
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 183
CHLOROTHALONIL
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. M.H. Litchfield, Arundel, United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1996
The issue of this document does not constitute formal publication.
It should not be reviewed, abstracted, or quoted without the written
permission of the Manager, International Programme on Chemical Safety,
WHO, Geneva, Switzerland.
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme (UNEP), the
International Labour Organisation (ILO), and the World Health
Organization (WHO). The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include the
development of epidemiological, experimental laboratory, and risk-
assessment methods that could produce internationally comparable
results, and the development of manpower in the field of toxicology.
Other activities carried out by the IPCS include the development of
know-how for coping with chemical accidents, coordination of
laboratory testing and epidemiological studies, and promotion of
research on the mechanisms of the biological action of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Chlorothalonil
(Environmental health criteria ; 183)
1.Fungicides, Industrial 2.Pesticides 3.Agrochemicals
4.Environmental exposure I.Series
ISBN 92 4 157183 7 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
Preamble
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity, physical and chemical properties, and
analytical methods
1.1.2. Sources of human and environmental exposure
1.1.3. Environmental transport, distribution and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in laboratory animals
1.1.6. Effects on laboratory mammals and in vitro test
systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory and
field
1.2. Evaluation
1.2.1. Evaluation of human health risks
1.2.2. Evaluation of effects on the environment
1.2.2.1 Transport, distribution and
transformation
1.2.2.2 Aquatic organisms
1.2.2.3 Terrestrial organisms
1.2.3. Toxicological criteria for setting guidance values
1.3. Conclusions and recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
2.3.1. Sample preparation
2.3.2. Analytical determination
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Production levels and processes
3.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Transformation
4.2.1. Biodegradation
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation
4.3. Waste disposal
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Food crops
5.1.5. Dairy produce
5.1.6. Animal feed
5.2. General population exposure
5.2.1. Food
5.3. Occupational exposure
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.3.1. Rat
6.3.2. Dog
6.3.3. Monkey
6.4. Elimination and excretion
6.4.1. Rat
6.4.2. Mouse
6.4.3. Dog
6.4.4. Monkey
6.5. Reaction with body components
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term exposure
7.2.1. Oral
7.2.1.1 Rat
7.2.1.2 Mouse
7.2.1.3 Dog
7.2.2. Dermal: Rabbit
7.3. Long-term exposure
7.3.1. Rat
7.3.2. Mouse
7.3.3. Dog
7.3.4. Summary of key dietary studies
7.4. Skin and eye irritation; sensitization
7.5. Reproductive and developmental toxicity
7.6. Mutagenicity
7.7. Carcinogenicity
7.8. Other special studies
7.9. Toxicity of metabolites
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.1.1 Aquatic microorganisms
9.1.1.2 Soil microorganisms
9.1.2. Aquatic organisms
9.1.3. Terrestrial organisms
9.1.3.1 Plants
9.1.3.2 Earthworms
9.1.3.3 Earwigs and honey-bees
9.1.3.4 Birds
9.2. Field observations
9.2.1. Soil microorganisms
9.2.2. Plants
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01 ES02617-
15 from the National Institute of Environmental Health Sciences,
National Institutes of Health, USA, and by financial support from the
European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure
to environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects
of pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
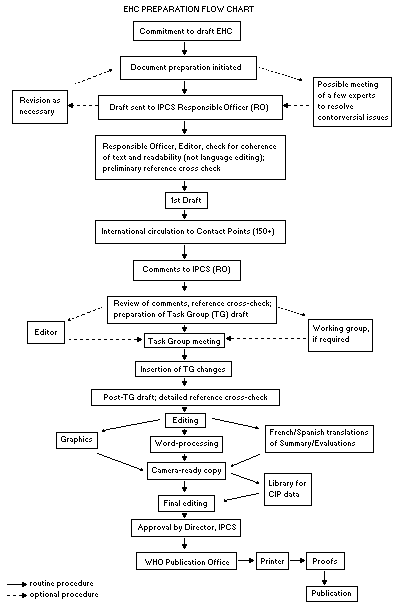
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
 additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom (Vice-
Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine,
University of Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate
School of Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist,
The Netherlands
Representatives of GIFAPa (Groupement International des
Associations Nationales de Fabricants de Produits Agrochimiques)
Dr M. Bliss, Jr., ISK Biosciences Corporation, Mentor, Ohio, USA
Dr A.C. Dykstra, Registration Department BPID, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr H. Frazier, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr R. Gardiner, GIFAP, Brussels, Belgium
Dr B. Julin, Regulatory Affairs, Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels, Belgium
Dr S.M. Kennedy (Environmental Science), Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels, Belgium
Dr J. Killeen, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr Th. S.M. Koopman, Toxicology Department, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr R.L. Mull, Du Pont Agricultural Products, Wilmington, Delaware, USA
Dr J.L.G. Thus, Environmental Research Department, Solvay-Duphar BV,
CP Weesp, The Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
a Participated as required for exchange of information.
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Pletina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides met in Geneva from 25 October to 3 November 1994. Dr W.
Kreisel of the WHO welcomed the participants on behalf of WHO, and
Dr M. Mercier, Director, IPCS, on behalf of the IPCS and its
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to chlorothalonil.
The first draft of the monograph was prepared by Dr M.H.
Litchfield, Arundel, United Kingdom. The second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs, was
prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The fact that ISK Biosciences Corporation made available to the
IPCS its proprietary toxicological information on chlorothalonil is
gratefully acknowledged. This allowed the CAG to make its evaluation
on a more complete database.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
BCF bioconcentration factor
BUN blood urea nitrogen
ECD electron capture detector
EDB 1,2-dibromoethane (ethylene dibromide)
FID flame ionization detector
GC gas chromatography
GSH glutathione
gamma-GT gamma-glutamyltranspeptidase
HECD Hall electron capture detector
LOEL lowest-observed-effect level
MS mass spectrometry
NADPH reduced nicotinamide adenine dinucleotide phosphate
NOEL no-observed-effect level
PIB piperonyl butoxide
SGOT serum glutamic-oxalic transaminase
SGPT serum glutamic-pyruvic transaminase
TEAM total exposure assessment methodology
TWA time-weighted average
UDS unscheduled DNA synthesis
VHH volatile halogenated hydrocarbon
VOC volatile organic carbon compound
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Chlorothalonil is a colourless, odourless, crystalline solid with
a melting point of 250°C and a vapour pressure of 7.63 × 10-5 Pa
(5.72 × 10-7 mmHg) at 25°C. It has low water solubility
(0.6-1.2 mg/litre at 25°C) and an octanol/water partition coefficient
(log Kow) of 2.882. It is hydrolysed in water slowly at pH 9 but is
stable at pH 7 or below (at 25°C).
The most prevalent analytical method, after sample extraction and
clean-up, is gas-liquid chromatography using an electron-capture
detector.
1.1.2 Sources of human and environmental exposure
Chlorothalonil has been produced commercially since 1969 by
chlorination of isophthalonitrile or by treatment of
tetrachloroisophthaloyl amide with phosphorus oxychloride. It is a
fungicide with a broad spectrum of activity used mainly in agriculture
but also on turf, lawns and ornamental plants. Crops protected
include pome and stone fruit, citrus, currants, berries, bananas,
tomatoes, green vegetables, coffee, peanuts, potatoes, onions and
cereals. In addition, it is used in wood preservatives and in paints.
The three main formulations are a suspension concentrate, a water
dispersible granule and a wettable powder. They are readily diluted
with water and applied by ground spray systems or by air. Typical
active ingredient rates are 1.2-2.5 kg/ha for crops such as beans,
celery and onions. The main sources of human exposure will be during
preparation and application of the products and from ingestion of crop
residues in foodstuff (see section 1.1.4).
1.1.3 Environmental transport, distribution and transformation
Chlorothalonil is removed from aqueous media by strong adsorption
on suspended matter. Modelled data suggest little or no partition to
bottom sediment. Biodegradation may occur in natural waters with
enzyme processes being involved. Chlorothalonil is rapidly degraded
in soil, and degradation may occur in water with the production of the
4-hydroxy metabolite, 4-hydroxy-2,5,6-trichloroisophthalonitrile.
Half-lives for dissipation of the 4-hydroxy metabolite in soils range
between 6 and 43 days.
Chlorothalonil does not translocate from the site of application
to other parts of a plant. It is metabolized only to a limited extent
on plants and the 4-hydroxy metabolite is usually < 5% of the
residue.
Chlorothalonil is metabolized in fish via glutathione conjugation
to give more polar excretory products. The enzyme glutathione-
S-transferase is involved with this conversion. High concentrations
of radiolabel found in the gall bladder and bile, after exposure of
rainbow trout to 14C-chlorothalonil, are consistent with the
excretion of the compound as glutathione conjugates. The
concentrations of radiolabel accumulating in the gall bladder and
other organs fell rapidly when the fish were placed in clean water.
Chlorothalonil does not bioaccumulate in aquatic organisms.
1.1.4 Environmental levels and human exposure
In a potato crop study, a small stream was oversprayed with
chlorothalonil. Subsequent sampling/analysis of down-stream water
demonstrated rapid disappearance of chlorothalonil (i.e. 450 µg/litre
at 30 min post-spraying to 2-6 µg/litre at 12 h post-spraying). The
routine spraying of irrigated field crops such as potatoes and barley
gave rise to low concentrations of chlorothalonil (0.04-3.6 µg/litre)
in tile drain water on a small number of sampling occasions.
Crop residues are composed mainly of chlorothalonil itself. The
residue levels are dependent upon the applied rate, time interval
between the last application and harvest, and the type of crop.
Residue levels at harvest can be derived from the numerous supervised
trials that have taken place on many crops worldwide and reported to
FAO/WHO. Residues of chlorothalonil in dairy products are expected to
be undetectable or very low. Dairy cows given high concentrations (up
to 250 mg/kg) of chlorothalonil in their feed for 30 days showed no
detectable residue in milk and only very low levels in tissues.
Total diet and individual food analysis in several countries have
shown undetectable or low concentrations of chlorothalonil in sampling
surveys. Residue levels on foodstuffs are further reduced by
preparation processes such as washing, peeling and cooking.
1.1.5 Kinetics and metabolism in laboratory animals
About 30% of an oral dose of chlorothalonil is absorbed within
48 h in rats at doses up to 50 mg/kg body weight. At higher doses,
absorption is lower, indicating a saturation process. When
14C-chlorothalonil is given orally the radioactivity is distributed
into blood and tissues within 2 h. The greatest concentration is
found in the kidney, followed by liver and blood. The kidneys contain
0.3% of a 5 mg/kg body weight dose after 24 h.
Most of an oral dose of chlorothalonil in rats is found in faeces
(> 82% within 48-72 h, regardless of dose). Biliary excretion is
rapid, peaking within 2 h after a 5 mg/kg body weight oral dose, and
is saturated at 50 mg/kg body weight and above. Urinary excretion
accounts for 5-10% of the dose in rats. Faecal excretion is the main
route in dogs and monkeys but urinary excretion (< 4%) is less than
in rats.
Metabolic studies in rats indicate that chlorothalonil is
conjugated with glutathione in the liver as well as in the
gastrointestinal tract. Some of the glutathione conjugates may be
absorbed from the intestine and transported to the kidneys, where they
are converted by cytosolic ß-lyase to thiol analogues that are
excreted in the urine. When germ-free rats are dosed with
chlorothalonil, the thiol metabolites appear in urine in much smaller
amounts than with normal rats, indicating the involvement of
intestinal microflora in the metabolism of chlorothalonil. Dogs or
monkeys dosed orally with chlorothalonil excrete little or no thiol
derivatives in urine.
When 14C-chlorothalonil was applied to rat skin, approximately
28% of the dose was absorbed within 120 h. About 18% of the dose was
found in faeces and 6% in urine within 120 h.
1.1.6 Effects on laboratory mammals and in vitro test systems
Chlorothalonil has low acute oral and dermal toxicity in rats and
rabbits, respectively (acute oral and dermal LD50 values are
> 10 000 mg/kg body weight). Hammer-milled technical chlorothalonil
(MMAD 5-8 µm) exhibited high toxicity in rats in an inhalation study,
with a 4-h LC50 of 0.1 mg/litre.
Chlorothalonil is a skin and eye irritant in the rabbit. Skin
sensitization studies in the guinea-pig were inconclusive.
The main effects of repeated oral dosing in rats are on the
stomach and kidney. Groups of 25 rats of each sex per group were fed
chlorothalonil at 0, 1.5, 3, 10 or 40 mg/kg body weight per day in the
diet for 13 weeks, and this was followed by a 13-week recovery period.
Increased incidences of hyperplasia and hyperkeratosis of the
forestomach occurred at 10 and 40 mg/kg; these reversed when treatment
ceased. At 40 mg/kg, there was an increased incidence of hyperplasia
of kidney proximal tubular epithelium in males at 13 weeks and after
the recovery period. The NOEL was 3 mg/kg body weight per day based
upon lack of forestomach lesions. The onset of the forestomach and
kidney changes was shown to be rapid, with the lesions developing
within 4-7 days in male rats at a dietary level of 175 mg/kg body
weight per day.
In a 13-week study on mice (0, 7.5, 15, 50, 275 or 750 mg/kg in
the diet), increased incidences of hyperplasia and hyperkeratosis of
the squamous epithelial cells of the forestomach occurred in males and
females at 50 mg/kg diet and above. The NOEL, based upon these
changes, was 15 mg/kg chlorothalonil in the diet, equivalent to
3 mg/kg body weight per day.
A 16-week study in dogs with dietary levels of 0, 250, 500 or
750 mg/kg showed no treatment-related changes.
The forestomach and kidney lesions were investigated further in
2-year studies on rats, mice and dogs. In a study on rats (0, 1.8,
3.8, 15 or 175 mg/kg body weight per day), the effects were
characterized histologically as an increase in the incidence and
severity of hyperplasia, hyperkeratosis, and ulcers and erosions of
the squamous mucosa of the forestomach, and as epithelial hyperplasia
of the kidney proximal convoluted tubules at 3.8 mg/kg and above. The
NOEL for non-neoplastic effects was therefore 1.8 mg/kg. The
incidence of renal tumours (adenomas and carcinomas) and forestomach
tumours (papillomas and carcinomas) was markedly increased at
175 mg/kg. There was evidence for an increased incidence of kidney
tumours in males at 15 mg/kg and of stomach tumours at 3.8 and
15 mg/kg in males and females. The NOEL for neoplastic effects was
therefore 1.8 mg/kg body weight per day based upon changes in
forestomach tumour incidence. Supporting evidence for the
carcinogenic potential of chlorothalonil in the kidney and forestomach
of rats was provided by the results from other 2-year studies at
higher dose levels.
In a study on mice (0, 15, 40, 175 or 750 mg/kg in the diet), an
increased incidence of renal tubular hyperplasia occurred at 175 mg/kg
and above and of hyperplasia and hyperkeratosis of the forestomach at
40 mg/kg and above. The incidence of squamous tumours of the
forestomach was slightly increased at 750 mg/kg. The NOELs for
neoplastic and non-neoplastic changes were therefore 175 and 15 mg/kg
in the diet (equivalent to 17.5 and 1.6 mg/kg body weight per day,
respectively). Supporting evidence for these effects in the mouse was
provided in another study at higher dose levels, but a study in
B6C3F1 mice did not show any evidence for carcinogenic potential at
high dose levels.
In a 2-year study on dogs (60 and 120 mg/kg in the diet), no
effects attributable to chlorothalonil were found. The NOEL was
therefore 120 mg/kg in the diet (equivalent to 3 mg/kg body weight per
day).
Chlorothalonil was not mutagenic in several in vitro and in
vivo tests, although it was positive in a small number of assays.
The monothio, dithio, trithio, dicysteine, tricysteine and
monoglutathione derivatives of chlorothalonil, which are potential
nephrotoxicants, were shown to be negative in the Ames assay.
Chlorothalonil was not teratogenic in rats or rabbits at doses up
to 400 and 50 mg/kg body weight per day, respectively. Reproductive
parameters such as mating, fertility and gestation length were not
affected by chlorothalonil at levels up to 1500 mg/kg in the diet in a
two-generation study in rats.
The acute oral toxicity of the 4-hydroxy metabolite is greater
than that of chlorothalonil itself (acute oral LD50 of 332 mg/kg body
weight versus > 10 000 mg/kg body weight). Several studies have been
undertaken to characterize the toxicological profile of this
metabolite and to establish NOELs.
1.1.7 Effects on humans
Contact dermatitis has been reported for personnel working in
chlorothalonil manufacturing and in farmers and horticultural workers.
Workers in the manufacture of wood products have also developed
contact dermatitis on the hands and face when wood preservatives
containing chlorothalonil were used.
1.1.8 Effects on other organisms in the laboratory and field
Chlorothalonil is highly toxic to fish and aquatic invertebrates
in laboratory studies, the LC50 values being below 0.5 mg/litre. The
maximum acceptable toxicant concentration (MATC) in a two-generation
reproduction study in Daphnia magna was 35 µg/litre.
With minor exceptions, chlorothalonil is not phytotoxic.
The LC50 of a suspension concentrate formulation (500 g
chlorothalonil/litre) in artificial soil for earthworms was
> 1000 mg/kg soil (14 days). Earwigs suffered increased mortality
when in contact with chlorothalonil residues on peanut foliage or
ingesting it as a food source in laboratory tests; there was no other
indication of insecticidal action.
Chlorothalonil is of low toxicity to birds with a reported acute
oral LD50 of 4640 mg/kg diet in the mallard duck. No significant
reproductive effects were reported.
A field study of aquatic organisms exposed following
chlorothalonil application suggests that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physicochemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field. There have been no
reported incidents of kills in the environment. However, despite the
short residence time of chlorothalonil in environmental media, kills
would be expected to occur. Linking kills to the compound would be
difficult given that residues would not persist long enough for
chlorothalonil to be identified.
1.2 Evaluation
1.2.1 Evaluation of human health risks
The review of the toxicological data for chlorothalonil revealed
that the most important studies for human risk estimation were the
long-term studies in rodents and dogs.
In the rodent studies, chlorothalonil caused lesions in the
forestomach and kidney. The lesions in the forestomach were
characterized as hyperplasia and hyperkeratosis of the squamous
epithelial cells. These occurred soon after dosing and were shown to
be reversible after dosing ceased. Long-term administration led to
the formation of tumours (papilloma and carcinoma). The renal lesions
in rodents were of rapid onset and characterized as hyperplasia of the
proximal tubular epithelium. On longer-term administration, renal
tumours (adenoma and carcinoma) occurred in the rat and in one study
on mice.
In order to interpret the significance of these findings, the
results of the mutagenic studies were taken into account.
Chlorothalonil gave negative results in in vitro and in vivo
mutagenic assays in which a variety of end-points were studied. Thiol
derivatives of chlorothalonil were negative in the Ames test, and
14C-chlorothalonil did not bind to rat kidney DNA in vivo. The
compound does not appear to have genotoxic potential on this basis,
indicating that it probably exerts its carcinogenic effect in rodents
via a non-genotoxic mechanism. The initial forestomach lesions in
rodents were attributed to the irritant action of chlorothalonil, and,
where this does not occur, a NOEL can be attained. The irritant action
on rodent forestomach in conjunction with the relatively long
residence time of the compound in this organ were seen to be factors
presenting the opportunity for the initiation of the lesions and
leading to carcinogenic action on prolonged administration. It was
concluded that, since humans do not possess a comparable organ,
rodents are probably not representative of the action of this compound
in man in this respect. This reasoning is also supported by the fact
that another animal species, the dog, is not affected by the compound
at similar or higher doses.
In the assessment of the relevance of the rodent renal lesions,
the metabolic conversion of chlorothalonil to metabolites which act
directly upon the kidney was seen to be a major factor. In the kidney
glutathione conjugates are converted by ß-lyase to chlorothalonil
thiol derivatives. Chlorothalonil is thought to be conjugated with
glutathione (GSH) mostly in the gastrointestinal tract prior to
absorption, although there is evidence of glutathione conjugation at
other sites. After absorption the conjugates pass to the kidney where
they are converted to chlorothalonil thiol derivatives following the
action of ß-lyase. It has been shown in vitro that the di- and
trithiol metabolites inhibit the function of renal cortical
mitochondria. Therefore, a cycle of cell death and regenerative renal
hyperplasia may be initiated.
In adducing the relevance of these findings for humans, the
species differences in the metabolic pathway for chlorothalonil were
taken into account. It was noted that the formation of the thiol
metabolites, as determined by urinary excretion, was considerably
diminished when chlorothalonil was fed to germ-free rats. This
indicates that the type and/or quantity of gut microflora has a
determining role in the production of the thiol derivatives. Studies
in dogs and monkeys showed that the excretion of the thiol derivatives
was barely detectable after oral administration of chlorothalonil.
This suggests that the rat is rather different from other species in
this respect. Furthermore there is some evidence that ß-lyase
activity in the kidney varies among species, being an order of
magnitude lower in humans than in rats.
For all the reasons stated above it was concluded that the rodent
was not the most relevant species for evaluating the long-term effect
of chlorothalonil in humans and that the dog was a more representative
species for this purpose. The NOEL of 120 mg/kg in the diet in the
2-year study on dogs, equivalent to 3 mg/kg body weight per day,
should therefore be used for the purpose of human risk estimation.
1.2.2 Evaluation of effects on the environment
Chlorothalonil is algicidal for a number of algal species. The
fungicide does not inhibit bacterial growth except at very high
concentrations in laboratory culture. Field and laboratory evidence
shows no effects on nitrogen fixation or nitrification at recommended
application rates and minimal effects at higher application rates in
temperate soils. There was insufficient information to assess effects
on the nitrogen cycle in tropical soils.
Laboratory acute toxicity tests show chlorothalonil to be very
highly toxic to many aquatic animals including fish and Daphnia,
although molluscs appear to be insensitive. The LC50 concentrations
for a range of fish and invertebrates are similar and below
0.5 mg/litre.
A single study indicated reproductive effects in fish following
continuous exposure for 35 days. Since the compound both adsorbs to
suspended material and is degraded rapidly, the significance of this
finding was considered to be questionable.
A field study of aquatic organisms exposed following
chlorothalonil application suggests that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physicochemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field. There have been no
reported incidents of kills in the environment. However, despite the
short residence time of chlorothalonil in environmental media, kills
would be expected to occur immediately after application. Linking
kills to the compound would be difficult given that residues would not
persist long enough for chlorothalonil to be identified.
With minor exceptions, chlorothalonil is not phytotoxic.
Several studies have shown no toxicity of chlorothalonil to
earthworms at recommended application rates. At an exposure of five
times the maximum recommended rate, the compound severely reduced worm
reproduction.
Chlorothalonil is classified as "relatively non-toxic" to
honey-bees. Earwigs exposed to residues topically and via food showed
some mortality (20-55%), but there is no other evidence of
insecticidal action.
Chlorothalonil has low toxicity to birds in acute or dietary
tests. The low acute toxicity of chlorothalonil to laboratory mammals
tempered with its short persistence in the environment suggests
minimal hazard to wild mammal species.
1.2.2.1 Transport, distribution and transformation
Chlorothalonil adsorbs strongly to organic matter in soil and
suspended material in water. It is not, therefore, leached from soil
to groundwater. It is removed rapidly from surface water to suspended
material and to a lesser extent to bottom sediment. Chlorothalonil is
not translocated in plants from the site of application.
Abiotic degradation of chlorothalonil in water through photolysis
does not occur. Some hydrolysis does take place at higher pH.
Microbial degradation is the major cause of dissipation in soil
and may take place to some extent in water; this involves several
parallel processes, one of which leads to formation of the 4-hydroxy
metabolite. Half-lives for dissipation of this metabolite from non-
sterile soils range between 6 and 43 days. Biodegradation on plants
is limited and the 4-hydroxy metabolite comprises less than 5% of the
total residues.
During exposure, fish bioconcentrate chlorothalonil, but almost
total degradation occurs within 2 weeks after termination of exposure.
Chlorothalonil is metabolized in fish through glutathione conjugation
and the conjugates are excreted through the bile.
1.2.2.2 Aquatic organisms
The results of a single field study measuring concentrations of
chlorothalonil in water following overspray of the water were
available; corresponding data on concentrations in suspended and
bottom sediment were unreliable. Output from the EXAMS II fate model
using the same application scenario produced estimated water
concentrations which closely corresponded to the measured ones.
Little or no chlorothalonil was predicted in bottom sediment.
Based on this combination of measured and modelled data, the
ratio between a "toxic" concentration (the rainbow trout LC50) and
expected concentration is less than 1 for up to 5 h after overspray
and increases rapidly thereafter. Similar results were obtained for
daphnids. Therefore, despite its rapid removal from water and
degradation, the high toxicity of chlorothalonil is expected to cause
deaths of aquatic organisms in the period immediately after spraying.
This is the worst case situation of direct water overspray.
There were no data to extend this quantitative evaluation to
other field situations or climates.
1.2.2.3 Terrestrial organisms
A calculated maximum soil concentration, based on application of
chlorothalonil at 2.5 kg a.i./ha and complete bioavailability, is 3
orders of magnitude higher than the lowest estimate of LC50 for
earthworms.
For grazing birds (ducks and geese) total daily intake is at
least a factor of 100 below the NOEL for oral toxicity. For rabbits,
total daily intake is also at least 2 orders of magnitude lower than
the reported NOEL. This is based on a maximum recommended application
rate of 2.5 kg a.i./ha, an estimated worst case value for residues on
grass, no degradation of the compound, consumption of the total daily
intake at a single time and no choice but to eat contaminated food.
Table 1 contains a summary of risk quotients for birds, fish and
aquatic invertebrates.
Table 1. Toxicity/exposure ratios for birds, fish and aquatic invertebrates based
on application rates of 2.5 kg a.i./ha of chlorothalonil to soybeans
(worst case)
Risk category LC50 (mg/litre Estimated exposure Toxicity/exposure
or mg/kg diet) (mg/litre or ratio (TER)c
mg/kg diet)a,b
Acute bird 4640 73.7-535.7 63.0-8.7
Acute fish (stream) 0.01 0.009-0.04 1.1-0.25
Acute fish (pond) 0.01 0.01 1.0
Acute aquatic
invertebrate (stream) 0.07 0.009-0.04 7.8-1.8
Acute aquatic
invertebrate (pond) 0.07 0.01 7.0
a Estimated environmental concentration in the terrestrial environment (for bird exposure)
is based on the stated application rate and the assumption of deposition on short grass
using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STREAM model based on a single
application and estimated run-off into water; no direct overspray is included.
c TER is the toxicity (as LC50) divided by the exposure; values at or below 1.0 indicate
likely exposure to toxic concentrations by organisms in the different risk categories.
1.2.3 Toxicological criteria for setting guidance values
The toxicological studies on chlorothalonil of relevance for
setting guidance values are displayed in Table 2. The study results
and their significance are described briefly and gaps in test
requirements are indicated.
Table 2. Toxicological criteria for setting guidance values for chlorothalonil
Exposure Relevant route/effect/ Result/remarks
scenario species
Short-term skin, irritation, rabbit irritant
(1-7 days)
eye, irritation, rabbit irritant
skin, sensitization, tests were inconclusive
guinea-pig
evidence in humans of contact
dermatitis
inhalation, lethality, high toxicity in 4-h study with
rat hammermilled technical chlorothalonil
(MMAD 5-8 µm); not relevant for most
human exposure situations
Medium-term repeat dermal, rabbit 21-day study; irritant at 2.5 mg/kg
(1-26 weeks) body weight per day and above; no
systemic effects at 50 mg/kg body
weight per day
repeat oral, mice and 13-22 week studies; NOEL = 3 mg/kg
rats body weight per day in rats and mice
maternal, oral, rabbit teratology study; maternal toxicity
NOEL = 10 mg/kg body weight per day
by gavage; no fetotoxic or teratogenic
effect
Long-term repeat oral, dog 2-year study; NOEL = 3 mg/kg body
weight per day
1.3 Conclusions and recommendations
Considering the toxicological characteristics of chlorothalonil,
both qualitatively and quantitatively, it was concluded, on the basis
of the NOEL of 3 mg/kg body weight per day derived in the 2-year study
on dogs and applying a 100-fold uncertainty factor, that 0.03 mg/kg
body weight per day will probably not cause adverse effects in humans
by any route of exposure.
A study to assess the skin irritation potential is needed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom (Vice-
Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine,
University of Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate
School of Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist,
The Netherlands
Representatives of GIFAPa (Groupement International des
Associations Nationales de Fabricants de Produits Agrochimiques)
Dr M. Bliss, Jr., ISK Biosciences Corporation, Mentor, Ohio, USA
Dr A.C. Dykstra, Registration Department BPID, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr H. Frazier, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr R. Gardiner, GIFAP, Brussels, Belgium
Dr B. Julin, Regulatory Affairs, Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels, Belgium
Dr S.M. Kennedy (Environmental Science), Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels, Belgium
Dr J. Killeen, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr Th. S.M. Koopman, Toxicology Department, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr R.L. Mull, Du Pont Agricultural Products, Wilmington, Delaware, USA
Dr J.L.G. Thus, Environmental Research Department, Solvay-Duphar BV,
CP Weesp, The Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
a Participated as required for exchange of information.
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Pletina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides met in Geneva from 25 October to 3 November 1994. Dr W.
Kreisel of the WHO welcomed the participants on behalf of WHO, and
Dr M. Mercier, Director, IPCS, on behalf of the IPCS and its
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to chlorothalonil.
The first draft of the monograph was prepared by Dr M.H.
Litchfield, Arundel, United Kingdom. The second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs, was
prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The fact that ISK Biosciences Corporation made available to the
IPCS its proprietary toxicological information on chlorothalonil is
gratefully acknowledged. This allowed the CAG to make its evaluation
on a more complete database.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
BCF bioconcentration factor
BUN blood urea nitrogen
ECD electron capture detector
EDB 1,2-dibromoethane (ethylene dibromide)
FID flame ionization detector
GC gas chromatography
GSH glutathione
gamma-GT gamma-glutamyltranspeptidase
HECD Hall electron capture detector
LOEL lowest-observed-effect level
MS mass spectrometry
NADPH reduced nicotinamide adenine dinucleotide phosphate
NOEL no-observed-effect level
PIB piperonyl butoxide
SGOT serum glutamic-oxalic transaminase
SGPT serum glutamic-pyruvic transaminase
TEAM total exposure assessment methodology
TWA time-weighted average
UDS unscheduled DNA synthesis
VHH volatile halogenated hydrocarbon
VOC volatile organic carbon compound
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Chlorothalonil is a colourless, odourless, crystalline solid with
a melting point of 250°C and a vapour pressure of 7.63 × 10-5 Pa
(5.72 × 10-7 mmHg) at 25°C. It has low water solubility
(0.6-1.2 mg/litre at 25°C) and an octanol/water partition coefficient
(log Kow) of 2.882. It is hydrolysed in water slowly at pH 9 but is
stable at pH 7 or below (at 25°C).
The most prevalent analytical method, after sample extraction and
clean-up, is gas-liquid chromatography using an electron-capture
detector.
1.1.2 Sources of human and environmental exposure
Chlorothalonil has been produced commercially since 1969 by
chlorination of isophthalonitrile or by treatment of
tetrachloroisophthaloyl amide with phosphorus oxychloride. It is a
fungicide with a broad spectrum of activity used mainly in agriculture
but also on turf, lawns and ornamental plants. Crops protected
include pome and stone fruit, citrus, currants, berries, bananas,
tomatoes, green vegetables, coffee, peanuts, potatoes, onions and
cereals. In addition, it is used in wood preservatives and in paints.
The three main formulations are a suspension concentrate, a water
dispersible granule and a wettable powder. They are readily diluted
with water and applied by ground spray systems or by air. Typical
active ingredient rates are 1.2-2.5 kg/ha for crops such as beans,
celery and onions. The main sources of human exposure will be during
preparation and application of the products and from ingestion of crop
residues in foodstuff (see section 1.1.4).
1.1.3 Environmental transport, distribution and transformation
Chlorothalonil is removed from aqueous media by strong adsorption
on suspended matter. Modelled data suggest little or no partition to
bottom sediment. Biodegradation may occur in natural waters with
enzyme processes being involved. Chlorothalonil is rapidly degraded
in soil, and degradation may occur in water with the production of the
4-hydroxy metabolite, 4-hydroxy-2,5,6-trichloroisophthalonitrile.
Half-lives for dissipation of the 4-hydroxy metabolite in soils range
between 6 and 43 days.
Chlorothalonil does not translocate from the site of application
to other parts of a plant. It is metabolized only to a limited extent
on plants and the 4-hydroxy metabolite is usually < 5% of the
residue.
Chlorothalonil is metabolized in fish via glutathione conjugation
to give more polar excretory products. The enzyme glutathione-
S-transferase is involved with this conversion. High concentrations
of radiolabel found in the gall bladder and bile, after exposure of
rainbow trout to 14C-chlorothalonil, are consistent with the
excretion of the compound as glutathione conjugates. The
concentrations of radiolabel accumulating in the gall bladder and
other organs fell rapidly when the fish were placed in clean water.
Chlorothalonil does not bioaccumulate in aquatic organisms.
1.1.4 Environmental levels and human exposure
In a potato crop study, a small stream was oversprayed with
chlorothalonil. Subsequent sampling/analysis of down-stream water
demonstrated rapid disappearance of chlorothalonil (i.e. 450 µg/litre
at 30 min post-spraying to 2-6 µg/litre at 12 h post-spraying). The
routine spraying of irrigated field crops such as potatoes and barley
gave rise to low concentrations of chlorothalonil (0.04-3.6 µg/litre)
in tile drain water on a small number of sampling occasions.
Crop residues are composed mainly of chlorothalonil itself. The
residue levels are dependent upon the applied rate, time interval
between the last application and harvest, and the type of crop.
Residue levels at harvest can be derived from the numerous supervised
trials that have taken place on many crops worldwide and reported to
FAO/WHO. Residues of chlorothalonil in dairy products are expected to
be undetectable or very low. Dairy cows given high concentrations (up
to 250 mg/kg) of chlorothalonil in their feed for 30 days showed no
detectable residue in milk and only very low levels in tissues.
Total diet and individual food analysis in several countries have
shown undetectable or low concentrations of chlorothalonil in sampling
surveys. Residue levels on foodstuffs are further reduced by
preparation processes such as washing, peeling and cooking.
1.1.5 Kinetics and metabolism in laboratory animals
About 30% of an oral dose of chlorothalonil is absorbed within
48 h in rats at doses up to 50 mg/kg body weight. At higher doses,
absorption is lower, indicating a saturation process. When
14C-chlorothalonil is given orally the radioactivity is distributed
into blood and tissues within 2 h. The greatest concentration is
found in the kidney, followed by liver and blood. The kidneys contain
0.3% of a 5 mg/kg body weight dose after 24 h.
Most of an oral dose of chlorothalonil in rats is found in faeces
(> 82% within 48-72 h, regardless of dose). Biliary excretion is
rapid, peaking within 2 h after a 5 mg/kg body weight oral dose, and
is saturated at 50 mg/kg body weight and above. Urinary excretion
accounts for 5-10% of the dose in rats. Faecal excretion is the main
route in dogs and monkeys but urinary excretion (< 4%) is less than
in rats.
Metabolic studies in rats indicate that chlorothalonil is
conjugated with glutathione in the liver as well as in the
gastrointestinal tract. Some of the glutathione conjugates may be
absorbed from the intestine and transported to the kidneys, where they
are converted by cytosolic ß-lyase to thiol analogues that are
excreted in the urine. When germ-free rats are dosed with
chlorothalonil, the thiol metabolites appear in urine in much smaller
amounts than with normal rats, indicating the involvement of
intestinal microflora in the metabolism of chlorothalonil. Dogs or
monkeys dosed orally with chlorothalonil excrete little or no thiol
derivatives in urine.
When 14C-chlorothalonil was applied to rat skin, approximately
28% of the dose was absorbed within 120 h. About 18% of the dose was
found in faeces and 6% in urine within 120 h.
1.1.6 Effects on laboratory mammals and in vitro test systems
Chlorothalonil has low acute oral and dermal toxicity in rats and
rabbits, respectively (acute oral and dermal LD50 values are
> 10 000 mg/kg body weight). Hammer-milled technical chlorothalonil
(MMAD 5-8 µm) exhibited high toxicity in rats in an inhalation study,
with a 4-h LC50 of 0.1 mg/litre.
Chlorothalonil is a skin and eye irritant in the rabbit. Skin
sensitization studies in the guinea-pig were inconclusive.
The main effects of repeated oral dosing in rats are on the
stomach and kidney. Groups of 25 rats of each sex per group were fed
chlorothalonil at 0, 1.5, 3, 10 or 40 mg/kg body weight per day in the
diet for 13 weeks, and this was followed by a 13-week recovery period.
Increased incidences of hyperplasia and hyperkeratosis of the
forestomach occurred at 10 and 40 mg/kg; these reversed when treatment
ceased. At 40 mg/kg, there was an increased incidence of hyperplasia
of kidney proximal tubular epithelium in males at 13 weeks and after
the recovery period. The NOEL was 3 mg/kg body weight per day based
upon lack of forestomach lesions. The onset of the forestomach and
kidney changes was shown to be rapid, with the lesions developing
within 4-7 days in male rats at a dietary level of 175 mg/kg body
weight per day.
In a 13-week study on mice (0, 7.5, 15, 50, 275 or 750 mg/kg in
the diet), increased incidences of hyperplasia and hyperkeratosis of
the squamous epithelial cells of the forestomach occurred in males and
females at 50 mg/kg diet and above. The NOEL, based upon these
changes, was 15 mg/kg chlorothalonil in the diet, equivalent to
3 mg/kg body weight per day.
A 16-week study in dogs with dietary levels of 0, 250, 500 or
750 mg/kg showed no treatment-related changes.
The forestomach and kidney lesions were investigated further in
2-year studies on rats, mice and dogs. In a study on rats (0, 1.8,
3.8, 15 or 175 mg/kg body weight per day), the effects were
characterized histologically as an increase in the incidence and
severity of hyperplasia, hyperkeratosis, and ulcers and erosions of
the squamous mucosa of the forestomach, and as epithelial hyperplasia
of the kidney proximal convoluted tubules at 3.8 mg/kg and above. The
NOEL for non-neoplastic effects was therefore 1.8 mg/kg. The
incidence of renal tumours (adenomas and carcinomas) and forestomach
tumours (papillomas and carcinomas) was markedly increased at
175 mg/kg. There was evidence for an increased incidence of kidney
tumours in males at 15 mg/kg and of stomach tumours at 3.8 and
15 mg/kg in males and females. The NOEL for neoplastic effects was
therefore 1.8 mg/kg body weight per day based upon changes in
forestomach tumour incidence. Supporting evidence for the
carcinogenic potential of chlorothalonil in the kidney and forestomach
of rats was provided by the results from other 2-year studies at
higher dose levels.
In a study on mice (0, 15, 40, 175 or 750 mg/kg in the diet), an
increased incidence of renal tubular hyperplasia occurred at 175 mg/kg
and above and of hyperplasia and hyperkeratosis of the forestomach at
40 mg/kg and above. The incidence of squamous tumours of the
forestomach was slightly increased at 750 mg/kg. The NOELs for
neoplastic and non-neoplastic changes were therefore 175 and 15 mg/kg
in the diet (equivalent to 17.5 and 1.6 mg/kg body weight per day,
respectively). Supporting evidence for these effects in the mouse was
provided in another study at higher dose levels, but a study in
B6C3F1 mice did not show any evidence for carcinogenic potential at
high dose levels.
In a 2-year study on dogs (60 and 120 mg/kg in the diet), no
effects attributable to chlorothalonil were found. The NOEL was
therefore 120 mg/kg in the diet (equivalent to 3 mg/kg body weight per
day).
Chlorothalonil was not mutagenic in several in vitro and in
vivo tests, although it was positive in a small number of assays.
The monothio, dithio, trithio, dicysteine, tricysteine and
monoglutathione derivatives of chlorothalonil, which are potential
nephrotoxicants, were shown to be negative in the Ames assay.
Chlorothalonil was not teratogenic in rats or rabbits at doses up
to 400 and 50 mg/kg body weight per day, respectively. Reproductive
parameters such as mating, fertility and gestation length were not
affected by chlorothalonil at levels up to 1500 mg/kg in the diet in a
two-generation study in rats.
The acute oral toxicity of the 4-hydroxy metabolite is greater
than that of chlorothalonil itself (acute oral LD50 of 332 mg/kg body
weight versus > 10 000 mg/kg body weight). Several studies have been
undertaken to characterize the toxicological profile of this
metabolite and to establish NOELs.
1.1.7 Effects on humans
Contact dermatitis has been reported for personnel working in
chlorothalonil manufacturing and in farmers and horticultural workers.
Workers in the manufacture of wood products have also developed
contact dermatitis on the hands and face when wood preservatives
containing chlorothalonil were used.
1.1.8 Effects on other organisms in the laboratory and field
Chlorothalonil is highly toxic to fish and aquatic invertebrates
in laboratory studies, the LC50 values being below 0.5 mg/litre. The
maximum acceptable toxicant concentration (MATC) in a two-generation
reproduction study in Daphnia magna was 35 µg/litre.
With minor exceptions, chlorothalonil is not phytotoxic.
The LC50 of a suspension concentrate formulation (500 g
chlorothalonil/litre) in artificial soil for earthworms was
> 1000 mg/kg soil (14 days). Earwigs suffered increased mortality
when in contact with chlorothalonil residues on peanut foliage or
ingesting it as a food source in laboratory tests; there was no other
indication of insecticidal action.
Chlorothalonil is of low toxicity to birds with a reported acute
oral LD50 of 4640 mg/kg diet in the mallard duck. No significant
reproductive effects were reported.
A field study of aquatic organisms exposed following
chlorothalonil application suggests that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physicochemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field. There have been no
reported incidents of kills in the environment. However, despite the
short residence time of chlorothalonil in environmental media, kills
would be expected to occur. Linking kills to the compound would be
difficult given that residues would not persist long enough for
chlorothalonil to be identified.
1.2 Evaluation
1.2.1 Evaluation of human health risks
The review of the toxicological data for chlorothalonil revealed
that the most important studies for human risk estimation were the
long-term studies in rodents and dogs.
In the rodent studies, chlorothalonil caused lesions in the
forestomach and kidney. The lesions in the forestomach were
characterized as hyperplasia and hyperkeratosis of the squamous
epithelial cells. These occurred soon after dosing and were shown to
be reversible after dosing ceased. Long-term administration led to
the formation of tumours (papilloma and carcinoma). The renal lesions
in rodents were of rapid onset and characterized as hyperplasia of the
proximal tubular epithelium. On longer-term administration, renal
tumours (adenoma and carcinoma) occurred in the rat and in one study
on mice.
In order to interpret the significance of these findings, the
results of the mutagenic studies were taken into account.
Chlorothalonil gave negative results in in vitro and in vivo
mutagenic assays in which a variety of end-points were studied. Thiol
derivatives of chlorothalonil were negative in the Ames test, and
14C-chlorothalonil did not bind to rat kidney DNA in vivo. The
compound does not appear to have genotoxic potential on this basis,
indicating that it probably exerts its carcinogenic effect in rodents
via a non-genotoxic mechanism. The initial forestomach lesions in
rodents were attributed to the irritant action of chlorothalonil, and,
where this does not occur, a NOEL can be attained. The irritant action
on rodent forestomach in conjunction with the relatively long
residence time of the compound in this organ were seen to be factors
presenting the opportunity for the initiation of the lesions and
leading to carcinogenic action on prolonged administration. It was
concluded that, since humans do not possess a comparable organ,
rodents are probably not representative of the action of this compound
in man in this respect. This reasoning is also supported by the fact
that another animal species, the dog, is not affected by the compound
at similar or higher doses.
In the assessment of the relevance of the rodent renal lesions,
the metabolic conversion of chlorothalonil to metabolites which act
directly upon the kidney was seen to be a major factor. In the kidney
glutathione conjugates are converted by ß-lyase to chlorothalonil
thiol derivatives. Chlorothalonil is thought to be conjugated with
glutathione (GSH) mostly in the gastrointestinal tract prior to
absorption, although there is evidence of glutathione conjugation at
other sites. After absorption the conjugates pass to the kidney where
they are converted to chlorothalonil thiol derivatives following the
action of ß-lyase. It has been shown in vitro that the di- and
trithiol metabolites inhibit the function of renal cortical
mitochondria. Therefore, a cycle of cell death and regenerative renal
hyperplasia may be initiated.
In adducing the relevance of these findings for humans, the
species differences in the metabolic pathway for chlorothalonil were
taken into account. It was noted that the formation of the thiol
metabolites, as determined by urinary excretion, was considerably
diminished when chlorothalonil was fed to germ-free rats. This
indicates that the type and/or quantity of gut microflora has a
determining role in the production of the thiol derivatives. Studies
in dogs and monkeys showed that the excretion of the thiol derivatives
was barely detectable after oral administration of chlorothalonil.
This suggests that the rat is rather different from other species in
this respect. Furthermore there is some evidence that ß-lyase
activity in the kidney varies among species, being an order of
magnitude lower in humans than in rats.
For all the reasons stated above it was concluded that the rodent
was not the most relevant species for evaluating the long-term effect
of chlorothalonil in humans and that the dog was a more representative
species for this purpose. The NOEL of 120 mg/kg in the diet in the
2-year study on dogs, equivalent to 3 mg/kg body weight per day,
should therefore be used for the purpose of human risk estimation.
1.2.2 Evaluation of effects on the environment
Chlorothalonil is algicidal for a number of algal species. The
fungicide does not inhibit bacterial growth except at very high
concentrations in laboratory culture. Field and laboratory evidence
shows no effects on nitrogen fixation or nitrification at recommended
application rates and minimal effects at higher application rates in
temperate soils. There was insufficient information to assess effects
on the nitrogen cycle in tropical soils.
Laboratory acute toxicity tests show chlorothalonil to be very
highly toxic to many aquatic animals including fish and Daphnia,
although molluscs appear to be insensitive. The LC50 concentrations
for a range of fish and invertebrates are similar and below
0.5 mg/litre.
A single study indicated reproductive effects in fish following
continuous exposure for 35 days. Since the compound both adsorbs to
suspended material and is degraded rapidly, the significance of this
finding was considered to be questionable.
A field study of aquatic organisms exposed following
chlorothalonil application suggests that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physicochemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field. There have been no
reported incidents of kills in the environment. However, despite the
short residence time of chlorothalonil in environmental media, kills
would be expected to occur immediately after application. Linking
kills to the compound would be difficult given that residues would not
persist long enough for chlorothalonil to be identified.
With minor exceptions, chlorothalonil is not phytotoxic.
Several studies have shown no toxicity of chlorothalonil to
earthworms at recommended application rates. At an exposure of five
times the maximum recommended rate, the compound severely reduced worm
reproduction.
Chlorothalonil is classified as "relatively non-toxic" to
honey-bees. Earwigs exposed to residues topically and via food showed
some mortality (20-55%), but there is no other evidence of
insecticidal action.
Chlorothalonil has low toxicity to birds in acute or dietary
tests. The low acute toxicity of chlorothalonil to laboratory mammals
tempered with its short persistence in the environment suggests
minimal hazard to wild mammal species.
1.2.2.1 Transport, distribution and transformation
Chlorothalonil adsorbs strongly to organic matter in soil and
suspended material in water. It is not, therefore, leached from soil
to groundwater. It is removed rapidly from surface water to suspended
material and to a lesser extent to bottom sediment. Chlorothalonil is
not translocated in plants from the site of application.
Abiotic degradation of chlorothalonil in water through photolysis
does not occur. Some hydrolysis does take place at higher pH.
Microbial degradation is the major cause of dissipation in soil
and may take place to some extent in water; this involves several
parallel processes, one of which leads to formation of the 4-hydroxy
metabolite. Half-lives for dissipation of this metabolite from non-
sterile soils range between 6 and 43 days. Biodegradation on plants
is limited and the 4-hydroxy metabolite comprises less than 5% of the
total residues.
During exposure, fish bioconcentrate chlorothalonil, but almost
total degradation occurs within 2 weeks after termination of exposure.
Chlorothalonil is metabolized in fish through glutathione conjugation
and the conjugates are excreted through the bile.
1.2.2.2 Aquatic organisms
The results of a single field study measuring concentrations of
chlorothalonil in water following overspray of the water were
available; corresponding data on concentrations in suspended and
bottom sediment were unreliable. Output from the EXAMS II fate model
using the same application scenario produced estimated water
concentrations which closely corresponded to the measured ones.
Little or no chlorothalonil was predicted in bottom sediment.
Based on this combination of measured and modelled data, the
ratio between a "toxic" concentration (the rainbow trout LC50) and
expected concentration is less than 1 for up to 5 h after overspray
and increases rapidly thereafter. Similar results were obtained for
daphnids. Therefore, despite its rapid removal from water and
degradation, the high toxicity of chlorothalonil is expected to cause
deaths of aquatic organisms in the period immediately after spraying.
This is the worst case situation of direct water overspray.
There were no data to extend this quantitative evaluation to
other field situations or climates.
1.2.2.3 Terrestrial organisms
A calculated maximum soil concentration, based on application of
chlorothalonil at 2.5 kg a.i./ha and complete bioavailability, is 3
orders of magnitude higher than the lowest estimate of LC50 for
earthworms.
For grazing birds (ducks and geese) total daily intake is at
least a factor of 100 below the NOEL for oral toxicity. For rabbits,
total daily intake is also at least 2 orders of magnitude lower than
the reported NOEL. This is based on a maximum recommended application
rate of 2.5 kg a.i./ha, an estimated worst case value for residues on
grass, no degradation of the compound, consumption of the total daily
intake at a single time and no choice but to eat contaminated food.
Table 1 contains a summary of risk quotients for birds, fish and
aquatic invertebrates.
Table 1. Toxicity/exposure ratios for birds, fish and aquatic invertebrates based
on application rates of 2.5 kg a.i./ha of chlorothalonil to soybeans
(worst case)
Risk category LC50 (mg/litre Estimated exposure Toxicity/exposure
or mg/kg diet) (mg/litre or ratio (TER)c
mg/kg diet)a,b
Acute bird 4640 73.7-535.7 63.0-8.7
Acute fish (stream) 0.01 0.009-0.04 1.1-0.25
Acute fish (pond) 0.01 0.01 1.0
Acute aquatic
invertebrate (stream) 0.07 0.009-0.04 7.8-1.8
Acute aquatic
invertebrate (pond) 0.07 0.01 7.0
a Estimated environmental concentration in the terrestrial environment (for bird exposure)
is based on the stated application rate and the assumption of deposition on short grass
using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STREAM model based on a single
application and estimated run-off into water; no direct overspray is included.
c TER is the toxicity (as LC50) divided by the exposure; values at or below 1.0 indicate
likely exposure to toxic concentrations by organisms in the different risk categories.
1.2.3 Toxicological criteria for setting guidance values
The toxicological studies on chlorothalonil of relevance for
setting guidance values are displayed in Table 2. The study results
and their significance are described briefly and gaps in test
requirements are indicated.
Table 2. Toxicological criteria for setting guidance values for chlorothalonil
Exposure Relevant route/effect/ Result/remarks
scenario species
Short-term skin, irritation, rabbit irritant
(1-7 days)
eye, irritation, rabbit irritant
skin, sensitization, tests were inconclusive
guinea-pig
evidence in humans of contact
dermatitis
inhalation, lethality, high toxicity in 4-h study with
rat hammermilled technical chlorothalonil
(MMAD 5-8 µm); not relevant for most
human exposure situations
Medium-term repeat dermal, rabbit 21-day study; irritant at 2.5 mg/kg
(1-26 weeks) body weight per day and above; no
systemic effects at 50 mg/kg body
weight per day
repeat oral, mice and 13-22 week studies; NOEL = 3 mg/kg
rats body weight per day in rats and mice
maternal, oral, rabbit teratology study; maternal toxicity
NOEL = 10 mg/kg body weight per day
by gavage; no fetotoxic or teratogenic
effect
Long-term repeat oral, dog 2-year study; NOEL = 3 mg/kg body
weight per day
1.3 Conclusions and recommendations
Considering the toxicological characteristics of chlorothalonil,
both qualitatively and quantitatively, it was concluded, on the basis
of the NOEL of 3 mg/kg body weight per day derived in the 2-year study
on dogs and applying a 100-fold uncertainty factor, that 0.03 mg/kg
body weight per day will probably not cause adverse effects in humans
by any route of exposure.
A study to assess the skin irritation potential is needed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure
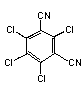 Molecular formula C8Cl4N2
Relative molecular mass 265.9
CAS chemical name 2,4,5,6,-tetrachloro-1,3-benzenedi
carbonitrile
CAS registry number 1897-45-6
RTECS registry number NT2600000
Common name chlorothalonil
IUPAC name tetrachloroisophthalonitrile
Synonyms m-TCPN; 2,4,5,6-tetrachloro-3-cyano
benzonitrile
Trade names Bravo (ISK Biotech)
(manufacturers & Daconil (ISK Biotech)
suppliers) Faber (Tripart Farm Chemicals)
Repulse (ICI); Exotherm (Alto Elite)
Nopocide (a preservative in paints and
adhesives)
Technical product > 97%
purity
Technical product tetrachlorophthalonitrile (< 0.1),
impurities (%) tetrachloroterephthalonitrile (0.1-1.6),
pentachlorobenzonitrile (0.5-2.5),
partially chlorinated dicyanobenzenes
(0.2-1.0), unchlorinated dicyano
benzenes (0.1-1.6), HCB (0.03),
insoluble in xylene (0.1-1.0)
2.2 Physical and chemical properties
The physical properties of chlorothalonil are listed in Table 3.
Table 3. Physical properties of chlorothalonil
Physical state crystalline solid
Colour colourless
Odour odourless
Melting point (°C) 250-251
Boiling point (°C) 350 (760 mmHg)
Vapour pressure at 25°C 5.72 × 10-7
Relative density 1.8
Octanol-water partition coefficient 2.88-3.86
(log Kow)
Solubility in water (mg/litre) at 25°C 0.6-1.2
Solubility in organic solvents (g/litre) acetone 20, dimethylformamide 30,
dimethylsulfoxide 20, xylene 80, readily
soluble in benzene
Chlorothalonil is non-flammable and non-explosive. It is
thermally stable under normal storage conditions and to UV radiation,
and it is chemically stable in neutral or acidic aqueous solutions.
It breaks down at pH 9, the rate following first-order kinetics at
1.8% per day (at 25°C) (Szalkowski & Stallard, 1977). It has been
shown that chlorothalonil is unstable to light when dissolved in
benzene and that 2,3,5-trichloro-4,6-dicyanobiphenyl is a condensation
product (Kawamura et al., 1978). Chlorothalonil is not corrosive.
2.3 Analytical methods
Analytical methods for determining chlorothalonil in
formulations, fruit, vegetables, soil and water are summarized in
Table 4. In general, the methods also detect the principal metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile.
2.3.1 Sample preparation
Samples are extracted initially with an organic solvent such as
acetone. For samples where interference with the analytical method is
expected, e.g., plant material, further partitioning with organic
solvents is required, followed by clean-up on alumina or Florisil
columns if necessary. The sample extracts are submitted for
analytical determination.
2.3.2 Analytical determination
In most cases the cleaned-up sample extracts are analysed by
gas-liquid chromatography using an electron capture detector. This
provides sufficient sensitivity for the analysis of trace quantities
of chlorothalonil residues at detection limits down to 0.01 mg/kg in
many cases.
Where less sensitive determination is required, e.g., for
formulation analysis, a flame ionization detector gives sufficient
sensitivity. A method for formulation analysis using infrared
spectroscopy after dichloromethane extraction has been reported (US
EPA, 1976).
The Joint FAO/WHO Codex Alimentarius Commission has given
recommendations for the methods of analysis to be used for the
determination of chlorothalonil residues (FAO/WHO, 1989).
Table 4. Methods for the determination of chlorothalonil
Sample type Sample preparation Analytical Limit of detection Reference
extraction/clean-up methoda (µg/kg or µg/litre)
Formulation extract (1,4-dioxane or methylethylketone/ GC/TCD or - Ballee et al. (1976)
carbon disulfide/1,2-dimethoxyethane) GC/FID -
Fruit & vegetable Strip (dichloromethane) GC/ECD 10 Ballee et al. (1976)
surfaces evaporate, dilute (benzene)
Green leafy extract (acidified acetone), evaporate, GC/ECD 10 Ballee et al. (1976)
vegetables dissolve (aqueous NaHCO3), adjust pH,
extract (diisopropyl ether), evaporate,
dilute (benzene), chromatograph (alumina)
Fruit and extract (acetone), evaporate, acidify GC/ECD 20 Burchfield & Storrs
vegetables and extract (ether), evaporate, chromatograph (1977)
(Florisil), elute (acetone/dichloromethane)
Non-fatty products extract (toluene/isopropanol), aqueous GC/ECD 10-50 Holmes & Wood
especially with separation, evaporate, chromatograph (1972)
S interference, (alumina/AgNO3), elute (hexane)
onion, cabbage,
celery
Potatoes extract (acidified acetone), chromatograph GC/ECD 10 Markus & Puma
(Florisil) derivatize (diazomethane) GC/MCD 20 (1973)
Table 4. (Cont'd)
Sample type Sample preparation Analytical Limit of detection Reference
extraction/clean-up methoda (µg/kg or µg/litre)
Apples rinse (acidified acetone), adjust pH, partition GC/ECD 50 Suzuki & Oda (1977)
(hexane), extract tissue (acidified acetone),
concentrate, partition (hexane), acidify
aqueous fraction, partition (diisopropyl ether)
Cranberries extract (acetone), filter, (Celite 545), adsorb GC/ECD not quoted Camoni et al. (1991)
(Extrelut-20), elute (petroleum ether),
evaporate, dissolve (benzene)
Fresh fruit extract (acetone), partition (petroleum ether HPLC/UV (232 nm) < 50 Gidvydis & Walters
and methylene chloride), concentrate and HPLC/ (1988)
photoconductivity
detection (PC)
Soil extract (acidified acetone), extract GC/ECD 10 Ballee et al. (1976)
(acetonitrile/hexane), partition (aqueous
layer) extract (diisopropyl ether) concentrate,
dilute (benzene) chromatograph (alumina)
extract (acetone: sulfuric acid), partition GC/ECD 10 Kenyon & Wiedmann
(petroleum ether), evaporate, redissolve in (1992b)
hexane/methylene chloride, elute, concentrate
Table 4. (Cont'd)
Sample type Sample preparation Analytical Limit of detection Reference
extraction/clean-up methoda (µg/kg or µg/litre)
Water adjust pH to 4.5, extract (diisopropyl ether), GC/ECD 10 Ballee et al. (1976)
concentrate, dilute (benzene)
adjust pH, extract (petroleum ether), add GC/ECD 0.05 Kenyon & Wiedmann
keeper, concentrate, redissolve (hexane/ (1992a)
methylene chloride), elute (methylene
chloride/hexane/acetonitrile)
Air samples, extraction (methional 2-propanol, n-hexane) HPLC with UV 0.5 Jongen et al. (1991)
dislodgeable detection at 254
residues or 325 nm
a GC = gas chromatography; ECD = electron capture detector; FID = flame ionization detector; HPLC = high performance liquid
chromatography; MCD = microcoulometric detection; TCD = thermal conductivity detection
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Chlorothalonil does not occur naturally in the environment.
3.2 Production levels and processes
Chlorothalonil is produced by the chlorination of
isophthalonitrile or by treatment of tetrachloroisophthaloyl amide
with phosphorus oxychloride. It has been produced commercially in the
USA since 1969. No data on production are available but it has been
estimated at 5000 tonnes annually (IARC, 1983). The annual production
in Japan has been estimated to be 3000 tonnes (IARC, 1983).
Imports into the USA were 1650 tonnes in 1976 and 175 tonnes in
1980 (IARC, 1983).
No data are available on possible releases to the environment
from production processes or transportation.
3.3 Uses
Chlorothalonil is a fungicide with a broad spectrum of activity
used mainly in agriculture but also on turf, lawns and ornamental
plants. It protects plants against a variety of fungal infections
such as rusts, downy mildew, leaf spot, scabs, blossom blight and
black pod. Crops protected include pome fruit, stone fruit, citrus,
currants, cranberries, strawberries, bananas, vines, hops, tomatoes,
green vegetables, tobacco, coffee, tea, soya bean, groundnuts,
potatoes, onions, cereals and sugar beet. In addition, it is used in
wood preservatives, fish net coatings and anti-fouling paints.
Global estimates of chlorothalonil use for these purposes are not
available. The extent of use in various countries on an annual basis
is shown in Table 5.
Chlorothalonil is used in agriculture in formulated products.
The three main formulations are a suspension concentrate containing
500 g chlorothalonil/litre, a water dispersible granule and a wettable
powder containing 75% chlorothalonil. The formulations mix readily
with water and are diluted to give a spray mixture which can be
applied by ground spray systems or by air, and as dilute or
concentrated sprays.
The dose rates recommended for crop protection have been derived
from efficacy studies conducted in a variety of climatic conditions in
various parts of the world. The label recommendations are designed to
give satisfactory fungal disease control and to keep residues within
national and international limits. Typical active ingredient rates
are 1.25-2.5 kg/ha for crops such as beans, celery and onions. Rates
Table 5. Quantities of chlorothalonil used in various countries
Country Year Consumption Usage Reference
(tonnes)
Canada 1982 5.1 potatoes O'Neill (1991)
(New Brunswick)
Colombia 1980 14.5 fruit, flowers, ornamentals IRPTC (1989)
1981 22.2 fruit, flowers, ornamentals IRPTC (1989)
1982 12.5 fruit, flowers, ornamentals IRPTC (1989)
Mexico 1983 250 broccoli, potatoes, etc. IRPTC (1989)
Sweden 1981 30 agricultural crops IRPTC (1989)
3 paint, wood
Thailand 1976 6 agriculture IRPTC (1989)
1982 10.4
United Republic 1981-2 640 coffee beans, tomatoes IRPTC (1989)
of Tanzania
USA 1976 2000 by farmers on major crops IARC (1983)
1978 300 mildewcide in paint IARC (1983)
1980 5000 53% peanuts, 31% vegetables, IARC (1983)
12% turf, 5% potatoes
of use for a variety of purposes are shown in Table 6. Spray volumes
usually range from about 200 to 400 litres/ha for dilute sprays and 45
to 95 litres/ha for concentrated sprays. Applications should commence
when weather conditions favour disease, e.g., high humidity, and prior
to initial infection. Repeat applications may be needed as directed
on the label for the country concerned. Examples of crops, diseases
controlled, agronomic importance, application rates, timing of
treatment and pre-harvest intervals on a variety of crops in the
Netherlands have been given by FAO (1982). A summary of approved uses
for grapes, including formulation used, application rates, number of
treatments and pre-harvest interval for a variety of countries, has
been given by FAO/WHO (1986a).
Chlorothalonil formulations are compatible in use with many other
fungicides and insecticides and combined formulations are registered
and available for use in many countries.
Table 6. Ranges of application rates for chlorothalonil
Application rate
(kg active ingredient per ha)
Agronomic crops:
Corn, lentils, peanuts, potatoes, 0.875-2.0
soybeans, wheat, barley, rice
Tree fruit crops:
Stone, citrus, nut, pome 1.25-3.5
Small fruit:
Cranberry, blackberry, grape 1.25-5.85
Vegetable crops 0.875-2.5
Ornamentals 1.25-2.5
Turf 4.5-25.0
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The sorptive characteristics of chlorothalonil have been
investigated to estimate its potential for contamination of aquifers
after application to a cranberry bog (Reduker et al., 1988). The soil
studied was mainly sandy in character. The studies included a kinetic
and an absorption equilibrium assessment, the soil being shaken with
chlorothalonil in water for periods up to 48 h, and a soil column
study with 2.8 mg chlorothalonil/100 ml at a flow rate of 642 ml/day
for 64 days. A linear adsorption relationship was established with a
partition coefficient for chlorothalonil of 74.4 ml/g for this soil.
Very little (< 22%) of the adsorbed chemical was recovered. The soil
column study produced a dispersion coefficient of 100 cm2/day. Only
a small proportion (less than 2.8%) of chlorothalonil appeared in the
effluent or was extracted from the soil, indicating either
irreversible adsorption, degradation, or both.
The movement of chlorothalonil in a sandy soil was observed on a
commercial farm with a high water table and a tile drain system in
Manitoba, Canada. Chlorothalonil was routinely sprayed on irrigated
crops such as potatoes and barley. In one season it was detected in
the tile drain water on 4 out of 66 sampling days at concentrations of
0.04-3.66 µg/litre. In the same period chlorothalonil was also found
in groundwater from a well on the site at levels of 10-272 µg/litre.
There was some evidence of a small amount of carry-over into the
following season (Krawchuk & Webster, 1987). They also reported
serious background contamination problems due to the autosampler.
When these problems were corrected (i.e., 1983), the residue levels in
the well ranged from 0.9 to 8.6 µg/litre. In this report, the authors
interpreted their data to demonstrate both leaching and potential
carry-over. However, it should be noted that an initial tile water
outflow sample, taken in 1981, showed no detectable chlorothalonil
(i.e., < 0.02 µg/litre), although chlorothalonil was applied to the
site that year.
Water/sediment measurements were made after aerial spraying of a
potato crop in Canada (O'Neill, 1991). The area oversprayed included
a small water course with a pond. The results showed a rapidly
decreasing chlorothalonil content in the water phase after
overspraying, little or no compound being found in the sediment
(63-91% sand). The author indicated that sediments with greater clay
or silt content would play a greater role in chlorothalonil transport.
Analysis of stream water samples containing chlorothalonil showed
significant binding to suspended material, with an average log
partition coefficient (log PSm/w) of 5.695 and an average of 81%
chlorothalonil being bound to the suspended matter. Algal growths on
stream pebbles played a dominant role in chlorothalonil removal by
absorption and biodegradation. It was also shown that Galaxias
auratus enhanced chlorothalonil loss in fish tanks by a factor of 25
times (Davies, 1988).
Chlorothalonil does not translocate from the site of
application to other parts of a plant. For example, ring-labelled
14C-chlorothalonil does not translocate when applied topically to
cucumber, bean or tomato leaves. It was not translocated into the
aerial parts of corn or tomato plants when they were cultivated for 23
days in soil treated with 14C-chlorothalonil. There was no movement
or translocation of radioactivity within the root systems of sweet
corn, cucumber or tomato grown in soil treated with ring-labelled
chlorothalonil. This also indicated that the major 4-hydroxy
metabolite in soil was not translocated (Kunkel, 1967a,b).
Chlorothalonil residues remaining on food crops at harvest may
enter the human food chain. Residues in foodstuffs may be further
reduced by processing and cooking (see sections 5.1 and 5.2).
4.2 Transformation
4.2.1 Biodegradation
Studies with river water from two sources in Tasmania showed that
loss of chlorothalonil was slow in still water. Comparison of loss
rates at 5 and 15°C indicated involvement of enzymic processes.
Uptake by algal growths also indicated biodegradation with the
appearance of polar metabolites. However, biodegradation is unlikely
to play a major role in the fate of chlorothalonil in moderate to fast
flowing streams, where volatilization and adsorption are liable to be
dominant factors (Davies, 1988).
Chlorothalonil is rapidly degraded in soil under both laboratory
and field conditions. In laboratory experiments its half-life ranged
from 4 to 40 days in various types of soil. The rate increased with
increasing organic matter content, moisture and temperature. It
appeared that little was lost due to volatilization. On turf plots at
three locations in the USA, the half-life of chlorothalonil ranged
from 26 to 45 days after treatments (Stallard & Wolfe, 1967). The
major soil degradation product is the 4-hydroxy metabolite,
4-hydroxy-2,5,6-trichloroisophthalonitrile. Laboratory studies in
five soils showed half-lives for the 4-hydroxy metabolite of 36 days
in a sandy loam and up to 220 days in clay type soil (Wolfe &
Stallard, 1968). It has been shown that bacteria isolated from soil
are capable of metabolizing chlorothalonil in culture media. It can
be deduced that soil microorganisms play a role in the rapid
degradation of chlorothalonil in soil (Duane, 1970).
Degradation of chlorothalonil in soil involves a series of
parallel processes, one of which involves formation and dissipation of
4-hydroxy-2,5,6-trichloroisophthalonitrile (SDS-3701). Chlorothalonil
dissipation data were re-analysed to obtain half-life estimates for
SDS-3701 soil dissipation. Assuming first order kinetics, non-linear
least-squares regression modelling was used to estimate the values of
the model parameters. For SDS-3701, half-lives between 6 and 43 days
were determined for the various non-sterile soils. An alternative
method of data analysis, utilizing a transformation and a linearizing
approximation, was also used and gave a similar range of half-lives
(Jacobson & Schollenberger, 1992).
The dissipation of chlorothalonil in soils was suppressed by the
repeated applications of this fungicide to the soils. The dissipation
was due to microbial action, since chlorothalonil disappeared in a
nonsterile soil but not in an autoclaved soil. The amendments of the
soil with easily decomposable organic materials recovered the
suppressed dissipation ability of the soil. The results suggested
that easily decomposable organic materials play an important role in
the microbial degradation of chlorothalonil in soil (Katayama, et al.,
1991).
Fig. 1 lists the structure and identification code of the five
soil metabolites that have been identified in aerobic soil studies
involving 14C-chlorothalonil in the laboratory. Identifications were
based on independent synthesis of authentic standard and GLC or
HPLC/MS confirmations. It should be noted that the scheme is a
suggested pathway (Frazier, 1993). There is no direct evidence that
any of the five soil metabolites are converted directly to "bound"
residue. Typical dissipation curves (Figs. 2, 3, 4) show the
dissipation of chlorothalonil and the formation/dissipation of the
4-hydroxy-metabolite (SDS-3701); note that the scale for time is not
linear. These same dissipation curves show the formation of bound
residue. Attempts to liberate and characterize this bound residue have
produced limited characterization data and no definitive structure
identifications.
A complete picture of all of the known transformations which
occur with chlorothalonil under various environmental conditions is
given in Fig. 5 (ISK Biosciences, 1995).
Molecular formula C8Cl4N2
Relative molecular mass 265.9
CAS chemical name 2,4,5,6,-tetrachloro-1,3-benzenedi
carbonitrile
CAS registry number 1897-45-6
RTECS registry number NT2600000
Common name chlorothalonil
IUPAC name tetrachloroisophthalonitrile
Synonyms m-TCPN; 2,4,5,6-tetrachloro-3-cyano
benzonitrile
Trade names Bravo (ISK Biotech)
(manufacturers & Daconil (ISK Biotech)
suppliers) Faber (Tripart Farm Chemicals)
Repulse (ICI); Exotherm (Alto Elite)
Nopocide (a preservative in paints and
adhesives)
Technical product > 97%
purity
Technical product tetrachlorophthalonitrile (< 0.1),
impurities (%) tetrachloroterephthalonitrile (0.1-1.6),
pentachlorobenzonitrile (0.5-2.5),
partially chlorinated dicyanobenzenes
(0.2-1.0), unchlorinated dicyano
benzenes (0.1-1.6), HCB (0.03),
insoluble in xylene (0.1-1.0)
2.2 Physical and chemical properties
The physical properties of chlorothalonil are listed in Table 3.
Table 3. Physical properties of chlorothalonil
Physical state crystalline solid
Colour colourless
Odour odourless
Melting point (°C) 250-251
Boiling point (°C) 350 (760 mmHg)
Vapour pressure at 25°C 5.72 × 10-7
Relative density 1.8
Octanol-water partition coefficient 2.88-3.86
(log Kow)
Solubility in water (mg/litre) at 25°C 0.6-1.2
Solubility in organic solvents (g/litre) acetone 20, dimethylformamide 30,
dimethylsulfoxide 20, xylene 80, readily
soluble in benzene
Chlorothalonil is non-flammable and non-explosive. It is
thermally stable under normal storage conditions and to UV radiation,
and it is chemically stable in neutral or acidic aqueous solutions.
It breaks down at pH 9, the rate following first-order kinetics at
1.8% per day (at 25°C) (Szalkowski & Stallard, 1977). It has been
shown that chlorothalonil is unstable to light when dissolved in
benzene and that 2,3,5-trichloro-4,6-dicyanobiphenyl is a condensation
product (Kawamura et al., 1978). Chlorothalonil is not corrosive.
2.3 Analytical methods
Analytical methods for determining chlorothalonil in
formulations, fruit, vegetables, soil and water are summarized in
Table 4. In general, the methods also detect the principal metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile.
2.3.1 Sample preparation
Samples are extracted initially with an organic solvent such as
acetone. For samples where interference with the analytical method is
expected, e.g., plant material, further partitioning with organic
solvents is required, followed by clean-up on alumina or Florisil
columns if necessary. The sample extracts are submitted for
analytical determination.
2.3.2 Analytical determination
In most cases the cleaned-up sample extracts are analysed by
gas-liquid chromatography using an electron capture detector. This
provides sufficient sensitivity for the analysis of trace quantities
of chlorothalonil residues at detection limits down to 0.01 mg/kg in
many cases.
Where less sensitive determination is required, e.g., for
formulation analysis, a flame ionization detector gives sufficient
sensitivity. A method for formulation analysis using infrared
spectroscopy after dichloromethane extraction has been reported (US
EPA, 1976).
The Joint FAO/WHO Codex Alimentarius Commission has given
recommendations for the methods of analysis to be used for the
determination of chlorothalonil residues (FAO/WHO, 1989).
Table 4. Methods for the determination of chlorothalonil
Sample type Sample preparation Analytical Limit of detection Reference
extraction/clean-up methoda (µg/kg or µg/litre)
Formulation extract (1,4-dioxane or methylethylketone/ GC/TCD or - Ballee et al. (1976)
carbon disulfide/1,2-dimethoxyethane) GC/FID -
Fruit & vegetable Strip (dichloromethane) GC/ECD 10 Ballee et al. (1976)
surfaces evaporate, dilute (benzene)
Green leafy extract (acidified acetone), evaporate, GC/ECD 10 Ballee et al. (1976)
vegetables dissolve (aqueous NaHCO3), adjust pH,
extract (diisopropyl ether), evaporate,
dilute (benzene), chromatograph (alumina)
Fruit and extract (acetone), evaporate, acidify GC/ECD 20 Burchfield & Storrs
vegetables and extract (ether), evaporate, chromatograph (1977)
(Florisil), elute (acetone/dichloromethane)
Non-fatty products extract (toluene/isopropanol), aqueous GC/ECD 10-50 Holmes & Wood
especially with separation, evaporate, chromatograph (1972)
S interference, (alumina/AgNO3), elute (hexane)
onion, cabbage,
celery
Potatoes extract (acidified acetone), chromatograph GC/ECD 10 Markus & Puma
(Florisil) derivatize (diazomethane) GC/MCD 20 (1973)
Table 4. (Cont'd)
Sample type Sample preparation Analytical Limit of detection Reference
extraction/clean-up methoda (µg/kg or µg/litre)
Apples rinse (acidified acetone), adjust pH, partition GC/ECD 50 Suzuki & Oda (1977)
(hexane), extract tissue (acidified acetone),
concentrate, partition (hexane), acidify
aqueous fraction, partition (diisopropyl ether)
Cranberries extract (acetone), filter, (Celite 545), adsorb GC/ECD not quoted Camoni et al. (1991)
(Extrelut-20), elute (petroleum ether),
evaporate, dissolve (benzene)
Fresh fruit extract (acetone), partition (petroleum ether HPLC/UV (232 nm) < 50 Gidvydis & Walters
and methylene chloride), concentrate and HPLC/ (1988)
photoconductivity
detection (PC)
Soil extract (acidified acetone), extract GC/ECD 10 Ballee et al. (1976)
(acetonitrile/hexane), partition (aqueous
layer) extract (diisopropyl ether) concentrate,
dilute (benzene) chromatograph (alumina)
extract (acetone: sulfuric acid), partition GC/ECD 10 Kenyon & Wiedmann
(petroleum ether), evaporate, redissolve in (1992b)
hexane/methylene chloride, elute, concentrate
Table 4. (Cont'd)
Sample type Sample preparation Analytical Limit of detection Reference
extraction/clean-up methoda (µg/kg or µg/litre)
Water adjust pH to 4.5, extract (diisopropyl ether), GC/ECD 10 Ballee et al. (1976)
concentrate, dilute (benzene)
adjust pH, extract (petroleum ether), add GC/ECD 0.05 Kenyon & Wiedmann
keeper, concentrate, redissolve (hexane/ (1992a)
methylene chloride), elute (methylene
chloride/hexane/acetonitrile)
Air samples, extraction (methional 2-propanol, n-hexane) HPLC with UV 0.5 Jongen et al. (1991)
dislodgeable detection at 254
residues or 325 nm
a GC = gas chromatography; ECD = electron capture detector; FID = flame ionization detector; HPLC = high performance liquid
chromatography; MCD = microcoulometric detection; TCD = thermal conductivity detection
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Chlorothalonil does not occur naturally in the environment.
3.2 Production levels and processes
Chlorothalonil is produced by the chlorination of
isophthalonitrile or by treatment of tetrachloroisophthaloyl amide
with phosphorus oxychloride. It has been produced commercially in the
USA since 1969. No data on production are available but it has been
estimated at 5000 tonnes annually (IARC, 1983). The annual production
in Japan has been estimated to be 3000 tonnes (IARC, 1983).
Imports into the USA were 1650 tonnes in 1976 and 175 tonnes in
1980 (IARC, 1983).
No data are available on possible releases to the environment
from production processes or transportation.
3.3 Uses
Chlorothalonil is a fungicide with a broad spectrum of activity
used mainly in agriculture but also on turf, lawns and ornamental
plants. It protects plants against a variety of fungal infections
such as rusts, downy mildew, leaf spot, scabs, blossom blight and
black pod. Crops protected include pome fruit, stone fruit, citrus,
currants, cranberries, strawberries, bananas, vines, hops, tomatoes,
green vegetables, tobacco, coffee, tea, soya bean, groundnuts,
potatoes, onions, cereals and sugar beet. In addition, it is used in
wood preservatives, fish net coatings and anti-fouling paints.
Global estimates of chlorothalonil use for these purposes are not
available. The extent of use in various countries on an annual basis
is shown in Table 5.
Chlorothalonil is used in agriculture in formulated products.
The three main formulations are a suspension concentrate containing
500 g chlorothalonil/litre, a water dispersible granule and a wettable
powder containing 75% chlorothalonil. The formulations mix readily
with water and are diluted to give a spray mixture which can be
applied by ground spray systems or by air, and as dilute or
concentrated sprays.
The dose rates recommended for crop protection have been derived
from efficacy studies conducted in a variety of climatic conditions in
various parts of the world. The label recommendations are designed to
give satisfactory fungal disease control and to keep residues within
national and international limits. Typical active ingredient rates
are 1.25-2.5 kg/ha for crops such as beans, celery and onions. Rates
Table 5. Quantities of chlorothalonil used in various countries
Country Year Consumption Usage Reference
(tonnes)
Canada 1982 5.1 potatoes O'Neill (1991)
(New Brunswick)
Colombia 1980 14.5 fruit, flowers, ornamentals IRPTC (1989)
1981 22.2 fruit, flowers, ornamentals IRPTC (1989)
1982 12.5 fruit, flowers, ornamentals IRPTC (1989)
Mexico 1983 250 broccoli, potatoes, etc. IRPTC (1989)
Sweden 1981 30 agricultural crops IRPTC (1989)
3 paint, wood
Thailand 1976 6 agriculture IRPTC (1989)
1982 10.4
United Republic 1981-2 640 coffee beans, tomatoes IRPTC (1989)
of Tanzania
USA 1976 2000 by farmers on major crops IARC (1983)
1978 300 mildewcide in paint IARC (1983)
1980 5000 53% peanuts, 31% vegetables, IARC (1983)
12% turf, 5% potatoes
of use for a variety of purposes are shown in Table 6. Spray volumes
usually range from about 200 to 400 litres/ha for dilute sprays and 45
to 95 litres/ha for concentrated sprays. Applications should commence
when weather conditions favour disease, e.g., high humidity, and prior
to initial infection. Repeat applications may be needed as directed
on the label for the country concerned. Examples of crops, diseases
controlled, agronomic importance, application rates, timing of
treatment and pre-harvest intervals on a variety of crops in the
Netherlands have been given by FAO (1982). A summary of approved uses
for grapes, including formulation used, application rates, number of
treatments and pre-harvest interval for a variety of countries, has
been given by FAO/WHO (1986a).
Chlorothalonil formulations are compatible in use with many other
fungicides and insecticides and combined formulations are registered
and available for use in many countries.
Table 6. Ranges of application rates for chlorothalonil
Application rate
(kg active ingredient per ha)
Agronomic crops:
Corn, lentils, peanuts, potatoes, 0.875-2.0
soybeans, wheat, barley, rice
Tree fruit crops:
Stone, citrus, nut, pome 1.25-3.5
Small fruit:
Cranberry, blackberry, grape 1.25-5.85
Vegetable crops 0.875-2.5
Ornamentals 1.25-2.5
Turf 4.5-25.0
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
The sorptive characteristics of chlorothalonil have been
investigated to estimate its potential for contamination of aquifers
after application to a cranberry bog (Reduker et al., 1988). The soil
studied was mainly sandy in character. The studies included a kinetic
and an absorption equilibrium assessment, the soil being shaken with
chlorothalonil in water for periods up to 48 h, and a soil column
study with 2.8 mg chlorothalonil/100 ml at a flow rate of 642 ml/day
for 64 days. A linear adsorption relationship was established with a
partition coefficient for chlorothalonil of 74.4 ml/g for this soil.
Very little (< 22%) of the adsorbed chemical was recovered. The soil
column study produced a dispersion coefficient of 100 cm2/day. Only
a small proportion (less than 2.8%) of chlorothalonil appeared in the
effluent or was extracted from the soil, indicating either
irreversible adsorption, degradation, or both.
The movement of chlorothalonil in a sandy soil was observed on a
commercial farm with a high water table and a tile drain system in
Manitoba, Canada. Chlorothalonil was routinely sprayed on irrigated
crops such as potatoes and barley. In one season it was detected in
the tile drain water on 4 out of 66 sampling days at concentrations of
0.04-3.66 µg/litre. In the same period chlorothalonil was also found
in groundwater from a well on the site at levels of 10-272 µg/litre.
There was some evidence of a small amount of carry-over into the
following season (Krawchuk & Webster, 1987). They also reported
serious background contamination problems due to the autosampler.
When these problems were corrected (i.e., 1983), the residue levels in
the well ranged from 0.9 to 8.6 µg/litre. In this report, the authors
interpreted their data to demonstrate both leaching and potential
carry-over. However, it should be noted that an initial tile water
outflow sample, taken in 1981, showed no detectable chlorothalonil
(i.e., < 0.02 µg/litre), although chlorothalonil was applied to the
site that year.
Water/sediment measurements were made after aerial spraying of a
potato crop in Canada (O'Neill, 1991). The area oversprayed included
a small water course with a pond. The results showed a rapidly
decreasing chlorothalonil content in the water phase after
overspraying, little or no compound being found in the sediment
(63-91% sand). The author indicated that sediments with greater clay
or silt content would play a greater role in chlorothalonil transport.
Analysis of stream water samples containing chlorothalonil showed
significant binding to suspended material, with an average log
partition coefficient (log PSm/w) of 5.695 and an average of 81%
chlorothalonil being bound to the suspended matter. Algal growths on
stream pebbles played a dominant role in chlorothalonil removal by
absorption and biodegradation. It was also shown that Galaxias
auratus enhanced chlorothalonil loss in fish tanks by a factor of 25
times (Davies, 1988).
Chlorothalonil does not translocate from the site of
application to other parts of a plant. For example, ring-labelled
14C-chlorothalonil does not translocate when applied topically to
cucumber, bean or tomato leaves. It was not translocated into the
aerial parts of corn or tomato plants when they were cultivated for 23
days in soil treated with 14C-chlorothalonil. There was no movement
or translocation of radioactivity within the root systems of sweet
corn, cucumber or tomato grown in soil treated with ring-labelled
chlorothalonil. This also indicated that the major 4-hydroxy
metabolite in soil was not translocated (Kunkel, 1967a,b).
Chlorothalonil residues remaining on food crops at harvest may
enter the human food chain. Residues in foodstuffs may be further
reduced by processing and cooking (see sections 5.1 and 5.2).
4.2 Transformation
4.2.1 Biodegradation
Studies with river water from two sources in Tasmania showed that
loss of chlorothalonil was slow in still water. Comparison of loss
rates at 5 and 15°C indicated involvement of enzymic processes.
Uptake by algal growths also indicated biodegradation with the
appearance of polar metabolites. However, biodegradation is unlikely
to play a major role in the fate of chlorothalonil in moderate to fast
flowing streams, where volatilization and adsorption are liable to be
dominant factors (Davies, 1988).
Chlorothalonil is rapidly degraded in soil under both laboratory
and field conditions. In laboratory experiments its half-life ranged
from 4 to 40 days in various types of soil. The rate increased with
increasing organic matter content, moisture and temperature. It
appeared that little was lost due to volatilization. On turf plots at
three locations in the USA, the half-life of chlorothalonil ranged
from 26 to 45 days after treatments (Stallard & Wolfe, 1967). The
major soil degradation product is the 4-hydroxy metabolite,
4-hydroxy-2,5,6-trichloroisophthalonitrile. Laboratory studies in
five soils showed half-lives for the 4-hydroxy metabolite of 36 days
in a sandy loam and up to 220 days in clay type soil (Wolfe &
Stallard, 1968). It has been shown that bacteria isolated from soil
are capable of metabolizing chlorothalonil in culture media. It can
be deduced that soil microorganisms play a role in the rapid
degradation of chlorothalonil in soil (Duane, 1970).
Degradation of chlorothalonil in soil involves a series of
parallel processes, one of which involves formation and dissipation of
4-hydroxy-2,5,6-trichloroisophthalonitrile (SDS-3701). Chlorothalonil
dissipation data were re-analysed to obtain half-life estimates for
SDS-3701 soil dissipation. Assuming first order kinetics, non-linear
least-squares regression modelling was used to estimate the values of
the model parameters. For SDS-3701, half-lives between 6 and 43 days
were determined for the various non-sterile soils. An alternative
method of data analysis, utilizing a transformation and a linearizing
approximation, was also used and gave a similar range of half-lives
(Jacobson & Schollenberger, 1992).
The dissipation of chlorothalonil in soils was suppressed by the
repeated applications of this fungicide to the soils. The dissipation
was due to microbial action, since chlorothalonil disappeared in a
nonsterile soil but not in an autoclaved soil. The amendments of the
soil with easily decomposable organic materials recovered the
suppressed dissipation ability of the soil. The results suggested
that easily decomposable organic materials play an important role in
the microbial degradation of chlorothalonil in soil (Katayama, et al.,
1991).
Fig. 1 lists the structure and identification code of the five
soil metabolites that have been identified in aerobic soil studies
involving 14C-chlorothalonil in the laboratory. Identifications were
based on independent synthesis of authentic standard and GLC or
HPLC/MS confirmations. It should be noted that the scheme is a
suggested pathway (Frazier, 1993). There is no direct evidence that
any of the five soil metabolites are converted directly to "bound"
residue. Typical dissipation curves (Figs. 2, 3, 4) show the
dissipation of chlorothalonil and the formation/dissipation of the
4-hydroxy-metabolite (SDS-3701); note that the scale for time is not
linear. These same dissipation curves show the formation of bound
residue. Attempts to liberate and characterize this bound residue have
produced limited characterization data and no definitive structure
identifications.
A complete picture of all of the known transformations which
occur with chlorothalonil under various environmental conditions is
given in Fig. 5 (ISK Biosciences, 1995).
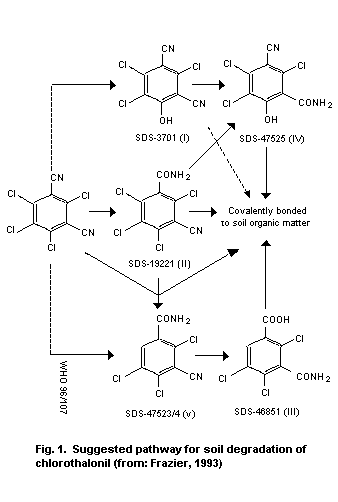
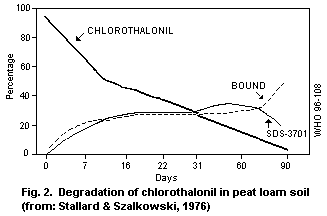
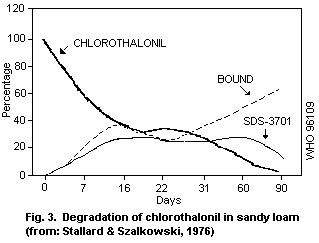
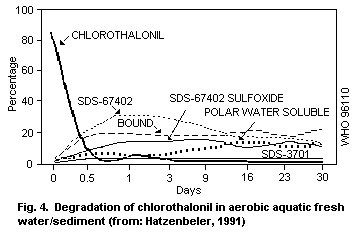
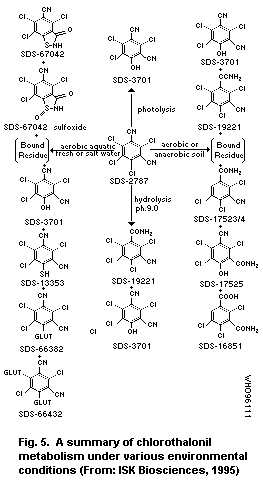 On plants, chlorothalonil is metabolized only to a limited extent
to the 4-hydroxy metabolite. The majority of the residue remains as
the parent compound. Generally less than 5% of the total residue is
present as the 4-hydroxy metabolite. A review of plant residues
worldwide showed that the 4-hydroxy metabolite level was < 0.1 mg/kg
in most of the crops analysed. It accounted for approximately 10% of
the total residue in lima beans, 5% in cantaloupes, 2% in peaches and
onions, 1% in celery and 0.1% in peanuts (FAO/WHO, 1985). The decline
of chlorothalonil residues and the appearance and decline of the
4-hydroxy metabolite on onions is shown in Table 7 (personal
communication to the IPCS by the Government of Canada, 1979). The
chlorothalonil residue decayed with a half-life of about 3 days.
Studies with corn silage showed that 90% of chlorothalonil
degraded within 18 days (30 to 3 mg/kg). The half-life was
approximately 4 days. In a second experiment, the 4-hydroxy
metabolite formation was very low in the bound materials (which were
converted to an extractable form), representing only about 2% of the
chlorothalonil on the first day of ensiling (FAO/WHO, 1978).
After chlorothalonil was applied to growing peanut foliage at
1.26 kg/ha its half-life was 13.6 days (range 7-19 days) under the
field conditions of use (Elliott & Spurr, 1993).
In the excretion of 14C-chlorothalonil metabolites from rainbow
trout (Salmo gairdneri), the almost complete absence of
chlorothalonil itself and the accumulation of 14C entities in the
bile indicated the possibility of glutathione conjugation as the first
step in chlorothalonil metabolism (Davies & White, 1985). Further
studies showed the existence of mono- and diglutathione conjugates of
chlorothalonil in the bile of rainbow trout exposed to
14C-chlorothalonil (Davies, 1985a).
Studies with liver cytosol from five fish species showed that the
enzyme glutathione- S-transferase (GST) is involved in the conversion
of chlorothalonil to polar conjugates. Comparisons of GST activity in
rainbow trout organs revealed that the potential for chlorothalonil
transformation was in the order liver » kidney > spleen, with no
activity in bile. Low concentrations of chlorothalonil in water
induced fish GST activity for its biotransformation. Hepatic
glutathione (GSH) and GST activity for chlorothalonil transformation
were compared in three species of fish (Oncorhynchus mykiss,
Galaxias maculatus and Galaxias auratus). The order of their
asymptotic LC50 values agreed with that of their hepatic GST
activities for chlorothalonil transformation and was consistent with a
detoxification role for GSH-chlorothalonil conjugation (Davies,
1985b). A study involving co-exposure to zinc and chlorothalonil
indicated that metallothionein does not play a significant role in
chlorothalonil detoxification in fish at sublethal exposures (Davies,
1985c).
Small amounts of the 4-hydroxy metabolite were found in the milk
and kidney of a cow fed 250 mg chlorothalonil/kg in its feed. Only
0.2% of the ingested chlorothalonil was eliminated in the milk as the
4-hydroxy metabolite (Ladd et al., 1971).
4.2.2 Abiotic degradation
Chlorothalonil does not break down in aqueous solution
(0.5 mg/litre) in the dark at pH 5 or 7. It is hydrolysed at pH 9,
over 50% disappearing in 49 days, with the formation of 4-hydroxy-
2,5,6-trichloroisophthalonitrile and 3-cyano-2,4,5,6-tetrachloro-
benzamide (Szalkowski & Stallard, 1977).
Chlorothalonil degrades very slowly under aqueous photolytic
conditions to the 4-hydroxy metabolite. The half-life was found to be
approximately 65 days (ISK Biotech proprietary information).
4.2.3 Bioaccumulation
In a study of the uptake and elimination of 14C-chlorothalonil
in rainbow trout, two groups of fish were exposed to 10 µg/litre of
the compound in flow-through tanks for 96 h (Davies & White, 1985).
After exposure was discontinued, the depuration rate was followed for
96 h. There was a very high uptake in the gall bladder and bile
(concentration factors up to 4.4 × 105). Uptake was also high in the
hind gut, liver, fat and kidney with concentration factors of
2-11 × 103. After 96 h of exposure, the concentration factor in
muscle was 940 and 740, respectively, for the two groups of fish, a
level which may give an indication of the magnitude of the whole body
bioconcentration factor (BCF) for rainbow trout (not measured).
After transfer to clean water, gall bladder levels dropped
rapidly, and so did gill and blood levels. In one group of fish,
concentrations in both liver and kidney doubled until 24 h after
transfer and thereafter dropped to the levels in the other group.
Concentrations in the spleen in both groups continued to increase
throughout the depuration period. Muscle levels dropped only slowly
and remained around 1 µg/g. The high concentrations found in the gall
bladder and bile are consistent with the fact that chlorothalonil is
excreted from fish as glutathione conjugates (Davies & White, 1985).
Bluegill sunfish exposed to 8 µg 14C-chlorothalonil/litre in a
flow-through system for 30 days showed a plateau of 14C uptake within
14 days. The residues in whole fish at 30 days were 264 times the
water concentration. When the fish were placed in clean water, 80% of
the radioactive residues were lost within 14 days. Bioaccumulation in
catfish, in a static system, showed a 16-fold concentration at 26
days. In this case 90% of the 14C residues were depurated in 14 days
after removal from the treated water. The 4-hydroxy metabolite did
not bioaccumulate in fish (SDS Biotech Corporation, 1972).
In tanks containing stream water with chlorothalonil at
20 µg/litre, uptake of the compound occurred in algal growths attached
to bottom pebbles. Analysis of the algal growths showed a
concentration factor for chlorothalonil of 270 times after 14 days of
static exposure. Since this represented only 9.5% of the initial dose
it seems that the removal of chlorothalonil from the water is enhanced
by its conversion to polar metabolites in addition to bioconcentration
(Davies, 1988).
4.3 Waste disposal
Chlorothalonil can be incinerated in units operating at 850°C
fitted with off-gas scrubbing equipment (Lawless et al., 1975).
The disposal methods for waste pesticides and containers
advocated by FAO and GIFAP should be applied to unused chlorothalonil
products and their empty packages (FAO, 1985; GIFAP, 1987).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Chlorothalonil was detected (amongst other pesticides) in 3 out
of 9 outdoor and indoor samples and 1 out of 9 personal monitoring
samples in 9 homes in Jacksonville, Florida, USA. No actual figures
were reported (Lewis et al., 1988).
Average exposures to chlorothalonil of 173 persons in
Jacksonville, Florida and Springfield, Massachusetts, USA were
0.7 ng/m3 (personal exposure) and 0.5 ng/m3 (outdoor air
concentrations) (Wallace, 1991).
Chlorothalonil was not detected in 51 samples in an Environmental
Survey of Chemicals in Japan in 1991 (personal communication by the
Office of Health Studies Environment Agency, Tokyo, 1992).
5.1.2 Water
O'Neil (1991) studied concentrations of chlorothalonil in water
and sediment following overspraying of a pond (0.2 ha and 0.5 m deep)
at 875 g a.i./ha (Ernst, 1991). Water and sediment were monitored in
a stream flowing out of the pond at the outlet and 30 m downstream.
The stream was approximately 1 m wide and 0.5 m deep and ran at
0.033 m3/sec for the first overspray and at 0.015 m3/sec for the
repeat spray. Whole water samples were filtered for separate
measurement of chlorothalonil in water and sediment. Following the
first spraying, samples were taken at the downstream site at 30 min
intervals up to 6 h after spraying and further samples were collected
10 and 24 h after spraying. Initial water concentrations of up to
60 µg/litre fell rapidly to around 15 µg/litre 2 h after spraying.
The water concentration was 1.9 µg/litre 10 h after spraying, and at
24 h there was no measurable chlorothalonil. In the second spraying
at the lower stream flow rate, whole water samples were taken more
frequently over the 2 h following application. Concentrations peaked
at 350 to 450 µg/litre at the pond outlet and 30 m downstream,
respectively, 20 to 30 min after application, falling to between 50
and 100 µg/litre at 2 h. A concentration of 6.3 µg/litre was found
12 h after spraying. Total chlorothalonil mass was measured on
suspended sediment following the first spraying and showed 10 µg
persisting for 1.5 h after spraying and thereafter falling to
approximately 0.01 µg at 10 and 24 h. The report did not make clear
the volume of water filtered, which appears, however, to have been
1 litre. Environmental conditions such as total organic carbon (TOC),
pH, temperature and water hardness were not reported; consequently
their impacts on degradation could not be evaluated.
Chlorothalonil was detected on occasions at concentrations up to
3.6 µg/litre in a tile drainage system from a farm in Manitoba,
Canada, where the fungicide was sprayed routinely. It was detected on
one occasion (0.06 µg/litre) in the sump well outflow draining to a
municipal ditch (Krawchuk & Webster, 1987).
Over a 5-year period (1986-1990), water was sampled and analysed
from 1300 community water systems and rural domestic wells for 101
pesticides, including chlorothalonil. Chlorothalonil was not detected
in any of these samples although the reporting limit was 0.12
µg/litre, which represented the minimum quantification limits for this
particular pesticide in the study (US EPA, 1990).
Chlorothalonil was not detected in 57 water samples, 30 sediment
samples and 30 fish samples in an Environmental Survey of Chemicals in
Japan in 1991 (personal communication by the Office of Health Studies,
Environment Agency, Tokyo, 1992).
5.1.3 Soil
Levels of chlorothalonil and its metabolite SDS-3701 (see section
4.2.1) in soil were reported after three annual treatments (Kenyon &
Ballee, 1990; King et al., 1991, 1992). Four plots were established
of bare untreated and treated, winter wheat treated and untreated at
two different sites, Osterwede and Rohlstof (Germany). Treatment
consisted of an annual chlorothalonil application of 2.2 kg a.i./ha.
Soil samples were taken before and after each treatment. No
chlorothalonil was detected in any of the untreated samples.
Consistently there was no carry over from one year to another. Levels
in soil were highest 2 or 3 days after the treatment (sampling
depended on the sites), with mean levels in the bare plots around 0.40
mg/kg and in those with wheat around 0.34 mg/kg (values ranging
between 0.07 and 0.64 mg/kg). Between 52 and 60 days after each
treatment, levels were 0.02-0.03 mg/kg in plots with wheat while in
bare plots they were generally below the detection limit of 0.01
mg/kg. Before each treatment in the previously treated plots the
level of metabolite SDS-3701 ranged from the limit of detection (0.01)
to 0.03 mg/kg, which was the same as the level 2 or 3 days after
treatment. However, between 52 and 60 days after treatment (depending
on the site) levels rose at the Osterwede site to 0.07 mg/kg for the
bare treated plot. One year after the last treatment, levels of
SDS-3701 ranged from the detection limit to 0.03 mg/kg.
5.1.4 Food crops
Chlorothalonil is used as a broad spectrum fungicide on
vegetables, fruit trees, small fruit bushes and other agricultural and
horticultural food crops. Its use is intended to protect crops up to
harvesting, hence small residues will be present at that time. The
residue levels expected in crops at harvest can be derived from the
numerous supervized trials that have taken place on many crops in
countries all over the world (FAO/WHO, 1975, 1978, 1979, 1980, 1982,
1985a, 1986a, 1990a).
The amount of residue at harvest depends upon factors such as the
application rate, time interval between the last application and
harvest, and the type of crop. Residues are composed mainly of
chlorothalonil, and only negligible amounts of the metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile (SDS-3701) are present (see
Table 7 for example).
The decline of chlorothalonil residues on food crops after
application is shown by the field treatment of apples and pears
against Botryris cynerea by spraying with a chlorothalonil flowable
formulation and then harvesting at intervals after treatment (Camoni
et al., 1991). The results are shown in Table 8.
Table 8. Decline of chlorothalonil residues
Days after treatment Pears Apples
(mg/kg) (mg/kg)
0 3.85 2.35
7 2.48 1.73
14 2.00 0.92
28 1.35 0.98
From: Camoni et al. (1991)
Similar examples of the decline of chlorothalonil residues have
been given for grapes in Australia, Germany and South Africa (FAO/WHO,
1985a). The decline of residues in onions is shown in Table 7. The
distribution of the residues on this plant showed that the levels in
the older outer leaves were about 5 times above those in the younger
leaves (2.4 and 0.51 mg/kg, respectively).
Pre-harvest intervals are set on the basis of supervized trials,
e.g., 7 days for apricots, and cherries in Australia, 7-14 days for
grapes in Australia and 7 days for onions in the Netherlands (FAO/WHO,
1990a).
One of two samples of currants from growers in the United Kingdom
had a residue level of 7.5 mg/kg 54 days after the last of three
treatments at half the recommended rate of application. The residue
level on the second sample was < 0.5 mg/kg 76 days after two
applications at the maximum rate (UK, 1985).
5.1.5 Dairy produce
There have been no reports of chlorothalonil residues in dairy
produce. However, some indication can be gained from studies on dairy
cattle fed high levels of the compound. In one cow fed 250 mg
chlorothalonil/kg feed for 44 days, no chlorothalonil was detected in
the milk and only 0.2% of the dose appeared as the 4-hydroxy
metabolite. Neither compound could be detected in muscle or fat and
only a low level of the 4-hydroxy metabolite (0.7 mg/kg) was found in
the kidney (Ladd et al., 1971; Wolfe & Stallard, 1971). In another
study, groups of four cows were fed chlorothalonil combined with the
4-hydroxy metabolite at levels up to 250 and 0.6 mg/kg, respectively,
for 30 days. At the end of the period half the cows were sacrificed
and half continued for a 32-day recovery period. No chlorothalonil
(< 0.02 mg/kg) was found in milk. Small residues of chlorothalonil
and the 4-hydroxy metabolite were detected in muscle, fat, liver and
kidney after 30 days administration but none were detected in these
organs after the 32-day recovery period (FAO/WHO, 1975). No
chlorothalonil (< 0.03 mg/kg) was detected in milk from a cow fed the
compound at 5 mg/kg in its rations for 4 days (Gutenmann & Lisk,
1966).
5.1.6 Animal feed
Dry cannery waste (tomato pommace), sometimes used for animal
feed, contained < 1 mg/kg chlorothalonil plus its 4-hydroxy
metabolite (in the ratio 6:1) as a residue (FAO/WHO, 1978).
5.2 General population exposure
5.2.1 Food
In a study of imported fruit and vegetables in Finland, chlorothalonil
levels of 0.02-0.15 mg/kg in strawberries, 0.01-0.86 mg/kg in Chinese
lettuce and 0.12-1.2 mg/kg in peaches were found (personal
communication to the IPCS by the Government of Finland, 1979).
No chlorothalonil (< 0.01 mg/kg) was detected in a US Food and
Drug Administration (FDA) total diet study in the USA in 1976 or 1977
(personal communication to the IPCS by J.R. Wessel, 1979). In a
Canadian total diet survey, chlorothalonil was detected in one out of
six composite samples of garden fruits at the detection level (0.02
mg/kg). On the basis of this one sample, a dietary intake of 0.04 µg
per person per day was estimated (McLeod et al., 1980).
Chlorothalonil was detected (0.001-1.35 mg/kg) in most samples of
apples, peaches and other fruit and vegetables marketed in Tokyo
(Koseki et al., 1980).
No chlorothalonil (< 0.005 mg/kg) was detected in samples of
potatoes in Sweden in 1979. During 1981-1983, 1070 out of 1085
samples of domestic and imported commodities in Sweden had
chlorothalonil residues below 0.21 mg/kg. Samples having higher
residues included one of cauliflowers (out of 165) at 0.41 mg/kg, one
of cucumbers (out of 580) at 0.23 mg/kg and two of strawberries (out
of 143) at 2.9 mg/kg (personal communication: data submitted to the
IPCS by the Government of Sweden and entitled "Chlorothalonil residues
in imported and domestic commodities - 1981 to 1983").
In 1982, analysis at the point of retail in the United Kingdom
showed chlorothalonil residues below 0.5 mg/kg in 41 samples of
strawberries, 15 of gooseberries, 13 of currants and 9 of berries.
Other analyses during 1981-3 showed that only one out of 30 samples of
imported strawberries, 2 out of 15 samples of domestic celery and 5
out of 40 of gooseberries had chlorothalonil residues above 0.1 mg/kg
(UK, 1985).
In the United Kingdom, chlorothalonil residues in bananas
(imported), chinese cabbage (all origins) and parsnips (United Kingdom
origin) were below the reporting levels of 0.2, 1.0 and 0.01 mg/kg,
respectively, in 1988-1989. During the same period, one sample out of
ten of imported strawberries contained 0.1 mg/kg (UK MAFF & HSE,
1990).
Residues of chlorothalonil in foodstuffs are decreased by
processes such as washing. For example, it was shown that 94% of the
residue could be removed by washing tomatoes and that there was no
detectable residue in canned tomato pulp, paste or juice. Peaches
washed in water followed by a caustic rinse showed a 97% removal of
field residues. No chlorothalonil was detected in canned peach puree
(FAO/WHO, 1978).
In a Honduran study, unwashed bananas had a maximum residue level
of 0.17 mg/kg and a mean of 0.08 mg/kg. This was reduced to 0.02
mg/kg after washing. No chlorothalonil was found in the edible pulp
(< 0.01 mg/kg). Similar results were obtained in the Philippines
(FAO/WHO, 1980).
Trimming and peeling also removes a large proportion of residues
from some foodstuffs. For example there are significant reductions
after trimming the outer leaves from cabbages and lettuces. Most of
the residue is removed when cucumbers, melons, peanuts and potatoes
are peeled (Diamond Shamrock, 1974).
As much as 85-98% of chlorothalonil added to tomatoes or green
beans was lost during cooking in open vessels. Only 2.4% was
converted to the 4-hydroxy metabolite, which was stable to cooking
(SDS Biotech Corporation, 1983a).
5.3 Occupational exposure
The exposure of a tractor driver applying chlorothalonil to
ornamental plants in Florida, USA, was assessed. Total-body exposure
rates, estimated from external exposure pads and air sampling, were
low (approximately 5 mg a.i./h) (Stamper et al., 1989a). In the case
of a greenhouse drencher, this exposure was approximately 100 mg
a.i./h (Stamper et al., 1989b).
Occupational exposure to four insecticides and two fungicides was
measured for 151 commercial tree and shrub applicators in the USA who
used hand-held equipment when spraying pesticides. The study was
conducted for 3 consecutive years: 1985, 1986 and 1987. Worker
exposure was determined by collecting full-shift, breathing zone air
samples. Sampling was conducted with battery-operated constant-flow
air-sampling devices. Chlorothalonil was detected in only one out of
14 samples at 0.011 mg/m3 (Leonard & Yeary, 1990).
Spencer et al. (1991) estimated the dermal exposure of workers on
mechanical tomato harvesters to residues of chlorothalonil. An
average of 499.6 µg/h was obtained by gauze pad dosimeters placed
outside the workers' clothing, whereas 43.4 µg/h was obtained by
undershirt dosimetry. The results showed that regular work clothing
provides an excellent protection (90% reduction in dermal exposure)
against chlorothalonil. Air concentrations in the field were also
determined and averaged 0.002 to 0.02 µg/litre, which contributed 8 to
28% to the total exposure.
The exposure of 11 pesticide operators mixing, loading or
applying chlorothalonil fungicide formulations by aerial or ground
applicators has been assessed. The highest exposure was on the hands
(1.7 mg/m2 per h) (Diamond Shamrock, 1980).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Biliary excretion of radioactivity was studied in groups of six
male Sprague-Dawley rats administered a single oral dose of 1.5, 5, 50
or 200 mg/kg body weight 14C-chlorothalonil (98% radiochemically
pure), uniformly labelled in the aromatic ring, as a suspension with a
mean particle size of 3.6 to 5.0 µm in 0.75% methylcellulose in water.
The bile duct was cannulated and bile was collected in 1-h fractions
for 48 h after dosing. Blood, urine and faeces were also collected at
various times after dosing and at termination. During the 48 h after
a single dose of 1.5, 5, 50 and 200 mg/kg body weight, biliary
excretion was 22.5, 16.4, 16.3, and 7.7% of the administered dose,
respectively. Profiles of radioactivity excretion after the two low
doses were quantitatively different from those obtained after the two
high doses. The authors interpreted these results as indicative of a
change in metabolism occurring between 5 and 50 mg/kg body weight,
possibly due to saturation of biliary excretion. Mean urinary
excretion during the 48 h after dosing was 8.0, 8.2 and 7.6% of the
administered dose at 1.5, 5 and 50 mg/kg body weight, respectively,
and only 4.7% at the high dose level of 200 mg/kg body weight.
Excretion of radioactivity in the urine within 6 h after dosing was
inversely related to the dose administered. Total recovery of
radioactivity in this study was 89-99% in the three low-dose groups
and 74% in the high-dose group. After doses of 1.5, 5, 50 and
200 mg/kg body weight, rats absorbed 32, 25.7, 25.9 and 15.5% of the
administered dose, respectively. It was concluded by the authors that
enterohepatic circulation or reabsorption of biliary metabolites from
the gastrointestinal tract did not contribute significantly to the
amount of radiolabel in the kidney. Based on a one-compartment model
for chlorothalonil absorption and excretion and using several
assumptions, it was calculated that the rate of absorption of the
200 mg/kg body weight dose was only twice as fast as that of the
50 mg/kg body weight dose (Marciniszyn et al., 1986).
In a study by Chin et al. (1981), absorption was compared by the
oral, dermal and endotracheal routes with a 1 mg/kg body weight dose
of 14C-chlorothalonil in male Sprague-Dawley rats. The comparisons
were made on the basis of blood levels and urine excretion. In each
case, absorption was highest by endotracheal administration and lowest
by the dermal route. Less than 6% of the administered dose was
recovered in blood and urine within 48 h after dosing.
When 14C-chlorothalonil was introduced into sacs formed from the
upper section of rat small intestine, no unchanged substance passed
through the mucosa and was transferred to the serosal side of the sac.
These data suggest that chlorothalonil is very rapidly conjugated,
since in vivo studies have not identified chlorothalonil itself in
body fluids or tissues after oral administration to rats (Savides et
al., 1986e).
14C-chlorothalonil was applied to the skin of male rats at an
averaged dose of 1167 µg/rat (= 5 mg/kg) on an area of 25 cm2. The
amount absorbed was deduced from the amount remaining on the treated
skin area and the amount of radioactivity found at each time interval
in urine, faeces and carcass. Approximately 28% of the applied dose
was absorbed over the experimental exposure period of 120 h. The
absorption appeared to be time-dependent, about 6.3% of the applied
dose being absorbed during each 24 h period. Radioactivity appeared
quickly in blood and rose steadily up to 72 h, when it reached a
plateau (Marciniszyn et al., 1984a).
In a study by Magee et al. (1990), four monkeys were treated
dermally with 5 mg/kg body weight of 14C-chlorothalonil under a
non-occlusive patch. After 48 h the patch was taken off and the skin
was washed. About 90% of the dose was recovered from the surface and
about 2.26% was completely absorbed through the skin. The urine
contained 1% of the dose, but methylated mono-, di- and trithiols were
not detectable in the urine.
6.2 Distribution
Groups of male and female rats were administered
14C-chlorothalonil orally, in microparticulate suspension, as single
doses of 5, 50 or 200 mg/kg, and tissue activity was determined after
2, 9, 24, 96 and 168 h (Marciniszyn et al., 1984b, 1985a). With the
exception of gastrointestinal tract tissues the greatest concentration
of radioactivity was found in the kidneys, at each dose level,
followed by those in liver and whole blood. The peak concentrations
in kidney occurred at 2 h after 5 mg/kg, 9 h after 50 mg/kg and 24 h
after 200 mg/kg. Similar shifts in peak time with dose occurred in
the liver and blood. In terms of the original dose, kidneys, liver
and blood each contained 0.7% of the label 2 h after 5 mg/kg and 0.3%
(kidney), 0.14% (liver) and 0.23% (blood) after 24 h in female rats.
Distribution of radioactivity was also studied after repeated
oral administration of 14C-chlorothalonil to male rats. Five doses
were given at 24 h intervals at concentrations of 1.5, 5, 50 or
160 mg/kg. The rats were killed 2, 9, 24, 96 and 168 h after the last
dose. The distribution of activity showed a similar profile to that
after single dosing, i.e. the highest concentrations occurred in
kidneys, followed by liver and blood, at all doses and times. At all
dose levels, the concentrations peaked 2 h after the last dose. The
percentage of the dose found in the kidney at this time was 0.28% and
0.20% at the 1.5 and 5 mg/kg dose levels, which was significantly
higher than that found at the higher doses (about 0.09%). At dose
levels up to 50 mg/kg there was significant depletion of radioactivity
from the blood during the 24 h between doses. In the kidney there was
a trend to slower overall depletion with increase in dose (Savides et
al., 1986a).
A study in mice showed that the distribution of activity in
non-gastrointestinal tract tissues was similar to that in rats after a
single oral dose of 14C-chlorothalonil. The kidney had the highest
concentration of radioactivity after doses of 1.5, 15 or 105 mg/kg
(Ribovich et al., 1983).
6.3 Metabolic transformation
6.3.1 Rat
Male Sprague-Dawley rats were administered, via oral gavage,
14C-chlorothalonil (purity 99.7%) at a dose level of 200 mg/kg in
order to isolate and identify the urinary metabolites. Urine was
collected 17, 24 and 48 h after dosing. Urinary metabolites accounted
for 2.4% of the administered dose and, except for 30% of the
radiolabel which was non-extractable from the urine, were found to be
trimethylthiomonochloro-isophthalonitrile and dimethylthiodichloro-
isophthalonitrile. These thiols were excreted in urine both as free
thiols and as their methylated derivatives. The authors suggested a
metabolic pathway such that hepatic metabolism proceeds through
conjugation with GSH followed by enzymatic degradation. The smaller
conjugates are then transported via the bloodstream to the kidney,
where they are converted to thiol metabolites and excreted in the
urine (Marciniszyn et al., 1985b).
A study was also carried out in rats given five daily oral doses
of 14C-chlorothalonil (1.5, 5, 50 or 160 mg/kg per day). Urine
samples, acidified and extracted with ethyl acetate, showed decreasing
extractability of radioactivity with increasing dose. GC/MS analysis
identified methylated or partly methylated dithiol and trithiol
derivatives of chlorothalonil from the first dose onwards. The
percentage of the trithiol derivative excreted was constant with
increasing dose while the dithiol increased with dose. Multiple
dosing resulted in a decreasing daily excretion of total thiol
derivatives. These results emphasize the probable involvement of
glutathione in the metabolic pathway for chlorothalonil (Savides et
al., 1986b).
A group of three rats, pretreated with the gamma-glutamyl
transpeptidase inhibitor AT-125, were dosed with 50 mg/kg
14C-chlorothalonil, while three other rats were given chlorothalonil
only. Urine samples were acidified and extracted with ethyl acetate.
The group of rats pre-treated with AT-125 showed only 15% of
radioactivity extractable after 12 h, while the other group showed 75%
extractability. The non-extractable fraction from the
inhibitor-treated rats contained glutathione conjugates of
chlorothalonil. The kidneys contained 2-3 times more radioactivity
than those of the untreated rats. These results gave further support
to the hypothesis that glutathione is involved with chlorothalonil
metabolism (Marciniszyn et al., 1988).
The production of metabolites was also studied in groups of rats
following dermal administration. 14C-chlorothalonil (4.6 mg/kg) was
applied to a shaved area of the dorsal region. The area was covered
and exposure continued for 48 h. Urine samples collected at 24 and
48 h were acidified and extracted with ethyl acetate. The extracts
were submitted to reverse-phase HPLC/LSC followed by methylation and
further clean-up. The trithiol derivative of chlorothalonil was the
major metabolite in all samples. The excretion of total thiol
metabolites was at least 20-fold less than that resulting from oral
dosing at the same dose level (Savides et al., 1987a).
The radiolabelled monoglutathione derivative of chlorothalonil
was administered to male rats (115 mg/kg) as a single oral or
intraperitoneal dose. Six hours after intraperitoneal dosing the
blood level was 10 times higher than after oral dosing. The
proportion of the administered intraperitoneal dose in the kidney was
16 times higher than after oral dosing. Urine from the orally dosed
rats contained 9% trithiol derivative and 5% dithiol, while
intraperitoneally dosed rats showed < 1% dithiol derivative and none
of the trithiol in urine. This indicates that the orally administered
monoglutathione conjugate is further conjugated with glutathione in
the gastrointestinal tract prior to absorption (Savides et al.,
1986f).
Nine germ-free male rats each received approximately 56 µCi
14C-chlorothalonil in a single oral dose of 50 mg/kg. Urine and
faeces were collected over a 96-h period, and the urine was processed
to identify and quantify thiol derivatives of chlorothalonil. These
derivatives were detected in only three of the nine rats and
represented < 0.03% of the dose. This is fifty times less than that
obtained for normal rats. There is therefore strong evidence that
intestinal microflora make a significant contribution to the
metabolism of chlorothalonil after oral administration in the rat
(Savides et al., 1990a).
The HPLC analysis of faecal extracts from rats dosed with 200 mg
chlorothalonil/kg showed that 28% was excreted unchanged and 5% was
converted to 4-hydroxy 2,3,5-trichloroisophthalonitrile. The amounts
after a dose of 5 mg/kg were 1.6 and 6.2%, respectively (Ignatoski et
al., 1983).
The HPLC analysis of faeces from rats given 14C-chlorothalonil
orally at 5, 50 and 200 mg/kg showed the presence of at least seven
radioactive components. Two of the peaks had the same retention times
as chlorothalonil and its 4-hydroxy metabolite. A higher proportion
of the metabolite was present after the 5 mg/kg dose than after the
higher doses. The majority of the activity was unextractable and was
therefore bound to faecal components (Lee et al., 1982).
6.3.2 Dog
Male beagle dogs were given 14C-chlorothalonil at a dose level
of 50 mg/kg either by gelatin capsule or by gavage. In each case the
urinary excretion of radioactivity was very small and none of the
methylated thiol derivatives of chlorothalonil were detected (Savides
et al., 1989, 1990b).
6.3.3 Monkey
Four male Chinese rhesus monkeys were dosed with
14C-chlorothalonil by gavage at 50 mg/kg body weight suspended in
0.75% aqueous methylcellulose. Extraction of urine, collected over
48 h, with acidified ethyl acetate showed that 32-65% of the
radioactivity was extractable. The total amount of chlorothalonil
thiol derivatives excreted was 0.001-0.01% of the administered dose,
mainly as the trimethylthiol entity. This was more than 100 times
less than that excreted from the rat (Savides et al., 1990c).
6.4 Elimination and excretion
6.4.1 Rat
The main route of elimination of chlorothalonil from the rat
after oral dosing is via the faeces. The percentage eliminated was
consistent for males and females, at doses of 1.5-200 mg/kg and from
single or repeated doses. The amount eliminated was consistently
above 82%, the majority appearing in the first 48 h at low doses and
within 72 h at high doses (Marciniszyn et al., 1984b, 1985a).
Biliary excretion at dose levels up to 5 mg/kg is rapid, peaking
at 2 h, and is more prolonged at levels of 50 mg/kg or more.
Excretion decreases with increasing dose, from 22.5% at 1.5 mg/kg to
7.7% at 200 mg/kg over 48 h. Studies using bile duct cannulation
indicate that the excretion is saturated at 50 mg/kg or more.
Comparison with non-cannulated rats showed that there was no
difference in the radioactive concentration found in the kidney,
indicating that enterohepatic circulation of biliary metabolites did
not play a significant role (Savides et al., 1986c).
The fate of orally administered 14C-chlorothalonil (purity
99.7%) at three dose levels (5, 50 and 200 mg/kg) was investigated in
Sprague-Dawley rats to determine the effects of increasing doses of
the test material. Four animals of each sex at each dose level were
killed 2, 9, 24, 96 and 168 h after dosing and urine, faeces and
selected tissues were assayed for radioactivity. The average recovery
of the radiolabel at each of the dose levels was approximately 89% for
males and 96% for females. The major route of elimination was via the
faeces (83-87%) and was essentially complete by 48 h in low-dose
females and low/mid-dose males, and by 72 h in the mid/high-dose
females and high-dose males. A delay in stomach-emptying time was
observed for mid- and high-dose males and females. Urinary excretion
was 92-93% complete for low-dose rats within 24 h, mid-dose rats
within 48 h, and 95% complete for high-dose rats within 72 h. Urinary
excretion of the radiolabel at the three dose levels was 5-7% of the
administered dose in males and 5-11.5% in females. Urinary excretion
was essentially saturated as the dose level increased. The highest
concentrations of radiolabelled material in non-gastrointestinal
tissues were found in the kidney, being approximately 0.7% of the dose
per gram of kidney for males and 0.4% in females at peak concentration
(2 h) for the 5 mg/kg dose level. Kidney concentrations were greatest
at 2, 9 and 24 h for the low, mid and high doses, respectively
(Marciniszyn et al., 1984b, 1985a).
When 14C-chlorothalonil was applied dermally to male rats at
5 mg/kg the major route of excretion was the faeces. Approximately 18%
of the dose was excreted by this route in 120 h compared to about 6%
via urine (Marciniszyn et al., 1984a).
After administration of 1 mg chlorothalonil/kg by the oral,
dermal or endotracheal routes, the excretion in urine during 24 h was
2.9, 0.9 and 5.7%, respectively (Chin et al., 1981).
A study involving intubation of the 4-hydroxy metabolite of
chlorothalonil to rats at 4 or 43 mg/kg showed that the majority of
the dose was excreted in the faeces and a small amount in the urine
(Jarrett et al., 1978).
6.4.2 Mouse
In male mice, dosed orally with 1.5, 15 or 105 mg
14C-chlorothalonil/kg, the major route of elimination was via the
faeces. This was complete by 24 h for the two lower doses and by 96 h
for the highest dose. Urinary excretion at all doses varied between 5
and 10% of the administered dose (Ribovich et al., 1983).
6.4.3 Dog
Most of an oral dose of chlorothalonil was excreted in the faeces
of beagle dogs. Over 12 days, 99.6% was excreted from two dogs given
50 mg 14C-chlorothalonil/kg by gelatin capsule, and 76-98% was
excreted over 24 h when three dogs were given the same dose by gavage.
In both studies the amount excreted in urine was very small, and this
occurred mostly within the first 10 h (Savides et al., 1989, 1990b).
6.4.4 Monkey
Oral administration of 14C-chlorothalonil to four male Chinese
rhesus monkeys showed faecal elimination to be the main route of
excretion, 52-92% of the dose (50 mg/kg) being excreted in 96 h.
Urinary excretion amounted to 1.8-4.1% of the dose. Most of the
radiolabel was eliminated in the first 48 h (Savides et al., 1990c).
6.5 Reaction with body components
Incubation of 14C-chlorothalonil with glutathione in aqueous
medium in the presence or absence of glutathione- S-transferase
resulted in the rapid disappearance of chlorothalonil and the
appearance of more polar compounds. These were identified as
conjugates of chlorothalonil with glutathione, their formation
following a step-wise process, i.e., from mono->di->triglutathione
conjugates (Savides et al., 1985). This reaction with glutathione
parallels similar findings with chlorothalonil in other biological
systems such as Saccharomyces pastorianus (Tillman et al., 1973).
The incubation of chlorothalonil with rat stomach or intestinal
mucosal cells indicated that polar metabolites were formed which were
chromatographically similar to glutathione conjugates of
chlorothalonil (Savides et al., 1986d).
The in vivo action of chlorothalonil on glutathione (GSH) was
shown in a rat study where chlorothalonil was dosed orally at
5000 mg/kg. Hepatic GSH was decreased by 40% 18 h later but recovered
to a normal value after 48 h. Kidney GSH increased to two times its
control level after 48 h (Sadler et al., 1985).
Chlorothalonil has been shown to bind to calf thymus histones,
the rate and amount depending upon pH and type of histones. There was
little binding to DNA. Treatment of rat liver nuclei indicated
similar binding patterns to those for histones (Rosanoff & Siegel,
1981).
Groups of four rats were administered 50 mg 14C-chlorothalonil
per kg orally, killed after 6 h and their kidneys excised. The kidney
tissue was homogenized, and protein and DNA were isolated. Radiolabel
was found to be bound to kidney protein but not to DNA (Savides et
al., 1987b). Kidney tissue from rats dosed 50 mg
14C-chlorothalonil/kg orally was separated by ultracentrifugation
into subcellular organelles. The kidneys contained about 0.38% of the
original dose, the majority of this activity (81%) being in the
soluble fraction. About 10% of the remaining activity was contained
in the mitochondrial subfractions (Savides et al., 1987c).
Studies with rat liver and kidney mitochondrial preparations
showed that the dithiol derivative of chlorothalonil completely
inhibited state 3 mitrochondrial respiration. The monothiol
derivative affected oxygen uptake by liver mitochondria but not by
kidney mitochondria. The mono- and diglutathione conjugates of
chlorothalonil did not affect oxygen uptake by mitochondria. Since
cleavage of the glutathione conjugates to give the thiol derivatives
takes place in the kidney, this may explain the toxic action of
chlorothalonil in this organ (Savides et al., 1988; see also section
7.8).
Available evidence indicates that the enzyme ß-lyase is required
for the formation of thiol derivatives from cysteine conjugates. The
activity of this enzyme has been assessed in rat, mouse and human
kidney cytosolic fractions using the perchloroethylene metabolite
S-(1,2,2-trichlorovinyl)-l-cysteine as substrate. The activity of
renal ß-lyase in human kidney was comparable to that of mouse, but was
an order of magnitude lower than that in the rat (Green et al., 1990).
The proposed metabolic pathway for the production of thiol
derivatives from chlorothalonil in the kidney is shown in Fig. 6.
On plants, chlorothalonil is metabolized only to a limited extent
to the 4-hydroxy metabolite. The majority of the residue remains as
the parent compound. Generally less than 5% of the total residue is
present as the 4-hydroxy metabolite. A review of plant residues
worldwide showed that the 4-hydroxy metabolite level was < 0.1 mg/kg
in most of the crops analysed. It accounted for approximately 10% of
the total residue in lima beans, 5% in cantaloupes, 2% in peaches and
onions, 1% in celery and 0.1% in peanuts (FAO/WHO, 1985). The decline
of chlorothalonil residues and the appearance and decline of the
4-hydroxy metabolite on onions is shown in Table 7 (personal
communication to the IPCS by the Government of Canada, 1979). The
chlorothalonil residue decayed with a half-life of about 3 days.
Studies with corn silage showed that 90% of chlorothalonil
degraded within 18 days (30 to 3 mg/kg). The half-life was
approximately 4 days. In a second experiment, the 4-hydroxy
metabolite formation was very low in the bound materials (which were
converted to an extractable form), representing only about 2% of the
chlorothalonil on the first day of ensiling (FAO/WHO, 1978).
After chlorothalonil was applied to growing peanut foliage at
1.26 kg/ha its half-life was 13.6 days (range 7-19 days) under the
field conditions of use (Elliott & Spurr, 1993).
In the excretion of 14C-chlorothalonil metabolites from rainbow
trout (Salmo gairdneri), the almost complete absence of
chlorothalonil itself and the accumulation of 14C entities in the
bile indicated the possibility of glutathione conjugation as the first
step in chlorothalonil metabolism (Davies & White, 1985). Further
studies showed the existence of mono- and diglutathione conjugates of
chlorothalonil in the bile of rainbow trout exposed to
14C-chlorothalonil (Davies, 1985a).
Studies with liver cytosol from five fish species showed that the
enzyme glutathione- S-transferase (GST) is involved in the conversion
of chlorothalonil to polar conjugates. Comparisons of GST activity in
rainbow trout organs revealed that the potential for chlorothalonil
transformation was in the order liver » kidney > spleen, with no
activity in bile. Low concentrations of chlorothalonil in water
induced fish GST activity for its biotransformation. Hepatic
glutathione (GSH) and GST activity for chlorothalonil transformation
were compared in three species of fish (Oncorhynchus mykiss,
Galaxias maculatus and Galaxias auratus). The order of their
asymptotic LC50 values agreed with that of their hepatic GST
activities for chlorothalonil transformation and was consistent with a
detoxification role for GSH-chlorothalonil conjugation (Davies,
1985b). A study involving co-exposure to zinc and chlorothalonil
indicated that metallothionein does not play a significant role in
chlorothalonil detoxification in fish at sublethal exposures (Davies,
1985c).
Small amounts of the 4-hydroxy metabolite were found in the milk
and kidney of a cow fed 250 mg chlorothalonil/kg in its feed. Only
0.2% of the ingested chlorothalonil was eliminated in the milk as the
4-hydroxy metabolite (Ladd et al., 1971).
4.2.2 Abiotic degradation
Chlorothalonil does not break down in aqueous solution
(0.5 mg/litre) in the dark at pH 5 or 7. It is hydrolysed at pH 9,
over 50% disappearing in 49 days, with the formation of 4-hydroxy-
2,5,6-trichloroisophthalonitrile and 3-cyano-2,4,5,6-tetrachloro-
benzamide (Szalkowski & Stallard, 1977).
Chlorothalonil degrades very slowly under aqueous photolytic
conditions to the 4-hydroxy metabolite. The half-life was found to be
approximately 65 days (ISK Biotech proprietary information).
4.2.3 Bioaccumulation
In a study of the uptake and elimination of 14C-chlorothalonil
in rainbow trout, two groups of fish were exposed to 10 µg/litre of
the compound in flow-through tanks for 96 h (Davies & White, 1985).
After exposure was discontinued, the depuration rate was followed for
96 h. There was a very high uptake in the gall bladder and bile
(concentration factors up to 4.4 × 105). Uptake was also high in the
hind gut, liver, fat and kidney with concentration factors of
2-11 × 103. After 96 h of exposure, the concentration factor in
muscle was 940 and 740, respectively, for the two groups of fish, a
level which may give an indication of the magnitude of the whole body
bioconcentration factor (BCF) for rainbow trout (not measured).
After transfer to clean water, gall bladder levels dropped
rapidly, and so did gill and blood levels. In one group of fish,
concentrations in both liver and kidney doubled until 24 h after
transfer and thereafter dropped to the levels in the other group.
Concentrations in the spleen in both groups continued to increase
throughout the depuration period. Muscle levels dropped only slowly
and remained around 1 µg/g. The high concentrations found in the gall
bladder and bile are consistent with the fact that chlorothalonil is
excreted from fish as glutathione conjugates (Davies & White, 1985).
Bluegill sunfish exposed to 8 µg 14C-chlorothalonil/litre in a
flow-through system for 30 days showed a plateau of 14C uptake within
14 days. The residues in whole fish at 30 days were 264 times the
water concentration. When the fish were placed in clean water, 80% of
the radioactive residues were lost within 14 days. Bioaccumulation in
catfish, in a static system, showed a 16-fold concentration at 26
days. In this case 90% of the 14C residues were depurated in 14 days
after removal from the treated water. The 4-hydroxy metabolite did
not bioaccumulate in fish (SDS Biotech Corporation, 1972).
In tanks containing stream water with chlorothalonil at
20 µg/litre, uptake of the compound occurred in algal growths attached
to bottom pebbles. Analysis of the algal growths showed a
concentration factor for chlorothalonil of 270 times after 14 days of
static exposure. Since this represented only 9.5% of the initial dose
it seems that the removal of chlorothalonil from the water is enhanced
by its conversion to polar metabolites in addition to bioconcentration
(Davies, 1988).
4.3 Waste disposal
Chlorothalonil can be incinerated in units operating at 850°C
fitted with off-gas scrubbing equipment (Lawless et al., 1975).
The disposal methods for waste pesticides and containers
advocated by FAO and GIFAP should be applied to unused chlorothalonil
products and their empty packages (FAO, 1985; GIFAP, 1987).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Chlorothalonil was detected (amongst other pesticides) in 3 out
of 9 outdoor and indoor samples and 1 out of 9 personal monitoring
samples in 9 homes in Jacksonville, Florida, USA. No actual figures
were reported (Lewis et al., 1988).
Average exposures to chlorothalonil of 173 persons in
Jacksonville, Florida and Springfield, Massachusetts, USA were
0.7 ng/m3 (personal exposure) and 0.5 ng/m3 (outdoor air
concentrations) (Wallace, 1991).
Chlorothalonil was not detected in 51 samples in an Environmental
Survey of Chemicals in Japan in 1991 (personal communication by the
Office of Health Studies Environment Agency, Tokyo, 1992).
5.1.2 Water
O'Neil (1991) studied concentrations of chlorothalonil in water
and sediment following overspraying of a pond (0.2 ha and 0.5 m deep)
at 875 g a.i./ha (Ernst, 1991). Water and sediment were monitored in
a stream flowing out of the pond at the outlet and 30 m downstream.
The stream was approximately 1 m wide and 0.5 m deep and ran at
0.033 m3/sec for the first overspray and at 0.015 m3/sec for the
repeat spray. Whole water samples were filtered for separate
measurement of chlorothalonil in water and sediment. Following the
first spraying, samples were taken at the downstream site at 30 min
intervals up to 6 h after spraying and further samples were collected
10 and 24 h after spraying. Initial water concentrations of up to
60 µg/litre fell rapidly to around 15 µg/litre 2 h after spraying.
The water concentration was 1.9 µg/litre 10 h after spraying, and at
24 h there was no measurable chlorothalonil. In the second spraying
at the lower stream flow rate, whole water samples were taken more
frequently over the 2 h following application. Concentrations peaked
at 350 to 450 µg/litre at the pond outlet and 30 m downstream,
respectively, 20 to 30 min after application, falling to between 50
and 100 µg/litre at 2 h. A concentration of 6.3 µg/litre was found
12 h after spraying. Total chlorothalonil mass was measured on
suspended sediment following the first spraying and showed 10 µg
persisting for 1.5 h after spraying and thereafter falling to
approximately 0.01 µg at 10 and 24 h. The report did not make clear
the volume of water filtered, which appears, however, to have been
1 litre. Environmental conditions such as total organic carbon (TOC),
pH, temperature and water hardness were not reported; consequently
their impacts on degradation could not be evaluated.
Chlorothalonil was detected on occasions at concentrations up to
3.6 µg/litre in a tile drainage system from a farm in Manitoba,
Canada, where the fungicide was sprayed routinely. It was detected on
one occasion (0.06 µg/litre) in the sump well outflow draining to a
municipal ditch (Krawchuk & Webster, 1987).
Over a 5-year period (1986-1990), water was sampled and analysed
from 1300 community water systems and rural domestic wells for 101
pesticides, including chlorothalonil. Chlorothalonil was not detected
in any of these samples although the reporting limit was 0.12
µg/litre, which represented the minimum quantification limits for this
particular pesticide in the study (US EPA, 1990).
Chlorothalonil was not detected in 57 water samples, 30 sediment
samples and 30 fish samples in an Environmental Survey of Chemicals in
Japan in 1991 (personal communication by the Office of Health Studies,
Environment Agency, Tokyo, 1992).
5.1.3 Soil
Levels of chlorothalonil and its metabolite SDS-3701 (see section
4.2.1) in soil were reported after three annual treatments (Kenyon &
Ballee, 1990; King et al., 1991, 1992). Four plots were established
of bare untreated and treated, winter wheat treated and untreated at
two different sites, Osterwede and Rohlstof (Germany). Treatment
consisted of an annual chlorothalonil application of 2.2 kg a.i./ha.
Soil samples were taken before and after each treatment. No
chlorothalonil was detected in any of the untreated samples.
Consistently there was no carry over from one year to another. Levels
in soil were highest 2 or 3 days after the treatment (sampling
depended on the sites), with mean levels in the bare plots around 0.40
mg/kg and in those with wheat around 0.34 mg/kg (values ranging
between 0.07 and 0.64 mg/kg). Between 52 and 60 days after each
treatment, levels were 0.02-0.03 mg/kg in plots with wheat while in
bare plots they were generally below the detection limit of 0.01
mg/kg. Before each treatment in the previously treated plots the
level of metabolite SDS-3701 ranged from the limit of detection (0.01)
to 0.03 mg/kg, which was the same as the level 2 or 3 days after
treatment. However, between 52 and 60 days after treatment (depending
on the site) levels rose at the Osterwede site to 0.07 mg/kg for the
bare treated plot. One year after the last treatment, levels of
SDS-3701 ranged from the detection limit to 0.03 mg/kg.
5.1.4 Food crops
Chlorothalonil is used as a broad spectrum fungicide on
vegetables, fruit trees, small fruit bushes and other agricultural and
horticultural food crops. Its use is intended to protect crops up to
harvesting, hence small residues will be present at that time. The
residue levels expected in crops at harvest can be derived from the
numerous supervized trials that have taken place on many crops in
countries all over the world (FAO/WHO, 1975, 1978, 1979, 1980, 1982,
1985a, 1986a, 1990a).
The amount of residue at harvest depends upon factors such as the
application rate, time interval between the last application and
harvest, and the type of crop. Residues are composed mainly of
chlorothalonil, and only negligible amounts of the metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile (SDS-3701) are present (see
Table 7 for example).
The decline of chlorothalonil residues on food crops after
application is shown by the field treatment of apples and pears
against Botryris cynerea by spraying with a chlorothalonil flowable
formulation and then harvesting at intervals after treatment (Camoni
et al., 1991). The results are shown in Table 8.
Table 8. Decline of chlorothalonil residues
Days after treatment Pears Apples
(mg/kg) (mg/kg)
0 3.85 2.35
7 2.48 1.73
14 2.00 0.92
28 1.35 0.98
From: Camoni et al. (1991)
Similar examples of the decline of chlorothalonil residues have
been given for grapes in Australia, Germany and South Africa (FAO/WHO,
1985a). The decline of residues in onions is shown in Table 7. The
distribution of the residues on this plant showed that the levels in
the older outer leaves were about 5 times above those in the younger
leaves (2.4 and 0.51 mg/kg, respectively).
Pre-harvest intervals are set on the basis of supervized trials,
e.g., 7 days for apricots, and cherries in Australia, 7-14 days for
grapes in Australia and 7 days for onions in the Netherlands (FAO/WHO,
1990a).
One of two samples of currants from growers in the United Kingdom
had a residue level of 7.5 mg/kg 54 days after the last of three
treatments at half the recommended rate of application. The residue
level on the second sample was < 0.5 mg/kg 76 days after two
applications at the maximum rate (UK, 1985).
5.1.5 Dairy produce
There have been no reports of chlorothalonil residues in dairy
produce. However, some indication can be gained from studies on dairy
cattle fed high levels of the compound. In one cow fed 250 mg
chlorothalonil/kg feed for 44 days, no chlorothalonil was detected in
the milk and only 0.2% of the dose appeared as the 4-hydroxy
metabolite. Neither compound could be detected in muscle or fat and
only a low level of the 4-hydroxy metabolite (0.7 mg/kg) was found in
the kidney (Ladd et al., 1971; Wolfe & Stallard, 1971). In another
study, groups of four cows were fed chlorothalonil combined with the
4-hydroxy metabolite at levels up to 250 and 0.6 mg/kg, respectively,
for 30 days. At the end of the period half the cows were sacrificed
and half continued for a 32-day recovery period. No chlorothalonil
(< 0.02 mg/kg) was found in milk. Small residues of chlorothalonil
and the 4-hydroxy metabolite were detected in muscle, fat, liver and
kidney after 30 days administration but none were detected in these
organs after the 32-day recovery period (FAO/WHO, 1975). No
chlorothalonil (< 0.03 mg/kg) was detected in milk from a cow fed the
compound at 5 mg/kg in its rations for 4 days (Gutenmann & Lisk,
1966).
5.1.6 Animal feed
Dry cannery waste (tomato pommace), sometimes used for animal
feed, contained < 1 mg/kg chlorothalonil plus its 4-hydroxy
metabolite (in the ratio 6:1) as a residue (FAO/WHO, 1978).
5.2 General population exposure
5.2.1 Food
In a study of imported fruit and vegetables in Finland, chlorothalonil
levels of 0.02-0.15 mg/kg in strawberries, 0.01-0.86 mg/kg in Chinese
lettuce and 0.12-1.2 mg/kg in peaches were found (personal
communication to the IPCS by the Government of Finland, 1979).
No chlorothalonil (< 0.01 mg/kg) was detected in a US Food and
Drug Administration (FDA) total diet study in the USA in 1976 or 1977
(personal communication to the IPCS by J.R. Wessel, 1979). In a
Canadian total diet survey, chlorothalonil was detected in one out of
six composite samples of garden fruits at the detection level (0.02
mg/kg). On the basis of this one sample, a dietary intake of 0.04 µg
per person per day was estimated (McLeod et al., 1980).
Chlorothalonil was detected (0.001-1.35 mg/kg) in most samples of
apples, peaches and other fruit and vegetables marketed in Tokyo
(Koseki et al., 1980).
No chlorothalonil (< 0.005 mg/kg) was detected in samples of
potatoes in Sweden in 1979. During 1981-1983, 1070 out of 1085
samples of domestic and imported commodities in Sweden had
chlorothalonil residues below 0.21 mg/kg. Samples having higher
residues included one of cauliflowers (out of 165) at 0.41 mg/kg, one
of cucumbers (out of 580) at 0.23 mg/kg and two of strawberries (out
of 143) at 2.9 mg/kg (personal communication: data submitted to the
IPCS by the Government of Sweden and entitled "Chlorothalonil residues
in imported and domestic commodities - 1981 to 1983").
In 1982, analysis at the point of retail in the United Kingdom
showed chlorothalonil residues below 0.5 mg/kg in 41 samples of
strawberries, 15 of gooseberries, 13 of currants and 9 of berries.
Other analyses during 1981-3 showed that only one out of 30 samples of
imported strawberries, 2 out of 15 samples of domestic celery and 5
out of 40 of gooseberries had chlorothalonil residues above 0.1 mg/kg
(UK, 1985).
In the United Kingdom, chlorothalonil residues in bananas
(imported), chinese cabbage (all origins) and parsnips (United Kingdom
origin) were below the reporting levels of 0.2, 1.0 and 0.01 mg/kg,
respectively, in 1988-1989. During the same period, one sample out of
ten of imported strawberries contained 0.1 mg/kg (UK MAFF & HSE,
1990).
Residues of chlorothalonil in foodstuffs are decreased by
processes such as washing. For example, it was shown that 94% of the
residue could be removed by washing tomatoes and that there was no
detectable residue in canned tomato pulp, paste or juice. Peaches
washed in water followed by a caustic rinse showed a 97% removal of
field residues. No chlorothalonil was detected in canned peach puree
(FAO/WHO, 1978).
In a Honduran study, unwashed bananas had a maximum residue level
of 0.17 mg/kg and a mean of 0.08 mg/kg. This was reduced to 0.02
mg/kg after washing. No chlorothalonil was found in the edible pulp
(< 0.01 mg/kg). Similar results were obtained in the Philippines
(FAO/WHO, 1980).
Trimming and peeling also removes a large proportion of residues
from some foodstuffs. For example there are significant reductions
after trimming the outer leaves from cabbages and lettuces. Most of
the residue is removed when cucumbers, melons, peanuts and potatoes
are peeled (Diamond Shamrock, 1974).
As much as 85-98% of chlorothalonil added to tomatoes or green
beans was lost during cooking in open vessels. Only 2.4% was
converted to the 4-hydroxy metabolite, which was stable to cooking
(SDS Biotech Corporation, 1983a).
5.3 Occupational exposure
The exposure of a tractor driver applying chlorothalonil to
ornamental plants in Florida, USA, was assessed. Total-body exposure
rates, estimated from external exposure pads and air sampling, were
low (approximately 5 mg a.i./h) (Stamper et al., 1989a). In the case
of a greenhouse drencher, this exposure was approximately 100 mg
a.i./h (Stamper et al., 1989b).
Occupational exposure to four insecticides and two fungicides was
measured for 151 commercial tree and shrub applicators in the USA who
used hand-held equipment when spraying pesticides. The study was
conducted for 3 consecutive years: 1985, 1986 and 1987. Worker
exposure was determined by collecting full-shift, breathing zone air
samples. Sampling was conducted with battery-operated constant-flow
air-sampling devices. Chlorothalonil was detected in only one out of
14 samples at 0.011 mg/m3 (Leonard & Yeary, 1990).
Spencer et al. (1991) estimated the dermal exposure of workers on
mechanical tomato harvesters to residues of chlorothalonil. An
average of 499.6 µg/h was obtained by gauze pad dosimeters placed
outside the workers' clothing, whereas 43.4 µg/h was obtained by
undershirt dosimetry. The results showed that regular work clothing
provides an excellent protection (90% reduction in dermal exposure)
against chlorothalonil. Air concentrations in the field were also
determined and averaged 0.002 to 0.02 µg/litre, which contributed 8 to
28% to the total exposure.
The exposure of 11 pesticide operators mixing, loading or
applying chlorothalonil fungicide formulations by aerial or ground
applicators has been assessed. The highest exposure was on the hands
(1.7 mg/m2 per h) (Diamond Shamrock, 1980).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Biliary excretion of radioactivity was studied in groups of six
male Sprague-Dawley rats administered a single oral dose of 1.5, 5, 50
or 200 mg/kg body weight 14C-chlorothalonil (98% radiochemically
pure), uniformly labelled in the aromatic ring, as a suspension with a
mean particle size of 3.6 to 5.0 µm in 0.75% methylcellulose in water.
The bile duct was cannulated and bile was collected in 1-h fractions
for 48 h after dosing. Blood, urine and faeces were also collected at
various times after dosing and at termination. During the 48 h after
a single dose of 1.5, 5, 50 and 200 mg/kg body weight, biliary
excretion was 22.5, 16.4, 16.3, and 7.7% of the administered dose,
respectively. Profiles of radioactivity excretion after the two low
doses were quantitatively different from those obtained after the two
high doses. The authors interpreted these results as indicative of a
change in metabolism occurring between 5 and 50 mg/kg body weight,
possibly due to saturation of biliary excretion. Mean urinary
excretion during the 48 h after dosing was 8.0, 8.2 and 7.6% of the
administered dose at 1.5, 5 and 50 mg/kg body weight, respectively,
and only 4.7% at the high dose level of 200 mg/kg body weight.
Excretion of radioactivity in the urine within 6 h after dosing was
inversely related to the dose administered. Total recovery of
radioactivity in this study was 89-99% in the three low-dose groups
and 74% in the high-dose group. After doses of 1.5, 5, 50 and
200 mg/kg body weight, rats absorbed 32, 25.7, 25.9 and 15.5% of the
administered dose, respectively. It was concluded by the authors that
enterohepatic circulation or reabsorption of biliary metabolites from
the gastrointestinal tract did not contribute significantly to the
amount of radiolabel in the kidney. Based on a one-compartment model
for chlorothalonil absorption and excretion and using several
assumptions, it was calculated that the rate of absorption of the
200 mg/kg body weight dose was only twice as fast as that of the
50 mg/kg body weight dose (Marciniszyn et al., 1986).
In a study by Chin et al. (1981), absorption was compared by the
oral, dermal and endotracheal routes with a 1 mg/kg body weight dose
of 14C-chlorothalonil in male Sprague-Dawley rats. The comparisons
were made on the basis of blood levels and urine excretion. In each
case, absorption was highest by endotracheal administration and lowest
by the dermal route. Less than 6% of the administered dose was
recovered in blood and urine within 48 h after dosing.
When 14C-chlorothalonil was introduced into sacs formed from the
upper section of rat small intestine, no unchanged substance passed
through the mucosa and was transferred to the serosal side of the sac.
These data suggest that chlorothalonil is very rapidly conjugated,
since in vivo studies have not identified chlorothalonil itself in
body fluids or tissues after oral administration to rats (Savides et
al., 1986e).
14C-chlorothalonil was applied to the skin of male rats at an
averaged dose of 1167 µg/rat (= 5 mg/kg) on an area of 25 cm2. The
amount absorbed was deduced from the amount remaining on the treated
skin area and the amount of radioactivity found at each time interval
in urine, faeces and carcass. Approximately 28% of the applied dose
was absorbed over the experimental exposure period of 120 h. The
absorption appeared to be time-dependent, about 6.3% of the applied
dose being absorbed during each 24 h period. Radioactivity appeared
quickly in blood and rose steadily up to 72 h, when it reached a
plateau (Marciniszyn et al., 1984a).
In a study by Magee et al. (1990), four monkeys were treated
dermally with 5 mg/kg body weight of 14C-chlorothalonil under a
non-occlusive patch. After 48 h the patch was taken off and the skin
was washed. About 90% of the dose was recovered from the surface and
about 2.26% was completely absorbed through the skin. The urine
contained 1% of the dose, but methylated mono-, di- and trithiols were
not detectable in the urine.
6.2 Distribution
Groups of male and female rats were administered
14C-chlorothalonil orally, in microparticulate suspension, as single
doses of 5, 50 or 200 mg/kg, and tissue activity was determined after
2, 9, 24, 96 and 168 h (Marciniszyn et al., 1984b, 1985a). With the
exception of gastrointestinal tract tissues the greatest concentration
of radioactivity was found in the kidneys, at each dose level,
followed by those in liver and whole blood. The peak concentrations
in kidney occurred at 2 h after 5 mg/kg, 9 h after 50 mg/kg and 24 h
after 200 mg/kg. Similar shifts in peak time with dose occurred in
the liver and blood. In terms of the original dose, kidneys, liver
and blood each contained 0.7% of the label 2 h after 5 mg/kg and 0.3%
(kidney), 0.14% (liver) and 0.23% (blood) after 24 h in female rats.
Distribution of radioactivity was also studied after repeated
oral administration of 14C-chlorothalonil to male rats. Five doses
were given at 24 h intervals at concentrations of 1.5, 5, 50 or
160 mg/kg. The rats were killed 2, 9, 24, 96 and 168 h after the last
dose. The distribution of activity showed a similar profile to that
after single dosing, i.e. the highest concentrations occurred in
kidneys, followed by liver and blood, at all doses and times. At all
dose levels, the concentrations peaked 2 h after the last dose. The
percentage of the dose found in the kidney at this time was 0.28% and
0.20% at the 1.5 and 5 mg/kg dose levels, which was significantly
higher than that found at the higher doses (about 0.09%). At dose
levels up to 50 mg/kg there was significant depletion of radioactivity
from the blood during the 24 h between doses. In the kidney there was
a trend to slower overall depletion with increase in dose (Savides et
al., 1986a).
A study in mice showed that the distribution of activity in
non-gastrointestinal tract tissues was similar to that in rats after a
single oral dose of 14C-chlorothalonil. The kidney had the highest
concentration of radioactivity after doses of 1.5, 15 or 105 mg/kg
(Ribovich et al., 1983).
6.3 Metabolic transformation
6.3.1 Rat
Male Sprague-Dawley rats were administered, via oral gavage,
14C-chlorothalonil (purity 99.7%) at a dose level of 200 mg/kg in
order to isolate and identify the urinary metabolites. Urine was
collected 17, 24 and 48 h after dosing. Urinary metabolites accounted
for 2.4% of the administered dose and, except for 30% of the
radiolabel which was non-extractable from the urine, were found to be
trimethylthiomonochloro-isophthalonitrile and dimethylthiodichloro-
isophthalonitrile. These thiols were excreted in urine both as free
thiols and as their methylated derivatives. The authors suggested a
metabolic pathway such that hepatic metabolism proceeds through
conjugation with GSH followed by enzymatic degradation. The smaller
conjugates are then transported via the bloodstream to the kidney,
where they are converted to thiol metabolites and excreted in the
urine (Marciniszyn et al., 1985b).
A study was also carried out in rats given five daily oral doses
of 14C-chlorothalonil (1.5, 5, 50 or 160 mg/kg per day). Urine
samples, acidified and extracted with ethyl acetate, showed decreasing
extractability of radioactivity with increasing dose. GC/MS analysis
identified methylated or partly methylated dithiol and trithiol
derivatives of chlorothalonil from the first dose onwards. The
percentage of the trithiol derivative excreted was constant with
increasing dose while the dithiol increased with dose. Multiple
dosing resulted in a decreasing daily excretion of total thiol
derivatives. These results emphasize the probable involvement of
glutathione in the metabolic pathway for chlorothalonil (Savides et
al., 1986b).
A group of three rats, pretreated with the gamma-glutamyl
transpeptidase inhibitor AT-125, were dosed with 50 mg/kg
14C-chlorothalonil, while three other rats were given chlorothalonil
only. Urine samples were acidified and extracted with ethyl acetate.
The group of rats pre-treated with AT-125 showed only 15% of
radioactivity extractable after 12 h, while the other group showed 75%
extractability. The non-extractable fraction from the
inhibitor-treated rats contained glutathione conjugates of
chlorothalonil. The kidneys contained 2-3 times more radioactivity
than those of the untreated rats. These results gave further support
to the hypothesis that glutathione is involved with chlorothalonil
metabolism (Marciniszyn et al., 1988).
The production of metabolites was also studied in groups of rats
following dermal administration. 14C-chlorothalonil (4.6 mg/kg) was
applied to a shaved area of the dorsal region. The area was covered
and exposure continued for 48 h. Urine samples collected at 24 and
48 h were acidified and extracted with ethyl acetate. The extracts
were submitted to reverse-phase HPLC/LSC followed by methylation and
further clean-up. The trithiol derivative of chlorothalonil was the
major metabolite in all samples. The excretion of total thiol
metabolites was at least 20-fold less than that resulting from oral
dosing at the same dose level (Savides et al., 1987a).
The radiolabelled monoglutathione derivative of chlorothalonil
was administered to male rats (115 mg/kg) as a single oral or
intraperitoneal dose. Six hours after intraperitoneal dosing the
blood level was 10 times higher than after oral dosing. The
proportion of the administered intraperitoneal dose in the kidney was
16 times higher than after oral dosing. Urine from the orally dosed
rats contained 9% trithiol derivative and 5% dithiol, while
intraperitoneally dosed rats showed < 1% dithiol derivative and none
of the trithiol in urine. This indicates that the orally administered
monoglutathione conjugate is further conjugated with glutathione in
the gastrointestinal tract prior to absorption (Savides et al.,
1986f).
Nine germ-free male rats each received approximately 56 µCi
14C-chlorothalonil in a single oral dose of 50 mg/kg. Urine and
faeces were collected over a 96-h period, and the urine was processed
to identify and quantify thiol derivatives of chlorothalonil. These
derivatives were detected in only three of the nine rats and
represented < 0.03% of the dose. This is fifty times less than that
obtained for normal rats. There is therefore strong evidence that
intestinal microflora make a significant contribution to the
metabolism of chlorothalonil after oral administration in the rat
(Savides et al., 1990a).
The HPLC analysis of faecal extracts from rats dosed with 200 mg
chlorothalonil/kg showed that 28% was excreted unchanged and 5% was
converted to 4-hydroxy 2,3,5-trichloroisophthalonitrile. The amounts
after a dose of 5 mg/kg were 1.6 and 6.2%, respectively (Ignatoski et
al., 1983).
The HPLC analysis of faeces from rats given 14C-chlorothalonil
orally at 5, 50 and 200 mg/kg showed the presence of at least seven
radioactive components. Two of the peaks had the same retention times
as chlorothalonil and its 4-hydroxy metabolite. A higher proportion
of the metabolite was present after the 5 mg/kg dose than after the
higher doses. The majority of the activity was unextractable and was
therefore bound to faecal components (Lee et al., 1982).
6.3.2 Dog
Male beagle dogs were given 14C-chlorothalonil at a dose level
of 50 mg/kg either by gelatin capsule or by gavage. In each case the
urinary excretion of radioactivity was very small and none of the
methylated thiol derivatives of chlorothalonil were detected (Savides
et al., 1989, 1990b).
6.3.3 Monkey
Four male Chinese rhesus monkeys were dosed with
14C-chlorothalonil by gavage at 50 mg/kg body weight suspended in
0.75% aqueous methylcellulose. Extraction of urine, collected over
48 h, with acidified ethyl acetate showed that 32-65% of the
radioactivity was extractable. The total amount of chlorothalonil
thiol derivatives excreted was 0.001-0.01% of the administered dose,
mainly as the trimethylthiol entity. This was more than 100 times
less than that excreted from the rat (Savides et al., 1990c).
6.4 Elimination and excretion
6.4.1 Rat
The main route of elimination of chlorothalonil from the rat
after oral dosing is via the faeces. The percentage eliminated was
consistent for males and females, at doses of 1.5-200 mg/kg and from
single or repeated doses. The amount eliminated was consistently
above 82%, the majority appearing in the first 48 h at low doses and
within 72 h at high doses (Marciniszyn et al., 1984b, 1985a).
Biliary excretion at dose levels up to 5 mg/kg is rapid, peaking
at 2 h, and is more prolonged at levels of 50 mg/kg or more.
Excretion decreases with increasing dose, from 22.5% at 1.5 mg/kg to
7.7% at 200 mg/kg over 48 h. Studies using bile duct cannulation
indicate that the excretion is saturated at 50 mg/kg or more.
Comparison with non-cannulated rats showed that there was no
difference in the radioactive concentration found in the kidney,
indicating that enterohepatic circulation of biliary metabolites did
not play a significant role (Savides et al., 1986c).
The fate of orally administered 14C-chlorothalonil (purity
99.7%) at three dose levels (5, 50 and 200 mg/kg) was investigated in
Sprague-Dawley rats to determine the effects of increasing doses of
the test material. Four animals of each sex at each dose level were
killed 2, 9, 24, 96 and 168 h after dosing and urine, faeces and
selected tissues were assayed for radioactivity. The average recovery
of the radiolabel at each of the dose levels was approximately 89% for
males and 96% for females. The major route of elimination was via the
faeces (83-87%) and was essentially complete by 48 h in low-dose
females and low/mid-dose males, and by 72 h in the mid/high-dose
females and high-dose males. A delay in stomach-emptying time was
observed for mid- and high-dose males and females. Urinary excretion
was 92-93% complete for low-dose rats within 24 h, mid-dose rats
within 48 h, and 95% complete for high-dose rats within 72 h. Urinary
excretion of the radiolabel at the three dose levels was 5-7% of the
administered dose in males and 5-11.5% in females. Urinary excretion
was essentially saturated as the dose level increased. The highest
concentrations of radiolabelled material in non-gastrointestinal
tissues were found in the kidney, being approximately 0.7% of the dose
per gram of kidney for males and 0.4% in females at peak concentration
(2 h) for the 5 mg/kg dose level. Kidney concentrations were greatest
at 2, 9 and 24 h for the low, mid and high doses, respectively
(Marciniszyn et al., 1984b, 1985a).
When 14C-chlorothalonil was applied dermally to male rats at
5 mg/kg the major route of excretion was the faeces. Approximately 18%
of the dose was excreted by this route in 120 h compared to about 6%
via urine (Marciniszyn et al., 1984a).
After administration of 1 mg chlorothalonil/kg by the oral,
dermal or endotracheal routes, the excretion in urine during 24 h was
2.9, 0.9 and 5.7%, respectively (Chin et al., 1981).
A study involving intubation of the 4-hydroxy metabolite of
chlorothalonil to rats at 4 or 43 mg/kg showed that the majority of
the dose was excreted in the faeces and a small amount in the urine
(Jarrett et al., 1978).
6.4.2 Mouse
In male mice, dosed orally with 1.5, 15 or 105 mg
14C-chlorothalonil/kg, the major route of elimination was via the
faeces. This was complete by 24 h for the two lower doses and by 96 h
for the highest dose. Urinary excretion at all doses varied between 5
and 10% of the administered dose (Ribovich et al., 1983).
6.4.3 Dog
Most of an oral dose of chlorothalonil was excreted in the faeces
of beagle dogs. Over 12 days, 99.6% was excreted from two dogs given
50 mg 14C-chlorothalonil/kg by gelatin capsule, and 76-98% was
excreted over 24 h when three dogs were given the same dose by gavage.
In both studies the amount excreted in urine was very small, and this
occurred mostly within the first 10 h (Savides et al., 1989, 1990b).
6.4.4 Monkey
Oral administration of 14C-chlorothalonil to four male Chinese
rhesus monkeys showed faecal elimination to be the main route of
excretion, 52-92% of the dose (50 mg/kg) being excreted in 96 h.
Urinary excretion amounted to 1.8-4.1% of the dose. Most of the
radiolabel was eliminated in the first 48 h (Savides et al., 1990c).
6.5 Reaction with body components
Incubation of 14C-chlorothalonil with glutathione in aqueous
medium in the presence or absence of glutathione- S-transferase
resulted in the rapid disappearance of chlorothalonil and the
appearance of more polar compounds. These were identified as
conjugates of chlorothalonil with glutathione, their formation
following a step-wise process, i.e., from mono->di->triglutathione
conjugates (Savides et al., 1985). This reaction with glutathione
parallels similar findings with chlorothalonil in other biological
systems such as Saccharomyces pastorianus (Tillman et al., 1973).
The incubation of chlorothalonil with rat stomach or intestinal
mucosal cells indicated that polar metabolites were formed which were
chromatographically similar to glutathione conjugates of
chlorothalonil (Savides et al., 1986d).
The in vivo action of chlorothalonil on glutathione (GSH) was
shown in a rat study where chlorothalonil was dosed orally at
5000 mg/kg. Hepatic GSH was decreased by 40% 18 h later but recovered
to a normal value after 48 h. Kidney GSH increased to two times its
control level after 48 h (Sadler et al., 1985).
Chlorothalonil has been shown to bind to calf thymus histones,
the rate and amount depending upon pH and type of histones. There was
little binding to DNA. Treatment of rat liver nuclei indicated
similar binding patterns to those for histones (Rosanoff & Siegel,
1981).
Groups of four rats were administered 50 mg 14C-chlorothalonil
per kg orally, killed after 6 h and their kidneys excised. The kidney
tissue was homogenized, and protein and DNA were isolated. Radiolabel
was found to be bound to kidney protein but not to DNA (Savides et
al., 1987b). Kidney tissue from rats dosed 50 mg
14C-chlorothalonil/kg orally was separated by ultracentrifugation
into subcellular organelles. The kidneys contained about 0.38% of the
original dose, the majority of this activity (81%) being in the
soluble fraction. About 10% of the remaining activity was contained
in the mitochondrial subfractions (Savides et al., 1987c).
Studies with rat liver and kidney mitochondrial preparations
showed that the dithiol derivative of chlorothalonil completely
inhibited state 3 mitrochondrial respiration. The monothiol
derivative affected oxygen uptake by liver mitochondria but not by
kidney mitochondria. The mono- and diglutathione conjugates of
chlorothalonil did not affect oxygen uptake by mitochondria. Since
cleavage of the glutathione conjugates to give the thiol derivatives
takes place in the kidney, this may explain the toxic action of
chlorothalonil in this organ (Savides et al., 1988; see also section
7.8).
Available evidence indicates that the enzyme ß-lyase is required
for the formation of thiol derivatives from cysteine conjugates. The
activity of this enzyme has been assessed in rat, mouse and human
kidney cytosolic fractions using the perchloroethylene metabolite
S-(1,2,2-trichlorovinyl)-l-cysteine as substrate. The activity of
renal ß-lyase in human kidney was comparable to that of mouse, but was
an order of magnitude lower than that in the rat (Green et al., 1990).
The proposed metabolic pathway for the production of thiol
derivatives from chlorothalonil in the kidney is shown in Fig. 6.
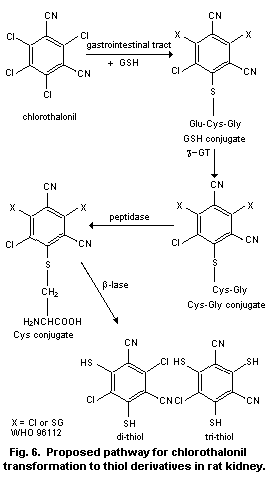 7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of chlorothalonil by oral, dermal and
inhalation routes of administration is shown in Table 9. Signs of
poisoning include depression, diarrhoea and unkempt appearance. An
oral LD50 could not be attained in the dog because of emesis.
Chlorothalonil administered orally (1 g/kg) to mice increased
intestinal motility. The laxative action was reduced by pre-treatment
with corn oil (Teeters, 1966).
Table 9. The acute toxicity of chlorothalonil
Species LD50 Route Reference
(mg/kg)
Rat > 10 000 oral Powers (1965)
Dog > 5000 oral Paynter (1965a)
Mouse 6000 oral Yoshikawa & Kawai (1966)
Rat 332 oral Wazeter (1971)
(4 hydroxy
metabolite)
Rabbit > 10 000 dermal Doyle & Elsea (1963)
Rat (f & m)a 0.22 mg/litre inhalation Danks & Fowler (1988)
Rat (f & m)b 0.52 mg/litre inhalation Shults et al. (1991)
Rat (f & m)c 0.10 mg/litre inhalation Shults et al. (1993)
a LC50 actual concentration
b Hammermilled technical chlorothalonil - 1 h exposure
c Hammermilled technical chlorothalonil - 4 h exposure
The acute oral toxicity of the metabolite 4-hydroxy-2,5,6-
trichloroisophthalonitrile is greater than that of chlorothalonil
itself, the acute oral LD50 values being 332 and 10 000 mg/kg,
respectively).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rat
When groups of 35 male and 35 female rats were fed chlorothalonil
in the diet at dose levels of 0, 250, 500, 750 or 1500 mg/kg diet for
22 weeks, growth was slightly reduced at all levels in males and in
the highest two levels in females. Liver and kidney weight was
increased in the two highest dose groups in males. Kidney changes,
more evident in males than females, occurred at all dose levels and
included irregular swelling of the tubular epithelium, epithelial
degeneration and tubular dilation (Blackmore & Shott, 1968). In a
separate study, no compound-related effects were seen in the kidneys
of rats fed chlorothalonil at seven dietary levels from 1-120 mg/kg
diet for 17 weeks (Busey, 1975).
When groups of 20 male and 20 female rats were fed chlorothalonil
in the diet (0, 40, 80, 175, 375 and 1500 mg/kg body weight per day)
for 90 days, a significant dose-related reduction in body weight gain
was seen at levels of 375 mg/kg per day or more which was allied to
cathartic action at the two highest doses. Male urine output
decreased and specific gravity increased at the two highest dose
levels. Relative kidney weight was increased in both sexes at all
dose levels. A histopathological re-evaluation of the kidneys showed
the presence of epithelial hyperplasia of the proximal convoluted
tubules at all levels in males and at levels of 175 mg/kg per day or
more in females (Wilson, 1981; Wilson et al., 1985c).
Groups of 25 male and 25 female rats were fed chlorothalonil for
13 weeks at 0, 1.5, 3, 10 and 40 mg/kg body weight per day. Five rats
of each sex per group were killed at 6 weeks, ten at 13 weeks and the
survivors were killed after a further 13-week recovery period. There
was no effect on mortality, clinical condition, body weight, food and
water consumption, haematology or urinalysis. Increases in kidney
(> 3 mg/kg per day) and liver (highest dose) weights and the
incidence of hyperplasia and hyperkeratosis of the forestomach
squamous epithelium (at 10 and 40 mg/kg per day) returned to normal
when treatment ceased. Histopathological re-examination of the kidney
revealed (in males at the highest dose level) an increased incidence
of hyperplasia of the epithelium of the proximal tubules at 6 and
13 weeks and after a 13-week recovery period. In a few animals this
resulted in an increase in the size of the tubules. An overall
no-observed-effect level of 3 mg/kg body weight per day was
established, based upon the lack of lesions in the squamous epithelium
of the forestomach (Wilson et al., 1983a, 1985a).
The kidney and stomach changes were investigated further in a
study where 90 male rats were fed chlorothalonil (175 mg/kg body
weight per day) for up to 91 days. Groups of ten rats were killed
after 4 or 7 days and 2, 4, 6, 8, 10 or 12 weeks, the kidney and
stomach taken for histopathological examination. Groups of ten rats
at each interval were taken from a control group and examined. The
forestomach effects of chlorothalonil were characterized initially as
multifocal ulceration and erosion of the mucosa, developing to
squamous epithelial hyperplasia and hyperkeratosis. Within the first
week there was vacuolar degeneration, cell death, karyomegaly and
regeneration of the proximal tubular epithelium. There was apparent
recovery at day 14 with few lesions present in treated animals.
Continued administration led to tubular epithelial vascularization,
regeneration, hyperplasia and hypertrophy (Ford et al., 1987a).
Chlorothalonil or the monoglutathione conjugate of chlorothalonil
was administered in the diet to male Fischer-344 rats in a 90-day
study. A third group of rats received only the vehicle (0.5%
methylcellulose). The oral administration of approximately equimolar
doses of chlorothalonil (75 mg/kg per day) or the monoglutathione
conjugate of chlorothalonil (150 mg/kg per day) resulted in
significantly increased kidney weight and in treatment-related
histopathological changes in the kidney. The primary changes observed
were morphologically similar for both compounds and were characterized
by proximal tubular hyperplasia, tubular dilation (hypertrophy),
vacuolar degeneration and interstitial fibrosis. The oral
administration of chlorothalonil resulted in gross and microscopic
changes in the non-glandular portion of the forestomach. The oral
administration of the monoglutathione conjugate of chlorothalonil did
not produce alterations in the rat forestomach. The gross and
microscopic changes observed in the forestomach of animals given
chlorothalonil were considered to be due to the local irritational
effects of chlorothalonil. The alteration of the molecule by the
addition of a single glutathione residue appeared to eliminate the
irritation to the forestomach. Thiol metabolites of chlorothalonil
were detected in the urine of animals given either the monoglutathione
conjugate of chlorothalonil or chlorothalonil itself. The presence of
the thiol metabolites, coupled with the similar histopathological
changes in the kidney, suggests a common metabolic pathway. The data
support the conclusion that the nephrotoxicity produced by
chlorothalonil is associated with conjugation with glutathione (Ford
et al., 1987b; Wilson et al., 1990).
7.2.1.2 Mouse
Groups of 15 male and 15 female CD-1 mice were administered
chlorothalonil in diets at levels of 0, 7.5, 15, 50, 275 or 750 mg/kg
diet for 13 weeks. Five animals per group were killed at 6 weeks.
There was no effect on clinical condition, mortality, body weight gain
or food consumption. Kidney weight was increased in females at 275
and 750 mg/kg. Microscopic re-examination revealed hyperplasia of the
epithelium of the proximal convoluted tubules, minimal or slight in
severity, in only 4 out of 15 males. This was not considered to be a
clear treatment-related effect. There was an increased incidence of
hyperplasia and hyperkeratosis of the squamous epithelial cells of the
stomach in males and females at levels of 50 mg/kg or more. Mucosal
ulceration and submucosal inflammation were observed in some treated
animals. The no-observed-effect level was considered to be 15 mg/kg
(3 mg/kg body weight per day) (Shults et al., 1983; Busey, 1985).
7.2.1.3 Dog
In a 30-day feeding study, encapsulated chlorothalonil (97.9%
purity) was administered daily to groups of two male and two female
beagle dogs at 0, 50, 150, or 500 mg/kg body weight per day. The
following tissues were examined macroscopically and microscopically at
necropsy: brain, liver, kidneys, testes with epididymis, ovaries,
adrenals, heart, thyroid, and parathyroid. During treatment the
high-dose dogs exhibited emesis and weight loss and reduced food
consumption (males only). Female dogs had slightly reduced body
weight gains at all doses. At necropsy, the liver weights of
high-dose females were slightly increased. There were no microscopic
changes in the tissues examined. Due to the reduced body-weight gains
of treated females, a no-observed-adverse-effect level (NOAEL) was not
established in this study (Fullmore & Laveglia, 1992; FAO/WHO, 1993b).
In a 16-week dietary study, chlorothalonil (purity unspecified)
was fed at 0, 250, 500 or 750 mg/kg to groups of four beagle dogs.
There were no compound-related effects on appearance, behaviour,
appetite or body weight. No changes in haematological parameters were
found at weeks 0, 4, 13 or 16. At termination, protein-bound iodine
was found to be increased in all treated dogs. Urinalysis at weeks 6,
9, 13 and 16 was unremarkable. No compound-related macroscopic or
microscopic changes were found at necropsy. In particular, only
incidental changes were observed in liver and kidneys. A NOAEL was
not established in this study (Paynter & Murphy, 1967; FAO/WHO,
1993b).
7.2.2 Dermal: Rabbit
Chlorothalonil (75% formulation) was applied to the intact or
abraded skin of groups of rabbits at 0, 500 or 1000 mg/kg per day, 5
days/week, for 3 weeks. The treated groups comprised five males and
five females per group, with two males and two females as controls.
Treatment resulted in dose-related irritation which was more severe
with abraded skin. Histopathological examination revealed a moderate
degree of acanthosis, hyperkeratosis and slight to moderate leucocytic
infiltration. No abnormality was detected in other tissues (Paynter,
1965b).
Chlorothalonil was applied dermally each day for 21 days to
groups of six male and six female rabbits at 0.1, 2.5 and 50 mg/kg per
day. The fungicide was suspended in 0.125% aqueous methylcellulose
and covered 10% of the body surface when applied to the back. Contact
was for 6 h daily. The only effect revealed by a wide range of
observations and examinations was dermal irritation at 2.5 and 50
mg/kg per day. The histopathological changes seen were minimal to
slight acanthosis and hyperkeratosis. The no-observed-effect level
(NOEL) for dermal irritation was 0.1 mg/kg per day (Shults et al.,
1986).
7.3 Long-term exposure
7.3.1 Rat
An early study, lasting 76 weeks, produced evidence of kidney
changes in rats (15 male and 15 female per group) fed high levels of
chlorothalonil at 500, 1000 and 5000 mg/kg diet. There was no
overall effect on mortality, clinical condition or growth. The
compound-related changes in the kidney, at all levels, consisted of
tubular hypertrophy, epithelial irregularities and vacuolation. Males
were more affected than females (Paynter & Busey, 1967).
A 2-year study, reported in 1967, was initiated with
chlorothalonil levels of 1500, 15 000 and 30 000 mg/kg diet with
groups of 35 male and 35 female rats (70 of each sex in control
group). Because of cathartic effects, the top dose administration was
curtailed after 15 weeks. The rats in the two remaining treatment
groups continued for the full 2 years with evaluations including
haematology, biochemistry and histopathology. There was no effect on
survival, but the effect on growth was dose-related. The relative
organ weights of liver and kidney were increased at 15 000 mg/kg.
Microscopic changes in the forestomach were described as acanthosis
and hyperkeratosis of the squamous epithelium at 15 000 mg/kg and, in
the kidney, as tubular hypertrophy and hyperplasia at both 1500 and
15 000 mg/kg diet (Paynter, 1967a).
A supplementary study, run concurrently with the previous study,
assessed chronic toxicity at 5000 mg/kg diet. Growth rate was
depressed and a cathartic action was expressed as increased water
consumption and faecal excretion. Relative organ weights were
increased for caecum and kidneys. Histopathological examination
showed kidney changes as tubular hypertrophy and occasional
degeneration of the proximal tubular epithelium (Paynter & Crews,
1967).
A 2-year study at six chlorothalonil dose levels between 4 and 60
mg/kg diet was designed to determine an NOEL (50 males and 50 females
per group). No effects were seen on survival, clinical observations,
growth, haematological or biochemical parameters. No changes of
toxicological significance were found for organ weights or during
gross and microscopic examination of tissues. The highest dose level
(3 mg/kg body weight per day) was considered the NOEL (Holsing &
Shott, 1970).
A long-term study was undertaken to evaluate the carcinogenic
potential of chlorothalonil in Fischer-344 rats. Males were studied
for 27 months and females for 30 months. Chlorothalonil was
administered in the diet to groups of 60 males and 60 females at dose
levels of 0, 40, 80 and 175 mg/kg body weight per day. There was no
effect on survival of females or males up to 2 years. However, at the
highest dose level, there was increased mortality after 2 years, but
only in males. Decreases in body weight gain were dose-related at 80
and 175 mg/kg per day. Treatment-related effects on other parameters
appeared to be related to nephrotoxicity. These included increases in
serum urea nitrogen and creatinine, increased urine volume and
decreased specific gravity, and increased kidney weight.
Histopathological examination of the kidney showed a dose-related
increase in chronic glomerulonephritis (nephropathy) compared with
controls. A re-examination of the kidneys also revealed tubular
hyperplasia (a sign of preneoplastic change) and chronic progressive
nephropathy in the treated groups. Secondary lesions in other organs
included periarteritis and parathyroid hyperplasia. There was
increased incidence or severity of hyperplasia and hyperkeratosis of
the squamous mucosa of the oesophagus and forestomach in all dosed
groups, which was probably the result of the irritant effect of
chlorothalonil. Neoplastic changes in the kidney and forestomach are
evaluated in section 7.7 (Wilson et al., 1985b, 1986a).
A further study was carried out to evaluate the neoplastic
findings in the kidney and stomach seen in the previous study and to
determine a NOEL for non-neoplastic effects. Groups of 65 male and
65 female rats were administered chlorothalonil in the diet at doses
of 0, 1.8, 3.8, 15 and 175 mg/kg body weight per day. Males were
killed at 23 months (highest dose) and 26 months and females at
29 months. The effects at 175 mg/kg per day were similar to those
described above, i.e. changes associated with the kidney and stomach.
At 15 mg/kg per day there was elevated serum urea nitrogen and
slightly increased kidney weight. At 3.8 mg/kg per day there was a
small increase in kidney weight, but there were no effects at 1.8
mg/kg per day. Microscopic examination revealed an increased
incidence and severity of epithelial hyperplasia in the proximal
convoluted tubules at 3.8 mg/kg per day or more. Clear cell
hyperplasia of the proximal convoluted tubules was increased at
15 mg/kg per day in females and at 175 mg/kg per day in both sexes.
In addition, at 3.8 mg/kg per day or more, there was an increased
incidence and severity of hyperplasia, hyperkeratosis, ulcers and
erosions of the squamous mucosa of the forestomach. At 175 mg/kg per
day, the incidence of erosions of the glandular stomach was
significantly increased compared to controls. Renal tumours at levels
of 15 and 175 mg/kg per day and stomach tumours at 3.8 mg/kg per day
or more are evaluated in section 7.7. An NOEL of 1.8 mg/kg body
weight per day was established for non-neoplastic effects seen in the
study (Wilson et al., 1989a).
7.3.2 Mouse
A 2-year mouse study was carried out with 60 males and 60 females
per group at 0, 750, 1500 and 3000 mg chlorothalonil/kg in diet.
There was a slightly increased mortality in males at the highest dose
level but no effect was seen on body weight, food consumption,
clinical condition or haematological parameters. Kidney weight was
increased in all treated groups compared to controls. Non-neoplastic
changes in the kidney were characterized as glomerulonephritis,
cortical tubular degeneration and cysts. The incidence of these
changes, found at all treatment levels, was not dose-related but was
considered to be due to treatment. A histopathological re-evaluation
of the kidneys revealed a high incidence of tubular hyperplasia in all
male groups and a lower incidence in females. Non-neoplastic effects
in the stomach and oesophagus included hyperplasia and hyperkeratosis
of the squamous mucosa, probably due to the irritant action of
chlorothalonil. The evaluation of kidney and stomach tumours found in
this study is described in section 7.7 (Wilson et al., 1983b, 1986b).
A second mouse study was undertaken to determine the NOEL for
stomach and kidney changes in male mice. Sixty males per group were
fed chlorothalonil at levels of 0, 15, 40, 175 and 750 mg/kg diet for
2 years. Kidney weight and incidence of tubular hyperplasia were
increased at 750 mg/kg and, very slightly, at 175 mg/kg. The
increased incidence of hyperplasia and hyperkeratosis of the
forestomach was dose-related between 40 and 750 mg/kg. The dietary
NOEL for non-neoplastic effects was determined to be 15 mg/kg (1.6
mg/kg body weight per day). The tumour evaluation is considered in
section 7.7 (Wilson et al., 1987).
7.3.3 Dog
In a 2-year study, chlorothalonil (93.6% purity) was fed to
groups of four beagle dogs at dietary concentrations of 0, 1500,
15 000 or 30 000 mg/kg (equivalent to 0, 37.5, 375, or 750 mg/kg body
weight per day). Eight dogs, one of each sex and of each group, were
killed at 12 months and the remainder at 24 months. One dog of each
treatment group lost weight during the study. There was a tendency
towards mild anaemia in four of the mid-dose dogs at 2 years and at
earlier intervals in two of the high-dose dogs. Biochemical and urine
analyses were unremarkable. Absolute and relative thyroid and kidney
weights, and liver to body weight ratios were increased at the
mid- and high-dose levels. Histopathological treatment-related
changes occurred in the liver, thyroid, kidney and stomach of mid- and
high-dose dogs; changes in low-dose dogs were equivocal. In the
liver, the findings were similar in nature (though slightly more
pronounced) at low-dose levels to those in the control dogs, but they
increased in severity at mid- and high-dose levels. They included
pericholangitis with associated portal fibrosis, bile duct hyperplasia
and pigmentation of hepatic cytoplasm and of macrophages of sinusoids
and portal triads. Renal glomerulosclerosis and degenerative renal
tubular changes (tubular hypertrophy and dilation) were found in the
kidneys of mid- and high-dose dogs. In the thyroid, markedly
increased pigmentation of follicular epithelia occurred in mid- and
high-dose dogs. Moderate to severe gastritis was found irregularly in
mid- and high-dose animals. In summary, administration of
chlorothalonil in the diet of dogs at concentrations of 15 000 and
30 000 mg/kg caused irregular body weight reduction, borderline
anaemia and histopathological changes in the liver, kidney, thyroid
and stomach. At 1500 mg/kg, the histopathological changes found in
the liver were qualitatively similar but minimally to slightly
increased in comparison to those found in control animals.
Histopathological changes to the other tissues were unremarkable at
the low dose. A NOAEL was not established in this study (Paynter &
Busey, 1966; FAO/WHO, 1993b).
Groups of beagle dogs (eight males and eight females per group)
were fed chlorothalonil in the diet at dose levels of 0, 60 and
120 mg/kg. Four dogs of each sex per group were killed at one year
and the remaining animals at 2 years. There were no effects on
behaviour or growth over the course of the study. Clinical chemistry
values, including haematological, biochemical and urine analyses, were
comparable to the controls at all dose levels. Gross and microscopic
examination of tissues and organs performed on animals killed at 12
months indicated a slight increase in the severity of renal tubule
vacuolation in high-dose males. Examination of tissues and organs at
24 months showed a slight degree of renal tubule vacuolation in two
out of four males at 120 mg/kg. In the absence of other changes
(urinalyses values) this finding was considered questionable,
especially as a slight degree of vacuolation was noted in controls as
well as other treated animals. The NOAEL was considered to be
120 mg/kg diet, equivalent to 3 mg/kg body weight per day (Holsing &
Voelker, 1970; FAO/WHO, 1991, 1993b).
7.3.4 Summary of key dietary studies
A summary of the key dietary studies with chlorothalonil is given
in Table 10.
7.4 Skin and eye irritation; sensitization
Chlorothalonil is an irritant to rabbit skin, as shown by
repeated dose studies (5 days/week for 3 weeks at 500 or 1000 mg/kg
per day or daily for 21 days at 2.5 or 50 mg/kg per day; details in
section 7.2.2).
The influence of the vehicle on the skin irritant potential of
chlorothalonil was shown by a rabbit study with 0.1% chlorothalonil in
saline, petrolatum or acetone. Compared to the vehicle alone,
chlorothalonil did not cause a significant increase in irritation in
Table 10. Summary of key dietary studies with chlorothalonil
Species Duration Dose levels NOEL LOEL Key effects Reference
Rat 13 weeks 0, 1.5, 3, 10, 3 mg/kg per 10 mg/kg per 40 mg/kg per day: increased kidney and Wilson et
40 mg/kg body day day liver weight, incidence of hyperplasia al. (1983a,
weight per day and hyperkeratosis of forestomach, 1985a)
incidence of epithelial hyperplasia in
proximal tubules 10 mg/kg per day:
increased kidney weight, hyperplasia
and hyperkeratosis of forestomach
Rat 23-29 months 0, 1.8, 3.8, 15, 1.8 mg/kg 3.8 mg/kg 175 mg/kg per day: increases in serum Wilson et
175 mg/kg body per day per day urea nitrogen, urine volume, kidney al. (1989a)
weight per day weight, renal tubular hyperplasia,
hyperplasia and hyperkeratosis of
forestomach; increased incidence of
renal and forestomach tumours
15 mg/kg per day: similar but less
intense changes to those shown at
highest dose level 3.8 mg/kg per day:
small increase in kidney weight, renal
tubular hyperplasia, hyperplasia
and hyperkeratosis of forestomach and
forestomach tumours
Mouse 13 weeks 0, 7.5, 15, 50, 15 mg/kg diet 50 mg/kg diet 275 and 750 mg/kg: increased kidney Shults et
275, 750 mg/kg (= 3 mg/kg (= 10 mg/kg weight 50, 275 and 750 mg/kg: al. (1983);
diet per day) body weight dose-related increased incidence Busey (1985)
per day) of hyperplasia and hyperkeratosis
of forestomach
Table 10. (Cont'd)
Species Duration Dose levels NOEL LOEL Key effects Reference
Mouse 2 years 0, 15, 40, 175, 15 mg/kg diet 40 mg/kg diet 750 mg/kg: increased kidney weight, Wilson et
750 mg/kg diet (= 1.6 mg/kg (= 4.5 mg/kg renal tubular hyperplasia, forestomach al. (1987)
body weight body weight hyperplasia and hyperkeratosis,
per day) per day) slightly increased incidence of
forestomach tumours 175 mg/kg: slightly
increased incidence of renal tubular
hyperplasia, increased forestomach
hyperplasia and hyperkeratosis
40 mg/kg: increased incidence of
forestomach hyperplasia and
hyperkeratosis
Dog 2 years 0, 1500, 15 000, - 1500 mg/kg in 15 000 and 30 000 mg/kg: increased Paynter &
30 000 mg/kg diet (= 37.5 kidney, liver and thyroid weights and Busey (1966)
diet mg/kg body histopathological changes, gastritis
weight per 1500 mg/kg: slightly increased incidence
day) hepatic findings
Dog 2 years 0, 60, 120 mg/kg 120 mg/kg diet - no changes of toxicological significance Holsing &
diet (= 3 mg/kg Voelker
body weight (1970);
per day FAO/WHO
(1991, 1993)
saline, but doubled the mild irritation caused by petrolatum. Acetone
itself caused no irritation but the addition of chlorothalonil
produced mild skin irritation (Flannigan & Tucker, 1985).
A further study in rabbits using a cumulative irritation assay
confirmed the irritant properties of 0.1% chlorothalonil in acetone.
A concentration of 0.01% gave evidence of mild irritation, probably of
no clinical significance, and 0.001% was not irritant to the skin
(Flannigan et al., 1986).
Chlorothalonil irritancy to the eye was evaluated in a modified
Draize system, 0.1 mg being instilled into one eye of each of three
male and three female rabbits. The eyes were examined and the results
scored after 24, 48 and 72 h, 7 and 14 days. Ocular irritation
occurred in all animals and corneal opacity persisted to day 14 (Major
et al., 1982).
Skin sensitization was tested in a guinea-pig maximization test
using 10 Hartley female guinea-pigs. Topical concentrations of 0.5
and 5% chlorothalonil were used. Upon challenge, chlorothalonil was
shown to be a strong sensitizer. Moderate cross-sensitization with
benomyl was also demonstrated (Matsushita & Aoyama, 1981). By
contrast, chlorothalonil did not produce skin sensitization in a
Draize test. The test substance (0.2 g) was applied to the shaved
backs of Hartley-derived guinea-pigs. The material was occluded for
24 h and then removed. This procedure was performed 3 times a week
for a total of 10 applications. On day 36 of the study a challenge
application of 0.2 g chlorothalonil was applied to the shaved flanks
and occluded. The skin was assessed 24 h later after removal of the
occlusive dressing. The positive control substance DNCB showed the
expected dermal sensitization but chlorothalonil was shown not to be a
sensitizer in this test (Wilson et al., 1982).
Luperi & Forster (1988) studied the ability of chlorothalonil to
induce delayed contact hypersensitivity in the guinea-pig using the
maximization test of Magnusson and Kligman. There was no evidence of
an induced sensitization response, but adequate evaluation was impeded
by a diffuse irritant reaction following the challenge.
7.5 Reproductive and developmental toxicity
Teratological evaluations have been carried out in the rat and
rabbit.
Chlorothalonil was administered orally via gavage to pregnant
Sprague-Dawley rats (25 per group) on days 6-15 of gestation at dose
levels of 0, 25, 100 or 400 mg/kg body weight per day, and the animals
were killed on day 20. Clinical signs of maternal toxicity were
evident at the highest dose level. There were 3 deaths and lowered
body weight during the dosing period. There was a slight increase in
the number of early embryonic deaths at the highest dose level,
probably associated with the maternal toxicity. There were no
compound-related incidences of external, internal or skeletal
malformations in the fetuses in treated animals. It was concluded
that chlorothalonil is not teratogenic to the rat (Mizens et al.,
1983).
Chlorothalonil was given orally to pregnant rabbits on days 8-16
of gestation at dose levels of 0, 180 or 375 mg/kg per day (days 8 and
9) and 0, 62.5 or 31.25 mg/kg per day (day 10 to day 16). There were
marked effects on food intake and maternal mortality occurred in both
treated groups, which imposed limitations on the evaluation of the
study. However no teratogenic effect was observed (Paynter, 1966b).
Rabbits were dosed with chlorothalonil (0, 5, or 50 mg/kg per
day) during days 6-18 of pregnancy (8 in control group, 9 in each dose
group) and killed on day 29. Four out of nine does aborted at the
highest dose level, and body weight was reduced in this group.
Although the incidence of fetal deaths appeared to increase with dose,
the difference was not statistically significant. The number of
implants and live fetuses per pregnancy and the fetal weights were
reduced in the high-dose group compared to controls. No treatment-
related effects were seen during external, internal or skeletal
examinations. It was concluded that chlorothalonil was not
teratogenic to the rabbit (Shirasu & Teramoto, 1975).
Chlorothalonil was administered by gavage to groups of 20
pregnant rabbits on days 7-19 of gestation at dose levels of 0, 5, 10
or 20 mg/kg per day. All survivors were weighed, killed on day 30 and
examined for live, dead or resorbed fetuses. Live and dead fetuses
were weighed and examined for external, visceral and skeletal
abnormalities. The highest dose level caused maternal body weight
loss and decreased food consumption. The pregnancy rate was > 95%
in each group. Examinations revealed no fetotoxicity or
teratogenicity due to chlorothalonil (Wilson et al., 1988).
A three-generation reproduction study was undertaken in rats at
dietary levels of 0, 1500 or 15 000 mg chlorothalonil/kg diet. A
supplementary study with 0 or 5000 mg/kg was also carried out 6 months
later. Groups consisted of 10 males and 20 females which were fed the
test diet for 11 or 12 weeks prior to mating. The study design was
for three generations with two matings per generation to give A and B
litters. Growth suppression occurred in the nursing A and B litters
in all generations at all dose levels and the pups appeared smaller
than the controls. There were no malformations due to treatment.
However, there were difficulties in execution, and this study is not
considered adequate by present-day standards (Paynter, 1967b).
Reproductive performance was also assessed in a one-generation
study on rats at dietary levels of 0, 200, 375, 750, 1500 or 3000
mg/kg diet with groups of 15 males and 15 females. The parents were
treated for 10 weeks prior to mating. No clinical signs of toxicity
were evident in the parents, but male body weight gain was reduced at
1500 and 3000 mg/kg and the kidneys were enlarged at 3000 mg/kg.
Reproductive parameters such as mating, fertility and gestation length
were not affected. There were no treatment-related abnormalities in
the offspring. The only effect on the offspring was lower body weight
at 3000 mg/kg on lactation days 14 and 21 (Wilson et al., 1989b).
In a two-generation reproduction study with two litters per
generation, technical chlorothalonil was administered to Charles River
CD rats by dietary admixture at concentrations of 0, 500, 1500 and
3000 mg/kg diet. There were 35 rats of each sex in each group. The
parental animals from each generation were treated continuously during
the growth period prior to mating and then throughout the mating,
gestation, lactation and resting phases of the study. The growth
period was 10 weeks for the F0 animals and 14 weeks for the F1
animals. Each generation of parental animals was mated twice to
produce the F1a, F1b, F2a and F2b litters. The offspring were
exposed to the test diets throughout the lactation period. Thirty-
five F1b males and females per group were selected to become the F1
generation after weaning. No mortalities or clinical signs of
toxicity associated with administration of chlorothalonil were
observed in the F0 or F1 parent animals. There was a
treatment-related effect towards lowered body weight in both males and
females in the F0 and F1 adults. The NOEL for body weight was 500
mg/kg in diet. Increased relative food consumption was observed in
the groups that showed lower body weights. The anticipated
treatment-related lesions in the kidneys and stomachs of adult animals
were observed in the F0 and F1 generations by gross and microscopic
pathology. These effects were observed in the kidney at all dose
levels in males and at 1500 and 3000 mg/kg in females. Stomach
effects were observed at all dose levels in both sexes. Reproductive
parameters in F0 and F1 animals, including mating and fertility
indices and gestation length, were not affected by treatment with
chlorothalonil. No gross malformations which were considered
treatment-related were observed in offspring in any of the groups
throughout the study. Litters were culled at day 4 to 8 pups/litter.
No effects on the number of live and stillborn pups, pup sex ratio,
pup survival and physical condition of the pups during lactation were
observed. No findings which were considered treatment-related were
observed during necropsy of the pups. The only effect observed in
pups in this study was lowered body weight compared to the controls on
day 21 of lactation. The NOEL for this effect was considered to be
1500 mg/kg diet, equal to 75 mg/kg body weight per day (Lucas & Benz,
1990).
7.6 Mutagenicity
Chlorothalonil has been assessed for mutagenic potential in a
wide range of in vitro and in vivo assays as shown in Tables 11
and 12. Most of the tests showed chlorothalonil not to be mutagenic
or clastogenic. A positive result in the DNA repair test with
Salmonella typhimurium was not reproduced in Bacillus subtilis,
nor was DNA binding shown in an in vivo study (see section 6.5). A
positive result in Chinese hamster ovary cells without metabolic
activation was not seen in the presence of activation nor confirmed by
in vivo chromosomal tests in rats and mice. Equivocal results were
obtained with Chinese hamster bone marrow in vivo.
In addition to the results shown in the Tables 11 and 12, a
series of studies was reported by IARC (1983). The results, which
were all negative, included those with S. typhimurium in the
presence and absence of a metabolic activating system and
Saccharomyces cerevisiae and Aspergillus nidulans in the presence
of activation. Chlorothalonil also failed to induce mutations in
silkworms and chromosomal aberrations in barley shoot tips or hamster
lung fibroblasts.
Chlorothalonil is not a transforming agent in Fischer rat embryo
cell lines (Price, 1978a).
The monothio, dithio, trithio, dicysteine, tricysteine and
monoglutathione metabolites of chlorothalonil have been shown to be
negative in the Ames assay with or without rat kidney metabolic
activation (see section 7.9).
Considering all the results of mutagenicity testing, it is
unlikely that chlorothalonil will show mutagenic activity in intact
mammalian systems.
7.7 Carcinogenicity
Several long-term rodent studies have been carried out on
chlorothalonil and have included carcinogenic evaluation. The design
of these studies and the chronic toxicity results have been described
in section 7.3. Some of the earlier rat studies did not show any
carcinogenic effect but it is probable that their design was not
sufficient to provide a critical test. The carcinogenic evaluation
in this section therefore concentrates upon the more recent rat and
mouse studies. Detailed evaluations of these studies have already
appeared in the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
reviews of 1983, 1985 and 1990 (FAO/WHO, 1985, 1986b, 1990b).
Therefore, for most of these studies, only the salient points will be
described here.
Table 11. In vitro mutagenicity tests
Test Organism Metabolic Dose range Mutagenic Reference
activation (+ or -) potential
Prokaryotes
Point mutation Salmonella typhimurium + and - 0.33-6.6 µg/plate negative Banzer (1977a)
(5 strains)
Point mutation S. typhimurium + and - 0.16-50 µg/plate negative Jones et al. (1984)
(5 strains) (renal)
Point mutation S. typhimurium - 1-10 µg/plate negative Shirasu et al.
(5 strains) + 2-10 µg/plate negative (1977)
Escherichia coli WP2 - 10-500 µg/plate negative
+ 10-100 µg/plate negative
Point mutation S. typhimurium TA98, + and - 0.76, 7.6, 76 µg/plate negative Wei (1982)
TA100, TA1535, TA1537, (hepatic and
TA1538 renal)
DNA repair tests S. typhimurium + and - 2, 10, 20 µg positive Banzer (1977b)
TA1978, TA1538
Bacillus subtilis - 2-200 µg negative Shirasu et al.
H17/M45 rec-assay (1977)
Table 11. (Cont'd)
Test Organism Metabolic Dose range Mutagenic Reference
activation (+ or -) potential
Mammalian cells
Gene mutation Chinese hamster V79 + and - 0.3 µg/ml negative Banzer (1977c)
mouse fibroblast + and - 0.03 µg/ml negative
BALB/3T3
Chromosome Chinese hamster - 0.03-0.3 µg/ml positive Mizens et al.
aberration ovary cells + 0.6-6.0 µg/ml negative (1986a)
Chromosome Human lymphocytes - 0.54-2.5 µg/ml negative Mosesso & Forster
aberrations + 1.16-5.38 µg/ml negative (1988)
Table 12. In vivo mutagenicity tests
Species Test Dose Mutagenic potential Reference
Mouse in vivo 6.5 mg/kg per day negative Legator (1974a)
cytogenetics 5 days orally
Mouse host-mediated assay; 8 strains 6.5 mg/kg per day negative Legator (1974a)
Salmonella typhimurium 5 days orally
Mouse dominant lethal assay, 5 days 6.5 mg/kg per day negative Legator (1974a)
dosing, 8 weeks mating 5 days orally
Mouse micronucleus 4-2500 mg/kg orally, twice with negative Siou (1981a)
(polychromatic erythrocytes) 24 h interval
Mouse chromosome aberration (bone 4-2500 mg/kg orally, twice with negative Siou (1981b)
marrow 6 h after last dose) 24 h interval 250, 1250, 2500 negative Mizens et al.
(bone marrow at 6, 24 and 48 h) mg/kg oral single dose (1985a)
Rat micronucleus 8-5000 mg/kg orally, twice with negative Siou (1981a)
(polychromatic erythrocytes) 24 h interval
Rat chromosome aberration (bone doses as above negative Siou (1981b)
marrow 6 h after last dose)
(bone marrow 6, 24 and 48 h) 500, 2500, 5000 mg/kg oral negative Mizens et al. (1985b)
single dose
Table 12. (Cont'd)
Species Test Dose Mutagenic potential Reference
Chinese micronucleus 4-2500 mg/kg orally, twice with negative Siou (1981a)
hamster (polychromatic erythrocytes) 24 h interval
Chinese chromosome aberration 8-5000 mg/kg for 2 days inconclusive Siou (1981b)
hamster (bone marrow 6 h after last dose)
(bone marrow at 6, 24 and 48 h) 500, 2500, 5000 mg/kg oral equivocal Mizens et al. (1985c)
single dose (± at 48 h)
(bone marrow 6 h after last dose) 50, 125, 250 mg/kg per day weak response Mizens et al. (1985c)
orally for 5 days not dose-related
A bioassay of technical grade chlorothalonil for possible
carcinogenicity was conducted by administering the test chemical in
the diet to Osborne-Mendel rats and B6C3F1 mice. Groups of 50 rats
of each sex were administered chlorothalonil at one of two dose levels
for 80 weeks and then observed for 30-31 weeks. Time-weighted average
doses for both males and females were 5063 or 10 126 mg/kg diet.
Matched controls consisted of groups of 10 untreated rats of each sex;
pooled controls consisted of the matched control groups combined with
55 untreated male or female rats from similar bioassays of five other
test chemicals. All surviving rats were killed at 110-111 weeks.
Groups of 50 mice of each sex were administered chlorothalonil at one
of two dose levels for 80 weeks, then observed for 11-12 weeks. Time-
weighted average doses for males were 2688 or 5375 mg/kg diet, and for
females, 3000 or 6000 mg/kg diet. Matched controls consisted of
groups of 10 untreated mice of each sex; pooled controls consisted of
the matched control groups combined with 50 untreated male or female
mice from similar bioassays of five other test chemicals. All
surviving mice were killed at 91-92 weeks. Clinical signs that
appeared with increasing frequency in dosed rats included haematuria
and, from week 72 until termination of the study, bright yellow urine.
Since the dosed female mice did not have depression in mean body
weights or decreased survival compared with the controls, they may
have been able to tolerate a higher dose. In rats, adenomas and
carcinomas of the renal tubular epithelium occurred with a significant
dose-related trend in both the males and females (males: pooled
controls 0/62, low dose 3/46, high dose 4/49; females: pooled controls
0/62, low dose 1/48, high dose 5/50). These tumours included both
adenomas and carcinomas which are considered to be histogenically
related. In mice, no tumours were found to occur at a greater
incidence among dosed animals than among controls. It was concluded
that under the conditions of this bioassay, technical grade
chlorothalonil was carcinogenic to Osborne-Mendel rats, producing
tumours of the kidney. However, chlorothalonil was not carcinogenic
for B6C3F1 mice (US NCI, 1978).
In a lifetime study on Fischer-344 rats at dietary doses of 0,
40, 80 or 175 mg/kg body weight per day (see section 7.3), a higher
incidence of primary renal tumours of epithelial origin (adenomas and
carcinomas) was seen in the treated groups (0/60, 7/60, 7/60 and 19/60
for males and 0/60, 3/60, 6/60 and 23/60 for females). It was
considered that the increased incidence of renal hyperplasia seen in
this study was associated with the formation of these tumours and
constituted a pre-neoplastic change. Papillomas and carcinomas of the
squamous mucosa of the forestomach were found in rats in the treated
groups (0/60, 1/60, 1/60, 3/60 for males and 0/60, 1/60, 2/60, 7/60
for females). These are probably related to the proliferative non-
neoplastic effects on the squamous mucosa as a result of the chronic
irritation by chlorothalonil (Wilson et al., 1985a, 1986b).
A further dietary study evaluated the carcinogenicity of
chlorothalonil at lower doses of 1.8, 3.8 and 15 mg/kg body weight per
day as well as at 175 mg/kg body weight per day (see section 7.3).
Rats with renal tumours (adenomas and carcinomas) occurred at 1/55,
1/54, 1/54, 4/54, 23/55 (male groups) and 0/55, 0/54, 0/55, 0/53,
32/55 (female groups). This confirmed the effect at 175 mg/kg per day
and an NOEL of 3.8 mg/kg per day was determined for these tumours.
Animals with papillomas and carcinomas of the forestomach occurred at
0/55, 0/54, 3/54, 2/54, 5/55 (male groups) and 1/55, 1/54, 2/55, 5/53,
9/55 (female groups) giving an NOEL of 1.8 mg/kg per day (Wilson et
al., 1989a).
A 2-year study with Charles River CD-1 mice at 750, 1500 or 3000
mg chlorothalonil/kg diet (section 7.3) showed increased incidences of
gastric and renal tumours in the treated groups. Mice with tumours of
the squamous epithelium of the forestomach occurred at 0/60, 2/60,
5/60, 2/60 (male groups) and 0/60, 2/60, 4/60, 5/59 (female groups).
Although not strictly dose-related, these results were considered to
be a treatment effect and linked to the irritant properties of
chlorothalonil. There was also a low incidence of renal tubular
tumours in male treated groups, not seen in controls, at 0/60, 6/60,
4/60, 5/60 (not dose-related). These were probably linked to the high
incidence of renal tubular hyperplasia seen in male mice in the
treated groups (Wilson et al., 1983b, 1986b).
A second study at 0, 15, 40, 175 and 750 mg/kg diet was
undertaken to establish an NOEL for kidney and stomach changes in male
mice (section 7.3). Only two renal tumours (one at 40 mg/kg and one
at 175 mg/kg) were found. There was a slightly higher incidence of
squamous tumours of the forestomach at 750 mg/kg. Taking account of
the overall results of the two studies it was considered that the
tumorigenic NOEL was at least 175 mg/kg diet. In this study, the NOEL
for tubular hyperplasia was 40 mg/kg (equal to 4.5 mg/kg per day) and
the NOEL for hyperplasia/hyperkeratosis in the forestomach was 1.6
mg/kg per day (Wilson et al., 1987).
7.8 Other special studies
Rats fed 0, 1500 or 15 000 mg chlorothalonil/kg diet showed a
dose-related decrease in the retention of a dye, indicating a laxative
effect. A more detailed study attempted to determine the effect of
chlorothalonil on the absorption and utilization of proteins, fats and
amino acids during a 10-week feeding study on a group of 10 male and
10 female rats. It was concluded that the compound did not interfere
directly with the absorption and utilization and that the depressed
weight gain was probably due to catharsis (Paynter, 1967c).
In a study by Andre et al. (1991), mitochondria were obtained
from fresh kidney cortical tissue by homogenization and differential
centrifugation. The mitochondria were incubated in the presence or
absence of sulfur-containing analogues of chlorothalonil and the
degree of mitochondrial respiratory control was evaluated by
polarographic techniques. The following sulfur-containing analogues
of chlorothalonil were tested: the mono-, di-, and tri-thiol analogues
and the mono-, di-, and tri-glutathione analogues. Kidney
mitochondria were incubated with succinate, a site 2 substrate, or
with glutamate, a site 1 substrate, in the presence or absence of the
test material. Mitochondrial respiratory control, expressed as the
acceptor control ratio (ACR), was determined by taking the ratio of
the rate of oxygen consumption in the presence of ADP (state 3) to the
rate of oxygen consumption after the ADP had been consumed (state 4).
When the mono-thiol, mono-, di-, or tri-glutathione analogues of
chlorothalonil and succinate were added to kidney mitochondria, no
significant differences were found in the ACR from the controls.
Incubation of the di- or tri-thiol analogues of chlorothalonil and
succinate with kidney mitochondria resulted in significant differences
of the experimental ACR from the control ACR. When glutamate was used
as the substrate for the electron transport system in kidney
mitochondria, no significant differences from the control were
detected for any of the six test materials. These data suggest that
the effects of the di- or tri-thiol analogues of chlorothalonil may
impair the respiratory control of kidney mitochondria by inhibiting
the transfer of reducing equivalents from succinate to coenzyme Q.
The effects on mitochondrial respiration may be due to the formation
of disulfide bonds between the thiol analogues and proteins.
7.9 Toxicity of metabolites
Most studies have centred on the 4-hydroxy-2,5,6-
trichloroisophthalonitrile metabolite. This is found as a small
proportion of chlorothalonil plant residues (section 4.2.1) and is
also a breakdown product of chlorothalonil in the environment. It has
been identified in faeces of laboratory animals after chlorothalonil
dosing. It is more acutely toxic than chlorothalonil itself (acute
oral LD50 values are 332 and 10 000 mg/kg, respectively).
Several laboratory animal studies have been undertaken with the
4-hydroxy metabolite and have been described in some detail in JMPR
reviews (FAO/WHO, 1978, 1982, 1985). The following is a brief summary
of the studies and their results.
Various effects were noted in rats fed the 4-hydroxy metabolite
at eight dose levels (10 to 750 mg/kg body weight per day) for 61-69
days. Mortality was increased in males at 125 mg/kg per day or more,
and in females at 75 mg/kg per day or more. Body weight was depressed
in both sexes at > 40 mg/kg per day. Anaemia was evident at 75
mg/kg per day or more in males and 40 mg/kg per day or more in
females. Histopathological examination revealed treatment-related
effects in bone marrow and spleen (in the form of erythroid
hyperplasia and depressed granulopoiesis) at > 40 mg/kg per day and
in the liver (haemosiderosis, centrilobular hepatitis) and kidney
(cortical atrophy) at > 75 mg/kg per day. The overall NOEL was 20
mg/kg body weight per day (Murchison, 1979).
A rabbit teratology study was undertaken with oral doses of 0, 1,
2.5 and 5 mg/kg per day during days 6-18 of gestation, with necropsy
at day 28. There was a marginal effect on dams at day 5 but no
evidence of a teratogenic effect in the study (Wazeter & Goldenthal,
1976).
The 4-hydroxy metabolite was evaluated in a three-generation, two
litters/generation study in groups of 15 male and 30 female rats at
dose levels of 0, 10, 60 and 125 mg/kg diet. There were no
treatment-related changes except for body weight reductions at 60 and
125 mg/kg (FAO/WHO, 1982).
In a one-generation follow-up study, groups of rats were fed
diets containing the 4-hydroxy metabolite at 0, 10, 20, 30, 60 and 120
mg/kg diet for 18 weeks before mating (12 males and 24 females per
group). Two sets of mating were undertaken. There was some effect on
live pup weights at 60 and 120 mg/kg. The clear NOEL was considered
to be 30 mg/kg diet (Ford, 1982).
The metabolite was assessed for chronic toxicity and
carcinogenicity in long-term rodent studies. A 2-year rat study was
undertaken at dose levels of 0, 0.5, 3 and 10 mg/kg body weight per
day with groups of 75 males and 75 females. Anaemia was evident at
the highest dose. The NOEL was determined to be 3 mg/kg body weight
per day. There was no evidence for a carcinogenic effect. In the
mouse study, the dietary dose levels were 0, 375, 750 and 1500 mg/kg
diet using groups of 60 males and 60 females. The study was
terminated at 20-22 months because of increasing and high mortality.
A series of effects, including amyloidosis, haemosiderin in the spleen
and increases in reticulocyte counts and red cell haemolysis,
precluded the establishment of an NOEL. No carcinogenic effect was
evident (Hozan & Auletta, 1981; McGee, 1983).
The 4-hydroxy metabolite was not mutagenic in a number of in
vitro and in vivo assays. These were the Salmonella mutagenicity
assay with and without metabolic activation (Banzer, 1977d), a
host-mediated assay in mice given a single intraperitoneal dose of
6.5 mg/kg (Legator, 1974b), Chinese hamster (V-79) and mouse
fibroblast (Balb/3T3) cells in culture with and without activation
(Banzer, 1977e), a micronucleus test in mice at 6.5 mg/kg per day for
5 days (Legator, 1974b), a dominant lethal study in male rats given
single oral doses (0, 2, 4 or 8 mg/kg) singly or daily for 5 days
(Hastings & Clifford, 1975), a dominant lethal study in male mice
given 1, 3 or 6.5 mg/kg per day for 5 days (Legator, 1974b), a DNA
repair assay using S. typhimurium in a spot test with or without
activation (Banzer, 1977f), and a cell transformation assay with rat
embryo cell lines in culture at 0.1, 1 or 10 µg/ml (Price 1978b).
A series of in vitro gene mutation assays with S. typhimurium
tester strains with and without renal metabolic activation were
undertaken with chlorothalonil, four manufacturing impurities and
eight known or potential metabolites. No mutagenic potential was
shown by any of the compounds. Full details were given in the 1985
JMPR evaluation (FAO/WHO, 1986b).
The mutagenic potential of the thiol and cysteine derivatives of
chlorothalonil has been evaluated in the Ames test with and without
metabolic activation with S9 from the kidney of male Fischer rats.
These compounds were 2,5-dichloro-4,6-bismercaptoiso-phthalonitrile,
5-(2,4-dicyano-3,5,6-trichlorophenyl) glutathione, 5-chloro-2,4,6-
trimercaptoisophthalonitrile, S,S'-(2,4-dicyano-3,6-dichloro
phenyl)dicysteine and S,S',S"-(2,4-dicyano-6-chlorophenyl)-
tricysteine (purity ranging from 90.5 to 97.5%). Four other compounds
were used as positive controls. The Salmonella typhimurium tester
strains TA98, TA100, TA1535, TA1537 and TA1538 were used. In all
these studies, there was no significant increase (doubling) over
solvent control values in the number of revertants for any of the five
tester strains used either with or without metabolic activation
(Mizens et al., 1985d,e, 1986b,c, 1987).
A description of a 90-day rat study on the monoglutathione
conjugate of chlorothalonil is given in section 7.3.
8. EFFECTS ON HUMANS
8.1 General population exposure
A case of acute facial dermatitis in a 53-year-old man, caused by
staying in a summer cottage, has been reported. Patch testing
revealed contact allergy to the paint that was applied to all the
window-frames, and to the chlorothalonil contained in the paint.
After removal of the frames, there were no further recurrences of
facial dermatitis. The authors suggested that products containing
chlorothalonil are not suitable for indoor use (Liden, 1990; Eilrich &
Chelsky, 1991).
8.2 Occupational exposure
Chlorothalonil contact dermatitis was observed in a number of
employees in a chlorothalonil manufacturing plant. There were 19
cases out of 103 employees. About 60% of the employees showed some
kind of skin abnormality compared with 18.5% of employees not working
with chlorothalonil. When the hygiene conditions of the plant were
improved the overall proportion of skin abnormalities fell to about
20% and there were no cases of chlorothalonil contact dermatitis
(Diamond Shamrock, 1980).
Wood preservatives containing chlorothalonil have also been
implicated in the appearance of allergic contact dermatitis. One
report concerned a Danish cabinet maker who developed dermatitis on
his hands after 9 months of painting furniture with preservative
containing the compound. This was possibly caused by contact via the
wood dust after sandpapering (Bach & Pedersen, 1980). Another report
referred to three cases, two with erythema on the face, particularly
periorbitally, and one with eczema of the hands, in people engaged in
similar work (Spindeldreier & Deichmann, 1980). The four people in
these cases showed a positive reaction to patch tests with 0.01%
chlorothalonil in acetone.
A further case of contact dermatitits was described by Meding
(1986) in a 33-year-old male painter, who regularly worked with paint
containing chlorothalonil. A patch test with chlorothalonil was
positive.
Work-related skin complaints occurred in a Norwegian factory
producing wooden window frames. The wood preservative used was white
spirit containing 0.5% chlorothalonil. Fourteen out of 20 workers
experienced some kind of skin reaction including pruritus, erythema
and oedema of the eyelids and other facial regions, and eruptions on
arms and hands. Seven of these 14 subjects yielded a positive patch
test reaction with 0.01% chlorothalonil in acetone compared with 1 out
of 14 controls (Johnsson et al., 1983).
Allergic contact dermatitis has also been described in Japanese
farmers (Horiuchi & Ando, 1980) and in Dutch horticultural workers
(Bruynzeel & van Ketel, 1986) using chlorothalonil fungicide
formulations.
In a group of 84 tea growers, two showed a positive skin patch
test with 0.02% chlorothalonil in petrolatum (Fujita, 1985).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Aquatic microorganisms
The algicidal activity of chlorothalonil was examined by Goulding
(1971). It was shown that this compound is effective against a range
of algae including Chlorella, Chlamydomonas, Ulothrix , Anabaena,
Oscillatoria and Microcystis at low concentrations, often
less than 1 µg/litre. It was also effective on natural populations
of algae obtained from lakes, rivers, and reservoirs. The effect
was generally less after 300 h than after 150 h and was dependent upon
the size of the initial cell inoculum.
Walker et al. (1984) describe a simple shake-flask screening test
to evaluate pesticide persistence and aquatic toxicity in the
laboratory. Four systems were used: active sediment, sterile
sediment, active water and sterile water. Chlorothalonil at
162 µg/litre did not increase the mortality of Mysidopsis above the
control level within 96 h. The authors concluded that degradation of
chlorothalonil involved microorganisms and that the degradation
products did not enhance the aquatic toxicity of chlorothalonil.
9.1.1.2 Soil microorganisms
Chlorothalonil, at dose levels up to 5000 mg/litre in bacterial
suspensions, inhibited the growth of three strains of Rhizobium
japonicum (Tu, 1980).
Chlorothalonil, at concentrations up to 1000 mg/kg medium, did
not inhibit or stimulate the growth of any one of 25 strains of
Rhizobium bacteria isolated from red clover root nodules
(Heinonen-Tanski et al., 1982).
Several studies were conducted with chlorothalonil in soil to
determine the effects (if any) on normal soil processes such as
nitrogen fixation, nitrification and degradation of substrates such as
protein, pectin, cellulose and starch. These studies were conducted
at two rates: use rate (2.5 mg/kg) and 10 times the use rate
(25 mg/kg). In general, any inhibitory effects observed were
temporary in nature and more pronounced at the high rate. No effects
on the use of protein, pectin or cellulose by soil microorganisms were
observed, but there was increased utilization of starch (Szalkowski et
al., 1980).
The results obtained by Szalkowski et al. (1981a), who studied
the effect of chlorothalonil on non-symbiotic nitrogen-fixing soil
microorganisms, are given in Table 13.
Table 13. The effect of chlorothalonil on non-symbiotic nitrogen-fixing soil microorganisms
Sandy loam Clay loam
Application Ten times Application Ten times
rate application rate application
rate rate
Aerobic nitrogen initial inhibition inhibition at no effect stimulation
fixation days 0, 21 and
28
Anaerobic nitrogen general stimulation inhibition up no effect stimulation
fixation to day 21
Szalkowski et al. (1981b) studied the effect of chlorothalonil on
nitrogen transformation in sandy loam and clay loam soils at two
rates: one equivalent to the application rate and one equivalent to
ten times this rate. In sandy loam soil, there was a consistent
inhibitory effect at the higher rate, which was reduced with time. In
clay loam soil, at the higher rate, inhibition was no longer observed
after day 21. In both types of soil at the normal rate, there was
little if any inhibition.
9.1.2 Aquatic organisms
The acute toxicity of chlorothalonil to various aquatic species
is shown in Table 14. Some of these toxicity tests were carried out
in static or semi-static systems and others in flow-through systems.
Table 14. Acute toxicity of chlorothalonil to aquatic organisms
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Rainbow trout
(Oncorhynchus mykiss)
6-11 g flow through freshwater 80% 14 acetone > 99% 19.0 17.1 Davies &
6-11 g flow through freshwater 53% 16 acetone > 99% 18.8 10.5 White
6-11 g semi-static freshwater 90% 10 acetone > 99% 18.0 (1985)
(24 h)
- static freshwater - 12 acetone 96% 56 49 SDS Biotech
(soft) Corporation
(1980a)
3.5-4.0 g static freshwater 6.5-7.4 8.4-11.2 12.5-15.5 acetone 97.8% 76 Ernst et
(12.3 mg/litre) mg/litre al. (1991)
3.5-4.0 g static freshwater 6.5-7.4 8.4-11.2 12.5-15.5 Bravo 500 69 Ernst et
(12.3 mg/litre) mg/litre al. (1991)
Bluegill
(Lepomis macrochirus)
- static freshwater 22 acetone 96% 46-77 62 SDS Biotech
(soft) Corporation
(1979)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Common jolly tail
(Galaxias maculatus)
7-10 g flow through freshwater 75% 16 acetone 99% 18.2 16.3 Davies &
White (1985)
Spotted galaxis
(G. fruttaceus)
8-20 g flow through freshwater 75% 16 acetone 99% 25.8 18.9 Davies &
White (1985)
Golden galaxias
(G. auratus)
7-11 g flow through freshwater 75% 13 acetone 99% 46.6 29.2 Davies &
White (1985)
Three spine stickleback
(Gasterosteus aculeatus)
0.3 g static freshwater 7.7-8.0 9.2-9.5 9-10 Bravo 500 < 73 Ernst et
mg/litre al. (1991)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Channel catfish
(Ictalurus punctalus)
40-80 g semi-static, freshwater 7.0-7.2 5.0-6.0 23 acetone 99% 62 52 Gallagher et
24 h (30 mg/litre) mg/litre al. (1992)
- static freshwater 22 acetone 96% 55 44 SDS Biotech
(soft) Corporation
(1980b)
Spot
(Leiosfomus xanthurus)
- flow through brackish water 11 technical 32 Mayer (1987)
(22 ppt)
Sheepshead minnow
(Cyprinodor variegatus)
3-7 days static marine technical 32 SDS Biotech
Corporation
(1982b)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Water flea
(Daphnia magna)
- static freshwater 7.7-8.1 9.1-9.3 20-22 Bravo 500 97a Ernst et
(250 mg/litre) mg/litre al. (1991)
Dungeness crab
(Cancer magister)
larvae semistatic, marine 13 Bravo, 75% 560 140 Armstrong
24h (25 ppt) et al. (1976)
Clam
(Mya arenaria)
5.2 cm static marine 7.3-8.0 8.5-9.9 10.5-12 Bravo 500 35 000 Ernst et
(30-31 ppt) mg/litre al. (1991)
Blue mussel
(Mytilus edulis)
5.9 cm static marine 7.3-8.0 8.5-9.9 10.5-12 Bravo 500 5940 Ernst et
(30-31 ppt) mg/litre al. (1991)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Eastern oyster
(Crassostrea virginica)
- flow through marine 29 technical 26b Mayer (1987)
(27 ppt)
a EC50 - immobility
b EC50 - shell deposition
In view of the strong adsorption and degradation characteristics of
chlorothalonil, nominal concentrations in static systems are likely to
underestimate the toxicity (overestimate the LC50) of chlorothalonil.
At the same time, it should be pointed out that the static test
systems more closely resemble the field situation.
Davies & White (1985) described the reactions of fish to
exposure. Oncorhynchus mykiss and Galaxias sp showed marked
lethargy, the degree increasing with time and concentration of
exposure. O. mykiss showed normal startle reactions at
concentrations below 8.7 µg/litre, and G. maculatus, G. truttaceus
and G. auratus at 8.8, 9.0 and 13.3 µg/litre, respectively, over
96 h. In O. mykiss, loss of startle reaction was followed by
reduction of activity, and permanent lethargy was followed by loss of
righting ability and death. In Galaxias sp, the onset of lethargy
was accompanied by varying degrees of fin collapse.
Davies & White (1985) commented on the fact that most acute
toxicity values have been obtained in tests where solvent was added to
the chlorothalonil.
A 48-h EC50 has been reported for the pink shrimp ( Penaeus
duorarum) at 320 µg/litre and a 96-h EC50 for the eastern oyster
( Crassostrea virginica) at 26 µg/litre. The value for the pink
shrimp was based on immobility or loss of equilibrium and that for the
eastern oyster on shell deposition (Mayer, 1987). Oysters suffered a
42% mortality when exposed to 1000 µg chlorothalonil per litre for
96 h and a 25% reduction in growth at 10 µg/litre (SDS Biotech
Corporation, 1983b).
Ernst et al. (1991) used both laboratory bioassay and field
treatments of a pond system to determine the toxic effects of
chlorothalonil on aquatic fauna. The acute toxicity of technical
chlorothalonil and a commercial formulation on five species, including
mussel and clams, was determined (Table 14). In the field study also
reported by O'Neill (1991) (see section 5.1.2 for measured
concentrations after spraying), the mortality of caged invertebrate
and vertebrate species was monitored. Seven species were caged in the
stream flowing from the treated pond: water boatman (Sigara
alternata), caddisfly larva ( Limnephilus sp), freshwater clam
( Pisidium, sp), crawling water beetle ( Haliplus sp), scud
( Gammarus spp.), stickleback (Gasterosteus aculeatus) and midge
larva (Chironomidae). The floating cages were placed near the
surface of the water. The water boatman suffered the highest
mortality, ranging from 49 to 84% in replicate cages; mortality in a
control pond was 16-20% over the same period (24 h). Midge larva
mortality (69%) was judged by the authors to be a consequence of
handling. Stickleback mortality was 37 to 56% in the treated pond,
compared to 2 to 6% in the controls. Caddisfly larvae, clams, beetles
and scud showed no deaths during the 24 h. Rainbow trout
(Oncorhynchus mykiss) also showed no mortality following spraying.
An estimate of total invertebrate numbers before and after spraying in
the pond showed a slight increase following the first spray and a
slight reduction after the second (not statistically significant).
The control pond also showed fluctuations. The total was heavily
influenced by Chironomid midge larvae, which was by far the most
frequently occurring species. This study showed that a lower
toxicological effect was observed in the field study than in the
laboratory bioassays, indicating a reduction in exposure to the
available chlorothalonil and thus less severe impacts in the pond
system through physical and chemical processes.
Gallagher et al. (1992) showed that sublethal chlorothalonil
exposure may cause acute necrosis of the intestinal epithelial lining
in channel catfish. Exposure to 13 µg/litre for 72 h resulted in
increased tissue GSH concentrations in liver, posterior kidney and
gills, which suggests a protective role for tissue GSH against
chlorothalonil exposure.
When two generations of Daphnia magna were exposed to technical
chlorothalonil at levels of 6.2, 12, 25, 50 and 100 µg/litre for 21
consecutive days during each generation, adverse effects on adult
survival and reproduction were observed at a nominal concentration of
100 µg/litre. The maximum acceptable toxicant concentration (MATC)
for technical chlorothalonil, based upon nominal concentrations, was
50 µg/litre (35 µg/litre was the measured concentration) (Shults et
al., 1982).
Fathead minnows (Pimephales promelas) were continuously exposed
in duplicate aquaria to nominal concentrations of 25, 12.5, 6.3, 3.1
and 1.5 µg/litre (measured concentrations 16, 6.5, 3.0, 1.4 and
0.6 µg/litre, respectively) technical chlorothalonil, a diluent water
control, and a solvent (acetone) control throughout a complete (egg to
egg) life cycle. No significant effects were observed in either
generation at mean measured concentrations < 3.0 µg/litre. The
first generation (F0) eggs exhibited a significantly reduced
hatchability and survival of fry after 35 days when exposed to a mean
measured concentration of 16 µg/litre. The reproductive success of
Fo fish was adversely affected (reduction in the number of eggs per
spawn) by exposure to concentrations > 6.5 µg/litre. The second
generation (F1) eggs exhibited a significantly reduced hatchability
when exposed to a mean measured concentration of 6.5 µg/litre. The
survival of fry at this concentration was not affected. Based on
these data, the MATC (mean measured) of technical chlorothalonil in
water for fathead minnows was estimated to be in the range of 3.0 to
6.5 µg/litre (Shults et al., 1980).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
In a study by Stephenson et al. (1980), 30-day-old tomato plants
were treated with chlorothalonil (at 2.5 kg/ha) or chlorothalonil in
combination with metribuzine. The effect was assessed as the tomato
shoot dry weight (as a percentage of control plant weight). On this
basis, the weight of the chlorothalonil-treated plants was 89% of the
control plant weight, a difference which was statistically significant
(P > 0.05). The effect was additive when chlorothalonil was used in
combination with metribuzine.
9.1.3.2 Earthworms
When chlorothalonil suspension concentrate (500 g/litre) was
added to artificial soil containing earthworms (Eisenia foetida),
the treated soil was non-toxic after 7 and 14 days. The LC50 was
found to be > 1000 mg/kg soil (on a dry weight basis) (Wuthrich,
1990).
When earthworms (Eisenia foetida) were immersed for 1 min in
solutions of chlorothalonil (0.1, 1 and 2% w/v); there was no effect
on survival. Bermuda grass clippings were air dried and ground; 15 g
samples were then stirred into 100 ml of 0.1% chlorothalonil and
subsequently fed to earthworms after filtration of excess liquid.
There was no effect on longevity. Worms reared in soil in which
chlorothalonil had been incorporated showed reduction in longevity of
about 50% compared to controls 52-84 days after the beginning of
treatment. The amount of chlorothalonil added was equivalent to 5
times the recommended application rate at 0.9 g in 4700 cm3 of soil,
and reproduction was virtually eliminated (Roark & Dale, 1979).
9.1.3.3 Earwigs and honey-bees
Earwigs (Labidura riparia) were exposed to chlorothalonil in
three ways: a) on glass; b) as a residue on peanut foliage; and c) as
a residue on a food source (i.e. 7-day-old armyworms). In the first
treatment, chlorothalonil at a rate of 0.72 kg/ha produced up to 20%
mortality in 24 h and up to 30% in 48 h. In the second experiment,
chlorothalonil was applied, at the same rate, to peanut foliage which
was then used for the test. There was a 10% mortality within 24 h and
20% in 48 h on 4-day-old residues and no mortality on 8-day-old
residues. In the food source experiment there was 25-30% mortality
within 24 h of the larvae being consumed and up to 55% mortality
within 48 h (DeRivero & Poe, 1981).
Atkins et al. (1975) found no contact toxicity of chlorothalonil
(11 µg/bee), and classified it as relatively non-toxic to honey-bees.
The oral LD50 for chlorothalonil in 20% sucrose solution for
honey-bees was > 0.2 µg/bee and the contact LD50 > 65 µg/bee
(Davies, 1986).
9.1.3.4 Birds
The following toxicity values have been reported for birds, but
without descriptions of the experimental methodology. The acute oral
LD50 in the mallard duck was reported to be > 4640 mg/kg. The 8-day
dietary LC50 in the same species and in the bobwhite quail (Colinus
virginianus) was given as > 10 000 mg/kg diet in each case (SDS
Biotech Corporation, 1981a,b). Dietary 8-day LC50 values were also
reported as > 21 500 mg/kg diet for the mallard duck and 5200 mg/kg
diet for the bobwhite quail (Worthing, 1991).
Shults et al. (1988a) evaluated the effect of chlorothalonil on
reproduction in the bobwhite quail. Four groups (16 pairs per group)
of quail were administered chlorothalonil in the diet at levels of
1000, 5000 and 10 000 mg/kg for a period of 21 weeks. Quail were fed
the amended diet for 11 weeks prior to egg laying and for the duration
of the egg laying period. Adult and offspring were examined for body
weight, general health, adult food consumption, egg production,
eggshell thickness, embryo viability, hatching success survivability
of offspring and gross pathology. At a dietary concentration of
10 000 mg/kg, the birds experienced reproductive impairment, which
included mortality, general health, body weight, food consumption,
gross pathology and other reproductive end-points. Hatching survival
was also affected at the highest dosage. General health and
reproductive parameters were also affected at 5000 mg/kg. Other
effects observed at this dose level included decreased body weight
gain and survivability of offspring. The lowest test dosage showed no
apparent affects on either adult quail or offspring. A
no-observed-effect concentration (NOEC) of 1000 mg/kg diet was
established for chlorothalonil regarding reproductive effects.
In a separate but similar reproductive study with mallard ducks
(Anas platyrhynchos), Shults et al. (1988b) reported that
10 000 mg/kg diet reduced egg production and the percentage of
hatchlings per incubated egg. No reproductive impairments were
observed for ducks dosed at 1000 or 5000 mg/kg. Shults et al. (1988b)
reported no effect on eggshell thickness at these chlorothalonil
concentrations. The NOEC for reproductive effects in mallard duck was
5000 mg/kg diet.
9.2 Field observations
9.2.1 Soil microorganisms
Smiley & Craven (1979) applied chlorothalonil (as Daconil 2787)
at the normal rate (actual dose not given) every 21 days between April
and September (9 applications for 3 consecutive years) to an
experimental plot of Sward Kentucky blue grass (Pia pratensis).
Treated plots were 1 × 5 m and were replicated. Samples of soil cores
2.54 cm in diameter and 3 cm deep were taken (n = 5) from each
replicate plot. The cores included thatch and were chopped and mixed
well, and 10 g was suspended in sterile distilled water. A dilution
series was plated out to estimate bacterial, actinomycete and fungal
populations. None of the organisms were affected by chlorothalonil.
The fungicide did not affect numbers of Nitrosomonas or Nitrobacter
bacteria and had no effect on the disappearance of added ammonium by
nitrification. Treatments alternating different fungicides had a
greater effect than one fungicide alone.
9.2.2 Plants
Many crop plants are tolerant of chlorothalonil and do not suffer
any phytotoxicity when sprays of chlorothalonil formulations are
applied according to recommended practices. However, a few phytotoxic
effects have been observed with certain species as a direct result of
chlorothalonil applications and also as a result of physiologically
incompatible mixtures of chlorothalonil formulations with additives or
other pesticides.
Chlorothalonil applied at a rate of 2.52 kg/ha did not affect
yields of tomato plants grown in field conditions. In addition,
chlorothalonil did not enhance metribuzin damage when the two
compounds were used in combination on tomato plants. These results
are contrary to those obtained in laboratory studies (section 9.1.3)
and indicate that under field conditions phytotoxic reactions between
chlorothalonil and metribuzin are unlikely to occur (Stephenson et
al., 1980).
Clear results were not obtained concerning the effect of
chlorothalonil on perennial rye grass (Lolium perenne) when it was
sprayed regularly for the control of leaf-spotting disease. In the
first year of treatment the grass yield was increased by 15% at the
third harvest, but at the following harvest, when disease incidence
was greater, the yield was not increased. The authors concluded that
chlorothalonil did not have a direct stimulatory effect on grass
growth (Lam & Lewis, 1983).
Chlorothalonil was used to treat onion beds at a rate of
2.34 litres formulation/ha with 10 weekly applications. The onions
(two short-day cultivars) were harvested either 146 or 167 days after
planting. Chlorothalonil reduced the weight and size of marketable
bulbs by 44 and 32%, respectively, but did not influence the
maturation rate. Alternate application of chlorothalonil and mancozeb
reduced these values by 27 and 33%, respectively. The disease
incidence was nil in all treatments (Stoffella & Sonoda, 1982).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed
and evaluated chlorothalonil at its meetings in 1974, 1977, 1978,
1979, 1981, 1983, 1985, 1987, 1990 and 1992 (FAO/WHO, 1975, 1978,
1979, 1980, 1982, 1985, 1986a,b, 1988, 1990a,b, 1993b). In 1990 an
acceptable daily intake (ADI) of 0-0.03 mg/kg body weight was
established. This ADI was confirmed in 1992, based on the NOAEL of 3
mg/kg body weight/day established in the two-year dog study (FAO/WHO,
1993a,c).
The Joint FAO/WHO Codex Alimentarius Commission has established
maximum residue limits (MRLs) for chlorothalonil in various
commodities (FAO/WHO, 1993c).
WHO has classified chlorothalonil as a technical product unlikely
to present an acute hazard in normal use (WHO, 1992).
On the basis of data available at the time, the International
Agency for Research on Cancer evaluated chlorothalonil as showing
limited evidence of carcinogenicity in animal studies and categorized
it as an agent not classifiable as to its carcinogenicity to humans
(IARC, 1987).
REFERENCES
André JC, Marciniszyn JP, & Killeen JC Jr (1991) Evaluation of
mitochondrial function in the presence and absence of sulphur-
containing analogs of chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 3113-88-0107-AM-001,
submitted to WHO by ISK-Biotech, Mentor, Ohio, USA).
Armstrong DA, Buchanan DV, & Caldwell RS (1976) A mycosis caused by
Lagenidium sp.in laboratory-reared larvae of the dungeness crab,
cancer magister, and possible chemical treatments. Department of
Fisheries and Wildlife, Oregon State University, Marine Science
Center, Newport, Oregon 97365. J Invertebr Pathol, 28: 329-336.
Atkins EL, Greywood EA, & MacDonald RL (1975) Toxicity of pesticides
and other agricultural chemicals to honey bees. Riverside, California,
University of California, Division of Agricultural Sciences, 37 pp
(Report No. 2287).
Bach B & Pedersen NB (1980) Contact dermatitis from a wood
preservative containing tetrachloroisophthalonitrile. Contact
Dermatitis, 6: 142.
Ballee DL, Duane WC, Stallard DE, & Wolfe AL (1976) Chlorothalonil.
In: Zweig G & Sherma J ed. Analytical methods for pesticides and plant
growth regulators. New York, Academic Press, vol VIII, pp 266-274.
Banzer CB (1977a) Activity of chlorothalonil in the Salmonella/
microsomal assay for bacterial mutagenicity. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 000-5TX-77-0035-001).
Banzer CB (1977b) Activity of chlorothalonil in a test for
differential inhibition of repair deficient and repair competent
strains of Salmonella typhimurium: Repair test. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 000-5TX-77-0033-001).
Banzer CB (1977c) Activity of chlorothalonil in an in vitro
mammalian cell point mutation assay. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 000-5TX-77-0034-001).
Banzer CB (1977d) Activity of DTX-0038 in the Salmonella/microsomal
assay for bacterial mutagenicity. Bethesda, Maryland, Microbiological
Associates Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Banzer CB (1977e) Activity of DTX-77-0040 in an in vitro mammalian
cell point mutation assay. Bethesda, Maryland, Microbiological
Associates Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Banzer CB (1977f) Activity of DTX-77-0039 in a test for differential
inhibition of repair deficient and repair competent strains of
Salmonella typhimurium: Repair test. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Blackmore R & Shott L (1968) Final report four-month feeding study -
rats. Hazleton Laboratories, Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Bruynzeel DP & van Ketel WG (1986) Contact dermatitis due to
chlorothalonil in floriculture. Contact Dermatitis, 14: 67-68.
Burchfield HP & Storrs EE (1977) Residue analysis. In: Seigel MR &
Sisler HD ed. Antifungal compounds. New York, Marcel Dekker, Inc., vol
1, pp 463-505.
Busey WM (1975) Histopathological incidence data - kidney. Herndon,
Virginia, Experimental Pathology Laboratory, Inc. (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Busey WM (1985) A 90-day feeding study in mice with T-117-11.
Experimental Pathology Laboratories, Inc. (Unpublished revised
pathology report No. SDS 753-5TX-85-0053-002, submitted to WHO by SDS
Biotech, Mentor, USA).
Camoni I, DiMuccio A, Pontecorvo D, Rubbiani M, Vergori L, & Lugaresi
C (1991) Residue levels in apples and pears field-treated with two
experimental chlorothalonil formulations. Bull Environ Contam Toxicol,
46: 361-367.
Chin BH, McGloin JB, Spangler NL, & Heilman RD (1981) Chlorothalonil
equivalents in the blood and urine of rats following oral,
endotracheal and dermal administration of 14C-chlorothalonil. Bull
Environ Contam Toxicol, 26: 258-261.
Danks A & Fowler JSL (1988) Chlorothalonil technical: acute inhalation
toxicity study in the rat. Eye, Suffolk, United Kingdom, Life Science
Research Ltd (Report No. 88/CFA 00I/85, submitted to WHO by ISK-
Biotech, Mentor, USA).
Davies PE (1985a) The toxicology and metabolism of chlorothalonil in
fish. II. Glutathione conjugates and protein binding. Aquat Toxicol,
7: 265-275.
Davies PE (1985b) The toxicology and metabolism of chlorothalonil in
fish. III. Metabolism, enzymatics and detoxication in Salmo spp and
Galaxias spp. Aquat Toxicol, 7: 277-299.
Davies PE (1985c) The toxicology and metabolism of chlorothalonil in
fish. IV. Zinc coexposure and the significance of metallothionein in
detoxication in Salmo Gairdneri. Aquat Toxicol, 7: 301-306.
Davies LG (1986) Report on a laboratory investigation into the
toxicity of Sipcam chlorothalonil to honey bees (Apis mellifera).
Nottingham, Trent Polytechnic, Department of Sciences (Report
submitted to WHO by ISK-Biotech, Mentor, USA).
Davies PE (1988) Disappearance rates of chlorothalonil (TCIN) in the
aquatic environment. Bull Environ Contam Toxicol, 40: 405-409.
Davies PE & White RWG (1985) The toxicology and metabolism of
chlorothalonil in fish I. Lethal levels for Salmo gairdneri,
Galaxias maculatus, G. truttaceus and G. auratus and the fate of
14C-TCIN in S. gairdneri. Aquat Toxicol, 7: 93-105.
DeRivero NA & Poe SL (1981) Response of Labidura riparia (Pallas) to
residues of pesticides used on peanuts. Peanut Sci, 8: 93-96.
Diamond Shamrock (1974) Unpublished report on the pesticide
chlorothalonil, submitted to FAO/WHO by Diamond Shamrock Corporation,
Mentor, Ohio, USA.
Diamond Shamrock (1980) Unpublished toxicological report, submitted to
the 1981 JMPR by Diamond Shamrock Corporation, Mentor, Ohio, USA.
Doyle R & Elsea J (1963) Acute oral, dermal, and eye toxicity and
irritation studies on DAC-2787. Hill Top Research Institute Inc.
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
Duane WC (1970) Biodegradation of Daconil 2787. Mentor, Ohio, Diamond
Shamrock Corporation (Unpublished report).
Eilrich GL & Chelsky M (1991) Letters to the editor : Facial
dermatitis caused by chlorothalonil in paint. Contact Dermatitis, 25:
141-144.
Elliott VJ & Spurr HW (1993) Temporal dynamics of chlorothalonil
residues on peanut foliage and the influence of weather factors and
plant growth. Plant Dis, 77: 455-460.
Ernst W, Doe K, Jonah P, Young J, Julien G, & Hennigar P (1991) The
toxicity of chlorothalonil to aquatic fauna and the impact of its
operational use on a pond ecosystem. Arch Environ Toxicol, 21: 1-9.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO (1985) Guidelines for the disposal of waste pesticide and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1975) 1974 Evaluations of some pesticide residues in food:
The monographs. Geneva, World Health Organization, pp 101-148 (WHO
Pesticide Residues Series, No. 4).
FAO/WHO (1978) Pesticide residues in food - 1977. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Sup.).
FAO/WHO (1979) Pesticide residues in food - 1978. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Sup.).
FAO/WHO (1980) Pesticide residues in food - 1979. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Sup.).
FAO/WHO (1982) Pesticide residues in food - 1981. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1985) Pesticide residues in food - 1983. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 61).
FAO/WHO (1986a) Pesticide residues in food - 1985. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 72/1).
FAO/WHO (1986b) Pesticide residues in food - 1985. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 72/2).
FAO/WHO (1988) Pesticide residues in food - 1987. Evaluations: Part II
- Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 86/2).
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Codex Committee on Pesticide
Residues, Food and Agriculture Organization of the United Nations
(CAC/PR8-1989).
FAO/WHO (1990a) Pesticide residues in food - 1990. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 103/1).
FAO/WHO (1990b) Pesticide residues in food - 1990. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 103/2).
FAO/WHO (1991) Pesticide residues in food - 1990. Evaluations: Part II
- Toxicology. Geneva, World Health Organization (WHO/PCS/91.47).
FAO/WHO (1993a) Pesticide residues in food - 1992. Report. Rome, Food
and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper 116).
FAO/WHO (1993b) Pesticide residues in food - 1992. Evaluations: Part
II - Toxicology. Geneva, World Health Organization (WHO/PCS/93.34).
FAO/WHO (1993c) Codex Alimentarius. Volume 2: Pesticide residues in
food. Rome, Food and Agriculture Organisation of the United Nations.
Flannigan SA & Tucker SB (1985) Influence of the vehicle on irritant
contact dermatitis. Contact Dermatitis, 12: 177-178.
Flannigan SA, Tucker SB, & Calderon V (1986) Irritant dermatitis from
tetrachloroisophthalonitrile. Contact Dermatitis, 14: 258-259.
Ford WH (1982) A one-generation reproduction study in rats with
DS-3701. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Ford WH, Killeen JC, & Baxter RA (1987a) A 90-day feeding study in
rats with chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 1115-85-0079-TX-006).
Ford WH, Killeen JC, & Baxter RA (1987b) A 90-day study in rats with
the monoglutathione conjugate of chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1108-85-0078-TX-006).
Frazier HW (1993) Chlorothalonil: A response to Environmental Fate and
Ground Water Branch (EFGWG) review. Painesville, Ohio, Ricerca, Inc.
(Document No. 3254-93-0280-EF-001, submitted to WHO by ISK Biosciences
Corporation, Mentor, USA).
Fujita Y (1985) Studies on contact dermatitis from pesticides in tea
growers. Acta Med Univ Kagoshima, 27(1): 17-37.
Fullmore GE & Leveglia J (1992) A thirty-day toxicity study in dogs
with T-117-2. Painesville, Ohio, Ricerca, Inc. (Unpublished report
No. 5092-91-0554-TX003, submitted to WHO by ISK-Biotech Corporation,
Mentor, USA).
Gallagher EP, Cattley RC, & Di Giulio RT (1992) The acute toxicity and
sublethal effects of chlorothalonil in channel catfish (Ictalurus
punctatus). Chemosphere 24: 3-10.
GIFAP (1987) Guidelines for the avoidance, limitation and disposal of
pesticide waste on the farm. Brussels, International Group of National
Associations of Manufacturers of Agrochemical Products.
Gilvydis DM & Walters SM (1988) Rapid liquid chromatographic analysis
of chlorothalonil in fresh produce using photoconductivity and UV
detectors in tandem. J Agric Food Chem, 36: 957-961.
Goulding KH (1971) A preliminary investigation of a potential new
algicide. Proceedings of the 6th British Insecticides and Fungicides
Conference, pp 621-629.
Green T, Odum J, Nash JA, & Foster JR (1990) Perchloroethylene-induced
rat kidney tumours: An investigation of the mechanisms involved and
their relevance to humans. Toxicol Appl Pharmacol, 103: 77-89.
Gutenmann WH & Lisk DJ (1966) Metabolism of Daconil and Dacthal
pesticides in lactating cows. J Dairy Sci, 49(10): 1272-1276.
Hastings TF & Clifford D (1975) Eight-week dominant lethal study of
DAC-3701 in rats. Bio/Tox Research Laboratories, Inc. (Unpublished
report submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Hatzenbeler CJ (1991) An aerobic aquatic metabolism study with
14C-chlorothalonil. Painesville, Ohio, Ricerca, Inc. (Document No.
3163-90-0240-EF-001, submitted to WHO by ISK Biosciences Corporation,
Mentor, USA).
Heinonen-Tanski H, Oros G, & Kecskes M (1982) The effect of soil
pesticides on the growth of red clover rhizobia. Acta Agric Scand, 32:
283-288.
Holmes DC & Wood NF (1972) Removal of interfering substances from
vegetable extracts prior to the determination of organochlorine
pesticide residues. J Chromatogr, 67: 173-174.
Holsing G & Shott L (1970) Two-year dietary administration - rats.
Vienna, Virginia, Hazleton Laboratories, Inc. (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Holsing GC & Voelker RW (1970) 104-Week dietary administration - dogs,
Daconil 2787 (technical). Hazleton Laboratories, Inc. (Unpublished
report submitted to WHO by Biotech Corporation, Mentor, USA).
Horiuchi N & Ando S (1980) Contact dermatitis due to pesticides for
agricultural use. Jpn J Dermatol, 90: 289.
Hozan GK & Auletta CS (1981) A chronic dietary study in mice with
T-114 (DS-3701). East Millstone, New Jersey, BioDynamics, Inc.
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
IARC (1983) Chlorothalonil. In: Miscellaneous pesticides. Lyon,
International Agency for Research on Cancer, pp 319-328 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 30).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 60 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Ignatoski JA, Killenen JC, & Marciniscyn JP (1983) Distribution of
radioactivity following oral administration of 14C-chlorothalonil to
rats: extraction and analysis of 14C-materials in excreta. Mentor,
Ohio, Diamond Shamrock Corporation (Unpublished report).
IRPTC (1989) Data profile on chlorothalonil. Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
ISK Biosciences Corporation (1995) A summary of chlorothalonil
metabolism under various environmental conditions in the
chlorothalonil summary dossiers for the inclusion of active substances
in Annex 1 of Directive 91/414/EEC. Mentor, Ohio, ISK Biosciences
Corporation.
Jacobson BM & Schollenberger JM (1992) Dissipation of 4-hydroxy-2,4,5-
trichloroisophthalonitrile (SOS-3701) in soil: A re-analysis of
chlorothalonil soil dissipation data. Painesville, Ohio, Ricerca, Inc.
(Proprietary report No. 1874-T, submitted to WHO by ISK-Biotech,
Mentor, USA).
Jarrett RD, Stallard DE, & Bachand RT (1978) Absorption, excretion and
tissue distribution of orally administered 14C-hydroxy 2,5,6-
trichloroisophthalonitrile (14C-DAC 3701) in male Sprague-Dawley
rats. Painsville, Ohio, T.R. Evans Research Centre (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Johnsson M, Buhagen M, Leira HL, & Solvang S (1983) Fungicide induced
contact dermatitis. Contact Dermatitis, 9: 285-288.
Jones RE, Killeen JC Jr, & Ignatoski JA (1984) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 694-5TX-84-0064-002).
Jongen NJ, Engel R, & Leenheers LH (1991) Determination of the
pesticide chlorothalonil by HPLC and UV detection for occupational
exposure assessment in greenhouse carnation culture. J Anal Toxicol,
15: 30-34.
Katayama A, Isemura H, & Kuwatsuka S (1991) Suppression of
chlorothalonil dissipation in soil by repeated applications. J Pestic
Sci, 16: 233-238.
Kawamura Y, Takeda M, & Uchiyama M (1978) Photolysis of chlorothalonil
in benzene. J Pestic Sci, 3: 397-400.
Kenyon RG & Ballee DL (1990) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1989). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-007).
Kenyon RG & Wiedmann JL (1992a) Analytical methods including recovery
rates and the limits of determination for residues in water. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 5221-92-0072-MD-001).
Kenyon RG & Wiedmann JL (1992b) Analytical methods including recovery
rates and the limits of determination for residues in soil. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 5221-92-0073-MD-001).
King C, Prince PM, & Ballee DL (1991) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1990). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-008).
King C, Prince PM, & Ballee DL (1992) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1991). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-010).
Koseki M, Shimamura Y, Maki T, Tamura Y, & Naoi Y (1980) Residues of
TPN (tetrachloroisophthalonitrile), captan, diazinon and
chinomethionate in vegetables and fruits. Tokyo Toriotu Eisei
Kenkyusho Kenkyu Nempo, 31:(1) 170-173.
Krawchuk BP & Webster GRB (1987) Movement of pesticides to ground
water in an irrigated soil. Water Pollut Res J Can, 22(1): 129-145.
Kunkel JF (1967a) Absence of 14C movement in crop plant organs after
topical application and soil amendment studies with isotopic Daconil
2787. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished report).
Kunkel JF (1967b Movement of 14C in or on roots of crop species grown
in soil amended with isotopic Daconil 2787. Mentor, Ohio, Diamond
Shamrock Corporation (Unpublished report).
Ladd K, Jenkins DH, & Kepplinger ML (1971) Meat and milk residues
study with technical Daconil 2787 - 97% and pure DAC-3701 in dairy
cattle. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Lam A & Lewis GC (1983) Chemical control of foliar diseases of
perennial rye grass (Lolium perenne L.) and their effects on yield
and quality of the crop. Crop Prot, 2(1): 75-83.
Lawless EW, Ferguson TL, & Meiners AF (1975) Guidelines for the
disposal of small quantities of unused pesticides. Cincinnati, Ohio,
US Environmental Protection Agency (EPA 670/2-75-057).
Lee SS, Marciniscyn JP, Marks AF, & Ignatoski JA (1982) Balance study
of the distribution of radioactivity following oral administration of
14C-chlorothalonil (14C-DS 2787) to rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 000-4AM-81-0209-001).
Legator MS (1974a) Mutagenic testing with DAC 2787. Providence, Rhode
Island, Roger Williams General Hospital, Division of Genetics and
Brown University, Division of Biological and Medical Sciences
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
Legator MS (1974b) Mutagenic testing with DAC 3701. Mentor, Ohio,
Diamond Shamrock Corporation (Unpublished report).
Leonard JA & Yeary RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51(11): 605-609.
Lewis RG, Bond AE, Johnson DE, & Hsu JP (1988) Measurement of
atmospheric concentrations of common household pesticides : a pilot
study. Environ Monit Assess, 10: 59-73.
Liden C (1990) Facial dermatitis caused by chlorothalonil in paint.
Contact Dermatitis, 22: 206-211.
Lucas F & Benz G (1990) A two generation reproduction study in rats
with technical chlorothalonil. Painesville, Ohio, Ricerda, Inc.
(Proprietary report No. 1722-87-0121-TX-003, submitted to WHO by
ISK-Biotech, Mentor, USA).
Luperi L & Forster R (1988) Delayed contact hypersensitivity study in
the Guinea pig. Rome, Life Science Research, Toxicology Centre S.P.A.
(Report No. 128005-ST-02687, submitted to WHO by ISK-Biotech, Mentor,
USA).
McGee (1983) A two-year toxicity and tumourigenicity study of T-114 in
rats. Mattawan, Michigan, International Research and Development
Corporation (Unpublished report submitted to WHO by Diamond Shamrock
Corporation, Mentor, USA).
McLeod HA, Smith DC, & Bluman N (1980) Pesticide residues in the total
diet in Canada, V: 1976 to 1978. J Food Saf, 2: 141-164.
Magee TA, Marciniszyn JP, & Killeen JC Jr (1990) Study to evaluate the
urinary metabolites of chlorothalonil following dermal application to
male Rhesus monkeys. Painesville, Ohio, Ricerca, Inc. (Report No.
3382-89-02-AM-001, submitted to WHO by ISK-Biotech, Mentor, USA).
Major D, Killeen JC, & Ignatoski JA (1982) Primary eye irritation
study in albino rabbits with technical chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 104-5TX-80-0037-002).
Marciniszyn JP, Savides MC, Killeen JC, & Ignatoski JA (1984a) Study
of the dermal absorption of 14C-chlorothalonil (14C-DS-2787) by male
rats. Mentor, Ohio, Fermenta ASC (Unpublished report No.
649-4AM-84-0010-001).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1984b) Study of the
distribution of radioactivity following oral administration of
(14C-DS-2787) to male Sprague Dawley rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 631-4AM-83-0011-002).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1985a) Study of the
distribution of radioactivity following oral administration of
(14C-SDS-2787) to female Sprague Dawley rats. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 631-4AM-84-0078-002).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1985b) Identification of
metabolites in urine and blood following oral administration of
14C-chlorothalonil (14C-SDS-2787) to male rats: the thiol metabolites
in urine. Mentor, Ohio, Fermenta ASC (Unpublished report No.
621-4AM-83-0061-001).
Marciniszyn JP, Savides MC, Killeen JC, & Ignatoski JA (1986) Study of
the biliary excretion of radioactivity following oral administration
of 14C-SDS-2787 to male Sprague-Dawley rats. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 633-4AM-85-0012-002).
Marciniszyn JP, Killeen JC, & Baxter RA (1988) Pilot study of the
effect of the gamma-glutamyl transpeptidase inhibitor AT-125 on the
metabolism of 14C-chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1376-86-0072-AM-002).
Markus JR & Puma B ed. (1973) Pesticide analytical manual: Methods for
individual pesticide residues: Volume 2 - Pesticide registration
section 120.275. Washington, DC, US Food and Drug Administration.
Matsushita T & Aoyama K (1981) Cross reactions between some pesticides
and the fungicide benomyl in contact allergy. Ind Health, 19: 77-82.
Mayer FL ed. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Gulf Breeze, Florida. Washington, DC, US Environmental
Protection Agency (EPA-600/8-017; NTIS PB 87-188686).
Meding B (1986) Contact dermatitis from tetrachloroisopathalonitrile
in paint. Contact Dermatitis, 15: 187.
Mizens M, Wilson NH, Laveglia J, Killeen JC, & Ignatoski JA (1983) A
teratology study in rats with technical chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 517-5TX-82-0011-003).
Mizens M, Killeen JC, Claudio G, & Ignatoski JA (1985a) In vivo bone
marrow chromosomal aberration assay in mice with a single dose of
technical chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 625-5TX-83-0029-002).
Mizens M, Killeen JC, Claudio G, & Ignatoski JA (1985b) In vivo bone
marrow aberration assay in rats with a single dose of technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 625-5TX-83-0028-002).
Mizens M, Killeen JC, & Ignatoski JA (1985c) Acute and subchronic in
vivo bone marrow chromosomal aberration assay in Chinese hamsters
with technical chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 625-5TX-83-0014-003).
Mizens M, Killeen JC Jr, & Ignatoski JA (1985d) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with 2,5-dichloro-4,6-bismercaptoisoph-
thalonitrile (SDS-3939) (Study No. T4147.501 performed by
Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
SDS Biotech Corporation (Unpublished report No. 694-5TX-85-0042-002,
submitted to WHO by Fermenta Plant Protection Co., Painesville, USA).
Mizens M, Killeen JC Jr, & Ignatoski JA (1985e) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test)
with and without renal activation with 5-(2,4-dicyano-3,5,6-
trichlorophenyl) Glutathione (SDS-66382) (Study No. T4147.501
performed by Microbiological Associates Inc., Bethesda, USA).
Painesville, Ohio, SDS Biotech Corporation (Unpublished report
No. 694-5TX-85-0043-002, submitted to WHO by Fermenta Plant
Protection Co., Painesville, USA).
Mizens M, Killeen JC, & Ignatoski JA (1986a) In vitro chromosomal
aberration assay in Chinese hamster ovary (CHO) cells with technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1109-85-0082-TX-002).
Mizens M, Killeen JC Jr, & Baxter RA (1986b) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with 5-chloro-2,4,6-
trimercaptoisophthalonitrile (SDS-66471) (Study No. T5079.1505
performed by Microbiological Associates Inc., Bethesda, USA).
Painesville, Ohio, Ricerca, Inc., Department of Toxicology and Animal
Metabolism (Unpublished report No. 1097-86-0037-TX-002, submitted to
WHO by Fermenta Plant Protection Co., Painesville, USA).
Mizens M, Killeen JC Jr, & Baxter RA (1986c) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with S,S',S''--(2,4-dicyano-6-
dichlorophenyl)tricysteine (SDS-66473) (Study No. T5081.1505 performed
by Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
Ricerca, Inc., Department of Toxicology and Animal Metabolism
(Unpublished report No. 1097-86-0039-TX-002, submitted to WHO by
Fermenta Plant Protection Co., Painesville, USA.)
Mizens M, Killeen JC Jr, & Baxter RA (1987) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with S,S'(2,4-dicyano-3,6-dichlorophenyl)-
dicysteine (SDS-66474) (Study No. T5080.1505 performed by
Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
Ricerca, Inc., Department of Toxicology and Animal Metabolism
(Unpublished report No. 1097-86-0038-TX-002, submitted to WHO by
Fermenta Plant Protection Co., Painesville, USA).
Modesso P & Forster R (1988) Chromosome aberrations in human
lymphocytes cultured in vitro. Test substance: Chlorothalonil
technical. Rome, Life Science Research, Toxicology Centre S.P.A.
(Report No. 128008-M-10787, submitted to WHO by ISK-Biotech, Mentor,
USA).
Murchison TE (1979) A short-term dietary study in rats with T-114-2
(DS 3701). Dawson Research Corporation (Unpublished report submitted
to WHO by Diamond Shamrock Corporation, Mentor, USA).
O'Neill HJ (1991) Transport of the fungicide chlorothalonil from its
operational use on a pond ecosystem. Bull Environ Contam Toxicol,
46:822-828.
Paynter OE (1965a) Oral dose range: dogs - Final report. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE (1965b) Repeated dermal application: Rabbits. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE (1966a) Two-year dietary administration: dogs DAC 2787.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-66-0002-
001).
Paynter OE (1966b) Reproduction rabbits DAC 2787. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 000-5TX-66-003-001).
Paynter OE (1967a) Final report - Two-year dietary feeding: rats.
Hazleton Laboratories, Inc. (Unpublished report submitted to WHO by
Diamond Shamrock Corporation, Mentor, USA).
Paynter OE (1967b) Three-generation reproduction study: rats DAC 2787.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-67-0005-
001). USA.
Paynter OE (1967c) Ten-week amino acid feeding study: rats. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE & Busey WM (1966) Two-year dietary administration: dogs
DAC 2787. Hazelton Laboratories, Inc. (Unpublished report submitted to
WHO by Biotech Corporation, Mentor, USA).
Paynter OE & Busey W (1967) Long-term (76 weeks) feeding study: rats
DAC 2787. Hazleton Laboratories, Inc. (Unpublished report submitted to
WHO by Diamond Shamrock Corporation, Mentor, USA).
Paynter OE & Crews L (1967) Final report - Two-year dietary feeding:
rats. Hazleton Laboratories, Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Paynter OE & Murphy JC (1967) 16-Week dietary administration: dogs
DAC 2787. Hazelton Laboratories, Inc. (Unpublished report submitted to
WHO by Biotech Corporation, Mentor, USA).
Powers M (1965) Acute oral administration: rats. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Price P (1978a) The activity of compound DTX-77-0037 in the Fischer
rat embryo transformation assay system. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Price P (1978b) The activity of compound DTX-77-0041 in the Fischer
rat embryo transformation assay system. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Reduker S, Uchrin CG, & Winnett G (1988) Characteristics of the
sorption of chlorothalonil and azinphos-methyl to a soil from a
commercial cranberry bog. Bull Environ Contam Toxicol, 41: 633-641.
Ribovich ML, Pollock GA, Marciniszyn JP, Killeen JC, & Ignatoski JA
(1983) Balance study of the distribution of radioactivity following
oral administration of 14C-chlorothalonil (14C-DS-2787) to male mice.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 613-4AM-82-0178-
001).
Roark JH & Dale JL (1979) The effect of turf fungicides on earthworms.
Arkansas Acad Sci Proc, 33: 71-74.
Rosanoff KA & Siegel MR (1981) Mechanism of action and fate of the
fungicide chlorothalonil in biological systems. 3. Interaction with
mammalian DNA, histones and isolated rat liver nuclei. Pestic Biochem
Physiol, 16: 120-128.
Sadler EM, Wilson NH, Killeen JC, & Ignatoski JA (1985) Time course of
the acute effect of technical chlorothalonil on hepatic and renal
glutathione content in male rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 751-5TX-85-0032-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1985)
Isolation and identification of metabolites in the bile of rats orally
administered 14C-chlorothalonil I. Synthesis and characterisation of
glutathione conjugates of chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 633-4AM-84-0104-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986a) Study
of the distribution of radioactivity following repeated oral
administration of 14C-chlorothalonil (14C-SDS-2787) to male
Sprague-Dawley rats. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1173-84-0079-AM-003).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986b)
Identification of metabolites in urine and blood following oral
administration of 14C-chlorothalonil to male rats. II. Effects of
multiple dose administration on the excretion of thiol metabolites in
urine. Mentor, Ohio, Fermenta ASC (Unpublished report No. 621-4AM-83-
0061-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986c) Study
of the biliary excretion of radioactivity following oral
administration of (14C-SDS-2787) to male Sprague-Dawley rats. Mentor,
Ohio, Fermenta ASC (Unpublished report No. 633-4AM-85-0012-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986d) Animal
metabolism method development studies. II. In vitro incubations of
14C-chlorothalonil with stomach and intestinal mucosal cells. Mentor,
Ohio, Fermenta ASC (Unpublished report No. 1172-85-0081-AM-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986e) In
vitro studies on the transfer of 14C-chlorothalonil and/or its
metabolites from the mucosal to the serosal surface of the
gastrointestinal tract. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1179-86-0020-AM-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986f) Pilot
study to determine the concentration of radiolabel in kidneys
following oral administration of the mono-glutathione conjugate of
14C-chlorothalonil to male rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 631-4AM-85-0064-001).
Savides MC, Marciniszyn JP, Killeen JC, & Baxter RA (1987a) Study to
determine the metabolic pathway for chlorothalonil following dermal
application to rats. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1625-87-0057-AM-000).
Savides MC, Marciniszyn JP, Killeen JC, & Baxter RA (1987b)
Determination of the covalent binding of radiolabel to DNA in the
kidneys of male rats administered 14C-chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1173-86-0096-AM-002).
Savides MC, Kidon BJ, Marciniszyn JP, Killeen JC, & Baxter RA (1987c)
Subcellular fractionation of kidneys from male rats administered
14C-chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1178-86-0016-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1988) A study to evaluate
the effects of sulphur-containing analogs of chlorothalonil on
mitochondrial function. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1479-87-0037-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1989) Study to compare the
metabolism of chlorothalonil in dogs with its metabolism in rats
following oral administration of 14C-chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1626-88-0008-AM-001).
Savides MC, Magee TA, Marciniszyn JP, Killeen JC, & Baxter RA (1990a)
Study to evaluate the metabolic pathway of chlorothalonil
(14C-ASC-2787) in germ-free rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 3060-88-0219-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1990b) Study of the urinary
excretion of radiolabel by catheterised dogs following oral
administration of 14C-chlorothalonil by gavage. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 3086-89-0041-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1990c) Study to evaluate
urinary metabolites of chlorothalonil from male Rhesus monkeys.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 3349-89-0179-AM-
001).
SDS Biotech Corporation (1972) Exposure of fish to 14C-labelled
DAC-2787-Tech.: Accumulation, distribution and elimination of
residues. Mentor, Ohio, ISK-Biotech (Proprietary report No. 000-5TX-
72-0006/8-001).
SDS Biotech Corporation (1979) Acute toxicity of DS-3701 to Bluegill
sunfish. Mentor, Ohio, ISK-Biotech (Proprietary report No. 102-5TX-79-
0044-002).
SDS Biotech Corporation (1980a) Technical chlorothalonil - Acute
toxicity (LC50) study in Rainbow trout. Mentor, Ohio, ISK-Biotech
(Proprietary report No. 102-5TX-79-0045-002).
SDS Biotech Corporation (1980b) Technical chlorothalonil - Acute
toxicity (LC50) study in Channel catfish. Mentor, Ohio, ISK-Biotech
(Proprietary report No. 102-5TX-79-0046-002).
SDS Biotech Corporation (1981a) Dietary study (LC50) in Mallard ducks
with DS-3701. Mentor, Ohio, ISK-Biotech (Proprietary report No.
449-5TX-81-0008-002).
SDS Biotech Corporation (1981b) Dietary study (LC50) in Bobwhite
quail with DS-3701. Mentor, Ohio, ISK-Biotech (Proprietary report
No. 448-5TX-81-0007-002).
SDS Biotech Corporation (1982a) Static, acute toxicity study in
Penaeid (pink) shrimp with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0054-002).
SDS Biotech Corporation (1982b) Static, acute toxicity study in
Sheepshead Minnows with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0053-002).
SDS Biotech Corporation (1983a) The effects of cooking 2,4,5,6-
tetrachloroisoph-thalonitrile (chlorothalonil, DS-2787) with
vegetables. Mentor, Ohio, ISK-Biotech (Unpublished report No.
372-3EF-83-0004-001, submitted to WHO by SDS Biotech Corporation,
Mentor, USA).
SDS Biotech Corporation (1983b) Flow-through, acute oyster shell
deposition study with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0133-003).
Shirasu Y & Teramoto S (1975) Teratogenicity study of Daconil in
rabbits. Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-
75-2077-001).
Shirasu Y, Moriya M, & Watanabe K (1977) Mutagenicity testing on
Daconil in microbial systems. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 00-5TX-61-0002-001).
Shults SK, Killeen JC, & Heilman RD (1980) A chronic study in the
fathead minnow (Pimephales promelas) with technical chlorothalonil.
Cleveland, Ohio, Diamond Shamrock Corporation, 16 pp (Proprietary
report No. 090-5TX-79-0049-002, submitted to WHO by ISK-Biotech,
Mentor, USA.
Shults SK, Killeen JC, & Ignatoski JA (1982) Chronic toxicity study in
Daphnia magna with technical chlorothalonil. Cleveland, Ohio,
Diamond Shamrock Corporation, 25 pp (Proprietary report No.
447-5TX-81-0006-002, submitted to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Laveglia J, Killeen JC, & Ignatoski JA (1983) A 90-day
feeding study in mice with technical chlorothalonil. Mentor, Ohio, SDS
Biotech Corporation (Unpublished report No. 618-5TX-83-0007-004).
Shults SK, Wilson NH, Killeen JC, & Ignatoski JA (1986) 21-Day
repeated dose dermal toxicity study in albino rabbits with technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report No.
754-5TX-85-0023-007).
Shults SK, Wilson NH, & Killeen JC (1988a) Reproduction study in
bobwhite quail with technical chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 1469-87-0006-TX-002, submitted
to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Wilson NH, & Killeen JC (1988b) Reproduction study in
mallard ducks with technical chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 1469-87-0004-TX-002, submitted
to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Brock AW, & Killeen JC (1991) Acute (one-hour) inhalation
toxicity (LC50) study in rats with hammer milled technical
chlorothalonil. Painesville, Ohio, Ricerca, Inc. (Proprietary report
No. 3593-90-0168-TX-002, submitted to WHO by ISK-Biotech, Mentor,
USA).
Shults SK, Brock AW, & Laveglia J (1993) Acute (four-hour) inhalation
toxicity (LC50) study in rats with hammer milled technical
chlorothalonil (T-117-15). Painesville, Ohio, Ricerca, Inc.
(Proprietary report No. 5290-92-0160-TX-002, submitted to WHO by
ISK-Biotech, Mentor, USA).
Siou G (1981a) The micronucleus test in the rat, mouse and hamster
using chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 000-5TX-81-0024-004).
Siou G (1981b) The chromosomal aberration test in the rat, mouse and
hamster using chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 000-5TX-81-0025-004).
Smiley RW & Craven MM (1979) Microflora of turfgrass treated with
fungicides. Soil Biol Biochem, 11: 349-353.
Spencer JR, Bissell SR, Sanborn JR, Schneider FA, Margetich SS, &
Krieger RI (1991) Chlorothalonil exposure of workers on mechanical
tomato harvesters. Toxicol Lett, 55: 99-107.
Spindeldreier A & Deichmann B (1980) [Contact dermatitis against a
wood preservative with a new fungicidal agent.] Dermatosen, 28(3):
88-90 (in German).
Stallard DE & Wolfe AL (1967) The fate of 2,4,5,6-
tetrachloroisophthalonitrile (Daconil 2787) in soil. Mentor, Ohio,
Diamond Shamrock Corporation (Unpublished report).
Stallard DE & Szalkowski ME (1976) Effect of microorganisms upon the
soil metabolism of Daconil (i.e., chlorothalonil) and 4-hydroxy-2,5,6-
trichloroisophthalonitrile. Painesville, Ohio, Ricerca, Inc. (Report
No. 1000-3EF-76-2087-001, submitted to WHO by ISK Biosciences
Corporation, Mentor, USA).
Stamper JH, Nigg HN, Mahon WD, Nielsen AP, & Royer MD (1989a)
Applicator exposure to fluvalinate, chlorpyrifos, captan, and
chlorothalonil in Florida ornamentals. J Agric Food Chem, 37: 240-244.
Stamper JH, Nigg HN, Mahon WD, Nielsen AP, & Royer MD (1989b)
Pesticide exposure to a greenhouse drencher. Bull Environ Contam
Toxicol, 42: 209-217.
Stephenson GR, Phatak SC, Makowski RI, & Bouw WJ (1980) Phytotoxic
interactions involving metribuzin and other pesticides in tomatoes.
Can J Plant Sci, 60: 167-175.
Stoffella PJ & Sonoda RM (1982) Reduction of onion yields by
chlorothalonil. Hortscience, 17(4): 628-629.
Suzuki S & Oda M (1977) Simultaneous determination of chlorothalonil,
captan and diazinon in apples. Bull Agric Chem Insp Stn (Tokyo),
17:43-45.
Szalkowski MB & Stallard DE (1977) Effect of pH on the hydrolysis of
chlorothalonil. J Agric Food Chem, 25(1): 208-210.
Szalkowski MB, Stallard DE, & Ignatoski JA (1980) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon the
microbial utilization of selected macromolecules in soil. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 326-3EI-79-0009-001).
Szalkowski MB, Stallard DE, & Ignatoski JA (1981a) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon
non-symbiotic nitrogen fixing soil microorganisms. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 327-3EI-79-0010-001).
Szalkowski MB, Stallard DE, & Ignatoski JA (1981b) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon nitrogen
transformation in soil. Mentor, Ohio, ISK-Biotech (Proprietary report
No. 328-3EI-79-0011-001).
Teeters W (1966) In vivo assay of spasmodic activity, mice, DAC
2787. Hazelton Laboratories (Unpublished report submitted to WHO by
Diamond Shamrock Corporation, Mentor, USA).
Tillman RW, Siegel MR, & Long JW (1973) Mechanism of action and fate
of the fungicide chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile)
in biological systems I. Reactions with cells and subcellular
components of Saccharomyces pastorianus. Pestic Biochem Physiol,
3:160-167.
Tu CM (1980) Effect of fungicides on growth of Rhizobium japonicum
in vitro. Bull Environ Contam Toxicol, 25: 364-368.
UK (1985) Annex 1 - Monitoring of fruits and vegetables (at point of
retail sale) 1981-1983. Annex 2 - Chlorothalonil, soft fruit sampled
directly from growers 1982; soft fruit sampled at retail outlets, 1982
(no treatment histories). Submission to FAO by the UK Ministry of
Agriculture, June 14, 1985.
UK-MAFF & HSE (1990) Report of the working party on pesticide
residues 1988-1989. London, Ministry of Agriculture, Fisheries and
Food and Health and Safety Executive.
US EPA (1976) Manual of chemical methods for pesticides and devices.
Washington DC, US environmental Protection Agency, Office of Pesticide
Programs (Chlorothalonil EPA-1).
US EPA (1990) National survey of pesticides in drinking water wells.
Washington, DC, US Environmental Protection Agency (EPA 570/9-90-015).
US NCI (1978) Bioassay of chlorothalonil for possible carcinogenicity.
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
78-841).
Walker WW, Cripe CR, Pritchard PH, & Bourquin AW (1984) Biological and
abiotic degradation of xenobiotic compounds in in vitro estuarine
water and sediment/water systems. Chemosphere, 17(12): 2255-2270.
Wallace LA (1991) Comparison of risks from outdoor and indoor exposure
to toxic chemicals. Environ Health Perspect, 95: 7-13.
Wazeter FX (1971) Acute oral LD50 in male albino rats. International
Research and Development Corporation (Unpublished report submitted to
WHO by Diamond Shamrock Corporation, Mentor, USA).
Wazeter FX & Goldenthal EI (1976) Teratology study in rabbits.
International Research and Development Corporation (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, Ohio, USA).
Wei CI (1982) Lack of mutagenicity of the fungicide 2,4,5,6-
tetrachloroisophthalonitrile in the Ames Salmonella microsome test.
Appl Environ Microbiol, 43(1): 252-254.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997. Geneva, World Health
Organization. (WHO/PCS/96.3).
Wilson NH (1981) A 90-day toxicity study of technical chlorothalonil
in rats. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Wilson NH, Killeen JC, & Ignatoski JA (1982) Dermal sensitization
study in Hartley-derived guinea-pigs with technical chlorothalonil.
Painesville, Ohio, Ricerca, Inc. (Report No. 394-5TX-81-0132-002,
submitted to WHO by ISK Biosciences Corporation, Mentor, USA).
Wilson NH, Laveglia J, Killeen JC, & Ignatoski JA (1983a) A subchronic
toxicity study of technical chlorothalonil in rats. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 562-5TX-81-0213-004).
Wilson NH, Killeen JC, & Ignatoski JA (1983b) A chronic dietary study
in mice with technical chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 108-5TX-79-0102-004).
Wilson NH, Killeen JC, & Ignatoski JA (1985a) Histopathological
re-evaluation of renal tissue from a sub-chronic toxicity study of
technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 753-5TX-85-0056-002).
Wilson NH, Killeen JC, & Ignatoski JA (1985b) A tumorigenicity study
of technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 099-5TX-80-0234-008).
Wilson NH, Killeen JC, & Ignatoski JA (1985c) Histopathologic
re-evaluation of renal tissue from a 90-day toxicity study of
technical chlorothalonil in rats (5TX-80-0200). Mentor, Ohio, SDS
Biotech (Unpublished report No. 753-5TX-85-0055-002).
Wilson NH, Killeen JC, & Ignatoski JA (1986a) Histopathological
re-evaluation of renal tissue from a rat tumorigenicity study with
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report No.
764-5TX-85-0071-002).
Wilson NH, Killeen JC, & Ignatoski JA (1986b) Histopathological
re-evaluation on renal tissue from a mouse tumorigenicity study with
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report).
Wilson NH, Killeen JC, & Baxter RA (1987) A tumorigenicity study of
technical chlorothalonil in male mice - final report. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1099-84-0077-TX-006).
Wilson NH, Killeen JC, & Baxter RA (1988) A teratology study in
rabbits with technical chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1544-87-0060-TX-002).
Wilson NH, Killeen JC, & Baxter RA (1989a) A tumorigenicity study of
technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1102-84-0103-TX-007).
Wilson NH, Chun JS, Killeen JC, & Baxter RA (1989b) Reproduction
dose-range finding study in rats with technical chlorothalonil.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 1722-87-0120-TX-
001).
Wilson NH, Killeen JC Jr, Ford WH, Siou G, Busey WM, & Eilrich GL
(1990) A 90-day study in rats with the monoglutathione conjugate of
chlorothalonil. Toxicol Lett, 53: 155-156.
Wolfe AL & Stallard DE (1968) The fate of DAC-3701 (4-hydroxy-2,5,6,-
trichloroisophthalonitrile) in soil. Mentor, Ohio, Diamond Shamrock
Corporation (Unpublished report).
Wolfe AL & Stallard DE (1971) Supplementary milk and meat study.
Mentor, Ohio, Diamond Shamrock Corporation (Unpublished report).
Worthing CR ed. (1991) The pesticide manual: A world compendium, 9th
ed. Farnham, British Crop Protection Council.
Wüthrich V (1990) Acute-toxicity (LC50) study of Daconil 2787 extra
to earthworms (Project No. 282971). Itingen, Switzerland, RCC
Umweltchemie A.G. (Proprietary report submitted to WHO by ISK Biotech,
Mentor, USA).
Yoshikawa H & Kawai K (1966) Toxicity of phthalonitrile and
tetrachlorophthalodinitrile: I. Acute toxicity in mice. Ind Health,
4:11-15.
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le chlorothalonil est un solide cristallin incolore et inodore,
dont le point de fusion est de 250°C et la tension de vapeur de 7,63 ×
10-5 Pa (5,72 × 10-7 mmHg) à 25°C. Il est peu soluble dans l'eau
(0,6-1,2 mg/litre à 25°C) et son coefficient de partage entre
l'octanol et l'eau (log Kow) est de 2,882. Dans l'eau, il
s'hydrolyse lentement à pH 9 mais il est stable à pH < 7 (à 25°C).
La méthode d'analyse la plus courante, après extraction et
purification, est la chromatographie gaz-liquide avec détection par
capture d'électrons.
2. Sources d'exposition humaine et environnementale
Le chlorothalonil est produit depuis 1969 à des fins
commerciales, soit par chloration de l'isophtalonitrile, soit en
traitant le tétrachlorisophtaloylamide par l'oxychlorure de phosphore.
C'est un fongicide à large spectre utilisé non seulement en
agriculture, mais aussi pour traiter le gazon et les plantes
ornementales. On l'utilise pour protéger les fruits à pépins et à
noyaux, les agrumes, les groseilles, les baies, les bananes, les
tomates, les légumes verts, le café, les arachides, les pommes de
terre, les oignons et les céréales. En outre, il entre dans la
composition de certains produits pour la conservation du bois et de
certaines peintures.
Il existe en trois formulations principales, un concentré pour
suspensions, des granulés dispersables dans l'eau et une poudre
mouillable.Ces produits sont facilement dilués dans l'eau et épandus
par pulvérisations au sol ou aériennes. La dose d'emploi est
habituellement de 1,2 à 2,5 kg de matière active par hectare pour le
traitement des haricots, des céleris et des oignons. L'exposition
humaine a lieu principalement pendant la préparation et l'épandage du
produit ou lors de l'ingestion de résidus présents dans certaines
denrées alimentaires (voir section 1.1.4).
3. Transport, distribution et transformation dans l'environnement
Le chlorothalonil s'élimine des milieux aqueux par une forte
adsorption sur les particules en suspension. La modélisation des
données disponibles montre qu'il ne migre pratiquement pas vers les
sédiments du fond. Il est possible qu'il subisse une biodégradation
enzymatique dans les eaux naturelles. Dans le sol, il est rapidement
dégradé, cette dégradation pouvant également avoir lieu dans l'eau
avec production du métabolite hydroxylé en position 4, c'est-à-dire le
4-hydroxy-2,5,6-trichlorisophtalonitrile. La demi-vie de dissipation
du métabolite 4-hydroxy dans le sol est comprise entre 6 et 43 jours.
Une fois entré en contact avec une plante, le chlorothalonil ne
migre pas vers d'autres zones du végétal. Les végétaux ne le
métabolisent que dans une proportion limitée et le métabolite
4-hydroxy constitue en général moins de 5% du résidu.
Chez les poissons, la métabolisation du chlorothalonil comporte
une conjugaison avec le glutathion, qui aboutit à des produits
d'excrétion plus polaires. Elle s'effectue sous l'action de la
glutathion- S-transférase. L'excrétion du composé sous forme de
conjugué avec le glutathion est corroborée par le fait que, chez la
truite arc-en-ciel, on trouve une forte concentration de marqueur dans
la vésicule biliaire et dans la bile après exposition à du
14C-chlorothalonil.Une fois les poissons replacés en eau propre, on a
constaté une chute rapide de la concentration du marqueur qui s'était
accumulé dans la vésicule biliaire et les autres organes.
Le chlorothalonil ne s'accumule pas chez les organismes
aquatiques.
4. Concentrations dans l'environnement et exposition humaine
Lors d'une étude sur des cultures de pommes de terre, on a
pulvérisé du chlorothalonil sur un petit cours d'eau. Après
prélèvement et analyse de l'eau en aval du secteur traité, on a
constaté que le composé disparaissait rapidement (par ex. la
concentration passait de 450 µg/litre 30 minutes après le traitement à
2-6 µg/litre 12 h après le traitement). Les traitements de routine
effectués sur des cultures irriguées de plein champ, comme les pommes
de terre ou l'orge, n'ont donné lieu qu'à de faibles concentrations de
chlorothalonil (0,04-3,6 µg/litre), comme l'ont montré un certain
nombre d'analyses pratiquées sur de l'eau prélevée à quelques
occcasions dans des drains en grès vernissé.
Les résidus qui subsistent sur les récoltes sont essentiellement
constitués par le chlorothalonil lui-même. Leur concentration est
fonction de la dose d'emploi, du temps écoulé depuis le dernier
épandage et la dernière récolte ainsi que du type de culture. A
partir des nombreux essais effectués sous contrôle un peu partout dans
le monde et dont les résultats ont été communiqués à la FAO et à
l'OMS, il est possible de déterminer les concentrations de résidus
présentes au moment de la récolte. En ce qui concerne les produits
laitiers, il est vraisemblable que les résidus sont soit
indétectables, soit très faibles. Dans le lait de vaches laitières
qui avaient reçu pendant 30 jours du chlorothalonil mêlé à leur
nourriture (jusqu'à 250 mg/kg), on n'a pas trouvé trace du composé,
mais celui-ci était présent dans les tissus à très faible
concentration.
Des analyses pratiquées dans plusieurs pays, soit sur la ration
totale, soit sur tel ou tel alliment, ont montré, à l'occasions
d'enquêtes par sondage, que le chlorothalonil n'était présent qu'en
quantités indétectables ou du moins très faibles. Les diverses
préparations que subissent les produits alimentaires, comme le pelage,
le lavage et la cuisine en général contribuent d'ailleurs à abaisser
encore leur teneur en résidus.
5. Cinétique et métabolisme chez les animaux de laboratoire
Chez des rats qui en recevaient par voie orale des doses allant
jusqu'à 50 mg/kg de poids corporel, le composé a été absorbé à hauteur
d'environ 30% en l'espace de 48 h. A plus forte dose, l'absorption
est moindre, ce qui est le signe d'un processus de saturation. Après
administration de 14C-chlorothalonil par voie orale, on a observé une
répartition tissulaire et sanguine de la radioactivité en 2 h. C'est
au niveau des reins, du foie et du sang - dans cet ordre - qu'ont été
relevées les concentrations les plus imporantes. Au bout de 24 h, on
a mesuré, au niveau des reins, une concentration égale à 0.3% d'une
dose initiale de 5 mg/kg de poids corporel.
La majeure partie d'une dose administrée par voie orale à des
rats a été retrouvée dans leurs matières fécales (> 82% en l'espace
de 48-72 h, quelle que soit la dose initiale). L'excrétion biliaire
est rapide, culminant au bout de 2 h après ingestion d'une dose de 5
mg/kg de poids corporel et la saturation est atteinte à partir de 50
mg/kg de poids corporel. Chez le rat, la dose est excrétée à hauteur
de 5-10% par la voie urinaire. Chez le chien et le singe, la
prncipale voie d'excrétion est la voie fécale, la voie urinaire étant
moins importante que chez le rat (< 4%).
Les études métaboliques menées sur des rats montrent que le
chlorothalonil est conjugué avec le glutathion dans le foie ainsi que
dans les voies digestives. Certains de ces conjugués peuvent être
absorbés dans l'intestin et parvenir jusqu'aux reins où ils sont
transformés par la ß-lyase du cytosol en analogues thioliques,
excrétés ensuite par la voie urinaire. Lorsqu'on administre du
chlorothalonil à des rats axéniques, les métabolites thioliques
apparaissent dans l'urine en quantité bien moindre que chez des rats
normaux, ce qui indique que la flore intestinale intervient dans le
métabolisme de ce composé. Des chiens et des singes à qui on
administre du chlorothalonil par voie orale, n'excrètent que peu ou
pas de métabolites thioliques dans leurs urines.
Après application cutanée de 14C-chlorothalonil à des rats,
environ 28% de la dose ont été absorbés en 120 h. On a retrouvé
environ 18% de la dose dans les matières fécales et 6% dans les urines
au bout de 120 h.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Chez le rat et le lapin, la toxicité aiguë du chlorothalonil est
faible, que ce soit par voie orale ou en applications cutanée (DL50
> 10 000 mg/kg de poids corporel). Du chlorothalonil broyé au
mortier (D médian des particules égal à 5-8 µm) s'est révélé très
toxique pour des rats lors d'une étude toxicologique par inhalation,
avec une CL50 à 4 h de 0,1 mg/litre.
Le chlorothalonil est irritant pour la peau et les yeux chez le
lapin. Les études de sensibilisation cutanée effectuées sur des
cobayes n'ont pas été concluantes.
Chez le rat, les principaux effets de doses orales répétées
s'exercent au niveau des reins et de l'estomac.Pendant 13 semaines, on
fait ingérer quotidiennement à des rats, répartis en groupes de 25
animaux de chaque sexe, du chlorothalonil mêlé à leur nourriture aux
doses de 0, 1,5, 3, 10, ou 40 mg/kg de poids corporel. Après une
période de récupération de 13 semaines, les rats ont été sacrifiés et
l'on a observé une hyperplasie et une hyperkératose au niveau de la
portion cardiaque de l'estomac aux doses de 10 et 40 mg/kg; ces
lésions ont regressé lorsque le traitement a cessé. A la dose de 40
mg/kg, on notait chez les mâles une augmentation de l'incidence des
hyperplasies épithéliales au niveau des tubules proximaux du rein au
bout des 13 semaines de traitement ainsi qu'après la période de
récupération. La dose sans effet observable a été évaluée à 3 mg/kg
de poids corporel par jour, le critère retenu étant l'absence de
lésions au niveau de la portion cardiaque de l'estomac. Les lésions
interessant cette partie de l'eatomac ainsi que les reins sont
apparues rapidement, à savoir en 4 à 7 jours chez les mâles lorsqu'on
a porté la dose alimentaire quotidienne à 175 mg/kg de poids corporel.
Lors d'une étude de 13 semaines sur des souris (0, 7,5, 15, 50,
275, ou 750 mg/kg en mélange à la nourriture), on a constaté une
incidence accrue des hyperplasies et des hyperkératoses de
l'épithélium pavimenteux au niveau de la portion cardiaque de
l'estomac. Ces lésions ont été observées chez les mâles comme chez
les femelles à partir de 50 mg/kg de nourriture. En se basant sur la
présence ou l'absence de ces lésions, on a évalué à 15 mg/kg de
nourriture la dose de chlorothalonil sans effet observable, soit
l'équivalent quotidien de 3 mg/kg de poids corporel.
Une étude de 16 semaines sur des chiens aux doses alimentaires de
0, 250, 500, ou 750 mg/kg n'a pas révélé de d'effets qui soient
imputables au traitement.
Les lésions observées au niveau des reins et de la portion
cardiaque de l'estomac ont fait l'objet, pendant 2 ans, d'études plus
approfondies sur des souris et des chiens. Une autre étude, portant
cette fois sur des rats (aux doses quotidiennes de 0, 1,8, 3,8, 15 ou
175 mg/kg de poids corporel), a permis de caractériser histo-
logiquement les effets observés: il s'agissait d'une part, d'une
augmentation de l'incidence des hyperplasies, des hyperkératoses, des
ulcérations et des abrasions de l'épithélium pavimenteux au niveau de
la portion cardiaque de l'estomac et, d'autre part, de la présence
d'une hyperplasie au niveau des tubules contournés proximaux du rein.
Ces anomalies ont été observées à partir de 3,8 mg/kg. A la dose de
175 mg/kg, on notait un accroisssement sensible de l'incidence des
tumeurs rénales (adénomes et carcinomes) et des tumeurs interessant la
portion cardiaque de l'estomac (papillomes et carcinomes). On est
fondé à penser que l'incidence des tumeurs rénales était augmentée
chez les mâles à partir de la dose de 15 mg/kg, de même que celle des
tumeurs gastriques chez les deux sexes aux doses de 3,8 mg/kg et de 15
mg/kg. La dose sans effets néoplasiques observables a donc été prise
égale à 1,8 mg/kg, en prenant comme critère l' incidence des tumeurs
au niveau de la portion cardiaque de l'estomac. Une autre étude de 2
ans, au cours de laquelle des doses plus élevées ont été utilisées, a
confirmé le pouvoir cancérogène du chlorothalonil, tant au niveau du
rein que de la portion cardiaque de l'estomac.
Une étude sur des souris (doses de 0, 15, 40, 175, ou 750 mg/kg
de nourriture) a révélé une augmentation de l'incidence des
hyperplasies au niveau des tubules rénaux à partir de 175 mg/kg, le
même phénomène étant observé à partir de 40 mg/kg au niveau de la
portion cardiaque de l'estomac, avec en outre une hyperkératose. A la
dose de 750 mg/kg, il y avait une légère augmentation des tumeurs
spinocellulaires au niveau de la portion cardiaque de l'estomac. On
en a donc conclu que les doses sans effets néoplasiques ou non
néoplasiques étaient respectivement égales à 175 et 15 mg/kg de
nourriture (soit l' équivalent quotidien de 17,5 et 1,6 mg/kg de poids
corporel, respectivement). Ces effets constatés chez la souris sont
corroborés par les résultats d'une autre étude avec des doses plus
élevées, mais une troisième investigation portant sur des souris
B6C3F1 n'a pas mis en évidence d'effets cancérogènes à dose élevée.
Lors d'une étude de 2 ans sur des chiens (60 et 120 mg/kg de
nourriture), aucun effet attribuable au chlorothalonil n'a été
observé. On en conclu que la dose sans effet observable était de 120
mg/kg de nourriture (soit l'équivalent quotidien de 3 mg/kg de poids
corporel).
Plusieurs épreuves de mutagénicité in vivo et in vitro se
sont révélées négatives, mais il y en a tout de même eu quelques unes
de positives.
Les dérivés monothio, dithio, trithio, dicystéinyl, tricystéinyl
et monoglutathionyl du chlorothalonil, qui sont potentiellement
néphrotoxiques, se sont révélés négatifs dans l'épreuve d'Ames.
Le chlorothalonil ne s'est pas montré tératogène pour le rat ou
le lapin à des doses quotidiennes atteignant respectivement 400 et 50
mg/kg de poids corporel. Lors d'une étude sur deux générations de
rats, on n'a pas constaté d'effets sur l'accouplement, la fécondité ou
la gestation jusqu'à des doses atteignant 1500 mg/kg de nourriture.
La toxicité aiguë par voie orale du métabolite 4-hydroxy est
supérieure à celle du chlorothalonil lui-même (DL50 aiguë par voie
orale égale à 332 mg/kg de poids corporel contre > 10 000 mg/kg de
poids corporel). Plusieurs études ont été entreprises pour
caractériser le profil toxicologique de ce métabolite et établir les
doses sans effets observables.
7. Effets sur l'homme
On a signalé des cas de dermatite de contact parmi des personnes
employées à la fabrication de chlorothalonil, chez des agriculteurs et
des horticulteurs. D'autres cas de dermatite siégeant au niveau des
mains et de la face ont été observés chez des personnes qui
utilisaient des produits de protection du bois à base de
chlorothalonil.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Le chlorothalonil est extrêmement toxique pour les poissons et
les invertébrés aquatiques, comme le montrent un certain nombre
d'études en laboratoire, avec des valeurs de la CL50 inférieures à
0,5 mg/litre. La concentration maximale acceptable de produit toxique
(MATC) s'est révélée être égale à 35 µg/litre lors d'une étude sur
deux générations de daphnies.
A quelques exceptions près, d'ailleurs sans grande importance, le
chlorothalonil n'est pas phytotoxique.
La CL50 d'un concentré pour suspension (500 g de chlorothalonil
par litre) répandu sur un sol artificiel pour lombrics, a été évaluée
à > 1000 mg/kg de terre (14 jours). On a observé une surmortalité
parmi des scolopendres qui s'étaient trouvés en contact avec des
résidus de chlorothalonil présents sur des feuilles d'arachide ou s'en
étaient nourris au laboratoire; il n'y a pas eu d'autre indice d'une
action insecticide.
Le chlorothalonil est peu toxique pour les oiseaux, comme le
montre la valeur de la DL50 aiguë par voie orale chez le colvert
(4640 mg/kg). Aucun effet important sur la reproduction n'a été
signalé.
D'après une étude effectuée sur le terrain, la toxicité du
chlorothalonil pour les organismes aquatiques est moindre que ne le
font craindre les expériences de laboratoire; ce résultat est en
accord avec les propriétés physico-chimiques de ce composé. On a tout
de même enregistré une mortalité chez des espèces exposées
expérimentalement sur le terrain. En revanche, on n'a pas signalé
d'accidents écologiques ayant entraîné une mortalité. Malgré la
faible persistance du chlorothalonil dans les divers compartiments du
milieu, il faut tout de même s'attendre à une certaine mortalité. En
pareil cas, il sera difficile d'établir un lien de cause à effet étant
donné que les résidus de chlorothalonil ne subsistent pas suffisamment
lontemps pour que l'on puisse identifier le composé.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El clorotalonilo es un sólido cristalino inodoro e incoloro con
un punto de fusión de 250°C y una presión de vapor de 7,63 × 105 Pa
(5,72 × 10-7 mmHg) a 25°C. Es poco soluble en agua (0,6 a 1,2 mg/litro
a 25°C) y tiene un coeficiente de partición octanol/agua (log
Koa) de 2,882. Se hidroliza lentamente en agua con un pH de 9, pero
es estable a un pH de 7 o inferior (a 25°C).
El método analítico más corriente, después de la extracción y
depuración de las muestras, es la cromatografía gas-liquido empleando
un detector de captura de electrones.
2. Fuentes de exposición del ser humano y del medio ambiente
El clorotalonilo se viene produciendo a escala comercial desde
1969 por cloración del isoftalonitrilo o mediante el tratamiento de la
amida tetracloroisoftalolil con oxicloruro de fósforo. Es un fungicida
con amplio espectro de actividad empleado principalmente en la
agricultura pero también en el césped, los pastos y las plantas
ornamentales. Los cultivos protegidos incluyen frutas de pepitas y de
hueso, cítricos, grosellas, fresas, bananas, tomates, verduras, café,
cacahuete, patatas, cebollas y cereales. Se emplea también en
sustancias protectoras de la madera y en pinturas.
Las tres formulaciones principales son una suspensión
concentrada, un gránulo hidrodispersible y un polvo humectable. Se
disuelven fácilmente en agua y se aplican empleando sistemas de
pulverización en los suelos o rociado aéreo. Las tasas típicas del
ingrediente activo oscilan entre 1,2 y 2,5 kg/ha en el caso de
cultivos tales como frijoles, apio y cebollas. Las principales fuentes
de exposición del ser humano son la preparación y aplicación de los
productos, así como la ingestión de residuos de las cosechas en los
alimentos (véase la sección 1.1.4).
3. Transporte, distribución y transformación en el medio ambiente
El clorotalonilo se elimina de los medios acuosos mediante
intensa adsorción en las materias en suspensión. Los datos de los
modelos parecen indicar poca, o ninguna, partición detectable en el
sedimento del fondo. Puede producirse biodegradación en aguas
naturales, con la participación de procesos enzimáticos. El
clorotalonilo se degrada rápidamente en el suelo, y puede haber
degradación en el agua con la producción del metabolito 4-hidroxi-
2,5,6-tricloroisoftalonitrilo. La semivida para la disipación en el
suelo de ese metabolito varía entre 6 y 43 días.
El clorotalonilo no se transloca del punto de aplicación a otras
partes de la planta. Su metabolización en las plantas es limitada y,
por o general, ese metabolito representa < 5% del residuo.
En cuanto a los peces, el clorotalonilo se metaboliza mediante la
conjugación con glutatión para producir productos de degradación más
polares. En esa conversión interviene la enzima glutatión-
S-transferasa. Las elevadas concentraciones del radioisótopo
marcador detectadas en la vesícula biliar y la bilis después de la
exposición de la trucha arco iris al 14C-clorotalonilo son
compatibles con la excreción del compuesto en forma de conjugados de
glutatión. Las concentraciones de los radioisótopos de trazado que se
acumulan en la vesícula biliar y otros órganos disminuyen rápidamente
al colocar los peces en agua no contaminada.
El clorotalonilo no experimenta bioacumulación en los organismos
acuáticos.
4. Niveles ambientales y exposición humana
En un estudio de un cultivo de patatas, se procedió a rociar un
pequeño arroyo con clorotalonilo. Muestreos y análisis posteriores del
agua río abajo demostraron la rápida desaparición del clorotalonilo
(las concentraciones eran de 450 µg/litro a los 30 minutos después del
rociado, y oscilaban entre 2 y 6 µg/litro a las 12 h después del
rociado). El rociado sistemático de los cultivos irrigados como, por
ejemplo, patatas y cebada, estuvo acompañado de bajas concentraciones
de clorotalonilo (0,04 a 3,6 µg/litro) en el agua de los tubos de
drenaje en un pequeño número de muestras.
Los residuos de las cosechas están compuestos principalmente de
clorotalonilo propiamente dicho. Las concentraciones residuales
dependen del nivel aplicado, del tiempo transcurrido entre la última
aplicación y la cosecha, y del tipo de cosecha. Los niveles residuales
en la cosecha pueden deducirse de los numerosos ensayos supervisados
realizados con muchas cosechas en todo el mundo y comunicados a la FAO
y la OMS. Se prevé que los residuos de clorotalonilo en los productos
lácteos serán imposibles de detectar, o las concentraciones muy bajas.
En un estudio con vacas lecheras alimentadas durante 30 días con
pienso al que se habían añadido elevadas concentraciones (hasta 250
mg/kg) de clorotalonilo no se observó ningún residuo detectable en la
leche y sólo niveles muy bajos en los tejidos.
Los análisis del régimen alimenticio total y de alimentos
específicos realizados en varios países han revelado concentraciones
no detectables, o muy bajas, de clorotalonilo en los estudios por
muestreo. Los procesos de preparación tales como el lavado, el pelado
y la cocción permiten reducir aún más los niveles residuales en los
alimentos.
5. Cinética y metabolismo en animales de laboratorio
En estudios con ratas a las que se administraron dosis de hasta
50 mg/kg de peso corporal, se observó que un 30% de la dosis oral de
clorotalonilo se absorbía dentro de las 48 horas. La absorción es
inferior con posologías más elevadas, lo que indica un proceso de
saturación. Cuando se administra oralmente 14C-clorotalonilo, la
radioactividad se distribuye en la sangre y los tejidos en menos de
dos horas. Las mayores concentraciones se encuentran en el riñón, el
hígado y la sangre, en ese orden. Con una posología de 5 mg/kg de peso
corporal, la concentración en los riñones es 0,3% a las 24 horas.
Por lo que respecta a las ratas, la mayor parte de la dosis oral
de clorotalonilo se encuentra en las heces (> 82% dentro de las 48 a
72 horas, independientemente de la dosis). Con una dosis oral de 5
mg/kg de peso corporal, la excreción biliar es rápida, alcanzando su
valor máximo dentro de las 2 horas, ocurriendo saturación con
posologías de 50 mg/kg de peso corporal y superiores. En el caso de
las ratas, la excreción urinaria representa entre un 5 y 10% de la
dosis. En perros y monos, la excreción fecal es la vía principal, pero
la excreción urinaria (< 4%) es inferior a la observada en las ratas.
Estudios metabólicos llevados a cabo con ratas indican que el
clorotalonilo se conjuga con el glutatión tanto en el hígado como en
el tracto gastrointestinal. Algunos de los conjugados del glutatión
pueden ser absorbidos en el intestino y transportados a los riñones
donde son convertidos por la ß-liasa citosólica en análogos del tiol
que se excretan por la orina. Cuando se dan dosis de clorotalonilo a
ratas axénicas, se observan en la orina metabolitos del tiol en
cantidades muy inferiores a las registradas en ratas normales, lo que
indica que la microflora intestinal interviene en el metabolismo del
clorotalonilo. En el caso de los perros o monos que reciben dosis
orales de clorotalonilo, la excreción de derivados del tiol por la
orina no es detectable, o es muy baja.
Cuando se aplicó 14C-clorotalonilo a la piel de la rata, un 28%
de la dosis fue absorbida en menos de 120 horas. Se observaron
concentraciones del 18% de la dosis en las heces y del 6% en la orina
al cabo de 120 horas.
6. Efectos en mamíferos de laboratorio y sistemas de pruebas
in vitro
El clorotalonilo tiene baja toxicidad oral y cutánea en ratas y
conejos, respectivamente (los valores agudos orales y cutáneos de la
DL50 son > 10 000 mg/kg de peso corporal). Por lo que respecta a
las ratas, en un estudio de inhalación se observó que el clorotalonilo
técnico pulverizado (MMAD 5 a 8 µm) presentaba elevada toxicidad, con
una CL50 de 0,1 mg/litro a las 4 h.
El clorotalonilo es un irritante de la piel y los ojos en el
conejo. Los estudios de sensibilización cutánea en el conejillo de
indias no arrojaron resultados concluyentes.
En el caso de las ratas, los efectos principales de dosis orales
repetidas de clorotalonilo se observan en el estómago y los riñones.
En un estudio con grupos de 25 ratas, en que se separaron los sexos,
se emplearon posologías de 0, 1,5, 3, 10 ó 40 mg/kg de peso corporal
por día en la dieta durante 13 semanas, lo que estuvo seguido de un
período de recuperación de 13 semanas. Se observó mayor frecuencia de
hiperplasis y hiperketarosis del preestómago con las posologías de 10
y 40 mg/kg; los efectos desaparecieron cuando cesó el tratamiento. Con
una concentración de 40 mg/kg, se registro mayor incidencia de
hiperplasia del epitelio tubular proximal del riñón en los machos a
las 13 semanas y después del período de recuperación. El nivel sin
efecto observado fue de 3 mg/kg de peso corporal por día en base a la
ausencia de lesiones en el preestómago. Se ha demostrado que los
cambios observados en el preestómago y los riñones son de rápida
aparición, presentándose las lesiones dentro de un período de 4 a 7
días en el caso de los machos, cuyo régimen alimenticio incluía una
concentración de 175 mg/kg de peso corporal al día.
En un estudio de 13 semanas de duración realizado con ratones
(empleando dosis de 0, 7,5, 15, 50, 275 ó 750 mg/kg en el alimento),
se observó mayor incidencia de hiperplasia e hiperkeratosis de las
células epiteliales escamosas del preestómago en el caso de los machos
y las hembras cuando se emplearon posologías de 50 mg/kg y superiores.
Atendiendo a esos cambios, el nivel sin efecto observado fue de 15
mg/kg de clorotalonilo en la dieta, lo que equivale a 3 mg/kg de peso
corporal por día.
Un estudio de 16 semanas de duración con perros cuyo alimento
contenía concentraciones de 0, 250, 500 ó 750 mg/kg no reveló cambios
relacionados con el tratamiento.
Se llevaron a cabo investigaciones adicionales de las lesiones en
el preestómago y los riñones, llevándose a cabo estudios con ratas,
ratones y perros durante un período de 2 años. En un estudio realizado
con ratas (empleando dosis de 0, 1,8, 3,8, 15 ó 175 mg/kg de peso
corporal al día), los efectos estuvieron caracterizados
histológicamente por una mayor incidencia e intensidad de hiperplasia,
hiperkeratosis, y úlceras y erosiones de la mucosa escamosa del
preestómago, y por hiperplasia del epitelio de los túbulos
contorneados proximales de los riñones con posologías de 3,8 mg/kg y
superiores. Por lo que respecta a los efectos no neoplásicos, el nivel
sin efecto observado fue, por lo tanto, de 1,8 mg/kg. La incidencia de
tumores renales (adenomas y carcinomas) y de tumores del preestómago
(papilomas y carcinomas) fue considerablemente superior, alcanzando
175 mg/kg. Hubo pruebas de mayor incidencia de tumores renales en los
machos con dosis de 15 mg/kg, así como de tumores estomacales en los
machos y las hembras con dosis de 3,8 y 15 mg/kg. Por lo que respecta
a los efectos neoplásicos, el nivel sin efecto observado fue, por lo
tanto, de 1,8 mg/kg de peso corporal por día sobre la base de los
cambios en la incidencia de tumores en el preestómago. El riesgo
carcinogénico del clorotalonilo en los riñones y preestómago de las
ratas se vio corroborado por los resultados de otros estudios de 2
años de duración en que emplearon dosis más elevadas.
En un estudio con ratones (empleando dosis de 0, 15, 40, 175 ó
750 mg/kg en el alimento), se observó mayor incidencia de hiperplasia
tubular renal con dosis de 175 mg/kg y superiores, así como de
hiperplasia y hiperkeratosis del preestómago con concentraciones de 40
mg/kg y superiores. La incidencia de tumores escamosos del preestómago
aumentó ligeramente con dosis de 750 mg/kg. Por consiguiente, por lo
que respecta a los cambios neoplásicos y no neoplásicos, los niveles
sin efecto observado fueron de 175 y 15 mg/kg en la dieta (lo que
equivale a 17,5 y 1,6 mg/kg de peso corporal por día respectivamente).
Otro estudio con posologías superiores corroboró esos efectos en el
ratón, pero un estudio con ratones B6C3F1 no señaló ningún riesgo
carcinogénico con dosis elevadas.
En un estudio de 2 años de duración con perros (empleando 60 y
120 mg/kg en el alimento), no se detectó ningún efecto atribuible al
clorotalonilo. Por lo tanto, el nivel sin efecto observado fue de 120
mg/kg en el alimento (lo que equivale a 3 mg/kg de peso corporal al
día).
El clorotalonilo no resultó mutagénico en varias pruebas in
vitro e in vivo, aunque fue positivo en un pequeño número de
valoraciones.
Los derivados monotio, ditio, tritio, dicisteina, tricisteina y
monoglutatión del clorotalonilo, que son posibles substancias
nefrotóxicas, arrojaron resultados negativos en el análisis de Ames.
El clorotalonilo no resultó teratogénico en las ratas o conejos
con dosis de hasta 400 y 50 mg/kg de peso corporal al día,
respectivamente. En un estudio realizado con dos generaciones de
ratas, los parámetros reproductivos tales como el apareamiento, la
fertilidad y el período de gestación no se vieron afectados por el
clorotalonilo con concentraciones de hasta 1500 mg/kg en la dieta.
La toxicidad oral aguda del metabolito 4-hidróxido es superior a
la del clorotalonilo propiamente dicho (DL50 oral aguda de 332 mg/kg
de peso corporal en comparación con > 10 000 mg/kg de peso corporal).
Se han llevado a cabo varios estudios destinados a caracterizar el
perfil toxicológico de ese metabolito y a establecer los niveles sin
efecto observado.
7. Efectos en el ser humano
Se ha informado de dermatitis por contacto en el caso de personas
que trabajan en la producción de clorotalonilo, así como en el de
agricultores y hortelanos. Se tienen noticias también de trabajadores
de fábricas de productos de la madera que han contraído dermatitis por
contacto en las manos y el rostro cuando empleaban preservativos de la
madera que contenían clorotalonilo.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
El clorotalonilo es sumamente tóxico para los peces y los
invertebrados acuáticos en los estudios de laboratorio, siendo los
valores de CL50 inferiores a 0,5 mg/litro. En un estudio sobre
reproducción realizado con dos generaciones de Daphnia magna, la
concentración tóxica máxima aceptable (CTMA) fue de 35 µg/litro.
Con pocas excepciones, el clorotalonilo no es fitotóxico.
En lombrices, la CL50 de una formulación de una suspensión
concentrada (500 g de clorotalonilo/litro) en suelo artificial fue
> 1000 mg/kg de suelo (14 días). Las tijeretas experimentaron mayor
mortalidad cuando estaban en contacto con residuos de clorotalonilo en
las hojas del cacahuete o lo ingerían en su fuente de alimentos en las
pruebas de laboratorio; no hubo ningún otro indicio de efecto
insecticida.
El clorotalonilo tiene poca toxicidad para las aves, habiéndose
informado de una DL50 oral aguda de 4640 mg/kg de alimento en el
pato real. No se informó de ningún efecto considerable sobre la
reproducción.
Un estudio sobre el terreno de organismos acuáticos expuestos
después de la aplicación de clorotalonilo parece indicar que la
toxicidad es inferior a la que cabría esperar atendiendo a los
estudios de laboratorio; esto es también compatible con las
propiedades fisicoquímicas de los compuestos. Se observaron muertes en
algunas especies expuestas experimentalmente en el campo. No se ha
informado de incidentes de muertes en el medio ambiente. Sin embargo,
a pesar del corto tiempo de presencia del clorotalonilo en el medio
ambiente, cabría esperar que ocurran muertes. Resultaría difícil
establecer vínculos entre las muertes y los compuestos ya que la
persistencia de los residuos no seria suficientemente prolongada para
poder identificar el clorotalonilo.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of chlorothalonil by oral, dermal and
inhalation routes of administration is shown in Table 9. Signs of
poisoning include depression, diarrhoea and unkempt appearance. An
oral LD50 could not be attained in the dog because of emesis.
Chlorothalonil administered orally (1 g/kg) to mice increased
intestinal motility. The laxative action was reduced by pre-treatment
with corn oil (Teeters, 1966).
Table 9. The acute toxicity of chlorothalonil
Species LD50 Route Reference
(mg/kg)
Rat > 10 000 oral Powers (1965)
Dog > 5000 oral Paynter (1965a)
Mouse 6000 oral Yoshikawa & Kawai (1966)
Rat 332 oral Wazeter (1971)
(4 hydroxy
metabolite)
Rabbit > 10 000 dermal Doyle & Elsea (1963)
Rat (f & m)a 0.22 mg/litre inhalation Danks & Fowler (1988)
Rat (f & m)b 0.52 mg/litre inhalation Shults et al. (1991)
Rat (f & m)c 0.10 mg/litre inhalation Shults et al. (1993)
a LC50 actual concentration
b Hammermilled technical chlorothalonil - 1 h exposure
c Hammermilled technical chlorothalonil - 4 h exposure
The acute oral toxicity of the metabolite 4-hydroxy-2,5,6-
trichloroisophthalonitrile is greater than that of chlorothalonil
itself, the acute oral LD50 values being 332 and 10 000 mg/kg,
respectively).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rat
When groups of 35 male and 35 female rats were fed chlorothalonil
in the diet at dose levels of 0, 250, 500, 750 or 1500 mg/kg diet for
22 weeks, growth was slightly reduced at all levels in males and in
the highest two levels in females. Liver and kidney weight was
increased in the two highest dose groups in males. Kidney changes,
more evident in males than females, occurred at all dose levels and
included irregular swelling of the tubular epithelium, epithelial
degeneration and tubular dilation (Blackmore & Shott, 1968). In a
separate study, no compound-related effects were seen in the kidneys
of rats fed chlorothalonil at seven dietary levels from 1-120 mg/kg
diet for 17 weeks (Busey, 1975).
When groups of 20 male and 20 female rats were fed chlorothalonil
in the diet (0, 40, 80, 175, 375 and 1500 mg/kg body weight per day)
for 90 days, a significant dose-related reduction in body weight gain
was seen at levels of 375 mg/kg per day or more which was allied to
cathartic action at the two highest doses. Male urine output
decreased and specific gravity increased at the two highest dose
levels. Relative kidney weight was increased in both sexes at all
dose levels. A histopathological re-evaluation of the kidneys showed
the presence of epithelial hyperplasia of the proximal convoluted
tubules at all levels in males and at levels of 175 mg/kg per day or
more in females (Wilson, 1981; Wilson et al., 1985c).
Groups of 25 male and 25 female rats were fed chlorothalonil for
13 weeks at 0, 1.5, 3, 10 and 40 mg/kg body weight per day. Five rats
of each sex per group were killed at 6 weeks, ten at 13 weeks and the
survivors were killed after a further 13-week recovery period. There
was no effect on mortality, clinical condition, body weight, food and
water consumption, haematology or urinalysis. Increases in kidney
(> 3 mg/kg per day) and liver (highest dose) weights and the
incidence of hyperplasia and hyperkeratosis of the forestomach
squamous epithelium (at 10 and 40 mg/kg per day) returned to normal
when treatment ceased. Histopathological re-examination of the kidney
revealed (in males at the highest dose level) an increased incidence
of hyperplasia of the epithelium of the proximal tubules at 6 and
13 weeks and after a 13-week recovery period. In a few animals this
resulted in an increase in the size of the tubules. An overall
no-observed-effect level of 3 mg/kg body weight per day was
established, based upon the lack of lesions in the squamous epithelium
of the forestomach (Wilson et al., 1983a, 1985a).
The kidney and stomach changes were investigated further in a
study where 90 male rats were fed chlorothalonil (175 mg/kg body
weight per day) for up to 91 days. Groups of ten rats were killed
after 4 or 7 days and 2, 4, 6, 8, 10 or 12 weeks, the kidney and
stomach taken for histopathological examination. Groups of ten rats
at each interval were taken from a control group and examined. The
forestomach effects of chlorothalonil were characterized initially as
multifocal ulceration and erosion of the mucosa, developing to
squamous epithelial hyperplasia and hyperkeratosis. Within the first
week there was vacuolar degeneration, cell death, karyomegaly and
regeneration of the proximal tubular epithelium. There was apparent
recovery at day 14 with few lesions present in treated animals.
Continued administration led to tubular epithelial vascularization,
regeneration, hyperplasia and hypertrophy (Ford et al., 1987a).
Chlorothalonil or the monoglutathione conjugate of chlorothalonil
was administered in the diet to male Fischer-344 rats in a 90-day
study. A third group of rats received only the vehicle (0.5%
methylcellulose). The oral administration of approximately equimolar
doses of chlorothalonil (75 mg/kg per day) or the monoglutathione
conjugate of chlorothalonil (150 mg/kg per day) resulted in
significantly increased kidney weight and in treatment-related
histopathological changes in the kidney. The primary changes observed
were morphologically similar for both compounds and were characterized
by proximal tubular hyperplasia, tubular dilation (hypertrophy),
vacuolar degeneration and interstitial fibrosis. The oral
administration of chlorothalonil resulted in gross and microscopic
changes in the non-glandular portion of the forestomach. The oral
administration of the monoglutathione conjugate of chlorothalonil did
not produce alterations in the rat forestomach. The gross and
microscopic changes observed in the forestomach of animals given
chlorothalonil were considered to be due to the local irritational
effects of chlorothalonil. The alteration of the molecule by the
addition of a single glutathione residue appeared to eliminate the
irritation to the forestomach. Thiol metabolites of chlorothalonil
were detected in the urine of animals given either the monoglutathione
conjugate of chlorothalonil or chlorothalonil itself. The presence of
the thiol metabolites, coupled with the similar histopathological
changes in the kidney, suggests a common metabolic pathway. The data
support the conclusion that the nephrotoxicity produced by
chlorothalonil is associated with conjugation with glutathione (Ford
et al., 1987b; Wilson et al., 1990).
7.2.1.2 Mouse
Groups of 15 male and 15 female CD-1 mice were administered
chlorothalonil in diets at levels of 0, 7.5, 15, 50, 275 or 750 mg/kg
diet for 13 weeks. Five animals per group were killed at 6 weeks.
There was no effect on clinical condition, mortality, body weight gain
or food consumption. Kidney weight was increased in females at 275
and 750 mg/kg. Microscopic re-examination revealed hyperplasia of the
epithelium of the proximal convoluted tubules, minimal or slight in
severity, in only 4 out of 15 males. This was not considered to be a
clear treatment-related effect. There was an increased incidence of
hyperplasia and hyperkeratosis of the squamous epithelial cells of the
stomach in males and females at levels of 50 mg/kg or more. Mucosal
ulceration and submucosal inflammation were observed in some treated
animals. The no-observed-effect level was considered to be 15 mg/kg
(3 mg/kg body weight per day) (Shults et al., 1983; Busey, 1985).
7.2.1.3 Dog
In a 30-day feeding study, encapsulated chlorothalonil (97.9%
purity) was administered daily to groups of two male and two female
beagle dogs at 0, 50, 150, or 500 mg/kg body weight per day. The
following tissues were examined macroscopically and microscopically at
necropsy: brain, liver, kidneys, testes with epididymis, ovaries,
adrenals, heart, thyroid, and parathyroid. During treatment the
high-dose dogs exhibited emesis and weight loss and reduced food
consumption (males only). Female dogs had slightly reduced body
weight gains at all doses. At necropsy, the liver weights of
high-dose females were slightly increased. There were no microscopic
changes in the tissues examined. Due to the reduced body-weight gains
of treated females, a no-observed-adverse-effect level (NOAEL) was not
established in this study (Fullmore & Laveglia, 1992; FAO/WHO, 1993b).
In a 16-week dietary study, chlorothalonil (purity unspecified)
was fed at 0, 250, 500 or 750 mg/kg to groups of four beagle dogs.
There were no compound-related effects on appearance, behaviour,
appetite or body weight. No changes in haematological parameters were
found at weeks 0, 4, 13 or 16. At termination, protein-bound iodine
was found to be increased in all treated dogs. Urinalysis at weeks 6,
9, 13 and 16 was unremarkable. No compound-related macroscopic or
microscopic changes were found at necropsy. In particular, only
incidental changes were observed in liver and kidneys. A NOAEL was
not established in this study (Paynter & Murphy, 1967; FAO/WHO,
1993b).
7.2.2 Dermal: Rabbit
Chlorothalonil (75% formulation) was applied to the intact or
abraded skin of groups of rabbits at 0, 500 or 1000 mg/kg per day, 5
days/week, for 3 weeks. The treated groups comprised five males and
five females per group, with two males and two females as controls.
Treatment resulted in dose-related irritation which was more severe
with abraded skin. Histopathological examination revealed a moderate
degree of acanthosis, hyperkeratosis and slight to moderate leucocytic
infiltration. No abnormality was detected in other tissues (Paynter,
1965b).
Chlorothalonil was applied dermally each day for 21 days to
groups of six male and six female rabbits at 0.1, 2.5 and 50 mg/kg per
day. The fungicide was suspended in 0.125% aqueous methylcellulose
and covered 10% of the body surface when applied to the back. Contact
was for 6 h daily. The only effect revealed by a wide range of
observations and examinations was dermal irritation at 2.5 and 50
mg/kg per day. The histopathological changes seen were minimal to
slight acanthosis and hyperkeratosis. The no-observed-effect level
(NOEL) for dermal irritation was 0.1 mg/kg per day (Shults et al.,
1986).
7.3 Long-term exposure
7.3.1 Rat
An early study, lasting 76 weeks, produced evidence of kidney
changes in rats (15 male and 15 female per group) fed high levels of
chlorothalonil at 500, 1000 and 5000 mg/kg diet. There was no
overall effect on mortality, clinical condition or growth. The
compound-related changes in the kidney, at all levels, consisted of
tubular hypertrophy, epithelial irregularities and vacuolation. Males
were more affected than females (Paynter & Busey, 1967).
A 2-year study, reported in 1967, was initiated with
chlorothalonil levels of 1500, 15 000 and 30 000 mg/kg diet with
groups of 35 male and 35 female rats (70 of each sex in control
group). Because of cathartic effects, the top dose administration was
curtailed after 15 weeks. The rats in the two remaining treatment
groups continued for the full 2 years with evaluations including
haematology, biochemistry and histopathology. There was no effect on
survival, but the effect on growth was dose-related. The relative
organ weights of liver and kidney were increased at 15 000 mg/kg.
Microscopic changes in the forestomach were described as acanthosis
and hyperkeratosis of the squamous epithelium at 15 000 mg/kg and, in
the kidney, as tubular hypertrophy and hyperplasia at both 1500 and
15 000 mg/kg diet (Paynter, 1967a).
A supplementary study, run concurrently with the previous study,
assessed chronic toxicity at 5000 mg/kg diet. Growth rate was
depressed and a cathartic action was expressed as increased water
consumption and faecal excretion. Relative organ weights were
increased for caecum and kidneys. Histopathological examination
showed kidney changes as tubular hypertrophy and occasional
degeneration of the proximal tubular epithelium (Paynter & Crews,
1967).
A 2-year study at six chlorothalonil dose levels between 4 and 60
mg/kg diet was designed to determine an NOEL (50 males and 50 females
per group). No effects were seen on survival, clinical observations,
growth, haematological or biochemical parameters. No changes of
toxicological significance were found for organ weights or during
gross and microscopic examination of tissues. The highest dose level
(3 mg/kg body weight per day) was considered the NOEL (Holsing &
Shott, 1970).
A long-term study was undertaken to evaluate the carcinogenic
potential of chlorothalonil in Fischer-344 rats. Males were studied
for 27 months and females for 30 months. Chlorothalonil was
administered in the diet to groups of 60 males and 60 females at dose
levels of 0, 40, 80 and 175 mg/kg body weight per day. There was no
effect on survival of females or males up to 2 years. However, at the
highest dose level, there was increased mortality after 2 years, but
only in males. Decreases in body weight gain were dose-related at 80
and 175 mg/kg per day. Treatment-related effects on other parameters
appeared to be related to nephrotoxicity. These included increases in
serum urea nitrogen and creatinine, increased urine volume and
decreased specific gravity, and increased kidney weight.
Histopathological examination of the kidney showed a dose-related
increase in chronic glomerulonephritis (nephropathy) compared with
controls. A re-examination of the kidneys also revealed tubular
hyperplasia (a sign of preneoplastic change) and chronic progressive
nephropathy in the treated groups. Secondary lesions in other organs
included periarteritis and parathyroid hyperplasia. There was
increased incidence or severity of hyperplasia and hyperkeratosis of
the squamous mucosa of the oesophagus and forestomach in all dosed
groups, which was probably the result of the irritant effect of
chlorothalonil. Neoplastic changes in the kidney and forestomach are
evaluated in section 7.7 (Wilson et al., 1985b, 1986a).
A further study was carried out to evaluate the neoplastic
findings in the kidney and stomach seen in the previous study and to
determine a NOEL for non-neoplastic effects. Groups of 65 male and
65 female rats were administered chlorothalonil in the diet at doses
of 0, 1.8, 3.8, 15 and 175 mg/kg body weight per day. Males were
killed at 23 months (highest dose) and 26 months and females at
29 months. The effects at 175 mg/kg per day were similar to those
described above, i.e. changes associated with the kidney and stomach.
At 15 mg/kg per day there was elevated serum urea nitrogen and
slightly increased kidney weight. At 3.8 mg/kg per day there was a
small increase in kidney weight, but there were no effects at 1.8
mg/kg per day. Microscopic examination revealed an increased
incidence and severity of epithelial hyperplasia in the proximal
convoluted tubules at 3.8 mg/kg per day or more. Clear cell
hyperplasia of the proximal convoluted tubules was increased at
15 mg/kg per day in females and at 175 mg/kg per day in both sexes.
In addition, at 3.8 mg/kg per day or more, there was an increased
incidence and severity of hyperplasia, hyperkeratosis, ulcers and
erosions of the squamous mucosa of the forestomach. At 175 mg/kg per
day, the incidence of erosions of the glandular stomach was
significantly increased compared to controls. Renal tumours at levels
of 15 and 175 mg/kg per day and stomach tumours at 3.8 mg/kg per day
or more are evaluated in section 7.7. An NOEL of 1.8 mg/kg body
weight per day was established for non-neoplastic effects seen in the
study (Wilson et al., 1989a).
7.3.2 Mouse
A 2-year mouse study was carried out with 60 males and 60 females
per group at 0, 750, 1500 and 3000 mg chlorothalonil/kg in diet.
There was a slightly increased mortality in males at the highest dose
level but no effect was seen on body weight, food consumption,
clinical condition or haematological parameters. Kidney weight was
increased in all treated groups compared to controls. Non-neoplastic
changes in the kidney were characterized as glomerulonephritis,
cortical tubular degeneration and cysts. The incidence of these
changes, found at all treatment levels, was not dose-related but was
considered to be due to treatment. A histopathological re-evaluation
of the kidneys revealed a high incidence of tubular hyperplasia in all
male groups and a lower incidence in females. Non-neoplastic effects
in the stomach and oesophagus included hyperplasia and hyperkeratosis
of the squamous mucosa, probably due to the irritant action of
chlorothalonil. The evaluation of kidney and stomach tumours found in
this study is described in section 7.7 (Wilson et al., 1983b, 1986b).
A second mouse study was undertaken to determine the NOEL for
stomach and kidney changes in male mice. Sixty males per group were
fed chlorothalonil at levels of 0, 15, 40, 175 and 750 mg/kg diet for
2 years. Kidney weight and incidence of tubular hyperplasia were
increased at 750 mg/kg and, very slightly, at 175 mg/kg. The
increased incidence of hyperplasia and hyperkeratosis of the
forestomach was dose-related between 40 and 750 mg/kg. The dietary
NOEL for non-neoplastic effects was determined to be 15 mg/kg (1.6
mg/kg body weight per day). The tumour evaluation is considered in
section 7.7 (Wilson et al., 1987).
7.3.3 Dog
In a 2-year study, chlorothalonil (93.6% purity) was fed to
groups of four beagle dogs at dietary concentrations of 0, 1500,
15 000 or 30 000 mg/kg (equivalent to 0, 37.5, 375, or 750 mg/kg body
weight per day). Eight dogs, one of each sex and of each group, were
killed at 12 months and the remainder at 24 months. One dog of each
treatment group lost weight during the study. There was a tendency
towards mild anaemia in four of the mid-dose dogs at 2 years and at
earlier intervals in two of the high-dose dogs. Biochemical and urine
analyses were unremarkable. Absolute and relative thyroid and kidney
weights, and liver to body weight ratios were increased at the
mid- and high-dose levels. Histopathological treatment-related
changes occurred in the liver, thyroid, kidney and stomach of mid- and
high-dose dogs; changes in low-dose dogs were equivocal. In the
liver, the findings were similar in nature (though slightly more
pronounced) at low-dose levels to those in the control dogs, but they
increased in severity at mid- and high-dose levels. They included
pericholangitis with associated portal fibrosis, bile duct hyperplasia
and pigmentation of hepatic cytoplasm and of macrophages of sinusoids
and portal triads. Renal glomerulosclerosis and degenerative renal
tubular changes (tubular hypertrophy and dilation) were found in the
kidneys of mid- and high-dose dogs. In the thyroid, markedly
increased pigmentation of follicular epithelia occurred in mid- and
high-dose dogs. Moderate to severe gastritis was found irregularly in
mid- and high-dose animals. In summary, administration of
chlorothalonil in the diet of dogs at concentrations of 15 000 and
30 000 mg/kg caused irregular body weight reduction, borderline
anaemia and histopathological changes in the liver, kidney, thyroid
and stomach. At 1500 mg/kg, the histopathological changes found in
the liver were qualitatively similar but minimally to slightly
increased in comparison to those found in control animals.
Histopathological changes to the other tissues were unremarkable at
the low dose. A NOAEL was not established in this study (Paynter &
Busey, 1966; FAO/WHO, 1993b).
Groups of beagle dogs (eight males and eight females per group)
were fed chlorothalonil in the diet at dose levels of 0, 60 and
120 mg/kg. Four dogs of each sex per group were killed at one year
and the remaining animals at 2 years. There were no effects on
behaviour or growth over the course of the study. Clinical chemistry
values, including haematological, biochemical and urine analyses, were
comparable to the controls at all dose levels. Gross and microscopic
examination of tissues and organs performed on animals killed at 12
months indicated a slight increase in the severity of renal tubule
vacuolation in high-dose males. Examination of tissues and organs at
24 months showed a slight degree of renal tubule vacuolation in two
out of four males at 120 mg/kg. In the absence of other changes
(urinalyses values) this finding was considered questionable,
especially as a slight degree of vacuolation was noted in controls as
well as other treated animals. The NOAEL was considered to be
120 mg/kg diet, equivalent to 3 mg/kg body weight per day (Holsing &
Voelker, 1970; FAO/WHO, 1991, 1993b).
7.3.4 Summary of key dietary studies
A summary of the key dietary studies with chlorothalonil is given
in Table 10.
7.4 Skin and eye irritation; sensitization
Chlorothalonil is an irritant to rabbit skin, as shown by
repeated dose studies (5 days/week for 3 weeks at 500 or 1000 mg/kg
per day or daily for 21 days at 2.5 or 50 mg/kg per day; details in
section 7.2.2).
The influence of the vehicle on the skin irritant potential of
chlorothalonil was shown by a rabbit study with 0.1% chlorothalonil in
saline, petrolatum or acetone. Compared to the vehicle alone,
chlorothalonil did not cause a significant increase in irritation in
Table 10. Summary of key dietary studies with chlorothalonil
Species Duration Dose levels NOEL LOEL Key effects Reference
Rat 13 weeks 0, 1.5, 3, 10, 3 mg/kg per 10 mg/kg per 40 mg/kg per day: increased kidney and Wilson et
40 mg/kg body day day liver weight, incidence of hyperplasia al. (1983a,
weight per day and hyperkeratosis of forestomach, 1985a)
incidence of epithelial hyperplasia in
proximal tubules 10 mg/kg per day:
increased kidney weight, hyperplasia
and hyperkeratosis of forestomach
Rat 23-29 months 0, 1.8, 3.8, 15, 1.8 mg/kg 3.8 mg/kg 175 mg/kg per day: increases in serum Wilson et
175 mg/kg body per day per day urea nitrogen, urine volume, kidney al. (1989a)
weight per day weight, renal tubular hyperplasia,
hyperplasia and hyperkeratosis of
forestomach; increased incidence of
renal and forestomach tumours
15 mg/kg per day: similar but less
intense changes to those shown at
highest dose level 3.8 mg/kg per day:
small increase in kidney weight, renal
tubular hyperplasia, hyperplasia
and hyperkeratosis of forestomach and
forestomach tumours
Mouse 13 weeks 0, 7.5, 15, 50, 15 mg/kg diet 50 mg/kg diet 275 and 750 mg/kg: increased kidney Shults et
275, 750 mg/kg (= 3 mg/kg (= 10 mg/kg weight 50, 275 and 750 mg/kg: al. (1983);
diet per day) body weight dose-related increased incidence Busey (1985)
per day) of hyperplasia and hyperkeratosis
of forestomach
Table 10. (Cont'd)
Species Duration Dose levels NOEL LOEL Key effects Reference
Mouse 2 years 0, 15, 40, 175, 15 mg/kg diet 40 mg/kg diet 750 mg/kg: increased kidney weight, Wilson et
750 mg/kg diet (= 1.6 mg/kg (= 4.5 mg/kg renal tubular hyperplasia, forestomach al. (1987)
body weight body weight hyperplasia and hyperkeratosis,
per day) per day) slightly increased incidence of
forestomach tumours 175 mg/kg: slightly
increased incidence of renal tubular
hyperplasia, increased forestomach
hyperplasia and hyperkeratosis
40 mg/kg: increased incidence of
forestomach hyperplasia and
hyperkeratosis
Dog 2 years 0, 1500, 15 000, - 1500 mg/kg in 15 000 and 30 000 mg/kg: increased Paynter &
30 000 mg/kg diet (= 37.5 kidney, liver and thyroid weights and Busey (1966)
diet mg/kg body histopathological changes, gastritis
weight per 1500 mg/kg: slightly increased incidence
day) hepatic findings
Dog 2 years 0, 60, 120 mg/kg 120 mg/kg diet - no changes of toxicological significance Holsing &
diet (= 3 mg/kg Voelker
body weight (1970);
per day FAO/WHO
(1991, 1993)
saline, but doubled the mild irritation caused by petrolatum. Acetone
itself caused no irritation but the addition of chlorothalonil
produced mild skin irritation (Flannigan & Tucker, 1985).
A further study in rabbits using a cumulative irritation assay
confirmed the irritant properties of 0.1% chlorothalonil in acetone.
A concentration of 0.01% gave evidence of mild irritation, probably of
no clinical significance, and 0.001% was not irritant to the skin
(Flannigan et al., 1986).
Chlorothalonil irritancy to the eye was evaluated in a modified
Draize system, 0.1 mg being instilled into one eye of each of three
male and three female rabbits. The eyes were examined and the results
scored after 24, 48 and 72 h, 7 and 14 days. Ocular irritation
occurred in all animals and corneal opacity persisted to day 14 (Major
et al., 1982).
Skin sensitization was tested in a guinea-pig maximization test
using 10 Hartley female guinea-pigs. Topical concentrations of 0.5
and 5% chlorothalonil were used. Upon challenge, chlorothalonil was
shown to be a strong sensitizer. Moderate cross-sensitization with
benomyl was also demonstrated (Matsushita & Aoyama, 1981). By
contrast, chlorothalonil did not produce skin sensitization in a
Draize test. The test substance (0.2 g) was applied to the shaved
backs of Hartley-derived guinea-pigs. The material was occluded for
24 h and then removed. This procedure was performed 3 times a week
for a total of 10 applications. On day 36 of the study a challenge
application of 0.2 g chlorothalonil was applied to the shaved flanks
and occluded. The skin was assessed 24 h later after removal of the
occlusive dressing. The positive control substance DNCB showed the
expected dermal sensitization but chlorothalonil was shown not to be a
sensitizer in this test (Wilson et al., 1982).
Luperi & Forster (1988) studied the ability of chlorothalonil to
induce delayed contact hypersensitivity in the guinea-pig using the
maximization test of Magnusson and Kligman. There was no evidence of
an induced sensitization response, but adequate evaluation was impeded
by a diffuse irritant reaction following the challenge.
7.5 Reproductive and developmental toxicity
Teratological evaluations have been carried out in the rat and
rabbit.
Chlorothalonil was administered orally via gavage to pregnant
Sprague-Dawley rats (25 per group) on days 6-15 of gestation at dose
levels of 0, 25, 100 or 400 mg/kg body weight per day, and the animals
were killed on day 20. Clinical signs of maternal toxicity were
evident at the highest dose level. There were 3 deaths and lowered
body weight during the dosing period. There was a slight increase in
the number of early embryonic deaths at the highest dose level,
probably associated with the maternal toxicity. There were no
compound-related incidences of external, internal or skeletal
malformations in the fetuses in treated animals. It was concluded
that chlorothalonil is not teratogenic to the rat (Mizens et al.,
1983).
Chlorothalonil was given orally to pregnant rabbits on days 8-16
of gestation at dose levels of 0, 180 or 375 mg/kg per day (days 8 and
9) and 0, 62.5 or 31.25 mg/kg per day (day 10 to day 16). There were
marked effects on food intake and maternal mortality occurred in both
treated groups, which imposed limitations on the evaluation of the
study. However no teratogenic effect was observed (Paynter, 1966b).
Rabbits were dosed with chlorothalonil (0, 5, or 50 mg/kg per
day) during days 6-18 of pregnancy (8 in control group, 9 in each dose
group) and killed on day 29. Four out of nine does aborted at the
highest dose level, and body weight was reduced in this group.
Although the incidence of fetal deaths appeared to increase with dose,
the difference was not statistically significant. The number of
implants and live fetuses per pregnancy and the fetal weights were
reduced in the high-dose group compared to controls. No treatment-
related effects were seen during external, internal or skeletal
examinations. It was concluded that chlorothalonil was not
teratogenic to the rabbit (Shirasu & Teramoto, 1975).
Chlorothalonil was administered by gavage to groups of 20
pregnant rabbits on days 7-19 of gestation at dose levels of 0, 5, 10
or 20 mg/kg per day. All survivors were weighed, killed on day 30 and
examined for live, dead or resorbed fetuses. Live and dead fetuses
were weighed and examined for external, visceral and skeletal
abnormalities. The highest dose level caused maternal body weight
loss and decreased food consumption. The pregnancy rate was > 95%
in each group. Examinations revealed no fetotoxicity or
teratogenicity due to chlorothalonil (Wilson et al., 1988).
A three-generation reproduction study was undertaken in rats at
dietary levels of 0, 1500 or 15 000 mg chlorothalonil/kg diet. A
supplementary study with 0 or 5000 mg/kg was also carried out 6 months
later. Groups consisted of 10 males and 20 females which were fed the
test diet for 11 or 12 weeks prior to mating. The study design was
for three generations with two matings per generation to give A and B
litters. Growth suppression occurred in the nursing A and B litters
in all generations at all dose levels and the pups appeared smaller
than the controls. There were no malformations due to treatment.
However, there were difficulties in execution, and this study is not
considered adequate by present-day standards (Paynter, 1967b).
Reproductive performance was also assessed in a one-generation
study on rats at dietary levels of 0, 200, 375, 750, 1500 or 3000
mg/kg diet with groups of 15 males and 15 females. The parents were
treated for 10 weeks prior to mating. No clinical signs of toxicity
were evident in the parents, but male body weight gain was reduced at
1500 and 3000 mg/kg and the kidneys were enlarged at 3000 mg/kg.
Reproductive parameters such as mating, fertility and gestation length
were not affected. There were no treatment-related abnormalities in
the offspring. The only effect on the offspring was lower body weight
at 3000 mg/kg on lactation days 14 and 21 (Wilson et al., 1989b).
In a two-generation reproduction study with two litters per
generation, technical chlorothalonil was administered to Charles River
CD rats by dietary admixture at concentrations of 0, 500, 1500 and
3000 mg/kg diet. There were 35 rats of each sex in each group. The
parental animals from each generation were treated continuously during
the growth period prior to mating and then throughout the mating,
gestation, lactation and resting phases of the study. The growth
period was 10 weeks for the F0 animals and 14 weeks for the F1
animals. Each generation of parental animals was mated twice to
produce the F1a, F1b, F2a and F2b litters. The offspring were
exposed to the test diets throughout the lactation period. Thirty-
five F1b males and females per group were selected to become the F1
generation after weaning. No mortalities or clinical signs of
toxicity associated with administration of chlorothalonil were
observed in the F0 or F1 parent animals. There was a
treatment-related effect towards lowered body weight in both males and
females in the F0 and F1 adults. The NOEL for body weight was 500
mg/kg in diet. Increased relative food consumption was observed in
the groups that showed lower body weights. The anticipated
treatment-related lesions in the kidneys and stomachs of adult animals
were observed in the F0 and F1 generations by gross and microscopic
pathology. These effects were observed in the kidney at all dose
levels in males and at 1500 and 3000 mg/kg in females. Stomach
effects were observed at all dose levels in both sexes. Reproductive
parameters in F0 and F1 animals, including mating and fertility
indices and gestation length, were not affected by treatment with
chlorothalonil. No gross malformations which were considered
treatment-related were observed in offspring in any of the groups
throughout the study. Litters were culled at day 4 to 8 pups/litter.
No effects on the number of live and stillborn pups, pup sex ratio,
pup survival and physical condition of the pups during lactation were
observed. No findings which were considered treatment-related were
observed during necropsy of the pups. The only effect observed in
pups in this study was lowered body weight compared to the controls on
day 21 of lactation. The NOEL for this effect was considered to be
1500 mg/kg diet, equal to 75 mg/kg body weight per day (Lucas & Benz,
1990).
7.6 Mutagenicity
Chlorothalonil has been assessed for mutagenic potential in a
wide range of in vitro and in vivo assays as shown in Tables 11
and 12. Most of the tests showed chlorothalonil not to be mutagenic
or clastogenic. A positive result in the DNA repair test with
Salmonella typhimurium was not reproduced in Bacillus subtilis,
nor was DNA binding shown in an in vivo study (see section 6.5). A
positive result in Chinese hamster ovary cells without metabolic
activation was not seen in the presence of activation nor confirmed by
in vivo chromosomal tests in rats and mice. Equivocal results were
obtained with Chinese hamster bone marrow in vivo.
In addition to the results shown in the Tables 11 and 12, a
series of studies was reported by IARC (1983). The results, which
were all negative, included those with S. typhimurium in the
presence and absence of a metabolic activating system and
Saccharomyces cerevisiae and Aspergillus nidulans in the presence
of activation. Chlorothalonil also failed to induce mutations in
silkworms and chromosomal aberrations in barley shoot tips or hamster
lung fibroblasts.
Chlorothalonil is not a transforming agent in Fischer rat embryo
cell lines (Price, 1978a).
The monothio, dithio, trithio, dicysteine, tricysteine and
monoglutathione metabolites of chlorothalonil have been shown to be
negative in the Ames assay with or without rat kidney metabolic
activation (see section 7.9).
Considering all the results of mutagenicity testing, it is
unlikely that chlorothalonil will show mutagenic activity in intact
mammalian systems.
7.7 Carcinogenicity
Several long-term rodent studies have been carried out on
chlorothalonil and have included carcinogenic evaluation. The design
of these studies and the chronic toxicity results have been described
in section 7.3. Some of the earlier rat studies did not show any
carcinogenic effect but it is probable that their design was not
sufficient to provide a critical test. The carcinogenic evaluation
in this section therefore concentrates upon the more recent rat and
mouse studies. Detailed evaluations of these studies have already
appeared in the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
reviews of 1983, 1985 and 1990 (FAO/WHO, 1985, 1986b, 1990b).
Therefore, for most of these studies, only the salient points will be
described here.
Table 11. In vitro mutagenicity tests
Test Organism Metabolic Dose range Mutagenic Reference
activation (+ or -) potential
Prokaryotes
Point mutation Salmonella typhimurium + and - 0.33-6.6 µg/plate negative Banzer (1977a)
(5 strains)
Point mutation S. typhimurium + and - 0.16-50 µg/plate negative Jones et al. (1984)
(5 strains) (renal)
Point mutation S. typhimurium - 1-10 µg/plate negative Shirasu et al.
(5 strains) + 2-10 µg/plate negative (1977)
Escherichia coli WP2 - 10-500 µg/plate negative
+ 10-100 µg/plate negative
Point mutation S. typhimurium TA98, + and - 0.76, 7.6, 76 µg/plate negative Wei (1982)
TA100, TA1535, TA1537, (hepatic and
TA1538 renal)
DNA repair tests S. typhimurium + and - 2, 10, 20 µg positive Banzer (1977b)
TA1978, TA1538
Bacillus subtilis - 2-200 µg negative Shirasu et al.
H17/M45 rec-assay (1977)
Table 11. (Cont'd)
Test Organism Metabolic Dose range Mutagenic Reference
activation (+ or -) potential
Mammalian cells
Gene mutation Chinese hamster V79 + and - 0.3 µg/ml negative Banzer (1977c)
mouse fibroblast + and - 0.03 µg/ml negative
BALB/3T3
Chromosome Chinese hamster - 0.03-0.3 µg/ml positive Mizens et al.
aberration ovary cells + 0.6-6.0 µg/ml negative (1986a)
Chromosome Human lymphocytes - 0.54-2.5 µg/ml negative Mosesso & Forster
aberrations + 1.16-5.38 µg/ml negative (1988)
Table 12. In vivo mutagenicity tests
Species Test Dose Mutagenic potential Reference
Mouse in vivo 6.5 mg/kg per day negative Legator (1974a)
cytogenetics 5 days orally
Mouse host-mediated assay; 8 strains 6.5 mg/kg per day negative Legator (1974a)
Salmonella typhimurium 5 days orally
Mouse dominant lethal assay, 5 days 6.5 mg/kg per day negative Legator (1974a)
dosing, 8 weeks mating 5 days orally
Mouse micronucleus 4-2500 mg/kg orally, twice with negative Siou (1981a)
(polychromatic erythrocytes) 24 h interval
Mouse chromosome aberration (bone 4-2500 mg/kg orally, twice with negative Siou (1981b)
marrow 6 h after last dose) 24 h interval 250, 1250, 2500 negative Mizens et al.
(bone marrow at 6, 24 and 48 h) mg/kg oral single dose (1985a)
Rat micronucleus 8-5000 mg/kg orally, twice with negative Siou (1981a)
(polychromatic erythrocytes) 24 h interval
Rat chromosome aberration (bone doses as above negative Siou (1981b)
marrow 6 h after last dose)
(bone marrow 6, 24 and 48 h) 500, 2500, 5000 mg/kg oral negative Mizens et al. (1985b)
single dose
Table 12. (Cont'd)
Species Test Dose Mutagenic potential Reference
Chinese micronucleus 4-2500 mg/kg orally, twice with negative Siou (1981a)
hamster (polychromatic erythrocytes) 24 h interval
Chinese chromosome aberration 8-5000 mg/kg for 2 days inconclusive Siou (1981b)
hamster (bone marrow 6 h after last dose)
(bone marrow at 6, 24 and 48 h) 500, 2500, 5000 mg/kg oral equivocal Mizens et al. (1985c)
single dose (± at 48 h)
(bone marrow 6 h after last dose) 50, 125, 250 mg/kg per day weak response Mizens et al. (1985c)
orally for 5 days not dose-related
A bioassay of technical grade chlorothalonil for possible
carcinogenicity was conducted by administering the test chemical in
the diet to Osborne-Mendel rats and B6C3F1 mice. Groups of 50 rats
of each sex were administered chlorothalonil at one of two dose levels
for 80 weeks and then observed for 30-31 weeks. Time-weighted average
doses for both males and females were 5063 or 10 126 mg/kg diet.
Matched controls consisted of groups of 10 untreated rats of each sex;
pooled controls consisted of the matched control groups combined with
55 untreated male or female rats from similar bioassays of five other
test chemicals. All surviving rats were killed at 110-111 weeks.
Groups of 50 mice of each sex were administered chlorothalonil at one
of two dose levels for 80 weeks, then observed for 11-12 weeks. Time-
weighted average doses for males were 2688 or 5375 mg/kg diet, and for
females, 3000 or 6000 mg/kg diet. Matched controls consisted of
groups of 10 untreated mice of each sex; pooled controls consisted of
the matched control groups combined with 50 untreated male or female
mice from similar bioassays of five other test chemicals. All
surviving mice were killed at 91-92 weeks. Clinical signs that
appeared with increasing frequency in dosed rats included haematuria
and, from week 72 until termination of the study, bright yellow urine.
Since the dosed female mice did not have depression in mean body
weights or decreased survival compared with the controls, they may
have been able to tolerate a higher dose. In rats, adenomas and
carcinomas of the renal tubular epithelium occurred with a significant
dose-related trend in both the males and females (males: pooled
controls 0/62, low dose 3/46, high dose 4/49; females: pooled controls
0/62, low dose 1/48, high dose 5/50). These tumours included both
adenomas and carcinomas which are considered to be histogenically
related. In mice, no tumours were found to occur at a greater
incidence among dosed animals than among controls. It was concluded
that under the conditions of this bioassay, technical grade
chlorothalonil was carcinogenic to Osborne-Mendel rats, producing
tumours of the kidney. However, chlorothalonil was not carcinogenic
for B6C3F1 mice (US NCI, 1978).
In a lifetime study on Fischer-344 rats at dietary doses of 0,
40, 80 or 175 mg/kg body weight per day (see section 7.3), a higher
incidence of primary renal tumours of epithelial origin (adenomas and
carcinomas) was seen in the treated groups (0/60, 7/60, 7/60 and 19/60
for males and 0/60, 3/60, 6/60 and 23/60 for females). It was
considered that the increased incidence of renal hyperplasia seen in
this study was associated with the formation of these tumours and
constituted a pre-neoplastic change. Papillomas and carcinomas of the
squamous mucosa of the forestomach were found in rats in the treated
groups (0/60, 1/60, 1/60, 3/60 for males and 0/60, 1/60, 2/60, 7/60
for females). These are probably related to the proliferative non-
neoplastic effects on the squamous mucosa as a result of the chronic
irritation by chlorothalonil (Wilson et al., 1985a, 1986b).
A further dietary study evaluated the carcinogenicity of
chlorothalonil at lower doses of 1.8, 3.8 and 15 mg/kg body weight per
day as well as at 175 mg/kg body weight per day (see section 7.3).
Rats with renal tumours (adenomas and carcinomas) occurred at 1/55,
1/54, 1/54, 4/54, 23/55 (male groups) and 0/55, 0/54, 0/55, 0/53,
32/55 (female groups). This confirmed the effect at 175 mg/kg per day
and an NOEL of 3.8 mg/kg per day was determined for these tumours.
Animals with papillomas and carcinomas of the forestomach occurred at
0/55, 0/54, 3/54, 2/54, 5/55 (male groups) and 1/55, 1/54, 2/55, 5/53,
9/55 (female groups) giving an NOEL of 1.8 mg/kg per day (Wilson et
al., 1989a).
A 2-year study with Charles River CD-1 mice at 750, 1500 or 3000
mg chlorothalonil/kg diet (section 7.3) showed increased incidences of
gastric and renal tumours in the treated groups. Mice with tumours of
the squamous epithelium of the forestomach occurred at 0/60, 2/60,
5/60, 2/60 (male groups) and 0/60, 2/60, 4/60, 5/59 (female groups).
Although not strictly dose-related, these results were considered to
be a treatment effect and linked to the irritant properties of
chlorothalonil. There was also a low incidence of renal tubular
tumours in male treated groups, not seen in controls, at 0/60, 6/60,
4/60, 5/60 (not dose-related). These were probably linked to the high
incidence of renal tubular hyperplasia seen in male mice in the
treated groups (Wilson et al., 1983b, 1986b).
A second study at 0, 15, 40, 175 and 750 mg/kg diet was
undertaken to establish an NOEL for kidney and stomach changes in male
mice (section 7.3). Only two renal tumours (one at 40 mg/kg and one
at 175 mg/kg) were found. There was a slightly higher incidence of
squamous tumours of the forestomach at 750 mg/kg. Taking account of
the overall results of the two studies it was considered that the
tumorigenic NOEL was at least 175 mg/kg diet. In this study, the NOEL
for tubular hyperplasia was 40 mg/kg (equal to 4.5 mg/kg per day) and
the NOEL for hyperplasia/hyperkeratosis in the forestomach was 1.6
mg/kg per day (Wilson et al., 1987).
7.8 Other special studies
Rats fed 0, 1500 or 15 000 mg chlorothalonil/kg diet showed a
dose-related decrease in the retention of a dye, indicating a laxative
effect. A more detailed study attempted to determine the effect of
chlorothalonil on the absorption and utilization of proteins, fats and
amino acids during a 10-week feeding study on a group of 10 male and
10 female rats. It was concluded that the compound did not interfere
directly with the absorption and utilization and that the depressed
weight gain was probably due to catharsis (Paynter, 1967c).
In a study by Andre et al. (1991), mitochondria were obtained
from fresh kidney cortical tissue by homogenization and differential
centrifugation. The mitochondria were incubated in the presence or
absence of sulfur-containing analogues of chlorothalonil and the
degree of mitochondrial respiratory control was evaluated by
polarographic techniques. The following sulfur-containing analogues
of chlorothalonil were tested: the mono-, di-, and tri-thiol analogues
and the mono-, di-, and tri-glutathione analogues. Kidney
mitochondria were incubated with succinate, a site 2 substrate, or
with glutamate, a site 1 substrate, in the presence or absence of the
test material. Mitochondrial respiratory control, expressed as the
acceptor control ratio (ACR), was determined by taking the ratio of
the rate of oxygen consumption in the presence of ADP (state 3) to the
rate of oxygen consumption after the ADP had been consumed (state 4).
When the mono-thiol, mono-, di-, or tri-glutathione analogues of
chlorothalonil and succinate were added to kidney mitochondria, no
significant differences were found in the ACR from the controls.
Incubation of the di- or tri-thiol analogues of chlorothalonil and
succinate with kidney mitochondria resulted in significant differences
of the experimental ACR from the control ACR. When glutamate was used
as the substrate for the electron transport system in kidney
mitochondria, no significant differences from the control were
detected for any of the six test materials. These data suggest that
the effects of the di- or tri-thiol analogues of chlorothalonil may
impair the respiratory control of kidney mitochondria by inhibiting
the transfer of reducing equivalents from succinate to coenzyme Q.
The effects on mitochondrial respiration may be due to the formation
of disulfide bonds between the thiol analogues and proteins.
7.9 Toxicity of metabolites
Most studies have centred on the 4-hydroxy-2,5,6-
trichloroisophthalonitrile metabolite. This is found as a small
proportion of chlorothalonil plant residues (section 4.2.1) and is
also a breakdown product of chlorothalonil in the environment. It has
been identified in faeces of laboratory animals after chlorothalonil
dosing. It is more acutely toxic than chlorothalonil itself (acute
oral LD50 values are 332 and 10 000 mg/kg, respectively).
Several laboratory animal studies have been undertaken with the
4-hydroxy metabolite and have been described in some detail in JMPR
reviews (FAO/WHO, 1978, 1982, 1985). The following is a brief summary
of the studies and their results.
Various effects were noted in rats fed the 4-hydroxy metabolite
at eight dose levels (10 to 750 mg/kg body weight per day) for 61-69
days. Mortality was increased in males at 125 mg/kg per day or more,
and in females at 75 mg/kg per day or more. Body weight was depressed
in both sexes at > 40 mg/kg per day. Anaemia was evident at 75
mg/kg per day or more in males and 40 mg/kg per day or more in
females. Histopathological examination revealed treatment-related
effects in bone marrow and spleen (in the form of erythroid
hyperplasia and depressed granulopoiesis) at > 40 mg/kg per day and
in the liver (haemosiderosis, centrilobular hepatitis) and kidney
(cortical atrophy) at > 75 mg/kg per day. The overall NOEL was 20
mg/kg body weight per day (Murchison, 1979).
A rabbit teratology study was undertaken with oral doses of 0, 1,
2.5 and 5 mg/kg per day during days 6-18 of gestation, with necropsy
at day 28. There was a marginal effect on dams at day 5 but no
evidence of a teratogenic effect in the study (Wazeter & Goldenthal,
1976).
The 4-hydroxy metabolite was evaluated in a three-generation, two
litters/generation study in groups of 15 male and 30 female rats at
dose levels of 0, 10, 60 and 125 mg/kg diet. There were no
treatment-related changes except for body weight reductions at 60 and
125 mg/kg (FAO/WHO, 1982).
In a one-generation follow-up study, groups of rats were fed
diets containing the 4-hydroxy metabolite at 0, 10, 20, 30, 60 and 120
mg/kg diet for 18 weeks before mating (12 males and 24 females per
group). Two sets of mating were undertaken. There was some effect on
live pup weights at 60 and 120 mg/kg. The clear NOEL was considered
to be 30 mg/kg diet (Ford, 1982).
The metabolite was assessed for chronic toxicity and
carcinogenicity in long-term rodent studies. A 2-year rat study was
undertaken at dose levels of 0, 0.5, 3 and 10 mg/kg body weight per
day with groups of 75 males and 75 females. Anaemia was evident at
the highest dose. The NOEL was determined to be 3 mg/kg body weight
per day. There was no evidence for a carcinogenic effect. In the
mouse study, the dietary dose levels were 0, 375, 750 and 1500 mg/kg
diet using groups of 60 males and 60 females. The study was
terminated at 20-22 months because of increasing and high mortality.
A series of effects, including amyloidosis, haemosiderin in the spleen
and increases in reticulocyte counts and red cell haemolysis,
precluded the establishment of an NOEL. No carcinogenic effect was
evident (Hozan & Auletta, 1981; McGee, 1983).
The 4-hydroxy metabolite was not mutagenic in a number of in
vitro and in vivo assays. These were the Salmonella mutagenicity
assay with and without metabolic activation (Banzer, 1977d), a
host-mediated assay in mice given a single intraperitoneal dose of
6.5 mg/kg (Legator, 1974b), Chinese hamster (V-79) and mouse
fibroblast (Balb/3T3) cells in culture with and without activation
(Banzer, 1977e), a micronucleus test in mice at 6.5 mg/kg per day for
5 days (Legator, 1974b), a dominant lethal study in male rats given
single oral doses (0, 2, 4 or 8 mg/kg) singly or daily for 5 days
(Hastings & Clifford, 1975), a dominant lethal study in male mice
given 1, 3 or 6.5 mg/kg per day for 5 days (Legator, 1974b), a DNA
repair assay using S. typhimurium in a spot test with or without
activation (Banzer, 1977f), and a cell transformation assay with rat
embryo cell lines in culture at 0.1, 1 or 10 µg/ml (Price 1978b).
A series of in vitro gene mutation assays with S. typhimurium
tester strains with and without renal metabolic activation were
undertaken with chlorothalonil, four manufacturing impurities and
eight known or potential metabolites. No mutagenic potential was
shown by any of the compounds. Full details were given in the 1985
JMPR evaluation (FAO/WHO, 1986b).
The mutagenic potential of the thiol and cysteine derivatives of
chlorothalonil has been evaluated in the Ames test with and without
metabolic activation with S9 from the kidney of male Fischer rats.
These compounds were 2,5-dichloro-4,6-bismercaptoiso-phthalonitrile,
5-(2,4-dicyano-3,5,6-trichlorophenyl) glutathione, 5-chloro-2,4,6-
trimercaptoisophthalonitrile, S,S'-(2,4-dicyano-3,6-dichloro
phenyl)dicysteine and S,S',S"-(2,4-dicyano-6-chlorophenyl)-
tricysteine (purity ranging from 90.5 to 97.5%). Four other compounds
were used as positive controls. The Salmonella typhimurium tester
strains TA98, TA100, TA1535, TA1537 and TA1538 were used. In all
these studies, there was no significant increase (doubling) over
solvent control values in the number of revertants for any of the five
tester strains used either with or without metabolic activation
(Mizens et al., 1985d,e, 1986b,c, 1987).
A description of a 90-day rat study on the monoglutathione
conjugate of chlorothalonil is given in section 7.3.
8. EFFECTS ON HUMANS
8.1 General population exposure
A case of acute facial dermatitis in a 53-year-old man, caused by
staying in a summer cottage, has been reported. Patch testing
revealed contact allergy to the paint that was applied to all the
window-frames, and to the chlorothalonil contained in the paint.
After removal of the frames, there were no further recurrences of
facial dermatitis. The authors suggested that products containing
chlorothalonil are not suitable for indoor use (Liden, 1990; Eilrich &
Chelsky, 1991).
8.2 Occupational exposure
Chlorothalonil contact dermatitis was observed in a number of
employees in a chlorothalonil manufacturing plant. There were 19
cases out of 103 employees. About 60% of the employees showed some
kind of skin abnormality compared with 18.5% of employees not working
with chlorothalonil. When the hygiene conditions of the plant were
improved the overall proportion of skin abnormalities fell to about
20% and there were no cases of chlorothalonil contact dermatitis
(Diamond Shamrock, 1980).
Wood preservatives containing chlorothalonil have also been
implicated in the appearance of allergic contact dermatitis. One
report concerned a Danish cabinet maker who developed dermatitis on
his hands after 9 months of painting furniture with preservative
containing the compound. This was possibly caused by contact via the
wood dust after sandpapering (Bach & Pedersen, 1980). Another report
referred to three cases, two with erythema on the face, particularly
periorbitally, and one with eczema of the hands, in people engaged in
similar work (Spindeldreier & Deichmann, 1980). The four people in
these cases showed a positive reaction to patch tests with 0.01%
chlorothalonil in acetone.
A further case of contact dermatitits was described by Meding
(1986) in a 33-year-old male painter, who regularly worked with paint
containing chlorothalonil. A patch test with chlorothalonil was
positive.
Work-related skin complaints occurred in a Norwegian factory
producing wooden window frames. The wood preservative used was white
spirit containing 0.5% chlorothalonil. Fourteen out of 20 workers
experienced some kind of skin reaction including pruritus, erythema
and oedema of the eyelids and other facial regions, and eruptions on
arms and hands. Seven of these 14 subjects yielded a positive patch
test reaction with 0.01% chlorothalonil in acetone compared with 1 out
of 14 controls (Johnsson et al., 1983).
Allergic contact dermatitis has also been described in Japanese
farmers (Horiuchi & Ando, 1980) and in Dutch horticultural workers
(Bruynzeel & van Ketel, 1986) using chlorothalonil fungicide
formulations.
In a group of 84 tea growers, two showed a positive skin patch
test with 0.02% chlorothalonil in petrolatum (Fujita, 1985).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Aquatic microorganisms
The algicidal activity of chlorothalonil was examined by Goulding
(1971). It was shown that this compound is effective against a range
of algae including Chlorella, Chlamydomonas, Ulothrix , Anabaena,
Oscillatoria and Microcystis at low concentrations, often
less than 1 µg/litre. It was also effective on natural populations
of algae obtained from lakes, rivers, and reservoirs. The effect
was generally less after 300 h than after 150 h and was dependent upon
the size of the initial cell inoculum.
Walker et al. (1984) describe a simple shake-flask screening test
to evaluate pesticide persistence and aquatic toxicity in the
laboratory. Four systems were used: active sediment, sterile
sediment, active water and sterile water. Chlorothalonil at
162 µg/litre did not increase the mortality of Mysidopsis above the
control level within 96 h. The authors concluded that degradation of
chlorothalonil involved microorganisms and that the degradation
products did not enhance the aquatic toxicity of chlorothalonil.
9.1.1.2 Soil microorganisms
Chlorothalonil, at dose levels up to 5000 mg/litre in bacterial
suspensions, inhibited the growth of three strains of Rhizobium
japonicum (Tu, 1980).
Chlorothalonil, at concentrations up to 1000 mg/kg medium, did
not inhibit or stimulate the growth of any one of 25 strains of
Rhizobium bacteria isolated from red clover root nodules
(Heinonen-Tanski et al., 1982).
Several studies were conducted with chlorothalonil in soil to
determine the effects (if any) on normal soil processes such as
nitrogen fixation, nitrification and degradation of substrates such as
protein, pectin, cellulose and starch. These studies were conducted
at two rates: use rate (2.5 mg/kg) and 10 times the use rate
(25 mg/kg). In general, any inhibitory effects observed were
temporary in nature and more pronounced at the high rate. No effects
on the use of protein, pectin or cellulose by soil microorganisms were
observed, but there was increased utilization of starch (Szalkowski et
al., 1980).
The results obtained by Szalkowski et al. (1981a), who studied
the effect of chlorothalonil on non-symbiotic nitrogen-fixing soil
microorganisms, are given in Table 13.
Table 13. The effect of chlorothalonil on non-symbiotic nitrogen-fixing soil microorganisms
Sandy loam Clay loam
Application Ten times Application Ten times
rate application rate application
rate rate
Aerobic nitrogen initial inhibition inhibition at no effect stimulation
fixation days 0, 21 and
28
Anaerobic nitrogen general stimulation inhibition up no effect stimulation
fixation to day 21
Szalkowski et al. (1981b) studied the effect of chlorothalonil on
nitrogen transformation in sandy loam and clay loam soils at two
rates: one equivalent to the application rate and one equivalent to
ten times this rate. In sandy loam soil, there was a consistent
inhibitory effect at the higher rate, which was reduced with time. In
clay loam soil, at the higher rate, inhibition was no longer observed
after day 21. In both types of soil at the normal rate, there was
little if any inhibition.
9.1.2 Aquatic organisms
The acute toxicity of chlorothalonil to various aquatic species
is shown in Table 14. Some of these toxicity tests were carried out
in static or semi-static systems and others in flow-through systems.
Table 14. Acute toxicity of chlorothalonil to aquatic organisms
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Rainbow trout
(Oncorhynchus mykiss)
6-11 g flow through freshwater 80% 14 acetone > 99% 19.0 17.1 Davies &
6-11 g flow through freshwater 53% 16 acetone > 99% 18.8 10.5 White
6-11 g semi-static freshwater 90% 10 acetone > 99% 18.0 (1985)
(24 h)
- static freshwater - 12 acetone 96% 56 49 SDS Biotech
(soft) Corporation
(1980a)
3.5-4.0 g static freshwater 6.5-7.4 8.4-11.2 12.5-15.5 acetone 97.8% 76 Ernst et
(12.3 mg/litre) mg/litre al. (1991)
3.5-4.0 g static freshwater 6.5-7.4 8.4-11.2 12.5-15.5 Bravo 500 69 Ernst et
(12.3 mg/litre) mg/litre al. (1991)
Bluegill
(Lepomis macrochirus)
- static freshwater 22 acetone 96% 46-77 62 SDS Biotech
(soft) Corporation
(1979)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Common jolly tail
(Galaxias maculatus)
7-10 g flow through freshwater 75% 16 acetone 99% 18.2 16.3 Davies &
White (1985)
Spotted galaxis
(G. fruttaceus)
8-20 g flow through freshwater 75% 16 acetone 99% 25.8 18.9 Davies &
White (1985)
Golden galaxias
(G. auratus)
7-11 g flow through freshwater 75% 13 acetone 99% 46.6 29.2 Davies &
White (1985)
Three spine stickleback
(Gasterosteus aculeatus)
0.3 g static freshwater 7.7-8.0 9.2-9.5 9-10 Bravo 500 < 73 Ernst et
mg/litre al. (1991)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Channel catfish
(Ictalurus punctalus)
40-80 g semi-static, freshwater 7.0-7.2 5.0-6.0 23 acetone 99% 62 52 Gallagher et
24 h (30 mg/litre) mg/litre al. (1992)
- static freshwater 22 acetone 96% 55 44 SDS Biotech
(soft) Corporation
(1980b)
Spot
(Leiosfomus xanthurus)
- flow through brackish water 11 technical 32 Mayer (1987)
(22 ppt)
Sheepshead minnow
(Cyprinodor variegatus)
3-7 days static marine technical 32 SDS Biotech
Corporation
(1982b)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Water flea
(Daphnia magna)
- static freshwater 7.7-8.1 9.1-9.3 20-22 Bravo 500 97a Ernst et
(250 mg/litre) mg/litre al. (1991)
Dungeness crab
(Cancer magister)
larvae semistatic, marine 13 Bravo, 75% 560 140 Armstrong
24h (25 ppt) et al. (1976)
Clam
(Mya arenaria)
5.2 cm static marine 7.3-8.0 8.5-9.9 10.5-12 Bravo 500 35 000 Ernst et
(30-31 ppt) mg/litre al. (1991)
Blue mussel
(Mytilus edulis)
5.9 cm static marine 7.3-8.0 8.5-9.9 10.5-12 Bravo 500 5940 Ernst et
(30-31 ppt) mg/litre al. (1991)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Eastern oyster
(Crassostrea virginica)
- flow through marine 29 technical 26b Mayer (1987)
(27 ppt)
a EC50 - immobility
b EC50 - shell deposition
In view of the strong adsorption and degradation characteristics of
chlorothalonil, nominal concentrations in static systems are likely to
underestimate the toxicity (overestimate the LC50) of chlorothalonil.
At the same time, it should be pointed out that the static test
systems more closely resemble the field situation.
Davies & White (1985) described the reactions of fish to
exposure. Oncorhynchus mykiss and Galaxias sp showed marked
lethargy, the degree increasing with time and concentration of
exposure. O. mykiss showed normal startle reactions at
concentrations below 8.7 µg/litre, and G. maculatus, G. truttaceus
and G. auratus at 8.8, 9.0 and 13.3 µg/litre, respectively, over
96 h. In O. mykiss, loss of startle reaction was followed by
reduction of activity, and permanent lethargy was followed by loss of
righting ability and death. In Galaxias sp, the onset of lethargy
was accompanied by varying degrees of fin collapse.
Davies & White (1985) commented on the fact that most acute
toxicity values have been obtained in tests where solvent was added to
the chlorothalonil.
A 48-h EC50 has been reported for the pink shrimp ( Penaeus
duorarum) at 320 µg/litre and a 96-h EC50 for the eastern oyster
( Crassostrea virginica) at 26 µg/litre. The value for the pink
shrimp was based on immobility or loss of equilibrium and that for the
eastern oyster on shell deposition (Mayer, 1987). Oysters suffered a
42% mortality when exposed to 1000 µg chlorothalonil per litre for
96 h and a 25% reduction in growth at 10 µg/litre (SDS Biotech
Corporation, 1983b).
Ernst et al. (1991) used both laboratory bioassay and field
treatments of a pond system to determine the toxic effects of
chlorothalonil on aquatic fauna. The acute toxicity of technical
chlorothalonil and a commercial formulation on five species, including
mussel and clams, was determined (Table 14). In the field study also
reported by O'Neill (1991) (see section 5.1.2 for measured
concentrations after spraying), the mortality of caged invertebrate
and vertebrate species was monitored. Seven species were caged in the
stream flowing from the treated pond: water boatman (Sigara
alternata), caddisfly larva ( Limnephilus sp), freshwater clam
( Pisidium, sp), crawling water beetle ( Haliplus sp), scud
( Gammarus spp.), stickleback (Gasterosteus aculeatus) and midge
larva (Chironomidae). The floating cages were placed near the
surface of the water. The water boatman suffered the highest
mortality, ranging from 49 to 84% in replicate cages; mortality in a
control pond was 16-20% over the same period (24 h). Midge larva
mortality (69%) was judged by the authors to be a consequence of
handling. Stickleback mortality was 37 to 56% in the treated pond,
compared to 2 to 6% in the controls. Caddisfly larvae, clams, beetles
and scud showed no deaths during the 24 h. Rainbow trout
(Oncorhynchus mykiss) also showed no mortality following spraying.
An estimate of total invertebrate numbers before and after spraying in
the pond showed a slight increase following the first spray and a
slight reduction after the second (not statistically significant).
The control pond also showed fluctuations. The total was heavily
influenced by Chironomid midge larvae, which was by far the most
frequently occurring species. This study showed that a lower
toxicological effect was observed in the field study than in the
laboratory bioassays, indicating a reduction in exposure to the
available chlorothalonil and thus less severe impacts in the pond
system through physical and chemical processes.
Gallagher et al. (1992) showed that sublethal chlorothalonil
exposure may cause acute necrosis of the intestinal epithelial lining
in channel catfish. Exposure to 13 µg/litre for 72 h resulted in
increased tissue GSH concentrations in liver, posterior kidney and
gills, which suggests a protective role for tissue GSH against
chlorothalonil exposure.
When two generations of Daphnia magna were exposed to technical
chlorothalonil at levels of 6.2, 12, 25, 50 and 100 µg/litre for 21
consecutive days during each generation, adverse effects on adult
survival and reproduction were observed at a nominal concentration of
100 µg/litre. The maximum acceptable toxicant concentration (MATC)
for technical chlorothalonil, based upon nominal concentrations, was
50 µg/litre (35 µg/litre was the measured concentration) (Shults et
al., 1982).
Fathead minnows (Pimephales promelas) were continuously exposed
in duplicate aquaria to nominal concentrations of 25, 12.5, 6.3, 3.1
and 1.5 µg/litre (measured concentrations 16, 6.5, 3.0, 1.4 and
0.6 µg/litre, respectively) technical chlorothalonil, a diluent water
control, and a solvent (acetone) control throughout a complete (egg to
egg) life cycle. No significant effects were observed in either
generation at mean measured concentrations < 3.0 µg/litre. The
first generation (F0) eggs exhibited a significantly reduced
hatchability and survival of fry after 35 days when exposed to a mean
measured concentration of 16 µg/litre. The reproductive success of
Fo fish was adversely affected (reduction in the number of eggs per
spawn) by exposure to concentrations > 6.5 µg/litre. The second
generation (F1) eggs exhibited a significantly reduced hatchability
when exposed to a mean measured concentration of 6.5 µg/litre. The
survival of fry at this concentration was not affected. Based on
these data, the MATC (mean measured) of technical chlorothalonil in
water for fathead minnows was estimated to be in the range of 3.0 to
6.5 µg/litre (Shults et al., 1980).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
In a study by Stephenson et al. (1980), 30-day-old tomato plants
were treated with chlorothalonil (at 2.5 kg/ha) or chlorothalonil in
combination with metribuzine. The effect was assessed as the tomato
shoot dry weight (as a percentage of control plant weight). On this
basis, the weight of the chlorothalonil-treated plants was 89% of the
control plant weight, a difference which was statistically significant
(P > 0.05). The effect was additive when chlorothalonil was used in
combination with metribuzine.
9.1.3.2 Earthworms
When chlorothalonil suspension concentrate (500 g/litre) was
added to artificial soil containing earthworms (Eisenia foetida),
the treated soil was non-toxic after 7 and 14 days. The LC50 was
found to be > 1000 mg/kg soil (on a dry weight basis) (Wuthrich,
1990).
When earthworms (Eisenia foetida) were immersed for 1 min in
solutions of chlorothalonil (0.1, 1 and 2% w/v); there was no effect
on survival. Bermuda grass clippings were air dried and ground; 15 g
samples were then stirred into 100 ml of 0.1% chlorothalonil and
subsequently fed to earthworms after filtration of excess liquid.
There was no effect on longevity. Worms reared in soil in which
chlorothalonil had been incorporated showed reduction in longevity of
about 50% compared to controls 52-84 days after the beginning of
treatment. The amount of chlorothalonil added was equivalent to 5
times the recommended application rate at 0.9 g in 4700 cm3 of soil,
and reproduction was virtually eliminated (Roark & Dale, 1979).
9.1.3.3 Earwigs and honey-bees
Earwigs (Labidura riparia) were exposed to chlorothalonil in
three ways: a) on glass; b) as a residue on peanut foliage; and c) as
a residue on a food source (i.e. 7-day-old armyworms). In the first
treatment, chlorothalonil at a rate of 0.72 kg/ha produced up to 20%
mortality in 24 h and up to 30% in 48 h. In the second experiment,
chlorothalonil was applied, at the same rate, to peanut foliage which
was then used for the test. There was a 10% mortality within 24 h and
20% in 48 h on 4-day-old residues and no mortality on 8-day-old
residues. In the food source experiment there was 25-30% mortality
within 24 h of the larvae being consumed and up to 55% mortality
within 48 h (DeRivero & Poe, 1981).
Atkins et al. (1975) found no contact toxicity of chlorothalonil
(11 µg/bee), and classified it as relatively non-toxic to honey-bees.
The oral LD50 for chlorothalonil in 20% sucrose solution for
honey-bees was > 0.2 µg/bee and the contact LD50 > 65 µg/bee
(Davies, 1986).
9.1.3.4 Birds
The following toxicity values have been reported for birds, but
without descriptions of the experimental methodology. The acute oral
LD50 in the mallard duck was reported to be > 4640 mg/kg. The 8-day
dietary LC50 in the same species and in the bobwhite quail (Colinus
virginianus) was given as > 10 000 mg/kg diet in each case (SDS
Biotech Corporation, 1981a,b). Dietary 8-day LC50 values were also
reported as > 21 500 mg/kg diet for the mallard duck and 5200 mg/kg
diet for the bobwhite quail (Worthing, 1991).
Shults et al. (1988a) evaluated the effect of chlorothalonil on
reproduction in the bobwhite quail. Four groups (16 pairs per group)
of quail were administered chlorothalonil in the diet at levels of
1000, 5000 and 10 000 mg/kg for a period of 21 weeks. Quail were fed
the amended diet for 11 weeks prior to egg laying and for the duration
of the egg laying period. Adult and offspring were examined for body
weight, general health, adult food consumption, egg production,
eggshell thickness, embryo viability, hatching success survivability
of offspring and gross pathology. At a dietary concentration of
10 000 mg/kg, the birds experienced reproductive impairment, which
included mortality, general health, body weight, food consumption,
gross pathology and other reproductive end-points. Hatching survival
was also affected at the highest dosage. General health and
reproductive parameters were also affected at 5000 mg/kg. Other
effects observed at this dose level included decreased body weight
gain and survivability of offspring. The lowest test dosage showed no
apparent affects on either adult quail or offspring. A
no-observed-effect concentration (NOEC) of 1000 mg/kg diet was
established for chlorothalonil regarding reproductive effects.
In a separate but similar reproductive study with mallard ducks
(Anas platyrhynchos), Shults et al. (1988b) reported that
10 000 mg/kg diet reduced egg production and the percentage of
hatchlings per incubated egg. No reproductive impairments were
observed for ducks dosed at 1000 or 5000 mg/kg. Shults et al. (1988b)
reported no effect on eggshell thickness at these chlorothalonil
concentrations. The NOEC for reproductive effects in mallard duck was
5000 mg/kg diet.
9.2 Field observations
9.2.1 Soil microorganisms
Smiley & Craven (1979) applied chlorothalonil (as Daconil 2787)
at the normal rate (actual dose not given) every 21 days between April
and September (9 applications for 3 consecutive years) to an
experimental plot of Sward Kentucky blue grass (Pia pratensis).
Treated plots were 1 × 5 m and were replicated. Samples of soil cores
2.54 cm in diameter and 3 cm deep were taken (n = 5) from each
replicate plot. The cores included thatch and were chopped and mixed
well, and 10 g was suspended in sterile distilled water. A dilution
series was plated out to estimate bacterial, actinomycete and fungal
populations. None of the organisms were affected by chlorothalonil.
The fungicide did not affect numbers of Nitrosomonas or Nitrobacter
bacteria and had no effect on the disappearance of added ammonium by
nitrification. Treatments alternating different fungicides had a
greater effect than one fungicide alone.
9.2.2 Plants
Many crop plants are tolerant of chlorothalonil and do not suffer
any phytotoxicity when sprays of chlorothalonil formulations are
applied according to recommended practices. However, a few phytotoxic
effects have been observed with certain species as a direct result of
chlorothalonil applications and also as a result of physiologically
incompatible mixtures of chlorothalonil formulations with additives or
other pesticides.
Chlorothalonil applied at a rate of 2.52 kg/ha did not affect
yields of tomato plants grown in field conditions. In addition,
chlorothalonil did not enhance metribuzin damage when the two
compounds were used in combination on tomato plants. These results
are contrary to those obtained in laboratory studies (section 9.1.3)
and indicate that under field conditions phytotoxic reactions between
chlorothalonil and metribuzin are unlikely to occur (Stephenson et
al., 1980).
Clear results were not obtained concerning the effect of
chlorothalonil on perennial rye grass (Lolium perenne) when it was
sprayed regularly for the control of leaf-spotting disease. In the
first year of treatment the grass yield was increased by 15% at the
third harvest, but at the following harvest, when disease incidence
was greater, the yield was not increased. The authors concluded that
chlorothalonil did not have a direct stimulatory effect on grass
growth (Lam & Lewis, 1983).
Chlorothalonil was used to treat onion beds at a rate of
2.34 litres formulation/ha with 10 weekly applications. The onions
(two short-day cultivars) were harvested either 146 or 167 days after
planting. Chlorothalonil reduced the weight and size of marketable
bulbs by 44 and 32%, respectively, but did not influence the
maturation rate. Alternate application of chlorothalonil and mancozeb
reduced these values by 27 and 33%, respectively. The disease
incidence was nil in all treatments (Stoffella & Sonoda, 1982).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed
and evaluated chlorothalonil at its meetings in 1974, 1977, 1978,
1979, 1981, 1983, 1985, 1987, 1990 and 1992 (FAO/WHO, 1975, 1978,
1979, 1980, 1982, 1985, 1986a,b, 1988, 1990a,b, 1993b). In 1990 an
acceptable daily intake (ADI) of 0-0.03 mg/kg body weight was
established. This ADI was confirmed in 1992, based on the NOAEL of 3
mg/kg body weight/day established in the two-year dog study (FAO/WHO,
1993a,c).
The Joint FAO/WHO Codex Alimentarius Commission has established
maximum residue limits (MRLs) for chlorothalonil in various
commodities (FAO/WHO, 1993c).
WHO has classified chlorothalonil as a technical product unlikely
to present an acute hazard in normal use (WHO, 1992).
On the basis of data available at the time, the International
Agency for Research on Cancer evaluated chlorothalonil as showing
limited evidence of carcinogenicity in animal studies and categorized
it as an agent not classifiable as to its carcinogenicity to humans
(IARC, 1987).
REFERENCES
André JC, Marciniszyn JP, & Killeen JC Jr (1991) Evaluation of
mitochondrial function in the presence and absence of sulphur-
containing analogs of chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 3113-88-0107-AM-001,
submitted to WHO by ISK-Biotech, Mentor, Ohio, USA).
Armstrong DA, Buchanan DV, & Caldwell RS (1976) A mycosis caused by
Lagenidium sp.in laboratory-reared larvae of the dungeness crab,
cancer magister, and possible chemical treatments. Department of
Fisheries and Wildlife, Oregon State University, Marine Science
Center, Newport, Oregon 97365. J Invertebr Pathol, 28: 329-336.
Atkins EL, Greywood EA, & MacDonald RL (1975) Toxicity of pesticides
and other agricultural chemicals to honey bees. Riverside, California,
University of California, Division of Agricultural Sciences, 37 pp
(Report No. 2287).
Bach B & Pedersen NB (1980) Contact dermatitis from a wood
preservative containing tetrachloroisophthalonitrile. Contact
Dermatitis, 6: 142.
Ballee DL, Duane WC, Stallard DE, & Wolfe AL (1976) Chlorothalonil.
In: Zweig G & Sherma J ed. Analytical methods for pesticides and plant
growth regulators. New York, Academic Press, vol VIII, pp 266-274.
Banzer CB (1977a) Activity of chlorothalonil in the Salmonella/
microsomal assay for bacterial mutagenicity. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 000-5TX-77-0035-001).
Banzer CB (1977b) Activity of chlorothalonil in a test for
differential inhibition of repair deficient and repair competent
strains of Salmonella typhimurium: Repair test. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 000-5TX-77-0033-001).
Banzer CB (1977c) Activity of chlorothalonil in an in vitro
mammalian cell point mutation assay. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 000-5TX-77-0034-001).
Banzer CB (1977d) Activity of DTX-0038 in the Salmonella/microsomal
assay for bacterial mutagenicity. Bethesda, Maryland, Microbiological
Associates Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Banzer CB (1977e) Activity of DTX-77-0040 in an in vitro mammalian
cell point mutation assay. Bethesda, Maryland, Microbiological
Associates Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Banzer CB (1977f) Activity of DTX-77-0039 in a test for differential
inhibition of repair deficient and repair competent strains of
Salmonella typhimurium: Repair test. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Blackmore R & Shott L (1968) Final report four-month feeding study -
rats. Hazleton Laboratories, Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Bruynzeel DP & van Ketel WG (1986) Contact dermatitis due to
chlorothalonil in floriculture. Contact Dermatitis, 14: 67-68.
Burchfield HP & Storrs EE (1977) Residue analysis. In: Seigel MR &
Sisler HD ed. Antifungal compounds. New York, Marcel Dekker, Inc., vol
1, pp 463-505.
Busey WM (1975) Histopathological incidence data - kidney. Herndon,
Virginia, Experimental Pathology Laboratory, Inc. (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Busey WM (1985) A 90-day feeding study in mice with T-117-11.
Experimental Pathology Laboratories, Inc. (Unpublished revised
pathology report No. SDS 753-5TX-85-0053-002, submitted to WHO by SDS
Biotech, Mentor, USA).
Camoni I, DiMuccio A, Pontecorvo D, Rubbiani M, Vergori L, & Lugaresi
C (1991) Residue levels in apples and pears field-treated with two
experimental chlorothalonil formulations. Bull Environ Contam Toxicol,
46: 361-367.
Chin BH, McGloin JB, Spangler NL, & Heilman RD (1981) Chlorothalonil
equivalents in the blood and urine of rats following oral,
endotracheal and dermal administration of 14C-chlorothalonil. Bull
Environ Contam Toxicol, 26: 258-261.
Danks A & Fowler JSL (1988) Chlorothalonil technical: acute inhalation
toxicity study in the rat. Eye, Suffolk, United Kingdom, Life Science
Research Ltd (Report No. 88/CFA 00I/85, submitted to WHO by ISK-
Biotech, Mentor, USA).
Davies PE (1985a) The toxicology and metabolism of chlorothalonil in
fish. II. Glutathione conjugates and protein binding. Aquat Toxicol,
7: 265-275.
Davies PE (1985b) The toxicology and metabolism of chlorothalonil in
fish. III. Metabolism, enzymatics and detoxication in Salmo spp and
Galaxias spp. Aquat Toxicol, 7: 277-299.
Davies PE (1985c) The toxicology and metabolism of chlorothalonil in
fish. IV. Zinc coexposure and the significance of metallothionein in
detoxication in Salmo Gairdneri. Aquat Toxicol, 7: 301-306.
Davies LG (1986) Report on a laboratory investigation into the
toxicity of Sipcam chlorothalonil to honey bees (Apis mellifera).
Nottingham, Trent Polytechnic, Department of Sciences (Report
submitted to WHO by ISK-Biotech, Mentor, USA).
Davies PE (1988) Disappearance rates of chlorothalonil (TCIN) in the
aquatic environment. Bull Environ Contam Toxicol, 40: 405-409.
Davies PE & White RWG (1985) The toxicology and metabolism of
chlorothalonil in fish I. Lethal levels for Salmo gairdneri,
Galaxias maculatus, G. truttaceus and G. auratus and the fate of
14C-TCIN in S. gairdneri. Aquat Toxicol, 7: 93-105.
DeRivero NA & Poe SL (1981) Response of Labidura riparia (Pallas) to
residues of pesticides used on peanuts. Peanut Sci, 8: 93-96.
Diamond Shamrock (1974) Unpublished report on the pesticide
chlorothalonil, submitted to FAO/WHO by Diamond Shamrock Corporation,
Mentor, Ohio, USA.
Diamond Shamrock (1980) Unpublished toxicological report, submitted to
the 1981 JMPR by Diamond Shamrock Corporation, Mentor, Ohio, USA.
Doyle R & Elsea J (1963) Acute oral, dermal, and eye toxicity and
irritation studies on DAC-2787. Hill Top Research Institute Inc.
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
Duane WC (1970) Biodegradation of Daconil 2787. Mentor, Ohio, Diamond
Shamrock Corporation (Unpublished report).
Eilrich GL & Chelsky M (1991) Letters to the editor : Facial
dermatitis caused by chlorothalonil in paint. Contact Dermatitis, 25:
141-144.
Elliott VJ & Spurr HW (1993) Temporal dynamics of chlorothalonil
residues on peanut foliage and the influence of weather factors and
plant growth. Plant Dis, 77: 455-460.
Ernst W, Doe K, Jonah P, Young J, Julien G, & Hennigar P (1991) The
toxicity of chlorothalonil to aquatic fauna and the impact of its
operational use on a pond ecosystem. Arch Environ Toxicol, 21: 1-9.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO (1985) Guidelines for the disposal of waste pesticide and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1975) 1974 Evaluations of some pesticide residues in food:
The monographs. Geneva, World Health Organization, pp 101-148 (WHO
Pesticide Residues Series, No. 4).
FAO/WHO (1978) Pesticide residues in food - 1977. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Sup.).
FAO/WHO (1979) Pesticide residues in food - 1978. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Sup.).
FAO/WHO (1980) Pesticide residues in food - 1979. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Sup.).
FAO/WHO (1982) Pesticide residues in food - 1981. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1985) Pesticide residues in food - 1983. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 61).
FAO/WHO (1986a) Pesticide residues in food - 1985. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 72/1).
FAO/WHO (1986b) Pesticide residues in food - 1985. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 72/2).
FAO/WHO (1988) Pesticide residues in food - 1987. Evaluations: Part II
- Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 86/2).
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Codex Committee on Pesticide
Residues, Food and Agriculture Organization of the United Nations
(CAC/PR8-1989).
FAO/WHO (1990a) Pesticide residues in food - 1990. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 103/1).
FAO/WHO (1990b) Pesticide residues in food - 1990. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 103/2).
FAO/WHO (1991) Pesticide residues in food - 1990. Evaluations: Part II
- Toxicology. Geneva, World Health Organization (WHO/PCS/91.47).
FAO/WHO (1993a) Pesticide residues in food - 1992. Report. Rome, Food
and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper 116).
FAO/WHO (1993b) Pesticide residues in food - 1992. Evaluations: Part
II - Toxicology. Geneva, World Health Organization (WHO/PCS/93.34).
FAO/WHO (1993c) Codex Alimentarius. Volume 2: Pesticide residues in
food. Rome, Food and Agriculture Organisation of the United Nations.
Flannigan SA & Tucker SB (1985) Influence of the vehicle on irritant
contact dermatitis. Contact Dermatitis, 12: 177-178.
Flannigan SA, Tucker SB, & Calderon V (1986) Irritant dermatitis from
tetrachloroisophthalonitrile. Contact Dermatitis, 14: 258-259.
Ford WH (1982) A one-generation reproduction study in rats with
DS-3701. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Ford WH, Killeen JC, & Baxter RA (1987a) A 90-day feeding study in
rats with chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 1115-85-0079-TX-006).
Ford WH, Killeen JC, & Baxter RA (1987b) A 90-day study in rats with
the monoglutathione conjugate of chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1108-85-0078-TX-006).
Frazier HW (1993) Chlorothalonil: A response to Environmental Fate and
Ground Water Branch (EFGWG) review. Painesville, Ohio, Ricerca, Inc.
(Document No. 3254-93-0280-EF-001, submitted to WHO by ISK Biosciences
Corporation, Mentor, USA).
Fujita Y (1985) Studies on contact dermatitis from pesticides in tea
growers. Acta Med Univ Kagoshima, 27(1): 17-37.
Fullmore GE & Leveglia J (1992) A thirty-day toxicity study in dogs
with T-117-2. Painesville, Ohio, Ricerca, Inc. (Unpublished report
No. 5092-91-0554-TX003, submitted to WHO by ISK-Biotech Corporation,
Mentor, USA).
Gallagher EP, Cattley RC, & Di Giulio RT (1992) The acute toxicity and
sublethal effects of chlorothalonil in channel catfish (Ictalurus
punctatus). Chemosphere 24: 3-10.
GIFAP (1987) Guidelines for the avoidance, limitation and disposal of
pesticide waste on the farm. Brussels, International Group of National
Associations of Manufacturers of Agrochemical Products.
Gilvydis DM & Walters SM (1988) Rapid liquid chromatographic analysis
of chlorothalonil in fresh produce using photoconductivity and UV
detectors in tandem. J Agric Food Chem, 36: 957-961.
Goulding KH (1971) A preliminary investigation of a potential new
algicide. Proceedings of the 6th British Insecticides and Fungicides
Conference, pp 621-629.
Green T, Odum J, Nash JA, & Foster JR (1990) Perchloroethylene-induced
rat kidney tumours: An investigation of the mechanisms involved and
their relevance to humans. Toxicol Appl Pharmacol, 103: 77-89.
Gutenmann WH & Lisk DJ (1966) Metabolism of Daconil and Dacthal
pesticides in lactating cows. J Dairy Sci, 49(10): 1272-1276.
Hastings TF & Clifford D (1975) Eight-week dominant lethal study of
DAC-3701 in rats. Bio/Tox Research Laboratories, Inc. (Unpublished
report submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Hatzenbeler CJ (1991) An aerobic aquatic metabolism study with
14C-chlorothalonil. Painesville, Ohio, Ricerca, Inc. (Document No.
3163-90-0240-EF-001, submitted to WHO by ISK Biosciences Corporation,
Mentor, USA).
Heinonen-Tanski H, Oros G, & Kecskes M (1982) The effect of soil
pesticides on the growth of red clover rhizobia. Acta Agric Scand, 32:
283-288.
Holmes DC & Wood NF (1972) Removal of interfering substances from
vegetable extracts prior to the determination of organochlorine
pesticide residues. J Chromatogr, 67: 173-174.
Holsing G & Shott L (1970) Two-year dietary administration - rats.
Vienna, Virginia, Hazleton Laboratories, Inc. (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Holsing GC & Voelker RW (1970) 104-Week dietary administration - dogs,
Daconil 2787 (technical). Hazleton Laboratories, Inc. (Unpublished
report submitted to WHO by Biotech Corporation, Mentor, USA).
Horiuchi N & Ando S (1980) Contact dermatitis due to pesticides for
agricultural use. Jpn J Dermatol, 90: 289.
Hozan GK & Auletta CS (1981) A chronic dietary study in mice with
T-114 (DS-3701). East Millstone, New Jersey, BioDynamics, Inc.
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
IARC (1983) Chlorothalonil. In: Miscellaneous pesticides. Lyon,
International Agency for Research on Cancer, pp 319-328 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 30).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 60 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Ignatoski JA, Killenen JC, & Marciniscyn JP (1983) Distribution of
radioactivity following oral administration of 14C-chlorothalonil to
rats: extraction and analysis of 14C-materials in excreta. Mentor,
Ohio, Diamond Shamrock Corporation (Unpublished report).
IRPTC (1989) Data profile on chlorothalonil. Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
ISK Biosciences Corporation (1995) A summary of chlorothalonil
metabolism under various environmental conditions in the
chlorothalonil summary dossiers for the inclusion of active substances
in Annex 1 of Directive 91/414/EEC. Mentor, Ohio, ISK Biosciences
Corporation.
Jacobson BM & Schollenberger JM (1992) Dissipation of 4-hydroxy-2,4,5-
trichloroisophthalonitrile (SOS-3701) in soil: A re-analysis of
chlorothalonil soil dissipation data. Painesville, Ohio, Ricerca, Inc.
(Proprietary report No. 1874-T, submitted to WHO by ISK-Biotech,
Mentor, USA).
Jarrett RD, Stallard DE, & Bachand RT (1978) Absorption, excretion and
tissue distribution of orally administered 14C-hydroxy 2,5,6-
trichloroisophthalonitrile (14C-DAC 3701) in male Sprague-Dawley
rats. Painsville, Ohio, T.R. Evans Research Centre (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Johnsson M, Buhagen M, Leira HL, & Solvang S (1983) Fungicide induced
contact dermatitis. Contact Dermatitis, 9: 285-288.
Jones RE, Killeen JC Jr, & Ignatoski JA (1984) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 694-5TX-84-0064-002).
Jongen NJ, Engel R, & Leenheers LH (1991) Determination of the
pesticide chlorothalonil by HPLC and UV detection for occupational
exposure assessment in greenhouse carnation culture. J Anal Toxicol,
15: 30-34.
Katayama A, Isemura H, & Kuwatsuka S (1991) Suppression of
chlorothalonil dissipation in soil by repeated applications. J Pestic
Sci, 16: 233-238.
Kawamura Y, Takeda M, & Uchiyama M (1978) Photolysis of chlorothalonil
in benzene. J Pestic Sci, 3: 397-400.
Kenyon RG & Ballee DL (1990) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1989). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-007).
Kenyon RG & Wiedmann JL (1992a) Analytical methods including recovery
rates and the limits of determination for residues in water. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 5221-92-0072-MD-001).
Kenyon RG & Wiedmann JL (1992b) Analytical methods including recovery
rates and the limits of determination for residues in soil. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 5221-92-0073-MD-001).
King C, Prince PM, & Ballee DL (1991) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1990). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-008).
King C, Prince PM, & Ballee DL (1992) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1991). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-010).
Koseki M, Shimamura Y, Maki T, Tamura Y, & Naoi Y (1980) Residues of
TPN (tetrachloroisophthalonitrile), captan, diazinon and
chinomethionate in vegetables and fruits. Tokyo Toriotu Eisei
Kenkyusho Kenkyu Nempo, 31:(1) 170-173.
Krawchuk BP & Webster GRB (1987) Movement of pesticides to ground
water in an irrigated soil. Water Pollut Res J Can, 22(1): 129-145.
Kunkel JF (1967a) Absence of 14C movement in crop plant organs after
topical application and soil amendment studies with isotopic Daconil
2787. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished report).
Kunkel JF (1967b Movement of 14C in or on roots of crop species grown
in soil amended with isotopic Daconil 2787. Mentor, Ohio, Diamond
Shamrock Corporation (Unpublished report).
Ladd K, Jenkins DH, & Kepplinger ML (1971) Meat and milk residues
study with technical Daconil 2787 - 97% and pure DAC-3701 in dairy
cattle. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Lam A & Lewis GC (1983) Chemical control of foliar diseases of
perennial rye grass (Lolium perenne L.) and their effects on yield
and quality of the crop. Crop Prot, 2(1): 75-83.
Lawless EW, Ferguson TL, & Meiners AF (1975) Guidelines for the
disposal of small quantities of unused pesticides. Cincinnati, Ohio,
US Environmental Protection Agency (EPA 670/2-75-057).
Lee SS, Marciniscyn JP, Marks AF, & Ignatoski JA (1982) Balance study
of the distribution of radioactivity following oral administration of
14C-chlorothalonil (14C-DS 2787) to rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 000-4AM-81-0209-001).
Legator MS (1974a) Mutagenic testing with DAC 2787. Providence, Rhode
Island, Roger Williams General Hospital, Division of Genetics and
Brown University, Division of Biological and Medical Sciences
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
Legator MS (1974b) Mutagenic testing with DAC 3701. Mentor, Ohio,
Diamond Shamrock Corporation (Unpublished report).
Leonard JA & Yeary RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51(11): 605-609.
Lewis RG, Bond AE, Johnson DE, & Hsu JP (1988) Measurement of
atmospheric concentrations of common household pesticides : a pilot
study. Environ Monit Assess, 10: 59-73.
Liden C (1990) Facial dermatitis caused by chlorothalonil in paint.
Contact Dermatitis, 22: 206-211.
Lucas F & Benz G (1990) A two generation reproduction study in rats
with technical chlorothalonil. Painesville, Ohio, Ricerda, Inc.
(Proprietary report No. 1722-87-0121-TX-003, submitted to WHO by
ISK-Biotech, Mentor, USA).
Luperi L & Forster R (1988) Delayed contact hypersensitivity study in
the Guinea pig. Rome, Life Science Research, Toxicology Centre S.P.A.
(Report No. 128005-ST-02687, submitted to WHO by ISK-Biotech, Mentor,
USA).
McGee (1983) A two-year toxicity and tumourigenicity study of T-114 in
rats. Mattawan, Michigan, International Research and Development
Corporation (Unpublished report submitted to WHO by Diamond Shamrock
Corporation, Mentor, USA).
McLeod HA, Smith DC, & Bluman N (1980) Pesticide residues in the total
diet in Canada, V: 1976 to 1978. J Food Saf, 2: 141-164.
Magee TA, Marciniszyn JP, & Killeen JC Jr (1990) Study to evaluate the
urinary metabolites of chlorothalonil following dermal application to
male Rhesus monkeys. Painesville, Ohio, Ricerca, Inc. (Report No.
3382-89-02-AM-001, submitted to WHO by ISK-Biotech, Mentor, USA).
Major D, Killeen JC, & Ignatoski JA (1982) Primary eye irritation
study in albino rabbits with technical chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 104-5TX-80-0037-002).
Marciniszyn JP, Savides MC, Killeen JC, & Ignatoski JA (1984a) Study
of the dermal absorption of 14C-chlorothalonil (14C-DS-2787) by male
rats. Mentor, Ohio, Fermenta ASC (Unpublished report No.
649-4AM-84-0010-001).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1984b) Study of the
distribution of radioactivity following oral administration of
(14C-DS-2787) to male Sprague Dawley rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 631-4AM-83-0011-002).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1985a) Study of the
distribution of radioactivity following oral administration of
(14C-SDS-2787) to female Sprague Dawley rats. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 631-4AM-84-0078-002).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1985b) Identification of
metabolites in urine and blood following oral administration of
14C-chlorothalonil (14C-SDS-2787) to male rats: the thiol metabolites
in urine. Mentor, Ohio, Fermenta ASC (Unpublished report No.
621-4AM-83-0061-001).
Marciniszyn JP, Savides MC, Killeen JC, & Ignatoski JA (1986) Study of
the biliary excretion of radioactivity following oral administration
of 14C-SDS-2787 to male Sprague-Dawley rats. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 633-4AM-85-0012-002).
Marciniszyn JP, Killeen JC, & Baxter RA (1988) Pilot study of the
effect of the gamma-glutamyl transpeptidase inhibitor AT-125 on the
metabolism of 14C-chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1376-86-0072-AM-002).
Markus JR & Puma B ed. (1973) Pesticide analytical manual: Methods for
individual pesticide residues: Volume 2 - Pesticide registration
section 120.275. Washington, DC, US Food and Drug Administration.
Matsushita T & Aoyama K (1981) Cross reactions between some pesticides
and the fungicide benomyl in contact allergy. Ind Health, 19: 77-82.
Mayer FL ed. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Gulf Breeze, Florida. Washington, DC, US Environmental
Protection Agency (EPA-600/8-017; NTIS PB 87-188686).
Meding B (1986) Contact dermatitis from tetrachloroisopathalonitrile
in paint. Contact Dermatitis, 15: 187.
Mizens M, Wilson NH, Laveglia J, Killeen JC, & Ignatoski JA (1983) A
teratology study in rats with technical chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 517-5TX-82-0011-003).
Mizens M, Killeen JC, Claudio G, & Ignatoski JA (1985a) In vivo bone
marrow chromosomal aberration assay in mice with a single dose of
technical chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 625-5TX-83-0029-002).
Mizens M, Killeen JC, Claudio G, & Ignatoski JA (1985b) In vivo bone
marrow aberration assay in rats with a single dose of technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 625-5TX-83-0028-002).
Mizens M, Killeen JC, & Ignatoski JA (1985c) Acute and subchronic in
vivo bone marrow chromosomal aberration assay in Chinese hamsters
with technical chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 625-5TX-83-0014-003).
Mizens M, Killeen JC Jr, & Ignatoski JA (1985d) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with 2,5-dichloro-4,6-bismercaptoisoph-
thalonitrile (SDS-3939) (Study No. T4147.501 performed by
Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
SDS Biotech Corporation (Unpublished report No. 694-5TX-85-0042-002,
submitted to WHO by Fermenta Plant Protection Co., Painesville, USA).
Mizens M, Killeen JC Jr, & Ignatoski JA (1985e) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test)
with and without renal activation with 5-(2,4-dicyano-3,5,6-
trichlorophenyl) Glutathione (SDS-66382) (Study No. T4147.501
performed by Microbiological Associates Inc., Bethesda, USA).
Painesville, Ohio, SDS Biotech Corporation (Unpublished report
No. 694-5TX-85-0043-002, submitted to WHO by Fermenta Plant
Protection Co., Painesville, USA).
Mizens M, Killeen JC, & Ignatoski JA (1986a) In vitro chromosomal
aberration assay in Chinese hamster ovary (CHO) cells with technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1109-85-0082-TX-002).
Mizens M, Killeen JC Jr, & Baxter RA (1986b) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with 5-chloro-2,4,6-
trimercaptoisophthalonitrile (SDS-66471) (Study No. T5079.1505
performed by Microbiological Associates Inc., Bethesda, USA).
Painesville, Ohio, Ricerca, Inc., Department of Toxicology and Animal
Metabolism (Unpublished report No. 1097-86-0037-TX-002, submitted to
WHO by Fermenta Plant Protection Co., Painesville, USA).
Mizens M, Killeen JC Jr, & Baxter RA (1986c) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with S,S',S''--(2,4-dicyano-6-
dichlorophenyl)tricysteine (SDS-66473) (Study No. T5081.1505 performed
by Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
Ricerca, Inc., Department of Toxicology and Animal Metabolism
(Unpublished report No. 1097-86-0039-TX-002, submitted to WHO by
Fermenta Plant Protection Co., Painesville, USA.)
Mizens M, Killeen JC Jr, & Baxter RA (1987) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with S,S'(2,4-dicyano-3,6-dichlorophenyl)-
dicysteine (SDS-66474) (Study No. T5080.1505 performed by
Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
Ricerca, Inc., Department of Toxicology and Animal Metabolism
(Unpublished report No. 1097-86-0038-TX-002, submitted to WHO by
Fermenta Plant Protection Co., Painesville, USA).
Modesso P & Forster R (1988) Chromosome aberrations in human
lymphocytes cultured in vitro. Test substance: Chlorothalonil
technical. Rome, Life Science Research, Toxicology Centre S.P.A.
(Report No. 128008-M-10787, submitted to WHO by ISK-Biotech, Mentor,
USA).
Murchison TE (1979) A short-term dietary study in rats with T-114-2
(DS 3701). Dawson Research Corporation (Unpublished report submitted
to WHO by Diamond Shamrock Corporation, Mentor, USA).
O'Neill HJ (1991) Transport of the fungicide chlorothalonil from its
operational use on a pond ecosystem. Bull Environ Contam Toxicol,
46:822-828.
Paynter OE (1965a) Oral dose range: dogs - Final report. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE (1965b) Repeated dermal application: Rabbits. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE (1966a) Two-year dietary administration: dogs DAC 2787.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-66-0002-
001).
Paynter OE (1966b) Reproduction rabbits DAC 2787. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 000-5TX-66-003-001).
Paynter OE (1967a) Final report - Two-year dietary feeding: rats.
Hazleton Laboratories, Inc. (Unpublished report submitted to WHO by
Diamond Shamrock Corporation, Mentor, USA).
Paynter OE (1967b) Three-generation reproduction study: rats DAC 2787.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-67-0005-
001). USA.
Paynter OE (1967c) Ten-week amino acid feeding study: rats. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE & Busey WM (1966) Two-year dietary administration: dogs
DAC 2787. Hazelton Laboratories, Inc. (Unpublished report submitted to
WHO by Biotech Corporation, Mentor, USA).
Paynter OE & Busey W (1967) Long-term (76 weeks) feeding study: rats
DAC 2787. Hazleton Laboratories, Inc. (Unpublished report submitted to
WHO by Diamond Shamrock Corporation, Mentor, USA).
Paynter OE & Crews L (1967) Final report - Two-year dietary feeding:
rats. Hazleton Laboratories, Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Paynter OE & Murphy JC (1967) 16-Week dietary administration: dogs
DAC 2787. Hazelton Laboratories, Inc. (Unpublished report submitted to
WHO by Biotech Corporation, Mentor, USA).
Powers M (1965) Acute oral administration: rats. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Price P (1978a) The activity of compound DTX-77-0037 in the Fischer
rat embryo transformation assay system. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Price P (1978b) The activity of compound DTX-77-0041 in the Fischer
rat embryo transformation assay system. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Reduker S, Uchrin CG, & Winnett G (1988) Characteristics of the
sorption of chlorothalonil and azinphos-methyl to a soil from a
commercial cranberry bog. Bull Environ Contam Toxicol, 41: 633-641.
Ribovich ML, Pollock GA, Marciniszyn JP, Killeen JC, & Ignatoski JA
(1983) Balance study of the distribution of radioactivity following
oral administration of 14C-chlorothalonil (14C-DS-2787) to male mice.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 613-4AM-82-0178-
001).
Roark JH & Dale JL (1979) The effect of turf fungicides on earthworms.
Arkansas Acad Sci Proc, 33: 71-74.
Rosanoff KA & Siegel MR (1981) Mechanism of action and fate of the
fungicide chlorothalonil in biological systems. 3. Interaction with
mammalian DNA, histones and isolated rat liver nuclei. Pestic Biochem
Physiol, 16: 120-128.
Sadler EM, Wilson NH, Killeen JC, & Ignatoski JA (1985) Time course of
the acute effect of technical chlorothalonil on hepatic and renal
glutathione content in male rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 751-5TX-85-0032-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1985)
Isolation and identification of metabolites in the bile of rats orally
administered 14C-chlorothalonil I. Synthesis and characterisation of
glutathione conjugates of chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 633-4AM-84-0104-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986a) Study
of the distribution of radioactivity following repeated oral
administration of 14C-chlorothalonil (14C-SDS-2787) to male
Sprague-Dawley rats. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1173-84-0079-AM-003).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986b)
Identification of metabolites in urine and blood following oral
administration of 14C-chlorothalonil to male rats. II. Effects of
multiple dose administration on the excretion of thiol metabolites in
urine. Mentor, Ohio, Fermenta ASC (Unpublished report No. 621-4AM-83-
0061-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986c) Study
of the biliary excretion of radioactivity following oral
administration of (14C-SDS-2787) to male Sprague-Dawley rats. Mentor,
Ohio, Fermenta ASC (Unpublished report No. 633-4AM-85-0012-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986d) Animal
metabolism method development studies. II. In vitro incubations of
14C-chlorothalonil with stomach and intestinal mucosal cells. Mentor,
Ohio, Fermenta ASC (Unpublished report No. 1172-85-0081-AM-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986e) In
vitro studies on the transfer of 14C-chlorothalonil and/or its
metabolites from the mucosal to the serosal surface of the
gastrointestinal tract. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1179-86-0020-AM-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986f) Pilot
study to determine the concentration of radiolabel in kidneys
following oral administration of the mono-glutathione conjugate of
14C-chlorothalonil to male rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 631-4AM-85-0064-001).
Savides MC, Marciniszyn JP, Killeen JC, & Baxter RA (1987a) Study to
determine the metabolic pathway for chlorothalonil following dermal
application to rats. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1625-87-0057-AM-000).
Savides MC, Marciniszyn JP, Killeen JC, & Baxter RA (1987b)
Determination of the covalent binding of radiolabel to DNA in the
kidneys of male rats administered 14C-chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1173-86-0096-AM-002).
Savides MC, Kidon BJ, Marciniszyn JP, Killeen JC, & Baxter RA (1987c)
Subcellular fractionation of kidneys from male rats administered
14C-chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1178-86-0016-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1988) A study to evaluate
the effects of sulphur-containing analogs of chlorothalonil on
mitochondrial function. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1479-87-0037-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1989) Study to compare the
metabolism of chlorothalonil in dogs with its metabolism in rats
following oral administration of 14C-chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1626-88-0008-AM-001).
Savides MC, Magee TA, Marciniszyn JP, Killeen JC, & Baxter RA (1990a)
Study to evaluate the metabolic pathway of chlorothalonil
(14C-ASC-2787) in germ-free rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 3060-88-0219-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1990b) Study of the urinary
excretion of radiolabel by catheterised dogs following oral
administration of 14C-chlorothalonil by gavage. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 3086-89-0041-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1990c) Study to evaluate
urinary metabolites of chlorothalonil from male Rhesus monkeys.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 3349-89-0179-AM-
001).
SDS Biotech Corporation (1972) Exposure of fish to 14C-labelled
DAC-2787-Tech.: Accumulation, distribution and elimination of
residues. Mentor, Ohio, ISK-Biotech (Proprietary report No. 000-5TX-
72-0006/8-001).
SDS Biotech Corporation (1979) Acute toxicity of DS-3701 to Bluegill
sunfish. Mentor, Ohio, ISK-Biotech (Proprietary report No. 102-5TX-79-
0044-002).
SDS Biotech Corporation (1980a) Technical chlorothalonil - Acute
toxicity (LC50) study in Rainbow trout. Mentor, Ohio, ISK-Biotech
(Proprietary report No. 102-5TX-79-0045-002).
SDS Biotech Corporation (1980b) Technical chlorothalonil - Acute
toxicity (LC50) study in Channel catfish. Mentor, Ohio, ISK-Biotech
(Proprietary report No. 102-5TX-79-0046-002).
SDS Biotech Corporation (1981a) Dietary study (LC50) in Mallard ducks
with DS-3701. Mentor, Ohio, ISK-Biotech (Proprietary report No.
449-5TX-81-0008-002).
SDS Biotech Corporation (1981b) Dietary study (LC50) in Bobwhite
quail with DS-3701. Mentor, Ohio, ISK-Biotech (Proprietary report
No. 448-5TX-81-0007-002).
SDS Biotech Corporation (1982a) Static, acute toxicity study in
Penaeid (pink) shrimp with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0054-002).
SDS Biotech Corporation (1982b) Static, acute toxicity study in
Sheepshead Minnows with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0053-002).
SDS Biotech Corporation (1983a) The effects of cooking 2,4,5,6-
tetrachloroisoph-thalonitrile (chlorothalonil, DS-2787) with
vegetables. Mentor, Ohio, ISK-Biotech (Unpublished report No.
372-3EF-83-0004-001, submitted to WHO by SDS Biotech Corporation,
Mentor, USA).
SDS Biotech Corporation (1983b) Flow-through, acute oyster shell
deposition study with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0133-003).
Shirasu Y & Teramoto S (1975) Teratogenicity study of Daconil in
rabbits. Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-
75-2077-001).
Shirasu Y, Moriya M, & Watanabe K (1977) Mutagenicity testing on
Daconil in microbial systems. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 00-5TX-61-0002-001).
Shults SK, Killeen JC, & Heilman RD (1980) A chronic study in the
fathead minnow (Pimephales promelas) with technical chlorothalonil.
Cleveland, Ohio, Diamond Shamrock Corporation, 16 pp (Proprietary
report No. 090-5TX-79-0049-002, submitted to WHO by ISK-Biotech,
Mentor, USA.
Shults SK, Killeen JC, & Ignatoski JA (1982) Chronic toxicity study in
Daphnia magna with technical chlorothalonil. Cleveland, Ohio,
Diamond Shamrock Corporation, 25 pp (Proprietary report No.
447-5TX-81-0006-002, submitted to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Laveglia J, Killeen JC, & Ignatoski JA (1983) A 90-day
feeding study in mice with technical chlorothalonil. Mentor, Ohio, SDS
Biotech Corporation (Unpublished report No. 618-5TX-83-0007-004).
Shults SK, Wilson NH, Killeen JC, & Ignatoski JA (1986) 21-Day
repeated dose dermal toxicity study in albino rabbits with technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report No.
754-5TX-85-0023-007).
Shults SK, Wilson NH, & Killeen JC (1988a) Reproduction study in
bobwhite quail with technical chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 1469-87-0006-TX-002, submitted
to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Wilson NH, & Killeen JC (1988b) Reproduction study in
mallard ducks with technical chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 1469-87-0004-TX-002, submitted
to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Brock AW, & Killeen JC (1991) Acute (one-hour) inhalation
toxicity (LC50) study in rats with hammer milled technical
chlorothalonil. Painesville, Ohio, Ricerca, Inc. (Proprietary report
No. 3593-90-0168-TX-002, submitted to WHO by ISK-Biotech, Mentor,
USA).
Shults SK, Brock AW, & Laveglia J (1993) Acute (four-hour) inhalation
toxicity (LC50) study in rats with hammer milled technical
chlorothalonil (T-117-15). Painesville, Ohio, Ricerca, Inc.
(Proprietary report No. 5290-92-0160-TX-002, submitted to WHO by
ISK-Biotech, Mentor, USA).
Siou G (1981a) The micronucleus test in the rat, mouse and hamster
using chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 000-5TX-81-0024-004).
Siou G (1981b) The chromosomal aberration test in the rat, mouse and
hamster using chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 000-5TX-81-0025-004).
Smiley RW & Craven MM (1979) Microflora of turfgrass treated with
fungicides. Soil Biol Biochem, 11: 349-353.
Spencer JR, Bissell SR, Sanborn JR, Schneider FA, Margetich SS, &
Krieger RI (1991) Chlorothalonil exposure of workers on mechanical
tomato harvesters. Toxicol Lett, 55: 99-107.
Spindeldreier A & Deichmann B (1980) [Contact dermatitis against a
wood preservative with a new fungicidal agent.] Dermatosen, 28(3):
88-90 (in German).
Stallard DE & Wolfe AL (1967) The fate of 2,4,5,6-
tetrachloroisophthalonitrile (Daconil 2787) in soil. Mentor, Ohio,
Diamond Shamrock Corporation (Unpublished report).
Stallard DE & Szalkowski ME (1976) Effect of microorganisms upon the
soil metabolism of Daconil (i.e., chlorothalonil) and 4-hydroxy-2,5,6-
trichloroisophthalonitrile. Painesville, Ohio, Ricerca, Inc. (Report
No. 1000-3EF-76-2087-001, submitted to WHO by ISK Biosciences
Corporation, Mentor, USA).
Stamper JH, Nigg HN, Mahon WD, Nielsen AP, & Royer MD (1989a)
Applicator exposure to fluvalinate, chlorpyrifos, captan, and
chlorothalonil in Florida ornamentals. J Agric Food Chem, 37: 240-244.
Stamper JH, Nigg HN, Mahon WD, Nielsen AP, & Royer MD (1989b)
Pesticide exposure to a greenhouse drencher. Bull Environ Contam
Toxicol, 42: 209-217.
Stephenson GR, Phatak SC, Makowski RI, & Bouw WJ (1980) Phytotoxic
interactions involving metribuzin and other pesticides in tomatoes.
Can J Plant Sci, 60: 167-175.
Stoffella PJ & Sonoda RM (1982) Reduction of onion yields by
chlorothalonil. Hortscience, 17(4): 628-629.
Suzuki S & Oda M (1977) Simultaneous determination of chlorothalonil,
captan and diazinon in apples. Bull Agric Chem Insp Stn (Tokyo),
17:43-45.
Szalkowski MB & Stallard DE (1977) Effect of pH on the hydrolysis of
chlorothalonil. J Agric Food Chem, 25(1): 208-210.
Szalkowski MB, Stallard DE, & Ignatoski JA (1980) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon the
microbial utilization of selected macromolecules in soil. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 326-3EI-79-0009-001).
Szalkowski MB, Stallard DE, & Ignatoski JA (1981a) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon
non-symbiotic nitrogen fixing soil microorganisms. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 327-3EI-79-0010-001).
Szalkowski MB, Stallard DE, & Ignatoski JA (1981b) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon nitrogen
transformation in soil. Mentor, Ohio, ISK-Biotech (Proprietary report
No. 328-3EI-79-0011-001).
Teeters W (1966) In vivo assay of spasmodic activity, mice, DAC
2787. Hazelton Laboratories (Unpublished report submitted to WHO by
Diamond Shamrock Corporation, Mentor, USA).
Tillman RW, Siegel MR, & Long JW (1973) Mechanism of action and fate
of the fungicide chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile)
in biological systems I. Reactions with cells and subcellular
components of Saccharomyces pastorianus. Pestic Biochem Physiol,
3:160-167.
Tu CM (1980) Effect of fungicides on growth of Rhizobium japonicum
in vitro. Bull Environ Contam Toxicol, 25: 364-368.
UK (1985) Annex 1 - Monitoring of fruits and vegetables (at point of
retail sale) 1981-1983. Annex 2 - Chlorothalonil, soft fruit sampled
directly from growers 1982; soft fruit sampled at retail outlets, 1982
(no treatment histories). Submission to FAO by the UK Ministry of
Agriculture, June 14, 1985.
UK-MAFF & HSE (1990) Report of the working party on pesticide
residues 1988-1989. London, Ministry of Agriculture, Fisheries and
Food and Health and Safety Executive.
US EPA (1976) Manual of chemical methods for pesticides and devices.
Washington DC, US environmental Protection Agency, Office of Pesticide
Programs (Chlorothalonil EPA-1).
US EPA (1990) National survey of pesticides in drinking water wells.
Washington, DC, US Environmental Protection Agency (EPA 570/9-90-015).
US NCI (1978) Bioassay of chlorothalonil for possible carcinogenicity.
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
78-841).
Walker WW, Cripe CR, Pritchard PH, & Bourquin AW (1984) Biological and
abiotic degradation of xenobiotic compounds in in vitro estuarine
water and sediment/water systems. Chemosphere, 17(12): 2255-2270.
Wallace LA (1991) Comparison of risks from outdoor and indoor exposure
to toxic chemicals. Environ Health Perspect, 95: 7-13.
Wazeter FX (1971) Acute oral LD50 in male albino rats. International
Research and Development Corporation (Unpublished report submitted to
WHO by Diamond Shamrock Corporation, Mentor, USA).
Wazeter FX & Goldenthal EI (1976) Teratology study in rabbits.
International Research and Development Corporation (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, Ohio, USA).
Wei CI (1982) Lack of mutagenicity of the fungicide 2,4,5,6-
tetrachloroisophthalonitrile in the Ames Salmonella microsome test.
Appl Environ Microbiol, 43(1): 252-254.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997. Geneva, World Health
Organization. (WHO/PCS/96.3).
Wilson NH (1981) A 90-day toxicity study of technical chlorothalonil
in rats. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Wilson NH, Killeen JC, & Ignatoski JA (1982) Dermal sensitization
study in Hartley-derived guinea-pigs with technical chlorothalonil.
Painesville, Ohio, Ricerca, Inc. (Report No. 394-5TX-81-0132-002,
submitted to WHO by ISK Biosciences Corporation, Mentor, USA).
Wilson NH, Laveglia J, Killeen JC, & Ignatoski JA (1983a) A subchronic
toxicity study of technical chlorothalonil in rats. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 562-5TX-81-0213-004).
Wilson NH, Killeen JC, & Ignatoski JA (1983b) A chronic dietary study
in mice with technical chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 108-5TX-79-0102-004).
Wilson NH, Killeen JC, & Ignatoski JA (1985a) Histopathological
re-evaluation of renal tissue from a sub-chronic toxicity study of
technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 753-5TX-85-0056-002).
Wilson NH, Killeen JC, & Ignatoski JA (1985b) A tumorigenicity study
of technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 099-5TX-80-0234-008).
Wilson NH, Killeen JC, & Ignatoski JA (1985c) Histopathologic
re-evaluation of renal tissue from a 90-day toxicity study of
technical chlorothalonil in rats (5TX-80-0200). Mentor, Ohio, SDS
Biotech (Unpublished report No. 753-5TX-85-0055-002).
Wilson NH, Killeen JC, & Ignatoski JA (1986a) Histopathological
re-evaluation of renal tissue from a rat tumorigenicity study with
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report No.
764-5TX-85-0071-002).
Wilson NH, Killeen JC, & Ignatoski JA (1986b) Histopathological
re-evaluation on renal tissue from a mouse tumorigenicity study with
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report).
Wilson NH, Killeen JC, & Baxter RA (1987) A tumorigenicity study of
technical chlorothalonil in male mice - final report. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1099-84-0077-TX-006).
Wilson NH, Killeen JC, & Baxter RA (1988) A teratology study in
rabbits with technical chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1544-87-0060-TX-002).
Wilson NH, Killeen JC, & Baxter RA (1989a) A tumorigenicity study of
technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1102-84-0103-TX-007).
Wilson NH, Chun JS, Killeen JC, & Baxter RA (1989b) Reproduction
dose-range finding study in rats with technical chlorothalonil.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 1722-87-0120-TX-
001).
Wilson NH, Killeen JC Jr, Ford WH, Siou G, Busey WM, & Eilrich GL
(1990) A 90-day study in rats with the monoglutathione conjugate of
chlorothalonil. Toxicol Lett, 53: 155-156.
Wolfe AL & Stallard DE (1968) The fate of DAC-3701 (4-hydroxy-2,5,6,-
trichloroisophthalonitrile) in soil. Mentor, Ohio, Diamond Shamrock
Corporation (Unpublished report).
Wolfe AL & Stallard DE (1971) Supplementary milk and meat study.
Mentor, Ohio, Diamond Shamrock Corporation (Unpublished report).
Worthing CR ed. (1991) The pesticide manual: A world compendium, 9th
ed. Farnham, British Crop Protection Council.
Wüthrich V (1990) Acute-toxicity (LC50) study of Daconil 2787 extra
to earthworms (Project No. 282971). Itingen, Switzerland, RCC
Umweltchemie A.G. (Proprietary report submitted to WHO by ISK Biotech,
Mentor, USA).
Yoshikawa H & Kawai K (1966) Toxicity of phthalonitrile and
tetrachlorophthalodinitrile: I. Acute toxicity in mice. Ind Health,
4:11-15.
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le chlorothalonil est un solide cristallin incolore et inodore,
dont le point de fusion est de 250°C et la tension de vapeur de 7,63 ×
10-5 Pa (5,72 × 10-7 mmHg) à 25°C. Il est peu soluble dans l'eau
(0,6-1,2 mg/litre à 25°C) et son coefficient de partage entre
l'octanol et l'eau (log Kow) est de 2,882. Dans l'eau, il
s'hydrolyse lentement à pH 9 mais il est stable à pH < 7 (à 25°C).
La méthode d'analyse la plus courante, après extraction et
purification, est la chromatographie gaz-liquide avec détection par
capture d'électrons.
2. Sources d'exposition humaine et environnementale
Le chlorothalonil est produit depuis 1969 à des fins
commerciales, soit par chloration de l'isophtalonitrile, soit en
traitant le tétrachlorisophtaloylamide par l'oxychlorure de phosphore.
C'est un fongicide à large spectre utilisé non seulement en
agriculture, mais aussi pour traiter le gazon et les plantes
ornementales. On l'utilise pour protéger les fruits à pépins et à
noyaux, les agrumes, les groseilles, les baies, les bananes, les
tomates, les légumes verts, le café, les arachides, les pommes de
terre, les oignons et les céréales. En outre, il entre dans la
composition de certains produits pour la conservation du bois et de
certaines peintures.
Il existe en trois formulations principales, un concentré pour
suspensions, des granulés dispersables dans l'eau et une poudre
mouillable.Ces produits sont facilement dilués dans l'eau et épandus
par pulvérisations au sol ou aériennes. La dose d'emploi est
habituellement de 1,2 à 2,5 kg de matière active par hectare pour le
traitement des haricots, des céleris et des oignons. L'exposition
humaine a lieu principalement pendant la préparation et l'épandage du
produit ou lors de l'ingestion de résidus présents dans certaines
denrées alimentaires (voir section 1.1.4).
3. Transport, distribution et transformation dans l'environnement
Le chlorothalonil s'élimine des milieux aqueux par une forte
adsorption sur les particules en suspension. La modélisation des
données disponibles montre qu'il ne migre pratiquement pas vers les
sédiments du fond. Il est possible qu'il subisse une biodégradation
enzymatique dans les eaux naturelles. Dans le sol, il est rapidement
dégradé, cette dégradation pouvant également avoir lieu dans l'eau
avec production du métabolite hydroxylé en position 4, c'est-à-dire le
4-hydroxy-2,5,6-trichlorisophtalonitrile. La demi-vie de dissipation
du métabolite 4-hydroxy dans le sol est comprise entre 6 et 43 jours.
Une fois entré en contact avec une plante, le chlorothalonil ne
migre pas vers d'autres zones du végétal. Les végétaux ne le
métabolisent que dans une proportion limitée et le métabolite
4-hydroxy constitue en général moins de 5% du résidu.
Chez les poissons, la métabolisation du chlorothalonil comporte
une conjugaison avec le glutathion, qui aboutit à des produits
d'excrétion plus polaires. Elle s'effectue sous l'action de la
glutathion- S-transférase. L'excrétion du composé sous forme de
conjugué avec le glutathion est corroborée par le fait que, chez la
truite arc-en-ciel, on trouve une forte concentration de marqueur dans
la vésicule biliaire et dans la bile après exposition à du
14C-chlorothalonil.Une fois les poissons replacés en eau propre, on a
constaté une chute rapide de la concentration du marqueur qui s'était
accumulé dans la vésicule biliaire et les autres organes.
Le chlorothalonil ne s'accumule pas chez les organismes
aquatiques.
4. Concentrations dans l'environnement et exposition humaine
Lors d'une étude sur des cultures de pommes de terre, on a
pulvérisé du chlorothalonil sur un petit cours d'eau. Après
prélèvement et analyse de l'eau en aval du secteur traité, on a
constaté que le composé disparaissait rapidement (par ex. la
concentration passait de 450 µg/litre 30 minutes après le traitement à
2-6 µg/litre 12 h après le traitement). Les traitements de routine
effectués sur des cultures irriguées de plein champ, comme les pommes
de terre ou l'orge, n'ont donné lieu qu'à de faibles concentrations de
chlorothalonil (0,04-3,6 µg/litre), comme l'ont montré un certain
nombre d'analyses pratiquées sur de l'eau prélevée à quelques
occcasions dans des drains en grès vernissé.
Les résidus qui subsistent sur les récoltes sont essentiellement
constitués par le chlorothalonil lui-même. Leur concentration est
fonction de la dose d'emploi, du temps écoulé depuis le dernier
épandage et la dernière récolte ainsi que du type de culture. A
partir des nombreux essais effectués sous contrôle un peu partout dans
le monde et dont les résultats ont été communiqués à la FAO et à
l'OMS, il est possible de déterminer les concentrations de résidus
présentes au moment de la récolte. En ce qui concerne les produits
laitiers, il est vraisemblable que les résidus sont soit
indétectables, soit très faibles. Dans le lait de vaches laitières
qui avaient reçu pendant 30 jours du chlorothalonil mêlé à leur
nourriture (jusqu'à 250 mg/kg), on n'a pas trouvé trace du composé,
mais celui-ci était présent dans les tissus à très faible
concentration.
Des analyses pratiquées dans plusieurs pays, soit sur la ration
totale, soit sur tel ou tel alliment, ont montré, à l'occasions
d'enquêtes par sondage, que le chlorothalonil n'était présent qu'en
quantités indétectables ou du moins très faibles. Les diverses
préparations que subissent les produits alimentaires, comme le pelage,
le lavage et la cuisine en général contribuent d'ailleurs à abaisser
encore leur teneur en résidus.
5. Cinétique et métabolisme chez les animaux de laboratoire
Chez des rats qui en recevaient par voie orale des doses allant
jusqu'à 50 mg/kg de poids corporel, le composé a été absorbé à hauteur
d'environ 30% en l'espace de 48 h. A plus forte dose, l'absorption
est moindre, ce qui est le signe d'un processus de saturation. Après
administration de 14C-chlorothalonil par voie orale, on a observé une
répartition tissulaire et sanguine de la radioactivité en 2 h. C'est
au niveau des reins, du foie et du sang - dans cet ordre - qu'ont été
relevées les concentrations les plus imporantes. Au bout de 24 h, on
a mesuré, au niveau des reins, une concentration égale à 0.3% d'une
dose initiale de 5 mg/kg de poids corporel.
La majeure partie d'une dose administrée par voie orale à des
rats a été retrouvée dans leurs matières fécales (> 82% en l'espace
de 48-72 h, quelle que soit la dose initiale). L'excrétion biliaire
est rapide, culminant au bout de 2 h après ingestion d'une dose de 5
mg/kg de poids corporel et la saturation est atteinte à partir de 50
mg/kg de poids corporel. Chez le rat, la dose est excrétée à hauteur
de 5-10% par la voie urinaire. Chez le chien et le singe, la
prncipale voie d'excrétion est la voie fécale, la voie urinaire étant
moins importante que chez le rat (< 4%).
Les études métaboliques menées sur des rats montrent que le
chlorothalonil est conjugué avec le glutathion dans le foie ainsi que
dans les voies digestives. Certains de ces conjugués peuvent être
absorbés dans l'intestin et parvenir jusqu'aux reins où ils sont
transformés par la ß-lyase du cytosol en analogues thioliques,
excrétés ensuite par la voie urinaire. Lorsqu'on administre du
chlorothalonil à des rats axéniques, les métabolites thioliques
apparaissent dans l'urine en quantité bien moindre que chez des rats
normaux, ce qui indique que la flore intestinale intervient dans le
métabolisme de ce composé. Des chiens et des singes à qui on
administre du chlorothalonil par voie orale, n'excrètent que peu ou
pas de métabolites thioliques dans leurs urines.
Après application cutanée de 14C-chlorothalonil à des rats,
environ 28% de la dose ont été absorbés en 120 h. On a retrouvé
environ 18% de la dose dans les matières fécales et 6% dans les urines
au bout de 120 h.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Chez le rat et le lapin, la toxicité aiguë du chlorothalonil est
faible, que ce soit par voie orale ou en applications cutanée (DL50
> 10 000 mg/kg de poids corporel). Du chlorothalonil broyé au
mortier (D médian des particules égal à 5-8 µm) s'est révélé très
toxique pour des rats lors d'une étude toxicologique par inhalation,
avec une CL50 à 4 h de 0,1 mg/litre.
Le chlorothalonil est irritant pour la peau et les yeux chez le
lapin. Les études de sensibilisation cutanée effectuées sur des
cobayes n'ont pas été concluantes.
Chez le rat, les principaux effets de doses orales répétées
s'exercent au niveau des reins et de l'estomac.Pendant 13 semaines, on
fait ingérer quotidiennement à des rats, répartis en groupes de 25
animaux de chaque sexe, du chlorothalonil mêlé à leur nourriture aux
doses de 0, 1,5, 3, 10, ou 40 mg/kg de poids corporel. Après une
période de récupération de 13 semaines, les rats ont été sacrifiés et
l'on a observé une hyperplasie et une hyperkératose au niveau de la
portion cardiaque de l'estomac aux doses de 10 et 40 mg/kg; ces
lésions ont regressé lorsque le traitement a cessé. A la dose de 40
mg/kg, on notait chez les mâles une augmentation de l'incidence des
hyperplasies épithéliales au niveau des tubules proximaux du rein au
bout des 13 semaines de traitement ainsi qu'après la période de
récupération. La dose sans effet observable a été évaluée à 3 mg/kg
de poids corporel par jour, le critère retenu étant l'absence de
lésions au niveau de la portion cardiaque de l'estomac. Les lésions
interessant cette partie de l'eatomac ainsi que les reins sont
apparues rapidement, à savoir en 4 à 7 jours chez les mâles lorsqu'on
a porté la dose alimentaire quotidienne à 175 mg/kg de poids corporel.
Lors d'une étude de 13 semaines sur des souris (0, 7,5, 15, 50,
275, ou 750 mg/kg en mélange à la nourriture), on a constaté une
incidence accrue des hyperplasies et des hyperkératoses de
l'épithélium pavimenteux au niveau de la portion cardiaque de
l'estomac. Ces lésions ont été observées chez les mâles comme chez
les femelles à partir de 50 mg/kg de nourriture. En se basant sur la
présence ou l'absence de ces lésions, on a évalué à 15 mg/kg de
nourriture la dose de chlorothalonil sans effet observable, soit
l'équivalent quotidien de 3 mg/kg de poids corporel.
Une étude de 16 semaines sur des chiens aux doses alimentaires de
0, 250, 500, ou 750 mg/kg n'a pas révélé de d'effets qui soient
imputables au traitement.
Les lésions observées au niveau des reins et de la portion
cardiaque de l'estomac ont fait l'objet, pendant 2 ans, d'études plus
approfondies sur des souris et des chiens. Une autre étude, portant
cette fois sur des rats (aux doses quotidiennes de 0, 1,8, 3,8, 15 ou
175 mg/kg de poids corporel), a permis de caractériser histo-
logiquement les effets observés: il s'agissait d'une part, d'une
augmentation de l'incidence des hyperplasies, des hyperkératoses, des
ulcérations et des abrasions de l'épithélium pavimenteux au niveau de
la portion cardiaque de l'estomac et, d'autre part, de la présence
d'une hyperplasie au niveau des tubules contournés proximaux du rein.
Ces anomalies ont été observées à partir de 3,8 mg/kg. A la dose de
175 mg/kg, on notait un accroisssement sensible de l'incidence des
tumeurs rénales (adénomes et carcinomes) et des tumeurs interessant la
portion cardiaque de l'estomac (papillomes et carcinomes). On est
fondé à penser que l'incidence des tumeurs rénales était augmentée
chez les mâles à partir de la dose de 15 mg/kg, de même que celle des
tumeurs gastriques chez les deux sexes aux doses de 3,8 mg/kg et de 15
mg/kg. La dose sans effets néoplasiques observables a donc été prise
égale à 1,8 mg/kg, en prenant comme critère l' incidence des tumeurs
au niveau de la portion cardiaque de l'estomac. Une autre étude de 2
ans, au cours de laquelle des doses plus élevées ont été utilisées, a
confirmé le pouvoir cancérogène du chlorothalonil, tant au niveau du
rein que de la portion cardiaque de l'estomac.
Une étude sur des souris (doses de 0, 15, 40, 175, ou 750 mg/kg
de nourriture) a révélé une augmentation de l'incidence des
hyperplasies au niveau des tubules rénaux à partir de 175 mg/kg, le
même phénomène étant observé à partir de 40 mg/kg au niveau de la
portion cardiaque de l'estomac, avec en outre une hyperkératose. A la
dose de 750 mg/kg, il y avait une légère augmentation des tumeurs
spinocellulaires au niveau de la portion cardiaque de l'estomac. On
en a donc conclu que les doses sans effets néoplasiques ou non
néoplasiques étaient respectivement égales à 175 et 15 mg/kg de
nourriture (soit l' équivalent quotidien de 17,5 et 1,6 mg/kg de poids
corporel, respectivement). Ces effets constatés chez la souris sont
corroborés par les résultats d'une autre étude avec des doses plus
élevées, mais une troisième investigation portant sur des souris
B6C3F1 n'a pas mis en évidence d'effets cancérogènes à dose élevée.
Lors d'une étude de 2 ans sur des chiens (60 et 120 mg/kg de
nourriture), aucun effet attribuable au chlorothalonil n'a été
observé. On en conclu que la dose sans effet observable était de 120
mg/kg de nourriture (soit l'équivalent quotidien de 3 mg/kg de poids
corporel).
Plusieurs épreuves de mutagénicité in vivo et in vitro se
sont révélées négatives, mais il y en a tout de même eu quelques unes
de positives.
Les dérivés monothio, dithio, trithio, dicystéinyl, tricystéinyl
et monoglutathionyl du chlorothalonil, qui sont potentiellement
néphrotoxiques, se sont révélés négatifs dans l'épreuve d'Ames.
Le chlorothalonil ne s'est pas montré tératogène pour le rat ou
le lapin à des doses quotidiennes atteignant respectivement 400 et 50
mg/kg de poids corporel. Lors d'une étude sur deux générations de
rats, on n'a pas constaté d'effets sur l'accouplement, la fécondité ou
la gestation jusqu'à des doses atteignant 1500 mg/kg de nourriture.
La toxicité aiguë par voie orale du métabolite 4-hydroxy est
supérieure à celle du chlorothalonil lui-même (DL50 aiguë par voie
orale égale à 332 mg/kg de poids corporel contre > 10 000 mg/kg de
poids corporel). Plusieurs études ont été entreprises pour
caractériser le profil toxicologique de ce métabolite et établir les
doses sans effets observables.
7. Effets sur l'homme
On a signalé des cas de dermatite de contact parmi des personnes
employées à la fabrication de chlorothalonil, chez des agriculteurs et
des horticulteurs. D'autres cas de dermatite siégeant au niveau des
mains et de la face ont été observés chez des personnes qui
utilisaient des produits de protection du bois à base de
chlorothalonil.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Le chlorothalonil est extrêmement toxique pour les poissons et
les invertébrés aquatiques, comme le montrent un certain nombre
d'études en laboratoire, avec des valeurs de la CL50 inférieures à
0,5 mg/litre. La concentration maximale acceptable de produit toxique
(MATC) s'est révélée être égale à 35 µg/litre lors d'une étude sur
deux générations de daphnies.
A quelques exceptions près, d'ailleurs sans grande importance, le
chlorothalonil n'est pas phytotoxique.
La CL50 d'un concentré pour suspension (500 g de chlorothalonil
par litre) répandu sur un sol artificiel pour lombrics, a été évaluée
à > 1000 mg/kg de terre (14 jours). On a observé une surmortalité
parmi des scolopendres qui s'étaient trouvés en contact avec des
résidus de chlorothalonil présents sur des feuilles d'arachide ou s'en
étaient nourris au laboratoire; il n'y a pas eu d'autre indice d'une
action insecticide.
Le chlorothalonil est peu toxique pour les oiseaux, comme le
montre la valeur de la DL50 aiguë par voie orale chez le colvert
(4640 mg/kg). Aucun effet important sur la reproduction n'a été
signalé.
D'après une étude effectuée sur le terrain, la toxicité du
chlorothalonil pour les organismes aquatiques est moindre que ne le
font craindre les expériences de laboratoire; ce résultat est en
accord avec les propriétés physico-chimiques de ce composé. On a tout
de même enregistré une mortalité chez des espèces exposées
expérimentalement sur le terrain. En revanche, on n'a pas signalé
d'accidents écologiques ayant entraîné une mortalité. Malgré la
faible persistance du chlorothalonil dans les divers compartiments du
milieu, il faut tout de même s'attendre à une certaine mortalité. En
pareil cas, il sera difficile d'établir un lien de cause à effet étant
donné que les résidus de chlorothalonil ne subsistent pas suffisamment
lontemps pour que l'on puisse identifier le composé.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El clorotalonilo es un sólido cristalino inodoro e incoloro con
un punto de fusión de 250°C y una presión de vapor de 7,63 × 105 Pa
(5,72 × 10-7 mmHg) a 25°C. Es poco soluble en agua (0,6 a 1,2 mg/litro
a 25°C) y tiene un coeficiente de partición octanol/agua (log
Koa) de 2,882. Se hidroliza lentamente en agua con un pH de 9, pero
es estable a un pH de 7 o inferior (a 25°C).
El método analítico más corriente, después de la extracción y
depuración de las muestras, es la cromatografía gas-liquido empleando
un detector de captura de electrones.
2. Fuentes de exposición del ser humano y del medio ambiente
El clorotalonilo se viene produciendo a escala comercial desde
1969 por cloración del isoftalonitrilo o mediante el tratamiento de la
amida tetracloroisoftalolil con oxicloruro de fósforo. Es un fungicida
con amplio espectro de actividad empleado principalmente en la
agricultura pero también en el césped, los pastos y las plantas
ornamentales. Los cultivos protegidos incluyen frutas de pepitas y de
hueso, cítricos, grosellas, fresas, bananas, tomates, verduras, café,
cacahuete, patatas, cebollas y cereales. Se emplea también en
sustancias protectoras de la madera y en pinturas.
Las tres formulaciones principales son una suspensión
concentrada, un gránulo hidrodispersible y un polvo humectable. Se
disuelven fácilmente en agua y se aplican empleando sistemas de
pulverización en los suelos o rociado aéreo. Las tasas típicas del
ingrediente activo oscilan entre 1,2 y 2,5 kg/ha en el caso de
cultivos tales como frijoles, apio y cebollas. Las principales fuentes
de exposición del ser humano son la preparación y aplicación de los
productos, así como la ingestión de residuos de las cosechas en los
alimentos (véase la sección 1.1.4).
3. Transporte, distribución y transformación en el medio ambiente
El clorotalonilo se elimina de los medios acuosos mediante
intensa adsorción en las materias en suspensión. Los datos de los
modelos parecen indicar poca, o ninguna, partición detectable en el
sedimento del fondo. Puede producirse biodegradación en aguas
naturales, con la participación de procesos enzimáticos. El
clorotalonilo se degrada rápidamente en el suelo, y puede haber
degradación en el agua con la producción del metabolito 4-hidroxi-
2,5,6-tricloroisoftalonitrilo. La semivida para la disipación en el
suelo de ese metabolito varía entre 6 y 43 días.
El clorotalonilo no se transloca del punto de aplicación a otras
partes de la planta. Su metabolización en las plantas es limitada y,
por o general, ese metabolito representa < 5% del residuo.
En cuanto a los peces, el clorotalonilo se metaboliza mediante la
conjugación con glutatión para producir productos de degradación más
polares. En esa conversión interviene la enzima glutatión-
S-transferasa. Las elevadas concentraciones del radioisótopo
marcador detectadas en la vesícula biliar y la bilis después de la
exposición de la trucha arco iris al 14C-clorotalonilo son
compatibles con la excreción del compuesto en forma de conjugados de
glutatión. Las concentraciones de los radioisótopos de trazado que se
acumulan en la vesícula biliar y otros órganos disminuyen rápidamente
al colocar los peces en agua no contaminada.
El clorotalonilo no experimenta bioacumulación en los organismos
acuáticos.
4. Niveles ambientales y exposición humana
En un estudio de un cultivo de patatas, se procedió a rociar un
pequeño arroyo con clorotalonilo. Muestreos y análisis posteriores del
agua río abajo demostraron la rápida desaparición del clorotalonilo
(las concentraciones eran de 450 µg/litro a los 30 minutos después del
rociado, y oscilaban entre 2 y 6 µg/litro a las 12 h después del
rociado). El rociado sistemático de los cultivos irrigados como, por
ejemplo, patatas y cebada, estuvo acompañado de bajas concentraciones
de clorotalonilo (0,04 a 3,6 µg/litro) en el agua de los tubos de
drenaje en un pequeño número de muestras.
Los residuos de las cosechas están compuestos principalmente de
clorotalonilo propiamente dicho. Las concentraciones residuales
dependen del nivel aplicado, del tiempo transcurrido entre la última
aplicación y la cosecha, y del tipo de cosecha. Los niveles residuales
en la cosecha pueden deducirse de los numerosos ensayos supervisados
realizados con muchas cosechas en todo el mundo y comunicados a la FAO
y la OMS. Se prevé que los residuos de clorotalonilo en los productos
lácteos serán imposibles de detectar, o las concentraciones muy bajas.
En un estudio con vacas lecheras alimentadas durante 30 días con
pienso al que se habían añadido elevadas concentraciones (hasta 250
mg/kg) de clorotalonilo no se observó ningún residuo detectable en la
leche y sólo niveles muy bajos en los tejidos.
Los análisis del régimen alimenticio total y de alimentos
específicos realizados en varios países han revelado concentraciones
no detectables, o muy bajas, de clorotalonilo en los estudios por
muestreo. Los procesos de preparación tales como el lavado, el pelado
y la cocción permiten reducir aún más los niveles residuales en los
alimentos.
5. Cinética y metabolismo en animales de laboratorio
En estudios con ratas a las que se administraron dosis de hasta
50 mg/kg de peso corporal, se observó que un 30% de la dosis oral de
clorotalonilo se absorbía dentro de las 48 horas. La absorción es
inferior con posologías más elevadas, lo que indica un proceso de
saturación. Cuando se administra oralmente 14C-clorotalonilo, la
radioactividad se distribuye en la sangre y los tejidos en menos de
dos horas. Las mayores concentraciones se encuentran en el riñón, el
hígado y la sangre, en ese orden. Con una posología de 5 mg/kg de peso
corporal, la concentración en los riñones es 0,3% a las 24 horas.
Por lo que respecta a las ratas, la mayor parte de la dosis oral
de clorotalonilo se encuentra en las heces (> 82% dentro de las 48 a
72 horas, independientemente de la dosis). Con una dosis oral de 5
mg/kg de peso corporal, la excreción biliar es rápida, alcanzando su
valor máximo dentro de las 2 horas, ocurriendo saturación con
posologías de 50 mg/kg de peso corporal y superiores. En el caso de
las ratas, la excreción urinaria representa entre un 5 y 10% de la
dosis. En perros y monos, la excreción fecal es la vía principal, pero
la excreción urinaria (< 4%) es inferior a la observada en las ratas.
Estudios metabólicos llevados a cabo con ratas indican que el
clorotalonilo se conjuga con el glutatión tanto en el hígado como en
el tracto gastrointestinal. Algunos de los conjugados del glutatión
pueden ser absorbidos en el intestino y transportados a los riñones
donde son convertidos por la ß-liasa citosólica en análogos del tiol
que se excretan por la orina. Cuando se dan dosis de clorotalonilo a
ratas axénicas, se observan en la orina metabolitos del tiol en
cantidades muy inferiores a las registradas en ratas normales, lo que
indica que la microflora intestinal interviene en el metabolismo del
clorotalonilo. En el caso de los perros o monos que reciben dosis
orales de clorotalonilo, la excreción de derivados del tiol por la
orina no es detectable, o es muy baja.
Cuando se aplicó 14C-clorotalonilo a la piel de la rata, un 28%
de la dosis fue absorbida en menos de 120 horas. Se observaron
concentraciones del 18% de la dosis en las heces y del 6% en la orina
al cabo de 120 horas.
6. Efectos en mamíferos de laboratorio y sistemas de pruebas
in vitro
El clorotalonilo tiene baja toxicidad oral y cutánea en ratas y
conejos, respectivamente (los valores agudos orales y cutáneos de la
DL50 son > 10 000 mg/kg de peso corporal). Por lo que respecta a
las ratas, en un estudio de inhalación se observó que el clorotalonilo
técnico pulverizado (MMAD 5 a 8 µm) presentaba elevada toxicidad, con
una CL50 de 0,1 mg/litro a las 4 h.
El clorotalonilo es un irritante de la piel y los ojos en el
conejo. Los estudios de sensibilización cutánea en el conejillo de
indias no arrojaron resultados concluyentes.
En el caso de las ratas, los efectos principales de dosis orales
repetidas de clorotalonilo se observan en el estómago y los riñones.
En un estudio con grupos de 25 ratas, en que se separaron los sexos,
se emplearon posologías de 0, 1,5, 3, 10 ó 40 mg/kg de peso corporal
por día en la dieta durante 13 semanas, lo que estuvo seguido de un
período de recuperación de 13 semanas. Se observó mayor frecuencia de
hiperplasis y hiperketarosis del preestómago con las posologías de 10
y 40 mg/kg; los efectos desaparecieron cuando cesó el tratamiento. Con
una concentración de 40 mg/kg, se registro mayor incidencia de
hiperplasia del epitelio tubular proximal del riñón en los machos a
las 13 semanas y después del período de recuperación. El nivel sin
efecto observado fue de 3 mg/kg de peso corporal por día en base a la
ausencia de lesiones en el preestómago. Se ha demostrado que los
cambios observados en el preestómago y los riñones son de rápida
aparición, presentándose las lesiones dentro de un período de 4 a 7
días en el caso de los machos, cuyo régimen alimenticio incluía una
concentración de 175 mg/kg de peso corporal al día.
En un estudio de 13 semanas de duración realizado con ratones
(empleando dosis de 0, 7,5, 15, 50, 275 ó 750 mg/kg en el alimento),
se observó mayor incidencia de hiperplasia e hiperkeratosis de las
células epiteliales escamosas del preestómago en el caso de los machos
y las hembras cuando se emplearon posologías de 50 mg/kg y superiores.
Atendiendo a esos cambios, el nivel sin efecto observado fue de 15
mg/kg de clorotalonilo en la dieta, lo que equivale a 3 mg/kg de peso
corporal por día.
Un estudio de 16 semanas de duración con perros cuyo alimento
contenía concentraciones de 0, 250, 500 ó 750 mg/kg no reveló cambios
relacionados con el tratamiento.
Se llevaron a cabo investigaciones adicionales de las lesiones en
el preestómago y los riñones, llevándose a cabo estudios con ratas,
ratones y perros durante un período de 2 años. En un estudio realizado
con ratas (empleando dosis de 0, 1,8, 3,8, 15 ó 175 mg/kg de peso
corporal al día), los efectos estuvieron caracterizados
histológicamente por una mayor incidencia e intensidad de hiperplasia,
hiperkeratosis, y úlceras y erosiones de la mucosa escamosa del
preestómago, y por hiperplasia del epitelio de los túbulos
contorneados proximales de los riñones con posologías de 3,8 mg/kg y
superiores. Por lo que respecta a los efectos no neoplásicos, el nivel
sin efecto observado fue, por lo tanto, de 1,8 mg/kg. La incidencia de
tumores renales (adenomas y carcinomas) y de tumores del preestómago
(papilomas y carcinomas) fue considerablemente superior, alcanzando
175 mg/kg. Hubo pruebas de mayor incidencia de tumores renales en los
machos con dosis de 15 mg/kg, así como de tumores estomacales en los
machos y las hembras con dosis de 3,8 y 15 mg/kg. Por lo que respecta
a los efectos neoplásicos, el nivel sin efecto observado fue, por lo
tanto, de 1,8 mg/kg de peso corporal por día sobre la base de los
cambios en la incidencia de tumores en el preestómago. El riesgo
carcinogénico del clorotalonilo en los riñones y preestómago de las
ratas se vio corroborado por los resultados de otros estudios de 2
años de duración en que emplearon dosis más elevadas.
En un estudio con ratones (empleando dosis de 0, 15, 40, 175 ó
750 mg/kg en el alimento), se observó mayor incidencia de hiperplasia
tubular renal con dosis de 175 mg/kg y superiores, así como de
hiperplasia y hiperkeratosis del preestómago con concentraciones de 40
mg/kg y superiores. La incidencia de tumores escamosos del preestómago
aumentó ligeramente con dosis de 750 mg/kg. Por consiguiente, por lo
que respecta a los cambios neoplásicos y no neoplásicos, los niveles
sin efecto observado fueron de 175 y 15 mg/kg en la dieta (lo que
equivale a 17,5 y 1,6 mg/kg de peso corporal por día respectivamente).
Otro estudio con posologías superiores corroboró esos efectos en el
ratón, pero un estudio con ratones B6C3F1 no señaló ningún riesgo
carcinogénico con dosis elevadas.
En un estudio de 2 años de duración con perros (empleando 60 y
120 mg/kg en el alimento), no se detectó ningún efecto atribuible al
clorotalonilo. Por lo tanto, el nivel sin efecto observado fue de 120
mg/kg en el alimento (lo que equivale a 3 mg/kg de peso corporal al
día).
El clorotalonilo no resultó mutagénico en varias pruebas in
vitro e in vivo, aunque fue positivo en un pequeño número de
valoraciones.
Los derivados monotio, ditio, tritio, dicisteina, tricisteina y
monoglutatión del clorotalonilo, que son posibles substancias
nefrotóxicas, arrojaron resultados negativos en el análisis de Ames.
El clorotalonilo no resultó teratogénico en las ratas o conejos
con dosis de hasta 400 y 50 mg/kg de peso corporal al día,
respectivamente. En un estudio realizado con dos generaciones de
ratas, los parámetros reproductivos tales como el apareamiento, la
fertilidad y el período de gestación no se vieron afectados por el
clorotalonilo con concentraciones de hasta 1500 mg/kg en la dieta.
La toxicidad oral aguda del metabolito 4-hidróxido es superior a
la del clorotalonilo propiamente dicho (DL50 oral aguda de 332 mg/kg
de peso corporal en comparación con > 10 000 mg/kg de peso corporal).
Se han llevado a cabo varios estudios destinados a caracterizar el
perfil toxicológico de ese metabolito y a establecer los niveles sin
efecto observado.
7. Efectos en el ser humano
Se ha informado de dermatitis por contacto en el caso de personas
que trabajan en la producción de clorotalonilo, así como en el de
agricultores y hortelanos. Se tienen noticias también de trabajadores
de fábricas de productos de la madera que han contraído dermatitis por
contacto en las manos y el rostro cuando empleaban preservativos de la
madera que contenían clorotalonilo.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
El clorotalonilo es sumamente tóxico para los peces y los
invertebrados acuáticos en los estudios de laboratorio, siendo los
valores de CL50 inferiores a 0,5 mg/litro. En un estudio sobre
reproducción realizado con dos generaciones de Daphnia magna, la
concentración tóxica máxima aceptable (CTMA) fue de 35 µg/litro.
Con pocas excepciones, el clorotalonilo no es fitotóxico.
En lombrices, la CL50 de una formulación de una suspensión
concentrada (500 g de clorotalonilo/litro) en suelo artificial fue
> 1000 mg/kg de suelo (14 días). Las tijeretas experimentaron mayor
mortalidad cuando estaban en contacto con residuos de clorotalonilo en
las hojas del cacahuete o lo ingerían en su fuente de alimentos en las
pruebas de laboratorio; no hubo ningún otro indicio de efecto
insecticida.
El clorotalonilo tiene poca toxicidad para las aves, habiéndose
informado de una DL50 oral aguda de 4640 mg/kg de alimento en el
pato real. No se informó de ningún efecto considerable sobre la
reproducción.
Un estudio sobre el terreno de organismos acuáticos expuestos
después de la aplicación de clorotalonilo parece indicar que la
toxicidad es inferior a la que cabría esperar atendiendo a los
estudios de laboratorio; esto es también compatible con las
propiedades fisicoquímicas de los compuestos. Se observaron muertes en
algunas especies expuestas experimentalmente en el campo. No se ha
informado de incidentes de muertes en el medio ambiente. Sin embargo,
a pesar del corto tiempo de presencia del clorotalonilo en el medio
ambiente, cabría esperar que ocurran muertes. Resultaría difícil
establecer vínculos entre las muertes y los compuestos ya que la
persistencia de los residuos no seria suficientemente prolongada para
poder identificar el clorotalonilo.
 additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom (Vice-
Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine,
University of Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate
School of Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist,
The Netherlands
Representatives of GIFAPa (Groupement International des
Associations Nationales de Fabricants de Produits Agrochimiques)
Dr M. Bliss, Jr., ISK Biosciences Corporation, Mentor, Ohio, USA
Dr A.C. Dykstra, Registration Department BPID, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr H. Frazier, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr R. Gardiner, GIFAP, Brussels, Belgium
Dr B. Julin, Regulatory Affairs, Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels, Belgium
Dr S.M. Kennedy (Environmental Science), Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels, Belgium
Dr J. Killeen, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr Th. S.M. Koopman, Toxicology Department, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr R.L. Mull, Du Pont Agricultural Products, Wilmington, Delaware, USA
Dr J.L.G. Thus, Environmental Research Department, Solvay-Duphar BV,
CP Weesp, The Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
a Participated as required for exchange of information.
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Pletina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides met in Geneva from 25 October to 3 November 1994. Dr W.
Kreisel of the WHO welcomed the participants on behalf of WHO, and
Dr M. Mercier, Director, IPCS, on behalf of the IPCS and its
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to chlorothalonil.
The first draft of the monograph was prepared by Dr M.H.
Litchfield, Arundel, United Kingdom. The second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs, was
prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The fact that ISK Biosciences Corporation made available to the
IPCS its proprietary toxicological information on chlorothalonil is
gratefully acknowledged. This allowed the CAG to make its evaluation
on a more complete database.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
BCF bioconcentration factor
BUN blood urea nitrogen
ECD electron capture detector
EDB 1,2-dibromoethane (ethylene dibromide)
FID flame ionization detector
GC gas chromatography
GSH glutathione
gamma-GT gamma-glutamyltranspeptidase
HECD Hall electron capture detector
LOEL lowest-observed-effect level
MS mass spectrometry
NADPH reduced nicotinamide adenine dinucleotide phosphate
NOEL no-observed-effect level
PIB piperonyl butoxide
SGOT serum glutamic-oxalic transaminase
SGPT serum glutamic-pyruvic transaminase
TEAM total exposure assessment methodology
TWA time-weighted average
UDS unscheduled DNA synthesis
VHH volatile halogenated hydrocarbon
VOC volatile organic carbon compound
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Chlorothalonil is a colourless, odourless, crystalline solid with
a melting point of 250°C and a vapour pressure of 7.63 × 10-5 Pa
(5.72 × 10-7 mmHg) at 25°C. It has low water solubility
(0.6-1.2 mg/litre at 25°C) and an octanol/water partition coefficient
(log Kow) of 2.882. It is hydrolysed in water slowly at pH 9 but is
stable at pH 7 or below (at 25°C).
The most prevalent analytical method, after sample extraction and
clean-up, is gas-liquid chromatography using an electron-capture
detector.
1.1.2 Sources of human and environmental exposure
Chlorothalonil has been produced commercially since 1969 by
chlorination of isophthalonitrile or by treatment of
tetrachloroisophthaloyl amide with phosphorus oxychloride. It is a
fungicide with a broad spectrum of activity used mainly in agriculture
but also on turf, lawns and ornamental plants. Crops protected
include pome and stone fruit, citrus, currants, berries, bananas,
tomatoes, green vegetables, coffee, peanuts, potatoes, onions and
cereals. In addition, it is used in wood preservatives and in paints.
The three main formulations are a suspension concentrate, a water
dispersible granule and a wettable powder. They are readily diluted
with water and applied by ground spray systems or by air. Typical
active ingredient rates are 1.2-2.5 kg/ha for crops such as beans,
celery and onions. The main sources of human exposure will be during
preparation and application of the products and from ingestion of crop
residues in foodstuff (see section 1.1.4).
1.1.3 Environmental transport, distribution and transformation
Chlorothalonil is removed from aqueous media by strong adsorption
on suspended matter. Modelled data suggest little or no partition to
bottom sediment. Biodegradation may occur in natural waters with
enzyme processes being involved. Chlorothalonil is rapidly degraded
in soil, and degradation may occur in water with the production of the
4-hydroxy metabolite, 4-hydroxy-2,5,6-trichloroisophthalonitrile.
Half-lives for dissipation of the 4-hydroxy metabolite in soils range
between 6 and 43 days.
Chlorothalonil does not translocate from the site of application
to other parts of a plant. It is metabolized only to a limited extent
on plants and the 4-hydroxy metabolite is usually < 5% of the
residue.
Chlorothalonil is metabolized in fish via glutathione conjugation
to give more polar excretory products. The enzyme glutathione-
S-transferase is involved with this conversion. High concentrations
of radiolabel found in the gall bladder and bile, after exposure of
rainbow trout to 14C-chlorothalonil, are consistent with the
excretion of the compound as glutathione conjugates. The
concentrations of radiolabel accumulating in the gall bladder and
other organs fell rapidly when the fish were placed in clean water.
Chlorothalonil does not bioaccumulate in aquatic organisms.
1.1.4 Environmental levels and human exposure
In a potato crop study, a small stream was oversprayed with
chlorothalonil. Subsequent sampling/analysis of down-stream water
demonstrated rapid disappearance of chlorothalonil (i.e. 450 µg/litre
at 30 min post-spraying to 2-6 µg/litre at 12 h post-spraying). The
routine spraying of irrigated field crops such as potatoes and barley
gave rise to low concentrations of chlorothalonil (0.04-3.6 µg/litre)
in tile drain water on a small number of sampling occasions.
Crop residues are composed mainly of chlorothalonil itself. The
residue levels are dependent upon the applied rate, time interval
between the last application and harvest, and the type of crop.
Residue levels at harvest can be derived from the numerous supervised
trials that have taken place on many crops worldwide and reported to
FAO/WHO. Residues of chlorothalonil in dairy products are expected to
be undetectable or very low. Dairy cows given high concentrations (up
to 250 mg/kg) of chlorothalonil in their feed for 30 days showed no
detectable residue in milk and only very low levels in tissues.
Total diet and individual food analysis in several countries have
shown undetectable or low concentrations of chlorothalonil in sampling
surveys. Residue levels on foodstuffs are further reduced by
preparation processes such as washing, peeling and cooking.
1.1.5 Kinetics and metabolism in laboratory animals
About 30% of an oral dose of chlorothalonil is absorbed within
48 h in rats at doses up to 50 mg/kg body weight. At higher doses,
absorption is lower, indicating a saturation process. When
14C-chlorothalonil is given orally the radioactivity is distributed
into blood and tissues within 2 h. The greatest concentration is
found in the kidney, followed by liver and blood. The kidneys contain
0.3% of a 5 mg/kg body weight dose after 24 h.
Most of an oral dose of chlorothalonil in rats is found in faeces
(> 82% within 48-72 h, regardless of dose). Biliary excretion is
rapid, peaking within 2 h after a 5 mg/kg body weight oral dose, and
is saturated at 50 mg/kg body weight and above. Urinary excretion
accounts for 5-10% of the dose in rats. Faecal excretion is the main
route in dogs and monkeys but urinary excretion (< 4%) is less than
in rats.
Metabolic studies in rats indicate that chlorothalonil is
conjugated with glutathione in the liver as well as in the
gastrointestinal tract. Some of the glutathione conjugates may be
absorbed from the intestine and transported to the kidneys, where they
are converted by cytosolic ß-lyase to thiol analogues that are
excreted in the urine. When germ-free rats are dosed with
chlorothalonil, the thiol metabolites appear in urine in much smaller
amounts than with normal rats, indicating the involvement of
intestinal microflora in the metabolism of chlorothalonil. Dogs or
monkeys dosed orally with chlorothalonil excrete little or no thiol
derivatives in urine.
When 14C-chlorothalonil was applied to rat skin, approximately
28% of the dose was absorbed within 120 h. About 18% of the dose was
found in faeces and 6% in urine within 120 h.
1.1.6 Effects on laboratory mammals and in vitro test systems
Chlorothalonil has low acute oral and dermal toxicity in rats and
rabbits, respectively (acute oral and dermal LD50 values are
> 10 000 mg/kg body weight). Hammer-milled technical chlorothalonil
(MMAD 5-8 µm) exhibited high toxicity in rats in an inhalation study,
with a 4-h LC50 of 0.1 mg/litre.
Chlorothalonil is a skin and eye irritant in the rabbit. Skin
sensitization studies in the guinea-pig were inconclusive.
The main effects of repeated oral dosing in rats are on the
stomach and kidney. Groups of 25 rats of each sex per group were fed
chlorothalonil at 0, 1.5, 3, 10 or 40 mg/kg body weight per day in the
diet for 13 weeks, and this was followed by a 13-week recovery period.
Increased incidences of hyperplasia and hyperkeratosis of the
forestomach occurred at 10 and 40 mg/kg; these reversed when treatment
ceased. At 40 mg/kg, there was an increased incidence of hyperplasia
of kidney proximal tubular epithelium in males at 13 weeks and after
the recovery period. The NOEL was 3 mg/kg body weight per day based
upon lack of forestomach lesions. The onset of the forestomach and
kidney changes was shown to be rapid, with the lesions developing
within 4-7 days in male rats at a dietary level of 175 mg/kg body
weight per day.
In a 13-week study on mice (0, 7.5, 15, 50, 275 or 750 mg/kg in
the diet), increased incidences of hyperplasia and hyperkeratosis of
the squamous epithelial cells of the forestomach occurred in males and
females at 50 mg/kg diet and above. The NOEL, based upon these
changes, was 15 mg/kg chlorothalonil in the diet, equivalent to
3 mg/kg body weight per day.
A 16-week study in dogs with dietary levels of 0, 250, 500 or
750 mg/kg showed no treatment-related changes.
The forestomach and kidney lesions were investigated further in
2-year studies on rats, mice and dogs. In a study on rats (0, 1.8,
3.8, 15 or 175 mg/kg body weight per day), the effects were
characterized histologically as an increase in the incidence and
severity of hyperplasia, hyperkeratosis, and ulcers and erosions of
the squamous mucosa of the forestomach, and as epithelial hyperplasia
of the kidney proximal convoluted tubules at 3.8 mg/kg and above. The
NOEL for non-neoplastic effects was therefore 1.8 mg/kg. The
incidence of renal tumours (adenomas and carcinomas) and forestomach
tumours (papillomas and carcinomas) was markedly increased at
175 mg/kg. There was evidence for an increased incidence of kidney
tumours in males at 15 mg/kg and of stomach tumours at 3.8 and
15 mg/kg in males and females. The NOEL for neoplastic effects was
therefore 1.8 mg/kg body weight per day based upon changes in
forestomach tumour incidence. Supporting evidence for the
carcinogenic potential of chlorothalonil in the kidney and forestomach
of rats was provided by the results from other 2-year studies at
higher dose levels.
In a study on mice (0, 15, 40, 175 or 750 mg/kg in the diet), an
increased incidence of renal tubular hyperplasia occurred at 175 mg/kg
and above and of hyperplasia and hyperkeratosis of the forestomach at
40 mg/kg and above. The incidence of squamous tumours of the
forestomach was slightly increased at 750 mg/kg. The NOELs for
neoplastic and non-neoplastic changes were therefore 175 and 15 mg/kg
in the diet (equivalent to 17.5 and 1.6 mg/kg body weight per day,
respectively). Supporting evidence for these effects in the mouse was
provided in another study at higher dose levels, but a study in
B6C3F1 mice did not show any evidence for carcinogenic potential at
high dose levels.
In a 2-year study on dogs (60 and 120 mg/kg in the diet), no
effects attributable to chlorothalonil were found. The NOEL was
therefore 120 mg/kg in the diet (equivalent to 3 mg/kg body weight per
day).
Chlorothalonil was not mutagenic in several in vitro and in
vivo tests, although it was positive in a small number of assays.
The monothio, dithio, trithio, dicysteine, tricysteine and
monoglutathione derivatives of chlorothalonil, which are potential
nephrotoxicants, were shown to be negative in the Ames assay.
Chlorothalonil was not teratogenic in rats or rabbits at doses up
to 400 and 50 mg/kg body weight per day, respectively. Reproductive
parameters such as mating, fertility and gestation length were not
affected by chlorothalonil at levels up to 1500 mg/kg in the diet in a
two-generation study in rats.
The acute oral toxicity of the 4-hydroxy metabolite is greater
than that of chlorothalonil itself (acute oral LD50 of 332 mg/kg body
weight versus > 10 000 mg/kg body weight). Several studies have been
undertaken to characterize the toxicological profile of this
metabolite and to establish NOELs.
1.1.7 Effects on humans
Contact dermatitis has been reported for personnel working in
chlorothalonil manufacturing and in farmers and horticultural workers.
Workers in the manufacture of wood products have also developed
contact dermatitis on the hands and face when wood preservatives
containing chlorothalonil were used.
1.1.8 Effects on other organisms in the laboratory and field
Chlorothalonil is highly toxic to fish and aquatic invertebrates
in laboratory studies, the LC50 values being below 0.5 mg/litre. The
maximum acceptable toxicant concentration (MATC) in a two-generation
reproduction study in Daphnia magna was 35 µg/litre.
With minor exceptions, chlorothalonil is not phytotoxic.
The LC50 of a suspension concentrate formulation (500 g
chlorothalonil/litre) in artificial soil for earthworms was
> 1000 mg/kg soil (14 days). Earwigs suffered increased mortality
when in contact with chlorothalonil residues on peanut foliage or
ingesting it as a food source in laboratory tests; there was no other
indication of insecticidal action.
Chlorothalonil is of low toxicity to birds with a reported acute
oral LD50 of 4640 mg/kg diet in the mallard duck. No significant
reproductive effects were reported.
A field study of aquatic organisms exposed following
chlorothalonil application suggests that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physicochemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field. There have been no
reported incidents of kills in the environment. However, despite the
short residence time of chlorothalonil in environmental media, kills
would be expected to occur. Linking kills to the compound would be
difficult given that residues would not persist long enough for
chlorothalonil to be identified.
1.2 Evaluation
1.2.1 Evaluation of human health risks
The review of the toxicological data for chlorothalonil revealed
that the most important studies for human risk estimation were the
long-term studies in rodents and dogs.
In the rodent studies, chlorothalonil caused lesions in the
forestomach and kidney. The lesions in the forestomach were
characterized as hyperplasia and hyperkeratosis of the squamous
epithelial cells. These occurred soon after dosing and were shown to
be reversible after dosing ceased. Long-term administration led to
the formation of tumours (papilloma and carcinoma). The renal lesions
in rodents were of rapid onset and characterized as hyperplasia of the
proximal tubular epithelium. On longer-term administration, renal
tumours (adenoma and carcinoma) occurred in the rat and in one study
on mice.
In order to interpret the significance of these findings, the
results of the mutagenic studies were taken into account.
Chlorothalonil gave negative results in in vitro and in vivo
mutagenic assays in which a variety of end-points were studied. Thiol
derivatives of chlorothalonil were negative in the Ames test, and
14C-chlorothalonil did not bind to rat kidney DNA in vivo. The
compound does not appear to have genotoxic potential on this basis,
indicating that it probably exerts its carcinogenic effect in rodents
via a non-genotoxic mechanism. The initial forestomach lesions in
rodents were attributed to the irritant action of chlorothalonil, and,
where this does not occur, a NOEL can be attained. The irritant action
on rodent forestomach in conjunction with the relatively long
residence time of the compound in this organ were seen to be factors
presenting the opportunity for the initiation of the lesions and
leading to carcinogenic action on prolonged administration. It was
concluded that, since humans do not possess a comparable organ,
rodents are probably not representative of the action of this compound
in man in this respect. This reasoning is also supported by the fact
that another animal species, the dog, is not affected by the compound
at similar or higher doses.
In the assessment of the relevance of the rodent renal lesions,
the metabolic conversion of chlorothalonil to metabolites which act
directly upon the kidney was seen to be a major factor. In the kidney
glutathione conjugates are converted by ß-lyase to chlorothalonil
thiol derivatives. Chlorothalonil is thought to be conjugated with
glutathione (GSH) mostly in the gastrointestinal tract prior to
absorption, although there is evidence of glutathione conjugation at
other sites. After absorption the conjugates pass to the kidney where
they are converted to chlorothalonil thiol derivatives following the
action of ß-lyase. It has been shown in vitro that the di- and
trithiol metabolites inhibit the function of renal cortical
mitochondria. Therefore, a cycle of cell death and regenerative renal
hyperplasia may be initiated.
In adducing the relevance of these findings for humans, the
species differences in the metabolic pathway for chlorothalonil were
taken into account. It was noted that the formation of the thiol
metabolites, as determined by urinary excretion, was considerably
diminished when chlorothalonil was fed to germ-free rats. This
indicates that the type and/or quantity of gut microflora has a
determining role in the production of the thiol derivatives. Studies
in dogs and monkeys showed that the excretion of the thiol derivatives
was barely detectable after oral administration of chlorothalonil.
This suggests that the rat is rather different from other species in
this respect. Furthermore there is some evidence that ß-lyase
activity in the kidney varies among species, being an order of
magnitude lower in humans than in rats.
For all the reasons stated above it was concluded that the rodent
was not the most relevant species for evaluating the long-term effect
of chlorothalonil in humans and that the dog was a more representative
species for this purpose. The NOEL of 120 mg/kg in the diet in the
2-year study on dogs, equivalent to 3 mg/kg body weight per day,
should therefore be used for the purpose of human risk estimation.
1.2.2 Evaluation of effects on the environment
Chlorothalonil is algicidal for a number of algal species. The
fungicide does not inhibit bacterial growth except at very high
concentrations in laboratory culture. Field and laboratory evidence
shows no effects on nitrogen fixation or nitrification at recommended
application rates and minimal effects at higher application rates in
temperate soils. There was insufficient information to assess effects
on the nitrogen cycle in tropical soils.
Laboratory acute toxicity tests show chlorothalonil to be very
highly toxic to many aquatic animals including fish and Daphnia,
although molluscs appear to be insensitive. The LC50 concentrations
for a range of fish and invertebrates are similar and below
0.5 mg/litre.
A single study indicated reproductive effects in fish following
continuous exposure for 35 days. Since the compound both adsorbs to
suspended material and is degraded rapidly, the significance of this
finding was considered to be questionable.
A field study of aquatic organisms exposed following
chlorothalonil application suggests that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physicochemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field. There have been no
reported incidents of kills in the environment. However, despite the
short residence time of chlorothalonil in environmental media, kills
would be expected to occur immediately after application. Linking
kills to the compound would be difficult given that residues would not
persist long enough for chlorothalonil to be identified.
With minor exceptions, chlorothalonil is not phytotoxic.
Several studies have shown no toxicity of chlorothalonil to
earthworms at recommended application rates. At an exposure of five
times the maximum recommended rate, the compound severely reduced worm
reproduction.
Chlorothalonil is classified as "relatively non-toxic" to
honey-bees. Earwigs exposed to residues topically and via food showed
some mortality (20-55%), but there is no other evidence of
insecticidal action.
Chlorothalonil has low toxicity to birds in acute or dietary
tests. The low acute toxicity of chlorothalonil to laboratory mammals
tempered with its short persistence in the environment suggests
minimal hazard to wild mammal species.
1.2.2.1 Transport, distribution and transformation
Chlorothalonil adsorbs strongly to organic matter in soil and
suspended material in water. It is not, therefore, leached from soil
to groundwater. It is removed rapidly from surface water to suspended
material and to a lesser extent to bottom sediment. Chlorothalonil is
not translocated in plants from the site of application.
Abiotic degradation of chlorothalonil in water through photolysis
does not occur. Some hydrolysis does take place at higher pH.
Microbial degradation is the major cause of dissipation in soil
and may take place to some extent in water; this involves several
parallel processes, one of which leads to formation of the 4-hydroxy
metabolite. Half-lives for dissipation of this metabolite from non-
sterile soils range between 6 and 43 days. Biodegradation on plants
is limited and the 4-hydroxy metabolite comprises less than 5% of the
total residues.
During exposure, fish bioconcentrate chlorothalonil, but almost
total degradation occurs within 2 weeks after termination of exposure.
Chlorothalonil is metabolized in fish through glutathione conjugation
and the conjugates are excreted through the bile.
1.2.2.2 Aquatic organisms
The results of a single field study measuring concentrations of
chlorothalonil in water following overspray of the water were
available; corresponding data on concentrations in suspended and
bottom sediment were unreliable. Output from the EXAMS II fate model
using the same application scenario produced estimated water
concentrations which closely corresponded to the measured ones.
Little or no chlorothalonil was predicted in bottom sediment.
Based on this combination of measured and modelled data, the
ratio between a "toxic" concentration (the rainbow trout LC50) and
expected concentration is less than 1 for up to 5 h after overspray
and increases rapidly thereafter. Similar results were obtained for
daphnids. Therefore, despite its rapid removal from water and
degradation, the high toxicity of chlorothalonil is expected to cause
deaths of aquatic organisms in the period immediately after spraying.
This is the worst case situation of direct water overspray.
There were no data to extend this quantitative evaluation to
other field situations or climates.
1.2.2.3 Terrestrial organisms
A calculated maximum soil concentration, based on application of
chlorothalonil at 2.5 kg a.i./ha and complete bioavailability, is 3
orders of magnitude higher than the lowest estimate of LC50 for
earthworms.
For grazing birds (ducks and geese) total daily intake is at
least a factor of 100 below the NOEL for oral toxicity. For rabbits,
total daily intake is also at least 2 orders of magnitude lower than
the reported NOEL. This is based on a maximum recommended application
rate of 2.5 kg a.i./ha, an estimated worst case value for residues on
grass, no degradation of the compound, consumption of the total daily
intake at a single time and no choice but to eat contaminated food.
Table 1 contains a summary of risk quotients for birds, fish and
aquatic invertebrates.
Table 1. Toxicity/exposure ratios for birds, fish and aquatic invertebrates based
on application rates of 2.5 kg a.i./ha of chlorothalonil to soybeans
(worst case)
Risk category LC50 (mg/litre Estimated exposure Toxicity/exposure
or mg/kg diet) (mg/litre or ratio (TER)c
mg/kg diet)a,b
Acute bird 4640 73.7-535.7 63.0-8.7
Acute fish (stream) 0.01 0.009-0.04 1.1-0.25
Acute fish (pond) 0.01 0.01 1.0
Acute aquatic
invertebrate (stream) 0.07 0.009-0.04 7.8-1.8
Acute aquatic
invertebrate (pond) 0.07 0.01 7.0
a Estimated environmental concentration in the terrestrial environment (for bird exposure)
is based on the stated application rate and the assumption of deposition on short grass
using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STREAM model based on a single
application and estimated run-off into water; no direct overspray is included.
c TER is the toxicity (as LC50) divided by the exposure; values at or below 1.0 indicate
likely exposure to toxic concentrations by organisms in the different risk categories.
1.2.3 Toxicological criteria for setting guidance values
The toxicological studies on chlorothalonil of relevance for
setting guidance values are displayed in Table 2. The study results
and their significance are described briefly and gaps in test
requirements are indicated.
Table 2. Toxicological criteria for setting guidance values for chlorothalonil
Exposure Relevant route/effect/ Result/remarks
scenario species
Short-term skin, irritation, rabbit irritant
(1-7 days)
eye, irritation, rabbit irritant
skin, sensitization, tests were inconclusive
guinea-pig
evidence in humans of contact
dermatitis
inhalation, lethality, high toxicity in 4-h study with
rat hammermilled technical chlorothalonil
(MMAD 5-8 µm); not relevant for most
human exposure situations
Medium-term repeat dermal, rabbit 21-day study; irritant at 2.5 mg/kg
(1-26 weeks) body weight per day and above; no
systemic effects at 50 mg/kg body
weight per day
repeat oral, mice and 13-22 week studies; NOEL = 3 mg/kg
rats body weight per day in rats and mice
maternal, oral, rabbit teratology study; maternal toxicity
NOEL = 10 mg/kg body weight per day
by gavage; no fetotoxic or teratogenic
effect
Long-term repeat oral, dog 2-year study; NOEL = 3 mg/kg body
weight per day
1.3 Conclusions and recommendations
Considering the toxicological characteristics of chlorothalonil,
both qualitatively and quantitatively, it was concluded, on the basis
of the NOEL of 3 mg/kg body weight per day derived in the 2-year study
on dogs and applying a 100-fold uncertainty factor, that 0.03 mg/kg
body weight per day will probably not cause adverse effects in humans
by any route of exposure.
A study to assess the skin irritation potential is needed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom (Vice-
Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine,
University of Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate
School of Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist,
The Netherlands
Representatives of GIFAPa (Groupement International des
Associations Nationales de Fabricants de Produits Agrochimiques)
Dr M. Bliss, Jr., ISK Biosciences Corporation, Mentor, Ohio, USA
Dr A.C. Dykstra, Registration Department BPID, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr H. Frazier, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr R. Gardiner, GIFAP, Brussels, Belgium
Dr B. Julin, Regulatory Affairs, Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels, Belgium
Dr S.M. Kennedy (Environmental Science), Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels, Belgium
Dr J. Killeen, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr Th. S.M. Koopman, Toxicology Department, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr R.L. Mull, Du Pont Agricultural Products, Wilmington, Delaware, USA
Dr J.L.G. Thus, Environmental Research Department, Solvay-Duphar BV,
CP Weesp, The Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Châtelaine,
Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
a Participated as required for exchange of information.
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Pletina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR CHLOROTHALONIL
The Core Assessment Group (CAG) of the Joint Meeting on
Pesticides met in Geneva from 25 October to 3 November 1994. Dr W.
Kreisel of the WHO welcomed the participants on behalf of WHO, and
Dr M. Mercier, Director, IPCS, on behalf of the IPCS and its
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to chlorothalonil.
The first draft of the monograph was prepared by Dr M.H.
Litchfield, Arundel, United Kingdom. The second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs, was
prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The fact that ISK Biosciences Corporation made available to the
IPCS its proprietary toxicological information on chlorothalonil is
gratefully acknowledged. This allowed the CAG to make its evaluation
on a more complete database.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
BCF bioconcentration factor
BUN blood urea nitrogen
ECD electron capture detector
EDB 1,2-dibromoethane (ethylene dibromide)
FID flame ionization detector
GC gas chromatography
GSH glutathione
gamma-GT gamma-glutamyltranspeptidase
HECD Hall electron capture detector
LOEL lowest-observed-effect level
MS mass spectrometry
NADPH reduced nicotinamide adenine dinucleotide phosphate
NOEL no-observed-effect level
PIB piperonyl butoxide
SGOT serum glutamic-oxalic transaminase
SGPT serum glutamic-pyruvic transaminase
TEAM total exposure assessment methodology
TWA time-weighted average
UDS unscheduled DNA synthesis
VHH volatile halogenated hydrocarbon
VOC volatile organic carbon compound
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Chlorothalonil is a colourless, odourless, crystalline solid with
a melting point of 250°C and a vapour pressure of 7.63 × 10-5 Pa
(5.72 × 10-7 mmHg) at 25°C. It has low water solubility
(0.6-1.2 mg/litre at 25°C) and an octanol/water partition coefficient
(log Kow) of 2.882. It is hydrolysed in water slowly at pH 9 but is
stable at pH 7 or below (at 25°C).
The most prevalent analytical method, after sample extraction and
clean-up, is gas-liquid chromatography using an electron-capture
detector.
1.1.2 Sources of human and environmental exposure
Chlorothalonil has been produced commercially since 1969 by
chlorination of isophthalonitrile or by treatment of
tetrachloroisophthaloyl amide with phosphorus oxychloride. It is a
fungicide with a broad spectrum of activity used mainly in agriculture
but also on turf, lawns and ornamental plants. Crops protected
include pome and stone fruit, citrus, currants, berries, bananas,
tomatoes, green vegetables, coffee, peanuts, potatoes, onions and
cereals. In addition, it is used in wood preservatives and in paints.
The three main formulations are a suspension concentrate, a water
dispersible granule and a wettable powder. They are readily diluted
with water and applied by ground spray systems or by air. Typical
active ingredient rates are 1.2-2.5 kg/ha for crops such as beans,
celery and onions. The main sources of human exposure will be during
preparation and application of the products and from ingestion of crop
residues in foodstuff (see section 1.1.4).
1.1.3 Environmental transport, distribution and transformation
Chlorothalonil is removed from aqueous media by strong adsorption
on suspended matter. Modelled data suggest little or no partition to
bottom sediment. Biodegradation may occur in natural waters with
enzyme processes being involved. Chlorothalonil is rapidly degraded
in soil, and degradation may occur in water with the production of the
4-hydroxy metabolite, 4-hydroxy-2,5,6-trichloroisophthalonitrile.
Half-lives for dissipation of the 4-hydroxy metabolite in soils range
between 6 and 43 days.
Chlorothalonil does not translocate from the site of application
to other parts of a plant. It is metabolized only to a limited extent
on plants and the 4-hydroxy metabolite is usually < 5% of the
residue.
Chlorothalonil is metabolized in fish via glutathione conjugation
to give more polar excretory products. The enzyme glutathione-
S-transferase is involved with this conversion. High concentrations
of radiolabel found in the gall bladder and bile, after exposure of
rainbow trout to 14C-chlorothalonil, are consistent with the
excretion of the compound as glutathione conjugates. The
concentrations of radiolabel accumulating in the gall bladder and
other organs fell rapidly when the fish were placed in clean water.
Chlorothalonil does not bioaccumulate in aquatic organisms.
1.1.4 Environmental levels and human exposure
In a potato crop study, a small stream was oversprayed with
chlorothalonil. Subsequent sampling/analysis of down-stream water
demonstrated rapid disappearance of chlorothalonil (i.e. 450 µg/litre
at 30 min post-spraying to 2-6 µg/litre at 12 h post-spraying). The
routine spraying of irrigated field crops such as potatoes and barley
gave rise to low concentrations of chlorothalonil (0.04-3.6 µg/litre)
in tile drain water on a small number of sampling occasions.
Crop residues are composed mainly of chlorothalonil itself. The
residue levels are dependent upon the applied rate, time interval
between the last application and harvest, and the type of crop.
Residue levels at harvest can be derived from the numerous supervised
trials that have taken place on many crops worldwide and reported to
FAO/WHO. Residues of chlorothalonil in dairy products are expected to
be undetectable or very low. Dairy cows given high concentrations (up
to 250 mg/kg) of chlorothalonil in their feed for 30 days showed no
detectable residue in milk and only very low levels in tissues.
Total diet and individual food analysis in several countries have
shown undetectable or low concentrations of chlorothalonil in sampling
surveys. Residue levels on foodstuffs are further reduced by
preparation processes such as washing, peeling and cooking.
1.1.5 Kinetics and metabolism in laboratory animals
About 30% of an oral dose of chlorothalonil is absorbed within
48 h in rats at doses up to 50 mg/kg body weight. At higher doses,
absorption is lower, indicating a saturation process. When
14C-chlorothalonil is given orally the radioactivity is distributed
into blood and tissues within 2 h. The greatest concentration is
found in the kidney, followed by liver and blood. The kidneys contain
0.3% of a 5 mg/kg body weight dose after 24 h.
Most of an oral dose of chlorothalonil in rats is found in faeces
(> 82% within 48-72 h, regardless of dose). Biliary excretion is
rapid, peaking within 2 h after a 5 mg/kg body weight oral dose, and
is saturated at 50 mg/kg body weight and above. Urinary excretion
accounts for 5-10% of the dose in rats. Faecal excretion is the main
route in dogs and monkeys but urinary excretion (< 4%) is less than
in rats.
Metabolic studies in rats indicate that chlorothalonil is
conjugated with glutathione in the liver as well as in the
gastrointestinal tract. Some of the glutathione conjugates may be
absorbed from the intestine and transported to the kidneys, where they
are converted by cytosolic ß-lyase to thiol analogues that are
excreted in the urine. When germ-free rats are dosed with
chlorothalonil, the thiol metabolites appear in urine in much smaller
amounts than with normal rats, indicating the involvement of
intestinal microflora in the metabolism of chlorothalonil. Dogs or
monkeys dosed orally with chlorothalonil excrete little or no thiol
derivatives in urine.
When 14C-chlorothalonil was applied to rat skin, approximately
28% of the dose was absorbed within 120 h. About 18% of the dose was
found in faeces and 6% in urine within 120 h.
1.1.6 Effects on laboratory mammals and in vitro test systems
Chlorothalonil has low acute oral and dermal toxicity in rats and
rabbits, respectively (acute oral and dermal LD50 values are
> 10 000 mg/kg body weight). Hammer-milled technical chlorothalonil
(MMAD 5-8 µm) exhibited high toxicity in rats in an inhalation study,
with a 4-h LC50 of 0.1 mg/litre.
Chlorothalonil is a skin and eye irritant in the rabbit. Skin
sensitization studies in the guinea-pig were inconclusive.
The main effects of repeated oral dosing in rats are on the
stomach and kidney. Groups of 25 rats of each sex per group were fed
chlorothalonil at 0, 1.5, 3, 10 or 40 mg/kg body weight per day in the
diet for 13 weeks, and this was followed by a 13-week recovery period.
Increased incidences of hyperplasia and hyperkeratosis of the
forestomach occurred at 10 and 40 mg/kg; these reversed when treatment
ceased. At 40 mg/kg, there was an increased incidence of hyperplasia
of kidney proximal tubular epithelium in males at 13 weeks and after
the recovery period. The NOEL was 3 mg/kg body weight per day based
upon lack of forestomach lesions. The onset of the forestomach and
kidney changes was shown to be rapid, with the lesions developing
within 4-7 days in male rats at a dietary level of 175 mg/kg body
weight per day.
In a 13-week study on mice (0, 7.5, 15, 50, 275 or 750 mg/kg in
the diet), increased incidences of hyperplasia and hyperkeratosis of
the squamous epithelial cells of the forestomach occurred in males and
females at 50 mg/kg diet and above. The NOEL, based upon these
changes, was 15 mg/kg chlorothalonil in the diet, equivalent to
3 mg/kg body weight per day.
A 16-week study in dogs with dietary levels of 0, 250, 500 or
750 mg/kg showed no treatment-related changes.
The forestomach and kidney lesions were investigated further in
2-year studies on rats, mice and dogs. In a study on rats (0, 1.8,
3.8, 15 or 175 mg/kg body weight per day), the effects were
characterized histologically as an increase in the incidence and
severity of hyperplasia, hyperkeratosis, and ulcers and erosions of
the squamous mucosa of the forestomach, and as epithelial hyperplasia
of the kidney proximal convoluted tubules at 3.8 mg/kg and above. The
NOEL for non-neoplastic effects was therefore 1.8 mg/kg. The
incidence of renal tumours (adenomas and carcinomas) and forestomach
tumours (papillomas and carcinomas) was markedly increased at
175 mg/kg. There was evidence for an increased incidence of kidney
tumours in males at 15 mg/kg and of stomach tumours at 3.8 and
15 mg/kg in males and females. The NOEL for neoplastic effects was
therefore 1.8 mg/kg body weight per day based upon changes in
forestomach tumour incidence. Supporting evidence for the
carcinogenic potential of chlorothalonil in the kidney and forestomach
of rats was provided by the results from other 2-year studies at
higher dose levels.
In a study on mice (0, 15, 40, 175 or 750 mg/kg in the diet), an
increased incidence of renal tubular hyperplasia occurred at 175 mg/kg
and above and of hyperplasia and hyperkeratosis of the forestomach at
40 mg/kg and above. The incidence of squamous tumours of the
forestomach was slightly increased at 750 mg/kg. The NOELs for
neoplastic and non-neoplastic changes were therefore 175 and 15 mg/kg
in the diet (equivalent to 17.5 and 1.6 mg/kg body weight per day,
respectively). Supporting evidence for these effects in the mouse was
provided in another study at higher dose levels, but a study in
B6C3F1 mice did not show any evidence for carcinogenic potential at
high dose levels.
In a 2-year study on dogs (60 and 120 mg/kg in the diet), no
effects attributable to chlorothalonil were found. The NOEL was
therefore 120 mg/kg in the diet (equivalent to 3 mg/kg body weight per
day).
Chlorothalonil was not mutagenic in several in vitro and in
vivo tests, although it was positive in a small number of assays.
The monothio, dithio, trithio, dicysteine, tricysteine and
monoglutathione derivatives of chlorothalonil, which are potential
nephrotoxicants, were shown to be negative in the Ames assay.
Chlorothalonil was not teratogenic in rats or rabbits at doses up
to 400 and 50 mg/kg body weight per day, respectively. Reproductive
parameters such as mating, fertility and gestation length were not
affected by chlorothalonil at levels up to 1500 mg/kg in the diet in a
two-generation study in rats.
The acute oral toxicity of the 4-hydroxy metabolite is greater
than that of chlorothalonil itself (acute oral LD50 of 332 mg/kg body
weight versus > 10 000 mg/kg body weight). Several studies have been
undertaken to characterize the toxicological profile of this
metabolite and to establish NOELs.
1.1.7 Effects on humans
Contact dermatitis has been reported for personnel working in
chlorothalonil manufacturing and in farmers and horticultural workers.
Workers in the manufacture of wood products have also developed
contact dermatitis on the hands and face when wood preservatives
containing chlorothalonil were used.
1.1.8 Effects on other organisms in the laboratory and field
Chlorothalonil is highly toxic to fish and aquatic invertebrates
in laboratory studies, the LC50 values being below 0.5 mg/litre. The
maximum acceptable toxicant concentration (MATC) in a two-generation
reproduction study in Daphnia magna was 35 µg/litre.
With minor exceptions, chlorothalonil is not phytotoxic.
The LC50 of a suspension concentrate formulation (500 g
chlorothalonil/litre) in artificial soil for earthworms was
> 1000 mg/kg soil (14 days). Earwigs suffered increased mortality
when in contact with chlorothalonil residues on peanut foliage or
ingesting it as a food source in laboratory tests; there was no other
indication of insecticidal action.
Chlorothalonil is of low toxicity to birds with a reported acute
oral LD50 of 4640 mg/kg diet in the mallard duck. No significant
reproductive effects were reported.
A field study of aquatic organisms exposed following
chlorothalonil application suggests that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physicochemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field. There have been no
reported incidents of kills in the environment. However, despite the
short residence time of chlorothalonil in environmental media, kills
would be expected to occur. Linking kills to the compound would be
difficult given that residues would not persist long enough for
chlorothalonil to be identified.
1.2 Evaluation
1.2.1 Evaluation of human health risks
The review of the toxicological data for chlorothalonil revealed
that the most important studies for human risk estimation were the
long-term studies in rodents and dogs.
In the rodent studies, chlorothalonil caused lesions in the
forestomach and kidney. The lesions in the forestomach were
characterized as hyperplasia and hyperkeratosis of the squamous
epithelial cells. These occurred soon after dosing and were shown to
be reversible after dosing ceased. Long-term administration led to
the formation of tumours (papilloma and carcinoma). The renal lesions
in rodents were of rapid onset and characterized as hyperplasia of the
proximal tubular epithelium. On longer-term administration, renal
tumours (adenoma and carcinoma) occurred in the rat and in one study
on mice.
In order to interpret the significance of these findings, the
results of the mutagenic studies were taken into account.
Chlorothalonil gave negative results in in vitro and in vivo
mutagenic assays in which a variety of end-points were studied. Thiol
derivatives of chlorothalonil were negative in the Ames test, and
14C-chlorothalonil did not bind to rat kidney DNA in vivo. The
compound does not appear to have genotoxic potential on this basis,
indicating that it probably exerts its carcinogenic effect in rodents
via a non-genotoxic mechanism. The initial forestomach lesions in
rodents were attributed to the irritant action of chlorothalonil, and,
where this does not occur, a NOEL can be attained. The irritant action
on rodent forestomach in conjunction with the relatively long
residence time of the compound in this organ were seen to be factors
presenting the opportunity for the initiation of the lesions and
leading to carcinogenic action on prolonged administration. It was
concluded that, since humans do not possess a comparable organ,
rodents are probably not representative of the action of this compound
in man in this respect. This reasoning is also supported by the fact
that another animal species, the dog, is not affected by the compound
at similar or higher doses.
In the assessment of the relevance of the rodent renal lesions,
the metabolic conversion of chlorothalonil to metabolites which act
directly upon the kidney was seen to be a major factor. In the kidney
glutathione conjugates are converted by ß-lyase to chlorothalonil
thiol derivatives. Chlorothalonil is thought to be conjugated with
glutathione (GSH) mostly in the gastrointestinal tract prior to
absorption, although there is evidence of glutathione conjugation at
other sites. After absorption the conjugates pass to the kidney where
they are converted to chlorothalonil thiol derivatives following the
action of ß-lyase. It has been shown in vitro that the di- and
trithiol metabolites inhibit the function of renal cortical
mitochondria. Therefore, a cycle of cell death and regenerative renal
hyperplasia may be initiated.
In adducing the relevance of these findings for humans, the
species differences in the metabolic pathway for chlorothalonil were
taken into account. It was noted that the formation of the thiol
metabolites, as determined by urinary excretion, was considerably
diminished when chlorothalonil was fed to germ-free rats. This
indicates that the type and/or quantity of gut microflora has a
determining role in the production of the thiol derivatives. Studies
in dogs and monkeys showed that the excretion of the thiol derivatives
was barely detectable after oral administration of chlorothalonil.
This suggests that the rat is rather different from other species in
this respect. Furthermore there is some evidence that ß-lyase
activity in the kidney varies among species, being an order of
magnitude lower in humans than in rats.
For all the reasons stated above it was concluded that the rodent
was not the most relevant species for evaluating the long-term effect
of chlorothalonil in humans and that the dog was a more representative
species for this purpose. The NOEL of 120 mg/kg in the diet in the
2-year study on dogs, equivalent to 3 mg/kg body weight per day,
should therefore be used for the purpose of human risk estimation.
1.2.2 Evaluation of effects on the environment
Chlorothalonil is algicidal for a number of algal species. The
fungicide does not inhibit bacterial growth except at very high
concentrations in laboratory culture. Field and laboratory evidence
shows no effects on nitrogen fixation or nitrification at recommended
application rates and minimal effects at higher application rates in
temperate soils. There was insufficient information to assess effects
on the nitrogen cycle in tropical soils.
Laboratory acute toxicity tests show chlorothalonil to be very
highly toxic to many aquatic animals including fish and Daphnia,
although molluscs appear to be insensitive. The LC50 concentrations
for a range of fish and invertebrates are similar and below
0.5 mg/litre.
A single study indicated reproductive effects in fish following
continuous exposure for 35 days. Since the compound both adsorbs to
suspended material and is degraded rapidly, the significance of this
finding was considered to be questionable.
A field study of aquatic organisms exposed following
chlorothalonil application suggests that the toxicity is less than
that predicted from laboratory studies; this is again consistent with
the physicochemical properties of the compound. Deaths were seen in
some species exposed experimentally in the field. There have been no
reported incidents of kills in the environment. However, despite the
short residence time of chlorothalonil in environmental media, kills
would be expected to occur immediately after application. Linking
kills to the compound would be difficult given that residues would not
persist long enough for chlorothalonil to be identified.
With minor exceptions, chlorothalonil is not phytotoxic.
Several studies have shown no toxicity of chlorothalonil to
earthworms at recommended application rates. At an exposure of five
times the maximum recommended rate, the compound severely reduced worm
reproduction.
Chlorothalonil is classified as "relatively non-toxic" to
honey-bees. Earwigs exposed to residues topically and via food showed
some mortality (20-55%), but there is no other evidence of
insecticidal action.
Chlorothalonil has low toxicity to birds in acute or dietary
tests. The low acute toxicity of chlorothalonil to laboratory mammals
tempered with its short persistence in the environment suggests
minimal hazard to wild mammal species.
1.2.2.1 Transport, distribution and transformation
Chlorothalonil adsorbs strongly to organic matter in soil and
suspended material in water. It is not, therefore, leached from soil
to groundwater. It is removed rapidly from surface water to suspended
material and to a lesser extent to bottom sediment. Chlorothalonil is
not translocated in plants from the site of application.
Abiotic degradation of chlorothalonil in water through photolysis
does not occur. Some hydrolysis does take place at higher pH.
Microbial degradation is the major cause of dissipation in soil
and may take place to some extent in water; this involves several
parallel processes, one of which leads to formation of the 4-hydroxy
metabolite. Half-lives for dissipation of this metabolite from non-
sterile soils range between 6 and 43 days. Biodegradation on plants
is limited and the 4-hydroxy metabolite comprises less than 5% of the
total residues.
During exposure, fish bioconcentrate chlorothalonil, but almost
total degradation occurs within 2 weeks after termination of exposure.
Chlorothalonil is metabolized in fish through glutathione conjugation
and the conjugates are excreted through the bile.
1.2.2.2 Aquatic organisms
The results of a single field study measuring concentrations of
chlorothalonil in water following overspray of the water were
available; corresponding data on concentrations in suspended and
bottom sediment were unreliable. Output from the EXAMS II fate model
using the same application scenario produced estimated water
concentrations which closely corresponded to the measured ones.
Little or no chlorothalonil was predicted in bottom sediment.
Based on this combination of measured and modelled data, the
ratio between a "toxic" concentration (the rainbow trout LC50) and
expected concentration is less than 1 for up to 5 h after overspray
and increases rapidly thereafter. Similar results were obtained for
daphnids. Therefore, despite its rapid removal from water and
degradation, the high toxicity of chlorothalonil is expected to cause
deaths of aquatic organisms in the period immediately after spraying.
This is the worst case situation of direct water overspray.
There were no data to extend this quantitative evaluation to
other field situations or climates.
1.2.2.3 Terrestrial organisms
A calculated maximum soil concentration, based on application of
chlorothalonil at 2.5 kg a.i./ha and complete bioavailability, is 3
orders of magnitude higher than the lowest estimate of LC50 for
earthworms.
For grazing birds (ducks and geese) total daily intake is at
least a factor of 100 below the NOEL for oral toxicity. For rabbits,
total daily intake is also at least 2 orders of magnitude lower than
the reported NOEL. This is based on a maximum recommended application
rate of 2.5 kg a.i./ha, an estimated worst case value for residues on
grass, no degradation of the compound, consumption of the total daily
intake at a single time and no choice but to eat contaminated food.
Table 1 contains a summary of risk quotients for birds, fish and
aquatic invertebrates.
Table 1. Toxicity/exposure ratios for birds, fish and aquatic invertebrates based
on application rates of 2.5 kg a.i./ha of chlorothalonil to soybeans
(worst case)
Risk category LC50 (mg/litre Estimated exposure Toxicity/exposure
or mg/kg diet) (mg/litre or ratio (TER)c
mg/kg diet)a,b
Acute bird 4640 73.7-535.7 63.0-8.7
Acute fish (stream) 0.01 0.009-0.04 1.1-0.25
Acute fish (pond) 0.01 0.01 1.0
Acute aquatic
invertebrate (stream) 0.07 0.009-0.04 7.8-1.8
Acute aquatic
invertebrate (pond) 0.07 0.01 7.0
a Estimated environmental concentration in the terrestrial environment (for bird exposure)
is based on the stated application rate and the assumption of deposition on short grass
using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STREAM model based on a single
application and estimated run-off into water; no direct overspray is included.
c TER is the toxicity (as LC50) divided by the exposure; values at or below 1.0 indicate
likely exposure to toxic concentrations by organisms in the different risk categories.
1.2.3 Toxicological criteria for setting guidance values
The toxicological studies on chlorothalonil of relevance for
setting guidance values are displayed in Table 2. The study results
and their significance are described briefly and gaps in test
requirements are indicated.
Table 2. Toxicological criteria for setting guidance values for chlorothalonil
Exposure Relevant route/effect/ Result/remarks
scenario species
Short-term skin, irritation, rabbit irritant
(1-7 days)
eye, irritation, rabbit irritant
skin, sensitization, tests were inconclusive
guinea-pig
evidence in humans of contact
dermatitis
inhalation, lethality, high toxicity in 4-h study with
rat hammermilled technical chlorothalonil
(MMAD 5-8 µm); not relevant for most
human exposure situations
Medium-term repeat dermal, rabbit 21-day study; irritant at 2.5 mg/kg
(1-26 weeks) body weight per day and above; no
systemic effects at 50 mg/kg body
weight per day
repeat oral, mice and 13-22 week studies; NOEL = 3 mg/kg
rats body weight per day in rats and mice
maternal, oral, rabbit teratology study; maternal toxicity
NOEL = 10 mg/kg body weight per day
by gavage; no fetotoxic or teratogenic
effect
Long-term repeat oral, dog 2-year study; NOEL = 3 mg/kg body
weight per day
1.3 Conclusions and recommendations
Considering the toxicological characteristics of chlorothalonil,
both qualitatively and quantitatively, it was concluded, on the basis
of the NOEL of 3 mg/kg body weight per day derived in the 2-year study
on dogs and applying a 100-fold uncertainty factor, that 0.03 mg/kg
body weight per day will probably not cause adverse effects in humans
by any route of exposure.
A study to assess the skin irritation potential is needed.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure
 Molecular formula C8Cl4N2
Relative molecular mass 265.9
CAS chemical name 2,4,5,6,-tetrachloro-1,3-benzenedi
carbonitrile
CAS registry number
Molecular formula C8Cl4N2
Relative molecular mass 265.9
CAS chemical name 2,4,5,6,-tetrachloro-1,3-benzenedi
carbonitrile
CAS registry number 



 On plants, chlorothalonil is metabolized only to a limited extent
to the 4-hydroxy metabolite. The majority of the residue remains as
the parent compound. Generally less than 5% of the total residue is
present as the 4-hydroxy metabolite. A review of plant residues
worldwide showed that the 4-hydroxy metabolite level was < 0.1 mg/kg
in most of the crops analysed. It accounted for approximately 10% of
the total residue in lima beans, 5% in cantaloupes, 2% in peaches and
onions, 1% in celery and 0.1% in peanuts (FAO/WHO, 1985). The decline
of chlorothalonil residues and the appearance and decline of the
4-hydroxy metabolite on onions is shown in Table 7 (personal
communication to the IPCS by the Government of Canada, 1979). The
chlorothalonil residue decayed with a half-life of about 3 days.
Studies with corn silage showed that 90% of chlorothalonil
degraded within 18 days (30 to 3 mg/kg). The half-life was
approximately 4 days. In a second experiment, the 4-hydroxy
metabolite formation was very low in the bound materials (which were
converted to an extractable form), representing only about 2% of the
chlorothalonil on the first day of ensiling (FAO/WHO, 1978).
After chlorothalonil was applied to growing peanut foliage at
1.26 kg/ha its half-life was 13.6 days (range 7-19 days) under the
field conditions of use (Elliott & Spurr, 1993).
In the excretion of 14C-chlorothalonil metabolites from rainbow
trout (Salmo gairdneri), the almost complete absence of
chlorothalonil itself and the accumulation of 14C entities in the
bile indicated the possibility of glutathione conjugation as the first
step in chlorothalonil metabolism (Davies & White, 1985). Further
studies showed the existence of mono- and diglutathione conjugates of
chlorothalonil in the bile of rainbow trout exposed to
14C-chlorothalonil (Davies, 1985a).
Studies with liver cytosol from five fish species showed that the
enzyme glutathione- S-transferase (GST) is involved in the conversion
of chlorothalonil to polar conjugates. Comparisons of GST activity in
rainbow trout organs revealed that the potential for chlorothalonil
transformation was in the order liver » kidney > spleen, with no
activity in bile. Low concentrations of chlorothalonil in water
induced fish GST activity for its biotransformation. Hepatic
glutathione (GSH) and GST activity for chlorothalonil transformation
were compared in three species of fish (Oncorhynchus mykiss,
Galaxias maculatus and Galaxias auratus). The order of their
asymptotic LC50 values agreed with that of their hepatic GST
activities for chlorothalonil transformation and was consistent with a
detoxification role for GSH-chlorothalonil conjugation (Davies,
1985b). A study involving co-exposure to zinc and chlorothalonil
indicated that metallothionein does not play a significant role in
chlorothalonil detoxification in fish at sublethal exposures (Davies,
1985c).
Small amounts of the 4-hydroxy metabolite were found in the milk
and kidney of a cow fed 250 mg chlorothalonil/kg in its feed. Only
0.2% of the ingested chlorothalonil was eliminated in the milk as the
4-hydroxy metabolite (Ladd et al., 1971).
4.2.2 Abiotic degradation
Chlorothalonil does not break down in aqueous solution
(0.5 mg/litre) in the dark at pH 5 or 7. It is hydrolysed at pH 9,
over 50% disappearing in 49 days, with the formation of 4-hydroxy-
2,5,6-trichloroisophthalonitrile and 3-cyano-2,4,5,6-tetrachloro-
benzamide (Szalkowski & Stallard, 1977).
Chlorothalonil degrades very slowly under aqueous photolytic
conditions to the 4-hydroxy metabolite. The half-life was found to be
approximately 65 days (ISK Biotech proprietary information).
4.2.3 Bioaccumulation
In a study of the uptake and elimination of 14C-chlorothalonil
in rainbow trout, two groups of fish were exposed to 10 µg/litre of
the compound in flow-through tanks for 96 h (Davies & White, 1985).
After exposure was discontinued, the depuration rate was followed for
96 h. There was a very high uptake in the gall bladder and bile
(concentration factors up to 4.4 × 105). Uptake was also high in the
hind gut, liver, fat and kidney with concentration factors of
2-11 × 103. After 96 h of exposure, the concentration factor in
muscle was 940 and 740, respectively, for the two groups of fish, a
level which may give an indication of the magnitude of the whole body
bioconcentration factor (BCF) for rainbow trout (not measured).
After transfer to clean water, gall bladder levels dropped
rapidly, and so did gill and blood levels. In one group of fish,
concentrations in both liver and kidney doubled until 24 h after
transfer and thereafter dropped to the levels in the other group.
Concentrations in the spleen in both groups continued to increase
throughout the depuration period. Muscle levels dropped only slowly
and remained around 1 µg/g. The high concentrations found in the gall
bladder and bile are consistent with the fact that chlorothalonil is
excreted from fish as glutathione conjugates (Davies & White, 1985).
Bluegill sunfish exposed to 8 µg 14C-chlorothalonil/litre in a
flow-through system for 30 days showed a plateau of 14C uptake within
14 days. The residues in whole fish at 30 days were 264 times the
water concentration. When the fish were placed in clean water, 80% of
the radioactive residues were lost within 14 days. Bioaccumulation in
catfish, in a static system, showed a 16-fold concentration at 26
days. In this case 90% of the 14C residues were depurated in 14 days
after removal from the treated water. The 4-hydroxy metabolite did
not bioaccumulate in fish (SDS Biotech Corporation, 1972).
In tanks containing stream water with chlorothalonil at
20 µg/litre, uptake of the compound occurred in algal growths attached
to bottom pebbles. Analysis of the algal growths showed a
concentration factor for chlorothalonil of 270 times after 14 days of
static exposure. Since this represented only 9.5% of the initial dose
it seems that the removal of chlorothalonil from the water is enhanced
by its conversion to polar metabolites in addition to bioconcentration
(Davies, 1988).
4.3 Waste disposal
Chlorothalonil can be incinerated in units operating at 850°C
fitted with off-gas scrubbing equipment (Lawless et al., 1975).
The disposal methods for waste pesticides and containers
advocated by FAO and GIFAP should be applied to unused chlorothalonil
products and their empty packages (FAO, 1985; GIFAP, 1987).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Chlorothalonil was detected (amongst other pesticides) in 3 out
of 9 outdoor and indoor samples and 1 out of 9 personal monitoring
samples in 9 homes in Jacksonville, Florida, USA. No actual figures
were reported (Lewis et al., 1988).
Average exposures to chlorothalonil of 173 persons in
Jacksonville, Florida and Springfield, Massachusetts, USA were
0.7 ng/m3 (personal exposure) and 0.5 ng/m3 (outdoor air
concentrations) (Wallace, 1991).
Chlorothalonil was not detected in 51 samples in an Environmental
Survey of Chemicals in Japan in 1991 (personal communication by the
Office of Health Studies Environment Agency, Tokyo, 1992).
5.1.2 Water
O'Neil (1991) studied concentrations of chlorothalonil in water
and sediment following overspraying of a pond (0.2 ha and 0.5 m deep)
at 875 g a.i./ha (Ernst, 1991). Water and sediment were monitored in
a stream flowing out of the pond at the outlet and 30 m downstream.
The stream was approximately 1 m wide and 0.5 m deep and ran at
0.033 m3/sec for the first overspray and at 0.015 m3/sec for the
repeat spray. Whole water samples were filtered for separate
measurement of chlorothalonil in water and sediment. Following the
first spraying, samples were taken at the downstream site at 30 min
intervals up to 6 h after spraying and further samples were collected
10 and 24 h after spraying. Initial water concentrations of up to
60 µg/litre fell rapidly to around 15 µg/litre 2 h after spraying.
The water concentration was 1.9 µg/litre 10 h after spraying, and at
24 h there was no measurable chlorothalonil. In the second spraying
at the lower stream flow rate, whole water samples were taken more
frequently over the 2 h following application. Concentrations peaked
at 350 to 450 µg/litre at the pond outlet and 30 m downstream,
respectively, 20 to 30 min after application, falling to between 50
and 100 µg/litre at 2 h. A concentration of 6.3 µg/litre was found
12 h after spraying. Total chlorothalonil mass was measured on
suspended sediment following the first spraying and showed 10 µg
persisting for 1.5 h after spraying and thereafter falling to
approximately 0.01 µg at 10 and 24 h. The report did not make clear
the volume of water filtered, which appears, however, to have been
1 litre. Environmental conditions such as total organic carbon (TOC),
pH, temperature and water hardness were not reported; consequently
their impacts on degradation could not be evaluated.
Chlorothalonil was detected on occasions at concentrations up to
3.6 µg/litre in a tile drainage system from a farm in Manitoba,
Canada, where the fungicide was sprayed routinely. It was detected on
one occasion (0.06 µg/litre) in the sump well outflow draining to a
municipal ditch (Krawchuk & Webster, 1987).
Over a 5-year period (1986-1990), water was sampled and analysed
from 1300 community water systems and rural domestic wells for 101
pesticides, including chlorothalonil. Chlorothalonil was not detected
in any of these samples although the reporting limit was 0.12
µg/litre, which represented the minimum quantification limits for this
particular pesticide in the study (US EPA, 1990).
Chlorothalonil was not detected in 57 water samples, 30 sediment
samples and 30 fish samples in an Environmental Survey of Chemicals in
Japan in 1991 (personal communication by the Office of Health Studies,
Environment Agency, Tokyo, 1992).
5.1.3 Soil
Levels of chlorothalonil and its metabolite SDS-3701 (see section
4.2.1) in soil were reported after three annual treatments (Kenyon &
Ballee, 1990; King et al., 1991, 1992). Four plots were established
of bare untreated and treated, winter wheat treated and untreated at
two different sites, Osterwede and Rohlstof (Germany). Treatment
consisted of an annual chlorothalonil application of 2.2 kg a.i./ha.
Soil samples were taken before and after each treatment. No
chlorothalonil was detected in any of the untreated samples.
Consistently there was no carry over from one year to another. Levels
in soil were highest 2 or 3 days after the treatment (sampling
depended on the sites), with mean levels in the bare plots around 0.40
mg/kg and in those with wheat around 0.34 mg/kg (values ranging
between 0.07 and 0.64 mg/kg). Between 52 and 60 days after each
treatment, levels were 0.02-0.03 mg/kg in plots with wheat while in
bare plots they were generally below the detection limit of 0.01
mg/kg. Before each treatment in the previously treated plots the
level of metabolite SDS-3701 ranged from the limit of detection (0.01)
to 0.03 mg/kg, which was the same as the level 2 or 3 days after
treatment. However, between 52 and 60 days after treatment (depending
on the site) levels rose at the Osterwede site to 0.07 mg/kg for the
bare treated plot. One year after the last treatment, levels of
SDS-3701 ranged from the detection limit to 0.03 mg/kg.
5.1.4 Food crops
Chlorothalonil is used as a broad spectrum fungicide on
vegetables, fruit trees, small fruit bushes and other agricultural and
horticultural food crops. Its use is intended to protect crops up to
harvesting, hence small residues will be present at that time. The
residue levels expected in crops at harvest can be derived from the
numerous supervized trials that have taken place on many crops in
countries all over the world (FAO/WHO, 1975, 1978, 1979, 1980, 1982,
1985a, 1986a, 1990a).
The amount of residue at harvest depends upon factors such as the
application rate, time interval between the last application and
harvest, and the type of crop. Residues are composed mainly of
chlorothalonil, and only negligible amounts of the metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile (SDS-3701) are present (see
Table 7 for example).
The decline of chlorothalonil residues on food crops after
application is shown by the field treatment of apples and pears
against Botryris cynerea by spraying with a chlorothalonil flowable
formulation and then harvesting at intervals after treatment (Camoni
et al., 1991). The results are shown in Table 8.
Table 8. Decline of chlorothalonil residues
Days after treatment Pears Apples
(mg/kg) (mg/kg)
0 3.85 2.35
7 2.48 1.73
14 2.00 0.92
28 1.35 0.98
From: Camoni et al. (1991)
Similar examples of the decline of chlorothalonil residues have
been given for grapes in Australia, Germany and South Africa (FAO/WHO,
1985a). The decline of residues in onions is shown in Table 7. The
distribution of the residues on this plant showed that the levels in
the older outer leaves were about 5 times above those in the younger
leaves (2.4 and 0.51 mg/kg, respectively).
Pre-harvest intervals are set on the basis of supervized trials,
e.g., 7 days for apricots, and cherries in Australia, 7-14 days for
grapes in Australia and 7 days for onions in the Netherlands (FAO/WHO,
1990a).
One of two samples of currants from growers in the United Kingdom
had a residue level of 7.5 mg/kg 54 days after the last of three
treatments at half the recommended rate of application. The residue
level on the second sample was < 0.5 mg/kg 76 days after two
applications at the maximum rate (UK, 1985).
5.1.5 Dairy produce
There have been no reports of chlorothalonil residues in dairy
produce. However, some indication can be gained from studies on dairy
cattle fed high levels of the compound. In one cow fed 250 mg
chlorothalonil/kg feed for 44 days, no chlorothalonil was detected in
the milk and only 0.2% of the dose appeared as the 4-hydroxy
metabolite. Neither compound could be detected in muscle or fat and
only a low level of the 4-hydroxy metabolite (0.7 mg/kg) was found in
the kidney (Ladd et al., 1971; Wolfe & Stallard, 1971). In another
study, groups of four cows were fed chlorothalonil combined with the
4-hydroxy metabolite at levels up to 250 and 0.6 mg/kg, respectively,
for 30 days. At the end of the period half the cows were sacrificed
and half continued for a 32-day recovery period. No chlorothalonil
(< 0.02 mg/kg) was found in milk. Small residues of chlorothalonil
and the 4-hydroxy metabolite were detected in muscle, fat, liver and
kidney after 30 days administration but none were detected in these
organs after the 32-day recovery period (FAO/WHO, 1975). No
chlorothalonil (< 0.03 mg/kg) was detected in milk from a cow fed the
compound at 5 mg/kg in its rations for 4 days (Gutenmann & Lisk,
1966).
5.1.6 Animal feed
Dry cannery waste (tomato pommace), sometimes used for animal
feed, contained < 1 mg/kg chlorothalonil plus its 4-hydroxy
metabolite (in the ratio 6:1) as a residue (FAO/WHO, 1978).
5.2 General population exposure
5.2.1 Food
In a study of imported fruit and vegetables in Finland, chlorothalonil
levels of 0.02-0.15 mg/kg in strawberries, 0.01-0.86 mg/kg in Chinese
lettuce and 0.12-1.2 mg/kg in peaches were found (personal
communication to the IPCS by the Government of Finland, 1979).
No chlorothalonil (< 0.01 mg/kg) was detected in a US Food and
Drug Administration (FDA) total diet study in the USA in 1976 or 1977
(personal communication to the IPCS by J.R. Wessel, 1979). In a
Canadian total diet survey, chlorothalonil was detected in one out of
six composite samples of garden fruits at the detection level (0.02
mg/kg). On the basis of this one sample, a dietary intake of 0.04 µg
per person per day was estimated (McLeod et al., 1980).
Chlorothalonil was detected (0.001-1.35 mg/kg) in most samples of
apples, peaches and other fruit and vegetables marketed in Tokyo
(Koseki et al., 1980).
No chlorothalonil (< 0.005 mg/kg) was detected in samples of
potatoes in Sweden in 1979. During 1981-1983, 1070 out of 1085
samples of domestic and imported commodities in Sweden had
chlorothalonil residues below 0.21 mg/kg. Samples having higher
residues included one of cauliflowers (out of 165) at 0.41 mg/kg, one
of cucumbers (out of 580) at 0.23 mg/kg and two of strawberries (out
of 143) at 2.9 mg/kg (personal communication: data submitted to the
IPCS by the Government of Sweden and entitled "Chlorothalonil residues
in imported and domestic commodities - 1981 to 1983").
In 1982, analysis at the point of retail in the United Kingdom
showed chlorothalonil residues below 0.5 mg/kg in 41 samples of
strawberries, 15 of gooseberries, 13 of currants and 9 of berries.
Other analyses during 1981-3 showed that only one out of 30 samples of
imported strawberries, 2 out of 15 samples of domestic celery and 5
out of 40 of gooseberries had chlorothalonil residues above 0.1 mg/kg
(UK, 1985).
In the United Kingdom, chlorothalonil residues in bananas
(imported), chinese cabbage (all origins) and parsnips (United Kingdom
origin) were below the reporting levels of 0.2, 1.0 and 0.01 mg/kg,
respectively, in 1988-1989. During the same period, one sample out of
ten of imported strawberries contained 0.1 mg/kg (UK MAFF & HSE,
1990).
Residues of chlorothalonil in foodstuffs are decreased by
processes such as washing. For example, it was shown that 94% of the
residue could be removed by washing tomatoes and that there was no
detectable residue in canned tomato pulp, paste or juice. Peaches
washed in water followed by a caustic rinse showed a 97% removal of
field residues. No chlorothalonil was detected in canned peach puree
(FAO/WHO, 1978).
In a Honduran study, unwashed bananas had a maximum residue level
of 0.17 mg/kg and a mean of 0.08 mg/kg. This was reduced to 0.02
mg/kg after washing. No chlorothalonil was found in the edible pulp
(< 0.01 mg/kg). Similar results were obtained in the Philippines
(FAO/WHO, 1980).
Trimming and peeling also removes a large proportion of residues
from some foodstuffs. For example there are significant reductions
after trimming the outer leaves from cabbages and lettuces. Most of
the residue is removed when cucumbers, melons, peanuts and potatoes
are peeled (Diamond Shamrock, 1974).
As much as 85-98% of chlorothalonil added to tomatoes or green
beans was lost during cooking in open vessels. Only 2.4% was
converted to the 4-hydroxy metabolite, which was stable to cooking
(SDS Biotech Corporation, 1983a).
5.3 Occupational exposure
The exposure of a tractor driver applying chlorothalonil to
ornamental plants in Florida, USA, was assessed. Total-body exposure
rates, estimated from external exposure pads and air sampling, were
low (approximately 5 mg a.i./h) (Stamper et al., 1989a). In the case
of a greenhouse drencher, this exposure was approximately 100 mg
a.i./h (Stamper et al., 1989b).
Occupational exposure to four insecticides and two fungicides was
measured for 151 commercial tree and shrub applicators in the USA who
used hand-held equipment when spraying pesticides. The study was
conducted for 3 consecutive years: 1985, 1986 and 1987. Worker
exposure was determined by collecting full-shift, breathing zone air
samples. Sampling was conducted with battery-operated constant-flow
air-sampling devices. Chlorothalonil was detected in only one out of
14 samples at 0.011 mg/m3 (Leonard & Yeary, 1990).
Spencer et al. (1991) estimated the dermal exposure of workers on
mechanical tomato harvesters to residues of chlorothalonil. An
average of 499.6 µg/h was obtained by gauze pad dosimeters placed
outside the workers' clothing, whereas 43.4 µg/h was obtained by
undershirt dosimetry. The results showed that regular work clothing
provides an excellent protection (90% reduction in dermal exposure)
against chlorothalonil. Air concentrations in the field were also
determined and averaged 0.002 to 0.02 µg/litre, which contributed 8 to
28% to the total exposure.
The exposure of 11 pesticide operators mixing, loading or
applying chlorothalonil fungicide formulations by aerial or ground
applicators has been assessed. The highest exposure was on the hands
(1.7 mg/m2 per h) (Diamond Shamrock, 1980).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Biliary excretion of radioactivity was studied in groups of six
male Sprague-Dawley rats administered a single oral dose of 1.5, 5, 50
or 200 mg/kg body weight 14C-chlorothalonil (98% radiochemically
pure), uniformly labelled in the aromatic ring, as a suspension with a
mean particle size of 3.6 to 5.0 µm in 0.75% methylcellulose in water.
The bile duct was cannulated and bile was collected in 1-h fractions
for 48 h after dosing. Blood, urine and faeces were also collected at
various times after dosing and at termination. During the 48 h after
a single dose of 1.5, 5, 50 and 200 mg/kg body weight, biliary
excretion was 22.5, 16.4, 16.3, and 7.7% of the administered dose,
respectively. Profiles of radioactivity excretion after the two low
doses were quantitatively different from those obtained after the two
high doses. The authors interpreted these results as indicative of a
change in metabolism occurring between 5 and 50 mg/kg body weight,
possibly due to saturation of biliary excretion. Mean urinary
excretion during the 48 h after dosing was 8.0, 8.2 and 7.6% of the
administered dose at 1.5, 5 and 50 mg/kg body weight, respectively,
and only 4.7% at the high dose level of 200 mg/kg body weight.
Excretion of radioactivity in the urine within 6 h after dosing was
inversely related to the dose administered. Total recovery of
radioactivity in this study was 89-99% in the three low-dose groups
and 74% in the high-dose group. After doses of 1.5, 5, 50 and
200 mg/kg body weight, rats absorbed 32, 25.7, 25.9 and 15.5% of the
administered dose, respectively. It was concluded by the authors that
enterohepatic circulation or reabsorption of biliary metabolites from
the gastrointestinal tract did not contribute significantly to the
amount of radiolabel in the kidney. Based on a one-compartment model
for chlorothalonil absorption and excretion and using several
assumptions, it was calculated that the rate of absorption of the
200 mg/kg body weight dose was only twice as fast as that of the
50 mg/kg body weight dose (Marciniszyn et al., 1986).
In a study by Chin et al. (1981), absorption was compared by the
oral, dermal and endotracheal routes with a 1 mg/kg body weight dose
of 14C-chlorothalonil in male Sprague-Dawley rats. The comparisons
were made on the basis of blood levels and urine excretion. In each
case, absorption was highest by endotracheal administration and lowest
by the dermal route. Less than 6% of the administered dose was
recovered in blood and urine within 48 h after dosing.
When 14C-chlorothalonil was introduced into sacs formed from the
upper section of rat small intestine, no unchanged substance passed
through the mucosa and was transferred to the serosal side of the sac.
These data suggest that chlorothalonil is very rapidly conjugated,
since in vivo studies have not identified chlorothalonil itself in
body fluids or tissues after oral administration to rats (Savides et
al., 1986e).
14C-chlorothalonil was applied to the skin of male rats at an
averaged dose of 1167 µg/rat (= 5 mg/kg) on an area of 25 cm2. The
amount absorbed was deduced from the amount remaining on the treated
skin area and the amount of radioactivity found at each time interval
in urine, faeces and carcass. Approximately 28% of the applied dose
was absorbed over the experimental exposure period of 120 h. The
absorption appeared to be time-dependent, about 6.3% of the applied
dose being absorbed during each 24 h period. Radioactivity appeared
quickly in blood and rose steadily up to 72 h, when it reached a
plateau (Marciniszyn et al., 1984a).
In a study by Magee et al. (1990), four monkeys were treated
dermally with 5 mg/kg body weight of 14C-chlorothalonil under a
non-occlusive patch. After 48 h the patch was taken off and the skin
was washed. About 90% of the dose was recovered from the surface and
about 2.26% was completely absorbed through the skin. The urine
contained 1% of the dose, but methylated mono-, di- and trithiols were
not detectable in the urine.
6.2 Distribution
Groups of male and female rats were administered
14C-chlorothalonil orally, in microparticulate suspension, as single
doses of 5, 50 or 200 mg/kg, and tissue activity was determined after
2, 9, 24, 96 and 168 h (Marciniszyn et al., 1984b, 1985a). With the
exception of gastrointestinal tract tissues the greatest concentration
of radioactivity was found in the kidneys, at each dose level,
followed by those in liver and whole blood. The peak concentrations
in kidney occurred at 2 h after 5 mg/kg, 9 h after 50 mg/kg and 24 h
after 200 mg/kg. Similar shifts in peak time with dose occurred in
the liver and blood. In terms of the original dose, kidneys, liver
and blood each contained 0.7% of the label 2 h after 5 mg/kg and 0.3%
(kidney), 0.14% (liver) and 0.23% (blood) after 24 h in female rats.
Distribution of radioactivity was also studied after repeated
oral administration of 14C-chlorothalonil to male rats. Five doses
were given at 24 h intervals at concentrations of 1.5, 5, 50 or
160 mg/kg. The rats were killed 2, 9, 24, 96 and 168 h after the last
dose. The distribution of activity showed a similar profile to that
after single dosing, i.e. the highest concentrations occurred in
kidneys, followed by liver and blood, at all doses and times. At all
dose levels, the concentrations peaked 2 h after the last dose. The
percentage of the dose found in the kidney at this time was 0.28% and
0.20% at the 1.5 and 5 mg/kg dose levels, which was significantly
higher than that found at the higher doses (about 0.09%). At dose
levels up to 50 mg/kg there was significant depletion of radioactivity
from the blood during the 24 h between doses. In the kidney there was
a trend to slower overall depletion with increase in dose (Savides et
al., 1986a).
A study in mice showed that the distribution of activity in
non-gastrointestinal tract tissues was similar to that in rats after a
single oral dose of 14C-chlorothalonil. The kidney had the highest
concentration of radioactivity after doses of 1.5, 15 or 105 mg/kg
(Ribovich et al., 1983).
6.3 Metabolic transformation
6.3.1 Rat
Male Sprague-Dawley rats were administered, via oral gavage,
14C-chlorothalonil (purity 99.7%) at a dose level of 200 mg/kg in
order to isolate and identify the urinary metabolites. Urine was
collected 17, 24 and 48 h after dosing. Urinary metabolites accounted
for 2.4% of the administered dose and, except for 30% of the
radiolabel which was non-extractable from the urine, were found to be
trimethylthiomonochloro-isophthalonitrile and dimethylthiodichloro-
isophthalonitrile. These thiols were excreted in urine both as free
thiols and as their methylated derivatives. The authors suggested a
metabolic pathway such that hepatic metabolism proceeds through
conjugation with GSH followed by enzymatic degradation. The smaller
conjugates are then transported via the bloodstream to the kidney,
where they are converted to thiol metabolites and excreted in the
urine (Marciniszyn et al., 1985b).
A study was also carried out in rats given five daily oral doses
of 14C-chlorothalonil (1.5, 5, 50 or 160 mg/kg per day). Urine
samples, acidified and extracted with ethyl acetate, showed decreasing
extractability of radioactivity with increasing dose. GC/MS analysis
identified methylated or partly methylated dithiol and trithiol
derivatives of chlorothalonil from the first dose onwards. The
percentage of the trithiol derivative excreted was constant with
increasing dose while the dithiol increased with dose. Multiple
dosing resulted in a decreasing daily excretion of total thiol
derivatives. These results emphasize the probable involvement of
glutathione in the metabolic pathway for chlorothalonil (Savides et
al., 1986b).
A group of three rats, pretreated with the gamma-glutamyl
transpeptidase inhibitor AT-125, were dosed with 50 mg/kg
14C-chlorothalonil, while three other rats were given chlorothalonil
only. Urine samples were acidified and extracted with ethyl acetate.
The group of rats pre-treated with AT-125 showed only 15% of
radioactivity extractable after 12 h, while the other group showed 75%
extractability. The non-extractable fraction from the
inhibitor-treated rats contained glutathione conjugates of
chlorothalonil. The kidneys contained 2-3 times more radioactivity
than those of the untreated rats. These results gave further support
to the hypothesis that glutathione is involved with chlorothalonil
metabolism (Marciniszyn et al., 1988).
The production of metabolites was also studied in groups of rats
following dermal administration. 14C-chlorothalonil (4.6 mg/kg) was
applied to a shaved area of the dorsal region. The area was covered
and exposure continued for 48 h. Urine samples collected at 24 and
48 h were acidified and extracted with ethyl acetate. The extracts
were submitted to reverse-phase HPLC/LSC followed by methylation and
further clean-up. The trithiol derivative of chlorothalonil was the
major metabolite in all samples. The excretion of total thiol
metabolites was at least 20-fold less than that resulting from oral
dosing at the same dose level (Savides et al., 1987a).
The radiolabelled monoglutathione derivative of chlorothalonil
was administered to male rats (115 mg/kg) as a single oral or
intraperitoneal dose. Six hours after intraperitoneal dosing the
blood level was 10 times higher than after oral dosing. The
proportion of the administered intraperitoneal dose in the kidney was
16 times higher than after oral dosing. Urine from the orally dosed
rats contained 9% trithiol derivative and 5% dithiol, while
intraperitoneally dosed rats showed < 1% dithiol derivative and none
of the trithiol in urine. This indicates that the orally administered
monoglutathione conjugate is further conjugated with glutathione in
the gastrointestinal tract prior to absorption (Savides et al.,
1986f).
Nine germ-free male rats each received approximately 56 µCi
14C-chlorothalonil in a single oral dose of 50 mg/kg. Urine and
faeces were collected over a 96-h period, and the urine was processed
to identify and quantify thiol derivatives of chlorothalonil. These
derivatives were detected in only three of the nine rats and
represented < 0.03% of the dose. This is fifty times less than that
obtained for normal rats. There is therefore strong evidence that
intestinal microflora make a significant contribution to the
metabolism of chlorothalonil after oral administration in the rat
(Savides et al., 1990a).
The HPLC analysis of faecal extracts from rats dosed with 200 mg
chlorothalonil/kg showed that 28% was excreted unchanged and 5% was
converted to 4-hydroxy 2,3,5-trichloroisophthalonitrile. The amounts
after a dose of 5 mg/kg were 1.6 and 6.2%, respectively (Ignatoski et
al., 1983).
The HPLC analysis of faeces from rats given 14C-chlorothalonil
orally at 5, 50 and 200 mg/kg showed the presence of at least seven
radioactive components. Two of the peaks had the same retention times
as chlorothalonil and its 4-hydroxy metabolite. A higher proportion
of the metabolite was present after the 5 mg/kg dose than after the
higher doses. The majority of the activity was unextractable and was
therefore bound to faecal components (Lee et al., 1982).
6.3.2 Dog
Male beagle dogs were given 14C-chlorothalonil at a dose level
of 50 mg/kg either by gelatin capsule or by gavage. In each case the
urinary excretion of radioactivity was very small and none of the
methylated thiol derivatives of chlorothalonil were detected (Savides
et al., 1989, 1990b).
6.3.3 Monkey
Four male Chinese rhesus monkeys were dosed with
14C-chlorothalonil by gavage at 50 mg/kg body weight suspended in
0.75% aqueous methylcellulose. Extraction of urine, collected over
48 h, with acidified ethyl acetate showed that 32-65% of the
radioactivity was extractable. The total amount of chlorothalonil
thiol derivatives excreted was 0.001-0.01% of the administered dose,
mainly as the trimethylthiol entity. This was more than 100 times
less than that excreted from the rat (Savides et al., 1990c).
6.4 Elimination and excretion
6.4.1 Rat
The main route of elimination of chlorothalonil from the rat
after oral dosing is via the faeces. The percentage eliminated was
consistent for males and females, at doses of 1.5-200 mg/kg and from
single or repeated doses. The amount eliminated was consistently
above 82%, the majority appearing in the first 48 h at low doses and
within 72 h at high doses (Marciniszyn et al., 1984b, 1985a).
Biliary excretion at dose levels up to 5 mg/kg is rapid, peaking
at 2 h, and is more prolonged at levels of 50 mg/kg or more.
Excretion decreases with increasing dose, from 22.5% at 1.5 mg/kg to
7.7% at 200 mg/kg over 48 h. Studies using bile duct cannulation
indicate that the excretion is saturated at 50 mg/kg or more.
Comparison with non-cannulated rats showed that there was no
difference in the radioactive concentration found in the kidney,
indicating that enterohepatic circulation of biliary metabolites did
not play a significant role (Savides et al., 1986c).
The fate of orally administered 14C-chlorothalonil (purity
99.7%) at three dose levels (5, 50 and 200 mg/kg) was investigated in
Sprague-Dawley rats to determine the effects of increasing doses of
the test material. Four animals of each sex at each dose level were
killed 2, 9, 24, 96 and 168 h after dosing and urine, faeces and
selected tissues were assayed for radioactivity. The average recovery
of the radiolabel at each of the dose levels was approximately 89% for
males and 96% for females. The major route of elimination was via the
faeces (83-87%) and was essentially complete by 48 h in low-dose
females and low/mid-dose males, and by 72 h in the mid/high-dose
females and high-dose males. A delay in stomach-emptying time was
observed for mid- and high-dose males and females. Urinary excretion
was 92-93% complete for low-dose rats within 24 h, mid-dose rats
within 48 h, and 95% complete for high-dose rats within 72 h. Urinary
excretion of the radiolabel at the three dose levels was 5-7% of the
administered dose in males and 5-11.5% in females. Urinary excretion
was essentially saturated as the dose level increased. The highest
concentrations of radiolabelled material in non-gastrointestinal
tissues were found in the kidney, being approximately 0.7% of the dose
per gram of kidney for males and 0.4% in females at peak concentration
(2 h) for the 5 mg/kg dose level. Kidney concentrations were greatest
at 2, 9 and 24 h for the low, mid and high doses, respectively
(Marciniszyn et al., 1984b, 1985a).
When 14C-chlorothalonil was applied dermally to male rats at
5 mg/kg the major route of excretion was the faeces. Approximately 18%
of the dose was excreted by this route in 120 h compared to about 6%
via urine (Marciniszyn et al., 1984a).
After administration of 1 mg chlorothalonil/kg by the oral,
dermal or endotracheal routes, the excretion in urine during 24 h was
2.9, 0.9 and 5.7%, respectively (Chin et al., 1981).
A study involving intubation of the 4-hydroxy metabolite of
chlorothalonil to rats at 4 or 43 mg/kg showed that the majority of
the dose was excreted in the faeces and a small amount in the urine
(Jarrett et al., 1978).
6.4.2 Mouse
In male mice, dosed orally with 1.5, 15 or 105 mg
14C-chlorothalonil/kg, the major route of elimination was via the
faeces. This was complete by 24 h for the two lower doses and by 96 h
for the highest dose. Urinary excretion at all doses varied between 5
and 10% of the administered dose (Ribovich et al., 1983).
6.4.3 Dog
Most of an oral dose of chlorothalonil was excreted in the faeces
of beagle dogs. Over 12 days, 99.6% was excreted from two dogs given
50 mg 14C-chlorothalonil/kg by gelatin capsule, and 76-98% was
excreted over 24 h when three dogs were given the same dose by gavage.
In both studies the amount excreted in urine was very small, and this
occurred mostly within the first 10 h (Savides et al., 1989, 1990b).
6.4.4 Monkey
Oral administration of 14C-chlorothalonil to four male Chinese
rhesus monkeys showed faecal elimination to be the main route of
excretion, 52-92% of the dose (50 mg/kg) being excreted in 96 h.
Urinary excretion amounted to 1.8-4.1% of the dose. Most of the
radiolabel was eliminated in the first 48 h (Savides et al., 1990c).
6.5 Reaction with body components
Incubation of 14C-chlorothalonil with glutathione in aqueous
medium in the presence or absence of glutathione- S-transferase
resulted in the rapid disappearance of chlorothalonil and the
appearance of more polar compounds. These were identified as
conjugates of chlorothalonil with glutathione, their formation
following a step-wise process, i.e., from mono->di->triglutathione
conjugates (Savides et al., 1985). This reaction with glutathione
parallels similar findings with chlorothalonil in other biological
systems such as Saccharomyces pastorianus (Tillman et al., 1973).
The incubation of chlorothalonil with rat stomach or intestinal
mucosal cells indicated that polar metabolites were formed which were
chromatographically similar to glutathione conjugates of
chlorothalonil (Savides et al., 1986d).
The in vivo action of chlorothalonil on glutathione (GSH) was
shown in a rat study where chlorothalonil was dosed orally at
5000 mg/kg. Hepatic GSH was decreased by 40% 18 h later but recovered
to a normal value after 48 h. Kidney GSH increased to two times its
control level after 48 h (Sadler et al., 1985).
Chlorothalonil has been shown to bind to calf thymus histones,
the rate and amount depending upon pH and type of histones. There was
little binding to DNA. Treatment of rat liver nuclei indicated
similar binding patterns to those for histones (Rosanoff & Siegel,
1981).
Groups of four rats were administered 50 mg 14C-chlorothalonil
per kg orally, killed after 6 h and their kidneys excised. The kidney
tissue was homogenized, and protein and DNA were isolated. Radiolabel
was found to be bound to kidney protein but not to DNA (Savides et
al., 1987b). Kidney tissue from rats dosed 50 mg
14C-chlorothalonil/kg orally was separated by ultracentrifugation
into subcellular organelles. The kidneys contained about 0.38% of the
original dose, the majority of this activity (81%) being in the
soluble fraction. About 10% of the remaining activity was contained
in the mitochondrial subfractions (Savides et al., 1987c).
Studies with rat liver and kidney mitochondrial preparations
showed that the dithiol derivative of chlorothalonil completely
inhibited state 3 mitrochondrial respiration. The monothiol
derivative affected oxygen uptake by liver mitochondria but not by
kidney mitochondria. The mono- and diglutathione conjugates of
chlorothalonil did not affect oxygen uptake by mitochondria. Since
cleavage of the glutathione conjugates to give the thiol derivatives
takes place in the kidney, this may explain the toxic action of
chlorothalonil in this organ (Savides et al., 1988; see also section
7.8).
Available evidence indicates that the enzyme ß-lyase is required
for the formation of thiol derivatives from cysteine conjugates. The
activity of this enzyme has been assessed in rat, mouse and human
kidney cytosolic fractions using the perchloroethylene metabolite
S-(1,2,2-trichlorovinyl)-l-cysteine as substrate. The activity of
renal ß-lyase in human kidney was comparable to that of mouse, but was
an order of magnitude lower than that in the rat (Green et al., 1990).
The proposed metabolic pathway for the production of thiol
derivatives from chlorothalonil in the kidney is shown in Fig. 6.
On plants, chlorothalonil is metabolized only to a limited extent
to the 4-hydroxy metabolite. The majority of the residue remains as
the parent compound. Generally less than 5% of the total residue is
present as the 4-hydroxy metabolite. A review of plant residues
worldwide showed that the 4-hydroxy metabolite level was < 0.1 mg/kg
in most of the crops analysed. It accounted for approximately 10% of
the total residue in lima beans, 5% in cantaloupes, 2% in peaches and
onions, 1% in celery and 0.1% in peanuts (FAO/WHO, 1985). The decline
of chlorothalonil residues and the appearance and decline of the
4-hydroxy metabolite on onions is shown in Table 7 (personal
communication to the IPCS by the Government of Canada, 1979). The
chlorothalonil residue decayed with a half-life of about 3 days.
Studies with corn silage showed that 90% of chlorothalonil
degraded within 18 days (30 to 3 mg/kg). The half-life was
approximately 4 days. In a second experiment, the 4-hydroxy
metabolite formation was very low in the bound materials (which were
converted to an extractable form), representing only about 2% of the
chlorothalonil on the first day of ensiling (FAO/WHO, 1978).
After chlorothalonil was applied to growing peanut foliage at
1.26 kg/ha its half-life was 13.6 days (range 7-19 days) under the
field conditions of use (Elliott & Spurr, 1993).
In the excretion of 14C-chlorothalonil metabolites from rainbow
trout (Salmo gairdneri), the almost complete absence of
chlorothalonil itself and the accumulation of 14C entities in the
bile indicated the possibility of glutathione conjugation as the first
step in chlorothalonil metabolism (Davies & White, 1985). Further
studies showed the existence of mono- and diglutathione conjugates of
chlorothalonil in the bile of rainbow trout exposed to
14C-chlorothalonil (Davies, 1985a).
Studies with liver cytosol from five fish species showed that the
enzyme glutathione- S-transferase (GST) is involved in the conversion
of chlorothalonil to polar conjugates. Comparisons of GST activity in
rainbow trout organs revealed that the potential for chlorothalonil
transformation was in the order liver » kidney > spleen, with no
activity in bile. Low concentrations of chlorothalonil in water
induced fish GST activity for its biotransformation. Hepatic
glutathione (GSH) and GST activity for chlorothalonil transformation
were compared in three species of fish (Oncorhynchus mykiss,
Galaxias maculatus and Galaxias auratus). The order of their
asymptotic LC50 values agreed with that of their hepatic GST
activities for chlorothalonil transformation and was consistent with a
detoxification role for GSH-chlorothalonil conjugation (Davies,
1985b). A study involving co-exposure to zinc and chlorothalonil
indicated that metallothionein does not play a significant role in
chlorothalonil detoxification in fish at sublethal exposures (Davies,
1985c).
Small amounts of the 4-hydroxy metabolite were found in the milk
and kidney of a cow fed 250 mg chlorothalonil/kg in its feed. Only
0.2% of the ingested chlorothalonil was eliminated in the milk as the
4-hydroxy metabolite (Ladd et al., 1971).
4.2.2 Abiotic degradation
Chlorothalonil does not break down in aqueous solution
(0.5 mg/litre) in the dark at pH 5 or 7. It is hydrolysed at pH 9,
over 50% disappearing in 49 days, with the formation of 4-hydroxy-
2,5,6-trichloroisophthalonitrile and 3-cyano-2,4,5,6-tetrachloro-
benzamide (Szalkowski & Stallard, 1977).
Chlorothalonil degrades very slowly under aqueous photolytic
conditions to the 4-hydroxy metabolite. The half-life was found to be
approximately 65 days (ISK Biotech proprietary information).
4.2.3 Bioaccumulation
In a study of the uptake and elimination of 14C-chlorothalonil
in rainbow trout, two groups of fish were exposed to 10 µg/litre of
the compound in flow-through tanks for 96 h (Davies & White, 1985).
After exposure was discontinued, the depuration rate was followed for
96 h. There was a very high uptake in the gall bladder and bile
(concentration factors up to 4.4 × 105). Uptake was also high in the
hind gut, liver, fat and kidney with concentration factors of
2-11 × 103. After 96 h of exposure, the concentration factor in
muscle was 940 and 740, respectively, for the two groups of fish, a
level which may give an indication of the magnitude of the whole body
bioconcentration factor (BCF) for rainbow trout (not measured).
After transfer to clean water, gall bladder levels dropped
rapidly, and so did gill and blood levels. In one group of fish,
concentrations in both liver and kidney doubled until 24 h after
transfer and thereafter dropped to the levels in the other group.
Concentrations in the spleen in both groups continued to increase
throughout the depuration period. Muscle levels dropped only slowly
and remained around 1 µg/g. The high concentrations found in the gall
bladder and bile are consistent with the fact that chlorothalonil is
excreted from fish as glutathione conjugates (Davies & White, 1985).
Bluegill sunfish exposed to 8 µg 14C-chlorothalonil/litre in a
flow-through system for 30 days showed a plateau of 14C uptake within
14 days. The residues in whole fish at 30 days were 264 times the
water concentration. When the fish were placed in clean water, 80% of
the radioactive residues were lost within 14 days. Bioaccumulation in
catfish, in a static system, showed a 16-fold concentration at 26
days. In this case 90% of the 14C residues were depurated in 14 days
after removal from the treated water. The 4-hydroxy metabolite did
not bioaccumulate in fish (SDS Biotech Corporation, 1972).
In tanks containing stream water with chlorothalonil at
20 µg/litre, uptake of the compound occurred in algal growths attached
to bottom pebbles. Analysis of the algal growths showed a
concentration factor for chlorothalonil of 270 times after 14 days of
static exposure. Since this represented only 9.5% of the initial dose
it seems that the removal of chlorothalonil from the water is enhanced
by its conversion to polar metabolites in addition to bioconcentration
(Davies, 1988).
4.3 Waste disposal
Chlorothalonil can be incinerated in units operating at 850°C
fitted with off-gas scrubbing equipment (Lawless et al., 1975).
The disposal methods for waste pesticides and containers
advocated by FAO and GIFAP should be applied to unused chlorothalonil
products and their empty packages (FAO, 1985; GIFAP, 1987).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Chlorothalonil was detected (amongst other pesticides) in 3 out
of 9 outdoor and indoor samples and 1 out of 9 personal monitoring
samples in 9 homes in Jacksonville, Florida, USA. No actual figures
were reported (Lewis et al., 1988).
Average exposures to chlorothalonil of 173 persons in
Jacksonville, Florida and Springfield, Massachusetts, USA were
0.7 ng/m3 (personal exposure) and 0.5 ng/m3 (outdoor air
concentrations) (Wallace, 1991).
Chlorothalonil was not detected in 51 samples in an Environmental
Survey of Chemicals in Japan in 1991 (personal communication by the
Office of Health Studies Environment Agency, Tokyo, 1992).
5.1.2 Water
O'Neil (1991) studied concentrations of chlorothalonil in water
and sediment following overspraying of a pond (0.2 ha and 0.5 m deep)
at 875 g a.i./ha (Ernst, 1991). Water and sediment were monitored in
a stream flowing out of the pond at the outlet and 30 m downstream.
The stream was approximately 1 m wide and 0.5 m deep and ran at
0.033 m3/sec for the first overspray and at 0.015 m3/sec for the
repeat spray. Whole water samples were filtered for separate
measurement of chlorothalonil in water and sediment. Following the
first spraying, samples were taken at the downstream site at 30 min
intervals up to 6 h after spraying and further samples were collected
10 and 24 h after spraying. Initial water concentrations of up to
60 µg/litre fell rapidly to around 15 µg/litre 2 h after spraying.
The water concentration was 1.9 µg/litre 10 h after spraying, and at
24 h there was no measurable chlorothalonil. In the second spraying
at the lower stream flow rate, whole water samples were taken more
frequently over the 2 h following application. Concentrations peaked
at 350 to 450 µg/litre at the pond outlet and 30 m downstream,
respectively, 20 to 30 min after application, falling to between 50
and 100 µg/litre at 2 h. A concentration of 6.3 µg/litre was found
12 h after spraying. Total chlorothalonil mass was measured on
suspended sediment following the first spraying and showed 10 µg
persisting for 1.5 h after spraying and thereafter falling to
approximately 0.01 µg at 10 and 24 h. The report did not make clear
the volume of water filtered, which appears, however, to have been
1 litre. Environmental conditions such as total organic carbon (TOC),
pH, temperature and water hardness were not reported; consequently
their impacts on degradation could not be evaluated.
Chlorothalonil was detected on occasions at concentrations up to
3.6 µg/litre in a tile drainage system from a farm in Manitoba,
Canada, where the fungicide was sprayed routinely. It was detected on
one occasion (0.06 µg/litre) in the sump well outflow draining to a
municipal ditch (Krawchuk & Webster, 1987).
Over a 5-year period (1986-1990), water was sampled and analysed
from 1300 community water systems and rural domestic wells for 101
pesticides, including chlorothalonil. Chlorothalonil was not detected
in any of these samples although the reporting limit was 0.12
µg/litre, which represented the minimum quantification limits for this
particular pesticide in the study (US EPA, 1990).
Chlorothalonil was not detected in 57 water samples, 30 sediment
samples and 30 fish samples in an Environmental Survey of Chemicals in
Japan in 1991 (personal communication by the Office of Health Studies,
Environment Agency, Tokyo, 1992).
5.1.3 Soil
Levels of chlorothalonil and its metabolite SDS-3701 (see section
4.2.1) in soil were reported after three annual treatments (Kenyon &
Ballee, 1990; King et al., 1991, 1992). Four plots were established
of bare untreated and treated, winter wheat treated and untreated at
two different sites, Osterwede and Rohlstof (Germany). Treatment
consisted of an annual chlorothalonil application of 2.2 kg a.i./ha.
Soil samples were taken before and after each treatment. No
chlorothalonil was detected in any of the untreated samples.
Consistently there was no carry over from one year to another. Levels
in soil were highest 2 or 3 days after the treatment (sampling
depended on the sites), with mean levels in the bare plots around 0.40
mg/kg and in those with wheat around 0.34 mg/kg (values ranging
between 0.07 and 0.64 mg/kg). Between 52 and 60 days after each
treatment, levels were 0.02-0.03 mg/kg in plots with wheat while in
bare plots they were generally below the detection limit of 0.01
mg/kg. Before each treatment in the previously treated plots the
level of metabolite SDS-3701 ranged from the limit of detection (0.01)
to 0.03 mg/kg, which was the same as the level 2 or 3 days after
treatment. However, between 52 and 60 days after treatment (depending
on the site) levels rose at the Osterwede site to 0.07 mg/kg for the
bare treated plot. One year after the last treatment, levels of
SDS-3701 ranged from the detection limit to 0.03 mg/kg.
5.1.4 Food crops
Chlorothalonil is used as a broad spectrum fungicide on
vegetables, fruit trees, small fruit bushes and other agricultural and
horticultural food crops. Its use is intended to protect crops up to
harvesting, hence small residues will be present at that time. The
residue levels expected in crops at harvest can be derived from the
numerous supervized trials that have taken place on many crops in
countries all over the world (FAO/WHO, 1975, 1978, 1979, 1980, 1982,
1985a, 1986a, 1990a).
The amount of residue at harvest depends upon factors such as the
application rate, time interval between the last application and
harvest, and the type of crop. Residues are composed mainly of
chlorothalonil, and only negligible amounts of the metabolite
4-hydroxy-2,5,6-trichloroisophthalonitrile (SDS-3701) are present (see
Table 7 for example).
The decline of chlorothalonil residues on food crops after
application is shown by the field treatment of apples and pears
against Botryris cynerea by spraying with a chlorothalonil flowable
formulation and then harvesting at intervals after treatment (Camoni
et al., 1991). The results are shown in Table 8.
Table 8. Decline of chlorothalonil residues
Days after treatment Pears Apples
(mg/kg) (mg/kg)
0 3.85 2.35
7 2.48 1.73
14 2.00 0.92
28 1.35 0.98
From: Camoni et al. (1991)
Similar examples of the decline of chlorothalonil residues have
been given for grapes in Australia, Germany and South Africa (FAO/WHO,
1985a). The decline of residues in onions is shown in Table 7. The
distribution of the residues on this plant showed that the levels in
the older outer leaves were about 5 times above those in the younger
leaves (2.4 and 0.51 mg/kg, respectively).
Pre-harvest intervals are set on the basis of supervized trials,
e.g., 7 days for apricots, and cherries in Australia, 7-14 days for
grapes in Australia and 7 days for onions in the Netherlands (FAO/WHO,
1990a).
One of two samples of currants from growers in the United Kingdom
had a residue level of 7.5 mg/kg 54 days after the last of three
treatments at half the recommended rate of application. The residue
level on the second sample was < 0.5 mg/kg 76 days after two
applications at the maximum rate (UK, 1985).
5.1.5 Dairy produce
There have been no reports of chlorothalonil residues in dairy
produce. However, some indication can be gained from studies on dairy
cattle fed high levels of the compound. In one cow fed 250 mg
chlorothalonil/kg feed for 44 days, no chlorothalonil was detected in
the milk and only 0.2% of the dose appeared as the 4-hydroxy
metabolite. Neither compound could be detected in muscle or fat and
only a low level of the 4-hydroxy metabolite (0.7 mg/kg) was found in
the kidney (Ladd et al., 1971; Wolfe & Stallard, 1971). In another
study, groups of four cows were fed chlorothalonil combined with the
4-hydroxy metabolite at levels up to 250 and 0.6 mg/kg, respectively,
for 30 days. At the end of the period half the cows were sacrificed
and half continued for a 32-day recovery period. No chlorothalonil
(< 0.02 mg/kg) was found in milk. Small residues of chlorothalonil
and the 4-hydroxy metabolite were detected in muscle, fat, liver and
kidney after 30 days administration but none were detected in these
organs after the 32-day recovery period (FAO/WHO, 1975). No
chlorothalonil (< 0.03 mg/kg) was detected in milk from a cow fed the
compound at 5 mg/kg in its rations for 4 days (Gutenmann & Lisk,
1966).
5.1.6 Animal feed
Dry cannery waste (tomato pommace), sometimes used for animal
feed, contained < 1 mg/kg chlorothalonil plus its 4-hydroxy
metabolite (in the ratio 6:1) as a residue (FAO/WHO, 1978).
5.2 General population exposure
5.2.1 Food
In a study of imported fruit and vegetables in Finland, chlorothalonil
levels of 0.02-0.15 mg/kg in strawberries, 0.01-0.86 mg/kg in Chinese
lettuce and 0.12-1.2 mg/kg in peaches were found (personal
communication to the IPCS by the Government of Finland, 1979).
No chlorothalonil (< 0.01 mg/kg) was detected in a US Food and
Drug Administration (FDA) total diet study in the USA in 1976 or 1977
(personal communication to the IPCS by J.R. Wessel, 1979). In a
Canadian total diet survey, chlorothalonil was detected in one out of
six composite samples of garden fruits at the detection level (0.02
mg/kg). On the basis of this one sample, a dietary intake of 0.04 µg
per person per day was estimated (McLeod et al., 1980).
Chlorothalonil was detected (0.001-1.35 mg/kg) in most samples of
apples, peaches and other fruit and vegetables marketed in Tokyo
(Koseki et al., 1980).
No chlorothalonil (< 0.005 mg/kg) was detected in samples of
potatoes in Sweden in 1979. During 1981-1983, 1070 out of 1085
samples of domestic and imported commodities in Sweden had
chlorothalonil residues below 0.21 mg/kg. Samples having higher
residues included one of cauliflowers (out of 165) at 0.41 mg/kg, one
of cucumbers (out of 580) at 0.23 mg/kg and two of strawberries (out
of 143) at 2.9 mg/kg (personal communication: data submitted to the
IPCS by the Government of Sweden and entitled "Chlorothalonil residues
in imported and domestic commodities - 1981 to 1983").
In 1982, analysis at the point of retail in the United Kingdom
showed chlorothalonil residues below 0.5 mg/kg in 41 samples of
strawberries, 15 of gooseberries, 13 of currants and 9 of berries.
Other analyses during 1981-3 showed that only one out of 30 samples of
imported strawberries, 2 out of 15 samples of domestic celery and 5
out of 40 of gooseberries had chlorothalonil residues above 0.1 mg/kg
(UK, 1985).
In the United Kingdom, chlorothalonil residues in bananas
(imported), chinese cabbage (all origins) and parsnips (United Kingdom
origin) were below the reporting levels of 0.2, 1.0 and 0.01 mg/kg,
respectively, in 1988-1989. During the same period, one sample out of
ten of imported strawberries contained 0.1 mg/kg (UK MAFF & HSE,
1990).
Residues of chlorothalonil in foodstuffs are decreased by
processes such as washing. For example, it was shown that 94% of the
residue could be removed by washing tomatoes and that there was no
detectable residue in canned tomato pulp, paste or juice. Peaches
washed in water followed by a caustic rinse showed a 97% removal of
field residues. No chlorothalonil was detected in canned peach puree
(FAO/WHO, 1978).
In a Honduran study, unwashed bananas had a maximum residue level
of 0.17 mg/kg and a mean of 0.08 mg/kg. This was reduced to 0.02
mg/kg after washing. No chlorothalonil was found in the edible pulp
(< 0.01 mg/kg). Similar results were obtained in the Philippines
(FAO/WHO, 1980).
Trimming and peeling also removes a large proportion of residues
from some foodstuffs. For example there are significant reductions
after trimming the outer leaves from cabbages and lettuces. Most of
the residue is removed when cucumbers, melons, peanuts and potatoes
are peeled (Diamond Shamrock, 1974).
As much as 85-98% of chlorothalonil added to tomatoes or green
beans was lost during cooking in open vessels. Only 2.4% was
converted to the 4-hydroxy metabolite, which was stable to cooking
(SDS Biotech Corporation, 1983a).
5.3 Occupational exposure
The exposure of a tractor driver applying chlorothalonil to
ornamental plants in Florida, USA, was assessed. Total-body exposure
rates, estimated from external exposure pads and air sampling, were
low (approximately 5 mg a.i./h) (Stamper et al., 1989a). In the case
of a greenhouse drencher, this exposure was approximately 100 mg
a.i./h (Stamper et al., 1989b).
Occupational exposure to four insecticides and two fungicides was
measured for 151 commercial tree and shrub applicators in the USA who
used hand-held equipment when spraying pesticides. The study was
conducted for 3 consecutive years: 1985, 1986 and 1987. Worker
exposure was determined by collecting full-shift, breathing zone air
samples. Sampling was conducted with battery-operated constant-flow
air-sampling devices. Chlorothalonil was detected in only one out of
14 samples at 0.011 mg/m3 (Leonard & Yeary, 1990).
Spencer et al. (1991) estimated the dermal exposure of workers on
mechanical tomato harvesters to residues of chlorothalonil. An
average of 499.6 µg/h was obtained by gauze pad dosimeters placed
outside the workers' clothing, whereas 43.4 µg/h was obtained by
undershirt dosimetry. The results showed that regular work clothing
provides an excellent protection (90% reduction in dermal exposure)
against chlorothalonil. Air concentrations in the field were also
determined and averaged 0.002 to 0.02 µg/litre, which contributed 8 to
28% to the total exposure.
The exposure of 11 pesticide operators mixing, loading or
applying chlorothalonil fungicide formulations by aerial or ground
applicators has been assessed. The highest exposure was on the hands
(1.7 mg/m2 per h) (Diamond Shamrock, 1980).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Biliary excretion of radioactivity was studied in groups of six
male Sprague-Dawley rats administered a single oral dose of 1.5, 5, 50
or 200 mg/kg body weight 14C-chlorothalonil (98% radiochemically
pure), uniformly labelled in the aromatic ring, as a suspension with a
mean particle size of 3.6 to 5.0 µm in 0.75% methylcellulose in water.
The bile duct was cannulated and bile was collected in 1-h fractions
for 48 h after dosing. Blood, urine and faeces were also collected at
various times after dosing and at termination. During the 48 h after
a single dose of 1.5, 5, 50 and 200 mg/kg body weight, biliary
excretion was 22.5, 16.4, 16.3, and 7.7% of the administered dose,
respectively. Profiles of radioactivity excretion after the two low
doses were quantitatively different from those obtained after the two
high doses. The authors interpreted these results as indicative of a
change in metabolism occurring between 5 and 50 mg/kg body weight,
possibly due to saturation of biliary excretion. Mean urinary
excretion during the 48 h after dosing was 8.0, 8.2 and 7.6% of the
administered dose at 1.5, 5 and 50 mg/kg body weight, respectively,
and only 4.7% at the high dose level of 200 mg/kg body weight.
Excretion of radioactivity in the urine within 6 h after dosing was
inversely related to the dose administered. Total recovery of
radioactivity in this study was 89-99% in the three low-dose groups
and 74% in the high-dose group. After doses of 1.5, 5, 50 and
200 mg/kg body weight, rats absorbed 32, 25.7, 25.9 and 15.5% of the
administered dose, respectively. It was concluded by the authors that
enterohepatic circulation or reabsorption of biliary metabolites from
the gastrointestinal tract did not contribute significantly to the
amount of radiolabel in the kidney. Based on a one-compartment model
for chlorothalonil absorption and excretion and using several
assumptions, it was calculated that the rate of absorption of the
200 mg/kg body weight dose was only twice as fast as that of the
50 mg/kg body weight dose (Marciniszyn et al., 1986).
In a study by Chin et al. (1981), absorption was compared by the
oral, dermal and endotracheal routes with a 1 mg/kg body weight dose
of 14C-chlorothalonil in male Sprague-Dawley rats. The comparisons
were made on the basis of blood levels and urine excretion. In each
case, absorption was highest by endotracheal administration and lowest
by the dermal route. Less than 6% of the administered dose was
recovered in blood and urine within 48 h after dosing.
When 14C-chlorothalonil was introduced into sacs formed from the
upper section of rat small intestine, no unchanged substance passed
through the mucosa and was transferred to the serosal side of the sac.
These data suggest that chlorothalonil is very rapidly conjugated,
since in vivo studies have not identified chlorothalonil itself in
body fluids or tissues after oral administration to rats (Savides et
al., 1986e).
14C-chlorothalonil was applied to the skin of male rats at an
averaged dose of 1167 µg/rat (= 5 mg/kg) on an area of 25 cm2. The
amount absorbed was deduced from the amount remaining on the treated
skin area and the amount of radioactivity found at each time interval
in urine, faeces and carcass. Approximately 28% of the applied dose
was absorbed over the experimental exposure period of 120 h. The
absorption appeared to be time-dependent, about 6.3% of the applied
dose being absorbed during each 24 h period. Radioactivity appeared
quickly in blood and rose steadily up to 72 h, when it reached a
plateau (Marciniszyn et al., 1984a).
In a study by Magee et al. (1990), four monkeys were treated
dermally with 5 mg/kg body weight of 14C-chlorothalonil under a
non-occlusive patch. After 48 h the patch was taken off and the skin
was washed. About 90% of the dose was recovered from the surface and
about 2.26% was completely absorbed through the skin. The urine
contained 1% of the dose, but methylated mono-, di- and trithiols were
not detectable in the urine.
6.2 Distribution
Groups of male and female rats were administered
14C-chlorothalonil orally, in microparticulate suspension, as single
doses of 5, 50 or 200 mg/kg, and tissue activity was determined after
2, 9, 24, 96 and 168 h (Marciniszyn et al., 1984b, 1985a). With the
exception of gastrointestinal tract tissues the greatest concentration
of radioactivity was found in the kidneys, at each dose level,
followed by those in liver and whole blood. The peak concentrations
in kidney occurred at 2 h after 5 mg/kg, 9 h after 50 mg/kg and 24 h
after 200 mg/kg. Similar shifts in peak time with dose occurred in
the liver and blood. In terms of the original dose, kidneys, liver
and blood each contained 0.7% of the label 2 h after 5 mg/kg and 0.3%
(kidney), 0.14% (liver) and 0.23% (blood) after 24 h in female rats.
Distribution of radioactivity was also studied after repeated
oral administration of 14C-chlorothalonil to male rats. Five doses
were given at 24 h intervals at concentrations of 1.5, 5, 50 or
160 mg/kg. The rats were killed 2, 9, 24, 96 and 168 h after the last
dose. The distribution of activity showed a similar profile to that
after single dosing, i.e. the highest concentrations occurred in
kidneys, followed by liver and blood, at all doses and times. At all
dose levels, the concentrations peaked 2 h after the last dose. The
percentage of the dose found in the kidney at this time was 0.28% and
0.20% at the 1.5 and 5 mg/kg dose levels, which was significantly
higher than that found at the higher doses (about 0.09%). At dose
levels up to 50 mg/kg there was significant depletion of radioactivity
from the blood during the 24 h between doses. In the kidney there was
a trend to slower overall depletion with increase in dose (Savides et
al., 1986a).
A study in mice showed that the distribution of activity in
non-gastrointestinal tract tissues was similar to that in rats after a
single oral dose of 14C-chlorothalonil. The kidney had the highest
concentration of radioactivity after doses of 1.5, 15 or 105 mg/kg
(Ribovich et al., 1983).
6.3 Metabolic transformation
6.3.1 Rat
Male Sprague-Dawley rats were administered, via oral gavage,
14C-chlorothalonil (purity 99.7%) at a dose level of 200 mg/kg in
order to isolate and identify the urinary metabolites. Urine was
collected 17, 24 and 48 h after dosing. Urinary metabolites accounted
for 2.4% of the administered dose and, except for 30% of the
radiolabel which was non-extractable from the urine, were found to be
trimethylthiomonochloro-isophthalonitrile and dimethylthiodichloro-
isophthalonitrile. These thiols were excreted in urine both as free
thiols and as their methylated derivatives. The authors suggested a
metabolic pathway such that hepatic metabolism proceeds through
conjugation with GSH followed by enzymatic degradation. The smaller
conjugates are then transported via the bloodstream to the kidney,
where they are converted to thiol metabolites and excreted in the
urine (Marciniszyn et al., 1985b).
A study was also carried out in rats given five daily oral doses
of 14C-chlorothalonil (1.5, 5, 50 or 160 mg/kg per day). Urine
samples, acidified and extracted with ethyl acetate, showed decreasing
extractability of radioactivity with increasing dose. GC/MS analysis
identified methylated or partly methylated dithiol and trithiol
derivatives of chlorothalonil from the first dose onwards. The
percentage of the trithiol derivative excreted was constant with
increasing dose while the dithiol increased with dose. Multiple
dosing resulted in a decreasing daily excretion of total thiol
derivatives. These results emphasize the probable involvement of
glutathione in the metabolic pathway for chlorothalonil (Savides et
al., 1986b).
A group of three rats, pretreated with the gamma-glutamyl
transpeptidase inhibitor AT-125, were dosed with 50 mg/kg
14C-chlorothalonil, while three other rats were given chlorothalonil
only. Urine samples were acidified and extracted with ethyl acetate.
The group of rats pre-treated with AT-125 showed only 15% of
radioactivity extractable after 12 h, while the other group showed 75%
extractability. The non-extractable fraction from the
inhibitor-treated rats contained glutathione conjugates of
chlorothalonil. The kidneys contained 2-3 times more radioactivity
than those of the untreated rats. These results gave further support
to the hypothesis that glutathione is involved with chlorothalonil
metabolism (Marciniszyn et al., 1988).
The production of metabolites was also studied in groups of rats
following dermal administration. 14C-chlorothalonil (4.6 mg/kg) was
applied to a shaved area of the dorsal region. The area was covered
and exposure continued for 48 h. Urine samples collected at 24 and
48 h were acidified and extracted with ethyl acetate. The extracts
were submitted to reverse-phase HPLC/LSC followed by methylation and
further clean-up. The trithiol derivative of chlorothalonil was the
major metabolite in all samples. The excretion of total thiol
metabolites was at least 20-fold less than that resulting from oral
dosing at the same dose level (Savides et al., 1987a).
The radiolabelled monoglutathione derivative of chlorothalonil
was administered to male rats (115 mg/kg) as a single oral or
intraperitoneal dose. Six hours after intraperitoneal dosing the
blood level was 10 times higher than after oral dosing. The
proportion of the administered intraperitoneal dose in the kidney was
16 times higher than after oral dosing. Urine from the orally dosed
rats contained 9% trithiol derivative and 5% dithiol, while
intraperitoneally dosed rats showed < 1% dithiol derivative and none
of the trithiol in urine. This indicates that the orally administered
monoglutathione conjugate is further conjugated with glutathione in
the gastrointestinal tract prior to absorption (Savides et al.,
1986f).
Nine germ-free male rats each received approximately 56 µCi
14C-chlorothalonil in a single oral dose of 50 mg/kg. Urine and
faeces were collected over a 96-h period, and the urine was processed
to identify and quantify thiol derivatives of chlorothalonil. These
derivatives were detected in only three of the nine rats and
represented < 0.03% of the dose. This is fifty times less than that
obtained for normal rats. There is therefore strong evidence that
intestinal microflora make a significant contribution to the
metabolism of chlorothalonil after oral administration in the rat
(Savides et al., 1990a).
The HPLC analysis of faecal extracts from rats dosed with 200 mg
chlorothalonil/kg showed that 28% was excreted unchanged and 5% was
converted to 4-hydroxy 2,3,5-trichloroisophthalonitrile. The amounts
after a dose of 5 mg/kg were 1.6 and 6.2%, respectively (Ignatoski et
al., 1983).
The HPLC analysis of faeces from rats given 14C-chlorothalonil
orally at 5, 50 and 200 mg/kg showed the presence of at least seven
radioactive components. Two of the peaks had the same retention times
as chlorothalonil and its 4-hydroxy metabolite. A higher proportion
of the metabolite was present after the 5 mg/kg dose than after the
higher doses. The majority of the activity was unextractable and was
therefore bound to faecal components (Lee et al., 1982).
6.3.2 Dog
Male beagle dogs were given 14C-chlorothalonil at a dose level
of 50 mg/kg either by gelatin capsule or by gavage. In each case the
urinary excretion of radioactivity was very small and none of the
methylated thiol derivatives of chlorothalonil were detected (Savides
et al., 1989, 1990b).
6.3.3 Monkey
Four male Chinese rhesus monkeys were dosed with
14C-chlorothalonil by gavage at 50 mg/kg body weight suspended in
0.75% aqueous methylcellulose. Extraction of urine, collected over
48 h, with acidified ethyl acetate showed that 32-65% of the
radioactivity was extractable. The total amount of chlorothalonil
thiol derivatives excreted was 0.001-0.01% of the administered dose,
mainly as the trimethylthiol entity. This was more than 100 times
less than that excreted from the rat (Savides et al., 1990c).
6.4 Elimination and excretion
6.4.1 Rat
The main route of elimination of chlorothalonil from the rat
after oral dosing is via the faeces. The percentage eliminated was
consistent for males and females, at doses of 1.5-200 mg/kg and from
single or repeated doses. The amount eliminated was consistently
above 82%, the majority appearing in the first 48 h at low doses and
within 72 h at high doses (Marciniszyn et al., 1984b, 1985a).
Biliary excretion at dose levels up to 5 mg/kg is rapid, peaking
at 2 h, and is more prolonged at levels of 50 mg/kg or more.
Excretion decreases with increasing dose, from 22.5% at 1.5 mg/kg to
7.7% at 200 mg/kg over 48 h. Studies using bile duct cannulation
indicate that the excretion is saturated at 50 mg/kg or more.
Comparison with non-cannulated rats showed that there was no
difference in the radioactive concentration found in the kidney,
indicating that enterohepatic circulation of biliary metabolites did
not play a significant role (Savides et al., 1986c).
The fate of orally administered 14C-chlorothalonil (purity
99.7%) at three dose levels (5, 50 and 200 mg/kg) was investigated in
Sprague-Dawley rats to determine the effects of increasing doses of
the test material. Four animals of each sex at each dose level were
killed 2, 9, 24, 96 and 168 h after dosing and urine, faeces and
selected tissues were assayed for radioactivity. The average recovery
of the radiolabel at each of the dose levels was approximately 89% for
males and 96% for females. The major route of elimination was via the
faeces (83-87%) and was essentially complete by 48 h in low-dose
females and low/mid-dose males, and by 72 h in the mid/high-dose
females and high-dose males. A delay in stomach-emptying time was
observed for mid- and high-dose males and females. Urinary excretion
was 92-93% complete for low-dose rats within 24 h, mid-dose rats
within 48 h, and 95% complete for high-dose rats within 72 h. Urinary
excretion of the radiolabel at the three dose levels was 5-7% of the
administered dose in males and 5-11.5% in females. Urinary excretion
was essentially saturated as the dose level increased. The highest
concentrations of radiolabelled material in non-gastrointestinal
tissues were found in the kidney, being approximately 0.7% of the dose
per gram of kidney for males and 0.4% in females at peak concentration
(2 h) for the 5 mg/kg dose level. Kidney concentrations were greatest
at 2, 9 and 24 h for the low, mid and high doses, respectively
(Marciniszyn et al., 1984b, 1985a).
When 14C-chlorothalonil was applied dermally to male rats at
5 mg/kg the major route of excretion was the faeces. Approximately 18%
of the dose was excreted by this route in 120 h compared to about 6%
via urine (Marciniszyn et al., 1984a).
After administration of 1 mg chlorothalonil/kg by the oral,
dermal or endotracheal routes, the excretion in urine during 24 h was
2.9, 0.9 and 5.7%, respectively (Chin et al., 1981).
A study involving intubation of the 4-hydroxy metabolite of
chlorothalonil to rats at 4 or 43 mg/kg showed that the majority of
the dose was excreted in the faeces and a small amount in the urine
(Jarrett et al., 1978).
6.4.2 Mouse
In male mice, dosed orally with 1.5, 15 or 105 mg
14C-chlorothalonil/kg, the major route of elimination was via the
faeces. This was complete by 24 h for the two lower doses and by 96 h
for the highest dose. Urinary excretion at all doses varied between 5
and 10% of the administered dose (Ribovich et al., 1983).
6.4.3 Dog
Most of an oral dose of chlorothalonil was excreted in the faeces
of beagle dogs. Over 12 days, 99.6% was excreted from two dogs given
50 mg 14C-chlorothalonil/kg by gelatin capsule, and 76-98% was
excreted over 24 h when three dogs were given the same dose by gavage.
In both studies the amount excreted in urine was very small, and this
occurred mostly within the first 10 h (Savides et al., 1989, 1990b).
6.4.4 Monkey
Oral administration of 14C-chlorothalonil to four male Chinese
rhesus monkeys showed faecal elimination to be the main route of
excretion, 52-92% of the dose (50 mg/kg) being excreted in 96 h.
Urinary excretion amounted to 1.8-4.1% of the dose. Most of the
radiolabel was eliminated in the first 48 h (Savides et al., 1990c).
6.5 Reaction with body components
Incubation of 14C-chlorothalonil with glutathione in aqueous
medium in the presence or absence of glutathione- S-transferase
resulted in the rapid disappearance of chlorothalonil and the
appearance of more polar compounds. These were identified as
conjugates of chlorothalonil with glutathione, their formation
following a step-wise process, i.e., from mono->di->triglutathione
conjugates (Savides et al., 1985). This reaction with glutathione
parallels similar findings with chlorothalonil in other biological
systems such as Saccharomyces pastorianus (Tillman et al., 1973).
The incubation of chlorothalonil with rat stomach or intestinal
mucosal cells indicated that polar metabolites were formed which were
chromatographically similar to glutathione conjugates of
chlorothalonil (Savides et al., 1986d).
The in vivo action of chlorothalonil on glutathione (GSH) was
shown in a rat study where chlorothalonil was dosed orally at
5000 mg/kg. Hepatic GSH was decreased by 40% 18 h later but recovered
to a normal value after 48 h. Kidney GSH increased to two times its
control level after 48 h (Sadler et al., 1985).
Chlorothalonil has been shown to bind to calf thymus histones,
the rate and amount depending upon pH and type of histones. There was
little binding to DNA. Treatment of rat liver nuclei indicated
similar binding patterns to those for histones (Rosanoff & Siegel,
1981).
Groups of four rats were administered 50 mg 14C-chlorothalonil
per kg orally, killed after 6 h and their kidneys excised. The kidney
tissue was homogenized, and protein and DNA were isolated. Radiolabel
was found to be bound to kidney protein but not to DNA (Savides et
al., 1987b). Kidney tissue from rats dosed 50 mg
14C-chlorothalonil/kg orally was separated by ultracentrifugation
into subcellular organelles. The kidneys contained about 0.38% of the
original dose, the majority of this activity (81%) being in the
soluble fraction. About 10% of the remaining activity was contained
in the mitochondrial subfractions (Savides et al., 1987c).
Studies with rat liver and kidney mitochondrial preparations
showed that the dithiol derivative of chlorothalonil completely
inhibited state 3 mitrochondrial respiration. The monothiol
derivative affected oxygen uptake by liver mitochondria but not by
kidney mitochondria. The mono- and diglutathione conjugates of
chlorothalonil did not affect oxygen uptake by mitochondria. Since
cleavage of the glutathione conjugates to give the thiol derivatives
takes place in the kidney, this may explain the toxic action of
chlorothalonil in this organ (Savides et al., 1988; see also section
7.8).
Available evidence indicates that the enzyme ß-lyase is required
for the formation of thiol derivatives from cysteine conjugates. The
activity of this enzyme has been assessed in rat, mouse and human
kidney cytosolic fractions using the perchloroethylene metabolite
S-(1,2,2-trichlorovinyl)-l-cysteine as substrate. The activity of
renal ß-lyase in human kidney was comparable to that of mouse, but was
an order of magnitude lower than that in the rat (Green et al., 1990).
The proposed metabolic pathway for the production of thiol
derivatives from chlorothalonil in the kidney is shown in Fig. 6.
 7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of chlorothalonil by oral, dermal and
inhalation routes of administration is shown in Table 9. Signs of
poisoning include depression, diarrhoea and unkempt appearance. An
oral LD50 could not be attained in the dog because of emesis.
Chlorothalonil administered orally (1 g/kg) to mice increased
intestinal motility. The laxative action was reduced by pre-treatment
with corn oil (Teeters, 1966).
Table 9. The acute toxicity of chlorothalonil
Species LD50 Route Reference
(mg/kg)
Rat > 10 000 oral Powers (1965)
Dog > 5000 oral Paynter (1965a)
Mouse 6000 oral Yoshikawa & Kawai (1966)
Rat 332 oral Wazeter (1971)
(4 hydroxy
metabolite)
Rabbit > 10 000 dermal Doyle & Elsea (1963)
Rat (f & m)a 0.22 mg/litre inhalation Danks & Fowler (1988)
Rat (f & m)b 0.52 mg/litre inhalation Shults et al. (1991)
Rat (f & m)c 0.10 mg/litre inhalation Shults et al. (1993)
a LC50 actual concentration
b Hammermilled technical chlorothalonil - 1 h exposure
c Hammermilled technical chlorothalonil - 4 h exposure
The acute oral toxicity of the metabolite 4-hydroxy-2,5,6-
trichloroisophthalonitrile is greater than that of chlorothalonil
itself, the acute oral LD50 values being 332 and 10 000 mg/kg,
respectively).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rat
When groups of 35 male and 35 female rats were fed chlorothalonil
in the diet at dose levels of 0, 250, 500, 750 or 1500 mg/kg diet for
22 weeks, growth was slightly reduced at all levels in males and in
the highest two levels in females. Liver and kidney weight was
increased in the two highest dose groups in males. Kidney changes,
more evident in males than females, occurred at all dose levels and
included irregular swelling of the tubular epithelium, epithelial
degeneration and tubular dilation (Blackmore & Shott, 1968). In a
separate study, no compound-related effects were seen in the kidneys
of rats fed chlorothalonil at seven dietary levels from 1-120 mg/kg
diet for 17 weeks (Busey, 1975).
When groups of 20 male and 20 female rats were fed chlorothalonil
in the diet (0, 40, 80, 175, 375 and 1500 mg/kg body weight per day)
for 90 days, a significant dose-related reduction in body weight gain
was seen at levels of 375 mg/kg per day or more which was allied to
cathartic action at the two highest doses. Male urine output
decreased and specific gravity increased at the two highest dose
levels. Relative kidney weight was increased in both sexes at all
dose levels. A histopathological re-evaluation of the kidneys showed
the presence of epithelial hyperplasia of the proximal convoluted
tubules at all levels in males and at levels of 175 mg/kg per day or
more in females (Wilson, 1981; Wilson et al., 1985c).
Groups of 25 male and 25 female rats were fed chlorothalonil for
13 weeks at 0, 1.5, 3, 10 and 40 mg/kg body weight per day. Five rats
of each sex per group were killed at 6 weeks, ten at 13 weeks and the
survivors were killed after a further 13-week recovery period. There
was no effect on mortality, clinical condition, body weight, food and
water consumption, haematology or urinalysis. Increases in kidney
(> 3 mg/kg per day) and liver (highest dose) weights and the
incidence of hyperplasia and hyperkeratosis of the forestomach
squamous epithelium (at 10 and 40 mg/kg per day) returned to normal
when treatment ceased. Histopathological re-examination of the kidney
revealed (in males at the highest dose level) an increased incidence
of hyperplasia of the epithelium of the proximal tubules at 6 and
13 weeks and after a 13-week recovery period. In a few animals this
resulted in an increase in the size of the tubules. An overall
no-observed-effect level of 3 mg/kg body weight per day was
established, based upon the lack of lesions in the squamous epithelium
of the forestomach (Wilson et al., 1983a, 1985a).
The kidney and stomach changes were investigated further in a
study where 90 male rats were fed chlorothalonil (175 mg/kg body
weight per day) for up to 91 days. Groups of ten rats were killed
after 4 or 7 days and 2, 4, 6, 8, 10 or 12 weeks, the kidney and
stomach taken for histopathological examination. Groups of ten rats
at each interval were taken from a control group and examined. The
forestomach effects of chlorothalonil were characterized initially as
multifocal ulceration and erosion of the mucosa, developing to
squamous epithelial hyperplasia and hyperkeratosis. Within the first
week there was vacuolar degeneration, cell death, karyomegaly and
regeneration of the proximal tubular epithelium. There was apparent
recovery at day 14 with few lesions present in treated animals.
Continued administration led to tubular epithelial vascularization,
regeneration, hyperplasia and hypertrophy (Ford et al., 1987a).
Chlorothalonil or the monoglutathione conjugate of chlorothalonil
was administered in the diet to male Fischer-344 rats in a 90-day
study. A third group of rats received only the vehicle (0.5%
methylcellulose). The oral administration of approximately equimolar
doses of chlorothalonil (75 mg/kg per day) or the monoglutathione
conjugate of chlorothalonil (150 mg/kg per day) resulted in
significantly increased kidney weight and in treatment-related
histopathological changes in the kidney. The primary changes observed
were morphologically similar for both compounds and were characterized
by proximal tubular hyperplasia, tubular dilation (hypertrophy),
vacuolar degeneration and interstitial fibrosis. The oral
administration of chlorothalonil resulted in gross and microscopic
changes in the non-glandular portion of the forestomach. The oral
administration of the monoglutathione conjugate of chlorothalonil did
not produce alterations in the rat forestomach. The gross and
microscopic changes observed in the forestomach of animals given
chlorothalonil were considered to be due to the local irritational
effects of chlorothalonil. The alteration of the molecule by the
addition of a single glutathione residue appeared to eliminate the
irritation to the forestomach. Thiol metabolites of chlorothalonil
were detected in the urine of animals given either the monoglutathione
conjugate of chlorothalonil or chlorothalonil itself. The presence of
the thiol metabolites, coupled with the similar histopathological
changes in the kidney, suggests a common metabolic pathway. The data
support the conclusion that the nephrotoxicity produced by
chlorothalonil is associated with conjugation with glutathione (Ford
et al., 1987b; Wilson et al., 1990).
7.2.1.2 Mouse
Groups of 15 male and 15 female CD-1 mice were administered
chlorothalonil in diets at levels of 0, 7.5, 15, 50, 275 or 750 mg/kg
diet for 13 weeks. Five animals per group were killed at 6 weeks.
There was no effect on clinical condition, mortality, body weight gain
or food consumption. Kidney weight was increased in females at 275
and 750 mg/kg. Microscopic re-examination revealed hyperplasia of the
epithelium of the proximal convoluted tubules, minimal or slight in
severity, in only 4 out of 15 males. This was not considered to be a
clear treatment-related effect. There was an increased incidence of
hyperplasia and hyperkeratosis of the squamous epithelial cells of the
stomach in males and females at levels of 50 mg/kg or more. Mucosal
ulceration and submucosal inflammation were observed in some treated
animals. The no-observed-effect level was considered to be 15 mg/kg
(3 mg/kg body weight per day) (Shults et al., 1983; Busey, 1985).
7.2.1.3 Dog
In a 30-day feeding study, encapsulated chlorothalonil (97.9%
purity) was administered daily to groups of two male and two female
beagle dogs at 0, 50, 150, or 500 mg/kg body weight per day. The
following tissues were examined macroscopically and microscopically at
necropsy: brain, liver, kidneys, testes with epididymis, ovaries,
adrenals, heart, thyroid, and parathyroid. During treatment the
high-dose dogs exhibited emesis and weight loss and reduced food
consumption (males only). Female dogs had slightly reduced body
weight gains at all doses. At necropsy, the liver weights of
high-dose females were slightly increased. There were no microscopic
changes in the tissues examined. Due to the reduced body-weight gains
of treated females, a no-observed-adverse-effect level (NOAEL) was not
established in this study (Fullmore & Laveglia, 1992; FAO/WHO, 1993b).
In a 16-week dietary study, chlorothalonil (purity unspecified)
was fed at 0, 250, 500 or 750 mg/kg to groups of four beagle dogs.
There were no compound-related effects on appearance, behaviour,
appetite or body weight. No changes in haematological parameters were
found at weeks 0, 4, 13 or 16. At termination, protein-bound iodine
was found to be increased in all treated dogs. Urinalysis at weeks 6,
9, 13 and 16 was unremarkable. No compound-related macroscopic or
microscopic changes were found at necropsy. In particular, only
incidental changes were observed in liver and kidneys. A NOAEL was
not established in this study (Paynter & Murphy, 1967; FAO/WHO,
1993b).
7.2.2 Dermal: Rabbit
Chlorothalonil (75% formulation) was applied to the intact or
abraded skin of groups of rabbits at 0, 500 or 1000 mg/kg per day, 5
days/week, for 3 weeks. The treated groups comprised five males and
five females per group, with two males and two females as controls.
Treatment resulted in dose-related irritation which was more severe
with abraded skin. Histopathological examination revealed a moderate
degree of acanthosis, hyperkeratosis and slight to moderate leucocytic
infiltration. No abnormality was detected in other tissues (Paynter,
1965b).
Chlorothalonil was applied dermally each day for 21 days to
groups of six male and six female rabbits at 0.1, 2.5 and 50 mg/kg per
day. The fungicide was suspended in 0.125% aqueous methylcellulose
and covered 10% of the body surface when applied to the back. Contact
was for 6 h daily. The only effect revealed by a wide range of
observations and examinations was dermal irritation at 2.5 and 50
mg/kg per day. The histopathological changes seen were minimal to
slight acanthosis and hyperkeratosis. The no-observed-effect level
(NOEL) for dermal irritation was 0.1 mg/kg per day (Shults et al.,
1986).
7.3 Long-term exposure
7.3.1 Rat
An early study, lasting 76 weeks, produced evidence of kidney
changes in rats (15 male and 15 female per group) fed high levels of
chlorothalonil at 500, 1000 and 5000 mg/kg diet. There was no
overall effect on mortality, clinical condition or growth. The
compound-related changes in the kidney, at all levels, consisted of
tubular hypertrophy, epithelial irregularities and vacuolation. Males
were more affected than females (Paynter & Busey, 1967).
A 2-year study, reported in 1967, was initiated with
chlorothalonil levels of 1500, 15 000 and 30 000 mg/kg diet with
groups of 35 male and 35 female rats (70 of each sex in control
group). Because of cathartic effects, the top dose administration was
curtailed after 15 weeks. The rats in the two remaining treatment
groups continued for the full 2 years with evaluations including
haematology, biochemistry and histopathology. There was no effect on
survival, but the effect on growth was dose-related. The relative
organ weights of liver and kidney were increased at 15 000 mg/kg.
Microscopic changes in the forestomach were described as acanthosis
and hyperkeratosis of the squamous epithelium at 15 000 mg/kg and, in
the kidney, as tubular hypertrophy and hyperplasia at both 1500 and
15 000 mg/kg diet (Paynter, 1967a).
A supplementary study, run concurrently with the previous study,
assessed chronic toxicity at 5000 mg/kg diet. Growth rate was
depressed and a cathartic action was expressed as increased water
consumption and faecal excretion. Relative organ weights were
increased for caecum and kidneys. Histopathological examination
showed kidney changes as tubular hypertrophy and occasional
degeneration of the proximal tubular epithelium (Paynter & Crews,
1967).
A 2-year study at six chlorothalonil dose levels between 4 and 60
mg/kg diet was designed to determine an NOEL (50 males and 50 females
per group). No effects were seen on survival, clinical observations,
growth, haematological or biochemical parameters. No changes of
toxicological significance were found for organ weights or during
gross and microscopic examination of tissues. The highest dose level
(3 mg/kg body weight per day) was considered the NOEL (Holsing &
Shott, 1970).
A long-term study was undertaken to evaluate the carcinogenic
potential of chlorothalonil in Fischer-344 rats. Males were studied
for 27 months and females for 30 months. Chlorothalonil was
administered in the diet to groups of 60 males and 60 females at dose
levels of 0, 40, 80 and 175 mg/kg body weight per day. There was no
effect on survival of females or males up to 2 years. However, at the
highest dose level, there was increased mortality after 2 years, but
only in males. Decreases in body weight gain were dose-related at 80
and 175 mg/kg per day. Treatment-related effects on other parameters
appeared to be related to nephrotoxicity. These included increases in
serum urea nitrogen and creatinine, increased urine volume and
decreased specific gravity, and increased kidney weight.
Histopathological examination of the kidney showed a dose-related
increase in chronic glomerulonephritis (nephropathy) compared with
controls. A re-examination of the kidneys also revealed tubular
hyperplasia (a sign of preneoplastic change) and chronic progressive
nephropathy in the treated groups. Secondary lesions in other organs
included periarteritis and parathyroid hyperplasia. There was
increased incidence or severity of hyperplasia and hyperkeratosis of
the squamous mucosa of the oesophagus and forestomach in all dosed
groups, which was probably the result of the irritant effect of
chlorothalonil. Neoplastic changes in the kidney and forestomach are
evaluated in section 7.7 (Wilson et al., 1985b, 1986a).
A further study was carried out to evaluate the neoplastic
findings in the kidney and stomach seen in the previous study and to
determine a NOEL for non-neoplastic effects. Groups of 65 male and
65 female rats were administered chlorothalonil in the diet at doses
of 0, 1.8, 3.8, 15 and 175 mg/kg body weight per day. Males were
killed at 23 months (highest dose) and 26 months and females at
29 months. The effects at 175 mg/kg per day were similar to those
described above, i.e. changes associated with the kidney and stomach.
At 15 mg/kg per day there was elevated serum urea nitrogen and
slightly increased kidney weight. At 3.8 mg/kg per day there was a
small increase in kidney weight, but there were no effects at 1.8
mg/kg per day. Microscopic examination revealed an increased
incidence and severity of epithelial hyperplasia in the proximal
convoluted tubules at 3.8 mg/kg per day or more. Clear cell
hyperplasia of the proximal convoluted tubules was increased at
15 mg/kg per day in females and at 175 mg/kg per day in both sexes.
In addition, at 3.8 mg/kg per day or more, there was an increased
incidence and severity of hyperplasia, hyperkeratosis, ulcers and
erosions of the squamous mucosa of the forestomach. At 175 mg/kg per
day, the incidence of erosions of the glandular stomach was
significantly increased compared to controls. Renal tumours at levels
of 15 and 175 mg/kg per day and stomach tumours at 3.8 mg/kg per day
or more are evaluated in section 7.7. An NOEL of 1.8 mg/kg body
weight per day was established for non-neoplastic effects seen in the
study (Wilson et al., 1989a).
7.3.2 Mouse
A 2-year mouse study was carried out with 60 males and 60 females
per group at 0, 750, 1500 and 3000 mg chlorothalonil/kg in diet.
There was a slightly increased mortality in males at the highest dose
level but no effect was seen on body weight, food consumption,
clinical condition or haematological parameters. Kidney weight was
increased in all treated groups compared to controls. Non-neoplastic
changes in the kidney were characterized as glomerulonephritis,
cortical tubular degeneration and cysts. The incidence of these
changes, found at all treatment levels, was not dose-related but was
considered to be due to treatment. A histopathological re-evaluation
of the kidneys revealed a high incidence of tubular hyperplasia in all
male groups and a lower incidence in females. Non-neoplastic effects
in the stomach and oesophagus included hyperplasia and hyperkeratosis
of the squamous mucosa, probably due to the irritant action of
chlorothalonil. The evaluation of kidney and stomach tumours found in
this study is described in section 7.7 (Wilson et al., 1983b, 1986b).
A second mouse study was undertaken to determine the NOEL for
stomach and kidney changes in male mice. Sixty males per group were
fed chlorothalonil at levels of 0, 15, 40, 175 and 750 mg/kg diet for
2 years. Kidney weight and incidence of tubular hyperplasia were
increased at 750 mg/kg and, very slightly, at 175 mg/kg. The
increased incidence of hyperplasia and hyperkeratosis of the
forestomach was dose-related between 40 and 750 mg/kg. The dietary
NOEL for non-neoplastic effects was determined to be 15 mg/kg (1.6
mg/kg body weight per day). The tumour evaluation is considered in
section 7.7 (Wilson et al., 1987).
7.3.3 Dog
In a 2-year study, chlorothalonil (93.6% purity) was fed to
groups of four beagle dogs at dietary concentrations of 0, 1500,
15 000 or 30 000 mg/kg (equivalent to 0, 37.5, 375, or 750 mg/kg body
weight per day). Eight dogs, one of each sex and of each group, were
killed at 12 months and the remainder at 24 months. One dog of each
treatment group lost weight during the study. There was a tendency
towards mild anaemia in four of the mid-dose dogs at 2 years and at
earlier intervals in two of the high-dose dogs. Biochemical and urine
analyses were unremarkable. Absolute and relative thyroid and kidney
weights, and liver to body weight ratios were increased at the
mid- and high-dose levels. Histopathological treatment-related
changes occurred in the liver, thyroid, kidney and stomach of mid- and
high-dose dogs; changes in low-dose dogs were equivocal. In the
liver, the findings were similar in nature (though slightly more
pronounced) at low-dose levels to those in the control dogs, but they
increased in severity at mid- and high-dose levels. They included
pericholangitis with associated portal fibrosis, bile duct hyperplasia
and pigmentation of hepatic cytoplasm and of macrophages of sinusoids
and portal triads. Renal glomerulosclerosis and degenerative renal
tubular changes (tubular hypertrophy and dilation) were found in the
kidneys of mid- and high-dose dogs. In the thyroid, markedly
increased pigmentation of follicular epithelia occurred in mid- and
high-dose dogs. Moderate to severe gastritis was found irregularly in
mid- and high-dose animals. In summary, administration of
chlorothalonil in the diet of dogs at concentrations of 15 000 and
30 000 mg/kg caused irregular body weight reduction, borderline
anaemia and histopathological changes in the liver, kidney, thyroid
and stomach. At 1500 mg/kg, the histopathological changes found in
the liver were qualitatively similar but minimally to slightly
increased in comparison to those found in control animals.
Histopathological changes to the other tissues were unremarkable at
the low dose. A NOAEL was not established in this study (Paynter &
Busey, 1966; FAO/WHO, 1993b).
Groups of beagle dogs (eight males and eight females per group)
were fed chlorothalonil in the diet at dose levels of 0, 60 and
120 mg/kg. Four dogs of each sex per group were killed at one year
and the remaining animals at 2 years. There were no effects on
behaviour or growth over the course of the study. Clinical chemistry
values, including haematological, biochemical and urine analyses, were
comparable to the controls at all dose levels. Gross and microscopic
examination of tissues and organs performed on animals killed at 12
months indicated a slight increase in the severity of renal tubule
vacuolation in high-dose males. Examination of tissues and organs at
24 months showed a slight degree of renal tubule vacuolation in two
out of four males at 120 mg/kg. In the absence of other changes
(urinalyses values) this finding was considered questionable,
especially as a slight degree of vacuolation was noted in controls as
well as other treated animals. The NOAEL was considered to be
120 mg/kg diet, equivalent to 3 mg/kg body weight per day (Holsing &
Voelker, 1970; FAO/WHO, 1991, 1993b).
7.3.4 Summary of key dietary studies
A summary of the key dietary studies with chlorothalonil is given
in Table 10.
7.4 Skin and eye irritation; sensitization
Chlorothalonil is an irritant to rabbit skin, as shown by
repeated dose studies (5 days/week for 3 weeks at 500 or 1000 mg/kg
per day or daily for 21 days at 2.5 or 50 mg/kg per day; details in
section 7.2.2).
The influence of the vehicle on the skin irritant potential of
chlorothalonil was shown by a rabbit study with 0.1% chlorothalonil in
saline, petrolatum or acetone. Compared to the vehicle alone,
chlorothalonil did not cause a significant increase in irritation in
Table 10. Summary of key dietary studies with chlorothalonil
Species Duration Dose levels NOEL LOEL Key effects Reference
Rat 13 weeks 0, 1.5, 3, 10, 3 mg/kg per 10 mg/kg per 40 mg/kg per day: increased kidney and Wilson et
40 mg/kg body day day liver weight, incidence of hyperplasia al. (1983a,
weight per day and hyperkeratosis of forestomach, 1985a)
incidence of epithelial hyperplasia in
proximal tubules 10 mg/kg per day:
increased kidney weight, hyperplasia
and hyperkeratosis of forestomach
Rat 23-29 months 0, 1.8, 3.8, 15, 1.8 mg/kg 3.8 mg/kg 175 mg/kg per day: increases in serum Wilson et
175 mg/kg body per day per day urea nitrogen, urine volume, kidney al. (1989a)
weight per day weight, renal tubular hyperplasia,
hyperplasia and hyperkeratosis of
forestomach; increased incidence of
renal and forestomach tumours
15 mg/kg per day: similar but less
intense changes to those shown at
highest dose level 3.8 mg/kg per day:
small increase in kidney weight, renal
tubular hyperplasia, hyperplasia
and hyperkeratosis of forestomach and
forestomach tumours
Mouse 13 weeks 0, 7.5, 15, 50, 15 mg/kg diet 50 mg/kg diet 275 and 750 mg/kg: increased kidney Shults et
275, 750 mg/kg (= 3 mg/kg (= 10 mg/kg weight 50, 275 and 750 mg/kg: al. (1983);
diet per day) body weight dose-related increased incidence Busey (1985)
per day) of hyperplasia and hyperkeratosis
of forestomach
Table 10. (Cont'd)
Species Duration Dose levels NOEL LOEL Key effects Reference
Mouse 2 years 0, 15, 40, 175, 15 mg/kg diet 40 mg/kg diet 750 mg/kg: increased kidney weight, Wilson et
750 mg/kg diet (= 1.6 mg/kg (= 4.5 mg/kg renal tubular hyperplasia, forestomach al. (1987)
body weight body weight hyperplasia and hyperkeratosis,
per day) per day) slightly increased incidence of
forestomach tumours 175 mg/kg: slightly
increased incidence of renal tubular
hyperplasia, increased forestomach
hyperplasia and hyperkeratosis
40 mg/kg: increased incidence of
forestomach hyperplasia and
hyperkeratosis
Dog 2 years 0, 1500, 15 000, - 1500 mg/kg in 15 000 and 30 000 mg/kg: increased Paynter &
30 000 mg/kg diet (= 37.5 kidney, liver and thyroid weights and Busey (1966)
diet mg/kg body histopathological changes, gastritis
weight per 1500 mg/kg: slightly increased incidence
day) hepatic findings
Dog 2 years 0, 60, 120 mg/kg 120 mg/kg diet - no changes of toxicological significance Holsing &
diet (= 3 mg/kg Voelker
body weight (1970);
per day FAO/WHO
(1991, 1993)
saline, but doubled the mild irritation caused by petrolatum. Acetone
itself caused no irritation but the addition of chlorothalonil
produced mild skin irritation (Flannigan & Tucker, 1985).
A further study in rabbits using a cumulative irritation assay
confirmed the irritant properties of 0.1% chlorothalonil in acetone.
A concentration of 0.01% gave evidence of mild irritation, probably of
no clinical significance, and 0.001% was not irritant to the skin
(Flannigan et al., 1986).
Chlorothalonil irritancy to the eye was evaluated in a modified
Draize system, 0.1 mg being instilled into one eye of each of three
male and three female rabbits. The eyes were examined and the results
scored after 24, 48 and 72 h, 7 and 14 days. Ocular irritation
occurred in all animals and corneal opacity persisted to day 14 (Major
et al., 1982).
Skin sensitization was tested in a guinea-pig maximization test
using 10 Hartley female guinea-pigs. Topical concentrations of 0.5
and 5% chlorothalonil were used. Upon challenge, chlorothalonil was
shown to be a strong sensitizer. Moderate cross-sensitization with
benomyl was also demonstrated (Matsushita & Aoyama, 1981). By
contrast, chlorothalonil did not produce skin sensitization in a
Draize test. The test substance (0.2 g) was applied to the shaved
backs of Hartley-derived guinea-pigs. The material was occluded for
24 h and then removed. This procedure was performed 3 times a week
for a total of 10 applications. On day 36 of the study a challenge
application of 0.2 g chlorothalonil was applied to the shaved flanks
and occluded. The skin was assessed 24 h later after removal of the
occlusive dressing. The positive control substance DNCB showed the
expected dermal sensitization but chlorothalonil was shown not to be a
sensitizer in this test (Wilson et al., 1982).
Luperi & Forster (1988) studied the ability of chlorothalonil to
induce delayed contact hypersensitivity in the guinea-pig using the
maximization test of Magnusson and Kligman. There was no evidence of
an induced sensitization response, but adequate evaluation was impeded
by a diffuse irritant reaction following the challenge.
7.5 Reproductive and developmental toxicity
Teratological evaluations have been carried out in the rat and
rabbit.
Chlorothalonil was administered orally via gavage to pregnant
Sprague-Dawley rats (25 per group) on days 6-15 of gestation at dose
levels of 0, 25, 100 or 400 mg/kg body weight per day, and the animals
were killed on day 20. Clinical signs of maternal toxicity were
evident at the highest dose level. There were 3 deaths and lowered
body weight during the dosing period. There was a slight increase in
the number of early embryonic deaths at the highest dose level,
probably associated with the maternal toxicity. There were no
compound-related incidences of external, internal or skeletal
malformations in the fetuses in treated animals. It was concluded
that chlorothalonil is not teratogenic to the rat (Mizens et al.,
1983).
Chlorothalonil was given orally to pregnant rabbits on days 8-16
of gestation at dose levels of 0, 180 or 375 mg/kg per day (days 8 and
9) and 0, 62.5 or 31.25 mg/kg per day (day 10 to day 16). There were
marked effects on food intake and maternal mortality occurred in both
treated groups, which imposed limitations on the evaluation of the
study. However no teratogenic effect was observed (Paynter, 1966b).
Rabbits were dosed with chlorothalonil (0, 5, or 50 mg/kg per
day) during days 6-18 of pregnancy (8 in control group, 9 in each dose
group) and killed on day 29. Four out of nine does aborted at the
highest dose level, and body weight was reduced in this group.
Although the incidence of fetal deaths appeared to increase with dose,
the difference was not statistically significant. The number of
implants and live fetuses per pregnancy and the fetal weights were
reduced in the high-dose group compared to controls. No treatment-
related effects were seen during external, internal or skeletal
examinations. It was concluded that chlorothalonil was not
teratogenic to the rabbit (Shirasu & Teramoto, 1975).
Chlorothalonil was administered by gavage to groups of 20
pregnant rabbits on days 7-19 of gestation at dose levels of 0, 5, 10
or 20 mg/kg per day. All survivors were weighed, killed on day 30 and
examined for live, dead or resorbed fetuses. Live and dead fetuses
were weighed and examined for external, visceral and skeletal
abnormalities. The highest dose level caused maternal body weight
loss and decreased food consumption. The pregnancy rate was > 95%
in each group. Examinations revealed no fetotoxicity or
teratogenicity due to chlorothalonil (Wilson et al., 1988).
A three-generation reproduction study was undertaken in rats at
dietary levels of 0, 1500 or 15 000 mg chlorothalonil/kg diet. A
supplementary study with 0 or 5000 mg/kg was also carried out 6 months
later. Groups consisted of 10 males and 20 females which were fed the
test diet for 11 or 12 weeks prior to mating. The study design was
for three generations with two matings per generation to give A and B
litters. Growth suppression occurred in the nursing A and B litters
in all generations at all dose levels and the pups appeared smaller
than the controls. There were no malformations due to treatment.
However, there were difficulties in execution, and this study is not
considered adequate by present-day standards (Paynter, 1967b).
Reproductive performance was also assessed in a one-generation
study on rats at dietary levels of 0, 200, 375, 750, 1500 or 3000
mg/kg diet with groups of 15 males and 15 females. The parents were
treated for 10 weeks prior to mating. No clinical signs of toxicity
were evident in the parents, but male body weight gain was reduced at
1500 and 3000 mg/kg and the kidneys were enlarged at 3000 mg/kg.
Reproductive parameters such as mating, fertility and gestation length
were not affected. There were no treatment-related abnormalities in
the offspring. The only effect on the offspring was lower body weight
at 3000 mg/kg on lactation days 14 and 21 (Wilson et al., 1989b).
In a two-generation reproduction study with two litters per
generation, technical chlorothalonil was administered to Charles River
CD rats by dietary admixture at concentrations of 0, 500, 1500 and
3000 mg/kg diet. There were 35 rats of each sex in each group. The
parental animals from each generation were treated continuously during
the growth period prior to mating and then throughout the mating,
gestation, lactation and resting phases of the study. The growth
period was 10 weeks for the F0 animals and 14 weeks for the F1
animals. Each generation of parental animals was mated twice to
produce the F1a, F1b, F2a and F2b litters. The offspring were
exposed to the test diets throughout the lactation period. Thirty-
five F1b males and females per group were selected to become the F1
generation after weaning. No mortalities or clinical signs of
toxicity associated with administration of chlorothalonil were
observed in the F0 or F1 parent animals. There was a
treatment-related effect towards lowered body weight in both males and
females in the F0 and F1 adults. The NOEL for body weight was 500
mg/kg in diet. Increased relative food consumption was observed in
the groups that showed lower body weights. The anticipated
treatment-related lesions in the kidneys and stomachs of adult animals
were observed in the F0 and F1 generations by gross and microscopic
pathology. These effects were observed in the kidney at all dose
levels in males and at 1500 and 3000 mg/kg in females. Stomach
effects were observed at all dose levels in both sexes. Reproductive
parameters in F0 and F1 animals, including mating and fertility
indices and gestation length, were not affected by treatment with
chlorothalonil. No gross malformations which were considered
treatment-related were observed in offspring in any of the groups
throughout the study. Litters were culled at day 4 to 8 pups/litter.
No effects on the number of live and stillborn pups, pup sex ratio,
pup survival and physical condition of the pups during lactation were
observed. No findings which were considered treatment-related were
observed during necropsy of the pups. The only effect observed in
pups in this study was lowered body weight compared to the controls on
day 21 of lactation. The NOEL for this effect was considered to be
1500 mg/kg diet, equal to 75 mg/kg body weight per day (Lucas & Benz,
1990).
7.6 Mutagenicity
Chlorothalonil has been assessed for mutagenic potential in a
wide range of in vitro and in vivo assays as shown in Tables 11
and 12. Most of the tests showed chlorothalonil not to be mutagenic
or clastogenic. A positive result in the DNA repair test with
Salmonella typhimurium was not reproduced in Bacillus subtilis,
nor was DNA binding shown in an in vivo study (see section 6.5). A
positive result in Chinese hamster ovary cells without metabolic
activation was not seen in the presence of activation nor confirmed by
in vivo chromosomal tests in rats and mice. Equivocal results were
obtained with Chinese hamster bone marrow in vivo.
In addition to the results shown in the Tables 11 and 12, a
series of studies was reported by IARC (1983). The results, which
were all negative, included those with S. typhimurium in the
presence and absence of a metabolic activating system and
Saccharomyces cerevisiae and Aspergillus nidulans in the presence
of activation. Chlorothalonil also failed to induce mutations in
silkworms and chromosomal aberrations in barley shoot tips or hamster
lung fibroblasts.
Chlorothalonil is not a transforming agent in Fischer rat embryo
cell lines (Price, 1978a).
The monothio, dithio, trithio, dicysteine, tricysteine and
monoglutathione metabolites of chlorothalonil have been shown to be
negative in the Ames assay with or without rat kidney metabolic
activation (see section 7.9).
Considering all the results of mutagenicity testing, it is
unlikely that chlorothalonil will show mutagenic activity in intact
mammalian systems.
7.7 Carcinogenicity
Several long-term rodent studies have been carried out on
chlorothalonil and have included carcinogenic evaluation. The design
of these studies and the chronic toxicity results have been described
in section 7.3. Some of the earlier rat studies did not show any
carcinogenic effect but it is probable that their design was not
sufficient to provide a critical test. The carcinogenic evaluation
in this section therefore concentrates upon the more recent rat and
mouse studies. Detailed evaluations of these studies have already
appeared in the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
reviews of 1983, 1985 and 1990 (FAO/WHO, 1985, 1986b, 1990b).
Therefore, for most of these studies, only the salient points will be
described here.
Table 11. In vitro mutagenicity tests
Test Organism Metabolic Dose range Mutagenic Reference
activation (+ or -) potential
Prokaryotes
Point mutation Salmonella typhimurium + and - 0.33-6.6 µg/plate negative Banzer (1977a)
(5 strains)
Point mutation S. typhimurium + and - 0.16-50 µg/plate negative Jones et al. (1984)
(5 strains) (renal)
Point mutation S. typhimurium - 1-10 µg/plate negative Shirasu et al.
(5 strains) + 2-10 µg/plate negative (1977)
Escherichia coli WP2 - 10-500 µg/plate negative
+ 10-100 µg/plate negative
Point mutation S. typhimurium TA98, + and - 0.76, 7.6, 76 µg/plate negative Wei (1982)
TA100, TA1535, TA1537, (hepatic and
TA1538 renal)
DNA repair tests S. typhimurium + and - 2, 10, 20 µg positive Banzer (1977b)
TA1978, TA1538
Bacillus subtilis - 2-200 µg negative Shirasu et al.
H17/M45 rec-assay (1977)
Table 11. (Cont'd)
Test Organism Metabolic Dose range Mutagenic Reference
activation (+ or -) potential
Mammalian cells
Gene mutation Chinese hamster V79 + and - 0.3 µg/ml negative Banzer (1977c)
mouse fibroblast + and - 0.03 µg/ml negative
BALB/3T3
Chromosome Chinese hamster - 0.03-0.3 µg/ml positive Mizens et al.
aberration ovary cells + 0.6-6.0 µg/ml negative (1986a)
Chromosome Human lymphocytes - 0.54-2.5 µg/ml negative Mosesso & Forster
aberrations + 1.16-5.38 µg/ml negative (1988)
Table 12. In vivo mutagenicity tests
Species Test Dose Mutagenic potential Reference
Mouse in vivo 6.5 mg/kg per day negative Legator (1974a)
cytogenetics 5 days orally
Mouse host-mediated assay; 8 strains 6.5 mg/kg per day negative Legator (1974a)
Salmonella typhimurium 5 days orally
Mouse dominant lethal assay, 5 days 6.5 mg/kg per day negative Legator (1974a)
dosing, 8 weeks mating 5 days orally
Mouse micronucleus 4-2500 mg/kg orally, twice with negative Siou (1981a)
(polychromatic erythrocytes) 24 h interval
Mouse chromosome aberration (bone 4-2500 mg/kg orally, twice with negative Siou (1981b)
marrow 6 h after last dose) 24 h interval 250, 1250, 2500 negative Mizens et al.
(bone marrow at 6, 24 and 48 h) mg/kg oral single dose (1985a)
Rat micronucleus 8-5000 mg/kg orally, twice with negative Siou (1981a)
(polychromatic erythrocytes) 24 h interval
Rat chromosome aberration (bone doses as above negative Siou (1981b)
marrow 6 h after last dose)
(bone marrow 6, 24 and 48 h) 500, 2500, 5000 mg/kg oral negative Mizens et al. (1985b)
single dose
Table 12. (Cont'd)
Species Test Dose Mutagenic potential Reference
Chinese micronucleus 4-2500 mg/kg orally, twice with negative Siou (1981a)
hamster (polychromatic erythrocytes) 24 h interval
Chinese chromosome aberration 8-5000 mg/kg for 2 days inconclusive Siou (1981b)
hamster (bone marrow 6 h after last dose)
(bone marrow at 6, 24 and 48 h) 500, 2500, 5000 mg/kg oral equivocal Mizens et al. (1985c)
single dose (± at 48 h)
(bone marrow 6 h after last dose) 50, 125, 250 mg/kg per day weak response Mizens et al. (1985c)
orally for 5 days not dose-related
A bioassay of technical grade chlorothalonil for possible
carcinogenicity was conducted by administering the test chemical in
the diet to Osborne-Mendel rats and B6C3F1 mice. Groups of 50 rats
of each sex were administered chlorothalonil at one of two dose levels
for 80 weeks and then observed for 30-31 weeks. Time-weighted average
doses for both males and females were 5063 or 10 126 mg/kg diet.
Matched controls consisted of groups of 10 untreated rats of each sex;
pooled controls consisted of the matched control groups combined with
55 untreated male or female rats from similar bioassays of five other
test chemicals. All surviving rats were killed at 110-111 weeks.
Groups of 50 mice of each sex were administered chlorothalonil at one
of two dose levels for 80 weeks, then observed for 11-12 weeks. Time-
weighted average doses for males were 2688 or 5375 mg/kg diet, and for
females, 3000 or 6000 mg/kg diet. Matched controls consisted of
groups of 10 untreated mice of each sex; pooled controls consisted of
the matched control groups combined with 50 untreated male or female
mice from similar bioassays of five other test chemicals. All
surviving mice were killed at 91-92 weeks. Clinical signs that
appeared with increasing frequency in dosed rats included haematuria
and, from week 72 until termination of the study, bright yellow urine.
Since the dosed female mice did not have depression in mean body
weights or decreased survival compared with the controls, they may
have been able to tolerate a higher dose. In rats, adenomas and
carcinomas of the renal tubular epithelium occurred with a significant
dose-related trend in both the males and females (males: pooled
controls 0/62, low dose 3/46, high dose 4/49; females: pooled controls
0/62, low dose 1/48, high dose 5/50). These tumours included both
adenomas and carcinomas which are considered to be histogenically
related. In mice, no tumours were found to occur at a greater
incidence among dosed animals than among controls. It was concluded
that under the conditions of this bioassay, technical grade
chlorothalonil was carcinogenic to Osborne-Mendel rats, producing
tumours of the kidney. However, chlorothalonil was not carcinogenic
for B6C3F1 mice (US NCI, 1978).
In a lifetime study on Fischer-344 rats at dietary doses of 0,
40, 80 or 175 mg/kg body weight per day (see section 7.3), a higher
incidence of primary renal tumours of epithelial origin (adenomas and
carcinomas) was seen in the treated groups (0/60, 7/60, 7/60 and 19/60
for males and 0/60, 3/60, 6/60 and 23/60 for females). It was
considered that the increased incidence of renal hyperplasia seen in
this study was associated with the formation of these tumours and
constituted a pre-neoplastic change. Papillomas and carcinomas of the
squamous mucosa of the forestomach were found in rats in the treated
groups (0/60, 1/60, 1/60, 3/60 for males and 0/60, 1/60, 2/60, 7/60
for females). These are probably related to the proliferative non-
neoplastic effects on the squamous mucosa as a result of the chronic
irritation by chlorothalonil (Wilson et al., 1985a, 1986b).
A further dietary study evaluated the carcinogenicity of
chlorothalonil at lower doses of 1.8, 3.8 and 15 mg/kg body weight per
day as well as at 175 mg/kg body weight per day (see section 7.3).
Rats with renal tumours (adenomas and carcinomas) occurred at 1/55,
1/54, 1/54, 4/54, 23/55 (male groups) and 0/55, 0/54, 0/55, 0/53,
32/55 (female groups). This confirmed the effect at 175 mg/kg per day
and an NOEL of 3.8 mg/kg per day was determined for these tumours.
Animals with papillomas and carcinomas of the forestomach occurred at
0/55, 0/54, 3/54, 2/54, 5/55 (male groups) and 1/55, 1/54, 2/55, 5/53,
9/55 (female groups) giving an NOEL of 1.8 mg/kg per day (Wilson et
al., 1989a).
A 2-year study with Charles River CD-1 mice at 750, 1500 or 3000
mg chlorothalonil/kg diet (section 7.3) showed increased incidences of
gastric and renal tumours in the treated groups. Mice with tumours of
the squamous epithelium of the forestomach occurred at 0/60, 2/60,
5/60, 2/60 (male groups) and 0/60, 2/60, 4/60, 5/59 (female groups).
Although not strictly dose-related, these results were considered to
be a treatment effect and linked to the irritant properties of
chlorothalonil. There was also a low incidence of renal tubular
tumours in male treated groups, not seen in controls, at 0/60, 6/60,
4/60, 5/60 (not dose-related). These were probably linked to the high
incidence of renal tubular hyperplasia seen in male mice in the
treated groups (Wilson et al., 1983b, 1986b).
A second study at 0, 15, 40, 175 and 750 mg/kg diet was
undertaken to establish an NOEL for kidney and stomach changes in male
mice (section 7.3). Only two renal tumours (one at 40 mg/kg and one
at 175 mg/kg) were found. There was a slightly higher incidence of
squamous tumours of the forestomach at 750 mg/kg. Taking account of
the overall results of the two studies it was considered that the
tumorigenic NOEL was at least 175 mg/kg diet. In this study, the NOEL
for tubular hyperplasia was 40 mg/kg (equal to 4.5 mg/kg per day) and
the NOEL for hyperplasia/hyperkeratosis in the forestomach was 1.6
mg/kg per day (Wilson et al., 1987).
7.8 Other special studies
Rats fed 0, 1500 or 15 000 mg chlorothalonil/kg diet showed a
dose-related decrease in the retention of a dye, indicating a laxative
effect. A more detailed study attempted to determine the effect of
chlorothalonil on the absorption and utilization of proteins, fats and
amino acids during a 10-week feeding study on a group of 10 male and
10 female rats. It was concluded that the compound did not interfere
directly with the absorption and utilization and that the depressed
weight gain was probably due to catharsis (Paynter, 1967c).
In a study by Andre et al. (1991), mitochondria were obtained
from fresh kidney cortical tissue by homogenization and differential
centrifugation. The mitochondria were incubated in the presence or
absence of sulfur-containing analogues of chlorothalonil and the
degree of mitochondrial respiratory control was evaluated by
polarographic techniques. The following sulfur-containing analogues
of chlorothalonil were tested: the mono-, di-, and tri-thiol analogues
and the mono-, di-, and tri-glutathione analogues. Kidney
mitochondria were incubated with succinate, a site 2 substrate, or
with glutamate, a site 1 substrate, in the presence or absence of the
test material. Mitochondrial respiratory control, expressed as the
acceptor control ratio (ACR), was determined by taking the ratio of
the rate of oxygen consumption in the presence of ADP (state 3) to the
rate of oxygen consumption after the ADP had been consumed (state 4).
When the mono-thiol, mono-, di-, or tri-glutathione analogues of
chlorothalonil and succinate were added to kidney mitochondria, no
significant differences were found in the ACR from the controls.
Incubation of the di- or tri-thiol analogues of chlorothalonil and
succinate with kidney mitochondria resulted in significant differences
of the experimental ACR from the control ACR. When glutamate was used
as the substrate for the electron transport system in kidney
mitochondria, no significant differences from the control were
detected for any of the six test materials. These data suggest that
the effects of the di- or tri-thiol analogues of chlorothalonil may
impair the respiratory control of kidney mitochondria by inhibiting
the transfer of reducing equivalents from succinate to coenzyme Q.
The effects on mitochondrial respiration may be due to the formation
of disulfide bonds between the thiol analogues and proteins.
7.9 Toxicity of metabolites
Most studies have centred on the 4-hydroxy-2,5,6-
trichloroisophthalonitrile metabolite. This is found as a small
proportion of chlorothalonil plant residues (section 4.2.1) and is
also a breakdown product of chlorothalonil in the environment. It has
been identified in faeces of laboratory animals after chlorothalonil
dosing. It is more acutely toxic than chlorothalonil itself (acute
oral LD50 values are 332 and 10 000 mg/kg, respectively).
Several laboratory animal studies have been undertaken with the
4-hydroxy metabolite and have been described in some detail in JMPR
reviews (FAO/WHO, 1978, 1982, 1985). The following is a brief summary
of the studies and their results.
Various effects were noted in rats fed the 4-hydroxy metabolite
at eight dose levels (10 to 750 mg/kg body weight per day) for 61-69
days. Mortality was increased in males at 125 mg/kg per day or more,
and in females at 75 mg/kg per day or more. Body weight was depressed
in both sexes at > 40 mg/kg per day. Anaemia was evident at 75
mg/kg per day or more in males and 40 mg/kg per day or more in
females. Histopathological examination revealed treatment-related
effects in bone marrow and spleen (in the form of erythroid
hyperplasia and depressed granulopoiesis) at > 40 mg/kg per day and
in the liver (haemosiderosis, centrilobular hepatitis) and kidney
(cortical atrophy) at > 75 mg/kg per day. The overall NOEL was 20
mg/kg body weight per day (Murchison, 1979).
A rabbit teratology study was undertaken with oral doses of 0, 1,
2.5 and 5 mg/kg per day during days 6-18 of gestation, with necropsy
at day 28. There was a marginal effect on dams at day 5 but no
evidence of a teratogenic effect in the study (Wazeter & Goldenthal,
1976).
The 4-hydroxy metabolite was evaluated in a three-generation, two
litters/generation study in groups of 15 male and 30 female rats at
dose levels of 0, 10, 60 and 125 mg/kg diet. There were no
treatment-related changes except for body weight reductions at 60 and
125 mg/kg (FAO/WHO, 1982).
In a one-generation follow-up study, groups of rats were fed
diets containing the 4-hydroxy metabolite at 0, 10, 20, 30, 60 and 120
mg/kg diet for 18 weeks before mating (12 males and 24 females per
group). Two sets of mating were undertaken. There was some effect on
live pup weights at 60 and 120 mg/kg. The clear NOEL was considered
to be 30 mg/kg diet (Ford, 1982).
The metabolite was assessed for chronic toxicity and
carcinogenicity in long-term rodent studies. A 2-year rat study was
undertaken at dose levels of 0, 0.5, 3 and 10 mg/kg body weight per
day with groups of 75 males and 75 females. Anaemia was evident at
the highest dose. The NOEL was determined to be 3 mg/kg body weight
per day. There was no evidence for a carcinogenic effect. In the
mouse study, the dietary dose levels were 0, 375, 750 and 1500 mg/kg
diet using groups of 60 males and 60 females. The study was
terminated at 20-22 months because of increasing and high mortality.
A series of effects, including amyloidosis, haemosiderin in the spleen
and increases in reticulocyte counts and red cell haemolysis,
precluded the establishment of an NOEL. No carcinogenic effect was
evident (Hozan & Auletta, 1981; McGee, 1983).
The 4-hydroxy metabolite was not mutagenic in a number of in
vitro and in vivo assays. These were the Salmonella mutagenicity
assay with and without metabolic activation (Banzer, 1977d), a
host-mediated assay in mice given a single intraperitoneal dose of
6.5 mg/kg (Legator, 1974b), Chinese hamster (V-79) and mouse
fibroblast (Balb/3T3) cells in culture with and without activation
(Banzer, 1977e), a micronucleus test in mice at 6.5 mg/kg per day for
5 days (Legator, 1974b), a dominant lethal study in male rats given
single oral doses (0, 2, 4 or 8 mg/kg) singly or daily for 5 days
(Hastings & Clifford, 1975), a dominant lethal study in male mice
given 1, 3 or 6.5 mg/kg per day for 5 days (Legator, 1974b), a DNA
repair assay using S. typhimurium in a spot test with or without
activation (Banzer, 1977f), and a cell transformation assay with rat
embryo cell lines in culture at 0.1, 1 or 10 µg/ml (Price 1978b).
A series of in vitro gene mutation assays with S. typhimurium
tester strains with and without renal metabolic activation were
undertaken with chlorothalonil, four manufacturing impurities and
eight known or potential metabolites. No mutagenic potential was
shown by any of the compounds. Full details were given in the 1985
JMPR evaluation (FAO/WHO, 1986b).
The mutagenic potential of the thiol and cysteine derivatives of
chlorothalonil has been evaluated in the Ames test with and without
metabolic activation with S9 from the kidney of male Fischer rats.
These compounds were 2,5-dichloro-4,6-bismercaptoiso-phthalonitrile,
5-(2,4-dicyano-3,5,6-trichlorophenyl) glutathione, 5-chloro-2,4,6-
trimercaptoisophthalonitrile, S,S'-(2,4-dicyano-3,6-dichloro
phenyl)dicysteine and S,S',S"-(2,4-dicyano-6-chlorophenyl)-
tricysteine (purity ranging from 90.5 to 97.5%). Four other compounds
were used as positive controls. The Salmonella typhimurium tester
strains TA98, TA100, TA1535, TA1537 and TA1538 were used. In all
these studies, there was no significant increase (doubling) over
solvent control values in the number of revertants for any of the five
tester strains used either with or without metabolic activation
(Mizens et al., 1985d,e, 1986b,c, 1987).
A description of a 90-day rat study on the monoglutathione
conjugate of chlorothalonil is given in section 7.3.
8. EFFECTS ON HUMANS
8.1 General population exposure
A case of acute facial dermatitis in a 53-year-old man, caused by
staying in a summer cottage, has been reported. Patch testing
revealed contact allergy to the paint that was applied to all the
window-frames, and to the chlorothalonil contained in the paint.
After removal of the frames, there were no further recurrences of
facial dermatitis. The authors suggested that products containing
chlorothalonil are not suitable for indoor use (Liden, 1990; Eilrich &
Chelsky, 1991).
8.2 Occupational exposure
Chlorothalonil contact dermatitis was observed in a number of
employees in a chlorothalonil manufacturing plant. There were 19
cases out of 103 employees. About 60% of the employees showed some
kind of skin abnormality compared with 18.5% of employees not working
with chlorothalonil. When the hygiene conditions of the plant were
improved the overall proportion of skin abnormalities fell to about
20% and there were no cases of chlorothalonil contact dermatitis
(Diamond Shamrock, 1980).
Wood preservatives containing chlorothalonil have also been
implicated in the appearance of allergic contact dermatitis. One
report concerned a Danish cabinet maker who developed dermatitis on
his hands after 9 months of painting furniture with preservative
containing the compound. This was possibly caused by contact via the
wood dust after sandpapering (Bach & Pedersen, 1980). Another report
referred to three cases, two with erythema on the face, particularly
periorbitally, and one with eczema of the hands, in people engaged in
similar work (Spindeldreier & Deichmann, 1980). The four people in
these cases showed a positive reaction to patch tests with 0.01%
chlorothalonil in acetone.
A further case of contact dermatitits was described by Meding
(1986) in a 33-year-old male painter, who regularly worked with paint
containing chlorothalonil. A patch test with chlorothalonil was
positive.
Work-related skin complaints occurred in a Norwegian factory
producing wooden window frames. The wood preservative used was white
spirit containing 0.5% chlorothalonil. Fourteen out of 20 workers
experienced some kind of skin reaction including pruritus, erythema
and oedema of the eyelids and other facial regions, and eruptions on
arms and hands. Seven of these 14 subjects yielded a positive patch
test reaction with 0.01% chlorothalonil in acetone compared with 1 out
of 14 controls (Johnsson et al., 1983).
Allergic contact dermatitis has also been described in Japanese
farmers (Horiuchi & Ando, 1980) and in Dutch horticultural workers
(Bruynzeel & van Ketel, 1986) using chlorothalonil fungicide
formulations.
In a group of 84 tea growers, two showed a positive skin patch
test with 0.02% chlorothalonil in petrolatum (Fujita, 1985).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Aquatic microorganisms
The algicidal activity of chlorothalonil was examined by Goulding
(1971). It was shown that this compound is effective against a range
of algae including Chlorella, Chlamydomonas, Ulothrix , Anabaena,
Oscillatoria and Microcystis at low concentrations, often
less than 1 µg/litre. It was also effective on natural populations
of algae obtained from lakes, rivers, and reservoirs. The effect
was generally less after 300 h than after 150 h and was dependent upon
the size of the initial cell inoculum.
Walker et al. (1984) describe a simple shake-flask screening test
to evaluate pesticide persistence and aquatic toxicity in the
laboratory. Four systems were used: active sediment, sterile
sediment, active water and sterile water. Chlorothalonil at
162 µg/litre did not increase the mortality of Mysidopsis above the
control level within 96 h. The authors concluded that degradation of
chlorothalonil involved microorganisms and that the degradation
products did not enhance the aquatic toxicity of chlorothalonil.
9.1.1.2 Soil microorganisms
Chlorothalonil, at dose levels up to 5000 mg/litre in bacterial
suspensions, inhibited the growth of three strains of Rhizobium
japonicum (Tu, 1980).
Chlorothalonil, at concentrations up to 1000 mg/kg medium, did
not inhibit or stimulate the growth of any one of 25 strains of
Rhizobium bacteria isolated from red clover root nodules
(Heinonen-Tanski et al., 1982).
Several studies were conducted with chlorothalonil in soil to
determine the effects (if any) on normal soil processes such as
nitrogen fixation, nitrification and degradation of substrates such as
protein, pectin, cellulose and starch. These studies were conducted
at two rates: use rate (2.5 mg/kg) and 10 times the use rate
(25 mg/kg). In general, any inhibitory effects observed were
temporary in nature and more pronounced at the high rate. No effects
on the use of protein, pectin or cellulose by soil microorganisms were
observed, but there was increased utilization of starch (Szalkowski et
al., 1980).
The results obtained by Szalkowski et al. (1981a), who studied
the effect of chlorothalonil on non-symbiotic nitrogen-fixing soil
microorganisms, are given in Table 13.
Table 13. The effect of chlorothalonil on non-symbiotic nitrogen-fixing soil microorganisms
Sandy loam Clay loam
Application Ten times Application Ten times
rate application rate application
rate rate
Aerobic nitrogen initial inhibition inhibition at no effect stimulation
fixation days 0, 21 and
28
Anaerobic nitrogen general stimulation inhibition up no effect stimulation
fixation to day 21
Szalkowski et al. (1981b) studied the effect of chlorothalonil on
nitrogen transformation in sandy loam and clay loam soils at two
rates: one equivalent to the application rate and one equivalent to
ten times this rate. In sandy loam soil, there was a consistent
inhibitory effect at the higher rate, which was reduced with time. In
clay loam soil, at the higher rate, inhibition was no longer observed
after day 21. In both types of soil at the normal rate, there was
little if any inhibition.
9.1.2 Aquatic organisms
The acute toxicity of chlorothalonil to various aquatic species
is shown in Table 14. Some of these toxicity tests were carried out
in static or semi-static systems and others in flow-through systems.
Table 14. Acute toxicity of chlorothalonil to aquatic organisms
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Rainbow trout
(Oncorhynchus mykiss)
6-11 g flow through freshwater 80% 14 acetone > 99% 19.0 17.1 Davies &
6-11 g flow through freshwater 53% 16 acetone > 99% 18.8 10.5 White
6-11 g semi-static freshwater 90% 10 acetone > 99% 18.0 (1985)
(24 h)
- static freshwater - 12 acetone 96% 56 49 SDS Biotech
(soft) Corporation
(1980a)
3.5-4.0 g static freshwater 6.5-7.4 8.4-11.2 12.5-15.5 acetone 97.8% 76 Ernst et
(12.3 mg/litre) mg/litre al. (1991)
3.5-4.0 g static freshwater 6.5-7.4 8.4-11.2 12.5-15.5 Bravo 500 69 Ernst et
(12.3 mg/litre) mg/litre al. (1991)
Bluegill
(Lepomis macrochirus)
- static freshwater 22 acetone 96% 46-77 62 SDS Biotech
(soft) Corporation
(1979)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Common jolly tail
(Galaxias maculatus)
7-10 g flow through freshwater 75% 16 acetone 99% 18.2 16.3 Davies &
White (1985)
Spotted galaxis
(G. fruttaceus)
8-20 g flow through freshwater 75% 16 acetone 99% 25.8 18.9 Davies &
White (1985)
Golden galaxias
(G. auratus)
7-11 g flow through freshwater 75% 13 acetone 99% 46.6 29.2 Davies &
White (1985)
Three spine stickleback
(Gasterosteus aculeatus)
0.3 g static freshwater 7.7-8.0 9.2-9.5 9-10 Bravo 500 < 73 Ernst et
mg/litre al. (1991)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Channel catfish
(Ictalurus punctalus)
40-80 g semi-static, freshwater 7.0-7.2 5.0-6.0 23 acetone 99% 62 52 Gallagher et
24 h (30 mg/litre) mg/litre al. (1992)
- static freshwater 22 acetone 96% 55 44 SDS Biotech
(soft) Corporation
(1980b)
Spot
(Leiosfomus xanthurus)
- flow through brackish water 11 technical 32 Mayer (1987)
(22 ppt)
Sheepshead minnow
(Cyprinodor variegatus)
3-7 days static marine technical 32 SDS Biotech
Corporation
(1982b)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Water flea
(Daphnia magna)
- static freshwater 7.7-8.1 9.1-9.3 20-22 Bravo 500 97a Ernst et
(250 mg/litre) mg/litre al. (1991)
Dungeness crab
(Cancer magister)
larvae semistatic, marine 13 Bravo, 75% 560 140 Armstrong
24h (25 ppt) et al. (1976)
Clam
(Mya arenaria)
5.2 cm static marine 7.3-8.0 8.5-9.9 10.5-12 Bravo 500 35 000 Ernst et
(30-31 ppt) mg/litre al. (1991)
Blue mussel
(Mytilus edulis)
5.9 cm static marine 7.3-8.0 8.5-9.9 10.5-12 Bravo 500 5940 Ernst et
(30-31 ppt) mg/litre al. (1991)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Eastern oyster
(Crassostrea virginica)
- flow through marine 29 technical 26b Mayer (1987)
(27 ppt)
a EC50 - immobility
b EC50 - shell deposition
In view of the strong adsorption and degradation characteristics of
chlorothalonil, nominal concentrations in static systems are likely to
underestimate the toxicity (overestimate the LC50) of chlorothalonil.
At the same time, it should be pointed out that the static test
systems more closely resemble the field situation.
Davies & White (1985) described the reactions of fish to
exposure. Oncorhynchus mykiss and Galaxias sp showed marked
lethargy, the degree increasing with time and concentration of
exposure. O. mykiss showed normal startle reactions at
concentrations below 8.7 µg/litre, and G. maculatus, G. truttaceus
and G. auratus at 8.8, 9.0 and 13.3 µg/litre, respectively, over
96 h. In O. mykiss, loss of startle reaction was followed by
reduction of activity, and permanent lethargy was followed by loss of
righting ability and death. In Galaxias sp, the onset of lethargy
was accompanied by varying degrees of fin collapse.
Davies & White (1985) commented on the fact that most acute
toxicity values have been obtained in tests where solvent was added to
the chlorothalonil.
A 48-h EC50 has been reported for the pink shrimp ( Penaeus
duorarum) at 320 µg/litre and a 96-h EC50 for the eastern oyster
( Crassostrea virginica) at 26 µg/litre. The value for the pink
shrimp was based on immobility or loss of equilibrium and that for the
eastern oyster on shell deposition (Mayer, 1987). Oysters suffered a
42% mortality when exposed to 1000 µg chlorothalonil per litre for
96 h and a 25% reduction in growth at 10 µg/litre (SDS Biotech
Corporation, 1983b).
Ernst et al. (1991) used both laboratory bioassay and field
treatments of a pond system to determine the toxic effects of
chlorothalonil on aquatic fauna. The acute toxicity of technical
chlorothalonil and a commercial formulation on five species, including
mussel and clams, was determined (Table 14). In the field study also
reported by O'Neill (1991) (see section 5.1.2 for measured
concentrations after spraying), the mortality of caged invertebrate
and vertebrate species was monitored. Seven species were caged in the
stream flowing from the treated pond: water boatman (Sigara
alternata), caddisfly larva ( Limnephilus sp), freshwater clam
( Pisidium, sp), crawling water beetle ( Haliplus sp), scud
( Gammarus spp.), stickleback (Gasterosteus aculeatus) and midge
larva (Chironomidae). The floating cages were placed near the
surface of the water. The water boatman suffered the highest
mortality, ranging from 49 to 84% in replicate cages; mortality in a
control pond was 16-20% over the same period (24 h). Midge larva
mortality (69%) was judged by the authors to be a consequence of
handling. Stickleback mortality was 37 to 56% in the treated pond,
compared to 2 to 6% in the controls. Caddisfly larvae, clams, beetles
and scud showed no deaths during the 24 h. Rainbow trout
(Oncorhynchus mykiss) also showed no mortality following spraying.
An estimate of total invertebrate numbers before and after spraying in
the pond showed a slight increase following the first spray and a
slight reduction after the second (not statistically significant).
The control pond also showed fluctuations. The total was heavily
influenced by Chironomid midge larvae, which was by far the most
frequently occurring species. This study showed that a lower
toxicological effect was observed in the field study than in the
laboratory bioassays, indicating a reduction in exposure to the
available chlorothalonil and thus less severe impacts in the pond
system through physical and chemical processes.
Gallagher et al. (1992) showed that sublethal chlorothalonil
exposure may cause acute necrosis of the intestinal epithelial lining
in channel catfish. Exposure to 13 µg/litre for 72 h resulted in
increased tissue GSH concentrations in liver, posterior kidney and
gills, which suggests a protective role for tissue GSH against
chlorothalonil exposure.
When two generations of Daphnia magna were exposed to technical
chlorothalonil at levels of 6.2, 12, 25, 50 and 100 µg/litre for 21
consecutive days during each generation, adverse effects on adult
survival and reproduction were observed at a nominal concentration of
100 µg/litre. The maximum acceptable toxicant concentration (MATC)
for technical chlorothalonil, based upon nominal concentrations, was
50 µg/litre (35 µg/litre was the measured concentration) (Shults et
al., 1982).
Fathead minnows (Pimephales promelas) were continuously exposed
in duplicate aquaria to nominal concentrations of 25, 12.5, 6.3, 3.1
and 1.5 µg/litre (measured concentrations 16, 6.5, 3.0, 1.4 and
0.6 µg/litre, respectively) technical chlorothalonil, a diluent water
control, and a solvent (acetone) control throughout a complete (egg to
egg) life cycle. No significant effects were observed in either
generation at mean measured concentrations < 3.0 µg/litre. The
first generation (F0) eggs exhibited a significantly reduced
hatchability and survival of fry after 35 days when exposed to a mean
measured concentration of 16 µg/litre. The reproductive success of
Fo fish was adversely affected (reduction in the number of eggs per
spawn) by exposure to concentrations > 6.5 µg/litre. The second
generation (F1) eggs exhibited a significantly reduced hatchability
when exposed to a mean measured concentration of 6.5 µg/litre. The
survival of fry at this concentration was not affected. Based on
these data, the MATC (mean measured) of technical chlorothalonil in
water for fathead minnows was estimated to be in the range of 3.0 to
6.5 µg/litre (Shults et al., 1980).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
In a study by Stephenson et al. (1980), 30-day-old tomato plants
were treated with chlorothalonil (at 2.5 kg/ha) or chlorothalonil in
combination with metribuzine. The effect was assessed as the tomato
shoot dry weight (as a percentage of control plant weight). On this
basis, the weight of the chlorothalonil-treated plants was 89% of the
control plant weight, a difference which was statistically significant
(P > 0.05). The effect was additive when chlorothalonil was used in
combination with metribuzine.
9.1.3.2 Earthworms
When chlorothalonil suspension concentrate (500 g/litre) was
added to artificial soil containing earthworms (Eisenia foetida),
the treated soil was non-toxic after 7 and 14 days. The LC50 was
found to be > 1000 mg/kg soil (on a dry weight basis) (Wuthrich,
1990).
When earthworms (Eisenia foetida) were immersed for 1 min in
solutions of chlorothalonil (0.1, 1 and 2% w/v); there was no effect
on survival. Bermuda grass clippings were air dried and ground; 15 g
samples were then stirred into 100 ml of 0.1% chlorothalonil and
subsequently fed to earthworms after filtration of excess liquid.
There was no effect on longevity. Worms reared in soil in which
chlorothalonil had been incorporated showed reduction in longevity of
about 50% compared to controls 52-84 days after the beginning of
treatment. The amount of chlorothalonil added was equivalent to 5
times the recommended application rate at 0.9 g in 4700 cm3 of soil,
and reproduction was virtually eliminated (Roark & Dale, 1979).
9.1.3.3 Earwigs and honey-bees
Earwigs (Labidura riparia) were exposed to chlorothalonil in
three ways: a) on glass; b) as a residue on peanut foliage; and c) as
a residue on a food source (i.e. 7-day-old armyworms). In the first
treatment, chlorothalonil at a rate of 0.72 kg/ha produced up to 20%
mortality in 24 h and up to 30% in 48 h. In the second experiment,
chlorothalonil was applied, at the same rate, to peanut foliage which
was then used for the test. There was a 10% mortality within 24 h and
20% in 48 h on 4-day-old residues and no mortality on 8-day-old
residues. In the food source experiment there was 25-30% mortality
within 24 h of the larvae being consumed and up to 55% mortality
within 48 h (DeRivero & Poe, 1981).
Atkins et al. (1975) found no contact toxicity of chlorothalonil
(11 µg/bee), and classified it as relatively non-toxic to honey-bees.
The oral LD50 for chlorothalonil in 20% sucrose solution for
honey-bees was > 0.2 µg/bee and the contact LD50 > 65 µg/bee
(Davies, 1986).
9.1.3.4 Birds
The following toxicity values have been reported for birds, but
without descriptions of the experimental methodology. The acute oral
LD50 in the mallard duck was reported to be > 4640 mg/kg. The 8-day
dietary LC50 in the same species and in the bobwhite quail (Colinus
virginianus) was given as > 10 000 mg/kg diet in each case (SDS
Biotech Corporation, 1981a,b). Dietary 8-day LC50 values were also
reported as > 21 500 mg/kg diet for the mallard duck and 5200 mg/kg
diet for the bobwhite quail (Worthing, 1991).
Shults et al. (1988a) evaluated the effect of chlorothalonil on
reproduction in the bobwhite quail. Four groups (16 pairs per group)
of quail were administered chlorothalonil in the diet at levels of
1000, 5000 and 10 000 mg/kg for a period of 21 weeks. Quail were fed
the amended diet for 11 weeks prior to egg laying and for the duration
of the egg laying period. Adult and offspring were examined for body
weight, general health, adult food consumption, egg production,
eggshell thickness, embryo viability, hatching success survivability
of offspring and gross pathology. At a dietary concentration of
10 000 mg/kg, the birds experienced reproductive impairment, which
included mortality, general health, body weight, food consumption,
gross pathology and other reproductive end-points. Hatching survival
was also affected at the highest dosage. General health and
reproductive parameters were also affected at 5000 mg/kg. Other
effects observed at this dose level included decreased body weight
gain and survivability of offspring. The lowest test dosage showed no
apparent affects on either adult quail or offspring. A
no-observed-effect concentration (NOEC) of 1000 mg/kg diet was
established for chlorothalonil regarding reproductive effects.
In a separate but similar reproductive study with mallard ducks
(Anas platyrhynchos), Shults et al. (1988b) reported that
10 000 mg/kg diet reduced egg production and the percentage of
hatchlings per incubated egg. No reproductive impairments were
observed for ducks dosed at 1000 or 5000 mg/kg. Shults et al. (1988b)
reported no effect on eggshell thickness at these chlorothalonil
concentrations. The NOEC for reproductive effects in mallard duck was
5000 mg/kg diet.
9.2 Field observations
9.2.1 Soil microorganisms
Smiley & Craven (1979) applied chlorothalonil (as Daconil 2787)
at the normal rate (actual dose not given) every 21 days between April
and September (9 applications for 3 consecutive years) to an
experimental plot of Sward Kentucky blue grass (Pia pratensis).
Treated plots were 1 × 5 m and were replicated. Samples of soil cores
2.54 cm in diameter and 3 cm deep were taken (n = 5) from each
replicate plot. The cores included thatch and were chopped and mixed
well, and 10 g was suspended in sterile distilled water. A dilution
series was plated out to estimate bacterial, actinomycete and fungal
populations. None of the organisms were affected by chlorothalonil.
The fungicide did not affect numbers of Nitrosomonas or Nitrobacter
bacteria and had no effect on the disappearance of added ammonium by
nitrification. Treatments alternating different fungicides had a
greater effect than one fungicide alone.
9.2.2 Plants
Many crop plants are tolerant of chlorothalonil and do not suffer
any phytotoxicity when sprays of chlorothalonil formulations are
applied according to recommended practices. However, a few phytotoxic
effects have been observed with certain species as a direct result of
chlorothalonil applications and also as a result of physiologically
incompatible mixtures of chlorothalonil formulations with additives or
other pesticides.
Chlorothalonil applied at a rate of 2.52 kg/ha did not affect
yields of tomato plants grown in field conditions. In addition,
chlorothalonil did not enhance metribuzin damage when the two
compounds were used in combination on tomato plants. These results
are contrary to those obtained in laboratory studies (section 9.1.3)
and indicate that under field conditions phytotoxic reactions between
chlorothalonil and metribuzin are unlikely to occur (Stephenson et
al., 1980).
Clear results were not obtained concerning the effect of
chlorothalonil on perennial rye grass (Lolium perenne) when it was
sprayed regularly for the control of leaf-spotting disease. In the
first year of treatment the grass yield was increased by 15% at the
third harvest, but at the following harvest, when disease incidence
was greater, the yield was not increased. The authors concluded that
chlorothalonil did not have a direct stimulatory effect on grass
growth (Lam & Lewis, 1983).
Chlorothalonil was used to treat onion beds at a rate of
2.34 litres formulation/ha with 10 weekly applications. The onions
(two short-day cultivars) were harvested either 146 or 167 days after
planting. Chlorothalonil reduced the weight and size of marketable
bulbs by 44 and 32%, respectively, but did not influence the
maturation rate. Alternate application of chlorothalonil and mancozeb
reduced these values by 27 and 33%, respectively. The disease
incidence was nil in all treatments (Stoffella & Sonoda, 1982).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed
and evaluated chlorothalonil at its meetings in 1974, 1977, 1978,
1979, 1981, 1983, 1985, 1987, 1990 and 1992 (FAO/WHO, 1975, 1978,
1979, 1980, 1982, 1985, 1986a,b, 1988, 1990a,b, 1993b). In 1990 an
acceptable daily intake (ADI) of 0-0.03 mg/kg body weight was
established. This ADI was confirmed in 1992, based on the NOAEL of 3
mg/kg body weight/day established in the two-year dog study (FAO/WHO,
1993a,c).
The Joint FAO/WHO Codex Alimentarius Commission has established
maximum residue limits (MRLs) for chlorothalonil in various
commodities (FAO/WHO, 1993c).
WHO has classified chlorothalonil as a technical product unlikely
to present an acute hazard in normal use (WHO, 1992).
On the basis of data available at the time, the International
Agency for Research on Cancer evaluated chlorothalonil as showing
limited evidence of carcinogenicity in animal studies and categorized
it as an agent not classifiable as to its carcinogenicity to humans
(IARC, 1987).
REFERENCES
André JC, Marciniszyn JP, & Killeen JC Jr (1991) Evaluation of
mitochondrial function in the presence and absence of sulphur-
containing analogs of chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 3113-88-0107-AM-001,
submitted to WHO by ISK-Biotech, Mentor, Ohio, USA).
Armstrong DA, Buchanan DV, & Caldwell RS (1976) A mycosis caused by
Lagenidium sp.in laboratory-reared larvae of the dungeness crab,
cancer magister, and possible chemical treatments. Department of
Fisheries and Wildlife, Oregon State University, Marine Science
Center, Newport, Oregon 97365. J Invertebr Pathol, 28: 329-336.
Atkins EL, Greywood EA, & MacDonald RL (1975) Toxicity of pesticides
and other agricultural chemicals to honey bees. Riverside, California,
University of California, Division of Agricultural Sciences, 37 pp
(Report No. 2287).
Bach B & Pedersen NB (1980) Contact dermatitis from a wood
preservative containing tetrachloroisophthalonitrile. Contact
Dermatitis, 6: 142.
Ballee DL, Duane WC, Stallard DE, & Wolfe AL (1976) Chlorothalonil.
In: Zweig G & Sherma J ed. Analytical methods for pesticides and plant
growth regulators. New York, Academic Press, vol VIII, pp 266-274.
Banzer CB (1977a) Activity of chlorothalonil in the Salmonella/
microsomal assay for bacterial mutagenicity. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 000-5TX-77-0035-001).
Banzer CB (1977b) Activity of chlorothalonil in a test for
differential inhibition of repair deficient and repair competent
strains of Salmonella typhimurium: Repair test. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 000-5TX-77-0033-001).
Banzer CB (1977c) Activity of chlorothalonil in an in vitro
mammalian cell point mutation assay. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 000-5TX-77-0034-001).
Banzer CB (1977d) Activity of DTX-0038 in the Salmonella/microsomal
assay for bacterial mutagenicity. Bethesda, Maryland, Microbiological
Associates Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Banzer CB (1977e) Activity of DTX-77-0040 in an in vitro mammalian
cell point mutation assay. Bethesda, Maryland, Microbiological
Associates Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Banzer CB (1977f) Activity of DTX-77-0039 in a test for differential
inhibition of repair deficient and repair competent strains of
Salmonella typhimurium: Repair test. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Blackmore R & Shott L (1968) Final report four-month feeding study -
rats. Hazleton Laboratories, Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Bruynzeel DP & van Ketel WG (1986) Contact dermatitis due to
chlorothalonil in floriculture. Contact Dermatitis, 14: 67-68.
Burchfield HP & Storrs EE (1977) Residue analysis. In: Seigel MR &
Sisler HD ed. Antifungal compounds. New York, Marcel Dekker, Inc., vol
1, pp 463-505.
Busey WM (1975) Histopathological incidence data - kidney. Herndon,
Virginia, Experimental Pathology Laboratory, Inc. (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Busey WM (1985) A 90-day feeding study in mice with T-117-11.
Experimental Pathology Laboratories, Inc. (Unpublished revised
pathology report No. SDS 753-5TX-85-0053-002, submitted to WHO by SDS
Biotech, Mentor, USA).
Camoni I, DiMuccio A, Pontecorvo D, Rubbiani M, Vergori L, & Lugaresi
C (1991) Residue levels in apples and pears field-treated with two
experimental chlorothalonil formulations. Bull Environ Contam Toxicol,
46: 361-367.
Chin BH, McGloin JB, Spangler NL, & Heilman RD (1981) Chlorothalonil
equivalents in the blood and urine of rats following oral,
endotracheal and dermal administration of 14C-chlorothalonil. Bull
Environ Contam Toxicol, 26: 258-261.
Danks A & Fowler JSL (1988) Chlorothalonil technical: acute inhalation
toxicity study in the rat. Eye, Suffolk, United Kingdom, Life Science
Research Ltd (Report No. 88/CFA 00I/85, submitted to WHO by ISK-
Biotech, Mentor, USA).
Davies PE (1985a) The toxicology and metabolism of chlorothalonil in
fish. II. Glutathione conjugates and protein binding. Aquat Toxicol,
7: 265-275.
Davies PE (1985b) The toxicology and metabolism of chlorothalonil in
fish. III. Metabolism, enzymatics and detoxication in Salmo spp and
Galaxias spp. Aquat Toxicol, 7: 277-299.
Davies PE (1985c) The toxicology and metabolism of chlorothalonil in
fish. IV. Zinc coexposure and the significance of metallothionein in
detoxication in Salmo Gairdneri. Aquat Toxicol, 7: 301-306.
Davies LG (1986) Report on a laboratory investigation into the
toxicity of Sipcam chlorothalonil to honey bees (Apis mellifera).
Nottingham, Trent Polytechnic, Department of Sciences (Report
submitted to WHO by ISK-Biotech, Mentor, USA).
Davies PE (1988) Disappearance rates of chlorothalonil (TCIN) in the
aquatic environment. Bull Environ Contam Toxicol, 40: 405-409.
Davies PE & White RWG (1985) The toxicology and metabolism of
chlorothalonil in fish I. Lethal levels for Salmo gairdneri,
Galaxias maculatus, G. truttaceus and G. auratus and the fate of
14C-TCIN in S. gairdneri. Aquat Toxicol, 7: 93-105.
DeRivero NA & Poe SL (1981) Response of Labidura riparia (Pallas) to
residues of pesticides used on peanuts. Peanut Sci, 8: 93-96.
Diamond Shamrock (1974) Unpublished report on the pesticide
chlorothalonil, submitted to FAO/WHO by Diamond Shamrock Corporation,
Mentor, Ohio, USA.
Diamond Shamrock (1980) Unpublished toxicological report, submitted to
the 1981 JMPR by Diamond Shamrock Corporation, Mentor, Ohio, USA.
Doyle R & Elsea J (1963) Acute oral, dermal, and eye toxicity and
irritation studies on DAC-2787. Hill Top Research Institute Inc.
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
Duane WC (1970) Biodegradation of Daconil 2787. Mentor, Ohio, Diamond
Shamrock Corporation (Unpublished report).
Eilrich GL & Chelsky M (1991) Letters to the editor : Facial
dermatitis caused by chlorothalonil in paint. Contact Dermatitis, 25:
141-144.
Elliott VJ & Spurr HW (1993) Temporal dynamics of chlorothalonil
residues on peanut foliage and the influence of weather factors and
plant growth. Plant Dis, 77: 455-460.
Ernst W, Doe K, Jonah P, Young J, Julien G, & Hennigar P (1991) The
toxicity of chlorothalonil to aquatic fauna and the impact of its
operational use on a pond ecosystem. Arch Environ Toxicol, 21: 1-9.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO (1985) Guidelines for the disposal of waste pesticide and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1975) 1974 Evaluations of some pesticide residues in food:
The monographs. Geneva, World Health Organization, pp 101-148 (WHO
Pesticide Residues Series, No. 4).
FAO/WHO (1978) Pesticide residues in food - 1977. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Sup.).
FAO/WHO (1979) Pesticide residues in food - 1978. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Sup.).
FAO/WHO (1980) Pesticide residues in food - 1979. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Sup.).
FAO/WHO (1982) Pesticide residues in food - 1981. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1985) Pesticide residues in food - 1983. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 61).
FAO/WHO (1986a) Pesticide residues in food - 1985. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 72/1).
FAO/WHO (1986b) Pesticide residues in food - 1985. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 72/2).
FAO/WHO (1988) Pesticide residues in food - 1987. Evaluations: Part II
- Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 86/2).
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Codex Committee on Pesticide
Residues, Food and Agriculture Organization of the United Nations
(CAC/PR8-1989).
FAO/WHO (1990a) Pesticide residues in food - 1990. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 103/1).
FAO/WHO (1990b) Pesticide residues in food - 1990. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 103/2).
FAO/WHO (1991) Pesticide residues in food - 1990. Evaluations: Part II
- Toxicology. Geneva, World Health Organization (WHO/PCS/91.47).
FAO/WHO (1993a) Pesticide residues in food - 1992. Report. Rome, Food
and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper 116).
FAO/WHO (1993b) Pesticide residues in food - 1992. Evaluations: Part
II - Toxicology. Geneva, World Health Organization (WHO/PCS/93.34).
FAO/WHO (1993c) Codex Alimentarius. Volume 2: Pesticide residues in
food. Rome, Food and Agriculture Organisation of the United Nations.
Flannigan SA & Tucker SB (1985) Influence of the vehicle on irritant
contact dermatitis. Contact Dermatitis, 12: 177-178.
Flannigan SA, Tucker SB, & Calderon V (1986) Irritant dermatitis from
tetrachloroisophthalonitrile. Contact Dermatitis, 14: 258-259.
Ford WH (1982) A one-generation reproduction study in rats with
DS-3701. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Ford WH, Killeen JC, & Baxter RA (1987a) A 90-day feeding study in
rats with chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 1115-85-0079-TX-006).
Ford WH, Killeen JC, & Baxter RA (1987b) A 90-day study in rats with
the monoglutathione conjugate of chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1108-85-0078-TX-006).
Frazier HW (1993) Chlorothalonil: A response to Environmental Fate and
Ground Water Branch (EFGWG) review. Painesville, Ohio, Ricerca, Inc.
(Document No. 3254-93-0280-EF-001, submitted to WHO by ISK Biosciences
Corporation, Mentor, USA).
Fujita Y (1985) Studies on contact dermatitis from pesticides in tea
growers. Acta Med Univ Kagoshima, 27(1): 17-37.
Fullmore GE & Leveglia J (1992) A thirty-day toxicity study in dogs
with T-117-2. Painesville, Ohio, Ricerca, Inc. (Unpublished report
No. 5092-91-0554-TX003, submitted to WHO by ISK-Biotech Corporation,
Mentor, USA).
Gallagher EP, Cattley RC, & Di Giulio RT (1992) The acute toxicity and
sublethal effects of chlorothalonil in channel catfish (Ictalurus
punctatus). Chemosphere 24: 3-10.
GIFAP (1987) Guidelines for the avoidance, limitation and disposal of
pesticide waste on the farm. Brussels, International Group of National
Associations of Manufacturers of Agrochemical Products.
Gilvydis DM & Walters SM (1988) Rapid liquid chromatographic analysis
of chlorothalonil in fresh produce using photoconductivity and UV
detectors in tandem. J Agric Food Chem, 36: 957-961.
Goulding KH (1971) A preliminary investigation of a potential new
algicide. Proceedings of the 6th British Insecticides and Fungicides
Conference, pp 621-629.
Green T, Odum J, Nash JA, & Foster JR (1990) Perchloroethylene-induced
rat kidney tumours: An investigation of the mechanisms involved and
their relevance to humans. Toxicol Appl Pharmacol, 103: 77-89.
Gutenmann WH & Lisk DJ (1966) Metabolism of Daconil and Dacthal
pesticides in lactating cows. J Dairy Sci, 49(10): 1272-1276.
Hastings TF & Clifford D (1975) Eight-week dominant lethal study of
DAC-3701 in rats. Bio/Tox Research Laboratories, Inc. (Unpublished
report submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Hatzenbeler CJ (1991) An aerobic aquatic metabolism study with
14C-chlorothalonil. Painesville, Ohio, Ricerca, Inc. (Document No.
3163-90-0240-EF-001, submitted to WHO by ISK Biosciences Corporation,
Mentor, USA).
Heinonen-Tanski H, Oros G, & Kecskes M (1982) The effect of soil
pesticides on the growth of red clover rhizobia. Acta Agric Scand, 32:
283-288.
Holmes DC & Wood NF (1972) Removal of interfering substances from
vegetable extracts prior to the determination of organochlorine
pesticide residues. J Chromatogr, 67: 173-174.
Holsing G & Shott L (1970) Two-year dietary administration - rats.
Vienna, Virginia, Hazleton Laboratories, Inc. (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Holsing GC & Voelker RW (1970) 104-Week dietary administration - dogs,
Daconil 2787 (technical). Hazleton Laboratories, Inc. (Unpublished
report submitted to WHO by Biotech Corporation, Mentor, USA).
Horiuchi N & Ando S (1980) Contact dermatitis due to pesticides for
agricultural use. Jpn J Dermatol, 90: 289.
Hozan GK & Auletta CS (1981) A chronic dietary study in mice with
T-114 (DS-3701). East Millstone, New Jersey, BioDynamics, Inc.
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
IARC (1983) Chlorothalonil. In: Miscellaneous pesticides. Lyon,
International Agency for Research on Cancer, pp 319-328 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 30).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 60 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Ignatoski JA, Killenen JC, & Marciniscyn JP (1983) Distribution of
radioactivity following oral administration of 14C-chlorothalonil to
rats: extraction and analysis of 14C-materials in excreta. Mentor,
Ohio, Diamond Shamrock Corporation (Unpublished report).
IRPTC (1989) Data profile on chlorothalonil. Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
ISK Biosciences Corporation (1995) A summary of chlorothalonil
metabolism under various environmental conditions in the
chlorothalonil summary dossiers for the inclusion of active substances
in Annex 1 of Directive 91/414/EEC. Mentor, Ohio, ISK Biosciences
Corporation.
Jacobson BM & Schollenberger JM (1992) Dissipation of 4-hydroxy-2,4,5-
trichloroisophthalonitrile (SOS-3701) in soil: A re-analysis of
chlorothalonil soil dissipation data. Painesville, Ohio, Ricerca, Inc.
(Proprietary report No. 1874-T, submitted to WHO by ISK-Biotech,
Mentor, USA).
Jarrett RD, Stallard DE, & Bachand RT (1978) Absorption, excretion and
tissue distribution of orally administered 14C-hydroxy 2,5,6-
trichloroisophthalonitrile (14C-DAC 3701) in male Sprague-Dawley
rats. Painsville, Ohio, T.R. Evans Research Centre (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Johnsson M, Buhagen M, Leira HL, & Solvang S (1983) Fungicide induced
contact dermatitis. Contact Dermatitis, 9: 285-288.
Jones RE, Killeen JC Jr, & Ignatoski JA (1984) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 694-5TX-84-0064-002).
Jongen NJ, Engel R, & Leenheers LH (1991) Determination of the
pesticide chlorothalonil by HPLC and UV detection for occupational
exposure assessment in greenhouse carnation culture. J Anal Toxicol,
15: 30-34.
Katayama A, Isemura H, & Kuwatsuka S (1991) Suppression of
chlorothalonil dissipation in soil by repeated applications. J Pestic
Sci, 16: 233-238.
Kawamura Y, Takeda M, & Uchiyama M (1978) Photolysis of chlorothalonil
in benzene. J Pestic Sci, 3: 397-400.
Kenyon RG & Ballee DL (1990) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1989). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-007).
Kenyon RG & Wiedmann JL (1992a) Analytical methods including recovery
rates and the limits of determination for residues in water. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 5221-92-0072-MD-001).
Kenyon RG & Wiedmann JL (1992b) Analytical methods including recovery
rates and the limits of determination for residues in soil. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 5221-92-0073-MD-001).
King C, Prince PM, & Ballee DL (1991) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1990). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-008).
King C, Prince PM, & Ballee DL (1992) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1991). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-010).
Koseki M, Shimamura Y, Maki T, Tamura Y, & Naoi Y (1980) Residues of
TPN (tetrachloroisophthalonitrile), captan, diazinon and
chinomethionate in vegetables and fruits. Tokyo Toriotu Eisei
Kenkyusho Kenkyu Nempo, 31:(1) 170-173.
Krawchuk BP & Webster GRB (1987) Movement of pesticides to ground
water in an irrigated soil. Water Pollut Res J Can, 22(1): 129-145.
Kunkel JF (1967a) Absence of 14C movement in crop plant organs after
topical application and soil amendment studies with isotopic Daconil
2787. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished report).
Kunkel JF (1967b Movement of 14C in or on roots of crop species grown
in soil amended with isotopic Daconil 2787. Mentor, Ohio, Diamond
Shamrock Corporation (Unpublished report).
Ladd K, Jenkins DH, & Kepplinger ML (1971) Meat and milk residues
study with technical Daconil 2787 - 97% and pure DAC-3701 in dairy
cattle. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Lam A & Lewis GC (1983) Chemical control of foliar diseases of
perennial rye grass (Lolium perenne L.) and their effects on yield
and quality of the crop. Crop Prot, 2(1): 75-83.
Lawless EW, Ferguson TL, & Meiners AF (1975) Guidelines for the
disposal of small quantities of unused pesticides. Cincinnati, Ohio,
US Environmental Protection Agency (EPA 670/2-75-057).
Lee SS, Marciniscyn JP, Marks AF, & Ignatoski JA (1982) Balance study
of the distribution of radioactivity following oral administration of
14C-chlorothalonil (14C-DS 2787) to rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 000-4AM-81-0209-001).
Legator MS (1974a) Mutagenic testing with DAC 2787. Providence, Rhode
Island, Roger Williams General Hospital, Division of Genetics and
Brown University, Division of Biological and Medical Sciences
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
Legator MS (1974b) Mutagenic testing with DAC 3701. Mentor, Ohio,
Diamond Shamrock Corporation (Unpublished report).
Leonard JA & Yeary RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51(11): 605-609.
Lewis RG, Bond AE, Johnson DE, & Hsu JP (1988) Measurement of
atmospheric concentrations of common household pesticides : a pilot
study. Environ Monit Assess, 10: 59-73.
Liden C (1990) Facial dermatitis caused by chlorothalonil in paint.
Contact Dermatitis, 22: 206-211.
Lucas F & Benz G (1990) A two generation reproduction study in rats
with technical chlorothalonil. Painesville, Ohio, Ricerda, Inc.
(Proprietary report No. 1722-87-0121-TX-003, submitted to WHO by
ISK-Biotech, Mentor, USA).
Luperi L & Forster R (1988) Delayed contact hypersensitivity study in
the Guinea pig. Rome, Life Science Research, Toxicology Centre S.P.A.
(Report No. 128005-ST-02687, submitted to WHO by ISK-Biotech, Mentor,
USA).
McGee (1983) A two-year toxicity and tumourigenicity study of T-114 in
rats. Mattawan, Michigan, International Research and Development
Corporation (Unpublished report submitted to WHO by Diamond Shamrock
Corporation, Mentor, USA).
McLeod HA, Smith DC, & Bluman N (1980) Pesticide residues in the total
diet in Canada, V: 1976 to 1978. J Food Saf, 2: 141-164.
Magee TA, Marciniszyn JP, & Killeen JC Jr (1990) Study to evaluate the
urinary metabolites of chlorothalonil following dermal application to
male Rhesus monkeys. Painesville, Ohio, Ricerca, Inc. (Report No.
3382-89-02-AM-001, submitted to WHO by ISK-Biotech, Mentor, USA).
Major D, Killeen JC, & Ignatoski JA (1982) Primary eye irritation
study in albino rabbits with technical chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 104-5TX-80-0037-002).
Marciniszyn JP, Savides MC, Killeen JC, & Ignatoski JA (1984a) Study
of the dermal absorption of 14C-chlorothalonil (14C-DS-2787) by male
rats. Mentor, Ohio, Fermenta ASC (Unpublished report No.
649-4AM-84-0010-001).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1984b) Study of the
distribution of radioactivity following oral administration of
(14C-DS-2787) to male Sprague Dawley rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 631-4AM-83-0011-002).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1985a) Study of the
distribution of radioactivity following oral administration of
(14C-SDS-2787) to female Sprague Dawley rats. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 631-4AM-84-0078-002).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1985b) Identification of
metabolites in urine and blood following oral administration of
14C-chlorothalonil (14C-SDS-2787) to male rats: the thiol metabolites
in urine. Mentor, Ohio, Fermenta ASC (Unpublished report No.
621-4AM-83-0061-001).
Marciniszyn JP, Savides MC, Killeen JC, & Ignatoski JA (1986) Study of
the biliary excretion of radioactivity following oral administration
of 14C-SDS-2787 to male Sprague-Dawley rats. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 633-4AM-85-0012-002).
Marciniszyn JP, Killeen JC, & Baxter RA (1988) Pilot study of the
effect of the gamma-glutamyl transpeptidase inhibitor AT-125 on the
metabolism of 14C-chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1376-86-0072-AM-002).
Markus JR & Puma B ed. (1973) Pesticide analytical manual: Methods for
individual pesticide residues: Volume 2 - Pesticide registration
section 120.275. Washington, DC, US Food and Drug Administration.
Matsushita T & Aoyama K (1981) Cross reactions between some pesticides
and the fungicide benomyl in contact allergy. Ind Health, 19: 77-82.
Mayer FL ed. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Gulf Breeze, Florida. Washington, DC, US Environmental
Protection Agency (EPA-600/8-017; NTIS PB 87-188686).
Meding B (1986) Contact dermatitis from tetrachloroisopathalonitrile
in paint. Contact Dermatitis, 15: 187.
Mizens M, Wilson NH, Laveglia J, Killeen JC, & Ignatoski JA (1983) A
teratology study in rats with technical chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 517-5TX-82-0011-003).
Mizens M, Killeen JC, Claudio G, & Ignatoski JA (1985a) In vivo bone
marrow chromosomal aberration assay in mice with a single dose of
technical chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 625-5TX-83-0029-002).
Mizens M, Killeen JC, Claudio G, & Ignatoski JA (1985b) In vivo bone
marrow aberration assay in rats with a single dose of technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 625-5TX-83-0028-002).
Mizens M, Killeen JC, & Ignatoski JA (1985c) Acute and subchronic in
vivo bone marrow chromosomal aberration assay in Chinese hamsters
with technical chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 625-5TX-83-0014-003).
Mizens M, Killeen JC Jr, & Ignatoski JA (1985d) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with 2,5-dichloro-4,6-bismercaptoisoph-
thalonitrile (SDS-3939) (Study No. T4147.501 performed by
Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
SDS Biotech Corporation (Unpublished report No. 694-5TX-85-0042-002,
submitted to WHO by Fermenta Plant Protection Co., Painesville, USA).
Mizens M, Killeen JC Jr, & Ignatoski JA (1985e) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test)
with and without renal activation with 5-(2,4-dicyano-3,5,6-
trichlorophenyl) Glutathione (SDS-66382) (Study No. T4147.501
performed by Microbiological Associates Inc., Bethesda, USA).
Painesville, Ohio, SDS Biotech Corporation (Unpublished report
No. 694-5TX-85-0043-002, submitted to WHO by Fermenta Plant
Protection Co., Painesville, USA).
Mizens M, Killeen JC, & Ignatoski JA (1986a) In vitro chromosomal
aberration assay in Chinese hamster ovary (CHO) cells with technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1109-85-0082-TX-002).
Mizens M, Killeen JC Jr, & Baxter RA (1986b) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with 5-chloro-2,4,6-
trimercaptoisophthalonitrile (SDS-66471) (Study No. T5079.1505
performed by Microbiological Associates Inc., Bethesda, USA).
Painesville, Ohio, Ricerca, Inc., Department of Toxicology and Animal
Metabolism (Unpublished report No. 1097-86-0037-TX-002, submitted to
WHO by Fermenta Plant Protection Co., Painesville, USA).
Mizens M, Killeen JC Jr, & Baxter RA (1986c) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with S,S',S''--(2,4-dicyano-6-
dichlorophenyl)tricysteine (SDS-66473) (Study No. T5081.1505 performed
by Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
Ricerca, Inc., Department of Toxicology and Animal Metabolism
(Unpublished report No. 1097-86-0039-TX-002, submitted to WHO by
Fermenta Plant Protection Co., Painesville, USA.)
Mizens M, Killeen JC Jr, & Baxter RA (1987) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with S,S'(2,4-dicyano-3,6-dichlorophenyl)-
dicysteine (SDS-66474) (Study No. T5080.1505 performed by
Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
Ricerca, Inc., Department of Toxicology and Animal Metabolism
(Unpublished report No. 1097-86-0038-TX-002, submitted to WHO by
Fermenta Plant Protection Co., Painesville, USA).
Modesso P & Forster R (1988) Chromosome aberrations in human
lymphocytes cultured in vitro. Test substance: Chlorothalonil
technical. Rome, Life Science Research, Toxicology Centre S.P.A.
(Report No. 128008-M-10787, submitted to WHO by ISK-Biotech, Mentor,
USA).
Murchison TE (1979) A short-term dietary study in rats with T-114-2
(DS 3701). Dawson Research Corporation (Unpublished report submitted
to WHO by Diamond Shamrock Corporation, Mentor, USA).
O'Neill HJ (1991) Transport of the fungicide chlorothalonil from its
operational use on a pond ecosystem. Bull Environ Contam Toxicol,
46:822-828.
Paynter OE (1965a) Oral dose range: dogs - Final report. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE (1965b) Repeated dermal application: Rabbits. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE (1966a) Two-year dietary administration: dogs DAC 2787.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-66-0002-
001).
Paynter OE (1966b) Reproduction rabbits DAC 2787. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 000-5TX-66-003-001).
Paynter OE (1967a) Final report - Two-year dietary feeding: rats.
Hazleton Laboratories, Inc. (Unpublished report submitted to WHO by
Diamond Shamrock Corporation, Mentor, USA).
Paynter OE (1967b) Three-generation reproduction study: rats DAC 2787.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-67-0005-
001). USA.
Paynter OE (1967c) Ten-week amino acid feeding study: rats. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE & Busey WM (1966) Two-year dietary administration: dogs
DAC 2787. Hazelton Laboratories, Inc. (Unpublished report submitted to
WHO by Biotech Corporation, Mentor, USA).
Paynter OE & Busey W (1967) Long-term (76 weeks) feeding study: rats
DAC 2787. Hazleton Laboratories, Inc. (Unpublished report submitted to
WHO by Diamond Shamrock Corporation, Mentor, USA).
Paynter OE & Crews L (1967) Final report - Two-year dietary feeding:
rats. Hazleton Laboratories, Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Paynter OE & Murphy JC (1967) 16-Week dietary administration: dogs
DAC 2787. Hazelton Laboratories, Inc. (Unpublished report submitted to
WHO by Biotech Corporation, Mentor, USA).
Powers M (1965) Acute oral administration: rats. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Price P (1978a) The activity of compound DTX-77-0037 in the Fischer
rat embryo transformation assay system. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Price P (1978b) The activity of compound DTX-77-0041 in the Fischer
rat embryo transformation assay system. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Reduker S, Uchrin CG, & Winnett G (1988) Characteristics of the
sorption of chlorothalonil and azinphos-methyl to a soil from a
commercial cranberry bog. Bull Environ Contam Toxicol, 41: 633-641.
Ribovich ML, Pollock GA, Marciniszyn JP, Killeen JC, & Ignatoski JA
(1983) Balance study of the distribution of radioactivity following
oral administration of 14C-chlorothalonil (14C-DS-2787) to male mice.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 613-4AM-82-0178-
001).
Roark JH & Dale JL (1979) The effect of turf fungicides on earthworms.
Arkansas Acad Sci Proc, 33: 71-74.
Rosanoff KA & Siegel MR (1981) Mechanism of action and fate of the
fungicide chlorothalonil in biological systems. 3. Interaction with
mammalian DNA, histones and isolated rat liver nuclei. Pestic Biochem
Physiol, 16: 120-128.
Sadler EM, Wilson NH, Killeen JC, & Ignatoski JA (1985) Time course of
the acute effect of technical chlorothalonil on hepatic and renal
glutathione content in male rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 751-5TX-85-0032-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1985)
Isolation and identification of metabolites in the bile of rats orally
administered 14C-chlorothalonil I. Synthesis and characterisation of
glutathione conjugates of chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 633-4AM-84-0104-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986a) Study
of the distribution of radioactivity following repeated oral
administration of 14C-chlorothalonil (14C-SDS-2787) to male
Sprague-Dawley rats. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1173-84-0079-AM-003).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986b)
Identification of metabolites in urine and blood following oral
administration of 14C-chlorothalonil to male rats. II. Effects of
multiple dose administration on the excretion of thiol metabolites in
urine. Mentor, Ohio, Fermenta ASC (Unpublished report No. 621-4AM-83-
0061-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986c) Study
of the biliary excretion of radioactivity following oral
administration of (14C-SDS-2787) to male Sprague-Dawley rats. Mentor,
Ohio, Fermenta ASC (Unpublished report No. 633-4AM-85-0012-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986d) Animal
metabolism method development studies. II. In vitro incubations of
14C-chlorothalonil with stomach and intestinal mucosal cells. Mentor,
Ohio, Fermenta ASC (Unpublished report No. 1172-85-0081-AM-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986e) In
vitro studies on the transfer of 14C-chlorothalonil and/or its
metabolites from the mucosal to the serosal surface of the
gastrointestinal tract. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1179-86-0020-AM-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986f) Pilot
study to determine the concentration of radiolabel in kidneys
following oral administration of the mono-glutathione conjugate of
14C-chlorothalonil to male rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 631-4AM-85-0064-001).
Savides MC, Marciniszyn JP, Killeen JC, & Baxter RA (1987a) Study to
determine the metabolic pathway for chlorothalonil following dermal
application to rats. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1625-87-0057-AM-000).
Savides MC, Marciniszyn JP, Killeen JC, & Baxter RA (1987b)
Determination of the covalent binding of radiolabel to DNA in the
kidneys of male rats administered 14C-chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1173-86-0096-AM-002).
Savides MC, Kidon BJ, Marciniszyn JP, Killeen JC, & Baxter RA (1987c)
Subcellular fractionation of kidneys from male rats administered
14C-chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1178-86-0016-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1988) A study to evaluate
the effects of sulphur-containing analogs of chlorothalonil on
mitochondrial function. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1479-87-0037-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1989) Study to compare the
metabolism of chlorothalonil in dogs with its metabolism in rats
following oral administration of 14C-chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1626-88-0008-AM-001).
Savides MC, Magee TA, Marciniszyn JP, Killeen JC, & Baxter RA (1990a)
Study to evaluate the metabolic pathway of chlorothalonil
(14C-ASC-2787) in germ-free rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 3060-88-0219-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1990b) Study of the urinary
excretion of radiolabel by catheterised dogs following oral
administration of 14C-chlorothalonil by gavage. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 3086-89-0041-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1990c) Study to evaluate
urinary metabolites of chlorothalonil from male Rhesus monkeys.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 3349-89-0179-AM-
001).
SDS Biotech Corporation (1972) Exposure of fish to 14C-labelled
DAC-2787-Tech.: Accumulation, distribution and elimination of
residues. Mentor, Ohio, ISK-Biotech (Proprietary report No. 000-5TX-
72-0006/8-001).
SDS Biotech Corporation (1979) Acute toxicity of DS-3701 to Bluegill
sunfish. Mentor, Ohio, ISK-Biotech (Proprietary report No. 102-5TX-79-
0044-002).
SDS Biotech Corporation (1980a) Technical chlorothalonil - Acute
toxicity (LC50) study in Rainbow trout. Mentor, Ohio, ISK-Biotech
(Proprietary report No. 102-5TX-79-0045-002).
SDS Biotech Corporation (1980b) Technical chlorothalonil - Acute
toxicity (LC50) study in Channel catfish. Mentor, Ohio, ISK-Biotech
(Proprietary report No. 102-5TX-79-0046-002).
SDS Biotech Corporation (1981a) Dietary study (LC50) in Mallard ducks
with DS-3701. Mentor, Ohio, ISK-Biotech (Proprietary report No.
449-5TX-81-0008-002).
SDS Biotech Corporation (1981b) Dietary study (LC50) in Bobwhite
quail with DS-3701. Mentor, Ohio, ISK-Biotech (Proprietary report
No. 448-5TX-81-0007-002).
SDS Biotech Corporation (1982a) Static, acute toxicity study in
Penaeid (pink) shrimp with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0054-002).
SDS Biotech Corporation (1982b) Static, acute toxicity study in
Sheepshead Minnows with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0053-002).
SDS Biotech Corporation (1983a) The effects of cooking 2,4,5,6-
tetrachloroisoph-thalonitrile (chlorothalonil, DS-2787) with
vegetables. Mentor, Ohio, ISK-Biotech (Unpublished report No.
372-3EF-83-0004-001, submitted to WHO by SDS Biotech Corporation,
Mentor, USA).
SDS Biotech Corporation (1983b) Flow-through, acute oyster shell
deposition study with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0133-003).
Shirasu Y & Teramoto S (1975) Teratogenicity study of Daconil in
rabbits. Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-
75-2077-001).
Shirasu Y, Moriya M, & Watanabe K (1977) Mutagenicity testing on
Daconil in microbial systems. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 00-5TX-61-0002-001).
Shults SK, Killeen JC, & Heilman RD (1980) A chronic study in the
fathead minnow (Pimephales promelas) with technical chlorothalonil.
Cleveland, Ohio, Diamond Shamrock Corporation, 16 pp (Proprietary
report No. 090-5TX-79-0049-002, submitted to WHO by ISK-Biotech,
Mentor, USA.
Shults SK, Killeen JC, & Ignatoski JA (1982) Chronic toxicity study in
Daphnia magna with technical chlorothalonil. Cleveland, Ohio,
Diamond Shamrock Corporation, 25 pp (Proprietary report No.
447-5TX-81-0006-002, submitted to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Laveglia J, Killeen JC, & Ignatoski JA (1983) A 90-day
feeding study in mice with technical chlorothalonil. Mentor, Ohio, SDS
Biotech Corporation (Unpublished report No. 618-5TX-83-0007-004).
Shults SK, Wilson NH, Killeen JC, & Ignatoski JA (1986) 21-Day
repeated dose dermal toxicity study in albino rabbits with technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report No.
754-5TX-85-0023-007).
Shults SK, Wilson NH, & Killeen JC (1988a) Reproduction study in
bobwhite quail with technical chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 1469-87-0006-TX-002, submitted
to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Wilson NH, & Killeen JC (1988b) Reproduction study in
mallard ducks with technical chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 1469-87-0004-TX-002, submitted
to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Brock AW, & Killeen JC (1991) Acute (one-hour) inhalation
toxicity (LC50) study in rats with hammer milled technical
chlorothalonil. Painesville, Ohio, Ricerca, Inc. (Proprietary report
No. 3593-90-0168-TX-002, submitted to WHO by ISK-Biotech, Mentor,
USA).
Shults SK, Brock AW, & Laveglia J (1993) Acute (four-hour) inhalation
toxicity (LC50) study in rats with hammer milled technical
chlorothalonil (T-117-15). Painesville, Ohio, Ricerca, Inc.
(Proprietary report No. 5290-92-0160-TX-002, submitted to WHO by
ISK-Biotech, Mentor, USA).
Siou G (1981a) The micronucleus test in the rat, mouse and hamster
using chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 000-5TX-81-0024-004).
Siou G (1981b) The chromosomal aberration test in the rat, mouse and
hamster using chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 000-5TX-81-0025-004).
Smiley RW & Craven MM (1979) Microflora of turfgrass treated with
fungicides. Soil Biol Biochem, 11: 349-353.
Spencer JR, Bissell SR, Sanborn JR, Schneider FA, Margetich SS, &
Krieger RI (1991) Chlorothalonil exposure of workers on mechanical
tomato harvesters. Toxicol Lett, 55: 99-107.
Spindeldreier A & Deichmann B (1980) [Contact dermatitis against a
wood preservative with a new fungicidal agent.] Dermatosen, 28(3):
88-90 (in German).
Stallard DE & Wolfe AL (1967) The fate of 2,4,5,6-
tetrachloroisophthalonitrile (Daconil 2787) in soil. Mentor, Ohio,
Diamond Shamrock Corporation (Unpublished report).
Stallard DE & Szalkowski ME (1976) Effect of microorganisms upon the
soil metabolism of Daconil (i.e., chlorothalonil) and 4-hydroxy-2,5,6-
trichloroisophthalonitrile. Painesville, Ohio, Ricerca, Inc. (Report
No. 1000-3EF-76-2087-001, submitted to WHO by ISK Biosciences
Corporation, Mentor, USA).
Stamper JH, Nigg HN, Mahon WD, Nielsen AP, & Royer MD (1989a)
Applicator exposure to fluvalinate, chlorpyrifos, captan, and
chlorothalonil in Florida ornamentals. J Agric Food Chem, 37: 240-244.
Stamper JH, Nigg HN, Mahon WD, Nielsen AP, & Royer MD (1989b)
Pesticide exposure to a greenhouse drencher. Bull Environ Contam
Toxicol, 42: 209-217.
Stephenson GR, Phatak SC, Makowski RI, & Bouw WJ (1980) Phytotoxic
interactions involving metribuzin and other pesticides in tomatoes.
Can J Plant Sci, 60: 167-175.
Stoffella PJ & Sonoda RM (1982) Reduction of onion yields by
chlorothalonil. Hortscience, 17(4): 628-629.
Suzuki S & Oda M (1977) Simultaneous determination of chlorothalonil,
captan and diazinon in apples. Bull Agric Chem Insp Stn (Tokyo),
17:43-45.
Szalkowski MB & Stallard DE (1977) Effect of pH on the hydrolysis of
chlorothalonil. J Agric Food Chem, 25(1): 208-210.
Szalkowski MB, Stallard DE, & Ignatoski JA (1980) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon the
microbial utilization of selected macromolecules in soil. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 326-3EI-79-0009-001).
Szalkowski MB, Stallard DE, & Ignatoski JA (1981a) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon
non-symbiotic nitrogen fixing soil microorganisms. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 327-3EI-79-0010-001).
Szalkowski MB, Stallard DE, & Ignatoski JA (1981b) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon nitrogen
transformation in soil. Mentor, Ohio, ISK-Biotech (Proprietary report
No. 328-3EI-79-0011-001).
Teeters W (1966) In vivo assay of spasmodic activity, mice, DAC
2787. Hazelton Laboratories (Unpublished report submitted to WHO by
Diamond Shamrock Corporation, Mentor, USA).
Tillman RW, Siegel MR, & Long JW (1973) Mechanism of action and fate
of the fungicide chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile)
in biological systems I. Reactions with cells and subcellular
components of Saccharomyces pastorianus. Pestic Biochem Physiol,
3:160-167.
Tu CM (1980) Effect of fungicides on growth of Rhizobium japonicum
in vitro. Bull Environ Contam Toxicol, 25: 364-368.
UK (1985) Annex 1 - Monitoring of fruits and vegetables (at point of
retail sale) 1981-1983. Annex 2 - Chlorothalonil, soft fruit sampled
directly from growers 1982; soft fruit sampled at retail outlets, 1982
(no treatment histories). Submission to FAO by the UK Ministry of
Agriculture, June 14, 1985.
UK-MAFF & HSE (1990) Report of the working party on pesticide
residues 1988-1989. London, Ministry of Agriculture, Fisheries and
Food and Health and Safety Executive.
US EPA (1976) Manual of chemical methods for pesticides and devices.
Washington DC, US environmental Protection Agency, Office of Pesticide
Programs (Chlorothalonil EPA-1).
US EPA (1990) National survey of pesticides in drinking water wells.
Washington, DC, US Environmental Protection Agency (EPA 570/9-90-015).
US NCI (1978) Bioassay of chlorothalonil for possible carcinogenicity.
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
78-841).
Walker WW, Cripe CR, Pritchard PH, & Bourquin AW (1984) Biological and
abiotic degradation of xenobiotic compounds in in vitro estuarine
water and sediment/water systems. Chemosphere, 17(12): 2255-2270.
Wallace LA (1991) Comparison of risks from outdoor and indoor exposure
to toxic chemicals. Environ Health Perspect, 95: 7-13.
Wazeter FX (1971) Acute oral LD50 in male albino rats. International
Research and Development Corporation (Unpublished report submitted to
WHO by Diamond Shamrock Corporation, Mentor, USA).
Wazeter FX & Goldenthal EI (1976) Teratology study in rabbits.
International Research and Development Corporation (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, Ohio, USA).
Wei CI (1982) Lack of mutagenicity of the fungicide 2,4,5,6-
tetrachloroisophthalonitrile in the Ames Salmonella microsome test.
Appl Environ Microbiol, 43(1): 252-254.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997. Geneva, World Health
Organization. (WHO/PCS/96.3).
Wilson NH (1981) A 90-day toxicity study of technical chlorothalonil
in rats. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Wilson NH, Killeen JC, & Ignatoski JA (1982) Dermal sensitization
study in Hartley-derived guinea-pigs with technical chlorothalonil.
Painesville, Ohio, Ricerca, Inc. (Report No. 394-5TX-81-0132-002,
submitted to WHO by ISK Biosciences Corporation, Mentor, USA).
Wilson NH, Laveglia J, Killeen JC, & Ignatoski JA (1983a) A subchronic
toxicity study of technical chlorothalonil in rats. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 562-5TX-81-0213-004).
Wilson NH, Killeen JC, & Ignatoski JA (1983b) A chronic dietary study
in mice with technical chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 108-5TX-79-0102-004).
Wilson NH, Killeen JC, & Ignatoski JA (1985a) Histopathological
re-evaluation of renal tissue from a sub-chronic toxicity study of
technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 753-5TX-85-0056-002).
Wilson NH, Killeen JC, & Ignatoski JA (1985b) A tumorigenicity study
of technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 099-5TX-80-0234-008).
Wilson NH, Killeen JC, & Ignatoski JA (1985c) Histopathologic
re-evaluation of renal tissue from a 90-day toxicity study of
technical chlorothalonil in rats (5TX-80-0200). Mentor, Ohio, SDS
Biotech (Unpublished report No. 753-5TX-85-0055-002).
Wilson NH, Killeen JC, & Ignatoski JA (1986a) Histopathological
re-evaluation of renal tissue from a rat tumorigenicity study with
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report No.
764-5TX-85-0071-002).
Wilson NH, Killeen JC, & Ignatoski JA (1986b) Histopathological
re-evaluation on renal tissue from a mouse tumorigenicity study with
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report).
Wilson NH, Killeen JC, & Baxter RA (1987) A tumorigenicity study of
technical chlorothalonil in male mice - final report. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1099-84-0077-TX-006).
Wilson NH, Killeen JC, & Baxter RA (1988) A teratology study in
rabbits with technical chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1544-87-0060-TX-002).
Wilson NH, Killeen JC, & Baxter RA (1989a) A tumorigenicity study of
technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1102-84-0103-TX-007).
Wilson NH, Chun JS, Killeen JC, & Baxter RA (1989b) Reproduction
dose-range finding study in rats with technical chlorothalonil.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 1722-87-0120-TX-
001).
Wilson NH, Killeen JC Jr, Ford WH, Siou G, Busey WM, & Eilrich GL
(1990) A 90-day study in rats with the monoglutathione conjugate of
chlorothalonil. Toxicol Lett, 53: 155-156.
Wolfe AL & Stallard DE (1968) The fate of DAC-3701 (4-hydroxy-2,5,6,-
trichloroisophthalonitrile) in soil. Mentor, Ohio, Diamond Shamrock
Corporation (Unpublished report).
Wolfe AL & Stallard DE (1971) Supplementary milk and meat study.
Mentor, Ohio, Diamond Shamrock Corporation (Unpublished report).
Worthing CR ed. (1991) The pesticide manual: A world compendium, 9th
ed. Farnham, British Crop Protection Council.
Wüthrich V (1990) Acute-toxicity (LC50) study of Daconil 2787 extra
to earthworms (Project No. 282971). Itingen, Switzerland, RCC
Umweltchemie A.G. (Proprietary report submitted to WHO by ISK Biotech,
Mentor, USA).
Yoshikawa H & Kawai K (1966) Toxicity of phthalonitrile and
tetrachlorophthalodinitrile: I. Acute toxicity in mice. Ind Health,
4:11-15.
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le chlorothalonil est un solide cristallin incolore et inodore,
dont le point de fusion est de 250°C et la tension de vapeur de 7,63 ×
10-5 Pa (5,72 × 10-7 mmHg) à 25°C. Il est peu soluble dans l'eau
(0,6-1,2 mg/litre à 25°C) et son coefficient de partage entre
l'octanol et l'eau (log Kow) est de 2,882. Dans l'eau, il
s'hydrolyse lentement à pH 9 mais il est stable à pH < 7 (à 25°C).
La méthode d'analyse la plus courante, après extraction et
purification, est la chromatographie gaz-liquide avec détection par
capture d'électrons.
2. Sources d'exposition humaine et environnementale
Le chlorothalonil est produit depuis 1969 à des fins
commerciales, soit par chloration de l'isophtalonitrile, soit en
traitant le tétrachlorisophtaloylamide par l'oxychlorure de phosphore.
C'est un fongicide à large spectre utilisé non seulement en
agriculture, mais aussi pour traiter le gazon et les plantes
ornementales. On l'utilise pour protéger les fruits à pépins et à
noyaux, les agrumes, les groseilles, les baies, les bananes, les
tomates, les légumes verts, le café, les arachides, les pommes de
terre, les oignons et les céréales. En outre, il entre dans la
composition de certains produits pour la conservation du bois et de
certaines peintures.
Il existe en trois formulations principales, un concentré pour
suspensions, des granulés dispersables dans l'eau et une poudre
mouillable.Ces produits sont facilement dilués dans l'eau et épandus
par pulvérisations au sol ou aériennes. La dose d'emploi est
habituellement de 1,2 à 2,5 kg de matière active par hectare pour le
traitement des haricots, des céleris et des oignons. L'exposition
humaine a lieu principalement pendant la préparation et l'épandage du
produit ou lors de l'ingestion de résidus présents dans certaines
denrées alimentaires (voir section 1.1.4).
3. Transport, distribution et transformation dans l'environnement
Le chlorothalonil s'élimine des milieux aqueux par une forte
adsorption sur les particules en suspension. La modélisation des
données disponibles montre qu'il ne migre pratiquement pas vers les
sédiments du fond. Il est possible qu'il subisse une biodégradation
enzymatique dans les eaux naturelles. Dans le sol, il est rapidement
dégradé, cette dégradation pouvant également avoir lieu dans l'eau
avec production du métabolite hydroxylé en position 4, c'est-à-dire le
4-hydroxy-2,5,6-trichlorisophtalonitrile. La demi-vie de dissipation
du métabolite 4-hydroxy dans le sol est comprise entre 6 et 43 jours.
Une fois entré en contact avec une plante, le chlorothalonil ne
migre pas vers d'autres zones du végétal. Les végétaux ne le
métabolisent que dans une proportion limitée et le métabolite
4-hydroxy constitue en général moins de 5% du résidu.
Chez les poissons, la métabolisation du chlorothalonil comporte
une conjugaison avec le glutathion, qui aboutit à des produits
d'excrétion plus polaires. Elle s'effectue sous l'action de la
glutathion- S-transférase. L'excrétion du composé sous forme de
conjugué avec le glutathion est corroborée par le fait que, chez la
truite arc-en-ciel, on trouve une forte concentration de marqueur dans
la vésicule biliaire et dans la bile après exposition à du
14C-chlorothalonil.Une fois les poissons replacés en eau propre, on a
constaté une chute rapide de la concentration du marqueur qui s'était
accumulé dans la vésicule biliaire et les autres organes.
Le chlorothalonil ne s'accumule pas chez les organismes
aquatiques.
4. Concentrations dans l'environnement et exposition humaine
Lors d'une étude sur des cultures de pommes de terre, on a
pulvérisé du chlorothalonil sur un petit cours d'eau. Après
prélèvement et analyse de l'eau en aval du secteur traité, on a
constaté que le composé disparaissait rapidement (par ex. la
concentration passait de 450 µg/litre 30 minutes après le traitement à
2-6 µg/litre 12 h après le traitement). Les traitements de routine
effectués sur des cultures irriguées de plein champ, comme les pommes
de terre ou l'orge, n'ont donné lieu qu'à de faibles concentrations de
chlorothalonil (0,04-3,6 µg/litre), comme l'ont montré un certain
nombre d'analyses pratiquées sur de l'eau prélevée à quelques
occcasions dans des drains en grès vernissé.
Les résidus qui subsistent sur les récoltes sont essentiellement
constitués par le chlorothalonil lui-même. Leur concentration est
fonction de la dose d'emploi, du temps écoulé depuis le dernier
épandage et la dernière récolte ainsi que du type de culture. A
partir des nombreux essais effectués sous contrôle un peu partout dans
le monde et dont les résultats ont été communiqués à la FAO et à
l'OMS, il est possible de déterminer les concentrations de résidus
présentes au moment de la récolte. En ce qui concerne les produits
laitiers, il est vraisemblable que les résidus sont soit
indétectables, soit très faibles. Dans le lait de vaches laitières
qui avaient reçu pendant 30 jours du chlorothalonil mêlé à leur
nourriture (jusqu'à 250 mg/kg), on n'a pas trouvé trace du composé,
mais celui-ci était présent dans les tissus à très faible
concentration.
Des analyses pratiquées dans plusieurs pays, soit sur la ration
totale, soit sur tel ou tel alliment, ont montré, à l'occasions
d'enquêtes par sondage, que le chlorothalonil n'était présent qu'en
quantités indétectables ou du moins très faibles. Les diverses
préparations que subissent les produits alimentaires, comme le pelage,
le lavage et la cuisine en général contribuent d'ailleurs à abaisser
encore leur teneur en résidus.
5. Cinétique et métabolisme chez les animaux de laboratoire
Chez des rats qui en recevaient par voie orale des doses allant
jusqu'à 50 mg/kg de poids corporel, le composé a été absorbé à hauteur
d'environ 30% en l'espace de 48 h. A plus forte dose, l'absorption
est moindre, ce qui est le signe d'un processus de saturation. Après
administration de 14C-chlorothalonil par voie orale, on a observé une
répartition tissulaire et sanguine de la radioactivité en 2 h. C'est
au niveau des reins, du foie et du sang - dans cet ordre - qu'ont été
relevées les concentrations les plus imporantes. Au bout de 24 h, on
a mesuré, au niveau des reins, une concentration égale à 0.3% d'une
dose initiale de 5 mg/kg de poids corporel.
La majeure partie d'une dose administrée par voie orale à des
rats a été retrouvée dans leurs matières fécales (> 82% en l'espace
de 48-72 h, quelle que soit la dose initiale). L'excrétion biliaire
est rapide, culminant au bout de 2 h après ingestion d'une dose de 5
mg/kg de poids corporel et la saturation est atteinte à partir de 50
mg/kg de poids corporel. Chez le rat, la dose est excrétée à hauteur
de 5-10% par la voie urinaire. Chez le chien et le singe, la
prncipale voie d'excrétion est la voie fécale, la voie urinaire étant
moins importante que chez le rat (< 4%).
Les études métaboliques menées sur des rats montrent que le
chlorothalonil est conjugué avec le glutathion dans le foie ainsi que
dans les voies digestives. Certains de ces conjugués peuvent être
absorbés dans l'intestin et parvenir jusqu'aux reins où ils sont
transformés par la ß-lyase du cytosol en analogues thioliques,
excrétés ensuite par la voie urinaire. Lorsqu'on administre du
chlorothalonil à des rats axéniques, les métabolites thioliques
apparaissent dans l'urine en quantité bien moindre que chez des rats
normaux, ce qui indique que la flore intestinale intervient dans le
métabolisme de ce composé. Des chiens et des singes à qui on
administre du chlorothalonil par voie orale, n'excrètent que peu ou
pas de métabolites thioliques dans leurs urines.
Après application cutanée de 14C-chlorothalonil à des rats,
environ 28% de la dose ont été absorbés en 120 h. On a retrouvé
environ 18% de la dose dans les matières fécales et 6% dans les urines
au bout de 120 h.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Chez le rat et le lapin, la toxicité aiguë du chlorothalonil est
faible, que ce soit par voie orale ou en applications cutanée (DL50
> 10 000 mg/kg de poids corporel). Du chlorothalonil broyé au
mortier (D médian des particules égal à 5-8 µm) s'est révélé très
toxique pour des rats lors d'une étude toxicologique par inhalation,
avec une CL50 à 4 h de 0,1 mg/litre.
Le chlorothalonil est irritant pour la peau et les yeux chez le
lapin. Les études de sensibilisation cutanée effectuées sur des
cobayes n'ont pas été concluantes.
Chez le rat, les principaux effets de doses orales répétées
s'exercent au niveau des reins et de l'estomac.Pendant 13 semaines, on
fait ingérer quotidiennement à des rats, répartis en groupes de 25
animaux de chaque sexe, du chlorothalonil mêlé à leur nourriture aux
doses de 0, 1,5, 3, 10, ou 40 mg/kg de poids corporel. Après une
période de récupération de 13 semaines, les rats ont été sacrifiés et
l'on a observé une hyperplasie et une hyperkératose au niveau de la
portion cardiaque de l'estomac aux doses de 10 et 40 mg/kg; ces
lésions ont regressé lorsque le traitement a cessé. A la dose de 40
mg/kg, on notait chez les mâles une augmentation de l'incidence des
hyperplasies épithéliales au niveau des tubules proximaux du rein au
bout des 13 semaines de traitement ainsi qu'après la période de
récupération. La dose sans effet observable a été évaluée à 3 mg/kg
de poids corporel par jour, le critère retenu étant l'absence de
lésions au niveau de la portion cardiaque de l'estomac. Les lésions
interessant cette partie de l'eatomac ainsi que les reins sont
apparues rapidement, à savoir en 4 à 7 jours chez les mâles lorsqu'on
a porté la dose alimentaire quotidienne à 175 mg/kg de poids corporel.
Lors d'une étude de 13 semaines sur des souris (0, 7,5, 15, 50,
275, ou 750 mg/kg en mélange à la nourriture), on a constaté une
incidence accrue des hyperplasies et des hyperkératoses de
l'épithélium pavimenteux au niveau de la portion cardiaque de
l'estomac. Ces lésions ont été observées chez les mâles comme chez
les femelles à partir de 50 mg/kg de nourriture. En se basant sur la
présence ou l'absence de ces lésions, on a évalué à 15 mg/kg de
nourriture la dose de chlorothalonil sans effet observable, soit
l'équivalent quotidien de 3 mg/kg de poids corporel.
Une étude de 16 semaines sur des chiens aux doses alimentaires de
0, 250, 500, ou 750 mg/kg n'a pas révélé de d'effets qui soient
imputables au traitement.
Les lésions observées au niveau des reins et de la portion
cardiaque de l'estomac ont fait l'objet, pendant 2 ans, d'études plus
approfondies sur des souris et des chiens. Une autre étude, portant
cette fois sur des rats (aux doses quotidiennes de 0, 1,8, 3,8, 15 ou
175 mg/kg de poids corporel), a permis de caractériser histo-
logiquement les effets observés: il s'agissait d'une part, d'une
augmentation de l'incidence des hyperplasies, des hyperkératoses, des
ulcérations et des abrasions de l'épithélium pavimenteux au niveau de
la portion cardiaque de l'estomac et, d'autre part, de la présence
d'une hyperplasie au niveau des tubules contournés proximaux du rein.
Ces anomalies ont été observées à partir de 3,8 mg/kg. A la dose de
175 mg/kg, on notait un accroisssement sensible de l'incidence des
tumeurs rénales (adénomes et carcinomes) et des tumeurs interessant la
portion cardiaque de l'estomac (papillomes et carcinomes). On est
fondé à penser que l'incidence des tumeurs rénales était augmentée
chez les mâles à partir de la dose de 15 mg/kg, de même que celle des
tumeurs gastriques chez les deux sexes aux doses de 3,8 mg/kg et de 15
mg/kg. La dose sans effets néoplasiques observables a donc été prise
égale à 1,8 mg/kg, en prenant comme critère l' incidence des tumeurs
au niveau de la portion cardiaque de l'estomac. Une autre étude de 2
ans, au cours de laquelle des doses plus élevées ont été utilisées, a
confirmé le pouvoir cancérogène du chlorothalonil, tant au niveau du
rein que de la portion cardiaque de l'estomac.
Une étude sur des souris (doses de 0, 15, 40, 175, ou 750 mg/kg
de nourriture) a révélé une augmentation de l'incidence des
hyperplasies au niveau des tubules rénaux à partir de 175 mg/kg, le
même phénomène étant observé à partir de 40 mg/kg au niveau de la
portion cardiaque de l'estomac, avec en outre une hyperkératose. A la
dose de 750 mg/kg, il y avait une légère augmentation des tumeurs
spinocellulaires au niveau de la portion cardiaque de l'estomac. On
en a donc conclu que les doses sans effets néoplasiques ou non
néoplasiques étaient respectivement égales à 175 et 15 mg/kg de
nourriture (soit l' équivalent quotidien de 17,5 et 1,6 mg/kg de poids
corporel, respectivement). Ces effets constatés chez la souris sont
corroborés par les résultats d'une autre étude avec des doses plus
élevées, mais une troisième investigation portant sur des souris
B6C3F1 n'a pas mis en évidence d'effets cancérogènes à dose élevée.
Lors d'une étude de 2 ans sur des chiens (60 et 120 mg/kg de
nourriture), aucun effet attribuable au chlorothalonil n'a été
observé. On en conclu que la dose sans effet observable était de 120
mg/kg de nourriture (soit l'équivalent quotidien de 3 mg/kg de poids
corporel).
Plusieurs épreuves de mutagénicité in vivo et in vitro se
sont révélées négatives, mais il y en a tout de même eu quelques unes
de positives.
Les dérivés monothio, dithio, trithio, dicystéinyl, tricystéinyl
et monoglutathionyl du chlorothalonil, qui sont potentiellement
néphrotoxiques, se sont révélés négatifs dans l'épreuve d'Ames.
Le chlorothalonil ne s'est pas montré tératogène pour le rat ou
le lapin à des doses quotidiennes atteignant respectivement 400 et 50
mg/kg de poids corporel. Lors d'une étude sur deux générations de
rats, on n'a pas constaté d'effets sur l'accouplement, la fécondité ou
la gestation jusqu'à des doses atteignant 1500 mg/kg de nourriture.
La toxicité aiguë par voie orale du métabolite 4-hydroxy est
supérieure à celle du chlorothalonil lui-même (DL50 aiguë par voie
orale égale à 332 mg/kg de poids corporel contre > 10 000 mg/kg de
poids corporel). Plusieurs études ont été entreprises pour
caractériser le profil toxicologique de ce métabolite et établir les
doses sans effets observables.
7. Effets sur l'homme
On a signalé des cas de dermatite de contact parmi des personnes
employées à la fabrication de chlorothalonil, chez des agriculteurs et
des horticulteurs. D'autres cas de dermatite siégeant au niveau des
mains et de la face ont été observés chez des personnes qui
utilisaient des produits de protection du bois à base de
chlorothalonil.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Le chlorothalonil est extrêmement toxique pour les poissons et
les invertébrés aquatiques, comme le montrent un certain nombre
d'études en laboratoire, avec des valeurs de la CL50 inférieures à
0,5 mg/litre. La concentration maximale acceptable de produit toxique
(MATC) s'est révélée être égale à 35 µg/litre lors d'une étude sur
deux générations de daphnies.
A quelques exceptions près, d'ailleurs sans grande importance, le
chlorothalonil n'est pas phytotoxique.
La CL50 d'un concentré pour suspension (500 g de chlorothalonil
par litre) répandu sur un sol artificiel pour lombrics, a été évaluée
à > 1000 mg/kg de terre (14 jours). On a observé une surmortalité
parmi des scolopendres qui s'étaient trouvés en contact avec des
résidus de chlorothalonil présents sur des feuilles d'arachide ou s'en
étaient nourris au laboratoire; il n'y a pas eu d'autre indice d'une
action insecticide.
Le chlorothalonil est peu toxique pour les oiseaux, comme le
montre la valeur de la DL50 aiguë par voie orale chez le colvert
(4640 mg/kg). Aucun effet important sur la reproduction n'a été
signalé.
D'après une étude effectuée sur le terrain, la toxicité du
chlorothalonil pour les organismes aquatiques est moindre que ne le
font craindre les expériences de laboratoire; ce résultat est en
accord avec les propriétés physico-chimiques de ce composé. On a tout
de même enregistré une mortalité chez des espèces exposées
expérimentalement sur le terrain. En revanche, on n'a pas signalé
d'accidents écologiques ayant entraîné une mortalité. Malgré la
faible persistance du chlorothalonil dans les divers compartiments du
milieu, il faut tout de même s'attendre à une certaine mortalité. En
pareil cas, il sera difficile d'établir un lien de cause à effet étant
donné que les résidus de chlorothalonil ne subsistent pas suffisamment
lontemps pour que l'on puisse identifier le composé.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El clorotalonilo es un sólido cristalino inodoro e incoloro con
un punto de fusión de 250°C y una presión de vapor de 7,63 × 105 Pa
(5,72 × 10-7 mmHg) a 25°C. Es poco soluble en agua (0,6 a 1,2 mg/litro
a 25°C) y tiene un coeficiente de partición octanol/agua (log
Koa) de 2,882. Se hidroliza lentamente en agua con un pH de 9, pero
es estable a un pH de 7 o inferior (a 25°C).
El método analítico más corriente, después de la extracción y
depuración de las muestras, es la cromatografía gas-liquido empleando
un detector de captura de electrones.
2. Fuentes de exposición del ser humano y del medio ambiente
El clorotalonilo se viene produciendo a escala comercial desde
1969 por cloración del isoftalonitrilo o mediante el tratamiento de la
amida tetracloroisoftalolil con oxicloruro de fósforo. Es un fungicida
con amplio espectro de actividad empleado principalmente en la
agricultura pero también en el césped, los pastos y las plantas
ornamentales. Los cultivos protegidos incluyen frutas de pepitas y de
hueso, cítricos, grosellas, fresas, bananas, tomates, verduras, café,
cacahuete, patatas, cebollas y cereales. Se emplea también en
sustancias protectoras de la madera y en pinturas.
Las tres formulaciones principales son una suspensión
concentrada, un gránulo hidrodispersible y un polvo humectable. Se
disuelven fácilmente en agua y se aplican empleando sistemas de
pulverización en los suelos o rociado aéreo. Las tasas típicas del
ingrediente activo oscilan entre 1,2 y 2,5 kg/ha en el caso de
cultivos tales como frijoles, apio y cebollas. Las principales fuentes
de exposición del ser humano son la preparación y aplicación de los
productos, así como la ingestión de residuos de las cosechas en los
alimentos (véase la sección 1.1.4).
3. Transporte, distribución y transformación en el medio ambiente
El clorotalonilo se elimina de los medios acuosos mediante
intensa adsorción en las materias en suspensión. Los datos de los
modelos parecen indicar poca, o ninguna, partición detectable en el
sedimento del fondo. Puede producirse biodegradación en aguas
naturales, con la participación de procesos enzimáticos. El
clorotalonilo se degrada rápidamente en el suelo, y puede haber
degradación en el agua con la producción del metabolito 4-hidroxi-
2,5,6-tricloroisoftalonitrilo. La semivida para la disipación en el
suelo de ese metabolito varía entre 6 y 43 días.
El clorotalonilo no se transloca del punto de aplicación a otras
partes de la planta. Su metabolización en las plantas es limitada y,
por o general, ese metabolito representa < 5% del residuo.
En cuanto a los peces, el clorotalonilo se metaboliza mediante la
conjugación con glutatión para producir productos de degradación más
polares. En esa conversión interviene la enzima glutatión-
S-transferasa. Las elevadas concentraciones del radioisótopo
marcador detectadas en la vesícula biliar y la bilis después de la
exposición de la trucha arco iris al 14C-clorotalonilo son
compatibles con la excreción del compuesto en forma de conjugados de
glutatión. Las concentraciones de los radioisótopos de trazado que se
acumulan en la vesícula biliar y otros órganos disminuyen rápidamente
al colocar los peces en agua no contaminada.
El clorotalonilo no experimenta bioacumulación en los organismos
acuáticos.
4. Niveles ambientales y exposición humana
En un estudio de un cultivo de patatas, se procedió a rociar un
pequeño arroyo con clorotalonilo. Muestreos y análisis posteriores del
agua río abajo demostraron la rápida desaparición del clorotalonilo
(las concentraciones eran de 450 µg/litro a los 30 minutos después del
rociado, y oscilaban entre 2 y 6 µg/litro a las 12 h después del
rociado). El rociado sistemático de los cultivos irrigados como, por
ejemplo, patatas y cebada, estuvo acompañado de bajas concentraciones
de clorotalonilo (0,04 a 3,6 µg/litro) en el agua de los tubos de
drenaje en un pequeño número de muestras.
Los residuos de las cosechas están compuestos principalmente de
clorotalonilo propiamente dicho. Las concentraciones residuales
dependen del nivel aplicado, del tiempo transcurrido entre la última
aplicación y la cosecha, y del tipo de cosecha. Los niveles residuales
en la cosecha pueden deducirse de los numerosos ensayos supervisados
realizados con muchas cosechas en todo el mundo y comunicados a la FAO
y la OMS. Se prevé que los residuos de clorotalonilo en los productos
lácteos serán imposibles de detectar, o las concentraciones muy bajas.
En un estudio con vacas lecheras alimentadas durante 30 días con
pienso al que se habían añadido elevadas concentraciones (hasta 250
mg/kg) de clorotalonilo no se observó ningún residuo detectable en la
leche y sólo niveles muy bajos en los tejidos.
Los análisis del régimen alimenticio total y de alimentos
específicos realizados en varios países han revelado concentraciones
no detectables, o muy bajas, de clorotalonilo en los estudios por
muestreo. Los procesos de preparación tales como el lavado, el pelado
y la cocción permiten reducir aún más los niveles residuales en los
alimentos.
5. Cinética y metabolismo en animales de laboratorio
En estudios con ratas a las que se administraron dosis de hasta
50 mg/kg de peso corporal, se observó que un 30% de la dosis oral de
clorotalonilo se absorbía dentro de las 48 horas. La absorción es
inferior con posologías más elevadas, lo que indica un proceso de
saturación. Cuando se administra oralmente 14C-clorotalonilo, la
radioactividad se distribuye en la sangre y los tejidos en menos de
dos horas. Las mayores concentraciones se encuentran en el riñón, el
hígado y la sangre, en ese orden. Con una posología de 5 mg/kg de peso
corporal, la concentración en los riñones es 0,3% a las 24 horas.
Por lo que respecta a las ratas, la mayor parte de la dosis oral
de clorotalonilo se encuentra en las heces (> 82% dentro de las 48 a
72 horas, independientemente de la dosis). Con una dosis oral de 5
mg/kg de peso corporal, la excreción biliar es rápida, alcanzando su
valor máximo dentro de las 2 horas, ocurriendo saturación con
posologías de 50 mg/kg de peso corporal y superiores. En el caso de
las ratas, la excreción urinaria representa entre un 5 y 10% de la
dosis. En perros y monos, la excreción fecal es la vía principal, pero
la excreción urinaria (< 4%) es inferior a la observada en las ratas.
Estudios metabólicos llevados a cabo con ratas indican que el
clorotalonilo se conjuga con el glutatión tanto en el hígado como en
el tracto gastrointestinal. Algunos de los conjugados del glutatión
pueden ser absorbidos en el intestino y transportados a los riñones
donde son convertidos por la ß-liasa citosólica en análogos del tiol
que se excretan por la orina. Cuando se dan dosis de clorotalonilo a
ratas axénicas, se observan en la orina metabolitos del tiol en
cantidades muy inferiores a las registradas en ratas normales, lo que
indica que la microflora intestinal interviene en el metabolismo del
clorotalonilo. En el caso de los perros o monos que reciben dosis
orales de clorotalonilo, la excreción de derivados del tiol por la
orina no es detectable, o es muy baja.
Cuando se aplicó 14C-clorotalonilo a la piel de la rata, un 28%
de la dosis fue absorbida en menos de 120 horas. Se observaron
concentraciones del 18% de la dosis en las heces y del 6% en la orina
al cabo de 120 horas.
6. Efectos en mamíferos de laboratorio y sistemas de pruebas
in vitro
El clorotalonilo tiene baja toxicidad oral y cutánea en ratas y
conejos, respectivamente (los valores agudos orales y cutáneos de la
DL50 son > 10 000 mg/kg de peso corporal). Por lo que respecta a
las ratas, en un estudio de inhalación se observó que el clorotalonilo
técnico pulverizado (MMAD 5 a 8 µm) presentaba elevada toxicidad, con
una CL50 de 0,1 mg/litro a las 4 h.
El clorotalonilo es un irritante de la piel y los ojos en el
conejo. Los estudios de sensibilización cutánea en el conejillo de
indias no arrojaron resultados concluyentes.
En el caso de las ratas, los efectos principales de dosis orales
repetidas de clorotalonilo se observan en el estómago y los riñones.
En un estudio con grupos de 25 ratas, en que se separaron los sexos,
se emplearon posologías de 0, 1,5, 3, 10 ó 40 mg/kg de peso corporal
por día en la dieta durante 13 semanas, lo que estuvo seguido de un
período de recuperación de 13 semanas. Se observó mayor frecuencia de
hiperplasis y hiperketarosis del preestómago con las posologías de 10
y 40 mg/kg; los efectos desaparecieron cuando cesó el tratamiento. Con
una concentración de 40 mg/kg, se registro mayor incidencia de
hiperplasia del epitelio tubular proximal del riñón en los machos a
las 13 semanas y después del período de recuperación. El nivel sin
efecto observado fue de 3 mg/kg de peso corporal por día en base a la
ausencia de lesiones en el preestómago. Se ha demostrado que los
cambios observados en el preestómago y los riñones son de rápida
aparición, presentándose las lesiones dentro de un período de 4 a 7
días en el caso de los machos, cuyo régimen alimenticio incluía una
concentración de 175 mg/kg de peso corporal al día.
En un estudio de 13 semanas de duración realizado con ratones
(empleando dosis de 0, 7,5, 15, 50, 275 ó 750 mg/kg en el alimento),
se observó mayor incidencia de hiperplasia e hiperkeratosis de las
células epiteliales escamosas del preestómago en el caso de los machos
y las hembras cuando se emplearon posologías de 50 mg/kg y superiores.
Atendiendo a esos cambios, el nivel sin efecto observado fue de 15
mg/kg de clorotalonilo en la dieta, lo que equivale a 3 mg/kg de peso
corporal por día.
Un estudio de 16 semanas de duración con perros cuyo alimento
contenía concentraciones de 0, 250, 500 ó 750 mg/kg no reveló cambios
relacionados con el tratamiento.
Se llevaron a cabo investigaciones adicionales de las lesiones en
el preestómago y los riñones, llevándose a cabo estudios con ratas,
ratones y perros durante un período de 2 años. En un estudio realizado
con ratas (empleando dosis de 0, 1,8, 3,8, 15 ó 175 mg/kg de peso
corporal al día), los efectos estuvieron caracterizados
histológicamente por una mayor incidencia e intensidad de hiperplasia,
hiperkeratosis, y úlceras y erosiones de la mucosa escamosa del
preestómago, y por hiperplasia del epitelio de los túbulos
contorneados proximales de los riñones con posologías de 3,8 mg/kg y
superiores. Por lo que respecta a los efectos no neoplásicos, el nivel
sin efecto observado fue, por lo tanto, de 1,8 mg/kg. La incidencia de
tumores renales (adenomas y carcinomas) y de tumores del preestómago
(papilomas y carcinomas) fue considerablemente superior, alcanzando
175 mg/kg. Hubo pruebas de mayor incidencia de tumores renales en los
machos con dosis de 15 mg/kg, así como de tumores estomacales en los
machos y las hembras con dosis de 3,8 y 15 mg/kg. Por lo que respecta
a los efectos neoplásicos, el nivel sin efecto observado fue, por lo
tanto, de 1,8 mg/kg de peso corporal por día sobre la base de los
cambios en la incidencia de tumores en el preestómago. El riesgo
carcinogénico del clorotalonilo en los riñones y preestómago de las
ratas se vio corroborado por los resultados de otros estudios de 2
años de duración en que emplearon dosis más elevadas.
En un estudio con ratones (empleando dosis de 0, 15, 40, 175 ó
750 mg/kg en el alimento), se observó mayor incidencia de hiperplasia
tubular renal con dosis de 175 mg/kg y superiores, así como de
hiperplasia y hiperkeratosis del preestómago con concentraciones de 40
mg/kg y superiores. La incidencia de tumores escamosos del preestómago
aumentó ligeramente con dosis de 750 mg/kg. Por consiguiente, por lo
que respecta a los cambios neoplásicos y no neoplásicos, los niveles
sin efecto observado fueron de 175 y 15 mg/kg en la dieta (lo que
equivale a 17,5 y 1,6 mg/kg de peso corporal por día respectivamente).
Otro estudio con posologías superiores corroboró esos efectos en el
ratón, pero un estudio con ratones B6C3F1 no señaló ningún riesgo
carcinogénico con dosis elevadas.
En un estudio de 2 años de duración con perros (empleando 60 y
120 mg/kg en el alimento), no se detectó ningún efecto atribuible al
clorotalonilo. Por lo tanto, el nivel sin efecto observado fue de 120
mg/kg en el alimento (lo que equivale a 3 mg/kg de peso corporal al
día).
El clorotalonilo no resultó mutagénico en varias pruebas in
vitro e in vivo, aunque fue positivo en un pequeño número de
valoraciones.
Los derivados monotio, ditio, tritio, dicisteina, tricisteina y
monoglutatión del clorotalonilo, que son posibles substancias
nefrotóxicas, arrojaron resultados negativos en el análisis de Ames.
El clorotalonilo no resultó teratogénico en las ratas o conejos
con dosis de hasta 400 y 50 mg/kg de peso corporal al día,
respectivamente. En un estudio realizado con dos generaciones de
ratas, los parámetros reproductivos tales como el apareamiento, la
fertilidad y el período de gestación no se vieron afectados por el
clorotalonilo con concentraciones de hasta 1500 mg/kg en la dieta.
La toxicidad oral aguda del metabolito 4-hidróxido es superior a
la del clorotalonilo propiamente dicho (DL50 oral aguda de 332 mg/kg
de peso corporal en comparación con > 10 000 mg/kg de peso corporal).
Se han llevado a cabo varios estudios destinados a caracterizar el
perfil toxicológico de ese metabolito y a establecer los niveles sin
efecto observado.
7. Efectos en el ser humano
Se ha informado de dermatitis por contacto en el caso de personas
que trabajan en la producción de clorotalonilo, así como en el de
agricultores y hortelanos. Se tienen noticias también de trabajadores
de fábricas de productos de la madera que han contraído dermatitis por
contacto en las manos y el rostro cuando empleaban preservativos de la
madera que contenían clorotalonilo.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
El clorotalonilo es sumamente tóxico para los peces y los
invertebrados acuáticos en los estudios de laboratorio, siendo los
valores de CL50 inferiores a 0,5 mg/litro. En un estudio sobre
reproducción realizado con dos generaciones de Daphnia magna, la
concentración tóxica máxima aceptable (CTMA) fue de 35 µg/litro.
Con pocas excepciones, el clorotalonilo no es fitotóxico.
En lombrices, la CL50 de una formulación de una suspensión
concentrada (500 g de clorotalonilo/litro) en suelo artificial fue
> 1000 mg/kg de suelo (14 días). Las tijeretas experimentaron mayor
mortalidad cuando estaban en contacto con residuos de clorotalonilo en
las hojas del cacahuete o lo ingerían en su fuente de alimentos en las
pruebas de laboratorio; no hubo ningún otro indicio de efecto
insecticida.
El clorotalonilo tiene poca toxicidad para las aves, habiéndose
informado de una DL50 oral aguda de 4640 mg/kg de alimento en el
pato real. No se informó de ningún efecto considerable sobre la
reproducción.
Un estudio sobre el terreno de organismos acuáticos expuestos
después de la aplicación de clorotalonilo parece indicar que la
toxicidad es inferior a la que cabría esperar atendiendo a los
estudios de laboratorio; esto es también compatible con las
propiedades fisicoquímicas de los compuestos. Se observaron muertes en
algunas especies expuestas experimentalmente en el campo. No se ha
informado de incidentes de muertes en el medio ambiente. Sin embargo,
a pesar del corto tiempo de presencia del clorotalonilo en el medio
ambiente, cabría esperar que ocurran muertes. Resultaría difícil
establecer vínculos entre las muertes y los compuestos ya que la
persistencia de los residuos no seria suficientemente prolongada para
poder identificar el clorotalonilo.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of chlorothalonil by oral, dermal and
inhalation routes of administration is shown in Table 9. Signs of
poisoning include depression, diarrhoea and unkempt appearance. An
oral LD50 could not be attained in the dog because of emesis.
Chlorothalonil administered orally (1 g/kg) to mice increased
intestinal motility. The laxative action was reduced by pre-treatment
with corn oil (Teeters, 1966).
Table 9. The acute toxicity of chlorothalonil
Species LD50 Route Reference
(mg/kg)
Rat > 10 000 oral Powers (1965)
Dog > 5000 oral Paynter (1965a)
Mouse 6000 oral Yoshikawa & Kawai (1966)
Rat 332 oral Wazeter (1971)
(4 hydroxy
metabolite)
Rabbit > 10 000 dermal Doyle & Elsea (1963)
Rat (f & m)a 0.22 mg/litre inhalation Danks & Fowler (1988)
Rat (f & m)b 0.52 mg/litre inhalation Shults et al. (1991)
Rat (f & m)c 0.10 mg/litre inhalation Shults et al. (1993)
a LC50 actual concentration
b Hammermilled technical chlorothalonil - 1 h exposure
c Hammermilled technical chlorothalonil - 4 h exposure
The acute oral toxicity of the metabolite 4-hydroxy-2,5,6-
trichloroisophthalonitrile is greater than that of chlorothalonil
itself, the acute oral LD50 values being 332 and 10 000 mg/kg,
respectively).
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Rat
When groups of 35 male and 35 female rats were fed chlorothalonil
in the diet at dose levels of 0, 250, 500, 750 or 1500 mg/kg diet for
22 weeks, growth was slightly reduced at all levels in males and in
the highest two levels in females. Liver and kidney weight was
increased in the two highest dose groups in males. Kidney changes,
more evident in males than females, occurred at all dose levels and
included irregular swelling of the tubular epithelium, epithelial
degeneration and tubular dilation (Blackmore & Shott, 1968). In a
separate study, no compound-related effects were seen in the kidneys
of rats fed chlorothalonil at seven dietary levels from 1-120 mg/kg
diet for 17 weeks (Busey, 1975).
When groups of 20 male and 20 female rats were fed chlorothalonil
in the diet (0, 40, 80, 175, 375 and 1500 mg/kg body weight per day)
for 90 days, a significant dose-related reduction in body weight gain
was seen at levels of 375 mg/kg per day or more which was allied to
cathartic action at the two highest doses. Male urine output
decreased and specific gravity increased at the two highest dose
levels. Relative kidney weight was increased in both sexes at all
dose levels. A histopathological re-evaluation of the kidneys showed
the presence of epithelial hyperplasia of the proximal convoluted
tubules at all levels in males and at levels of 175 mg/kg per day or
more in females (Wilson, 1981; Wilson et al., 1985c).
Groups of 25 male and 25 female rats were fed chlorothalonil for
13 weeks at 0, 1.5, 3, 10 and 40 mg/kg body weight per day. Five rats
of each sex per group were killed at 6 weeks, ten at 13 weeks and the
survivors were killed after a further 13-week recovery period. There
was no effect on mortality, clinical condition, body weight, food and
water consumption, haematology or urinalysis. Increases in kidney
(> 3 mg/kg per day) and liver (highest dose) weights and the
incidence of hyperplasia and hyperkeratosis of the forestomach
squamous epithelium (at 10 and 40 mg/kg per day) returned to normal
when treatment ceased. Histopathological re-examination of the kidney
revealed (in males at the highest dose level) an increased incidence
of hyperplasia of the epithelium of the proximal tubules at 6 and
13 weeks and after a 13-week recovery period. In a few animals this
resulted in an increase in the size of the tubules. An overall
no-observed-effect level of 3 mg/kg body weight per day was
established, based upon the lack of lesions in the squamous epithelium
of the forestomach (Wilson et al., 1983a, 1985a).
The kidney and stomach changes were investigated further in a
study where 90 male rats were fed chlorothalonil (175 mg/kg body
weight per day) for up to 91 days. Groups of ten rats were killed
after 4 or 7 days and 2, 4, 6, 8, 10 or 12 weeks, the kidney and
stomach taken for histopathological examination. Groups of ten rats
at each interval were taken from a control group and examined. The
forestomach effects of chlorothalonil were characterized initially as
multifocal ulceration and erosion of the mucosa, developing to
squamous epithelial hyperplasia and hyperkeratosis. Within the first
week there was vacuolar degeneration, cell death, karyomegaly and
regeneration of the proximal tubular epithelium. There was apparent
recovery at day 14 with few lesions present in treated animals.
Continued administration led to tubular epithelial vascularization,
regeneration, hyperplasia and hypertrophy (Ford et al., 1987a).
Chlorothalonil or the monoglutathione conjugate of chlorothalonil
was administered in the diet to male Fischer-344 rats in a 90-day
study. A third group of rats received only the vehicle (0.5%
methylcellulose). The oral administration of approximately equimolar
doses of chlorothalonil (75 mg/kg per day) or the monoglutathione
conjugate of chlorothalonil (150 mg/kg per day) resulted in
significantly increased kidney weight and in treatment-related
histopathological changes in the kidney. The primary changes observed
were morphologically similar for both compounds and were characterized
by proximal tubular hyperplasia, tubular dilation (hypertrophy),
vacuolar degeneration and interstitial fibrosis. The oral
administration of chlorothalonil resulted in gross and microscopic
changes in the non-glandular portion of the forestomach. The oral
administration of the monoglutathione conjugate of chlorothalonil did
not produce alterations in the rat forestomach. The gross and
microscopic changes observed in the forestomach of animals given
chlorothalonil were considered to be due to the local irritational
effects of chlorothalonil. The alteration of the molecule by the
addition of a single glutathione residue appeared to eliminate the
irritation to the forestomach. Thiol metabolites of chlorothalonil
were detected in the urine of animals given either the monoglutathione
conjugate of chlorothalonil or chlorothalonil itself. The presence of
the thiol metabolites, coupled with the similar histopathological
changes in the kidney, suggests a common metabolic pathway. The data
support the conclusion that the nephrotoxicity produced by
chlorothalonil is associated with conjugation with glutathione (Ford
et al., 1987b; Wilson et al., 1990).
7.2.1.2 Mouse
Groups of 15 male and 15 female CD-1 mice were administered
chlorothalonil in diets at levels of 0, 7.5, 15, 50, 275 or 750 mg/kg
diet for 13 weeks. Five animals per group were killed at 6 weeks.
There was no effect on clinical condition, mortality, body weight gain
or food consumption. Kidney weight was increased in females at 275
and 750 mg/kg. Microscopic re-examination revealed hyperplasia of the
epithelium of the proximal convoluted tubules, minimal or slight in
severity, in only 4 out of 15 males. This was not considered to be a
clear treatment-related effect. There was an increased incidence of
hyperplasia and hyperkeratosis of the squamous epithelial cells of the
stomach in males and females at levels of 50 mg/kg or more. Mucosal
ulceration and submucosal inflammation were observed in some treated
animals. The no-observed-effect level was considered to be 15 mg/kg
(3 mg/kg body weight per day) (Shults et al., 1983; Busey, 1985).
7.2.1.3 Dog
In a 30-day feeding study, encapsulated chlorothalonil (97.9%
purity) was administered daily to groups of two male and two female
beagle dogs at 0, 50, 150, or 500 mg/kg body weight per day. The
following tissues were examined macroscopically and microscopically at
necropsy: brain, liver, kidneys, testes with epididymis, ovaries,
adrenals, heart, thyroid, and parathyroid. During treatment the
high-dose dogs exhibited emesis and weight loss and reduced food
consumption (males only). Female dogs had slightly reduced body
weight gains at all doses. At necropsy, the liver weights of
high-dose females were slightly increased. There were no microscopic
changes in the tissues examined. Due to the reduced body-weight gains
of treated females, a no-observed-adverse-effect level (NOAEL) was not
established in this study (Fullmore & Laveglia, 1992; FAO/WHO, 1993b).
In a 16-week dietary study, chlorothalonil (purity unspecified)
was fed at 0, 250, 500 or 750 mg/kg to groups of four beagle dogs.
There were no compound-related effects on appearance, behaviour,
appetite or body weight. No changes in haematological parameters were
found at weeks 0, 4, 13 or 16. At termination, protein-bound iodine
was found to be increased in all treated dogs. Urinalysis at weeks 6,
9, 13 and 16 was unremarkable. No compound-related macroscopic or
microscopic changes were found at necropsy. In particular, only
incidental changes were observed in liver and kidneys. A NOAEL was
not established in this study (Paynter & Murphy, 1967; FAO/WHO,
1993b).
7.2.2 Dermal: Rabbit
Chlorothalonil (75% formulation) was applied to the intact or
abraded skin of groups of rabbits at 0, 500 or 1000 mg/kg per day, 5
days/week, for 3 weeks. The treated groups comprised five males and
five females per group, with two males and two females as controls.
Treatment resulted in dose-related irritation which was more severe
with abraded skin. Histopathological examination revealed a moderate
degree of acanthosis, hyperkeratosis and slight to moderate leucocytic
infiltration. No abnormality was detected in other tissues (Paynter,
1965b).
Chlorothalonil was applied dermally each day for 21 days to
groups of six male and six female rabbits at 0.1, 2.5 and 50 mg/kg per
day. The fungicide was suspended in 0.125% aqueous methylcellulose
and covered 10% of the body surface when applied to the back. Contact
was for 6 h daily. The only effect revealed by a wide range of
observations and examinations was dermal irritation at 2.5 and 50
mg/kg per day. The histopathological changes seen were minimal to
slight acanthosis and hyperkeratosis. The no-observed-effect level
(NOEL) for dermal irritation was 0.1 mg/kg per day (Shults et al.,
1986).
7.3 Long-term exposure
7.3.1 Rat
An early study, lasting 76 weeks, produced evidence of kidney
changes in rats (15 male and 15 female per group) fed high levels of
chlorothalonil at 500, 1000 and 5000 mg/kg diet. There was no
overall effect on mortality, clinical condition or growth. The
compound-related changes in the kidney, at all levels, consisted of
tubular hypertrophy, epithelial irregularities and vacuolation. Males
were more affected than females (Paynter & Busey, 1967).
A 2-year study, reported in 1967, was initiated with
chlorothalonil levels of 1500, 15 000 and 30 000 mg/kg diet with
groups of 35 male and 35 female rats (70 of each sex in control
group). Because of cathartic effects, the top dose administration was
curtailed after 15 weeks. The rats in the two remaining treatment
groups continued for the full 2 years with evaluations including
haematology, biochemistry and histopathology. There was no effect on
survival, but the effect on growth was dose-related. The relative
organ weights of liver and kidney were increased at 15 000 mg/kg.
Microscopic changes in the forestomach were described as acanthosis
and hyperkeratosis of the squamous epithelium at 15 000 mg/kg and, in
the kidney, as tubular hypertrophy and hyperplasia at both 1500 and
15 000 mg/kg diet (Paynter, 1967a).
A supplementary study, run concurrently with the previous study,
assessed chronic toxicity at 5000 mg/kg diet. Growth rate was
depressed and a cathartic action was expressed as increased water
consumption and faecal excretion. Relative organ weights were
increased for caecum and kidneys. Histopathological examination
showed kidney changes as tubular hypertrophy and occasional
degeneration of the proximal tubular epithelium (Paynter & Crews,
1967).
A 2-year study at six chlorothalonil dose levels between 4 and 60
mg/kg diet was designed to determine an NOEL (50 males and 50 females
per group). No effects were seen on survival, clinical observations,
growth, haematological or biochemical parameters. No changes of
toxicological significance were found for organ weights or during
gross and microscopic examination of tissues. The highest dose level
(3 mg/kg body weight per day) was considered the NOEL (Holsing &
Shott, 1970).
A long-term study was undertaken to evaluate the carcinogenic
potential of chlorothalonil in Fischer-344 rats. Males were studied
for 27 months and females for 30 months. Chlorothalonil was
administered in the diet to groups of 60 males and 60 females at dose
levels of 0, 40, 80 and 175 mg/kg body weight per day. There was no
effect on survival of females or males up to 2 years. However, at the
highest dose level, there was increased mortality after 2 years, but
only in males. Decreases in body weight gain were dose-related at 80
and 175 mg/kg per day. Treatment-related effects on other parameters
appeared to be related to nephrotoxicity. These included increases in
serum urea nitrogen and creatinine, increased urine volume and
decreased specific gravity, and increased kidney weight.
Histopathological examination of the kidney showed a dose-related
increase in chronic glomerulonephritis (nephropathy) compared with
controls. A re-examination of the kidneys also revealed tubular
hyperplasia (a sign of preneoplastic change) and chronic progressive
nephropathy in the treated groups. Secondary lesions in other organs
included periarteritis and parathyroid hyperplasia. There was
increased incidence or severity of hyperplasia and hyperkeratosis of
the squamous mucosa of the oesophagus and forestomach in all dosed
groups, which was probably the result of the irritant effect of
chlorothalonil. Neoplastic changes in the kidney and forestomach are
evaluated in section 7.7 (Wilson et al., 1985b, 1986a).
A further study was carried out to evaluate the neoplastic
findings in the kidney and stomach seen in the previous study and to
determine a NOEL for non-neoplastic effects. Groups of 65 male and
65 female rats were administered chlorothalonil in the diet at doses
of 0, 1.8, 3.8, 15 and 175 mg/kg body weight per day. Males were
killed at 23 months (highest dose) and 26 months and females at
29 months. The effects at 175 mg/kg per day were similar to those
described above, i.e. changes associated with the kidney and stomach.
At 15 mg/kg per day there was elevated serum urea nitrogen and
slightly increased kidney weight. At 3.8 mg/kg per day there was a
small increase in kidney weight, but there were no effects at 1.8
mg/kg per day. Microscopic examination revealed an increased
incidence and severity of epithelial hyperplasia in the proximal
convoluted tubules at 3.8 mg/kg per day or more. Clear cell
hyperplasia of the proximal convoluted tubules was increased at
15 mg/kg per day in females and at 175 mg/kg per day in both sexes.
In addition, at 3.8 mg/kg per day or more, there was an increased
incidence and severity of hyperplasia, hyperkeratosis, ulcers and
erosions of the squamous mucosa of the forestomach. At 175 mg/kg per
day, the incidence of erosions of the glandular stomach was
significantly increased compared to controls. Renal tumours at levels
of 15 and 175 mg/kg per day and stomach tumours at 3.8 mg/kg per day
or more are evaluated in section 7.7. An NOEL of 1.8 mg/kg body
weight per day was established for non-neoplastic effects seen in the
study (Wilson et al., 1989a).
7.3.2 Mouse
A 2-year mouse study was carried out with 60 males and 60 females
per group at 0, 750, 1500 and 3000 mg chlorothalonil/kg in diet.
There was a slightly increased mortality in males at the highest dose
level but no effect was seen on body weight, food consumption,
clinical condition or haematological parameters. Kidney weight was
increased in all treated groups compared to controls. Non-neoplastic
changes in the kidney were characterized as glomerulonephritis,
cortical tubular degeneration and cysts. The incidence of these
changes, found at all treatment levels, was not dose-related but was
considered to be due to treatment. A histopathological re-evaluation
of the kidneys revealed a high incidence of tubular hyperplasia in all
male groups and a lower incidence in females. Non-neoplastic effects
in the stomach and oesophagus included hyperplasia and hyperkeratosis
of the squamous mucosa, probably due to the irritant action of
chlorothalonil. The evaluation of kidney and stomach tumours found in
this study is described in section 7.7 (Wilson et al., 1983b, 1986b).
A second mouse study was undertaken to determine the NOEL for
stomach and kidney changes in male mice. Sixty males per group were
fed chlorothalonil at levels of 0, 15, 40, 175 and 750 mg/kg diet for
2 years. Kidney weight and incidence of tubular hyperplasia were
increased at 750 mg/kg and, very slightly, at 175 mg/kg. The
increased incidence of hyperplasia and hyperkeratosis of the
forestomach was dose-related between 40 and 750 mg/kg. The dietary
NOEL for non-neoplastic effects was determined to be 15 mg/kg (1.6
mg/kg body weight per day). The tumour evaluation is considered in
section 7.7 (Wilson et al., 1987).
7.3.3 Dog
In a 2-year study, chlorothalonil (93.6% purity) was fed to
groups of four beagle dogs at dietary concentrations of 0, 1500,
15 000 or 30 000 mg/kg (equivalent to 0, 37.5, 375, or 750 mg/kg body
weight per day). Eight dogs, one of each sex and of each group, were
killed at 12 months and the remainder at 24 months. One dog of each
treatment group lost weight during the study. There was a tendency
towards mild anaemia in four of the mid-dose dogs at 2 years and at
earlier intervals in two of the high-dose dogs. Biochemical and urine
analyses were unremarkable. Absolute and relative thyroid and kidney
weights, and liver to body weight ratios were increased at the
mid- and high-dose levels. Histopathological treatment-related
changes occurred in the liver, thyroid, kidney and stomach of mid- and
high-dose dogs; changes in low-dose dogs were equivocal. In the
liver, the findings were similar in nature (though slightly more
pronounced) at low-dose levels to those in the control dogs, but they
increased in severity at mid- and high-dose levels. They included
pericholangitis with associated portal fibrosis, bile duct hyperplasia
and pigmentation of hepatic cytoplasm and of macrophages of sinusoids
and portal triads. Renal glomerulosclerosis and degenerative renal
tubular changes (tubular hypertrophy and dilation) were found in the
kidneys of mid- and high-dose dogs. In the thyroid, markedly
increased pigmentation of follicular epithelia occurred in mid- and
high-dose dogs. Moderate to severe gastritis was found irregularly in
mid- and high-dose animals. In summary, administration of
chlorothalonil in the diet of dogs at concentrations of 15 000 and
30 000 mg/kg caused irregular body weight reduction, borderline
anaemia and histopathological changes in the liver, kidney, thyroid
and stomach. At 1500 mg/kg, the histopathological changes found in
the liver were qualitatively similar but minimally to slightly
increased in comparison to those found in control animals.
Histopathological changes to the other tissues were unremarkable at
the low dose. A NOAEL was not established in this study (Paynter &
Busey, 1966; FAO/WHO, 1993b).
Groups of beagle dogs (eight males and eight females per group)
were fed chlorothalonil in the diet at dose levels of 0, 60 and
120 mg/kg. Four dogs of each sex per group were killed at one year
and the remaining animals at 2 years. There were no effects on
behaviour or growth over the course of the study. Clinical chemistry
values, including haematological, biochemical and urine analyses, were
comparable to the controls at all dose levels. Gross and microscopic
examination of tissues and organs performed on animals killed at 12
months indicated a slight increase in the severity of renal tubule
vacuolation in high-dose males. Examination of tissues and organs at
24 months showed a slight degree of renal tubule vacuolation in two
out of four males at 120 mg/kg. In the absence of other changes
(urinalyses values) this finding was considered questionable,
especially as a slight degree of vacuolation was noted in controls as
well as other treated animals. The NOAEL was considered to be
120 mg/kg diet, equivalent to 3 mg/kg body weight per day (Holsing &
Voelker, 1970; FAO/WHO, 1991, 1993b).
7.3.4 Summary of key dietary studies
A summary of the key dietary studies with chlorothalonil is given
in Table 10.
7.4 Skin and eye irritation; sensitization
Chlorothalonil is an irritant to rabbit skin, as shown by
repeated dose studies (5 days/week for 3 weeks at 500 or 1000 mg/kg
per day or daily for 21 days at 2.5 or 50 mg/kg per day; details in
section 7.2.2).
The influence of the vehicle on the skin irritant potential of
chlorothalonil was shown by a rabbit study with 0.1% chlorothalonil in
saline, petrolatum or acetone. Compared to the vehicle alone,
chlorothalonil did not cause a significant increase in irritation in
Table 10. Summary of key dietary studies with chlorothalonil
Species Duration Dose levels NOEL LOEL Key effects Reference
Rat 13 weeks 0, 1.5, 3, 10, 3 mg/kg per 10 mg/kg per 40 mg/kg per day: increased kidney and Wilson et
40 mg/kg body day day liver weight, incidence of hyperplasia al. (1983a,
weight per day and hyperkeratosis of forestomach, 1985a)
incidence of epithelial hyperplasia in
proximal tubules 10 mg/kg per day:
increased kidney weight, hyperplasia
and hyperkeratosis of forestomach
Rat 23-29 months 0, 1.8, 3.8, 15, 1.8 mg/kg 3.8 mg/kg 175 mg/kg per day: increases in serum Wilson et
175 mg/kg body per day per day urea nitrogen, urine volume, kidney al. (1989a)
weight per day weight, renal tubular hyperplasia,
hyperplasia and hyperkeratosis of
forestomach; increased incidence of
renal and forestomach tumours
15 mg/kg per day: similar but less
intense changes to those shown at
highest dose level 3.8 mg/kg per day:
small increase in kidney weight, renal
tubular hyperplasia, hyperplasia
and hyperkeratosis of forestomach and
forestomach tumours
Mouse 13 weeks 0, 7.5, 15, 50, 15 mg/kg diet 50 mg/kg diet 275 and 750 mg/kg: increased kidney Shults et
275, 750 mg/kg (= 3 mg/kg (= 10 mg/kg weight 50, 275 and 750 mg/kg: al. (1983);
diet per day) body weight dose-related increased incidence Busey (1985)
per day) of hyperplasia and hyperkeratosis
of forestomach
Table 10. (Cont'd)
Species Duration Dose levels NOEL LOEL Key effects Reference
Mouse 2 years 0, 15, 40, 175, 15 mg/kg diet 40 mg/kg diet 750 mg/kg: increased kidney weight, Wilson et
750 mg/kg diet (= 1.6 mg/kg (= 4.5 mg/kg renal tubular hyperplasia, forestomach al. (1987)
body weight body weight hyperplasia and hyperkeratosis,
per day) per day) slightly increased incidence of
forestomach tumours 175 mg/kg: slightly
increased incidence of renal tubular
hyperplasia, increased forestomach
hyperplasia and hyperkeratosis
40 mg/kg: increased incidence of
forestomach hyperplasia and
hyperkeratosis
Dog 2 years 0, 1500, 15 000, - 1500 mg/kg in 15 000 and 30 000 mg/kg: increased Paynter &
30 000 mg/kg diet (= 37.5 kidney, liver and thyroid weights and Busey (1966)
diet mg/kg body histopathological changes, gastritis
weight per 1500 mg/kg: slightly increased incidence
day) hepatic findings
Dog 2 years 0, 60, 120 mg/kg 120 mg/kg diet - no changes of toxicological significance Holsing &
diet (= 3 mg/kg Voelker
body weight (1970);
per day FAO/WHO
(1991, 1993)
saline, but doubled the mild irritation caused by petrolatum. Acetone
itself caused no irritation but the addition of chlorothalonil
produced mild skin irritation (Flannigan & Tucker, 1985).
A further study in rabbits using a cumulative irritation assay
confirmed the irritant properties of 0.1% chlorothalonil in acetone.
A concentration of 0.01% gave evidence of mild irritation, probably of
no clinical significance, and 0.001% was not irritant to the skin
(Flannigan et al., 1986).
Chlorothalonil irritancy to the eye was evaluated in a modified
Draize system, 0.1 mg being instilled into one eye of each of three
male and three female rabbits. The eyes were examined and the results
scored after 24, 48 and 72 h, 7 and 14 days. Ocular irritation
occurred in all animals and corneal opacity persisted to day 14 (Major
et al., 1982).
Skin sensitization was tested in a guinea-pig maximization test
using 10 Hartley female guinea-pigs. Topical concentrations of 0.5
and 5% chlorothalonil were used. Upon challenge, chlorothalonil was
shown to be a strong sensitizer. Moderate cross-sensitization with
benomyl was also demonstrated (Matsushita & Aoyama, 1981). By
contrast, chlorothalonil did not produce skin sensitization in a
Draize test. The test substance (0.2 g) was applied to the shaved
backs of Hartley-derived guinea-pigs. The material was occluded for
24 h and then removed. This procedure was performed 3 times a week
for a total of 10 applications. On day 36 of the study a challenge
application of 0.2 g chlorothalonil was applied to the shaved flanks
and occluded. The skin was assessed 24 h later after removal of the
occlusive dressing. The positive control substance DNCB showed the
expected dermal sensitization but chlorothalonil was shown not to be a
sensitizer in this test (Wilson et al., 1982).
Luperi & Forster (1988) studied the ability of chlorothalonil to
induce delayed contact hypersensitivity in the guinea-pig using the
maximization test of Magnusson and Kligman. There was no evidence of
an induced sensitization response, but adequate evaluation was impeded
by a diffuse irritant reaction following the challenge.
7.5 Reproductive and developmental toxicity
Teratological evaluations have been carried out in the rat and
rabbit.
Chlorothalonil was administered orally via gavage to pregnant
Sprague-Dawley rats (25 per group) on days 6-15 of gestation at dose
levels of 0, 25, 100 or 400 mg/kg body weight per day, and the animals
were killed on day 20. Clinical signs of maternal toxicity were
evident at the highest dose level. There were 3 deaths and lowered
body weight during the dosing period. There was a slight increase in
the number of early embryonic deaths at the highest dose level,
probably associated with the maternal toxicity. There were no
compound-related incidences of external, internal or skeletal
malformations in the fetuses in treated animals. It was concluded
that chlorothalonil is not teratogenic to the rat (Mizens et al.,
1983).
Chlorothalonil was given orally to pregnant rabbits on days 8-16
of gestation at dose levels of 0, 180 or 375 mg/kg per day (days 8 and
9) and 0, 62.5 or 31.25 mg/kg per day (day 10 to day 16). There were
marked effects on food intake and maternal mortality occurred in both
treated groups, which imposed limitations on the evaluation of the
study. However no teratogenic effect was observed (Paynter, 1966b).
Rabbits were dosed with chlorothalonil (0, 5, or 50 mg/kg per
day) during days 6-18 of pregnancy (8 in control group, 9 in each dose
group) and killed on day 29. Four out of nine does aborted at the
highest dose level, and body weight was reduced in this group.
Although the incidence of fetal deaths appeared to increase with dose,
the difference was not statistically significant. The number of
implants and live fetuses per pregnancy and the fetal weights were
reduced in the high-dose group compared to controls. No treatment-
related effects were seen during external, internal or skeletal
examinations. It was concluded that chlorothalonil was not
teratogenic to the rabbit (Shirasu & Teramoto, 1975).
Chlorothalonil was administered by gavage to groups of 20
pregnant rabbits on days 7-19 of gestation at dose levels of 0, 5, 10
or 20 mg/kg per day. All survivors were weighed, killed on day 30 and
examined for live, dead or resorbed fetuses. Live and dead fetuses
were weighed and examined for external, visceral and skeletal
abnormalities. The highest dose level caused maternal body weight
loss and decreased food consumption. The pregnancy rate was > 95%
in each group. Examinations revealed no fetotoxicity or
teratogenicity due to chlorothalonil (Wilson et al., 1988).
A three-generation reproduction study was undertaken in rats at
dietary levels of 0, 1500 or 15 000 mg chlorothalonil/kg diet. A
supplementary study with 0 or 5000 mg/kg was also carried out 6 months
later. Groups consisted of 10 males and 20 females which were fed the
test diet for 11 or 12 weeks prior to mating. The study design was
for three generations with two matings per generation to give A and B
litters. Growth suppression occurred in the nursing A and B litters
in all generations at all dose levels and the pups appeared smaller
than the controls. There were no malformations due to treatment.
However, there were difficulties in execution, and this study is not
considered adequate by present-day standards (Paynter, 1967b).
Reproductive performance was also assessed in a one-generation
study on rats at dietary levels of 0, 200, 375, 750, 1500 or 3000
mg/kg diet with groups of 15 males and 15 females. The parents were
treated for 10 weeks prior to mating. No clinical signs of toxicity
were evident in the parents, but male body weight gain was reduced at
1500 and 3000 mg/kg and the kidneys were enlarged at 3000 mg/kg.
Reproductive parameters such as mating, fertility and gestation length
were not affected. There were no treatment-related abnormalities in
the offspring. The only effect on the offspring was lower body weight
at 3000 mg/kg on lactation days 14 and 21 (Wilson et al., 1989b).
In a two-generation reproduction study with two litters per
generation, technical chlorothalonil was administered to Charles River
CD rats by dietary admixture at concentrations of 0, 500, 1500 and
3000 mg/kg diet. There were 35 rats of each sex in each group. The
parental animals from each generation were treated continuously during
the growth period prior to mating and then throughout the mating,
gestation, lactation and resting phases of the study. The growth
period was 10 weeks for the F0 animals and 14 weeks for the F1
animals. Each generation of parental animals was mated twice to
produce the F1a, F1b, F2a and F2b litters. The offspring were
exposed to the test diets throughout the lactation period. Thirty-
five F1b males and females per group were selected to become the F1
generation after weaning. No mortalities or clinical signs of
toxicity associated with administration of chlorothalonil were
observed in the F0 or F1 parent animals. There was a
treatment-related effect towards lowered body weight in both males and
females in the F0 and F1 adults. The NOEL for body weight was 500
mg/kg in diet. Increased relative food consumption was observed in
the groups that showed lower body weights. The anticipated
treatment-related lesions in the kidneys and stomachs of adult animals
were observed in the F0 and F1 generations by gross and microscopic
pathology. These effects were observed in the kidney at all dose
levels in males and at 1500 and 3000 mg/kg in females. Stomach
effects were observed at all dose levels in both sexes. Reproductive
parameters in F0 and F1 animals, including mating and fertility
indices and gestation length, were not affected by treatment with
chlorothalonil. No gross malformations which were considered
treatment-related were observed in offspring in any of the groups
throughout the study. Litters were culled at day 4 to 8 pups/litter.
No effects on the number of live and stillborn pups, pup sex ratio,
pup survival and physical condition of the pups during lactation were
observed. No findings which were considered treatment-related were
observed during necropsy of the pups. The only effect observed in
pups in this study was lowered body weight compared to the controls on
day 21 of lactation. The NOEL for this effect was considered to be
1500 mg/kg diet, equal to 75 mg/kg body weight per day (Lucas & Benz,
1990).
7.6 Mutagenicity
Chlorothalonil has been assessed for mutagenic potential in a
wide range of in vitro and in vivo assays as shown in Tables 11
and 12. Most of the tests showed chlorothalonil not to be mutagenic
or clastogenic. A positive result in the DNA repair test with
Salmonella typhimurium was not reproduced in Bacillus subtilis,
nor was DNA binding shown in an in vivo study (see section 6.5). A
positive result in Chinese hamster ovary cells without metabolic
activation was not seen in the presence of activation nor confirmed by
in vivo chromosomal tests in rats and mice. Equivocal results were
obtained with Chinese hamster bone marrow in vivo.
In addition to the results shown in the Tables 11 and 12, a
series of studies was reported by IARC (1983). The results, which
were all negative, included those with S. typhimurium in the
presence and absence of a metabolic activating system and
Saccharomyces cerevisiae and Aspergillus nidulans in the presence
of activation. Chlorothalonil also failed to induce mutations in
silkworms and chromosomal aberrations in barley shoot tips or hamster
lung fibroblasts.
Chlorothalonil is not a transforming agent in Fischer rat embryo
cell lines (Price, 1978a).
The monothio, dithio, trithio, dicysteine, tricysteine and
monoglutathione metabolites of chlorothalonil have been shown to be
negative in the Ames assay with or without rat kidney metabolic
activation (see section 7.9).
Considering all the results of mutagenicity testing, it is
unlikely that chlorothalonil will show mutagenic activity in intact
mammalian systems.
7.7 Carcinogenicity
Several long-term rodent studies have been carried out on
chlorothalonil and have included carcinogenic evaluation. The design
of these studies and the chronic toxicity results have been described
in section 7.3. Some of the earlier rat studies did not show any
carcinogenic effect but it is probable that their design was not
sufficient to provide a critical test. The carcinogenic evaluation
in this section therefore concentrates upon the more recent rat and
mouse studies. Detailed evaluations of these studies have already
appeared in the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
reviews of 1983, 1985 and 1990 (FAO/WHO, 1985, 1986b, 1990b).
Therefore, for most of these studies, only the salient points will be
described here.
Table 11. In vitro mutagenicity tests
Test Organism Metabolic Dose range Mutagenic Reference
activation (+ or -) potential
Prokaryotes
Point mutation Salmonella typhimurium + and - 0.33-6.6 µg/plate negative Banzer (1977a)
(5 strains)
Point mutation S. typhimurium + and - 0.16-50 µg/plate negative Jones et al. (1984)
(5 strains) (renal)
Point mutation S. typhimurium - 1-10 µg/plate negative Shirasu et al.
(5 strains) + 2-10 µg/plate negative (1977)
Escherichia coli WP2 - 10-500 µg/plate negative
+ 10-100 µg/plate negative
Point mutation S. typhimurium TA98, + and - 0.76, 7.6, 76 µg/plate negative Wei (1982)
TA100, TA1535, TA1537, (hepatic and
TA1538 renal)
DNA repair tests S. typhimurium + and - 2, 10, 20 µg positive Banzer (1977b)
TA1978, TA1538
Bacillus subtilis - 2-200 µg negative Shirasu et al.
H17/M45 rec-assay (1977)
Table 11. (Cont'd)
Test Organism Metabolic Dose range Mutagenic Reference
activation (+ or -) potential
Mammalian cells
Gene mutation Chinese hamster V79 + and - 0.3 µg/ml negative Banzer (1977c)
mouse fibroblast + and - 0.03 µg/ml negative
BALB/3T3
Chromosome Chinese hamster - 0.03-0.3 µg/ml positive Mizens et al.
aberration ovary cells + 0.6-6.0 µg/ml negative (1986a)
Chromosome Human lymphocytes - 0.54-2.5 µg/ml negative Mosesso & Forster
aberrations + 1.16-5.38 µg/ml negative (1988)
Table 12. In vivo mutagenicity tests
Species Test Dose Mutagenic potential Reference
Mouse in vivo 6.5 mg/kg per day negative Legator (1974a)
cytogenetics 5 days orally
Mouse host-mediated assay; 8 strains 6.5 mg/kg per day negative Legator (1974a)
Salmonella typhimurium 5 days orally
Mouse dominant lethal assay, 5 days 6.5 mg/kg per day negative Legator (1974a)
dosing, 8 weeks mating 5 days orally
Mouse micronucleus 4-2500 mg/kg orally, twice with negative Siou (1981a)
(polychromatic erythrocytes) 24 h interval
Mouse chromosome aberration (bone 4-2500 mg/kg orally, twice with negative Siou (1981b)
marrow 6 h after last dose) 24 h interval 250, 1250, 2500 negative Mizens et al.
(bone marrow at 6, 24 and 48 h) mg/kg oral single dose (1985a)
Rat micronucleus 8-5000 mg/kg orally, twice with negative Siou (1981a)
(polychromatic erythrocytes) 24 h interval
Rat chromosome aberration (bone doses as above negative Siou (1981b)
marrow 6 h after last dose)
(bone marrow 6, 24 and 48 h) 500, 2500, 5000 mg/kg oral negative Mizens et al. (1985b)
single dose
Table 12. (Cont'd)
Species Test Dose Mutagenic potential Reference
Chinese micronucleus 4-2500 mg/kg orally, twice with negative Siou (1981a)
hamster (polychromatic erythrocytes) 24 h interval
Chinese chromosome aberration 8-5000 mg/kg for 2 days inconclusive Siou (1981b)
hamster (bone marrow 6 h after last dose)
(bone marrow at 6, 24 and 48 h) 500, 2500, 5000 mg/kg oral equivocal Mizens et al. (1985c)
single dose (± at 48 h)
(bone marrow 6 h after last dose) 50, 125, 250 mg/kg per day weak response Mizens et al. (1985c)
orally for 5 days not dose-related
A bioassay of technical grade chlorothalonil for possible
carcinogenicity was conducted by administering the test chemical in
the diet to Osborne-Mendel rats and B6C3F1 mice. Groups of 50 rats
of each sex were administered chlorothalonil at one of two dose levels
for 80 weeks and then observed for 30-31 weeks. Time-weighted average
doses for both males and females were 5063 or 10 126 mg/kg diet.
Matched controls consisted of groups of 10 untreated rats of each sex;
pooled controls consisted of the matched control groups combined with
55 untreated male or female rats from similar bioassays of five other
test chemicals. All surviving rats were killed at 110-111 weeks.
Groups of 50 mice of each sex were administered chlorothalonil at one
of two dose levels for 80 weeks, then observed for 11-12 weeks. Time-
weighted average doses for males were 2688 or 5375 mg/kg diet, and for
females, 3000 or 6000 mg/kg diet. Matched controls consisted of
groups of 10 untreated mice of each sex; pooled controls consisted of
the matched control groups combined with 50 untreated male or female
mice from similar bioassays of five other test chemicals. All
surviving mice were killed at 91-92 weeks. Clinical signs that
appeared with increasing frequency in dosed rats included haematuria
and, from week 72 until termination of the study, bright yellow urine.
Since the dosed female mice did not have depression in mean body
weights or decreased survival compared with the controls, they may
have been able to tolerate a higher dose. In rats, adenomas and
carcinomas of the renal tubular epithelium occurred with a significant
dose-related trend in both the males and females (males: pooled
controls 0/62, low dose 3/46, high dose 4/49; females: pooled controls
0/62, low dose 1/48, high dose 5/50). These tumours included both
adenomas and carcinomas which are considered to be histogenically
related. In mice, no tumours were found to occur at a greater
incidence among dosed animals than among controls. It was concluded
that under the conditions of this bioassay, technical grade
chlorothalonil was carcinogenic to Osborne-Mendel rats, producing
tumours of the kidney. However, chlorothalonil was not carcinogenic
for B6C3F1 mice (US NCI, 1978).
In a lifetime study on Fischer-344 rats at dietary doses of 0,
40, 80 or 175 mg/kg body weight per day (see section 7.3), a higher
incidence of primary renal tumours of epithelial origin (adenomas and
carcinomas) was seen in the treated groups (0/60, 7/60, 7/60 and 19/60
for males and 0/60, 3/60, 6/60 and 23/60 for females). It was
considered that the increased incidence of renal hyperplasia seen in
this study was associated with the formation of these tumours and
constituted a pre-neoplastic change. Papillomas and carcinomas of the
squamous mucosa of the forestomach were found in rats in the treated
groups (0/60, 1/60, 1/60, 3/60 for males and 0/60, 1/60, 2/60, 7/60
for females). These are probably related to the proliferative non-
neoplastic effects on the squamous mucosa as a result of the chronic
irritation by chlorothalonil (Wilson et al., 1985a, 1986b).
A further dietary study evaluated the carcinogenicity of
chlorothalonil at lower doses of 1.8, 3.8 and 15 mg/kg body weight per
day as well as at 175 mg/kg body weight per day (see section 7.3).
Rats with renal tumours (adenomas and carcinomas) occurred at 1/55,
1/54, 1/54, 4/54, 23/55 (male groups) and 0/55, 0/54, 0/55, 0/53,
32/55 (female groups). This confirmed the effect at 175 mg/kg per day
and an NOEL of 3.8 mg/kg per day was determined for these tumours.
Animals with papillomas and carcinomas of the forestomach occurred at
0/55, 0/54, 3/54, 2/54, 5/55 (male groups) and 1/55, 1/54, 2/55, 5/53,
9/55 (female groups) giving an NOEL of 1.8 mg/kg per day (Wilson et
al., 1989a).
A 2-year study with Charles River CD-1 mice at 750, 1500 or 3000
mg chlorothalonil/kg diet (section 7.3) showed increased incidences of
gastric and renal tumours in the treated groups. Mice with tumours of
the squamous epithelium of the forestomach occurred at 0/60, 2/60,
5/60, 2/60 (male groups) and 0/60, 2/60, 4/60, 5/59 (female groups).
Although not strictly dose-related, these results were considered to
be a treatment effect and linked to the irritant properties of
chlorothalonil. There was also a low incidence of renal tubular
tumours in male treated groups, not seen in controls, at 0/60, 6/60,
4/60, 5/60 (not dose-related). These were probably linked to the high
incidence of renal tubular hyperplasia seen in male mice in the
treated groups (Wilson et al., 1983b, 1986b).
A second study at 0, 15, 40, 175 and 750 mg/kg diet was
undertaken to establish an NOEL for kidney and stomach changes in male
mice (section 7.3). Only two renal tumours (one at 40 mg/kg and one
at 175 mg/kg) were found. There was a slightly higher incidence of
squamous tumours of the forestomach at 750 mg/kg. Taking account of
the overall results of the two studies it was considered that the
tumorigenic NOEL was at least 175 mg/kg diet. In this study, the NOEL
for tubular hyperplasia was 40 mg/kg (equal to 4.5 mg/kg per day) and
the NOEL for hyperplasia/hyperkeratosis in the forestomach was 1.6
mg/kg per day (Wilson et al., 1987).
7.8 Other special studies
Rats fed 0, 1500 or 15 000 mg chlorothalonil/kg diet showed a
dose-related decrease in the retention of a dye, indicating a laxative
effect. A more detailed study attempted to determine the effect of
chlorothalonil on the absorption and utilization of proteins, fats and
amino acids during a 10-week feeding study on a group of 10 male and
10 female rats. It was concluded that the compound did not interfere
directly with the absorption and utilization and that the depressed
weight gain was probably due to catharsis (Paynter, 1967c).
In a study by Andre et al. (1991), mitochondria were obtained
from fresh kidney cortical tissue by homogenization and differential
centrifugation. The mitochondria were incubated in the presence or
absence of sulfur-containing analogues of chlorothalonil and the
degree of mitochondrial respiratory control was evaluated by
polarographic techniques. The following sulfur-containing analogues
of chlorothalonil were tested: the mono-, di-, and tri-thiol analogues
and the mono-, di-, and tri-glutathione analogues. Kidney
mitochondria were incubated with succinate, a site 2 substrate, or
with glutamate, a site 1 substrate, in the presence or absence of the
test material. Mitochondrial respiratory control, expressed as the
acceptor control ratio (ACR), was determined by taking the ratio of
the rate of oxygen consumption in the presence of ADP (state 3) to the
rate of oxygen consumption after the ADP had been consumed (state 4).
When the mono-thiol, mono-, di-, or tri-glutathione analogues of
chlorothalonil and succinate were added to kidney mitochondria, no
significant differences were found in the ACR from the controls.
Incubation of the di- or tri-thiol analogues of chlorothalonil and
succinate with kidney mitochondria resulted in significant differences
of the experimental ACR from the control ACR. When glutamate was used
as the substrate for the electron transport system in kidney
mitochondria, no significant differences from the control were
detected for any of the six test materials. These data suggest that
the effects of the di- or tri-thiol analogues of chlorothalonil may
impair the respiratory control of kidney mitochondria by inhibiting
the transfer of reducing equivalents from succinate to coenzyme Q.
The effects on mitochondrial respiration may be due to the formation
of disulfide bonds between the thiol analogues and proteins.
7.9 Toxicity of metabolites
Most studies have centred on the 4-hydroxy-2,5,6-
trichloroisophthalonitrile metabolite. This is found as a small
proportion of chlorothalonil plant residues (section 4.2.1) and is
also a breakdown product of chlorothalonil in the environment. It has
been identified in faeces of laboratory animals after chlorothalonil
dosing. It is more acutely toxic than chlorothalonil itself (acute
oral LD50 values are 332 and 10 000 mg/kg, respectively).
Several laboratory animal studies have been undertaken with the
4-hydroxy metabolite and have been described in some detail in JMPR
reviews (FAO/WHO, 1978, 1982, 1985). The following is a brief summary
of the studies and their results.
Various effects were noted in rats fed the 4-hydroxy metabolite
at eight dose levels (10 to 750 mg/kg body weight per day) for 61-69
days. Mortality was increased in males at 125 mg/kg per day or more,
and in females at 75 mg/kg per day or more. Body weight was depressed
in both sexes at > 40 mg/kg per day. Anaemia was evident at 75
mg/kg per day or more in males and 40 mg/kg per day or more in
females. Histopathological examination revealed treatment-related
effects in bone marrow and spleen (in the form of erythroid
hyperplasia and depressed granulopoiesis) at > 40 mg/kg per day and
in the liver (haemosiderosis, centrilobular hepatitis) and kidney
(cortical atrophy) at > 75 mg/kg per day. The overall NOEL was 20
mg/kg body weight per day (Murchison, 1979).
A rabbit teratology study was undertaken with oral doses of 0, 1,
2.5 and 5 mg/kg per day during days 6-18 of gestation, with necropsy
at day 28. There was a marginal effect on dams at day 5 but no
evidence of a teratogenic effect in the study (Wazeter & Goldenthal,
1976).
The 4-hydroxy metabolite was evaluated in a three-generation, two
litters/generation study in groups of 15 male and 30 female rats at
dose levels of 0, 10, 60 and 125 mg/kg diet. There were no
treatment-related changes except for body weight reductions at 60 and
125 mg/kg (FAO/WHO, 1982).
In a one-generation follow-up study, groups of rats were fed
diets containing the 4-hydroxy metabolite at 0, 10, 20, 30, 60 and 120
mg/kg diet for 18 weeks before mating (12 males and 24 females per
group). Two sets of mating were undertaken. There was some effect on
live pup weights at 60 and 120 mg/kg. The clear NOEL was considered
to be 30 mg/kg diet (Ford, 1982).
The metabolite was assessed for chronic toxicity and
carcinogenicity in long-term rodent studies. A 2-year rat study was
undertaken at dose levels of 0, 0.5, 3 and 10 mg/kg body weight per
day with groups of 75 males and 75 females. Anaemia was evident at
the highest dose. The NOEL was determined to be 3 mg/kg body weight
per day. There was no evidence for a carcinogenic effect. In the
mouse study, the dietary dose levels were 0, 375, 750 and 1500 mg/kg
diet using groups of 60 males and 60 females. The study was
terminated at 20-22 months because of increasing and high mortality.
A series of effects, including amyloidosis, haemosiderin in the spleen
and increases in reticulocyte counts and red cell haemolysis,
precluded the establishment of an NOEL. No carcinogenic effect was
evident (Hozan & Auletta, 1981; McGee, 1983).
The 4-hydroxy metabolite was not mutagenic in a number of in
vitro and in vivo assays. These were the Salmonella mutagenicity
assay with and without metabolic activation (Banzer, 1977d), a
host-mediated assay in mice given a single intraperitoneal dose of
6.5 mg/kg (Legator, 1974b), Chinese hamster (V-79) and mouse
fibroblast (Balb/3T3) cells in culture with and without activation
(Banzer, 1977e), a micronucleus test in mice at 6.5 mg/kg per day for
5 days (Legator, 1974b), a dominant lethal study in male rats given
single oral doses (0, 2, 4 or 8 mg/kg) singly or daily for 5 days
(Hastings & Clifford, 1975), a dominant lethal study in male mice
given 1, 3 or 6.5 mg/kg per day for 5 days (Legator, 1974b), a DNA
repair assay using S. typhimurium in a spot test with or without
activation (Banzer, 1977f), and a cell transformation assay with rat
embryo cell lines in culture at 0.1, 1 or 10 µg/ml (Price 1978b).
A series of in vitro gene mutation assays with S. typhimurium
tester strains with and without renal metabolic activation were
undertaken with chlorothalonil, four manufacturing impurities and
eight known or potential metabolites. No mutagenic potential was
shown by any of the compounds. Full details were given in the 1985
JMPR evaluation (FAO/WHO, 1986b).
The mutagenic potential of the thiol and cysteine derivatives of
chlorothalonil has been evaluated in the Ames test with and without
metabolic activation with S9 from the kidney of male Fischer rats.
These compounds were 2,5-dichloro-4,6-bismercaptoiso-phthalonitrile,
5-(2,4-dicyano-3,5,6-trichlorophenyl) glutathione, 5-chloro-2,4,6-
trimercaptoisophthalonitrile, S,S'-(2,4-dicyano-3,6-dichloro
phenyl)dicysteine and S,S',S"-(2,4-dicyano-6-chlorophenyl)-
tricysteine (purity ranging from 90.5 to 97.5%). Four other compounds
were used as positive controls. The Salmonella typhimurium tester
strains TA98, TA100, TA1535, TA1537 and TA1538 were used. In all
these studies, there was no significant increase (doubling) over
solvent control values in the number of revertants for any of the five
tester strains used either with or without metabolic activation
(Mizens et al., 1985d,e, 1986b,c, 1987).
A description of a 90-day rat study on the monoglutathione
conjugate of chlorothalonil is given in section 7.3.
8. EFFECTS ON HUMANS
8.1 General population exposure
A case of acute facial dermatitis in a 53-year-old man, caused by
staying in a summer cottage, has been reported. Patch testing
revealed contact allergy to the paint that was applied to all the
window-frames, and to the chlorothalonil contained in the paint.
After removal of the frames, there were no further recurrences of
facial dermatitis. The authors suggested that products containing
chlorothalonil are not suitable for indoor use (Liden, 1990; Eilrich &
Chelsky, 1991).
8.2 Occupational exposure
Chlorothalonil contact dermatitis was observed in a number of
employees in a chlorothalonil manufacturing plant. There were 19
cases out of 103 employees. About 60% of the employees showed some
kind of skin abnormality compared with 18.5% of employees not working
with chlorothalonil. When the hygiene conditions of the plant were
improved the overall proportion of skin abnormalities fell to about
20% and there were no cases of chlorothalonil contact dermatitis
(Diamond Shamrock, 1980).
Wood preservatives containing chlorothalonil have also been
implicated in the appearance of allergic contact dermatitis. One
report concerned a Danish cabinet maker who developed dermatitis on
his hands after 9 months of painting furniture with preservative
containing the compound. This was possibly caused by contact via the
wood dust after sandpapering (Bach & Pedersen, 1980). Another report
referred to three cases, two with erythema on the face, particularly
periorbitally, and one with eczema of the hands, in people engaged in
similar work (Spindeldreier & Deichmann, 1980). The four people in
these cases showed a positive reaction to patch tests with 0.01%
chlorothalonil in acetone.
A further case of contact dermatitits was described by Meding
(1986) in a 33-year-old male painter, who regularly worked with paint
containing chlorothalonil. A patch test with chlorothalonil was
positive.
Work-related skin complaints occurred in a Norwegian factory
producing wooden window frames. The wood preservative used was white
spirit containing 0.5% chlorothalonil. Fourteen out of 20 workers
experienced some kind of skin reaction including pruritus, erythema
and oedema of the eyelids and other facial regions, and eruptions on
arms and hands. Seven of these 14 subjects yielded a positive patch
test reaction with 0.01% chlorothalonil in acetone compared with 1 out
of 14 controls (Johnsson et al., 1983).
Allergic contact dermatitis has also been described in Japanese
farmers (Horiuchi & Ando, 1980) and in Dutch horticultural workers
(Bruynzeel & van Ketel, 1986) using chlorothalonil fungicide
formulations.
In a group of 84 tea growers, two showed a positive skin patch
test with 0.02% chlorothalonil in petrolatum (Fujita, 1985).
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Aquatic microorganisms
The algicidal activity of chlorothalonil was examined by Goulding
(1971). It was shown that this compound is effective against a range
of algae including Chlorella, Chlamydomonas, Ulothrix , Anabaena,
Oscillatoria and Microcystis at low concentrations, often
less than 1 µg/litre. It was also effective on natural populations
of algae obtained from lakes, rivers, and reservoirs. The effect
was generally less after 300 h than after 150 h and was dependent upon
the size of the initial cell inoculum.
Walker et al. (1984) describe a simple shake-flask screening test
to evaluate pesticide persistence and aquatic toxicity in the
laboratory. Four systems were used: active sediment, sterile
sediment, active water and sterile water. Chlorothalonil at
162 µg/litre did not increase the mortality of Mysidopsis above the
control level within 96 h. The authors concluded that degradation of
chlorothalonil involved microorganisms and that the degradation
products did not enhance the aquatic toxicity of chlorothalonil.
9.1.1.2 Soil microorganisms
Chlorothalonil, at dose levels up to 5000 mg/litre in bacterial
suspensions, inhibited the growth of three strains of Rhizobium
japonicum (Tu, 1980).
Chlorothalonil, at concentrations up to 1000 mg/kg medium, did
not inhibit or stimulate the growth of any one of 25 strains of
Rhizobium bacteria isolated from red clover root nodules
(Heinonen-Tanski et al., 1982).
Several studies were conducted with chlorothalonil in soil to
determine the effects (if any) on normal soil processes such as
nitrogen fixation, nitrification and degradation of substrates such as
protein, pectin, cellulose and starch. These studies were conducted
at two rates: use rate (2.5 mg/kg) and 10 times the use rate
(25 mg/kg). In general, any inhibitory effects observed were
temporary in nature and more pronounced at the high rate. No effects
on the use of protein, pectin or cellulose by soil microorganisms were
observed, but there was increased utilization of starch (Szalkowski et
al., 1980).
The results obtained by Szalkowski et al. (1981a), who studied
the effect of chlorothalonil on non-symbiotic nitrogen-fixing soil
microorganisms, are given in Table 13.
Table 13. The effect of chlorothalonil on non-symbiotic nitrogen-fixing soil microorganisms
Sandy loam Clay loam
Application Ten times Application Ten times
rate application rate application
rate rate
Aerobic nitrogen initial inhibition inhibition at no effect stimulation
fixation days 0, 21 and
28
Anaerobic nitrogen general stimulation inhibition up no effect stimulation
fixation to day 21
Szalkowski et al. (1981b) studied the effect of chlorothalonil on
nitrogen transformation in sandy loam and clay loam soils at two
rates: one equivalent to the application rate and one equivalent to
ten times this rate. In sandy loam soil, there was a consistent
inhibitory effect at the higher rate, which was reduced with time. In
clay loam soil, at the higher rate, inhibition was no longer observed
after day 21. In both types of soil at the normal rate, there was
little if any inhibition.
9.1.2 Aquatic organisms
The acute toxicity of chlorothalonil to various aquatic species
is shown in Table 14. Some of these toxicity tests were carried out
in static or semi-static systems and others in flow-through systems.
Table 14. Acute toxicity of chlorothalonil to aquatic organisms
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Rainbow trout
(Oncorhynchus mykiss)
6-11 g flow through freshwater 80% 14 acetone > 99% 19.0 17.1 Davies &
6-11 g flow through freshwater 53% 16 acetone > 99% 18.8 10.5 White
6-11 g semi-static freshwater 90% 10 acetone > 99% 18.0 (1985)
(24 h)
- static freshwater - 12 acetone 96% 56 49 SDS Biotech
(soft) Corporation
(1980a)
3.5-4.0 g static freshwater 6.5-7.4 8.4-11.2 12.5-15.5 acetone 97.8% 76 Ernst et
(12.3 mg/litre) mg/litre al. (1991)
3.5-4.0 g static freshwater 6.5-7.4 8.4-11.2 12.5-15.5 Bravo 500 69 Ernst et
(12.3 mg/litre) mg/litre al. (1991)
Bluegill
(Lepomis macrochirus)
- static freshwater 22 acetone 96% 46-77 62 SDS Biotech
(soft) Corporation
(1979)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Common jolly tail
(Galaxias maculatus)
7-10 g flow through freshwater 75% 16 acetone 99% 18.2 16.3 Davies &
White (1985)
Spotted galaxis
(G. fruttaceus)
8-20 g flow through freshwater 75% 16 acetone 99% 25.8 18.9 Davies &
White (1985)
Golden galaxias
(G. auratus)
7-11 g flow through freshwater 75% 13 acetone 99% 46.6 29.2 Davies &
White (1985)
Three spine stickleback
(Gasterosteus aculeatus)
0.3 g static freshwater 7.7-8.0 9.2-9.5 9-10 Bravo 500 < 73 Ernst et
mg/litre al. (1991)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Channel catfish
(Ictalurus punctalus)
40-80 g semi-static, freshwater 7.0-7.2 5.0-6.0 23 acetone 99% 62 52 Gallagher et
24 h (30 mg/litre) mg/litre al. (1992)
- static freshwater 22 acetone 96% 55 44 SDS Biotech
(soft) Corporation
(1980b)
Spot
(Leiosfomus xanthurus)
- flow through brackish water 11 technical 32 Mayer (1987)
(22 ppt)
Sheepshead minnow
(Cyprinodor variegatus)
3-7 days static marine technical 32 SDS Biotech
Corporation
(1982b)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Water flea
(Daphnia magna)
- static freshwater 7.7-8.1 9.1-9.3 20-22 Bravo 500 97a Ernst et
(250 mg/litre) mg/litre al. (1991)
Dungeness crab
(Cancer magister)
larvae semistatic, marine 13 Bravo, 75% 560 140 Armstrong
24h (25 ppt) et al. (1976)
Clam
(Mya arenaria)
5.2 cm static marine 7.3-8.0 8.5-9.9 10.5-12 Bravo 500 35 000 Ernst et
(30-31 ppt) mg/litre al. (1991)
Blue mussel
(Mytilus edulis)
5.9 cm static marine 7.3-8.0 8.5-9.9 10.5-12 Bravo 500 5940 Ernst et
(30-31 ppt) mg/litre al. (1991)
Table 14. (Cont'd)
Stage (weight Test Freshwater/ pH O2 Temperature Solvent Purity 48-h LC50 96-h LC50 Reference
or length) systems marine (°C) (µg/litre) (µg/litre)
(hardness)
Eastern oyster
(Crassostrea virginica)
- flow through marine 29 technical 26b Mayer (1987)
(27 ppt)
a EC50 - immobility
b EC50 - shell deposition
In view of the strong adsorption and degradation characteristics of
chlorothalonil, nominal concentrations in static systems are likely to
underestimate the toxicity (overestimate the LC50) of chlorothalonil.
At the same time, it should be pointed out that the static test
systems more closely resemble the field situation.
Davies & White (1985) described the reactions of fish to
exposure. Oncorhynchus mykiss and Galaxias sp showed marked
lethargy, the degree increasing with time and concentration of
exposure. O. mykiss showed normal startle reactions at
concentrations below 8.7 µg/litre, and G. maculatus, G. truttaceus
and G. auratus at 8.8, 9.0 and 13.3 µg/litre, respectively, over
96 h. In O. mykiss, loss of startle reaction was followed by
reduction of activity, and permanent lethargy was followed by loss of
righting ability and death. In Galaxias sp, the onset of lethargy
was accompanied by varying degrees of fin collapse.
Davies & White (1985) commented on the fact that most acute
toxicity values have been obtained in tests where solvent was added to
the chlorothalonil.
A 48-h EC50 has been reported for the pink shrimp ( Penaeus
duorarum) at 320 µg/litre and a 96-h EC50 for the eastern oyster
( Crassostrea virginica) at 26 µg/litre. The value for the pink
shrimp was based on immobility or loss of equilibrium and that for the
eastern oyster on shell deposition (Mayer, 1987). Oysters suffered a
42% mortality when exposed to 1000 µg chlorothalonil per litre for
96 h and a 25% reduction in growth at 10 µg/litre (SDS Biotech
Corporation, 1983b).
Ernst et al. (1991) used both laboratory bioassay and field
treatments of a pond system to determine the toxic effects of
chlorothalonil on aquatic fauna. The acute toxicity of technical
chlorothalonil and a commercial formulation on five species, including
mussel and clams, was determined (Table 14). In the field study also
reported by O'Neill (1991) (see section 5.1.2 for measured
concentrations after spraying), the mortality of caged invertebrate
and vertebrate species was monitored. Seven species were caged in the
stream flowing from the treated pond: water boatman (Sigara
alternata), caddisfly larva ( Limnephilus sp), freshwater clam
( Pisidium, sp), crawling water beetle ( Haliplus sp), scud
( Gammarus spp.), stickleback (Gasterosteus aculeatus) and midge
larva (Chironomidae). The floating cages were placed near the
surface of the water. The water boatman suffered the highest
mortality, ranging from 49 to 84% in replicate cages; mortality in a
control pond was 16-20% over the same period (24 h). Midge larva
mortality (69%) was judged by the authors to be a consequence of
handling. Stickleback mortality was 37 to 56% in the treated pond,
compared to 2 to 6% in the controls. Caddisfly larvae, clams, beetles
and scud showed no deaths during the 24 h. Rainbow trout
(Oncorhynchus mykiss) also showed no mortality following spraying.
An estimate of total invertebrate numbers before and after spraying in
the pond showed a slight increase following the first spray and a
slight reduction after the second (not statistically significant).
The control pond also showed fluctuations. The total was heavily
influenced by Chironomid midge larvae, which was by far the most
frequently occurring species. This study showed that a lower
toxicological effect was observed in the field study than in the
laboratory bioassays, indicating a reduction in exposure to the
available chlorothalonil and thus less severe impacts in the pond
system through physical and chemical processes.
Gallagher et al. (1992) showed that sublethal chlorothalonil
exposure may cause acute necrosis of the intestinal epithelial lining
in channel catfish. Exposure to 13 µg/litre for 72 h resulted in
increased tissue GSH concentrations in liver, posterior kidney and
gills, which suggests a protective role for tissue GSH against
chlorothalonil exposure.
When two generations of Daphnia magna were exposed to technical
chlorothalonil at levels of 6.2, 12, 25, 50 and 100 µg/litre for 21
consecutive days during each generation, adverse effects on adult
survival and reproduction were observed at a nominal concentration of
100 µg/litre. The maximum acceptable toxicant concentration (MATC)
for technical chlorothalonil, based upon nominal concentrations, was
50 µg/litre (35 µg/litre was the measured concentration) (Shults et
al., 1982).
Fathead minnows (Pimephales promelas) were continuously exposed
in duplicate aquaria to nominal concentrations of 25, 12.5, 6.3, 3.1
and 1.5 µg/litre (measured concentrations 16, 6.5, 3.0, 1.4 and
0.6 µg/litre, respectively) technical chlorothalonil, a diluent water
control, and a solvent (acetone) control throughout a complete (egg to
egg) life cycle. No significant effects were observed in either
generation at mean measured concentrations < 3.0 µg/litre. The
first generation (F0) eggs exhibited a significantly reduced
hatchability and survival of fry after 35 days when exposed to a mean
measured concentration of 16 µg/litre. The reproductive success of
Fo fish was adversely affected (reduction in the number of eggs per
spawn) by exposure to concentrations > 6.5 µg/litre. The second
generation (F1) eggs exhibited a significantly reduced hatchability
when exposed to a mean measured concentration of 6.5 µg/litre. The
survival of fry at this concentration was not affected. Based on
these data, the MATC (mean measured) of technical chlorothalonil in
water for fathead minnows was estimated to be in the range of 3.0 to
6.5 µg/litre (Shults et al., 1980).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
In a study by Stephenson et al. (1980), 30-day-old tomato plants
were treated with chlorothalonil (at 2.5 kg/ha) or chlorothalonil in
combination with metribuzine. The effect was assessed as the tomato
shoot dry weight (as a percentage of control plant weight). On this
basis, the weight of the chlorothalonil-treated plants was 89% of the
control plant weight, a difference which was statistically significant
(P > 0.05). The effect was additive when chlorothalonil was used in
combination with metribuzine.
9.1.3.2 Earthworms
When chlorothalonil suspension concentrate (500 g/litre) was
added to artificial soil containing earthworms (Eisenia foetida),
the treated soil was non-toxic after 7 and 14 days. The LC50 was
found to be > 1000 mg/kg soil (on a dry weight basis) (Wuthrich,
1990).
When earthworms (Eisenia foetida) were immersed for 1 min in
solutions of chlorothalonil (0.1, 1 and 2% w/v); there was no effect
on survival. Bermuda grass clippings were air dried and ground; 15 g
samples were then stirred into 100 ml of 0.1% chlorothalonil and
subsequently fed to earthworms after filtration of excess liquid.
There was no effect on longevity. Worms reared in soil in which
chlorothalonil had been incorporated showed reduction in longevity of
about 50% compared to controls 52-84 days after the beginning of
treatment. The amount of chlorothalonil added was equivalent to 5
times the recommended application rate at 0.9 g in 4700 cm3 of soil,
and reproduction was virtually eliminated (Roark & Dale, 1979).
9.1.3.3 Earwigs and honey-bees
Earwigs (Labidura riparia) were exposed to chlorothalonil in
three ways: a) on glass; b) as a residue on peanut foliage; and c) as
a residue on a food source (i.e. 7-day-old armyworms). In the first
treatment, chlorothalonil at a rate of 0.72 kg/ha produced up to 20%
mortality in 24 h and up to 30% in 48 h. In the second experiment,
chlorothalonil was applied, at the same rate, to peanut foliage which
was then used for the test. There was a 10% mortality within 24 h and
20% in 48 h on 4-day-old residues and no mortality on 8-day-old
residues. In the food source experiment there was 25-30% mortality
within 24 h of the larvae being consumed and up to 55% mortality
within 48 h (DeRivero & Poe, 1981).
Atkins et al. (1975) found no contact toxicity of chlorothalonil
(11 µg/bee), and classified it as relatively non-toxic to honey-bees.
The oral LD50 for chlorothalonil in 20% sucrose solution for
honey-bees was > 0.2 µg/bee and the contact LD50 > 65 µg/bee
(Davies, 1986).
9.1.3.4 Birds
The following toxicity values have been reported for birds, but
without descriptions of the experimental methodology. The acute oral
LD50 in the mallard duck was reported to be > 4640 mg/kg. The 8-day
dietary LC50 in the same species and in the bobwhite quail (Colinus
virginianus) was given as > 10 000 mg/kg diet in each case (SDS
Biotech Corporation, 1981a,b). Dietary 8-day LC50 values were also
reported as > 21 500 mg/kg diet for the mallard duck and 5200 mg/kg
diet for the bobwhite quail (Worthing, 1991).
Shults et al. (1988a) evaluated the effect of chlorothalonil on
reproduction in the bobwhite quail. Four groups (16 pairs per group)
of quail were administered chlorothalonil in the diet at levels of
1000, 5000 and 10 000 mg/kg for a period of 21 weeks. Quail were fed
the amended diet for 11 weeks prior to egg laying and for the duration
of the egg laying period. Adult and offspring were examined for body
weight, general health, adult food consumption, egg production,
eggshell thickness, embryo viability, hatching success survivability
of offspring and gross pathology. At a dietary concentration of
10 000 mg/kg, the birds experienced reproductive impairment, which
included mortality, general health, body weight, food consumption,
gross pathology and other reproductive end-points. Hatching survival
was also affected at the highest dosage. General health and
reproductive parameters were also affected at 5000 mg/kg. Other
effects observed at this dose level included decreased body weight
gain and survivability of offspring. The lowest test dosage showed no
apparent affects on either adult quail or offspring. A
no-observed-effect concentration (NOEC) of 1000 mg/kg diet was
established for chlorothalonil regarding reproductive effects.
In a separate but similar reproductive study with mallard ducks
(Anas platyrhynchos), Shults et al. (1988b) reported that
10 000 mg/kg diet reduced egg production and the percentage of
hatchlings per incubated egg. No reproductive impairments were
observed for ducks dosed at 1000 or 5000 mg/kg. Shults et al. (1988b)
reported no effect on eggshell thickness at these chlorothalonil
concentrations. The NOEC for reproductive effects in mallard duck was
5000 mg/kg diet.
9.2 Field observations
9.2.1 Soil microorganisms
Smiley & Craven (1979) applied chlorothalonil (as Daconil 2787)
at the normal rate (actual dose not given) every 21 days between April
and September (9 applications for 3 consecutive years) to an
experimental plot of Sward Kentucky blue grass (Pia pratensis).
Treated plots were 1 × 5 m and were replicated. Samples of soil cores
2.54 cm in diameter and 3 cm deep were taken (n = 5) from each
replicate plot. The cores included thatch and were chopped and mixed
well, and 10 g was suspended in sterile distilled water. A dilution
series was plated out to estimate bacterial, actinomycete and fungal
populations. None of the organisms were affected by chlorothalonil.
The fungicide did not affect numbers of Nitrosomonas or Nitrobacter
bacteria and had no effect on the disappearance of added ammonium by
nitrification. Treatments alternating different fungicides had a
greater effect than one fungicide alone.
9.2.2 Plants
Many crop plants are tolerant of chlorothalonil and do not suffer
any phytotoxicity when sprays of chlorothalonil formulations are
applied according to recommended practices. However, a few phytotoxic
effects have been observed with certain species as a direct result of
chlorothalonil applications and also as a result of physiologically
incompatible mixtures of chlorothalonil formulations with additives or
other pesticides.
Chlorothalonil applied at a rate of 2.52 kg/ha did not affect
yields of tomato plants grown in field conditions. In addition,
chlorothalonil did not enhance metribuzin damage when the two
compounds were used in combination on tomato plants. These results
are contrary to those obtained in laboratory studies (section 9.1.3)
and indicate that under field conditions phytotoxic reactions between
chlorothalonil and metribuzin are unlikely to occur (Stephenson et
al., 1980).
Clear results were not obtained concerning the effect of
chlorothalonil on perennial rye grass (Lolium perenne) when it was
sprayed regularly for the control of leaf-spotting disease. In the
first year of treatment the grass yield was increased by 15% at the
third harvest, but at the following harvest, when disease incidence
was greater, the yield was not increased. The authors concluded that
chlorothalonil did not have a direct stimulatory effect on grass
growth (Lam & Lewis, 1983).
Chlorothalonil was used to treat onion beds at a rate of
2.34 litres formulation/ha with 10 weekly applications. The onions
(two short-day cultivars) were harvested either 146 or 167 days after
planting. Chlorothalonil reduced the weight and size of marketable
bulbs by 44 and 32%, respectively, but did not influence the
maturation rate. Alternate application of chlorothalonil and mancozeb
reduced these values by 27 and 33%, respectively. The disease
incidence was nil in all treatments (Stoffella & Sonoda, 1982).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed
and evaluated chlorothalonil at its meetings in 1974, 1977, 1978,
1979, 1981, 1983, 1985, 1987, 1990 and 1992 (FAO/WHO, 1975, 1978,
1979, 1980, 1982, 1985, 1986a,b, 1988, 1990a,b, 1993b). In 1990 an
acceptable daily intake (ADI) of 0-0.03 mg/kg body weight was
established. This ADI was confirmed in 1992, based on the NOAEL of 3
mg/kg body weight/day established in the two-year dog study (FAO/WHO,
1993a,c).
The Joint FAO/WHO Codex Alimentarius Commission has established
maximum residue limits (MRLs) for chlorothalonil in various
commodities (FAO/WHO, 1993c).
WHO has classified chlorothalonil as a technical product unlikely
to present an acute hazard in normal use (WHO, 1992).
On the basis of data available at the time, the International
Agency for Research on Cancer evaluated chlorothalonil as showing
limited evidence of carcinogenicity in animal studies and categorized
it as an agent not classifiable as to its carcinogenicity to humans
(IARC, 1987).
REFERENCES
André JC, Marciniszyn JP, & Killeen JC Jr (1991) Evaluation of
mitochondrial function in the presence and absence of sulphur-
containing analogs of chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 3113-88-0107-AM-001,
submitted to WHO by ISK-Biotech, Mentor, Ohio, USA).
Armstrong DA, Buchanan DV, & Caldwell RS (1976) A mycosis caused by
Lagenidium sp.in laboratory-reared larvae of the dungeness crab,
cancer magister, and possible chemical treatments. Department of
Fisheries and Wildlife, Oregon State University, Marine Science
Center, Newport, Oregon 97365. J Invertebr Pathol, 28: 329-336.
Atkins EL, Greywood EA, & MacDonald RL (1975) Toxicity of pesticides
and other agricultural chemicals to honey bees. Riverside, California,
University of California, Division of Agricultural Sciences, 37 pp
(Report No. 2287).
Bach B & Pedersen NB (1980) Contact dermatitis from a wood
preservative containing tetrachloroisophthalonitrile. Contact
Dermatitis, 6: 142.
Ballee DL, Duane WC, Stallard DE, & Wolfe AL (1976) Chlorothalonil.
In: Zweig G & Sherma J ed. Analytical methods for pesticides and plant
growth regulators. New York, Academic Press, vol VIII, pp 266-274.
Banzer CB (1977a) Activity of chlorothalonil in the Salmonella/
microsomal assay for bacterial mutagenicity. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 000-5TX-77-0035-001).
Banzer CB (1977b) Activity of chlorothalonil in a test for
differential inhibition of repair deficient and repair competent
strains of Salmonella typhimurium: Repair test. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 000-5TX-77-0033-001).
Banzer CB (1977c) Activity of chlorothalonil in an in vitro
mammalian cell point mutation assay. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 000-5TX-77-0034-001).
Banzer CB (1977d) Activity of DTX-0038 in the Salmonella/microsomal
assay for bacterial mutagenicity. Bethesda, Maryland, Microbiological
Associates Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Banzer CB (1977e) Activity of DTX-77-0040 in an in vitro mammalian
cell point mutation assay. Bethesda, Maryland, Microbiological
Associates Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Banzer CB (1977f) Activity of DTX-77-0039 in a test for differential
inhibition of repair deficient and repair competent strains of
Salmonella typhimurium: Repair test. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Blackmore R & Shott L (1968) Final report four-month feeding study -
rats. Hazleton Laboratories, Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Bruynzeel DP & van Ketel WG (1986) Contact dermatitis due to
chlorothalonil in floriculture. Contact Dermatitis, 14: 67-68.
Burchfield HP & Storrs EE (1977) Residue analysis. In: Seigel MR &
Sisler HD ed. Antifungal compounds. New York, Marcel Dekker, Inc., vol
1, pp 463-505.
Busey WM (1975) Histopathological incidence data - kidney. Herndon,
Virginia, Experimental Pathology Laboratory, Inc. (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Busey WM (1985) A 90-day feeding study in mice with T-117-11.
Experimental Pathology Laboratories, Inc. (Unpublished revised
pathology report No. SDS 753-5TX-85-0053-002, submitted to WHO by SDS
Biotech, Mentor, USA).
Camoni I, DiMuccio A, Pontecorvo D, Rubbiani M, Vergori L, & Lugaresi
C (1991) Residue levels in apples and pears field-treated with two
experimental chlorothalonil formulations. Bull Environ Contam Toxicol,
46: 361-367.
Chin BH, McGloin JB, Spangler NL, & Heilman RD (1981) Chlorothalonil
equivalents in the blood and urine of rats following oral,
endotracheal and dermal administration of 14C-chlorothalonil. Bull
Environ Contam Toxicol, 26: 258-261.
Danks A & Fowler JSL (1988) Chlorothalonil technical: acute inhalation
toxicity study in the rat. Eye, Suffolk, United Kingdom, Life Science
Research Ltd (Report No. 88/CFA 00I/85, submitted to WHO by ISK-
Biotech, Mentor, USA).
Davies PE (1985a) The toxicology and metabolism of chlorothalonil in
fish. II. Glutathione conjugates and protein binding. Aquat Toxicol,
7: 265-275.
Davies PE (1985b) The toxicology and metabolism of chlorothalonil in
fish. III. Metabolism, enzymatics and detoxication in Salmo spp and
Galaxias spp. Aquat Toxicol, 7: 277-299.
Davies PE (1985c) The toxicology and metabolism of chlorothalonil in
fish. IV. Zinc coexposure and the significance of metallothionein in
detoxication in Salmo Gairdneri. Aquat Toxicol, 7: 301-306.
Davies LG (1986) Report on a laboratory investigation into the
toxicity of Sipcam chlorothalonil to honey bees (Apis mellifera).
Nottingham, Trent Polytechnic, Department of Sciences (Report
submitted to WHO by ISK-Biotech, Mentor, USA).
Davies PE (1988) Disappearance rates of chlorothalonil (TCIN) in the
aquatic environment. Bull Environ Contam Toxicol, 40: 405-409.
Davies PE & White RWG (1985) The toxicology and metabolism of
chlorothalonil in fish I. Lethal levels for Salmo gairdneri,
Galaxias maculatus, G. truttaceus and G. auratus and the fate of
14C-TCIN in S. gairdneri. Aquat Toxicol, 7: 93-105.
DeRivero NA & Poe SL (1981) Response of Labidura riparia (Pallas) to
residues of pesticides used on peanuts. Peanut Sci, 8: 93-96.
Diamond Shamrock (1974) Unpublished report on the pesticide
chlorothalonil, submitted to FAO/WHO by Diamond Shamrock Corporation,
Mentor, Ohio, USA.
Diamond Shamrock (1980) Unpublished toxicological report, submitted to
the 1981 JMPR by Diamond Shamrock Corporation, Mentor, Ohio, USA.
Doyle R & Elsea J (1963) Acute oral, dermal, and eye toxicity and
irritation studies on DAC-2787. Hill Top Research Institute Inc.
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
Duane WC (1970) Biodegradation of Daconil 2787. Mentor, Ohio, Diamond
Shamrock Corporation (Unpublished report).
Eilrich GL & Chelsky M (1991) Letters to the editor : Facial
dermatitis caused by chlorothalonil in paint. Contact Dermatitis, 25:
141-144.
Elliott VJ & Spurr HW (1993) Temporal dynamics of chlorothalonil
residues on peanut foliage and the influence of weather factors and
plant growth. Plant Dis, 77: 455-460.
Ernst W, Doe K, Jonah P, Young J, Julien G, & Hennigar P (1991) The
toxicity of chlorothalonil to aquatic fauna and the impact of its
operational use on a pond ecosystem. Arch Environ Toxicol, 21: 1-9.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO (1985) Guidelines for the disposal of waste pesticide and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO/WHO (1975) 1974 Evaluations of some pesticide residues in food:
The monographs. Geneva, World Health Organization, pp 101-148 (WHO
Pesticide Residues Series, No. 4).
FAO/WHO (1978) Pesticide residues in food - 1977. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 10 Sup.).
FAO/WHO (1979) Pesticide residues in food - 1978. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 15 Sup.).
FAO/WHO (1980) Pesticide residues in food - 1979. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 20 Sup.).
FAO/WHO (1982) Pesticide residues in food - 1981. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1985) Pesticide residues in food - 1983. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 61).
FAO/WHO (1986a) Pesticide residues in food - 1985. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 72/1).
FAO/WHO (1986b) Pesticide residues in food - 1985. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 72/2).
FAO/WHO (1988) Pesticide residues in food - 1987. Evaluations: Part II
- Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 86/2).
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Codex Committee on Pesticide
Residues, Food and Agriculture Organization of the United Nations
(CAC/PR8-1989).
FAO/WHO (1990a) Pesticide residues in food - 1990. Evaluations: Part I
- Residues. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 103/1).
FAO/WHO (1990b) Pesticide residues in food - 1990. Evaluations: Part
II - Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 103/2).
FAO/WHO (1991) Pesticide residues in food - 1990. Evaluations: Part II
- Toxicology. Geneva, World Health Organization (WHO/PCS/91.47).
FAO/WHO (1993a) Pesticide residues in food - 1992. Report. Rome, Food
and Agriculture Organization of the United Nations (FAO Plant
Production and Protection Paper 116).
FAO/WHO (1993b) Pesticide residues in food - 1992. Evaluations: Part
II - Toxicology. Geneva, World Health Organization (WHO/PCS/93.34).
FAO/WHO (1993c) Codex Alimentarius. Volume 2: Pesticide residues in
food. Rome, Food and Agriculture Organisation of the United Nations.
Flannigan SA & Tucker SB (1985) Influence of the vehicle on irritant
contact dermatitis. Contact Dermatitis, 12: 177-178.
Flannigan SA, Tucker SB, & Calderon V (1986) Irritant dermatitis from
tetrachloroisophthalonitrile. Contact Dermatitis, 14: 258-259.
Ford WH (1982) A one-generation reproduction study in rats with
DS-3701. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Ford WH, Killeen JC, & Baxter RA (1987a) A 90-day feeding study in
rats with chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 1115-85-0079-TX-006).
Ford WH, Killeen JC, & Baxter RA (1987b) A 90-day study in rats with
the monoglutathione conjugate of chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1108-85-0078-TX-006).
Frazier HW (1993) Chlorothalonil: A response to Environmental Fate and
Ground Water Branch (EFGWG) review. Painesville, Ohio, Ricerca, Inc.
(Document No. 3254-93-0280-EF-001, submitted to WHO by ISK Biosciences
Corporation, Mentor, USA).
Fujita Y (1985) Studies on contact dermatitis from pesticides in tea
growers. Acta Med Univ Kagoshima, 27(1): 17-37.
Fullmore GE & Leveglia J (1992) A thirty-day toxicity study in dogs
with T-117-2. Painesville, Ohio, Ricerca, Inc. (Unpublished report
No. 5092-91-0554-TX003, submitted to WHO by ISK-Biotech Corporation,
Mentor, USA).
Gallagher EP, Cattley RC, & Di Giulio RT (1992) The acute toxicity and
sublethal effects of chlorothalonil in channel catfish (Ictalurus
punctatus). Chemosphere 24: 3-10.
GIFAP (1987) Guidelines for the avoidance, limitation and disposal of
pesticide waste on the farm. Brussels, International Group of National
Associations of Manufacturers of Agrochemical Products.
Gilvydis DM & Walters SM (1988) Rapid liquid chromatographic analysis
of chlorothalonil in fresh produce using photoconductivity and UV
detectors in tandem. J Agric Food Chem, 36: 957-961.
Goulding KH (1971) A preliminary investigation of a potential new
algicide. Proceedings of the 6th British Insecticides and Fungicides
Conference, pp 621-629.
Green T, Odum J, Nash JA, & Foster JR (1990) Perchloroethylene-induced
rat kidney tumours: An investigation of the mechanisms involved and
their relevance to humans. Toxicol Appl Pharmacol, 103: 77-89.
Gutenmann WH & Lisk DJ (1966) Metabolism of Daconil and Dacthal
pesticides in lactating cows. J Dairy Sci, 49(10): 1272-1276.
Hastings TF & Clifford D (1975) Eight-week dominant lethal study of
DAC-3701 in rats. Bio/Tox Research Laboratories, Inc. (Unpublished
report submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Hatzenbeler CJ (1991) An aerobic aquatic metabolism study with
14C-chlorothalonil. Painesville, Ohio, Ricerca, Inc. (Document No.
3163-90-0240-EF-001, submitted to WHO by ISK Biosciences Corporation,
Mentor, USA).
Heinonen-Tanski H, Oros G, & Kecskes M (1982) The effect of soil
pesticides on the growth of red clover rhizobia. Acta Agric Scand, 32:
283-288.
Holmes DC & Wood NF (1972) Removal of interfering substances from
vegetable extracts prior to the determination of organochlorine
pesticide residues. J Chromatogr, 67: 173-174.
Holsing G & Shott L (1970) Two-year dietary administration - rats.
Vienna, Virginia, Hazleton Laboratories, Inc. (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Holsing GC & Voelker RW (1970) 104-Week dietary administration - dogs,
Daconil 2787 (technical). Hazleton Laboratories, Inc. (Unpublished
report submitted to WHO by Biotech Corporation, Mentor, USA).
Horiuchi N & Ando S (1980) Contact dermatitis due to pesticides for
agricultural use. Jpn J Dermatol, 90: 289.
Hozan GK & Auletta CS (1981) A chronic dietary study in mice with
T-114 (DS-3701). East Millstone, New Jersey, BioDynamics, Inc.
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
IARC (1983) Chlorothalonil. In: Miscellaneous pesticides. Lyon,
International Agency for Research on Cancer, pp 319-328 (IARC
Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 30).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer, p 60 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Supplement 7).
Ignatoski JA, Killenen JC, & Marciniscyn JP (1983) Distribution of
radioactivity following oral administration of 14C-chlorothalonil to
rats: extraction and analysis of 14C-materials in excreta. Mentor,
Ohio, Diamond Shamrock Corporation (Unpublished report).
IRPTC (1989) Data profile on chlorothalonil. Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
ISK Biosciences Corporation (1995) A summary of chlorothalonil
metabolism under various environmental conditions in the
chlorothalonil summary dossiers for the inclusion of active substances
in Annex 1 of Directive 91/414/EEC. Mentor, Ohio, ISK Biosciences
Corporation.
Jacobson BM & Schollenberger JM (1992) Dissipation of 4-hydroxy-2,4,5-
trichloroisophthalonitrile (SOS-3701) in soil: A re-analysis of
chlorothalonil soil dissipation data. Painesville, Ohio, Ricerca, Inc.
(Proprietary report No. 1874-T, submitted to WHO by ISK-Biotech,
Mentor, USA).
Jarrett RD, Stallard DE, & Bachand RT (1978) Absorption, excretion and
tissue distribution of orally administered 14C-hydroxy 2,5,6-
trichloroisophthalonitrile (14C-DAC 3701) in male Sprague-Dawley
rats. Painsville, Ohio, T.R. Evans Research Centre (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, USA).
Johnsson M, Buhagen M, Leira HL, & Solvang S (1983) Fungicide induced
contact dermatitis. Contact Dermatitis, 9: 285-288.
Jones RE, Killeen JC Jr, & Ignatoski JA (1984) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 694-5TX-84-0064-002).
Jongen NJ, Engel R, & Leenheers LH (1991) Determination of the
pesticide chlorothalonil by HPLC and UV detection for occupational
exposure assessment in greenhouse carnation culture. J Anal Toxicol,
15: 30-34.
Katayama A, Isemura H, & Kuwatsuka S (1991) Suppression of
chlorothalonil dissipation in soil by repeated applications. J Pestic
Sci, 16: 233-238.
Kawamura Y, Takeda M, & Uchiyama M (1978) Photolysis of chlorothalonil
in benzene. J Pestic Sci, 3: 397-400.
Kenyon RG & Ballee DL (1990) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1989). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-007).
Kenyon RG & Wiedmann JL (1992a) Analytical methods including recovery
rates and the limits of determination for residues in water. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 5221-92-0072-MD-001).
Kenyon RG & Wiedmann JL (1992b) Analytical methods including recovery
rates and the limits of determination for residues in soil. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 5221-92-0073-MD-001).
King C, Prince PM, & Ballee DL (1991) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1990). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-008).
King C, Prince PM, & Ballee DL (1992) Determination of residues of
tetrachloroisophthalonitrile (chlorothalonil, SDS-2787), its
degradation-products and manufacturing impurities in soil from West
Germany (Ostenwede and Rohlstof - 1991). Mentor, Ohio, ISK-Biotech
(Proprietary report No. 1667-87-0118-CR-010).
Koseki M, Shimamura Y, Maki T, Tamura Y, & Naoi Y (1980) Residues of
TPN (tetrachloroisophthalonitrile), captan, diazinon and
chinomethionate in vegetables and fruits. Tokyo Toriotu Eisei
Kenkyusho Kenkyu Nempo, 31:(1) 170-173.
Krawchuk BP & Webster GRB (1987) Movement of pesticides to ground
water in an irrigated soil. Water Pollut Res J Can, 22(1): 129-145.
Kunkel JF (1967a) Absence of 14C movement in crop plant organs after
topical application and soil amendment studies with isotopic Daconil
2787. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished report).
Kunkel JF (1967b Movement of 14C in or on roots of crop species grown
in soil amended with isotopic Daconil 2787. Mentor, Ohio, Diamond
Shamrock Corporation (Unpublished report).
Ladd K, Jenkins DH, & Kepplinger ML (1971) Meat and milk residues
study with technical Daconil 2787 - 97% and pure DAC-3701 in dairy
cattle. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Lam A & Lewis GC (1983) Chemical control of foliar diseases of
perennial rye grass (Lolium perenne L.) and their effects on yield
and quality of the crop. Crop Prot, 2(1): 75-83.
Lawless EW, Ferguson TL, & Meiners AF (1975) Guidelines for the
disposal of small quantities of unused pesticides. Cincinnati, Ohio,
US Environmental Protection Agency (EPA 670/2-75-057).
Lee SS, Marciniscyn JP, Marks AF, & Ignatoski JA (1982) Balance study
of the distribution of radioactivity following oral administration of
14C-chlorothalonil (14C-DS 2787) to rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 000-4AM-81-0209-001).
Legator MS (1974a) Mutagenic testing with DAC 2787. Providence, Rhode
Island, Roger Williams General Hospital, Division of Genetics and
Brown University, Division of Biological and Medical Sciences
(Unpublished report submitted to WHO by Diamond Shamrock Corporation,
Mentor, USA).
Legator MS (1974b) Mutagenic testing with DAC 3701. Mentor, Ohio,
Diamond Shamrock Corporation (Unpublished report).
Leonard JA & Yeary RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51(11): 605-609.
Lewis RG, Bond AE, Johnson DE, & Hsu JP (1988) Measurement of
atmospheric concentrations of common household pesticides : a pilot
study. Environ Monit Assess, 10: 59-73.
Liden C (1990) Facial dermatitis caused by chlorothalonil in paint.
Contact Dermatitis, 22: 206-211.
Lucas F & Benz G (1990) A two generation reproduction study in rats
with technical chlorothalonil. Painesville, Ohio, Ricerda, Inc.
(Proprietary report No. 1722-87-0121-TX-003, submitted to WHO by
ISK-Biotech, Mentor, USA).
Luperi L & Forster R (1988) Delayed contact hypersensitivity study in
the Guinea pig. Rome, Life Science Research, Toxicology Centre S.P.A.
(Report No. 128005-ST-02687, submitted to WHO by ISK-Biotech, Mentor,
USA).
McGee (1983) A two-year toxicity and tumourigenicity study of T-114 in
rats. Mattawan, Michigan, International Research and Development
Corporation (Unpublished report submitted to WHO by Diamond Shamrock
Corporation, Mentor, USA).
McLeod HA, Smith DC, & Bluman N (1980) Pesticide residues in the total
diet in Canada, V: 1976 to 1978. J Food Saf, 2: 141-164.
Magee TA, Marciniszyn JP, & Killeen JC Jr (1990) Study to evaluate the
urinary metabolites of chlorothalonil following dermal application to
male Rhesus monkeys. Painesville, Ohio, Ricerca, Inc. (Report No.
3382-89-02-AM-001, submitted to WHO by ISK-Biotech, Mentor, USA).
Major D, Killeen JC, & Ignatoski JA (1982) Primary eye irritation
study in albino rabbits with technical chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 104-5TX-80-0037-002).
Marciniszyn JP, Savides MC, Killeen JC, & Ignatoski JA (1984a) Study
of the dermal absorption of 14C-chlorothalonil (14C-DS-2787) by male
rats. Mentor, Ohio, Fermenta ASC (Unpublished report No.
649-4AM-84-0010-001).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1984b) Study of the
distribution of radioactivity following oral administration of
(14C-DS-2787) to male Sprague Dawley rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 631-4AM-83-0011-002).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1985a) Study of the
distribution of radioactivity following oral administration of
(14C-SDS-2787) to female Sprague Dawley rats. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 631-4AM-84-0078-002).
Marciniszyn JP, Killeen JC, & Ignatoski JA (1985b) Identification of
metabolites in urine and blood following oral administration of
14C-chlorothalonil (14C-SDS-2787) to male rats: the thiol metabolites
in urine. Mentor, Ohio, Fermenta ASC (Unpublished report No.
621-4AM-83-0061-001).
Marciniszyn JP, Savides MC, Killeen JC, & Ignatoski JA (1986) Study of
the biliary excretion of radioactivity following oral administration
of 14C-SDS-2787 to male Sprague-Dawley rats. Mentor, Ohio, Fermenta
ASC (Unpublished report No. 633-4AM-85-0012-002).
Marciniszyn JP, Killeen JC, & Baxter RA (1988) Pilot study of the
effect of the gamma-glutamyl transpeptidase inhibitor AT-125 on the
metabolism of 14C-chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1376-86-0072-AM-002).
Markus JR & Puma B ed. (1973) Pesticide analytical manual: Methods for
individual pesticide residues: Volume 2 - Pesticide registration
section 120.275. Washington, DC, US Food and Drug Administration.
Matsushita T & Aoyama K (1981) Cross reactions between some pesticides
and the fungicide benomyl in contact allergy. Ind Health, 19: 77-82.
Mayer FL ed. (1987) Acute toxicity handbook of chemicals to estuarine
organisms, Gulf Breeze, Florida. Washington, DC, US Environmental
Protection Agency (EPA-600/8-017; NTIS PB 87-188686).
Meding B (1986) Contact dermatitis from tetrachloroisopathalonitrile
in paint. Contact Dermatitis, 15: 187.
Mizens M, Wilson NH, Laveglia J, Killeen JC, & Ignatoski JA (1983) A
teratology study in rats with technical chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 517-5TX-82-0011-003).
Mizens M, Killeen JC, Claudio G, & Ignatoski JA (1985a) In vivo bone
marrow chromosomal aberration assay in mice with a single dose of
technical chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 625-5TX-83-0029-002).
Mizens M, Killeen JC, Claudio G, & Ignatoski JA (1985b) In vivo bone
marrow aberration assay in rats with a single dose of technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 625-5TX-83-0028-002).
Mizens M, Killeen JC, & Ignatoski JA (1985c) Acute and subchronic in
vivo bone marrow chromosomal aberration assay in Chinese hamsters
with technical chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 625-5TX-83-0014-003).
Mizens M, Killeen JC Jr, & Ignatoski JA (1985d) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with 2,5-dichloro-4,6-bismercaptoisoph-
thalonitrile (SDS-3939) (Study No. T4147.501 performed by
Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
SDS Biotech Corporation (Unpublished report No. 694-5TX-85-0042-002,
submitted to WHO by Fermenta Plant Protection Co., Painesville, USA).
Mizens M, Killeen JC Jr, & Ignatoski JA (1985e) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test)
with and without renal activation with 5-(2,4-dicyano-3,5,6-
trichlorophenyl) Glutathione (SDS-66382) (Study No. T4147.501
performed by Microbiological Associates Inc., Bethesda, USA).
Painesville, Ohio, SDS Biotech Corporation (Unpublished report
No. 694-5TX-85-0043-002, submitted to WHO by Fermenta Plant
Protection Co., Painesville, USA).
Mizens M, Killeen JC, & Ignatoski JA (1986a) In vitro chromosomal
aberration assay in Chinese hamster ovary (CHO) cells with technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1109-85-0082-TX-002).
Mizens M, Killeen JC Jr, & Baxter RA (1986b) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with 5-chloro-2,4,6-
trimercaptoisophthalonitrile (SDS-66471) (Study No. T5079.1505
performed by Microbiological Associates Inc., Bethesda, USA).
Painesville, Ohio, Ricerca, Inc., Department of Toxicology and Animal
Metabolism (Unpublished report No. 1097-86-0037-TX-002, submitted to
WHO by Fermenta Plant Protection Co., Painesville, USA).
Mizens M, Killeen JC Jr, & Baxter RA (1986c) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with S,S',S''--(2,4-dicyano-6-
dichlorophenyl)tricysteine (SDS-66473) (Study No. T5081.1505 performed
by Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
Ricerca, Inc., Department of Toxicology and Animal Metabolism
(Unpublished report No. 1097-86-0039-TX-002, submitted to WHO by
Fermenta Plant Protection Co., Painesville, USA.)
Mizens M, Killeen JC Jr, & Baxter RA (1987) Salmonella/
mammalian-microsome plate incorporation assay (Ames Test) with and
without renal activation with S,S'(2,4-dicyano-3,6-dichlorophenyl)-
dicysteine (SDS-66474) (Study No. T5080.1505 performed by
Microbiological Associates Inc., Bethesda, USA). Painesville, Ohio,
Ricerca, Inc., Department of Toxicology and Animal Metabolism
(Unpublished report No. 1097-86-0038-TX-002, submitted to WHO by
Fermenta Plant Protection Co., Painesville, USA).
Modesso P & Forster R (1988) Chromosome aberrations in human
lymphocytes cultured in vitro. Test substance: Chlorothalonil
technical. Rome, Life Science Research, Toxicology Centre S.P.A.
(Report No. 128008-M-10787, submitted to WHO by ISK-Biotech, Mentor,
USA).
Murchison TE (1979) A short-term dietary study in rats with T-114-2
(DS 3701). Dawson Research Corporation (Unpublished report submitted
to WHO by Diamond Shamrock Corporation, Mentor, USA).
O'Neill HJ (1991) Transport of the fungicide chlorothalonil from its
operational use on a pond ecosystem. Bull Environ Contam Toxicol,
46:822-828.
Paynter OE (1965a) Oral dose range: dogs - Final report. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE (1965b) Repeated dermal application: Rabbits. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE (1966a) Two-year dietary administration: dogs DAC 2787.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-66-0002-
001).
Paynter OE (1966b) Reproduction rabbits DAC 2787. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 000-5TX-66-003-001).
Paynter OE (1967a) Final report - Two-year dietary feeding: rats.
Hazleton Laboratories, Inc. (Unpublished report submitted to WHO by
Diamond Shamrock Corporation, Mentor, USA).
Paynter OE (1967b) Three-generation reproduction study: rats DAC 2787.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-67-0005-
001). USA.
Paynter OE (1967c) Ten-week amino acid feeding study: rats. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Paynter OE & Busey WM (1966) Two-year dietary administration: dogs
DAC 2787. Hazelton Laboratories, Inc. (Unpublished report submitted to
WHO by Biotech Corporation, Mentor, USA).
Paynter OE & Busey W (1967) Long-term (76 weeks) feeding study: rats
DAC 2787. Hazleton Laboratories, Inc. (Unpublished report submitted to
WHO by Diamond Shamrock Corporation, Mentor, USA).
Paynter OE & Crews L (1967) Final report - Two-year dietary feeding:
rats. Hazleton Laboratories, Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Paynter OE & Murphy JC (1967) 16-Week dietary administration: dogs
DAC 2787. Hazelton Laboratories, Inc. (Unpublished report submitted to
WHO by Biotech Corporation, Mentor, USA).
Powers M (1965) Acute oral administration: rats. Hazleton
Laboratories, Inc. (Unpublished report submitted to WHO by Diamond
Shamrock Corporation, Mentor, USA).
Price P (1978a) The activity of compound DTX-77-0037 in the Fischer
rat embryo transformation assay system. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Price P (1978b) The activity of compound DTX-77-0041 in the Fischer
rat embryo transformation assay system. Bethesda, Maryland,
Microbiological Associates Inc. (Unpublished report submitted to WHO
by Diamond Shamrock Corporation, Mentor, USA).
Reduker S, Uchrin CG, & Winnett G (1988) Characteristics of the
sorption of chlorothalonil and azinphos-methyl to a soil from a
commercial cranberry bog. Bull Environ Contam Toxicol, 41: 633-641.
Ribovich ML, Pollock GA, Marciniszyn JP, Killeen JC, & Ignatoski JA
(1983) Balance study of the distribution of radioactivity following
oral administration of 14C-chlorothalonil (14C-DS-2787) to male mice.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 613-4AM-82-0178-
001).
Roark JH & Dale JL (1979) The effect of turf fungicides on earthworms.
Arkansas Acad Sci Proc, 33: 71-74.
Rosanoff KA & Siegel MR (1981) Mechanism of action and fate of the
fungicide chlorothalonil in biological systems. 3. Interaction with
mammalian DNA, histones and isolated rat liver nuclei. Pestic Biochem
Physiol, 16: 120-128.
Sadler EM, Wilson NH, Killeen JC, & Ignatoski JA (1985) Time course of
the acute effect of technical chlorothalonil on hepatic and renal
glutathione content in male rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 751-5TX-85-0032-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1985)
Isolation and identification of metabolites in the bile of rats orally
administered 14C-chlorothalonil I. Synthesis and characterisation of
glutathione conjugates of chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 633-4AM-84-0104-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986a) Study
of the distribution of radioactivity following repeated oral
administration of 14C-chlorothalonil (14C-SDS-2787) to male
Sprague-Dawley rats. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1173-84-0079-AM-003).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986b)
Identification of metabolites in urine and blood following oral
administration of 14C-chlorothalonil to male rats. II. Effects of
multiple dose administration on the excretion of thiol metabolites in
urine. Mentor, Ohio, Fermenta ASC (Unpublished report No. 621-4AM-83-
0061-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986c) Study
of the biliary excretion of radioactivity following oral
administration of (14C-SDS-2787) to male Sprague-Dawley rats. Mentor,
Ohio, Fermenta ASC (Unpublished report No. 633-4AM-85-0012-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986d) Animal
metabolism method development studies. II. In vitro incubations of
14C-chlorothalonil with stomach and intestinal mucosal cells. Mentor,
Ohio, Fermenta ASC (Unpublished report No. 1172-85-0081-AM-002).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986e) In
vitro studies on the transfer of 14C-chlorothalonil and/or its
metabolites from the mucosal to the serosal surface of the
gastrointestinal tract. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1179-86-0020-AM-001).
Savides MC, Marciniszyn JP, Killeen JC, & Ignatoski JA (1986f) Pilot
study to determine the concentration of radiolabel in kidneys
following oral administration of the mono-glutathione conjugate of
14C-chlorothalonil to male rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 631-4AM-85-0064-001).
Savides MC, Marciniszyn JP, Killeen JC, & Baxter RA (1987a) Study to
determine the metabolic pathway for chlorothalonil following dermal
application to rats. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1625-87-0057-AM-000).
Savides MC, Marciniszyn JP, Killeen JC, & Baxter RA (1987b)
Determination of the covalent binding of radiolabel to DNA in the
kidneys of male rats administered 14C-chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1173-86-0096-AM-002).
Savides MC, Kidon BJ, Marciniszyn JP, Killeen JC, & Baxter RA (1987c)
Subcellular fractionation of kidneys from male rats administered
14C-chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1178-86-0016-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1988) A study to evaluate
the effects of sulphur-containing analogs of chlorothalonil on
mitochondrial function. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 1479-87-0037-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1989) Study to compare the
metabolism of chlorothalonil in dogs with its metabolism in rats
following oral administration of 14C-chlorothalonil. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1626-88-0008-AM-001).
Savides MC, Magee TA, Marciniszyn JP, Killeen JC, & Baxter RA (1990a)
Study to evaluate the metabolic pathway of chlorothalonil
(14C-ASC-2787) in germ-free rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 3060-88-0219-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1990b) Study of the urinary
excretion of radiolabel by catheterised dogs following oral
administration of 14C-chlorothalonil by gavage. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 3086-89-0041-AM-001).
Savides MC, Marciniszyn JP, & Killeen JC (1990c) Study to evaluate
urinary metabolites of chlorothalonil from male Rhesus monkeys.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 3349-89-0179-AM-
001).
SDS Biotech Corporation (1972) Exposure of fish to 14C-labelled
DAC-2787-Tech.: Accumulation, distribution and elimination of
residues. Mentor, Ohio, ISK-Biotech (Proprietary report No. 000-5TX-
72-0006/8-001).
SDS Biotech Corporation (1979) Acute toxicity of DS-3701 to Bluegill
sunfish. Mentor, Ohio, ISK-Biotech (Proprietary report No. 102-5TX-79-
0044-002).
SDS Biotech Corporation (1980a) Technical chlorothalonil - Acute
toxicity (LC50) study in Rainbow trout. Mentor, Ohio, ISK-Biotech
(Proprietary report No. 102-5TX-79-0045-002).
SDS Biotech Corporation (1980b) Technical chlorothalonil - Acute
toxicity (LC50) study in Channel catfish. Mentor, Ohio, ISK-Biotech
(Proprietary report No. 102-5TX-79-0046-002).
SDS Biotech Corporation (1981a) Dietary study (LC50) in Mallard ducks
with DS-3701. Mentor, Ohio, ISK-Biotech (Proprietary report No.
449-5TX-81-0008-002).
SDS Biotech Corporation (1981b) Dietary study (LC50) in Bobwhite
quail with DS-3701. Mentor, Ohio, ISK-Biotech (Proprietary report
No. 448-5TX-81-0007-002).
SDS Biotech Corporation (1982a) Static, acute toxicity study in
Penaeid (pink) shrimp with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0054-002).
SDS Biotech Corporation (1982b) Static, acute toxicity study in
Sheepshead Minnows with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0053-002).
SDS Biotech Corporation (1983a) The effects of cooking 2,4,5,6-
tetrachloroisoph-thalonitrile (chlorothalonil, DS-2787) with
vegetables. Mentor, Ohio, ISK-Biotech (Unpublished report No.
372-3EF-83-0004-001, submitted to WHO by SDS Biotech Corporation,
Mentor, USA).
SDS Biotech Corporation (1983b) Flow-through, acute oyster shell
deposition study with technical chlorothalonil. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 537-5TX-82-0133-003).
Shirasu Y & Teramoto S (1975) Teratogenicity study of Daconil in
rabbits. Mentor, Ohio, Fermenta ASC (Unpublished report No. 000-5TX-
75-2077-001).
Shirasu Y, Moriya M, & Watanabe K (1977) Mutagenicity testing on
Daconil in microbial systems. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 00-5TX-61-0002-001).
Shults SK, Killeen JC, & Heilman RD (1980) A chronic study in the
fathead minnow (Pimephales promelas) with technical chlorothalonil.
Cleveland, Ohio, Diamond Shamrock Corporation, 16 pp (Proprietary
report No. 090-5TX-79-0049-002, submitted to WHO by ISK-Biotech,
Mentor, USA.
Shults SK, Killeen JC, & Ignatoski JA (1982) Chronic toxicity study in
Daphnia magna with technical chlorothalonil. Cleveland, Ohio,
Diamond Shamrock Corporation, 25 pp (Proprietary report No.
447-5TX-81-0006-002, submitted to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Laveglia J, Killeen JC, & Ignatoski JA (1983) A 90-day
feeding study in mice with technical chlorothalonil. Mentor, Ohio, SDS
Biotech Corporation (Unpublished report No. 618-5TX-83-0007-004).
Shults SK, Wilson NH, Killeen JC, & Ignatoski JA (1986) 21-Day
repeated dose dermal toxicity study in albino rabbits with technical
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report No.
754-5TX-85-0023-007).
Shults SK, Wilson NH, & Killeen JC (1988a) Reproduction study in
bobwhite quail with technical chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 1469-87-0006-TX-002, submitted
to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Wilson NH, & Killeen JC (1988b) Reproduction study in
mallard ducks with technical chlorothalonil. Painesville, Ohio,
Ricerca, Inc. (Proprietary report No. 1469-87-0004-TX-002, submitted
to WHO by ISK-Biotech, Mentor, USA).
Shults SK, Brock AW, & Killeen JC (1991) Acute (one-hour) inhalation
toxicity (LC50) study in rats with hammer milled technical
chlorothalonil. Painesville, Ohio, Ricerca, Inc. (Proprietary report
No. 3593-90-0168-TX-002, submitted to WHO by ISK-Biotech, Mentor,
USA).
Shults SK, Brock AW, & Laveglia J (1993) Acute (four-hour) inhalation
toxicity (LC50) study in rats with hammer milled technical
chlorothalonil (T-117-15). Painesville, Ohio, Ricerca, Inc.
(Proprietary report No. 5290-92-0160-TX-002, submitted to WHO by
ISK-Biotech, Mentor, USA).
Siou G (1981a) The micronucleus test in the rat, mouse and hamster
using chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report
No. 000-5TX-81-0024-004).
Siou G (1981b) The chromosomal aberration test in the rat, mouse and
hamster using chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished
report No. 000-5TX-81-0025-004).
Smiley RW & Craven MM (1979) Microflora of turfgrass treated with
fungicides. Soil Biol Biochem, 11: 349-353.
Spencer JR, Bissell SR, Sanborn JR, Schneider FA, Margetich SS, &
Krieger RI (1991) Chlorothalonil exposure of workers on mechanical
tomato harvesters. Toxicol Lett, 55: 99-107.
Spindeldreier A & Deichmann B (1980) [Contact dermatitis against a
wood preservative with a new fungicidal agent.] Dermatosen, 28(3):
88-90 (in German).
Stallard DE & Wolfe AL (1967) The fate of 2,4,5,6-
tetrachloroisophthalonitrile (Daconil 2787) in soil. Mentor, Ohio,
Diamond Shamrock Corporation (Unpublished report).
Stallard DE & Szalkowski ME (1976) Effect of microorganisms upon the
soil metabolism of Daconil (i.e., chlorothalonil) and 4-hydroxy-2,5,6-
trichloroisophthalonitrile. Painesville, Ohio, Ricerca, Inc. (Report
No. 1000-3EF-76-2087-001, submitted to WHO by ISK Biosciences
Corporation, Mentor, USA).
Stamper JH, Nigg HN, Mahon WD, Nielsen AP, & Royer MD (1989a)
Applicator exposure to fluvalinate, chlorpyrifos, captan, and
chlorothalonil in Florida ornamentals. J Agric Food Chem, 37: 240-244.
Stamper JH, Nigg HN, Mahon WD, Nielsen AP, & Royer MD (1989b)
Pesticide exposure to a greenhouse drencher. Bull Environ Contam
Toxicol, 42: 209-217.
Stephenson GR, Phatak SC, Makowski RI, & Bouw WJ (1980) Phytotoxic
interactions involving metribuzin and other pesticides in tomatoes.
Can J Plant Sci, 60: 167-175.
Stoffella PJ & Sonoda RM (1982) Reduction of onion yields by
chlorothalonil. Hortscience, 17(4): 628-629.
Suzuki S & Oda M (1977) Simultaneous determination of chlorothalonil,
captan and diazinon in apples. Bull Agric Chem Insp Stn (Tokyo),
17:43-45.
Szalkowski MB & Stallard DE (1977) Effect of pH on the hydrolysis of
chlorothalonil. J Agric Food Chem, 25(1): 208-210.
Szalkowski MB, Stallard DE, & Ignatoski JA (1980) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon the
microbial utilization of selected macromolecules in soil. Mentor,
Ohio, ISK-Biotech (Proprietary report No. 326-3EI-79-0009-001).
Szalkowski MB, Stallard DE, & Ignatoski JA (1981a) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon
non-symbiotic nitrogen fixing soil microorganisms. Mentor, Ohio,
ISK-Biotech (Proprietary report No. 327-3EI-79-0010-001).
Szalkowski MB, Stallard DE, & Ignatoski JA (1981b) Effect of 2,4,5,6-
tetrachloroisophthalonitrile (chlorothalonil, DS-2787) upon nitrogen
transformation in soil. Mentor, Ohio, ISK-Biotech (Proprietary report
No. 328-3EI-79-0011-001).
Teeters W (1966) In vivo assay of spasmodic activity, mice, DAC
2787. Hazelton Laboratories (Unpublished report submitted to WHO by
Diamond Shamrock Corporation, Mentor, USA).
Tillman RW, Siegel MR, & Long JW (1973) Mechanism of action and fate
of the fungicide chlorothalonil (2,4,5,6-tetrachloroisophthalonitrile)
in biological systems I. Reactions with cells and subcellular
components of Saccharomyces pastorianus. Pestic Biochem Physiol,
3:160-167.
Tu CM (1980) Effect of fungicides on growth of Rhizobium japonicum
in vitro. Bull Environ Contam Toxicol, 25: 364-368.
UK (1985) Annex 1 - Monitoring of fruits and vegetables (at point of
retail sale) 1981-1983. Annex 2 - Chlorothalonil, soft fruit sampled
directly from growers 1982; soft fruit sampled at retail outlets, 1982
(no treatment histories). Submission to FAO by the UK Ministry of
Agriculture, June 14, 1985.
UK-MAFF & HSE (1990) Report of the working party on pesticide
residues 1988-1989. London, Ministry of Agriculture, Fisheries and
Food and Health and Safety Executive.
US EPA (1976) Manual of chemical methods for pesticides and devices.
Washington DC, US environmental Protection Agency, Office of Pesticide
Programs (Chlorothalonil EPA-1).
US EPA (1990) National survey of pesticides in drinking water wells.
Washington, DC, US Environmental Protection Agency (EPA 570/9-90-015).
US NCI (1978) Bioassay of chlorothalonil for possible carcinogenicity.
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
78-841).
Walker WW, Cripe CR, Pritchard PH, & Bourquin AW (1984) Biological and
abiotic degradation of xenobiotic compounds in in vitro estuarine
water and sediment/water systems. Chemosphere, 17(12): 2255-2270.
Wallace LA (1991) Comparison of risks from outdoor and indoor exposure
to toxic chemicals. Environ Health Perspect, 95: 7-13.
Wazeter FX (1971) Acute oral LD50 in male albino rats. International
Research and Development Corporation (Unpublished report submitted to
WHO by Diamond Shamrock Corporation, Mentor, USA).
Wazeter FX & Goldenthal EI (1976) Teratology study in rabbits.
International Research and Development Corporation (Unpublished report
submitted to WHO by Diamond Shamrock Corporation, Mentor, Ohio, USA).
Wei CI (1982) Lack of mutagenicity of the fungicide 2,4,5,6-
tetrachloroisophthalonitrile in the Ames Salmonella microsome test.
Appl Environ Microbiol, 43(1): 252-254.
WHO (1996) The WHO Recommended Classification of Pesticides by Hazard
and Guidelines to Classification 1996-1997. Geneva, World Health
Organization. (WHO/PCS/96.3).
Wilson NH (1981) A 90-day toxicity study of technical chlorothalonil
in rats. Mentor, Ohio, Diamond Shamrock Corporation (Unpublished
report).
Wilson NH, Killeen JC, & Ignatoski JA (1982) Dermal sensitization
study in Hartley-derived guinea-pigs with technical chlorothalonil.
Painesville, Ohio, Ricerca, Inc. (Report No. 394-5TX-81-0132-002,
submitted to WHO by ISK Biosciences Corporation, Mentor, USA).
Wilson NH, Laveglia J, Killeen JC, & Ignatoski JA (1983a) A subchronic
toxicity study of technical chlorothalonil in rats. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 562-5TX-81-0213-004).
Wilson NH, Killeen JC, & Ignatoski JA (1983b) A chronic dietary study
in mice with technical chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 108-5TX-79-0102-004).
Wilson NH, Killeen JC, & Ignatoski JA (1985a) Histopathological
re-evaluation of renal tissue from a sub-chronic toxicity study of
technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 753-5TX-85-0056-002).
Wilson NH, Killeen JC, & Ignatoski JA (1985b) A tumorigenicity study
of technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 099-5TX-80-0234-008).
Wilson NH, Killeen JC, & Ignatoski JA (1985c) Histopathologic
re-evaluation of renal tissue from a 90-day toxicity study of
technical chlorothalonil in rats (5TX-80-0200). Mentor, Ohio, SDS
Biotech (Unpublished report No. 753-5TX-85-0055-002).
Wilson NH, Killeen JC, & Ignatoski JA (1986a) Histopathological
re-evaluation of renal tissue from a rat tumorigenicity study with
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report No.
764-5TX-85-0071-002).
Wilson NH, Killeen JC, & Ignatoski JA (1986b) Histopathological
re-evaluation on renal tissue from a mouse tumorigenicity study with
chlorothalonil. Mentor, Ohio, Fermenta ASC (Unpublished report).
Wilson NH, Killeen JC, & Baxter RA (1987) A tumorigenicity study of
technical chlorothalonil in male mice - final report. Mentor, Ohio,
Fermenta ASC (Unpublished report No. 1099-84-0077-TX-006).
Wilson NH, Killeen JC, & Baxter RA (1988) A teratology study in
rabbits with technical chlorothalonil. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1544-87-0060-TX-002).
Wilson NH, Killeen JC, & Baxter RA (1989a) A tumorigenicity study of
technical chlorothalonil in rats. Mentor, Ohio, Fermenta ASC
(Unpublished report No. 1102-84-0103-TX-007).
Wilson NH, Chun JS, Killeen JC, & Baxter RA (1989b) Reproduction
dose-range finding study in rats with technical chlorothalonil.
Mentor, Ohio, Fermenta ASC (Unpublished report No. 1722-87-0120-TX-
001).
Wilson NH, Killeen JC Jr, Ford WH, Siou G, Busey WM, & Eilrich GL
(1990) A 90-day study in rats with the monoglutathione conjugate of
chlorothalonil. Toxicol Lett, 53: 155-156.
Wolfe AL & Stallard DE (1968) The fate of DAC-3701 (4-hydroxy-2,5,6,-
trichloroisophthalonitrile) in soil. Mentor, Ohio, Diamond Shamrock
Corporation (Unpublished report).
Wolfe AL & Stallard DE (1971) Supplementary milk and meat study.
Mentor, Ohio, Diamond Shamrock Corporation (Unpublished report).
Worthing CR ed. (1991) The pesticide manual: A world compendium, 9th
ed. Farnham, British Crop Protection Council.
Wüthrich V (1990) Acute-toxicity (LC50) study of Daconil 2787 extra
to earthworms (Project No. 282971). Itingen, Switzerland, RCC
Umweltchemie A.G. (Proprietary report submitted to WHO by ISK Biotech,
Mentor, USA).
Yoshikawa H & Kawai K (1966) Toxicity of phthalonitrile and
tetrachlorophthalodinitrile: I. Acute toxicity in mice. Ind Health,
4:11-15.
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le chlorothalonil est un solide cristallin incolore et inodore,
dont le point de fusion est de 250°C et la tension de vapeur de 7,63 ×
10-5 Pa (5,72 × 10-7 mmHg) à 25°C. Il est peu soluble dans l'eau
(0,6-1,2 mg/litre à 25°C) et son coefficient de partage entre
l'octanol et l'eau (log Kow) est de 2,882. Dans l'eau, il
s'hydrolyse lentement à pH 9 mais il est stable à pH < 7 (à 25°C).
La méthode d'analyse la plus courante, après extraction et
purification, est la chromatographie gaz-liquide avec détection par
capture d'électrons.
2. Sources d'exposition humaine et environnementale
Le chlorothalonil est produit depuis 1969 à des fins
commerciales, soit par chloration de l'isophtalonitrile, soit en
traitant le tétrachlorisophtaloylamide par l'oxychlorure de phosphore.
C'est un fongicide à large spectre utilisé non seulement en
agriculture, mais aussi pour traiter le gazon et les plantes
ornementales. On l'utilise pour protéger les fruits à pépins et à
noyaux, les agrumes, les groseilles, les baies, les bananes, les
tomates, les légumes verts, le café, les arachides, les pommes de
terre, les oignons et les céréales. En outre, il entre dans la
composition de certains produits pour la conservation du bois et de
certaines peintures.
Il existe en trois formulations principales, un concentré pour
suspensions, des granulés dispersables dans l'eau et une poudre
mouillable.Ces produits sont facilement dilués dans l'eau et épandus
par pulvérisations au sol ou aériennes. La dose d'emploi est
habituellement de 1,2 à 2,5 kg de matière active par hectare pour le
traitement des haricots, des céleris et des oignons. L'exposition
humaine a lieu principalement pendant la préparation et l'épandage du
produit ou lors de l'ingestion de résidus présents dans certaines
denrées alimentaires (voir section 1.1.4).
3. Transport, distribution et transformation dans l'environnement
Le chlorothalonil s'élimine des milieux aqueux par une forte
adsorption sur les particules en suspension. La modélisation des
données disponibles montre qu'il ne migre pratiquement pas vers les
sédiments du fond. Il est possible qu'il subisse une biodégradation
enzymatique dans les eaux naturelles. Dans le sol, il est rapidement
dégradé, cette dégradation pouvant également avoir lieu dans l'eau
avec production du métabolite hydroxylé en position 4, c'est-à-dire le
4-hydroxy-2,5,6-trichlorisophtalonitrile. La demi-vie de dissipation
du métabolite 4-hydroxy dans le sol est comprise entre 6 et 43 jours.
Une fois entré en contact avec une plante, le chlorothalonil ne
migre pas vers d'autres zones du végétal. Les végétaux ne le
métabolisent que dans une proportion limitée et le métabolite
4-hydroxy constitue en général moins de 5% du résidu.
Chez les poissons, la métabolisation du chlorothalonil comporte
une conjugaison avec le glutathion, qui aboutit à des produits
d'excrétion plus polaires. Elle s'effectue sous l'action de la
glutathion- S-transférase. L'excrétion du composé sous forme de
conjugué avec le glutathion est corroborée par le fait que, chez la
truite arc-en-ciel, on trouve une forte concentration de marqueur dans
la vésicule biliaire et dans la bile après exposition à du
14C-chlorothalonil.Une fois les poissons replacés en eau propre, on a
constaté une chute rapide de la concentration du marqueur qui s'était
accumulé dans la vésicule biliaire et les autres organes.
Le chlorothalonil ne s'accumule pas chez les organismes
aquatiques.
4. Concentrations dans l'environnement et exposition humaine
Lors d'une étude sur des cultures de pommes de terre, on a
pulvérisé du chlorothalonil sur un petit cours d'eau. Après
prélèvement et analyse de l'eau en aval du secteur traité, on a
constaté que le composé disparaissait rapidement (par ex. la
concentration passait de 450 µg/litre 30 minutes après le traitement à
2-6 µg/litre 12 h après le traitement). Les traitements de routine
effectués sur des cultures irriguées de plein champ, comme les pommes
de terre ou l'orge, n'ont donné lieu qu'à de faibles concentrations de
chlorothalonil (0,04-3,6 µg/litre), comme l'ont montré un certain
nombre d'analyses pratiquées sur de l'eau prélevée à quelques
occcasions dans des drains en grès vernissé.
Les résidus qui subsistent sur les récoltes sont essentiellement
constitués par le chlorothalonil lui-même. Leur concentration est
fonction de la dose d'emploi, du temps écoulé depuis le dernier
épandage et la dernière récolte ainsi que du type de culture. A
partir des nombreux essais effectués sous contrôle un peu partout dans
le monde et dont les résultats ont été communiqués à la FAO et à
l'OMS, il est possible de déterminer les concentrations de résidus
présentes au moment de la récolte. En ce qui concerne les produits
laitiers, il est vraisemblable que les résidus sont soit
indétectables, soit très faibles. Dans le lait de vaches laitières
qui avaient reçu pendant 30 jours du chlorothalonil mêlé à leur
nourriture (jusqu'à 250 mg/kg), on n'a pas trouvé trace du composé,
mais celui-ci était présent dans les tissus à très faible
concentration.
Des analyses pratiquées dans plusieurs pays, soit sur la ration
totale, soit sur tel ou tel alliment, ont montré, à l'occasions
d'enquêtes par sondage, que le chlorothalonil n'était présent qu'en
quantités indétectables ou du moins très faibles. Les diverses
préparations que subissent les produits alimentaires, comme le pelage,
le lavage et la cuisine en général contribuent d'ailleurs à abaisser
encore leur teneur en résidus.
5. Cinétique et métabolisme chez les animaux de laboratoire
Chez des rats qui en recevaient par voie orale des doses allant
jusqu'à 50 mg/kg de poids corporel, le composé a été absorbé à hauteur
d'environ 30% en l'espace de 48 h. A plus forte dose, l'absorption
est moindre, ce qui est le signe d'un processus de saturation. Après
administration de 14C-chlorothalonil par voie orale, on a observé une
répartition tissulaire et sanguine de la radioactivité en 2 h. C'est
au niveau des reins, du foie et du sang - dans cet ordre - qu'ont été
relevées les concentrations les plus imporantes. Au bout de 24 h, on
a mesuré, au niveau des reins, une concentration égale à 0.3% d'une
dose initiale de 5 mg/kg de poids corporel.
La majeure partie d'une dose administrée par voie orale à des
rats a été retrouvée dans leurs matières fécales (> 82% en l'espace
de 48-72 h, quelle que soit la dose initiale). L'excrétion biliaire
est rapide, culminant au bout de 2 h après ingestion d'une dose de 5
mg/kg de poids corporel et la saturation est atteinte à partir de 50
mg/kg de poids corporel. Chez le rat, la dose est excrétée à hauteur
de 5-10% par la voie urinaire. Chez le chien et le singe, la
prncipale voie d'excrétion est la voie fécale, la voie urinaire étant
moins importante que chez le rat (< 4%).
Les études métaboliques menées sur des rats montrent que le
chlorothalonil est conjugué avec le glutathion dans le foie ainsi que
dans les voies digestives. Certains de ces conjugués peuvent être
absorbés dans l'intestin et parvenir jusqu'aux reins où ils sont
transformés par la ß-lyase du cytosol en analogues thioliques,
excrétés ensuite par la voie urinaire. Lorsqu'on administre du
chlorothalonil à des rats axéniques, les métabolites thioliques
apparaissent dans l'urine en quantité bien moindre que chez des rats
normaux, ce qui indique que la flore intestinale intervient dans le
métabolisme de ce composé. Des chiens et des singes à qui on
administre du chlorothalonil par voie orale, n'excrètent que peu ou
pas de métabolites thioliques dans leurs urines.
Après application cutanée de 14C-chlorothalonil à des rats,
environ 28% de la dose ont été absorbés en 120 h. On a retrouvé
environ 18% de la dose dans les matières fécales et 6% dans les urines
au bout de 120 h.
6. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
Chez le rat et le lapin, la toxicité aiguë du chlorothalonil est
faible, que ce soit par voie orale ou en applications cutanée (DL50
> 10 000 mg/kg de poids corporel). Du chlorothalonil broyé au
mortier (D médian des particules égal à 5-8 µm) s'est révélé très
toxique pour des rats lors d'une étude toxicologique par inhalation,
avec une CL50 à 4 h de 0,1 mg/litre.
Le chlorothalonil est irritant pour la peau et les yeux chez le
lapin. Les études de sensibilisation cutanée effectuées sur des
cobayes n'ont pas été concluantes.
Chez le rat, les principaux effets de doses orales répétées
s'exercent au niveau des reins et de l'estomac.Pendant 13 semaines, on
fait ingérer quotidiennement à des rats, répartis en groupes de 25
animaux de chaque sexe, du chlorothalonil mêlé à leur nourriture aux
doses de 0, 1,5, 3, 10, ou 40 mg/kg de poids corporel. Après une
période de récupération de 13 semaines, les rats ont été sacrifiés et
l'on a observé une hyperplasie et une hyperkératose au niveau de la
portion cardiaque de l'estomac aux doses de 10 et 40 mg/kg; ces
lésions ont regressé lorsque le traitement a cessé. A la dose de 40
mg/kg, on notait chez les mâles une augmentation de l'incidence des
hyperplasies épithéliales au niveau des tubules proximaux du rein au
bout des 13 semaines de traitement ainsi qu'après la période de
récupération. La dose sans effet observable a été évaluée à 3 mg/kg
de poids corporel par jour, le critère retenu étant l'absence de
lésions au niveau de la portion cardiaque de l'estomac. Les lésions
interessant cette partie de l'eatomac ainsi que les reins sont
apparues rapidement, à savoir en 4 à 7 jours chez les mâles lorsqu'on
a porté la dose alimentaire quotidienne à 175 mg/kg de poids corporel.
Lors d'une étude de 13 semaines sur des souris (0, 7,5, 15, 50,
275, ou 750 mg/kg en mélange à la nourriture), on a constaté une
incidence accrue des hyperplasies et des hyperkératoses de
l'épithélium pavimenteux au niveau de la portion cardiaque de
l'estomac. Ces lésions ont été observées chez les mâles comme chez
les femelles à partir de 50 mg/kg de nourriture. En se basant sur la
présence ou l'absence de ces lésions, on a évalué à 15 mg/kg de
nourriture la dose de chlorothalonil sans effet observable, soit
l'équivalent quotidien de 3 mg/kg de poids corporel.
Une étude de 16 semaines sur des chiens aux doses alimentaires de
0, 250, 500, ou 750 mg/kg n'a pas révélé de d'effets qui soient
imputables au traitement.
Les lésions observées au niveau des reins et de la portion
cardiaque de l'estomac ont fait l'objet, pendant 2 ans, d'études plus
approfondies sur des souris et des chiens. Une autre étude, portant
cette fois sur des rats (aux doses quotidiennes de 0, 1,8, 3,8, 15 ou
175 mg/kg de poids corporel), a permis de caractériser histo-
logiquement les effets observés: il s'agissait d'une part, d'une
augmentation de l'incidence des hyperplasies, des hyperkératoses, des
ulcérations et des abrasions de l'épithélium pavimenteux au niveau de
la portion cardiaque de l'estomac et, d'autre part, de la présence
d'une hyperplasie au niveau des tubules contournés proximaux du rein.
Ces anomalies ont été observées à partir de 3,8 mg/kg. A la dose de
175 mg/kg, on notait un accroisssement sensible de l'incidence des
tumeurs rénales (adénomes et carcinomes) et des tumeurs interessant la
portion cardiaque de l'estomac (papillomes et carcinomes). On est
fondé à penser que l'incidence des tumeurs rénales était augmentée
chez les mâles à partir de la dose de 15 mg/kg, de même que celle des
tumeurs gastriques chez les deux sexes aux doses de 3,8 mg/kg et de 15
mg/kg. La dose sans effets néoplasiques observables a donc été prise
égale à 1,8 mg/kg, en prenant comme critère l' incidence des tumeurs
au niveau de la portion cardiaque de l'estomac. Une autre étude de 2
ans, au cours de laquelle des doses plus élevées ont été utilisées, a
confirmé le pouvoir cancérogène du chlorothalonil, tant au niveau du
rein que de la portion cardiaque de l'estomac.
Une étude sur des souris (doses de 0, 15, 40, 175, ou 750 mg/kg
de nourriture) a révélé une augmentation de l'incidence des
hyperplasies au niveau des tubules rénaux à partir de 175 mg/kg, le
même phénomène étant observé à partir de 40 mg/kg au niveau de la
portion cardiaque de l'estomac, avec en outre une hyperkératose. A la
dose de 750 mg/kg, il y avait une légère augmentation des tumeurs
spinocellulaires au niveau de la portion cardiaque de l'estomac. On
en a donc conclu que les doses sans effets néoplasiques ou non
néoplasiques étaient respectivement égales à 175 et 15 mg/kg de
nourriture (soit l' équivalent quotidien de 17,5 et 1,6 mg/kg de poids
corporel, respectivement). Ces effets constatés chez la souris sont
corroborés par les résultats d'une autre étude avec des doses plus
élevées, mais une troisième investigation portant sur des souris
B6C3F1 n'a pas mis en évidence d'effets cancérogènes à dose élevée.
Lors d'une étude de 2 ans sur des chiens (60 et 120 mg/kg de
nourriture), aucun effet attribuable au chlorothalonil n'a été
observé. On en conclu que la dose sans effet observable était de 120
mg/kg de nourriture (soit l'équivalent quotidien de 3 mg/kg de poids
corporel).
Plusieurs épreuves de mutagénicité in vivo et in vitro se
sont révélées négatives, mais il y en a tout de même eu quelques unes
de positives.
Les dérivés monothio, dithio, trithio, dicystéinyl, tricystéinyl
et monoglutathionyl du chlorothalonil, qui sont potentiellement
néphrotoxiques, se sont révélés négatifs dans l'épreuve d'Ames.
Le chlorothalonil ne s'est pas montré tératogène pour le rat ou
le lapin à des doses quotidiennes atteignant respectivement 400 et 50
mg/kg de poids corporel. Lors d'une étude sur deux générations de
rats, on n'a pas constaté d'effets sur l'accouplement, la fécondité ou
la gestation jusqu'à des doses atteignant 1500 mg/kg de nourriture.
La toxicité aiguë par voie orale du métabolite 4-hydroxy est
supérieure à celle du chlorothalonil lui-même (DL50 aiguë par voie
orale égale à 332 mg/kg de poids corporel contre > 10 000 mg/kg de
poids corporel). Plusieurs études ont été entreprises pour
caractériser le profil toxicologique de ce métabolite et établir les
doses sans effets observables.
7. Effets sur l'homme
On a signalé des cas de dermatite de contact parmi des personnes
employées à la fabrication de chlorothalonil, chez des agriculteurs et
des horticulteurs. D'autres cas de dermatite siégeant au niveau des
mains et de la face ont été observés chez des personnes qui
utilisaient des produits de protection du bois à base de
chlorothalonil.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Le chlorothalonil est extrêmement toxique pour les poissons et
les invertébrés aquatiques, comme le montrent un certain nombre
d'études en laboratoire, avec des valeurs de la CL50 inférieures à
0,5 mg/litre. La concentration maximale acceptable de produit toxique
(MATC) s'est révélée être égale à 35 µg/litre lors d'une étude sur
deux générations de daphnies.
A quelques exceptions près, d'ailleurs sans grande importance, le
chlorothalonil n'est pas phytotoxique.
La CL50 d'un concentré pour suspension (500 g de chlorothalonil
par litre) répandu sur un sol artificiel pour lombrics, a été évaluée
à > 1000 mg/kg de terre (14 jours). On a observé une surmortalité
parmi des scolopendres qui s'étaient trouvés en contact avec des
résidus de chlorothalonil présents sur des feuilles d'arachide ou s'en
étaient nourris au laboratoire; il n'y a pas eu d'autre indice d'une
action insecticide.
Le chlorothalonil est peu toxique pour les oiseaux, comme le
montre la valeur de la DL50 aiguë par voie orale chez le colvert
(4640 mg/kg). Aucun effet important sur la reproduction n'a été
signalé.
D'après une étude effectuée sur le terrain, la toxicité du
chlorothalonil pour les organismes aquatiques est moindre que ne le
font craindre les expériences de laboratoire; ce résultat est en
accord avec les propriétés physico-chimiques de ce composé. On a tout
de même enregistré une mortalité chez des espèces exposées
expérimentalement sur le terrain. En revanche, on n'a pas signalé
d'accidents écologiques ayant entraîné une mortalité. Malgré la
faible persistance du chlorothalonil dans les divers compartiments du
milieu, il faut tout de même s'attendre à une certaine mortalité. En
pareil cas, il sera difficile d'établir un lien de cause à effet étant
donné que les résidus de chlorothalonil ne subsistent pas suffisamment
lontemps pour que l'on puisse identifier le composé.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El clorotalonilo es un sólido cristalino inodoro e incoloro con
un punto de fusión de 250°C y una presión de vapor de 7,63 × 105 Pa
(5,72 × 10-7 mmHg) a 25°C. Es poco soluble en agua (0,6 a 1,2 mg/litro
a 25°C) y tiene un coeficiente de partición octanol/agua (log
Koa) de 2,882. Se hidroliza lentamente en agua con un pH de 9, pero
es estable a un pH de 7 o inferior (a 25°C).
El método analítico más corriente, después de la extracción y
depuración de las muestras, es la cromatografía gas-liquido empleando
un detector de captura de electrones.
2. Fuentes de exposición del ser humano y del medio ambiente
El clorotalonilo se viene produciendo a escala comercial desde
1969 por cloración del isoftalonitrilo o mediante el tratamiento de la
amida tetracloroisoftalolil con oxicloruro de fósforo. Es un fungicida
con amplio espectro de actividad empleado principalmente en la
agricultura pero también en el césped, los pastos y las plantas
ornamentales. Los cultivos protegidos incluyen frutas de pepitas y de
hueso, cítricos, grosellas, fresas, bananas, tomates, verduras, café,
cacahuete, patatas, cebollas y cereales. Se emplea también en
sustancias protectoras de la madera y en pinturas.
Las tres formulaciones principales son una suspensión
concentrada, un gránulo hidrodispersible y un polvo humectable. Se
disuelven fácilmente en agua y se aplican empleando sistemas de
pulverización en los suelos o rociado aéreo. Las tasas típicas del
ingrediente activo oscilan entre 1,2 y 2,5 kg/ha en el caso de
cultivos tales como frijoles, apio y cebollas. Las principales fuentes
de exposición del ser humano son la preparación y aplicación de los
productos, así como la ingestión de residuos de las cosechas en los
alimentos (véase la sección 1.1.4).
3. Transporte, distribución y transformación en el medio ambiente
El clorotalonilo se elimina de los medios acuosos mediante
intensa adsorción en las materias en suspensión. Los datos de los
modelos parecen indicar poca, o ninguna, partición detectable en el
sedimento del fondo. Puede producirse biodegradación en aguas
naturales, con la participación de procesos enzimáticos. El
clorotalonilo se degrada rápidamente en el suelo, y puede haber
degradación en el agua con la producción del metabolito 4-hidroxi-
2,5,6-tricloroisoftalonitrilo. La semivida para la disipación en el
suelo de ese metabolito varía entre 6 y 43 días.
El clorotalonilo no se transloca del punto de aplicación a otras
partes de la planta. Su metabolización en las plantas es limitada y,
por o general, ese metabolito representa < 5% del residuo.
En cuanto a los peces, el clorotalonilo se metaboliza mediante la
conjugación con glutatión para producir productos de degradación más
polares. En esa conversión interviene la enzima glutatión-
S-transferasa. Las elevadas concentraciones del radioisótopo
marcador detectadas en la vesícula biliar y la bilis después de la
exposición de la trucha arco iris al 14C-clorotalonilo son
compatibles con la excreción del compuesto en forma de conjugados de
glutatión. Las concentraciones de los radioisótopos de trazado que se
acumulan en la vesícula biliar y otros órganos disminuyen rápidamente
al colocar los peces en agua no contaminada.
El clorotalonilo no experimenta bioacumulación en los organismos
acuáticos.
4. Niveles ambientales y exposición humana
En un estudio de un cultivo de patatas, se procedió a rociar un
pequeño arroyo con clorotalonilo. Muestreos y análisis posteriores del
agua río abajo demostraron la rápida desaparición del clorotalonilo
(las concentraciones eran de 450 µg/litro a los 30 minutos después del
rociado, y oscilaban entre 2 y 6 µg/litro a las 12 h después del
rociado). El rociado sistemático de los cultivos irrigados como, por
ejemplo, patatas y cebada, estuvo acompañado de bajas concentraciones
de clorotalonilo (0,04 a 3,6 µg/litro) en el agua de los tubos de
drenaje en un pequeño número de muestras.
Los residuos de las cosechas están compuestos principalmente de
clorotalonilo propiamente dicho. Las concentraciones residuales
dependen del nivel aplicado, del tiempo transcurrido entre la última
aplicación y la cosecha, y del tipo de cosecha. Los niveles residuales
en la cosecha pueden deducirse de los numerosos ensayos supervisados
realizados con muchas cosechas en todo el mundo y comunicados a la FAO
y la OMS. Se prevé que los residuos de clorotalonilo en los productos
lácteos serán imposibles de detectar, o las concentraciones muy bajas.
En un estudio con vacas lecheras alimentadas durante 30 días con
pienso al que se habían añadido elevadas concentraciones (hasta 250
mg/kg) de clorotalonilo no se observó ningún residuo detectable en la
leche y sólo niveles muy bajos en los tejidos.
Los análisis del régimen alimenticio total y de alimentos
específicos realizados en varios países han revelado concentraciones
no detectables, o muy bajas, de clorotalonilo en los estudios por
muestreo. Los procesos de preparación tales como el lavado, el pelado
y la cocción permiten reducir aún más los niveles residuales en los
alimentos.
5. Cinética y metabolismo en animales de laboratorio
En estudios con ratas a las que se administraron dosis de hasta
50 mg/kg de peso corporal, se observó que un 30% de la dosis oral de
clorotalonilo se absorbía dentro de las 48 horas. La absorción es
inferior con posologías más elevadas, lo que indica un proceso de
saturación. Cuando se administra oralmente 14C-clorotalonilo, la
radioactividad se distribuye en la sangre y los tejidos en menos de
dos horas. Las mayores concentraciones se encuentran en el riñón, el
hígado y la sangre, en ese orden. Con una posología de 5 mg/kg de peso
corporal, la concentración en los riñones es 0,3% a las 24 horas.
Por lo que respecta a las ratas, la mayor parte de la dosis oral
de clorotalonilo se encuentra en las heces (> 82% dentro de las 48 a
72 horas, independientemente de la dosis). Con una dosis oral de 5
mg/kg de peso corporal, la excreción biliar es rápida, alcanzando su
valor máximo dentro de las 2 horas, ocurriendo saturación con
posologías de 50 mg/kg de peso corporal y superiores. En el caso de
las ratas, la excreción urinaria representa entre un 5 y 10% de la
dosis. En perros y monos, la excreción fecal es la vía principal, pero
la excreción urinaria (< 4%) es inferior a la observada en las ratas.
Estudios metabólicos llevados a cabo con ratas indican que el
clorotalonilo se conjuga con el glutatión tanto en el hígado como en
el tracto gastrointestinal. Algunos de los conjugados del glutatión
pueden ser absorbidos en el intestino y transportados a los riñones
donde son convertidos por la ß-liasa citosólica en análogos del tiol
que se excretan por la orina. Cuando se dan dosis de clorotalonilo a
ratas axénicas, se observan en la orina metabolitos del tiol en
cantidades muy inferiores a las registradas en ratas normales, lo que
indica que la microflora intestinal interviene en el metabolismo del
clorotalonilo. En el caso de los perros o monos que reciben dosis
orales de clorotalonilo, la excreción de derivados del tiol por la
orina no es detectable, o es muy baja.
Cuando se aplicó 14C-clorotalonilo a la piel de la rata, un 28%
de la dosis fue absorbida en menos de 120 horas. Se observaron
concentraciones del 18% de la dosis en las heces y del 6% en la orina
al cabo de 120 horas.
6. Efectos en mamíferos de laboratorio y sistemas de pruebas
in vitro
El clorotalonilo tiene baja toxicidad oral y cutánea en ratas y
conejos, respectivamente (los valores agudos orales y cutáneos de la
DL50 son > 10 000 mg/kg de peso corporal). Por lo que respecta a
las ratas, en un estudio de inhalación se observó que el clorotalonilo
técnico pulverizado (MMAD 5 a 8 µm) presentaba elevada toxicidad, con
una CL50 de 0,1 mg/litro a las 4 h.
El clorotalonilo es un irritante de la piel y los ojos en el
conejo. Los estudios de sensibilización cutánea en el conejillo de
indias no arrojaron resultados concluyentes.
En el caso de las ratas, los efectos principales de dosis orales
repetidas de clorotalonilo se observan en el estómago y los riñones.
En un estudio con grupos de 25 ratas, en que se separaron los sexos,
se emplearon posologías de 0, 1,5, 3, 10 ó 40 mg/kg de peso corporal
por día en la dieta durante 13 semanas, lo que estuvo seguido de un
período de recuperación de 13 semanas. Se observó mayor frecuencia de
hiperplasis y hiperketarosis del preestómago con las posologías de 10
y 40 mg/kg; los efectos desaparecieron cuando cesó el tratamiento. Con
una concentración de 40 mg/kg, se registro mayor incidencia de
hiperplasia del epitelio tubular proximal del riñón en los machos a
las 13 semanas y después del período de recuperación. El nivel sin
efecto observado fue de 3 mg/kg de peso corporal por día en base a la
ausencia de lesiones en el preestómago. Se ha demostrado que los
cambios observados en el preestómago y los riñones son de rápida
aparición, presentándose las lesiones dentro de un período de 4 a 7
días en el caso de los machos, cuyo régimen alimenticio incluía una
concentración de 175 mg/kg de peso corporal al día.
En un estudio de 13 semanas de duración realizado con ratones
(empleando dosis de 0, 7,5, 15, 50, 275 ó 750 mg/kg en el alimento),
se observó mayor incidencia de hiperplasia e hiperkeratosis de las
células epiteliales escamosas del preestómago en el caso de los machos
y las hembras cuando se emplearon posologías de 50 mg/kg y superiores.
Atendiendo a esos cambios, el nivel sin efecto observado fue de 15
mg/kg de clorotalonilo en la dieta, lo que equivale a 3 mg/kg de peso
corporal por día.
Un estudio de 16 semanas de duración con perros cuyo alimento
contenía concentraciones de 0, 250, 500 ó 750 mg/kg no reveló cambios
relacionados con el tratamiento.
Se llevaron a cabo investigaciones adicionales de las lesiones en
el preestómago y los riñones, llevándose a cabo estudios con ratas,
ratones y perros durante un período de 2 años. En un estudio realizado
con ratas (empleando dosis de 0, 1,8, 3,8, 15 ó 175 mg/kg de peso
corporal al día), los efectos estuvieron caracterizados
histológicamente por una mayor incidencia e intensidad de hiperplasia,
hiperkeratosis, y úlceras y erosiones de la mucosa escamosa del
preestómago, y por hiperplasia del epitelio de los túbulos
contorneados proximales de los riñones con posologías de 3,8 mg/kg y
superiores. Por lo que respecta a los efectos no neoplásicos, el nivel
sin efecto observado fue, por lo tanto, de 1,8 mg/kg. La incidencia de
tumores renales (adenomas y carcinomas) y de tumores del preestómago
(papilomas y carcinomas) fue considerablemente superior, alcanzando
175 mg/kg. Hubo pruebas de mayor incidencia de tumores renales en los
machos con dosis de 15 mg/kg, así como de tumores estomacales en los
machos y las hembras con dosis de 3,8 y 15 mg/kg. Por lo que respecta
a los efectos neoplásicos, el nivel sin efecto observado fue, por lo
tanto, de 1,8 mg/kg de peso corporal por día sobre la base de los
cambios en la incidencia de tumores en el preestómago. El riesgo
carcinogénico del clorotalonilo en los riñones y preestómago de las
ratas se vio corroborado por los resultados de otros estudios de 2
años de duración en que emplearon dosis más elevadas.
En un estudio con ratones (empleando dosis de 0, 15, 40, 175 ó
750 mg/kg en el alimento), se observó mayor incidencia de hiperplasia
tubular renal con dosis de 175 mg/kg y superiores, así como de
hiperplasia y hiperkeratosis del preestómago con concentraciones de 40
mg/kg y superiores. La incidencia de tumores escamosos del preestómago
aumentó ligeramente con dosis de 750 mg/kg. Por consiguiente, por lo
que respecta a los cambios neoplásicos y no neoplásicos, los niveles
sin efecto observado fueron de 175 y 15 mg/kg en la dieta (lo que
equivale a 17,5 y 1,6 mg/kg de peso corporal por día respectivamente).
Otro estudio con posologías superiores corroboró esos efectos en el
ratón, pero un estudio con ratones B6C3F1 no señaló ningún riesgo
carcinogénico con dosis elevadas.
En un estudio de 2 años de duración con perros (empleando 60 y
120 mg/kg en el alimento), no se detectó ningún efecto atribuible al
clorotalonilo. Por lo tanto, el nivel sin efecto observado fue de 120
mg/kg en el alimento (lo que equivale a 3 mg/kg de peso corporal al
día).
El clorotalonilo no resultó mutagénico en varias pruebas in
vitro e in vivo, aunque fue positivo en un pequeño número de
valoraciones.
Los derivados monotio, ditio, tritio, dicisteina, tricisteina y
monoglutatión del clorotalonilo, que son posibles substancias
nefrotóxicas, arrojaron resultados negativos en el análisis de Ames.
El clorotalonilo no resultó teratogénico en las ratas o conejos
con dosis de hasta 400 y 50 mg/kg de peso corporal al día,
respectivamente. En un estudio realizado con dos generaciones de
ratas, los parámetros reproductivos tales como el apareamiento, la
fertilidad y el período de gestación no se vieron afectados por el
clorotalonilo con concentraciones de hasta 1500 mg/kg en la dieta.
La toxicidad oral aguda del metabolito 4-hidróxido es superior a
la del clorotalonilo propiamente dicho (DL50 oral aguda de 332 mg/kg
de peso corporal en comparación con > 10 000 mg/kg de peso corporal).
Se han llevado a cabo varios estudios destinados a caracterizar el
perfil toxicológico de ese metabolito y a establecer los niveles sin
efecto observado.
7. Efectos en el ser humano
Se ha informado de dermatitis por contacto en el caso de personas
que trabajan en la producción de clorotalonilo, así como en el de
agricultores y hortelanos. Se tienen noticias también de trabajadores
de fábricas de productos de la madera que han contraído dermatitis por
contacto en las manos y el rostro cuando empleaban preservativos de la
madera que contenían clorotalonilo.
8. Efectos en otros organismos en el laboratorio y sobre el terreno
El clorotalonilo es sumamente tóxico para los peces y los
invertebrados acuáticos en los estudios de laboratorio, siendo los
valores de CL50 inferiores a 0,5 mg/litro. En un estudio sobre
reproducción realizado con dos generaciones de Daphnia magna, la
concentración tóxica máxima aceptable (CTMA) fue de 35 µg/litro.
Con pocas excepciones, el clorotalonilo no es fitotóxico.
En lombrices, la CL50 de una formulación de una suspensión
concentrada (500 g de clorotalonilo/litro) en suelo artificial fue
> 1000 mg/kg de suelo (14 días). Las tijeretas experimentaron mayor
mortalidad cuando estaban en contacto con residuos de clorotalonilo en
las hojas del cacahuete o lo ingerían en su fuente de alimentos en las
pruebas de laboratorio; no hubo ningún otro indicio de efecto
insecticida.
El clorotalonilo tiene poca toxicidad para las aves, habiéndose
informado de una DL50 oral aguda de 4640 mg/kg de alimento en el
pato real. No se informó de ningún efecto considerable sobre la
reproducción.
Un estudio sobre el terreno de organismos acuáticos expuestos
después de la aplicación de clorotalonilo parece indicar que la
toxicidad es inferior a la que cabría esperar atendiendo a los
estudios de laboratorio; esto es también compatible con las
propiedades fisicoquímicas de los compuestos. Se observaron muertes en
algunas especies expuestas experimentalmente en el campo. No se ha
informado de incidentes de muertes en el medio ambiente. Sin embargo,
a pesar del corto tiempo de presencia del clorotalonilo en el medio
ambiente, cabría esperar que ocurran muertes. Resultaría difícil
establecer vínculos entre las muertes y los compuestos ya que la
persistencia de los residuos no seria suficientemente prolongada para
poder identificar el clorotalonilo.
