox D (1995) Uptake of
trace amounts of aluminium into the brain from drinking water.
Neurotoxicology, 16: 187-190.
Ward MK (1991) Aluminium toxicity and people with impaired renal
function. In: Lord Walton of Detchant ed. Alzheimer's disease and the
environment. London, Royal Society of Medicine Services Ltd,
pp 106-110 (Round Table Series No. 26).
Ward MK, Feest TG, Ellis HA, Parkinson IS, Kerr DNS, Herrington J, &
Goode GL (1978) Osteomalacic dialysis osteodystrophy: Evidence for a
water-borne aetiological agent, probably aluminium. Lancet,
1: 841-845.
Watt WD, Scott CD, & White WJ (1983) Evidence of acidification of some
Nova Scotian rivers and its impact on Atlantic salmon, Salmo salar.
Can J Fish Aquat Sci, 40: 462-473.
Weatherley NS, Ormerod SJ, Thomas SP, & Edwards RW (1988) The response
of macroinvertebrates to experimental episodes of low pH with
different forms of aluminium, during a natural spate. Hydrobiologia,
169: 225-232.
Weatherley NS, Rogers AP, Goenaga X, & Ormerod SJ (1990) The survival
of early life stages of brown trout ( Salmo trutta L.) in relation
to aluminium speciation in upland Welsh streams. Aquat Toxicol,
17: 213-230.
Wedrychowski A, Schmidt WN, & Hnilica LS (1986) The in-vivo
cross-linking of proteins and DNA by heavy metals. J Biol Chem,
261: 3370.
Wefers K & Bell GM (1972) Oxides and hydroxides of aluminum. East St.
Louis, Illinois, ALCOA (Aluminium Company of America), Research
Laboratories (Technical Paper No. 19).
Wettstein A, Aeppli J, Gautschi K, & Peter M (1991) Failure to find a
relationship beetwen mnestic skills of octogenarians and aluminium in
drinking water. Int Arch Occup Environ Health, 63: 97-103.
Wheeler DM, Power IL, & Edmeades DC (1993) Effect of various metal
ions on growth of two wheat lines known to differ in aluminium
tolerance. Plant Soil, 155/156: 489-492.
White DM, Longstreth WT, Rosenstock L, Claypoole KHJ, Brodkin CA, &
Townes BD (1992) Neurologic syndrome in 25 workers from an aluminum
smelting plant. Arch Intern Med, 152: 1443-1448.
Whitehead PG, Bird S, Hornung M, Cosby J, Neal C, & Paricos P (1988)
Stream acidification trends in the Welsh uplands: a modelling study of
the Llyn Brianne catchments. J Hydrol, 101: 191-212.
Whitehead PG, Musgrove TJ, & Cosby BJ (1990) Hydrochemical modelling
of acidification in Wales. In: Edwards RW, Gee AS, & Stoner JH ed.
Acid waters in Wales. Dordrecht, Boston, London, Kluwer Academic
Publishers, pp 255-277.
WHO (1993) Guidelines for drinking-water quality, 2nd ed. - Volume 1:
Recommendations. Geneva, World Health Organization, pp 39-40.
Wicklung Glynn A, Norrgren L, & Malmborg O (1992) The influence of
calcium and humic substances on aluminium accumulation and toxicity in
the minnow, Phoxinus phoxinus, at low pH. Comp Biochem Physiol,
102C(3): 427-432.
Wild A ed. (1988) Russell's soil conditions and plant growth, 11th ed.
Harlow, Longman Scientific & Technical.
Wilhelm M & Idel H (1995) [Aluminium in drinking water: a risk factor
for Alzheimer's disease?] Forum St�dte-Hyg, 46: 255-258 (in German
with English summary).
Wilhelm M, Passlick J, Busch T, Szydlik M, & Ohnesorge FK (1989) Scalp
hair as an indicator of aluminium exposure: comparison to bone and
plasma. Hum Toxicol, 8: 5-9.
Wilhelm M, J�ger E, & Ohnesorge FK (1990) Aluminium toxicokinetics.
Pharmacol Toxicol, 66: 4-9.
Wilhelm M, Zhang X-J, Hafner D, & Ohnesorge FK (1992) Single-dose
toxicokinetics of aluminum in the rat. Arch Toxicol, 66: 700-705.
Wilhelm M, Lombeck I, Kouros B, Wuthe J, & Ohnesorge FK (1995)
[Duplicate study on the dietary intake of some metals/metalloids by
German children: Part II. Aluminium, cadmium and lead.] Zent.bl Hyg,
197: 357-368 (in German with English summary).
Wills MR & Savory J (1983) Aluminium poisoning: Dialysis
encephalopathy, osteomalacia, and anaemia. Lancet, 2: 29-33.
Wills MR & Savory J (1989) Aluminum and chronic renal failure:
Sources, absorption, transport, and toxicity. CRC Crit Rev Clin Lab
Sci, 27: 59-107.
Wilson RW, Bergman HL, & Wood CM (1994a) Metabolic costs and
physiological consequences of acclimation to aluminum in juvenile
rainbow trout ( Oncorhynchus mykiss): 1. Acclimation specificity,
resting physiology, feeding, and growth. Can J Fish Aquat Sci,
51: 527-535.
Wilson RW, Bergman HL, & Wood CM (1994b) Metabolic costs and
physiological consequences of acclimation to aluminum in juvenile
rainbow trout ( Oncorhynchus mykiss): 1. Gill morphology, swimming
performance, and aerobic scope. Can J Fish Aquat Sci, 51: 536-544.
Windom HL (1981) Comparison of atmospheric and riverine transport of
trace elements to the continental shelf environment. In: Eisma MB ed.
River inputs to the ocean systems: Proceedings of the UNEP/IOC/SCDR
Workshops, Rome, 1981. New York, United Nations, pp 360-369.
Wischik CM, Harrington CR, Mukaetova-Ladinska EB, Novak M, Edwards PC,
& McArthur FK (1992) Molecular characterization and measurement of
Alzheimer`s disease pathology: implications for genetic and
environmental aetiology. In: Aluminium in biology and medicine. New
York, Chichester, Brisbane, Toronto, John Wiley and Sons, pp 268-302
(Ciba Foundation Symposium No. 169).
Wisniewski HM & Kozlowski PB (1982) Evidence for blood-brain barrier
changes in senile dementia of the Alzheimer type (SDAT). Ann NY Acad
Sci, 396: 119-129.
Wisniewski HM & Rabe A (1992) Differences between aluminum
encephalopathy and Alzheimer's disease. In: Gitelman HJ ed.
Proceedings of the Second International Conference on Aluminum and
Health. Washington, DC, Aluminium Association, pp 193-194.
Wisniewski HM & Wen GY (1992) Aluminium and Alzheimer`s disease. In:
Aluminium in biology and medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 142-164 (Ciba Foundation Symposium
No. 169).
Wisniewski HM, Sturman JA, & Shek JW (1982) Chronic model of
neurofibrillary changes induced in mature rabbits by metallic
aluminum. Neurobiol Aging, 3: 11-22.
Wisser LA, Heinrichs BS, & Leach RM (1990) Effect of aluminum on
performance and mineral metabolism in young chicks and laying hens. J
Nutr, 120: 493-498.
Witters HE (1986) Acute acid exposure of rainbow trout, Salmo
gairdneri Richardson: effects of aluminium and calcium on ion
balance and haematology. Aquat Toxicol, 8: 197-210.
Witters H, Vangenechten JHD, Van Puymbroeck S, & Vanderborght OLJ
(1984) Interference of aluminium and pH on the Na-influx in an aquatic
insect Corixa punctata (Illig.). Bull Environ Contam Toxicol,
32: 575-579.
World Bureau of Metal Statistics (1994) World bauxite production.
World Met Stat, 47: 6.
Wood CM & McDonald DG (1987) The physiology of acid/aluminium stress
in trout. Ann Soc Zool Belg, 117(suppl 1): 399-410.
Wood DJ, Cooper C, Stevens J, & Edwardson J (1988) Bone mass and
dementia in hip fracture patients from areas with different aluminium
concentrations in water supplies. Age Ageing, 17: 415-419.
Wren CD, MacCrimmon HR, & Loescher BR (1983) Examination of
bioaccumulation and biomagnification of metals in a Precambrian shield
lake. Water Air Soil Pollut, 19: 277-291.
Wyttenbach A, Bajo S, Tobler L, & Keller T (1985) Major and trace
element concentrations in needles of Picea abies: levels,
distribution functions, correlations and environmental influences.
Plant Soil, 85: 313-325.
Yagi M, Mizukawa K, Kajino M, Tatumi S, Akiyama M, & Ando M (1992)
Behaviour of aluminium in water purification process at purification
plants in the River Yodo system. Water Supply, 10(4): 65-75.
Yeats PA & Bewers JM (1982) Discharge of metals from the St Lawrence
River. Can J Earth Sci, 19: 982-992.
Yen-Koo HC (1992) The effect of aluminum on conditioned avoidance
response (CAR) in mice. Toxicol Ind Health, 8: 1-7.
Yokel RA (1983) Repeated systemic aluminum exposure effects on
classical conditioning of the rabbit. Neurobehav Toxicol Teratol,
5: 41-46.
Yokel RA (1984) Toxicity of aluminum exposure during lactation to the
maternal and suckling rabbit. Toxicol Appl Pharmacol, 75: 35-43.
Yokel RA (1985) Toxicity of gestational aluminum exposure to the
maternal rabbit and offspring. Toxicol Appl Pharmacol, 79: 121-133.
Yokel RA (1987) Toxicity of aluminum exposure to the neonatal and
immature rabbit. Fundam Appl Toxicol, 9: 795-806.
Yokel RA & McNamara PJ (1985) Aluminum bioavailability and deposition
in adult and immature rabbits. Toxicol Appl Pharmacol, 77: 344-352.
Yokel RA & McNamara PJ (1989) Elevated aluminium persists in serum and
tissues of rabbits after a 6-hour infusion. Toxicol Appl Pharmacol,
99: 133-138.
Yukawa M, Suzuki-Yasumoto M, Amano K, & Terai M (1980) Distribution of
trace elements in the human body determined by neutron activation
analysis. Arch Environ Health, 35: 36-44.
Zelenov KK (1965) Aluminum and titanium in Kava Ijen volcano crater
lake (Indonesia). Int Geol Rev, 11(1): 84-93.
Zietz JR (1985) Aluminum compounds, organic. In: Gerhartz W, Yamamoto
YS, Campbell FT, Pfefferkorn R, & Rounsaville JF ed. Ullmann's
encyclopedia of industrial chemistry - Volume A1: Abrasives to
aluminum oxide, 5th revised ed. Weinheim, Verlag Chemie, pp 543-556.
Zoller WH, Gladney ES, & Duce RA (1974) Atmospheric concentrations and
sources of trace metals at the South Pole. Science, 183: 198-200.
Zwarun AA & Thomas GW (1973) Effect of soluble and exchangeable
aluminum on Pseudomonas stutzeri. Soil Sci Soc Am Proc,
37: 386-387.
Zwarun AA, Bloomfield BJ, & Thomas GW (1971) Effect of soluble and
exchangeable aluminum on a soil Bacillus. Soil Sci Soc Am Proc,
35: 460-463.
RESUME ET CONCLUSIONS
1. Identit�, propri�t�s chimiques et physiques
L'aluminium est un m�tal ductile et mall�able de couleur blanc
argent�. Il appartient au groupe IIIA de la classification p�riodique
et se trouve g�n�ralement au degr� d'oxydation III dans ses d�riv�s
usuels. Il constitue environ 8% de l'�corce terrestre et c'est l'un
des m�taux usuels les plus r�actifs. En pr�sence d'eau, d'oxyg�ne et
d'autres oxydants, il se recouvre d'une couche superficielle d'oxyde
qui lui conf�re une grande r�sistance � la corrosion. L'oxyde
d'aluminium est soluble dans les acides min�raux ainsi que dans les
bases fortes, mais il est insoluble dans l'eau. Par contre, le
chlorure, le nitrate et le sulfate sont solubles dans l'eau. Les
halog�nures, l'hydrure et les premiers termes de la s�rie des
alkylaluminiums r�agissent violemment avec l'eau.
L'aluminium a une forte conductivit� thermique et �lectrique,
sa densit� est faible et sa r�sistance � la corrosion �lev�e. On
l'utilise souvent en alliage avec d'autres m�taux. Ces alliages sont
l�gers, r�sistants et faciles � usiner.
2. M�thodes d'analyse
On a mis au point diverses m�thodes d'analyse pour la recherche
et le dosage de l'aluminium dans les milieux biologiques et les
�chantillons pr�lev�s dans l'environnement. Les plus fr�quemment
utilis�es sont la spectrom�trie d'absorption atomique avec four �
�lectrode de graphite (GF-AAS) et la spectrom�trie d'�mission atomique
avec plasma � couplage inductif (ICP-AES). La principale source
d'erreur est la contamination des �chantillons, lors du pr�l�vement
et de la pr�paration de la prise d'essai, par l'aluminium pr�sent
dans l'air, les r�cipients et les r�actifs. Selon les traitements
pr�liminaires subis par l'�chantillon et les techniques de s�paration
et de concentration utilis�es, les limites de d�tection vont de 1,9 �
4 �g/litre dans les liquides biologiques et de 0, 005 � 0,5 �g/g de
poids sec dans les tissus quand on proc�de par GF-AAS, et de 5 �g/m3
d'air ou de 3 �g/litre d'eau quand on proc�de par ICP-AES.
3. Sources d'exposition humaine et environnementale
De l'aluminium est lib�r� dans l'environnement tant � la faveur
de processus naturels que d'activit�s humaines. Il est pr�sent sous
une forme tr�s concentr�e dans les poussi�res que produisent les
exploitations mini�res et agricoles ainsi que dans les particules
produites par la combustion du charbon. Les silicates d'aluminium
(argiles), qui sont des constituants majeurs des sols, contribuent
pour une large part � la teneur des poussi�res en silicates. L'appport
d'aluminium � l'environnement trouve beaucoup plus son origine dans
des processus naturels que dans l'activit� humaine. La mobilisation de
l'aluminium du fait d'activit�s humaine est, pour une large part,
indirecte et provient de l'�mission de substances acidifiantes. D'une
fa�on g�n�rale, une diminution du pH entra�ne une augmentation de
la mobilit� et de la biodisponibilit� des formes monom�res de
l'aluminium. La mati�re premi�re la plus importante pour la production
de l'aluminium est la bauxite, qui contient jusqu'� 55% d'alumine
(oxyde d'aluminium). La production mondiale de bauxite a �t� de 106
millions de tonnes en 1992. Le m�tal a des usages tr�s vari�s, dans
les industries du b�timent, de l'automobile et de l'a�ronautique, et
peut �galement �tre utilis� sous la forme d'alliages. Les d�riv�s de
l'aluminium ont �galement des usages tr�s divers, notamment dans
l'industrie du verre, de la c�ramique et du caoutchouc ainsi que pour
la confection de produits destin�s � la protection du bois, de
produits pharmaceutiques et de tissus imperm�ables. Les min�raux
naturels contenant de l'aluminium, en particulier la bentonite et la
z�olite, sont utilis�s pour la purification de l'eau, ainsi que dans
les sucreries, les brasseries et l'industrie papeti�re.
4. Transport, distribution et transformation dans l'environnement
L'aluminium est omnipr�sent dans l'environnement sous forme de
silicates, d'oxydes et d'hydroxydes, combin� � d'autres �l�ments comme
le sodium et le fluor ou encore sous forme de complexes organiques. On
ne le rencontre pas � l'�tat natif du fait de sa r�activit�. Dans la
nature, il n'existe qu'au degr� d'oxydation (+3) ; son transport et sa
distribution ne d�pendent donc que de sa chimie de coordination et des
caract�ristiques physico-chimiques de l'environnement local. Aux
valeurs du pH sup�rieures � 5,5, les d�riv�s de l'aluminium d'origine
naturelle existent principalement sous forme non dissoute, par exemple
sous forme de gibbsite (Al(OH)3) ou d'aluminosilicates, sauf en
pr�sence de grandes quantit�s de mati�res organiques en solution qui
se lient � l'aluminium et peuvent conduire � une augmentation de la
teneur en aluminium dissous, dans les cours d'eau et les lacs, par
exemple. Plusieurs facteurs influent sur la mobilit� de l'aluminium
et, par voie de cons�quence, sur son transport dans l'environnement.
Il s'agit en particulier de la nature des esp�ces chimiques en cause,
des trajectoires d'�coulement, des interactions entre le sol et l'eau
et de la composition des mat�riaux g�ologiques sous-jacents. La
solubilit� de l'aluminium en �quilibre avec Al (OH)3 en phase solide
d�pend fortement du pH et de la pr�sence d'agents complexants comme
les fluorures, les silicates, les phosphates et les mati�res
organiques. On peut consid�rer que la chimie de l'aluminium
inorganique dans les sols acides et les cours d'eau fait intervenir
les processus suivants: solubilit� des esp�ces min�rales, �change
d'ions et miscibilit� � l'eau. Lorsqu'il y a acidification du sol,
l'aluminium peut passer en solution et �tre ainsi transport� dans les
cours d'eau. La mobilisation de l'aluminium par pr�cipitation en
milieu acide permet aux plantes de fixer une plus grande quantit� de
cet �l�ment.
5. Concentrations dans l'environnement et exposition humaine
L'aluminium est pr�sent en proportion importante dans un certain
nombre de constituants de l'atmosph�re, en particulier des poussi�res
arrach�es au sol (qu'elles soient d'origine naturelle ou qu'elles
r�sultent de l'activit� humaine) et des particules issues de la
combustion du charbon. En milieu urbain, la concentration de
l'aluminium dans la poussi�re des rues varie de 3,7 � 11,6 �g/kg. La
teneur de l'air en aluminium va de 0,5 ng/m3 au-dessus de
l'Antarctique � plus de 1000 ng/m3 dans les zones industrialis�es.
Dans les eaux douces superficielles ou souterraines, la
concentration de l'aluminium peut varier dans des proportions
importantes, en fonction de facteurs physico-chimiques et g�ologiques.
L'aluminium peut se trouver en suspension ou en solution. Il peut �tre
li� � des coordinats organiques ou inorganiques ou exister � l'�tat
d'ion libre. En milieu aqueux naturel, les esp�ces chimiques dans
lesquelles l'aluminium est engag� existent � l'�tat de monom�res
ou de polym�res. La nature de ces esp�ces d�pend du pH et de la
concentration en carbone organique dissous, fluorures, sulfates,
phosphates et particules en suspension. Au voisinage de la neutralit�,
la concentration de l'aluminium dissous est g�n�ralement assez faible,
de l'ordre de 1,0 � 50 �g/litre. Lorsque l'acidit� de l'eau augmente,
elle peut monter jusqu'� 500-1000 �g/litre. Dans les eaux rendues
extr�mement acides par les liquides de drainage de mines, on a mesur�
des concentrations d'aluminium en solution qui atteignaient
90 mg/litre.
L'exposition humaine non professionnelle � l'aluminium pr�sent
dans l'environnement se produit principalement lors de l'ingestion
d'eau ou de nourriture, la nourriture �tant essentiellement en cause.
L'apport journalier d'aluminium par les aliments et la boisson se
situe, chez l'adulte, entre 2,5 et 13 mg, ce qui repr�sente 90 � 95%
de l'apport total. Compte tenu de la valeur-guide internationale
actuellement en vigueur, l'apport par l'eau de boisson pourrait
se situer aux environs de 0,4 mg par jour, mais il est plus
probablement de l'ordre de 0,2 mg. La contribution de la voie
d'exposition respiratoire peut atteindre 0,04 mg/jour. Dans certaines
circonstances, par exemple en cas d'exposition professionnelle ou lors
de l'utlisation d'antiacides, l'exposition pourra �tre beaucoup plus
intense. Par exemple, deux comprim�s d'antiacide moyennement dos�s
peuvent apporter plus de 500 mg d'aluminium. Les �tudes effectu�es
avec de l'aluminium-26 indiquent que le citrate constitue la forme
la plus disponible biologiquement et que sous cette forme,
l'aluminium pourrait �tre absorb� � hauteur de 1%. L'absorption par
l'interm�diaire de l'eau de boisson ne repr�senterait en revanche que
3% de l'apport quotidien total, cette source �tant relativement
mineure par rapport � l'apport alimentaire. Il n'est pas tr�s facile
de d�terminer quelle est la quantit� effectivement fix�e � la suite
d'une telle exposition en raison des probl�mes d'analyse et
d'�chantillonnage que cela pose.
6. Cin�tique et m�tabolisme
6.1 Chez l'homme
L'aluminium et ses d�riv�s sont mal absorb�s par l'organisme
humain, encore que la vitesse et le taux d'absorption n'aient pas �t�
suffisamment �tudi�s. Un moyen facile d'�valuer la r�sorption de
l'aluminium consiste � en mesurer la concentration dans l'urine et le
sang; on a d'ailleurs constat� une augmentation de la concentration
urinaire chez des soudeurs � l'aluminium et des ouvriers travaillant �
la production de poudre d'aluminium.
Le m�canisme de r�sorption de l'aluminium au niveau
gastrointestinal n'est pas encore parfaitement �lucid�. Les variations
observ�es r�sultent des propri�t�s chimiques de cet �l�ment et de la
formation de diverses esp�ces chimiques, sous l'influence du pH, de la
force ionique, de la comp�tition avec d'autres �l�ments (silicium) ou
agents complexants pr�sents dans les voies digestives (des citrates,
par ex.).
On a �tudi� le comportement biologique de l'aluminium et son
absorption dans les voies digestives humaines au moyen de l'isotope
radioactif Al-26. Des diff�rences interindividuelles importantes ont
�t� mises en �vidence. La litt�rature donne ainsi des taux de
r�sorption de 5 � 10-3 pour le citrate, de 1,04 � 10-4 pour
l'hydroxyde et de 1,3 � 10-3 pour l'hydroxyde ing�r� avec du citrate.
Une �tude de la r�sorption fractionn�e de l'aluminium pr�sent dans
l'eau de boisson a donn� une valeur de 2,35 � 10-3. On en a conclu
que les membres de la population g�n�rale qui boivent quotidiennement
1,5 litre d'eau contenant 100 �g/litre d'aluminium, absorberaient de
la sorte 3% de leur apport total d'aluminium, selon les concentrations
pr�sentes dans leur nourriture et leur utilisation �ventuelle
d'antiacides.
La proportion d'Al3+ normalement li� aux prot�ines chez
l'Homme peut atteindre 70-90% chez les malades h�modialys�s, avec une
augmentation mod�r�e de l'aluminium plasmatique. C'est dans les
poumons que l'on peut trouver les taux les plus �lev�s d'aluminium,
parfois pr�sent sous formes de particules inhal�es insolubles.
C'est l'urine qui constitue la voie d'excr�tion la plus
importante de l'aluminium. Apr�s administration par voie orale d'une
dose unique d'aluminium, 83% ont �t� excr�t�s dans l'urine en l'espace
de 13 jours, contre 1,8% dans les mati�res f�cales. La demi-vie de
l'aluminium urinaire chez des soudeurs expos�s pendant plus de 10 ans
s'est r�v�l�e �tre d'au moins 6 mois. Chez des ouvriers � la retraite
qui avaient �t� expos�s � de la poudre d'aluminium, le calcul de la
demi-vie a donn� une valeur comprise entre 0,7 et 8 ans.
6.2 Chez l'animal
La r�sorption dans les voies digestives est en g�n�ral inf�rieure
� 1%. Elle d�pend principalement de l'esp�ce chimique en cause et de
sa solubilit� ainsi que du pH. Les agents complexants organiques comme
les citrates, augmentent la r�sorption. Il peut �galement y avoir
interaction avec le calcium et le syst�me de transport du fer.
L'absorption par la voie respiratoire et la voie percutan�e n'a pas
�t� �tudi�e en d�tail. L'aluminium se r�partit dans la plupart des
organes en s'accumulant principalement dans les os, du moins
lorsque les doses sont fortes. Dans une proportion limit�e encore
qu'ind�termin�e, il franchit la barri�re h�mato-enc�phalique et peut
parvenir jusqu'au foetus. Il est efficacement �limin� par la voie
urinaire. Sa demi-vie plasmatique est d'environ 1 h chez les rongeurs.
7. Effets sur les mammif�res de laboratoire et les syst�mes d'�preuve
in vitro
L'aluminium et ses compos�s n'ont qu'une faible toxicit� aigu�,
les valeurs publi�es de la DL50 par voie orale �tant de l'ordre de
quelques centaines de mg � 1000 mg d'aluminium par kg de poids
corporel et par jour. On n'a pas connaissance de la valeur de la Cl50
par inhalation.
Lors des �tudes toxicologiques � court terme qui ont �t�
effectu�es sur des rats, des souris ou des chiens avec un eventail
suffisant de points d'aboutissement et divers compos�s de l'aluminium
(phosphate sodique, hydroxyde et nitrate) incorpor�s � la nourriture
ou � l'eau de boisson,on n'a constat� que des effets minimes
(r�duction du gain de poids g�n�ralement associ�e � une moindre
consommation de nourriture ou effets histopathologiques mod�r�s aux
doses les plus �lev�es, c'est-�-dire 70 � 300 mg d'aluminium par kg de
poids corporel et par jour). Parmi les effets g�n�raux observ�s apr�s
administration parent�rale, on peut citer une insuffisance r�nale.
On n'a pas eu connaissance de l'existence d'�tudes d'inhalation
satisfaisantes. Apr�s administration intratrach�enne d'oxyde
d'aluminium, on a observ� une fibrose induite par les particules
d'oxyde qui �tait analogue � celle que les �tudes sur la poussi�re de
silice et de charbon ont mise en �vidence.
Apr�s avoir gav� des rats avec des rations quotidiennes contenant
respectivement 13, 26 ou 52 mg d'aluminium (sous forme de nitrate)
par kg de poids corporel, on n'a constat� aucune foetotoxicit�
manifeste ni effet d�l�t�re sur l'ensemble des param�tres g�n�siques.
Toutefois, on a not� un retard de croissance li� � la dose dans la
descendance de ces animaux, retard qui s'observait � la dose de
13 mg/kg chez les femelles et � la dose de 26 mg/kg chez les m�les.
La dose la plus faible produisant un effet d�l�t�re observable
(LOAEL) sur le d�veloppement, � savoir, une mauvaise ossification,
une augmentation de la fr�quence des malformations vert�brales
et stern�brales, ou encore une r�duction du poids des foetus,
s'�tablissait � 13 mg/kg (nitrate d'aluminium). Ces effets n'ont pas
�t� observ�s apr�s administration d'hydroxyde d'aluminium, � des doses
pourtant beaucoup plus fortes. A la dose de 13 mg/kg de nitrate
d'aluminium, on a observ� une r�duction de la croissance postnatale
mais on n'a pas cherch� � relever la pr�sence �ventuelle d'effets
toxiques chez les m�res. Les �tudes portant sur le d�veloppement
c�r�bral ont mis en �vidence une diminution de la force d'agrippement
dans la descendance de rattes qui avaient re�u 100 mg d'aluminium
par kg de poids corporel sous forme de lactate incorpor� � leur
nourriture, sans pr�senter elles-m�mes d'effets toxiques.
Rien n'indique que l'aluminium soit canc�rog�ne. Il peut former
des complexes avec l'ADN et provoquer la r�ticulation des prot�ines et
de l'ADN chromosomiques, mais il ne se montre pas mutag�ne pour les
bact�ries et n'induit pas de mutations ou de transformations dans des
cultures de cellules mammaliennes in vitro. Toutefois, des
aberrations chromosomiques ont �t� observ�es dans les cellules de la
moelle osseuse, chez des souris et des rats expos�s.
Une somme tr�s importante de faits exp�rimentaux incite � penser
que l'aluminium est neurotoxique chez l'animal d'exp�rience, encore
qu'il y ait des diff�rences consid�rables entre les esp�ces. Chez
celles qui sont sensibles, les effets toxiques cons�cutifs �
l'administration parent�rale d'aluminium se caract�risent par des
alt�rations neurologiques progressives qui conduisent � la mort dans
un �tat de mal �pileptique (DL50 = 6 �g d'aluminium/g de tissu
c�r�bral sec). Sur le plan morphologique, l'enc�phalopathie
progressive est associ�e, au niveau de la moelle �pini�re, du tronc
c�r�bral et de certaines parties de l'hippocampe, � une atteinte des
neurofibrilles dans les neurones de grande taille et de taille
moyenne. Ces l�sions de d�g�n�rescence neurofibrillaire sont
morphologiquement et biochimiquement distinctes de celles que l'on
observe dans la maladie d'Alzheimer. Des troubles comportementaux ont
�t� observ�s, en l'absence d'enc�phalopathie ou de l�sions du tissu
c�r�bral, chez des animaux ayant re�u des sels solubles d'aluminium
(chlorure, lactate, par ex.) dans leur alimentation ou leur eau de
boisson � des doses quotidiennes �gales ou sup�rieures � 50 mg
d'aluminium par kg de poids corporel.
L'ost�omalacie, sous la forme o� elle se manifeste chez l'homme,
s'observe r�guli�rement chez les gros animaux (par exemple, le chien
ou le porc) expos�s � l'aluminium; une pathologie du m�me genre
s'observe chez les rongeurs. A ce qu'il para�t, ces effets se
produisent chez toutes les esp�ces, y compris l'Homme, � des
concentrations d'aluminium de 100 � 200 �g/g de cendres d'os.
8. Effets sur l'Homme
On n'a pas d�crit d'effets pathog�nes aigus cons�cutifs � une
exposition de la population g�n�rale � l'aluminium.
En Angleterre, une population d'environ 20 000 personnes a �t�
expos�e pendant au moins 5 jours � des concentrations excessives de
sulfate d'aluminium qui avait �t� d�pos� accidentellement dans une
installation fournissant de l'eau potable. On a fait �tat � cette
occasion de cas de naus�e, de vomissements, de diarrh�e, d'ulc�rations
buccales et cutan�es, d'�ruptions et d'arthralgies. A l'examen, ces
sympt�mes se sont r�v�l�s pour la plupart b�nins et de br�ve dur�e.
Aucun effet pathologique durable n'a pu �tre attribu� � des cas pr�cis
d'exposition � l'aluminium pr�sent dans l'eau de boisson.
On a �mis l'hypoth�se que la pr�sence d'aluminium dans l'eau de
boisson serait responsable de la survenue ou de l'�volution plus
rapide de la maladie d'Alzheimer ou encore de la d�t�rioration des
fonctions intellectuelles chez les personnes �g�es. On a �galement
avanc� que les fum�es d�gag�es lors de la compression de l'aluminium
pulv�rulent pouvaient �galement avoir une responsabilit� dans la
d�t�rioration des fonctions intellectuelles et les pneumopathies
observ�es chez certaines professions.
Une vingtaine d'�tudes �pid�miologiques ont �t� effectu�es pour
v�rifier si la pr�sence d'aluminium dans l'eau de boisson �tait
effectivement un facteur de risque de la maladie d'Alzheimer et deux
d'entre elles ont consist� � �valuer l'association entre ce facteur et
la d�t�rioration des fonctions intellectuelles. Ces �tudes allaient
des enqu�tes �cologiques aux �tudes cas-t�moins. Huit �tudes
effectu�es sur des populations de Norv�ge, du Canada, de France, de
Suisse et d'Angleterre ont �t� jug�es d'une qualit� suffisante pour
satisfaire aux crit�res retenus pour l'�valuation de la relation
exposition-effet avec correction pour tenir compte d'au moins quelques
facteurs de confusion. Sur les six �tudes consacr�es � l'existence
�ventuelle d'une relation entre l'aluminium de l'eau de boisson et la
d�mence s�nile ou la maladie d'Alzheimer, trois seulement ont conclu
par l'affirmative. Il est toutefois � noter que chacune de ces �tudes
pr�sentait des d�fauts de conception (par exemple, dans l'�valuation
de l'exposition environnementale, pas de prise en compte de
l'exposition � l'aluminium de toutes origines, ni de correction pour
tenir compte des importants facteurs de confusion que sont le niveau
d'instruction, la situation socio-�conomique et les ant�c�dents
familiaux, le recours � des crit�res de jugement diff�rents pour la
maladie d'Alzheimer et le biais de s�lection). En g�n�ral le calcul a
donn� une valeur de 2 pour le risque relatif, avec un large intervalle
de confiance, lorsque la concentration totale de l'aluminium dans
l'eau de boisson �tait �gale ou sup�rieure � 100 �g/litre. Compte
tenu des connaissances actuelles sur la maladie d'Alzheimer et de
l'ensemble des r�sultats fournis par ces �tudes �pid�miologiques, on a
conclu que les donn�es �pid�miologiques ne militent pas en faveur de
l'existence d'une relation causale entre la maladie d'Alzheimer et la
pr�sence d'aluminium dans l'eau de boisson.
Outre les �tudes �pid�miologiques pr�cit�es qui ont port� sur la
relation entre la maladie d'Alzheimer et la pr�sence d'aluminium dans
l'eau de boisson, deux autres �tudes ont �t� consacr�es � ce m�me type
de relation chez les populations �g�es. L� encore, on a obtenu des
r�sultats contradictoires. La premi�re �tude, qui a port� sur 800
octog�naires du sexe masculin qui consommaient une eau contenant
jusqu'� 98 �g d'aluminium par litre, n'a pas mis en �vidence de
relation de cause � effet. La seconde �tude, pour laquelle le crit�re
de jugement retenu �tait "tout signe de d�t�rioration des fonctions
mentales", a �valu� � 1,72 le risque relatif pour des teneurs en
aluminium sup�rieures � 85 �g/litre chez 250 sujets du sexe masculin.
Ces donn�es ne permettent pas de conclure que l'aluminium est �
l'origine d'une d�t�rioration des fonctions intellectuelles chez les
personnes �g�es.
Comme on vient de le voir, les conclusions des travaux relatifs
aux effets de l'aluminium sur les fonctions intellectuelles sont
contradictoires. La plupart des �tudes ont �t� effectu�es sur des
populations de faible effectif et la m�thodologie utilis�e est
discutable, eu �gard � l'ampleur de l'effet constat�, � l'appr�ciation
de l'exposition efective et aux facteurs de confusion. Lors d'une
�tude au cours de laquelle on a compar� la d�t�rioration des fonctions
intellectuelles chez des mineurs expos�s � une poudre contenant 85%
d'aluminium finement pulv�ris� et 15% d'oxyde d'aluminium (� titre de
prophylaxie contre la silice) � un groupe t�moin constitu� de mineurs
non expos�s, les r�sultats aux tests et le nombre de sujets pr�sentant
un d�ficit dans au moins un test ont mis en �vidence un d�savantage
chez les mineurs expos�s. On a constat� l'existence d'une tendance �
l'accroissement du risque qui �tait li�e � la dose.
Dans toutes les �tudes relatives � l'exposition professionnelle
qui ont �t� publi�es, l'ampleur des effets constat�s, la pr�sence de
facteurs de confusion, les probl�mes pos�s par l'appr�ciation de
l'exposition effective et le fait qu'on ne peut exclure l'exposition �
plusieurs substances, ne permettent gu�re de conclure que l'aluminium
provoque une d�t�rioration des fonctions intellectuelles chez les
personnes expos�es de par leur profession.
Lors d'�tudes limit�es sur des travailleurs expos�s � des vapeurs
d'aluminium, des syndromes neurologiques et en particulier une
d�t�rioration des fonction intellectuelles, des troubles moteurs et
des neuropathies p�riph�riques ont �t� constat�s. En comparant un
petit groupe de soudeurs d'aluminium � un groupe de soudeurs de fer,
on a constat� chez les premiers un l�ger d�ficit de l'activit� motrice
lors de t�ches r�p�titives. Dans une autre �tude, une enqu�te par
questionnaire a permis de mettre en �vidence une augmentation des
sympt�mes neuropsychiatriques.
Les insuffisants r�naux chroniques qui sont expos�s � l'aluminium
pr�sent dans les liquides de dialyse et re�oivent des m�dicaments qui
en contiennent, peuvent souffrir de troubles iatrog�nes tels
qu'enc�phalopathies, ost�omalacies r�sistantes � la vitamine D et
an�mies microcytaires. Ces syndromes cliniques peuvent �tre �vit�s en
r�duisant l'exposition � l'aluminium.
Chez les pr�matur�s, m�me ceux dont l'insuffisance r�nale n'est
pas suffisamment grave pour provoquer une augmentation du taux sanguin
de cr�atinine, il peut y avoir accumulation tissulaire d'aluminium,
notamment au niveau des os, en cas d'exposition iatrog�ne �
l'aluminium. A partir d'un certain degr� d'insuffsance r�nale, on peut
craindre des convulsions et une enc�phalopathie.
L'exposition humaine � l'aluminium est tr�s courante, mais seuls
quelques cas d'hypersensibilit� ont �t� signal�s � la suite de
l'application sur la peau ou de l'administration parent�rale de
certains compos�s de l'aluminium.
Des cas de fibrose pulmonaire ont �t� rapport�s chez quelques
ouvriers employ�s � la fabrication d'explosifs et de feux d'artifice
et expos�s � de tr�s fines poudres d'aluminium comprim�es. Dans
presque tous les cas, il y avait eu exposition � des particules
d'aluminium enrob�es d'huile min�rale. Ce proc�d� n'est plus utilis�.
D'autres cas de fibrose pulmonaire ont pu �tre �galement attribu�s �
la pr�sence d'autres min�raux comme la silice et l'amiante;
l'aluminium n'�tait donc pas seul en cause.
On a pu attribuer l'apparition d'un asthme d'irritation �
l'inhalation de compos�s tels que le sulfate et le fluorure
d'aluminium, le t�trafluoraluminate de potassium ainsi qu'au s�jour
dans l'environnement complexe des ateliers de production de
l'aluminium.
On ne dispose pas de donn�es suffisantes pour d�terminer dans
quelle cat�gorie de risque canc�rog�ne pour l'Homme se situent
l'aluminium et ses d�riv�s. Quoi qu'il en soit, les �tudes men�es sur
l'animal n'attribuent aucun pouvoir canc�rog�ne � l'aluminium ou � ses
compos�s.
9. Effets sur les autres �tres vivants au laboratoire ou dans leur
milieu naturel
Chez les algues unicellulaires, on observe un effet toxique plus
important aux faibles valeurs du pH, lorsque la biodisponibilit� de
l'aluminium augmente. Elles sont plus sensibles que les autres
microorganismes, la plupart des 19 esp�ces lacustres test�es
pr�sentant une inhibition totale de la croissance � la concentration
de 200 �g/litre d'aluminium total (pH 5,5). Il peut y avoir s�lection
des souches tol�rantes � l'aluminium. Ainsi, on a isol� des algues
capables de se d�velopper en pr�sence d'une concentration d'aluminium
de 48 �g/litre et d'un pH de 4,6. Dans le cas des invert�br�s
aquatiques, on a obtenu des valeurs de la Cl50 qui vont de
0,48 mg/litre (polych�tes) � 59,6 mg/litre (daphnies). En ce qui
concerne les poissons, les valeurs vont de 0,095 mg/litre
( Jordanella floridae) � 235 mg/litre (gambusies). il faut
cependant interpr�ter ces r�sultats avec prudence du fait que la
biodisponibililt� de l'aluminium d�pend fortement du pH. La grande
dispersion des valeurs de la Cl50 traduit probablement les variations
de la biodisponibilit�. L'adjonction d'agents ch�latants, comme le NTA
ou l'EDTA, r�duit la toxicit� de l'aluminium pour les poissons.
Les macroinvert�br�s r�agissent de mani�re variable � la pr�sence
d'aluminium. Lorsque le pH se situe dans les limites normales, la
toxicit� de l'aluminium augmente � mesure que le pH diminue;
toutefois, dans les eaux tr�s acides, l'aluminium peut att�nuer
l'effet de l'acidit�. Certains invert�br�s sont tr�s r�sistants �
l'acidit� et peuvent abonder dans les eaux acides. On a constat� une
augmentation de la d�rive des invert�br�s dans les cours d'eau o� ils
sont soumis � un stress d� au pH ou � la pr�sence d'aluminium. Il
s'agit l� d'une r�action commune � divers facteurs de stress. Lors
d'un test, des invert�br�s lacustres ont en g�n�ral surv�cu � une
exposition � l'aluminium mais ont souffert de la diminution des
phosphates dans des conditions rendues oligotrophiques par la
pr�cipitation de l'aluminium. Les �tudes toxicologiques � court et �
long terme effectu�es sur les poissons se sont d�roul�es dans des
conditions tr�s diverses et, ce qui est beaucoup plus important, dans
des milieux de pH vari�. Il ressort des donn�es disponibles que des
effets non n�gligeables ont �t� observ�s � des concentrations en
aluminium inorganique sous forme de compos�s monom�res ne d�passant
pas 25 �g/litre. Toutefois, les relations complexes qui existent entre
l'acidit� et la biodisponibilit� de l'aluminium rendent difficile
l'interpr�tation des donn�es toxicologiques. Lorsque le pH est tr�s
bas, c'est-�-dire dans des conditions qui ne sont normalement pas
celles qui r�gnent dans les eaux naturelles, c'est la concentration en
ions hydrog�ne qui constitue le facteur toxique, l'addition
d'aluminium ayant tendance � r�duire la toxicit�. C'est pour un pH
compris entre 4,5 et 6,0 que l'aluminium en �quililbre exerce son
effet toxique maximal. On a �galement montr� que la toxicit� augmente
avec le pH lorsque celui-ci est alcalin. On explique la toxicit� de
l'aluminium pour les poissons par le fait qu'ils ne peuvent plus
assurer leur osmor�gulation ainsi que par des probl�mes respiratoires
dus � la pr�cipitation de l'aluminium sur le mucus branchial. Le
premier de ces effets est associ� � des valeurs basses du pH. Ces
r�sultats de laboratoire ont �t� confirm�s par des observation en
milieu naturel, notamment dans des conditions d'acidit� excessive.
Les oeufs et les larves d'amphibiens souffrent de l'acidit� et de
la pr�sence d'aluminium, ces deux facteurs �tant en interaction. On a
fait �tat d'effets tels que r�duction de l'�closion, �closion
retard�e, m�tamorphose retard�e ou � petite taille, et mortalit� pour
des teneurs en aluminium inf�rieures � 1 mg/litre.
L'exposition des racines des plantes terrestres � l'aluminium
peut r�duire la croissance de ces racines, diminuer la fixation des
nutriments et provoquer un rabougrissement du v�g�tal. On a cependant
montr� au laboratoire comme sur le terrain que les v�g�taux pouvaient
manifester une tol�rance � l'aluminium.
10. Conclusions
10.1 Population g�n�rale
Des �tudes sur l'animal ont permis de d�finir les risques que
l'exposition � l'aluminium pr�sente pour le d�veloppement neurologique
et les fonctions c�r�brales. Toutefois, il n'appara�t pas que
l'aluminium menace la sant� des personnes en bonne sant� qui ne sont
pas soumises � une exposition professionnelle.
Rien n'indique que l'aluminium puisse jouer un r�le important
dans l'�tiologie de la maladie d'Alzheimer et en tout �tat de cause,
il ne provoque chez aucune esp�ce animale ou chez l'Homme des
pathologies de type Alzheimer.
On ne dispose pas de donn�es suffisantes pour donner corps �
l'hypoth�se selon laquelle l'exposition � l'aluminium des personnes
vivant dans des r�gions o� l'eau a une forte teneur en cet �l�ment
pourrait exacerber la maladie d'Alzheimer ou en acc�l�rer l'�volution.
On ne dispose pas d'�l�ments d'appr�ciation suffisants, sur le
plan sanitaire, pour entreprendre une r�vision des valeurs-guides de
l'OMS concernant l'exposition � l'aluminium des personnes en bonne
sant� non expos�es professionnellement. Par exemple, il n'existe pas
de base scientifique suffisante pour que l'on puisse �tablir une norme
de concentration de l'aluminium dans l'eau de boisson.
10.2 Sous-groupes de population expos�s � un risque particulier
On a montr� que chez des sujets de tous �ges atteints
d'insuffisance r�nale, l'accumulation d'aluminium provoquait un
syndrome clinique d'enc�phalopathie, une ost�omalacie r�sistante � la
vitamine D et une an�mie microcytaire. Cet aluminium peut provenir
du liquide d'h�modialyse ou de produits pharmaceutiques qui en
contiennent (par exemple les capteurs d'ions phosphate). La prise de
produits contenant des citrates peut provoquer une exacerbation de
l'absorption intestinale. Les insuffisants r�naux sont donc expos�s au
risque de neurotoxicit� aluminique.
L'exposition iatrog�ne � l'aluminium constitue une menace pour
les insuffisants r�naux chroniques. Chez les pr�matur�s, la charge de
l'organisme en aluminium est plus �lev�e que chez les autres
nourrissons. On s'efforcera donc de limiter ce genre d'exposition chez
tous ces groupes vuln�rables.
10.3 Populations professionnellement expos�es
Le risque est plus important pour les travailleurs expos�s
pendant de longues p�riodes � de fines particules d'aluminium.
Cependant, on ne dispose pas de donn�es suffisantes pour fixer avec
quelque certitude des limites d'exposition qui prot�gent contre les
effets ind�sirables de l'aluminium.
On conna�t des cas de fibrose pulmonaire dus � une exposition �
de la poudre d'aluminium � usage pyrotechnique, tr�s souvent enrob�e
d'huile min�rale .En revanche, il n'est pas prouv� que l'exposition �
l'aluminium sous d'autres formes puisse �galement provoquer ce type de
fibrose. Dans la plupart des cas de fibrose observ�s, il y avait eu
exposition simultan�e � d'autres agents fibrog�nes.
On a attribu� un certain nombre de cas d'asthme d'irritation �
l'inhalation de sulfate, de fluorure d'aluminium ou de
t�trafluoraluminate de potassium ou encore � un s�jour dans
l'environnement complexe des ateliers de production de l'aluminium.
10.4 Effets sur l'environnement
L'aluminium pr�sent dans l'environnement en phase solide est
relativement insoluble, en particulier lorsque le pH est voisin de la
neutralit�, d'o� les tr�s faibles concentrations d'aluminium en
solution dans la plupart des eaux naturelles.
Dans les milieux acides ou peu tamponn�s o� l'apport d'acide est
important, la concentration de l'aluminium peut atteindre des valeurs
nocives pour les organismes aquatiques et les plantes terrestres.
Toutefois, la sensibilit� � ce m�tal varie largement selon les
esp�ces, les souches et les stades de d�veloppement.
Les effets biologiques d�l�t�res de fortes teneurs en aluminium
inorganique sous forme monom�re peuvent �tre att�nu�s par la pr�sence
d'acides organiques, de fluorures, de silicates et de concentrations
�lev�es de calcium et de magn�sium.
Dans les eaux excessivement acides, il y a une r�duction sensible
de la diversit� biologique due � la mobilisation de l'aluminium sous
des formes plus toxiques. Cette r�duction de la diversit� biologique
s'observe � tous les niveaux trophiques.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades f�sicas y qu�micas
El aluminio es un metal blanco plateado, d�ctil y maleable.
Pertenece al grupo IIIA de la Tabla Peri�dica, y en los compuestos
suele encontrarse como AlIII. Forma cerca del 8% de la corteza
terrestre y es uno de los metales comunes m�s reactivos. La exposici�n
al agua, al ox�geno o a otros oxidantes conduce a la formaci�n de una
capa superficial de �xido de aluminio que confiere al metal una gran
resistencia a la corrosi�n. El �xido de aluminio es soluble en �cidos
minerales y �lcalis fuertes, pero insoluble en agua, mientras que el
cloruro, el nitrato y el sulfato de aluminio son solubles en agua. Los
halogenuros, los hidruros y los alquilos m�s cortos de aluminio
reaccionan violentamente con el agua.
El aluminio posee una elevada conductividad t�rmica y el�ctrica,
baja densidad y gran resistencia a la corrosi�n. A menudo se alea con
otros metales. Las aleaciones de aluminio son fuertes, ligeras y
f�cilmente susceptibles de conformaci�n a m�quina.
2. M�todos anal�ticos
Se han desarrollado diversos m�todos anal�ticos para determinar
la presencia de aluminio en muestras biol�gicas y ambientales. Los dos
m�todos m�s utilizados son la espectrometr�a de absorci�n at�mica en
horno de grafito (GF-AAS) y la espectrometr�a de emisi�n at�mico-
plasm�tica inductivamente acoplada (ICP-AES). La contaminaci�n de las
muestras por el aluminio del aire, de los recipientes o de los
reactivos durante el muestreo y preparaci�n constituye la principal
fuente de error anal�tico. En funci�n del tratamiento previo de la
muestra y de los procedimientos de concentraci�n y separaci�n, los
l�mites de detecci�n son de 1,9-4 �g/litro en los l�quidos biol�gicos
y 0,005-0,5 �g/g de peso seco en los tejidos al usar la GF-AAS, y de
5 �g/m3 en el aire y 3 �g/litro en el agua cuando se emplea la
ICP-AES.
3. Fuentes de exposici�n humana y ambiental
El aluminio se libera en el medio ambiente tanto a trav�s de
procesos naturales como de fuentes antropog�nicas. Est� muy
concentrado en el polvo de suelos dedicados a actividades tales como
la miner�a y la agricultura, y en las part�culas generadas por la
combusti�n del carb�n. Los silicatos de aluminio (arcillas), un
importante componente de los suelos, contribuyen a los niveles de
aluminio hallados en el polvo. Los procesos naturales superan con
mucho la contribuci�n antropog�nica directa al medio ambiente. El
aluminio movilizado por el hombre se forma en su mayor�a de forma
indirecta como resultado de la emisi�n de sustancias acidificantes.
En general, un descenso del pH ocasiona un aumento de la movilidad y
biodisponibilidad de las formas monom�ricas de aluminio. La principal
materia prima utilizada para producir aluminio es la bauxita, que
contiene hasta un 55% de al�mina (�xido de aluminio). La producci�n
mundial de bauxita fue de 106 millones de toneladas en 1992. El
metal de aluminio tiene una gran variedad de usos, entre ellos la
fabricaci�n de materiales estructurales para los sectores de la
construcci�n, el autom�vil y la aviaci�n, y la producci�n de
aleaciones met�licas. Los compuestos y materiales de aluminio tienen
tambi�n una amplia gama de usos, que incluyen la producci�n de vidrio,
cer�mica, caucho, conservantes de la madera, preparaciones
farmac�uticas y tejidos impermeabilizantes. Los minerales de aluminio
naturales, especialmente la bentonita y la zeolita, se emplean para
depurar el agua, para refinar el az�car, y en las industrias papeleras
y de la fermentaci�n.
4. Transporte, distribuci�n y transformaci�n en el medio ambiente
El aluminio es un metal ubicuo en el medio ambiente, donde se
encuentra en forma de silicatos, �xidos e hidr�xidos, combinado con
otros elementos como el sodio y el fl�or, y formando complejos con
materia org�nica. No se halla como metal libre debido a su
reactividad. S�lo tiene un estado de oxidaci�n (+3) en la naturaleza,
por lo que su transporte y distribuci�n en el medio ambiente dependen
tan s�lo de su qu�mica de coordinaci�n y de las caracter�sticas
f�sico-qu�micas del sistema ambiental concreto. A valores de pH
superiores a 5,5 los compuestos naturales de aluminio aparecen
predominantemente en forma no disuelta como gibbsita (Al(OH)3) o como
aluminosilicatos, excepto en presencia de grandes cantidades de
material org�nico disuelto, que se une al aluminio y puede dar lugar a
un aumento de la concentraci�n del aluminio disuelto en lagos y cursos
de agua. La movilidad del aluminio y su ulterior transporte en el
medio ambiente dependen de varios factores, entre los que cabe
mencionar el tipo de especies qu�micas, los cursos hidrol�gicos, las
interacciones suelo-agua y la composici�n del sustrato geol�gico. La
solubilidad del aluminio en equilibrio con la fase s�lida Al(OH)3
depende en gran medida del pH y de agentes complejantes tales como
fluoruros, silicatos, fosfatos y materia org�nica. La qu�mica del
aluminio inorg�nico en los suelos y los cursos de agua �cidos depende
de la solubilidad de los minerales y de los procesos de intercambio y
de mezcla de las aguas.
La acidificaci�n del suelo libera aluminio disolvi�ndolo, y el
metal llega as� a las corrientes de agua. La movilizaci�n del aluminio
por precipitaci�n �cida permite que haya m�s aluminio disponible para
captaci�n por las plantas.
5. Niveles ambientales y exposici�n humana
El aluminio es uno de los principales constituyentes de varios
componentes de la atm�sfera, en particular del polvo procedente de
suelos (tanto de fuentes naturales como de origen humano) y de
part�culas generadas por la combusti�n del carb�n. En las zonas
urbanas los niveles de aluminio en el polvo de la calle van de 3,7 a
11,6 �g/kg. Los niveles de aluminio en el aire var�an desde 0,5 ng/m3
sobre la Ant�rtida hasta m�s de 1000 ng/m3 en las zonas
industrializadas.
Las concentraciones de aluminio en las aguas superficiales y
subterr�neas son muy variables, dependiendo de factores geol�gicos y
f�sico-qu�micos. El aluminio puede estar en suspensi�n o disuelto.
Puede estar en forma de ligandos org�nicos o inorg�nicos o de ion
aluminio libre. En las aguas naturales el aluminio existe tanto en
forma monom�rica como polim�rica. Las especies de aluminio dependen
del pH y de las concentraciones de carbono org�nico disuelto (DOC)
fluoruros, sulfatos, fosfatos y part�culas en suspensi�n. Las
concentraciones de aluminio disuelto en las aguas de pH
aproximadamente neutro suelen ser bastante bajas, entre 1,0 y
50 �g/litro. En aguas m�s �cidas se alcanzan valores de hasta
500-1000 �g/litro. En condiciones de acidez extrema provocadas por el
avenamiento �cido de minas se han medido concentraciones de aluminio
disuelto de hasta 90 mg/litro.
La exposici�n humana no ocupacional al aluminio en el medio
ambiente se produce principalmente a trav�s de la ingesti�n de agua y
alimentos, sobre todo de estos �ltimos. La ingesta diaria de aluminio
con los alimentos y bebidas es en los adultos de entre 2,5 y 13 mg.
Esto representa un 90%-95% de la ingesta total. El agua de bebida
puede contribuir con unos 0,4 mg diariamente, seg�n los valores de las
actuales directrices internacionales, pero m�s probablemente se sit�a
en torno a los 0,2 mg/d�a. La exposici�n pulmonar puede contribuir
hasta con 0,04 mg/d�a. En algunas circunstancias, como la exposici�n
ocupacional y el uso de anti�cidos, los niveles de exposici�n pueden
ser mucho mayores. Con dos tabletas anti�cido de tama�o medio, por
ejemplo, se puede consumir m�s de 500 mg de aluminio. La evaluaci�n de
la captaci�n consecutiva a ese tipo de exposiciones plantea algunos
problemas debido a dificultades anal�ticas y de muestreo. Las
investigaciones isot�picas realizadas con Al26 indican que una de las
formas m�s biodisponibles del aluminio es el citrato, y que cuando el
aluminio est� en dicha forma podr�a darse hasta un 1% de absorci�n. No
obstante, los seres humanos absorben al parecer s�lo el 3% de la
cantidad total de aluminio ingerida diariamente con el agua de bebida,
una fuente relativamente secundaria en comparaci�n con los alimentos.
6. Cin�tica y metabolismo
6.1 Ser humano
El aluminio y sus compuestos parecen ser mal absorbidos por los
seres humanos, pero no hay estudios adecuados sobre la velocidad y el
grado de absorci�n. Las concentraciones de aluminio en la sangre y la
orina se han empleado como una medida f�cilmente obtenible de la
captaci�n de aluminio, habi�ndose observado niveles elevados en la
orina de soldadores de aluminio y de productores de polvo de escamas
de aluminio.
El mecanismo de absorci�n gastrointestinal del aluminio todav�a
no ha sido totalmente dilucidado. La variabilidad se debe a las
propiedades qu�micas del elemento y a las diversas especies qu�micas
formadas en funci�n del pH, la fuerza i�nica, la presencia de
elementos competitivos (silicio) y la presencia de agentes
complejantes en el interior del tracto digestivo (p.ej., citrato).
Se ha empleado el is�topo radiactivo Al26 para estudiar el
destino biol�gico y la absorci�n gastrointestinal del aluminio en el
hombre tras la ingesti�n de compuestos de aluminio. Se ha detectado
una variabilidad significativa entre los individuos. Se han notificado
fracciones de captaci�n de 5 � 10-3 para el aluminio como citrato,
1,04 � 10-4 para el hidr�xido de aluminio y 1,36 � 10-3 para el
hidr�xido administrado con citrato. Un estudio sobre la captaci�n del
aluminio del agua de bebida revel� una fracci�n de captaci�n de
2,35 � 10-3, lleg�ndose a la conclusi�n de que en la poblaci�n
general los individuos que consumen 1,5 litros/d�a de agua de bebida
con un contenido de 100 �g aluminio/litro, absorben un 3% del aluminio
total ingerido diariamente a partir de esa fuente, dependiendo de los
niveles presentes en los alimentos y de la frecuencia del uso de
anti�cidos.
La proporci�n de Al3+ plasm�tico normalmente unido a prote�nas
en el ser humano puede ser hasta del 70%-90% en los pacientes
sometidos a hemodi�lisis con niveles moderadamente altos de aluminio
en el plasma. Los niveles m�s elevados de aluminio suelen encontrarse
en los pulmones, donde puede hallarse en forma de part�culas inhaladas
insolubles.
La orina es la v�a m�s importante de excreci�n del aluminio. Tras
la administraci�n por v�a oral de una dosis �nica de aluminio, pasados
13 d�as se hab�a excretado un 83% por la orina y un 1,8% por las
heces. La semivida de las concentraciones urinarias en soldadores
expuestos durante m�s de 10 a�os fue de 6 meses o superior. Entre
trabajadores jubilados expuestos al polvo de escamas de aluminio, las
semividas calculadas se situaban entre 0,7 y 8 a�os.
6.2 Animales
La absorci�n por v�a gastrointestinal es normalmente menor
del 1%. Los principales factores que influyen en la absorci�n son
la solubilidad, el pH y las especies qu�micas. Los compuestos
complejantes org�nicos, sobre todo el citrato, aumentan la absorci�n.
La absorci�n del aluminio puede interferir en los sistemas de
transporte del calcio y el hierro. La absorci�n cut�nea y por
inhalaci�n no han sido estudiadas con detalle. El aluminio se
distribuye en la mayor�a de los �rganos del cuerpo, y cuando las dosis
son altas se acumula principalmente en los huesos. De forma limitada
pero a�n no determinada con precisi�n, atraviesa la barrera
hematoencef�lica, y llega tambi�n al feto. El aluminio se elimina
eficazmente por la orina. El periodo de semieliminaci�n plasm�tica es
de aproximadamente 1 h en los roedores.
7. Efectos en mam�feros de laboratorio y en sistemas de pruebas
in vitro
La toxicidad aguda del aluminio met�lico y de los compuestos de
aluminio es baja; los valores notificados de la DL50 oral est�n
comprendidas entre varios cientos y 1000 mg aluminio/kg de peso
corporal. Los valores de la CL50 por inhalaci�n no han sido
establecidos.
En estudios a corto plazo en los que se examin� una gama adecuada
de variables de evaluaci�n despu�s de exponer ratas, ratones o perros
a diversos compuestos de aluminio (fosfato s�dico de aluminio,
hidr�xido de aluminio, nitrato de aluminio) a trav�s de los alimentos
o del agua, s�lo se observaron efectos muy discretos (disminuciones
del aumento de peso corporal, asociadas generalmente a un descenso del
consumo de comida, o efectos histopatol�gicos leves) a las dosis m�s
altas (70 a 300 mg aluminio/kg de peso corporal al d�a). Los efectos
sist�micos que siguieron a la administraci�n parenteral incluyeron
tambi�n disfunci�n renal.
No se han hallado estudios de inhalaci�n adecuados. Despu�s de la
administraci�n intratraqueal de �xido de aluminio se observ� fibrosis
asociada a part�culas, parecida a la hallada en otros estudios sobre
el s�lice y el polvo de carb�n.
No se observaron signos manifiestos de fetotoxicidad, ni
alteraciones de los par�metros reproductivos generales despu�s del
tratamiento de ratas con 13, 26 � 52 mg aluminio/kg de peso corporal
al d�a (en forma de nitrato de aluminio) mediante alimentaci�n
forzada. Sin embargo, se observ� un retraso dosis-dependiente del
crecimiento en la descendencia, a dosis de 13 mg/kg en el caso de las
hembras y de 26 mg/kg en el caso de los machos. El nivel m�nimo con
efectos adversos observados (LOAEL) en el desarrollo (disminuci�n de
la osificaci�n, aumento de la incidencia de defectos cong�nitos en las
v�rtebras y el estern�n y peso fetal reducido) fue de 13 mg/kg
(nitrato de aluminio). Estos efectos no se observaron a dosis mucho
m�s altas de hidr�xido de aluminio. Se detect� una reducci�n del
crecimiento posnatal al emplear 13 mg/kg (nitrato de aluminio), aunque
no se analiz� la toxicidad materna. En estudios sobre el desarrollo
cerebral se detect� una disminuci�n de la fuerza de aprehensi�n en la
descendencia de hembras a las que se administraron con los alimentos
100 mg aluminio/kg de peso corporal en forma de lactato de aluminio,
no observ�ndose signos de toxicidad materna.
No existen indicios de que el aluminio sea carcin�geno. Puede
formar complejos con el ADN, as� como enlaces entre las prote�nas
cromos�micas y el ADN, pero no se ha demostrado que sea mut�geno en
bacterias o que induzca mutaciones o transformaciones en c�lulas de
mam�fero in vitro. Se han observado aberraciones cromos�micas en
c�lulas de la medula �sea de ratas y ratones expuestos.
Hay indicios fundados de que el aluminio es neurot�xico en
animales de experimentaci�n, aunque se da una considerable
variabilidad entre especies. En especies susceptibles, la toxicidad
que sigue a la administraci�n parenteral se caracteriza por un
deterioro neurol�gico progresivo, conducente a un estado epil�ptico
que acarrea la muerte (DL50 = 6 �g Al/g de peso seco del cerebro).
Morfol�gicamente, la encefalopat�a progresiva se asocia a una
patolog�a neurofibrilar de las neuronas grandes y medianas, sobre todo
en la m�dula espinal, el tallo encef�lico y determinadas zonas del
hipocampo. Estos ovillos neurofibrilares son morfol�gica y
bioqu�micamente diferentes de los que caracterizan la enfermedad de
Alzheimer. En animales de experimentaci�n expuestos a sales solubles
de aluminio (p.ej., lactato y cloruro) a trav�s de los alimentos o el
agua, a dosis de 50 mg aluminio/kg de peso al d�a o mayores, se ha
observado un deterioro de la conducta sin signos concomitantes de
encefalopat�a o cambios neurohistopatol�gicos manifiestos.
La osteomalacia que afecta al hombre se observa tambi�n
sistem�ticamente en las especies de cierto tama�o (p.ej., el perro
o el cerdo) expuestas al aluminio; en los roedores se observan
manifestaciones parecidas. Estos efectos parecen darse en todas las
especies, incluido el hombre, a niveles de aluminio de 100 a 200 �g/g
de cenizas �seas.
8. Efectos en el hombre
No se han descrito efectos pat�genos agudos en la poblaci�n
general como consecuencia de la exposici�n al aluminio.
En Inglaterra una poblaci�n de aproximadamente 20 000 individuos
se vio expuesta durante por lo menos 5 d�as a niveles elevados de
sulfato de aluminio, debido a la contaminaci�n accidental de unas
instalaciones de agua de bebida. Se inform� de casos de n�useas,
v�mitos, diarrea, �lceras bucales, �lceras y erupciones cut�neas y
dolores artr�ticos. Se comprob� que los s�ntomas eran en su mayor�a
leves y de corta duraci�n. No se pudieron atribuir efectos duraderos
sobre la salud a las exposiciones conocidas al aluminio del agua de
bebida.
Se ha propuesto la hip�tesis de que el aluminio presente en
el agua potable es un factor de riesgo por lo que se refiere al
desarrollo o la aceleraci�n de la enfermedad de Alzheimer y al
deterioro senil de la funci�n cognitiva. Se ha sugerido tambi�n que el
polvo y los vapores de aluminio fino apisonado son quiz� factores de
riesgo que propician el deterioro de las funciones cognitivas y la
aparici�n de enfermedades pulmonares en determinados trabajos.
Se han llevado a cabo unos 20 estudios epidemiol�gicos para
comprobar la hip�tesis de que el aluminio del agua de bebida es un
factor de riesgo de la enfermedad de Alzheimer, y dos estudios han
evaluado la asociaci�n entre el aluminio del agua de bebida y el
deterioro de las funciones cognitivas. El dise�o de estos estudios
abarcaba desde el control ecol�gico hasta el control de los casos. Se
consider� que 8 estudios realizados en poblaciones de Noruega, el
Canad�, Francia, Suiza e Inglaterra ten�an la calidad necesaria para
satisfacer los criterios generales establecidos a fin de evaluar la
exposici�n y los resultados y de poder efectuar ajustes por lo menos
en algunas variables de confusi�n. De los seis estudios que analizaron
la relaci�n entre el aluminio del agua de bebida y la demencia o la
enfermedad de Alzheimer, tres hallaron una relaci�n positiva, pero no
as� los otros tres. Sin embargo, todos los estudios presentaban
algunos fallos de dise�o (relacionados por ejemplo con la evaluaci�n
de la exposici�n ecol�gica, la no consideraci�n de todas las fuentes
de exposici�n al aluminio y de factores de confusi�n importantes como
la educaci�n, la situaci�n socioecon�mica y los antecedentes
familiares, el uso de medidas indirectas de la evoluci�n de la
enfermedad de Alzheimer, o unos m�todos sesgados de selecci�n). En
general, los riesgos relativos determinados eran inferiores a 2, con
grandes intervalos de confianza, a concentraciones totales de aluminio
de 100 �g/litro o superiores en el agua de bebida. Sobre la base de
los conocimientos disponibles acerca de la patog�nesis de la
enfermedad de Alzheimer y de todas las pruebas aportadas por estos
estudios epidemiol�gicos, se lleg� a la conclusi�n de que los datos
epidemiol�gicos actuales no respaldan la hip�tesis de una relaci�n
causal entre la enfermedad de Alzheimer y el aluminio del agua de
bebida.
Adem�s de los estudios epidemiol�gicos realizados para analizar
la relaci�n entre la enfermedad de Alzheimer y el aluminio del agua
de bebida en otros dos estudios se examin� la relaci�n entre los casos
de disfunci�n cognitiva y de enfermedad de Alzheimer en poblaciones
de ancianos y los niveles de aluminio en el agua de bebida. Los
resultados fueron una vez m�s contradictorios. En un estudio realizado
en 800 octogenarios varones que consum�an agua de bebida con
concentraciones de aluminio de hasta 98 �g/litro no se hall� relaci�n
alguna. El segundo estudio utiliz� "cualquier indicio de deterioro
mental" como criterio de seguimiento, calculando as� un riesgo
relativo de 1,72 para concentraciones de aluminio superiores a
85 �g/litro en 250 varones. Tales datos son insuficientes para
demostrar que el aluminio sea una causa de deterioro cognitivo en los
ancianos.
Los datos sobre el deterioro de la funci�n cognitiva en relaci�n
con la exposici�n al aluminio son contradictorios. La mayor�a de los
estudios se han hecho en poblaciones reducidas, y la metodolog�a
utilizada es cuestionable en lo que respecta a la magnitud del efecto
estudiado, la evaluaci�n de la exposici�n y los factores de confusi�n.
En un estudio comparativo del deterioro cognitivo entre mineros no
expuestos y mineros expuestos a un polvo que conten�a un 85% de
aluminio molido muy fino y un 15% de �xido de aluminio (como
profilaxis contra el s�lice), los resultados de las pruebas cognitivas
y la proporci�n de personas con dificultades en al menos una de las
pruebas fueron peores entre los mineros expuestos. Se detect� una
tendencia al aumento del riesgo relacionada con la exposici�n.
En todos los estudios ocupacionales de los que se tiene noticia,
la magnitud de los efectos observados, la presencia de factores de
confusi�n, los problemas relacionados con la evaluaci�n de la
exposici�n y la probabilidad de exposiciones mixtas hacen que, en
conjunto, los datos sean insuficientes como para extraer la conclusi�n
de que el aluminio es una causa de deterioro cognitivo en los
trabajadores expuestos al aluminio en su trabajo.
En estudios limitados con trabajadores expuestos a vapores de
aluminio se ha informado de s�ndromes neurol�gicos que incluyen el
deterioro de la funci�n cognitiva, disfunciones motoras y neuropat�a
perif�rica. Se ha informado de una peque�a poblaci�n de soldadores de
aluminio que, comparados con soldadores de hierro, presentaron un
ligero deterioro de la funci�n motora repetitiva. En otros estudios
realizado con cuestionarios se detect� un aumento de los s�ntomas
neuropsiqui�tricos.
En los pacientes con insuficiencia renal cr�nica, la exposici�n
iatrog�nica a l�quidos de di�lisis o productos farmac�uticos con
aluminio puede producir encefalopat�a, osteomalacia resistente a la
vitamina D y anemia microc�tica. Estos s�ndromes cl�nicos pueden
prevenirse reduciendo la exposici�n al aluminio.
En los ni�os prematuros expuestos a fuentes iatrog�nicas de
aluminio puede producirse un incremento del contenido de aluminio
de los tejidos, particularmente en los huesos, incluso cuando la
disfunci�n renal no es lo suficientemente grave como para provocar
un aumento de los niveles de creatinina en sangre. En caso de
insuficiencia renal pueden aparecer convulsiones y encefalopat�a.
Aunque la exposici�n humana al aluminio est� muy extendida,
s�lo se ha informado de unos cuantos casos de hipersensibilidad
consecutivos a la aplicaci�n cut�nea o la administraci�n parenteral
de algunos compuestos de aluminio.
Se ha informado de casos de fibrosis pulmonar en algunos
trabajadores expuestos a polvo muy fino de aluminio apisonado
utilizado en la fabricaci�n de explosivos y material pirot�cnico. Casi
todos los casos estaban relacionados con la exposici�n a part�culas de
aluminio cubiertas de aceite mineral. Dicho procedimiento ya no se
utiliza. Otros casos de fibrosis pulmonar se han relacionado con
exposiciones a otros agentes minerales como el s�lice y el asbesto y
no pueden atribuirse exclusivamente al aluminio.
Algunos casos de asma inducida por sustancias irritantes se han
asociado a la inhalaci�n de sulfato de aluminio, fluoruro de aluminio
o tetrafluoruro pot�sico de aluminio, as� como al ambiente cargado de
los cuartos de calderas utilizados en la producci�n de aluminio.
La informaci�n disponible es insuficiente para poder clasificar
el riesgo de c�ncer asociado a las distintas formas de exposici�n
humana al aluminio y sus compuestos. Los estudios en animales no
indican que el aluminio o los compuestos del aluminio sean
carcin�genos.
9. Efectos en otros organismos en el laboratorio y en el campo
En las algas acu�ticas unicelulares se ha detectado una mayor
toxicidad a pH bajo, circunstancia que aumenta la biodisponibilidad
del aluminio. Estas algas son m�s sensibles que otros microorganismos,
y de 19 especies lacustres la mayor parte presentaron una inhibici�n
completa del crecimiento a una concentraci�n total de aluminio de
200 �g/litro (pH 5,5). Es posible seleccionar cepas tolerantes al
aluminio; se han aislado algas verdes capaces de crecer en presencia
de 48 mg/litro a pH 4,6.
En los invertebrados acu�ticos la CL50 est� comprendida entre
0,48 mg/litro (poliquetos) y 59,6 mg/litro (d�fnidos). En los peces,
la CL50 a las 96 horas va desde 0,095 mg/litro (pez estandarte
americano) hasta 235 mg/litro (gambusia com�n). Sin embargo, estos
resultados deben interpretarse con cautela dados los importantes
efectos del pH en la disponibilidad del aluminio. El amplio margen de
valores de la CL50 se debe probablemente a la distinta
disponibilidad. La adici�n de agentes quelantes, como el NTA y el
EDTA, reduce la toxicidad aguda del aluminio para los peces.
Los macroinvertebrados responden de diversa forma al aluminio. En
el margen normal de pH la toxicidad del aluminio aumenta al disminuir
el pH, sin embargo en aguas muy �cidas el aluminio puede reducir los
efectos del estr�s �cido. Algunos invertebrados son muy resistentes al
estr�s �cido y pueden llegar a ser muy abundantes en aguas �cidas. Se
ha informado de un aumento de la tasa de deriva de los invertebrados
en cursos de agua en condiciones de estr�s por pH o por pH/aluminio;
esa es una respuesta com�n a diversos agentes estresantes.
Invertebrados lacustres sobrevivieron en general a la exposici�n al
aluminio en la naturaleza, pero sufrieron en condiciones oligotr�ficas
debido a la disminuci�n del fosfato inducida por la precipitaci�n con
el aluminio.
Se han llevado a cabo pruebas de toxicidad en peces a corto y
largo plazo en muy diversas condiciones y, lo que es m�s importante,
a distintos valores de pH. Los datos muestran efectos significativos a
niveles de aluminio inorg�nico monom�rico de tan s�lo 25 �g/litro. No
obstante, la compleja relaci�n entre la acidez y la biodisponibilidad
del aluminio dificulta la interpretaci�n de los datos de toxicidad. A
niveles de pH muy bajos (inhabituales en aguas naturales) la
concentraci�n de hidrogeniones parece ser el factor t�xico, y la
adici�n de aluminio tiende a reducir la toxicidad. En el margen de pH
4,5 a 6,0 el aluminio en equilibrio ejerce su m�ximo efecto t�xico. Se
ha visto que la toxicidad tambi�n aumenta a niveles crecientes de pH
en la regi�n de pH alcalino. El mecanismo propuesto para explicar la
toxicidad del aluminio en los peces es la incapacidad de los animales
a mantener el equilibrio osmorregulador, y los problemas respiratorios
asociados a la precipitaci�n del aluminio en el moco de las branquias.
El primero de estos efectos se asocia a niveles bajos de pH. Estos
datos de laboratorio han sido confirmados por estudios realizados en
la naturaleza, especialmente en zonas sometidas a estr�s �cido.
Los huevos y las larvas de los anfibios se ven afectados por
la acidez y el aluminio, y se da una interacci�n entre estos dos
factores. Se ha informado de fen�menos de disminuci�n de la
incubaci�n, retraso de la eclosi�n, retraso de la metamorfosis,
metamorfosis con tama�o reducido y aumento de la mortalidad en
diversas especies a concentraciones de aluminio inferiores a
1 mg/litro.
La exposici�n de las ra�ces de plantas terrestres al aluminio
puede frenar el crecimiento radicular, la captaci�n de nutrientes y el
desarrollo de la planta. Se ha demostrado la tolerancia al aluminio
tanto en el laboratorio como en la naturaleza.
10. Conclusiones
10.1 Poblaci�n general
Los estudios realizados en animales acerca de la exposici�n al
aluminio han revelado riesgos para el desarrollo neurol�gico y las
funciones cerebrales. Sin embargo, no se ha demostrado que el aluminio
entra�e riesgos para la salud de las personas sanas y no expuestas en
el trabajo.
No existen pruebas de que el aluminio tenga un papel causal
primordial en la enfermedad de Alzheimer, patolog�a que el metal no
induce in vivo en ninguna especie, incluido el hombre.
La hip�tesis de que la exposici�n de la poblaci�n anciana de
algunas regiones a niveles elevados de aluminio en el agua de bebida
puede exacerbar o acelerar la enfermedad de Alzheimer no est� avalada
por datos v�lidos.
Tampoco hay datos v�lidos que corroboren la hip�tesis de que
determinadas exposiciones, ya sean ocupacionales o a trav�s del agua
de bebida, pueden asociarse a un deterioro inespec�fico de la funci�n
cognitiva.
No existen datos suficientes relacionados con la salud que
justifiquen la revisi�n de las actuales directrices de la OMS respecto
a la exposici�n al aluminio de individuos sanos no expuestos
ocupacionalmente. As�, por ejemplo, no hay una base cient�fica s�lida
para establecer una norma basada en criterios de salud sobre el
aluminio en el agua de bebida.
10.2 Subpoblaciones con un riesgo especial
En pacientes con trastornos de la funci�n renal, cualquiera que
sea su edad, la acumulaci�n de aluminio provoca un s�ndrome cl�nico
caracterizado por encefalopat�a, osteomalacia resistente a la vitamina
D y anemia microc�tica. Las fuentes de aluminio son los l�quidos
de hemodi�lisis y las preparaciones famarc�uticas que contienen
aluminio (p.ej., aglutinantes de fosfato). Los productos que contienen
citrato pueden aumentar la absorci�n intestinal. Los pacientes con
insuficiencia renal corren por tanto el riesgo de sufrir
neurotoxicidad por aluminio.
La exposici�n iatrog�nica al aluminio entra�a riesgos para los
pacientes con insuficiencia renal cr�nica. Los lactantes prematuros
tienen en su organismo una mayor carga de aluminio que los otros
lactantes. Deben tomarse toda clase de precauciones para limitar la
exposici�n de esos grupos.
10.3 Poblaciones expuestas ocupacionalmente
Los trabajadores que han estado expuestos durante largo tiempo a
niveles altos de part�culas finas de aluminio corren quiz� un mayor
riesgo de sufrir efectos nocivos para su salud. Sin embargo, no hay
datos suficientes para fijar con cierto grado de fiabilidad unos
l�mites de exposici�n ocupacional en relaci�n con los efectos adversos
del aluminio.
La exposici�n al polvo de aluminio pirot�cnico apisonado,
recubierto casi siempre de aceites lubricantes minerales, ha provocado
fibrosis pulmonar (aluminosis), pero no se ha demostrado que la
exposici�n a otras formas de aluminio produzca fibrosis pulmonar. En
la mayor parte de los casos notificados hab�a habido tambi�n
exposici�n a otros agentes potencialmente fibrog�nicos.
Algunos casos de asma inducida por sustancias irritantes se han
asociado a la inhalaci�n de sulfato de aluminio, fluoruro de aluminio
o tetrafluoruro pot�sico de aluminio, as� como al ambiente cargado del
interior de los cuartos de calderas utilizados en la producci�n de
aluminio.
10.4 Efectos ambientales en el medio ambiente
Las fases s�lidas que contienen aluminio en el medio ambiente son
relativamente insolubles, en particular a un pH aproximadamente
neutro, de ah� las bajas concentraciones de aluminio disuelto que
tienen la mayor�a de las aguas naturales.
En los entornos �cidos o escasamente tamponados que reciben
aportes muy acidificantes, las concentraciones de aluminio pueden
aumentar hasta niveles perjudiciales tanto para los organismos
acu�ticos como para las plantas terrestres. No obstante, existen
grandes diferencias en la sensibilidad a este metal seg�n la especie,
la cepa y la etapa de la vida.
Los efectos biol�gicos adversos de las concentraciones elevadas
de aluminio monom�rico inorg�nico son menores en presencia de �cidos
org�nicos, fluoruros, silicatos y niveles altos de calcio y magnesio.
En las aguas sometidas a estr�s �cido se produce una reducci�n
sustancial de la diversidad de especies, asociada a una movilizaci�n
de las formas m�s t�xicas de aluminio. Dicha p�rdida de diversidad se
observa en todos los niveles tr�ficos.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
Members
Dr T.M. Florence, Centre for Environmental Health Sciences, Oyster
Bay, New South Wales, Australia
Dr M. Golub, California Regional Primate Research Center, University
of California, Davis, California, USA
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
( Co-Rapporteur)
Professor D.R. McLachlan ( Retired from: Centre for Research in
Neurogenerative Diseases, University of Toronto, Toronto,
Ontario, Canada
Dr M. Moore, National Health and Medical Research Council, National
Research Centre for Environmental Toxicology, Coopers Plains,
Brisbane, Australia ( Chairman)
Dr T.V. O'Donnell, University of Otago, Wellington South, New Zealand
( Vice-Chairman)
Professor B. Rosseland, Norwegian Institute of Water Research (NIVA),
Oslo, Norway
Dr B. Simon, Fraunhofer Institute, Hanover, Germany ( Co-Rapporteur)
Dr B. Sjogren, Department of Occupational Medicine, Swedish National
Institute for Working Life, Solna, Sweden
Dr L. Smith, Disease Control Service, Public Health Branch, Ontario
Ministry of Health, North York, Ontario, Canada
Dr E. Storey, Royal Melbourne Hospital, Department of Pathology,
University of Melbourne, Parkville, Victoria, Australia
Dr H. Temmink, Department of Toxicology, Agricultural University,
Wageningen, The Netherlands ( Vice-Chairman)
Dr M.K. Ward, Department of Renal Medicine, Royal Victoria Infirmary,
Newcastle-upon-Tyne, United Kingdom
Dr M. Wilhelm, Health Institute, University of Dusseldorf, Dusseldorf,
Germany
Professor H.M. Wisniewski, New York State Institute for Basic
Research in Developmental Disabilities, Staten Island, New York,
USA
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute
of Occupational Medicine, Ministry of Health, Beijing, China
Observers
Dr K. Bentley, Environmental Health Assessment and Criteria, Human
Services and Health, Woden, Australia
Dr O.C. B�ckman, Norsk Hydro, Porsgrunn Research Centre, Porsgrunn,
Norway
Dr J. Borak, Occupational and Environmental Health, Jonathan Borak &
Co., New Haven, Connecticut, USA
Dr I. Calder, Occupational and Environmental Health, South Australian
Health Commission, Adelaide, Australia
Dr J.N. Fisher, ALCOA of Australia Ltd, Point Henry Works, Geelong,
Victoria, Australia
Mr D. Hughes, Environment, Mount Isa Mine Holdings, Brisbane,
Australia
Dr P. Imray, Environmental Health Branch, Queensland Health, Brisbane,
Australia
Ms M.E. Meek, Environmental Health Directorate, Health Canada,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr N. Priest, AEA Technology, Harwell, Didcot, Oxfordshire, United
Kingdom
Dr D. Wilcox, Medical Section, Health Services, Sydney Water, Sydney,
Australia
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety
Inter-regional Research Unit, World Health Organization, Research
Triangle Park, North Carolina, USA ( Secretary)
Dr D. Johns, DPIE, Coal and Mineral Division, Canberra, Australia
( Temporary Adviser)
Mr D. Wagner, Chemicals Safety Unit, Human Services and Health,
Canberra, Australia ( Temporary Adviser)
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
A WHO Task Group on Environmental Health Criteria for Aluminium
met in Brisbane, Australia, from 24 to 28 April 1995. The meeting
was sponsored by a consortium of Australian Commonwealth and State
Governments through a national steering committee chaired by
Dr K. Bentley, Director, Health and Environmental Policy, Department
of Health and Family Services, Canberra. The meeting was hosted and
organized by the NHMRC National Research Centre for Environmental
Toxicology (NRCET), Dr M. Moore, Director, being responsible for
the arrangements. Dr D. Lange, Chief Health Officer, welcomed
participants on behalf of Queensland Health, and Professor L. Roy
Webb, Vice-chancellor, Griffith University, welcomed them on behalf of
NRCET. Dr G.C. Becking, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS and the three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft criteria monograph and made an evaluation of the risks to human
health and the environment from exposure to aluminium.
The first draft was prepared under the coordination of Dr G.
Rosner, Fraunhofer Institute of Toxicology and Aerosol Research,
Germany, and Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood,
United Kingdom. The draft reviewed by the Task Group, incorporating
the comments received following review by the IPCS Contact Points, was
prepared through the cooperative effort of the Fraunhofer Institute,
Institute of Terrestrial Ecology and the Secretariat.
Dr G.C. Becking (IPCS Central Unit, Inter-regional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively, of
this monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
ABBREVIATIONS
AD Alzheimer's disease
AIBD aluminium-induced bone disease
cAMP cyclic adenosine monophosphate
CI confidence interval
1,25-(OH)2-D3 1,25-dihydroxy-vitamin D3
DOC dissolved organic carbon
EDTA ethylenediaminetetraacetic acid
i.p. intraperitoneal
i.v. intravenous
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
LTP long-term potentiation
NFT neurofibrillary tangle
NIOSH National Institute for Occupational Safety and
Health (USA)
NOEC no-observed-effect concentration
NOEL no-observed-effect level
NTA nitrilotriacetic acid
OR odds ratio
PHF paired helical filaments
Pt platinum unit (1 unit equals the colour produced by
lung chloroplatinate in 1 litre of water)
PTH parathyroid hormone
s.c. subcutaneous
WAIS Weschler Adult Intelligence Scale
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties
Aluminium is a silvery-white, ductile and malleable metal. It
belongs to group IIIA of the Periodic Table, and in compounds it is
usually found as AlIII. It forms about 8% of the earth's crust and is
one of the most reactive of the common metals. Exposure to water,
oxygen or other oxidants leads to the formation of a superficial
coating of aluminium oxide, which provides the metal with a high
resistance to corrosion. Aluminium oxide is soluble in mineral acids
and strong alkalis but insoluble in water, whereas aluminium chloride,
nitrate and sulfate are water soluble. Aluminium halogenides, hydride
and lower aluminium alkyls react violently with water.
Aluminium possesses high electrical and thermal conductivity, low
density and great resistance to corrosion. It is often alloyed with
other metals. Aluminium alloys are light, strong and readily machined
into shapes.
1.2 Analytical methods
Various analytical methods have been developed to determine
aluminium in biological and environmental samples. Graphite furnace
- atomic-absorption spectrometry (GF-AAS) and inductively coupled
plasma - atomic-emission spectrometry (ICP-AES) are the most
frequently used methods. Contamination of the samples with aluminium
from air, vessels or reagents during sampling and preparation is the
main source of analytical error. Depending on sample pretreatment,
separation and concentration procedures, detection limits are
1.9-4 �g/litre in biological fluids and 0.005-0.5 �g/g dry weight in
tissues using GF-AAS, and 5 �g/m3 in air and 3 �g/litre in water
using ICP-AES.
1.3 Sources of human and environmental exposure
Aluminium is released to the environment both by natural
processes and from anthropogenic sources. It is highly concentrated in
soil-derived dusts from such activities as mining and agriculture, and
in particulate matter from coal combustion. Aluminium silicates
(clays), a major component of soils, contribute to the aluminium
levels of dust. Natural processes far outweigh direct anthropogenic
contributions to the environment. Mobilization of aluminium through
human actions is mostly indirect and occurs as a result of emission of
acidifying substances. In general, decreasing pH results in an
increase in mobility and bioavailability for monomeric forms of
aluminium. The most important raw material for the production of
aluminium is bauxite, which contains up to 55% alumina (aluminium
oxide). World bauxite production was 106 million tonnes in 1992.
Aluminium metal has a vide variety of uses, including structural
materials in construction, automobiles and aircraft, and the
production of metal alloys. Aluminium compounds and materials also
have a wide variety of uses, including production of glass, ceramics,
rubber, wood preservatives, pharmaceuticals and waterproofing
textiles. Natural aluminium minerals, especially bentonite and
zeolite, are used in water purification, sugar refining, brewing and
paper industries.
1.4 Environmental transport, distribution and transformation
Aluminium occurs ubiquitously in the environment in the form of
silicates, oxides and hydroxides, combined with other elements such as
sodium and fluorine and as complexes with organic matter. It is not
found as a free metal because of its reactivity. It has only one
oxidation state (+3) in nature; therefore, its transport and
distribution in the environment depend only upon its coordination
chemistry and the chemical-physical characteristics of the local
environmental system. At pH values greater than 5.5, naturally
occurring aluminium compounds exist predominantly in an undissolved
form such as gibbsite (Al(OH)3) or as aluminosilicates, except in the
presence of high amounts of dissolved organic material, which binds
with aluminium and can lead to increased concentrations of dissolved
aluminium in streams and lakes. Several factors influence aluminium
mobility and subsequent transport within the environment. These
include chemical speciation, hydrological flow paths, soil-water
interactions, and the composition of the underlying geological
materials. The solubility of aluminium in equilibrium with solid phase
Al(OH)3 is highly dependent on pH and on complexing agents such as
fluoride, silicate, phosphate and organic matter. The chemistry of
inorganic aluminium in acid soil and stream water can be considered in
terms of mineral solubility, ion exchange and water mixing processes.
Upon acidification of soils, aluminium can be released into
solution for transport to streams. Mobilization of aluminium by acid
precipitation results in more aluminium being available for plant
uptake.
1.5 Environmental levels and human exposure
Aluminium is a major constituent of a number of atmospheric
components particularly in soil-derived dusts (both from natural
sources and human activity) and particulates from coal combustion. In
urban areas aluminium levels in street dust range from 3.7 to
11.6 �g/kg. Airborne aluminium levels vary from 0.5 ng/m3 over
Antarctica to more than 1000 ng/m3 in industrialized areas.
Surface freshwater and soil water aluminium concentrations can
vary substantially, being dependent on physico-chemical and geological
factors. Aluminium can be suspended or dissolved. It can be bound with
organic or inorganic ligands, or it can exist as a free aluminium ion.
In natural waters aluminium exists in both monomeric and polymeric
forms. Aluminium speciation is determined by pH and the concentrations
of dissolved organic carbon (DOC), fluoride, sulfate, phosphate and
suspended particulates. Dissolved aluminium concentrations for water
in the circumneutral pH range are usually quite low, ranging from
1.0 to 50 �g/litre. This rises to 500-1000 �g/litre in more acidic
water. At the extreme acidity of water affected by acid mine drainage,
dissolved aluminium concentrations of up to 90 mg/litre have been
measured.
Non-occupational human exposure to aluminium in the environment
is primarily through ingestion of food and water. Of these, food is
the principal contributor. The daily intake of aluminium from food and
beverages in adults ranges between 2.5 and 13 mg. This is between 90
and 95% of total intake. Drinking-water may contribute around 0.4 mg
daily at present international guideline values, but is more likely
to be around 0.2 mg/day. Pulmonary exposure may contribute up to
0.04 mg/day. In some circumstances, such as occupational exposure and
antacid use, the levels of exposure will be much greater. For example,
> 500 mg of aluminium may be consumed in two average-sized antacid
tablets. There are some difficulties in assessing uptake from these
exposures because of analytical and sampling difficulties. Isotopic
investigations with Al26 indicate that one of the most bioavailable
forms of aluminium is the citrate and that there could be as much as
1% absorption when aluminium is in this form. However, humans would
absorb only 3% of their total daily uptake of aluminium from drinking-
water, a relatively minor source compared to food.
1.6 Kinetics and metabolism
1.6.1 Humans
Aluminium and its compounds appear to be poorly absorbed in
humans, although the rate and extent of absorption have not been
adequately studied. Concentrations of aluminium in blood and urine
have been used as a readily available measure of aluminium uptake,
increased urine levels having been observed among aluminium welders
and aluminium flake-powder producers.
The mechanism of gastrointestinal absorption of aluminium has not
yet been fully elucidated. Variability results from the chemical
properties of the element and the formation of various chemical
species, which is dependent upon the pH, ionic strength, presence of
competing elements (silicon), and the presence of complexing agents
within the gastrointestinal tract (e.g., citrate).
The biological behaviour and gastrointestinal absorption of
aluminium in humans ingesting aluminium compounds has been studied by
using the radioactive isotope Al26. Significant intersubject
variability has been demonstrated. Measured fractional uptakes of 5 �
10-3 for aluminium as citrate, 1.04 � 10-4 for aluminium hydroxide
and 1.36 � 10-3 for the hydroxide given with citrate were reported. A
study of the fractional uptake of aluminium from drinking-water showed
an uptake fraction of 2.35 � 10-3. It was concluded that members of
the general population consuming 1.5 litres/day of drinking-water
containing 100 �g aluminium/litre would absorb about 3% of their total
daily intake of aluminium from this source depending upon the levels
found in food and the frequency of antacid use.
The proportion of plasma Al3+ normally bound to protein in
humans may be as high as 70-90% in haemodialysis patients with
moderately increased plasma aluminium. The highest levels of aluminium
may be found in the lungs, where it may be present as inhaled
insoluble particles.
The urine is the most important route of aluminium excretion.
After peroral administration of a single dose of aluminium, 83% was
excreted in urine after 13 days and 1.8% in the faeces. The half-life
of urinary concentration among welders exposed for more than 10 years
was 6 months or longer. Among retired workers exposed to aluminium
flake powders, the calculated half-lives were between 0.7 and 8 years.
1.6.2 Animals
Absorption via the gastrointestinal tract is usually less than
1%. The main factors influencing absorption are solubility, pH and
chemical species. Organic complexing compounds, notably citrate,
increase absorption. The aluminium absorption may interact with
calcium and iron transport systems. Dermal and inhalation absorption
has not been studied in detail. Aluminium is distributed in most
organs within the body with accumulation occurring mainly in bone at
high dose levels. To a limited but as yet undetermined extent,
aluminium passes the blood-brain barrier and is also distributed to
the fetus. Aluminium is eliminated effectively by urine. Plasma half-
life is about 1 h in rodents.
1.7 Effects on laboratory mammals and in vitro test systems
The acute toxicity of metallic aluminium and aluminium compounds
is low, the reported oral LD50 values being in the range of several
hundred to 1000 mg aluminium/kg body weight per day. However, the
LC50 values for inhalation have not been identified.
In short-term studies in which an adequate range of end-points
was examined following exposure of rats, mice or dogs to various
aluminium compounds (sodium aluminium phosphate, aluminium hydroxide,
aluminium nitrate) in the diet or drinking-water, only minimal effects
(decreases in body weight gain generally associated with decreases in
food consumption or mild histopathological effects) have been observed
at the highest administered doses (70 to 300 mg aluminium/kg body
weight per day). Systemic effects following parenteral administration
also included kidney dysfunction.
Adequate inhalation studies were not identified. Following
intratracheal administration of aluminium oxide, particle-associated
fibrosis was observed, similar to that found in other studies on
silica and coal dust.
No overt fetotoxicity was noted, nor were general reproductive
parameters noted after gavage treatment of rats with 13, 26 or 52 mg
aluminium/kg body weight per day (as aluminium nitrate). However, a
dose-dependent delay in the growth of offspring was noted with females
administered 13 mg/kg and in male offspring at 26 mg/kg. The lowest-
observed-adverse-effect level (LOAEL) for developmental effects
(decreased ossification, increased incidence of vertebral and
sternebrae terata and reduced fetal weight) was 13 mg/kg (aluminium
nitrate). These effects were not observed at much higher doses of
aluminium hydroxide. There were reductions in postnatal growth at
13 mg/kg (aluminium nitrate), although maternal toxicity was not
examined. In studies on brain development, grip strength was impaired
in offspring of dams fed 100 mg aluminium/kg body weight as aluminium
lactate in the diet, in the absence of maternal toxicity.
There is no indication that aluminium is carcinogenic. It can
form complexes with DNA and cross-link chromosomal proteins and DNA,
but it has not been shown to be mutagenic in bacteria or induce
mutation or transformation in mammalian cells in vitro. Chromosomal
aberrations have been observed in bone marrow cells of exposed mice
and rats.
There is considerable evidence that aluminium is neurotoxic in
experimental animals, although there is considerable variation among
species. In susceptible species, toxicity following parenteral
administration is characterized by progressive neurological
impairment, resulting in death with status epilepticus (LD50 =
6 �g Al/g dry weight of brain). Morphologically, the progressive
encephalopathy is associated with neurofibrillary pathology in
large and medium size neurons predominantly in the spinal cord,
brainstem and selected areas of the hippocampus. These tangles are
morphologically and biochemically different from those that occur in
Alzheimer's disease (AD). Behavioural impairment has been observed in
the absence of overt encephalopathy or neurohistopathology in
experimental animals exposed to soluble aluminium salts (e.g.,
lactate, chloride) in the diet or drinking-water at doses of 50 mg
aluminium/kg body weight per day or more.
Osteomalacia, as it presents in man, is observed consistently in
larger species (e.g., dogs and pigs) exposed to aluminium; a similar
condition is observed in rodents. These effects appear to occur in all
species, including humans, at aluminium levels of 100 to 200 �g/g bone
ash.
1.8 Effects on humans
No acute pathogenic effects in the general population have been
described after exposure to aluminium.
In England, a population of about 20 000 individuals was exposed
for at least 5 days to increased levels of aluminium sulfate,
accidentally placed in a drinking-water facility. Case reports of
nausea, vomiting, diarrhoea, mouth ulcers, skin ulcers, skin rashes
and arthritic pain were noted. It was concluded that the symptoms were
mostly mild and short-lived. No lasting effects on health could be
attributed to the known exposures from aluminium in the drinking-
water.
It has been hypothesized that aluminium in the drinking-water is
a risk factor for the development or acceleration of AD as well as for
impaired cognitive function in the elderly. It has also been suggested
that stamped fine aluminium powder and fume may be risk factors for
impaired cognitive function and pulmonary disease in certain
occupations.
Some 20 epidemiological studies have been carried out to test the
hypothesis that aluminium in drinking-water is a risk factor for AD,
and two studies have evaluated the association between aluminiun in
drinking-water and impaired cognitive function. Study designs ranged
from ecological to case control. Eight studies in populations in
Norway, Canada, France, Switzerland and England were considered
of sufficiently high quality to meet the general criteria for
exposure and outcome assessment and the adjustment for at least
some confounding variables. Of the six studies that examined the
relationship between aluminium in drinking-water and dementia or AD,
three found a positive relationship but three did not. However, each
of the studies had some deficiencies in the study design (e.g.,
ecological exposure assessment, failure to consider aluminium exposure
from all sources and to control for important confounders such as
education, socioeconomic status and family history, the use of
surrogate outcome measures for AD, and selection bias). In general,
the relative rists determined were less than 2, with large confidence
intervals, when the total aluminium concentration in drinking-water
was 100 �g/litre or higher. Based on current knowledge on the
pathogenesis of AD and the totality of evidence from these
epidemiological studies, it was concluded that the present
epidemiological evidence does not support a causal association between
AD and aluminium in drinking-water.
In addition to the epidemiological studies that examined the
relationship between AD and aluminium in drinking-water, two studies
examined cognitive dysfunction and AD in elderly populations in
relation to the levels of aluminium in drinking-water. The results
were again conflicting. One study of 800 male octogenarians consuming
drinking-water with aluminium concentrations up to 98 �g/litre found
no relationship. The second study used "any evidence of mental
impairment" as an outcome measure and found a relative risk of 1.72 at
aluminium concentrations greater than 85 �g/litre in 250 males. Such
data are insufficient to show that aluminium is a cause of cognitive
impairment in the elderly.
Reports of impaired cognitive function related to aluminium
exposure are conflicting. Most studies are on small populations, and
the methodology used in these studies is open to question with respect
to magnitude of effect reported, exposure assessment and confounding
factors. In a comparative study of cognitive impairment in miners
exposed to a powder containing 85% finely ground aluminium and 15%
aluminium oxide (as prophylaxis against silica) and unexposed miners,
the cognitive test scores and the proportion impaired in at least one
test indicated a disadvantage for the exposed miners. A positive
exposure-related trend of increased risk was noted.
In all occupational studies reported, the magnitude of effects
found, presence of confounding factors, problems with exposure
assessment and the probability of mixed exposures all make the data
insufficient to conclude that aluminium is a cause of cognitive
impairment in workers exposed occupationally to aluminium.
Neurological syndromes including impairment of cognitive
function, motor dysfunction and peripheral neuropathy have been
reported in limited studies of workers exposed to aluminium fume. A
small population of aluminium welders who were compared with iron
welders were reported to show a small decrement in repetitive motor
function. When a questionnaire methodology was used in another study,
an increase in neuropsychiatric symptoms was reported.
Iatrogenic exposure in patients with chronic renal failure,
exposed to aluminium-containing dialysis fluids and pharmaceutical
products, may cause encephalopathy, vitamin-D-resistant osteomalacia
and microcytic anaemia. These clinical syndromes can be prevented by
reduction in exposure to aluminium.
Premature infants, even where kidney impairment is not severe
enough to cause raised blood creatinine levels, may develop increased
tissue loading of aluminium, particularly in bone, when exposed to
iatrogenic sources of aluminium. Where there is kidney failure,
seizures and encephalopathy may occur.
Although human exposure to aluminium is widespread, in only a few
cases has hypersensitivity been reported following exposure to some
aluminium compounds after dermal application or parenteral
administration.
Pulmonary fibrosis was reported in some workers exposed to very
fine stamped aluminium powder in the manufacture of explosives and
fireworks. Nearly all cases involved exposure to aluminium particles
coated with mineral oil. That process is no longer used. Other cases
of pulmonary fibrosis have related to mineral exposures to other
agents such as silica and asbestos and cannot be attributed solely to
aluminium.
Irritant-induced asthma has been associated with inhalation
of aluminium sulfate, aluminium fluoride, potassium aluminium
tetrafluoride and with the complex environment of the potrooms during
aluminium production.
There is insufficient information to allow for classification of
the cancer risk from human exposures to aluminium and its compounds.
Animal studies do not indicate that aluminium or aluminium compounds
are carcinogenic.
1.9 Effects on other organisms in the laboratory and field
Aquatic unicellular algae showed increased toxic effect at low
pH, where bioavailability of aluminium is increased. They are more
sensitive than other microorganisms, the majority of 19 lake species
showing complete growth inhibition at 200 �g/litre total aluminium
(pH 5.5). Selection of aluminium-tolerant strains is possible; green
algae capable of growing in the presence of 48 mg/litre at pH 4.6 have
been isolated.
For aquatic invertebrates, LC50 values range from 0.48 mg/litre
(polychaete) to 59.6 mg/litre (daphnid). For fish, 96-h LC50 values
range from 0.095 mg/litre (American flagfish) to 235 mg/litre
(mosquito fish). However, care must be taken when interpreting the
results because of the significant effects of pH on the availability
of aluminium. The wide range of LC50 values probably reflects
variable availability. The addition of chelating agents, such as NTA
and EDTA, reduces the acute toxicity of aluminium to fish.
Responses to aluminium by macroinvertebrates are variable. In the
normal pH range aluminium toxicity increases with decreasing pH;
however, in very acidic waters aluminium can reduce the effects of
acid stress. Some invertebrates are very resistant to acid stress and
can be very numerous in acidic waters. Increased drift rate of
invertebrates has been reported in streams suffering either pH or
pH/aluminium stress; this is a common response to a variety of
stressors. Lake invertebrates generally survived field exposure to
aluminium but suffered as a result of phosphate reduction in
oligotrophic conditions induced by precipitation with aluminium.
Short- and long-term toxicity tests on fish have been carried out
under a variety of conditions and, most importantly, at a range of pH
values. The data show that significant effects have been observed at
monomeric inorganic aluminium levels as low as 25 �g/litre. However,
the complex relationship between acidity and aluminium bioavailability
makes interpretation of the toxicity data more difficult. At very
low pH (not normally found in natural waters) the hydrogen ion
concentration appears to be the toxic factor, with the addition of
aluminium tending to reduce toxicity. In the pH range 4.5 to 6.0
aluminium in equilibrium exerts its maximum toxic effect. Toxicity has
also been shown to increase with increasing pH levels in the alkaline
pH region. The mechanism of aluminium toxicity to fish has been
attributed to the inability of fish to maintain their osmoregulatory
balance, as well as respiratory problems associated with precipitation
of aluminium on the gill mucus. The former effect is associated with
lower pH levels. These laboratory findings have been confirmed by
field studies especially in areas under acid stress.
Amphibian eggs and larvae are affected by acidity and aluminium,
with interaction between the two factors. Reduced hatching, delayed
hatching, delayed metamorphosis, metamorphosis at small size, and
mortality have been reported in various species and at aluminium
concentrations below 1 mg/litre.
Exposure of roots of terrestrial plants to aluminium can cause
diminished root growth, reduced uptake of plant nutrients and stunted
plant development. Tolerance to aluminium has been demonstrated both
in the laboratory and the field.
1.10 Conclusions
1.10.1 General population
Hazards to neurological development and brain function from
exposure to aluminium have been identified through animal studies.
However, aluminium has not been demonstrated to pose a health risk to
healthy, non-occupationally exposed humans.
There is no evidence to support a primary causative role of
aluminium in Alzheimer's disease (AD), and aluminium does not induce
AD pathology in vivo in any species, including humans.
The hypothesis that exposure of the elderly population in some
regions to elevated levels of aluminium in drinking-water may
exacerbate or accelerate AD lacks adequate supporting data.
The data in support of the hypothesis that particular exposures,
either occupational or via drinking-water, may be associated with non-
specific impaired cognitive function are also inadequate.
There is insufficient health-related evidence to justify
revisions to existing WHO Guidelines for aluminium exposure in
healthy, non-occupationally exposed humans. As an example, there is an
inadequate scientific basis for setting a health-based standard for
aluminium in drinking-water.
1.10.2 Subpopulations at special risk
In people of all ages with impaired renal function, aluminium
accumulation has been shown to cause the clinical syndrome of
encephalopathy, vitamin-D-resistant osteomalacia and microcytic
anaemia. The sources of aluminium are haemodialysis fluid and
aluminium-containing pharmaceutical agents (e.g., phosphate binders).
Intestinal absorption can be exacerbated by the use of citrate-
containing products. Patients with renal failure are thus at risk of
neurotoxicity from aluminium.
Iatrogenic aluminium exposure poses a hazard to patients with
chronic renal failure. Premature infants have higher body burdens of
aluminium than other infants. Every effort should be made to limit
such exposure in these groups.
1.10.3 Occupationally exposed populations
Workers having long-term, high-level exposure to fine aluminium
particulates may be at increased risk of adverse health effects.
However, there are insufficient data from which to develop with any
degree of certainty occupational exposure limits with regards to the
adverse effects of aluminium.
Exposure to stamped pyrotechnic aluminium powder most often
coated with mineral oil lubricants has caused pulmonary fibrosis
(aluminosis), whereas exposure to other forms of aluminium has not
been proven to cause pulmonary fibrosis. Most reported cases had
exposure to other potentially fibrogenic agents.
Irritant-induced asthma has been associated with inhalation of
aluminium sulfate, aluminium fluoride or potassium aluminium
tetrafluoride, and with the complex environment within the potrooms
during aluminium production.
1.10.4 Environmental effects
Aluminium-bearing solid phases in the environment are relatively
insoluble, particularly at circumneutral pH values, resulting in low
concentrations of dissolved aluminium in most natural water.
In acidic or poorly buffered environments subjected to strong
acidifying inputs, concentrations of aluminium can increase to levels
resulting in adverse effects on both aquatic organisms and terrestrial
plants. However, there exist large species, strain and life history
stage differences in sensitivity to this metal.
The detrimental biological effects from elevated concentrations
of inorganic monomeric aluminium can be mitigated in the presence of
organic acids, fluorides, silicate and high levels of calcium and
magnesium.
There is a substantial reduction in species richness associated
with the mobilization of the more toxic forms of aluminium in acid-
stressed waters. This loss of species diversity is observed at all
trophic levels.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
The element aluminium (Al) was first obtained in an impure form
by Oersted in 1825, and pure aluminium was prepared by Woehler two
years later. The name aluminium is derived from alum, which the
ancient Greeks used as an astringent in medicine (Lide, 1991).
Aluminium is the most abundant metallic element and constitutes
8.13% of the earth's crust. Owing to its high reactivity, it is always
found combined with other elements and does not occur in its pure
state. Combined with oxygen, silicon, the alkali and alkaline-earth
metals, and fluorine, and as hydroxides, sulfates and phosphates,
aluminium appears in a wide variety of minerals (Frank et al., 1985;
Hudson et al., 1985; Lide, 1991).
Some aluminium compounds, synonyms and molecular formulae are
listed in Table 1. The most abundant natural aluminium ores are shown
in Table 2.
2.1 Identity
Pure aluminium is a silvery-white, malleable, ductile metal with
the atomic number of 13 and the relative atomic mass of 26.98. With
few exceptions aluminium is found in chemical compounds as AlIII.
Aluminium occurs naturally as 27Al; eight radioactive isotopes are
known, of which 26Al is the most stable with a half-life of 7.4 �
105 years (Frank et al., 1985).
2.2 Physical and chemical properties
2.2.1 Aluminium metal
Elemental aluminium possesses many desirable characteristics and
is therefore widely used in commerce (Sax & Lewis, 1987; Lide, 1991).
Aluminium crystallizes in a face-centered cubic lattice that is stable
from 4 K to melting point; the coordination number is 12, it is light
and malleable, and thus is easily formed into a variety of shapes
(Frank et al., 1985).
Owing to the high charge/radius ratio of Al3+ in aqueous
solutions, the ion proteolyses part of the water envelope and forms
hydroxo complexes. It can also complex with electron-rich species,
such as fluoride and chloride. The chemical properties of aluminium
resemble those of beryllium and silicon. Because of its amphoteric
character, it reacts with mineral acids and strong alkalis (Sax &
Lewis, 1987). Although aluminium is one of the most reactive of the
common metals used commercially, it has excellent resistance to
Table 1. Chemical names, synonyms and molecular formulae of elemental aluminium and aluminium compoundsa
Chemical name CAS registry number Synonyms Formula
Aluminium 7429-90-5 Aluminium, metana Al
Aluminium chloride 7446-70-0 Aluminium trichloride AlCl3
Aluminium chlorohydrate 1327-41-9 Aluminium chlorohydroxide, AlCl(OH)5
11097-68-0 aluminium chloride, basic,
84861-98-3 chlorhydrol, polyaluminium chlorideb Al2Cl(OH)52H2O
Aluminium fluoride 7784-18-1 Aluminium trifluoride AlF3
Aluminium lactate 18917-91-4 Aluctyl Al(C3H5O3)3
Aluminium oxidec 1302-74-5 alpha-Alumina, corundum Al2O3
Aluminium oxide hydroxidec 14457-84-2 Diaspore alpha-AlO(OH) or alpha-Al2O3H2O
Aluminium oxide hydroxidec 1318-23-6 Boehmite gamma-AlO(OH) or gamma-Al2O3H2O
Aluminium oxide, trihydratec 20257-20-9 Bayerite, alpha-aluminium trihydroxide alpha-Al(OH)3 or alpha-Al2O33H2O
Aluminium oxide, trihydratec 13840-05-6 Nordstrandite, �-aluminium trihydroxide �-Al(OH)3 or �-Al2O33H2O
Aluminium oxide, trihydratec 14762-49-3 Gibbsite, hydrargillite, gamma-aluminium gamma-Al(OH)3 or gamma-Al2O33H2O
trihydroxide
Nitric acid, aluminium salt 13473-90-0 Aluminium trinitrate, aluminium nitrate Al(NO3)3
Table 1. (Con't)
Chemical name CAS registry number Synonyms Formula
Phosphoric acid,
aluminium salt 7784-30-7 Aluminium orthophosphate AlPO4
Sodium aluminate 1302-42-7 NaAlO2, Na2OAl2O3 or
Na2Al2O4
Sulfuric acid, aluminium salt 10043-01-3 Alum, aluminium trisulfate, cake alum Al2(SO4)3
Trimethylaluminiumb 75-24-1 Al(CH3)3
2-Propanol, aluminium saltb 555-31-7 Aluminium isopropoxide, aluminium Al(OCH(CH3)2)3
isopropylate
2-Butanol, aluminium saltb 2269-22-9 Aluminium sec-butoxide, aluminium Al(OC4H9)3
butylate
a adapted from ATSDR (1992)
b Zietz (1985)
c Hudson et al. (1985)
Table 2. CAS chemical names and registry numbers, synonyms, trade names, content and molecular formula of aluminium oresa
Chemical name CAS registry Synonyms and trade Composition Formula
number names
Aluminium magnesium - Magnesium aluminium 48.8% O MgAl2(SiO4)2
silicate silicate 21.4% Si
20.6% Al
9.3% Mg
Aluminium silicate, hydrate - Kaolinite 40% Al2O3b Al2Si2O5(OH)4 or
46% SiO2 Al2O3SiO2H2O
14% H2O
Aluminium silicofluoride - Topaz 71.2% F 2Al2O32Al(F,OH)33SiO2
17.6% Si
11.2% Al
Ammonium aluminium 7784-26-1 Ammonium alum, - NH4Al(SO4)212H2O or
sulfate, hydrate ammonium Al2O3(NH4)2O24HOH
aluminium sulfate
Bauxite 1318-16-7 - 30-75% Al2O3 -
3-25% Fe2O3
9-31% H2O
2-9% SiO2
1-3% TiO2
Table 2. (Con't)
Chemical name CAS registry Synonyms and trade Composition Formula
number names
Potassium aluminium 7784-24-9 Potash alum, potassium 37% Al2O3 K(AlO)3(SO4)212H2O or
sulfate, hydrate aluminium sulfate 11% K2O Al2(SO4)3K2SO424HOH
39% SO3
13% H2O
Sodium aluminium 15096-52-3 Cryolite, greenland - Na3AlF6 or 3NaFAlF3
fluoride spar, isestone
Sodium aluminium 7784-28-3 Sodium alum, sodium - NaAl(SO4)212H2O or
sulfate, hydrate aluminium sulfate Al2(SO4)2Na2SO424HOH
Sodium calcium - Anorthosite, soda-lime 26-35% Al2O3b Na2OAl2O36SiO2 &
silicoaluminate feldspar 46-59% SiO2 CaOAl2O32SiO2
8-18% CaO
1-7% Na2O
a From: Sax & Lewis (1987)
b US Bureau of Mines (1967)
corrosion. Exposed to oxygen, water or other oxidants, a continuous
film of aluminium oxide (Al2O3) grows rapidly on the nascent
aluminium surface, providing the metal with a high resistance to
corrosion. The oxide film dissolves in alkaline solutions with
evolution of hydrogen and formation of soluble alkali-metal aluminates
(Sax & Lewis, 1987).
The oxide film on the solid metal is resistant to some acids
(e.g., nitric acid), and prevents further chemical attack on the
metal. However, the protective oxide film dissolves in some acids
(e.g., hydrochloric or hot sulfuric acids) and also in alkaline
solutions, exposing the metal to further reactions. At elevated
temperatures, aluminium metal reacts with water (above 180�C),
producing Al(OH)3 and H2, and with many metal oxides producing
Al2O3 and the metal. This reaction is used to produce certain
metals, for example, manganese and alloys (e.g., ferro-titanium).
Finely divided aluminium dust can ignite and cause explosions
(Wade & Banister, 1973; Frank et al., 1985).
Many applications of aluminium and its alloys are based upon its
inherent properties of high electrical and thermal conductivity, low
density, and great resistance to corrosion. Pure aluminium is soft and
lacks strength, but it can be alloyed with small amounts of Cu, Mg,
Si, Mn and other elements to impart greater strength and a variety of
other useful properties. Aluminium alloys are light, strong and
readily worked into a variety of shapes (Frank et al., 1985; Lide,
1991).
2.2.2 Aluminium compounds
The aluminium compounds of the greatest industrial importance are
aluminium oxide, aluminium sulfate and aluminium silicate. Some
physical and chemical data of aluminium and selected aluminium
compounds are summarized in Table 3.
Aluminium oxide is a white powder that is found as balls or
lump of various mesh sizes. Owing to its amphoteric character, it is
soluble in mineral acids and strong alkali. Aluminium oxide is
found in different modifications. The hexagonally closest-packed
alpha-modification "corundum" (alpha-Al2O3) is the most stable
oxide. Emery is an abrasive containing corundum, and ruby and sapphire
are impure crystalline varieties of gem quality (Hudson et al., 1985).
Formation of aluminium oxide by dehydration of the hydroxides produces
a series of alumina types still containing a small proportion of
hydroxyl groups and retaining some chemical reactivity. All oxides
produced at low temperatures are collectively referred to as
transitional oxides. Those formed by dehydration below 600�C are known
Table 3. Physical and chemical properties of aluminium and some of its compoundsa
Chemical name Relative atomic/ Melting Boiling Relative density Crystalline Solubilityd
molecular mass point (�C) point (�C) (g/cm3)b form
Aluminium 26.98 660 2450c 2.708 silver-white cubic sol alkali, HCl, H2SO4;
insol H2O, HNO3e
alpha-Aluminium 77.99 300 (-H2O) 2.420 monoclinic, powder sol acid; insol H2O,
hydroxide (bayerite) alcohole
Aluminium nitrate 213.00 74 135 - rhombic delinq. sol H2O, alkali,
(decomposes) acetone, HNO3
Aluminium oxide 101.94 2072 2980 3.965 (25) hexagonal very sl sol benzene;
insol H2O
gamma-Aluminium oxide 59.99 - - 3.440 orthorhomic sol acid; sl sol
hydroxide (boehmite) alkali; insol H2O,
alcohole
Aluminium phosphate 121.95 1500 - 2.566 rhombic platelets sol acid, alkali; insol H2O
Aluminium sulfate, 342.14 700 - 2.710 powder sol H2O, dil acid; sl
anhydrate (decomposes) sol alkali
Aluminium sulfate, 666.41 87 - 1.690 (17) monoclinic sol H2O, dil. acid; sl
hydrate (decomposes) sol alkali
Aluminium 204.25 119 141 1.035 (20) crystals sol alcohol, benzene,
isopropoxidee chloroform
a Compiled from ATSDR (1992)
b Temperature is given in parentheses
c Sax & Lewis (1987)
d Sol = soluble; insol = insoluble; sl = slightly
e Lide (1991)
as gamma-aluminas or activated aluminas, while the aluminas formed by
dehydration at higher temperatures (900-1000�C), the rho-aluminas, are
nearly anhydrous Al2O3 (Wade & Banister, 1973). At 1400�C all
transitional alumina converts to alpha-alumina (Hudson et al., 1985).
The structural and compositional differences among various forms of
alumina are associated with differing particulate size, particulate
surface area, surface reactivity and catalytic activity.
Various forms of aluminium hydroxides are known. The best defined
forms are the trihydroxides (Al(OH)3) and the oxide-hydroxides
(AlO(OH)). Besides these well-defined crystalline forms, several other
hydroxides have been described in the literature (Wefers & Bell,
1972). The aluminium hydroxides found abundantly in nature are
gibbsite (Al(OH)3), diaspore �-(AlO(OH)), and boehmite
alpha-(AlO(OH)). They all convert to aluminium oxide when heated
(Hudson et al., 1985).
Aluminium sulfate can exist with varying proportions of water,
the common form being Al2(SO4)3�18H2O. It is almost insoluble
in anhydrous alcohol, but readily soluble in water. Above 770�C
decomposition to aluminium oxide is observed. Aluminium sulfate is
mainly used in water treatment, dyeing, leather tanning and in the
production of other aluminium compounds. Alums are crystalline double
salts composed of aluminium, sulfate and a monovalent cation, such
as potassium, sodium or ammonium, and have the general formula
M+Al3+(SO4)2�12H2O. In aqueous solution, alums show all the
chemical properties that their components show separately (Helmboldt
et al., 1985).
Clays are aluminium silicates. They have cation-exchange capacity
and the amounts and types of clay minerals in a soil largely determine
its physical properties and suitability for agriculture (Wild, 1988).
Aluminium halogenides, hydrides and lower aluminium alkyls react
violently with molecular oxygen, and are spontaneously inflammable in
air and explosive with water. Industrially these compounds are used as
co-catalysts for organometallic and organic synthesis, and as
intermediates in various production processes (Stokinger, 1987;
Budavari, 1989).
Further compounds of industrial interest are aluminium antimonide
(AlSb) and selenide (AlSe), which are employed in the semiconductor
technology industry (Budavari, 1989). Aluminium phosphide (AlP) is
used as a rodenticide and pesticide, but it is not discussed in this
monograph since its biocidal activity is due to the phosphide moiety
and not to the aluminium.
2.3 Analytical methods
Various methods for sampling, sample preparation and
determination of aluminium in biological and environmental samples
have been developed and described. An overview of standard methods is
given in Table 4.
2.3.1 Sampling and sample preparation
Because of the ubiquitous distribution of aluminium in nature,
care must be taken during sampling and sample preparation to avoid
contamination. Most analytical errors are due to contamination of the
sample with aluminium from air, vessels and reagents during sampling
and preparation for analysis. To prevent aluminium contamination, the
use of aluminium-free polyethylene, polypropylene, teflon or quartz
materials is recommended. Containers and laboratory materials have to
be washed with warm, dilute nitric acid and subsequently rinsed with
de-ionized water prior to use (Andersen, 1987).
Air is sampled with high volume samplers using low-ash cellulose
or cellulose ester filters for particulate aluminium (NIOSH, 1984).
Biological samples need to be preserved by cooling, freezing or
lyophilization. Preservation with 10% formalin is not recommended
because of a high risk of aluminium contamination (Bouman et al.,
1986).
Homogeneity of the samples is an absolute prerequisite for
accurate analysis. To prepare samples for analysis, inorganic samples
are usually dissolved in nitric acid or extracted with water.
Solutions are filtered with a membrane filter and the particulate
residue is analysed separately (Dunemann & Schwedt, 1984).
Water (DIN, 1993) and urine should be acidified with HNO3 or HCl
to pH < 2 to prevent adsorption effects and the precipitation of
salts. This ensures that aluminium remains in solution. Water samples
for speciation analysis should be stored, without acidification, in
high-density polyethylene bottles (Berden et al., 1994; Fairman et
al., 1994). Prior to analysis biological tissues must be homogenized
and separated or extracted. Blood and urine samples may be separated
by centrifugation and diluted, or, if appropriate, analysed directly
without pretreatment.
Table 4. Analytical methods for aluminium and aluminium compoundsa
Medium Sample preparation Analytical method Detection limit Recovery Reference
Environmental
samples
Air Sample collection on cellulose FAAS 500 �g/m3 (100-litre n.g. NIOSH (1984)
filter, ashing with HNO3 sample)
Sample collection on cellulose ICP-AES 5 �g/m3 (500-litre n.g. NIOSH (1984)
filter, ashing with HNO3 sample)
Water Reaction with sulfonitrazo DAF Spectrophotometry 4 �g/litre n.g. Ermolenko &
Dedkov (1988)
Reaction with Chromazurol S Spectrophotometry 0.0005 �g/0.5 ml- n.g. Schwedt (1989)
sample
Reaction with alizarin S Spectrophotometry 10 �g/litre; 50 �g/litre n.g. DIN (1993)
(after digestion)
Digestion with HNO3 and ICP-AES 100 �g/litre n.g. DIN (1993)
H2O2
Filtration, digestion with HNO3, Spectrophotometry 6-13 �g/litre range 98-100% van Benschoten &
reaction with 8-hydroxyquinoline ICP-AES 3 �g/litre limit detection Edzwald (1990)
Soil Extraction with H2O, filtration, GF-AAS n.g. Gardinier et
high-performance size exclusion al. (1987)
chromatography
Soil Extraction with H2O, filtration, Spectrophotometry 0.005 �g (absolute) n.g. Dunemann &
gel chromatography, reaction Schwedt (1984)
with Chromazurol
Fly ash Vacuum dried NAA n.g. Fleming &
Lindstrom (1987)
Table 4. (Con't)
Medium Sample preparation Analytical method Detection limit Recovery Reference
Rock, soil, Dried, digestion with ICP-AES 1-5 �g/litre > 57% Que Hee &
paint, HNO3/HCl Boyle (1988)
citrus leaves
Biological
samples
Serum Centrifugation, dilution with GF-AAS 14.3-150 �g/litre 97-102% Bettinelli et
Mg(NO3)2 (analytical range) al. (1985)
Plasma, Centrifugation, dilution with GF-AAS 4 �g/litre 90-102% Gardinier et
serum water al. (1981)
Whole blood, Dilution with Triton X-100 GF-AAS 1.9 �g/litre (serum), n.g. van der Voet
plasma, serum 1.8 �g/litre (plasma), et al. (1985)
2.3 �g/litre (blood)
Biological Wet-digestion, complexation NAA 2.1 �g/litre (liver) n.g. Blotcky et
tissue, urine with Tiron, anion-exchange 0.18 �g/ml (urine) al. (1992)
chromatography
Urine, blood Dilution with water ICP-AES 6 �g/litre n.g. Sanz-Medel et
al. (1987)
Dilution with water ICP-AES 0.3 �g/litre n.g. Mauras &
Allain (1985)
Table 4. (Con't)
Medium Sample preparation Analytical method Detection limit Recovery Reference
Biological Freeze dry, grind NAA n.g. Yukawa et
tissues al. (1980)
Dried, digestion with HNO3, GF-AAS 0.5 �g/g dry tissue 80-117% Bouman et
dilution with water al. (1986)
Dried, digestion with HNO3, AMS 106 atoms 26Al n.g. Kobayashi et
cation-exchange al. (1990)
chromatography
Digestion, high-performance Spectrophotometry 7 �g/litre 87-94% Dean (1989)
ion-exchange chromatography,
reaction with Tiron
Hair Washed with 2-propanol, GF-AAS 0.65 �g/g dry weight 84-105% Chappuis et
digestion with HNO3 al. (1988)
Body fluids Dilution with HNO3/HCl ICP-AES 1-5 �g/litre > 57% Que Hee &
Boyle (1988)
Haemodialysis Dilution with HNO3 and GF-AAS 3 �g/litre 93-108% Andersen (1987)
concentrates Triton X-100 (Zeeman-corrected)
Haemodialysis Reaction with ferron in Phosphorimetry 5.4 �g/litre n.g. De La Campa
fluids CTAB et al. (1988)
a AMS = accelerator mass spectrometry; CTAB = cetyltriammonium bromide; EDTA = ethylenediaminetetraacetic acid; FAAS = flame
atomic-absorption spectrophotometry; ferron = 7-iodo-8-quinolinol-5-sulfonic acid; GF-AAS = graphite furnace - atomic-absorption
spectrophotometry; ICP-AES = inductively coupled plasma - atomic-emission spectrophotometry; NAA = neutron activation analysis;
n.g. = not given; Tiron = 4,5-dihydroxy-1,3-benzenedisulfonic acid
Free aluminium may be determined directly from the samples or the
sample extracts. To determine insoluble aluminium compounds and
organically bound species, the samples (organic matter, air-filters,
water, soil, etc.) need to be subjected to wet ashing (digestion) or
dry ashing. Wet ashing, i.e. heating with nitric acid under reflux, is
suitable for most organic and biological samples. The residues are
dissolved in acids before analysis (NIOSH, 1984; Kobayashi et al.,
1990; DIN, 1993). After digestion, differentiation between free metal
species and kinetically labile and stable complexes is not possible.
2.3.2 Separation and concentration
A fractionation procedure for aluminium species in water using an
0.22 �m size filter has been proposed by van Benschoten & Edzwald
(1990). Total reactive aluminium is determined in the unfiltered,
acidified sample. Dissolved monomeric aluminium is analysed in the
unfiltered sample without acidification. Analysis of total dissolved
aluminium is performed after filtration and acidification of the
sample. Dissolved organically bound aluminium is analysed after
separation of the filtered sample on a column of cation exchange
resin. The eluate is acidified and analysed colorimetrically. For the
determination of dissolved organic monomeric aluminium, samples are
passed through a cation exchange column and are analysed with no
acidification.
In order to carry out long-term characterization of the highly
acute toxicity during the initial phase of aluminium polymerization in
"mixing zones" (Rosseland et al., 1992), in situ fractionation
techniques such as ultrafiltration (Lydersen et al., 1987) are
recommended (see section 9.1.2.3).
For the extraction of aluminium bound to fulvic acids, soil
samples may be extracted with copper chloride solution (Gardinier et
al., 1987). The clean-up of aqueous extracts of soil samples can be
performed by gel chromatography (Dunemann & Schwedt, 1984) or by size
exclusion chromatography. These methods are very mild and thus
suitable for the determination of labile aluminium species (Gardinier
et al., 1987).
Water samples may be concentrated by careful evaporation (DIN,
1993). Macro quantities of aluminium can be separated from small
amounts of interfering elements by precipitation of aluminium as
its hydroxide or phosphate. Chelating agents, such as EDTA,
8-hydroxyquinoline, and 2,2'-dihydroxyazobenzene, can be used to
extract aluminium into an organic solvent (Alderman & Gitelman, 1980).
Biological materials contain a variety of compounds that
can severely interfere with aluminium determinations. Hence,
chromatographic methods are often employed for sample purification.
Biological tissue samples may be cleaned-up by cation-exchange
chromatography after acid digestion (Dean, 1989; Kobayashi et al.,
1990). Blotcky et al. (1992) proposed the chelating of aluminium prior
to anion-exchange chromatography. Precolumn derivatization coupled
with reversed-phase high performance liquid chromatography (RP-HPLC)
is an effective method for the separation of the chelates of
different interfering metal ions (Nagaosa et al., 1991). Solvent
extraction of aluminium chelate complexes, e.g., 2,4-pentanedione and
4-methyl-2-pentanone, has been described as a separation and pre-
concentration step in the analysis of body fluids (Buratti et al.,
1984).
2.3.3 Detection and measurement
Spectrophotometric methods for aluminium analysis are simple
and quick, and are most often used for the determination of aluminium
in water. Samples are treated with inorganic or organic reagents to
form coloured soluble complexes that can be measured by absorption
spectrometry. Disadvantages of these methods are the narrowness of
the pH range of the reaction, the instability of the complexes, the
low selectivity, and the low sensitivity (Bettinelli et al., 1985).
The working range for the aluminium determination with chromazurol C
is 25-1000 �g/litre (Schwedt, 1989), with alizarin S it is 10-500
�g/litre (DIN, 1993), and with Tiron it is 7-5000 �g/litre (Dean,
1989). Detection limits of 1 �g/litre can be achieved. Chromatographic
separation of chelates of interfering metals increases the selectivity
of spectrophotometric methods.
De La Campa et al. (1988) and Garc�a et al. (1991) reported a
room temperature phosphorimetric method for aluminium analysis.
Aluminium reacts with 7-iodo-8-quinolinol-5-sulfonic acid (ferron) in
cetyltrimethylammonium bromide micelles to form a highly
phosphorescent complex. The method is used to determine aluminium in
water and dialysis fluids. The given detection limits are 5.4 �g/litre
and 2 �g/litre, respectively.
Instrumental methods applied to the determination of aluminium
include neutron activation, X-ray fluorescence, flame atomic-
absorption spectrophotometry, inductively coupled plasma - atomic-
emission spectrophotometry (ICP-AES) and graphite furnace - atomic-
absorption spectrophotometry (GF-AAS). However, neither X-ray
fluorescence nor flame absorption methods are sensitive enough to
measure trace levels in biological samples (Bettinelli et al., 1985).
The NIOSH procedure for aluminium analysis in air is applicable over a
working range of 50-5000 �g per sample or 0.5-10 mg/m3 for a
100-litre sample (NIOSH, 1984).
Neutron activation analysis produces excellent results but the
methods are time consuming and the facilities are not always readily
available. The method is used for determining aluminium in fly ash
(Fleming & Lindstrom, 1987) and biological tissues (Yukawa et al.,
1980; Blotcky et al., 1992). After digestion and concentration of the
biological samples, a detection limit of 2.1 �g/g was found for bovine
liver (Blotcky et al. 1992).
GF-AAS is the most frequently used technique to determine
aluminium at low concentrations. Detection limits between 0.5 and 4
�g/litre or �g/g are achieved with the analysis of various
environmental and biological samples (Gardinier et al., 1981; van der
Voet et al., 1985; Bettinelli et al., 1985; Andersen, 1987). Most
liquid samples can be injected directly after dilution into GF-AAS.
Dilution is necessary because most biological fluids have high salt
contents (in the order of 30%) (Andersen, 1987). To prevent
precipitation of aluminium and the formation of carbon residues, EDTA
or Triton X can serve as diluents. Ammonia may be added to convert
aluminium to aluminate and thus avoid loss of aluminium as its
chloride (Gardinier et al., 1981). Triton X-100 is used to reduce the
viscosity of the samples, and MgNO3 is added as a matrix modifier to
improve the volatility of aluminium (Bettinelli et al., 1985).
ICP-AES is used for the determination of aluminium in various
biological and environmental samples, allowing the simultaneous
determination of different elements at low levels of interference
(Mauras & Allain, 1985; Sanz-Medel et al., 1987). The NIOSH method for
aluminium determination in air samples is recommended for a working
range of 5-2000 �g/m3 for a 500-litre sample (NIOSH, 1984). A
detection limit of 1 �g/litre in biological and environmental samples
has been reported by Que Hee & Boyle (1988). ICP can also be combined
with a mass spectrometer to further increase the sensitivity of the
method. As a multi-element detector for reversed-phase liquid
chromatography, ICP-MS offers the ability to measure isotope ratios on
eluting peaks and to remove troublesome matrices on-line (Thompson &
Houk, 1986).
For 26Al tracer experiments (Kobayashi et al. 1990), the
application of accelerator mass spectrometry (AMS) has been described.
The limit of detection is 106 atoms; thus the sensitivity of AMS is
105 times greater than that of gamma-ray counting techniques.
Aluminium concentrations in human brain can be investigated by
laser multipoint microprobe mass analysis (LAMMA) using focussed laser
ionization with time-of-flight mass spectrometry (Stern et al., 1986).
27Al nuclear magnetic resonance (NMR) may be used to ascertain the
coordination of aluminium in soil solutions (Schierl, 1985).
Aluminium in natural water samples has been determined using
reversed-phase liquid chromatography of the 8-quinolinol complex using
spectrophotometric detection. A detection limit of 2 �g/litre was
reported (Nagaose et al., 1991).
2.3.4 Speciation analysis of aluminium in water
Speciation analysis aims to distinguish and determine
quantitatively different groups of physico-chemical species present
in a water sample. All speciation methods, with the exception of
potentiometric techniques and direct spectroscopic methods (e.g.,
NMR), will alter the speciation of the sample during measurement. This
may not be a disadvantage, particularly if, as is usual, the
speciation analysis is being carried out in order to estimate the
toxicity of the sample to aquatic biota. Toxicity itself is a dynamic
process, and the interaction of aluminium species in water with a
biomembrane (e.g., a fish gill) will change the aluminium species
distribution in the solution close to the biomembrane. The best
speciation probe is one that reacts with aluminium in a water sample
to a similar extent and at a similar rate to the reaction of a
biomembrane with the aluminium in the samples.
Speciation analysis of aluminium in a water sample is usually
carried out after first filtering the sample through a 0.45 �m
membrane filter to remove particulate matter. The filtrate can then be
analysed for groups of species by several different techniques,
including kinetic spectrophotometry (Parker & Bertsch, 1992a,b), ion
exchange (Driscoll, 1984) and ion chromatography (Jones, 1991).
Combinations of methods such as sample acidification, kinetic
spectrophotometry and ion exchange are frequently used to determine a
variety of species (Driscoll, 1984; Courtijn et al., 1990; van
Benschoten & Edzwald, 1990). These speciation schemes provide
information on various speciation groups, including total dissolved
aluminium, acid-soluble aluminium, total monomeric aluminium, reactive
monomeric aluminium, non-reactive monomeric aluminium, aluminium
fluoride complexes, organic monomeric aluminium and inorganic
monomeric aluminium. The terms "reactive" and "labile", as applied to
aluminium species, are operationally defined and refer to species that
react rapidly with an analytical probe such as a cation exchange resin
or a chromogenic reagent.
The aluminium species that are most toxic to aquatic organisms
are believed to reside in the reactive monomeric inorganic aluminium
fraction and to consist principally of aluminium hydroxy complexes
(Helliwell et al., 1983; Fairman et al., 1994; Parent & Cambell,
1994). Although the fluoro complex is toxic, it is less so than the
aluminium hydroxy complexes (Helliwell et al., 1983).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Aluminium is released to the environment both by natural
processes and from anthropogenic sources. Natural processes far
outweigh the contribution of anthropogenic sources because aluminium
is a major constituent of the earth's crust, making up about 8% of the
earth's surface (Lantzy & Mackenzie, 1979). Anthropogenic releases
are mostly indirect, for example, through emission of acidifying
substances such as sulfur dioxide and nitrogen oxides to the
atmosphere. These acidify rain and soil and contribute to dissolution
of aluminium from the soil. The largest anthropogenic impact on
aluminium movement in the environment is through enhanced wind and
water erosion from cultivated land, notably when fallow. Aluminium is
the third most abundant element. It does not occur naturally in the
metallic, elemental state, but is widely distributed in the earth's
crust in combination with oxygen, fluorine, silicon and other
constituents. Aluminium occurs ubiquitously in silicates such as
feldspars and micas, complexed with sodium and fluoride as cryolite,
and in bauxite rock, which is composed of hydrous aluminium oxides,
aluminium hydroxides and impurities such as free silica. In general,
decreasing pH as a result of acid rain or the release of acid mine
drainage results in increased mobility of the monomeric forms of
aluminium (ATSDR, 1992). Chemical speciation in soil and water
affecting the bioavailability of aluminium to organisms is discussed
in chapter 4.
3.2 Anthropogenic sources
Direct anthropogenic releases of aluminium compounds are
primarily to the atmosphere and are associated with industrial
processes such as smelting. However, the use of aluminium and
aluminium compounds in processing, packaging, storage of food products
and as flocculants in the treatment of drinking-water may contribute
to its presence in drinking-water and food stuffs (ATSDR, 1992).
3.2.1 Production levels and processes
The most important raw material for the production of aluminium
is bauxite, which contains up to 55% alumina (aluminium oxide). The
commercial deposits of bauxite are mainly gibbsite (Al2O3�3H2O) and
boehmite (Al2O3�H2O). The bauxite is extracted by open-cast mining
(Dinman, 1983).
The production of the metal comprises two basic steps: refining
and reduction. Refining involves the production of alumina from
bauxite by the Bayer process in which bauxite is digested at high
temperature and pressure in a strong solution of caustic soda. The
resultant hydrate is crystallized and calcined to the oxide. Reduction
involves the reduction of alumina to virgin aluminium metal by the
Hall-Heroult electrolytic process using carbon electrodes and a
cryolite flux (Dinman, 1983).
World bauxite production was 106 million tonnes in 1992. A
comparison of the quarterly average figures for 1993 and 1994 with
this figure shows that production in major producing countries is
remaining fairly constant (World Bureau of Metal Statistics, 1994).
The total primary aluminium production for 1992 is summarized in Table
5. The amount of aluminium recovered from purchased or tolled scrap in
1992 was 14% of the total primary production figure. The total alumina
production for 1992 is summarized in Table 6. The total alumina
production figure includes 30 million tonnes for metallurgical uses
and 3 million tonnes for non-metallurgical uses. The total figures for
primary aluminium and alumina production have not changed greatly
since 1988.
3.2.2 Uses
Aluminium metal has a wide variety of uses including structural
material for construction, automobiles and aircraft, and the
production of metal alloys. Other uses include die-cast motor parts,
cooking utensils, decorations, road signs, fencing, beverage cans,
food packaging, foil, corrosion-resistant chemical equipment, solid
fuel rocket propellents and explosives, dental crowns, and denture
materials. In the electrical industry aluminium is used for power
lines, electrical conductors, insulated cables and wiring (ATSDR,
1992).
Table 5. Primary aluminium production in 1992 (from: IPAI, 1993)
Geographical area Thousands of tonnes
Africa 617
North America 6016
Latin America 1949
East and South Asia 1379
Europe 3319
Oceania 1483
Total 14 763
Table 6. Alumina production in 1992 (from: IPAI, 1993)
Geographical area Thousands of tonnes
Africa 604
North America 5812
Latin America 7627
East and South Asia 2360
Europe 5565
Oceania 11 803
Total 33 771
Aluminium compounds and materials also have a wide variety of
uses, some of which are listed in Table 7. Aluminium powder is used in
paints, protective coatings and fireworks. Natural aluminium minerals
especially bentonite and zeolite are used in water purification, sugar
refining, brewing and paper industries. Aluminium sulfate is used for
water purification, as a mordant in dyeing, and in paper production.
Other aluminium compounds are used as tanning agents in the leather
industry, and as components of human and veterinary medicines, glues,
disinfectants, and in toothpaste, styptic pencils, deodorants,
antacids and food additives. Clays (aluminium silicates) are used as
industrial raw materials (e.g., production of ceramics), and
aluminates are constituents of cement. Alkyl aluminium products are
used as catalysts for the production of low pressure polyethylene
(ATSDR, 1992).
Table 7. Main uses of aluminium compoundsa
Aluminium compounds Uses
alums hardening agent and setting accelerator for gypsum
plaster, in tanning and dyeing, and (formerly) in styptic
pencils
aluminas in water treatment and as accelerator for concrete
solidification (high alumina cements)
alkoxides in varnishes, for textile impregnation, in cosmetics and
as an intermediate in pharmaceutical production
borate production of glass and ceramics
carbonate antacid
chlorides production of rubber, lubricants and wood preservatives,
and in cosmetics as an astringent; the anhydrous
product is used as a catalyst and raw material in the
chemical and petrochemical industries; active ingredient
in antiperspirants
hydroxide stomach antacid, other pharmaceuticals
isopropoxide used in the soap and paint industries; waterproofing
textiles
phosphate antacid
silicate component of dental cement; antacid, food additives
sulfate used in water purification as a flocculent, in paper
production, as a mordant in dyeing, and as a starting
material for the production of other aluminium
compounds
trioxide used as an absorbent, abrasive and refractory material
sodium aluminium food additives
phosphate
a From: Helmbolt et al. (1985); ATSDR (1992)
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Aluminium occurs ubiquitously in silicates such as feldspars and
micas, complexed with sodium and fluoride as cryolite, and in bauxite
rock composed of hydrous aluminium oxides, aluminium hydroxides, and
impurities such as free silica (ATSDR, 1992). Aluminium is not
found as a free metal because of its reactivity. It has only one
oxidation state (+3); therefore, its transport and distribution in
the environment depend upon its coordination chemistry and the
characteristics of the local environmental system. Aluminium
partitions between solid and liquid phases by reacting and complexing
with water molecules and electron-rich anions, such as chloride,
fluoride, sulfate, nitrate, phosphate and negatively charged
functional groups on humic materials and clay.
At a pH greater than 5.5, naturally occurring aluminium compounds
exist predominantly in an undissolved form such as gibbsite (Al(OH)3)
or as aluminosilicates, except in the presence of high amounts of
dissolved organic material such as fulvic acid, which binds with
aluminium and can cause an increase in dissolved aluminium
concentrations in streams and lakes (ATSDR, 1992). Several processes
influence aluminium mobility and its subsequent transport within the
environment; these include chemical speciation, hydrological flow
paths, other spatial and temporal factors related to soil-water
interactions, and the composition of the underlying geological
materials (Grant et al., 1990). Watersheds with shallow, acidic soils
and poorly buffered surface waters mobilize aluminium when exposed to
acidic deposition (Driscoll et al., 1988).
4.1.1 Air
Aluminium enters the atmosphere as a major constituent of a
number of atmospheric particulates, such as soil-derived dusts from
erosion and particulates from coal combustion (Grant et al., 1990).
Eisenreich (1980) studied the atmospheric loading of aluminium to Lake
Michigan, USA. It was found that aluminium was generally associated
with large particles (> 2 �m diameter) and that these were deposited
near the source. The total atmospheric loading of aluminium to the
lake was calculated to be 0.86 kg/ha per year. The more industrialized
area south of the lake contributes 75% of this total loading. Cambray
et al. (1975) calculated the dry deposition flux of aluminium to the
North Sea to be 51 000 tonnes/year. Ottley & Harrison (1993)
calculated the flux to be 7 300 tonnes/year; they suggest that the
lower estimate is due to more spatially appropriate and extensive air
monitoring since 1975. Rahn (1981) calculated the input of aluminium
from the atmosphere to the Arctic Ocean at 30 000 tonnes/year. The
input was significantly less than those of oceanic and riverine inputs
(140 000 and 110 000 tonnes/year, respectively).
Guieu et al. (1991) compared atmospheric inputs with river inputs
of aluminium for the Golfe du Lion, France. Atmospheric inputs were
found to be 11% of total inputs of aluminium. Rainwater was analysed
for aluminium and only 19% was found in the dissolved fraction
(< 0.4 �m). Losno et al. (1993) monitored rainwater and snow for
aluminium and found large variations in the solubility of aluminium.
The variations seem to be largely due to pH, lower pH values
increasing the solubility of aluminium. Thermodynamic calculations
reveal that, at pH values higher than 5, equilibrium with gibbsite or
an insoluble trivalent alkaline form of aluminium acts to limit
solubility, whereas, at lower pH values, aluminium could be in
equilibrium with a hydroxysulfate salt.
4.1.2 Freshwater
4.1.2.1 Dissolved aluminium
In groundwater or surface water systems an equilibrium is formed
that controls the extent to which aluminium dissolution can occur. The
solubility of aluminium in equilibrium with solid phase Al(OH)3 is
highly pH-dependent. Aquo complex Al(H2O)63+ predominates at low
pH values (e.g., pH < 4), but as the pH of the solution increases
(e.g., pH 4-6) and/or the temperature rises, the positive charge
of aluminium forces hydrolysis of a water ligand producing the
Al(OH)(H2O)52+ ion. The degree of hydrolysis increases as the
solution pH increases, resulting in a series of Al-OH complexes such
as Al(OH)2+, Al(OH)2+, Al(OH) 4 - (Schecher & Driscoll, 1987).
Fluoride ions, being similar in size to hydroxyl ions, will readily
substitute in these complexes. At pH < 5.5, molar concentrations of
aluminium in certain areas exceed concentrations of fluoride ions and
form low ligand number complexes. The concentration of Al-F complexes
under those conditions is limited by the total concentration of
fluoride ions. At pH > 7.0, Al-OH complexes predominate in waters
that are low in dissolved organic matter and silicate. Under acidic
conditions sulfate also forms complexes with aluminium. Even though
sulfate concentrations are typically higher than those of fluoride in
surface waters, Al-SO42- complexes are significant only at high
sulfate concentrations and low pH values (Courtijn et al., 1987).
The chemical speciation of aluminium in natural water regulates
its mobility, bioavailability and toxicity. The concentration of
aluminium in some natural water as a function of pH can be estimated
by thermodynamic calculations. The actual geochemical mobility of
aluminium is very complex and difficult to predict in areas affected
by acid deposition, as non-equilibrium processes usually predominate
during episodic events associated with aluminium release and transport
(Bull & Hall, 1986; Lawrence et al., 1988; Seip et al., 1989).
The chemistry of inorganic aluminium in acid soil and stream
water can be considered in terms of mineral solubility, ion exchange
and water mixing processes (Neal et al., 1990). The minerals that
determine stream water Al3+ activity in acidic and acid-sensitive
systems are kaoline (Al2Si2O5(OH)4), various forms of aluminium
hydroxide (Al(OH)3), aluminium hydroxy sulfate (Al(OH)SO 4) and
aluminium hydroxy silicate (Seip et al., 1984; Neal & Williams, 1988;
Nealet al., 1990).
Goenaga & Williams (1988) found that the concentrations of Al-F
and Al-SO4 complexes in Welsh upland water were < 40 �g/litre and
accounted for less than 20% of the inorganic aluminium at pH < 5.0.
Organic complexes were significant (16 to 49 �g/litre) even in samples
with a low total organic carbon content. Organic monomeric aluminium
increased during high flows as total organic carbon (TOC) and free
ionic aluminium concentrations increased. LaZerte (1984) analysed
streams and lakes during spring snow melt in acidified catchments in
Ontario, Canada (pH 4.1-6.9, and TOC 1-24 mg/litre). Very little
polymeric and amorphous aluminium was found, and most of the inorganic
monomeric fraction was in the fluoride complexes.
Neal (1988) found that Plynlimon (Wales) stream water was
saturated or over-saturated with respect to some crystalline form of
Al(OH)3, and saturated or under-saturated with respect to amorphous
Al(OH)3: the level of saturation became less as pH decreased. The
solubility relationship for the kaolin mineral group is that at low pH
the waters are saturated with respect to crystalline kaolin
(kaolinite) and under-saturated with respect to poorly crystalline
kaolin (low crystallinity hallyosite). At higher pH the waters become
progressively more over-saturated with respect to crystalline forms
and near to saturation with respect to poorly crystalline forms. It
appears that at high pH the waters are in approximate equilibrium with
amorphous Al(OH)3 whilst at low pH values the waters are in
equilibrium with an aluminium hydroxy sulfate phase (equilibrium
constant 10-4.9).
Aluminium begins to polymerize when the pH of an acidic solution
increases notably over pH 4.5. Polymerization implies that in the
first step, two hydroxyls are shared by two aluminium atoms, e.g.,
2 Al(OH)(H2O)52+ -> Al2(OH)2(H2O)84+ + 2 H2O
Polymerization gradually proceeds to larger structures, eventually
leading to the formation of the Al13 "polycation" (Hem & Robertson,
1967; Parker & Bertsch, 1992a,b). As polymers coalesce, they increase
in relative molecular mass, eventually becoming large enough to
precipitate aluminium hydroxide from solution. As the precipitate ages
the solubility decreases (Chow, 1992). Tipping et al. (1988b) found
that if precipitation occurs at pH 4 to 6 it involves the formation of
aluminium (oxy)hydroxide and not aluminosilicates or basic aluminium
sulfates. The solubility of the (oxy)hydroxide was highly temperature
dependent and decreased in the presence of sulfate and more
particularly humate. L�kewille & van Breemen (1992) analysed
precipitates from stream bottoms in the Senne area of northern Germany
and found them to consist of amorphous aluminium hydroxide,
co-precipitated with minor amounts of sulfate, phosphate and silica.
Aluminium is predominantly cationic under acidic conditions and
strongly binds with negatively charged organic functional groups such
as fulvic acid and humic acid (Chow, 1992). Aluminium-humate complex
formation has been modelled by several researchers. For example,
Tipping et al. (1988a,c) developed a model using data collected from
acidified streams in northern England and Scotland. This model
describes the equilibria between humic acid substances, aluminium
species, calcium ions and hydrogen ions under acidic conditions (pH <
6) (Tipping et al., 1989a). Tipping & Backes (1988) reported on two
models of aluminium-humic acid complexation. Reasonable results were
obtained only at pH 4.0 to 5.0. Plankey & Patterson (1987) used a
fluorescence technique to study the complex formation kinetics of
aluminium with a single metal-free fulvic acid isolated from an
Adirondack mountain soil (USA). At pH 3.0 to 4.5 two types of
aluminium binding sites were identified.
4.1.2.2 Aluminium adsorbed on particles
Goenaga et al. (1987) collected stream water from tributaries of
Llyn Brianne reservoir, Wales. Analysis of the freshwater samples
showed total aluminium and monomeric aluminium concentrations to be
positively correlated with suspended solid content. The levels of
total (acid digestible) aluminium that were detected in filtrates of
freshwater samples were affected by the pore diameter of the membrane
filter used. Between 13% and 50% of this form of aluminium initially
present in the sample could be removed by a 0.4 �m pore diameter
membrane, depending on the level of suspended solid originally
present. Goenaga & Williams (1988) calculated the amount of aluminium
adsorbed onto suspended solids by measuring monomeric aluminium before
and after separation of suspended solids by filtration (0.015 �m pore
diameter). Adsorbed aluminium was low (< 20 �g/litre) during dry
weather periods; however, the adsorbed fraction was very significant
(> 40 �g/litre) for samples with high suspended solids (>
20 mg/litre) collected from a Welsh upland area during a storm
episode. Goenaga & Williams (1990) found that aluminium associated
with particles > 0.015 �m was negligible (< 20 �g/litre) in spot
samples with a suspended solid content of < 5 mg/litre but
significant (< 200 �g/litre) for episode samples with high suspended
solids (> 20 mg/litre); between 40% and 60% of the adsorbed aluminium
was found to be associated with particles > 0.4 �m. Tipping et al.
(1989b) studied the adsorption of aluminium in water from various
acidified streams in northern England. The adsorption of aluminium by
particles was found to increase with total aluminium, pH and particle
concentration. Calculations using an adsorption equation, and taking
competition by dissolved humic substances into account, suggest that
adsorbed aluminium may commonly account for a significant proportion
(> 10%) of total monomeric aluminium in such water.
4.1.2.3 Aluminium in acidified waters
Fisher et al. (1968) monitored aluminium concentrations in
acidified streams of the Hubbard Brook Experimental Forest, New
Hampshire, USA from 1964 to 1966. Annual loads of aluminium were
calculated to be between 0.9 and 2.4 kg/ha for three streams. The
acidic deposition at the Hubbard Brook ecosystem has induced a series
of geochemical responses. Firstly, hydrogen ion acidity is neutralized
by the dissolution of alumina primarily found in the soil zone.
Secondly, hydrogen ion acidity and aluminium acidity are neutralized
by the chemical weathering of silicate materials (Johnson et al.,
1981). Hall et al. (1980) acidified a stream in the Hubbard Brook
Experimental Forest. The pH of the control stream ranged from 5.7 to
6.4 and in the acidified stream from 3.9 to 4.5. Dissolved aluminium
increased significantly in the acidified stream water by an average of
181%compared to the control stream. Lawrence et al. (1988) reported
that at high elevations the increased stream flow was associated with
reduced surface water acidity and decreased inorganic aluminium
concentrations. At low elevations, increased stream flow was
associated with increases in stream acidity and concentrations of
inorganic aluminium. The contributions of flow from the more acidic
upper region of the watershed during high-flow conditions appear to be
the major hydrological influence on stream chemistry. In the acid-
affected Experimental Forest system, acidity of low-order stream water
was high due to elevated inputs of strong acids (sulfuric and nitric)
relative to the releases of basic cations from soil. Concentrations of
aluminium were also high and predominantly in labile (inorganic)
monomeric form. For comparison, samples were collected from the
Jamieson Creek watershed, British Columbia, Canada. For this watershed
the low-order stream water was acidic due to low concentrations of
basic cations coupled with the presence of organic acids.
Concentrations of aluminium were relatively low and largely associated
with organic solutes (Driscoll et al., 1988).
Large temporal and spatial variations in aluminium concentration
occur in Welsh stream water due to hydrological and land use controls.
The most acidic streams have the highest aluminium concentration.
Variations in stream and soil water aluminium concentrations in the
order semi-natural moorland < conifer forested moorland < recently
harvested forest can be explained by ion exchange reactions related to
changes in the anion concentrations passing through the system and
weathering (Neal et al., 1990). Lawrence et al. (1986) found that
observed altitude trends in stream aluminium chemistry may be related
to spatial variations in vegetation type and mineral soil depth.
Mobilization of aluminium was studied in streams at the Hubbard Brook
Experimental Forest, New Hampshire, USA. At the highest altitudes
maximum densities of spruce and fir vegetation occur, and aluminium
appears to be mobilized by transformations involving dissolved organic
matter. At mid-altitudes hardwood vegetation predominates and the
mechanism of aluminium mobilization shifts to dissolution by strong
acids within the mineral soil. At the lowest altitudes relatively
thick mineral soil seems to limit aluminium mobility resulting in low
concentrations in stream water. During 1983 and 1984 an experimental
watershed at Hubbard Brook was commercially whole-tree harvested.
Whole-tree harvesting resulted in a large increase in stream nitrate
concentrations, followed by a decrease in pH and concomitant increase
in inorganic aluminium (Lawrence et al., 1987). Ormerod et al. (1989)
studied the spatial patterns in aluminium and pH data from 113 Welsh
catchments of contrasting land use. It was found that pH declined and
aluminium increased significantly with increasing forest cover. The
percentage contribution of labile aluminium to the total filterable
concentration ranged from 39% to 90%, the highest levels being
associated with streams draining forest. Neal et al. (1992) found that
felling conifers led to decreases in pH and increases in aluminium
concentrations in streams and soils at Plynlimon, Wales, for the first
2 years. The major changes were found to occur during the winter storm
flow periods. The trends were reversed after the first two years. The
short-term effects (2-3 years) of forest harvesting on soil and stream
water inorganic aluminium chemistry were predominantly controlled by
the nitrogen dynamics of the site. A reduction in inorganic aluminium
was observed concomitant with declines in nitrate and total inorganic
anions. However, 4-5 years after harvesting, inorganic aluminium
concentration in soil and stream water of Welsh and Cumbrian study
sites was still greater than that expected in moorland catchments
(Reynolds et al., 1992).
Bird et al. (1990) compared the effects of a winter "rain on
snow" episode with a summer storm episode on the pH and dissolved
aluminium levels in a moorland stream and a conifer forest stream. The
pre-episode conditions were broadly similar in both streams. In
winter, following the snow melt, both moorland and forest stream
showed reduced pH accompanied by increases in dissolved aluminium
concentrations as buffering capacity provided by the calcium was
exceeded by anions such as sulfate. The timing of flow changes was
similar, but flush of solutes to the moorland stream was more rapid.
Both streams had a similar buffering capacity, but the changes in the
forest stream were much greater (pH reduction of 2 units and dissolved
aluminium levels exceeding 1 mg/litre), reflecting a greater flux of
anions. The surface run-off and reduced buffering capacity from frozen
soils led to the changes in both streams. During the summer episode,
again the forest stream showed a greater reduction in pH and higher
increase in dissolved aluminium; however, the concentrations of
sulfate were much lower in both streams and less aluminium was
mobilized.
Mach & Brezonik (1989) studied the biogeochemical cycling of
aluminium in acidified and reference basins of Little Rock Lake,
Wisconsin, USA. Background dissolved lake water aluminium
concentrations were 7 �g/litre (0.4 �m pore-size). The acidified basin
was acidified in a step-wise manner. Acidification to pH 5.1 resulted
in a 45% elevation of dissolved aluminium levels (15.5 �g/litre) over
reference basin levels (10.7 �g/litre). Analysis of suspended
particulate matter collected from both basins revealed lower levels of
particulate aluminium in the acidified basin, demonstrating that there
is reduced affinity for particulate matter at the lower pH. Brezonik
et al. (1990) studied the effects of acidification of Little Rock Lake
on the dissolved concentrations of aluminium. Aluminium desorbed from
sediments of the lake basin at pH 4 and below in laboratory studies.
In littoral enclosures, dissolved aluminium was elevated above control
levels at pH 4.5, and elevated levels were observed in pelagic
enclosures at both pH 5.0 and 4.5. Dissolved aluminium levels remained
constant in the acidified basin at pH 5.6 and 5.1 (16 �g/litre, while
concentrations in the reference basin declined to 11 �g/litre during
the study period. The authors stated that these levels of aluminium
were low compared with other reported values because the lake was
hydrologically isolated.
Cosby et al. (1985) presented a mathematical model (MAGIC; Model
Acidification of Groundwater In Catchments) that uses quantitative
descriptions of soil chemical processes to estimate the long-term
chemical changes that occur in soil, soil water and surface waters of
catchments in response to changes in atmospheric deposition. The model
is based on soil base cation exchange, dissolution of aluminium
hydroxide and solution of carbon dioxide. The model uses "average" or
lumped representations of these spatially distributed catchment
processes. The long-term responses of the model are controlled by
sulfate adsorption and primary weathering of base cations in the
catchment soils. The model was applied to the Shenandoah National
Park, Virginia, USA, and indicated that the alkalinity of surface
waters had been reduced by as much as 50% over the last 140 years.
Cosby et al. (1986) applied the MAGIC model to a sub-catchment in
southwestern Scotland. Assuming that deposition rates are maintained
in the future at 1984 levels, the model indicated that stream pH was
likely to decline. Neal et al. (1986) reported that the model predicts
increases in pH (reductions in acidity) with conifer deforestation.
In the Welsh uplands the model simulates quite accurately the
acidification of catchments (Whitehead et al., 1990). The model shows
that atmospheric deposition is the primary cause of stream
acidification with conifer afforestation enhancing stream acidity.
Historical trends determined by the model indicate that acidification
has been present since the turn of the century (Whitehead et al.,
1988). Ormerod et al. (1990) compared the predicted effects of reduced
acidic deposition and liming on stream acidification with actual
treatments. The results indicate that liming and 90% reduction in
sulfate deposition reduce concentrations of soluble aluminium to
similar levels. However, calcium concentrations and pH were increased
by liming to values that were high by comparison with conditions
simulated under low acid deposition.
A dynamic model has been developed that reproduces major trends
in chemical and hydrological behaviour in Norwegian catchments.
Christophersen & Seip (1982) reported that a simple two-reservoir
model incorporating a small number of physically realistic processes
accounts for the major short-term variations in stream water chemistry
during the snow-free season at a 0.41 km2 catchment in coniferous
forest on granite bedrock at Birkenes, Norway. The model incorporates
both hydrolytic and sulfate sub-models, and a cation sub-model that
includes hydrogen, aluminium, calcium and magnesium ions. Typical
characteristics predicted by the model include positive correlations
between hydrogen ions and aluminium concentrations and discharge, and
negative correlations between these factors and the calcium and
magnesium concentrations. Seip et al. (1989) attempted to model
episodic changes in stream water chemistry of hydrogen and aluminium
ions. However, only partial success was achieved. Trends were correct
for hydrogen ions but there were discrepancies at peak heights. There
were correct predictions for aluminium concentrations in the autumn
but not in the spring.
4.1.3 Seawater
In contrast to fresh water, seawater (salinity > 32%) has a
constant pH of approximately 8.2. Hydes (1977) found that bottom and
suspended clay sediments probably act as a source of dissolved
aluminium to seawater. However, removal below predictions of clay
solubility is probably the result of biological activity.
Hydes & Liss (1977) reported that approximately 30% of the
dissolved aluminium entering the Conwy Estuary, Wales, appears to be
removed during mixing with seawater. The removal occurs during the
early stages of mixing and is virtually complete by the time the
salinity reaches 8%. The authors concluded that the most likely
mechanism involves the trapping of aluminium adsorbed onto the surface
of fine clay particles entering with the freshwater as the particles
are irreversibly coagulated on mixing with saline water.
Mackenzie et al. (1978) measured the concentration of aluminium
in a vertical hydrographic profile of the Mediterranean Sea. They
found that the concentrations did not correspond to seasonal
thermocline, nitrate minimum and an oxygen maximum, thus supporting
the hypothesis that aluminium cycles in the oceans are associated with
the activity of diatoms. Stoffyn (1979) stated that experimental
evidence using the diatom Skeletonema costatum supports the
hypothesis that the concentration and distribution of dissolved
aluminium in ocean water is controlled by biological activity in the
surface waters. However, Hydes (1979) reported that the distribution
of dissolved aluminium in open ocean waters is probably controlled by
the solution of aluminium from atmospherically derived particles and
bottom sediments balanced against scavenging by siliceous shells of
dead organisms. Chou & Wollast (1989) studied eight vertical profiles
of dissolved aluminium in the Mediterranean Sea. They found that
dissolved aluminium is depleted in surface waters as compared with
deep waters. The high concentrations of aluminium in deep water may
result from fluxes from pore water in sediments to the overlying
water. These authors suggested that aluminium is possibly removed by
biological processes in the euphotic zone.
4.1.4 Soil
In soil, aluminium is released into solution for transport to
streams upon acidification. The chemistry of inorganic aluminium in
acid soil and, in particular, the solubility controls are very similar
to those given in section 4.1.2 for fresh water (Furrer, 1993).
However, the extrapolation of stream water chemistry to soil is
difficult because of the complexity of hydrological pathways and
chemical reactivity during water mixing (Neal, 1988).
Water mixing processes may be important in determining the
relationship between aluminium (inorganic) and hydrogen ions.
Consequently there is a need to model how these species vary as a
function of mixing so that comparisons can be made with field
observation. In order to resolve the effects of mixing, a basic
calculation is made to allow soil to mix and degas carbon dioxide in
the stream. To do this it is assumed that pCO2 in soil/groundwater
and the stream is 25 and 2 times the atmospheric value, respectively,
and that bicarbonate principally controls the acid buffering (Neal et
al., 1990).
In soils, solid-phase aluminium occurs in the lattice structure
of minerals, in inter-layer sites of expanding clay minerals, and in
poorly ordered minerals (allophane and hydrous oxides) of variable
composition. Natural acidification processes result in increasing
solubility of aluminium. At moderately acidic levels (pH 5.5)
aluminium appears as the exchangeable cation that dominates in the
lower mineral horizons initially as poly-nuclear hydroxy ions but
subsequently as the simple mono-nuclear ions. Aluminium ions displace
calcium at permanent-charge exchange sites (Bache, 1980). The cation
exchange system of acid soils provides a large reserve of ionic
aluminium, which can be brought into solution when soluble salts
percolate through soil. Ligands, such as fluoride and organic anions,
which form aluminium complexes, combine with aluminium and maintain
higher concentrations of aluminium than might be expected, especially
at pH 5-7 (Bache, 1986). In soil the most soluble form of aluminium
under acidic conditions is non-silicate organically bound aluminium,
while the amorphous aluminium hydroxy forms are more soluble than the
crystalline forms (ATSDR, 1992; Sj�str�m, 1994).
Bloom et al. (1979) found that hydrolysis of organically bound
aluminium is a major source of buffering in the pH range 4 to 5 for
dilute salt suspensions of acid soils. The exchange of aluminium ions
from organic matter exchange sites controls the relationship between
pH and Al3+ activity in acid soils that have a low amount of
permanent-charge cation exchange capacity relative to the quantity of
organic matter. Walker et al. (1990) studied the influence of organic
matter on the solubility of aluminium in organic soil horizons from
different geographical regions of North America. The equilibrium
solubility of aluminium was dependent on pH and the degree to which
soil organic matter was saturated with aluminium. Soluble aluminium
increased with decreasing pH and increased with increasing surface-
bound aluminium at each pH level. Temperature dependence and rate
studies suggested that aluminium solubility was governed by an ion-
exchange reaction between H+ and aluminium and the organic matter.
Litaor (1987) studied the aluminium chemistry in an alpine
watershed, Front Range, Colorado, USA. It was found that the aluminium
solubility in the interstitial water is complex and controlled by
organic solutes, H4SiO4 and pH. However, neither pH nor sulfate
concentrations correlated with aluminium concentrations. The chemical
equilibrium of aluminium was controlled by amorphous aluminosilicate.
The most important inorganic aluminium complexes are the
mononuclear and to a lesser extent the polynuclear hydroxo species.
The formation of these complexes is directly coupled to pH and also,
to a lesser extent, to ionic strength. The hexa aqua-aluminium cation
(Al(H2O)63+) predominates at low pH, whereas the mononuclear
(Al(OH)2+) and dihydroxo-mononuclear species (Al(OH)2+) become
important in the circumneutral pH range. The tetrahydroxo aluminium
anion (aluminate, Al(OH)4-) is the predominant species at higher pH,
and is responsible for the increasing solubility of aluminium above pH
6.2. Polynuclear aluminium complexes can be the predominant species in
solution over a wide pH range, although, being formed under non-
equilibrium conditions, they are difficult to predict (Grant et al.,
1990). Dahlgren & Ugolini (1989) collected leachates from subalpine
spodosol located in the Cascade Range, Washington, USA. The ability of
organic acids to complex aluminium in these subalpine soils increases
from pH 3.8 to 5.0. Walker et al. (1988) found that adsorption of
aluminium by aluminosilicate clay minerals, such as montmorillonite,
kaolinite and vermiculite, is controlled by a simple electrostatic
cation exchange involving outer sphere complexes. Adsorption to
vermiculite may also be controlled by internal ion diffusion.
Equilibrium constants (Ka) for the formation of an adsorbed
aluminium clay complex were high (approx. 105) for the three minerals,
suggesting that they play a significant role in controlling aluminium
concentrations in soil solutions.
Blume & Br�mmer (1991) studied the influence of soil acidity on
aluminium binding in sandy soils with a low humus content (< 2%).
Binding was found to decrease steadily from very strong at pH 5.5-7.0
to very weak at pH 2.5. The binding of aluminium was found to be
increased by increasing the organic matter content. In loam or clay
soils binding was increased compared with sandy soils at all levels of
pH.
Driscoll et al. (1989) studied the chemistry and transfer of
aluminium in a forested watershed of the Adirondack region, New York,
USA. The drainage waters from the watershed were highly acidic due to
elevated inputs of both sulfuric and nitric acids compared with the
release of basic cations. The conditions facilitated the mobilization
of aluminium. Alumino-organic solutes were mainly released from the
soil organic horizon with inorganic monomeric aluminium derived
predominantly from the mineral soil and to a lesser extent from the
soil organic horizon. Inorganic monomeric aluminium predominated in
the drainage water. The deposition of organic monomeric aluminium in
the stream bed coincided with the dissolved organic content retention
whilst the deposition of inorganic monomeric aluminium appeared to be
facilitated by nitrate retention.
Nilsson & Bergkvist (1983) studied soil acidification and
aluminium chemistry in three adjacent catchments on the Swedish west
coast. The concentration of organic aluminium was linearly correlated
with concentrations of carbon. The percentage of organic species in
the dissolved aluminium decreased with increasing depth from > 90% in
the upper layers to < 10% below 55 cm. The average concentration
of total aluminium increased with increasing depth from 3.3 to
9.8 �mole/litre at 5 cm to 95.3 to 115 �mole/litre below 55 cm.
Atmospheric acid inputs have a strong impact on the aluminium
chemistry of acidic sandy soils with low concentrations of basic
cations. The base saturation of such soils is low and the rate of
basic cation weathering does not increase with rate of acid input. Any
additional acidic deposition is neutralized by aluminium. Dissolution
of aluminium is the major acid sink in such soils, neutralizing 30% to
95% of the total acid load (Mulder et al., 1989).
Bergkvist (1987) studied the leaching of aluminium in a brown
forest soil and a podzol with adjacent stands of spruce, beech and an
open regeneration area in South Sweden using lysimeter techniques. The
leaching of aluminium was greatest from the podzol. The solubility of
aluminium increased suddenly within a small pH range (4.5-4.0) in the
B horizon (15 to 55 cm). The concentration of aluminium in soil
solution increased from 2 to 10 mg/litre when the pH decreased from
4.4 to 4.2. Berggren et al. (1990) reported that forest soils of south
Sweden are losing base cations, such as aluminium, owing to increased
leaching rates following soil acidification. The processes controlling
the mobilization of aluminium in podzols and cambisols of southern
Sweden were investigated by Berggren (1992). Podzols in spruce and
beech stands had a high release of organic compounds from the upper 5
to 7 cm (O/Ah horizons), which resulted in high organic complexation
of aluminium in soil solution at a depth of 15 cm (E horizon). Organic
complexes were mainly adsorbed or precipitated at 20 to 40 cm (upper
Bh horizon) and the overall transport of aluminium at 50 cm was
governed by a pH-dependent dissolution of solid-phase aluminium. In
the cambisols inorganic aluminium predominated at both 15 cm and 50 cm
with solubility being closely related to solution pH. The results
indicate that the relatively large organically bound solid-phase
aluminium pools in both soil types give rise to the measured solution
aluminium activities. The authors also found that aluminium in
solution efficiently competed for exchange sites and played an
important role in the mobilization of cadmium in these soils.
In laboratory studies simulating snow melt leaching of forest
soils, nitric acid leached more aluminium than did sulfuric acid
from soil columns representative of high elevation forest soils
and watersheds thought to be sensitive to acidification by acid
precipitation. Increasing the nitric acid concentration 100-fold
(pH 5 to 3) increased the total aluminium concentration in the
leachate from 0.70 to 0.85 mmol/litre, while increasing the sulfuric
acid had no effect. Similar experiments with albic and ochric mineral
soil horizons revealed no difference between the acids and no effect
of increasing acid concentrations (James & Riha, 1989).
The acidity produced by soil nitrification is buffered by
exchange with base cations. In acidified soils aluminium will be
dissolved or the pH of the soil will decrease. Aluminium release from
soil is also dependent on the sulfate supply and the capacity of the
soil to provide aluminium hydroxides. The molar ratio between
aluminium and nitrate is important in evaluating the effects of
nitrification. During dry summers the release of aluminium ions may be
entirely controlled by the acidification caused by nitrification
(Gundersen & Rasmussen, 1990).
4.1.5 Vegetation and wildlife
Mobilization of aluminium by acid rain results in more aluminium
being available to plants (ATSDR, 1992).
Henriksen et al. (1988a) found during acidification experiments
in Norwegian streams that the buffering capacity was 20 times higher
than that associated with the water alone and a reduction of the pH to
5 resulted in large releases of aluminium (up to 2500 �g/litre).
The source of the buffering and the reservoir of aluminium was
hypothesized to be dense growths of liverwort. A second experiment
confirmed that liverworts are involved in the ion exchange of base
cations and aluminium during acid episodes.
Vogt et al. (1987) studied the aluminium concentrations of above-
and below-ground tissues of a white fir ( Abies amabilis) stand in
the Cascade mountains, Washington, USA. It was found that 97% of the
total detrital cycling of aluminium was below ground. When compared
with the other elements analysed, aluminium showed the highest
proportion of total annual element pool circulated (82%). The large
root biomasses of these stands allows large amounts of aluminium to be
accumulated and immobilized. The high root turnover observed for these
stands appears to be due to the root senescence occurring in response
to high aluminium accumulation. However, there is little impact on
short-term elemental cycling because the roots decay very slowly
(99% decay = 456 years).
4.2 Biotransformation
4.2.1 Biodegradation and abiotic degradation
Elemental aluminium does not degrade in the environment. In the
trivalent oxidation state, it can complex with electron-rich species
(ATSDR, 1992).
4.2.1 Bioaccumulation
4.2.2.1 Plants
Plants differ in their ability to take up aluminium; some
accumulate aluminium whereas others are able to immobilize it at the
root surface (Roy et al., 1988). Exposure to aluminium in nutrient
solution leads to accumulation, especially in the roots (Lee, 1972;
Boxman et al., 1991).
Aluminium taken up by roots is mainly found in the mucilage layer
on the root tip surface (Horst et al., 1982) and in the walls of the
epidermis and cortex cells (Huett & Menary, 1980). In the cell wall
pectins, aluminium ions compete with calcium ions for the same
absorption sites (Wagatsuma, 1983). Some aluminium is taken up in the
cytoplasm and bound to nucleic acids and acid-soluble phosphates
(Wagatsuma, 1983). Aluminium is translocated only to a small extent to
shoots.
The concentrations of aluminium in leaf tissue of a variety of
plants growing on limestone soils in Sweden (pH around 8) were found
to be similar to those in plants growing on an acid silicate (granite;
pH 4.1-4.9) site, although the aluminium concentration in the topsoil
solution was at least one order of magnitude lower in the limestone
than in the acid silicate soils (Tyler, 1994).
4.2.2.2 Invertebrates
Ryther et al. (1979) cultured soft shell clams ( Mya arenaria),
hard shell clams ( Mercenaria mercenaria), American oysters
( Crassostrea virginica) and sand worms ( Nereis virens) in tanks
containing fly ash from a coal-burning power station. The fly ash
contained a wide range of elements including aluminium at 105.8 g/kg.
After a period of 4 months the sand worms and the edible parts of the
clams and oyster were analysed. Aluminium concentrations were
9645 mg/kg (dry weight) for the sand worms and 8218, 268 and
1373 mg/kg for soft shell clams, hard shell clams and oysters,
respectively.
Crayfish ( Orconectes virilis) from a lake with an average total
aluminium concentration of 36 �g/litre were placed in caged tubes
spiked with 40 �g/litre total aluminium and transferred to a lake with
background levels of 8 �g/litre total aluminium. Half of the tubes
were acidified to pH 5.3 and the others remained at pH 6.7. None of
the crayfish accumulated aluminium. Controls had lower aluminium
concentrations in the hepatopancreas and abdominal muscle after 25 to
27 days. Crayfish under acidified conditions retained aluminium in the
hepatopancreas and not in the muscle whereas those at pH 6.7 retained
aluminium in the muscle and not the hepatopancreas. The same authors
also carried out a laboratory experiment with crayfish obtained
from the original lake. Crayfish were maintained in a solution of
500 �g/litre for 14 days. No tissues showed an increase in aluminium
levels. However, crayfish transferred back to the original lake water
for 16 days retained aluminium only in the carapace and gills (Malley
et al., 1987).
Havas (1985) exposed water fleas ( Daphnia magna) to total
aluminium concentrations of 0.02, 0.32 and 1.02 mg/litre for 24 h.
Bioconcentration was related to pH, the highest concentration factors
occurring at pH 6.5 and the lowest at pH 4.5; there was no effect of
increasing the calcium concentration from 2.5 to 12.5 mg/litre. Mean
bioaccumulation factors ranged from 11 000 to 18 000 at pH 6.5, 3000
to 9000 at pH 5.0, and 1200 to 4300 at pH 4.5.
Frick & Herrmann (1990) studied the accumulation of aluminium
by nymphs of the mayfly ( Heptagenia sulphurea) exposed to
concentrations of 0.2 and 2 mg inorganic monomeric aluminium/litre at
pH 4.5 for up to 4 weeks. The highest mean concentrations found in
mayflies were 1.24 and 2.34 mg/g aluminium (dry weight) for the higher
treatment groups, which did not undergo moulting. The major part of
the aluminium was deposited on/in the exuviae of the nymphs, as
aluminium determinations revealed a 70% decrease in content after
moulting.
4.2.2.3 Fish
Cleveland et al. (1986) exposed brook trout ( Salvelinus
fontinalis) eggs, larvae and juveniles to 300 �g/litre total
aluminium at three pH levels. At 30 days post-hatch for larvae and
for an exposure period of 30 days for juveniles (37 to 67 days),
significantly more aluminium was accumulated at pH 5.28 than at either
pH 7.24 or 4.44. Aluminium levels at pH 5.28 were 398 and 112 mg/kg
for the larvae and juveniles, respectively. At pH 7.24 residues were
12 and 33 mg/kg, and at pH 4.44, 71 and 17 mg/kg, respectively.
Cleveland et al. (1991) maintained brook trout in water containing
200 �g/litre total aluminium at pH values of 5.0, 6.0 and 7.2 for 56
days. Estimated steady state bioconcentration factors for aluminium,
which were inversely related to pH, were 215 at pH 5.3, 123 at pH 6.1
and 36 at pH 7.2. The estimated time to 90% steady state was 1.5 days
at pH 5.3, 4.2 days at pH 6.1 and 1.7 days at pH 7.2. Elimination
during the 28-day depuration phase was more rapid at pH 5.3 than at pH
6.1 or 7.2. Karlsson-Norrgren et al. (1986b) found that brown trout
( Salmo trutta) accumulated significantly more aluminium in gill
tissue at pH 5.5 than at pH 7.0 (60-160 �g/kg and 10-40 �g/kg dry
weight, respectively) when exposed to 200-500 �g total
aluminium/litre. Skogheim et al. (1984) found a gill aluminium
accumulation of 70 to 341 �g/g fresh weight in dying Atlantic salmon
( Salmo salar) during an episodic fish kill in the river Ogna,
Norway, at pH 5.4-5.5 and total aluminium and labile aluminium
concentrations of 160 and 130 �g/litre, respectively.
Segner et al. (1988) exposed young brown trout ( Salmo trutta)
to total aluminium (230 �g/litre) at pH 5.0 in high calcium water at a
temperature of 12�C for 5 days. Whole body aluminium concentrations
were 230 mg/kg dry weight in aluminium-exposed fish, as compared to
75 mg/kg (pH 5.0) and 44 mg/kg (pH 7.2) for fish in aluminium-free
water.
Wicklund Glynn et al. (1992) exposed minnows ( Phoxinus
phoxinus) to acidic water (pH 5.0) with and without total aluminium
(150 �g/litre) at varying calcium (0, 0.07 and 2 mmol/litre) and humus
(5 and 25 Pt) concentrations for 15 days. Aluminium concentrations in
the gills were highest in the lower calcium level groups with or
without humus. In the absence of calcium the median aluminium level in
the gills was 109 mg/kg wet weight, and at 2 mmol/litre calcium the
aluminium level was 50 mg/kg.
4.2.2.4 Birds
Carri�re et al. (1986) fed ring doves ( Streptopelia risoria) on
a diet containing 0.1% aluminium sulfate with reduced calcium and
phosphorus (0.9% Ca; 0.5% P) for a period of 4 months. Analysis of
the tissues of breeding adult doves revealed that there was no
accumulation of aluminium in kidney, brain or male femurae; however,
the femurae of female doves showed a significant increase from a mean
of 7.42 mg/kg dry weight in controls to 15.87 mg/kg in treated birds.
Juvenile doves fed on diets containing 500, 1000 and 1500 mg/kg
aluminium sulfate did not accumulate aluminium in leg and wing bones
but did show a significant tendency to accumulate in the sternum.
Sparling (1991) fed black ducks ( Anas rubripes) and mallard
( Anas platyrhynchos) on a diet containing 200, 1000 or 5000 mg
aluminium/kg with varying amounts of calcium (3600 and 15 100 mg/kg)
and phosphorus (6200, 13 500 and 21 500 mg/kg) for a period of 10
weeks. All of the diets produced a dose-related and significant
increase in the aluminium content of the femur. Black ducks maintained
on normal calcium and phosphorus levels showed femur aluminium
concentrations of 5.42, 13.6 and 19.5 mg/kg after 10 weeks at the
three dose levels, respectively. Mallard femurs contained aluminium
levels of 9.49, 12.1 and 18 mg/kg, respectively.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Aluminium is ubiquitous in the environment and its chemistry is
controlled by pH, mineralogical composition, and the quantity and
qualitative nature of the organic constituents present. It is,
therefore, difficult to provide generalized estimates of natural
background concentrations (Grant et al., 1990). Aluminium is released
to the environment by both natural processes and anthropogenic
sources. It is a major constituent of the earth's crust, and natural
mobilization of aluminium far outweighs the direct contribution from
anthropogenic sources (Lantzy & Mackenzie, 1979). The concentrations
of aluminium in the different environmental compartments are dependent
on its speciation and mobilization (see section 4.1). Jones & Bennett
(1985) summarized the data on aluminium concentrations in the
environment and produced a list of representative values as follows:
urban air 1000 ng/m3 (160-7000 ng/m3), rural air 200 ng/m3
(150-325 ng/m3), agricultural soil 70 000 mg/kg (10 000-300 000
mg/kg), fresh water (dissolved) 50 �g/litre (1-2250 �g/litre), ocean
(dissolved) 2 �g/litre (1-5 �g/litre), and terrestrial plants
100 mg/kg (50-600 mg/kg).
5.1.1 Air
Aluminium is a major constituent of a number of atmospheric
components, being highly concentrated in soil-derived dusts and in
particulates from coal combustion (Grant et al., 1990). The sources of
soil-derived dust are both natural (Sorenson et al., 1974) and from
human activity such as mining and agriculture (Eisenreich, 1980).
Leharne et al. (1992) monitored street dust from the inner London
area, United Kingdom, and found aluminium levels ranging from 3.7 to
11.6 �g/kg. The largest sources of particle-borne aluminium are the
flux of dust from soil and rock materials in the earth's crust and
from volcanic eruptions (Lee & von Lehmden, 1973; Sorenson et al.,
1974; Lantzy & Mackenzie, 1979). Atmospheric aluminium concentrations
show widespread temporal and spatial variations. The concentrations of
aluminium in air are summarized in Table 8 and range from 0.5 ng/m3
over Antarctica to > 1000 ng/m3 in industrialized areas.
Table 8. Concentrations of aluminium in air
Area Year Particle Aluminium Reference
size concentration
(�m) a (ng/m3)
Antarctic 1970 NR 0.57 Zoller et al.
(0.32-0.81) (1974)
Arctic 1976-1978 NR 25 Rahn (1981)
(Barrow, Alaska)
Hawaii 1967 > 0.15 2-40 Hoffman et al.
(1969)
Atlantic Ocean NR 8-370 Duce et al.
(1975)
NR 95 (41-160) Windom (1981)
Atlantic Ocean NR 102-184 Windom (1981)
near coast of USA
North Sea 1988-1989 NR 294.5 Chester &
(21-887) Bradshaw (1991)
1988-1989 NR 197 Ottley &
(17-903) Harrison (1993)
1985-1986 NR 210 Kersten et al.
(64-600) (1988)
Baltic Sea 1985 NR 218 H�s�nen et al.
(47-800) (1990)
Kiel Bight, 1981-1983 NR 394 Schneider (1987)
Germany (68-720)
USA cities & 1975-1977 < 3.5 48-1983 Stevens et al.
industrial areas (1978)
1975-1977 > 3.5 331-8678 Stevens et al.
(1978)
Buffalo, 1968-1969 NR 1000-8000 Pillay & Thomas
New York, USA (1971)
Southern Arizona 1974 NR 5700 Moyers et al.
(urban) (1977)
Table 8. (Con't)
Area Year Particle Aluminium Reference
size concentration
(�m) a (ng/m3)
Southern Arizona 1974 NR 1200 Moyers et al.
(rural) (1977)
Charleston, 1976 < 3.5 74 Lewis & Macias
West Virginia (1980)
1976 > 3.5 1100 Lewis & Macias
(1980)
UK (non-urban 1972-1973 NR 27-640 ng/kg Cawse (1974)
sites)
Birkenes, 1978-1979 NR 80 Amundsen et al.
S. Norway (1992)
1985-1986 NR 73 Amundsen et al.
(1992)
a NR = not reported
Amundsen et al. (1992) analysed air samples from Birkenes,
southern Norway, for aluminium and found concentrations to be
highest in the spring period from March to May. The authors
concluded that this was due to soil dust from wind erosion and
agricultural activities because soils in Europe are likely to be dry
during this period. Windom (1981) measured aluminium concentrations of
28 000 ng/m3 during a dust storm.
Aluminium was found to be concentrated up to 2650 ng/m3 in the
Baltimore harbour tunnel, a two-fold increase on the air intake levels
(Ondov et al., 1982).
5.1.2 Precipitation
Aluminium has been measured in atmospheric precipitation in the
USA at concentrations of up to 1200 �g/litre (Feth et al., 1964;
Fisher et al., 1968; Norton, 1971).
Feth et al. (1964) analysed snow samples from the northern Sierra
Nevada, USA, in 1959. Aluminium was detected in 7 out of 8 samples at
a mean concentration of 30 �g/litre. Ecker et al. (1990) measured
aluminium in wet-deposited snow at several sites in Japan, both
urban and rural. Mean aluminium concentrations ranged from 9.6 to
25.8 �g/litre. Average aluminium concentrations of freshly deposited
snow at Shiramine (a mountain site) were 2.4 �g/litre in the insoluble
fraction (> 0.45 �m) and 15.0 �g/litre in the soluble fraction (<
0.45 �m).
Rainwater collected in southern central Florida, USA, between
1967 and 1969 contained aluminium concentrations ranging from not
detectable to 900 �g/litre (Dantzman & Breland, 1969). Guieu et al.
(1991) monitored rainfall at 45 sites in the south of France and found
mean aluminium concentrations of 487 �g/litre and 55 �g/litre in the
particulate (> 0.4 �m) and dissolved fractions (< 0.4 �m),
respectively. Cawse (1974) measured aluminium in rainfall and dry
deposition from seven non-urban United Kingdom sites in 1972 and 1973.
Aluminium concentrations ranged from 56 to 14 800 �g/litre for
rainfall and from 3.9 to 42 �g/cm2 per year for dry deposition.
Aluminium concentrations in rainfall, total and dry deposition were
measured for Bagauda, Nigeria, in 1976. Aluminium concentrations were
1700 �g/litre for rainfall and 220 and 145 �g/cm2 per year for total
and dry deposition, respectively (Beavington & Cawse, 1979).
5.1.3 Water
5.1.3.1 Freshwater
Surface freshwater aluminium concentrations can vary
significantly, being dependent on the various physicochemical and
mineralogical factors described in section 4.1. Aluminium can occur in
a number of different forms in freshwater. It can be suspended or
dissolved. It can be bound with organic or inorganic ligands, or it
can exist as a free aluminium ion. It can exist as a monomer in
natural water, but tends to polymerize with time (see section 2.3.4).
Aluminium speciation is determined by pH, dissolved organic carbon
(DOC), fluoride, sulfate, phosphate, silicate and suspended
particulate matter. Dissolved aluminium concentrations for water in
the circumneutral pH range are usually quite low, ranging from 1.0 to
50 �g/litre, and rise to 500 to 1000 �g/litre in more acidic waters.
At the extreme acidity of waters affected by acid mine drainage,
dissolved aluminium concentrations of up to 90 mg/litre can be
measured (Filipek et al., 1987). Aluminium can also be leached from
landfill containing coal combustion ash and aluminium smelting wastes
(Sorenson et al., 1974). The concentrations of aluminium in freshwater
are summarized in Table 9.
Table 9. Concentrations of aluminium in freshwater
Area Year pH Particle Aluminium Detection Reference
size concentration limit
(�m) (�g/litre) (�g/litre)
Lake Gardsjon catchment, 1981 4.0-6.4 < 0.45 300-2500 Lee (1985)
Sweden
Swedish lakes 1980 5.7-8.8 69 (10-243) 10 Borg (1987)
Loch Ard Forest and 1979-1980 4.63 < 1.0 400 Caines et al. (1985)
Galloway streams, Scotland 6.62 < 1.0 25 Caines et al. (1985)
Llyn Brianne catchment, Wales 1984-1985 4.6-5.3 < 0.45 120-430 Goenaga & Williams (1990)
1984-1985 4.87 42 �Eq/litre Whitehead et al. (1988)
1984-1985 5.2 18 �Eq/litre Whitehead et al. (1988)
1984-1985 6.9 7 �Eq/litre Whitehead et al. (1988)
Rivers Esk and Duddon,
Cumbria, UK
(moderate flow) 1983-1984 4.3-7.2 20-940 Bull & Hall (1986)
(low flow) 1983-1984 4.8-7.5 5-245 Bull & Hall (1986)
Vosges mountain streams, 1990 6.96 64 Mersch et al. (1993)
France 1990 4.64-5.74 185-351 Mersch et al. (1993)
Boglakes, NE Belgium 1984-1986 3.4-3.9 < 0.4 150-3770 20 Courtijn et al. (1987)
1984-1985 3.4-3.9 > 0.4 3200-65 000 Courtijn et al. (1987)
1984-1986 6.0 < 0.4 < 20 20 Courtijn et al. (1987)
1984-1985 6.0 > 0.4 78 100-145 100 Courtijn et al. (1987)
Table 9. (Con't)
Area Year pH Particle Aluminium Detection Reference
size concentration limit
(�m) (�g/litre) (�g/litre)
Stream water, California, USA < 0.45 15 7 Silvey (1967)
St Lawrence river, USA 1974-1976 7.6-8.0 < 0.4 64 Yeats & Bewers (1982)
1974-1976 7.6-8.0 > 0.4 964 Yeats & Bewers (1982)
South central Florida, streams 1969 200-300 Dantzman & Breland
(1969)
Highway drains, Louisiana 1990 412 (250-1270) Madigosky et al. (1992)
Northern California streams 1972 5-10 Jones et al. (1974)
Shield lakes, Ontario and 1982 4.4-7.1 46-372 Stokes et al. (1985)
Quebec
Zaire river 1976 6.8 < 0.45 28-44 van Bennekom & Jager
(1978)
Niger river 1976 6.7 < 0.45 3-6 van Bennekom & Jager
(1978)
Reservoirs, Madras, India 1991 14 (5-210) 2 Pitchai et al. (1992)
Orange river, Vioolsdrif, 1958-1959 6.4-8.1 36-1080 de Villiers (1962)
South Africa
River Yodo, Japan 1981-1990 10-1150 Yagi et al. (1992)
Aluminium occurs ubiquitously in natural waters. Aluminium levels
in surface waters can be increased by intense urban and industrial
activity (Eisenreich, 1980). Kopp & Kroner (1970) monitored rivers and
lakes in the USA from 1962 to 1967 and detected aluminium in 31%
of samples. Mean dissolved aluminium levels ranged from 11 to
333 �g/litre, the highest levels of 2760 �g/litre being measured in
the Missouri river. Aluminium was found to be predominantly in the
suspended sediment fraction (> 0.45) with a mean concentration of
3860 �g/litre (compared with the dissolved phase at 74 �g/litre).
Filipek et al. (1987) reported that weathering of sulfide ores
exposed to the atmosphere in inactive mines and tailings dumps
released large quantities of sulfuric acid and metals such as
aluminium (up to 90 mg/litre). Boult et al. (1994) measured aluminium
in the Afon Goch, Anglesey, Wales, a stream polluted by mine drainage.
Sampling sites with pH 2.40, 5.99 and 6.49 gave mean soluble aluminium
concentrations (< 0.45 �m) of 55.56, 1.14 and 0.12 mg/litre,
respectively; mean aluminium concentrations in the particulate phase
(> 0.45 �m) were 0.31, 2.15 and 0.69 mg/litre, respectively. Koga
(1967) sampled water discharged from Wairakei drill holes in 1965.
Total aluminium concentrations in filtered water ranged from 0.023 to
0.05 mg/litre. Zelenov (1965) found elevated levels (4913 mg/litre) of
aluminium in water of a volcanic crater lake in Indonesia.
The concentrations of dissolved aluminium in water vary with pH
levels and the humic-derived acid content of the water (ATSDR, 1992).
Watt et al. (1983) compared Nova Scotian river water samples from
1954-1955 with those from 1980-1981 and found that significant
decreases in the pH corresponded to significant increases in dissolved
aluminium. High aluminium concentrations occur in surface waters when
the pH is less than 5 (Sorenson et al., 1974; Filipek et al., 1987).
In general, aluminium levels in surface waters at pH levels above 5.5
will be less than 0.1 mg/litre (Sorenson et al., 1974). However, even
at neutral pHs, higher aluminium levels have been detected where the
humic acid content is high (ATSDR, 1992).
In the Thousand Lake Survey in Norway (Henriksen et al., 1988b),
90% of the lakes with pH below 5.4 had a concentration of inorganic
monomeric aluminium above 60 �g/litre. Lakes with a pH of 4.6-4.8
and 4.8-5.0 had concentrations of 146-170 and 101-135 �g/litre,
respectively.
Generally, the data indicate that total aluminium concentrations
in surface waters are elevated during periods of high flow, following
episodic storm events, and/or during spring snow melt. Many studies
also reported corresponding increases in the labile or inorganic
aluminium fraction during these periods (LaZerte, 1984; Bull & Hall,
1986; Henriksen et al., 1988c; Lawrence et al., 1988).
Jones et al. (1974) monitored streams in Northern California and
found that samples collected during low flow periods contained
aluminium concentrations of between 1 and 3 �g/litre, whereas those
collected during moderate flow periods contained 10 �g/litre. Values
of aluminium that were higher than expected were associated with storm
run-off.
Caines et al. (1985) monitored streams in the Loch Ard Forest
and Galloway areas of Scotland during 1978, 1979 and 1980. The
concentrations of aluminium were found to be very closely dependent on
the hydrogen ion concentrations in the stream water. The maximum
concentration of aluminium in a stream with an average pH of 6.62 was
25 �g/litre, compared with almost 400 �g/litre in a stream with an
average pH of 4.63. The seasonal variation that occurred in the most
acid stream had a maximum of 394 �g/litre in February, which declined
rapidly to 35 �g/litre in May, during 1980. The authors stated that
the sharp decrease coincided with a period of low rainfall which
resulted in a rise in stream pH and greatly reduced leaching of
aluminium from the catchment.
Bull & Hall (1986) measured aluminium in the rivers Esk and
Duddon, Cumbria, United Kingdom, and their tributaries. The findings
showed a relationship between inorganic aluminium and pH, while
organic aluminium was generally low in these rivers. In general, lower
pHs and higher aluminium concentrations occurred at higher river flow
rates.
Ecker et al. (1990) monitored aluminium in the first and last
meltwater run-off from snowfields at Shiramine, a mountainous area in
Japan. Average aluminium concentrations were non-detectable and
445.6 �g/litre in the insoluble fraction (> 0 45 �m) of the first and
last run-offs, respectively, and 25.0 and 19.9 �g/litre, respectively,
in the soluble fraction (< 0.45 �m).
5.1.3.2 Seawater
The concentrations of aluminium in seawater are summarized in
Table 10. The concentration of aluminium is dependent on the salinity
of the water. Concentrations in open seawater are typically around
1 to 2 �g/litre in the dissolved fraction (< 0.45 �m). Bruland (1983)
stated that the concentration of aluminium in surface seawater in the
open ocean reflects atmospheric input and scavenging processes; the
concentration is low at high latitudes in the North Atlantic because
of a low atmospheric input and a higher scavenging rate resulting from
intensified biological activity in these waters. Aluminium is higher
in surface waters of mid-latitudes due to the higher atmospheric input
and lower scavenging rate in these oligotrophic waters.
Table 10. Concentrations of aluminium in seawater
Area Year Depth Salinity Particle Aluminium Reference
(m) (%) size concentration
(�m) (�g/litre)
Open Atlantic Ocean 1980-1982 15.2 and Kremling (1985)
25.4 nmol/kg
North-East Atlantic 1982 < 150 35-37 16-32 nmol/litre Hydes (1983)
Ocean 1982 > 1000 35-37 6-11 nmol/litre
Atlantic Ocean near 1974 300-1100 0.3-4.26 Alberts et al. (1976)
Carribean islands 1977-1978 0-2730 33-37 0.51-2.41 Stoffyn & Mackenzie (1982)
Atlantic Ocean near 1951-1952 0-10 Simons et al. (1953)
USA coast
Gulf of Mexico > 0.45 2.0 (0.2-10.2) Feely et al. (1971)
1951-1952 2-5 Simons et al. (1953)
Pacific Ocean near < 0.45 1 Sackett & Arrhenius (1962)
USA coast > 0.45 0.2-27 Sackett & Arrhenius (1962)
0.3 < 0.45 9.8 (3.7-166) Silvey (1967)
Weddel Sea, Antarctica < 0.45 1 Sackett & Arrhenius (1962)
> 0.45 4-120 Sackett & Arrhenius (1962)
North Sea 1988 6 34-35 < 0.4 10.2-49.2 Hydes & Kremling (1993)
nmol/litre
Mediterranean Sea 1976-1977 0-1500 35-38 1.0-4.8 Stoffyn & Mackenzie (1982)
5.1.4 Soil and sediment
Aluminium partitions from water to sediment and particulate
matter especially at circumneutral pH. The concentrations of aluminium
in sediment are summarized in Table 11. Mean aluminium concentrations
range from 20 000 to 80 000 mg/kg. Subramanian et al. (1988) measured
heavy metals in the bed sediments and particulate matter of the Ganges
Estuary, India. Average aluminium concentrations were 56 526 mg/kg for
bed sediments and 70 222 mg/kg for suspended sediments. Sanin et al.
(1992) measured elements in the sediments of the river Goksu and the
Tasucu Delta, Turkey. Mean aluminium concentrations ranged from
20 700 to 26 800 mg/kg for the river and from 30 150 to 42 875 mg/kg
for the delta. Benninger & Wells (1993) sampled sediment from the
Neuse river estuary, North Carolina, USA, between 1982 and 1990.
Aluminium concentrations ranged from 2.1 to 4.9 mmol/g, equivalent to
10.7% to 25.0% aluminium oxide. Fileman et al. (1991) found a mean
aluminium level of 2490 mg/kg in suspended particulate material from
the Dogger Bank region of the central North Sea.
Aluminium is one of the most abundant elements in soil and
concentrations vary widely. Shacklette & Boerngen (1984) collated the
aluminium concentrations measured by the US Geological Survey; levels
ranged from 700 to 100 000 mg/kg with an average of 72 000 mg/kg.
Beavington & Cawse (1979) analysed soil from Baguada, Nigeria, and
found aluminium levels of 24 000 �g/g (dry weight).
Table 11. Concentrations of aluminium in sediment
Area Yeara Aluminium Reference
concentration (mg/kg)
River Goksu, NR 20 800 to 26 600 Sanin et al.
Turkey (1992)
Tasucu Delta, NR 30 150 to 42 875 Sanin et al.
Turkey (1992)
Tadenac Lake, 1979 31 000 to 64 800 Wren et al.
Ontario (1983)
Turkey Lakes, 1981-1982 31 000 to 56 300 Johnson et al.
Ontario (1986)
Hamilton, 1986-1987 25 941 to 68 870 Irvine et al.
Ontario (1992)
(urban run-off)
Fontana Lake, 1978 36 400 to 84 600 Abernathy et al.
North Carolina (1984)
a NR = not reported
Aluminium is found in soil interstitial water at levels similar
to those reported for freshwater. Litaor (1987) measured a mean
aluminium concentration of 24.8 �mole/litre for the interstitial water
(pH 6.0) of soil from the Green Lakes Valley Front Range in Colorado,
USA.
5.1.5 Terrestrial and aquatic organisms
Mason & MacDonald (1988) monitored aluminium levels in aquatic
moss ( Fontinalis squamosa) from the River Mawddach catchment, Wales
(polluted with drainage water from disused mines) in 1984 and 1985.
Mean aluminium concentrations ranged from 1970 to 26 800 mg/kg (dry
weight). Mersch et al. (1993) transplanted aquatic moss ( Amblystegium
riparium) from a non-acidified stream to streams with pH values
ranging from 4.64 to 5.74. The moss accumulated aluminium, those
exposed to acidified streams containing aluminium ranging from 10 390
to 12 700 �g/g (dry weight) and those in a control stream (pH 6.96)
containing 7750 �g/g. Caines et al. (1985) collected aquatic
liverworts ( Nardia compressa and Scapania undulata) from streams
in the Loch Ard and Galloway areas of Scotland. Mean liverwort
aluminium concentrations were 3148, 6166 and 8532 mg/kg (dry weight)
from streams containing 195, 71 and 24 �g/litre, respectively.
Bioconcentration of aluminium occurred in all streams; however,
increased hydrogen ion concentrations were associated with decreased
liverwort aluminium concentrations. Albers & Camardese (1993a)
monitored aquatic plants from acidified and non-acidified constructed
wetlands. Aluminium concentrations for bur-reed ( Sparganium
americnum) and bladderwort ( Utricularia spp.) were 167 and 533 �g/g
(dry weight), respectively, for acidified wetlands and 104 and
487 �g/g for non-acidified wetlands. Duckweed ( Lemna spp.) and green
algae ( Oedogonium spp.) contained 998 �g aluminium/g at the non-
acidified sites; acidified wetlands did not contain duckweed or green
algae. Bur-reed (Sparganium spp.), bladderwort and pondweed collected
from sites in Maryland and Maine contained aluminium concentrations of
74.6, 1740 and 296 �g/g, respectively. The accumulation of aluminium
by these aquatic plants correlated poorly with the water concentration
(Albers & Camardese, 1993b).
Leinonen (1989) collected leaves of Vaccinium myrtillus from
untreated forest, clear-cut untilled forest and clear-cut tilled land
in Kuru, southern Finland in 1987. Aluminium levels were significantly
higher in the tilled area, with levels of approximately 140 mg/kg dry
weight in untilled areas and 185 mg/kg in the tilled area. Moomaw et
al. (1959) collected a wide selection of Hawaiian plant species from
highly leached latosol soils of low pH and high aluminium content.
Aluminium concentrations ranged from 59 to 16 000 mg/kg dry weight.
Thirteen of the 23 species contained aluminium levels in excess of
1000 mg/kg; the highest levels were found in the pteridophyte
Polypodium phymatoides and the dicotyledon Melastoma malahathricum.
Beavington & Cawse (1979) analysed sorghum grain from Bagauda,
Nigeria, and found aluminium levels of 90 �g/g dry weight.
Wyttenbach et al. (1985) collected needles of Picea abies from
around the city of Winterthur, Switzerland. The washed needles were
analysed for a wide range of elements including aluminium. The mean
aluminium content of the needles was 19 mg/kg (10-64 mg/kg). It was
found that washing the needles had removed more than 80% of the
aluminium residue. Landolt et al. (1989) sampled spruce needles from
locations throughout Switzerland in 1983 to study the distribution of
elements. The mean aluminium concentration was found to be 61.4 mg/kg
with a range of 12.88 to 344.5 mg/kg. H�s�nen & Huttunen (1989)
measured the aluminium content of the annual rings of pine trees
( Pinus sylvestris). The mean concentration for the period 1920 to
1980 was 4.2 mg/kg (3.4-5.1 mg/kg). In the areas associated with
higher sulfur deposition there had been increases in aluminium uptake
since 1950.
Malley et al. (1987) collected crayfish ( Orconectes virilis)
from a lake in northwestern Ontario containing a total aluminium
concentration of 36 �g/litre. Mean aluminium concentrations in the
crayfish were highest in the gut tissue (774 mg/kg) and there were
levels of 65.2, 84.4 and 50.4 mg/kg in the carapace, green gland and
ovary, respectively. Madigosky et al. (1991) monitored red swamp
crayfish ( Procambarus clarkii) from roadside drainage ditches
in Louisiana, USA. Aluminium concentrations ranged from 1.75
to 981.50 mg/kg dry weight in the order abdominal muscle
< hepatopancreas < exoskeleton < alimentary canal tissue. The
crayfish contained significantly higher levels of aluminium than those
found in control crayfish sampled from a commercial crayfish farm.
Madigosky et al. (1992) collected crayfish during 1990 from a site
near to a Louisiana highway intersection. Aluminium concentrations
were 2409 and 2342 mg/kg for intestinal tissue and contents,
respectively, while concentrations of 527 and 27 388 mg/kg were found
for stomach tissue and contents, respectively. It was found that
purging the crayfish in 1.5% sodium chloride for 6 h did not
significantly reduce aluminium in the gut tissue. However, there was
an increase in the water concentration of aluminium probably caused by
its release from exterior tissue sites.
Albers & Camardese (1993a) collected aquatic insects from both
acidified and non-acidified constructed wetlands; aluminium
concentrations were 94.3 and 158 �g/g (dry weight) for the two types
of wetland, respectively. Albers & Camardese (1993b) analysed aquatic
insects from sites in Maryland (224 �g/g) and in Maine (102 �g/g). The
same authors analysed crayfish and snails from sites in Maryland and
Maine in 1987. Whole body aluminium concentrations were found to be 66
to 542 �g/g (dry weight) and 27 to 398 �g/g for the two species,
respectively.
Brumbaugh & Kane (1985) collected smallmouth bass ( Micropterus
dolomieui) from the Chatuge reservoir on the border between Georgia
and North Carolina, USA. The reservoir receives run-off from poorly
buffered, forested watersheds, and the average pH of the reservoir was
6.3. Mean aluminium concentrations were 58 �g/g wet weight for gills
and 3.0, 2.5, 1.5 and < 1.0 �g/g for the carcass, gut, liver, and
kidney, respectively. Fish collected from the vicinity of a liquid
waste site in North Carolina, USA contained mean aluminium levels
ranging from 10.9 to 18.2 mg/kg (wet weight - based on whole gutted
fish) (Loehle & Paller, 1990). Buergel & Soltero (1983) analysed
plankton and fish ( Oncorhynchus mykiss) from a lake in Washington
State, USA, that had been treated with aluminium sulfate to reduce
high phosphorus concentrations. Total and dissolved aluminium in the
lake water ranged from 0.16 to 0.75 mg/litre, and from 0.09 to
0.42 mg/litre, respectively. Aluminium concentrations in plankton
ranged from 6.53 to 49.81 mg/kg, while those in various fish tissues
ranged from 0.07 to 6.25 mg/kg with the highest levels concentrated in
the gills. The aluminium concentrations measured in the fish were not
significantly different from those analysed in fish from untreated
lakes. Berg & Burns (1985) compared the aluminium concentrations in
fish tissues from a lake receiving water treatment plant sludge
containing aluminium hydroxide with a control lake. Both lakes had pH
values in the range 7.0 to 8.0. Dissolved aluminium was 0.1 mg/litre
in the treated lake and < 0.1 mg/litre in the control lake. Aluminium
was found in all tissues of all fish analysed. Liver, kidney and gill
samples from channel catfish ( Ictalurus punctatus) taken from the
polluted lake contained significantly more aluminium than those from
the control lake. For catfish brain and muscle, and for all tissues
from largemouth bass ( Micropterus salmoides) and gizzard shad
( Dorosoma cepedianum) there were no significant differences.
Aluminium concentrations ranged from 60.8 to 1808.9 mg/kg; the highest
concentrations were found in the liver and brain.
Karlsson-Norrgren et al. (1986a) collected and analysed brown
trout ( Salmo trutta) from two fish farms within acid-susceptible
areas in Sweden using lime-treated waters. Preliming, the water had a
pH of 4.6 to 4.7, with total and labile aluminium concentrations of
390-516 and 270-300 �g/litre, respectively. The post-liming water
quality (to the hatchery) was 208-261 �g/litre as total aluminium and
12-80 �g/litre as labile aluminium. Trout from a third non-acidified
location (pH 6.9; total aluminium levels in water 35 �g/litre) were
also analysed. Aluminium concentrations ranged from 89.3 mg/kg (wet
weight) for gills to 0.8 mg/kg for muscle in fish from acid-
susceptible areas. Fish from the control area contained aluminium
ranging from 2.6 mg/kg in the intestine to 0.6 mg/kg in muscle, while
levels in the gills were 1.9 mg/kg.
Hellou et al. (1992a) analysed muscle samples from the bluefin
tuna ( Thunnus thynnus) collected off the cost of Newfoundland,
Canada, in 1990. Aluminium concentrations ranged from 0.4 to 1.9 �g/g
dry weight with a mean value of 1.0 �g/g. Hellou et al. (1992b) found
aluminium concentrations of < 1 to 8 �g/g dry weight in muscle, liver
and ovaries of cod ( Gadus morhua) sampled from several sites off the
coast of Newfoundland during 1990 and 1991.
Wren et al. (1983) analysed fish, bird and mammal muscle from
Tadenac Lake (a Precambrian Shield lake), Ontario, Canada, and its
surrounding area. The lake had a pH of 7.1 and contained 47 400 mg
aluminium/kg in the sediment. Mean aluminium concentrations ranged
from 1.7 to 2.8 mg/kg (wet weight) for fish and from 2.5 to 5.2 mg/kg
for birds and mammals.
5.2 Occupational exposure
The levels of aluminium to which workers are exposed vary greatly
according to the type of industry and whether adequate industrial
hygiene practices are adhered to. Most studies have dealt with
inhalation of aluminium-containing dust particles rather than
aluminium per se. Some, however, have utilized urinary aluminium
determinations as an indicator of exposure (Sj�gren et al., 1983;
Gitelman et al., 1995). Utilizing such a technique for exposure is
essential, since it is rare for a worker to be exposed solely to
aluminium but rather to a mixture of aluminium-containing dusts and
chemicals.
Occupational exposure limits for aluminium fumes and dust have
been developed in many countries. Time-weighted averages of 5 mg/m3
(respirable dust) and 10 mg/m3 (total dust) have generally been
accepted. However, an occupational exposure limit of 1 mg/m3
calculated as aluminium has been proposed in Sweden regarding
aluminium-containing respirable fumes (Sj�gren & Ekinder, 1992).
Given the minimal amount of data on actual aluminium levels in
workplace air, it is difficult to estimate a daily exposure from the
occupational setting. Based on a recent publication, aluminium process
and production workers are generally exposed to less than 1 mg per 8-h
shift, assuming 10 m3 inhaled per shift (Gitelman et al., 1995). It
should be noted that, in some occupations and under less than optimal
industrial hygiene practices, occupational exposures to aluminium
could be higher. Welders performing metal-inert gas welding have been
exposed to 4 mg/m3 (calculated as aluminium) and these particles are
generally less than 1 �m (Sj�gren & Ulfvarson, 1985; Sj�gren et al.,
1985). Assuming 10 m3 inhaled per shift, this implies an exposure of
40 mg per shift.
Occupational exposures have been reported as total dust or
particulate matter: e.g., potroom workers, 1.67 mg/m3 (Kongerud &
Samuelsen, 1991); production of abrasives, 0.2 to 44.6 mg aluminium
oxide/m3 (Jederlinic et al., 1990); MIG welders, 10 mg/m3; TIG
welders, 1 mg/m3; respirable particles with a mean aluminium content
of 39% (Ulfvarson, 1981; Sj�gren et al., 1985) and aluminium soldering
of aluminium cables, 1.1 mg/m3 respirable dust decreasing to
0.7 mg/m3 after installation of a vacuum collection system
(Hjortsbert, 1994).
5.3 General population exposures
5.3.1 Air
Pulmonary exposure to aluminium is determined by air
concentration, particulate size and ventilatory volume. Air
concentrations vary between low levels in rural settings
(20-500 ng/m3) and higher levels in urban settings
(1000-6000 ng/m3) (see Table 8). Particles larger than 5-10 �m
diameter tend to be removed from inhaled air and penetrate poorly into
the lungs. Humans living in an urban area with ambient aluminium
concentrations of about 2000 ng/m3, particle size < 5 �m and a
ventilatory volume of 20 m3/day would be exposed to 40 �g
aluminium/day by inhalation.
5.3.2 Food and beverages
Since aluminium is a major component of the earth's crust, it is
naturally present in varying amounts in most food-stuffs consumed. The
actual concentration in food and beverages from various countries will
vary widely depending upon the food product, the type of processing
used and, in particular, the levels of aluminium-containing food
additives permitted and the geographical area in which food crops are
grown. In general, the foods highest in aluminium are those than
contain aluminium additives (e.g., grain products (flour), processed
dairy products, infant formulae, etc.). Foods naturally high in
aluminium include baked potato (skin on), spinach, prune juice and tea
(Pennington & Schoen, 1995).
The preparation and storage of food in aluminium vessels, foil or
cans, may increase the aluminium content, particularly in the case of
foods that are acidic, salty or alkaline (Greger et al., 1985b; Nagy &
Nikdel, 1986; Baxter et al., 1988). Preparing acidic foods such as
tomatoes and rhubarb in aluminium pans was found to lead to a
significant increase in the level of aluminium in the food (0.5 mg/kg
wet weight raw tomatoes to 3.3 mg/kg wet weight cooked), whereas only
a slight increase was noted in similarly prepared rice or potatoes
(Greger et al., 1985b). Although individual foodstuffs may leach
aluminium from the vessel, there are indications that aluminium from
cookware represents only a small fraction of the total dietary intake
(Kupchella & Syty, 1980; Savory et al., 1987).
The total intake of aluminium from food and beverages (excluding
drinking-water) in several countries is given in Table 12. All
estimates are less than 15 mg/day, with the lower values probably
reflecting a lower use of aluminium additives in the preparation of
cereal grain products (bread, etc.) (UK MAFF, 1993).
Table 12. Estimated average dietary intake of aluminium in various countries
Country Method of Estimated intake of Reference
samplinga aluminium
(mg/day)
Australia MB 2.4 (male) NFA (1993)
1.9 (female)
Canada MB 0.08-0.69b (infants) Dabeka &
McKenzie (1992)
Finland TD 6.7 Varo &
Koivistoinen (1980)
Germany MB 11.0 (males) Treptow &
MB 8.0 (females) Askar (1987)
DD 0.78 (5-8 years old) Wilhelm et al.
(1995)
Japan TD 4.5 Teraoka et al.
(1981)
Netherlands DD 3.1 (mean male and female) Ellen et al. (1990)
Sweden DD 13.0 (female) Jorhem &
Haegglund (1992)
Switzerland DD 4.4 Knutti &
Zimmerli (1985)
UK TD 0.03-0.05 (4-month infant)c UK MAFF (1993)
0.27-0.53 (4-month infant)d
TD 3.9
USA TD 0.7 (6-11 month old infant) Pennington &
6.5 (6 years old) Schoen (1995)
11.5 (14-16 year old male)
7.1 (adult female)
8.2 (adult male)
a MB = Market basket survey; TD = Total diet study; DD = Duplicate diet study
b Range represents intake of an infant (0-1 month old) fed cow's milk to that
for an infant (1-3 month old) fed exclusively soya-based formulae
c Range for infants fed cow's milk-based formulae
d Range for infants fed soya-based formulae
5.3.3 Drinking-water
Aluminium levels in drinking-water, whether distributed through
household plumbing or as bottled water, vary according to the natural
levels found in the source and whether aluminium flocculants were used
during the purification process. An international drinking-water
guideline for aluminium was based on aesthetic rather than health
grounds (WHO, 1993).
Results from extensive monitoring of drinking-water supplies have
been obtained from Germany (Wilhelm & Idel, 1995), Ontario Canada
(OMEE, 1995), and the United Kingdom (UK MAFF, 1993).
In Germany, levels of aluminium in public water supplies averaged
(median) 10 �g/litre in the western region while 2.7% of public
supplies in the eastern region exceeded 200 �g/litre. It was estimated
that 500 000 people were exposed to these high levels. Aluminium
levels of up to 10 000 �g/litre were reported in drinking-water from
private wells in areas where the soil had low buffering capacity and
was subjected to high acidic stress (M�hlenberg, 1990; Wilhelm & Idel,
1995).
In a province-wide survey of the aluminium content of public
water supplies in Ontario, Canada, approximately 75% of all average
levels in 1993 and 1994 were less than 100 �g/litre, the present
operational guideline for Ontario (OMEE, 1995). The range of average
values was 40 to 851 �g/litre.
A large monitoring programme in 1991 by the water companies in
the United Kingdom (75 305 samples) reported that only 553 (0.7%)
exceeded the United Kingdom aluminium standard of 200 �g/litre (UK
MAFF, 1993). Drinking-water would add about 400 �g aluminium to the
daily intake, assuming a consumption of 2 litres water daily at the
aesthetic guideline value of 200 �g/litre (WHO, 1993). From the
monitoring data discussed above and normal intakes of water, a more
realistic intake would be at or below 200 �g/day from monitored
municipal supplies.
5.3.4 Miscellaneous exposures
The use of antacids and buffered analgesics may result in large
intakes of aluminium, far in excess of that normally consumed in food
(Shore & Wyatt, 1983; Lione, 1983; Schenck et al., 1989). It has been
estimated that daily doses of aluminium in antacids and buffered
analgesics range from 840 to 5000 mg and 130 to 730 mg per day,
respectively (Lione, 1983). These are approximately two to three
orders of magnitude greater than normal dietary intakes (see Table 12)
and well in excess of the recommended provisional tolerable weekly
intake (PTWI) of 420 mg for a 60-kg adult (FAO/WHO, 1989).
Aluminium compounds are widely used in the preparation of
cosmetics, particularly in antiperspirants (Sorenson et al., 1974).
However, there are no reliable data supporting dermal absorption from
such products.
5.3.5 Total human intake of aluminium from all environmental pathways
In calculating total human exposures one must be aware of the
quality of the sampling and analytical procedures, particularly when
using data from earlier studies. Total intake of aluminium must
consider all routes of exposure, i.e. inhalation, oral and dermal.
For humans, non-occupationally exposed to aluminium, oral intake
of aluminium represents the major route of exposure. As shown in Table
12 the total daily intake of aluminium in adults ranges from 2.5 to
13 mg/day, depending upon the country of origin as well as the age and
sex of the subject. The variation reflects different dietary habits as
well as the level of additives used in food processing. For infants
(under 6 months) daily intakes range from 0.27 to 0.53 mg/day for
those consuming soya-based formulae and 0.03 to 0.05 mg/day for
infants consuming cow's milk formulae (UK MAFF, 1993). Similar values
were reported from Canada (respectively, 0.08 and 0.69) (Dabeka &
McKenzie, 1990). Aluminium intake from breast milk has been calculated
to be < 0.04 mg/day (UK MAFF, 1993).
In conclusion, the total intake of aluminium by the general
population varies between 2.5 and 13 mg/day. In most countries over
95% of this comes from food and less than 1% from airborne aluminium.
As noted in section 5.3.4, these intakes can be increased greatly
(10 to 100 times) through the use of aluminium-containing antacids and
buffered analgesics. Total daily exposure to aluminium from all
sources, other than medicines, and for all age groups has been shown
to be less than the PTWI of 1 mg/kg per day (WHO, 1993).
5.3.6 Aluminium uptake
In view of the fact that over 95% of the normal daily intake of
aluminium comes from food and water, uptake from the gastrointestinal
tract will play a major role in determining tissue levels of the
metal. The ratio of intake to uptake will be a major determinant in
the risk of orally ingested aluminium to humans. Factors affecting
gastrointestinal absorption of aluminium are discussed in section
6.1.2.
Recent studies of the bioavailability and uptake of aluminium in
human volunteers have employed the radioactive isotope 26Al, which
may be detected at very low masses, i.e. 5 � 10-15 g using accelerator
mass spectrometry (AMS). The first of these was a study by Day et al.
(1991), who measured the uptake of aluminium in one volunteer
following the ingestion of 1.1 �g of the isotope in sodium citrate.
For this study aluminium uptake was assessed by extrapolation from a
single measurement of 26Al in blood plasma 6 h after administration.
The fraction of absorbed aluminium was estimated to be 1%. Later, the
same technique was employed by Day et al. (1994) to estimate aluminium
uptake from orange juice (with or without added silicate) in control
subjects and Down�s syndrome patients. For the normal subjects uptake
factors ranging from 0.04 to 1.5 � 10-4 were calculated. The addition
of silica reduced the uptake by a factor of about 7. In the Down�s
syndrome patients, many of whom develop AD, uptake was approximately 5
times higher than in controls (4.7 � 10-4 compared with 0.91 � 10-4).
Most recently, human bioavailability studies have been undertaken
by Priest (1994) using a more vigorous methodology, employing the
collection of blood samples and total excreta for a period of up to a
week after a single administration of the aluminium compound. The
results obtained showed significant intersubject variability in the
extent and timing of aluminium absorption and indicated that the
method employed by Day et al. (1994) was of limited utility. Two main
studies were undertaken. The first was a study of the uptake of
aluminium, as aluminium citrate, aluminium hydroxide and aluminium
hydroxide in the presence of citrate, from the gut following the
administration of 100 mg aluminium by gastric tube (Priest, 1994). The
measured fractional uptakes were as follows: 5 � 10-3 for aluminium
as citrate; 1.04 � 10-4 for aluminium hydroxide; 1.36 � 10-3 for
hydroxide in the presence of sodium citrate. This study demonstrated
the greater bioavailability of the citrate complex and the ability of
citrate to enhance the uptake of aluminium taken in another chemical
form. The second study measured the fractional uptake of aluminium
from drinking-water using a similar technique, but different
volunteers (Priest et al., 1995a,b,c). The measured uptake fraction
was 2.2 � 10-4. It was concluded that members of the public, drinking
1.5 litres per day of water containing 100 �g aluminium/litre, would
absorb from this source about 3% of their total daily aluminium
uptake. This result suggests that drinking-water, under most
circumstances, is likely to be a minor source of aluminium for humans.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
Investigations into the kinetics of aluminium include estimation
of typical toxicokinetic parameters as well as issues specifically
related to the chemistry of aluminium and its compounds. Many studies
have been performed at high-dose levels. Since there are indications
that the toxicokinetics of aluminium are dose-dependent, these results
should be interpreted cautiously with respect to their relevance to
humans (Wilhelm et al., 1990). In addition, owing to large variations
in experimental protocols employed, many data-sets are not comparable,
making the interpretation of these data very difficult.
6.1 Absorption
6.1.1 Animal studies
6.1.1.1 Inhalation exposure
Reports of systematic studies of the pulmonary absorption of
aluminium in experimental animals have not been identified. However,
aluminium has been detected in organs other than the lung following
some inhalation experiments.
In rats and guinea-pigs exposed for 24 months to 0.25-25 mg/m3
aluminium chlorohydrate, aluminium was present primarily in the lungs.
The only other organs with significant concentrations of aluminium
were the peribronchial lymph nodes in guinea-pigs and the adrenal
glands in rats (Stone et al., 1979).
In New Zealand rabbits exposed to 0.56 mg aluminium/m3 for
5 months, there was a 2.5 fold increase in the aluminium content of
the brain (10.1 mg/kg dry weight) compared to control (4.1 mg/kg dry
weight) animals, while the concentration of aluminium in serum was
only slightly increased (R�llin et al., 1991a).
6.1.1.2 Oral administration
The gastrointestinal tract is the most important port of entry.
In addition, inhaled aluminium aerosols that are cleared from the
surface of the mucous membranes of the respiratory tract by action of
the mucociliary escalator are swallowed and thus may be absorbed from
the gastrointestinal tract.
Based on available data, absorption via the gastrointestinal
tract in experimental animals is generally less than 1%. However,
estimates of the proportion absorbed vary considerably, in part, as a
result of the different conditions of exposure (i.e., use of citrate
versus hydroxide salts, etc.) to various compounds (see Table 13).
Values from balance studies are probably overestimates, since the
amount of aluminium retained in the gut was probably calculated as
absorbed aluminium. The
rather high value obtained by Gupta et al. (1986) has not been
confirmed. Some studies on aluminium uptake after oral administration
of various compounds are summarized in Table 14 and Table 15, where
uptake has been measured by blood aluminium levels or tissue levels.
Results from studies on isolated intestinal organ systems
support the findings of low absorption rates of aluminium from the
gastrointestinal tract (J�ger et al., 1991). Also, although not
directly applicable to the human situation, experiments where
aluminium salts have been given to rats and mice by interperitoneal
injection further support the low amount of aluminium absorbed from
the gastrointestinal tract (Leblondel & Allain, 1980; Muller et al.,
1992; Greger & Powers, 1992). For example, blood aluminium levels in
rats given 10 mg aluminium chloride/kg body weight per day for 11 days
were about 15 times greater than controls (20 �g/litre compared to
300 �g/litre) (Muller et al., 1992). In contrast, in rats fed 0.1%
aluminium chloride in the diet for up to 25 days there was only a 22%
increase in blood aluminium levels (0.91 mg/litre compared to
1.11 mg/litre) (Mayor et al., 1977).
The mechanism of intestinal absorption of aluminium is fairly
complex and not yet fully elucidated (van der Voet, 1992). This
complexity results from the very particular chemical properties of the
element, i.e. (1) great variability of solubility at different pH
values, amphoteric character, and formation of various chemical
species depending on the pH, the ionic strength and the presence of
complexing agents in the intestine (Martin, 1992), and (2) the complex
organisation of the mammalian digestive tract where the chyme passes
through a sequence of chemical environments differing in pH, presence
of secretory products, etc. In addition, the different parts of the
intestine may be distinct with regard to their resorptive properties
and may be influenced by variation in physiological conditions. There
are indications that aluminium interacts with the gastrointestinal
calcium transport system (Adler & Berlyne, 1985; Provan & Yokel, 1988)
and with transferrin-mediated iron uptake (van der Voet & de Wolff,
1987; J�ger et al., 1991). There is consistent evidence that
absorption of aluminium increases in the presence of citrate (Slanina
et al., 1986; Froment et al., 1989a,b). There are some data suggesting
that uptake increases after fasting (Walton et al., 1994).
6.1.1.3 Dermal
Aluminium absorption via the skin in animals has not been
studied.
Table 13. Gastrointestinal absorption of aluminium compoundsa
Species Dose Form f (%)b Methodc Remarks References
Rat 8.1 mg/kg AlCl3 27 3 Gupta et al. (1986)
Rat 1; 12 mg Al/kg lactate 0.18 2 Wilhelm et al. (1992)
Rat 1; 12 mg Al/kg lactate 0.02 3 Wilhelm et al. (1992)
Rat 35 mg Al/kg sucralfate, lactate 0.015 2 Froment et al. (1989a)
Rat 35 mg Al/kg AlCl3 0.037 2 Froment et al. (1989a)
Rat 1.20 mmol Al/kg lactate 0.037 2 Froment et al. (1989a)
Rat 3.8 ng 26Al and 0.02 2 Jouhanneau et al.
63 ng 27Al in citrate (1993)
and citrate-free 0.02 2
solutions
Rat 1; 12 mg Al/kg lactate 0.18 2 Wilhelm et al. (1992)
Rat 1; 12 mg Al/kg lactate 0.02 3 Wilhelm et al. (1992)
Rat 35 mg Al/kg sucralfate 0.015 2 Froment et al. (1989a,b)
Rat 35 mg Al/kg Al(OH)3 0.015 2 Froment et al. (1989a,b)
Rat 35 mg Al/kg AlCl3 0.037 2 Froment et al. (1989a,b)
Table 13. (Con't)
Species Dose Form f (%)b Methodc Remarks References
Rabbit 10.8, 540 mg lactate 0.70-1.9 3 no significant influence Yokel & McNamara
Al/kg of dose (1985)
Rabbit 2.5-10 mmol/kg various 0.3-2.2 3 absorption: soluble>insoluble; Yokel & McNamara
minor differences between (1989)
organic and inorganic forms;
best bioavailability, citrate;
minor influence of renal
impairment
Sheep 1-2 g/day Al2(SO4)3; 2-15 1 order of absorption: Allen & Fontenot
Al-citrate; Al2(SO4)3>Al citrate>AlCl3 (1984)
AlCl3
a Modified from: Wilhelm et al. (1990)
b f = mass Al absorbed + mass Al ingested
c 1 = balance study; 2 = estimation based on urinary excretion; 3 = comparison of areas under plasma aluminium concentration after oral
and intravenous application
Table 14. Tissue aluminium concentrations in experimental animals administered aluminium compounds orallya
Species Treatment Bone Brain Reference
Mouse (BALB/c, AlCl3, gavage n.d. n.d. Cranmer et al. (1986)
5-10/group) 200 mg/kg per day
300 mg/kg per day
Mouse (Swiss, 6/group) Al lactate, 25 mg Al/kg (5.3 mg/kg w.w.) 35.3 mg/kg w.w. Golub et al. (1989)
(= control),
500 mg Al/kg diet 5.0 mg/kg w.w. 38.3 mg/kg w.w.
1000 mg Al/kg diet 6.5 mg/kg w.w. 108.7 mg/kg w.w.
Rat (8 Wistar) 2835 mg Al/kg in feed, femur Ondreicka et al. (1966)
(as Al2(SO4)3), 24 days (702 mg/kg w.w.) (7.1 mg/kg w.w.)
912 mg/kg w.w. 10.8 mg/kg w.w.
Rat (juvenile, aluminium in water n.d. n.d. Cann et al. (1979)
male SD, 8/group) (1) control
(2) 0.32 g Al/litre, 29 days
(3) low Ca2+ + Al
Rat (male SD, 8/group) 100 mg Al/kg b.w. cortex: Slanina et al. (1984)
6 d/w; by gavage (0.36 mg/kg w.w.) (0.013 mg/kg w.w.)
Al(OH)3 (9 weeks); 0.41 mg/kg w.w. 0.013 mg/kg w.w.
Al citrate (4 weeks); x 40 increased 0.057 mg/kg w.w.
citric acid (4 weeks) x 20 increased 0.028 mg/kg w.w.
Rat (male SD, 7/group) gavage; 3 d/w; 11 week (0.22 mg/kg w.w.) (0.016 mg/kg w.w.) Slanina et al. (1985)
Al(OH)3 0.89 mg/kg w.w. 0.012 mg/kg w.w.
Al citrate 10.7 mg/kg w.w. 0.048 mg/kg w.w.
Al(OH)3 + citrate 26.6 mg/kg w.w. 0.092 mg/kg w.w.
Table 14. (Con't)
Species Treatment Bone Brain Reference
Rat (weanling, male 270 mg Al/kg diet, 18 days tibia: (1.9 mg/kg w.w.) (0.0 mg/kg w.w.) Greger et al. (1985a)
SD, 6/group) Al(OH)3 15.6 mg/kg w.w. 2.2 mg/kg w.w.
Al palmitate 15.0 mg/kg w.w. 0.6 mg/kg w.w.
Al lactate 13.0 mg/kg w.w. 1.6 mg/kg w.w.
AlPO4 14.5 mg/kg w.w. 1.3 mg/kg w.w.
Rat (weanling, male Al(OH)3 in diet, 67 days tibia: (4.04 mg/kg) n.d. Greger et al. (1986)
SD, 9/group) 257 mg Al/kg diet 11.3 mg/kg (3.13 mg/kg)
1075 mg Al/kg diet 10.4 mg/kg
Rat (male SD, 10/group) Al(NO3)3, in water, 4 w (5.75 mg/kg w.w.) (1.4 mg/kg w.w.) G�mez et al. (1986)
375 mg/kg/d 11.4 mg/kg w.w. 7.7 mg/kg w.w.
750 mg/kg/d 8.5 mg/kg w.w. 10.1 mg/kg w.w.
1500 mg/kg/d 17.7 mg/kg w.w. 7.9 mg/kg w.w.
Rat (female SD, Al(NO3)3, oral, 100 d (17.15 mg/kg w.w.) (< 0.5 mg/kg w.w.) Domingo et al. (1987b)
10/group) 360 mg/kg w.w. 75.08 mg/kg w.w. 4.93 mg/kg w.w.
720 mg/kg w.w. 79.18 mg/kg w.w. 2.09 mg/kg w.w.
3600 mg/kg w.w. 56.39 mg/kg w.w. 4.28 mg/kg w.w.
Rat (SD, weanling Al(OH)3, in feed, 10 d femur: (6.8 mg/kg d.w.) n.d. Chan et al. (1988)
6/group) 50-60 mg/kg b.w. 8.4 mg/kg d.w.
Rat (male, weanling SD) Al(OH)3, 28 d tibia: n.d. Ecelbarger & Greger
13 mg Al/kg diet 36 mmol/kg w.w. (1991)
+ 5 mmol/kg citrate 36 mmol/kg w.w.
41 mg Al/kg diet 50 mmol/kg w.w.
+ 5 mmol/kg citrate 69 mmol/kg w.w.
Table 14. (Con't)
Species Treatment Bone Brain Reference
Rat (18 male, Al(OH)3 in feed, 29 days tibia: (28.9 mmol/kg w.w.) n.d. Greger & Powers
weanling SD) 0.39 �mol Al/g diet 52.6 mmol/kg w.w. (1992)
aluminium + 4% citrate 74.4 mmol/kg w.w.
100 �mol Al/g diet 79.6 mmol/kg w.w.
+ 4% citrate
Rabbit (female NZ, inhalation exposure (18.2 mg/kg d.w.) (4.1 mg/kg d.w.) R�llin et al. (1991a)
8/group) 0.56 mg Al/m3 as Al2O3 22.2 mg/kg d.w. 10.1 mg/kg d.w.
8 h/d; 5 d/w; 5 months
Rabbit (male NZ, 50 g/kg AlCl3, in feed (n.d.) cortex, gray matter: Thornton et al.
3-4/group) 1 month (n.d.) 3.1 mg/kg d.w. (1983)
ethanol 1.3 mg/kg d.w.
aluminium + ethanol 3.0 mg/kg d.w.
Dog Al(OH)3 in feed, 3 g/d; n.d. cerebral cortex: Arieff et al. (1979)
5 months 0.77 mg/kg d.w.
2.4 mg/kg d.w.
Cattle (steer, 6/group) AlCl3 in feed, 84 d (n.d.) (6.4 mg/kg w.w.) Valdivia et al.
300 mg/kg 7.6 mg/kg w.w. (1978)
600 mg/kg 5.5 mg/kg w.w.
1200 mg/kg 7.7 mg/kg w.w.
Values in parentheses are normal control values in unexposed animals; b.w. = body weight; d = day; d.w. = dry weight;
n.d. = not detected; NZ = New Zealand; SD = Sprague-Dawley; w = week; w.w. = wet weight
Table 15. Blood aluminium concentrations in experimental animals exposed orally to aluminium compoundsa
Species Sample Dose Duration Compound Aluminium concentration Reference
(control value)
Rat (8 Wistar) blood 2835 mg Al/kg feed 24 days Al2(SO4)3 (6.5 mg/kg w.w.) Ondreicka et al.
10.8 mg/kg w.w. (1966)
Rat (male albino) serum Al(OH)3 (0.24 mg/litre) Berlyne et
150 mg Al/kg/d, gavage 0.99 mg/litre al. (1972)
Rat (male SD, serum AlCl3 (0.91 mg/litre) Mayor et al.
8/group) 0.1% aluminium in feed day 10: 1.12 mg/litre, (1977)
day 25: 1.09 mg/litre
Rat (male SD, blood 11 weeks (0.005 mg/kg w.w.) Slanina et al.
7/group) 3 d/w, gavage Al(OH)3 0.009 mg/kg w.w. (1985)
Al citrate 0.014 mg/kg w.w.
Al(OH)3 + citrate 0.039 mg/kg w.w.
Rat (male SD, blood Al(NO3)3 (3.7 mg/kg w.w.) G�mez et al.
10/group) 375 mg/kg/d 3.1 mg/kg w.w. (1986)
750 mg/kg/d 2.5 mg/kg w.w.
1500 mg/kg/d, in water 3.0 mg/kg w.w.
Rat (female SD, blood 100 days Al(NO3)3 (< 0.5 mg/kg) Domingo et al.
10/group) 360 mg/kg w.w. < 0.5 mg/kg (1987b)
720 mg/kg w.w. < 0.5 mg/kg
3600 mg/kg w.w., oral < 0.5 mg/kg
Rat (18 male, serum 29 days Al(OH)3 (0.28 �mol/litre) Greger &
weanling SD) 0.39 mmol Al/kg diet 0.98 �mol/litre Powers (1992)
aluminium + 4% citrate 1.15 �mol/litre
100 mmol Al/kg diet
+ 4% citrate, in feed 1.09 �mol/litre
Table 15. (Con't)
Species Sample Dose Duration Compound Aluminium concentration Reference
(control value)
Rat (weanling serum 160 mg Al/kg d, gavage, 10 days (18.8 �g/litre) Santos et al.
SD, 4/group) 1,25-(OH)2-D3 24.3 �g/litre (1987)
1,25-(OH)2-D3 + Al(OH)3 29.5 �g/litre
1,25-(OH)2-D3 + Al citrate 16.3 �g/litre
Rabbit (male serum 1 month (5 �g/litre) Thornton et al.
NZ, 3-4/group) 50 g/kg in feed AlCl3 14 �g/litre (1983)
Al+ethanol 24 �g/litre
Cattle (steer, blood 84 days AlCl3 (0.103 mg/litre) Valdivia et al.
6/group) 300 mg/kg 0.118 mg/litre (1978)
600 mg/kg 0.100 mg/litre
1200 mg/kg, in feed 0.120 mg/litre
a 1,25-(OH)2-D3 = 1,25-dihydroxy-vitamin D3; d = day; NZ = New Zealand; SD = Sprague-Dawley; w = week; w.w. = wet weight
6.1.2 Studies in humans
6.1.2.1 Inhalation exposures
Studies dealing with the absorption of aluminium compounds in
humans usually use the blood aluminium concentration or the urinary
aluminium excretion as a marker of uptake (Schaller & Valentin, 1984;
Ganrot, 1986). However, part of the aluminium-containing particulates
deposited in the respiratory tract is cleared from the organ by
mucociliary action and, when swallowed, enters the digestive tract.
This means that after inhalation exposure to aluminium compounds not
all aluminium appearing in the systemic circulation or in the urine
necessarily arises solely from absorption in the respiratory tract.
"Insoluble" particulates may be slowly dissolved and thus enter
the blood circulation. Owing to the chemical properties of aluminium,
the absorption of aluminium metal or its compounds by the respiratory
system depends on the aluminium species inhaled and the biological
environment in the tissue compartment where they are deposited
(Martin, 1992).
There is evidence from a number of reports that even aluminium
compounds that are almost insoluble in water are bioavailable when
introduced into the respiratory system. For example, increased urinary
concentrations have been observed in aluminium welders and aluminium
flake and powder producers after exposure to relatively insoluble
particulate matter and metallic fumes and dusts. The levels of tissue
aluminium after inhalation exposures are given in Table 16. When
comparing the analytical results given in these tables, one has to
keep in mind, however, that the analytical precision of the aluminium
determination has been hampered by the potential for contamination
during sampling and processing in view of the ubiquitous presence of
the element (Steinegger et al., 1990). Analytical techniques for
determination of aluminium have been improved considerably during
recent years (see Chapter 2).
6.1.2.2 Oral administration
In view of the fact that over 95% of the normal daily intake of
aluminium comes from food and water, uptake from the gastrointestinal
tract will play a major role in determining tissue levels of the
metal.
The mechanism of gastrointestinal absorption of aluminium is
fairly complex and has not yet been fully elucidated (van der Voet,
1992). This complexity results from (1) the unique chemical properties
of the element, particularly its amphoteric character, leading to
marked variability in solubility at different pH values and the
formation of various chemical species in the gut depending on the pH,
the ionic
strength and the presence of complexing agents (Martin, 1992), and (2)
the complex organization of the mammalian digestive tract where the
chyme passes through a sequence of chemical environments differing
greatly in pH, presence of secretory products, etc. In addition, the
different parts of the intestine may be distinct with regard to their
absorptive and resorptive properties with respect to aluminium.
Aluminium species may be modified in the gut prior to absorption
(Skalsky & Carchman, 1983; Ganrot, 1986; Martin, 1986, 1992).
Quantitatively the intraluminal absorption depends upon the amount of
the chemical species present in the gut lumen, in the blood, and in
the interstitial fluid. Absorption is influenced by the presence of
other complexing ligands (citrate, lactate, etc.) and competing ions
(e.g., iron, silicon). Other factors proposed to influence absorption
include: age; renal function; and iron and calcium status (Birchall,
1991; van der Voet, 1992; Edwardson, 1993).
To date, research concerning the intestinal absorption of
aluminium in humans has been mainly guided by clinical problems and
has used a variety of physiological states and chemical conditions.
Owing to the large variations in experimental conditions, many results
are not comparable and interpretation of their relevance to the health
population becomes very difficult or impossible.
Gastrointestinal absorption of aluminium in humans ingesting
antacids or phosphate binders is well documented. Variable quantities
of Al(OH)3 or Al2(CO3)3 given to volunteers or patients for
different periods of time resulted in significant increases in plasma
and/or urinary aluminium concentrations (Cam et al., 1976; Kaehny et
al., 1977a; Recker et al., 1977; Gorsky et al., 1979; Mauras et al.,
1982; Herzog et al., 1982; Greger & Baier, 1983a). Administration of
the insoluble AlPO4 did not significantly alter blood and urinary
aluminium levels (Kaehny et al., 1977a). The results of these studies
are summarized in Tables 16 and 17. It must be emphasized, however,
where compounds are poorly bioavailable, the expected incremental
increase in the plasma aluminium level after exposure may be lower
than can be detected against normal plasma aluminium levels.
Recent studies of the bioavailability and uptake of aluminium in
human volunteers have employed the radioactive isotope 26Al, which
may be detected at very low concentrations (5 � 10-15 g) using
accelerator mass spectrometry (AMS). The first of these was a study by
Day et al. (1991) who measured the uptake of aluminium in one
volunteer following the ingestion of 1.1 �g of the isotope in sodium
citrate. For this study aluminium uptake was assessed by extrapolation
from a single measurement of 26Al in blood plasma 6 h after
Table 16. Blood and urine aluminium concentrations in humans after oral ingestion of aluminium compoundsa
Subjects and Aluminium concentration Aluminium concentration Remarks Reference
treatment in blood (�g/litre) in urine (�g/litre)
5 normal subjects, not specified not specified Al absorption Cam et al.(1976)
2 patients with CRF;
Al(OH)3 antacids, normal: 0.3-3.6 mmol/day
86-91 mmol/day CRF: 3.3-9.1 mmol/day
Normal subjects, not detected (85.8 �g/day) Recker et al. (1977)
Al(OH)3, oral, 3.8 g increased by 4-10 times
Al/day for 3 days
Normal subjects, plasma: (8-16) cumulative increase in Kaehny et al. (1977a)
2.2 g Al for 3 days (6-7) excretion (�g)
Al(OH)3 17 176-325 730 � 487
Al2(CO3)3 14 51-355 567 � 437
Al(OH)2-aminoacetate 17 243-726 1430 � 1157
AlPO4 9 52-60 123 � 77
Normal subjects 6.2 (serum) not detected Marsden et al.
CRF 13.4 (serum) (1979)
CRF + Al 34.1
Normal subjects, antacids plasma Al: urinary Al excretion: Al balance positive during Gorsky et al. (1979)
taken orally, 23-313 2-fold increase 2- to 6-fold increase Al administration
mg/day for 18-30 days
Al-hydrocarbonate (before Al: 8.35) (before Al: 6.35) serum aluminium in dialysed Mauras et al.
(Lithiagel), oral, 1.84 g 3-day Al: 15.9 3-day Al: 430.8 patients treated with Aludrox: (1982)
Al/day; 5 days 5-day Al: 14.8 5-day Al: 262.5 6-254 �g/litre
after 1 week Al: 8.0 after 1 week Al: 12.2
Table 16. (Con't)
Subjects and Aluminium concentration Aluminium concentration Remarks Reference
treatment in blood (�g/litre) in urine (�g/litre)
Al-supplemented diet serum (before: 4) urinary excretion: normalized to creatinine Greger & Baier
control: 4.6 mg Al/day control: 4 control: 35-36 �g/day control: 20 mg/kg (1983a)
test: 125 mg Al/day test: 7 test: 105-129 �g/day test: 57-72 mg/kg
12 subjects serum Al Herzog et al.
(normal young) given placebo: 0.3-0.9 placebo: 1.0-3.0 (1982)
280 nM Mg + 190 nM test: 0.8-1.1 test: 3.6-20.2
Al/day for 4 weeks
CRF, (3.4 plasma) no correlation with Al Wilhelm et al.
on home dialysis 37.7-68.7 concentration in hair (1989)
on CAPD 33.9-45.0
a control values are given in parentheses
CAPD = continuous ambulatory peritoneal dialysis; CRF = chronic renal failure
Table 17. Tissue aluminium concentrations (mg/kg) in humans exposed to aluminium compoundsa
Subjects and Bone Muscle Kidney Liver Lung Brain Remarks Reference
treatment
Normal n.d. 1.55 d.w. 2.02 d.w. 2.4 d.w. 122.5 d.w. 1.4 d.w. adrenal: 4.8 d.w. Tipton &
adult spleen: 3.7 d.w. Cook
duod.: 4.56 d.w. (1963)
jejun.: 2.84 d.w.
ileum: 9.86 d.w.
Healthy hard water area: 0.5 w.w. whole 2.6 w.w. 18.2 w.w. whole brain: Hamilton
human 73.4 w.w. kidney: 0.5 w.w. et al.
controls soft water area: 0.4 w.w. frontal lobe: (1973)
60 w.w. cortex: 0.05 w.w.
0.4 w.w. basal ganglia:
medulla: 0.07 w.w.
0.3 w.w.
Normal males < 15 d.w. n.d. 11 d.w. 19 d.w. 230 d.w. n.d. spleen: (22 d.w.) Teraoka
Stonemason n.d. n.d. 16 d.w. 130 d.w. 2000 d.w. 520 d.w. (1981)
heart: (11 d.w.)
2.0 d.w.
adrenal: (37 d.w.)
n.d.
Ball-mill room 30 w.w. n.d. n.d. 90 w.w. upper lobe: 5 w.w. McLaughlin
worker in 430 w.w. et al.
aluminium powder lower lobe: (1962)
factory 340 w.w.
Surgical and n.d. no Al n.d. n.d. n.d. n.d. no Al intake: Cann et
autopsy specimen intake: PT: 13 d.w. al. (1979)
(hyperparathyroidism) 2.0 d.w. thy: 3.5 d.w.
Al intake: Al intake:
7.6 d.w. PT: 78 d.w.
thy: 8.8 d.w.
Table 17. (Con't)
Subjects and Bone Muscle Kidney Liver Lung Brain Remarks Reference
treatment
Normal controls: 10.6 23.6 17.5 15.8 97.2 11.9 spleen: 17.2 Flendrig
uraemia, non-dial: 6.4 24.7 33.8 19.7 142.3 n.d. 25.1 et al.
uraemia, dial: 23.5 39.6 44.1 32.9 127.1 12.1 37.9 (1976)
DES: 272.7 13.8 156.5 610.2 99.6 66.1 454.5
DES cortical bone: n.d. n.d. n.d. grey matter: brain white Alfrey et
normal control (3.88) (1.22) (2.18) matter: (2.00) al.
uraemia/dial 46.83 DES: 23.6 non-DES: 6.5 non-DES: 3.81 (1976)b
uraemia/non-dial 8.4 non-DES: DES: 24.98 DES: 5.59
trabecular 10.24
bone: (2.39)
98.48
37.4
Iliac bone n.d. n.d. n.d. n.d. n.d. correlation of Ellis et
(biopsy or duration of al.
autopsy spec.) dialysis and (1979)
control 5.7 ash bone Al
uraemia, non-dial 13.6 ash
dial 151.8 ash
dial + transpl 92 ash
Patients n.d. grey matter: spleen: Alfrey
healthy controls 3.3 d.w. 1.2 d.w. 4.0 d.w. 56 d.w. 2.2 d.w. 3.8 d.w. (1980)
uraemia, non-dial 27 d.w. 2.6 d.w. 25.5 d.w. 75 d.w. 4.1 d.w. 35 d.w.
uraemia, dial 115 d.w. 9.1 d.w. 160 d.w. 89 d.w. 8.5 d.w. 243 d.w.
DES 281 d.w. 15 d.w. 301 d.w. 215 d.w. 24.5 d.w. 493 d.w.
Table 17. (Con't)
Subjects and Bone Muscle Kidney Liver Lung Brain Remarks Reference
treatment
Uraemia + dial n.d. n.d. n.d. n.d. n.d. Hodsman
normal control 2.4 d.w. et al.
osteomalacia 175 d.w. (1982)
osteitis fibrosa 46 d.w.
mixed lesions 81 d.w.
mild lesions 67 d.w.
Normal, necropsy n.d. n.d. n.d. n.d. n.d. cortex: Crapper
0.23-2.7 d.w. et al.
white matter: (1973)
0.6-1.1 d.w.
Normal adult n.d. n.d. n.d. n.d. n.d. 1.9 d.w. Crapper
infant 0.7 d.w. et al.
fetus 0.7 d.w. (1976)
Normal controls n.d. n.d. n.d. n.d. n.d. 2.5 d.w. whole brain McDermott
5.6 d.w. hippocampus et al.
2.4 d.w. frontal cortex (1979)
1.4 d.w. temporal cortex
2.9 d.w. parietal cortex
2.9 d.w. occipital cortex
2.6 d.w. cerebellum
1.5 d.w. corpus callosum
4.1 d.w. mininges
1.3 d.w. isolated neurons
Normal adult n.d. n.d. n.d. n.d. n.d. 0.467 w.w. Markesbery
normal infant 0.298 d.w. et al.
(1981)
Table 17. (Con't)
Subjects and Bone Muscle Kidney Liver Lung Brain Remarks Reference
treatment
Al welders (2) (0.6-5 d.w.) n.d. n.d. n.d. n.d. n.d. also increased: Elinder et
welding fumes 18-29 d.w. blood and al. (1991)
urinary Al
concentration
CRF, no correlation Wilhelm
cumulative (median values) with Al et al.
oral Al intake: concentration (1989)
0 kg 5.3 in hair:
< 0.25 kg 47.5 control: 2.6
0.25 to 0.5 kg 56.7 dial: 1.6-5.5
0.5 to 1.0 kg 62.6
1.0 to 5.0 kg 133.4
Controls 18.8 w.w. n.d. n.d. n.d. n.d. n.d. no differences Burnel et
(surgical between cortical al. (1982)
specimens) and medullary
CRF 6-130 w.w. bone;
(biopsies; no correlation
intake of with age
variable
amounts of Al)
a Normal control values are given in parentheses
DES = dialysis encephalopathy syndrome; dial= on haemodialysis;
w.w. = wet weight; non-dial= not on haemodialysis;
d.w. = dry weight; transpl= transplantation;
n.d. = no data; PT= parathyroid gland;
ARF = acute renal failure; thy= thyroid gland
CRF = chronic renal failure;
b All concentrations expressed as mg Al/kg fat-free solid
administration. Day estimated the fraction of absorbed aluminium to be
1%. Later, the same technique was employed by Day et al. (1994) to
estimate aluminium uptake from orange juice (with or without added
silicate) in control subjects and patients with Down�s syndrome.
Results were expressed as the gastrointestinal absorption factor
(F1), defined as the ratio of mass of aluminium absorbed to mass of
aluminium ingested. For the normal subjects uptake factors ranging
from 0.04 to 1.5 � 10-4 were calculated. The addition of silica
reduced the uptake by a factor of about 7. In the Down�s syndrome
patients uptake was apparently 5 times higher than in controls (4.7 �
10-4 compared to an average of 0.91 � 10-4 in controls).
Most recently, human bioavailability studies have been undertaken
by Priest and his co-workers using a methodology employing the
collection of blood samples and total excreta for a period of up to a
week after a single administration of aluminium compound. The results
of these experiments showed significant intersubject variability in
the extent and timing of aluminium absorption, indicating the
shortcomings of the methods employed by Day et al. (1991, 1994). Two
main studies were undertaken. The first was a study of the uptake from
the gut of aluminium, as aluminium citrate, aluminium hydroxide, and
aluminium hydroxide in the presence of citrate, following the
administration of 100 mg aluminium by gastric tube (Priest, 1994). The
absorption fractions obtained were as follows: 5 � 10-3 for aluminium
as citrate; 1.04 � 10-4 for aluminium hydroxide; and 1.36 � 10-3 for
hydroxide in the presence of sodium citrate. This study demonstrated
the greater bioavailability of the citrate complex and the ability of
citrate to enhance the uptake of aluminium taken in another chemical
form. The second study measured the fractional uptake of aluminium
from drinking-water using a similar technique, but different
volunteers (Priest et al., 1995a). The measured uptake fraction was
2.2 � 10-4. It was concluded that members of the public, drinking
1.5 litres/day of water containing 100 �g of aluminium/litre, would
absorb about 3% of their total daily aluminium uptake from this
source. This result suggests that drinking-water, under most
circumstances, is likely to be a minor source of aluminium for humans.
6.1.2.3 Dermal exposure
There is no direct evidence that aluminium is absorbed through
the intact skin of humans.
6.2 Distribution
6.2.1 Animal studies
After absorption aluminium is bound in the plasma primarily to
transferrin and, to a lesser extent, also to albumin (Trapp, 1983;
Bertholf et al., 1984; Martin, 1986). There are indications that
aluminium binding to protein is dose-dependent, with low binding rates
at unexposed plasma levels (H�hr et al., 1989; Wilhelm et al., 1990).
Aluminium distribution depends on the animal species used, route of
administration and the aluminium compound administered. Volumes of
distribution for aluminium have been estimated only following
parenteral administration (Wilhelm et al., 1990) and are thus not
relevant to the exposure of the general population. After a single
dose of aluminium lactate (1 mg/kg body weight) in rats, no tissue
uptake could be detected, whereas at 12 000 mg/kg body weight the only
significant increase in tissue aluminium occurred in bone (Wilhelm et
al., 1992). In other oral studies summarized in Table 17, more or less
significant increases in tissue levels after aluminium ingestion were
found, these increases generally being dose-dependent. In animals
receiving aluminium, increases in tissue levels were most marked in
bone.
It has to be considered that at high-dose levels aluminium is
toxic to the tissue of the gastrointestinal tract, thus inducing
pathological changes that might be followed by increased uptake (J�ger
et al., 1991). Gut tissue pathology has not been investigated in most
distribution studies.
Two studies have reported on aluminium accumulation following
administration in drinking-water. Fulton et al. (1989) administered 0,
0.1, 2.0 or 100 mg/litre Al(OH)3 or AlCl3 (equivalent to 0, 0.01,
0.2 or 5.5 mg Al/kg body weight per day) in drinking-water containing
acetate or citrate at various pH levels to Sprague-Dawley rats
(6 rats per group) for 10 weeks. In the highest-dose group, aluminium
accumulated in intestinal cells but not in other tissues investigated.
The effect was more pronounced when citrate was added and when water
with an acidic pH was used.
In a recent study, Walton et al. (1995) administered to eight
fasted adult rats by gavage 4 ml of aluminium-free drinking-water
containing 1.0 �g 27Al and 70 becquerel (0.1 �g) 26Al. Two
experimental animals had brain 26Al/27Al ratios similar to the two
controls while the remaining six animals had ratios of 26Al/27Al in
brain tissue that were substantially higher than background. However,
the variation between animals was marked (148 � 19 to 5220 � 208).
Studies in mice (Golub et al., 1993), rats (Muller et al., 1992)
and rabbits (Yokel, 1984) indicate that aluminium is not readily
transferred from the dam to offspring via nursing.
Studies in mice (Golub et al., 1993) and rabbits (Yokel, 1985)
indicate that aluminium compounds that are bioavailable and therefore
available to the dam also reach the fetus. However, a study in rats
reported elevated levels of aluminium in maternal tissue, but not in
the fetus, after exposure to aluminium lactate in the diet during
gestation (Muller et al., 1993). Limited data in mice (Cranmer et al.,
1986) and rabbits (Yokel, 1985) show higher concentrations of
aluminium in the placenta than in maternal or fetal tissues after
administration of aluminium to dams.
6.2.2 Human studies
6.2.2.1 Transport in blood
The extent to which plasma aluminium is normally bound to
proteins may be as high as 70 to 90% in haemodialysis patients with
moderately increased plasma aluminium levels (25 to 200 �g/litre).
Studies using 26Al (Day et al., 1994) have shown that one day
after injection, 99% of aluminium in blood is present in the plasma
fraction and 1% in the erythrocytes. By contrast, 880 days after
injection, 14% was associated with the erythrocyte fraction,
indicating the probable incorporation of aluminium into the
erythrocytes during erythropoiesis. One day after injection, 80% of
the plasma fraction was found to be associated with transferrin, 10%
with albumin, 5% with low molecular weight proteins (5000-50 000
molecular weight) and about 4% with a lower molecular weight fraction.
No details of the plasma speciation at 880 days were given. Priest et
al. (1996) found that only about 50% of injected aluminium was
recoverable from blood at 15 min after injection. It was suggested
that aluminium may pass through blood vessel walls to establish an
equilibrium between aluminium in blood and aluminium in the
extravascular tissue fluids. In contrast, the same study showed that
gallium remained in the blood. This and other observations suggested
that gallium is an inappropriate surrogate for aluminium in
bioavailability and kinetic studies (Priest et al., 1991).
6.2.2.2 Plasma aluminium concentrations in humans
The role of aluminium in the etiology of dialysis-related
disorders such as encephalopathy, vitamin-D-resistant osteomalacia,
and normochromic microcytic anaemia has drawn attention to the
possible mechanisms of aluminium uptake through the parenteral and
intestinal routes.
Normal human plasma or serum aluminium values reported vary
largely, mainly due to methodological problems in the analytical
technique and with sample contamination. Ganrot (1986) emphasized this
in his extensive review.
As methods have improved, suggested reference values for plasma
levels have been revised downwards, and it was suggested by Nieboer et
al. (1995) that the actual value in normal subjects lies in the range
of 0.04 to 0.07 �mol/litre (1.1 to 1.9 �g/litre).
Seasonal variations in serum aluminium concentrations in patients
with moderate chronic renal failure were observed by Nordal et al.
(1988), with peak levels occurring in the autumn. These variations
were presumed to be related to an increased gastrointestinal
absorption due to waterborne factors.
The data summarized in Tables 16 and 17 indicate that, as is the
case with experimental animals, the aluminium concentrations in human
blood and selected tissues are increased after ingestion or inhalation
of aluminium compounds.
6.2.2.3 Tissue aluminium concentrations in humans
(a) Normal concentrations
The available data relating to the aluminium concentrations found
in various human tissues are summarized in Table 17. It should be
noted that the measurement of tissue concentrations is difficult. In
particular, where the average concentrations for a tissue have been
reconstructed, by extrapolation, from the analysis of small samples of
the tissues concerned, they may be significantly affected by lack of
homogeneity in the distribution of the metal in the organ and will
also amplify errors due to sample contamination. In this respect the
reconstruction of average bone concentrations is particularly
difficult, given that small samples of bone collected from disparate
skeletal sites will contain very different levels of aluminium as a
result of the different amounts of bone surface to which the metal
binds. Owing to the above difficulties it is commonly prudent to
ignore measurements that indicate very high levels of aluminium in a
particular tissue without additional biological data to explain the
findings. This approach has been taken by a Canadian group (Nieboer et
al., 1995), which based its conclusions on the lowest levels
consistently reported for the tissues considered (bone and brain). It
was concluded that normal levels of aluminium in bone are in the order
of 1-3 �g/g (wet weight) and that background levels in brain tissue
(mostly in the grey matter) are around 1-3 �g/g (dry weight) or <
0.5 �g/g (wet weight), based on the lowest levels consistently
reported. The authors also showed that the bone and brain aluminium
levels are significantly elevated in patients with renal failure who
are treated with either aluminium-containing phosphate scavengers or
who received total parenteral nutrition with aluminium-contaminated
intravenous nutrient solutions.
With respect to other tissues, measurements suggest that the
highest levels of aluminium are found in the lung (56-215 mg/kg dry
weight) (Alfrey et al., 1980), presumably as inhaled, undissolved,
aluminium-containing particles. Similarly, inhaled particles may
relocate in the regional (hilar) lymph nodes and in the organs
comprising the reticulo-endothelial system, i.e. liver, spleen and
bone marrow. This may explain some or, in the case of the lymph nodes,
most of the aluminium present in these organs. For example, Teraoka
(1981) reported the levels of aluminium in the lungs and reticulo-
endothelial organs of a stone mason dying from silicosis and
corpulmonale: lungs (2 g/kg dry weight); hilar lymph nodes (3.2 g/kg
dry weight); spleen (520 mg/kg dry weight) and liver (130 mg/kg dry
weight). Excluding particulate aluminium, it is likely that of all the
extrapulmonary tissues only the skeleton contains significant levels
of aluminium. This is the conclusion of Priest (1993), who based his
conclusion on a theoretical consideration of ion size of Al3+,
supported by measurements of the distribution of the sources of 26Al
gamma-emission in a human volunteer at earlier times after injection
(using the same techniques gallium uptake in the liver was estimated
to be about 30%). The possibility that at later times liver levels
build up would be consistent with rather high levels for this tissue
(Flendrig et al., 1976) and with the observation of Day et al. (1994)
that some aluminium is taken up by red blood cells - considering the
role of the liver in the breakdown of old red blood cells.
(b) Concentrations after aluminium exposure
Data on the tissue burden of aluminium-exposed humans are mostly
derived from patients with chronic uraemia as well from occupationally
exposed workers. In these patients, the aluminium intake may come from
more than one source and is difficult to follow.
More than 40 years after exposure, the cerebrospinal fluid of an
aluminium powder worker exposed heavily during 1944-1946 contained 250
�g aluminium/litre. Aluminium-associated pulmonary fibrosis
(aluminosis) was diagnosed in 1946 (Sj�gren et al., 1994a,b). The
normal value for cerebrospinal fluid is less than 10 �g/litre.
In two heavily exposed aluminium welders, bone aluminium
concentrations were 18 mg/kg dry weight and 29 mg/kg dry weight,
clearly above the reference range for the investigators (0.6-5 mg/kg
dry weight) (Elinder et al., 1991). In a worker producing fine
aluminium powder, who developed encephalopathy and pulmonary fibrosis
after 13.5 years of work, the aluminium concentration in lung and
brain was increased 20-fold and in the liver 122-fold (McLaughlin et
al., 1962).
In patients with impaired renal function, a wide range of tissue
aluminium concentrations have been reported (bone, 8.4-281 mg/kg;
muscle, 2-23.6 mg/kg; brain, 2.2-25 mg/kg; Table 16). This wide range
reflects the variable exposure to aluminium-containing phosphate-
binding pharmaceutical agents and the duration of haemodialysis. The
increased tissue concentrations are associated with the clinical
syndromes of encephalopathy, osteomalacia and microcytic anaemia.
Channon et al.
(1988) reported a positive correlation between the dose of aluminium-
containing phosphate-binding pharmaceutical agent prescribed and bone
aluminium content, but no correlation with the serum aluminium
concentration for the 6 months preceding bone biopsy.
In a worker exposed to aluminium dust and powder in a ball-mill
room for 13.5 years, the aluminium concentration in lung and brain was
increased 20 times and that of the liver 122 times over the normal
value (Table 16). Teraoka (1981) published data indicating that
aluminium concentrations were increased in the lungs (2000 mg/kg dry
weight), hilar lymph nodes (3200 mg/kg dry weight), spleen (520 mg/kg
dry weight), and liver (130 mg/kg dry weight) in a stone-mason dying
from silicosis. The average organ concentrations in normal unexposed
control males were 230, 2000, 22 and 19 mg/kg, respectively
(Table 16).
6.3 Elimination and excretion
6.3.1 Animal studies
In animals aluminium is eliminated effectively by urine.
Following single intravenous doses of up to 100 �g/kg body weight in
rats, aluminium was quantitatively recovered from urine (Wilhelm et
al., 1992). It is difficult to obtain an accurate half-life for low
oral doses, since the rate of aluminium absorption is low. Data on
plasma half-life and on renal clearance have been mainly obtained from
parenteral administration generally using high doses of aluminium
(Wilhelm et al., 1990). It seems that at doses comparable with human
exposure the plasma half-life is less than 1 h.
Based on stop-flow experiments conducted in pigs, Monteagudo et
al. (1988) concluded that aluminium excretion occurs in the distal
tubule of the kidney and is situated close to the sites of maximal
calcium and sodium ion reabsorption.
6.3.2 Human studies
6.3.2.1 Urinary excretion
The biokinetics of aluminium in man has been evaluated by Priest
and his co-workers (Priest et al., 1991, 1995b, 1996; Talbot et al.,
1995) using 26Al injected into human volunteers. These authors
described the pattern of urinary excretion of the isotope following
its intravenous injection as citrate, the effect of excretion on body
retention and the relationship between aluminium levels in blood and
in urine/faeces. In a first study using a single volunteer (Priest et
al., 1991, 1995b, 1996), the authors confirmed that aluminium is
overwhelmingly excreted by the urinary route and that most blood
aluminium is cleared to excretion. More than half of the 26Al had
left the blood within 15 min and the decline continued, leaving < 1%
in the blood after 2 days. Total excretion up to 13 days was 83%
(urine) and 1.8% (faeces), leaving 15% in the body. After 4 months
more than 90% was excreted. With increasing time the rate of urinary
excretion, as indicated by the fraction of retained aluminium (Rt),
decreased with time (t) according to the power function:
Rt = 35.4 t-0.32(t > 1)
At 1178 days after injection about 4% remained in the body; an
estimated 94% had been excreted by the urinary route and 2% in the
faeces. Faecal excretion most likely represented aluminium that had
entered the gastrointestinal tract in bile. The power function
calculated for the fraction of aluminium excreted by the urinary route
(Ut) at time (t) after intake was:
Ut = 0.47 t-1.36
The excretory clearance rate (ECR) from whole blood (in kg/day)
during the first two weeks after injection was expressed as:
ECR = 42 t-0.14
In a second study (Talbot et al., 1995) of shorter duration, but
using six male volunteers, inter-subject variability was examined.
This showed significant inter-subject variation in the pattern of
aluminium excretion. For example, after 5 days an average of
71.8% � 7.3% (SD) of the injected activity had been excreted in urine
(range 62.4-82.9%). Of this total, an average of 59.1% was excreted in
the first day, 7.2% in day two, 2.6% in day three, 1.7% in day four
and 1.1% in the fifth day. In the same period an average of 1.2% was
excreted in the faeces. With respect to blood clearance to urine, the
study showed a gradual decrease in the fraction of blood aluminium
excreted per unit time, indicating a changing speciation of aluminium
in the blood. Overall, the results of the study were wholly consistent
with those generated in the single volunteer study, with the first
volunteer showing aluminium biokinetics in the middle of the range of
results generated by the multi-volunteer study.
Sj�gren et al. (1985) studied previously unexposed volunteers and
individuals previously exposed to welding fumes containing aluminium
at their workplaces for different periods of time. All subjects were
exposed to welding fumes containing aluminium during a working day for
about 8 h. In previously unexposed individuals urinary aluminium
concentration after exposure was increased but decreased to pre-
exposure levels after a few days. The half-life of the first phase of
excretion was approximately 8 h. In welders exposed for less than 2
years in the aluminium concentration decreased during the weekend, the
half-life being about 9 days. In welders exposed for more than 10
years urinary concentration did not change despite cessation of
exposure. In these welders, the urine half-life was more than 6 months
(Sj�gren et al., 1988).
Elinder et al. (1991) analysed urinary, blood and bone aluminium
content in two aluminium welders exposed to welding fumes for more
than 20 years. The urinary values varied from 107 to 351 �g
aluminium/litre. The daily urinary aluminium elimination was estimated
to be 0.06% of the estimated body burden (on the basis of bone
aluminium content), which corresponds to a half-life of about 3 years.
Ljunggren et al. (1991) studied workers occupationally exposed to
aluminium flake powder after acute exposure and after periods of non-
exposure of varying length. The calculated half-life during 4-5 weeks
of exposure-free vacations was 6.8 weeks. In workers retired (for 6
months to 14 years) after exposure periods of 9 to 50 years, the
calculated half-life varied between 0.7 and 7.9 years, depending on
the length of the exposure-free period.
6.3.2.2 Biliary excretion
There is insufficient information to comment on biliary excretion
of aluminium in humans.
6.4 Biological indices of exposure, body burden and organ
concentration
Aluminium levels in blood and urine have been used to determine
exposure levels. However, this relation is relatively weak. Blood
aluminium levels are a poor indicator of tissue stores, rather
indicating acute intake or rate of tissue store mobilization. In
patients with impaired renal function, blood and urine levels are poor
indicators of tissue levels.
High occupational exposure levels seem to be reflected better by
urine levels than by blood levels, but the quantitative relation is
not well established. Balance studies by current methodologies are not
possible. Increased blood and urine concentrations have been observed
in several groups of occupationally exposed workers and a quantitative
relationship between the amount inhaled and the urinary aluminium
concentration has been suggested (Sj�gren et al., 1983).
A linear relationship has been observed between the air levels of
aluminium exposure, the number of exposure years and the post-shift
urine level in aluminium welders (Sj�gren et al., 1988):
UAl = 41.7 � AAl + 6.7 � E � 4.6
where UAl = urine aluminium level (�g/litre)
AAl = air aluminium level (mg/m3)
E = years of exposure
Owing to intra-individual variation, this equation can only be
used for groups, not individuals. There have been no adequate
investigations of the relation between air levels of exposure and
urine or blood levels in workers apart from welders.
No information exists regarding the use of biological exposure
indices in general populations.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity is influenced by the solubility and
bioavailability of the aluminium compounds administered. Aluminium
compounds are only poorly absorbed after exposure by the
gastrointestinal, respiratory and dermal routes. The acute toxicity of
aluminium metal and aluminium compounds is relatively low. No LC50
has been identified in inhalation studies.
Lethal doses of soluble aluminium compounds, such as AlCl3,
Al(NO3)3 and Al2(SO4)3, have been determined by the oral or
parenteral routes. Some LD50 values are given in Table 18.
Table 18. LD50 values for various aluminium compounds
Compound Species Route of LD50 Reference
administration (mg Al/kg b.w.)
AlCl3 mouse (male oral (gavage) 770 Ondreicka
Al2(SO4)3 Dobr� Voda) 980 et al. (1966)
Al(NO3)3 mouse (Swiss; oral (gavage) 286 Llobet et al.
20/sex) i.p. 133 (1987)
AlCl3 oral (gavage) 222
i.p. 105
Al2(SO4)3 oral (gavage) > 730
i.p. 40
AlBr3 oral (gavage) 164
i.p. 108
Al(NO3)3 rat (Sprague- oral (gavage) 261 Llobet et al.
Dawley, 20/sex) i.p. 65 (1987)
AlCl3 oral (gavage) 370
i.p. 81
Al2(SO4)3 oral (gavage) > 730
i.p. 25
AlBr3 oral (gavage) 162
i.p. 82
7.2 Short- and long-term exposure
7.2.1 Oral administration
Available data on the toxicity of aluminium compounds following
repeated oral administration are presented in Table 19. Few of
the studies reviewed are adequate to serve as a basis for the
determination of effect levels, since many were designed to address
principally aspects such as effect of citrate and decrements in kidney
function on the body burden of aluminium; a very limited range of
toxicological end-points was also examined in these studies
(Ecelbarger & Greger, 1991; Greger & Powers, 1992).
There have been several repeated dose toxicity studies in which a
wide range of end-points, including clinical signs, food and water
consumption, growth, haematological and serum analyses, tissue and
plasma concentrations of aluminium, histopathology, has been examined
following oral exposure to various aluminium compounds. There were no
treatment-related effects in rats fed up to 288 mg Al/kg body weight
per day as sodium aluminium phosphate or 302 mg Al/kg body weight per
day as aluminium hydroxide in the diet for 28 days (Hicks et al.,
1987). In a subchronic study in which aluminium nitrate was
administered in drinking-water to rats, the only effect observed was a
significant decrease in body weight gain associated with a decrease in
food consumption at 261 mg Al/kg body weight per day. (NOEL = 52 mg
Al/kg body weight per day) (Domingo et al., 1987b).
When small groups of Beagle dogs were given sodium aluminium
phosphate for 6 months in the diet, there were no treatment-related
effects except for a decrease in food consumption not associated with
a decrease in body weight (NOEL = approximately 70 mg Al/kg body
weight) (Katz et al., 1984). Similarly, in small groups of Beagle dogs
administered up to 80 mg Al/kg body weight per day as sodium aluminium
phosphate for 26 weeks, the only treatment-related effect was a sharp,
transient decrease in food consumption and concomitant decrease in
body weight in males (LOEL = 75 to 80 mg Al/kg body weight per day;
Pettersen et al., 1990).
Table 19. Toxicity of aluminium compounds after repeated oral administration
Protocol descriptiona End-points examined Results Reference
5-10 male rats (Weizman strain) clinical signs, histopathology periorbital bleeding in 3 of 5 animals at 350 Berlyne
per group; (appears to have been mg/kg body weight per day Al2(SO4); tissue and et al.
Al2(SO4)3 (200 or 350 mg Al/kg limited to animals that serum levels highest in nephrectomized animals; (1972)
body weight per day in drinking died during study), tissue clinical signs of toxicity and death in all groups
water), AlC13 (250 mg Al/kg concentrations of nephrectomized animals; comments: protocol and
body weight per day in drinking results poorly documented; inadequate for
water), Al(OH3) (150 mg Al/kg establishment of effect levels
body weight per day by gavage)
for an unspecified period; groups
of animals that were 5/6
nephrectomized similarly exposed
Controls: 56 male Sprague-Dawley aluminium concentrations in five of these measures (concentrations in tibia, Greger &
rats 10.5 mg Al/kg exposed groups tibia, liver, kidney and serum; liver, and serum and urinary excretion with and Powers
16 animals, ingesting: aluminium serum aluminium concentrations without DFO treatment); were highly correlated (1992)
hydroxide, 1079 mg Al/kg diet after DFO; urinary with oral exposure; changes induced by DFO were
(29 days) or 1012 mg Al/kg diet aluminium excretion with and very small; ingestion of citrate had small but
plus 4% citrate (29 days), 2688 without DFO treatment; body significant effects on aluminium retention; rats
mg/kg and 4% citrate (12 or 29 and organ weight gain and fed citrate weighed less (not associated with
days); 24 h prior to sacrifice, haematological status differences in intake of feed) and had significantly
all animals injected i.p. with enlarged kidneys and livers and significantly smaller
desferrioxamine (DFO) or buffer tibias; haematocrits were inversely correlated to
tissue concentrations of aluminium - more evident
with oral than parenteral exposure; hepatic iron
was elevated with the citrate-containing diets but
was only weakly correlated with hepatic aluminium
concentrations;
Table 19. (Con't)
Protocol descriptiona End-points examined Results Reference
comments: comparisons of aluminium exposure in Greger &
tibias and sera of rats exposed parenterally and Powers
orally indicated that 0.01 to 0.04% of dietary (1992)
aluminium was absorbed; inadequate for establishment
of effect levels owing to limited range of end-points
examined; primarily an investigation to monitor
aluminium body burdens
Administration to groups of 6 rats tissue concentrations of ingestion of citrate increased retention of aluminium Ecelbarger
exposed in a 2x2x2x2 factorial aluminium; body and in bone of rats fed 1000 mg Al/kg diet and increased & Greger
design of diets containing 13 or organ weight changes apparent absorption of zinc; when dietary calcium (1991)
1112 mg Al/kg as aluminium intake was increased from 67 to 250 mmol/kg diet,
hydroxide, citrate (0 or 5.2 mmol/kg aluminium concentrations in bone were reduced without
diet) and calcium (2.7 or 10.0 g/kg a change in growth of rats; reduction in kidney
diet) for 30 days; groups of 6 rats function (by removal of one kidney), which was
exposed in a 4x2 factorial design insufficient to alter growth, increased aluminium
to 14 or 904 mg Al/kg diet and one retention in bone by 13% in rats fed aluminium; rats
of 4 levels of citrate (0, 10, 21 or fed aluminium retained only 0.01 to 0.5% as much as
31 mmol/kg diet) for 28 days; those injected; comments:authors concluded that
groups of 7 rats exposed in 2x2x2 tissue concentrations and, presumably, toxicity can
factorial design to 9 or 1044 mg be altered by moderate changes in diet and kidney
Al/kg diet and citrate (0 or function even though overall retention of orally
21 mmole/kg diet) sham-operated administered aluminium is low, inadequate for
or with one kidney removed for establishment of effect levels owing to limited
28 days range of end-points examined; designed primarily to
investigate effect of dietary citrate, calcium
and decreases in kidney function on absorption
of aluminium
Table 19. (Con't)
Protocol descriptiona End-points examined Results Reference
Groups of 10 female Sprague- clinical signs, food and water no effects on any end-points examined except for G�mez
Dawley receiving 1, 26, 52 or consumption, growth, mild histopathological changes in the spleen and et al.
104 mg Al/kg body weight per haematological and serum liver of high dose group (hyperaemia in the red pulp (1986)
day as Al(NO3)3 in drinking analyses, tissue and plasma of the spleen and liver; periportal lymphomonocytic
water for 28 days concentrations of aluminium, infiltrate in the liver); dose-dependent accumulation
histopathology of aluminium in spleen, heart and gastrointestinal
tract; comments: well-conducted repeated dose
toxicity in which a wide range of end-points was
examined, though short term; NOEL = 52 mg Al/kg
body weight per day; LOEL = 104 mg Al/kg body
weight per day (aluminium nitrate)
Groups of female Sprague-Dawley clinical signs, food and water significant decrease in body weight gain at highest Domingo
rats receiving 0, 26, 52 or 261 mg consumption, organ and body dose associated with decrease in food consumption; et al.
Al/kg body weight per day as weights, haematological and no dose-dependent accumulation of aluminium in (1987b)
Al(NO3)3 in drinking-water for serum analyses, tissue and tissues; comments: well-conducted repeated dose
100 days plasma concentrations of toxicity in which a wide range of end-points;
aluminium, histopathology LOEL = 261 mg Al/kg body weight per day; NOEL =
52 mg Al/kg body weight per day for subchronic
period of exposure
Groups of Beagle dogs (4/sex) clinical signs, food and water statistically significant decreases in food Katz et
administered 0, 0.3, 1.0 or 3.0% consumption, organ and body consumption in females but no associated decrease al.
sodium aluminium phosphate in weights, haematological and in body weight; no other treatment-related effects; (1984)
the diet for 6 months (118, 317 serum analyses, urinalysis, comments: range of end-points examined; subchronic
and 1034 mg/kg body weight per ophthalmological examinations, period of exposure for dogs; small group sizes;
day in males and 112, 361 and tissue and plasma NOEL = 1087 mg/kg body weight per day (approximately
1087 mg/kg body weight per day concentrations of aluminium, 70 mg Al/kg body weight per day)
in females histopathology
Table 19. (Con't)
Protocol descriptiona End-points examined Results Reference
Groups of Beagle dogs (4/sex) clinical signs, food and water sharp, transient decrease in food consumption and Pettersen
administered 0, 10, 22 to 27 or consumption, organ and body concomitant decrease in body weight in high-dose et al.
75 to 80 mg Al/kg body weight weights, haematological and males; in same group, decrease in testes weight and (1990)
per day as sodium aluminium serum analyses, urinalysis, histopathological changes in the liver and kidney
phosphate in the diet for ophthalmological examinations, considered to be secondary to decreased food
26 weeks tissue and plasma consumption; slight increase in concentration of
concentrations of aluminium, aluminium in the brain in high-dose females but not
histopathology males; comments: wide range of end-points examined;
subchronic period of exposure for dogs; small group
sizes; minimal toxicity at the highest dose;
LOEL = 75 to 80 mg Al/kg body weight per day
Groups of 25 male Sprague-Dawley
rats fed control diets or
30 000 mg/kg
KASAL I (6% aluminium-sodium clinical signs, food and water no treatment-related effects or significant Hicks et
aluminium phosphate), 7000 or consumption, organ and body deposition of aluminium in bone; comments: wide al.
30 000 mg/kg KASAL II (13% weights, haematology and range of end-points examined; short-term exposure; (1987)
aluminium-sodium aluminium clinical chemistry urinalysis, no effects at 141 mg Al/kg body weight per day
phosphate) or 14 470 mg/kg ophthalmological examinations, KASAL I; NOEL for KASAL II = 288 mg Al/kg body
aluminium hydroxide for 28 days concentrations of weight per day; no effects at 302 mg Al/kg body
(5, 141, 67, 288, 302 mg Al/kg aluminium in the femur, weight per day aluminium hydroxide
body weight per day, respectively) histopathology
Table 19. (Con't)
Protocol descriptiona End-points examined Results Reference
Groups of 15 albino male rats histopathological examination dose-related cytotoxic effect in the liver - Roy et
administered aluminium sulfate of heart, liver, kidney, cytoplasmic degeneration at 17 to 29 mg Al/kg body al.
(0, 17, 22, 29, 43, 86 and brain, testes, stomach and weight with multifocal degeneration and fibrous (1991b)
172 mg Al/kg body weight) and femur tissue proliferation at higher doses. Dose-related
potassium aluminium sulfate effects in the kidney at 17 mg Al/kg body weight
(43 mg Al/kg body weight, 29 mg per day as aluminium sulfate, slight swelling of
Al/kg body weight) in deionized the tubules. With increased dose, increased swelling
water for 21 days; 5 rats killed and degeneration of the cortical tubules.
weekly Degeneration of the nerve cell at 29 and 43 mg Al/kg
body weight per day aluminium sulfate and
potassium aluminium sulfate, respectively, which
was more severe at higher doses. Some evidence of
spermatological cell decrease at doses of 43 mg Al/kg
body weight per day and above. Multifocal degeneration
and decalcification at 43 mg Al/kg body weight
per day and above for both salts, which increased with
increasing dose; degeneration of calcified bone and
irregularity of osteoblasts in animals exposed to 86
and 171 mg Al/kg body weight as aluminium sulfate.
Hyperplasia and ulceration of stomach at highest doses.
Comments: difficult to verify reported effect levels
based on limited information presented in paper.
a Strain, number of animals/group and vehicle specified, where available; doses reported as mg/kg body weight, unless specified
In a study that was not well reported, mild histopathological
effects on the kidney and liver of rats, which increased in severity
with dose, were reported at doses as low as 17.2 mg Al/kg body weight
(as aluminium sulfate) administered by gavage for 21 days (Roy et al.,
1991b). Data presented in the report were inadequate to verify the
reported effect levels.
7.2.2 Inhalation exposure
Many inhalation/intra-tracheal instillation studies have
been conducted using aluminium compounds, including chloride,
chlorohydrate, oxyhydrate and oxides (Stacy et al., 1959; Corrin,
1963; Christie et al., 1963; Gross et al., 1973; Drew et al., 1974;
Steinhagen et al., 1978; Stone et al., 1979; Finelli et al., 1981;
Thompson et al., 1986). However, in the case of the inhalation
studies, little data is available concerning exposure conditions and
the size of the ambient aerosol, and some studies were of relatively
short duration compared with the life-span of the animal employed.
Consequently, although no toxic effects were reported in nearly all
cases, it is not possible to assess how much, if any, of the compound
was deposited in the lungs of the experimental animal, and the time-
span of the experiment may have been too short to demonstrate delayed
effects. The only inhalation study that demonstrated an effect was
that of Finelli et al. (1981) who described increased lung size. Where
aluminium oxide particles were administered by intra-tracheal
instillation, fibrosis has been consistently reported (Stacy et al.,
1959; Corrin, 1963). Dinman (1988) described this disease as "alumina-
related pulmonary disease". However, such fibrosis is a common
consequence of the inhalation of many particles, including silica and
coal; it is unlikely to be related to aluminium per se, but rather
to the physical properties of the particle inhaled.
7.2.3 Parenteral administration
Aluminium compounds were found to possess an increased toxicity
when administered parenterally rather than orally. The effect depends
on the dose, the aluminium compound used and the particular animal
model. It can vary from death to behavioural alteration (loss of
memory), loss of weight or minor changes in aluminium accumulation in
bone. A LOAEL of approximately 1 mg/kg body weight per day can be
obtained by this route for osteomalacia or for deterioration of renal
function (Chan et al., 1983; Henry et al., 1984; Quarles et al., 1985;
Br�unlich et al., 1986). Partially nephrectomized animals exhibited
greater susceptibility to aluminium (Ittel et al., 1992).
7.3 Reproductive and developmental toxicity
7.3.1 Reproductive effects
The limited number of studies are not able to provide adequate
information on reproductive toxicity (Domingo, 1995). Of these, few
provide direct and complete evaluations of reproduction.
Domingo et al. (1987a) administered aluminium nitrate at 0, 13,
26 or 52 mg Al/kg body weight per day by intubation to male rats for
60 days prior to mating and to virgin females treated for 14 days
prior to mating. The same doses were administered by gavage to
pregnant animals from 14 days gestation to 21 days of lactation in a
separate study (Domingo et al., 1987c). Domingo et al. (1987a)
reported no reproductive effects on fertility (number of litters
produced), litter size, or intrauterine or postnatal offspring
mortality. Numbers of corpus lutea on day 13 of gestation were
significantly lower at 52 mg Al/kg body weight per day. However a
dose-dependant delay in the growth of the pups was observed in all
treatment groups; female offspring were affected at 13 mg Al/kg body
weight per day and males at 26 and 52 mg Al/kg body weight per day.
Because of the design of the study by Domingo et al. (1987a) it is not
clear whether the postnatal growth effects in offspring represented
general toxicity to male or female parents, or represented specific
effects on reproduction or development. However, the reported LOAEL
for adverse effects in females in this study was 13 mg Al/kg body
weight per day.
Dosing of pregnant animals from 14 days gestation to 21 days
lactation with 13, 26 or 52 mg Al/kg body weight per day did not
produce overt fetotoxicity (Domingo et al., 1987c), but growth of
offspring was significantly delayed (body weight, body length and tail
length) from birth to weaning.
7.3.2 Developmental effects
Details of the study designs of oral or gavage dosing studies of
developmental toxicity are given in Table 20. Aluminium chloride
administered i.p. to rats (Benett et al., 1975) or i.v. to mice (Wide,
1984) during embryogenesis produced a syndrome characterized by
delayed and incomplete ossification of skull and vertebrae, skeletal
variations and malformations, internal haemorrhage and reduced fetal
growth. Abnormal digits were also noted in rats. Administration of
aluminium chloride in the diet with accompanying parathyroid hormone
(PTH) injection (McCormack et al., 1979) also produced reduced
skeletal ossification and increased incidence of skeletal variations.
Maternal toxicity in the study of Benett et al. (1975) included
reduced weight gain, hepatic granulomas and necrosis and maternal
death at the highest dose levels (100 and 200 mg Al/kg per day).
Resorption and embryolethality were seen primarily at highly
maternally toxic doses (200 mg Al/kg per day). Maternal monitoring and
reduced gestational weight gain were not seen at the LOAEL for
developmental toxicity (75 mg Al/kg per day; day 9-13 of gestation).
At this dose mean fetal weight and crown rump were reduced, and the
number of resorptions increased. The use of i.p. administration makes
it difficult to interpret these results with regards to human health
effects.
The severity of developmental aluminium toxicity by the oral
route is highly dependent on the form of aluminium and the presence of
organic chelators that influence bioavailability, as demonstrated in a
series of studies by Domingo (1995) using gavage administration during
embryogenesis. Aluminium nitrate (nonahydrate) produced developmental
effects in rats (Paternain et al., 1988) similar to the effects seen
after i.p. injections (Benett et al., 1975), including skeletal
variations, poor ossification, haemorrhage, oligodactyly and some soft
tissue malformations. Aluminium hydroxide did not produce either
maternal or developmental toxicity when it was administered by gavage
during embryogenesis to rats (G�mez et al., 1990) or mice (Domingo et
al., 1989). When aluminium hydroxide was administered with ascorbate
(Colomina et al., 1994), no maternal or developmental toxicity was
seen in mice in spite of elevated maternal tissue concentrations of
aluminium, whereas aluminium hydroxide given with citrate produced
maternal and fetal toxicity in rats (G�mez et al., 1991). Aluminium
hydroxide given with lactate was not toxic, but aluminium lactate
administration produced developmental toxicity, in mice including poor
ossification, skeletal variations and cleft palate (Colomina et al.,
1992).
In summary, developmental toxicology syndromes described above
commonly included growth retardation, such as lower fetal weights and
length (Bennet et al., 1975; Paternain et al., 1988; G�mez et al.,
1990; Colomina et al., 1992). A study using s.c. administration of
aluminium lactate did not demonstrate any developmental effects other
than lower fetal length (Golub et al., 1987). Postnatal growth
retardation has also been demonstrated in rats exposed in late
gestation to aluminium nitrate (Domingo, 1987c) and in rabbits treated
postnatally with aluminium lactate s.c. (Yokel, 1984). Reduced bone
formation demonstrated when postnatal aluminium lactate was
administered subcutaneously in rabbits (Yokel, 1987) supports the
importance of bone as a target organ for developmental effects of
aluminium.
Table 20. Developmental toxicity after oral administration of aluminium saltsa
Species Route of application Compound Dose Duration Reference
Mouse (Swiss, oral (gavage) Al(OH)3 66.5, 133, 266 mg/kg gest. day 6-15 Domingo et al.
20/group) b.w./day (1989)
Mouse (BALB/c, oral (gavage) AlCl3 oral: 200, 300 mg/kg gest. day 7-16 Cranmer et al.
6-7 mice/group) b.w./day (1986)
Mouse (Swiss oral (feed) Al lactate 500, 1000 mg Al/kg diet gest. day 0 to day Golub et al.
Webster, 15/group) 21 p.p. (1987)
Mouse (16 Swiss oral (feed) Al lactate 25, 500, 1000 mg Al/kg diet gest. day 0, until Donald et al.
Webster) weaning (1989)
Mouse (Swiss albino oral (gavage) Al(OH)3 57.5 mg/kg b.w./day gest. day 6-15 Colomina et
CD-1, 10-13/group) Al lactate 166 mg/kg/day al. (1992)
Al(OH)3 + lactic acid 627 mg/kg/day
Mouse (Swiss oral (feed) Al lactate 25, 1000 mg/kg diet/day gest. day 0 - day Golub et al.
Webster) 20 p.p. (1992)
Mouse (CBA) oral (water) Al2(SO4)3 750 mg/litre gest. day 10-17 Clayton et al.
(1992)
Rat (Sprague-Dawley) oral (feed) AlCl3 50 mg/kg b.w. gest. day 6-19 McCormack
et al. (1979)
Rat (Wistar, 14/group) oral (feed) Al lactate 100, 200, 300 mg Al/kg b.w. gest. day 8 to Bernuzzi et al.
parturition (1986, 1989a,b)
Rat (Sprague-Dawley) oral (gavage) Al(NO3)3 180, 360, 720 mg/kg day gest. day 14-21 Domingo et al.
(1987a)
Table 20. (Con't)
Species Route of application Compound Dose Duration Reference
Rat (Sprague-Dawley, oral (gavage) Al(NO3)3 180, 360, 720 mg/kg b.w. gest. day 6-14 Paternain et
10/group) al. (1988)
Rat (Sprague-Dawley, oral (gavage) Al(OH)3 384 mg/kg/day gest. day 6-15 G�mez et al.
15-19/group) Al citrate 1064 mg/kg/day (1991)
Al(OH)3 + citric acid
Rat (Wistar, oral (gavage) Al(OH)3 192, 384, 768 mg/kg/day gest. day 6-15 G�mez et al.
18-19/group) (1990)
Rat (Wistar, oral (feed) Al lactate 400 mg Al/kg diet gest. day 1-7 Muller et al.
6-9/group) gest. day 1-14 (1990)
gest. day 1-20
a b.w. = body weight; gest. = gestational; p.p. = postpartum
Effects of aluminium exposures on brain development have also
been studied in mice (Donald et al., 1989; Golub et al., 1992, 1994,
1995; Clayton et al., 1992) and rats (Bernuzzi et al., 1986, 1989a,b;
Muller et al., 1990; Cherroret et al., 1992) by the oral route, and in
rabbits (Yokel, 1984, 1985, 1987) using subcutaneous injections.
Effects recorded in immature animals in more than one study in rats
and mice included impaired performance of reflexes and simple
behaviours (e.g., righting reflex, grasping, negative geotaxis, rod
climbing). Other effects included footsplay and temperature
sensitivity (tail withdrawal); (Donald et al., 1989; Golub et al.,
1992) and auditory startle (Golub et al., 1994). Postnatal mortality
and growth were also affected at the higher doses in only some of
these studies. Studies of cognitive parameters in immature animals are
limited to evaluation of classical conditioning of the nictating
membrane response in rabbits. No effect of aluminium was seen after
postnatal subcutaneous injection of aluminium lactate (Yokel, 1987);
both enhancement and impairment of conditioning were seen after
exposure during gestation to aluminium lactate, depending on the dose
(Yokel, 1985).
Adult rats and mice have also been evaluated for brain function
after developmental exposures. Reduced grip strength and startle
responsiveness were found to persist up to 150 days of age in mice
(Golub et al., 1995). No effect was found on a light avoidance task in
rats after gestational (Bernuzzi et al., 1989b) or postnatal exposure
(Cherroret et al., 1992). Radial maze learning/performance was also
unaffected by postnatal exposure (Cherroret et al., 1992). No effects
on delayed alternation or discrimination reversals were recorded in
mice after dietary exposure during gestation and lactation (Golub et
al., 1995).
The lowest-observed-adverse-effect level (LOAEL) for
developmental effects after oral dosing was 13 mg Al/kg body weight
per day by oral gavage of aluminium nitrate (Paternian et al., 1988).
A dose-response relationship was noted with the highest dose of this
extremely soluble aluminium salts (52 mg Al/kg body weight)
representing 1/5 the LD50 (see Table 18). At 13 mg Al/kg per day,
decreased ossification of skull bones, increased incidence of
vertebral and sternebrae, and reduced fetal weight and tail length
were reported with higher incidence of these effects at the higher
doses (26 and 52 mg Al/kg body weight). Maternal toxicity (reduced
weight gain during pregnancy) was reported to occur in a dose-
dependent manner. No developmental toxicity was noted using much
higher doses of aluminium hydroxide (266 mg Al/kg per day) in a
similarly designed study (G�mez et al., 1990).
After administration of aluminium lactate in the diet of mice and
rats a LOAEL of 100 mg Al/kg body weight per day was reported (Donald
et al., 1989; Bernuzzi et al., 1989a,b).
The effect reported at 100 mg Al/kg per day by Bernuzzi et al.
(1989a,b) in rats was impaired grip strength in the 6-day-old
offspring of dams fed aluminium lactate in diet throughout gestation.
Dose response was indicated in this study; at higher doses (200 and
400 mg Al/kg per day) impaired grip strength was reported along with
impaired righting reflex and locomotor coordination. No effects on
maternal or offspring weight were reported at 100 mg Al/kg although
they occurred at the higher doses. Litters were not culled to a
standard size at birth in this study. The litter was used as the unit
of statistical analysis to avoid litter effect.
The effects reported at 100 mg Al/kg per day by Donald et al.
(1989) in mice were increased landing foot splay, increased hindlimb
grip strength and decreased temperature sensitivity in 21-day-old
offspring of mice fed aluminium lactate in the diet throughout
gestation and lactation. There was no effect on negative geotaxis or
startle reflexes in the offspring. Dose response was not indicated in
this study; similar effects were reported at a higher dose (200 mg
Al/kg). No effects on maternal or offspring weights were found at
either dose. It is not stated whether litters were culled to a
standard number at birth. To address litter effects, litter was nested
under treatment group in the statistical analysis. In this study,
daily aluminium intake was estimated based on food intake at the
beginning of pregnancy.
7.4 Mutagenicity and related end-points
7.4.1 Interactions with DNA
A number of observations indicate that aluminium is able to form
complexes with DNA and can cross-link chromosomal proteins and DNA. In
thermal denaturation, circular dichroism and fluorescence studies,
Karlik et al. (1980) found that aluminium had a stabilizing effect
upon the DNA double helix at a pH > 6, while at lower pH levels,
binding of aluminium de-stabilized the DNA double helix. A recent
investigation of NMR spectra and circular dichroism of DNA-aluminium
complexes indicated that Al3+ binds to the phosphate oxygen while
hydroxylated aluminium-species probably prefer other sites such as DNA
bases (Rao & Divakar, 1993).
Cross-linking of various cytoplasmic proteins to DNA was
investigated in live Novikoff ascites hepatoma cells exposed to
aluminium in vitro (Wedrychowski et al., 1986). Cross-linking agents
frequently produce clastogenic effects, owing to conformational
distortions that prohibit proper replication of the DNA. More recently
it was shown that AlF4- stimulates the glycation of the histone H1
in the proximity of its nucleotide-binding site, thus interfering with
nucleoside triphosphate hydrolysis by H1 and with nucleotide
modulation of H1 DNA binding (Tarkka et al., 1993).
In addition, it has been shown that micro-molar levels of
aluminium reduce 3H-thymidine incorporation in a transformed cell
line (UMR 106-01) by impeding the cell cycle progression (Blair et
al., 1989). More specifically, aluminium was shown to inhibit the
ADP-ribosylation, a mechanism important for DNA repair, in vivo and
in vitro (Crapper McLachlan et al., 1983).
7.4.2 Mutations
The rec-assay using Bacillus subtilis strains failed to show
mutagenic activity for Al2O3, AlCl3 or Al2(SO4)3 at
concentrations of 1-10 mM (Nishioka, 1975; Kada et al., 1980;
Kanematsu et al., 1980; L�onard & Gerber, 1988; Bhamra & Costa,
1992). No reverse mutations were observed in the Ames test using
Salmonella typhimurium strain TA102 with AlCl3 (concentration
range 10-100 nM per plate; Marzin & Phi, 1985). No morphological
transformations were seen in Syrian hamster embryo cells after
application of aluminium salts (no further specification) (Di Paolo &
Casto, 1979). No induction of forward mutations were observed at the
thymidine kinase locus in L5178Y mouse lymphoma assay with AlCl3 when
tested at concentrations up to 625 �g AlCl3/ml (Oberly et al., 1982).
7.4.3 Chromosomal effects
A significant increase of chromatid-type aberrations (including
gaps, breaks, translocation and ring formations), with non-random
distribution over the chromosome complement, was found in bone marrow
cells from mice that were dosed interperitoneally with AlCl3 (Manna &
Das, 1972). Prolonged treatment of rats with Al2(SO4)3 or
KAl(SO4)2 caused a dose-dependent inhibition of dividing cells (bone
marrow) and an increase in chromosomal aberrations (Roy et al.,
1991a). Chromosomal aberrations were also induced in peritoneal cells
from rats, mice and Chinese hamsters (Bhamra & Costa, 1992) as well as
in human leukocyte cultures (Roy et al., 1990). Aluminium caused a
concentration-dependent bimodal change in the number of sister
chromatid exchange in cultured human lymphocytes and increased the
unscheduled DNA synthesis in cultured human astrocytes (De Boni et
al., 1980).
7.5 Carcinogenicity
Based on limited early studies, there is little indication that
aluminium is carcinogenic (Leonard & Gerber, 1988; Bhamra & Costa,
1992). Some studies indicated that inhalation of aluminium-containing
fibres and particles may induce carcinomas in the lung. However, in
these cases it is likely that the toxicity reflects the physical
properties of the particles/fibres (3.5 �m median diameter).
Similarly, aluminium implanted subcutaneously has induced soft tissue
carcinomas at the site of implantation, but in these cases also the
effects are probably related to a chronic foreign body reaction rather
than to the aluminium ion itself (Stanton, 1974; Pigott & Ishmael,
1981; Pigott et al., 1981; Krueger et al., 1984).
7.6 Neurotoxicity
Considerable evidence indicates that aluminium is neurotoxic to
experimental animals, but species variation exists. In susceptible
animals, the toxicity is characterized by progressive neurological
impairment resulting in death associated with status epilepticus.
Morphologically, the progressive encephalopathy is associated with
neurofibrillary pathology in large and medium size neurons
predominantly in the spinal cord, brain stem, and selected areas of
cortex (hippocampus and cingulate gyrus). However the nature of the
accumulated fibrils at the light microscopic level and under the
electron microscope differ from those found in AD. The tangles are not
birefringent and are composed of 10 nm neurofilaments. The proteins
involved in the aluminium-induced neurofibrillary tangles also differ
from those found in the human diseases (see section 8.1.3.1). However,
aluminium is the only known trace element capable of inducing this
type of mylo-encephalopathy in susceptible animals (rabbit, cat,
guinea-pig, ferret). The epileptogenic property of aluminium, in
contrast to the progressive encephalopathy, occurs in all species
studied (e.g., primates, rodents, fish). Routes of administration of
aluminium sufficient to induce the encephalopathy include intrathecal
intracerebral and subcutaneous injections. There have been no reports
of progressive encephalopathy or epilepsy when aluminium compounds
were given orally.
The brain aluminium concentration necessary to achieve LD50 in
rabbits is about 6 �g aluminium/g dry weight (Crapper McLachlan et
al., 1989; McLachlan & Massiah, 1992). The normal brain aluminium
concentration in healthy rabbits is approximately 1.1 �g/dry weight.
7.6.1 Impairments of cognitive and motor function
Cats and adult or infant rabbits given intracerebral injections
of soluble aluminium compounds revealed a progressive impairment in
learning and memory performance after an asymptomatic period of 8 to
10 days (Crapper & Dalton, 1973a,b; Petit et al., 1980; Rabe et al.,
1982; Solomon et al., 1990). Repeated subcutaneous injections of
aluminium in rabbits affected classical conditioning (Yokel, 1983).
The intracisternal injection of a single or repeated low doses of
metallic aluminium in rabbits resulted in altered motor function
(Wisniewski et al., 1982; Bugiani & Ghetti, 1982; Strong et al., 1991;
Strong & Garruto, 1991b). The animals developed progressive myelopathy
and topographically specific motor neuron degeneration. They exhibited
myoclonic jerks and muscular weakness. Histopathologically a
neurofibrillary degeneration with swelling of the proximal axonal
processes of anterior horn neurons was present. The authors proposed
these preparations as possible models of human amyotrophic lateral
sclerosis.
Behavioural impairment has been reported in laboratory animals
exposed to aluminium in the diet or drinking-water in the absence of
overt encephalopathy or neurohistopathology. Both rats (Commissaris et
al., 1982; Thorne et al., 1987; Connor et al., 1988) and mice
(Yen-Koo, 1992) have demonstrated such impairments at doses exceeding
200 mg Al/kg body weight. While significant alterations in acquisition
and retention of learned behaviour were documented, the possible role
of organ damage (kidney, liver, immunological) due to aluminium was
incompletely evaluated.
7.6.2 Alterations in electrophysiological properties
The progressive encephalopathy and morphological alterations are
also associated with electrophysiological changes. The epileptic
seizures are associated with slowing of the EEG and epileptic
activity. The mechanisms that evoke the neuronal hyperexcitability
have not yet been completely elucidated but may involve altered
membrane electrotonic properties, K+ conductance, and synaptic
processes (Franceschetti et al., 1990). Associative long-term
potentiation (LTP), describes strengthening of a previously weak
synaptic input by concomitant activation of a strong synaptic input.
LTP can last up to several weeks and has been used as a model for the
hippocampal contribution to memory. LTP was not sustained normally in
hippocampal slices from rabbits exposed to intracranial injections of
aluminium about 7 days prior to sacrifice. The occurrence of this
electrophysiological alteration corresponds to the onset of
behavioural changes but is not necessarily accompanied by
neurofibrillary pathology in the hippocampal neurons exhibiting
impaired LTP. The loss of LTP can be partially reversed by an increase
in the calcium concentration in the bath (Farnell et al., 1985;
Crapper McLachlan & Farnell, 1986).
In summary, aluminium exposure has been used as an animal model
for the study of epilepsy, information processing, cognitive
dysfunction and motor neuron disease.
7.6.3 Metabolic effects in the nervous system
Considerable experimental evidence implicates aluminium in
alterations in the second messenger systems of cAMP and G proteins
(Steinweis & Gilman, 1982; Johnson & Jope, 1987; Johnson et al., 1990,
1992). An increased cAMP concentration in brain tissue is a
prerequisite for an increase in the phosphorylation of proteins. An
elevation of protein kinase C activity and in the basal activity of
cAMP-dependent protein kinase resulted in hyperphosphorylation of 12
proteins in rats chronically treated with aluminium (Johnson et al.,
1990). In rats chronically treated with low oral doses of aluminium,
hyperphosphorylation of MAP-2 was increased by 150% and the
neurofilament H subunit by 150-200%, while the phosphorylation of
several other proteins including tau was not different from that of
control rats (Johnson et al., 1990; Jope & Johnson, 1992). It was
suggested that abnormal phosphorylation may impair the axonal
transport of cytoskeletal proteins.
Ohtawa et al. (1983) showed that Al3+ binding to ferritin
reduced the binding of Fe2+ in rats fed aluminium. The free
intracellular Fe 2+ augmented the peroxidation of membrane lipids.
Lipid peroxidation in kidney, lung, liver and spleen were not
affected. The increased lipid peroxidation is at least, in part, due
to inhibition of superoxide dismutase in the brain but not in other
organ systems. An increase in lipid peroxidation was also shown in
chickens fed with aluminium sulfate (Chainy et al., 1993). It is
presumed that an increase in lipid peroxidation may be part of the
mechanisms underlying aluminium neurotoxicity.
Aluminium is not equally distributed among chromatin fractions
within the nucleus in control and aluminium-treated preparations.
Concentrations of aluminium on highly condensed, non-transcribed
chromatin are 15 to 20 times higher than those on active, decondensed
chromatin (Crapper et al., 1980). Several experimental models have
demonstrated that aluminium inhibits RNA synthesis and therefore may
have an effect on gene expression.
Transient change in the blood-brain barrier to [14C]sucrose have
been observed following low-dose intraperitoneal injection of
aluminium compounds (Kim et al., 1986). Intraperitoneal administration
of aluminium compounds increased the permeability of the blood-brain
barrier for a number of peptide and steroid hormones, such as
prolactin, growth hormone, luteinizing hormone, thyroxine and
cortisol. The greatest increase in penetration was observed for
thyroxine, which is transported by a carrier-mediated mechanism (Banks
& Kastin, 1985). From further studies on other transport systems, it
was suggested that aluminium selectively alters the transport systems
of the blood-brain barrier (Banks et al., 1988a; Vorbrodt et al.,
1994).
7.7 Effects on bone
The skeleton is the principal site of aluminium deposition in the
body. Aluminium deposited in this organ is important both because of
its toxic effects on bone tissues and because the deposits act as a
reservoir. Aluminium continues to be released from this reservoir to
the blood stream for a long time after intake as a consequence of bone
turnover, commonly referred to as bone remodelling.
Within the skeleton aluminium, in common with most other
polyvalent metal ions, deposits on bone surfaces within a very thin
layer (van de Vyver & Visser, 1990). How metal ions deposit in this
way is unclear, but three modes of uptake have been suggested.
Firstly, the metal may become trapped within the hydration shell of
the bone mineral crystal, secondly, it may become incorporated into
new bone crystals as they form at sites of bone accretion, and,
finally, they may become bound by acidic organic components of the
bone matrix, such as phosphoproteins (Priest, 1990). Subsequently,
much of the aluminium may remain on surfaces until it back-exchanges
into tissue fluids or may become locked into the bone matrix. "Locked
in" ions may then become buried to form volume deposits below bone
surfaces, as a result of bone accretion, or may be released by the
bone resorption process. Resorbed aluminium first enters osteoclasts
and macrophages and then returns to tissue fluids, including blood,
for recycling or excretion. The burial and resorption processes take
many years in man, where only a minority of surfaces show remodelling
activity, but occur rapidly in most experimental animals. As the rate
of bone turnover is largely under hormonal control, it follows that
hormones also regulate the retention by and release from the skeleton
of the more permanent deposits of aluminium. In this respect the most
important hormones are calcitonin and parathyroid hormone (PTH), which
act to increase either the rate of bone formation or the rate of bone
resorption in response to serum levels of calcium.
7.7.1 Toxic effects of aluminium in the skeleton
Excess deposits of aluminium in the skeleton may result in a
syndrome commonly referred to as "aluminium-induced bone disease"
(AIBD). This has been reviewed by Goodman (1986, 1990), van de Vyver &
Visser (1990) and Quarles (1991). A summary of some of the animal
models used to study osteomalacia induced by aluminium is given in
Table 21. It should be noted that all models utilize intraperitoneal
or intravenous routes of administration, making it impossible to
extrapolate to the risk in humans exposed to aluminium primarily by
the oral route. AIBD presents as a moderate to severe low bone
turnover osteomalacia, which is often insensitive to the vitamin D
complexes that reverse the osteomalacia of rickets. It may also result
in the de-coupling of the bone resorption and bone accretion processes
producing an excess volume of structurally incompetent bone (neo-
osteogenesis) and in disturbances in the normal processes of
endochondral ossification in the long-bone metaphyses. In osteomalacic
bones, osteoid (the unmineralized bone matrix) fails to mineralize or
is increased (Goodman et al., 1984a,b; Sedman et al., 1987; Quarles et
al., 1988), tetracycline markers of bone mineralization are not
incorporated (Ellis et al., 1979) and bone resorption is reduced,
resulting in an increase in the volume of unmineralized bone.
Associated with these changes is a reduction in the number of
osteoblasts (bone-forming cells) (Robertson et al., 1983) and a
reduction in the level of circulatory osteocalcin produced by these
cells. If osteomalacic bones are stained for aluminium then the metal
may be demonstrated as being present at the mineralized bone/osteoid
interface. Where present it would seem that the aluminium inhibits the
mineralization of osteoid, this amounting to a complete block in
severe cases. However, the mechanism for this block is unknown.
Goodman (1990) has suggested that it results from an impairment of the
movement of calcium and phosphate ions from the tissue fluids to the
face of the forming hydroxyapatite bone-mineral crystals.
Aluminium, like some other metals, e.g., strontium, when present
in bone crystals reduces the ability of the osteoclast (the bone
resorbing cells) to resorb the mineral. As expected, reduced bone
turnover may result in a reduced level of calcium in blood
(hypocalcaemia), which, in turn, might be expected to produce changes
in the levels of bone-active hormones in the circulation. In
particular aluminium-induced hypocalcaemia would be expected to result
in increased production of parathyroid hormone (PTH), in an attempt to
stimulate bone resorption and restore normal blood calcium levels.
However, the available evidence suggests that this does not occur and
that the levels of circulating PTH are normal or even reduced (Goodman
et al., 1984a). Rodriguez et al. (1990) found that PTH is able to
stimulate osteoblasts in the presence of aluminium, but that it cannot
improve mineralization. Similarly, there is also much evidence to
suggest that fluoride (a potent stimulator of osteoblast numbers)
interacts with aluminium in the skeleton by antagonizing the
aluminium-induced reduction in osteoblast numbers, but does not
ameliorate the aluminium-induced decrease in mineralization (Ittel et
al., 1992).
Of the available animal models, only the larger species, e.g.,
dogs and pigs, consistently show osteomalacia as it presents in man
(Goodman, 1990). In rodents bone remodelling is an unimportant feature
of normal bone turnover (bone growth continues throughout life), and
in these animals aluminium does not produce classical osteomalacia,
although the changes in bone accretion, mineralization and resorption
seen in the larger species are also seen in rats and mice. In these
species, the disturbances seen in the ossification and resorption of
bone under the growth cartilages may indicate a risk of similar
occurrences in aluminium-contaminated children. Animal studies have
shown that osteomalacia in trabecular bone is induced faster than in
cortical bone, a result of the lower bone turnover in the latter
(Goodman et al., 1984a,b).
Table 21. Experimental animal models of aluminium-induced osteomalaciaa
Species Route of Compound Dose Duration of Al concentration Histomorphometry Reference
application treatment in boneb
Rat (20 i.p. AlCl3 0.27-2.7 mg once daily; 5 days/ (15.4 �g/g), n.d. Ellis et
Wistar) Al/day week; 48-85 days 109.3-176 �g/g al. (1979)
Rat i.p. AlCl3 N (control), once daily; 5 days/ n.d. % osteoid: N: 6.6; Robertson
week; 90-120 days LD: 7.8; HD: 1.8; et al.
LD: 0.1 mg osteoid width (�m): N: 3.3; (1983)
Al/day; LD: 3.7; HD: 14.9;
HD: 1.0 mg tetracycline uptake:
Al/day irregular, attenuated in HD
Rat (74 i.p. AlCl3 1.5 mg Al/kg 5 days/week; 55 mg/kg d.w. Alfrey et
male SD) b.w. per day 35-79 days al. (1985)
Rat i.p. Al, 2 mg/day 5 days/week, not specified decreased bone formation Goodman
(weanling, elemental 4 weeks and osteoid maturation et al.
male H, in Al-treated animals (1984b)
10/group)
Rat i.p. Al, 2 mg/day 5 days/week, not specified formation of periosteal bone Goodman
(weanling elemental 44 days and matrix reduced (1984)
male H,
10/group)
Rat (male i.p. AlCl3 1.5 mg Al/kg 5 days/week, control: osteoid width (�m): normal: Chan et al.
W, 23) day; 9 weeks 1.8 mg/kg d.w.; 3.5; Al-treated: 3.0 (1983)
Al-treated:
47.0 mg/kg d.w.
Table 21. (Con't)
Species Route of Compound Dose Duration of Al concentration Histomorphometry Reference
application treatment in boneb
Rat (male i.p. AlCl3 10 mg/kg 5 days/week 3, 6, 9 weeks, 9 w: trabecular and Ott et al.
H, 18/group) per day 3 weeks recovery endosteal bone formation (1987)
weanling, decreased;
adult periosteal bone formation
normal, recovery to
normal level
Dog i.v. AlCl3 1 mg/kg b.w. 3 times weekly control: 10.5 % osteoid surface: Quarles
(Beagle, 3 weeks mg/kg d.w. control: 35.6% et al.
6/group) Al-treated: 73.6 Al-treated: 35.8% (1985)
mg/kg d.w.
Dog (6 i.v. AlCl3 1 mg Al/kg 3-5 weeks (1.3 mg/kg), % osteoid: (2.8)b 7.0 Goodman
female b.w. per day 94 mg/kg d.w. osteoid width: (5.7)b 8.0 et al.
mongrel) poor tetracycline uptake (1984a)
Dog i.v. AlCl3 0.75 mg Al/kg 5 days/week, (7.4 mg/kg d.w.) number of osteoblasts Galceran
(female b.w. 3 months 202.6 mg/kg d.w. 8-fold decreased et al.
mongrel, (1987)
7/group)
Dog i.v. AlCl3 0.75 mg Al/kg 3 days/week, (2.2 �g/g) low dose, 8 weeks; reduced Quarles
(18 male b.w. 16 weeks low dose, bone resorption and et al.
Beagles) 8 weeks: osteoblastic surfaces; low (1988)
1.2 mg Al/kg 65.8 mg/kg low turnover: low dose, 16 weeks;
b.w. dose, 16 weeks: increased trabecular number;
161.7 mg/kg uncoupled bone formation
Table 21. (Con't)
Species Route of Compound Dose Duration of Al concentration Histomorphometry Reference
application treatment in boneb
high dose, high dose, 8 and 16 weeks:
8 weeks: uncoupled bone formation
125.2 mg/kg
high dose,
16 weeks:
152.2 mg/kg
Dog i.v. AlCl3 1.25 mg/kg 3 days/week: (4.2 ng/litre) sham op. + Al bone volume, Quarles
(8 male b.w. per day 8 weeks sham op. + Al: tabecular number increased et al.
Beagles), 147 ng/litre (1989)
normal
Piglets i.v. AlCl3 1.5 mg/kg 8 weeks (1.6 mg/kg d.w.) osteoid seam width, osteoid Sedman
(8 Yorkshire) per day 241 mg/kg d.w. volume, mineralization lag et al.
time: increased; reduction of (1987)
active bone forming surface
a b.w = body weight; d.w. = dry weight; H = Holtzman; i.p. = intraperitoneally; i.v. = intravenous; n.d. = no data; SD = Sprague-Dawley;
W = Wistar; N = control; LD = low dose; HD = high dose; op = operation
b Control values are given in parentheses
Neo-osteogenesis, resulting in large increases in the volume of
trabecular bone, following aluminium administration has been observed
in a number of animal studies (Galceran et al., 1987; Quarles et al.,
1988, 1990) and has even been suggested as a possible treatment for
post-menopausal osteoporosis in women. In these studies a dose and
time-dependent effect was seen, lower levels of aluminium suppressing
bone formation but higher doses for long periods of time resulting in
an increased bone volume. Under such conditions it is likely that bone
apposition has become uncoupled from bone resorption processes - a
pathological change.
7.7.2 Dose response
As indicated above, the extent of pathological changes in bone
produced by aluminium after parenteral administration is dose-
dependent (Goodman, 1986), being barely perceptible at low doses, but
marked or severe at high doses. However, dose response will be much
affected by the period of aluminium accumulation, so that a single
high intake of aluminium may produce transient changes that are more
marked than in the case of higher accumulations over a long period. In
general, the available evidence suggests that, as in humans, bone
aluminium levels in the order of 100-200 �g/kg bone ash are required
to produce these changes. The study of Ellis et al. (1979) showed that
levels of aluminium in bone in the order of 100 �g/g bone ash produced
few bone changes in rats, but that higher levels produced marked
changes in the rat metaphysis and osteomalacic changes, these being
most marked in rats with excess aluminium levels of about 200 �g/g
bone ash. The authors found that osteotoxic doses of aluminium were
similar in humans and rats. Longer term studies in dogs (see Table 21)
show that low exposures to aluminium for short times produced few
morphological changes in bone, but that similar or higher dose levels
for a longer period did result in observable changes. Uncoupled bone
formation (neo-osteogenesis) was produced after 16 weeks at a final
bone aluminium level of 170 �g/g dry weight (Quarles et al., 1988). An
earlier study by the same authors showed no observable bone changes
after 9 weeks at a final bone aluminium content of 70 �g/g dry weight
(Quarles et al., 1985). Similarly, human aluminium levels of this
order are unlikely to result in osteomalacia.
7.8 Effects on mineral metabolism
Oral application of AlCl3 (300 to 1200 mg/kg diet) to cattle for
84 days did not change plasma or tissue levels of calcium, phosphorus,
magnesium or iron, although increased levels of zinc were seen in
liver and kidney (Valdivia et al., 1978). In sheep (20 lambs) fed a
diet supplemented with AlCl3 (2000 mg/kg for 56 days), Valdivia et
al. (1982) observed a reduction of the apparent calcium absorption and
lower plasma/serum phosphate levels. The ingestion of moderate
concentrations of aluminium (aluminium lactate, aluminium palmitate,
aluminium phosphate, aluminium hydroxide; 5-272 �g Al/g diet) also had
no effect on tissue calcium, magnesium or iron levels in male Sprague-
Dawley rats (Greger et al., 1985a). Small effects were seen on tissue
levels of phosphorus, zinc and copper. In rats receiving high
intraperitoneal doses of aluminium (AlCl3, 2.7 mg Al/day) repeatedly
for 10 days, the calcium concentration was increased in brain, liver
and spleen, but not in the heart, and serum calcium levels were not
significantly affected (Burnatowska-Hledin & Mayor, 1984). No changes
in plasma magnesium levels were seen in this study.
Reduced plasma magnesium concentrations were observed in cows
(Kappel et al., 1983; Allen et al., 1986) and sheep (Valdivia et al.,
1982) after feeding a diet supplemented with various amounts of
aluminium chloride, sulfate or citrate. In sheep (24 lambs) fed an
aluminium-rich diet (1450 �g/g), the magnesium content of kidney and
bone was reduced (Rosa et al., 1982).
From their experiments in dogs (female mongrel) receiving i.v.
injections of AlCl3 (1 mg Al/kg body weight, 5 days/week, for 3-5
weeks), Henry et al. (1984) concluded that the increased serum calcium
concentration was due to an increased liberation from bone.
In cows fed a diet supplemented with aluminium citrate
(1730 mg Al/kg dry weight for 56 days), serum and urinary calcium
concentrations were increased (Allen et al., 1986).
The effect of vitamin D and its metabolites on calcium metabolism
following aluminium intoxication was studied by Hodsman et al. (1984)
and by Henry & Norman (1985). In vitamin-D-deficient rats receiving
repeated intraperitoneal injections of AlCl3 (9.25 mg aluminium for
33 days), serum calcium levels were increased regardless of the
vitamin D status of the animals (Hodsman et al., 1984). Treatment of
chickens with AlCl3 (5 mg Al/kg body weight intraperitoneally for 5
days) partially blocked the intestinal calcium absorption response to
vitamin D in vitamin-D-deficient animals, although serum calcium
levels were elevated (Henry & Norman, 1985). No consistent effects of
aluminium on the bone calcium mobilization response to vitamin D or
1,25(OH)2D3 were noted. The authors concluded that the ability of
the intestine to respond normally to 1,25(OH)2D3 may be compromised
by the aluminium application.
A depression of the serum phosphate level was also observed in
vitamin-D-deficient rats receiving repeated intraperitoneal injections
of AlCl3 (9.25 mg aluminium for 33 days; Hodsman et al., 1984).
Aluminium forms complexes with fluoride, which are considerably
more stable than the respective Fe3+ complexes. Ingestion of
relatively small amounts of aluminium decreases the fluoride
concentration available in the intestinal lumen by complexation and
thus fluoride absorption from the intestine. Fluoride and AlF4-
stimulate the enzyme adenylate cyclase (Sternweis & Gilman, 1982).
This effect presumably proceeds by complexation of Al3+ that is
present as a contaminant of the substrate ATP, thus raising the
effective ATP concentration (Martin, 1986).
Aluminium exerts its protective effect from fluorine toxicosis,
which has been reported in hens (Hahn & Guenter, 1986), turkeys (Cakir
et al., 1977) and sheep (Saia et al., 1977), also by formation of a
stable complex between Al3+ and F-, thus increasing the fecal
fluoride excretion.
8. EFFECTS ON HUMANS
8.1 General population exposure
Aluminium is a potential neurotoxic agent in humans (Steinegger
et al., 1990). Humans have highly efficient natural barriers to limit
aluminium concentration within the central nervous system except under
specific conditions such as renal failure. Encephalopathy attributed
to aluminium intoxication in patients receiving treatment for chronic
renal failure is discussed in section 8.3.1.
8.1.1 Acute toxicity
There is little indication that aluminium is acutely toxic by
oral exposure despite its widespread occurrence in foods, drinking-
water and many antacid preparations.
8.1.2 Effects of short-term exposure
In 1988 a population of perhaps 20 000 local residents and
numerous tourists were exposed for 5 days or more to unknown levels of
aluminium sulfate, subsequent to 20 tonnes of concentrated aluminium
sulfate being accidentally placed in the Lowermoor water treatment
plants in Camelford, England. The drinking-water also contained
elevated concentrations of lead and copper, which leached from the
plumbing systems due to increased water acidity. In view of the
anecdotal reports of nausea, mouth ulcers, skin rashes and increased
arthritic pain, some lasting for months after the exposure, the
Cornwall District Health Authority convened a Health Advisory Group
which prepared an official report on the incident (Clayton, 1989). The
report described the exposure scenario and made conclusions based on
expert knowledge of aluminium toxicity and interviews with local
residences and tourists claiming long-lasting adverse health effects.
No one was exposed to levels of aluminium over 100 mg/litre since such
water is unpalatable. Levels between 10 and 50 mg/litre were found for
only 1-3 days and water levels for the next month were above 0.2 mg/
litre but below 1 mg/litre. The report concluded that such exposures
should not pose a hazard to human health. Furthermore, the report
concluded that there is no evidence to support the causation by the
levels of aluminium, zinc, lead and sulfate of joint and muscle pain,
memory loss, hypersensitivity or gastrointestinal disorders reported
by residents and tourists some months after the incident. The report
stated: "In our view it is not possible to attribute the very real
current health complaints to the toxic effects of the incident, except
insofar as they are the consequence of the sustained anxiety naturally
felt by many people". Other reports, such as that of McMillan et al.
(1993), despite containing major scientific deficiencies, do not
provide evidence contrary to this conclusion.
8.1.3 Neurotoxic effects
8.1.3.1 Aluminium and Alzheimer's disease (AD)
It has been suggested that aluminium exposure is a risk factor
for the development or acceleration of onset of Alzheimer's disease
(AD) in humans (Crapper McLachlan, 1986; Crapper McLachlan et al.,
1989). The precise pathogenic role of aluminium in AD is judged
controversial and remains to be defined (Wisniewski & Wen, 1992;
Wischik et al., 1992; Edwardson, 1992).
The purported association is based on six points:
1. The experimental induction of neurofibrillary changes in the
neurons of certain species of animals, which suffer a unique
progressive neurological impairment after parenteral administration of
aluminium salts (see chapter 7). However, these neurofibrillary
changes differ from those seen in AD in staining properties and
ultrastructural and biochemical composition (Wisniewski & Wen, 1992).
2. The presence of elevated aluminium levels in bulk grey matter of
AD-affected brains, which have been found by most investigators using
various techniques, including graphite furnace atomic absorption
spectroscopy (Crapper et al., 1973, 1976), neutron activation
techniques, and inductively coupled mass spectroscopy. However, some
investigators using the same techniques have failed to find elevated
aluminium levels in the brains of patients with AD (McDermott et al.,
1979; Jacobs et al., 1989).
3. The reported detection of aluminium in the amyloid core of
classical plaques, neurofibrillary tangles and neuronal nuclei
affected by neurofibrillary pathology in AD. Increased aluminium
levels have been found within the neurofibrillary tangles of AD using
laser microprobe mass spectrometry (LMMS) (Good et al., 1992).
However, using the same technology, others have been unable to
replicate these results (Lovell et al., 1993). These techniques are
difficult. There may be technical reasons for the disparity in the
results, or some of the differences may relate to aluminium
contamination of fixatives and stains (Landsberg et al., 1992). AD
patients may have an altered blood-brain barrier that allows excess
aluminium to accumulate in the brain (Wisniewski & Kozlowski, 1982;
Liss & Thornton, 1986; Banks et al., 1988b), although this may in turn
be secondary to deposition of amyloid fibrils in the walls of large
and small cerebral vessels (amyloid angiopathy) (Wisniewski et al.,
1992). If it is accepted that aluminium levels are raised in classical
plaques and neurofibrillary tangles in AD, it has been proposed that
this may be a secondary phenomenon rather than the primary etiological
agent.
4. Epidemiological studies showing an association between aluminium
intake in drinking-water and an increase in the prevalence of AD. The
methodology and interpretation of these studies is still under debate,
and is considered further in section 8.1.3.2.
5. The reported decrease in the rate of disease progression in
clinically diagnosed AD patients in one, single-blind, oral-versus-
placebo controlled trial of desferrioxamine, a trivalent ion chelator
administered by intramuscular injection (McLachlan et al., 1992).
However, this study has been criticized on three grounds: a)
desferrioxamine also chelates iron, which has been linked to free
radical damage in AD (Andorn et al., 1990; Crapper McLachlan et al.,
1991); b) desferrioxamine may have acted through an anti-inflammatory
effect in this (at least partly) inflammatory disease (Crapper
McLachlan et al., 1991); c) the absence of a double-blind placebo
controlled design may have resulted in differential treatment of
patients in the active arm (Wisniewski & Rabe, 1992).
6. The reported interactions of aluminium with �-amyloid protein
(the major component of AD plaques) and with purified paired helical
filament tau protein (the major component of neurofibrillary tangles,
NFTs). The neurotoxicity of �-amyloid and the formation of plaque
deposits is dependent on its aggregation, which has been found to be
promoted by low millimolar concentrations of aluminium, iron and zinc
(Mantyh et al., 1993). However, this pattern of results has been
attributed to iodination-induced alteration of the �-protein structure
by Bush et al. (1994), who reported that zinc is a much more potent
metallic ion aggregator of native �-protein than aluminium, being
active at low micromolar concentrations. In vitro studies have
failed to induce Alzheimer-type paired helical filaments (PHFs) in any
cellular system. However, human neuroblastoma cells in tissue culture,
exposed to aluminium, exhibit epitopes found in AD tangles. Mesco et
al. (1991) reported aluminium induction of the well-known Alz50
epitope-recognizing NFT, and Guy et al. (1991) reported the
development of an epitope, recognized by an antibody staining for NFT
and neuropil threads. Alz50 expression is also observed in
experimental aluminium encephalopathy. Shin et al. (1994) have found
that aluminium binds to and stabilizes paired helical filament tau,
both in vitro and in vivo. Although aluminium-stabilized PHF tau
induced co-deposition of �-protein in vivo, the relevance of these
recent unconfirmed findings to AD is as yet unclear.
It seems likely that the causation and pathogenesis of AD is
multi-factorial (i.e. it may be regarded as a syndrome rather than a
disease) and that genetic factors and environmental factors each
contribute to a greater or lesser extent in the individual case.
Recent genetic studies show that in a small proportion of dominant
familial cases, a single point mutation near or in the �-protein
segment of the amyloid precursor protein is necessary to cause the
disease - a situation of great theoretical importance despite its
rarity (Goate et al., 1991).
Other familial AD pedigrees have been linked to a locus on
chromosome 14 (St. George-Hyslop et al., 1992; van Broeckhoven et al.,
1992). Apo-E allele status is also a major risk factor, the presence
of one E4 allele conveying a relative risk of about 3 (Saunders et
al., 1993). Other environmental risk factors include low educational
and socio-economic status, and head injury (van Duijin et al., 1991).
It is against this knowledge base that the possible contribution of
aluminium to AD must be evaluated.
8.1.3.2 Epidemiological studies on AD and environmental aluminium
levels
Many studies have examined risk factors for AD. Among the many
case control studies that have been carried out, head trauma, family
history, thyroid status, maternal age, child with Down syndrome all
stand out as important risk factors (van Duijn et al., 1991).
Aluminium exposure as a single risk factor began to be examined in the
early 1980s, when reports of the increased level of aluminium in
brains of AD patients suggested that this might also be a factor. The
availability of water-borne aluminium measurements in many public
water supplies and of readily available vital statistics made the
study of this exposure relatively accessible.
Studies that examine the relationship between aluminium in
drinking-water and AD have been carried out in five separate
populations: Norway (Flaten, 1990), Ontario, Canada (Neri & Hewitt,
1991; McLachlan, 1996), France (Michel et al., 1991; Jaqmin et al.,
1994), Switzerland (Wettstein et al., 1991) and England (Martyn et
al., 1989). These are summarized in Table 22. Several studies examined
the water aluminium-AD relationship in the course of investigating
other associations (Wood et al., 1988; Frecker, 1991). In addition,
exposure from aluminium-containing antiperspirants (Graves et al.,
1990) and aluminium-containing antacids (Flaten et al., 1991) have
also been explored as risk factors for dementia and/or AD.
Each of the studies that relate aluminium in drinking-water to
AD can be assessed systematically for comparability of exposed and
control groups, precision of exposure assessment and outcome
definition. Ideally, exposed and control groups should be controlled
for age, sex, socio-economic status and other variables that can
confound results (e.g., education, family history, etc.). Exposure
should include concentration and duration for each member of the study
group and include a dose range which can be used to assess dose-
response relationships. The outcome should be measured preferably by
standard criteria, not by surrogates (e.g., dementia for AD), and
latency should be incorporated into the analysis (Smith, 1995).
Table 22. Summary of epidemiological studies of aluminium in drinking-water and dementia or Alzheimer's disease (AD)a
Type of study Exposure measure of Outcome measure/ Results Reference
aluminum intake data source RR
Ecological aluminum in drinking-water mention of dementia AD only Flaten
(concurrent) ICD9 290, 290.1 (dementia) (1990)
4 seasonal samples 342.0 (Parkinson's disease) Al Males Females
348.0 (ALS); sex-adjusted < 0.05 1.00 1.00
death certificate 0.05-0.2 1.15 1.19
> 0.2 1.32 1.42
PD and ALS - no gradient
Morbidity aluminum in finished drinking- dementia by diagnostic category all males and females Martyn et
prevalence water; historical (not standard) RR 1.3-1.5, no dose-response al. (1989)
CT scan center records < 65 males and females
age-sex-adjusted 1.4-1.7b, dose response
Morbidity finished drinking-water "cases" were hospital discharges RR from OR, gradient for AD Neri &
prevalence aluminum; historical with Dx of AD (ICD9 331.0), Hewitt
case control presenile dementia (ICD9 290). Al RR from OR (1991)
age/sex/residence-matched < 0.01 1.00
controls with other Dx 0.01-0.099 1.13
0.10-0.199 1.26
HMRI data base - Ontario > 0.2 1.46
Morbidity aluminum in finished drinking- mnemic skills in octogenerarians no difference in mean scores of Wettstein
prevalence water; residence >15 years urinary and serum Al tests for cognitive function et al.
(1991)
Table 22. (Con't)
Type of study Exposure measure of Outcome measure/ Results Reference
aluminum intake data source RR
Morbidity urinary aluminum and serum sample of 800 residents in high slightly higher serum aluminium Wettstein
aluminum; historical and & low aluminum areas 10 AD in AD in low aluminium areas; et al.
concurrent patients & controls in each area similar urinary excretion in AD (1991)
and controls
age, sex, education
hypothesis of association not
population based supported
Morbidity aluminum in drinking-water; cognitive function in sample of probable AD - gradient-adjusted Michel
prevalence historical > 65 years by test battery for age, education, residence et al.
(DSM III) (1991)
RR 4.53/100 �g/litre aluminium
population-based (2792); age, sex, (NSS); RR corrected to NS with
education, ses, Al in water - many current aluminium measurement
sources for the data
Case control aluminium in drinking-water; pathological; confirmation of RR 1.7 aluminium > 100 �g/litre McLachlan
residence-weighted diagnosis in all cases and controls RR 2.5 aluminium > 100 �g/litre et al.
historical no age-sex-education adjustment based adjustment for 10-year (1996)
weighted exposure history
Morbidity aluminum in water, pH, cognitive function calcium protective Jacqmin
prevalence calcium RR = 1.2 with pH < 7.3 et al.
case control NS /all other pH values (1994)
a AD = Alzheimer's disease; PD = Parkinson's disease; RR = relative; OR = odds ratio; NS = not significant; ses = socioeconomic status
b Significant (P < 0.05) of highest exposure only
In addition, a variety of study designs from least powerful to
most powerful will allow a progressive assessment of the relationship
under study (e.g., ecological, cross-sectional, case control, cohort).
Nearly 20 studies that examine the relationship between AD and
drinking-water aluminium levels have been published.
Studies in five populations using different design are of
sufficient quality and meet the general criteria for exposure and
outcome assessment and for the adjustment of at least some confounding
factors (e.g., age and sex) in order to be used here to evaluate the
relationship between water-borne aluminium and AD.
Results of four studies are consistent for a positive
relationship between water-borne aluminium and AD (Martyn et al.,
1989; Neri & Hewitt, 1990; Flaten et al., 1991; McLachlan et al.,
1996). Three found a "dose-response" relationship (McLachlan, 1989;
Flaten, 1990; Neri & Hewitt, 1991) and one found a significant
relationship between high and low aluminium exposure (Martyn et al.,
1989). Adjustment for sex was performed in two of these studies
(Martyn et al., 1989; Flaten, 1990). One study (McLachlan et al.,
1996) did not adjust for age, sex or any other confounding factor but
did correct for "during life" exposure.
With regard to exposure assessment, all of the positive studies
used ecological assessment of exposure but from only one source, the
public water supply. Total exposure to aluminium was not determined.
It is therefore impossible to determine if the relationship observed
is due to water aluminium alone without explicit adjustment for, and
information about, other sources of aluminium intake.
Studies that showed no association between water aluminium and AD
(Wettstein et al., 1991; Michel et al., 1991) were more precise in
their outcome measure. However, the water aluminium levels were very
low and corresponded to the water aluminium levels found as the lowest
concentrations of the studies that showed a positive association.
Initial results reported by Michel et al. (1991) in the Bordeaux
cohort study showed a high, though not significant, risk (4.5) for
exposure to water aluminium levels greater than 0.10 mg/litre when
10- to 15-year historical analyses of water were used. When current
analyses of water were used, the relationship disappeared (Jacqmin et
al., 1994). However, other relationships appeared, such as the
increase in risk of cognitive impairment when the pH was below 7.3, a
decrease in risk with a pH greater than 7.3, and no elevated risk when
pH was not considered. An inverse relationship was found between
cognitive impairment and calcium concentration (Jacqmin et al., 1994).
The initial observation of elevated risk for cognitive impairment
with a set of numbers that were possibly random (Flaten, 1990) and the
changes in risk level with other water quality parameters such as
calcium and pH are difficult to interpret and require further
evaluation.
Studies with more precise outcome and exposure measures would be
expected to show the highest relative risks or odds ratios.
McLachlan et al. (1996) examined the relationship between
autopsy-confirmed AD and aluminium in drinking-water. The odds ratio
of exposure to water above 100 �g/litre was calculated for confirmed
cases of AD and a combination of AD and other neuropathology, compared
to controls. Cases and controls were obtained from brains donated to a
tissue bank supported by lay organizations. Control neuropathology
included normal brains and brains with non-AD neuropathology. The
aluminium level in water was obtained from a data bank of water
measurements for public water supplies. A next-of-kin interview was
used to refine exposure by weighing for length of residence by
assigning exposure to residence not to place of death.
The odds ratio of exposure to water with an aluminium
concentration of > 100 �g/litre for AD alone compared to controls
was 1.7 (1.2-2.5). The use of weighted exposure measures (residential
group levels of aluminium in water) increased the odds ratios for the
same comparison groups to 2.5 or more.
The use of pathologically confirmed outcome measures and of
accurate exposure measures brings added strength to the studies
examining the relationship between aluminium and AD. Notwithstanding,
this study is subject to selection bias of the sample (voluntary
donation to a tissue bank), the possibility of misclassification of
the ecological measure of exposure and a failure to account for
confounding factors.
The study of McLachlan et al. (1996) does show the highest
relative risks of any of these studies. However, this study suffers
from lack of adjustment for very important known risk factors, such as
age, sex, education and socioeconomic status.
8.1.3.3 Epidemiological studies relating aluminium concentrations in
water to cognitive dysfunction
Wettstein et al. (1991) looked for a relationship between water
aluminium levels up to 98 �g/litre and cognitive function in a male
population (800 men) of octogenarians but found none (OR = 0.92)
(CI = 0.66-1.29).
Forbes et al. (1994) examined a cohort of 2000 men followed since
1959 in a longitudinal study of ageing. The outcome measure examined
was "any evidence of mental impairment" as measured by skills of daily
living assessment. In the survivors of the cohort for whom information
was available (290 individuals), exposure was linked to the level of
aluminium in their drinking-water obtained from the Ontario Canada
Drinking Water Surveillance database. In an analysis that considered
fluoride and aluminium in drinking-water, the odds ratio after
exposure to water with high aluminium and low fluoride levels was 3.98
[CI = 1.72-9.19]. In a multi-variate analysis, which adjusted for a
variety of confounding factors, the adjusted odds ratio for high
aluminium level (> 85 �g/litre) was 1.72 (1.08-2.75).
8.1.3.4 Other neurological conditions in the general population
Other severe neurological diseases, such as amyotrophic lateral
sclerosis, Parkinsonism and the dementia complex of Guam, have been
related to aluminium accumulation in the brain (Gajdusek & Salazar,
1982; Perl et al., 1982; Garruto et al., 1984). However, the role of
aluminium in these conditions is still under considerable scientific
debate.
8.1.3.5 Conclusions regarding neurological effects of aluminium
The positive relationship between aluminium in drinking-water and
AD, which has been demonstrated in several epidemiological studies,
cannot be totally dismissed. However, strong reservations about
inferring a causal relationship are warranted in view of the failure
of studies to account for demonstrated confounding factors and for
aluminium intake from all sources.
Taken together, the relative risks for AD from exposure to
aluminium in drinking-water at levels above 100 �g/litre as determined
in these studies are low. But, because the risk estimates are
imprecise for a variety of methodological reasons, a population-
attributable risk cannot be calculated with precision. Such
predictions may, however, be useful in making decisions about the need
to control the exposure to aluminium in the general population.
In light of the above studies, which consider water-borne
aluminium as the sole risk factor, and the recent findings that water
accounts for less than 5% of daily uptake of aluminium, it is
difficult to reconcile a presumable impact on cognition. Several lines
of investigation should be pursued to elucidate further the nature of
the relationship found in these studies (see Chapter 12).
8.1.4 Allergic effects
Although human exposure to aluminium is widespread,
hypersensitivity has been reported following exposure to some
aluminium compounds in only a few cases, either after dermal
application or parenteral administration.
A case of contact sensitivity to aluminium was reported in
Sweden. The patient had regularly been using an aluminium chloride
roll-on antiperspirant and developed an itchy dermatitis in the
axillae. Patch-tests with aluminium chloride were positive (Fischer &
Rystedt, 1982). Contact allergy to aluminium also occurred in a
patient hyposensitized with aluminium-precipitated grass pollen
(Clemmensen & Knudsen, 1980). Two cases of contact allergy to
aluminium after use of topical medications containing aluminium
acetotartrate have been reported (Meding et al., 1984).
Childhood immunization with an aluminium-bound vaccine can lead
to delayed hypersensitivity to aluminium. Children who had had
previous injections with these vaccines showed positive patch-tests to
aluminium chloride (B�hler-Sommeregger & Lindemayr, 1986; Veien et
al., 1986).
In Denmark a follow-up study was made of 202 children (age
6-15 years) who had received hyposensitization therapy with various
aluminium-containing extracts (subcutaneous application) for
an average of 3 years. One to three years after cessation of
hyposensitization, 4% (13 children) still had severely pruriginous
treatment-resistant subcutaneous nodules in their forearm (application
site). Six of these 13 children were patch-tested and four reacted
positively on aluminium chloride administration (Frost et al., 1985).
8.2 Occupational exposure
This section deals with the effects observed in occupations where
workers are exposed to aluminium metal and aluminium compounds. Where
exposures are to mixed dusts and/or chemical mixtures, one cannot
infer causality between aluminium exposure and effects from studies on
such workers.
8.2.1 Respiratory tract effects
Respiratory disorders among workers in the aluminium industry
have been reviewed in detail (Dinman, 1988b; Abramson et al., 1989).
8.2.1.1 Restrictive pulmonary disease
Historically, pulmonary fibrosis has been associated with various
jobs within the aluminium industry. Shaver's disease (described in the
1940s) was a form of silicosis associated with the production of
corundum abrasives (Shaver & Riddell, 1947). Another historically
important occupational exposure associated with pulmonary fibrosis was
experienced by "pyro powder" workers, who were exposed to very fine
stamped aluminium powder (generally < 1 �m), including that used in
the manufacture of explosives and fireworks (Doese, 1938; Meyer &
Kasper, 1942; Mitchell et al., 1961; Jordan, 1961; McLaughlin et al.,
1962; Gross et al., 1970). In that process, oils and solvents were
used to coat particles to prevent naturally occurring oxidation, and
nearly all cases of fibrosis were reported in workers exposed to
mineral-oil-coated particles. That process is no longer used (Dinman,
1988a) and only one case has been reported since 1960 (McLaughlin et
al., 1962). This syndrome indicates the potential pulmonary effect of
non-oxidized aluminium metal, but such exposures do not occur in
nature.
In a report of nine cases of workers exposed to aluminium oxide
(mean duration of exposure 25 years), abnormal chest roentgenograms
were described, as well as pathological lung functions in three of the
cases (Jederlinic et al., 1990). Biopsies were taken from these three
patients and analysed by electron microscopy and microprobe analysis.
Interstitial fibrosis was the main histological finding. Metals
occurred in amounts several orders of magnitude above background
levels and the majority was aluminium oxide. The authors stated that
aluminium oxide was the most likely cause for the development of
interstitial fibrosis in these workers and that asbestos could be
ruled out. Exposure to a "mixed dust", including free silica, also
seemed to be a possible explanation.
With the exception of this exposure, pathological findings
associated with aluminium exposure listed in Table 23 refers to mixed
exposures, and cannot be solely attributed to aluminium. Other
exposures, such as to silica or other metals, must be considered.
8.2.1.2 Obstructive pulmonary disease
a) Asthma
A potentially persistent form of occupational asthma related to
primary aluminium smelting (pot room asthma) has been reported over
the past 35 years; reversible symptoms, airflow limitation and
increased bronchial responsiveness have been described (O'Donnell et
al., 1989). The likely causes are irritant airborne particulate and
fumes contributed by cryolite (sodium aluminium fluoride), gaseous
hydrogen fluoride and other agents that may be adsorbed onto
aluminium. A close relationship in aluminium potroom workers between
levels of exposure to fluoride, which may be one of a number of
Table 23. Clinical and pathological pulmonary findings in aluminium-exposed workersa
Exposure Clinical effects Pathological changes Confounding exposures Reference
Aluminium powder cough, DOE, abnormal pulmonary alveolar proteinosis iron, kaolinite, mica, rutile, Miller et al.
grinder for 6 years X-ray, restrictive PFTs calcium at biopsy (1984)
Polisher for cough, DOE bronchogenic cancer; diffuse stainless steel, chromium, de Vuyst et
24 years interstitial fibrosis nickel, cigarettes (45 packet- al. (1986)
years), silica (biopsy)
Catalyst fabrication cough, DOE, mild restriction non-caseating granuloma; iron, copper, zinc, nickel, de Vuyst et
no clinical evidence T-lymphocyte alveolitis chromium, manganese, cobalt, al. (1987)
of sarcoid molybdenum, vanadium,
palladium, silica, nobellium
on biopsy; cigarettes
Welding fumes mild ventilatory restriction diffuse and focal fibrosis; iron, cigarettes Vallyathan
pigmented content of macrophages et al. (1982)
Welding fumes, X-ray interstitial pattern; interstitial granuloma, smoker, no TB Chen et
intermittent welder dyspnoea macrophages, foreign body giant al. (1978)
(1965-1970) cells, crystals, EDA indicated
aluminium crystals
Welder for 16 years dyspnoea, X-ray bilateral, lung biopsy, diffuse chronic ex-smoker Herbert et
hazy basal infiltrates, interstitial pneumonia, al. (1982)
reduced TLC (3.5/6.8) predominantly desquamative
DOE = dyspnoea on exertion; TLC = total lung capacity (measured/predicted (6.8 litres)); EDA = energy dispersive analysis;
PFT = pulmonary function test; TB = tuberculosis
general inhalant irritants, and the work-related asthmatic symptoms
has been shown (Kongerud et al., 1990; Kongerud, 1991). A positive
association between plasma levels of fluoride and increased bronchial
responsiveness has also been reported (S�yseth et al., 1994).
A similar occupational asthma ascribed to irritant particulate
has also been described among workers following technical failure in
plants producing aluminium fluoride and aluminium sulfate (Simonsson
et al., 1985) and in solderers working with potassium aluminium
tetrafluoride flux (Hjortsbert et al., 1994).
b) Chronic bronchitis
Aluminium production and processing may lead to high levels of
workplace exposure to dusts and particulate.
In Italy the possible association of aluminium exposure and
pneumoconiosis was investigated (Saia et al., 1981). Chronic
bronchitis symptoms were found in 39% of the 119 exposed workers and
in 13% of the 119 control subjects. The X-ray findings showed one kind
of pneumoconiosis with small irregular opacities or accentuation of
broncopulmonary markings in 29% of the exposed workers and in 15% of
the controls.
A case study of 2086 employees at the Arkansas operations of a
large aluminium production company was performed (Townsend et al.,
1985). The study indicated that long-term high accumulative dust
exposure was associated with decreased levels of pulmonary function in
active workers at a bauxite refinery and aluminium-based chemical
products plant. A follow-up study of this cohort (Townsend et al.,
1985) supported the conclusion regarding respiratory effects of dust
in the workplace related to lung function.
In a cross-sectional study (Sj�gren & Ulfvarson, 1985) on 64
aluminium welders and 64 age-matched controls (non-welding industrial
workers), an increased prevalence of chronic bronchitis was observed
but there were no effects on pulmonary function. The prevalence of
chronic bronchitis among aluminium welders was similar to that of
welders working with stainless steel or iron.
8.2.2 Central nervous system effects
A number of neurological effects have been associated with
occupational exposure to aluminium, including impairment of cognitive
function, motor dysfunction and peripheral neuropathy.
Welders exposed to aluminium fumes for about 13 years had
significantly more neuropsychiatric symptoms (ascertained from
positive answers in a questionnaire) than railway track welders not
exposed to aluminium (Sj�gren et al., 1990). Despite the potential
bias associated with the questionnaire methodology used in this study,
a dose-response effect was seen.
In a further study (Sj�gren et al., 1994b) 38 aluminium-exposed
welders (median urinary aluminium level, 22 �g/litre; median exposure
time, 4.5 years) were compared with a group of 39 iron-exposed
welders. Small decrements in the speed of repetitive motor functions
were found, but there were no differences in other neurophysiological
or neuropsychological parameters.
A mixture of finely ground aluminium (85%) and aluminium oxide
(15%) powder was used between 1944 and 1979 as a prophylactic against
silicosis. Underground gold and uranium miners were exposed to an
aluminium dust concentration of 20 000-34 000 particles/ml air
(approximately 30 mg/m3) in their changing room before each shift for
10 min (Rifat et al., 1990). Exposure to aluminium powder in the
cohort ranged from 6 months to 36 years. A yearly deposition in each
miner of about 375 mg of aluminium powder has been calculated.
From the 29 000 underground miners examined in provincial chest
clinics between 1955 and 1979, a sampling frame was constructed
containing a cohort of 6604. Two samples were drawn from this cohort.
One sample consisted of 369 exposed and 369 unexposed matched miners
adjusted for age and year of their first mining experience in Ontario,
Canada, and total mining time. The second sample consisted of 678
randomly drawn miners in equal numbers from the exposed and unexposed
populations. Between 1988 and 1989, miners who could be traced were
interviewed and psychometric testing was performed. Cognitive test
scores and proportions impaired in at least one test indicated a
disadvantage for exposed miners. A positive exposure-related trend in
increased risk was described.
A group of 87 workers (average age 40.7 years) from an aluminium
foundry exposed to workplace aluminium concentrations ranging between
4.6 and 11.5 mg/m3 air, with an exposure time of at least 6 years,
was studied by Hosovski et al. (1990). Sixty non-exposed workers
matched for age, job, seniority and social status served as control.
Psychomotor and psychometric tests were performed, except on workers
who consumed alcohol or who had taken psychotropic drugs within a
month prior to the test. A significant difference in complex reaction
time, oculomotor coordination and the sum of manipulative tests was
noted in exposed workers compared to controls. In the Weschler Adult
Intelligence Scale (WAIS), the most significant differences were found
in the memory subtest.
In contrast, Bast-Petersen et al. (1994) did not find any
impairments in small groups of foundry workers (8) or potroom workers
(14) in a broad battery of psychometric tests. A cluster of aluminium
potroom workers exposed to unhooded pots for 4 or more years displayed
an increased incidence of impairment of cognitive function and/or
defects in motor control. However, insufficient biochemical
investigations were undertaken to determine whether aluminium or other
potential neurotoxins were the causative agent (Longstreth et al.,
1985; White et al., 1992).
In a case where a man was exposed to ultrafine aluminium powder
for 13.5 years in the ballmill area of an aluminium factory, the
individual died following a rapidly progressive encephalopathy, and
his brain was found to contain elevated aluminium levels (McLaughlin
et al., 1962).
8.3 Cancer
There is insufficient information to allow for the classification
of the cancer risk from human exposures to aluminium and its
compounds.
8.4 Genotoxicity
In an abstract, Haugen et al. (1983) reported no increase in the
number of sister chromatid exchanges in peripheral blood lymphocytes
of workers employed in an aluminium factory. There have been no
reports concerning genetic effects of aluminium in humans following
oral exposure to aluminium.
8.5 Reproductive toxicity
There is no information regarding reproductive toxicity in humans
following exposure to aluminium.
8.6 Subpopulations at special risk
Aluminium intoxication developed over weeks or months in patients
with chronic renal failure when dialysis fluids or parenteral
solutions contained aluminium (Alfrey et al., 1972; Klein, 1991), or
when the main source was aluminium-containing oral phosphate binders.
In patients suffering from renal failure, increases in serum and
tissue aluminium concentration were observed. The increased aluminium
content in brains of patients with renal failure seems to be the major
etiological factor in the development of the neurological syndrome
termed either dialysis encephalopathy or dialysis dementia. The
development of a specific form of osteomalacia and of microcytic,
hypochromic anaemia is also attributed to aluminium (Ward, 1991).
Aluminium intoxication is caused by using haemodialysis fluids
made from tap water without removal of the aluminium (Elliot et al.,
1978). After the introduction of water treatment with a combination of
filtration, softening, carbon absorption, reverse osmosis and
de-ionization, these clinical syndromes were prevented. Nephrologists
limit the exposure to aluminium from dialysis fluids and drugs. This
follows the introduction of guidelines in the USA, Canada, Japan and
the EEC. As a consequence, in most dialysis centres the dialysis
fluids are monitored and the aluminium level is kept below
0.4 �mol/litre (10 �g/litre). Aluminium-free phosphate-binding agents
such as calcium carbonate are preferably used for oral medication. The
same clinical syndromes have been described in patients with renal
impairments, including premature infants who have not been dialyzed,
and are a consequence of aluminium accumulation from aluminium-
containing pharmaceutical products and parenteral solutions (Finberg
et al., 1986).
8.6.1 Encephalopathy
Dialysis encephalopathy is a complication of prolonged
haemodialysis first described in 1972 (Alfrey et al., 1972). The main
symptoms are speech disorder followed by the development of dementia,
convulsions and myoclonus. The mean duration of dialysis was 48 months
and the dialysis fluids were made with untreated tap water. Elevated
aluminium contents were found in the brain, muscle and bone tissues
of the affected patients. The same findings were reported from
other dialysis centres in Europe and the USA. Many outbreaks of
encephalopathy have been described in association with the use of
dialysis fluids containing a high concentration of aluminium, usually
above 200 �g/litre (Flendrig et al., 1976; McDermott et al., 1978;
Alfrey, 1978).
In a study with 55 patients suffering from dialysis
encephalopathy in six dialysis centres using a uniform clinical
classification, the incidence of dialysis encephalopathy rose
significantly with increasing cumulative exposure to aluminium via the
dialysate (Schreeder et al., 1983).
Epidemiological studies of dialysis centres in England showed
that encephalopathy was almost non-existent in those centres using
water with aluminium concentrations less than 50 �g/litre to prepare
dialysis fluids. The incidence of encephalopathy rose progressively
with higher water concentrations of aluminium. The Registration
Committee of the European Dialysis and Transplant Association made a
European survey, which showed clusters of encephalopathy in certain
areas of Britain, Spain, Greece and Scandinavia. In Britain, 92% of
the patients in these areas had been treated with dialysis fluids made
from softened tap water (Kerr & Ward, 1988). No signs of overt
aluminium toxicity were observed in 27 long-term haemodialysis
patients on dialysis fluids containing low aluminium concentrations
(Altmann et al., 1989) and these subjects had only mildly elevated
serum aluminium levels. However, defects in several tests of
psychomotor function, including digit coding, were found.
8.6.2 Osteomalacia
Osteomalacia has also been observed in patients with chronic
renal failure, exposed to aluminium in dialysis fluids, or in infants
with renal failure treated with aluminium hydroxide to control
hyperphosphataemia (Ward et al., 1978; Andreoli et al., 1984). Bone
pain, myopathy, pathological fractures and poor response to vitamin D
therapy are the characteristic symptoms of osteomalacia, accompanied
by radiological changes, including partial and complete non-healing
fractures, osteopenia, and reduction in calcified bone area (Simpson
et al., 1973). When aluminium was removed from fluids used for
dialysis, the incidence of osteomalacia diminished. The level without
undue risk was estimated to be 30 �g/litre or less (Platts et al.,
1984). The aluminium content of the bone is increased in patients with
renal disease, treated by haemodialysis, and this aluminium may remain
in the bone even after successful renal transplantation (Ellis et al.,
1979). The aluminium in patients with osteomalacia was found to be
mainly localized at the interface between the osteoid and the
calcified matrix (Cournot-Witmer et al., 1981). Vitamin-D-resistant
osteomalacia due to aluminium is a progressive metabolic bone disease.
The mechanism for the disordered bone formation remains to be
clarified.
8.6.3 Microcytic anaemia
In a study of ten aluminium-intoxicated dialysis patients,
microcytic anaemia was observed. The disease was reversible after
deionization of the dialysis water (Touam et al., 1983). The mechanism
by which an excess of aluminium induces microcytic anaemia remains to
be clarified (Wills & Savory, 1983, 1989).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Den Dooren de Jong (1971) inoculated a medium containing
aluminium chloride (10-4 mol/litre) with three Azobacter strains.
After incubation for 1 to 2 days, the aluminium had produced an
inhibition zone of 14 mm with increased pigmentation at the edges. The
inhibition zone, when compared with that produced by other metals, was
found to indicate intermediate toxicity.
9.1.1.1 Water
Sheets (1957) studied the effect of shock loadings of aluminium
sulfate on the biochemical oxygen demand of sewage sludge and found
that a concentration of 18 mg/litre caused a 50% reduction.
Panasenkov (1987) found that aluminium sulfate at concentrations
of 0.1 and 1 mg/litre had no effect on the heterotrophic fixation of
14CO2 or the number of saprophytes of a natural bacterial coenosis.
However, at 10 mg/litre aluminium sulfate reduced these two parameters
by 50% and 60%, respectively.
Dobbs et al. (1989) studied the effect of complexation on the
toxicity of aluminium using the Microtox system based on measurement
of the light output of a luminescent bacterium. Aluminium citrate
complexes were essentially non-toxic up to levels of at least
25 mg Al/litre. The one-hour EC50, defined as a 50% reduction in
light output, was 300 �g/litre in the absence of citrate. Complexation
by fluoride was complicated by a toxic response to fluoride itself.
Correction for this effect still revealed an appreciable toxicity of
aluminium fluoride complexes. The toxic response in the presence of 9
and 36 mg/litre fulvic acid was reduced by 24% and 61%, respectively.
Hoke et al. (1992) reported 15-min EC50 values (Microtox tests) for
aluminium chloride (as aluminium) of 5.57 and 3.31 mg/litre for
solutions osmotically adjusted with 22% sodium chloride and 20.4%
sucrose, respectively.
9.1.1.2 Soil
Zwarun et al. (1971) found the soil bacterium Bacillus sp. to
be resistant to aluminium. Increasing acidity from pH 6.6 to pH 4.5
reduced the number of surviving cells suspended for 3 h in an acetate
buffer. Addition of 80 mg Al/litre produced no further reduction, even
though the cell walls were saturated with aluminium. Zwarun & Thomas
(1973) exposed the soil bacterium Pseudomonas stutzeri to acidic
conditions at aluminium concentrations of 1, 10 and 80 mg/litre for
3 h. Increasing the acidity reduced the number of surviving cells. The
addition of aluminium (10 mg/litre) at pH 4.5 significantly reduced
the survival rate of cells, and 80 mg/litre was lethal.
Thompson & Medve (1984) studied the effects of aluminium
(0-500 �g/ml) on the growth of the ectomycorrhizal fungi Cenococcum
graniforme, Suillus luteus, Thelephora terrestris, and three
isolates of Pisolithus tinctorius (all four species are commonly
found on spoil tips). T. terrestris was the most sensitive species
showing no growth at aluminium concentrations > 150 �g/ml, whereas
S. luteus was the most tolerant, being unaffected at aluminium
concentrations < 350 �g/ml. The other species showed reduced growth
at all aluminium concentrations. However, the results obtained were
not consistent with field observations.
The severity of the incidence of the fungal disease potato scab
was reduced by the addition of aluminium (Mizuno & Yoshida, 1993).
9.1.2 Aquatic organisms
9.1.2.1 Plants
Algal assays are often carried out in culture media containing
high concentrations of nutrients, including phosphate. These nutrients
ameliorate the toxicity of aluminium, and limit the application of the
results to natural waters. For example, in culture medium the green
alga Chlorella pyrenoidosa successfully grew at concentrations of
up to 12 mg total aluminium/litre at pH 4.6, but an aluminium
concentration of 24 mg/litre was toxic. By selecting those algae which
tolerated high levels of aluminium, the authors were able to develop
algal cultures that grew at up to 48 mg/litre (Foy & Gerloff, 1972).
However, Helliwell et al. (1983) found that maximum toxicity of
aluminium to algal growth was achieved at pH 5.8 to 6.2, 5 �g labile
Al/litre significantly inhibiting the growth of the alga Chlorella
pyrenoidosa in a synthetic hard water. The assay showed that
toxicity is a function of the labile, rather than the total,
aluminium. It was found that Al (OH)2+ was the aluminium species
most toxic to the alga. Similar results were reported by Parent &
Campbell (1994), although they found that polymerized aluminium
species were also toxic.
Bringmann & K�hn (1959) found a toxic threshold of 1.5 to
2 mg/litre aluminium chloride for the green alga Scenedesmus
quadricauda. Rueter et al. (1987) found that a concentration of
60 �mol aluminium/litre at pH 5.7 is required to produce any
inhibitory effect on the growth rate of the S. quadricauda. In an
experiment studying the effect of both copper and aluminium on the
growth of Scenedesmus it was found that the majority of the toxic
action was due to indirect chemical interactions that result in higher
cupric ion activities.
H�rnstr�m et al. (1984) studied the effects of aluminium on 19
freshwater algal species in lake water at pH 5.5. The biotests showed
that 13 of the species, including all of the desmoids and diatoms,
experienced complete growth inhibition at 200 �g/litre. In fact, two
of the four diatom species ( Nitschia actinastroides and Synedra
nana) showed 47% inhibition at 100 �g/litre. The most insensitive
group was the Chrysophyceae, where three out of five species were
unaffected at 400 �g/litre.
Lindemann et al. (1990) studied the impact of aluminium
(4-220 �mol/litre) on green algae ( Scenedesmus sp. and Chlorella
sp.) isolated from acidic and alkaline headwater streams in a semi-
continuous culture media at pH 5. The threshold for inhibition was
found to be less than 4 �mol aluminium/litre when the aluminium was
added to the medium abruptly. When the aluminium was added gradually
there was a lag period, which can be explained by the formation of
polymeric aluminium hydroxy compounds and the precipitation of
aluminium hydroxides and phosphates; this reduces the amount of
aluminium bioavailable to the algae. Folsom et al. (1986) studied
the toxicity of aluminium (0, 10, 25 and 50 mg/litre) to the acid-
tolerant green alga Chlorella saccarophila at pH 3.0 and found a
concentration-dependent growth inhibition. Addition of the fast-
exchange ions Ca2+, Mg2+, Na+ and K+ (150 mg/litre) caused some
reversal of the toxic effects of aluminium.
Exley et al. (1993) studied the effect of aluminium on the
freshwater diatom Navicula pelliculosa and the amelioration of this
toxicity with silicon. An aluminium concentration of 10 �mol/litre
significantly inhibited diatom growth at all but the highest silicic
acid concentration (100 �mol/litre). The mechanism of action was found
to be independent of a direct effect on cell biomass, chlorophyll
a content per cell or the protein content of each cell. The toxicity
of aluminium was mitigated by increasing the nominal phosphorus
concentration from 1 to 10 �mol/litre. Further growth experiments
were carried out on the non-silicon-requiring green alga Chlorella
vulgaris. The rate of growth was significantly inhibited at
48 �mol/litre. The inhibitory effects were again removed at the
highest silicic acid concentration. The authors concluded that the
mechanism of aluminium toxicity was a reduction in the bioavailability
of phosphorus.
Genter & Amyot (1994) exposed freshwater benthic algal
populations to aluminium concentrations of 50, 100 and 500 �g Al/litre
(as aluminium sulfate) at pH 4.8 in artificial streams. During the
28-day test, aluminium in acidified water inhibited the abundance of
diatoms and cyanobacteria (blue-green algae) more than the acidity
alone. Aluminium decreased chlorophyll abundance beyond the effects of
acidity alone at 500 �g/litre.
Nalewajko & Paul (1985) studied the effect of aluminium on
phytoplankton collected from a circumneutral and an acid-stressed
lake. Addition of aluminium (50 �g/litre) caused significant decreases
in microbial phosphate uptake and photosynthesis. The effects were
more pronounced at pH 5.2-6.9 (lake water pH range) than at pH 4.5,
there being larger decreases in phytoplankton from the acid-stressed
lake. The authors reported that the toxicity was due to both the
precipitation of phosphate as particles after addition of aluminium
and the direct effect of aluminium.
Stanley (1974) grew the aquatic angiosperm Eurasian milfoil
( Myriophyllum spicatum) in solutions of aluminium for 32 days. There
was a 50% inhibition of root dry weight, shoot dry weight, root length
and shoot length at aluminium concentrations of 2.5, 7.6, 5.1 and
12.7 mg/litre, respectively.
9.1.2.2 Invertebrates
a) Acute toxicity
The acute toxicity of aluminium to aquatic invertebrates is
summarized in Table 24; 48-h and 96-h LC50 values range from
0.48 mg/litre (polychaete) to 59.6 mg/litre (daphnid). However, care
must be taken when interpreting the results because of the significant
effects of pH on the availability of aluminium.
Bringmann & K�hn (1959) found no effect on Daphnia magna
immobilization at aluminium chloride concentrations of up to
1000 mg/litre over a 48 h exposure period at pH 7.5.
Havens (1990) exposed two acid-sensitive cladocerans ( Daphnia
galeata and D. retrocurva) and one acid-tolerant cladoceran
( Bosmina longirostris) to aluminium concentrations of 200 �g/litre
at pH 5.0 for 24 h. The exposure consistently resulted in nearly 100%
mortality for D. galeata and D. retrocurva, but mortality rates
for B. longirostris were not significantly different from those of
controls (2-6%). Haematoxylin staining procedures revealed that the
daphnids showed marked aluminium binding at the maxillary glands (the
site of ion exchange), whereas B. longirostris showed no noticeable
aluminium binding.
Havas & Hutchinson (1982) studied the tolerance of crustaceans,
collected from acid (pH 2.8) and alkaline (pH 8.2) tundra ponds, to
low pH and elevated levels of aluminium. When adjusted to pH 4.5,
water from acid ponds was more toxic than water from alkaline ponds,
probably due to elevated concentrations of aluminium (up to 20 mg
Al/litre). Removal of heavy metals and aluminium by co-precipitation
significantly reduced the toxicity of adjusted pond
Table 24. Toxicity of aluminium (LC50) to aquatic invertebrates
Organism Size/age Stat/flowa Temperature Hardnessb pH Salt Duration LC50c Reference
(�C) (mg/litre) (h) (mg/litre)
Bivalve stat 20-25 3.5 96 > 1.0 m Mackie
Pisidium stat 20-25 4.5 96 > 0.4 m (1989)
casertanum
Bivalve stat 20-25 3.5 96 > 1.0 m Mackie
Pisidium stat 20-25 4.5 96 > 0.4 m (1989)
compressum
Gastropod stat 20-25 3.5 96 > 1.0 m Mackie
Amnicola stat 20-25 4.5 96 > 0.4 m (1989)
limosa
Hyallela stat 20-25 3.5 96 > 1.0 m Mackie
azteca stat 20-25 4.5 96 > 0.4 m (1989)
Enallagma sp. stat 20-25 3.5 96 > 1.0 m Mackie
stat 20-25 4.5 96 > 0.4 m (1989)
Polychaete stat 20-25 7.6-8.0 chloride 96 > 2.0 n Petrich &
Neanthes Reish
arenaceodentata (1979)
Polychaete stat 7.6-8.0 chloride 96 2.0 n Petrich &
Polychaete stat 7.6-8.0 chloride 96 0.48 n Reish (1979)
Ctenodrilus
serratus
Copepod adult stat 20 7d 8.0 chloride 96 10 Bengtsson
Nitocra (7.5-13.4) (1978)
spinipes
Table 24. (Con't)
Organism Size/age Stat/flowa Temperature Hardnessb pH Salt Duration LC50c Reference
(�C) (mg/litre) (h) (mg/litre)
Water flea < 24 h stat 17-19 44-53 7.4-8.2 chloride 48 3.9 n Biesinger &
Daphnia Christensen
magna (1972)
stat 12-15 240 7.2-7.8 ammonium 48 59.6 Khangarot
sulfate (45.8-73.3) ne & Ray
(1989)
a Stat = static conditions (water unchanged for duration of test)
b Hardness expressed as mg CaCO3/litre
c n = based on nominal concentrations; m = based on measured concentrations
d Salinity (%)
e EC50 based on immobilization
water to crustaceans. The subsequent addition of 20 mg/litre aluminium
resulted in 100% mortality of Daphnia within 20 h, whereas the
addition of other metals (iron, nickel and zinc) did not restore the
toxicity.
Havas (1985) studied the effect of aluminium chloride (0.02, 0.32
and 1.02 mg Al/litre), pH (6.5, 5.0 and 4.5) and calcium (2.5 and
12.5 mg/litre) on the survival of Daphnia magna during a 48-h
exposure period. Maximum aluminium toxicity was observed at pH 6.5 and
a calcium concentration of 2.5 mg/litre. A pH of 5.0 was toxic to
D. magna in soft water, 50% of the daphnids being immobilized within
24 h. Aluminium marginally increased the toxicity of water at pH 5.0.
At pH 4.5, high concentrations of aluminium significantly reduced the
hydrogen ion toxicity. However, this amelioration was short lived and
all of the Daphnia had died within 24 h. Havas & Likens (1985)
exposed the crustaceans Daphnia catawba and Holopedium gibberum,
and the insect larvae Chaoborus punctipennis and Chironomus
anthrocinus to the same aluminium concentrations (0.02, 0.32 and
1.02 mg Al/litre) at pH levels of 3.5, 4.0, 4.5, 5.0 and 6.5. The
crustaceans were exposed for 72 h and the insect larvae for 168 h.
D. catawba was the most acid-sensitive species, mortality being
significantly increased at and below pH 5.0; high concentrations
of aluminium significantly increased mortality only at pH 6.5.
H. gibberum was less sensitive to both hydrogen ions and aluminium
than D. catawba. The highest aluminium concentration was moderately
toxic at pH 6.5; however, as with D. catawba, the effect of
aluminium at lower pH was completely masked by hydrogen ion toxicity.
Neither aluminium nor hydrogen ions affected the mortality of
C. punctipennis or C. anthrocinus.
Lamb & Bailey (1981) studied the effects of aluminium sulfate on
larvae of the midge Tanytarsus dissimilis at pH 7.8. There was no
apparent effect of aluminium on either second or third instar larvae
at aluminium sulfate doses of between 80 and 960 mg/litre after 96 h.
Owing to the polymeric, coagulant nature of aluminium sulfate, a white
grey precipitate (up to 3-4 mm) formed in all solutions.
Six common macro-invertebrates were exposed to 200 �g/litre
aluminium sulfate at pH 4.5 and a calcium concentration of
2.45 mg/litre. The order of acid sensitivity (mean 48-h survival is
given in parentheses) for the species tested was: Caenis sp. (2%)
> Hyalella azteca (12%) > Enallagma sp. (20%) > Gyraulus sp.
(55%) > Chironomidae (94%) > Hydracarina (99%). Aluminium
significantly reduced survival still further in H. azteca, Gyraulus
sp. and Chironomidae. However, the addition of aluminium significantly
increased survival for Enallagma sp. and Caenis sp. when compared
with the acid-only group (Havens, 1993).
b) Long-term toxicity
France & Stokes (1987) studied the effect of nominal aluminium
concentrations of 0.05 to 0.70 mg/litre on the hydrogen ion toxicity
to the amphipod Hyalella azteca over an 8-day period. Aluminium
concentrations of 0.25 and 0.40 mg/litre at pH 4.8 and 0.40 mg/litre
at pH 4.3 significantly increased the mortality of H. azteca
compared with that in reference aluminium concentrations of
0.05 mg/litre. Mortality rates remained unchanged with the addition of
0.25 mg Al/litre at pH 4.3 or 5.3 and with either 0.40 mg Al/litre or
0.70 mg Al/litre at pH 4.0. The authors predicted from these results
that mortality of this amphipod from springmelt pulses will be
determined primarily by hydrogen ions and only secondarily by
aluminium in the pH range 4.3 to 5.3. Berrill et al. (1985) found no
effect of aluminium (up to 200 �Eq/litre) on the accumulated 10-day
mortality caused by hydrogen ions in the crayfish Orconectes
rusticus, O. propinquus and Cambarus robustus.
Biesinger & Christensen (1972) exposed water fleas ( Daphnia
magna) to aluminium chloride for a period of three weeks in Lake
Superior water (pH 7.4-8.2). An LC50 of 1.4 mg total aluminium/litre
was calculated and the EC50, based on reproductive impairment, was
found to be 0.68 mg/litre.
Burton & Allan (1986) exposed three species of stream
invertebrates ( Nemoura, Asellus and Physella) to aluminium
concentrations of 250 or 500 �g/litre at pH 4, 5 and 7 for 28 days in
experimental streams. Survival of all species was significantly
decreased at pH 4; the addition of aluminium at 15�C did not cause
additional mortality. However, at 2�C or with low organic matter the
addition of 500 �g/litre caused a significant additional mortality for
both Nemoura and Asellus. Addition of citrate reduced the effect
of aluminium in low-organic treatments.
Petrich & Reish (1979) studied the effect of aluminium chloride
(pH 7.6-8.0) on the polychaetes Neanthes arenaceodenata, Capitella
capitata and Ctenodrilus serratus. Neither Capitella nor Neanthes
were affected by a 7-day exposure to 2 mg/litre aluminium chloride
(the maximum concentration that could be used without precipitation in
seawater). Ctenodrilus showed significant reproductive suppression
during a 28-day exposure to aluminium chloride concentrations of
0.5 mg/litre or more.
c) Physiological and biochemical effects
Herrmann & Andersson (1986) exposed the nymphs of three mayfly
species Heptagenia fuscogrisea, H. sulphurea and Ephemera danica
to total inorganic monomeric aluminium levels of 500 and 2000 �g/litre
at pH 4.0 and 4.8 for 10 days. The oxygen consumption rate of nymphs
was monitored. The rate showed a tendency to increase at 500 �g/litre
for H. sulphurea and E. danica. At 2000 �g/litre there were
significant increases in the oxygen consumption rate for all three
species at both pH levels. E. danica, which is restricted to less
heavily acidified regions, was the most severely affected by the
aluminium treatments. Exposure of E. danica and H. sulphurea to
the same aluminium and pH regimes for 14 days caused significant
aluminium-related decreases in sodium levels (Herrmann, 1987).
Malley & Chang (1985) studied calcium-45 uptake by postmoult
crayfish ( Orconectes virilis) exposed to aluminium chloride
concentrations of 200, 500 and 1000 �g Al/litre for 2 to 3 h at pH 5.3
to 7.2. An aluminium concentration of 200 �g/litre had no effect on
calcium uptake at neutral pH. However, reducing the pH to 5.5 caused
an inhibition of calcium uptake. Exposure of crayfish to 500 �g/litre,
under acidic conditions, also caused a significant reduction in
calcium uptake. However, exposure to acidic conditions alone revealed
that most of the reduction was due to acidic conditions rather than
aluminium. In fact, transferring the crayfish from 500 �g Al/litre to
1000 �g Al/litre, under acidic conditions, had no significant effect.
Witters et al. (1984) maintained the air-breathing water bugs
( Corixa punctata) at aluminium chloride concentrations of 0.15, 0.3
(the natural level), 2.5, 5, 10 and 50 mg Al/litre at pH 3 and 4. A
dose-related decrease in sodium-influx was observed and there was a
significant 50% decrease when comparing the lowest concentration with
10 mg/litre.
d) Population studies
Havens (1991) studied the effect of aluminium on the survival
of littoral zooplankton species collected from alkaline lakes.
Toxicity tests were performed at pH 4.5 with or without aluminium
(500 �g/litre) for 24 h. The four cladocerans Simocephalus
serrulatus, Diaphanosoma birgii, Acantholeberis curvirostris and
Chydorus sphaericus were unaffected by either the acidic conditions
or aluminium. The cladoceran Eurycercus lamellatus and the copepod
Acanthocyclops vernalis suffered 100% mortality at pH 4.5 with or
without aluminium. The cladocerans Camptocercus rectirostris, Alona
costata and Pleuroxus denticulatus and the copepod Mesocyclops
edax showed decreased survival at pH 4.5 and a significantly greater
decrease in survival under acid conditions and aluminium exposure.
Havens & Heath (1989) carried out an in situ mesocosm study of
zooplankton responses to acidification and aluminium. Large plastic
enclosures were acidified (pH 4.5) with or without the addition of
aluminium, giving an inorganic monomeric aluminium concentration of
180 �g/litre. The populations of acid-sensitive species declined more
rapidly in the acid-plus-aluminium treatment than in the acid-alone
treatment. Two cladocerans ( Bosmina longirostris and Chydorus
sphaericus) were tolerant to acidity and aluminium. Havens & Decosta
(1987) performed bioassays using in situ enclosures to expose
zooplankton to acidified waters (pH 4.7) with and without the addition
of aluminium (300 �g/litre) for up to 49 days. Acidification did not
affect abundance of zooplankton or succession because all species were
acid-tolerant. However, addition of aluminium resulted in a reduction
in zooplankton abundance.
9.1.2.3 Fish
The bioavailability and toxicity of aluminium varies with its
chemical speciation. In the case of fish, higher polymers are less
toxic than monomers and polymers of low relative molecular mass.
Polymerization is a slow process, hence the biological activity of
aluminium in water depends not only on aluminium concentration and
conditions such as pH, temperature and the presence of complexing
ions, but can also depend on the pre-history of the water. The various
aluminium species differ in their effects on fish gills, either
disturbing the ion balance or interfering with respiration. The
toxicity diminishes if the aluminium is inactivated by complexation
with organic ligands, fluoride or silicate, or by extensive
polymerization to large molecules in the water (Rosseland & Staurnes,
1994).
a) Acute toxicity
The acute toxicity of aluminium to fish is summarized in Table
25. The 96-h LC50 values range from 0.095 mg/litre (American
flagfish) to 235 mg/litre (mosquito fish). However, care must be taken
when interpreting these results because of the significant effects of
pH on the availability of aluminium. The wide range of LC50 values
probably reflects this variable availability. LT50 values for
salmonids are also summarized in Table 25. Muramoto (1981) found that
addition of the complexans NTA and EDTA reduced the acute (48-h)
toxicity of aluminium to carp ( Cyprinus carpio).
Rosseland & Skogheim (1984) exposed three salmonid species,
Atlantic salmon ( Salmo salar), brown trout ( Salmo trutta) and
brook trout ( Salvelinus fontinalis), to inorganic monomeric
aluminium concentrations of 120, 225 and 415 �g/litre (as aluminium
sulfate) under flow-through conditions. Owing to the acidity of the
aluminium sulfate the pH decreased from 6.6 to 4.9. Pre-smolt salmon
were the most sensitive, showing 100% mortality within 48 h at
245 �g/litre. Brook trout were the least sensitive, mortalities only
Table 25. Toxicity of aluminium to fish (laboratory studies)
Organism Size/ Stat/flowa Temperature Hardnessb Calcium pH Salt LT50 (mg 96-h LC50c Reference
age (�C) (mg/litre) concentration inorganic (mg Al/litre)
(mg/litre) monomeric
(Al/litre)
Atlantic 1 + flow 5.2 5 2.0 4.95 sulfate 59 0.245 m Rosseland &
salmon 2 + 4.95 33 0.245 m Skogheim
(Salmo salar) 1 + flow 5.2 5 2.0 4.94 sulfate 57 0.313 m (1984)
2 + 4.94 sulfate 22 0.313 m
1 + 4.90 27 0.463 m
2 + 4.90 15 0.463 m
Brown trout 2 + flow 5.2 5 2.0 4.94 sulfate 40 0.313 m
(Salmo trutta) 1 + 4.90 57 0.463
2 + 4.80 30 0.463
Atlantic 2 + stat 3.7 1.3 5.06 chloride 108 0.075 Skogheim &
salmon (38 g) 1.3 4.92 38 0.137 m Rosseland
(Salmo salar) 1.3 4.90 32 0.177 m (1986)
Mummichog 2.7 g stat 20 6.6d ammonium 3.6 n Dorfman
(Fundulus sulfate (1977)
heteroclitus) 2.7 g stat 20 17d ammonium 27.5 n
sulfate
2.7 g stat 20 7.9d chloride 3.6 n Dorfman
2.7 g stat 20 18.8d chloride 31.5 n (1977)
Mosquito fish stat 20-21 4.3-7.2 chloride 133 n Wallen et al.
(Gambusia stat 19-22 4.4-7.7 sulfate 235 n (1957)
affinis)
Table 25. (Con't)
Organism Size/ Stat/flowa Temperature Hardnessb Calcium pH Salt LT50 (mg 96-h LC50c Reference
age (�C) (mg/litre) concentration inorganic (mg Al/litre)
(mg/litre) monomeric
(Al/litre)
Fathead 0.45 g stat 22 38 7.4 nitratee 4.25 Mayer &
minnow (3.3-5.5) Ellersieck
(Pimephales 0.45 g stat 22 38 7.4 sulfatef 4.4 (1986)
promelas) (3.4-5.6)
American 2-3 stat 25 6.0 5.8 0.095 m Hutchinson &
flagfish days Sprague
(Jordanella (1986)
floridae)
a Stat = static conditions (water unchanged for duration of test) d Salinity (%)
b Hardness expressed as mg CaCO3/litre e 7.2% technical material
c n = based on nominal concentrations; m = based on measured concentrations f 8.1% technical material
occurring at 463 �g/litre (less than 25% over the 64-h exposure). The
authors reported that whenever aluminium sulfate was added excessive
mucus was observed between the gill lamellae; or all species mucus
clogging increased with an increased addition of aluminium. However,
there was no excessive mucus on gills of fish that died in acid brook
water with naturally occurring aluminium concentrations.
Schofield & Trojnar (1980) exposed brook trout ( Salvelinus
fontinalis) fry to aluminium (0.1-0.5 mg/litre) at various pH levels
(4.0-5.2). At pH 4.0 survival of fish was not related to aluminium
concentration, the LT50 values ranging from 2.8 to 5.2 days. However,
at pH levels of > 4.4, mortality increased with increasing
aluminium concentration. At pH 4.9 and 5.2 neither acidity nor 0.1 mg
Al/litre affected fish mortality; 0.5 mg Al/litre produced LT50
values ranging from 1.6 to 3.3 days. Symptoms of stress were darkening
of skin coloration and cessation of feeding. All fish at pH 4.0 and
4.4 showed these symptoms, although they took longer to develop at pH
4.4 with 0 or 0.1 mg/litre aluminium. No symptoms were observed at pH
4.9 and 5.2 for aluminium concentrations of 0.1 mg/litre; however,
stress symptoms were seen in all groups exposed to > 0.25 mg/litre
aluminium at any pH level. Heavy accumulations of mucous and cellular
debris on the gills were found in trout exposed to > 0.25 mg/litre
aluminium at pH levels of > 4.4. Histopathological changes
observed in sections of gills from fish exposed to aluminium levels
> 0.5 mg/litre included cell proliferation at the distal ends of
gill filaments, lamellar oedema and fusion, epithelial desquamation,
filament collapse, and general loss of gill structure.
Gundersen et al. (1994) exposed rainbow trout ( Oncorhynchus
mykiss) to aluminium at pH values ranging from 7.97 to 8.56 in 96-h
tests. No significant mortality was observed at pH 8.33 or less and
filterable aluminium concentrations of 0.52 mg/litre or less. However,
100% mortality was found at pH 8.58 and a filterable aluminium
concentration of 1 mg/litre. The 96-h LC50 values ranged from 0.36 to
0.79 mg filterable aluminium/litre at weakly alkaline pH levels.
Young brown trout ( Salmo trutta) exposed for 5 days to pH 5 in
high calcium water at temperatures of 4 and 12�C showed no alterations
in growth or in mucous cell concentration and volume. However,
exposure to aluminium (230 �g/litre) under the same testing regime
resulted in significant growth depression but no changes to mucous
cell morphometrics (Segner et al., 1988).
Freeman & Everhart (1971) found that the toxicity of aluminium
hydroxide complexes (5.2 mg/litre) to rainbow trout ( Oncorhynchus
mykiss) increased with the amount of aluminium dissolved. At pH 6.8,
8.0, 8.5 and 9.0 the amounts of aluminium dissolved were 1%, 10%, 31%
and 97%, respectively, and the respective LT50 values were 38.90,
31.96, 7.46 and 2.98 days. Surviving fish recovered rapidly in all
groups, except those exposed at pH 8.0, with normal growth being
resumed within 2 weeks (Freeman, 1973).
b) Long-term toxicity
Hickie et al. (1993) exposed rainbow trout ( Oncorhynchus
mykiss) to aluminium for 23-26 days after hatching at pH 5.8 and
4.9. The 144-h LC50 for total aluminium was found to be > 1050
and 91 �g/litre at the two pH levels, respectively. An LC50 of
1.17 �g/litre was calculated for fish exposed from 16 to 19 days after
hatching at pH 4.9.
Neville & Campbell (1988) exposed juvenile rainbow trout
( Oncorhynchus mykiss) to aluminium (2.8 �mol/litre nominal
concentration) in a flow-through system over a pH range of 4.0 to 6.5
for up to 11 days. The response of trout to aluminium was most severe
at pH 4.5 (electrolyte loss) and 6.1 (asphyxia). At pH 4.0 there was
competition between hydrogen ions and aluminium for binding at the
gill surface which reduced toxicity. However, the toxic response at pH
6.1 appeared to be more complex being either a bimodal response to two
different aluminium species or a physical response to precipitation on
the gill surface.
Driscoll et al. (1980) studied the toxic effect of aluminium on
brook trout ( Salvelinus fontinalis) fry. At pH values of 4.4 and 5.2
there was no effect on survival during the 14-day exposure period. The
addition of inorganic monomeric aluminium (0.42-0.48 mg/litre)
produced an LT50 of 115 h at pH 5.2 and 256 h at pH 4.4. Treatment
with excess fluoride or citrate reduced the toxicity of aluminium.
Skogheim & Rosseland (1986) exposed Atlantic salmon ( Salmo salar) to
aluminium at varying pH levels. No mortality occurred during a 20-day
exposure to pH 5.07 alone. At pH 5.06 and 75 �g Al/litre the LT50 was
108 h, at pH 4.92 and 137 �g Al/litre the LT50 was 38 h, and at pH
4.9 and 177 �g Al/litre the LT50 was 32 h. Brown (1983) exposed brown
trout ( Salmo trutta) to aluminium (0, 0.25 and 0.5 mg/litre) at pH
values ranging from 4.5 to 5.4 and calcium concentrations ranging from
0.5 to 2.0 mg/litre for 16 days. Survival was relatively unaffected by
pH except at a calcium level of 0.25 mg/litre and a pH of 4.5. High
mortality was observed at both aluminium exposure levels at calcium
levels of 0.25 and 0.5 mg/litre. At calcium levels of 1.0 and
2.0 mg/litre there was increased mortality at the highest aluminium
concentration.
Gundersen et al. (1994) studied the effects of aluminium on
rainbow trout ( Oncorhynchus mykiss) in 16-day tests. Growth rates
were higher at weakly alkaline pH (7.97-8.10) than at near-neutral pH
(7.30-7.35). The authors concluded that polymeric and colloidal forms
of aluminium are more potent than soluble forms in restricting growth.
Trout exposed to aluminium at 0.53 to 2.56 mg/litre and humic acid at
4.31 to 5.23 mg/litre had higher specific growth rates and lower
mortality than those exposed to aluminium and no humic acid at all the
pH values tested. When exposed to sub-lethal concentrations of
aluminium (38 �g/litre nominal concentration) in a synthetic soft
water of pH 5.2, rainbow trout became acclimated to aluminium and
showed increased resistance when exposed to lethal levels of aluminium
(162 �g/litre nominal concentration) in the same soft water.
Acclimation was associated with reduced disturbances of ionoregulation
and respiration (Wilson et al., 1994a). Acclimation to 38 �g/litre
(nominal concentration) also caused a 4-fold increase in gill mucous
density and a reduction in apparent lamellar surface area (Wilson et
al., 1994b). Acclimation to sub-lethal levels of aluminium could
explain the continued presence of fish populations in acidified lakes
and rivers containing more than 100 �g Al/litre. Wicklund Glynn et al.
(1992) exposed minnows ( Phoxinus phoxinus) to acidic water (pH 5.0)
with and without aluminium (150 �g/litre) at various calcium (0, 0.07
and 2 mmol/litre) and humus (5 and 25 Pt) concentrations for 15 days.
Mortality among fish exposed to aluminium was higher than among
unexposed fish but was less at the highest calcium level. At 0.07 mmol
calcium/litre, the aluminium-induced mortality was reduced by the
presence of humus. Gill morphology was altered after exposure to
aluminium at pH 5.0, but was not affected by different concentrations
of calcium or humus.
Juvenile brook trout ( Salvelinus fontinalis) were
intermittently or continuously exposed to aluminium (0.2 to
1.2 mg/litre) at pH 4.4 or 4.9 for 24 days. There was 100% survival of
fish at both pH levels in the absence of aluminium, regardless of
exposure regime. Aluminium significantly reduced survival at
0.2 mg/litre or more for all exposure regimes except the intermittent
exposure at pH 4.4 where significant mortality was observed at
0.4 mg/litre or more. When aluminium concentration was expressed as
the 24-day mean, it was shown that intermittent exposure was more
toxic than continuous exposure (Siddens et al., 1986). Ingersoll et
al. (1990) exposed 1-year-old brook trout ( Salvelinus fontinalis) to
combinations of aluminium, pH and calcium during a 28-day experiment.
Survival was reduced at inorganic monomeric aluminium concentrations
of 29 �g/litre at pH 5.2 and > 228 �g/litre at pH 4.4 or 4.8. Fish
weight was reduced at an aluminium concentration of > 34 �g/litre
and pH < 4.8. The gills sampled from low pH groups showed lifting of
the outer epithelium and hypertrophy of chloride and epithelial cells.
These effects were more pronounced at low pH with elevated aluminium
concentrations. Effects such as vacuolation and degeneration of
epithelial and chloride cells and the presence of dense cells were
also observed at low pH and elevated aluminium concentration.
No mortality of lake trout ( Salvelinus namaycush) embryos
occurred during 5-day exposures to aluminium sulfate (0, 100 and
200 �g Al/litre) at pH 5.0 or during 21- and 32-day recovery periods.
None of the embryos or later alevins displayed erratic swimming
behaviour or mucus accumulation around the mouth or gills. After
21-day (late embryos) and 32-day (early embryos) recovery periods,
fish at the highest aluminium concentrations were significantly
smaller in length, had reduced whole body concentrations of calcium
and potassium, and were significantly less successful as predators on
Daphnia magna (Gunn & Noakes, 1987).
Cleveland et al. (1991) exposed brook trout ( Salvelinus
fontinalis) to a nominal aluminium concentration of 200 �g/litre
for 56 days under flow-through conditions at pH 5.3, 6.1 and 7.2.
The weights of trout exposed to pH 5.3 and 6.1 did not differ
significantly throughout the study. After day 3 fish exposed to pH 7.2
weighed significantly more than those at pH 5.3 and 6.1. Mortality was
significantly higher in brook trout exposed to pH 5.3 than in those
exposed to pH 6.1 (except on day 56) or 7.2.
c) Lifestage effects
Not only do species differences in response to a given pH and
aluminium concentration exist but great differences in sensitivity
also exist between strains of the same species as well as between
different life-history stages (Rosseland et al., 1990; Rosseland &
Staurnes, 1994).
Fivelstad & Leivestad (1984) studied the toxicity of aluminium to
different life-stages of Atlantic salmon ( Salmo salar) and brown
trout ( Salmo trutta). To study the effect of acidity citrate was
added. Only 1 of 200 swim-up salmon larvae died during a 108-h
exposure at pH 4.9; no behavioural responses were observed. However,
when exposed to aluminium concentrations ranging from 110 to
300 �g/litre, salmon swim-up larvae were more sensitive than the
postlarval stage. Toxicity was found to be most significantly
correlated with inorganic monomeric aluminium concentration, survival
time decreasing with increasing aluminium concentration. The LT50 at
an inorganic monomeric aluminium concentration of 148 �g/litre was
26 h for swim-up larvae. Exposure of salmon parr to natural aluminium
variations (50-180 �g/litre) at pH 5.3 induced hyperventilatory
responses together with increases in haematocrit and small decreases
in chloride. The authors concluded that coughing, hyperventilation and
excessive mucous clogging on the gill surface was due to an irritant
effect of aluminium. Brown trout exposed at pH 5 to the same aluminium
regime showed no sublethal stress symptoms.
Rosseland & Skogheim (1984) demonstrated the increased
sensitivity of Atlantic salmon undergoing smoltification compared to
younger year classes. In a laboratory study at pH 4.9-5.0 and
inorganic monomeric Al concentrations of 130-463 �g/litre, pre-smolt
(age 2 years) were more sensitive than parr (age 1 year) in all
combinations of pH and aluminium. For instance, at pH 4.95 and 245 �g
Al/litre, the LT50 for pre-smolt was 35 h whereas the LT50 for parr
was 60 h.
Early life-stage (fertilized eggs, alevins and swim-up fry)
golden trout ( Oncorhynchus aguabonita aguabonita) were exposed to
low pH (4.5-6.5) and aluminium (50-300 �g Al/litre, nominal
concentration) for 7 days. Significant mortality occurred at pH 4.5
in the absence of aluminium, at pH 5.5 in the presence of 100 �g
aluminium/litre for larvae and at pH 5.0 with 300 �g aluminium/litre
for alevins. The duration of swimming and feeding activity was
unaffected by treatment in golden trout exposed as eggs. Locomotory
behaviour of alevins was severely inhibited at both pH 5.0 and 5.5
irrespective of treatment and at pH 4.5 and 6.0 in aluminium-exposed
fish. Feeding activity was reduced at pH 4.5, at pH 5.0 with
> 50 �g aluminium/litre and at pH 5.5 with 100 �g/litre. Swimming
activity was not greatly affected among fish exposed as swim-up
larvae. Feeding activity was greatly inhibited at all aluminium
concentrations and at pH 4.5 (DeLonay et al., 1993).
Farag et al. (1993) studied the effect of aluminium (50-300 �g
Al/litre, nominal concentration) on eggs, eyed embryos, alevins and
swim-up larvae of cutthroat trout ( Oncorhynchus clarki bouvieri) at
pH values ranging from 4.5 to 6.5 for either 7 days or during
continuous exposure until 40 days after hatching. Fish survival
decreased when pH was lowered to 5.0 or 4.5 for 7 days during the egg
stage. Alevin and swim-up larval stages were less sensitive to low
pH and more sensitive to aluminium, with 100 �g/litre at pH 5.0
significantly decreasing survival. The eyed embryo stage was the most
resistant; there was > 90% survival in all groups except in the
presence of > 100 �g/litre at both pH 5.0 and 5.5. During continuous
exposure, survival decreased with time and individuals died earlier in
each life-stage when exposed to combinations of pH and aluminium than
did those exposed to pH alone. Swim-up larvae were the most sensitive
group with regard to growth and all larvae exposed to 50 �g/litre
showed significantly reduced growth.
Buckler et al. (1987) exposed striped bass ( Morone saxatilis)
of different ages to total aluminium (up to 400 �g/litre) at various
pH levels (pH 5.0-7.2) in a flow-through diluter system for 7 days.
Eleven-day-old fish showed significant mortality at pH 6.0
irrespective of aluminium exposure; significant mortality was observed
at 25 �g/litre for pH 6.5 and at 400 �g/litre for pH 7.2. Older fish
(160 days) were less sensitive, showing significant mortality at 50 �g
Al/litre for pH 6.0, 200 �g/litre for pH 6.5, and 400 �g/litre for pH
7.2. In a similar test, 300 �g/litre was lethal to 100% of both 159-
and 195-day-old bass at pH 5.5, but produced no observable adverse
effects at pH 6.5 or 7.2. Mortality among 159-day-old fish held in
water at pH 5.5 without aluminium was 22% after 7 days, there being no
deaths among control fish. No mortality was observed among 195-day-old
fish exposed to pH 5.5 alone.
Eggs, larvae and post-larvae of white suckers ( Catostomus
commersoni) and brook trout ( Salvelinus fontinalis) were exposed
to pH levels of 4.2 to 5.6 and inorganic monomeric aluminium
concentrations ranging from 0 to 0.5 mg/litre. White sucker embryos
were very sensitive to low pH levels, with none surviving to the eyed
stage at pH levels of 5.0 or less. The addition of aluminium increased
embryo survival through the eyed stage but did not increase hatching.
At pH levels of 5.4 and 5.6 survival to eyed stage increased to 38% to
69% and was 74% to 81% in controls. At pH levels above 5.2 the
presence of aluminium resulted in embryo deformities. Survival of
trout eggs through the eyed stage was unaffected at pH 4.6 or more
irrespective of aluminium treatment. However, in the absence of
aluminium at pH levels of 4.4 survival decreased and at pH 4.2 no
embryos survived. The addition of aluminium to low pH groups
significantly increased survival through the eyed stage to hatching.
All white sucker larvae died within 146 h at pH levels below 5.0 with
or without aluminium. In the absence of aluminium at pH levels greater
than 5.0, more than 80% survived the 13-day experiment; however, the
addition of aluminium further decreased the survival of larvae. At pH
levels of 4.4 or more > 97% of trout larvae survived without
aluminium. The addition of more than 0.1 mg Al/litre at all pH levels
decreased the survival of larvae. White sucker post-larvae were
sensitive to low pH levels, only 16 to 68% surviving at pH 4.6. The
addition of aluminium to acidic solutions further decreased survival.
In the presence of high levels of aluminium (0.3 or 0.5 mg/litre) at
all pH levels, all post-larvae died within 75 h. At aluminium levels
of 0.1 and 0.2 mg/litre at low pH levels all post-larvae died within
145 h. Brook trout post-larvae were tolerant of low pH levels. All
post-larvae survived at pH levels ranging from 6.99 to 4.22 without
aluminium. At aluminium levels of 0.2 mg/litre or more survival was
decreased at all pH levels (Baker & Schofield, 1982).
Cleveland et al. (1986) carried out a partial life-cycle toxicity
study on brook trout ( Salvelinus fontinalis) for 60 days in a flow-
through proportional diluter. Eyed brook trout eggs and the resultant
larvae were exposed in water containing 3 mg calcium/litre at
nominal pH values of 7.2, 5.5 and 4.5 with and without aluminium
(300 �g Al/litre, nominal concentration) until 30 days after hatching.
Mortality of trout eggs was not influenced by aluminium but was
significantly increased by low pH. Larval growth and mortality was
unaffected by aluminium at pH 7.2 and 4.5, but mortality was
significantly increased and growth decreased by aluminium at pH 5.5.
DNA and RNA content was significantly reduced by aluminium at pH 5.5.
In general swimming and feeding behaviour were unaffected by aluminium
at pH 7.2 and significantly reduced by aluminium at pH 5.5. At pH 4.5
behaviour was inhibited to such an extent that possible effects of
aluminium were masked. In a second experiment 37-day-old trout were
exposed to similar conditions. Mortality was significantly increased
by aluminium at pH 5.5 and 4.5. Aluminium significantly reduced growth
at pH 7.2 and 5.5. DNA and RNA content was significantly increased by
aluminium at pH 5.5. Juvenile trout behaviour was less affected by
acid conditions than in the case of larvae; there were significant
decreases in behaviour in the presence of aluminium at pH 5.5 and 4.5.
Hunn et al. (1987) utilizing a similar experimental set-up found that
embryo mortality exceeded 80% at pH 4.5, averaged 15% to 18% at pH 5.5
and was less than 2% at pH 7.5. Aluminium significantly increased
mortality at pH 4.5 but did not affect mortality at pH 5.5 or 7.5.
Hatching success was pH-dependent and was not influenced by aluminium
exposure. Brook trout larvae suffered 100% mortality at pH 4.5, 20%
mortality at pH 7.5 with or without aluminium, 69% mortality at pH 5.5
without aluminium and 100% mortality within 15 days with aluminium.
Cleveland et al. (1989) reported that 60-day no-observed-effect
nominal concentrations for aluminium at pH 5.6 to 5.7 were 29 �g/litre
for swimming capacity, 68 �g/litre for weight and 142 �g/litre for
frequency of movement (2 min period), strike frequency (directed at
prey), fry mortality and length. At pH 6.5 to 6.6 no-observed-effect
concentration (NOEC) values were 88 �g/litre for length and weight,
169 �g/litre for fry mortality and > 350 �g/litre for movement,
strike frequency and swimming capacity.
d) Physiological and biochemical effects
Physiological and biochemical effects have recently been reviewed
by Rosseland & Staurnes (1994).
Witters (1986) studied the effect of total aluminium
(350 �g/litre), pH (4.1 and 6.1) and calcium concentration (38 and
190 �Eq/litre) on ion balance and haematology in rainbow trout
( Oncorhynchus mykiss) exposed for 3.5 h. None of the treatment
combinations affected the number of erythrocytes or the haemoglobin
content. However, exposure to aluminium under acidic conditions
significantly reduced plasma osmolarity and increased plasma potassium
levels. Plasma sodium and chloride levels were significantly reduced
under acidic conditions with or without aluminium. The haematocrit
value was significantly increased and plasma ammonium decreased by
aluminium only under acidic conditions and at low calcium
concentration.
Muniz & Leivestad (1980) reported that brown trout ( Salmo
trutta) exposed to acidic conditions (pH 4.3 to 5.5) showed plasma
losses of both chloride and sodium. These losses were enhanced by the
addition of total aluminium at 900 �g/litre. Fish showing signs of
stress exhibited hyperventilation, coughing and excessive mucus
clogging of the gills. The authors reported that the changes in blood
electrolytes indicate that aluminium toxicity is similar to that seen
with hydrogen ion stress. However, aluminium can cause such effects at
pH levels that are not physiologically harmful.
Wood & McDonald (1987) studied the physiology of brook trout and
rainbow trout exposed to inorganic monomeric aluminium concentrations
ranging from 111 to 1000 �g/litre (as aluminium chloride) at pHs
ranging from 4.4 to 6.5. Acid stress alone for 10 days was not lethal
to adult brook trout, but there was a net loss of sodium and chloride
ions. The addition of aluminium resulted in an increased loss of ions
and severe mortality. At pH 4.8, low calcium level (25 �Eq/litre) and
an aluminium concentration of 333 �g/litre, the LT50 was found to be
39 h. At a lower pH (4.4) the average survival time was twice as long.
The cause of death was ionoregulatory failure. However, increasing the
calcium levels (400 �Eq/litre) still killed fish almost as quickly but
the cause of death was respiratory disturbance. Rainbow trout were
more sensitive to both acid and acid/aluminium than brook trout.
Respiratory disturbances were found to be the cause of death in both
high and low calcium groups exposed to acid/aluminium conditions. For
both species there was a correlation between toxic effects and
aluminium accumulation in gills.
Dalziel et al. (1986) exposed brown trout to nominal aluminium
concentrations of 8 �mol/litre at pH levels ranging from 7.0 to 4.0
and calcium levels of 10 or 50 �mol/litre. Low pH had little effect on
the influx of sodium, but the addition of aluminium significantly
reduced influx at pH 4.5 and 4.0. Efflux of sodium tended to be
increased by low pH, but no further effect was caused by aluminium.
Aluminium at higher pH values appeared to have no effect on sodium
fluxes. Dalziel et al. (1987) found that reduced pH levels had no
effect on the sodium influx in brown trout ( Salmo trutta). However,
the presence of aluminium at concentrations of 2 �mol/litre at pH 4.5
and 4.0 significantly decreased sodium influx. At pH 5.4 there was no
effect of aluminium on influx. Sodium efflux was significantly
increased at low pH. Increasing the aluminium concentration at pH 5.4,
and to a smaller extent at pH 4.5, tended to increase efflux. There
was no effect of aluminium on sodium efflux at pH 4.0.
Booth et al. (1988) studied the effects of total aluminium at
concentrations of 333 and 1000 �g/litre and at low pH (5.2-4.4) on
net ion fluxes and ion balance in the brook trout ( Salvelinus
fontinalis) over a period of 11 days. Low pH caused a pH-dependent
net loss of sodium and chloride ions and the addition of aluminium
increased this loss. The authors reported that any fish losing more
than 4% of total sodium ions during the initial 24 h of aluminium
exposure was 90% more likely to die. All fish exposed to aluminium
accumulated it on gill surfaces; fish that died accumulated more
aluminium than survivors.
Leivestad et al. (1987) studied the effect of inorganic monomeric
aluminium on Atlantic salmon ( Salmo salar) exposed for 28 weeks at
pH levels of 4.8-6.5 and aluminium concentrations of 50-350 �g/litre.
The authors found that failure in ionic regulation was the primary
cause for mortality and that Na-K-ATPase activity was reduced at toxic
aluminium levels. The symptoms were correlated with ion-exchangeable
aluminium, precipitating aluminium hydroxide having low toxicity.
Staurnes et al. (1993) maintained smolting Atlantic salmon in soft
water at pH 5 both with and without 50 �g/litre total aluminium.
Exposure to acid water resulted in osmoregulatory failure and high
mortality, and aluminium greatly enhanced the toxicity. Sensitivity to
acid and acid-aluminium increased when fish had developed to seawater-
tolerant smolts. Gill carbonic anhydrase activity was reduced by
aluminium exposure. Fish in both treatment groups had low seawater
tolerance and this was related to a decline in Na+/K+-ATPase
activity.
Hutchinson et al. (1987) studied the effects of total aluminium
(0-1000 �g/litre) at pH levels ranging from 3.8 to 6.0 on the early
lifestages of lake trout ( Salvelinus namaycush), brook trout
( Salvelinus fontinalis) and pumpkin seed sunfish ( Lepomis
gibbosus). Three different responses were observed: a) aluminium
toxicity at pH < 5.0 represented joint action with hydrogen ions
producing ionoregulatory failure; b) at pH 5.0-6.0 aluminium toxicity
required concentrations of inorganic forms that greatly exceeded
theoretical gibbsite solubility; c) at acutely lethal levels of pH and
ionic strength, aluminium increased the resistance time of eggs, fry
and adults.
Ogilvie & Stechey (1983) studied the respiratory responses of
rainbow trout ( Oncorhynchus mykiss) to aluminium exposure (50 to
500 �g/litre) at a pH of 6.0 and an exposure period of 26 h. Mean
opercular rate was significantly increased at 500 �g aluminium/litre
and mean cough rate was significantly increased at both 200 and
500 �g/litre. Spontaneous locomotion and mean activity levels were
variable in all groups.
Neurotoxic effects on the olfactory organ of rainbow trout were
demonstrated by Klaprat et al. (1988), who exposed the fish to pH 7.7
and pH 4.7 both with (5.0, 9.5 and 20.0 �mol total Al/litre) and
without aluminium. At pH 4.7 alone, increased mucous was observed over
parts of the olfactory epithelium. After Al additions, however, loss
of receptor cell cilia, irregular shaped olfactory knobs, changed
microvilli and swellings of microridge cells were observed. Electrical
response from the olfactory nerve to L-serin was not changed by pH
alone, but was depressed by aluminium additives. Since sensory organs
play a very important role in the behavioural ecology of fish
populations (feeding, alarm signals, pheromones, imprinting, spawning,
etc), neurotoxic effects on the olfactory organ can have great adverse
effects in nature (Rosseland & Staurnes, 1994).
e) Pathological effects
Hunter et al. (1980) noted that rainbow trout ( Oncorhynchus
mykiss) which survived exposure to 50 mg/litre aluminium at pH 8.0
to 9.0 showed several pathological signs of toxicity. These included
proliferative changes in the gills and congestion of the secondary
lamellae, slight demyelination of the brain, extensive necrosis of the
liver, severe inflammatory glomerular necrosis of the kidney and some
evidence of skin hyperplasia. Karlsson-Norrgren et al. (1986b) exposed
brown trout ( Salmo trutta) to aluminium sulfate (50, 200 and 500 �g
total aluminium/litre) at pH 5.5 and 7.0 for up to 6 weeks. Advanced
gill lesions (enlargement of secondary lamellae due to the increased
number of chloride cells in the epithelia) were observed in fish
exposed to aluminium at pH 5.5 and a temperature of 2.5�C. The lesions
contained cytoplasmic aluminium precipitates. The addition of humus or
increasing the pH to 7.0 reduced or inhibited the effects of
aluminium. A water temperature of 15�C reduced the gill lesions
observed at 2.5�C. However, prolonged exposure to higher water
temperatures produced gill alterations even in controls. Eggs and the
resulting fry of Atlantic salmon ( Salmo salar) exposed to aluminium
(38-300 �g/litre) at pH 5.5 were investigated. Scanning electron
microscopy revealed gill abnormalities, which included poorly
developed or absent secondary lamellae, fused primary lamellae,
proliferation of epithelial cells and increased numbers of surface
pits. These effects were not noted when fish were raised in aluminium-
free water at pH values ranging from 4.5 to 7.2 (Jagoe et al., 1987).
9.1.2.4 Amphibians
Clark & LaZerte (1985) exposed eggs and tadpoles of the American
toad ( Bufo americanus) and the wood frog ( Rana sylvatica) to total
aluminium concentrations of 10, 20, 50, 100 and 200 �g/litre at pH
ranging from 4.14 to 5.75. A nominal pH of 4.14 significantly reduced
hatching in the absence of aluminium. Aluminium had no effect on
hatching at pH 4.75 or pH 5.75. However, at pH 4.14 there was a
significant reduction in hatching for eggs exposed to any aluminium
concentration compared with eggs without aluminium at the same pH
level. Tadpoles that hatched out were not affected by any aluminium
concentration or pH level.
Clark & Hall (1985) studied the effects of total aluminium
(7-210 �g/litre), pH (pH 4.41-6.29) and dissolved organic content
(2.2-9.9 mg/litre) at calcium concentrations of 2 mg/litre on
B. americanus, R. sylvatica and the spotted salamander ( Ambystoma
maculatum). High aluminium, low pH and high dissolved organic
content (DOC) significantly reduced hatching success of B.
americanus. For R. sylvatica neither aluminium nor pH correlated
significantly with hatching success over the range of pH tested.
Hatching success for A. maculatum ranged from 41% at pH 4.4 to 68%
at pH 6.1. Although pH was not significantly correlated with hatching
success, a greater number of eggs hatched above pH 5.0 than below, the
difference being significant. Decreased hatching success was
correlated with high aluminium and high DOC. In a second experiment
the authors studied the effects of aluminium (total aluminium
54-75 �g/litre) and pH (pH 4.23-5.8) at calcium concentrations of
2 mg/litre. Hatching success of B. americanus and R. sylvatica was
unaffected at pH 4.8 and 5.8 with total aluminium concentrations of 54
to 63 �g/litre. However, there were significant reductions in hatching
at a pH of 4.3 and total aluminium concentration of 75 �g/litre.
Hatching success of A. maculatum was not correlated significantly
with pH. However, hatching success was only 57% at the highest pH
value and lowest aluminium concentration.
Gascon et al. (1987) studied the affects of total dissolved
aluminium (7.4 �mol/litre), pH (4.5 and 6.2) and calcium (25 and
500 �Eq/litre) on the eggs and tadpoles of Rana sylvatica. There was
no egg mortality in any group. Hatching was significantly delayed in
groups exposed to aluminium under acidic conditions. Tadpoles exposed
to aluminium at pH 4.5 and calcium at 25 �Eq/litre suffered 100%
mortality. In a similar group where the pH was allowed to drift up to
5.3, mortality was not significant. Metamorphosis in surviving
tadpoles was significantly delayed by acidic conditions and aluminium
exposure. Growth, as measured by dry weight, was significantly
depressed in the group suffering 100% mortality. In the groups exposed
to aluminium with either pH rising to 5.3 or calcium at 500 �Eq/litre,
growth was significantly increased over controls.
Freda & McDonald (1990) exposed embryos (4 to 5 days) and
tadpoles (96 h) of the leopard frog ( Rana pipiens) to a range of
total aluminium concentrations (250-1000 �g/litre) and pHs (4.2-6.5).
The pH and the aluminium concentration had a significant effect on the
survival of embryos. All control embryos hatched whereas 94% of
embryos at pH 4.2 failed to hatch. At pH 4.2 and 4.4 the addition
of aluminium ameliorated the effects of low pH, increasing
hatchability to 78-99%. At pH 4.6 and 4.8 aluminium was found to be
toxic. The LC50 values for aluminium at pH 4.6 and 4.8 were 811 and
403 �g/litre, respectively. Tadpoles (pre-stage 25) were less
sensitive to low pH than embryos, showing 20% mortality at pH 4.2.
However, they were much more sensitive to aluminium, all the tadpoles
dying at aluminium concentrations > 500 �g/litre and pH 4.4 or 4.6,
and at > 250 �g/litre and pH 4.8. Three-week-old tadpoles were less
sensitive to lowered pH and elevated aluminium than embryos or newly
hatched tadpoles. Low pH (4.2) had no effect on the survival of
tadpoles, and aluminium was only toxic at pH 4.8 with 40% mortality at
1000 �g/litre.
Common frog ( Rana temporaria) tadpoles were raised to
metamorphosis at total aluminium concentrations of 800 and
1600 �g/litre and at pH 4.4. Decreasing pH reduced maximum body size
and delayed metamorphosis. Growth was depressed and metamorphosis
delayed at 800 �g Al/litre; at 1600 �g/litre small tadpoles had
arrested growth and development and subsequently died, whereas large
tadpoles metamorphosed at a very small size (Cummins, 1986).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Numerous studies exist of plants exposed to aluminium in nutrient
solution or sand culture. They show that exposure causes diminished
root growth and development, reduced uptake of plant nutrients
(notably phosphorus, calcium and magnesium) and stunted plant growth
(Bartlett & Riego, 1972a,b; G�ransson & Eldhuset, 1987; Boxman et al.,
1991; Keltjens & Tan (1993). The effect of aluminium on plants is
complex. It can act directly on plant cell processes (Taylor, 1991) or
indirectly by interfering with plant nutrition (Roy et al., 1988;
Taylor, 1991).
Plant species vary in their response to aluminium (Roy et al.,
1988; Taylor, 1995). Even within species (e.g., wheat, Triticum
aestivum), aluminium sensitive and tolerant varieties exist (Taylor
& Foy, 1985; Kinraide et al., 1992; Huang et al., 1992a; Wheeler et
al., 1993). There are reports that aluminium can benefit plants
(Hackett, 1962, 1964, 1967). Various proposed mechanisms are listed in
the review by Roy et al. (1988). However, it seems that exposure to
excessive concentrations of aluminium is detrimental to plants, but
the level that is excessive is highly variable.
9.1.3.2 Invertebrates
No data have been reported on the effects of aluminium on
terrestrial invertebrates.
9.1.3.3 Birds
Hussein et al. (1988) fed Japanese quail ( Coturnix coturnix
japonica) on diets containing 0.05, 0.1, 0.15 and 0.3% aluminium (as
aluminium sulfate) for 4 weeks. Egg production was significantly
decreased at 0.1% and body weight gain at 0.15%. Feed intake was
significantly depressed temporarily at 0.1 and 0.15% and permanently
at 0.3%. Eggshell breaking strength was temporarily reduced (after 1
week only) at 0.1, 0.15 and 0.3%.
Hussein et al. (1989a) fed white leghorn laying hens on a diet
containing 0.05, 0.1 or 0.15% aluminium (as aluminium sulfate) for 28
days. Feed intake, body weight, tibia breaking strength and plasma
inorganic phosphorus were significantly reduced at 0.15%. Egg
production was only significantly depressed after 21 days at 0.15%.
Eggshell breaking strength was unaffected by the treatment. In a
second experiment hens were exposed to diets containing up to 0.3%
aluminium for 42 days. Feeding 0.3% aluminium significantly decreased
plasma inorganic phosphorus in samples collected immediately following
oviposition (10 to 42 days). Plasma calcium, tibia weight and tibia
breaking strength were unaffected. Egg production and feed intake were
significantly reduced during days 1 to 21 but not during days 22 to
42. The effects of 0.3% aluminium on the egg production and shell
quality of laying hens are similar to those obtained with conventional
force-moulting procedures using feed restriction (Hussein et al.,
1989b). White leghorn laying hens were maintained on a diet containing
0, 0.15 or 0.3% aluminium for 17 weeks. Hatchability of eggs was
unaffected while fertility and body weight of chicks were
significantly depressed at both aluminium treatments. Total egg
production and feed consumption both were significantly reduced at the
highest aluminium dose (Wisser et al., 1990).
Carri�re et al. (1986) fed ring doves ( Streptopelia risoria)
on a diet containing 0.1% aluminium sulfate with reduced calcium
and phosphorus levels (0.9% Ca; 0.5% P) for a period of 4 months.
There were no significant effects on egg production, fertility,
hatchability, growth or final weight of chicks. Egg permeability was
initially decreased but subsequently recovered to normal levels. The
diet had no effect on plasma calcium, phosphorus or magnesium. There
was no effect on weight or growth rate in juvenile doves fed diets
containing 500, 1000 or 1500 mg/kg aluminium sulfate from day 21 to
day 63.
9.2 Field observations
9.2.1 Microorganisms
No data have been reported regarding effects in the field of
aluminium on microorganisms.
9.2.2 Aquatic organisms
9.2.2.1 Plants
H�rnstr�m et al. (1984) studied the effects of pH and different
levels of aluminium on lake phytoplankton from the Swedish west coast
area. They concluded that the absence of several phytoplankton species
in acid lakes was not caused by the low pH but rather a raised
aluminium supply from the surrounding land, which produced
oligotrophic waters through precipitation of phosphorus. Comparing
phytoplankton communities in strongly acid lakes with low and high
levels of aluminium revealed that aluminium toxicity alone contributed
significantly to the reduced numbers of phytoplankton species.
9.2.2.2 Invertebrates
Aston et al. (1987) found that for streams in Wales and the Peak
district, United Kingdom, the population density and biomass of
freshwater invertebrates were generally lowest in streams with low pH
and high aluminium content. H�rnstr�m et al. (1984) reported that most
zooplankton species found in acidic lakes of the Swedish west coast
were relatively resistant to acidity but were more susceptible to the
oligotrophication process caused by the precipitation of phosphorus by
aluminium. There was also an inverse correlation between the number of
invertebrates and the aluminium concentration.
Hall et al. (1985) added aluminium chloride (0.28-3.8 mg total
Al/litre) to a stream to simulate episodic release during acidic
snowmelt. Significant decreases in pH and dissolved oxygen content
accompanied increases in aluminium. There was an increased drift of
invertebrates with increasing aluminium concentration. The gradually
increasing drift rate of benthic macro-invertebrates appeared to be a
stress response to the aluminium/hydrogen ion concentrations. Drift of
terrestrial insects feeding at the water surface appeared to be due to
the reduction in surface tension of the aluminium-treated portion of
the stream. Hall et al. (1987) found that during second-order stream
experiments, overall more aquatic invertebrates drifted at pH 5.0
during aluminium chloride addition (> 0.28 mg total Al/litre) than at
pH 5.0 during hydrochloric acid addition (0.012 mg total Al/litre).
McCahon et al. (1987) subdivided a stream into sections of low pH
(4.3) and low aluminium concentration (0.052 mg total Al/litre) by
adding sulfuric acid and high aluminium concentration (0.35 mg total
Al/litre; pH 5.0) by adding aluminium sulfate. In the acid zone,
mayfly mortalities of 20% and 5.3% after 24 h were observed for
Baetis rhodani and Ecdyonurus venosus, respectively. Mortality
rose to 52.6% after 48 h for E. venosus. Mayflies killed during the
exposure did not stain for aluminium or mucus. Similar mortalities
were observed for both species in the acid/aluminium zone. However,
both species gave an aluminium-positive reaction for all parts of the
body examined, aluminium being concentrated in the gut, within and
surrounding the gill plates, and on the outer surface of the abdomen.
Ormerod et al. (1987a) created simultaneous episodes of low pH
(4.28), and low pH (5.02) with increased aluminium content (347 �g
Al/litre) in a soft-water stream in upland Wales . In situ toxicity
tests were performed, and Chironomus riparius, Hydropsyche
angustipennis and Dinocras cephalotes were found to have suffered
no mortality. Ecdyonurus venosus, Baetis rhodani and Gammarus
pulex showed up to 25% mortality in both treatment zones. Drift
densities increased, especially in the aluminium-treated zone,
Baetis rhodani showing an increase of 8 times. Baetis rhodani was
the only invertebrate to show a significant decline in benthic density
(in the aluminium zone), which was due mostly to drift. Weatherley et
al. (1988) created 24-h experimental episodes by adding acid,
aluminium and citric acid to different treatment zones of an upland
stream. Drift density was only observed for the ephemerophteran Baetis
rhodani, and was found to be unaffected by flow at pH 7 or
organically-bound aluminium. Both acidity (pH 4.9) and labile
aluminium (0.11 mg/litre) increased drift density. Benthic density was
significantly decreased by both labile and organically bound
aluminium.
9.2.2.3 Vertebrates
Grahn (1980) reported two fish kills in lakes Ransj�n and �mten,
two pristine acid lakes in Sweden, during 1978 and 1979. In 1978 the
main part of the ciscoe ( Coregonus albula) population was wiped out.
One week later the pH was found to be 5.4 at the surface and 4.9 at
the bottom, with maximum aluminium levels of 0.91 mg/litre in
groundwater and 0.31 mg/litre in stream water. One year later a
similar incident occurred when the pH was 6.0 in surface water and 5.4
in bottom water. Total aluminium concentrations ranged from 0.36 to
0.52 mg/litre. Analysis of fish gills revealed that aluminium
concentrations were at least 6 to 7 times higher than those found in
fish gills from reference lakes in the area. The authors conclude that
weather patterns caused an increase in phytoplankton, which in turn
increased pH levels near the surface. Aluminium hydroxide was
precipitated out in the epilimnion water. Ciscoes migrate to the
surface to feed where the flocculated aluminium hydroxide caused fish
gills to clog and fish to die of suffocation.
Hunter et al. (1980) investigated Black Cart water, a tributary
of the River Clyde, Scotland, following sporadic occurrences of fish
kills (brown trout Salmo trutta) during 1974 and 1975. Aluminium
levels were 475 mg/kg (dry weight) in the lateral muscle of dead fish
and 358 mg/kg in live fish. Effluent waters from an anodizing factory
(pH 6.3-7.3; total aluminium 12-530 �g/litre and soluble aluminium
20-360 �g/litre) were mixed with waters of pH 8.35-8.70 and total and
soluble aluminium concentrations of 190-220 �g/litre and < 20
�g/litre, respectively. Downstream of the confluent, which was the
area of fish kills, the waters varied in pH between 8.1 and 8.5, and
in total (200-1300 �g/litre) and soluble (20-33 �g/litre) aluminium.
The authors suggested that when a high pH river receives high loadings
of aluminium from water-treatment works soluble aluminium
concentrations lethal to fish can occur.
Schofield & Trojnar (1980) reported that lakes in the Adirondack
mountains, USA, in which stocked brook trout ( Salvelinus fontinalis)
were not recovered in netting surveys during 1975, were those with
significantly lower mean pH (4.79 versus 5.25), calcium level (1.62
versus 1.87 mg/litre) and magnesium level (0.30 versus 0.39 mg/litre),
and higher mean aluminium concentrations (0.29 versus 0.11 mg/litre).
The covariance analysis of water quality suggested that aluminium
intoxication may have been a primary factor in determining the success
or failure of fish stocking, whereas both pH and calcium level showed
a lack of significance.
Henriksen et al. (1984) showed the acid-aluminium-rich (pH 5.1;
inorganic monomeric aluminium 30 �g/litre) waters of the river
Vikedal, in southwestern Norway, during a period of increased water
flow, to be toxic to pre-smolt Atlantic salmon ( Salmo salar).
Skogheim et al. (1984) reported massive deaths of adult Atlantic
salmon in the River Ogna in south-west Norway in 1982. Newly run
migrating spawners were killed when acid tributary water (pH 4.7;
inorganic monomeric Al 340-360 �g/litre) was mixed into the falling
main stream flow of better water quality (pH 5.96-6.18; inorganic
monomeric Al 12-13 �g/litre). A near total mortality of salmon
occurred for several km downstream of the confluent (pH 4.76-5.41;
inorganic monomeric Al 110-307 �g/litre). Blood samples from highly
stressed fish (plasma chloride 103-113 mEq/litre) demonstrated
osmoregulative failure (normal values 120-135 mEq/litre), and gill
aluminium accumulation (70-341 �g Al/g fresh weight) was around 10
times higher than normal values.
Harriman et al. (1987) studied the long-term fish population
changes in streams and lochs of south-west Scotland during 1978, 1979
and 1984. Trout were not caught in areas known to have contained them
in the past. Fish-less lochs and streams contained the levels of
acidity (pH 4-5) and aluminium (up to 300 �g labile monoesic Al/litre)
known to be toxic to fish. The authors concluded that acidic
deposition was probably the major cause of the change in the fish
population in this area.
Eaton et al. (1992) compared laboratory exposure of four embryo-
larval fish species to aluminium (< 6.0-70 �g/litre) and pH levels of
4.5 to 7.0 with field observations. Laboratory results showed that
monomeric aluminium concentrations of approximately 50 �g/litre, which
were comparable to aluminium concentrations in the acidified half of a
lake, increased low pH toxicity. However, the laboratory tests
for largemouth bass ( Micropterus salmoides) and rock bass
( Ambloplites rupestris) underestimated field toxicity, those for
black crappie ( Pomoxis nigromaculatus) overestimated field toxicity
and those for yellow perch ( Perca flavescens) were similar to those
observed in the field.
Karlsson-Norrgren et al. (1986a) collected and examined brown
trout ( Salmo trutta) from two fish farms within acid-susceptible
areas in Sweden. The total aluminium concentrations in the water were
200 to 300 �g/litre. Trout from a third non-acidified location
(aluminium levels in water 35 �g/litre) were also examined. Light and
electron microscopic examination of fish from the acid-susceptible
areas revealed two major types of gill lesion characterized by
chloride cell hyperplasia, and enlargement of the intercellular
spaces, in the secondary lamellar epithelium. The gills from the
control area appeared normal, exhibiting features characteristic of
salmonids.
Norrgren & Degerman (1993) transferred eyed eggs of Atlantic
salmon ( Salmo salar) and brown trout ( Salmo trutta) in plastic
boxes to various locations on the Morrum river, Sweden. The pH levels
ranged from 5.1 to 6.7 and total mean aluminium levels ranged from 933
to 569 �g/litre (mean reactive aluminium concentrations ranged from
8 to 203 �g/litre). No significant effects were observed on hatching
(13 days) or fry survival (77 days) at pH > 5.7 and reactive
aluminium concentrations of up to 49 �g/litre. At pH 5.1 and a total
aluminium concentration of 569 �g/litre (reactive aluminium =
203 �g/litre), there was a significant reduction in the salmon hatch
but not the trout hatch; total fry mortality after 77 days was 100%
for salmon and 94% for trout. Histochemical studies revealed aluminium
precipitates in the gills of dead fish; precipitates were associated
with the apical plasma membrane.
Stoner et al. (1984) carried out 17-day survival studies of caged
brown trout ( Salmo trutta) in streams of the river Tywi catchment,
Wales. All streams where fish died had concurrent mean total aluminium
concentrations exceeding 21 �Eq/litre and mean pH levels of less than
6. There was a high positive correlation between fish mortality
(expressed as the inverse of LT50) and mean filterable aluminium
concentration. Differences in LT50 values reflected differences in
aluminium at similar pH levels. There were considerable differences in
gill aluminium concentrations between "control" fish (61 �g/g) and
those that died most rapidly (3505 �g/g). There was also a positive
correlation between mortality rate and gill aluminium concentration.
Weatherley et al. (1990) studied the survival of eggs, alevins and
parr of brown trout ( Salmo trutta) in streams of different acidity
(pH 4.7-6.9). Egg survival from fertilization to hatching was
independent of the mean total monomeric aluminium concentration, which
ranged from 3 to 397 �g/litre. However, the survival of alevins was
strongly related to both aluminium and pH; LC50 values for 28- and
42-day exposures were approximately 19 and 15 �g total monomeric
aluminium/litre, respectively (equivalent to 79 and 72 �g/litre of
0.45 �m filterable aluminium). The 21-day LC50 for parr (3 months)
was between 84 and 105 �g/litre filterable aluminium.
Ormerod et al. (1987a) created an acidic episode in a softwater
stream in upland Wales. Three stretches were identified: (a) upper
reach with natural waters of pH 7.0 (40 �g total Al/litre), (b)
addition of sulfuric acid to pH 4.2-4.5 (52 �g total Al/litre), and
(c) pH 5.2 (347 �g total Al/litre). Caged salmon ( Salmo salar) and
brown trout ( Salmo trutta) showed 7-10% mortality in the acid zone
(b) and 50-87% in the aluminium zone (c). Salmon (14.7 h) had a
significantly shorter LT50 than trout (23.5 h) in the aluminium zone
(c). Ormerod et al. (1987b) reported that brown trout ( Salmo trutta)
exposed to similar episodes showed reduced plasma sodium at low pH
(4.2), which appeared to be decreased by the addition of aluminium;
however, at pH 4.8 to 5.1 aluminium increased the effect. At pH
4.8-5.1 with the addition of aluminium, fish showed an increased
frequency of ventilation. At lower pH effects on ventilation were less
consistent.
McCahon et al. (1987) reported that trout and salmon exposed to
acid conditions (pH 4.3) alone and low aluminium concentration
(0.052 mg/litre) did not stain for aluminium, although increased mucus
production was demonstrated in the gills. Fish exposed to aluminium
(0.35 mg/litre) at low pH (5.0) exhibited extensive aluminium and
mucus coating of the secondary gill lamellae. Fish that died in the
acid/aluminium zone showed large significant increases in gill
aluminium content compared with controls or with fish from the acid-
only zone. Mean aluminium concentrations in fish gills from the
acid/aluminium zone were 2950 and 3050 �g/g (dry weight) for trout and
salmon, respectively.
Rosseland et al. (1992) exposed in situ caged smolts of
Atlantic salmon ( Salmo salar) and sea trout ( Salmo trutta) to
waters in an acid tributary (pH 4.8; inorganic monomeric aluminium
236 �g/litre) and the mixing zone with the limed river Audna (Norway)
(pH 7.0; inorganic monomeric aluminium 17 �g/litre). In the acid
tributary LT50 values were 22 h and 40 h for the two species,
respectively. However, in the mixing zone, between 1 and 3 min after
mixing (pH 5.8-5.9; inorganic monomeric Al 78-111 �g/litre), both
species gave an LT50 of 7 h. Fish showed osmoregulatory failure (loss
of plasma ions and reduced gill Na/K-ATPase) and gill lesions. No
mortality occurred 5 min after mixing or in the limed water. The
authors concluded that the increased toxicity in the mixing zone was
due to the transformation of aluminium from a low to a high molecular
weight precipitating species. The ecological importance of these
mixing zones was pointed out (especially being related to seasonal
changes) as low temperatures (longer polymerization period) and high
flows both would increase the river stretch being affected by these
processes.
Pol�o et al. (1994) used the same water sources to a channel
experiment with control mixing of the waters. Caged Atlantic salmon
were exposed to acid stream water (pH 4.89; inorganic monomeric
Al 139 �g/litre), limed stream water (pH 6.41; inorganic monomeric
Al 14 �g/litre) and the mixture of the two (pH 5.64; inorganic
monomeric Al 70 �g/litre). No mortality occurred in the limed water,
but there was a 27% mortality in the acid stream water after 72 h
exposure. In the mixed water, mortality of up to 90% (mean of 75%)
occurred in the first two metres and up to 1 min after mixing, the
LT50 being 60 h. Within the next 3 min of reaction a total mortality
of 16% was found. Fish in the most toxic zone lost plasma ions at a
slower rate than in the acid, less toxic zone, apparently
demonstrating a different mechanism of toxicity. Although no mortality
occurred towards the end of the channel (4-7 min after mixing), loss
of plasma ions and increased haematocrit were found, indicating
suboptimal conditions.
Lydersen et al. (1994) exposed caged one-year-old parr of brown
trout to mixing zone conditions in a channel, using as input water
from an acid lake (pH 5.3; total Al 379 �g/litre and inorganic
monomeric Al 350 �g/litre) and a neutral lake of pH 7.0, total Al
38 �g/litre. After mixing, a constant pH of 6.1-6.2 was achieved all
along the channel. Total mortality in the lake water was 15% after
24 h. Downstream of the confluent (9 seconds of mixing) mortality
started after 5 h, reaching 100% after 17 h. The LT50 values after 9,
18, 45, 136, 180 and 225 seconds of mixing were 7.5, 9, 12, 17, 23 and
26 h, respectively. Through the use of improved in situ aluminium-
separation techniques, the authors could for the first time
demonstrate the increase in high molecular Al forms (polymerization)
at stations along the channel and relate this directly to toxicity.
Freda & McDonald (1993) transplanted embryos and tadpoles of the
wood frog ( Rana sylvatica), the American toad ( Bufo americanus)
and the spotted salamander ( Ambystoma maculatum) to acidified ponds.
Statistical analysis revealed that pH was the only significant
parameter in determining mortality; the concentrations of total and
labile aluminium were not correlated with the survival of any of these
species.
9.2.3 Terrestrial organisms
9.2.3.1 Plants
Aluminium toxicity to plants is well known in agriculture and
forestry (Wild, 1988). Direct and indirect effects of enhanced
aluminium availability in soil due to soil acidification may be a
cause of current problems in some European forests (Abrahamsen et al.,
1994).
Reich et al. (1994) monitored the light-saturated net
photosynthesis, dark respiration and foliar nutrient content of Scots
pine ( Pinus sylvestris) growing in polluted and non-polluted sites.
At the polluted site soil pH and calcium and magnesium contents were
10 times lower and aluminium content 10 times higher than at the
control site. The rate of photosynthesis was lower and that of dark
respiration was higher at the polluted site. Photosynthesis was found
to be negatively correlated to the aluminium:calcium ratio across both
sites. Dark respiration rate increased with nitrogen and aluminium
contents. Based on the data, the authors concluded that at the
polluted site there were excessive soil aluminium levels and deficient
levels of magnesium. Low needle magnesium level and high aluminium
concentrations and aluminium:calcium ratios resulted in reduced
photosynthetic capacity and increased respiration.
Cronan et al. (1987) reported that observed field total
monomeric aluminium concentrations and aluminium ion activities were
considerably lower than laboratory-based toxic thresholds for several
tree species. However, analysis suggests that calcium/aluminium ratios
for fine roots were near to critical levels. Aluminium toxicity could
possibly have been acting as a stress factor either directly or
indirectly by nutritional interference.
Joslin et al. (1988) sampled roots and foliage of spruce trees
from five sites in North America and Europe. Aluminium concentrations
in fine roots from B horizons were highly correlated with soil
solution monomeric aluminium. Sites with higher levels of plant-
available aluminium had spruce trees with lower foliage levels of
calcium and magnesium. The authors concluded that this may be evidence
of aluminium interference in calcium and magnesium uptake and
transport. There was no obvious interference of aluminium in the
uptake and translocation of phosphorus.
Godbold et al. (1988b) studied the effects of aluminium on
nutrient fluxes in Picea abies. The authors reported that, using
X-ray microanalysis, the distribution of aluminium, magnesium, calcium
and potassium was found to be similar in roots to those collected from
declining spruce stands in Solling, Germany.
Cuenca & Herrera (1987) reported that trees analysed from a
tropical cloud forest growing in acid/aluminium-rich soil were found
to have two distinct strategies for survival: accumulator plants, with
aluminium levels of > 1000 mg/kg, and non-accumulators. Both types
contained large amounts of aluminium in the roots, but in accumulators
the plasmalemma in endodermic cells was permeable to aluminium. The
authors found that both groups were able to accumulate aluminium in
cell walls, alkalinize the surrounding rhizosphere and produce
chelating agents, but all at a high ecological cost and with low
growth rates as a result.
Most cultivated plants are non-accumulators and efficiently
exclude aluminium from the harvested parts. A notable exception is tea
( Camellia sinensis) where aluminium concentration in mature leaves
can reach 1.7% Al/dry weight (Chenery, 1955; Coriat & Gillard, 1986).
Clarkson (1967) studied the aluminium tolerance of four species
of Agrostris from different sites in the United Kingdom. The most
sensitive species was A. stolanifera from a site with no detectable
soil aluminium (as exchangeable ions) and the most tolerant was
A. setacea from a site with soil levels of 3.6 mEq Al/kg. Growing
the species in soils from each of the different sites revealed that
peak growth was achieved in soil from the site where the plant was
collected.
9.2.3.2 Invertebrates
No information has been reported regarding the field effects of
aluminium on terrestrial invertebrates.
9.2.3.3 Vertebrates
Nyholm (1981) found aluminium in the bone marrow tissue of the
humeri of pied flycatchers ( Ficedula hypoleuca) with impaired
breeding from the shores of Lake Tjultr�sk, Sweden. The impairments
included production of small clutches, defective eggshell formation
and intrauterine bleeding. The authors stated that these impairments
compare with the symptoms of aluminium intoxication in mammals.
However, the effects may be indirect, the result of phosphate binding
in the intestinal tract rather than direct toxicity of aluminium.
Ormerod et al. (1986) reported that sites without breeding
dippers ( Cinclus cinclus) had significantly higher mean
concentrations of filterable aluminium, lower mean pH, fewer
trichopteran larvae and ephemeropteran nymphs, and were on rivers with
more conifer afforestation on their catchments than sites where
dippers were present. Ormerod et al. (1988) found no evidence that
aluminium (0.47-2.13 mg/g; dry weight) in the invertebrate prey of
dippers ( Cinclus cinclus) collected from streams of different pH
from Wales and Scotland adversely affected shell thickness or mass of
eggs.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Health effects
The overall assessment of the risks of aluminium exposure to
healthy, non-occupationally exposed humans is rendered uncertain by
several deficiencies in the database, namely:
i) the questionable relevance to humans of animal experiments
designed for hazard identification, in which massive doses were
administered by parenteral routes, and the lack of data based on
oral dosing at relevant exposure levels;
ii) the dubious relevance to humans of the many biochemical findings
from cell culture experiments and other in vitro models;
iii) the uncertainty in the analytical and estimated cumulative
exposure data in epidemiological studies of the relationship of
aluminium in drinking-water to Alzheimer's disease (AD), and the
less than robust dose-response data obtained from such studies;
iv) the complete lack of epidemiological studies relating to total
aluminium exposure, when it is considered that aluminium derived
from drinking-water is responsible for only a small fraction of
such exposure.
It should be noted, however, that animal models employing oral
aluminium administration provide some limited support for cognitive
and motor impairment in the absence of neuropathological changes.
Adverse health effects from aluminium exposure have been
definitely established in one special group, i.e. patients with
severely impaired renal function. These patients are at risk from
severe neurological dysfunction and are also at risk of vitamin-D-
resistant osteomalacia and microcytic anaemia from iatrogenic exposure
to aluminium-containing preparations. The toxicity of oral aluminium-
containing preparations is exacerbated by concurrent use of oral
citrate-containing preparations under these circumstances.
An increased tissue loading of aluminium, especially in bone, has
been shown in premature infants without azotaemia. Current paediatric
practice is to warn about the potential risk that may be associated
with increased tissue levels of aluminium following parenteral
administration or oral intake of products with elevated aluminium
content.
10.1.1 Exposure assessment
Human exposure to aluminium can vary greatly depending on diet,
use of specific medications, sources of drinking-water and exposure to
other ambient and occupational sources of aluminium. In many countries
non-occupationally exposed adults are exposed to between 2.5 and 13 mg
aluminium/day from air, water and food (0.08-0.18 mg/kg body weight
per day for a 60-kg individual). However, large variations in daily
intake can occur as a consequence of differing intakes of foods
containing commonly encountered food additives. Infants consuming
formulae based on cow's milk have very low aluminium intakes
(0.1-0.8 mg/litre), whereas those fed soya-based formulae have much
greater aluminium intakes (3.1-4.3 mg/litre). Moreover, use of
aluminium containing antacids and/or buffered analgesics can increase
daily intake between 10 and 1000 times. Actual exposures will vary
from country to country, but the above figures can be used as a first
approximation for assessing human health risks from aluminium
exposure.
Occupationally exposed populations have background exposure
levels similar to those of non-occupationally exposed populations, but
their work can lead to significantly increased exposure. Actual levels
of exposure depends on the specific work tasks performed, the type and
form of aluminium compound encountered, and the adequacy of workplace
hygiene practices. Inhalation is the most important route of
occupational exposure, but the extent of pulmonary uptake and
retention has not been determined in most occupational settings. Based
on limited data, daily occupational aluminium exposure can range from
<1 mg to 40 mg per 8-h shift.
10.1.2 Evaluation of animal data
Information from toxicokinetic studies in animals is consistent
with and complementary to that obtained in humans. Available studies
indicate that oral bioavailability is low (< 1%) and they provide
valuable information on dose-related concentrations and effects in
target tissues.
In general, the animal studies evaluated were not designed for
risk assessment purposes. However, these studies provide information
relevant to hazard identification and some data on dose-response.
The target tissues in animals are similar to those in humans and
include the bone and nervous system.
In developmental studies in rodents, reduced intrauterine
weight gains were noted in the female offspring of dams gavaged with
aluminium nitrate at doses as low as 13 mg Al/kg body weight per
day, whereas male offspring were not affected at this dose level.
Neurobehavioural and motor development were affected at 100 and
200 mg Al/kg body weight per day when aluminium lactate was
administered to mice in the feed.
There is no indication that aluminium is carcinogenic.
10.1.3 Evaluation of human data
There is no indication that aluminium is acutely toxic after oral
intake. A causal relationship between short-term exposure to high
levels of aluminium in drinking-water and adverse health effects is
not supported by available data.
The information available is insufficient to classify the
carcinogenic risk to humans from exposure to aluminium and aluminium
compounds.
No information is available associating aluminium exposures with
adverse reproductive effects.
Although human exposure to aluminium is widespread, only a few
reports of hypersensitivity exist. In all cases the hypersensitivity
followed dermal or parenteral administration.
In the healthy general population the major health concern
relates to the purported association between the intake of aluminium
and the development and/or acceleration of the onset of AD and other
neurotoxic effects. After an in-depth analysis of the available
epidemiological data (section 8.1.3 - Table 22), considering study
design, exposure measurements and relative risks reported, it was
concluded that:
* On the whole, the positive relationship between aluminium in
drinking-water and AD, which was demonstrated in several
epidemiological studies, cannot be totally dismissed. However,
strong reservations about inferring a causal relationship are
warranted in view of the failure of these studies to account for
demonstrated confounding factors and for total aluminium intake
from all sources.
* Taken together, the relative risks for AD from exposure to
aluminium in drinking-water above 100 �g/litre, as determined in
these studies, are low (less than 2.0). But, because the risk
estimates are imprecise for a variety of methodological reasons,
a population-attributable risk cannot be calculated with
precision. Such imprecise predictions may, however, be useful in
making decisions about the need to control exposures to aluminium
in the general population.
* In light of the above studies, which consider water-borne
aluminium as the sole risk factor, and the recent findings that
water accounts for less than 5% of daily intake of aluminium, it
is difficult to reconcile this with the presumed impact on
cognition. Several lines of investigation should be pursued to
further elucidate the nature of the relationship found in these
studies.
Data from many clinical studies have firmly established the
causal relationship between aluminium exposure in patients with
chronic renal failure (including premature infants with kidney
failure) and the development of encephalopathy (dementia), vitamin-D-
resistant osteomalacia, and microcytic anaemia. The major sources of
aluminium exposure have been indicated as the level of aluminium in
the water used to prepare the dialysis fluids and treatment with
aluminium-containing phosphate binders. Dialysis encephalopathy was
found to be rare when the aluminium level in the dialysis fluid was
maintained below 50 �g/litre. The use of aluminium-free phosphate-
binding agents will also decrease the risk of developing
encephalopathy in patients treated for kidney failure.
In many occupational settings workers are exposed not simply to
aluminium fumes and dust, but rather to a complex mixture of chemicals
and dusts, of which only some contain aluminium. Therefore, it is
essential that urinary aluminium levels be determined as a measure of
exposure (dose), in addition to workplace levels of particulates, if a
causal association between aluminium and effects on the respiratory
and nervous systems is to be developed with any degree of certainty.
Only a few of the published surveys contain such information.
In the past, exposure to stamped pyrotechnic aluminium powder,
usually coated with mineral oil lubricants, has caused pulmonary
fibrosis. However, exposure to other forms of aluminium has not proved
to be a cause of pulmonary fibrosis.
There is no evidence of specific toxicity of any immunological
reaction to the element aluminium as a cause of occupational asthma.
This disorder is considered to be irritant-induced following exposure
during the production of compounds such as aluminium sulfate,
aluminium fluoride and potassium aluminium tetrafluoride, or in
production areas of primary aluminium smelting. Exposure to dust and
particulate in workers fabricating (welding) aluminium have been
associated with an increased level of chronic bronchitis or decreased
lung function. A causative role of non-aluminium fume or dusts, e.g.,
fluorides, cannot be ruled out. It was reported that the chronic
bronchitis noted in aluminium welders was similar to that in welders
working with stainless steel or iron.
There is some evidence that aluminium exposure in welders, miners
and foundry workers can lead to an impairment of cognitive and motor
functions. However, owing to the small cohorts used in some studies,
methodological deficiencies in most studies, and conflicting results
from different studies, there is considerable uncertainty in ascribing
a causative role for aluminium in the neurological effects reported.
In one large study of miners exposed to aluminium powder, the
difficulties in adequately controlling for confounding factors also
lead to much uncertainty in accepting the positive effects observed on
cognition. There is a need for additional studies to clarify the
uncertainties in these data.
10.2 Evaluation of effects on the environment
10.2.1 Exposure
Aluminium is among the least mobile of the major elements of the
geological sedimentary cycle. In air it is present mainly as wind-
blown soil particles. Aluminium-bearing solid phases are relatively
insoluble, particularly in the circumneutral pH range, and so most
natural waters contain low concentrations of dissolved aluminium.
However, the solubility of aluminium is highly dependent on pH,
increasing at both low and high pH values, which reflects the
amphoteric nature of the element. This fact, coupled with the large
reservoir of aluminium in soils and sediments, means that aluminium
concentrations can be substantially enhanced in acidic or poorly
buffered environments subjected to sustained or periodic exposure to
strong acidifying inputs. Under such conditions aluminium can be
transported from soil to surface waters. Afforestation, the cessation
of liming, and sulfide oxidation all contribute to acidification and
thus to the release of previously bound aluminium. However, a major
cause of acidification is acidifying deposition from the atmosphere.
10.2.2 Effects
Laboratory and field studies have revealed that the inorganic
mononuclear aluminium complexes are more toxic to both aquatic
organisms and terrestrial plant species than the organically complexed
forms. Aluminium has been shown to affect adversely both aquatic
organisms and terrestrial plants at concentrations found in acidic or
poorly buffered environments. However, there exist large species,
strain and life-history stage differences in sensitivity. Elevated
concentrations of inorganic monomeric aluminium have detrimental
biological effects that are mitigated in the presence of organic
acids, fluoride, silicate, and high concentrations of calcium and
magnesium. There is a substantial reduction in species richness
associated with the mobilization of the more toxic forms of aluminium
in acid-stressed waters; this loss of species diversity is reflected
at all trophic levels. In waters with non-steady-state aluminium
chemistry (ongoing polymerization), toxicity can become extreme in pH
ranges not normally associated with toxicity.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
In reaching the following conclusions with regards to the risks
from aluminium exposure the Task Group felt it was appropriate to
consider separately the human populations at risk as well as the
environment. The conclusions are, therefore, grouped as to the risk to
the healthy general population, healthy workers and individuals with
impaired kidney function. This is followed by conclusions regarding
the risk to the environment from aluminium.
11.1 Conclusions
11.1.1 Healthy general population
Hazards posed by aluminium to intrauterine and neurological
development and brain function have been identified through animal
studies. However, aluminium has not been shown to pose a health risk
to healthy, non-occupationally exposed humans.
There is no evidence to support a primary causative role of
aluminium in Alzheimer's disease (AD). Aluminium does not induce AD
pathology in vivo in any species, including humans.
The hypothesis that exposure of the elderly population in some
regions to high levels of aluminium in drinking-water may exacerbate
or accelerate AD is not supported by available data.
It has also been hypothesized that particular exposures, either
occupational or via drinking-water, may be associated with non-
specific impaired cognitive function. The data in support of this
hypothesis are currently inadequate.
There is insufficient health-related evidence to justify
revisions to existing WHO Guidelines for aluminium exposure in
healthy, non-occupationally exposed humans. As an example, there is an
inadequate scientific basis for setting a health-based standard for
aluminium in drinking-water.
11.1.2 Subpopulations at special risk
In people of all ages with impaired renal function, aluminium
accumulation has been shown to cause the clinical syndrome of
encephalopathy, vitamin-D-resistant osteomalacia and microcytic
anaemia. The sources of aluminium are haemodialysis fluid and
aluminium-containing pharmaceutical agents (e.g., phosphate binders).
Intestinal absorption can be exacerbated by the use of citrate-
containing products. Patients with renal failure are thus at risk of
neurotoxicity from aluminium.
Iatrogenic aluminium exposure poses a hazard to patients with
chronic renal failure and to premature infants. Every effort should be
made to limit such exposure in these groups.
11.1.3 Occupationally exposed populations
Workers having long-term, high-level exposure to fine aluminium
particulates may be at increased risk of adverse health effects.
However, there are insufficient data from which to develop, with any
degree of certainty, occupational exposure limits with regards to the
adverse effects of aluminium.
Exposure to stamped pyrotechnic aluminium powder, usually
coated with mineral oil lubricants, has caused pulmonary fibrosis
(aluminosis), whereas exposure to other forms of aluminium has not
been proved to cause pulmonary fibrosis. Most reported cases involved
exposure to other potentially fibrogenic agents.
Irritant-induced asthma has been shown to be associated with
inhalation of aluminium sulfate, aluminium fluoride and potassium
aluminium tetrafluoride, and found to occur within the complex
environment of primary aluminium production, especially in potrooms.
11.1.4 Environmental risk
Aluminium-bearing solid phases in the environment are relatively
insoluble, particularly at circumneutral pH values, resulting in low
concentrations of dissolved aluminium in most natural waters.
In acidic or poorly buffered environments subjected to strong
acidifying inputs, concentrations of aluminium can increase to levels
resulting in adverse effects on both aquatic organisms and terrestrial
plants. However, there exist large species, strain and life-history
stage differences in sensitivity to this metal.
The detrimental biological effects from elevated concentrations
of inorganic monomeric aluminium can be mitigated in the presence of
organic acids, fluorides, silicate and high levels of calcium and
magnesium.
A substantial reduction in species richness is associated with
the mobilization of the more toxic forms of aluminium in acid-stressed
waters. This loss of species diversity is reflected at all trophic
levels.
11.2 Recommendations
11.2.1 Public health protection
a) Strategies should be developed to limit exposure for patients
with severly impaired renal function who are exposed to aluminium
from pharmaceutical products.
b) For patients with end-stage renal failure treated by dialysis,
exposure to aluminium should be limited by treatment of the water
and elimination of aluminium contamination of the chemicals used
to prepare dialysis fluid. It is suggested that dialysis fluids
should contain less than 15 �g aluminium/litre.
11.2.2 Recommendations for protection of the environment
a) Soil and water acidification can mobilize aluminium and have
detrimental effects on aquatic and terrestrial lifeforms. Actions
that cause enhanced acidification, e.g., emissions of SO2, NOx
and NH3, should continue to be restricted.
b) When waters are limed to mitigate aluminium toxicity to fish, it
should be done in such a manner that sufficient time is available
for establishment of equilibrium between the aluminium species
before the water reaches the most biologically sensitive or
valuable parts of the system.
12. FURTHER RESEARCH
In order to improve the scientific database on aluminium for the
assessment of the risks to human health, thus decreasing the present
level of uncertainty, the Task Group considered that additional
research in several areas was essential.
12.1 Bioavailability and kinetics
(a) Future studies should address the issue of whether aluminium in
drinking-water is more bioavailable than that from other sources
such as food, consumer products and food additives paying
particular attention to the study of factors that can modify
aluminium uptake by the body. This question is best answered by
studies in humans using 26Al although appropriately designed
animal experiments would provide useful data.
(b) Once aluminium has gained access to the brain, at what rate is it
mobilized or excreted? Is the brain a "one-way sink for
aluminium"? These questions can only be addressed successfully in
an animal model where serial cortical biopsies can be performed.
(c) In view of the importance of achieving international concordance
of accurate chemical analysis for aluminium in environmental and
biological samples, it is recommended that the development of
expert reference laboratories and systems of quality control
should be facilitated and supported.
12.2 Toxicological data
(a) It is thought unlikely that further studies of parenteral
aluminium exposure to normal animals will improve the database
significantly. There is, however, a need for well-designed animal
studies focusing on neurotoxicity and reproductive and
developmental end-points using protocols developed for the
assessment of dose-response relationships for exposures by either
the oral or inhalation routes.
(b) Research in experimental animals on the factors related to the
development of Alzheimer's disease (AD) is hampered by the lack
of a suitable model. However, aged primates or transgenic mice
bearing the human APP gene with familial AD point mutation could
potentially be used for this purpose (with some reservations).
The advantage of these models is that total aluminium intake can
be controlled during the relevant portion of the animal's life
span (about 3-10 years and 1-2 years for primates and mice,
respectively).
(c) There is a requirement for further studies to determine the
effects of diminished renal function on aluminium toxicokinetics.
Such studies should be designed to assess the impact of early-
stage renal disease (varying degrees) and age-related decreases
in renal function on aluminium retention and toxicity.
12.3 Research on the relationship between aluminium exposure and
Alzheimer's disease
(a) Further studies of aluminium levels in bulk tissues from brains
of patients with AD are unlikely to be of value in resolving the
current controversy or enable the distinction to be made between
a pathogenic factor and an epiphenomenon.
(b) The knowledge base is unlikely to be advanced significantly by
further ecological retrospective studies of exposure to aluminium
in drinking-water and AD. Rather, prospective studies of matched
cohorts of elderly people from stable populations living in areas
with high and low levels of aluminium in drinking-water are
required, with special attention to known confounding factors.
Exposure should preferably be assessed through monitoring of
aluminium in the tap water of all cohort members. Although as an
outcome measure it is preferable to compare the pathological
configuration of AD to reference populations, this may be
impractical, and standardized clinical assessments have to be
relied on using carefully considered criteria for the diagnosis
of Alzheimer-type dementia.
(c) As Alzheimer-type neuropathology is almost universal in older
Down's syndrome subjects, and as decline in cognitive function,
as assessed by decline in activities of daily living skills, is
relatively common (25-35), a point study of rates of cognitive
impairment in Down's syndrome patients over 40 years of age from
high and low water aluminium areas might be performed relatively
quickly and with small numbers of subjects. Unbiased subject
recruitment would, however, present problems.
(d) If the hypothesis that higher drinking-water aluminium levels are
an accelerating or exacerbating factor for AD is correct, a
neuropathological assessment of elderly subjects dying of an
unrelated cause requiring routine postmortem examination (e.g.,
passengers in road traffic accident) ought to reveal a greater
burden of Alzheimer's pathology in those from areas with high
aluminium levels in drinking-water than those from areas with low
aluminium levels.
12.4 Occupational exposure
The possible adverse effects of occupational aluminium exposure
on the health of workers should be a specific priority for future
research.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
At the Thirty-third Meeting of the Joint FAO/WHO Expert Committee
on Food Additives and Food Contaminants, a provisional tolerable
weekly intake (PTWI) of 7.0 mg/kg body weight was recommended. This
value included the intake of aluminium from its use as a food additive
(FAO/WHO, 1989).
On the basis of non-health-related criteria, a WHO Drinking-Water
Guideline of 0.2 mg/litre has been proposed (WHO, 1993). No health-
based guideline was recommended.
The carcinogenic risk from aluminium and its compounds has not
been evaluated by the International Agency for Research on Cancer
(IARC). However, that there is sufficient evidence that certain
exposures occurring during aluminium production cause cancer in
humans. Most epidemiological studies suggest pitch volatiles to be
the causative agent (IARC, 1987).
Regulatory standards for aluminium and some aluminium compounds
established by national bodies in several countries and the European
Union are summarized in the legal file of the International Register
of Potentially Toxic Chemicals (IRPTC, 1993).
REFERENCES
Abernathy AR, Larson GL, & Mathews RC (1984) Heavy metals in the
surficial sediments of Fontana Lake, North Carolina. Water Res,
18(3): 351-354.
Abrahamsen G, Stuanes AO, & Tveite B (1994) Long-term experiments with
acid rain in Norwegian forest ecosystems. Berlin, Heidelberg, New
York, Springer-Verlag, pp 297-331.
Abramson MJ, Wlodarczyk JH, Saunders NA, & Hensley MJ (1989) Does
aluminum smelting cause lung disease? Am Rev Respir Dis, 139:
1042-1057.
Adler AJ & Berlyne GM (1985) Duodenal aluminum absorption in the rat:
effect of vitamin D. Am J Physiol, 249: G/209-G/213.
Albers PH & Camardese MB (1993a) Effects of acidification on metal
accumulation by aquatic plants and invertebrates. 1. Constructed
wetlands. Environ Toxicol Chem, 12: 959-967.
Albers PH & Camardese MB (1993b) Effects of acidification on metal
accumulation by aquatic plants and invertebrates. 2. Wetlands, ponds
and small lakes. Environ Toxicol Chem, 12: 969-976.
Alberts JJ, Leyden DE, & Patterson TA (1976) Distribution of total Al,
Cd, Co, Cu, Ni and Zn in the tongue of the ocean and the northwestern
Atlantic Ocean. Mar Chem, 4: 51-56.
Alderman FR & Gitelman HJ (1980) Improved electrothermal determination
of aluminum in serum by atomic absorption spectroscopy. Clin Chem,
26: 258-260.
Alfrey AC (1978) Dialysis encephalopathy syndrome. Annu Rev Med,
29: 93-98.
Alfrey AC (1980) Aluminum metabolism in uremia. Neurotoxicology,
1: 43-53.
Alfrey AC, Mishell JM, Burks J, Contiguglia SR, Rudolph H, Lewin E, &
Holmes JH (1972) Syndrome of dyspraxia and multifocal seizures
associated with chronic hemodialysis. Trans Am Soc Artif Intern
Organs, 18: 257-261.
Alfrey AC, Legendre GR, & Kaehny WD (1976) The dialysis encephalopathy
syndrome: Possible aluminum intoxication. N Engl J Med, 294:
184-188.
Alfrey AC, Hegg A, & Craswell P (1980) Metabolism and toxicity of
aluminium in renal failure. Am J Clin Nutr, 33: 1509-1516.
Alfrey AC, Sedman A, & Chan YL (1985) The compartmentalization and
metabolism of aluminum in uremic rats. J Lab Clin Med, 105: 227-233.
Allen VG & Fontenot JP (1984) Influence of aluminium as sulfate,
chloride and citrate on magnesium and calcium metabolism in sheep.
J Anim Sci, 59: 798-804.
Allen VG, Horn FP, & Fontenot JP (1986) Influence of ingestion of
aluminium, citric acid and soil on mineral metabolism of lactating
beef cows. J Anim Sci, 62: 1396-1403.
Altmann P, Dhanesha U, Hamon C, Cunningham J, Blair J, & Marsh F
(1989) Disturbance of cerebral function by aluminium in haemodialysis
patients without overt aluminium toxicity. Lancet, 2: 7-12.
Amundsen CE, Hanssen JE, Semb A, & Steinnes E (1992) Long-range
atmospheric transport of trace elements to southern Norway. Atmos
Environ, A26(7): 1309-1324.
Andersen JR (1987) Graphite furnace atomic absorption spectrometric
screening method for the determination of aluminium in haemodialysis
concentrates. J Anal Atmos Spectrom, 2: 257-259.
Andorn MC, Britton RS, & Bacon BR (1990) Evidence that lipid
peroxidation and total iron are increased in Alzheimer's brain.
Neurobiol Aging, 11: 316.
Andreoli SP, Bergstein JM, & Sherrard DJ (1984) Aluminum intoxication
from aluminum-containing phosphate binders in children with azotemia
not undergoing dialysis. N Engl J Med, 310: 1079-1084.
Arieff AI, Cooper JD, Armstrong D, & Lazarowitz VC (1979) Dementia,
renal failure, and brain aluminum. Ann Int Med, 90: 741-747.
Aston RJ, Sadler K, Milner AGP, & Lynam S (1987) The effects of pH and
related factors on stream invertebrates. Leatherhead, Central
Electricity Generating Board, Technology Planning and Research
Division, Central Electricity Research Laboratories (Report No.
TPRD/L/2792/N84).
ATSDR (1992) Toxicological profile for aluminum and compounds.
Atlanta, Georgia, Agency for Toxic Substances and Disease Registry,
136 pp (TP-91/01).
Bache BW (1980) The acidification of soils. In: Hutchinson TC & Havas
M ed. Effects of acid precipitation on terrestrial ecosystems. New
York, London, Plenum Press, pp 183-202.
Bache BW (1986) Aluminium mobilization in soils and waters. J Geol Soc
(Lond), 143: 699-706.
Baker JP & Schofield CL (1982) Aluminum toxicity to fish in acidic
waters. Water Air Soil Pollut, 18: 289-309.
Banks WA & Kastin AJ (1985) Aluminum alters the permeability of the
blood-brain barrier to some non-peptides. Neuropharmacology, 24:
407-412.
Banks WA, Kastin AJ, Fasold MB, Barrera CM, & Augereau G (1988a)
Studies of the slow bidirectional transport of iron and transferrin
across the blood-brain barrier. Brain Res Bull, 21: 881-885.
Banks WA, Kastin AJ, & Fasold MB (1988b) Differential effect of
aluminum on the blood-brain barrier transport of peptides, technetium
and albumin. J Pharmacol Exp Ther, 244: 579-585.
Bartlett RJ & Riego DC (1972a) Toxicity of hydroxy aluminum in
relation to pH and phosphorus. Soil Sci, 114(3): 194-200.
Bartlett RJ & Riego DC (1972b) Effect of chelation on the toxicity of
aluminum. Plant Soil, 37: 419-423.
Bast-Pettersen R, Drablos PA, Goffeng LO, Thomassen Y, & Torres CG
(1994) Neuropsychological deficit among elderly workers in aluminium
production. Am J Ind Med, 25: 649-662.
Baxter MJ, Burrell JA, Crews HM, & Massey RC (1988) The effects of
fluoride on the leaching of aluminium saucepans during cooking. Food
Addit Contam, 5: 651-656.
Beavington F & Cawse PA (1979) The deposition of trace elements and
major nutrients in dust and rainwater in northern Nigeria. Sci Total
Environ, 13: 263-274.
Benett RW, Persaud TVN, & Moore KL (1975) Experimental studies on the
effects of aluminum on pregnancy and fetal development. Anat Anz,
138: 365-378.
Bengtsson B-E (1978) Use of a harpacticoid copepod in toxicity tests.
Mar Pollut Bull, 9(9): 238-241.
Benninger LK & Wells JT (1993) Sources of sediment to the Neuse River
estuary, North Carolina. Mar Chem, 43: 137-156.
Berd�n M, Clarke N, Danielsson LG, & Spar�n A (1994) Aluminium
speciation: variations caused by the choice of analytical method and
by sample storage. Water Air Soil Pollut, 72: 213-233.
Berg DJ & Burns TA (1985) The distribution of aluminum in the tissues
of three fish species. J Freshw Ecol, 3: 113-120.
Berggren D (1992) Speciation and mobilization of aluminium and cadmium
in podzols and cambisols of S. Sweden. Water Air Soil Pollut,
62: 125-156.
Bergkvist B (1987) Leaching of metals from forest soils as influenced
by tree species and management. For Ecol Manage, 22: 29-56.
Berlyne GM, Yagil R, Ben-Ari J, Weinberger G, Knopf E, & Danovitch GM
(1972) Aluminium toxicity in rats. Lancet, 1: 564-567.
Bernuzzi V, Desor D, & Lehr PR (1986) Effects of prenatal aluminium
exposure on neuromotor maturation in the rat. Neurobehav Toxicol
Teratol, 8: 115-119.
Bernuzzi V, Desor D, & Lehr PR (1989a) Effects of postnatal aluminium
lactate exposure on neuromotor maturation in the rat. Bull Environ
Contam Toxicol, 42: 451-455.
Bernuzzi V, Desor D, & Lehr PR (1989b) Developmental alterations in
offspring of female rats orally intoxicated by aluminum chloride or
lactate during gestation. Teratology, 40: 21-27.
Berrill M, Hollett L, Margosian A, & Hudson J (1985) Variation in
tolerance to low environmental pH by the crayfish Orconectes
rusticus, O. propinquus, and Cambarus robustus. Can J Zool, 63:
2586-2589.
Bertholf RL, Wills MR, & Savory J (1984) Quantitative study of
aluminum binding to human serum albumin and transferrin by chelex
competitive binding assay. Biochem Biophys Res Commun, 125: 1020-
1024.
Bettinelli M, Baroni U, Fontana F, & Poisetti P (1985) Evaluation of
the L'vov platform and matric modification for the determination of
aluminium in serum. Analyst, 110: 19-22.
Bhamra RK & Costa M (1992) Trace elements aluminum, arsenic, cadmium,
mercury, and nickel. In: Lippmann M ed. Environmental toxicants: Human
exposures and their health effects. New York, Van Nostrand Reinhold
Company, pp 575-632.
Biesinger KE & Christensen GM (1972) Effects of various metals on
survival, growth, reproduction, and metabolism of Daphnia magna.
J Fish Res Board Can, 29: 1691-1700.
Birchall JD (1991) The role of silicon in aluminium toxicity. In: Lord
Walton of Detchant ed. Alzheimer's disease and the environment.
London, Royal Society of Medicine Services Ltd, pp 70-77 (Round Table
Series No. 26).
Bird SC, Brown SJ, & Vaughan E (1990) The influence of land management
on stream water chemistry. In: Edwards RW, Gee AS, & Stoner JH ed.
Acid waters in Wales. Dordrecht, Boston, London, Kluwer Academic
Publishers, pp 241-253.
Blotcky AJ, Claassen JP, Roman FR, Rack EP, & Badakhsh S (1992)
Determination of aluminum by chemical and instrumental neutron
activation analysis in biological standard reference material and
human brain tissue. Anal Chem, 64: 2910-2913.
Blume H-P & Br�mmer G (1991) Prediction of heavy metal behavior in
soil by means of simple field tests. Ecotoxicol Environ Saf, 22:
164-174.
B�hler-Sommeregger K & Lindemayr H (1986) Contact sensitivity to
aluminium. Contact Dermatitis, 15: 278-281.
Booth CE, McDonald DG, Simons BP, & Wood CM (1988) Effects of aluminum
and low pH on net ion fluxes and ion balance in the brook trout
( Salvelinus fontinalis). Can J Fish Aquat Sci, 45: 1563-1574.
Borg H (1987) Trace metals and water chemistry of forest lakes in
northern Sweden. Water Res, 21(1): 65-72.
Boult S, Collins DN, White KN, & Curtis CD (1994) Metal transport in a
stream polluted by acid mine drainage - the Afon Goch, Anglesey, UK.
Environ Pollut, 84: 279-284.
Bouman AA, Platenkamp AJ, & Posma FD (1986) Determination of aluminium
in human tissues by flameless atomic absorption spectroscopy and
comparison of reference values. Ann Clin Biochem, 23: 97-101.
Boxman AW, Krabbendam H, Bellemakers MJS, & Roelofs JGM (1991) Effects
of ammonium and aluminium on the development and nutrition of Pinus
nigra in hydroculture. Environ Pollut, 73: 119-136.
Brady DJ, Edwards DG, Asher CJ, & Blamey FPC (1993) Calcium
amelioration of aluminium toxicity effects on root hair development in
soybean [ Glycine max (L.) Merr.]. New Phytol, 123: 531-538.
Br�unlich H, Fleck C, Kersten L, Stein G, Laske V, M�ller A, & Keil E
(1986) Renal effects of aluminium in uraemic rats and rats with intact
kidney function. J Appl Toxicol, 6: 55-59.
Brezonik PL, Mach CE, Downing G, Richardson N, & Brighman M (1990)
Effects of acidification on minor and trace metal chemistry in Little
Rock Lake, Wisconsin. Environ Toxicol Chem, 9: 871-885.
Bringmann G & K�hn R (1959) [The toxic effects of wastewater on
aquatic bacteria, algae, and small crustacea.] Gesundheits-Ingenieur,
80(4): 115-120 (in German).
Brown DJA (1983) Effect of calcium and aluminum concentrations on the
survival of brown trout ( Salmo trutta) at low pH. Bull Environ
Contam Toxicol, 30: 582-587.
Bruland KW (1983) Trace elements in sea-water. In: Riley JP & Chester
R ed. Chemical oceanography. New York, London, Academic Press, vol 8,
pp 157-220.
Brumbaugh WG & Kane DA (1985) Variability of aluminum concentrations
in organs and whole bodies of smallmouth bass ( Micropterus
dolimieui). Environ Sci Technol, 19: 828-831.
Buckler DR, Mehrle PM, Cleveland L, & Dwyer FJ (1987) Influence of pH
on the toxicity of aluminium and other inorganic contaminants to east
coast striped bass. Water Air Soil Pollut, 35: 97-106.
Budavari S ed. (1989) The Merck index: An encyclopedia of chemicals,
drugs and biologicals, 11th ed. Rahway, New Jersey, Merck & Co.,
pp 54-60.
Buergel PM & Soltero RA (1983) The distribution and accumulation of
aluminum in rainbow trout following a whole-lake alum treatment.
J Freshw Ecol, 2(1): 37-44.
Bugiani O & Ghetti B (1982) Progressing encephalomyelopathy with
muscular athropy, induced by aluminum powder. Neurobiol Aging,
3: 209-222.
Bull KR & Hall JR (1986) Aluminium in the rivers Esk and Duddon,
Cumbria, and their tributaries. Environ Pollut, B12: 165-193.
Buratti M, Caravelli G, Calzaferri G, & Colombi A (1984) Determination
of aluminium in body fluids by solvent extraction and atomic
absorption spectroscopy with electro-thermal atomization. Clin Chim
Acta, 141: 253-259.
Burnatowska-Hledin MA & Mayor GH (1984) The effects of aluminum
loading on selected tissue calcium and magnesium concentrations in
rats. Biol Trace Elem Res, 6: 531-535.
Burnel D, Hutin MF, Netter P, Kessler M, Huriet C, & Gaucher A (1982)
Int�r�t du dosage de l'aluminium osseux par polarographie
impulsionelle. Pathol Biol, 30: 27-32.
Burton TM & Allan JW (1986) Influence of pH, aluminum, and organic
matter on stream invertebrates. Can J Fish Aquat Sci, 43: 1285-1289.
Bush AI, Pettingell WH, Multhaup G, Paradis MD, Vonsattel J-P, Gusella
JF, Beyreuther K, Masters CL, & Tanzi RE (1994) Rapid induction of
Alzheimer A� amyloid formation by zinc. Science, 265: 1464-1467.
Caines LA, Watt AW, & Wells DE (1985) The uptake and release of some
trace metals by aquatic bryophytes in acidified waters in Scotland.
Environ Pollut, B10: 1-18.
Cakir A, Sullivan TW, & Struwe FJ (1977) Effect of supplemental
aluminum on the value of fertilizer phosphates in turkey diets. Poult
Sci, 56: 544-549.
Cam JM, Luck VA, Eastwood JB, & De Wardener HE (1976) The effect of
aluminium hydroxide orally on calcium, phosphorus and aluminium in
normal subjects. Clin Sci Mol Med, 51: 407-414.
Cann CE, Prussin SG, & Gordan GS (1979) Aluminum uptake by the
parathyroid glands. J Clin Endocrinol Metab, 49: 543-545.
Carri�re D, Fischer KL, Peakall DB, & Anghern P (1986) Effects of
dietary aluminum sulphate on reproductive success and growth of ringed
turtle doves ( Streptopelia risoria). Can J Zool, 64: 1500-1505.
Cawse PA (1974) A survey of atmospheric trace elements in the U.K.
(1972-73). Harwell, United Kingdom Atomic Energy Authority, 84 pp
(Report No. AERE-R 7669).
Cawse PA (1987) Trace and major elements in the atmosphere at rural
locations in Great Britain, 1972-1981. In: Coughtrey PJ, Martin MH, &
Unsworth MH ed. Pollutant transport and fate in ecosystems. Oxford,
London, Blackwell Scientific Publications, pp 89-112 (British
Ecological Society Special Publication No. 6).
Chadwick DJ & Whelan J (1992) Aluminium in biology and medicine. New
York, Chichester, Brisbane, Toronto, John Wiley and Sons, 138 pp
(Ciba Foundation Symposium No. 169).
Chainy GBN, Sahoo A, & Swain C (1993) Effect of aluminum on lipid
peroxidation of cerebral hemisphere of chick. Bull Environ Contam
Toxicol, 50: 85-91.
Chan YL, Alfrey AC, Posen S, Lissner D, Hills E, Dunstan CR, & Evans
RA (1983) Effect of aluminum on normal and uremic rats: tissue
distribution, vitamin D metabolites, and quantitative bone histology.
Calcif Tissue Int, 35: 344-351.
Chan JC, Jacob M, Brown S, Savory J, & Wills MR (1988) Aluminum
metabolism in rats: effects of vitamin D, dihydrotachysterol,
1,25-dihydroxyvitamin D and phosphate binders. Nephron, 48: 61-64.
Channon SM, Arfeen S, & Ward MK (1988) Long-term accumulation of
aluminium in patients with renal failure. Trace Elem Med, 5:
154-157.
Chappuis P, Duhaux L, Paolaggi F, De Vernejoul MC, & Rousselet F
(1988) Analytical problems encountered in determining aluminum status
from hair in controls and hemodialyzed patients. Clin Chem, 34:
2253-2255.
Chen W, Monnat RJ, Chen M, & Mottet K (1978) Aluminum induced
pulmonary granulomatosis. Hum Pathol, 9: 705-712.
Chenery EM (1955) A preliminary study of aluminium and the tea bush.
Plant Soil, VI(2): 174-200.
Cherroret G, Bernuzzi V, Desor D, Hutin M-F, Burnel D, & Lehr PR
(1992) Effects of postnatal aluminum exposure on choline
acetyltransferase activity and learning abilities in the rat.
Neurotoxicol Teratol, 14: 259-264.
Chester R & Bradshaw GF (1991) Source control on the distribution of
particulate trace metals in the North Sea atmosphere. Mar Pollut Bull,
22(1): 30-36.
Chou L & Wollast R (1989) Distribution and geochemistry of aluminium
in the Mediterranean Sea. Water Pollut Res Rep, 13: 232-240.
Chow WM (1992) Behaviour of aluminium and its ecological significance
in natural waters. In: Schulhof P ed. IWSA International Workshop on
Aluminium in Drinking Water, Hong Kong, 15-17 January 1992. Oxford,
London, Blackwell Scientific Publications, pp 1-10.
Christie H, Mackay RJ, & Fisher AM (1963) Pulmonary effects of
inhalation of aluminum by rats and hamsters. Ind Hyg J, 24: 47-56.
Christophersen N & Seip HM (1982) A model for streamwater chemistry at
Birkenes, Norway. Water Resour Res, 18(4): 977-996.
Clark KL & Hall RJ (1985) Effects of elevated hydrogen ion and
aluminum concentrations on the survival of amphibian embryos and
larvae. Can J Zool, 63: 116-123.
Clark KL & Lazerte BD (1985) A laboratory study of the effects of
aluminum and pH on amphibian eggs and tadpoles. Can J Fish Aquat Sci,
42: 1544-1551.
Clarkson DT (1967) Aluminium tolerance in species within the genus
Agrostis. J Ecol, 54: 167-178.
Clayton DB (1989) Water polution at Lowermoore North Cornwall: Report
of the Lowermoor incident health advisory committee. Truro, United
Kingdom, Cornwall District Health Authority, 22 pp.
Clayton RM, Sedowofia SKA, Rankin JM, & Manning A (1992) Long-term
effects of aluminium on the fetal mouse brain. Life Sci, 51:
1921-1928.
Clemmensen O & Knudsen HE (1980) Contact sensitivity to aluminium in a
patient hyposensitized with aluminium precipitated grass pollen.
Contact Dermatitis, 6: 305-308.
Cleveland L, Little EE, Hamilton SJ, Buckler DR, & Huhn JB (1986)
Interactive toxicity of aluminum and acidity to early life stages of
brook trout. Trans Am Fish Soc, 115: 610-620.
Cleveland L, Little EE, Wiedmeyer RH, & Buckler DR (1989) Chronic
no-observed-effect concentrations of aluminum for brook trout exposed
in low-calcium, dilute acidic water. In: Lewis TE ed. Environmental
chemistry and toxicology of aluminum. Proceedings of the 194th Annual
Meeting of the American Chemical Society, New Orleans (LA), 30
August-4 September 1987. Chelsea, Michigan, Lewis Publishers Inc.,
pp 229-246.
Cleveland L, Buckler DR, & Brumbaugh WG (1991) Residue dynamics and
effects of aluminum on growth and mortality in brook trout. Environ
Toxicol Chem, 10: 243-248.
Colomina MT, Gomez M, Domingo JL, Llobet JM, & Corbella J (1992)
Concurrent ingestion of lactate and aluminum can result in development
toxicity in mice. Res Commun Chem Pathol Pharmacol, 77: 95-106.
Colomina MT, Gomez M, Domingo JL, Llobet JM, & Corbella J (1994) Lack
of maternal and developmental toxicity in mice given high doses of
aluminium hydroxide and ascorbic acid during gestation. Pharmacol
Toxicol, 74: 236-239.
Commisaris RL, Cordon JJ, Sprague S, Keiser J, Mayov G, & Rich R
(1982) Behavioral changes in rats after chronic aluminium and
parathyroid hormone administration. Neurobehav Toxicol Teratol,
4: 403-410.
Connor DJ, Jope RS, & Harrell LE (1988) Chronic, oral aluminum
administration to rats: Cognition and cholinergic parameters.
Pharmacol Biochem Behav, 31: 467-474.
Coriat A-M & Gillard RD (1986) Beware the cups that cheer. Nature
(Lond), 321: 570.
Corrin B (1963) Aluminium pneumoconiosis. II. Effect on the rat lung
of intratracheal injections of stamped aluminium powders containing
different lubricating agents and of a granular aluminium powder.
Br J Ind Med, 20: 268-276.
Cosby BJ, Wright RF, Hornberger GM, & Galloway JN (1985) Modeling the
effects of acid deposition: estimation of long-term water quality
responses in a small forested catchment. Water Resour Res, 21(11):
1591-1601.
Cosby BJ, Whitehead PG, & Neale R (1986) A preliminary model of
long-term changes in stream acidity in southwestern Scotland. J
Hydrol, 84: 381-401.
Cournot-Witmer G, Zinngraff J, Plachot JJ, Escaig F, Lefevre R,
Boumati P, Bourdeau A, Garab�dian M, Galle P, Bourdon R, Dr�eke T, &
Balsan S (1981) Aluminium localization in bone from hemodialyzed
patients: Relationship to matrix mineralization. Kidney Int, 20:
375-385.
Courtijn E, Vandecasteele C, Yu ZQ, Nagels M, & Dams R (1987)
Determination and speciation of aluminium in acidified surface waters.
In: Witters H & Vanderborght O ed. Ecophysiology of acid stress in
aquatic organisms: Proceedings of an International Symposium, Antwerp,
13-16 January 1987. Antwerp, Belgian Royal Zoological Society,
pp 33-44.
Courtijn E, Vandecasteele C, & Dams R (1990) Speciation of aluminium
in surface water. Sci Total Environ, 90: 191-202.
Cranmer JM, Wilkins JD, Cannon DJ, & Smith L (1986) Fetal-placental-
maternal uptake of aluminum in mice following gestational exposure:
Effect of dose and route of administration. Neurotoxicology, 7:
601-608.
Crapper DR & Dalton AJ (1973a) Alterations in short term retention,
conditioned avoidance response acquisition and motivation following
aluminum induced neurofibrillary degeneration. Physiol Behav, 10:
925-933.
Crapper DR & Dalton AJ (1973b) Aluminum induced neurofibrillary
degeneration, brain electrical activity and alterations in acquisition
and retention. Physiol Behav, 10: 935-945.
Crapper DR, Krishnan SS, & Dalton AJ (1973) Brain aluminum
distribution in Alzheimer's disease and experimental neurofibrillary
degeneration. Science, 180: 511-513.
Crapper DR, Krishnan SS, & Quittkat S (1976) Aluminum, neurofibrillar
degeneration and Alzheimer's disease. Brain, 99: 67-80.
Crapper DR, Quittkat S, Krishnan SS, Dalton AJ, & De Boni U (1980)
Intranuclear aluminum content in Alzheimer's disease, dialysis
encephalopathy and experimental aluminum encephalopathy. Acta
Neuropathol, 50: 19-24.
Crapper McLachlan DR (1986) Aluminum and Alzheimer's disease.
Neurobiol Aging, 7: 525-532.
Crapper McLachlan DR & Farnell BJ (1986) Cellular mechanisms of
aluminium toxicity. Ann Ist Super Sanita, 22: 697-702.
Crapper McLachlan DR, Dam TV, Farnell BJ, & Lewis PN (1983) Aluminum
inhibition of ADP-ribosylation in vivo and in vitro. Neurobehav
Toxicol Teratol, 5: 645-647.
Crapper McLachlan DR, Lukiw WJ, & Kruck TPA (1989) New evidence for
an active role of aluminum in Alzheimer's disease. Can J Neurol Sci,
16: 490-497.
Crapper McLachlan DR, Dalton AJ, Kruck TPA, Bell MY, Smith WL, Kalow
W, & Andrews DF (1991) Intramuscular desferrioxamine in patients with
Alzheimer's disease. Lancet, 337: 1304-1308.
Cronan CS, Kelly JM, Schofield CL, & Goldstein RA (1987) Aluminium
geochemistry and tree toxicity in forests exposed to acidic
deposition. In: Perry R ed. Acid rain: Scientific and technical
advances. London, Selper, pp 649-656.
Cuenca G & Herrera R (1987) Ecophysiology of aluminium in terrestrial
plants, growing in acid and aluminium-rich tropical soils. In: Witters
H & Vanderborght O ed. Ecophysiology of acid stress in aquatic
organisms: Proceedings of an International Symposium, Antwerp, 13-16
January 1987. Antwerp, Belgian Royal Zoological Society, pp 57-73.
Cummins CP (1986) Effects of aluminium and low pH on growth and
development in Rana temporaria tadpoles. Oecologia, 69: 248-252.
Dabeka RW & McKenzie AD (1990) Aluminium levels in Canadian infant
formulae and estimation of aluminium intakes from formulae by infants
0-3 months old. Food Addit Contam, 7(2): 275-282.
Dahlgren RA & Ugolini FC (1989) Aluminum fractionation of soil
solutions from unperturbed and tephra-treated spodosols, Cascade
Range, Washington, USA. Soil Sci Soc Am J, 53: 559-566.
Dalziel TRK, Morris R, & Brown DJA (1986) The effects of low pH, low
calcium concentrations and elevated aluminium concentrations on sodium
fluxes in brown trout, Salmo trutta L. Water Air Soil Pollut, 30:
569-577.
Dalziel TRK, Morris R, & Brown DJA (1987) Sodium uptake inhibition
in brown trout, Salmo trutta exposed to elevated aluminium
concentrations at low pH. In: Witters H & Vanderborght O ed.
Ecophysiology of acid stress in aquatic organisms: Proceedings of
an International Conference, Antwerp, 13-16 January 1987. Antwerp,
Belgian Royal Zoological Society, pp 421-434.
Dantzman CL & Breland HL (1969) Chemical status of some water sources
in south central Florida. Soil Crop Sci Soc Fla Proc, 29: 18-28.
Day JP, Barker J, Evans LJA, Perks J, Seabright PJ, Ackrill P, Lilley
JS, Drumm PV, & Newton GWA (1991) Aluminium absorption studied by
26Al tracer. Lancet, 337: 1345.
Day JP, Barker J, King SJ, Miller RV, Templar J, Lilley JS, Drumm PV,
Newton GWA, Fifield LK, Stone JOH, Allan GL, Edwardson JA, Moore PB,
Ferrier IN, Priest ND, Newton D, Talbot RJ, Brock JH, Sanchez L,
Dobson CB, Itzhaki RF, Radunovic A, & Bradbury MWB (1994) Biological
chemistry of aluminium studied using 26Al and accelerator mass
spectrometry. Nucl Instrum Methods Phys Res, B92: 463-468.
Dean JR (1989) Ion chromatographic determination of aluminium with
ultraviolet spectrophotometric detection. Analyst, 114: 165-168.
De Boni U, Seger M, & Crapper McLachlan DR (1980) Functional
consequences of chromatin bound aluminum in cultured human cells.
In: Liss L ed. Aluminum neurotoxicity. Park Forest, South Illinois,
Pathotox Publishers, Inc., pp 65-81.
De La Campa MRF, Garcia MED, & Sanz-Medel A (1988) Room-temperature
liquid phosphorimetry of the aluminium-ferron chelate in micellar
media. Determination of aluminium. Anal Chim Acta, 212: 235-243.
Delonay AJ, Little EE, Woodward DF, Brumbauch WG, Farag AM, & Rabeni
CF (1993) Sensitivity of early-life-stage golden trout to low pH and
elevated aluminum. Environ Toxicol Chem, 12: 1223-1232.
Den Dooren De Jong LE (1971) Tolerance of Azotobacter for metallic
and non-metallic ions. Antonie van Leeuwenhoek, 37: 119-124.
De Villiers PR (1962) The chemical composition of the water of the
Orange River at Vioolsdrif, Cape Province. Ann Geol Opname Repub
S Afr, 1: 197-208.
De Vuyst P, Dumortier P, Rickaert F, Van De Weyer R, Lenclud C, &
Yernault JC (1986) Occupational lung fibrosis in an aluminium
polisher. Eur J Respir Dis, 68: 131-140.
De Vuyst P, Dumortier P, Schanden� L, Estenne M, Verhest A, & Yernault
JC (1987) Sarcoid-like lung granulomatosis induced by aluminum dust.
Am Rev Respir Dis, 135: 493-497.
DIN (1993) [German standard procedures for testing water, sewage water
and sludge: Physical, chemical, biological and bacterial procedures.]
Society of German Chemists, Expert Group on Water Chemistry and German
Institute for Standardization (DIN), Standardization Committee for
Water (NAW), vol II, pp E9/1-11, E22/1-21 (DIN 38 406, Sections 9
and 22) (in German).
Dinman BD (1983) Aluminium, alloys and compounds. In: Parmeggiani
L ed. Encyclopedia of occupational health and safety. Geneva,
International Labour Organisation, vol 1, pp 131-135.
Dinman BD (1988a) Aluminium in the lung: the pyropowder conundrum.
J Occup Med, 29(11): 869-876.
Dinman BD (1988b) Alumina-related pulmonary disease. J Occup Med,
30: 328-335.
Di Paolo JA & Casto BC (1979) Quantitative studies of in vitro
morphological transformation of Syrian hamster cells by inorganic
metal salts. Cancer Res, 39: 1008-1013.
Dobbs AJ, French P, Gunn AM, Hunt DTE, & Winnard DA (1989) Aluminum
speciation and toxicity in upland waters. In: Lewis TE ed.
Environmental chemistry and toxicology of aluminum. Proceedings of the
194th Annual Meeting of the American Chemical Society, New Orleans,
LA, 30 August-4 September 1987. Chelsea, Michigan, Lewis Publishers
Inc., pp 209-228.
Doese M (1938) [Industrial medicine studies on injury to health caused
by aluminium, in particular lung disease from aluminium dust.] Arch
Gewerbepathol, 8: 501-531 (in German).
Domingo JL (1995) Reproductive and developmental toxicity of
aluminium: A review. Neurotoxicol Teratol, 17(4): 515-521.
Domingo JL, Paternain JL, Llobet JM, & Corbella J (1987a) Effects of
oral aluminum administration on perinatal and postnatal development in
rats. Res Commun Chem Pathol Pharmacol, 57: 129-132.
Domingo JL, Llobet JM, Gomez M, Tomas JM, & Corbella J (1987b)
Nutritional and toxicological effects of short-term ingestion of
aluminum by the rat. Res Commun Chem Pathol Pharmacol, 56: 409-419.
Domingo JL, Paternain JL, Llobet JM, & Corbella J (1987c) The effects
of aluminium ingestion on reproduction and postnatal survival in rats.
Life Sci, 41: 1127-1131.
Domingo JL, Gomez M, Bosque MA, & Corbella J (1989) Lack of
teratogenicity of aluminum hydroxide in rats. Life Sci, 45: 243-247.
Donald JM, Golub MS, Gershwin ME, & Keen CL (1989) Neurobehavioral
effects in offspring of mice given excess aluminum in diet during
gestation and lactation. Neurotoxicol Teratol, 11: 345-351.
Dorfman D (1977) Tolerance of Fundulus heteroclitus to different
metals in salt waters. Bull NJ Acad Sci, 22(2): 21-23.
Drew RT, Gupta BN, Bend JR, & Hook GER (1974) Inhalation studies with
a glycol complex of aluminum-chloride-hydroxide. Arch Environ Health,
28: 321-326.
Driscoll CT (1984) A procedure for the fractionation of aqueous
aluminum in dilute acidic waters. Int J Environ Anal Chem, 16:
267-283.
Driscoll CT, Baker JP, Bisogni JJ, & Schofield CL (1980) Effect of
aluminium speciation on fish in dilute acidified waters. Nature
(Lond), 284: 161-164.
Driscoll CT, Johnson NM, Likens GE, & Feller MC (1988) Effects of
acidic deposition on the chemistry of headwater streams: a comparison
between Hubbard Brook, New Hampshire, and Jamieson Creek, British
Columbia. Water Resour Res, 24(2): 195-200.
Driscoll CT, Wyskowski BJ, Destaffan P, & Newton RM (1989) Chemistry
and transfer of aluminum in a forested watershed in the Adirondack
region of New York, USA. In: Lewis TE ed. Environmental chemistry and
toxicology of aluminum. Proceedings of the 194th Annual Meeting of the
American Chemical Society, New Orleans, LA, 30 August-4 September
1987. Chelsea, Michigan, Lewis Publishers Inc., pp 83-105.
Duce RA, Hoffman GL, & Zoller WH (1975) Atmospheric trace metals at
remote northern and southern hemisphere sites: pollution or natural?
Science, 187: 59-61.
Dunemann L & Schwedt G (1984) [Element species analysis in soil
solutions by gel chromatography and chemical reaction detectors.]
Fresenius Z Anal Chem, 317: 394-399 (in German).
Eaton JG, Swenson WA, McCormick JH, Simonson TD, & Jensen KM (1992) A
field and laboratory investigation of acid effects on largemouth bass,
rock bass, black crappie, and yellow perch. Trans Am Fish Soc, 121:
644-658.
Ecelbarger CA & Greger JL (1991) Dietary citrate and kidney function
affect aluminum, zinc, and iron utilization in rats. J Nutr, 121:
1755-1762.
Ecker F-J, Hirai E, & Chohji T (1990) The release of heavy metals from
snowpacks on the Japan Sea side of Japan. Environ Pollut, 65:
141-153.
Edwardson JA (1992) Alzheimer's disease and brain mineral metabolism.
In: Munro H & Schlierf G ed. Nutrition of the elderly. New York,
Raven Press, pp 193-202 (Nestl� Nutrition Workshop Series, Volume 29).
Edwardson JA, Candy JM, Ince PG, McArthur FK, Morris CM, Oakley AE,
Taylor GA, & Bjertness E (1992) Aluminium accumulation, beta-amyloid
deposition and neurofibrillary changes in the central nervous system.
In: Aluminium in biology and medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 165-185 (Ciba Foundation Symposium
No. 169).
Edwardson JA, Moore PB, Ferrier IN, Lilley JS, Newton GWA, Barker J,
Templar J, & Day JP (1993) Effect of silicon on gastrointestinal
absorption of aluminium. Lancet, 342(7): 211-212.
Eisenreich SJ (1980) Atmospheric input of trace metals to Lake
Michigan. Water Air Soil Pollut, 13: 287-301.
Elinder CG, Ahrengart L, Lidums V, Pettersson E, & Sj�gren B (1991)
Evidence of aluminium accumulation in aluminium welders. Br J Ind Med,
48: 735-738.
Ellen G, Egmond E, Van Loon JW, Sahertian ET, & Tolsma K (1990)
Dietary intakes of some essential and non-essential trace elements,
nitrate, nitrite and N-nitrosamines by Dutch adults estimated in a
24-hour duplicate portion study. Food Addit Contam, 7: 207-221.
Elliot HL, Dryburgh F, Fell GS, Sabet S, & MacDougall AI (1978)
Aluminium toxicity during regular haemodialysis. Br Med J,
1: 1101-1103.
Ellis HA, McCarthy JH, & Herrington J (1979) Bone aluminium in
haemodialysed patients and in rats injected with aluminium chloride:
relationship to impaired bone mineralisation. J Clin Pathol,
32: 832-844.
Ermolenko LV & Dedkov YM (1988) Photometric determination of aluminum
in water with the sulfonitrazo DAF reagent. J Anal Chem (USSR),
43: 815-820.
Exley C, Tollervey A, Gray G, Roberts S, & Birchall JD (1993) Silicon,
aluminium and the biological availability of phosphorus in algae. Proc
R Soc (Lond), B253: 93-99.
Fairman B, Sanz-Medel A, Gallego M, Quintela MJ, Jones P, & Benson R
(1994) Method comparison for the determination of labile aluminium
species in natural waters. Anal Chim Acta, 286: 401-409.
FAO/WHO (1989) Aluminium. In: Evaluation of certain food additives and
contaminants. Thirty-third report of the Joint FAO/WHO Expert
Committee on Food Additives. Geneva, World Health Organization,
pp 28-31 (WHO Technical Report Series No. 776).
Farag AM, Woodward DF, Little EE, Steadman B, & Vertucci FA (1993) The
effects of low pH and elevated aluminum on yellowstone cutthroat trout
( Oncorhynchus clarki bouvieri). Environ Toxicol Chem, 12: 719-731.
Farnell BJ, Crapper McLachlan DR, Baimbridge K, De Boni U, Wong L,
& Wood PL (1985) Calcium metabolism in aluminum encephalopathy. Exp
Neurol, 88: 68-83.
Feely RA, Sackett WM, & Harris JE (1971) Distribution of particulate
aluminum in the Gulf of Mexico. J Geophys Res, 76(24): 5893-5902.
Feth JH, Rogers SM, & Roberson CE (1964) Chemical composition of snow
in the northern Sierra Nevada and other areas. Washington, DC, US
Department of Interior, Geological Survey, 39 pp (Geological Survey-
Water Supply Paper No. 1535-J).
Fileman CF, Althaus M, Law RJ, & Haslam I (1991) Dissolved and
particulate trace metals in surface waters over the Dogger Bank,
Central North Sea. Mar Pollut Bull, 22(5): 241-244.
Filipek LH, Nordstrom DK, & Ficklin WH (1987) Interaction of acid
mine drainage with waters and sediments of West Squaw Creek in the
West Shasta mining district, California. Environ Sci Technol,
21: 388-396.
Finberg L, Dweck HS, Holmes F, Kretchmer N, Mauer AM, Reynolds JW,
& Suskind RM (1986) Aluminum toxicity in infants and children.
Pediatrics, 78: 1150-1154.
Finelli VN, Que Hee SS, & Niemeier RW (1981) Influence of exposure to
aluminium chloride and fluoride dusts on some biochemical and
physiological parameters in rats. In: Brown SS & Davies DS ed. Organ-
directed toxicity chemical indices and mechanisms (IUPAC). New York,
Pergamon Press, pp 291-295.
Fischer T & Rystedt I (1982) A case of contact sensitivity to
aluminium. Contact Dermatitis, 8: 343.
Fisher DW, Gambell AW, Likens GE, & Bormann FH (1968) Atmospheric
contributions to water quality of streams in the Hubbard Brook
Experimental Forest, New Hampshire. Water Resour Res, 4: 1115-1126.
Fivelstad S & Leivestad H (1984) Aluminium toxicity to Atlantic salmon
( Salmo salar L.) and brown trout ( Salmo trutta L.): mortality and
physiological response. Oslo, Norway, Institute of Fresh Water
Ecology, pp 69-77 (Research Report No. 61).
Flaten TP (1990) Geographical associations between aluminium in
drinking water and death rates with dementia (including Alzheimer's
disease), Parkinson's disease and amyotrophic lateral sclerosis in
Norway. Environ Geochem Health, 12: 152-167.
Flaten TP, Glattre E, Viste A, & S�reide O (1991) Mortality from
dementia among gastro-duodenal ulcer patients. J Epidemiol Community
Health, 45: 203-206.
Fleming RF & Lindstrom RM (1987) Precise determination of aluminum by
instrumental neutron activation. J Radioanal Nucl Chem, 113: 35-42.
Flendrig JA, Kruis H, & Das HA (1976) Aluminium and dialysis dementia.
Lancet, 1: 1235.
Flora SJS, Dhawan M, & Tandon SK (1991) Effects of combined exposure
to aluminium and ethanol on aluminium body burden and some neuronal,
hepatic and haematopoietic biochemical variables in the rat. Hum Exp
Toxicol, 10: 45-48.
Folsom BR, Popescu NA, & Wood JM (1986) Comparative study of aluminum
and copper transport and toxicity in an acid-tolerant freshwater green
alga. Environ Sci Technol, 20: 616-620.
Forbes WF, McAiney CA, Hayward LM, & Agwani N (1994) Geochemical risk
factors for mental functioning, based on the Ontario longitudinal
study of aging (LSA): II. The role of pH. Can J Aging, 13: 249-267.
Foy CD & Gerloff GC (1972) Response of Chlorella pyrenoidosa to
aluminum and low pH. J Phycol, 8: 268-271.
Fraga CG, Oteiza PI, Golub MS, Gershwin ME, & Keen CL (1990) Effects
of aluminum on brain lipid peroxidation. Toxicol Lett, 51: 213-219.
France RL & Stokes PM (1987) Influence of manganese, calcium, and
aluminum on hydrogen ion toxicity to the amphipod Hyalella azteca.
Can J Zool, 65: 3071-3078.
Franceschetti S, Bugiani O, Panzica F, Tagliavini F, & Avanzini G
(1990) Changes in excitability of CA1 pyramidal neurons in slices
prepared from AlCl3-treated rabbits. Epilepsy Res, 6: 39-48.
Frank WB, Haupin WE, Dawless RK, Granger DA, Wei MW, Calhoun KJ, &
Bonney TB (1985) Aluminum. In: Gerhartz W, Yamamoto YS, Campbell FT,
Pfefferkorn R, & Rounsaville JF ed. Ullmann's encyclopedia of
industrial chemistry - Volume A1: Abrasives to aluminium oxide, 5th
revised ed. Weinheim, Verlag Chemie, pp 459-480.
Frecker MF (1991) Dementia in Newfoundlands: Identification of a
geographical isolate? J Epidemiol Community Health, 45: 307-311.
Freda J & McDonald DG (1990) Effects of aluminum on the leopard
frog, Rana pipiens: life stage comparisons and aluminum uptake. Can
J Fish Aquat Sci, 47: 210-216.
Freda J & McDonald DG (1993) Toxicity of amphibian breeding ponds in
the Sudbury region. Can J Fish Aquat Sci, 50: 1497-1503.
Freeman RA (1973) Recovery of rainbow trout from aluminum poisoning.
Trans Am Fish Soc, 102(1): 152-154.
Freeman RA & EverharT WH (1971) Toxicity of aluminum hydroxide
complexes in neutral and basic media to rainbow trout. Trans Am Fish
Soc, 100(4): 644-658.
Frick KG & Herrmann J (1990) Aluminum accumulation in a lotic mayfly
at low pH - a laboratory study. Ecotoxicol Environ Saf, 19: 81-88.
Froment DH, Buddington B, Miller NL, & Alfrey AC (1989a) Effect of
solubility on the gastrointestinal absorption of aluminum from various
aluminum compounds in the rat. J Lab Clin Med, 114: 237-242.
Froment DPH, Molitoris BA, Buddington B, Miller N, & Alfrey AC (1989b)
Site and mechanism of enhanced gastrointestinal absorption of aluminum
by citrate. Kidney Int, 36: 978-984.
Frost L, Johansen P, Pedersen S, Veien N, Aabel �stergaard P, &
Nielsen MH (1985) Persistant subcutaneous nodules in children
hyposensitized with aluminium-containing allergen extracts. Allergy,
40: 368-372.
Fulton B, Jaw S, & Jeffery EH (1989) Bioavailability of aluminum from
drinking water. Fundam Appl Toxicol, 12: 144-150.
Furrer G (1993) New aspects on the chemistry of aluminum in soils.
Aquat Sci, 55(4): 281-290.
Gajdusek DC & Salazar AM (1982) Amyotrophic lateral sclerosis and
parkinsonian syndromes in high incidence among the Auyu and Jakai
people of West New Guinea. Neurology, 32: 107-126.
Galceran T, Finch J, Bergfeld M, Coburn JW, Martin K, Teitelbaum S, &
Slatopolsky E (1987) Biological effects of aluminum on normal dogs:
studies on the isolated perfused bone. Endocrinology (Baltimore),
121: 406-413.
Ganrot PO (1986) Metabolism and possible health effects of aluminum.
Environ Health Perspect, 65: 363-441.
Garcia RP, Liu YM, Garcia MED, & Sanz-Medel A (1991) Solid-surface
room-temperature phosphorescence optosensing in continuous flow
systems: an approach for ultratrace metal ion determination. Anal
Chem, 63: 1759-1763.
Gardinier PE, Ottaway JM, Fell GS, & Halls DJ (1981) Determination of
aluminium in blood plasma or serum by electrothermal atomic absorption
spectrometry. Anal Chim Acta, 128: 57-66.
Gardinier PE, Schierl R, & Kreutzer K (1987) Aluminium speciation in
soil solutions as studied by size exclusion chromatography. Plant
Soil, 103: 151-154.
Garruto RM, Fukatsu R, Yanagihara R, Gajdusek DC, Hook G, & Fiori CE
(1984) Imaging of calcium and aluminum in neurofibrillary tangle-
bearing neurons in parkinsonism-dementia of Guam. Proc Natl Acad Sci
(USA), 81: 1875-1879.
Gascon C, Planas D, & Moreau G (1987) The interaction of pH, calcium
and aluminium concentrations on the survival and development of
wood frog ( Rana sylvatica) eggs and tadpoles. In: Witters H &
Vanderborght O ed. Ecophysiology of acid stress in aquatic organisms:
Proceedings of an International Symposium, Antwerp, 13-16 January
1987. Antwerp, Belgian Royal Zoological Society, pp 189-199.
Genter RB & Amyot DJ (1994) Freshwater benthic algal population and
community changes due to acidity and aluminum-acid mixtures in
artificial streams. Environ Toxicol Chem, 13(3): 369-380.
Gitelman HJ, Alderman FR, Kurs-Lasky M, & Rockette HE (1995) Serum and
urinary aluminium levels of workers in the aluminium industry. Ann
Occup Hyg, 39: 181-191.
Goate AM, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L,
Guiffral L, Haynes A, & Hardy JA (1991) Segregation of a mis-sense
mutation in the amyloid precursor protein gene with familial Alzheimer
disease. Nature (Lond), 349: 704-706.
Godbold DL, Dictus K, & H�ttermann A (1988a) Influence of aluminium
and nitrate on root growth and mineral nutrition of Norway spruce
( Picea abies) seedlings. Can J For Res, 18: 1167-1171.
Godbold DL, Fritz E, & H�ttermann A (1988b) Aluminum toxicity and
forest decline. Proc Natl Acad Sci (USA), 85: 3888-3892.
Goenaga X & Williams DJA (1988) Aluminium speciation in surface
waters from a Welsh upland area. Environ Pollut, 52: 131-149.
Goenaga X & Williams DJA (1990) Determination of aluminium speciation
in acid waters. In: Edwards RW, Gee AS, & Stoner JH ed. Acid waters in
Wales. Dordrecht, Boston, London, Kluwer Academic Publishers,
pp 189-201.
Goenaga X, Bryant R, & Williams DJA (1987) Influence of sorption
processes on aluminum determinations in acidic waters. Anal Chem,
59: 2673-2678.
Golub MS, Gershwin ME, Donald JM, Negri S, & Keen CL (1987) Maternal
and developmental toxicity of chronic aluminum exposure in mice.
Fundam Appl Toxicol, 8: 346-357.
Golub MS, Donald JM, Gershwin ME, & Keen CL (1989) Effects of aluminum
ingestion on spontaneous motor activity of mice. Neurotoxicol Teratol,
11: 231-235.
Golub MS, Keen CL, & Gershwin ME (1992) Neurodevelopmental effect
of aluminum in mice: Fostering studies. Neurotoxicol Teratol,
14: 177-182.
Golub MS, Han B, Keen CL, & Gershwin ME (1993) Developmental patterns
of aluminium in mouse brain and interactions between dietary aluminium
excess and manganese deficiency. Toxicology, 81: 33-47.
Golub MS, Han B, Keen CL, & Gershwin ME (1994) Auditory startle in
Swiss Webster mice fed excess aluminium in diet. Neurotoxicol Teratol,
16: 423-425.
Golub MS, Han B, Keen CL, Gershwin ME, & Tarara RP (1995) Behavioral
performance of Swiss Webster mice exposed to excess dietary aluminum
during development or during development and as adults. Toxicol Appl
Pharmacol, 133: 64-72.
Gomez M, Domingo JL, Llobet JM, Tomas JM, & Corbella J (1986) Short-
term oral toxicity study of aluminium in rats. Arch Pharmacol Toxicol,
12: 145-151.
Gomez M, Bosque MA, Domingo JL, Llobet JM, & Corbella J (1990)
Evaluation of the maternal and developmental toxicity of aluminum
from high doses of aluminum hydroxide in rats. Vet Hum Toxicol,
32: 545-548.
Gomez M, Domingo JL, & Llobet JM (1991) Developmental toxicity
evaluation of oral aluminum in rats: Influence of citrate.
Neurotoxicol Teratol, 13: 323-328.
Good PF, Perl DP, Bierer LM, & Schmeidler J (1992) Selective
accumulation of aluminium and iron in the neurofibrillary tangles of
Alzheimer's disease: a laser microprobe (LAMMA) study. Ann Neurol,
31: 286-292.
Goodman WG (1984) Short-term aluminum administration in the rat:
reductions in formation without osteomalacia. J Lab Clin Med,
103: 749-757.
Goodman WG (1986) Experimental aluminum-induced bone disease: Studies
in vivo. Kidney Int, 18(suppl): S32-S36.
Goodman WG (1990) Pathophysiologic mechanisms of aluminum toxicity:
Aluminum-induced bone disease. In: de Broe ME & Coburn JW ed. Aluminum
and renal failure. Dordrecht, Boston, London, Kluwer Academic
Publishers, pp 87-108.
Goodman WG, Henry DA, Horst R, Nudelman RK, Alfrey AC, & Coburn JW
(1984a) Parenteral aluminum administration in the dog: II. Induction
osteomalacia and effects on vitamin D metabolism. Kidney Int,
25: 370-375.
Goodman WG, Gilligan J, & Horst R (1984b) Short-term aluminum
administration in the rat: effects on bone formation and relationship
with renal osteomalacia. J Clin Invest, 73: 171-181.
G�ransson A & Eldhuset TD (1987) Effects of aluminium on growth and
nutrient uptake of Betula pendula seedlings. Physiol Plant,
69: 193-199.
Grahn O (1980) Fishkills in two moderately acid lakes due to high
aluminum concentration. In: Drabl�s D & Tollan A ed. Ecological impact
of acid precipitation: Proceedings of an International Conference.
Sandefjord, Norway, 11-14 March 1980. Oslo-�s, SNSF, pp 310-311.
Grant LD, Elias R, Goyer R, Nicholson W, & Olem H (1990) Indirect
health effects associated with acidic deposition. Washington, DC,
National Acid Precipitation Assessment Program, 173 pp (NAPAP Report
No. 23).
Graves AB, White E, Koepsell TD, Reifler BV, Van Belle G, & Larson EB
(1990) The association between aluminum-containing products and
Alzheimer's disease. J Clin Epidemiol, 43: 35-44.
Greger JL & Baier MJ (1983a) Excretion and retention of low or
moderate levels of aluminium by human subjects. Food Chem Toxicol,
21: 473-477.
Greger JL & Baier MJ (1983b) Effect of dietary aluminum on mineral
metabolism of adult males. Am J Clin Nutr, 38: 411-419.
Greger JL & Powers CF (1992) Assessment of exposure to parenteral and
oral aluminum with and without citrate using a desferrioxamine test in
rats. Toxicology, 76: 119-132.
Greger JL, Bula EN, & Gum ET (1985a) Mineral metabolism of rats fed
moderate levels of various aluminum compounds for short periods of
time. J Nutr, 115: 1708-1716.
Greger JL, Goetz W, & Sullivan D (1985b) Aluminium levels in foods
cooked and stored in aluminium pans, trays and foil. J Food Prot,
48: 772-777.
Greger JL, Gum ET, & Bula EN (1986) Mineral metabolism of rats fed
various levels of aluminum hydroxide. Biol Trace Elem Res, 9: 67-77.
Gross P, De Treville RTP, Cralley LJ, Grandquist WT, & Pundsack FL
(1970) The pulmonary response to fibrous dusts of diverse
compositions. Am Ind Hyg Assoc J, 31: 125-132.
Gross P, Harley RA, & Detreville RTP (1973) Pulmonary reaction to
metallic aluminum powders. Arch Environ Health, 26: 227-236.
Guieu C, Martin JM, Thomas AJ, & Elbaz-Poulichet F (1991) Atmospheric
versus river inputs of metals to the Gulf of Lions: Total
concentrations, partitioning and fluxes. Mar Pollut Bull, 22(4):
176-183.
Gundersen P & Rasmussen L (1990) Nitrification in forest soils:
effects from nitrogen deposition on soil acidification and aluminum
release. Rev Environ Contam Toxicol, 113: 1-45.
Gundersen DT, Bustaman S, Seim WK, & Curtis LR (1994) pH, hardness,
and humic acid influence aluminum toxicity to rainbow trout
( Oncorhynchus mykiss) in weakly alkaline waters. Can J Fish Aquat
Sci, 51: 1345-1355.
Gunn JM & Noakes DLG (1987) Latent effects of pulse exposure to
aluminum and low pH on size, ionic composition, and feeding efficiency
of lake trout ( Salvelinus namaycush) alevins. Can J Fish Aquat Sci,
44: 1418-1424.
Gupta SK, Waters DH, & Gwilt PR (1986) Absorption and disposition of
aluminium in the rat. J Pharm Sci, 75: 586-589.
Guy SP, Jones D, Mann DMA, & Itzhaki RF (1991) Human neuroblastoma
cells treated with aluminium express an epitope associated with
Alzheimer's disease neurofibrillary tangles. Neurosci Lett,
121: 166-168.
Hackett C (1962) Stimulative effects of aluminium on plant growth.
Nature (Lond), 195(4840): 471-472.
Hackett C (1964) Ecological aspects of the nutrition of Deschampsia
flexuosa (L.) Trin: I. The effect of aluminium, manganese and pH on
germination. J Ecol, 52: 159-167.
Hackett C (1967) Ecological aspects of the nutrition of Deschampsia
flexuosa (L.) Trin: III. Investigation of phosphorus requirement and
response to aluminium in water culture, and a study of growth in soil.
J Ecol, 55: 831-840.
Hahn PHB & Guenter W (1986) Effect of dietary fluoride and aluminum on
laying hen performance and fluoride concentration in blood, soft
tissue, bone, and egg. Poult Sci, 65: 1343-1349.
Hall RJ, Likens GE, Fiance SB, & Hendrey GR (1980) Experimental
acidification of a stream in the Hubbard Brook Experimental Forest,
New Hampshire. Ecology, 61(4): 976-989.
Hall RJ, Driscoll CT, Likens GE, & Pratt JM (1985) Physical, chemical,
and biological consequences of episodic aluminum additions to a
stream. Limnol Oceanogr, 30(1): 212-220.
Hall RJ, Driscoll CT, & Likens GE (1987) Importance of hydrogen ions
and aluminium in regulating the structure and function of stream
ecosystems: an experimental test. Freshw Biol, 18: 17-43.
Harriman R, Morrison BRS, Caines LA, Collen P, & Watt AW (1987)
Long-term changes in fish populations of acid streams and lochs in
Galloway, south west Scotland. Water Air Soil Pollut, 32: 89-112.
H�s�nen E & Huttunen S (1989) Acid deposition and the element
composition of pine tree rings. Chemosphere, 18(9/10): 1913-1920.
H�s�nen E, Lipponen M, Minkkinen P, Kattainen R, Markkanen K, &
Brjukhanov P (1990) Elemental concentrations of aerosol samples from
the Baltic Sea area. Chemosphere, 21(3): 339-347.
Haugen AA, Becher G, & Bj�rseth A (1983) Biological monitoring of
occupational exposure to polycyclic aromatic hydrocarbons (PAHs) in an
aluminium plant (Abstract). Eur J Cancer Clin Oncol, 19: 1287.
Havas M (1985) Aluminum bioaccumulation and toxicity to Daphnia
magna in soft water at low pH. Can J Fish Aquat Sci, 42:
1741-1748.
Havas M & Hutchinson TC (1982) Aquatic invertebrates from the Smoking
Hills, N.W.T.: effect of pH and metals on mortality. Can J Fish Aquat
Sci, 39: 890-903.
Havas M & Likens GE (1985) Toxicity of aluminum and hydrogen ions to
Daphnia catawba, Holopedium gibberum, Chaoborus punctipennis, and
Chironomus anthrocinus from Mirror Lake, New Hampshire. Can J Zool,
63: 1114-1119.
Havens KE (1990) Aluminum binding to ion exchange sites in
acid-sensitive versus acid-tolerant cladocerans. Environ Pollut,
64: 133-141.
Havens KE (1991) Littoral zooplankton responses to acid and aluminum
stress during short-term laboratory bioassays. Environ Pollut,
73: 71-84.
Havens KE (1993) Acid and aluminum effects on the survival of
littoral macro-invertebrates during acute bioassays. Environ Pollut,
80: 95-100.
Havens KE & Heath RT (1989) Acid and aluminum effects on freshwater
zooplankton: an in situ mesocosm study. Environ Pollut,
62: 195-211.
Helliwell S, Batley GE, Florence TM, & Lumsden BG (1983) Speciation
and toxicity of aluminium in a model fresh water. Environ Technol
Lett, 4: 141-144.
Hellou J, Fancey LL, & Payne JF (1992a) Concentrations of twenty-four
elements in bluefin tuna Thunnus thynnus from the northwest
Atlantic. Chemosphere, 24(2): 211-218.
Hellou J, Warren WG, Payne JF, Belkhode S, & Lobel P (1992b) Heavy
metals and other elements in three tissues of cod, Gadus morhua from
the northwest Atlantic. Mar Pollut Bull, 24(9): 452-458.
Helmboldt O, Hudson LK, Misra C, Wefers K, Stark H, & Danner M (1985)
Aluminum compounds, inorganic. In: Gerhartz W, Yamamoto YS, Campbell
FT, Pfefferkorn R, & Rounsaville JF ed. Ullmann's encyclopedia of
industrial chemistry - Volume A1: Abrasives to aluminum oxide, 5th
revised ed. Weinheim, Verlag Chemie, pp 527-541.
Hem JD & Roberson CE (1967) Form and stability of aluminum hydroxide
complexes in dilute solution. In: Chemistry of aluminum in natural
water. Washington, DC, US Department of Interior, Geological Survey
(Geological Survey-Water Supply Paper No. 1827-A).
Henriksen A, Skogheim OK, & Rosseland BO (1984) Episodic changes in pH
and aluminium-speciation kill fish in a Norwegian salmon river.
Vatten, 40: 255-260.
Henriksen A, Lien L, Traaen TS, Sevaldrud IS, & Brakke DF (1988a) Lake
acidification in Norway: present and predicted fish status. Ambio,
18(6): 314-321.
Henriksen A, Lien L, Traaen TS, Sevaldrud IS, & Brakke DF (1988b) Lake
acidification in Norway - present and predicted chemical status.
Ambio, 17(4): 259-266.
Henriksen A, Wathne BM, R�geberg EJS, Norton SA, & Brakke DF (1988c)
The role of stream substrates in aluminium mobility and acid
neutralization. Water Res, 22(8): 1069-1073.
Henry HL & Norman AW (1985) Interactions between aluminium and the
actions and metabolism of vitamin D3 in the chick. Calcif Tissue Int,
37: 484-490.
Henry DA, Goodman WG, Nudelman RK, Didomenico NC, Alfrey AC,
Slatopolsky E, Stanley TM, & Coburn JW (1984) Parenteral aluminum
administration in the dog: I. Plasma kinetics, tissue levels, calcium
metabolism, and para-thyroid hormone. Kidney Int, 25: 362-369.
Herbert A, Sterling G, Abraham J, & Corrin B (1982) Desquamative
interstitial pneumonia in an aluminum welder. Hum Pathol,
13: 694-699.
Herrmann J (1987) Sodium levels of lotic mayfly nymphs being exposed
to aluminium at low pH. A preliminary report. Ann Soc R Zool Belg,
117: 181-188.
Herrmann J & Andersson KG (1986) Aluminium impact on respiration of
lotic mayflies at low pH. Water Air Soil Pollut, 30: 703-709.
Herzog P, Schmitt KF, Grendhal T, Van der Linden J, & Holterm�ller KH
(1982) VI.5. Evaluation of serum and urine electrolyte changes during
therapy with a magnesium-aluminum containing antacid: results of a
prospective study. In: Halter F ed. Antacids in the eighties:
Symposium on Antacids, Hamburg, June 1980. M�nchen, Urban &
Schwarzenberg, pp 123-135.
Hickie BE, Hutchinson NJ, Dixon DG, & Hodson PV (1993) Toxicity of
trace metal mixtures to alevin rainbow trout ( Oncorhynchus mykiss)
and larval fathead minnow ( Pimephales promelas) in soft, acidic
water. Can J Fish Aquat Sci, 50: 1348-1355.
Hicks JS, Hackett DS, & Sprague GL (1987) Toxicity and aluminium
concentration in bone following dietary administration of two
sodium aluminium phosphate formulations in rats. Food Chem Toxicol,
25(7): 533-538.
Hjortsbert U, Orbaek P, Arborelius M Jr, & Karlsson J-E (1994) Upper
airway irritation and small airways hyperreactivity due to exposure to
potassium aluminium tetrafluoride flux: an extended case report.
Occup Environ Med, 51: 706-709.
Hodsman AB, Sherrard DJ, Alfrey AC, Ott S, Brickman AS, Miller NL,
Maloney NA, & Coburn JW (1982) Bone aluminum and histomorphometric
features of renal osteodystrophy. J Clin Endocrinol Metab,
54: 539-546.
Hodsman AB, Anderson C, & Leung FY (1984) Accelerated accumulation of
aluminum by osteoid matrix in vitamin D deficiency. Miner Electrolyte
Metab, 10: 309-315.
H�hr D, Abel J, & Wilhelm M (1989) Renal clearance of aluminium:
studies in the isolated perfused rat kidney. Toxicol Lett,
45: 165-174.
Hoke RA, Giesy JP, & Kreis RG (1992) Sediment pore water toxicity
identification in the lower Fox River and Green Bay, Wisconsin, using
the microtox assay. Ecotoxicol Environ Saf, 23: 343-354.
H�rnstr�m E, Ekstr�m C, & Duraini MO (1984) Effects of pH and
different levels of aluminium on lake plankton in the Swedish west
coast area. Oslo, Norway, Institute of Fresh Water Ecology, pp 115-127
(Research Report No. 61).
Horst WJ, Wagner A, & Marschner H (1982) Mucilage protects root
meristems from aluminium injury. Z Pflanzenphysiol Bd, 105: 435-444.
Hosovski E, Mastelica Z, Sunderic D, & Radulovic D (1990) Mental
abilities of workers exposed to aluminium. Med Lav, 81: 119-123.
Huang JW, Grunes DL, & Kochian LV (1992a) Aluminum effects on the
kinetics of calcium uptake into cells of the wheat root apex:
Quantification of calcium fluxes using a calcium-selective vibrating
microelectrode. Planta, 188: 414-421.
Huang JW, Shaff JE, Grunes DL, & Kochian LV (1992b) Aluminum effects
on calcium fluxes at the root apex of aluminum-tolerant and
aluminum-sensitive wheat cultivars. Plant Physiol, 98: 230-237.
Hudson LK, Misra C, & Wefers K (1985) Aluminum oxide. In: Gerhartz W,
Yamamoto YS, Campbell FT, Pfefferkorn R, & Rounsaville JF ed.
Ullmann's encyclopedia of industrial chemistry - Volume A1: Abrasives
to aluminum oxide, 5th revised ed. Weinheim, Verlag Chemie,
pp 557-594.
Huett DO & Menary RC (1980) Aluminium distribution in freeze-dried
roots of cabbage, lettuce and kikuyu grass by energy-dispersive x-ray
analysis. Aust J Plant Physiol, 7: 101-111.
Hunn JB, Cleveland L, & Little EE (1987) Influence of pH and aluminum
on developing brook trout in a low calcium water. Environ Pollut,
43: 63-73.
Hunter JB, Ross SL, & Tannahill J (1980) Aluminium pollution and fish
toxicity. J Water Pollut Control Fed, 79: 413-420.
Hussein AS, Cantor AH, & Johnson TH (1988) Use of high levels of
dietary aluminum and zinc for inducing pauses in egg production of
Japanese quail. Poult Sci, 67: 1157-1165.
Hussein AS, Cantor AH, & Johnson TH (1989a) Effect of dietary aluminum
on calcium and phosphorus metabolism and performance of laying hens.
Poult Sci, 68: 706-714.
Hussein AS, Cantor AH, & Johnson TH (1989b) Comparison of the use of
dietary aluminum with the use of feed restriction for force-molting
laying hens. Poult Sci, 68: 891-896.
Hutchinson NJ & Sprague JB (1986) Toxicity of trace metal mixtures
to American flagfish ( Jordanella floridae) in soft, acidic water and
implications for cultural acidification. Can J Fish Aquat Sci, 43:
647-655.
Hydes DJ (1977) Dissolved aluminium concentration in sea water. Nature
(Lond), 268: 136-137.
Hydes DJ (1979) Aluminum in seawater: control by inorganic processes.
Science, 205: 1260-1262.
Hydes DJ (1983) Distribution of aluminium in waters of the north east
Atlantic 250N to 350N. Geochim Cosmochim Acta, 47: 967-973.
Hydes DJ & Kremling K (1993) Patchiness in dissolved metals (Al, Cd,
Co, Cu, Mn, Ni) in North Sea surface waters: seasonal differences and
influence of suspended sediment. Cont Shelf Res, 13(10): 1083-1101.
Hydes DJ & Liss PS (1977) The behaviour of dissolved aluminium in
estuarine and coastal waters. Estuar Coast Mar Sci, 5: 755-769.
IARC (1987) Cadmium and cadmium compounds. In: Overall evaluations of
carcinogenicity: An updating of IARC Monographs, Volumes 1 to 42.
Lyon, International Agency for Research on Cancer, pp 139-142 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Supplement 7).
Ingersoll CG, Gulley DD, Mount DR, Mueller ME, Fernandez JD, Hockett
JR, & Bergman HL (1990) Aluminum and acid toxicity to two strains
of brook trout ( Salvelinus fontinalis). Can J Fish Aquat Sci,
47: 1641-1648.
IPAI (1993) Statistical summary - Volume 5. London, International
Primary Aluminium Institute, 36 pp.
IRPTC (1993) IRPTC legal file 1986. Geneva, International Register of
Potentially Toxic Chemicals, United Nations Environment Programme.
Irvine KN, Drake JJ, & James W (1992) Particulate trace element
concentrations and loadings associated with erosion of pervious urban
land. Environ Technol, 13: 231-237.
Ittel TH, Gruber E, Heinrichs A, Handt S, Hofst�dter F, & Sieberth HG
(1992) Effect of fluoride on aluminum-induced bone disease in rats
with renal failure. Kidney Int, 41: 1340-1348.
Jacobs RW, Duong T, Jones RE, Trapp GA, & Scheibel AB (1989) A
re-examination of aluminium in Alzheimer's disease: Analysis by
energy dispersive x-ray microprobe and flameless atomic absorbtion
spectrophotometry. Can J Neurol Sci, 16: 498-503.
Jacqmin H, Commenges D, Letenneur L, Barberger-Gateau P, & Dartigues
JF (1994) Components of drinking water and risk of cognitive
impairment in the elderly. Am J. Epidemiol, 139: 48-57.
J�ger DE, Wilhelm M, Witte G, & Ohnesorge FK (1991) Intestinal
absorption of aluminium: studies in the isolated perfused rat
intestinal preparation. J Trace Elem Electrolytes Health Dis,
5(2): 81-85.
Jagoe CH, Haines TA, & Buckler DR (1987) Abnormal gill development in
Atlantic salmon ( Salmo salar) fry exposed to aluminium at low pH.
In: Witters H & Vanderborght O ed. Ecophysiology of acid stress in
aquatic organisms: Proceedings of an International Symposium, Antwerp,
13-16 January 1987. Antwerp, Belgian Royal Zoological Society,
pp 375-386.
James BR & Riha SJ (1989) Aluminum leaching by mineral acids in
forest soils: I. Nitric-sulfuric acid differences. Soil Sci Soc Am J,
53: 259-264.
Jederlinic PJ, Abraham JL, Churg A, Himmelstein JS, Epler GR, &
Gaensler EA (1990) Pulmonary fibrosis in aluminum oxide workers. Am
Rev Respir Dis, 142: 1179-1184.
Johnson GVM & Jope RS (1987) Aluminum alters cyclic AMP and cyclic
GMP levels but not presynaptic cholinergic markers in rat brain
in vivo. Brain Res, 403: 1-6.
Johnson NM, Driscoll CT, Eaton JS, Likens GE, & McDowell WH (1981)
'Acid rain' dissolved aluminum and chemical weathering at the Hubbard
Brook Experimental Forest, New Hampshire. Geochim Cosmochim Acta,
45: 1421-1437.
Johnson MG, Culp LR, & George SE (1986) Temporal and spatial trends in
metal loadings to sediments of the Turkey Lakes, Ontario. Can J Fish
Aquat Sci, 43: 754-762.
Johnson GVW, Cogdill KW, & Jope RS (1990) Oral aluminum alters
in vitro protein phosphorylation and kinase activities in rat brain.
Neurobiol Aging, 11: 209-216.
Johnson GVW, Watson AL Jr, Lartius R, Uemura E, & Jope RS (1992)
Dietary aluminum selectively decreases MAP-2 in brains of developing
and adult rats. Neurotoxicology, 13: 463-474.
Jones P (1991) The investigation of aluminium speciation in natural
and potable waters using short-column ion chromatography. Int J
Environ Anal Chem, 44: 1-10.
Jones KC & Bennett BG (1985) Exposure commitment assessments of
environmental pollutants. London, University of London, King's
College, Monitoring and Assessment Research Centre, 33 pp (MARC
Technical Report 33).
Jones BF, Kennedy VC, & Zellweger GW (1974) Comparison of observed and
calculated concentrations of dissolved Al and Fe in stream water.
Water Resour Res, 10(4): 791-793.
Jope RS & Johnson GVW (1992) Neurotoxic effects of dietary aluminium.
In: Aluminium in biology and medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 254-267 (Ciba Foundation Symposium
No. 169.
Jorhem L & Haegglund G (1992) Aluminium in foodstuffs and diets in
Sweden. Z Lebensm.unters Forsch, 194: 38-42.
Joslin JD, Kelly JM, Wolfe MH, & Rustad LE (1988) Elemental patterns
in roots and foliage of mature spruce across a gradient of soil
aluminium. Water Air Soil Pollut, 40: 375-390.
Jouhanneau P, Lacour B, Raisbeck G, Yiou F, Banide H, Brown E, &
Dr�eke T (1993) Gastrointestinal absorption of aluminium in rats
using 26Al and accelerator mass spectrometry. Clin Nephrol,
40(4): 244-248.
Kabra SG & Narayanan R (1988) Aluminium phosphide: worse than Bhopal.
Lancet, 1: 1333.
Kada T, Hirano K, & Shirasu Y (1980) Screening of environmental
chemical mutagens by the rec(-) assay system with Bacillus
subtilis. In: Hollaender A & de Serres FJ ed. Chemical mutagens:
Principles and methods for their detection. New York, London, Plenum
Press, pp 149-173.
Kaehny WD, Hegg AP, & Alfrey AC (1977a) Gastrointestinal absorption
of aluminum from aluminum- containing antacids. N Engl J Med,
296: 1389-1390.
Kaehny WD, Alfrey AC, Holman RE, & Shorr WJ (1977b) Aluminium transfer
during hemodialysis. Kidney Int, 12: 361-365.
Kanematsu N, Hara M, & Kada T (1980) Rec assay and mutagenicity
studies on metal compounds. Mutat Res, 77: 109-116.
Kappel LC, Youngberg H, Ingraham RH, Hembry FG, Robinson DL, & Cherney
JH (1983) Effects of dietary aluminum on magnesium status of cows. Am
J Vet Res, 44: 770-773.
Karlik SJ, Eichhorn GL, & Crapper McLachlan DR (1980) Molecular
interactions of aluminum with DNA. Neurotoxicology, 1: 83-88.
Karlsson-Norrgren L, Dickson W, Ljungberg O, & Runn P (1986a) Acid
water and aluminium exposure: gill lesions and aluminium accumulation
in farmed brown trout, Salmo trutta L. J Fish Dis, 9: 1-9.
Karlsson-Norrgren L, Bj�rklund I, Ljungberg O, & Runn P (1986b) Acid
water and aluminium exposure: experimentally induced gill lesions in
brown trout, Salmo trutta L. J Fish Dis, 9: 11-25.
Katz AC, Frank DW, Sauerhoff MW, Zwicker GM, & Freudenthal RI (1984) A
6-month dietary toxicity study of acidic sodium aluminium phosphate in
beagle dogs. Food Chem Toxicol, 22: 7-9.
Kerr DNS & Ward MK (1988) Aluminum intoxication: History of its
clinical recognition and management. In: Sigel H & Sigel A ed. Metal
ions in biological systems - Volume 24: Aluminum and its role in
biology. New York, Basel, Marcel Dekker, Inc., pp 217-258.
Kersten M, Dicke M, Kriews M, Naumann K, Schmidt D, Schulz M,
Schwikowski M, & Steiger M (1988) Distribution and fate of heavy
metals in the North Sea. In: Salomons W ed. Pollution of the North
Sea: An assessment. Berlin, Heidelberg, New York, Springer Verlag,
pp 300-347.
Khangarot BS & Ray PK (1989) Investigation of correlation between
physicochemical properties of metals and their toxicity to the water
flea Daphnia magna Straus. Ecotoxicol Environ Saf, 18: 109-120.
Kim YS, Lee MH, & Wisniewski HM (1986) Aluminum induced reversible
change in permeability of the blood-brain barrier to [14C]sucrose.
Brain Res, 377: 286-291.
King RG, Sharp JA, & Boura ALA (1983) The effects of Al3+, Cd2+ and
Mn2+ on human erythrocyte choline transport. Biochem Pharmacol,
32: 3611-3617.
Kinraide TB, Ryan PR, & Kochian LV (1992) Interactive effects of
Al3+, H+, and other cations on root elongation considered in terms
of cell-surface electrical potential. Plant Physiol, 99: 1461-1468.
Klaprat DA, Brown SB, & Hara TJ (1988) The effect of low pH and
aluminum on the olfactory organ of rainbow trout, Salmo gairdneri.
Environ Biol Fish, 22(1): 69-77.
Klein GL (1991) The aluminum content of parenteral solutions: Current
status. Nutr Rev, 49: 74-79.
Knutti R & Zimmerli B (1985) [Investigation of the daily portions of
Swiss food establishments: III. Lead, cadmium, mercury, nickel and
aluminium. Mitt Geb Lebensm.hyg, 76: 206-232 (in German).
Kobayashi K, Yumoto S, Nagai H, Hosoyama Y, Imamura M, Masuzawa S,
Koizumi Y, & Yamashita H (1990) 26Al tracer experiment by accelerator
mass spectrometry and its application to the studies for amyotrophic
lateral sclerosis and Alzheimer's disease. I. Proc Jpn Acad, B66:
189-192.
Koga A (1967) Iron, aluminium and manganese concentrations in waters
discharged from Wairakei drillholes. N Z J Sci, 10: 979-987.
Kongerud J (1991) Occupational exposure and asthma: An epidemioligic
study of aluminium potroom workers. Nor J Occup Med, 2(suppl):
1-192.
Kongerud J & Samuelsen SO (1991) A longitudinal study of respiratory
symptoms in aluminium potroom workers. Am Rev Respir Dis, 144:
10-16.
Kongerud J, Groennesby JK, & Magnus P (1990) Respiratory symptoms and
lung function of aluminium potroom workers. Scand J Work Environ
Health, 16: 270-277.
Kopp JF & Kroner RC (1970) Trace metals in the waters of the United
States. Washington, DC, US Department of Interior, Federal Water
Pollution Control Administration (Report No. 9208-4).
Kremling K (1985) The distribution of cadmium, copper, nickel,
manganese, and aluminium in surface waters of the open Atlantic and
European shelf area. Deep-Sea Res, 32(5): 531-555.
Krueger GL, Morris TK, Suskind RR, & Widner EM (1984) The health
effects of aluminum compounds in mammals. CRC Crit Rev Toxicol,
13: 1-24.
Kupchella L & Syty A (1980) Determination of nickel, manganese, copper
and aluminium in chewing gum by nonflame atomic absorption
spectrometry. J Agric Food Chem, 28: 1035-1036.
Lamb DS & Bailey GC (1981) Acute and chronic effects of alum to midge
larva ( Diptera: Chironomidae). Bull Environ Contam Toxicol,
27: 59-67.
Landolt W, Guecheva M, & Bucher JB (1989) The spatial distribution of
different elements in and on the foliage of Norway spruce growing in
Switzerland. Environ Pollut, 56: 155-167.
Landsberg JP, McDonald B, & Watt F (1992) Absence of aluminium in
neuritic plaque cores in Alzheimer's disease. Nature (Lond),
360: 65-68.
Lantzy RJ & MacKenzie FT (1979) Atmospheric trace metals: global
cycles and assessment of man's impact. Geochim Cosmochim Acta,
43: 511-525.
Lawrence GB, Fuller RD, & Driscoll CT (1986) Spatial relationships of
aluminum chemistry in the streams of the Hubbard Brook Experimental
Forest, New Hampshire. Biogeochemistry, 2: 115-135.
Lawrence GB, Fuller RD, & Driscoll CT (1987) Release of aluminum
following whole-tree harvesting at the Hubbard Brook Experimental
Forest, New Hampshire. J Environ Qual, 16(4): 383-390.
Lawrence GB, Driscoll CT, & Fuller RD (1988) Hydrologic control of
aluminum chemistry in an acidic headwater stream. Water Resour Res,
24(5): 659-669.
Lazerte BD (1984) Forms of aqueous aluminum in acidified catchments of
central Ontario: a methodological analysis. Can J Fish Aquat Sci,
41: 766-776.
Leblondel G & Allain P (1980) Blood and brain aluminium concentrations
in mice after intraperitoneal injection of different aluminium
compounds. Res Commun Chem Pathol Pharmacol, 27: 579-586.
Lee CR (1972) Interrelationships of aluminum and manganese on the
potato plant. Agron J, 64: 546-549.
Lee YH (1985) Aluminium speciation in different water types. Ecol
Bull, 37: 109-119.
Lee RE & Von Lehmden DJ (1973) Trace metal pollution in the
environment. J Air Pollut Control Assoc, 23(10): 853-857.
Leharne S, Charlesworth D, & Chowdhry B (1992) A survey of metal
levels in street dusts in an inner London neighbourhood. Environ Int,
18: 263-270.
Leinonen KP (1989) The influence of soil preparation on the levels of
aluminium, manganese, iron, copper, zinc, cadmium and mercury in
Vaccinium myrtillus. Chemosphere, 18(7/8): 1581-1587.
Leivestad H, Jensen E, Kjartansson H, & Xingfu L (1987) Aqueous
speciation of aluminium and toxic effects on Atlantic salmon. In:
Witters H & Vanderborght O ed. Ecophysiology of acid stress in aquatic
organisms: Proceedings of an International Symposium, Antwerp, 13-16
January 1987. Antwerp, Belgian Royal Zoological Society, pp 387-398.
L�onard A & Gerber GB (1988) Mutagenicity, carcinogenicity, and
teratogenicity of aluminum. Mutat Res, 196: 247-257.
Lewis CW & Macias ES (1980) Composition of size-fractionated aerosol
in Charleston, West Virginia. Atmos Environ, 14: 185-194.
Lide DR (1991) CRC handboook of chemistry and physics: A ready-
reference book of chemical and physical data, 71st ed. Boca Raton,
Florida, CRC Press, pp 4/3, 4/41-4/42.
Lindemann J, Holtkamp E, & Herrmann R (1990) The impact of aluminium
on green algae isolated from two hydrochemically different headwater
streams, Bavaria, Germany. Environ Pollut, 67: 61-77.
Lione A (1983) The prophylactic reduction of aluminium intake. Food
Chem Toxicol, 21: 103-109.
Liss L & Thornton DJ (1986) The rationale for aluminum absorption
control in early stages of Alzheimer disease. Neurobiol Aging,
7: 552-554.
Litaor MI (1987) Aluminum chemistry: fractionation, speciation, and
mineral equilibria of soil interstitial waters of an alpine watershed,
Front Range, Colorado. Geochim Cosmochim Acta, 51: 1285-1295.
Ljunggren KG, Lidums V, & Sj�gren B (1991) Blood and urine levels of
aluminium among workers exposed to aluminium flakes. Br J Ind Med,
48: 106-109.
Llobet JM, Domingo JL, Gomez M, Tomas JM, & Corbella J (1987) Acute
toxicity studies of aluminium compounds: antidotal efficacy of several
chelating agents. Pharmacol Toxicol, 60: 280-283.
Longstreth WT, Rosenstock L, & Heyer NJ (1985) Potroom palsy?
Neurologic disorder in three aluminum smelter workers. Arch Int Med,
145: 1972-1975.
Losno R, Colin JL, Le Bris N, Bergametti G, Jickells T, & Lim B (1993)
Aluminium solubility in rainwater and molten snow. J Atmos Chem,
17: 29-43.
Lovell MA, Ehmann WD, & Markesbery WR (1993) Laser microprobe analysis
of brain aluminium in Alzheimer's disease. Ann Neurol, 33: 36-42.
L�kewille A & Van Breemen N (1992) Aluminium precipitates from
groundwater of an aquifer affected by acid atmospheric deposition in
the Senne, northern Germany. Water Air Soil Pollut, 63: 411-416.
Lydersen E, Bj�rnstad HE, Salbu B, & Pappas AC (1987) Trace element
speciation in natural waters using hollow-fiber ultrafiltration. In:
Ladner L ed. Speciation of metals in water, sediments and soil
systems: Lecture notes in earth sciences. Berlin, Heidelberg, New
York, Springer Verlag, vol 11, pp 58-97.
Lydersen E, Salbu B, Poleo ABS, & Muniz IP (1990) The influence of
temperature on aqueous aluminium chemistry. Water Air Soil Pollut,
51: 203-215.
Lydersen E, Poleo ABS, Nandrup Pettersen M, Riise G, Salbu B, Kroglund
F, & Rosseland BO (1994) The importance of "in situ" measurements to
relate toxicity and chemistry in dynamic aluminium freshwater systems.
J Ecol Chem, 3: 357-365.
McCahon CP, Pascoe D, & McKavanagh C (1987) Histochemical observations
on the salmonids Salmo salar L. and Salmo trutta L. and the
ephemeropterans Baetis rhodani(Pict.) and Ecdyonurus venosus
(Fabr.) following a simulated episode of acidity in an upland stream.
Hydrobiologia, 153: 3-12.
McCormack KM, Ottosen LD, Sanger VL, Sprague S, Mayor GH, & Hook JB
(1979) Effect on prenatal administration of aluminum and parathyroid
hormone on fetal development in the rat. Proc Soc Exp Biol Med,
161: 74-77.
McDermott JR, Smith A, Ward MK, Parkinson IS, & Kerr DNS (1978)
Brain-aluminium concentration in dialyses encephalopathy. Lancet,
1: 901-904.
McDermott JR, Smith I, Iqbal K, & Wisniewski HM (1979) Brain aluminum
in aging and Alzheimer disease. Neurology, 29: 809-814.
Mach CE & Brezonik PL (1989) Trace metal research at Little Rock Lake,
Wisconsin: background data, enclosure experiments, and the first three
years of acidification. Sci Total Environ, 87/88: 269-285.
MacKenzie FT, Stoffyn M, & Wollast R (1978) Aluminum in seawater:
control by biological activity. Science, 199: 680-682.
Mackie GL (1989) Tolerances of five benthic invertebrates to hydrogen
ions and metals (Cd, Pb, Al). Arch Environ Contam Toxicol,
18: 215-223.
McLachlan DRC (1989) Aluminium neurotoxicity: Criteria for assigning a
role in Alzheimer's disease. In: Lewis TE ed. Environmental chemistry
and toxicology of aluminium. Chelsea, Michigan, Lewis Publishers Inc.,
pp 299-315.
McLachlan DRC (in press) Aluminium and the risk for Alzheimer's
disease. Environmetrics.
McLachlan DR & Massiah J (1992) Aluminum ingestion: A risk factor for
Alzheimer's disease? Chapter 4. In: Isaacson RL & Jensen KF ed. The
vulnerable brain and environmental risks - Volume 2. Toxins in food.
New York, London, Plenum Press, chapter 4, pp 49-60.
McLachlan DR, Bergeron C, Smith JE, Boomer D, & Rifat SL (1996) Risk
for neuropathologically confirmed Alzheimer's disease and residual
aluminium in municipal drinking water employing weighted residential
histories. Neurology, 46(2): 401-405.
McLaughlin AIG, Kazantzis G, King E, Teare D, Porter RJ, & Owen R
(1962) Pulmonary fibrosis and encephalopathy associated with the
inhalation of aluminium dust. Br J Ind Med, 19: 253-263.
McMillan TM, Freemont AJ, Herxheimer A, Denton J, Taylor AP, Pazianas
M, Cummin ARC, & Eastwood JB (1993) Camelford water poisoning
accident: serial neuropsychological assessments and further
observations on bone aluminium. Hum Exp Toxicol, 12: 37-42.
Madigosky SR, Alvarez-Hernandez X, & Glass J (1991) Lead, cadmium, and
aluminum accumulation in the red swamp crayfish Procambarus clarkii
G. collected from roadside drainage ditches in Louisiana. Arch Environ
Contam Toxicol, 20: 253-258.
Madigosky SR, Alvarez-Hernandez X, & Glass J (1992) Concentrations of
aluminum in gut tissue of crayfish ( Procambarus clarkii), purged in
sodium chloride. Bull Environ Contam Toxicol, 49: 626-632.
Malley DF & Chang PSS (1985) Effects of aluminum and acid on calcium
uptake by the crayfish Orconectes virilis. Arch Environ Contam
Toxicol, 14: 739-747.
Malley DF, Chang PSS, & Moore CM (1987) Changes in the aluminum
content of tissues of crayfish held in the laboratory and in
experimental field enclosures. Can Tech Rep Fish Aquat Sci,
1480: 54-68.
Manna GK & Das RK (1972) Chromosome aberration in mice induced by
aluminum chloride. Nucleus, 15: 180-186.
Mantyh PW, Ghilardi JR, Rogers S, Demaster E, Clark JA, Stimson ER, &
Maggio JE (1993) Aluminum, iron, and zinc ions promote aggregation of
physiological concentrations of �-amyloid peptide. J Neurochem,
61: 1171-1174.
Markesbery WR, Ehmann WD, Hossain TIM, Alauddin M, & Goodin DT (1981)
Instrumental neutron activation analysis of brain aluminum in
Alzheimer disease and aging. Ann Neurol, 10: 511-516.
Marsden SNE, Parkinson IS, Ward MK, Ellsi HA, & Kerr DNS (1979)
Evidence for aluminium accumulation in renal failure. Proc Eur Dial
Transplant Assoc, 16: 588-596.
Martin RB (1986) The chemistry of aluminium as related to biology and
medicine. Clin Chem, 32: 1797-1806.
Martin RB (1992) Aluminium speciation in biology. In: Aluminium in
biology and medicine. New York, Chichester, Brisbane, Toronto, John
Wiley and Sons, pp 5-25 (Ciba Foundation Symposium No. 169).
Martyn CN, Osmond C, Edwardson JA, Barker DJP, Harris EC, & Lacey RF
(1989) Geographical relation between Alzheimer's disease and aluminium
in drinking water. Lancet, 1: 59-62.
Marzin DR & Phi HV (1985) Study of the mutagenicity of metal
derivatives with Salmonella typhimurium TA102. Mutat Res,
155: 49-51.
Mason CF & MacDonald SM (1988) Metal contamination in mosses and otter
distribution in a rural Welsh river receiving mine drainage.
Chemosphere, 17(6): 1159-1166.
Mauras Y & Allain P (1985) Automatic determination of aluminum in
biological samples by inductively coupled plasma emission
spectrometry. Anal Chem, 57: 1706.
Mauras Y, Allain P, & Riberi P (1982) Etude de l'absorption digestive
de l'hydrocarbonate d'aluminium chez l'individu sain. Th�rapie,
37: 598-600.
Mayor GH, Keiser JA, Makdani D, & Ku PK (1977) Aluminum absorption
and distribution: Effect of parathyroid hormone. Science,
197: 1187-1189.
Meding B, Augustsson A, & Hansson C (1984) Short communications. Patch
test reactions to aluminium. Contact Dermatitis, 10: 107.
Mersch J, Gu�rold F, Rousselle P, & Pihan J-C (1993) Transplanted
aquatic mosses for monitoring trace metal mobilization in acidified
streams of the Vosges Mountains, France. Bull Environ Contam Toxicol,
51: 255-259.
Mesco ER, Kachen C, & Timiras PS (1991) Effects of aluminium on tau
proteins in human neuroblastoma cells. Mol Chem Neuropathol,
14(3): 199-212.
Meyer FA & Kasper W (1942) [Examination of the effects of aluminium on
the lung.] Dtsch Arch Klin Med, 189: 471-495 (in German).
Michel P, Commenges D, Dartigues JF, Gagnon M, Barberger-Gateau P,
Letenneur L, & Paquid Research Group (1991) Study of the relationship
between aluminium concentration in drinking water and risk of
Alzheimer's disease. In: Iqbal K, McLachlan DRC, Winblad B, &
Wisniewski HM ed. Alzheimer's disease: Basic mechanisms, diagnosis and
therapeutic strategies. New York, John Wiley, pp 387-391.
Miller RR, Churg AM, Hutcheon M, & Lam S (1984) Pulmonary alveolar
proteinosis and aluminum dust exposure. Am Rev Respir Dis,
130: 312-315.
Mitchell J, Manning GB, Molyneux M, & Lane RE (1961) Pulmonary
fibrosis in workers exposed to finely powdered aluminium. Br J Ind
Med, 18: 10-20.
Mizuno N & Yoshida H (1993) Effects of exchangeable aluminium on the
reduction of potato scab. Plant Soil, 155/156: 505-508.
Monteagudo FSE, Isaacson LC, Wilson G, Hickman R, & Folb PI (1988)
Aluminium exretion by the distal tubule of the pig kidney. Nephron,
49: 245-250.
Moomaw JC, Nakamura MT, & Sherman GD (1959) Aluminium in some Hawaiian
plants. Pac Sci, 13: 335-341.
M�hlenberg W (1990) [High aluminium levels in wells of the southern
sandy health-land of lower Saxony by acid precipitation:
Interpretation from hygienic and ecological points of view.]
�ffentl Gesundh.wes, 52: 1-8 (in German with English summary).
Mulder J, Van Breemen N, Rasmussen L, & Driscoll CT (1989) Aluminum
chemistry of acidic sandy soils with various inputs of acidic
deposition in the Netherlands and in Denmark In: Lewis TE ed.
Environmental chemistry and toxicology of aluminum. Proceedings of the
194th Annual Meeting of the American Chemical Society, New Orleans,
LA, 30 August-4 September 1987. Chelsea, Michigan, Lewis Publishers
Inc., pp 171-169.
Muller G, Bernuzzi V, Desor D, Hutin M-F, Burnel D, & Lehr PR (1990)
Developmental alterations in offspring of female rats orally
intoxicated by aluminum lactate at different gestation periods.
Teratology, 42: 253-261.
Muller G, Hutin MF, Burnel D, & Lehr PR (1992) Aluminum transfer
through milk in female rats intoxicated by aluminum chloride. Biol
Trace Elem Res, 34: 79-87.
Muller G, Burnel D, Gery A, & Lehr PR (1993) Element variations in
pregnant and nonpregnant female rats orally intoxicated by aluminium
lactate. Biol Trace Elem Res, 39: 211-219.
Muniz IP & Leivestad H (1980) Toxic effects of aluminium on the brown
trout, Salmo trutta L. In: Drabl�s D & Tollan A ed. Ecological
impact of acid precipitation: Proceedings of an International
Conference, Sandefjord, Norway, 11-14 March 1980. Oslo-�s, SNSF,
pp 320-321.
Muramoto S (1981) Influence of complexans (NTA, EDTA) on the toxicity
of aluminum chloride and sulfate to fish at high concentrations. Bull
Environ Contam Toxicol, 27: 221-225.
Nagy S & Nikdel S (1986) Tin, iron and aluminum contents of
commercially canned single-strength grapefruit juice stored at
varying temperatures. J Agric Food Chem, 34: 588-593.
Nalewajko C & Paul B (1985) Effects of manipulations of aluminum
concentrations and pH on phosphate uptake and photosynthesis of
planktonic communities in two Precambrian shield lakes. Can J Fish
Aquat Sci, 42: 1946-1953.
Neal C (1988) Aluminium solubility relationships in acid waters: a
practical example of the need for a radical reappraisal. J Hydrol,
104: 141-159.
Neal C & Williams RJ (1988) Towards establishing aluminium hydroxy
silicate solubility relationships for natural waters. J Hydrol,
97: 347-352.
Neal C, Whitehead P, Neale R, & Cosby J (1986) Modelling the effects
of acidic deposition and conifer afforestation on stream acidity in
the British uplands. J Hydrol, 86: 15-26.
Neal C, Reynolds B, Stevens PA, Hornung M, & Brown SJ (1990) Dissolved
inorganic aluminium in acidic stream and soil waters in Wales. In:
Edwards RW, Gee AS, & Stoner JH ed. Acid waters in Wales. Dordrecht,
Boston, London, Kluwer Academic Publishers, pp 173-188.
Neal C, Reynolds B, Smith CJ, Hill S, Neal M, Conway T, Ryland GP,
Jeffrey H, Robson AJ, & Fisher R (1992) The impact of conifer
harvesting on stream water pH, alkalinity and aluminium concentrations
for the British uplands: an example for an acidic and acid sensitive
catchment in mid-Wales. Sci Total Environ, 126: 75-87.
Neri LC & Hewitt D (1991) Aluminium, Alzheimer`s disease, and drinking
water. Lancet, 338: 390.
Neville CM & Campbell PGC (1988) Possible mechanisms of aluminum
toxicity in a dilute, acidic environment to fingerlings and older life
stages of salmonids. Water Air Soil Pollut, 42: 311-327.
NFA (1993) The 1992 Australian market basket survey - A total diet
survey of pesticides and contaminants. Canberra, Australian National
Food Authority, 96 pp.
Nieboer E, Gibson BL, Oxman AD, & Kramer JR (1995) Health effects of
aluminium: A Critical review with emphasis in drinking water. Environ
Rev, 3: 29-85.
Nilsson SI & Bergkvist B (1983) Aluminium chemistry and acidification
processes in a shallow podzol on the Swedish westcoast. Water Air Soil
Pollut, 20: 311-329.
NIOSH (1984) Aluminium and compounds: Method 7300. In: NIOSH manual.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, vol 5, p 173/1.
Nishioka H (1975) Mutagenic activities of metal compounds in bacteria.
Mutat Res, 31: 185-189.
Nordal KP, Dahl E, Thomassen Y, Brodwall EK, & Halse J (1988)
Seasonal variations in serum aluminium concentrations. Pharmacol
Toxicol, 62: 80-83.
Norrgren L & Degerman E (1993) Effects of different water qualities
on the early development of Atlantic salmon and brown trout exposed
in situ. Ambio, 22(4): 213-218.
Norton SA (1971) Geochemical cycles involving flora, lake water and
bottom sediments. Orono, Maine, University of Maine, Water Resources
Research Center (NTIS PB-206 197).
Nyholm NEI (1981) Evidence of involvement of aluminum in causation of
defective formation of eggshells and of impaired breeding in wild
passerine birds. Environ Res, 26: 363-371.
Oberly TJ, Piper CE, & McDonald DS (1982) Mutagenicity of metal
salts in the L5178Y mouse lymphoma assay. J Toxicol Environ Health,
9: 367-376.
O'Donnell TV, Welford B, & Coleman ED (1989) Potroom asthma: New
Zealand experience and follow-up. Am J Ind Med, 15: 43-49.
Ogilvie DM & Stechey DM (1983) Effects of aluminum on respiratory
responses and spontaneous activity of rainbow trout, Salmo
gairdneri. Environ Toxicol Chem, 2: 43-48.
Ohtawa M, Seko M, & Takayama F (1983) Effect of aluminum ingestion on
lipid peroxidation in rats. Chem Pharm Bull, 31: 1415-1418.
OMEE (1995) Drinking water surveillance programme - Province of
Ontario. Toronto, Canada, Ontario Ministry of Environment and Energy.
Ondov JM, Zoller WH, & Gordon GE (1982) Trace element emissions on
aerosols from motor vehicles. Environ Sci Technol, 16(6): 318-328.
Ondreicka R, Ginter E, & Kortus J (1966) Chronic toxicity of aluminium
in rats and mice and its effects on phosphorus metabolism. Br J Ind
Med, 23: 305-312.
Ormerod SJ, Allinson N, Hudson D, & Tyler SJ (1986) The distribution
of breeding dippers ( Cinclus cinclus (L.); Aves) in relation to
stream acidity in upland Wales. Freshw Biol, 16: 501-507.
Ormerod SJ, Boole P, McCahon CP, Weatherley NS, Pascoe D, & Edwards RW
(1987a) Short-term experimental acidification of a Welsh stream:
comparing the biological effects of hydrogen ions and aluminium.
Freshw Biol, 17: 341-356.
Ormerod SJ, Weatherly NS, French P, Blake S, & Jones WM (1987b) The
physiological response of brown trout Salmo trutta to induced
episodes of low pH and elevated aluminium in a Welsh hill-stream In:
Witters H & Vanderborght O ed. Ecophysiology of acid stress in aquatic
organisms: Proceedings of an International Symposium, Antwerp, 13-16
January 1987. Antwerp, Belgian Royal Zoological Society, pp 435-447.
Ormerod SJ, Bull KR, Cummins CP, Tyler SJ, & Vickery JA (1988) Egg
mass and shell thickness in dippers Cinclus cinclus in relation to
stream acidity in Wales and Scotland. Environ Pollut, 55: 107-121.
Ormerod SJ, Donald AP, & Brown SJ (1989) The influence of plantation
forestry on the pH and aluminium concentration of upland Welsh
streams: a re-examination. Environ Pollut, 62: 47-62.
Ormerod SJ, Weatherley NS, Merrett WJ, Gee AS, & Whitehead PG (1990)
Restoring acidified streams in upland Wales: a modelling comparison of
the chemical and biological effects of liming and reduced sulphate
deposition. Environ Pollut, 64: 67-85.
Ott SM, Feist E, Andress DL, Liu CC, Sherrard DJ, Alfrey AC,
Slatopolsky E, & Howard GA (1987) Development and reversibility
of aluminum-induced bone lesions in the rat. J Lab Clin Med,
109: 40-47.
Ottley CJ & Harrison RM (1993) Atmospheric dry deposition flux of
metallic species to the North Sea. Atmos Environ, 27A(5): 685-695.
Panasenkov YV (1987) Determination of the effect of aluminium sulphate
on natural microbial coenoses in experiment. J Hyg Epidemiol Microbiol
Immunol, 31(3): 279-286.
Parent L & Campbell PGC (1994) Aluminum bioavailability to the green
alga Chlorella pyrenoidosa in acidified synthetic soft water.
Environ Toxicol Chem, 13(4): 587-598.
Parker DR & Bertsch PM (1992a) Identification and quantification of
the "Al13" tridecameric polycation using ferron. Environ Sci Technol,
26(5): 908-914.
Parker DR & Bertsch PM (1992b) Formation of the "Al13" tridecameric
polycation under diverse synthesis conditions. Environ Sci Technol,
26(5): 914-916.
Paternain JL, Domingo JL, Llobet JM, & Corbella J (1988) Embryotoxic
and teratogenic effects of aluminum nitrate in rats upon oral
administration. Teratology, 38: 253-257.
Pennington JAT & Schoen SA (1995) Estimates of dietary exposure to
aluminium. Food Addit Contam, 12(1): 119-128.
Perl DP, Gajdusek DC, Garruto RM, Yanagihara RT, & Gibbs CJ (1982)
Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis
and parkinsonism-dementia of Guam. Science, 217: 1053-1055.
Petit TL, Biedermann GB, & McMullen PA (1980) Neurofibrillary
degeneration, dying back and learning-memory deficits after aluminum
administration, implications for brain aging. Exp Neurol,
67: 152-162.
Petrich SM & Reish DJ (1979) Effects of aluminium and nickel on
survival and reproduction in polychaetous annelids. Bull Environ
Contam Toxicol, 23: 698-702.
Pettersen JC, Hackett DS, & Zwicker GM (1990) Twenty-six week toxicity
study with kasal (basic sodium aluminum phosphate) in beagle dogs.
Environ Geochem Health, 12: 121-123.
Pigott GH & Ishmael J (1981) An assessment of the fibrogenic potential
of two refractory fibres by intraperitoneal injection in rats. Toxicol
Lett, 8: 153.
Pigott GH, Gaskell BA, & Ishmael J (1981) Effects of long term
inhalation of alumina fibres in rats. Br J Exp Pathol, 62: 323-331.
Pillay KKS & Thomas CC (1971) Determination of the trace element
levels in atmospheric pollutants by neutron activation analysis.
J Radioanal Chem, 7: 107-118.
Pitchai R, Subramanian R, Selvapathy P, & Elangovan R (1992) Aluminium
content of drinking water in Madras. Water Supply, 10(4): 83-86.
Plankey BJ & Patterson HH (1987) Kinetics of aluminum-fulvic acid
complexation in acidic waters. Environ Sci Technol, 21: 595-601.
Platts MM, Owen G, & Smith S (1984) Water purification and the
incidence of fractures in patients receiving home haemodialysis
supervised by a single centre: evidence for "safe" upper limit of
aluminium in water. Br Med J, 288: 969-972.
Pol�o ABS, Lydersen E, Rosseland BO, Kroglund F, Salbu B, Vogt RD, &
Kvellestad A (1994) Increased mortality of fish due to changing
Al-chemistry of mixing zones between limed streams and acidic
tributaries. Water Air Soil Pollut, 75: 339-351.
Priest ND (1990) The distribution and behaviour of heavy metals in the
skeleton and body: studies with bone-seeking radionuclides. In: Priest
ND & Van de Vyver F ed. Trace metals and fluoride in bones and teeth.
Boca Raton, Florida, CRC Press, pp 83-139.
Priest ND (1993) The bioavailability and metabolism of aluminium
compounds in man. Proc Nutr Soc, 52: 231-240.
Priest ND (1994) A human volunteer feeding study using aluminium-26
labelled aluminium citrate, aluminium hydroxide and aluminium
hydroxide in the presence of citrate. Harwell, Oxfordshire, AEA
Technology (Report No. AEA-TPD-268).
Priest ND, Newton, D, & Talbot RJ (1991) Metabolism of aluminium-26
and gallium-67 in a volunteer following their injections as citrates.
Harwell, Oxfordshire, AEA Technology (Report No. AEA-EE-0106).
Priest N, Newton D, & Talbot R (1992) Metabolism of Al-26 and Ga-67
following injection as citrates. In: Gitelman HJ ed. Proceedings of
the Second International Conference on Aluminium and Health.
Washington, DC, Alumunium Association, pp 7-10.
Priest ND, Talbot RJ, & Austin JG (1995a) The bioavailability in young
adults of aluminium ingested in drinking water. Harwell, Oxfordshire,
AEA Technology (Report No. AEA-TPD-269).
Priest ND, Newton D, Day JP, Talbot RJ, & Warner AJ (1995b) Human
metabolism of aluminium-26 and gallium-67 injected as citrates. Hum
Exp Toxicol, 14(3): 287-293.
Priest N, Newton D, & Talbot R (1996) Studies on the metabolism of
injected and ingested aluminium using 26Al. In: Proceedings of the
Third International Conference on Aluminium and Health, Miami,
Florida. Washington, DC, Aluminium Association, pp 30-34.
Provan SD & Yokel RA (1988) Aluminum uptake by the in situ rat gut
preparation. J Pharmacol Exp Ther, 245: 928-931.
Quarles LD (1991) Paradoxical toxic and trophic osseous actions
of aluminum: Potential explanations. Miner Electrolyte Metab,
17: 233-239.
Quarles LD, Dennis VW, Gitelman HJ, Harrelson JM, & Drezner MK (1985)
Aluminum deposition at the osteoid-bone interface. J Clin Invest,
75: 1441-1447.
Quarles LD, Gitelman HJ, & Drezner MK (1988) Induction of bone
formation in the beagle: a novel effect of aluminum. J Clin Invest,
81: 1056-1066.
Quarles LD, Gitelman HJ, & Drezner MK (1989) Aluminium-induced de novo
bone formation in the beagle: a parathyroid hormone-dependent event.
J Clin Invest, 83: 1644-1650.
Quarles LD, Murphy G, Vogler JB, & Drezner MK (1990) Aluminum-induced
neo-osteogenesis: a generalized process affecting trabecular
networking in the axial skeleton. J Bone Miner Metab, 5: 625-635.
Que Hee SS & Boyle JR (1988) Simultaneous multielemental analysis of
some environmental and biological samples by inductively coupled
plasma atomic emission spectrometry. Anal Chem, 60: 1033-1042.
Rabe A, Lee MH, Shek J, & Wisniewski HM (1982) Learning deficit in
immature rabbits with aluminum-induced neurofibrillary changes. Exp
Neurol, 76: 441-446.
Rahn KA (1981) Atmospheric, riverine and oceanic sources of
seven trace constituents to the Arctic Ocean. Atmos Environ,
15(8): 1507-1516.
Rao KSJ & Divakar S (1993) Spectroscopic studies on the effects of
aluminum ion on calf-thymus DNA. Bull Environ Contam Toxicol,
50: 92-99.
Recker RR, Blotcky AJ, Leffler JA, & Rack EP (1977) Evidence for
aluminum absorption from the gastrointestinal tract and bone
deposition by aluminum carbonate ingestion with normal renal function.
J Lab Clin Med, 90: 810-815.
Reich PB, Oleskyn J, & Tjoelker MG (1994) Relationship of aluminium
and calcium to net CO2 exchange among diverse Scots pine provenances
under pollution stress in Poland. Oecologia, 97: 82-92.
Reynolds B, Stevens PA, Adamson JK, Hughes S, & Roberts JD (1992)
Effects of clearfelling on stream and soil water aluminium chemistry
in three UK forests. Environ Pollut, 77: 157-165.
Rifat SL, Eastwood MR, Crapper McLachlan DR, & Corey PN (1990) Effect
of exposure of miners to aluminium powder. Lancet, 336: 1162-1165.
Robertson JA, Felsenfeld AJ, Haygood CC, Wilson P, Clarke C, & Llach F
(1983) Animal model of aluminum-induced osteomalacia: Role of chronic
renal failure. Kidney Int, 23: 327-335.
Rodriguez M, Felsenfeld AJ, & Llach F (1990) Aluminum administration
in the rat separately affects the osteoblast and bone mineralization.
J Bone Miner Res, 5: 59-68.
R�llin HB, Theodorou P, & Kilroe-Smith TA (1991a) Deposition of
aluminium in tissues of rabbits exposed to inhalations of low
concentration of Al2O3 dust. Br J Ind Med, 48: 389-391.
R�llin HB, Theodorou P, & Kilroe-Smith TA (1991b) The effect of
exposure to aluminium on concentrations of essential metals in serum
of foundry workers. Br J Ind Med, 48: 243-246.
Ronneberg A & Langmark F (1992) Epidemiologic evidence of cancer in
aluminium reduction plant workers. Am J Ind Med, 22: 573-590.
Rosa IV, Henry PR, & Ammermann CB (1982) Interrelationship of dietary
phosphorus, aluminium and iron on performance and tissue mineral
composition in lambs. J Anim Sci, 1982: 1231-1240.
Rosseland BO & Skogheim OK (1984) A comparative study on salmonid fish
species in acid aluminium-rich water. II. Physiological stress and
mortality of one- and two-year-old fish. Oslo, Norway, Institute of
Fresh Water Ecology, pp 186-194 (Research Report No. 61).
Rosseland BO & Staurnes M (1994) Physiological mechanisms for toxic
effects and resistance to acidic water: an ecophysiological and
ecotoxicological approach. In: Steinberg CEW & wright RF ed.
Acidification of freshwater ecosystems: Implications for the future.
New York, Chichester, Brisbane, Toronto, John Wiley and Sons,
pp 228-246.
Rosseland BO, Eldhuset TD, & Staurnes M (1990) Environmental effects
of aluminium. Environ Geochem Health, 12(1-2): 17-27.
Rosseland BO, Blakar IA, Bulger A, Kroglund F, Kvellstad A, Lydersen
E, Oughton DH, Salbu B, Staurnes M, & Vogt R (1992) The mixing zone
between limed and acidic river waters: complex aluminium chemistry and
extreme toxicity for salmonids. Environ Pollut, 78(1/3): 3-8.
Roy AK, Sharma A, & Talukder G (1988) Some aspects of aluminum
toxicity in plants. Bot Rev, 54(2): 145-178.
Roy AK, Talukder G, & Sharma A (1990) Effects of aluminium sulphate on
human leukocyte chromosomes in vitro. Mutat Res, 244: 179-184.
Roy AK, Sharma A, & Talukder G (1991a) Effects of aluminum salts on
bone marrow chromosomes in rats in vivo. Cytobios, 66: 105-112.
Roy AK, Talukder G, & Sharma A (1991b) Similar effects in vivo of
two aluminum salts on the liver, kidney, bone, and brain of
Rattus norvegicus. Bull Environ Contam Toxicol, 47: 288-295.
Rueter JG, O'Reilly KT, & Petersen RR (1987) Indirect aluminum
toxicity to the green alga Scenedesmus through increased cupric ion
activity. Environ Sci Technol, 21(5): 435-438.
Ryther J, Losordo TM, Furr AK, Parkinson TF, Gutenman WH, Pakkala IS,
& Lisk DJ (1979) Concentration of elements in marine organisms
cultured in seawater flowing through coal-fly ash. Bull Environ Contam
Toxicol, 23: 207-210.
Sackett W & Arrhenius G (1962) Distribution of aluminum species in
the hydrosphere. I. Aluminum in the ocean. Geochim Cosmochim Acta,
26: 955-968.
Saia AN, Slagsvold P, Bergh H, & Laksesvela B (1977) High fluorine
water to weather sheep maintained in pens. Nord Vet Med,
29: 172-180.
Saia B, Cortese S, Piazza G, Camposampietro A, & Clonfero E (1981)
Chest x-ray findings among aluminium production plant workers. Med
Lav, 4: 323-329.
St George-Hyslop P, Haines J, Rogaev E, Mortilla M, Vaula G, Pericak-
Vance M, Foncin JF, Montesi M, Bruni A, Sorbi S, Raincro I, Pinessi I,
Pollen D, Polinski R, Nee I, Kennedy J, Macciardi F, Rogaeva E, Liang
Y, Alexandrova N, Lukiw W, Schlumpf K, Tanzi R, Tsuda T, Farrer L,
Cantu J-M, Duara R, Amaducci L, Bergamini I, Gusella J, Roses A, &
Crapper McLachlan D (1992) Genetic evidence for a novel familial
Alzheimer disease gene on chromosome 14. Nature (Genet), 2: 330-334.
Sanin S, Tuncel G, Gaines AF, & Balkas TI (1992) Concentrations and
distributions of some major and minor elements in the sediments of
the River G�ksu and Tasucu Delta, Turkey. Mar Pollut Bull,
24(3): 167-169.
Santos F, Chan JCM, Yang MS, Savory J, & Wills MR (1987) Aluminum
deposition in the central nervous system: Preferential accumulation in
the hippocampus in weenling rats. Med Biol, 65: 53-55.
Sanz-Medel A, Roza RR, Alonson RG, Vallina AN, & Cannata J (1987)
Atomic spectrometric methods (atomic absorption and inductively
coupled plasma atomic emission) for the determination of aluminium at
the parts per billion level in biological fluids. J Anal Atmos Spec,
2: 177-184.
Saunders A, Strittmatter WJ, Schmechel St George-Hyslop P, Pericak-
Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper MacLachlan DR, Alberts
MJ, Hulette C, Crain B, Goldgaber D, & Roses AD (1993) Association of
apolipoprotein E allelle E4 with late onset familial and sporadic
Alzheimer disease. Neurology, 43: 1467-1472.
Savory J, Nicholson JR, & Wills MR (1987) Is aluminium leaching
enhanced by fluoride? Nature (Lond), 327: 107-108.
Sax NI & Lewis RJSR (1987) Hawley's condensed chemical dictionary,
11th ed. New York, Van Nostrand Reinhold Company, pp 42-51.
Schaller KH & Valentin H (1984) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Commission of the European Communities, pp 21-29.
Schecher WD & Driscoll CT (1987) An evaluation of uncertainty
associated with aluminum equilibrium calculations. Water Resour Res,
23(4): 525-534.
Schenck RU, Bjorksten J, & Yaeger L (1989) Composition and
consequences of aluminum in water, beverages, and other ingestibles.
In: Lewis TE ed. Environmental chemistry and toxicology of aluminum.
Chelsa, Michigan, Lewis Publishers Inc., pp 247-269.
Schofield CL & Trojnar JR (1980) Aluminum toxicity to brook trout
( Salvelinus fontinalis) in acidified waters. In: Toribara TY, Miller
MW, & Marrow PE ed. Polluted rain: Proceedings of the 12th Rochester
International Conference on Environmental Toxicity, Rochester, May
1979. New York, London, Plenum Press, pp 341-366.
Schreeder MT, Favero MS, Hughes JR, Petersen NJ, Bennett PH, & Maynard
JE (1983) Dialysis encephalopathy and aluminum exposure: An
epidemiologic analysis. J Chronic Dis, 36: 581-593.
Schwedt G (1989) [Photometric determination of aluminium.] Stuttgart,
Wissenschaftliche Verlagsgesellschaft mbH, pp IV/A1/1-4, II/A1/1-3 (in
German).
Sedman AB, Alfrey AC, Miller NL, & Goodman WG (1987) Tissue and
cellular basis for impaired bone formation in aluminum-related
osteomalacia in the pig. J Clin Invest, 79: 86-92.
Segner H, Marthaler R, & Linnenbach M (1988) Growth, aluminium uptake
and mucous cell morphometrics of early life stages of brown trout,
Salmo trutta, in low pH water. Environ Biol Fish, 21(2): 153-159.
Seip HM, M�ller L, & Naas A (1984) Aluminium speciation: comparison of
two spectrophotometric analytical methods and observed concentrations
in some acidic aquatic systems in southern Norway. Water Air Soil
Pollut, 23: 81-95.
Seip HM, Christophersen N, & Sullivan TJ (1989) Episodic variations in
streamwater aluminum chemistry at Birkenes, southernmost Norway. In:
Lewis TE ed. Environmental chemistry and toxicology of aluminum.
Proceedings of the 194th Annual Meeting of the American Chemical
Society, New Orleans, LA, 30 August-4 September 1987. Chelsea,
Michigan, Lewis Publishers Inc., pp 159-169.
Shacklette HT & Boerngen JG (1984) Element concentrations in soils and
other surficial materials of the conterminous United States.
Washington, DC, US Department of Interior, Geological Survey, 105 pp
(Geological Survey Professional Paper No. 1270).
Shaver CG & Riddell AR (1947) Lung changes associated with the
manufacture of alumina abrasives. J Ind Hyg Toxicol, 29: 145-157.
Sheets WD (1957) Toxicity studies of metal-finishing wastes. Sew Ind
Wastes, 29(12): 1380-1384.
Shin R-W, Lee VM-Y, & Trojanowski JQ (1994) Aluminum modifies the
properties of Alzheimer's disease PHF(tau) proteins in vivo and
in vitro. J Neurosci, 14(11): 7221-7233.
Shore D & Wyatt RJ (1983) Aluminum and Alzheimer's disease. J Nerv
Ment Dis, 71: 553-558.
Siddens LK, Seim WK, Curtis LR, & Chapman GA (1986) Comparison of
continuous and episodic exposure to acidic, aluminum-contaminated
waters of brook trout ( Salvelinus fontinalis). Can J Fish Aquat Sci,
43: 2036-2040.
Silvey WD (1967) Occurrence of selected minor elements in the waters
of California. Washington, DC, US Department of Interior, Geological
Survey, 25 pp (Geological Survey-Water Supply Paper No. 1535-L).
Simons LH, Monaghan PH, & Taggart MS (1953) Aluminum and iron in
Atlantic and Gulf of Mexico surface waters. Anal Chem, 25: 989-990.
Simonsson Bg, Sj�berg A, Rolf C, & Haeger-Aronsen B (1985) Acute and
long-term airway hyperreactivity in aluminium-salt exposed workers
with nocturnal asthma. Eur J Respir Dis, 66: 105-118.
Simpson W, Kerr DNS, Hill AVL, & Siddiqui JY (1973) Skeletal changes
in patients on regular hemodialysis. Diagn Radiol, 107: 313-320.
Sj�gren B & Elinder CG (1992) Proposal of a dose-response relationship
between aluminium welding fume exposure and effect on the central
nervous system. Med Lav, 83(5): 484-488.
Sj�gren B & Ulfvarson U (1985) Respiratory symtoms and pulmonary
function among welders working with aluminum, stainless steel and
railroad tracks. Scand J Work Environ Health, 11: 27-32.
Sj�gren B, Lundberg I, & Lidums V (1983) Aluminium in the blood and
urine of industrially exposed workers. Br J Ind Med, 40: 301-304.
Sj�gren B, Lidums V, Hakansson M, & Hedstrom L (1985) Exposure and
urinary excretion of aluminum during welding. Scand J Work Environ
Health, 11: 39-43.
Sj�gren B, Elinder CG, Lidums V, & Chang G (1988) Uptake and urinary
excretion of aluminum among welders. Int Arch Occup Environ Health,
60: 77-79.
Sj�gren B, Gustavsson P, & Hogstedt C (1990) Neuropsychiatric
symptoms among welders exposed to neurotoxic metals. Br J Ind Med,
47: 704-707.
Sj�gren B, Ljunggren KG, Almkvist O, Frech W, & Basun H (1994a)
Aluminosis and dementia. Lancet, 344(8930): 1154.
Sj�gren B, Iregren A, Frech W, Hagman M, Johansson L, Tesarz M, &
Wennberg A (1994b) [Effects on the nervous system among welders
exposed to aluminum or manganese.] Solna, Sweden, National Institute
of Occupational Health, 27 pp (Work and Health Scientific Publication
Series) (in Swedish with English summary).
Sj�str�m J (1994) Aluminium and sulphate in acidic soils and
groundwaters on the Swedish west coast. Water Air Soil Pollut,
75: 127-139.
Skalsky HL & Carchman RA (1983) Aluminum homeostasis in man. J Am Coll
Toxicol, 2: 405-423.
Skogheim OK & Rosseland BO (1986) Mortality of smolt of Atlantic
salmon, Salmo salar L., at low levels of aluminium in acidic
softwater. Bull Environ Contam Toxicol, 37: 258-265.
Skogheim OK, Rosseland BO, & Sevaldrud IH (1984) Deaths of spawners of
Atlantic salmon ( Salmo salar L.) in River Ogna, SW Norway, caused by
acidified aluminium-rich water. Oslo, Norway, Institute of Fresh Water
Ecology, pp 195-202 (Research Report No. 61).
Slanina P, Falkeborn Y, Frech W, & Cedergren A (1984) Aluminium
concentrations in the brain and bone of rats fed citric acid,
aluminium citrate or aluminium hydroxide. Food Chem Toxicol,
22: 391-397.
Slanina P, Frech W, Bernhardson A, Cedergren A, & Mattsson P (1985)
Influence of dietary factors on aluminium absorption and retention in
the brain and bone rats. Acta Pharmacol Toxicol, 56: 331-336.
Slanina P, Frech W, Ekstr�m L-G, L��f L, Slorach S, & Cedergren A
(1986) Dietary citric acid enhances absorption of aluminium in
antacids. Clin Chem, 32: 539-541.
Smith LF (1995) Public health role, aluminium and Alzheimer's disease.
Environmetrics, 6: 277-286.
Solomon PR, Koota D, & Pendlebury WW (1990) Disrupted retention of the
classically conditioned nictitating membrane response in the aluminum-
intoxicated rabbit using electrical stimulation of the brain as a
conditioned stimulus. Neurobiol Aging, 11: 523-528.
Sorenson JRJ, Campbell IR, Tepper LB, & Lingg RD (1974) Aluminum in
the environment and human health. Environ Health Perspect, 8: 3-95.
S�yseth V, Kingerud J, Ekstrand J, & Boe J (1994) Relation between
exposure to fluoride and bronchial responsiveness in aluminium potroom
workers with work-related asthma-like symptoms. Thorax, 49: 984-989.
Sparling DW (1991) Acid precipitation and food quality: effects of
dietary Al, Ca, and P on bone and liver characteristics in American
black ducks and mallards. Arch Environ Contam Toxicol, 21: 281-288.
Stacy BD, King EJ, Harrison CV, Nagelschmidt G, & Nelson S (1959)
Tissue changes in rats' lungs caused by hydroxides, oxides and
phosphates of aluminium and iron. Pathol Bacteriol, 77: 417-426.
Stanley RA (1974) Toxicity of heavy metals and salts to eurasian
watermilfoil ( Myriophyllum spicatum L.). Arch Environ Contam
Toxicol, 2(4): 331-341.
Stanton MF (1974) Fiber carcinogenesis: is asbestos the only hazard?
(editorial). J Natl Cancer Inst, 52: 633.
Staurnes M, Blix P, & Reite OB (1993) Effects of acid water and
aluminum on parr-smolt transformation and seawater tolerance in
Atlantic salmon, Salmo salar. Can J Fish Aquat Sci, 50: 1816-1827.
Steinegger A, Rickenbacher U, & Schlatter C (1990) Aluminium. In:
Hutzinger O ed. The handbook of environmental chemistry. Berlin,
Heidelberg, New York, Springer Verlag, vol 3, part E, pp 155-184.
Steinhagen WH, Cavender FL, & Cockrell BY (1978) Six month inhalation
exposures of rats and guinea pigs to aluminum chlorhydrate. J Environ
Pathol Toxicol, 1: 267-277.
Stern AJ, Perl DP, Monoz-Garcia D, Good PF, Abraham C, & Selkoe DJ
(1986) Investigation of silicon and aluminum content in isolated
senile plaque cores by laser microprobe mass analysis (LAMMA).
J Neuropathol Exp Neurol, 45: 361.
Sternweis PC & Gilman AG (1982) Aluminum: A requirement for activation
of the regulatory component of adenylate cyclase by fluoride. Proc
Natl Acad Sci (USA), 79: 4888-4891.
Stoffyn M (1979) Biological control of dissolved aluminum in seawater:
experimental evidence. Science, 203: 651-653.
Stoffyn M & MacKenzie FT (1982) Fate of dissolved aluminum in the
oceans. Mar Chem, 11: 105-127.
Stokinger HE (1987) The metals. In: Clayton GD & Clayton FE ed.
Patty's industrial hygiene and toxicology, 3rd revised ed. - Volume
2A: Toxicology. New York, Chichester, Brisbane, Toronto, John Wiley
and Sons, pp 1493-1505.
Stone CJ, McLaurin DA, Steinhagen WH, Cavender FL, & Haseman JK (1979)
Tissue deposition patterns after chronic inhalation exposures of rats
and guinea pigs to aluminum chlorhydrate. Toxicol Appl Pharmacol,
49: 71-76.
Stoner JH, Gee AS, & Wade KR (1984) The effects of acidification on
the ecology of streams in the Upper Tywi catchment in west Wales.
Environ Pollut, A35: 125-157.
Strong MJ & Garruto RM (1991a) Neuron-specific thresholds of aluminum
toxicity in vitro: A comparative analysis of dissociated fetal
rabbit hippocampal and motor neuron-enriched cultures. Lab Invest,
65: 243-249.
Strong MJ & Garruto RM (1991b) Chronic aluminum-induced motor neuron
degeneration: Clinical, neuropathological and molecular biological
aspects. Can J Neurol Sci, 18: 428-431.
Strong MJ, Wolff AV, Wakayama I, & Garruto RM (1991) Aluminum-induced
chronic myelopathy in rabbits. Neurotoxicology, 12: 9-22.
Subramanian V, Jha PK, & Van Grieken R (1988) Heavy metals in the
Ganges estuary. Mar Pollut Bull, 19(6): 290-293.
Talbot RJ, Newton D, Priest ND, Austin JG, & Day JP (1995)
Inter-subject variability in the metabolism of aluminium following
intravenous injection as citrate. Hum Exp Toxicol, 14(7): 595-599.
Tarkka T, Yli-M�yry N, Mannermaa RM, Majamaa K, & Oikarinen J (1993)
Specific non-enzymatic glycation of the rat histone H1 nucleotide
binding site in vitro in the presence of AlF4-. A putative
mechanism for impaired chromatin function. Biochim Biophys Acta,
1180: 294-298.
Taylor GJ (1991) Current views of the aluminum stress response: the
physiological basis of tolerance. Curr Top Plant Biochem Physiol,
10: 57-93.
Taylor GJ (1995) Overcoming barriers to understanding the cellular
basis of aluminium resistance. Plant Soil, 171(1): 89-103.
Taylor GJ & Foy CD (1985) Mechanisms of aluminum tolerance in
Triticum aestivum L. (wheat): I. Differential pH induced by winter
cultivars in nutrient solutions. Am J Bot, 72(5): 695-701.
Teraoka H (1981) Distribution of 24 elements in the internal organs
of normal males and metallic workers in Japan. Arch Environ Health,
36: 155-165.
Teraoka H, Morii F, & Kobayashi J (1981) The concentrations of 24
elements in foodstuffs and the estimate of their daily intake. J Jpn
Soc Food Nutr, 34: 221-239.
Thompson JJ & Houk RS (1986) Inductively coupled plasma mass
spectrometric detection for multielement flow injection analysis and
elemental speciation by reversed-phase liquid chromatography. Anal
Chem, 58: 2541-2548.
Thompson GW & Medve RJ (1984) Effects of aluminum and manganese
on the growth of ectomycorrhizal fungi. Appl Environ Microbiol,
48(3): 556-560.
Thomson SM, Burnett DC, Bergmann JD, & Hixson CJ (1986) Comparative
inhalation hazards of aluminum and brass powders using
bronchopulmonary lavage as an indicator of lung damage. J Appl
Toxicol, 6: 197-209.
Thorne BM, Cook A, Donohoe T, Lyon S, Medeiros DM, & Moutzoukis C
(1987) Aluminum toxicity and behavior in the weanling Long-Evans rat.
Bull Psychon Soc, 25: 129-132.
Thornton DJ, Liss L, & Lott JA (1983) Effect of ethanol on the uptake
and distribution of aluminum in rats. Clin Chem, 29: 1281.
Tipping E & Backes CA (1988) Organic complexation of Al in acid
waters: model-testing by titration of a streamwater sample. Water Res,
22(5): 593-595.
Tipping E, Woof C, Backes CA, & Ohnstad M (1988a) Aluminium speciation
in acidic natural waters: testing of a model for Al-humic
complexation. Water Res, 22(3): 321-326.
Tipping E, Woof C, Walters PB, & Ohnstad M (1988b) Conditions required
for the precipitation of aluminium in acidic natural waters. Water
Res, 22(5): 585-592.
Tipping E, Backes CA, & Hurley MA (1988c) The complexation of protons,
aluminium and calcium by aquatic humic substances: a model
incorporating binding-site heterogeneity and macroionic effects. Water
Res, 22(5): 597-611.
Tipping E, Backes CA, & Hurley MA (1989a) Modeling the interactions of
Al species, protons and Ca2+ with humic substances in acid waters and
soils In: Lewis TE ed. Environmental chemistry and toxicology of
aluminum. Proceedings of the 194th Annual Meeting of the American
Chemical Society, New Orleans, LA, 30 August-4 September 1987.
Chelsea, Michigan, Lewis Publishers Inc., pp 59-82.
Tipping E, Ohnstad M, & Woof C (1989b) Adsorption of aluminium by
stream particulates. Environ Pollut, 57: 85-96.
Tipton IH & Cook MJ (1963) Trace elements in human tissue: Part II.
Adult subjects from the United States. Health Phys, 9: 103-145.
Townsend MC, Enterline PE, Sussman NB, Bonney TB, & Rippey LL (1985)
Pulmonary function in relation to total dust exposure at a bauxite
refinery and alumina-based chemical products plant. Am Rev Respir Dis,
132: 1174-1180.
Trapp GA (1983) Plasma aluminum is bound to transferrin. Life Sci,
33: 311.
Tyler G (1994) Plant uptake of aluminium from calcareous soils.
Experientia (Basel), 50: 701-703.
Ulfvarson U (1981) Survey of air contaminants from welding. Scand J
Work Environ Health, 7(suppl 2): 1-28.
UK MAFF (1993) Aluminium in food - 39th report of the Steering Group
on Chemical Aspects of Food Surveillance. London, UK Ministry of
Agriculture, Fisheries and Food, 52 pp.
US Bureau of Mines (1967) Potential sources of aluminium. Washington,
DC, US Government Printing Office, pp 3-4.
Valdivia R, Ammerman CB, Wilcox CJ, & Henry PR (1978) Effect of
dietary aluminum on animal performance and tissue mineral levels in
growing steers. J Anim Sci, 47: 1351-1356.
Valdivia R, Ammermann CB, Henry PR, Feaster JP, & Wilcox CJ (1982)
Effect of dietary aluminium and phosphorus on performance, phosphorus
utilization and tissue mineral composition in sheep. J Anim Sci,
55: 402-410.
Vallyathan V, Bergeron WN, Robichaux PA, & Craighead JE (1982)
Pulmonary fibrosis in an aluminum arc welder. Chest, 81: 372-374.
Van Bennekom AJ & Jager JE (1978) Dissolved aluminium in the Zaire
river plume. Neth J Sea Res, 12(3/4): 358-367.
Van Benschoten JE & Edzwald JK (1990) Measuring aluminum during
water treatment: methodology and application. J Am Water Works Assoc,
82: 71-78.
Van Broeckhoven C, Backhovens H, Cruts M, De Winter G, Bruyland M,
Cras P, Martin J-J (1992) Mapping of a gene predisposing to early
onset Alzheimer's disease to chromosome 14q24.3. Nature (Genet),
2: 335-339.
Van de Vyver F & Visser WJ (1990) Aluminium accumulation in bone.
In: Priest ND & Van de Vyver F Trace metals and fluoride in bones and
teeth. Boca Raton, Florida, CRC Press, pp 41-81.
Van der Voet GB (1992) Intestinal absorption of aluminium. In:
Aluminium in biology and medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 109-122 (Ciba Foundation Symposium
No. 169).
Van der Voet GB & De Wolff FA (1984) A method of studying the
intestinal absorption of aluminium in the rat. Arch Toxicol,
55: 168-172.
Van der Voet GB, De Haas EJM, & De Wolff FA (1985) Monitoring of
aluminum in whole blood, plasma, serum, and water by a single
procedure using flameless atomic absorption spectrophotometry. J Anal
Toxicol, 9: 97-100.
Van Duijn CM, Stijnen T, & Hofman A (1991) Risk factors for
Alzheimer's disease: overview of the EURODEM collaborative re-analysis
of case-control studies. Int J Epidemiol, 20(2): S/4-S/12.
Varo P & Koivistoinen P (1980) Mineral element composition of Finnish
foods: XII. General discussion and nutritional evaluation. Acta Agric
Scand, 22(suppl): 165-171.
Veien NK, Hattel T, Justesen O, & Noerholm A (1986) Aluminium allergy.
Contact Dermatitis, 15: 295-297.
Vogt KA, Dahlgren R, Ugolini F, Zabowski D, Moore EE, & Zasoski R
(1987) Aluminum, Fe, Ca, Mg, K, Mn, Cu, Zn, and P in above- and
below-ground biomass. II. Pools and circulation in a subalpine
Abies amabilis stand. Biogeochemistry, 4: 295-311.
Vorbrodt AW, Dobrogowska DH, & Lossinsky AS (1994) Ultracytochemical
studies of the effects of aluminum on the blood-brain barrier of mice.
J Microchem Cytochem, 42(2): 203-212.
Wade K & Banister AJ (1973) The chemistry of aluminium, gallium,
indium and thallium: Comprehensive inorganic chemistry. Oxford, New
York, Pergamon Press, chapter 12, pp 993-1036.
Wagatsuma T (1983) Characterization of absorption sites for aluminum
in the roots. Soil Sci Plant Nutr, 29(4): 499-515.
Walker WJ, Cronan CS, & Patterson HH (1988) A kinetic study of
aluminum adsorption by aluminosilicate clay minerals. Geochim
Cosmochim Acta, 52: 55-62.
Walker WJ, Cronan CS, & Bloom PR (1990) Aluminum solubility in organic
soil horizons from northern and southern forested watersheds. Soil Sci
Soc Am J, 54: 369-374.
Wallen IE, Greer WC, & Lasater R (1957) Toxicity to Gambusia
affinis of certain pure chemicals in turbid waters. Sew Ind Wastes,
29: 695-711.
Walton J, Hams G, & Wilcox D (1994) Bioavailability of aluminium from
drinking water: Co-exposure with foods and beverages. Melbourne,
Australia, Urban Water Research Association of Australia, Melbourne
Water Corporation, 38 pp (Research Report No. 83).
Walton J, Tuniz C, Fink D, Jacobsen G, & Wilcox D (1995) Uptake of
trace amounts of aluminium into the brain from drinking water.
Neurotoxicology, 16: 187-190.
Ward MK (1991) Aluminium toxicity and people with impaired renal
function. In: Lord Walton of Detchant ed. Alzheimer's disease and the
environment. London, Royal Society of Medicine Services Ltd,
pp 106-110 (Round Table Series No. 26).
Ward MK, Feest TG, Ellis HA, Parkinson IS, Kerr DNS, Herrington J, &
Goode GL (1978) Osteomalacic dialysis osteodystrophy: Evidence for a
water-borne aetiological agent, probably aluminium. Lancet,
1: 841-845.
Watt WD, Scott CD, & White WJ (1983) Evidence of acidification of some
Nova Scotian rivers and its impact on Atlantic salmon, Salmo salar.
Can J Fish Aquat Sci, 40: 462-473.
Weatherley NS, Ormerod SJ, Thomas SP, & Edwards RW (1988) The response
of macroinvertebrates to experimental episodes of low pH with
different forms of aluminium, during a natural spate. Hydrobiologia,
169: 225-232.
Weatherley NS, Rogers AP, Goenaga X, & Ormerod SJ (1990) The survival
of early life stages of brown trout ( Salmo trutta L.) in relation
to aluminium speciation in upland Welsh streams. Aquat Toxicol,
17: 213-230.
Wedrychowski A, Schmidt WN, & Hnilica LS (1986) The in-vivo
cross-linking of proteins and DNA by heavy metals. J Biol Chem,
261: 3370.
Wefers K & Bell GM (1972) Oxides and hydroxides of aluminum. East St.
Louis, Illinois, ALCOA (Aluminium Company of America), Research
Laboratories (Technical Paper No. 19).
Wettstein A, Aeppli J, Gautschi K, & Peter M (1991) Failure to find a
relationship beetwen mnestic skills of octogenarians and aluminium in
drinking water. Int Arch Occup Environ Health, 63: 97-103.
Wheeler DM, Power IL, & Edmeades DC (1993) Effect of various metal
ions on growth of two wheat lines known to differ in aluminium
tolerance. Plant Soil, 155/156: 489-492.
White DM, Longstreth WT, Rosenstock L, Claypoole KHJ, Brodkin CA, &
Townes BD (1992) Neurologic syndrome in 25 workers from an aluminum
smelting plant. Arch Intern Med, 152: 1443-1448.
Whitehead PG, Bird S, Hornung M, Cosby J, Neal C, & Paricos P (1988)
Stream acidification trends in the Welsh uplands: a modelling study of
the Llyn Brianne catchments. J Hydrol, 101: 191-212.
Whitehead PG, Musgrove TJ, & Cosby BJ (1990) Hydrochemical modelling
of acidification in Wales. In: Edwards RW, Gee AS, & Stoner JH ed.
Acid waters in Wales. Dordrecht, Boston, London, Kluwer Academic
Publishers, pp 255-277.
WHO (1993) Guidelines for drinking-water quality, 2nd ed. - Volume 1:
Recommendations. Geneva, World Health Organization, pp 39-40.
Wicklung Glynn A, Norrgren L, & Malmborg O (1992) The influence of
calcium and humic substances on aluminium accumulation and toxicity in
the minnow, Phoxinus phoxinus, at low pH. Comp Biochem Physiol,
102C(3): 427-432.
Wild A ed. (1988) Russell's soil conditions and plant growth, 11th ed.
Harlow, Longman Scientific & Technical.
Wilhelm M & Idel H (1995) [Aluminium in drinking water: a risk factor
for Alzheimer's disease?] Forum St�dte-Hyg, 46: 255-258 (in German
with English summary).
Wilhelm M, Passlick J, Busch T, Szydlik M, & Ohnesorge FK (1989) Scalp
hair as an indicator of aluminium exposure: comparison to bone and
plasma. Hum Toxicol, 8: 5-9.
Wilhelm M, J�ger E, & Ohnesorge FK (1990) Aluminium toxicokinetics.
Pharmacol Toxicol, 66: 4-9.
Wilhelm M, Zhang X-J, Hafner D, & Ohnesorge FK (1992) Single-dose
toxicokinetics of aluminum in the rat. Arch Toxicol, 66: 700-705.
Wilhelm M, Lombeck I, Kouros B, Wuthe J, & Ohnesorge FK (1995)
[Duplicate study on the dietary intake of some metals/metalloids by
German children: Part II. Aluminium, cadmium and lead.] Zent.bl Hyg,
197: 357-368 (in German with English summary).
Wills MR & Savory J (1983) Aluminium poisoning: Dialysis
encephalopathy, osteomalacia, and anaemia. Lancet, 2: 29-33.
Wills MR & Savory J (1989) Aluminum and chronic renal failure:
Sources, absorption, transport, and toxicity. CRC Crit Rev Clin Lab
Sci, 27: 59-107.
Wilson RW, Bergman HL, & Wood CM (1994a) Metabolic costs and
physiological consequences of acclimation to aluminum in juvenile
rainbow trout ( Oncorhynchus mykiss): 1. Acclimation specificity,
resting physiology, feeding, and growth. Can J Fish Aquat Sci,
51: 527-535.
Wilson RW, Bergman HL, & Wood CM (1994b) Metabolic costs and
physiological consequences of acclimation to aluminum in juvenile
rainbow trout ( Oncorhynchus mykiss): 1. Gill morphology, swimming
performance, and aerobic scope. Can J Fish Aquat Sci, 51: 536-544.
Windom HL (1981) Comparison of atmospheric and riverine transport of
trace elements to the continental shelf environment. In: Eisma MB ed.
River inputs to the ocean systems: Proceedings of the UNEP/IOC/SCDR
Workshops, Rome, 1981. New York, United Nations, pp 360-369.
Wischik CM, Harrington CR, Mukaetova-Ladinska EB, Novak M, Edwards PC,
& McArthur FK (1992) Molecular characterization and measurement of
Alzheimer`s disease pathology: implications for genetic and
environmental aetiology. In: Aluminium in biology and medicine. New
York, Chichester, Brisbane, Toronto, John Wiley and Sons, pp 268-302
(Ciba Foundation Symposium No. 169).
Wisniewski HM & Kozlowski PB (1982) Evidence for blood-brain barrier
changes in senile dementia of the Alzheimer type (SDAT). Ann NY Acad
Sci, 396: 119-129.
Wisniewski HM & Rabe A (1992) Differences between aluminum
encephalopathy and Alzheimer's disease. In: Gitelman HJ ed.
Proceedings of the Second International Conference on Aluminum and
Health. Washington, DC, Aluminium Association, pp 193-194.
Wisniewski HM & Wen GY (1992) Aluminium and Alzheimer`s disease. In:
Aluminium in biology and medicine. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 142-164 (Ciba Foundation Symposium
No. 169).
Wisniewski HM, Sturman JA, & Shek JW (1982) Chronic model of
neurofibrillary changes induced in mature rabbits by metallic
aluminum. Neurobiol Aging, 3: 11-22.
Wisser LA, Heinrichs BS, & Leach RM (1990) Effect of aluminum on
performance and mineral metabolism in young chicks and laying hens. J
Nutr, 120: 493-498.
Witters HE (1986) Acute acid exposure of rainbow trout, Salmo
gairdneri Richardson: effects of aluminium and calcium on ion
balance and haematology. Aquat Toxicol, 8: 197-210.
Witters H, Vangenechten JHD, Van Puymbroeck S, & Vanderborght OLJ
(1984) Interference of aluminium and pH on the Na-influx in an aquatic
insect Corixa punctata (Illig.). Bull Environ Contam Toxicol,
32: 575-579.
World Bureau of Metal Statistics (1994) World bauxite production.
World Met Stat, 47: 6.
Wood CM & McDonald DG (1987) The physiology of acid/aluminium stress
in trout. Ann Soc Zool Belg, 117(suppl 1): 399-410.
Wood DJ, Cooper C, Stevens J, & Edwardson J (1988) Bone mass and
dementia in hip fracture patients from areas with different aluminium
concentrations in water supplies. Age Ageing, 17: 415-419.
Wren CD, MacCrimmon HR, & Loescher BR (1983) Examination of
bioaccumulation and biomagnification of metals in a Precambrian shield
lake. Water Air Soil Pollut, 19: 277-291.
Wyttenbach A, Bajo S, Tobler L, & Keller T (1985) Major and trace
element concentrations in needles of Picea abies: levels,
distribution functions, correlations and environmental influences.
Plant Soil, 85: 313-325.
Yagi M, Mizukawa K, Kajino M, Tatumi S, Akiyama M, & Ando M (1992)
Behaviour of aluminium in water purification process at purification
plants in the River Yodo system. Water Supply, 10(4): 65-75.
Yeats PA & Bewers JM (1982) Discharge of metals from the St Lawrence
River. Can J Earth Sci, 19: 982-992.
Yen-Koo HC (1992) The effect of aluminum on conditioned avoidance
response (CAR) in mice. Toxicol Ind Health, 8: 1-7.
Yokel RA (1983) Repeated systemic aluminum exposure effects on
classical conditioning of the rabbit. Neurobehav Toxicol Teratol,
5: 41-46.
Yokel RA (1984) Toxicity of aluminum exposure during lactation to the
maternal and suckling rabbit. Toxicol Appl Pharmacol, 75: 35-43.
Yokel RA (1985) Toxicity of gestational aluminum exposure to the
maternal rabbit and offspring. Toxicol Appl Pharmacol, 79: 121-133.
Yokel RA (1987) Toxicity of aluminum exposure to the neonatal and
immature rabbit. Fundam Appl Toxicol, 9: 795-806.
Yokel RA & McNamara PJ (1985) Aluminum bioavailability and deposition
in adult and immature rabbits. Toxicol Appl Pharmacol, 77: 344-352.
Yokel RA & McNamara PJ (1989) Elevated aluminium persists in serum and
tissues of rabbits after a 6-hour infusion. Toxicol Appl Pharmacol,
99: 133-138.
Yukawa M, Suzuki-Yasumoto M, Amano K, & Terai M (1980) Distribution of
trace elements in the human body determined by neutron activation
analysis. Arch Environ Health, 35: 36-44.
Zelenov KK (1965) Aluminum and titanium in Kava Ijen volcano crater
lake (Indonesia). Int Geol Rev, 11(1): 84-93.
Zietz JR (1985) Aluminum compounds, organic. In: Gerhartz W, Yamamoto
YS, Campbell FT, Pfefferkorn R, & Rounsaville JF ed. Ullmann's
encyclopedia of industrial chemistry - Volume A1: Abrasives to
aluminum oxide, 5th revised ed. Weinheim, Verlag Chemie, pp 543-556.
Zoller WH, Gladney ES, & Duce RA (1974) Atmospheric concentrations and
sources of trace metals at the South Pole. Science, 183: 198-200.
Zwarun AA & Thomas GW (1973) Effect of soluble and exchangeable
aluminum on Pseudomonas stutzeri. Soil Sci Soc Am Proc,
37: 386-387.
Zwarun AA, Bloomfield BJ, & Thomas GW (1971) Effect of soluble and
exchangeable aluminum on a soil Bacillus. Soil Sci Soc Am Proc,
35: 460-463.
RESUME ET CONCLUSIONS
1. Identit�, propri�t�s chimiques et physiques
L'aluminium est un m�tal ductile et mall�able de couleur blanc
argent�. Il appartient au groupe IIIA de la classification p�riodique
et se trouve g�n�ralement au degr� d'oxydation III dans ses d�riv�s
usuels. Il constitue environ 8% de l'�corce terrestre et c'est l'un
des m�taux usuels les plus r�actifs. En pr�sence d'eau, d'oxyg�ne et
d'autres oxydants, il se recouvre d'une couche superficielle d'oxyde
qui lui conf�re une grande r�sistance � la corrosion. L'oxyde
d'aluminium est soluble dans les acides min�raux ainsi que dans les
bases fortes, mais il est insoluble dans l'eau. Par contre, le
chlorure, le nitrate et le sulfate sont solubles dans l'eau. Les
halog�nures, l'hydrure et les premiers termes de la s�rie des
alkylaluminiums r�agissent violemment avec l'eau.
L'aluminium a une forte conductivit� thermique et �lectrique,
sa densit� est faible et sa r�sistance � la corrosion �lev�e. On
l'utilise souvent en alliage avec d'autres m�taux. Ces alliages sont
l�gers, r�sistants et faciles � usiner.
2. M�thodes d'analyse
On a mis au point diverses m�thodes d'analyse pour la recherche
et le dosage de l'aluminium dans les milieux biologiques et les
�chantillons pr�lev�s dans l'environnement. Les plus fr�quemment
utilis�es sont la spectrom�trie d'absorption atomique avec four �
�lectrode de graphite (GF-AAS) et la spectrom�trie d'�mission atomique
avec plasma � couplage inductif (ICP-AES). La principale source
d'erreur est la contamination des �chantillons, lors du pr�l�vement
et de la pr�paration de la prise d'essai, par l'aluminium pr�sent
dans l'air, les r�cipients et les r�actifs. Selon les traitements
pr�liminaires subis par l'�chantillon et les techniques de s�paration
et de concentration utilis�es, les limites de d�tection vont de 1,9 �
4 �g/litre dans les liquides biologiques et de 0, 005 � 0,5 �g/g de
poids sec dans les tissus quand on proc�de par GF-AAS, et de 5 �g/m3
d'air ou de 3 �g/litre d'eau quand on proc�de par ICP-AES.
3. Sources d'exposition humaine et environnementale
De l'aluminium est lib�r� dans l'environnement tant � la faveur
de processus naturels que d'activit�s humaines. Il est pr�sent sous
une forme tr�s concentr�e dans les poussi�res que produisent les
exploitations mini�res et agricoles ainsi que dans les particules
produites par la combustion du charbon. Les silicates d'aluminium
(argiles), qui sont des constituants majeurs des sols, contribuent
pour une large part � la teneur des poussi�res en silicates. L'appport
d'aluminium � l'environnement trouve beaucoup plus son origine dans
des processus naturels que dans l'activit� humaine. La mobilisation de
l'aluminium du fait d'activit�s humaine est, pour une large part,
indirecte et provient de l'�mission de substances acidifiantes. D'une
fa�on g�n�rale, une diminution du pH entra�ne une augmentation de
la mobilit� et de la biodisponibilit� des formes monom�res de
l'aluminium. La mati�re premi�re la plus importante pour la production
de l'aluminium est la bauxite, qui contient jusqu'� 55% d'alumine
(oxyde d'aluminium). La production mondiale de bauxite a �t� de 106
millions de tonnes en 1992. Le m�tal a des usages tr�s vari�s, dans
les industries du b�timent, de l'automobile et de l'a�ronautique, et
peut �galement �tre utilis� sous la forme d'alliages. Les d�riv�s de
l'aluminium ont �galement des usages tr�s divers, notamment dans
l'industrie du verre, de la c�ramique et du caoutchouc ainsi que pour
la confection de produits destin�s � la protection du bois, de
produits pharmaceutiques et de tissus imperm�ables. Les min�raux
naturels contenant de l'aluminium, en particulier la bentonite et la
z�olite, sont utilis�s pour la purification de l'eau, ainsi que dans
les sucreries, les brasseries et l'industrie papeti�re.
4. Transport, distribution et transformation dans l'environnement
L'aluminium est omnipr�sent dans l'environnement sous forme de
silicates, d'oxydes et d'hydroxydes, combin� � d'autres �l�ments comme
le sodium et le fluor ou encore sous forme de complexes organiques. On
ne le rencontre pas � l'�tat natif du fait de sa r�activit�. Dans la
nature, il n'existe qu'au degr� d'oxydation (+3) ; son transport et sa
distribution ne d�pendent donc que de sa chimie de coordination et des
caract�ristiques physico-chimiques de l'environnement local. Aux
valeurs du pH sup�rieures � 5,5, les d�riv�s de l'aluminium d'origine
naturelle existent principalement sous forme non dissoute, par exemple
sous forme de gibbsite (Al(OH)3) ou d'aluminosilicates, sauf en
pr�sence de grandes quantit�s de mati�res organiques en solution qui
se lient � l'aluminium et peuvent conduire � une augmentation de la
teneur en aluminium dissous, dans les cours d'eau et les lacs, par
exemple. Plusieurs facteurs influent sur la mobilit� de l'aluminium
et, par voie de cons�quence, sur son transport dans l'environnement.
Il s'agit en particulier de la nature des esp�ces chimiques en cause,
des trajectoires d'�coulement, des interactions entre le sol et l'eau
et de la composition des mat�riaux g�ologiques sous-jacents. La
solubilit� de l'aluminium en �quilibre avec Al (OH)3 en phase solide
d�pend fortement du pH et de la pr�sence d'agents complexants comme
les fluorures, les silicates, les phosphates et les mati�res
organiques. On peut consid�rer que la chimie de l'aluminium
inorganique dans les sols acides et les cours d'eau fait intervenir
les processus suivants: solubilit� des esp�ces min�rales, �change
d'ions et miscibilit� � l'eau. Lorsqu'il y a acidification du sol,
l'aluminium peut passer en solution et �tre ainsi transport� dans les
cours d'eau. La mobilisation de l'aluminium par pr�cipitation en
milieu acide permet aux plantes de fixer une plus grande quantit� de
cet �l�ment.
5. Concentrations dans l'environnement et exposition humaine
L'aluminium est pr�sent en proportion importante dans un certain
nombre de constituants de l'atmosph�re, en particulier des poussi�res
arrach�es au sol (qu'elles soient d'origine naturelle ou qu'elles
r�sultent de l'activit� humaine) et des particules issues de la
combustion du charbon. En milieu urbain, la concentration de
l'aluminium dans la poussi�re des rues varie de 3,7 � 11,6 �g/kg. La
teneur de l'air en aluminium va de 0,5 ng/m3 au-dessus de
l'Antarctique � plus de 1000 ng/m3 dans les zones industrialis�es.
Dans les eaux douces superficielles ou souterraines, la
concentration de l'aluminium peut varier dans des proportions
importantes, en fonction de facteurs physico-chimiques et g�ologiques.
L'aluminium peut se trouver en suspension ou en solution. Il peut �tre
li� � des coordinats organiques ou inorganiques ou exister � l'�tat
d'ion libre. En milieu aqueux naturel, les esp�ces chimiques dans
lesquelles l'aluminium est engag� existent � l'�tat de monom�res
ou de polym�res. La nature de ces esp�ces d�pend du pH et de la
concentration en carbone organique dissous, fluorures, sulfates,
phosphates et particules en suspension. Au voisinage de la neutralit�,
la concentration de l'aluminium dissous est g�n�ralement assez faible,
de l'ordre de 1,0 � 50 �g/litre. Lorsque l'acidit� de l'eau augmente,
elle peut monter jusqu'� 500-1000 �g/litre. Dans les eaux rendues
extr�mement acides par les liquides de drainage de mines, on a mesur�
des concentrations d'aluminium en solution qui atteignaient
90 mg/litre.
L'exposition humaine non professionnelle � l'aluminium pr�sent
dans l'environnement se produit principalement lors de l'ingestion
d'eau ou de nourriture, la nourriture �tant essentiellement en cause.
L'apport journalier d'aluminium par les aliments et la boisson se
situe, chez l'adulte, entre 2,5 et 13 mg, ce qui repr�sente 90 � 95%
de l'apport total. Compte tenu de la valeur-guide internationale
actuellement en vigueur, l'apport par l'eau de boisson pourrait
se situer aux environs de 0,4 mg par jour, mais il est plus
probablement de l'ordre de 0,2 mg. La contribution de la voie
d'exposition respiratoire peut atteindre 0,04 mg/jour. Dans certaines
circonstances, par exemple en cas d'exposition professionnelle ou lors
de l'utlisation d'antiacides, l'exposition pourra �tre beaucoup plus
intense. Par exemple, deux comprim�s d'antiacide moyennement dos�s
peuvent apporter plus de 500 mg d'aluminium. Les �tudes effectu�es
avec de l'aluminium-26 indiquent que le citrate constitue la forme
la plus disponible biologiquement et que sous cette forme,
l'aluminium pourrait �tre absorb� � hauteur de 1%. L'absorption par
l'interm�diaire de l'eau de boisson ne repr�senterait en revanche que
3% de l'apport quotidien total, cette source �tant relativement
mineure par rapport � l'apport alimentaire. Il n'est pas tr�s facile
de d�terminer quelle est la quantit� effectivement fix�e � la suite
d'une telle exposition en raison des probl�mes d'analyse et
d'�chantillonnage que cela pose.
6. Cin�tique et m�tabolisme
6.1 Chez l'homme
L'aluminium et ses d�riv�s sont mal absorb�s par l'organisme
humain, encore que la vitesse et le taux d'absorption n'aient pas �t�
suffisamment �tudi�s. Un moyen facile d'�valuer la r�sorption de
l'aluminium consiste � en mesurer la concentration dans l'urine et le
sang; on a d'ailleurs constat� une augmentation de la concentration
urinaire chez des soudeurs � l'aluminium et des ouvriers travaillant �
la production de poudre d'aluminium.
Le m�canisme de r�sorption de l'aluminium au niveau
gastrointestinal n'est pas encore parfaitement �lucid�. Les variations
observ�es r�sultent des propri�t�s chimiques de cet �l�ment et de la
formation de diverses esp�ces chimiques, sous l'influence du pH, de la
force ionique, de la comp�tition avec d'autres �l�ments (silicium) ou
agents complexants pr�sents dans les voies digestives (des citrates,
par ex.).
On a �tudi� le comportement biologique de l'aluminium et son
absorption dans les voies digestives humaines au moyen de l'isotope
radioactif Al-26. Des diff�rences interindividuelles importantes ont
�t� mises en �vidence. La litt�rature donne ainsi des taux de
r�sorption de 5 � 10-3 pour le citrate, de 1,04 � 10-4 pour
l'hydroxyde et de 1,3 � 10-3 pour l'hydroxyde ing�r� avec du citrate.
Une �tude de la r�sorption fractionn�e de l'aluminium pr�sent dans
l'eau de boisson a donn� une valeur de 2,35 � 10-3. On en a conclu
que les membres de la population g�n�rale qui boivent quotidiennement
1,5 litre d'eau contenant 100 �g/litre d'aluminium, absorberaient de
la sorte 3% de leur apport total d'aluminium, selon les concentrations
pr�sentes dans leur nourriture et leur utilisation �ventuelle
d'antiacides.
La proportion d'Al3+ normalement li� aux prot�ines chez
l'Homme peut atteindre 70-90% chez les malades h�modialys�s, avec une
augmentation mod�r�e de l'aluminium plasmatique. C'est dans les
poumons que l'on peut trouver les taux les plus �lev�s d'aluminium,
parfois pr�sent sous formes de particules inhal�es insolubles.
C'est l'urine qui constitue la voie d'excr�tion la plus
importante de l'aluminium. Apr�s administration par voie orale d'une
dose unique d'aluminium, 83% ont �t� excr�t�s dans l'urine en l'espace
de 13 jours, contre 1,8% dans les mati�res f�cales. La demi-vie de
l'aluminium urinaire chez des soudeurs expos�s pendant plus de 10 ans
s'est r�v�l�e �tre d'au moins 6 mois. Chez des ouvriers � la retraite
qui avaient �t� expos�s � de la poudre d'aluminium, le calcul de la
demi-vie a donn� une valeur comprise entre 0,7 et 8 ans.
6.2 Chez l'animal
La r�sorption dans les voies digestives est en g�n�ral inf�rieure
� 1%. Elle d�pend principalement de l'esp�ce chimique en cause et de
sa solubilit� ainsi que du pH. Les agents complexants organiques comme
les citrates, augmentent la r�sorption. Il peut �galement y avoir
interaction avec le calcium et le syst�me de transport du fer.
L'absorption par la voie respiratoire et la voie percutan�e n'a pas
�t� �tudi�e en d�tail. L'aluminium se r�partit dans la plupart des
organes en s'accumulant principalement dans les os, du moins
lorsque les doses sont fortes. Dans une proportion limit�e encore
qu'ind�termin�e, il franchit la barri�re h�mato-enc�phalique et peut
parvenir jusqu'au foetus. Il est efficacement �limin� par la voie
urinaire. Sa demi-vie plasmatique est d'environ 1 h chez les rongeurs.
7. Effets sur les mammif�res de laboratoire et les syst�mes d'�preuve
in vitro
L'aluminium et ses compos�s n'ont qu'une faible toxicit� aigu�,
les valeurs publi�es de la DL50 par voie orale �tant de l'ordre de
quelques centaines de mg � 1000 mg d'aluminium par kg de poids
corporel et par jour. On n'a pas connaissance de la valeur de la Cl50
par inhalation.
Lors des �tudes toxicologiques � court terme qui ont �t�
effectu�es sur des rats, des souris ou des chiens avec un eventail
suffisant de points d'aboutissement et divers compos�s de l'aluminium
(phosphate sodique, hydroxyde et nitrate) incorpor�s � la nourriture
ou � l'eau de boisson,on n'a constat� que des effets minimes
(r�duction du gain de poids g�n�ralement associ�e � une moindre
consommation de nourriture ou effets histopathologiques mod�r�s aux
doses les plus �lev�es, c'est-�-dire 70 � 300 mg d'aluminium par kg de
poids corporel et par jour). Parmi les effets g�n�raux observ�s apr�s
administration parent�rale, on peut citer une insuffisance r�nale.
On n'a pas eu connaissance de l'existence d'�tudes d'inhalation
satisfaisantes. Apr�s administration intratrach�enne d'oxyde
d'aluminium, on a observ� une fibrose induite par les particules
d'oxyde qui �tait analogue � celle que les �tudes sur la poussi�re de
silice et de charbon ont mise en �vidence.
Apr�s avoir gav� des rats avec des rations quotidiennes contenant
respectivement 13, 26 ou 52 mg d'aluminium (sous forme de nitrate)
par kg de poids corporel, on n'a constat� aucune foetotoxicit�
manifeste ni effet d�l�t�re sur l'ensemble des param�tres g�n�siques.
Toutefois, on a not� un retard de croissance li� � la dose dans la
descendance de ces animaux, retard qui s'observait � la dose de
13 mg/kg chez les femelles et � la dose de 26 mg/kg chez les m�les.
La dose la plus faible produisant un effet d�l�t�re observable
(LOAEL) sur le d�veloppement, � savoir, une mauvaise ossification,
une augmentation de la fr�quence des malformations vert�brales
et stern�brales, ou encore une r�duction du poids des foetus,
s'�tablissait � 13 mg/kg (nitrate d'aluminium). Ces effets n'ont pas
�t� observ�s apr�s administration d'hydroxyde d'aluminium, � des doses
pourtant beaucoup plus fortes. A la dose de 13 mg/kg de nitrate
d'aluminium, on a observ� une r�duction de la croissance postnatale
mais on n'a pas cherch� � relever la pr�sence �ventuelle d'effets
toxiques chez les m�res. Les �tudes portant sur le d�veloppement
c�r�bral ont mis en �vidence une diminution de la force d'agrippement
dans la descendance de rattes qui avaient re�u 100 mg d'aluminium
par kg de poids corporel sous forme de lactate incorpor� � leur
nourriture, sans pr�senter elles-m�mes d'effets toxiques.
Rien n'indique que l'aluminium soit canc�rog�ne. Il peut former
des complexes avec l'ADN et provoquer la r�ticulation des prot�ines et
de l'ADN chromosomiques, mais il ne se montre pas mutag�ne pour les
bact�ries et n'induit pas de mutations ou de transformations dans des
cultures de cellules mammaliennes in vitro. Toutefois, des
aberrations chromosomiques ont �t� observ�es dans les cellules de la
moelle osseuse, chez des souris et des rats expos�s.
Une somme tr�s importante de faits exp�rimentaux incite � penser
que l'aluminium est neurotoxique chez l'animal d'exp�rience, encore
qu'il y ait des diff�rences consid�rables entre les esp�ces. Chez
celles qui sont sensibles, les effets toxiques cons�cutifs �
l'administration parent�rale d'aluminium se caract�risent par des
alt�rations neurologiques progressives qui conduisent � la mort dans
un �tat de mal �pileptique (DL50 = 6 �g d'aluminium/g de tissu
c�r�bral sec). Sur le plan morphologique, l'enc�phalopathie
progressive est associ�e, au niveau de la moelle �pini�re, du tronc
c�r�bral et de certaines parties de l'hippocampe, � une atteinte des
neurofibrilles dans les neurones de grande taille et de taille
moyenne. Ces l�sions de d�g�n�rescence neurofibrillaire sont
morphologiquement et biochimiquement distinctes de celles que l'on
observe dans la maladie d'Alzheimer. Des troubles comportementaux ont
�t� observ�s, en l'absence d'enc�phalopathie ou de l�sions du tissu
c�r�bral, chez des animaux ayant re�u des sels solubles d'aluminium
(chlorure, lactate, par ex.) dans leur alimentation ou leur eau de
boisson � des doses quotidiennes �gales ou sup�rieures � 50 mg
d'aluminium par kg de poids corporel.
L'ost�omalacie, sous la forme o� elle se manifeste chez l'homme,
s'observe r�guli�rement chez les gros animaux (par exemple, le chien
ou le porc) expos�s � l'aluminium; une pathologie du m�me genre
s'observe chez les rongeurs. A ce qu'il para�t, ces effets se
produisent chez toutes les esp�ces, y compris l'Homme, � des
concentrations d'aluminium de 100 � 200 �g/g de cendres d'os.
8. Effets sur l'Homme
On n'a pas d�crit d'effets pathog�nes aigus cons�cutifs � une
exposition de la population g�n�rale � l'aluminium.
En Angleterre, une population d'environ 20 000 personnes a �t�
expos�e pendant au moins 5 jours � des concentrations excessives de
sulfate d'aluminium qui avait �t� d�pos� accidentellement dans une
installation fournissant de l'eau potable. On a fait �tat � cette
occasion de cas de naus�e, de vomissements, de diarrh�e, d'ulc�rations
buccales et cutan�es, d'�ruptions et d'arthralgies. A l'examen, ces
sympt�mes se sont r�v�l�s pour la plupart b�nins et de br�ve dur�e.
Aucun effet pathologique durable n'a pu �tre attribu� � des cas pr�cis
d'exposition � l'aluminium pr�sent dans l'eau de boisson.
On a �mis l'hypoth�se que la pr�sence d'aluminium dans l'eau de
boisson serait responsable de la survenue ou de l'�volution plus
rapide de la maladie d'Alzheimer ou encore de la d�t�rioration des
fonctions intellectuelles chez les personnes �g�es. On a �galement
avanc� que les fum�es d�gag�es lors de la compression de l'aluminium
pulv�rulent pouvaient �galement avoir une responsabilit� dans la
d�t�rioration des fonctions intellectuelles et les pneumopathies
observ�es chez certaines professions.
Une vingtaine d'�tudes �pid�miologiques ont �t� effectu�es pour
v�rifier si la pr�sence d'aluminium dans l'eau de boisson �tait
effectivement un facteur de risque de la maladie d'Alzheimer et deux
d'entre elles ont consist� � �valuer l'association entre ce facteur et
la d�t�rioration des fonctions intellectuelles. Ces �tudes allaient
des enqu�tes �cologiques aux �tudes cas-t�moins. Huit �tudes
effectu�es sur des populations de Norv�ge, du Canada, de France, de
Suisse et d'Angleterre ont �t� jug�es d'une qualit� suffisante pour
satisfaire aux crit�res retenus pour l'�valuation de la relation
exposition-effet avec correction pour tenir compte d'au moins quelques
facteurs de confusion. Sur les six �tudes consacr�es � l'existence
�ventuelle d'une relation entre l'aluminium de l'eau de boisson et la
d�mence s�nile ou la maladie d'Alzheimer, trois seulement ont conclu
par l'affirmative. Il est toutefois � noter que chacune de ces �tudes
pr�sentait des d�fauts de conception (par exemple, dans l'�valuation
de l'exposition environnementale, pas de prise en compte de
l'exposition � l'aluminium de toutes origines, ni de correction pour
tenir compte des importants facteurs de confusion que sont le niveau
d'instruction, la situation socio-�conomique et les ant�c�dents
familiaux, le recours � des crit�res de jugement diff�rents pour la
maladie d'Alzheimer et le biais de s�lection). En g�n�ral le calcul a
donn� une valeur de 2 pour le risque relatif, avec un large intervalle
de confiance, lorsque la concentration totale de l'aluminium dans
l'eau de boisson �tait �gale ou sup�rieure � 100 �g/litre. Compte
tenu des connaissances actuelles sur la maladie d'Alzheimer et de
l'ensemble des r�sultats fournis par ces �tudes �pid�miologiques, on a
conclu que les donn�es �pid�miologiques ne militent pas en faveur de
l'existence d'une relation causale entre la maladie d'Alzheimer et la
pr�sence d'aluminium dans l'eau de boisson.
Outre les �tudes �pid�miologiques pr�cit�es qui ont port� sur la
relation entre la maladie d'Alzheimer et la pr�sence d'aluminium dans
l'eau de boisson, deux autres �tudes ont �t� consacr�es � ce m�me type
de relation chez les populations �g�es. L� encore, on a obtenu des
r�sultats contradictoires. La premi�re �tude, qui a port� sur 800
octog�naires du sexe masculin qui consommaient une eau contenant
jusqu'� 98 �g d'aluminium par litre, n'a pas mis en �vidence de
relation de cause � effet. La seconde �tude, pour laquelle le crit�re
de jugement retenu �tait "tout signe de d�t�rioration des fonctions
mentales", a �valu� � 1,72 le risque relatif pour des teneurs en
aluminium sup�rieures � 85 �g/litre chez 250 sujets du sexe masculin.
Ces donn�es ne permettent pas de conclure que l'aluminium est �
l'origine d'une d�t�rioration des fonctions intellectuelles chez les
personnes �g�es.
Comme on vient de le voir, les conclusions des travaux relatifs
aux effets de l'aluminium sur les fonctions intellectuelles sont
contradictoires. La plupart des �tudes ont �t� effectu�es sur des
populations de faible effectif et la m�thodologie utilis�e est
discutable, eu �gard � l'ampleur de l'effet constat�, � l'appr�ciation
de l'exposition efective et aux facteurs de confusion. Lors d'une
�tude au cours de laquelle on a compar� la d�t�rioration des fonctions
intellectuelles chez des mineurs expos�s � une poudre contenant 85%
d'aluminium finement pulv�ris� et 15% d'oxyde d'aluminium (� titre de
prophylaxie contre la silice) � un groupe t�moin constitu� de mineurs
non expos�s, les r�sultats aux tests et le nombre de sujets pr�sentant
un d�ficit dans au moins un test ont mis en �vidence un d�savantage
chez les mineurs expos�s. On a constat� l'existence d'une tendance �
l'accroissement du risque qui �tait li�e � la dose.
Dans toutes les �tudes relatives � l'exposition professionnelle
qui ont �t� publi�es, l'ampleur des effets constat�s, la pr�sence de
facteurs de confusion, les probl�mes pos�s par l'appr�ciation de
l'exposition effective et le fait qu'on ne peut exclure l'exposition �
plusieurs substances, ne permettent gu�re de conclure que l'aluminium
provoque une d�t�rioration des fonctions intellectuelles chez les
personnes expos�es de par leur profession.
Lors d'�tudes limit�es sur des travailleurs expos�s � des vapeurs
d'aluminium, des syndromes neurologiques et en particulier une
d�t�rioration des fonction intellectuelles, des troubles moteurs et
des neuropathies p�riph�riques ont �t� constat�s. En comparant un
petit groupe de soudeurs d'aluminium � un groupe de soudeurs de fer,
on a constat� chez les premiers un l�ger d�ficit de l'activit� motrice
lors de t�ches r�p�titives. Dans une autre �tude, une enqu�te par
questionnaire a permis de mettre en �vidence une augmentation des
sympt�mes neuropsychiatriques.
Les insuffisants r�naux chroniques qui sont expos�s � l'aluminium
pr�sent dans les liquides de dialyse et re�oivent des m�dicaments qui
en contiennent, peuvent souffrir de troubles iatrog�nes tels
qu'enc�phalopathies, ost�omalacies r�sistantes � la vitamine D et
an�mies microcytaires. Ces syndromes cliniques peuvent �tre �vit�s en
r�duisant l'exposition � l'aluminium.
Chez les pr�matur�s, m�me ceux dont l'insuffisance r�nale n'est
pas suffisamment grave pour provoquer une augmentation du taux sanguin
de cr�atinine, il peut y avoir accumulation tissulaire d'aluminium,
notamment au niveau des os, en cas d'exposition iatrog�ne �
l'aluminium. A partir d'un certain degr� d'insuffsance r�nale, on peut
craindre des convulsions et une enc�phalopathie.
L'exposition humaine � l'aluminium est tr�s courante, mais seuls
quelques cas d'hypersensibilit� ont �t� signal�s � la suite de
l'application sur la peau ou de l'administration parent�rale de
certains compos�s de l'aluminium.
Des cas de fibrose pulmonaire ont �t� rapport�s chez quelques
ouvriers employ�s � la fabrication d'explosifs et de feux d'artifice
et expos�s � de tr�s fines poudres d'aluminium comprim�es. Dans
presque tous les cas, il y avait eu exposition � des particules
d'aluminium enrob�es d'huile min�rale. Ce proc�d� n'est plus utilis�.
D'autres cas de fibrose pulmonaire ont pu �tre �galement attribu�s �
la pr�sence d'autres min�raux comme la silice et l'amiante;
l'aluminium n'�tait donc pas seul en cause.
On a pu attribuer l'apparition d'un asthme d'irritation �
l'inhalation de compos�s tels que le sulfate et le fluorure
d'aluminium, le t�trafluoraluminate de potassium ainsi qu'au s�jour
dans l'environnement complexe des ateliers de production de
l'aluminium.
On ne dispose pas de donn�es suffisantes pour d�terminer dans
quelle cat�gorie de risque canc�rog�ne pour l'Homme se situent
l'aluminium et ses d�riv�s. Quoi qu'il en soit, les �tudes men�es sur
l'animal n'attribuent aucun pouvoir canc�rog�ne � l'aluminium ou � ses
compos�s.
9. Effets sur les autres �tres vivants au laboratoire ou dans leur
milieu naturel
Chez les algues unicellulaires, on observe un effet toxique plus
important aux faibles valeurs du pH, lorsque la biodisponibilit� de
l'aluminium augmente. Elles sont plus sensibles que les autres
microorganismes, la plupart des 19 esp�ces lacustres test�es
pr�sentant une inhibition totale de la croissance � la concentration
de 200 �g/litre d'aluminium total (pH 5,5). Il peut y avoir s�lection
des souches tol�rantes � l'aluminium. Ainsi, on a isol� des algues
capables de se d�velopper en pr�sence d'une concentration d'aluminium
de 48 �g/litre et d'un pH de 4,6. Dans le cas des invert�br�s
aquatiques, on a obtenu des valeurs de la Cl50 qui vont de
0,48 mg/litre (polych�tes) � 59,6 mg/litre (daphnies). En ce qui
concerne les poissons, les valeurs vont de 0,095 mg/litre
( Jordanella floridae) � 235 mg/litre (gambusies). il faut
cependant interpr�ter ces r�sultats avec prudence du fait que la
biodisponibililt� de l'aluminium d�pend fortement du pH. La grande
dispersion des valeurs de la Cl50 traduit probablement les variations
de la biodisponibilit�. L'adjonction d'agents ch�latants, comme le NTA
ou l'EDTA, r�duit la toxicit� de l'aluminium pour les poissons.
Les macroinvert�br�s r�agissent de mani�re variable � la pr�sence
d'aluminium. Lorsque le pH se situe dans les limites normales, la
toxicit� de l'aluminium augmente � mesure que le pH diminue;
toutefois, dans les eaux tr�s acides, l'aluminium peut att�nuer
l'effet de l'acidit�. Certains invert�br�s sont tr�s r�sistants �
l'acidit� et peuvent abonder dans les eaux acides. On a constat� une
augmentation de la d�rive des invert�br�s dans les cours d'eau o� ils
sont soumis � un stress d� au pH ou � la pr�sence d'aluminium. Il
s'agit l� d'une r�action commune � divers facteurs de stress. Lors
d'un test, des invert�br�s lacustres ont en g�n�ral surv�cu � une
exposition � l'aluminium mais ont souffert de la diminution des
phosphates dans des conditions rendues oligotrophiques par la
pr�cipitation de l'aluminium. Les �tudes toxicologiques � court et �
long terme effectu�es sur les poissons se sont d�roul�es dans des
conditions tr�s diverses et, ce qui est beaucoup plus important, dans
des milieux de pH vari�. Il ressort des donn�es disponibles que des
effets non n�gligeables ont �t� observ�s � des concentrations en
aluminium inorganique sous forme de compos�s monom�res ne d�passant
pas 25 �g/litre. Toutefois, les relations complexes qui existent entre
l'acidit� et la biodisponibilit� de l'aluminium rendent difficile
l'interpr�tation des donn�es toxicologiques. Lorsque le pH est tr�s
bas, c'est-�-dire dans des conditions qui ne sont normalement pas
celles qui r�gnent dans les eaux naturelles, c'est la concentration en
ions hydrog�ne qui constitue le facteur toxique, l'addition
d'aluminium ayant tendance � r�duire la toxicit�. C'est pour un pH
compris entre 4,5 et 6,0 que l'aluminium en �quililbre exerce son
effet toxique maximal. On a �galement montr� que la toxicit� augmente
avec le pH lorsque celui-ci est alcalin. On explique la toxicit� de
l'aluminium pour les poissons par le fait qu'ils ne peuvent plus
assurer leur osmor�gulation ainsi que par des probl�mes respiratoires
dus � la pr�cipitation de l'aluminium sur le mucus branchial. Le
premier de ces effets est associ� � des valeurs basses du pH. Ces
r�sultats de laboratoire ont �t� confirm�s par des observation en
milieu naturel, notamment dans des conditions d'acidit� excessive.
Les oeufs et les larves d'amphibiens souffrent de l'acidit� et de
la pr�sence d'aluminium, ces deux facteurs �tant en interaction. On a
fait �tat d'effets tels que r�duction de l'�closion, �closion
retard�e, m�tamorphose retard�e ou � petite taille, et mortalit� pour
des teneurs en aluminium inf�rieures � 1 mg/litre.
L'exposition des racines des plantes terrestres � l'aluminium
peut r�duire la croissance de ces racines, diminuer la fixation des
nutriments et provoquer un rabougrissement du v�g�tal. On a cependant
montr� au laboratoire comme sur le terrain que les v�g�taux pouvaient
manifester une tol�rance � l'aluminium.
10. Conclusions
10.1 Population g�n�rale
Des �tudes sur l'animal ont permis de d�finir les risques que
l'exposition � l'aluminium pr�sente pour le d�veloppement neurologique
et les fonctions c�r�brales. Toutefois, il n'appara�t pas que
l'aluminium menace la sant� des personnes en bonne sant� qui ne sont
pas soumises � une exposition professionnelle.
Rien n'indique que l'aluminium puisse jouer un r�le important
dans l'�tiologie de la maladie d'Alzheimer et en tout �tat de cause,
il ne provoque chez aucune esp�ce animale ou chez l'Homme des
pathologies de type Alzheimer.
On ne dispose pas de donn�es suffisantes pour donner corps �
l'hypoth�se selon laquelle l'exposition � l'aluminium des personnes
vivant dans des r�gions o� l'eau a une forte teneur en cet �l�ment
pourrait exacerber la maladie d'Alzheimer ou en acc�l�rer l'�volution.
On ne dispose pas d'�l�ments d'appr�ciation suffisants, sur le
plan sanitaire, pour entreprendre une r�vision des valeurs-guides de
l'OMS concernant l'exposition � l'aluminium des personnes en bonne
sant� non expos�es professionnellement. Par exemple, il n'existe pas
de base scientifique suffisante pour que l'on puisse �tablir une norme
de concentration de l'aluminium dans l'eau de boisson.
10.2 Sous-groupes de population expos�s � un risque particulier
On a montr� que chez des sujets de tous �ges atteints
d'insuffisance r�nale, l'accumulation d'aluminium provoquait un
syndrome clinique d'enc�phalopathie, une ost�omalacie r�sistante � la
vitamine D et une an�mie microcytaire. Cet aluminium peut provenir
du liquide d'h�modialyse ou de produits pharmaceutiques qui en
contiennent (par exemple les capteurs d'ions phosphate). La prise de
produits contenant des citrates peut provoquer une exacerbation de
l'absorption intestinale. Les insuffisants r�naux sont donc expos�s au
risque de neurotoxicit� aluminique.
L'exposition iatrog�ne � l'aluminium constitue une menace pour
les insuffisants r�naux chroniques. Chez les pr�matur�s, la charge de
l'organisme en aluminium est plus �lev�e que chez les autres
nourrissons. On s'efforcera donc de limiter ce genre d'exposition chez
tous ces groupes vuln�rables.
10.3 Populations professionnellement expos�es
Le risque est plus important pour les travailleurs expos�s
pendant de longues p�riodes � de fines particules d'aluminium.
Cependant, on ne dispose pas de donn�es suffisantes pour fixer avec
quelque certitude des limites d'exposition qui prot�gent contre les
effets ind�sirables de l'aluminium.
On conna�t des cas de fibrose pulmonaire dus � une exposition �
de la poudre d'aluminium � usage pyrotechnique, tr�s souvent enrob�e
d'huile min�rale .En revanche, il n'est pas prouv� que l'exposition �
l'aluminium sous d'autres formes puisse �galement provoquer ce type de
fibrose. Dans la plupart des cas de fibrose observ�s, il y avait eu
exposition simultan�e � d'autres agents fibrog�nes.
On a attribu� un certain nombre de cas d'asthme d'irritation �
l'inhalation de sulfate, de fluorure d'aluminium ou de
t�trafluoraluminate de potassium ou encore � un s�jour dans
l'environnement complexe des ateliers de production de l'aluminium.
10.4 Effets sur l'environnement
L'aluminium pr�sent dans l'environnement en phase solide est
relativement insoluble, en particulier lorsque le pH est voisin de la
neutralit�, d'o� les tr�s faibles concentrations d'aluminium en
solution dans la plupart des eaux naturelles.
Dans les milieux acides ou peu tamponn�s o� l'apport d'acide est
important, la concentration de l'aluminium peut atteindre des valeurs
nocives pour les organismes aquatiques et les plantes terrestres.
Toutefois, la sensibilit� � ce m�tal varie largement selon les
esp�ces, les souches et les stades de d�veloppement.
Les effets biologiques d�l�t�res de fortes teneurs en aluminium
inorganique sous forme monom�re peuvent �tre att�nu�s par la pr�sence
d'acides organiques, de fluorures, de silicates et de concentrations
�lev�es de calcium et de magn�sium.
Dans les eaux excessivement acides, il y a une r�duction sensible
de la diversit� biologique due � la mobilisation de l'aluminium sous
des formes plus toxiques. Cette r�duction de la diversit� biologique
s'observe � tous les niveaux trophiques.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades f�sicas y qu�micas
El aluminio es un metal blanco plateado, d�ctil y maleable.
Pertenece al grupo IIIA de la Tabla Peri�dica, y en los compuestos
suele encontrarse como AlIII. Forma cerca del 8% de la corteza
terrestre y es uno de los metales comunes m�s reactivos. La exposici�n
al agua, al ox�geno o a otros oxidantes conduce a la formaci�n de una
capa superficial de �xido de aluminio que confiere al metal una gran
resistencia a la corrosi�n. El �xido de aluminio es soluble en �cidos
minerales y �lcalis fuertes, pero insoluble en agua, mientras que el
cloruro, el nitrato y el sulfato de aluminio son solubles en agua. Los
halogenuros, los hidruros y los alquilos m�s cortos de aluminio
reaccionan violentamente con el agua.
El aluminio posee una elevada conductividad t�rmica y el�ctrica,
baja densidad y gran resistencia a la corrosi�n. A menudo se alea con
otros metales. Las aleaciones de aluminio son fuertes, ligeras y
f�cilmente susceptibles de conformaci�n a m�quina.
2. M�todos anal�ticos
Se han desarrollado diversos m�todos anal�ticos para determinar
la presencia de aluminio en muestras biol�gicas y ambientales. Los dos
m�todos m�s utilizados son la espectrometr�a de absorci�n at�mica en
horno de grafito (GF-AAS) y la espectrometr�a de emisi�n at�mico-
plasm�tica inductivamente acoplada (ICP-AES). La contaminaci�n de las
muestras por el aluminio del aire, de los recipientes o de los
reactivos durante el muestreo y preparaci�n constituye la principal
fuente de error anal�tico. En funci�n del tratamiento previo de la
muestra y de los procedimientos de concentraci�n y separaci�n, los
l�mites de detecci�n son de 1,9-4 �g/litro en los l�quidos biol�gicos
y 0,005-0,5 �g/g de peso seco en los tejidos al usar la GF-AAS, y de
5 �g/m3 en el aire y 3 �g/litro en el agua cuando se emplea la
ICP-AES.
3. Fuentes de exposici�n humana y ambiental
El aluminio se libera en el medio ambiente tanto a trav�s de
procesos naturales como de fuentes antropog�nicas. Est� muy
concentrado en el polvo de suelos dedicados a actividades tales como
la miner�a y la agricultura, y en las part�culas generadas por la
combusti�n del carb�n. Los silicatos de aluminio (arcillas), un
importante componente de los suelos, contribuyen a los niveles de
aluminio hallados en el polvo. Los procesos naturales superan con
mucho la contribuci�n antropog�nica directa al medio ambiente. El
aluminio movilizado por el hombre se forma en su mayor�a de forma
indirecta como resultado de la emisi�n de sustancias acidificantes.
En general, un descenso del pH ocasiona un aumento de la movilidad y
biodisponibilidad de las formas monom�ricas de aluminio. La principal
materia prima utilizada para producir aluminio es la bauxita, que
contiene hasta un 55% de al�mina (�xido de aluminio). La producci�n
mundial de bauxita fue de 106 millones de toneladas en 1992. El
metal de aluminio tiene una gran variedad de usos, entre ellos la
fabricaci�n de materiales estructurales para los sectores de la
construcci�n, el autom�vil y la aviaci�n, y la producci�n de
aleaciones met�licas. Los compuestos y materiales de aluminio tienen
tambi�n una amplia gama de usos, que incluyen la producci�n de vidrio,
cer�mica, caucho, conservantes de la madera, preparaciones
farmac�uticas y tejidos impermeabilizantes. Los minerales de aluminio
naturales, especialmente la bentonita y la zeolita, se emplean para
depurar el agua, para refinar el az�car, y en las industrias papeleras
y de la fermentaci�n.
4. Transporte, distribuci�n y transformaci�n en el medio ambiente
El aluminio es un metal ubicuo en el medio ambiente, donde se
encuentra en forma de silicatos, �xidos e hidr�xidos, combinado con
otros elementos como el sodio y el fl�or, y formando complejos con
materia org�nica. No se halla como metal libre debido a su
reactividad. S�lo tiene un estado de oxidaci�n (+3) en la naturaleza,
por lo que su transporte y distribuci�n en el medio ambiente dependen
tan s�lo de su qu�mica de coordinaci�n y de las caracter�sticas
f�sico-qu�micas del sistema ambiental concreto. A valores de pH
superiores a 5,5 los compuestos naturales de aluminio aparecen
predominantemente en forma no disuelta como gibbsita (Al(OH)3) o como
aluminosilicatos, excepto en presencia de grandes cantidades de
material org�nico disuelto, que se une al aluminio y puede dar lugar a
un aumento de la concentraci�n del aluminio disuelto en lagos y cursos
de agua. La movilidad del aluminio y su ulterior transporte en el
medio ambiente dependen de varios factores, entre los que cabe
mencionar el tipo de especies qu�micas, los cursos hidrol�gicos, las
interacciones suelo-agua y la composici�n del sustrato geol�gico. La
solubilidad del aluminio en equilibrio con la fase s�lida Al(OH)3
depende en gran medida del pH y de agentes complejantes tales como
fluoruros, silicatos, fosfatos y materia org�nica. La qu�mica del
aluminio inorg�nico en los suelos y los cursos de agua �cidos depende
de la solubilidad de los minerales y de los procesos de intercambio y
de mezcla de las aguas.
La acidificaci�n del suelo libera aluminio disolvi�ndolo, y el
metal llega as� a las corrientes de agua. La movilizaci�n del aluminio
por precipitaci�n �cida permite que haya m�s aluminio disponible para
captaci�n por las plantas.
5. Niveles ambientales y exposici�n humana
El aluminio es uno de los principales constituyentes de varios
componentes de la atm�sfera, en particular del polvo procedente de
suelos (tanto de fuentes naturales como de origen humano) y de
part�culas generadas por la combusti�n del carb�n. En las zonas
urbanas los niveles de aluminio en el polvo de la calle van de 3,7 a
11,6 �g/kg. Los niveles de aluminio en el aire var�an desde 0,5 ng/m3
sobre la Ant�rtida hasta m�s de 1000 ng/m3 en las zonas
industrializadas.
Las concentraciones de aluminio en las aguas superficiales y
subterr�neas son muy variables, dependiendo de factores geol�gicos y
f�sico-qu�micos. El aluminio puede estar en suspensi�n o disuelto.
Puede estar en forma de ligandos org�nicos o inorg�nicos o de ion
aluminio libre. En las aguas naturales el aluminio existe tanto en
forma monom�rica como polim�rica. Las especies de aluminio dependen
del pH y de las concentraciones de carbono org�nico disuelto (DOC)
fluoruros, sulfatos, fosfatos y part�culas en suspensi�n. Las
concentraciones de aluminio disuelto en las aguas de pH
aproximadamente neutro suelen ser bastante bajas, entre 1,0 y
50 �g/litro. En aguas m�s �cidas se alcanzan valores de hasta
500-1000 �g/litro. En condiciones de acidez extrema provocadas por el
avenamiento �cido de minas se han medido concentraciones de aluminio
disuelto de hasta 90 mg/litro.
La exposici�n humana no ocupacional al aluminio en el medio
ambiente se produce principalmente a trav�s de la ingesti�n de agua y
alimentos, sobre todo de estos �ltimos. La ingesta diaria de aluminio
con los alimentos y bebidas es en los adultos de entre 2,5 y 13 mg.
Esto representa un 90%-95% de la ingesta total. El agua de bebida
puede contribuir con unos 0,4 mg diariamente, seg�n los valores de las
actuales directrices internacionales, pero m�s probablemente se sit�a
en torno a los 0,2 mg/d�a. La exposici�n pulmonar puede contribuir
hasta con 0,04 mg/d�a. En algunas circunstancias, como la exposici�n
ocupacional y el uso de anti�cidos, los niveles de exposici�n pueden
ser mucho mayores. Con dos tabletas anti�cido de tama�o medio, por
ejemplo, se puede consumir m�s de 500 mg de aluminio. La evaluaci�n de
la captaci�n consecutiva a ese tipo de exposiciones plantea algunos
problemas debido a dificultades anal�ticas y de muestreo. Las
investigaciones isot�picas realizadas con Al26 indican que una de las
formas m�s biodisponibles del aluminio es el citrato, y que cuando el
aluminio est� en dicha forma podr�a darse hasta un 1% de absorci�n. No
obstante, los seres humanos absorben al parecer s�lo el 3% de la
cantidad total de aluminio ingerida diariamente con el agua de bebida,
una fuente relativamente secundaria en comparaci�n con los alimentos.
6. Cin�tica y metabolismo
6.1 Ser humano
El aluminio y sus compuestos parecen ser mal absorbidos por los
seres humanos, pero no hay estudios adecuados sobre la velocidad y el
grado de absorci�n. Las concentraciones de aluminio en la sangre y la
orina se han empleado como una medida f�cilmente obtenible de la
captaci�n de aluminio, habi�ndose observado niveles elevados en la
orina de soldadores de aluminio y de productores de polvo de escamas
de aluminio.
El mecanismo de absorci�n gastrointestinal del aluminio todav�a
no ha sido totalmente dilucidado. La variabilidad se debe a las
propiedades qu�micas del elemento y a las diversas especies qu�micas
formadas en funci�n del pH, la fuerza i�nica, la presencia de
elementos competitivos (silicio) y la presencia de agentes
complejantes en el interior del tracto digestivo (p.ej., citrato).
Se ha empleado el is�topo radiactivo Al26 para estudiar el
destino biol�gico y la absorci�n gastrointestinal del aluminio en el
hombre tras la ingesti�n de compuestos de aluminio. Se ha detectado
una variabilidad significativa entre los individuos. Se han notificado
fracciones de captaci�n de 5 � 10-3 para el aluminio como citrato,
1,04 � 10-4 para el hidr�xido de aluminio y 1,36 � 10-3 para el
hidr�xido administrado con citrato. Un estudio sobre la captaci�n del
aluminio del agua de bebida revel� una fracci�n de captaci�n de
2,35 � 10-3, lleg�ndose a la conclusi�n de que en la poblaci�n
general los individuos que consumen 1,5 litros/d�a de agua de bebida
con un contenido de 100 �g aluminio/litro, absorben un 3% del aluminio
total ingerido diariamente a partir de esa fuente, dependiendo de los
niveles presentes en los alimentos y de la frecuencia del uso de
anti�cidos.
La proporci�n de Al3+ plasm�tico normalmente unido a prote�nas
en el ser humano puede ser hasta del 70%-90% en los pacientes
sometidos a hemodi�lisis con niveles moderadamente altos de aluminio
en el plasma. Los niveles m�s elevados de aluminio suelen encontrarse
en los pulmones, donde puede hallarse en forma de part�culas inhaladas
insolubles.
La orina es la v�a m�s importante de excreci�n del aluminio. Tras
la administraci�n por v�a oral de una dosis �nica de aluminio, pasados
13 d�as se hab�a excretado un 83% por la orina y un 1,8% por las
heces. La semivida de las concentraciones urinarias en soldadores
expuestos durante m�s de 10 a�os fue de 6 meses o superior. Entre
trabajadores jubilados expuestos al polvo de escamas de aluminio, las
semividas calculadas se situaban entre 0,7 y 8 a�os.
6.2 Animales
La absorci�n por v�a gastrointestinal es normalmente menor
del 1%. Los principales factores que influyen en la absorci�n son
la solubilidad, el pH y las especies qu�micas. Los compuestos
complejantes org�nicos, sobre todo el citrato, aumentan la absorci�n.
La absorci�n del aluminio puede interferir en los sistemas de
transporte del calcio y el hierro. La absorci�n cut�nea y por
inhalaci�n no han sido estudiadas con detalle. El aluminio se
distribuye en la mayor�a de los �rganos del cuerpo, y cuando las dosis
son altas se acumula principalmente en los huesos. De forma limitada
pero a�n no determinada con precisi�n, atraviesa la barrera
hematoencef�lica, y llega tambi�n al feto. El aluminio se elimina
eficazmente por la orina. El periodo de semieliminaci�n plasm�tica es
de aproximadamente 1 h en los roedores.
7. Efectos en mam�feros de laboratorio y en sistemas de pruebas
in vitro
La toxicidad aguda del aluminio met�lico y de los compuestos de
aluminio es baja; los valores notificados de la DL50 oral est�n
comprendidas entre varios cientos y 1000 mg aluminio/kg de peso
corporal. Los valores de la CL50 por inhalaci�n no han sido
establecidos.
En estudios a corto plazo en los que se examin� una gama adecuada
de variables de evaluaci�n despu�s de exponer ratas, ratones o perros
a diversos compuestos de aluminio (fosfato s�dico de aluminio,
hidr�xido de aluminio, nitrato de aluminio) a trav�s de los alimentos
o del agua, s�lo se observaron efectos muy discretos (disminuciones
del aumento de peso corporal, asociadas generalmente a un descenso del
consumo de comida, o efectos histopatol�gicos leves) a las dosis m�s
altas (70 a 300 mg aluminio/kg de peso corporal al d�a). Los efectos
sist�micos que siguieron a la administraci�n parenteral incluyeron
tambi�n disfunci�n renal.
No se han hallado estudios de inhalaci�n adecuados. Despu�s de la
administraci�n intratraqueal de �xido de aluminio se observ� fibrosis
asociada a part�culas, parecida a la hallada en otros estudios sobre
el s�lice y el polvo de carb�n.
No se observaron signos manifiestos de fetotoxicidad, ni
alteraciones de los par�metros reproductivos generales despu�s del
tratamiento de ratas con 13, 26 � 52 mg aluminio/kg de peso corporal
al d�a (en forma de nitrato de aluminio) mediante alimentaci�n
forzada. Sin embargo, se observ� un retraso dosis-dependiente del
crecimiento en la descendencia, a dosis de 13 mg/kg en el caso de las
hembras y de 26 mg/kg en el caso de los machos. El nivel m�nimo con
efectos adversos observados (LOAEL) en el desarrollo (disminuci�n de
la osificaci�n, aumento de la incidencia de defectos cong�nitos en las
v�rtebras y el estern�n y peso fetal reducido) fue de 13 mg/kg
(nitrato de aluminio). Estos efectos no se observaron a dosis mucho
m�s altas de hidr�xido de aluminio. Se detect� una reducci�n del
crecimiento posnatal al emplear 13 mg/kg (nitrato de aluminio), aunque
no se analiz� la toxicidad materna. En estudios sobre el desarrollo
cerebral se detect� una disminuci�n de la fuerza de aprehensi�n en la
descendencia de hembras a las que se administraron con los alimentos
100 mg aluminio/kg de peso corporal en forma de lactato de aluminio,
no observ�ndose signos de toxicidad materna.
No existen indicios de que el aluminio sea carcin�geno. Puede
formar complejos con el ADN, as� como enlaces entre las prote�nas
cromos�micas y el ADN, pero no se ha demostrado que sea mut�geno en
bacterias o que induzca mutaciones o transformaciones en c�lulas de
mam�fero in vitro. Se han observado aberraciones cromos�micas en
c�lulas de la medula �sea de ratas y ratones expuestos.
Hay indicios fundados de que el aluminio es neurot�xico en
animales de experimentaci�n, aunque se da una considerable
variabilidad entre especies. En especies susceptibles, la toxicidad
que sigue a la administraci�n parenteral se caracteriza por un
deterioro neurol�gico progresivo, conducente a un estado epil�ptico
que acarrea la muerte (DL50 = 6 �g Al/g de peso seco del cerebro).
Morfol�gicamente, la encefalopat�a progresiva se asocia a una
patolog�a neurofibrilar de las neuronas grandes y medianas, sobre todo
en la m�dula espinal, el tallo encef�lico y determinadas zonas del
hipocampo. Estos ovillos neurofibrilares son morfol�gica y
bioqu�micamente diferentes de los que caracterizan la enfermedad de
Alzheimer. En animales de experimentaci�n expuestos a sales solubles
de aluminio (p.ej., lactato y cloruro) a trav�s de los alimentos o el
agua, a dosis de 50 mg aluminio/kg de peso al d�a o mayores, se ha
observado un deterioro de la conducta sin signos concomitantes de
encefalopat�a o cambios neurohistopatol�gicos manifiestos.
La osteomalacia que afecta al hombre se observa tambi�n
sistem�ticamente en las especies de cierto tama�o (p.ej., el perro
o el cerdo) expuestas al aluminio; en los roedores se observan
manifestaciones parecidas. Estos efectos parecen darse en todas las
especies, incluido el hombre, a niveles de aluminio de 100 a 200 �g/g
de cenizas �seas.
8. Efectos en el hombre
No se han descrito efectos pat�genos agudos en la poblaci�n
general como consecuencia de la exposici�n al aluminio.
En Inglaterra una poblaci�n de aproximadamente 20 000 individuos
se vio expuesta durante por lo menos 5 d�as a niveles elevados de
sulfato de aluminio, debido a la contaminaci�n accidental de unas
instalaciones de agua de bebida. Se inform� de casos de n�useas,
v�mitos, diarrea, �lceras bucales, �lceras y erupciones cut�neas y
dolores artr�ticos. Se comprob� que los s�ntomas eran en su mayor�a
leves y de corta duraci�n. No se pudieron atribuir efectos duraderos
sobre la salud a las exposiciones conocidas al aluminio del agua de
bebida.
Se ha propuesto la hip�tesis de que el aluminio presente en
el agua potable es un factor de riesgo por lo que se refiere al
desarrollo o la aceleraci�n de la enfermedad de Alzheimer y al
deterioro senil de la funci�n cognitiva. Se ha sugerido tambi�n que el
polvo y los vapores de aluminio fino apisonado son quiz� factores de
riesgo que propician el deterioro de las funciones cognitivas y la
aparici�n de enfermedades pulmonares en determinados trabajos.
Se han llevado a cabo unos 20 estudios epidemiol�gicos para
comprobar la hip�tesis de que el aluminio del agua de bebida es un
factor de riesgo de la enfermedad de Alzheimer, y dos estudios han
evaluado la asociaci�n entre el aluminio del agua de bebida y el
deterioro de las funciones cognitivas. El dise�o de estos estudios
abarcaba desde el control ecol�gico hasta el control de los casos. Se
consider� que 8 estudios realizados en poblaciones de Noruega, el
Canad�, Francia, Suiza e Inglaterra ten�an la calidad necesaria para
satisfacer los criterios generales establecidos a fin de evaluar la
exposici�n y los resultados y de poder efectuar ajustes por lo menos
en algunas variables de confusi�n. De los seis estudios que analizaron
la relaci�n entre el aluminio del agua de bebida y la demencia o la
enfermedad de Alzheimer, tres hallaron una relaci�n positiva, pero no
as� los otros tres. Sin embargo, todos los estudios presentaban
algunos fallos de dise�o (relacionados por ejemplo con la evaluaci�n
de la exposici�n ecol�gica, la no consideraci�n de todas las fuentes
de exposici�n al aluminio y de factores de confusi�n importantes como
la educaci�n, la situaci�n socioecon�mica y los antecedentes
familiares, el uso de medidas indirectas de la evoluci�n de la
enfermedad de Alzheimer, o unos m�todos sesgados de selecci�n). En
general, los riesgos relativos determinados eran inferiores a 2, con
grandes intervalos de confianza, a concentraciones totales de aluminio
de 100 �g/litro o superiores en el agua de bebida. Sobre la base de
los conocimientos disponibles acerca de la patog�nesis de la
enfermedad de Alzheimer y de todas las pruebas aportadas por estos
estudios epidemiol�gicos, se lleg� a la conclusi�n de que los datos
epidemiol�gicos actuales no respaldan la hip�tesis de una relaci�n
causal entre la enfermedad de Alzheimer y el aluminio del agua de
bebida.
Adem�s de los estudios epidemiol�gicos realizados para analizar
la relaci�n entre la enfermedad de Alzheimer y el aluminio del agua
de bebida en otros dos estudios se examin� la relaci�n entre los casos
de disfunci�n cognitiva y de enfermedad de Alzheimer en poblaciones
de ancianos y los niveles de aluminio en el agua de bebida. Los
resultados fueron una vez m�s contradictorios. En un estudio realizado
en 800 octogenarios varones que consum�an agua de bebida con
concentraciones de aluminio de hasta 98 �g/litro no se hall� relaci�n
alguna. El segundo estudio utiliz� "cualquier indicio de deterioro
mental" como criterio de seguimiento, calculando as� un riesgo
relativo de 1,72 para concentraciones de aluminio superiores a
85 �g/litro en 250 varones. Tales datos son insuficientes para
demostrar que el aluminio sea una causa de deterioro cognitivo en los
ancianos.
Los datos sobre el deterioro de la funci�n cognitiva en relaci�n
con la exposici�n al aluminio son contradictorios. La mayor�a de los
estudios se han hecho en poblaciones reducidas, y la metodolog�a
utilizada es cuestionable en lo que respecta a la magnitud del efecto
estudiado, la evaluaci�n de la exposici�n y los factores de confusi�n.
En un estudio comparativo del deterioro cognitivo entre mineros no
expuestos y mineros expuestos a un polvo que conten�a un 85% de
aluminio molido muy fino y un 15% de �xido de aluminio (como
profilaxis contra el s�lice), los resultados de las pruebas cognitivas
y la proporci�n de personas con dificultades en al menos una de las
pruebas fueron peores entre los mineros expuestos. Se detect� una
tendencia al aumento del riesgo relacionada con la exposici�n.
En todos los estudios ocupacionales de los que se tiene noticia,
la magnitud de los efectos observados, la presencia de factores de
confusi�n, los problemas relacionados con la evaluaci�n de la
exposici�n y la probabilidad de exposiciones mixtas hacen que, en
conjunto, los datos sean insuficientes como para extraer la conclusi�n
de que el aluminio es una causa de deterioro cognitivo en los
trabajadores expuestos al aluminio en su trabajo.
En estudios limitados con trabajadores expuestos a vapores de
aluminio se ha informado de s�ndromes neurol�gicos que incluyen el
deterioro de la funci�n cognitiva, disfunciones motoras y neuropat�a
perif�rica. Se ha informado de una peque�a poblaci�n de soldadores de
aluminio que, comparados con soldadores de hierro, presentaron un
ligero deterioro de la funci�n motora repetitiva. En otros estudios
realizado con cuestionarios se detect� un aumento de los s�ntomas
neuropsiqui�tricos.
En los pacientes con insuficiencia renal cr�nica, la exposici�n
iatrog�nica a l�quidos de di�lisis o productos farmac�uticos con
aluminio puede producir encefalopat�a, osteomalacia resistente a la
vitamina D y anemia microc�tica. Estos s�ndromes cl�nicos pueden
prevenirse reduciendo la exposici�n al aluminio.
En los ni�os prematuros expuestos a fuentes iatrog�nicas de
aluminio puede producirse un incremento del contenido de aluminio
de los tejidos, particularmente en los huesos, incluso cuando la
disfunci�n renal no es lo suficientemente grave como para provocar
un aumento de los niveles de creatinina en sangre. En caso de
insuficiencia renal pueden aparecer convulsiones y encefalopat�a.
Aunque la exposici�n humana al aluminio est� muy extendida,
s�lo se ha informado de unos cuantos casos de hipersensibilidad
consecutivos a la aplicaci�n cut�nea o la administraci�n parenteral
de algunos compuestos de aluminio.
Se ha informado de casos de fibrosis pulmonar en algunos
trabajadores expuestos a polvo muy fino de aluminio apisonado
utilizado en la fabricaci�n de explosivos y material pirot�cnico. Casi
todos los casos estaban relacionados con la exposici�n a part�culas de
aluminio cubiertas de aceite mineral. Dicho procedimiento ya no se
utiliza. Otros casos de fibrosis pulmonar se han relacionado con
exposiciones a otros agentes minerales como el s�lice y el asbesto y
no pueden atribuirse exclusivamente al aluminio.
Algunos casos de asma inducida por sustancias irritantes se han
asociado a la inhalaci�n de sulfato de aluminio, fluoruro de aluminio
o tetrafluoruro pot�sico de aluminio, as� como al ambiente cargado de
los cuartos de calderas utilizados en la producci�n de aluminio.
La informaci�n disponible es insuficiente para poder clasificar
el riesgo de c�ncer asociado a las distintas formas de exposici�n
humana al aluminio y sus compuestos. Los estudios en animales no
indican que el aluminio o los compuestos del aluminio sean
carcin�genos.
9. Efectos en otros organismos en el laboratorio y en el campo
En las algas acu�ticas unicelulares se ha detectado una mayor
toxicidad a pH bajo, circunstancia que aumenta la biodisponibilidad
del aluminio. Estas algas son m�s sensibles que otros microorganismos,
y de 19 especies lacustres la mayor parte presentaron una inhibici�n
completa del crecimiento a una concentraci�n total de aluminio de
200 �g/litro (pH 5,5). Es posible seleccionar cepas tolerantes al
aluminio; se han aislado algas verdes capaces de crecer en presencia
de 48 mg/litro a pH 4,6.
En los invertebrados acu�ticos la CL50 est� comprendida entre
0,48 mg/litro (poliquetos) y 59,6 mg/litro (d�fnidos). En los peces,
la CL50 a las 96 horas va desde 0,095 mg/litro (pez estandarte
americano) hasta 235 mg/litro (gambusia com�n). Sin embargo, estos
resultados deben interpretarse con cautela dados los importantes
efectos del pH en la disponibilidad del aluminio. El amplio margen de
valores de la CL50 se debe probablemente a la distinta
disponibilidad. La adici�n de agentes quelantes, como el NTA y el
EDTA, reduce la toxicidad aguda del aluminio para los peces.
Los macroinvertebrados responden de diversa forma al aluminio. En
el margen normal de pH la toxicidad del aluminio aumenta al disminuir
el pH, sin embargo en aguas muy �cidas el aluminio puede reducir los
efectos del estr�s �cido. Algunos invertebrados son muy resistentes al
estr�s �cido y pueden llegar a ser muy abundantes en aguas �cidas. Se
ha informado de un aumento de la tasa de deriva de los invertebrados
en cursos de agua en condiciones de estr�s por pH o por pH/aluminio;
esa es una respuesta com�n a diversos agentes estresantes.
Invertebrados lacustres sobrevivieron en general a la exposici�n al
aluminio en la naturaleza, pero sufrieron en condiciones oligotr�ficas
debido a la disminuci�n del fosfato inducida por la precipitaci�n con
el aluminio.
Se han llevado a cabo pruebas de toxicidad en peces a corto y
largo plazo en muy diversas condiciones y, lo que es m�s importante,
a distintos valores de pH. Los datos muestran efectos significativos a
niveles de aluminio inorg�nico monom�rico de tan s�lo 25 �g/litro. No
obstante, la compleja relaci�n entre la acidez y la biodisponibilidad
del aluminio dificulta la interpretaci�n de los datos de toxicidad. A
niveles de pH muy bajos (inhabituales en aguas naturales) la
concentraci�n de hidrogeniones parece ser el factor t�xico, y la
adici�n de aluminio tiende a reducir la toxicidad. En el margen de pH
4,5 a 6,0 el aluminio en equilibrio ejerce su m�ximo efecto t�xico. Se
ha visto que la toxicidad tambi�n aumenta a niveles crecientes de pH
en la regi�n de pH alcalino. El mecanismo propuesto para explicar la
toxicidad del aluminio en los peces es la incapacidad de los animales
a mantener el equilibrio osmorregulador, y los problemas respiratorios
asociados a la precipitaci�n del aluminio en el moco de las branquias.
El primero de estos efectos se asocia a niveles bajos de pH. Estos
datos de laboratorio han sido confirmados por estudios realizados en
la naturaleza, especialmente en zonas sometidas a estr�s �cido.
Los huevos y las larvas de los anfibios se ven afectados por
la acidez y el aluminio, y se da una interacci�n entre estos dos
factores. Se ha informado de fen�menos de disminuci�n de la
incubaci�n, retraso de la eclosi�n, retraso de la metamorfosis,
metamorfosis con tama�o reducido y aumento de la mortalidad en
diversas especies a concentraciones de aluminio inferiores a
1 mg/litro.
La exposici�n de las ra�ces de plantas terrestres al aluminio
puede frenar el crecimiento radicular, la captaci�n de nutrientes y el
desarrollo de la planta. Se ha demostrado la tolerancia al aluminio
tanto en el laboratorio como en la naturaleza.
10. Conclusiones
10.1 Poblaci�n general
Los estudios realizados en animales acerca de la exposici�n al
aluminio han revelado riesgos para el desarrollo neurol�gico y las
funciones cerebrales. Sin embargo, no se ha demostrado que el aluminio
entra�e riesgos para la salud de las personas sanas y no expuestas en
el trabajo.
No existen pruebas de que el aluminio tenga un papel causal
primordial en la enfermedad de Alzheimer, patolog�a que el metal no
induce in vivo en ninguna especie, incluido el hombre.
La hip�tesis de que la exposici�n de la poblaci�n anciana de
algunas regiones a niveles elevados de aluminio en el agua de bebida
puede exacerbar o acelerar la enfermedad de Alzheimer no est� avalada
por datos v�lidos.
Tampoco hay datos v�lidos que corroboren la hip�tesis de que
determinadas exposiciones, ya sean ocupacionales o a trav�s del agua
de bebida, pueden asociarse a un deterioro inespec�fico de la funci�n
cognitiva.
No existen datos suficientes relacionados con la salud que
justifiquen la revisi�n de las actuales directrices de la OMS respecto
a la exposici�n al aluminio de individuos sanos no expuestos
ocupacionalmente. As�, por ejemplo, no hay una base cient�fica s�lida
para establecer una norma basada en criterios de salud sobre el
aluminio en el agua de bebida.
10.2 Subpoblaciones con un riesgo especial
En pacientes con trastornos de la funci�n renal, cualquiera que
sea su edad, la acumulaci�n de aluminio provoca un s�ndrome cl�nico
caracterizado por encefalopat�a, osteomalacia resistente a la vitamina
D y anemia microc�tica. Las fuentes de aluminio son los l�quidos
de hemodi�lisis y las preparaciones famarc�uticas que contienen
aluminio (p.ej., aglutinantes de fosfato). Los productos que contienen
citrato pueden aumentar la absorci�n intestinal. Los pacientes con
insuficiencia renal corren por tanto el riesgo de sufrir
neurotoxicidad por aluminio.
La exposici�n iatrog�nica al aluminio entra�a riesgos para los
pacientes con insuficiencia renal cr�nica. Los lactantes prematuros
tienen en su organismo una mayor carga de aluminio que los otros
lactantes. Deben tomarse toda clase de precauciones para limitar la
exposici�n de esos grupos.
10.3 Poblaciones expuestas ocupacionalmente
Los trabajadores que han estado expuestos durante largo tiempo a
niveles altos de part�culas finas de aluminio corren quiz� un mayor
riesgo de sufrir efectos nocivos para su salud. Sin embargo, no hay
datos suficientes para fijar con cierto grado de fiabilidad unos
l�mites de exposici�n ocupacional en relaci�n con los efectos adversos
del aluminio.
La exposici�n al polvo de aluminio pirot�cnico apisonado,
recubierto casi siempre de aceites lubricantes minerales, ha provocado
fibrosis pulmonar (aluminosis), pero no se ha demostrado que la
exposici�n a otras formas de aluminio produzca fibrosis pulmonar. En
la mayor parte de los casos notificados hab�a habido tambi�n
exposici�n a otros agentes potencialmente fibrog�nicos.
Algunos casos de asma inducida por sustancias irritantes se han
asociado a la inhalaci�n de sulfato de aluminio, fluoruro de aluminio
o tetrafluoruro pot�sico de aluminio, as� como al ambiente cargado del
interior de los cuartos de calderas utilizados en la producci�n de
aluminio.
10.4 Efectos ambientales en el medio ambiente
Las fases s�lidas que contienen aluminio en el medio ambiente son
relativamente insolubles, en particular a un pH aproximadamente
neutro, de ah� las bajas concentraciones de aluminio disuelto que
tienen la mayor�a de las aguas naturales.
En los entornos �cidos o escasamente tamponados que reciben
aportes muy acidificantes, las concentraciones de aluminio pueden
aumentar hasta niveles perjudiciales tanto para los organismos
acu�ticos como para las plantas terrestres. No obstante, existen
grandes diferencias en la sensibilidad a este metal seg�n la especie,
la cepa y la etapa de la vida.
Los efectos biol�gicos adversos de las concentraciones elevadas
de aluminio monom�rico inorg�nico son menores en presencia de �cidos
org�nicos, fluoruros, silicatos y niveles altos de calcio y magnesio.
En las aguas sometidas a estr�s �cido se produce una reducci�n
sustancial de la diversidad de especies, asociada a una movilizaci�n
de las formas m�s t�xicas de aluminio. Dicha p�rdida de diversidad se
observa en todos los niveles tr�ficos.
 All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
Members
Dr T.M. Florence, Centre for Environmental Health Sciences, Oyster
Bay, New South Wales, Australia
Dr M. Golub, California Regional Primate Research Center, University
of California, Davis, California, USA
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
( Co-Rapporteur)
Professor D.R. McLachlan ( Retired from: Centre for Research in
Neurogenerative Diseases, University of Toronto, Toronto,
Ontario, Canada
Dr M. Moore, National Health and Medical Research Council, National
Research Centre for Environmental Toxicology, Coopers Plains,
Brisbane, Australia ( Chairman)
Dr T.V. O'Donnell, University of Otago, Wellington South, New Zealand
( Vice-Chairman)
Professor B. Rosseland, Norwegian Institute of Water Research (NIVA),
Oslo, Norway
Dr B. Simon, Fraunhofer Institute, Hanover, Germany ( Co-Rapporteur)
Dr B. Sjogren, Department of Occupational Medicine, Swedish National
Institute for Working Life, Solna, Sweden
Dr L. Smith, Disease Control Service, Public Health Branch, Ontario
Ministry of Health, North York, Ontario, Canada
Dr E. Storey, Royal Melbourne Hospital, Department of Pathology,
University of Melbourne, Parkville, Victoria, Australia
Dr H. Temmink, Department of Toxicology, Agricultural University,
Wageningen, The Netherlands ( Vice-Chairman)
Dr M.K. Ward, Department of Renal Medicine, Royal Victoria Infirmary,
Newcastle-upon-Tyne, United Kingdom
Dr M. Wilhelm, Health Institute, University of Dusseldorf, Dusseldorf,
Germany
Professor H.M. Wisniewski, New York State Institute for Basic
Research in Developmental Disabilities, Staten Island, New York,
USA
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute
of Occupational Medicine, Ministry of Health, Beijing, China
Observers
Dr K. Bentley, Environmental Health Assessment and Criteria, Human
Services and Health, Woden, Australia
Dr O.C. B�ckman, Norsk Hydro, Porsgrunn Research Centre, Porsgrunn,
Norway
Dr J. Borak, Occupational and Environmental Health, Jonathan Borak &
Co., New Haven, Connecticut, USA
Dr I. Calder, Occupational and Environmental Health, South Australian
Health Commission, Adelaide, Australia
Dr J.N. Fisher, ALCOA of Australia Ltd, Point Henry Works, Geelong,
Victoria, Australia
Mr D. Hughes, Environment, Mount Isa Mine Holdings, Brisbane,
Australia
Dr P. Imray, Environmental Health Branch, Queensland Health, Brisbane,
Australia
Ms M.E. Meek, Environmental Health Directorate, Health Canada,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr N. Priest, AEA Technology, Harwell, Didcot, Oxfordshire, United
Kingdom
Dr D. Wilcox, Medical Section, Health Services, Sydney Water, Sydney,
Australia
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety
Inter-regional Research Unit, World Health Organization, Research
Triangle Park, North Carolina, USA ( Secretary)
Dr D. Johns, DPIE, Coal and Mineral Division, Canberra, Australia
( Temporary Adviser)
Mr D. Wagner, Chemicals Safety Unit, Human Services and Health,
Canberra, Australia ( Temporary Adviser)
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
A WHO Task Group on Environmental Health Criteria for Aluminium
met in Brisbane, Australia, from 24 to 28 April 1995. The meeting
was sponsored by a consortium of Australian Commonwealth and State
Governments through a national steering committee chaired by
Dr K. Bentley, Director, Health and Environmental Policy, Department
of Health and Family Services, Canberra. The meeting was hosted and
organized by the NHMRC National Research Centre for Environmental
Toxicology (NRCET), Dr M. Moore, Director, being responsible for
the arrangements. Dr D. Lange, Chief Health Officer, welcomed
participants on behalf of Queensland Health, and Professor L. Roy
Webb, Vice-chancellor, Griffith University, welcomed them on behalf of
NRCET. Dr G.C. Becking, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS and the three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft criteria monograph and made an evaluation of the risks to human
health and the environment from exposure to aluminium.
The first draft was prepared under the coordination of Dr G.
Rosner, Fraunhofer Institute of Toxicology and Aerosol Research,
Germany, and Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood,
United Kingdom. The draft reviewed by the Task Group, incorporating
the comments received following review by the IPCS Contact Points, was
prepared through the cooperative effort of the Fraunhofer Institute,
Institute of Terrestrial Ecology and the Secretariat.
Dr G.C. Becking (IPCS Central Unit, Inter-regional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively, of
this monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
ABBREVIATIONS
AD Alzheimer's disease
AIBD aluminium-induced bone disease
cAMP cyclic adenosine monophosphate
CI confidence interval
1,25-(OH)2-D3 1,25-dihydroxy-vitamin D3
DOC dissolved organic carbon
EDTA ethylenediaminetetraacetic acid
i.p. intraperitoneal
i.v. intravenous
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
LTP long-term potentiation
NFT neurofibrillary tangle
NIOSH National Institute for Occupational Safety and
Health (USA)
NOEC no-observed-effect concentration
NOEL no-observed-effect level
NTA nitrilotriacetic acid
OR odds ratio
PHF paired helical filaments
Pt platinum unit (1 unit equals the colour produced by
lung chloroplatinate in 1 litre of water)
PTH parathyroid hormone
s.c. subcutaneous
WAIS Weschler Adult Intelligence Scale
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties
Aluminium is a silvery-white, ductile and malleable metal. It
belongs to group IIIA of the Periodic Table, and in compounds it is
usually found as AlIII. It forms about 8% of the earth's crust and is
one of the most reactive of the common metals. Exposure to water,
oxygen or other oxidants leads to the formation of a superficial
coating of aluminium oxide, which provides the metal with a high
resistance to corrosion. Aluminium oxide is soluble in mineral acids
and strong alkalis but insoluble in water, whereas aluminium chloride,
nitrate and sulfate are water soluble. Aluminium halogenides, hydride
and lower aluminium alkyls react violently with water.
Aluminium possesses high electrical and thermal conductivity, low
density and great resistance to corrosion. It is often alloyed with
other metals. Aluminium alloys are light, strong and readily machined
into shapes.
1.2 Analytical methods
Various analytical methods have been developed to determine
aluminium in biological and environmental samples. Graphite furnace
- atomic-absorption spectrometry (GF-AAS) and inductively coupled
plasma - atomic-emission spectrometry (ICP-AES) are the most
frequently used methods. Contamination of the samples with aluminium
from air, vessels or reagents during sampling and preparation is the
main source of analytical error. Depending on sample pretreatment,
separation and concentration procedures, detection limits are
1.9-4 �g/litre in biological fluids and 0.005-0.5 �g/g dry weight in
tissues using GF-AAS, and 5 �g/m3 in air and 3 �g/litre in water
using ICP-AES.
1.3 Sources of human and environmental exposure
Aluminium is released to the environment both by natural
processes and from anthropogenic sources. It is highly concentrated in
soil-derived dusts from such activities as mining and agriculture, and
in particulate matter from coal combustion. Aluminium silicates
(clays), a major component of soils, contribute to the aluminium
levels of dust. Natural processes far outweigh direct anthropogenic
contributions to the environment. Mobilization of aluminium through
human actions is mostly indirect and occurs as a result of emission of
acidifying substances. In general, decreasing pH results in an
increase in mobility and bioavailability for monomeric forms of
aluminium. The most important raw material for the production of
aluminium is bauxite, which contains up to 55% alumina (aluminium
oxide). World bauxite production was 106 million tonnes in 1992.
Aluminium metal has a vide variety of uses, including structural
materials in construction, automobiles and aircraft, and the
production of metal alloys. Aluminium compounds and materials also
have a wide variety of uses, including production of glass, ceramics,
rubber, wood preservatives, pharmaceuticals and waterproofing
textiles. Natural aluminium minerals, especially bentonite and
zeolite, are used in water purification, sugar refining, brewing and
paper industries.
1.4 Environmental transport, distribution and transformation
Aluminium occurs ubiquitously in the environment in the form of
silicates, oxides and hydroxides, combined with other elements such as
sodium and fluorine and as complexes with organic matter. It is not
found as a free metal because of its reactivity. It has only one
oxidation state (+3) in nature; therefore, its transport and
distribution in the environment depend only upon its coordination
chemistry and the chemical-physical characteristics of the local
environmental system. At pH values greater than 5.5, naturally
occurring aluminium compounds exist predominantly in an undissolved
form such as gibbsite (Al(OH)3) or as aluminosilicates, except in the
presence of high amounts of dissolved organic material, which binds
with aluminium and can lead to increased concentrations of dissolved
aluminium in streams and lakes. Several factors influence aluminium
mobility and subsequent transport within the environment. These
include chemical speciation, hydrological flow paths, soil-water
interactions, and the composition of the underlying geological
materials. The solubility of aluminium in equilibrium with solid phase
Al(OH)3 is highly dependent on pH and on complexing agents such as
fluoride, silicate, phosphate and organic matter. The chemistry of
inorganic aluminium in acid soil and stream water can be considered in
terms of mineral solubility, ion exchange and water mixing processes.
Upon acidification of soils, aluminium can be released into
solution for transport to streams. Mobilization of aluminium by acid
precipitation results in more aluminium being available for plant
uptake.
1.5 Environmental levels and human exposure
Aluminium is a major constituent of a number of atmospheric
components particularly in soil-derived dusts (both from natural
sources and human activity) and particulates from coal combustion. In
urban areas aluminium levels in street dust range from 3.7 to
11.6 �g/kg. Airborne aluminium levels vary from 0.5 ng/m3 over
Antarctica to more than 1000 ng/m3 in industrialized areas.
Surface freshwater and soil water aluminium concentrations can
vary substantially, being dependent on physico-chemical and geological
factors. Aluminium can be suspended or dissolved. It can be bound with
organic or inorganic ligands, or it can exist as a free aluminium ion.
In natural waters aluminium exists in both monomeric and polymeric
forms. Aluminium speciation is determined by pH and the concentrations
of dissolved organic carbon (DOC), fluoride, sulfate, phosphate and
suspended particulates. Dissolved aluminium concentrations for water
in the circumneutral pH range are usually quite low, ranging from
1.0 to 50 �g/litre. This rises to 500-1000 �g/litre in more acidic
water. At the extreme acidity of water affected by acid mine drainage,
dissolved aluminium concentrations of up to 90 mg/litre have been
measured.
Non-occupational human exposure to aluminium in the environment
is primarily through ingestion of food and water. Of these, food is
the principal contributor. The daily intake of aluminium from food and
beverages in adults ranges between 2.5 and 13 mg. This is between 90
and 95% of total intake. Drinking-water may contribute around 0.4 mg
daily at present international guideline values, but is more likely
to be around 0.2 mg/day. Pulmonary exposure may contribute up to
0.04 mg/day. In some circumstances, such as occupational exposure and
antacid use, the levels of exposure will be much greater. For example,
> 500 mg of aluminium may be consumed in two average-sized antacid
tablets. There are some difficulties in assessing uptake from these
exposures because of analytical and sampling difficulties. Isotopic
investigations with Al26 indicate that one of the most bioavailable
forms of aluminium is the citrate and that there could be as much as
1% absorption when aluminium is in this form. However, humans would
absorb only 3% of their total daily uptake of aluminium from drinking-
water, a relatively minor source compared to food.
1.6 Kinetics and metabolism
1.6.1 Humans
Aluminium and its compounds appear to be poorly absorbed in
humans, although the rate and extent of absorption have not been
adequately studied. Concentrations of aluminium in blood and urine
have been used as a readily available measure of aluminium uptake,
increased urine levels having been observed among aluminium welders
and aluminium flake-powder producers.
The mechanism of gastrointestinal absorption of aluminium has not
yet been fully elucidated. Variability results from the chemical
properties of the element and the formation of various chemical
species, which is dependent upon the pH, ionic strength, presence of
competing elements (silicon), and the presence of complexing agents
within the gastrointestinal tract (e.g., citrate).
The biological behaviour and gastrointestinal absorption of
aluminium in humans ingesting aluminium compounds has been studied by
using the radioactive isotope Al26. Significant intersubject
variability has been demonstrated. Measured fractional uptakes of 5 �
10-3 for aluminium as citrate, 1.04 � 10-4 for aluminium hydroxide
and 1.36 � 10-3 for the hydroxide given with citrate were reported. A
study of the fractional uptake of aluminium from drinking-water showed
an uptake fraction of 2.35 � 10-3. It was concluded that members of
the general population consuming 1.5 litres/day of drinking-water
containing 100 �g aluminium/litre would absorb about 3% of their total
daily intake of aluminium from this source depending upon the levels
found in food and the frequency of antacid use.
The proportion of plasma Al3+ normally bound to protein in
humans may be as high as 70-90% in haemodialysis patients with
moderately increased plasma aluminium. The highest levels of aluminium
may be found in the lungs, where it may be present as inhaled
insoluble particles.
The urine is the most important route of aluminium excretion.
After peroral administration of a single dose of aluminium, 83% was
excreted in urine after 13 days and 1.8% in the faeces. The half-life
of urinary concentration among welders exposed for more than 10 years
was 6 months or longer. Among retired workers exposed to aluminium
flake powders, the calculated half-lives were between 0.7 and 8 years.
1.6.2 Animals
Absorption via the gastrointestinal tract is usually less than
1%. The main factors influencing absorption are solubility, pH and
chemical species. Organic complexing compounds, notably citrate,
increase absorption. The aluminium absorption may interact with
calcium and iron transport systems. Dermal and inhalation absorption
has not been studied in detail. Aluminium is distributed in most
organs within the body with accumulation occurring mainly in bone at
high dose levels. To a limited but as yet undetermined extent,
aluminium passes the blood-brain barrier and is also distributed to
the fetus. Aluminium is eliminated effectively by urine. Plasma half-
life is about 1 h in rodents.
1.7 Effects on laboratory mammals and in vitro test systems
The acute toxicity of metallic aluminium and aluminium compounds
is low, the reported oral LD50 values being in the range of several
hundred to 1000 mg aluminium/kg body weight per day. However, the
LC50 values for inhalation have not been identified.
In short-term studies in which an adequate range of end-points
was examined following exposure of rats, mice or dogs to various
aluminium compounds (sodium aluminium phosphate, aluminium hydroxide,
aluminium nitrate) in the diet or drinking-water, only minimal effects
(decreases in body weight gain generally associated with decreases in
food consumption or mild histopathological effects) have been observed
at the highest administered doses (70 to 300 mg aluminium/kg body
weight per day). Systemic effects following parenteral administration
also included kidney dysfunction.
Adequate inhalation studies were not identified. Following
intratracheal administration of aluminium oxide, particle-associated
fibrosis was observed, similar to that found in other studies on
silica and coal dust.
No overt fetotoxicity was noted, nor were general reproductive
parameters noted after gavage treatment of rats with 13, 26 or 52 mg
aluminium/kg body weight per day (as aluminium nitrate). However, a
dose-dependent delay in the growth of offspring was noted with females
administered 13 mg/kg and in male offspring at 26 mg/kg. The lowest-
observed-adverse-effect level (LOAEL) for developmental effects
(decreased ossification, increased incidence of vertebral and
sternebrae terata and reduced fetal weight) was 13 mg/kg (aluminium
nitrate). These effects were not observed at much higher doses of
aluminium hydroxide. There were reductions in postnatal growth at
13 mg/kg (aluminium nitrate), although maternal toxicity was not
examined. In studies on brain development, grip strength was impaired
in offspring of dams fed 100 mg aluminium/kg body weight as aluminium
lactate in the diet, in the absence of maternal toxicity.
There is no indication that aluminium is carcinogenic. It can
form complexes with DNA and cross-link chromosomal proteins and DNA,
but it has not been shown to be mutagenic in bacteria or induce
mutation or transformation in mammalian cells in vitro. Chromosomal
aberrations have been observed in bone marrow cells of exposed mice
and rats.
There is considerable evidence that aluminium is neurotoxic in
experimental animals, although there is considerable variation among
species. In susceptible species, toxicity following parenteral
administration is characterized by progressive neurological
impairment, resulting in death with status epilepticus (LD50 =
6 �g Al/g dry weight of brain). Morphologically, the progressive
encephalopathy is associated with neurofibrillary pathology in
large and medium size neurons predominantly in the spinal cord,
brainstem and selected areas of the hippocampus. These tangles are
morphologically and biochemically different from those that occur in
Alzheimer's disease (AD). Behavioural impairment has been observed in
the absence of overt encephalopathy or neurohistopathology in
experimental animals exposed to soluble aluminium salts (e.g.,
lactate, chloride) in the diet or drinking-water at doses of 50 mg
aluminium/kg body weight per day or more.
Osteomalacia, as it presents in man, is observed consistently in
larger species (e.g., dogs and pigs) exposed to aluminium; a similar
condition is observed in rodents. These effects appear to occur in all
species, including humans, at aluminium levels of 100 to 200 �g/g bone
ash.
1.8 Effects on humans
No acute pathogenic effects in the general population have been
described after exposure to aluminium.
In England, a population of about 20 000 individuals was exposed
for at least 5 days to increased levels of aluminium sulfate,
accidentally placed in a drinking-water facility. Case reports of
nausea, vomiting, diarrhoea, mouth ulcers, skin ulcers, skin rashes
and arthritic pain were noted. It was concluded that the symptoms were
mostly mild and short-lived. No lasting effects on health could be
attributed to the known exposures from aluminium in the drinking-
water.
It has been hypothesized that aluminium in the drinking-water is
a risk factor for the development or acceleration of AD as well as for
impaired cognitive function in the elderly. It has also been suggested
that stamped fine aluminium powder and fume may be risk factors for
impaired cognitive function and pulmonary disease in certain
occupations.
Some 20 epidemiological studies have been carried out to test the
hypothesis that aluminium in drinking-water is a risk factor for AD,
and two studies have evaluated the association between aluminiun in
drinking-water and impaired cognitive function. Study designs ranged
from ecological to case control. Eight studies in populations in
Norway, Canada, France, Switzerland and England were considered
of sufficiently high quality to meet the general criteria for
exposure and outcome assessment and the adjustment for at least
some confounding variables. Of the six studies that examined the
relationship between aluminium in drinking-water and dementia or AD,
three found a positive relationship but three did not. However, each
of the studies had some deficiencies in the study design (e.g.,
ecological exposure assessment, failure to consider aluminium exposure
from all sources and to control for important confounders such as
education, socioeconomic status and family history, the use of
surrogate outcome measures for AD, and selection bias). In general,
the relative rists determined were less than 2, with large confidence
intervals, when the total aluminium concentration in drinking-water
was 100 �g/litre or higher. Based on current knowledge on the
pathogenesis of AD and the totality of evidence from these
epidemiological studies, it was concluded that the present
epidemiological evidence does not support a causal association between
AD and aluminium in drinking-water.
In addition to the epidemiological studies that examined the
relationship between AD and aluminium in drinking-water, two studies
examined cognitive dysfunction and AD in elderly populations in
relation to the levels of aluminium in drinking-water. The results
were again conflicting. One study of 800 male octogenarians consuming
drinking-water with aluminium concentrations up to 98 �g/litre found
no relationship. The second study used "any evidence of mental
impairment" as an outcome measure and found a relative risk of 1.72 at
aluminium concentrations greater than 85 �g/litre in 250 males. Such
data are insufficient to show that aluminium is a cause of cognitive
impairment in the elderly.
Reports of impaired cognitive function related to aluminium
exposure are conflicting. Most studies are on small populations, and
the methodology used in these studies is open to question with respect
to magnitude of effect reported, exposure assessment and confounding
factors. In a comparative study of cognitive impairment in miners
exposed to a powder containing 85% finely ground aluminium and 15%
aluminium oxide (as prophylaxis against silica) and unexposed miners,
the cognitive test scores and the proportion impaired in at least one
test indicated a disadvantage for the exposed miners. A positive
exposure-related trend of increased risk was noted.
In all occupational studies reported, the magnitude of effects
found, presence of confounding factors, problems with exposure
assessment and the probability of mixed exposures all make the data
insufficient to conclude that aluminium is a cause of cognitive
impairment in workers exposed occupationally to aluminium.
Neurological syndromes including impairment of cognitive
function, motor dysfunction and peripheral neuropathy have been
reported in limited studies of workers exposed to aluminium fume. A
small population of aluminium welders who were compared with iron
welders were reported to show a small decrement in repetitive motor
function. When a questionnaire methodology was used in another study,
an increase in neuropsychiatric symptoms was reported.
Iatrogenic exposure in patients with chronic renal failure,
exposed to aluminium-containing dialysis fluids and pharmaceutical
products, may cause encephalopathy, vitamin-D-resistant osteomalacia
and microcytic anaemia. These clinical syndromes can be prevented by
reduction in exposure to aluminium.
Premature infants, even where kidney impairment is not severe
enough to cause raised blood creatinine levels, may develop increased
tissue loading of aluminium, particularly in bone, when exposed to
iatrogenic sources of aluminium. Where there is kidney failure,
seizures and encephalopathy may occur.
Although human exposure to aluminium is widespread, in only a few
cases has hypersensitivity been reported following exposure to some
aluminium compounds after dermal application or parenteral
administration.
Pulmonary fibrosis was reported in some workers exposed to very
fine stamped aluminium powder in the manufacture of explosives and
fireworks. Nearly all cases involved exposure to aluminium particles
coated with mineral oil. That process is no longer used. Other cases
of pulmonary fibrosis have related to mineral exposures to other
agents such as silica and asbestos and cannot be attributed solely to
aluminium.
Irritant-induced asthma has been associated with inhalation
of aluminium sulfate, aluminium fluoride, potassium aluminium
tetrafluoride and with the complex environment of the potrooms during
aluminium production.
There is insufficient information to allow for classification of
the cancer risk from human exposures to aluminium and its compounds.
Animal studies do not indicate that aluminium or aluminium compounds
are carcinogenic.
1.9 Effects on other organisms in the laboratory and field
Aquatic unicellular algae showed increased toxic effect at low
pH, where bioavailability of aluminium is increased. They are more
sensitive than other microorganisms, the majority of 19 lake species
showing complete growth inhibition at 200 �g/litre total aluminium
(pH 5.5). Selection of aluminium-tolerant strains is possible; green
algae capable of growing in the presence of 48 mg/litre at pH 4.6 have
been isolated.
For aquatic invertebrates, LC50 values range from 0.48 mg/litre
(polychaete) to 59.6 mg/litre (daphnid). For fish, 96-h LC50 values
range from 0.095 mg/litre (American flagfish) to 235 mg/litre
(mosquito fish). However, care must be taken when interpreting the
results because of the significant effects of pH on the availability
of aluminium. The wide range of LC50 values probably reflects
variable availability. The addition of chelating agents, such as NTA
and EDTA, reduces the acute toxicity of aluminium to fish.
Responses to aluminium by macroinvertebrates are variable. In the
normal pH range aluminium toxicity increases with decreasing pH;
however, in very acidic waters aluminium can reduce the effects of
acid stress. Some invertebrates are very resistant to acid stress and
can be very numerous in acidic waters. Increased drift rate of
invertebrates has been reported in streams suffering either pH or
pH/aluminium stress; this is a common response to a variety of
stressors. Lake invertebrates generally survived field exposure to
aluminium but suffered as a result of phosphate reduction in
oligotrophic conditions induced by precipitation with aluminium.
Short- and long-term toxicity tests on fish have been carried out
under a variety of conditions and, most importantly, at a range of pH
values. The data show that significant effects have been observed at
monomeric inorganic aluminium levels as low as 25 �g/litre. However,
the complex relationship between acidity and aluminium bioavailability
makes interpretation of the toxicity data more difficult. At very
low pH (not normally found in natural waters) the hydrogen ion
concentration appears to be the toxic factor, with the addition of
aluminium tending to reduce toxicity. In the pH range 4.5 to 6.0
aluminium in equilibrium exerts its maximum toxic effect. Toxicity has
also been shown to increase with increasing pH levels in the alkaline
pH region. The mechanism of aluminium toxicity to fish has been
attributed to the inability of fish to maintain their osmoregulatory
balance, as well as respiratory problems associated with precipitation
of aluminium on the gill mucus. The former effect is associated with
lower pH levels. These laboratory findings have been confirmed by
field studies especially in areas under acid stress.
Amphibian eggs and larvae are affected by acidity and aluminium,
with interaction between the two factors. Reduced hatching, delayed
hatching, delayed metamorphosis, metamorphosis at small size, and
mortality have been reported in various species and at aluminium
concentrations below 1 mg/litre.
Exposure of roots of terrestrial plants to aluminium can cause
diminished root growth, reduced uptake of plant nutrients and stunted
plant development. Tolerance to aluminium has been demonstrated both
in the laboratory and the field.
1.10 Conclusions
1.10.1 General population
Hazards to neurological development and brain function from
exposure to aluminium have been identified through animal studies.
However, aluminium has not been demonstrated to pose a health risk to
healthy, non-occupationally exposed humans.
There is no evidence to support a primary causative role of
aluminium in Alzheimer's disease (AD), and aluminium does not induce
AD pathology in vivo in any species, including humans.
The hypothesis that exposure of the elderly population in some
regions to elevated levels of aluminium in drinking-water may
exacerbate or accelerate AD lacks adequate supporting data.
The data in support of the hypothesis that particular exposures,
either occupational or via drinking-water, may be associated with non-
specific impaired cognitive function are also inadequate.
There is insufficient health-related evidence to justify
revisions to existing WHO Guidelines for aluminium exposure in
healthy, non-occupationally exposed humans. As an example, there is an
inadequate scientific basis for setting a health-based standard for
aluminium in drinking-water.
1.10.2 Subpopulations at special risk
In people of all ages with impaired renal function, aluminium
accumulation has been shown to cause the clinical syndrome of
encephalopathy, vitamin-D-resistant osteomalacia and microcytic
anaemia. The sources of aluminium are haemodialysis fluid and
aluminium-containing pharmaceutical agents (e.g., phosphate binders).
Intestinal absorption can be exacerbated by the use of citrate-
containing products. Patients with renal failure are thus at risk of
neurotoxicity from aluminium.
Iatrogenic aluminium exposure poses a hazard to patients with
chronic renal failure. Premature infants have higher body burdens of
aluminium than other infants. Every effort should be made to limit
such exposure in these groups.
1.10.3 Occupationally exposed populations
Workers having long-term, high-level exposure to fine aluminium
particulates may be at increased risk of adverse health effects.
However, there are insufficient data from which to develop with any
degree of certainty occupational exposure limits with regards to the
adverse effects of aluminium.
Exposure to stamped pyrotechnic aluminium powder most often
coated with mineral oil lubricants has caused pulmonary fibrosis
(aluminosis), whereas exposure to other forms of aluminium has not
been proven to cause pulmonary fibrosis. Most reported cases had
exposure to other potentially fibrogenic agents.
Irritant-induced asthma has been associated with inhalation of
aluminium sulfate, aluminium fluoride or potassium aluminium
tetrafluoride, and with the complex environment within the potrooms
during aluminium production.
1.10.4 Environmental effects
Aluminium-bearing solid phases in the environment are relatively
insoluble, particularly at circumneutral pH values, resulting in low
concentrations of dissolved aluminium in most natural water.
In acidic or poorly buffered environments subjected to strong
acidifying inputs, concentrations of aluminium can increase to levels
resulting in adverse effects on both aquatic organisms and terrestrial
plants. However, there exist large species, strain and life history
stage differences in sensitivity to this metal.
The detrimental biological effects from elevated concentrations
of inorganic monomeric aluminium can be mitigated in the presence of
organic acids, fluorides, silicate and high levels of calcium and
magnesium.
There is a substantial reduction in species richness associated
with the mobilization of the more toxic forms of aluminium in acid-
stressed waters. This loss of species diversity is observed at all
trophic levels.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
The element aluminium (Al) was first obtained in an impure form
by Oersted in 1825, and pure aluminium was prepared by Woehler two
years later. The name aluminium is derived from alum, which the
ancient Greeks used as an astringent in medicine (Lide, 1991).
Aluminium is the most abundant metallic element and constitutes
8.13% of the earth's crust. Owing to its high reactivity, it is always
found combined with other elements and does not occur in its pure
state. Combined with oxygen, silicon, the alkali and alkaline-earth
metals, and fluorine, and as hydroxides, sulfates and phosphates,
aluminium appears in a wide variety of minerals (Frank et al., 1985;
Hudson et al., 1985; Lide, 1991).
Some aluminium compounds, synonyms and molecular formulae are
listed in Table 1. The most abundant natural aluminium ores are shown
in Table 2.
2.1 Identity
Pure aluminium is a silvery-white, malleable, ductile metal with
the atomic number of 13 and the relative atomic mass of 26.98. With
few exceptions aluminium is found in chemical compounds as AlIII.
Aluminium occurs naturally as 27Al; eight radioactive isotopes are
known, of which 26Al is the most stable with a half-life of 7.4 �
105 years (Frank et al., 1985).
2.2 Physical and chemical properties
2.2.1 Aluminium metal
Elemental aluminium possesses many desirable characteristics and
is therefore widely used in commerce (Sax & Lewis, 1987; Lide, 1991).
Aluminium crystallizes in a face-centered cubic lattice that is stable
from 4 K to melting point; the coordination number is 12, it is light
and malleable, and thus is easily formed into a variety of shapes
(Frank et al., 1985).
Owing to the high charge/radius ratio of Al3+ in aqueous
solutions, the ion proteolyses part of the water envelope and forms
hydroxo complexes. It can also complex with electron-rich species,
such as fluoride and chloride. The chemical properties of aluminium
resemble those of beryllium and silicon. Because of its amphoteric
character, it reacts with mineral acids and strong alkalis (Sax &
Lewis, 1987). Although aluminium is one of the most reactive of the
common metals used commercially, it has excellent resistance to
Table 1. Chemical names, synonyms and molecular formulae of elemental aluminium and aluminium compoundsa
Chemical name CAS registry number Synonyms Formula
Aluminium
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
Members
Dr T.M. Florence, Centre for Environmental Health Sciences, Oyster
Bay, New South Wales, Australia
Dr M. Golub, California Regional Primate Research Center, University
of California, Davis, California, USA
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
( Co-Rapporteur)
Professor D.R. McLachlan ( Retired from: Centre for Research in
Neurogenerative Diseases, University of Toronto, Toronto,
Ontario, Canada
Dr M. Moore, National Health and Medical Research Council, National
Research Centre for Environmental Toxicology, Coopers Plains,
Brisbane, Australia ( Chairman)
Dr T.V. O'Donnell, University of Otago, Wellington South, New Zealand
( Vice-Chairman)
Professor B. Rosseland, Norwegian Institute of Water Research (NIVA),
Oslo, Norway
Dr B. Simon, Fraunhofer Institute, Hanover, Germany ( Co-Rapporteur)
Dr B. Sjogren, Department of Occupational Medicine, Swedish National
Institute for Working Life, Solna, Sweden
Dr L. Smith, Disease Control Service, Public Health Branch, Ontario
Ministry of Health, North York, Ontario, Canada
Dr E. Storey, Royal Melbourne Hospital, Department of Pathology,
University of Melbourne, Parkville, Victoria, Australia
Dr H. Temmink, Department of Toxicology, Agricultural University,
Wageningen, The Netherlands ( Vice-Chairman)
Dr M.K. Ward, Department of Renal Medicine, Royal Victoria Infirmary,
Newcastle-upon-Tyne, United Kingdom
Dr M. Wilhelm, Health Institute, University of Dusseldorf, Dusseldorf,
Germany
Professor H.M. Wisniewski, New York State Institute for Basic
Research in Developmental Disabilities, Staten Island, New York,
USA
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute
of Occupational Medicine, Ministry of Health, Beijing, China
Observers
Dr K. Bentley, Environmental Health Assessment and Criteria, Human
Services and Health, Woden, Australia
Dr O.C. B�ckman, Norsk Hydro, Porsgrunn Research Centre, Porsgrunn,
Norway
Dr J. Borak, Occupational and Environmental Health, Jonathan Borak &
Co., New Haven, Connecticut, USA
Dr I. Calder, Occupational and Environmental Health, South Australian
Health Commission, Adelaide, Australia
Dr J.N. Fisher, ALCOA of Australia Ltd, Point Henry Works, Geelong,
Victoria, Australia
Mr D. Hughes, Environment, Mount Isa Mine Holdings, Brisbane,
Australia
Dr P. Imray, Environmental Health Branch, Queensland Health, Brisbane,
Australia
Ms M.E. Meek, Environmental Health Directorate, Health Canada,
Tunney's Pasture, Ottawa, Ontario, Canada
Dr N. Priest, AEA Technology, Harwell, Didcot, Oxfordshire, United
Kingdom
Dr D. Wilcox, Medical Section, Health Services, Sydney Water, Sydney,
Australia
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety
Inter-regional Research Unit, World Health Organization, Research
Triangle Park, North Carolina, USA ( Secretary)
Dr D. Johns, DPIE, Coal and Mineral Division, Canberra, Australia
( Temporary Adviser)
Mr D. Wagner, Chemicals Safety Unit, Human Services and Health,
Canberra, Australia ( Temporary Adviser)
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ALUMINIUM
A WHO Task Group on Environmental Health Criteria for Aluminium
met in Brisbane, Australia, from 24 to 28 April 1995. The meeting
was sponsored by a consortium of Australian Commonwealth and State
Governments through a national steering committee chaired by
Dr K. Bentley, Director, Health and Environmental Policy, Department
of Health and Family Services, Canberra. The meeting was hosted and
organized by the NHMRC National Research Centre for Environmental
Toxicology (NRCET), Dr M. Moore, Director, being responsible for
the arrangements. Dr D. Lange, Chief Health Officer, welcomed
participants on behalf of Queensland Health, and Professor L. Roy
Webb, Vice-chancellor, Griffith University, welcomed them on behalf of
NRCET. Dr G.C. Becking, IPCS, welcomed the participants on behalf of
Dr M. Mercier, Director of the IPCS and the three cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft criteria monograph and made an evaluation of the risks to human
health and the environment from exposure to aluminium.
The first draft was prepared under the coordination of Dr G.
Rosner, Fraunhofer Institute of Toxicology and Aerosol Research,
Germany, and Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood,
United Kingdom. The draft reviewed by the Task Group, incorporating
the comments received following review by the IPCS Contact Points, was
prepared through the cooperative effort of the Fraunhofer Institute,
Institute of Terrestrial Ecology and the Secretariat.
Dr G.C. Becking (IPCS Central Unit, Inter-regional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively, of
this monograph.
The efforts of all who helped in the preparation and finalization
of this publication are gratefully acknowledged.
ABBREVIATIONS
AD Alzheimer's disease
AIBD aluminium-induced bone disease
cAMP cyclic adenosine monophosphate
CI confidence interval
1,25-(OH)2-D3 1,25-dihydroxy-vitamin D3
DOC dissolved organic carbon
EDTA ethylenediaminetetraacetic acid
i.p. intraperitoneal
i.v. intravenous
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
LTP long-term potentiation
NFT neurofibrillary tangle
NIOSH National Institute for Occupational Safety and
Health (USA)
NOEC no-observed-effect concentration
NOEL no-observed-effect level
NTA nitrilotriacetic acid
OR odds ratio
PHF paired helical filaments
Pt platinum unit (1 unit equals the colour produced by
lung chloroplatinate in 1 litre of water)
PTH parathyroid hormone
s.c. subcutaneous
WAIS Weschler Adult Intelligence Scale
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties
Aluminium is a silvery-white, ductile and malleable metal. It
belongs to group IIIA of the Periodic Table, and in compounds it is
usually found as AlIII. It forms about 8% of the earth's crust and is
one of the most reactive of the common metals. Exposure to water,
oxygen or other oxidants leads to the formation of a superficial
coating of aluminium oxide, which provides the metal with a high
resistance to corrosion. Aluminium oxide is soluble in mineral acids
and strong alkalis but insoluble in water, whereas aluminium chloride,
nitrate and sulfate are water soluble. Aluminium halogenides, hydride
and lower aluminium alkyls react violently with water.
Aluminium possesses high electrical and thermal conductivity, low
density and great resistance to corrosion. It is often alloyed with
other metals. Aluminium alloys are light, strong and readily machined
into shapes.
1.2 Analytical methods
Various analytical methods have been developed to determine
aluminium in biological and environmental samples. Graphite furnace
- atomic-absorption spectrometry (GF-AAS) and inductively coupled
plasma - atomic-emission spectrometry (ICP-AES) are the most
frequently used methods. Contamination of the samples with aluminium
from air, vessels or reagents during sampling and preparation is the
main source of analytical error. Depending on sample pretreatment,
separation and concentration procedures, detection limits are
1.9-4 �g/litre in biological fluids and 0.005-0.5 �g/g dry weight in
tissues using GF-AAS, and 5 �g/m3 in air and 3 �g/litre in water
using ICP-AES.
1.3 Sources of human and environmental exposure
Aluminium is released to the environment both by natural
processes and from anthropogenic sources. It is highly concentrated in
soil-derived dusts from such activities as mining and agriculture, and
in particulate matter from coal combustion. Aluminium silicates
(clays), a major component of soils, contribute to the aluminium
levels of dust. Natural processes far outweigh direct anthropogenic
contributions to the environment. Mobilization of aluminium through
human actions is mostly indirect and occurs as a result of emission of
acidifying substances. In general, decreasing pH results in an
increase in mobility and bioavailability for monomeric forms of
aluminium. The most important raw material for the production of
aluminium is bauxite, which contains up to 55% alumina (aluminium
oxide). World bauxite production was 106 million tonnes in 1992.
Aluminium metal has a vide variety of uses, including structural
materials in construction, automobiles and aircraft, and the
production of metal alloys. Aluminium compounds and materials also
have a wide variety of uses, including production of glass, ceramics,
rubber, wood preservatives, pharmaceuticals and waterproofing
textiles. Natural aluminium minerals, especially bentonite and
zeolite, are used in water purification, sugar refining, brewing and
paper industries.
1.4 Environmental transport, distribution and transformation
Aluminium occurs ubiquitously in the environment in the form of
silicates, oxides and hydroxides, combined with other elements such as
sodium and fluorine and as complexes with organic matter. It is not
found as a free metal because of its reactivity. It has only one
oxidation state (+3) in nature; therefore, its transport and
distribution in the environment depend only upon its coordination
chemistry and the chemical-physical characteristics of the local
environmental system. At pH values greater than 5.5, naturally
occurring aluminium compounds exist predominantly in an undissolved
form such as gibbsite (Al(OH)3) or as aluminosilicates, except in the
presence of high amounts of dissolved organic material, which binds
with aluminium and can lead to increased concentrations of dissolved
aluminium in streams and lakes. Several factors influence aluminium
mobility and subsequent transport within the environment. These
include chemical speciation, hydrological flow paths, soil-water
interactions, and the composition of the underlying geological
materials. The solubility of aluminium in equilibrium with solid phase
Al(OH)3 is highly dependent on pH and on complexing agents such as
fluoride, silicate, phosphate and organic matter. The chemistry of
inorganic aluminium in acid soil and stream water can be considered in
terms of mineral solubility, ion exchange and water mixing processes.
Upon acidification of soils, aluminium can be released into
solution for transport to streams. Mobilization of aluminium by acid
precipitation results in more aluminium being available for plant
uptake.
1.5 Environmental levels and human exposure
Aluminium is a major constituent of a number of atmospheric
components particularly in soil-derived dusts (both from natural
sources and human activity) and particulates from coal combustion. In
urban areas aluminium levels in street dust range from 3.7 to
11.6 �g/kg. Airborne aluminium levels vary from 0.5 ng/m3 over
Antarctica to more than 1000 ng/m3 in industrialized areas.
Surface freshwater and soil water aluminium concentrations can
vary substantially, being dependent on physico-chemical and geological
factors. Aluminium can be suspended or dissolved. It can be bound with
organic or inorganic ligands, or it can exist as a free aluminium ion.
In natural waters aluminium exists in both monomeric and polymeric
forms. Aluminium speciation is determined by pH and the concentrations
of dissolved organic carbon (DOC), fluoride, sulfate, phosphate and
suspended particulates. Dissolved aluminium concentrations for water
in the circumneutral pH range are usually quite low, ranging from
1.0 to 50 �g/litre. This rises to 500-1000 �g/litre in more acidic
water. At the extreme acidity of water affected by acid mine drainage,
dissolved aluminium concentrations of up to 90 mg/litre have been
measured.
Non-occupational human exposure to aluminium in the environment
is primarily through ingestion of food and water. Of these, food is
the principal contributor. The daily intake of aluminium from food and
beverages in adults ranges between 2.5 and 13 mg. This is between 90
and 95% of total intake. Drinking-water may contribute around 0.4 mg
daily at present international guideline values, but is more likely
to be around 0.2 mg/day. Pulmonary exposure may contribute up to
0.04 mg/day. In some circumstances, such as occupational exposure and
antacid use, the levels of exposure will be much greater. For example,
> 500 mg of aluminium may be consumed in two average-sized antacid
tablets. There are some difficulties in assessing uptake from these
exposures because of analytical and sampling difficulties. Isotopic
investigations with Al26 indicate that one of the most bioavailable
forms of aluminium is the citrate and that there could be as much as
1% absorption when aluminium is in this form. However, humans would
absorb only 3% of their total daily uptake of aluminium from drinking-
water, a relatively minor source compared to food.
1.6 Kinetics and metabolism
1.6.1 Humans
Aluminium and its compounds appear to be poorly absorbed in
humans, although the rate and extent of absorption have not been
adequately studied. Concentrations of aluminium in blood and urine
have been used as a readily available measure of aluminium uptake,
increased urine levels having been observed among aluminium welders
and aluminium flake-powder producers.
The mechanism of gastrointestinal absorption of aluminium has not
yet been fully elucidated. Variability results from the chemical
properties of the element and the formation of various chemical
species, which is dependent upon the pH, ionic strength, presence of
competing elements (silicon), and the presence of complexing agents
within the gastrointestinal tract (e.g., citrate).
The biological behaviour and gastrointestinal absorption of
aluminium in humans ingesting aluminium compounds has been studied by
using the radioactive isotope Al26. Significant intersubject
variability has been demonstrated. Measured fractional uptakes of 5 �
10-3 for aluminium as citrate, 1.04 � 10-4 for aluminium hydroxide
and 1.36 � 10-3 for the hydroxide given with citrate were reported. A
study of the fractional uptake of aluminium from drinking-water showed
an uptake fraction of 2.35 � 10-3. It was concluded that members of
the general population consuming 1.5 litres/day of drinking-water
containing 100 �g aluminium/litre would absorb about 3% of their total
daily intake of aluminium from this source depending upon the levels
found in food and the frequency of antacid use.
The proportion of plasma Al3+ normally bound to protein in
humans may be as high as 70-90% in haemodialysis patients with
moderately increased plasma aluminium. The highest levels of aluminium
may be found in the lungs, where it may be present as inhaled
insoluble particles.
The urine is the most important route of aluminium excretion.
After peroral administration of a single dose of aluminium, 83% was
excreted in urine after 13 days and 1.8% in the faeces. The half-life
of urinary concentration among welders exposed for more than 10 years
was 6 months or longer. Among retired workers exposed to aluminium
flake powders, the calculated half-lives were between 0.7 and 8 years.
1.6.2 Animals
Absorption via the gastrointestinal tract is usually less than
1%. The main factors influencing absorption are solubility, pH and
chemical species. Organic complexing compounds, notably citrate,
increase absorption. The aluminium absorption may interact with
calcium and iron transport systems. Dermal and inhalation absorption
has not been studied in detail. Aluminium is distributed in most
organs within the body with accumulation occurring mainly in bone at
high dose levels. To a limited but as yet undetermined extent,
aluminium passes the blood-brain barrier and is also distributed to
the fetus. Aluminium is eliminated effectively by urine. Plasma half-
life is about 1 h in rodents.
1.7 Effects on laboratory mammals and in vitro test systems
The acute toxicity of metallic aluminium and aluminium compounds
is low, the reported oral LD50 values being in the range of several
hundred to 1000 mg aluminium/kg body weight per day. However, the
LC50 values for inhalation have not been identified.
In short-term studies in which an adequate range of end-points
was examined following exposure of rats, mice or dogs to various
aluminium compounds (sodium aluminium phosphate, aluminium hydroxide,
aluminium nitrate) in the diet or drinking-water, only minimal effects
(decreases in body weight gain generally associated with decreases in
food consumption or mild histopathological effects) have been observed
at the highest administered doses (70 to 300 mg aluminium/kg body
weight per day). Systemic effects following parenteral administration
also included kidney dysfunction.
Adequate inhalation studies were not identified. Following
intratracheal administration of aluminium oxide, particle-associated
fibrosis was observed, similar to that found in other studies on
silica and coal dust.
No overt fetotoxicity was noted, nor were general reproductive
parameters noted after gavage treatment of rats with 13, 26 or 52 mg
aluminium/kg body weight per day (as aluminium nitrate). However, a
dose-dependent delay in the growth of offspring was noted with females
administered 13 mg/kg and in male offspring at 26 mg/kg. The lowest-
observed-adverse-effect level (LOAEL) for developmental effects
(decreased ossification, increased incidence of vertebral and
sternebrae terata and reduced fetal weight) was 13 mg/kg (aluminium
nitrate). These effects were not observed at much higher doses of
aluminium hydroxide. There were reductions in postnatal growth at
13 mg/kg (aluminium nitrate), although maternal toxicity was not
examined. In studies on brain development, grip strength was impaired
in offspring of dams fed 100 mg aluminium/kg body weight as aluminium
lactate in the diet, in the absence of maternal toxicity.
There is no indication that aluminium is carcinogenic. It can
form complexes with DNA and cross-link chromosomal proteins and DNA,
but it has not been shown to be mutagenic in bacteria or induce
mutation or transformation in mammalian cells in vitro. Chromosomal
aberrations have been observed in bone marrow cells of exposed mice
and rats.
There is considerable evidence that aluminium is neurotoxic in
experimental animals, although there is considerable variation among
species. In susceptible species, toxicity following parenteral
administration is characterized by progressive neurological
impairment, resulting in death with status epilepticus (LD50 =
6 �g Al/g dry weight of brain). Morphologically, the progressive
encephalopathy is associated with neurofibrillary pathology in
large and medium size neurons predominantly in the spinal cord,
brainstem and selected areas of the hippocampus. These tangles are
morphologically and biochemically different from those that occur in
Alzheimer's disease (AD). Behavioural impairment has been observed in
the absence of overt encephalopathy or neurohistopathology in
experimental animals exposed to soluble aluminium salts (e.g.,
lactate, chloride) in the diet or drinking-water at doses of 50 mg
aluminium/kg body weight per day or more.
Osteomalacia, as it presents in man, is observed consistently in
larger species (e.g., dogs and pigs) exposed to aluminium; a similar
condition is observed in rodents. These effects appear to occur in all
species, including humans, at aluminium levels of 100 to 200 �g/g bone
ash.
1.8 Effects on humans
No acute pathogenic effects in the general population have been
described after exposure to aluminium.
In England, a population of about 20 000 individuals was exposed
for at least 5 days to increased levels of aluminium sulfate,
accidentally placed in a drinking-water facility. Case reports of
nausea, vomiting, diarrhoea, mouth ulcers, skin ulcers, skin rashes
and arthritic pain were noted. It was concluded that the symptoms were
mostly mild and short-lived. No lasting effects on health could be
attributed to the known exposures from aluminium in the drinking-
water.
It has been hypothesized that aluminium in the drinking-water is
a risk factor for the development or acceleration of AD as well as for
impaired cognitive function in the elderly. It has also been suggested
that stamped fine aluminium powder and fume may be risk factors for
impaired cognitive function and pulmonary disease in certain
occupations.
Some 20 epidemiological studies have been carried out to test the
hypothesis that aluminium in drinking-water is a risk factor for AD,
and two studies have evaluated the association between aluminiun in
drinking-water and impaired cognitive function. Study designs ranged
from ecological to case control. Eight studies in populations in
Norway, Canada, France, Switzerland and England were considered
of sufficiently high quality to meet the general criteria for
exposure and outcome assessment and the adjustment for at least
some confounding variables. Of the six studies that examined the
relationship between aluminium in drinking-water and dementia or AD,
three found a positive relationship but three did not. However, each
of the studies had some deficiencies in the study design (e.g.,
ecological exposure assessment, failure to consider aluminium exposure
from all sources and to control for important confounders such as
education, socioeconomic status and family history, the use of
surrogate outcome measures for AD, and selection bias). In general,
the relative rists determined were less than 2, with large confidence
intervals, when the total aluminium concentration in drinking-water
was 100 �g/litre or higher. Based on current knowledge on the
pathogenesis of AD and the totality of evidence from these
epidemiological studies, it was concluded that the present
epidemiological evidence does not support a causal association between
AD and aluminium in drinking-water.
In addition to the epidemiological studies that examined the
relationship between AD and aluminium in drinking-water, two studies
examined cognitive dysfunction and AD in elderly populations in
relation to the levels of aluminium in drinking-water. The results
were again conflicting. One study of 800 male octogenarians consuming
drinking-water with aluminium concentrations up to 98 �g/litre found
no relationship. The second study used "any evidence of mental
impairment" as an outcome measure and found a relative risk of 1.72 at
aluminium concentrations greater than 85 �g/litre in 250 males. Such
data are insufficient to show that aluminium is a cause of cognitive
impairment in the elderly.
Reports of impaired cognitive function related to aluminium
exposure are conflicting. Most studies are on small populations, and
the methodology used in these studies is open to question with respect
to magnitude of effect reported, exposure assessment and confounding
factors. In a comparative study of cognitive impairment in miners
exposed to a powder containing 85% finely ground aluminium and 15%
aluminium oxide (as prophylaxis against silica) and unexposed miners,
the cognitive test scores and the proportion impaired in at least one
test indicated a disadvantage for the exposed miners. A positive
exposure-related trend of increased risk was noted.
In all occupational studies reported, the magnitude of effects
found, presence of confounding factors, problems with exposure
assessment and the probability of mixed exposures all make the data
insufficient to conclude that aluminium is a cause of cognitive
impairment in workers exposed occupationally to aluminium.
Neurological syndromes including impairment of cognitive
function, motor dysfunction and peripheral neuropathy have been
reported in limited studies of workers exposed to aluminium fume. A
small population of aluminium welders who were compared with iron
welders were reported to show a small decrement in repetitive motor
function. When a questionnaire methodology was used in another study,
an increase in neuropsychiatric symptoms was reported.
Iatrogenic exposure in patients with chronic renal failure,
exposed to aluminium-containing dialysis fluids and pharmaceutical
products, may cause encephalopathy, vitamin-D-resistant osteomalacia
and microcytic anaemia. These clinical syndromes can be prevented by
reduction in exposure to aluminium.
Premature infants, even where kidney impairment is not severe
enough to cause raised blood creatinine levels, may develop increased
tissue loading of aluminium, particularly in bone, when exposed to
iatrogenic sources of aluminium. Where there is kidney failure,
seizures and encephalopathy may occur.
Although human exposure to aluminium is widespread, in only a few
cases has hypersensitivity been reported following exposure to some
aluminium compounds after dermal application or parenteral
administration.
Pulmonary fibrosis was reported in some workers exposed to very
fine stamped aluminium powder in the manufacture of explosives and
fireworks. Nearly all cases involved exposure to aluminium particles
coated with mineral oil. That process is no longer used. Other cases
of pulmonary fibrosis have related to mineral exposures to other
agents such as silica and asbestos and cannot be attributed solely to
aluminium.
Irritant-induced asthma has been associated with inhalation
of aluminium sulfate, aluminium fluoride, potassium aluminium
tetrafluoride and with the complex environment of the potrooms during
aluminium production.
There is insufficient information to allow for classification of
the cancer risk from human exposures to aluminium and its compounds.
Animal studies do not indicate that aluminium or aluminium compounds
are carcinogenic.
1.9 Effects on other organisms in the laboratory and field
Aquatic unicellular algae showed increased toxic effect at low
pH, where bioavailability of aluminium is increased. They are more
sensitive than other microorganisms, the majority of 19 lake species
showing complete growth inhibition at 200 �g/litre total aluminium
(pH 5.5). Selection of aluminium-tolerant strains is possible; green
algae capable of growing in the presence of 48 mg/litre at pH 4.6 have
been isolated.
For aquatic invertebrates, LC50 values range from 0.48 mg/litre
(polychaete) to 59.6 mg/litre (daphnid). For fish, 96-h LC50 values
range from 0.095 mg/litre (American flagfish) to 235 mg/litre
(mosquito fish). However, care must be taken when interpreting the
results because of the significant effects of pH on the availability
of aluminium. The wide range of LC50 values probably reflects
variable availability. The addition of chelating agents, such as NTA
and EDTA, reduces the acute toxicity of aluminium to fish.
Responses to aluminium by macroinvertebrates are variable. In the
normal pH range aluminium toxicity increases with decreasing pH;
however, in very acidic waters aluminium can reduce the effects of
acid stress. Some invertebrates are very resistant to acid stress and
can be very numerous in acidic waters. Increased drift rate of
invertebrates has been reported in streams suffering either pH or
pH/aluminium stress; this is a common response to a variety of
stressors. Lake invertebrates generally survived field exposure to
aluminium but suffered as a result of phosphate reduction in
oligotrophic conditions induced by precipitation with aluminium.
Short- and long-term toxicity tests on fish have been carried out
under a variety of conditions and, most importantly, at a range of pH
values. The data show that significant effects have been observed at
monomeric inorganic aluminium levels as low as 25 �g/litre. However,
the complex relationship between acidity and aluminium bioavailability
makes interpretation of the toxicity data more difficult. At very
low pH (not normally found in natural waters) the hydrogen ion
concentration appears to be the toxic factor, with the addition of
aluminium tending to reduce toxicity. In the pH range 4.5 to 6.0
aluminium in equilibrium exerts its maximum toxic effect. Toxicity has
also been shown to increase with increasing pH levels in the alkaline
pH region. The mechanism of aluminium toxicity to fish has been
attributed to the inability of fish to maintain their osmoregulatory
balance, as well as respiratory problems associated with precipitation
of aluminium on the gill mucus. The former effect is associated with
lower pH levels. These laboratory findings have been confirmed by
field studies especially in areas under acid stress.
Amphibian eggs and larvae are affected by acidity and aluminium,
with interaction between the two factors. Reduced hatching, delayed
hatching, delayed metamorphosis, metamorphosis at small size, and
mortality have been reported in various species and at aluminium
concentrations below 1 mg/litre.
Exposure of roots of terrestrial plants to aluminium can cause
diminished root growth, reduced uptake of plant nutrients and stunted
plant development. Tolerance to aluminium has been demonstrated both
in the laboratory and the field.
1.10 Conclusions
1.10.1 General population
Hazards to neurological development and brain function from
exposure to aluminium have been identified through animal studies.
However, aluminium has not been demonstrated to pose a health risk to
healthy, non-occupationally exposed humans.
There is no evidence to support a primary causative role of
aluminium in Alzheimer's disease (AD), and aluminium does not induce
AD pathology in vivo in any species, including humans.
The hypothesis that exposure of the elderly population in some
regions to elevated levels of aluminium in drinking-water may
exacerbate or accelerate AD lacks adequate supporting data.
The data in support of the hypothesis that particular exposures,
either occupational or via drinking-water, may be associated with non-
specific impaired cognitive function are also inadequate.
There is insufficient health-related evidence to justify
revisions to existing WHO Guidelines for aluminium exposure in
healthy, non-occupationally exposed humans. As an example, there is an
inadequate scientific basis for setting a health-based standard for
aluminium in drinking-water.
1.10.2 Subpopulations at special risk
In people of all ages with impaired renal function, aluminium
accumulation has been shown to cause the clinical syndrome of
encephalopathy, vitamin-D-resistant osteomalacia and microcytic
anaemia. The sources of aluminium are haemodialysis fluid and
aluminium-containing pharmaceutical agents (e.g., phosphate binders).
Intestinal absorption can be exacerbated by the use of citrate-
containing products. Patients with renal failure are thus at risk of
neurotoxicity from aluminium.
Iatrogenic aluminium exposure poses a hazard to patients with
chronic renal failure. Premature infants have higher body burdens of
aluminium than other infants. Every effort should be made to limit
such exposure in these groups.
1.10.3 Occupationally exposed populations
Workers having long-term, high-level exposure to fine aluminium
particulates may be at increased risk of adverse health effects.
However, there are insufficient data from which to develop with any
degree of certainty occupational exposure limits with regards to the
adverse effects of aluminium.
Exposure to stamped pyrotechnic aluminium powder most often
coated with mineral oil lubricants has caused pulmonary fibrosis
(aluminosis), whereas exposure to other forms of aluminium has not
been proven to cause pulmonary fibrosis. Most reported cases had
exposure to other potentially fibrogenic agents.
Irritant-induced asthma has been associated with inhalation of
aluminium sulfate, aluminium fluoride or potassium aluminium
tetrafluoride, and with the complex environment within the potrooms
during aluminium production.
1.10.4 Environmental effects
Aluminium-bearing solid phases in the environment are relatively
insoluble, particularly at circumneutral pH values, resulting in low
concentrations of dissolved aluminium in most natural water.
In acidic or poorly buffered environments subjected to strong
acidifying inputs, concentrations of aluminium can increase to levels
resulting in adverse effects on both aquatic organisms and terrestrial
plants. However, there exist large species, strain and life history
stage differences in sensitivity to this metal.
The detrimental biological effects from elevated concentrations
of inorganic monomeric aluminium can be mitigated in the presence of
organic acids, fluorides, silicate and high levels of calcium and
magnesium.
There is a substantial reduction in species richness associated
with the mobilization of the more toxic forms of aluminium in acid-
stressed waters. This loss of species diversity is observed at all
trophic levels.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
The element aluminium (Al) was first obtained in an impure form
by Oersted in 1825, and pure aluminium was prepared by Woehler two
years later. The name aluminium is derived from alum, which the
ancient Greeks used as an astringent in medicine (Lide, 1991).
Aluminium is the most abundant metallic element and constitutes
8.13% of the earth's crust. Owing to its high reactivity, it is always
found combined with other elements and does not occur in its pure
state. Combined with oxygen, silicon, the alkali and alkaline-earth
metals, and fluorine, and as hydroxides, sulfates and phosphates,
aluminium appears in a wide variety of minerals (Frank et al., 1985;
Hudson et al., 1985; Lide, 1991).
Some aluminium compounds, synonyms and molecular formulae are
listed in Table 1. The most abundant natural aluminium ores are shown
in Table 2.
2.1 Identity
Pure aluminium is a silvery-white, malleable, ductile metal with
the atomic number of 13 and the relative atomic mass of 26.98. With
few exceptions aluminium is found in chemical compounds as AlIII.
Aluminium occurs naturally as 27Al; eight radioactive isotopes are
known, of which 26Al is the most stable with a half-life of 7.4 �
105 years (Frank et al., 1985).
2.2 Physical and chemical properties
2.2.1 Aluminium metal
Elemental aluminium possesses many desirable characteristics and
is therefore widely used in commerce (Sax & Lewis, 1987; Lide, 1991).
Aluminium crystallizes in a face-centered cubic lattice that is stable
from 4 K to melting point; the coordination number is 12, it is light
and malleable, and thus is easily formed into a variety of shapes
(Frank et al., 1985).
Owing to the high charge/radius ratio of Al3+ in aqueous
solutions, the ion proteolyses part of the water envelope and forms
hydroxo complexes. It can also complex with electron-rich species,
such as fluoride and chloride. The chemical properties of aluminium
resemble those of beryllium and silicon. Because of its amphoteric
character, it reacts with mineral acids and strong alkalis (Sax &
Lewis, 1987). Although aluminium is one of the most reactive of the
common metals used commercially, it has excellent resistance to
Table 1. Chemical names, synonyms and molecular formulae of elemental aluminium and aluminium compoundsa
Chemical name CAS registry number Synonyms Formula
Aluminium 