
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 189
Di-n-butyl Phthalate
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 189
First draft prepared by Dr G. Long and Dr E. Meek, Health and Welfare,
Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1997
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Di-n-butyl phthalate.
(Environmental health criteria ; 189)
1.Phthalic acids - adverse effects 2.Phthalic acids - toxicity
3.Plasticizers - adverse effects 4.Plasticizers - toxicity
5.Occupational exposure I.Series
ISBN 92 4 157189 6 (NLM Classification: QV 612)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1997
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL PHTHALATE
Preamble
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels
3.2.2. Uses
3.2.3. Emissions
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Transformation
4.2.1. Abiotic degradation
4.2.2. Biodegradation
4.2.3. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.2.1 Surface water
5.1.2.2 Groundwater
5.1.2.3 Seawater
5.1.2.4 Precipitation
5.1.2.5 Effluent and wastewater
5.1.3. Sewage sludge
5.1.4. Soil
5.1.5. Sediment
5.1.6. Aquatic organisms
5.1.7. Terrestrial organisms
5.2. General population exposure
5.2.1. Ambient air
5.2.2. Indoor air
5.2.3. Drinking-water
5.2.4. Food
5.2.5. Consumer products
5.2.6. Medical devices
5.2.7. Levels in human tissue
5.3. Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption, distribution and excretion
6.1.1. Dermal
6.1.2. Ingestion
6.1.2.1 In vivo studies
6.1.2.2 In vitro studies
6.1.3. Inhalation
6.2. Metabolic transformation
6.2.1. In vivo studies
6.2.2. In vitro studies
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term exposure
7.3. Long-term exposure
7.4. Irritation and sensitization
7.5. Reproductive and developmental toxicity
7.5.1. Reproductive effects
7.5.1.1 Testicular effects
7.5.1.2 Effects on fertility
7.5.2. Developmental effects
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.8. Special studies
7.8.1. Induction of metabolizing enzymes
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
8.2.1. Acute toxicity
8.2.2. Epidemiological studies
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.2. Aquatic organisms
9.1.2.1 Algae
9.1.2.2 Invertebrates
9.1.2.3 Vertebrates
9.1.3. Terrestrial organisms
9.1.3.1 Plants
9.1.3.2 Invertebrates
9.1.3.3 Vertebrates
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure
10.1.2. Health effects
10.1.3. Guidance values
10.2. Evaluation of effects in the environment
10.2.1. Exposure
10.2.2. Effects
10.2.3. Risk evaluation
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
12. FURTHER RESEARCH
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01 ES02617-
15 from the National Institute of Environmental Health Sciences,
National Institutes of Health, USA, and by financial support from the
European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
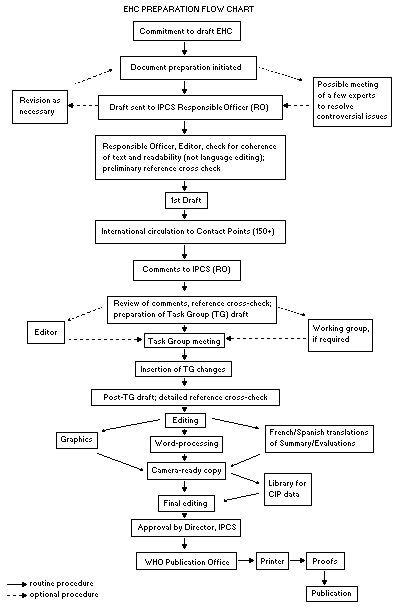
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL
PHTHALATE
Members
Dr B. Butterworth, Chemical Industry Institute of Toxicology Research
Triangle Park, North Carolina, USA (Chairman)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon Cambridgeshire,
United Kingdom (Co-Rapporteur)
Mr G. Long, Health and Welfare Canada, Environmental Health
Centre, Tunney's Pasture, Ottawa, Ontario, Canada
(Co-Rapporteur)
Dr R. Maronpot, Laboratory of Experimental Pathology, National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA
Dr E. Meek, Health and Welfare Canada, Environmental Health Centre,
Tunney's Pasture, Ottawa, Ontario, Canada
(Co-Rapporteur)
Dr S. Oishi, Department of Toxicology, Tokyo Metropolitan Research
Laboratory of Public Health, Tokyo, Japan
Dr Choon-Nam Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
Dr S.A. Soliman, Department of Pesticide Chemistry, Faculty of
Agriculture, Alexandria University, El-Shatby, Alexandria, Egypt*
Dr S.P. Srivastava, Industrial Toxicology Research Center, Lucknow,
India
Dr F. Sullivan, Division of Pharmacology and Toxicology, St. Thomas's
Hospital, London, United Kingdom
Dr C. Weber, Federal Environmental Agency, Berlin, Germany
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
*Invited but unable to attend
ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL PHTHALATE
A WHO Task Group on Environmental Health Criteria for
Di- n-butyl Phthalate (DBP) met in Geneva from 30 October to
3 November 1995. Dr B.H. Chen, IPCS, opened the meeting and welcomed
the participants on behalf of the Director, IPCS, and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to DBP.
The first draft of this monograph was prepared by Dr G. Long and
Dr E. Meek, Health and Welfare, Canada. The second draft was prepared
by Dr E. Meek incorporating comments received following the
circulation of the first draft to the IPCS Contact Points for
Environmental Health Criteria monographs. Dr E. Meek, Mr P. Howe and
Dr F. Sullivan contributed to the final text of this monograph.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
AP alkaline phosphatase
DBP di- n-butyl phthalate
DEHP diethylhexyl phthalate
GOT glutamic-oxaloacetic transaminase
GPT glutamic-pyruvic transaminase
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MBP monobutyl phthalate
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
1. SUMMARY
Di- n-butyl phthalate (DBP) is an inert, colourless, oily
liquid, with a low vapour pressure, which is soluble in most organic
solvents, but only slightly soluble in water. The most sensitive and
selective analytical determinations of phthalic acid esters, including
DBP, in environmental media are achieved by gas chromatography with
electron capture detection or mass spectrometry. Since phthalates
frequently occur as plasticizers in analytical equipment and as
contaminants in laboratory air and solvents, a great deal of care is
needed to prevent contamination during the collection, storage and
analysis of samples.
DBP is used mainly as a speciality plasticizer for nitro-
cellulose, polyvinyl acetate and polyvinyl chloride, a lubricant for
aerosol valves, an antifoaming agent, a skin emollient and a
plasticizer in nail polish, fingernail elongators and hair spray.
In the atmosphere, DBP has been measured in both the vapour and
the particulate phases. Washout via rainfall or dry deposition is
believed to play a significant role in the removal of DBP from the
atmosphere. In surface water, most of the DBP is present in the water
fraction rather than in the suspended solids. Volatilization of DBP
from soil is not expected to be significant because of its low vapour
pressure and moderate adsorption to soil.
DBP is relatively non-persistent in air and surface waters, and
has a half-life in these compartments of only a few days. Complete
biodegradation of DBP is rapid under aerobic conditions but much
slower under anaerobic conditions. For soil, similar half-lives to
air and water have been predicted; however, some studies suggest that
DBP may be more persistent in soil. DBP would be expected to
bioaccumulate as a result of its high octanol-water partition
coefficient. However, it is quite readily metabolized in fish and,
consequently, bioconcentration factors tend to be lower then
predicted. The highest bioconcentration factor, based on the parent
compound (DBP), is 590 for the fathead minnow. Biomagnification is
unlikely in terrestrial animals, based upon limited data on birds and
the rapid metabolism and excretion observed in laboratory mammals.
Steps taken to avoid contamination are rarely described in
reports of concentrations of DBP in the environment published before
1980 and, consequently, the reliability of the early monitoring data
often cannot be assessed. Limited data on concentrations in ambient
air indicate that mean levels are generally less than 5 ng/m3. In
recent studies, mean rainwater concentrations ranged from 0.2 to
1.4 µg/litre; much lower values have been measured in remote
areas. Mean concentrations in surface water tend to be less than
1 µg/litre; however, levels in polluted rivers are much higher (12 to
34 µg/litre). There are only a few data on groundwater concentrations
of DBP, mean values being 0.15 to 0.46 µg/litre. DBP concentrations
in effluents range up to 100 µg/litre, whilst concentrations in sewage
sludge range from 0.2 to 200 mg/kg dry weight. Levels in sediment are
generally less than 1 mg/kg dry weight; however, in polluted areas
concentrations of up to 20 mg/kg have been measured. In studies on
aquatic biota, mean concentrations of DBP tend to be less than
0.2 mg/kg wet weight; however, in polluted areas, concentrations of up
to 35 mg/kg have been measured.
In a survey of 125 homes in California, USA, in 1990, the median
daytime concentration of DBP in indoor air was 420 ng/m3. DBP has
rarely been detected in drinking-water supplies (< 1.0 µg/litre),
according to limited data from Canada. In a small number of samples
of drinking-water in Toronto, Canada, the mean concentration was
14 ng/litre; concentrations in seven brands of bottled spring water
ranged from 21 to 55 ng/litre.
In addition to entry through environmental contamination, DBP may
be present in foodstuffs as a result of migration from packaging, and
this was investigated in a number of studies conducted in the late
1980s. In many countries, precautions were introduced to reduce
leaching of plasticizers from packaging and as a result, levels of DBP
in foodstuffs have declined over time. In a Canadian market-basket
survey of 98 different food type samples in Halifax in 1986, DBP was
detected in butter (1.5 µg/g), freshwater fish (0.5 µg/g), cereal
products (range from undetectable to 0.62 µg/g), baked potatoes
(0.63 µg/g), coleslaw (0.11 µg/g), bananas (0.12 µg/g), blueberries
(0.09 µg/g), pineapples (0.05 µg/g), margarine (0.64 µg/g), white
sugar (0.2 µg/g) and gelatin dessert (0.09 µg/g).
On the basis of the limited data available, the principal media
of exposure to DBP for the general population, listed in order of
their relative importance based upon estimated intake, are as follows:
food, indoor air and drinking-water. Estimated intakes from food and
indoor air are 7 µg/kg body weight per day and 0.42 µg/kg body weight
per day, respectively. Estimated intakes from drinking-water and
ambient air are considerably less, < 0.02 µg/kg body weight per day
and 0.26-0.36 ng/kg body weight per day, respectively. Based on these
intakes, it is estimated that the total average daily intake from air,
drinking-water and food is 7.4 µg/kg body weight per day. It
should be noted, however, that intake of DBP in the diet can vary
considerably, depending upon the nature and extent of packaged food
consumed and the nature of use of food wrapping in food preparation.
For the United Kingdom, the maximum likely human intake of DBP from
food sources has been estimated to be approximately 2 mg per person
per day (approximately 31 µg/kg body weight per day, assuming a mean
body weight of 64 kg). There is also potential for exposure to DBP in
cosmetics, although available data are inadequate to quantify intake
from this source.
The most recent provisional data from the NIOSH National
Occupational Exposure Survey indicates that in the USA over 500 000
workers, including 200 000 women, are potentially exposed to DBP.
Based on determinations at a limited number of worksites in the USA,
concentrations are generally less than the limit of detection (i.e.,
0.01 to 0.02 mg/m3), although higher levels have been reported in
some countries.
In studies on rats, DBP is absorbed through the skin, although in
in vitro studies human skin has been found to be less permeable than
rat skin to this compound. Studies in laboratory animals indicate that
DBP is rapidly absorbed from the gastrointestinal tract, distributed
primarily to the liver and kidneys of rats and excreted in urine as
metabolites following oral or intravenous administration. Following
inhalation, it was consistently detected at low concentrations in the
brain.
Available data indicate that in rats, following ingestion, DBP is
metabolized by nonspecific esterases mainly in the small intestine
to yield mono- n-butyl phthalate (MBP) with limited subsequent
biochemical oxidation of the alkyl side chain of MBP. MBP is stable
and resistant to hydrolysis of the second ester group. The MBP and
other metabolites are excreted in the urine mainly as glucuronide
conjugates. Species differences in the excretion of conjugates and
unconjugated metabolites of DBP in the urine of rats and hamsters have
been observed, with more free MBP being present in rats than hamsters.
Accumulation has not been observed in any organ.
The profile of effects following exposure to DBP is similar to
that of other phthalate esters, which, in susceptible species, can
induce hepatomegaly, increased numbers of hepatic peroxisomes,
fetotoxicity, teratogenicity and testicular damage.
The acute toxicity of DBP in rats and mice is low. Reported
LD50 values following oral administration to rats range from
approximately 8 g/kg body weight to at least 20 g/kg body weight; in
mice, values are approximately 5 g/kg body weight to 16 g/kg body
weight. The dermal LD50 in rabbits is > 4 g/kg body weight.
Reports of acute toxicity following inhalation of DBP have not been
identified. Signs of acute toxicity in laboratory animals include
depression of activity, laboured breathing and lack of coordination.
In a case of accidental poisoning of a worker who ingested
approximately 10 grams of DBP, recovery was gradual within two weeks
and complete after 1 month.
In short-term repeated-dose toxicity studies, effects at lowest
levels in rats after oral administration for 5 to 21 days included
peroxisome proliferation and hepatomegaly at doses of 420 mg/kg body
weight per day or more.
In longer-term studies, the effects in rats observed following
ingestion of DBP for periods up to 7 months included reduced rate of
weight gain at doses of 250 mg/kg body weight per day or more.
Increase in relative liver weight has been observed at doses of
120 mg/kg body weight or more. Peroxisomal proliferation with
increased peroxisomal enzyme activity has been observed at doses of
279 mg/kg body weight per day or more. Necrotic hepatic changes in
Wistar rats have been reported at doses of 250 mg/kg body weight per
day or more but not in F-344 or Sprague-Dawley rats exposed to up to
2500 mg/kg body weight per day. Alteration in testicular enzymes and
degeneration of testicular germinal cells of rats have been observed
at doses of 250 and 571 mg/kg body weight per day. There are
considerable species differences in effects on the testes following
exposure to DBP, minimal effects being observed in mice and hamsters
at doses as high as 2000 mg/kg body weight per day. In mice, effects
on body and organ weights and histological alterations in the liver
indicative of metabolic stress have been reported in a recent
subchronic bioassay, for which the no-observed-effect-level (NOEL) was
353 mg/kg body weight per day.
On the basis of limited available data in animal species, DBP
appears to have little potential to irritate skin or eyes or to induce
sensitization. In humans, a few cases of sensitization after exposure
to DBP have been reported, although this was not confirmed in
controlled studies of larger numbers of individuals reported only in
secondary accounts.
In a continuous breeding protocol, which included cross-over
mating and offspring assessment phases, rats were exposed to 0, 1000,
5000 or 10 000 mg DBP/kg in the diet (equivalent to 0, 66, 320 and
651 mg/kg body weight per day). In the first generation the reduction
in pup weight in the mid-dose group, in the absence of any adverse
effect on maternal weight, could be regarded as a developmental
toxicity effect. There was also a significant reduction of live
litter numbers at all three dose levels. The effects in the second
generation were more severe, with reduced pup weight in all groups
including the low-dose group, structural defects (such as prepucial/
penile malformations, seminiferous tubule degeneration, and absence or
underdevelopment of the epididymides) in the mid- and high-dose
groups, and severe effects on spermatogenesis in the high-dose group
that were not seen in the parent animals. These results suggest that
the adverse effects of DBP are more marked in animals exposed during
development and maturation than in animals exposed as adults only. No
clear NOEL was established in this study. The lowest-observed-
adverse-effect-level (LOAEL) was considered to be 66 mg/kg body weight
per day.
The available studies show that DBP generally induces fetotoxic
effects in the absence of maternal toxicity. Available data also
indicate that DBP is teratogenic at high doses and that susceptibility
to teratogenesis varies with developmental stage and period of
administration. In mice, DBP caused dose-dependent increases in the
number of resorptions and dead fetuses at oral doses of 400 mg/kg body
weight per day or more. Dose-dependent decreases in fetal weights and
number of viable litters were also observed in mice at these doses.
Adequate carcinogenesis bioassays for DBP have not been
conducted. The weight of the available evidence indicates that DBP is
not genotoxic.
As a class, chemicals which cause peroxisome proliferation are
often hepatocarcinogenic via a non-genotoxic mode of action. Although
the mechanism of action remains unknown, tumour formation is preceded
by peroxisomal proliferation and hepatomegaly. Since DBP causes
peroxisomal proliferation, it is possible that it might be a rodent
liver carcinogen, although it is much weaker in inducing hepatomegaly
and peroxisome proliferation than DEHP. To the degree that
hepatomegaly and peroxisomal proliferation correlate with carcinogenic
potency, DBP would be expected to be a less potent carcinogen than
DEHP and would probably exhibit no activity as measured by current
cancer bioassay methodologies.
Identified epidemiological investigations are limited to those of
workers exposed to mixtures of phthalates. These studies do not
contribute to our understanding of the effects associated with DBP
alone.
Since DBP is not genotoxic and is expected to be a less potent
carcinogen than DEHP, it would probably exhibit no activity as
measured by current cancer bioassay methodologies. Thus, it is
unlikely that DBP presents any significantly increased risk of cancer
at concentrations generally present in the environment.
Ingestion is by far the principal route of exposure to DBP;
moreover, the toxicological data for other routes of administration
are insufficient for evaluation. A guidance value has, therefore, been
developed for the oral route, although the ultimate objective should
be reduction of total exposure from all sources to less than the
tolerable daily intake.
No clear no-observed-adverse-effect-level (NOAEL) for the
end-points considered to be most appropriate for derivation of
guidance values (i.e., developmental and reproductive toxicity) was
established. The LOAEL for developmental and reproductive toxicity
from a continuous breeding study was considered to be 66 mg/kg body
weight per day, although the effects observed at this dose level were
moderate and probably reversible. On the basis of these data, a
tolerable daily intake of 66 g/kg body weight per day has been
derived, incorporating an uncertainty factor of 1000 (× 10 for
interspecies variation, × 10 for inter-individual variation, and × 10
for extrapolation from LOAEL to NOAEL).
Information on the ecotoxicity of DBP includes acute and chronic
data for a number of species from various trophic levels in the
aquatic environment. For freshwater algae the lowest reported 96-h
EC50 was 750 µg DBP/litre. The lowest reported values in acute
toxicity tests on aquatic invertebrates were a 96-h LC50 of
750 µg/litre (mysid shrimp) and a 48-h EC50 of 760 µg/litre (midge
larvae). In chronic studies, the most sensitive invertebrate species
was Daphnia magna, with a 21-day NOEC (parent survival) of
500 µg/litre. In a non-standard test with the scud (Gammarus pulex)
a 10-day LOEC of 500 µg/litre and a NOEC of 100 µg/litre, both based
on reduced locomotor activity, were reported. In acute toxicity tests
with fish the lowest reported 96-h LC50 for a freshwater species was
350 µg/litre (yellow perch) and for a marine species 600 µg/litre
(sheepshead minnow). The most sensitive chronic study was based on
the rainbow trout with a 99-day NOEC (growth) of 100 µg/litre and a
99-day LOEC of 190 µg/litre (growth reduced by about 27%).
The acute toxicity of DBP to birds is low.
The risk to aquatic organisms associated with the present mean
concentrations of DBP in surface water is low. However, in highly
polluted rivers the safety margin is much smaller. There is
inadequate data to assess the risk of DBP to sediment-dwelling
organisms. At current levels of exposure, it can be concluded that
the risk to fish-eating birds and mammals is low.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Di- n-butyl phthalate (DBP), a phthalic acid ester, has the CAS
(Chemical Abstracts Service) Registry Number 84-74-2, the molecular
formula C16H22O4, and a relative molecular mass of 278.4. Synonyms
and trade names are presented in Table 1.
2.2 Physical and chemical properties
DBP is an inert colourless oily liquid, with a vapour pressure of
about 0.01 Pa at 25°C (CMA, 1984), Henry's law constant of 4.6 × 10-7
atmÊm3/mol at 25°C (Howard, 1989) and an octanol-water partition
coefficient (log Kow) between 4.31 and 4.79 (Montgomery & Welkom,
1990). The solubility in water is about 10 mg/litre (McKone & Layton,
1986), although higher values have also been reported (Montgomery &
Welkom, 1990). The determination of the water solubility of phthalic
acid esters is complicated since these compounds easily form colloidal
dispersions (Klöpfer et al., 1982) and are subject to "molecular
folding" (Callahan et al., 1979). DBP is soluble in most of the
organic solvents (BUA, 1987). Additional chemical and physical
properties of DBP are presented in Table 1.
2.3 Conversion factors
1 ppm = 11.4 mg/m3
1 mg/m3 = 0.088 ppm
2.4 Analytical methods
The most sensitive and selective analytical determinations of
phthalic acid esters, including DBP, in environmental media are
achieved by gas chromatography (GC) with electron-capture detection
(ECD), with or without derivatization (Kohli et al., 1989). In the
analysis of environmental samples it is imperative to note that peaks
of other components can interfere with determinations of DBP. This
problem is particularly serious when ECD is used, because of its high
sensitivity towards halogenated aromatics, PCBs etc. The US
Environmental Protection Agency has standardized sample preparation
and analysis for municipal and industrial wastewater using GC with ECD
(Method 606, detection limit 0.36 µg/litre) and GC/mass spectrometry
(MS) (Method 625, detection limit 2.5 µg/litre) (US EPA, 1982b).
Thin-layer chromatography may be used to separate phthalates from
other solvent-extracted organic compounds. Analysis can also be
carried out by using high-performance liquid chromatography with
ultraviolet detection (HPLC-UV) (Poole & Wibberley, 1977).
Table 1. Physical properties of di- n-butyl phthalate
(Adapted and modified from: USEPA, 1981; ATSDR, 1990)
Chemical formula C16H22O4
Structure
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL
PHTHALATE
Members
Dr B. Butterworth, Chemical Industry Institute of Toxicology Research
Triangle Park, North Carolina, USA (Chairman)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon Cambridgeshire,
United Kingdom (Co-Rapporteur)
Mr G. Long, Health and Welfare Canada, Environmental Health
Centre, Tunney's Pasture, Ottawa, Ontario, Canada
(Co-Rapporteur)
Dr R. Maronpot, Laboratory of Experimental Pathology, National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA
Dr E. Meek, Health and Welfare Canada, Environmental Health Centre,
Tunney's Pasture, Ottawa, Ontario, Canada
(Co-Rapporteur)
Dr S. Oishi, Department of Toxicology, Tokyo Metropolitan Research
Laboratory of Public Health, Tokyo, Japan
Dr Choon-Nam Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
Dr S.A. Soliman, Department of Pesticide Chemistry, Faculty of
Agriculture, Alexandria University, El-Shatby, Alexandria, Egypt*
Dr S.P. Srivastava, Industrial Toxicology Research Center, Lucknow,
India
Dr F. Sullivan, Division of Pharmacology and Toxicology, St. Thomas's
Hospital, London, United Kingdom
Dr C. Weber, Federal Environmental Agency, Berlin, Germany
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
*Invited but unable to attend
ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL PHTHALATE
A WHO Task Group on Environmental Health Criteria for
Di- n-butyl Phthalate (DBP) met in Geneva from 30 October to
3 November 1995. Dr B.H. Chen, IPCS, opened the meeting and welcomed
the participants on behalf of the Director, IPCS, and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to DBP.
The first draft of this monograph was prepared by Dr G. Long and
Dr E. Meek, Health and Welfare, Canada. The second draft was prepared
by Dr E. Meek incorporating comments received following the
circulation of the first draft to the IPCS Contact Points for
Environmental Health Criteria monographs. Dr E. Meek, Mr P. Howe and
Dr F. Sullivan contributed to the final text of this monograph.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
AP alkaline phosphatase
DBP di- n-butyl phthalate
DEHP diethylhexyl phthalate
GOT glutamic-oxaloacetic transaminase
GPT glutamic-pyruvic transaminase
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MBP monobutyl phthalate
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
1. SUMMARY
Di- n-butyl phthalate (DBP) is an inert, colourless, oily
liquid, with a low vapour pressure, which is soluble in most organic
solvents, but only slightly soluble in water. The most sensitive and
selective analytical determinations of phthalic acid esters, including
DBP, in environmental media are achieved by gas chromatography with
electron capture detection or mass spectrometry. Since phthalates
frequently occur as plasticizers in analytical equipment and as
contaminants in laboratory air and solvents, a great deal of care is
needed to prevent contamination during the collection, storage and
analysis of samples.
DBP is used mainly as a speciality plasticizer for nitro-
cellulose, polyvinyl acetate and polyvinyl chloride, a lubricant for
aerosol valves, an antifoaming agent, a skin emollient and a
plasticizer in nail polish, fingernail elongators and hair spray.
In the atmosphere, DBP has been measured in both the vapour and
the particulate phases. Washout via rainfall or dry deposition is
believed to play a significant role in the removal of DBP from the
atmosphere. In surface water, most of the DBP is present in the water
fraction rather than in the suspended solids. Volatilization of DBP
from soil is not expected to be significant because of its low vapour
pressure and moderate adsorption to soil.
DBP is relatively non-persistent in air and surface waters, and
has a half-life in these compartments of only a few days. Complete
biodegradation of DBP is rapid under aerobic conditions but much
slower under anaerobic conditions. For soil, similar half-lives to
air and water have been predicted; however, some studies suggest that
DBP may be more persistent in soil. DBP would be expected to
bioaccumulate as a result of its high octanol-water partition
coefficient. However, it is quite readily metabolized in fish and,
consequently, bioconcentration factors tend to be lower then
predicted. The highest bioconcentration factor, based on the parent
compound (DBP), is 590 for the fathead minnow. Biomagnification is
unlikely in terrestrial animals, based upon limited data on birds and
the rapid metabolism and excretion observed in laboratory mammals.
Steps taken to avoid contamination are rarely described in
reports of concentrations of DBP in the environment published before
1980 and, consequently, the reliability of the early monitoring data
often cannot be assessed. Limited data on concentrations in ambient
air indicate that mean levels are generally less than 5 ng/m3. In
recent studies, mean rainwater concentrations ranged from 0.2 to
1.4 µg/litre; much lower values have been measured in remote
areas. Mean concentrations in surface water tend to be less than
1 µg/litre; however, levels in polluted rivers are much higher (12 to
34 µg/litre). There are only a few data on groundwater concentrations
of DBP, mean values being 0.15 to 0.46 µg/litre. DBP concentrations
in effluents range up to 100 µg/litre, whilst concentrations in sewage
sludge range from 0.2 to 200 mg/kg dry weight. Levels in sediment are
generally less than 1 mg/kg dry weight; however, in polluted areas
concentrations of up to 20 mg/kg have been measured. In studies on
aquatic biota, mean concentrations of DBP tend to be less than
0.2 mg/kg wet weight; however, in polluted areas, concentrations of up
to 35 mg/kg have been measured.
In a survey of 125 homes in California, USA, in 1990, the median
daytime concentration of DBP in indoor air was 420 ng/m3. DBP has
rarely been detected in drinking-water supplies (< 1.0 µg/litre),
according to limited data from Canada. In a small number of samples
of drinking-water in Toronto, Canada, the mean concentration was
14 ng/litre; concentrations in seven brands of bottled spring water
ranged from 21 to 55 ng/litre.
In addition to entry through environmental contamination, DBP may
be present in foodstuffs as a result of migration from packaging, and
this was investigated in a number of studies conducted in the late
1980s. In many countries, precautions were introduced to reduce
leaching of plasticizers from packaging and as a result, levels of DBP
in foodstuffs have declined over time. In a Canadian market-basket
survey of 98 different food type samples in Halifax in 1986, DBP was
detected in butter (1.5 µg/g), freshwater fish (0.5 µg/g), cereal
products (range from undetectable to 0.62 µg/g), baked potatoes
(0.63 µg/g), coleslaw (0.11 µg/g), bananas (0.12 µg/g), blueberries
(0.09 µg/g), pineapples (0.05 µg/g), margarine (0.64 µg/g), white
sugar (0.2 µg/g) and gelatin dessert (0.09 µg/g).
On the basis of the limited data available, the principal media
of exposure to DBP for the general population, listed in order of
their relative importance based upon estimated intake, are as follows:
food, indoor air and drinking-water. Estimated intakes from food and
indoor air are 7 µg/kg body weight per day and 0.42 µg/kg body weight
per day, respectively. Estimated intakes from drinking-water and
ambient air are considerably less, < 0.02 µg/kg body weight per day
and 0.26-0.36 ng/kg body weight per day, respectively. Based on these
intakes, it is estimated that the total average daily intake from air,
drinking-water and food is 7.4 µg/kg body weight per day. It
should be noted, however, that intake of DBP in the diet can vary
considerably, depending upon the nature and extent of packaged food
consumed and the nature of use of food wrapping in food preparation.
For the United Kingdom, the maximum likely human intake of DBP from
food sources has been estimated to be approximately 2 mg per person
per day (approximately 31 µg/kg body weight per day, assuming a mean
body weight of 64 kg). There is also potential for exposure to DBP in
cosmetics, although available data are inadequate to quantify intake
from this source.
The most recent provisional data from the NIOSH National
Occupational Exposure Survey indicates that in the USA over 500 000
workers, including 200 000 women, are potentially exposed to DBP.
Based on determinations at a limited number of worksites in the USA,
concentrations are generally less than the limit of detection (i.e.,
0.01 to 0.02 mg/m3), although higher levels have been reported in
some countries.
In studies on rats, DBP is absorbed through the skin, although in
in vitro studies human skin has been found to be less permeable than
rat skin to this compound. Studies in laboratory animals indicate that
DBP is rapidly absorbed from the gastrointestinal tract, distributed
primarily to the liver and kidneys of rats and excreted in urine as
metabolites following oral or intravenous administration. Following
inhalation, it was consistently detected at low concentrations in the
brain.
Available data indicate that in rats, following ingestion, DBP is
metabolized by nonspecific esterases mainly in the small intestine
to yield mono- n-butyl phthalate (MBP) with limited subsequent
biochemical oxidation of the alkyl side chain of MBP. MBP is stable
and resistant to hydrolysis of the second ester group. The MBP and
other metabolites are excreted in the urine mainly as glucuronide
conjugates. Species differences in the excretion of conjugates and
unconjugated metabolites of DBP in the urine of rats and hamsters have
been observed, with more free MBP being present in rats than hamsters.
Accumulation has not been observed in any organ.
The profile of effects following exposure to DBP is similar to
that of other phthalate esters, which, in susceptible species, can
induce hepatomegaly, increased numbers of hepatic peroxisomes,
fetotoxicity, teratogenicity and testicular damage.
The acute toxicity of DBP in rats and mice is low. Reported
LD50 values following oral administration to rats range from
approximately 8 g/kg body weight to at least 20 g/kg body weight; in
mice, values are approximately 5 g/kg body weight to 16 g/kg body
weight. The dermal LD50 in rabbits is > 4 g/kg body weight.
Reports of acute toxicity following inhalation of DBP have not been
identified. Signs of acute toxicity in laboratory animals include
depression of activity, laboured breathing and lack of coordination.
In a case of accidental poisoning of a worker who ingested
approximately 10 grams of DBP, recovery was gradual within two weeks
and complete after 1 month.
In short-term repeated-dose toxicity studies, effects at lowest
levels in rats after oral administration for 5 to 21 days included
peroxisome proliferation and hepatomegaly at doses of 420 mg/kg body
weight per day or more.
In longer-term studies, the effects in rats observed following
ingestion of DBP for periods up to 7 months included reduced rate of
weight gain at doses of 250 mg/kg body weight per day or more.
Increase in relative liver weight has been observed at doses of
120 mg/kg body weight or more. Peroxisomal proliferation with
increased peroxisomal enzyme activity has been observed at doses of
279 mg/kg body weight per day or more. Necrotic hepatic changes in
Wistar rats have been reported at doses of 250 mg/kg body weight per
day or more but not in F-344 or Sprague-Dawley rats exposed to up to
2500 mg/kg body weight per day. Alteration in testicular enzymes and
degeneration of testicular germinal cells of rats have been observed
at doses of 250 and 571 mg/kg body weight per day. There are
considerable species differences in effects on the testes following
exposure to DBP, minimal effects being observed in mice and hamsters
at doses as high as 2000 mg/kg body weight per day. In mice, effects
on body and organ weights and histological alterations in the liver
indicative of metabolic stress have been reported in a recent
subchronic bioassay, for which the no-observed-effect-level (NOEL) was
353 mg/kg body weight per day.
On the basis of limited available data in animal species, DBP
appears to have little potential to irritate skin or eyes or to induce
sensitization. In humans, a few cases of sensitization after exposure
to DBP have been reported, although this was not confirmed in
controlled studies of larger numbers of individuals reported only in
secondary accounts.
In a continuous breeding protocol, which included cross-over
mating and offspring assessment phases, rats were exposed to 0, 1000,
5000 or 10 000 mg DBP/kg in the diet (equivalent to 0, 66, 320 and
651 mg/kg body weight per day). In the first generation the reduction
in pup weight in the mid-dose group, in the absence of any adverse
effect on maternal weight, could be regarded as a developmental
toxicity effect. There was also a significant reduction of live
litter numbers at all three dose levels. The effects in the second
generation were more severe, with reduced pup weight in all groups
including the low-dose group, structural defects (such as prepucial/
penile malformations, seminiferous tubule degeneration, and absence or
underdevelopment of the epididymides) in the mid- and high-dose
groups, and severe effects on spermatogenesis in the high-dose group
that were not seen in the parent animals. These results suggest that
the adverse effects of DBP are more marked in animals exposed during
development and maturation than in animals exposed as adults only. No
clear NOEL was established in this study. The lowest-observed-
adverse-effect-level (LOAEL) was considered to be 66 mg/kg body weight
per day.
The available studies show that DBP generally induces fetotoxic
effects in the absence of maternal toxicity. Available data also
indicate that DBP is teratogenic at high doses and that susceptibility
to teratogenesis varies with developmental stage and period of
administration. In mice, DBP caused dose-dependent increases in the
number of resorptions and dead fetuses at oral doses of 400 mg/kg body
weight per day or more. Dose-dependent decreases in fetal weights and
number of viable litters were also observed in mice at these doses.
Adequate carcinogenesis bioassays for DBP have not been
conducted. The weight of the available evidence indicates that DBP is
not genotoxic.
As a class, chemicals which cause peroxisome proliferation are
often hepatocarcinogenic via a non-genotoxic mode of action. Although
the mechanism of action remains unknown, tumour formation is preceded
by peroxisomal proliferation and hepatomegaly. Since DBP causes
peroxisomal proliferation, it is possible that it might be a rodent
liver carcinogen, although it is much weaker in inducing hepatomegaly
and peroxisome proliferation than DEHP. To the degree that
hepatomegaly and peroxisomal proliferation correlate with carcinogenic
potency, DBP would be expected to be a less potent carcinogen than
DEHP and would probably exhibit no activity as measured by current
cancer bioassay methodologies.
Identified epidemiological investigations are limited to those of
workers exposed to mixtures of phthalates. These studies do not
contribute to our understanding of the effects associated with DBP
alone.
Since DBP is not genotoxic and is expected to be a less potent
carcinogen than DEHP, it would probably exhibit no activity as
measured by current cancer bioassay methodologies. Thus, it is
unlikely that DBP presents any significantly increased risk of cancer
at concentrations generally present in the environment.
Ingestion is by far the principal route of exposure to DBP;
moreover, the toxicological data for other routes of administration
are insufficient for evaluation. A guidance value has, therefore, been
developed for the oral route, although the ultimate objective should
be reduction of total exposure from all sources to less than the
tolerable daily intake.
No clear no-observed-adverse-effect-level (NOAEL) for the
end-points considered to be most appropriate for derivation of
guidance values (i.e., developmental and reproductive toxicity) was
established. The LOAEL for developmental and reproductive toxicity
from a continuous breeding study was considered to be 66 mg/kg body
weight per day, although the effects observed at this dose level were
moderate and probably reversible. On the basis of these data, a
tolerable daily intake of 66 g/kg body weight per day has been
derived, incorporating an uncertainty factor of 1000 (× 10 for
interspecies variation, × 10 for inter-individual variation, and × 10
for extrapolation from LOAEL to NOAEL).
Information on the ecotoxicity of DBP includes acute and chronic
data for a number of species from various trophic levels in the
aquatic environment. For freshwater algae the lowest reported 96-h
EC50 was 750 µg DBP/litre. The lowest reported values in acute
toxicity tests on aquatic invertebrates were a 96-h LC50 of
750 µg/litre (mysid shrimp) and a 48-h EC50 of 760 µg/litre (midge
larvae). In chronic studies, the most sensitive invertebrate species
was Daphnia magna, with a 21-day NOEC (parent survival) of
500 µg/litre. In a non-standard test with the scud (Gammarus pulex)
a 10-day LOEC of 500 µg/litre and a NOEC of 100 µg/litre, both based
on reduced locomotor activity, were reported. In acute toxicity tests
with fish the lowest reported 96-h LC50 for a freshwater species was
350 µg/litre (yellow perch) and for a marine species 600 µg/litre
(sheepshead minnow). The most sensitive chronic study was based on
the rainbow trout with a 99-day NOEC (growth) of 100 µg/litre and a
99-day LOEC of 190 µg/litre (growth reduced by about 27%).
The acute toxicity of DBP to birds is low.
The risk to aquatic organisms associated with the present mean
concentrations of DBP in surface water is low. However, in highly
polluted rivers the safety margin is much smaller. There is
inadequate data to assess the risk of DBP to sediment-dwelling
organisms. At current levels of exposure, it can be concluded that
the risk to fish-eating birds and mammals is low.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Di- n-butyl phthalate (DBP), a phthalic acid ester, has the CAS
(Chemical Abstracts Service) Registry Number 84-74-2, the molecular
formula C16H22O4, and a relative molecular mass of 278.4. Synonyms
and trade names are presented in Table 1.
2.2 Physical and chemical properties
DBP is an inert colourless oily liquid, with a vapour pressure of
about 0.01 Pa at 25°C (CMA, 1984), Henry's law constant of 4.6 × 10-7
atmÊm3/mol at 25°C (Howard, 1989) and an octanol-water partition
coefficient (log Kow) between 4.31 and 4.79 (Montgomery & Welkom,
1990). The solubility in water is about 10 mg/litre (McKone & Layton,
1986), although higher values have also been reported (Montgomery &
Welkom, 1990). The determination of the water solubility of phthalic
acid esters is complicated since these compounds easily form colloidal
dispersions (Klöpfer et al., 1982) and are subject to "molecular
folding" (Callahan et al., 1979). DBP is soluble in most of the
organic solvents (BUA, 1987). Additional chemical and physical
properties of DBP are presented in Table 1.
2.3 Conversion factors
1 ppm = 11.4 mg/m3
1 mg/m3 = 0.088 ppm
2.4 Analytical methods
The most sensitive and selective analytical determinations of
phthalic acid esters, including DBP, in environmental media are
achieved by gas chromatography (GC) with electron-capture detection
(ECD), with or without derivatization (Kohli et al., 1989). In the
analysis of environmental samples it is imperative to note that peaks
of other components can interfere with determinations of DBP. This
problem is particularly serious when ECD is used, because of its high
sensitivity towards halogenated aromatics, PCBs etc. The US
Environmental Protection Agency has standardized sample preparation
and analysis for municipal and industrial wastewater using GC with ECD
(Method 606, detection limit 0.36 µg/litre) and GC/mass spectrometry
(MS) (Method 625, detection limit 2.5 µg/litre) (US EPA, 1982b).
Thin-layer chromatography may be used to separate phthalates from
other solvent-extracted organic compounds. Analysis can also be
carried out by using high-performance liquid chromatography with
ultraviolet detection (HPLC-UV) (Poole & Wibberley, 1977).
Table 1. Physical properties of di- n-butyl phthalate
(Adapted and modified from: USEPA, 1981; ATSDR, 1990)
Chemical formula C16H22O4
Structure
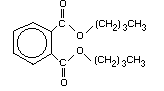 Relative molecular mass 278.34
Synonyms butylphthalate; dibutylphthalate; DBP;
1,2-benzenedicarboxylic acid dibutyl ester;
o-benzenedicarboxylic acid, dibutyl ester;
dibutyl 1,2-benzene dicarboxylate;
dibutyl- o-phthalate
CAS name 1,2-benzenedicarboxylic acid, dibutyl ester
CAS registry number 84-74-2
Trade names Caswell No. 292; Uniflex DBP; Celluflex DBP;
Ergoplast FDB; Polycizer DBP; Genoplast B;
Staflex DBP; Palatinol C; Hexaplast M/B; PX
104; RC Plasticizer DBP
Physical state Oily liquid
Colour Colourless
Odour Mild, aromatic
Melting point -35°C
Boiling point 340°C
Flashpoint 171°C
Table 1. contd.
Vapour pressure at 25°C 0.01 Pa (1.0 × 10-5 mmHg)
Density at 20°C 1.047
Partition coefficients
Log octanol/water 4.31-4.79
Log Koc 5.23
Solubility
Water at 25°C 10 mg/litre
Organic solvents Soluble in alcohol, ether, benzene
Henry's law constant 4.6 × 10-7 atmÊm3/mol
Phthalates frequently occur as plasticizers in analytical
equipment and as contaminants in laboratory air and solvents. This
can result in overestimation of their concentration in environmental
samples. For example, Ishida et al. (1980) detected DBP in laboratory
solvents at concentrations as high as 0.17 mg/kg (in benzene)
and in solid reagents at concentrations up to 9.89 mg/kg (in
carboxymethylcellulose), while polyvinyl tubing contained 20% DBP.
Therefore, a great deal of care is needed to prevent contamination
during the collection, storage and analysis of samples (Mathur, 1974;
US EPA, 1982b; Kohli et al., 1989; Hites & Budde, 1991). A summary of
analytical methods for the determination of DBP in environmental
samples and biological materials is presented in Tables 2 and 3,
respectively.
Table 2. Analytical methods for determining di- n-butyl phthalate in environmental samplesa
Sample matrix Sample preparation Analytical Sample detection
methodsb limit Accuracy Reference
Air Adsorption/solvent extraction HRGC/MS No data 115 ± 5%c Ligocki & Pankow
with polyurethane foam plug (1985)
Rainwater Adsorb on Tenax-GC columns, GC/MS < 34 ng/litre No data Ligocki et al.
thermally desorb (1985)
Water Extract with dichloromethane, GC/ECD 0.36 µg/litre 80 ± 6%c US EPA (1982a)
exchange to hexane, concentrate
Water Extract with dichloromethane at GC/MS 2.5 µg/litre 80 ± 6%c US EPA (1982b)
pH 11 and 2, concentrate
Water Adsorb on small bed volume GC/MS No data No data Pankow et al.
Tenax cartridges, thermally (1988)
desorb
Soil Extract with dichloromethane, GC/ECD 240 ng/kg 96% US EPA (1986a)
clean up, exchange to hexane
Waste, Extract with dichloromethane, GC/ECD 36 mg/kg 96% US EPA (1986a)
non-water-miscible clean up, exchange to hexane
Soil Extract from sample, clean up GC/MS 1.7 mg/kg 96% US EPA (1986b)
Waste, Extract from sample, clean up GC/MS 350 mg/kg 76% US EPA (1986b)
non-water-miscible
Soil/sediment Extract from sample, clean up HRGC/MS 660 µg/kg 76% US EPA (1986c)
Table 2. Continued
Sample matrix Sample preparation Analytical Sample detection
methodsb limit Accuracy Reference
Waste, Extract from sample, clean up HRGC/MS 50 mg/kg 76% US EPA (1986c)
non-water-miscible
Soil/sediment Extract from sample, clean up HRGC/FTIR 10 µg/litred No data US EPA (1986d)
Wastes, Extract from sample, cleanup HRGC/FTIR 10 µg/litred No data US EPA (1986d)
non-water-miscible
a From: Agency for Toxic Substances and Diseases Registry (1990).
b HRGC = high-resolution gas chromatography;
MS = mass spectrometry;
GC = gas chromatography;
ECD = electron-capture detector;
FTIR = Fourier transform infrared spectrometry.
c Relative recovery, percentage ± standard deviation.
d Identification limit. Detection limits for actual samples are several orders of magnitude higher depending upon the sample
matrix and extraction procedure employed.
Table 3. Analytical methods for determining di- n-butyl phthalate in biological materials
Sample matrix Sample preparation Analytical Sample detection Accuracy Reference
methoda limit (% recovery)
Aquatic organisms Extract with acetonitrile HRGC/ECD 0.1 µg/kg 68 Thuren (1986)
and petroleum ether
Adipose tissue Extraction, bulk lipid HRGC/MS 10 µg/kg No data Stanley (1986)
removal, Florisil
fractionation
Blood serum Extraction, bulk lipid HRGC/MS 10 µg/kg No data Stanley (1986)
removal, Florisil
fractionation
Blood serum Extraction with organic GC/MS No data No data Ching et al. (1981)
solvents (propanol,
heptane)
Cooked meat Remove with nitrogen gas GC/MS No data No data Ho (1983)
trap, extract with diethyl
ether
a HRGC High-resolution gas chromatography;
ECD Electron-capture detector;
MS Mass spectrometry;
GC Gas chromatography
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
The occurrence of naturally produced phthalates in biological and
geochemical samples has been suggested, but in most cases the
possibility of contamination during sampling or analysis could not be
ruled out (Mathur, 1974). However, it is unlikely that the amounts of
phthalates produced naturally would be significant compared with those
from anthropogenic sources (IPCS, 1992).
3.2 Anthropogenic sources
3.2.1 Production levels
Total DBP production in western Europe in 1994 was estimated to
be 49 000 tonnes (personal communication by the European Council for
Plasticisers and Intermediates to the IPCS, 1996). In Germany, the
average annual production was 20 000 tonnes for 1982-1986 (BUA, 1987).
DBP is produced by 36 companies in the USA, with total production of
7720 tonnes in 1977 and 11 400 tonnes in 1987 (ATSDR, 1990; NTP,
1995). Annual production in Japan in 1994 was about 17 000 tonnes
(JPIF, 1995).
3.2.2 Uses
DBP is used mainly as a speciality plasticizer for nitrocellulose
polyvinyl acetate and polyvinyl chloride (PVC) (ATSDR, 1990). In
1991, approximately 54% of the total supply of DBP in Canada was used
in adhesives, while about 15% was used in coatings (including
lacquers), and the rest in miscellaneous applications, including paper
coating (Camford Information Services Inc., 1992).
In Germany, approximately 25% of the DBP produced served as
plasticizer and adjuvant for the processing of PVC and about 20% was
used in adhesives (BUA, 1987).
DBP is one of the most commonly used plasticizers in regenerated
cellulose film, being present mainly in nitrocellulose coatings which
are applied to the films (average content, 2.5% of the weight of the
film) (MAFF, 1987).
DBP is used in cosmetics as a perfume solvent and fixative, a
suspension agent for solids in aerosols, a lubricant for aerosol
valves, an antifoaming agent, a skin emollient and a plasticizer in
nail polish, fingernail elongators and hair spray (Brandt, 1985).
3.2.3 Emissions
Although DBP has low volatility, its widespread use in many thin
polymeric sheets and coatings provides large surface areas for
volatization during manufacture, use and disposal of these products.
Disposal at dump sites and disintegration or incineration of the
plastics allow for dispersal of small particulates into the air
(ATSDR, 1990) Perwak et al. (1981) estimated that about 300 tonnes of
DBP were released into the air in 1977 in the USA.
Based on a production of 22 100 tonnes in Germany in 1986,
the release into the environment was estimated to be about
500 tonnes/year. Release associated with the production of DBP was
estimated to be about 0.1 tonnes/year, whereas emission related
to end usage was 400 tonnes/year. It was estimated that about
100 tonnes/year were released by further processing activities, such
as manufacture of plastic and other materials (BUA, 1987).
DBP may be released into surface water. It is estimated that
300 tonnes of DBP were released to water in 1977 in the USA (Perwak et
al., 1981).
No specific release of DBP to soils has been reported. However,
it may seep into soil from DBP coating sewage sludge that is deposited
on land (ATSDR, 1990).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
In the atmosphere, DBP has been measured in both the vapour and
the particulate phases. In various studies, the proportion of total
DBP present in the vapour form in the atmosphere has been reported to
range from 68% (32% in the particulate phase) in the Gulf of Mexico
(Giam et al., 1980) to 78% (22% in the particulate phase) in Antwerp,
Belgium (Cautreels & van Cauwenberghe, 1978). Hoff & Chan (1987),
however, reported that in the Niagara River region of North America,
more than 57% of atmospheric DBP occurs in the suspended particulate
phase.
Washout via rainfall or dry deposition is believed to play a
significant role in the removal of DBP from the atmosphere.
Eisenreich et al. (1981) predicted that atmospheric deposition is a
significant source of DBP in the Great Lakes, North America, with a
calculated total deposition of 48 tonnes/year to the five Great Lakes
and values for each ranging from 3.7 tonnes/year for Lake Ontario to
16 tonnes/year for Lake Superior. Based on levels of DBP in airborne
fallout at 14 locations in Sweden, the total deposition was estimated
to be 90 tonnes per year (Thurén & Larsson, 1990).
In surface water, most of the DBP (> 75%) is present in the
water fraction rather than in the suspended solids (Niagara River Data
Interpretation Group, 1990). Sullivan et al. (1982) reported that DBP
was rapidly adsorbed onto and desorbed from three clay minerals,
sediment and glass test tubes. During the experiments no more than
11% of the total DBP was adsorbed. Al-Omran & Preston (1987) found
that DBP reached an adsorption equilibria within 30 min, the degree of
adsorption being most closely correlated to the lipid content of
suspended particles. The adsorption was enhanced by the presence of
salt.
DBP is moderately adsorbed to soil (Howard, 1989; Zurmühl et al.,
1991), but it forms a complex with water-soluble fulvic acid and this
may increase its mobilization and reactivity in soil to some degree
(Matsuda & Schnitzer, 1971). Volatilization of DBP from soil is not
expected to be significant because of its low vapour pressure and
moderate adsorption to soil (Howard, 1989).
Using the Exposure Analysis Modelling System (EXAMS), Wolfe et
al. (1980) calculated that at equilibrium the loss of DBP from a pond
was 3.3% hydrolysis, 1.2% photolysis, 31.8% biodegradation and 6.2%
volatilization.
4.2 Transformation
4.2.1 Abiotic degradation
Howard et al. (1991) estimated the photo-oxidation half-life of
DBP in air to range from 7.4 h to 3.1 days.
The photolytic half-life of DBP in water has been estimated to be
144 days (Howard, 1989; calculated from Wolfe et al., 1980).
4.2.2 Biodegradation
DBP is biodegradable in natural surface waters, with an estimated
half-life in the range of 1 to 14 days (Schouten et al., 1979; Johnson
et al., 1984; Walker et al., 1984; Howard, 1989; Howard et al., 1991).
Primary degradation exceeded 95% in 24 h in the Semi-Continuous
Activated Sludge (SCAS) test, while ultimate biodegradation to CO2
amounted to 57.4% (half-life of 15.4 days) in the shake flask test
(CMA, 1984). Sugatt et al. (1984) reported 90% primary degradation of
DBP in the 28-day shake flask test using mixed populations of
microorganisms from natural sources.
Howard et al. (1991) predicted a DBP half-life of 2-23 days in
groundwater, based upon aerobic and anaerobic degradation rates.
Sediment from the upper 5 cm of a test pond served as the
inoculum in tests of aerobic and anaerobic degradation of DBP (Johnson
& Lulves, 1975). The samples contained 1 mg/litre of 14C-labelled
DBP. The extent of aerobic degradation was 53% within 24 h and 98%
within 5 days. The anaerobic solutions still contained 69% of the
initial amount after 5 days and only 2% after 30 days.
O'Connor et al. (1989) found > 85% mineralization of DBP during
incubation of anaerobic sludge for 90 days at a concentration of
200 mg DBP/litre. In anaerobic sludge, degradation of DBP proceeded
through mono- n-butyl phthalate to phthalic acid, followed by ring
cleavage and mineralization (Shelton et al., 1984).
In an experiment with batch anaerobic digestion of sewage sludge
spiked with DBP at a concentration range of 0.5-10 mg/litre, DBP was
degraded rapidly with a degradation rate following first-order
kinetics. More than 90% was removed in under 8 days without any lag
phase (Ziogou et al., 1989). The degradation rate can vary with
sludge source and sampling time. DBP was found to be degraded from an
activated sludge system very efficiently (Iturbe et al., 1991).
In a series of studies, Kurane et al. (1979a,b) demonstrated that
DBP is efficiently removed from wastewater by inoculating viable cells
of Nocardia erythropolis, a bacterium capable of rapidly degrading
phthalate esters in activated sludge. When the wastewater containing
3000 mg DBP/litre was treated with the activated sludge inoculated
with N. erythropolis, the DBP was found to be removed at a rate of
94.2% in one day and 100% after the 5th day (as measured by gas
chromatography) (Kurane et al., 1979a,b). Phthalate ester-utilizing
microoganism species isolated from the inoculated and uninoculated
activated sludge were N. erythropolis, N. restricta, Pseudomonas
capacia, P. fluorescens and P. acidovorans (Kurane et al.,
1979a,b).
Pseudomonas pseudoalcaligenes B20b1 (a denitrifying strain) was
enriched from the effluent of a biological sewage plant with DBP as
the sole carbon source (Benckiser & Ottow, 1982). After 20 days
at 30°C, TLC and MS analysis of the culture extracts showed
mono- n-butyl phthalate and phthalic acid as the only products,
suggesting that an n-butanol moiety served essentially as the carbon
source for growth and denitrification. A Micrococcus sp. (strain
12B) was also isolated by enriching with DBP as sole carbon and energy
source, and a metabolic pathway for DBP by this strain was proposed
(Eaton & Ribbons 1982). In this pathway, DBP is converted to mono-
n-butyl phthalate and then to 3,4-dihydro-3,4-dihydroxy phthalate,
which is in turn converted to 3,4-dihydroxy phthalate and then to
protocatechuate (3,4-dihydroxy benzoate). Protocatechuate is
metabolized by a meta-cleavage pathway to pyruvate and oxaloacetate
and by an ortho-cleavage pathway to beta-keto-adipate (Eaton &
Ribbons, 1982).
Wang et al. (1995) isolated five strains of DBP-degrading
microorganisms from coke-plant wastewater treatment plant sludge.
All strains were capable of achieving complete degradation of DBP
(100 mg/litre). One strain was able to completely degrade DBP within
40 h. Further experimental studies revealed that the rate of DBP
degradation was higher with immobilized cells than with free cells.
Chauret et al. (1995) have isolated a psychrotrophic denitrifying
Pseudomonas fluorescens from DBP-spiked microcosms, which is
capable of transforming DBP at 10°C under both aerobic and anaerobic
conditions. The isolated pseudomonad did not grow with phthalic acid
as the sole source of carbon, indicating that DBP was not mineralized
by this bacterium.
Howard et al. (1991) predicted a half-life for DBP in soil of 2
to 23 days. Inman et al. (1984) reported that DBP was almost
completely metabolized within 100 days in non-sterile soils of various
types (silt loam, sand, mixture of silica sand and peaty muck).
Overcash et al. (1982), however, reported half-lives of > 26 weeks in
loam and sand at application rates of 800 mg DBP/kg or more, while, at
a lower application rate (200 mg/kg), the half-life of DBP in loam and
sand was about 12 weeks.
Shanker et al. (1985) incubated garden soil containing DBP at a
concentration of 500 mg/kg. Within 10 days, 91% of the DBP had been
degraded and, after 15 days, 100% of the parent compound had been
degraded. No degradation was detected when sterilized soil was used.
Degradation of DBP was much slower in anaerobic soil, flooded with
sterile water to reduce oxygen tension. After a 30-day incubation,
66% of the DBP had been degraded, compared with 100% degradation
within 15 days under aerobic conditions.
Yan et al. (1995) reported that algae are capable of degrading
DBP. An average biodegradation rate of 2.1 mg/litre per day was found
when the alga Chlorella pyrenoidosa was exposed to 7 mg DBP/litre.
Degradation of the parent compound was complete within 72 h.
4.2.3 Bioaccumulation
The log octanol-water partition coefficient for DBP is between
4.31 and 4.79, which indicates a potential for the chemical to
bioaccumulate. However, the accumulation of DBP is influenced by the
capability of an organism to metabolize it, and several authors have
shown the ability of fish to metabolize DBP. Stalling et al. (1973)
found that radioactively-labelled DBP was metabolized by microsomal
preparations from fish (channel catfish) liver to mono- n-butyl
phthalate (55%) and three other unidentified metabolites (42%) within
2 h. Only 3% of the parent compound was recovered. All of the values
are expressed as percentage of radioactivity. The hepatic microsomes
taken from male channel catfish degraded DBP 16 times more rapidly
than diethylhexyl phthalate (DEHP). When Wofford et al. (1981)
exposed sheepshead minnow to 14C-DBP for 24 h, the distribution of
metabolites was as follows: 13% diester; 28.2% monoester; 47.8%
phthalic acid; and 11% of the radioactivity in the residue.
Bioconcentration factors for a number of organisms are presented
in Table 4. A wide variety of bioconcentration factors have been
reported reflecting not only the capability of organisms to accumulate
DBP but also the variety of exposure concentrations and test
conditions. Care must be taken when interpreting data based on the
accumulation of radioactivity because of the metabolism of the parent
compound (DBP). The highest bioconcentration factor quoted, based on
the parent compound, is 590 for the fathead minnow ( Pimephales
promelas) at an exposure concentration of 34.8 µg/litre. The
bioconcentration factor was a mean value based on the percentage of
DBP in the measured radioactivity over an 11-day period. The
percentage of DBP ranged from 50% on day 3 to 8% on day 11 (Call et
al., 1983).
Lokke & Bro-Rasmussen (1981) applied DBP, in a mixture that also
contained DEHP and di-iso-butyl phthalate, at a concentration of
2.5 µg/cm2 to the leaves of Sinapis alba. The residue level of
DBP on the leaves immediately after application was 2.4 µg/cm2.
There was rapid elimination of DBP and after 15 days DBP levels had
decreased to only 0.03 µg/cm2.
Belisle et al. (1975) fed mallard ducks ( Anas platyrhynchos)
on a diet containing 10 mg DBP/kg for a period of 5 months. No DBP
was detected in fat, heart, lung or breast tissue (detection limit =
0.1 mg/kg in a 2-g sample). The exposure concentration was equivalent
to a dose of 0.56 mg/kg body weight per day, assuming a body weight of
1.1 kg/bird and a food consumption rate of 0.0619 kg dry weight per
day (Nagy, 1987). There appears to have been no biomagnification of
DBP in this study. In fact, it would seem unlikely that terrestrial
animals will biomagnify DBP, based upon the rapid metabolism and
excretion observed in laboratory mammals (see Chapter 6).
Table 4. DBP bioconcentration (BCF) factors for various aquatic organisms
Species Water Duration BCFa Reference
concentration (days)
(µg/litre)
Oyster 100 1 21.1b Wofford et al.
(Crassostrea (1981)
virginica)
Oyster 500 1 41.6b Wofford et al.
(Crassostrea (1981)
virginica)
Water flea 0.08 14 400c Mayer & Sanders
(Daphnia magna) (1973)
Scud 0.10 14 1400c Mayer & Sanders
(Gammarus (1973)
pseudolimnaeus)
Scud 100 10 140 Thurén & Woin (1991)
(Gammarus pulex) (accumulated)
Scud 100 10 45 Thurén & Woin (1991)
(Gammarus pulex) (adsorbed)
Scud 500 10 64 Thurén & Woin (1991)
(Gammarus pulex) (accumulated)
Scud 500 10 8.4 Thurén & Woin (1991)
(Gammarus pulex) (adsorbed)
Table 4. Continued
Species Water Duration BCFa Reference
concentration (days)
(µg/litre)
Brown shrimp 100 1 2.9 Wofford et al. (1981)
(Penaeus aztecus)
Brown shrimp 500 1 30.6 Wofford et al. (1981)
(Penaeus aztecus)
Midge 0.18 7 720c Mayer & Sanders (1973)
(Chironomus
plumosus)
Mayfly 0.008 7 430c Mayer & Sanders (1973)
(Hexagenia
bilineata)
Fathead minnow 4.83 11 570d Call et al. (1983)
(Pimephales
promelas)
Fathead minnow 34.8 11 590d Call et al. (1983)
(Pimephales
promelas)
Sheepshead minnow 100 1 11.7 Wofford et al. (1981)
(Cyprinodon
variegatus)
a BCF based on whole-body concentrations, unless otherwise indicated
b BCF based on concentration in muscle
c Based on radioactivity
d Based on a mean for the % DBP in the radioactivity measured on days 1, 3 and 11
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Identified data on concentrations of DBP in various media
are presented in Table 5. Data from the surveys considered to be
most representative are addressed in the text.
In interpreting this data, it should be noted that steps
taken to avoid contamination are rarely described in the reports
published before 1980 and, consequently, the reliability of the
early data often cannot be assessed. The more recent available
data have therefore been emphasized.
5.1.1 Air
The levels of DBP in air are summarized in Table 5.
Giam et al. (1978) reported mean concentrations of
0.3 ng/m3 over the Gulf of Mexico (n = 8) and 1.0 ng/m3 over
the North Atlantic Ocean (n = 5). No other information was
provided.
DBP was detected in samples of air taken in 1982 (n = 5)
along the Niagara River in Ontario, Canada, with mean
concentrations of 1.9 ± 1.3 ng/m3 in the gas phase and
4.0 ± 2.2 ng/m3 in the particulate phase (Hoff & Chan, 1987).
In 1983, mean levels were 4.5 ± 3.5 ng/m3 in 15 samples of
the gas phase and 6.2 ± 2.6 ng/m3 in 19 samples of the
particulate phase. Eisenreich et al. (1981) reported that
atmospheric concentrations of DBP in the Great Lakes area ranged
from 0.5 to 5 ng/m3; however, no sampling or analytical
details were given.
DBP has been identified in ambient air in Barcelona, Spain;
concentrations of 3.0 and 17 ng/m3 were reported in winter, and
1.1 and 10 ng/m3 in summer for coarse (> 7.2 µm) and fine
(> 0.5 µm) particulates, respectively (Aceves & Grimalt 1993).
Cautreels et al. (1977) reported a range of concentrations
of DBP from 24 to 74 ng/m3 in the suspended particulate phase of
the air in a residential area of Antwerp, Belgium, in contrast to
19 to 36 ng/m3 in samples from a rural area in Bolivia. Atlas &
Giam (1981) reported atmospheric concentrations of DBP as high as
18.5 ng/m3 at Pigeon Key, Florida. Bove et al. (1978) reported
mean concentrations of DBP ranging from 3.28 ng/m3 at Staten
Island to 5.69 ng/m3 at Brooklyn, New York. Weschler (1981)
reported DBP in the Arctic aerosol at Barrow, Alaska, at a
concentration of about 1 ng/m3. In Japan, in 1985, DBP was
detected in 56 out of 63 samples of ambient air at levels
ranging from 17 to 370 ng/m3 (detection limits, 5 to
70 ng/m3) (Environment Agency, Japan, 1995).
5.1.2 Water
5.1.2.1 Surface water
The levels of DBP in surface water are summarized in
Table 5. Information on concentrations of DBP in surface water
in a national database in Canada is limited to 73 records for
Alberta and two records for British Columbia dating from 1985 to
1988. Concentrations were above the detection limit for only
eight records and reported values ranged from < 1 to 2 µg/litre
(NAQUADAT, 1993). For water samples collected in 1988 and 1989,
mean concentrations of 12.2 ng/litre at Fort Erie, Ontario (all
of 26 samples contained DBP at concentrations above the
detection limit of 0.29 ng/litre; maximum 26.78 ng/litre) and
15.16 ng/litre at Niagara-on-the-Lake, Ontario (all of 25 samples
contained DBP at concentrations above the detection limit of
0.29 ng/litre; maximum 72.93 ng/litre) were reported (Niagara
River Data Interpretation Group, 1990).
In Japan, for the years 1974, 1975 and 1982, levels of DBP
in surface water ranged from 0.013 to 36 µg/litre (detected in 55
to 93% of samples; detection limits, 0.01 to 40 µg/litre).
(Environment Agency, Japan, 1995).
In 1991 and 1992; DBP concentrations were measured in
unfiltered water samples of the River Rhine (4 locations) and six
of its tributaries. DBP was detected in 99% of 217 samples with
a detection limit of 0.03 µg/litre. The mean concentration in
the Rhine was 0.18 µg/litre, and the maximum value was
1.3 µg/litre. Mean values in the tributaries were in the same
range (LWA, 1993; Furtmann, 1994). The concentrations in the
particulate fraction of R. Rhine water were reported to be in the
range of 1.2 to 7.8 mg/kg dry weight. Schouten et al. (1979)
reported that DBP concentrations in rivers in the Netherlands
ranged from < 0.1 to 2.8 µg/litre. Other measurements of DBP
concentrations in the Netherlands revealed a mean value of
0.1 µg/litre in the Rhine (maximum = 1.1 µg/litre, 53 samples) in
1991 (RIWA, 1991) and 1.0 µg/litre in the Ijssel Sea (maximum =
6.9 µg/litre; 7 samples) in 1992 (RIWA, 1992). In both reports a
mean value of 0.1 µg/litre was given for the River Lek.
In 1984, DBP was detected in the Rivers Irwell (12.1 and
33.5 g/litre) and Etherow (32.5 and 23.5 g/litre) in
Manchester, United Kingdom (Fatoki & Vernon, 1990). Both rivers
received discharges from factories making plastic products.
5.1.2.2 Groundwater
At four sites in woodland areas of Germany, which are not
directly influenced by industry or agriculture, DBP
concentrations were measured monthly in wellwater and groundwater
in 1988 and 1989 (Schleyer et al., 1991). Mean concentrations
were 0.15 to 0.46 µg/litre.
5.1.2.3 Seawater
The levels of DBP in seawater are summarized in Table 5. In
an early study, concentrations of DBP up to 0.47 µg/litre in
water from the Gulf of Mexico were reported (Chan, 1975).
Reported maximum concentrations of DBP in seawater range from
0.203 µg/litre in the Kiel Bight (Baltic Sea) (Ehrhardt &
Derenbach, 1980) and 0.230 µg/litre (Ray et al., 1983a) in Nueces
Estuary, Texas, up to 4.8 µg/litre in United Kingdom estuaries in
industrial areas (North and Irish Seas) (Law et al., 1991) and
24.1 µg/litre in the Baltic and North Seas off the coast of Germany
(von Westernhagen et al., 1987).
5.1.2.4 Precipitation
Atlas & Giam (1981) reported concentrations of DBP in
rainwater ranging from 0.0026 to 0.0725 µg/litre at the Enewetak
Atoll in the North Pacific Ocean. Eisenreich et al. (1981)
reported that concentrations of DBP in rainwater in the Great
Lakes area ranged from 0.004 to 0.01 µg/litre; however, no
sampling or analytical details were given. In Japan in 1974
levels of DBP in rainwater ranged from 0.13 to 52 µg/litre
(detected in 68 out of 111 samples; detection limits ranged from
0.1 to 4 µg/litre) (personal communication by the Environment
Agency, Japan, to the IPCS 1995).
In 1992 DBP concentrations were measured in rainwater
samples from 3 sites in industrial areas of Germany (LWA 1993).
Mean values of 0.8 to 1.4 µg/litre and maximum values of 1.1 to
4.5 µg/litre were determined. In woodland areas of Germany that
are not directly influenced by industry or agriculture, DBP
concentrations in rainwater were measured at four sites in 1988
and 1989 (Schleyer et al., 1991). Outside the forest, mean
concentrations of 0.21 to 0.35 µg/litre were found. The
precipipitation sampled below the trees contained nearly the same
amount of DBP; at one site the concentration was slightly higher
with 0.52 µg/litre. A minimum concentration of 0.06 µg/litre
and a maximum concentration of 1 µg/litre were found.
5.1.2.5 Effluent and wastewater
Concentrations of DBP in effluent ranged from not detectable
to 61 µg/litre for five Canadian organic chemical plants (number
of samples unspecified), from not detectable to 94 µg/litre for
industrial and municipal plants in Cornwall, Ontario (number of
samples unspecified) and from 1.0 to 100 µg/litre for petro-
chemical refineries along the St. Clair River (n= 28) (CCREM,
1987). The detection limit for this study was 1.0 µg/litre.
Concentrations of DBP in fifteen 24-h composite samples of
process waters collected in 1981 from Canadian refineries
(unspecified locations) ranged from traces (detection limit,
2 µg/litre) to 56 µg/litre (PACE, 1985). However, DBP was not
detected in 19 samples of effluent discharge of non-chlorinated
primary-treated municipal wastewater collected in Vancouver in
1983 (Rogers et al., 1986).
The concentration in sewage treatment plant effluent from
Manchester, United Kingdom, sampled during 1984, was 6.0 g
DBP/litre (Fatoki & Vernon, 1990).
5.1.3 Sewage sludge
DBP has been detected in sludge from municipal wastewater
plants in Canada (Webber & Lesage, 1989). Concentrations ranged
from 0.2 to 161 mg/kg dry weight in Winnipeg in 1981 and 1982.
In Hamilton, the concentrations ranged from 14 mg/kg dry weight
in 1983 to 57 mg/kg dry weight in 1981. The authors noted that
recovery of phthalate esters was erratic, possibly due to
laboratory contamination or lack of sample homogeneity.
DBP concentrations were investigated in anaerobic digester
sludge from nine German municipal wastewater treatment plants
(Zurmühl, 1990). In eight plants concentrations were in the
range of 2.3 to 26 mg/kg dry weight (detection limit =
1.9 mg/kg). A level of 236 mg/kg dry weight was found as the
maximum value. Sewage sludge from another municipal wastewater
plant contained 0.87 mg DBP/kg dry weight (Kördel & Müller 1992).
5.1.4 Soil
DBP levels of < 0.1 to 1.4 µg/g were detected in 13 out of
30 samples (detection limit, 0.1 µg/g) of soils in urban areas of
Port Credit and Oakville/Burlington, Ontario (Golder Associates,
1987). Concentrations in the background samples on- and off-site
were similar (Golder Associates, SENES Consultants Limited and
CanTox, 1987).
Kördel & Müller (1992, 1993) investigated the DBP
concentrations in soil in the vicinity of phthalate-emitting
plants and compared them to a remote area. There was a great
deal of variability in the concentrations at the different
sampling sites, resulting in the fact that no influence of the
phthalate-emitting plants on soil DBP levels could be derived.
The concentrations for the remote site were in the range of <
0.005 mg/kg to 0.185 mg/kg dry weight. In the vicinity of the
industrial sites the values were < 0.005 to 0.560 mg/kg dry
weight.
5.1.5 Sediment
The levels of DBP in sediment are summarized in Table 5.
Samples of sediment collected from the Detroit River in 1982
contained concentrations of DBP ranging from < 0.1 to 0.65 mg/kg
dry weight (Fallon & Horvath, 1985). Concentrations of DBP in
sediment samples taken in 1982 from the Fraser Estuary, British
Columbia, ranged from 0.07 to 0.45 mg/kg dry weight (Rogers &
Hall, 1987). The concentration of DBP decreased from 0.204 mg/kg
dry weight in sediment 0.5 km from a large sewage outfall in the
estuary to 0.060 mg/kg in sediment 1.0 km from the outfall
(Rogers & Hall, 1987). Concentrations of DBP up to 0.3 mg/kg were
reported in samples of sediment collected from Lake Superior and
Lake Huron in the 1970s (CCREM, 1987). Concentrations of DBP in
sediment from the Neckar River in Germany ranged from 0.09 to
0.3 mg/kg (Malisch et al., 1981). Higher concentrations (0.028
to 0.9 mg/kg) were reported in sediment in Maryland, USA
(Peterson & Freeman, 1984). Marine sediment from the Crouch
Estuary United Kingdom contained 0.0039 to 0.0145 mg/kg
(Waldock, 1983). Reported concentrations of DBP from marine
sediments in the USA ranged from 0.0042 mg/kg dry weight in
Nueces Estuary, Texas (Ray et al., 1983a) to 0.355 mg/kg dry
weight at Los Angeles (Swartz et al., 1985). In Japan, levels in
1974 and 1982 ranged from 0.001 to 2.3 mg/kg (detected in 41 -
86% of total of 415 samples; detection limits, 0.0007 to
0.28 mg/kg).
DBP concentrations in Rhine sediments were measured in 1991.
In seven samples concentrations ranged from 0.14 to 2.2 mg/kg dry
weight. In 9 out of 10 samples of sediments of the River Weser,
DBP was detected at concentrations of 0.03 to 0.34 mg/kg dry
weight with one maximum value of 9.1 mg/kg. The detection limit
was 0.02 mg/kg (LWA, 1993). In Sweden sediment samples from
different types of enviornment were taken in 1994 (Parkman &
Remberger, 1995). DBP concentrations in samples from remote sites
were in the range from 1 to 8 µg/kg dry weight, with one outlier
of 56 µg/kg (average of three samples per site). Concentrations
in industrialized areas were 0 to 182 µg/kg dry weight (detection
limit = 1.9 µg/kg).
5.1.6 Aquatic organisms
In early studies, the concentrations of DBP in aquatic biota
from the Great Lakes and other areas in Canada were less than
10 mg/kg (Williams, 1973; Glass et al., 1977; Swain, 1978;
Burns et al., 1981). The highest concentrations were reported
for skinless fillets from long-nose suckers, Catostomus
catostomus, (8.1 µg DBP/g) and rainbow trout, Oncorhynchus
mykiss, (5.4 µg/g) from Lake Superior (Glass et al., 1977).
In fish from various US Great Lakes harbours and tributary mouths
in the USA, the concentrations of DBP in the majority of the
samples ranged from < 0.02 to 0.16 µg/g wet weight; however,
there were some higher values ranging up to 35 µg/g in more
polluted areas (DeVault, 1985). Ray et al. (1983b) reported
concentrations of DBP in the marine polychaete worm Neanthes
virens from Portland, Maine, USA, ranging from 0.070 to
0.180 mg/kg.
5.1.7 Terrestrial organisms
Data on phthalate levels in wild birds and mammals are very
sparse. In an early study, Zitko (1972) detected DBP in egg
yolks of the double-crested cormorant, Phalacrocorax auritus,
(14.1 µg/g lipid) and herring gull, Larus argentatus, (10.9,
17.1 and 19.1 µg/g lipid).
5.2 General population exposure
5.2.1 Ambient air
Data on concentrations of DBP in ambient air are extremely
limited. The most extensive information available is the range
of concentrations of 4.5 (mean of 15 samples; gas phase) to
6.2 ng/m3 (mean of 19 samples; particulate phase) in air sampled
along the Niagara River in 1983 (Hoff & Chan, 1987). These
values are similar to those determined more recently in a small
number of ambient air samples from Barcelona, Spain (Aceves &
Grimalt, 1993). Based upon a daily inhalation volume for adults
of 22 m3, a mean body weight for males and females of 64 kg, the
assumption that 4 of 24 h are spent outdoors (IPCS, 1993) and the
above range of concentrations in ambient air, the mean intake of
DBP via ambient air for the general population is estimated to
range from 0.26 to 0.36 ng/kg body weight per day.
5.2.2 Indoor air
The maximum concentration of DBP in indoor air in nine homes
in Montreal, Canada, sampled for three consecutive periods of 20
days each, was 2.85 µg/m3 (nominal quantification limit,
0.50 µg/m3) (Otson & Benoit, 1985). No other information on
measured concentrations (e.g., mean concentrations) was
presented. In a survey of 125 homes in California in 1990, the
median daytime concentration of DBP in indoor air was 420 ng/m3
(California Environmental Protection Agency, 1992).
Based upon a daily inhalation volume for adults of 22 m3, a
mean body weight for males and females of 64 kg, the assumption
that 20 of 24 h are spent indoors (IPCS, 1993) and the median
concentration of DBP reported in a survey of a large number of
homes in California (420 ng/m3), the daily intake of DBP in
indoor air for the general population is estimated to be
120 ng/kg body weight per day.
5.2.3 Drinking-water
Data on concentrations of DBP in drinking-water are limited.
In an early survey (1974), DBP was detected (detection limit
unspecified) in six out of ten city water supplies in the USA.
The concentrations of DBP ranged from 0.01 to 0.1 µg/litre for
five cities and was 5.0 µg/litre for one city (Keith et al.,
1976). Concentrations in two samples of tap water from the
Shizuoka Prefecture in Japan taken in 1974 were 1.0 and
0.8 µg/litre (Shibuya, 1979). In samples of tap and well water
in Japan, levels were 1.9 and 2.5 µg/litre, respectively (Ishida
et al., 1980). In a survey of an unspecified number of samples
of the municipal drinking-water supplies of seven cities in the
Niagara region and in the vicinity of Lake Ontario conducted in
1984 (MOE, 1984), DBP was not detected (detection limit,
1.0 µg/litre).
In a small number of samples of drinking-water in Toronto,
Canada, the mean concentration was 14 ng/litre; concentrations in
seven brands of bottled spring water ranged from 21 to
55 ng/litre (City of Toronto, 1990).
Based upon a daily water consumption for adults of 1.4
litres, a mean body weight for males and females of 64 kg (IPCS,
1993) and a mean concentration of < 1.0 µg/litre, the estimated
mean intake of DBP from drinking-water for the general population
is <0.02 µg/kg body weight per day.
5.2.4 Food
In addition to entry through environmental contamination,
DBP may be present in foodstuffs as a result of migration from
packaging. This has been investigated in a number of studies
conducted in the late 1980s. In many countries, on the basis of
the results of these studies, precautions were introduced to
reduce leaching of plasticizers from packaging. As a result,
levels of DBP in foodstuffs have declined over time. In this
section, studies designed to investigate the presence of DBP in
foodstuffs due to leaching from packaging are presented, followed
by data from more broadly based market-basket surveys.
Concentrations of DBP ranged from 0.13 to 1.62 mg/kg in
three brands of aluminum foil in Japan (Ishida et al., 1980).
In the first of several studies conducted in the United
Kingdom to investigate the impact of packaging on the DBP content
of foodstuffs, foods were purchased at retail stores and stored
in their packaging until their "sell by" or "best before" date
(British Ministry of Agriculture, Fisheries and Food, 1987).
Mean concentrations of DBP were 8 to 32 mg/kg in chocolate
confectionery, 13 mg/kg in sugar confectionery, 11 mg/kg in
cakes, 3.9 to 11 mg/kg in baked savouries, 6 to 10 mg/kg in meat
pies and 2 mg/kg in sandwiches.
In a survey of plastic-packaged Italian foodstuffs, DBP was
detected in cheese (0.84 g/g), salted meat (1.09 mg/kg),
vegetable soups (2.06 mg/kg), potato chips (2.80 mg/kg) and
pasteurized milk (0.07 mg/kg) (Cocchieri, 1986).
Levels of DBP ranged from 0.5 to 30.8 mg/kg in nougat and
chocolate, respectively, in a wide range of foodstuffs in the
United Kingdom, which were wrapped in a range of different
packaging including nitrocellulose-coated regenerated cellulose
film (RCF). Levels of plasticizers were 0.5 to 1.5%, on a total
film-weight basis (Castle et al.,1988). In a later study, Castle
et al. (1989) reported that DBP in the ink on the outer surface
of film can transfer onto the inner food contact surface. The
level of DBP in a chocolate-covered confectionery product
increased from 0.2 to 6.7 mg/kg over a storage period of 180
days. DBP levels in 47 samples of confectionery, snack products
and biscuits purchased in the United Kingdom, wrapped in printed
polypropylene film, ranged from 0.02 to 14.1 mg/kg.
In a more recent reported retail survey in the United
Kingdom (MAFF, 1990), ranges in up to 30 samples each of plastic
wrapped foods were 0.09 to 0.13 mg/kg in biscuits, 0.02 to
14.1 mg/kg in potato snacks, 0.15 to 5.6 mg/kg in chocolate-
covered bars and 2.6 to 9.2 mg/kg in candy-coated chocolate
sweets. In the same report, results of sequential analysis of a
few foods were also reported. Concentrations in potato snacks,
candy-coated individual sweets and chocolate bars increased
approximately 2- to 3-fold over a 6-month period.
Page & Lacroix (1992) reported that retail samples of
packaged butter and margarine sold in Canada contained up to
10.6 mg DBP/kg.
Nerin et al. (1993) analysed plastic-wrapped food products
for DBP from both Spain and the United Kingdom and reported (for
an average of three determinations) up to 0.81 mg/kg in chocolate
bars and 0.60 mg/kg in biscuits.
In an early Canadian study (Williams, 1973), DBP was
determined in 21 samples of fish. DBP was detected in one sample
of canned tuna at a concentration of 78 µg/kg while the levels in
one sample of canned salmon was 37 µg/kg. Concentrations of DBP
in the muscle of fish (n = 10 samples from five species) from the
lower Fraser River in British Columbia ranged from 0.07 to
0.15 mg/kg wet weight (Swain & Walton, 1989). The authors
considered 0.07 mg/kg as the background level, owing to
contamination; the detection limit was not reported. Elevated
concentrations of DBP have occasionally been reported in fish in
polluted areas (see section 5.1).
Based upon residue analysis of commercial eggs collected
throughout Japan, 0.098 mg DBP/kg (trace - 0.15 mg/kg was
present in egg whites (Ishida et al., 1981). No phthalate
residues were found in the egg yolks. In an early study of 2 to
14 samples each of various foodstuffs in Japan, DBP was detected
in meat (100 µg/kg), fish (180 µg/kg), eggs (80 µg/kg), but not
in milk (detection limit, 50 µg/kg) (Howard, 1989). In another
study (Tomita et al., 1977), DBP was determined by gas-liquid
chromatography (detection limit, 0.01 mg/kg) in 22 kinds of
Japanese foods (17 samples of fatty foods and 38 samples of non-
fatty foods mostly in plastic containers). DBP was detected in
tempura (frying) powder (0.39 to 17.70 mg/kg), instant cream soup
(1.73 to 60.37 mg/kg), fried potato cake (not detected to
1.11 mg/kg), orange juice (0.35 mg/kg) and pickles (0.11 mg/kg).
Ito et al. (1993) reported that 2 out of 15 samples of
imported vodka in Japan contained up to 0.2 mg DBP/litre. In the
USA, DBP was detected in 18 out of 50 samples of vodka (maximum
concentration: 204 µg/litre; limit of detection: 20 µg/litre)
(Leibowitz et al., 1995). DBP was detected in 1 out of 60 samples
of Russian vodka (0.7 mg/litre) and in 1 out of 7 samples of
European vodka (1.1 mg/litre) (Saito et al., 1993).
In a Canadian market-basket survey of 98 different food
types sampled in Halifax in 1986 (Page & Lacroix, 1995), DBP was
detected in butter (1.5 mg/kg), freshwater fish (0.5 mg/g),
cereal products (ranged from not detected to 0.62 mg/kg), baked
potatoes (0.63 mg/kg), coleslaw (0.11 mg/kg), bananas,
blueberries and pineapples (0.12, 0.09 and 0.05 mg/kg,
respectively), margarine (0.64 mg/kg), white sugar (0.2 mg/kg)
and gelatin dessert (0.09 mg/kg). The detection limits varied
(ranging from 0.01 to 0.5 mg/kg) according to the reagent blank
values (interferences arising from coextracted food components)
and the fat content of the food.
Exposure of the general population to DBP in food has been
estimated on the basis of data from the only study identified in
which there was a sufficiently wide variety of foodstuffs to
serve as a basis, i.e., those from a market-basket survey in
Canadaa. Based upon the average daily consumption of various
foodstuffs by adultsb, a mean body weight for males and females
of 64 kg (IPCS, 1993) and concentrations of DBP reported in the
Canadian market basket survey, the estimated daily intake from
food is 7 µg/kg body weight per day. It should be noted,
however, that intake of DBP in the diet can vary considerably,
depending upon the nature and amount of packaged food that is
consumed and the nature of use of food wrapping in food
preparation. In the United Kingdom, the Ministry of Agriculture,
Fisheries and Food has estimated that the maximum likely human
intake of DBP from food sources is approximately 2 mg per person
per day (approximately 31 g/kg body weight per day, assuming a
mean body weight of 64 kg).
5.2.5 Consumer products
In 1981, DBP was reported as an ingredient in a total of 590
cosmetic formulations in the USA, at concentrations ranging from
less than 0.1% to between 10 and 25% (Brandt, 1985). There is
potential for exposure to DBP in cosmetics, but available data
are inadequate to quantify intake from this source.
The "new car smell" in automobiles has been attributed to
DBP and other phthalic acid esters (Shea, 1971). Levels of total
phthalic acid esters in the µg/m3 range have been identified in
samples of air taken from new cars in an early study (Graham,
1973).
5.2.6 Medical devices
Plastic tubing used in hospitals for oral/nasal feeding of
patients, has been reported to contain 54 mg DBP/g (Khaliq et
a Data from the Canadian market-basket survey used in
calculating the estimated average daily intake include
concentrations of DBP in the following foodstuffs: butter,
1.5 mg/kg; freshwater fish, 0.5 mg/kg; cereal products,
0.62 mg/kg, baked potatoes, 0.63 mg/kg; bananas, 0.12 mg/kg;
white sugar, 0.2 mg/kg.
b Dietary intakes consist of: cereals, 323 g/day; starchy
roots, 225 g/day; sugar (excludes syrups and honey),
72 g/day; pulses and nuts, 33 g/day; vegetables and fruits,
325 g/day; meat, 125 g/day, eggs, 19 g/day; fish, 23 g/day;
milk products (excludes butter), 360 g/day; fats and oils
(includes butter), 31 g/day (IPCS, 1993).
al., 1992). DBP leached from tubing into distilled water and
solutions of ethanol, acetic acid and sodium bicarbonate, in
concentrations which increased with temperature and duration of
contact.
5.2.7 Levels in human tissue
In an early study, concentrations of DBP in 25 samples of
human adipose tissue collected from Vancouver (n = 2), Toronto (n
= 22) and Montreal (n = 1) at autopsies of accident victims,
ranged from 0.01 to 0.3 mg/kg (detection limit not reported) (Mes
et al., 1974).
Levels of DBP in the blood collected from 13 individuals
(mean, 0.10 mg/litre) following ingestion of food that had been
in contact with unspecified flexible plastics packaging materials
containing DBP were higher than those collected from nine
individuals before meals (mean levels in blood, 0.02 mg/litre)
(Tomita et al., 1977).
5.3 Occupational exposure
Identified data on levels of DBP in the occupational
environment are limited. Based on a survey conducted by the
National Institute of Occupational Safety and Health (NIOSH) in
1981-1983, it was estimated that there were 229 000 workers in
the USA with potential exposure to DBP (Howard, 1989). The most
recent provisional data from the National Occupational Exposure
Survey indicates that over 500 000 workers, including 200 000
women are potentially exposed to DBP (NIOSH, 1994).
In 1986, NIOSH conducted a health hazard evaluation of a
silkscreening area in a Department of Highways sign shop (NIOSH,
1987). Concentrations of DBP were below the limit of detection
(less than 0.01 mg per sample), i.e., less than 0.02 mg/m3.
Only trace quantities of DBP were detected in a 1975 survey
of a Goodyear Tire and Rubber Company plant in areas involved in
the production of rubber sleeve stock (NIOSH, 1976).
In 1981, an environmental survey was conducted at a US army
ammunition plant, in an area where DBP-containing propellant was
processed (NIOSH, 1982). Four samples (1 breathing zone, 3 area)
were collected. One area sample contained DBP in an amount
corresponding to a concentration of 0.08 mg DBP/m3. The other
three samples contained less than the detection limit
(0.01 mg/sample).
An industrial hygiene survey was conducted in a plastic pipe
fabricating plant in the USA in 1988. Six personal breathing
zone air samples collected for DBP were below the level of
detection, corresponding to < 0.01 mg/m3 (NIOSH, 1989).
Fischer et al. (1993) reported that concentrations of DBP
ranged from 1.3 to 8.2 mg/m3 in a plant in the Czech Republic
that produced PVC products.
Thus, based on determinations at a limited number of
worksites in the USA, concentrations have generally been less
than the limit of detection (i.e., 0.01 to 0.02 mg/m3), although
levels of up to 8 mg/m3 were reported in a PVC plant in the
Czech Republic.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Data on kinetics and metabolism in mammals are presented in
this chapter. Information on metabolism in invertebrates is
presented in Chapter 4.
6.1 Absorption, distribution and excretion
6.1.1 Dermal
A study was conducted by Elsisi et al. (1989) in which
157 µmol/kg (43.7 mg/kg) of 14C-DBP (uniformly labelled on the
ring) was applied to the back of male F-344 rats and the area of
application was covered with a perforated cap for a 7-day
period). Approximately 10 to 12% of the administered dose was
excreted in the urine each day for several days (total of 60%
after 1 week). Only small amounts of radioactivity were detected
in tissues in the exposed rats. About 33% of the dose remained
at the site of application; all other tissues combined contained
less than 0.5% of the applied dose.
Based on results observed in vitro, Scott et al. (1987)
reported that DBP was slowly absorbed through both rat and human
skin, with rat skin being more permeable.
6.1.2 Ingestion
6.1.2.1 In vivo studies
Levels of DBP in the blood collected from 13 individuals
(mean, 0.10 mg/litre) 2 h following ingestion of food, which had
been in contact with unspecified flexible plastic packaging
materials containing DBP, were higher than those collected from
nine individuals before meals (mean level in blood,
0.02 mg/litre) (Tomita et al., 1977).
Studies in experimental animals indicate that DBP or its
metabolites are rapidly absorbed from the gastrointestinal tract.
In a study conducted by Williams & Blanchfield (1975), following
administration of a single oral dose of about 0.1 g/kg body
weight 7-14C-DBP to male Wistar rats, 96% of the radioactivity
was excreted in the urine at 48 h; less than 0.1% was exhaled as
14CO2. In addition, blood and tissue levels and urine output
were determined at 4, 8, 24 and 48 h following administration of
single oral doses of 7-14C-DBP (0.27 or 2.31 g/kg body weight).
The radioactivity was distributed more or less evenly throughout
the tissues except that the level in the brain was about one
third to one tenth that in the other tissues. Excretion in the
urine was rapid, with 46% of the low dose and 20% of the high
dose being present in the urine at 8 h, 85 and 61%, respectively,
at 24 h, and 92 and 83%, respectively, at 48 h. Based on
analysis of the urine, 80 to 90% of the dose was metabolized and
excreted in the urine in 48 h as phthalic acid (2%), mono-
n-butyl phthalate (88%), mono 3-hydroxy butyl phthalate (8%)
and mono-4-hydroxy butyl phthalate (2%). These authors also
reported that there was no evidence of accumulation in any
tissues in rats fed 0.1% DBP in the diet for 4, 8 or 12 weeks.
Twenty four hours following gavage (in 3% DMSO solution)
administration of a single dose of 60 mg/kg body weight 14C-DBP
to small groups (n=3) of male Wistar rats, radioactivity was
detected in the liver, kidney, blood, muscle, adipose tissue,
stomach and intestine (the latter probably associated with
biliary excretion). There was no significant retention of DBP
within tissues; more than 90% of the administered radioactivity
was recovered in the urine within 48 h (Tanaka et al., 1978).
In DSN hamsters, 79% of a single oral dose of 2 g/kg body
weight (10 µCi of 14C-DBP/kg body weight) administered by gavage
was excreted in the urine within 24 h, mainly as mono- n-butyl
phthalate (Foster et al., 1982).
6.1.2.2 In vitro studies
Mono- n-butyl phthalate (MBP) was absorbed in significantly
greater quantity than DBP in an in vitro study in an everted
gut-sac preparation from the small intestine of male Sprague
Dawley rats (White et al., 1980). DBP was actively hydrolysed by
esterases within the mucosal epithelium during absorption; 95.5%
of DBP was hydrolysed to MBP. When the esterase activity of the
mucosa was reduced by intragastric exposure of the rats to S,S,S-
tributylphosphorotrithioate (8 mg/kg body weight), the absorption
of DBP, but not of MBP, was significantly reduced (from 0.62 to
0.15 µmol/mg per h).
6.1.3 Inhalation
Following inhalation by rats of 50 mg/m3 for various
periods up to 6 months (Kawano, 1980b), DBP was detected by GC/MS
at relatively low concentrations in the brain (0.53 µg/g), lung
(0.17 µg/g) and liver (0.25 µg/g) of small groups of male Wistar
rats. Levels in the testes were lower (mean 0.13 g/g).
Following exposure to 0.5 mg/m3 (0.044 ppm), DBP was
consistently detected only in the brain of exposed rats.
6.2 Metabolic transformation
6.2.1 In vivo studies
Available data indicate that in rats DBP is metabolized by
nonspecific esterases, mainly by hydrolysis, to yield MBP, with
subsequent oxidation of the alkyl side chain of MBP.
Interestingly, MBP is stable and resistant to hydrolysis of the
second ester group (Cater et al., 1977; Rowland et al., 1977).
Following oral administration of DBP to rats, metabolic products
identified in the urine were mainly MBP, various oxidation
products of MBP (2-3%), and a small amount of the free phthalic
acid (Albro & Moore, 1974; Williams & Blanchfield, 1975; Foster
et al., 1982). The MBP and other metabolites are excreted in the
urine mainly as glucuronide conjugates; species differences in
the excretion of conjugated and unconjugated metabolites of DBP
in the urine of Wistar rats and DSN hamsters have been observed.
In hamsters, 53% was excreted as the conjugate and 3.5% as free
monoester. In rats, 38% was excreted as conjugate and 14% as
free monoester, following administration of an oral dose of 2
g/kg body weight (10 µCi of 14C-DBP/kg body weight per day) by
gavage. No free DBP was detected in the urine in either species
(Foster et al., 1982).
6.2.2 In vitro studies
In in vitro studies, DBP was hydrolysed to MBP by cell
preparations from the small intestine (rat, baboon, man), the
liver (rat, baboon) and kidneys (rats) (Lake et al., 1977; Tanaka
et al., 1978; Kaneshima et al., 1978).
Rowland et al. (1977) incubated the contents of the male
Wistar rat stomach, small intestine and caecum with 14C-labelled
DBP for 16 h. About 0.5, 80 and 23% of the DBP was hydrolysed to
MBP by the contents of the stomach, small intestine and caecum,
respectively. The metabolism of DBP by the small intestinal
contents was very rapid, 38% of a dose of 1 mg DBP/ml and 70% of
a dose of 200 g/ml being metabolized in 30 min. Thus, it would
appear that DBP is relatively quickly converted to MBP in the
intestines, this being the principal metabolite. Activity in the
female rat small intestine was only slightly less than that for
the male. Suspensions prepared from human faeces also had modest
DBP hydrolytic activity (6% in 16 h) (Rowland et al., 1977).
Because activity did not decrease when antibiotics were present
during the incubation, the author concluded that the enzymatic
hydrolytic activity was of mammalian origin (possibly pancreatic
and mucosal lipases).
Using 14C-DBP as substrate, the rate of esterase activity
was comparable in small intestinal tissue of rats and hamsters,
whereas the liver of hamsters had approximately double the
activity of rats. In contrast, the ß-glucuronidase activity of
testicular homogenates in the rat was much higher than that in
the hamster ( p-nitrophenyl glucuronide and phenolphthalein
glucuronide were used as substrates) (Foster et al., 1982).
In in vitro assays of rat liver, kidney, pancreas, small
intestine and blood, structural analogues of DBP (di- n-butyl
isophthalate and di- n-butyl terephthalate) were hydrolysed to
their corresponding acids, whereas phthalic acid was not formed
from DBP (Takahashi & Tanaka, 1989). The authors concluded that
nonionic esters are hydrolysed at a much higher rate than charged
analogues and that esterase activities are strikingly different
for different substrates.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of DBP in mice and rats is low. Reported
LD50 values following oral administration to rats range from
approximately 8 g/kg body weight to at least 20 g/kg body weight
(Smith, 1953; Lehman, 1955; White et al., 1983; Brandt, 1985); in
mice, values are approximately 5 to 16 g/kg body weight
(Woodward, 1988; Brandt, 1985; Yamada, 1974). Reported LD50
values following intraperitoneal administration range from 4 to
7 g/kg body weight in rats and approximately 3 to 6 g/kg body
weight in mice (Woodward, 1988). The dermal LD50 in rabbits is
> 4000 mg/kg body weight (Lehman, 1955). Signs of toxicity
include general depression of activity, laboured breathing and
lack of coordination. Reports of acute toxicity of DBP following
inhalation have not been identified.
Following intraperitoneal injection, MBP (the principal
metabolite of DBP) appeared to be somewhat more acutely toxic
than DBP; the LD50 was 1.0 g/kg in the mouse (Chambon et al.
1971).
7.2 Short-term exposure
The short-term toxicity of DBP has been investigated in
rodents following oral administration. The available data are
summarized in Table 6.
In most of these studies, animals were exposed to only one
dose level. Effects in rats after oral administration for 5 to
21 days include those on liver enzymes (Aitio & Parkki, 1978;
Bell et al., 1978; Kawashima et al., 1983; BIBRA, 1986; Barber et
al., 1987) and hepatomegaly at doses of >420 mg/kg body weight
per day (Yamada, 1974; Bell et al., 1978; Oishi & Hiraga, 1980a;
BIBRA, 1986; Barber et al., 1987), a reduction in the rate of
weight gain at doses of >5 ml/kg body weight per day
(5235 mg/kg body weight per day) (Yamada, 1974) and splenomegaly
after intragastric intubation of 1.0 ml/kg body weight per day
(1047 mg/kg body weight per day) (Yamada, 1974). Peroxisome
proliferation, based on increased oxidation of cyanide-
insensitive CoA oxidation, in the liver of male F-344 rats was
observed after administration of 2100 mg/kg body weight per day
in the diet for 21 days (Barber et al., 1987) and also in male
Wistar rats after exposure for 34 to 36 days to 2500 mg/kg body
weight per day in the diet (Murakami et al., 1986a).
Proliferation at lower levels has also been reported in an
investigation summarized in an abstract by Lake et al. (1991). A
slight but insignificant increase in kidney weight was reported
in JCL:Wistar rats exposed to 2060 mg/kg body weight per day for
7 days by Oishi & Hiraga (1980a).
Table 6. Short-term repeated dose toxicity of DBP
Species Protocol Results Effect Levels Reference
Rat (Wistar, 1047 or 5235 mg/kg The rate of b.w. gain was slightly reduced at the high LOAEL = 1047 Yamada (1974)
groups of 5 b.w. per day by dose. One rat administered the high dose died during mg/kg b.w.
females) stomach tube daily the study. Hepatomegaly and marked splenomegaly noted per day
for 3 weeks. at necropsy in both exposed groups; relative kidney
Controls were weight of high-dose group 76% greater than that in
administered controls.
10 ml/kg distilled
water in the same
manner.
Rat (Wistar, 2% DBP in the diet Marked increases in stearoyl-CoA desaturation, One dose group Kawashima
groups of (equivalent to 1000 palmitoyl-CoA oxidation and catalase activity; only (effects et al. (1983)
3 males) mg/kg b.w. per day) increases in microsomal octadecanoic acid in liver, observed at
for 7 days hepatic homogenates and serum. The increases in the 1000 mg/kg b.w.
stearoyl-CoA desaturation appeared to be due to the per day)
increased activity (4 fold) of the terminal
desaturase and not to increases in the activities
of NADH cytochrome-C-reductase or in cytochrome b5
content.
Rat (JCL:Wistar, 2% DBP in the diet Mean b.w.s of exposed rats were slightly but not One dose group Oishi & Hiraga
groups of 10 equivalent to 2060 significantly lower than that of the controls. only (effects (1980a)
males) mg/kg b.w. per day Significant decrease in absolute and relative observed at
for 7 days testicular weights, but the absolute and relative 2060 mg/kg b.w.
liver weights were significantly increased. per day)
Slight but insignificant increase in kidney weight
in exposed rats.
Table 6. Continued
Species Protocol Results Effect Levels Reference
Rat (Fischer-344, dietary Males at mid and high dose and females at high dose LOEL = 624 BIBRA (1986),
5 animals per administration for gained less weight than controls. Absolute and mg/kg b.w. Barber et al.
sex per dose) 21 days at levels relative liver weight increased in all exposed per day (1987)
of 0, 0.6%, 1.2% groups. Lower testis weight in high-dose males;
or 2.5% DBP; severe atrophy observed upon histopathological
examination. Serum triglyceride and cholesterol
a positive control levels decreased in all exposed males and cholesterol
group was level reduced in all exposed females, in a
administered 1.2% non-dose-related manner. Slight reduction in
di(2-ethylhexyl) hepatocyte cytoplasmic basophilia in all rats at
phthalate; highest doses and in males at 1.2%.
Cyanide-insensitive palmitoyl CoA oxidation
dose levels increased in both sexes at the highest dose and at
(calculated by the 1.2% dose in males.
investigators and Lauric acid 11 and 12 hydroxylase activities were
presented in BIBRA significantly increased in all exposed males and
(1986)); in females in the high-dose group.
males: 0, 624,
1234, 2156 mg/kg
b.w. per day
females: 0, 632,
1261, 2107 mg/kg
b.w. per day
Table 6. Continued
Species Protocol Results Effect Levels Reference
Rat (F-344, 0.05, 0.1, 0.5, 1.0 A dose-related liver enlargement and induction of NOAEL = 104 Lake et al.
male, groups or 2.5% DBP in the palmitoyl-CoA oxidation activity were reported. mg/kg b.w. (1991) (abstract)
of 5 males) diet for 28 days Based on the enzyme activity, the no-effect level for per day
(not possible to induction of hepatic peroxisome proliferation was
present doses on a determined to be 104 mg/kg b.w. per day by the authors.
b.w. basis since
food consumption was
determined but not
reported)
Rat 0.7% DBP in the diet Hepatomegaly was noted in exposed rats. Reduction One dose group Bell et al.
(Sprague-Dawley, (equivalent to 420 in serum cholesterol levels in exposed animals and only (effects (1978)
groups of 9 mg/kg b.w. per day) inhibition in hepatic sterologenesis reducing the observed at 420
males) for 21 days uptake of 14C-mevalonate and 14C-acetate by the liver mg/kg b.w.
minces of the exposed rats. There was no effect on per day)
hepatic cholesterol levels.
Rat (Wistar, 5 mmol/kg b.w. per Increases in hepatic cytochrome P-450 levels and One dose group Aitio & Parkki
groups of 7 day (1390 mg/kg b.w. in the activities of epoxide hydratase and only (effects (1978)
males) per day) in corn oil glutathione-S-transferase. No statistically observed at
by gavage for 6 days significant increase in the catalytic activities 1390 mg/kg b.w.
dependent on cytochrome P-450 (ethoxycoumarin per day)
de-ethylation and benzo(a)pyrene hydroxylation).
Mouse (ICR, 2% DBP in the diet Food consumption was affected (data not presented) One dose group Oishi & Hiraga
groups of 10 (2400 mg/kg b.w. and b.w. gain was significantly decreased. The only (effects (1980b)
males) per day) for 1 week relative liver weight was significantly increased observed at
whereas the relative kidney weight was significantly 2400 mg/kg b.w.
reduced. The zinc concentration in the liver was per day)
reduced to 88 ± 3.14% of the control value while
that of the kidney remained unchanged.
For mice, identified data on short-term toxicity are limited
to one investigation, in which there was a significant decrease
in the relative kidney weight when ICR male mice were fed a diet
containing 2% (equivalent to 2400 mg/kg body weight per day) DBP
for 1 week (Oishi & Hiraga, 1980b). Results of histopathological
examinations were not reported.
In a short-term study, for which only an abstract was
published, Lake et al. (1991) compared the relative potentials of
several phthalates, including DBP and DEHP, to induce peroxisome
proliferation and testicular atrophy in rats. DEHP was more
potent than DBP in inducing palmitoyl-CoA oxidation activity.
The NOEL values for induction of peroxisome proliferation
activity were considered to be 52 and 104 mg/kg body weight per
day for DEHP and DBP, respectively. In contrast, the values for
testicular atrophy were 1093 and 515 mg/kg body weight per day
for DEHP and DBP, respectively.
In another study reported by Barber et al. (1987), in which
groups of five male and five female rats were administered 2.5%
DBP in the diet (1250 mg/kg body weight per day) for 21 days, the
extent of peroxisome proliferation in the males, on the basis of
electron micrographs, was equivalent to that produced by 0.6%
DEHP (300 mg/kg body weight per day). A scale of peroxisome
proliferation activity in the male rat was drawn up by the
investigators based on their own short-term results and work
published by other investigators. Relative values, based on
these studies conducted by various investigators for fenofibrate,
ciprofibrate, Wy 14,643, DEHP, DBP and aspirin were 304, 66, 44,
15, 3 and 1, respectively.
7.3 Long-term exposure
The effects of long-term exposure to DBP have been
investigated in several studies on rodents following oral
exposure; however, only limited information concerning effects
following inhalation was identified. Details of study design and
results are presented in Table 7.
In the study by Nikonorow et al. (1973), groups of 20 Wistar
rats (10 males and 10 females) were administered 120 and
1200 mg/kg body weight per day for 3 months by gavage in olive
oil. At both doses, there was a statistically significant
increase in the relative liver weight. No particular
alterations in the liver, kidneys and spleen of any rats
administered DBP were seen during gross or histological
examination. The LOEL was considered to be 120 mg/kg body weight
per day, based on the increase in relative liver weights.
A series of three subchronic dietary studies have been
published recently (NTP, 1995):
Table 7. Long-term toxicity of DBP
Species Protocol Results Effect Levels Reference
Oral
Rat (Wistar, 120 or 1200 mg/kg b.w. per Clinical signs of toxicity were not described. LOEL = 120 Nikonorow et
groups of 10 day in olive oil by gavage One rat from the high-dose group died but the mg/kg b.w. al. (1973)
males and 10 daily for 3 months death was not considered to be treatment-related per day
females) (sex not specified). At necropsy, the only
effect noted was hepatomegaly at both doses.
No gross or microscopic changes were noted in
the spleen or kidneys.
Rat (strain 2300 mg/kg b.w. per day There was a reduction in the rate of weight one dose group Radeva &
and sex administered by gavage gain from day 1 onwards, but no other only (reduction Dinoyeva
unspecified, (vehicle unspecified) for clinical signs of oxicity were noted and no in weight gain (1966)
groups of 8; 50 days deaths occurred. No other end-points were at 2300 mg/kg
5 as controls) reported. b.w. per day)
Rat (strain diets containing levels There were no clinical signs of toxicity and cannot be Radeva &
not specified, equivalent to 0.1, 1 and no haematological abnormalities. Urine determined Dinoyeva
groups of 8 10 mg/kg b.w. per day for analyses for hippuric acid, albumin and (1966)
males) 7 months; controls sediment contents were normal. Marked
received the vehicle used venous congestion in some exposed rats at
to produce the feed mix, necropsy was reported, but the organ and
sunflower oil, in the diet dose group(s) in which it occurred were not
specified. No other compound-related lesions
were noted.
Table 7. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, 5% DBP in the diet Reduction in b.w. gain during the first week, one dose group Murakami
groups of 5 (equivalent to a dose of followed by a plateau, which was 65% of the only (effects et al.
males) 2500 mg/kg b.w. per day) control value on the 35th day. There were observed at 2500 (1986b)
for 35 to 45 days significant increases in relative liver and mg/kg b.w. per
spleen weights but no significant changes in day)
absolute weight. In addition, there was
marked atrophy of the testicles. There was
also depressed respiration in liver
mitochondria when succinate or pyruvate was
used as a substrate. Hepatic glutamate
dehydrogenase activity was decreased by 73%
of the control value but this was not
significant. Succinate and pyruvate
dehydrogenase activities were significantly
decreased (by 59% and 38% of the control
values, respectively).
Table 7. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, 0.5% or 5% in the diet B.w., expressed as percentage of weight in LOAEL = 250 Murakami
groups of 5 (equivalent to 250 or the control group, decreased gradually in mg/kg b.w. et al.
males) 2500 mg/kg b.w. per day, both groups. There were significant per day (1986a)
respectively) for 34 to increases in the relative weights of the
36 days liver, kidney and spleen, and decreases in
the weight of the testicles in the high-dose
group. The succinate and pyruvate
dehydrogenase activities in liver
mitochondria were significantly inhibited at
the high dose, but not the glutamate
dehydrogenase activity. The activities of
AP, GOT and GPT increased in rats that
received the high dose. Decreased globulin
and increased albumin/globulin ratio were
observed in both dose groups. In the
liver, cytotoxic injury including single
cell necrosis, zonal necrosis and degeneration
with ballooning were observed in many of the
rats that received the high dose. Zonal
necrosis and liver atrophy were observed in
the low-dose group. Ultrastructural
examination of liver cells revealed that the
effects of exposure to the high dose were more
extensive than the low dose in increasing
peroxisomes, lysosomes and mitochondria.
Table 7. Continued
Species Protocol Results Effect Levels Reference
F-344 rat; This study has been designated Final b.w.: reduction in males at >10 000 LOEL = 356 NTP (1995)
10 per sex "NTP Study 2"; administration mg/kg and in females at >20 000 mg/kg mg/kg b.w.
per group in diet for 13 weeks at 0, Organ weights: hepatomegaly in males at per day
2500, 5000, 10 000, 20 000 or >5000 mg/kg and in females at >10 000 mg/kg; (peroxisomal
40 000 mg/kg; mean equivalent testis and epididymal weights lower at 20 000 proliferation);
dose levels based on and 40 000 mg/kg 720 mg/kg b.w.
consumption and b.w.s: Haematology: minimal anaemia in males at per day based
females: 0, 177, 356, 712, >5000 mg/kg on germinal
1413 or 2943 mg/kg b.w. Clinical chemistry: hypocholesterolaemia in epithelial
males: 0, 176, 359, 720, 1540 both sexes at 20 000 and 40 000 mg/kg; atrophy in the
or 2964 mg/kg b.w. hypotriglyceridaemia in all exposed males and testes and
females at >10 000 mg/kg; elevations in histopathological
alkaline phosphatase activity and bile acid lesions in the
concentration in both sexes was considered liver
indicative of cholestasis
NOEL = 177
mg/kg b.w.
per day
Table 7. Continued
Species Protocol Results Effect Levels Reference
F-344 rat; Histopathology: NTP (1995)
10 per sex Liver: hepatocellular cytoplasmic alterations
per group consistent with glycogen depletion in both sexes
at >10 000 mg/kg; in both sexes at
40 000 mg/kg, small, fine,
eosinophilic granules in cytoplasm of
hepatocytes; ultrastructural examination
showed the presence of increased numbers
of peroxisomes, and peroxisomal enzyme
activity was elevated in liver of both
sexes at >5000 mg/kg (enzyme activity at
40 000 mg/kg was 13-fold greater than
controls for males and 32-fold greater
than controls for females)
Testes: degeneration of germinal epithelium,
mild to marked focal lesions at 10 000 and
20 000 mg/kg and marked, diffuse lesion in all
animals at 40 000 mg/kg; almost complete loss
of germinal epithelium at 40 000 mg/kg;
testicular zinc concentrations lower at 20 000
and 40 000 mg/kg; serum testosterone lower
than controls at 20 000 and 40 000 mg/kg; at
20 000 mg/kg, spermatid heads per testis and
per gram testis, epididymal spermatozoal
motility, and the number of epididymal
spermatozoa per gram epididymis were lower than
controls.
Table 7. Continued
Species Protocol Results Effect Levels Reference
F-344 rat This study has been designated 10 control and 10 exposed pups per sex were LOEL for hepatic NTP (1995)
"NTP Study 3" examined at weaning; hepatomegaly and peroxisomal
markedly increased peroxisomal enzyme proliferation and
Combined perinatal and activities (19-fold greater than control hepatomegaly is
subchronic exposure. An values) were observed. 279 mg/kg b.w. per
unspecified number of dams was Body weight gain: dose-related decrease day for males and
administered 10 000 mg/kg in significant in males in all dose groups and 593 mg/kg b.w. per
the diet beginning at day one in females at >20 000 mg/kg day for females;
of gestation, throughout Organ weights: hepatomegaly in males at NOEL is 138 mg/kg
gestation, and until weaning. >5000 mg/kg and in females at >2500 mg/kg; b.w. per day for
Pups had no exposure for four lower testes weight at >20 000 mg/kg; lower males and 294 mg/kg
weeks post-weaning. Ten pups epididymal weight at 20 000 mg/kg b.w. per day for
per sex per group were then Haematology: mild anaemia in males at females.
administered 2500, 5000, >10 000 mg/kg and in females at 40 000 mg/kg
10 000, 20 000 or 40 000 mg/kg Clinical chemistry: hypocholesterolaemia in
in the diet for 13 weeks. There both sexes at higher concentrations;
were two control groups - hypotriglyceridaemia in females at 20 000
animals which had been and 40 000 mg/kg and in males at >10 000
perinatally exposed and those mg/kg; elevations in alkaline phosphatase
with no exposure. activity and bile acid concentrations in
both sexes at 20 000 and 40 000 mg/kg were
indicative of cholestasis
Table 7. Continued
Species Protocol Results Effect Levels Reference
F-344 rat Mean equivalent dose levels Histopathology, liver: hepatocellular testicular NTP (1995)
based on consumption and body cytoplasmic alteration, consistent with germinal
weights: glycogen depletion, in both sexes at epithelium
females: 0, 147, 294, 593, 1182 >10 000 mg/kg; small, fine, eosinophilic degeneration
and 2445 mg/kg b.w. per day granules in cytoplasm of hepatocytes in observed at 571
males: 0, 138, 279, 571, 1262 males at 40 000 mg/kg; ultrastructural mg/kg b.w. per
and 2495 mg/kg b.w. per day examination showed the presence of day
increased numbers of peroxisomes at the
highest dose; peroxisomal enzyme activity
increased in males at >5000 mg/kg and in
females at >10 000 mg/kg (at 40 000 mg/kg,
activities were 20-fold higher than in
controls) testes: degeneration of germinal
epithelium, mild to moderate focal lesion
at 10 000 and 20 000 mg/kg, and marked,
diffuse lesion at 40 000 mg/kg; almost
complete loss of germinal epithelium at
40 000 mg/kg; testicular zinc concentrations
lower at 40 000 mg/kg; at 20 000 mg/kg,
there were fewer spermatid heads per testis
than unexposed controls and epididymal
spermatozoal concentrations were less than
in perinatally exposed controls
Rat, ingestion of diets containing half of the animals at the highest dose died Smith (1953)
Sprague-Dawley, 0.01, 0.05, 0.25 or 1.25% DBP during the first week
10 males per for 1 year
group haematology: no abnormalities at 3, 6, 9
equivalent doses: 6, 30, 150 months or at necropsy
or 750 mg/kg b.w. per day
no gross or microscopic changes in lung,
heart, liver, spleen, adrenals, kidneys,
stomach, small intestine, thyroid or brain.
Table 7. Continued
Species Protocol Results Effect Levels Reference
Rat, Wistar, ingestion of diet containing 0 15% of exposed rats died Nikonorow
20 males and 20 or 0.125% DBP for 1 year; et al. (1973)
females per no gross or histological changes in liver,
group equivalent dose: 0 or 75 kidney or spleen
mg/kg/b.w. per day
Mouse (ddy, 0.25% or 2.5% in diet for 86 remarkable vacuolar degeneration and LOAEL = 500 Ota et al.
groups of 3 or 90 days; (500 or 5000 mg/kg necrosis of single cells in the liver, and mg/kg b.w. (1973, 1974)
males and 12 b.w. per day) cysts and degeneration of epithelial cells per day
females) in the renal tubules in the high-dose group;
in the low-dose group, histological changes
were slight in the liver and kidney but
degeneration of parenchyma was observed
B6C3F1 mice; 10 administration in diet for 13 Mean body weight and body weight gain: decreased LOEL = 812 NTP (1994a,b;
per sex per weeks (0, 1250, 2500, 5000, in both sexes in a dose-related manner; decreases mg/kg b.w. 1995)
group 10 000 or 20 000 mg/kg); were significant at >5000 mg/kg per day, based
mean equivalent dose levels Relative liver weight: greater in both sexes at on decreases
based on consumption and body >5000 mg/kg in body weight
weights: Haematology: minimal anaemia was suggested in in both sexes
females: 0, 238, 486, 971, females at 20 000 mg/kg and increases
2137, or 4278 mg/kg b.w. per Histopathology: hepatocellular cytoplasmic in relative
day alterations, consistent with glycogen depletion, liver weight
males: 0, 163, 353, 812, 1601 in males at 10 000 and 20 000 mg/kg and females (NTP, 1995)
or 3689 mg/kg b.w. per day at 20 000 mg/kg; small, fine, eosinophilic
granules consistent with peroxisome proliferation NOEL = 486
were observed in the cytoplasm of hepatocytes in mg/kg b.w. per
both sexes at 20 000 mg/kg. day
Table 7. Continued
Species Protocol Results Effect Levels Reference
Inhalation
Rat (strain, 900 ± 80 mg/m3, 6 h per day Reduced body weights in exposed animals at the one dose group Antonyuk &
number and sex for 35 days end of the study (initial 173 g, final 151 g) only (effects Aldyreva
not specified) compared with controls (initial 174 g; final observed at (1973)
223 g). A decrease in haemoglobin content of 900 mg/m3)
peripheral blood was observed on day 10 but there
was a slight increase over control values by day
30. A decrease in phagocytic ability of
peripheral blood neutrophils was also noted.
There were statistically significant increases
in the weight of the liver, lungs, kidneys,
adrenals and brain, but data were not presented.
Mouse (strain range of 20 to 85 mg/m3 for 86 At the end of the study period, pulmonary could not be Spasovski
and sex not days. For a further 6 days, oedema was observed. No other end-points determined (1964)
specified, the concentration was increased were reported.
groups of 15) to a range of 170 to 420 mg/m3
Table 7. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, 0.5 mg/m3 (0.04 mg/kg) or A reduced rate of body weight gain was noted LOEL = 0.5 Kawano
groups of 11 to 50 mg/m3 (4.4 mg/kg) vapour, in rats administered the high concentration. mg/m3 (1980a)
14 males) 6 h per day except 1 day per There were no major differences between controls
week when exposure was for and exposed animals with respect to red cells,
3 h, 6 days/week for up to 6 platelets, haemoglobin concentration, haematocrit,
months. The levels of DBP lymphocytes, and neutrophils at 3 months but there
were checked throughout the was a reduction in lymphocyte numbers with an
study and varied between 45.0 increase in neutrophil numbers in exposed animals
and 59.2 mg/m3 in the high at 6 months. There were slight increases in
concentration and 0.31 and serum aspartate aminotransferase, alanine
0.56 mg/m3 in the low aminotransferase and alkaline phosphatase levels
concentration chambers. at 1, 3 and 6 months, and in blood glucose level
at 6 months in animals exposed to both
concentrations. At 6 months, serum cholesterol
had fallen slightly but serum triglyceride levels
had risen. The effects in the rats in the
low-concentration group were similar, but less
pronounced. In the high-concentration group, the
relative organ weights (brain, lung, liver,
kidney, testes) were normal at the end of the
first month, but after 6 months the relative
weights of the brain, lung, kidney and testes had
all increased.
Rat (strain 0.095, 0.25 and 1 mg/m3, 24 h No clinical signs of toxicity were noted during NOEL = 1 mg/m3 Men'shikova
unspecified, per day for 93 days the study; animals appeared healthy and the rate (1971)
groups of 15 of body weight gain for treated animals was
males) similar to controls. No abnormalities in
haemoglobin or red blood cell counts based on
haematological examinations, which were carried
out monthly. White cell counts fell during
exposure and these did not return to normal in a
group of animals observed for 6 months after
termination of exposure.
NTP study 1: prenatal/postnatal dose range-finding study in F-344
rats (described in section 7.5)
NTP study 2: conventional 13 week study in F-344 rats
NTP study 3: prenatal/postnatal exposure followed by a 13-week
exposure with two control groups (one with and one without
prenatal/postnatal exposure) in F-344 rats
NTP study 4: continuous breeding protocol in Sprague Dawley rats
(described in section 7.5)
No deaths occurred in the 13-week dietary study (NTP study 2
in rats) in which groups of 10 F-344 rats received 0, 2500, 5000,
10 000, 20 000 or 40 000 mg/kg diet. Average equivalent doses
were 0, 177, 356, 712, 1413 or 2943 mg/kg body weight per day for
females and 0, 176, 359, 720, 1540 or 2964 mg/kg body weight per
day for males (NTP, 1995). The final body weights of males
receiving approximately > 720 mg/kg body weight per day and
females receiving approximately > 1413 mg/kg body weight per
day were less than those of controls. No overt hepatic necrosis
or inflammation was observed at any dose. Hepatomegaly was
observed in males exposed to approximately > 359 mg/kg body
weight per day and females exposed to > 712 mg/kg body weight
per day. Testis and epididymal weights were less than those of
controls in animals exposed to > 1540 mg/kg body weight.
Histopathological examination of the liver revealed
hepatocellular cytoplasmic alterations, consistent with glycogen
depletion, in both sexes at >10 000 mg/kg diet. At 40 000
mg/kg diet, eosinophilic granules were observed in hepatocellular
cytoplasm. Upon ultrastructural examination, increased numbers of
peroxisomes were observed, and peroxisomal enzyme activity was
elevated in the liver of both sexes at > 5000 mg/kg diet.
Hepatic peroxisomal enzyme activity at the highest dose was 13-
and 32- fold greater than controls in males and females,
respectively. Examination of the testes revealed degeneration of
the germinal epithelium, mild to marked focal lesions at
> 720 mg/kg body weight, and a marked, diffuse lesion in all
males at 2964 mg/kg body weight per day. There was an almost
complete loss of the germinal epithelium at this dose.
Concentrations of testicular zinc and serum testosterone were
less than those of controls at > 1540 mg/kg body weight per
day. At 2400 mg/kg body weight per day, spermatid heads per
testis and per gram testis, epididymal spermatozoal motility, and
the number of epididymal spermatozoa per gram epididymis were
less than that in controls.
The only target tissues identified, therefore, in this study
were the liver and testes. The LOEL and NOEL values in this study
for hepatic peroxisomal proliferation and hepatomegaly were 356
and 176 mg/kg body weight per day, respectively. Testicular
germinal epithelial degeneration was observed at higher doses
(720 mg/kg body weight per day).
In a NTP study 3, pregnant F-344 rats were administered 0 or
10 000 mg/kg in the diet during gestation and lactation, and
weaned pups were administered the same diets as their dams
received for an additional 4 weeks until the beginning of the 13-
week exposure phase (NTP, 1995). The offspring then received 0,
2500, 5000, 10 000, 20 000 or 40 000 mg/kg diet (equivalent to
mean doses of 0, 147, 294, 593, 1182 and 2445 mg/kg body weight
per day for females and 0, 138, 279, 571, 1262 and 2495 mg/kg
body weight per day for males) for 13 weeks.
Ten control and 10 exposed pups of each sex were examined at
weaning. Hepatomegaly was observed in exposed pups, and
peroxisomal enzyme activity was 19-fold greater than in controls.
The body weight of prenatally/perinatally exposed pups was less
than that of controls throughout the 4-week period prior to the
13-week adult exposures. At the end of the 13-week exposure,
statistically significant changes in prenatally/perinatally
exposed controls versus unexposed controls were limited to
increased relative testis weight and lower final body weight in
males. The Task Group felt that the appropriate comparison was
to the pretreated control groups.
At the end of the 13-week exposure, body weights of males in
all exposed groups and of females at > 593 mg/kg body weight
per day were less than those in unexposed controls. No overt
hepatic necrosis or inflammation was observed at any dose. In
adult rats, hepatomegaly was observed in males at > 279 mg/kg
body weight per day and in females at > 593 mg/kg body weight
per day. There was hepatocellular cytoplasmic alteration
consistent with glycogen depletion in both sexes at >
10 000 mg/kg diet. Marked elevations of peroxisomal enzyme
activity were detected in males receiving > 279 mg/kg body
weight per day and in females receiving > 593 mg/kg body
weight per day.
At > 1262 mg/kg body weight per day, testis weight was
lower than that in controls. There was mild to moderate
degeneration of the germinal epithelium at > 571 mg/kg body
weight per day and marked diffuse germinal epithelial
degeneration at 2495 mg/kg body weight per day, at which dose an
almost complete loss of the germinal epithelium resulted. At the
highest dose, testicular zinc concentration was reduced, there
were fewer spermatid heads per testis than in unexposed controls,
and epididymal spermatozoal concentration was less than that in
the prenatally perinatally exposed controls.
The only target tissues identified, therefore, in this study
were the liver and testes. The LOEL and NOEL values in this study
for hepatic peroxisomal proliferation and hepatomegaly were 279
and 138 mg/kg body weight per day in males and 593 and 294 mg/kg
body weight per day in females. Testicular germinal epithelial
degeneration was observed at higher doses (571 mg/kg body weight
per day).
In other studies, male Wistar rats were administered 5% DBP
in the diet (equivalent to 2500 mg/kg body weight per day) for 35
to 45 days (Murakami et al., 1986b) or 0.5 or 5% (equivalent to
250 or 2500 mg/kg body weight per day) for 34 to 36 days
(Murakami et al., 1986a). In the rats ingesting the diet
containing 5% DBP, there was growth depression, liver
enlargement, testicular atrophy, decreased activities of
succinate and pyruvate dehydrogenase in liver mitochrondria and
abnormal changes in biochemical tests of serum and in
histological examinations of the liver and testes. Hepatic
lesions (including necrosis and atrophy) were also observed in
rats fed the diet containing 0.5% DBP, although these lesions
were less severe than those reported in the high-dose group.
There were changes in hepatocellular ultrastructure in rats
exposed to DBP, which were related to increases in peroxisomes,
lysosomes and mitochondria. A NOEL could not be established on
the basis of this study; the LOAEL was 250 mg/kg body weight per
day, based on liver pathology (Murakami et al., 1986b).
Additional mechanistic studies into DBP-related effects on
hepatic cell proliferation, peroxisomal enzyme activities,
clinical chemistry, haematology and gene expression are being
undertaken in a 90-day feed study by the National Toxicology
Program, but published reports are not yet available (personal
communication by R.R. Maronpot, National Institute of
Environmental Health Sciences, to the IPCS, 1995).
Little information on repeated dose toxicity in rats
following ingestion for periods longer than 3 months has been
identified. In an early study (Smith, 1953), groups of 10 male
Sprague-Dawley rats ingested diets containing 0.01, 0.05, 0.25 or
1.25% DBP (equivalent to 6, 30, 150 or 750 mg/kg body weight per
day) for 1 year. There were no effects on growth, but half of
the exposed animals administered the highest dietary level
(750 mg/kg body weight per day) died during the first week of the
study. No abnormalities were seen during examination of
haematological parameters at 3, 6 and 9 months, and at necropsy
there were no abnormal gross or microscopic findings in any of
the organs examined, which included the lung, heart, liver,
spleen, adrenals, kidneys, stomach, small intestine, thyroid and
the brain.
In another 12-month dietary study (Nikonorow et al., 1973),
groups of 20 male and 20 female Wistar rats ingested a diet
containing 0.125% of DBP (equivalent to 75 mg/kg body weight per
day). There were marked differences in food intake in exposed
animals, compared to controls, and 15% of the exposed rats died.
No alterations in the liver, kidneys or spleen were seen during
gross and histological examination.
In an inhalation study, effects at lowest levels were
observed by Kawano (1980b). In this investigation, rats were
exposed to 0.5 or 50 mg/m3 of DBP mist for 6 h/day, 5 days a
week, for up to 6 months (except for one day/week when rats were
exposed for only 3 h). There was a reduction in the rate of body
weight gain and increases in the relative weights of the brain
and lung. An increase in the percentage of neutrophils was
observed in both exposed groups. High levels of urea nitrogen
and low levels of cholesterol and triglyceride in the serum of
rats exposed to the high concentration were observed, indicating
hypolipidaemic activity of DBP. Similar, but less pronounced,
effects were observed in the group exposed to the low
concentration. In other identified studies, no effects were
observed following exposure for 93 days to 1 mg/m3 (Men'shikova,
1971), whereas effects on body weight gain, organ weights and
haematological parameters were observed at a high concentration
(900 mg/m3) following exposure for 35 days (Antonyuk &
Aldyreva, 1973).
In small groups of male and female ddY mice given a diet
containing 500 or 5000 mg/kg body weight per day for 3 months,
there were marked lesions in the liver and kidney (Ota et al.,
1973, 1974). In the high-dose group, there was remarkable
vacuolar degeneration and necrosis of single cells in the liver,
and cysts and degeneration of tubular epithelial cells in the
kidney. In the low-dose group, histological changes were slight
in the liver and kidney but degeneration of the parenchyma was
observed. The LOAEL was considered to be 500 mg/kg body weight
per day on the basis of histological changes in the liver and
kidneys.
B6C3F1 mice received dietary concentrations of 0, 1250,
2500, 5000, 10 000 or 20 000 mg/kg diet for 13 weeks (equivalent
to mean doses of 0, 238, 486, 971, 2137, or 4278 mg/kg body
weight per day in females and 0, 163, 353, 812, 1601 or
3689 mg/kg body weight per day in males (NTP, 1995). Body weight
and body weight gain were significantly decreased and relative
liver weight was significantly increased in both sexes at
> 5000 mg/kg diet. No overt hepatic necrosis was observed.
Histopathological examination revealed hepatocellular cytoplasmic
alterations, consistent with glycogen depletion, in males at
>1601 mg/kg body weight per day and in females at 4278 mg/kg
body weight per day. Eosinophilic granules, consistent with
peroxisomal proliferation, were observed in the cytoplasm of
hepatocytes in both sexes at 20 000 mg/kg diet. The LOEL in this
study was 812 mg/kg body weight per day, based on decreases in
body weight in both sexes and increases in relative liver weight
(NOEL = 353 mg/kg body weight per day). In contrast to rats,
there were no histological alterations in the testes of mice
exposed to any dose of DBP.
In summary, the effects in rats following ingestion of DBP
for periods of up to 13 weeks include reduced rate of weight gain
at doses of >250 mg/kg body weight per day (Radeva & Dinoyeva,
1966; Murakami et al., 1986a; NTP, 1995). Increase in relative
liver weights have been observed at doses of >120 mg/kg body
weight per day (Nikonorow et al., 1973; Murakami et al., 1986a;
1986b; NTP, 1995). Peroxisomal proliferation as determined by
increased peroxisomal enzyme activity has been observed at doses
of >279 mg/kg body weight per day (NTP, 1995). Although in
Fischer rats, no overt pathological effects on the liver were
observed (NTP, 1995), necrotic hepatic changes in Wistar rats
have been reported at doses of >250 mg/kg body weight per day
(Murakami et al., 1986a). Effects on the testes of male rats
have been observed at doses of >571 mg/kg body weight per day
(NTP, 1995).
In summary, histopathological lesions in the kidney and
liver were observed in mice in a limited study at DBP doses of
> 500 mg/kg body weight per day for 3 months (Ota et al.,
1973, 1974). Effects on body and organ weights and histological
alterations in the liver were also reported at higher doses in a
subchronic bioassay on mice (NTP, 1995) for which the NOEL was
353 mg/kg body weight per day, which was the lower of the two
NOELs in males and females (NTP, 1995).
In rats given a diet containing 5% MBP (the principal
metabolite of DBP), growth depression, liver enlargement,
testicular atrophy, decreased activities of succinate and
pyruvate dehydrogenase in liver mitochrondria, biochemical
effects on serum and histopathological effects on the liver and
testes were noted (Murakami et al., 1986a). Hepatic necrosis was
also observed in rats fed a diet containing 0.5% MBP (250 mg/kg
body weight per day), although it was less severe than in the
rats administered the higher concentration. The changes in
hepatocellular ultrastructure were more prominent in rats exposed
to DBP than in those administered MBP.
7.4 Irritation and sensitization
DBP appears to have little potential to irritate skin. Very
slight skin irritation, but no skin sensitization, was seen when
over 4 mg/kg body weight per day was applied to the skin of
rabbits for 90 days (Lehman, 1955). The ability of DBP to induce
an inflammatory response based on extravasation of trypan blue
has also been examined in rabbits (Calley et al., 1966). An
inflammatory response was observed but no details were given as
to whether there was any clinical irritancy.
No irritation was noted after DBP was instilled into the
eyes of rabbits examined at intervals up to 48 h after
application (concentration or amount not specified) (Lawrence
et al., 1975).
7.5 Reproductive and developmental toxicity
7.5.1 Reproductive effects
7.5.1.1 Testicular effects
Studies on the reproductive effects of DBP are summarized in
Table 8. Repeated oral exposure to concentrations of DBP for 4
to 90 days (250 to 2600 mg/kg body weight per day) affects the
reproductive system of male rodents. However, there are
considerable interspecies differences in response. Observed
effects in the available studies, most of which only used one
dose level (generally in the range 1200 to 2400 mg/kg body weight
per day), included marked reductions in the weights of the testes
and accessory sex glands, decreased numbers of spermatocytes,
degeneration of the seminiferous tubules of the testes, a
reduction in testicular zinc and iron levels and serum
testosterone levels, an increase in testosterone levels in the
testes, sloughing of germ cells, decreased activity of succinate
dehydrogenase in Sertoli cells, and an increase in urinary zinc
excretion at doses of >250 mg/kg body weight per day (Cater et
al., 1977; Gray & Butterworth, 1980; Oishi & Hiraga, 1980a,
1980b; Gray et al., 1982; Ikemoto et al., 1983; Fukuoka et al.,
1989, 1990; Zhou et al., 1990; Srivastava et al., 1990a,b; Lake
et al., 1991; Fukuoka et al., 1993; NTP, 1995).
The lowest reported effect levels in sufficiently well-
documented studies were those in a multi-dose investigation in
which DBP in groundnut oil (250, 500 or 1000 mg/kg body weight
per day) was administered to young male rats by gavage for 15
days (Srivastava et al., 1990a,b). A significant decrease in the
weight of the testes was observed at 500 and 1000 mg/kg body
weight per day. At these two doses, histopathological
examination revealed marked degeneration of the seminiferous
tubules. In all exposed groups, the activities of testicular
enzymes associated with post-meiotic spermatogenic cells, such as
sorbitol dehydrogenase and acid phosphatase, were decreased
significantly (P < 0.05), while that of testicular specific
lactate dehydrogenase was significantly increased, coincident
with degeneration of spermatogenic cells. The activities of
enzymes associated with pre-meiotic spermatogenic cells, Sertoli
cells or interstitial cells, and of ß-glucuronidase,
gamma-glutamyl-transpeptidase and glucose-6-phosphate
dehydrogenase were significantly increased (P < 0.05).
Therefore, the LOEL was 250 mg/kg body weight per day based on
enzyme changes in the testes; this value was also the NOAEL for
testicular weight and histopathological changes.
Table 8. Reproductive effects of DBP
Species Protocol Results Effect Levels Reference
Rat 500, 1000 or 2000 mg/kg In the first study, decreases in testicular LOEL = 500 mg/kg Cater et al.
(Sprague-Dawley, b.w. per day DBP by weight at the two highest doses (p<0.01 and b.w. per day in (1977)
groups of 6 gavage in corn oil daily p<0.001) after 4 days; the weight decreased one experiment.
males, 3 to 4 for 4 or 6 days in one further after 8 days at all 3 doses (p<0.05 One dose group
weeks old) experiment. 2000 mg/kg at 500 mg/kg b.w. per day and p<0.001 at the only in the other
b.w. per day by gavage two highest doses). In the second study, body experiment
in corn oil daily for weight gain also declined but the change was not (effects observed
periods of up to 14 days significant. Diminution of both spermatocyte at 2000 mg/kg
in a second experiment. and spermatogonia counts upon histological b.w. per day)
For urinary zinc examination of testes after 4 days of exposure
measurements, zinc-65 to 2000 mg/kg b.w. per day. At 2000 mg/kg b.w.
chloride was administered per day, urinary zinc excretion was increased
and the zinc-65 content by 34 to 43% over the first 4 days and then
was estimated by returned to normal. In the testes, the turnover
radioactive counting. rate of zinc was increased, the half-life was
reduced from 14 to 5 days and the zinc levels
were significantly reduced in the testes.
There was no change in zinc half-life or content
in liver or kidneys. Specific activity of
testicular alcohol dehydrogenase (or
zinc-dependent enzyme) decreased to 20 to 40%
of control after 5 days of exposure. A 3-day
pretreatment with 2000 mg/kg b.w. per day
caused a 25% decrease (p<0.001) in testicular
zinc uptake in vivo. Simultaneous ingestion of
zinc sulfate resulted in no testicular atrophy,
as measured by relative testicular weight (no
histopathological examinations).
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat 2% in the diet Mean body weights were slightly but not One dose group Oishi &
(JCL:Wistar, (equivalent to 2060 significantly lower than that of the controls. only (effects Hiraga
groups of 10 mg/kg b.w. per day) Absolute and relative testicular weights were observed at 2060 (1980a)
males, 5 weeks for 1 week. significantly decreased, and both absolute and mg/kg b.w. per
old) relative liver weights were significantly day)
increased. Histological examination of the
testes revealed a decrease in both spermatocyte
and spermatogonia counts. Zinc concentrations
in the testes and the liver were significantly
decreased. Testosterone concentration in the
testes was significantly increased.
Rat (Wistar, 2000 mg/kg b.w. per day Decreases in the relative weight of the testes, One dose group Gray &
groups of 10 by oral intubation in corn prostate and seminal vesicles were reported only (effects Butterworth
males, 4 weeks oil daily for 10 days. (data not presented). Testicular atrophy was observed at 2000 (1980)
old observed. mg/kg b.w. per day)
Rat 2000 mg/kg b.w. per day There was no change in body weight. Weight of One dose group Gray et al.
(Sprague-Dawley, by oral intubation in the testes was reduced to 45% of control only (effects (1982)
groups of 6 corn oil daily for 9 days. (p<0.001), and >90% tubular atrophy was seen observed at 2000
males, 4 to 6 in all animals upon histological examination. mg/kg b.w. per day)
weeks old)
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, 2400 mg/kg b.w. per day Severe testicular atrophy was evident; the weight One dose group Ikemoto et
groups of 5 administered by gavage of the testes was 0.59 g compared with 0.97 g in only (effects al. (1983)
rats, 5-week (vehicle unspecified) controls. Relative organ weights were not observed at 2400 (English
old males) daily for 7 days. presented and body weights were presented mg/kg b.w. per day) abstract)
Animals were killed graphically, only. Based on these graphs, the
on the day after relative weight of the testes was approximately
administration terminated. 0.29% compared with 0.48% for controls. Upon
microscopic examination, there was an almost
complete absence of germ cells in the
seminiferous tubules, with enlargement and
vacuolation of the Sertoli cells. These cells
had increased numbers of lipid droplets. The
Leydig cells also appeared atrophied. The gross
and microscopic changes were accompanied by a
decrease in the serum testosterone levels to 82%
of control values.
Rat (Wistar, 2400 mg/kg b.w. per day Decreases in testicular fructose and glucose One dose group Fukuoka et
28 adult males) administered by gavage levels and a sloughing of the germ cells on the only (effects al. (1989)
(neat DBP) daily for 7 first day of exposure. On day 2, more severe observed at 2400
days after acclimatization sloughing, accompanied by decreases in testicular mg/kg b.w. per day)
for 1 week. Groups of 6 iron and zinc levels and increases in the level
exposed rats were killed at of inositol and cholesterol. The sloughing was
24, 48, 120 or 168 h and 2 followed by atrophy, accompanied by dissociation
were killed at 72 and 96 h. of the germ cells from the Sertoli cells and
reduction of triglycerides, cholesterol and
phospholipids containing choline and ethanolamine
residues in the testis.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, Single oral dose of 2400 Based on histological examination, DBP caused One dose group Fukuoka et
27 adult males) mg/kg b.w. per day sloughing of the germ cells at 6 h. On days 1 only (effects al. (1990)
administered by gavage and 2, there was more severe sloughing, followed observed at 2400
(neat DBP) after by atrophy and the dissociation of the germ cells mg/kg b.w. per day)
acclimatization for 1 week. from the Sertoli cells and the spermatogonia.
Groups of 3 exposed rats Biochemically, there was an elevation of
were killed at 3, 6, 12, 24, gamma-glutamyl transferase, a decrease in sorbitol
48, 72, 96, 120 and 168 h. levels at 3 h up to the 7th day and a decrease in
the activity of aldose reductase at 6 h in the
testes of treated rats. This was followed by
decreases in fructose levels and increases in the
activity of lactate dehydrogenase (LDH) and in
lactate levels at 12 h, and decreases in the
activities of sorbitol dehydrogenase and succinate
dehydrogenase on day 2. LDH isoenzymes 4 and 5
increased at 6 h prior to the increase in lactate
levels. The data are consistent with DBP-induced
testicular toxicity being associated with a
disturbance of the activity of the enzymes that
are linked with Sertoli cell function and
replication and germ cell maturation.
Rat (Wistar All treated groups Mono-n-butyl phthalate (MBP) (metabolite of DBP) One dose group Zhou et
adult male) administered neat DBP as was transported through the blood-tubular barrier only (effects al. (1990)
a single oral dose of 2400 onto the seminiferous lumen; it was incorporated observed at 2400
mg/kg b.w. Control rats into the lumen at a maximum rate between 1 and 3 h mg/kg b.w.)
administered 0.9% saline after dosing. MBP caused decreases in the
activities of succinate dehydrogenase in the
Rat (18) Experiment A Sertoli cells and sorbitol dehydrogenase in the
Study of transportation of germ cells, an increase in the activity of lactate
DBP from interstitial cell dehydrogenase in the germ cells and in the
fraction to Sertoli cells seminiferous lumen and a decrease in testicular
and germ cells. Three rats iron levels.
sacrificed at 1, 3, 6, 12,
24 and 48 h.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (15 exposed Experiment B Zhou et
and 15 control) Determination of enzyme al. (1990)
activities in separated
cell fractions. Three rats
in each group sacrificed at
3, 6, 12, 24 and 48 h
Rat (27 exposed Experiment C
and 15 control) Measurement of metal ions in
testes. Three exposed rats
sacrificed at 3, 6, 12, 24,
48, 72, 96, 120 and 168 h.
Three control rats sacrificed
at 3, 24, 46, 96 and 168 h.
Rat (Wistar, Single oral dose, 2400 At 6 h: sloughing of germ cells; decrease in One dose group Fukuota et
adult male) mg/kg b.w. Control rats activity of succinate dehydrogenase in the only (effects al. (1993)
received 0.9% saline. Sertoli cells and in the Sertoli-germ connection; observed at 2400
Serial sacrifice of 3 rats increase in activity of lactate dehydrogenase in mg/kg b.w.)
at each of 1, 3 and 6 h. germ cells. Increases in transferrin
concentrations in Sertoli cells, Sertoli-germ
connection, epididymus-ductus deferens and liver.
Decrease in transferrin in seminal vesicle.
Decrease in ferritin in seminiferous lumen.
Increase in flavin adenine dinucleotide level in
interstitial cells.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar 0, 250, 500 or 1000 mg/kg Significant decrease in testicular weight at 500 LOAEL = 250 mg/kg Srivastava
albino, groups b.w. per day by gavage in and 1000 mg/kg b.w. per day. Histopathological b.w. per day et al.
of 6 males, ground nut oil daily for examination revealed marked degeneration of (1990a,b)
5 weeks old) 15 days. seminiferous tubules at these doses. In all
exposed groups, the activities of testicular
enzymes associated with post-meiotic spermatogenic
cells, such as sorbitol dehydrogenase and acid
phosphatase, were decreased significantly, while
that of lactate dehydrogenase was significantly
increased, coincident with degeneration of
spermatogenic cells. The activities of enzymes
associated with premeiotic spermatogenic cells,
Sertoli cells or interstitial cells,
-glucuronidase, gamma-glutamyl transpeptidase and
glucose-6-phosphate dehydrogenase were also
significantly increased in all exposed groups.
Rat (F-344, 0, 0.05, 0.1, 0.5, 1.0 or Based on histological changes and data on organ NOEL = 515 mg/kg Lake et al.
groups of 5 2.5% in the diet for 28 weights, the authors concluded that the NOEL for b.w. per day (1991)
males, 6 weeks days (conversions to dose testicular atrophy was 515 mg/kg b.w. per day. (abstract)
old) on a body weight basis not No other information was reported.
available since food
consumption was determined
but not fully reported)
Rats (males; Oral administration by Some regeneration of seminiferous tubules 2 weeks One dose group Tanino et
strain and gavage (vehicle after discontinuation of the administration. only (effects al. (1987)
number of unspecified) of 2400 Active spermatogenesis in almost all tubules observed at 2400
animals mg/kg b.w. per day DBP though vacuolation of germinal epithelium and mg/kg b.w. per day)
unspecified) daily for 7 days. decreased number of sperm were still evident 3
weeks after exposure was terminated.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (males and 500 or 1000 mg/kg b.w. per In the first experiment, there were no effects In the first Gray et
females, strain day from 20 to 55 days of on the female reproductive system while male rats experiment: LOAEL al. (1983)
and number age in one experiment and were severely affected at both doses. Exposed = 500 mg/kg b.w. (abstract)
unspecified) 250 or 500 mg/kg b.w. per rats had smaller testes and seminal vesicles and per day (males);
day from 20 to 75 days of no sperm in the vas deferens. In the second NOEL = 1000 mg/kg
age in a second experiment experiment, rats were unaffected at 250 mg/kg b.w. b.w. per day
(nature, vehicle and per day but half of the pairs did not breed in the (females)
pattern of administration high-dose group. No other information was In the second
unspecified) reported. experiment:
NOEL=250 mg/kg
b.w. per day
Rat (strain 0.52 g/kg b.w. per day by There were no effects on conception rate or litter One dose group Bornmann &
unspecified, gavage daily (vehicle sizes in exposed animals when compared with only (no effects Loeser (1956)
groups of 8 unspecified) for 6 weeks controls. Neonatal growth rates in the F1 were observed at 520
females) and then mated with also comparable with those of control animals. mg/kg b.w. per day)
untreated males to produce The weights of endocrine organs in this generation
an F1 generation. were within normal limits and the onset of estrus
Interbreeding of untreated was similar to that in control rats. There were
F1 rats produced an F2 also no abnormalities in the F2 and F3 generations.
generation. An F3
generation was similarly
produced.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (CD This has been designated the final dose levels selected were 0.1, 0.5 and NTP concluded that NTP (1991,
Sprague-Dawley) "NTP Study 4" Continuous 1.0%, on the basis of clinical signs, body weight these effects 1995); Wine
breeding protocol which and food consumption. document et al. (1997)
included cross-over mating reproductive and
and offspring assessment developmental
phases. Preceded by a toxicity of DBP
range-finding study in F0 rats at all
("Task 1") [5 doses (0.1, dose levels and
0.5, 1.0, 1.5 and 2.0% in more severe
diet) and control; 8 toxicity to F1
rats/sex/group] offspring.
NOAEL not
identified
LOAEL =
approximately 66
mg/kg b.w. per day
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (CD "Task 2", continuous All control and exposed pairs were fertile. No NOAEL NTP (1991,
Sprague-Dawley) breeding phase. Based upon Dose-related decrease in number of live pups per established for 1995); Wine
food consumption data, the litter (significant at all doses); absolute and decreased litter et al. (1997)
authors estimated that the adjusted live pup weight significantly decreased size, LOAEL = 66
intakes for the exposed in mid- and high-dose groups. Dam weights at mg/kg b.w. per day.
groups were 52, 256 and 509 delivery were significantly decreased at each NOAEL for pup body
mg/kg b.w. for males and litter in the high dose-group. weight 66 mg/kg1
80, 385 and 794 mg/kg b.w. In the mid-dose group, reduction in pup body b.w. per day.
for females, giving average weight was not accompanied by reduced dam body NOAEL for fertility
intakes of 66, 320 and 651 weight. 651 mg/kg b.w. per
mg/kg b.w. per day. 40 day.
breeding pairs as controls
and 3 dose groups of 20
pairs each. Animals housed
as breeding pairs for 112
days. End-points were
clinical signs, parental
body weight, feed
consumption, fertility
(number producing a
litter/number of breeding
pairs), numbers of litters
per pair, number of live
pups per litter, proportion
of live pups, sex ratio of
live pups, body weight of
pups. The last litter born
during "Task 2" was reared
for "Task 4". litters
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (CD "Task 3"; since an adverse No overall difference with respect to mating, One dose group NTP (1991,
Sprague-Dawley) effect on reproduction was pregnancy or fertility indices. Live pup weight only. Effects 1995); Wine
detected during Task 2, a was adversely affected in Group C, which suggested seen in treated et al. (1997)
1-week cross-over mating that DBP was a reproductive toxicant in females. females at 665
trial was performed to However the authors noted that it was not possible mg/kg b.w. per day.
determine the affected sex. to distinguish between an effect on dam body
weight and direct toxicity to the fetus.
Consisted of 3 groups of 20 Significant increase in organ to body weight
pairs each: ratios for liver and kidneys in males and F0
- control males × control females. No effect upon sperm concentration,
females (Group A) motility, percent abnormal forms or testicular
- high-dose males × spermatid head count. No apparent effects upon
control females (Group B) estrual cyclicity or average estrous cycle length.
- control males ×
high-dose females (Group C)
End-points as for Task 2,
with addition of checking
for presence of vaginal
copulatory plug or sperm.
Estimated average daily
intakes of exposed animals
were 410 and 665 mg/kg b.w.
for males and females
respectively.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (CD "Task 4"; the last litter In the F1 high-dose group, body weight was No NOAEL NTP (1991,
Sprague-Dawley) born following the significantly lower at weaning and at necropsy established for 1995); Wine
continuous breeding phase for both sexes. reduced pup weight. et al. (1997)
("Task 2") was reared by Mating, pregnancy and fertility indices were LOEL = 66 mg/kg
the dam until weaning, at significantly lower in the high-dose group (1 b.w. per day.
which time the F1 animals litter born versus 19 in control group). No NOAEL
were exposed similarly to Absolute and ajusted live F2 pup weights were established for
the parents until 13 weeks significantly lower in all exposed groups. testis tubule
of age. Estimated average In high-dose males, significant decrease in the degeneration.
daily intakes for exposed absolute weight and relative weight of prostate, LOAEL = 322 mg/kg
groups were 50, 247 and 498 right testis and seminal vesicles; significant b.w. per day (low
mg/kg b.w. for males and 83, increase in liver and kidney weight. dose group not
397 and 828 mg/kg b.w. for In high-dose males, adverse effect upon epididymal examined)
females. At sexual maturity, sperm count and concentration and testicular
groups of 20 males and 20 spermatid head count and concentration. No
females from the same apparent effects upon estrual cyclicity or average
treatment groups cohabited estrous cycle length.
for 7 days, then housed Epididymides absent or poorly developed in 5/10
singly until delivery. high-dose, 0/10 mid-dose males.
End-points same as for Histological examination of control, mid- and
"Task 2", followed by high-dose groups showed testicular lesions
necropsy. consisting of degeneration of seminiferous tubules
(8/10 in high-dose and 3/10 in mid-dose group),
interstitial cell hyperplasia (7/10 in high-dose
group) and underdeveloped or defective
epididymides (5/10 in high dose group).
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (F-344, DBP administered in diet to Weight gain: decreased in dams at 20 000 mg/kg 10 000 mg/kg diet NTP (1995)
number dams during gestation and during gestation and in dams at 10 000 mg/kg was recommended
unspecified) lactation and to pups during lactation. Mean b.w. of pups reduced as the maximum
postweaning for 4 weeks, at during lactation and at end of 4 weeks of dietary perinatal exposure
concentrations of 0, 1250, exposure. concentration for
2500, 5000, 7500, 10 000 or Gestation index: (number of live pups per male and female
20 000 mg/kg diet. breeding female) was significantly lower in the rats.
20 000 mg/kg group [pup mortality in this group
was 100% by day 1 of lactation]; pup survival was
89% or more in all other treatment groups.
Organ weight: increased relative liver weight in
all exposed males and in females at >2500 mg/kg.
Histopathology: moderate hypospermia of the
epididymis in all males at 7500 and 10 000 mg/kg;
mild hypospermia in 2 out of 10 males at 5000
mg/kg; no degeneration of germinal epithelium was
detected.
Mouse (B6C3F1, Dietary concentrations of 0, Only 5 dams at 10 000 mg/kg delivered live pups, Developmental NTP (1995)
pregnant, 20 1250, 2500, 5000, 7500, and none at 20 000 mg/kg. Only 1 pup at 10 000 toxicity and pup
per group) 10 000 or 20 000 mg/kg mg/kg survived past lactation day one; number of mortality were
during gestation and live pups per litter at 7500 mg/kg remained low suggested at
lactation; pups were weaned throughout lactation; no deaths occurred in pups concentrations as
onto same diet as dams and after weaning. low as 7500 mg/kg
exposed for an additional Postweaning and final body weights of males at and 5000 mg/kg was
4 weeks >2500 mg/kg were significantly less than controls. considered to be
Organ weights: absolute liver weight of males at the maximum
7500 mg/kg was greater than controls. perinatal exposure
The one surviving male pup at 10 000 mg/kg had concentration.
cytoplasmic alteration in liver, consistent with
peroxisome proliferation.
Developmental toxicity and fetal and pup mortality
were suggested as low as 7500 mg/kg.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Mouse (ICR, 2% in the diet (equivalent Food consumption was affected, though relevant One dose group Oishi &
groups of 10 to 2400 mg/kg b.w. per day) data were not presented and body weight gain was only (effects Hiraga (1980b)
males) for 1 week. significantly decreased. The relative weights of observed at 2400
the testes and liver were significantly mg/kg b.w. per day)
increased, whereas the relative weight of the
kidney was significantly reduced. The zinc
concentration in the testes and liver was reduced
to 81 ± 3.14% and 88 ± 3.14% of the control,
respectively (p<0.05), but not in the kidney.
The concentration of testosterone in the testes
was unaltered and there was no testicular atrophy.
Mouse (T O 2000 mg/kg b.w. per day by There was a significant depression of the weight One dose group Gray et
strain, groups oral intubation in corn oil of the testis but no effect on body weight. 4 only (effects al. (1982)
of 10 males, 4 daily for 9 days. out of 10 animals had isolated atrophic tubules observed at 2000
to 6 weeks old) while in the other 6 mice, only 10 to 20% of the mg/kg b.w. per day)
tubules showed pronounced atrophy.
Mouse (dd; 5 3, 9, 27, 50, 100 or 200 Reduction in relative testicular weight and NOEL = 27 mg/kg Sajiki
males and 5 mg/kg b.w. per day in olive increases in leukocyte counts and serum lactate b.w. per day (1975a,b)
females per oil by gavage, daily for 30 dehydrogenase activity were observed. LOEL = 50 mg/kg
group) days Congestion, oedema and congestive oedema in lung, b.w. per day
and loss of spermatogenic cells and spermatogonia
in testes were observed at doses of >50 mg/kg
b.w. per day.
Guinea-pig 2000 mg/kg b.w. per day There was a decrease in body weight (p<0.05) and One dose group Gray et
(Dunkin-Hartley, by oral intubation in corn weight of the testes (p<0.001). Based on only (effects al. (1982)
groups of 5 oil daily for 7 days. histological examination of the testes, there was observed at 2000
males, 4 to 6 severe tubular atrophy with loss of spermatids mg/kg b.w. per day)
weeks old) and a reduction in primary spermatocytes and
spermatogonia.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Hamster (males 500 or 1000 mg/kg b.w. per In the first experiment, the testes, the seminal In the first Gray et
and females, day from 20 to 55 days of vesicles and the epididymis of the high-dose experiment: al. (1983)
strain and age in one experiment and group were smaller. There were no effects on NOEL = 500 mg/kg (abstract)
number 1000 mg/kg b.w. per day from the female reproductive system. In the second b.w. per day
unspecified) 20 to 75 days of age in a experiment, the exposed hamsters had smaller (males); NOEL =
second experiment (nature, testes; in their offspring, there was decreased 1000 mg/kg b.w. per
vehicle and pattern of viability and growth was retarded. day (females)
administration unspecified) In the second
experiment: one
dose group only,
effects observed
at 1000 mg/kg b.w.
per day)
Hamster 2000 mg/kg b.w. per day by There was no effect on body weight, weight of the One dose group Gray et
(Syrian DSN, oral intubation in corn oil testes or testicular histology. only (no effects al. (1982)
groups of 7 for 9 days. observed at 2000
males, 4 to 6 mg/kg b.w. per day)
weeks old)
Table 8. Continued
Species Protocol Results Effect Levels Reference
Mouse (Swiss Continous breeding protocol DBP exposure resulted in a reduction in the NOEL = 0.3% in the NTP (1984,
CD-1 albino, with cross-over mating. numbers of litters per pair and of live pups diet = 390 mg/kg 1995); Lamb
groups of 20 0.03, 0.3 or 1.0% DBP in per litter, and in the proportion of pups born b.w. per day. et al. (1987);
of each sex) the diet (equivalent to 39, alive at the 1.0% amount, but not at lower dose LOAEL = 1.0% in
390, 1300 mg/kg b.w. per levels. A cross-over mating trial with the the diet = 1300
day) for a 7-day pre-mating control and the high dose F0 mice demonstrated mg/kg b.w. per day
period randomly grouped as that female mice, but not males, were affected
mating pairs which were by DBP, as shown by significant decreases in the
exposed during a 98-day percentage of fertile pairs, the number of live
period of cohabitation. pups per litter, the proportion of pups born
alive, and live pup weight in the control male
and exposed female pairing. In the F0 females,
absolute and relative liver weights were
significantly increased and uterine weight was
significantly decreased at the high dose, which
suggested that this dose was maternally toxic.
There were no significant differences in the %
motile sperm, sperm concentration, or % abnormal
sperm in the cauda epididymis between male mice
exposed to 0 or 1.0% DBP in the diet. No
treatment-related gross or histopathological
lesions were noted for the testis, epididymis,
prostate or seminal vesicles in male mice, or
for the ovary, oviduct, uterus or vagina in the
female mice.
In a recently reported NTP subchronic study on F-344 rats
(see NTP rat study 2 in section 7.3), histopathological effects
in the testes (degeneration of the germinal epithelium) were
observed at doses of > 720 mg/kg body weight. The NOEL for
these effects was 359 mg/kg body weight per day. In a study with
combined prenatal perinatal and subchronic exposure (see NTP rat
study 3 in Section 7.3), similar effects were observed at doses
of > 571 mg/kg body weight (NOEL = 279 mg/kg body weight per
day) (NTP, 1995). In Task 4 of the continous breeding study (see
Table 8), in Sprague Dawley rats, testicular tubular degeneration
was observed in the F1 offspring that had been treated during
the pre- and postnatal period with 322 mg/kg body weight per day,
and no NOAEL was determined (NTP, 1995; Wine et al., 1997). The
F1 offspring showed much more severe damage to the testes and
secondary sexual organs than did the parent F0 generation at the
same dose levels.
The effects on the testes of short-term exposure of rats to
DBP appear to be at least in part reversible. Tanino et al.
(1987) reported that, 2 weeks after discontinuation of the
administration of 2400 mg/kg body weight per day for 7 days, some
regeneration of the seminiferous tubules had occurred. Active
spermatogenesis was observed in almost all tubules, although
vacuolation of germinal epithelium and decreased numbers of sperm
were still evident 3 weeks after exposure had ceased.
Based on the results of Cater et al. (1977), zinc appears to
play a role in DBP-induced testicular atrophy in rats. After 4
days of exposure of rats to 500, 1000 or 2000 mg DBP/kg body
weight per day, the weight of the testes was decreased at
1000 mg/kg (P < 0.01) and 2000 mg/kg (P < 0.001), and decreased
further after 6 days (P < 0.05 at 500 mg/kg body weight per day
and P < 0.001 at the two highest doses) (LOEL = 500 mg/kg body
weight per day on the basis of decreased testicular weight).
Based on histological examination of the testes after 4 days of
exposure to 2000 mg/kg body weight per day, there was a
diminution of both spermato-cytes and spermatogonia. In
addition, at this dose, urinary zinc excretion was increased by
34 to 43% over the first 4 days and then returned to normal
levels. In the testes, the turnover rate of zinc was enhanced,
the half-life was decreased from 14 to 5 days and zinc levels
were significantly reduced; values were 88 - 93% of those of
controls after 2 days and 64 - 71% after 6 days. There was no
change in the turnover rate or zinc content in liver or kidneys.
The decrease in the activity of testicular alcohol dehydrogenase
(a zinc-dependent enzyme) ranged from 20 to 40% of control values
following 5 days of exposure. A 3-day pretreatment with
2000 mg/kg body weight per day caused a 25% decrease (P < 0.001)
in testicular zinc uptake in vivo. Concomitant intraperitoneal
administration of zinc sulfate (50 mg/kg body weight per day)
resulted in no testicular atrophy (based only on relative testes
weight rather than on microscopic appearance of the testes).
Mice and hamsters appear to be somewhat more resistant than
rats and guinea-pigs to DBP-induced testicular atrophy. For
example, testicular effects were observed in rats but not mice in
NTP subchronic toxicity studies (NTP, 1995). Following
administration of 2000 mg DBP/kg body weight per day by gavage in
corn oil for 7 to 9 days, only isolated tubular atrophy was
observed in 40% of the mice; no effects on testicular histology
were observed in hamsters, but oral doses of 2000 mg/kg body
weight per day administered to rats and guinea-pigs produced
severe tubular atrophy with loss of spermatids and reductions in
primary spermatocytes and spermatogonia (Gray et al., 1982). In
a study reported in the form of an abstract (Gray et al., 1983a),
pronounced effects were observed in male rats following
administration of 500 or 1000 mg/kg body weight per day from 22
to 55 days of age, while effects in hamsters were observed at the
high dose only. Exposed rats and hamsters both had smaller
testes and seminal vesicles than controls. The rats also had no
sperm in the vas deferens and the size of the epididymis of the
hamster was reduced. In a separate experiment reported in the
same abstract (Gray et al., 1983a), half of the rats exposed to
500 mg/kg body weight per day from 20 to 75 days of age did not
breed, while hamsters exposed to 1000 mg/kg body weight per day
had smaller testes but bred, although the viability of their
offspring was decreased and growth was retarded. In other
studies in which ICR male mice ingested a diet containing 2% DBP
(equivalent to 2400 mg/kg body weight per day), there was a
decrease in testicular levels of zinc but no testicular atrophy
was apparent (Oishi & Hiraga, 1980b), whereas testicular atrophy
and decreased testicular zinc levels were observed in male Wistar
rats exposed under the same conditions (2% in the diet equivalent
to 2060 mg/kg body weight per day) (Oishi & Hiraga, 1980a). In
an early study, the results of which have not been confirmed at
such low doses in mice by other investigators, Sajiki (1975a,b)
reported loss of spermatogenic cells and spermatogonia at doses
of >50 mg/kg body weight per day administered for 30 days by
stomach intubation.
In summary, alteration in testicular enzymes and
degeneration of testicular germinal cells in rats have been
observed at doses of 250, 322 and 571 mg/kg body weight per day,
respectively. Effects on a second generation may be more severe
than on the first generation (Srivastava et al., 1990a, 1990b;
NTP, 1995). There are considerable species differences in
effects on the testes following exposure to DBP, minimal effects
being observed in mice and none in hamsters at doses as high as
2000 mg/kg body weight per day (Gray et al., 1982).
It has been suggested (Foster et al., 1982) that part of the
difference in sensitivity of the rat and hamster to the
testicular toxicity of DBP relates to the higher levels of free
MBP in the rat (with lower levels of conjugate) than in the
hamster, coupled with the increased testicular ß-glucuronidase
activity in the rat. Both of these could lead to higher levels
of MBP in the rat testis. No data have been identified on
metabolism or tissue levels in mice or humans.
Results of available studies on the effects of MBP (the
principle metabolite of DBP) on the testes are presented in
Table 9. MBP induces testicular damage at doses similar to those
of DBP. Clear signs of testicular atrophy were observed after
oral administration of MBP to rats (Table 9). There were
reductions in testicular weight in rats ingesting 400 to 800 mg
MBP/kg body weight per day for periods of 5 or 6 days (Cater et
al., 1977; Gray et al., 1980). Histologically, the majority of
seminiferous tubules in animals administered 800 mg/kg body
weight per day for 6 days were atrophied, and there were
reductions in numbers of spermatocytes and spermatogonia (Foster
et al., 1981). Reductions in testicular zinc and relative testes
weights were observed in rats administered 2% MBP in the diet for
1 week (Oishi & Hiraga, 1980c).
In vitro exposure of human spermatozoa to 278 mg DBP/litre
resulted in a 25% reduction in motility (Fredricsson et al.,
1993).
7.5.1.2 Effects on fertility
Available data concerning the effects of DBP on fertility
are presented in Table 8. Corresponding data for MBP are
presented in Table 9.
Cummings & Gray (1987) reported that in rats, DBP had no
effect on early pregnancy during short-term exposure following
ovulation and continuing throughout the period of implantation
during pregnancy and pseudopregnancy at doses up to 2000 mg/kg
body weight per day; neither the number of implantation sites,
uterine weight, ovarian weight nor serum progesterone
concentrations were affected (relative to vehicle-exposed
controls). In addition, there were no significant effects on the
decidual cell response. These data indicated that DBP did not
affect any maternal parameter of progestational physiology
including the ability of the uterus to undergo deciduation.
Table 9. Reproductive effects of monobutyl phthalate
Species Protocol Results Effect levels Reference
Rat (Wistar, 800 mg/kg b.w. per day Loss of weight in testes, seminal vesicle and One dose group Ikemoto et
groups of 5 by gavage (vehicle prostate was evident; an almost complete absence only; effects al. (1983)
males, 5 weeks unspecified), daily for of germ cells in seminiferous tubules. observed at 800
old) 7 days mg/kg b.w. per day
Rat (Wistar, 800 mg/kg b.w. per day by Decreases in relative weights of testes, seminal One dose only; Ikemote
groups of 30 gavage in dimethyl sulfoxide vesicle and prostate were evident. effects observed (1985)
males, 35 days for 1 week; animals were Histopathologically, germ cells had almost at 800 mg/kg b.w.
old) sacrificed one day, 2 weeks disappeared in seminiferous tubules with per day
and 4 weeks after final vacuolation and enlargement. Concentration of
dosing. testosterone in serum was decreased but FSH and
LH concentration were not changed. Four weeks
after end of dosing, spermatogenesis had
recovered.
Rat 800 mg/kg b.w. per day by Testicular weight was reduced to 57% of control One dose group Grey et
(Sprague-Dawley) oral intubation value (p<0.001). Upon microscopic examination, only; effects al. (1982)
tubular atrophy was observed in all treated rats. observed at 800
mg/kg b.w. per day
Rat 800 mg/kg b.w. per day for Relative weights of testes and seminal vesicles One dose group Foster et
(Sprague-Dawley, 6 days by oral intubation were decreased. Urinary zinc excretion was only; effects al. (1981)
groups of 6 increased. observed at 800
males) mg/kg b.w. per day
Rat 400 or 800 mg/kg b.w. per Decrease in testicular weight at both doses after LOEL = 400 mg/kg Cater et
(Sprague-Dawley, day for 4 or 6 days by 4 days; the weight decreased further after 6 b.w. per day al. (1977)
groups of 6 gavage days.
males)
Table 9. Continued
Species Protocol Results Effect levels Reference
Rat (JCL:Wistar, 2% in the diet for 7 days Mean body weight was significantly lower than One dose group Oishi &
groups of 10 (2000 mg/kg b.w. per day) that of control. Absolute and testicular only; effects Hiraga
males, 5 weeks weights were decreased. Zinc concentration in observed at 2000 (1980c)
old) the testis was decreased. Testosterone mg/kg b.w. per day
concentration in the testis was increased but
that in serum was not changed.
Mouse (JCL:ICR, 2% in the diet for 7 days Body weight gain decreased. Relative weight of One dose group Oishi &
groups of 10 (2500 mg/kg b.w. per day) testes was increased. Concentrations of zinc and only; effects Hiraga
males, 5 weeks testosterone in the testis were decreased. observed at 2500 (1980d)
old) mg/kg b.w. per day
Hamster (DSN, 1600 mg/kg b.w. per day for Occasional tubular atrophy observed in 2 Gray et
groups of 7 9 days by oral intubation hamsters al. (1982)
males)
In NTP study 4 (section 7.3), DBP was administered in the
diet (0, 1000, 5000 or 10 000 mg/kg diet) to Sprague-Dawley rats
in a continuous breeding protocol, which included cross-over
mating and offspring assessment phases (NTP, 1995; Wine et al.,
1997). Additional details on the protocol and levels at which
effects occurred are presented in Table 8. Average equivalent
dose levels on a body weight basis were 66, 320 and 651 mg/kg
body weight (NTP, 1991). Mean body weights of exposed dams
generally decreased with increasing dose and, in the high-dose
group, were 6 - 13% lower than those of controls at delivery and
during lactation. In the F0 generation, the average number of
live pups per litter (all groups) and mean pup weight at birth
and during lactation (mid- and high-dose groups) were less than
in controls. Cross-over mating trials in the F0 generation
revealed no effects on the fertility of male or female rats
receiving the highest dose, although the live pup weight, when
adjusted for litter size, was significantly less for litters from
exposed dams. The absolute liver weight of exposed male rats and
relative liver and kidney weights of exposed male and female F0
rats of the high-dose group were significantly greater than those
in controls. In contrast to the F0 rats, mating, pregnancy and
fertility indices of F1 rats were lower in the high-dose group
than in controls (1/20 pregnant versus 19/20 in the controls).
In the high-dose group of F1 males, absolute and relative
epidydimal, right caudal epididymal, right testis, seminal
vesicle and prostate gland weights were reduced; germinal
epithelial degeneration of the testes, absence or
underdevelopment of the epididymides and interstitial cell
hyperplasia were also observed. Epididymal sperm count and
concentration and testicular spermatid head count and
concentration were also significantly decreased in the high-dose
group of males. Seminiferous tubule degeneration was observed in
1/10, 3/10 and 8/10 in the controls, mid- and high-dose groups,
respectively. In F1 females, the right ovary weights were
unchanged. Total and adjusted live pup weights were lower in all
exposed groups than in the controls. No clear NOEL was
established in this study. In the first generation (F0) the
reduction in pup weight in the mid-dose group, in the absence of
any adverse effect on maternal weight, can be regarded as a
developmental toxicity effect. There was also a significant
reduction of live litter numbers at all three dose levels. The
effects in the second generation were more severe, with reduced
pup weight in all groups including the low-dose group, structural
defects in the mid- and high-dose groups, and severe effects on
spermatogenesis in the high-dose group that were not seen in the
parent animals. These results suggest that the adverse effects
of DBP are more marked in animals exposed during development and
maturation than in animals exposed as adults only (Wine et al.,
1997).
In NTP rat study 1, DBP was administered in the diet to
F-344 rat dams during gestation and lactation and to the pups
postweaning for four additional weeks, at concentrations of 0,
1250, 2500, 5000, 7500, 10 000 and 20 000 mg/kg diet. Based on
decreased weight gains in the dams at >10 000 mg/kg, decreased
gestation index and increased pup mortality at 20 000 mg/kg,
decreased body weight of pups at 10 000 mg/kg and mild to marked
epidydimal hypospermia at >7500 mg/kg, 10 000 mg/kg was
recommended as the maximum perinatal exposure concentration for
male and female rats for subsequent studies (NTP, 1995).
DBP was administered in the diet (0, 300, 3 000 or
10 000 mg/kg diet) to Swiss (CD-1) mice (NTP, 1995). Average
equivalent dose levels on a body weight basis were 0, 39, 390 or
1300 mg/kg body weight per day (NTP, 1984). In F0 mice in the
high-dose group that received DBP during the continuous breeding
phase, the fertility index, average number of litters per
breeding pair, live male and female pups, and live pups per
litter were significantly lower than in the controls. The ratio
of live male pups to total live pups in the high-dose group was
greater than in the controls. In the cross-over mating trial,
the fertility index, numbers of live male, female and total pups
per litter, and total and adjusted live pup weights were
significantly lower for F0 females (bred with control males) in
the high-dose group than for the control females bred with
unexposed males. The female pup weights in litters from control
females bred with exposed males were also lower than those of
control females bred with unexposed males. Fertility was not
affected, though the pup weights were lower. In females in the
10 000 mg/kg group, the liver weight was greater and the uterine
weight was less than in control females. Based on comparison
with a similar study in rats, mice therefore appear to be less
sensitive than rats to reproductive effects of DBP, effects only
being seen at the highest dose level (NTP, 1995).
In a prenatal/perinatal range-finding study, 20 pregnant
B6C3F1 mice per group were exposed to 0, 1250, 2500, 5000, 7500,
10 000 or 20 000 mg DBP/kg diet throughout gestation and
lactation. Pups were weaned onto the same diet as their dams and
exposed for a further 4 weeks (NTP, 1995). Developmental
toxicity and pup mortality were suggested at concentrations as
low as 7500 mg/kg.
In a study reported only as an abstract, Gray et al.
(1983a), reported no effects on the female reproductive system in
an unspecified number of hamsters exposed to 500 or 1000 mg/kg
body weight per day from 20 to 55 days of age. In a second
experiment in the same report, however, half of the breeding
pairs of rats exposed to 500 mg/kg body weight per day from 20 to
75 days of age did not breed (NOEL = 250 mg/kg body weight
per day). At similar doses (LOAEL = 500 mg/kg body weight per
day; NOEL = 250 mg/kg body weight per day), breeding in rats was
adversely affected (Gray et al., 1983a,b).
Heindel et al. (1989) reported the results of a reproductive
study for diethylhexyl phthalate in mice. Though data on the
other phthalates were not presented in the published report, the
authors concluded, on the basis of similar studies for these
compounds, that the relative order of reproductive toxicity for
the various phthalates was diethylhexyl, dihexyl, dipentyl, di-
n-butyl and dipropyl; diethyl and dioctyl phthalates were
considered non-toxic.
7.5.2 Developmental effects
The developmental effects of DBP have been examined in rats
and mice following oral and intraperitoneal administration (the
latter considered less relevant for assessment of dose-related
effects), as summarized in Table 10. DBP generally induced
fetotoxic effects in the absence of maternal toxicity, and
teratogenic effects only at high maternally toxic doses.
Ema et al. (1993) administered DBP by gavage to Wistar rats
on days 7-15 of gestation at dose levels of 0, 500, 630, 750 and
1000 mg/kg body weight per day. No effects in either dams or
offspring were reported at 500 mg/kg body weight. At the LOEL of
630 mg/kg body weight per day, there was a significant increase
in maternal body weight gain, significantly increased incidence
of postimplantation loss, and significant decrease in fetal
weight and increased malformations. The NOEL was 500 mg/kg body
weight per day.
Results of a recent study indicate that susceptibility to
teratogenesis varies with the developmental stage during the
period of DBP administration, based on exposure of Wistar rats to
0, 750, 1000 or 1500 mg/kg on either days 7 to 9, days 10 to 12,
or days 13 to 15 of gestation (Ema et al., 1994). When DBP was
administered on days 10 to 12 of gestation, there was no
evidence of teratogenicity. Following administration on days 7
to 9 and 13 to 15, the frequency of malformations increased with
dose level, and was highest when DBP was administered on days 13
to 15 (information on maternal toxicity was not reported).
Malformations were also observed during the postnatal development
of the rats of the final litter in the Continuous Breeding
protocol study (Task 4, NTP, 1995; Wine et al., 1997) (Table 8).
Three out of 20 males of the high-dose group that had been
exposed pre- and postnatally to 650 mg DBP/kg body weight had
small and malformed prepuces and/or penises and non-palpable
testes. Five out of ten rats examined histologically had
underdeveloped or defective epididymides (Wine et al., 1997).
Table 10. Developmental effects of DBP
Species Protocol Results Effect levels Reference
Rat (Holtzman, Pseudopregnant rats No effect on the decidual cell response, NOEL = 2000 mg/kg Cummings &
groups of 6 received 0, 250, 500, pregnant uterine weight, number of b.w. per day Gray (1987)
pseudopregnant 1000 or 2000 mg/kg b.w. implantation sites, ovarian weight, or serum
females and per day while pregnant progesterone concentration during early
groups of 6 to rats received 0, 500, pregnancy or pseudopregnancy. These data
8 pregnant 1000 or 2000 mg/kg b.w. indicated that short-term exposure to DBP had
females) per day by gavage in no direct maternal effects in the rat and
sesame oil from day 1 suggested that the viability of preimplantation
through day 8 of embryos was not compromised.
pseudopregnancy or
pregnancy.
Rat (females, 250 mg/kg b.w. per day A large increase in total embryonal death, One dose group only Aldyreva
strain and by gavage daily (vehicle owing to high preimplantation losses, was (effects observed at et al. (1975)
number unspecified) at various noted. Data on maternal toxicity were not 250 mg/kg b.w. per
unspecified) stages of gestation or presented. day)
over the first 22 days of
gestation.
Rat (Wistar, 120 or 600 mg/kg b.w. per No effects on ossification, bone development of NOEL = 120 mg/kg b.w. Nikonorow
groups of 10 day by gavage in corn oil the base of the skull, paws of the front and per day (offspring) et al. (1973)
or 20 females) to groups of 20 females hind extremities or rib fusion in fetuses. LOEL = 600 mg/kg b.w.
prior to or during mating. Increased number of resorptions and decreased per day (offspring)
120 or 600 mg/kg b.w. per fetal body weights were observed at the high
day by gavage in olive oil dose. However, these abnormalities were not
daily throughout gestation observed when DBP was administered prior to and
(21 days). during mating. Maternal toxicity was not
addressed.
Table 10. Continued
Species Protocol Results Effect levels Reference
Rat, (Wistar, Administered DBP by Significant decrease in maternal body weight NOEL = 500 mg/kg b.w. Ema et al.
groups of 11 gavage in olive oil, days gain at >630 mg/kg b.w. Maternal deaths at per day (1993)
or 12 females) 7-15 of gestation, 0, 500, 1000 mg/kg b.w. per day. Significant increase
630, 750 or 1000 mg/kg in postimplantation loss at 630 mg/kg b.w. per
b.w. per day day with complete resorption of implanted
embryos in surviving dams at 1000 mg/kg b.w.
per day. Significant decrease in fetal weight
at >630 mg/kg b.w. Increased incidence of
malformed fetuses (predominantly cleft palate)
at >630 mg/kg b.w. (significant at 750 mg/kg
b.w).
Rat (Wistar, Pregnant rats were housed Ema et al.
groups of 11 individually and dosed by (1994)
or 12 females) gastric intubation with DBP
(99% pure) in olive oil.
All rats killed on day 20.
Dosing on days 7 to 9 of Significant increase in number of resorptions, LOAEL = 750 mg/kg b.w.
pregnancy - 0, 750, 1000 or dead fetuses per litter; postimplantation per day (teratogenic
1500 mg/kg b.w. per day loss at >0.75 g/kg b.w. per day (100% effects)
postimplantation loss at 1.5 g/kg b.w. per
day). Reduction in body weight of male and
female fetuses at >750 mg/kg b.w. per day.
Significant increase of fetuses with skeletal
malformations, fusion or absence of cervical
vertebral arches or ribs at 750 mg/kg b.w.
per day.
Table 10. Continued
Species Protocol Results Effect levels Reference
Rat (Wistar, Dosing on days 10 to 12 Significant increase in number of resorptions LOAEL = 750 mg/kg Ema et al.
groups of 11 of pregnancy - 0, 750, and dead fetuses per litter; and b.w. per day (no (1994)
or 12 females) 1000 or 1500 mg/kg b.w. postimplantation loss per litter at >750 mg/kg teratogenic effects)
per day b.w. per day (100% postimplantation loss at
1500 mg/kg b.w. per day). Reduction in body
weight of live female fetuses at 750 mg/kg b.w.
per day and change in sex ratio of live fetuses
at 1000 mg/kg b.w. per day.
Dosing on days 13 to 15 Significant increase in postimplantation loss LOAEL = 750 mg/kg b.w.
of pregnancy - 0, 750, per litter at >750 mg/kg b.w. per day (100% per day (teratogenic
1000 or 1500 mg/kg b.w. postimplantation loss at 1500 mg/kg b.w. per effects)
per day day) and number of resorptions and dead fetuses
per litter at 1000 mg/kg b.w. per day.
Increase in fetuses with malformations, cleft
palate, skeletal malformations and fusion of
sternebrae at >750 mg/kg b.w. per day.
Mouse (ICR-JCL, 0.05, 0.1, 0.2, 0.4 or There was a significant reduction in body NOEL = 370 mg/kg b.w. Shiota et al.
groups of 7 to 1.0% in the food throughout weight at day 18 in mothers administered the per day (offspring) (1980);
15 females) gestation (18 days) highest dose. The total number of implants was LOEL = 660 mg/kg b.w. Shiota &
corresponding to 80, 180, similar in exposed and control animals but the per day (offspring) Nishimura
370, 660 and 2100 mg/kg numbers of resorptions and dead fetuses were NOEL = 660 mg/kg b.w. (1982)
b.w. per day based on data much higher in high-dose animals. There was a per day (mothers)
on food consumption. dose-dependent decline in fetal body weights
but this was only significant at the two higher
doses. There were no abnormalities except in
the group exposed to the highest concentration,
in which there were 2 fetuses (75%) which had
exencephaly and myeloschisis. No malformations
of internal organs were observed in the fetuses
examined by the microdissection method.
Table 10. Continued
Species Protocol Results Effect levels Reference
Mouse (ICR-JCL, 0, 0.005, 0.05, 0.5% DBP The number of pregnant animals, the incidences NOEL = 0.05% (100 Hamano et
groups of 15 to in the diet of spontaneous abortion and maternal deaths, mg/kg b.w. per day) al. (1977)
18 females) and the number of mice with live offspring (offspring)
Based upon food intake were similar in the exposed groups and LOAEL = 0.5% (400
data, the two highest controls. No effects were noted on maternal mg/kg b.w. per day)
doses were calculated to liver and spleen weights. A statistically (offspring)
be 100 and 400 mg/kg b.w. significant increase in kidney weight was NOEL = 0.05% (100
per day observed in the high-dose group. An mg/kg b.w. per day)
embryotoxic effect was noted at the highest (mothers)
concentration, resulting in a lower number
of live offspring. The incidence of external
anomalies was also significantly higher in the
high-dose group. At the high dose, these
anomalies were non-closing eyelid (3),
encephalocoele (6), cleft palate (1), spina
bifida (1), non-closing eyelid + encephalocoele
(3). The rate of ossification for all dosed
groups appeared to be within normal limits.
The incidence of skeletal anomalies, especially
of the sternum, was higher (but not
statisticaal significant) in the high-dose
group with respect to the controls.
Mouse (CD-1, 2500 mg/kg b.w. per day by 5 exposed mice died and there were no viable One dose group only Hardin et al.
group of 50 gavage in corn oil on days litters. Maternal toxicity was not addressed. (effects observed at (1987)
females) 6 to 13 of gestation. 2500 mg/kg b.w. per
day)
Table 10. Continued
Species Protocol Results Effect levels Reference
Rat 0, 0.32, 0.64 or 1.06 g/kg At all doses, the number of resorptions in LOAEL = 320 mg/kg b.w. Singh et al.
(Sprague-Dawley, b.w. intraperitoneally on exposed animals was higher than in controls per day (offspring) (1972)
groups of 5 days 5, 10 and 15 of and there was a corresponding decrease in the
females) gestation. number of live fetuses. Fetal weight was
significantly lower than in controls at all
doses. There was a higher incidence (not
analysed statistically) of skeletal anomalies
in exposed animals when compared with
unexposed controls. These were mainly rib
abnormalities, absence of tail bones,
incomplete skull bones and incomplete or
missing leg bones. Maternal toxicity was
not addressed.
Rat 2 ml/kg b.w. (2080 mg/kg One rat administered the highest dose died. LOEL = 2080 mg/kg b.w. Peters &
(Sprague-Dawley, b.w.) or 4 ml/kg b.w. There were no significant effects on Cook (1973)
groups of 5 (4170 mg/kg b.w.) implantation. The average number of pups
females) intraperitoneally on days weaned per litter was significantly lower in
3, 6 and 9 of gestation. exposed animals compared with controls. Fetal
abnormalities were not addressed.
In the study reported by Hamano et al. (1977), JCL:ICR mice
were administered 0.005, 0.05 or 0.5% DBP in food throughout 18
days of gestation (the two highest doses were calculated on the
basis of food intake to correspond to 100 and 400 mg/kg body
weight per day). There were no significant differences in the
mortality of maternal mice, the rate of spontaneous abortions or
the rate of premature births between the control and exposed
groups. The highest dose was embryotoxic, resulting in a lower
number of live offspring. At this highest dose, an increase in
kidney weight in mothers was reported, although there were no
effects on the weights of other organs, body weight gain or
survival in the mothers. The frequency of offspring with
external anomalies was also significantly higher in the high-dose
group than in controls. The anomalies consisted mainly of spina
bifida, exencephaly, cleft palate and open eye. A small but non-
significant increase in skeletal anomalies was also seen in the
high-dose group. Therefore, the NOEL and LOEL values in this
study were considered to be 100 and 400 mg/kg body weight per
day, respectively, on the basis of embryotoxic and teratogenic
effects.
In summary, the lowest reported LOAEL for developmental
effects of DBP was that reported by Hamano et al. (1977), i.e.
400 mg/kg body weight per day for increases in the number of
resorptions and dead fetuses in JCL:ICR mice. The NOEL in this
study was 100 mg/kg body weight per day.
7.6 Mutagenicity and related end-points
The weight of the available evidence indicates that DBP is
not genotoxic (Table 11). There are no structural alerts
indicative of potential reactivity with DNA. The major metabolic
pathway involves hydrolysis of one ester linkage to yield MBP and
n-butyl alcohol, neither of which react with DNA.
DBP (100 to 10 000 g/plate) was not mutagenic in any of
four tester strains of Salmonella tymphimurium in the presence
or absence of Arochlor-induced rat or hamster liver S-9 (Zieger
et al., 1985). These data are consistent with earlier negative
Ames test studies (Yagi et al., 1976; 1978; Florin et al., 1980;
Kozumbo et al., 1982). In two studies, very weak positive
responses were reported in the absence of an S-9 metabolic
activation system (Seed, 1982; Agarwal et al., 1985). These
results are questionable because the parent compound clearly does
not react with DNA. Similarly, an increase in mutant frequency
was seen without metabolic activation and at very high cytotoxic
doses in the L5178Y mouse lymphoma cell assay (NTP, 1995). It
should be noted, however, that false positive results are common
in this assay at cytotoxic concentrations.
Table 11. Mutagenicity of DBP from HSE, 1986
Species Protocol Results Reference
Salmonella typhimurium; Levels of up to 1000 µg/plate in the Negative. Full data not reported. Kozumbo et al.
TA98, TA100 presence and absence of S-9. (1982)
S. typhimurium; With and without Aroclor S-9. Tested Negative. Complete data not reported. Florin et al.
TA98, TA100, TA1535, TA1537 up to levels that precipitated. (1980)
S. typhimurium Not specified. Negative. No other information provided. Yagi et al.
(strain not reported) (1976, 1978)
S. typhimurium; Levels of 100-10 000 µg/plate in Negative. Full data were not provided. Zeiger et al.
TA98, TA100, TA1535, TA1537 DMSO, with and without S-9 in a (1982)
preincubation-type assay.
S. typhimurium; Levels of 0.013, 0.03 and 0.05 mg/ml Small dose-related increase in Seed (1982)
TA100 in the 8-azaguanine resistance assay mutation frequency in the absence of
using a preincubation assay with and S-9, statistically significant at the
without S-9. two highest doses. Values were
increased 1.5 times control levels
at the highest dose.
S. typhimurium; Test for base-pair substitution or Spot tests yielded negative responses Agarwal et al.
TA98, TA100, TA1535, TA1537, frameshift-type mutations; spot tests for all strains. (1985)
TA1538 and TA2637. with 500 µg per plate. "Mildly positive" response in TA100
Dose-response test with 100 to 2000 µg and TA1535, but not in presence of S9.
per plate, with and without S9
metabolic activation.
Bacillus subtilis; H17 62.5 µg/ml (limit of solubility) No inhibition indicative of DNA Sato et al.
(Rec+) and M45 (Rec-) damage; positive controls produced (1975)
clear zones of inhibition. Test did
not appear to have been carried out
using S-9.
Table 11. Mutagenicity of DBP from HSE, 1986
Species Protocol Results Reference
Pseudo-diploid Chinese Concentrations of 0.28, 2.78 and Negative for SCE and chromosomal Abe & Sasaki
hamster cell line (Don) 27.8 mg/ml were tested for ability aberrations. (1977)
to induce chromosome aberrations
and sister chromatid exchange
(SCE). Ethanol solvent.
Clonal sub-line of a Chinese Concentrations up to 0.03 mg/ml Suspicious or equivocal results for Ishidate &
hamster fibroblast cell line. dissolved in aqueous bovine albumin, induced chromosomal aberrations. Odashima (1977)
tested for induction of chromosomal
aberrations.
Human leucocytes (male Chromosomal aberrations determined No increase in frequency of Tsuchiya &
derived) in 100 human leucocytes following chromosomal aberrations. Hattori (1976)
8-h exposure to 0.03 mg/ml DBP in
whole human blood culture. This
concentration had been previously
shown to inhibit growth of the
culture cells by 20 to 50%.
Mouse lymphoma cell line Cells exposed in suspension to DBP Increased mutant frequency under NTP (1995)
L5178Y for 4 h in the presence and absence non-activation conditions with high
of rat liver S9 metabolic activation. cytotoxicity.
Mouse Balb/3T3 cells In vitro transformation assay. DBP did not induce the appearance Litton Bionetics
Concentrations of DBP were 3.4, of a significant number of Inc. (1985)
13.7, 27.5, 55.0 and 82.3 nl/ml. transformed foci.
Protocol included both positive and
negative controls.
DBP did not induce sister-chromatid exchanges (SCE) or
chromosome aberrations in CHO cells (Abe & Sasaki, 1977) but an
equivocal result was reported for induction of chromosome
aberrations in a Chinese hamster fibroblast cell line in the
absence of metabolic activation (Ishidate & Ohashima, 1977).
DBP was inactive in the Balb/C-3T3 in vitro
transformation assay (Litton Bionetics Inc., 1985).
In the only identified in vivo study, analysis of
peripheral blood samples from male and female mice at the end of
the 13-week feeding study did not reveal any micronuclei (NTP,
1995).There was no increase in the numbers of revertants in
Salmonella typhimurium strains TA98 and TA100 exposed to 50 to
2000 µg/plate MBP, the principal metabolite of DBP, in the
presence and absence of S9 (Yoshikawa et al., 1983). Similar
concentrations were also non-mutagenic in two Escherichia coli
strains (WP2 uvr A+ and uvr A-).
7.7 Carcinogenicity
A long-term carcinogenicity study for DBP has not been
conducted, although no tumours were observed in two one-year
bioassays (Smith, 1953; Nikonorow et al., 1973).
7.8 Special studies
7.8.1 Induction of metabolizing enzymes
Following daily oral administration of 0.01, 0.1 or
1.0 mmol/kg body weight (2.78, 27.8 or 278 mg/kg body weight) DBP
by gavage in corn oil to male Sprague Dawley rats (n=20) for
5 days, there was a 48% increase in the hepatic microsomal
concentration of cytochrome P-450 at 0.01 mmol/kg body weight and
28-29% increase in NADPH-cytochrome-reductase activity at 0.01
and 0.1 mmol/kg body weight (Walseth & Nilsen, 1986). The
authors concluded that DBP is a moderate to weak inducer of
several microsomal enzymes though the reasons for increased
enzyme activity observed at the lower doses but not at the high
dose (1.0 mmol/kg body weight per day) were not addressed.
There were no changes in liver, lung or body weights in male
Sprague Dawley rats (n=15) exposed to 5.7, 28.5 or 79.8 mg
DPB/m3 air (0.5, 2.5 or 7.0 mg/kg) 6 h per day for 5 days
(Walseth & Nilsen, 1984). There was a small but significant
increase in the activity of hepatic NADPH-cytochrome-c reductase
in the group exposed to 5.7 mg/m3. In contrast to the study of
Walseth & Nilsen, 1986, effects on the hepatic liver microsomal
enzymes were not observed. However, the concentration of
cytochrome P-450 in the lung decreased in a dose-dependent manner
to a level of 37% of the control.
In Sprague Dawley rats (n=5) administered intraperitoneally
3.8 mmol/kg body weight per day (1058 mg/kg body weight per day)
for 5 days (Walseth et al., 1982), increases in relative liver
and lung weights and hepatic microsomal cytochrome P-450 content
were observed; however there was a decrease in pulmonary
microsomal cytochrome P-450. In another study in which albino
male rats (n=5) were intraperitoneally administered 3.05 ml
DBP/kg body weight per day (3190 mg/kg body weight per day) and
killed 18 h or 7 days after the treatment (Seth et al., 1981),
the activity of aniline hydroxylase was inhibited after 18 h.
There was also mild inhibition of aminopyrine- N-demethylase
activity, but no effects on the activities of glucose-6-
phosphatase or NADPH-cytochrome-c reductase were observed. There
was no effect on the activity of hepatic tyrosine
aminotransferase activity following the single exposure, but
there was an increase in the activity of this enzyme following
daily administration.
8. EFFECTS ON HUMANS
8.1 General population exposure
Cases of sensitization after exposure to DBP have been
reported. A 30-year-old woman developed an axillary dermatitis
after the use of an anti-perspirant containing DBP (Calnan,
1975). Patch testing of the skin was positive with both the
formulation and with DBP, but not with any of the other
constituents of the formulation.
In another reported case, a 32-year-old woman noted pruritis
and redness in the axillae after changing from her usual
deodorant spray to a new one (Sneddon, 1972). Patch testing with
the original formulation, an alternative deodorant spray, 1%
paraphenylene-diamine and 3% formalin, was negative, but patch
testing with the new deodorant and DBP, but not the other
constituents, was positive.
In a case reported by Husain (1975), a 44-year-old architect
noticed a patch of eczema under a plastic watch strap on the left
wrist and after transferring the watch to the right wrist. The
results of patch tests were positive for the plastic watch strip,
20% colophony, 1% paratertiary butylphenol formaldehyde resin and
5% DBP.
Cosmetic products containing 4.5-9% DBP were patch tested on
50 to 250 individuals per sample and no skin sensitization was
observed (Brandt, 1985). No other details were provided.
8.2 Occupational exposure
8.2.1 Acute toxicity
Sandmeyer & Kirwin (1981) reported a case of accidental
poisoning in which a 23-year-old healthy male worker ingested
10 g of DBP. Delayed symptoms were nausea, vomiting, and
dizziness, followed by headache, pain and irritation of the eyes,
lacrimation, photophobia and conjunctivitis. Urinalysis was
abnormal; the urine was dark yellow in colour with sediment and
contained numerous erythrocytes and leucocytes with moderate
numbers of oxalate crystals. Recovery was gradual within 2 weeks
and complete after 1 month.
8.2.2 Epidemiological studies
Identified data are limited to studies of workers exposed to
mixtures of phthalates. These include two cross-section studies
in which similar neurological symptoms were reported (Milkov et
al., 1973) (Gilioli et al., 1978) and a cross-sectional
investigation of reproductive effects (Aldyreva et al., 1975).
Neurological effects were examined based on clinical
examinations and self-reported symptoms in a cross-sectional
study of workers employed in the manufacture of artificial
leather (Milkov et al., 1973). The workers were exposed and to
DBP and also to di(2-ethylhexyl) phthalate, di-iso-octyl-
phthalate and small amounts of di- n-butyl sebacate, di(2-
ethylhexyl) sebacate and their respective adipates. Tricresyl
phosphate was also present in 10-20% of machines used by various
workers. The study group consisted of 147 workers (87 females
and 60 males), the majority (75%) of whom were less than 40 years
old. Pain in the upper and lower extremities, accompanied by
spasms and numbness, was reported in 57% of those employed for 6
to 10 years (28 persons) and 82% of those employed for more than
10 years (65 persons). These symptoms generally developed after
6-7 years of employment and the pain became continuous with
increasing length of employment. Weakness and pain in the legs
were usually more noticeable on exercise. Polyneuritis was noted
in 47 workers (32 with an autonomic-sensory form and 15 with a
mixed form) predominantly among those with greater length of
employment. Another 22 workers (15%) were reported to have
"functional disturbance of the nervous system". Approximately
50% of the workforce was considered normal by the authors. The
study was limited, however, by the lack of comparison of effects
in the exposed workers with those in an appropriate control
group. Moreover, it is difficult to attribute the observed
effects due to DBP since workers were exposed to a mixture of
phthalates and other compounds, including tricresyl phosphate
which is believed to induce polyneuritis.
A cross sectional study of neurological symptoms based on
clinical examination was carried out on three groups of male
workers in Italy who were involved in the production of phthalate
esters (Gilioli et al., 1978). The first group of workers was
exposed to phthalates (23 subjects), while the second and third
groups were exposed to alcohols (9 subjects) and phthalic
anhydride (6 subjects), both chemical precursors of phthalate
esters. The phthalates involved were di- n-butyl, diisobutyl,
di(2-ethylhexyl) and dioctyl phthalates. Mean concentrations of
phthalates varied from 1 to 5 mg/m3; peak levels were as high as
61 mg/m3. Phthalate-exposed workers frequently complained of
paraesthesia of the upper and lower limbs. These symptoms became
continuous with increasing length of employment. Excessive
perspiration of the hands and feet and vasomotor irregularity
indicative of autonomic effects were observed in 3 workers.
Neurological examination revealed polyneuropathy in 12 (57%) of
the workers exposed to phthalates. In seven workers, bilateral
painful decreased sensitivity of skin or senses of the hands and
feet were noted; three had decreased sense of vibrations.
Sensory neuropathy was observed in two workers with long-term
exposure (13 and 18 years) in the alcohol department;
hyporeflexia was observed in one worker in the phthalic anhydride
department. However, the authors suggested that no definite
conclusions could be drawn from this study because of the small
number of workers examined.
Only one study on the reproductive effects of DBP in humans
has been reported. In this cross-sectional investigation
(Aldyreva et al., 1975), workers were reported to have been
exposed to levels of DBP in excess of the Maximum Allowable
Concentration (0.5 mg/m3); however, quantitative data were not
provided. Based on gynaecological examinations of 189 women
working in processes involving exposure to DBP, approximately 33%
were considered to be healthy while 33% were reported to have
"deviations of the uterus". The health status of the remaining
34% was not disclosed. Decreases in the frequency of pregnancy
and births were reported in women exposed to phthalates, when
compared with controls; however, quantitative data on the
prevalence of effects and the composition of the control group
were not specified. There were decreases in the frequency of
miscarriages (no quantitative data was reported), although this
probably reflected the decreased frequency of pregnancy. Based
on colpocytological examination of 19 of the 189 women, 3 women
had normal biphasic (oestrogen/progesterone) vaginal cycles,
2 women had biphasic cycles but with insufficient progesterone
activity and 3 also had biphasic cycles which were hypohormonal.
Anovulatory hypoestrogenous cycles in 10 women and an anovulatory
hyperestrogenous cycles in 1 woman were observed. In a control
group, the composition of which was not described, single-phase
hyperestrogenous cycles and 2-phase hypoprogesterone cycles were
common (total incidence 21/28). The authors believed that these
results indicated a general increase in progesterone levels in
exposed women though specific data were not provided to support
this contention. It is possible that exposure to DBP may
contribute to the induction of hormonal changes reflected in
reduced fertility and changes in the vaginal cycle. However, on
the basis of the results from this study, it is difficult to draw
meaningful conclusions owing to inadequate documentation and lack
of confirmation of these observations. In addition, quantitative
data on exposure of the workers (who were also exposed to a
variety of other unspecified compounds) to DBP were not provided.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
The results of toxicity studies in various organisms are
presented in Tables 12 (microorganisms and algae), 13 (aquatic
invertebrates) and 14 (fish). Those in which effects were
observed at lowest concentrations are summarized in the following
sections.
9.1.1 Microorganisms
The toxicity of DBP to microorganisms is summarized in
Table 12.
Concentrations of DBP up to 300 mg/litre did not inhibit
methanogenesis in an anaerobic toxicity assay using secondary
sludge as the source of a heterogeneous anaerobic population
(O'Connor et al., 1989).
In water, the 5- and 30-min EC50 value for DBP was
10.9 mg/litre in the Microtox Test (Tarkpea et al., 1986).
Yoshioka et al. (1985) reported a 24-h EC50 (cell proliferation)
of 2.2 mg DBP/litre for the protozoan, Tetrahymena pyriformis.
Based upon the Microtox test, in which reduction in light
emitted by the luminescent marine bacteria Photobacterium
phosphoreum is determined, the 15-min apparent effects
threshold (AET)a was estimated to be 1.4 mg DBP/kg dry weight
for Puget Sound sediment (Tetra Tech Inc., 1986).
a The AET is defined as the concentration above which
statistically significant adverse effects are always
expected relative to appropriate reference conditions. This
approach involves comparison of data on the composition of
sediments collected in contaminated areas to measures of
biological effects associated with these sediments. The
site specificity and lack of assessment of cause-effect
relationships should be borne in mind when interpreting
apparent effects thresholds.
Table 12. Toxicity of DBP to microorganisms and algae
Species Study Type End Point Valuea Reference
Microorganisms
Bacterium
(Photobacterium) Microtox 15-min EC50 1400 µg/kg dw sediment (m) Tetra Tech Inc. (1986)
(reduced luminescence)
Bacterium
(Photobacterium phosphoreum) Microtox 5-min EC50 10 900 µg/litre (n) Tarkpea et al. (1986)
Bacterium
(Photobacterium phosphoreum) Microtox 15-min EC50 11 100 µg/litre (n) Tarkpea et al. (1986)
5-min & 30-min EC50 10 900 µg/litre (n)
Protozoan
(Tetrahymena pyriformis) Acute, static 24-h EC50 2200 µg/litre (n) Yoshioka et al. (1985)
(cell proliferation)
Table 12. Continued
Species Study Type End Point Valuea Reference
Plants
Green alga
(Scenedesmus subspicatus) Acute, static 48-h EC10 (biomass) 1400 µg/litre (n) Kühn & Pattard (1990)
48-h EC50 (biomass) 3500 µg/litre (n)
48-h EC10 (growth rate) 2600 µg/litre (n)
48-h EC50 (growth rate) 9000 µg/litre (n)
Green alga
(Selenastrum capricornutum) Acute, static 96-h EC50 (growth inhibition) 750 µg/litre (m) CMA (1984)
96-h EC50 (TLM) (survival rate) 20-600 µg/litre (n) Wilson et al. (1978)
96-h EC50 (growth) 3.4-200 µg/litre (n)
Green alga
(Selenastrum capricornutum) Chronic, static 10-day EC30 (dec. cell 750 µg/litre (m) Springborn Bionomics
numbers) (1984a)
Green alga
(Selenastrum capricornutum) Chronic, static 7-day NOEL (dec. biomass) 2800 µg/litre (n) Melin & Egnéus (1983)
7-day LOEL (dec. biomass) 28 000 µg/litre (n)
a n = nominal concentration; m = measured concentration
9.1.2 Aquatic organisms
9.1.2.1 Algae
The toxicity of DBP to algae is summarized in Table 12.
A 96-h EC50 (decreased cell numbers) of 750 µg DBP/litre
was reported for the green alga Selenastrum capricornutum
(Springborn Bionomics, 1984a). Kühn & Pattard (1990) reported
48-h EC10 and EC50 values for DBP of 1400 µg/litre and
500 µg/litre, respectively, for Scenedesmus subspicatus, based
on biomass. The 96-h EC50 values for the marine dinoflagellate
Gymnodinium breve were 3.4-200 µg DBP/litre based on growth and
20-600 µg DBP/litre based on survival (Wilson et al., 1978).
However, care must be taken when interpreting these data because
the two sets of values are not ranges but each are two replicates
with large variations.
Yan et al. (1995) report 96-h EC50 values for dimethyl and
diethyl phthalate, based on inhibition of growth of Chlorella
pyrenoidosa; however, an EC50 for DBP could not be generated
within the range of its water solubility (13 mg/litre).
At concentrations of about 7000 µg/litre and 2800 µg/litre,
DBP reduced the growth rates of the monodispersed marine plankton
(i.e. non-aggregated) Thalassiosira pseudomona (diatom) and
Dunaliella parva (green algae), respectively (Acey et al.,
1987).
9.1.2.2 Invertebrates
Acute and chronic toxicity data for aquatic invertebrates
are summarized in Table 13.
The most sensitive aquatic invertebrates, based on acute
toxicity tests, are the Mysid shrimp, Mysidopsis bahia, with a
96-h LC50 of 750 µg/litre (EG&G Bionomics, 1984a), and the midge
Chironomus plumosus, with a 48-h EC50 (based on
immobilization) of 760 µg DBP/litre (Streufert et al., 1980).
For chronic studies the most sensitive species in a standard
test is Daphnia magna with a 21- day NOEC, based on parent
survival, of 500 µg/litre (measured value) (Kühn et al., 1989).
Another sensitive invertebrate species is the scud, Gammarus
pulex, with a 10-day LOEL of 500 µg DBP/litre and a NOEC of
100 µg/litre, based on reduced locomotor activity (Thurén & Woin,
1991). A 7-day EC50 of 540 µg DBP/litre has been reported for
the planarian Dugesia japonica, based on reduced head
regeneration (Yoshioka et al., 1986).
Table 13. Toxicity of DBP to aquatic invertebrates
Species Study type End-point Concentrationsa Reference
Water flea
(Daphnia magna) Acute, static 48-h LC50 5200 µg/litre McCarthy & Whitmore (1985)
Acute, static 48-h EC50 3400 µg/litreb CMA (1984)
Acute, static 48-h LC50 3700 µg/litreb Call et al. (1983)
renewal
Acute, static 24-h EC50 17 000 µg/litre Kühn et al. (1989)
Acute, static 24-h EC0 8900 µg/litre
Grass shrimp
(Palaemonetes pugio) Acute, static 96-h LC0 1000 µg/litre Clark et al. (1987)
(water
exposure)
96-h LC50 >1000 µg/litre
(water
exposure)
96-h LC0 10 mg/kg
(sediment
exposure)
96-h LC50 > 10 mg/kg Clark et al. (1987)
(sediment
exposure)
Table 13. Continued
Species Study type End-point Concentrationsa Reference
Grass shrimp
(Palaemonetes pugio) 10-day LC0 10 mg/kg
(sediment
exposure)
10-day LC50 > 10 mg/kg
(sediment
exposure)
Mysid shrimp Acute, static 96-h LC50 750 µg/litre EG & G Bionomics (1984a)
(Mysidopsis bahia)
Scud Acute, static 24-h LC50 7000 µg/litre Mayer & Sanders (1973)
(Gammarus pseudolimnaeus)
96-h LC50 2100 µg/litre
Brine shrimp Sublethal, static 72-h NOEL < 10 000 µg/litre Sugawara (1974a, 1974b)
(Artemia salina) (egg hatching,
larvae survival)
Acute, static 24-h LC50 8000 µg/litre Hudson et al. (1981)
Acute, static 24-h LC50 5600 µg/litre Hudson & Bagshaw (1978)
Crayfish Acute, static 24-h LC50 + > 10 000 µg/litre Mayer & Ellersieck (1986)
(Orconectes nais) 96 h LC50
Harpacticoid Acute, static 96-h LC50 1700 µg/litre Lindén et al. (1979)
(Nitocra spinipes)
Table 13. Continued
Species Study type End-point Concentrationsa Reference
Midge larvae Acute, static 48-h LC50 5400 µg/litre Mayer & Ellersieck (1986)
(Chironomus plumosus)
48-h LC50 4000 µg/litre
48-h EC50 760 µg/litre Streufert et al. (1980)
(immobilization)
Midge Acute, static 48-h EC50 5800 µg/litre EG & G Bionomics (1984b)
(Paratanytarsus parthenogenica)
Benthic community composition Chronic, 14-day LOEL 340 µg/litre Tagatz et al. (1983)
flow-through (decrease number
of amphipods)
Planarian Acute, static 7-day EC50 (head 540 µg/litre Yoshioka et al. (1986)
(Dugesia japonica) regeneration)
Acute, static 7-day LC50 840 µg/litre
(increased
abnormalities)
Water flea Chronic, 21-day NOEL 960 µg/litreb CMA (1984)
(Daphnia magna) flow through (mortality)
21-day LOEL 2500 µg/litreb
(mortality)
Chronic, static 16-day LOEL 1800 µg/litre McCarthy & Whitmore (1985)
renewal (survival and
reproduction)
Table 13. Continued
Species Study type End-point Concentrationsa Reference
Water flea 16-day NOEL 560 µg/litre
(Daphnia magna) (survaval and
reproduction)
21-day LC50 1920 µg/litreb DeFoe et al. (1990)
21-day EC50 1640 µg/litreb
(reproduction)
21-day EC50 1050 µg/litreb
(reproduction)
Chronic, static 21-day NOEL 500 µg/litreb Kühn et al. (1989)
renewal (parent survival)
Chronic, static 21-day LOEL 2500 µg/litreb Springborn Bionomics (1984b)
renewal (survival and
reproduction)
Grass shrimp Chronic, static 10-day LC0 10 mg/kg Clark et al. (1987)
(Palaemonetes pugio) (sediment
exposure)
10-day LC50 > 10 mg/kg
(sediment
exposure)
Chronic, 28-day LOEL 1000 µg/litre Laughlin et al. (1978)
semi-static (survival)
28-day NOEL 500 µg/litre
(survival)
Table 13. Continued
Species Study type End-point Concentrationsa Reference
Scud (Gammarus pulex) Chronic, 10-day LOEL > 500 µg/litre Thurén & Woin (1991)
flow-through (survival)
10-day LOEL 500 µg/litre
(decreased
locomotor activity)
10-day NOEL 100 µg/litre
(decreased
locomotor activity)
Midge (Chironomus plumosus) Chronic, 30-day LOEL > 560 µg/litre Streufert & Sanders (1977)
flow-through (larval emergence)
a all concentrations are nominal unless stated otherwise
b measured concentration
In both laboratory and field studies with estuarine benthic
systems, DBP had statistically significant effects on 8-week
colonization at 1000 mg/kg sediment, the highest nominal
concentration tested (Tagatz et al., 1986). In the laboratory
study, the total number of species per box was significantly
decreased by DBP, while in the field study, only the total number
of individual molluscs was affected. The actual exposure
concentrations were lower than the nominal concentrations, as
only 48% and 19% of the original concentration persisted in the
laboratory and field systems, respectively, during the last two
weeks of the study. In an earlier study in which DBP was
introduced into the water rather than into the sediment, Tagatz
et al. (1983) reported that colonization was significantly
reduced at concentrations of 3700 and 3800 µg/litre in
laboratory- and field-colonized communities, respectively. At
340 µg/litre, there was no statistically significant effect on
the total numbers of species or individuals in the laboratory-
colonized community, except that the number of Corophium
acherusicum (amphipods) was significantly reduced. At
450 µg/litre, DBP did not have a statistically significant effect
on the field-colonized community.
9.1.2.3 Vertebrates
Acute and chronic toxicity data for fish are summarized in
Table 14.
In acute toxicity tests the yellow perch ( Perca flavescens)
and the channel catfish ( Ictalurus punctatus) were the most
sensitive freshwater fish with 96-h LC50 values of 350 and
460 µg DBP/litre respectively (Mayer & Ellersieck, 1986). The
sheepshead minnow, Cyprinodon variegatus, for which a 96-h
LC50 of 600 µg/litre has been reported (CMA, 1984), was the most
sensitive marine fish species identified.
Yoshioka et al. (1986) reported a 48-h LC50 of 630 µg
DBP/litre for the red killifish, Oryzias latipes, while the
96-h LC50 values were 730 µg DBP/litre in the bluegill
( Lepomis macrochirus) (Mayer & Sanders, 1973) and 850 µg
DBP/litre in the fathead minnow ( Pimephales promelas) (DeFoe
et al., 1990).
The most sensitive chronic study was based on the rainbow
trout ( Oncorhynchus mykiss) in an early life stage test with a
99-day no-observed-effect concentration (NOEC) (growth) of
100 µg/litre, a 99-day LOEC of 190 µg/litre (growth reduced by
about 27%) and 100% mortality on day 40 at 400 µg/litre (Ward &
Boeri, 1991). In an early life stage test on fathead minnows
( Pimephales promelas) a 20-day NOEC, based on hatching rate
and larval survival, of 560 µg/litre was reported (McCarthy &
Whitmore, 1985).
Table 14. Toxicity of DBP to fish
Species Study type End-Point Concentration References
(µg/litre)a
Fathead minnow Acute, static 24-h LC50 3300 Mayer & Ellersieck (1986)
(Pimephales promelas)
Acute, static 24-h LC50 3000 EG & Bionomics (1983a)
Acute, flow-through 24-h LC50 4800 Mayer & Ellersieck (1986)
Acute, flow-through 24-h LC50 1600 EG & G Bionomics (1983b)
Acute 48-h LC50 1490 Mayer & Sanders (1973)
Acute, static 48-h LC50 + 96 h LC50 3000 EG & Bionomics (1983a)
Acute, flow-through 48-h LC50 1200 EG & Bionomics (1983b)
Acute, static 96-h LC50 2020 McCarthy & Whitmore (1985)
Acute, static 96-h LC50 1300 Mayer & Ellersieck (1986)
Acute, flow-through 96-h LC50 850b DeFoe et al. (1990)
commercial phthalate
Acute, flow-through 96-h LC50 1100b DeFoe et al. (1990)
syntherised phthalate
Acute, flow-through 96-h LC50 3950 Mayer & Ellersieck (1986)
Acute, flow-through 96-h LC50 920 EG & Bionomics (1983b)
Acute, flow-through 96-h LOEL 1800 McCarthy & Whitmore (1985)
(embryo survival)
96-h NOEL 1000
(embryo survival)
Table 14. Continued
Species Study type End-Point Concentration References
(µg/litre)a
Yellow perch Acute, flow-through 24-h LC50 >1240 Mayer & Ellersieck (1986)
(Perca flavescens)
96-h LC50 350
Bluegill Acute, static 24-h LC50 1230 Mayer & Sanders (1973)
(Lepomis macrochirus)
24-h LC50 2100 Buccafusco et al. (1981)
24-h LC50 >3000 Mayer & Ellersieck (1986)
24-h LC50 1000 EG & G Bionomics (1983c)
48-h LC50 1200
96-h LC50 1200 Buccafusco et al. (1981)
96-h LC50 730 Mayer & Sanders (1973)
96-h LC50 2100 at pH 6.5 Mayer & Ellersieck (1986)
96-h LC50 1580 at pH 7.5
96-h LC50 2050 at pH 9.0 Mayer & Ellersieck (1986)
96-h LC50 850 EG & G Bionomics (1983c)
96-h LC50 1550 Mayer & Ellersieck (1986)
Table 14. Continued
Species Study type End-Point Concentration References
(µg/litre)a
Channel catfish Acute 24-h LC50 3720 Mayer & Sanders (1973)
(Ictalurus punctatus)
96-h LC50 2910 Mayer & Sanders (1973)
Acute, flow-through 96-h LC50 460 Mayer & Ellersieck (1986)
Rainbow trout
(Oncorhynchus mykiss) Acute, static 24-h LC50 > 16 000 Mayer & Ellersieck (1986)
Acute, static 24-h LC50 2800
Acute, flow-through 24-h LC50 + 48-h LC50 1600 EG & G Bionomics (1983d)
24-h LC50 4200 Mayer & Ellersieck (1986)
Acute, static 96-h LC50 2560
96-h LC50 1200 Hrudey et al. (1976)
96-h LC50 6470 Mayer & Sanders (1973)
Acute, flow-through 96-h LC50 1480 Mayer & Ellersieck (1986)
Acute, flow-through 96-h LC50 1600 EG & G Bionomics (1983d)
Acute, flow-through 24-h LC50 + 96-h LC50 > 1240 Mayer & Ellersieck (1986)
(yolk-sac fry)
Red killifish Acute, static 48-h LC50 630 Yoshioka et al. (1986)
(Orizias latipes)
Table 14. Continued
Species Study type End-Point Concentration References
(µg/litre)a
Sheephead minnow Acute, flow-through 96-h LC50 600b CMA (1984)
(Cyprinodon variegatus)
Fathead minnow Chronic, flow-through 20-day EC100 1800 McCarthy & Whitmore (1985)
(Pimephales promelas) (embryo mortality)
20-day LOEL (hatching 1000
rate and larval survival)
20-day NOEL (hatching 560
rate and larval survival)
Rainbow trout Chronic, flow-through 99-day NOEL (growth) 100b Ward & Boeri (1991)
(Oncorhynchus mykiss)
Chronic, flow-through 99-day LOEL (growth) 190b
Chronic, flow-through 40-day LC100 400b Ward & Boeri (1991)
Cyprinodontiform fish Chronic, static 147-day 13% reduction 2000 Davis (1988)
(Rivulus marmoratus) in embryonic viability
Chronic, static 147-day 155% increase 1000
in skeletal abnormalities
in progeny of exposed fish
Post-exposure 63-day NOEL 1000
(reproduction)
Post-exposure 63-day LOEL 2000
(reproduction)
a Concentrations are nominal unless stated otherwise
b Measured concentrations
9.1.3 Terrestrial organisms
9.1.3.1 Plants
DBP vapour from flexible plastics (e.g., glazing strips)
used in greenhouses has been implicated in development of damage
to plants. The threshold concentration for visible damage in
summer cabbage, Brassica oleracea L. cv. Derby Day was between
0.141 and 0.360 µg DBP/m3, the latter figure determined in a
4-week laboratory experiment in which growth restriction,
chlorosis and cotyledon death were observed (Hardwick et al.,
1984). However, it is unclear whether other phthalates
influenced the observed toxicity.
At higher concentrations in air, DBP caused damage in other
plant species. In strong light, leaves of radish seedlings
( Raphanus sativus) faded to pale green due to the
disappearance of carotenoid and chlorophyll pigments after 6 days
exposure to 41.3 to 62.3 µg DBP/m3 air and to white after 9 days
exposure to 56.5 to 90.7 µg DBP/m3 (Virgin, 1988). Such effects
were not observed in wheat seedlings ( Triticum aestivum) when
exposed to DBP vapour alone, but they did develop when the
seedlings were also treated with DBP-saturated water.
DBP inhibited photosynthesis in radish plants ( Raphanus
sativus) exposed to 120 µg DBP/m3 at a rate of 0.003 m3/min
for 13 days (Millar and Hannay, 1986). Concentrations of DBP as
low as 10 µmoles/m3 (approximately 2800 µg/m3) reduced
uncoupled electron transport in isolated spinach thylakoids by
about 13%, while 44 µmoles/m3 (approx. 12 250 µg/m3) caused a
50% reduction. Basal electron transport rates were reduced by
50% at 87 µmoles/m3 (approx. 24 200 µg/m3).
Application of DBP to leaves of white mustard ( Sinapis
alba) at a rate of 1.5 µg/cm2 caused chlorosis in new leaves
as they appeared on the third day after treatment (Lokke &
Bro-Rasmussen, 1981). This effect did not occur with DBP
application to nipplewort ( Lapsana communis) or to milfoil
( Achillea millefolium). Plants can also be adversely affected
by exposure to DBP in the soil. DBP concentrations in soil of
200 mg/kg or more reduced the germination of soybeans ( Glycine
max) by > 33% and decreased the growth of corn ( Zea mays)
and soybeans by 29 to 80% (Overcash et al., 1982). Plant height
and shoot weight were significantly reduced by 17 and 25%,
respectively, when corn seeds were planted in soil containing
2000 mg DBP/kg and grown for 3 weeks. Growth was not affected at
a concentration in soil of 200 mg DBP/kg (Shea et al., 1982).
A concentration of 1000 mg DBP/litre (added as a methanol
solution) reduced seed germination by 48% in peas ( Pisum
sativum) and by 58% in spinach ( Spinacia oleracea) grown in
tap water, but there was no observable effect on subsequent
development of the seeds that did germinate (Herring & Bering,
1988). It should be noted, however, that this concentration is
many times higher than the saturation concentration of DBP in
water (about 10 mg/litre).
9.1.3.2 Invertebrates
The LC50 for DBP in the earthworm Eisenia fetida was
1360 µg/cm2 in a 2-day contact test in which the chemical was
applied to filter paper (the toxic units referring to the amount
of chemical per cm2 of paper). In comparison, the LC50 of
dimethylphthalate was 550 µg/m2 and that of 2,4-dinitrophenol
was 0.6 µg/m2 (Neuhauser et al., 1985, 1986).
DBP applied to female house flies topically or by injection
at a concentration of 20 µg/fly (1000 µg/g body weight) was not
toxic, causing a mortality of less than 16% after 24 h (Al-Badry
& Knowles, 1980). Antagonistic interactions were observed when
flies were treated simultaneously with DBP and various
organophosphate insecticides, while synergistic interactions were
observed when flies were pretreated with the phthalate 30 min
before exposure to the pesticides. DBP inhibited the metabolism
of organophosphate pesticides, accounting for the synergistic
effects. When the phthalate and insecticides were applied
simultaneously, the resulting increase in the total lipophilic
pool by DBP may have resulted in an internal concentration of
insecticide below the toxicity threshold.
9.1.3.3 Vertebrates
Hill et al. (1975) found no deaths among 10-day-old mallard
( Anas platyrhynchos) fed up to 5000 mg DBP/kg for 5 days,
followed by 3 days on a normal diet.
In a study in which ring doves ( Streptophelia risoria)
were fed a diet containing 10 mg DBP/kg (1.1 mg DBP/kg body
weight per day) for a period of 3 weeks prior to mating through
completion of a clutch of two eggs, there was a 23% increase in
water permeability and a 10% decrease in egg- shell thickness
(Peakall, 1974). A 15% decrease in shell thickness is considered
significant for reproductive effects. Rapid recovery occurred
upon cessation of exposure.
Korhonen et al. (1983) studied the embryotoxicity of DBP to
white leghorn chicken eggs. On the third day of incubation, DBP
was injected on the inner shell membrane at doses of 13 and
26 µmol per egg (3.62 and 7.24 mg/egg, respectively). At 26 µmol
per egg, 30 eggs were tested and there were 6 early deaths (2
days after injection) and 4 non-malformed and 1 malformed late
deaths (between 3 and 11 days after injection). An approximate
ED50 of 33 µmol (9.19 mg) per egg was calculated for DBP.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Based on the limited data available, the principal media of
exposure to DBP for the general population, listed in order of
their relative importance based upon estimated intake, are as
follows: food, indoor air and drinking-water. Estimated intakes
from food and indoor air are 7 µg/kg body weight per day and
0.42 µg/kg body weight per day, respectively. Estimated intakes
from drinking-water and ambient air are considerably less,
< 0.02 µg/kg body weight per day and 0.26-0.36 ng/kg body weight
per day, respectively. Based on these intakes, it is estimated
that the total average daily intake from air, drinking-water and
food is 7.4 µg/kg body weight per day. It should be noted,
however, that intake of DBP in the diet can vary considerably,
depending upon the nature and extent of packaged food consumed
and the nature of use of food wrapping in food preparation. In
the United Kingdom, the Ministry of Agriculture, Fisheries and
Food has estimated that the maximum likely human intake of DBP
from food sources is approximately 2 mg per person per day
(approximately 31 g/kg body weight per day, assuming a mean body
weight of 64 kg). There is also potential for exposure to DBP in
cosmetics, although available data are inadequate to quantify
intake from this source.
The most recent provisional data from the NIOSH National
Occupational Exposure Survey indicate that in the USA over
500 000 workers, including 200 000 women, are potentially exposed
to DBP. At a limited number of worksites in the USA,
concentrations were generally less than the limit of detection
(i.e., 0.01 to 0.02 mg/m3).
10.1.2 Health effects
The acute toxicity of DBP in mice and rats is low.
In a case of accidental poisoning of a worker who ingested
approximately 10 grams of DBP, recovery was gradual within 2
weeks and complete after 1 month.
Based on limited available data in animal species, DBP
appears to have little potential to irritate skin or eyes,
although in humans a few cases of sensitization after exposure
have been reported.
Available data on the effects of DBP in humans are limited
to those of workers exposed to mixtures of phthalates and are
inadequate to serve as a basis for assessment of effects of DBP.
The remainder of this evaluation is, therefore, based on studies
in animals.
The profile of effects following exposure to DBP is similar
to that of other phthalate esters, which, in susceptible species,
induce hepatomegaly and increase numbers of hepatic peroxisomes,
are fetotoxic, have teratogenic potential and produce testicular
damage.
Adequate carcinogenesis bioassays for DBP have not been
conducted. The weight of available evidence indicates that DBP
is not genotoxic.
As a class, chemicals which cause peroxisome proliferation
are often hepatocarcinogenic via a non-genotoxic mode of action.
Although the mechanism of action remains unknown, tumour
formation is preceded by peroxisomal proliferation and
hepatomegaly. As a chemical causing peroxisomal proliferation,
it is possible that DBP might be a rodent liver carcinogen,
although it is much weaker in inducing hepatomegaly and
peroxisome proliferation than DEHP. To the degree that
hepatomegaly and peroxisomal proliferation correlate with
carcinogenic potency, DBP would be anticipated to be a less
potent carcinogen than DEHP and would probably exhibit no
activity as measured by current cancer bioassay methodologies.
Thus, it is unlikely that DBP presents any significantly
increased risk of cancer at concentrations generally present in
the environment.
Effects of DBP observed at lowest doses in repeated dose
toxicity studies are those on the liver and testes of the rat and
include hepatomegaly and peroxisome proliferation. In one study,
hepatic necrotic changes were also reported. Effects on the
testes include decreases in the activities of testicular enzymes
and, at higher doses, degeneration of the germinal epithelium and
reductions in testicular zinc levels. DBP also induces adverse
effects on fertility, is fetotoxic and induces teratogenic
effects at high concentrations that are toxic to the dams.
Toxicity to the testes is more marked when exposure to DBP occurs
during development and maturation than when adults only are
exposed. Lowest reported effect levels in adequate studies for
these various effects and their associated no-observed-(adverse)-
effect levels (NOEL/NOAEL) are summarized in Table 15.
Table 15. Effect levels of DBP
No- Lowest-
observed- observed-
(adverse)- (adverse)-
effect levela effect
levelb
End-point Species (mg/kg b.w. per day) Reference
Liver:
Organ weight rat, - 120 Nikonorow
(relative) Wistar et al. (1973)
Peroxisomal rat, 176 356 NTP (1995),
proliferation F-344 Study No. 2
and hepatomegaly
rat, 138 279 NTP (1995),
F-344 Study No. 3
Necrosis (not rat, 250 Murakami et
confirmed) Wistar al. (1986a)
Testis:
Enzymes rat, 250 Srivastava
Wistar et al.
(1990a,b)
Histopathological rat, 359 720 NTP (1995),
lesions F-344 Study No. 2
rat, 279 571 NTP (1995),
F-344 Study No. 3
Table 15. contd.
No- Lowest-
observed- observed-
(adverse)- (adverse)-
effect levela effect
levelb
End-point Species (mg/kg b.w. per day) Reference
Reproduction/fertility/ rat, NI c 66NTP (1995;
developmental Sprague- Wine et al.,
Dawley 1997),
Study No. 4
Developmental mouse, 100 400 Hamano et al.
JCL:ICR (1977)
a Each value in this column is either a NOEL or NOAEL
b Each value in this column is either a LOEL or LOAEL
c NI = not identified
10.1.3 Guidance values
The following guidance is provided as a potential basis for
derivation of limits of exposure by relevant authorities. Since
ingestion is by far the principal route of exposure to DBP and
since the toxicological data for other routes of administration
are insufficient for evaluation, only the oral route is
addressed here. However, the ultimate objective should be
reduction of total exposure from all sources to less than the
tolerable daily intake presented below.
The Task Group considered that the testicular and
reproductive/developmental effects are the most relevant for
derivation of guidance values for protection of human health.
Increases in liver weight, hepatomegaly and peroxisome
proliferation were regarded by the Task Group as being
functional, relating most likely to the metabolism of the
material, rather than pathological. Moreover, although hepatic
necrosis was observed in one strain of rats at 250 mg/kg body
weight per day, it was not observed in two other strains at much
higher doses.
The NOAEL/NOEL values for the end-points considered to be
most appropriate for derivation of guidance values (i.e.
developmental and reproductive toxicity) have not been identified
in the Continuous Breeding study (NTP study 4); the lowest dose
studied (66 mg/kg body weight per day) is a LOAEL (NTP, 1995;
Wine et al., 1997). On the basis of these data, an acceptable
daily intake (ADI) is derived as follows:
ADI = 66 mg/kg body weight per day
1000
= 0.066 mg/kg body weight per day
= 66 g/kg body weight per day
where:
* 66 mg/kg body weight per day is the approximate LOAEL for
developmental and reproductive effects in rats observed in
the most sensitive studies conducted to date
* 1000 is the uncertainty factor (×10 for interspecies
variation, ×10 for interindividual variation, ×10 for lack
of data on a NOAEL. A factor of 10 for lack of a NOAEL was
considered adequate since the effects observed at the lowest
dose levels were moderate and probably reversible. The
severe, possibly irreversible, teratogenic, testicular and
epididymal effects were only observed at the highest dose
level tested, which also produced other signs of toxicity.
Because DBP is rapidly metabolized and eliminated, with no
evidence of accumulation in tissues, no additional factor
was incorporated for lack of data on chronic effects.
10.2 Evaluation of effects in the environment
10.2.1 Exposure
DBP exists widely in the environment, being released during
production, processing, usage and disposal. However, it is
relatively non-persistent in air and surface water. The most
important process leading to the elimination of DBP is biological
breakdown, aerobic degradation being rapid and complete. It would
be expected to be more persistent in anaerobic sediments. It is
moderately adsorbed to soil.
DBP would be expected to bioaccumulate, based on a log Kow
of 4.3 to 4.7. However, it tends to be readily metabolized
leading to bioconcentration factors lower than predicted.
Biomagnification in terrestrial animals is unlikely.
Mean concentrations of DBP in surface water tend to be less
than 1 µg/litre. However, levels in polluted rivers are much
higher, with values of 12 to 34 µg/litre. Levels in sediment are
generally less than 1 mg/kg dry weight although in polluted areas
concentrations of up to 10 mg/kg have been measured. DBP
concentrations in sewage sludge range from 0.2 to 200 mg/kg dry
weight.
10.2.2 Effects
A comparison of the results of acute and long-term tests on
aquatic organisms shows that there is no increase in toxic
effects with increasing duration of exposure.
In acute toxicity tests the sensitivity of the different
trophic levels is similar. The 48-h and 96-h LC50 and EC50
values for the most sensitive species are in the range of 350 to
760 µg/litre for freshwater organisms and 600 to 750 µg/litre for
marine organisms.
The most sensitive chronic study was based on the rainbow
trout; the 99-day no-observed-effect concentration (NOEC) based
on growth was 100 µg/litre and the lowest-observed-effect
concentration (LOEC) 190 µg/litre.
The acute toxicity of DBP to birds is low.
10.2.3 Risk evaluation
The lowest reported chronic effect level for dissolved DBP
in aquatic organisms was 190 µg/litre (99-day LOEC for growth)
and the lowest NOEC was 100 µg/litre in the same test. These
values are at least factors of 190 and 100, respectively, greater
than the mean surface water concentration of DBP. Therefore, the
risk to aquatic organisms from mean DBP concentrations in surface
water is low. However, in highly polluted rivers where surface
water concentrations have been found to be up to 34 µg/litre, the
ratio between the concentration and the NOEC is only 3.
There is inadequate data to assess the risk of DBP to
sediment-dwelling organisms.
The most likely route of exposure for higher organisms,
e.g., birds and mammals, is through food intake, in particular
fish.
The only acute toxicity test on birds was carried out on
10-day-old mallards, where a 5-day LC50 of > 5000 mg/kg diet
was found. Based on food consumption and body weight, an LD50
for the mallard of > 2043.5 mg/kg body weight can be calculated.
Using this data an estimated LC50 for a fish-eating bird (e.g.,
kingfisher), based on body weight and food consumption, can be
calculated.
LC50 (mg/kg dry weight of diet)
test species LD50 (mg/kg) body wt (kg)
=
food consumption (kg)
The estimated LC50 for the fish-eating bird is >
9350 mg/kg diet. The highest water concentration (34 µg/litre)
multiplied by the highest bioconcentration factor (590) gives a
residue level in fish of 20 mg/kg. Comparing this value to the
estimated LC50 value gives a Toxicity Exposure Ratio (TER) of
> 470. A TER of less than 1 would give cause for concern; but a
value of > 470 indicates that the risk to fish-eating birds from
DBP is very low.
For mammals, the mink, a terrestrial mammal with a diet
consisting predominantly of aquatic prey, can be used. The
estimated intake for a "worst case" scenario is 3.1 mg/kg body
weight per day. This is based on an ingestion rate of 155 g per
day and assumes a diet of 75% fish, a maximum measured
bioconcentration factor of 590 for the fathead minnow, and a
maximum concentration of DBP in water of 34 µg/litre. The
estimated intake is considerably less than the no-observed-
adverse-effect levels in toxicity studies in laboratory mammals
(i.e. 250 mg/kg body weight per day).
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
In laboratory animals, the critical toxic effects of DBP
were those on development and reproduction at concentrations well
above those to which people are normally exposed in the general
environment. DBP is readily broken down in the environment and
in the body and shows no tendency to accumulate or to persist in
any specific tissues or organs. It is unlikely that there is any
risk to human health at present levels of exposure in the general
environment.
There is inadequate information to assess exposure from use
in cosmetics.
The risk to aquatic organisms associated with the present
mean concentrations of DBP in surface waters is low. However, in
highly polluted rivers the safety margin is much smaller. There
is inadequate data to assess the risk of DBP to sediment-dwelling
organisms. At current levels of exposure, it can be concluded
that the risk to fish-eating birds and mammals is low.
The current measures being taken to limit the release of DBP
into the environment and to control its use in food-packaging
materials should be maintained.
12. FURTHER RESEARCH
The most sensitive end-points used in determining the
guidance value were effects on reproduction, both fertility and
development. No NOAEL was identified for these effects and the
results suggest that the adverse effects of DBP are more marked
in animals exposed during development and maturation than in
animals exposed as adults only. Data on effects of exposure
during the developmental period are very limited and further work
to identify a NOEL for such exposure is urgently needed.
The number and quality of studies describing the profile of
toxicity of DBP and its behaviour in the environment are
sufficient to make reasonable assessments of potential health
effects, environmental fate and to set a guidance value for
limiting human exposure to preclude adverse health effects.
Therefore, additional research in these areas is of low priority,
relative to that for other substances.
However, one area of concern is that the potential for
exposure in cosmetics is largely unknown. It is recommended,
therefore, that additional data on the use and levels of DBP in
cosmetics be acquired. If there is a potential for considerable
additional exposure from this source, it is recommended that
controlled studies be conducted to examine the rate of skin
absorption, dosimetry, metabolism and excretion of DBP in humans.
In view of the large species differences in some toxic
effects of DBP on laboratory animals, additional studies on
kinetics and metabolism in humans or human cells are desirable.
More research is needed on the effects of DBP on sediment-
dwelling organisms.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
DBP may be used for the manufacture of regenerated cellulose
film which is intended to or does come into contact with food-
stuffs. It may be used as a plasticizer in total amount of
12.5 mg/dm2 on the side of the film in contact with foodstuffs
(EEC, 1987).
REFERENCES
Abe S & Sasaki M (1977) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster cells exposed to various
chemicals. J Natl Cancer Inst, 58(6): 1635-1641.
Aceves M & Grimalt JO (1993) Large and small particle size
screening of organic cmpounds in urban air. Atmos Environ,
B27(2): 251-263.
Acey R, Healy P, Unger TF, Ford CE, & Hudson RA (1987) Growth and
aggregation behavior of representative phytoplankton as affected
by the environmental contaminant di-n-butyl phthalate. Bull
Environ Contam Toxicol, 39: 1-6.
Adams WJ, Kimerle RA, & Barnett JW (1992) Sediment quality and
aquatic life assessment. Environ Sci Technol, 26: 1864-1875.
Agarwal DK, Lawrence WH, Nunez LJ, & Autian J (1985) Mutagenicity
evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J Toxicol Environ Health,
16(1): 61-69.
Aitio A & Parkki M (1978) Effect of phthalate esters on drug
metabolising enzyme activities in rat liver. Arch Int
Pharmacodyn, 235: 187-195.
Al-Badry MS & Knowles CO (1980) Phthalate-organophosphate
interactions: toxicity, penetration, and metabolism studies with
house flies. Arch Environ Contam Toxicol, 9(2): 147-161.
Al-Omran LA & Preston MR (1987) The interactions of phthalate
esters with suspended particulate material in fresh and marine
waters. Environ Pollut, 46: 117-186.
Albro PW & & Moore B (1974) Identification of the metabolites of
simple phthalate diesters in rat urine. J Chromatogr, 94:
209-218.
Aldyreva MV, Klimova TS, Izyumova AS, & Timofievskaya LA (1975)
[Effect of plasticisers on reproductive function.] Gig Tr Prof
Zabol, 12: 25-29 (in Russian).
Antonyuk OK & Aldyreva MV (1973) [Substantiation of the maximum
allowable concentration of dibutyl phthalate in the air of
industrial premises.] Gig Trud Prof Zabol, 12: 26-30 (in
Russian).
Ariyoshi T, Koga Y, Yoshitake S, Takada K, & Tenda N (1976)
Metabolism of dibutyl phthalate and the effects of its
metabolites on animals. Kyushu Yakugokkai Kaiho, 30: 17-22.
Atlas E & Giam CS (1981) Global transport of organic pollutants:
ambient concentrations in the remote marine atmosphere. Science,
211: 163-165.
ATSDR (1990) Toxicological profile for di-n-butylphthalate.
Atlanta, Georgia, Agency for Toxic Substances and Disease
Registry, 109 pp.
Atwater JW, Jasper SE, Parkinson PD, & Mavinic DS (1990) Organic
contaminants in Canadian coal wastewaters and associated
sediments. Water Pollut Res J Can, 25: 187-200.
Barber ED, Astill BD, Moran EJ, Schneider BF, Gray TJB, Lake BG,
& Evans JG (1987) Peroxisome induction studies on seven phthalate
esters. Toxicol Ind Health, 3(2): 7-24.
Barrick R, Becker S, Brown L, Beller H, & Pastrook R (1988)
Sediment quality values refinement: 1988 update and evaluation of
Puget Sound AET. Bellevue, Washington, PTI Environmental
Services.
Belisle AA, Reichel WL, & Spann JW (1975) Analysis of tissues of
mallard ducks fed two phthalate esters. Bull Environ Contam
Toxicol, 13(2): 129-132.
Bell FP, Patt CS, Brundage B, Gillies PJ, & Phillips WA (1978)
Studies on lipid biosynthesis and cholesterol content of liver
and serum lipoproteins in rats fed various phthalate esters.
Lipids, 13: 66-74.
Benckiser G & Ottow JCG (1982) Metabolism of the plasticizer
di-normal-butylphthalate by Pseudomonas pseudoalcaligenes under
anaerobic conditions, with nitrate as the only electron-acceptor.
Appl Environ Microbiol, 44(3): 576-578.
BIBRA (1986) A 21 day feeding study of di-n-butyl phthalate to
rats: Effects on the liver and liver lipids. Carshalton, Surrey,
The British Industrial Biological Research Association (Report to
Chemical Manufacturers Association, Washington, DC) (CMA
reference PE 28.0-BT-BIB).
Bornmann G & Loeser A (1956) The reaction of the body to the
action of various plasticizers. Z Lebensm.unters Forsch, 103:
413-434.
Bove JL, Dalven P, & Kukreja VP (1978) Airborne di-butyl and di-
(2-ethylhexyl)-phthalate at three New York City air sampling
stations. Int J Environ Anal Chem, 5: 189-194.
Brandt K (1985) Final report on the safety assessment of dibutyl
phthalate, and diethyl phthalate. J Am Coll Toxicol, 4(3):
267-303.
BUA (1987) Dibutyl phthalate. Frankfurt am Main, German Chemical
Society, Advisory Committee on Existing Chemicals of
Environmental Relevance, 71 pp (BUA Report No. 22).
Buccafusco RJ, Ellis SJ, & LeBlanc GA (1981) Acute toxicity of
priority pollutants to bluegill ( Lepomis macrochirus). Bull
Environ Contam Toxicol, 26: 446-452.
Burns BG, Musial CJ, & Uthe JF (1981) Novel cleanup method for
quantitative gas chromatographic determination of trace amounts
of di-2-ethylhexyl phthalate in fish lipid. J Assoc Off Anal Chem,
64(2): 282-286.
California Environmental Protection Agency (1992) PTEAM:
Monitoring of phthalates and PAHS in indoor and outdoor air
samples in Riverside, California. Final Report, Volume II
(Contract No. A933-144). Research Triangle Park, North Carolina,
Research Triangle Institute (Submitted to California Air
Resources Board).
Call DJ, Brooke LT, Ahmad N, & Richter JE (1983) Toxicity and
metabolism studies with EPA Priority Pollutants and related
chemicals in freshwater organisms. Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research
Laboratory, Office of Research and Development, 120 pp (EPA
600/3-83-095).
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR,
& Gould C (1979) Water-related environmental fate of 129 priority
pollutants. Volume II: Halogenated aliphatic hydrocarbons,
halogenated ethers, monocyclic aromatics, phthalate esters,
polycyclic aromatic hydrocarbons, nitrosamines, miscellaneous
compounds. Washington, DC, US Environmental Protection Agency
(EPA 440/4-79-029b).
Calley D, Autian J, & Guess WL (1966) Toxicology of a series of
phthalate esters. J Pharm Sci, 55: 158-162.
Calnan CD (1975) Dibutyl phthalate. Contact Dermatitis, 1: 388.
Camford Information Services Inc. (1992) CPI product profiles -
Di- n-butyl phthalate. Dons Mills, Ontario, Camford Information
Services Inc., 3pp.
Castle L, Mercer AJ, Startin JR, & Gilbert J (1988) Migration
from plasticized films into foods. 3. Migration of phthalate,
sebacate, citrate and phosphate esters from films used for retail
food packaging. Food Addit Contam, 5(1):-9-20.
Castle L, Mayo A, & Gilbert J (1989) Migration of plasticizers
from printing inks into foods. Food Addit Contam, 6(4):
437-443.
Cater BR, Cook MW, Gangolli SD, & Grasso P (1977) Studies on
dibutyl phthalate-induced testicular atrophy in the rat: Effect
on zinc metabolism. Toxicol Appl Pharmacol, 41(3): 609-618.
Cautreels W & van Cauwenberghe K (1978) Experiments on the
distribution of organic pollutants between airborne particulate
matter and the corresponding gas phase. Atmos Environ, 12:
1133-1141.
Cautreels W, van Cauwenberghe K, & Guzman LA (1977) Comparison
between the organic fraction of suspended matter at a background
and an urban station. Sci Total Environ, 8(1): 79-88.
CCREM (Canadian Council of Resource and Environment Ministers)
(1987) Canadian water quality guidelines. Winnipeg, Manitoba,
Task Force on Water Quality Guidelines of the Canadian Council of
Resources and Environment Ministers (now part of the Canadian
Council of Environment Ministers), pp 3/37, 6/182-6/187.
Chambon P, Riotte M, Daudon M, Chambon-Mougenot R and Bringuier R
(1971) Etude du métabolisme des phthalates de dibutyle et de
diéthyle chez le rat. C R Acad Sci Paris, 273: 2165-2168.
Chan HS (1975) A study of the transfer processes of phthalate
esters to the marine environment. Houston, Texas, Graduate
College of Texas A&M University (Ph.D. Dissertation).
Chapman P (1989) Current approaches to developing sediment
quality criteria. Environ Toxicol Chem, 8: 589-599.
Chauret C, Mayfield CI, & Inniss WE (1995) Biotransformation of
di- n-butyl phthalate by a psychotrophic Pseudomonas
fluorescens (BGW) isolated from subsurface environment. Can J
Microbiol, 41: 54-63.
Ching NP, Jham GN, & Subbarayan C (1981) Gas chromatographic-mass
spectrometric detection of circulating plasticizers in surgical
patients. J Chromatogr, 222: 171-177.
City of Toronto (1990) The quality of drinking-water in Toronto:
Summary report. Toronto, City of Toronto, Department of Health.
Clark JR, Patrick JM Jr, Moore JC, & Lores EM (1987) Waterborne
and sediment-source toxicities of six organic chemicals to grass
shrimp ( Palaemonetes pugio) and amphioxus ( Branchiostoma
caribaeum). Arch Environ Contam Toxicol, 16(4): 401-407.
CMA (1984) Generation of environmental fate and effects data base
on 14 phthalate esters. Summary Report - Environmental studies:
Phase I. Washington, DC, Chemicals Manufacturers Association,
Phthalate Esters Program Panel.
Cocchieri RA (1986) Occurrence of phthalate esters in Italian
packaged foods. J Food Prot, 49(4): 265-266.
Cummings AM & Gray LE Jr (1987) Dibutyl phthalate: maternal
effects versus fetotoxicity. Toxicol Lett, 39(1): 43-50.
Davis WP (1988) Reproductive and developmental responses in the
self-fertilizing fish, Rivulus marmoratus, induced by the
plasticizer, di- n-butylphthalate. Environ Biol Fishes, 21(2):
81-90.
Davis BJ, Maronpot RR, & Heindel JJ (1994a) Di-(2-
ethylhexyl)phthlate suppresses estradiol and ovulation in cycling
rats. Toxicol Appl Pharmacol, 128: 216-223.
Davis BJ, Weaver R, Gaines LJ, & Heindel JJ (1994b) Mono-(2-
ethylhexyl)phthalate suppresses estradiol production independent
of FSH-cAMP stimulation in rat granulosa cells. Toxicol Appl
Pharmacol, 128: 224-228.
DeFoe DL, Holcombe GW, Hammermeister DE, & Biesinger KE (1990)
Solubility and toxicity of eight phthalate esters to four aquatic
organisms. Environ Toxicol Chem, 9: 623-636.
DeVault DS (1985) Contaminants in fish from Great Lakes harbors
and tributary mouths. Arch Environ Contam Toxicol, 14(5):
587-594.
Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE,
Pavlou SP, Allen HE, Thomas NA, & Paquin PR (1991) Technical
basis for establishing sediment quality criteria for nonionic
organic chemicals using equilibrium partitioning. Environ Toxicol
Chem, 10: 1541-1583.
Eaton RW & Ribbons DW (1982) Metabolism of dibutyl phthalate and
phthalate by Micrococcus sp. strain 12B. J Bacteriol, 151:
48-57.
EEC (European Economic Community) (1987) Council directive of 25
April 1983 on the approximation of the laws of the Member States
relating to materials and articles made of regenerated cellulose
film intended to come into contact with foodstuffs.
EG&G Bionomics (1983a) Acute toxicity of fourteen phthalate
esters to fathead minnows ( Pimephales promelas). Penascola,
Florida, EG&G Bionomics (Toxicity test report submitted to
Chemical Manufacturers Association, Washington, DC).
EG&G Bionomics (1983b) Acute toxicity of thirteen phthalate
esters to fathead minnows ( Pimephales promelas) under flow-
through conditions. Penascola, Florida, EG&G Bionomics (Toxicity
test report submitted to Chemical Manufacturers Association,
Washington, DC).
EG&G Bionomics (1983c) Acute toxicity of thirteen phthalate
esters to bluegill ( Lepomis macrochirus). Penascola, Florida,
EG&G Bionomics (Toxicity test report submitted to Chemical
Manufacturers Association, Washington, DC).
EG&G Bionomics (1983d) Acute toxicity of fourteen phthalate
esters to rainbow trout ( Salmo gairdneri) under flow-through
conditions. Penascola, Florida, EG&G Bionomics (Toxicity test
report submitted to Chemical Manufacturers Association,
Washington, DC).
EG&G Bionomics (1984a) Acute toxicity of twelve phthalate esters
to mysid shrimp ( Mysidopsis bahia). Penascola, Florida, EG&G
Bionomics (Toxicity test report submitted to Chemical
Manufacturers Association, Washington, DC).
EG&G Bionomics (1984b) Acute toxicity of twelve phthalate esters
to Paratanytarsus parthenogenica. Penascola, Florida, EG&G
Bionomics (Toxicity test report submitted to Chemical
Manufacturers Association, Washington, DC).
Eglinton G, Simoneit BRT, & Zoro JA (1975) he recognition of
organic pollutants in aquatic sediments. Proc R Soc (Lond),
B189: 415-442.
Ehrhardt M & Derenbach J (1980) Phthalate esters in the Kiel
Bight. Mar Chem, 8: 339-346.
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol,
15: 30-38.
Elsisi AE, Carter DE, & Spies IG (1989) Dermal absorption of
phthalate diesters in rats. Fundam Appl Toxicol, 12(1): 70-77.
Ema M, Amano H, Itami T, & Kawasaki H (1993) Teratogenic
evaluation of di-n-butyl phthalate in rats. Toxicol Lett,
69(2): 197-203.
Ema M, Amano H, & Ogawa Y (1994) Characterization of the
developmental toxicity of di-n-butyl phthalate in rats.
Toxicology, 86(3): 163-174.
Fallon ME & Horvath FJ (1985) Preliminary assessment of
contaminants in soft sediments of the Detroit River. J Great
Lakes Res, 11: 373-378.
Fatoki OS & Vernon F (1990) Phthalate esters in rivers of the
Greater Manchester area, U.K. Sci Total Environ, 95: 227-232.
Fishbein L & Albro PW (1972) Chromatographic and biological
aspects of the phthalate esters. J Chromatogr, 70: 365-412.
Fischer J, Ventura K, Prokes B, & Janderaa P (1993) Methods for
determination of plasticizers in industrial emissions.
Chromatographia, 37(1/2): 47-50.
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15: 219-232.
Foster PMD, Lake BG, Thomas LV, Cook MW, & Gangolli D (1981)
Studies on the testicular effects and zinc excretion produced by
various isomers of monobutyl-o-phthalate in the rat. Chem-Biol
Interact, 34: 233-238.
Foster PM, Cook MW, Thomas LV, Walters DG, & Gangolli SD (1982)
Differences in urinary metabolic profile from di-n-butyl
phthalate-treated rats and hamsters: A possible explanation for
species differences in susceptibility to testicular atrophy. Drug
Metab Dispos, 11(1): 59-61.
Fredricsson B, Mller L, Pousette Å, & Westerholm R (1993) Human
sperm motility is affected by plasticizers and diesel particle
extracts. Pharmacol Toxicol, 72(2): 128-133.
Fukuoka M, Tanimoto T, Zhou Y, Kawasaki N, Tanaka A, Ikemoto I, &
Machida T (1989) Mechanism of testicular atrophy induced by
di-n-butyl phthalate in rats. Part 1. J Appl Toxicol, 9(4):
277-283.
Fukuoka M, Zhou Y, Tanaka A, Ikemoto I, & Machida T (1990)
Mechanism of testicular atrophy induced by di-n-butyl phthalate
in rats. Part 2. The effects on some testicular enzymes. J Appl
Toxicol, 10(4):
285-293.
Fukuoka M, Kobayashi T, Zhou Y, & Hayakawa T (1993) Mechanisms of
testicular atrophy induced by di- n-butyl phthalate in rats.
Part 4. Changes in the activity of succinate dehydrogenase and
the levels of transferrin and ferritin in the Sertoli and germ
cells. J Appl Toxicol, 13(4): 241-246.
Furtmann K (1994) Phthalate in surface water - a method for
routine trace level analysis. Fresenius J Anal Chem, 348:
291-296.
Giam CS & Atlas E (1980) Accumulation of phthalate ester
plasticizers in Lake Constance sediments. Nat.wiss, 67:
508-510.
Giam CS, Chan HS, Neff GS, & Atlas EL (1978) Phthalate ester
plasticizers: a new class of marine pollutant. Science, 199:
419-421.
Giam CS, Atlas E, & Chan HS (1980) Phthalate esters, PCB and DDT
residues in the Gulf of Mexico atmosphere. Atmos Environ, 14:
65-69.
Gilioli R, Bulgherain C, Terrana T, Filippini G, Massette N, &
Boeri R (1978) Horizontal and longitudinal study of a population
employed in the production of phthalates. Med Lav, 69: 620-631.
Glass GE, Strachan WMI, Willford WA, Armstrong FAI, Kaiser KLE, &
Lutz A (1977) Organic contaminants. In: The waters of Lake Huron
and Lake Superior. Volume III, Part B: Lake Superior. Detroit,
Michigan, The Upper Lakes Reference Group, pp 417-429, 499-502
(Report to the International Joint Commission) (EPA 600/J-77-
042).
Golder Associates (1987) Testing of specific organic compounds in
soils in background in urban areas, Port Credit and
Oakville/Burlington, Ontario. Mississauga, Ontario, Canada,
Golder Associates (Working paper to Shell Canada Limited and
Texaco Canada Limited).
Golder Associates, SENES Consultants Limited and CanTox (1987)
Summary interpretation of the influence of soil conditions on the
decommissioning of the Oakville refinery site. Mississauga,
Ontario, Canada, Golder Associates (Working paper No. 4 to Shell
Canada Limited).
Government of Canada (1993) Canadian Environmental Protection Act
- Priority substances list. Supporting document: Dibutyl
phthalate. Ottawa, Ontario, Environment Canada.
Government of Canada (1994) Canadian Environmental Protection Act
- Priority substances list. Assessment report: Dibutyl phthalate.
Ottawa, Ontario, Environment Canada and Health Canada.
Graham PR (1973) Phthalate ester plasticizers - Why and how they
are used. Environ Health Perspect, 3: 3-12.
Gray TJB & Butterworth KR (1980) Testicular atrophy produced by
phthalate esters. Arch Toxicol, 4(Suppl): 452-455.
Gray TJB, Rowland IR, Foster PMD, & Gangolli SD (1982) Species
differences in the testicular toxicity of phthalate esters.
Toxicol Lett, 11: 141-147.
Gray LE Jr, Laskey JW, Ostby J, & Ferrell J (1983a) The effects
of dibutyl phthlate on the reproductive tract of the male and
female rat and hamster. Toxicologist, 3: 22 (Abstract No. 87).
Gray TJB, Lake B, Beamand A, Foster JR, & Gangolli SD (1983b)
Peroxisomal effects of phthlate esters in primary cultures of rat
hepatocytes. Toxicology, 28: 167-179.
Guardiola J, Ventura F, & Rivera J (1989) Occurrence of
industrial organic pollution in a groundwater supply: screening,
monitoring and evaluation of treatment processes. Water Supply,
7: 11-16.
Hamano Y, Kuwano A, Inoue K, Oda Y, Yamamoto H, Mitsuda B, &
Kunita N (1977) Studies on toxicity of phthalic acid esters.
First report: Teratogenic effects in mice administered orally.
Osaka-furitsu Koshu Esei kenkyusho Kenkyu Hokoka Shokukhim Eisei
Hen, 8: 29-33.
Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie
KM, Piccirillo VJ, & Smith KN (1987) Evaluation of 60 chemicals
in a preliminary developmental toxicity test. Teratogen
Carcinogen Mutagen, 7: 29-48.
Hardwick RC, Cole RA, & Fyfield TP (1984) Injury to and death of
cabbage ( Brassica oleracea) seedlings caused by vapours of di
butyl phthalate emitted from certain plastics. Ann Appl Biol,
105: 97-105.
Hazleton Biotechnologies Company (1986) Mutagenicity of 1C in a
mouse lymphoma mutation assay. Final Report (Genetics assay No.
7156 - HB Project No. 20989). Kensington, Maryland, Hazleton
Biotechnologies Company (Submitted by Chemical Manufacturers
Association to US Environmental Protection Agency) (EPA Document
No. 40-8626225; microfiche No. OTS0510527).
Heindel JJ, Gulati DK, Mounce RC, Russell SR, & Lamb JC IV (1989)
Reproductive toxicity of three phthalic acid esters in a
continuous breeding protocol. Fundam Appl Toxicol, 12(3):
508-518.
Herring R & Bering CL (1988) Effects of phthalate esters on plant
seedlings and reversal by a soil microorganism. Bull Environ
Contam Toxicol, 40(4): 626-632.
Hibino M, Matsuda H, Sato T, Ose Y, Nagase H, & Kito H (1992)
Generation of mutagenicity by ozonation of humic substances'
components. Sci Total Environ, 16: 1-13.
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC,
US Department of the Interior, Fish and Wildlife Service (Special
Scientific Report - Wildlife No. 191).
Hites RA & Budde WL (1991) EPA's analytical methods for water:
the next generation. Environ Sci Technol, 25(6): 998-1006.
Ho CT, Lee KN, & Jin QZ (1983) Isolation and identification of
volatile flavor compounds in fried bacon. J Agric Food Chem,
31: 336-342.
Hoff RM & Chan KW (1987) Measurement of polycyclic aromatic
hydrocarbons in air along the Niagara river. Environ Sci Technol,
21(6): 556-561.
Howard PH ed. (1989) Handbook of environmental fate and exposure
data for organic chemicals. Chelsea, Michigan, Lewis Publishers
Inc., 574 pp.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM
(1991) Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers Inc.
Hrudey SE, Sergy GA, & Thackeray T (1976) Toxicity of oil sands
plant wastewaters and associated organic contaminants. In: Water
pollution research in Canada: Proceedings of the 11th Canadian
Symposium. Ottawa, Ontario, Water Pollution Research Canada, pp
34-45.
HSE (Health and Safety Executive) (1986) Review of the toxicity
of the esters of o-phthalic acid (phthalate esters). Sudbury,
United Kingdom, HSE Books, 183 pp (Toxicity Review 14).
Hudson RA & Bagshaw JC (1978) Toxicity of di- n-butyl phthalate
for developing larvae of the brine shrimp, Artemia salina. Fed
Proc, 37: 1702 (Abstract No. 2393).
Hudson RA, Austerberry CF, & Bagshaw JC (1981) Phthalate ester
hydrolases and phthalate ester toxicity in synchronously
developing larvae of the brine shrimp ( Artemia). Life Sci,
29: 1865-1872.
Husain SL (1975) Dibutyl phthalate sensitivity. Contact
Dermatitis, 1: 395.
Ikemoto I (1985) Experimental studies on testicular damage
induced by phthalic acid esters. Tokyo Jikeikai Med J, 100:
1115-1127.
Ikemoto I, Kotera S, Katsurai K, Inaba Y, Machida T, & Tanaka A
(1983) Experimental testicular damage induced by dibutyl
phthalate and monobutyl phthalate. Jpn J Fertil Steril, 28(7):
159-165.
Inman JC, Strachan SD, Sommers LE, & Nelson DW (1984) The
decomposition of phthalate esters in soil. J Environ Sci Health,
B19: 245-257.
IPCS (1992) Environmental health criteria 131: Diethylhexyl
phthalate. Geneva, World Health Organization, International
Programme on Chemical Safety, 141 pp.
IPCS (1993) Environmental health criteria 170: Assessing human
health risks of chemicals: Derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization,
International Programme on Chemical Safety, 73 pp.
Ishida M, Suyama K, & Adachi S (1980) Background contamination by
phthalates commonly encountered in the chromatographic analysis
of lipid samples. J Chromatogr, 189: 421-424.
Ishida M, Suyama K, & Adachi S (1981) Occurrence of dibutyl and
di(2-ethylhexyl) phthalate in chicken eggs. J Agric Food Chem,
29: 72-74.
Ishidate M & Odashima S (1977) Chromosome tests with 134
compounds on Chinese hamster cells in vitro - a screening for
chemical carcinogens. Mutat Res, 48(3/4): 337-354.
Ito S, Takeda H, Kobayashi A, Sakurai H, Tada Y, Aoki G, Hosogai
T, Yamanaka T, & Ishiwata H (1993) A simple and rapid method for
determination of n-dibutylphthalate in imported vodka by FID-GC
and GC/MS. J Food Hyg Soc Jpn, 34(3): 254-256.
Iturbe R, Elefsiniotis P, & Moreno G (1991) Efficiency of a
phthalate ester in an activated sludge system. Environ Technol,
19(9): 783-796.
Johnson BT & Lulves W (1975) Biodegradation in di- n-butyl
phthalate and di-(2-ethylhexyl) phthalate in freshwater
hydrosoil. J Fish Res Board Can, 32(3): 333.
Johnson BT, Stalling DL, Hogan JW, & Schoettger RA (1977)
Dynamics of phthalate acid esters in aquatic organisms. In:
Suffet IH ed. Fate of pollutants in the air and water
environment. Part 2. New York, Wiley Interscience, pp 283-300
(Advances in Environmental Science and Technology, Volume 8).
Johnson BT, Heitkamp MA, & Jones JR (1984) Environmental and
chemical factors influencing the biodegradation of phthalic acid
esters in freshwater sediments. Environ Pollut, B8: 101-118.
JPIF (1995) Production of plastics. Plastics, 46: 104.
Kaneshima H, Yamaguchi T, Okui T, & Naitoh M (1978) Studies on
the effects of phthalate esters on the biological system. Part 2:
In vitro metabolism and biliary excretion of phthalate esters
in rats. Bull Environ Contam Toxicol, 19: 502-509.
Kawano M (1980a) Toxicological studies on phthlate esters. 2.
Metabolism, accumulation and excretion of phthlate esters in
rats. Jpn J Hyg, 35: 693-701.
Kawano M (1980b) Toxicological studies on phthlate esters. 1.
Inhalation effects of dibutyl phthalate (DBP) on rats. Jpn J Hyg,
35: 684-692.
Kawashima Y, Hanioka N, Matsumura M, & Kozuka H (1983) Induction
of microsomal stearoyl-CoA desaturation by the administration of
various peroxisome proliferators. Biochim Biophys Acta, 752:
259-264.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD, &
Thruston AD (1976) Identification of organic compounds in
drinking water from thirteen U.S. cities. In: Keith LH ed.
Identification and analysis of organic pollutants in water. Ann
Arbor, Michigan, Ann Arbor Science Publishers Inc., pp 329-373.
Khaliq MA, Alam MS, & Srivastava SP (1992) Implications of
physico-chemical factors on the migration of phthalate esters
from tubing commonly used for oral/nasal feeding. Bull Environ
Contam Toxicol, 48: 572-578.
Klöpfer W, Kaufmann G, Rippen G, & Poremski HJ (1982) A
laboratory method for testing the volatility from aqueous
solution: first results and comparison with theory. Ecotoxicol
Environ Saf, 6: 545-559.
Kohli J, Ryan JF, & Afghan BK (1989) Phthalate esters in the
aquatic environment. In: Chau BK & Chau ASY ed. Analysis of trace
organics in the aquatic environment. Boca Raton, Florida, CRC
Press Inc., pp 243-281.
Kördel W & Müller J (1992) Occurrence of phthalic acid esters in
soil and plants nearby phthalate-emitting sources and by sewage
sludge treatment. Schmallenberg-Grafshaft, Germany, Fraunhofer
Institute for Environmental Chemistry and Ecotoxicology.
Korhonen A, Hemminki K, & Vanio H (1983) Embryotoxic effects of
phthalic-acid derivatives phosphates and aromatic oils used in
the manufacturing of rubber on 3 day chicken embryos. Drug Chem
Toxicol, 6(2): 191-208.
Kozumbo WJ, Kroll R, & Rubin RJ (1982) Assessment of the
mutagenicity of phthalate esters. Environ Health Perspect, 45:
103-109.
Krauskopf LG (1973) Studies of the toxicity of phthalates via
ingestion. Environ Health Perspect, 3: 61-72.
Kühn R & Pattard M (1990) Results of the harmful effects of water
pollutants to green algae ( Scenedesmus subspicatus) in the
cell multiplication inhibition test. Water Res, 24(1): 31-38.
Kühn R, Pattard M, Pernak, KD, & Winter A (1989) Results of the
harmful effects of water pollutants to Daphnia magna in the 21
day reproduction test. Water Res, 23(4): 501-510.
Kurane R, Suzuki T, & Takahara Y (1979a) Microbial population and
identification of phthalate ester-utilizing microorganisms. Agric
Biol Chem, 43: 907-917.
Kurane R, Suzuki T, & Takara Y (1979b) Microbial degradation of
phthalic esters. Agric Biol Chem, 43(3): 421-427.
Kveseth NJ (1980) Chlorinated hydrocarbons in sewage sludge from
a plant in Oslo. Nord Vet Med, 32: 341-347.
Lake BG, Phillips JC, Linnell JC, & Gangolli SD (1977) The
in vitro hydrolysis of some phthalate diesters by hepatic and
intestinal preparations from various species. Toxicol Appl
Pharmacol, 39: 239-248.
Lake BG, Cook WM, Worrell NR, Cunninghame ME, Evans JG, Price RJ,
Young PJ, & Carpanini FMB (1991) Dose-response relationships for
induction of hepatic peroxisome proliferation and testicular
atrophy by phthalate esters in the rat. Hum Exp Toxicol, 10:
67-68 (Abstract).
Lamb JC, Chapin RE, Teague J, Lawton AD, & Reel JR (1987)
Reproductive effects of four phthalic acid esters in the mouse.
Toxicol Appl Pharmacol, 88(2): 255-269.
Laughlin RB Jr, Neff JM, Hrung YC, Goodwin TC, & Giam CS (1978)
The effects of three phthalate esters on the larval development
of the grass shrimp Palaemonetes pugio (Holthuis). Water Air
Soil Pollut, 9: 323-336.
Law RJ, Fileman TW, & Matthiessen P (1991) Phthalate esters and
other industrial organic chemicals in the North and Irish Seas.
Water Sci Technol, 24: 127-134.
Lawrence WH, Malik M, Turner JE, Singh AR, & Autian J (1975) A
toxicological investigation of some acute, short-term and chronic
effects of administering di(2-ethylhexyl) phthalate (DEHP) and
other phthalate esters. Environ Res, 9: 1-11.
Lehman AJ (1955) Insect repellents. Assoc Food Drug Off, 19:
87-99.
Leibowitz JN, Sarmiento R, Dugar SM, & Ethridge MW (1995)
Determination of six common phthalate plasticisers in grain
neutral spirits and vodka. J Assoc Off Anal Chem Int 78(3):
730-735.
Lesage S (1991) Characterization of groundwater contaminants
using dynamic thermal stripping and adsorption/thermal
desorption-GC-MS. Fresenius J Anal Chem, 339: 516-527.
Lewis RJ (1991) Reproductively active chemicals: A reference
guide. New York, Van Nostrand Reinhold Co.
Leyder F & Boulanger P (1983) Ultraviolet absorption, aqueous
solubility, and octanol-water partition for several phthalates.
Bull Environ Contam Toxicol, 30(2): 152-157.
Ligocki MP & Pankow JF (1985) Assessment of absorption/solvent
extraction with polyurethane foam and adsorption/thermal
desorption with Tenax-GC for the collection and analysis of
ambient organic vapours. Anal Chem, 57: 1138-1144.
Ligocki MP, Leuenberger C, & Pankow JF (1985) Trace organic
compounds in rain-II. Gas scavenging of neutral organic
compounds. Atmos Environ, 19: 1609-1617.
Lindén E, Bengtsson BE, Svanberg O, & Sundström G (1979) The
acute toxicity of 78 chemicals and pesticide formulations against
two brackish water organisms, the bleak ( Alburnus alburnus)
and the harpacticoid ( Nitocra spinipes). Chemosphere,
8(11/12): 843-851.
Litton Bionetics Inc. (1985) Evaluation of 1C in the in vitro
transformation of Balb/3T3 cellsaAssay. Final report (Genetics
assay No. 7156 - LBI Project No. 20992). Kensington, Maryland,
Litton Bionetics Inc. (Submitted by Chemical Manufacturers
Association to US Environmental Protection Agency (EPA document
No. 40+8526206; microfiche No. OTS0509537).
Lokke H & Bro-Rasmussen F (1981) Studies of mobility of di-iso-
butyl phthalate (DiBP), di-n-butyl phthalate (DBP), and di-(2-
ethyl hexyl) phthalate (DEHP) by plant foliage treatment in a
closed terrestrial simulation chamber. Chemosphere, 10(11/12):
1223-1235.
Lunde G, Gether J, GjØs N, & StØbet Lande MB (1977) Organic
micropollutants in precipitation in Norway. Atmos Environ, 11:
1007-1014.
LWA (1993) Report on the quality of the Rhine. Düsseldorf,
Northrhine-Westfalia Office for Water and Waste.
McCarthy JF & Whitmore DK (1985) Chronic toxicity of di-n-butyl
and di-n-octyl phthalate to Daphnia magna and the fathead
minnow. Environ Toxicol Chem, 4: 167-179.
McKone TE & Layton DW (1986) Exposure and risk assessment of
toxic waste in a multimedia context. 79th Annual Meeting of the
Air Pollution Control Association, Minneapolis, Minnesota, 22-27
June 1986. Pittsburgh, Pennsylvania, Air Pollution Control
Association, vol 1, 16 pp.
MAFF (Ministry of Agriculture, Fisheries and Food, United
Kingdom) (1987) Survey of plasticiser levels in food contact
materials and in foods. The 21st report of the Steering Group on
Food Surveillance/The Working Party on Chemical Contaminants from
Food Contact Materials / Sub Group on Plasticisers. London, Her
Majesty's Stationery Office, 105 pp (Food Surveillance Paper
No. 21).
MAFF (Ministry of Agriculture, Fisheries and Food, United
Kingdom) (1990) Plasticizers: Continuing surveillance. The 31st
report of the Steering Group on Food Surveillance/The Working
Party on Chemical Contaminants from Food Contact Materials / Sub
Group on Plasticisers. London, Her Majesty's Stationery Office,
53 pp (Food Surveillance Paper No. 30).
Malisch R, Schulte E, & Acker L (1981) [Organochlorine
pesticides, polychlorinated biphenyls and phthalate in Rhine and
Neckar sediments.] Chem Ztg, 105: 187-194 (in German).
Mathur SP (1974) Phthalate esters in the environment: pollutants
or natural products? J Environ Qual, 3(3): 189-197.
Matsuda K & Schnitzer M (1971) Reactions between fulvic acid, a
soil humic material, and dialkyl phthalates. Bull Environ Contam
Toxicol, 6(3): 200-204.
Mayer FL Jr & Sanders HO (1973) Toxicology of phthalic acid
esters in aquatic organisms. Environ Health Perspect, 3:
153-157.
Mayer FL & and Ellersieck MR (1986) Manual of acute toxicity:
interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the
Interior, Fish and Wildlife Service (Resource Publication 160;
NTIS Publication No. PB86-239878).
Melin C & Egnéus H (1983) Effects of di- n-octyl phthalate on
growth and photosynthesis in algae and on isolated organelles
from higher plants. Physiol Plant, 59: 461-466.
Men'shikova TA (1971) Hygienic evaluation of dibutyl phthlate in
relation to the use of polymer finishes in shipboard living
quarters. Hyg Sanit, 36: 349-353.
Mes J, Coffin DE, & Campbell DS (1974) Di-n-butyl- and di-2-
ethylhexyl phthalate in human adipose tissue. Bull Environ Contam
Toxicol, 12(6): 721-725.
Milkov LE, Aldyrova MV, Popova TB, Lopukhova KA, Makarenko YL,
Malyar LM, & Shakhova TK (1973) Health status of workers exposed
to phthalate plasticizers in the manufacture of artificial
leather and films based on PVC resins. Environ Health Perspect,
3: 175-178.
Millar DJ & Hannay JW (1986) Phytotoxicity of phthalate
plasticisers. 2. Site and mode of action. J Exp Bot, 37(179):
898-908.
MOE (Ontario Ministry of the Environment) (1984) Drinking water
survey of selected municipalities in the Niagara area and Lake
Ontario. Ottawa, Ontario, Ontario Ministry of the Environment.
Montgomery JH & Welkom LM (1990) Groundwater chemicals desk
reference. Chelsea, Michigan, Lewis Publishers Inc.
Murakami K, Nishiyama K, & Higuti T (1986a) Toxicity of dibutyl
phthalate and its metabolites in rats. Jpn J Hyg, 41(4):
775-780.
Murakami K, Nishiyama K, & Higuti T (1986b) Mitochrondrial effect
of orally administered dibutyl phthalate in rats. Jpn J Hyg,
41(4): 769-774.
Nagy KA (1987) Field metabolic rate and food requirement scaling
in mammals and birds. Ecol Monogr, 57(2): 111-128.
NAQUADAT (1993) National water quality data bank. Ottawa,
Ontario, Environment Canada, Inland Waters Directorate, Water
Quality Branch.
Nerin C, Cacho J, & Gancedo P (1993) Plasticizers from printing
inks in a selection of food packagings and their migration to
food. Food Addit Contam, 10(4): 453-460.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR
(1985) The toxicity of selected organic chemicals to the
earthworm Eisenia fetida. J Environ Qual, 14(3): 383-388.
Neuhauser EF, Loehr RC, & Malecki MR (1986) Contact and
artificial soil tests using earthworms to evaluate the impact of
wastes in soil. In: Petros JK, Lacy WJ, & Conway RA ed. Fourth
Symposium on Hazardous and Industrial Solid Waste Testing.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 192-203 (ASTM STP 886).
Niagara River Data Interpretation Group (1990) Joint evaluation
of upstream/downstream Niagara River monitoring data, 1988-1989.
Prepared by Data Interpretation Group, River Monitoring
Committee. Joint publication of Environment Canada, US
Environmental Protection Agency, Ontario Ministry of the
Environment, and New York State Department of Environmental
Conservation.
Nikonorow M, Mazur H, & Piekacz H (1973) Effect of orally
administered plasticizers and polyvinyl chloride stabilizers in
the rat. Toxicol Appl Pharmacol, 26: 253-259.
NIOSH (1976) Health hazard evaluation determination report
No. 74-120-260: Goodyear Tire and Rubber Company, Gadsden,
Alabama. Cincinnati, Ohio, National Institute for Occupational
Safety and Health (NTIS Publication No. PB89-166078).
NIOSH (1982) Health hazard evaluation report No. HETA-81-277-
1089: Indiana Army Ammunition Plant, Charlestown, Indiana.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (NTIS Publication No. PB83-198697).
NIOSH (1987) Health hazard evaluation report No. MHETA 86-191-
1836: West Virginia Department of Highways, Charleston, West
Virginia. Cincinnati, Ohio, National Institute for Occupational
Safety and Health (NTIS Publication No. PB88-162698).
NIOSH (1989) Health hazard evaluation report No. MHETA 88-214-
1952: Flying W Plastics Company, Glenville, West Virginia.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (NTIS Publication No. PB89-230544).
NTP (1984) Di(n-butyl) phthalate: Reproduction and fertility
assessment in CD-1 mice when administered in the feed. Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program, 100 pp (NTIS Publication
No. PB85-144798).
NTP(1991) Final report on the reproductive toxicity of di-n-butyl
phthalate (CAS No. 84-74-2) in Sprague-Dawley rats. Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program (Report No. T-0035C; NTIS
Publication No. PB92-111996).
NTP (1994a) Chairperson's report. Pathology Working Group (PWG)
review - Dibutylphthalate (C62022B) and (C62022): A 13-week
subchronic toxicity study (Phases II and III) administered by
dose feed in F344 rats and B6C3F1 mice conducted at Battelle
Columbus. Research Triangle Park, North Carolina, US Department
of Health and Human Services, National Toxicology
Program/Environmental Toxicology Program.
NTP (1994b) Prechronic study, dibutyl phthalate diet study in
mice. Research Triangle Park, North Carolina, US Department of
Health and Human Services, National Toxicology
Program/Environmental Toxicology Program.
NTP (1995) NTP technical report on toxicity studies of dibutyl
phthalate (CAS No. 84-74-2) aministered in feed to F344/N rats
and B6C3F1 mice. Research Triangle Park, North Carolina, US
Department of Health and Human Services, National Toxicology
Program (Toxicity Series No. 30).
O'Connor OA, Rivera MD, & Young LY (1989) Toxicity and
biodegradation of phthalic acid esters under methanogenic
conditions. Environ Toxicol Chem, 8: 569-574.
Ogura N, Ambe Y, Ogura K, Ishiwatari R, Mizutani T, Satoh Y,
Matsushima H, Katase T, Ochiai M, Tadokoro K, Takada T, Sugihara
K, Matsumoto G, Nakamoto N, Funakoshi M, & Hanya T (1975)
Chemical composition of organic compounds present in water of the
Tamagawa River. Jpn J Limnol, 36: 23-30.
Oishi S & Hiraga K (1980a) Testicular atrophy induced by phthalic
acid esters. Effect on testosterone and zinc concentrations.
Toxicol Appl Pharmacol, 53: 35-41.
Oishi S & Hiraga K (1980b) Effect of phthalic acid esters on
mouse testes. Toxicol Lett, 5: 413-416.
Oishi S & Hiraga K (1980c) Testicular atrophy induced by phthalic
acid monoesters: Effects of zinc and testosterone concentrations.
Toxicology, 15: 197-202.
Oishi S & Hiraga K (1980d) Effects of phthalic acid monoesters on
mouse testes. Toxicol Lett, 6: 239-242.
Oishi S & Hiraga K (1982) Effects of monoesters of o-phthalic
acid on serum lipid composition of rats. Toxicol Lett, 14:
79-84.
Ota H, Takashima K, Takashima Y, Onda H, Kodama H, & Yamada N
(1973) Biological effects of phthalate esters. (I)
Histopathological findings from experiments in mice. Nippon
Byorigakkai Kaishi, 62: 119-120.
Ota H, Onda H, Kodama H, & Yamada N (1974) Histopathological
studies on the effect of phthalic acid esters on the biological
system of mice. Nippon Eiseigaku Kaishi, 29: 519-524.
Otson R & Benoit FM (1985) Surveys of selected organics in
residential air. In: Walkinshaw DS ed. Indoor air quality in
cold climates: An Air Pollution Control Association speciality
conference. Pittsburgh, Pennsylvania, Air Pollution Control
Association, pp 224-236.
Overcash MR, Weber JB, & Miles ML (1982) Behavior of organic
priority pollutants in the terrestrial system: di-n-butyl
phthalate ester, toluene, and 2,4-dinitrophenol. Raleign, North
Carolina, University of North Carolina, Water Resources Research
Institute (Report No. 171).
PACE (Petroleum Association for the Conservation of the Canadian
Environment) (1985) Evaluation of the variability of trace
organic substances in petroleum refinery effluents. Ottawa,
Ontario, Canadian Petroleum Products Institute (PACE Report
No. 85-6).
Page BD & Lacroix GM (1992) Studies into the transfer and
migration of phthalate esters from aluminum foil-paper laminates
to butter and margarine. Food Addit Contam, 9(3): 197-212.
Page BD & Lacroix GM (1995) The occurrence of phthalate ester and
di-2-ethylhexyl adipate plasticizers in Canadian packaging and
food sampled in 1985-1989: a survey. Food Addit Contam, 12(1):
129-151.
Pankow JF, Ligocki MP, & Rosen ME (1988) Adsorption/thermal
desorption with small cartridges for the determination of trace
aqueous semivolatile organic compounds. Anal Chem, 60: 40-47.
Parkman H & Remberger M (1995) Phthalates in Swedish sediments.
Stockholm, Swedish Environmental Research Institute (IVL-Report).
Peakall DB (1974) Effects of di-n-butyl and di-2-ethylhexyl
phthalate on the eggs of ring doves. Bull Environ Contam Toxicol,
12: 698-702.
Peakall DB (1975) Phthalate esters: occurrence and biological
effects. Residue Rev, 54: 1-41.
Perwak J, Goyer M, & Schimke G (1981) An exposure and risk
assessment for phthalate esters: Di(2-ethylhexyl) phthalate, di-
n-butyl phthalate, dimethyl phthalate, diethyl phthalate, di-n-
octyl phthalate, butyl benzyl phthalate. Washington, DC, US
Environmental Protection Agency, Office of Water Regulations and
Standards (EPA-440/4-81-020;NTIS Publication No. PB85-211936).
Peters JW & Cook RM (1973) Effect of phthalate esters on
reproduction in rats. Environ Health Perspect, 3: 91-94.
Peterson JC & Freeman DH (1984) Variations of phthalate ester
concentrations in sediments from the Chester River, Maryland. Int
J Environ Anal Chem, 18(4): 237-252.
Pierce RC, Mathur SP, Williams DT, & Boddington MJ (1980)
Phthalate esters in the aquatic environment. Ottawa, Ontario,
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality (Report No. NRCC
17583).
Poole CF & Vibberley DG (1977) Determination of DEHP ih human
placenta. J Chromatogr, 132: 511.
Radeva M & Dinoyeva S (1966) [The toxicity of the plasticiser
dibutyl phthlate with oral administration to white rats.] Khig i
Zdrav, 9: 510-515 (in Russian).
Ray LE, Murray HE, & Giam CS (1983a) Analysis of water and
sediment from the Nueces Estuary/Corpus Christi Bay (Texas) for
selected organic pollutants. Chemosphere, 12(7/8): 1039-1045.
Ray LE, Murray HE, & Giam CS 1983b) Organic pollutants in marine
samples from Portland, Maine. Chemosphere, 12(7/8): 1031-1038.
RIWA (1991) Annual Report of the Working Party on the Rhine and
the Maas. Part A: The Rhine. Amsterdam, National Institute for
Water and Waste (RIWA).
RIWA (1992) Annual Report of the Working Party on the Rhine and
the Maas. Part A: The Rhine. Amsterdam, National Institute for
Water and Waste (RIWA).
Rogers IH & Hall KJ (1987) Chlorophenols and chlorinated
hydrocarbons in starry flounder ( Platichthys stellatus) and
contaminants in estuarine sediments near a large municipal
outfall. Water Pollut Res J Can, 22(2): 197-210.
Rogers IH, Birtell IK, & Kruzynski GM (1986) Organic extractables
in municipal wastewater, Vancouver, British Columbia. Water
Pollut Res J Can, 21(2): 187-204.
Rowland IR, Cottrell RC, & Phillips JC (1977) Hydrolysis of
phthalate esters by the gastro-intestinal contents of the rat.
Food Cosmet Toxicol, 15: 17-21.
Saito K, Nakazato M, Ishikawa F, Fujinuma K, Moriyasu T, Nagayama
T, Kobayashi M, Shioda H, & Kamimura K (1993) [Determination of
methyl isocyanate in wine and dibutyl phthalate in vodka.] Kenkyu
Nempo Tokyo-Toritsu Eisei Kenkyusho, 44: 119-127 (in Japanese).
Sajiki J (1975a) [Pathological observations of phthalate ester
(di- n-butyl phthalate) for mice with orally administrations.]
Chibaken Eiseikenkyusho Nenpo, 24: 57-63 (in Japanese).
Sajiki J (1975b) Biological effects of phthalate ester (di- n-
butyl phthalate) orally administered in mice.] Chibaken
Eiseikenkyusho Nenpo, 24: 64-72 (in Japanese).
Sandmeyer EE & Kirwin CJ (1981) Esters. In: Clayton GD & Clayton
FE ed. Patty's industrial hygiene and toxicology. Volume 2A:
Toxicology, 3rd rev ed. New York, John Wiley and Sons Inc.,
pp 2345-2346.
Sato H, Sato N, & Ichihara N (1975) Rec-assay application for
mutagenicity testing of phthalic acid esters. Hokkaido-ritsu
Eisei Kenkyushcho, 1975: 146-147.
Schacht RA (1974) Pesticides in the Illinois waters of Lake
Michigan. Washington, DC, US Environmental Protection Agency,
Office of Research and Development (EPA 660/3-74-002).
Schleyer R, Renner I, & Mühlhausen D (1991) [Immission load -
Consequences for grounwater quality.] Bonn, Germany, Federal
Ministry for Environment, Nature Conservation and Nuclear Safety
(in German).
Schouten MJ, Copius Peereboom JW, & Brinkman UA Th (1979) Liquid
chromatographic analysis of phthalate esters in Dutch river
water. Int J Environ Anal Chem, 7: 13-23.
Schwartz HE, Anzion CJM, Van Vliets HPM, Copius Peerbooms JW, &
UATh Brinkman (1979) Analysis of phthalate esters in sediments
from Dutch rivers by means of high performance liquid
chromatography. Int J Environ Anal Chem, 6: 133-144.
Scott RC, Dugard PH, Ramsey JD, & Rhodes C (1987) In vitro
absorption of some o-phthalate diesters through human and rat
skin. Environ Health Perspect, 74: 223-227.
Seed JL (1982) Mutagenic activity of phthalate esters in
bacterial liquid suspension assays. Environ Health Perspect,
45: 111-114.
Seth PK, Agarwal DK, & Agarwal S (1981) Effect of phthalic acid
esters on drug metabolizing enzymes. Bull Environ Contam Toxicol,
26: 764-768.
Shahin MM & von Borstel RC (1977) Mutagenic and lethal effects of
alpha-dibutyl phthalate and trichloroethylene in Saccharomyces
cerevisiae. Mutat Res, 48: 173-180.
Shanker R, Ramakrishna C, & Seth PK (1985) Degradation of some
phthalic acid esters in soil. Environ Pollut, A39: 1-7.
Shea KP (1971) The new-car smell. Environment, 13(8): 2-9.
Shea PJ, Weber JB, & Overcash MR (1982) Uptake and phytotoxicity
of di- n-butyl phthalate in corn ( Zea mays). Bull Environ
Contam Toxicol, 29: 153-158.
Sheldon LS & Hites RA (1978) Organic compounds in the Delaware
River. Environ Sci Technol, 12(10): 1188-1194.
Sheldon LS & Hites RA (1979) Sources and movement of organic
chemicals in the Delaware River. Environ Sci Technol, 13(5):
574-579.
Shelton DR, Boyd SA, & Tiedje JM (1984) Anaerobic biodegradation
of phthalic acid esters in sludge. Environ Sci Technol, 18(2):
93-97.
Shibuya S (1979) Phthalic acid esters as one of the marker
environmental pollutants - occurrence in the water and aquatic
environment in Shizuoka Prefecture. Numazu Kogyo Kota Semmon
Gakko Kenkyu Hokoku, 14: 63-72.
Shiota K & Nishimura H (1982) Teratogenicity of di(2-ethylhexyl)
phthalate (DEHP) and di-n-butyl phthalate (DBP) in mice. Environ
Health Perspect, 45: 65-70.
Shiota K, Chou MJ, & Nishimura H (1980) Embryotoxic effects of
di(2-ethylhexyl)phthalate (DEHP) and di-n-butyl phthalate (DBP)
in mice. Environ Res, 22: 245-253.
Singh AR, Lawrence WH, & Autian J (1972) Teratogenicity of
phthalate esters in rats. J Pharm Sci, 61(1): 51-55.
Smith CC (1953) Toxicity of butyl stearate, dibutyl sebacate,
dibutyl phthalate and methoxyethyl oleate. Ind Hyg Occup Med,
7: 310-318.
Sneddon IB (1972) Dermatitis from dibutyl phthalate in an aerosol
antiperspirant and deodorant. Contact Dermatitis, 1972: 308.
Spasovski M (1964) Experimental data to establish a maximum
allowable concentration for dibutyl phthalate. Higiena, 7:
38-44.
Springborn Bionomics (1984a) Toxicity of fourteen phthalate
esters to the freshwater green algae, Selenastrum
capricornutum. Wareham, Massachusetts, Springborn Bionomics, 52
pp (Toxicity test report submitted to the Chemical Manufacturers
Association, Washington, DC).
Springborn Bionomics (1984b) Chronic toxicity of fourteen
phthalate esters to Daphnia magna. Wareham, Massachusetts,
Springborn Bionomics (Toxicity test report No. BW-84-5-1567
submitted to the Chemical Manufacturers Association, Washington,
DC).
Srivastava SP, Srivastava S, Saxena DK, Chandra SV, & Seth PK
(1990a) Testicular effects of di-n-butyl phthalate (DBP):
Biochemical and histopathological alterations. Arch Toxicol,
64(2): 148-152.
Srivastava S, Singh GB, Srivastava SP, & Seth PK (1990b)
Testicular toxicity of di-n-butyl phthalate in adult rats: Effect
on marker enzymes of spermatogenesis. Ind J Exp Biol, 28(1):
67-70.
Stalling DL, Hogan JW, & Johnson JL (1973) Phthalate ester
residues - their metabolism and analysis in fish. Environ Health
Perspect, 3: 159-173.
Stanley JS (1986) Broad scan analysis of the FY82 national human
adipose tissue survey specimens - Volume I: Executive summary.
Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA 560/5-86-035).
Streufert JM & Sanders HO (1977) Chronic effects of two phthalic
acid esters on midge Chironomus plumosus. TransMo Acad Sci,
10/11: 297.
Streufert JM, Jones JR, & Sanders HO (1980) Toxicity and
biological effects of phthalate esters on midges ( Chironomus
plumosus). TransMo Acad Sci, 14: 33-40.
Sugatt RH, O'Grady DP, Banerjee S, Howard PH, & Gledhill WE
(1984) Shake flask biodegradation of 14 commercial phthalate
esters. Appl Environ Microbiol, 47: 601-606.
Sugawara N (1974a) Toxic effect of a normal series of phthalate
esters on the hatching of shrimp eggs. Toxicol Appl Pharmacol,
30(1): 87-89.
Sugawara N (1974b) Effect of phthalate esters on shrimp. Bull
Environ Contam Toxicol, 12(4): 421-424.
Sullivan KF, Atlas EL, & Giam CS (1982) Adsorption of phthalic
acid esters from seawater. Environ Sci Technol, 16: 428-432.
Swain WR (1978) Chlorinated organic residues in fish, water, and
precipitation from the vicinity of Isle Royale, Lake Superior. J
Great Lakes Res, 4: 398-407.
Swain LG & Walton DG (1989) Report on the 1988 Fish Monitoring
Program. British Columbia, Ministry of the Environment.
Swartz RC, Schults DW, Ditsworth GR, DeBen WA, & Cole FA (1985)
Sediment toxicity, contamination, and macrobenthic communities
near a large sewage outfall. In: Boyle TD ed. Validation and
predictabolity of laboratory methods for assessing the fate and
effects of contaminants in aquatic ecosystems. Philadelphia,
Pennsylvania, American Society for Testing and Materials,
pp 152-175 (ASTM STP 865).
Tagatz ME, Deans CH, Moore JC, & Plaia GR (1983) Alterations in
composition of field- and laboratory-developed estuarine benthic
communities exposed to di-n-butyl phthalate. Aquatic Toxicol,
3: 239-248.
Tagatz ME, Plaia GR, & Deans CH (1986) Toxicity of dibutyl
phthalate-contaminated sediment to laboratory-and field-colonized
estuarine benthic communities. Bull Environ Contam Toxicol,
37(1): 141-149.
Takahashi T & Tanaka A (1989) Biochemical studies on phthalic
esters.V. Comparative studies on in vitro hydrolysis of di-n-
butyl phthalate isomers in rats. Arch Toxicol, 63: 72-74.
Tanaka A, Matsumoto A, & Yamaha T (1978) Biochemical studies on
phthalic esters. III. Metabolism of dibutyl phthalate (DBP) in
animals. Toxicology, 9: 109-123.
Tanino M, Ikemoto I, & Tanaka A (1987) Enzyme levels in rat
testis damaged experimentally with dibutyl phthalate. Jikeikai
Med J, 34: 245-252.
Tarkpea M, Hansson M, & Samuelsson B (1986) Comparison of the
Microtox test with the 96-hr LC50 test for the harpacticoid
Nitocra spinipes. Ecotoxicol Environ Saf, 11: 127-143.
Tetra Tech Inc. (1986) Development of sediment quality values for
puget sound. Bellevue, Washington, Tetra Tech Inc., vol 1,
128 pp.
Thurén A (1986) Determination of phthalates in aquatic
environments. Bull Environ Contam Toxciol, 36(1): 33-40.
Thurén A & Larsson P (1990) Phthalate esters in the Swedish
atmosphere. Environ Sci Technol, 24(4): 554-559.
Thurén A & Woin P (1991) Effects of phthalate esters on the
locomotor activity of the freshwater amphipod Gammarus pulex.
Bull Environ Contam Toxicol, 46(1): 159-166.
Tomita I, Nakamura Y, & Yagi Y (1977) Phthalic acid esters in
various foodstuffs and biological materials. Ecotoxicol Environ
Saf, 1: 275-287.
Tsuchiya K & Hattori K (1976) [Chromosomal study on human
leucocyte cultures treated with phthalate acid ester.] Japan,
Hokkaido Institute of Public Health (in Japanese).
US EPA (1981) An exposure and risk assessment for phthalate
esters. Washington, DC, US Environmental Protection Agency,
Office of Water Regulations and Standards (EPA 440/4-81-020; NTIS
Publication No. PB85-211936).
US EPA (1982a) Aquatic fate process data for organic priority
pollutants. Washington, DC, US Environmental Protection Agency
(EPA 440/4-81-014; NTIS Publication No. PB87-169090).
US EPA (1982b) Methods for organic chemical analysis of municipal
and industrial wastewater. Cincinnati, Ohio, US Environmental
Protection Agency, Environmental Monitoring and Support
Laboratory (EPA 600/4-82-057; NTIS Publication No. PB83-201798).
US EPA (1986a) Method 8060: Phthalate esters. In: Test methods
for evaluating solid wastes, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and
Emergency Response.
US EPA (1986b) Method 8250: Gas chromatography/mass spectrometry
for semivolatile organics - packed column technique. In: Test
methods for evaluating solid wastes, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and
Emergency Response.
US EPA (1986c) Method 8270: Gas chromatography/mass spectrometry
for semivolatile organics - capillary column technique. In: Test
methods for evaluating solid wastes, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and
Emergency Response.
US EPA (1986d) Method 8410: Capillary column analysis of
semivolatile organic compounds by gas chromatography/fourier
transform infrared (GC/FT-IR) spectrometry. In: Test methods for
evaluating solid wastes, 3rd ed. Washington, DC, US Environmental
Protection Agency, Office of Solid Waste and Emergency Response.
Virgin HI (1988) Accumulation of di-n-butylphthalate in plants
and its effect on pigment and protein content. Physiol Plant,
72(1): 190-196.
Von Westernhagen H, Landolt M, Kocan R, Fürstenberg G, Janssen
D, & Kremling K (1987) Toxicity of sea-surface microlayer:
effects on herring and turbot embryos. Mar Environ Res, 23:
273-290.
Waldock MJ (1983) Determination of phthalate esters in samples
from the marine environment using gas chromatography mass
spectrometry. Chem Ecol, 1: 261-277.
Walker WW, Cripe CR, Pritchard PH, & Bourquin AW (1984) Dibutyl-
phthalate degradation in estuarine and freshwater sites.
Chemosphere, 13(12): 1283-1294.
Walseth F & Nilsen OG (1984) Phthalate esters. II. Effects of
inhaled dibutylphthalate on cytochrome P-450 mediated metabolism
in rat liver and lung. Arch Toxicol, 55: 132-136.
Walseth F & Nilsen OG (1986) Phthalate esters: Effects of orally
administered dibutylphthalate on cytochrome P-450 mediated
metabolism in rat liver and lung. Acta Pharmacol Toxicol, 59:
263-269.
Walseth F, Toftgard R, & Nilsen OG (1982) Phthalate esters I:
Effects on cytochrome P-450 mediated metabolism in rat liver and
lung, serum enzymatic activities and serum protein levels.
Arch Toxicol, 50: 1-10.
Wang JL, Liu P, & Qian Y (1995) Microbial degradation of di-n-
butyl phthalate. Chemosphere, 31(9): 4051-4059.
Ward TJ & Boeri RL (1991) Early life stage toxicity of di-n-butyl
phthalate (DnBP) to the rainbow trout ( Oncorhynchus mykiss)
under flow-through conditions. Hampton, New Hampshire, Resource
Analysts, Inc., EnviroSystems Division (Toxicity report submitted
to the Chemical Manufacturers Association, Washington, DC).
Webber MD & Lesage S (1989) Organic contaminants in Canadian
municipal sludges. Waste Manage Res, 7: 63-82.
Weschler C (1981) Identification of selected organics in the
Arctic aerosol. Atmos Environ, 15(8): 1365-1369.
White RD, Carter DE, Earnest D, & Mueller J (1980) bsorption and
metabolism of three phthalate diesters by the rat small
intestine. Food Cosmet Toxicol, 18: 383-386.
White RD, Earnest DL, & Carter DE (1983) The effect of intestinal
esterase inhibition on the in vivo absorption and toxicity of
di-n-butyl phthalate. Food Cosmet Toxicol, 21: 99-101.
Williams DT (1973) Dibutyl- and di-(2-ethylhexyl)phthalate in
fish. J Agric Food Chem, 21(6): 1128-1129.
Williams DT & Blanchfield BJ (1975) The retention, distribution,
excretion and metabolism of dibutyl phthalate-7-14C in the rat.
J Agric Food Chem, 23(5): 854-858.
Wilson WB, Giam CS, Goodwin TE, Aldrich A, Carpenter V, & Hrung
YC (1978) The toxicity of phthalates to the marine dinoflagellate
Gymnodinium breve. Bull Environ Contam Toxicol, 20(2):
149-154.
Wilson LG, Osborn MD, Olson KL, Maida SM, & Katz LT (1990) The
ground water recharge and pollution potential of dry wells in
Pima County, Arizona. Ground Water Monit Rev, 10: 114-121.
Wine RN, Li LH, Barnes LH, Gulati OK, & Chapin RE (1977)
Reproductive toxicity of di- n-butylphthalate in a continuous
breeding protocol in SD rats. Environ Hlth Persp, 105(1):
102-107.
Wofford HW, Wilsey CD, Neff GS, Giam CS, & Neff JM (1981)
Bioaccumulation and metabolism of phthalate esters by oysters,
brown shrimp, and sheepshead minnows. Ecotoxicol Environ Saf,
5: 202-210.
Wolfe NL, Burns LA, & Steen WC (1980) Use of linear free energy
relationships and an evaluative model to assess the fate and
transport of phthalate esters in the aquatic environment.
Chemosphere, 9: 393-402.
Woodward KN (1988) Phthalate esters: Toxicity and metabolism,
Volumes I and II. Boca Raton, Florida, CRC Press Inc.
Woodward KN (1990) Phthalate esters, cystic kidney disease in
animals and possible effects on human health: A review. Hum Exp
Toxicol, 9: 397-401.
Wu C, Pei X, Cao J, & Xue H (1993) A new pharmacological activity
of dibutyl phthalate (DBP) on selective elimination of tumor
cells from bone marrow. Leuk Res, 17(7): 557-560.
Yagi Y, Tutikawa K, & Shimoi N (1976) Teratogenicity and
mutagenicity of a phthalate ester. Teratology, 14: 259-260.
Yagi Y, Nakamura Y, Tomita I, Tutikawa K, & Shimoi N (1978)
Embryotoxicity of phthalate esters in mice. In: Plaa GL & Duncan
WAM ed. Proceedings of the First International Congress on
Toxicology. New York, London, San Francisco, Academic Press, pp
590-591.
Yamada A (1974) [Toxicity of phthalic acid esters and
hepatotoxicity of di-(2-ehtyl hexyl) phthalate.] J Food Hyg Soc
Jpn, 15: 147-152 (in Japanese, with English summary).
Yan H, Ye C, & Yin C (1995) Kinetics of phthalate ester
biodegradation by Chlorella pyrenoidosa. Environ Toxicol Chem,
14(6): 931-938.
Yoshikawa K, Tanaka A, Yamada T, & Kurata H (1983) Mutagenicity
study of nine monoalkyl phthalates and a dialkyl phthalate using
Salmonella typhimurium and Escherichia coli. Food Chem
Toxicol, 21(2): 221-223.
Yoshioka Y, Ose Y, & Sato T (1985) Testing for the toxicity of
chemicals with Tetrahymena pyriformis. Sci Total Environ,
43(1/2): 149-157.
Yoshioka Y, Ose Y, & Sato T (1986) Correlation of the five test
methods to assess chemical toxicity and relation to physical
properties. Ecotoxicol Environ Saf, 12:15-21.
Zeiger E, Haworth S, Speck W, & Mortelmans K (1982) Phthalate
ester testing in the National Toxicology Program's environmental
mutagenesis test development program. Environ Health Perspect,
45: 99-101.
Zeiger E, Haworth S, Mortelmans K, & Speck W (1985) Mutagenicity
testing of di(2-ethylhexyl)phthalate and related chemicals in
Salmonella. Environ Mutagen, 7: 213-232.
Zhou Y, Fukuoka M, & Tanaka A (1990) Mechanisms of testicular
atrophy induced by di- n-butyl phthalate in rats. Part 3.
Changes in the activity of some enzymes in the Sertoli and germ
cells, and in the levels of metal ions. J Appl Toxicol, 10(6):
447-453.
Ziogou K, Kirk PWW, & Lester JN (1989) Behaviour of phthalic acid
esters during batch anaerobic digestion of sludge. Water Res,
23(6): 743-748.
Zitko V (1972) Determination, fate, and environmental levels of
phthalate plasticizers. St. Andrews, N.B., Fisheries Research
Board of Canada, Biological Station (Technical Report No. 344).
Zurmühl T (1990) Development of a method for the determination of
phthalate esters in sewage sludge including chromatographic
separation from polychlorinated biphenyls, pesticides and
polyaromatic hydrocarbons. Analyst, 115: 117.
Zurmühl T, Durner W, & Herrmann R (1991) Transport of phthalate-
esters in undisturbed and unsaturated soil columns. J Contam
Hydrol, 8: 111-133.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
Le phtalate de di- n-butyle (DBP) est un liquide inerte,
incolore et de consistance huileuse, qui présente une faible
tension de vapeur. Soluble dans la plupart des solvants
organiques, il n'est que légérement soluble dans l'eau.
L'analyse la plus sélective et la plus sensible des prélèvements
effectués dans l'environnement pour la recherche et le dosage du
DBP et, plus généralement, des esters phtaliques, se fait par
chromatographie en phase gazeuse avec détection par capture
d'électrons ou spectrométrie de masse. Etant donné que des
phtalates peuvent être présents sous la forme de plastifiants
dans certaines pièces des instruments de mesure ou
encore contaminer l'air du laboratoire, il faudra veiller tout
particulièrement à éviter la contamination lors du prélèvement,
de la conservation et de l'analyse des échantillons.
Le DBP est principalement utilisé comme plastifiant de la
nitrocellulose, de l'acétate et du chlorure de polyvinyle, comme
lubrifiant des buses de bombes aérosol, comme agent antimoussant,
comme adoucissant de la peau, comme plastifiant dans le vernis à
ongles et les faux ongles ou encore dans les aérosols
capillaires. Le dosage du DBP présent dans l'atmosphère
s'effectue en phase vapeur ou particulaire. On pense que, pour
une part non négligeable, le DBP est évacué de l'atmosphère par
les précipitations ou par dépôt à sec. Dans les eaux de surface,
la majeure partie du DBP est présente dans la phase liquide
plutôt que dans les solides en suspension. Il ne semble pas que
le composé puisse s'évaporer du sol en quantités appréciables en
raison de sa faible tension de vapeur et du fait qu'il est
modérément adsorbé sur les particules du sol.
La persistance du DBP dans l'air et les eaux de surface est
relativement faible et sa demi-vie dans ces compartiments du
milieu n'est que de quelques jours. Il est rapidement biodégradé
en aérobiose et beaucoup plus lentement en anaérobiose. On a
estimé que sa demi-vie dans le sol était du même ordre que dans
l'air et l'eau, mais il semble, selon certaines études, que le
DBP persiste plus longtemps dans le sol. On peut s'attendre à
une bioaccumulation importante du fait de la valeur élevée du
coefficient de partage entre l'octanol et l'eau. Toutefois, sa
métabolisation est rapide chez les poissons, aussi le facteur de
bioconcentration a-t-il tendance à être plus faible que prévu.
La valeur la plus élevée (relativement au composé initial), à
savoir 590, a été observée chez un cyprinidé d'Amérique du Nord,
Pimephales promelas. Chez les animaux terrestres, il est peu
probable qu'il y ait une bioamplification notable, à en juger
d'après quelques données concernant les oiseaux et compte tenu du
fait que la métabolisation et l'excrétion sont rapides chez les
mammifères de laboratoire.
Il n'est pas possible d'apprécier dans quelle mesure les
anciennes données de surveillance peuvent être considérées comme
fiables car, dans la littérature antérieure à 1980, on ne trouve
que rarement mention des dispositions prises pour éviter la
contamination des échantillons prélevés dans l'environnement.
Les données limitées dont on dispose au sujet des concentrations
dans l'air ambiant indiquent que les valeurs moyennes sont
généralement inférieures à 5 ng/m3. Des études récentes ont
montré que dans l'eau de pluie, la concentration moyenne allait
de 0,2 à 1,4 µg/litre; des valeurs beaucoup plus faibles ont été
mesurées dans des régions reculées. Dans les eaux de surface, la
concentration a tendance à être inférieure à 1 µg/litre;
cependant on a relevé des valeurs beaucoup plus élevées dans des
cours d'eau pollués (12 à 34 µg/litre). On ne possède que
quelques données sur la concentration dans les eaux souterraines,
les valeurs se situant entre 0,15 et 0,46 µg/litre. Dans les
effluents, la concentration du DBP peut aller jusqu'à 100
µg/litre; elle varie de 0,2 à 200 µg/kg de poids sec dans les
boues résiduaires. Dans les sédiments, la concentration est en
général inférieure à 1 mg/kg de poids sec; toutefois, dans les
zones polluées, on a mesuré des concentrations pouvant aller
jusqu'à 20 mg/kg. Selon les études portant sur la faune et la
flore aquatiques, les concentrations auraient tendance à se
situer à moins de 0,2 mg/kg de poids humide; néanmoins, des
valeurs atteignant 35 mg/kg ont été relevées dans des secteurs
pollués.
Lors d'une enquête menée en Californie sur plus de 125
résidences au cours de l'année 1990, on a relevé une
concentration diurne moyenne de 420 ng/m3 dans l'air intérieur.
Quelques données canadiennes indiquent que la présence de DBP
dans l'eau de boisson est plutôt rare et que sa concentration est
inférieure à 1,0 µg/litre. A Toronto, l'analyse d'un petit
nombre d'échantillons a montré que la concentration du DBP y
était de 14 ng/litre; on en a trouvé de 21 à 55 ng/litre dans des
échantillons de plusieurs marques d'eau minérale en bouteille.
Du DBP peut pénétrer dans les aliments par suite d'une
contamination de l'environnement, mais la présence de ce composé
dans une denrée alimentaire peut également être due à la
migration du DBP de l'emballage vers son contenu. Ce problème a
été étudié à plusieurs reprises vers la fin des années 80. Dans
de nombreux pays, des précautions ont été prises pour réduire le
passage, par lixiviation, des plastifiants de l'emballage dans le
produit alimentaire. Ces mesures ont eu pour effet de réduire peu
à peu la teneur des aliments en DBP. En 1986, on a effectué à
Halifax une enquête sur le panier de la ménagère au cours de
laquelle 98 produits alimentaires ont été étudiés. La présence
de DBP a été décelée dans du beurre (1,5 µg/g), du poisson d'eau
douce (0,5 µg/g), des produits céréaliers (de non décelable à
0,62 µg/g), des pommes de terre rôties (0,63 µg/g), de la salade
de chou (0,11 µg/g), des bananes (0,12 µg/g), des airelles
(0,09µg/g), des ananas (0,05 µg/g), de la margarine (0,64 µg/g),
du sucre raffiné (0,2 µg/g), et des desserts à la gélatine
(0,09 µg/g).
Sur la base des données limitées dont on dispose, on peut
dresser la liste suivante des principaux milieux par lesquels la
population est exposée au DBP (par ordre d'importance
décroissante et en fonction de la dose absorbée estimative):
alimentation, air intérieur et eau de boisson. On estime que la
dose absorbée quotidiennement à partir de l'alimentation et de
l'air intérieur est respectivement égale à 7 µg/kg et 0,42 µg/kg
de poids corporel. Les doses absorbées journellement à partir de
l'eau de boisson et de l'air ambiant sont très inférieures à ces
valeurs, à savoir <0,02 µg/kg de poids corporel et 0,26 -
0,36 ng/kg depoids corporel, respectivement. En se basant sur
ces valeurs, on peut calculer que la dose moyenne totale absorbée
en une journée à partir de l'air, de l'eau de boisson et des
aliments est égale à 7,4 µg/kg de poids corporel. Toutefois, il
est à noter que la dose absorbée à partir des aliments peu varier
dans de larges proportions selon la nature et la quantité du
produit emballé qui est consommé et également, selon les
modalités d'utilisation de tel ou tel emballage au cours de la
préparation du produit. On estime qu'au Royaume-Uni, la dose
maximale ainsi ingérée est probablement de 2 mg environ par
personne et par jour (soit approximativement 31 µg/kg de poids
corporel en une journée, pour une personne d'un poids moyen de
64 kg). Il y a également un risque d'exposition au DBP présent
dans les cosmétiques, encore que dans ce cas, on ne dispose pas
de données suffisantes pour évaluer la dose absorbée de cette
manière.
Les données provisoires les plus récentes fournies par
l'enquête nationale sur l'exposition professionnelle qu'a menée
le NIOSH indiquent qu'aux Etats-Unis, plus de 500 000
travailleurs, dont 200 000 femmes, courent un risque d'exposition
au DBP. D'après les mesures effectuées sur un nombre limité de
sites de ce pays, la concentration du DBP est généralement
inférieure à la limite de détection (soit de 0,01 à 0,02 mg/m3),
mais des valeurs plus élevées ont été relevées dans d'autres
pays.
Les études sur le rat montrent que le DBP est absorbé par la
voie percutanée mais des travaux au cours desquels de la peau
humaine a été exposée in vitro ont révélé que cette dernière
était moins perméable au DBP que la peau du rat.
L'expérimentation animale (sur le rat) indique qu'une fois
administré par voie orale ou intraveineuse, le DBP est rapidement
résorbé au niveau des voies digestives et se répartit
principalement dans le foie et les reins, avant d'être éliminé
dans les urines sous forme de métabolites. Après inhalation, on
le retrouve systématiquement à faible concentration dans
l'encéphale.
Les données disponibles montrent qu'après ingestion par des
rats de laboratoire, le DBP est métabolisé par des esterases non
spécifiques, principalement dans l'intestin grêle, pour donner du
phtalate de mono- n-butyle (MBP) qui subit ensuite une oxydation
limitée de sa chaîne latérale alkyle. Le MBP est stable et le
deuxième goupement ester résiste à l'hydrolyse. Ce composé ainsi
que les autres métabolites sont excrétés dans les urines sous
forme de glucuro-conjugués. On observe des différences
interspécifiques relativement à l'excrétion des métabolites
conjugués et non conjugués, notamment entre hamster et le rat,
l'urine de ce dernier contenant davantage de MBP libre. Aucune
accumulation n'a été observée au niveau des divers organes.
La palette des effets de l'exposition au DBP est analogue à
celle que l'on observe avec les autres esters phtaliques, qui,
chez les espèces sensibles, peuvent se réveler foetotoxiques et
tératogènes et provoquer également une hépatomégalie, un
accroissement du nombre des peroxysomes hépatiques et des lésions
testiculaires.
Le DBP présente une faible toxicité aiguë pour le rat et la
souris. Après administration par voie orale à des rats on a
obtenu, pour la DL50, des valeurs allant d'environ 8 g/kg à au
moins 20 g/kg de poids corporel. Chez la souris, les valeurs
vont d'environ 5 g/kg à environ 16 g/kg de poids corporel. Chez
le lapin, la DL50 cutanée est supérieure à 4 g/kg de poids
corporel. L'existence d'effets toxiques aigus consécutifs à
l'inhalation de DBP n'a pu être documentée. Chez les animaux de
laboratoire, l'intoxication aiguë se manifeste par les signes
suivants: réduction de l'activité, respiration difficile et perte
de coordination. Un travailleur qui avait été intoxiqué par
suite de l'ingestion accidentelle de 10 g de DBP, s'est remis
progressivement de son intoxication en l'espace de quinze jours,
la récupération étant totale au bout d'un mois.
Lors d'études toxicologiques au cours desquelles des rats
ont reçu DBP à plusieurs reprises, on a observé, au bout d'une
période de 5 à 21 jours, une prolifération des peroxysomes et une
hépatomégalie aux doses supérieures ou égales à 420 mg/kg de
poids corporel sur une journée.
Lors d'études à plus long terme, les effets observés sur des
rats ayant ingéré du DBP pendant des périodes allant jusqu'à 7
mois consistaient notamment en une réduction du gain de poids à
des doses quotidiennes supérieures ou égales à 250 mg/kg de poids
corporel. A des doses supérieures ou égales à 120 mg/kg de poids
corporel, il y avait augmentation du poids relatif du foie.
Lorsque la dose quotidienne dépassait 279 mg/kg de poids
corporel, on observait également une prolifération des
peroxysomes et un accroisssement de l'activité des enzymes
correspondantes. Chez des rats Wistar qui avaient reçu des doses
quotidiennes supérieures ou égales à 250 mg/kg de poids corporel,
on a observé des signes de nécrose hépatique; en revanche ,
aucune lésion de ce genre n'a été relevée chez des rats
F-344 ou Sprague-Dawley soumis à des doses quotidiennes égales ou
supérieures à 2 500 mg/kg de poids corporel. Aux doses
quotidiennes de 250 et 571 mg/kg de poids corporel, on a observé
chez le rat un certain nombre d'anomalies au niveau testiculaire,
notamment des modifications affectant les enzymes et une
dégénérescence des cellules germinales. Les anomalies
testiculaires observées varient considérablement d'une espèce à
l'autre, les effets étant minimaux chez le hamster et la souris à
des doses quotidiennes qui peuvent atteindre 2 000 mg/kg. Une
récente étude consistant en une exposition subchronique a permis
de mettre en évidence, chez la souris, des effets sur le poids du
corps et le poids des organes, de même que des modifications
histologiques au niveau du foie, qui trahissent l'existence d'un
stress métabolique; la dose sans effet observable pour ce type
d'anomalie a été évaluée à 353 mg/kg de poids corporel.
D'après les quelques données d'expérimentation animale dont
on dispose, il semble que le DBP ne puisse guère provoquer
d'irritation cutanée ou oculaire ni entraîner une
sensibilisation. Chez l'homme, on connaît quelques cas de
sensibilisation après exposition à du DBP, mais ces observations
n'ont pas été confirmées par des études contrôlées sur un plus
grand nombre d'individus.
Dans le cadre d'un protocole d'élevage en continu, au cours
duquel on a procédé à des croisements et à l'examen de la
progéniture obtenue, des rats ont reçu une alimentation conentant
0, 1000, 5000 ou 10 000 mg de DBP par kg de nourriture (soit
l'équivalent quotidien de 0, 66, 320 et 651 mg de composé par kg
de poids corporel). Dans la première génération, on a pu
considérer comme un effet négatif sur le développement la
réduction du poids corporel observée chez les ratons ayant reçu
la dose médiane. On constatait également une réduction sensible
du nombre de portées viables à toutes les doses. Dans la deuxième
génération, les effets étaient plus graves, et consistaient en
une réduction du poids des ratons dans tous les groupes, y
compris celui qui avait reçu la dose la plus faible, en anomalies
morphologiques (malformations du prépuce et du pénis,
dégénérescence des tubes séminifères, et enfin, absence ou
développement insuffisant de l'épididyme) dans les groupes soumis
aux doses moyennes et fortes. Dans le groupe soumis à la dose la
plus forte, on notait de graves effets sur la spermatogénèse,
effets que l'on n'observait pas, en revanche, dans la génération
parentale. Ces résultats donnent à penser que les effets nocifs
du DBP sont plus marqués chez les animaux exposés au cours de
leur phase de développement et de maturation que lorsqu'ils le
sont uniquement à l'âge adulte. Aucune valeur bien nette de la
dose sans effet nocif observable (NOEL) n'a été tirée de cette
étude. On estime en revanche que la dose la plus faible
produisant un effet nocif (LOAEL) était égale à 66 mg/kg de poids
corporel par jour.
Les études dont on dispose montrent que le DBP est
généralement foetotoxique, sans pour autant qu'il y ait atteinte
de la mère. Les données existantes indiquent également que ce
composé est tératogène à forte dose, la sensibilité à cet effet
dépendant du stade de développement et de la période
d'administration. Chez la souris, on constaté que le DBP
provoquait une augmentation des résorptions et des morts foetales
à partir de 400 mg/kg de poids corporel, cet effet étant lié à la
dose. Pour ces valeurs de la dose, on a également constaté chez
la souris une réduction, liée à la dose, du poids foetal et du
nombre de portées viables.
On n'a pas effectué d'épreuves de cancérogénicité qui soient
satisfaisantes. A la lumière des données disponibles, on peut
penser que le DBP n'est pas génotoxique.
Les produits chimiques qui provoquent la prolifération des
peroxysomes constituent un groupe de substances souvent
génératrices de cancers du foie, selon un mécanisme qui
n'implique pas d'action toxique au niveau génique. Leur mode
d'action n'est pas encore élucidé, mais l'on sait cependant que
l'apparition de la tumeur est précédée par une prolifération des
peroxysomes et par une hépatomégalie. Comme le DBP provoque la
prolifération des peroxysomes, il n'est pas exclu qu'il puisse
également provoquer des cancers du foie chez les rongeurs, encore
qu'en ce qui concerne ces deux effets - prolifération des
peroxysomes et hépatomégalie - il soit beaucoup moins actif que
le DEHP. Dans la mesure où il y a corrélation entre ces deux
effets et le pouvoir cancérogène, on peut s'attendre à ce que le
DBP soit un cancérogène beaucoup moins puissant que le DHEP et
les méthodes actuelles de détermination biologique du pouvoir
cancérogène ne permettraient probablement pas de mettre une telle
activité en évidence.
Les enquêtes épidémiologiques dont on a connaissance se
limitent à l'étude du cas de travailleurs exposés à des mélanges
de phtalates. Elles ne nous permettent pas de progresser dans
l'élucidation des effets dus au seul DBP.
On a vu que le DBP n'étant pas génotoxique et ayant un
pouvoir cancérogène moindre que celui du DEHP, les méthodes
actuelles de mesure du pouvoir cancérogène ne révèleraient
vraisemblablement aucune activité de ce type. Il est donc peu
probable qu'à la concentration où il se trouve dans
l'environnement, ce composé contribue notablement à accroître le
risque de cancer.
C'est la voie alimentaire qui est, de loin, la principale
voie d'exposition au DBP. D'ailleurs, les données toxicologiques
relatives aux autres voies sont insuffisantes pour permettre une
évaluation. On a donc établi une valeur-guide pour la voie
orale, même si l'objectif final doit être de ramener l'exposition
totale de toutes origines à une valeur inférieure à la dose
journalière tolérable.
On n'a pas pu établir de valeur bien nette pour la dose sans
effet nocif observable (NOAEL) pour les points d'aboutissement
toxicologique jugés les plus appropriés à l'établissment de
valeurs-guides (en l'occurence, les effets néfastes sur la
reproduction et le développement). La dose la plus faible sans
effet nocif observable (LOAEL) sur la reproduction et le
développement a été fixée à 66 mg/kg de poids corporel par jour à
la suite d'une étude au cours de laquelle les animaux étaient
élevés en continu, avec cette réserve qu'à cette dose, les effets
observés étaient modérés et probablement réversibles. En se
basant sur ces données, on modérés et probablement réversibles.
En se basant sur ces données, on a fixé à 66 µg/kg p.c. la dose
journalière tolérable, compte tenu d'un facteur d'incertitude de
1000 (un facteur 10 pour les variations interspécifiques, un
facteur 10 pour les variations interindividuelles et un facteur
10 pour l'extrapolation de la LOAEL à la NOAEL).
Les renseignements dont on dispose sur l'écotoxicité du DBP
comportent des données de toxicité aiguë et de toxicité chronique
obtenues sur diverses espèces aquatiques à différents stades de
la chaîne alimentaire. Pour les algues d'eau douce, la valeur la
plus faible de la CE50 à 96 h qui ait été obtenue est égale à
750 µg de DBP par litre. La valeur la plus faible de la CL50
obtenue pour un invertébré aquatique (mysidé) est de 750 µg/litre
et on a relevé une CE50 à 48 h de 760 µg/litre pour des larves
de moucherons. Les études de toxicité chronique ont montré que
l'espèce d'invertébré la plus sensible était Daphnia magna,
avec une concentration sans effet observable à 21 jours (survie
parentale) de 500 µg/litre. Une épreuve non conventionnelle
effectuée sur Gammarus pulex a donné, pour la valeur de la
concentration la plus faible produisant un effet observable à 10
jours, le chiffre de 500 µg/litre et, pour la valeur de la
concentration sans effet observable, le chiffre de 100 µg/litre,
le critère retenu étant, dans les deux cas, la réduction de
l'activité locomotrice. Des épreuves de toxicité aiguë
pratiquées sur des poissons ont permis de constater que la valeur
la plus faible de la CL50 à 96 h était de 350 µg/litre pour une
espèce dulçaquicole, la perche jaune Perca flavescens, et de
600 µg/litre pour un sparidé marin. L'étude de toxicité
chronique la plus sensible qui ait été pratiquée utilisait la
truite arc-en-ciel et alle a donné une valeur de 100 µg/litre
pour la concentration sans effet observable à 99 jours
(croissance) et une valeur de 190 µg/litre pour la concentration
la plus faible produisant un effet observable à 99 jours, le
critère toxicologique retenu étant une réduction d'environ 27% de
la croissance.
La toxicité aiguë du DBP est faible pour les oiseaux.
La concentration moyenne actuelle du DBP dans l'eau ne
représente qu'un faible risque pour les organismes aquatiques.
Cependant, dans les cours d'eau très pollués, la marge de
sécurité est beaucoup plus faible. On ne dispose pas de données
suffisantes pour évaluer le risque encouru par les organismes
sédimenticoles. Compte tenu du niveau d'exposition actuel, le
risque reste faible pour les oiseaux et les mammifères
piscivores.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
El di- n-butil ftalato (DBF) es un líquido oleoso, incoloro
e inerte, con una presión de vapor baja, soluble en la mayor
parte de los disolventes orgánicos, pero sólo ligeramente en
agua. Las determinaciones analíticas más sensibles y selectivas
de los ésteres del ácido ftálico en el medio ambiente, incluido
el DBF, se logran mediante cromatografía de gases con detección
por captura de electrones o espectrometría de masas. Habida
cuenta de que con frecuencia los ftalatos se encuentran como
plastificantes en el equipo analítico y como contaminantes en el
aire y los disolventes del laboratorio, hay que tener una gran
precaución para evitar la contaminación durante la recogida, el
almacenamiento y el análisis de las muestras.
El DBF se utiliza principalmente como plastificante especial
para la nitrocelulosa, el acetato de polivinilo y el cloruro de
polivinilo, lubricante de válvulas de aerosoles, agente
antiespumante, emoliente de la piel y plastificante de esmaltes y
alargadores de uñas y pulverizadores para el pelo.
Se ha determinado la concentración de DBF en la atmósfera,
tanto en la fase de vapor como en la de partículas. Se considera
que el arrastre por la lluvia y la precipitación en seco ejercen
una función importante en su eliminación de la atmósfera. En las
aguas superficiales, la mayor parte del DBF está presente en la
fracción de agua más que en los sólidos suspendidos. La
volatilización a partir del suelo se supone insignificante,
puesto que su presión de vapor es baja y la adsorción en el suelo
moderada.
El DBF es relativamente no persistente en el aire y las
aguas superficiales y tiene una semivida en estos compartimentos
de sólo unos días. La biodegradación total es rápida en
condiciones aerobias, pero mucho más lenta en anaerobiosis. Para
el suelo se ha pronosticado una semivida semejante a las del aire
y el agua; sin embargo, algunos estudios indican que el DBF puede
ser más persistente en el suelo. Sería de esperar que el DBF se
bioacumulara, debido a su elevado coeficiente de reparto
octanol/agua. No obstante, los peces lo metabolizan bastante
fácilmente y, por consiguiente, los factores de bioconcentración
tienden a ser más bajos de lo previsto. El factor de
bioconcentración máximo, basado en el compuesto precursor, es 590
para Pimephales promelas. No es probable la bioamplificación
en los animales terrestres, de acuerdo con los datos limitados en
aves y con la rapidez del metabolismo y excreción que se ha
observado en mamíferos de laboratorio.
Raramente se han descrito medidas adoptadas para evitar la
contaminación en los informes sobre las concentraciones de DBF en
el medio ambiente publicados antes de 1980, por lo que no se
puede evaluar la fiabilidad de los primeros datos de vigilancia.
Hay datos limitados sobre concentraciones en el aire que indican
que los niveles medios suelen ser inferiores a 5 ng/m3. En
estudios recientes se ha observado que las concentraciones medias
en el agua de lluvia oscilaban entre 0,2 y 1,4 µg/litro; en zonas
remotas se han detectado valores muchos más bajos. Las
concentraciones medias en el agua superficial tienden a ser
inferiores a 1 µg/litro; sin embargo, los niveles en ríos
contaminados son mucho más elevados (12 a 34 µg/litro). Se
dispone de pocos datos sobre las concentraciones de DBF en el
aguas freática, con valores medios de 0,15 a 0,46 µg/litro. La
concentración en efluentes alcanza hasta 100 µg/litro, mientras
que en las aguas residuales varía de 0,2 a 200 mg/kg de peso
seco. Los niveles en los sedimentos son en general inferiores a
1 mg/kg de peso seco; sin embargo, en zonas contaminadas se han
medido concentraciones de hasta 20 mg/kg. En estudios realizados
en la biota acuática se ha comprobado que las concentraciones
medias de DBF tienden a ser menores de 0,2 mg/kg de peso seco;
sin embargo, en zonas contaminadas se han medido concentraciones
de hasta 35 mg/kg.
En un estudio realizado en 125 hogares de California,
Estados Unidos, en 1990, la concentración media durante el día en
el aire de la casa era de 420 ng/m3. Raramente se ha detectado
DBF en el agua de bebida (<1,0 µg/litro), según datos limitados
procedentes del Canadá. En un pequeño número de muestras de agua
de bebida de Toronto, Canadá, la concentración media era
14 ng/litro; las concentraciones en siete marcas de agua de
manantial embotellada oscilaban entre 21 y 55 ng/litro.
Además de su entrada mediante la contaminación del medio
ambiente, el DBF puede estar presente en productos alimenticios
como consecuencia de la migración desde el envase, aspecto que se
investigó en varios estudios realizados a finales del decenio de
1980. En muchos países, se adoptaron precauciones para reducir
la lixiviación de plastificantes de los envases y gracias a ello
los niveles de DBF en los productos alimenticios han disminuido a
lo largo del tiempo. En un estudio sobre la cesta de la compra
canadiense con 98 de muestras de tipos diferentes de alimentos
realizado en Halifax en 1986, se detectó DBF en la mantequilla
(1,5 µg/g), el pescado de agua dulce (0,5 µg/g), los productos a
base de cereales (entre indetectable y 0,62 µg/g), las papas
cocidas (0,63 µg/g), la ensalada de col (0,11 µg/g), los bananos
(0,12 µg/g), los arándanos (0,09 µg/g), las piñas (0,05 µg/g), la
margarina (0,64 µg/g), el azúcar blanco (0,2 µg/g) y el postre de
gelatina (0,09 µg/g).
Teniendo en cuenta los limitados datos disponibles, los
medios de exposición principales al DBF para la población
general, enumerados en orden de su importancia relativa según la
ingestión estimada son los siguientes: alimentos, aire de
espacios cerrados y agua de bebida. La ingesta estimada en
alimentos y en el aire de espacios cerrados es de 7 µg/kg de peso
corporal al día y 0,42 µg/kg de peso corporal al día,
respectivamente. Las ingestas con el agua de bebida y el aire del
medio ambiente son considerablemente inferiores, <0,02 µg/kg de
peso corporal al día y 0,26-0,36 ng/kg de peso corporal al día,
respectivamente. Habida cuenta de estas ingestas, se estima que
la cantidad media total diaria ingerida con el aire, el agua de
bebida y los alimentos es de 7,4 µg/kg de peso corporal al día.
Hay que señalar, sin embargo, que la ingestión de DBF en la
alimentación puede variar considerablemente, en función de la
naturaleza y la cantidad de los alimentos envasados consumidos y
el tipo de uso de los envoltorios de los alimentos en la
preparación de la comida. Para el Reino Unido, la ingestión
humana máxima probable de DBF de fuentes alimenticias se ha
estimado en unos 2 mg/persona/día (aproximadamente 31 µg/kg de
peso corporal/día, suponiendo un peso corporal medio de 64 kg).
Existe también la posibilidad de exposición al DBF a través de
los cosméticos, aunque los datos disponibles son insuficientes
para cuantificar la ingestión a partir de esta fuente.
Los datos provisionales más recientes de la Encuesta
Nacional de Exposición Profesional NIOSH señalan que en los
Estados Unidos hay más de 500 000 trabajadores, incluidas 200 000
mujeres, potencialmente expuestos al DBF. Teniendo en cuenta las
determinaciones en un número limitado de puestos de trabajo en
los Estados Unidos, las concentraciones son en general inferiores
al límite de detección (es decir, 0,01-0,02 mg/m3), si bien se
ha informado de niveles más elevados en algunos países.
En estudios con ratas, se ha observado que el DBF se absorbe
a través de la piel, aunque en estudios in vitro la piel humana
ha resultado menos permeable que la de rata a este compuesto. En
estudios con animales de laboratorio se ha advertido que, tras la
administración oral o intravenosa, el DBF se absorbe rápidamente
del tracto gastrointestinal, se distribuye fundamentalmente en el
hígado y los riñones y se excreta en la orina como metabolitos.
Tras la inhalación se detectaron constantemente concentraciones
bajas en el cerebro.
Los datos disponibles indican que en ratas, tras la
ingestión, el DBF se metaboliza mediante la acción de esterasas
inespecíficas, sobre todo en el intestino delgado, para producir
mono- n-butil ftalato (MBP), con la posterior oxidación
bioquímica limitada de la cadena alcalina lateral del MBP. Este
compuesto es estable y resistente a la hidrólisis del segundo
grupo éster. El MBP y otros metabolitos se excretan en la orina,
principalmente como conjugados de glucurónidos. Se han observado
especies diferentes en la excreción de metabolitos conjugados y
no conjugados del DBF en la orina de rata y hámster, con más MBP
libre en la rata que en el hámster. No se ha observado
acumulación en ningún órgano.
El perfil de los efectos tras la exposición al DBF es
semejante al de otros ésteres de ftalatos, que en especies
susceptibles puede inducir hepatomegalia, aumento del número de
peroxisomas hepáticos, fetotoxicidad, teratogenicidad y daños
testiculares.
La toxicidad aguda del DBF en ratas y ratones es baja. Los
valores de la DL50 notificados tras la administración oral a
ratas oscilan entre alrededor de 8 g/kg de peso corporal y por lo
menos 20 g/kg de peso corporal; en ratones, los valores son
aproximadamente de 5 g/kg de peso corporal y 16 g/kg de peso
corporal. La DL50 por vía cutánea en conejos es >4 g/kg de
peso corporal. No hay datos de toxicidad aguda tras la
inhalación de DBF. Los signos de toxicidad aguda observados en
los animales de laboratorio incluyen depresión de la actividad,
respiración fatigosa y falta de coordinación. En un caso de
intoxicación accidental de un trabajador que ingirió alrededor de
10 g de DBF, la recuperación fue gradual en un plazo de dos
semanas y completa al cabo de un mes.
En estudios de toxicidad de corta duración con dosis
repetidas, los efectos en ratas tras la administración oral
durante un período de 5 a 21 días con los niveles más bajos
fueron proliferación de peroxisomas y hepatomegalia a dosis de
420 mg/kg de peso corporal al día o más.
En estudios más prolongados, los efectos observados en ratas
tras la ingestión de DBF durante períodos de hasta siete meses
fueron un aumento reducido de peso a dosis de 250 mg/kg de peso
corporal al día o más. Se ha observado un aumento en el peso
relativo del hígado a dosis de 120 mg/kg de peso corporal o más.
A dosis de 279 mg/kg de peso corporal o más se ha registrado
proliferación de peroxisomas, con aumento de su actividad
enzimática. Se ha informado de cambios hepáticos necróticos en
ratas Wistar a dosis de 250 mg/kg de peso corporal por día o más,
pero no en ratas F-344 o Sprague-Dawley expuestas a dosis de
hasta 2500 mg/kg de peso corporal al día. Se han advertido
alteraciones en las enzimas testiculares y degeneración de las
células germinales testiculares de ratas con dosis de 250 y
571 mg/kg de peso corporal al día. Los efectos en los testículos
tras la exposición al DBF son muy diferentes en las distintas
especies, habiéndose observado efectos mínimos en ratones y
hámster a dosis de hasta 2000 mg/kg de peso corporal al día. En
un bioensayo subcrónico reciente realizado en ratones, se han
descrito efectos en el peso del cuerpo y los órganos y
alteraciones histológicas del hígado, factor indicativo de
tensión metabólica, por lo cual el NOEL fue 353 mg/kg de peso
corporal al día.
Teniendo en cuenta los limitados datos disponibles en
especies animales, el DBF parece tener escaso potencial para
irritar la piel o los ojos o inducir sensibilización. En el ser
humano se ha informado de un pequeño número de casos de
sensibilización tras la exposición al DBF, aunque esto no se vio
confirmado en estudios controlados realizados con un número más
elevado de personas, notificados solamente en resultados
secundarios.
En un protocolo continuo de reproducción, que comprendió
fases de apareamiento cruzado y de evaluación de la descendencia,
se expusieron ratas a 0, 1000, 5000 o 10 000 mg de DBF en la
alimentación (dosis equivalentes a 0, 66, 320 y 651 mg/kg de peso
corporal por día). En la primera generación, la reducción del
peso de las crías en el grupo expuesto a la dosis intermedia, en
ausencia de cualquier efecto adverso en el peso materno, pudo
considerarse como efecto de toxicidad sobre el desarrollo. Hubo
también una reducción significativa del número de crías vivas en
cada camada para los tres niveles de dosis. En la segunda
generación los efectos fueron más serios: reducción del peso de
las crías en todos los grupos, incluido el grupo expuesto a la
dosis baja; defectos estructurales (tales como malformaciones
prepuciales/peneanas, degeneración de los túbulos seminíferos y
ausencia o subdesarrollo de los epidídimos) en los grupos
sometidos a la dosis intermedia/ alta; y efectos graves sobre la
espermatogénesis en el grupo expuesto a la dosis alta, que no se
observaron en los progenitores. Estos resultados dan a entender
que los efectos adversos del DBF son más acusados en los animales
expuestos durante las fases de desarrollo y maduración que en los
expuestos solo en la edad adulta. No se estableció ningún NOEL
en ese estudio. El nivel inferior con efectos adversos observados
(LOAEL) se consideró que fue 66 mg/kg de peso corporal por día.
En los estudios disponibles se ha puesto de manifiesto que
el DBF suele inducir efectos fetotóxicos en ausencia de toxicidad
materna. Los datos existentes indican asimismo que el DBF es
teratogénico a dosis elevadas y que la susceptibilidad a la
teratogénesis varía en función de la fase de desarrollo y del
período de administración. La administración oral de DBF a
ratones en dosis de 400 mg/kg de peso corporal o superiores
produjo un aumento dependiente de la dosis en el número de
reabsorciones y de muertes fetales. Con estas mismas dosis se
observó también en ratones una disminución dependiente de la
dosis del peso fetal y del número de crías viable.
No se han realizado bioensayos adecuados de carcinogénesis
para el DBF. Las pruebas disponibles indican que el DBF no es
genotóxico.
En general, los productos químicos que causan proliferación
de los peroxisomas son con frecuencia hepatocarcinógenos mediante
una acción no genotóxica. Si bien no se conoce todavía el
mecanismo de acción, la formación de tumores va precedida de
proliferación de peroxisomas y hepatomegalia. Habida cuenta de
que el DBF produce proliferación de peroxisomas, es posible que
pudiera ser un carcinógeno hepático en roedores, aunque como
inductor de hepatomegalia y proliferación de peroxisomas es mucho
más débil que el DEHF. En la medida en que existe correlación
entre la hepatomegalia y la formación de peroxisomas por una
parte y la capacidad carcinógena por otra, cabe prever que el DBF
será un carcinógeno menos potente que el DEHF y probablemente no
mostrará actividad si la medición se realiza con las metodologías
actuales de bioensayo del cáncer.
Las investigaciones epidemiológicas identificadas se limitan
a las de trabajadores expuestos a mezclas de ftalatos. Estos
estudios no contribuyen a mejorar nuestros conocimientos sobre
los efectos asociados al DBF aislado.
Puesto que el DBF no es genotóxico y se supone que será
menos carcinógeno que el DEHF, probablemente no mostraría
actividad si la medición se realizara utilizando las metodologías
actuales de bioensayo del cáncer. Así pues, no es probable que
el DBF presente un aumento significativo del riesgo de cáncer en
las concentraciones a las que habitualmente se encuentra en el
medio ambiente.
La ingestión es con diferencia la vía principal de
exposición al DBF; además, los datos toxicológicos de las demás
vías de administración son insuficientes para su evaluación. Por
consiguiente, se ha preparado un valor orientativo para la vía
oral, aunque el objetivo último debería ser la reducción de la
exposición total a todas las fuentes para lograr una ingesta
diaria inferior a la tolerable.
No se estableció ningún claro nivel sin efectos adversos
observados (NOAEL) para los puntos finales considerados los más
adecuados para obtener los valores de orientación (es decir, la
toxicidad en el desarrollo y la reproducción). Se consideró que
el LOAEL resultante de un estudio continuo de reproducción para
la toxicidad en el desarrollo y la reproducción fue 66 mg/kg de
peso corporal al día, aunque los efectos observados a ese nivel
de dosis fueron moderados y probablemente reversibles. A partir
de esos datos, se ha obtenido una ingesta diaria tolerable de
66 µg/kg de peso corporal al día, incorporando un factor de
incertidumbre de 1000 (× 10 para la variación entre especies, ×
10 para la variación entre individuos, y × 10 para la
extrapolación del LOAEL al NOAEL).
La información sobre la ecotoxicidad del DBF consiste en
datos sobre toxicidad aguda y crónica para varias especies de
distintos niveles tróficos del medio ambiente acuático. La CE50
más baja descrita para algas de agua dulce a las 96 horas fue de
750 µg de DBF/litro. Los valores más pequeños obtenidos en las
pruebas de toxicidad aguda en invertebrados acuáticos fueron una
DL50 a las 96 horas de 750 µg/litro (mísidos) y una CE50 a las
48 horas de 760 µg/litro (larvas de mosca enana). En estudios de
toxicidad crónica, la especie más sensible de invertebrado fue
Daphnia magna, con una NOEC (supervivencia de los padres) a
los 21 días de 500 µg/litro. En una prueba no normalizada con un
antípodo ( Gammarus pulex) se obtuvo una LOEC a los 10 días de
500 µg/litro y una NOEC de 100 µg/litro, basados ambos en una
reducción de la actividad locomotriz. En pruebas de toxicidad
aguda con peces, la CL50 más baja a las 96 horas notificada para
una especie de agua dulce fue de 350 µg/litro (perca canadiense)
y para una especie marina de 600 µg/litro (sargo chopa). El
estudio de toxicidad crónica más sensible se basó en la trucha
irisada, con una NOEC (crecimiento) a los 99 días de 100 µg/litro
y una LOEC a los 99 días de 190 µg/litro (reducción del
crecimiento de alrededor del 27 por ciento).
La toxicidad aguda del DBF para las aves es baja.
El riesgo para los organismos acuáticos asociado a las
concentraciones medias presentes en las aguas superficiales es
bajo. Sin embargo, el margen de inocuidad en ríos muy
contaminados es mucho más pequeño. No se dispone de datos
adecuados que permitan evaluar el riesgo del DBF para los
organismos que viven en sedimentos. Se puede concluir que, con
los niveles actuales, el riesgo para las aves y los mamíferos que
se alimentan de peces es bajo.
Relative molecular mass 278.34
Synonyms butylphthalate; dibutylphthalate; DBP;
1,2-benzenedicarboxylic acid dibutyl ester;
o-benzenedicarboxylic acid, dibutyl ester;
dibutyl 1,2-benzene dicarboxylate;
dibutyl- o-phthalate
CAS name 1,2-benzenedicarboxylic acid, dibutyl ester
CAS registry number 84-74-2
Trade names Caswell No. 292; Uniflex DBP; Celluflex DBP;
Ergoplast FDB; Polycizer DBP; Genoplast B;
Staflex DBP; Palatinol C; Hexaplast M/B; PX
104; RC Plasticizer DBP
Physical state Oily liquid
Colour Colourless
Odour Mild, aromatic
Melting point -35°C
Boiling point 340°C
Flashpoint 171°C
Table 1. contd.
Vapour pressure at 25°C 0.01 Pa (1.0 × 10-5 mmHg)
Density at 20°C 1.047
Partition coefficients
Log octanol/water 4.31-4.79
Log Koc 5.23
Solubility
Water at 25°C 10 mg/litre
Organic solvents Soluble in alcohol, ether, benzene
Henry's law constant 4.6 × 10-7 atmÊm3/mol
Phthalates frequently occur as plasticizers in analytical
equipment and as contaminants in laboratory air and solvents. This
can result in overestimation of their concentration in environmental
samples. For example, Ishida et al. (1980) detected DBP in laboratory
solvents at concentrations as high as 0.17 mg/kg (in benzene)
and in solid reagents at concentrations up to 9.89 mg/kg (in
carboxymethylcellulose), while polyvinyl tubing contained 20% DBP.
Therefore, a great deal of care is needed to prevent contamination
during the collection, storage and analysis of samples (Mathur, 1974;
US EPA, 1982b; Kohli et al., 1989; Hites & Budde, 1991). A summary of
analytical methods for the determination of DBP in environmental
samples and biological materials is presented in Tables 2 and 3,
respectively.
Table 2. Analytical methods for determining di- n-butyl phthalate in environmental samplesa
Sample matrix Sample preparation Analytical Sample detection
methodsb limit Accuracy Reference
Air Adsorption/solvent extraction HRGC/MS No data 115 ± 5%c Ligocki & Pankow
with polyurethane foam plug (1985)
Rainwater Adsorb on Tenax-GC columns, GC/MS < 34 ng/litre No data Ligocki et al.
thermally desorb (1985)
Water Extract with dichloromethane, GC/ECD 0.36 µg/litre 80 ± 6%c US EPA (1982a)
exchange to hexane, concentrate
Water Extract with dichloromethane at GC/MS 2.5 µg/litre 80 ± 6%c US EPA (1982b)
pH 11 and 2, concentrate
Water Adsorb on small bed volume GC/MS No data No data Pankow et al.
Tenax cartridges, thermally (1988)
desorb
Soil Extract with dichloromethane, GC/ECD 240 ng/kg 96% US EPA (1986a)
clean up, exchange to hexane
Waste, Extract with dichloromethane, GC/ECD 36 mg/kg 96% US EPA (1986a)
non-water-miscible clean up, exchange to hexane
Soil Extract from sample, clean up GC/MS 1.7 mg/kg 96% US EPA (1986b)
Waste, Extract from sample, clean up GC/MS 350 mg/kg 76% US EPA (1986b)
non-water-miscible
Soil/sediment Extract from sample, clean up HRGC/MS 660 µg/kg 76% US EPA (1986c)
Table 2. Continued
Sample matrix Sample preparation Analytical Sample detection
methodsb limit Accuracy Reference
Waste, Extract from sample, clean up HRGC/MS 50 mg/kg 76% US EPA (1986c)
non-water-miscible
Soil/sediment Extract from sample, clean up HRGC/FTIR 10 µg/litred No data US EPA (1986d)
Wastes, Extract from sample, cleanup HRGC/FTIR 10 µg/litred No data US EPA (1986d)
non-water-miscible
a From: Agency for Toxic Substances and Diseases Registry (1990).
b HRGC = high-resolution gas chromatography;
MS = mass spectrometry;
GC = gas chromatography;
ECD = electron-capture detector;
FTIR = Fourier transform infrared spectrometry.
c Relative recovery, percentage ± standard deviation.
d Identification limit. Detection limits for actual samples are several orders of magnitude higher depending upon the sample
matrix and extraction procedure employed.
Table 3. Analytical methods for determining di- n-butyl phthalate in biological materials
Sample matrix Sample preparation Analytical Sample detection Accuracy Reference
methoda limit (% recovery)
Aquatic organisms Extract with acetonitrile HRGC/ECD 0.1 µg/kg 68 Thuren (1986)
and petroleum ether
Adipose tissue Extraction, bulk lipid HRGC/MS 10 µg/kg No data Stanley (1986)
removal, Florisil
fractionation
Blood serum Extraction, bulk lipid HRGC/MS 10 µg/kg No data Stanley (1986)
removal, Florisil
fractionation
Blood serum Extraction with organic GC/MS No data No data Ching et al. (1981)
solvents (propanol,
heptane)
Cooked meat Remove with nitrogen gas GC/MS No data No data Ho (1983)
trap, extract with diethyl
ether
a HRGC High-resolution gas chromatography;
ECD Electron-capture detector;
MS Mass spectrometry;
GC Gas chromatography
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
The occurrence of naturally produced phthalates in biological and
geochemical samples has been suggested, but in most cases the
possibility of contamination during sampling or analysis could not be
ruled out (Mathur, 1974). However, it is unlikely that the amounts of
phthalates produced naturally would be significant compared with those
from anthropogenic sources (IPCS, 1992).
3.2 Anthropogenic sources
3.2.1 Production levels
Total DBP production in western Europe in 1994 was estimated to
be 49 000 tonnes (personal communication by the European Council for
Plasticisers and Intermediates to the IPCS, 1996). In Germany, the
average annual production was 20 000 tonnes for 1982-1986 (BUA, 1987).
DBP is produced by 36 companies in the USA, with total production of
7720 tonnes in 1977 and 11 400 tonnes in 1987 (ATSDR, 1990; NTP,
1995). Annual production in Japan in 1994 was about 17 000 tonnes
(JPIF, 1995).
3.2.2 Uses
DBP is used mainly as a speciality plasticizer for nitrocellulose
polyvinyl acetate and polyvinyl chloride (PVC) (ATSDR, 1990). In
1991, approximately 54% of the total supply of DBP in Canada was used
in adhesives, while about 15% was used in coatings (including
lacquers), and the rest in miscellaneous applications, including paper
coating (Camford Information Services Inc., 1992).
In Germany, approximately 25% of the DBP produced served as
plasticizer and adjuvant for the processing of PVC and about 20% was
used in adhesives (BUA, 1987).
DBP is one of the most commonly used plasticizers in regenerated
cellulose film, being present mainly in nitrocellulose coatings which
are applied to the films (average content, 2.5% of the weight of the
film) (MAFF, 1987).
DBP is used in cosmetics as a perfume solvent and fixative, a
suspension agent for solids in aerosols, a lubricant for aerosol
valves, an antifoaming agent, a skin emollient and a plasticizer in
nail polish, fingernail elongators and hair spray (Brandt, 1985).
3.2.3 Emissions
Although DBP has low volatility, its widespread use in many thin
polymeric sheets and coatings provides large surface areas for
volatization during manufacture, use and disposal of these products.
Disposal at dump sites and disintegration or incineration of the
plastics allow for dispersal of small particulates into the air
(ATSDR, 1990) Perwak et al. (1981) estimated that about 300 tonnes of
DBP were released into the air in 1977 in the USA.
Based on a production of 22 100 tonnes in Germany in 1986,
the release into the environment was estimated to be about
500 tonnes/year. Release associated with the production of DBP was
estimated to be about 0.1 tonnes/year, whereas emission related
to end usage was 400 tonnes/year. It was estimated that about
100 tonnes/year were released by further processing activities, such
as manufacture of plastic and other materials (BUA, 1987).
DBP may be released into surface water. It is estimated that
300 tonnes of DBP were released to water in 1977 in the USA (Perwak et
al., 1981).
No specific release of DBP to soils has been reported. However,
it may seep into soil from DBP coating sewage sludge that is deposited
on land (ATSDR, 1990).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
In the atmosphere, DBP has been measured in both the vapour and
the particulate phases. In various studies, the proportion of total
DBP present in the vapour form in the atmosphere has been reported to
range from 68% (32% in the particulate phase) in the Gulf of Mexico
(Giam et al., 1980) to 78% (22% in the particulate phase) in Antwerp,
Belgium (Cautreels & van Cauwenberghe, 1978). Hoff & Chan (1987),
however, reported that in the Niagara River region of North America,
more than 57% of atmospheric DBP occurs in the suspended particulate
phase.
Washout via rainfall or dry deposition is believed to play a
significant role in the removal of DBP from the atmosphere.
Eisenreich et al. (1981) predicted that atmospheric deposition is a
significant source of DBP in the Great Lakes, North America, with a
calculated total deposition of 48 tonnes/year to the five Great Lakes
and values for each ranging from 3.7 tonnes/year for Lake Ontario to
16 tonnes/year for Lake Superior. Based on levels of DBP in airborne
fallout at 14 locations in Sweden, the total deposition was estimated
to be 90 tonnes per year (Thurén & Larsson, 1990).
In surface water, most of the DBP (> 75%) is present in the
water fraction rather than in the suspended solids (Niagara River Data
Interpretation Group, 1990). Sullivan et al. (1982) reported that DBP
was rapidly adsorbed onto and desorbed from three clay minerals,
sediment and glass test tubes. During the experiments no more than
11% of the total DBP was adsorbed. Al-Omran & Preston (1987) found
that DBP reached an adsorption equilibria within 30 min, the degree of
adsorption being most closely correlated to the lipid content of
suspended particles. The adsorption was enhanced by the presence of
salt.
DBP is moderately adsorbed to soil (Howard, 1989; Zurmühl et al.,
1991), but it forms a complex with water-soluble fulvic acid and this
may increase its mobilization and reactivity in soil to some degree
(Matsuda & Schnitzer, 1971). Volatilization of DBP from soil is not
expected to be significant because of its low vapour pressure and
moderate adsorption to soil (Howard, 1989).
Using the Exposure Analysis Modelling System (EXAMS), Wolfe et
al. (1980) calculated that at equilibrium the loss of DBP from a pond
was 3.3% hydrolysis, 1.2% photolysis, 31.8% biodegradation and 6.2%
volatilization.
4.2 Transformation
4.2.1 Abiotic degradation
Howard et al. (1991) estimated the photo-oxidation half-life of
DBP in air to range from 7.4 h to 3.1 days.
The photolytic half-life of DBP in water has been estimated to be
144 days (Howard, 1989; calculated from Wolfe et al., 1980).
4.2.2 Biodegradation
DBP is biodegradable in natural surface waters, with an estimated
half-life in the range of 1 to 14 days (Schouten et al., 1979; Johnson
et al., 1984; Walker et al., 1984; Howard, 1989; Howard et al., 1991).
Primary degradation exceeded 95% in 24 h in the Semi-Continuous
Activated Sludge (SCAS) test, while ultimate biodegradation to CO2
amounted to 57.4% (half-life of 15.4 days) in the shake flask test
(CMA, 1984). Sugatt et al. (1984) reported 90% primary degradation of
DBP in the 28-day shake flask test using mixed populations of
microorganisms from natural sources.
Howard et al. (1991) predicted a DBP half-life of 2-23 days in
groundwater, based upon aerobic and anaerobic degradation rates.
Sediment from the upper 5 cm of a test pond served as the
inoculum in tests of aerobic and anaerobic degradation of DBP (Johnson
& Lulves, 1975). The samples contained 1 mg/litre of 14C-labelled
DBP. The extent of aerobic degradation was 53% within 24 h and 98%
within 5 days. The anaerobic solutions still contained 69% of the
initial amount after 5 days and only 2% after 30 days.
O'Connor et al. (1989) found > 85% mineralization of DBP during
incubation of anaerobic sludge for 90 days at a concentration of
200 mg DBP/litre. In anaerobic sludge, degradation of DBP proceeded
through mono- n-butyl phthalate to phthalic acid, followed by ring
cleavage and mineralization (Shelton et al., 1984).
In an experiment with batch anaerobic digestion of sewage sludge
spiked with DBP at a concentration range of 0.5-10 mg/litre, DBP was
degraded rapidly with a degradation rate following first-order
kinetics. More than 90% was removed in under 8 days without any lag
phase (Ziogou et al., 1989). The degradation rate can vary with
sludge source and sampling time. DBP was found to be degraded from an
activated sludge system very efficiently (Iturbe et al., 1991).
In a series of studies, Kurane et al. (1979a,b) demonstrated that
DBP is efficiently removed from wastewater by inoculating viable cells
of Nocardia erythropolis, a bacterium capable of rapidly degrading
phthalate esters in activated sludge. When the wastewater containing
3000 mg DBP/litre was treated with the activated sludge inoculated
with N. erythropolis, the DBP was found to be removed at a rate of
94.2% in one day and 100% after the 5th day (as measured by gas
chromatography) (Kurane et al., 1979a,b). Phthalate ester-utilizing
microoganism species isolated from the inoculated and uninoculated
activated sludge were N. erythropolis, N. restricta, Pseudomonas
capacia, P. fluorescens and P. acidovorans (Kurane et al.,
1979a,b).
Pseudomonas pseudoalcaligenes B20b1 (a denitrifying strain) was
enriched from the effluent of a biological sewage plant with DBP as
the sole carbon source (Benckiser & Ottow, 1982). After 20 days
at 30°C, TLC and MS analysis of the culture extracts showed
mono- n-butyl phthalate and phthalic acid as the only products,
suggesting that an n-butanol moiety served essentially as the carbon
source for growth and denitrification. A Micrococcus sp. (strain
12B) was also isolated by enriching with DBP as sole carbon and energy
source, and a metabolic pathway for DBP by this strain was proposed
(Eaton & Ribbons 1982). In this pathway, DBP is converted to mono-
n-butyl phthalate and then to 3,4-dihydro-3,4-dihydroxy phthalate,
which is in turn converted to 3,4-dihydroxy phthalate and then to
protocatechuate (3,4-dihydroxy benzoate). Protocatechuate is
metabolized by a meta-cleavage pathway to pyruvate and oxaloacetate
and by an ortho-cleavage pathway to beta-keto-adipate (Eaton &
Ribbons, 1982).
Wang et al. (1995) isolated five strains of DBP-degrading
microorganisms from coke-plant wastewater treatment plant sludge.
All strains were capable of achieving complete degradation of DBP
(100 mg/litre). One strain was able to completely degrade DBP within
40 h. Further experimental studies revealed that the rate of DBP
degradation was higher with immobilized cells than with free cells.
Chauret et al. (1995) have isolated a psychrotrophic denitrifying
Pseudomonas fluorescens from DBP-spiked microcosms, which is
capable of transforming DBP at 10°C under both aerobic and anaerobic
conditions. The isolated pseudomonad did not grow with phthalic acid
as the sole source of carbon, indicating that DBP was not mineralized
by this bacterium.
Howard et al. (1991) predicted a half-life for DBP in soil of 2
to 23 days. Inman et al. (1984) reported that DBP was almost
completely metabolized within 100 days in non-sterile soils of various
types (silt loam, sand, mixture of silica sand and peaty muck).
Overcash et al. (1982), however, reported half-lives of > 26 weeks in
loam and sand at application rates of 800 mg DBP/kg or more, while, at
a lower application rate (200 mg/kg), the half-life of DBP in loam and
sand was about 12 weeks.
Shanker et al. (1985) incubated garden soil containing DBP at a
concentration of 500 mg/kg. Within 10 days, 91% of the DBP had been
degraded and, after 15 days, 100% of the parent compound had been
degraded. No degradation was detected when sterilized soil was used.
Degradation of DBP was much slower in anaerobic soil, flooded with
sterile water to reduce oxygen tension. After a 30-day incubation,
66% of the DBP had been degraded, compared with 100% degradation
within 15 days under aerobic conditions.
Yan et al. (1995) reported that algae are capable of degrading
DBP. An average biodegradation rate of 2.1 mg/litre per day was found
when the alga Chlorella pyrenoidosa was exposed to 7 mg DBP/litre.
Degradation of the parent compound was complete within 72 h.
4.2.3 Bioaccumulation
The log octanol-water partition coefficient for DBP is between
4.31 and 4.79, which indicates a potential for the chemical to
bioaccumulate. However, the accumulation of DBP is influenced by the
capability of an organism to metabolize it, and several authors have
shown the ability of fish to metabolize DBP. Stalling et al. (1973)
found that radioactively-labelled DBP was metabolized by microsomal
preparations from fish (channel catfish) liver to mono- n-butyl
phthalate (55%) and three other unidentified metabolites (42%) within
2 h. Only 3% of the parent compound was recovered. All of the values
are expressed as percentage of radioactivity. The hepatic microsomes
taken from male channel catfish degraded DBP 16 times more rapidly
than diethylhexyl phthalate (DEHP). When Wofford et al. (1981)
exposed sheepshead minnow to 14C-DBP for 24 h, the distribution of
metabolites was as follows: 13% diester; 28.2% monoester; 47.8%
phthalic acid; and 11% of the radioactivity in the residue.
Bioconcentration factors for a number of organisms are presented
in Table 4. A wide variety of bioconcentration factors have been
reported reflecting not only the capability of organisms to accumulate
DBP but also the variety of exposure concentrations and test
conditions. Care must be taken when interpreting data based on the
accumulation of radioactivity because of the metabolism of the parent
compound (DBP). The highest bioconcentration factor quoted, based on
the parent compound, is 590 for the fathead minnow ( Pimephales
promelas) at an exposure concentration of 34.8 µg/litre. The
bioconcentration factor was a mean value based on the percentage of
DBP in the measured radioactivity over an 11-day period. The
percentage of DBP ranged from 50% on day 3 to 8% on day 11 (Call et
al., 1983).
Lokke & Bro-Rasmussen (1981) applied DBP, in a mixture that also
contained DEHP and di-iso-butyl phthalate, at a concentration of
2.5 µg/cm2 to the leaves of Sinapis alba. The residue level of
DBP on the leaves immediately after application was 2.4 µg/cm2.
There was rapid elimination of DBP and after 15 days DBP levels had
decreased to only 0.03 µg/cm2.
Belisle et al. (1975) fed mallard ducks ( Anas platyrhynchos)
on a diet containing 10 mg DBP/kg for a period of 5 months. No DBP
was detected in fat, heart, lung or breast tissue (detection limit =
0.1 mg/kg in a 2-g sample). The exposure concentration was equivalent
to a dose of 0.56 mg/kg body weight per day, assuming a body weight of
1.1 kg/bird and a food consumption rate of 0.0619 kg dry weight per
day (Nagy, 1987). There appears to have been no biomagnification of
DBP in this study. In fact, it would seem unlikely that terrestrial
animals will biomagnify DBP, based upon the rapid metabolism and
excretion observed in laboratory mammals (see Chapter 6).
Table 4. DBP bioconcentration (BCF) factors for various aquatic organisms
Species Water Duration BCFa Reference
concentration (days)
(µg/litre)
Oyster 100 1 21.1b Wofford et al.
(Crassostrea (1981)
virginica)
Oyster 500 1 41.6b Wofford et al.
(Crassostrea (1981)
virginica)
Water flea 0.08 14 400c Mayer & Sanders
(Daphnia magna) (1973)
Scud 0.10 14 1400c Mayer & Sanders
(Gammarus (1973)
pseudolimnaeus)
Scud 100 10 140 Thurén & Woin (1991)
(Gammarus pulex) (accumulated)
Scud 100 10 45 Thurén & Woin (1991)
(Gammarus pulex) (adsorbed)
Scud 500 10 64 Thurén & Woin (1991)
(Gammarus pulex) (accumulated)
Scud 500 10 8.4 Thurén & Woin (1991)
(Gammarus pulex) (adsorbed)
Table 4. Continued
Species Water Duration BCFa Reference
concentration (days)
(µg/litre)
Brown shrimp 100 1 2.9 Wofford et al. (1981)
(Penaeus aztecus)
Brown shrimp 500 1 30.6 Wofford et al. (1981)
(Penaeus aztecus)
Midge 0.18 7 720c Mayer & Sanders (1973)
(Chironomus
plumosus)
Mayfly 0.008 7 430c Mayer & Sanders (1973)
(Hexagenia
bilineata)
Fathead minnow 4.83 11 570d Call et al. (1983)
(Pimephales
promelas)
Fathead minnow 34.8 11 590d Call et al. (1983)
(Pimephales
promelas)
Sheepshead minnow 100 1 11.7 Wofford et al. (1981)
(Cyprinodon
variegatus)
a BCF based on whole-body concentrations, unless otherwise indicated
b BCF based on concentration in muscle
c Based on radioactivity
d Based on a mean for the % DBP in the radioactivity measured on days 1, 3 and 11
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
Identified data on concentrations of DBP in various media
are presented in Table 5. Data from the surveys considered to be
most representative are addressed in the text.
In interpreting this data, it should be noted that steps
taken to avoid contamination are rarely described in the reports
published before 1980 and, consequently, the reliability of the
early data often cannot be assessed. The more recent available
data have therefore been emphasized.
5.1.1 Air
The levels of DBP in air are summarized in Table 5.
Giam et al. (1978) reported mean concentrations of
0.3 ng/m3 over the Gulf of Mexico (n = 8) and 1.0 ng/m3 over
the North Atlantic Ocean (n = 5). No other information was
provided.
DBP was detected in samples of air taken in 1982 (n = 5)
along the Niagara River in Ontario, Canada, with mean
concentrations of 1.9 ± 1.3 ng/m3 in the gas phase and
4.0 ± 2.2 ng/m3 in the particulate phase (Hoff & Chan, 1987).
In 1983, mean levels were 4.5 ± 3.5 ng/m3 in 15 samples of
the gas phase and 6.2 ± 2.6 ng/m3 in 19 samples of the
particulate phase. Eisenreich et al. (1981) reported that
atmospheric concentrations of DBP in the Great Lakes area ranged
from 0.5 to 5 ng/m3; however, no sampling or analytical
details were given.
DBP has been identified in ambient air in Barcelona, Spain;
concentrations of 3.0 and 17 ng/m3 were reported in winter, and
1.1 and 10 ng/m3 in summer for coarse (> 7.2 µm) and fine
(> 0.5 µm) particulates, respectively (Aceves & Grimalt 1993).
Cautreels et al. (1977) reported a range of concentrations
of DBP from 24 to 74 ng/m3 in the suspended particulate phase of
the air in a residential area of Antwerp, Belgium, in contrast to
19 to 36 ng/m3 in samples from a rural area in Bolivia. Atlas &
Giam (1981) reported atmospheric concentrations of DBP as high as
18.5 ng/m3 at Pigeon Key, Florida. Bove et al. (1978) reported
mean concentrations of DBP ranging from 3.28 ng/m3 at Staten
Island to 5.69 ng/m3 at Brooklyn, New York. Weschler (1981)
reported DBP in the Arctic aerosol at Barrow, Alaska, at a
concentration of about 1 ng/m3. In Japan, in 1985, DBP was
detected in 56 out of 63 samples of ambient air at levels
ranging from 17 to 370 ng/m3 (detection limits, 5 to
70 ng/m3) (Environment Agency, Japan, 1995).
5.1.2 Water
5.1.2.1 Surface water
The levels of DBP in surface water are summarized in
Table 5. Information on concentrations of DBP in surface water
in a national database in Canada is limited to 73 records for
Alberta and two records for British Columbia dating from 1985 to
1988. Concentrations were above the detection limit for only
eight records and reported values ranged from < 1 to 2 µg/litre
(NAQUADAT, 1993). For water samples collected in 1988 and 1989,
mean concentrations of 12.2 ng/litre at Fort Erie, Ontario (all
of 26 samples contained DBP at concentrations above the
detection limit of 0.29 ng/litre; maximum 26.78 ng/litre) and
15.16 ng/litre at Niagara-on-the-Lake, Ontario (all of 25 samples
contained DBP at concentrations above the detection limit of
0.29 ng/litre; maximum 72.93 ng/litre) were reported (Niagara
River Data Interpretation Group, 1990).
In Japan, for the years 1974, 1975 and 1982, levels of DBP
in surface water ranged from 0.013 to 36 µg/litre (detected in 55
to 93% of samples; detection limits, 0.01 to 40 µg/litre).
(Environment Agency, Japan, 1995).
In 1991 and 1992; DBP concentrations were measured in
unfiltered water samples of the River Rhine (4 locations) and six
of its tributaries. DBP was detected in 99% of 217 samples with
a detection limit of 0.03 µg/litre. The mean concentration in
the Rhine was 0.18 µg/litre, and the maximum value was
1.3 µg/litre. Mean values in the tributaries were in the same
range (LWA, 1993; Furtmann, 1994). The concentrations in the
particulate fraction of R. Rhine water were reported to be in the
range of 1.2 to 7.8 mg/kg dry weight. Schouten et al. (1979)
reported that DBP concentrations in rivers in the Netherlands
ranged from < 0.1 to 2.8 µg/litre. Other measurements of DBP
concentrations in the Netherlands revealed a mean value of
0.1 µg/litre in the Rhine (maximum = 1.1 µg/litre, 53 samples) in
1991 (RIWA, 1991) and 1.0 µg/litre in the Ijssel Sea (maximum =
6.9 µg/litre; 7 samples) in 1992 (RIWA, 1992). In both reports a
mean value of 0.1 µg/litre was given for the River Lek.
In 1984, DBP was detected in the Rivers Irwell (12.1 and
33.5 g/litre) and Etherow (32.5 and 23.5 g/litre) in
Manchester, United Kingdom (Fatoki & Vernon, 1990). Both rivers
received discharges from factories making plastic products.
5.1.2.2 Groundwater
At four sites in woodland areas of Germany, which are not
directly influenced by industry or agriculture, DBP
concentrations were measured monthly in wellwater and groundwater
in 1988 and 1989 (Schleyer et al., 1991). Mean concentrations
were 0.15 to 0.46 µg/litre.
5.1.2.3 Seawater
The levels of DBP in seawater are summarized in Table 5. In
an early study, concentrations of DBP up to 0.47 µg/litre in
water from the Gulf of Mexico were reported (Chan, 1975).
Reported maximum concentrations of DBP in seawater range from
0.203 µg/litre in the Kiel Bight (Baltic Sea) (Ehrhardt &
Derenbach, 1980) and 0.230 µg/litre (Ray et al., 1983a) in Nueces
Estuary, Texas, up to 4.8 µg/litre in United Kingdom estuaries in
industrial areas (North and Irish Seas) (Law et al., 1991) and
24.1 µg/litre in the Baltic and North Seas off the coast of Germany
(von Westernhagen et al., 1987).
5.1.2.4 Precipitation
Atlas & Giam (1981) reported concentrations of DBP in
rainwater ranging from 0.0026 to 0.0725 µg/litre at the Enewetak
Atoll in the North Pacific Ocean. Eisenreich et al. (1981)
reported that concentrations of DBP in rainwater in the Great
Lakes area ranged from 0.004 to 0.01 µg/litre; however, no
sampling or analytical details were given. In Japan in 1974
levels of DBP in rainwater ranged from 0.13 to 52 µg/litre
(detected in 68 out of 111 samples; detection limits ranged from
0.1 to 4 µg/litre) (personal communication by the Environment
Agency, Japan, to the IPCS 1995).
In 1992 DBP concentrations were measured in rainwater
samples from 3 sites in industrial areas of Germany (LWA 1993).
Mean values of 0.8 to 1.4 µg/litre and maximum values of 1.1 to
4.5 µg/litre were determined. In woodland areas of Germany that
are not directly influenced by industry or agriculture, DBP
concentrations in rainwater were measured at four sites in 1988
and 1989 (Schleyer et al., 1991). Outside the forest, mean
concentrations of 0.21 to 0.35 µg/litre were found. The
precipipitation sampled below the trees contained nearly the same
amount of DBP; at one site the concentration was slightly higher
with 0.52 µg/litre. A minimum concentration of 0.06 µg/litre
and a maximum concentration of 1 µg/litre were found.
5.1.2.5 Effluent and wastewater
Concentrations of DBP in effluent ranged from not detectable
to 61 µg/litre for five Canadian organic chemical plants (number
of samples unspecified), from not detectable to 94 µg/litre for
industrial and municipal plants in Cornwall, Ontario (number of
samples unspecified) and from 1.0 to 100 µg/litre for petro-
chemical refineries along the St. Clair River (n= 28) (CCREM,
1987). The detection limit for this study was 1.0 µg/litre.
Concentrations of DBP in fifteen 24-h composite samples of
process waters collected in 1981 from Canadian refineries
(unspecified locations) ranged from traces (detection limit,
2 µg/litre) to 56 µg/litre (PACE, 1985). However, DBP was not
detected in 19 samples of effluent discharge of non-chlorinated
primary-treated municipal wastewater collected in Vancouver in
1983 (Rogers et al., 1986).
The concentration in sewage treatment plant effluent from
Manchester, United Kingdom, sampled during 1984, was 6.0 g
DBP/litre (Fatoki & Vernon, 1990).
5.1.3 Sewage sludge
DBP has been detected in sludge from municipal wastewater
plants in Canada (Webber & Lesage, 1989). Concentrations ranged
from 0.2 to 161 mg/kg dry weight in Winnipeg in 1981 and 1982.
In Hamilton, the concentrations ranged from 14 mg/kg dry weight
in 1983 to 57 mg/kg dry weight in 1981. The authors noted that
recovery of phthalate esters was erratic, possibly due to
laboratory contamination or lack of sample homogeneity.
DBP concentrations were investigated in anaerobic digester
sludge from nine German municipal wastewater treatment plants
(Zurmühl, 1990). In eight plants concentrations were in the
range of 2.3 to 26 mg/kg dry weight (detection limit =
1.9 mg/kg). A level of 236 mg/kg dry weight was found as the
maximum value. Sewage sludge from another municipal wastewater
plant contained 0.87 mg DBP/kg dry weight (Kördel & Müller 1992).
5.1.4 Soil
DBP levels of < 0.1 to 1.4 µg/g were detected in 13 out of
30 samples (detection limit, 0.1 µg/g) of soils in urban areas of
Port Credit and Oakville/Burlington, Ontario (Golder Associates,
1987). Concentrations in the background samples on- and off-site
were similar (Golder Associates, SENES Consultants Limited and
CanTox, 1987).
Kördel & Müller (1992, 1993) investigated the DBP
concentrations in soil in the vicinity of phthalate-emitting
plants and compared them to a remote area. There was a great
deal of variability in the concentrations at the different
sampling sites, resulting in the fact that no influence of the
phthalate-emitting plants on soil DBP levels could be derived.
The concentrations for the remote site were in the range of <
0.005 mg/kg to 0.185 mg/kg dry weight. In the vicinity of the
industrial sites the values were < 0.005 to 0.560 mg/kg dry
weight.
5.1.5 Sediment
The levels of DBP in sediment are summarized in Table 5.
Samples of sediment collected from the Detroit River in 1982
contained concentrations of DBP ranging from < 0.1 to 0.65 mg/kg
dry weight (Fallon & Horvath, 1985). Concentrations of DBP in
sediment samples taken in 1982 from the Fraser Estuary, British
Columbia, ranged from 0.07 to 0.45 mg/kg dry weight (Rogers &
Hall, 1987). The concentration of DBP decreased from 0.204 mg/kg
dry weight in sediment 0.5 km from a large sewage outfall in the
estuary to 0.060 mg/kg in sediment 1.0 km from the outfall
(Rogers & Hall, 1987). Concentrations of DBP up to 0.3 mg/kg were
reported in samples of sediment collected from Lake Superior and
Lake Huron in the 1970s (CCREM, 1987). Concentrations of DBP in
sediment from the Neckar River in Germany ranged from 0.09 to
0.3 mg/kg (Malisch et al., 1981). Higher concentrations (0.028
to 0.9 mg/kg) were reported in sediment in Maryland, USA
(Peterson & Freeman, 1984). Marine sediment from the Crouch
Estuary United Kingdom contained 0.0039 to 0.0145 mg/kg
(Waldock, 1983). Reported concentrations of DBP from marine
sediments in the USA ranged from 0.0042 mg/kg dry weight in
Nueces Estuary, Texas (Ray et al., 1983a) to 0.355 mg/kg dry
weight at Los Angeles (Swartz et al., 1985). In Japan, levels in
1974 and 1982 ranged from 0.001 to 2.3 mg/kg (detected in 41 -
86% of total of 415 samples; detection limits, 0.0007 to
0.28 mg/kg).
DBP concentrations in Rhine sediments were measured in 1991.
In seven samples concentrations ranged from 0.14 to 2.2 mg/kg dry
weight. In 9 out of 10 samples of sediments of the River Weser,
DBP was detected at concentrations of 0.03 to 0.34 mg/kg dry
weight with one maximum value of 9.1 mg/kg. The detection limit
was 0.02 mg/kg (LWA, 1993). In Sweden sediment samples from
different types of enviornment were taken in 1994 (Parkman &
Remberger, 1995). DBP concentrations in samples from remote sites
were in the range from 1 to 8 µg/kg dry weight, with one outlier
of 56 µg/kg (average of three samples per site). Concentrations
in industrialized areas were 0 to 182 µg/kg dry weight (detection
limit = 1.9 µg/kg).
5.1.6 Aquatic organisms
In early studies, the concentrations of DBP in aquatic biota
from the Great Lakes and other areas in Canada were less than
10 mg/kg (Williams, 1973; Glass et al., 1977; Swain, 1978;
Burns et al., 1981). The highest concentrations were reported
for skinless fillets from long-nose suckers, Catostomus
catostomus, (8.1 µg DBP/g) and rainbow trout, Oncorhynchus
mykiss, (5.4 µg/g) from Lake Superior (Glass et al., 1977).
In fish from various US Great Lakes harbours and tributary mouths
in the USA, the concentrations of DBP in the majority of the
samples ranged from < 0.02 to 0.16 µg/g wet weight; however,
there were some higher values ranging up to 35 µg/g in more
polluted areas (DeVault, 1985). Ray et al. (1983b) reported
concentrations of DBP in the marine polychaete worm Neanthes
virens from Portland, Maine, USA, ranging from 0.070 to
0.180 mg/kg.
5.1.7 Terrestrial organisms
Data on phthalate levels in wild birds and mammals are very
sparse. In an early study, Zitko (1972) detected DBP in egg
yolks of the double-crested cormorant, Phalacrocorax auritus,
(14.1 µg/g lipid) and herring gull, Larus argentatus, (10.9,
17.1 and 19.1 µg/g lipid).
5.2 General population exposure
5.2.1 Ambient air
Data on concentrations of DBP in ambient air are extremely
limited. The most extensive information available is the range
of concentrations of 4.5 (mean of 15 samples; gas phase) to
6.2 ng/m3 (mean of 19 samples; particulate phase) in air sampled
along the Niagara River in 1983 (Hoff & Chan, 1987). These
values are similar to those determined more recently in a small
number of ambient air samples from Barcelona, Spain (Aceves &
Grimalt, 1993). Based upon a daily inhalation volume for adults
of 22 m3, a mean body weight for males and females of 64 kg, the
assumption that 4 of 24 h are spent outdoors (IPCS, 1993) and the
above range of concentrations in ambient air, the mean intake of
DBP via ambient air for the general population is estimated to
range from 0.26 to 0.36 ng/kg body weight per day.
5.2.2 Indoor air
The maximum concentration of DBP in indoor air in nine homes
in Montreal, Canada, sampled for three consecutive periods of 20
days each, was 2.85 µg/m3 (nominal quantification limit,
0.50 µg/m3) (Otson & Benoit, 1985). No other information on
measured concentrations (e.g., mean concentrations) was
presented. In a survey of 125 homes in California in 1990, the
median daytime concentration of DBP in indoor air was 420 ng/m3
(California Environmental Protection Agency, 1992).
Based upon a daily inhalation volume for adults of 22 m3, a
mean body weight for males and females of 64 kg, the assumption
that 20 of 24 h are spent indoors (IPCS, 1993) and the median
concentration of DBP reported in a survey of a large number of
homes in California (420 ng/m3), the daily intake of DBP in
indoor air for the general population is estimated to be
120 ng/kg body weight per day.
5.2.3 Drinking-water
Data on concentrations of DBP in drinking-water are limited.
In an early survey (1974), DBP was detected (detection limit
unspecified) in six out of ten city water supplies in the USA.
The concentrations of DBP ranged from 0.01 to 0.1 µg/litre for
five cities and was 5.0 µg/litre for one city (Keith et al.,
1976). Concentrations in two samples of tap water from the
Shizuoka Prefecture in Japan taken in 1974 were 1.0 and
0.8 µg/litre (Shibuya, 1979). In samples of tap and well water
in Japan, levels were 1.9 and 2.5 µg/litre, respectively (Ishida
et al., 1980). In a survey of an unspecified number of samples
of the municipal drinking-water supplies of seven cities in the
Niagara region and in the vicinity of Lake Ontario conducted in
1984 (MOE, 1984), DBP was not detected (detection limit,
1.0 µg/litre).
In a small number of samples of drinking-water in Toronto,
Canada, the mean concentration was 14 ng/litre; concentrations in
seven brands of bottled spring water ranged from 21 to
55 ng/litre (City of Toronto, 1990).
Based upon a daily water consumption for adults of 1.4
litres, a mean body weight for males and females of 64 kg (IPCS,
1993) and a mean concentration of < 1.0 µg/litre, the estimated
mean intake of DBP from drinking-water for the general population
is <0.02 µg/kg body weight per day.
5.2.4 Food
In addition to entry through environmental contamination,
DBP may be present in foodstuffs as a result of migration from
packaging. This has been investigated in a number of studies
conducted in the late 1980s. In many countries, on the basis of
the results of these studies, precautions were introduced to
reduce leaching of plasticizers from packaging. As a result,
levels of DBP in foodstuffs have declined over time. In this
section, studies designed to investigate the presence of DBP in
foodstuffs due to leaching from packaging are presented, followed
by data from more broadly based market-basket surveys.
Concentrations of DBP ranged from 0.13 to 1.62 mg/kg in
three brands of aluminum foil in Japan (Ishida et al., 1980).
In the first of several studies conducted in the United
Kingdom to investigate the impact of packaging on the DBP content
of foodstuffs, foods were purchased at retail stores and stored
in their packaging until their "sell by" or "best before" date
(British Ministry of Agriculture, Fisheries and Food, 1987).
Mean concentrations of DBP were 8 to 32 mg/kg in chocolate
confectionery, 13 mg/kg in sugar confectionery, 11 mg/kg in
cakes, 3.9 to 11 mg/kg in baked savouries, 6 to 10 mg/kg in meat
pies and 2 mg/kg in sandwiches.
In a survey of plastic-packaged Italian foodstuffs, DBP was
detected in cheese (0.84 g/g), salted meat (1.09 mg/kg),
vegetable soups (2.06 mg/kg), potato chips (2.80 mg/kg) and
pasteurized milk (0.07 mg/kg) (Cocchieri, 1986).
Levels of DBP ranged from 0.5 to 30.8 mg/kg in nougat and
chocolate, respectively, in a wide range of foodstuffs in the
United Kingdom, which were wrapped in a range of different
packaging including nitrocellulose-coated regenerated cellulose
film (RCF). Levels of plasticizers were 0.5 to 1.5%, on a total
film-weight basis (Castle et al.,1988). In a later study, Castle
et al. (1989) reported that DBP in the ink on the outer surface
of film can transfer onto the inner food contact surface. The
level of DBP in a chocolate-covered confectionery product
increased from 0.2 to 6.7 mg/kg over a storage period of 180
days. DBP levels in 47 samples of confectionery, snack products
and biscuits purchased in the United Kingdom, wrapped in printed
polypropylene film, ranged from 0.02 to 14.1 mg/kg.
In a more recent reported retail survey in the United
Kingdom (MAFF, 1990), ranges in up to 30 samples each of plastic
wrapped foods were 0.09 to 0.13 mg/kg in biscuits, 0.02 to
14.1 mg/kg in potato snacks, 0.15 to 5.6 mg/kg in chocolate-
covered bars and 2.6 to 9.2 mg/kg in candy-coated chocolate
sweets. In the same report, results of sequential analysis of a
few foods were also reported. Concentrations in potato snacks,
candy-coated individual sweets and chocolate bars increased
approximately 2- to 3-fold over a 6-month period.
Page & Lacroix (1992) reported that retail samples of
packaged butter and margarine sold in Canada contained up to
10.6 mg DBP/kg.
Nerin et al. (1993) analysed plastic-wrapped food products
for DBP from both Spain and the United Kingdom and reported (for
an average of three determinations) up to 0.81 mg/kg in chocolate
bars and 0.60 mg/kg in biscuits.
In an early Canadian study (Williams, 1973), DBP was
determined in 21 samples of fish. DBP was detected in one sample
of canned tuna at a concentration of 78 µg/kg while the levels in
one sample of canned salmon was 37 µg/kg. Concentrations of DBP
in the muscle of fish (n = 10 samples from five species) from the
lower Fraser River in British Columbia ranged from 0.07 to
0.15 mg/kg wet weight (Swain & Walton, 1989). The authors
considered 0.07 mg/kg as the background level, owing to
contamination; the detection limit was not reported. Elevated
concentrations of DBP have occasionally been reported in fish in
polluted areas (see section 5.1).
Based upon residue analysis of commercial eggs collected
throughout Japan, 0.098 mg DBP/kg (trace - 0.15 mg/kg was
present in egg whites (Ishida et al., 1981). No phthalate
residues were found in the egg yolks. In an early study of 2 to
14 samples each of various foodstuffs in Japan, DBP was detected
in meat (100 µg/kg), fish (180 µg/kg), eggs (80 µg/kg), but not
in milk (detection limit, 50 µg/kg) (Howard, 1989). In another
study (Tomita et al., 1977), DBP was determined by gas-liquid
chromatography (detection limit, 0.01 mg/kg) in 22 kinds of
Japanese foods (17 samples of fatty foods and 38 samples of non-
fatty foods mostly in plastic containers). DBP was detected in
tempura (frying) powder (0.39 to 17.70 mg/kg), instant cream soup
(1.73 to 60.37 mg/kg), fried potato cake (not detected to
1.11 mg/kg), orange juice (0.35 mg/kg) and pickles (0.11 mg/kg).
Ito et al. (1993) reported that 2 out of 15 samples of
imported vodka in Japan contained up to 0.2 mg DBP/litre. In the
USA, DBP was detected in 18 out of 50 samples of vodka (maximum
concentration: 204 µg/litre; limit of detection: 20 µg/litre)
(Leibowitz et al., 1995). DBP was detected in 1 out of 60 samples
of Russian vodka (0.7 mg/litre) and in 1 out of 7 samples of
European vodka (1.1 mg/litre) (Saito et al., 1993).
In a Canadian market-basket survey of 98 different food
types sampled in Halifax in 1986 (Page & Lacroix, 1995), DBP was
detected in butter (1.5 mg/kg), freshwater fish (0.5 mg/g),
cereal products (ranged from not detected to 0.62 mg/kg), baked
potatoes (0.63 mg/kg), coleslaw (0.11 mg/kg), bananas,
blueberries and pineapples (0.12, 0.09 and 0.05 mg/kg,
respectively), margarine (0.64 mg/kg), white sugar (0.2 mg/kg)
and gelatin dessert (0.09 mg/kg). The detection limits varied
(ranging from 0.01 to 0.5 mg/kg) according to the reagent blank
values (interferences arising from coextracted food components)
and the fat content of the food.
Exposure of the general population to DBP in food has been
estimated on the basis of data from the only study identified in
which there was a sufficiently wide variety of foodstuffs to
serve as a basis, i.e., those from a market-basket survey in
Canadaa. Based upon the average daily consumption of various
foodstuffs by adultsb, a mean body weight for males and females
of 64 kg (IPCS, 1993) and concentrations of DBP reported in the
Canadian market basket survey, the estimated daily intake from
food is 7 µg/kg body weight per day. It should be noted,
however, that intake of DBP in the diet can vary considerably,
depending upon the nature and amount of packaged food that is
consumed and the nature of use of food wrapping in food
preparation. In the United Kingdom, the Ministry of Agriculture,
Fisheries and Food has estimated that the maximum likely human
intake of DBP from food sources is approximately 2 mg per person
per day (approximately 31 g/kg body weight per day, assuming a
mean body weight of 64 kg).
5.2.5 Consumer products
In 1981, DBP was reported as an ingredient in a total of 590
cosmetic formulations in the USA, at concentrations ranging from
less than 0.1% to between 10 and 25% (Brandt, 1985). There is
potential for exposure to DBP in cosmetics, but available data
are inadequate to quantify intake from this source.
The "new car smell" in automobiles has been attributed to
DBP and other phthalic acid esters (Shea, 1971). Levels of total
phthalic acid esters in the µg/m3 range have been identified in
samples of air taken from new cars in an early study (Graham,
1973).
5.2.6 Medical devices
Plastic tubing used in hospitals for oral/nasal feeding of
patients, has been reported to contain 54 mg DBP/g (Khaliq et
a Data from the Canadian market-basket survey used in
calculating the estimated average daily intake include
concentrations of DBP in the following foodstuffs: butter,
1.5 mg/kg; freshwater fish, 0.5 mg/kg; cereal products,
0.62 mg/kg, baked potatoes, 0.63 mg/kg; bananas, 0.12 mg/kg;
white sugar, 0.2 mg/kg.
b Dietary intakes consist of: cereals, 323 g/day; starchy
roots, 225 g/day; sugar (excludes syrups and honey),
72 g/day; pulses and nuts, 33 g/day; vegetables and fruits,
325 g/day; meat, 125 g/day, eggs, 19 g/day; fish, 23 g/day;
milk products (excludes butter), 360 g/day; fats and oils
(includes butter), 31 g/day (IPCS, 1993).
al., 1992). DBP leached from tubing into distilled water and
solutions of ethanol, acetic acid and sodium bicarbonate, in
concentrations which increased with temperature and duration of
contact.
5.2.7 Levels in human tissue
In an early study, concentrations of DBP in 25 samples of
human adipose tissue collected from Vancouver (n = 2), Toronto (n
= 22) and Montreal (n = 1) at autopsies of accident victims,
ranged from 0.01 to 0.3 mg/kg (detection limit not reported) (Mes
et al., 1974).
Levels of DBP in the blood collected from 13 individuals
(mean, 0.10 mg/litre) following ingestion of food that had been
in contact with unspecified flexible plastics packaging materials
containing DBP were higher than those collected from nine
individuals before meals (mean levels in blood, 0.02 mg/litre)
(Tomita et al., 1977).
5.3 Occupational exposure
Identified data on levels of DBP in the occupational
environment are limited. Based on a survey conducted by the
National Institute of Occupational Safety and Health (NIOSH) in
1981-1983, it was estimated that there were 229 000 workers in
the USA with potential exposure to DBP (Howard, 1989). The most
recent provisional data from the National Occupational Exposure
Survey indicates that over 500 000 workers, including 200 000
women are potentially exposed to DBP (NIOSH, 1994).
In 1986, NIOSH conducted a health hazard evaluation of a
silkscreening area in a Department of Highways sign shop (NIOSH,
1987). Concentrations of DBP were below the limit of detection
(less than 0.01 mg per sample), i.e., less than 0.02 mg/m3.
Only trace quantities of DBP were detected in a 1975 survey
of a Goodyear Tire and Rubber Company plant in areas involved in
the production of rubber sleeve stock (NIOSH, 1976).
In 1981, an environmental survey was conducted at a US army
ammunition plant, in an area where DBP-containing propellant was
processed (NIOSH, 1982). Four samples (1 breathing zone, 3 area)
were collected. One area sample contained DBP in an amount
corresponding to a concentration of 0.08 mg DBP/m3. The other
three samples contained less than the detection limit
(0.01 mg/sample).
An industrial hygiene survey was conducted in a plastic pipe
fabricating plant in the USA in 1988. Six personal breathing
zone air samples collected for DBP were below the level of
detection, corresponding to < 0.01 mg/m3 (NIOSH, 1989).
Fischer et al. (1993) reported that concentrations of DBP
ranged from 1.3 to 8.2 mg/m3 in a plant in the Czech Republic
that produced PVC products.
Thus, based on determinations at a limited number of
worksites in the USA, concentrations have generally been less
than the limit of detection (i.e., 0.01 to 0.02 mg/m3), although
levels of up to 8 mg/m3 were reported in a PVC plant in the
Czech Republic.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
Data on kinetics and metabolism in mammals are presented in
this chapter. Information on metabolism in invertebrates is
presented in Chapter 4.
6.1 Absorption, distribution and excretion
6.1.1 Dermal
A study was conducted by Elsisi et al. (1989) in which
157 µmol/kg (43.7 mg/kg) of 14C-DBP (uniformly labelled on the
ring) was applied to the back of male F-344 rats and the area of
application was covered with a perforated cap for a 7-day
period). Approximately 10 to 12% of the administered dose was
excreted in the urine each day for several days (total of 60%
after 1 week). Only small amounts of radioactivity were detected
in tissues in the exposed rats. About 33% of the dose remained
at the site of application; all other tissues combined contained
less than 0.5% of the applied dose.
Based on results observed in vitro, Scott et al. (1987)
reported that DBP was slowly absorbed through both rat and human
skin, with rat skin being more permeable.
6.1.2 Ingestion
6.1.2.1 In vivo studies
Levels of DBP in the blood collected from 13 individuals
(mean, 0.10 mg/litre) 2 h following ingestion of food, which had
been in contact with unspecified flexible plastic packaging
materials containing DBP, were higher than those collected from
nine individuals before meals (mean level in blood,
0.02 mg/litre) (Tomita et al., 1977).
Studies in experimental animals indicate that DBP or its
metabolites are rapidly absorbed from the gastrointestinal tract.
In a study conducted by Williams & Blanchfield (1975), following
administration of a single oral dose of about 0.1 g/kg body
weight 7-14C-DBP to male Wistar rats, 96% of the radioactivity
was excreted in the urine at 48 h; less than 0.1% was exhaled as
14CO2. In addition, blood and tissue levels and urine output
were determined at 4, 8, 24 and 48 h following administration of
single oral doses of 7-14C-DBP (0.27 or 2.31 g/kg body weight).
The radioactivity was distributed more or less evenly throughout
the tissues except that the level in the brain was about one
third to one tenth that in the other tissues. Excretion in the
urine was rapid, with 46% of the low dose and 20% of the high
dose being present in the urine at 8 h, 85 and 61%, respectively,
at 24 h, and 92 and 83%, respectively, at 48 h. Based on
analysis of the urine, 80 to 90% of the dose was metabolized and
excreted in the urine in 48 h as phthalic acid (2%), mono-
n-butyl phthalate (88%), mono 3-hydroxy butyl phthalate (8%)
and mono-4-hydroxy butyl phthalate (2%). These authors also
reported that there was no evidence of accumulation in any
tissues in rats fed 0.1% DBP in the diet for 4, 8 or 12 weeks.
Twenty four hours following gavage (in 3% DMSO solution)
administration of a single dose of 60 mg/kg body weight 14C-DBP
to small groups (n=3) of male Wistar rats, radioactivity was
detected in the liver, kidney, blood, muscle, adipose tissue,
stomach and intestine (the latter probably associated with
biliary excretion). There was no significant retention of DBP
within tissues; more than 90% of the administered radioactivity
was recovered in the urine within 48 h (Tanaka et al., 1978).
In DSN hamsters, 79% of a single oral dose of 2 g/kg body
weight (10 µCi of 14C-DBP/kg body weight) administered by gavage
was excreted in the urine within 24 h, mainly as mono- n-butyl
phthalate (Foster et al., 1982).
6.1.2.2 In vitro studies
Mono- n-butyl phthalate (MBP) was absorbed in significantly
greater quantity than DBP in an in vitro study in an everted
gut-sac preparation from the small intestine of male Sprague
Dawley rats (White et al., 1980). DBP was actively hydrolysed by
esterases within the mucosal epithelium during absorption; 95.5%
of DBP was hydrolysed to MBP. When the esterase activity of the
mucosa was reduced by intragastric exposure of the rats to S,S,S-
tributylphosphorotrithioate (8 mg/kg body weight), the absorption
of DBP, but not of MBP, was significantly reduced (from 0.62 to
0.15 µmol/mg per h).
6.1.3 Inhalation
Following inhalation by rats of 50 mg/m3 for various
periods up to 6 months (Kawano, 1980b), DBP was detected by GC/MS
at relatively low concentrations in the brain (0.53 µg/g), lung
(0.17 µg/g) and liver (0.25 µg/g) of small groups of male Wistar
rats. Levels in the testes were lower (mean 0.13 g/g).
Following exposure to 0.5 mg/m3 (0.044 ppm), DBP was
consistently detected only in the brain of exposed rats.
6.2 Metabolic transformation
6.2.1 In vivo studies
Available data indicate that in rats DBP is metabolized by
nonspecific esterases, mainly by hydrolysis, to yield MBP, with
subsequent oxidation of the alkyl side chain of MBP.
Interestingly, MBP is stable and resistant to hydrolysis of the
second ester group (Cater et al., 1977; Rowland et al., 1977).
Following oral administration of DBP to rats, metabolic products
identified in the urine were mainly MBP, various oxidation
products of MBP (2-3%), and a small amount of the free phthalic
acid (Albro & Moore, 1974; Williams & Blanchfield, 1975; Foster
et al., 1982). The MBP and other metabolites are excreted in the
urine mainly as glucuronide conjugates; species differences in
the excretion of conjugated and unconjugated metabolites of DBP
in the urine of Wistar rats and DSN hamsters have been observed.
In hamsters, 53% was excreted as the conjugate and 3.5% as free
monoester. In rats, 38% was excreted as conjugate and 14% as
free monoester, following administration of an oral dose of 2
g/kg body weight (10 µCi of 14C-DBP/kg body weight per day) by
gavage. No free DBP was detected in the urine in either species
(Foster et al., 1982).
6.2.2 In vitro studies
In in vitro studies, DBP was hydrolysed to MBP by cell
preparations from the small intestine (rat, baboon, man), the
liver (rat, baboon) and kidneys (rats) (Lake et al., 1977; Tanaka
et al., 1978; Kaneshima et al., 1978).
Rowland et al. (1977) incubated the contents of the male
Wistar rat stomach, small intestine and caecum with 14C-labelled
DBP for 16 h. About 0.5, 80 and 23% of the DBP was hydrolysed to
MBP by the contents of the stomach, small intestine and caecum,
respectively. The metabolism of DBP by the small intestinal
contents was very rapid, 38% of a dose of 1 mg DBP/ml and 70% of
a dose of 200 g/ml being metabolized in 30 min. Thus, it would
appear that DBP is relatively quickly converted to MBP in the
intestines, this being the principal metabolite. Activity in the
female rat small intestine was only slightly less than that for
the male. Suspensions prepared from human faeces also had modest
DBP hydrolytic activity (6% in 16 h) (Rowland et al., 1977).
Because activity did not decrease when antibiotics were present
during the incubation, the author concluded that the enzymatic
hydrolytic activity was of mammalian origin (possibly pancreatic
and mucosal lipases).
Using 14C-DBP as substrate, the rate of esterase activity
was comparable in small intestinal tissue of rats and hamsters,
whereas the liver of hamsters had approximately double the
activity of rats. In contrast, the ß-glucuronidase activity of
testicular homogenates in the rat was much higher than that in
the hamster ( p-nitrophenyl glucuronide and phenolphthalein
glucuronide were used as substrates) (Foster et al., 1982).
In in vitro assays of rat liver, kidney, pancreas, small
intestine and blood, structural analogues of DBP (di- n-butyl
isophthalate and di- n-butyl terephthalate) were hydrolysed to
their corresponding acids, whereas phthalic acid was not formed
from DBP (Takahashi & Tanaka, 1989). The authors concluded that
nonionic esters are hydrolysed at a much higher rate than charged
analogues and that esterase activities are strikingly different
for different substrates.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of DBP in mice and rats is low. Reported
LD50 values following oral administration to rats range from
approximately 8 g/kg body weight to at least 20 g/kg body weight
(Smith, 1953; Lehman, 1955; White et al., 1983; Brandt, 1985); in
mice, values are approximately 5 to 16 g/kg body weight
(Woodward, 1988; Brandt, 1985; Yamada, 1974). Reported LD50
values following intraperitoneal administration range from 4 to
7 g/kg body weight in rats and approximately 3 to 6 g/kg body
weight in mice (Woodward, 1988). The dermal LD50 in rabbits is
> 4000 mg/kg body weight (Lehman, 1955). Signs of toxicity
include general depression of activity, laboured breathing and
lack of coordination. Reports of acute toxicity of DBP following
inhalation have not been identified.
Following intraperitoneal injection, MBP (the principal
metabolite of DBP) appeared to be somewhat more acutely toxic
than DBP; the LD50 was 1.0 g/kg in the mouse (Chambon et al.
1971).
7.2 Short-term exposure
The short-term toxicity of DBP has been investigated in
rodents following oral administration. The available data are
summarized in Table 6.
In most of these studies, animals were exposed to only one
dose level. Effects in rats after oral administration for 5 to
21 days include those on liver enzymes (Aitio & Parkki, 1978;
Bell et al., 1978; Kawashima et al., 1983; BIBRA, 1986; Barber et
al., 1987) and hepatomegaly at doses of >420 mg/kg body weight
per day (Yamada, 1974; Bell et al., 1978; Oishi & Hiraga, 1980a;
BIBRA, 1986; Barber et al., 1987), a reduction in the rate of
weight gain at doses of >5 ml/kg body weight per day
(5235 mg/kg body weight per day) (Yamada, 1974) and splenomegaly
after intragastric intubation of 1.0 ml/kg body weight per day
(1047 mg/kg body weight per day) (Yamada, 1974). Peroxisome
proliferation, based on increased oxidation of cyanide-
insensitive CoA oxidation, in the liver of male F-344 rats was
observed after administration of 2100 mg/kg body weight per day
in the diet for 21 days (Barber et al., 1987) and also in male
Wistar rats after exposure for 34 to 36 days to 2500 mg/kg body
weight per day in the diet (Murakami et al., 1986a).
Proliferation at lower levels has also been reported in an
investigation summarized in an abstract by Lake et al. (1991). A
slight but insignificant increase in kidney weight was reported
in JCL:Wistar rats exposed to 2060 mg/kg body weight per day for
7 days by Oishi & Hiraga (1980a).
Table 6. Short-term repeated dose toxicity of DBP
Species Protocol Results Effect Levels Reference
Rat (Wistar, 1047 or 5235 mg/kg The rate of b.w. gain was slightly reduced at the high LOAEL = 1047 Yamada (1974)
groups of 5 b.w. per day by dose. One rat administered the high dose died during mg/kg b.w.
females) stomach tube daily the study. Hepatomegaly and marked splenomegaly noted per day
for 3 weeks. at necropsy in both exposed groups; relative kidney
Controls were weight of high-dose group 76% greater than that in
administered controls.
10 ml/kg distilled
water in the same
manner.
Rat (Wistar, 2% DBP in the diet Marked increases in stearoyl-CoA desaturation, One dose group Kawashima
groups of (equivalent to 1000 palmitoyl-CoA oxidation and catalase activity; only (effects et al. (1983)
3 males) mg/kg b.w. per day) increases in microsomal octadecanoic acid in liver, observed at
for 7 days hepatic homogenates and serum. The increases in the 1000 mg/kg b.w.
stearoyl-CoA desaturation appeared to be due to the per day)
increased activity (4 fold) of the terminal
desaturase and not to increases in the activities
of NADH cytochrome-C-reductase or in cytochrome b5
content.
Rat (JCL:Wistar, 2% DBP in the diet Mean b.w.s of exposed rats were slightly but not One dose group Oishi & Hiraga
groups of 10 equivalent to 2060 significantly lower than that of the controls. only (effects (1980a)
males) mg/kg b.w. per day Significant decrease in absolute and relative observed at
for 7 days testicular weights, but the absolute and relative 2060 mg/kg b.w.
liver weights were significantly increased. per day)
Slight but insignificant increase in kidney weight
in exposed rats.
Table 6. Continued
Species Protocol Results Effect Levels Reference
Rat (Fischer-344, dietary Males at mid and high dose and females at high dose LOEL = 624 BIBRA (1986),
5 animals per administration for gained less weight than controls. Absolute and mg/kg b.w. Barber et al.
sex per dose) 21 days at levels relative liver weight increased in all exposed per day (1987)
of 0, 0.6%, 1.2% groups. Lower testis weight in high-dose males;
or 2.5% DBP; severe atrophy observed upon histopathological
examination. Serum triglyceride and cholesterol
a positive control levels decreased in all exposed males and cholesterol
group was level reduced in all exposed females, in a
administered 1.2% non-dose-related manner. Slight reduction in
di(2-ethylhexyl) hepatocyte cytoplasmic basophilia in all rats at
phthalate; highest doses and in males at 1.2%.
Cyanide-insensitive palmitoyl CoA oxidation
dose levels increased in both sexes at the highest dose and at
(calculated by the 1.2% dose in males.
investigators and Lauric acid 11 and 12 hydroxylase activities were
presented in BIBRA significantly increased in all exposed males and
(1986)); in females in the high-dose group.
males: 0, 624,
1234, 2156 mg/kg
b.w. per day
females: 0, 632,
1261, 2107 mg/kg
b.w. per day
Table 6. Continued
Species Protocol Results Effect Levels Reference
Rat (F-344, 0.05, 0.1, 0.5, 1.0 A dose-related liver enlargement and induction of NOAEL = 104 Lake et al.
male, groups or 2.5% DBP in the palmitoyl-CoA oxidation activity were reported. mg/kg b.w. (1991) (abstract)
of 5 males) diet for 28 days Based on the enzyme activity, the no-effect level for per day
(not possible to induction of hepatic peroxisome proliferation was
present doses on a determined to be 104 mg/kg b.w. per day by the authors.
b.w. basis since
food consumption was
determined but not
reported)
Rat 0.7% DBP in the diet Hepatomegaly was noted in exposed rats. Reduction One dose group Bell et al.
(Sprague-Dawley, (equivalent to 420 in serum cholesterol levels in exposed animals and only (effects (1978)
groups of 9 mg/kg b.w. per day) inhibition in hepatic sterologenesis reducing the observed at 420
males) for 21 days uptake of 14C-mevalonate and 14C-acetate by the liver mg/kg b.w.
minces of the exposed rats. There was no effect on per day)
hepatic cholesterol levels.
Rat (Wistar, 5 mmol/kg b.w. per Increases in hepatic cytochrome P-450 levels and One dose group Aitio & Parkki
groups of 7 day (1390 mg/kg b.w. in the activities of epoxide hydratase and only (effects (1978)
males) per day) in corn oil glutathione-S-transferase. No statistically observed at
by gavage for 6 days significant increase in the catalytic activities 1390 mg/kg b.w.
dependent on cytochrome P-450 (ethoxycoumarin per day)
de-ethylation and benzo(a)pyrene hydroxylation).
Mouse (ICR, 2% DBP in the diet Food consumption was affected (data not presented) One dose group Oishi & Hiraga
groups of 10 (2400 mg/kg b.w. and b.w. gain was significantly decreased. The only (effects (1980b)
males) per day) for 1 week relative liver weight was significantly increased observed at
whereas the relative kidney weight was significantly 2400 mg/kg b.w.
reduced. The zinc concentration in the liver was per day)
reduced to 88 ± 3.14% of the control value while
that of the kidney remained unchanged.
For mice, identified data on short-term toxicity are limited
to one investigation, in which there was a significant decrease
in the relative kidney weight when ICR male mice were fed a diet
containing 2% (equivalent to 2400 mg/kg body weight per day) DBP
for 1 week (Oishi & Hiraga, 1980b). Results of histopathological
examinations were not reported.
In a short-term study, for which only an abstract was
published, Lake et al. (1991) compared the relative potentials of
several phthalates, including DBP and DEHP, to induce peroxisome
proliferation and testicular atrophy in rats. DEHP was more
potent than DBP in inducing palmitoyl-CoA oxidation activity.
The NOEL values for induction of peroxisome proliferation
activity were considered to be 52 and 104 mg/kg body weight per
day for DEHP and DBP, respectively. In contrast, the values for
testicular atrophy were 1093 and 515 mg/kg body weight per day
for DEHP and DBP, respectively.
In another study reported by Barber et al. (1987), in which
groups of five male and five female rats were administered 2.5%
DBP in the diet (1250 mg/kg body weight per day) for 21 days, the
extent of peroxisome proliferation in the males, on the basis of
electron micrographs, was equivalent to that produced by 0.6%
DEHP (300 mg/kg body weight per day). A scale of peroxisome
proliferation activity in the male rat was drawn up by the
investigators based on their own short-term results and work
published by other investigators. Relative values, based on
these studies conducted by various investigators for fenofibrate,
ciprofibrate, Wy 14,643, DEHP, DBP and aspirin were 304, 66, 44,
15, 3 and 1, respectively.
7.3 Long-term exposure
The effects of long-term exposure to DBP have been
investigated in several studies on rodents following oral
exposure; however, only limited information concerning effects
following inhalation was identified. Details of study design and
results are presented in Table 7.
In the study by Nikonorow et al. (1973), groups of 20 Wistar
rats (10 males and 10 females) were administered 120 and
1200 mg/kg body weight per day for 3 months by gavage in olive
oil. At both doses, there was a statistically significant
increase in the relative liver weight. No particular
alterations in the liver, kidneys and spleen of any rats
administered DBP were seen during gross or histological
examination. The LOEL was considered to be 120 mg/kg body weight
per day, based on the increase in relative liver weights.
A series of three subchronic dietary studies have been
published recently (NTP, 1995):
Table 7. Long-term toxicity of DBP
Species Protocol Results Effect Levels Reference
Oral
Rat (Wistar, 120 or 1200 mg/kg b.w. per Clinical signs of toxicity were not described. LOEL = 120 Nikonorow et
groups of 10 day in olive oil by gavage One rat from the high-dose group died but the mg/kg b.w. al. (1973)
males and 10 daily for 3 months death was not considered to be treatment-related per day
females) (sex not specified). At necropsy, the only
effect noted was hepatomegaly at both doses.
No gross or microscopic changes were noted in
the spleen or kidneys.
Rat (strain 2300 mg/kg b.w. per day There was a reduction in the rate of weight one dose group Radeva &
and sex administered by gavage gain from day 1 onwards, but no other only (reduction Dinoyeva
unspecified, (vehicle unspecified) for clinical signs of oxicity were noted and no in weight gain (1966)
groups of 8; 50 days deaths occurred. No other end-points were at 2300 mg/kg
5 as controls) reported. b.w. per day)
Rat (strain diets containing levels There were no clinical signs of toxicity and cannot be Radeva &
not specified, equivalent to 0.1, 1 and no haematological abnormalities. Urine determined Dinoyeva
groups of 8 10 mg/kg b.w. per day for analyses for hippuric acid, albumin and (1966)
males) 7 months; controls sediment contents were normal. Marked
received the vehicle used venous congestion in some exposed rats at
to produce the feed mix, necropsy was reported, but the organ and
sunflower oil, in the diet dose group(s) in which it occurred were not
specified. No other compound-related lesions
were noted.
Table 7. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, 5% DBP in the diet Reduction in b.w. gain during the first week, one dose group Murakami
groups of 5 (equivalent to a dose of followed by a plateau, which was 65% of the only (effects et al.
males) 2500 mg/kg b.w. per day) control value on the 35th day. There were observed at 2500 (1986b)
for 35 to 45 days significant increases in relative liver and mg/kg b.w. per
spleen weights but no significant changes in day)
absolute weight. In addition, there was
marked atrophy of the testicles. There was
also depressed respiration in liver
mitochondria when succinate or pyruvate was
used as a substrate. Hepatic glutamate
dehydrogenase activity was decreased by 73%
of the control value but this was not
significant. Succinate and pyruvate
dehydrogenase activities were significantly
decreased (by 59% and 38% of the control
values, respectively).
Table 7. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, 0.5% or 5% in the diet B.w., expressed as percentage of weight in LOAEL = 250 Murakami
groups of 5 (equivalent to 250 or the control group, decreased gradually in mg/kg b.w. et al.
males) 2500 mg/kg b.w. per day, both groups. There were significant per day (1986a)
respectively) for 34 to increases in the relative weights of the
36 days liver, kidney and spleen, and decreases in
the weight of the testicles in the high-dose
group. The succinate and pyruvate
dehydrogenase activities in liver
mitochondria were significantly inhibited at
the high dose, but not the glutamate
dehydrogenase activity. The activities of
AP, GOT and GPT increased in rats that
received the high dose. Decreased globulin
and increased albumin/globulin ratio were
observed in both dose groups. In the
liver, cytotoxic injury including single
cell necrosis, zonal necrosis and degeneration
with ballooning were observed in many of the
rats that received the high dose. Zonal
necrosis and liver atrophy were observed in
the low-dose group. Ultrastructural
examination of liver cells revealed that the
effects of exposure to the high dose were more
extensive than the low dose in increasing
peroxisomes, lysosomes and mitochondria.
Table 7. Continued
Species Protocol Results Effect Levels Reference
F-344 rat; This study has been designated Final b.w.: reduction in males at >10 000 LOEL = 356 NTP (1995)
10 per sex "NTP Study 2"; administration mg/kg and in females at >20 000 mg/kg mg/kg b.w.
per group in diet for 13 weeks at 0, Organ weights: hepatomegaly in males at per day
2500, 5000, 10 000, 20 000 or >5000 mg/kg and in females at >10 000 mg/kg; (peroxisomal
40 000 mg/kg; mean equivalent testis and epididymal weights lower at 20 000 proliferation);
dose levels based on and 40 000 mg/kg 720 mg/kg b.w.
consumption and b.w.s: Haematology: minimal anaemia in males at per day based
females: 0, 177, 356, 712, >5000 mg/kg on germinal
1413 or 2943 mg/kg b.w. Clinical chemistry: hypocholesterolaemia in epithelial
males: 0, 176, 359, 720, 1540 both sexes at 20 000 and 40 000 mg/kg; atrophy in the
or 2964 mg/kg b.w. hypotriglyceridaemia in all exposed males and testes and
females at >10 000 mg/kg; elevations in histopathological
alkaline phosphatase activity and bile acid lesions in the
concentration in both sexes was considered liver
indicative of cholestasis
NOEL = 177
mg/kg b.w.
per day
Table 7. Continued
Species Protocol Results Effect Levels Reference
F-344 rat; Histopathology: NTP (1995)
10 per sex Liver: hepatocellular cytoplasmic alterations
per group consistent with glycogen depletion in both sexes
at >10 000 mg/kg; in both sexes at
40 000 mg/kg, small, fine,
eosinophilic granules in cytoplasm of
hepatocytes; ultrastructural examination
showed the presence of increased numbers
of peroxisomes, and peroxisomal enzyme
activity was elevated in liver of both
sexes at >5000 mg/kg (enzyme activity at
40 000 mg/kg was 13-fold greater than
controls for males and 32-fold greater
than controls for females)
Testes: degeneration of germinal epithelium,
mild to marked focal lesions at 10 000 and
20 000 mg/kg and marked, diffuse lesion in all
animals at 40 000 mg/kg; almost complete loss
of germinal epithelium at 40 000 mg/kg;
testicular zinc concentrations lower at 20 000
and 40 000 mg/kg; serum testosterone lower
than controls at 20 000 and 40 000 mg/kg; at
20 000 mg/kg, spermatid heads per testis and
per gram testis, epididymal spermatozoal
motility, and the number of epididymal
spermatozoa per gram epididymis were lower than
controls.
Table 7. Continued
Species Protocol Results Effect Levels Reference
F-344 rat This study has been designated 10 control and 10 exposed pups per sex were LOEL for hepatic NTP (1995)
"NTP Study 3" examined at weaning; hepatomegaly and peroxisomal
markedly increased peroxisomal enzyme proliferation and
Combined perinatal and activities (19-fold greater than control hepatomegaly is
subchronic exposure. An values) were observed. 279 mg/kg b.w. per
unspecified number of dams was Body weight gain: dose-related decrease day for males and
administered 10 000 mg/kg in significant in males in all dose groups and 593 mg/kg b.w. per
the diet beginning at day one in females at >20 000 mg/kg day for females;
of gestation, throughout Organ weights: hepatomegaly in males at NOEL is 138 mg/kg
gestation, and until weaning. >5000 mg/kg and in females at >2500 mg/kg; b.w. per day for
Pups had no exposure for four lower testes weight at >20 000 mg/kg; lower males and 294 mg/kg
weeks post-weaning. Ten pups epididymal weight at 20 000 mg/kg b.w. per day for
per sex per group were then Haematology: mild anaemia in males at females.
administered 2500, 5000, >10 000 mg/kg and in females at 40 000 mg/kg
10 000, 20 000 or 40 000 mg/kg Clinical chemistry: hypocholesterolaemia in
in the diet for 13 weeks. There both sexes at higher concentrations;
were two control groups - hypotriglyceridaemia in females at 20 000
animals which had been and 40 000 mg/kg and in males at >10 000
perinatally exposed and those mg/kg; elevations in alkaline phosphatase
with no exposure. activity and bile acid concentrations in
both sexes at 20 000 and 40 000 mg/kg were
indicative of cholestasis
Table 7. Continued
Species Protocol Results Effect Levels Reference
F-344 rat Mean equivalent dose levels Histopathology, liver: hepatocellular testicular NTP (1995)
based on consumption and body cytoplasmic alteration, consistent with germinal
weights: glycogen depletion, in both sexes at epithelium
females: 0, 147, 294, 593, 1182 >10 000 mg/kg; small, fine, eosinophilic degeneration
and 2445 mg/kg b.w. per day granules in cytoplasm of hepatocytes in observed at 571
males: 0, 138, 279, 571, 1262 males at 40 000 mg/kg; ultrastructural mg/kg b.w. per
and 2495 mg/kg b.w. per day examination showed the presence of day
increased numbers of peroxisomes at the
highest dose; peroxisomal enzyme activity
increased in males at >5000 mg/kg and in
females at >10 000 mg/kg (at 40 000 mg/kg,
activities were 20-fold higher than in
controls) testes: degeneration of germinal
epithelium, mild to moderate focal lesion
at 10 000 and 20 000 mg/kg, and marked,
diffuse lesion at 40 000 mg/kg; almost
complete loss of germinal epithelium at
40 000 mg/kg; testicular zinc concentrations
lower at 40 000 mg/kg; at 20 000 mg/kg,
there were fewer spermatid heads per testis
than unexposed controls and epididymal
spermatozoal concentrations were less than
in perinatally exposed controls
Rat, ingestion of diets containing half of the animals at the highest dose died Smith (1953)
Sprague-Dawley, 0.01, 0.05, 0.25 or 1.25% DBP during the first week
10 males per for 1 year
group haematology: no abnormalities at 3, 6, 9
equivalent doses: 6, 30, 150 months or at necropsy
or 750 mg/kg b.w. per day
no gross or microscopic changes in lung,
heart, liver, spleen, adrenals, kidneys,
stomach, small intestine, thyroid or brain.
Table 7. Continued
Species Protocol Results Effect Levels Reference
Rat, Wistar, ingestion of diet containing 0 15% of exposed rats died Nikonorow
20 males and 20 or 0.125% DBP for 1 year; et al. (1973)
females per no gross or histological changes in liver,
group equivalent dose: 0 or 75 kidney or spleen
mg/kg/b.w. per day
Mouse (ddy, 0.25% or 2.5% in diet for 86 remarkable vacuolar degeneration and LOAEL = 500 Ota et al.
groups of 3 or 90 days; (500 or 5000 mg/kg necrosis of single cells in the liver, and mg/kg b.w. (1973, 1974)
males and 12 b.w. per day) cysts and degeneration of epithelial cells per day
females) in the renal tubules in the high-dose group;
in the low-dose group, histological changes
were slight in the liver and kidney but
degeneration of parenchyma was observed
B6C3F1 mice; 10 administration in diet for 13 Mean body weight and body weight gain: decreased LOEL = 812 NTP (1994a,b;
per sex per weeks (0, 1250, 2500, 5000, in both sexes in a dose-related manner; decreases mg/kg b.w. 1995)
group 10 000 or 20 000 mg/kg); were significant at >5000 mg/kg per day, based
mean equivalent dose levels Relative liver weight: greater in both sexes at on decreases
based on consumption and body >5000 mg/kg in body weight
weights: Haematology: minimal anaemia was suggested in in both sexes
females: 0, 238, 486, 971, females at 20 000 mg/kg and increases
2137, or 4278 mg/kg b.w. per Histopathology: hepatocellular cytoplasmic in relative
day alterations, consistent with glycogen depletion, liver weight
males: 0, 163, 353, 812, 1601 in males at 10 000 and 20 000 mg/kg and females (NTP, 1995)
or 3689 mg/kg b.w. per day at 20 000 mg/kg; small, fine, eosinophilic
granules consistent with peroxisome proliferation NOEL = 486
were observed in the cytoplasm of hepatocytes in mg/kg b.w. per
both sexes at 20 000 mg/kg. day
Table 7. Continued
Species Protocol Results Effect Levels Reference
Inhalation
Rat (strain, 900 ± 80 mg/m3, 6 h per day Reduced body weights in exposed animals at the one dose group Antonyuk &
number and sex for 35 days end of the study (initial 173 g, final 151 g) only (effects Aldyreva
not specified) compared with controls (initial 174 g; final observed at (1973)
223 g). A decrease in haemoglobin content of 900 mg/m3)
peripheral blood was observed on day 10 but there
was a slight increase over control values by day
30. A decrease in phagocytic ability of
peripheral blood neutrophils was also noted.
There were statistically significant increases
in the weight of the liver, lungs, kidneys,
adrenals and brain, but data were not presented.
Mouse (strain range of 20 to 85 mg/m3 for 86 At the end of the study period, pulmonary could not be Spasovski
and sex not days. For a further 6 days, oedema was observed. No other end-points determined (1964)
specified, the concentration was increased were reported.
groups of 15) to a range of 170 to 420 mg/m3
Table 7. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, 0.5 mg/m3 (0.04 mg/kg) or A reduced rate of body weight gain was noted LOEL = 0.5 Kawano
groups of 11 to 50 mg/m3 (4.4 mg/kg) vapour, in rats administered the high concentration. mg/m3 (1980a)
14 males) 6 h per day except 1 day per There were no major differences between controls
week when exposure was for and exposed animals with respect to red cells,
3 h, 6 days/week for up to 6 platelets, haemoglobin concentration, haematocrit,
months. The levels of DBP lymphocytes, and neutrophils at 3 months but there
were checked throughout the was a reduction in lymphocyte numbers with an
study and varied between 45.0 increase in neutrophil numbers in exposed animals
and 59.2 mg/m3 in the high at 6 months. There were slight increases in
concentration and 0.31 and serum aspartate aminotransferase, alanine
0.56 mg/m3 in the low aminotransferase and alkaline phosphatase levels
concentration chambers. at 1, 3 and 6 months, and in blood glucose level
at 6 months in animals exposed to both
concentrations. At 6 months, serum cholesterol
had fallen slightly but serum triglyceride levels
had risen. The effects in the rats in the
low-concentration group were similar, but less
pronounced. In the high-concentration group, the
relative organ weights (brain, lung, liver,
kidney, testes) were normal at the end of the
first month, but after 6 months the relative
weights of the brain, lung, kidney and testes had
all increased.
Rat (strain 0.095, 0.25 and 1 mg/m3, 24 h No clinical signs of toxicity were noted during NOEL = 1 mg/m3 Men'shikova
unspecified, per day for 93 days the study; animals appeared healthy and the rate (1971)
groups of 15 of body weight gain for treated animals was
males) similar to controls. No abnormalities in
haemoglobin or red blood cell counts based on
haematological examinations, which were carried
out monthly. White cell counts fell during
exposure and these did not return to normal in a
group of animals observed for 6 months after
termination of exposure.
NTP study 1: prenatal/postnatal dose range-finding study in F-344
rats (described in section 7.5)
NTP study 2: conventional 13 week study in F-344 rats
NTP study 3: prenatal/postnatal exposure followed by a 13-week
exposure with two control groups (one with and one without
prenatal/postnatal exposure) in F-344 rats
NTP study 4: continuous breeding protocol in Sprague Dawley rats
(described in section 7.5)
No deaths occurred in the 13-week dietary study (NTP study 2
in rats) in which groups of 10 F-344 rats received 0, 2500, 5000,
10 000, 20 000 or 40 000 mg/kg diet. Average equivalent doses
were 0, 177, 356, 712, 1413 or 2943 mg/kg body weight per day for
females and 0, 176, 359, 720, 1540 or 2964 mg/kg body weight per
day for males (NTP, 1995). The final body weights of males
receiving approximately > 720 mg/kg body weight per day and
females receiving approximately > 1413 mg/kg body weight per
day were less than those of controls. No overt hepatic necrosis
or inflammation was observed at any dose. Hepatomegaly was
observed in males exposed to approximately > 359 mg/kg body
weight per day and females exposed to > 712 mg/kg body weight
per day. Testis and epididymal weights were less than those of
controls in animals exposed to > 1540 mg/kg body weight.
Histopathological examination of the liver revealed
hepatocellular cytoplasmic alterations, consistent with glycogen
depletion, in both sexes at >10 000 mg/kg diet. At 40 000
mg/kg diet, eosinophilic granules were observed in hepatocellular
cytoplasm. Upon ultrastructural examination, increased numbers of
peroxisomes were observed, and peroxisomal enzyme activity was
elevated in the liver of both sexes at > 5000 mg/kg diet.
Hepatic peroxisomal enzyme activity at the highest dose was 13-
and 32- fold greater than controls in males and females,
respectively. Examination of the testes revealed degeneration of
the germinal epithelium, mild to marked focal lesions at
> 720 mg/kg body weight, and a marked, diffuse lesion in all
males at 2964 mg/kg body weight per day. There was an almost
complete loss of the germinal epithelium at this dose.
Concentrations of testicular zinc and serum testosterone were
less than those of controls at > 1540 mg/kg body weight per
day. At 2400 mg/kg body weight per day, spermatid heads per
testis and per gram testis, epididymal spermatozoal motility, and
the number of epididymal spermatozoa per gram epididymis were
less than that in controls.
The only target tissues identified, therefore, in this study
were the liver and testes. The LOEL and NOEL values in this study
for hepatic peroxisomal proliferation and hepatomegaly were 356
and 176 mg/kg body weight per day, respectively. Testicular
germinal epithelial degeneration was observed at higher doses
(720 mg/kg body weight per day).
In a NTP study 3, pregnant F-344 rats were administered 0 or
10 000 mg/kg in the diet during gestation and lactation, and
weaned pups were administered the same diets as their dams
received for an additional 4 weeks until the beginning of the 13-
week exposure phase (NTP, 1995). The offspring then received 0,
2500, 5000, 10 000, 20 000 or 40 000 mg/kg diet (equivalent to
mean doses of 0, 147, 294, 593, 1182 and 2445 mg/kg body weight
per day for females and 0, 138, 279, 571, 1262 and 2495 mg/kg
body weight per day for males) for 13 weeks.
Ten control and 10 exposed pups of each sex were examined at
weaning. Hepatomegaly was observed in exposed pups, and
peroxisomal enzyme activity was 19-fold greater than in controls.
The body weight of prenatally/perinatally exposed pups was less
than that of controls throughout the 4-week period prior to the
13-week adult exposures. At the end of the 13-week exposure,
statistically significant changes in prenatally/perinatally
exposed controls versus unexposed controls were limited to
increased relative testis weight and lower final body weight in
males. The Task Group felt that the appropriate comparison was
to the pretreated control groups.
At the end of the 13-week exposure, body weights of males in
all exposed groups and of females at > 593 mg/kg body weight
per day were less than those in unexposed controls. No overt
hepatic necrosis or inflammation was observed at any dose. In
adult rats, hepatomegaly was observed in males at > 279 mg/kg
body weight per day and in females at > 593 mg/kg body weight
per day. There was hepatocellular cytoplasmic alteration
consistent with glycogen depletion in both sexes at >
10 000 mg/kg diet. Marked elevations of peroxisomal enzyme
activity were detected in males receiving > 279 mg/kg body
weight per day and in females receiving > 593 mg/kg body
weight per day.
At > 1262 mg/kg body weight per day, testis weight was
lower than that in controls. There was mild to moderate
degeneration of the germinal epithelium at > 571 mg/kg body
weight per day and marked diffuse germinal epithelial
degeneration at 2495 mg/kg body weight per day, at which dose an
almost complete loss of the germinal epithelium resulted. At the
highest dose, testicular zinc concentration was reduced, there
were fewer spermatid heads per testis than in unexposed controls,
and epididymal spermatozoal concentration was less than that in
the prenatally perinatally exposed controls.
The only target tissues identified, therefore, in this study
were the liver and testes. The LOEL and NOEL values in this study
for hepatic peroxisomal proliferation and hepatomegaly were 279
and 138 mg/kg body weight per day in males and 593 and 294 mg/kg
body weight per day in females. Testicular germinal epithelial
degeneration was observed at higher doses (571 mg/kg body weight
per day).
In other studies, male Wistar rats were administered 5% DBP
in the diet (equivalent to 2500 mg/kg body weight per day) for 35
to 45 days (Murakami et al., 1986b) or 0.5 or 5% (equivalent to
250 or 2500 mg/kg body weight per day) for 34 to 36 days
(Murakami et al., 1986a). In the rats ingesting the diet
containing 5% DBP, there was growth depression, liver
enlargement, testicular atrophy, decreased activities of
succinate and pyruvate dehydrogenase in liver mitochrondria and
abnormal changes in biochemical tests of serum and in
histological examinations of the liver and testes. Hepatic
lesions (including necrosis and atrophy) were also observed in
rats fed the diet containing 0.5% DBP, although these lesions
were less severe than those reported in the high-dose group.
There were changes in hepatocellular ultrastructure in rats
exposed to DBP, which were related to increases in peroxisomes,
lysosomes and mitochondria. A NOEL could not be established on
the basis of this study; the LOAEL was 250 mg/kg body weight per
day, based on liver pathology (Murakami et al., 1986b).
Additional mechanistic studies into DBP-related effects on
hepatic cell proliferation, peroxisomal enzyme activities,
clinical chemistry, haematology and gene expression are being
undertaken in a 90-day feed study by the National Toxicology
Program, but published reports are not yet available (personal
communication by R.R. Maronpot, National Institute of
Environmental Health Sciences, to the IPCS, 1995).
Little information on repeated dose toxicity in rats
following ingestion for periods longer than 3 months has been
identified. In an early study (Smith, 1953), groups of 10 male
Sprague-Dawley rats ingested diets containing 0.01, 0.05, 0.25 or
1.25% DBP (equivalent to 6, 30, 150 or 750 mg/kg body weight per
day) for 1 year. There were no effects on growth, but half of
the exposed animals administered the highest dietary level
(750 mg/kg body weight per day) died during the first week of the
study. No abnormalities were seen during examination of
haematological parameters at 3, 6 and 9 months, and at necropsy
there were no abnormal gross or microscopic findings in any of
the organs examined, which included the lung, heart, liver,
spleen, adrenals, kidneys, stomach, small intestine, thyroid and
the brain.
In another 12-month dietary study (Nikonorow et al., 1973),
groups of 20 male and 20 female Wistar rats ingested a diet
containing 0.125% of DBP (equivalent to 75 mg/kg body weight per
day). There were marked differences in food intake in exposed
animals, compared to controls, and 15% of the exposed rats died.
No alterations in the liver, kidneys or spleen were seen during
gross and histological examination.
In an inhalation study, effects at lowest levels were
observed by Kawano (1980b). In this investigation, rats were
exposed to 0.5 or 50 mg/m3 of DBP mist for 6 h/day, 5 days a
week, for up to 6 months (except for one day/week when rats were
exposed for only 3 h). There was a reduction in the rate of body
weight gain and increases in the relative weights of the brain
and lung. An increase in the percentage of neutrophils was
observed in both exposed groups. High levels of urea nitrogen
and low levels of cholesterol and triglyceride in the serum of
rats exposed to the high concentration were observed, indicating
hypolipidaemic activity of DBP. Similar, but less pronounced,
effects were observed in the group exposed to the low
concentration. In other identified studies, no effects were
observed following exposure for 93 days to 1 mg/m3 (Men'shikova,
1971), whereas effects on body weight gain, organ weights and
haematological parameters were observed at a high concentration
(900 mg/m3) following exposure for 35 days (Antonyuk &
Aldyreva, 1973).
In small groups of male and female ddY mice given a diet
containing 500 or 5000 mg/kg body weight per day for 3 months,
there were marked lesions in the liver and kidney (Ota et al.,
1973, 1974). In the high-dose group, there was remarkable
vacuolar degeneration and necrosis of single cells in the liver,
and cysts and degeneration of tubular epithelial cells in the
kidney. In the low-dose group, histological changes were slight
in the liver and kidney but degeneration of the parenchyma was
observed. The LOAEL was considered to be 500 mg/kg body weight
per day on the basis of histological changes in the liver and
kidneys.
B6C3F1 mice received dietary concentrations of 0, 1250,
2500, 5000, 10 000 or 20 000 mg/kg diet for 13 weeks (equivalent
to mean doses of 0, 238, 486, 971, 2137, or 4278 mg/kg body
weight per day in females and 0, 163, 353, 812, 1601 or
3689 mg/kg body weight per day in males (NTP, 1995). Body weight
and body weight gain were significantly decreased and relative
liver weight was significantly increased in both sexes at
> 5000 mg/kg diet. No overt hepatic necrosis was observed.
Histopathological examination revealed hepatocellular cytoplasmic
alterations, consistent with glycogen depletion, in males at
>1601 mg/kg body weight per day and in females at 4278 mg/kg
body weight per day. Eosinophilic granules, consistent with
peroxisomal proliferation, were observed in the cytoplasm of
hepatocytes in both sexes at 20 000 mg/kg diet. The LOEL in this
study was 812 mg/kg body weight per day, based on decreases in
body weight in both sexes and increases in relative liver weight
(NOEL = 353 mg/kg body weight per day). In contrast to rats,
there were no histological alterations in the testes of mice
exposed to any dose of DBP.
In summary, the effects in rats following ingestion of DBP
for periods of up to 13 weeks include reduced rate of weight gain
at doses of >250 mg/kg body weight per day (Radeva & Dinoyeva,
1966; Murakami et al., 1986a; NTP, 1995). Increase in relative
liver weights have been observed at doses of >120 mg/kg body
weight per day (Nikonorow et al., 1973; Murakami et al., 1986a;
1986b; NTP, 1995). Peroxisomal proliferation as determined by
increased peroxisomal enzyme activity has been observed at doses
of >279 mg/kg body weight per day (NTP, 1995). Although in
Fischer rats, no overt pathological effects on the liver were
observed (NTP, 1995), necrotic hepatic changes in Wistar rats
have been reported at doses of >250 mg/kg body weight per day
(Murakami et al., 1986a). Effects on the testes of male rats
have been observed at doses of >571 mg/kg body weight per day
(NTP, 1995).
In summary, histopathological lesions in the kidney and
liver were observed in mice in a limited study at DBP doses of
> 500 mg/kg body weight per day for 3 months (Ota et al.,
1973, 1974). Effects on body and organ weights and histological
alterations in the liver were also reported at higher doses in a
subchronic bioassay on mice (NTP, 1995) for which the NOEL was
353 mg/kg body weight per day, which was the lower of the two
NOELs in males and females (NTP, 1995).
In rats given a diet containing 5% MBP (the principal
metabolite of DBP), growth depression, liver enlargement,
testicular atrophy, decreased activities of succinate and
pyruvate dehydrogenase in liver mitochrondria, biochemical
effects on serum and histopathological effects on the liver and
testes were noted (Murakami et al., 1986a). Hepatic necrosis was
also observed in rats fed a diet containing 0.5% MBP (250 mg/kg
body weight per day), although it was less severe than in the
rats administered the higher concentration. The changes in
hepatocellular ultrastructure were more prominent in rats exposed
to DBP than in those administered MBP.
7.4 Irritation and sensitization
DBP appears to have little potential to irritate skin. Very
slight skin irritation, but no skin sensitization, was seen when
over 4 mg/kg body weight per day was applied to the skin of
rabbits for 90 days (Lehman, 1955). The ability of DBP to induce
an inflammatory response based on extravasation of trypan blue
has also been examined in rabbits (Calley et al., 1966). An
inflammatory response was observed but no details were given as
to whether there was any clinical irritancy.
No irritation was noted after DBP was instilled into the
eyes of rabbits examined at intervals up to 48 h after
application (concentration or amount not specified) (Lawrence
et al., 1975).
7.5 Reproductive and developmental toxicity
7.5.1 Reproductive effects
7.5.1.1 Testicular effects
Studies on the reproductive effects of DBP are summarized in
Table 8. Repeated oral exposure to concentrations of DBP for 4
to 90 days (250 to 2600 mg/kg body weight per day) affects the
reproductive system of male rodents. However, there are
considerable interspecies differences in response. Observed
effects in the available studies, most of which only used one
dose level (generally in the range 1200 to 2400 mg/kg body weight
per day), included marked reductions in the weights of the testes
and accessory sex glands, decreased numbers of spermatocytes,
degeneration of the seminiferous tubules of the testes, a
reduction in testicular zinc and iron levels and serum
testosterone levels, an increase in testosterone levels in the
testes, sloughing of germ cells, decreased activity of succinate
dehydrogenase in Sertoli cells, and an increase in urinary zinc
excretion at doses of >250 mg/kg body weight per day (Cater et
al., 1977; Gray & Butterworth, 1980; Oishi & Hiraga, 1980a,
1980b; Gray et al., 1982; Ikemoto et al., 1983; Fukuoka et al.,
1989, 1990; Zhou et al., 1990; Srivastava et al., 1990a,b; Lake
et al., 1991; Fukuoka et al., 1993; NTP, 1995).
The lowest reported effect levels in sufficiently well-
documented studies were those in a multi-dose investigation in
which DBP in groundnut oil (250, 500 or 1000 mg/kg body weight
per day) was administered to young male rats by gavage for 15
days (Srivastava et al., 1990a,b). A significant decrease in the
weight of the testes was observed at 500 and 1000 mg/kg body
weight per day. At these two doses, histopathological
examination revealed marked degeneration of the seminiferous
tubules. In all exposed groups, the activities of testicular
enzymes associated with post-meiotic spermatogenic cells, such as
sorbitol dehydrogenase and acid phosphatase, were decreased
significantly (P < 0.05), while that of testicular specific
lactate dehydrogenase was significantly increased, coincident
with degeneration of spermatogenic cells. The activities of
enzymes associated with pre-meiotic spermatogenic cells, Sertoli
cells or interstitial cells, and of ß-glucuronidase,
gamma-glutamyl-transpeptidase and glucose-6-phosphate
dehydrogenase were significantly increased (P < 0.05).
Therefore, the LOEL was 250 mg/kg body weight per day based on
enzyme changes in the testes; this value was also the NOAEL for
testicular weight and histopathological changes.
Table 8. Reproductive effects of DBP
Species Protocol Results Effect Levels Reference
Rat 500, 1000 or 2000 mg/kg In the first study, decreases in testicular LOEL = 500 mg/kg Cater et al.
(Sprague-Dawley, b.w. per day DBP by weight at the two highest doses (p<0.01 and b.w. per day in (1977)
groups of 6 gavage in corn oil daily p<0.001) after 4 days; the weight decreased one experiment.
males, 3 to 4 for 4 or 6 days in one further after 8 days at all 3 doses (p<0.05 One dose group
weeks old) experiment. 2000 mg/kg at 500 mg/kg b.w. per day and p<0.001 at the only in the other
b.w. per day by gavage two highest doses). In the second study, body experiment
in corn oil daily for weight gain also declined but the change was not (effects observed
periods of up to 14 days significant. Diminution of both spermatocyte at 2000 mg/kg
in a second experiment. and spermatogonia counts upon histological b.w. per day)
For urinary zinc examination of testes after 4 days of exposure
measurements, zinc-65 to 2000 mg/kg b.w. per day. At 2000 mg/kg b.w.
chloride was administered per day, urinary zinc excretion was increased
and the zinc-65 content by 34 to 43% over the first 4 days and then
was estimated by returned to normal. In the testes, the turnover
radioactive counting. rate of zinc was increased, the half-life was
reduced from 14 to 5 days and the zinc levels
were significantly reduced in the testes.
There was no change in zinc half-life or content
in liver or kidneys. Specific activity of
testicular alcohol dehydrogenase (or
zinc-dependent enzyme) decreased to 20 to 40%
of control after 5 days of exposure. A 3-day
pretreatment with 2000 mg/kg b.w. per day
caused a 25% decrease (p<0.001) in testicular
zinc uptake in vivo. Simultaneous ingestion of
zinc sulfate resulted in no testicular atrophy,
as measured by relative testicular weight (no
histopathological examinations).
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat 2% in the diet Mean body weights were slightly but not One dose group Oishi &
(JCL:Wistar, (equivalent to 2060 significantly lower than that of the controls. only (effects Hiraga
groups of 10 mg/kg b.w. per day) Absolute and relative testicular weights were observed at 2060 (1980a)
males, 5 weeks for 1 week. significantly decreased, and both absolute and mg/kg b.w. per
old) relative liver weights were significantly day)
increased. Histological examination of the
testes revealed a decrease in both spermatocyte
and spermatogonia counts. Zinc concentrations
in the testes and the liver were significantly
decreased. Testosterone concentration in the
testes was significantly increased.
Rat (Wistar, 2000 mg/kg b.w. per day Decreases in the relative weight of the testes, One dose group Gray &
groups of 10 by oral intubation in corn prostate and seminal vesicles were reported only (effects Butterworth
males, 4 weeks oil daily for 10 days. (data not presented). Testicular atrophy was observed at 2000 (1980)
old observed. mg/kg b.w. per day)
Rat 2000 mg/kg b.w. per day There was no change in body weight. Weight of One dose group Gray et al.
(Sprague-Dawley, by oral intubation in the testes was reduced to 45% of control only (effects (1982)
groups of 6 corn oil daily for 9 days. (p<0.001), and >90% tubular atrophy was seen observed at 2000
males, 4 to 6 in all animals upon histological examination. mg/kg b.w. per day)
weeks old)
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, 2400 mg/kg b.w. per day Severe testicular atrophy was evident; the weight One dose group Ikemoto et
groups of 5 administered by gavage of the testes was 0.59 g compared with 0.97 g in only (effects al. (1983)
rats, 5-week (vehicle unspecified) controls. Relative organ weights were not observed at 2400 (English
old males) daily for 7 days. presented and body weights were presented mg/kg b.w. per day) abstract)
Animals were killed graphically, only. Based on these graphs, the
on the day after relative weight of the testes was approximately
administration terminated. 0.29% compared with 0.48% for controls. Upon
microscopic examination, there was an almost
complete absence of germ cells in the
seminiferous tubules, with enlargement and
vacuolation of the Sertoli cells. These cells
had increased numbers of lipid droplets. The
Leydig cells also appeared atrophied. The gross
and microscopic changes were accompanied by a
decrease in the serum testosterone levels to 82%
of control values.
Rat (Wistar, 2400 mg/kg b.w. per day Decreases in testicular fructose and glucose One dose group Fukuoka et
28 adult males) administered by gavage levels and a sloughing of the germ cells on the only (effects al. (1989)
(neat DBP) daily for 7 first day of exposure. On day 2, more severe observed at 2400
days after acclimatization sloughing, accompanied by decreases in testicular mg/kg b.w. per day)
for 1 week. Groups of 6 iron and zinc levels and increases in the level
exposed rats were killed at of inositol and cholesterol. The sloughing was
24, 48, 120 or 168 h and 2 followed by atrophy, accompanied by dissociation
were killed at 72 and 96 h. of the germ cells from the Sertoli cells and
reduction of triglycerides, cholesterol and
phospholipids containing choline and ethanolamine
residues in the testis.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar, Single oral dose of 2400 Based on histological examination, DBP caused One dose group Fukuoka et
27 adult males) mg/kg b.w. per day sloughing of the germ cells at 6 h. On days 1 only (effects al. (1990)
administered by gavage and 2, there was more severe sloughing, followed observed at 2400
(neat DBP) after by atrophy and the dissociation of the germ cells mg/kg b.w. per day)
acclimatization for 1 week. from the Sertoli cells and the spermatogonia.
Groups of 3 exposed rats Biochemically, there was an elevation of
were killed at 3, 6, 12, 24, gamma-glutamyl transferase, a decrease in sorbitol
48, 72, 96, 120 and 168 h. levels at 3 h up to the 7th day and a decrease in
the activity of aldose reductase at 6 h in the
testes of treated rats. This was followed by
decreases in fructose levels and increases in the
activity of lactate dehydrogenase (LDH) and in
lactate levels at 12 h, and decreases in the
activities of sorbitol dehydrogenase and succinate
dehydrogenase on day 2. LDH isoenzymes 4 and 5
increased at 6 h prior to the increase in lactate
levels. The data are consistent with DBP-induced
testicular toxicity being associated with a
disturbance of the activity of the enzymes that
are linked with Sertoli cell function and
replication and germ cell maturation.
Rat (Wistar All treated groups Mono-n-butyl phthalate (MBP) (metabolite of DBP) One dose group Zhou et
adult male) administered neat DBP as was transported through the blood-tubular barrier only (effects al. (1990)
a single oral dose of 2400 onto the seminiferous lumen; it was incorporated observed at 2400
mg/kg b.w. Control rats into the lumen at a maximum rate between 1 and 3 h mg/kg b.w.)
administered 0.9% saline after dosing. MBP caused decreases in the
activities of succinate dehydrogenase in the
Rat (18) Experiment A Sertoli cells and sorbitol dehydrogenase in the
Study of transportation of germ cells, an increase in the activity of lactate
DBP from interstitial cell dehydrogenase in the germ cells and in the
fraction to Sertoli cells seminiferous lumen and a decrease in testicular
and germ cells. Three rats iron levels.
sacrificed at 1, 3, 6, 12,
24 and 48 h.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (15 exposed Experiment B Zhou et
and 15 control) Determination of enzyme al. (1990)
activities in separated
cell fractions. Three rats
in each group sacrificed at
3, 6, 12, 24 and 48 h
Rat (27 exposed Experiment C
and 15 control) Measurement of metal ions in
testes. Three exposed rats
sacrificed at 3, 6, 12, 24,
48, 72, 96, 120 and 168 h.
Three control rats sacrificed
at 3, 24, 46, 96 and 168 h.
Rat (Wistar, Single oral dose, 2400 At 6 h: sloughing of germ cells; decrease in One dose group Fukuota et
adult male) mg/kg b.w. Control rats activity of succinate dehydrogenase in the only (effects al. (1993)
received 0.9% saline. Sertoli cells and in the Sertoli-germ connection; observed at 2400
Serial sacrifice of 3 rats increase in activity of lactate dehydrogenase in mg/kg b.w.)
at each of 1, 3 and 6 h. germ cells. Increases in transferrin
concentrations in Sertoli cells, Sertoli-germ
connection, epididymus-ductus deferens and liver.
Decrease in transferrin in seminal vesicle.
Decrease in ferritin in seminiferous lumen.
Increase in flavin adenine dinucleotide level in
interstitial cells.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (Wistar 0, 250, 500 or 1000 mg/kg Significant decrease in testicular weight at 500 LOAEL = 250 mg/kg Srivastava
albino, groups b.w. per day by gavage in and 1000 mg/kg b.w. per day. Histopathological b.w. per day et al.
of 6 males, ground nut oil daily for examination revealed marked degeneration of (1990a,b)
5 weeks old) 15 days. seminiferous tubules at these doses. In all
exposed groups, the activities of testicular
enzymes associated with post-meiotic spermatogenic
cells, such as sorbitol dehydrogenase and acid
phosphatase, were decreased significantly, while
that of lactate dehydrogenase was significantly
increased, coincident with degeneration of
spermatogenic cells. The activities of enzymes
associated with premeiotic spermatogenic cells,
Sertoli cells or interstitial cells,
-glucuronidase, gamma-glutamyl transpeptidase and
glucose-6-phosphate dehydrogenase were also
significantly increased in all exposed groups.
Rat (F-344, 0, 0.05, 0.1, 0.5, 1.0 or Based on histological changes and data on organ NOEL = 515 mg/kg Lake et al.
groups of 5 2.5% in the diet for 28 weights, the authors concluded that the NOEL for b.w. per day (1991)
males, 6 weeks days (conversions to dose testicular atrophy was 515 mg/kg b.w. per day. (abstract)
old) on a body weight basis not No other information was reported.
available since food
consumption was determined
but not fully reported)
Rats (males; Oral administration by Some regeneration of seminiferous tubules 2 weeks One dose group Tanino et
strain and gavage (vehicle after discontinuation of the administration. only (effects al. (1987)
number of unspecified) of 2400 Active spermatogenesis in almost all tubules observed at 2400
animals mg/kg b.w. per day DBP though vacuolation of germinal epithelium and mg/kg b.w. per day)
unspecified) daily for 7 days. decreased number of sperm were still evident 3
weeks after exposure was terminated.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (males and 500 or 1000 mg/kg b.w. per In the first experiment, there were no effects In the first Gray et
females, strain day from 20 to 55 days of on the female reproductive system while male rats experiment: LOAEL al. (1983)
and number age in one experiment and were severely affected at both doses. Exposed = 500 mg/kg b.w. (abstract)
unspecified) 250 or 500 mg/kg b.w. per rats had smaller testes and seminal vesicles and per day (males);
day from 20 to 75 days of no sperm in the vas deferens. In the second NOEL = 1000 mg/kg
age in a second experiment experiment, rats were unaffected at 250 mg/kg b.w. b.w. per day
(nature, vehicle and per day but half of the pairs did not breed in the (females)
pattern of administration high-dose group. No other information was In the second
unspecified) reported. experiment:
NOEL=250 mg/kg
b.w. per day
Rat (strain 0.52 g/kg b.w. per day by There were no effects on conception rate or litter One dose group Bornmann &
unspecified, gavage daily (vehicle sizes in exposed animals when compared with only (no effects Loeser (1956)
groups of 8 unspecified) for 6 weeks controls. Neonatal growth rates in the F1 were observed at 520
females) and then mated with also comparable with those of control animals. mg/kg b.w. per day)
untreated males to produce The weights of endocrine organs in this generation
an F1 generation. were within normal limits and the onset of estrus
Interbreeding of untreated was similar to that in control rats. There were
F1 rats produced an F2 also no abnormalities in the F2 and F3 generations.
generation. An F3
generation was similarly
produced.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (CD This has been designated the final dose levels selected were 0.1, 0.5 and NTP concluded that NTP (1991,
Sprague-Dawley) "NTP Study 4" Continuous 1.0%, on the basis of clinical signs, body weight these effects 1995); Wine
breeding protocol which and food consumption. document et al. (1997)
included cross-over mating reproductive and
and offspring assessment developmental
phases. Preceded by a toxicity of DBP
range-finding study in F0 rats at all
("Task 1") [5 doses (0.1, dose levels and
0.5, 1.0, 1.5 and 2.0% in more severe
diet) and control; 8 toxicity to F1
rats/sex/group] offspring.
NOAEL not
identified
LOAEL =
approximately 66
mg/kg b.w. per day
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (CD "Task 2", continuous All control and exposed pairs were fertile. No NOAEL NTP (1991,
Sprague-Dawley) breeding phase. Based upon Dose-related decrease in number of live pups per established for 1995); Wine
food consumption data, the litter (significant at all doses); absolute and decreased litter et al. (1997)
authors estimated that the adjusted live pup weight significantly decreased size, LOAEL = 66
intakes for the exposed in mid- and high-dose groups. Dam weights at mg/kg b.w. per day.
groups were 52, 256 and 509 delivery were significantly decreased at each NOAEL for pup body
mg/kg b.w. for males and litter in the high dose-group. weight 66 mg/kg1
80, 385 and 794 mg/kg b.w. In the mid-dose group, reduction in pup body b.w. per day.
for females, giving average weight was not accompanied by reduced dam body NOAEL for fertility
intakes of 66, 320 and 651 weight. 651 mg/kg b.w. per
mg/kg b.w. per day. 40 day.
breeding pairs as controls
and 3 dose groups of 20
pairs each. Animals housed
as breeding pairs for 112
days. End-points were
clinical signs, parental
body weight, feed
consumption, fertility
(number producing a
litter/number of breeding
pairs), numbers of litters
per pair, number of live
pups per litter, proportion
of live pups, sex ratio of
live pups, body weight of
pups. The last litter born
during "Task 2" was reared
for "Task 4". litters
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (CD "Task 3"; since an adverse No overall difference with respect to mating, One dose group NTP (1991,
Sprague-Dawley) effect on reproduction was pregnancy or fertility indices. Live pup weight only. Effects 1995); Wine
detected during Task 2, a was adversely affected in Group C, which suggested seen in treated et al. (1997)
1-week cross-over mating that DBP was a reproductive toxicant in females. females at 665
trial was performed to However the authors noted that it was not possible mg/kg b.w. per day.
determine the affected sex. to distinguish between an effect on dam body
weight and direct toxicity to the fetus.
Consisted of 3 groups of 20 Significant increase in organ to body weight
pairs each: ratios for liver and kidneys in males and F0
- control males × control females. No effect upon sperm concentration,
females (Group A) motility, percent abnormal forms or testicular
- high-dose males × spermatid head count. No apparent effects upon
control females (Group B) estrual cyclicity or average estrous cycle length.
- control males ×
high-dose females (Group C)
End-points as for Task 2,
with addition of checking
for presence of vaginal
copulatory plug or sperm.
Estimated average daily
intakes of exposed animals
were 410 and 665 mg/kg b.w.
for males and females
respectively.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (CD "Task 4"; the last litter In the F1 high-dose group, body weight was No NOAEL NTP (1991,
Sprague-Dawley) born following the significantly lower at weaning and at necropsy established for 1995); Wine
continuous breeding phase for both sexes. reduced pup weight. et al. (1997)
("Task 2") was reared by Mating, pregnancy and fertility indices were LOEL = 66 mg/kg
the dam until weaning, at significantly lower in the high-dose group (1 b.w. per day.
which time the F1 animals litter born versus 19 in control group). No NOAEL
were exposed similarly to Absolute and ajusted live F2 pup weights were established for
the parents until 13 weeks significantly lower in all exposed groups. testis tubule
of age. Estimated average In high-dose males, significant decrease in the degeneration.
daily intakes for exposed absolute weight and relative weight of prostate, LOAEL = 322 mg/kg
groups were 50, 247 and 498 right testis and seminal vesicles; significant b.w. per day (low
mg/kg b.w. for males and 83, increase in liver and kidney weight. dose group not
397 and 828 mg/kg b.w. for In high-dose males, adverse effect upon epididymal examined)
females. At sexual maturity, sperm count and concentration and testicular
groups of 20 males and 20 spermatid head count and concentration. No
females from the same apparent effects upon estrual cyclicity or average
treatment groups cohabited estrous cycle length.
for 7 days, then housed Epididymides absent or poorly developed in 5/10
singly until delivery. high-dose, 0/10 mid-dose males.
End-points same as for Histological examination of control, mid- and
"Task 2", followed by high-dose groups showed testicular lesions
necropsy. consisting of degeneration of seminiferous tubules
(8/10 in high-dose and 3/10 in mid-dose group),
interstitial cell hyperplasia (7/10 in high-dose
group) and underdeveloped or defective
epididymides (5/10 in high dose group).
Table 8. Continued
Species Protocol Results Effect Levels Reference
Rat (F-344, DBP administered in diet to Weight gain: decreased in dams at 20 000 mg/kg 10 000 mg/kg diet NTP (1995)
number dams during gestation and during gestation and in dams at 10 000 mg/kg was recommended
unspecified) lactation and to pups during lactation. Mean b.w. of pups reduced as the maximum
postweaning for 4 weeks, at during lactation and at end of 4 weeks of dietary perinatal exposure
concentrations of 0, 1250, exposure. concentration for
2500, 5000, 7500, 10 000 or Gestation index: (number of live pups per male and female
20 000 mg/kg diet. breeding female) was significantly lower in the rats.
20 000 mg/kg group [pup mortality in this group
was 100% by day 1 of lactation]; pup survival was
89% or more in all other treatment groups.
Organ weight: increased relative liver weight in
all exposed males and in females at >2500 mg/kg.
Histopathology: moderate hypospermia of the
epididymis in all males at 7500 and 10 000 mg/kg;
mild hypospermia in 2 out of 10 males at 5000
mg/kg; no degeneration of germinal epithelium was
detected.
Mouse (B6C3F1, Dietary concentrations of 0, Only 5 dams at 10 000 mg/kg delivered live pups, Developmental NTP (1995)
pregnant, 20 1250, 2500, 5000, 7500, and none at 20 000 mg/kg. Only 1 pup at 10 000 toxicity and pup
per group) 10 000 or 20 000 mg/kg mg/kg survived past lactation day one; number of mortality were
during gestation and live pups per litter at 7500 mg/kg remained low suggested at
lactation; pups were weaned throughout lactation; no deaths occurred in pups concentrations as
onto same diet as dams and after weaning. low as 7500 mg/kg
exposed for an additional Postweaning and final body weights of males at and 5000 mg/kg was
4 weeks >2500 mg/kg were significantly less than controls. considered to be
Organ weights: absolute liver weight of males at the maximum
7500 mg/kg was greater than controls. perinatal exposure
The one surviving male pup at 10 000 mg/kg had concentration.
cytoplasmic alteration in liver, consistent with
peroxisome proliferation.
Developmental toxicity and fetal and pup mortality
were suggested as low as 7500 mg/kg.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Mouse (ICR, 2% in the diet (equivalent Food consumption was affected, though relevant One dose group Oishi &
groups of 10 to 2400 mg/kg b.w. per day) data were not presented and body weight gain was only (effects Hiraga (1980b)
males) for 1 week. significantly decreased. The relative weights of observed at 2400
the testes and liver were significantly mg/kg b.w. per day)
increased, whereas the relative weight of the
kidney was significantly reduced. The zinc
concentration in the testes and liver was reduced
to 81 ± 3.14% and 88 ± 3.14% of the control,
respectively (p<0.05), but not in the kidney.
The concentration of testosterone in the testes
was unaltered and there was no testicular atrophy.
Mouse (T O 2000 mg/kg b.w. per day by There was a significant depression of the weight One dose group Gray et
strain, groups oral intubation in corn oil of the testis but no effect on body weight. 4 only (effects al. (1982)
of 10 males, 4 daily for 9 days. out of 10 animals had isolated atrophic tubules observed at 2000
to 6 weeks old) while in the other 6 mice, only 10 to 20% of the mg/kg b.w. per day)
tubules showed pronounced atrophy.
Mouse (dd; 5 3, 9, 27, 50, 100 or 200 Reduction in relative testicular weight and NOEL = 27 mg/kg Sajiki
males and 5 mg/kg b.w. per day in olive increases in leukocyte counts and serum lactate b.w. per day (1975a,b)
females per oil by gavage, daily for 30 dehydrogenase activity were observed. LOEL = 50 mg/kg
group) days Congestion, oedema and congestive oedema in lung, b.w. per day
and loss of spermatogenic cells and spermatogonia
in testes were observed at doses of >50 mg/kg
b.w. per day.
Guinea-pig 2000 mg/kg b.w. per day There was a decrease in body weight (p<0.05) and One dose group Gray et
(Dunkin-Hartley, by oral intubation in corn weight of the testes (p<0.001). Based on only (effects al. (1982)
groups of 5 oil daily for 7 days. histological examination of the testes, there was observed at 2000
males, 4 to 6 severe tubular atrophy with loss of spermatids mg/kg b.w. per day)
weeks old) and a reduction in primary spermatocytes and
spermatogonia.
Table 8. Continued
Species Protocol Results Effect Levels Reference
Hamster (males 500 or 1000 mg/kg b.w. per In the first experiment, the testes, the seminal In the first Gray et
and females, day from 20 to 55 days of vesicles and the epididymis of the high-dose experiment: al. (1983)
strain and age in one experiment and group were smaller. There were no effects on NOEL = 500 mg/kg (abstract)
number 1000 mg/kg b.w. per day from the female reproductive system. In the second b.w. per day
unspecified) 20 to 75 days of age in a experiment, the exposed hamsters had smaller (males); NOEL =
second experiment (nature, testes; in their offspring, there was decreased 1000 mg/kg b.w. per
vehicle and pattern of viability and growth was retarded. day (females)
administration unspecified) In the second
experiment: one
dose group only,
effects observed
at 1000 mg/kg b.w.
per day)
Hamster 2000 mg/kg b.w. per day by There was no effect on body weight, weight of the One dose group Gray et
(Syrian DSN, oral intubation in corn oil testes or testicular histology. only (no effects al. (1982)
groups of 7 for 9 days. observed at 2000
males, 4 to 6 mg/kg b.w. per day)
weeks old)
Table 8. Continued
Species Protocol Results Effect Levels Reference
Mouse (Swiss Continous breeding protocol DBP exposure resulted in a reduction in the NOEL = 0.3% in the NTP (1984,
CD-1 albino, with cross-over mating. numbers of litters per pair and of live pups diet = 390 mg/kg 1995); Lamb
groups of 20 0.03, 0.3 or 1.0% DBP in per litter, and in the proportion of pups born b.w. per day. et al. (1987);
of each sex) the diet (equivalent to 39, alive at the 1.0% amount, but not at lower dose LOAEL = 1.0% in
390, 1300 mg/kg b.w. per levels. A cross-over mating trial with the the diet = 1300
day) for a 7-day pre-mating control and the high dose F0 mice demonstrated mg/kg b.w. per day
period randomly grouped as that female mice, but not males, were affected
mating pairs which were by DBP, as shown by significant decreases in the
exposed during a 98-day percentage of fertile pairs, the number of live
period of cohabitation. pups per litter, the proportion of pups born
alive, and live pup weight in the control male
and exposed female pairing. In the F0 females,
absolute and relative liver weights were
significantly increased and uterine weight was
significantly decreased at the high dose, which
suggested that this dose was maternally toxic.
There were no significant differences in the %
motile sperm, sperm concentration, or % abnormal
sperm in the cauda epididymis between male mice
exposed to 0 or 1.0% DBP in the diet. No
treatment-related gross or histopathological
lesions were noted for the testis, epididymis,
prostate or seminal vesicles in male mice, or
for the ovary, oviduct, uterus or vagina in the
female mice.
In a recently reported NTP subchronic study on F-344 rats
(see NTP rat study 2 in section 7.3), histopathological effects
in the testes (degeneration of the germinal epithelium) were
observed at doses of > 720 mg/kg body weight. The NOEL for
these effects was 359 mg/kg body weight per day. In a study with
combined prenatal perinatal and subchronic exposure (see NTP rat
study 3 in Section 7.3), similar effects were observed at doses
of > 571 mg/kg body weight (NOEL = 279 mg/kg body weight per
day) (NTP, 1995). In Task 4 of the continous breeding study (see
Table 8), in Sprague Dawley rats, testicular tubular degeneration
was observed in the F1 offspring that had been treated during
the pre- and postnatal period with 322 mg/kg body weight per day,
and no NOAEL was determined (NTP, 1995; Wine et al., 1997). The
F1 offspring showed much more severe damage to the testes and
secondary sexual organs than did the parent F0 generation at the
same dose levels.
The effects on the testes of short-term exposure of rats to
DBP appear to be at least in part reversible. Tanino et al.
(1987) reported that, 2 weeks after discontinuation of the
administration of 2400 mg/kg body weight per day for 7 days, some
regeneration of the seminiferous tubules had occurred. Active
spermatogenesis was observed in almost all tubules, although
vacuolation of germinal epithelium and decreased numbers of sperm
were still evident 3 weeks after exposure had ceased.
Based on the results of Cater et al. (1977), zinc appears to
play a role in DBP-induced testicular atrophy in rats. After 4
days of exposure of rats to 500, 1000 or 2000 mg DBP/kg body
weight per day, the weight of the testes was decreased at
1000 mg/kg (P < 0.01) and 2000 mg/kg (P < 0.001), and decreased
further after 6 days (P < 0.05 at 500 mg/kg body weight per day
and P < 0.001 at the two highest doses) (LOEL = 500 mg/kg body
weight per day on the basis of decreased testicular weight).
Based on histological examination of the testes after 4 days of
exposure to 2000 mg/kg body weight per day, there was a
diminution of both spermato-cytes and spermatogonia. In
addition, at this dose, urinary zinc excretion was increased by
34 to 43% over the first 4 days and then returned to normal
levels. In the testes, the turnover rate of zinc was enhanced,
the half-life was decreased from 14 to 5 days and zinc levels
were significantly reduced; values were 88 - 93% of those of
controls after 2 days and 64 - 71% after 6 days. There was no
change in the turnover rate or zinc content in liver or kidneys.
The decrease in the activity of testicular alcohol dehydrogenase
(a zinc-dependent enzyme) ranged from 20 to 40% of control values
following 5 days of exposure. A 3-day pretreatment with
2000 mg/kg body weight per day caused a 25% decrease (P < 0.001)
in testicular zinc uptake in vivo. Concomitant intraperitoneal
administration of zinc sulfate (50 mg/kg body weight per day)
resulted in no testicular atrophy (based only on relative testes
weight rather than on microscopic appearance of the testes).
Mice and hamsters appear to be somewhat more resistant than
rats and guinea-pigs to DBP-induced testicular atrophy. For
example, testicular effects were observed in rats but not mice in
NTP subchronic toxicity studies (NTP, 1995). Following
administration of 2000 mg DBP/kg body weight per day by gavage in
corn oil for 7 to 9 days, only isolated tubular atrophy was
observed in 40% of the mice; no effects on testicular histology
were observed in hamsters, but oral doses of 2000 mg/kg body
weight per day administered to rats and guinea-pigs produced
severe tubular atrophy with loss of spermatids and reductions in
primary spermatocytes and spermatogonia (Gray et al., 1982). In
a study reported in the form of an abstract (Gray et al., 1983a),
pronounced effects were observed in male rats following
administration of 500 or 1000 mg/kg body weight per day from 22
to 55 days of age, while effects in hamsters were observed at the
high dose only. Exposed rats and hamsters both had smaller
testes and seminal vesicles than controls. The rats also had no
sperm in the vas deferens and the size of the epididymis of the
hamster was reduced. In a separate experiment reported in the
same abstract (Gray et al., 1983a), half of the rats exposed to
500 mg/kg body weight per day from 20 to 75 days of age did not
breed, while hamsters exposed to 1000 mg/kg body weight per day
had smaller testes but bred, although the viability of their
offspring was decreased and growth was retarded. In other
studies in which ICR male mice ingested a diet containing 2% DBP
(equivalent to 2400 mg/kg body weight per day), there was a
decrease in testicular levels of zinc but no testicular atrophy
was apparent (Oishi & Hiraga, 1980b), whereas testicular atrophy
and decreased testicular zinc levels were observed in male Wistar
rats exposed under the same conditions (2% in the diet equivalent
to 2060 mg/kg body weight per day) (Oishi & Hiraga, 1980a). In
an early study, the results of which have not been confirmed at
such low doses in mice by other investigators, Sajiki (1975a,b)
reported loss of spermatogenic cells and spermatogonia at doses
of >50 mg/kg body weight per day administered for 30 days by
stomach intubation.
In summary, alteration in testicular enzymes and
degeneration of testicular germinal cells in rats have been
observed at doses of 250, 322 and 571 mg/kg body weight per day,
respectively. Effects on a second generation may be more severe
than on the first generation (Srivastava et al., 1990a, 1990b;
NTP, 1995). There are considerable species differences in
effects on the testes following exposure to DBP, minimal effects
being observed in mice and none in hamsters at doses as high as
2000 mg/kg body weight per day (Gray et al., 1982).
It has been suggested (Foster et al., 1982) that part of the
difference in sensitivity of the rat and hamster to the
testicular toxicity of DBP relates to the higher levels of free
MBP in the rat (with lower levels of conjugate) than in the
hamster, coupled with the increased testicular ß-glucuronidase
activity in the rat. Both of these could lead to higher levels
of MBP in the rat testis. No data have been identified on
metabolism or tissue levels in mice or humans.
Results of available studies on the effects of MBP (the
principle metabolite of DBP) on the testes are presented in
Table 9. MBP induces testicular damage at doses similar to those
of DBP. Clear signs of testicular atrophy were observed after
oral administration of MBP to rats (Table 9). There were
reductions in testicular weight in rats ingesting 400 to 800 mg
MBP/kg body weight per day for periods of 5 or 6 days (Cater et
al., 1977; Gray et al., 1980). Histologically, the majority of
seminiferous tubules in animals administered 800 mg/kg body
weight per day for 6 days were atrophied, and there were
reductions in numbers of spermatocytes and spermatogonia (Foster
et al., 1981). Reductions in testicular zinc and relative testes
weights were observed in rats administered 2% MBP in the diet for
1 week (Oishi & Hiraga, 1980c).
In vitro exposure of human spermatozoa to 278 mg DBP/litre
resulted in a 25% reduction in motility (Fredricsson et al.,
1993).
7.5.1.2 Effects on fertility
Available data concerning the effects of DBP on fertility
are presented in Table 8. Corresponding data for MBP are
presented in Table 9.
Cummings & Gray (1987) reported that in rats, DBP had no
effect on early pregnancy during short-term exposure following
ovulation and continuing throughout the period of implantation
during pregnancy and pseudopregnancy at doses up to 2000 mg/kg
body weight per day; neither the number of implantation sites,
uterine weight, ovarian weight nor serum progesterone
concentrations were affected (relative to vehicle-exposed
controls). In addition, there were no significant effects on the
decidual cell response. These data indicated that DBP did not
affect any maternal parameter of progestational physiology
including the ability of the uterus to undergo deciduation.
Table 9. Reproductive effects of monobutyl phthalate
Species Protocol Results Effect levels Reference
Rat (Wistar, 800 mg/kg b.w. per day Loss of weight in testes, seminal vesicle and One dose group Ikemoto et
groups of 5 by gavage (vehicle prostate was evident; an almost complete absence only; effects al. (1983)
males, 5 weeks unspecified), daily for of germ cells in seminiferous tubules. observed at 800
old) 7 days mg/kg b.w. per day
Rat (Wistar, 800 mg/kg b.w. per day by Decreases in relative weights of testes, seminal One dose only; Ikemote
groups of 30 gavage in dimethyl sulfoxide vesicle and prostate were evident. effects observed (1985)
males, 35 days for 1 week; animals were Histopathologically, germ cells had almost at 800 mg/kg b.w.
old) sacrificed one day, 2 weeks disappeared in seminiferous tubules with per day
and 4 weeks after final vacuolation and enlargement. Concentration of
dosing. testosterone in serum was decreased but FSH and
LH concentration were not changed. Four weeks
after end of dosing, spermatogenesis had
recovered.
Rat 800 mg/kg b.w. per day by Testicular weight was reduced to 57% of control One dose group Grey et
(Sprague-Dawley) oral intubation value (p<0.001). Upon microscopic examination, only; effects al. (1982)
tubular atrophy was observed in all treated rats. observed at 800
mg/kg b.w. per day
Rat 800 mg/kg b.w. per day for Relative weights of testes and seminal vesicles One dose group Foster et
(Sprague-Dawley, 6 days by oral intubation were decreased. Urinary zinc excretion was only; effects al. (1981)
groups of 6 increased. observed at 800
males) mg/kg b.w. per day
Rat 400 or 800 mg/kg b.w. per Decrease in testicular weight at both doses after LOEL = 400 mg/kg Cater et
(Sprague-Dawley, day for 4 or 6 days by 4 days; the weight decreased further after 6 b.w. per day al. (1977)
groups of 6 gavage days.
males)
Table 9. Continued
Species Protocol Results Effect levels Reference
Rat (JCL:Wistar, 2% in the diet for 7 days Mean body weight was significantly lower than One dose group Oishi &
groups of 10 (2000 mg/kg b.w. per day) that of control. Absolute and testicular only; effects Hiraga
males, 5 weeks weights were decreased. Zinc concentration in observed at 2000 (1980c)
old) the testis was decreased. Testosterone mg/kg b.w. per day
concentration in the testis was increased but
that in serum was not changed.
Mouse (JCL:ICR, 2% in the diet for 7 days Body weight gain decreased. Relative weight of One dose group Oishi &
groups of 10 (2500 mg/kg b.w. per day) testes was increased. Concentrations of zinc and only; effects Hiraga
males, 5 weeks testosterone in the testis were decreased. observed at 2500 (1980d)
old) mg/kg b.w. per day
Hamster (DSN, 1600 mg/kg b.w. per day for Occasional tubular atrophy observed in 2 Gray et
groups of 7 9 days by oral intubation hamsters al. (1982)
males)
In NTP study 4 (section 7.3), DBP was administered in the
diet (0, 1000, 5000 or 10 000 mg/kg diet) to Sprague-Dawley rats
in a continuous breeding protocol, which included cross-over
mating and offspring assessment phases (NTP, 1995; Wine et al.,
1997). Additional details on the protocol and levels at which
effects occurred are presented in Table 8. Average equivalent
dose levels on a body weight basis were 66, 320 and 651 mg/kg
body weight (NTP, 1991). Mean body weights of exposed dams
generally decreased with increasing dose and, in the high-dose
group, were 6 - 13% lower than those of controls at delivery and
during lactation. In the F0 generation, the average number of
live pups per litter (all groups) and mean pup weight at birth
and during lactation (mid- and high-dose groups) were less than
in controls. Cross-over mating trials in the F0 generation
revealed no effects on the fertility of male or female rats
receiving the highest dose, although the live pup weight, when
adjusted for litter size, was significantly less for litters from
exposed dams. The absolute liver weight of exposed male rats and
relative liver and kidney weights of exposed male and female F0
rats of the high-dose group were significantly greater than those
in controls. In contrast to the F0 rats, mating, pregnancy and
fertility indices of F1 rats were lower in the high-dose group
than in controls (1/20 pregnant versus 19/20 in the controls).
In the high-dose group of F1 males, absolute and relative
epidydimal, right caudal epididymal, right testis, seminal
vesicle and prostate gland weights were reduced; germinal
epithelial degeneration of the testes, absence or
underdevelopment of the epididymides and interstitial cell
hyperplasia were also observed. Epididymal sperm count and
concentration and testicular spermatid head count and
concentration were also significantly decreased in the high-dose
group of males. Seminiferous tubule degeneration was observed in
1/10, 3/10 and 8/10 in the controls, mid- and high-dose groups,
respectively. In F1 females, the right ovary weights were
unchanged. Total and adjusted live pup weights were lower in all
exposed groups than in the controls. No clear NOEL was
established in this study. In the first generation (F0) the
reduction in pup weight in the mid-dose group, in the absence of
any adverse effect on maternal weight, can be regarded as a
developmental toxicity effect. There was also a significant
reduction of live litter numbers at all three dose levels. The
effects in the second generation were more severe, with reduced
pup weight in all groups including the low-dose group, structural
defects in the mid- and high-dose groups, and severe effects on
spermatogenesis in the high-dose group that were not seen in the
parent animals. These results suggest that the adverse effects
of DBP are more marked in animals exposed during development and
maturation than in animals exposed as adults only (Wine et al.,
1997).
In NTP rat study 1, DBP was administered in the diet to
F-344 rat dams during gestation and lactation and to the pups
postweaning for four additional weeks, at concentrations of 0,
1250, 2500, 5000, 7500, 10 000 and 20 000 mg/kg diet. Based on
decreased weight gains in the dams at >10 000 mg/kg, decreased
gestation index and increased pup mortality at 20 000 mg/kg,
decreased body weight of pups at 10 000 mg/kg and mild to marked
epidydimal hypospermia at >7500 mg/kg, 10 000 mg/kg was
recommended as the maximum perinatal exposure concentration for
male and female rats for subsequent studies (NTP, 1995).
DBP was administered in the diet (0, 300, 3 000 or
10 000 mg/kg diet) to Swiss (CD-1) mice (NTP, 1995). Average
equivalent dose levels on a body weight basis were 0, 39, 390 or
1300 mg/kg body weight per day (NTP, 1984). In F0 mice in the
high-dose group that received DBP during the continuous breeding
phase, the fertility index, average number of litters per
breeding pair, live male and female pups, and live pups per
litter were significantly lower than in the controls. The ratio
of live male pups to total live pups in the high-dose group was
greater than in the controls. In the cross-over mating trial,
the fertility index, numbers of live male, female and total pups
per litter, and total and adjusted live pup weights were
significantly lower for F0 females (bred with control males) in
the high-dose group than for the control females bred with
unexposed males. The female pup weights in litters from control
females bred with exposed males were also lower than those of
control females bred with unexposed males. Fertility was not
affected, though the pup weights were lower. In females in the
10 000 mg/kg group, the liver weight was greater and the uterine
weight was less than in control females. Based on comparison
with a similar study in rats, mice therefore appear to be less
sensitive than rats to reproductive effects of DBP, effects only
being seen at the highest dose level (NTP, 1995).
In a prenatal/perinatal range-finding study, 20 pregnant
B6C3F1 mice per group were exposed to 0, 1250, 2500, 5000, 7500,
10 000 or 20 000 mg DBP/kg diet throughout gestation and
lactation. Pups were weaned onto the same diet as their dams and
exposed for a further 4 weeks (NTP, 1995). Developmental
toxicity and pup mortality were suggested at concentrations as
low as 7500 mg/kg.
In a study reported only as an abstract, Gray et al.
(1983a), reported no effects on the female reproductive system in
an unspecified number of hamsters exposed to 500 or 1000 mg/kg
body weight per day from 20 to 55 days of age. In a second
experiment in the same report, however, half of the breeding
pairs of rats exposed to 500 mg/kg body weight per day from 20 to
75 days of age did not breed (NOEL = 250 mg/kg body weight
per day). At similar doses (LOAEL = 500 mg/kg body weight per
day; NOEL = 250 mg/kg body weight per day), breeding in rats was
adversely affected (Gray et al., 1983a,b).
Heindel et al. (1989) reported the results of a reproductive
study for diethylhexyl phthalate in mice. Though data on the
other phthalates were not presented in the published report, the
authors concluded, on the basis of similar studies for these
compounds, that the relative order of reproductive toxicity for
the various phthalates was diethylhexyl, dihexyl, dipentyl, di-
n-butyl and dipropyl; diethyl and dioctyl phthalates were
considered non-toxic.
7.5.2 Developmental effects
The developmental effects of DBP have been examined in rats
and mice following oral and intraperitoneal administration (the
latter considered less relevant for assessment of dose-related
effects), as summarized in Table 10. DBP generally induced
fetotoxic effects in the absence of maternal toxicity, and
teratogenic effects only at high maternally toxic doses.
Ema et al. (1993) administered DBP by gavage to Wistar rats
on days 7-15 of gestation at dose levels of 0, 500, 630, 750 and
1000 mg/kg body weight per day. No effects in either dams or
offspring were reported at 500 mg/kg body weight. At the LOEL of
630 mg/kg body weight per day, there was a significant increase
in maternal body weight gain, significantly increased incidence
of postimplantation loss, and significant decrease in fetal
weight and increased malformations. The NOEL was 500 mg/kg body
weight per day.
Results of a recent study indicate that susceptibility to
teratogenesis varies with the developmental stage during the
period of DBP administration, based on exposure of Wistar rats to
0, 750, 1000 or 1500 mg/kg on either days 7 to 9, days 10 to 12,
or days 13 to 15 of gestation (Ema et al., 1994). When DBP was
administered on days 10 to 12 of gestation, there was no
evidence of teratogenicity. Following administration on days 7
to 9 and 13 to 15, the frequency of malformations increased with
dose level, and was highest when DBP was administered on days 13
to 15 (information on maternal toxicity was not reported).
Malformations were also observed during the postnatal development
of the rats of the final litter in the Continuous Breeding
protocol study (Task 4, NTP, 1995; Wine et al., 1997) (Table 8).
Three out of 20 males of the high-dose group that had been
exposed pre- and postnatally to 650 mg DBP/kg body weight had
small and malformed prepuces and/or penises and non-palpable
testes. Five out of ten rats examined histologically had
underdeveloped or defective epididymides (Wine et al., 1997).
Table 10. Developmental effects of DBP
Species Protocol Results Effect levels Reference
Rat (Holtzman, Pseudopregnant rats No effect on the decidual cell response, NOEL = 2000 mg/kg Cummings &
groups of 6 received 0, 250, 500, pregnant uterine weight, number of b.w. per day Gray (1987)
pseudopregnant 1000 or 2000 mg/kg b.w. implantation sites, ovarian weight, or serum
females and per day while pregnant progesterone concentration during early
groups of 6 to rats received 0, 500, pregnancy or pseudopregnancy. These data
8 pregnant 1000 or 2000 mg/kg b.w. indicated that short-term exposure to DBP had
females) per day by gavage in no direct maternal effects in the rat and
sesame oil from day 1 suggested that the viability of preimplantation
through day 8 of embryos was not compromised.
pseudopregnancy or
pregnancy.
Rat (females, 250 mg/kg b.w. per day A large increase in total embryonal death, One dose group only Aldyreva
strain and by gavage daily (vehicle owing to high preimplantation losses, was (effects observed at et al. (1975)
number unspecified) at various noted. Data on maternal toxicity were not 250 mg/kg b.w. per
unspecified) stages of gestation or presented. day)
over the first 22 days of
gestation.
Rat (Wistar, 120 or 600 mg/kg b.w. per No effects on ossification, bone development of NOEL = 120 mg/kg b.w. Nikonorow
groups of 10 day by gavage in corn oil the base of the skull, paws of the front and per day (offspring) et al. (1973)
or 20 females) to groups of 20 females hind extremities or rib fusion in fetuses. LOEL = 600 mg/kg b.w.
prior to or during mating. Increased number of resorptions and decreased per day (offspring)
120 or 600 mg/kg b.w. per fetal body weights were observed at the high
day by gavage in olive oil dose. However, these abnormalities were not
daily throughout gestation observed when DBP was administered prior to and
(21 days). during mating. Maternal toxicity was not
addressed.
Table 10. Continued
Species Protocol Results Effect levels Reference
Rat, (Wistar, Administered DBP by Significant decrease in maternal body weight NOEL = 500 mg/kg b.w. Ema et al.
groups of 11 gavage in olive oil, days gain at >630 mg/kg b.w. Maternal deaths at per day (1993)
or 12 females) 7-15 of gestation, 0, 500, 1000 mg/kg b.w. per day. Significant increase
630, 750 or 1000 mg/kg in postimplantation loss at 630 mg/kg b.w. per
b.w. per day day with complete resorption of implanted
embryos in surviving dams at 1000 mg/kg b.w.
per day. Significant decrease in fetal weight
at >630 mg/kg b.w. Increased incidence of
malformed fetuses (predominantly cleft palate)
at >630 mg/kg b.w. (significant at 750 mg/kg
b.w).
Rat (Wistar, Pregnant rats were housed Ema et al.
groups of 11 individually and dosed by (1994)
or 12 females) gastric intubation with DBP
(99% pure) in olive oil.
All rats killed on day 20.
Dosing on days 7 to 9 of Significant increase in number of resorptions, LOAEL = 750 mg/kg b.w.
pregnancy - 0, 750, 1000 or dead fetuses per litter; postimplantation per day (teratogenic
1500 mg/kg b.w. per day loss at >0.75 g/kg b.w. per day (100% effects)
postimplantation loss at 1.5 g/kg b.w. per
day). Reduction in body weight of male and
female fetuses at >750 mg/kg b.w. per day.
Significant increase of fetuses with skeletal
malformations, fusion or absence of cervical
vertebral arches or ribs at 750 mg/kg b.w.
per day.
Table 10. Continued
Species Protocol Results Effect levels Reference
Rat (Wistar, Dosing on days 10 to 12 Significant increase in number of resorptions LOAEL = 750 mg/kg Ema et al.
groups of 11 of pregnancy - 0, 750, and dead fetuses per litter; and b.w. per day (no (1994)
or 12 females) 1000 or 1500 mg/kg b.w. postimplantation loss per litter at >750 mg/kg teratogenic effects)
per day b.w. per day (100% postimplantation loss at
1500 mg/kg b.w. per day). Reduction in body
weight of live female fetuses at 750 mg/kg b.w.
per day and change in sex ratio of live fetuses
at 1000 mg/kg b.w. per day.
Dosing on days 13 to 15 Significant increase in postimplantation loss LOAEL = 750 mg/kg b.w.
of pregnancy - 0, 750, per litter at >750 mg/kg b.w. per day (100% per day (teratogenic
1000 or 1500 mg/kg b.w. postimplantation loss at 1500 mg/kg b.w. per effects)
per day day) and number of resorptions and dead fetuses
per litter at 1000 mg/kg b.w. per day.
Increase in fetuses with malformations, cleft
palate, skeletal malformations and fusion of
sternebrae at >750 mg/kg b.w. per day.
Mouse (ICR-JCL, 0.05, 0.1, 0.2, 0.4 or There was a significant reduction in body NOEL = 370 mg/kg b.w. Shiota et al.
groups of 7 to 1.0% in the food throughout weight at day 18 in mothers administered the per day (offspring) (1980);
15 females) gestation (18 days) highest dose. The total number of implants was LOEL = 660 mg/kg b.w. Shiota &
corresponding to 80, 180, similar in exposed and control animals but the per day (offspring) Nishimura
370, 660 and 2100 mg/kg numbers of resorptions and dead fetuses were NOEL = 660 mg/kg b.w. (1982)
b.w. per day based on data much higher in high-dose animals. There was a per day (mothers)
on food consumption. dose-dependent decline in fetal body weights
but this was only significant at the two higher
doses. There were no abnormalities except in
the group exposed to the highest concentration,
in which there were 2 fetuses (75%) which had
exencephaly and myeloschisis. No malformations
of internal organs were observed in the fetuses
examined by the microdissection method.
Table 10. Continued
Species Protocol Results Effect levels Reference
Mouse (ICR-JCL, 0, 0.005, 0.05, 0.5% DBP The number of pregnant animals, the incidences NOEL = 0.05% (100 Hamano et
groups of 15 to in the diet of spontaneous abortion and maternal deaths, mg/kg b.w. per day) al. (1977)
18 females) and the number of mice with live offspring (offspring)
Based upon food intake were similar in the exposed groups and LOAEL = 0.5% (400
data, the two highest controls. No effects were noted on maternal mg/kg b.w. per day)
doses were calculated to liver and spleen weights. A statistically (offspring)
be 100 and 400 mg/kg b.w. significant increase in kidney weight was NOEL = 0.05% (100
per day observed in the high-dose group. An mg/kg b.w. per day)
embryotoxic effect was noted at the highest (mothers)
concentration, resulting in a lower number
of live offspring. The incidence of external
anomalies was also significantly higher in the
high-dose group. At the high dose, these
anomalies were non-closing eyelid (3),
encephalocoele (6), cleft palate (1), spina
bifida (1), non-closing eyelid + encephalocoele
(3). The rate of ossification for all dosed
groups appeared to be within normal limits.
The incidence of skeletal anomalies, especially
of the sternum, was higher (but not
statisticaal significant) in the high-dose
group with respect to the controls.
Mouse (CD-1, 2500 mg/kg b.w. per day by 5 exposed mice died and there were no viable One dose group only Hardin et al.
group of 50 gavage in corn oil on days litters. Maternal toxicity was not addressed. (effects observed at (1987)
females) 6 to 13 of gestation. 2500 mg/kg b.w. per
day)
Table 10. Continued
Species Protocol Results Effect levels Reference
Rat 0, 0.32, 0.64 or 1.06 g/kg At all doses, the number of resorptions in LOAEL = 320 mg/kg b.w. Singh et al.
(Sprague-Dawley, b.w. intraperitoneally on exposed animals was higher than in controls per day (offspring) (1972)
groups of 5 days 5, 10 and 15 of and there was a corresponding decrease in the
females) gestation. number of live fetuses. Fetal weight was
significantly lower than in controls at all
doses. There was a higher incidence (not
analysed statistically) of skeletal anomalies
in exposed animals when compared with
unexposed controls. These were mainly rib
abnormalities, absence of tail bones,
incomplete skull bones and incomplete or
missing leg bones. Maternal toxicity was
not addressed.
Rat 2 ml/kg b.w. (2080 mg/kg One rat administered the highest dose died. LOEL = 2080 mg/kg b.w. Peters &
(Sprague-Dawley, b.w.) or 4 ml/kg b.w. There were no significant effects on Cook (1973)
groups of 5 (4170 mg/kg b.w.) implantation. The average number of pups
females) intraperitoneally on days weaned per litter was significantly lower in
3, 6 and 9 of gestation. exposed animals compared with controls. Fetal
abnormalities were not addressed.
In the study reported by Hamano et al. (1977), JCL:ICR mice
were administered 0.005, 0.05 or 0.5% DBP in food throughout 18
days of gestation (the two highest doses were calculated on the
basis of food intake to correspond to 100 and 400 mg/kg body
weight per day). There were no significant differences in the
mortality of maternal mice, the rate of spontaneous abortions or
the rate of premature births between the control and exposed
groups. The highest dose was embryotoxic, resulting in a lower
number of live offspring. At this highest dose, an increase in
kidney weight in mothers was reported, although there were no
effects on the weights of other organs, body weight gain or
survival in the mothers. The frequency of offspring with
external anomalies was also significantly higher in the high-dose
group than in controls. The anomalies consisted mainly of spina
bifida, exencephaly, cleft palate and open eye. A small but non-
significant increase in skeletal anomalies was also seen in the
high-dose group. Therefore, the NOEL and LOEL values in this
study were considered to be 100 and 400 mg/kg body weight per
day, respectively, on the basis of embryotoxic and teratogenic
effects.
In summary, the lowest reported LOAEL for developmental
effects of DBP was that reported by Hamano et al. (1977), i.e.
400 mg/kg body weight per day for increases in the number of
resorptions and dead fetuses in JCL:ICR mice. The NOEL in this
study was 100 mg/kg body weight per day.
7.6 Mutagenicity and related end-points
The weight of the available evidence indicates that DBP is
not genotoxic (Table 11). There are no structural alerts
indicative of potential reactivity with DNA. The major metabolic
pathway involves hydrolysis of one ester linkage to yield MBP and
n-butyl alcohol, neither of which react with DNA.
DBP (100 to 10 000 g/plate) was not mutagenic in any of
four tester strains of Salmonella tymphimurium in the presence
or absence of Arochlor-induced rat or hamster liver S-9 (Zieger
et al., 1985). These data are consistent with earlier negative
Ames test studies (Yagi et al., 1976; 1978; Florin et al., 1980;
Kozumbo et al., 1982). In two studies, very weak positive
responses were reported in the absence of an S-9 metabolic
activation system (Seed, 1982; Agarwal et al., 1985). These
results are questionable because the parent compound clearly does
not react with DNA. Similarly, an increase in mutant frequency
was seen without metabolic activation and at very high cytotoxic
doses in the L5178Y mouse lymphoma cell assay (NTP, 1995). It
should be noted, however, that false positive results are common
in this assay at cytotoxic concentrations.
Table 11. Mutagenicity of DBP from HSE, 1986
Species Protocol Results Reference
Salmonella typhimurium; Levels of up to 1000 µg/plate in the Negative. Full data not reported. Kozumbo et al.
TA98, TA100 presence and absence of S-9. (1982)
S. typhimurium; With and without Aroclor S-9. Tested Negative. Complete data not reported. Florin et al.
TA98, TA100, TA1535, TA1537 up to levels that precipitated. (1980)
S. typhimurium Not specified. Negative. No other information provided. Yagi et al.
(strain not reported) (1976, 1978)
S. typhimurium; Levels of 100-10 000 µg/plate in Negative. Full data were not provided. Zeiger et al.
TA98, TA100, TA1535, TA1537 DMSO, with and without S-9 in a (1982)
preincubation-type assay.
S. typhimurium; Levels of 0.013, 0.03 and 0.05 mg/ml Small dose-related increase in Seed (1982)
TA100 in the 8-azaguanine resistance assay mutation frequency in the absence of
using a preincubation assay with and S-9, statistically significant at the
without S-9. two highest doses. Values were
increased 1.5 times control levels
at the highest dose.
S. typhimurium; Test for base-pair substitution or Spot tests yielded negative responses Agarwal et al.
TA98, TA100, TA1535, TA1537, frameshift-type mutations; spot tests for all strains. (1985)
TA1538 and TA2637. with 500 µg per plate. "Mildly positive" response in TA100
Dose-response test with 100 to 2000 µg and TA1535, but not in presence of S9.
per plate, with and without S9
metabolic activation.
Bacillus subtilis; H17 62.5 µg/ml (limit of solubility) No inhibition indicative of DNA Sato et al.
(Rec+) and M45 (Rec-) damage; positive controls produced (1975)
clear zones of inhibition. Test did
not appear to have been carried out
using S-9.
Table 11. Mutagenicity of DBP from HSE, 1986
Species Protocol Results Reference
Pseudo-diploid Chinese Concentrations of 0.28, 2.78 and Negative for SCE and chromosomal Abe & Sasaki
hamster cell line (Don) 27.8 mg/ml were tested for ability aberrations. (1977)
to induce chromosome aberrations
and sister chromatid exchange
(SCE). Ethanol solvent.
Clonal sub-line of a Chinese Concentrations up to 0.03 mg/ml Suspicious or equivocal results for Ishidate &
hamster fibroblast cell line. dissolved in aqueous bovine albumin, induced chromosomal aberrations. Odashima (1977)
tested for induction of chromosomal
aberrations.
Human leucocytes (male Chromosomal aberrations determined No increase in frequency of Tsuchiya &
derived) in 100 human leucocytes following chromosomal aberrations. Hattori (1976)
8-h exposure to 0.03 mg/ml DBP in
whole human blood culture. This
concentration had been previously
shown to inhibit growth of the
culture cells by 20 to 50%.
Mouse lymphoma cell line Cells exposed in suspension to DBP Increased mutant frequency under NTP (1995)
L5178Y for 4 h in the presence and absence non-activation conditions with high
of rat liver S9 metabolic activation. cytotoxicity.
Mouse Balb/3T3 cells In vitro transformation assay. DBP did not induce the appearance Litton Bionetics
Concentrations of DBP were 3.4, of a significant number of Inc. (1985)
13.7, 27.5, 55.0 and 82.3 nl/ml. transformed foci.
Protocol included both positive and
negative controls.
DBP did not induce sister-chromatid exchanges (SCE) or
chromosome aberrations in CHO cells (Abe & Sasaki, 1977) but an
equivocal result was reported for induction of chromosome
aberrations in a Chinese hamster fibroblast cell line in the
absence of metabolic activation (Ishidate & Ohashima, 1977).
DBP was inactive in the Balb/C-3T3 in vitro
transformation assay (Litton Bionetics Inc., 1985).
In the only identified in vivo study, analysis of
peripheral blood samples from male and female mice at the end of
the 13-week feeding study did not reveal any micronuclei (NTP,
1995).There was no increase in the numbers of revertants in
Salmonella typhimurium strains TA98 and TA100 exposed to 50 to
2000 µg/plate MBP, the principal metabolite of DBP, in the
presence and absence of S9 (Yoshikawa et al., 1983). Similar
concentrations were also non-mutagenic in two Escherichia coli
strains (WP2 uvr A+ and uvr A-).
7.7 Carcinogenicity
A long-term carcinogenicity study for DBP has not been
conducted, although no tumours were observed in two one-year
bioassays (Smith, 1953; Nikonorow et al., 1973).
7.8 Special studies
7.8.1 Induction of metabolizing enzymes
Following daily oral administration of 0.01, 0.1 or
1.0 mmol/kg body weight (2.78, 27.8 or 278 mg/kg body weight) DBP
by gavage in corn oil to male Sprague Dawley rats (n=20) for
5 days, there was a 48% increase in the hepatic microsomal
concentration of cytochrome P-450 at 0.01 mmol/kg body weight and
28-29% increase in NADPH-cytochrome-reductase activity at 0.01
and 0.1 mmol/kg body weight (Walseth & Nilsen, 1986). The
authors concluded that DBP is a moderate to weak inducer of
several microsomal enzymes though the reasons for increased
enzyme activity observed at the lower doses but not at the high
dose (1.0 mmol/kg body weight per day) were not addressed.
There were no changes in liver, lung or body weights in male
Sprague Dawley rats (n=15) exposed to 5.7, 28.5 or 79.8 mg
DPB/m3 air (0.5, 2.5 or 7.0 mg/kg) 6 h per day for 5 days
(Walseth & Nilsen, 1984). There was a small but significant
increase in the activity of hepatic NADPH-cytochrome-c reductase
in the group exposed to 5.7 mg/m3. In contrast to the study of
Walseth & Nilsen, 1986, effects on the hepatic liver microsomal
enzymes were not observed. However, the concentration of
cytochrome P-450 in the lung decreased in a dose-dependent manner
to a level of 37% of the control.
In Sprague Dawley rats (n=5) administered intraperitoneally
3.8 mmol/kg body weight per day (1058 mg/kg body weight per day)
for 5 days (Walseth et al., 1982), increases in relative liver
and lung weights and hepatic microsomal cytochrome P-450 content
were observed; however there was a decrease in pulmonary
microsomal cytochrome P-450. In another study in which albino
male rats (n=5) were intraperitoneally administered 3.05 ml
DBP/kg body weight per day (3190 mg/kg body weight per day) and
killed 18 h or 7 days after the treatment (Seth et al., 1981),
the activity of aniline hydroxylase was inhibited after 18 h.
There was also mild inhibition of aminopyrine- N-demethylase
activity, but no effects on the activities of glucose-6-
phosphatase or NADPH-cytochrome-c reductase were observed. There
was no effect on the activity of hepatic tyrosine
aminotransferase activity following the single exposure, but
there was an increase in the activity of this enzyme following
daily administration.
8. EFFECTS ON HUMANS
8.1 General population exposure
Cases of sensitization after exposure to DBP have been
reported. A 30-year-old woman developed an axillary dermatitis
after the use of an anti-perspirant containing DBP (Calnan,
1975). Patch testing of the skin was positive with both the
formulation and with DBP, but not with any of the other
constituents of the formulation.
In another reported case, a 32-year-old woman noted pruritis
and redness in the axillae after changing from her usual
deodorant spray to a new one (Sneddon, 1972). Patch testing with
the original formulation, an alternative deodorant spray, 1%
paraphenylene-diamine and 3% formalin, was negative, but patch
testing with the new deodorant and DBP, but not the other
constituents, was positive.
In a case reported by Husain (1975), a 44-year-old architect
noticed a patch of eczema under a plastic watch strap on the left
wrist and after transferring the watch to the right wrist. The
results of patch tests were positive for the plastic watch strip,
20% colophony, 1% paratertiary butylphenol formaldehyde resin and
5% DBP.
Cosmetic products containing 4.5-9% DBP were patch tested on
50 to 250 individuals per sample and no skin sensitization was
observed (Brandt, 1985). No other details were provided.
8.2 Occupational exposure
8.2.1 Acute toxicity
Sandmeyer & Kirwin (1981) reported a case of accidental
poisoning in which a 23-year-old healthy male worker ingested
10 g of DBP. Delayed symptoms were nausea, vomiting, and
dizziness, followed by headache, pain and irritation of the eyes,
lacrimation, photophobia and conjunctivitis. Urinalysis was
abnormal; the urine was dark yellow in colour with sediment and
contained numerous erythrocytes and leucocytes with moderate
numbers of oxalate crystals. Recovery was gradual within 2 weeks
and complete after 1 month.
8.2.2 Epidemiological studies
Identified data are limited to studies of workers exposed to
mixtures of phthalates. These include two cross-section studies
in which similar neurological symptoms were reported (Milkov et
al., 1973) (Gilioli et al., 1978) and a cross-sectional
investigation of reproductive effects (Aldyreva et al., 1975).
Neurological effects were examined based on clinical
examinations and self-reported symptoms in a cross-sectional
study of workers employed in the manufacture of artificial
leather (Milkov et al., 1973). The workers were exposed and to
DBP and also to di(2-ethylhexyl) phthalate, di-iso-octyl-
phthalate and small amounts of di- n-butyl sebacate, di(2-
ethylhexyl) sebacate and their respective adipates. Tricresyl
phosphate was also present in 10-20% of machines used by various
workers. The study group consisted of 147 workers (87 females
and 60 males), the majority (75%) of whom were less than 40 years
old. Pain in the upper and lower extremities, accompanied by
spasms and numbness, was reported in 57% of those employed for 6
to 10 years (28 persons) and 82% of those employed for more than
10 years (65 persons). These symptoms generally developed after
6-7 years of employment and the pain became continuous with
increasing length of employment. Weakness and pain in the legs
were usually more noticeable on exercise. Polyneuritis was noted
in 47 workers (32 with an autonomic-sensory form and 15 with a
mixed form) predominantly among those with greater length of
employment. Another 22 workers (15%) were reported to have
"functional disturbance of the nervous system". Approximately
50% of the workforce was considered normal by the authors. The
study was limited, however, by the lack of comparison of effects
in the exposed workers with those in an appropriate control
group. Moreover, it is difficult to attribute the observed
effects due to DBP since workers were exposed to a mixture of
phthalates and other compounds, including tricresyl phosphate
which is believed to induce polyneuritis.
A cross sectional study of neurological symptoms based on
clinical examination was carried out on three groups of male
workers in Italy who were involved in the production of phthalate
esters (Gilioli et al., 1978). The first group of workers was
exposed to phthalates (23 subjects), while the second and third
groups were exposed to alcohols (9 subjects) and phthalic
anhydride (6 subjects), both chemical precursors of phthalate
esters. The phthalates involved were di- n-butyl, diisobutyl,
di(2-ethylhexyl) and dioctyl phthalates. Mean concentrations of
phthalates varied from 1 to 5 mg/m3; peak levels were as high as
61 mg/m3. Phthalate-exposed workers frequently complained of
paraesthesia of the upper and lower limbs. These symptoms became
continuous with increasing length of employment. Excessive
perspiration of the hands and feet and vasomotor irregularity
indicative of autonomic effects were observed in 3 workers.
Neurological examination revealed polyneuropathy in 12 (57%) of
the workers exposed to phthalates. In seven workers, bilateral
painful decreased sensitivity of skin or senses of the hands and
feet were noted; three had decreased sense of vibrations.
Sensory neuropathy was observed in two workers with long-term
exposure (13 and 18 years) in the alcohol department;
hyporeflexia was observed in one worker in the phthalic anhydride
department. However, the authors suggested that no definite
conclusions could be drawn from this study because of the small
number of workers examined.
Only one study on the reproductive effects of DBP in humans
has been reported. In this cross-sectional investigation
(Aldyreva et al., 1975), workers were reported to have been
exposed to levels of DBP in excess of the Maximum Allowable
Concentration (0.5 mg/m3); however, quantitative data were not
provided. Based on gynaecological examinations of 189 women
working in processes involving exposure to DBP, approximately 33%
were considered to be healthy while 33% were reported to have
"deviations of the uterus". The health status of the remaining
34% was not disclosed. Decreases in the frequency of pregnancy
and births were reported in women exposed to phthalates, when
compared with controls; however, quantitative data on the
prevalence of effects and the composition of the control group
were not specified. There were decreases in the frequency of
miscarriages (no quantitative data was reported), although this
probably reflected the decreased frequency of pregnancy. Based
on colpocytological examination of 19 of the 189 women, 3 women
had normal biphasic (oestrogen/progesterone) vaginal cycles,
2 women had biphasic cycles but with insufficient progesterone
activity and 3 also had biphasic cycles which were hypohormonal.
Anovulatory hypoestrogenous cycles in 10 women and an anovulatory
hyperestrogenous cycles in 1 woman were observed. In a control
group, the composition of which was not described, single-phase
hyperestrogenous cycles and 2-phase hypoprogesterone cycles were
common (total incidence 21/28). The authors believed that these
results indicated a general increase in progesterone levels in
exposed women though specific data were not provided to support
this contention. It is possible that exposure to DBP may
contribute to the induction of hormonal changes reflected in
reduced fertility and changes in the vaginal cycle. However, on
the basis of the results from this study, it is difficult to draw
meaningful conclusions owing to inadequate documentation and lack
of confirmation of these observations. In addition, quantitative
data on exposure of the workers (who were also exposed to a
variety of other unspecified compounds) to DBP were not provided.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
The results of toxicity studies in various organisms are
presented in Tables 12 (microorganisms and algae), 13 (aquatic
invertebrates) and 14 (fish). Those in which effects were
observed at lowest concentrations are summarized in the following
sections.
9.1.1 Microorganisms
The toxicity of DBP to microorganisms is summarized in
Table 12.
Concentrations of DBP up to 300 mg/litre did not inhibit
methanogenesis in an anaerobic toxicity assay using secondary
sludge as the source of a heterogeneous anaerobic population
(O'Connor et al., 1989).
In water, the 5- and 30-min EC50 value for DBP was
10.9 mg/litre in the Microtox Test (Tarkpea et al., 1986).
Yoshioka et al. (1985) reported a 24-h EC50 (cell proliferation)
of 2.2 mg DBP/litre for the protozoan, Tetrahymena pyriformis.
Based upon the Microtox test, in which reduction in light
emitted by the luminescent marine bacteria Photobacterium
phosphoreum is determined, the 15-min apparent effects
threshold (AET)a was estimated to be 1.4 mg DBP/kg dry weight
for Puget Sound sediment (Tetra Tech Inc., 1986).
a The AET is defined as the concentration above which
statistically significant adverse effects are always
expected relative to appropriate reference conditions. This
approach involves comparison of data on the composition of
sediments collected in contaminated areas to measures of
biological effects associated with these sediments. The
site specificity and lack of assessment of cause-effect
relationships should be borne in mind when interpreting
apparent effects thresholds.
Table 12. Toxicity of DBP to microorganisms and algae
Species Study Type End Point Valuea Reference
Microorganisms
Bacterium
(Photobacterium) Microtox 15-min EC50 1400 µg/kg dw sediment (m) Tetra Tech Inc. (1986)
(reduced luminescence)
Bacterium
(Photobacterium phosphoreum) Microtox 5-min EC50 10 900 µg/litre (n) Tarkpea et al. (1986)
Bacterium
(Photobacterium phosphoreum) Microtox 15-min EC50 11 100 µg/litre (n) Tarkpea et al. (1986)
5-min & 30-min EC50 10 900 µg/litre (n)
Protozoan
(Tetrahymena pyriformis) Acute, static 24-h EC50 2200 µg/litre (n) Yoshioka et al. (1985)
(cell proliferation)
Table 12. Continued
Species Study Type End Point Valuea Reference
Plants
Green alga
(Scenedesmus subspicatus) Acute, static 48-h EC10 (biomass) 1400 µg/litre (n) Kühn & Pattard (1990)
48-h EC50 (biomass) 3500 µg/litre (n)
48-h EC10 (growth rate) 2600 µg/litre (n)
48-h EC50 (growth rate) 9000 µg/litre (n)
Green alga
(Selenastrum capricornutum) Acute, static 96-h EC50 (growth inhibition) 750 µg/litre (m) CMA (1984)
96-h EC50 (TLM) (survival rate) 20-600 µg/litre (n) Wilson et al. (1978)
96-h EC50 (growth) 3.4-200 µg/litre (n)
Green alga
(Selenastrum capricornutum) Chronic, static 10-day EC30 (dec. cell 750 µg/litre (m) Springborn Bionomics
numbers) (1984a)
Green alga
(Selenastrum capricornutum) Chronic, static 7-day NOEL (dec. biomass) 2800 µg/litre (n) Melin & Egnéus (1983)
7-day LOEL (dec. biomass) 28 000 µg/litre (n)
a n = nominal concentration; m = measured concentration
9.1.2 Aquatic organisms
9.1.2.1 Algae
The toxicity of DBP to algae is summarized in Table 12.
A 96-h EC50 (decreased cell numbers) of 750 µg DBP/litre
was reported for the green alga Selenastrum capricornutum
(Springborn Bionomics, 1984a). Kühn & Pattard (1990) reported
48-h EC10 and EC50 values for DBP of 1400 µg/litre and
500 µg/litre, respectively, for Scenedesmus subspicatus, based
on biomass. The 96-h EC50 values for the marine dinoflagellate
Gymnodinium breve were 3.4-200 µg DBP/litre based on growth and
20-600 µg DBP/litre based on survival (Wilson et al., 1978).
However, care must be taken when interpreting these data because
the two sets of values are not ranges but each are two replicates
with large variations.
Yan et al. (1995) report 96-h EC50 values for dimethyl and
diethyl phthalate, based on inhibition of growth of Chlorella
pyrenoidosa; however, an EC50 for DBP could not be generated
within the range of its water solubility (13 mg/litre).
At concentrations of about 7000 µg/litre and 2800 µg/litre,
DBP reduced the growth rates of the monodispersed marine plankton
(i.e. non-aggregated) Thalassiosira pseudomona (diatom) and
Dunaliella parva (green algae), respectively (Acey et al.,
1987).
9.1.2.2 Invertebrates
Acute and chronic toxicity data for aquatic invertebrates
are summarized in Table 13.
The most sensitive aquatic invertebrates, based on acute
toxicity tests, are the Mysid shrimp, Mysidopsis bahia, with a
96-h LC50 of 750 µg/litre (EG&G Bionomics, 1984a), and the midge
Chironomus plumosus, with a 48-h EC50 (based on
immobilization) of 760 µg DBP/litre (Streufert et al., 1980).
For chronic studies the most sensitive species in a standard
test is Daphnia magna with a 21- day NOEC, based on parent
survival, of 500 µg/litre (measured value) (Kühn et al., 1989).
Another sensitive invertebrate species is the scud, Gammarus
pulex, with a 10-day LOEL of 500 µg DBP/litre and a NOEC of
100 µg/litre, based on reduced locomotor activity (Thurén & Woin,
1991). A 7-day EC50 of 540 µg DBP/litre has been reported for
the planarian Dugesia japonica, based on reduced head
regeneration (Yoshioka et al., 1986).
Table 13. Toxicity of DBP to aquatic invertebrates
Species Study type End-point Concentrationsa Reference
Water flea
(Daphnia magna) Acute, static 48-h LC50 5200 µg/litre McCarthy & Whitmore (1985)
Acute, static 48-h EC50 3400 µg/litreb CMA (1984)
Acute, static 48-h LC50 3700 µg/litreb Call et al. (1983)
renewal
Acute, static 24-h EC50 17 000 µg/litre Kühn et al. (1989)
Acute, static 24-h EC0 8900 µg/litre
Grass shrimp
(Palaemonetes pugio) Acute, static 96-h LC0 1000 µg/litre Clark et al. (1987)
(water
exposure)
96-h LC50 >1000 µg/litre
(water
exposure)
96-h LC0 10 mg/kg
(sediment
exposure)
96-h LC50 > 10 mg/kg Clark et al. (1987)
(sediment
exposure)
Table 13. Continued
Species Study type End-point Concentrationsa Reference
Grass shrimp
(Palaemonetes pugio) 10-day LC0 10 mg/kg
(sediment
exposure)
10-day LC50 > 10 mg/kg
(sediment
exposure)
Mysid shrimp Acute, static 96-h LC50 750 µg/litre EG & G Bionomics (1984a)
(Mysidopsis bahia)
Scud Acute, static 24-h LC50 7000 µg/litre Mayer & Sanders (1973)
(Gammarus pseudolimnaeus)
96-h LC50 2100 µg/litre
Brine shrimp Sublethal, static 72-h NOEL < 10 000 µg/litre Sugawara (1974a, 1974b)
(Artemia salina) (egg hatching,
larvae survival)
Acute, static 24-h LC50 8000 µg/litre Hudson et al. (1981)
Acute, static 24-h LC50 5600 µg/litre Hudson & Bagshaw (1978)
Crayfish Acute, static 24-h LC50 + > 10 000 µg/litre Mayer & Ellersieck (1986)
(Orconectes nais) 96 h LC50
Harpacticoid Acute, static 96-h LC50 1700 µg/litre Lindén et al. (1979)
(Nitocra spinipes)
Table 13. Continued
Species Study type End-point Concentrationsa Reference
Midge larvae Acute, static 48-h LC50 5400 µg/litre Mayer & Ellersieck (1986)
(Chironomus plumosus)
48-h LC50 4000 µg/litre
48-h EC50 760 µg/litre Streufert et al. (1980)
(immobilization)
Midge Acute, static 48-h EC50 5800 µg/litre EG & G Bionomics (1984b)
(Paratanytarsus parthenogenica)
Benthic community composition Chronic, 14-day LOEL 340 µg/litre Tagatz et al. (1983)
flow-through (decrease number
of amphipods)
Planarian Acute, static 7-day EC50 (head 540 µg/litre Yoshioka et al. (1986)
(Dugesia japonica) regeneration)
Acute, static 7-day LC50 840 µg/litre
(increased
abnormalities)
Water flea Chronic, 21-day NOEL 960 µg/litreb CMA (1984)
(Daphnia magna) flow through (mortality)
21-day LOEL 2500 µg/litreb
(mortality)
Chronic, static 16-day LOEL 1800 µg/litre McCarthy & Whitmore (1985)
renewal (survival and
reproduction)
Table 13. Continued
Species Study type End-point Concentrationsa Reference
Water flea 16-day NOEL 560 µg/litre
(Daphnia magna) (survaval and
reproduction)
21-day LC50 1920 µg/litreb DeFoe et al. (1990)
21-day EC50 1640 µg/litreb
(reproduction)
21-day EC50 1050 µg/litreb
(reproduction)
Chronic, static 21-day NOEL 500 µg/litreb Kühn et al. (1989)
renewal (parent survival)
Chronic, static 21-day LOEL 2500 µg/litreb Springborn Bionomics (1984b)
renewal (survival and
reproduction)
Grass shrimp Chronic, static 10-day LC0 10 mg/kg Clark et al. (1987)
(Palaemonetes pugio) (sediment
exposure)
10-day LC50 > 10 mg/kg
(sediment
exposure)
Chronic, 28-day LOEL 1000 µg/litre Laughlin et al. (1978)
semi-static (survival)
28-day NOEL 500 µg/litre
(survival)
Table 13. Continued
Species Study type End-point Concentrationsa Reference
Scud (Gammarus pulex) Chronic, 10-day LOEL > 500 µg/litre Thurén & Woin (1991)
flow-through (survival)
10-day LOEL 500 µg/litre
(decreased
locomotor activity)
10-day NOEL 100 µg/litre
(decreased
locomotor activity)
Midge (Chironomus plumosus) Chronic, 30-day LOEL > 560 µg/litre Streufert & Sanders (1977)
flow-through (larval emergence)
a all concentrations are nominal unless stated otherwise
b measured concentration
In both laboratory and field studies with estuarine benthic
systems, DBP had statistically significant effects on 8-week
colonization at 1000 mg/kg sediment, the highest nominal
concentration tested (Tagatz et al., 1986). In the laboratory
study, the total number of species per box was significantly
decreased by DBP, while in the field study, only the total number
of individual molluscs was affected. The actual exposure
concentrations were lower than the nominal concentrations, as
only 48% and 19% of the original concentration persisted in the
laboratory and field systems, respectively, during the last two
weeks of the study. In an earlier study in which DBP was
introduced into the water rather than into the sediment, Tagatz
et al. (1983) reported that colonization was significantly
reduced at concentrations of 3700 and 3800 µg/litre in
laboratory- and field-colonized communities, respectively. At
340 µg/litre, there was no statistically significant effect on
the total numbers of species or individuals in the laboratory-
colonized community, except that the number of Corophium
acherusicum (amphipods) was significantly reduced. At
450 µg/litre, DBP did not have a statistically significant effect
on the field-colonized community.
9.1.2.3 Vertebrates
Acute and chronic toxicity data for fish are summarized in
Table 14.
In acute toxicity tests the yellow perch ( Perca flavescens)
and the channel catfish ( Ictalurus punctatus) were the most
sensitive freshwater fish with 96-h LC50 values of 350 and
460 µg DBP/litre respectively (Mayer & Ellersieck, 1986). The
sheepshead minnow, Cyprinodon variegatus, for which a 96-h
LC50 of 600 µg/litre has been reported (CMA, 1984), was the most
sensitive marine fish species identified.
Yoshioka et al. (1986) reported a 48-h LC50 of 630 µg
DBP/litre for the red killifish, Oryzias latipes, while the
96-h LC50 values were 730 µg DBP/litre in the bluegill
( Lepomis macrochirus) (Mayer & Sanders, 1973) and 850 µg
DBP/litre in the fathead minnow ( Pimephales promelas) (DeFoe
et al., 1990).
The most sensitive chronic study was based on the rainbow
trout ( Oncorhynchus mykiss) in an early life stage test with a
99-day no-observed-effect concentration (NOEC) (growth) of
100 µg/litre, a 99-day LOEC of 190 µg/litre (growth reduced by
about 27%) and 100% mortality on day 40 at 400 µg/litre (Ward &
Boeri, 1991). In an early life stage test on fathead minnows
( Pimephales promelas) a 20-day NOEC, based on hatching rate
and larval survival, of 560 µg/litre was reported (McCarthy &
Whitmore, 1985).
Table 14. Toxicity of DBP to fish
Species Study type End-Point Concentration References
(µg/litre)a
Fathead minnow Acute, static 24-h LC50 3300 Mayer & Ellersieck (1986)
(Pimephales promelas)
Acute, static 24-h LC50 3000 EG & Bionomics (1983a)
Acute, flow-through 24-h LC50 4800 Mayer & Ellersieck (1986)
Acute, flow-through 24-h LC50 1600 EG & G Bionomics (1983b)
Acute 48-h LC50 1490 Mayer & Sanders (1973)
Acute, static 48-h LC50 + 96 h LC50 3000 EG & Bionomics (1983a)
Acute, flow-through 48-h LC50 1200 EG & Bionomics (1983b)
Acute, static 96-h LC50 2020 McCarthy & Whitmore (1985)
Acute, static 96-h LC50 1300 Mayer & Ellersieck (1986)
Acute, flow-through 96-h LC50 850b DeFoe et al. (1990)
commercial phthalate
Acute, flow-through 96-h LC50 1100b DeFoe et al. (1990)
syntherised phthalate
Acute, flow-through 96-h LC50 3950 Mayer & Ellersieck (1986)
Acute, flow-through 96-h LC50 920 EG & Bionomics (1983b)
Acute, flow-through 96-h LOEL 1800 McCarthy & Whitmore (1985)
(embryo survival)
96-h NOEL 1000
(embryo survival)
Table 14. Continued
Species Study type End-Point Concentration References
(µg/litre)a
Yellow perch Acute, flow-through 24-h LC50 >1240 Mayer & Ellersieck (1986)
(Perca flavescens)
96-h LC50 350
Bluegill Acute, static 24-h LC50 1230 Mayer & Sanders (1973)
(Lepomis macrochirus)
24-h LC50 2100 Buccafusco et al. (1981)
24-h LC50 >3000 Mayer & Ellersieck (1986)
24-h LC50 1000 EG & G Bionomics (1983c)
48-h LC50 1200
96-h LC50 1200 Buccafusco et al. (1981)
96-h LC50 730 Mayer & Sanders (1973)
96-h LC50 2100 at pH 6.5 Mayer & Ellersieck (1986)
96-h LC50 1580 at pH 7.5
96-h LC50 2050 at pH 9.0 Mayer & Ellersieck (1986)
96-h LC50 850 EG & G Bionomics (1983c)
96-h LC50 1550 Mayer & Ellersieck (1986)
Table 14. Continued
Species Study type End-Point Concentration References
(µg/litre)a
Channel catfish Acute 24-h LC50 3720 Mayer & Sanders (1973)
(Ictalurus punctatus)
96-h LC50 2910 Mayer & Sanders (1973)
Acute, flow-through 96-h LC50 460 Mayer & Ellersieck (1986)
Rainbow trout
(Oncorhynchus mykiss) Acute, static 24-h LC50 > 16 000 Mayer & Ellersieck (1986)
Acute, static 24-h LC50 2800
Acute, flow-through 24-h LC50 + 48-h LC50 1600 EG & G Bionomics (1983d)
24-h LC50 4200 Mayer & Ellersieck (1986)
Acute, static 96-h LC50 2560
96-h LC50 1200 Hrudey et al. (1976)
96-h LC50 6470 Mayer & Sanders (1973)
Acute, flow-through 96-h LC50 1480 Mayer & Ellersieck (1986)
Acute, flow-through 96-h LC50 1600 EG & G Bionomics (1983d)
Acute, flow-through 24-h LC50 + 96-h LC50 > 1240 Mayer & Ellersieck (1986)
(yolk-sac fry)
Red killifish Acute, static 48-h LC50 630 Yoshioka et al. (1986)
(Orizias latipes)
Table 14. Continued
Species Study type End-Point Concentration References
(µg/litre)a
Sheephead minnow Acute, flow-through 96-h LC50 600b CMA (1984)
(Cyprinodon variegatus)
Fathead minnow Chronic, flow-through 20-day EC100 1800 McCarthy & Whitmore (1985)
(Pimephales promelas) (embryo mortality)
20-day LOEL (hatching 1000
rate and larval survival)
20-day NOEL (hatching 560
rate and larval survival)
Rainbow trout Chronic, flow-through 99-day NOEL (growth) 100b Ward & Boeri (1991)
(Oncorhynchus mykiss)
Chronic, flow-through 99-day LOEL (growth) 190b
Chronic, flow-through 40-day LC100 400b Ward & Boeri (1991)
Cyprinodontiform fish Chronic, static 147-day 13% reduction 2000 Davis (1988)
(Rivulus marmoratus) in embryonic viability
Chronic, static 147-day 155% increase 1000
in skeletal abnormalities
in progeny of exposed fish
Post-exposure 63-day NOEL 1000
(reproduction)
Post-exposure 63-day LOEL 2000
(reproduction)
a Concentrations are nominal unless stated otherwise
b Measured concentrations
9.1.3 Terrestrial organisms
9.1.3.1 Plants
DBP vapour from flexible plastics (e.g., glazing strips)
used in greenhouses has been implicated in development of damage
to plants. The threshold concentration for visible damage in
summer cabbage, Brassica oleracea L. cv. Derby Day was between
0.141 and 0.360 µg DBP/m3, the latter figure determined in a
4-week laboratory experiment in which growth restriction,
chlorosis and cotyledon death were observed (Hardwick et al.,
1984). However, it is unclear whether other phthalates
influenced the observed toxicity.
At higher concentrations in air, DBP caused damage in other
plant species. In strong light, leaves of radish seedlings
( Raphanus sativus) faded to pale green due to the
disappearance of carotenoid and chlorophyll pigments after 6 days
exposure to 41.3 to 62.3 µg DBP/m3 air and to white after 9 days
exposure to 56.5 to 90.7 µg DBP/m3 (Virgin, 1988). Such effects
were not observed in wheat seedlings ( Triticum aestivum) when
exposed to DBP vapour alone, but they did develop when the
seedlings were also treated with DBP-saturated water.
DBP inhibited photosynthesis in radish plants ( Raphanus
sativus) exposed to 120 µg DBP/m3 at a rate of 0.003 m3/min
for 13 days (Millar and Hannay, 1986). Concentrations of DBP as
low as 10 µmoles/m3 (approximately 2800 µg/m3) reduced
uncoupled electron transport in isolated spinach thylakoids by
about 13%, while 44 µmoles/m3 (approx. 12 250 µg/m3) caused a
50% reduction. Basal electron transport rates were reduced by
50% at 87 µmoles/m3 (approx. 24 200 µg/m3).
Application of DBP to leaves of white mustard ( Sinapis
alba) at a rate of 1.5 µg/cm2 caused chlorosis in new leaves
as they appeared on the third day after treatment (Lokke &
Bro-Rasmussen, 1981). This effect did not occur with DBP
application to nipplewort ( Lapsana communis) or to milfoil
( Achillea millefolium). Plants can also be adversely affected
by exposure to DBP in the soil. DBP concentrations in soil of
200 mg/kg or more reduced the germination of soybeans ( Glycine
max) by > 33% and decreased the growth of corn ( Zea mays)
and soybeans by 29 to 80% (Overcash et al., 1982). Plant height
and shoot weight were significantly reduced by 17 and 25%,
respectively, when corn seeds were planted in soil containing
2000 mg DBP/kg and grown for 3 weeks. Growth was not affected at
a concentration in soil of 200 mg DBP/kg (Shea et al., 1982).
A concentration of 1000 mg DBP/litre (added as a methanol
solution) reduced seed germination by 48% in peas ( Pisum
sativum) and by 58% in spinach ( Spinacia oleracea) grown in
tap water, but there was no observable effect on subsequent
development of the seeds that did germinate (Herring & Bering,
1988). It should be noted, however, that this concentration is
many times higher than the saturation concentration of DBP in
water (about 10 mg/litre).
9.1.3.2 Invertebrates
The LC50 for DBP in the earthworm Eisenia fetida was
1360 µg/cm2 in a 2-day contact test in which the chemical was
applied to filter paper (the toxic units referring to the amount
of chemical per cm2 of paper). In comparison, the LC50 of
dimethylphthalate was 550 µg/m2 and that of 2,4-dinitrophenol
was 0.6 µg/m2 (Neuhauser et al., 1985, 1986).
DBP applied to female house flies topically or by injection
at a concentration of 20 µg/fly (1000 µg/g body weight) was not
toxic, causing a mortality of less than 16% after 24 h (Al-Badry
& Knowles, 1980). Antagonistic interactions were observed when
flies were treated simultaneously with DBP and various
organophosphate insecticides, while synergistic interactions were
observed when flies were pretreated with the phthalate 30 min
before exposure to the pesticides. DBP inhibited the metabolism
of organophosphate pesticides, accounting for the synergistic
effects. When the phthalate and insecticides were applied
simultaneously, the resulting increase in the total lipophilic
pool by DBP may have resulted in an internal concentration of
insecticide below the toxicity threshold.
9.1.3.3 Vertebrates
Hill et al. (1975) found no deaths among 10-day-old mallard
( Anas platyrhynchos) fed up to 5000 mg DBP/kg for 5 days,
followed by 3 days on a normal diet.
In a study in which ring doves ( Streptophelia risoria)
were fed a diet containing 10 mg DBP/kg (1.1 mg DBP/kg body
weight per day) for a period of 3 weeks prior to mating through
completion of a clutch of two eggs, there was a 23% increase in
water permeability and a 10% decrease in egg- shell thickness
(Peakall, 1974). A 15% decrease in shell thickness is considered
significant for reproductive effects. Rapid recovery occurred
upon cessation of exposure.
Korhonen et al. (1983) studied the embryotoxicity of DBP to
white leghorn chicken eggs. On the third day of incubation, DBP
was injected on the inner shell membrane at doses of 13 and
26 µmol per egg (3.62 and 7.24 mg/egg, respectively). At 26 µmol
per egg, 30 eggs were tested and there were 6 early deaths (2
days after injection) and 4 non-malformed and 1 malformed late
deaths (between 3 and 11 days after injection). An approximate
ED50 of 33 µmol (9.19 mg) per egg was calculated for DBP.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Based on the limited data available, the principal media of
exposure to DBP for the general population, listed in order of
their relative importance based upon estimated intake, are as
follows: food, indoor air and drinking-water. Estimated intakes
from food and indoor air are 7 µg/kg body weight per day and
0.42 µg/kg body weight per day, respectively. Estimated intakes
from drinking-water and ambient air are considerably less,
< 0.02 µg/kg body weight per day and 0.26-0.36 ng/kg body weight
per day, respectively. Based on these intakes, it is estimated
that the total average daily intake from air, drinking-water and
food is 7.4 µg/kg body weight per day. It should be noted,
however, that intake of DBP in the diet can vary considerably,
depending upon the nature and extent of packaged food consumed
and the nature of use of food wrapping in food preparation. In
the United Kingdom, the Ministry of Agriculture, Fisheries and
Food has estimated that the maximum likely human intake of DBP
from food sources is approximately 2 mg per person per day
(approximately 31 g/kg body weight per day, assuming a mean body
weight of 64 kg). There is also potential for exposure to DBP in
cosmetics, although available data are inadequate to quantify
intake from this source.
The most recent provisional data from the NIOSH National
Occupational Exposure Survey indicate that in the USA over
500 000 workers, including 200 000 women, are potentially exposed
to DBP. At a limited number of worksites in the USA,
concentrations were generally less than the limit of detection
(i.e., 0.01 to 0.02 mg/m3).
10.1.2 Health effects
The acute toxicity of DBP in mice and rats is low.
In a case of accidental poisoning of a worker who ingested
approximately 10 grams of DBP, recovery was gradual within 2
weeks and complete after 1 month.
Based on limited available data in animal species, DBP
appears to have little potential to irritate skin or eyes,
although in humans a few cases of sensitization after exposure
have been reported.
Available data on the effects of DBP in humans are limited
to those of workers exposed to mixtures of phthalates and are
inadequate to serve as a basis for assessment of effects of DBP.
The remainder of this evaluation is, therefore, based on studies
in animals.
The profile of effects following exposure to DBP is similar
to that of other phthalate esters, which, in susceptible species,
induce hepatomegaly and increase numbers of hepatic peroxisomes,
are fetotoxic, have teratogenic potential and produce testicular
damage.
Adequate carcinogenesis bioassays for DBP have not been
conducted. The weight of available evidence indicates that DBP
is not genotoxic.
As a class, chemicals which cause peroxisome proliferation
are often hepatocarcinogenic via a non-genotoxic mode of action.
Although the mechanism of action remains unknown, tumour
formation is preceded by peroxisomal proliferation and
hepatomegaly. As a chemical causing peroxisomal proliferation,
it is possible that DBP might be a rodent liver carcinogen,
although it is much weaker in inducing hepatomegaly and
peroxisome proliferation than DEHP. To the degree that
hepatomegaly and peroxisomal proliferation correlate with
carcinogenic potency, DBP would be anticipated to be a less
potent carcinogen than DEHP and would probably exhibit no
activity as measured by current cancer bioassay methodologies.
Thus, it is unlikely that DBP presents any significantly
increased risk of cancer at concentrations generally present in
the environment.
Effects of DBP observed at lowest doses in repeated dose
toxicity studies are those on the liver and testes of the rat and
include hepatomegaly and peroxisome proliferation. In one study,
hepatic necrotic changes were also reported. Effects on the
testes include decreases in the activities of testicular enzymes
and, at higher doses, degeneration of the germinal epithelium and
reductions in testicular zinc levels. DBP also induces adverse
effects on fertility, is fetotoxic and induces teratogenic
effects at high concentrations that are toxic to the dams.
Toxicity to the testes is more marked when exposure to DBP occurs
during development and maturation than when adults only are
exposed. Lowest reported effect levels in adequate studies for
these various effects and their associated no-observed-(adverse)-
effect levels (NOEL/NOAEL) are summarized in Table 15.
Table 15. Effect levels of DBP
No- Lowest-
observed- observed-
(adverse)- (adverse)-
effect levela effect
levelb
End-point Species (mg/kg b.w. per day) Reference
Liver:
Organ weight rat, - 120 Nikonorow
(relative) Wistar et al. (1973)
Peroxisomal rat, 176 356 NTP (1995),
proliferation F-344 Study No. 2
and hepatomegaly
rat, 138 279 NTP (1995),
F-344 Study No. 3
Necrosis (not rat, 250 Murakami et
confirmed) Wistar al. (1986a)
Testis:
Enzymes rat, 250 Srivastava
Wistar et al.
(1990a,b)
Histopathological rat, 359 720 NTP (1995),
lesions F-344 Study No. 2
rat, 279 571 NTP (1995),
F-344 Study No. 3
Table 15. contd.
No- Lowest-
observed- observed-
(adverse)- (adverse)-
effect levela effect
levelb
End-point Species (mg/kg b.w. per day) Reference
Reproduction/fertility/ rat, NI c 66NTP (1995;
developmental Sprague- Wine et al.,
Dawley 1997),
Study No. 4
Developmental mouse, 100 400 Hamano et al.
JCL:ICR (1977)
a Each value in this column is either a NOEL or NOAEL
b Each value in this column is either a LOEL or LOAEL
c NI = not identified
10.1.3 Guidance values
The following guidance is provided as a potential basis for
derivation of limits of exposure by relevant authorities. Since
ingestion is by far the principal route of exposure to DBP and
since the toxicological data for other routes of administration
are insufficient for evaluation, only the oral route is
addressed here. However, the ultimate objective should be
reduction of total exposure from all sources to less than the
tolerable daily intake presented below.
The Task Group considered that the testicular and
reproductive/developmental effects are the most relevant for
derivation of guidance values for protection of human health.
Increases in liver weight, hepatomegaly and peroxisome
proliferation were regarded by the Task Group as being
functional, relating most likely to the metabolism of the
material, rather than pathological. Moreover, although hepatic
necrosis was observed in one strain of rats at 250 mg/kg body
weight per day, it was not observed in two other strains at much
higher doses.
The NOAEL/NOEL values for the end-points considered to be
most appropriate for derivation of guidance values (i.e.
developmental and reproductive toxicity) have not been identified
in the Continuous Breeding study (NTP study 4); the lowest dose
studied (66 mg/kg body weight per day) is a LOAEL (NTP, 1995;
Wine et al., 1997). On the basis of these data, an acceptable
daily intake (ADI) is derived as follows:
ADI = 66 mg/kg body weight per day
1000
= 0.066 mg/kg body weight per day
= 66 g/kg body weight per day
where:
* 66 mg/kg body weight per day is the approximate LOAEL for
developmental and reproductive effects in rats observed in
the most sensitive studies conducted to date
* 1000 is the uncertainty factor (×10 for interspecies
variation, ×10 for interindividual variation, ×10 for lack
of data on a NOAEL. A factor of 10 for lack of a NOAEL was
considered adequate since the effects observed at the lowest
dose levels were moderate and probably reversible. The
severe, possibly irreversible, teratogenic, testicular and
epididymal effects were only observed at the highest dose
level tested, which also produced other signs of toxicity.
Because DBP is rapidly metabolized and eliminated, with no
evidence of accumulation in tissues, no additional factor
was incorporated for lack of data on chronic effects.
10.2 Evaluation of effects in the environment
10.2.1 Exposure
DBP exists widely in the environment, being released during
production, processing, usage and disposal. However, it is
relatively non-persistent in air and surface water. The most
important process leading to the elimination of DBP is biological
breakdown, aerobic degradation being rapid and complete. It would
be expected to be more persistent in anaerobic sediments. It is
moderately adsorbed to soil.
DBP would be expected to bioaccumulate, based on a log Kow
of 4.3 to 4.7. However, it tends to be readily metabolized
leading to bioconcentration factors lower than predicted.
Biomagnification in terrestrial animals is unlikely.
Mean concentrations of DBP in surface water tend to be less
than 1 µg/litre. However, levels in polluted rivers are much
higher, with values of 12 to 34 µg/litre. Levels in sediment are
generally less than 1 mg/kg dry weight although in polluted areas
concentrations of up to 10 mg/kg have been measured. DBP
concentrations in sewage sludge range from 0.2 to 200 mg/kg dry
weight.
10.2.2 Effects
A comparison of the results of acute and long-term tests on
aquatic organisms shows that there is no increase in toxic
effects with increasing duration of exposure.
In acute toxicity tests the sensitivity of the different
trophic levels is similar. The 48-h and 96-h LC50 and EC50
values for the most sensitive species are in the range of 350 to
760 µg/litre for freshwater organisms and 600 to 750 µg/litre for
marine organisms.
The most sensitive chronic study was based on the rainbow
trout; the 99-day no-observed-effect concentration (NOEC) based
on growth was 100 µg/litre and the lowest-observed-effect
concentration (LOEC) 190 µg/litre.
The acute toxicity of DBP to birds is low.
10.2.3 Risk evaluation
The lowest reported chronic effect level for dissolved DBP
in aquatic organisms was 190 µg/litre (99-day LOEC for growth)
and the lowest NOEC was 100 µg/litre in the same test. These
values are at least factors of 190 and 100, respectively, greater
than the mean surface water concentration of DBP. Therefore, the
risk to aquatic organisms from mean DBP concentrations in surface
water is low. However, in highly polluted rivers where surface
water concentrations have been found to be up to 34 µg/litre, the
ratio between the concentration and the NOEC is only 3.
There is inadequate data to assess the risk of DBP to
sediment-dwelling organisms.
The most likely route of exposure for higher organisms,
e.g., birds and mammals, is through food intake, in particular
fish.
The only acute toxicity test on birds was carried out on
10-day-old mallards, where a 5-day LC50 of > 5000 mg/kg diet
was found. Based on food consumption and body weight, an LD50
for the mallard of > 2043.5 mg/kg body weight can be calculated.
Using this data an estimated LC50 for a fish-eating bird (e.g.,
kingfisher), based on body weight and food consumption, can be
calculated.
LC50 (mg/kg dry weight of diet)
test species LD50 (mg/kg) body wt (kg)
=
food consumption (kg)
The estimated LC50 for the fish-eating bird is >
9350 mg/kg diet. The highest water concentration (34 µg/litre)
multiplied by the highest bioconcentration factor (590) gives a
residue level in fish of 20 mg/kg. Comparing this value to the
estimated LC50 value gives a Toxicity Exposure Ratio (TER) of
> 470. A TER of less than 1 would give cause for concern; but a
value of > 470 indicates that the risk to fish-eating birds from
DBP is very low.
For mammals, the mink, a terrestrial mammal with a diet
consisting predominantly of aquatic prey, can be used. The
estimated intake for a "worst case" scenario is 3.1 mg/kg body
weight per day. This is based on an ingestion rate of 155 g per
day and assumes a diet of 75% fish, a maximum measured
bioconcentration factor of 590 for the fathead minnow, and a
maximum concentration of DBP in water of 34 µg/litre. The
estimated intake is considerably less than the no-observed-
adverse-effect levels in toxicity studies in laboratory mammals
(i.e. 250 mg/kg body weight per day).
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
In laboratory animals, the critical toxic effects of DBP
were those on development and reproduction at concentrations well
above those to which people are normally exposed in the general
environment. DBP is readily broken down in the environment and
in the body and shows no tendency to accumulate or to persist in
any specific tissues or organs. It is unlikely that there is any
risk to human health at present levels of exposure in the general
environment.
There is inadequate information to assess exposure from use
in cosmetics.
The risk to aquatic organisms associated with the present
mean concentrations of DBP in surface waters is low. However, in
highly polluted rivers the safety margin is much smaller. There
is inadequate data to assess the risk of DBP to sediment-dwelling
organisms. At current levels of exposure, it can be concluded
that the risk to fish-eating birds and mammals is low.
The current measures being taken to limit the release of DBP
into the environment and to control its use in food-packaging
materials should be maintained.
12. FURTHER RESEARCH
The most sensitive end-points used in determining the
guidance value were effects on reproduction, both fertility and
development. No NOAEL was identified for these effects and the
results suggest that the adverse effects of DBP are more marked
in animals exposed during development and maturation than in
animals exposed as adults only. Data on effects of exposure
during the developmental period are very limited and further work
to identify a NOEL for such exposure is urgently needed.
The number and quality of studies describing the profile of
toxicity of DBP and its behaviour in the environment are
sufficient to make reasonable assessments of potential health
effects, environmental fate and to set a guidance value for
limiting human exposure to preclude adverse health effects.
Therefore, additional research in these areas is of low priority,
relative to that for other substances.
However, one area of concern is that the potential for
exposure in cosmetics is largely unknown. It is recommended,
therefore, that additional data on the use and levels of DBP in
cosmetics be acquired. If there is a potential for considerable
additional exposure from this source, it is recommended that
controlled studies be conducted to examine the rate of skin
absorption, dosimetry, metabolism and excretion of DBP in humans.
In view of the large species differences in some toxic
effects of DBP on laboratory animals, additional studies on
kinetics and metabolism in humans or human cells are desirable.
More research is needed on the effects of DBP on sediment-
dwelling organisms.
13. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
DBP may be used for the manufacture of regenerated cellulose
film which is intended to or does come into contact with food-
stuffs. It may be used as a plasticizer in total amount of
12.5 mg/dm2 on the side of the film in contact with foodstuffs
(EEC, 1987).
REFERENCES
Abe S & Sasaki M (1977) Chromosome aberrations and sister
chromatid exchanges in Chinese hamster cells exposed to various
chemicals. J Natl Cancer Inst, 58(6): 1635-1641.
Aceves M & Grimalt JO (1993) Large and small particle size
screening of organic cmpounds in urban air. Atmos Environ,
B27(2): 251-263.
Acey R, Healy P, Unger TF, Ford CE, & Hudson RA (1987) Growth and
aggregation behavior of representative phytoplankton as affected
by the environmental contaminant di-n-butyl phthalate. Bull
Environ Contam Toxicol, 39: 1-6.
Adams WJ, Kimerle RA, & Barnett JW (1992) Sediment quality and
aquatic life assessment. Environ Sci Technol, 26: 1864-1875.
Agarwal DK, Lawrence WH, Nunez LJ, & Autian J (1985) Mutagenicity
evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J Toxicol Environ Health,
16(1): 61-69.
Aitio A & Parkki M (1978) Effect of phthalate esters on drug
metabolising enzyme activities in rat liver. Arch Int
Pharmacodyn, 235: 187-195.
Al-Badry MS & Knowles CO (1980) Phthalate-organophosphate
interactions: toxicity, penetration, and metabolism studies with
house flies. Arch Environ Contam Toxicol, 9(2): 147-161.
Al-Omran LA & Preston MR (1987) The interactions of phthalate
esters with suspended particulate material in fresh and marine
waters. Environ Pollut, 46: 117-186.
Albro PW & & Moore B (1974) Identification of the metabolites of
simple phthalate diesters in rat urine. J Chromatogr, 94:
209-218.
Aldyreva MV, Klimova TS, Izyumova AS, & Timofievskaya LA (1975)
[Effect of plasticisers on reproductive function.] Gig Tr Prof
Zabol, 12: 25-29 (in Russian).
Antonyuk OK & Aldyreva MV (1973) [Substantiation of the maximum
allowable concentration of dibutyl phthalate in the air of
industrial premises.] Gig Trud Prof Zabol, 12: 26-30 (in
Russian).
Ariyoshi T, Koga Y, Yoshitake S, Takada K, & Tenda N (1976)
Metabolism of dibutyl phthalate and the effects of its
metabolites on animals. Kyushu Yakugokkai Kaiho, 30: 17-22.
Atlas E & Giam CS (1981) Global transport of organic pollutants:
ambient concentrations in the remote marine atmosphere. Science,
211: 163-165.
ATSDR (1990) Toxicological profile for di-n-butylphthalate.
Atlanta, Georgia, Agency for Toxic Substances and Disease
Registry, 109 pp.
Atwater JW, Jasper SE, Parkinson PD, & Mavinic DS (1990) Organic
contaminants in Canadian coal wastewaters and associated
sediments. Water Pollut Res J Can, 25: 187-200.
Barber ED, Astill BD, Moran EJ, Schneider BF, Gray TJB, Lake BG,
& Evans JG (1987) Peroxisome induction studies on seven phthalate
esters. Toxicol Ind Health, 3(2): 7-24.
Barrick R, Becker S, Brown L, Beller H, & Pastrook R (1988)
Sediment quality values refinement: 1988 update and evaluation of
Puget Sound AET. Bellevue, Washington, PTI Environmental
Services.
Belisle AA, Reichel WL, & Spann JW (1975) Analysis of tissues of
mallard ducks fed two phthalate esters. Bull Environ Contam
Toxicol, 13(2): 129-132.
Bell FP, Patt CS, Brundage B, Gillies PJ, & Phillips WA (1978)
Studies on lipid biosynthesis and cholesterol content of liver
and serum lipoproteins in rats fed various phthalate esters.
Lipids, 13: 66-74.
Benckiser G & Ottow JCG (1982) Metabolism of the plasticizer
di-normal-butylphthalate by Pseudomonas pseudoalcaligenes under
anaerobic conditions, with nitrate as the only electron-acceptor.
Appl Environ Microbiol, 44(3): 576-578.
BIBRA (1986) A 21 day feeding study of di-n-butyl phthalate to
rats: Effects on the liver and liver lipids. Carshalton, Surrey,
The British Industrial Biological Research Association (Report to
Chemical Manufacturers Association, Washington, DC) (CMA
reference PE 28.0-BT-BIB).
Bornmann G & Loeser A (1956) The reaction of the body to the
action of various plasticizers. Z Lebensm.unters Forsch, 103:
413-434.
Bove JL, Dalven P, & Kukreja VP (1978) Airborne di-butyl and di-
(2-ethylhexyl)-phthalate at three New York City air sampling
stations. Int J Environ Anal Chem, 5: 189-194.
Brandt K (1985) Final report on the safety assessment of dibutyl
phthalate, and diethyl phthalate. J Am Coll Toxicol, 4(3):
267-303.
BUA (1987) Dibutyl phthalate. Frankfurt am Main, German Chemical
Society, Advisory Committee on Existing Chemicals of
Environmental Relevance, 71 pp (BUA Report No. 22).
Buccafusco RJ, Ellis SJ, & LeBlanc GA (1981) Acute toxicity of
priority pollutants to bluegill ( Lepomis macrochirus). Bull
Environ Contam Toxicol, 26: 446-452.
Burns BG, Musial CJ, & Uthe JF (1981) Novel cleanup method for
quantitative gas chromatographic determination of trace amounts
of di-2-ethylhexyl phthalate in fish lipid. J Assoc Off Anal Chem,
64(2): 282-286.
California Environmental Protection Agency (1992) PTEAM:
Monitoring of phthalates and PAHS in indoor and outdoor air
samples in Riverside, California. Final Report, Volume II
(Contract No. A933-144). Research Triangle Park, North Carolina,
Research Triangle Institute (Submitted to California Air
Resources Board).
Call DJ, Brooke LT, Ahmad N, & Richter JE (1983) Toxicity and
metabolism studies with EPA Priority Pollutants and related
chemicals in freshwater organisms. Duluth, Minnesota, US
Environmental Protection Agency, Environmental Research
Laboratory, Office of Research and Development, 120 pp (EPA
600/3-83-095).
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR,
Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR,
& Gould C (1979) Water-related environmental fate of 129 priority
pollutants. Volume II: Halogenated aliphatic hydrocarbons,
halogenated ethers, monocyclic aromatics, phthalate esters,
polycyclic aromatic hydrocarbons, nitrosamines, miscellaneous
compounds. Washington, DC, US Environmental Protection Agency
(EPA 440/4-79-029b).
Calley D, Autian J, & Guess WL (1966) Toxicology of a series of
phthalate esters. J Pharm Sci, 55: 158-162.
Calnan CD (1975) Dibutyl phthalate. Contact Dermatitis, 1: 388.
Camford Information Services Inc. (1992) CPI product profiles -
Di- n-butyl phthalate. Dons Mills, Ontario, Camford Information
Services Inc., 3pp.
Castle L, Mercer AJ, Startin JR, & Gilbert J (1988) Migration
from plasticized films into foods. 3. Migration of phthalate,
sebacate, citrate and phosphate esters from films used for retail
food packaging. Food Addit Contam, 5(1):-9-20.
Castle L, Mayo A, & Gilbert J (1989) Migration of plasticizers
from printing inks into foods. Food Addit Contam, 6(4):
437-443.
Cater BR, Cook MW, Gangolli SD, & Grasso P (1977) Studies on
dibutyl phthalate-induced testicular atrophy in the rat: Effect
on zinc metabolism. Toxicol Appl Pharmacol, 41(3): 609-618.
Cautreels W & van Cauwenberghe K (1978) Experiments on the
distribution of organic pollutants between airborne particulate
matter and the corresponding gas phase. Atmos Environ, 12:
1133-1141.
Cautreels W, van Cauwenberghe K, & Guzman LA (1977) Comparison
between the organic fraction of suspended matter at a background
and an urban station. Sci Total Environ, 8(1): 79-88.
CCREM (Canadian Council of Resource and Environment Ministers)
(1987) Canadian water quality guidelines. Winnipeg, Manitoba,
Task Force on Water Quality Guidelines of the Canadian Council of
Resources and Environment Ministers (now part of the Canadian
Council of Environment Ministers), pp 3/37, 6/182-6/187.
Chambon P, Riotte M, Daudon M, Chambon-Mougenot R and Bringuier R
(1971) Etude du métabolisme des phthalates de dibutyle et de
diéthyle chez le rat. C R Acad Sci Paris, 273: 2165-2168.
Chan HS (1975) A study of the transfer processes of phthalate
esters to the marine environment. Houston, Texas, Graduate
College of Texas A&M University (Ph.D. Dissertation).
Chapman P (1989) Current approaches to developing sediment
quality criteria. Environ Toxicol Chem, 8: 589-599.
Chauret C, Mayfield CI, & Inniss WE (1995) Biotransformation of
di- n-butyl phthalate by a psychotrophic Pseudomonas
fluorescens (BGW) isolated from subsurface environment. Can J
Microbiol, 41: 54-63.
Ching NP, Jham GN, & Subbarayan C (1981) Gas chromatographic-mass
spectrometric detection of circulating plasticizers in surgical
patients. J Chromatogr, 222: 171-177.
City of Toronto (1990) The quality of drinking-water in Toronto:
Summary report. Toronto, City of Toronto, Department of Health.
Clark JR, Patrick JM Jr, Moore JC, & Lores EM (1987) Waterborne
and sediment-source toxicities of six organic chemicals to grass
shrimp ( Palaemonetes pugio) and amphioxus ( Branchiostoma
caribaeum). Arch Environ Contam Toxicol, 16(4): 401-407.
CMA (1984) Generation of environmental fate and effects data base
on 14 phthalate esters. Summary Report - Environmental studies:
Phase I. Washington, DC, Chemicals Manufacturers Association,
Phthalate Esters Program Panel.
Cocchieri RA (1986) Occurrence of phthalate esters in Italian
packaged foods. J Food Prot, 49(4): 265-266.
Cummings AM & Gray LE Jr (1987) Dibutyl phthalate: maternal
effects versus fetotoxicity. Toxicol Lett, 39(1): 43-50.
Davis WP (1988) Reproductive and developmental responses in the
self-fertilizing fish, Rivulus marmoratus, induced by the
plasticizer, di- n-butylphthalate. Environ Biol Fishes, 21(2):
81-90.
Davis BJ, Maronpot RR, & Heindel JJ (1994a) Di-(2-
ethylhexyl)phthlate suppresses estradiol and ovulation in cycling
rats. Toxicol Appl Pharmacol, 128: 216-223.
Davis BJ, Weaver R, Gaines LJ, & Heindel JJ (1994b) Mono-(2-
ethylhexyl)phthalate suppresses estradiol production independent
of FSH-cAMP stimulation in rat granulosa cells. Toxicol Appl
Pharmacol, 128: 224-228.
DeFoe DL, Holcombe GW, Hammermeister DE, & Biesinger KE (1990)
Solubility and toxicity of eight phthalate esters to four aquatic
organisms. Environ Toxicol Chem, 9: 623-636.
DeVault DS (1985) Contaminants in fish from Great Lakes harbors
and tributary mouths. Arch Environ Contam Toxicol, 14(5):
587-594.
Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE,
Pavlou SP, Allen HE, Thomas NA, & Paquin PR (1991) Technical
basis for establishing sediment quality criteria for nonionic
organic chemicals using equilibrium partitioning. Environ Toxicol
Chem, 10: 1541-1583.
Eaton RW & Ribbons DW (1982) Metabolism of dibutyl phthalate and
phthalate by Micrococcus sp. strain 12B. J Bacteriol, 151:
48-57.
EEC (European Economic Community) (1987) Council directive of 25
April 1983 on the approximation of the laws of the Member States
relating to materials and articles made of regenerated cellulose
film intended to come into contact with foodstuffs.
EG&G Bionomics (1983a) Acute toxicity of fourteen phthalate
esters to fathead minnows ( Pimephales promelas). Penascola,
Florida, EG&G Bionomics (Toxicity test report submitted to
Chemical Manufacturers Association, Washington, DC).
EG&G Bionomics (1983b) Acute toxicity of thirteen phthalate
esters to fathead minnows ( Pimephales promelas) under flow-
through conditions. Penascola, Florida, EG&G Bionomics (Toxicity
test report submitted to Chemical Manufacturers Association,
Washington, DC).
EG&G Bionomics (1983c) Acute toxicity of thirteen phthalate
esters to bluegill ( Lepomis macrochirus). Penascola, Florida,
EG&G Bionomics (Toxicity test report submitted to Chemical
Manufacturers Association, Washington, DC).
EG&G Bionomics (1983d) Acute toxicity of fourteen phthalate
esters to rainbow trout ( Salmo gairdneri) under flow-through
conditions. Penascola, Florida, EG&G Bionomics (Toxicity test
report submitted to Chemical Manufacturers Association,
Washington, DC).
EG&G Bionomics (1984a) Acute toxicity of twelve phthalate esters
to mysid shrimp ( Mysidopsis bahia). Penascola, Florida, EG&G
Bionomics (Toxicity test report submitted to Chemical
Manufacturers Association, Washington, DC).
EG&G Bionomics (1984b) Acute toxicity of twelve phthalate esters
to Paratanytarsus parthenogenica. Penascola, Florida, EG&G
Bionomics (Toxicity test report submitted to Chemical
Manufacturers Association, Washington, DC).
Eglinton G, Simoneit BRT, & Zoro JA (1975) he recognition of
organic pollutants in aquatic sediments. Proc R Soc (Lond),
B189: 415-442.
Ehrhardt M & Derenbach J (1980) Phthalate esters in the Kiel
Bight. Mar Chem, 8: 339-346.
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol,
15: 30-38.
Elsisi AE, Carter DE, & Spies IG (1989) Dermal absorption of
phthalate diesters in rats. Fundam Appl Toxicol, 12(1): 70-77.
Ema M, Amano H, Itami T, & Kawasaki H (1993) Teratogenic
evaluation of di-n-butyl phthalate in rats. Toxicol Lett,
69(2): 197-203.
Ema M, Amano H, & Ogawa Y (1994) Characterization of the
developmental toxicity of di-n-butyl phthalate in rats.
Toxicology, 86(3): 163-174.
Fallon ME & Horvath FJ (1985) Preliminary assessment of
contaminants in soft sediments of the Detroit River. J Great
Lakes Res, 11: 373-378.
Fatoki OS & Vernon F (1990) Phthalate esters in rivers of the
Greater Manchester area, U.K. Sci Total Environ, 95: 227-232.
Fishbein L & Albro PW (1972) Chromatographic and biological
aspects of the phthalate esters. J Chromatogr, 70: 365-412.
Fischer J, Ventura K, Prokes B, & Janderaa P (1993) Methods for
determination of plasticizers in industrial emissions.
Chromatographia, 37(1/2): 47-50.
Florin I, Rutberg L, Curvall M, & Enzell CR (1980) Screening of
tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15: 219-232.
Foster PMD, Lake BG, Thomas LV, Cook MW, & Gangolli D (1981)
Studies on the testicular effects and zinc excretion produced by
various isomers of monobutyl-o-phthalate in the rat. Chem-Biol
Interact, 34: 233-238.
Foster PM, Cook MW, Thomas LV, Walters DG, & Gangolli SD (1982)
Differences in urinary metabolic profile from di-n-butyl
phthalate-treated rats and hamsters: A possible explanation for
species differences in susceptibility to testicular atrophy. Drug
Metab Dispos, 11(1): 59-61.
Fredricsson B, Mller L, Pousette Å, & Westerholm R (1993) Human
sperm motility is affected by plasticizers and diesel particle
extracts. Pharmacol Toxicol, 72(2): 128-133.
Fukuoka M, Tanimoto T, Zhou Y, Kawasaki N, Tanaka A, Ikemoto I, &
Machida T (1989) Mechanism of testicular atrophy induced by
di-n-butyl phthalate in rats. Part 1. J Appl Toxicol, 9(4):
277-283.
Fukuoka M, Zhou Y, Tanaka A, Ikemoto I, & Machida T (1990)
Mechanism of testicular atrophy induced by di-n-butyl phthalate
in rats. Part 2. The effects on some testicular enzymes. J Appl
Toxicol, 10(4):
285-293.
Fukuoka M, Kobayashi T, Zhou Y, & Hayakawa T (1993) Mechanisms of
testicular atrophy induced by di- n-butyl phthalate in rats.
Part 4. Changes in the activity of succinate dehydrogenase and
the levels of transferrin and ferritin in the Sertoli and germ
cells. J Appl Toxicol, 13(4): 241-246.
Furtmann K (1994) Phthalate in surface water - a method for
routine trace level analysis. Fresenius J Anal Chem, 348:
291-296.
Giam CS & Atlas E (1980) Accumulation of phthalate ester
plasticizers in Lake Constance sediments. Nat.wiss, 67:
508-510.
Giam CS, Chan HS, Neff GS, & Atlas EL (1978) Phthalate ester
plasticizers: a new class of marine pollutant. Science, 199:
419-421.
Giam CS, Atlas E, & Chan HS (1980) Phthalate esters, PCB and DDT
residues in the Gulf of Mexico atmosphere. Atmos Environ, 14:
65-69.
Gilioli R, Bulgherain C, Terrana T, Filippini G, Massette N, &
Boeri R (1978) Horizontal and longitudinal study of a population
employed in the production of phthalates. Med Lav, 69: 620-631.
Glass GE, Strachan WMI, Willford WA, Armstrong FAI, Kaiser KLE, &
Lutz A (1977) Organic contaminants. In: The waters of Lake Huron
and Lake Superior. Volume III, Part B: Lake Superior. Detroit,
Michigan, The Upper Lakes Reference Group, pp 417-429, 499-502
(Report to the International Joint Commission) (EPA 600/J-77-
042).
Golder Associates (1987) Testing of specific organic compounds in
soils in background in urban areas, Port Credit and
Oakville/Burlington, Ontario. Mississauga, Ontario, Canada,
Golder Associates (Working paper to Shell Canada Limited and
Texaco Canada Limited).
Golder Associates, SENES Consultants Limited and CanTox (1987)
Summary interpretation of the influence of soil conditions on the
decommissioning of the Oakville refinery site. Mississauga,
Ontario, Canada, Golder Associates (Working paper No. 4 to Shell
Canada Limited).
Government of Canada (1993) Canadian Environmental Protection Act
- Priority substances list. Supporting document: Dibutyl
phthalate. Ottawa, Ontario, Environment Canada.
Government of Canada (1994) Canadian Environmental Protection Act
- Priority substances list. Assessment report: Dibutyl phthalate.
Ottawa, Ontario, Environment Canada and Health Canada.
Graham PR (1973) Phthalate ester plasticizers - Why and how they
are used. Environ Health Perspect, 3: 3-12.
Gray TJB & Butterworth KR (1980) Testicular atrophy produced by
phthalate esters. Arch Toxicol, 4(Suppl): 452-455.
Gray TJB, Rowland IR, Foster PMD, & Gangolli SD (1982) Species
differences in the testicular toxicity of phthalate esters.
Toxicol Lett, 11: 141-147.
Gray LE Jr, Laskey JW, Ostby J, & Ferrell J (1983a) The effects
of dibutyl phthlate on the reproductive tract of the male and
female rat and hamster. Toxicologist, 3: 22 (Abstract No. 87).
Gray TJB, Lake B, Beamand A, Foster JR, & Gangolli SD (1983b)
Peroxisomal effects of phthlate esters in primary cultures of rat
hepatocytes. Toxicology, 28: 167-179.
Guardiola J, Ventura F, & Rivera J (1989) Occurrence of
industrial organic pollution in a groundwater supply: screening,
monitoring and evaluation of treatment processes. Water Supply,
7: 11-16.
Hamano Y, Kuwano A, Inoue K, Oda Y, Yamamoto H, Mitsuda B, &
Kunita N (1977) Studies on toxicity of phthalic acid esters.
First report: Teratogenic effects in mice administered orally.
Osaka-furitsu Koshu Esei kenkyusho Kenkyu Hokoka Shokukhim Eisei
Hen, 8: 29-33.
Hardin BD, Schuler RL, Burg JR, Booth GM, Hazelden KP, MacKenzie
KM, Piccirillo VJ, & Smith KN (1987) Evaluation of 60 chemicals
in a preliminary developmental toxicity test. Teratogen
Carcinogen Mutagen, 7: 29-48.
Hardwick RC, Cole RA, & Fyfield TP (1984) Injury to and death of
cabbage ( Brassica oleracea) seedlings caused by vapours of di
butyl phthalate emitted from certain plastics. Ann Appl Biol,
105: 97-105.
Hazleton Biotechnologies Company (1986) Mutagenicity of 1C in a
mouse lymphoma mutation assay. Final Report (Genetics assay No.
7156 - HB Project No. 20989). Kensington, Maryland, Hazleton
Biotechnologies Company (Submitted by Chemical Manufacturers
Association to US Environmental Protection Agency) (EPA Document
No. 40-8626225; microfiche No. OTS0510527).
Heindel JJ, Gulati DK, Mounce RC, Russell SR, & Lamb JC IV (1989)
Reproductive toxicity of three phthalic acid esters in a
continuous breeding protocol. Fundam Appl Toxicol, 12(3):
508-518.
Herring R & Bering CL (1988) Effects of phthalate esters on plant
seedlings and reversal by a soil microorganism. Bull Environ
Contam Toxicol, 40(4): 626-632.
Hibino M, Matsuda H, Sato T, Ose Y, Nagase H, & Kito H (1992)
Generation of mutagenicity by ozonation of humic substances'
components. Sci Total Environ, 16: 1-13.
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC,
US Department of the Interior, Fish and Wildlife Service (Special
Scientific Report - Wildlife No. 191).
Hites RA & Budde WL (1991) EPA's analytical methods for water:
the next generation. Environ Sci Technol, 25(6): 998-1006.
Ho CT, Lee KN, & Jin QZ (1983) Isolation and identification of
volatile flavor compounds in fried bacon. J Agric Food Chem,
31: 336-342.
Hoff RM & Chan KW (1987) Measurement of polycyclic aromatic
hydrocarbons in air along the Niagara river. Environ Sci Technol,
21(6): 556-561.
Howard PH ed. (1989) Handbook of environmental fate and exposure
data for organic chemicals. Chelsea, Michigan, Lewis Publishers
Inc., 574 pp.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM
(1991) Handbook of environmental degradation rates. Chelsea,
Michigan, Lewis Publishers Inc.
Hrudey SE, Sergy GA, & Thackeray T (1976) Toxicity of oil sands
plant wastewaters and associated organic contaminants. In: Water
pollution research in Canada: Proceedings of the 11th Canadian
Symposium. Ottawa, Ontario, Water Pollution Research Canada, pp
34-45.
HSE (Health and Safety Executive) (1986) Review of the toxicity
of the esters of o-phthalic acid (phthalate esters). Sudbury,
United Kingdom, HSE Books, 183 pp (Toxicity Review 14).
Hudson RA & Bagshaw JC (1978) Toxicity of di- n-butyl phthalate
for developing larvae of the brine shrimp, Artemia salina. Fed
Proc, 37: 1702 (Abstract No. 2393).
Hudson RA, Austerberry CF, & Bagshaw JC (1981) Phthalate ester
hydrolases and phthalate ester toxicity in synchronously
developing larvae of the brine shrimp ( Artemia). Life Sci,
29: 1865-1872.
Husain SL (1975) Dibutyl phthalate sensitivity. Contact
Dermatitis, 1: 395.
Ikemoto I (1985) Experimental studies on testicular damage
induced by phthalic acid esters. Tokyo Jikeikai Med J, 100:
1115-1127.
Ikemoto I, Kotera S, Katsurai K, Inaba Y, Machida T, & Tanaka A
(1983) Experimental testicular damage induced by dibutyl
phthalate and monobutyl phthalate. Jpn J Fertil Steril, 28(7):
159-165.
Inman JC, Strachan SD, Sommers LE, & Nelson DW (1984) The
decomposition of phthalate esters in soil. J Environ Sci Health,
B19: 245-257.
IPCS (1992) Environmental health criteria 131: Diethylhexyl
phthalate. Geneva, World Health Organization, International
Programme on Chemical Safety, 141 pp.
IPCS (1993) Environmental health criteria 170: Assessing human
health risks of chemicals: Derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization,
International Programme on Chemical Safety, 73 pp.
Ishida M, Suyama K, & Adachi S (1980) Background contamination by
phthalates commonly encountered in the chromatographic analysis
of lipid samples. J Chromatogr, 189: 421-424.
Ishida M, Suyama K, & Adachi S (1981) Occurrence of dibutyl and
di(2-ethylhexyl) phthalate in chicken eggs. J Agric Food Chem,
29: 72-74.
Ishidate M & Odashima S (1977) Chromosome tests with 134
compounds on Chinese hamster cells in vitro - a screening for
chemical carcinogens. Mutat Res, 48(3/4): 337-354.
Ito S, Takeda H, Kobayashi A, Sakurai H, Tada Y, Aoki G, Hosogai
T, Yamanaka T, & Ishiwata H (1993) A simple and rapid method for
determination of n-dibutylphthalate in imported vodka by FID-GC
and GC/MS. J Food Hyg Soc Jpn, 34(3): 254-256.
Iturbe R, Elefsiniotis P, & Moreno G (1991) Efficiency of a
phthalate ester in an activated sludge system. Environ Technol,
19(9): 783-796.
Johnson BT & Lulves W (1975) Biodegradation in di- n-butyl
phthalate and di-(2-ethylhexyl) phthalate in freshwater
hydrosoil. J Fish Res Board Can, 32(3): 333.
Johnson BT, Stalling DL, Hogan JW, & Schoettger RA (1977)
Dynamics of phthalate acid esters in aquatic organisms. In:
Suffet IH ed. Fate of pollutants in the air and water
environment. Part 2. New York, Wiley Interscience, pp 283-300
(Advances in Environmental Science and Technology, Volume 8).
Johnson BT, Heitkamp MA, & Jones JR (1984) Environmental and
chemical factors influencing the biodegradation of phthalic acid
esters in freshwater sediments. Environ Pollut, B8: 101-118.
JPIF (1995) Production of plastics. Plastics, 46: 104.
Kaneshima H, Yamaguchi T, Okui T, & Naitoh M (1978) Studies on
the effects of phthalate esters on the biological system. Part 2:
In vitro metabolism and biliary excretion of phthalate esters
in rats. Bull Environ Contam Toxicol, 19: 502-509.
Kawano M (1980a) Toxicological studies on phthlate esters. 2.
Metabolism, accumulation and excretion of phthlate esters in
rats. Jpn J Hyg, 35: 693-701.
Kawano M (1980b) Toxicological studies on phthlate esters. 1.
Inhalation effects of dibutyl phthalate (DBP) on rats. Jpn J Hyg,
35: 684-692.
Kawashima Y, Hanioka N, Matsumura M, & Kozuka H (1983) Induction
of microsomal stearoyl-CoA desaturation by the administration of
various peroxisome proliferators. Biochim Biophys Acta, 752:
259-264.
Keith LH, Garrison AW, Allen FR, Carter MH, Floyd TL, Pope JD, &
Thruston AD (1976) Identification of organic compounds in
drinking water from thirteen U.S. cities. In: Keith LH ed.
Identification and analysis of organic pollutants in water. Ann
Arbor, Michigan, Ann Arbor Science Publishers Inc., pp 329-373.
Khaliq MA, Alam MS, & Srivastava SP (1992) Implications of
physico-chemical factors on the migration of phthalate esters
from tubing commonly used for oral/nasal feeding. Bull Environ
Contam Toxicol, 48: 572-578.
Klöpfer W, Kaufmann G, Rippen G, & Poremski HJ (1982) A
laboratory method for testing the volatility from aqueous
solution: first results and comparison with theory. Ecotoxicol
Environ Saf, 6: 545-559.
Kohli J, Ryan JF, & Afghan BK (1989) Phthalate esters in the
aquatic environment. In: Chau BK & Chau ASY ed. Analysis of trace
organics in the aquatic environment. Boca Raton, Florida, CRC
Press Inc., pp 243-281.
Kördel W & Müller J (1992) Occurrence of phthalic acid esters in
soil and plants nearby phthalate-emitting sources and by sewage
sludge treatment. Schmallenberg-Grafshaft, Germany, Fraunhofer
Institute for Environmental Chemistry and Ecotoxicology.
Korhonen A, Hemminki K, & Vanio H (1983) Embryotoxic effects of
phthalic-acid derivatives phosphates and aromatic oils used in
the manufacturing of rubber on 3 day chicken embryos. Drug Chem
Toxicol, 6(2): 191-208.
Kozumbo WJ, Kroll R, & Rubin RJ (1982) Assessment of the
mutagenicity of phthalate esters. Environ Health Perspect, 45:
103-109.
Krauskopf LG (1973) Studies of the toxicity of phthalates via
ingestion. Environ Health Perspect, 3: 61-72.
Kühn R & Pattard M (1990) Results of the harmful effects of water
pollutants to green algae ( Scenedesmus subspicatus) in the
cell multiplication inhibition test. Water Res, 24(1): 31-38.
Kühn R, Pattard M, Pernak, KD, & Winter A (1989) Results of the
harmful effects of water pollutants to Daphnia magna in the 21
day reproduction test. Water Res, 23(4): 501-510.
Kurane R, Suzuki T, & Takahara Y (1979a) Microbial population and
identification of phthalate ester-utilizing microorganisms. Agric
Biol Chem, 43: 907-917.
Kurane R, Suzuki T, & Takara Y (1979b) Microbial degradation of
phthalic esters. Agric Biol Chem, 43(3): 421-427.
Kveseth NJ (1980) Chlorinated hydrocarbons in sewage sludge from
a plant in Oslo. Nord Vet Med, 32: 341-347.
Lake BG, Phillips JC, Linnell JC, & Gangolli SD (1977) The
in vitro hydrolysis of some phthalate diesters by hepatic and
intestinal preparations from various species. Toxicol Appl
Pharmacol, 39: 239-248.
Lake BG, Cook WM, Worrell NR, Cunninghame ME, Evans JG, Price RJ,
Young PJ, & Carpanini FMB (1991) Dose-response relationships for
induction of hepatic peroxisome proliferation and testicular
atrophy by phthalate esters in the rat. Hum Exp Toxicol, 10:
67-68 (Abstract).
Lamb JC, Chapin RE, Teague J, Lawton AD, & Reel JR (1987)
Reproductive effects of four phthalic acid esters in the mouse.
Toxicol Appl Pharmacol, 88(2): 255-269.
Laughlin RB Jr, Neff JM, Hrung YC, Goodwin TC, & Giam CS (1978)
The effects of three phthalate esters on the larval development
of the grass shrimp Palaemonetes pugio (Holthuis). Water Air
Soil Pollut, 9: 323-336.
Law RJ, Fileman TW, & Matthiessen P (1991) Phthalate esters and
other industrial organic chemicals in the North and Irish Seas.
Water Sci Technol, 24: 127-134.
Lawrence WH, Malik M, Turner JE, Singh AR, & Autian J (1975) A
toxicological investigation of some acute, short-term and chronic
effects of administering di(2-ethylhexyl) phthalate (DEHP) and
other phthalate esters. Environ Res, 9: 1-11.
Lehman AJ (1955) Insect repellents. Assoc Food Drug Off, 19:
87-99.
Leibowitz JN, Sarmiento R, Dugar SM, & Ethridge MW (1995)
Determination of six common phthalate plasticisers in grain
neutral spirits and vodka. J Assoc Off Anal Chem Int 78(3):
730-735.
Lesage S (1991) Characterization of groundwater contaminants
using dynamic thermal stripping and adsorption/thermal
desorption-GC-MS. Fresenius J Anal Chem, 339: 516-527.
Lewis RJ (1991) Reproductively active chemicals: A reference
guide. New York, Van Nostrand Reinhold Co.
Leyder F & Boulanger P (1983) Ultraviolet absorption, aqueous
solubility, and octanol-water partition for several phthalates.
Bull Environ Contam Toxicol, 30(2): 152-157.
Ligocki MP & Pankow JF (1985) Assessment of absorption/solvent
extraction with polyurethane foam and adsorption/thermal
desorption with Tenax-GC for the collection and analysis of
ambient organic vapours. Anal Chem, 57: 1138-1144.
Ligocki MP, Leuenberger C, & Pankow JF (1985) Trace organic
compounds in rain-II. Gas scavenging of neutral organic
compounds. Atmos Environ, 19: 1609-1617.
Lindén E, Bengtsson BE, Svanberg O, & Sundström G (1979) The
acute toxicity of 78 chemicals and pesticide formulations against
two brackish water organisms, the bleak ( Alburnus alburnus)
and the harpacticoid ( Nitocra spinipes). Chemosphere,
8(11/12): 843-851.
Litton Bionetics Inc. (1985) Evaluation of 1C in the in vitro
transformation of Balb/3T3 cellsaAssay. Final report (Genetics
assay No. 7156 - LBI Project No. 20992). Kensington, Maryland,
Litton Bionetics Inc. (Submitted by Chemical Manufacturers
Association to US Environmental Protection Agency (EPA document
No. 40+8526206; microfiche No. OTS0509537).
Lokke H & Bro-Rasmussen F (1981) Studies of mobility of di-iso-
butyl phthalate (DiBP), di-n-butyl phthalate (DBP), and di-(2-
ethyl hexyl) phthalate (DEHP) by plant foliage treatment in a
closed terrestrial simulation chamber. Chemosphere, 10(11/12):
1223-1235.
Lunde G, Gether J, GjØs N, & StØbet Lande MB (1977) Organic
micropollutants in precipitation in Norway. Atmos Environ, 11:
1007-1014.
LWA (1993) Report on the quality of the Rhine. Düsseldorf,
Northrhine-Westfalia Office for Water and Waste.
McCarthy JF & Whitmore DK (1985) Chronic toxicity of di-n-butyl
and di-n-octyl phthalate to Daphnia magna and the fathead
minnow. Environ Toxicol Chem, 4: 167-179.
McKone TE & Layton DW (1986) Exposure and risk assessment of
toxic waste in a multimedia context. 79th Annual Meeting of the
Air Pollution Control Association, Minneapolis, Minnesota, 22-27
June 1986. Pittsburgh, Pennsylvania, Air Pollution Control
Association, vol 1, 16 pp.
MAFF (Ministry of Agriculture, Fisheries and Food, United
Kingdom) (1987) Survey of plasticiser levels in food contact
materials and in foods. The 21st report of the Steering Group on
Food Surveillance/The Working Party on Chemical Contaminants from
Food Contact Materials / Sub Group on Plasticisers. London, Her
Majesty's Stationery Office, 105 pp (Food Surveillance Paper
No. 21).
MAFF (Ministry of Agriculture, Fisheries and Food, United
Kingdom) (1990) Plasticizers: Continuing surveillance. The 31st
report of the Steering Group on Food Surveillance/The Working
Party on Chemical Contaminants from Food Contact Materials / Sub
Group on Plasticisers. London, Her Majesty's Stationery Office,
53 pp (Food Surveillance Paper No. 30).
Malisch R, Schulte E, & Acker L (1981) [Organochlorine
pesticides, polychlorinated biphenyls and phthalate in Rhine and
Neckar sediments.] Chem Ztg, 105: 187-194 (in German).
Mathur SP (1974) Phthalate esters in the environment: pollutants
or natural products? J Environ Qual, 3(3): 189-197.
Matsuda K & Schnitzer M (1971) Reactions between fulvic acid, a
soil humic material, and dialkyl phthalates. Bull Environ Contam
Toxicol, 6(3): 200-204.
Mayer FL Jr & Sanders HO (1973) Toxicology of phthalic acid
esters in aquatic organisms. Environ Health Perspect, 3:
153-157.
Mayer FL & and Ellersieck MR (1986) Manual of acute toxicity:
interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the
Interior, Fish and Wildlife Service (Resource Publication 160;
NTIS Publication No. PB86-239878).
Melin C & Egnéus H (1983) Effects of di- n-octyl phthalate on
growth and photosynthesis in algae and on isolated organelles
from higher plants. Physiol Plant, 59: 461-466.
Men'shikova TA (1971) Hygienic evaluation of dibutyl phthlate in
relation to the use of polymer finishes in shipboard living
quarters. Hyg Sanit, 36: 349-353.
Mes J, Coffin DE, & Campbell DS (1974) Di-n-butyl- and di-2-
ethylhexyl phthalate in human adipose tissue. Bull Environ Contam
Toxicol, 12(6): 721-725.
Milkov LE, Aldyrova MV, Popova TB, Lopukhova KA, Makarenko YL,
Malyar LM, & Shakhova TK (1973) Health status of workers exposed
to phthalate plasticizers in the manufacture of artificial
leather and films based on PVC resins. Environ Health Perspect,
3: 175-178.
Millar DJ & Hannay JW (1986) Phytotoxicity of phthalate
plasticisers. 2. Site and mode of action. J Exp Bot, 37(179):
898-908.
MOE (Ontario Ministry of the Environment) (1984) Drinking water
survey of selected municipalities in the Niagara area and Lake
Ontario. Ottawa, Ontario, Ontario Ministry of the Environment.
Montgomery JH & Welkom LM (1990) Groundwater chemicals desk
reference. Chelsea, Michigan, Lewis Publishers Inc.
Murakami K, Nishiyama K, & Higuti T (1986a) Toxicity of dibutyl
phthalate and its metabolites in rats. Jpn J Hyg, 41(4):
775-780.
Murakami K, Nishiyama K, & Higuti T (1986b) Mitochrondrial effect
of orally administered dibutyl phthalate in rats. Jpn J Hyg,
41(4): 769-774.
Nagy KA (1987) Field metabolic rate and food requirement scaling
in mammals and birds. Ecol Monogr, 57(2): 111-128.
NAQUADAT (1993) National water quality data bank. Ottawa,
Ontario, Environment Canada, Inland Waters Directorate, Water
Quality Branch.
Nerin C, Cacho J, & Gancedo P (1993) Plasticizers from printing
inks in a selection of food packagings and their migration to
food. Food Addit Contam, 10(4): 453-460.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR
(1985) The toxicity of selected organic chemicals to the
earthworm Eisenia fetida. J Environ Qual, 14(3): 383-388.
Neuhauser EF, Loehr RC, & Malecki MR (1986) Contact and
artificial soil tests using earthworms to evaluate the impact of
wastes in soil. In: Petros JK, Lacy WJ, & Conway RA ed. Fourth
Symposium on Hazardous and Industrial Solid Waste Testing.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 192-203 (ASTM STP 886).
Niagara River Data Interpretation Group (1990) Joint evaluation
of upstream/downstream Niagara River monitoring data, 1988-1989.
Prepared by Data Interpretation Group, River Monitoring
Committee. Joint publication of Environment Canada, US
Environmental Protection Agency, Ontario Ministry of the
Environment, and New York State Department of Environmental
Conservation.
Nikonorow M, Mazur H, & Piekacz H (1973) Effect of orally
administered plasticizers and polyvinyl chloride stabilizers in
the rat. Toxicol Appl Pharmacol, 26: 253-259.
NIOSH (1976) Health hazard evaluation determination report
No. 74-120-260: Goodyear Tire and Rubber Company, Gadsden,
Alabama. Cincinnati, Ohio, National Institute for Occupational
Safety and Health (NTIS Publication No. PB89-166078).
NIOSH (1982) Health hazard evaluation report No. HETA-81-277-
1089: Indiana Army Ammunition Plant, Charlestown, Indiana.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (NTIS Publication No. PB83-198697).
NIOSH (1987) Health hazard evaluation report No. MHETA 86-191-
1836: West Virginia Department of Highways, Charleston, West
Virginia. Cincinnati, Ohio, National Institute for Occupational
Safety and Health (NTIS Publication No. PB88-162698).
NIOSH (1989) Health hazard evaluation report No. MHETA 88-214-
1952: Flying W Plastics Company, Glenville, West Virginia.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (NTIS Publication No. PB89-230544).
NTP (1984) Di(n-butyl) phthalate: Reproduction and fertility
assessment in CD-1 mice when administered in the feed. Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program, 100 pp (NTIS Publication
No. PB85-144798).
NTP(1991) Final report on the reproductive toxicity of di-n-butyl
phthalate (CAS No. 84-74-2) in Sprague-Dawley rats. Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program (Report No. T-0035C; NTIS
Publication No. PB92-111996).
NTP (1994a) Chairperson's report. Pathology Working Group (PWG)
review - Dibutylphthalate (C62022B) and (C62022): A 13-week
subchronic toxicity study (Phases II and III) administered by
dose feed in F344 rats and B6C3F1 mice conducted at Battelle
Columbus. Research Triangle Park, North Carolina, US Department
of Health and Human Services, National Toxicology
Program/Environmental Toxicology Program.
NTP (1994b) Prechronic study, dibutyl phthalate diet study in
mice. Research Triangle Park, North Carolina, US Department of
Health and Human Services, National Toxicology
Program/Environmental Toxicology Program.
NTP (1995) NTP technical report on toxicity studies of dibutyl
phthalate (CAS No. 84-74-2) aministered in feed to F344/N rats
and B6C3F1 mice. Research Triangle Park, North Carolina, US
Department of Health and Human Services, National Toxicology
Program (Toxicity Series No. 30).
O'Connor OA, Rivera MD, & Young LY (1989) Toxicity and
biodegradation of phthalic acid esters under methanogenic
conditions. Environ Toxicol Chem, 8: 569-574.
Ogura N, Ambe Y, Ogura K, Ishiwatari R, Mizutani T, Satoh Y,
Matsushima H, Katase T, Ochiai M, Tadokoro K, Takada T, Sugihara
K, Matsumoto G, Nakamoto N, Funakoshi M, & Hanya T (1975)
Chemical composition of organic compounds present in water of the
Tamagawa River. Jpn J Limnol, 36: 23-30.
Oishi S & Hiraga K (1980a) Testicular atrophy induced by phthalic
acid esters. Effect on testosterone and zinc concentrations.
Toxicol Appl Pharmacol, 53: 35-41.
Oishi S & Hiraga K (1980b) Effect of phthalic acid esters on
mouse testes. Toxicol Lett, 5: 413-416.
Oishi S & Hiraga K (1980c) Testicular atrophy induced by phthalic
acid monoesters: Effects of zinc and testosterone concentrations.
Toxicology, 15: 197-202.
Oishi S & Hiraga K (1980d) Effects of phthalic acid monoesters on
mouse testes. Toxicol Lett, 6: 239-242.
Oishi S & Hiraga K (1982) Effects of monoesters of o-phthalic
acid on serum lipid composition of rats. Toxicol Lett, 14:
79-84.
Ota H, Takashima K, Takashima Y, Onda H, Kodama H, & Yamada N
(1973) Biological effects of phthalate esters. (I)
Histopathological findings from experiments in mice. Nippon
Byorigakkai Kaishi, 62: 119-120.
Ota H, Onda H, Kodama H, & Yamada N (1974) Histopathological
studies on the effect of phthalic acid esters on the biological
system of mice. Nippon Eiseigaku Kaishi, 29: 519-524.
Otson R & Benoit FM (1985) Surveys of selected organics in
residential air. In: Walkinshaw DS ed. Indoor air quality in
cold climates: An Air Pollution Control Association speciality
conference. Pittsburgh, Pennsylvania, Air Pollution Control
Association, pp 224-236.
Overcash MR, Weber JB, & Miles ML (1982) Behavior of organic
priority pollutants in the terrestrial system: di-n-butyl
phthalate ester, toluene, and 2,4-dinitrophenol. Raleign, North
Carolina, University of North Carolina, Water Resources Research
Institute (Report No. 171).
PACE (Petroleum Association for the Conservation of the Canadian
Environment) (1985) Evaluation of the variability of trace
organic substances in petroleum refinery effluents. Ottawa,
Ontario, Canadian Petroleum Products Institute (PACE Report
No. 85-6).
Page BD & Lacroix GM (1992) Studies into the transfer and
migration of phthalate esters from aluminum foil-paper laminates
to butter and margarine. Food Addit Contam, 9(3): 197-212.
Page BD & Lacroix GM (1995) The occurrence of phthalate ester and
di-2-ethylhexyl adipate plasticizers in Canadian packaging and
food sampled in 1985-1989: a survey. Food Addit Contam, 12(1):
129-151.
Pankow JF, Ligocki MP, & Rosen ME (1988) Adsorption/thermal
desorption with small cartridges for the determination of trace
aqueous semivolatile organic compounds. Anal Chem, 60: 40-47.
Parkman H & Remberger M (1995) Phthalates in Swedish sediments.
Stockholm, Swedish Environmental Research Institute (IVL-Report).
Peakall DB (1974) Effects of di-n-butyl and di-2-ethylhexyl
phthalate on the eggs of ring doves. Bull Environ Contam Toxicol,
12: 698-702.
Peakall DB (1975) Phthalate esters: occurrence and biological
effects. Residue Rev, 54: 1-41.
Perwak J, Goyer M, & Schimke G (1981) An exposure and risk
assessment for phthalate esters: Di(2-ethylhexyl) phthalate, di-
n-butyl phthalate, dimethyl phthalate, diethyl phthalate, di-n-
octyl phthalate, butyl benzyl phthalate. Washington, DC, US
Environmental Protection Agency, Office of Water Regulations and
Standards (EPA-440/4-81-020;NTIS Publication No. PB85-211936).
Peters JW & Cook RM (1973) Effect of phthalate esters on
reproduction in rats. Environ Health Perspect, 3: 91-94.
Peterson JC & Freeman DH (1984) Variations of phthalate ester
concentrations in sediments from the Chester River, Maryland. Int
J Environ Anal Chem, 18(4): 237-252.
Pierce RC, Mathur SP, Williams DT, & Boddington MJ (1980)
Phthalate esters in the aquatic environment. Ottawa, Ontario,
National Research Council of Canada, Associate Committee on
Scientific Criteria for Environmental Quality (Report No. NRCC
17583).
Poole CF & Vibberley DG (1977) Determination of DEHP ih human
placenta. J Chromatogr, 132: 511.
Radeva M & Dinoyeva S (1966) [The toxicity of the plasticiser
dibutyl phthlate with oral administration to white rats.] Khig i
Zdrav, 9: 510-515 (in Russian).
Ray LE, Murray HE, & Giam CS (1983a) Analysis of water and
sediment from the Nueces Estuary/Corpus Christi Bay (Texas) for
selected organic pollutants. Chemosphere, 12(7/8): 1039-1045.
Ray LE, Murray HE, & Giam CS 1983b) Organic pollutants in marine
samples from Portland, Maine. Chemosphere, 12(7/8): 1031-1038.
RIWA (1991) Annual Report of the Working Party on the Rhine and
the Maas. Part A: The Rhine. Amsterdam, National Institute for
Water and Waste (RIWA).
RIWA (1992) Annual Report of the Working Party on the Rhine and
the Maas. Part A: The Rhine. Amsterdam, National Institute for
Water and Waste (RIWA).
Rogers IH & Hall KJ (1987) Chlorophenols and chlorinated
hydrocarbons in starry flounder ( Platichthys stellatus) and
contaminants in estuarine sediments near a large municipal
outfall. Water Pollut Res J Can, 22(2): 197-210.
Rogers IH, Birtell IK, & Kruzynski GM (1986) Organic extractables
in municipal wastewater, Vancouver, British Columbia. Water
Pollut Res J Can, 21(2): 187-204.
Rowland IR, Cottrell RC, & Phillips JC (1977) Hydrolysis of
phthalate esters by the gastro-intestinal contents of the rat.
Food Cosmet Toxicol, 15: 17-21.
Saito K, Nakazato M, Ishikawa F, Fujinuma K, Moriyasu T, Nagayama
T, Kobayashi M, Shioda H, & Kamimura K (1993) [Determination of
methyl isocyanate in wine and dibutyl phthalate in vodka.] Kenkyu
Nempo Tokyo-Toritsu Eisei Kenkyusho, 44: 119-127 (in Japanese).
Sajiki J (1975a) [Pathological observations of phthalate ester
(di- n-butyl phthalate) for mice with orally administrations.]
Chibaken Eiseikenkyusho Nenpo, 24: 57-63 (in Japanese).
Sajiki J (1975b) Biological effects of phthalate ester (di- n-
butyl phthalate) orally administered in mice.] Chibaken
Eiseikenkyusho Nenpo, 24: 64-72 (in Japanese).
Sandmeyer EE & Kirwin CJ (1981) Esters. In: Clayton GD & Clayton
FE ed. Patty's industrial hygiene and toxicology. Volume 2A:
Toxicology, 3rd rev ed. New York, John Wiley and Sons Inc.,
pp 2345-2346.
Sato H, Sato N, & Ichihara N (1975) Rec-assay application for
mutagenicity testing of phthalic acid esters. Hokkaido-ritsu
Eisei Kenkyushcho, 1975: 146-147.
Schacht RA (1974) Pesticides in the Illinois waters of Lake
Michigan. Washington, DC, US Environmental Protection Agency,
Office of Research and Development (EPA 660/3-74-002).
Schleyer R, Renner I, & Mühlhausen D (1991) [Immission load -
Consequences for grounwater quality.] Bonn, Germany, Federal
Ministry for Environment, Nature Conservation and Nuclear Safety
(in German).
Schouten MJ, Copius Peereboom JW, & Brinkman UA Th (1979) Liquid
chromatographic analysis of phthalate esters in Dutch river
water. Int J Environ Anal Chem, 7: 13-23.
Schwartz HE, Anzion CJM, Van Vliets HPM, Copius Peerbooms JW, &
UATh Brinkman (1979) Analysis of phthalate esters in sediments
from Dutch rivers by means of high performance liquid
chromatography. Int J Environ Anal Chem, 6: 133-144.
Scott RC, Dugard PH, Ramsey JD, & Rhodes C (1987) In vitro
absorption of some o-phthalate diesters through human and rat
skin. Environ Health Perspect, 74: 223-227.
Seed JL (1982) Mutagenic activity of phthalate esters in
bacterial liquid suspension assays. Environ Health Perspect,
45: 111-114.
Seth PK, Agarwal DK, & Agarwal S (1981) Effect of phthalic acid
esters on drug metabolizing enzymes. Bull Environ Contam Toxicol,
26: 764-768.
Shahin MM & von Borstel RC (1977) Mutagenic and lethal effects of
alpha-dibutyl phthalate and trichloroethylene in Saccharomyces
cerevisiae. Mutat Res, 48: 173-180.
Shanker R, Ramakrishna C, & Seth PK (1985) Degradation of some
phthalic acid esters in soil. Environ Pollut, A39: 1-7.
Shea KP (1971) The new-car smell. Environment, 13(8): 2-9.
Shea PJ, Weber JB, & Overcash MR (1982) Uptake and phytotoxicity
of di- n-butyl phthalate in corn ( Zea mays). Bull Environ
Contam Toxicol, 29: 153-158.
Sheldon LS & Hites RA (1978) Organic compounds in the Delaware
River. Environ Sci Technol, 12(10): 1188-1194.
Sheldon LS & Hites RA (1979) Sources and movement of organic
chemicals in the Delaware River. Environ Sci Technol, 13(5):
574-579.
Shelton DR, Boyd SA, & Tiedje JM (1984) Anaerobic biodegradation
of phthalic acid esters in sludge. Environ Sci Technol, 18(2):
93-97.
Shibuya S (1979) Phthalic acid esters as one of the marker
environmental pollutants - occurrence in the water and aquatic
environment in Shizuoka Prefecture. Numazu Kogyo Kota Semmon
Gakko Kenkyu Hokoku, 14: 63-72.
Shiota K & Nishimura H (1982) Teratogenicity of di(2-ethylhexyl)
phthalate (DEHP) and di-n-butyl phthalate (DBP) in mice. Environ
Health Perspect, 45: 65-70.
Shiota K, Chou MJ, & Nishimura H (1980) Embryotoxic effects of
di(2-ethylhexyl)phthalate (DEHP) and di-n-butyl phthalate (DBP)
in mice. Environ Res, 22: 245-253.
Singh AR, Lawrence WH, & Autian J (1972) Teratogenicity of
phthalate esters in rats. J Pharm Sci, 61(1): 51-55.
Smith CC (1953) Toxicity of butyl stearate, dibutyl sebacate,
dibutyl phthalate and methoxyethyl oleate. Ind Hyg Occup Med,
7: 310-318.
Sneddon IB (1972) Dermatitis from dibutyl phthalate in an aerosol
antiperspirant and deodorant. Contact Dermatitis, 1972: 308.
Spasovski M (1964) Experimental data to establish a maximum
allowable concentration for dibutyl phthalate. Higiena, 7:
38-44.
Springborn Bionomics (1984a) Toxicity of fourteen phthalate
esters to the freshwater green algae, Selenastrum
capricornutum. Wareham, Massachusetts, Springborn Bionomics, 52
pp (Toxicity test report submitted to the Chemical Manufacturers
Association, Washington, DC).
Springborn Bionomics (1984b) Chronic toxicity of fourteen
phthalate esters to Daphnia magna. Wareham, Massachusetts,
Springborn Bionomics (Toxicity test report No. BW-84-5-1567
submitted to the Chemical Manufacturers Association, Washington,
DC).
Srivastava SP, Srivastava S, Saxena DK, Chandra SV, & Seth PK
(1990a) Testicular effects of di-n-butyl phthalate (DBP):
Biochemical and histopathological alterations. Arch Toxicol,
64(2): 148-152.
Srivastava S, Singh GB, Srivastava SP, & Seth PK (1990b)
Testicular toxicity of di-n-butyl phthalate in adult rats: Effect
on marker enzymes of spermatogenesis. Ind J Exp Biol, 28(1):
67-70.
Stalling DL, Hogan JW, & Johnson JL (1973) Phthalate ester
residues - their metabolism and analysis in fish. Environ Health
Perspect, 3: 159-173.
Stanley JS (1986) Broad scan analysis of the FY82 national human
adipose tissue survey specimens - Volume I: Executive summary.
Washington, DC, US Environmental Protection Agency, Office of
Toxic Substances (EPA 560/5-86-035).
Streufert JM & Sanders HO (1977) Chronic effects of two phthalic
acid esters on midge Chironomus plumosus. TransMo Acad Sci,
10/11: 297.
Streufert JM, Jones JR, & Sanders HO (1980) Toxicity and
biological effects of phthalate esters on midges ( Chironomus
plumosus). TransMo Acad Sci, 14: 33-40.
Sugatt RH, O'Grady DP, Banerjee S, Howard PH, & Gledhill WE
(1984) Shake flask biodegradation of 14 commercial phthalate
esters. Appl Environ Microbiol, 47: 601-606.
Sugawara N (1974a) Toxic effect of a normal series of phthalate
esters on the hatching of shrimp eggs. Toxicol Appl Pharmacol,
30(1): 87-89.
Sugawara N (1974b) Effect of phthalate esters on shrimp. Bull
Environ Contam Toxicol, 12(4): 421-424.
Sullivan KF, Atlas EL, & Giam CS (1982) Adsorption of phthalic
acid esters from seawater. Environ Sci Technol, 16: 428-432.
Swain WR (1978) Chlorinated organic residues in fish, water, and
precipitation from the vicinity of Isle Royale, Lake Superior. J
Great Lakes Res, 4: 398-407.
Swain LG & Walton DG (1989) Report on the 1988 Fish Monitoring
Program. British Columbia, Ministry of the Environment.
Swartz RC, Schults DW, Ditsworth GR, DeBen WA, & Cole FA (1985)
Sediment toxicity, contamination, and macrobenthic communities
near a large sewage outfall. In: Boyle TD ed. Validation and
predictabolity of laboratory methods for assessing the fate and
effects of contaminants in aquatic ecosystems. Philadelphia,
Pennsylvania, American Society for Testing and Materials,
pp 152-175 (ASTM STP 865).
Tagatz ME, Deans CH, Moore JC, & Plaia GR (1983) Alterations in
composition of field- and laboratory-developed estuarine benthic
communities exposed to di-n-butyl phthalate. Aquatic Toxicol,
3: 239-248.
Tagatz ME, Plaia GR, & Deans CH (1986) Toxicity of dibutyl
phthalate-contaminated sediment to laboratory-and field-colonized
estuarine benthic communities. Bull Environ Contam Toxicol,
37(1): 141-149.
Takahashi T & Tanaka A (1989) Biochemical studies on phthalic
esters.V. Comparative studies on in vitro hydrolysis of di-n-
butyl phthalate isomers in rats. Arch Toxicol, 63: 72-74.
Tanaka A, Matsumoto A, & Yamaha T (1978) Biochemical studies on
phthalic esters. III. Metabolism of dibutyl phthalate (DBP) in
animals. Toxicology, 9: 109-123.
Tanino M, Ikemoto I, & Tanaka A (1987) Enzyme levels in rat
testis damaged experimentally with dibutyl phthalate. Jikeikai
Med J, 34: 245-252.
Tarkpea M, Hansson M, & Samuelsson B (1986) Comparison of the
Microtox test with the 96-hr LC50 test for the harpacticoid
Nitocra spinipes. Ecotoxicol Environ Saf, 11: 127-143.
Tetra Tech Inc. (1986) Development of sediment quality values for
puget sound. Bellevue, Washington, Tetra Tech Inc., vol 1,
128 pp.
Thurén A (1986) Determination of phthalates in aquatic
environments. Bull Environ Contam Toxciol, 36(1): 33-40.
Thurén A & Larsson P (1990) Phthalate esters in the Swedish
atmosphere. Environ Sci Technol, 24(4): 554-559.
Thurén A & Woin P (1991) Effects of phthalate esters on the
locomotor activity of the freshwater amphipod Gammarus pulex.
Bull Environ Contam Toxicol, 46(1): 159-166.
Tomita I, Nakamura Y, & Yagi Y (1977) Phthalic acid esters in
various foodstuffs and biological materials. Ecotoxicol Environ
Saf, 1: 275-287.
Tsuchiya K & Hattori K (1976) [Chromosomal study on human
leucocyte cultures treated with phthalate acid ester.] Japan,
Hokkaido Institute of Public Health (in Japanese).
US EPA (1981) An exposure and risk assessment for phthalate
esters. Washington, DC, US Environmental Protection Agency,
Office of Water Regulations and Standards (EPA 440/4-81-020; NTIS
Publication No. PB85-211936).
US EPA (1982a) Aquatic fate process data for organic priority
pollutants. Washington, DC, US Environmental Protection Agency
(EPA 440/4-81-014; NTIS Publication No. PB87-169090).
US EPA (1982b) Methods for organic chemical analysis of municipal
and industrial wastewater. Cincinnati, Ohio, US Environmental
Protection Agency, Environmental Monitoring and Support
Laboratory (EPA 600/4-82-057; NTIS Publication No. PB83-201798).
US EPA (1986a) Method 8060: Phthalate esters. In: Test methods
for evaluating solid wastes, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and
Emergency Response.
US EPA (1986b) Method 8250: Gas chromatography/mass spectrometry
for semivolatile organics - packed column technique. In: Test
methods for evaluating solid wastes, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and
Emergency Response.
US EPA (1986c) Method 8270: Gas chromatography/mass spectrometry
for semivolatile organics - capillary column technique. In: Test
methods for evaluating solid wastes, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and
Emergency Response.
US EPA (1986d) Method 8410: Capillary column analysis of
semivolatile organic compounds by gas chromatography/fourier
transform infrared (GC/FT-IR) spectrometry. In: Test methods for
evaluating solid wastes, 3rd ed. Washington, DC, US Environmental
Protection Agency, Office of Solid Waste and Emergency Response.
Virgin HI (1988) Accumulation of di-n-butylphthalate in plants
and its effect on pigment and protein content. Physiol Plant,
72(1): 190-196.
Von Westernhagen H, Landolt M, Kocan R, Fürstenberg G, Janssen
D, & Kremling K (1987) Toxicity of sea-surface microlayer:
effects on herring and turbot embryos. Mar Environ Res, 23:
273-290.
Waldock MJ (1983) Determination of phthalate esters in samples
from the marine environment using gas chromatography mass
spectrometry. Chem Ecol, 1: 261-277.
Walker WW, Cripe CR, Pritchard PH, & Bourquin AW (1984) Dibutyl-
phthalate degradation in estuarine and freshwater sites.
Chemosphere, 13(12): 1283-1294.
Walseth F & Nilsen OG (1984) Phthalate esters. II. Effects of
inhaled dibutylphthalate on cytochrome P-450 mediated metabolism
in rat liver and lung. Arch Toxicol, 55: 132-136.
Walseth F & Nilsen OG (1986) Phthalate esters: Effects of orally
administered dibutylphthalate on cytochrome P-450 mediated
metabolism in rat liver and lung. Acta Pharmacol Toxicol, 59:
263-269.
Walseth F, Toftgard R, & Nilsen OG (1982) Phthalate esters I:
Effects on cytochrome P-450 mediated metabolism in rat liver and
lung, serum enzymatic activities and serum protein levels.
Arch Toxicol, 50: 1-10.
Wang JL, Liu P, & Qian Y (1995) Microbial degradation of di-n-
butyl phthalate. Chemosphere, 31(9): 4051-4059.
Ward TJ & Boeri RL (1991) Early life stage toxicity of di-n-butyl
phthalate (DnBP) to the rainbow trout ( Oncorhynchus mykiss)
under flow-through conditions. Hampton, New Hampshire, Resource
Analysts, Inc., EnviroSystems Division (Toxicity report submitted
to the Chemical Manufacturers Association, Washington, DC).
Webber MD & Lesage S (1989) Organic contaminants in Canadian
municipal sludges. Waste Manage Res, 7: 63-82.
Weschler C (1981) Identification of selected organics in the
Arctic aerosol. Atmos Environ, 15(8): 1365-1369.
White RD, Carter DE, Earnest D, & Mueller J (1980) bsorption and
metabolism of three phthalate diesters by the rat small
intestine. Food Cosmet Toxicol, 18: 383-386.
White RD, Earnest DL, & Carter DE (1983) The effect of intestinal
esterase inhibition on the in vivo absorption and toxicity of
di-n-butyl phthalate. Food Cosmet Toxicol, 21: 99-101.
Williams DT (1973) Dibutyl- and di-(2-ethylhexyl)phthalate in
fish. J Agric Food Chem, 21(6): 1128-1129.
Williams DT & Blanchfield BJ (1975) The retention, distribution,
excretion and metabolism of dibutyl phthalate-7-14C in the rat.
J Agric Food Chem, 23(5): 854-858.
Wilson WB, Giam CS, Goodwin TE, Aldrich A, Carpenter V, & Hrung
YC (1978) The toxicity of phthalates to the marine dinoflagellate
Gymnodinium breve. Bull Environ Contam Toxicol, 20(2):
149-154.
Wilson LG, Osborn MD, Olson KL, Maida SM, & Katz LT (1990) The
ground water recharge and pollution potential of dry wells in
Pima County, Arizona. Ground Water Monit Rev, 10: 114-121.
Wine RN, Li LH, Barnes LH, Gulati OK, & Chapin RE (1977)
Reproductive toxicity of di- n-butylphthalate in a continuous
breeding protocol in SD rats. Environ Hlth Persp, 105(1):
102-107.
Wofford HW, Wilsey CD, Neff GS, Giam CS, & Neff JM (1981)
Bioaccumulation and metabolism of phthalate esters by oysters,
brown shrimp, and sheepshead minnows. Ecotoxicol Environ Saf,
5: 202-210.
Wolfe NL, Burns LA, & Steen WC (1980) Use of linear free energy
relationships and an evaluative model to assess the fate and
transport of phthalate esters in the aquatic environment.
Chemosphere, 9: 393-402.
Woodward KN (1988) Phthalate esters: Toxicity and metabolism,
Volumes I and II. Boca Raton, Florida, CRC Press Inc.
Woodward KN (1990) Phthalate esters, cystic kidney disease in
animals and possible effects on human health: A review. Hum Exp
Toxicol, 9: 397-401.
Wu C, Pei X, Cao J, & Xue H (1993) A new pharmacological activity
of dibutyl phthalate (DBP) on selective elimination of tumor
cells from bone marrow. Leuk Res, 17(7): 557-560.
Yagi Y, Tutikawa K, & Shimoi N (1976) Teratogenicity and
mutagenicity of a phthalate ester. Teratology, 14: 259-260.
Yagi Y, Nakamura Y, Tomita I, Tutikawa K, & Shimoi N (1978)
Embryotoxicity of phthalate esters in mice. In: Plaa GL & Duncan
WAM ed. Proceedings of the First International Congress on
Toxicology. New York, London, San Francisco, Academic Press, pp
590-591.
Yamada A (1974) [Toxicity of phthalic acid esters and
hepatotoxicity of di-(2-ehtyl hexyl) phthalate.] J Food Hyg Soc
Jpn, 15: 147-152 (in Japanese, with English summary).
Yan H, Ye C, & Yin C (1995) Kinetics of phthalate ester
biodegradation by Chlorella pyrenoidosa. Environ Toxicol Chem,
14(6): 931-938.
Yoshikawa K, Tanaka A, Yamada T, & Kurata H (1983) Mutagenicity
study of nine monoalkyl phthalates and a dialkyl phthalate using
Salmonella typhimurium and Escherichia coli. Food Chem
Toxicol, 21(2): 221-223.
Yoshioka Y, Ose Y, & Sato T (1985) Testing for the toxicity of
chemicals with Tetrahymena pyriformis. Sci Total Environ,
43(1/2): 149-157.
Yoshioka Y, Ose Y, & Sato T (1986) Correlation of the five test
methods to assess chemical toxicity and relation to physical
properties. Ecotoxicol Environ Saf, 12:15-21.
Zeiger E, Haworth S, Speck W, & Mortelmans K (1982) Phthalate
ester testing in the National Toxicology Program's environmental
mutagenesis test development program. Environ Health Perspect,
45: 99-101.
Zeiger E, Haworth S, Mortelmans K, & Speck W (1985) Mutagenicity
testing of di(2-ethylhexyl)phthalate and related chemicals in
Salmonella. Environ Mutagen, 7: 213-232.
Zhou Y, Fukuoka M, & Tanaka A (1990) Mechanisms of testicular
atrophy induced by di- n-butyl phthalate in rats. Part 3.
Changes in the activity of some enzymes in the Sertoli and germ
cells, and in the levels of metal ions. J Appl Toxicol, 10(6):
447-453.
Ziogou K, Kirk PWW, & Lester JN (1989) Behaviour of phthalic acid
esters during batch anaerobic digestion of sludge. Water Res,
23(6): 743-748.
Zitko V (1972) Determination, fate, and environmental levels of
phthalate plasticizers. St. Andrews, N.B., Fisheries Research
Board of Canada, Biological Station (Technical Report No. 344).
Zurmühl T (1990) Development of a method for the determination of
phthalate esters in sewage sludge including chromatographic
separation from polychlorinated biphenyls, pesticides and
polyaromatic hydrocarbons. Analyst, 115: 117.
Zurmühl T, Durner W, & Herrmann R (1991) Transport of phthalate-
esters in undisturbed and unsaturated soil columns. J Contam
Hydrol, 8: 111-133.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
Le phtalate de di- n-butyle (DBP) est un liquide inerte,
incolore et de consistance huileuse, qui présente une faible
tension de vapeur. Soluble dans la plupart des solvants
organiques, il n'est que légérement soluble dans l'eau.
L'analyse la plus sélective et la plus sensible des prélèvements
effectués dans l'environnement pour la recherche et le dosage du
DBP et, plus généralement, des esters phtaliques, se fait par
chromatographie en phase gazeuse avec détection par capture
d'électrons ou spectrométrie de masse. Etant donné que des
phtalates peuvent être présents sous la forme de plastifiants
dans certaines pièces des instruments de mesure ou
encore contaminer l'air du laboratoire, il faudra veiller tout
particulièrement à éviter la contamination lors du prélèvement,
de la conservation et de l'analyse des échantillons.
Le DBP est principalement utilisé comme plastifiant de la
nitrocellulose, de l'acétate et du chlorure de polyvinyle, comme
lubrifiant des buses de bombes aérosol, comme agent antimoussant,
comme adoucissant de la peau, comme plastifiant dans le vernis à
ongles et les faux ongles ou encore dans les aérosols
capillaires. Le dosage du DBP présent dans l'atmosphère
s'effectue en phase vapeur ou particulaire. On pense que, pour
une part non négligeable, le DBP est évacué de l'atmosphère par
les précipitations ou par dépôt à sec. Dans les eaux de surface,
la majeure partie du DBP est présente dans la phase liquide
plutôt que dans les solides en suspension. Il ne semble pas que
le composé puisse s'évaporer du sol en quantités appréciables en
raison de sa faible tension de vapeur et du fait qu'il est
modérément adsorbé sur les particules du sol.
La persistance du DBP dans l'air et les eaux de surface est
relativement faible et sa demi-vie dans ces compartiments du
milieu n'est que de quelques jours. Il est rapidement biodégradé
en aérobiose et beaucoup plus lentement en anaérobiose. On a
estimé que sa demi-vie dans le sol était du même ordre que dans
l'air et l'eau, mais il semble, selon certaines études, que le
DBP persiste plus longtemps dans le sol. On peut s'attendre à
une bioaccumulation importante du fait de la valeur élevée du
coefficient de partage entre l'octanol et l'eau. Toutefois, sa
métabolisation est rapide chez les poissons, aussi le facteur de
bioconcentration a-t-il tendance à être plus faible que prévu.
La valeur la plus élevée (relativement au composé initial), à
savoir 590, a été observée chez un cyprinidé d'Amérique du Nord,
Pimephales promelas. Chez les animaux terrestres, il est peu
probable qu'il y ait une bioamplification notable, à en juger
d'après quelques données concernant les oiseaux et compte tenu du
fait que la métabolisation et l'excrétion sont rapides chez les
mammifères de laboratoire.
Il n'est pas possible d'apprécier dans quelle mesure les
anciennes données de surveillance peuvent être considérées comme
fiables car, dans la littérature antérieure à 1980, on ne trouve
que rarement mention des dispositions prises pour éviter la
contamination des échantillons prélevés dans l'environnement.
Les données limitées dont on dispose au sujet des concentrations
dans l'air ambiant indiquent que les valeurs moyennes sont
généralement inférieures à 5 ng/m3. Des études récentes ont
montré que dans l'eau de pluie, la concentration moyenne allait
de 0,2 à 1,4 µg/litre; des valeurs beaucoup plus faibles ont été
mesurées dans des régions reculées. Dans les eaux de surface, la
concentration a tendance à être inférieure à 1 µg/litre;
cependant on a relevé des valeurs beaucoup plus élevées dans des
cours d'eau pollués (12 à 34 µg/litre). On ne possède que
quelques données sur la concentration dans les eaux souterraines,
les valeurs se situant entre 0,15 et 0,46 µg/litre. Dans les
effluents, la concentration du DBP peut aller jusqu'à 100
µg/litre; elle varie de 0,2 à 200 µg/kg de poids sec dans les
boues résiduaires. Dans les sédiments, la concentration est en
général inférieure à 1 mg/kg de poids sec; toutefois, dans les
zones polluées, on a mesuré des concentrations pouvant aller
jusqu'à 20 mg/kg. Selon les études portant sur la faune et la
flore aquatiques, les concentrations auraient tendance à se
situer à moins de 0,2 mg/kg de poids humide; néanmoins, des
valeurs atteignant 35 mg/kg ont été relevées dans des secteurs
pollués.
Lors d'une enquête menée en Californie sur plus de 125
résidences au cours de l'année 1990, on a relevé une
concentration diurne moyenne de 420 ng/m3 dans l'air intérieur.
Quelques données canadiennes indiquent que la présence de DBP
dans l'eau de boisson est plutôt rare et que sa concentration est
inférieure à 1,0 µg/litre. A Toronto, l'analyse d'un petit
nombre d'échantillons a montré que la concentration du DBP y
était de 14 ng/litre; on en a trouvé de 21 à 55 ng/litre dans des
échantillons de plusieurs marques d'eau minérale en bouteille.
Du DBP peut pénétrer dans les aliments par suite d'une
contamination de l'environnement, mais la présence de ce composé
dans une denrée alimentaire peut également être due à la
migration du DBP de l'emballage vers son contenu. Ce problème a
été étudié à plusieurs reprises vers la fin des années 80. Dans
de nombreux pays, des précautions ont été prises pour réduire le
passage, par lixiviation, des plastifiants de l'emballage dans le
produit alimentaire. Ces mesures ont eu pour effet de réduire peu
à peu la teneur des aliments en DBP. En 1986, on a effectué à
Halifax une enquête sur le panier de la ménagère au cours de
laquelle 98 produits alimentaires ont été étudiés. La présence
de DBP a été décelée dans du beurre (1,5 µg/g), du poisson d'eau
douce (0,5 µg/g), des produits céréaliers (de non décelable à
0,62 µg/g), des pommes de terre rôties (0,63 µg/g), de la salade
de chou (0,11 µg/g), des bananes (0,12 µg/g), des airelles
(0,09µg/g), des ananas (0,05 µg/g), de la margarine (0,64 µg/g),
du sucre raffiné (0,2 µg/g), et des desserts à la gélatine
(0,09 µg/g).
Sur la base des données limitées dont on dispose, on peut
dresser la liste suivante des principaux milieux par lesquels la
population est exposée au DBP (par ordre d'importance
décroissante et en fonction de la dose absorbée estimative):
alimentation, air intérieur et eau de boisson. On estime que la
dose absorbée quotidiennement à partir de l'alimentation et de
l'air intérieur est respectivement égale à 7 µg/kg et 0,42 µg/kg
de poids corporel. Les doses absorbées journellement à partir de
l'eau de boisson et de l'air ambiant sont très inférieures à ces
valeurs, à savoir <0,02 µg/kg de poids corporel et 0,26 -
0,36 ng/kg depoids corporel, respectivement. En se basant sur
ces valeurs, on peut calculer que la dose moyenne totale absorbée
en une journée à partir de l'air, de l'eau de boisson et des
aliments est égale à 7,4 µg/kg de poids corporel. Toutefois, il
est à noter que la dose absorbée à partir des aliments peu varier
dans de larges proportions selon la nature et la quantité du
produit emballé qui est consommé et également, selon les
modalités d'utilisation de tel ou tel emballage au cours de la
préparation du produit. On estime qu'au Royaume-Uni, la dose
maximale ainsi ingérée est probablement de 2 mg environ par
personne et par jour (soit approximativement 31 µg/kg de poids
corporel en une journée, pour une personne d'un poids moyen de
64 kg). Il y a également un risque d'exposition au DBP présent
dans les cosmétiques, encore que dans ce cas, on ne dispose pas
de données suffisantes pour évaluer la dose absorbée de cette
manière.
Les données provisoires les plus récentes fournies par
l'enquête nationale sur l'exposition professionnelle qu'a menée
le NIOSH indiquent qu'aux Etats-Unis, plus de 500 000
travailleurs, dont 200 000 femmes, courent un risque d'exposition
au DBP. D'après les mesures effectuées sur un nombre limité de
sites de ce pays, la concentration du DBP est généralement
inférieure à la limite de détection (soit de 0,01 à 0,02 mg/m3),
mais des valeurs plus élevées ont été relevées dans d'autres
pays.
Les études sur le rat montrent que le DBP est absorbé par la
voie percutanée mais des travaux au cours desquels de la peau
humaine a été exposée in vitro ont révélé que cette dernière
était moins perméable au DBP que la peau du rat.
L'expérimentation animale (sur le rat) indique qu'une fois
administré par voie orale ou intraveineuse, le DBP est rapidement
résorbé au niveau des voies digestives et se répartit
principalement dans le foie et les reins, avant d'être éliminé
dans les urines sous forme de métabolites. Après inhalation, on
le retrouve systématiquement à faible concentration dans
l'encéphale.
Les données disponibles montrent qu'après ingestion par des
rats de laboratoire, le DBP est métabolisé par des esterases non
spécifiques, principalement dans l'intestin grêle, pour donner du
phtalate de mono- n-butyle (MBP) qui subit ensuite une oxydation
limitée de sa chaîne latérale alkyle. Le MBP est stable et le
deuxième goupement ester résiste à l'hydrolyse. Ce composé ainsi
que les autres métabolites sont excrétés dans les urines sous
forme de glucuro-conjugués. On observe des différences
interspécifiques relativement à l'excrétion des métabolites
conjugués et non conjugués, notamment entre hamster et le rat,
l'urine de ce dernier contenant davantage de MBP libre. Aucune
accumulation n'a été observée au niveau des divers organes.
La palette des effets de l'exposition au DBP est analogue à
celle que l'on observe avec les autres esters phtaliques, qui,
chez les espèces sensibles, peuvent se réveler foetotoxiques et
tératogènes et provoquer également une hépatomégalie, un
accroissement du nombre des peroxysomes hépatiques et des lésions
testiculaires.
Le DBP présente une faible toxicité aiguë pour le rat et la
souris. Après administration par voie orale à des rats on a
obtenu, pour la DL50, des valeurs allant d'environ 8 g/kg à au
moins 20 g/kg de poids corporel. Chez la souris, les valeurs
vont d'environ 5 g/kg à environ 16 g/kg de poids corporel. Chez
le lapin, la DL50 cutanée est supérieure à 4 g/kg de poids
corporel. L'existence d'effets toxiques aigus consécutifs à
l'inhalation de DBP n'a pu être documentée. Chez les animaux de
laboratoire, l'intoxication aiguë se manifeste par les signes
suivants: réduction de l'activité, respiration difficile et perte
de coordination. Un travailleur qui avait été intoxiqué par
suite de l'ingestion accidentelle de 10 g de DBP, s'est remis
progressivement de son intoxication en l'espace de quinze jours,
la récupération étant totale au bout d'un mois.
Lors d'études toxicologiques au cours desquelles des rats
ont reçu DBP à plusieurs reprises, on a observé, au bout d'une
période de 5 à 21 jours, une prolifération des peroxysomes et une
hépatomégalie aux doses supérieures ou égales à 420 mg/kg de
poids corporel sur une journée.
Lors d'études à plus long terme, les effets observés sur des
rats ayant ingéré du DBP pendant des périodes allant jusqu'à 7
mois consistaient notamment en une réduction du gain de poids à
des doses quotidiennes supérieures ou égales à 250 mg/kg de poids
corporel. A des doses supérieures ou égales à 120 mg/kg de poids
corporel, il y avait augmentation du poids relatif du foie.
Lorsque la dose quotidienne dépassait 279 mg/kg de poids
corporel, on observait également une prolifération des
peroxysomes et un accroisssement de l'activité des enzymes
correspondantes. Chez des rats Wistar qui avaient reçu des doses
quotidiennes supérieures ou égales à 250 mg/kg de poids corporel,
on a observé des signes de nécrose hépatique; en revanche ,
aucune lésion de ce genre n'a été relevée chez des rats
F-344 ou Sprague-Dawley soumis à des doses quotidiennes égales ou
supérieures à 2 500 mg/kg de poids corporel. Aux doses
quotidiennes de 250 et 571 mg/kg de poids corporel, on a observé
chez le rat un certain nombre d'anomalies au niveau testiculaire,
notamment des modifications affectant les enzymes et une
dégénérescence des cellules germinales. Les anomalies
testiculaires observées varient considérablement d'une espèce à
l'autre, les effets étant minimaux chez le hamster et la souris à
des doses quotidiennes qui peuvent atteindre 2 000 mg/kg. Une
récente étude consistant en une exposition subchronique a permis
de mettre en évidence, chez la souris, des effets sur le poids du
corps et le poids des organes, de même que des modifications
histologiques au niveau du foie, qui trahissent l'existence d'un
stress métabolique; la dose sans effet observable pour ce type
d'anomalie a été évaluée à 353 mg/kg de poids corporel.
D'après les quelques données d'expérimentation animale dont
on dispose, il semble que le DBP ne puisse guère provoquer
d'irritation cutanée ou oculaire ni entraîner une
sensibilisation. Chez l'homme, on connaît quelques cas de
sensibilisation après exposition à du DBP, mais ces observations
n'ont pas été confirmées par des études contrôlées sur un plus
grand nombre d'individus.
Dans le cadre d'un protocole d'élevage en continu, au cours
duquel on a procédé à des croisements et à l'examen de la
progéniture obtenue, des rats ont reçu une alimentation conentant
0, 1000, 5000 ou 10 000 mg de DBP par kg de nourriture (soit
l'équivalent quotidien de 0, 66, 320 et 651 mg de composé par kg
de poids corporel). Dans la première génération, on a pu
considérer comme un effet négatif sur le développement la
réduction du poids corporel observée chez les ratons ayant reçu
la dose médiane. On constatait également une réduction sensible
du nombre de portées viables à toutes les doses. Dans la deuxième
génération, les effets étaient plus graves, et consistaient en
une réduction du poids des ratons dans tous les groupes, y
compris celui qui avait reçu la dose la plus faible, en anomalies
morphologiques (malformations du prépuce et du pénis,
dégénérescence des tubes séminifères, et enfin, absence ou
développement insuffisant de l'épididyme) dans les groupes soumis
aux doses moyennes et fortes. Dans le groupe soumis à la dose la
plus forte, on notait de graves effets sur la spermatogénèse,
effets que l'on n'observait pas, en revanche, dans la génération
parentale. Ces résultats donnent à penser que les effets nocifs
du DBP sont plus marqués chez les animaux exposés au cours de
leur phase de développement et de maturation que lorsqu'ils le
sont uniquement à l'âge adulte. Aucune valeur bien nette de la
dose sans effet nocif observable (NOEL) n'a été tirée de cette
étude. On estime en revanche que la dose la plus faible
produisant un effet nocif (LOAEL) était égale à 66 mg/kg de poids
corporel par jour.
Les études dont on dispose montrent que le DBP est
généralement foetotoxique, sans pour autant qu'il y ait atteinte
de la mère. Les données existantes indiquent également que ce
composé est tératogène à forte dose, la sensibilité à cet effet
dépendant du stade de développement et de la période
d'administration. Chez la souris, on constaté que le DBP
provoquait une augmentation des résorptions et des morts foetales
à partir de 400 mg/kg de poids corporel, cet effet étant lié à la
dose. Pour ces valeurs de la dose, on a également constaté chez
la souris une réduction, liée à la dose, du poids foetal et du
nombre de portées viables.
On n'a pas effectué d'épreuves de cancérogénicité qui soient
satisfaisantes. A la lumière des données disponibles, on peut
penser que le DBP n'est pas génotoxique.
Les produits chimiques qui provoquent la prolifération des
peroxysomes constituent un groupe de substances souvent
génératrices de cancers du foie, selon un mécanisme qui
n'implique pas d'action toxique au niveau génique. Leur mode
d'action n'est pas encore élucidé, mais l'on sait cependant que
l'apparition de la tumeur est précédée par une prolifération des
peroxysomes et par une hépatomégalie. Comme le DBP provoque la
prolifération des peroxysomes, il n'est pas exclu qu'il puisse
également provoquer des cancers du foie chez les rongeurs, encore
qu'en ce qui concerne ces deux effets - prolifération des
peroxysomes et hépatomégalie - il soit beaucoup moins actif que
le DEHP. Dans la mesure où il y a corrélation entre ces deux
effets et le pouvoir cancérogène, on peut s'attendre à ce que le
DBP soit un cancérogène beaucoup moins puissant que le DHEP et
les méthodes actuelles de détermination biologique du pouvoir
cancérogène ne permettraient probablement pas de mettre une telle
activité en évidence.
Les enquêtes épidémiologiques dont on a connaissance se
limitent à l'étude du cas de travailleurs exposés à des mélanges
de phtalates. Elles ne nous permettent pas de progresser dans
l'élucidation des effets dus au seul DBP.
On a vu que le DBP n'étant pas génotoxique et ayant un
pouvoir cancérogène moindre que celui du DEHP, les méthodes
actuelles de mesure du pouvoir cancérogène ne révèleraient
vraisemblablement aucune activité de ce type. Il est donc peu
probable qu'à la concentration où il se trouve dans
l'environnement, ce composé contribue notablement à accroître le
risque de cancer.
C'est la voie alimentaire qui est, de loin, la principale
voie d'exposition au DBP. D'ailleurs, les données toxicologiques
relatives aux autres voies sont insuffisantes pour permettre une
évaluation. On a donc établi une valeur-guide pour la voie
orale, même si l'objectif final doit être de ramener l'exposition
totale de toutes origines à une valeur inférieure à la dose
journalière tolérable.
On n'a pas pu établir de valeur bien nette pour la dose sans
effet nocif observable (NOAEL) pour les points d'aboutissement
toxicologique jugés les plus appropriés à l'établissment de
valeurs-guides (en l'occurence, les effets néfastes sur la
reproduction et le développement). La dose la plus faible sans
effet nocif observable (LOAEL) sur la reproduction et le
développement a été fixée à 66 mg/kg de poids corporel par jour à
la suite d'une étude au cours de laquelle les animaux étaient
élevés en continu, avec cette réserve qu'à cette dose, les effets
observés étaient modérés et probablement réversibles. En se
basant sur ces données, on modérés et probablement réversibles.
En se basant sur ces données, on a fixé à 66 µg/kg p.c. la dose
journalière tolérable, compte tenu d'un facteur d'incertitude de
1000 (un facteur 10 pour les variations interspécifiques, un
facteur 10 pour les variations interindividuelles et un facteur
10 pour l'extrapolation de la LOAEL à la NOAEL).
Les renseignements dont on dispose sur l'écotoxicité du DBP
comportent des données de toxicité aiguë et de toxicité chronique
obtenues sur diverses espèces aquatiques à différents stades de
la chaîne alimentaire. Pour les algues d'eau douce, la valeur la
plus faible de la CE50 à 96 h qui ait été obtenue est égale à
750 µg de DBP par litre. La valeur la plus faible de la CL50
obtenue pour un invertébré aquatique (mysidé) est de 750 µg/litre
et on a relevé une CE50 à 48 h de 760 µg/litre pour des larves
de moucherons. Les études de toxicité chronique ont montré que
l'espèce d'invertébré la plus sensible était Daphnia magna,
avec une concentration sans effet observable à 21 jours (survie
parentale) de 500 µg/litre. Une épreuve non conventionnelle
effectuée sur Gammarus pulex a donné, pour la valeur de la
concentration la plus faible produisant un effet observable à 10
jours, le chiffre de 500 µg/litre et, pour la valeur de la
concentration sans effet observable, le chiffre de 100 µg/litre,
le critère retenu étant, dans les deux cas, la réduction de
l'activité locomotrice. Des épreuves de toxicité aiguë
pratiquées sur des poissons ont permis de constater que la valeur
la plus faible de la CL50 à 96 h était de 350 µg/litre pour une
espèce dulçaquicole, la perche jaune Perca flavescens, et de
600 µg/litre pour un sparidé marin. L'étude de toxicité
chronique la plus sensible qui ait été pratiquée utilisait la
truite arc-en-ciel et alle a donné une valeur de 100 µg/litre
pour la concentration sans effet observable à 99 jours
(croissance) et une valeur de 190 µg/litre pour la concentration
la plus faible produisant un effet observable à 99 jours, le
critère toxicologique retenu étant une réduction d'environ 27% de
la croissance.
La toxicité aiguë du DBP est faible pour les oiseaux.
La concentration moyenne actuelle du DBP dans l'eau ne
représente qu'un faible risque pour les organismes aquatiques.
Cependant, dans les cours d'eau très pollués, la marge de
sécurité est beaucoup plus faible. On ne dispose pas de données
suffisantes pour évaluer le risque encouru par les organismes
sédimenticoles. Compte tenu du niveau d'exposition actuel, le
risque reste faible pour les oiseaux et les mammifères
piscivores.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
El di- n-butil ftalato (DBF) es un líquido oleoso, incoloro
e inerte, con una presión de vapor baja, soluble en la mayor
parte de los disolventes orgánicos, pero sólo ligeramente en
agua. Las determinaciones analíticas más sensibles y selectivas
de los ésteres del ácido ftálico en el medio ambiente, incluido
el DBF, se logran mediante cromatografía de gases con detección
por captura de electrones o espectrometría de masas. Habida
cuenta de que con frecuencia los ftalatos se encuentran como
plastificantes en el equipo analítico y como contaminantes en el
aire y los disolventes del laboratorio, hay que tener una gran
precaución para evitar la contaminación durante la recogida, el
almacenamiento y el análisis de las muestras.
El DBF se utiliza principalmente como plastificante especial
para la nitrocelulosa, el acetato de polivinilo y el cloruro de
polivinilo, lubricante de válvulas de aerosoles, agente
antiespumante, emoliente de la piel y plastificante de esmaltes y
alargadores de uñas y pulverizadores para el pelo.
Se ha determinado la concentración de DBF en la atmósfera,
tanto en la fase de vapor como en la de partículas. Se considera
que el arrastre por la lluvia y la precipitación en seco ejercen
una función importante en su eliminación de la atmósfera. En las
aguas superficiales, la mayor parte del DBF está presente en la
fracción de agua más que en los sólidos suspendidos. La
volatilización a partir del suelo se supone insignificante,
puesto que su presión de vapor es baja y la adsorción en el suelo
moderada.
El DBF es relativamente no persistente en el aire y las
aguas superficiales y tiene una semivida en estos compartimentos
de sólo unos días. La biodegradación total es rápida en
condiciones aerobias, pero mucho más lenta en anaerobiosis. Para
el suelo se ha pronosticado una semivida semejante a las del aire
y el agua; sin embargo, algunos estudios indican que el DBF puede
ser más persistente en el suelo. Sería de esperar que el DBF se
bioacumulara, debido a su elevado coeficiente de reparto
octanol/agua. No obstante, los peces lo metabolizan bastante
fácilmente y, por consiguiente, los factores de bioconcentración
tienden a ser más bajos de lo previsto. El factor de
bioconcentración máximo, basado en el compuesto precursor, es 590
para Pimephales promelas. No es probable la bioamplificación
en los animales terrestres, de acuerdo con los datos limitados en
aves y con la rapidez del metabolismo y excreción que se ha
observado en mamíferos de laboratorio.
Raramente se han descrito medidas adoptadas para evitar la
contaminación en los informes sobre las concentraciones de DBF en
el medio ambiente publicados antes de 1980, por lo que no se
puede evaluar la fiabilidad de los primeros datos de vigilancia.
Hay datos limitados sobre concentraciones en el aire que indican
que los niveles medios suelen ser inferiores a 5 ng/m3. En
estudios recientes se ha observado que las concentraciones medias
en el agua de lluvia oscilaban entre 0,2 y 1,4 µg/litro; en zonas
remotas se han detectado valores muchos más bajos. Las
concentraciones medias en el agua superficial tienden a ser
inferiores a 1 µg/litro; sin embargo, los niveles en ríos
contaminados son mucho más elevados (12 a 34 µg/litro). Se
dispone de pocos datos sobre las concentraciones de DBF en el
aguas freática, con valores medios de 0,15 a 0,46 µg/litro. La
concentración en efluentes alcanza hasta 100 µg/litro, mientras
que en las aguas residuales varía de 0,2 a 200 mg/kg de peso
seco. Los niveles en los sedimentos son en general inferiores a
1 mg/kg de peso seco; sin embargo, en zonas contaminadas se han
medido concentraciones de hasta 20 mg/kg. En estudios realizados
en la biota acuática se ha comprobado que las concentraciones
medias de DBF tienden a ser menores de 0,2 mg/kg de peso seco;
sin embargo, en zonas contaminadas se han medido concentraciones
de hasta 35 mg/kg.
En un estudio realizado en 125 hogares de California,
Estados Unidos, en 1990, la concentración media durante el día en
el aire de la casa era de 420 ng/m3. Raramente se ha detectado
DBF en el agua de bebida (<1,0 µg/litro), según datos limitados
procedentes del Canadá. En un pequeño número de muestras de agua
de bebida de Toronto, Canadá, la concentración media era
14 ng/litro; las concentraciones en siete marcas de agua de
manantial embotellada oscilaban entre 21 y 55 ng/litro.
Además de su entrada mediante la contaminación del medio
ambiente, el DBF puede estar presente en productos alimenticios
como consecuencia de la migración desde el envase, aspecto que se
investigó en varios estudios realizados a finales del decenio de
1980. En muchos países, se adoptaron precauciones para reducir
la lixiviación de plastificantes de los envases y gracias a ello
los niveles de DBF en los productos alimenticios han disminuido a
lo largo del tiempo. En un estudio sobre la cesta de la compra
canadiense con 98 de muestras de tipos diferentes de alimentos
realizado en Halifax en 1986, se detectó DBF en la mantequilla
(1,5 µg/g), el pescado de agua dulce (0,5 µg/g), los productos a
base de cereales (entre indetectable y 0,62 µg/g), las papas
cocidas (0,63 µg/g), la ensalada de col (0,11 µg/g), los bananos
(0,12 µg/g), los arándanos (0,09 µg/g), las piñas (0,05 µg/g), la
margarina (0,64 µg/g), el azúcar blanco (0,2 µg/g) y el postre de
gelatina (0,09 µg/g).
Teniendo en cuenta los limitados datos disponibles, los
medios de exposición principales al DBF para la población
general, enumerados en orden de su importancia relativa según la
ingestión estimada son los siguientes: alimentos, aire de
espacios cerrados y agua de bebida. La ingesta estimada en
alimentos y en el aire de espacios cerrados es de 7 µg/kg de peso
corporal al día y 0,42 µg/kg de peso corporal al día,
respectivamente. Las ingestas con el agua de bebida y el aire del
medio ambiente son considerablemente inferiores, <0,02 µg/kg de
peso corporal al día y 0,26-0,36 ng/kg de peso corporal al día,
respectivamente. Habida cuenta de estas ingestas, se estima que
la cantidad media total diaria ingerida con el aire, el agua de
bebida y los alimentos es de 7,4 µg/kg de peso corporal al día.
Hay que señalar, sin embargo, que la ingestión de DBF en la
alimentación puede variar considerablemente, en función de la
naturaleza y la cantidad de los alimentos envasados consumidos y
el tipo de uso de los envoltorios de los alimentos en la
preparación de la comida. Para el Reino Unido, la ingestión
humana máxima probable de DBF de fuentes alimenticias se ha
estimado en unos 2 mg/persona/día (aproximadamente 31 µg/kg de
peso corporal/día, suponiendo un peso corporal medio de 64 kg).
Existe también la posibilidad de exposición al DBF a través de
los cosméticos, aunque los datos disponibles son insuficientes
para cuantificar la ingestión a partir de esta fuente.
Los datos provisionales más recientes de la Encuesta
Nacional de Exposición Profesional NIOSH señalan que en los
Estados Unidos hay más de 500 000 trabajadores, incluidas 200 000
mujeres, potencialmente expuestos al DBF. Teniendo en cuenta las
determinaciones en un número limitado de puestos de trabajo en
los Estados Unidos, las concentraciones son en general inferiores
al límite de detección (es decir, 0,01-0,02 mg/m3), si bien se
ha informado de niveles más elevados en algunos países.
En estudios con ratas, se ha observado que el DBF se absorbe
a través de la piel, aunque en estudios in vitro la piel humana
ha resultado menos permeable que la de rata a este compuesto. En
estudios con animales de laboratorio se ha advertido que, tras la
administración oral o intravenosa, el DBF se absorbe rápidamente
del tracto gastrointestinal, se distribuye fundamentalmente en el
hígado y los riñones y se excreta en la orina como metabolitos.
Tras la inhalación se detectaron constantemente concentraciones
bajas en el cerebro.
Los datos disponibles indican que en ratas, tras la
ingestión, el DBF se metaboliza mediante la acción de esterasas
inespecíficas, sobre todo en el intestino delgado, para producir
mono- n-butil ftalato (MBP), con la posterior oxidación
bioquímica limitada de la cadena alcalina lateral del MBP. Este
compuesto es estable y resistente a la hidrólisis del segundo
grupo éster. El MBP y otros metabolitos se excretan en la orina,
principalmente como conjugados de glucurónidos. Se han observado
especies diferentes en la excreción de metabolitos conjugados y
no conjugados del DBF en la orina de rata y hámster, con más MBP
libre en la rata que en el hámster. No se ha observado
acumulación en ningún órgano.
El perfil de los efectos tras la exposición al DBF es
semejante al de otros ésteres de ftalatos, que en especies
susceptibles puede inducir hepatomegalia, aumento del número de
peroxisomas hepáticos, fetotoxicidad, teratogenicidad y daños
testiculares.
La toxicidad aguda del DBF en ratas y ratones es baja. Los
valores de la DL50 notificados tras la administración oral a
ratas oscilan entre alrededor de 8 g/kg de peso corporal y por lo
menos 20 g/kg de peso corporal; en ratones, los valores son
aproximadamente de 5 g/kg de peso corporal y 16 g/kg de peso
corporal. La DL50 por vía cutánea en conejos es >4 g/kg de
peso corporal. No hay datos de toxicidad aguda tras la
inhalación de DBF. Los signos de toxicidad aguda observados en
los animales de laboratorio incluyen depresión de la actividad,
respiración fatigosa y falta de coordinación. En un caso de
intoxicación accidental de un trabajador que ingirió alrededor de
10 g de DBF, la recuperación fue gradual en un plazo de dos
semanas y completa al cabo de un mes.
En estudios de toxicidad de corta duración con dosis
repetidas, los efectos en ratas tras la administración oral
durante un período de 5 a 21 días con los niveles más bajos
fueron proliferación de peroxisomas y hepatomegalia a dosis de
420 mg/kg de peso corporal al día o más.
En estudios más prolongados, los efectos observados en ratas
tras la ingestión de DBF durante períodos de hasta siete meses
fueron un aumento reducido de peso a dosis de 250 mg/kg de peso
corporal al día o más. Se ha observado un aumento en el peso
relativo del hígado a dosis de 120 mg/kg de peso corporal o más.
A dosis de 279 mg/kg de peso corporal o más se ha registrado
proliferación de peroxisomas, con aumento de su actividad
enzimática. Se ha informado de cambios hepáticos necróticos en
ratas Wistar a dosis de 250 mg/kg de peso corporal por día o más,
pero no en ratas F-344 o Sprague-Dawley expuestas a dosis de
hasta 2500 mg/kg de peso corporal al día. Se han advertido
alteraciones en las enzimas testiculares y degeneración de las
células germinales testiculares de ratas con dosis de 250 y
571 mg/kg de peso corporal al día. Los efectos en los testículos
tras la exposición al DBF son muy diferentes en las distintas
especies, habiéndose observado efectos mínimos en ratones y
hámster a dosis de hasta 2000 mg/kg de peso corporal al día. En
un bioensayo subcrónico reciente realizado en ratones, se han
descrito efectos en el peso del cuerpo y los órganos y
alteraciones histológicas del hígado, factor indicativo de
tensión metabólica, por lo cual el NOEL fue 353 mg/kg de peso
corporal al día.
Teniendo en cuenta los limitados datos disponibles en
especies animales, el DBF parece tener escaso potencial para
irritar la piel o los ojos o inducir sensibilización. En el ser
humano se ha informado de un pequeño número de casos de
sensibilización tras la exposición al DBF, aunque esto no se vio
confirmado en estudios controlados realizados con un número más
elevado de personas, notificados solamente en resultados
secundarios.
En un protocolo continuo de reproducción, que comprendió
fases de apareamiento cruzado y de evaluación de la descendencia,
se expusieron ratas a 0, 1000, 5000 o 10 000 mg de DBF en la
alimentación (dosis equivalentes a 0, 66, 320 y 651 mg/kg de peso
corporal por día). En la primera generación, la reducción del
peso de las crías en el grupo expuesto a la dosis intermedia, en
ausencia de cualquier efecto adverso en el peso materno, pudo
considerarse como efecto de toxicidad sobre el desarrollo. Hubo
también una reducción significativa del número de crías vivas en
cada camada para los tres niveles de dosis. En la segunda
generación los efectos fueron más serios: reducción del peso de
las crías en todos los grupos, incluido el grupo expuesto a la
dosis baja; defectos estructurales (tales como malformaciones
prepuciales/peneanas, degeneración de los túbulos seminíferos y
ausencia o subdesarrollo de los epidídimos) en los grupos
sometidos a la dosis intermedia/ alta; y efectos graves sobre la
espermatogénesis en el grupo expuesto a la dosis alta, que no se
observaron en los progenitores. Estos resultados dan a entender
que los efectos adversos del DBF son más acusados en los animales
expuestos durante las fases de desarrollo y maduración que en los
expuestos solo en la edad adulta. No se estableció ningún NOEL
en ese estudio. El nivel inferior con efectos adversos observados
(LOAEL) se consideró que fue 66 mg/kg de peso corporal por día.
En los estudios disponibles se ha puesto de manifiesto que
el DBF suele inducir efectos fetotóxicos en ausencia de toxicidad
materna. Los datos existentes indican asimismo que el DBF es
teratogénico a dosis elevadas y que la susceptibilidad a la
teratogénesis varía en función de la fase de desarrollo y del
período de administración. La administración oral de DBF a
ratones en dosis de 400 mg/kg de peso corporal o superiores
produjo un aumento dependiente de la dosis en el número de
reabsorciones y de muertes fetales. Con estas mismas dosis se
observó también en ratones una disminución dependiente de la
dosis del peso fetal y del número de crías viable.
No se han realizado bioensayos adecuados de carcinogénesis
para el DBF. Las pruebas disponibles indican que el DBF no es
genotóxico.
En general, los productos químicos que causan proliferación
de los peroxisomas son con frecuencia hepatocarcinógenos mediante
una acción no genotóxica. Si bien no se conoce todavía el
mecanismo de acción, la formación de tumores va precedida de
proliferación de peroxisomas y hepatomegalia. Habida cuenta de
que el DBF produce proliferación de peroxisomas, es posible que
pudiera ser un carcinógeno hepático en roedores, aunque como
inductor de hepatomegalia y proliferación de peroxisomas es mucho
más débil que el DEHF. En la medida en que existe correlación
entre la hepatomegalia y la formación de peroxisomas por una
parte y la capacidad carcinógena por otra, cabe prever que el DBF
será un carcinógeno menos potente que el DEHF y probablemente no
mostrará actividad si la medición se realiza con las metodologías
actuales de bioensayo del cáncer.
Las investigaciones epidemiológicas identificadas se limitan
a las de trabajadores expuestos a mezclas de ftalatos. Estos
estudios no contribuyen a mejorar nuestros conocimientos sobre
los efectos asociados al DBF aislado.
Puesto que el DBF no es genotóxico y se supone que será
menos carcinógeno que el DEHF, probablemente no mostraría
actividad si la medición se realizara utilizando las metodologías
actuales de bioensayo del cáncer. Así pues, no es probable que
el DBF presente un aumento significativo del riesgo de cáncer en
las concentraciones a las que habitualmente se encuentra en el
medio ambiente.
La ingestión es con diferencia la vía principal de
exposición al DBF; además, los datos toxicológicos de las demás
vías de administración son insuficientes para su evaluación. Por
consiguiente, se ha preparado un valor orientativo para la vía
oral, aunque el objetivo último debería ser la reducción de la
exposición total a todas las fuentes para lograr una ingesta
diaria inferior a la tolerable.
No se estableció ningún claro nivel sin efectos adversos
observados (NOAEL) para los puntos finales considerados los más
adecuados para obtener los valores de orientación (es decir, la
toxicidad en el desarrollo y la reproducción). Se consideró que
el LOAEL resultante de un estudio continuo de reproducción para
la toxicidad en el desarrollo y la reproducción fue 66 mg/kg de
peso corporal al día, aunque los efectos observados a ese nivel
de dosis fueron moderados y probablemente reversibles. A partir
de esos datos, se ha obtenido una ingesta diaria tolerable de
66 µg/kg de peso corporal al día, incorporando un factor de
incertidumbre de 1000 (× 10 para la variación entre especies, ×
10 para la variación entre individuos, y × 10 para la
extrapolación del LOAEL al NOAEL).
La información sobre la ecotoxicidad del DBF consiste en
datos sobre toxicidad aguda y crónica para varias especies de
distintos niveles tróficos del medio ambiente acuático. La CE50
más baja descrita para algas de agua dulce a las 96 horas fue de
750 µg de DBF/litro. Los valores más pequeños obtenidos en las
pruebas de toxicidad aguda en invertebrados acuáticos fueron una
DL50 a las 96 horas de 750 µg/litro (mísidos) y una CE50 a las
48 horas de 760 µg/litro (larvas de mosca enana). En estudios de
toxicidad crónica, la especie más sensible de invertebrado fue
Daphnia magna, con una NOEC (supervivencia de los padres) a
los 21 días de 500 µg/litro. En una prueba no normalizada con un
antípodo ( Gammarus pulex) se obtuvo una LOEC a los 10 días de
500 µg/litro y una NOEC de 100 µg/litro, basados ambos en una
reducción de la actividad locomotriz. En pruebas de toxicidad
aguda con peces, la CL50 más baja a las 96 horas notificada para
una especie de agua dulce fue de 350 µg/litro (perca canadiense)
y para una especie marina de 600 µg/litro (sargo chopa). El
estudio de toxicidad crónica más sensible se basó en la trucha
irisada, con una NOEC (crecimiento) a los 99 días de 100 µg/litro
y una LOEC a los 99 días de 190 µg/litro (reducción del
crecimiento de alrededor del 27 por ciento).
La toxicidad aguda del DBF para las aves es baja.
El riesgo para los organismos acuáticos asociado a las
concentraciones medias presentes en las aguas superficiales es
bajo. Sin embargo, el margen de inocuidad en ríos muy
contaminados es mucho más pequeño. No se dispone de datos
adecuados que permitan evaluar el riesgo del DBF para los
organismos que viven en sedimentos. Se puede concluir que, con
los niveles actuales, el riesgo para las aves y los mamíferos que
se alimentan de peces es bajo.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL
PHTHALATE
Members
Dr B. Butterworth, Chemical Industry Institute of Toxicology Research
Triangle Park, North Carolina, USA (Chairman)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon Cambridgeshire,
United Kingdom (Co-Rapporteur)
Mr G. Long, Health and Welfare Canada, Environmental Health
Centre, Tunney's Pasture, Ottawa, Ontario, Canada
(Co-Rapporteur)
Dr R. Maronpot, Laboratory of Experimental Pathology, National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA
Dr E. Meek, Health and Welfare Canada, Environmental Health Centre,
Tunney's Pasture, Ottawa, Ontario, Canada
(Co-Rapporteur)
Dr S. Oishi, Department of Toxicology, Tokyo Metropolitan Research
Laboratory of Public Health, Tokyo, Japan
Dr Choon-Nam Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
Dr S.A. Soliman, Department of Pesticide Chemistry, Faculty of
Agriculture, Alexandria University, El-Shatby, Alexandria, Egypt*
Dr S.P. Srivastava, Industrial Toxicology Research Center, Lucknow,
India
Dr F. Sullivan, Division of Pharmacology and Toxicology, St. Thomas's
Hospital, London, United Kingdom
Dr C. Weber, Federal Environmental Agency, Berlin, Germany
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
*Invited but unable to attend
ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL PHTHALATE
A WHO Task Group on Environmental Health Criteria for
Di- n-butyl Phthalate (DBP) met in Geneva from 30 October to
3 November 1995. Dr B.H. Chen, IPCS, opened the meeting and welcomed
the participants on behalf of the Director, IPCS, and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to DBP.
The first draft of this monograph was prepared by Dr G. Long and
Dr E. Meek, Health and Welfare, Canada. The second draft was prepared
by Dr E. Meek incorporating comments received following the
circulation of the first draft to the IPCS Contact Points for
Environmental Health Criteria monographs. Dr E. Meek, Mr P. Howe and
Dr F. Sullivan contributed to the final text of this monograph.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
AP alkaline phosphatase
DBP di- n-butyl phthalate
DEHP diethylhexyl phthalate
GOT glutamic-oxaloacetic transaminase
GPT glutamic-pyruvic transaminase
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MBP monobutyl phthalate
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
1. SUMMARY
Di- n-butyl phthalate (DBP) is an inert, colourless, oily
liquid, with a low vapour pressure, which is soluble in most organic
solvents, but only slightly soluble in water. The most sensitive and
selective analytical determinations of phthalic acid esters, including
DBP, in environmental media are achieved by gas chromatography with
electron capture detection or mass spectrometry. Since phthalates
frequently occur as plasticizers in analytical equipment and as
contaminants in laboratory air and solvents, a great deal of care is
needed to prevent contamination during the collection, storage and
analysis of samples.
DBP is used mainly as a speciality plasticizer for nitro-
cellulose, polyvinyl acetate and polyvinyl chloride, a lubricant for
aerosol valves, an antifoaming agent, a skin emollient and a
plasticizer in nail polish, fingernail elongators and hair spray.
In the atmosphere, DBP has been measured in both the vapour and
the particulate phases. Washout via rainfall or dry deposition is
believed to play a significant role in the removal of DBP from the
atmosphere. In surface water, most of the DBP is present in the water
fraction rather than in the suspended solids. Volatilization of DBP
from soil is not expected to be significant because of its low vapour
pressure and moderate adsorption to soil.
DBP is relatively non-persistent in air and surface waters, and
has a half-life in these compartments of only a few days. Complete
biodegradation of DBP is rapid under aerobic conditions but much
slower under anaerobic conditions. For soil, similar half-lives to
air and water have been predicted; however, some studies suggest that
DBP may be more persistent in soil. DBP would be expected to
bioaccumulate as a result of its high octanol-water partition
coefficient. However, it is quite readily metabolized in fish and,
consequently, bioconcentration factors tend to be lower then
predicted. The highest bioconcentration factor, based on the parent
compound (DBP), is 590 for the fathead minnow. Biomagnification is
unlikely in terrestrial animals, based upon limited data on birds and
the rapid metabolism and excretion observed in laboratory mammals.
Steps taken to avoid contamination are rarely described in
reports of concentrations of DBP in the environment published before
1980 and, consequently, the reliability of the early monitoring data
often cannot be assessed. Limited data on concentrations in ambient
air indicate that mean levels are generally less than 5 ng/m3. In
recent studies, mean rainwater concentrations ranged from 0.2 to
1.4 µg/litre; much lower values have been measured in remote
areas. Mean concentrations in surface water tend to be less than
1 µg/litre; however, levels in polluted rivers are much higher (12 to
34 µg/litre). There are only a few data on groundwater concentrations
of DBP, mean values being 0.15 to 0.46 µg/litre. DBP concentrations
in effluents range up to 100 µg/litre, whilst concentrations in sewage
sludge range from 0.2 to 200 mg/kg dry weight. Levels in sediment are
generally less than 1 mg/kg dry weight; however, in polluted areas
concentrations of up to 20 mg/kg have been measured. In studies on
aquatic biota, mean concentrations of DBP tend to be less than
0.2 mg/kg wet weight; however, in polluted areas, concentrations of up
to 35 mg/kg have been measured.
In a survey of 125 homes in California, USA, in 1990, the median
daytime concentration of DBP in indoor air was 420 ng/m3. DBP has
rarely been detected in drinking-water supplies (< 1.0 µg/litre),
according to limited data from Canada. In a small number of samples
of drinking-water in Toronto, Canada, the mean concentration was
14 ng/litre; concentrations in seven brands of bottled spring water
ranged from 21 to 55 ng/litre.
In addition to entry through environmental contamination, DBP may
be present in foodstuffs as a result of migration from packaging, and
this was investigated in a number of studies conducted in the late
1980s. In many countries, precautions were introduced to reduce
leaching of plasticizers from packaging and as a result, levels of DBP
in foodstuffs have declined over time. In a Canadian market-basket
survey of 98 different food type samples in Halifax in 1986, DBP was
detected in butter (1.5 µg/g), freshwater fish (0.5 µg/g), cereal
products (range from undetectable to 0.62 µg/g), baked potatoes
(0.63 µg/g), coleslaw (0.11 µg/g), bananas (0.12 µg/g), blueberries
(0.09 µg/g), pineapples (0.05 µg/g), margarine (0.64 µg/g), white
sugar (0.2 µg/g) and gelatin dessert (0.09 µg/g).
On the basis of the limited data available, the principal media
of exposure to DBP for the general population, listed in order of
their relative importance based upon estimated intake, are as follows:
food, indoor air and drinking-water. Estimated intakes from food and
indoor air are 7 µg/kg body weight per day and 0.42 µg/kg body weight
per day, respectively. Estimated intakes from drinking-water and
ambient air are considerably less, < 0.02 µg/kg body weight per day
and 0.26-0.36 ng/kg body weight per day, respectively. Based on these
intakes, it is estimated that the total average daily intake from air,
drinking-water and food is 7.4 µg/kg body weight per day. It
should be noted, however, that intake of DBP in the diet can vary
considerably, depending upon the nature and extent of packaged food
consumed and the nature of use of food wrapping in food preparation.
For the United Kingdom, the maximum likely human intake of DBP from
food sources has been estimated to be approximately 2 mg per person
per day (approximately 31 µg/kg body weight per day, assuming a mean
body weight of 64 kg). There is also potential for exposure to DBP in
cosmetics, although available data are inadequate to quantify intake
from this source.
The most recent provisional data from the NIOSH National
Occupational Exposure Survey indicates that in the USA over 500 000
workers, including 200 000 women, are potentially exposed to DBP.
Based on determinations at a limited number of worksites in the USA,
concentrations are generally less than the limit of detection (i.e.,
0.01 to 0.02 mg/m3), although higher levels have been reported in
some countries.
In studies on rats, DBP is absorbed through the skin, although in
in vitro studies human skin has been found to be less permeable than
rat skin to this compound. Studies in laboratory animals indicate that
DBP is rapidly absorbed from the gastrointestinal tract, distributed
primarily to the liver and kidneys of rats and excreted in urine as
metabolites following oral or intravenous administration. Following
inhalation, it was consistently detected at low concentrations in the
brain.
Available data indicate that in rats, following ingestion, DBP is
metabolized by nonspecific esterases mainly in the small intestine
to yield mono- n-butyl phthalate (MBP) with limited subsequent
biochemical oxidation of the alkyl side chain of MBP. MBP is stable
and resistant to hydrolysis of the second ester group. The MBP and
other metabolites are excreted in the urine mainly as glucuronide
conjugates. Species differences in the excretion of conjugates and
unconjugated metabolites of DBP in the urine of rats and hamsters have
been observed, with more free MBP being present in rats than hamsters.
Accumulation has not been observed in any organ.
The profile of effects following exposure to DBP is similar to
that of other phthalate esters, which, in susceptible species, can
induce hepatomegaly, increased numbers of hepatic peroxisomes,
fetotoxicity, teratogenicity and testicular damage.
The acute toxicity of DBP in rats and mice is low. Reported
LD50 values following oral administration to rats range from
approximately 8 g/kg body weight to at least 20 g/kg body weight; in
mice, values are approximately 5 g/kg body weight to 16 g/kg body
weight. The dermal LD50 in rabbits is > 4 g/kg body weight.
Reports of acute toxicity following inhalation of DBP have not been
identified. Signs of acute toxicity in laboratory animals include
depression of activity, laboured breathing and lack of coordination.
In a case of accidental poisoning of a worker who ingested
approximately 10 grams of DBP, recovery was gradual within two weeks
and complete after 1 month.
In short-term repeated-dose toxicity studies, effects at lowest
levels in rats after oral administration for 5 to 21 days included
peroxisome proliferation and hepatomegaly at doses of 420 mg/kg body
weight per day or more.
In longer-term studies, the effects in rats observed following
ingestion of DBP for periods up to 7 months included reduced rate of
weight gain at doses of 250 mg/kg body weight per day or more.
Increase in relative liver weight has been observed at doses of
120 mg/kg body weight or more. Peroxisomal proliferation with
increased peroxisomal enzyme activity has been observed at doses of
279 mg/kg body weight per day or more. Necrotic hepatic changes in
Wistar rats have been reported at doses of 250 mg/kg body weight per
day or more but not in F-344 or Sprague-Dawley rats exposed to up to
2500 mg/kg body weight per day. Alteration in testicular enzymes and
degeneration of testicular germinal cells of rats have been observed
at doses of 250 and 571 mg/kg body weight per day. There are
considerable species differences in effects on the testes following
exposure to DBP, minimal effects being observed in mice and hamsters
at doses as high as 2000 mg/kg body weight per day. In mice, effects
on body and organ weights and histological alterations in the liver
indicative of metabolic stress have been reported in a recent
subchronic bioassay, for which the no-observed-effect-level (NOEL) was
353 mg/kg body weight per day.
On the basis of limited available data in animal species, DBP
appears to have little potential to irritate skin or eyes or to induce
sensitization. In humans, a few cases of sensitization after exposure
to DBP have been reported, although this was not confirmed in
controlled studies of larger numbers of individuals reported only in
secondary accounts.
In a continuous breeding protocol, which included cross-over
mating and offspring assessment phases, rats were exposed to 0, 1000,
5000 or 10 000 mg DBP/kg in the diet (equivalent to 0, 66, 320 and
651 mg/kg body weight per day). In the first generation the reduction
in pup weight in the mid-dose group, in the absence of any adverse
effect on maternal weight, could be regarded as a developmental
toxicity effect. There was also a significant reduction of live
litter numbers at all three dose levels. The effects in the second
generation were more severe, with reduced pup weight in all groups
including the low-dose group, structural defects (such as prepucial/
penile malformations, seminiferous tubule degeneration, and absence or
underdevelopment of the epididymides) in the mid- and high-dose
groups, and severe effects on spermatogenesis in the high-dose group
that were not seen in the parent animals. These results suggest that
the adverse effects of DBP are more marked in animals exposed during
development and maturation than in animals exposed as adults only. No
clear NOEL was established in this study. The lowest-observed-
adverse-effect-level (LOAEL) was considered to be 66 mg/kg body weight
per day.
The available studies show that DBP generally induces fetotoxic
effects in the absence of maternal toxicity. Available data also
indicate that DBP is teratogenic at high doses and that susceptibility
to teratogenesis varies with developmental stage and period of
administration. In mice, DBP caused dose-dependent increases in the
number of resorptions and dead fetuses at oral doses of 400 mg/kg body
weight per day or more. Dose-dependent decreases in fetal weights and
number of viable litters were also observed in mice at these doses.
Adequate carcinogenesis bioassays for DBP have not been
conducted. The weight of the available evidence indicates that DBP is
not genotoxic.
As a class, chemicals which cause peroxisome proliferation are
often hepatocarcinogenic via a non-genotoxic mode of action. Although
the mechanism of action remains unknown, tumour formation is preceded
by peroxisomal proliferation and hepatomegaly. Since DBP causes
peroxisomal proliferation, it is possible that it might be a rodent
liver carcinogen, although it is much weaker in inducing hepatomegaly
and peroxisome proliferation than DEHP. To the degree that
hepatomegaly and peroxisomal proliferation correlate with carcinogenic
potency, DBP would be expected to be a less potent carcinogen than
DEHP and would probably exhibit no activity as measured by current
cancer bioassay methodologies.
Identified epidemiological investigations are limited to those of
workers exposed to mixtures of phthalates. These studies do not
contribute to our understanding of the effects associated with DBP
alone.
Since DBP is not genotoxic and is expected to be a less potent
carcinogen than DEHP, it would probably exhibit no activity as
measured by current cancer bioassay methodologies. Thus, it is
unlikely that DBP presents any significantly increased risk of cancer
at concentrations generally present in the environment.
Ingestion is by far the principal route of exposure to DBP;
moreover, the toxicological data for other routes of administration
are insufficient for evaluation. A guidance value has, therefore, been
developed for the oral route, although the ultimate objective should
be reduction of total exposure from all sources to less than the
tolerable daily intake.
No clear no-observed-adverse-effect-level (NOAEL) for the
end-points considered to be most appropriate for derivation of
guidance values (i.e., developmental and reproductive toxicity) was
established. The LOAEL for developmental and reproductive toxicity
from a continuous breeding study was considered to be 66 mg/kg body
weight per day, although the effects observed at this dose level were
moderate and probably reversible. On the basis of these data, a
tolerable daily intake of 66 g/kg body weight per day has been
derived, incorporating an uncertainty factor of 1000 (× 10 for
interspecies variation, × 10 for inter-individual variation, and × 10
for extrapolation from LOAEL to NOAEL).
Information on the ecotoxicity of DBP includes acute and chronic
data for a number of species from various trophic levels in the
aquatic environment. For freshwater algae the lowest reported 96-h
EC50 was 750 µg DBP/litre. The lowest reported values in acute
toxicity tests on aquatic invertebrates were a 96-h LC50 of
750 µg/litre (mysid shrimp) and a 48-h EC50 of 760 µg/litre (midge
larvae). In chronic studies, the most sensitive invertebrate species
was Daphnia magna, with a 21-day NOEC (parent survival) of
500 µg/litre. In a non-standard test with the scud (Gammarus pulex)
a 10-day LOEC of 500 µg/litre and a NOEC of 100 µg/litre, both based
on reduced locomotor activity, were reported. In acute toxicity tests
with fish the lowest reported 96-h LC50 for a freshwater species was
350 µg/litre (yellow perch) and for a marine species 600 µg/litre
(sheepshead minnow). The most sensitive chronic study was based on
the rainbow trout with a 99-day NOEC (growth) of 100 µg/litre and a
99-day LOEC of 190 µg/litre (growth reduced by about 27%).
The acute toxicity of DBP to birds is low.
The risk to aquatic organisms associated with the present mean
concentrations of DBP in surface water is low. However, in highly
polluted rivers the safety margin is much smaller. There is
inadequate data to assess the risk of DBP to sediment-dwelling
organisms. At current levels of exposure, it can be concluded that
the risk to fish-eating birds and mammals is low.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Di- n-butyl phthalate (DBP), a phthalic acid ester, has the CAS
(Chemical Abstracts Service) Registry Number
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL
PHTHALATE
Members
Dr B. Butterworth, Chemical Industry Institute of Toxicology Research
Triangle Park, North Carolina, USA (Chairman)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon Cambridgeshire,
United Kingdom (Co-Rapporteur)
Mr G. Long, Health and Welfare Canada, Environmental Health
Centre, Tunney's Pasture, Ottawa, Ontario, Canada
(Co-Rapporteur)
Dr R. Maronpot, Laboratory of Experimental Pathology, National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA
Dr E. Meek, Health and Welfare Canada, Environmental Health Centre,
Tunney's Pasture, Ottawa, Ontario, Canada
(Co-Rapporteur)
Dr S. Oishi, Department of Toxicology, Tokyo Metropolitan Research
Laboratory of Public Health, Tokyo, Japan
Dr Choon-Nam Ong, Department of Community, Occupational and Family
Medicine, National University of Singapore, Singapore
Dr S.A. Soliman, Department of Pesticide Chemistry, Faculty of
Agriculture, Alexandria University, El-Shatby, Alexandria, Egypt*
Dr S.P. Srivastava, Industrial Toxicology Research Center, Lucknow,
India
Dr F. Sullivan, Division of Pharmacology and Toxicology, St. Thomas's
Hospital, London, United Kingdom
Dr C. Weber, Federal Environmental Agency, Berlin, Germany
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
*Invited but unable to attend
ENVIRONMENTAL HEALTH CRITERIA FOR DI- n-BUTYL PHTHALATE
A WHO Task Group on Environmental Health Criteria for
Di- n-butyl Phthalate (DBP) met in Geneva from 30 October to
3 November 1995. Dr B.H. Chen, IPCS, opened the meeting and welcomed
the participants on behalf of the Director, IPCS, and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to DBP.
The first draft of this monograph was prepared by Dr G. Long and
Dr E. Meek, Health and Welfare, Canada. The second draft was prepared
by Dr E. Meek incorporating comments received following the
circulation of the first draft to the IPCS Contact Points for
Environmental Health Criteria monographs. Dr E. Meek, Mr P. Howe and
Dr F. Sullivan contributed to the final text of this monograph.
Dr B.H. Chen and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
ABBREVIATIONS
AP alkaline phosphatase
DBP di- n-butyl phthalate
DEHP diethylhexyl phthalate
GOT glutamic-oxaloacetic transaminase
GPT glutamic-pyruvic transaminase
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MBP monobutyl phthalate
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
1. SUMMARY
Di- n-butyl phthalate (DBP) is an inert, colourless, oily
liquid, with a low vapour pressure, which is soluble in most organic
solvents, but only slightly soluble in water. The most sensitive and
selective analytical determinations of phthalic acid esters, including
DBP, in environmental media are achieved by gas chromatography with
electron capture detection or mass spectrometry. Since phthalates
frequently occur as plasticizers in analytical equipment and as
contaminants in laboratory air and solvents, a great deal of care is
needed to prevent contamination during the collection, storage and
analysis of samples.
DBP is used mainly as a speciality plasticizer for nitro-
cellulose, polyvinyl acetate and polyvinyl chloride, a lubricant for
aerosol valves, an antifoaming agent, a skin emollient and a
plasticizer in nail polish, fingernail elongators and hair spray.
In the atmosphere, DBP has been measured in both the vapour and
the particulate phases. Washout via rainfall or dry deposition is
believed to play a significant role in the removal of DBP from the
atmosphere. In surface water, most of the DBP is present in the water
fraction rather than in the suspended solids. Volatilization of DBP
from soil is not expected to be significant because of its low vapour
pressure and moderate adsorption to soil.
DBP is relatively non-persistent in air and surface waters, and
has a half-life in these compartments of only a few days. Complete
biodegradation of DBP is rapid under aerobic conditions but much
slower under anaerobic conditions. For soil, similar half-lives to
air and water have been predicted; however, some studies suggest that
DBP may be more persistent in soil. DBP would be expected to
bioaccumulate as a result of its high octanol-water partition
coefficient. However, it is quite readily metabolized in fish and,
consequently, bioconcentration factors tend to be lower then
predicted. The highest bioconcentration factor, based on the parent
compound (DBP), is 590 for the fathead minnow. Biomagnification is
unlikely in terrestrial animals, based upon limited data on birds and
the rapid metabolism and excretion observed in laboratory mammals.
Steps taken to avoid contamination are rarely described in
reports of concentrations of DBP in the environment published before
1980 and, consequently, the reliability of the early monitoring data
often cannot be assessed. Limited data on concentrations in ambient
air indicate that mean levels are generally less than 5 ng/m3. In
recent studies, mean rainwater concentrations ranged from 0.2 to
1.4 µg/litre; much lower values have been measured in remote
areas. Mean concentrations in surface water tend to be less than
1 µg/litre; however, levels in polluted rivers are much higher (12 to
34 µg/litre). There are only a few data on groundwater concentrations
of DBP, mean values being 0.15 to 0.46 µg/litre. DBP concentrations
in effluents range up to 100 µg/litre, whilst concentrations in sewage
sludge range from 0.2 to 200 mg/kg dry weight. Levels in sediment are
generally less than 1 mg/kg dry weight; however, in polluted areas
concentrations of up to 20 mg/kg have been measured. In studies on
aquatic biota, mean concentrations of DBP tend to be less than
0.2 mg/kg wet weight; however, in polluted areas, concentrations of up
to 35 mg/kg have been measured.
In a survey of 125 homes in California, USA, in 1990, the median
daytime concentration of DBP in indoor air was 420 ng/m3. DBP has
rarely been detected in drinking-water supplies (< 1.0 µg/litre),
according to limited data from Canada. In a small number of samples
of drinking-water in Toronto, Canada, the mean concentration was
14 ng/litre; concentrations in seven brands of bottled spring water
ranged from 21 to 55 ng/litre.
In addition to entry through environmental contamination, DBP may
be present in foodstuffs as a result of migration from packaging, and
this was investigated in a number of studies conducted in the late
1980s. In many countries, precautions were introduced to reduce
leaching of plasticizers from packaging and as a result, levels of DBP
in foodstuffs have declined over time. In a Canadian market-basket
survey of 98 different food type samples in Halifax in 1986, DBP was
detected in butter (1.5 µg/g), freshwater fish (0.5 µg/g), cereal
products (range from undetectable to 0.62 µg/g), baked potatoes
(0.63 µg/g), coleslaw (0.11 µg/g), bananas (0.12 µg/g), blueberries
(0.09 µg/g), pineapples (0.05 µg/g), margarine (0.64 µg/g), white
sugar (0.2 µg/g) and gelatin dessert (0.09 µg/g).
On the basis of the limited data available, the principal media
of exposure to DBP for the general population, listed in order of
their relative importance based upon estimated intake, are as follows:
food, indoor air and drinking-water. Estimated intakes from food and
indoor air are 7 µg/kg body weight per day and 0.42 µg/kg body weight
per day, respectively. Estimated intakes from drinking-water and
ambient air are considerably less, < 0.02 µg/kg body weight per day
and 0.26-0.36 ng/kg body weight per day, respectively. Based on these
intakes, it is estimated that the total average daily intake from air,
drinking-water and food is 7.4 µg/kg body weight per day. It
should be noted, however, that intake of DBP in the diet can vary
considerably, depending upon the nature and extent of packaged food
consumed and the nature of use of food wrapping in food preparation.
For the United Kingdom, the maximum likely human intake of DBP from
food sources has been estimated to be approximately 2 mg per person
per day (approximately 31 µg/kg body weight per day, assuming a mean
body weight of 64 kg). There is also potential for exposure to DBP in
cosmetics, although available data are inadequate to quantify intake
from this source.
The most recent provisional data from the NIOSH National
Occupational Exposure Survey indicates that in the USA over 500 000
workers, including 200 000 women, are potentially exposed to DBP.
Based on determinations at a limited number of worksites in the USA,
concentrations are generally less than the limit of detection (i.e.,
0.01 to 0.02 mg/m3), although higher levels have been reported in
some countries.
In studies on rats, DBP is absorbed through the skin, although in
in vitro studies human skin has been found to be less permeable than
rat skin to this compound. Studies in laboratory animals indicate that
DBP is rapidly absorbed from the gastrointestinal tract, distributed
primarily to the liver and kidneys of rats and excreted in urine as
metabolites following oral or intravenous administration. Following
inhalation, it was consistently detected at low concentrations in the
brain.
Available data indicate that in rats, following ingestion, DBP is
metabolized by nonspecific esterases mainly in the small intestine
to yield mono- n-butyl phthalate (MBP) with limited subsequent
biochemical oxidation of the alkyl side chain of MBP. MBP is stable
and resistant to hydrolysis of the second ester group. The MBP and
other metabolites are excreted in the urine mainly as glucuronide
conjugates. Species differences in the excretion of conjugates and
unconjugated metabolites of DBP in the urine of rats and hamsters have
been observed, with more free MBP being present in rats than hamsters.
Accumulation has not been observed in any organ.
The profile of effects following exposure to DBP is similar to
that of other phthalate esters, which, in susceptible species, can
induce hepatomegaly, increased numbers of hepatic peroxisomes,
fetotoxicity, teratogenicity and testicular damage.
The acute toxicity of DBP in rats and mice is low. Reported
LD50 values following oral administration to rats range from
approximately 8 g/kg body weight to at least 20 g/kg body weight; in
mice, values are approximately 5 g/kg body weight to 16 g/kg body
weight. The dermal LD50 in rabbits is > 4 g/kg body weight.
Reports of acute toxicity following inhalation of DBP have not been
identified. Signs of acute toxicity in laboratory animals include
depression of activity, laboured breathing and lack of coordination.
In a case of accidental poisoning of a worker who ingested
approximately 10 grams of DBP, recovery was gradual within two weeks
and complete after 1 month.
In short-term repeated-dose toxicity studies, effects at lowest
levels in rats after oral administration for 5 to 21 days included
peroxisome proliferation and hepatomegaly at doses of 420 mg/kg body
weight per day or more.
In longer-term studies, the effects in rats observed following
ingestion of DBP for periods up to 7 months included reduced rate of
weight gain at doses of 250 mg/kg body weight per day or more.
Increase in relative liver weight has been observed at doses of
120 mg/kg body weight or more. Peroxisomal proliferation with
increased peroxisomal enzyme activity has been observed at doses of
279 mg/kg body weight per day or more. Necrotic hepatic changes in
Wistar rats have been reported at doses of 250 mg/kg body weight per
day or more but not in F-344 or Sprague-Dawley rats exposed to up to
2500 mg/kg body weight per day. Alteration in testicular enzymes and
degeneration of testicular germinal cells of rats have been observed
at doses of 250 and 571 mg/kg body weight per day. There are
considerable species differences in effects on the testes following
exposure to DBP, minimal effects being observed in mice and hamsters
at doses as high as 2000 mg/kg body weight per day. In mice, effects
on body and organ weights and histological alterations in the liver
indicative of metabolic stress have been reported in a recent
subchronic bioassay, for which the no-observed-effect-level (NOEL) was
353 mg/kg body weight per day.
On the basis of limited available data in animal species, DBP
appears to have little potential to irritate skin or eyes or to induce
sensitization. In humans, a few cases of sensitization after exposure
to DBP have been reported, although this was not confirmed in
controlled studies of larger numbers of individuals reported only in
secondary accounts.
In a continuous breeding protocol, which included cross-over
mating and offspring assessment phases, rats were exposed to 0, 1000,
5000 or 10 000 mg DBP/kg in the diet (equivalent to 0, 66, 320 and
651 mg/kg body weight per day). In the first generation the reduction
in pup weight in the mid-dose group, in the absence of any adverse
effect on maternal weight, could be regarded as a developmental
toxicity effect. There was also a significant reduction of live
litter numbers at all three dose levels. The effects in the second
generation were more severe, with reduced pup weight in all groups
including the low-dose group, structural defects (such as prepucial/
penile malformations, seminiferous tubule degeneration, and absence or
underdevelopment of the epididymides) in the mid- and high-dose
groups, and severe effects on spermatogenesis in the high-dose group
that were not seen in the parent animals. These results suggest that
the adverse effects of DBP are more marked in animals exposed during
development and maturation than in animals exposed as adults only. No
clear NOEL was established in this study. The lowest-observed-
adverse-effect-level (LOAEL) was considered to be 66 mg/kg body weight
per day.
The available studies show that DBP generally induces fetotoxic
effects in the absence of maternal toxicity. Available data also
indicate that DBP is teratogenic at high doses and that susceptibility
to teratogenesis varies with developmental stage and period of
administration. In mice, DBP caused dose-dependent increases in the
number of resorptions and dead fetuses at oral doses of 400 mg/kg body
weight per day or more. Dose-dependent decreases in fetal weights and
number of viable litters were also observed in mice at these doses.
Adequate carcinogenesis bioassays for DBP have not been
conducted. The weight of the available evidence indicates that DBP is
not genotoxic.
As a class, chemicals which cause peroxisome proliferation are
often hepatocarcinogenic via a non-genotoxic mode of action. Although
the mechanism of action remains unknown, tumour formation is preceded
by peroxisomal proliferation and hepatomegaly. Since DBP causes
peroxisomal proliferation, it is possible that it might be a rodent
liver carcinogen, although it is much weaker in inducing hepatomegaly
and peroxisome proliferation than DEHP. To the degree that
hepatomegaly and peroxisomal proliferation correlate with carcinogenic
potency, DBP would be expected to be a less potent carcinogen than
DEHP and would probably exhibit no activity as measured by current
cancer bioassay methodologies.
Identified epidemiological investigations are limited to those of
workers exposed to mixtures of phthalates. These studies do not
contribute to our understanding of the effects associated with DBP
alone.
Since DBP is not genotoxic and is expected to be a less potent
carcinogen than DEHP, it would probably exhibit no activity as
measured by current cancer bioassay methodologies. Thus, it is
unlikely that DBP presents any significantly increased risk of cancer
at concentrations generally present in the environment.
Ingestion is by far the principal route of exposure to DBP;
moreover, the toxicological data for other routes of administration
are insufficient for evaluation. A guidance value has, therefore, been
developed for the oral route, although the ultimate objective should
be reduction of total exposure from all sources to less than the
tolerable daily intake.
No clear no-observed-adverse-effect-level (NOAEL) for the
end-points considered to be most appropriate for derivation of
guidance values (i.e., developmental and reproductive toxicity) was
established. The LOAEL for developmental and reproductive toxicity
from a continuous breeding study was considered to be 66 mg/kg body
weight per day, although the effects observed at this dose level were
moderate and probably reversible. On the basis of these data, a
tolerable daily intake of 66 g/kg body weight per day has been
derived, incorporating an uncertainty factor of 1000 (× 10 for
interspecies variation, × 10 for inter-individual variation, and × 10
for extrapolation from LOAEL to NOAEL).
Information on the ecotoxicity of DBP includes acute and chronic
data for a number of species from various trophic levels in the
aquatic environment. For freshwater algae the lowest reported 96-h
EC50 was 750 µg DBP/litre. The lowest reported values in acute
toxicity tests on aquatic invertebrates were a 96-h LC50 of
750 µg/litre (mysid shrimp) and a 48-h EC50 of 760 µg/litre (midge
larvae). In chronic studies, the most sensitive invertebrate species
was Daphnia magna, with a 21-day NOEC (parent survival) of
500 µg/litre. In a non-standard test with the scud (Gammarus pulex)
a 10-day LOEC of 500 µg/litre and a NOEC of 100 µg/litre, both based
on reduced locomotor activity, were reported. In acute toxicity tests
with fish the lowest reported 96-h LC50 for a freshwater species was
350 µg/litre (yellow perch) and for a marine species 600 µg/litre
(sheepshead minnow). The most sensitive chronic study was based on
the rainbow trout with a 99-day NOEC (growth) of 100 µg/litre and a
99-day LOEC of 190 µg/litre (growth reduced by about 27%).
The acute toxicity of DBP to birds is low.
The risk to aquatic organisms associated with the present mean
concentrations of DBP in surface water is low. However, in highly
polluted rivers the safety margin is much smaller. There is
inadequate data to assess the risk of DBP to sediment-dwelling
organisms. At current levels of exposure, it can be concluded that
the risk to fish-eating birds and mammals is low.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES AND ANALYTICAL METHODS
2.1 Identity
Di- n-butyl phthalate (DBP), a phthalic acid ester, has the CAS
(Chemical Abstracts Service) Registry Number  Relative molecular mass 278.34
Synonyms butylphthalate; dibutylphthalate; DBP;
1,2-benzenedicarboxylic acid dibutyl ester;
o-benzenedicarboxylic acid, dibutyl ester;
dibutyl 1,2-benzene dicarboxylate;
dibutyl- o-phthalate
CAS name 1,2-benzenedicarboxylic acid, dibutyl ester
CAS registry number
Relative molecular mass 278.34
Synonyms butylphthalate; dibutylphthalate; DBP;
1,2-benzenedicarboxylic acid dibutyl ester;
o-benzenedicarboxylic acid, dibutyl ester;
dibutyl 1,2-benzene dicarboxylate;
dibutyl- o-phthalate
CAS name 1,2-benzenedicarboxylic acid, dibutyl ester
CAS registry number 